
Title: The Cambridge natural history, Vol. 05 (of 10)
Editor: S. F. Harmer
Author: Adam Sedgwick
David Sharp
F. G. Sinclair
Editor: Sir A. E. Shipley
Release date: November 6, 2023 [eBook #72052]
Language: English
Original publication: London: Macmillan and Co, 1895
Credits: Keith Edkins, Peter Becker and the Online Distributed Proofreading Team at https://www.pgdp.net (This file was produced from images generously made available by The Internet Archive)
THE
CAMBRIDGE NATURAL HISTORY
EDITED BY
S. F. HARMER, M.A., Fellow of King's College, Cambridge; Superintendent of the University Museum of Zoology
AND
A. E. SHIPLEY, M.A., Fellow of Christ's College, Cambridge; University Lecturer on the Morphology of Invertebrates
VOLUME V
PERIPATUS
By Adam Sedgwick, M.A., F.R.S., Fellow and Lecturer of Trinity College, Cambridge
MYRIAPODS
By F. G. Sinclair, M.A., Trinity College, Cambridge
INSECTS
PART I. Introduction, Aptera, Orthoptera, Neuroptera, and a portion of Hymenoptera (Sessiliventres and Parasitica)
By David Sharp, M.A. (Cantab.), M.B. (Edinb.), F.R.S.
London
MACMILLAN AND CO.
AND NEW YORK
1895
All rights reserved
"Creavit in cœlo Angelos, in terra vermiculos: non superior in illis, non inferior in istis. Sicut enim nulla manus Angelum, ita nulla posset creare vermiculum."—Saint Augustine, Liber soliloquiorum animae ad Deum, Caput IX.
| PAGE | |
| Scheme of the Classification adopted in this book | ix |
| PERIPATUS | |
| CHAPTER I | |
| Introduction—External Features—Habits—Breeding—Anatomy—Alimentary Canal—Nervous System—The Body Wall—The Tracheal System—The Muscular System—The Vascular System—The Body Cavity—Nephridia—Generative Organs—Development—Synopsis of the Species—Summary of Distribution | 3 |
| MYRIAPODA | |
| CHAPTER II | |
| Introduction—Habits—Classification—Structure—Chilognatha—Chilopoda—Schizotarsia—Symphyla—Pauropoda—Embryology—Palaeontology | 29 |
| INSECTA | |
| CHAPTER III | |
| Characteristic Features of Insect Life—Social Insects—Definition of the Class Insecta—Composition of Insect Skeleton—Number of Segments—Nature of Sclerites—Head—Appendages of the Mouth—Eyes—Thorax—Entothorax—Legs—Wings—Abdomen or Hind Body—Spiracles—Systematic Orientation | 83 |
| {vi}
CHAPTER IV |
|
| Arrangement of Internal Organs—Muscles—Nervous System—Ganglionic Chain—Brain—Sense-Organs—Alimentary Canal—Malpighian Tubes—Respiration—Tracheal System—Function of Respiration—Blood or Blood-chyle—Dorsal Vessel or Heart—Fat-body—Ovaries—Testes—Parthenogenesis—Glands | 114 |
| CHAPTER V | |
| DEVELOPMENT | |
| Embryology—Eggs—Micropyles—Formation of Embryo—Ventral Plate—Ectoderm and Endoderm—Segmentation—Later Stages—Direct Observation of Embryo—Metamorphosis—Complete and Incomplete—Instar—Hypermetamorphosis—Metamorphosis of Internal Organs—Integument—Metamorphosis of Blowfly—Histolysis—Imaginal Discs—Physiology of Metamorphosis—Ecdysis | 143 |
| CHAPTER VI | |
| Classification—The Nine Orders of Insects—Their Characters—Packard's Arrangement—Brauer's Classification—Classifications based on Metamorphosis—Super-Orders—The Subdivisions of Orders | 171 |
| CHAPTER VII | |
| The Order Aptera—Definition—Chief Characteristics—Thysanura—Campodea—Japyx—Machilis—Lepisma—Diversity of Internal Structure in Thysanura—Ectotrophi and Entotrophi—Collembola—Lipuridae—Poduridae—Smynthuridae—The Spring—The Ventral Tube—Abdominal Appendages—Prostemmatic Organ—Tracheal System—Anurida maritima—Collembola on Snow—Life-Histories of Collembola—Fossil Aptera—Apterygogenea—Antiquity and Distribution of Campodea | 180 |
| CHAPTER VIII | |
| Orthoptera—Forficulidae, Earwigs—Hemimeridae | 198 |
| {vii}
CHAPTER IX |
|
| Orthoptera continued—Blattidae, Cockroaches | 220 |
| CHAPTER X | |
| Orthoptera continued—Mantidae, Soothsayers | 242 |
| CHAPTER XI | |
| Orthoptera continued—Phasmidae, Walking-Leaves, Stick-Insects | 260 |
| CHAPTER XII | |
| Orthoptera continued—Acridiidae, Locusts, Grasshoppers | 279 |
| CHAPTER XIII | |
| Orthoptera continued—Locustidae, Green Grasshoppers, Katydids | 311 |
| CHAPTER XIV | |
| Orthoptera continued—Gryllidae, Crickets | 330 |
| CHAPTER XV | |
| Neuroptera—Mallophaga—Embiidae | 341 |
| CHAPTER XVI | |
| Neuroptera continued—Termitidae, Termites or White Ants | 356 |
| {viii}
CHAPTER XVII |
|
| Neuroptera continued—Psocidae (Book-Lice and Death-Watches)—The First Family of Amphibious Neuroptera (Perlidae, Stone-Flies) | 390 |
| CHAPTER XVIII | |
| Amphibious Neuroptera continued—Odonata, Dragon-Flies | 409 |
| CHAPTER XIX | |
| Amphibious Neuroptera continued—Ephemeridae, May-Flies | 429 |
| CHAPTER XX | |
| Neuroptera Planipennia—Sialidae, Alder-Flies, Snake-Flies—Panorpidae, Scorpion-Flies—Hemerobiidae, Ant-Lions, Lacewings, etc. | 444 |
| CHAPTER XXI | |
| Neuroptera continued—Trichoptera, the Phryganeidae or Caddis-Flies | 473 |
| CHAPTER XXII | |
| Hymenoptera—Hymenoptera Sessiliventres—Cephidae—Oryssidae—Siricidae—Tenthredinidae or Sawflies | 487 |
| CHAPTER XXIII | |
| Hymenoptera Petiolata—Parasitic Hymenoptera—Cynipidae or Gall-Flies—Proctotrypidae—Chalcididae—Ichneumonidae—Braconidae—Stephanidae—Megalyridae—Evaniidae—Pelecinidae—Trigonalidae | 519 |
| Index | 567 |
| PROTOTRACHEATA |
| Peripatus (p. 1) |
| MYRIAPODA | |
| Order. | Family. |
| CHILOGNATHA (= DIPLOPODA) |
Polyxenidae (p. 43). Glomeridae (p. 43). Sphaerotheriidae (p. 43). Julidae (p. 43). Blanjulidae (p. 44). Chordeumidae (p. 44). Polydesmidae (p. 44). Polyzoniidae (p. 44). |
| CHILOPODA |
Lithobiidae (p. 45). Scolopendridae (p. 45). Notophilidae (p. 45). Geophilidae (p. 46). |
| SCHIZOTARSIA | Cermatiidae (= Scutigeridae) (p. 46). |
| SYMPHYLA. | Scolopendrellidae (p. 46). |
| PAUROPODA | Pauropidae (p. 47). |
| INSECTA | ||||
| Order. | Division, Series, or Sub-Order. | Family. | Tribe or Sub-Family. | Group. |
| APTERA (p. 180) | Thysanura (p. 182) |
Campodeidae (p. 183). Japygidae (p. 184). Machilidae (p. 184). Lepismidae (p. 185). |
||
| Collembola (p. 189) |
Lipuridae (p. 190). Poduridae (p. 190). Smynthuridae (p. 191). |
|||
| ORTHOPTERA (p. 198) | Orthoptera cursoria |
Forficulidae (p. 202). Hemimeridae (p. 217). |
||
| Blattidae (p. 220) |
Ectobiides. Phyllodromiides. Nyctiborides. Epilamprides. Periplanetides. Panchlorides. Blaberides. Corydiides. Oxyhaloides. Perisphaeriides. Panesthiides. ? Geoscapheusides. |
|||
| Mantidae (p. 242) |
Amorphoscelides. Orthoderides. Mantides. Harpagides. Vatides. Empusides. |
|||
| Phasmidae (p. 260) |
Lonchodides. Bacunculides. Bacteriides. Necroscides. Clitumnides. Acrophyllides. Cladomorphides. Anisomorphides. Phasmides. Aschipasmides. Bacillides. Phylliides. |
|||
| Orthoptera saltatoria |
Acridiidae (p. 279) |
Tettigides. Pneumorides. Mastacides. Proscopiides. Tryxalides. Oedipodides. Pyrgomorphides. Pamphagides. Acridiides. |
||
| Locustidae (p. 311) |
Phaneropterides. Meconemides. Mecopodides. Prochilides. Pseudophyllides. Conocephalides. Tympanophorides. Sagides. Locustides. Decticides. Callimenides. Ephippigerides. Hetrodides. Gryllacrides. Stenopelmatides. |
|||
| Gryllidae (p. 330) |
Tridactylides. Gryllotalpides. Myrmecophilides. Gryllides. Oecanthides. Trigonidiides. Eneopterides. |
|||
| NEUROPTERA (p. 341) | Mallophaga (p. 345) |
Leiotheides. Philopterides. |
||
| Pseudoneuroptera |
Embiidae (p. 351). Termitidae (p. 356). Psocidae (p. 390). |
|||
| Neuroptera Amphibiotica |
Perlidae (p. 398). | |||
| Odonata (p. 409) | Anisopterides |
Gomphinae. Cordulegasterinae. Aeschninae. Corduliinae. Libellulinae. |
||
| Zygopterides |
Calepteryginae. Agrioninae. |
|||
| Ephemeridae (p. 429). | ||||
| Neuroptera planipennia |
Sialidae (p. 444) |
Sialides. Raphidiides. |
||
| Panorpidae (p. 449). | ||||
| Hemerobiidae (p. 453) | Myrmeleonides (p. 454). | |||
| Ascalaphides (p. 459) |
Holophthalmi. Schizophthalmi. |
|||
|
Nemopterides (p. 462). Mantispides (p. 463). |
||||
| Hemerobiides (p. 465) |
Dilarina. Nymphidina. Osmylina. Hemerobiina. |
|||
|
Chrysopides (p. 469). Coniopterygides (p. 471). |
||||
| Trichoptera | Phryganeidae (p. 473) |
Phryganeides (p. 480). Limnophilides (p. 481). Sericostomatides (p. 482). Leptocerides (p. 482). Hydropsychides (p. 482). Rhyacophilides (p. 483). Hydroptilides (p. 484). |
||
| HYMENOPTERA (p. 487) | Hymenoptera Sessiliventres |
Cephidae (p. 504). Oryssidae (p. 506). Siricidae (p. 507). Tenthredinidae (p. 510). |
||
| Hymenoptera Petiolata (part) |
Cynipidae (p. 523). Proctotrypidae (p. 533). Chalcididae (p. 539). Ichneumonidae (p. 551). Braconidae (p. 558). Stephanidae (p. 561). Megalyridae (p. 562). Evaniidae (p. 562). Pelecinidae (p. 563). Trigonalidae (p. 564). |
|||
| (To be continued in Vol. VI.) | ||||
| PROTOTRACHEATA |
| Peripatus (p. 1) |
| MYRIAPODA | ||
| Order. | Family. | |
| CHILOGNATHA (= DIPLOPODA) |  |
Polyxenidae (p. 43). Glomeridae (p. 43). Sphaerotheriidae (p. 43). Julidae (p. 43). Blanjulidae (p. 44). Chordeumidae (p. 44). Polydesmidae (p. 44). Polyzoniidae (p. 44). |
| CHILOPODA |  |
Lithobiidae (p. 45). Scolopendridae (p. 45). Notophilidae (p. 45). Geophilidae (p. 46). |
| SCHIZOTARSIA | Cermatiidae (= Scutigeridae) (p. 46). | |
| SYMPHYLA. | Scolopendrellidae (p. 46). | |
| PAUROPODA | Pauropidae (p. 47). | |
| INSECTA | ||||||||
| Order. | Division, Series, or Sub-Order. |
Family. | Tribe or Sub-Family. | Group. | ||||
| APTERA (p. 180) |  |
Thysanura (p. 182) |  |
Campodeidae (p. 183). Japygidae (p. 184). Machilidae (p. 184). Lepismidae (p. 185). |
||||
| Collembola (p. 189) |  |
Lipuridae (p. 190). Poduridae (p. 190). Smynthuridae (p. 191). |
||||||
| {x}
ORTHOPTERA (p. 198) |
 |
Orthoptera cursoria |
 |
Forficulidae (p. 202). Hemimeridae (p. 217). |
||||
| Blattidae (p. 220) |  |
Ectobiides. Phyllodromiides. Nyctiborides. Epilamprides. Periplanetides. Panchlorides. Blaberides. Corydiides. Oxyhaloides. Perisphaeriides. Panesthiides. ? Geoscapheusides. |
||||||
| Mantidae (p. 242) |  |
Amorphoscelides. Orthoderides. Mantides. Harpagides. Vatides. Empusides. |
||||||
| Phasmidae (p. 260) |  |
Lonchodides. Bacunculides. Bacteriides. Necroscides. Clitumnides. Acrophyllides. Cladomorphides. Anisomorphides. Phasmides. Aschipasmides. Bacillides. Phylliides. |
||||||
| Orthoptera saltatoria |
 |
Acridiidae (p. 279) |  |
Tettigides. Pneumorides. Mastacides. Proscopiides. Tryxalides. Oedipodides. Pyrgomorphides. Pamphagides. Acridiides. |
||||
| Locustidae (p. 311) |  |
Phaneropterides. Meconemides. Mecopodides. Prochilides. Pseudophyllides. Conocephalides. Tympanophorides. Sagides. Locustides. Decticides. Callimenides. Ephippigerides. Hetrodides. Gryllacrides. Stenopelmatides. |
||||||
| {xi}
Gryllidae (p. 330) |
 |
Tridactylides. Gryllotalpides. Myrmecophilides. Gryllides. Oecanthides. Trigonidiides. Eneopterides. |
||||||
| NEUROPTERA (p. 341) |  |
Mallophaga (p. 345) |  |
 |
Leiotheides. Philopterides. |
|||
| Pseudoneuroptera |  |
Embiidae (p. 351). Termitidae (p. 356). Psocidae (p. 390). |
||||||
| Neuroptera Amphibiotica |
 |
Perlidae (p. 398). | ||||||
| Odonata (p. 409) |  |
Anisopterides |  |
Gomphinae. Cordulegasterinae. Aeschninae. Corduliinae. Libellulinae. |
||||
| Zygopterides |  |
Calepteryginae. Agrioninae. |
||||||
| Ephemeridae (p. 429). | ||||||||
| Neuroptera planipennia |
 |
Sialidae (p. 444) |  |
Sialides. Raphidiides. |
||||
| Panorpidae (p. 449). | ||||||||
| Hemerobiidae (p. 453) |  |
Myrmeleonides (p. 454). | ||||||
| Ascalaphides (p. 459) |  |
Holophthalmi. Schizophthalmi. |
||||||
|
Nemopterides (p. 462). Mantispides (p. 463). |
||||||||
| Hemerobiides (p. 465) |  |
Dilarina. Nymphidina. Osmylina. Hemerobiina. |
||||||
|
Chrysopides (p. 469). Coniopterygides (p. 471). |
||||||||
| Trichoptera |  |
Phryganeidae (p. 473) |  |
Phryganeides (p. 480). Limnophilides (p. 481). Sericostomatides (p. 482). Leptocerides (p. 482). Hydropsychides (p. 482). Rhyacophilides (p. 483). Hydroptilides (p. 484). |
||||
| HYMENOPTERA (p. 487) |  |
Hymenoptera Sessiliventres |
 |
Cephidae (p. 504). Oryssidae (p. 506). Siricidae (p. 507). Tenthredinidae (p. 510). |
||||
| Hymenoptera Petiolata (part) |
 |
Cynipidae (p. 523). Proctotrypidae (p. 533). Chalcididae (p. 539). Ichneumonidae (p. 551). Braconidae (p. 558). Stephanidae (p. 561). Megalyridae (p. 562). Evaniidae (p. 562). Pelecinidae (p. 563). Trigonalidae (p. 564). |
||||||
| (To be continued in Vol. VI.) | ||||||||
BY
ADAM SEDGWICK, M.A., F.R.S.
Fellow of Trinity College, Cambridge.
PERIPATUS
INTRODUCTION–EXTERNAL FEATURES–HABITS–BREEDING–ANATOMY–ALIMENTARY CANAL–NERVOUS SYSTEM–THE BODY WALL–THE TRACHEAL SYSTEM–THE MUSCULAR SYSTEM–THE VASCULAR SYSTEM–THE BODY CAVITY–NEPHRIDIA–GENERATIVE ORGANS–DEVELOPMENT–SYNOPSIS OF THE SPECIES–SUMMARY OF DISTRIBUTION.
The genus Peripatus was established in 1826 by Guilding,[1] who first obtained specimens of it from St. Vincent in the Antilles. He regarded it as a Mollusc, being no doubt deceived by the slug-like appearance given by the antennae. Specimens were subsequently obtained from other parts of the Neotropical region and from South Africa and Australia, and the animal was variously assigned by the zoologists of the day to the Annelida and Myriapoda. Its true place in the system, as a primitive member of the group Arthropoda, was first established in 1874 by Moseley,[2] who discovered the tracheae. The genus has been monographed by Sedgwick,[3] who has also written an account of the development of the Cape species.[4] A bibliography will be found in Sedgwick's Monograph.
There can be no doubt that Peripatus is an Arthropod, for it possesses the following features, all characteristic of that group, and all of first-class morphological importance: (1) The presence of appendages modified as jaws; (2) the presence of paired lateral ostia perforating the wall of the heart and putting its cavity in communication with the pericardium; (3) the presence of a vascular body cavity and pericardium (haemocoelic body cavity); (4) absence of a perivisceral section of the coelom. Finally, the tracheae, though not characteristic of all the classes of the Arthropoda, are found nowhere outside that group, and constitute a very important additional reason for uniting Peripatus with it.
Peripatus, though indubitably an Arthropod, differs in such important respects from all the old-established Arthropod classes, that a special class, equivalent in rank to the others, and called Prototracheata, has had to be created for its sole occupancy. This unlikeness to other Arthropoda is mainly due to the Annelidan affinities which it presents, but in part to the presence of the following peculiar features: (1) The number and diffusion of the tracheal apertures; (2) the restriction of the jaws to a single pair; (3) the disposition of the generative organs; (4) the texture of the skin; and (5) the simplicity and similarity of all the segments of the body behind the head.
The Annelidan affinities are superficially indicated in so marked a manner by the thinness of the cuticle, the dermo-muscular body wall, the hollow appendages, that, as already stated, many of the earlier zoologists who examined Peripatus placed it amongst the segmented worms; and the discovery that there is some solid morphological basis for this determination constitutes one of the most interesting points of the recent work on the genus. The Annelidan features are: (1) The paired nephridia in every segment of the body behind the first two (Saenger, Balfour[5]); (2) the presence of cilia in the generative tracts (Gaffron). It is true that neither of these features are absolutely distinctive of the Annelida, but when taken in conjunction with the Annelidan disposition of the chief systems of organs, viz. the central nervous system, and the main vascular trunk or heart, may be considered as indicating affinities in that {5}direction. Peripatus, therefore, is zoologically of extreme interest from the fact that, though in the main Arthropodan, it possesses features which are possessed by no other Arthropod, and which connect it to the group to which the Arthropoda are in the general plan of their organisation most closely related. It must, therefore, according to our present lights, be regarded as a very primitive form; and this view of it is borne out by its extreme isolation at the present day. Peripatus stands absolutely alone as a kind of half-way animal between the Arthropoda and Annelida. There is no gradation of structure within the genus; the species are very limited in number, and in all of them the peculiar features above mentioned are equally sharply marked.
Peripatus, though a lowly organised animal, and of remarkable sluggishness, with but slight development of the higher organs of sense, with eyes the only function of which is to enable it to avoid the light—though related to those animals most repulsive to the aesthetic sense of man, animals which crawl upon their bellies and spit at, or poison, their prey—is yet, strange to say, an animal of striking beauty. The exquisite sensitiveness and constantly changing form of the antennae, the well-rounded plump body, the eyes set like small diamonds on the side of the head, the delicate feet, and, above all, the rich colouring and velvety texture of the skin, all combine to give these animals an aspect of quite exceptional beauty. Of all the species which I have seen alive, the most beautiful are the dark green individuals of Capensis, and the species which I have called Balfouri. These animals, so far as skin is concerned, are not surpassed in the animal kingdom. I shall never forget my astonishment and delight when on bearing away the bark of a rotten tree-stump in the forest on Table Mountain, I first came upon one of these animals in its natural haunts, or when Mr. Trimen showed me in confinement at the South African Museum a fine fat, full-grown female, accompanied by her large family of thirty or more just-born but pretty young, some of which were luxuriously creeping about on the beautiful skin of their mother's back.
External Features.
The anterior part of the body may be called the head, though it is not sharply marked off from the rest of the body (Fig. 1).
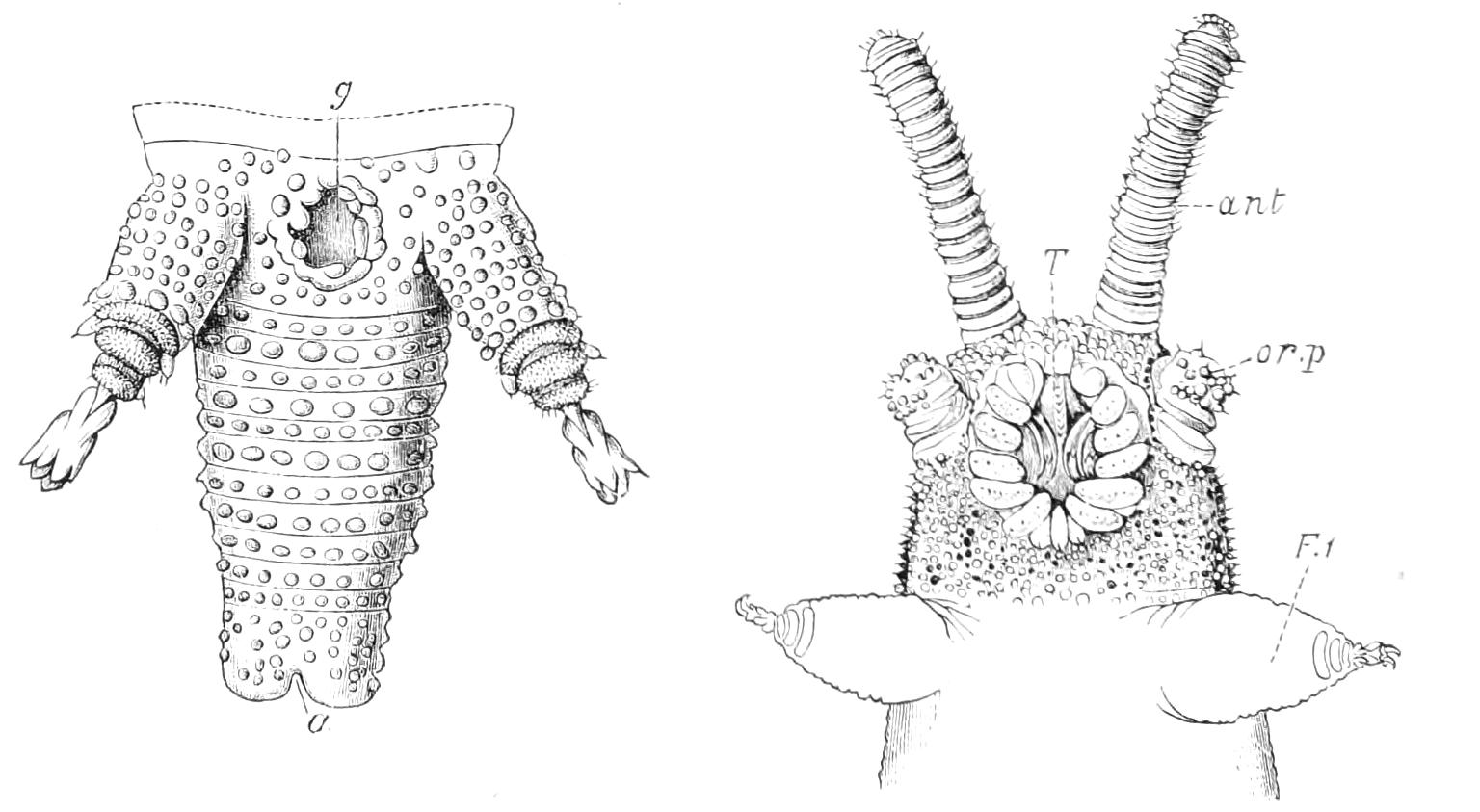
Fig. 2.—Ventral view of hind-end of P. Novae-Zealandiae. (After Sedgwick.) g, Generative opening; a, anus.
Fig. 3.—Ventral view of the head of P. capensis. (After Sedgwick.) ant, Antennae; or.p, oral papillae; F.1, first leg; T, tongue.
The head carries three pairs of appendages, a pair of simple eyes, and a ventrally placed mouth. The body is elongated and vermiform; it bears a number of paired appendages, each terminating in a pair of claws, and all exactly alike. The number varies in the different species. The anus is always at the posterior end of the body, and the generative opening is on the ventral surface just in front of the anus; it may be between the legs of the last pair (Fig. 2), or it may be behind them. There is in most species a thin median white line extending the whole length of the dorsal surface of the body, on each side of which the skin pigment is darker than elsewhere. The colour varies considerably in the different species, and even in different individuals of the same species. The ventral surface is nearly always flesh-coloured, while the dorsal surface has a darker colour. In the {7}South African species the colour of the dorsal surface varies from a dark green graduating to a bluish gray, to a brown varying to a red orange. The colour of the Australasian species varies in like manner, while that of the Neotropical species (S. American and W. Indian) is less variable. The skin is thrown into a number of transverse ridges, along which wart-like papillae are placed. The papillae, which are found everywhere, are specially developed on the dorsal surface, less so on the ventral. Each papilla carries at its extremity a well-marked spine.
The appendages of the head are the antennae, the jaws and the oral papillae.
The antennae, which are prolongations of the dorso-lateral parts of the head, are ringed, and taper slightly till near their termination, where they are slightly enlarged. The rings bear a number of spines, and the free end of the antennae is covered by a cap of spiniferous tissue like that of the rings.
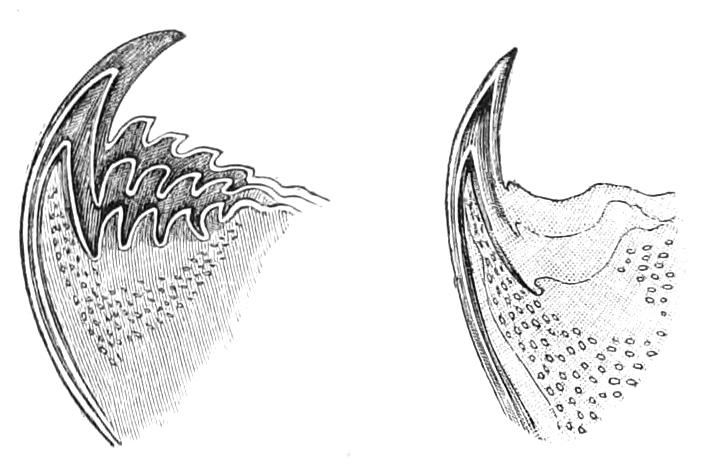
Fig. 4.—Inner jaw-claw of P. capensis. (After Balfour.)
Fig. 5.—Outer jaw-claw of P. capensis. (After Balfour.)
The mouth is at the hinder end of a depression called the buccal cavity, and is surrounded by an annular tumid lip, raised into papilliform ridges and bearing a few spines (Fig. 3). Within the buccal cavity are the two jaws. They are short, stump-like, muscular structures, armed at their free extremities by a pair of cutting blades or claws, and are placed one on each side of the mouth. In the median line of the buccal cavity in front is placed a thick muscular protuberance, which may be called the tongue, though attached to the dorsal instead of to the ventral wall of the mouth (Fig. 3). The tongue bears a row of small chitinous teeth. The jaw-claws (Figs. 4 and 5), which resemble in all essential points the claws borne by the feet, and like these are thickenings of the cuticle, are sickle-shaped. They have their convex edge directed forwards and their concave or cutting edge turned backwards. The inner cutting plate (Fig. 4) usually bears a number of cutting teeth. The jaws appear to be used for tearing the food, to which the mouth adheres by means of the tumid suctorial lips. The oral papillae are placed at the sides of the head (Fig. 3). The {8}ducts of the slime-glands open at their free end. They possess two main rings of projecting tissue, and their extremities bear papillae irregularly arranged.
The ambulatory appendages vary in number. There are seventeen pairs in P. capensis and eighteen in P. Balfouri, while in P. Edwardsii the number varies from twenty-nine to thirty-four pairs. They consist of two main divisions, which we may call the leg and the foot (Figs. 6 and 7). The leg (l) has the form of a truncated cone, the broad end of which is attached to the ventro-lateral wall of the body, of which it is a prolongation. It is marked by a number of rings of papillae placed transversely to its long axis, the dorsal of which are pigmented like the dorsal surface of the body, and the ventral like the ventral surface. At the narrow distal end of the leg there are on the ventral surface three spiniferous pads, each of which is continued dorsally into a row of papillae.
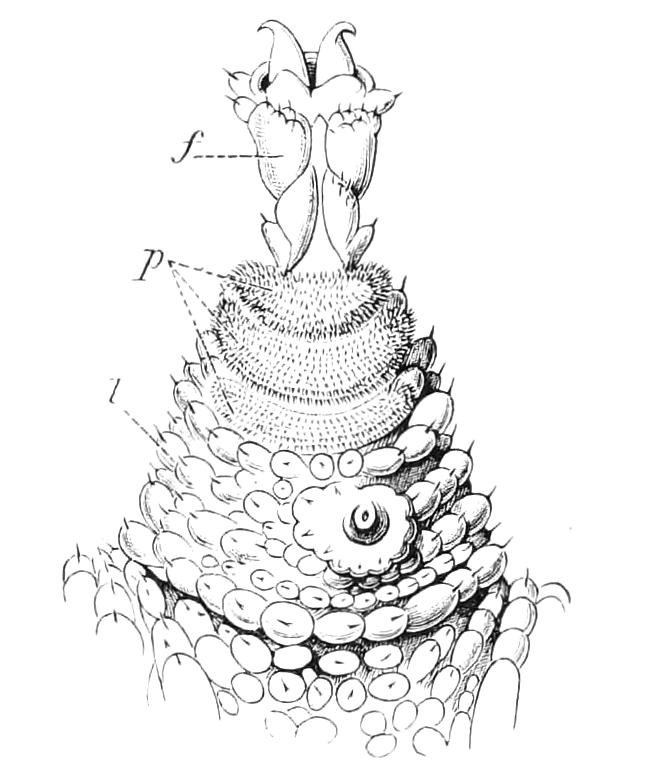
Fig. 6.—Ventral view of last leg of a male P. capensis. (After Sedgwick.) f, Foot; l, leg; p, spiniferous pads. The white papilla on the proximal part of this leg is characteristic of the male of this species.
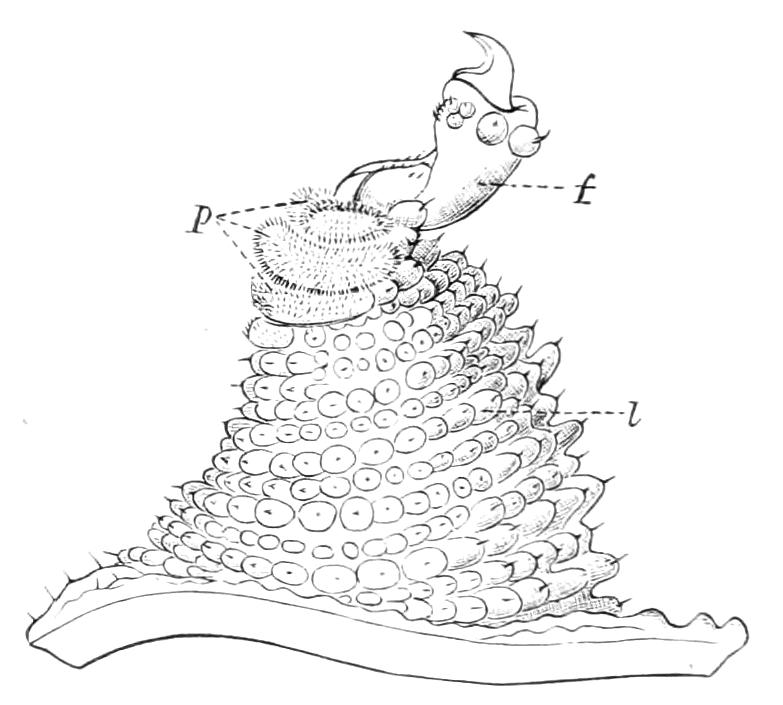
Fig. 7.—Leg of P. capensis seen from the front. (After Sedgwick.) f, Foot; l, leg; p, spiniferous pads.
The foot is attached to the distal end of the leg. It is slightly narrower at its attached extremity than at its free end. It bears two sickle-shaped claws and a few papillae. The part of the foot which carries the claws is especially retractile, and is generally found more or less telescoped into the proximal part. The legs of the fourth and fifth pairs differ from the others in {9}the fact that the proximal pad is broken up into three, a small central and two larger lateral. The enlarged nephridia of these legs open on the small central division.
The males are generally rather smaller than the females. In those species in which the number of legs varies, the male has a smaller number of legs than the female.
Habits.
They live beneath the bark of rotten stumps of trees, in the crevices of rock, and beneath stones. They require a moist atmosphere, and are exceedingly susceptible to drought. They avoid light, and are therefore rarely seen. They move with great deliberation, picking their course by means of their antennae and eyes. It is by the former that they acquire a knowledge of the ground over which they are travelling, and by the latter that they avoid the light. The antennae are extraordinarily sensitive, and so delicate, indeed, that they seem to be able to perceive the nature of objects without actual contact. When irritated they eject with considerable force the contents of their slime reservoirs from the oral papillae. The force is supplied by the sudden contraction of the muscular body wall. They can squirt the slime to the distance of almost a foot. The slime, which appears to be perfectly harmless, is extremely sticky, but it easily comes away from the skin of the animal itself.
I have never seen them use this apparatus for the capture of prey, but Hutton describes the New Zealand species as using it for this purpose. So far as I can judge, it is used as a defensive weapon; but this of course will not exclude its offensive use. They will turn their heads to any part of the body which is being irritated and violently discharge their slime at the offending object. Locomotion is effected entirely by means of the legs, with the body fully extended.
Of their food in the natural state we know little; but it is probably mainly, if not entirely, animal. Hutton describes his specimens as sucking the juices of flies which they had stuck down with their slime, and those which I kept in captivity eagerly devoured the entrails of their fellows, and the developing young from the uterus. They also like raw sheep's liver. They move their mouths in a suctorial manner, tearing the food with their jaws. They have the power of extruding their jaws from {10}the mouth, and of working them alternately backwards or forwards. This is readily observed in individuals immersed in water.
Breeding.
All species are viviparous. It has been lately stated that one of the Australian species is normally oviparous, but this has not been proved. The Australasian species come nearest to laying eggs, inasmuch as the eggs are large, full of yolk, and enclosed in a shell; but development normally takes place in the uterus, though, abnormally, incompletely developed eggs are extruded.
The young of P. capensis are born in April and May. They are almost colourless at birth, excepting the antennae, which are green, and their length is 10 to 15 mm. A large female will produce thirty to forty young in one year. The period of gestation is thirteen months, that is to say, the ova pass into the oviducts about one month before the young of the preceding year are born. They are born one by one, and it takes some time for a female to get rid of her whole stock of embryos; in fact, the embryos in any given female differ slightly in age, those next the oviduct being a little older (a few hours) than those next the vagina. The mother does not appear to pay any special attention to her young, which wander away and get their own food.
There does not appear to be any true copulation. The male deposits small, white, oval spermatophores, which consist of small bundles of spermatozoa cemented together by some glutinous substance, indiscriminately on any part of the body of the female. Such spermatophores are found on the bodies of both males and females from July to January, but they appear to be most numerous in our autumn. It seems probable that the spermatozoa make their way from the adherent spermatophore through the body wall into the body, and so by traversing the tissues reach the ovary. The testes are active from June to the following March. From March to June the vesiculae of the male are empty.
There are no other sexual differences except in some of the South African species, in which the last or penultimate leg of the male bears a small white papilla on its ventral surface (Fig. 6).
Whereas in the Cape species embryos in the same uterus are all practically of the same age (except in the month of April, when two broods overlap in P. capensis), and birth takes place at a fixed season; in the Neotropical species the uterus, which is {11}always pregnant, contains embryos of different ages, and births probably take place all the year round.
In all species of Peripatus the young are fully formed at birth, and differ from the adults only in size and colour.
ANATOMY
The Alimentary Canal (Fig. 8).
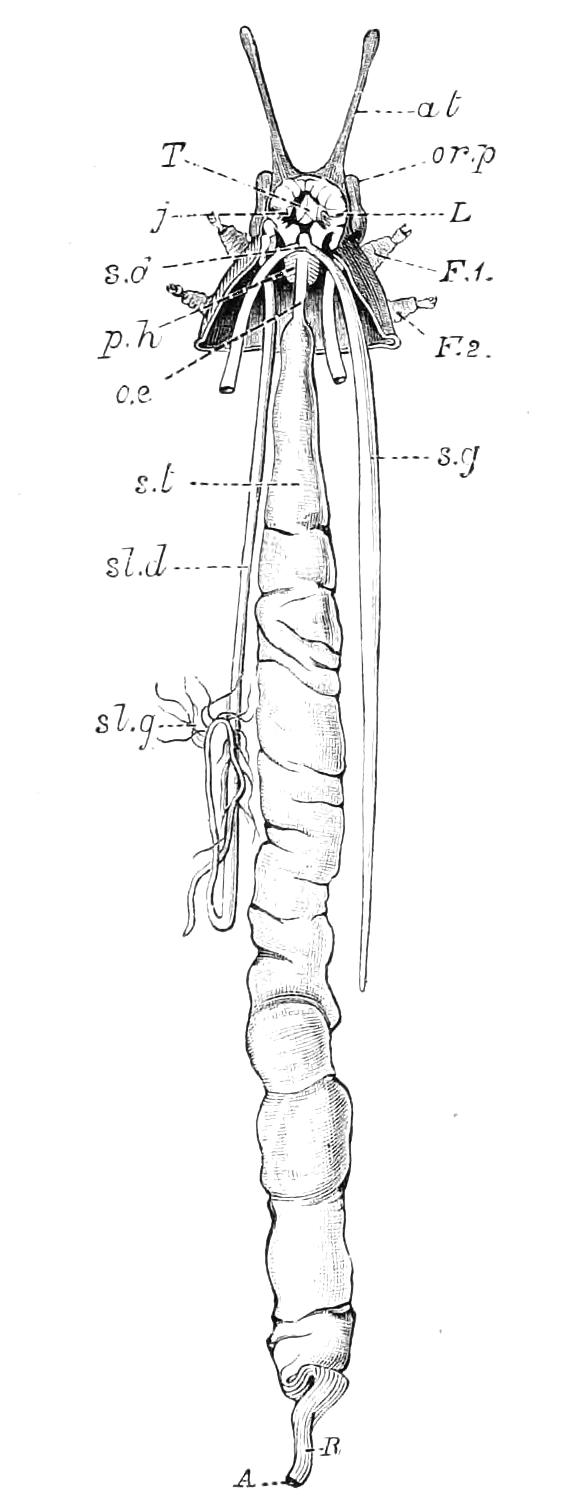
Fig. 8.—Peripatus capensis dissected so as to show the alimentary canal, slime glands, and salivary glands. (After Balfour.) The dissection is viewed from the ventral side, and the lips (L) have been cut through in the middle line behind and pulled outwards so as to expose the jaws (j), which have been turned outwards, and the tongue (T) bearing a median row of chitinous teeth, which branches behind into two. The muscular pharynx, extending back into the space between the first and second pairs of legs, is followed by a short tubular oesophagus. The latter opens into the large stomach with plicated walls, extending almost to the hind end of the animal. The stomach at its point of junction with the rectum presents an S-shaped ventro-dorsal curve. A, Anus; at, antenna; F.1, F.2, first and second feet; j, jaws; L, lips; oe, oesophagus; or.p, oral papilla; ph, pharynx; R, rectum; s.d, salivary duct; s.g, salivary gland; sl.d, slime reservoir; sl.g, portion of tubules of slime gland; st, stomach; T, tongue in roof of mouth.
The buccal cavity, as explained above, is a secondary formation around the true mouth, which is at its dorsal posterior end. It contains the tongue and the jaws, which have already been described, and into the hind end of it there opens ventrally by a median opening the salivary glands (s.g). The mouth leads into a muscular pharynx (ph), which is connected by a short oesophagus (oe) with a stomach (st). The stomach forms by far the {12}largest part of the alimentary canal. It is a dilated soft-walled tube, and leads behind into the short narrow rectum (R), which opens at the anus. There are no glands opening into the alimentary canal.
Nervous System.
The central nervous system consists of a pair of supra-oesophageal ganglia united in the middle line, and of a pair of widely divaricated ventral cords, continuous in front with the supra-oesophageal ganglia (Fig. 9).
The ventral cords at first sight appear to be without ganglionic thickenings, but on more careful examination they are found to be enlarged at each pair of legs (Fig. 9). These enlargements may be regarded as imperfect ganglia. There are, therefore, as many pairs of ganglia as there are pairs of legs. There is in addition a ganglionic enlargement at the commencement of the oesophageal commissures, where the nerves to the oral papillae are given off (Fig. 9, or.g).
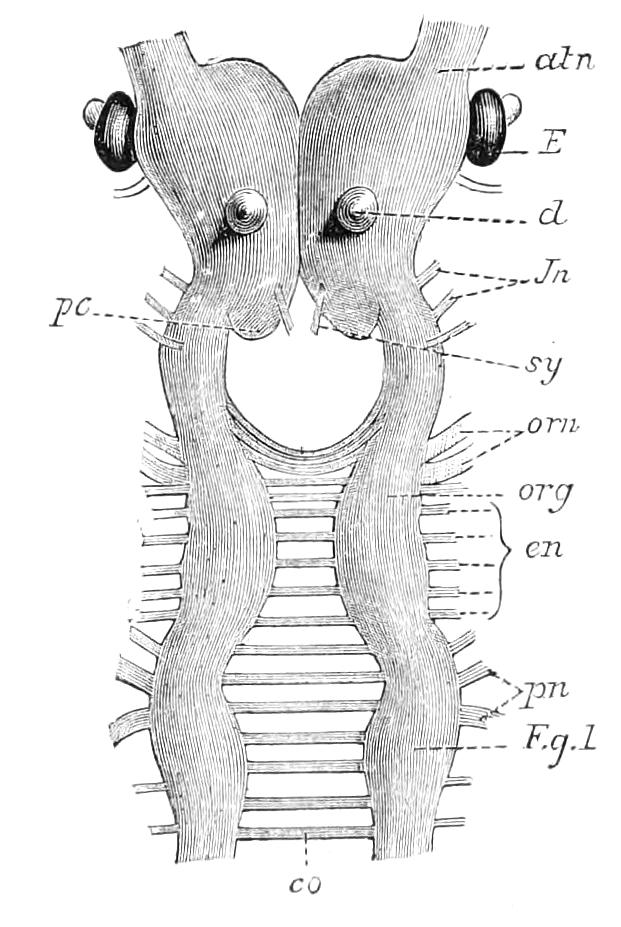
Fig. 9.—Brain and anterior part of the ventral nerve-cords of Peripatus capensis enlarged and viewed from the ventral surface. (After Balfour.) The paired appendages (d) of the ventral surface of the brain are seen, and the pair of sympathetic nerves (sy) arising from the ventral surface of the hinder part. From the commencement of the oesophageal commissures pass off on each side a pair of nerves to the jaws (Jn). The three anterior commissures between the ventral nerve-cords are placed close together; immediately behind them the nerve-cords are swollen, to form the ganglionic enlargements from which pass off to the oral papillae a pair of large nerves on each side (orn). Behind this the cords present a series of enlargements, one pair for each pair of feet, from which a pair of large nerves pass off on each side to the feet (pn). atn, Antennary nerves; co, commissures between ventral cords; d, ventral appendages of brain; E, eye; en, nerves passing outwards from ventral cord; F.g.1, ganglionic enlargements from which nerves to feet pass off; jn, nerves to jaws; org, ganglionic enlargement from which nerves to oral papillae pass off; orn, nerves to oral papillae; pc, posterior lobe of brain; pn, nerves to feet; sy, sympathetic nerves.
The ventral cords are placed each in the lateral compartments of the body cavity, immediately within the longitudinal layer of muscles. They are connected with each other, rather like the pedal nerves of Chiton and the lower Prosobranchiata, by a number of commissures. These commissures exhibit a {13}fairly regular arrangement from the region included between the first and the last pair of true feet. There are nine or ten of them between each pair of feet. They pass along the ventral wall of the body, perforating the ventral mass of longitudinal muscles. On their way they give off nerves which innervate the skin.
Posteriorly the two nerve-cords nearly meet immediately in front of the generative aperture, and then, bending upwards, fall into each other dorsally to the rectum. They give off a series of nerves from their outer borders, which present throughout the trunk a fairly regular arrangement. From each ganglion two large nerves (pn) are given off, which, diverging somewhat from each other, pass into the feet.
From the oesophageal commissures, close to their junction with the supra-oesophageal ganglia, a nerve arises on each side which passes to the jaws, and a little in front of this, apparently from the supra-oesophageal ganglion itself, a second nerve to the jaws also takes its origin.
The supra-oesophageal ganglia (Fig. 9) are large, somewhat oval masses, broader in front than behind, completely fused in the middle, but free at their extremities. Each of them is prolonged anteriorly into an antennary nerve, and is continuous behind with one of the oesophageal commissures. On the ventral surface of each, rather behind the level of the eye, is placed a hollow protuberance (Fig. 9, d), of which I shall say more in dealing with the development. About one-third of the way back the two large optic nerves take their origin, arising laterally, but rather from the dorsal surface (Fig. 9). Each of them joins a large ganglionic mass placed immediately behind the retina.
The histology of the ventral cords and oesophageal commissures is very simple and uniform. They consist of a cord almost wholly formed of nerve-fibres placed dorsally, and of a ventral layer of ganglion cells.
The Body Wall.
The skin is formed of three layers.
(1) The cuticle.
(2) The epidermis or hypodermis.
(3) The dermis.
The cuticle is a thin layer. The spines, jaws, and claws are special developments of it. Its surface is not, however, smooth, {14}but is everywhere, with the exception of the perioral region, raised into minute secondary papillae, which in most instances bear at their free extremity a somewhat prominent spine. The whole surface of each of the secondary papillae just described is in its turn covered by numerous minute spinous tubercles.
The epidermis, placed immediately within the cuticle, is composed of a single layer of cells, which vary, however, a good deal in size in different regions of the body. The cells excrete the cuticle, and they stand in a very remarkable relation to the secondary papillae of the cuticle just described. Each epidermis cell is in fact placed within one of these secondary papillae, so that the cuticle of each secondary papilla is the product of a single epidermis cell. The pigment which gives the characteristic colour to the skin is deposited in the protoplasm of the outer ends of the cells in the form of small granules.
At the apex of most, if not all, the primary wart-like papillae there are present oval aggregations, or masses of epidermis cells, each such mass being enclosed in a thickish capsule and bearing a long projecting spine. These structures are probably tactile organs. In certain regions of the body they are extremely numerous; more especially is this the case in the antennae, lips, and oral papillae. On the ventral surface of the peripheral rings of the thicker sections of the feet they are also very thickly set and fused together so as to form a kind of pad (Figs. 6 and 7). In the antennae they are thickly set side by side on the rings of skin which give such an Arthropodan appearance to these organs in Peripatus.
The Tracheal System.
The apertures of the tracheal system are placed in the depressions between the papillae or ridges of the skin. Each of them leads into a tube, which may be called the tracheal pit (Fig. 10), the walls of which are formed of epithelial cells bounded towards the lumen of the pit by a very delicate cuticular membrane continuous with the cuticle covering the surface of the body. The pits vary somewhat in depth; the pit figured was about 0.09 mm. It perforates the dermis and terminates in the subjacent muscular layer.
Internally it expands in the transverse plane and from the expanded portion the tracheal tubes arise in diverging bundles. Nuclei similar in character to those in the walls of the tracheal {15}pit are placed between the tracheae, and similar but slightly more elongated nuclei are found along the bundles. The tracheae are minute tubes exhibiting a faint transverse striation which is probably the indication of a spiral fibre. They appear to branch, but only exceptionally. The tracheal apertures are diffused over the surface of the body, but are especially developed in certain regions.
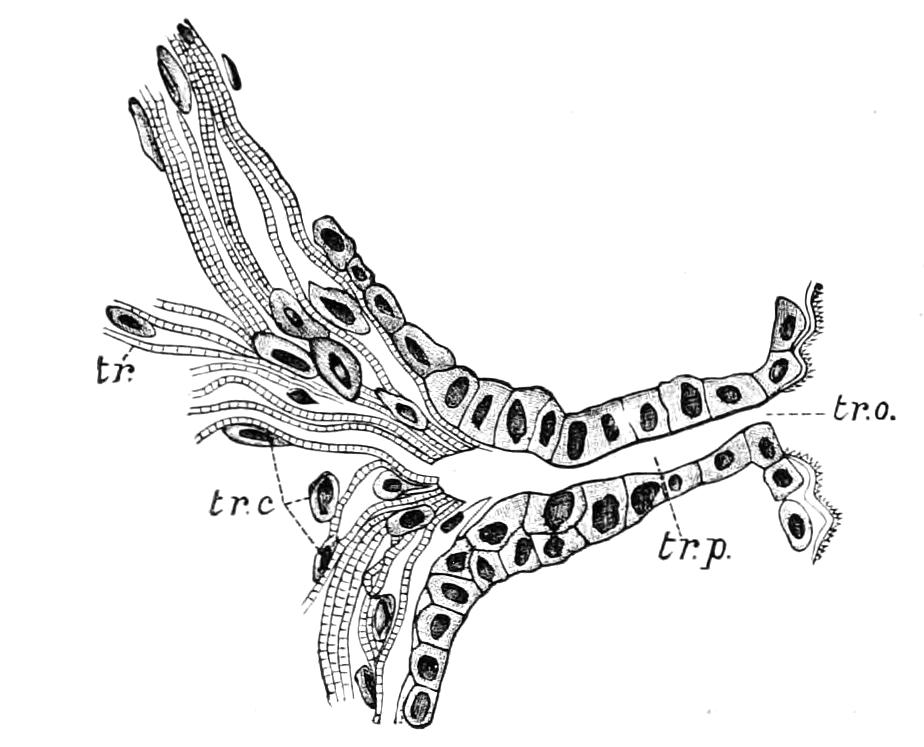
Fig. 10.—Section through a tracheal pit and diverging bundles of tracheal tubes taken transversely to the long axis of the body. (After Balfour.) tr, Tracheae, showing rudimentary spiral fibre; tr.c, cells resembling those lining the tracheal pits, which occur at intervals along the course of the tracheae; tr.o, tracheal stigma; tr.p, tracheal pit.
The Muscular System.
The general muscular system consists of—(1) the general wall of the body; (2) the muscles connected with the mouth, pharynx, and jaws; (3) the muscles of the feet; (4) the muscles of the alimentary tract.
The muscular wall of the body is formed of—(1) an external layer of circular fibres; (2) an internal layer of longitudinal muscles.
The main muscles of the body are unstriated and divided into fibres, each invested by a delicate membrane. The muscles of the jaws alone are transversely striated.
The Vascular System.
The vascular system consists of a dorsal tubular heart with paired ostia leading into it from the pericardium, of the pericardium, and the various other divisions of the perivisceral cavity (Fig. 14, D). As in all Arthropoda, the perivisceral cavity is a haemocoele; i.e. it contains blood and forms part of the vascular system. The heart extends from close to the hind end of the body to the head.
The Body Cavity.
The body cavity is formed of four compartments—one central, two lateral, and a pericardial (Fig. 14, D). The former is by far the largest, and contains the alimentary tract, the generative organs, and the slime glands. It is lined by a delicate endothelial layer, and is not divided into compartments nor traversed by muscular fibres. The lateral divisions are much smaller than the central, and are shut off from it by the inner transverse band of muscles. They are almost entirely filled with the nerve-cord and salivary gland in front and with the nerve-cord alone behind, and their lumen is broken up by muscular bands. They further contain the nephridia. They are prolonged into the feet, as is the embryonic body cavity of most Arthropoda. The pericardium contains a peculiar cellular tissue, probably, as suggested by Moseley, equivalent to the fat-bodies of insects.
Nephridia.
In Peripatus capensis nephridia are present in all the legs. In all of them (except the first three) the following parts may be recognised (Fig. 11):—
(1) A vesicular portion opening to the exterior on the ventral surface of the legs by a narrow passage.
(2) A coiled portion, which is again subdivided into several sections.
(3) A section with closely packed nuclei ending by a somewhat enlarged opening.
(4) The terminal portion, which consists of a thin-walled vesicle.
The last twelve pairs of these organs are all constructed in a very similar manner, while the two pairs situated in the fourth and fifth pairs of legs are considerably larger than those behind, and are in some respects very differently constituted.
It will be convenient to commence with one of the hinder nephridia. Such a nephridium from the ninth pair of legs is represented in Fig. 11. The external opening is placed at the outer end of a transverse groove at the base of one of the legs, while the main portion of the organ lies in the body cavity in the base of the leg, and extends into the trunk to about the level {17}of the outer edge of the nerve-cord of its side. The external opening (o.s) leads into a narrow tube (s.d), which gradually dilates into a large sac (s). The narrow part is lined by small epithelial cells, which are directly continuous with and perfectly similar to those of the epidermis. The sac itself, which forms a kind of bladder or collecting vesicle for the organ, is provided with an extremely thin wall, lined with very large flattened cells. The second section of the nephridium is formed by the coiled tube, the epithelial lining of which varies slightly in the different parts. The third section (s.o.t), constitutes the most distinct portion of the whole organ. Its walls are formed of columnar cells almost filled by oval nuclei, which absorb colouring matters with very great avidity, and thus render this part extremely conspicuous. The nuclei are arranged in several rows. It ends by opening into a vesicle (Fig. 14, D), the wall of which is so delicate that it is destroyed when the nephridium is removed from the body, and consequently is not shown in Fig. 11.
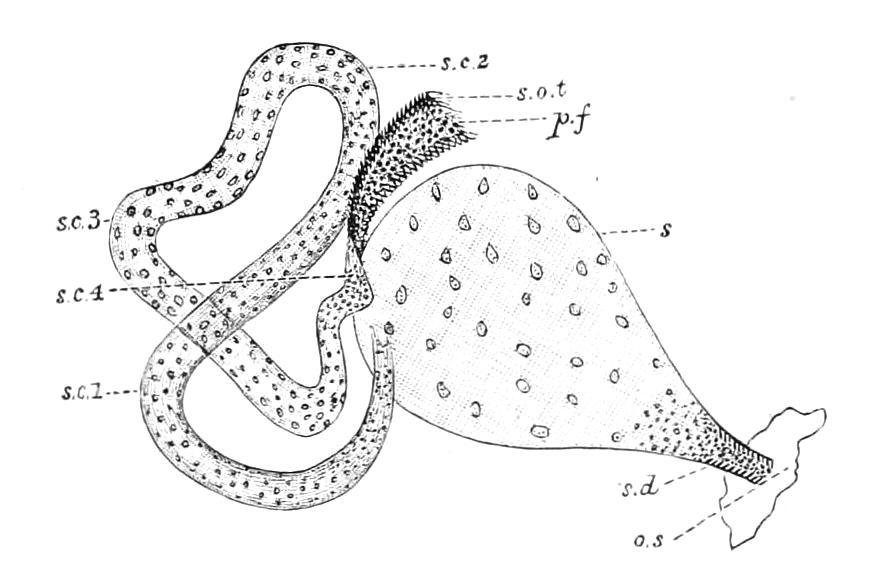
Fig. 11.—Nephridium from the 9th pair of legs of P. capensis. o.s, External opening of segmental organ; p.f, internal opening of nephridium into the body cavity (lateral compartment); s, vesicle of segmental organ; s.c.1, s.c.2, s.c.3, s.c.4, successive regions of coiled portion of nephridium; s.o.t, third portion of nephridium broken off at p.f from the internal vesicle, which is not shown.
The fourth and fifth pairs are very considerably larger than those behind, and are in other respects peculiar. The great mass of each organ is placed behind the leg on which the external opening is placed, immediately outside one of the lateral nerve-cords. The external opening, instead of being placed near the base of the leg, is placed on the ventral side of the third ring (counting from the outer end) of the thicker portion of the leg. It leads into a portion which clearly corresponds with the collecting vesicle of the hinder nephridia. This part is not, however, dilated into a vesicle. The three pairs of nephridia in the three foremost pairs of legs are rudimentary, consisting solely of a vesicle and duct. The salivary glands are the modified nephridia of the segment of the oral papillae.
Generative Organs.
Male.—The male organs (Fig. 12) consist of a pair of testes (te), a pair of vesicles (v), vasa deferentia (v.d), and accessory glandular tubules (f). All the above parts lie in the central compartment of the body cavity. In P. capensis the accessory glandular bodies or crural glands of the last (17th) pair of legs are enlarged and prolonged into an elongated tube placed in the lateral compartment of the body cavity (a.g).
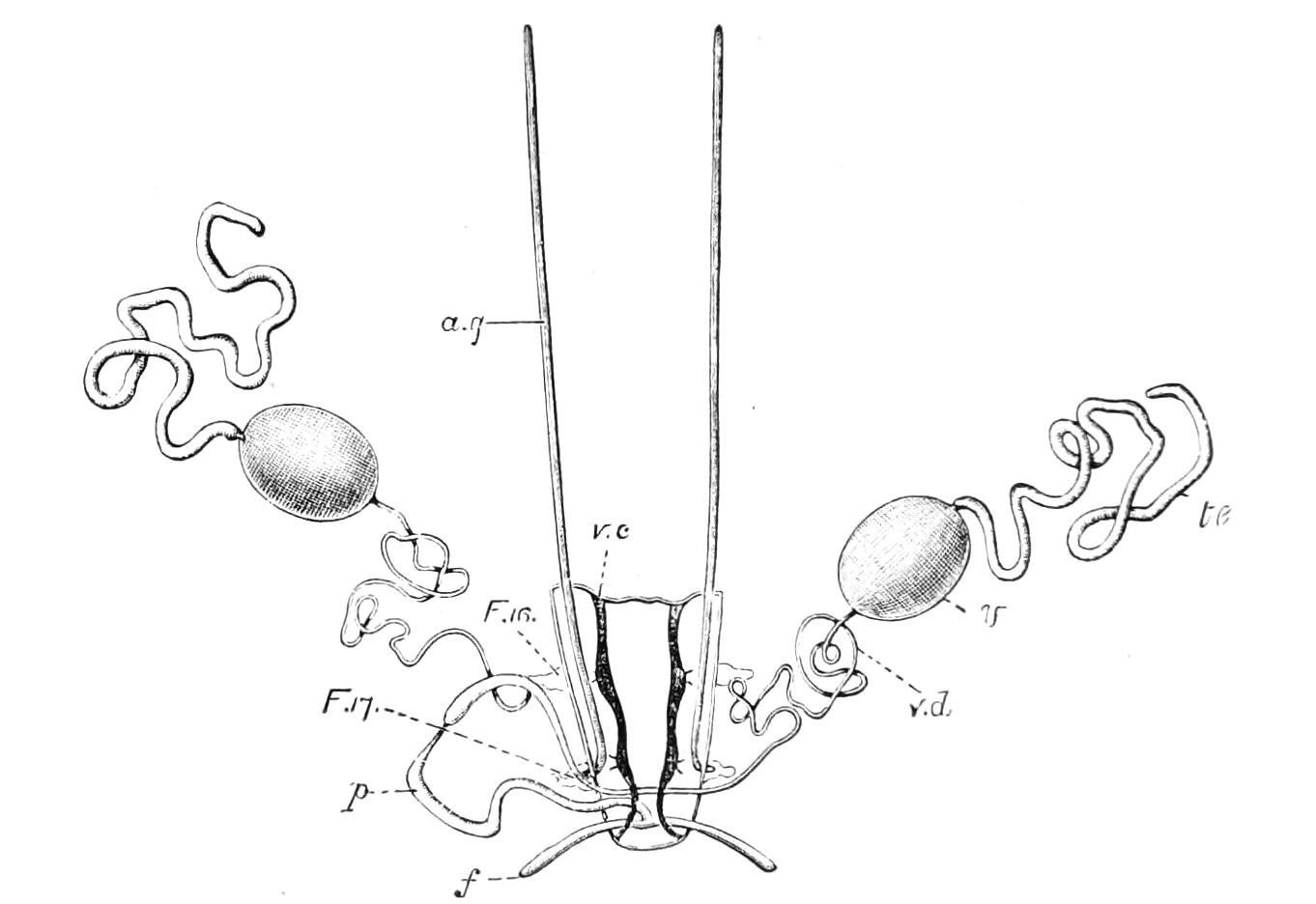
Fig. 12.—Male generative organs of Peripatus capensis, viewed from the dorsal surface. (After Balfour.) a.g, Enlarged crural glands of last pair of legs; F.16, 17, last pairs of legs; f, small accessory glandular tubes; p, common duct into which the vasa deferentia open; te, testis; v, seminal vesicle; v.c, nerve-cord; v.d, vas deferens.
The right vas deferens passes under both nerve-cords to join the left, and form the enlarged tube (p), which, passing beneath the nerve-cord of its side, runs to the external orifice. The enlarged terminal portion possesses thick muscular walls, and possibly constitutes a spermatophore maker, as has been shown to be the case in P. N. Zealandiae, by Moseley. In some specimens a different arrangement obtains, in that the left vas deferens passes under both nerve-cords to join the right.
Female.—The ovaries consist of a pair of tubes closely applied together, and continued posteriorly into the oviducts. The oviducts, after a short course, become dilated into the uteruses, which join behind and open to the exterior by a median {19}opening. The ovaries always contain spermatozoa, some of which project through the ovarian wall into the body cavity. Spermatozoa are not found in the uterus and oviducts, and it appears probable that they reach the ovary directly by boring through the skin and traversing the body cavity.[6] In the neotropical species there is a globular receptaculum seminis opening by two short ducts close together into the oviduct, and there is a small receptaculum ovorum with extremely thin walls opening into the oviduct by a short duct just in front of the receptaculum seminis. The epithelium of the latter structure is clothed with actively moving cilia. In the New Zealand species there is a receptaculum seminis with two ducts, but the receptacula ovorum has not been seen.
There appear to be present in most, if not all, the legs some accessory glandular structures opening just externally to the nephridia. They are called the crural glands.
DEVELOPMENT.
As stated at the outset, Peripatus is found in three of the great regions, viz. in Africa, in Australasia, and in South America and the West Indies. It is a curious and remarkable fact that although the species found in these various localities are really closely similar, the principal differences relating to the structure of the female generative organs and to the number of the legs, they do differ in the most striking manner in the structure of the ovum and in the early development. In all the Australasian species the egg is large and heavily charged with food-yolk, and is surrounded by a tough membrane. In the Cape species the eggs are smaller, though still of considerable size; the yolk is much less developed, and the egg membrane is thinner though dense. In the neotropical species the egg is minute and almost entirely devoid of yolk. The unsegmented uterine ovum of P. Novae-Zealandiae measures 1.5 mm. in length by .8 mm. in breadth; that of P. capensis is .56 mm. in length; and that of P. Trinidadensis .04 mm. in diameter. In correspondence with these differences in the ovum there are differences in the early development, though the later stages are closely similar.
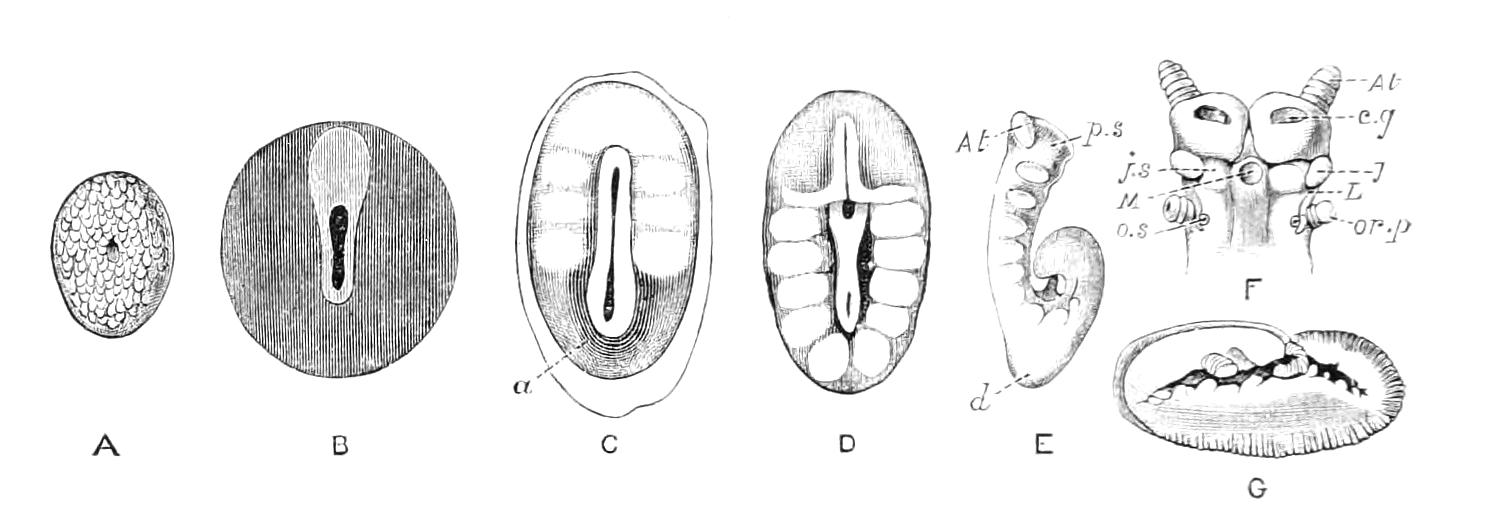
Fig. 13.—A series of embryos of P. capensis. The hind end of embryos B, C, D is uppermost in the figures, the primitive streak is the white patch behind the blastopore. (After Sedgwick.) A, Gastrula stage, ventral view, showing blastopore. B, Older gastrula stage, ventral view, showing elongated blastopore and primitive streak. C, Ventral view of embryo with three pairs of mesoblastic somites, dumb-bell-shaped blastopore and primitive streak. D, Ventral view of embryo, in which the blastopore has completely closed in its middle portion, and given rise to two openings, the embryonic mouth and anus. The anterior pair of somites have moved to the front end of the body, and the primitive groove has appeared on the primitive streak. E, Side view of embryo, in which the hind end of the body has begun to elongate in a spiral manner, and in which the appendages have begun. At, antenna; d, dorsal projection; p.s, preoral somite. F, Ventral view of head of embryo intermediate between E and G. The cerebral grooves are wide and shallow. The lips have appeared, and have extended behind the openings of the salivary glands, but have not yet joined in the middle line. At, antennae; c.g, cerebral groove; j, jaws; j.s, swelling at base of jaws; L, lips; M, mouth; or.p, oral papillae; o.s, opening of salivary gland. G, Side view of older embryo with the full number of appendages, to show the position in which the embryos lie in the uterus.
But unfortunately the development has only been fully worked out in one species, and to that species—P. capensis—the following description refers. The ova are apparently fertilised in the ovary, and they pass into the oviducts in April and May. In May the brood of the preceding year are born, and the new ova, which have meanwhile undergone cleavage, pass into the uterus. There are ten to twenty ova in each uterus. The segmentation is peculiar, and leads to the formation of a solid gastrula, consisting of a cortex of ectoderm nuclei surrounding a central endodermal mass, which consists of a much-vacuolated tissue with some irregularly-shaped nuclei. The endoderm mass is exposed at one point—the blastopore (gastrula mouth). The central vacuoles of the endoderm now unite and form the enteron of the embryo, and at the same time the embryo elongates into a markedly oval form, and an opacity—the primitive streak—appears at the hind end of the blastopore (Fig. 13, B). This elongation of the embryo is accompanied by an elongation of the blastopore, which soon becomes dumb-bell shaped (Fig. 13, C). At the same time the mesoblastic somites (embryonic segments of mesoderm) have made {21}their appearance in pairs at the hind end, and gradually travel forward on each side of the blastopore to the front end, where the somites of the anterior pair soon meet in front of the blastopore (Fig. 13, D). Meanwhile the narrow middle part of the blastopore has closed by a fusion of its lips, so that the blastopore is represented by two openings, the future mouth and anus. A primitive groove makes its appearance behind the blastopore (Fig. 13, D). At this stage the hind end of the body becomes curved ventrally into a spiral (Fig. 13, E), and at the same time the appendages appear as hollow processes of the body wall, a mesoblastic somite being prolonged into each of them. The first to appear are the antennae, into which the praeoral somites are prolonged. The remainder appear from before backwards in regular order, viz. jaw, oral papillae, legs 1-17. The full number of somites and their appendages is not, however, completed until a later stage. The nervous system is formed as an annular thickening of ectoderm passing in front of the mouth and behind the anus, and lying on each side of the blastopore along the lines of the somites. The praeoral part of this thickening, which gives rise to the cerebral ganglia, becomes pitted inwards on each side (Fig. 13, F, c.g). These pits are eventually closed, and form the hollow ventral appendages of the supra-pharyngeal ganglia of the adult (Fig. 9, d). The lips are formed as folds of the side wall of the body, extending from the praeoral lobes to just behind the jaw (Fig. 13, F, L). They enclose the jaws (j) mouth (M), and opening of the salivary glands (o.s), and so give rise to the buccal cavity. The embryo has now lost its spiral curvature, and becomes completely doubled upon itself, the hind end being in contact with the mouth (Fig. 13, G). It remains in this position until birth. The just-born young are from 10-15 mm. in length and have green antennae, but the rest of the body is either quite white or of a reddish colour. This red colour differs from the colour of the adult in being soluble in spirit.
The mesoblastic somites are paired sacs formed from the anterior lateral portions of the primitive streak (Fig. 13, C). As they are formed they become placed in pairs on each side of the blastopore. The somites of the first pair eventually obtain a position entirely in front of the blastopore (Fig. 13, D). They form the somites of the praeoral lobes. The full complement of somites is acquired at about the stage of Fig. 13, E.

Fig. 14.—A series of diagrams of transverse sections through Peripatus embryos to show the relations of the coelom at successive stages. (After Sedgwick.) A, Early stage: 1, gut; 2, mesoblastic somite; no trace of the vascular space; endoderm and ectoderm in contact. B, Endoderm has separated from the dorsal and ventral ectoderm. The somite is represented as having divided on the left side into a dorsal and ventral portion: 1, gut; 2, somite; 3, haemocoele. C, The haemocoele (3) has become divided up into a number of spaces, the arrangement of which is unimportant. The dorsal part of each somite has travelled dorsalwards, and now constitutes a small space (triangular in section) just dorsal to the gut. The ventral portion (2′) has assumed a tubular character, and has acquired an external opening. The internal vesicle is already indicated, and is shown in the diagram by the thinner black line: 1, gut; 2′, nephridial part of coelom; 3, haemocoele; 3′, part of haemocoele which will form the heart—the part of the haemocoele on each side of this will form the pericardium; 4, nerve-cord. D represents the conditions at the time of birth; numbers as in C, except 5, slime glands. The coelom is represented as surrounded by a thick black line, except in the part which forms the internal vesicle of the nephridium.
The relations of the somites is shown in Fig. 14, A, which represents a transverse section taken between the mouth and anus of an embryo of the stage of Fig. 13, D. The history of these somites is an exceedingly interesting one, and may be described shortly as follows:—They divide into two parts—a ventral part, which extends into the appendage, and a dorsal part (Fig. 14, B). The ventral part acquires an opening to the exterior just outside the nerve-cord, and becomes entirely transformed into a nephridium (Fig. 14, D, 2′). The dorsal part shifts dorsalwards and diminishes relatively in size (Fig. 14, C). Its fate differs in the different parts {23}of the body. In the anterior somites it dwindles and disappears, but in the posterior part it unites with the dorsal divisions of contiguous somites of the same side, and forms a tube—the generative tube (Fig. 14, D, 2). The last section of this tube retains its connexion with the ventral portion of the somite, and so acquires an external opening, which is at first lateral, but soon shifts to the middle line, and fuses with its fellow, to form the single generative opening. The praeoral somite develops the rudiment of a nephridium, but eventually entirely disappears. The jaw somite also disappears; the oral papilla somite forms ventrally the salivary glands, which are thus serially homologous with nephridia. The perivisceral cavity of Peripatus is, as in all Arthropoda, a haemocoele. Its various divisions develop as a series of spaces between the ectoderm and endoderm, and later in the mesoderm. The mesoderm seems to be formed entirely from the proliferation of the cells of the mesoblastic somites. It thus appears that in Peripatus the coelom does not develop a perivisceral portion, but gives rise only to the renal and reproductive organs.
Synopsis of the Species of Peripatus.
Peripatus, Guilding.
Soft-bodied vermiform animals, with one pair of ringed antennae, one pair of jaws, one pair of oral papillae, and a varying number of claw-bearing ambulatory legs. Dorsal surface arched and more darkly pigmented than the flat ventral surface. Skin transversely ridged and beset by wart-like spiniferous papillae. Mouth anterior, ventral; anus posterior, terminal. Generative opening single, median, ventral, and posterior. One pair of simple eyes. Brain large, with two ventral hollow appendages; ventral cords widely divaricated, without distinct ganglia. Alimentary canal simple, uncoiled. Segmentally arranged, paired nephridia are present. Body cavity is continuous with the vascular system, and does not communicate with the paired nephridia. Heart tubular, with paired ostia. Respiration by means of tracheae. Dioecious; males smaller and generally less numerous than females. Generative glands tubular, continuous with the ducts. Viviparous. Young born fully developed. They shun the light, and live in damp places beneath stones, leaves, and bark of rotten stumps. They eject when irritated a viscid fluid through openings at the apex of the oral papillae.
Distribution: South Africa, New Zealand, and Australia, South America and the West Indies [and in Sumatra?].
South African Species.
With three spinous pads on the legs and two primary papillae on the anterior side of the foot, and one accessory tooth on the outer blade of the jaw; with a white papilla on the ventral surface of the last fully developed leg of the male. Genital opening subterminal, behind the last pair of fully-developed legs. The terminal unpaired portion of vas deferens short. Ova of considerable size, but with only a small quantity of food-yolk. (Colour highly variable, number of legs constant in same species (?).)
P. capensis (Grube).—South African Peripatus, with seventeen pairs of claw-bearing ambulatory legs. Locality, Table Mountain.
P. Balfouri (Sedgwick).—South African Peripatus, with eighteen pairs of claw-bearing ambulatory legs, of which the last pair is rudimentary. With white papillae on the dorsal surface. Locality, Table Mountain.
P. brevis (De Blainville).—South African Peripatus, with fourteen pairs of ambulatory legs. Locality, Table Mountain. (I have not seen this species. Presumably it has the South African characters.)
P. Moseleyi (Wood Mason).—South African Peripatus, with twenty-one and twenty-two pairs of claw-bearing ambulatory legs. Locality, near Williamstown, Cape Colony; and Natal.[7]
Doubtful Species.
(1) South African Peripatus, with twenty pairs of claw-bearing ambulatory legs (Sedgwick). Locality, Table Mountain. (Also Peters, locality not stated.)
(2) South African Peripatus, with nineteen pairs of ambulatory legs (Trimen). Locality, Plettenberg Bay, Cape Colony. (Also Peters, locality not stated.)
Australasian Species.
With fifteen pairs of claw-bearing ambulatory legs, with three spinous pads on the legs, and a primary papilla projecting from the median dorsal portion of the feet. Genital opening between the legs of the last pair. Receptacula seminis present. Unpaired portion of vas deferens long and complicated. Ova large and heavily charged with yolk. (Colour variable, number of legs constant in same species (?).)
P. Novae Zealandiae (Hutton).—Australasian Peripatus, without an accessory tooth on the outer blade of the jaw, and without a white papilla on the base of the last leg of the male. New Zealand.
P. Leuckarti (Saenger).—Australasian Peripatus, with an accessory tooth on the outer blade of the jaw, and a white papilla on the base of the last leg of the male. Queensland.
Neotropical Species.
With four spinous pads on the legs, and the generative aperture between {25}the legs of the penultimate pair. Dorsal white line absent. Primary papillae divided into two portions. Inner blade of jaw with gap between the first minor tooth and the rest. Oviducts provided with receptacula ovorum and seminis. Unpaired part of vas deferens very long and complicated. Ova minute, without food-yolk. (Colour fairly constant, number of legs variable in same species (?).)
P. Edwardsii.[8]—Neotropical Peripatus from Caracas, with a variable number of ambulatory legs (twenty-nine to thirty-four). Males with twenty-nine or thirty legs, and tubercles on a varying number of the posterior legs. The basal part or the primary papilla is cylindrical.
P. Trinidadensis (n. sp.).—Neotropical Peripatus from Trinidad, with twenty-eight to thirty-one pairs of ambulatory legs, and a large number of teeth on the inner blade of the jaw. The basal portion of the primary papillae is conical.
P. torquatus (Kennel).— Neotropical Peripatus from Trinidad, with forty-one to forty-two pairs of ambulatory legs. With a transversely placed bright yellow band on the dorsal surface behind the head.
Doubtful Species.
The above are probably distinct species. Of the remainder we do not know enough to say whether they are distinct species or not. The following is a list of these doubtful species, with localities and principal characters:—
P. juliformis (Guilding).—Neotropical Peripatus from St. Vincent, with thirty-three pairs of ambulatory legs.
P. Chiliensis (Gay).—Neotropical Peripatus from Chili, with nineteen pairs of ambulatory legs.
P. demeraranus (Sclater).—Neotropical Peripatus from Maccasseema, Demerara, with twenty-seven to thirty-one pairs of ambulatory legs and conical primary papillae.
Peripatus from Cayenne (Audouin and Milne-Edwards).—With thirty pairs of legs. Named P. Edwardsii by Blanchard.
Peripatus from Valentia Lake, Columbia (Wiegmann).—With thirty pairs of legs.
Peripatus from St. Thomas (Moritz).—No description.
Peripatus from Colonia Towar, Venezuela (Grube).—With twenty-nine to thirty-one pairs of ambulatory legs. Named P. Edwardsii by Grube.
Peripatus from Santo Domingo, Nicaragua (Belt).—With thirty-one pairs of ambulatory legs.
Peripatus from Dominica (Angas).—Neotropical Peripatus, with twenty-six to thirty (Pollard) pairs of ambulatory legs.
Peripatus from Jamaica (Gosse).—With thirty-one and thirty-seven pairs of ambulatory legs.
Peripatus from Santaram.—Neotropical Peripatus, with thirty-one pairs of ambulatory legs.
Peripatus from Cuba.—No details.
Peripatus from Hoorubea Creek, Demerara (Quelch).—With thirty pairs of legs.
Peripatus from Marajo (Branner).—No details.
Peripatus from Utuado, Porto Rico (Peters).—With twenty-seven, thirty, thirty-one, and thirty-two pairs of legs.
Peripatus from Surinam (Peters).—No details.
Peripatus from Puerto Cabello, Venezuela (Peters).—With thirty and thirty-two pairs of legs.
Peripatus from Laguayra, Venezuela (Peters).—No details.
Peripatus Quitensis (Schmarda).—From Quito, with thirty-six pairs of legs.
Peripatus from Sumatra (?).
P. Sumatranus (Horst).—Peripatus from Sumatra, with twenty-four pairs of ambulatory legs, and four spinous pads on the legs. The primary papillae of the neotropical character with conical bases. Generative opening between the legs of the penultimate pair. Feet with only two papillae.[9]
Summary of Distribution
Distribution of the South African Species—
Slopes of Table Mountain, neighbourhood of Williamstown, Plettenberg Bay—Cape Colony—Natal.
Distribution of the Australasian Species—
Queensland—Australia.
North and South Islands—New Zealand.
Oriental Region (?)—
Sumatra.
Distribution of the Neotropical Species—
Nicaragua.
Valencia Lake, Caracas, Puerto Cabello, Laguayra, Colonia Towar—Venezuela.
Quito—Ecuador.
Maccasseema, Hoorubea Creek—Demerara.
Surinam (Peters).
Cayenne.
Santarem, Marajo, at the mouth of the Amazon—Brazil.
Chili.
And in the following West Indian Islands—Cuba, Dominica, Porto Rico (Peters), Jamaica, St. Thomas, St. Vincent, Trinidad.
BY
F. G. SINCLAIR, M.A.
(FORMERLY F. G. HEATHCOTE)
Trinity College, Cambridge.
MYRIAPODA
INTRODUCTION–HABITS–CLASSIFICATION–STRUCTURE–CHILOGNATHA–CHILOPODA–SCHIZOTARSIA–SYMPHYLA–PAUROPODA–EMBRYOLOGY–PALAEONTOLOGY.
Tracheata with separated head and numerous, fairly similar segments. They have one pair of antennae, two or three pairs of mouth appendages, and numerous pairs of legs.
The Myriapoda are a class of animals which are widely distributed, and are represented in almost every part of the globe. Heat and cold alike seem to offer favourable conditions for their existence, and they flourish both in the most fertile and the most barren countries.
They have not attracted much notice until comparatively recent times. Compared with Insects they have been but little known. The reason of this is not hard to find. The Myriapods do not exercise so much direct influence on human affairs as do some other classes of animals; for instance, Insects. They include no species which is of direct use to man, like the silkworm or the cochineal insect, and they are of no use to him as food. It is true that they are injurious to his crops. For instance, the species of Millepede known as the "wire worm"[10] is extremely harmful; but this has only attracted much notice in modern times, when land is of more value than formerly, and agriculture is pursued in a more scientific manner, and the constant endeavour to get the utmost amount of crop from the soil has caused a minute investigation into the various species of animals which are noxious to the growing crop. The species of {30}Myriapoda best known to the ancients were those which were harmful to man on account of their poisonous bite.
Some writers have supposed that the word which is translated "mole" in the Bible (Lev. xi. 30) is really Scolopendra (a genus of Centipede), and, if this is so, it is the earliest mention of the Myriapods. They were rarely noticed in the classical times; almost the only mention of them is by Ælian, who says that the whole population of a town called Rhetium were driven out by a swarm of Scolopendras. Pliny tells us of a marine Scolopendra, but this was most probably a species of marine worm.
Linnaeus included Myriapods among the Insects; and the writers after him till the beginning of this century classed them with all sorts of Insects, with Spiders, Scorpions, and even among Serpents. It was Leach who first raised them to the importance of a separate class, and Latreille first gave them the name of Myriapoda, which they have retained ever since.
Myriapods are terrestrial animals, crawling or creeping on the ground or on logs of wood, or even under the bark of trees. There is, however, a partial exception to this; various naturalists have from time to time given descriptions of marine Centipedes. These are not found in the sea, but crawl about on the shore, where they are submerged by each tide. Professor F. Plateau has given an account of the two species of Myriapods that are found thus living a semi-aquatic life. They are named Geophilus maritimus and Geophilus submarinus, and Plateau found that they could exist in sea water from twelve to seventy hours, and in fresh water from six to ten days. They thus offer a striking example of the power that their class possess of existing under unfavourable circumstances.
With regard to their habits the different species differ very considerably. On the one hand we have the Chilopoda, or Centipedes, as they are called in this country, active, swift, and ferocious; living for the most part in dark and obscure places, beneath stones, logs of wood, and dried leaves, etc., and feeding on living animals. On the other hand, we have the Chilognatha, or Millepedes, distinguished by their slow movements and vegetable diet; inoffensive to man, except by the destruction they occasion to his crops, and having as a means of defence no formidable weapon like the large poison claws of the Centipedes, but only a peculiarly offensive liquid secreted by special glands {31}known by the unpleasant though expressive name of "stink glands," or by the more euphonious Latin name of glandulae odoriferae.
As a general rule the larger species of Myriapods are found in the hotter climates, some of the tropical species being very large, and some, among the family of the Scolopendridae, extremely poisonous; and it is even said that their bite is fatal to man.
If, however, the Centipede is sometimes fatal to man, it does not always have it its own way, for we read of man making food of Centipedes. It is hard to believe that any human being could under any circumstances eat Centipedes, which have been described by one naturalist as "a disgusting tribe loving the darkness." Nevertheless, Humboldt informs us that he has seen the Indian children drag out of the earth Centipedes eighteen inches long and more than half an inch wide and devour them.
This, I believe, is the only account of human beings using the Myriapoda as food, if we except the accounts of the religious fanatics among the African Arabs, who are said to devour Centipedes alive; though this is not a case of eating for pleasure, for the Scolopendras are devoured in company with leaves of the prickly pear, broken glass, etc., as a test of the unpleasant things which may be eaten under the influence of religious excitement.
A cold climate, however, is not fatal to some fairly large species of Centipedes. A striking instance of this came under my own observation some years ago. In 1886 I was travelling in the island of Cyprus—the "Enchanted Island," as Mr. Mallock calls it in his book written about the same time—with the intention of observing its natural history. This island consists of a broad flat country crossed by two mountain ranges of considerable height, thus offering the contrast of a hot climate in the plains and a cold climate in the mountains. On the plain country I found among the Myriapoda that the most common species were a large Scolopendra and a large Lithobius. The Scolopendra was fairly common, living for the most part under large stones, and it was a pleasant task to search for them in a ruined garden near Larnaca.
This garden was made for the public, and is situated about a quarter of a mile from the old town of Larnaca. It has been suffered to fall into decay, and is now quite neglected. Mr. Mallock has described many beautiful scenes in his book, but I think he could have found few more beautiful than this old garden with its deserted gardener's house, now a heap of ruins, but overgrown with masses of luxuriant vegetation, with beautiful flowers peeping out here and there as if charitably endeavouring to hide the negligence of man, and to turn the desolation into a scene of beauty. I got several prizes in this garden, but found the Myriapods were principally represented by the species I have mentioned.
After leaving Larnaca I rode across the plain country through blazing heat, which was rapidly parching up the ground to a uniform brown colour. At every stopping-place I found the same species of Scolopendra and of Lithobius. After a few days I began to get up among the mountains of the northern range, and the burning heat of the treeless plain was gradually exchanged for the cool shade of the pine-trees and the fresh air of the mountains. As I ascended higher and higher the temperature grew cooler till I reached the top of Mount Troodos, the ancient Olympus. Here in the month of May the snow still lingered in white patches, and the air was clear and cold. I remained on the top of Troodos for a week, while I made a close examination of the fauna to be found there. I was much surprised to find the identical species of Scolopendra and {33}Lithobius with which I had become acquainted in the heat of the low country, quite at home among the snow, and as common as in, what I should have imagined to be, the more congenial climate. Nor were they any the less lively. Far from exhibiting any sort of torpor from the cold, the first one which I triumphantly seized in my forceps wriggled himself loose and fastened on my finger with a vigour which made me as anxious to get rid of him as I had formerly been to secure him. However, he eventually went into my collecting box.
On the whole, we may say that the Chilopoda are most largely represented in the hotter climates, where they find a more abundant diet in the rich insect life of the tropical and semi-tropical countries. The more brightly-coloured Myriapods, too, are for the most part inhabitants of the warmer countries. The ease with which they are introduced into a country in the earth round plants, and in boxes of fruit, may account to a great extent for the wide distribution of the various species in different countries. Mr. Pocock, who examined the Myriapods brought back from the "Challenger" Expedition, informs us that of ten species brought from Bermuda, four had been introduced from the West Indies. There is no doubt that animals which can bear changes of temperature and deprivation of food, and even a short immersion in the water, are well calculated to be introduced into strange countries in many unexpected ways.
As might be expected from a class of animals so widely distributed, Myriapods show an almost infinite variety of size and colour. We find them so small that we can hardly see them with the naked eye, as in the case of the tiny Polyxenus, the Pauropidae, and the Scolopendrellidae. We also find them more than six inches in length, as the larger species of Scolopendridae. I am afraid we must dismiss as an exaggeration an account of Centipedes in Carthagena a yard in length, and more than six inches in breadth. The giver of this account—Ulloa—informs us that the bite of this gigantic serpent-like creature is mortal if a timely remedy be not applied. It is certainly extremely probable that the bite of a Centipede of this size would be fatal to any one. Some Centipedes are short and broad, and composed of few segments, as Glomeris; some are long and thin, with more than a hundred segments, as Geophilus. They may be beautifully coloured with brilliant streaks of colour, as in some {34}of the Julidae or Polydesmidae, or may be of a dull and rusty iron colour, or quite black.
One of the strangest peculiarities found among Myriapods is that some of them (e.g. Geophilus electricus) are phosphorescent. As I was walking one summer evening near my home in Cambridgeshire I saw what I thought was a match burning. Looking more closely, I saw it move, and thinking it was a glow-worm I picked it up, and was surprised to find that it was a Geophilus shining with a brilliant phosphorescent light. I let it crawl over my hand, and it left a bright trail of light behind it, which lasted some time. I have been told that this species is common in Epping Forest; also in Cambridgeshire.[11]
Besides G. electricus, G. phosphoreus has been described as a luminous species by Linnaeus, on the authority of a Swedish sea captain, who asserted that it dropped from the air, shining like a glow-worm, upon his ship when he was sailing in the Indian Ocean a hundred miles from land.
What the use of this phosphorescence may be is not known with any degree of certainty. It may be either a defence against enemies, or else a means of attracting the two sexes to one another.
The places which the Myriapods select for their habitation vary as much as their colour and size, though, with a few exceptions, they chose dark and obscure places. A curious species of Myriapod is Pseudotremia cavernarum (Cope), which is found in certain caves in America. The peculiar life it leads in these caves seems to have a great influence on its colour, and also affects the development of its eyes. Mr. Packard's account of them is worth quoting: "Four specimens which I collected in Little Wyandotte cave were exactly the same size as those from Great Wyandotte cave. They were white tinged, dusky on the head and fore part of the body. The eyes are black and the eye-patch of the same size and shape, while the antennae are the same.
"Six specimens from Bradford cave, Ind. (which is a small grotto formed by a vertical fissure in the rock, and only 300 to 400 yards deep), showed more variation than those from the two Wyandotte caves. They are of the same size and form, but slightly longer and a little slenderer.... The antennae are much whiter than in those from the Wyandotte caves, and the {35}head and body are paler, more bleached out than most of the Wyandotte specimens.... It thus appears that the body is most bleached and the eyes the most rudimentary in the Bradford cave, the smallest and most accessible, and in which consequently there is the most variation in surroundings, temperature, access of light and changed condition of air. Under such circumstances as these we should naturally expect the most variation."[12]
A strong contrast to these animals is afforded us by the Scutigeridae (Schizotarsia). They are unknown in this country, but abound in some of the Mediterranean countries and in parts of Africa. They remind one strongly of spiders, with their long legs and their peculiar way of running on stones and about the walls of houses.
Some years ago I was in Malta, and I used to go and watch them on the slopes outside Valetta, where they were to be found in great numbers. They used to come out from beneath great stones and run about rapidly on the ground or on the stones and rubbish with which the ground was covered, now and again making a dart at some small insect which tempted them, and seemingly not minding the blazing sun at all. As might be expected from their habits, their eyes, far from being rudimentary, like those of the cave-living Pseudotremia, or absent {36}like those of the Polydesmidae, or of our own Cryptops, are highly developed, and form the only example among the Myriapods of what are known as facetted eyes. The Scutigeridae are also remarkable among Myriapods for the possession of a peculiar sense-organ which is found in no other Myriapod.
The Myriapods most numerous in our own country are Lithobius and Julus. Lithobius, which will be described later on, may be found in almost any garden under dried leaves, stones, etc. Julus, the common wire-worm, is found crawling on plants and leaves and under the bark of trees, and does a good deal of damage in a garden. Polydesmus is also frequently found in great numbers, and usually a great many of them together. Glomeris is also found, though it is not so common as the first two mentioned animals. Geophilus is also common, and especially in the south of England. Scolopendridae are only represented by a single genus, Cryptops, which is not very common, though by no means rare. The best place to find them is in manure heaps. The animals of this species are small compared to most Scolopendras, and have the peculiarity of being without any eyes.
Scutigera is unrepresented in this country. One was found in Scotland some years ago by Mr. Gibson Carmichael, but was shown to have been imported, and not bred in the place.
The means of defence possessed by these animals also differ very much in the different species of Myriapods. In the Centipedes the animals are provided with a powerful weapon in the great poison claws which lie just beneath the mouth, and which are provided with large poison glands, which supply a fluid which runs through a canal in the hard substance of the claw and passes into the wound made by the latter. The effect of this fluid is instantaneous on the small animals which form the food of the Centipedes. I have myself watched Lithobius in this country creep up to a blue-bottle fly and seize it between the poison claws. One powerful nip and the blue-bottle was dead, as if struck by lightning. I have also seen them kill worms and also other Lithobius in the same way. When another Lithobius is wounded by the poison claws it seems to be paralysed behind the wound. The Millepedes, on the other hand, have no such offensive and defensive weapon. They rely for protection on the fluid secreted by the stigmata repugnatoria (or glandulae odoriferae) mentioned before. This fluid has been shown to contain prussic acid, and has a very unpleasant odour.
Most of the Millepedes are provided with these glands; but in the cave Myriapods mentioned before, the animals have not to contend against so many adversaries, and these glands almost disappear. Other Myriapods defend themselves by means of the long and stiff bristles with which they are provided, e.g. the little Polyxenus. This means of defence seems to have been more common among the fossil Myriapods than among those still living. Variations in the shape and size of the limbs are numerous, as might be expected in so large a class of animals. One of the most curious of such variations is found in a Centipede of the Scolopendra tribe, called Eucorybas, in which the last limbs are flattened out and provided with paddle-shaped lobes. The use of these is unknown, but it is probable that they are concerned in some way with the breeding habits of the animal. The habits of the Myriapods connected with their breeding are most interesting, but have been very insufficiently investigated. There is no doubt that a full inquiry into all such habits would be of great interest, and would help to answer some of the problems which are still unsolved in these forms. My own observations refer to two forms—Julus terrestris among the Millepedes, and Lithobius forficatus among the Centipedes. Julus terrestris is one of the most common of the English Millepedes, and can be easily obtained. I kept them in large shallow glass vessels with a layer of earth at the bottom, and thus was able easily to watch the whole process. They breed in the months of May, June, and July. The female Julus when about to lay her eggs sets to work to form a kind of nest or receptacle for her eggs. She burrows down into the earth, and at some distance below the surface begins the work. She moistens small bits of earth with the sticky fluid secreted by her salivary glands, which become extraordinarily active in the spring. She works up these bits of earth with her jaws and front legs till they are of a convenient size and shape, and places them together. When complete, the nest is shaped like a hollow sphere, the inside being smooth and even, while the outside is rough and shows the shape of the small knobs of earth of which it is composed. {38}She leaves a small opening in the top. The size of the whole nest is about that of a small nut. When she is ready to lay her eggs she passes them through the hole in the top, and usually lays about 60 to 100 eggs at a time. The eggs, which are very small, are coated with a glutinous fluid which causes them to adhere together. When they are all laid she closes up the aperture with a piece of earth moistened with her saliva; and having thus hermetically sealed the nest, she leaves the whole to its fate. The eggs hatch in about twelve days.
A naturalist named Verloef has lately found that the males of some Julidae undergo certain changes in the form of the legs and other organs in autumn and spring. These changes are probably connected with the breeding of the animal, and remind us of the changes undergone during the breeding season by salmon among the fishes.
Julus breed very readily if carefully attended to and well supplied with food. If they cannot obtain the food they like they will not breed so well. I found that sliced apples with leaves and grass formed the best food for them.
The process in the case of Lithobius is much harder to watch. Lithobius is not so plentiful as Julus terrestris, and the animals are more impatient of captivity, more shy in their habits, and do not breed so readily.
In January 1889 I was given the use of a room in the New Museums at Cambridge, and was allowed to fit it up as I liked, so that I was able to try the effect of different degrees of light and darkness, and of different degrees of warmth. I succeeded in observing the whole process. The female Lithobius is furnished with two small movable hooks at the end of the under surface of the body close to the opening of the oviduct. These small hooks have been observed by many naturalists, but their use has, so far as I know, never been described before. They play an important part in the proceedings following the laying of the egg. The time of breeding in Lithobius is rather later than in Julus, and begins about June and continues till August. There are first of all some convulsive movements of the last segments of the body, and then in about ten minutes the egg appears at the entrance of the oviduct. The egg is a small sphere (about the size of a number five shot), rather larger than that of Julus, and is covered with a sticky slime {39}secreted by the large glands inside the body, usually called the accessory glands. When the egg falls out it is received by the little hooks, and is firmly clasped by them. This is the critical moment in the existence of the Lithobius into which the egg is destined to develop. If a male Lithobius sees the egg he makes a rush at the female, seizes the egg, and at once devours it. All the subsequent proceedings of the female seem to be directed to the frustration of this act of cannibalism. As soon as the egg is firmly clasped in the little hooks she rushes off to a convenient place away from the male, and uses her hooks to roll the egg round and round until it is completely covered by earth, which sticks to it owing to the viscous material with which it is coated; she also employs her hind legs, which have glands on the thighs, to effect her purpose. When the operation is complete the egg resembles a small round ball of mud, and is indistinguishable from the surrounding soil. It is thus safe from the voracious appetite of the male, and she leaves it to its fate. The number of eggs laid is small when compared with the number laid by Julus.
The food in the case of Lithobius consisted of worms and blue-bottles, which were put alive into the glass vessel containing the Lithobius. I tried raw meat chopped up, but they did not thrive on it in the same way that they did on the living animals. I also put into their vessel bits of rotten wood containing larvae of insects, etc.
I have succeeded in bringing back some specimens of Polydesmus alive from Madeira, and in getting them to breed in this country—of course in artificial warmth—and their way of laying eggs and making a nest resembles that of Julus. Geophilus has one curious habit in connexion with the fertilisation of the female. The male spins a web and deposits in the middle of it a single spermatophore, and the female comes to the web to be fertilised. The Scolopendridae are said to bring forth their young alive, but I think the evidence for this is unsatisfactory. What have been taken for the young Scolopendrae are perhaps the large spermatophores of the male, which are not unlike a larval Myriapod in size and shape. I have never been able to observe the process of breeding in this family. I have had the spermatophores sent me from Gibraltar as "eggs," but a little examination soon showed me their real character.
The mode of progression in the Myriapods differs considerably, as might be expected in a class in which the number of legs varies to such an extent. The swiftest among them are the Scutigeridae with their long spider-like legs. The Scolopendridae are also able to move with considerable rapidity, and are also able to move tail forward almost as well as in the ordinary manner. Where there are such a number of legs it becomes a curious question as to the order in which the animal moves them; and though several people have endeavoured to find this out, the number of legs to be moved and the rapid movements have rendered accurate observation impossible.
Some years ago Professor E. Ray Lankester tried to study the order in which the legs of Centipedes moved, and came to the conclusion (recorded in an amusing letter in Nature, 23rd May 1889) that if the animal had to study the question itself, it would not get on at all. He finishes his letter with the following verses:—
A Centipede was happy quite
Until a toad in fun
Said, "Pray which leg moves after which?"
This raised her doubts to such a pitch,
She fell exhausted in the ditch,
Not knowing how to run.
The progression of Millepedes is much slower than that of the Centipedes, and it is remarkable that when the animal is in motion a sort of wave runs down the long fringe-like row of feet. I have endeavoured to make out this motion, but have never been able to understand it satisfactorily. My belief was that the feet were moved in sets of five.
This wave-like peculiarity of motion is described in a curious old book, An Essay towards a Natural History of Serpents. Charles Owen, D.D. London, 1742: "The Ambua, so the natives of Brazil call the Millepedes and the Centipedes, are serpents. Those reptiles of thousand legs bend as they crawl along, and are reckoned very poisonous. In these Multipedes the mechanism of the body is very curious; in their going it is observable that on each side of their bodies every leg has its motion, one regularly after another, so that their legs, being numerous, form a kind of undulation, and thereby communicate to the body a swifter progression than one could imagine where {41}so many short feet are to take so many short steps, that follow one another rolling on like the waves of the sea."
Before proceeding to the classification of Myriapods, which will form the next part of this account, a few words on the common names for them may not be without interest.
In English we have the names Centipede and Millepede, and the Continental nations have similar names implying the possession of a hundred or a thousand legs, as the German "Tausendfüsse" and the French "Millepieds." Of course these are general words, simply implying the possession of a great number of legs. But we have also among the peasantry a name for Centipedes which conveys a much more accurate idea of the number. The people of the eastern counties (I daresay the term is more widely spread) call them "forty legs." This is not quite accurate, but as Lithobius has 17 legs on each side, and Scolopendra (Cryptops is the English species) has 21 on each side, it is a better approximation than Centipede. But another country has a still more accurate term. I found some Scolopendra in Beyrout, and asked my native servant what he called them. He gave them what I afterwards found was the common Arab name for them, "‘arba wál ‘arbarin," forty-four legs. Now the Scolopendras, which in hotter climates are the chief representatives of the Centipedes, have actually forty-two legs, or, if the poison claws are counted, forty-four. In looking up the Arab term for Centipede I came across a curious description given of them by Avicenna, the great Arabian physician: "This is an animal known for its habit of going into ears. For the most part it is a palm's length" [about four inches, which is the average length of many species]. "On each side of the body it has twenty-two feet, and moves equally well either backwards or forwards."
With regard to its alleged habit of going into ears, the learned Arabian has evidently made a false imputation on the character of our animal, and has probably relied too much on the stories told him. He has also exaggerated in stating that it goes equally well either backwards or forwards. Some Centipedes can go backwards very easily and well, though not so well as forwards. Perhaps he preferred examining dead specimens, which afford an easy opportunity of counting their legs, to experimenting with living animals, which might have resented liberties taken with them.
The Persians have several words for them, less accurate than the Arabs and more like our own terms. For instance, they call them "Hazarpa," or thousand feet, like our Millepedes; also "Sadpa," or hundred feet, equivalent to our Centipedes. Another term resembles our common term before mentioned, "Chehlpa," forty feet. A more figurative term is "tasbih dud," a worm resembling a rosary with a hundred beads; this word is translated in Richardson's Persian Dictionary as "a venomous insect having eight feet and a piked tail."
Classification of the Myriapoda.
Two of the principal writers on the classification of the Myriapods are Koch and Latzel, both of whom have classified the whole group. I do not wish for a moment to undervalue the many authors who have done excellent work on the classification of different groups and families, but I wish here to give an outline of a classification of the whole class, and I naturally have recourse to the authors who have treated the subject as a whole.
Koch's two works, the System der Myriapoden[13] and Die Myriapoden,[14] cover the whole range of the class, and his divisions are clearly marked out and are easily understood, but both works are comparatively old. He does not include the Scolopendrellidae or the Pauropidae, which are now included by all naturalists in the Myriapoda. Latzel is a more recent writer, and though his work is entitled The Myriapods of the Austro-Hungarian Empire,[15] he gives much information about Myriapods not found in Europe, and his work is fairly entitled to be considered as embracing the whole class. He divides the Myriapods into four Orders, including the Scolopendrellidae and Pauropidae. On the whole, I think it will be better here to take the classification of Koch, and to add to it the two Orders before mentioned, viz. Symphyla containing one family the Scolopendrellidae, and Pauropoda with one family the Pauropidae.
The Orders are as follows:—
Order I. Chilognatha (= Diplopoda)
Antennae 7 joints, three anterior body rings with one pair of legs to each ring. Posterior rings with two pairs of legs to each. Genital organs opening ventrally on the anterior rings of the posterior part of the body, i.e. on one of the anterior of the segments bearing two pairs of legs; usually the 7th.
This Order is divided into eight families:—
Family 1. Polyxenidae.
Ten body rings, not counting the neck-plate. Thirteen pairs of limbs. Eyes hard to find, on the lateral corner of the head (Fig. 18, p. 37).
Family 2. Glomeridae.
11 body rings. 17 pairs of legs. Eyes arranged in a row curved outwards.
Family 3. Sphaerotheriidae.
12 body rings. 19 pairs of legs. Eyes crowded together in a cluster.
Family 4. Julidae.
Body cylindrical. More than 30 body rings. Many eyes crowded together in a cluster.
Family 5. Blanjulidae.
Thin cylindrical body with more than 30 body rings. Eyes either absent or in a simple row beneath the edge of the forehead.
Family 6. Chordeumidae.
Resemble the Polydesmidae (Fam. 7), but the head is longer and less rounded in the forehead. The antennae are placed more at the side of the head. Eyes small and numerous, in a cluster. Body rings always 30 (Fig. 16).
Family 7. Polydesmidae.
Body cylindrical, with a lobe or keel on the posterior part of the upper surface of the body ring. Always 19 body rings. No eyes.
Family 8. Polyzoniidae.
Body with varying number of rings arched transversely downwards and sharp at the sides. The anterior part of the ring somewhat hidden. The eyes in a simple row. The stigmata very small and placed near the lateral corner of the body ring. Head small in proportion to the body.
Order II. Chilopoda (or Syngnatha).
Antennae with many joints, at least 14. Only one pair of legs to each body ring. The genital opening on the last ring of the body. Bases of the legs widely separate.
There are four families in this Order:—
Family 1. Lithobiidae.
Body with 9 principal and 6 subsidiary rings. On both principal and subsidiary rings one pair of legs, except on the last ring of the body. Many eyes; the posterior ones large and kidney-shaped. The antennae with many rings.
Family 2. Scolopendridae.
Body with 21 or 23 rings, no intermediate rings. Every ring with one pair of legs. The last pair very long. Last pair at the point of the last ring. Four or no eyes. Antennae with 17 or 20 joints. (Fig. 15, p. 31).
Family 3. Notophilidae.
Body very long, 200 to 350 rings; alternate principal and subsidiary rings. A pair of legs to each principal ring. No eyes. Maxillary palps {46}very thick. Compact or very short limbs. The terminal point of the last limb without claws.
Family 4. Geophilidae.
Body long, 80 to 180 rings, principal and subsidiary. No eyes. The maxillary palps not compact, and with first joint large. Last joint of the last pair of legs with a sharp claw.
Order III. Schizotarsia.
The tarsi of all the legs multiarticulate. The eyes facetted. Peculiar sense organ beneath the head.
Family 1. Cermatiidae (Scutigeridae)
Antennae with unequal number of joints. Body rings, each with one pair of legs. Dorsal scutes not so large as ventral. Limbs long and multiarticulate. (Fig. 17, p. 35).
Order IV. Symphyla.
Myriapods resembling Thysanura. A pair of limbs to each segment. The antennae are simple and multiarticulate with unequal joints. Eyes few. Mandibles short. One pair of maxillae. No maxillipedes. Genital orifice in the last segment of the body. A single pair of tracheae. Two abdominal glands on the posterior part of the body. Two caudal appendages. Free dorsal scutes. Ventral scutes often with parapodia.
Family 1. Scolopendrellidae.
With the characters of the Order.
Order V. Pauropoda.
A pair of limbs to each segment. Antennae branched. Eyes few or none. Labrum and labium indistinct. Genital orifice at the base of the second pair of limbs. Free dorsal scutes. Nine pairs of feet (always?). Some segments with sensitive hairs. Last segment the smallest.
Family 1. Pauropidae.
Body slender. Dorsal scutes smooth. Limbs long and projecting from the lateral margins of the body. Colour pale.
The Structure of the Myriapoda.
Having now given a short view of the classification of the Class, I will proceed to give a general account of their structure, the variations in which have led to the divisions into the various Orders and Families. Their structure shows resemblances to several widely different classes of animals. One cannot help being impressed with their likeness to the Worms, at the same time they have affinities with the Crustaceans, and still more with the Insects. In the latter class the likeness of the Thysanuridae to Scolopendrella and Pauropus have induced a celebrated Italian anatomist, Professor Grassi, to claim the former as the ancestors of the Myriapoda.
Myriapods have a body which is segmented, as it is termed; that is, composed of a number of more or less similar parts or segments joined together.
One of the most important characteristics which distinguish Myriapods from other Arthropoda is the fact that they possess on the posterior segments of the body true legs which are jointed and take part in locomotion. The head is in all cases quite distinct from the body, and may be regarded as a number of segments fused together into one mass. Their heads are always provided with a single pair of antennae and mouth appendages, consisting of an upper lip, a pair of mandibles or jaws, and one to two pairs of maxillae. The mandibles resemble those of Insects, and are strongly toothed. In the Chilognatha a pair of maxillae are fused so as to form a single oval appendage. In the Chilopoda they each consist of a single blade bearing a {48}short palp or feeler. The mouth parts may have the forms known as chewing, biting, or suctorial (Polyzonium) mouth appendages.
With the exception of the terminal segment, and in many cases the first or the seventh, each segment bears one or two pairs of limbs. These may be very long, as in Scutigera, or very short, as in Polyxenus. They may be attached close to one another near the ventral middle line of the body, or may have their bases far apart from each other, as in the Chilopoda. The exoskeleton or external armour is composed of chitin (Chilopoda) or of chitin with calcareous salts deposited in it (Chilognatha).
Their internal structure has a great likeness to that of Insects.
The general position of the internal organs may be seen from Fig. 28, which shows a Lithobius dissected so as to exhibit the digestive and nervous systems.
The digestive canal, which is a straight tube, extends throughout the whole length of the body, and terminates in the last segment of the body. It may be divided into the following parts:—
1. A narrow oesophagus, beginning with the mouth or buccal cavity, and receiving the contents of two or more salivary glands (d).
2. A wide mesenteron or mid-gut (n) extending throughout almost the whole length of the body.
3. A rectum which at its junction with the mid-gut receives the contents of two or four Malpighian tubes (g, h) which function as kidneys. Their function was for a long time unknown, but the discovery of crystals of uric acid in them placed the matter beyond doubt.
The heart has the form of a long pulsating dorsal vessel which extends through the whole length of the animal. It is divided into a number of chambers, which are attached to the dorsal wall of the body, and are furnished with muscles of a wing-like shape, which are known as the alary muscles, and which govern its pulsations. The chambers are furnished with valves and arteries for the exit of the blood, and slits known as ostia for the return of the blood to the heart. The blood enters the chambers of the heart from the body cavity through the {49}ostia, and passes out through the arteries to circulate through the organs of the body and to return by the ostia.
Fig. 28.—Lithobius dissected. (After Vogt and Yung.) a, antennae. b, poison claws. c, brain. d, salivary glands. e, legs. f, nerve cord. g, Malpighian tube. h, Malpighian tube. i, vesicula seminalis. j, accessory gland. k, accessory gland. l, testis. m, thigh gland. n, digestive tube.
The two figures below (Figs. 29 and 30) show the position of the arteries and the ostia in a single segment of the body. The heart is too small and delicate to be seen with the naked eye; it therefore requires the aid of the microscope. A freshly-killed animal was therefore taken and prepared in the manner known to all microscopists, and extremely thin slices or sections cut horizontally from its back. One of these sections cut the whole length of the heart in one segment, which was accordingly drawn under the microscope (Fig. 29), and shows a longitudinal {50}horizontal section through the whole length of the heart in a single segment, with the two ostia at each end of the segment and the two arteries in the middle.
The arteries, when they leave the body, pass into masses of fatty tissue on either side of the heart, and the other figure (Fig. 30) is intended to show the artery leaving the heart and penetrating into the fatty tissue. The figure is taken from the same section as the former one, but is much more highly magnified, so as to show more detail. The delicate coats of the heart are shown, the artery being covered with a clothing of large cells.
Fig. 30.—Heart of Julus terrestris showing structure of artery (Art.) and external coat of heart (ext.c), also fat body (Fb), highly magnified. Ht, The cavity of the heart. The circular muscle fibres which surrounds the heart are shown just below the external coat (ext.c). ogl, Oil globules of the fat body.
Myriapods breathe by means of tracheae, with the exception of the Scutigeridae, which have an elementary form of lung which resembles that of spiders, and will be mentioned further on. These tracheae, as in Insects, are tubes lined with chitin, which is arranged in spiral bands. The tracheae open to the exterior by openings called stigmata, through which they receive the external air, which passes into the main tracheal tubes and into their ramifications, and thus effects the aeration of the blood.
The nervous system of the Myriapods consists, as in Insects, of a brain, which may be more or less developed, a circumoesophageal ring embracing the oesophagus, and a ventral chain of ganglia, and in some cases (Newport) of a system of visceral {51}nerves. With the nervous system we may mention the sense organs, the eyes, which are present in most cases, though wanting, as has been already stated, in many groups. They are usually present as clusters of ocelli or eye spots closely packed together, or (in Scutigera) as peculiarly formed facetted eyes. The sensory hairs on the antennae must be reckoned as sense organs, as also the tufts of sense hairs on the head of Polyxenus. Scutigera has also a peculiar sense organ beneath the head, consisting of a sac opening on the under side of the head full of slender hairs, each of which is connected at its base with a nerve fibre. Except the eyes, the Myriapod sense organs have usually the form of hairs or groups of hairs connected with nerve fibres, which communicate with the central nervous system.
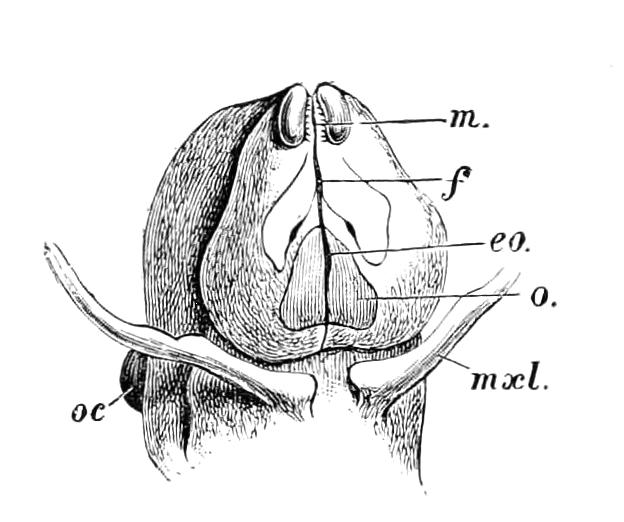
Fig. 31.—Under side of the head of Scutigera coleoptrata, with sense organ. eo, Opening of sense organ to the exterior; o, sense organ shown through the chitin; m, mouth; oc, eye; mxl, maxilla; f, furrow in the chitin. (Heathcote, Sense organ in Scutigera coleoptrata.)
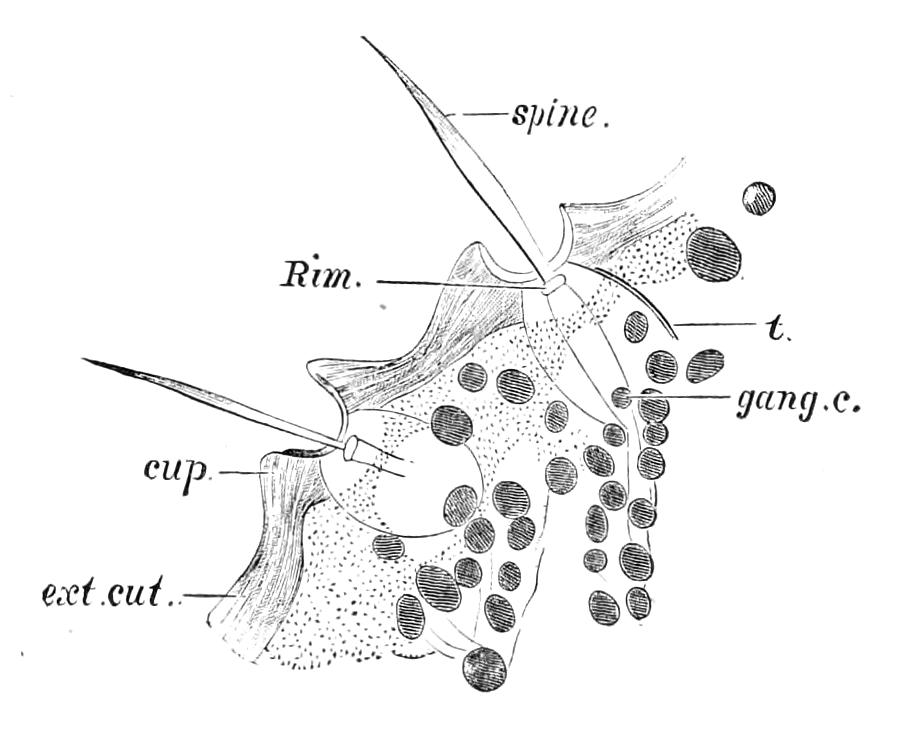
Fig. 32.—Highly magnified section through head of Polyxenus lagurus, showing sense organ. ext.cut, external cuticle; t, tube surrounding base of sense hair; gang.c, ganglion cell. (Heathcote, Anatomy of Polyxenus lagurus.)
These two sense organs are shown in Figs. 31 and 32. Fig. 31 shows the under side of the head of Scutigera (Fig. 17), with the position of the sense organ and its opening. Fig. 32 is part of a section through the head of Polyxenus with two of the sense hairs. Each spine or sense hair fits into a cup in the chitin of the head; and the lower or internal part, which is divided from the upper or external part by a rim, is joined to a ganglionic nerve cell (gang.c.).
The Myriapods are of separate sexes, and the generative organs in both cases usually have the form of a long unpaired {52}tube, which in the male is connected with accessory glands, and in the female is usually provided with double receptacula seminis. The generative openings usually lie near the base of the second pair of legs (Chilognatha), or at the posterior end of the body (Chilopoda). In the Chilognatha there is usually in the male an external copulatory organ at the base of the seventh pair of legs, remote from the genital opening.
The preceding account of the anatomy of the Myriapods has shown us the general characteristics of the whole group. I shall now take each of the five Orders into which the class is divided in the classification adopted in this account, and endeavour to explain the differences in anatomy which have led to the establishment of the Order. The first Order with which we have to do is that of the Chilognatha, which includes a large number of Myriapods; no less than eight families, some of them including a great number of forms.
Order I. Chilognatha.
The Chilognatha differ from other Orders in the shape of the body. This is in almost all cases, cylindrical or sub-cylindrical, instead of being more or less flattened as in the other Orders.
The body, as in all other Myriapods, is composed of segments, but in the Chilognatha these segments are composed, in almost all cases, of a complete ring of the substance of which the exoskeleton (as the shell of the animal is called) is composed. This substance is in the case of the Chilognatha chitin (a kind of horny substance, resembling, for instance, the outer case of a beetle's wing), containing a quantity of chalk salts and colouring matter; the substance thus formed is hard and tough. In other Orders the chitin of the exoskeleton is without chalky matter and is much more flexible. The length of the body, as may be seen from the classification, may be either very long, as in Julus, or very short, as in Glomeris.
The next anatomical character distinctive of the Order is the form of the appendages. First, the antennae. These are, as a general rule, much shorter than in the Chilopods, never reaching the length of half the body. They are, as a rule, club-shaped, the terminal half being thicker than the half adjoining the body.
The next appendages to be mentioned are the mouth parts. These differ in form from those of the other Orders, and their differences are connected very largely with the fact that the Chilognatha live on vegetable substances. Their mouth parts are adapted for chewing, except in the case of the Polyzoniidae, the eighth family of the Order, in which, according to Brandt, the mouth parts are adapted for sucking, and are prolonged into a kind of proboscis. The mouth parts of the Chilognatha consist of—
(1) An upper lip. A transversely-placed plate, which is fused with the rest of the head.
(2) A pair of powerful mandibles or jaws adapted for mastication, and moved by powerful muscles. f and g in Fig. 33 shows these mandibles, while the rest of the figure constitutes the broad plate (No. 3).
(3) A broad plate covering the under part of the head and partially enclosing the mouth. This structure, which, as we shall afterwards see, is formed by the fusion of two appendages which are distinct in the animal when just hatched, has been called the deutomalae, the jaws receiving the name of protomalae.
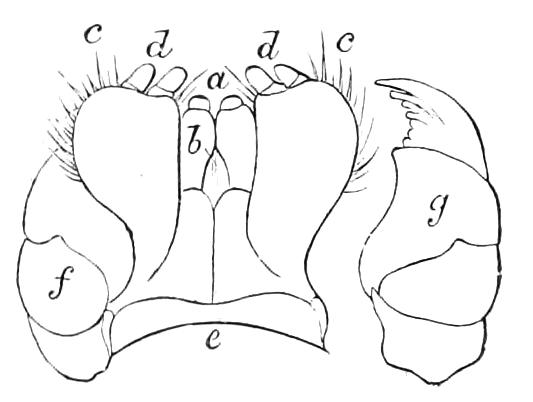
Fig. 33.—Mouth parts of Chilognatha. (From C. L. Koch, System der Myriapoden.) f and g, The mandibles. The parts marked a, b, c, d, e are firmly united and constitute the broad plate No. 3. They have received the following names—a, b, Internal stipes; c, external stipes; d, malellae; e, hypostoma.
After the mouth parts we come to the legs. We first notice the fact that the bases of the legs in each pair are closely approached to one another. They are so set into the body that the basal joints, or, as they are called, the coxal joints, nearly touch. This is the case in almost all Chilognatha, except in the Polyxenidae, and it is a fact connected with some important features in the internal anatomy. Then we have the peculiarity in the Chilognatha which has formed the basis of most classifications which have placed these animals in a group by themselves. This is the possession in most segments of two pairs of legs. This characteristic has caused the group to be called by some naturalists Diplopoda. As a general rule, the first four segments {54}have only three pairs of legs between them, one of them being without a pair of legs. This legless or apodal segment is usually the third. From the fifth segment to the end of the body all the segments have two pairs of legs each. The legs are shorter than those of the Chilopods, and are all nearly equal in size. This is not the case in the other Orders. The legs are commonly wanting in the seventh segment of the male, and are replaced by a copulatory organ. This peculiarity is related to the different position of the generative openings in the Chilognatha. Another anatomical feature peculiar to the Chilognatha is the possession of the stink glands—the glandulae odoriferae before mentioned. This, however, is a character which does not hold for all the Chilognatha, since the Polyxenidae have none of these glands. All the other families, however, possess them, and they are present in none of the other Orders.
As regards the internal anatomy of the Chilognatha, the digestive canal differs mainly in the glands which supply it with secretions. It receives the saliva from two long tubular salivary glands, which open at the base of the four-lobed plate which has been mentioned as the third of the mouth appendages. The secretion of these glands is used, as has already been said, in the process of preparing the nest for the eggs. We cannot fail to be reminded of a similar function of salivary glands in those swallows, which prepare the nests of which bird's-nest soup is made with the secretion of the salivary glands. Another feature in the form of the digestive tube is that in many cases, if not in all, it is marked with constrictions which correspond with the segments of the body.
The heart in the Chilognatha is not such a highly developed organ as in the other Orders. The muscles which have already been mentioned as the alary muscles (or wing-shaped muscles) are not so highly developed, and consist for the most part of a few muscular fibres. The muscular walls of the heart, which consist of three layers, have the muscles less strongly developed, and are in general adapted for a less energetic circulation.
The tracheae, which open into the stigmata, as has already been said, branch into tufts of fine tubes, but the ramifications of these tufts never join (or anastomose, as it is called), and consequently we never get, as in the other Orders, long tracheal trunks running along the body.
The nervous system, in addition to the existence of the visceral nerve system described by Newport, shows a marked peculiarity in the form of the ventral ganglionic chain. As has already been said, the nerve system consists of a brain or mass of ganglia fused together and connected with the ventral nervous cord by a collar of nervous substance surrounding the oesophagus, and generally known as the circumoesophageal collar. The ventral nerve cord is a stout cord of nervous substance passing along the whole length of the animal, and situated below (or ventral to) the digestive tube and the generative system. This cord is enlarged at certain points, and these enlargements or swellings are called ganglia, while from the ganglia pass off nerves which supply the different organs of the body. In the Chilognatha the cord has a compressed appearance as if the ganglia were pressed into one another in such a way that it is very hard to distinguish any ganglia at all. If we use the microscope and examine sections cut transversely through the cord, we see that it is not a simple cord. Even if we examine the nerve cord with a simple lens, we see that a furrow runs longitudinally down it, and the use of the compound microscope shows us that this furrow represents a division into two cords in such a way that the single stout cord as it appeared to the naked eye is in reality two cords running side by side, and so compressed together that the substance is partly fused together. The ganglia too are double, being swellings of the two cords and not a single enlargement on a single cord. As we shall see in the other Orders, this arrangement constitutes a characteristic distinction.
The generative organs consist of a long tubular ovary or testis lying along almost the whole length of the body and placed between the digestive organ and the nervous system. Near its exit from the body the long tube divides into two short tubes, the oviducts in the female or the vasa deferentia in the male. These ducts open in the third segment of the body, unlike those of Myriapods belonging to other Orders. The accessory glands present in most other Myriapods are not present in the Chilognatha.
The general arrangement of the organs of the Chilognatha may be seen from Fig. 34, which represents a transverse section through the body of Polyxenus (Fig. 18). A comparison of {56}these two figures (Figs. 34 and 18) will show the position of the organs mentioned in this account. The heart is shown with the suspensory and alary muscles attached.
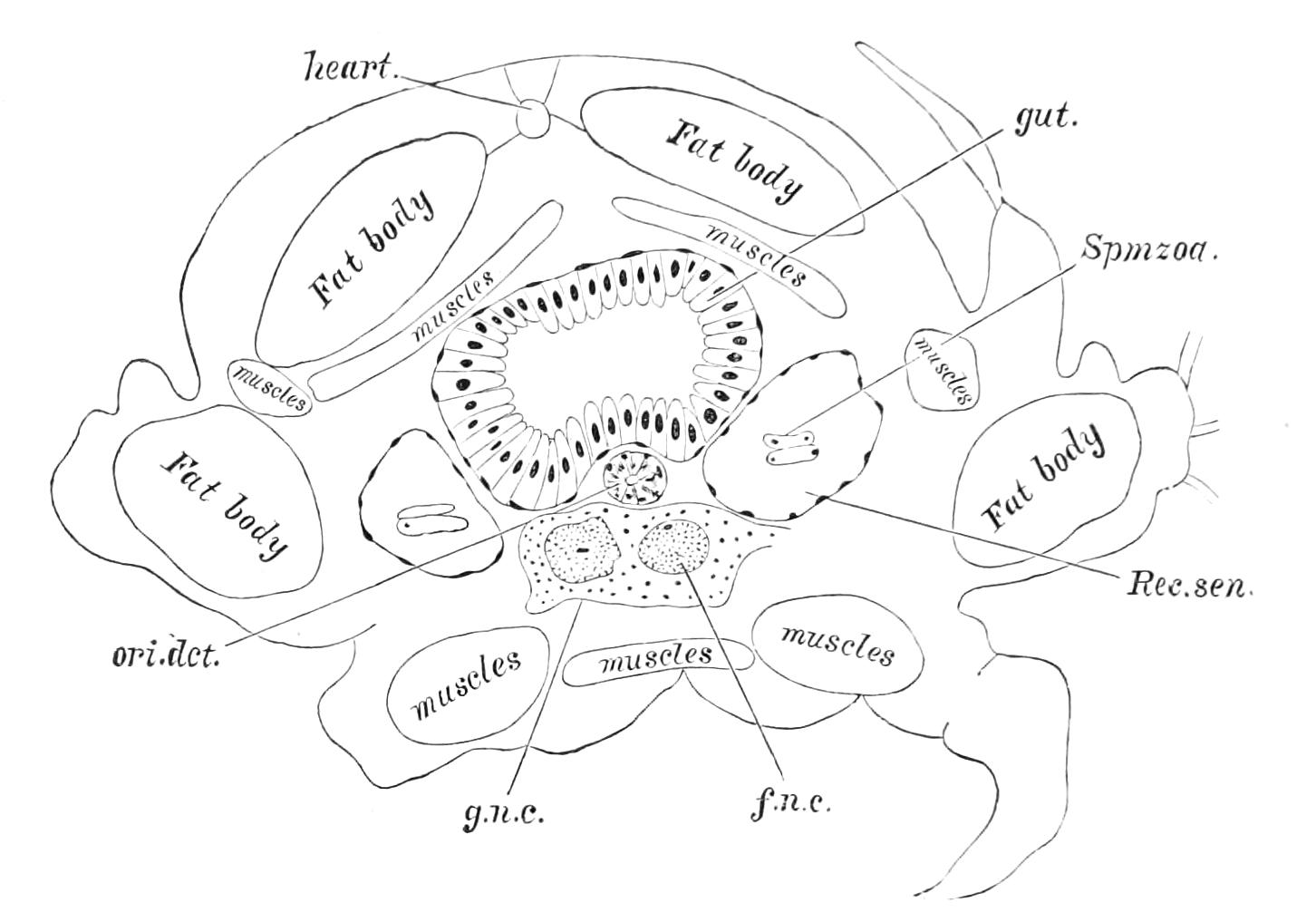
Fig. 34.—Transverse section through Polyxenus lagurus: g.n.c, f.n.c, ganglionic and fibrous parts of nerve cord; Rec.sen, receptaculum seminis; ori.dct, oviduct; Spmzoa, spermatoza. (From Heathcote, Anatomy of Polyxenus lagurus.)
Order II. Chilopoda.
The shape of the body differs from that of the Order which has been just described (Chilognatha), inasmuch as it is not cylindrical but flattened, the back, however, being more arched than the ventral surface. In this respect, however, it cannot be said to differ from the other Orders which we have yet to describe.
The segments are not formed by a single ring of the exoskeleton, which in this Order is formed of chitin, and is tough and flexible rather than hard and strong; but of two or three plates which form a covering to the segment. The back is covered by a large plate known as the tergum, the sides by two plates known as pleura, and the ventral part by a plate called the sternum. The pleura and sternum are, however, in most cases fused together or indistinguishable. In this, as in most of the anatomical peculiarities, there is a much greater difference between the two Orders Chilopoda and Chilognatha than between {57}the Chilopoda and the other three Orders which have still to be described.
The Chilopoda have only one pair of appendages to each segment of the body instead of two pairs like the Chilognatha.
The antennae of the Chilopoda are as a rule very long, and are always longer than in the Chilognatha which we have just described. They differ from those of the Schizotarsia (the third Order, which will be the next to be described) in having the basal joints nearer together; in other words, they are differently placed on the head. They differ from those of the Pauropoda (the fifth Order) in being straight and not branched. As a rule the antennae of the Chilopoda taper towards the extremity.
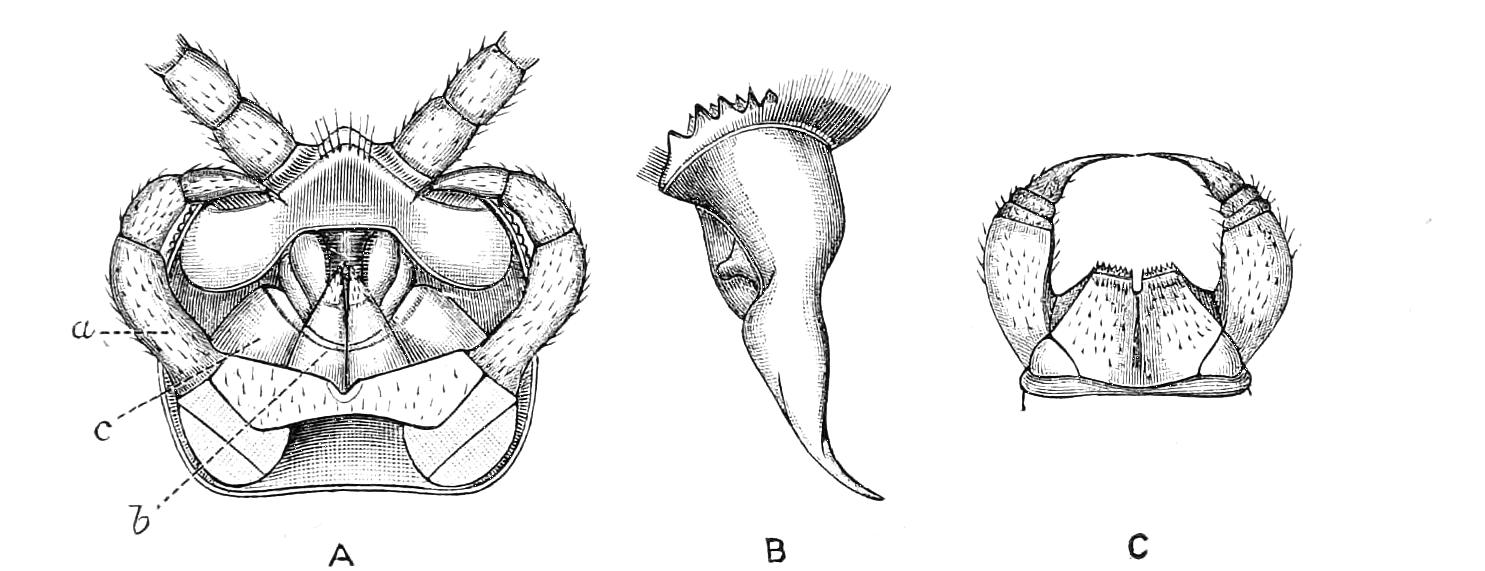
Fig. 35.—Mouth parts of Lithobius (Latzel). A, Head of Lithobius seen from the under surface after removal of poison claws; a, second maxilla; b, c, the two shafts of the first maxilla. B, One of the mandibles. C, The two poison claws.
The mouth parts are more numerous than in the Order we have just described (the Chilognatha). They consist of—
1. An upper lip. This is a transverse plate as just described in the case of the Chilognatha, but it is not always fused with the rest of the head. It is also usually composed of three pieces, two lateral and a middle piece.
2. A pair of jaws or mandibles, which are not of so simple a form as those of the Chilognatha, but rather resemble those of some of the Crustacea.
3 and 4. Two pairs of appendages called maxillae resembling feet, but used to aid the act of eating instead of locomotion. They are very different in different Chilopods, but are mostly slender and weak and usually provided with feelers (or palps) growing out of the main stem.
5. The next pair of appendages are the first pair of the legs of the body, which are also metamorphosed to serve a function different from the ambulatory function of the other limbs. These are the poison claws, and the possession of these forms another distinction between the Order we are now discussing and that of the Chilognatha. At the same time the third Order, that of the Schizotarsia, has poison claws, so that this feature does not separate the Chilopoda from all the other Orders. These poison claws are large curved claws connected with poison glands, the secretion of which flows through a canal which opens near the point.
The legs are longer than those of the Chilognatha, but not so long as those in the next Order to be described (the Schizotarsia). Their number is very various, from 15 pairs in Lithobius to 173 in the Geophilidae. Latzel notes a curious point in the number of the legs in this Order, namely, the number of pairs of legs is always an uneven one. There are always one pair to each segment. The last pair of legs is always longer than the other pairs, and this is a peculiarity of the Order.
The digestive tube resembles that of the other Orders, but the salivary glands are not long and tubular but short (Fig. 28, d). It is, moreover, not marked with constrictions corresponding with the segments of the body.
The tracheal system or the system of respiration may be said to be more highly developed in this Order than in any other. The tracheal branches anastomose with one another (that is, the branches join), and in some cases form long tracheal stems running along the body almost for its whole length. The number of the tracheal openings or stigmata varies and does not correspond with the number of segments.
The nervous system differs considerably from that in the Order Chilognatha; it resembles that in the Schizotarsia, and differs again from that in the other two Orders, Symphyla and Pauropoda. The brain shows some differences from other Orders chiefly in the development of the different lobes which are connected with the sense organs, the eyes and antennae, for instance; but the most marked difference is in the ventral ganglionic cord. First, the ganglionic swellings are much more clearly marked than in the Chilognatha. Secondly, the first three ganglia differ {59}from the others in being nearer to one another and forming a single mass when seen by the naked eye, though when examined by the aid of a microscope we can see all the different parts are there. Thirdly, the division into two cords mentioned in the Chilognatha is carried to a much greater extent. The ganglia in each segment can be seen plainly to be double, and the cords connecting the ganglia are two in number. We can plainly see that the ventral nervous system of the Chilopoda consists of two cords lying parallel to one another, and each having a ganglionic enlargement in every segment. Whether a visceral nervous system is present in the group is doubtful.
The eighth family of the Chilognatha, the Polyxenidae, show an approach to the Chilopod nervous system.
The generative system differs chiefly in the opening of the genital apparatus at the end of the body instead of in the third segment; though this difference only separates the Order from the Chilognatha and not from the other Orders. They also have two pairs of large accessory glands (as they are called) connected with the genital openings.
Order III. Schizotarsia.
The third Order of Myriapods, the Schizotarsia, show a much greater resemblance to the Chilopoda than to the first Order, the Chilognatha. There are, however, important differences to distinguish them from all the other Orders.
The shape of the body is short, thick, and very compact. The composition of the individual segments resembles that found in Chilopoda rather than that of Chilognatha.
The antennae are very long, longer than in any of the Chilopods, and are composed of a great number of very small joints. The mouth parts show a greater length and slenderness than do those of the other Orders mentioned as yet. They consist of—
1. An upper lip partly free, but fused at the sides with the rest of the head. The upper lip is in three parts, as in the Chilopoda, but with the middle part very small and the lateral pieces large.
2. A pair of jaws or mandibles. These are provided not only with teeth, as in the other Myriapods, but also with a sort of comb of stiff bristles.
3 and 4. Two pairs of maxillae or foot jaws distinguished by their length and slenderness.
5. The poison claws long, slender, and not sharply curved. The bases of the poison claws hardly fused together and short.
The respiratory system in the Schizotarsia differs from that in all other Myriapods in the fact before mentioned, that they breathe by means of lungs and not by tracheae. There are, as before mentioned, eight dorsal scales in these animals; each dorsal scale except the last bears one of the peculiar organs which I have called lungs. At the hinder end of the scale there is a slit which leads into an air sac, from which a number of short tubes project into the blood in the space round the heart and serve to aerate it before it enters the heart. The heart, therefore, sends aerated blood to the organs, while in the tracheal-breathing Myriapods the blood is aerated in the organs themselves by means of tracheae.
The poison claws are followed by segments bearing fifteen pairs of true ambulatory legs. These are covered by eight large dorsal plates, increasing in size from before to the middle of the body, the middle plate being the largest, and then diminishing in size.
The nervous system resembles that of the Chilopoda, but there is a special pair of nerves which supply the sense organ, which has been mentioned as peculiar to the Order. The ventral nerve cord shows a very clear division into two, the ganglia of the two cords being almost entirely separate. The first few ganglia are fused, as has been mentioned in the Chilopoda.
The digestive tube resembles that of the Chilopoda. The legs are very long and slender, and the joints are beset with bristles. Both sexes have small hook-like appendages at the sides of the genital openings.
The eyes have already been mentioned as being more highly developed than in other classes, in correspondence with the more active habits of the animal. The generative organs open at the hind end of the body, as in Chilopoda.
The heart is highly developed, quite as much so as the Chilopod heart, the alary muscles being strong and broad, and the arteries being quite as perfect as those in any Myriapod. The muscular coats which govern the pulsations by their contractions are powerful and well developed.
Order IV. Symphyla.
We next come to one of the last two Orders which have been recently added to the Myriapoda. These little animals have a great resemblance to the Thysanura among the Insects, and especially to Campodea among the Thysanura. It will be well, therefore, to begin our account with a few of the reasons which have induced naturalists to include them among the Myriapods rather than among the Thysanura.
1. Campodea has three pairs of mouth appendages, while Scolopendrella has only two.
2. Scolopendrella has broad plates covering the back, not only on the anterior (thoracic) segments, but on the whole body.
3. The terminal appendages of Scolopendrella differ from those in Campodea.
4. Scolopendrella has a sense organ which is absent in Campodea.
5. Campodea breathes by means of three stigmata in the anterior part of the body. The stigmata of Scolopendrella are hard to see, and are not in the same position.
6. Scolopendrella has twelve pairs of legs, and Campodea, like all Insects, has only three.
I will now go on to an account of their anatomy. The body is small and slender, and is covered with a delicate shell or exoskeleton of chitin, which is so thin as to be almost transparent.
The antennae are long, and are composed of many joints of equal size.
The mouth parts consist of—
1. An upper lip.
2. A pair of mandibles.
3. A pair of maxillae.
The segments are not all of equal size. Some are larger than others. The larger and smaller segments are arranged alternately, and the smaller do not bear legs. As before stated, there are twelve leg-bearing segments.
At the end of the body there are two hook-like appendages which are pierced by a canal, through which is poured the secretion of a pair of glands. Near the sides of these appendages are a pair of sense organs, consisting of long hairs connected with nerves.
The digestive canal is a long straight tube passing through the length of the body. In the middle it is much enlarged, so as to form a stomach with a glandular coat. Posterior to the stomach the digestive tube receives the contents of two Malpighian tubes which act as kidneys.
The tracheal system consists of a single pair of stigmata on the under surface of the head, and the tracheae connected with them.
Order V. Pauropoda.
The Pauropoda, which form the fifth Order of Myriapods, are as yet very imperfectly known. Pauropus was discovered by Sir John Lubbock, and its discovery was announced by him in 1866. He found this little Centipede in his kitchen garden among some Thysanura, and at first considered it as a larval form, but continued observation showed that it was a mature creature. He described it as a small, white, bustling, intelligent little creature about 1⁄25 inch in length.
The antennae are very curious and highly characteristic of the Order. They resemble those of Crustacea rather than those of Myriapoda. Each antenna is composed in the following manner. First there is a shaft of four joints. From the fourth joint of this shaft spring two branches; one of these two branches is narrower than the other, and ends in a long thin bristle composed of a great number of joints. The other and broader branch bears two such bristles, and between them a small pear-shaped or globular body, the function of which is unknown.
The mouth parts consist of two minute pairs of appendages, the anterior pair toothed and the posterior pointed. The body is rather narrower in front; the segment behind the head has one pair of legs, the second, third, fourth, and fifth behind the head two each. The posterior legs are the longest; the genital organs open at the base of the second pair of legs, between these and the third pair. The manner of breathing is as yet unknown, tracheae not having been discovered.
Pauropus at first looks most like a Chilopod, but differs from that Order—
1. In the form of the antennae.
2. In the absence of poison claws and in the form of the mouth parts.
3. The opening of the generative organs being in the front part of the body.
It differs from Chilognatha in the following respects:—
1. The legs are not of equal length, the posterior legs being the longest, as in Chilopods.
2. The mouth parts differ from those of Chilognaths almost as much as from those of Chilopods.
3. The form of the antennae.
Only a few Pauropoda have been discovered as yet.
Embryology.
The preceding account of the anatomy of Myriapods would be incomplete without some reference to the wonderful manner in which the different organs of the body are built up; the whole of the complex organism proceeding by a gradual and regulated process of development from a simple cell called the ovum derived from the female body, and united with a cell from the male body (called the spermatozoon). I hope to be able to give my readers some idea of the interest which the pursuit of the difficult study of embryology adds to anatomy, by offering us a key to the interpretation of the relations between our knowledge of the forms at present living on the earth and those which, we learn from Palaeontology, have inhabited our planet in past ages.
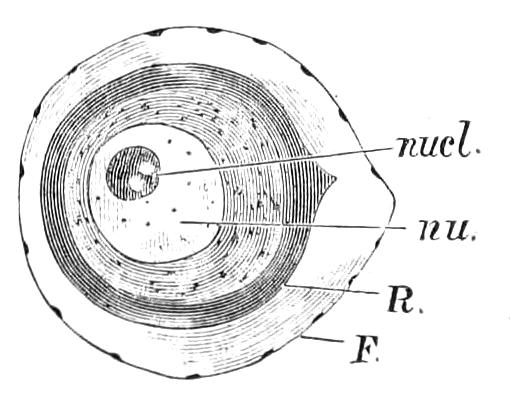
Fig. 36.—Young ovum of Julus terrestris: nucl, nucleolus; nu, nucleus; R, first appearance of yolk; F, follicle cells.
Like all living creatures with which we are acquainted, the starting-point of Myriapod life is the ovum, as it is called. This ovum is a cell resembling the cells of which the body of all living animals are built up, and which may be compared to the bricks of which a building is composed. This cell or ovum is a small sphere of living transparent substance called protoplasm, and it is nucleated—that is, it contains a small spot of denser protoplasm called the nucleus, and within that a still smaller spot of still more dense protoplasm called the nucleolus. In the process of impregnation the ovum unites with the male cell, and the cell so formed is called the impregnated ovum. This ovum has the property of dividing into two cells, each resembling the {64}parent cell from which it is derived; each of these cells has, like the parent cell, the same property of dividing into two more, and so on. Thus from this continual process of division or reproduction of every living cell, the materials are provided for the building up of the body.
The regularity of the process of the division of the ovum, or, as it is called, segmentation of the ovum, is interfered with by the presence of food yolk. The cells formed by the process of cell division just described need nourishment, and this nourishment is supplied to them by the food yolk formed in the body of the ovum before the process of segmentation begins. It is easy to understand that this yolk, which is not alive like the cells, cannot divide like them, and therefore the segmentation of the ovum in Myriapods is irregular, as it is called.
I will now go back a little and describe what happens to the ovum before the process of segmentation is complete. It increases in size and forms the supply of food yolk which is to provide the nutriment of the ovum. Then after impregnation the egg-shell is formed round it, and it becomes what we know as the egg. This egg is not a perfect sphere, but is oval (in most Myriapods) in shape. The egg is laid, and the process of segmentation begins shortly after it is laid, as has already been described.
When it has been laid for about 36 hours, if we take an egg and, after proper preparation, cut it into thin slices known to {65}microscopists by the name of sections, and examine it by means of the microscope, we shall see that segmentation has resulted in this. Just beneath the egg-shell there is a thin layer of cells, one cell thick, which completely surrounds the egg. Inside this coat of cells is the food yolk, with a few cells scattered about in it at rare intervals, something like the raisins in a plum-pudding.
With the next process the formation of the young Myriapod may be said to begin. A strip along the length of the oval-shaped egg is thickened, and this thick mass of cells represents the future ventral surface of the animal. The rest of the thin layer of cells already mentioned just below the shell will form the shell or exoskeleton of the future animal. The thick strip of cells at the ventral surface has by this time split into layers, so that, resorting to our microscope again, a section through the short axis of the oval-shaped egg—a transverse section—will show us—
1. The egg-shell.
2. A layer of cells completely surrounding the egg, thin everywhere but on the ventral surface. This layer is known to embryologists as the epiblast. The thick part of the epiblast on the ventral surface gives rise to the nervous system.
3 and 4. Two layers of cells connected in the middle, along the line of the thick strip, but separate elsewhere, and not extending round the whole of the inside. These layers constitute what is known as the mesoblast, and give rise to the muscles and most of the internal organs.
5. The scattered cells in the yolk. They are known as the hypoblast and give rise to the digestive canal.
After this point is reached the formation of the organs begins. The segments are formed in order from before backwards. First the head, then the next segment, and so on. When the number of segments with which the animal will be hatched are formed, another process begins, and the tail end of the animal, which can already be distinguished, is bent towards the head. This is a process that takes place in many animals besides Myriapods, and is called the formation of the ventral flexure. Shortly after this the animal bursts the shell and comes {66}into the outer world. The various processes may be understood by reference to the Figs. 36, 37, 38, 39, which are successive stages in the development of a Chilognath. Figs. 37, 38, are thin slices through the shorter diameter of the egg, which, as before mentioned, is an oval in shape. Fig. 39 is a section through the longer diameter of an egg in a more advanced stage of development, in fact just about to burst the shell. The body of the future animal is marked by constrictions, the future segments. Some of the organs are already formed, as the brain and the digestive tube, the openings of which will form the mouth (st) and the anus (pr).
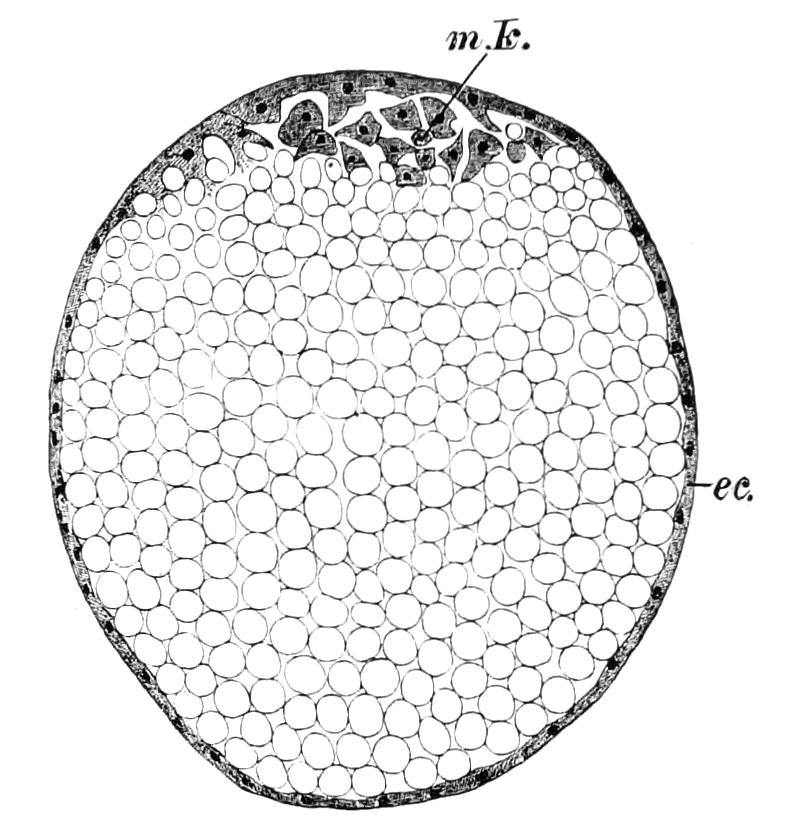
Fig. 38.—Transverse section through next stage: mk, keel-like mass of cells from which the mesoblast is produced; ec, epiblast. (From Heathcote, Post. Emb. Dev. of Julus terrestris; Phil. Trans. vol. 179, 1888, B.)
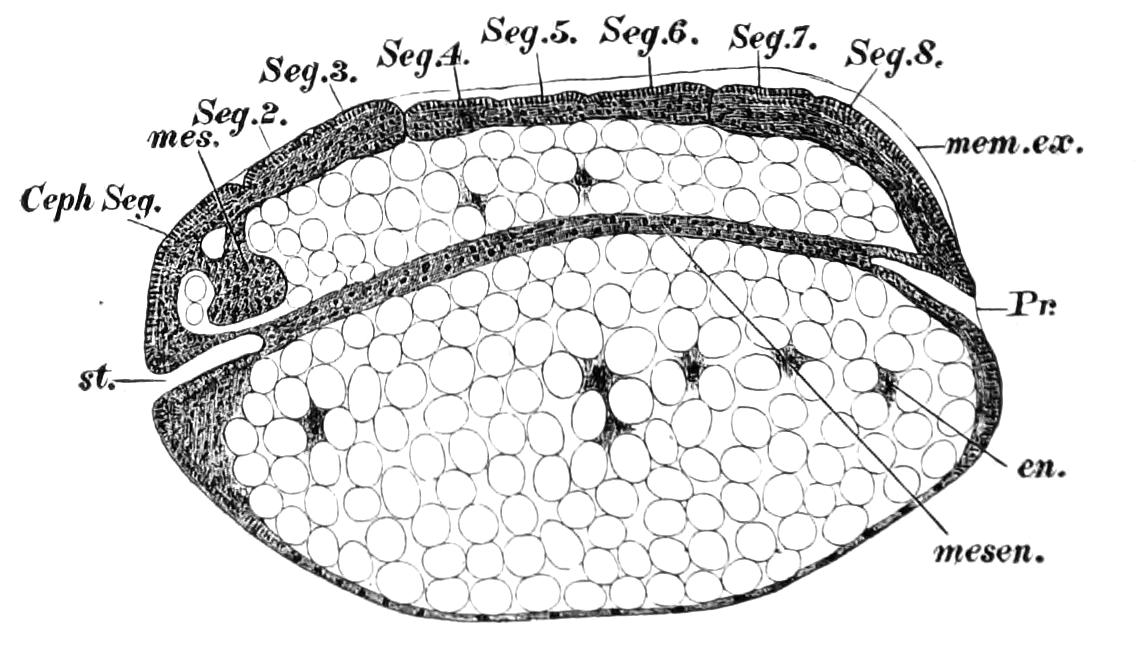
Fig. 39.—Longitudinal section through later stage: Segs. 2, 3, etc., segments; Ceph. Seg, head; mes, mesoblast; en, hypoblast; st, future mouth; pr, future anus; mesen, gut; mem.ex, as in Fig. 41. (From Heathcote, Post. Emb. Dev. of Julus terrestris.)
Myriapods are hatched at different stages of development. The Chilognatha have only three appendages, which are so little developed that they are only small shapeless stumps, while {67}the Chilopoda have the full number of legs in some cases; in others only a small number of legs, but yet more than the three pairs of legs of the Chilognatha, and fully developed instead of stump-like. The eyes are usually developed late in the life of the young animal. The bursting of the egg-shell is assisted in some Myriapods by a special kind of spike on the back part of the head.
The Fig. 40 shows a young Chilognath which has just burst the shell and come into the outer world. It is still surrounded with a membrane which has been formed by its skin or epiblast within the egg. One eye-spot has been formed.
Fig. 41 shows a longitudinal section through the young Chilognath shown in Fig. 40, and the next (Fig. 42) a transverse section through the same. In comparing the two Figs. 41 and 42 it must be remembered that they are sections in different planes through the animal shown in Fig. 40, and therefore they only show a small portion, a thin slice, of the organs.
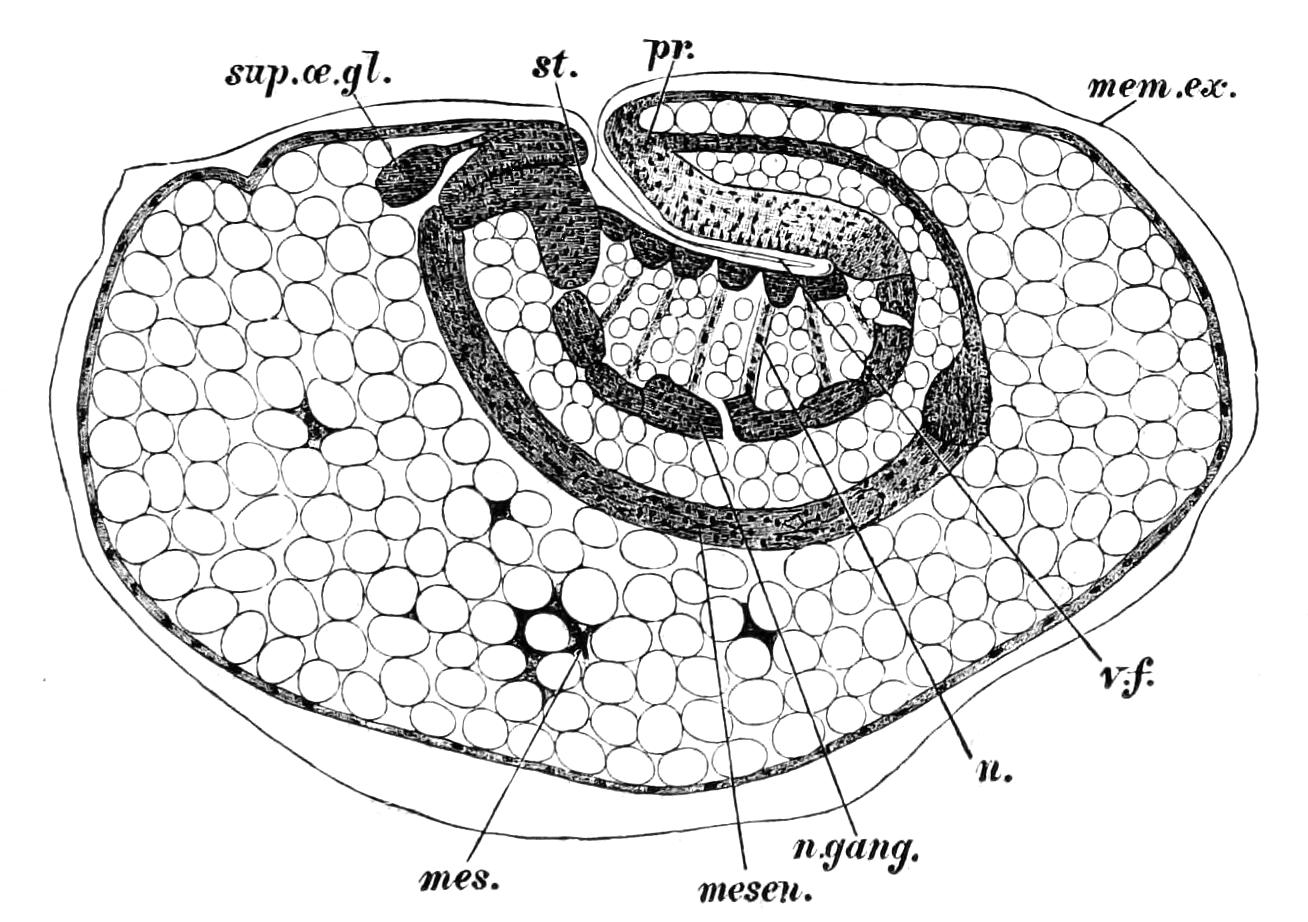
Fig. 41.—Longitudinal section through late stage: Sup.oe.gl, First appearance of brain; st, mouth; pr, anus; mesen, gut; n, nerve cord; n.gang, nerve ganglion; mem.ex, membrane surrounding the animal; v.f, ventral flexure; mes, mesoblast cells. (Heathcote, Post. Emb. Dev. of Julus terrestris.)
The first appearance of the mouth appendages has been already mentioned, and these are shown in Fig. 43, where the small stumps that later on change to jaws are shown. The figure shows the head of a young Chilognath seen from the lower side, and the second pair of stumps fuse together later on and produce the broad plate already mentioned as the characteristic mouth appendage of the Order.
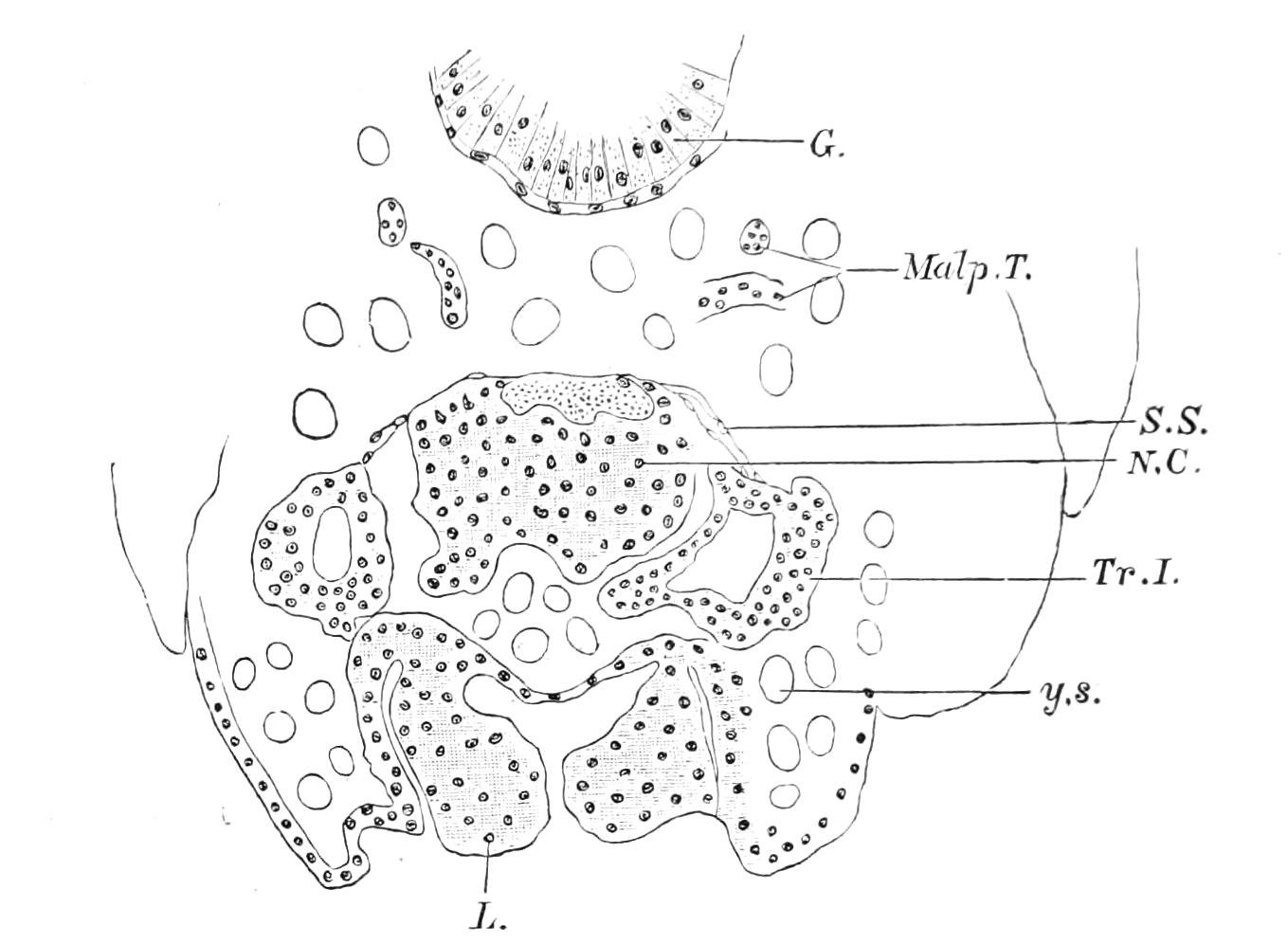
Fig. 42.—G, gut; Malp.T, Malpighian tube; N.C, nerve cord; Tr.I, deep invagination by which the tracheae are formed; y.s, yolk spherules still present; L, first appearance of legs; S.S, part of mesoblast. (Heathcote, Post. Emb. Dev. of Julus terrestris.)
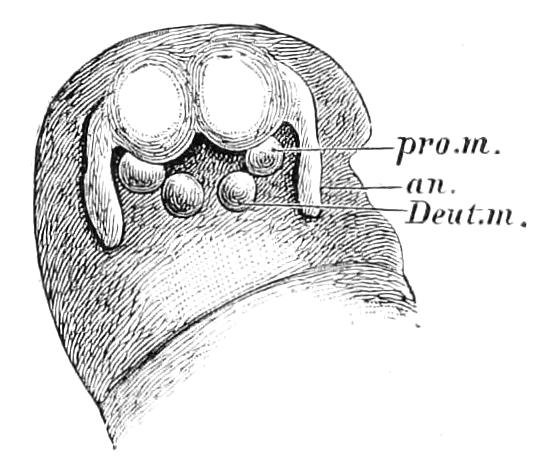
Fig. 43.—Under surface of the head of a young Julus terrestris: pro.m, rudimentary jaws; Deut.m, rudimentary mouth plate; an, antennae.
After the animal is hatched it has still, in the case of most Myriapods (those which are not hatched with all the segments complete), to undergo a further development, and in particular the eyes are still unformed. The process of development of the eye has only been followed out as yet in the Chilognatha, and in only one form, Julus, and is so curious that a short account may be of interest here. The development of the eye begins (in Julus) on the fourth day after hatching, and continues until the animal is full grown. A single {69}ocellus or eye-spot appears first, and the rest are added one by one until the full number are reached.
The first appearances connected with the formation of the eye take place in the cellular layer just beneath the chitinous exoskeleton. This layer, called the hypodermis, plays an important part in the organisation of the animal. It forms the inner layer of what we may call the skin of the animal, and the cells of which it is composed secrete the chitin of which the shell or exoskeleton of the animal is composed, and which is moulted every year.
The first process in the formation of the eye-spot is the thickening of the hypodermis beneath the chitin, just in the place where the eye will come. At the same time the cells of this thickened mass of hypodermis secrete a quantity of pigment of a dark red brown colour. Next the cells of the thick mass of hypodermis begin to separate from one another in such a way that a vesicle is formed. This vesicle is hollow inside, and the thick walls are formed from the cells of the thickened hypodermic mass. This can be seen from Fig. 44, which represents a section through an ocellus when it is partly formed. From this vesicle the eye is formed.
The wall of the vesicle nearest the exoskeleton gives rise to the lens of the eye, while the other walls of the vesicle form the retinal parts of the eye. The cells from the brain grow out and form the optic nerve connecting the retina with the brain. The whole eye spot is covered internally by a thin membrane, formed not from the hypodermis but by cells from the inside of the body (mesoblast cells).
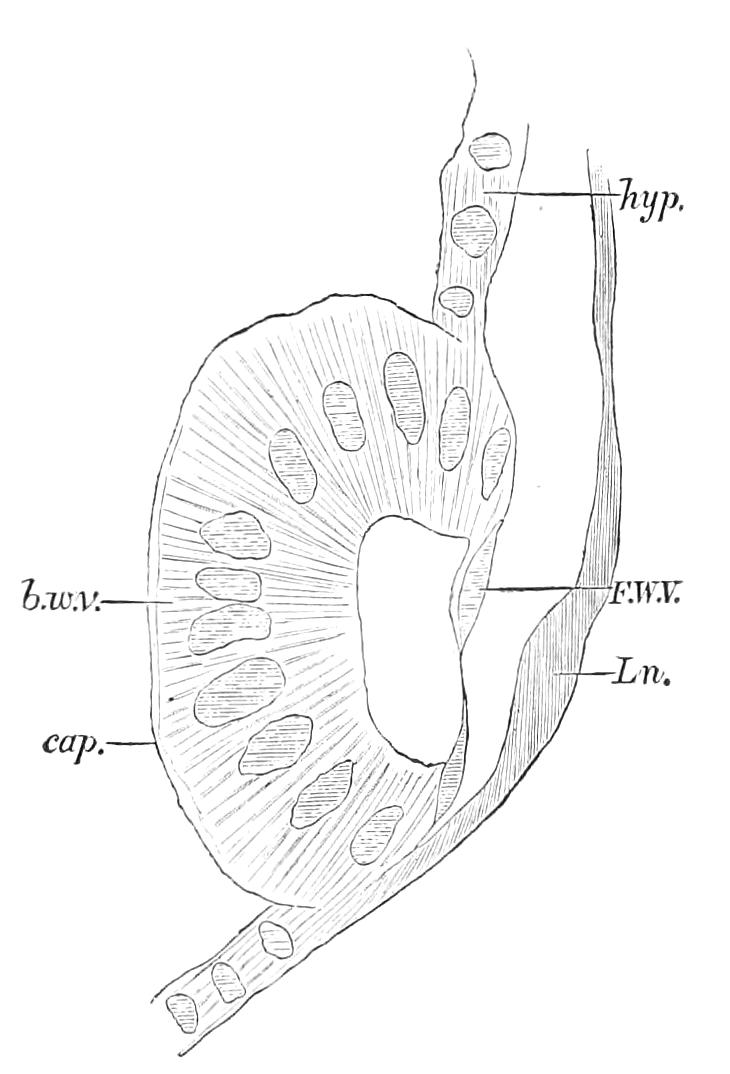
Fig. 44.—Section through eye when first forming: Hyp, hypodermis; Ln, lens; F.W.V, front wall of optic vesicle; b.w.v, back wall of vesicle; cap, capsule.
In the Chilognatha, the first Order of Myriapods, the young {70}animal leaves the egg with three pairs of appendages; the first have already the form of antennae, the second will form the jaws, but have not yet taken their proper form, while the third pair will fuse together and alter their shape so as to form the curious plate that has already been mentioned as forming the second pair of mouth appendages. Behind the mouth appendages will come the first three pairs of legs. The whole young animal on leaving the egg is enveloped in two membranes. These membranes are secreted by the outside layer of cells in the same way that the shell or exoskeleton of the animal will be eventually formed, and represent the first two moults of the animal, which continues to moult its shell every year throughout life.
Of the Chilopoda, the second Order of Myriapods, all the families leave the egg-shell with the full number of legs, with the exception of the Lithobiidae, which have seven pairs of legs including the poison-claws. The Schizotarsia, the third Order, also have seven pairs of legs when hatched.
The legs make their appearance not one by one but in batches (in Julus terrestris in batches of five). The addition of legs and segments to the body takes place, not at the end of the body, but between the end segment and the penultimate.
This is a short sketch of the gradual development of the Myriapoda from the ovum to the fully-grown animal. It is, I am aware, a short and insufficient account of all the beautiful processes by which the different organs take their rise, but space is insufficient here, and too much detail would be out of place in a work of this nature, which only aims at giving an outline sketch of the group, which shall be intelligible to the general reader who has not made a special study of such matters. Before leaving the subject, however, I must mention a few of the points of interest which are to be learned from the examination of the course of development which has been sketched here. One of the greatest puzzles in the natural history of the Order Chilognatha has always been the double segments, as they are called; that is, in fact, the possession of two pairs of legs to each segment, which is, as we have already said, a distinguishing characteristic of the Order. As we have seen, the Chilognatha at an early stage of existence do not possess this characteristic, which is only peculiar to the adult {71}and half-grown forms. Now what does this mean? Does each double segment in the full-grown Millepede represent two segments which have become fused together, or is each double segment, so called, a real segment resembling the segments present in the other Orders (for instance, Chilopoda), which has grown an extra pair of legs? Both these views have been advocated by distinguished naturalists. Neither of them is, in my opinion, quite right when viewed in the light cast on the subject by recent investigations into the life history of the Chilognatha.
A close examination into the minutiae of the growth of the different organs has shown us that the double characters of the double segments are more deeply seated than was imagined. The circulatory system, the nerve cord, and the first traces of segmentation in the mesoblast all show this double character, and the only single part about the segment is the broad plate covering the segment. Now in some of the most ancient of the fossil Myriapods this broad plate shows traces of a division, as if it were in reality two plates fused together. We have also to consider that the life history of the Chilognatha allows us to believe that the peculiar cylindrical shape of the body shown in the greatest degree in the Julidae is attained by the unequal development of the dorsal and ventral surfaces of the body; the ventral surface being compressed together till it is extremely narrow, and the dorsal surface, as it were, growing round it till the originally dorsal surface forms almost a complete ring round the body. Taking all this into consideration, we are justified, in my opinion, in concluding that each double segment in the Chilognatha is not two segments fused together, nor a single segment bearing two pairs of legs, but is two complete segments perfect in all particulars, but united by a large dorsal plate which was originally two plates which have been fused together, and which in most Chilognatha surrounds almost the whole of two segments in the form of a ring.
Again in the Chilopoda we see that a great distinctive feature that separates them from the Chilognatha is the character of the ventral nerve cord, the cord being double and not single, a character connected with the fact that the bases of the legs are widely separated from one another, and not closely approached to each other, as in the Chilognatha. As we before said, a more {72}minute anatomical examination showed us that this difference was not so great as appeared at first sight, the cord showing traces of a duplication. Well, are these traces superficial, or do they represent a state of affairs more or less similar to that in the Chilopoda? Embryology helps us to answer this question also. In the early stages of the Chilognatha we find that the nerve cord has exactly the form of that in Chilopoda, showing us that the appearances in the anatomy had led us to a right conclusion, and giving us a valuable confirmation of our views. These two examples will serve to show the kind of interest which attaches to embryology.
Palaeontology.
We have seen that embryology enables us to look at the structure of the Myriapods from a new standpoint, and to correct and supplement the knowledge gained from an examination of the adult animal. In the same way a study of the forms of Myriapods which have become extinct on the globe, and have been preserved to us in a fossil form, gives a further opportunity of considering the relations of one form to another, and again of the relations of our group to other groups of animals now existing on the earth. Myriapod fossils have been found in strata of great antiquity. The oldest of such fossils must have been among the first land animals. The figure below shows a fossil Myriapod found in America, belonging to the Order of the Protosyngnatha which are only found in the Palaeozoic strata. It is a good example of the manner in which Myriapods were protected by bundles of bristles in the same way as the Polyxenus of the present time.
The oldest fossil Myriapods which have been discovered at the present time are two species which have been found in the Old Red Sandstone in Scotland. To realise the antiquity of these Myriapods, it will be worth while recalling the typical fossils found in the Old Red Sandstone, so as to see what the contemporaries of these ancient Myriapods were like. Among the plants there were Algae, Ferns, and Conifers, belonging to the lower divisions of the plant tribe. Among the animals there were Sponges, Corals, Starfish, Worms, Shell-fish, and Fishes, but none of the more highly organised of the animal or vegetable tribe {73}had appeared on the earth. The Myriapods of the Old Red Sandstone, as has been before said, differ considerably from those of the present day, and as we proceed towards the species found in the more recent strata we find them more and more like the ones at present living, till we get to the Polyxenus and other species found in amber, which are hardly to be distinguished from living forms.
The next oldest fossil Myriapods are found in the coal measures, when both the animal and vegetable kingdoms were represented by more numerous and more specialised forms. The fossil fauna of this period is characterised by the number of gigantic Amphibia, many remains of which have been found. The great forests and the abundant vegetation of this time must have been favourable to the existence of our class, and accordingly we find no less than 32 species of fossil Myriapods. Of these most have been found in America, some in Great Britain, and some in Germany. One well-preserved fossil of Xylobius sigillariae was found by Dr. Dawson in America in the stump of a tree in the remains of a fossil forest. The eyes, head, and legs were plainly seen under the microscope. All these fossils belong to the earliest or Palaeozoic period.
The figure below (Fig. 46) shows a fossil also from the coal formations of Illinois, America, belonging to the family of the Euphoberiidae mentioned further on. It shows a nearer approach to the Julidae of the present time. The limbs, however, were of very curious shape, and may possibly have been adapted to locomotion in water as well as on land, and the small supposed branchiae on the ventral surface shown in Fig. 46, B, may possibly have been an arrangement to render respiration in the water possible.
In the secondary period the Myriapods were scantily represented, or, at any rate, geologists have failed to find their fossils. The class is represented by a single specimen found in the chalk in Greenland. This fossil, which has been included in the Julidae under the name of Julopsis cretacea, may perhaps belong to the Archipolypoda.
Passing on to the Tertiary or Recent period, we find the Myriapods again numerous, and more nearly resembling those living at the present time. They belong mostly to the Chilognatha and Chilopoda. They have been found in the fresh-water gypsum of Provence in France, the brown coal of Germany, and the green river formations of America. Several have been found in amber.
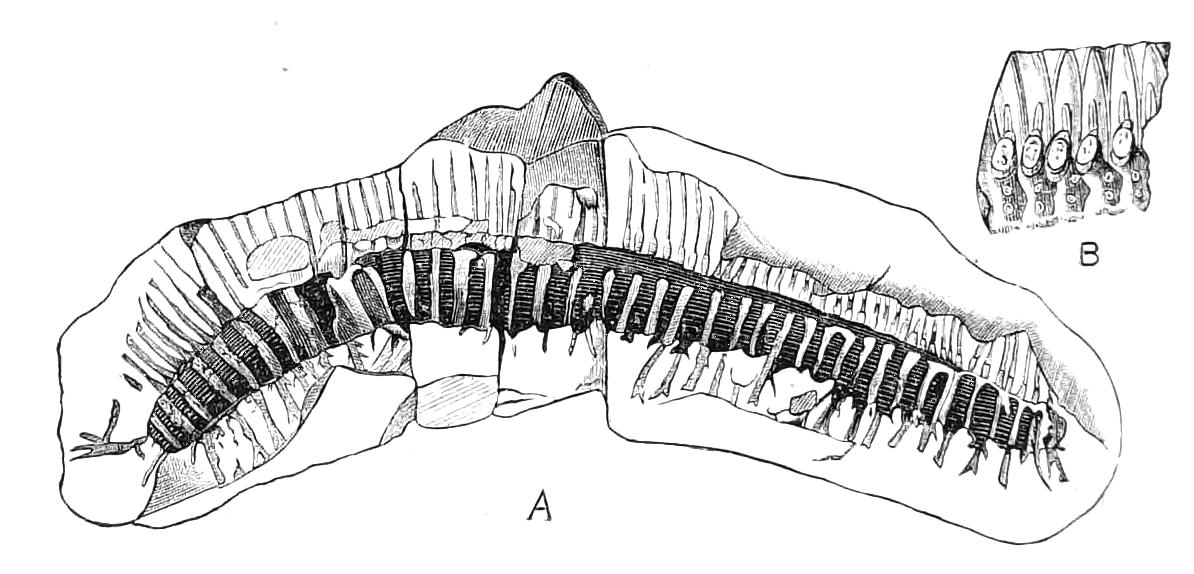
Fig. 46.—Acantherpestes major. (After Meek and Worth.) Mazon Creek, America. A, The whole animal; B, branchiae on the ventral surface.
Fossil Myriapods have been divided into four Orders, two of which coincide with the Orders of living Myriapods; the differences between the fossils and the living Myriapods having been held insufficient to warrant the establishment of a new Order. These two Orders are the Chilopoda and the Diplopoda or Chilognatha (Diplopoda is another name used by some writers for the group which we have hitherto called Chilognatha). The other two Orders have sufficient differences from living forms to render it necessary to include them in separate Orders.
The fossil Myriapods, then, are arranged as follows:—
| Order | I. | Protosyngnatha. |
| Order | II. | Chilopoda. |
| Order | III. | Archipolypoda. |
| Order | IV. | Chilognatha (or Diplopoda). |
The following table will show the species that have been discovered in the different strata:—
| Devonian, or Old Red Sandstone |
 |
2 species of Archipolypoda | ||
| Carboniferous |  |
01 species Protosyngnatha 31 species Archipolypoda |
||
| Permian (Rothliegendes of Germany), 4 specimens belonging to the Julidae or Archipolypoda. |
||||
| Cretaceous | 01 species |  |
Archipolypoda or Chilognatha |
|
| Oligocene |  |
17 species | Chilopoda | |
| 23 species |  |
Diplopoda (Chilognatha) |
||
| Miocene, | 01 species |  |
Diplopoda (Chilognatha) |
|
I will now give a short account of the different Orders, and the fossil forms which are included in them.
Order I. Protosyngnatha.
This Order is represented by a single fossil (Fig. 45), discovered in the coal at Mazon Creek, Illinois, America, by Meek and Worth. It differs greatly from any of those in existence at the present day. The body is cylindrical, and composed of ten segments. The cephalic appendages (that is, the antennae and mouth parts) are inserted into a single unsegmented cephalic mass (the head). Each segment behind the head bears a single dorsal and ventral plate of equal breadth and length. The limbs are placed in these plates with a wide space between the base of each leg and that of the opposite one of the pair. Along the back, bundles of bristles are arranged in longitudinal rows.
Order II. Chilopoda.
The fossil forms of this Order resemble those of the Chilopoda of the present day. The oldest of them are found in amber. The following families have been found:—
Lithobiidae. Several species have been found in amber.
Scolopendridae. One species in amber, several species in later Tertiary formations.
Geophilidae. Three species in amber.
Two species resembling the Schizotarsia of the present day have been found in amber.
Order III. Archipolypoda.
The most numerous of the fossil families. With a few exceptions, all the Palaeozoic (that is, the oldest) Myriapods belong to this Order. The Carboniferous Archipolypoda seem to be much more numerous in the coal of America than in that of England. They resemble for the most part the Myriapods of the present day, except that all the segments without exception bear legs.
The families are three in number.
Family 1. Archidesmidae.
Resemble the Polydesmidae of the present day. Two species have been found by Page in the Old Red Sandstone of Forfarshire. He named them Kampecaris. One found by Peach in the same formation is called Archidesmus.
Family 2. Euphoberiidae.
They show some resemblance to the Julidae of the present day, but the dorsal scutes, or plates of the back, are more or less perfectly divided into two divisions corresponding with the pairs of legs. The following are the principal fossils of this family:—
Acantherpestes. Found by Meek and Worth in the coal at Mazon Creek in America (Fig. 46).
Euphoberia. About 12 species found at the same place as the last named.
Amylispes. Found by Scudder, Mazon Creek, America.
Eileticus. Scudder, Mazon Creek, America.
Family 3. Archijulidae.
The dorsal plates nearly consolidated, but the division still apparent. Fossil forms are—
Trichijulus. Scudder, Mazon Creek, America.
Xylobius. Dawson. Found in the coal in Nova Scotia. Two species found at Mazon Creek, America.
Order IV. Chilognatha.
Families corresponding to those of the present day. The oldest specimens come from the chalk in Greenland; most of the others from amber.
Family 1. Glomeridae. One form, G. denticulata, has been found in amber.
Family 2. Polydesmidae. Two species in amber.
Family 3. Lysiopetalidae. A number of species, amongst which are 6 Craspedosoma, mostly from amber.
Family 4. Julidae. A number of species of this family have been found, some in amber, some in other Tertiary strata. Amongst the latter a probable example of Julus terrestris, living at the present time.
Family 5. Polyxenidae. Five species have been found in amber.
Now that we have considered the structure of the Myriapods and the groups into which they are subdivided or classified, we may proceed to consider what position they hold in the household of nature. That they present certain features of similarity to other classes has been already mentioned, and that this is the fact cannot be doubted when we look back at the way in which they have been classified in the works of early writers. For example, Lamarck, the great French naturalist, classifies them with spiders in his well-known work, La Philosophie Zoologique, under the name of Arachnides antennistes. Cuvier, the comparative anatomist, unites them with the Insects, making them the first Order, while the Thysanura is the second. We have already seen that one Order of Myriapods, the Symphyla, bears a great resemblance to the Thysanura. The English naturalist Leach was the first to establish Myriapods as a class, and his arrangement has been followed by all naturalists after his time. But while their peculiarities of structure and form are sufficiently marked to separate them as a class, it cannot be denied that the older naturalists were right to recognise that they have many essential characteristics in common with other classes of animals. And recent investigations have emphasised this fact. For instance, let us consider the recent discoveries of the Orders of Symphyla and Pauropoda, Orders which, while bearing so many of the characters of Myriapods that naturalists have agreed to place them in that class, yet resemble in many important points the Insect Order of Thysanura. This seems to justify Cuvier in claiming the close relationship for them that he did.
Recent investigations have also brought out more prominently the resemblances to the Worms. Of late, considerable attention has been directed to Peripatus (see pp. 1-26), and the resemblances to the Myriapods in its anatomy and development are such that Latzel has actually included it in the Myriapods as an Order, Malacopoda. Now Peripatus also shows resemblances to the annelid Worms, and thus affords us a connexion to the Worm type hardly less striking than that to the Insect. This {78}resemblance to the Worms, which Myriapods certainly bear, was noticed by the ancient writers, and as they had for the most part only external appearances to consider, they pushed this idea to extremes in actually including some of the marine Worms (Annelida) among the Centipedes. Pliny talks of a marine Scolopendra as a very poisonous animal, and there is little doubt that he meant one of the marine worms. An old German naturalist, Gesner, in a very curious book published in 1669 gives an account of an annelid sea-worm which he calls Scolopendra marina, and which is in all probability the sea Scolopendra which Pliny mentions. From Gesner's account it seems to have been used as a medicine (externally only). "The use of this animal in medicine. The animal soaked in oil makes the hair fall off. So do its ashes mixed in oil." It was also pounded up with honey.
This idea of Centipedes living in water survived among later naturalists. Charles Owen, the author before quoted, mentions them as amphibious in 1742. "The Scolopendra is a little venomous worm and amphibious. When it wounds any, there follows a blueness about the affected part and an itch all over the body like that caused by nettles. Its weapons of mischief are much the same with those of the spider, only larger; its bite is very tormenting, and produces not only pruriginous pain in the flesh, but very often distraction of mind. These little creatures make but a mean figure in the ranks of animals, yet have been terrible in their exploits, particularly in driving people out of their country. Thus the people of Rhytium, a city of Crete, were constrained to leave their quarters for them (Aelian, lib. xv. cap. 26)."
Myriapods have been considered to bear resemblances to the Crustacea, and this to a certain extent is true, though only to a certain extent, the resemblances being confined to the more general characteristics that they share with other groups of animals.
Of late years attempts have been made to speculate about the origin of the Myriapods—that is, to endeavour to obtain by means of investigation of their anatomy, embryology, and palaeontological history, some idea of the history of the group. Such attempts at research into the phylogeny, as it is called, of a group must be more or less speculative until our knowledge is much greater than {79}it is at present. But such inquiries have their value, and the schemes of descent and phylogenetic trees, at any rate, indicate a real relation to different groups, even if they do not provide us with a real and actual history of the animals.
There have been two main theories about the descent of the Myriapoda. One of these derives them directly from the Insecta through the forms known as the Thysanura, which resemble in such a degree the Myriapod Orders of Symphyla and Pauropoda. The other theory holds that the Myriapods, as well as the Insecta, have been derived from some ancestor bearing a resemblance to Peripatus. In other words, one theory claims that the relationship of Myriapoda to Insecta is that of father and son; the other that the relationship between the two is that of brother to brother. The arguments by which these theories are respectively supported consist for the most part of an analysis of the different characters of the anatomy and embryology and the determination of the most primitive among them. For example, the supporters of the theory that the Thysanura are the most nearly allied to the Myriapod ancestor lay great weight on the fact that some Myriapods are born with three pairs of legs only, and they compare this stage in the life history of the Myriapoda to the metamorphosis and larval stage of Insects. For the supporters of this view the Orders of Symphyla and Pauropoda are the most primitive of the Myriapods. On the other hand, the followers of the other theory do not allow that the characters in which the Myriapods are like Insects are primitive ones, but they lay more stress on the characters found in the early development, such as the character of the process of the formation of the body segments, the mesoblastic segmentation, and the origin of the various organs of the body.
It may be easily understood that such differences in the estimation of the primitive characters of the embryology of a group may arise. Embryology has been compared by one of the greatest of modern embryologists to "an ancient manuscript with many of the sheets lost, others displaced, and with spurious passages interpolated by a later hand." What wonder is it that different people examining such a record should come to different conclusions as to the more doubtful and difficult portions of it. It is this very difficulty which makes the principal interest in the study, and although our knowledge of the language in {80}which this manuscript is written is as yet imperfect, still we hope that constant study may teach us more and more, and enable us to read the great book of nature with more and more ease and certainty.
If any of my readers should wish for a more full account of the natural history of this group I must refer them to the following works, which I have used in compiling the above account. In the first of these there is an excellent bibliography of the subject:—
Latzel, Die Myriapoden der Oesterreichisch-Ungarischen Monarchie, Wien, 1880.
Zittel, Handbuch der Palaeontologie, 1 Abth, II. Bd., Leipzig, 1881-1885.
Korschelt and Heider, Lehrbuch der vergleichenden Entwicklungsgeschichte der wirbellosen Thiere, Jena 1891.
BY
DAVID SHARP, M.A., M.B., F.R.S.
CHARACTERISTIC FEATURES OF INSECT LIFE–SOCIAL INSECTS–DEFINITION OF THE CLASS INSECTA–COMPOSITION OF INSECT SKELETON–NUMBER OF SEGMENTS–NATURE OF SCLERITES–HEAD–APPENDAGES OF THE MOUTH–EYES–THORAX–ENTOTHORAX–LEGS–WINGS–ABDOMEN OR HIND BODY–SPIRACLES–SYSTEMATIC ORIENTATION.
Insects form by far the larger part of the land animals of the world; they outnumber in species all the other terrestrial animals together, while compared with the Vertebrates their numbers are simply enormous. Yet they attract but little attention from the ordinary observer, this being probably primarily due to the small size of the individual Insect, which leads the unreflecting to treat the creature as of little importance. "It can be crushed in a moment" is perhaps the unformulated idea that underlies the almost complete neglect of knowledge concerning Insects that prevails even in the educated classes of society. The largest Insects scarcely exceed in bulk a mouse or a wren, while the smallest are almost or quite imperceptible to the naked eye, and yet the larger part of the animal matter existing on the lands of the globe is in all probability locked up in the forms of Insects. Taken as a whole they are the most successful of all the forms of terrestrial animals.
In the waters of the globe the predominance of Insect life disappears. In the smaller collections of fresh water many Insects find a home during a portion of their lives, and some few contrive to pass their whole existence in such places; but of the larger bodies of fresh water they invade merely the fringes, and they make only the feeblest attempt at existence in the ocean; the genus Halobates containing, so far as we know, the sole Insects {84}that are capable of using the ocean as a medium of existence at a distance from the shore.
It will probably be asked, how has it come about that creatures so insignificant in size and strength have nevertheless been so successful in what we call the struggle for existence? And it is possible that the answer will be found in the peculiar relations that exist in Insects between the great functions of circulation and respiration; these being of such a nature that the nutrition of the organs of the body can be carried on very rapidly and very efficiently so long as a certain bulk is not exceeded.
Rapidity of growth is carried to an almost incredible extent in some Insects, and the powers of multiplication—which may be considered as equivalent to the growth of the species—even surpass the rapidity of the increase of the individual; while, as if to augment the favourable results attainable by the more usual routine of the physiological processes, "metamorphosis" has been adopted, as a consequence of which growth and development can be isolated from one another, thus allowing the former to go on unchecked or uncomplicated by the latter. A very simple calculation will show how favourable some of the chief features of Insect life are. Let it be supposed that growth of the individual takes time in proportion to the bulk attained, and let A be an animal that weighs one ounce, B a creature that weighs ten ounces, each having the power of producing 100 young when full grown; a simple calculation shows that after the lapse of a time necessary for the production of one generation of the larger creature the produce of the smaller animal will enormously outweigh that of its bulkier rival. Probably it was some consideration of this sort that led Linnaeus to make his somewhat paradoxical statement to the effect that three flies consume the carcase of a horse as quickly as a lion.[16]
Astonishing as may be the rapidity of the physiological processes of Insects, the results attained by them are, it must be admitted, scarcely less admirable: the structures of the Insect's body exhibit a perfection that, from a mechanical point of view, is unsurpassed, while the external beauty of some of the creatures makes them fit associates of the most delicate flowers or no mean rivals of the most gorgeous of the feathered world. The words {85}of Linnaeus, "Natura in minimis maxime miranda," are not a mere rhetorical effort, but the expression of a simple truth. Saint Augustine, too, though speaking from a point of view somewhat remote from that of the great Swedish naturalist, expressed an idea that leads to a similar conclusion when he said, "Creavit in coelum angelos, in terram vermiculos; nec major in illis nec minor in istis."
The formation of organised societies by some kinds of Insects is a phenomenon of great interest, for there are very few animals except man and Insects that display this method of existence. Particulars as to some of these societies will be given when we treat of the Termitidae, and of the Hymenoptera Aculeata; but we will take this opportunity of directing attention to some points of general interest in connexion with this subject. In Insect societies we find that not only do great numbers of separate individuals live together and adopt different modes of industrial action in accordance with the position they occupy in the association, but also that such individuals are profoundly modified in the structures of their body and in their physiological processes in such ways as to specially fit them for the parts they have to play. We may also see these societies in what may be considered different stages of evolution; the phenomena we are alluding to being in some species much less marked than they are in others, and these more primitive kinds of societies being composed of a smaller number of individuals, which are also much less different from one another. We, moreover, meet with complex societies exhibiting some remarkably similar features among Insects that are very different systematically. The true ants and the white ants belong to groups that are in structure and in the mode of growth of the individual essentially dissimilar, though their social lives are in several important respects analogous.
It should be remarked that the phenomena connected with the social life of Insects are still only very imperfectly known; many highly important points being quite obscure, and our ideas being too much based on fragments gathered from the lives of different species. The honey bee is the only social Insect of whose economy we have anything approaching to a wide knowledge, and even in the case of this Insect our information is neither so complete nor so precise as is desirable.
The various branches of knowledge connected with Insects {86}are called collectively Entomology. Although entomology is only a department of the great science of zoology, yet it is in practice a very distinct one; owing to its vast extent few of those who work at other branches of zoology also occupy themselves with entomology, while entomologists usually confine themselves to work in the vast field thus abandoned to them.
Before passing to the consideration of the natural history and structure of the members of the various Orders of Insects we will give a verbal diagrammatic sketch, if we may use such an expression, with a view to explaining the various terms that are ordinarily used. We shall make it as brief as possible, taking in succession (1) the external structure, (2) internal structure, (3) development of the individual, (4) classification.
In the course of this introductory sketch we shall find it necessary to mention the names of some of the Orders of Insects that will only be explained or defined in subsequent pages. We may therefore here state that the term "Orthoptera" includes grasshoppers, locusts, earwigs, cockroaches; "Neuroptera" comprises dragon-flies, May-flies, lacewings, stone-flies and caddis-flies; to the "Hymenoptera" belong bees, wasps, ants, sawflies, and a host of little creatures scarcely noticed by the ordinary observer: "Coleoptera" are beetles; "Lepidoptera," butterflies and moths; "Diptera," house-flies, blue-bottles, daddy-longlegs, and such; "Hemiptera" or "Rhynchota" are bugs, greenfly, etc.
Class Insecta: or Insecta Hexapoda.
Definition.—Insects are small animals, having the body divided into three regions placed in longitudinal succession—head, thorax, and abdomen: they take in air by means of tracheae, a system of tubes distributed throughout the body, and opening externally by means of orifices placed at the sides of the body. They have six legs, and a pair of antennae; these latter are placed on the head, while the legs are attached to the thorax, or second of the three great body divisions; the abdomen has no true legs, but not infrequently has terminal appendages and, on the under surface, protuberances which serve as feet. Very frequently there are two pairs of wings, sometimes only one pair, in other cases none: the wings are always placed on the thorax. Insects are transversely segmented—that is to say, the body has the form of a succession of {87}rings; but this condition is in many cases obscure; the number of these rings rarely, if ever, exceeds thirteen in addition to the head and to a terminal piece that sometimes exists. Insects usually change much in appearance in the course of their growth, the annulose or ringed condition being most evident in the early part of the individual's life. The legs are usually elongate and apparently jointed, but in the immature condition may be altogether absent, or very short; in the latter case the jointing is obscure. The number of jointed legs is always six.
External Structure.
The series of rings of which the external crust or skeleton of Insects is composed exhibits great modifications, not only in the various kinds of Insects but even in the different parts of the same individual, and at successive periods of its development; so that in the majority of mature Insects the separate rings are readily distinguished only in the hind body or abdomen. The total number of the visible rings, segments, somites, or arthromeres, as they are variously called by different writers, is frequently thirteen in addition to the head. This latter part is considered to be itself composed of the elements of several rings, but morphologists are not yet agreed as to their number, some thinking this is three while others place it as high as seven; three or four being, perhaps, the figures at present most in favour, though Viallanes, who has recently discussed[17] the subject, considers six, the number suggested by Huxley, as the most probable. Cholodkovsky is of a similar opinion. However this may be, the three rings behind the head constitute the thorax, which is always largely developed, though, like the head, its segmentation is usually very much obscured by unequal development of different parts, or by consolidation of some of them, or by both of these conditions. The third great division of the body, the abdomen, is also usually much modified by one or more of the terminal segments being changed in form, or even entirely withdrawn into the interior of the body. The existence of ten segments in the hind body can, however, be very frequently actually demonstrated, so that it is correct to speak of ten as the normal number.
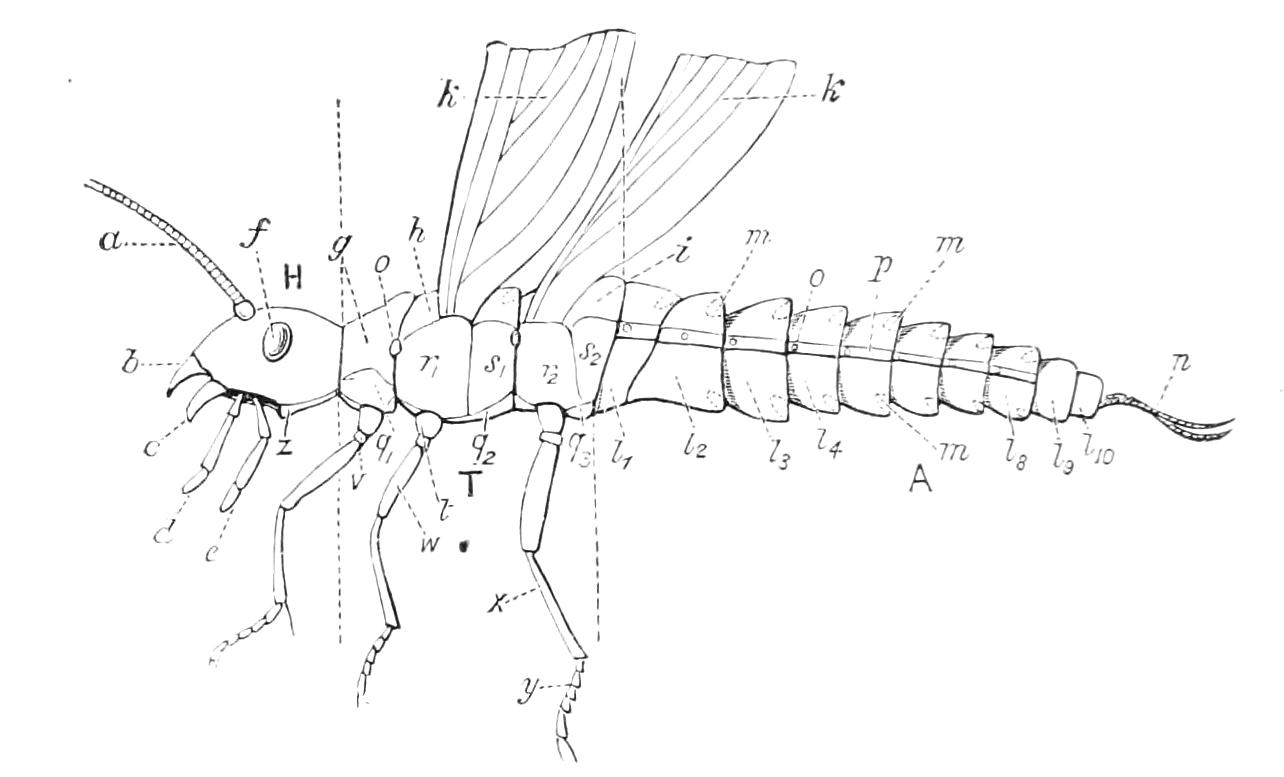
Fig. 47—Diagram of exterior of insect: the two vertical dotted lines indicate the divisions between H, head; T, thorax; and A, abdomen: a, antenna; b, labrum; c, mandible; d, maxillary palpus; e, labial palpus; f, facetted eye; g, pronotum; h, mesonotum; i, metanotum; k, wings; l1 to l10, abdominal segments; m, the internal membranous portions uniting the apparently separated segments; n, cerci; o, stigma; p, abdominal pleuron bearing small stigmata; q1, q2, q3, pro-, meso-, meta-sterna; r1, mesothoracic episternum; s1, epimeron, these two forming the mesopleuron; r2, s2, metathoracic episternum and epimeron; t, coxa; v, trochanter; w, femur; x, tibia; y, tarsus; z, gula.
It is no reproach to morphologists that they have not yet agreed as to the number of segments that may be taken as typical for an Insect, for all the branches of evidence bearing on the point are still imperfect. It may be well, therefore, to state the most extreme views that appear to be at all admissible. Hagen[18] has recently stated the opinion that each thoracic segment consists really of three segments—an anterior or wing-bearer, a middle or leg-bearer, and a posterior or stigma-bearer. There seems to be no reason for treating the stigma as being at all of the nature of an appendage, and the theory of a triple origin for these segments may be dismissed. There are, however, several facts that indicate a duplicity in these somites, among which we may specially mention the remarkable constancy of two pleural pieces on each side of each thoracic segment. The hypothesis of these rings being each the representative of two segments cannot therefore be at present considered entirely untenable, and in that case the maximum and minimum numbers that can be suggested appear to be twenty-four and eleven, distributed as follows:—
| Maximum. | Minimum. | |
| Head | 7 | 3 |
| Thorax | 6 | 3 |
| Abdomen | 11 | 5 |
| — | — | |
| Total | 24 | 11 |
Although it is not probable that ultimately so great a difference as these figures indicate will be found to prevail, it is certainly at present premature to say that all Insects are made up of the same number of primary segments.
A brief account of the structure of the integument will be found in the chapter dealing with the post-embryonic development.
The three great regions of the Insect body are functionally as well as anatomically distinct. The head bears the most important of the sense organs, viz. the antennae and ocular organs; it includes the greater of the nerve-centres, and carries the mouth as well as the appendages, the trophi, connected therewith. The thorax is chiefly devoted to the organs of locomotion, bearing externally the wings and legs, and including considerable masses of muscles, as well as the nerve centres by which they are innervated; through the thorax there pass, however, in the longitudinal direction, those structures by which the unity of the organisation is completed, viz. the alimentary canal, the dorsal vessel or "heart" for distributing the nutritive fluid, and also the nerve cords. The abdomen includes the greater part of the organs for carrying on the life of the individual and of the species; it also frequently bears externally, at or near its termination, appendages that are doubtless usually organs of sense of a tactile nature.
In the lower forms of Insect life there is little or no actual internal triple division of the body; but in the higher forms such separation becomes wonderfully complete, so that the head may communicate with the thorax only by a narrow isthmus, and the thorax with the abdomen only by a very slender link. This arrangement is carried to its greatest extreme in the Hymenoptera Aculeata. It may be looked on as possibly a means for separating the nutrition of the parts included in the three great body divisions.
Along each side of the body extends a series of orifices for the admission of air, the stigmata or spiracles; there are none of these on the head, but on each side of most of the other segments {90}there is one of these spiracles. This, however, is a rule subject to many exceptions, and it is doubtful whether there is ever a spiracle on the last abdominal segment. Even in the young stage of the Insect the number of these stigmata is variable; while in the perfect Insect the positions of some of the stigmata may be much modified correlatively with the unequal development or consolidation of parts, especially of the thorax when it is highly modified for bearing the wings.
The segments of the Insect are not separate parts connected with one another by joints and ligaments; the condition of the Insect crust is in fact that of a continuous long sac, in which there are slight constrictions giving rise to the segments, the interior of the sac being always traversed from end to end by a tube, or rather by the invaginated ends of the sac itself which connect with an included second sac, the stomach. The more prominent or exposed parts of the external sac are more or less hard, while the constricted parts remain delicate, and thus the continuous bag comes to consist of a series of more or less hard rings connected by more delicate membranes. This condition is readily seen in distended larvae, and is shown by our figure 48 which is taken from the same specimen, whose portrait, drawn during life, will be given when we come to the Coleoptera, family Cleridae. The nature of the concealed connexions between the apparently separate segments of Insects is shown at m, Fig. 47, p. 88.
As the number of segments in the adult Insect corresponds—except in the head—with the number of divisions that appear very early in the embryo, we conclude that the segmentation of the adult is, even in Insects which change their form very greatly during growth, due to the condition that existed in the embryo; but it must not be forgotten that important secondary changes occur in the somites during the growth and development of the individual. Hence in some cases there appear to be more than the usual number of segments, e.g. Cardiophorus larva, and in others the number of somites is diminished by {91}amalgamation, or by the extreme reduction in size of some of the parts.
Besides the division of the body into consecutive segments, another feature is usually conspicuous; the upper part, in many segments, being differentiated from the lower and the two being connected together by intervening parts in somewhat the same sort of way as the segments themselves are connected. Such a differentiation is never visible on the head, but may frequently be seen in the thorax, and almost always in the abdomen. A dorsal and a ventral aspect are thus separated, while the connecting bond on either side forms a pleuron. By this differentiation a second form of symmetry is introduced, for whereas there is but one upper and one lower aspect, and the two do not correspond, there are two lateral and similar areas. This bilateral symmetry is conspicuous in nearly all the external parts of the body, and extends to most of the internal organs. The pleura, or lateral regions of the sac, frequently remain membranous when the dorsal and ventral aspects are hard. The dorsal parts of the Insect's rings are also called by writers terga, or nota, and the ventral parts sterna.
The appendages of the body are:—(1) a pair of antennae; (2) the trophi, constituted by three pairs of mouth-parts; (3) three pairs of legs; (4) the wings[19]; (5) abdominal appendages of various kinds, but usually jointed. Before considering these in detail we shall do well to make ourselves more fully acquainted with the elementary details of the structure of the trunk.
In the adult Insect the integument or crust of the body is more or less hard or shell-like, sometimes, indeed, very hard, and on examination it will be seen that besides the divisions into segments and into dorsal, ventral, and pleural regions, there are lines indicating the existence of other divisions, and it will be found that by dissection along these lines distinct pieces can be readily separated. Each hard piece that can be so separated is called a sclerite, and the individual sclerites of a segment have received names from entomotomists. The sclerites are not really {92}quite separate pieces, though we are in the habit of speaking of them as if such were the case. If an Insect be distended by pressure from the interior, many of the sclerites can be forced apart, and it is then seen that they are connected by delicate membrane. The structure is thus made up of hard parts meeting one another along certain lines of union—sutures—so that the original membranous continuity may be quite concealed. In many Insects, or in parts of them, the sclerites do not come into apposition by sutures, and are thus, as it were, islands of hard matter surrounded by membrane. A brief consideration of some of the more important sclerites is all that is necessary for our present purpose: we will begin with the head.
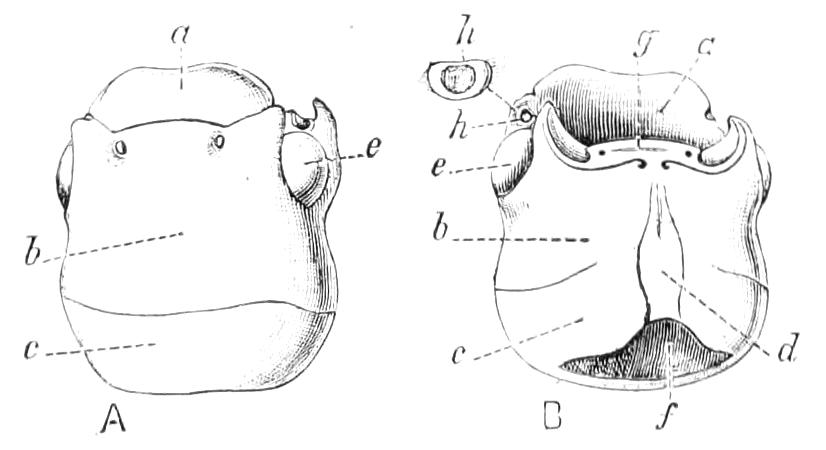
Fig. 49.—Capsule of head of beetle, Harpalus caliginosus: A, upper; B, under surface: a, clypeus; b, epicranium; c, protocranium; d, gula; e, facetted eye; f, occipital foramen; g, submentum; h, cavity for insertion of antenna.
The head is most variable in size and form; as a part of its surface is occupied by the eyes and as these organs differ in shape, extent, and position to a surprising degree, it is not a matter for astonishment that it is almost impossible to agree as to terms for the areas of the head. Of the sclerites of the head itself there are only three that are sufficiently constant and definite to be worthy of description here. These are the clypeus, the epicranium, and the gula. The clypeus is situate on the upper surface of the head-capsule, in front; it bears the labrum which may be briefly described as a sort of flap forming an upper lip. The labrum is usually possessed of some amount of mobility. The clypeus itself is excessively variable in size and form, and sometimes cannot be delimited owing to the obliteration of the suture of connexion with the more posterior part of the head; it is rarely or never a paired piece. Occasionally there is a more or less distinct piece interposed between the clypeus and the labrum, and which is the source of considerable difficulty, as it may be taken for the clypeus. Some authors call the clypeus the epistome, but it is better to use this latter term for the purpose of indicating the part that is immediately behind the labrum, whether that part be the clypeus, or some other sclerite; the {93}term is very convenient in those cases where the structure cannot be, or has not been, satisfactorily determined morphologically.
In Figure 50 the parts usually visible on the anterior aspect of the head and its appendages are shown so far as these latter can be seen when the mouth is closed; in the case of the Insect here represented the bases of the mandibles are clearly seen (g), while their apical portions are entirely covered by the labrum, just below the lower margin of which the tips of the maxillae are seen, looking as if they were the continuations of the mandibles.
The labrum is a somewhat perplexing piece, morphologists being not yet agreed as to its nature; it is usually placed quite on the front of the head, and varies extremely in form; it is nearly always a single or unpaired piece; the French morphologist Chatin considers that it is really a paired structure.
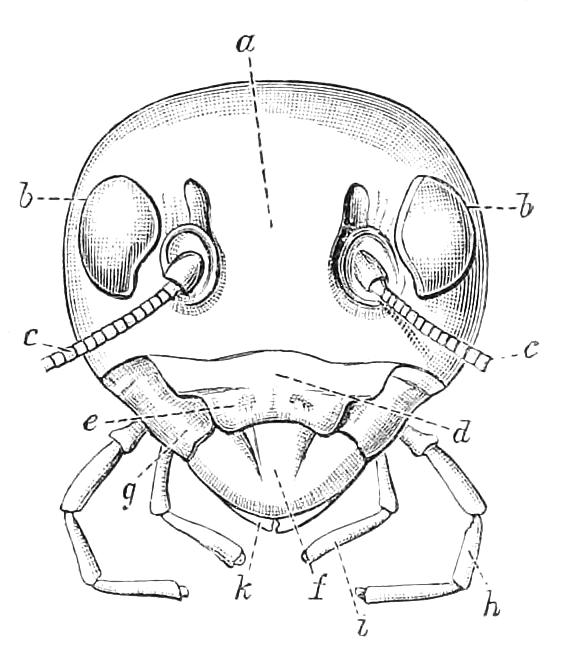
Fig. 50.—Front view of head of field-cricket (Gryllus): a, epicranium; b, compound eye; c, antenna; d, post-: e, ante-clypeus; f, labrum; g, base of mandible; h, maxillary palpus; i, labial palpus; k, apex of maxilla.
The gula (Fig. 49, B d, and Fig. 47, z) is a piece existing in the middle longitudinally of the under-surface of the head; in front it bears the mentum or the submentum, and extends backwards to the great occipital foramen, but in some Insects the gula is in front very distant from the edge of the buccal cavity. The epicranium forms the larger part of the head, and is consequently most inconstant in size and shape; it usually occupies the larger part of the upper-surface, and is reflected to the under-surface to meet the gula. Sometimes a transverse line exists (Fig. 49, A) dividing the epicranium into two parts, the posterior of which has been called the protocranium; which, however, is not a good term. The epicranium bears the antennae; these organs do not come out between the epicranium and the clypeus, the foramen for their insertion being seated entirely in the epicranium (see Fig. 50). In some Insects there are traces of the epicranium being divided longitudinally along the middle line. When this part is much modified the antennae may appear to be inserted on the lateral portions of the head, or even {94}on its under-side; this arises from extension of some part of the epicranium, as shown in Fig. 49, B, where h, the cavity of insertion of the antenna, appears to be situate on the under-surface of the epicranium, the appearance being due to an infolding of an angle of the part.
There is always a gap in the back of the head for the passage of the alimentary canal and other organs into the thorax; this opening is called the occipital foramen. Various terms, such as frons, vertex, occiput, temples, and cheeks, have been used for designating areas of the head. The only one of these which is of importance is the gena, and even this can only be defined as the anterior part of the lateral portion of the head-capsule. An extended study of the comparative anatomy of the head-capsule is still a desideratum in entomology. The appendages of the head that are engaged in the operations of feeding are frequently spoken of collectively as the trophi, a term which includes the labrum as well as the true buccal appendages.
The appendages forming the parts of the mouth are paired, and consist of the mandibles, the maxillae, and the labium, the pair in this latter part being combined to form a single body. The buccal appendages are frequently spoken of as gnathites. The gnathites are some, if not all, of them composed of apparently numerous parts, some of these being distinct sclerites, others membranous structures which may be either bare or pubescent—that is, covered with delicate short hair. In Insects the mouth functions in two quite different ways, by biting or by sucking. The Insects that bite are called Mandibulata, and those that suck Haustellata. In the mandibulate Insects the composition of the gnathites is readily comprehensible, so that in nearly the whole of the vast number of species of that type the corresponding parts can be recognised with something like certainty. This, however, is not the case with the sucking Insects; in them the parts of the mouth are very different indeed, so that in some cases morphologists are not agreed as to what parts really correspond with some of the structures of the Mandibulata. At present it will be sufficient for us to consider only the mandibulate mouth, leaving the various forms of sucking mouth to be discussed when we treat of the Orders of Haustellata in detail.
The upper or anterior pair of gnathites is the mandibles, (Fig. 50, g). There is no part of the body that varies more than {95}does the mandible, even in the mandibulate Insects. It can scarcely be detected in some, while in others, as in the male stag-beetle, it may attain the length of the whole of the rest of the body; its form, too, varies as much as its size; most usually, however, the pair of mandibles are somewhat of the form of callipers, and are used for biting, cutting, holding, or crushing purposes. The mandibles are frequently armed with processes spoken of as teeth, but which must not be in any way confounded with the teeth of Vertebrates. The only Insects that possess an articulated tooth are the Passalidae, beetles armed with a rather large mandible bearing a single mobile tooth among others that are not so. Wood Mason and Chatin consider the mandibles to be, morphologically, jointed appendages, and the latter authority states that in the mandible of Embia he has been able to distinguish the same elements as exist in the maxillae. In aculeate Hymenoptera the mandibles are used to a considerable extent for industrial purposes.
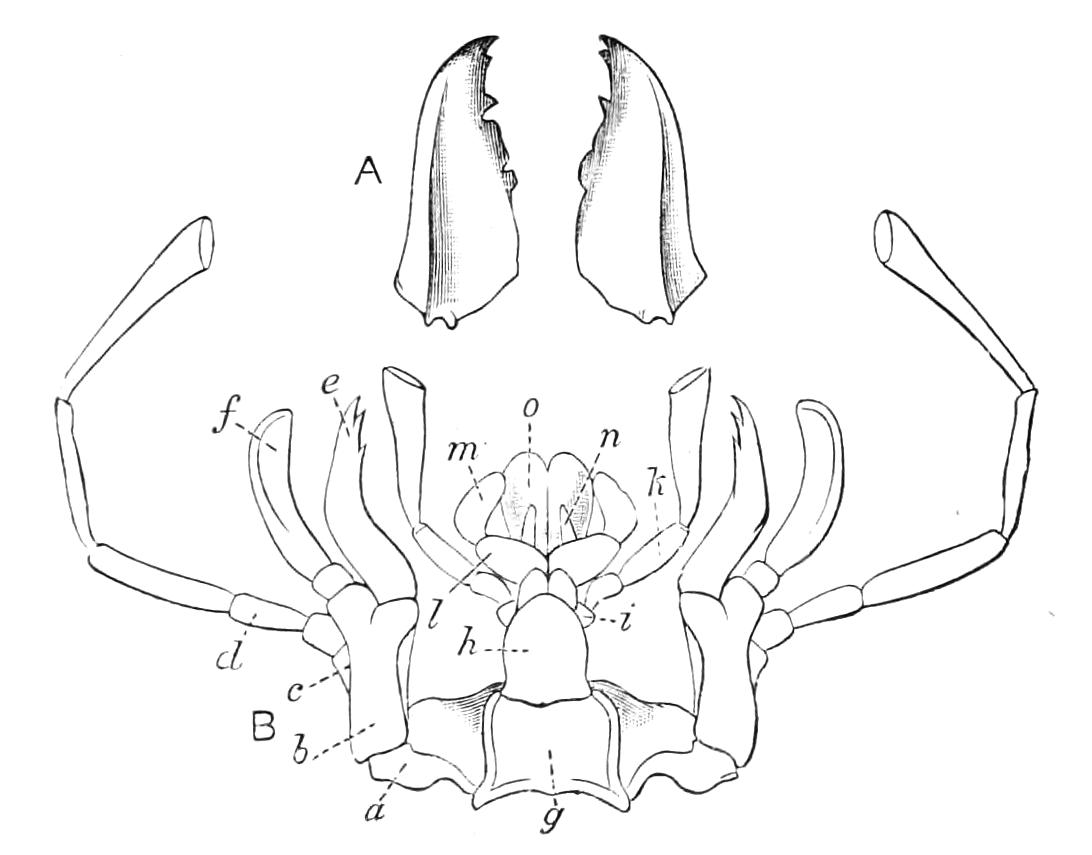
Fig. 51.—Mandibles, maxillae, and labium of Locusta viridissima: A, mandibles; B, maxillae (lateral parts) and labium (middle parts) united: a, cardo; b, stipes; c, palpiger; d, max. palp.; e, lacinia; f, galea; g, submentum; h, mentum; i, palpiger; k, labial palpus; l, ligula; m, paraglossa (galea); n, lacinia; o, lingua.
The maxilla is a complex organ consisting of numerous pieces, viz. cardo, stipes, palpiger, galea, lacinia, palpus. The galea and lacinia are frequently called the lobes of the maxilla. The maxilla no doubt acts as a sense organ as well as a mechanical apparatus for holding; this latter function being subordinate to the other. In Fig. 68, p. 122, we have represented a complex maxillary sense-organ.
The labium or lower lip has as its basal portion the {96}undivided mentum, and closes the mouth beneath or behind, according as the position of the head varies. In most Insects the labium appears very different from the maxilla, but in many cases several of the parts corresponding to those of the maxilla can be clearly traced in the labium.
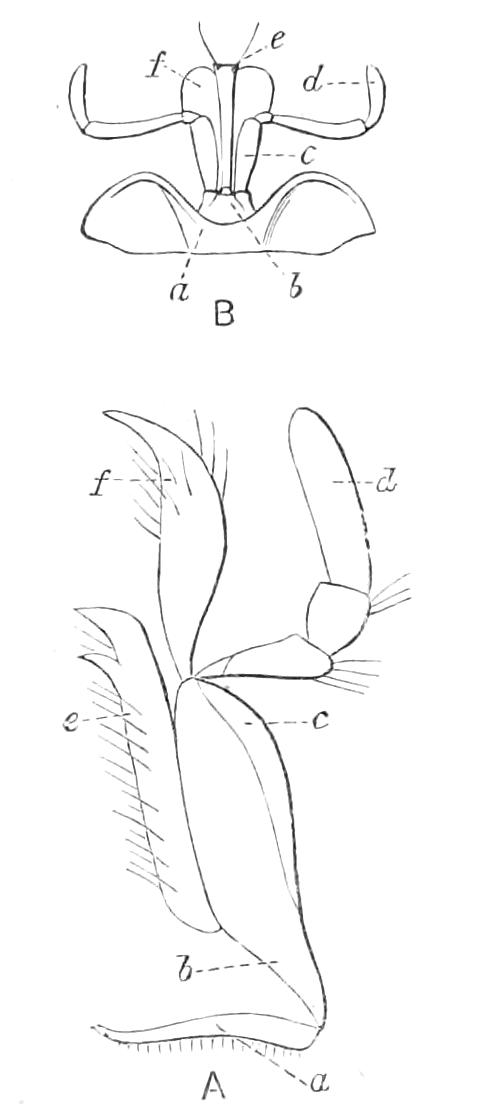
Fig. 52.—Maxilla and lower lip of Coleoptera. A, Maxilla of Passalus: a, cardo; b, stipes; c, palpiger; d, palpus; e, inner or inferior lobe or lacinia; f, outer or superior lobe or galea: B, Labium of Harpalus caliginosus: a, mentum; b, hypoglottis; c, palpiger (support of the labial palp); d, palp; e, ligula; f, paraglossa.
The mentum is an undivided, frequently very hard, piece, continuous with either the submentum or the gula, and anterior to this are placed the other parts, viz. the labial palpi and their supports, the palpigers; beyond and between these exists a central piece (Fig. 52, B, e), about whose name some difference of opinion prevails, but which may be called the ligula (languette of French authors), and on each side of this is a paraglossa. In the Orthoptera the single median piece—the ligula of Coleopterists—is represented by two divided parts. In some Insects (many Coleoptera) there is interposed between the mentum and the palpigers a piece called the hypoglottis (Fig. 52, B, b). It is not so well ascertained as it should be, that the pieces of the lower lip bearing the same names in different Orders are in all cases really homologous, and comparison suggests that the hypoglottis of Coleoptera may possibly represent the piece corresponding to the mentum of Orthopterists, the so-called mentum of beetles being in that case the submentum of Orthopterists.
There is another part of the mouth to which we may call special attention, as it has recently attracted more attention than it formerly did; it is a membranous lobe in the interior of the mouth, very conspicuous in Orthoptera, and called the tongue, lingua, or hypopharynx; it reposes, in the interior of the mouth (Fig. 51, o), on the middle parts of the front of the labium; it is probably not entirely lost in Coleoptera, but enters into the composition of the {97}complex middle part of the lip by amalgamation with the paraglossae. It has recently been proposed to treat this lingua as the morphological equivalent of the labium or of the maxillae, giving it the name of the endolabium, but the propriety of this course remains to be proved;[20] the view is apparently suggested chiefly by the structure of the mouth of Hemimerus, a very rare and most peculiar Insect that has not as yet been sufficiently studied.
As the maxillae and labium are largely used by taxonomists in the systematic arrangement of the mandibulate Insects, we give a figure of them as seen in Coleoptera, where the parts, though closely amalgamated, can nevertheless be distinguished. This Fig. 52 should be compared with Fig. 51.
In speaking of the segments of the body we pointed out that they were not separate parts but constituted an uninterrupted whole, and it is well to remark here that this is also true of the gnathites. Although the mouth parts are spoken of as separate pieces, they really form only projections from the great body wall. Fig. 51, B, shows the intimate connexion that exists between the maxillae and labium; the continuity of the mandibles with the membrane of the buccal cavity is capable of very easy demonstration.
The head bears, besides the pieces we have considered, a pair of antennae. These organs, though varying excessively in form, are always present in the adult Insect, and exist even in the majority of young Insects. They are very mobile, highly sensitive organs, situate on or near the front part of the head. The antennae arise in the embryo from the procephalic lobes, the morphological import of which parts is one of the most difficult points connected with Insect embryology.
The eyes of Insects are of two sorts, simple and compound. The simple eyes, or ocelli, vary in number from one to as many as eighteen or twenty; when thus numerous they are situated in groups on each side of the head. In their most perfect form, as found in adult aculeate Hymenoptera, in Orthoptera and Diptera, ocelli are usually two or three in number, and present the appearance of small, perfectly transparent lenses inserted in the integument. In their simplest form they are said to consist of some masses of pigment in connexion with a nerve.
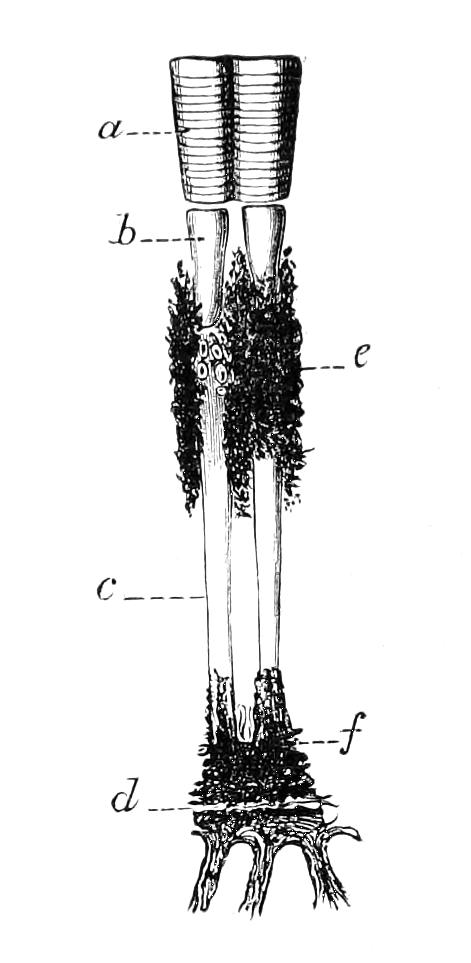
Fig. 53.—Two ommatidia from the eye of Colymbetes fuscus, × 160. (After Exner.) a, Cornea; b, crystalline cone; c, rhabdom; d, fenestrate membrane with nerve structures below it; e, iris-pigment; f, retina-pigment.
The compound, or facetted, eyes are the most remarkable of all the structures of the Insect, and in the higher and more active forms, such as the Dragon-flies and hovering Diptera, attain a complexity and delicacy of organisation that elicit the highest admiration from every one who studies them. They are totally different in structure and very distinct in function from the eyes of Vertebrata, and are seated on very large special lobes of the brain (see Fig. 65), which indeed are so large and so complex in structure that Insects may be described as possessing special ocular brains brought into relation with the lights, shades, and movements of the external world by a remarkably complex optical apparatus. This instrumental part of the eye is called the dioptric part in contradistinction from the percipient portion, and consists of an outer corneal lens (a, Fig. 53), whose exposed surface forms one of the facets of the eye; under the lens is placed the crystalline cone (b), this latter being borne on a rod-like object (c), called the rhabdom. There are two layers of pigment, the outer (e), called the iris-pigment, the inner (f), the retinal-pigment; underneath, or rather we should say more central than, the rhabdoms is the fenestrate membrane (d), beyond which there is an extremely complex mass of nerve-fibres; nerves also penetrate the fenestrate membrane, and their distal extremities are connected with the delicate sheaths by one of which each rhabdom is surrounded, the combination of sheath and nerves forming a retinula. Each set of the parts above the fenestrate membrane constitutes an ommatidium, and there may be many of these ommatidia in an eye; indeed, it is said that the eye of a small beetle, Mordella, contains as many as 25,000 ommatidia. As a rule the larvae of Insects with a complete metamorphosis bear only simple eyes. In the young of Dragon-flies, as well as of some other Insects having a less perfect metamorphosis, the compound eyes exist in the early stages, but they {99}have then an obscure appearance, and are probably functionally imperfect.
In the interior of the head there exists a horny framework called the tentorium, whose chief office apparently is to protect the brain. It is different in kind according to the species. The head shows a remarkable and unique relation to the following segments. It is the rule in Insect structure that the back of a segment overlaps the front part of the one following it; in other words, each segment receives within it the front of the one behind it. Though this is one of the most constant features of Insect anatomy, it is departed from in the case of the head, which may be either received into, or overlapped by, the segment following it, but never itself overlaps the latter. There is perhaps but a single Insect (Hypocephalus, an anomalous beetle) in which the relation between the head and thorax can be considered to be at all similar to that which exists between each of the other segments of the body and that following it; and even in Hypocephalus it is only the posterior angles of the head that overlap the thorax. Although the head usually appears to be very closely connected with the thorax, and is very frequently in repose received to a considerable extent within the latter, it nevertheless enjoys great freedom of motion; this is obtained by means of a large membrane, capable of much corrugation, and in which there are seated some sclerites, so arranged as to fold together and occupy little space when the head is retracted, but which help to prop and support it when extended for feeding or other purposes. These pieces are called the cervical sclerites or plates. They are very largely developed in Hymenoptera, in many Coleoptera, and in Blattidæ, and have not yet received from anatomists a sufficient amount of attention. Huxley suggested that they may be portions of head segments.
Fig. 54.—Extended head and front of thorax of a beetle, Euchroma: a, back of head; b, front of pronotum; c, chitinous retractile band; d, cervical sclerites.
Thorax.
The thorax, being composed of the three consecutive rings behind the head, falls naturally into three divisions—pro-, meso-, {100}and metathorax. These three segments differ greatly in their relative proportions in different Insects, and in different stages of the same Insect's life. In their more highly developed conditions each of the three divisions is of complex structure, and the sclerites of which it is externally made up are sufficiently constant in their numbers and relative positions to permit of their identification in a vast number of cases; hence the sclerites have received names, and their nomenclature is of practical importance, because some, if not all, of these parts are made use of in the classification of Insects. Each division of the thorax has an upper region, called synonymically dorsum, notum, or tergum; an inferior or ventral region, called sternum; and on each side a lateral region, the pleuron. These regions of each of the three thoracic divisions are further distinguished by joining to their name an indication of the segment spoken of, in the form of the prefixes pro-, meso-, and meta-; thus the pronotum, prosternum, and propleura make up the prothorax. The thoracic regions are each made up of sclerites whose nomenclature is due to Audouin.[21] He considered that every thoracic ring is composed of the pieces shown in Fig. 55, viz. (1) the sternum (B', a), an unpaired ventral piece; (2) the notum (A), composed of four pieces placed in consecutive longitudinal order (A'), and named praescutum (a), scutum (b), scutellum (c), and post-scutellum (d); (3) lateral pieces, of which he distinguished on each side an episternum (B', c), epimeron (e), and parapteron (d), these together forming the pleuron. We give Audouin's Figure, but we cannot enter on a full discussion of his views as to the thorax; they have become widely known, though the constancy of the parts is not so great as he supposed it would prove to be. Sometimes it is impossible to find all the elements he thought should be present in a thoracic ring, while in other cases too many sclerites exist. As a rule the notum of the meso- and metathoraces is in greater part composed of two pieces, the scutum and the scutellum; while in the pronotum only one dorsal piece can be satisfactorily distinguished, though a study of the development may show that really two are frequently, if not usually, present. On the other hand, one, or more, of the notal sclerites in some cases shows evidence of longitudinal division along the middle. The sternum or ventral piece, though varying greatly in form, is {101}the most constant element of a thoracic segment, but it has sometimes the appearance of consisting of two parts, an anterior and a posterior. The pleuron nearly always consists quite evidently of two parts, the episternum, the more anterior and inferior, and the epimeron.[22] The relations between these two parts vary much; in some cases the episternum is conspicuously the more anterior, while in others the epimeron is placed much above it, and may extend nearly as far forwards as it. It may be said, as a rule, that when the sternum extends farther backwards than the notum, the epimeron is above the episternum, as in many Coleoptera; but if the sternum be anterior to the notum, then the episternum is superior to the epimeron, as in dragon-flies. We would here again reiterate the fact that these "pieces" are really not separate parts, but are more or less indurated portions of a continuous integument, which is frequently entirely occupied by them; hence a portion of a sclerite that in one species is hard, may in an allied form be wholly or partly membranous, and in such case its delimitation may be very evident on some of its sides, and quite obscure on another.
Fig. 55.—Mesothorax of Dytiscus, after Audouin. A, notum; A', pieces of the notum separated: a, praescutum; b, scutum; c, scutellum; d, post-scutellum: B, the sternum and pleura united; B', their parts separated: a, sternum; c, episternum; d, parapteron; e, epimeron.
The parapteron of Audouin does not appear to be really a distinct portion of the pleuron; in the case of Dytiscus it is apparently merely a thickening of an edge. Audouin supposed this part to be specially connected with the wing-articulation, and the term has been subsequently used by other writers in connexion with several little pieces that exist in the pleural region of winged Insects.
The prothorax is even more subject to variation in its development than the other divisions of the thorax are. In the Hymenoptera the prosternum is disconnected from the pronotum and is capable, together with the first pair of legs, of movement independent of its corresponding dorsal part, the pronotum, which in this Order is always more or less completely united with the meso-thorax; in the Diptera the rule is that the three thoracic segments are closely consolidated into one mass. In the majority of Insects the prothorax is comparatively free, that is to say, it is not so closely united with the other two thoracic segments as they are with one another. The three thoracic rings are seen in a comparatively uniform state of development in a great number of larvae; also in the adult stages of some Aptera, and among winged insects in some Neuroptera such as the Embiidae, Termitidae, and Perlidae. In Lepidoptera the pronotum bears a pair of erectile processes called patagia; though frequently of moderately large size, they escape observation, being covered with scales and usually closely adpressed to the sides of the pronotum.
The two great divisions of the body—the mesothorax and the metathorax—are usually very intimately combined in winged Insects, and even when the prothorax is free, as in Coleoptera, these posterior two thoracic rings are very greatly amalgamated. In the higher forms of the Order just mentioned the mesosternum and mesopleuron become changed in direction, and form as it were a diaphragm closing the front of the metasternum. The meso- and meta-thorax frequently each bear a pair of wings.
We have described briefly and figured (Fig. 55) the sclerites of the mesothorax, and those of the metathorax correspond fairly well with them. In addition to the sclerites usually described as constituting these two thoracic divisions, there are some small pieces at the bases of the wings. Jurine discriminated and named no less than seven of these at the base of the anterior {103}wing of a Hymenopteron. One of them becomes of considerable size and importance in the Order just mentioned, and seems to be articulated so as to exert pressure on the base of the costa of the wing. This structure attains its maximum of development in a genus (? nondescript) of Scoliidae, as shown in Fig. 56. The best name for this sclerite seems to be that proposed by Kirby and Spence, tegula. Some writers call it paraptère, hypoptère, or squamule, and others have termed it patagium; this latter name is, however, inadmissible, as it is applied to a process of the prothorax we have already alluded to.
To complete our account of the structure of the thorax it is necessary to mention certain hard parts projecting into its interior, but of which there is usually little or no trace externally. A large process in many Insects projects upwards from the sternum in a forked manner. It was called by Audouin the entothorax; some modern authors prefer the term apophysis. Longitudinal partitions of very large size, descending from the dorsum into the interior, also exist; these are called phragmas, and are of great importance in some Insects with perfect flight, such as Hymenoptera, Lepidoptera, and Diptera. There is no phragma in connection with the pronotum, but behind this part there may be three. A phragma has the appearance of being a fold of the dorsum; it serves as an attachment for muscles, and may probably be of service in other ways. More insignificant projections into the interior are the little pieces called apodemes (Fig. 57, e); these are placed at the sides of the thorax near the wings. The apophyses are no doubt useful in preserving the delicate vital organs from shocks, or from derangement by the muscular movements and the changes of position of the body.
Fig. 57.—Transverse section of skeleton of metathorax of Goliathus druryi, seen from behind: a, metanotum; b, metasternum; c, phragma; d, entothorax (apophysis or furca); e, apodeme; f, tendon of articulation. (After Kolbe.)
The appendages of the thorax are (a) inferior, the legs; (b) {104}superior, the wings. The legs are always six in number, and are usually present even in larvae, though there exist many apodal larvae, especially in Diptera. The three pairs of legs form one of the most constant of the characters of Insects. They are jointed appendages and consist of foot, otherwise tarsus; tibia, femur, trochanter, and coxa; another piece, called trochantin more or less distinctly separated from the coxa, exists in many Insects. The legs are prolongations of the body sac, and are in closer relation with the epimera and with the episterna than with other parts of the crust, though they have a close relation with the sternum. If we look at the body and leg of a neuropterous Insect (Fig. 58) we see that the basal part of the leg—the coxa—is apparently a continuation of one of the two pleural pieces or of both; in the latter case one of the prolonged pieces forms the coxa proper, and the tip of the other forms a supporting piece, which may possibly be the homologue of the trochantin of some Insects. In some Orthoptera, especially in Blattidae, and in Termitidae, there is a transverse chitinised fold interposed between the sternum and the coxa, and this has the appearance of being the same piece as the trochantin of the anterior legs of Coleoptera.
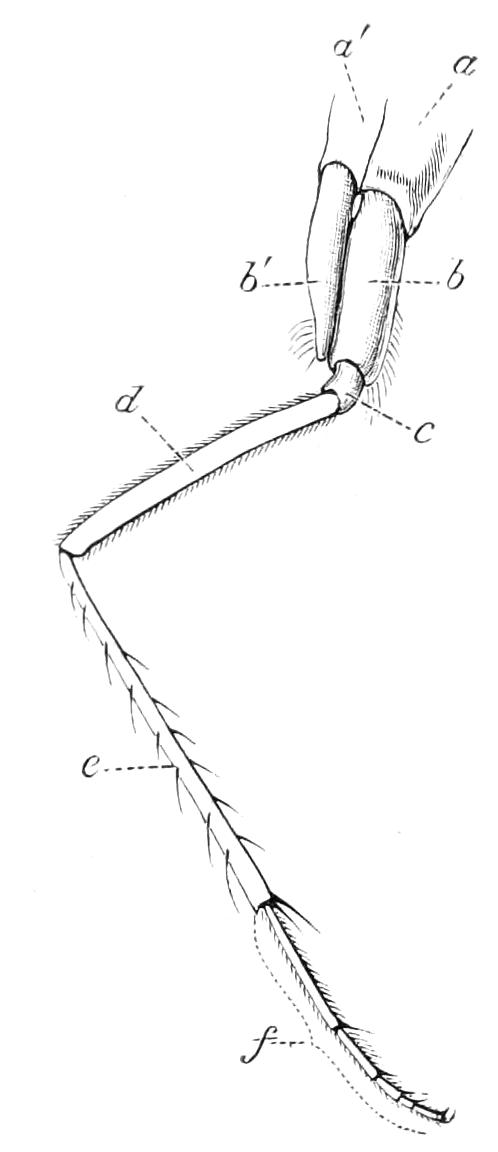
Fig. 58.—Hind leg of Panorpa: a, episternum; a′, epimeron; b, coxa; b′, coxal fold of epimeron; c, trochanter; d, femur; e, tibia; f, tarsus.
Beyond the coxa comes the trochanter; this in many Hymenoptera is a double piece, though in other Insects it is single; usually it is the most insignificant part of the leg. The femur is, on the whole, the least variable part of the leg; the tibia, which follows it, being frequently highly modified for industrial or other purposes. The joint between the femur and the tibia is usually bent, and is therefore the most conspicuous one in the leg; it is called the knee. The other joints have not corresponding names, though that between the tibia and the tarsus is of great importance. The spines at the tip of the tibia, projecting beyond it, are called spurs, or calcares. The tarsus or {105}foot is extremely variable; it is very rarely absent, but may consist of only one piece—joint, as it is frequently called[23]—or of any larger number up to five, which may be considered the characteristic number in the higher Insect forms. The terminal joint of the tarsus bears normally a pair of claws; between the claws there is frequently a lobe or process, according to circumstances very varied in different Insects, called empodium, arolium, palmula, plantula, pseudonychium, or pulvillus. This latter name should only be used in those cases in which the sole of the foot is covered with a dense pubescence. The form of the individual tarsal joints and the armature or vestiture of the lower surface are highly variable. The most remarkable tarsus is that found on the front foot of the male Dytiscus.
It has been suggested that the claws and the terminal appendage of the tarsus ought to be counted as forming a distinct joint; hence some authors state that the higher Insects have six joints to the feet. These parts, however, are never counted as separate joints by systematic entomologists, and it has recently been stated that they are not such originally.
The parts of the foot at the extremity of the last tarsal joint proper are of great importance to the creature, and vary greatly in different Insects. The most constant part of this apparatus is a pair of claws, or a single claw. Between the two claws there may exist the additional apparatus referred to above. This in some Insects—notably in the Diptera—reaches a very complex development. We figure these structures in Pelopaeus spinolae, a fossorial Hymenopteron, remarking that our figures exhibit the apparatus in a state of retraction (Fig. 59). According to the nomenclature of Dahl and Ockler[24] the plate (b) on the dorsal aspect is the pressure plate (Druck-Platte), and acts as an agent of pressure on the sole of the pad (C, e); c and d on the underside are considered to be extension-agents; c, extension-plate; d, extension-sole (Streck-Platte, Streck-Sohle). These agents are assisted in acting on the pad by means of an elastic bow placed in the interior of the latter. The pad (e) is a very remarkable structure, capable of much extension and retraction; {106}when extended it is seen that the pressure plate is bent twice at a right angle so as to form a step, the distal part of which runs along the upper face of the basal part of the pad; the apical portion of this latter consists of two large lobes, which in repose, as shown in our Figure (f), fall back on the pad, something in the fashion of the retracted claws of the cat, and conceal the pressure-plate.
The mode in which Insects are able to walk on smooth perpendicular surfaces has been much discussed, and it appears highly probable that the method by which this is accomplished is the exudation of moisture from the foot; there is still, however, much to be ascertained before the process can be satisfactorily comprehended. The theory to the effect that the method is the pressure of the atmosphere acting on the foot when the sole is in perfect apposition with the object walked on, or when a slight vacuum is created between the two, has apparently less to support it.
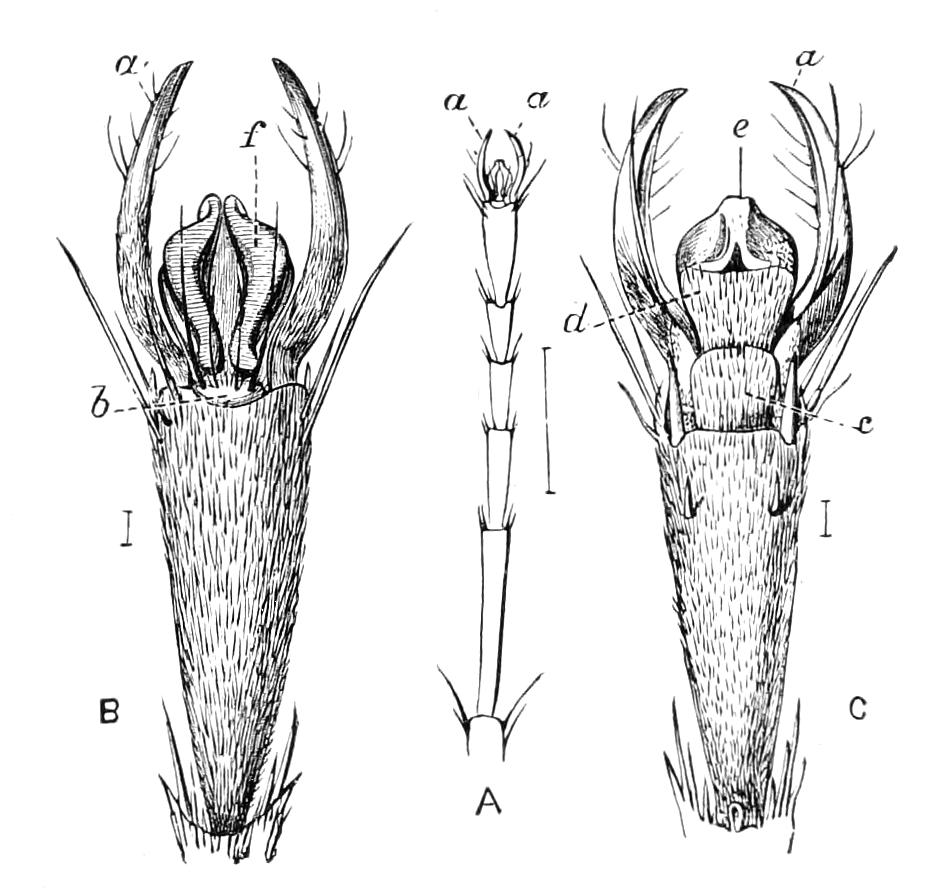
Fig. 59.—Foot of Pelopaeus, a fossorial wasp: A, tarsus entire; B, terminal joint, upper side; C, under side. a, claw; b, base of pressure-plate; c, extension-plate; d, extension-sole; e, pad; f, lobe of pad retracted.
The legs of the young Insect are usually more simple than those of the adult, and in caterpillars they are short appendages, and only imperfectly jointed. If a young larva, with feet, of a beetle, such as Crioceris asparagi be examined, it may be seen that the leg is formed by protuberance of the integument, which becomes divided into parts by simple creases; an observation suggesting that the more highly developed jointed leg is formed in a similar manner. This appears to be really the case, {107}for the actual continuity of the limb at the chief joint—the knee—can be demonstrated in many Insects by splitting the outer integument longitudinally and then pulling the pieces a little apart; while in other cases even this is not necessary, the knee along its inner face being membranous to a considerable extent, and the membrane continuous from femur to tibia.
Turning to the wings, we remark that there may be one or two pairs of these appendages. When there is but one pair it is nearly always mesothoracic, when there are two pairs one is invariably mesothoracic, the other metathoracic. The situation of the wing is always at the edge of the notum, but the attachment varies in other respects. It may be limited to a small spot, and this is usually the case with the anterior wing; or the attachment may extend for a considerable distance along the edge of the notum, a condition which frequently occurs, especially in the case of the posterior wings. The actual connexion of the wings with the thorax takes place by means of strong horny lines in them which come into very close relation with the little pieces in the thorax which we have already described, and which were styled by Audouin articulatory epidemes. There is extreme variety in the size, form, texture, and clothing of the wings, but there is so much resemblance in general characters amongst the members of each one of the Orders, that it is usually possible for an expert, seeing only a wing, to say with certainty what Order of Insects its possessor belonged to. We shall allude to these characters in treating of the Orders of Insects.
Each wing consists of two layers, an upper and a lower, and between them there may be tracheae and other structures, especially obvious when the wings are newly developed. It has been shown by Hagen that the two layers can be separated when the wings are recently formed, and it is then seen that each layer is traversed by lines of harder matter, the nervures. These ribs are frequently called wing-veins, or nerves, but as they have no relation to the anatomical structures bearing those names, it is better to make use of the term nervures. The strength, number, form and inter-relations of these nervures vary exceedingly; they are thus most important aids in the classification of Insects. Hence various efforts have been made to establish a system of nomenclature that shall be uniform throughout the different Orders, but at present success has not {108}attended these efforts, and it is probable that no real homology exists between the nervures of the different Orders of Insects. We shall not therefore discuss the question here. We may, however, mention that German savants have recently distinguished two forms of nervures which they consider essentially distinct, viz. convex and concave. These, to some extent, alternate with one another, but a fork given off by a convex one is not considered to be a concave one. The terms convex and concave are not happily chosen; they do not refer to the shape of the nervures, but appear to have been suggested by the fact that the surface of the wing being somewhat undulating the convex veins more usually run along the ridges, the concave veins along the depressions. The convex are the more important of the two, being the stronger, and more closely connected with the articulation of the wing.
The wings, broadly speaking, may be said to be three-margined: the margin that is anterior when the wings are extended is called the costa, and the edge that is then most distant from the body is the outer margin, while the limit that lies along the body when the wings are closed is the inner margin.
The only great Order of Insects provided with a single pair of wings is the Diptera, and in these the metathorax possesses, instead of wings, a pair of little capitate bodies called halteres or poisers. In the abnormal Strepsiptera, where a large pair of wings is placed on the metathorax, there are on the mesothorax some small appendages that are considered to represent the anterior wings. In the great Order Coleoptera, or beetles, the anterior wings are replaced by a pair of horny sheaths that close together over the back of the Insect, concealing the hind-wings, so that the beetle looks like a wingless Insect: in other four-winged Insects it is usually the front wings that are most useful in flight, but the elytra, as these parts are called in Coleoptera, take no active part in flight, and it has been recently suggested by Hoffbauer[25] that they are not the homologues of the front wings, but of the tegulae (see Fig. 56), of other Insects. In the Orthoptera the front wings also differ in consistence from the other pair over which they lie in repose, and are called tegmina. There are many Insects in which the wings {109}exist in a more or less rudimentary or vestigial condition, though they are never used for purposes of flight.
The abdomen, or hind body, is the least modified part of the body, though some of the numerous rings of which it is composed may be extremely altered from the usual simple form. Such change takes place at its two extremities, but usually to a much greater extent at the distal extremity than at the base. This latter part is attached to the thorax, and it is a curious fact that in many Insects the base of the abdomen is so closely connected with the thorax that it has all the appearance of being a portion of this latter division of the body; indeed it is sometimes difficult to trace the real division between the two parts. In such cases a further differentiation may occur, and the part of the abdomen that on its anterior aspect is intimately attached to the thorax may on its posterior aspect be very slightly connected with the rest of the abdomen. Under such circumstances it is difficult at first sight to recognise the real state of the case. When a segment is thus transferred from the abdomen to the metathorax, the part is called a median segment. The most remarkable median segment exists in those Hymenoptera which have a stalked abdomen, but a similar though less perfect condition exists in many Insects. When such a union occurs, it is usually most complete on the dorsal surface, and the first ventral plate may almost totally disappear: such an alteration may involve a certain amount of change in the sclerites of the next segment, so that the morphological determination of the parts at the back of the thorax and front of the abdomen is by no means a simple matter. A highly modified hind-body exists in the higher ants, Myrmicidae. In Fig. 60 we contrast the simple abdomen of Japyx with the highly modified state of the same part in an ant.
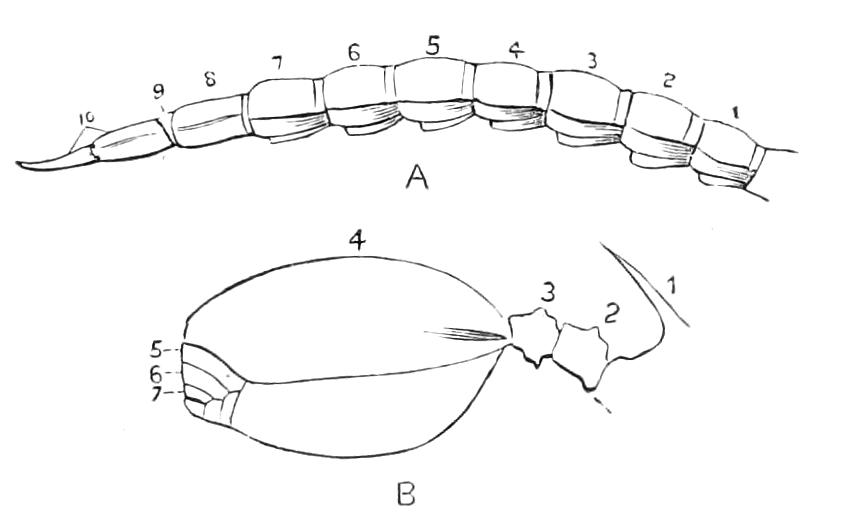
Fig. 60.—Simple abdomen of Japyx (A) contrasted with the highly modified one of an ant, Cryptocerus (B). The segments are numbered from before backwards.
Unlike the head and thorax, the abdomen is so loosely knitted together that it can undergo much expansion and contraction. {110}This is facilitated by an imbricated arrangement of the plates, and by their being connected by means of membranes admitting of much movement (Fig. 47, m, p. 88). In order to understand the structure of the abdomen it should be studied in its most distended state; it is then seen that there is a dorsal and a ventral hard plate to each ring, and there is also usually a stigma; there may be foldings or plications near the line of junction of the dorsal and ventral plates, but these margins are not really distinct pieces. The pleura, in fact, remain membranous in the abdominal region, contrasting strongly with the condition of these parts in the thorax. The proportions of the plates vary greatly; sometimes the ventral are very large in proportion to the dorsal, as is usually the case in Coleoptera, while in the Orthoptera the reverse condition prevails.
Cerci or other appendages frequently exist at the extremity of the abdomen (Fig. 47, n, p. 88); the former are sometimes like antennae, while in other cases they may be short compressed processes consisting of very few joints. The females of many Insects possess saws or piercing instruments concealed within the apical part of the abdomen; in other cases an elongate exserted organ, called ovipositor, used for placing the eggs in suitable positions, is present. Such organs consist, it is thought, either of modified appendages, called gonapophyses, or of dorsal, ventral, or pleural plates. The males frequently bear within the extremity of the body a more or less complicated apparatus called the genital armour. The term gonapophysis is at present a vague one, including stings, some ovipositors, portions of male copulatory apparatus, or other structures, of which the origin is more or less obscure.
The caterpillar, or larva, of the Lepidoptera and some other Insects, bears a greater number of legs than the three pairs we have mentioned as being the normal number in Insects, but the posterior feet are in this case very different from the anterior, and are called false legs or prolegs. These prolegs, which are placed on the hind body, bear a series of hooks in Lepidopterous larvae, but the analogous structures of Sawfly larvae are destitute of such hooks.
Placed along the sides of the body, usually quite visible in the larva, but more or less concealed in the perfect Insect, are little apertures for the admittance of air to the respiratory {111}system. They are called spiracles or stigmata. There is extreme variety in their structure and size; the largest and most remarkable are found on the prothorax of Coleoptera, especially in the groups Copridae and Cerambycidae.
The exact position of the stigmata varies greatly, as does also their number. In the Order Aptera there may be none, while the maximum number of eleven pairs is said by Grassi[26] to be attained in Japyx solifugus: in no other Insect have more than ten pairs been recorded, and this number is comparatively rare. Both position and number frequently differ in the early and later stages of the same Insect. The structure of the stigmata is quite as inconstant as the other points we have mentioned are.
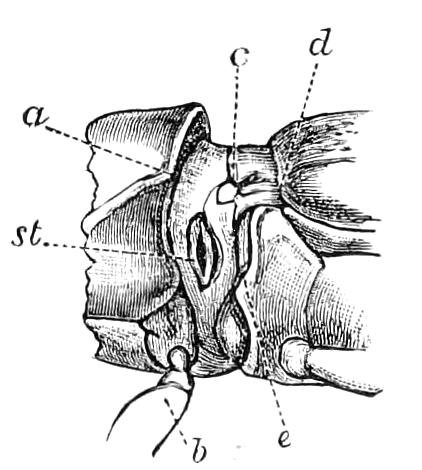
Fig. 61.—Membranous space between pro- and meso-thoraces of a beetle Euchroma, showing stigma (st); a, hind margin of pronotum; b, front leg; c, front margin of mesonotum; d, base of elytra; e, mesosternum.
The admission of air to the tracheal system and its confinement there, as well as the exclusion of foreign bodies, have to be provided for. The control of the air within the system is, according to Landois[27] and Krancher,[28] usually accomplished by means of an occluding apparatus placed on the tracheal trunk a little inside of the stigma, and in such case this latter orifice serves chiefly as a means for preventing the intrusion of foreign bodies. The occluding apparatus consists of muscular and mechanical parts, which differ much in their details in different Insects. Lowne supposes that the air is maintained in the tracheal system in a compressed condition, and if this be so, this apparatus must be of great importance in the Insect economy. Miall and Denny[29] state that in the anterior stigmata of the cockroach the valves act as the occluding agents, muscles being attached directly to the inner face of the valves, and in some other Insects the spiracular valves appear to act partially by muscular agency, but there are many stigmata having valves destitute of muscles. According to Lowne[30] there exist valves in the blowfly at the entrance to the trachea proper, and he gives the following as the arrangement of parts for the admission of air:—there is a spiracle {112}leading into a chamber, the atrium, which is limited inwardly by the occluding apparatus; and beyond this there is a second chamber, the vestibule, separated from the tracheae proper by a valvular arrangement. He considers that the vestibule acts as a pump to force the air into the tracheae.
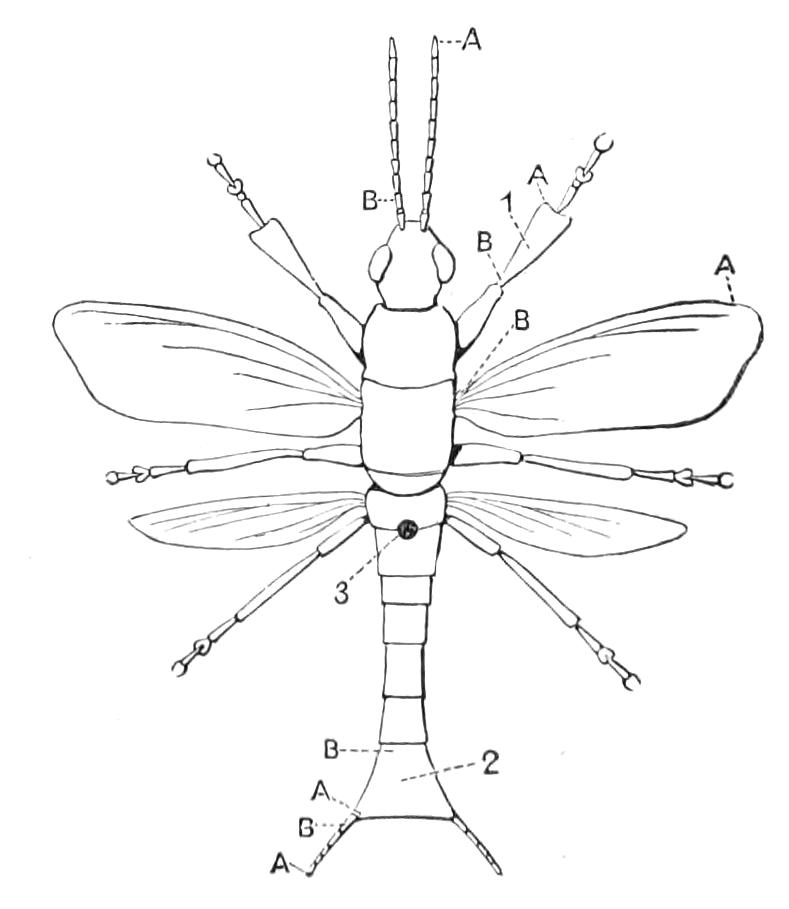
Fig. 62.—Diagrammatic Insect to explain terms of position. A, apex; B, base: 1, tibia; 2, last abdominal segment; 3, ideal centre.
Systematic Orientation.
Terms relating to position are unfortunately used by writers on entomology in various, even in opposite senses. Great confusion exists as to the application of such words as base, apex, transverse, longitudinal. We can best explain the way in which the relative positions and directions of parts should be described by reference to Figure 62. The spot 3 represents an imaginary centre, situated between the thorax and abdomen, to which all the parts of the body are supposed to be related. The Insect should always be described as if it were in the position shown in the Figure, and the terms used should not vary as the position is changed. The creature is placed with ventral surface beneath, and with the appendages extended, like the Insect itself, in a horizontal plane. In the Figure the legs are, for clearness, made to radiate, but in the proper position the anterior pair should be approximate in front, and the middle and hind pairs directed backwards under the body. The legs are not to be treated as if they were hanging from the body, though that is the position they frequently actually assume. The right and left sides, and the upper and lower faces (these latter are frequently also spoken of as sides), are still to retain the same nomenclature even when the position of the specimen is reversed. The base of an organ is that margin that is nearest to the ideal centre, the apex that which is most distant. {113}Thus in Fig. 62, where 1 indicates the front tibia, the apex (A) is broader than the base (B); in the antennae the apex is the front part, while in the cerci the apex is the posterior part; in the last abdominal segment (2) the base (B) is in front of the apex (A). The terms longitudinal and transverse should always be used with reference to the two chief axes of the body-surface; longitudinal referring to the axis extending from before backwards, and transverse to that going across, i.e. from side to side.
ARRANGEMENT OF INTERNAL ORGANS–MUSCLES–NERVOUS SYSTEM–GANGLIONIC CHAIN–BRAIN–SENSE-ORGANS–ALIMENTARY CANAL–MALPIGHIAN TUBES–RESPIRATION–TRACHEAL SYSTEM–FUNCTION OF RESPIRATION–BLOOD OR BLOOD-CHYLE–DORSAL VESSEL OR HEART–FAT-BODY–OVARIES–TESTES–PARTHENOGENESIS–GLANDS.
The internal anatomy of Insects may be conveniently dealt with under the following heads:—(1) Muscular system; (2) nervous system; (3) alimentary system (under which may be included secretion and excretion, about which in Insects very little is known); (4) respiratory organs; (5) circulatory system; (6) fat-body; (7) reproductive system.
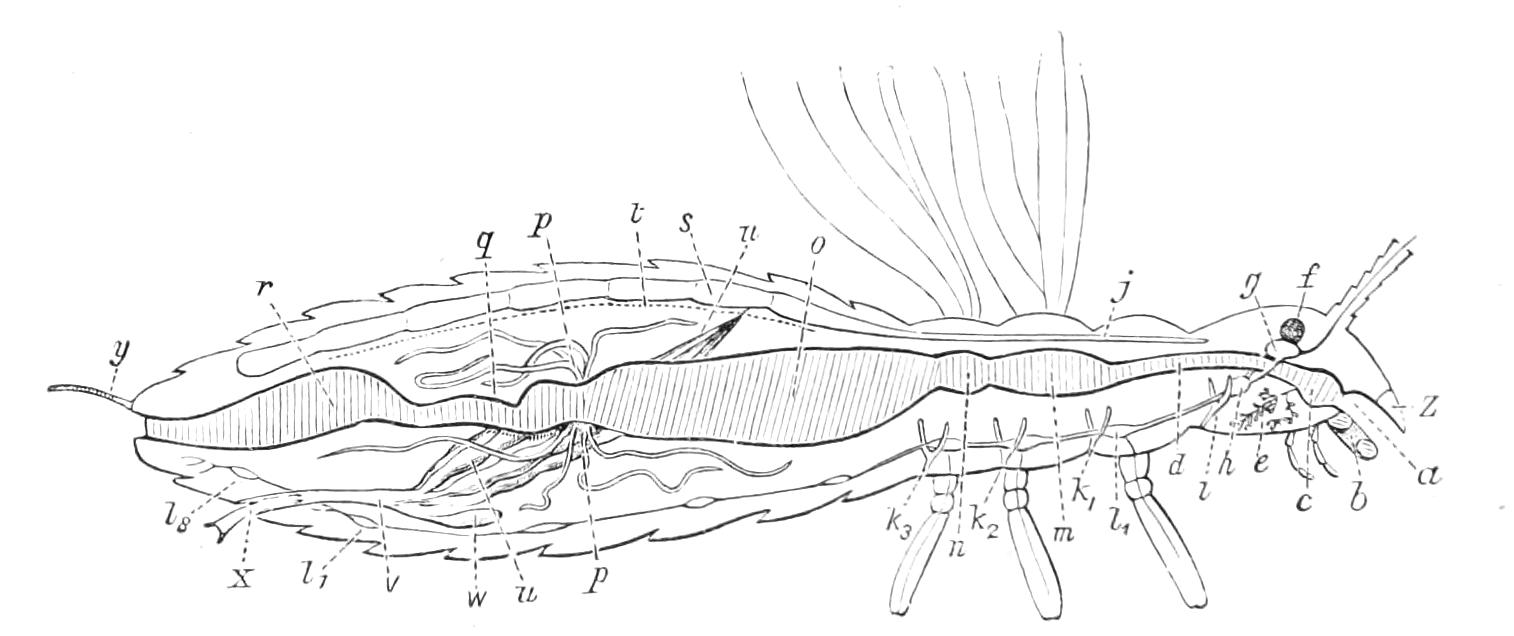
Fig. 63.—Diagram of arrangement of some of the internal organs of an Insect: a, mouth; b, mandible; c, pharynx; d, oesophagus; e, salivary glands (usually extending further backwards); f, eye; g, supra-oesophageal ganglion; h, sub-oesophageal ganglion; i, tentorium; j, aorta; k1, k2, k3, entothorax; l1-l8, ventral nervous chain; m, crop; n, proventriculus; o, stomach; p, Malpighian tubes; q, small intestine; r, large intestine; s, heart; t, pericardial septum; u, ovary composed of four egg-tubes; v, oviduct; w, spermatheca (or an accessory gland); x, retractile ovipositor; y, cercus; z, labrum.
Many of the anatomical structures have positions in the body that are fairly constant throughout the class. Parts of the respiratory and muscular systems and the fat-body occur in most of the districts of the body. The heart is placed just below the dorsal surface; the alimentary canal extends along the middle from the head to the end of the body. The chief parts of the nervous system are below the alimentary canal, except that the brain is placed above the beginning of the canal in the head. The reproductive system extends in the abdomen obliquely from above downwards, commencing anteriorly at the upper part and terminating posteriorly at the lower part of the body cavity.
In Fig. 63 we show the arrangement of some of the chief organs of the body, with the exception of the muscular and respiratory systems, and the fat-body. It is scarcely necessary to point out that the figure is merely diagrammatic, and does not show the shapes and sizes of the organs as they will be found in any one Insect.
Muscles.
The muscular system of Insects is very extensive, Lyonnet[31] having found, it is said, nearly 4000 muscles in the caterpillar of the goat-moth; a large part of this number are segmental repetitions, nevertheless the muscular system is really complex, as may be seen by referring to the study of the flight of dragon-flies by von Lendenfeld.[32]
The minute structure of the muscles does not differ essentially from what obtains in Vertebrate animals. The muscles are aggregations of minute fibrils which are transversely striated, though in variable degree. Those in the thorax are yellow or pale brown, but in other parts the colour is more nearly white. The muscles of flight are described as being penetrated by numerous tracheae, while those found elsewhere are merely surrounded by these aerating tubules.
The force brought into play by the contractions of Insect muscles is very great, and has been repeatedly stated to be much superior to that of Vertebrate animals; very little reliance can, however, be {116}placed on the assumptions and calculations that are supposed to prove this, and it is not supported by Camerano's recent researches.[33]
Some of the tendons to which the muscles are attached are very elaborate structures, and are as hard as the chitinous skeleton, so as to be like small bones in their nature. A very elaborate tendon of this kind is connected with the prothoracic trochantin in Coleoptera, and may be readily examined in Hydrophilus. It has been suggested that the entothorax is tendinous in its origin, but other morphologists treat it, with more reason, as an elaborate fold inwards of the integument.
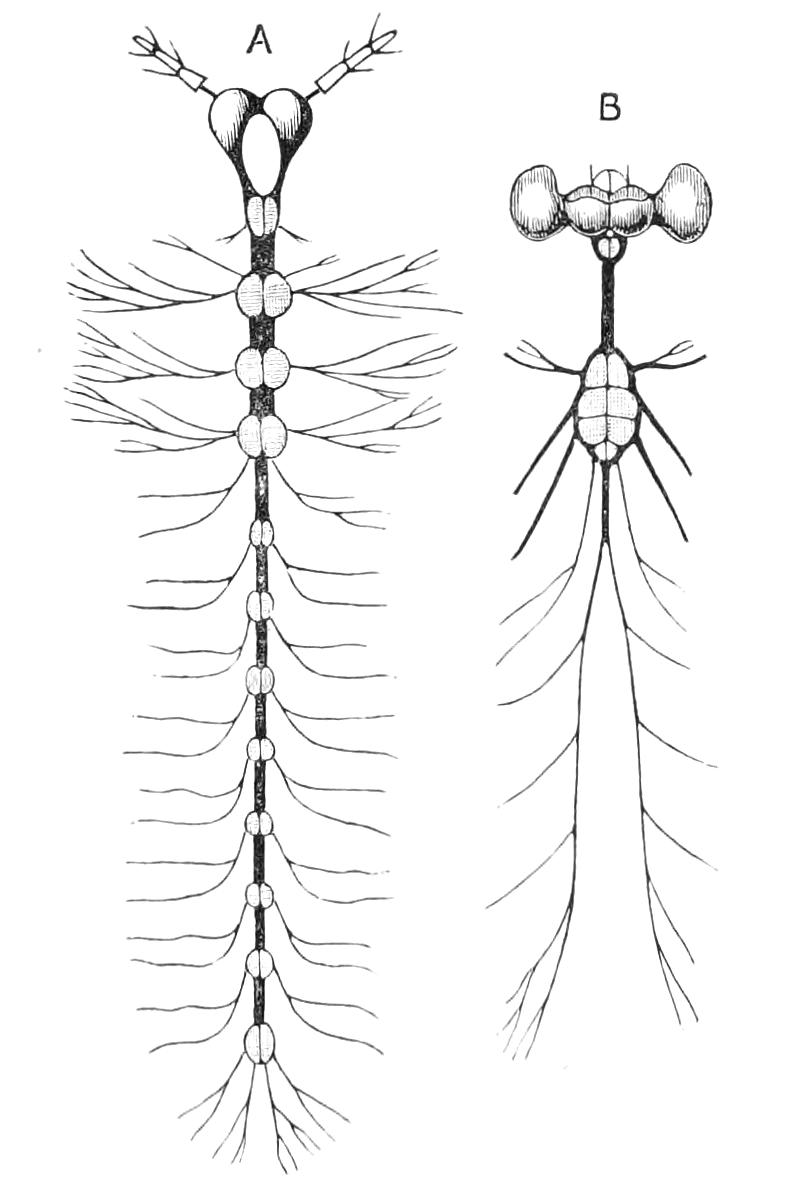
Fig. 64.—Cephalic and ventral chain of ganglia: A, larva of Chironomus; B, imago of Hippobosca. (After Brandt.)
Nervous System.
Insects are provided with a very complex nervous system, which may be treated as consisting of three divisions:—(1) The cephalic system; (2) the ventral, or ganglionic chain; (3) an accessory sympathetic system, or systems. All these divisions are intimately connected. We will consider first the most extensive, viz. the ventral chain. This consists of a series of small masses of nervous matter called ganglia which extend in the longitudinal direction of the body along the median line of the lower aspect, and are connected by longitudinal commissures, each ganglion being joined to that following it by two threads of nervous matter. Each of the ganglia of the ventral chain really consists of two ganglia placed side by side and connected by commissures as well as cellular matter. In larvae some of the ganglia may be contiguous, so that the commissures do not exist. From the ganglia motor nerves proceed to the various parts of the {117}body for the purpose of stimulating and co-ordinating the contractions of the muscles. The number of the ganglia in the ventral chain differs greatly in different Insects, and even in the different stages of metamorphosis of the same species, but never exceeds thirteen. As this number is that of the segments of the body, it has been considered that each segment had primitively a single ganglion. Thirteen ganglia for the ventral chain can, however, be only demonstrated in the embryonic state; in the later stages of life eleven appears to be the largest number that can be distinguished, and so many as this are found but rarely, and then chiefly in the larval stage. The diminution in number takes place by the amalgamation or coalescence of some of the ganglia, and hence those Insects in which the ganglia are few are said to have a highly concentrated nervous system. The modes in which these ganglia combine are very various; the most usual is perhaps that of the combination of the three terminal ganglia into one body. As a rule it may be said that concentration is the concomitant of a more forward position of the ganglia. As a result of this it is found that in some cases, as in Lamellicorn beetles, there are no ganglia situate in the abdomen. In the perfect state of the higher Diptera, the thoracic and abdominal ganglia are so completely concentrated in the thorax as to form a sort of thoracic brain. In Fig. 64 we represent a very diffuse and a very concentrated ganglionic chain; A being that of the larva of Chironomus, B that of the imago of Hippobosca. In both these sketches the cephalic ganglia as well as those of the ventral chain are shown.
Turning next to the cephalic masses, we find these in the perfect Insect to be nearly always two in number: a very large and complex one placed above the oesophagus, and therefore called the supra-oesophageal ganglion; and a smaller one, the sub- or infra-oesophageal, placed below the oesophagus. The latter ganglion is in many Insects so closely approximated to the supra-oesophageal ganglion that it appears to be a part thereof, and is sometimes spoken of as the lower brain. In other Insects these two ganglia are more remote, and the infra-oesophageal one then appears part of the ventral chain. In the embryo it is said that the mode of development of the supra-oesophageal ganglion lends support to the idea that it may be the equivalent of three ganglia; there being at one {118}time three lobes, which afterwards coalesce, on each side of the mouth. This is in accordance with the view formulated by Viallanes[34] to the effect that this great nerve-centre, or brain, as it is frequently called, consists essentially of three parts, viz. a Proto-, a Deuto-, and a Trito-cerebron. It is, however, only proper to say that though the brain and the ventral chain of ganglia may appear to be one system, and in the early embryonic condition to be actually continuous, these points cannot be considered to be fully established. Dr. L. Will has informed us[35] that in Aphididae the brain has a separate origin, and is only subsequently united with the ganglionic chain. Some authorities say that in the early condition the sub-oesophageal ganglion is formed from two, and the supra-oesophageal from the same number of ganglia; the division in that case being 2 and 2, not 3 and 1, as Viallanes' views would suggest. The inquiries that are necessary to establish such points involve very complex and delicate investigations, so that it is not a matter of surprise that it cannot yet be said whether each of these views may be in certain cases correct. The supra- and sub-oesophageal ganglia are always intimately connected by a commissure on each side of the oesophagus; when very closely approximated they look like one mass through which passes the oesophagus (Fig. 66, A). The large supra-oesophageal ganglion supplies the great nerves of the cephalic sense-organs, while the smaller sub-oesophageal centre gives off the nerves to the parts of the mouth. From the lower and anterior part of the supra-oesophageal ganglion a nervous filament extends as a ring round the anterior part of the oesophagus, and supplies a nerve to the upper lip.[36] This structure is not very well known, and has been chiefly studied by Liénard,[37] who considers that it will prove to be present in all Insects.
Whether the two cephalic ganglia be considered as really part of a single great ganglionic chain, or the reverse, they are at any rate always intimately connected with the ventral ganglia. We have already stated that the two cephalic masses are themselves closely approximated in many Insects, and may add that in some Hemiptera the first thoracic ganglion of the ventral chain is amalgamated into one body with the sub-oesophageal ganglion, {119}and further that there are a few Insects in which this latter centre is wanting. If the cephalic ganglia and ventral chain be looked on as part of one system, this may be considered as composed originally of seventeen ganglia, which number has been demonstrated in some embryos.
The anatomy of the supra-oesophageal ganglion is very complex; it has been recently investigated by Viallanes[38] in the wasp (Vespa) and in a grasshopper (Caloptenus italicus). The development and complication of its inner structure and of some of its outer parts appear to be proportional with the state of advancement of the instinct or intelligence of the Insect, and Viallanes found the brain of the grasshopper to be of a more simple nature than that of the wasp.
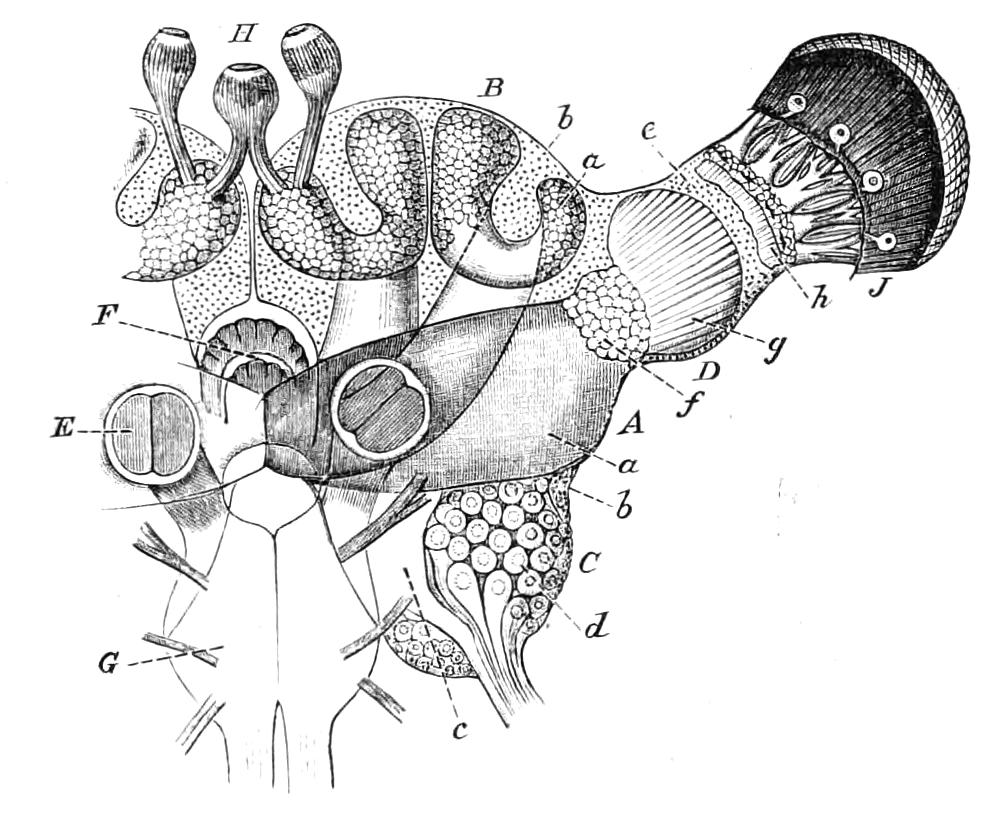
Fig. 65.—Brain of Worker Ant of Formica rufa. (After Leydig, highly magnified.) Explanation in text.
Brandt, to whom is due a large part of our knowledge of the anatomy of the nervous system in Insects, says that the supra-oesophageal ganglion varies greatly in size in various Insects, its mass being to a great extent proportional with the development of the compound eyes; hence the absolute size is not a criterion for the amount of intelligence, and we must rather look to the complication of the structure and to the development of certain parts for an index of this nature. The drone in the honey-bee has, correlatively with the superior development of its eyes, a larger brain than the worker, but the size of the hemispheres, and the development of the gyri cerebrales is superior in the latter. In other words, the mass of {120}those great lobes of the brain that are directly connected with the faceted eyes must not be taken into account in a consideration of the relation of the size and development of the brain to the intelligence of the individual. The weight of the brain in Insects is said by Lowne to vary from 1⁄150 to 1⁄2500 of the weight of the body.
Figure 65 gives a view of one side of the supra-oesophageal ganglion of the worker of an ant,—Formica rufa,—and is taken from Leydig, who gives the following elucidation of it: A, primary lobe, a, homogeneous granular inner substance, b, cellular envelope; B, stalked bodies (gyri cerebrales), a, b, as before; C, presumed olfactory lobes, c, inner substance, d, ganglionic masses; D, ocular lobes, e, f, g, h, various layers of the same; E, origin of lateral commissures; F, median commissure in interior of brain; G, lower brain (sub-oesophageal ganglion); H, ocelli; J, faceted eye.
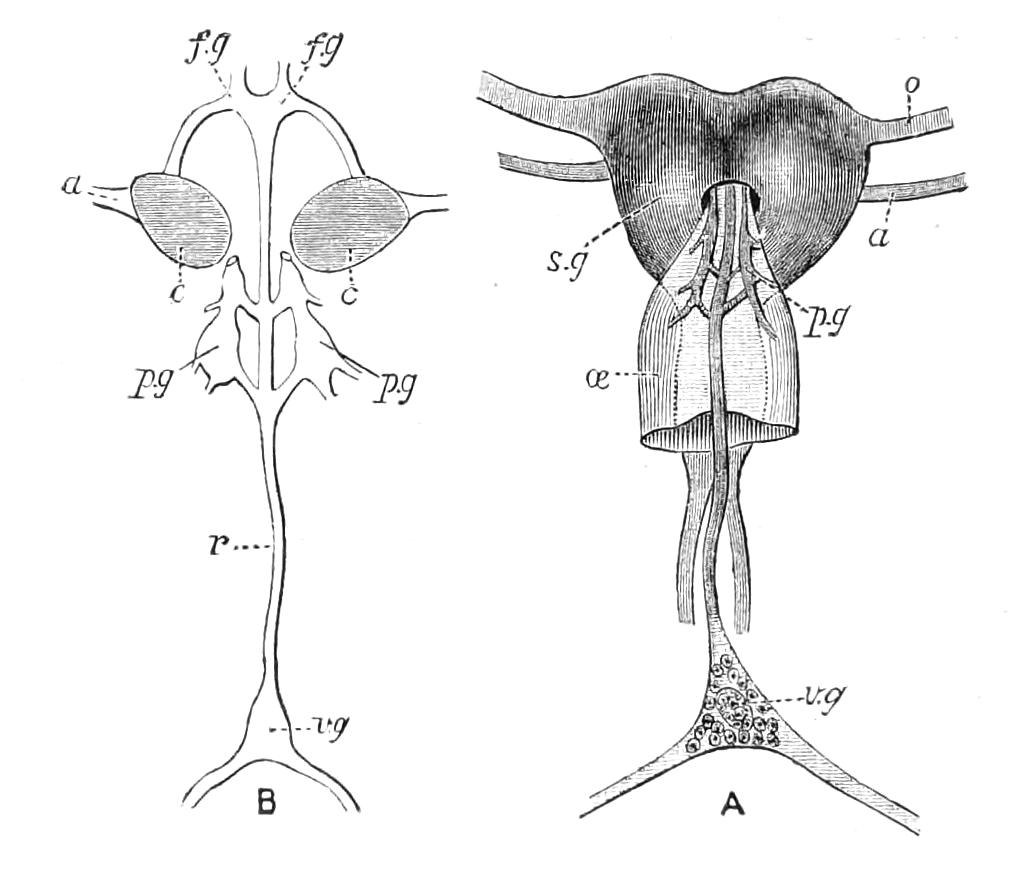
Fig. 66.—Stomato-gastric nerves of Cockroach: A, with brain in situ, after Koestler; B, with the brain removed, after Miall and Denny: s.g, supra-oesophageal ganglion; o, optic nerve; a, antennary nerve; f.g, frontal ganglion; oe, oesophagus; c, connective; p.g, paired ganglia; v.g, crop or ventricular ganglion; r, recurrent nerve.
Besides the brain and the great chain of ganglia there exists an accessory system, or systems, sometimes called the sympathetic, vagus, or visceral system. Although complex, these parts are delicate and difficult of dissection, and are consequently not so well known as is the ganglionic chain. There is a connecting or median nerve cord, communicating with the longitudinal commissures of each segment, and itself dilating into ganglia at intervals; this is sometimes called the unpaired system. There is another group of nerves having paired ganglia, {121}starting from a small ganglion in the forehead, then connecting with the brain, and afterwards extending along the oesophagus to the crop and proventriculus (Fig. 66). This is usually called the stomatogastric system. The oesophageal ring we have already spoken of.
By means of these accessory nervous systems all the organs of the body are brought into more or less direct relation with the brain and the ganglionic chain.
Our knowledge of these subsidiary nervous systems is by no means extensive, and their nomenclature is very unsettled; little is actually known as to their functions.
Organs of Sense.
Insects have most delicate powers of perception, indeed they are perhaps superior in this respect to the other classes of animals. Their senses, though probably on the whole analogous to those of the Vertebrata, are certainly far from corresponding therewith, and their sense organs seem to be even more different from those of what we call the higher animals than the functions themselves are. We have already briefly sketched the structure of the optical organs, which are invariably situate on the head. This is not the case with the ears, which certainly exist in one Order,—the Orthoptera,—and are placed either on the front legs below the knee, or at the base of the abdomen. Notwithstanding their strange situation, the structures alluded to are undoubtedly auditory, and somewhat approximate in nature to the ear of Vertebrates, being placed in proximity to the inner face of a tense membrane; we shall refer to them when considering the Orthoptera. Sir John Lubbock considers—no doubt with reason—that some ants have auditory organs in the tibia. Many Insects possess rod-like or bristle-like structures in various parts of the body, called chordotonal organs; they are considered by Graber[39] and others to have auditory functions, though they are not to be compared with the definite ears of the Orthoptera.
The other senses and sense organs of Insects are even less known, and have given rise to much perplexity; for though many structures have been detected that may with more or less probability be looked on as sense organs, it is difficult to assign a {122}particular function to any of them, except it be to the sensory hairs. These are seated on various parts of the body. The chitinous covering, being a dead, hard substance, has no nerves distributed in it, but it is pierced with orifices, and in some of these there is implanted a hair which at its base is in connexion with a nerve; such a structure may possibly be sensitive not only to contact with solid bodies, but even to various kinds of vibration. We give a figure (Fig. 67) of some of these hairs on the caudal appendage of a cricket, after Vom Rath. The small hairs on the outer surface of the chitin in this figure have no sensory function, but each of the others probably has; and these latter, being each accompanied by a different structure, must, though so closely approximated, be supposed to have a different function; but in what way those that have no direct connexion with a nerve may act it is difficult to guess.
The antennae of Insects are the seats of a great variety of sense organs, many of which are modifications of the hair, pit and nerve structure we have described above, but others cannot be brought within this category. Amongst these we may mention the pits covered with membrane (figured by various writers), perforations of the chitin without any hair, and membranous bodies either concealed in cavities or partially protruding therefrom.
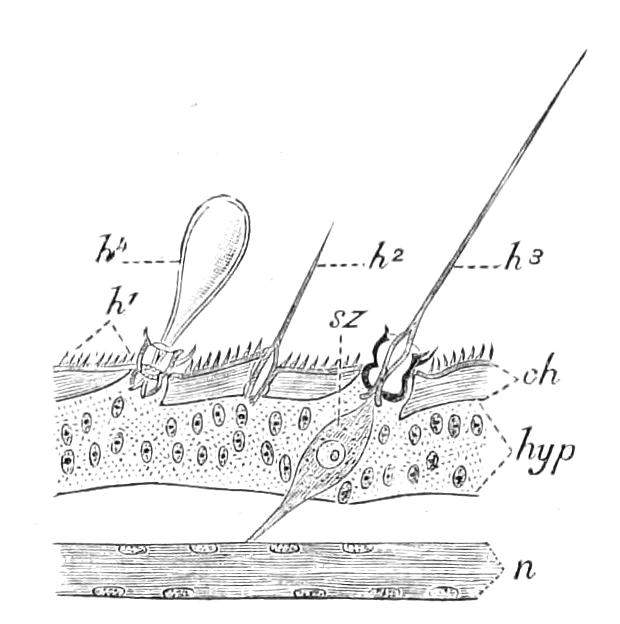
Fig. 67.—Longitudinal section of portion of caudal appendage of Acheta domestica (after Vom Rath): ch, chitin; hyp, hypodermis; n, nerve; h1, integumental hairs, not sensitive; h2, ordinary hair; h3, sensory hair; h4, bladder-like hair; sz, sense-cell.
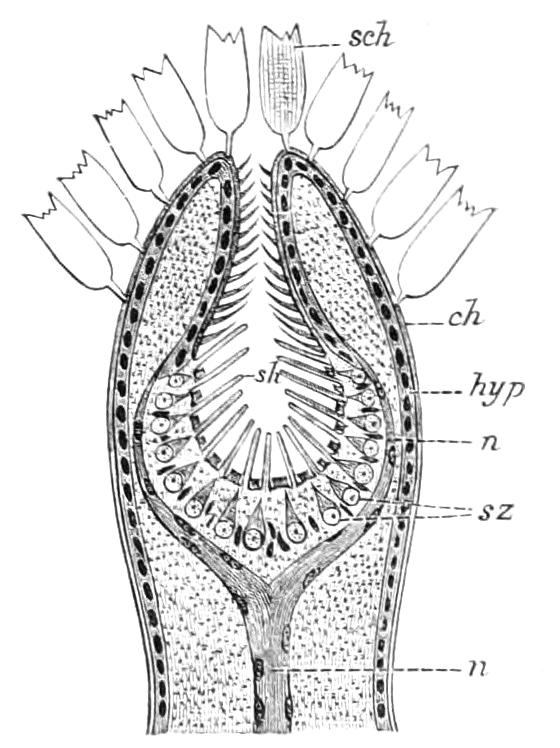
Fig. 68.—Longitudinal section of apex of palpus of Pieris brassicae: sch, scales; ch, chitin; hyp, hypodermis; n, nerve; sz, sense cells; sh, sense hairs. (After Vom Rath.)
Various parts of the mouth are also the seats of sense organs of different kinds, some of them of a compound character; in such cases there may be a considerable number of hairs seated on branches of a common nerve as figured {123}by Vom Rath[40] on the apex of the maxillary palp of Locusta viridissima, or a compound organ such as we represent in Fig. 68 may be located in the interior of the apical portion of the palp.
The functions of the various structures that have been detected are, as already remarked, very difficult to discover. Vom Rath thinks the cones he describes on the antennae and palpi are organs of smell, while he assigns to those on the maxillae, lower lip, epipharynx, and hypopharynx the rôle of taste organs, but admits he cannot draw any absolute line of distinction between the two forms. The opinions of Kraepelin, Hauser, and Will, as well as those of various earlier writers, are considered in Sir John Lubbock's book on this subject.[41]
Alimentary and Nutritive System.
The alimentary canal occupies the median longitudinal axis of the body, being situated below the dorsal vessel, and above the ventral nervous chain; it extends from the mouth to the opposite extremity of the body. It varies greatly in the different kinds of Insects, but in all its forms it is recognised as consisting essentially of three divisions: anterior, middle, and posterior. The first and last of these divisions are considered to be of quite different morphological nature from the middle part, or true stomach, and to be, as it were, invaginations of the extremities of a closed bag; it is ascertained that in the embryo these invaginations have really blind extremities (see Fig. 82, p. 151), and only subsequently become connected with the middle part of the canal. There are even some larvae of Insects in which the posterior portion of the canal is not opened till near the close of the larval life; this is the case with many Hymenoptera, and it is probable, though not as frequently stated certain, that the occlusion marks the point of junction of the proctodaeum with the stomach. The anterior and posterior parts of the canal are formed by the ectoderm of the embryo, and in embryological and morphological language are called respectively the stomodaeum and proctodaeum; the true stomach is formed from the endoderm, {124}and the muscular layer of the whole canal from the mesoderm.
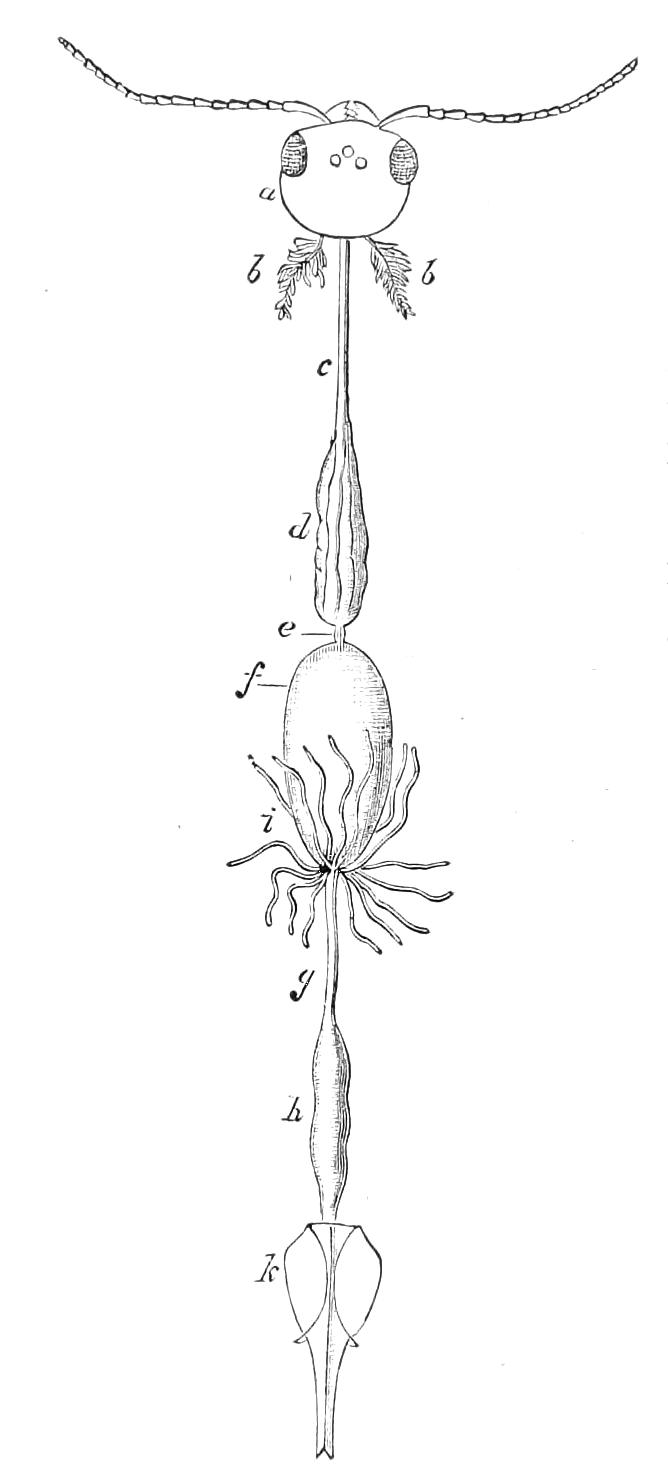
Fig. 69.—Digestive system of Xyphidria camelus (after Dufour): a, head capsule; b, salivary glands; c, oesophagus; d, crop; e, proventriculus; f, chyle, or true stomach; g, small intestine; h, large intestine; i, Malpighian tubes; k, termination of body.
The alimentary canal is more complex anatomically than it is morphologically, and various parts are distinguished, viz. the canal and its appendicula; the former consisting of oesophagus, crop, gizzard, true stomach, and an intestine divided into two or more parts. It should be remarked that though it is probable that the morphological distinctions correspond to a great extent with the anatomical lines of demarcation, yet this has not been sufficiently ascertained: the origin of the proctodaeum in Musca is indeed a point of special difficulty, and one on which there is considerable diversity of opinion. In some Hemiptera the division of the canal into three parts is very obscure, so that it would be more correct, as Dufour says, to define it as consisting in these Insects of two main divisions—one anterior to, the other posterior to, the insertion of the Malpighian tubes.
It should be borne in mind that the alimentary canal is very different in different Insects, so that the brief general description we must confine ourselves to will not be found to apply satisfactorily to any one Insect. The oesophagus is the part behind the mouth, and is usually narrow, as it has to pass through the most important nervous centres; extremely variable in length, it dilates behind to form the crop. It may, too, have a dilatation immediately behind the mouth, and in such case a pharynx is considered to exist. The crop is broader than the oesophagus, and must be looked on as a mere dilatation of the latter, as no line of {125}demarcation can be pointed out between the two, and the crop may be totally absent.
In some of the sucking Insects there is a lateral diverticulum, having a stalk of greater or less length, called the sucking-stomach; it is by no means certain that the function this name implies is correctly assigned to the organ.
The gizzard or proventriculus (French, gésier; German, Kaumagen) is a small body interposed in some Insects between the true stomach and the crop or oesophagus. It is frequently remarkable for the development of its chitinous lining into strong toothed or ridged processes that look as if they were well adapted for the comminution of food. The function of the proventriculus in some Insects is obscure; its structure is used by systematists in the classification of ants. The extremity of the proventriculus not infrequently projects into the cavity of the stomach.
The true stomach, or chylific ventricle (Magen or Mitteldarm of the Germans), is present in all the post-embryonic stages of the Insect's life, existing even in the imagines of those who live only for a few hours, and do not use the stomach for any alimentary purpose. It is so variable in shape and capacity that no general description of it can be given. Sometimes it is very elongate, so that it is coiled and like an intestine in shape; it very frequently bears diverticula or pouches, which are placed on the anterior part, and vary greatly in size, sometimes they are only two in number, while in other cases they are so numerous that a portion of the outside of the stomach looks as if it were covered with villi. A division of the stomach into two parts is in some cases very marked, and the posterior portion may, in certain cases, be mistaken for the intestine; but the position of the Malpighian tubes serves as a mark for the distinction of the two structures, the tubes being inserted just at the junction of the stomach with the intestine.
The intestine is very variable in length: the anterior part is the smaller, and is frequently spoken of as the colon; at the extremity of the body the gut becomes much larger, so as to form a rectum. There is occasionally a diverticulum or "caecum" connected with the rectum, and in some Insects stink-glands. In some Hemiptera there is no small intestine, the Malpighian tubes being inserted at the junction of the stomach with the {126}rectum. The total length of the alimentary canal is extremely variable; it is necessarily at least as long as the distance between the mouth and anal orifice, but sometimes it is five or six times as long as this, and some of its parts then form coils in the abdominal cavity.
The alimentary canal has two coats of muscles: a longitudinal and a transverse or annular. Both coexist in most of its parts. Internal to these coats there exists in the anterior and posterior parts of the canal a chitinous layer, which in the stomach is replaced by a remarkable epithelium, the cells of which are renewed, new ones growing while the old are still in activity. We figure a portion of this structure after Miall and Denny, and may remark that Oudemans[42] has verified the correctness of their representation. The layers below represent the longitudinal and transverse muscles.
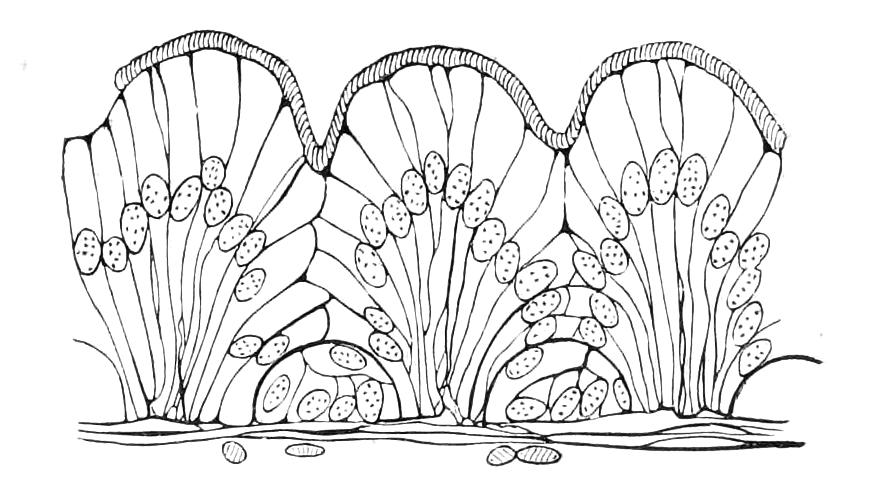
Fig. 70.—Epithelium of stomach of Cockroach (after Miall and Denny): the lower parts indicate the transverse and longitudinal muscular layers.
In addition to the various diverticula we have mentioned, there are two important sets of organs connected with the alimentary canal, viz. the salivary glands and the Malpighian tubes.
The salivary glands are present in many Insects, but are absent in others. They are situate in the anterior portion of the body, and are very variable in their development, being sometimes very extensive, in other cases inconspicuous. They consist either of simple tubes lined with cells, or of branched tubes, or of tubes dilated laterally into little acini or groups of bags, the arrangement then somewhat resembling that of a bunch of grapes. There are sometimes large sacs or reservoirs connected with the efferent tubes proceeding from the secreting portions of the glands. The salivary glands ultimately discharge into the mouth, so that the fluid secreted by them has to be {127}swallowed in the same manner as the food, not improbably along with it. The silk so copiously produced by some larvae comes from very long tubes similar in form and situation to the simple tubes of the salivary glands.
The Malpighian tubules are present in most Insects, though they are considered on good authority to be absent in many Collembola and in some Thysanura. They are placed near the posterior part of the body, usually opening into the alimentary canal just at the junction of the stomach and the intestine, at a spot called the pylorus. They vary excessively in length and in number,[43] being sometimes only two, while in other cases there may be a hundred or even more of them. In some cases they are budded off from the hind-gut of the embryo when this is still very small; in other cases they appear later; frequently their number is greater in the adult than it is in the young. In Gryllotalpa there is one tube or duct with a considerable number of finer tubes at the end of it. There is no muscular layer in the Malpighian tubes, they being lined with cells which leave a free canal in the centre. The tubes are now thought, on considerable evidence, to be organs for the excretion of uric acid or urates, but it is not known how they are emptied. Marchal has stated[44] that he has seen the Malpighian tubes, on extraction from the body, undergo worm-like movements; he suggests that their contents may be expelled by similar movements when they are in the body.
The functions of the different portions of the alimentary canal, and the extent to which the ingested food is acted on by their mechanical structures or their products is very obscure, and different opinions prevail on important points. It would appear that the saliva exercises a preparatory action on the food, and that the absorption of the nutritive matter into the body cavity takes place chiefly from the true stomach, while the Malpighian tubes perform an excretory function. Beyond these elementary, though but vaguely ascertained facts, little is known, though Plateau's[45] and Jousset's researches on the digestion of Insects throw some light on the subject.
Respiratory Organs.
The respiration of Insects is carried on by means of a system of vessels for the conveyance of air to all parts of the body; this system is most remarkably developed and elaborate, and contrasts strongly with the mechanism for the circulation of the blood, which is as much reduced as the air system is highly developed, as well as with the arrangement that exists in the Vertebrates. There are in Insects no lungs, but air is carried to every part of the body directly by means of tracheae. These tracheae connect with the spiracles—the orifices at the sides of the body we have already mentioned when describing the external structures—and the air thus finds its way into the most remote recesses of the Insect's body. The tracheae are all intimately connected. Large tubes connect the spiracles longitudinally, others pass from side to side of the body, and a set of tracheae for the lower part of the body is connected with another set on the upper surface by means of several descending tubes. From these main channels smaller branches extend in all directions, forking and giving off twigs, so that all the organs inside the body can be supplied with air in the most liberal manner. On opening a freshly deceased Insect the abundance of the tracheae is one of the peculiarities that most attracts the attention; and as these tubes have a peculiar white glistening appearance, they are recognised without difficulty. In Insects of active flight, possibly in some that are more passive, though never in larvae, there are air-sacs, of more than one kind, connected with the tracheae, and these are sufficiently capacious to have a considerable effect in diminishing the specific gravity of the Insect. The most usual situation for these sacs is the basal portion of the abdominal cavity, on the great lateral tracheal conduits. In speaking of the external structure we have remarked that the stigmata, or spiracles, by which the air is admitted are very various in their size and in the manner in which they open and close. Some spiracles have no power of opening; while others are provided with a muscular and valvular apparatus for the purpose of opening and closing effectually.
The structure of the tracheae is remarkable: they are elastic and consist of an outer cellular, and an inner chitinous layer; this latter is strengthened by a peculiar spiral fibre, which gives {129}to the tubes, when examined with the microscope, a transversely, closely striated appearance. Packard considers[46] that in some tracheae this fibre is not really spiral, but consists of a large number of closely placed rings. Such a condition has not, however, been recorded by any other observer. The spiral fibre is absent in the fine capillary twigs of the tracheal system, as well as from the expanded sacs. The mode of termination of the capillary branches is not clear. Some have supposed that the finest twigs anastomose with others; on the other hand it has been said that they terminate by penetrating cells, or that they simply come to an end with either open or closed extremities. Wistinghausen[47] states that in the silk-glands the tracheal twigs anastomose, and he is of opinion that the fine terminal portions contain fluid. However this may be, it is certain that all the organs are abundantly supplied with a capillary tracheal network, or arboreal ramification, and that in some cases the tubes enter the substance of tissues. Near their terminations they are said to be 1⁄30 to 1⁄60 millimetre in diameter.
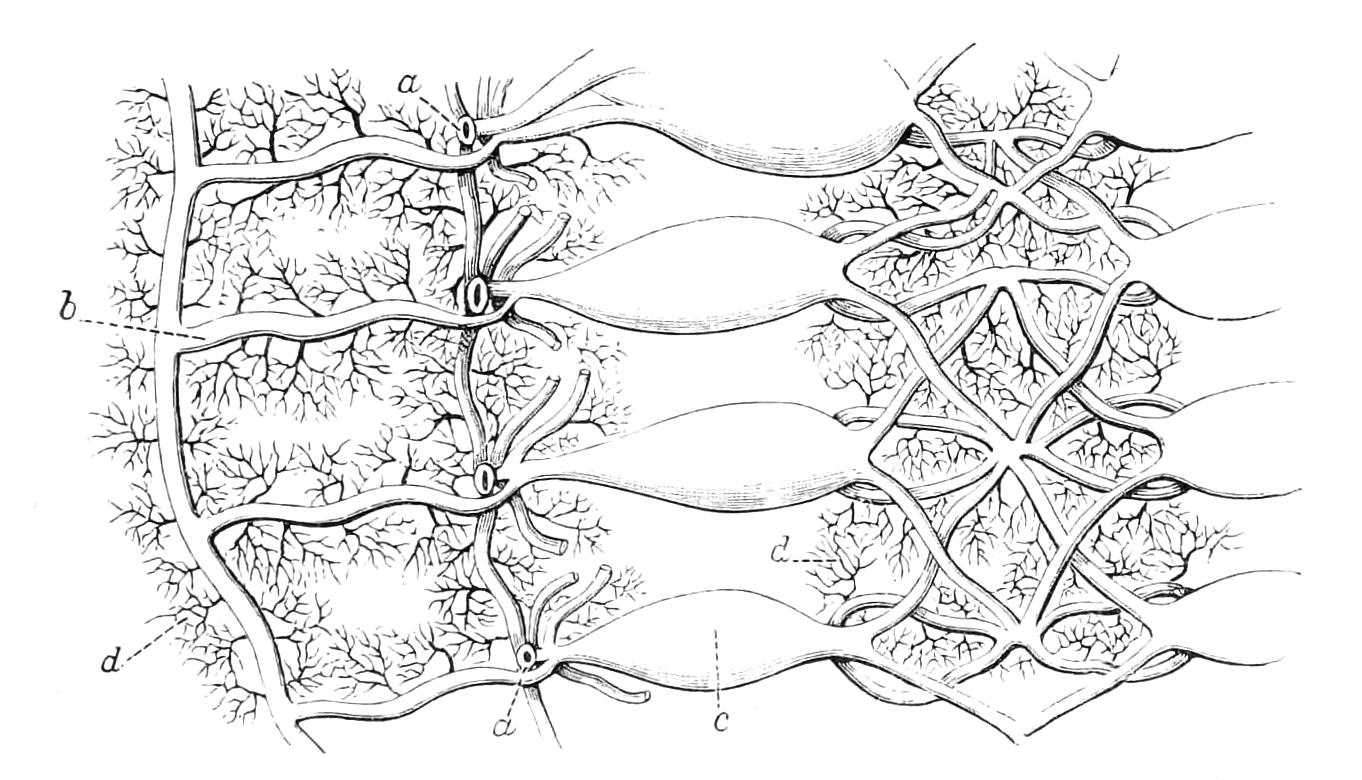
Fig. 71.—Portion of the abdominal part of tracheal system of a Locust (Oedipoda): a, spiracular orifices; b, tracheal tubes; c, vesicular dilatations; d, tracheal twigs or capillaries. (After Dufour.)
We must repeat that such a system as we have just sketched forms a striking contrast to the imperfect blood-vascular system, and that Insects differ profoundly in these respects from Vertebrate animals. In the latter the blood-vessels penetrate to all {130}the tissues and form capillaries, while the aerating apparatus is confined to one part of the body; in Insects the blood-circulating system is very limited, and air is carried directly by complex vessels to all parts; thus the tracheal system is universally recognised as one of the most remarkable of the characters of Insects. Many Insects have a very active respiratory system, as is shown by the rapidity with which they are affected by agents like chloroform; but the exact manner in which the breathing is carried on is unknown. In living Insects rapid movements of contraction and expansion of parts of the body, chiefly the abdomen, may be observed, and these body contractions are sometimes accompanied by opening and shutting the spiracular orifices: it has been inferred that these phenomena are respiratory. Although such movements are not always present, it is possible that when they occur they may force the air onwards to the tissues, though this is by no means certain. It is clear that the tracheal system is the usual means of supplying the organisation with oxygen, but it appears to be improbable that it can also act as the agent for removing the carbonaceous products of tissue-changes. It has been thought possible that carbonic acid might reach the spiracles from the remote capillaries by a process of diffusion,[48] but it should be recollected that as some Insects have no tracheal system, there must exist some other mode of eliminating carbonic acid, and it is possible that this mode may continue to operate as an important agent of purification, even when the tracheal system is, as a bearer of air to the tissues, highly developed. Eisig[49] has suggested that the formation of chitin is an act of excretion; if so this is capable of relieving the system of carbonic acid to some extent. Others have maintained that transpiration takes place through the delicate portions of the integument. Lubbock[50] has shown that Melolontha larvae breathe "partly by means of their skin." The mode in which the carbon of tissue-change, and the nitrogen of inspiration are removed, is still obscure; but it appears probable that the views expressed by Réaumur, Lyonnet, and Lowne[51] as to inspiration and expiration may prove to be nearer the truth than those which are more widely current. In {131}connexion with this it should be recollected that the outer integument consists of chitin, and is cast and renewed several times during the life of the individual. Now as chitin consists largely of carbon and nitrogen, it is evident that the moulting must itself serve as a carbonaceous and nitrogenous excretion. If, as is suggested by Bataillon's researches,[52] the condition accompanying metamorphosis be that of asphyxia, it is probable that the secretion of the new coat of chitin may figure as an act of excretion of considerable importance. If there be any truth in this suggestion it may prove the means of enabling us to comprehend some points in the development of Insects that have hitherto proved very perplexing.
Peyrou has shown[53] that the atmosphere extracted from the bodies of Insects (Melolontha) is much less rich in oxygen than the surrounding atmosphere is, and at ordinary temperatures always contains a much larger proportion of carbonic acid: he finds, too, that as in the leaves with which he makes a comparison, the proportion of oxygen augments as the protoplasmic activity diminishes. Were such an observation carried out so as to distinguish between the air in the tracheal system and the gas in other parts of the body the result would be still more interesting.
We know very little as to the animal heat produced by insects, but it is clear from various observations[54] that the amount evolved in repose is very small. In different conditions of activity the temperature of the insect may rise to be several degrees above that of the surrounding medium, but there seems to be at present no information as to the physiological mode of its production, and as to the channel by which the products—whether carbonic acid or other matters—may be disposed of.
In the order Aptera (Thysanura and Collembola) the tracheal system is highly peculiar. In some Collembola it apparently does not exist, and in this case we may presume with greater certainty that transpiration of gases occurs through the integument: in other members of this Order tracheae are present in a more or less imperfect state of development, but the tracheae of different segments do not communicate with one another, {132}thus forming a remarkable contrast to the amalgamated tracheal system of the other Orders of Insects, where, even when the tracheal system is much reduced in extent (as in Coccidae), it is nevertheless completely unified. Gryllotalpa is, however, said by Dohrn[55] to be exceptional in this respect; the tracheae connected with each spiracle remaining unconnected.
Water Insects have usually peculiarities in their respiratory systems, though these are not so great as might à priori have been anticipated. Some breathe by coming to the surface and taking in a supply of air in various manners, but some apparently obtain from the water itself the air necessary for their physiological processes. Aquatic Insects are frequently provided with gills, which may be either wing-like expansions of the integument containing some tracheae (Ephemeridae larvae), or bunches of tubes, or single tubes (Trichoptera larvae). Such Insects may either possess stigmata in addition to the gills, or be destitute of them. In other cases air is obtained by taking water into the posterior part of the alimentary canal (many dragon-flies), which part is then provided with special tracheae. Some water-larvae appear to possess neither stigmata nor gills (certain Perlidae and Diptera), and it is supposed that these obtain air through the integument; in such Insects tracheal twigs may frequently be seen on the interior of the skin. In the imago state it is the rule that Water Insects breathe by means of stigmata, and that they carry about with them a supply of air sufficient for a longer or shorter period. A great many Insects that live in water in their earlier stages and breathe there by peculiar means, in their perfect imago state live in the air and breathe in the usual manner. There are, in both terrestrial and aquatic Insects, a few cases of exsertile sacs without tracheae, but filled with blood (Pelobius larva, Machilis, etc.); and such organs are supposed to be of a respiratory nature, though there does not appear to be any positive evidence to that effect.
Blood and Blood-Circulation.
Owing to the great complexity of the tracheal system, and to its general diffusion in the body, the blood and its circulation are very different in Insects from what they are in Vertebrates, so {133}that it is scarcely conducive to the progress of physiological knowledge to call two fluids with such different functions by one name. The blood of Insects varies according to the species, and in all probability even in conformity with the stage of the life of the individual. Its primary office is that of feeding the tissues it bathes, and it cannot be considered as having any aerating function. It is frequently crowded with fatty substances. Graber says: "The richness of Insect blood in unsaponified or unelaborated fat shows in the plainest manner that it is more properly a mixture of blood and chyle; or indeed we might say with greater accuracy, leaving out of consideration certain matters to be eliminated from it, that it is a refined or distilled chyle." Connected in the most intimate manner with the blood there is a large quantity of material called vaguely the fat-body; the blood and its adjuncts of this kind being called by Wielowiejski[56] the blood-tissue. We shall return to the consideration of this tissue after sketching the apparatus for distributing the refined chyle, or blood as we must, using the ordinary term, call it.
There is in Insects no complete system of blood-vessels, though there is a pulsating vessel to ensure distribution of the nutritive fluid. This dorsal vessel, or heart as it is frequently called, may be distinguished and its pulsations watched, in transparent Insects when alive. It is situate at the upper part of the body, extending from the posterior extremity, or near it, to the head or thorax, and is an elongate tube, consisting as it were of a number of united chambers; it is closed behind, except in some larvae, but is open in front, and has several orifices at the sides; these orifices, or ostia, are frequently absent from the front part of the tube, which portion is also narrower, being called the aorta—by no means a suitable term. Near the lateral orifices there are delicate folds, which act to some extent as valves, facilitating, in conjunction with the mode of contraction of the vessel, a forward movement of the blood. The composition of the tube, or series of chambers, is that of a muscular layer, with internal and external membranous coverings, the intima and adventitia. Olga Poletajewa states[57] that in Bombus the dorsal vessel consists of five chambers placed in longitudinal succession, and not very intimately connected, and that there is but little {134}valvular structure. In Cimbex she finds a similar arrangement, but there are ten chambers, and no aorta.
The dorsal vessel is connected with the roof of the body by some short muscles, and is usually much surrounded by fat-body into which tracheae penetrate; by these various means it is kept in position, though only loosely attached; beneath it there is a delicate, incomplete or fenestrate, membrane, delimiting a sort of space called the pericardial chamber or sinus; connected with this membrane are some very delicate muscles, the alary muscles, extending inwards from the body wall (b, Fig. 72): the curtain formed by these muscles and the fenestrate membrane is called the pericardial diaphragm or septum. The alary muscles are not directly connected with the heart.
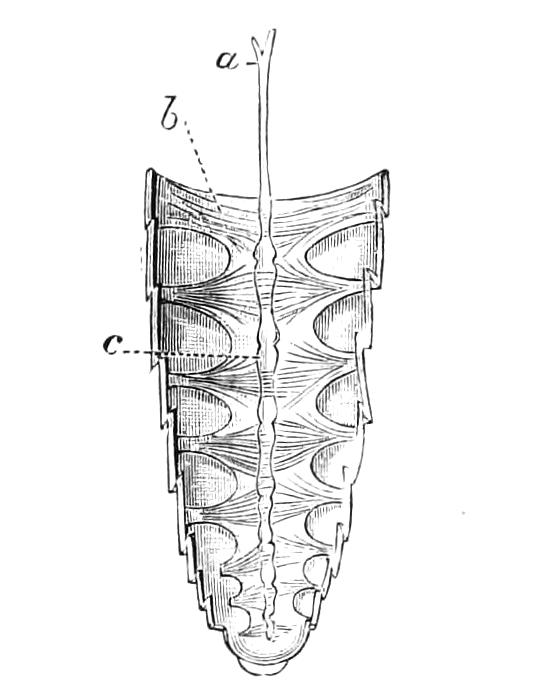
Fig. 72.—Dorsal vessel (c), and alary muscles (b), of Gryllotalpa (after Graber); a, aorta. N.B.—The ventral aspect is here dorsal, and nearly the whole of the body is removed to show these parts.
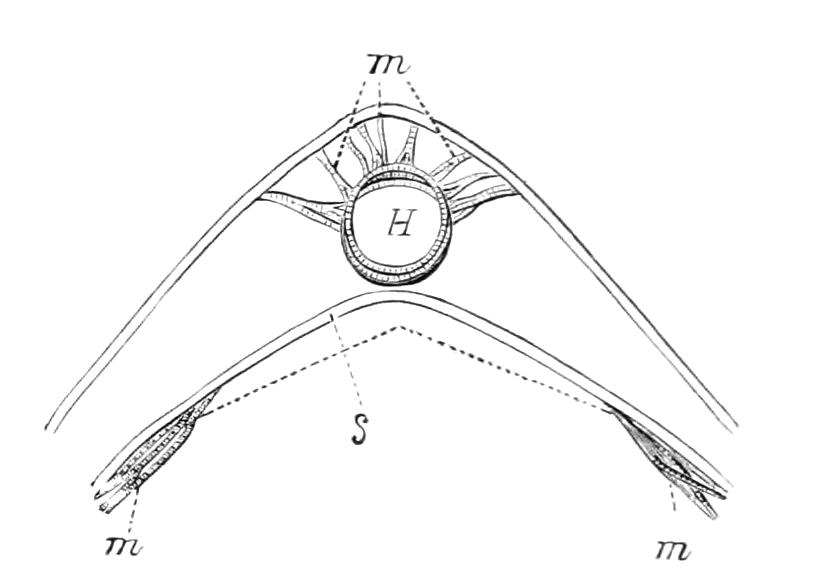
Fig. 73.—Diagram of transverse section of pericardial sinus of Oedipoda coerulescens. (After Graber, Arch. Mikr. Anat. ix.) H, heart; s, septum; m, muscles—the upper suspensory, the lower alary.
It has been thought by some that delicate vessels exist beyond the aorta through which the fluid is distributed in definite channels, but this does not appear to be really the case, although the fluid may frequently be seen to move in definite lines at some distance from the heart.
There is still much uncertainty as to some of the details of the action of the heart, and more especially as to the influence of the alary muscles. The effect of the contraction of these must be to increase the area of the pericardial chamber by rendering {135}its floor or septum less arched, as shown in our diagram (Fig. 73), representing a transverse section through the pericardial chamber, H being the dorsal vessel with m its suspensory muscles, and s its septum, with m the alary muscles. The contraction of these latter would draw the septum into the position of the dotted line, thus increasing the area of the sinus above; but as this floor or septum is a fenestrated structure, its contraction allows fluid to pass through it to the chamber above; thus this arrangement may be looked on as a means of keeping up a supply of fluid to the dorsal vessel, the perforated septum, when it contracts, exerting pressure on the tissues below; these are saturated with fluid, which passes through the apertures to the enlarged pericardial chamber.
Some misconception has prevailed, too, as to the function of the pericardial chamber. This space frequently contains a large quantity of fat-body—pericardial tissue—together with tracheae, and this has given rise to the idea that it might be lung-like in function; but, as Miall and Denny[58] have pointed out, this is erroneous; the tissues in Insects have their own ample supplies of air. It has also been supposed that the alary muscles cause the contraction of the heart, but this is not directly the case, for they are not attached to it, and it pulsates after they have been severed. It has been suggested that the contractions of this vessel are regulated by small ganglia placed on, or in, its substance. However this may be, these contractions vary enormously according to the condition of the Insect; they may be as many, it is said, as 100 or more in a minute, or they may be very slow and feeble, if not altogether absent, without the death of the Insect ensuing.
The expulsion of the blood from the front of the dorsal vessel seems to be due to the rhythm of the contraction of the vessel as well as to its mechanical structure. Bataillon says,[59] confirming an observation of Réaumur, that at the period when the silkworm is about to change to the chrysalis condition, the circulation undergoes periodical changes, the fluid moving during some intervals of about ten minutes' duration in a reversed direction, while at other times the blood is expelled in front and backwards simultaneously, owing apparently to a rhythmical change in the mode of contraction of the dorsal vessel.
As the dorsal vessel consists of a number of distinct chambers, it has been suggested that there is normally one of these for each segment of the body; and it appears that the total number is sometimes thirteen, which is frequently that of the segments of the body without the head. The number of chambers differs, however, greatly, as we have previously stated, and cannot be considered to support the idea of an original segmental arrangement of the chambers. The dorsal vessel, though in the adult a single organ, arises in the embryo from two lateral, widely separated parts which only in a subsequent stage of the embryonic development coalesce in the median line.
Fat-Body.
In discussing the tracheae we remarked on the importance of their function and on their abundant presence in the body. Equally conspicuous, and perhaps scarcely less important in function, is the fat-body, which on opening some Insects, especially such as are in the larval stage, at once attracts attention. It consists of masses of various size and indefinite form distributed throughout the body, loosely connected together, and more or less surrounding and concealing the different organs. The colour varies according to the species of Insect. This fat-body is much connected with fine tracheal twigs, so that an organisation extending throughout the body is thus formed. It may be looked on as a store of nutritious matter which may be added to or drawn on with great rapidity; and it is no doubt on this that many of the internal parasites, so common in the earlier stages of Insects' lives, subsist before attacking the more permanent tissues of their hosts. There is some reason to suppose that the fat-body may have some potency in determining the hunger of the Insect, for some parasitised larvae eat incessantly.
The matter extracted from the food taken into the stomach of the Insect, after undergoing some elaboration—on which point very little is known—finds its way into the body-cavity of the creature, and as it is not confined in any special vessels the fat-body has as unlimited a supply of the nutritive fluid as the other organs: if nutriment be present in much greater quantity than is required for the purposes of immediate activity, metamorphosis or reproduction, it is no doubt taken up by the {137}fat-body which thus maintains, as it were, an independent feeble life, subject to the demands of the higher parts of the organisation. It undoubtedly is very important in metamorphosis, indeed it is possible that one of the advantages of the larval state may be found in the fact that it facilitates, by means of the fat-body, the storage in the organisation of large quantities of material in a comparatively short period of time.
A considerable quantity of fat tissue is found in the pericardial sinus, where it is frequently of somewhat peculiar form, and is spoken of as pericardial cells, or pericardial tissue. Some large cells, frequently of pale yellow colour, and containing no fat, are called oenocytes by Wielowiejski. They are connected with the general fat-body, but are not entirely mingled with it; several kinds have been already distinguished, and they are probably generally present. The phagocytes, or leucocytes, the cells that institute the process of histolysis in the metamorphosis of Muscidae, are a form of blood cell; though these cells are amoeboid some writers derive them from the fat-body. The cells in the blood have no doubt generally an intimate relation with the fat-body, but very little accurate information has been obtained as to these important physiological points, though Graber has inaugurated their study.[60]
Organs of Sex.
The continuation of the species is effected in Insects by means of two sexes, each endowed with special reproductive organs. It has been stated that there are three sexes in some Insects—male, female, and neuter; but this is not correct, as the so-called neuters are truly sexed individuals,—generally females,—though, as a rule, they are not occupied with the direct physiological processes for continuing the species.
The offspring is usually produced in the shape of eggs, which are formed in ovaries. These organs consist of egg-tubes, a cluster of which is placed on each side of the body, and is suspended, according to Leydig[61] and others, to the tissue connected with the heart by means of the thread-like terminations of the tubes.
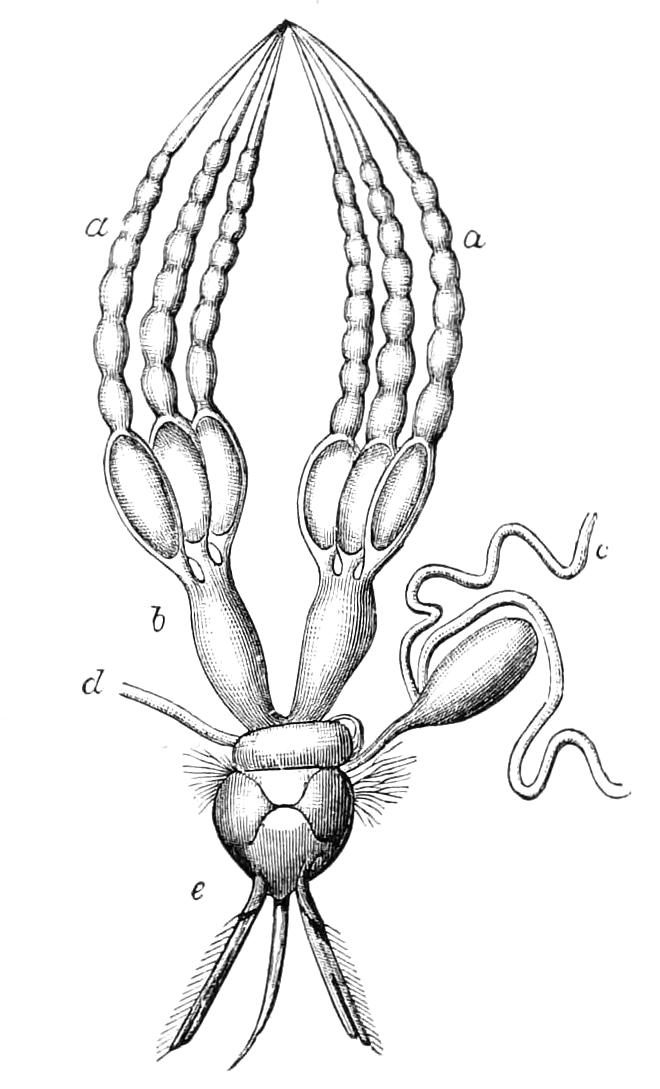
Fig. 74.—Sex organs of female of Scolia interrupta (after Dufour); a, egg-tubes; b, oviducts; c, poison glands; d, duct of accessory gland (or spermatheca); e, external terminal parts of body.
The number of egg-tubes varies greatly in different Insects; there may be only one to each ovary (Campodea), but usually the number is greater, and in the queen-bee it is increased to about 180. In the Queens of the Termitidae, or white ants, the ovaries take on an extraordinary development; they fill the whole of the greatly distended hind-body. Three thousand egg-tubes, each containing many hundred eggs, may be found in a Queen Termite, so that, as has been said by Hagen,[62] an offspring of millions in number is probable. There is considerable variety in the arrangements for the growth of the eggs in the egg-tubes. Speaking concisely, the tubes may be considered to be centres of attraction for nutritive material, of which they frequently contain considerable stores. Next to the terminal thread, of which we have already spoken, there is a greater or smaller enlargement of the tube, called the terminal chamber; and there may also be nutriment chambers, in addition to the dilatations which form the egg-chambers proper. Korschelt[63] distinguishes three principal forms of egg-tubes, viz. (1) there are no special nutriment chambers, a condition shown in Figure 74; (2) nutriment chambers alternate with the egg-chambers, as shown in our Figure of an egg-tube of Dytiscus marginalis; (3) the terminal chamber takes on an unusual development, acting as a large nutriment chamber, there being no other special nutriment chambers. This condition is found in Rhizotrogus solstitialis. The arrangements as to successive or simultaneous production of the eggs in the tubes seem to differ in different Insects. In some forms, such as the white ants, the process of egg-formation (oogenesis) attains a rapidity that is almost incredible, and is continued, it is said, for periods of many months. There is no point in which Insects differ more than in that of the number of eggs produced by one {139}female. The egg-tubes are connected with a duct for the conveyance of the eggs to the exterior, and the arrangements of the tubes with regard to the oviduct also vary much. An interesting condition is found in Machilis (see Fig. 94, p. 188), where the seven egg-tubes are not arranged in a bunch, but open at a distance from one another into the elongated duct. The two oviducts usually unite into one chamber, called the azygos portion or the uterus, near their termination. There are a few Insects (Ephemeridae) in which the two oviducts do not unite, but have a pair of orifices at the extremity of the body. Hatchett-Jackson has recently shown[64] that in Vanessa io of the Order Lepidoptera, the paired larval oviducts are solid, and are fixed ventrally so as to represent an Ephemeridean stage; that the azygos system of ducts and appended structures develop separately from the original oviducts, and that they pass through stages represented in other Orders of Insects to the stage peculiar to the Lepidoptera. Machilis, according to Oudemans, is a complete connecting link between the Insects with single and those with paired orifices.
There are in different Insects more than one kind of diverticula and accessory glands in connexion with the oviducts or uterus; a receptaculum seminis, also called spermatheca, is common. In the Lepidoptera there is added a remarkable structure, the bursa copulatrix, which is a pouch connected by a tubular isthmus with the common portion of the oviduct, but having at the same time a separate external orifice, so that there are two sexual orifices, the opening of the bursa copulatrix being the lower or more anterior. The organ called by Dufour in his various contributions glande sébifique, is now considered to be, in some cases at any rate, a spermatheca. The special functions of the accessory glands are still very obscure.
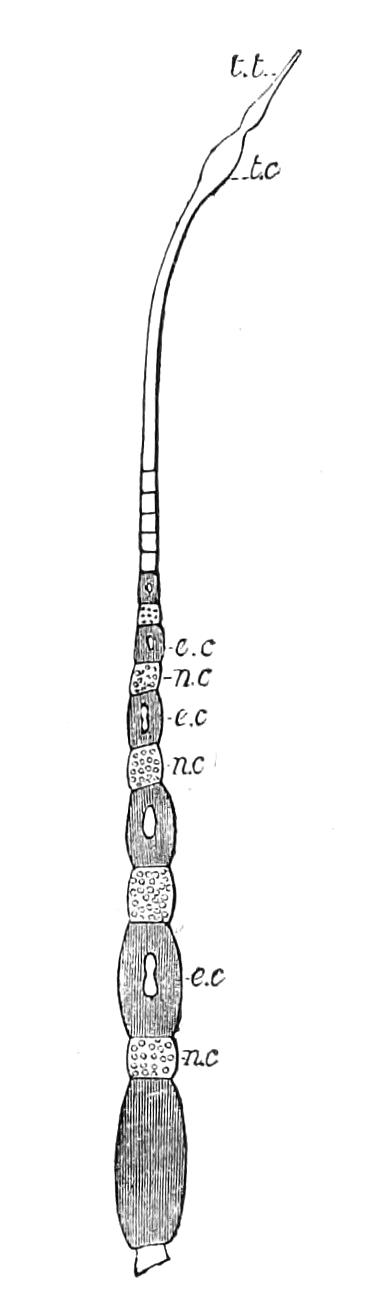
Fig. 75.—Egg-tube of Dytiscus marginalis; e.c, egg-chamber; n.c, nutriment chamber; t.c, terminal chamber; t.t, terminal thread. (After Korschelt.)
The ovaries of the female are replaced in the male by a pair {140}of testes, organs exhibiting much variety of form. The structure may consist of an extremely long and fine convoluted tube, packed into a small space and covered with a capsule; or there may be several shorter tubes. As another extreme may be mentioned the existence of a number of small follicles opening into a common tube, several of these small bodies forming together a testis. As a rule each testis has its own capsule, but cases occur—very frequently in the Lepidoptera—in which the two testes are enclosed in a common capsule; so that there then appears to be only one testis. The secretion of each testis is conveyed outwards by means of a slender tube, the vas deferens, and there are always two such tubes, even when the two testes are placed in one capsule. The vasa deferentia differ greatly in their length in different Insects, and are in some cases many times the length of the body; they open into a common duct, the ductus ejaculatorius. Usually at some part of the vas deferens there exists a reservoir in the form of a sac or dilatation, called the vesicula seminalis. There are in the male, as well as in the female, frequently diverticula, or glands, in connexion with the sexual passages; these sometimes exhibit very remarkable forms, as in the common cockroach, but their functions are quite obscure. There is, as we have already remarked, extreme variety in the details of the structure of the internal reproductive apparatus in the male, and there are a few cases in which the vasa deferentia do not unite behind, but terminate in a pair of separate orifices. The genus Machilis is as remarkable in the form of the sexual glands and ducts of the male as we have already mentioned it to be in the corresponding parts of the female.
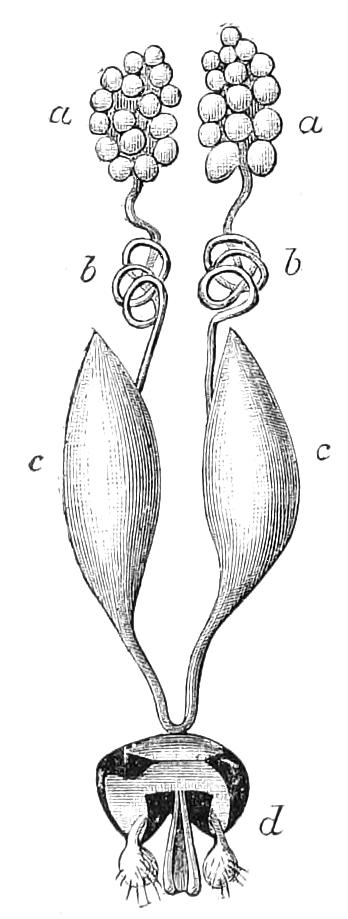
Fig. 76.—Tenthredo cincta. a, a, testes; b, b, vasa deferentia; c, c, vesiculæ seminales; d, extremity of body with copulatory armature. (After Dufour.)
Although the internal sexual organs are only fully developed in the imago or terminal stage of the individual life, yet in reality their rudiments appear very early, and may be detected from the embryo state onwards through the other preparatory stages.
The spermatozoa of a considerable number of Insects, especially of Coleoptera, have been examined by {141}Ballowitz;[65] they exhibit great variety; usually they are of extremely elongate form, thread-like, with curious sagittate or simply pointed heads, and are of a fibrillar structure, breaking up at various parts into finer threads.
External Sexual Organs.—The terminal segments of the body are usually very highly modified in connexion with the external sexual organs, and this modification occurs in such a great variety of forms as to render it impossible to give any general account thereof, or of the organs themselves. Some of these segments—or parts of the segments, for it may be dorsal plates or ventral plates, or both—may be withdrawn into the interior, and changed in shape, or may be doubled over, so that the true termination of the body may be concealed. The comparative anatomy of all these parts is especially complex in the males, and has been as yet but little elucidated, and as the various terms made use of by descriptive entomologists are of an unsatisfactory nature we may be excused from enumerating them. We may, however, mention that when a terminal chamber is found, with which both the alimentary canal and the sexual organs are connected, it is called a cloaca, as in other animals.
Parthenogenesis.
There are undoubted cases in Insects of the occurrence of parthenogenesis, that is, the production of young by a female without concurrence of a male. This phenomenon is usually limited to a small number of generations, as in the case of the Aphididae, or even to a single generation, as occurs in the alternation of generations of many Cynipidae, a parthenogenetic alternating with a sexual generation. There are, however, a few species of Insects of which no male is known (in Tenthredinidae, Cynipidae, Coccidae), and these must be looked on as perpetually parthenogenetic. It is a curious fact that the result of parthenogenesis in some species is the production of only one sex, which in some Insects is female, in others male; the phenomenon in the former case is called by Taschenberg[66] Thelyotoky, in the latter case Arrhenotoky; Deuterotoky being applied to the cases in which two sexes are produced. In some forms of {142}parthenogenesis the young are produced alive instead of in the form of eggs. A very rare kind of parthenogenesis, called paedogenesis, has been found to exist in two or three species of Diptera, young being produced by the immature Insect, either larva or pupa.
Glands.
Insects are provided with a variety of glands, some of which we have alluded to in describing the alimentary canal and the organs of sex; but in addition to these there are others in connexion with the outer integument; they may be either single cells, as described by Miall in Dicranota larva,[67] or groups of cells, isolated in tubes, or pouches. The minute structure of Insect glands has been to some extent described by Leydig;[68] they appear to be essentially of a simple nature, but their special functions are very problematic, it being difficult to obtain sufficient of their products for satisfactory examination.
DEVELOPMENT
EMBRYOLOGY–EGGS–MICROPYLES–FORMATION OF EMBRYO–VENTRAL PLATE–ECTODERM AND ENDODERM–SEGMENTATION–LATER STAGES–DIRECT OBSERVATION OF EMBRYO–METAMORPHOSIS–COMPLETE AND INCOMPLETE–INSTAR–HYPERMETAMORPHOSIS–METAMORPHOSIS OF INTERNAL ORGANS–INTEGUMENT–METAMORPHOSIS OF BLOWFLY–HISTOLYSIS–IMAGINAL DISCS–PHYSIOLOGY OF METAMORPHOSIS–ECDYSIS.
The processes for the maintenance of the life of the individual are in Insects of less proportional importance in comparison with those for the maintenance of the species than they are in Vertebrates. The generations of Insects are numerous, and the individuals produced in each generation are still more profuse. The individuals have as a rule only a short life; several successive generations may indeed make their appearances and disappear in the course of a single year.
Although eggs are laid by the great majority of Insects, a few species nevertheless increase their numbers by the production of living young, in a shape more or less closely similar to that of the parent. This is well known to take place in the Aphididae or green-fly Insects, whose rapid increase in numbers is such a plague to the farmer and gardener. These and some other cases are, however, exceptional, and only emphasise the fact that Insects are pre-eminently oviparous. Leydig, indeed, has found in the same Aphis, and even in the same ovary, an egg-tube producing eggs while a neighbouring tube is producing viviparous individuals.[69] In the Diptera pupipara the young are {144}produced one at a time, and are born in the pupal stage of their development, the earlier larval state being undergone in the body of the parent: thus a single large egg is laid, which is really a pupa.
The eggs are usually of rather large size in comparison with the parent, and are produced in numbers varying according to the species from a few—15 or even less in some fossorial Hymenoptera—to many thousands in the social Insects: somewhere between 50 and 100 may perhaps be taken as an average number for one female to produce. The whole number is frequently deposited with rapidity, and the parent then dies at once. Some of the migratory locusts are known to deposit batches of eggs after considerable intervals of time and change of locality. The social Insects present extraordinary anomalies as to the production of the eggs and the prolongation of the life of the female parent, who is in such cases called a queen.
The living matter contained in the egg of an Insect is protected by three external coats: (1) a delicate interior oolemm; (2) a stronger, usually shell-like, covering called the chorion; (3) a layer of material added to the exterior of the egg from glands, at or near the time when it is deposited, and of very various character, sometimes forming a coat on each egg and sometimes a common covering or capsule for a number of eggs. The egg-shell proper, or chorion, is frequently covered in whole or part with a complex minute sculpture, of a symmetrical character, and in some cases this is very highly developed, forming an ornamentation of much delicacy; hence some Insects' eggs are objects of admirable appearance, though the microscope is of course necessary to reveal their charms. One of the families of butterflies, the Lycaenidae, is remarkable for the complex forms displayed by the ornamentation of the chorion (see Fig. 78, B).
The egg-shell at one pole of the egg is perforated by one or more minute orifices for the admission to the interior of the spermatozoon, and it is the rule that the shell hereabouts is symmetrically sculptured (see Fig. 77), even when it is {145}unornamented elsewhere: the apertures in question are called micropyles. They are sometimes protected by a micropyle apparatus, consisting of raised processes, or porches: these are developed to an extraordinary extent in some eggs, especially in those of Hemiptera-Heteroptera (see Fig. 78, C). Some of these peculiar structures have been described and figured by Leuckart.[70] The purpose they serve is quite obscure.
Fig. 78.—Eggs of Insects: A, blowfly (after Henking); B, butterfly, Thecla (after Scudder); C, Hemipteron (Reduviid).
Formation of Embryo.
The mature, but unfertilised, egg is filled with matter that should ultimately become the future individual, and in the process of attaining this end is the seat of a most remarkable series of changes, which in some Insects are passed through with extreme rapidity. The egg-contents consist of a comparatively structureless matrix of a protoplasmic nature and of yolk, both of which are distributed throughout the egg in an approximately even manner. The yolk, however, is by no means of a simple nature, but consists, even in a single egg, of two or three kinds of spherular or granular constituents; and these vary much in their appearance and arrangement in the early stages of the development of an egg, the yolk of the same egg being either of a homogeneously granular nature, or consisting of granules and larger masses, as well as of particles of fatty matter; these latter when seen through the microscope looking sometimes like shining, nearly colourless, globules.
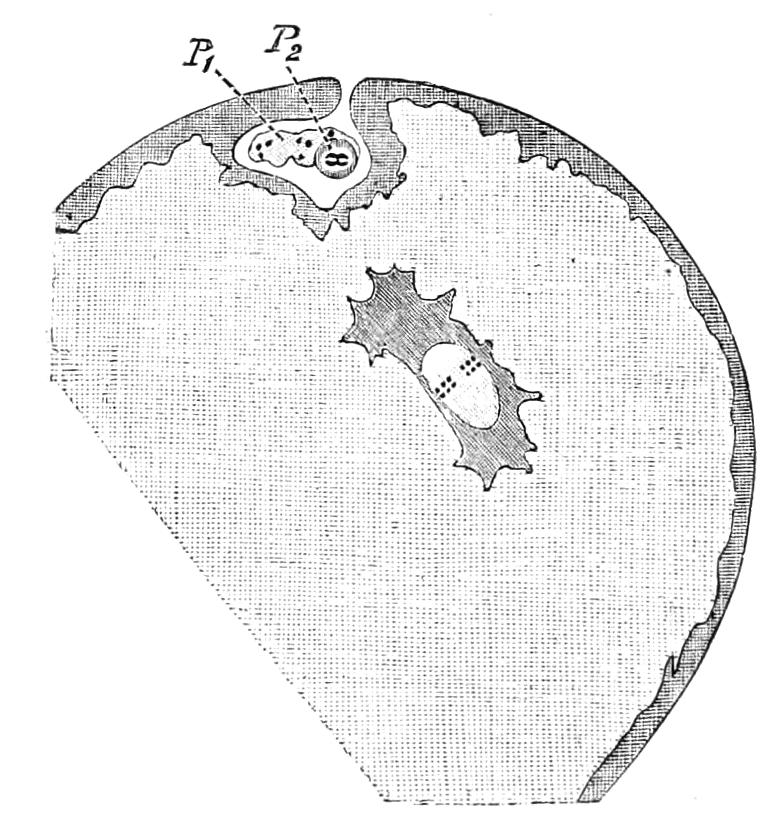
Fig. 79.—Showing the two extruded polar bodies P1, P2 now nearly fused and reincluded, and the formation of the spindle by junction of the male and female pronuclei. (After Henking.)
The nature of the matrix—which term we may apply to both the protoplasm and yolk as distinguished from the minute formative portions of the egg—and the changes that take place in it have been to some extent studied, and Kowalewsky, Dohrn,[71] Woodworth,[72] and others have given some particulars about them. The early changes in the formative parts of the mature egg have been observed by Henking in several Insects, and particularly in Pyrrhocoris, his observations being of considerable interest. When the egg is in the ovary and before it is quite mature,—at the time, in fact, when it is receiving nutriment from ovarian cells,—it contains a germinal vesicle including a germinal spot, but when the egg is mature the germinal vesicle has disappeared, and there exists in its place at one portion of the periphery of the egg-contents a cluster of minute bodies called chromosomes by Henking, whom we shall follow in briefly describing their changes. The group divides into two, each of which is arranged in a rod or spindle-like manner, and may then be called a directive rod or spindle. The outer of these two groups travels quite to the periphery of the egg, and there with some adjacent matter is extruded quite outside the egg-contents (not outside the egg-coverings), being in its augmented form called a polar or directive body. While this is going on the second directive spindle itself divides into two groups, the outer of which is then extruded in the manner we have already described in the case of the first polar body, thus completing the extrusion of two directive bodies. The essential parts of the bodies that are successively formed during these processes are the aggregates, called chromosomes; the number of these chromosomes appears to be constant in each species; their movements and dispositions are of a very interesting character, the systems they form in {147}the course of their development having polar and equatorial arrangements. These we cannot further allude to, but may mention that the extrusion of the directive bodies is only temporary, they being again included within the periphery of the egg by the growth and extension of adjacent parts which meet over and thus enclose the bodies.
The arrangements and movements we have briefly alluded to have been limited to the unfertilised condition of the egg (we should rather say, the fertilising element has taken no part in them), and have as their result the union of the chromosomes existing after the extrusion of the two polar bodies, into a small body called the female pronucleus or egg-nucleus (Eikern), while the position of the movements has been an extremely minute portion of the egg near to its outer surface or periphery. The introduction of a sperm, or male, element to the egg through the micropyle gives rise to the formation of another minute body placed more in the interior of the egg, and called the sperm-nucleus. The egg-nucleus, travelling more into the interior of the egg, meets the sperm-nucleus; the two amalgamate, forming a nucleus or body that goes through a series of changes resulting in its division into two daughter-bodies. These two again divide, and by repetitions of such division a large number of nuclei are formed which become arranged in a continuous manner so as to form an envelope enclosing a considerable part (if not quite the whole) of the egg-mass. This envelope is called the blastoderm, and together with its contents will form the embryo. We must merely allude to the fact that it has been considered that some of the nuclei forming the blastoderm arise directly from the egg-mass by a process of amalgamation, and if this prove to be correct it may be admitted that some portions of the embryo are not entirely the result of division or segmentation of combined germ and sperm-nuclei. Wheeler states[73] that some of the nuclei formed by the first differentiation go to form the vitellophags scattered throughout the yolk. We should also remark that, according to Henking, the blastoderm when completed shows at one part a thickening, immediately under which (i.e. included in the area the blastoderm encloses) are the two polar bodies, which, as we have seen, were formed by the germinating body at an earlier stage of its activity. Fig. 79 {148}represents a stage in the development of Pyrrhocoris, showing the interior of the egg after a body has been formed by the union of the sperm and egg-nuclei; this body is about to undergo division or segmentation, and the equatorial arrangement where this will take place is seen. The two polar bodies P1, P2, after having been excluded, are nearly reincluded in the egg.
The Ventral Plate.
The next important change after the formation of the blastoderm is the partial detachment of a part of its periphery to become placed in the interior of the other and larger portion. The way in which this takes place will be gathered from the accompanying diagrammatic figures taken from Graber: a thickened portion (a b) of the blastoderm becomes indrawn so as to leave a fold (c d) at each point of its withdrawal, and these folds afterwards grow and meet so as to enclose the thickened portion. The outer envelope, formed in part by the original blastoderm and in part by the new growth, is called the serosa (e f), the inner layer (g) of the conjoined new folds being termed the amnion: the part withdrawn to the interior and covered by the serosa and amnion is called the ventral plate, or germinal band (Keimstreif), and becomes developed into the future animal. The details of the withdrawal of the ventral plate to the interior are very different in the various Insects that have been investigated.
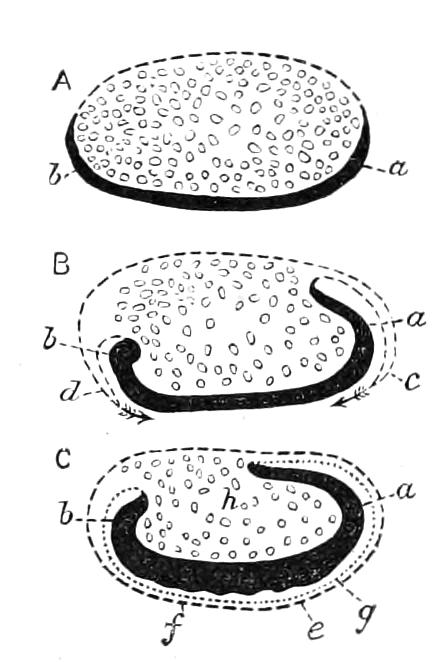
Fig. 80.—Stages of the enclosure of the ventral plate: A, a, b, ventral plate; B, c, d, folds of the blastoderm that form the commencement of the amnion and serosa; C, e, f, part of the serosa; g, amnion.
One of the earliest stages in the development is a differentiation of a portion of the ventral plate into layers from which the future parts of the organisation will be derived. This separation of endoderm from ectoderm takes place by a sort of invagination, analogous with that by which the ventral plate itself is formed. A longitudinal depression running along the middle of the ventral plate appears, and forms a groove or channel, which becomes obliterated as to its outer face by the meeting together of the two margins of the groove (except on the {149}anterior part, which remains open). The more internal layer of the periphery of this closed canal is the origin of the endoderm and its derivatives. Subsequently the ventral plate and its derivatives grow so as to form the ventral part and the internal organs of the Insect, the dorsal part being completed much later by growths that differ much in different Insects; Graber, who has specially investigated this matter, informing us[74] that an astonishing multifariousness is displayed. It would appear that the various modes of this development do not coincide with the divisions into Orders and Families adopted by any systematists.
We should observe that the terms ectoderm, mesoderm, and endoderm will probably be no longer applied to the layers of the embryo when embryologists shall have decided as to the nature of the derived layers, and shall have agreed as to names for them. According to the nomenclature of Graber[75] the blastoderm differentiates into Ectoblast and Endoblast; this latter undergoing a further differentiation into Coeloblast and Myoblast. This talented embryologist gives the following table of the relations of the embryonic layers and their nomenclature, the first term of each group being the one he proposed to use:—
Nussbaum considers[76] that "there are four layers in the cockroach-embryo, viz. (1) epiblast, from which the integument and nervous system are developed; (2) somatic layer of mesoblast, mainly converted into the muscles of the body-wall; (3) splanchnic layer of mesoblast, yielding the muscular coat of the alimentary canal; and (4) hypoblast, yielding the epithelium of the mesenteron."
Fig. 81.—Early stages of the segmentation of a beetle (Lina): A, segmentation not visible, 1 day; B, segmentation of head visible; C, segmentation still more advanced, 2¼ days; PC, procephalic lobes; g1, g2, g3, segments bearing appendages of the head; th, thorax; th1, th2, th3, segments of the thorax; a1, a2, anterior abdominal.
Turning our attention to the origin of the segmentation, that is so marked a feature of Insect structure, we find that evidence of division or arrangement of the body into segments appears very early, as shown in our Figure of some of the early stages of development of Lina (a beetle), Fig. 81. In A the segmentation of the ectoderm has not commenced, but the procephalic lobes (P C) are seen; in B the three head segments are distinct, while in C the thoracic segmentation has occurred, and that of the abdomen has commenced. Graber considers that in this species the abdomen consists of ten segmental lobes, and a terminal piece or telson. According to Graber[77] this is not a primitive condition, but is preceded by a division into three or four parts, corresponding with the divisions that will afterwards be head, thorax, and abdomen. This primary segmentation, he says, takes place in the Hypoblast (Endoderm) layer of the ventral plate; this layer being, in an early stage of the development of a common grasshopper (Stenobothrus variabilis), divided into four sections, two of which go to form the head, while the others become thorax and abdomen respectively. In Lina the primary segmentation is, Graber says, into three instead of four parts. Graber's opinion on the primary segmentation does not appear to be generally accepted, and Wheeler, who has studied[78] the {151}embryology of another Orthopteron, considers it will prove to be incorrect. When the secondary segmentation occurs the anterior of the two cephalic divisions remains intact, while the second divides into the three parts that afterwards bear the mouth parts as appendages. The thoracic mass subsequently segments into three parts, and still later the hind part of the ventral plate undergoes a similar differentiation so as to form the abdominal segments; what the exact number of these may be is, however, by no means easy to decide, the division being but vague, especially posteriorly, and not occurring all at once, but progressing from before backwards.
The investigations that have been made in reference to the segmentation of the ventral plate do not at present justify us in asserting that all Insects are formed from the same number of embryonic segments. The matter is summarised by Lowne, to the effect that posterior to the procephalic lobes there are three head segments and three thoracic segments, and a number of abdominal segments, "rarely less than nine or more than eleven." It will be seen by referring to Figure 81 that the segmentation appears, not simultaneously, but progressively from the head backwards; this of course greatly increases the difficulty of determining by means of a section the real number of segments.
Fig. 82.—Embryo of a moth (Zygaena) at the fifth day (after Graber): am, amnion; s, serosa; p, procephalic lobes; st, stomodaeum; pr, proctodaeum; g1, g2, g3, the mouth parts or head appendages; th1, th2, th3, appendages of the thoracic segments; a1-a10, abdominal segments; s.g, salivary gland.
The later stages in the development of Insects are already proved to be so various that it would be impossible to attempt to follow them in detail; but in Fig. 82 we represent a median section of the embryo of Zygaena filipendula at the fifth day. It shows well some of the more important of the general features of the development at a stage subsequent to those represented in Fig. 81, A, B, C. The very distinct stomodaeum (st) and proctodaeum (pr) are seen as inflexions of the external wall of the body; the segmentation and the development of the {152}ventral parts of the embryo are well advanced, while the dorsal part of the embryo is still quite incomplete.
The method of investigation by which embryologists chiefly carry on their researches is that of dividing the egg after proper preparation, into a large number of thin sections, which are afterwards examined in detail, so as to allow the arrangement to be completely inferred and described. Valuable as this method is, it is nevertheless clear that it should, if possible, be supplemented by direct observation of the processes as they take place in the living egg: this method was formerly used, and by its aid we may still hope to obtain exact knowledge as to the arrangements and rearrangements of particles by which the structures develop. Such questions as whether the whole formative power in the egg is absolutely confined to one or two small centres to which the whole of the other egg contents are merely, as it were, passive accessories, or whether an egg is a combination in which some portion of the powers of rearrangement is possessed by other particles, as well as the chromosomes, in virtue of their own nature or of their position at an early period in the whole, can scarcely be settled without the aid of direct observation of the processes during life.
The importance of the yolk is recognised by most of the recent writers. Nussbaum states (loc. cit.) that "scattered yolk-cells associate themselves with the mesoblast cells, so that the constituents of the mesoblast have a twofold origin." Wheeler finds[79] that amoeboid cells—he styles them vitellophags—traverse the yolk and assist in its rearrangement; he insists on the importance both as regards quantity and quality of the yolk.
The eggs of some insects are fairly transparent, and the process of development in them can, to a certain extent, be observed by simple inspection with the microscope; a method that was used by Weismann in his observations on the embryology of Chironomus. There is a moth (Limacodes testudo), that has no objection to depositing its eggs on glass microscope-slides. These eggs are about a millimetre long, somewhat more than half that width, are very flat, and the egg-shell or chorion is very thin and perfectly transparent. When first laid the contents of this egg appear nearly homogeneous and evenly distributed, a finely granular appearance being presented throughout; but in {153}twenty-four hours a great change is found to have taken place. The whole superficial contents of the egg are at that time arranged in groups, having the appearance of separate rounded or oval masses, pressed together so as to destroy much of their globular symmetry. The egg contents are also divided into very distinct forms, a granular matter, and a large number of transparent globules, these latter being the fatty portion of the yolk; these are present everywhere, though in the centre there is a space where they are very scanty, and they also do not extend quite to the circumference. But the most remarkable change that has taken place is the appearance in the middle of the field of an area different from the rest in several particulars; it occupies about one-third of the width and one-third of the length; it has a whiter and more opaque appearance, and the fat globules in it are fewer in number and more indistinct. This area is afterwards seen to be occupied by the developing embryo, the outlines of which become gradually more distinct. Fig. 83 gives an idea of the appearance of the egg about the middle period of the development. In warm weather the larva emerges from this egg ten or eleven days after it has been deposited.
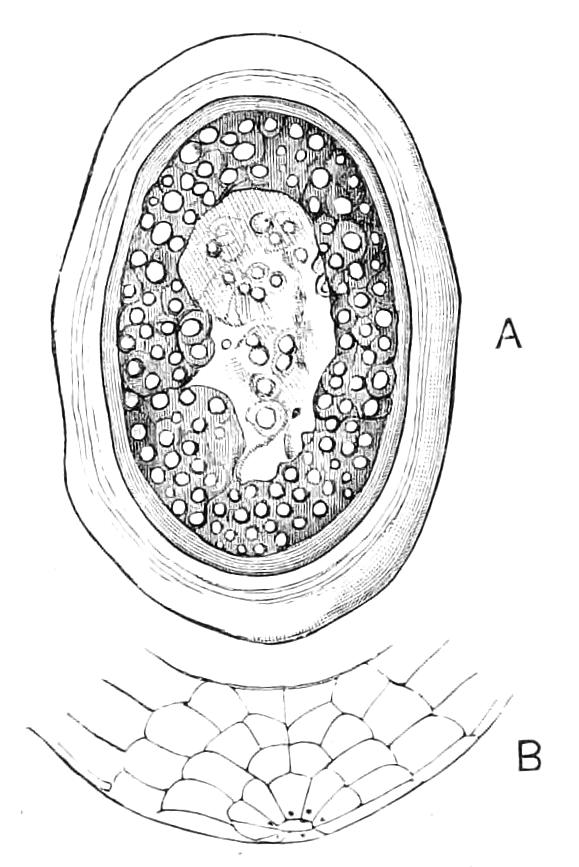
Fig. 83.—A, Egg of Limacodes testudo about the middle of the development of the embryo; B, micropyles and surrounding sculpture of chorion.
The period occupied by the development of the embryo is very different in the various kinds of Insects; the blowfly embryo is fully developed in less than twenty-four hours, while in some of the Orthoptera the embryonic stage may be prolonged through several months. According to Woodworth the blastoderm in Vanessa antiopa is complete in twenty-four hours after the deposition of the egg, and the involution of the ventral plate is accomplished within three days of deposition.
Metamorphosis.
The ontogeny, or life history of the individual, of Insects is peculiar, inasmuch as a very large part of the development takes {154}place only late in life and after growth has been completed. Insects leave the egg in a certain form, and in that condition they continue—with, however, a greater or less amount of change according to kind—till growth is completed, when, in many cases, a very great change of form takes place. Post-embryonic development, or change of form of this kind, is called metamorphosis. It is not a phenomenon peculiar to Insects, but exists to a greater or less extent in other groups of the Metazoa; while simpler post-embryonic development occurs in nearly all, as in scarcely any complex animals are all the organs completely formed at the time the individual becomes possessed of a separate existence. In many animals other than Insects the post-embryonic development assumes most remarkable and complex forms, though there are perhaps none in which the phenomenon is very similar to the metamorphosis of Insects. The essential features of metamorphosis, as exhibited in the great class we are writing of, appear to be the separation in time of growth and development, and the limitation of the reproductive processes to a short period at the end of the individual life. The peculiar phenomena of the post-embryonic development of the white ants show that there exists some remarkable correlation between the condition of the reproductive organs and the development of the other parts of the organisation. If we take it that the post-embryonic physiological processes of any individual Insect are of three kinds,—growth, development, and reproduction,—then we may say that in the higher Insects these three processes are almost completely separated, and go on consecutively, the order being,—first, growth; second, development; third, reproduction. While, if we complete the view by including the processes comprised in the formation of the egg and the development therein, the series will be—(1) oogenesis, or egg-growth; (2) development (embryonic); (3) growth (post-embryonic); (4) development (post-embryonic); (5) reproduction.
The metamorphosis of Insects is one of the most interesting parts of entomology. It is, however, as yet very little known from a scientific point of view, although the simpler of its external characters have for many ages past attracted the attention and elicited the admiration of lovers of nature. It may seem incorrect to say that little is yet known scientifically of a phenomenon concerning which references almost {155}innumerable are to be found in literature: nevertheless the observations that have been made as to metamorphosis, and the analysis that has been commenced of the facts are at present little more than sufficient to show us how vast and complex is the subject, and how great are the difficulties it presents.
There are three great fields of inquiry in regard to metamorphosis, viz. (1) the external form at the different stages; (2) the internal organs and their changes; (3) the physiological processes. Of these only the first has yet received any extensive attention, though it is the third that precedes or underlies the other two, and is the most important. We will say a few words about each of these departments of the inquiry. Taking first the external form—the instar. But before turning to this we must point out that in limiting the inquiry to the post-embryonic development, we are making one of those limitations that give rise to much misconception, though they are necessary for the acquisition of knowledge as to any complex set of phenomena. If we assume five well-marked stages as constituting the life of an Insect with extreme metamorphosis, viz. (1) the formation and growth of the egg; (2) the changes in the egg culminating in its hatching after fertilisation; (3) the period of growth; (4) the pupal changes; (5) the life of the perfect Insect; and if we limit our inquiry about development to the latter three, we are then shutting out of view a great preliminary question, viz. whether some Insects leave the egg in a different stage of development to others, and we are consequently exposing ourselves to the risk of forgetting that some of the distinctions we observe in the subsequent metamorphosis may be consequential on differences in the embryonic development.
Instar and Stadium.
Figs. 84 and 85 represent corresponding stages in the life of two different Insects, Fig. 84 showing a locust (Acridium), and Fig. 85 a white butterfly. In each A represents the newly-hatched individual; B, the insect just before its perfect state; C, the perfect or imago stage. On comparing the two sets of figures we see that the C stages correspond pretty well as regards the most important features (the position of the wings being unimportant), that the A stages are moderately different, {156}while the B states are not to be recognised as equivalent conditions.
Fig. 84.—Locust (Acridium peregrinum): A, newly hatched; B, just antecedent to last ecdysis; C, perfect Insect.
Fig. 85.—Butterfly (Pieris): A, the newly hatched young, or larva magnified; B, pupa (natural size) just antecedent to last ecdysis; C, perfect Insect.
Every Insect after leaving the egg undergoes during the process of growth castings of the skin, each of which is called a moult or ecdysis. Taking for our present purpose five as the number of ecdyses undergone by both the locust and butterfly, we may express the differences in the successions of change we portray in Figs. 84 and 85 by saying that previous to the first ecdysis the two Insects are moderately dissimilar, that the locust undergoes a moderate change before reaching the fifth ecdysis, and undergoes another moderate change at this moult, thus reaching its perfect condition by a slight, rather gradual series of {157}alterations of form. On the other hand, the butterfly undergoes but little modification, remaining much in the condition shown by A, Fig. 85, till the fourth, or penultimate, ecdysis, but then suffers a complete change of form and condition, which apparently is only inferior to another astonishing change that takes place at the fifth or final moult. The chief, though by no means the only, difference between the two series consists in the fact that the butterfly has interposed between the penultimate and the final ecdyses a completely quiescent helpless condition, in which it is deprived of external organs of sense, locomotion, and nutrition; while in the locust there is no loss of these organs, and such quiescent period as exists is confined to a short period just at the fifth ecdysis. The changes exhibited by the butterfly are called "complete metamorphosis," while this phenomenon in the locust is said to be "incomplete." The Insect with complete metamorphosis is in its early stage called a larva, and in the quiescent state a pupa. The adult state in both butterfly and locust is known as imago or perfect Insect.
The most conspicuous of the differences between Insects with complete and those with incomplete metamorphosis is, as we have remarked, the existence in the former of a pupa. The pupal state is by no means similar in all the Insects that possess it. The most anomalous conditions in regard to it occur in the Order Neuroptera. In some members of that Order—the Caddis-flies for instance—the pupa is at first quiescent, but becomes active before the last ecdysis; while in another division—the May-flies—the last ecdysis is not preceded by a formed pupa, nor is there even a distinct pupal period, but the penultimate ecdysis is accompanied by a change of form to the winged condition, the final ecdysis being merely a casting of the skin after the winged state has been assumed. In the Odonata or Dragon-flies there is no pupal stage, but the change of form occurring at the last ecdysis is very great. In those Insects where the interval between the last two moults is not accompanied by the creature's passing into a definite, quiescent pupa, the individual is frequently called then a nymph; but the term nymph has merely a distinctive meaning, and is not capable of accurate definition, owing to the variety of different conditions covered by the word. Eaton, in describing this term as it is used for Ephemeridae, says, "Nymphs are young which lead an {158}active life, quitting the egg at a tolerably advanced stage of morphological development, and having the mouth-parts formed after the same main type of construction as those of the adult insect."[80]
The intervals between the ecdyses are called stadia, the first stadium being the period between hatching and the first ecdysis. Unfortunately no term is in general use to express the form of the Insect at the various stadia; entomologists say, "the form assumed at the first moult," and so on. To avoid this circumlocution it may be well to adopt a term suggested by Fischer,[81] and call the Insect as it appears at hatching the first instar, what it is as it emerges from the first ecdysis the second instar, and so on; in that case the pupa of a Lepidopteron that assumed that condition at the fifth ecdysis would be the sixth instar, and the butterfly itself would be the seventh instar.
Various terms are used to express the differences that exist in the metamorphoses of Insects, and as these terms refer chiefly to the changes in the outer form, we will here mention them. As already stated, the locust is, in our own language, said to have an incomplete metamorphosis, the butterfly a complete one. The term Holometabola has been proposed for Insects with complete metamorphosis, while the appellations Ametabola, Hemimetabola, Heterometabola, and Paurometabola have been invented for the various forms of incomplete, or rather less complex, metamorphosis. Some writers use the term Ametabola for Insects that are supposed to exhibit no change of external form after quitting the egg, the contrasted series of all other Insects being then called Metabola. Westwood and others use the word Homomorpha for Insects in which the condition on hatching more or less resembles that attained at the close of the development, and Heteromorpha for those in which the form on emergence from the egg differs much from what it ultimately becomes.
Hypermetamorphosis.
There are certain unusual changes to which the term hypermetamorphosis has been applied; these we can here only briefly allude to.
Insects that have complete metamorphoses, and are not supplied with food by their parents or guardians, are provided during their larval life with special modifications of extremely various kinds to fit them for the period of life during which they are obtaining food and growing. Thus caterpillars possess numerous adaptations to fit them for the period during which they live on leaves, while maggots have modifications enabling them to live amongst decomposing flesh. Some larvae are greatly modified in this adaptive way, and when the adaptations change greatly during the life of the larva, hypermetamorphosis is said to exist. As an instance we may mention some beetle larvae that are born with legs by whose aid they can cling to a bee, and so get carried to its nest, where they will in future live on the stores of food the bee provides for its own young. In order that they may be accommodated to their totally different second circumstances, they change their first form, losing their legs, and becoming almost bladder-like creatures, fitted for floating on the honey without being injured by it. Such an occurrence has been described by Fabre[82] in the case of Sitaris humeralis, and his figures have been reproduced in Sir John Lubbock's book on the metamorphoses of Insects,[83] as well as in other works, yet they are of so much interest that we give them again, especially as the subject is still only in its infancy; we at present see no sufficient reason for the later of these larval states. Little is, we believe, known as to the internal anatomy of the various instars in these curious cases.
Fig. 86.—Preparatory stages of Sitaris humeralis: 9, 10, 11, 12, first, second, third, and fourth larval instars; 13, pupa. (After Lubbock and Fabre.)
There are certain minute Hymenoptera that deposit their eggs inside the eggs of other Insects, where the beings hatched from the parasitic eggs subsequently undergo their development and growth, finding their sustenance in the yolk or embryo contained in the host-egg. It is evident that such a life is very anomalous as regards both food and the conditions for respiration, and we consequently find that these tiny egg-parasites go through a series of changes of form of a most remarkable character.[84] It would appear that in these cases the embryonic and post-embryonic developments are not separated in the same way as they are in other Insects. We are not aware that any term has yet been proposed for this very curious kind of Insect development, which, as pointed out by Brauer,[85] is doubtless of a different nature from the hypermetamorphosis of Sitaris.
Changes in Internal Organs.
In relation to the post-embryonic development of the internal organs of the body there is but little exact generalisation to be made, the anatomical condition of these organs at the time of emergence from the egg having been ascertained in but few Insects. We know that in Holometabolous Insects the internal anatomy differs profoundly in the larval and imaginal instars. As to Insects with more imperfect metamorphosis very little information exists, but it appears probable that in many no extensive distinctions exist between the newly-hatched and the adult forms, except in the condition of the reproductive organs. Differences of minor importance doubtless exist, but there is almost no information as to their extent, or as to the periods at which the changes occur; so that we do not know to what extent they may be concentrated at the final ecdysis. In Insects with perfect metamorphosis the structures of the internal organs are, as we have said, in many cases totally different in the larval and imaginal periods of the life; but these changes are far from being uniform in all Holometabola. The nervous system in some cases undergoes a great concentration of the ganglia, in others does not, and important distinctions exist in this respect even within the limits of a single Order, such as the Coleoptera. {161}Some Insects take the same kind of food throughout their lives, but many others change totally in this respect, and their organs for the prehension and digestion of food undergo a corresponding change. Butterflies suck food in the form of liquid juices from flowers by means of a delicate and long proboscis, while the young butterfly—the caterpillar—disdains sweets, and consumes, by the assistance of powerful mandibles, a great bulk of leaves. Other Holometabola undergo no such total change of habits; the tiger-beetle, for instance, is as ferocious a consumer of the juices of Insects in its young stage as it is in the adult condition. Hence Brauer[86] divides Insects, as regards this point, into three categories. The forms in which both the young and adult take food by suction he calls Menorhyncha; those in which both the imago and immature forms feed by mandibles he calls Menognatha; while his Metagnatha consists of those insects that take food by jaws when young, but by suction with tubular mouths when mature. Besides these main divisions there are some exceptional cases to which we need not here allude, our present object being to indicate that in the Metagnatha the digestive organs are of a very different nature in the young and in the adult states of existence.
The internal organs for the continuance of the species are known to be present in a rudimentary stage in the embryo, and it is a rule that they do not attain their full development until growth has been completed; to this rule there may possibly be an exception in the case of the Aptera. But little information of a comparative character exists as to the dorsal vessel and the changes it undergoes during metamorphosis. There is considerable difficulty in connexion with the examination of this structure, but it appears probable that it is one of the organs that changes the least during the process of metamorphosis.
The exact nature of the internal changes that occur during metamorphosis is almost a modern subject. It is of course a matter of great difficulty to observe and record changes that go on in the interior of such small creatures as Insects, and when the phenomena occur with great rapidity, as is frequently the case in Insect metamorphosis, the difficulty is much increased. Nevertheless the subject is of such great interest that it has been investigated with a skill and perseverance that call for the {162}highest admiration. The greater part of the information obtained refers to a single Insect, the blowfly; and amongst those who have made important contributions to it we may mention Weismann,[87] Viallanes,[88] Ganin,[89] and Van Rees,[90] and it is at present under investigation by Lowne. A good deal, too, is becoming known about the processes in the case of the silkworm.
Integument and Ecdysis.
The integument consists of a cellular layer, usually called the hypodermis, situated on a basement membrane. The hypodermis, or layer of chitinogenous cells, excretes a matter which remains attached to the body, forming the hard outer layer of the skin. This layer consists of chitin and has no vitality, but its presence no doubt exerts a very important influence on the physiological processes of the Insect. The chitinous investment varies much in thickness and in other properties; in some Insects it is hard, even glassy, so as to be difficult to pierce with a pin, in others it is pliable, and in some very delicate. Chitin is a substance very difficult to investigate; according to the recent researches of Krawkow[91] it may prove to be of somewhat variable chemical composition.
After a time the hypodermis excretes a fresh supply of chitin, and, possibly by the commencement of this process, the older chitinous investment becomes separated and is shed. The details have, however, not been ascertained, though their importance has been suggested by Hatchett Jackson.[92] The newly exposed layer of integument is pallid, but afterwards becomes coloured in a manner varying according to the species, the process being possibly due to some secondary exudation permeating the freshly exposed chitin, or modifying some part of its exterior.
Lowne informs us that in the imago of the blowfly the great majority of the hypodermic cells themselves enter into the composition of the chitinous integument; and it is perhaps not a matter for surprise that the cells should die on the completion of their functional activity, and should form a part of the chitinous {163}investment. Some writers say that the chitinous layer may be shown to be covered by a delicate extima or outer coat.
The number of ecdyses varies greatly in Insects, but has been definitely ascertained in only a few forms outside the Order Lepidoptera. In Campodea Grassi says there is a single fragmentary moult, and in many Hymenoptera the skin that is cast is extremely delicate, and the process perhaps only occurs twice or three times previous to the pupal stage. In most Insects, however, ecdysis is a much more important affair, and the whole of the chitinous integument is cast off entire, even the linings of the tracheae, and of the alimentary canal and its adjuncts being parted with. Sir John Lubbock observed twenty-three moults in a May-fly of the genus Cloëon,[93] this being the maximum yet recorded, though Sommer states[94] that in Macrotoma plumbea moulting goes on as long as life lasts, even after the Insect has attained its full size.
Some Insects get quit of a considerable quantity of matter by their ecdyses, while in others the amount is comparatively slight. It has been thought that the moulting is effected in order to permit of increase of size of the Insect, but there are facts which point to the conclusion that this is only a factor of secondary importance in the matter. One of these is that many Insects make their first ecdysis almost immediately after they leave the egg; this is the case with the young larva of the blowfly, which, according to Lowne, moults within two hours of its emergence from the egg. We have already referred to the important suggestion made by Eisig[95] that, since chitin is a nitrogenous substance, the ecdyses may be a means of getting rid of waste nitrogenous matter; to which we have added that as chitin also consists largely of carbon, its excretion may be of importance in separating carbonaceous products from the blood.
Metamorphosis of Blowfly.
The phenomena of metamorphosis are displayed to their greatest extent in the transformations and physiological processes of the Muscid Diptera, of which the common blowfly is an {164}example. We will briefly consider the information that has been obtained on this subject.
The development of the embryo in the egg of the blowfly is unusually rapid, occupying only a period of twenty to twenty-four hours. After its first moult the blowfly larva grows rapidly during a period of about ten to fourteen days, during which it undergoes moults, the number of which appears not to be definitely ascertained. After becoming full-fed the larva loses its active state, and passes for a period into a condition of comparative quiescence, being spoken of in this state as a resting larva. This quiet period occurs in most full-grown larvae, and is remarkable for the great variation that may occur in its duration, it being in many Insects subject to prolongation for months, in some cases possibly even for years, though in favourable circumstances it may be very short. Lowne informs us that in the blowfly this period of the life is occupied by very great changes in the internal organs, which are undergoing very extensive processes of destruction and rebuilding. After some days the outer skin of the resting larva shrivels, and is detached from the internal living substances, round which it hardens and forms the sort of cocoon or capsule that is so well known. This using of the cast larval skin as a cocoon is, however, limited to certain of the two-winged flies, and perhaps a few other Insects, and so must be considered an exceptional condition. The capsule conceals from view a most remarkable state, known to the old naturalist Réaumur as the "spheroidal condition," but called by more recent writers the pronymph. The pronymphal state may be looked on as being to a great extent a return of the animal to the condition of an egg, the creature becoming an accumulation of soft creamy matter enclosed in a delicate skin. This spheroidal condition, however, really begins in the resting larva, and Van Rees and others think that the delicate membrane enclosing the substance of the pronymph is really the hypodermis of the integument of the larva. Although this seems probable, from the resemblance this condition would in that case present to the phenomena usual in ecdysis, it is not generally admitted, and there is much difficulty in settling the point. Lowne is of a contrary opinion, looking on the limiting membrane as a subsequent formation; he calls it the paraderm. The process of forming the various organs goes on in the pronymph, till the {165}"nymph" has completed its development, the creature having then again taken on a definite form which apparently corresponds to the pupa of Hymenoptera. Great doubt, however, exists as to this equivalence, and indeed as to any exact correspondence between the metamorphic stadia of different Insects, a view which long since was expressed by Sir John Lubbock[96] and Packard. The term nymph is used in this case not because there is any resemblance to the condition similarly named in Insects with less complete metamorphosis, but because the term pupa is applied to the outer case together with the contained nymph. The transformation of the nymph into the perfect blowfly occupies a period very variable according to the temperature.
Histolysis.—The processes by which the internal organs of the maggot are converted into those of the fly are of two kinds,—histolysis or breaking down, histogenesis or building up, of tissue. The intermediary agents in histolysis are phagocytes, cells similar to the leucocytes or white corpuscles of the blood: the intermediary agents in histogenesis are portions of tissue existing in the larval state incorporated with the different organs, or preserving a connexion therewith even when they are to a great extent separated therefrom. In this latter case they are called imaginal discs, though Professor Miall prefers to term them imaginal folds.[97] The two processes of histolysis and histogenesis, though to some extent mutually dependent (for the material to be built up has to be largely obtained by previous destruction), do not go on pari passu, though they are to a great extent contemporaneous. In the resting larva histolysis is predominant, while in the nymph histogenesis is more extensive. Microscopic observation shows that the phenomena connected with the histolysis of the muscular tissue are scarcely distinguishable from those of an inflammatory process, and Viallanes[98] dilates on this fact in an instructive manner. The phagocytes attach themselves to, or enter, the tissues which are to be disintegrated, and becoming distended, assume a granular appearance. By this pseudo-inflammatory process the larval structures are broken down into a creamy substance; the buds, or germs, from which the new organs are to be developed being exempt from the destruction. These buds, of which about sixty or upwards have already been detected, undergo {166}growth as they are liberated, and so the new creature is formed, the process of growth in certain parts going on while destruction is being accomplished in others. Considerable discrepancy prevails as to the extent to which the disintegration of some of the tissues is carried.
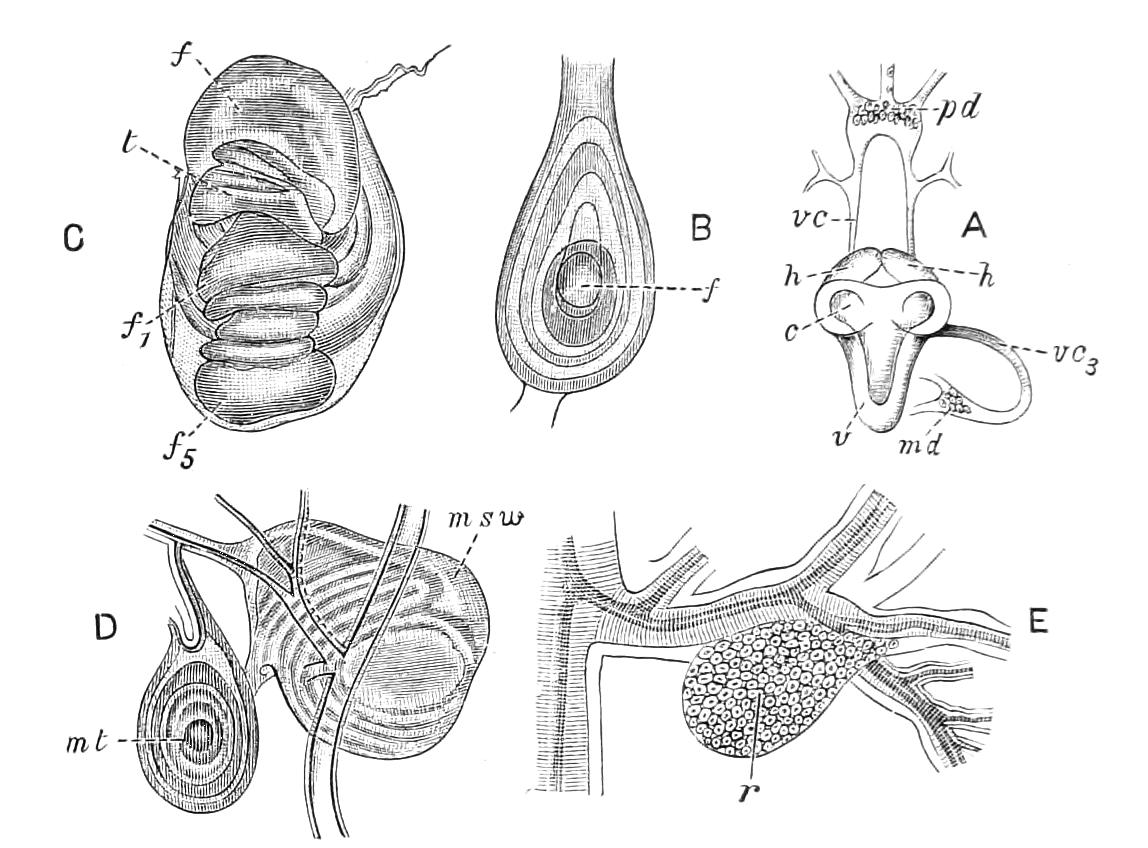
Fig. 87.—Imaginal discs of Muscidae in process of development: A, Brain and ventral ganglion of a larva 7 mm. long of M. vomitoria; v, ventral ganglion; c, cephalic ganglion; h, head rudiment; vc, portion of ventral chain; pd, prothoracic rudiment; vc3, third nerve; md, mesothoracic rudiment: B, mesothoracic rudiment, more advanced, in a pupa just formed of Sarcophaga carnaria, showing the base of the sternum and folds of the forming leg, the central part (f) representing the foot: C, the rudimentary leg of the same more advanced; f, femur; t, tibia; f1, f5, tarsal joints: D, two discs from a larva 20 mm. long of Sarcophaga, attached to tracheae; msw, mesonotal and wing-rudiment; mt, metathoracic rudiment: E, r, mesothoracic rudiment of a 7 mm. long larva attached to a tracheal twig. (After Weismann and Graber.)
According to Kowalevsky[99] it would appear that after the phagocytes have become loaded with granules they serve as nutriment for the growing tissues, and he thinks they become blood-cells in the imago. The process of histolysis has been chiefly studied in the blowfly, and not much is known of it in other Insects, yet it occurs to a considerable extent, according to Bugnion[100] and others, in the metamorphosis of Lepidoptera. Indeed it would almost seem that the processes of histolysis and histogenesis may be looked on as exaggerated forms of the phenomena of the ordinary life of tissues, due to greater rapidity and discontinuity of tissue nutrition.
Imaginal Discs.—The imaginal discs are portions of the larval hypoderm, detached from continuity with the main body of the integument, but connected therewith by strings or pedicels which may be looked on as portions of the basement membrane. Whether these discs, or histoblasts as they are called by Künckel d'Herculais,[101] are distinguished by any important character from other buds or portions of regenerative tissue that, according to Kowalevsky,[102] Korschelt and Heider,[103] and others, exist in other parts of the body, does not appear to be at present ascertained.
We give some figures, taken from Weismann and Graber, of the imaginal rudiments existing in the larvae of Muscidae. Although by no means good, they are the best for our purpose we can offer to the reader. Other figures will be found in Lowne's work on the blowfly now in course of publication. Weismann's paper[104] is now thirty years old, and, when it was written, he was not aware of the intimate connexion the rudiments have with the integument; this has, however, now been demonstrated by several observers. Pratt states[105] that the formation of the imaginal discs in Melophagus ovinus takes place in the later stages of the embryonic development, and after the manner formerly suggested by Balfour, viz. invagination of the ectoderm.
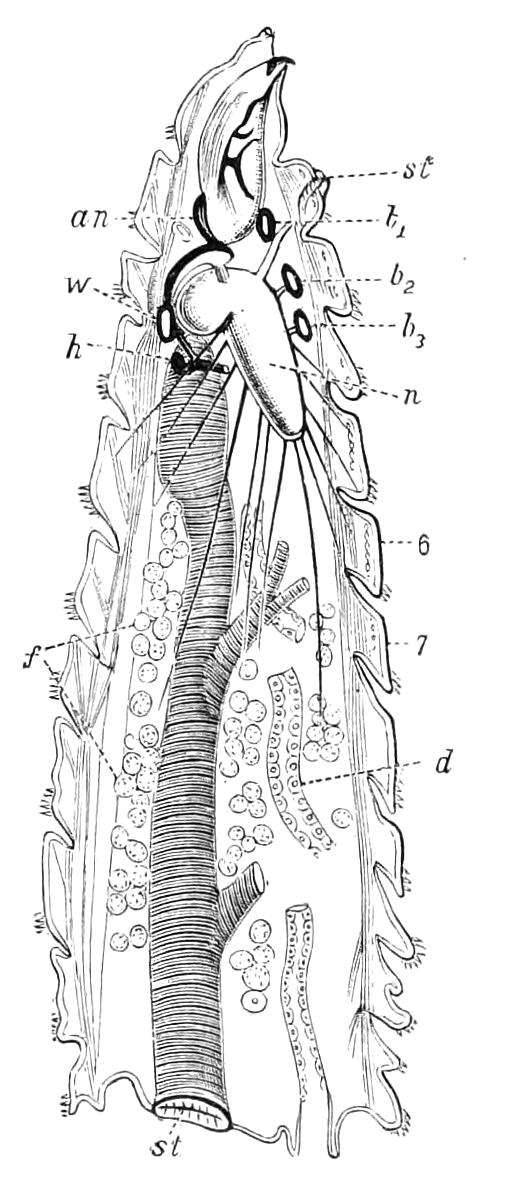
Fig. 88.—Median longitudinal section through larva of blowfly during the process of histolysis. (After Graber.) Explanation in text.
Both the regenerative buds and the rudimentary sexual glands are known to be derived directly from the embryo; neither of them undergoes any histolysis, so that we have in them embryonic structures which exist in a quiescent condition during the period in which the larva is growing with great rapidity, and which when the larva has attained its full growth and is disintegrating, then {168}appropriate the products of the disintegration so as to produce the perfect fly.
Our Fig. 88, taken from Graber, represents a longitudinal median section of a full-grown larva of Musca, in which the processes of metamorphosis are taking place. The position of some of the more important imaginal rudiments is shown by it: b1, b2, b3, rudiments of the three pairs of legs of the imago; an, of antennae; between an and w, rudiment of eye; w, of wings; h, of halteres; f, fat-body; d, middle of alimentary canal; n, ventral chain; st, stigma; 6, 7, sixth and seventh body segments.
Physiology of Metamorphosis.
Many years ago, Harvey perceived the probable existence of a physiological continuity between the earlier and later stages of the Insect's life. Modern investigation has shown that in the blowfly a remarkable analogy exists between the conditions of the pupa and the egg. The outer shell of the pupa corresponds to the chorion or egg-shell, and the delicate outer membrane of the pronymph to the oolemn or lining membrane of the egg; the creamy matter corresponds with the yolk, and the regenerative buds are analogous to the formative portions of the developing egg. The process of histolysis as carried out by the phagocytes of the later life appears also to find a parallel in the vitellophags of the embryonic life.[106] It appears probable that the physiological processes of the post-embryonic metamorphosis may be essentially a repetition—or an interrupted continuation—of those of the embryonic period.
The inquiry as to what are the determining causes of the metamorphic changes of the blowfly and other Insects has as yet but little advanced. Why does the larva grow up to a certain period with great rapidity, then cease its appropriating power and break up the parts that have been so rapidly and recently formed? And why do the imaginal buds remain quiescent till the other tissues are being disintegrated, and then, instead of sharing the general condition of disintegration, commence a career of development? To these questions no satisfactory answer has yet been given, though the remarkable studies, already referred to, of Bataillon on the later larval life {169}of the silkworm suggest the direction in which knowledge may be found, for they show that the physiological conditions of the later larval life are different from those of the earlier life, possibly as the direct result of the mere aggregation of matter, and the consequent different relations of the parts of the organism to atmospheric and aqueous conditions.
If we wish to understand metamorphosis, we must supplement the old opinion that ecdysis is merely an occurrence to facilitate expansion, by the more modern conception that it is also an important physiological process. That shedding the skin is done solely to permit of enlargement of size is a view rendered untenable by many considerations. The integument can increase and stretch to an enormous extent without the aid of moulting; witness the queen-termite, and the honey-bearers of the Myrmecocystus ants. Many moults are made when increase of size does not demand them, and the shedding of the skin at the time of pupation is accompanied by a decrease in size. And if moulting be merely connected with increase of size, it is impossible to see why Cloëon should require two dozen moults, while Campodea can do with one, or why a collembolon should go on moulting during the period of life subsequent to the cessation of growth.
The attention of entomologists has been chiefly directed to the ecdyses connected with the disclosure of the pupal and imaginal instars. Various important transformations may, however, occur previous to this, and when they do so it is always in connexion with ecdyses. Caterpillars frequently assume a different appearance and change their habits or character at a particular ecdysis; and in Orthoptera each ecdysis is accompanied by a change of form of the thoracic segments; this change is very considerable at one of the intermediate ecdyses.
The assumption of the pupa state is the concomitant of an ecdysis, and so also is the appearance of the imago; but the commencement of each of these two stages precedes the ecdysis, which is merely the outward mark of the physiological processes. The ecdysis by which the pupa is revealed occurs after the completion of growth and when great changes in the internal organs have occurred and are still taking place; the ecdysis by which the imago appears comes after development has been quite or nearly completed.
Although the existence of a pupa is to the eye the most {170}striking of the differences between Insects with perfect and those with imperfect metamorphosis, yet there is reason for supposing that the pupa and the pupal period are really of less importance than they at first sight appear to be. In Fig. 85 we showed how great is the difference in appearance between the pupa and the imago. The condition that precedes the appearance of the pupa is, however, really the period of the most important change. In Fig. 89 we represent the larva and pupa of a bee; it will be seen that the difference between the two forms is very great, while the further change that will be required to complete the perfect Insect is but slight. When the last skin of the larva of a bee or of a beetle is thrown off, it is, in fact, the imago that is revealed; the form thus displayed, though colourless and soft, is that of the perfect Insect; what remains to be done is a little shrinking of some parts and expansion of others, the development of the colour, the hardening of certain parts. The colour appears quite gradually and in a regular course, the eyes being usually the first parts to darken. After the coloration is more or less perfected—according to the species—a delicate pellicle is shed or rubbed off, and the bee or beetle assumes its final form, though usually it does not become active till after a farther period of repose.
Fig. 89.—Larva and pupa of a bee, Xylocopa violacea: A, larva; B, pupa, ventral aspect; C, pupa, dorsal aspect. (After Lucas.)
CLASSIFICATION—THE NINE ORDERS OF INSECTS—THEIR CHARACTERS—PACKARD'S ARRANGEMENT—BRAUER'S CLASSIFICATION—CLASSIFICATIONS BASED ON METAMORPHOSIS—SUPER-ORDERS—THE SUBDIVISIONS OF ORDERS.
Classification.
We have already alluded to the fact that Insects are the most numerous in species and individuals of all land animals: it is estimated that about 250,000 species have been already described and have had scientific names given to them, and it is considered that this is probably only about one-tenth of those that really exist. The classification in a comprehensible manner of such an enormous number of forms is, it will be readily understood, a matter of great difficulty. Several methods or schemes have since the time of Linnaeus been devised for the purpose, but we shall not trouble the reader to consider them, because most of them have fallen into disuse and have only a historical interest. Even at present there exists, however, considerable diversity of opinion on the question of classification, due in part to the fact that some naturalists take the structure of the perfect or adult Insect as the basis of their arrangement, while others prefer to treat the steps or processes by which the structure is attained, as being of primary importance. To consider the relative values of these two methods would be beyond our scope, but as in practice a knowledge of the structures themselves must precede an inquiry as to the phases of development by which the structures are reached; and as this latter kind of knowledge has been obtained in the case of a comparatively small portion of the known forms,—the embryology and metamorphosis having been investigated in but {172}few Insects,—it is clear that a classification on the basis of structure is the only one that can be at present of practical value. We shall therefore for the purposes of this work make use of an old and simple system, taking as of primary importance the nature of the organs of flight, and of the appendages for the introduction of food to the body by the perfect Insect. We do not attempt to disguise the fact that this method is open to most serious objections, but we believe that it is nevertheless at present the most simple and useful one, and is likely to remain such, at any rate as long as knowledge of development is in process of attainment.
Orders.
The great groups of Insects are called Orders, and of these we recognise nine, viz. (1) Aptera, (2) Orthoptera, (3) Neuroptera, (4) Hymenoptera, (5) Coleoptera, (6) Lepidoptera, (7) Diptera, (8) Thysanoptera, (9) Hemiptera. These names are framed to represent the nature of the wings; and there is some advantage in having the Orders named in a uniform and descriptive manner. The system we adopt differs but little from that proposed by Linnaeus.[107] The great Swedish naturalist did not, however, recognise the Orders Orthoptera and Thysanoptera; and his order Aptera was very different from ours.
These Orders may be briefly defined as follows,—the reader being asked to recall the fact that by a mandibulate mouth we understand one in which the mandibles, or the maxillæ, or both, are fitted for biting, crushing, or grasping food; while the term suctorial implies that some of the mouth parts are of a tubular form or are protrusible as a proboscis, which assists, or protects, a more minute and delicate sucking apparatus:—
1. Aptera (ἀ without, πτερόν a wing). Wingless[108] Insects; mouth mandibulate or very imperfectly suctorial. Metamorphosis very little.
2. Orthoptera (ὀρθός straight, πτερόν a wing). Four wings are present, the front pair being coriaceous (leather-like), usually smaller than the other pair, which are of more delicate texture, and contract in repose after the manner of a fan. Mouth mandibulate. Metamorphosis slight.
3. Neuroptera (νεῦρον nerve, πτερόν a wing). Four wings of membranous {173}consistency, frequently with much network; the front pair not much, if at all, harder than the other pair, the latter with but little or no fanlike action in closing. Mouth mandibulate. Metamorphosis variable, but rarely slight.
4. Hymenoptera (ὑμήν membrane, πτερόν a wing). Four wings of membranous consistency; the front pair larger than the hind, which are always small and do not fold up in repose. Mouth mandibulate, sometimes provided also with a tubular proboscis. Metamorphosis very great.
5. Coleoptera (κολεός sheath, πτερόν a wing). Four wings; the upper pair shell-like in consistency, and forming cases which meet together over the back in an accurate line of union, so as to entirely lose a winglike appearance, and to conceal the delicate membranous hind pair. Mouth mandibulate. Metamorphosis great.
6. Lepidoptera (λεπίς scale, πτερόν a wing). Four large wings covered with scales. Mouth suctorial. Metamorphosis great.
7. Diptera (δίς double, πτερόν a wing). Two membranous wings. Mouth suctorial, but varying greatly. Metamorphosis very great.
8. Thysanoptera (θύσανος fringe, πτερόν a wing). Four very narrow fringed wings. Mouth imperfectly suctorial. Metamorphosis slight.
9. Hemiptera (ἡμι half, πτερόν a wing). Four wings; the front pair either leather-like with more membranous apex, or entirely parchment-like or membranous. Mouth perfectly suctorial. Metamorphosis usually slight.
We must again ask the reader to bear in mind that numerous exceptions exist to these characters in most of the great Orders; for instance, wingless forms are not by any means rare in several of the Orders.
Before remarking further on this system we will briefly sketch two other arrangements of the Orders of Insects, for which we are indebted to Packard and Brauer.
Packard's Classification.
Packard has devoted much attention to the subject, and has published two or three successive schemes, of which the following is the most recent:[109] the definitions are those of the author himself, but the information in brackets is given to institute a concordance with the system we adopt:—
1. Thysanura. Wingless; often with a spring (equivalent to our Aptera).
2. Dermaptera. Front wings minute, elytra-like (= Forficulidae, a part of our Orthoptera).
3. Orthoptera. Wings net-veined; fore wings narrow, hind wings folded (= our Orthoptera after subtraction of Dermaptera).
4. Platyptera. Four net-veined wings; mouth parts adapted for biting (= Termitidae and Mallophaga, parts of our Neuroptera).
5. Odonata. Wings net-veined, equal (= Odonata, a division of our Neuroptera).
6. Plectoptera. Wings net-veined, unequal (= Ephemeridae, a part of our Neuroptera).
7. Thysanoptera. Mouth beaklike but with palpi (= our Thysanoptera).
8. Hemiptera. Mouth parts forming a beak for sucking. No palpi (= our Hemiptera).
The above eight Orders form the group Ametabola, while the following eight constitute the Metabola:—
9. Neuroptera. Wings net-veined; metamorphosis complete (= a small part of our Neuroptera).
10. Mecaptera. Wings long and narrow (for a small part of our Neuroptera; the Panorpatae of Brauer).
11. Trichoptera. Wings not net-veined (= our division of Neuroptera with the same name).
12. Coleoptera. Fore wings sheathing the hinder ones (= our Coleoptera).
13. Siphonaptera. Wingless, parasitic. Flea (= a division of Diptera).
14. Diptera. One pair of wings (= our Diptera after subtraction of Siphonaptera).
15. Lepidoptera. Four wings (and body) scaled (= our Lepidoptera).
16. Hymenoptera. Four clear wings; hinder pair small; a tongue (= our Hymenoptera).
Although this system of the Orders of Insects has some valuable features it is open to very serious objections, to which we can only briefly allude. The Order Hemiptera with its extensive divisions, Heteroptera, Homoptera, Coccidae, and Anoplura exhibiting great differences in structure and considerable divergence in metamorphosis, is treated as only equivalent to the little group Panorpatae (scorpion-flies); these latter being considered a distinct order, although they are not very different in structure or metamorphosis from the Orders he calls Neuroptera and Trichoptera. The arrangement appears to be specially designed with the view of making the Orders adopted in it fall into the two groups Ametabola and Metabola. The propriety of such a course is more than doubtful since very few of the Ametabola are really without metamorphosis, in the wide sense of that term, while the Metabola include Insects with various kinds of metamorphosis. Indeed if we substitute for the term Ametabola the more correct expression, "Insects with little metamorphosis," and for Metabola the definition, "Insects with more metamorphosis but of various kinds," we then recognise that the arrangement {175}is, like all others, a quite artificial one, while it is of little value, owing to the development of so few Insects being hitherto fully ascertained.
Brauer's Classification.
Professor Brauer has recently proposed[110] to adopt 17 Orders or chief groups of Insects, arranging them as follows:—
I. Apterygogenea (with one order).
1. Synaptera (= Aptera of our system).
II. Pterygogenea (= all the other Insects of our arrangement).
2. Dermaptera (= Orthoptera, Fam. Forficulidae in our arrangement).
3. Ephemeridae (= a division of Neuroptera in our arrangement).
4. Odonata (= a division of Neuroptera in our arrangement).
5. Plecoptera (= Neuroptera, Fam. Perlidae in our arrangement).
6. Orthoptera (= our Orthoptera - Forficulidae and + Embiidae).
7. Corrodentia (= the families Termitidae, Psocidae, and Mallophaga, of our Neuroptera).
8. Thysanoptera (as with us).
9. Rhynchota (= Hemiptera with us).
10. Neuroptera (= the families Hemerobiidæ and Sialidæ of our Neuroptera).
11. Panorpatae (= the family Panorpidae of our Neuroptera).
12. Trichoptera (= the division Trichoptera of Neuroptera).
13. Lepidoptera (= as with us).
14. Diptera (= our Diptera - Aphaniptera).
15. Siphonaptera (= Aphaniptera, a division of Diptera with us).
16. Coleoptera (= Coleoptera).
17. Hymenoptera (as with us).
The chief characters on which Brauer bases his system are: (1) The existence or absence of wings. (2) The condition of the mouth, and whether it undergoes radical changes in the ontogeny, arriving thus at the categories Menognatha, Metagnatha, and Menorhyncha, as we have mentioned on p. 161. (3) The metamorphosis; the grouping adopted being Ametabola, Hemimetabola, Metabola. (4) The number of the Malpighian tubules; Oligonephria, Polynephria. (5) The nature of the wings, the relative proportions of the thoracic segments, and some other characters.
Brauer's treatise is accompanied by a valuable and in many respects very sagacious consideration of the generalised characters of the Insecta; as a classification based partly on generalisations and partly on structures, it is, so far as the present {176}condition of our knowledge goes, a good one. But it is of little value as a practical guide, and as a basis for theoretical speculation it cannot be treated as of importance, because the generalisations it makes use of are premature, owing to the small proportion of the forms that have been examined. And even now the groups adopted are known to be subject to many exceptions.
Thus it begins by a division of Insecta into winged and wingless; but the winged division is made to comprehend an enormous number of wingless Insects, whole subdivisions of Orders such as the Mallophaga being placed in the winged series, although all are without wings. This first division is indeed entirely theoretical; and if a classification on generalisations were adopted, it would be more natural to begin with the old division into Homomorpha and Heteromorpha, and treat the Order Aptera as the first division of the Homomorpha, while the Heteromorpha would commence with the Ephemeridae and Odonata, in which, though the individual in the early part of the ontogeny is very different from the perfect Insect, there is no marked division of the later larval and the pupal stages. Brauer's system is also defective inasmuch as it takes no account of the embryological or oogenetic processes, though these are of equal importance with the later phases of the Ontogeny. Even as regards the division into Orders, it is far from being free from reproach; for instance, the separation of the Dermaptera from the Orthoptera, while Rhynchota remains intact, although including a more extensive series of heterogeneous forms; the division of the Neuroptera into widely separated groups, each of which is treated as equivalent to the great Orders, such as Coleoptera (in which Strepsiptera are included), Hymenoptera, and Diptera, is not reasonable. The association of Mallophaga and Termitidae, while Dermaptera are separated from Orthoptera, is also undeniably arbitrary, and other similar disparities are to be seen on scrutinising the details of the system.
On comparing the three arrangements we have outlined, it will be seen that the chief discrepancies they present come under two heads: (1) The treatment of the Neuroptera, opinions differing as to whether these Insects shall be grouped as a single Order, or shall be divided into numerous Orders; and as to what, if this latter course be adopted, the divisions shall be. (2) The treatment of the parasitic groups Mallophaga, Aphaniptera, etc. {177}It must be admitted that whichever of the three systems we have sketched be adopted, the result is, as regards both these points, open to criticism. The Order Neuroptera, if we take it in the broad sense, differs from the other Orders in the greater variety of metamorphosis exhibited by its members; while if, on the contrary, it be dismembered, we get a number of groups of very unequal extent and not distinguished from one another by the same decisive and important characters as are the other Orders of which they are considered equivalent. The discrepancy exists in nature, and can scarcely be evaded by any system. A similar observation may be made as to the parasitic groups, viz. Mallophaga, Anoplura, Aphaniptera, and Strepsiptera. If these be treated as separate Orders the result is not satisfactory; while, if they be associated with the larger groups to which they are respectively nearest allied, it is almost equally unsatisfactory.
We may mention that Packard and Brauer have in their treatises discussed the question of super-orders, and have gone so far as to propose names for them. These two authorities do not however agree in their conclusions; and as the names proposed are of little practical value, and are but rarely met with, we need not explain them or discuss the comparative merits of the two systems.
The divisions of inferior value to the Order are, after repeated scrutiny by many naturalists, becoming of a more satisfactory character, and notwithstanding various anomalies, may be, many of them, considered fairly natural.[111] Unfortunately entomologists have not been able to agree on a system of terminology, so that for these subdivisions terms such as sub-order, series, legion, section, tribe, etc., are used by different authorities in ways so various as to cause much confusion. In the following pages the terms sub-order and series will be used in a somewhat vague manner, the term sub-order being preferred where the group appears to be an important one and of a fairly natural character, while the word series will be adopted when the groups are connected in a conventional manner. The designation "family" will be used for groups of subordinate importance; and as regards this term we may remark that systematic entomologists are making genuine efforts to define the "families" in an accurate and comprehensible manner. The endeavour to make these systematic {178}families dependent throughout the Class Insecta on characters of similar morphological value has, however, scarcely been entered on, and it is perhaps not desirable, seeing how very small a portion of the Insects of the world have been critically examined, that much effort should be yet expended on an attempt of the kind. It must be admitted that the species of Insects should be obtained before they can be satisfactorily classified, and it is estimated[112] that at least nine-tenths of the Insects of the world are still unknown to entomologists.
Geological Record.—Although Insects have a very long pedigree, it is as yet a very imperfect one. The remains of creatures that can be referred to the Class Insecta have been found, it is said, in Silurian strata; only one or two of these very early forms are at present known, and the information about them is by no means satisfactory; if Insects at all—as to which some doubt exists—they apparently belong to very different forms, though, like all the earliest fossil Insects, they are winged. In the strata of the Carboniferous epoch numerous Insects have been detected, in both Europe and North America. These earlier Insects are by Scudder called Palaeodictyoptera, and separated from the Insects around us on the ground that he considers there existed amongst these palaeozoic Insects no ordinal distinctions such as obtain in the existing forms, but that the primeval creatures formed a single group of generalised Hexapods. Brauer does not accept this view, considering that the earlier Insects can be referred to families existing at the present time and forming parts of the Orthoptera, Neuroptera, and Hemiptera. The discrepancy between these two authorities depends to a great extent on the different classifications of existing Insects that they start from; Scudder treating the wings as of primary importance, while Brauer assigns to them only a subordinate value. From the point of view taken in the present work Scudder's view appears to be in the main correct, though his expression as to the primary fossil Insects forming a single homogeneous group is erroneous. The Neuroptera, still in existence, certainly form a heterogeneous group, and it is clear that the Palaeozoic fossils represent a more diverse assemblage than the present Neuroptera do.[113]
In the more recent rocks Insect remains become comparatively numerous, and in Mesozoic strata forms that can satisfactorily be referred to existing Orders are found, the Palaeodictyoptera of Goldenberg and Scudder having mostly disappeared; the Blattidae or cockroaches do not apparently present any great discontinuity between their Palaeozoic and Mesozoic forms. The Tertiary rocks afford us fairly satisfactory evidence to the effect that Insects were then more numerous in species than they are at the present day. At Florissant in Colorado the bed of an ancient lake has been discovered, and vast quantities of Insect remains have been found in it, the geographical conditions indicating that the creatures were not brought from a distance, but were the natural fauna of the locality; and if so we can only conclude that Insects must have been then more abundant in species than they are now.
Scudder has informed us[114] that not only were Insects abundant in the Tertiaries, but that their remains indicate conditions of existence very similar to what we find around us. "Certain peculiarities of secondary sexual dimorphism accompanying special forms of communistic life, such as the neuters and workers in Hymenoptera and the soldiers among the Termitina, are also found, as would be expected, among the fossils, at least through the whole series of the Tertiaries. The same may be said of other sexual characteristics, such as the stridulating organs of the Orthoptera, and of peculiarities of oviposition, as seen in the huge egg-capsules of an extinct Sialid of the early Tertiaries. The viviparity of the ancient Aphides is suggested, according to Buckton, by the appearance of one of the specimens from the Oligocene of Florissant, while some of the more extraordinary forms of parasitism are indicated at a time equally remote by the occurrence in amber of the triungulin larva of Meloe, already alluded to, and of a characteristic strepsipterous Insect; not only, too, are the present tribes of gall-making Insects abundant in the Tertiaries, but their galls as well have been found."
THE ORDER APTERA–DEFINITION–CHIEF CHARACTERISTICS–THYSANURA–CAMPODEA–JAPYX–MACHILIS–LEPISMA–DIVERSITY OF INTERNAL STRUCTURE IN THYSANURA–ECTOTROPHI AND ENTOTROPHI–COLLEMBOLA–LIPURIDAE–PODURIDAE–SMYNTHURIDAE–THE SPRING–THE VENTRAL TUBE–ABDOMINAL APPENDAGES–PROSTEMMATIC ORGAN–TRACHEAL SYSTEM–ANURIDA MARITIMA–COLLEMBOLA ON SNOW–LIFE-HISTORIES OF COLLEMBOLA–FOSSIL APTERA–APTERYGOGENEA–ANTIQUITY AND DISTRIBUTION OF CAMPODEA.
Order I. Aptera.
Small Insects with weak outer skin, destitute throughout life of wings or their rudiments, but with three pairs of legs; antennae large or moderate in size.
The above definition is the only one that can at present be framed to apply to all the Insects included in our Aptera. Unfortunately it is far from diagnostic, for it does not enable us to distinguish the Aptera from the larvae or young individuals of many Insects of other Orders. There are, however, certain characters existing in many species of Aptera that enable their possessors to be recognised with ease, though, as they are quite wanting in other members, they cannot correctly be included in a definition applying to the whole of the Order.
We are thus brought in view of two of the most important generalisations connected with the Aptera, viz. that these Insects in their external form remain throughout their life in a condition resembling the larval state of other Insects, and that they nevertheless exhibit extreme variety in structural characters.
The more important of the special characters alluded to above {181}as being possessed by some but not by all members of the Order are (1) a remarkable leaping apparatus, consisting of two elongate processes at the under side of the termination of the body; (2) a peculiar ventral tube, usually seen in the condition of a papilla with invaginated summit, and placed on the first abdominal segment (see Fig. 100, p. 194); (3) the scales covering the body; (4) the existence of abdominal appendages in the form of long cerci or processes at the termination of the body, or of short processes on the sides of the under surface of the abdominal segments.
Throughout the Order the general shape approximates to that of a larva; this is shown by the diagrammatic section of the body of Machilis (Fig. 90). There is a succession of rings differing little from one another, except so far as the head is concerned; even the division of thorax from abdomen is but little evident, and although in some of the forms the three thoracic segments may differ considerably among themselves, yet they never assume the consolidated form that they do to a greater or less extent in the imago stage of the other Orders. Fig. 90 shows the larva-like structure of the body, and also exhibits the inequalities in size between some of the dorsal and the corresponding ventral plates. This phenomenon is here displayed only to a small extent, so that the true relations of the dorsal and ventral plates can be readily detected; but in the higher Insects want of correspondence of this kind may be much more extensive.
The respiratory system is in many of these Insects very inferior in development, and may even be, so far as tracheae and spiracles are concerned, entirely absent, but in other members of the Aptera it is well developed. In the other internal organs there is also great variety, as there is in the external structure.
A brief explanation as to the term Aptera, which we have adopted as the name of this Order, is necessary. This name was used by Linnaeus for our Insects, but as he associated with them various other heterogeneous forms which were afterwards separated, his "Aptera" became completely broken up and ceased {182}to be recognised as an Order of Insects. The term was, however, revived by Haeckel and Balfour several years since, and applied quite properly to the Insects we have in view. Subsequently Packard and Brauer, recognising the claims of these Insects to an isolated position, proposed for them the names Synaptera and Apterygogenea, and Packard has also used the term Cinura. There is, however, clearly an advantage in retaining the termination "ptera" for each of the Orders of Insects; and as the fact that "Aptera" of Linnaeus included many Insects is not a sufficient reason for refusing to apply the term to a portion of the forms he used it for, we may, it is clear, make use of the Linnaean name with propriety, it being explicitly stated that the Order does not include by any means all the apterous forms of Insects.
The Order includes two sub-orders, viz. (1) Thysanura, in which the hind body (abdomen) is composed of ten segments, and there is no ventral tube on its first segment; and (2) Collembola, in which the hind body consists of not more than six segments, the first of which is furnished beneath with a peculiar tube or papilla.
Thysanura.
Our knowledge of this important sub-order has been recently much increased by the works of Grassi[115] and Oudemans.[116] Very little is known, however, of the extra-European forms, there being great difficulties in the way of collecting and preserving specimens of these Insects in such a way as to render them available for study and accurate comparison. Grassi and Rovelli[117] recognise four families among the few European species of Thysanura, viz. Campodeidae, Japygidae, Machilidae, Lepismidae. Campodeidae is perhaps limited to a single species, only one having been satisfactorily established, though several descriptions have been made of what are supposed to be other species.
This Insect (Campodea staphylinus) is, so far as external form goes, well known, from its having been figured in many works on natural history on account of its having been supposed to be {183}the nearest living representative of a primitive or ancestral Insect. The creature itself is but little known even to entomologists, although it is one of the commonest of Insects over a large part of Europe. It is numerous in the gardens and fields about London and Cambridge, and abounds in damp decaying wood in the New Forest; if there be only one species, it must possess an extraordinary capacity for adapting itself to extremes of climate, as we have found it at midsummer near the shores of the Mediterranean in company with the subtropical white ants, and within a day or two of the same time noticed it to be abundant on the actual summit of Mount Canigou, one of the higher Pyrenees, where the conditions were almost arctic, and it was nearly the only Insect to be found. The species is said to exist also in North America and in East India. It is a fragile, soft Insect of white colour, bending itself freely to either side like a Myriapod; the legs are rather long, the antennae are long and delicate, and the two processes, or cerci, at the other extremity of the body are remarkably similar to antennae. It has no eyes and shuns the light, disappearing very quickly in the earth after it has been exposed. If placed in a glass tube it usually dies speedily, and is so extremely delicate that it is difficult to pick it up even with a camel's hair brush without breaking it; so that we may fear it to be almost hopeless to get enough specimens from different parts of the world to learn what differences may exist amongst the individuals of this so-called primitive Insect. Meinert, a very able entomologist, considers that there is really more than one species of Campodea.
Campodeidae as a family may be briefly defined as Thysanura with the trophi buried in the head and with the body terminated by antenna-like processes. We shall consider some of the anatomical peculiarities of this interesting Insect after we have {184}briefly reviewed some of the external characters of the other Thysanura.
The second family (Japygidae) consists of one genus Japyx, of which there are, no doubt, several different species in various parts of the world, such having already been detected in tropical Africa, in Malasia, and in Mexico, as well as in Madeira and Europe. The commoner species of the latter continent, Japyx solifugus, lives in moss or in shady places on the edges of woods. It possesses a great resemblance to a newly-hatched earwig, and the writer has found it in France under a stone in company with a number of the tiny creatures it was so much like. This species has been found as far north as Paris, but has not been met with in Britain. The family Japygidae is, like the Campodeidae, entotrophous, and is distinguished by the body being terminated behind by a pair of forceps instead of antennary organs.
The other two families of Thysanura, Machilidae and Lepismidae, are ectotrophous—that is, the parts of the mouth are not buried in the head, but are arranged in the fashion usual in mandibulate Insects.
Only one genus of Machilidae is known, but it is no doubt very numerous in species, and probably is distributed over most of the globe. Machilis maritima is common in some places on the coast of England. Another species (M. polypoda) occurs amongst dead leaves in the New Forest, and we have also observed a species of the genus under the loose stones that frequently form the tops of the "dykes" or piled walls in Scotland. In more southern Europe species of Machilis are commonly met with on the perpendicular faces of very large stones or rocks, over which they glide with wonderful facility. The scales on the bodies of these rock-inhabiting species form pretty patterns, but are detached with such facility that it is almost impossible to obtain specimens in satisfactory condition for examination.
In Machilidae the dorsal plates of the hind body are reflexed to the under surface so as to form an imbrication covering the sides of the ventral plates, and the eyes are largely developed; by which characters the family is distinguished from the Lepismidae. The pair of large compound eyes (Fig. 92, O) is a remarkable feature, being indeed unique in the Aptera. The structures (o, o′) that Oudemans considers to be simple eyes have, in external appearance, a resemblance to the fenestrae of the {185}Blattidae; Grassi states, however, that not only are they eyes, but that they are of almost unique structure, being, in fact, intermediate between simple and compound eyes.
The mode of development of the compound eyes of Machilis is of considerable interest, but unfortunately very little is known about it, even the period at which the eyes appear being uncertain. Judging from analogy with the Orthoptera, we should suppose them to be present when the Insect leaves the egg, and Oudemans apparently considers this to be the case, but Bolivar states[118] that in the early stages of Machilis the eyes are only simple eyes; these being replaced by compound eyes in the later life. The writer has observed very young individuals of Machilis polypoda, and found the eyes to be evidently compound.
Fig. 92.—Head of Machilis maritima (after Oudemans): A, base of antenna; C, clypeus; F, vertex; P, fold; O, eye; o, o′, supposed simple eye; M, mandible; m, maxilla; L, upper lip; l, lower lip; T, portion of maxillary palp; t, of labial palp. × 20.
The remaining family of Thysanura, the Lepismidae, is in certain respects the most highly developed of the Order. The covering of scales found on the body is very remarkable in some of the species, especially in the genus Lepisma (Fig. 93, L. cincta); the thoracic segments are different from one another {186}and from those of the abdomen, and the tracheal system is more highly developed than it is in the Machilidae. Several genera are known, but only two members of the family have yet been detected in Britain. One of them (Lepisma saccharina), occurs only in houses, and is sometimes called the silver fish; it is, when full grown, less than half an inch long, and is covered with scales that give it a feebly metallic lustre. Like the other Thysanura, its movements are very perfect. It is said that it is occasionally injurious by nibbling paper, but the writer's observations lead him to doubt this; its usual food is doubtless farinaceous or saccharine matter. Thermobia furnorum, our other British Lepismid, has only recently been discovered; it is found in bakehouses at Cambridge and elsewhere. The bakers call these Insects fire-brats, apparently considering them to be fond of heat.
Much valuable information as to the anatomy of Thysanura has been obtained by Grassi and Oudemans, and is of great interest. Taking four genera, viz. Campodea, Japyx, Machilis, and Lepisma, to represent the four families constituting the sub-order, we will briefly enumerate some of the more remarkable of the characters of their internal anatomy. Campodea has a very inferior development of the tracheal system; there are three pairs of spiracles, which are situate on the thoracic region; the tracheae connected with each spiracle remain distinct, not uniting with those coming from another spiracle; there are thus six separate small tracheal systems, three on each side of the body. Japyx solifugus has eleven pairs of spiracles, of which four are thoracic; the tracheae are united into one system on each side by means of lateral tubes; thus there are two extensive tracheal systems situate one on each side of the body, there being a single transverse tube, placed near the posterior extremity, uniting the two lateral systems. In Machilis there are nine pairs of stigmata, two of them thoracic, seven abdominal; the tracheae from each spiracle remain unconnected, so that there are eighteen separate tracheal systems, some of which are considerably more developed than others. The Lepismidae have ten pairs of stigmata, and the tracheae connected with them are completely united into one system by longitudinal and transverse tubes. Besides these differences there are others, of considerable importance, in the position of the stigmata.
All the Thysanura possess salivary glands. In Campodea there are about sixteen extremely short Malpighian tubules, or perhaps glands representing these organs; Japyx is destitute of these structures; Machilis maritima has twenty elongate tubules; in Lepisma also they are long, and apparently vary in number from four to eight in different species. The proportions of the three divisions of the alimentary canal differ extremely; there is a very large proventriculus in Lepisma, but not in the other families; coecal diverticula are present on the anterior part of the true stomach in Machilis and in Lepisma, but are wanting in Campodea and in Japyx.
The dorsal vessel seems not to present any great differences in the sub-order. Grassi says there are no alary muscles present, but Oudemans describes them as existing in Machilis, but as being excessively delicate.
The ventral chain of nerve-ganglia consists in Campodea of one cephalic ganglion, one sub-oesophageal (which clearly belongs to the ventral series of ganglia), three thoracic, and seven abdominal. In the other families there are eight instead of seven abdominal ganglia.
The structure of the internal sexual organs is very remarkable in the Thysanura. In Campodea there is one extremely large, simple tube on each side of the body. In Japyx there are seven small tubes on each side, placed one in each of the successive abdominal segments, and opening into a common duct. In Machilis there are also seven tubes opening into a common duct, but the arrangement is no longer a distinctly segmental one. In Lepisma there are five egg-tubes on each side, the arrangement being segmental in the young state but not in the adult condition. In Campodea nutrient cells alternate with the eggs in the tubes, but this is not the case in the other families. Fig. 94 shows the ovaries in three of the Thysanura; in the drawing representing this part in Machilis (C), one of the two ovaries is cut away for the sake of clearness.
The male organs in Campodea are very similar in size and arrangement to the ovaries, there being a single large tube or sac and a short vas deferens on each side of the body. In Japyx there is a sac on each side, but it is rendered double by a coecum at its base, and there are long and tortuous vasa deferentia. In Lepisma there are three pairs of coeca on each {188}side, segmentally placed and opening into a common duct. In Machilis there are three retort-shaped sacs on each side opening near one another into a common duct, the vasa deferentia are elongate, and are very curiously formed, being each double for a considerable length, and the separated portions connected at intervals by five transverse commissural ducts.
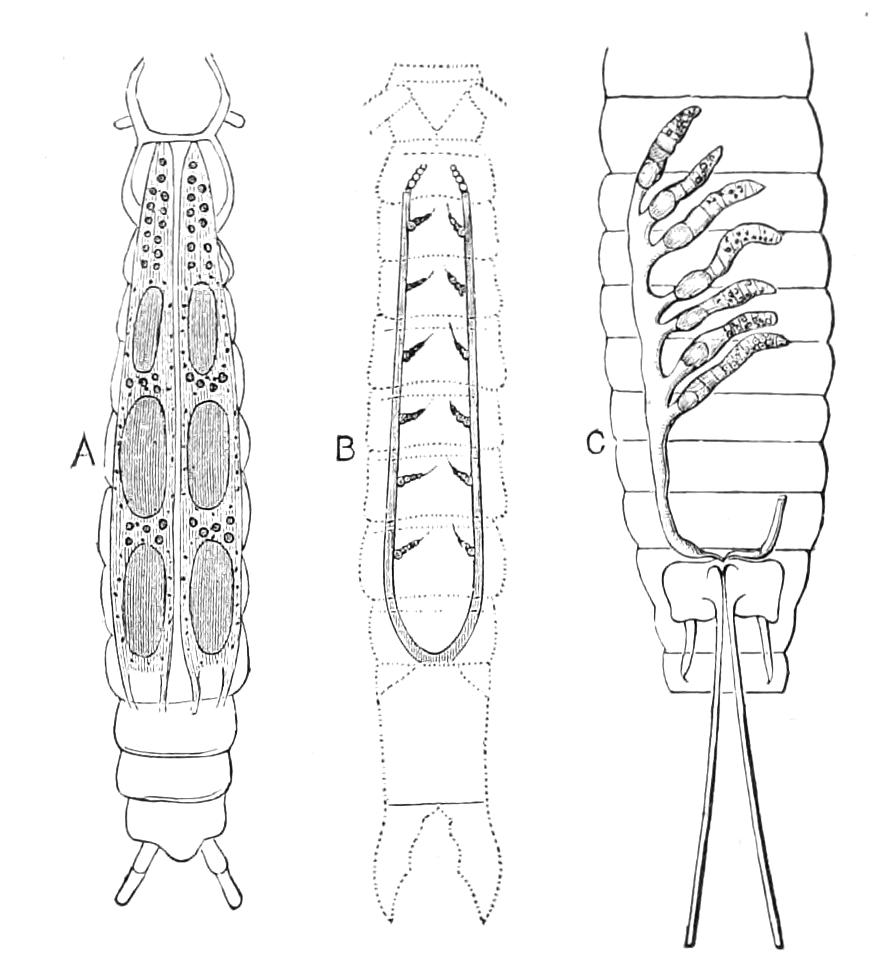
Fig. 94.—Ovaries of Thysanura: A, of Campodea; B, of Japyx; C, of Machilis. (After Grassi and Oudemans.)
One of the characteristic features of Insect structure is the restriction of articulated legs to the thoracic region. In the Thysanura there exist appendages occupying a position on the hind body somewhat similar to that of the legs on the thorax. These appendages are quite small bodies, and are placed at the hind margins of the ventral plates of the abdomen, one near each side; they are connected by a simple joint to the sternite and are provided with muscles. They are found in Campodea on segments 2 to 7; in Lepisma on 8 and 9, in the allied Nicoletia on 2 to 9; in Japyx on 1 to 7, being, however, more rudimentary than they are in Campodea. In Machilis they attain perhaps their greatest development and exist on segments 2 to 9; moreover, in this genus such appendages occur also on the coxae of the second and third pairs of thoracic legs. Oudemans thinks they help to support the abdomen, and that they also assist in leaping; Grassi considers that they are supporting agents to some extent, but that they are essentially tactile organs. He calls them false legs "Pseudozampe."
Still more remarkable and obscure in function are the vesicles found near the appendages; we figure a pair after Oudemans, showing them in the exserted state. In the retracted state the outer portion of the vesicles is withdrawn into the basal part P (Fig. 95), so that the vesicles are then only just visible, being {189}concealed by the ventral plate. The abdominal appendage is not retractile. In Machilis there are twenty-two of these vesicles, arranged either two or four on one ventral plate of the hind body. They are also present in Campodea, where there are six pairs. They are usually said to be absent in Japyx and in Lepisma, but Haase shows[119] that Japyx possess a pair placed behind the second ventral plate of the abdomen. The vesicles appear to be exserted by the entrance of blood into them, and to be retracted by muscular agency. Much difference of opinion prevails as to their function; it appears probable that they may be respiratory, as suggested by Oudemans.
The scales found on the bodies of the Ectotrophous Thysanura may be looked on as modified hairs, and are essentially similar to those of the Lepidoptera, and they drop off as readily as do those of the Lepidoptera.
Stummer-Traunfels, who has recently published[120] the results of his researches on the mouth-organs of Thysanura and Collembola, confirms the division of the Thysanura into Entotrophi and Ectotrophi, and considers that the Collembola agree with the former group. The German author therefore proposes to divide our Aptera, not into Thysanura and Collembola, but into Ectognathi and Entognathi, the former group consisting of Machilidae and Lepismidae, the latter of Campodeidae, Japygidae and the various families of Collembola. We think it far more natural, however, to retain the older division into Thysanura and Collembola.
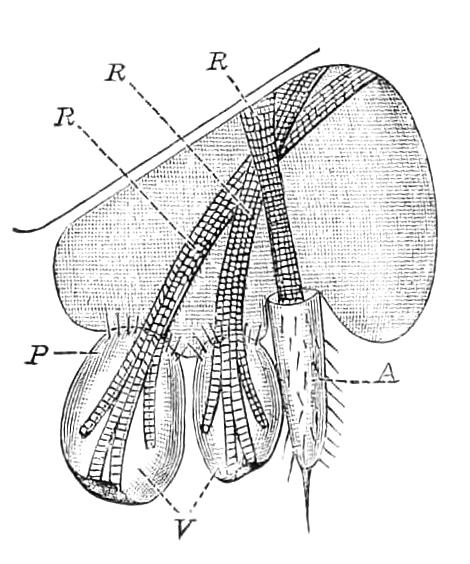
Fig. 95.—Abdominal appendage and exsertile vesicles of Machilis. A, appendage; V, vesicles protruded; P, basal portion; R, muscles, × 70.
Collembola.
The sub-order Collembola, which we have defined on p. 182, consists of small Insects, many of which possess the capacity of leaping, or springing suddenly, and when disturbed or alarmed naturally make use of this means of escaping. Their leaps, however, appear to be made quite at random, and very frequently do {190}not have the result of taking the creature into concealment, and in such circumstances they may be rapidly and frequently repeated until the Insect feels itself, as we may suppose, in a position of safety. Three families may be very readily distinguished, viz. (1) Lipuridae, in which no leaping apparatus is present; (2) Poduridae, a leaping apparatus exists near the extremity of the abdomen; the body is subcylindric and evidently segmented; (3) Smynthuridae, a leaping apparatus exists: the body is sub-globular with comparatively large head and abdomen, the intervening thoracic region being small; the segmentation of the body is obscure.
The study of the Collembola is much less advanced than that of the Thysanura, comparatively little having been added to our knowledge of the group since Lubbock's monograph of the British forms was published by the Ray Society in 1873. Why the Collembola should be neglected when the Thysanura attract so much attention is as inexplicable as many other fashions are.
The family Lipuridae consists of a few very small and obscure Insects of soft consistence. They move slowly, and, owing to the absence of any leaping power, attract attention less readily than the other Collembola do. Two genera are generally recognised, and they should probably form separate families; indeed, in Lubbock's arrangement they do so. In one of the genera (Anoura) the mouth is very imperfect, no mandibles or maxillae having been detected, while in the other genus (Lipura) these organs exist.
In the members of the family Poduridae, including the Degeeriidae of Lubbock, a saltatory apparatus is present in the form of appendages attached to the fifth abdominal segment (Degeeriides), or to the fourth (Podurides). These appendages are during life flexed beneath the body, but in dead specimens usually project backwards, having the appearance of a bifid tail. Poduridae are of elongate form, somewhat like small caterpillars, and are frequently prettily marked with variegate colours. Fig. 97 represents an arctic form closely allied to our native genus Isotoma.
The peculiar shape of the members of the Smynthuridae is sufficient for their identification. They possess a very convex abdomen, and very near to it a large head, the intervening chink being occupied by the small thorax. The segmentation of the body is not easily distinguished. Nicolet states that the thorax consists of three segments and the abdomen of the same number, and that when the Insect emerges from the egg these divisions can be perceived. In after life the posterior part of the thorax becomes amalgamated with the abdomen, so that it is difficult to trace the divisions, but there appears to be no information as to the manner in which this change occurs. Some of these minute Insects frequent trees and bushes, and their leaping powers are very perfect, so that it is difficult to capture them. The family includes both the Smynthuridae and the Papiriidae of Lubbock.
The two most characteristic organs of the Collembola are the spring and the ventral tube. The first of these is an elongate structure attached to the underside of the abdomen near its extremity, either on the penultimate or ante-penultimate segment. It consists of a basal part, and of two appendages attached thereto. It is carried under the Insect bent forwards, and is retained in this position by means of a catch which projects from the under surface of the third segment of the body, descending between the two branches of the spring, and passing under the extremity of its basal segment. It is considered that the spring is elastic, is flexed under the body by muscular action, and, being retained in this position {192}of restraint by the catch, when the latter is removed the spring extends by reason of its elasticity, and the leap is thus executed. Whether this is really the exact method of leaping is, however, doubtful, for Lubbock says that the catch "only exists in certain genera"; while in its structure it does not appear to be well calculated to retain in position an organ that by virtue of its elasticity is constantly exerting a considerable force.
Fig. 99.—Smynthurus fuscus, with exsertile vesicle (a) protruded from ventral tube; b, the spring extended.
The ventral tube is an anomalous and enigmatic structure. In the lower forms, such as Lipura or Anurida, it consists merely of a papilla (Fig. 100, A, a) more or less divided by fissure into two parts. In the Smynthuridae it is more highly developed, and protects two long delicate tubes that are capable of being protruded, as shown in the outline profile of Smynthurus fuscus (Fig. 99), which is taken from specimens preserved in balsam by Mr. J. J. Lister. The nature and use of this ventral tube have given rise to much discussion. Lubbock considered, and others have agreed with him, that it serves to attach the Insect to bodies to which it may be desirable the Insect should, when in the perpendicular position, adhere. Reuter[121] assigns a quite different function to this singular structure. He states that the hairs of the body are hygroscopic, and that the peculiar claws of the Insect having collected the moisture from the hairs, the ventral tube becomes the means of introducing the liquid into the body. These Insects possess, however, a mouth, and there seems to be no reason why a complex apparatus should be required in addition to it for so simple a purpose as the introduction of moisture to the interior of the body. Haase finds[122] that Collembola can crawl on glass without the aid of the ventral tube; he considers its function to be physiological, and that it may probably be respiratory as it has been suggested is the case with the vesicles of Thysanura. The function of the ventral tube is certainly not yet satisfactorily elucidated. The vesicles contained in it are said to be extruded by blood-pressure, and withdrawn by muscular action in a manner similar to that which we have described as occurring {193}in the case of the exsertile vesicles of the Thysanura. The processes in Smynthurus bear glandular structures at their extremities. It has been suggested that the ventral tube of Collembola is the homologue of a pair of ventral appendages. The term Collophore has been applied to it somewhat prematurely, seeing the doubt that still exists as to its function.
Some of the Collembola possess a very curious structure called the prostemmatic or ante-ocular organ; its nature and function have been very inadequately investigated. The ocular organs of the Collembola consist, when they are present, of isolated ocelli placed at the sides of the head like the corresponding organs of caterpillars; the prostemmate is placed slightly in front of the group of ocelli, and has a concentric arrangement of its parts, reminding one somewhat of the compound eyes of the higher Insects. This structure is represented in Fig. 100, B, C; it is said by Sir John Lubbock to be present in some of the Lipuridae that have no ocelli, and he therefore prefers to speak of it as the "post-antennal" organ.
A very characteristic feature in the Collembola is the slight development of the tracheal system. Although writers are far from being in accord as to details, it seems that stigmata and tracheae are usually absent. In Smynthurus there are, however, according to Lubbock,—whose statement is confirmed by Meinert and Tullberg,—a pair of stigmata situate on the head below the antennae, and from these there extends a tracheal system throughout the body. Such a position for stigmata is almost, if not quite unique in Insects; Grassi, however, seems to have found something of the kind existing in the embryo of the bee.
At present only a small number of species of the Order Aptera are known; Lubbock recognised about sixty British species, and Finot sixty-five as found in France. The North American forms have not received so much attention as the European, and the Aptera of other countries, though they are probably everywhere fairly numerous, are scarcely known at all. A few have been described from the Indo-Malayan region and some from Chili, and the writer has seen species from the West Indian and Sandwich Islands. All the exotic forms as yet detected are very similar to those of Europe.
The Thysanura are probably not very numerous in species, and appear to be in general intolerant of cold. With the Collembola {194}the reverse is the case. They are excessively numerous in individuals; they are found nearly everywhere on the surface of the ground in climatic conditions like those of our country, while no less than sixteen species have been found in Nova Zembla and one each in Kerguelen and South Georgia. One species, if not more, of Podura, lives on the surface of stagnant waters, on which the minute creatures may frequently be seen leaping about in great numbers after being disturbed.
In 1874 the plain of Gennevilliers in France was copiously irrigated; in the following year the soil was still very damp, and there existed numerous pools of stagnant water, on the surface of which Podura aquatica was developed in such prodigious quantity as to excite the astonishment of the inhabitants of the region.
Accounts have been frequently given of the occurrence on snow and glaciers of Insects spoken of as snow-fleas, or snow-worms. These mostly relate to Poduridae, which are sometimes found in countless number in such situations. The reason for this is not well understood. According to F. Löw,[123] on the 17th of March at St. Jacob in Carinthia, Parson Kaiser observed, on the occurrence of the first thaw-weather, enormous numbers of a Podura (? Achorutes murorum) on the surface of the snow for an extent of about half a mile, the snow being rendered black in appearance by them; eleven days afterwards they were found in diminished numbers on the snow, but in large quantity on the water left by its melting. This account suggests that the occurrence of the Insects on the snow was merely an incident during their passage from the land, where they had been hibernating, to the surface of the water.
One little member of the Lipuridae, Anurida maritima (Lipura maritima of Lubbock), has the habit, very unusual for an Insect, of frequenting salt water. It lives amongst the rocks on the shores of the English Channel, between high and low tide-marks. Its habits have been to some extent observed by Laboulbène[124] and Moniez[125]; it appears to be gregarious, and when the tide is high, to shelter itself against the commotions of the water in chinks of the rocks and other positions of advantage. When the tide is out the Insects apparently delight to {195}congregate in masses on the surface of the rock pools. This Anurida can endure prolonged immersion; but both the observers we are quoting say that it is, when submerged, usually completely covered with a coat of air so that the water does not touch it. The little creature can, however, it would appear, subsist for some time in the pools of salt water, even when it is not surrounded by its customary protecting envelope of the more congenial element. Its food is said, on very slender evidence, to consist of the remains of small marine animals, such as Molluscs. We reproduce some of Laboulbène's figures (Fig. 100); the under-surface shows at a the divided papilla of the ventral tube; B, C represent the peculiar prostemmatic organ, alluded to on p. 193, in its mature and immature states.
Fig. 100.—Anurida maritima: A, under-surface; a, papilla of ventral tube; B, prostemmatic organ of young; C, of adult. (After Laboulbène.)
Very little information exists as to the life-history of the Aptera; as for their food, it is generally considered to consist of refuse vegetable or animal matter. It is usual to say that they are completely destitute of metamorphosis, but Templeton says of Lepisma niveo-fasciata that "the young differ so much from the mature Insect that I took them at first for a distinct species; the thoracic plates are proportionately less broad, and the first is devoid of the white marginal band." As regards the moults, it would appear that in this, as in so many other points, great diversity prevails, Grassi stating that in Campodea there is a single fragmentary casting of the skin; and Sommer informing us that in Macrotoma plumbea the moults are not only numerous, but continue, after the creature has attained its full growth, throughout life.
A very marked feature of the Aptera is their intolerance of a dry atmosphere. Although Campodea can exist under very diverse conditions, it dies very soon after being placed in a dry closed tube; and the same susceptibility appears to be shared by all the other members of the Order, though it is not so extreme in all; possibly it may be due to some peculiarity in the structure {196}of the integument. So far as tolerance of heat and cold goes, the Aptera can apparently exist in any climate, for though some of the species extend to the Arctic regions, others are peculiar to the tropics.
Thysanura are recorded by Klebs and Scudder as occurring commonly in amber; the latter author has described a fossil, supposed to be a Lepisma, found in the Tertiary deposits at Florissant. Scudder has also described another fossil, likewise from Florissant, which he considers to form a special sub-order of Thysanura—Ballostoma—but it is extremely doubtful whether this anomalous creature should be assigned to the Order at all. A still older fossil, Dasyleptus lucasii Brongniart, from the Carboniferous strata in France, is considered to belong to the Order Aptera, but it must be admitted there is some doubt on this point.
The interest aroused in the minds of naturalists by the comparatively simple forms of these purely wingless and therefore anomalous Insects has been accompanied by much discussion as to their relations to other Insects, and as to whether they are really primitive forms, or whether they may perhaps be degenerate descendants from some less unusual states of Insect-life. Mayer and Brauer dissociated our Aptera entirely from other Insects, and proposed to consider the Hexapoda as being composed of two groups—(1) the Apterygogenea, consisting of the few species we have been specially considering; and (2) the Pterygogenea, including all the rest of the immense crowd of Insect forms. They were not, however, able to accompany their proposed division by any satisfactory characters of distinction, and the subsequent progress of knowledge has not supported their view, all the best investigators having found it necessary to recognise the extremely intimate relations of these Insects with the Orthoptera. Meinert thought that Lepisma must be included in the Orthoptera; Grassi proposes to consider the Thysanura as a distinct division of Orthoptera; and Oudemans recognises the close relations existing between Machilis and Orthoptera proper. Finot includes the Aptera in his Orthoptères de la France, and a species of Japyx has actually been described by a competent entomologist as an apterous earwig. At present, therefore, we must conclude that no good distinction has been found to justify the separation of the Aptera from all other Insects.
The taxonomy of the Collembola has not yet been adequately treated, and it is possible that more grounds will be found for separating them as a distinct Order from the Thysanura,—a course that was advocated by Lubbock,—than exist for dividing these latter from the Orthoptera proper. There are apparently no grounds for considering the Aptera to be degenerate Insects, and we may adopt the view of Grassi, that they are primitive, or rather little evolved forms. It must be admitted that there are not at present any sufficient reasons for considering these Insects to be "ancient" or "ancestral." The vague general resemblance of Campodea to many young Insects of very different kinds is clearly the correlative of its simple form, and is no more proof of actual ancestry to them than their resemblances inter se are proofs of ancestry to one another. But even if deprived of its claim to antiquity and to ancestral honours, it must be admitted that Campodea is an interesting creature. In its structure one of the most fragile of organisms, with a very feeble respiratory system, inadequate organs of sense, only one pair of ovarian tubes, very imperfect mouth-organs, and a simple alimentary canal, it nevertheless flourishes while highly-endowed Insects become extinct. In the suburban gardens of London, on the shores of the Mediterranean, on the summits of the higher Pyrenees, in North America even it is said in the caves of Kentucky, and in India, Campodea is at home, and will probably always be with us.
ORTHOPTERA—FORFICULIDAE, EARWIGS—HEMIMERIDAE
Order II.—Orthoptera.
Insects with the mouth parts conspicuous, formed for biting, the four palpi very distinct, the lower lip longitudinally divided in the middle. The tegmina (mesothoracic wings), of parchment-like consistence, in repose closed on the back of the Insect so as to protect it. The metathoracic wings, of more delicate consistence, ample, furnished with radiating or divergent nervures starting from the point of articulation, and with short cross nervules forming a sort of network; in repose collapsing like a fan, and more or less completely covered by the tegmina (except in certain Phasmidae, where, though the wings are ample, the tegmina are minute, so that the wings are uncovered). In a few forms (winged Forficulidae and some Blattidae) the metathoracic wings are, in addition to the longitudinal folding, contracted by means of one or two transverse folds. The mode of growth of each individual is a gradual increase of size, without any abrupt change of form, except that the wings are only fully developed in the final condition. There is no special pupal instar. Species in which the wings are absent or rudimentary are numerous.
The Orthoptera are Insects of comparatively large size. The Order, indeed, includes the largest of existing Insects, while none are so minute as many of the members of the other Orders are; three millimetres is the least length known for an Orthopterous Insect, and there are very few so small, though this is ten times the length of the smallest beetle. The Order includes earwigs, cockroaches, soothsayers or praying-insects, stick- and leaf-insects, grasshoppers, locusts, green grasshoppers, and crickets.
The changes of form that accompany the growth of the individual are much less abrupt and conspicuous than they are in most other Insects. The metamorphosis is therefore called Paurometabolous. It has been supposed by some naturalists that Orthoptera go through a larger portion of their development in the egg than other Insects do. This does not clearly appear to be the case, though it seems that there are distinctions of a general character in the embryology; the period of development in the egg is prolonged, and the yolk is said by Wheeler[126] to be more than usually abundant in comparison with the size of the young embryo. The embryonic development may in tropical countries be accomplished in three weeks (see Mantidae), but in countries where winter supervenes, the period may in some species be extended over seven or eight months.
The external features of the post-embryonic development—a term that is more convenient in connexion with Orthoptera than metamorphosis—are as follows: the wings are never present when the Insect is first hatched, but appear subsequently, and increase in size at the moults; the form and proportions of the segments of the body—especially of the thorax—undergo much change; an alteration of colour occurs at some of the moults, and the integument becomes harder in the adult condition. Neither the development of the internal organs, nor the physiological processes by which the changes of external form are effected, appear to have been studied to any great extent.
Many of the Orthoptera do not possess wings fit for flight, and some species even in the adult state have no trace whatever of such organs. Flight, indeed, appears to be of minor importance in the Order; in many cases where the wings exist they are purely musical organs, and are not of any use for flight. The apterous and the flightless conditions are not confined to one division of the Order, but are found in all the families and in many of their subdivisions. As the front pair of wings in Orthoptera do not really carry out the function of flight, and as they differ in several particulars from the hinder pair, or true wings, it is usual to call them tegmina. The musical powers of the Orthoptera are confined to the saltatorial group of families.
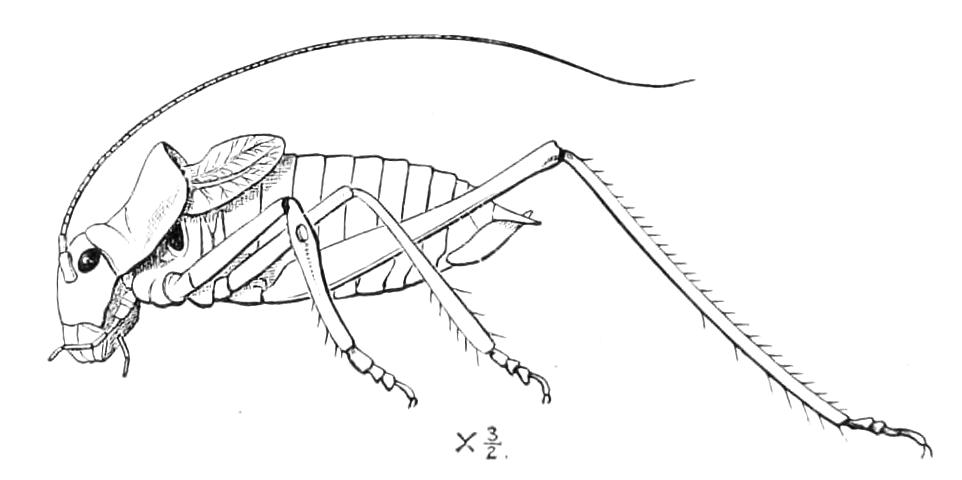
Fig. 101.—Poecilimon affinis ♂. Bulgaria. Alar organs serving only as musical organs. The ear on front tibia and aural orifice of prothorax are well shown.
The Cursoria are dumb or nearly so; it is a remarkable fact that also in this latter division the alar organs, though frequently present, have but little value for flight, and are in some cases devoted to what we may call purposes of ornament or concealment. This is specially the case in the Phasmidae and Mantidae, where the effectiveness of colour and pattern of these parts becomes truly astonishing. The tegmina frequently exhibit an extraordinary resemblance to vegetable structures, and this appearance is not superficial, for it may be seen that the nervures of the wings in their disposition and appearance resemble almost exactly the ribs of leaves. One of the most remarkable of the features of Orthoptera is that a great difference frequently exists between the colours of the tegmina and of the wings, i.e. the front and hind wings; the latter are concealed in the condition of repose, but when activity is entered on and they are displayed, the individual becomes in appearance a totally different creature. In some cases, contrary to what usually occurs in Insects, it is the female that is most remarkable; the male in Mantidae and Phasmidae being frequently a creature of quite inferior appearance and power in comparison with his consort. The musical powers of the saltatorial Orthoptera are, however, specially characteristic of the male sex. There is evidence that these powers are of great importance to the creatures, though in what way is far from clear. Some parts of the structures of the body are in many of these musical species clearly dominated by the musical organs, and are apparently specially directed to securing their efficiency. We find in some Locustidae that the tegmina are nothing but sound-producing instruments, while the pronotum is prolonged to form a hood that protects them without encumbering their action. In the males of the Pneumorides, where the phonetic organ is situated on the abdomen, this part of the body is inflated and tense, no doubt with the result of increasing the volume and quality of the sound. In the genus Methone (Fig. 185) we find a grasshopper whose great hind legs have no saltatorial function, and but little power of locomotion, but act as parts of a sound-producing {201}instrument, and as agents for protecting some parts of the body in repose. Further particulars of these cases must be looked for in our accounts of the different groups.
The eggs of many Orthoptera are deposited in capsules or cases; these capsules may contain only one egg, or a great many.
The Order includes many species of Insects, though in Britain it is poorly represented: we have only about forty species, and this small number includes some that are naturalised. Only a few of the forty extend their range to Scotland. A revision of the species found in Britain has recently been made by Mr. Eland Shaw.[127] In continental Europe, especially in the south, the species become more numerous; about 500 are known as inhabitants of geographical Europe. In countries where the face of nature has been less transformed by the operations of man, and especially in the tropical parts of the world, Orthoptera are much more abundant.
The lowest number at which the species now existing on the surface of the earth can be estimated is 10,000. This, however, is probably far under the mark, for the smaller and more obscure species of Orthoptera have never been thoroughly collected in any tropical continental region, while new forms of even the largest size are still frequently discovered in the tropics.
We shall treat the Order as composed of eight families:—
| Series, Cursoria: hind legs but little different from the others. |  |
1. Forficulidae—Tegmina short, wings complexly folded; body armed at the extremity with strong forceps. |
| 2. Hemimeridae—Apterous: head exserted, constricted behind. | ||
| 3. Blattidae—Coxae of the legs large, exserted, protecting the lower part of the body. | ||
| 4. Mantidae—Front legs very large, raptorial, armed with spines. | ||
| 5. Phasmidae—Mesothorax large as compared with the prothorax. | ||
| Series, Saltatoria: hind legs elongate, formed for leaping, their femora usually thickened. |  |
6. Acridiidae—Antennae short, not setaceous, of not more than 30 joints, tarsi three-jointed. |
| 7. Locustidae—Antennae very long, setaceous, composed of a large number of joints, tarsi four-jointed. | ||
| 8. Gryllidae—Antennae very long, setaceous, tarsi two- or three-jointed. |
The first five of these subdivisions are amongst the most distinct of any that exist in the Insecta, there being no connecting links between them. The three groups forming the {202}Saltatoria are much more intimately allied, and should, taken together, probably have only the same taxonomic value as any one of the other five groups.
Owing partly to the inherent difficulties of the subject, and partly to the fragmentary manner in which it has been treated by systematists, it has been impossible till recently to form any clear idea of the classification of Orthoptera. During the last twenty years Henri de Saussure and Brunner von Wattenwyl have greatly elucidated this subject. The latter of these two distinguished naturalists has recently published[128] a revision of the system of Orthoptera, which will be of great assistance to those who may wish to study these Insects. We therefore reproduce from it the characters of the tribes, placing the portion relating to each family at the end of our sketch thereof.
Fam. I. Forficulidae—Earwigs.
(DERMAPTERA OR DERMATOPTERA OF BRAUER AND OTHERS)
Insects of elongate form, with an imbricate arrangement of the segments of the body; bearing at the posterior extremity a pair of callipers or more distorted instruments. The hind wings (when present) folded in a complex manner, and covered, except at their tips, by a pair of short wing-covers (tegmina), of a leather-like consistence. Wingless forms are very numerous. The young is very similar to the adult.
Although earwigs are said to be rare in most parts of the world, yet in Europe no Insect is better known than Forficula auricularia, the common earwig, it being very abundant even in gardens and cultivated places. In certain seasons it not unfrequently enters our houses, in which case it too often falls a {203}victim to prejudices that have very little to justify them. This Insect is a good type of the winged earwigs. In the parts of the mouth it exhibits the structures usual in the Orthoptera; there is a large labrum, a pair of maxillae, each provided with two lobes and a palpus consisting of two very short basal joints and three longer joints beyond these; the mandibles are strong, with curvate pointed extremities; in the lower lip there is a ligula exposed in front of a very large mentum; it consists of two pieces, not joined together along the middle, but each bearing on its lateral edge a palpus with two elongate joints and a short basal one; this lip is completed by the lingua, which reposes on the upper face of the part, and completely overlaps and protects the chink left by the want of union along the middle line of the external parts of the lip. The antennae are elongate, filiform, and are borne very near the front of the exserted head. There are rather large facetted eyes, but no ocelli. The three segments of the thorax are distinct, the prothorax being quite free and capable of movement independent of the parts behind it: the meso- and meta-nota are covered by the tegmina and wings; these latter project slightly from underneath the former in the shape of small slips, that are often of rather lighter colour; the wing-covers are short, not extending beyond the insertion of the hind legs, and repose flat on the back, meeting together in a straight line along the middle. These peculiar flat, abbreviated wing-covers, with small slips (which are portions of the folded wings) projecting a little from underneath them, are distinctive marks of the winged Forficulidae.
The legs are inserted far from one another, the coxae being small; each sternum of the three thoracic segments projects backwards, forming a peculiar long, free fold, underlapping the front part of the following segment. The hind body or abdomen is elongate, and is formed of ten segments; the number readily visible being two less in the female than it is in the male. The segments are fitted together by a complex imbrication, which admits of great mobility and distension, while offering a remarkable power of resistance to external pressure: each segment is inserted far forward in the interior of that preceding it, and each also consists of separate upper and lower plates that much overlap where they meet at the sides (see Fig. 103). The body is always terminated by a pair of horny, pincer-like {204}processes, which are differently shaped according to the sex of the individual.
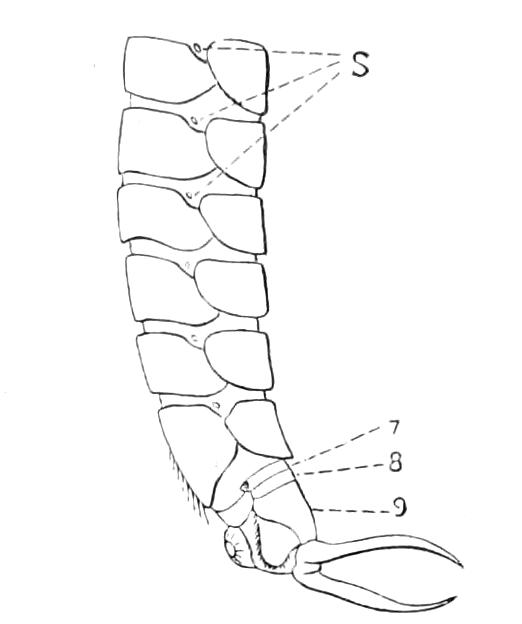
Fig. 103.—Lateral view of Forficula auricularia L. Female abdomen distended showing spiracles, S, and the small 8th and 9th dorsal plates (7 and 8 in Fig.).
The structure of the abdomen in the earwig has given rise to considerable discussion. In Fig. 103 we reproduce Westwood's diagram of it as seen fully distended in a female specimen; in this state the minute spiracles can be detected, though in the normal condition of the body they cannot be seen, being placed on the delicate membranes that connect the chitinous plates. Westwood's interpretation of the structure was not, however, quite correct, as the part which he considered to be the first dorsal plate is really the second; so that the segments numbered 7, 8, 9 in our figure are really 8, 9, 10. The common earwig is interesting as exhibiting, in an imperfect state, the union of the first dorsal plate of the abdomen with the thorax; a condition which is carried to so great an extent in the Hymenoptera as to quite obscure the nature of the parts, and which has consequently given rise to much perplexity and discussion. We represent this structure as seen in the common earwig in Fig. 104, where a represents the pronotum, b the mesonotum, c the metanotum, d the first, f the second abdominal segment; e being a delicate membrane of considerable size that intervenes between the two, and which is more exposed than are the corresponding membranes connecting the subsequent rings; a condition similar to that which is found in Cimbex, Cephus, and some other Hymenoptera.
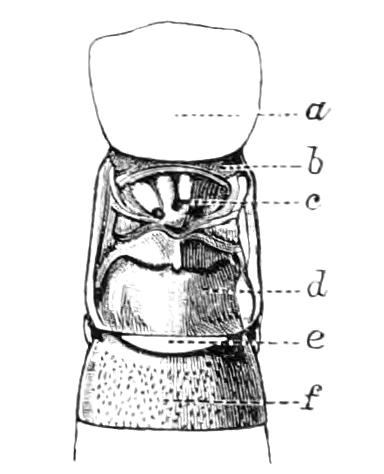
Fig. 104.—Dorsal portions of the middle segments of body of Forficula auricularia (tegmina and wings removed).
On the under surface of the abdomen of the earwig the full number of 10 plates cannot be superficially distinguished; but it is found by dissection that in the female the short eighth and ninth dorsal rings are joined on the ventral aspect by a delicate membrane, while the tenth ventral is of a less delicate {205}nature, and forms a triangular plate at the base of each half of the forceps. Between the branches of the forceps there is a perpendicular plate, the pygidium of Orthopterists, possibly the unpaired terminal portion of the body seen in some embryos, and called the telson. The pygidium is a separate sclerite, though it looks as if it were only a portion of the large tenth dorsal plate bent downwards, and in some descriptive works is erroneously described as being such.
Fig. 106.—Tegmina and wings (visible in part or invisible) of apterous earwigs. 1, Chelidura sp.; 2, Chelidura dilatata; 3, Anisolabis moesta; 4. A. maritima. a, First thoracic segment; b, second; c, third; d, basal portion of abdomen.
A very large number of species of Forficulidae have the organs of flight undeveloped. Fig. 105 represents Chelidura dilatata, an apterous form that is very common in the Eastern Pyrenees. The condition of the meso- and meta-nota—the parts from which the tegmina and wings are developed, and to which they are attached when present—is very remarkable in these forms, and exhibits much variety. In Fig. 106 we represent the conditions of these parts in a few apterous forms. The tegmina or the segment from which they are developed (b), are seen in the shape of a plate which may extend all across the middle and be undivided (No. 4); in which case the appearance indicates entire absence of the tegmina; these are, on the contrary, evidently present in the form of slips grafted one to each side of the second thoracic segment in Anisolabis (No. 3); or they may look like short broad slips extending all across the body, and marking off a piece frequently called a scutellum, but which is really the mesonotum (some species of Chelidura, as No. 2); or, again, they may be nearly free tegmina, somewhat similar to those of the winged forms; this is the case with some species of Chelidura, as represented by No. 1. This last figure is taken from a species from the Sierra Nevada, apparently undescribed, allied to C. bolivari.
In the cases we are considering no analogous structures exist on {206}the metanotum (the part of the body that in the winged forms bears the wings, and which is marked c in our diagrams, Fig. 106), so that the tegmina are to all appearance less rudimentary (or vestigial) than the wings. The metanotum forms a sort of flap, called by Fischer[129] "involucrum alarum"; he considered the part immediately behind this to be the metanotum; this piece is, however, no doubt really part of the abdomen (d in our Figure). This is apparently the view taken by Brunner.[130] The structure of these parts is important as bearing on the subject of the nature and origin of Insects' wings, a question to which no satisfactory answer has yet been given. The appearances we have remarked on are to some extent similar to the conditions existing in the immature state of the organs of flight in the common earwig (see Fig. 112, p. 212), but whether the varieties presented by the wingless forms have parallels in the immature conditions of the various winged forms is quite uncertain, the life-histories of earwigs being almost unknown.
Fig. 107.—Wing of Forficula auricularia. A, Wing expanded, explanation in text; B, wing folded and packed.
The developed wings of earwigs are worthy of attention, both as regards their actual structure and the manner in which they are folded up in repose. When expanded they have a shape curiously suggestive of the human ear. The chief parts of the wing, as shown in Fig. 107, A, are a, b, two portions of the horny piece that forms the scale which covers the more delicate parts of the wing when it is folded, and which, according to Brunner, represents the radial and ulnar fields of the wings of Acridiidae and Locustidae (see Fig. 167); c is the small apical field limited below by the vena dividens; d is the vena plicata which runs along the under side of the scale as far as the apical field, where it gives off the axillary nerves; e is a vena spuria, or adventitious vein such as exists in many other Orthoptera with delicate wings. On the front part of the scale, a, and on a different plane so that it is not shown in our figure, there is a very delicate small band which is supposed to {207}represent the marginal field of the wing of other Orthoptera. There are, however, grave difficulties in the way of accepting this view of the earwig's wing, amongst which we may mention the position of the vena dividens and its relation to the so-called radial and ulnar fields of the wing. The wings are remarkable for their delicacy; moreover, the way in which they fold up so as to be packed in the manner shown in B, Fig. 107, is very interesting, there being, in fact, no other Insects that fold up their wings in so complicated and compact a fashion as the earwigs do. The process is carried out somewhat as follows: the longer radii come a little nearer together, the delicate membrane between them falling into folds somewhat like those of a paper fan; a transverse fold, or turn-over, then occurs at the point where the radii, or axillary nerves, start from the vena plicata; then a second transverse fold, but in a reversed direction, occurs affecting the wing just close to the spots where the shorter radial nervures are dilated; then by a contraction close to the scale the whole series of complex folds and double are brought together and compressed.
It is quite a mystery why earwigs should fold their wings in this complex manner, and it is still more remarkable that the Insects very rarely use them. Indeed, though Forficula auricularia is scarcely surpassed in numbers by any British Insect, yet it is rarely seen on the wing; it is probable that the majority of the individuals of this species may never make use of their organs of flight or go through the complex process of unfolding and folding them. It should be remarked that no part of the delicate membranous expanse of the wing is exposed when the wings are packed in their position of repose; for the portion that projects from under the tegmina—and which, it will be remembered, is always present, for when wings exist in earwigs they are never entirely concealed by the tegmina—is, it is curious to note, of hard texture, and is frequently coloured and sculptured in harmony with the tegmen; in fact, one small part of the wing forms in colour and texture a most striking contrast to the rest of the organ, but agrees in these respects with the wing-covers. This condition is seen in Fig. 108, where B shows the sculpture of the tegmina t, and of the projecting tips of the wings w. There are numerous other instances in Orthoptera where one part of a wing or wing-case {208}is exposed and the other part concealed, and the exposed portion is totally different in colour and texture from the concealed portion.
The wings of earwigs are attached to the body in a very unusual manner; each wing is continued inwards on the upper surface of the metanotum, as if it were a layer of the integument meeting its fellow on the mesial line; the point of contact forming two angles just behind the metanotum.
Some writers have considered that the tegmina of earwigs are not the homologues of those of other Orthoptera, but are really tegulae (cf. Fig. 56, p. 103). We are not aware that any direct evidence has been produced in support of this view.
The pair of forceps with which the body is armed at its extremity forms another character almost peculiar to the earwigs, but which exists in the genus Japyx of the Thysanura. These forceps vary much in the different genera of the family; they sometimes attain a large size and assume very extraordinary and distorted shapes. They are occasionally used by the Insects as a means of completing the process of packing up the wings, but in many species it is not probable that they can be used for this purpose, because their great size and peculiarly distorted forms render them unsuitable for assisting in a delicate process of arrangement; they are, too, always present in the wingless forms of the family. Their importance to the creature is at present quite obscure; we can only compare them with the horns of Lamellicorn Coleoptera, which have hitherto proved inexplicable so far as utility is concerned. No doubt the callipers of the earwigs give them an imposing appearance, and may be of some little advantage on this account; they are not known to be used as offensive instruments for fighting, but they are occasionally brought into play for purposes of defence, the creatures using them for the infliction of nips, which, however, are by no means of a formidable character.
Fig. 108.—Anechura scabriuscula. Himalaya. A, Outline of the Insect; B, tegmina, t, and tips of wings, w, showing their similar sculpture.
These forceps are, in the case of the common earwig—and they have not been studied from this point of view in any other species—remarkable, because of the great variation in their development in the male, a character which again reminds us of the horns of Lamellicorn beetles: in the female they are comparatively invariable, as is also the case in the few species of Lamellicornia, which possess horned females. A and B in Figure 109 represent the forceps of different males of the common earwig, C showing those of the other sex. The subject of the variation of the male callipers of the earwig has been considered by Messrs. Bateson and Brindley,[131] who examined 1000 specimens captured on the same day on one of the Farne islands off the coast of Northumberland; 583 of these were mature males, and the pincers were found to vary in length from about 2½ mm. to 9 mm. (A and B in Fig. 109 represent two of the more extreme forms of this set of individuals.) Specimens of medium size were not, as it might perhaps have been expected they would be, the most common; there were, in fact, only about 12 individuals having the forceps of the medium length—4¾ to 5¼ mm., while there were no less than 90 individuals having forceps of a length of about 7 mm., and 120 with a length of from 2¾ to 3¼. Males with a medium large length of the organ and with a medium small length thereof were the most abundant, so that a sort of dimorphism was found to exist. Similar relations were detected in the length of the horns of the male of a Lamellicorn beetle examined by these gentlemen. In the case of the set of earwigs we have mentioned, very little variation existed in the length of the forceps in the female sex.
In many earwigs—including F. auricularia—there may be seen on each side of the dorsal aspect of the true fourth, or of the fourth and neighbouring segments of the hind body a small elevation, called by systematists a plica or fold, and on examination the fold will be found to possess a small orifice on its posterior aspect. These folds are shown in Figs. 105 and 108; {210}they have been made use of for purposes of classification, though no functional importance was attached to them. Meinert, however, discovered[132] that there are foetid glands in this situation, and Vosseler has recently shown[133] that the folds are connected with scent-glands, from which proceed, in all probability, the peculiar odour that is sometimes given off by the earwig. The forms destitute of the folds, e.g. Labidura, are considered to have no scent glands. There is a very peculiar series of smooth marks in the earwigs on the dorsal aspect of the abdominal segments, and these are present in the glandless forms as well as in the others.
The internal anatomy has been to some extent investigated by Dufour and Meinert. Dufour dissected F. auricularia and Labidura riparia, and found[134] that salivary glands exist in the latter Insect (called by him Forficula gigantea), though he was unable to discover them in the common earwig. According to Meinert,[135] there are, however, salivary glands affixed to the stipes of the maxillae in F. auricularia, while (in addition?) L. riparia possesses very elongate glands seated in the middle or posterior part of the breast. The alimentary canal is destitute of convolutions, but oesophagus, crop, and gizzard all exist, and the intestine behind the stomach consists of three divisions. The Malpighian tubes are numerous, 30 or 40, and elongate. The respiratory system is not highly developed. Earwigs—the European species at least—have, as already mentioned, very small powers of flight; the tracheal system is correspondingly small, and is destitute of the vesicular dilatations that are so remarkable in the migratory Locusts.
The three thoracic spiracles[136] are readily observed in living {211}individuals. There are seven pairs of abdominal spiracles, which, however, are very minute, and can only be found by distending the body as shown in Fig. 103. The ventral chain consists of nine ganglia (the sub-oesophageal centre is not alluded to by Dufour); the three thoracic are equidistant and rather small; the hindmost of the six abdominal ganglia is considerably larger than any one of the other five.
The ovaries of Labidura riparia and Forficula auricularia are extremely different. In L. riparia there are on each side five tubes, each terminating separately in an obliquely directed lateral part of the oviduct. In F. auricularia there is but one tube on each side, but it is covered by three longitudinal series of very short sub-sessile, grape-like bodies, each of the two tubes being much dilated behind the point where these bodies cease.
The testes in earwigs are peculiar and simple; they consist, on each side, of a pair of curvate tubular bodies, connected at their bases and prolonged outwards in the form of an elongate, slender vas deferens. The structures in the males of several species have been described at some length by Meinert,[137] who finds that in some species a double ejaculatory duct exists.
The young is similar to the adult in form; in the winged forms it is always easy to distinguish the adult by the full development of the wings, but in the wingless forms it can only be decided with certainty that a specimen is not adult by the softer and weaker condition of the integuments. Scarcely anything appears to be known as to the life-history, except a few observations that have been made on the common earwig; Camerano found[138] that this Insect has certainly three ecdyses, and possibly {212}an earlier one which he failed to notice, and his observation confirms the vague previous statement of Fischer. The eggs, in the neighbourhood of Turin, are deposited and hatched in the early spring; in one case they were laid on the 10th March, and the Insects issuing from them had completed their growth and were transformed into perfect Insects on the 22nd May. In the immature state the alar structures of the future imago may be detected. The tegmina-bearing sclerites, t, Fig. 112, look then somewhat like those of some of the apterous forms (Fig. 106) and, as shown in A and B, Fig. 112, do not differ greatly in the earlier and later stages. The wings, however, change much more than the tegmina do; at first (Fig. 112, A) there is but little difference between the two, though in the interior of the wing-flap some traces of a radiate arrangement can be seen, as shown at W in A, Fig. 112; in a subsequent condition the wing-pads are increased in size and are more divided, the appearance indicating that the wings themselves are present and packed about a centre, as shown in W of B, Fig. 112.
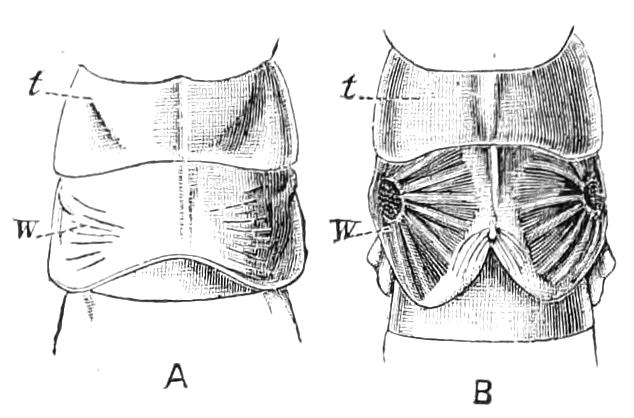
Fig. 112.—Notal plates from which the tegmina and wings of Forficula auricularia are developed in young, A, and more advanced, B, nymph.
In the young of the common earwig the number of joints[139] in the antennae increases with age. Camerano, l.c., says that before emergence from the egg there are apparently only 8 joints in the antennae, and Fischer states that the larvae of F. auricularia have at first only 8 antennal joints; later on 12 joints are commonly found, and, according to Bateson,[140] this number occasionally persists even in the adult individual. Meinert says[141] that the newly hatched Forficula has either 6 or 8 joints, and he adds that in the later portion of the preparatory stage the number is 12. Considerable discrepancy prevails in books as to the normal number of joints in the antennae of the adult F. auricularia, the statements varying from 13 to 15. The latter number may be set aside as erroneous, although it is, curiously {213}enough, the one given in the standard works of Fischer, Brunner, and Finot. Meinert gives without hesitation 14 as the number; Bateson, l.c., found that 14 joints occurred in 70 or 80 per cent of adult individuals, that 13 was not uncommon, that 12 or 11 occasionally occurred, and that the number may differ in the two antennae of the same individual. These variations, which seem at first sight very remarkable, may with probability be considered as due to the fact that in the young state the number of joints increases with age, and that the organs are so fragile that one or more of the joints is very frequently then lost, the loss being more or less completely repaired during the subsequent development. Thus a disturbing agency exists, so that the normal number of 14 joints is often departed from, though it appears to be really natural for this species. Bateson has also pointed out that when the normal number of articulations is not present, the relative proportions of joints 3 and 4 are much disturbed. It is, however, probable that the increase in number of the joints takes place by division of the third or third and fourth joints following previous growth thereof, as in Termitidae; so that the variations, as was suggested by Bateson, may be due to mutilation of the antennae, and consequent incompletion of the normal form of the parts from which the renovation takes place; growth preceding segmentation—in some cases the growth may be like that of the adult, while the segmentation remains more incomplete. In the young the forceps of the two sexes differ but slightly; the form of the abdominal rings is, on the contrary, according to Fischer, already different in the two sexes in the early stage.
The common earwig has a very bad reputation with gardeners, who consider it to be an injurious Insect, but it is probable that the little creature is sometimes made the scapegoat for damage done by other animals; it appears to be fond of sweets, for it often makes its way to the interior of fruits, and it no doubt nibbles the petals, or other delicate parts of flowers and vegetables. Camerano, however, states, l.c., that the specimens he kept in confinement preferred dead Insects rather than the fruits he offered them. Rühl considers the earwig to be fond of a carnivorous diet, eating larvae, small snails, etc., and only attacking flowers when these fail.[142] It has a great propensity for concealing {214}itself in places where there is only a small crevice for entry, and it is possible that its presence in fruits is due to this, rather than to any special fondness for the sweets. This habit of concealing itself in chinks and crannies in obscure places makes it an easy matter to trap the Insect by placing pieces of hollow stalks in the situations it affects; inverted flower-pots with a little hay, straw, or paper at the top are also effectual traps. We have remarked that it is very rarely seen on the wing, and though it has been supposed to fly more freely at night there is very little evidence of the fact. Another British species, Labia minor, a smaller Insect, is, however, very commonly seen flying.
Earwigs have the reputation of being fond of their young, and Camerano describes the female of the common earwig as carefully collecting its eggs when scattered, lifting them with its mandibles and placing them in a heap over which it afterwards brooded. De Geer[143] more than a century ago observed a fondness of the mother for the young. After the eggs were hatched, Camerano's individual, however, evinced no interest in the young. A larger species, Labidura riparia (Fig. 110) is said to move its eggs from place to place, so as to keep them in situations favourable for their development.
The name "earwig" is said to be due to an idea that these creatures are fond of penetrating into the ears of persons when asleep. Hence these Insects were formerly much dreaded, owing to a fear that they might penetrate even to the brain. There does not appear to be on record any occurrence that could justify such a dread, or the belief that they enter the ears. If they do not do so, it is certainly a curious fact that a superstition of the kind we have mentioned occurs in almost every country where the common earwig is abundant; for it has, in most parts of Europe, a popular name indicating the prevalence of some such idea. It is known as Ohren-wurm in German, as perce-oreille in French, and so on. The expanded wing of the earwig is in shape so very like the human ear, that one is tempted to suppose this resemblance may in former ages have given rise to the notion that the earwig has some connexion with the human ear; but this explanation is rendered very improbable by the fact that the earwig is scarcely ever seen with its wings expanded, and that it is a most difficult matter to unfold them {215}artificially, so that it is very unlikely that the shape of the wings should have been observed by untutored peoples.
The group Forficulidae seems to be most rich in species in warm and tropical regions; several unwinged species are met with in the mountainous districts of Europe; indeed, in some spots their individuals are extremely numerous under stones. In Britain we have a list of six species, but only two of these are to be met with; the others have probably been introduced by the agency of man, and it is doubtful whether more than one of these immigrants is actually naturalised here. One of these doubtfully native species is the fine Labidura riparia (Fig. 110), which was formerly found near Bournemouth. Altogether about 400 species of earwigs are known at the present time, and as they are usually much neglected by Insect collectors, it is certain that this number will be very largely increased, so that it would be a moderate estimate to put the number of existing species at about 2000 or 3000. None of them attain a very large size, Psalis americana being one of the largest and most robust of the family; a few display brilliant colours, and some exhibit a colour ornamentation of the surface; there are two or three species known that display a general resemblance to Insects of other Orders. The remarkable earwig represented in Fig. 102 (and which appears to be a nondescript form—either species or variety—closely allied to P. marmoricauda) was found by Baron von Hügel on the mountains of Java; the femora in this Insect have a broad face which is turned upwards instead of outwards, the legs taking a peculiar position; and it is curious that this exposed surface is ornamented with a pattern. The feature that most attracts attention on inspecting a collection of earwigs is, however, the forceps, and this is the most marked collective character of the group. These curious organs exhibit a very great variety; in some cases they are as long as the whole of the rest of the body, in others they are provided with tynes; sometimes they are quite asymmetrical, as in Anisolabis tasmanica (Fig. 113); in Opisthocosmia cervipyga, and many others they are curiously distorted in a variety of ways. The classification of the earwigs is still in a rudimentary state; the number of joints in the antennae, the form of the feet, and (in the terrestrial forms) the shape of the rudimentary wing-cases and wings being the characters that have been made most use of by {216}systematists; no arrangement into sub-families or groups of greater importance than genera is adopted.
The only particulars we have as to the embryological development of the earwig are due to Heymons.[144] The forceps spring from the eleventh abdominal segment, and represent the cerci of other Orthoptera. An egg-tooth is found to be present on the head for piercing the egg-shell. The embryo reverses its curved position during the development, as other Orthoptera have been observed to do, but in a somewhat different manner, analogous to that of the Myriapods.
Several fossil Forficulidae are known; specimens belonging to a peculiar genus have been described from the Lower Lias of Aargau and from the Jurassic strata in Eastern Siberia, but the examples apparently are not in a very satisfactory state of preservation. In the Tertiary formations earwigs have been found more frequently. Scudder has described eleven species of one peculiar genus from the Lower Miocene beds at Florissant in Colorado; some of these specimens have been found with the wings expanded, and no doubt that they were fully developed Forficulidae can exist. The fossil species of earwigs as yet known do not display so remarkable a development of the forceps as existing forms do.
Brauer and others treat the Forficulidae as a separate Order of Insects—Dermaptera—but the only structural characters that can be pointed out as special to the group are the peculiar form of the tegmina and hind wings—which latter, as we have said on p. 206, are considered by some to be formed on essentially the same plan as those of other Orthoptera—the imbrication of the segments, and the forceps terminating the body. The development, so far as it is known, is that of the normal Orthoptera. Thus the Forficulidae are a very distinct division of Orthoptera, the characters that separate them being comparatively slight, though there are no intermediate forms. Some of those who treat the Dermaptera as a sub-Order equivalent to the rest of the divisions of the Order, call the latter combination Euorthoptera.
Fam. II. Hemimeridae.
Apterous, blind Insects with exserted head, having a constricted neck, mouth placed quite inferiorly; the thoracic sterna large, imbricate. Hind body elongate, the segments imbricate, the dorsal plates being large and overlapping the ventral; the number of visible segments being different according to sex: a pair of long unsegmented cerci at the extremity. Coxae small, widely separated. Development intra-uterine.
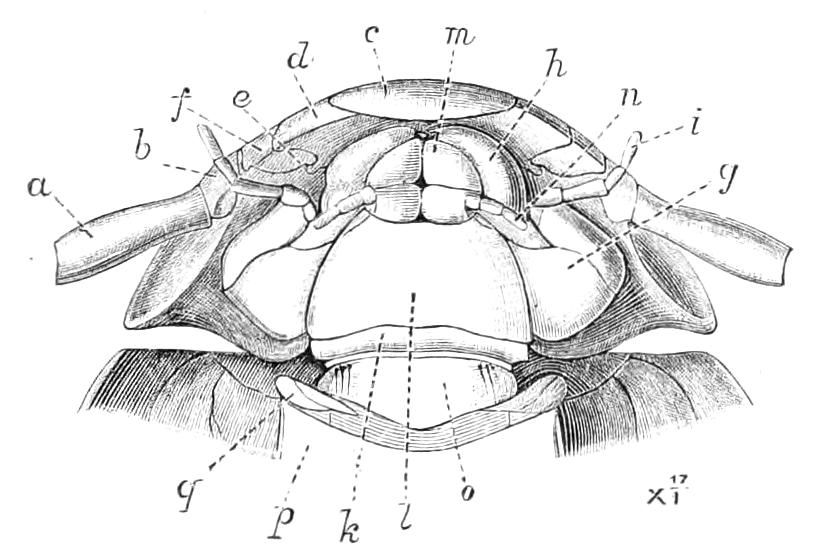
Fig. 115.—Under side of head and front of prothorax of Hemimerus. a, base of antenna; b, articulation of antenna; c, labrum; d, mandible; e, condyle of mandible; f, articular membrane of mandible; g, stipes of maxilla; h, exterior lobe; i, palpus of maxilla; k, submentum; l, mentum; m, terminal lobe of labium; n, labial palp; o, plate between submentum and sternum; p, prosternum; q, cervical sclerites. (After Hansen.)
In describing the labium of Mandibulata, p. 97, we alluded to the genus Hemimerus as reputed to possess a most peculiar mouth. When our remarks were made little was known about this Insect; but a very valuable paper[145] by Dr. H. J. Hansen on it has since appeared, correcting some errors and supplying us with information on numerous points. M. de Saussure described the Insect as possessing two lower lips, each bearing articulated palpi, and he therefore proposed to treat Hemimerus as the representative of a distinct Order of Insects, to be called Diploglossata. It now appears that the talented Swiss entomologist was in this case deceived by a bad preparation, and that the mouth shows but little departure from the ordinary mandibulate type. There is a large inflexed labrum; {218}the mandibles are concealed by the maxillae, but are large, compressed, and on their inner edge toothed. The maxillae are well developed, are surmounted by two lobes and bear five-jointed palpi. The ligula appears to be broad and short, and formed of two parts longitudinally divided; the short palpi consist of three segments. The mentum is very large. The lingua is present in the form of a free pubescent lobe with a smaller lobe on each side. The structure of the pleura is not fully understood; that of the abdomen seems to be very like the earwigs, with a similar difference in the sexes. The cerci are something like those of Gryllidae, being long, flexible, and unsegmented. The legs have rather small coxae, and three-jointed tarsi, two of which are densely studded with fine hairs beneath, as in Coleoptera. It is difficult to detect the stigmata, but Dr. Hansen believes there are ten pairs.
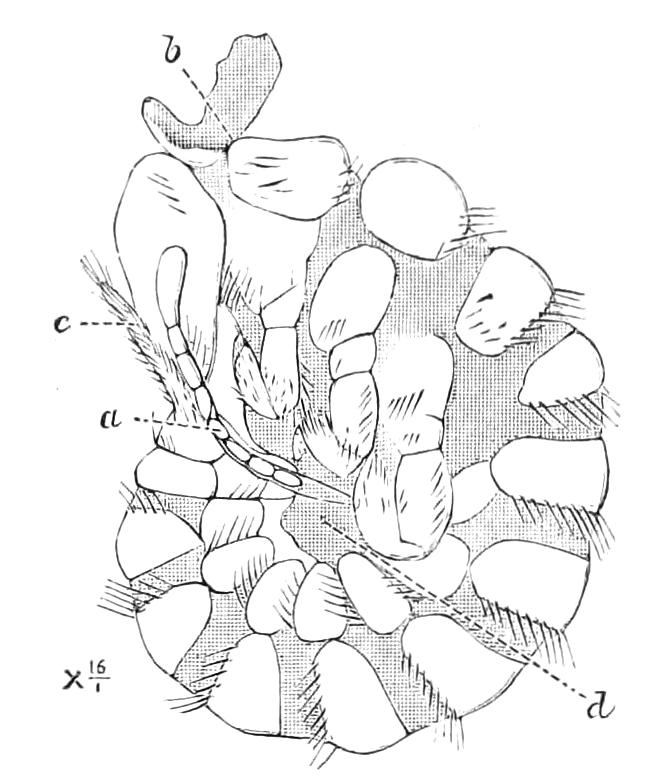
Fig. 116.—Foetus of Hemimerus. (After Hansen.) a, Antenna; b, organ from the neck; c, cerci; d, membrane (? cast skins).
The species described by Dr. Hansen as H. talpoides is probably distinct from that of Walker, though both come from equatorial West Africa. Dr. Hansen's species, which may be called H. hanseni, has been found living on the body of a large rat, Cricetomys gambianus; the Insect occurred on a few specimens only of the mammal, but when found was present in considerable numbers; it runs with rapidity among the hairs and apparently also springs. The nature of its food is by no means clear. Not the least remarkable fact in connexion with this peculiar Insect is its gestation. The young are borne inside the mother, {219}apparently about six at a time, the larger one being of course the nearest to the orifice. Dr. Hansen thinks the young specimens are connected with the walls of the maternal passages by means of a process from the neck of each. But the details of this and other points are insufficiently ascertained; it is, indeed, difficult to understand how, with a process of the kind of which a fragment is shown in Fig. 116, b, the Insect could fix itself after a detachment for change of position. The young is said to be very like the adult, but with a simpler structure of the antennae and abdomen. On the whole, it appears probable that Hemimerus is, as stated by Dr. Hansen, a special family of Orthoptera allied to Forficulidae; further information both as to structure and development are, however, required, as the material at the disposition of the Swedish entomologist was very small.
ORTHOPTERA CONTINUED—BLATTIDAE, COCKROACHES
Fam. III. Blattidae—Cockroaches.
Orthoptera with the head deflexed, in repose concealed from above, being flexed on to the under-surface with the anterior part directed backwards. All the coxae large, free, entirely covering the sternal surfaces of the three thoracic segments, as well as the base of the abdomen. The sternal sclerites of the thoracic segments little developed, being weak and consisting of pieces that do not form a continuous exo-skeleton; tegmina and wings extremely variable, sometimes entirely absent. The wings possess a definite anal region capable of fan-like folding; rarely the wing is also transversely folded. The three pairs of legs differ but little from one another.
The Blattidae, or cockroaches, are an extensive family of Insects, very much neglected by collectors, and known to the ordinary observer chiefly from the fact that a few species have {221}become naturalised in various parts of the world in the houses of man. One such species is abundant in Britain, and is the "black beetle" of popular language; the use of the word beetle in connexion with cockroaches is, however, entomologically incorrect. One or two members of the family are also well known, owing to their being used as the "corpora vilia" for students commencing anatomical investigation of the Arthropoda; for this purpose they are recommended by their comparatively large size and the ease with which an abundant supply of specimens may always be procured, but it must be admitted that in some respects they give but a poor idea of Insect-structure, and that to some persons they are very repulsive.
The inflexed position of the head is one of the most characteristic features of the Blattidae; in activity it is partially released from this posture, but the mouth does not appear to be capable of the full extension forwards that is found in other Insects that inflex the head in repose. The labium is deeply divided, the lingua forms a large lobe reposing on the cleft. The maxillary palpi have two basal short joints, and three longer joints beyond these; the labial palps consist of three joints of moderate length. The under-surface of the head is formed in large part by the submentum, which extends back to the occipital foramen.
The front of the head is the aspect that in repose looks directly downwards; the larger part of it is formed by the clypeus, which is separated from the epicranium by a very fine suture angulate in the middle; there is a large many-facetted eye on each side; near to the eye a circular space serves for the insertion of the antenna; close to this and to the eye there is a peculiar small area of paler colour, frequently membranous, called the fenestra, and which in the males of Corydia and {222}Heterogamia is replaced by an ocellus. The antennae are very elongate and consist of a large number of minute rings or joints, frequently about 100. The head is not inserted directly in the thorax, as is the case in so many Insects; but the front of the thorax has a very large opening, thus the neck between it and the head is of more than usual importance; it includes six cervical sclerites.
The pronotum is more or less like a shield in form, and frequently entirely conceals the head, and thus looks like the most anterior part of the body; usually it has no marked angles, but in some of the apterous forms the hind angles are sharp and project backwards. In contrast to the pronotum the prosternum is small and feeble, and consists of a slender lateral strip on each side, the two converging behind to unite with a median piece, the prosternum proper. None of these pieces of the ventral aspect of the prothorax are ordinarily visible, the side-pieces being covered by the inflexed head, and the median piece by the great coxae. In some of the winged Blattidae (Blabera, e.g.) there is at the base of each anterior coxa a small space covered by a more delicate membrane, that suggests the possibility of the existence of a sensory organ there (Fig. 120, i).[146] At the base of—above and behind—the front coxa the prothoracic spiracle is situate.
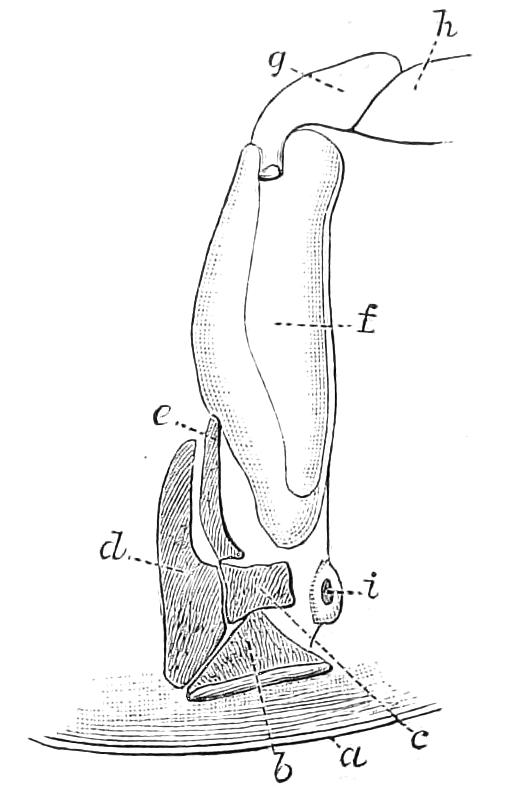
Fig. 120.—Base of front leg and portion of prothorax of Blabera gigantea. a, Under-side of pronotum; b, fold of pronotum?; c, epimeron?; d, episternum?; e, trochantin; f, coxa; g, trochanter; h, base of femur; i, presumed sense organ.
The meso- and meta-thoracic segments differ but slightly from one another; the notal or dorsal pieces are moderately large, while the sternal or ventral are remarkably rudimentary, and are frequently divided on the middle line. Connected with the posterior part of each sternum there is a piece, bent upwards, called by some anatomists the furca; when the sterna are divided the furca may extend forwards between them; in other {223}cases it is so obscure externally as to leave its existence in some doubt.
The sterna in Blattidae are remarkable for their rudimentary structure. This is probably correlated with the great development of the coxae, which serve as shields to the lower part of the body. The pieces of the sterna are not only small, but are also of feeble consistence—semi-membranous, in fact—and appear like thicker portions of the more extensive and delicate membrane in which they are situate; they sometimes differ considerably in the sexes of the same species. The coxae have very large bases, and between them and the sterna are some pieces that are grooved and plicate, so that it is not easy to decide as to their distinctions and homology (Fig. 120). The second breathing orifice is a slit placed in a horny area in the membrane between the middle and hind coxae.
The legs are remarkable for the large and numerous spines borne by the tibiae, and frequently also by the femora: the trochanters are distinct and of moderate size; the tarsi are five-jointed, frequently the basal four joints are furnished with a pad beneath; the fifth joint is elongate, bears two claws, and frequently between these a projecting lobe or arolium; this process scarcely exists in the young of Stilopyga orientalis, the common cockroach, though it is well developed in the adult. The hind body or abdomen is always large, and its division into rings is very visible, but the exact number of these that can be seen varies according to age, sex, species, and to whether the dorsal or ventral surface be examined. The differences are chiefly due to the retraction and inflexion of the apical segments; the details of the form of these parts differ in nearly every species. It is, however, considered that ten dorsal and ventral plates exist, though the latter are not so easily demonstrated as the former. The basal segment is often much diminished, the first dorsal plate being closely connected with the metanotum, while the first ventral may be still more rudimentary; much variety exists on this point. In the female two of the ventral terminal plates are frequently inflexed, so as to be quite invisible without dissection. From the sides of the tenth segment spring the cerci, flat or compressed processes very various in size, length, and form, usually more or less distinctly jointed. Systematists call the seventh ventral plate of the {224}female the "lamina subgenitalis," or the "lamina subgenitalis spuria," the concealed eighth plate being in this latter case considered the true subgenital plate. In the male this term is applied to the ventral plate of the ninth segment, the corresponding dorsal plate being called the "lamina supra-analis." These terms are much used in the systematic definitions of the genera and larger groups.
The males, in addition to the cerci alluded to as common to both sexes, are provided on the hind margin of the lamina subgenitalis with a pair of slender styles. These are wanting in the females, but in the common cockroach the young individuals of that sex are provided, like the male, with these peculiar organs. M. Peytoureau has described[147] the mode of their disappearance, viz. by a series of changes at the ecdyses. Cholodkovsky, who has examined the styles, considers them to be embryologically the homologues of true legs.[148] These styles are said not to be present in any shape in some species—Ectobia, Panesthia, etc.; this probably refers only to the adults. In some cases a curious condition occurs, inasmuch as one of the two styles is absent, and is replaced by a notch on the right side, thus causing an asymmetry—Phyllodromia, Temnopteryx, etc.
It has been found in several species that there are eight pairs of abdominal spiracles, making, with the two thoracic, ten pairs in all. The first of the abdominal spiracles is larger than the others, and in the winged species may be easily detected by raising the tegmina and wings, it being more dorsal in position than those following, which are in some species exposed on the ventral surface owing to the cutting away of the hind angles of the ventral plates; but the terminal spiracles are in all cases difficult to detect, and it is possible that the number may not be the same in all the species of the family. The cerci exhibit a great deal of variety. In the species with elongate tegmina and wings the cerci are elongate, and are like antennae in structure; in many of the purely apterous forms the cerci appear to be entirely absent (cf. Fig. 130, Gromphadorhina), but on examination may be found to exist in the form of a small plate, or papilla scarcely protuberant. In the males of Heterogamia they are, on the {225}contrary, very like little antennae; in the unwinged females of this genus they are concealed in a chink existing on the under-surface of the apex of the body.
The alar organs of Blattidae are of considerable interest from several points of view. They exist in various conditions as regards size and development, and in some forms are very large; each tegmen in some species of the genus Blabera (Fig. 132) may attain a length of nearly three inches; in other cases wings and tegmina are entirely absent, and various intermediate conditions are found. In Fig. 121 we give a diagram of the tegmen or front wing, A, and the hind wing, B, to explain the principal nervures and areas. The former are four in number, and, adopting Brunner's nomenclature[149] for them, are named proceeding from before backwards mediastinal, a; radial, b; infra-median (or ulnar), c; and dividens, d. An adventitious vein, vena spuria, existing in the hind wings of certain genera is marked sp in B.
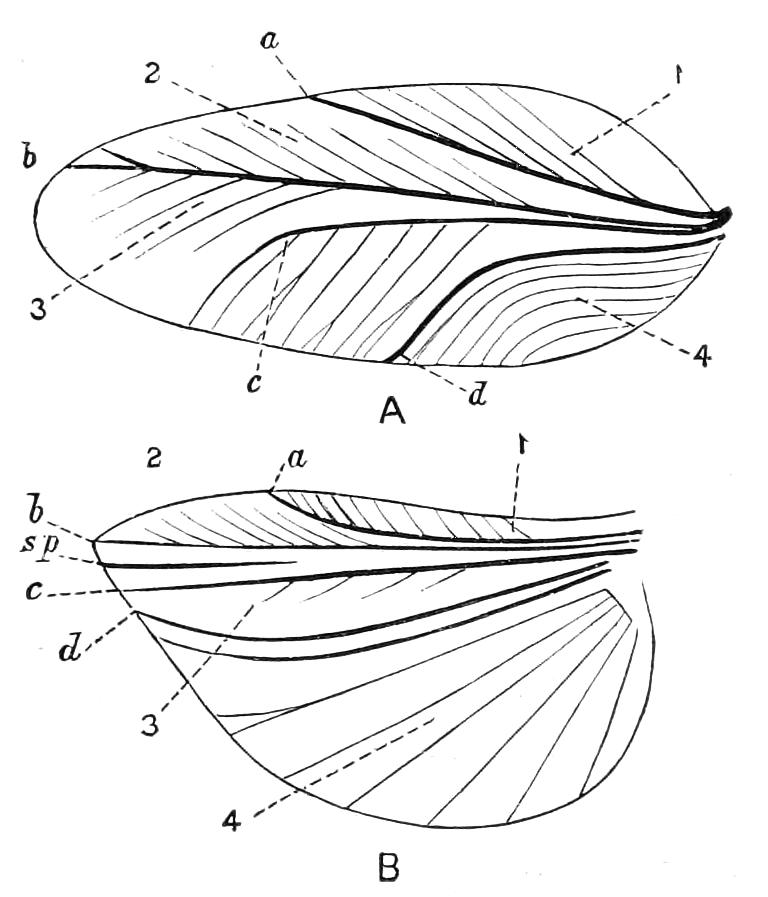
Fig. 121.—Diagram of tegmen, A, and wing, B, in Blattidae. Nervures: a, mediastinal; b, radial; c, ulnar or infra-median; d, dividens; sp, spuria. Areas: 1, mediastinal or marginal; 2, scapular or radial; 3, median; 4, anal or axillary.
The vena dividens is of great importance, as it marks off the anal or axillary field, which in both tegmen and wing has a different system of minor veins from what obtains in the rest of the organ; the veins being in the anterior region abundantly branching and dichotomous (Fig. 132), while in the anal field there is but little furcation, though the nervures converge much at the base. The mediastinal gives off minor veins towards the front only, the radial gives off veinlets at first towards the front, but nearer the tip of the wings sends off minor veins both backwards and forwards. The infra-median or ulnar vein is very variable; it is frequently {226}abbreviated, and on the whole is of subordinate importance to the other three. These latter thus form four chief areas or fields, viz.—1, mediastinal or marginal; 2, scapular or radial; 3, median; and 4, anal. These nervures and divisions may be traced in a large number of existing and fossil Blattidae, but there are forms existing at present which it is difficult to reduce to the same plan. In Euthyrhapha, found in the Pacific Islands, the hind wings are long and project beyond the tegmina, and have a very peculiar arrangement of the nervures; the species of Holocampsa also possess abnormal alar organs, while the structure of these parts in Diaphana (Fig. 122) is so peculiar that Brunner wisely refrains from attempting to homologise their nervures with those of the more normal Blattidae. The alar organs are frequently extremely different in the two sexes of the same species of Blattidae, and the hind wing may differ much from the tegmen as regards degree of departure from the normal. So that it is not a matter for surprise that the nervures in different genera cannot be satisfactorily homologised.
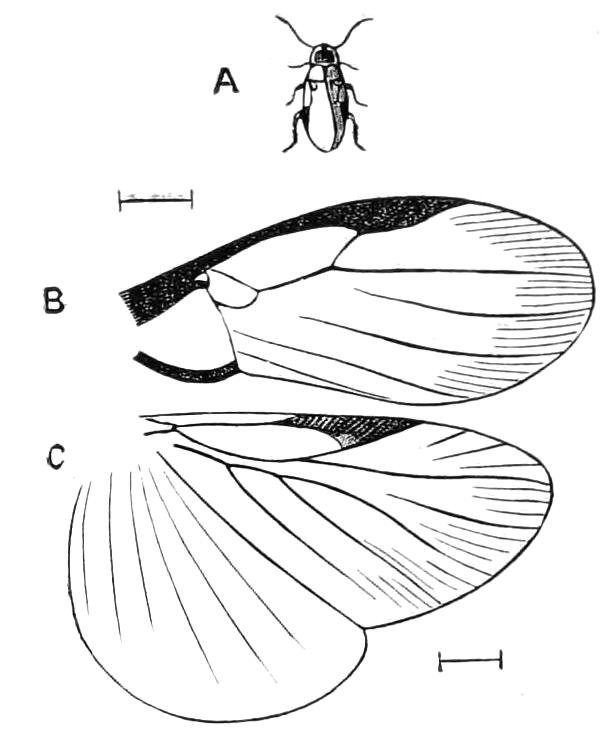
Fig. 122.—Diaphana fieberi. Brazil. A, The Insect, natural size; B, tegmen, and C, wing, magnified. (After Brunner.)
But the most peculiar wings in the family are the folded structures found in some forms of the groups Ectobiides and Oxyhaloides [Anaplectinae and Plectopterinae of de Saussure]. These have been studied by de Saussure,[150] and in Fig. 123 we reproduce some of his sketches, from which it will be seen that in B and C the wing is divided by an unusual cross-joint into two parts, the apical portion being also longitudinally divided into two pieces a and b. Such a form of wing as is here shown has no exact parallel in any of the other groups of Insects, though the earwigs and some of the Coleoptera make an approach to it. This structure permits a very perfect folding of the wing in repose. The peculiarities exhibited have been explained by de Saussure somewhat as follows. In the ordinary condition of Orthoptera the axillary or anal field (P) when the wings are {227}closed collapses like a fan, and also doubles under the anterior part (H) of the wing along the line a a, in Fig. 123, A, the result being similar to that shown by our Fig. 124. It will be noticed in Fig. 123, A, that a small triangular area (t) exists at the tip of the wing just where the fold takes place, so that when the wing is shut this little piece is liberated, as shown in t, Fig. 124. In many Blattidae, e.g. Blabera (Fig. 132), no trace of this little intercalated piece can be found, but in others it exists in various degrees of development intermediate between what is shown in Thorax porcellana (Fig. 123, A) and in Anaplecta azteca (123, B), so that a, b of the latter may be looked on as a greater development of the condition shown in A at t. It will be noticed that the superadded part of the wing of 123, B, possesses no venation, being traversed only by the line along which it folds; but in the wing of Diploptera silpha, 123, C, the corresponding part is complexly venated. This venation, as Brunner says,[151] is not an extension of the ordinary venation of the wing, but is sui generis. It is curious that though all the degrees of development between A and B exist in various forms of the tribes Ectobiides and Oxyhaloides, yet there is nothing to connect the veined apex of Diploptera with the unveined one of Anaplecta.
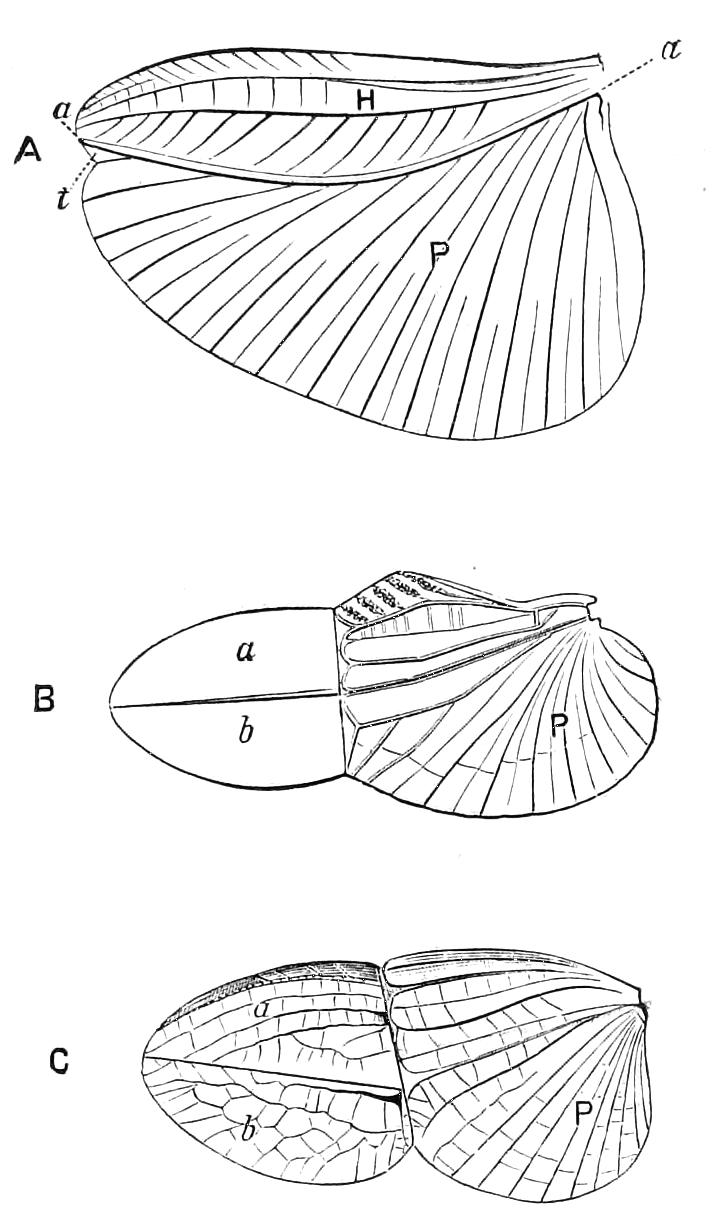
Fig. 123.—Hind wings of Blattidae. A, Thorax porcellana; B, Anaplecta azteca; C, Diploptera silpha. (After de Saussure.)
The internal anatomy of Blattids has been investigated in only one or two species. There are no great peculiarities, but some features of minor interest exist. The alimentary canal (Fig. 125) is remarkable {228}on account of the capacious crop, and the small gut-like, chylific ventricle; eight elongate pouches are situate on this latter part at its junction with the gizzard.
The Malpighian tubules are very numerous and delicate; there are extensive salivary glands and reservoirs; and on the anterior part of the true stomach there are eight caecal diverticula. The great chain of the nervous system consists in all of eleven ganglia—two cephalic, three thoracic, and six abdominal.
The ovaries in Stilopyga orientalis consist each of eight egg-tubes, placed at the periphery of a common receptacle or oviduct, the pair of receptacles themselves opening into a common chamber—the uterus—which is surrounded by a much branching serific or colleterial gland. In this chamber the egg-case is formed from the secretion of the gland just mentioned. According to Miall and Denny,[152] there is a spermatheca which opens not into the uterus but into the cloacal chamber behind it. Lowne doubts this diverticulum being a true spermatheca. The manner in which the eggs are fertilised and their capsule modelled is uncertain.[153]
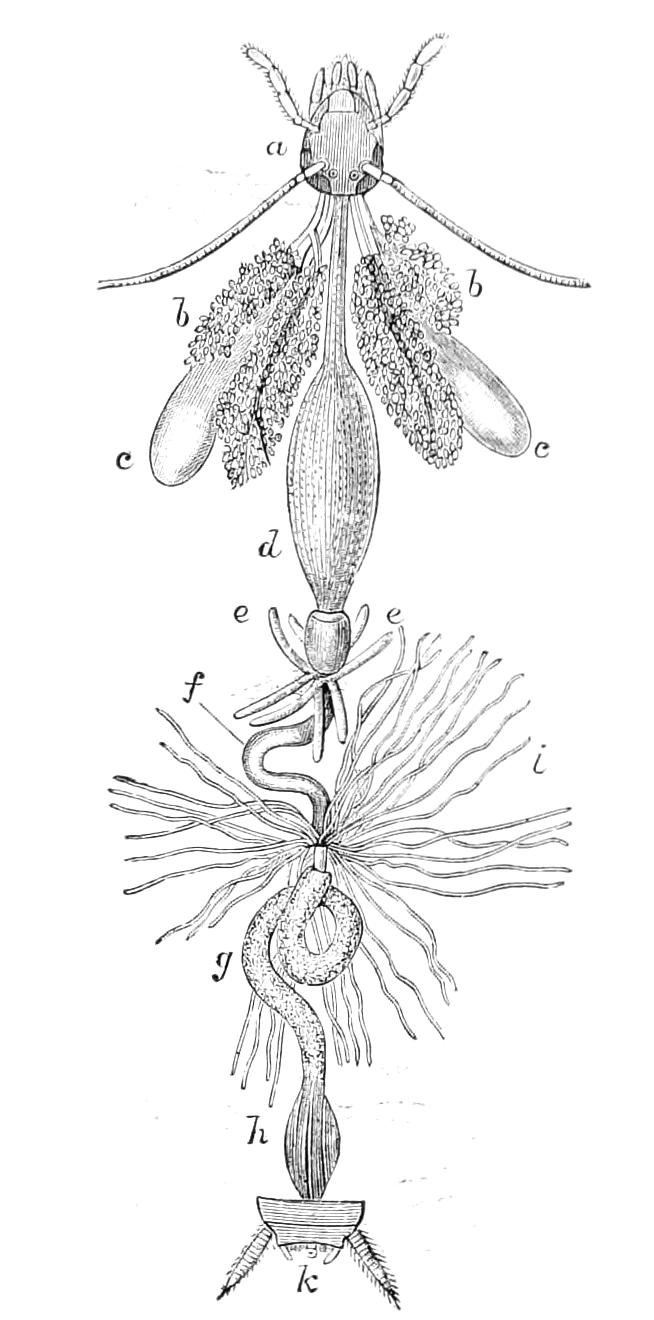
Fig. 125.—Alimentary canal of Stilopyga orientalis. (After Dufour.) a, Head; b, salivary glands; c, salivary reservoir; d, crop; e, diverticula placed below proventriculus; f, stomach; g, small intestine; h, rectum; i, Malpighian tubes; k, extremity of hind body.
The internal reproductive organs of the male are very complex in Stilopyga orientalis; each testis consists of a number (30 to 40) of vesicles placed on a tube which is prolonged to form the vas deferens. There is a very peculiar large complex gland consisting of longer and shorter utricles, opening into the vesiculae seminales, and forming a "mushroom-shaped gland."[154] {229}This gland is much larger than the testes proper, which, it is said, lose early their functional activity in the species in question, and shrivel. There is another important accessory gland, the conglobate gland of Miall and Denny, opening on a portion of the external copulatory armour.
Although some species of Blattidae are domesticated in our houses, and their bodies have been dissected by a generation of anatomists, very little is known as to their life histories. The common "black beetle" of the kitchen is said by Cornelius to be several years in attaining the adult state. Observations made at Cambridge by the writer, as well as others now being carried on there by Mr. H. H. Brindley, quite confirm this view, the extent of growth accomplished in several months being surprisingly little, and the amount of food consumed very small. It is therefore not improbable that the life of an individual of this species may extend to five years. Phyllodromia germanica, a species that is abundant in the dwellings of the peoples of north-eastern Europe, attains its full development in the course of a few months.
We have already alluded to the fact that in the Blattidae the eggs are laid in a capsule formed in the interior of the mother-Insect. This capsule is a horny case varying much in size and somewhat less in form in the different species; it is borne about for some time by the mother, who may not infrequently be seen running about with it protruding from the hinder part of the body. Sooner or later the capsule is deposited in a suitable situation, and the young cockroaches emerge; it is said that they are sometimes liberated by the aid of the mother. Mr. Brindley has found it very difficult to procure the hatching of the young from their capsules.
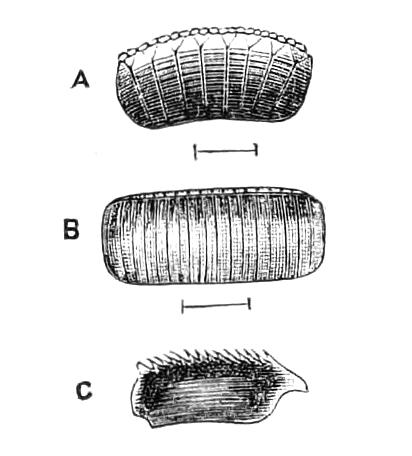
Fig. 126.—Egg-capsules of European Blattidae. A, Ectobia lapponica; B, Phyllodromia germanica; C, Heterogamia aegyptiaca. (After Brunner.)
It is known that some Blattidae are viviparous. In the case of one such species, Panchlora viridis, it appears probable that the egg-capsule is either wanting, or is present in only a very imperfect form.[155]
On emerging the young Blatta is in general form very similar to the parent, though usually much paler in colour. After casting {230}the skin an uncertain number of times—not less than five, probably as many as seven—it reaches the adult condition, the changes of outer form that it undergoes being of a gradual nature, except that at the last ecdysis the wings—in the case of the winged species—make their appearance, and the terminal segments of the body undergo a greater change of form. What mutations of shape may be undergone by the thoracic segments previous to the final production of the wings has not apparently been accurately recorded, Fischer's opinion being evidently based on very slight observation. The little that has been recorded as to the post-embryonic development since the observations of Hummel[156] and Cornelius[157] will be found in the works of Brunner.[158] According to this latter authority, in the wingless species the terminal segments of the body have the same form in the early stages as they have in the adult state, so that this latter condition can only be recognised by the greater hardness of the integument. When tegmina or wings are present in a well-developed form in a Blattid, it is certain that the Insect is adult; and when there can be seen at the side of the mesonotum or metanotum a piece, however small, separated by a distinct suture, it may be correctly assumed that the individual is an adult of a species having only rudimentary alar organs. The adult female of the common Stilopyga orientalis shows this phenomenon.
The cockroaches are remarkable for the excessive rapidity with which they run, or rather scurry, their gait being very peculiar. The common domestic forms, when alarmed, disappear with great agility, seeking obscure corners in which to hide themselves, it being part of their instinct to flee from light. Hence they are called lucifugous, and are most of them entirely nocturnal in their activities. In the South of Europe and other warmer regions many Blattidae may, however, be found on bushes and foliage in the daytime; these, when alarmed, fall down and run off with such speed and in so tortuous a manner, that it is a very difficult matter to seize them. It is recorded that the males of the genus Heterogamia are attracted by lights, though their apterous females keep themselves concealed underground in sandy places.
We may take this opportunity of alluding to the attraction that light exerts on Insects. Many species that conceal themselves during the daytime and shun light as if it were disagreeable, are at night-time so fascinated by it that it is the cause of their destruction. The quantity of Insects killed in this way by electric and other bright lights is now enormous; in many species the individuals immolate themselves by myriads. It would appear that only nocturnal and winged species are so attracted. So far as we know, light has no fascination for Insects except when they are on the wing. The phenomenon is not understood at present.
The food of Blattidae is believed to be of a very mixed character, though Brunner considers that dead animal matter is the natural nutriment of the members of this family. It is well known that the common cockroach eats a variety of peculiar substances; its individuals undoubtedly have the somewhat too economical habit of eating their own cast skins and empty egg-capsules, but in this they only act like many other much admired Insects. S. orientalis is gregarious, and the individuals are very amicable with one another; small specimens sit on, or run over the big individuals, and even nestle under them without their displaying the least resentment. The common cockroach is a rather amusing pet, as the creatures occasionally assume most comical attitudes, especially when cleaning their limbs; this they do somewhat after the fashion of cats, extending the head as far as they can in the desired direction, and then passing a leg or antenna through the mouth; or they comb other parts of the body with the spines on the legs, sometimes twisting and distorting themselves considerably in order to reach some not very accessible part of the body.
There is very little information extant as to the domestic Blattidae found in parts of the world outside Europe, but it seems that there are numerous species that prefer the dwellings of man, even though they only tolerate the owners. Belt says[159] "the cockroaches that infest the houses of the tropics are very wary, as they have numerous enemies—birds, rats, scorpions, and spiders; their long trembling antennae are ever stretched out, vibrating as if feeling the very texture of the air around them; and their long legs quickly take them out of danger. Sometimes {232}I tried to chase one of them up to a corner where on a wall a large cockroach-eating spider stood motionless looking out for his prey; the cockroach would rush away from me in the greatest fear, but as soon as it came within a foot of its mortal foe nothing would force it onwards, but back it would double, facing all the danger from me rather than advance nearer to its natural enemy." To this we may add that cockroaches are the natural prey of the fossorial Hymenoptera of the group Ampulicides, and that these wasps sometimes enter houses in search of the Insects.
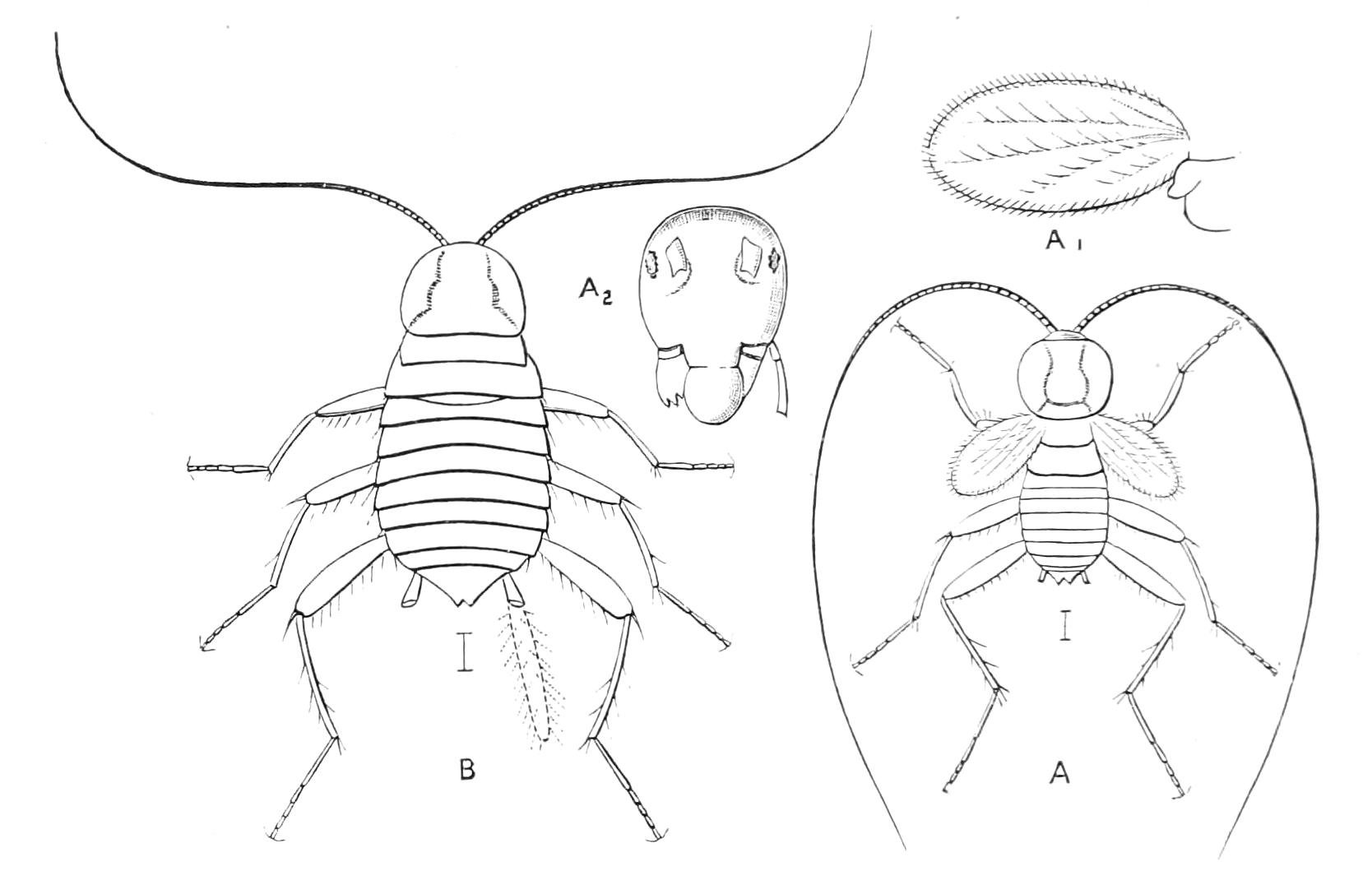
Fig. 127.—Nocticola simoni. A, male; A1, tegmen and rudiment of wing; A2, front of head; B, female. The cerci are broken, in B the right one is restored in outline. (After Bolivar.)
We have already noticed the considerable difference that exists in many cases between the sexes of the same species. This is sometimes carried to such an extent that nothing but direct observation could make us believe that the males and females are of one kin. Fig. 118 (p. 220) shows a case of this kind. Though the young as a rule are excessively similar to the adults, yet this is by no means invariably the case. In some of the more amply winged forms, such as Blabera, the young is about as different from the adult as the female of Heterogamia {233}is from its male. In Blattidae it is always the case—so far as is yet known—that when there is a difference as regards the alar organs between the two sexes, it is the male that has these structures most developed, and this even when they can be of little or no use for purposes of flight.
Among the most interesting forms of the family are the two species of the genus Nocticola, recently discovered by M. Simon in caves in the Philippine Islands.[160] They are amongst the smallest of the Orthoptera, the male being scarcely ⅛ of an inch long. In the larval state of N. simoni the ocular organs exist as three ocelli, or facets, on each side of the head, and in the perfect state the number is increased somewhat, as shown in Fig. 127, A2. In the second species of the genus the female is quite blind (the male being still undiscovered). The fenestræ in Nocticola are absent; the tegmina and wings are totally wanting in the female (Fig. 127, B), but are present in a very peculiar condition in the male (Fig. 127, A1). There are other anomalies in the structure of these cavernicolous Insects, the cerci being apparently of peculiar structure, and the spines of the legs more hair-like than usual. The condition of the eyes is remarkable; the peculiarity in their development is worthy of study.
To those who are acquainted with Blattidae only through our domestic "black beetle" it may seem absurd to talk of elegance in connexion with cockroaches. Yet there are numerous forms in which grace and beauty are attained, and some exhibit peculiarities of ornamentation that are worthy of attention. Corydia petiveriana (Fig. 128) is a common cockroach in East India. It has an effective system of coloration, the under wings and the sides of the body being vividly coloured with orange yellow; when the tegmina are closed the upper surface of the body is of a velvet-black colour, with cream-coloured marks; these spots are different {234}on the two tegmina, as shown in Fig. 128, A, but are so arranged that when the tegmina are closed (Fig. 128, B) a symmetrical pattern is produced by the combination of the marks of the two differently spotted tegmina. It is very curious to notice the great difference in the colour of the part of the right tegmen that is overlapped by the edge of the left one; this part of the tegmen being coloured orange yellow in harmony with the wings. The result of the remarkable differentiation of the colours of the two tegmina may be summarised by saying that on the right one the colour of a part is abruptly contrasted with that of the rest of the organ, so as to share the system of coloration of the under-wings and body, while the corresponding part of the other tegmen is very different, and completes the system of symmetrical ornamentation of the upper surface.
Many other members of the Blattidae have an elegant appearance, and depart more or less from their fellows in structural characters, with the result of adding to their graceful appearance; in such cases, so far as at present known, these Insects are brightly coloured. Thus Hypnorna amoena (Fig. 129) has the antennae banded in white, black, and red, while the overlapping part of the tegmina is arranged so as to bring the line of junction between them nearly straight along the middle line of the body, and thus produce a more symmetrical appearance than we find in other cockroaches. The head in this Insect is not so concealed as usual, and this undoubtedly adds somewhat to the effective appearance of this cockroach. This visibility of the front of the head in Hypnorna is not, as would be supposed, owing to its being less inflexed than usual. On the contrary, the head is quite as strongly inflexed as it is in other Blattidae, but the part just at the front of the thorax is unusually elongate, so that the eyes are exposed and the Insect has a larger field of vision. This interesting Insect belongs to the tribe Oxyhaloides [Plectopterinae Sauss.], in which group the most highly developed folded wings occur.
The wingless forms never exhibit the grace and elegance possessed by some of the more active of the winged Blattidae. {235}One of them, Gromphadorhina portentosa, found in Madagascar (Fig. 130), is a very robust Insect, and attains a length of 78 millim.—somewhat more than 3 inches. This Insect has projections on the thorax that remind us of the horns that exist in some of the Lamellicorn beetles.
Little has been yet written as to the resemblances of Blattidae to other species of their own family, or to other creatures, but it is probable that such similarities will be found to prevail to a considerable extent. W. A. Forbes has called attention[161] to the larva of a Blattid from Brazil as being remarkable for its superficial resemblance to an Isopod crustacean. Some of the wingless forms have a great resemblance to the small rolling-up Myriapods of the group Glomerides; Pseudoglomeris fornicata, of which we figure the female (Fig. 131), has received its name from this resemblance. The females of the S. African genus Derocalymma possess this Glomerid appearance, and have a peculiar structure of the prothorax, admitting of a more complete protection of the head. Brunner states that the wingless kinds of Derocalymma roll themselves up like wood-lice. In many of the forms of this tribe—Perisphaeriides—the males are winged, though the females are so like Myriapods. According to de Saussure[162] the gigantic Megaloblatta rufipes bears an extreme resemblance in appearance to the large cockroaches of the genus Blabera.
Some of the species of Holocompsa remind us strongly of Hemiptera of the family Capsidae; they have an arrangement of colours similar to what prevails in that group, and their tegmina and wings which, as being those of Blattids may be said to be abnormally formed, resemble in texture and the distribution of the venation those of the Hemiptera. These Insects are closely allied to Diaphana, of which genus we have figured a species (Fig. 122).
There is very little evidence on which to base an estimate of the number of species of Blattidae existing in the world at present. Probably the number extant in collections may amount to 1000 or thereabouts, and the total existing in the world may be as many as 5000. The species of Blattidae cannot tolerate cold, and are consequently only numerous in tropical regions. Europe possesses about twenty species, and in Britain there are only three that are truly native; these are all small Insects belonging to the genus Ectobia, and living out of doors, amongst leaves, under bushes, and in various other places. We have, however, several other species that have been introduced by the agency of man, and these all live under cover, where there is artificial warmth and they are protected from the inclemencies of the winter season. The commonest of these forms is Stilopyga orientalis, the "black beetle" of our kitchens and bakehouses. This Insect is said to have been brought to Europe from "Asia" about 200 years ago, but the evidence as to its introduction, and as to the country of which it is really a native, is very slight. It is indeed said[163] that S. orientalis has been found in peat in Schleswig-Holstein. Periplaneta americana is a larger Insect, and is common in some places; it is apparently the species that is most usually found on board ships, where it sometimes multiplies enormously, and entirely devours stores of farinaceous food to which it obtains access: it is known that sometimes a box or barrel supposed to contain biscuits, on being opened is found to have its edible contents entirely replaced by a mass of living cockroaches. Fortunately Periplaneta americana has not spread widely in this country, though it is found in great numbers in limited localities; one of the best known of which is the Zoological Gardens in the Regent's Park at London. Periplaneta australasiae is very similar to P. americana, but has a yellow mark on the shoulder of each tegmen. This has obtained a footing in some of the glass-houses in the Botanic Gardens at Cambridge and Kew; and it is said to be fairly well established in Belfast. Another of our introduced domestic cockroaches is Phyllodromia germanica, a much smaller Insect than the others we have mentioned. It has only established itself at a few places in this country, but it is extremely abundant in some parts of Northern and Eastern Europe. It has been increasing in numbers in Vienna, where, according to Brunner, it is {237}displacing Stilopyga orientalis. In addition to these, Rhyparobia maderae and species of the genus Blabera have been met with in our docks, and are possibly always to be found there. They are Insects of much larger size than those we have mentioned. We figure the alar organs of one of these species of Blabera of the natural size: the species in this genus are extremely similar to one another. Blaberae are known in the West Indies as drummers, it being supposed that they make a noise at night,[164] but details in confirmation of this statement are wanting.
It is a remarkable fact that no satisfactory reasons can be assigned for the prevalence of one rather than another of these domestic cockroaches in particular localities. It does not seem to depend at all on size, or on the period of development, for the three species Stilopyga orientalis, Periplaneta americana, and Phyllodromia germanica, which are the most abundant, differ much in these respects, and replace one another in particular localities, so that it does not appear that any one is gaining a permanent or widespread superiority as compared with another. There are, however, no sufficient records on these points, and further investigation may reveal facts of which we are at present ignorant, and which will throw some light on this subject. We may remark that Mr. Brindley has found it more difficult to obtain hatching of the young from the egg-capsules of Periplaneta americana and Phyllodromia germanica at Cambridge, than from those of Stilopyga orientalis.
Although much work has been done on the embryology of Blattidae, the subject is still very incomplete. The recent memoirs of Cholodkovsky[165] on Phyllodromia germanica contain so much of general interest as to the development of the external parts of the body that we may briefly allude to them. The earliest appearance of segmentation appears to be due to the centralisation of numerous {238}cells round certain points in the ventral plate. The segmentation of the anterior parts is first distinct, and the appearance of the appendages of the body takes place in regular order from before backwards, the antennae appearing first; the mandibles, however, become distinct only subsequent to the maxillae and thoracic appendages. There are in the course of the development appendages to each segment of the body (he counts eleven abdominal segments); the cerci develop in a similar manner to the antennae; the first pair of abdominal appendages—at first similar to the others—afterwards assume a peculiar stalked form. The abdominal appendages subsequently disappear, with the exception of the ninth pair, which form the ventral styles, and the eleventh pair, which become the cerci. The last ventral segment is said to be formed by the union of the tenth and eleventh embryonic ventral segments.
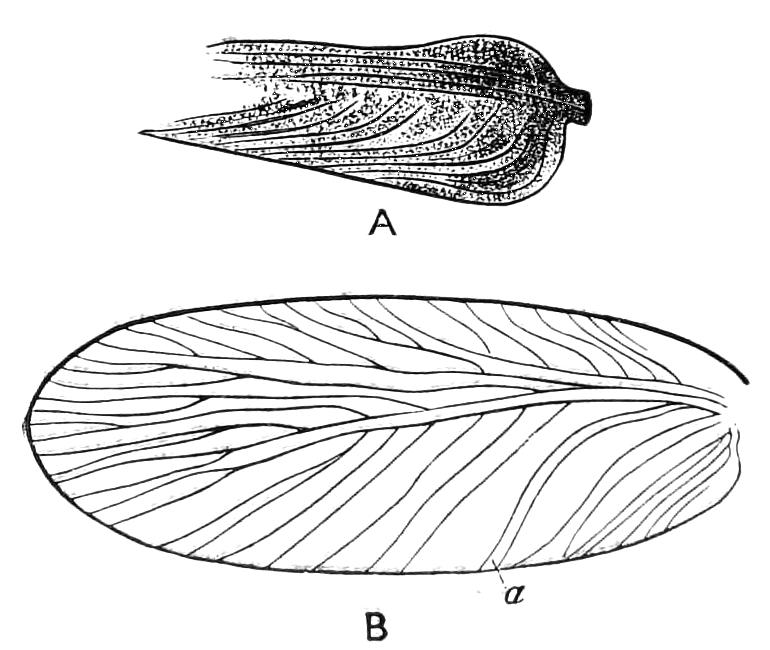
Fig. 133.—A, Tegmen(?) of Palaeoblattina douvillei; B, of Etoblattina manebachensis. (After Brauer and Scudder.)
As regards their Palaeontological forms Blattidae are amongst the most interesting of Insects, for it is certain that in the Carboniferous epoch they existed in considerable number and variety. A still earlier fossil has been found in the Silurian sandstone of Calvados; it consists of a fragment (Fig. 133, A), looking somewhat like an imperfect tegmen of a Blattid; it was described by Brongniart under the name of Palaeoblattina douvillei, and referred by him, with some doubt, to this family. Brauer has, however, expressed the opinion[166] that the fragment more probably belonged to an Insect like the mole-cricket, and in view of this discrepancy of authorities we may be pardoned for expressing our own opinion to the effect that the relic has no connexion with the Insecta. The figure given by Scudder[167] has not, however, so uninsect-like an appearance as that we have copied from Brauer. Whatever may prove to be the case with regard to Palaeoblattina, it is certain, as we {239}have already said, that in the Palaeozoic epoch Insects similar to our existing cockroaches were abundant, their remains being found in plenty in the coal-measures both of Europe and North America. Fig. 133, B, shows a fossil tegmen of Etoblattina manebachensis from the upper Carboniferous beds of Ilmenau in Germany. It will be noticed that the disposition of the nervures is very much like that which may be seen in some of our existing Blattidae (cf. the tegmen of Blabera, Fig. 132, A), the vena dividens (a) being similarly placed, as is also the mediastinal vein on the front part of the organ. The numerous carboniferous Blattidae have been separated as a distinct Order of Insects by Scudder under the name Palaeoblattariae, but apparently rather on theoretical grounds than because of any ascertained important structural distinctions. He also divided the Palaeoblattariae into two groups, Mylacridae and Blattinariae, the former of which was supposed to be peculiar to America. Brongniart has, however, recently discovered that in the Carboniferous deposits of Commentry in France Mylacridae are as common as in America. This latter authority also states that some of the females of these fossil Blattidae are distinguished by the presence of an elongate exserted organ at the end of the body. He considers this to have been an ovipositor by which the eggs were deposited in trees or other receptacles, after a manner that is common in certain Orthoptera at the present day. If this view be correct these Carboniferous Insects must have been very different from the Blattidae of our own epoch, one of whose marked characteristics is the deposition of the eggs in a capsule formed in the body of the parent.
In the strata of the secondary epoch remains of Blattidae have also been discovered in both Europe and America, in Oolitic, Liassic, and Triassic deposits. From the Tertiary strata, on the other hand, comparatively few species have been brought to light. A few have been discovered preserved in amber.
The classification of the Blattidae is attended with considerable difficulty on account of the numerous wingless forms, and of the {240}extreme difference in the organisation of the two sexes of many species. It has, however, been brought to a fairly satisfactory state by the reiterated labours of Brunner von Wattenwyl, and we reproduce his recently perfected exposition of their characters. His first division is made by means of a structure which is very easily observed, viz. whether the femora are armed with spines, as in Fig. 134, or not. The terms used in connexion with the wings and other parts of the body we have already explained.
Brunner's system is adopted by de Saussure,[168] who, however, proposes to replace the names Ectobiides and Oxyhaloides by Anaplectinae and Plectopterinae. He also proposes to apply the generic name Blatta to the Insect that is now so frequently called Phyllodromia germanica in zoological works. If that view be adopted, Brunner's group Phyllodromiides will be called Blattides.
Table of the tribes of Blattidae, after Brunner:—
1. Femora spiny beneath.[169]
2. The last ventral plate of the female large, without valves.
3. Supra-anal lamina of both male and female transverse, narrow. Wings, when present, furnished with a triangular apical field. Posterior femora unarmed beneath, or armed with two spines on the anterior margin. Egg-capsules furnished with a longitudinal suture. Tribe 1. Ectobiides. [Anaplectinae Saussure.]
3′. Supra-anal lamina of each sex more or less produced, triangular, or emarginate. Wings, when present, without apical field. Posterior femora with both edges spiny.
4. Supra-anal lamina of each sex triangular, not notched. Cerci projecting much beyond this lamina.
5. Pronotum and elytra smooth (i.e. without peculiarity of surface other than punctuation). The radial nervure of the wing giving off several parallel branches, pectinate on the anterior margin (except in the genus Abrodiaeta). Tarsal joints without pads. Tribe 2. Phyllodromiides. [Blattinae Saussure.]
5′. Pronotum and elytra holosericeous. Radial nervure of the wings giving off irregular branches on the anterior margin (ulnar vein many-branched). Tarsal joints furnished with pads. Tribe 3. Nyctiborides.
4′. Supra-anal lamina of males more or less four-sided, with obtuse angles, of females broad, rounded, or lobed. Cerci not projecting beyond the lamina. (Tarsal joints with distinct pads.) Ulnar nervure of the wings giving off parallel branches towards the vena dividens. Tribe 4. Epilamprides.
2′. The last ventral plate of the female furnished with valves. Tribe 5. Periplanetides.[170] (Fig. 119, Periplaneta australasiae.)
1′. Femora unarmed beneath. (In the tribe Panesthiides the anterior femora are frequently armed with two spines.)
2. Supra-anal lamina of each sex more or less produced, posterior margin notched.
3. A distinct pad between the claws. Tribe 6. Panchlorides.
3′. No pad between the claws, or only an excessively small one.
4. Wings with a folded fan-like anal field. Pronotum smooth. Tribe 7. Blaberides. (Fig. 132, Blabera sp. wings.)
4′. Anal field of the wing with a single fold. Pronotum more or less pilose. Tribe 8. Corydiides. (Fig. 128, Corydia petiveriana. Fig. 118, Heterogamia aegyptiaca.)
2′. Supra-anal lamina of each sex, short, transverse, posterior margin straight or rounded.
3. Subgenital lamina of the male somewhat produced, furnished with a single style. Tarsal claws with a distinct pad (except in the genus Paranauphoeta).
4. Anterior portion of the wings pointed, either the apical field of the wing very much produced, or the wings twice as long as the tegmina, folded in repose. Tribe 9. Oxyhaloides. [Plectopterinae Saussure.] (Fig. 129, Hypnorna amoena.)
4′. Anterior portion of wing, when present, rounded, with no apical field. Tribe 10. Perisphaeriides. (Fig. 130, Gromphadorhina portentosa; Fig. 131, Pseudoglomeris fornicata.)
3′. Subgenital lamina of males extremely small, without styles. No pad between claws. Tribe 11. Panesthiides.
To the above tribes another one—Geoscapheusides—has been recently added by Tepper,[171] for an extraordinary Australian Insect of fossorial habits, with front legs formed somewhat like those of Gryllotalpa.
ORTHOPTERA CONTINUED—MANTIDAE—SOOTHSAYERS
Fam. IV. Mantidae—Soothsayers or Praying Insects.
Orthoptera with exserted but deflexed head and elongate prothorax, the first pair of legs largely developed, raptorial, the coxae elongate, free, femora and tibiae armed with spines: second and third pair of legs simple and similar; the tarsi five-jointed, without a pad (arolium) between the claws; a pair of jointed cerci near the extremity of the body.
The Mantidae are an extensive family of Orthoptera, showing extreme variety in the shapes and outlines of the body, and characterised by the very remarkable front legs; the function of these legs being to seize and hold their prey, which consists of living Insects, Mantidae being carnivorous and highly voracious.
The labium is deeply divided, each half exhibiting a very near approach to the structure of a maxilla; there is a large membranous lingua reposing on the inner face of the lower lip. The head is quite free from the thorax, its front part being deflexed, and even somewhat inflexed, so that the mouth is directed downwards and somewhat backwards: it is very mobile, being connected to the thorax by a comparatively slender neck, which is, however, concealed by the pronotum. There are two large, prominent eyes, the antennae are frequently very slender, but they sometimes differ according to sex, and in some genera are pectinate in the male; just above and between their insertion are three ocelli placed in a triangle, two above, one below; between the antennae and the clypeus there is an interval called the scutellar space. In some forms of Mantidae the head assumes most extraordinary shapes; the eyes may become {243}elongate and horn-like; there may be a projection between them bearing the ocelli, and attaining occasionally a great length; the scutellar space also may have a remarkable development, the whole thus forming a peculiar ornamental structure, as in Fig. 136.
The prothorax is elongate, but there are a few genera, e.g. Eremiaphila, in which it is exceptionally short, and there are several others in which the elongate form is more or less masked by foliaceous expansions of the sides. The pronotum shows near the front a transverse depression or seam, which marks the position of an internal chitinous ridge. The anterior legs are {244}inserted near the front of the prosternum, which extends less far forwards than the pronotum does; the posterior part of the prosternum is very elongate, and is completely separated from the anterior part by the base of the coxae and the membranes attached to them; the pronotum and sternum are closely connected at the sides till near the posterior part where they diverge, the space so formed being occupied by a membrane in which the prothoracic stigma is situated. The mesothorax is as long as broad, and the front wings are attached to the whole length of the sides; the mesosternum is a triangular piece pointed behind, and bearing very large side-pieces, to the hinder portion of which the middle coxae are attached; these latter are large and quite free, and repose on the metasternum which they cover; the mesothoracic stigma may be detected as a slit situated on a slight prominence just behind and a little below the membranous hind-margin of the tegmen. The metathorax differs comparatively little in size and structure from the mesothorax; the membranous hind wings are attached to the sides of the notum along nearly the whole length of the latter. The abdomen is moderately long; in each sex ten dorsal plates may be detected, and there is a pair of ringed cerci projecting from beneath the sides of the tenth plate. The number of ventral plates is more difficult to verify, the first one being much reduced; eight other plates can be demonstrated in the male and six in the female.
The anterior legs are formed in a remarkable manner in the Mantidae, and are, in fact, the most characteristic feature of the family. Attached near the front of the thorax there is a very long coxa, to the apex of which is articulated the triangular trochanter; this bears the elongate femur, which is furnished on its lower face with sharp spines and teeth; the tibia which follows is much shorter and smaller than the femur; its lower face bears also an armature of teeth, and it is so articulated with {245}the femur that it can be completely closed thereon, its teeth fitting in among those of the femur (Fig. 137, B); the latter has one or more longer spines overlapping the apical part of the tibia when contracted. The tarsus is slender, five-jointed, without pad. The other two pairs of legs are simple; the hinder usually a little the longer, and in some species that possess powers of leaping (Ameles), with the femora a little thicker at the base.
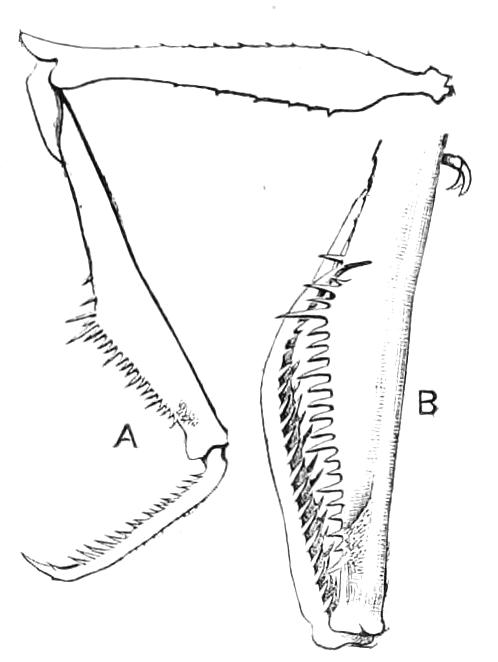
Fig. 137.—Front leg of Empusa pauperata, female: A, with tibia extended and tarsus wanting; B, more magnified (the basal parts removed), showing the mode of closure.
The alar organs of the Mantidae are as regards the nervures and areas fairly similar to those of the Blattidae. The tegmina are usually narrow, and exhibit three well-marked areas; the one in front or external (according as the wing is expanded or closed) is the mediastinal area; it is usually more elongate and occupies a larger portion of the surface of the tegmen than in Blattidae. The middle area, forming the larger part of the wing, is occupied by the branches of the radial and ulnar nervures. The third area, the anal, possesses a sort of appendage in the form of a small space of a more delicately membranous nature at the inner part of the base. The tegmina are often more or less leaf-like in texture and consistence; this character is as a rule not very marked, but there are a few species with the tegmina very like foliage, this being more marked in the female; in some, if not in all, of these cases the mediastinal area is considerably increased. One tegmen overlaps the other, as in Blattidae, but to a less extent, and the correlative asymmetry is but slight: there is frequently a pallid spot close to the main vein on the principal area, nearer to the base than to the extremity. The hind wings are more ample than the front, and of much more delicate consistence; they possess numerous veins converging to the base; the anterior part of the wing is firmer in consistence, and its veins are more numerously furcate; there are many more or less distinct minute cross-veinlets, and an elegant tinting is not infrequent. They close in a fan-like manner, transverse folding being unknown in the family.
But little has been written on the internal anatomy of the Mantidae. Dufour has described only very partially that of M. religiosa. The salivary glands are largely developed, salivary receptacles exist; the alimentary canal possesses eight elongate coecal diverticula placed on the chylific ventricle; there are about one hundred Malphigian tubules. In each ovary there are about 40 egg-tubes, and they are joined at their bases in clusters of about half a dozen; each cluster has a common sinus; these sinuses are placed at intervals along a tube, which is one of two branches whose union forms the oviduct; there are a large number of "serific glands" of two kinds in the female. The testes are unusually complex in their structure.
According to Schindler[172] the Malphigian tubes in Mantis are not inserted, as usual, at the base of the intestine, but on the intestine itself at about one-third of its length from the base. There is some doubt about this observation. Schindler considers the fact, if it be such, unique.
The eggs of the Mantidae are deposited in a singular manner: the female, placing the extremity of the body against a twig or stone, emits some foam-like matter in which the eggs are contained. This substance dries and forms the ootheca; whilst attaining a sufficient consistence it is maintained in position by the extremity of the body and the tips of the elytra, and it is shaped and fashioned by these parts. The eggs are not, as might be supposed, distributed at random through the case, but are lodged in symmetrically-arranged chambers, though how these chambers come into existence by the aid of so simple a mode of construction does not appear. The capsule is hard; it quite conceals the eggs, which might very naturally be supposed to be efficiently protected by their covering: this does not, however, appear to be the case, as it is recorded that they are subject to the attacks of Hymenopterous parasites. The time that elapses after the eggs are laid and before they hatch varies greatly according to circumstances. In France, Mantis religiosa deposits its eggs in September, but they do not hatch until the following June; while in E. India the young of another species of Mantis emerge from the eggs about twenty days after these have been deposited. Trimen has recorded some particulars as to the formation of its egg-case by a Mantis in S. Africa. This {247}specimen constructed four nests of eggs at intervals of about a fortnight, and Trimen states that the four were "as nearly as possible of the same size and of precisely similar shape." He also describes its mode of feeding, and says that it was fond of house-flies, and would eat "blue-bottles," i.e. Musca vomitoria, but if while eating one of the latter a house-fly were introduced, the "blue-bottle" was generally dropped, even though it might be in process of being devoured. The young have to escape from the chambers in which they are confined in these egg-cases; they do so in a most curious manner; not by the use of the feet, but by means of spines directed backwards on the cerci and legs, so that when the body is agitated advance is made in only one direction. The eggs last deposited are said to be the first to hatch. On reaching the exterior the young Mantids do not fall to the ground, but remain suspended, after the manner of spiders, to the ootheca by means of two threads attached to the extremities of the cerci; in this strange position they remain for some days until the first change of skin is effected, after which they commence the activity of their predatory life.
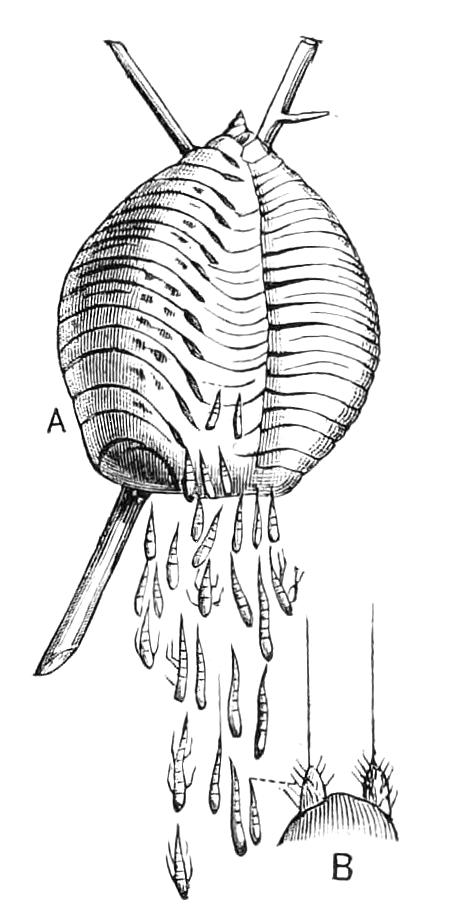
Fig. 138.—Egg-case of Mantis with young escaping: A, the case with young in their position of suspension; B, cerci magnified, showing the suspensory threads. (After Brongniart.)
Dr. Pagenstecher has given an account[173] of the development of Mantis religiosa, from which it would appear that the statements of Fischer and others as to the number of moults are erroneous, owing to the earliest stages not having been observed. When the young Mantis emerges from the egg it bears little resemblance to the future Insect, but looks more like a tiny pupa; the front legs, that will afterwards become so remarkable, are short and not different from the others, and the head is in a curious mummy-like state, with the mouth-parts undeveloped and is inflexed on the breast: there are, he says, nine abdominal segments. The first ecdysis soon takes place and the creature is thereafter recognisable as a young Mantis. Pagenstecher's specimens at first would only eat Aphididae, but at a later stage of the {248}development they devoured other Insects greedily: the number of ecdyses is seven or eight. The ocelli appear for the first time when the wing rudiments do so; the number of joints in the antennae increases at each moult. Dr. Pagenstecher considers that this Insect undergoes its chief metamorphosis immediately after leaving the egg, the earlier condition existing apparently to fit the Insect for escaping from the egg-case. In the immature stage of the Mantidae the alar organs appear (Fig. 139) as adjuncts of the sides of the meso- and meta-notum, projecting backwards and very deeply furrowed and ribbed in a wing-like manner. According to Pagenstecher, this wing-like appearance only commences in the fifth stadium, but he has not given particulars of the conditions of these parts in the preceding instars. According to de Saussure[174] the wings of the females of some species remain permanently in this undeveloped or nymphal state.
The Mantidae, as a rule, have a quiet unobtrusive mien, and were it not for their formidable front legs would look the picture of innocence; they, however, hold these legs in such manner as to greatly detract from the forbidding appearance thereof, stretching them out only partially so as to give rise to an appearance of supplication or prayer;[175] this effect is increased by their holding themselves in a semi-erect position, standing on the hind and middle legs with the upper parts of the body directed somewhat forwards, hence they are called by various names indicating prayer or supplication, and it is said that in some countries they are considered sacred. Some of the older {249}writers went so far as to say that a Mantis would indicate the road a child should take by stretching out one of its arms in the right direction. The traveller Burchell, speaking of a species since described by Westwood under the name of Tarachodes lucubrans, says: "I have become acquainted with a new species of Mantis, whose presence became afterwards sufficiently familiar to me by its never failing, on calm warm evenings, to pay me a visit as I was writing my journal, and sometimes to interrupt my lucubrations by putting out the lamp. All the Mantis tribe are very remarkable Insects; and this one, whose dusky sober colouring well suits the obscurity of night, is certainly so, by the very late hours it keeps. It often settled on my book, or on the press where I was writing, and remained still, as if considering some affair of importance, with an appearance of intelligence which had a wonderful effect in withholding my hand from doing it harm. Although hundreds have flown within my power, I never took more than five. I have given to this curious little creature the name of Mantis lucubrans; and having no doubt that he will introduce himself to every traveller who comes into this country [Southern Africa] in the months of November and December, I beg to recommend him as a harmless little companion, and entreat that kindness and mercy may be shown to him." This appearance of innocence and quietness must have struck all who have seen these Insects alive; nevertheless, it is of the most deceptive character, for the creature's activity consists of a series of wholesale massacres carried on day after day, the number of victims it sacrifices being enormous. The Mantis does not even spare its own kind; it is well known that the female not unfrequently devours its own mate. A very different picture to that of Burchell has been drawn by Potts, who observed the habits of a species in New Zealand.[176] He informs us that when about making an attack it approaches its intended prey with slow, deliberate movements, its anterior limbs folded in an innocent fashion, now and then raising itself or lifting the prothorax in a stealthy quiet manner, perhaps to judge accurately of its distance; when near enough, with one swift dart the victim is secured. The prey is held {250}firmly in the formidable trap formed by the anterior leg, and is thus brought near the mouth. The Mantis usually commences its feast by taking off some portions of the head of its wretched victim, and displays an absolute indifference to its struggling or kicking; the mandibles having seized a portion of the food, the legs holding it move away, thus leaving a fragment in the mouth. Portions only of a captured Insect are consumed, much being cast away; and Mr. Potts states that he has seen one of these voracious creatures kill and devour parts of fourteen small flies within a very brief space of time. This voracity and waste of animal food is very remarkable when we recollect that many Insects have such perfect powers of assimilation that during their whole period of growth they only consume a mass of food—and that vegetable—but little larger in size than the bulk they themselves attain. This fact is well known in the case of Bruchus, Caryoborus, and other seed-feeding Insects. Burmeister has stated good grounds for believing that some of the larger Mantidae do not confine themselves to Insect diet, but attack and devour small Vertebrates.[177] He has given a circumstantial account of a case at Buenos Ayres, where a small bird was secured by the wingless female of a large Mantis, which had commenced devouring its head when the observer took possession of the creature and its booty. Dubois states[178] that when a decapitated, but living, Mantis was suspending itself to a roll of drapery by its four posterior legs, a person could detach with the fingers the left anterior leg (of the four) and the right posterior, or conversely the left posterior and right anterior, without the interference producing any action on the part of the creature; but if one of the other legs was also interfered with, which would necessarily have changed the position of the body, then immediately one of the two unoccupied legs was placed by the creature in a proper position to assure its stability. This reflex action altogether resembled in appearance a conscious action, and was as effectually performed.
The combination in Mantidae of voracious and destructive instincts with helpless and inert attitudes gives rise to the idea that these latter are adopted for the purpose of deceiving the living prey and of thus more easily obtaining the means of subsistence. {251}It appears, however, more probable that the helpless attitudes have no such origin, but are due to the structure and form of the creature. The front legs being wonderfully well formed for raptorial purposes, have no capacity for locomotion or for supporting the Insect in the usual manner, so that the body has to be borne by the hinder two pairs of legs; at the same time the raptorial pair of limbs—which, it will be recollected, are of great size and attached to the anterior part of an unusually long prothorax—have to be held in such a position as will not derange the equilibrium maintained by the posterior part of the body; moreover, these large raptorial legs are entirely exserted, and have no trace of any articulatory cavity that might act as a mechanical aid to their support. Thus they could not be held extended without great muscular exhaustion; hence we can well believe that the sedentary and helpless attitudes of the creature are not the results of any guile.
A Mantis has been recorded as bearing a close resemblance to a Phasmid of the genus Bacillus and having only small front legs; it was suggested by Bates[179] that the Mantis would probably be found to feed on the Bacillus. Though the case is of considerable interest, no further information about it has been obtained.
The simplest forms of the family are found in the groups Amorphoscelides and Orthoderides. From our figure of one of these (Fig. 141, Mantoida luteola ♂), it will be seen that the peculiarities of the family can scarcely be detected, the raptorial legs being very little developed and the prothorax short. The sexes, too, differ but little in these simple forms. Most of them are very rare in collections, but Wood-Mason states[180] that Amorphoscelis annulicornis is frequently found about Calcutta on the trunks of trees, to the bark of which it is so similar that it is only discovered with difficulty. In its rapid movements it resembles the cockroaches or Machilis, more than it does the more differentiated forms of its own group.
In the genus Pyrgomantis (Fig. 142, P. singularis, female) the male has the tegmina and wings of normal size, while in the female they are rudimentary.
The variety of shape and external appearance in this family is very great; de Saussure considers it to be a mimetic group. In certain species some parts of the body—more especially the tegmina—have very much the appearance of foliage, and usually in such cases this appearance is confined to the female, the males in this family having, as we have said, the organs of flight more transparent and colourless; in the former sex the alar organs, when present, are frequently but little adapted for flying. In some species the prothorax is expanded at the sides (Fig. 135, Deroplatys sarawaca; and Fig. 143, Choeradodis cancellata), and in such cases the outline of the natural thorax—if we may use such an expression—may be detected occupying the middle of the unusual expansion. The European Mantis religiosa varies much in colour; in some examples the tegmina are leaf-green, while in others they are brown or gray. There is some evidence extant making it probable that in some species the colour of an individual changes at different times—Colonel Bowker saying of {253}Harpax ocellata that it "beats the Chameleon hollow in changing colour."
Some of the species of the old genus Eremiaphila (Fig. 144) are of very unusual form. De Saussure considers that some species of this genus are more highly modified than any other animals for maintaining their existence in desert regions. They are said to be found in places where no vegetation exists, and to assimilate in appearance with the sandy soil, the species varying in colour, so that the individuals agree in tint with the soil on which they dwell. These Insects are referred to the group Orthoderides, and have a short prothorax, the alar organs being unsuited for flight. What they live on is not actually known; although other Insects are the natural food of Mantids, it is said that these desert-frequenting species occur in spots where no other Insect life is known to exist. Lefebvre[181] met with these Eremiaphilas in the desert between the Nile and the Northern Oasis, El Bahryeh, but was quite unable to discover their mode of subsistence. These Insects are very rare in collections, and the information we possess about them is very meagre.
Mr. Graham Kerr found on the Pilcomayo river a species of Mantidae living on branches of trees amongst lichens, which it so exactly resembled that it was only detected by the movement of a limb; it was accompanied by a Phaneropterid grasshopper, which bore a similar resemblance to the lichens. One of the rarest and most remarkable forms of Mantidae is the genus Toxodera, in which the eyes project outwards as pointed cones (Fig. 145). These Insects offer an interesting problem for study, since we are entirely ignorant about them. Brunner places the Toxoderae in his tribe Harpagides, but with the remark that "these Insects of antediluvian shapes differ essentially from all other Mantidae."
Wood-Mason informs us[182] that the young of Hymenopus bicornis {254}beautifully simulate blossoms of different colours. And it has been stated by Dr. Wallace, on the authority of a communication made to him by Sir Charles Dilke, that a small Mantis found in Java exactly resembles a pink Orchis-flower, and this species "was not only said to attract Insects, but even the kind of Insects (butterflies) which it allures and devours was mentioned." We do not know of what species or genus this Insect may be, but Hymenopus bicornis is a peculiar form of the tribe Harpagides, and has, together with its younger state, been figured long ago by Caspar Stoll in his quaint and interesting old book.[183] Though it has very peculiar foliaceous expansions on the two hinder pairs of legs, these dilatations are very different from those seen in the curious Gongylus gongylodes, the female of which we figure (Fig. 146). This latter, according to the information we shall quote, is also a "floral simulator." Specimens of G. gongylodes were shown to the members of the Asiatic Society of Bengal in 1877 by Dr. J. Anderson,[184] who at the same gave some information about them which we shall reproduce in full, because, incomplete as it is, it is apparently almost the sole piece of definite information we possess as to this curious Insect, or any of its congeners:—
"These Insects all came from the same locality, having been {255}forwarded to Mr. Buckland by Mr. Larymore of the Central Jail at Midnapur. Mr. Larymore had procured them from the neighbouring country district, where Santál women and children had hunted them out and brought them in, hanging on branches or twigs of a bush, somewhat like a wild plum-tree. They are also said to be found upon rose-bushes, and in connexion with this it was observed that, in Midnapur, they were known as rose-leaf Insects, from the circumstance that when the Insect is more developed and furnished with wings, the foliaceous appendages are said greatly to increase in size, and exactly to resemble rose-leaves. Dr. Anderson, however, was disposed to think that more than one species might probably occur in the Midnapur district, and that these Insects with the larger foliaceous expansions might be distinct from the species now before the Society.
"Mr. Buckland had made over these Insects to Dr. Anderson, and since that time they have been regularly fed upon house-flies and grasshoppers; the latter, however, appear to be rather too strong for them, and they therefore prefer the flies. They have been tried with small fragments of plaintain and custard-apple, which they not only eat, but the juice of which they seem to suck with considerable avidity, Dr. Anderson, however, thought that it was the moisture of these fruits that was the chief attraction to these Insects, for the entire character of their organisation indicated a raptorial habit.
"Dr. Anderson went on to say that he had succeeded in identifying the three larger Insects by means of a single dried specimen in the Indian Museum, which, however, was fully mature and provided with wings. These remarkable Insects proved to be the pupae of a peculiar species of Mantis which was known to Aldrovandus, who figured it more than a century and a half before the first appearance of the Systema Naturae of Linnaeus, to whom it was known as Gryllus gongylodes, and also as Mantis gongylodes; and since the time of Aldrovandus it had been figured in a variety of works on Natural History, but apparently in every instance from mature, and seemingly dried specimens, so that the colours of the Insect during life had never been correctly described.
"So much by way of introduction to these remarkable pupal Mantises, the recognised scientific name of which is Gongylus gongylodes L.
"The reason which induced Dr. Anderson to bring them to the notice of the Society had now to be pointed out. On looking at the Insects from above, they did not exhibit any very striking features beyond the leaf-like expansion of the prothorax and the foliaceous appendages to the limbs, both of which, like the upper surface of the Insect, are coloured green, but on turning to the under surface the aspect is entirely different. The leaf-like expansion of the prothorax; instead of being green, is a clear, pale lavender-violet, with a faint pink bloom along the edges of the leaf, so that this portion of the Insect has the exact appearance of the corolla of a plant, a floral simulation which is perfected by the presence of a dark, blackish brown spot in the centre, over the prothorax, and which mimics the opening to the tube of a corolla. A favourite position of this Insect is to hang head {257}downwards among a mass of green foliage, and, when it does so, it generally remains almost motionless, but, at intervals, evinces a swaying movement as of a flower touched by a gentle breeze; and while in this attitude, with its fore-limbs banded violet and black, and drawn up in front of the centre of the corolla, the simulation of a papilionaceous flower is complete. The object of the bright colouring of the under surface of the prothoracic expansion is evident, its purpose being to act as a decoy to Insects, which, mistaking it for a corolla, fly directly into the expectant, serrated, sabre-like, raptorial arms of the simulator. It is no new fact that many Insects resemble the leaves of plants and trees, and that they manifest forms and colours which serve to protect them in the struggle for existence, but so far as Dr. Anderson had ascertained, this was the first recorded instance of an Insect simulating the corolla of a flower for the evident purpose of attracting Insects towards it for its sustenance. It is even more remarkable than this, for it is a localised adaptation for such a purpose, a portion of the Insect being so modified in form and colour that the appearance of the corolla of a plant is produced, in conjunction with the remainder of the long attenuated prothorax, which at a distance resembles the flower stem; the anterior limbs when in repose even adding to and heightening the deception."
That we should have no more precise information as to a large Insect of such remarkable habits and appearance, and one that has been known to naturalists for upwards of three centuries, is a matter for regret. Careful observation as to the habits, food, and variation of these floral simulators, and as to whether they seek for spots specially suitable to their coloration, would be of great interest. A European congener of this Insect, Empusa pauperata, has small foliaceous expansions on the legs, but its habits have not been noticed in detail.
The very curious Insect represented in Fig. 147, Stenophylla cornigera, is a member of the tribe Vatides; the form of the cerci at the end of the body is very peculiar. This extremely rare, if not absolutely unique, Insect is a native of the interior of Brazil.
Dufour has recorded that Mantis religiosa possesses the power of producing a mournful sound by rubbing the extremity of the body against the wings; it is stated that a hissing sound is {258}produced by other species, and Wood-Mason has suggested[185] that a special structure exists on the tegmina for the purpose.
There are probably about 600 species of Mantidae known; they are distributed over all the warmer parts of the earth, but there are none in the cooler regions. Europe possesses some twelve or fourteen species, most of them confined to the Mediterranean sub-region; a single species, Mantis religiosa, is frequently found in Central France, and has been recorded as occurring as far north as Havre. Although no species is a native of Britain, it is not difficult to keep them alive here. Denny records[186] that an egg-case of a Mantis was sent from Australia to England, and that the hatching of the eggs was completed after its arrival. The young fed readily on flies, and we are informed that in the neighbourhood of Melbourne, where this Mantis is plentiful, specimens are placed by the citizens on the window-blinds of their houses, so that the rooms may be cleared from flies by means of the indefatigable voracity of the Mantis.
The geological record as to Mantidae is very meagre and unsatisfactory. The genus Mantis is said to occur in amber, and Heer has referred to the same genus an ill-preserved fossil from the upper Miocene beds of Central Europe; a fragment {259}of a hind wing found in the Jurassic strata of Siberia has been assigned to the family; and until recently Lithomantis from the Carboniferous beds of Scotland was considered to belong to Mantidae. Scudder, however, has rejected it therefrom, placing it in the Neuropteroid division of Palaeodictyoptera, and Brongniart, adding another species to the genus from the Carboniferous strata in France, proposed to treat the two as a distinct family, which he called Palaeomantidae.[187] This naturalist has, however, since renewed his study[188] of these Insects, has become convinced that they have no relations with existing Mantidae, and has consequently removed them to the family Platypterides in the Order Neuroptera.
Six tribes of Mantidae are recognised by Brunner and de Saussure.
Table of the tribes of Mantidae:—
1. Anterior tibiae with the outer edge unarmed beneath or only furnished with very minute tubercles. (Pronotum not longer than the anterior coxae.) Tribe 1. Amorphoscelides. (Fig. 141, Mantoidea luteola.)
1′. Anterior tibiae with the outer edge spinose beneath.
2. Anterior femora having the inner edge armed beneath with equal spines, or with spines in which only the alternate are smaller. Antennae of the male simple, rarely unipectinate.
3. Tibiae and also the intermediate and hind femora even above.
4. Legs and body with no lobe-like processes. (Antennae simple in each sex.)
5. Pronotum not forming any dilatation above the insertion of the coxae, its lateral margins straight or (in the genus Choeradodis) strongly dilated with the anterior margin not rounded. Tribe 2. Orthoderides. (Fig. 142, Pyrgomantis; Fig. 143, Choeradodis; Fig. 144, Eremiaphila turcica.)
5′. Pronotum dilated above the insertion of the coxae, there with the lateral margins broadened in a round manner, the anterior margin rounded. Tribe 3. Mantides. (Fig. 140, Iris oratoria.)
4′. Legs or body furnished with lobes. (Posterior femora or segments of the body with lobes, or vertex of the head conically prolonged.) Tribe 4. Harpagides. (Fig. 136, Harpax variegatus; Fig. 135, Deroplatys sarawaca.)
3′. Tibiae as well as the intermediate and hind femora carinate above. (Pronotum elongate, with the posterior part, behind the transverse groove, three times as long as the anterior part.) Tribe 5. Vatides. (Fig. 147, Stenophylla cornigera.)
2′. Anterior femora beneath, with the inner edge armed between the longer teeth with shorter teeth, usually three in number. Antennae of the male bipectinate. (Vertex conically prolonged.) Tribe 6. Empusides. (Fig. 146, Gongylus gongylodes.)
ORTHOPTERA CONTINUED—PHASMIDAE—WALKING-LEAVES—STICK-INSECTS
Fam. V. Phasmidae—Stick and Leaf Insects.
Head exserted; prothorax small, not elongate; mesothorax very elongate; the six legs differing but little from one another, the front pair not raptorial, the hind pair not saltatorial. The cerci of the abdomen not jointed, consisting of only one piece; the tarsi five-jointed. Tegmina usually small, or entirely absent, even when the wings are present and ample. The sexes frequently very dissimilar. Absence of alar organs frequent.
These Insects are amongst the most curious of natural objects. They are frequently of large size, some attaining 9 inches in length (Fig. 162, Palophus centaurus, one-half natural length). Their variety of form could scarcely be surpassed; their resemblance to products of the vegetable kingdom is frequently very great: some of the more linear species (Fig. 148, Lonchodes nematodes) look like sticks or stems of grass; some have a moss-like appearance, while others resemble pieces of lichen-covered bark. The members of the tribe Phylliides are leaf-like. A certain number of other Phasmids are covered with strong spines, like thorns (Fig. 149). The plant-like appearance is greatest in the female sex. When there is a difference between the two sexes as to the organs of flight, these are more fully developed in the male.
The antennae are usually many-jointed, but the number of joints varies from 8 to more than 100; the head is exserted; the eyes are more or less prominent; ocelli are present in some cases. The prothorax is always small, and it is a remarkable fact that it undergoes but little elongation even in those species that are most linear and elongate in form (see Fig. 148, Lonchodes nematodes), and that have the meso- and metathoraces extremely long; it is very simple in structure, consisting apparently merely of a dorsal and of a sternal plate, nearly the whole of the side being occupied by the large space in which the coxae are inserted; the edges of the pronotum are not free. The mesothorax is frequently six times as long as the prothorax, though in the leaf-like and a few other forms it does not possess this great extension; still it is always of large size relatively to the other two thoracic segments. This is peculiar inasmuch as in other groups where the mesothorax is relatively large there are powerful mesothoracic wings; whereas the Phasmidae are remarkable for the obsolescence of the mesothoracic alar appendages. The middle legs and the tegmina or elytra, when present, are attached only to the posterior part of the mesothorax; the notum and the sternum are separated by two narrow slips on each side, the epimeron and episternum. The metathorax is formed like the mesothorax, except that the posterior part of the dorsal surface is considered to consist of the first ventral segment consolidated with the posterior part of the metanotum, the two being distinct enough in the winged forms. The hind body or abdomen is elongated except in the Phylliides; it consists of ten dorsal plates; the first frequently looks like a portion of the metanotum, and is treated as really such by Westwood, who describes the abdomen as consisting of nine segments. The flat apical appendages are attached behind the tenth dorsal plate. The ventral plates are similar to the dorsal in arrangement, except that in the female the eighth plate forms a sort of spoon-like or gutter-like process to assist in carrying or depositing the eggs, and that the two following segments are concealed by it, and are sometimes of more delicate texture. The legs vary greatly in the details of {262}their shape: the coxae are short, oval, or round, never large; the trochanter is small; the front femora often have the basal part narrower than the apical, and they are frequently so formed that they can be stretched out in front of the head, concealing its sides and outline and entirely encasing the antennae. There is an arolium or cushion between the claws of the five-jointed tarsi. The front legs are frequently longer than the others. Only a very slight study has been made of the alar organs of Phasmidae; but according to Redtenbacher and Brauer, they differ greatly from those of Blattidae and Mantidae, inasmuch as the costal vein is placed not on the actual margin of the wing but in the field thereof, and in this respect they more resemble the Orthoptera saltatoria.
Very little information exists as to the internal anatomy of the Phasmidae. Many years ago a memoir of a fragmentary and discursive nature was published on the subject by J. Müller,[189] but his conclusions require confirmation; the nervous system, according to his account, which refers to Arumatia ferula, has the anterior ganglia small, the supra-oesophageal ganglion being apparently not larger than those forming the ventral chain.
Joly's more recent memoir on the anatomy of Phyllium crurifolium[190] is also meagre; he states that the nervous system resembles that of the locusts (Acridiidae), though there are at least ten pairs of ganglia—one supra-, one infra-oesophageal, three thoracic, and five abdominal. He found no salivary glands; the Malpighian tubules are slender, elongate, and very numerous. The tracheal system has no air-vesicles. He found no distinction {263}of crop and proventriculus, but the true stomach appears to consist of two different parts, the anterior being remarkably uneven externally, though destitute of coeca, while on the posterior part there are peculiar vermiform processes. There are eighteen or twenty tubes in each ovary.
When the young Insect is in the egg, ready for emergence, the meso- and meta-thorax are not remarkably elongate, so that the femora are not very far apart, but by the time the creature has fairly emerged from the prison of its embryonic life the thoracic segments have attained their usual proportions; much expansion of the body takes place as the Insect leaves the egg, so that it appears a marvel how it could have been contained therein; this expansion affects the parts of the body unequally.
The records as to the post-embryonic development of Phasmidae are very scanty, but indicate great differences in the length of time occupied by it. Bacillus patellifer is said to moult several times, Diapheromera femorata only twice. This latter species becomes full grown in six weeks, while, according to Murray,[191] Phyllium scythe required fifteen or sixteen months for growth, and did not moult until ten months after hatching; the number of ecdyses in the case of the Phyllium was three. At each change of skin an immediate increase in size, similar to that we have noticed as occurring on leaving the egg, takes place; each limb on being freed becoming about a fourth longer and larger than the corresponding part of the envelope from which it has just been withdrawn. After the second moult of Phyllium the tegmina and wings made their appearance, but remained of very {264}small size until after the third moult, when they suddenly shot out to their full size; they came out of little cases about a quarter of an inch long, and in the course of a few minutes attained their full size of about two and a half inches of length. In the apterous species the difference between the young and adults in external characters is very slight.
Phasmidae are very sensitive to cold; both in North America and Australia their lives are terminated by the occurrence of frost. They are all vegetable feeders, the cannibalism that has been attributed to them by several writers being probably imaginary. They are, however, excessively voracious, so that a pair will destroy a great quantity of foliage; they are consequently in some parts of the world classed amongst injurious Insects. In Fiji and the Friendly Islands, Lopaphus cocophagus eats the cocoa-nut foliage and causes a scarcity of food, so that it becomes a matter of necessity to destroy these Insects. One writer has gone so far as to attribute the occurrence of cannibal habits amongst the inhabitants of some of these islands to the want of food caused by the ravages of this Insect. Some, if not all, of the Phasmidae have the habit of ejecting a stinking fluid, that is said to be very acrid, and occasionally, when it strikes the eye, to cause blindness; this liquid comes from glands placed in the thorax. Some Phasmidae are much relished as food by birds; Diapheromera femorata is sucked by several bugs as well as eaten by birds, and another species is recorded to have harboured Ichneumon-flies in its body without suffering any apparent inconvenience from their presence or from their emergence. Notwithstanding the great amount of food they consume and their want of activity, they produce comparatively few eggs. From twelve to twenty or thirty is frequently mentioned as about the {265}number, but in the case of Diapheromera femorata Riley speaks of upwards of one hundred. These eggs are not deposited in any careful way, but are discharged at random, simply dropping from the female; the noise caused by the dropping of the eggs of Diapheromera femorata from the trees on which the Insects are feeding to the ground is said to resemble the pattering of raindrops. The eggs of this species often remain till the second year before they hatch. The eggs in the Phasmidae generally are of a most remarkable nature, and nearly every one who mentions them speaks of their extreme resemblance to seeds. Göldi[192] has suggested that this is for the purpose of deceiving Ichneumons; it is, however, on record that the eggs are actually destroyed by Ichneumons. It is worthy of notice that the eggs are shed like seeds, being dropped loosely and, as we have said, remaining on the ground or elsewhere, sometimes for nearly two years, without other protection than that they derive from their coverings. Each egg is really a capsule containing an egg, reminding us thus of the capsule of the Blattidae, which contains, however, always a number of eggs. Not only do the eggs have a history like that of seeds, and resemble them in appearance, but their capsule in minute structure, as we shall subsequently show, greatly resembles vegetable tissue. The egg-capsule in Phasmidae is provided with a lid, which is pushed off when the Insect emerges (Fig. 157). This capsule induced Murray to suppose that the egg contained within is really a pupa, and he argued therefrom that in the Orthoptera the larval stages are passed in the egg, and that the Insect after its emergence should be looked on as an active pupa that takes food.
Fig. 152.—Eggs of Phasmidae: A, Lonchodes duivenbodi; B, Platycrania edulis; C, Haplopus grayi; D, Phyllium siccifolium. (After Kaup.)
The individuals of this group of Insects possess the power of reproducing a lost limb; and Scudder, who has made some experiments as to this,[193] states that if a leg be cut off beyond the {266}trochantero-femoral articulation, the parts remaining outside of this joint are dropped before the next moult, and are afterwards renewed either as a straight short stump in which the articulations are already observable, or as a miniature leg, the femur of which is straight and the tibia and tarsus curved into a nearly complete circle; in the former case, the leg assumes at the next moult the appearance that it has in the second case; this latter form is always changed at the succeeding moult into a leg resembling the normal limb in every respect excepting size, and the absence of the fourth tarsal joint (Fig. 153). If the leg be removed nearer to the body than the trochantero-femoral articulation the limb is not replaced.
The sexes are frequently extremely different; the female is usually very much larger than the male. This latter sex often possesses wings when they are quite wanting in the other sex; the resemblance to portions of plants is often very much greater in the female than it is in the male.
Fig. 153.—Cyphocrania aestuans; individual in which the right front leg has been renewed. Senegal. (After Westwood.)
We have pointed out that the tegmina or upper wings are usually of small size or absent (Fig. 150, Aschipasma catadromus), even in the species where the lower wings are very largely developed; in such cases the latter organs are folded in a complicated, fan-like manner, and repose on the back, looking as if they were really the tegmina (Fig. 159, Calvisia atrosignata); this appearance, moreover, is in some species enhanced much by the fact that the part of the wing which is outermost in the folded state is quite differently {267}coloured from the rest of the organ. The colour of the body in many Phasmidae is said to be very variable, and if the tints be owing to chlorophyll or other plant juices, finding their way amongst the Insect-tissues, this is readily understood; in Diapheromera the young Insect is brownish on hatching, becomes green after feeding, and turns brown again when the leaves do so. The ocelli, too, are said to be very variable, and M‘Coy goes so far as to state[194] that they may be either present or absent in different individuals though of the same species and sex,—a statement so remarkable as to require minute examination, though it is to some extent confirmed by the remarks of other entomologists.
The resemblance presented by different kinds of Orthoptera to leaves is so remarkable that it has attracted attention even in countries where Natural History is almost totally neglected; in many such places the inhabitants are firmly convinced that the Insects are truly transformed leaves, by which they understand a bud developing into a leaf and subsequently becoming a walking-leaf or Insect. To them the change is a kind of metamorphosis of habit; it grew as a leaf and then took to walking.[195] It is usually the tegmina that display this great resemblance to vegetable structures, and there is perhaps no case in which the phenomenon is more marked than it is in the genus Phyllium, the members of which occur only in the tropical regions of the Old World, where they extend from Mauritius and the Seychelles to the Fiji Islands—possibly even more to the East—and have, it would appear, a peculiar penchant for insular life.
The genus Phyllium constitutes by itself the tribe Phylliides. Although the characters and affinities of this group have been only very inadequately investigated, it will probably prove to be a very distinct and isolated one. The species are not well known, but are probably numerous, and the individuals are believed not to be rare, though the collections of entomologists are very badly supplied with them. The resemblance of the tegmina or front wings to leaves is certainly of the most remarkable nature. During the early life the Insect does not possess the tegmina, but it is said then to adapt itself to the appearance of the leaves it lives on, by the positions it assumes and the movements[196] it makes. When freshly hatched it is of a reddish-yellow colour. The colour varies at different periods of the life, but "always more or less resembles a leaf." After the young Insect has commenced eating the leaves it speedily becomes bright green; and when the metamorphosis is completed the female Insect is possessed of the leaf-like tegmina shown in Figs. 154, 156. Before its death the specimen described by Murray passed "through the different hues of a decaying leaf." Brongniart has had opportunities of observing one of these leaf-Insects, and has, with the aid of M. Becquerel, submitted their colouring matter to spectral analysis,[197] with the result of finding {269}that the spectrum exhibits slight distinctions from that of solutions of chlorophyll, but does not differ from that of living leaves. Mr. J. J. Lister when in the Seychelles brought away living specimens of Phyllium; and these becoming short of food, nibbled pieces out of one another just as they might have done out of leaves. The Phasmidae are purely vegetable feeders, and these specimens did not seriously injure one another, but confined their depredations to the leaf-like appendages and expansions.
The males of this genus are totally different from the females; the foliaceous tegmina being replaced by appendages that are not leaf-like, while the posterior wings, which are large and conspicuous parts of the body, have no leaf-like appearance (Fig. 155).
Fig. 156.—Alar organs and one side of thorax of Phyllium crurifolium: A, tegmen; B, rudiment of wing; C, pronotum; D, anterior division of mesonotum; E, posterior division; F, metanotum; a, b, c, d, e, chief wing-nervures; a, mediastinal; b, radial; c, ulnar; d, dividens?; e, plicata?.
In the female Phyllium the hind wings are not present, being represented by a minute process (Fig. 156, B). The tegmen of the female Phyllium is, from various points of view, a remarkable and exceptional structure. It is the rule that when there is in Insects a difference between the alar organs of the two sexes it is the male that has them largest; this is the case in Phyllium so far as the hind wings are concerned, but in the fore-wings the rule is departed from, the leaf-like tegmina of the female being very much larger than the rudimentary wing-covers of the male. In Phasmidae it is the rule that the tegmina are atrophied, even when the hind wings are largely developed. This is the case in the male of Phyllium, but in the female this normal condition is reversed. Although the alar organs of Phasmidae have received hitherto but a small amount of attention, it is probable that the female tegmen of Phyllium is as peculiar morphologically as it is in other respects. In Fig. 156 we give an accurate representation of the chief nervures in the tegmen of a female P. crurifolium. It is interesting to compare this with the diagrams we give of the tegmina of a Blattid (Fig. 121) and of an Acridiid {270}(Fig. 167); the tegmen of the Phyllium is very different, the radial vein and all the parts behind it being placed quite close to the posterior edge of the structure. A similar view is taken by both Redtenbacher and Brauer. The latter says,[198] "In Phyllium (the walking-leaf) almost the whole of the front wing is formed by the praecostal and subcostal fields; all the other fields with their nervures, including even the costa, are compressed towards the hind margin into a slender stripe. In the hind wing the costa is, however, marginal." Unfortunately no examination appears to have been made of the male tegmen, so that we do not know whether that of the female differs from it morphologically as strongly as it does anatomically. It is, however, clear that the tegmina of the female Phyllium not only violate a rule that is almost universal in the Insecta, but also depart widely from the same parts of its mate, and are totally different—and, for a Phasmid, in an almost if not quite unique fashion—from the other pair of alar organs of its own body.
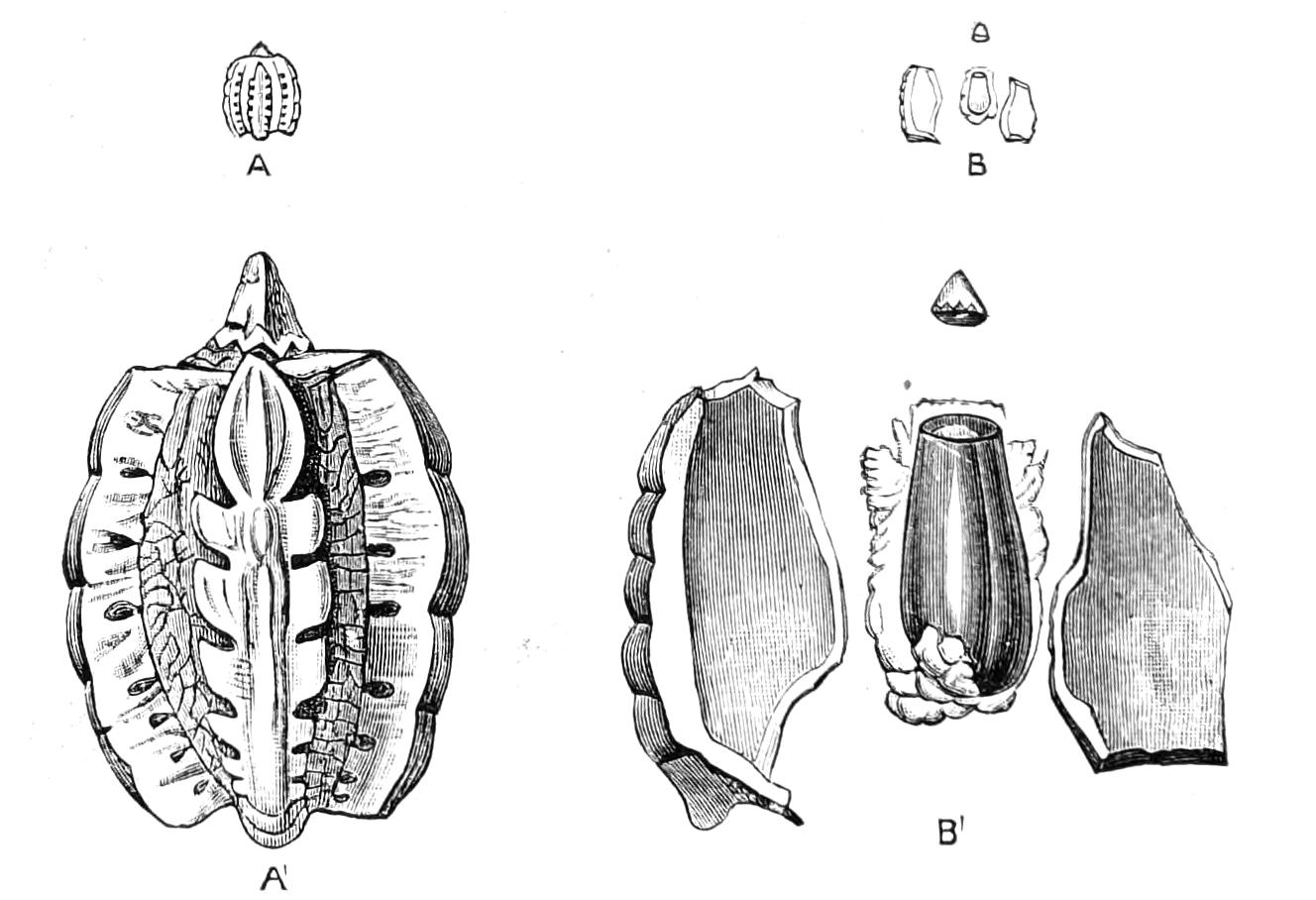
Fig. 157.—Egg of Phyllium scythe. (After Murray.) A, The whole egg, natural size; A', magnified; B, the capsule broken, showing the true egg inside, natural size; B', magnified.
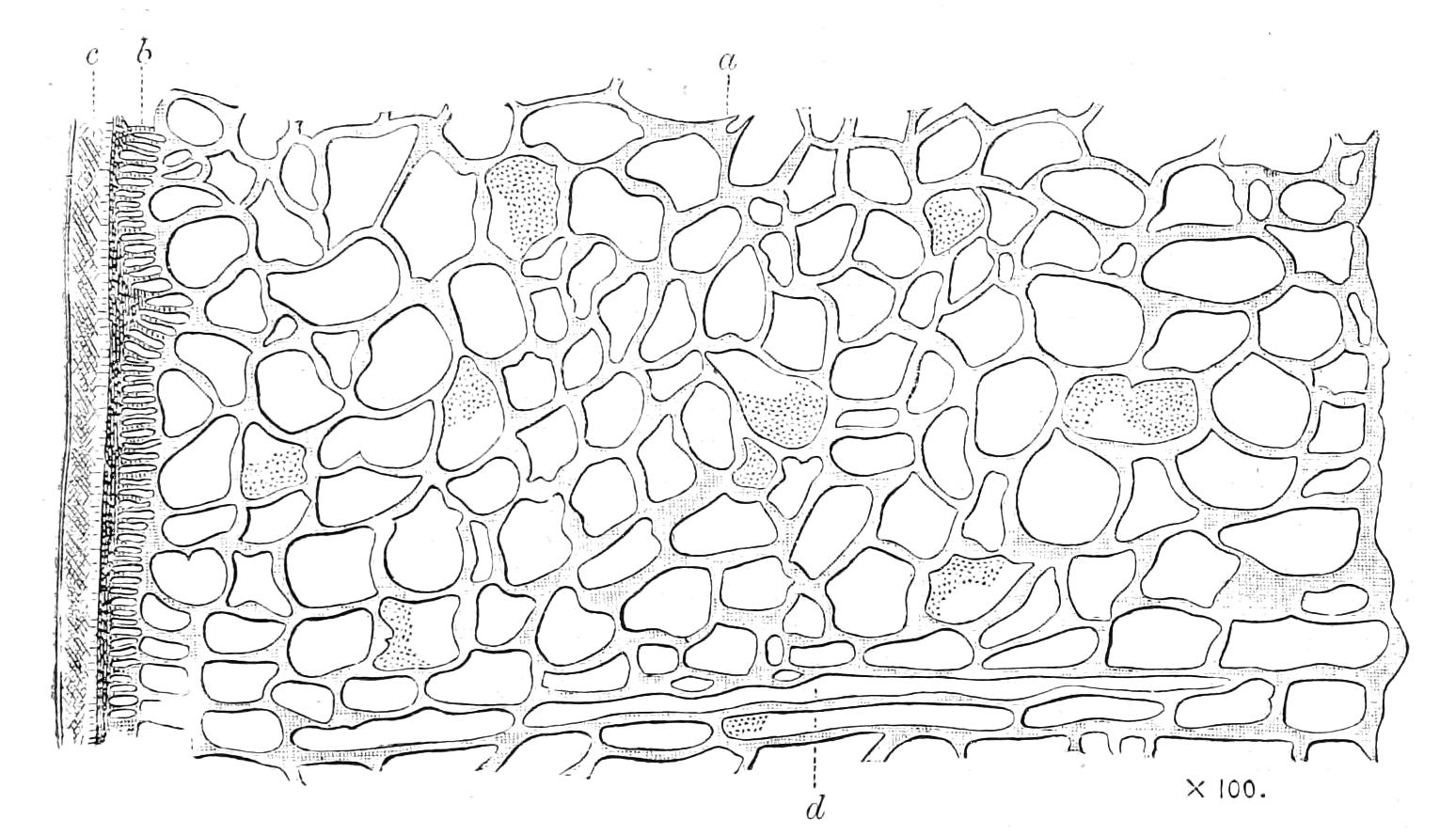
Fig. 158.—Portion of a longitudinal section of the egg capsule of Phyllium crurifolium: a, external; b, middle; c, inner zones; d, elongate alveoli. × 100. (After Henneguy.)
We have already alluded to the resemblance to seeds displayed by the eggs of Phasmidae. The eggs of Phyllium have been studied by several entomologists, and their resemblance to seeds excites general astonishment. Murray describes the egg-capsule of Phyllium scythe, and says: "It looks uncommonly like some seeds; if the edges of the seed of Mirabilis jalapa were rubbed off, the seed might be mistaken for the egg. The ribs are all placed at equal distances, except two, which are wider apart, and the space between them flatter, so that on the egg falling it rolls over till it comes to this flatter side, and there lies.... At the top there is a little conical lid, fitting very tightly to the mouth.... On removing the lid we see a beautiful porcelain chamber of a pale French-white colour, bearing a close resemblance to the texture of a hen's egg, but it is not calcareous, and has more the appearance of enamel." The eggs of P. crurifolium have been examined by Joly and Henneguy; their account confirms that of Murray. Henneguy adds that a prominent lozenge on the egg represents the surface by which the achene of an umbelliferous plant is united to the column, and that the micropyles are placed on this lozenge. The minute structure of the capsule has also been examined by several entomologists; and Henneguy,[199] who has described and figured some of the details of the capsule of P. crurifolium, says, "Almost every botanist, on examining for {272}the first time a section of this capsule, would declare that he is looking at a vegetable preparation."
We may remark that, although there is difference of opinion on the point, the evidence extant goes to show that the egg-capsules are formed in the egg-tubes, only one egg being produced at a time in a tube,[200] the others in it remaining quite rudimentary.
About 600 species of the family are known; there are only four or five kinds found in Europe, and they are all confined to the south, only one of them extending as far north as Central France. The males of these European Bacilli are extremely rare in comparison with the females, which are common Insects. Phasmidae are of almost universal distribution in the warm parts of the world, and even the species whose individuals are of large size seem to be able to continue their existence in comparatively small islands. Australia is perhaps the region where they are most largely developed at present. Macleay says of Podacanthus wilkinsoni that it is rare in any part of Australia to find in the summer season a gum-tree without a few of these Insects grazing on it; and occasionally this Insect has been so abundant there that the trees for miles around have been denuded of their foliage by it, and the dead and dying Insects have been found lying beneath the trees almost in heaps. There are several Phasmidae in New Zealand, all wingless forms, and different from those found in Australia. In Brazil a species of the genus Prisopus has the peculiar habit of seeking shelter under the stones submerged in the mountain streams; to enable it to do this it is remarkably constructed, the under side of the body being hollowed, and various parts set with a dense fringe of hairs; the Insect is supposed to expel the air from the body in order to adhere to the upper surface of a stone, where it sits with its fore legs extended in front of its head, which is directed against the current. Attention has been called to a still more remarkable form said to be allied to the Prisopi, by Wood-Mason,[201] who calls the Insect Cotylosoma dipneusticum. This Insect is apparently known only by a single example of the female sex; it is 3 or 4 inches in length, has rudimentary organs of flight, and along the lower margins of the metathorax there are said to be on each side five {273}conspicuous fringed plates of the nature of tracheal gills; these coexist with open stigmata for aerial respiration, as in the imago of Pteronarcys. The writer has examined this curious Insect, and thinks it very doubtful whether the plates are branchiae at all. The locality for this Insect is the island of Taviuni, not Borneo, as stated by Wood-Mason. These and one or two Acridiidae are the only Insects of the Order Orthoptera at present believed to possess aquatic habits.
Although the number of species of Phasmidae is small in comparison with what we find in many of the large families of Insecta, yet there is probably no other family that equals it in multiplicity of form and diversity of external appearance.
Karabidion (Fig. 160), a genus found in some of the islands of the southern hemisphere, has the hind legs enormously thickened in the male. Some Phasmids, e.g. Orxines zeuxis, have the hind wings marked and coloured after the manner of butterflies or moths. Lamponius laciniatus has an elaborately irregular outline, looking like a mass of moss, and some species of Bacteria are so very slender that the linear body is scarcely equal in size to one of the legs it bears. Among the most interesting forms are the Insects for which the genera Agathemera and Anisomorpha (Fig. 161) have been established; they are remarkably broad and short, have the mesothorax but little elongated, with the tegmina attached to it in the form of two short, thick, leathery lobes; while the wings are seen as marks on the metanotum looking like a mere sculpture of the surface; these Insects {275}have quite the appearance of larval forms, and it is worthy of note that the elongation of the mesothorax, which is one of the most marked features of the Phasmidae, is in these forms only very slight.
Fig. 162.—Palophus centaurus. Old Calabar. Half natural size. (After Westwood.[202])
Some Insects said to belong to the genera Phasma and Bacteria have been found in amber. A single Insect-fossil found in the Tertiary strata in North America has recently been referred by Scudder to the family, and even to a genus still existing in the New World—Agathemera; the fragment is, however, so defective, and the characteristic points of the Phasmidae are so little evident in it, that not much reliance can be placed on the determination. No Phasmid has been unearthed from Mesozoic strata, so that, with the exception of the fragment just mentioned, nothing that evidently belongs to the Phasmidae has been discovered older than the remains preserved in amber. In the Carboniferous layers of the Palaeozoic epoch there are found remains of gigantic Insects that may possibly be connected with our living Phasmidae. These fossils have been treated by Brongniart and Scudder as forming a distinct family called Protophasmidae. The first of these authors says[203] that our Phasmidae were represented in the Carboniferous {277}epoch by analogous types differing in the nature of the organs of flight: these ancient Insects were of larger size than their descendants, being 25 to 50 centimetres long, and as much as 70 in spread of wing. To this group are referred, on somewhat too inferential grounds, the fossil wings found in the Carboniferous layers, and called by Goldenberg Dictyoneura.
We reproduce from Zittel's handbook a figure (Fig. 162) of one of these gigantic Insects, and add an attempt at a restoration of the same after the fashion of Scudder (Fig. 163). From these figures it will be seen that the relation to our existing Phasmidae must at best have been very remote.[204] It will be noted that the larger of the two figures is on a ⅕ scale.
The classification of Phasmidae was left in a very involved state by Stål, but has recently been brought into a more satisfactory condition by Brunner von Wattenwyl. We give a translation of his table of the tribal characters:—
1. Tibiae beneath carinate to the apex, without an apical area.
2. Antennae much longer than the front femora, many jointed, the joints being above 30 in number and only distinct at the base and towards the apex.[205]
3. Median [true first abdominal] segment much shorter than the metanotum.[206] The species all apterous.
4. The anal segment of the males roof-like, more or less bilobate. The female has a supra-anal lamina. The species inhabit the Old World. Tribe 1. Lonchodides (Fig. 148, Lonchodes nematodes.)
4′. The anal segment of the males arched, straight behind. No supra-anal lamina in the female. The species are American. Tribe 2. Bacunculides.
3′. Median segment as long as, or longer than the metanotum. Species with the male or both sexes winged.
4. Females apterous or rarely possessed of short wings.[207] Males winged. Femora dentate beneath, or lobed, or at least armed with one tooth. Species occur both in America and in the Old World. Tribe 3. Bacteriides. (Fig. 162, Palophus centaurus.) {278}4′. Each sex winged. Femora smooth beneath. The species belong to the Old World. Tribe 4. Necroscides. (Fig. 159, Calvisia atrosignata.)
2′. Antennae (at any rate in the females) shorter than the front femora, the joints distinct, not more than 28 in number. The species belong to the Old World.
3. Median segment shorter than the metanotum. Apterous species. Cerci plump. Tribe 5. Clitumnides. (Fig. 160, Eurycantha australis.)
3′. Median segment longer than the metanotum. Species usually winged. Cerci (except in some genera of the group Platycraninae) flattened, elongate. Tribe 6. Acrophyllides. (Fig. 153, Cyphocrania aestuans.)
1′. Tibiae furnished beneath with a triangular apical area.
2. Antennae many jointed, longer than the front femora.
3. Median segment shorter than the metanotum. Apterous species.[208]
4. Either head, thorax, or legs spiny or lobed. Tribe 7. Cladomorphides. (Fig. 149, Heteropteryx grayi.)
4′. Head, thorax and legs unarmed. Tribe 8. Anisomorphides. (Fig. 161, Anisomorpha pardalina.)
3′. Median segment longer than the metanotum.
4. Claws unarmed. Tegmina lobe-like, either perfectly developed or entirely absent. The winged species are all American, the apterous are both African and Australian. Tribe 9. Phasmides.
4′. Claws toothed on the inner side. Tegmina spine-like. Wings well developed. The species are Asiatic. Tribe 10. Aschipasmides. (Fig. 150, Aschipasma catadromus.)
2′. Antennae shorter than the anterior femora,[209] formed of not more than 20 joints. Old World species.
3. Body slender. Apterous. Tribe 11. Bacillides.
3′. Body very broad, lamina-like. Either wings or tegmina present. Tribe 12. Phylliides. (Fig. 155, Phyllium scythe, male; Fig. 154, idem., female.)
ORTHOPTERA CONTINUED—ACRIDIIDAE
Fam. VI. Acridiidae—Locusts and Grasshoppers.
Orthoptera with the hind legs differing from the others by being more elongate and having their femora broader near the base. Antennae short, with less than 30 joints. No exserted ovipositor in female. Tarsi short, with three distinct joints. The auditory organ placed on the side of the upper part of the first abdominal segment.
We commence the consideration of the saltatorial Orthoptera with the family Acridiidae. It includes the grasshoppers of our native fields as well as the destructive migratory locusts of foreign countries, and is the most numerous in species and individuals of any of the Orthopterous families. Our native grasshoppers, though of small size, give a very good idea of the Acridiidae. Active little Insects, with large head, conspicuous {280}eyes, laterally somewhat compressed body, long hind legs with femur directed upwards and backwards, the knee-joint forming an acute angle, the organs of flight pressed to the sides of the body, our common grasshoppers represent the Acridiidae quite as truly as do the gigantic exotic forms, some of which measure 9 or 10 inches across the expanded wings.
The large head is immersed behind in the thorax; the front is deflexed, or even inflexed, so as to be placed in a plane at an acute angle with that of the vertex (Fig. 165); the compound eyes are placed at the sides of the head and rather widely separated; in front there are three small ocelli. Two of these are placed one on each side close to the eye between the eye and the base of the antenna; the third ocellus being in the middle just in front of the insertion of the antennae, between the edges of the margined space that usually runs down the middle of the front. The positions of these ocelli and the shape of the front and upper parts of the head are of importance in the classification of the family; the ocelli vary much in their development, being in some species beautifully clear and prominent (Fig. 166), while in others they are small, not easily detected, apparently functionally imperfect. The antennae are never very long, are sometimes compressed and pendent from the front of the head. The parts of the mouth are very large. The prothorax is much arched; it is often carinate or crested along the middle of the notum; this part is frequently prolonged backwards, forming a sort of hood over the base of the wings; the surface may be rugged or warty, forming in some species inexplicable structures; the legs are widely separated, all of them being placed at the sides of the body; the edge of the pronotum is distinct and situate close to the base of the leg; the prosternum frequently bears a large projection extending directly downwards between the front legs. The mesothorax is short, its chief sternal piece is very broad, the middle legs being very widely separated. The metathorax is larger; its sternal plate usually exhibits behind a sort of embrasure filled up by a portion of the first ventral plate.
Fig. 167.—Alar organs of Acridiidae (Bryodema tuberculata). A, Left tegmen; B, left wing: ar.med, area mediastina; ar.sc, area scapularis; ar.disc, area discoidalis; ar.an, anal area; v.m, vena mediastina; v.r, vena radialis; v.r.a, vena radialis anterior; v.r.m, vena radialis media; v.r.p, vena radialis posterior; v.i, vena intercalata; v.u.a, vena ulnaris anterior; v.u.p, vena ulnaris posterior; v.d, vena dividens; v.pl, vena plicata. (After Brunner.)
The hind body is elongate, and shows distinctly eight dorsal segments, behind which are the pieces forming—in the female, the fossorial organs which replace an ovipositor—in the male, the modified parts connected with the terminal segment. The alar organs (Fig. 167) exhibit, according to Brunner, the same areas as we have described in Blattidae. According, however, to Redtenbacher[210] the tegmina of the Acridiidae and other saltatorial Orthoptera differ from those of the cursorial group (with the exception of the Phasmidae) in that they possess a praecostal field, due to the fact that the vein which in the Cursoria is costal, i.e. forms the front margin, in the Saltatoria lies, on the contrary, in the field of the wing. If this view be correct the mediastinal area of Brunner is not homologous in the two divisions. The tegmina are long and comparatively narrow; they are of firm parchment-like texture, with several longitudinal veins, which divide beyond the middle, so as to become more numerous as they reach the extremity of the wing; there is much reticulation, dividing the surface into numerous small cells. The hind wings are much more ample, and of more delicate texture; the longitudinal veins fork but little, the numerous cross veinlets are fine. In repose the hind wings fold together in a fan-like manner, and are entirely concealed by the upper wings. The front and middle legs are similar and small, the coxae are quite small, and do not completely fill the articular cavities, which are partly covered by membrane; all the tarsi are three-jointed. The basal joint, when looked at beneath, is seen to bear three successively placed pads, so that from beneath the tarsi look as if they were five-jointed {282}(Fig. 185, C). The hind legs are occasionally very long; their femora, thicker towards the base, are generally peculiarly sculptured, bearing longitudinal ridges or grooves, which are more or less spinose, and are also very frequently marked with short parallel lines meeting a central longitudinal line at similar angles, so as to give rise to a well-marked pattern; where the legs are broader the pattern is more complex (Fig. 168). The long tibiae bear two rows of spines on their upper or posterior edge; this part of the hind leg can be completely bent in under the femur. The stigmata consist of one prothoracic, one metathoracic, and eight abdominal pairs.
In reference to the ocelli, which are shown in Fig. 166, we may remark that the Acridiidae is one of the large groups of Insects in which the coexistence of compound and single eyes is most constant, though in some of the wingless forms the ocelli are very imperfect. We know at present of nothing in the habits of Acridiidae to render two kinds of eyes specially necessary. We shall subsequently see that a similar condition in regard to the function of hearing is believed to exist in this family.
Acridiidae are remarkable amongst the Orthoptera for the possession of air sacs or vesicular dilatations in the interior of the Insect in connexion with the tracheae (Fig. 176). Such vesicles are found in many of the higher winged Insects, but not in larval forms, or in those that are destitute of powers of flight.[211] They, no doubt, assist the Insect in its movements in the air. The body of a large grasshopper or locust is naturally of considerable weight, and it is more than probable that true flight can only be accomplished when these vesicles are dilated and filled with air. The exact mode in which the sacs are dilated is not known; possibly it may be accomplished by the elasticity of the structure of the vesicles coming into action when the other contents of the {283}body are not completely developed, or are temporarily diminished. Although air vessels are absent in the neighbouring groups of Orthoptera, Dufour says they are present even in apterous forms of Acridiidae, but he gives no particulars.[212] Packard has given an account[213] of the arrangement of these remarkable sacs in the Rocky Mountain Locust. He finds that there are two sets: a thoracic group, consisting of a pair of very large size, with which are connected some smaller sacs placed in the head; and an abdominal set, which forms a very remarkable series. The figures we give (Fig. 176, A, B) show that these sacs are of such large size that if fully distended they must interfere with the development of the ovaries, and that they must be themselves greatly diminished, if not obliterated, by the distension of the alimentary canal. We may look on them as only coming into full play when the normal distension of the canal is prevented, and there is only small development of the reproductive organs. Under such circumstances the locust becomes a sort of balloon, and migrates. In addition to the air sacs there are many dilatable tracheae, placed chiefly in parts of the body where there is not space for the large air sacs. These are, for the sake of clearness, omitted from our figure.
The ganglia constituting the brain are simpler in Acridiidae than they are in the higher Insects, such as bees and wasps, and have been specially studied by Packard[214] and Viallanes.[215] The other ganglia of the nervous cord are eight in number, three thoracic and five abdominal.
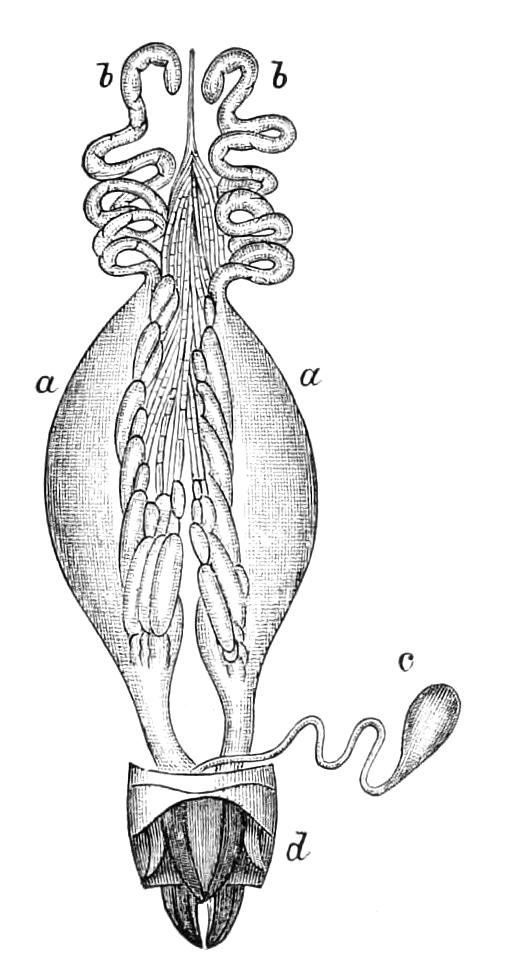
Fig. 169.—Ovaries of Oedipoda caerulescens: a, calyx; b, its gut-like appendage; c, sebific gland; d, termination of body. (After Dufour.)
The salivary glands are small. The alimentary canal is capacious but not coiled. It has no gizzard, but the crop has a peculiar structure, apparently as a substitute. There are diverticula connected with the true stomach. The Malpighian tubes are elongate {284}and extremely numerous. The pair of testes is united in a single envelope. The form and arrangement of the ovaries is remarkable (Fig. 169); the egg-tubes are united by the convergence of their terminal threads into a single mass; outside of each ovary there extends a large calyx, into which the tubes open; each calyx is prolonged at its extremity, and forms a long, convoluted tube.
Fig. 170.—Inner face of femur of Stenobothrus, male, showing line, a-a, of musical beads. (After Landois, magnified three times.)
Acridiidae possess structures for the production of sound, together with others that are, no doubt, for hearing. The chirping of grasshoppers is accomplished by rubbing together the outer face of the upper wing and the inner face of the hind femur. This latter part bears a series of small bead-like prominences placed on the upper of the two lower ridges that run along the side that is nearest to the body (Fig. 170); the tegmen or wing-case has projecting veins, one of which is slightly more prominent, and has a sharp edge; by scraping this edge over the beads of the femur the wing is thrown into a state of vibration and a musical sound is produced. The apparatus for producing sound was for long supposed to be confined to the male sex of grasshoppers; it was indeed known that females made the movements appropriate for producing music, but as they appeared to be destitute of instruments, and as no sound was known to follow from their efforts, it was concluded that these were merely imitative. Graber has, however, discovered[216] that rudimentary musical organs do exist in the females of various species of Stenobothrus (Fig. 171, B). It is true that in comparison with those of the male (Fig. 171, A) they are minute, but it would appear that they are really phonetic, though we can hear no sounds resulting from their use.
Fig. 171.—A, Some of the knobs projecting from the surface of the femur of Stenobothrus melanopterus, male; B, same of the female. Highly magnified. (After Graber.)
Graber considers that the musical pegs of Acridiidae are {285}modified hairs, and he states that in certain females the stages intermediate between hair and peg can be found. There is apparently much variety in the structure of these instruments in different species, and even in individuals of the same species. In Stenobothrus lineatus, instead of pegs, the instrument consists of raised folds.
In some of the aberrant forms of Acridiidae—certain Eremobiides and Pneumorides—the males are provided with sound-producing instruments different to those we have described, both as regards situation and structure.
Fig. 173.—Mecostethus grossus: A, Insect with wings expanded; B, profile of head and prothorax. (After Brunner.)
If the dorsal aspect of the first segment of the hind body of an Acridian Insect be carefully examined there may be seen in the majority of species an organ which has somewhat the appearance of an ear (Fig. 172), and which there is great reason for believing to be really an organ of that nature. It is situate a little over the articulation of the hind leg, very close to the spot where the sound is, as above described, produced. There are three forms of these Acridian ears as described by Brunner:[217] (1) a membrane surrounded by a rim; (2) the membrane somewhat depressed, a portion of the segment projecting a little over it; (3) the depression very strongly marked, and the sides projecting over it so much that all that is seen externally is a sort of broad slit with a cavity beneath it. This last is the condition in which the ear exists in the genera Mecostethus (Fig. 173) and Stenobothrus, which are among our few native grasshoppers. On minute examination this ear proves to consist of a tympanum supplied internally with nerve and ganglion in addition to {286}muscles, and tracheal apparatus of a complex nature; it is no doubt delicately sensitive to some forms of vibration. Unlike the stridulating organ, these ears exist in both sexes; they are found in a great majority of the species of Acridiidae. The forms in which the ears are absent are usually at the same time wingless and destitute of organs of stridulation; but, on the other hand, there are species—some of them wingless—that are, so far as is known, incapable of stridulation and yet possess these ears.
It is, indeed, a matter of great difficulty to decide as to the exact function of these ear-like acoustic organs, which, we may remind the reader, are peculiar to the saltatorial Orthoptera, and we must refer for a full discussion of the subject to Graber's masterly works,[218] contenting ourselves with a brief outline, which we may commence by saying that the Orthoptera with ears are believed to be sensitive to sounds by means other than these organs. This suggests that the latter exist for some purpose of perception of special sound. But if so what can this be? Only the males possess, so far as we know, effective sound-producing organs, but both sexes have the special ears; moreover, these structures are present in numerous species where we do not know of the existence of phonetic organs in either sex. Thus it appears at present impossible to accept these organs as being certainly special structures for the perception of the music of the species. It is generally thought that the females are charmed by the music of the males, and that these are stimulated to rivalry by the production of the sounds; and Dufour[219] has suggested that this process reacts on the physiological processes of the individual. There has not been a sufficient amount of observation to justify us in accepting these views, and they do not in any way dispose of the difficulty arising from the existence of the acoustic organs in species that do not, so far as we know, produce special sounds. It is possible that the solution of the difficulty may be found in the fact that these apparently dumb species do really produce some sound, though we are quite ignorant as to their doing so. It is well known that sounds inaudible to some human ears are perfectly distinct to others. Tyndall, in his work on Sound, has illustrated this by a fact that is of special interest from our present point of view. "Crossing {287}the Wengern Alp with a friend," he says, "the grass on each side of the path swarmed with Insects which to me rent the air with their shrill chirruping. My friend heard nothing of this, the Insect world lying beyond his limit of audition." If human ears are so different in their capacities for perceiving vibrations, it of course becomes more probable that auditory organs so differently constituted as are those of Insects from our own may hear sounds when the best human ear can detect nothing audible. On the whole, therefore, it would appear most probable that the Orthoptera provided with acoustic organs, and which we consider dumb, are not really so, but produce sounds we cannot hear, and do so in some manner unknown to us. If this be the case it is probable that these ears are special organs for hearing particular sounds.
Scudder, who has given considerable attention to the subject of Orthopteran music, says that in N. America "the uniformity with which each species of Stenobothrus plays its own song is quite remarkable. One kind, Stenobothrus curtipennis, produces about six notes per second, and continues them from one and a half to two and a half seconds; another, S. melanopleurus, makes from nine to twelve notes in about three seconds. In both cases the notes follow each other uniformly, and are slower in the shade than in the sun."
Some of the species of Acridiidae, it should be noticed, produce a noise during their flights through the air, due to the friction of the wings; whether this has a definite importance, or whether it may be entirely incidental, has scarcely yet been considered.
Information of a satisfactory kind as to the post-embryonic development of the Acridiidae is but scanty. We have represented in Fig. 84, A, the condition in which a migratory locust, Schistocerca peregrina, leaves the egg, and we will here complete the account of its growth; following Brongniart,[220] whose statement is confirmed by Lestage and other naturalists. Immediately on leaving the egg the young locust casts its skin, and is then of a clear green colour, but it rapidly becomes brown, and in twelve hours is black. At this early age the gregarious instinct, possessed by this and some other species of Acridiidae, becomes evident. In six days the individual undergoes a second moult, after which it is black, spotted and banded with white, and with a rose-coloured streak on each side of the hind body. The {288}third ecdysis occurs in six or eight days after the second; the rose colour becomes more distinct, and the head is of a brown tint instead of black. After eight days the fourth ecdysis occurs; the creature is then about 35 millimètres long; its colour has much changed, the position of the markings is the same, but the rose colour is replaced by citron yellow, the line of the spiracles is marked with white, and at this time the creature has the "first rudiments of wings," and is very voracious. In ten days another ecdysis takes place, the yellow colour is more vivid, the prothorax is definitely speckled with white, and the hind body is increasing much in size. In fifteen or twenty days the sixth moult occurs, and the Insect appears in its perfect form; the large tegmina now present are marked with black in the manner so well known, and the surface generally is variegated with bluish and rosy marks. Although this is the colour in Algeria, yet apparently it is not so farther south; the Insects that arrive thence in the French colony are on some occasions of a different colour, viz. reddish or yellowish, those of this latter tint being, it is believed, older specimens of the reddish kind. M. Brongniart points out that some Phasmidae—of the Phyllium group—undergo an analogous series of colour-changes in the course of the individual development, though other species do not.
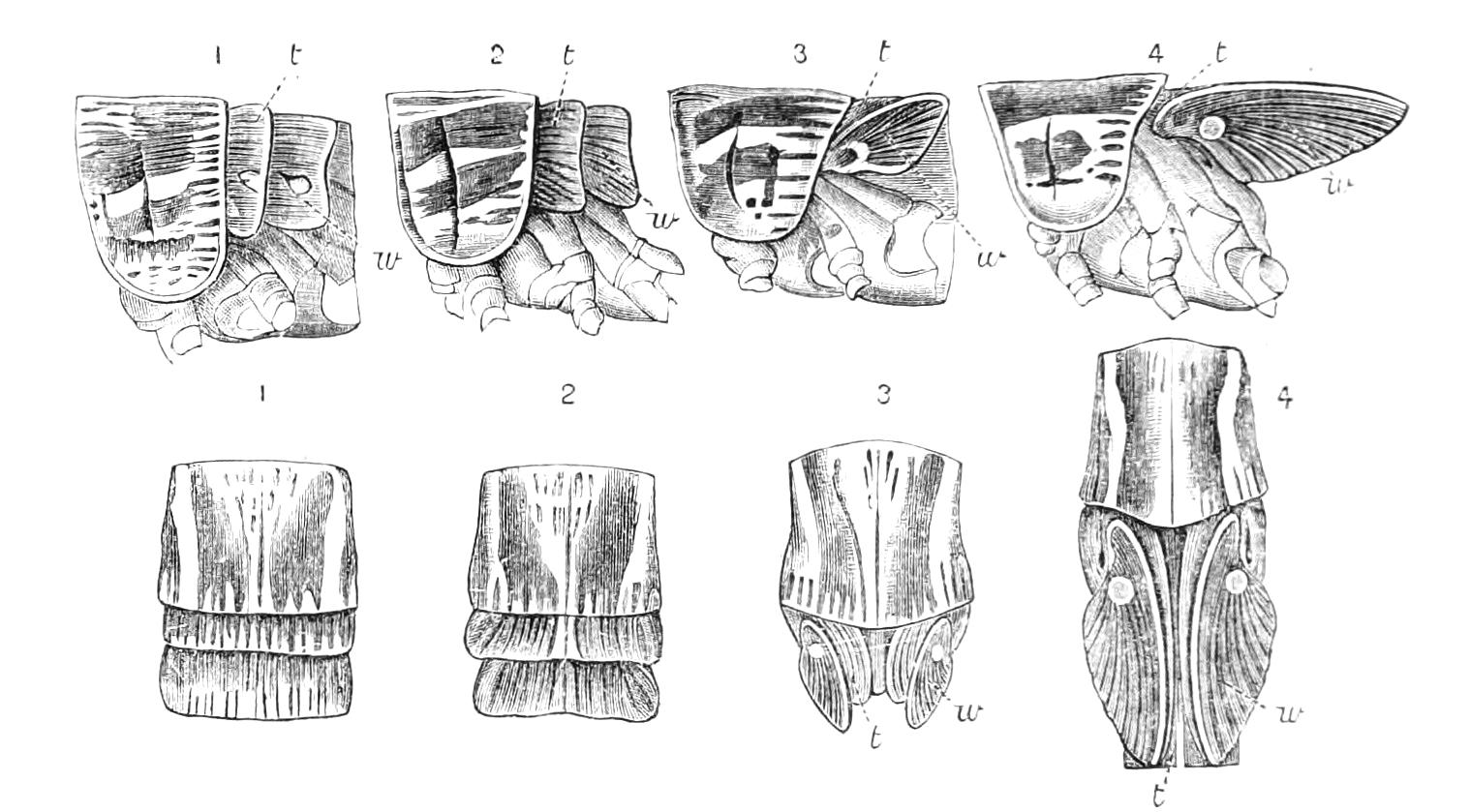
Fig. 174.—Development of wings in Caloptenus spretus: the upper row gives a lateral view of the thoracic segments, and the lower row a dorsal view of these segments; 1, second instar; 2, third instar; 3, fourth instar; 4, fifth instar. (After Riley.) t, tegmen; w, wing.
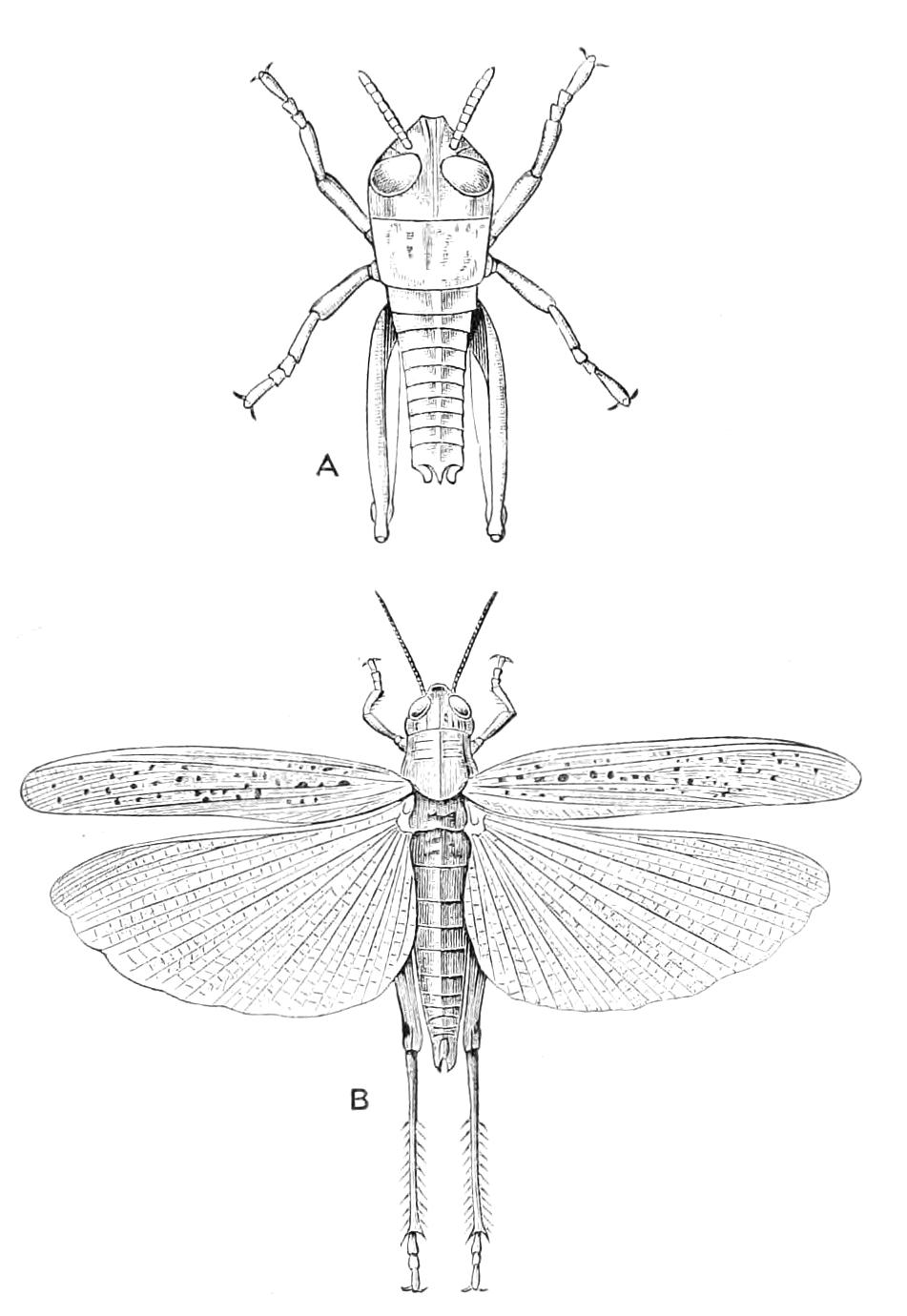
Fig. 175.—Caloptenus spretus. North America. A, Newly hatched, much magnified; B, adult, natural size. (After Riley.)
Riley and Packard have given an account[221] of some parts of the post-embryonic development of the Rocky Mountain Locust, which enables us to form a satisfactory conception of the stages of development of the wings. Fig. 175, A, represents the first instar, the young locust, just emerged from the egg and colourless. Fig. 174 shows some of the subsequent stages of development of the wings, the upper line of figures giving a profile view of the thoracic segments, and the lower line showing their dorsal aspects; 1 shows the condition of the parts in the second instar, the chief difference from the first instar being the development of colour; in the third instar there is an evident slight development of the future alar organs, exhibited chiefly in the outgrowth and lobing of the free posterior angles of the meso- and metanota, as shown in Fig. 174, 2. After the third moult there is a great difference; the instar then disclosed—the fourth—has undergone a considerable change in the position of the meso- and metathoraces, which are thrust forward under the pronotum; this has become more enlarged and hood-like (Fig. 174, 3); at the same time the wing-rudiments have become free and detached, the metathoracic pair being the larger, and overlapping the other pair. The fifth instar (Fig. 174, 4) differs but little from the fourth, except in the larger size of the pronotum and wing-rudiments. The sixth—shown in Fig. 175, {290}B—is the perfect Insect, with the alar organs free and large, the prothorax much changed in form, the colour different. From the above it will be seen that the chief changes occurred at the third and fifth ecdyses, after each of which a considerable difference in the form of the Insect was revealed. In the first three instars the sexes can scarcely be distinguished, in the fourth they are quite distinct, and in the fifth coupling is possible, though usually it does not occur till the final stage is attained.
The discovery that Orthoptera change their colours in the course of their development, and even after they have become adult, is important, not only from a physiological point of view, but because it throws some light on the questions as to the number of species and the geographical distribution of the migratory locusts, as to which there has existed a great confusion.
The Acridiidae are considered to be exclusively vegetable feeders, each individual consuming a very large quantity of food. The mode in which the female deposits her eggs has been described by Riley,[222] and is now widely known, his figures having been frequently reproduced. The female has no elongate ovipositor, but possesses instead some hard gonapophyses suitable for digging purposes; with these she excavates a hole in the ground, and then deposits the eggs, together with a quantity of fluid, in the hole. She prefers hard and compact soil to that which is loose, and when the operation is completed but little trace is left of it. The fluid deposited with the eggs hardens and forms a protection to them, corresponding to the more definite capsules of the cursorial Orthoptera.
The details of the process of oviposition and of the escape of the young from their imprisonment are of much interest. According to Künckel d'Herculais[223] the young Stauronotus maroccanus escapes from the capsule by putting into action an ampulla formed by the membrane between the head and the thorax; this ampulla is supposed to be dilated by fluid from the body cavity, and is maintained in the swollen condition by the Insect accumulating air in the crop beneath it. In order to dislodge the lid of the capsule, six or seven of the young ones inside combine their efforts to push it off by means of their ampullae. The ampulla {291}subsequently serves as a sort of reservoir, by the aid of which the Insect can diminish other parts of the body, and after emergence from the capsule, penetrate cracks in the earth so as to reach the surface. Immediately after doing this the young Stauronotus moults, the skin it casts being called by Künckel an amnios. The cervical ampulla reappears at subsequent moults, and enables the Insect to burst its skin and emerge from it.
The process is apparently different in Caloptenus spretus, which, according to Riley, ruptures the egg-shell and works its way out by the action of the spines at the apex of the tibiae. This latter Insect when it emerges moults a pellicle, which Riley considers to be part of the embryonic membranes.
Riley states that a female of Caloptenus spretus makes several egg-masses. Its period of ovipositing extends over about 62 days, the number of egg-masses being four and the total number of eggs deposited about 100. The French naturalists have recently observed a similar fact in Algeria, and have ascertained that one of the migratory locusts—Schistocerca peregrina—may make a deposit of eggs at more than one of the places it may alight on during its migration.
It has been ascertained that the eggs of Acridiidae are very nutritious and afford sustenance to a number of Insects, some of which indeed appear to find in them their sole means of subsistence. Beetles of the family Cantharidae frequent the localities where the eggs are laid and deposit their eggs in the egg-masses of the Orthoptera, which may thus be entirely devoured. Two-winged flies of the family Bombyliidae also avail themselves of these eggs for food, and a mite is said to be very destructive to them in North America. Besides being thus destroyed in enormous quantities by Insects, they are eaten by various birds and by some mammals.
Most of the Insects called locusts in popular language are members of the family Acridiidae, of which there are in different parts of the world very many species, probably 2000 being already known. To only a few of these can the term Locust be correctly applied. A locust is a species of grasshopper that occasionally increases greatly in number, and that moves about in swarms to seek fresh food. There are many Orthoptera that occasionally greatly increase in numbers, and that then extend their usual area more or less; and some Acridiidae multiply {292}locally to a great extent—very often for one or two seasons only,—and are then called locusts. The true migratory locusts are species that have gregarious habits strongly developed, and that move over considerable distances in swarms. Of these there are but few species, although we hear of their swarms in many parts of the world.
The migratory locusts do much more damage than the endemic species. In countries that are liable to their visitations they have a great influence on the prosperity of the inhabitants, for they appear suddenly on a spot in huge swarms, which, in the space of a few hours, clear off all the vegetable food that can be eaten, leaving no green thing for beast or man. It is difficult for those who have not witnessed a serious invasion to realise the magnitude of the event. Large swarms consist of an almost incalculable number of individuals. A writer in Nature[224] states that a flight of locusts that passed over the Red Sea in November 1889 was 2000 square miles in extent, and he estimated its weight at 42,850 millions of tons, each locust weighing 1⁄16 of an ounce. A second similar, perhaps even larger, flight was seen passing in the same direction the next day. That such an estimate may be no exaggeration is rendered probable by other testimony. From official accounts of locusts in Cyprus we find that in 1881,[225] up to the end of October, 1,600,000,000 egg-cases had been that season collected and destroyed, each case containing a considerable number of eggs. By the end of the season the weight of the eggs collected and made away with amounted to over 1300 tons, and, notwithstanding this, no less than 5,076,000,000 egg-cases were, it is believed, deposited in the island in 1883.
When we realise the enormous number of individuals of which a large swarm of locusts may consist we can see that famine is only a too probable sequence, and that pestilence may follow—as it often has done—from the decomposition of the bodies of the dead Insects. This latter result is said to have occurred on some occasions from locusts flying in a mass into the sea, and their dead bodies being afterwards washed ashore.
Locust swarms do not visit the districts that are subject to their invasions every year, but, as a rule, only after intervals of a considerable number of years. It has been satisfactorily {293}ascertained that in both Algeria and North America large swarms occur usually only at considerable intervals. In North America Riley thought[226] the average period was about eleven years. In Algeria the first invasion that occurred after the occupation of the country by the French was in 1845, the second in 1864, the third in 1866, since which 1874 and 1891 have been years of invasion. These breaks seem at first strange, for it would be supposed that as locusts have great powers of increase, when once they were established in any spot in large numbers, there would be a constant production of superfluous individuals which would have to migrate as regularly as is the case with swarms of bees. The irregularity seems to depend on three facts: viz. that the increase of locusts is kept in check by parasitic Insects; that the eggs may remain more than one year in the ground and yet hatch out when a favourable season occurs; and that the migratory instinct is only effective when great numbers of superfluous individuals are produced.
It is not known that the parasites have any power of remaining in abeyance as the locust eggs may do; and the bird destroyers of the locusts may greatly diminish in numbers during a year when the Insects are not numerous; so that a disproportion of numbers between the locusts and their destroyers may arise, and for a time the locusts may increase rapidly, while the parasites are much inferior to them in numbers. If there should come a year when very few of the locusts hatch, then the next year there will be very few parasites, and if there should then be a large hatching of locusts from eggs that have remained in abeyance, the parasites will not be present in sufficient quantity to keep the destructive Insects in check; consequently the next year the increase in number of the locusts may be so great as to give rise to a swarm.
It is well established that locusts of the migratory species exist in countries without giving rise to swarms, or causing any serious injuries; thus Pachytylus cinerascens—perhaps the most important of the migratory locusts—is always present in various localities in Belgium, and does not give rise to swarms. When migration of locusts does occur it is attended by remarkable manifestations of instinct. Although several generations may elapse without a migration, it is believed that the locusts when {294}they migrate do so in the direction taken by predecessors. Their movements are to a large extent dependent on the wind, and it is said that they make trial flights to ascertain its direction. When on the wing probably very little muscular effort is necessary. Their bodies contain elastic air sacs in communication with the tracheae, and at the time of flight it may be presumed that the body is comparatively empty, food being wanting, and the internal organs of reproduction, which occupy a large space when in activity, yet undeveloped, hence the sacs have full room for expansion, as explained on p. 283. Thus the Insects exert but little effort in their aerial movements, and are, it is believed, chiefly borne by the wind. Should this become unfavourable it is said that they alight and wait for a change.
The most obscure point in the natural history of the migratory locusts appears to be their disappearance from a spot they have invaded. A swarm will alight on a locality, deposit there a number of eggs, and then move on. But after a lapse of a season or two there will be few or none of the species present in the spot invaded. This appears to be partly due to the young locusts dying for want of food after hatching; but in other cases they again migrate after growth to the land of their ancestors. The latter fact is most remarkable, but it has been ascertained by the U.S. Entomological Commission that these return swarms do occur.
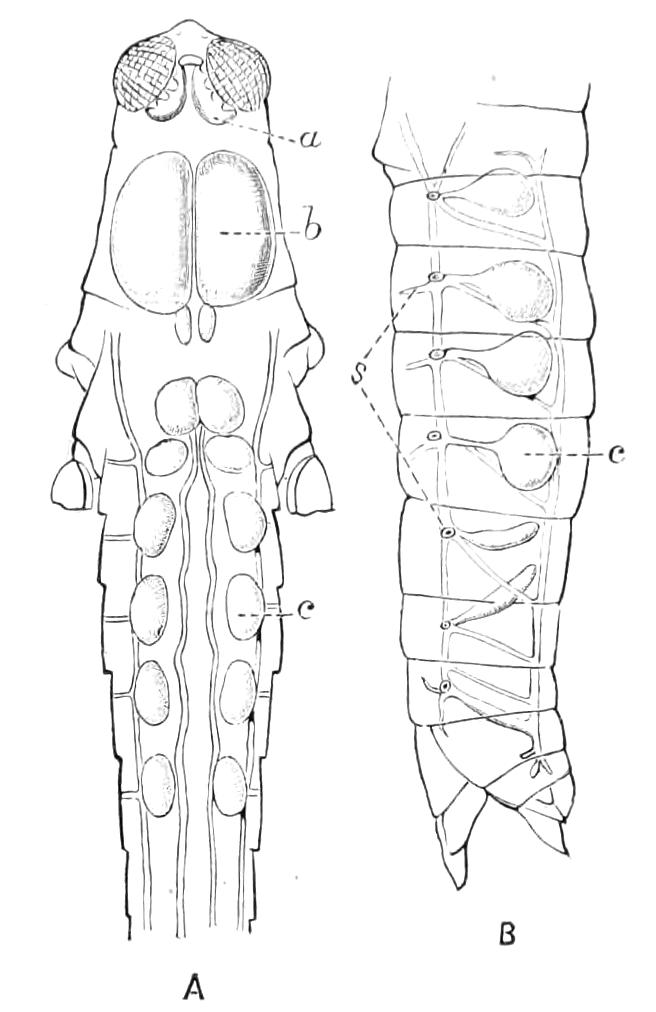
Fig. 176.—Portions of body of Caloptenus spretus to show some of the air-sacs. (Modified from Packard.) A, Dorsal aspect of anterior parts; B, lateral aspect of posterior parts of body; a, enlargements of tracheae in head; b, pair of large sacs in thorax; c, sacs on the tracheal trunks of abdomen; s, spiracles.
In South Africa it would appear that the movements of the migratory locusts are frequently made before the Insects have acquired their wings. Mrs. Barber, in an account of "Locusts and Locust-Birds in South Africa,"[227] has illustrated many points in the {295}Natural History of these Insects. The South African species manifests the gregarious and migratory disposition when the individuals are quite young, so that they travel in flocks on foot, and are called by the Dutch "Voetgangers." After hatching, the various families of young amalgamate, so that enormous numbers come together. Having denuded the neighbourhood of all its food-supplies, they move off in search of fresh crops and pastures new. They take advantage of roads, and sometimes a good many miles will be traversed in a day; they proceed by means of short leaps, rapidly repeated. When the "Voetgangers" are thus returning northwards towards the lands in the interior from which their progenitors departed, no obstacles can stay their course. Forests or rivers may intervene, diverting them for a while from their line of march, but they succeed ultimately in continuing their journey to the interior.
The manner in which these wingless locusts occasionally cross broad rivers is interesting, as it has some bearing on the difficult question of the possibility of winged locusts crossing seas of considerable width. Mrs. Barber refers to an instance that took place on the Vaal River in the spring of the year 1871, shortly after the discovery of the Diamond-fields. The country was at that time swarming with young locusts; every blade of grass was cleared off by them. One day a vast swarm of the "Voetgangers" made their appearance on the banks of the Vaal River; they appeared to be in search of a spot for crossing, which they could not find, the river being somewhat swollen. For several days the locusts travelled up the stream; in the course of doing this they paused for some time at an abrupt bend in the river where a number of rocks were cropping out, as if in doubt whether to attempt a passage at this place. They, however, passed on, as if with the hope of finding a better ford; in this apparently they were disappointed, for three days afterwards they returned to the same bend of the river, and there plunged in vast multitudes into the stream, where, assisted by a favourable current and the sedges and water-plants which grew upon the projecting rocks, they managed to effect a crossing, though great numbers were drowned and carried away by the flooded river. Mrs. Barber adds that "Voetgangers" have been known to attempt the passage of the Orange River when it was several hundred yards in breadth, pouring their vast swarms into the flooded stream regardless of the consequences, until they {296}became heaped upon each other in large bodies. As the living mass in the water accumulated, some portions of it were swept away by the strong current from the bank to which they were clinging, and as the living locusts tightly grasped each other and held together, they became floating islands, the individuals continually hopping and creeping over each other as they drifted away. Whether any of the locust-islands succeeded in reaching the opposite bank is unknown; probably some of them were drifted on land again. They are by no means rapid swimmers; they do not perish easily in the water when in masses, their habit of continually changing places and hopping and creeping round and round upon each other being very advantageous as a means of preservation. It is a common practice for the young locusts to form a bridge over a moderately broad stream by plunging indiscriminately into it and holding on to each other, grappling like drowning men at sticks or straws, or, in fact, anything that comes within their reach, and that will assist in floating them; meanwhile those from behind are eagerly pushing forward over the bodies of those that are already in the stream and hurrying on to the front, until at length by this process they reach the opposite bank of the river; thus a floating mass of living locusts is stretched across the stream, forming a bridge over which the whole swarm passes. In this manner few, comparatively speaking, are drowned, because the same individuals do not remain in the water during the whole of the time occupied by the swarm in crossing, the Insects continually changing places with each other; those that are beneath are endeavouring to reach the surface by climbing over others, whilst those above them are, in their turn, being forced below. Locusts are exceedingly tenacious of life, remaining under water for a considerable time without injury. An apparently drowned locust will revive beneath the warm rays of the sun, if by chance it reaches the bank or is cast on shore. Mrs. Barber relates an interesting case where the instinct of the "Voetgangers" was at fault, they plunging into a river from a steep sandy bank, only to find another similar sandy precipice on the other side. On this they could gain no footing, and all perished in the stream, where they putrefied, and caused the death of the fish, which floated likewise on the surface; so powerful were the effluvia produced that no one was able to approach the river.
Locusts are able to travel considerable distances, though how far is quite uncertain. Accounts vary as to their moving by night. It has, however, been recently proved that they do travel at night, but it is not ascertained how long they can remain in the air without descending. The ocean is undoubtedly a source of destruction to many swarms; nevertheless, they traverse seas of considerable width. They have been known to reach the Balearic Islands, and Scudder gives[228] a well-authenticated case of the occurrence of a swarm at sea. On the 2nd of November 1865 a ship on the voyage from Bordeaux to Boston, when 1200 miles from the nearest land, was invaded by a swarm of locusts, the air and the sails of the ship being filled with them for two days. The species proved to be Acridium (Schistocerca) peregrinum. This is an extraordinary case, for locusts do not fly with rapidity, being, indeed, as we have remarked, chiefly carried by the wind. Possibly some species may occasionally rest on the water at night, proceeding somewhat after the fashion of the "Voetgangers" when passing over rivers as described by Mrs. Barber. In Sir Hans Sloane's history of Jamaica an account of an occurrence of this kind is given on the authority of Colonel Needham, who states that in 1649 locusts devastated the island of Tenerife, that they were seen to come from Africa when the wind was blowing thence, that they flew as far as they could, then alighted on the water, one on the other, till they made a heap as big as the greatest ship, and that the next day, being refreshed by the sun, they took flight again and landed in clouds at Tenerife. De Saussure says[229] that the great oceans are, as a rule, impassable barriers, and that not a species of the tribe Oedipodides has passed from the Old World to the New. It is, however, possible that Acridium peregrinum, of the tribe Acridiides, may have originally been an inhabitant of America, and have passed from thence to the Old World.
The species of Acridiidae that have been ascertained to be migratory are not numerous.[230] The most abundant and widely distributed of them is Pachytylus cinerascens (Fig. 177), which has invaded a large part of the Eastern hemisphere, extending from the Atlantic Ocean to China. It exists in numerous spots in the Oriental region and the Asiatic Archipelago, and even in New Zealand. It is the commoner of the locusts of Europe. Its congener, P. migratorius, is much less widely distributed, its migrations being, according to de Saussure, limited to Turkestan and Eastern Europe. A third species, P. migratorioides, inhabits Eastern Africa, and a variety of it is the "Yolala" or locust of Madagascar. Mr. Distant has informed the writer that this migratory locust is found in South Africa. P. (Oedaleus) marmoratus has almost as wide a distribution in the Eastern hemisphere as P. cinerascens, except that it is more exclusively tropical; it is thus excluded from New Zealand. P. (Oedaleus) nigrofasciatus has a more northern distribution than its congener, but has extended to Africa and the Asiatic Archipelago. This Insect is so variable that the distinctions of its races from other species of the same genus are not yet clear. All the above-mentioned locusts belong to the tribe Oedipodides. Acridium peregrinum, now more frequently called Schistocerca peregrina, belongs to the tribe Acridiides. It is a large locust (Fig. 84), and has a wide distribution. It is the chief species in North Africa, and is probably the locust of the plagues of Egypt mentioned in the book of Exodus. It is also, according to Cotes,[231] the chief locust of North-West India. In this latter country Pachytylus cinerascens and some other species also occur. With the exception of S. peregrina, the species of the genus Schistocerca are confined to the New World. In North America locusts are more usually called grasshoppers. Several species of the genus Caloptenus are injurious in that country, but the chief migratory species is C. spretus (Fig. 175). This genus belongs to Acridiides. A large locust, Schistocerca americana, is also migratory to a small extent in the United States. In South America other species of Schistocerca are migratory; it is not known how many there may be, and it is possible that one or more may prove to be the S. peregrina of the Old World. A Chilian species, according to Mr. E. C. Reed,[232] {299}exhibits distinctions of colour similar to those that have been observed in S. peregrina in Algeria.
In Britain we are now exempt from the ravages of locusts, though swarms are said to have visited England in 1693 and 1748. Individuals of the migratory species are, however, still occasionally met with in England and the south of Scotland. P. cinerascens has been recorded from Kerry in Ireland, but erroneously, the Insect found being Mecostethus grossus (Fig. 173). According to Miss Ormerod,[233] large locusts are imported to this country in fodder in considerable numbers, but are usually dead; living individuals are, however, sometimes found among the others. In 1869 living specimens of Schistocerca peregrina were found in various parts of the country, having, in all probability, arrived here by crossing the German Ocean. Pachytylus cinerascens has also, it is believed, occurred here, the specimens that have been recorded at different times under the name of P. migratorius being more probably the former species.
Although the majority of the very large number of species included in Acridiidae are recognised with ease from their family likeness as belonging to the group, yet there are others that present an unusual aspect. This is specially the case with the members of the small tribes Tettigides, Proscopides, and Pneumorides, and with some of the apterous forms of the Oedipodides. The tribe Proscopides (Fig. 178, Cephalocoema lineata, female) includes some of the most curious of the Acridiidae. Breitenbach gives[234] a brief account of the habits of certain species which he met with near Porto Alegre in South America. On a stony hill there was some grass which, by several months' exposure to the sun's rays, had {300}become withered and brown. Apparently no live thing was to seen on this hillock except the ubiquitous ants, but after a while he noticed some "lightning-like" movements, which he found were due to specimens of Proscopia. The Insects exactly resemble the withered vegetation amongst which they sit, and when alarmed seek safety with a lengthy and most rapid leap. When attention was thus directed to them he found the Insects were really abundant, and was often able to secure fifty specimens on a single afternoon. These Insects bear a great general resemblance to the Phasmides, but there is no evidence at present to show that the two kinds of Insects live in company, as is the case with so many of the Insects that resemble one another in appearance. Although the linear form and the elongation of the body are common to the stick-Insects and the Proscopides, yet this structure is due to the growth of different parts in the two families. In the Phasmidae the prothorax is small, the mesothorax elongate, while in the Proscopides the reverse is the case. The elongation of the head is very curious in these Insects; the mouth is not thus brought any nearer to the front, but is placed on the under side of the head, quite close to the thorax. The tribe Tryxalides contains Insects (Fig. 165) that approach the Proscopides in the form of the head and other characters. In most cases the sexes of the Proscopides differ from one another so strongly that it is difficult to recognise them as being of the same species. Usually both sexes are entirely apterous, but the Chilian genus Astroma exhibits a remarkable exception and an almost unique condition of the alar organs, the mesonotum being in each sex entirely destitute of such appendages, while the female has on the metanotum rudiments of wings which are absent in the male.
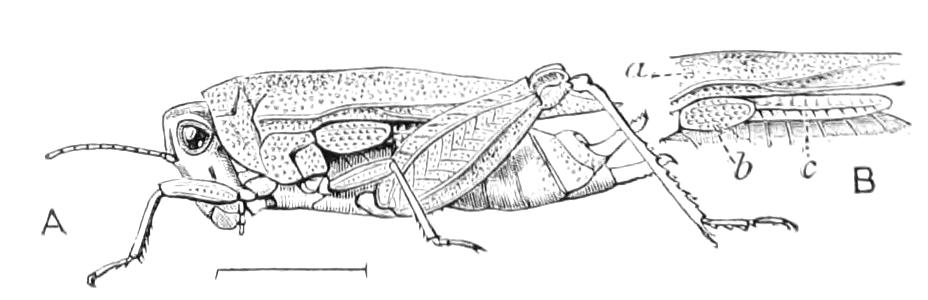
Fig. 179.—Tettix bipunctatus. Britain. A, The Insect magnified; B, part of the middle of the body; a, prolongation of pronotum; b, tegmen; c, wing.
The tribe Tettigides is a very extensive group of small Acridiidae, in which the pronotum extends backwards as a hood and covers the body, the tegmina and wings being more or less modified. In our British species (Fig. 179) this condition does not greatly modify the appearance of the Insect, but in many exotic species (Fig. 180) the hood assumes {301}remarkable developments, so that the Insects have no longer the appearance of Orthoptera. It would be impossible, without the aid of many figures, to give an idea of the variety of forms assumed by this prothoracic expansion. It is a repetition of what occurs in the Order Hemiptera, where the prothoracic hoods of the Membracides exhibit a similar, though even more extraordinary, series of monstrous forms. So great is the general similarity of the two groups that when the genus Xerophyllum (Fig. 180, A) was for the first time described, it was treated by the describer as being a bug instead of a grasshopper. This genus includes several species from Africa. The curious Cladonotus (Fig. 180, B) is a native of Ceylon, where it is said to live in sandy meadows, after the fashion of our indigenous species of Tettix (Fig. 179). Very little is known as to the habits of these curious Tettigides, but it has been ascertained that some of the genus Scelimena are amphibious, and do not hesitate to enter the water and swim about there; indeed it is said that they prefer plants growing under water as food. This habit has been observed both in Ceylon and the Himalayas. The species are said to have the hind legs provided with dilated foliaceous appendages useful for swimming.
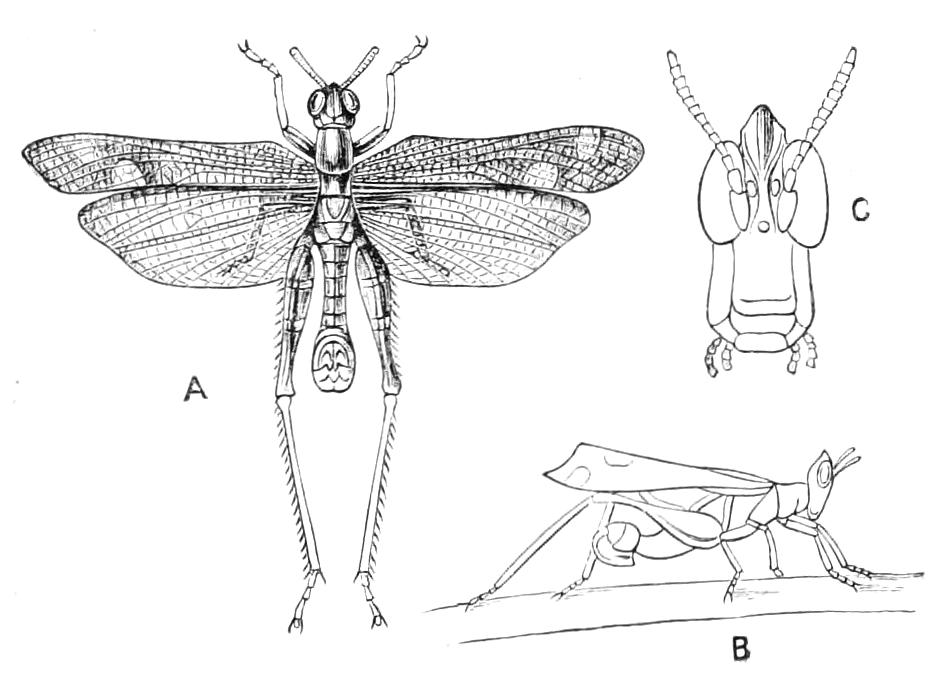
Fig. 181.—A, Mastax (Erianthus) guttatus, male. Sumatra. (After Westwood.) B, profile; C, front of head.
The tribe Mastacides includes thirty or forty species of Acridiidae with short antennae and vertical head (Fig. 181, Mastax guttatus); they are apparently all rare and little {302}known, but are widely distributed in the tropics of the Old and New Worlds. Nothing whatever seems to be known of their habits or of their development.
The tribe Pneumorides includes a still smaller number of species of very aberrant and remarkable grasshoppers, of large size, with short antennae, and with the pronotum prolonged and hood-like; they are peculiar to South Africa. Although amongst the most remarkable of Insects, we are not able to give any information as to their habits. It would appear from the form of their legs that they have but little power of hopping. The species of which we figure the female (Fig. 182) is very remarkable from the difference in colour of the sexes. The female is so extravagantly coloured that she has been said to look as if "got up" for a fancy-dress ball. She is of a gay green, with pearly white marks, each of which is surrounded by an edging of magenta; the white marks are very numerous, especially on the parts of the body not shown in our figure; the face has magenta patches and a large number of tiny pearly-white tubercles, each of which, when placed on a green part, is surrounded by a little ring of mauve colour. Though the female is certainly one of the most remarkably coloured of Insects, her consort is of a modest, almost unadorned green colour, and is considerably different in form. He is, however, provided with a musical apparatus, which it is possible may be a means of pleasing his gorgeous but dumb spouse. It consists of a series of ridges placed on each side of the inflated abdomen, which, as we have previously (p. 200) remarked, has every appearance of being inflated with the result of improving its resonance.
The Pyrgomorphides[235] is a small tribe of about 120 described species, two of which are found in the south of Europe (Fig. 183, Pyrgomorpha grylloides). The tribe includes a number of large and curious Insects, among them the species of Phymateus and Petasia, with peculiar excrescences on the pronotum and vivid colours on some parts of the body or its appendages, which are apparently common Insects in South Africa.
The tribe Tryxalides includes a great many species of grasshoppers. In them the front of the head joins the upper part at an acute angle (Figs. 165 and 173). This tribe and the Acridiides are the most numerous in species of the family. To the latter belong most of the migratory locusts of the New World (Fig. 175, Caloptenus spretus). A Spanish species of this tribe, Euprepocnemis plorans, though provided with well-developed wings, possesses the remarkable habit of seeking shelter by jumping into the water and attaching itself below the surface to the stems of plants.
The tribe Pamphagides[236] includes some 200 species, found chiefly in Africa and the arid regions near the Mediterranean Sea. They are mostly apterous forms, and this circumstance has, according to de Saussure, exercised a marked influence on the geographical distribution of the species. Although the tribe consists chiefly of apterous forms, several species possess {304}well-developed wings; sometimes this is the case of the male but not of the female. Some of the species are highly modified for a desert life, and exhibit a great variation in the colour of the individuals in conformity with the tint of the soil they inhabit. Xiphocera asina (Fig. 184) is thought by Péringuey to be the prey of the extraordinary South African tiger-beetles of the genus Manticora.
We have already mentioned the tribe Oedipodides[237] as including most of the species of migratory locusts of the Old World. Some striking cases of variation in colour occur amongst the winged Oedipodides. In certain species the hind wings may be either blue or rosaceous in colour; it is thought that the latter is the tint natural in the species, and that it is due to the mixture of a red pigment with the pale blue colour of the wing; hence the blue-coloured wings are analogous to cases of albinism. But the most remarkable fact is that this colour difference is correlative with locality. Brunner von Wattenwyl says[238] that the blue variety of Oe. variabilis occurs only in a few localities in Europe—he mentions Vienna and Sarepta,—and that where it occurs not a single red example can be met with. Similar phenomena occur in other species in both Europe and North America, and L. Bruner has suggested[239] that the phenomena in the latter country are correlative with climatic conditions.
The group Eremobiens, a subdivision of Oedipodides, includes some of the most interesting forms of Acridiidae. Its members have several modes of stridulation. Cuculligera flexuosa and other of the winged forms, according to Pantel,[240] produce sounds by the friction of the middle tibia against the wing, both of these parts being specially modified for the purpose in the male sex. The most peculiar members of the Eremobiens are some very large Insects, modified to an extraordinary extent for a sedentary life in deserts and arid places. Trimen says[241] that a South African species, Trachypetra bufo, which lives amongst stones, is so coloured that he had much difficulty in detecting it, and that he noticed in certain spots, often only a few square yards in extent, where the stones lying on the ground were darker, lighter, or more mottled than usual, that the individuals of the grasshopper were of a similar colour to the stones.
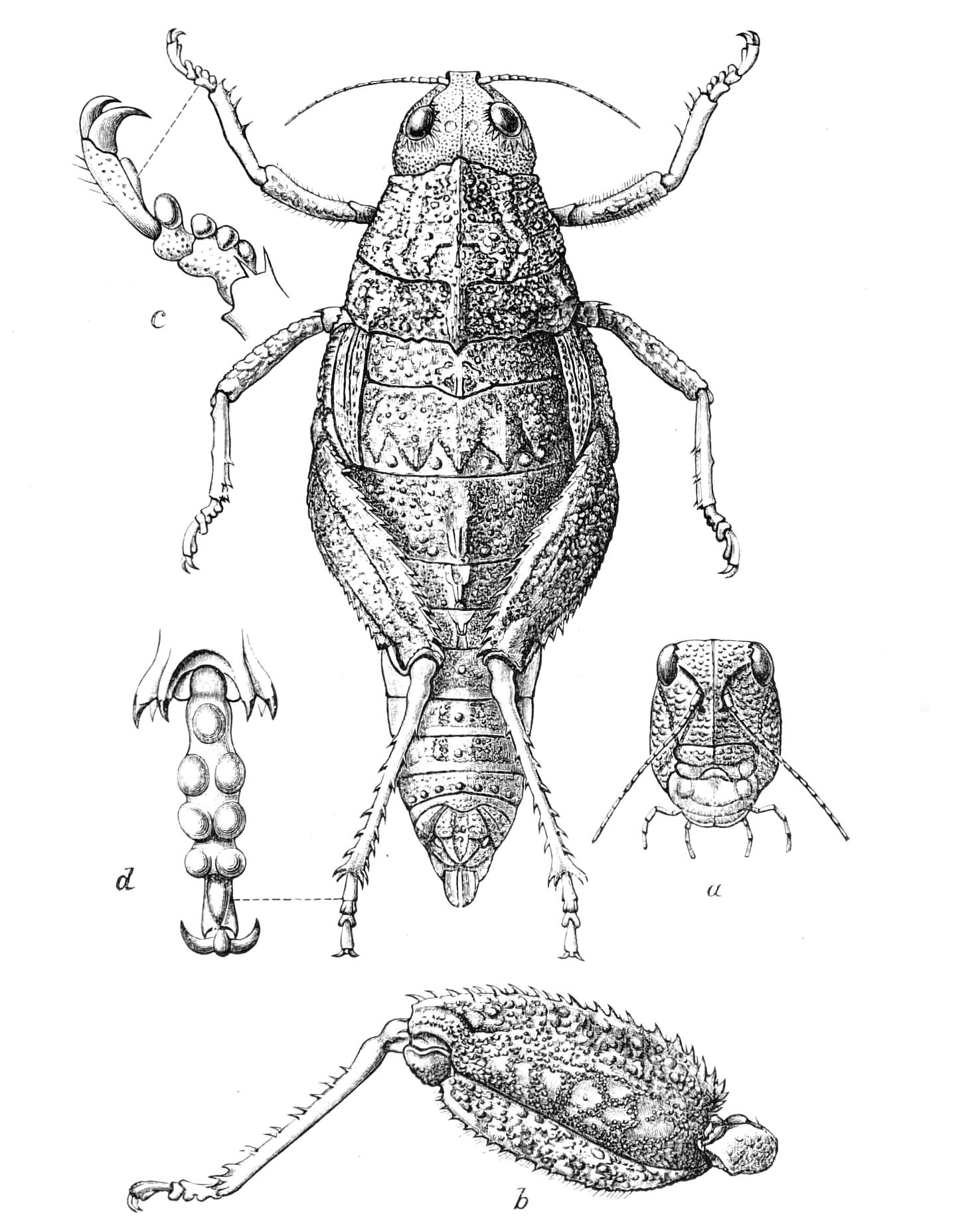
Fig. 185.—Methone anderssoni, female. S. Africa. a, Front of head; b, posterior leg; c, d, front and hind feet. (c and d magnified, the others natural size.)
The Insect referred to by Trimen is, we believe, the Batrachotettix whiti of de Saussure. In this species the alar organs are completely absent, and the pronotum forms a sort of hood that protects the base of the hind body. Some of the desert Eremobiens vary so much that the differences found among individuals of the same species {306}are said by Brunner and de Saussure to be so great as to affect even the generic characters, and give rise to the idea of an "uncompleted species-formation."
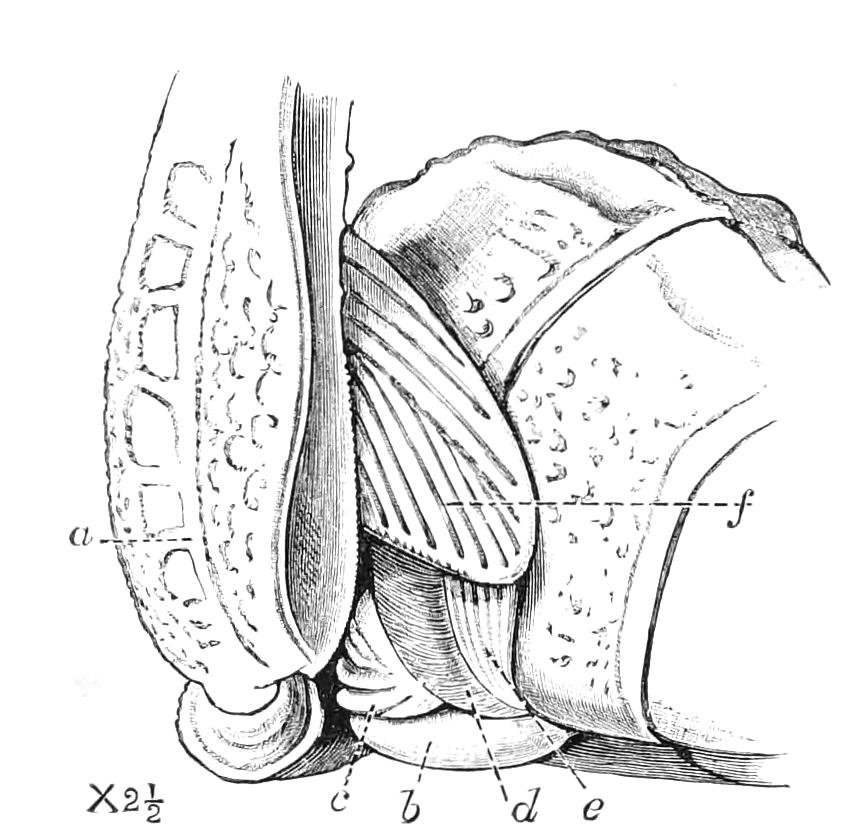
Fig. 186.—Portions of middle of the body and hind leg of Methone anderssoni ♂: a, femur; b, an inferior fold; c, rattling-plate; d, striated surface; e, the adjoining sculpture; f, grooved portion of tegmen. The part e is really, like d, a portion of the second abdominal segment, not of the third, as might be supposed from the figure.
Methone anderssoni, an inhabitant of the Karoo Desert of South Africa, is one of the largest of the Acridiidae. A female of this species is represented of the natural size in Fig. 185. This Insect is remarkable on account of the complex organs for producing sound, and for the great modification of the posterior legs (Fig. 185, b), which do not possess locomotive functions, but serve as a portion of the sound-producing apparatus, and as organs for protecting the sides of the body. This Insect is said to be very efficient in making a noise. The sexes differ considerably in their sound-producing organs, a portion of which are present in the female as well as in the male (Fig. 186). Connected with the first abdominal segment, but extending backwards on the second, there is a peculiar swelling bearing two or three strongly raised chitinous folds (Fig. 186, c). When the leg is rotated these folds are struck by some peg-like projections situate on the inner face of the base of the femur, and a considerable noise is thus produced. The pegs cannot be seen in our figure. This apparatus is equally well developed in female and male. On the second abdominal segment, immediately behind the creaking folds we have described, there is a prominent area, densely and finely striated (Fig. 186, d): this is rubbed by some fine asperities on the inner part of the femur near its base. Sound is produced by this friction on the striated surface, the sculpture of which is abruptly contrasted with that of the contiguous parts: these structures seem to be somewhat better developed in the male than they are in the female, and to be phonetic, at any rate in the former sex. {307}The male has the rudimentary tegmina (Fig. 186, f) much longer than they are in the female (Fig. 185), and their prolonged part is deeply grooved, so as to give rise to strong ridges, over which plays the edge of the denticulate and serrate femur. There is nothing to correspond to this in the female, and friction over the surface of this part of the male produces a different and louder sound. There can be little doubt that this is a phonetic structure peculiar to the male. It approximates in situation to the sound-producing apparatus of the males of the Stenobothri and other Acridiidae. Methone anderssoni has large tympanal organs: the small tegmina cover them up completely. In the female the tips of the tegmina seem to be adapted for forming covering-flaps for the tympana. In both sexes there is a sac (Fig. 186, b) adjoining the structures we have mentioned, but which is not directly phonetic, though it may be an adjunct of the apparatus.
There is no other Orthopteron in which the phonetic organs are so complex as they are in the male of Methone anderssoni, and it would appear probable that this Insect possesses the power of producing two, if not more, distinct sounds, one in common with the female, and peculiar to this and one or two other species; the other somewhat similar to that of other Acridiids, and more specially developed in the male, if not absolutely confined to it.
This Insect is of a very sedentary disposition, and when disturbed apparently seeks safety rather by the noise it can make than by flight. Its powers of locomotion indeed are very feeble. The alar organs are quite rudimentary, and of no assistance whatever for movement. The hind legs seem to be almost equally useless for this purpose; they are broader than they are in other Acridiidae, and have different functions. When Methone moves it does so by means of the anterior four legs, on which it walks propped up as if on stilts. When at rest the hind legs are pressed close to the body, and the tibiae are inflexed and not seen, the creature in this position greatly resembling a clod of earth. We know nothing of the life history of this Insect, except that the young resemble the adult in appearance, and are provided with the sound-producing apparatus, or some portion thereof.
The geographical distribution of the Eremobiens corresponds with that of the Pamphagides, with two important differences, viz. that in the Old World the former group occupies a somewhat {308}more restricted area, and that it is represented in the New World by two peculiar North American genera, Haldmanella and Brachystola. B. magna is an Insect nearly equal in size to Methone anderssoni. Its peculiar form and movements have procured for it in Texas and Colorado the popular names of "buffalo hopper" and "lubber grasshopper." This Insect has not—like Methone—the colours of the desert sands; it is of a green tint, with comparatively smooth body, and during the day rests concealed under tufts of grass. It has apparently no sound organs, though de Saussure thinks there are structures present that are vestiges or rudiments thereof.
The family Acridiidae includes a large part of the species that make up our meagre list of British Orthoptera. Indeed, the only native Orthoptera at the present time sufficiently common to attract general attention are, in addition to the earwig, the species of the genera Stenobothrus and Gomphocerus, whose musical instruments we have described previously. We have eight species of these Insects. They are the little grasshoppers, so common in our fields and gardens, the hunting of which is a source of much amusement to children. The Insect goes off with a sudden and long hop just as it is going to be seized, and this is appreciated by the child as very clever. The hunt, as a rule, does not result in much damage to the grasshoppers, the ingenious escape being the greater part of the pleasure. These Stenobothri are remarkable for their variation in colour, and it is thought by some that they frequent spots where they find themselves a match with their surroundings. There is, however, little or no information of importance on this point extant. Mecostethus grossus (Fig. 173), though larger, is very like the common field grasshoppers, but appears to have become rare since the fens were drained. The two curious little grasshoppers of the genus Tettix (Fig. 179) are not uncommon. In addition to these Acridiidae, three species of migratory locusts are occasionally met with in Britain, viz. Pachytylus cinerascens (Fig. 177), P. migratorius, and Schistocerca peregrina (Fig. 84); this latter we have already alluded to as being probably the locust mentioned in the book of Exodus.
Acridiidae have never been found in amber, owing possibly to their large size and strength. There are but few fossil forms known, and these do not extend farther back in time than the Mesozoic {309}epoch. Several forms, including three peculiar genera, have been found in the Tertiary strata at Florissant. The remains from the Mesozoic layers are apparently very fragmentary and obscure.
Brongniart has instituted a family of Insects under the name Palaeacrididae[242] for some fossil Insects from the Carboniferous strata at Commentry. He considers that these Insects were abundant in the epoch of the Carboniferous strata.
The very large number of genera and species of Acridiidae have been recently arranged in nine tribes by Brunner von Wattenwyl:—
1. Feet without a claw-pad.[243] [Pronotum covering all the body.] Tegmina lobe-like. Tribe 1. Tettigides. (Figs. 179, 180, Tettix, Xerophyllum, Cladonotus.)
1′. Feet with a claw-pad.
2. Antennae shorter than the anterior femora.
3. Head short, as if compressed from in front.
4. Body bladder-like, inflated.[244] [Pronotum covering half the abdomen.] South African species. Tribe 2. Pneumorides. (Fig. 182, Pneumora scutellaris.)
4′. Body ordinary. Tribe 3. Mastacides. (Fig. 181, Mastax guttatus.)
3′. Head very elongate. [Body apterous or sub-apterous.] Tribe 4. Proscopiides. (Fig. 178, Cephalocoema lineata.)
2′. Antennae longer than the anterior femora.
3. Prosternum unarmed.
4. The plane of the vertex of the head meeting the plane of the front of the head as an angle. The former produced or declivous. The face looking down. Tribe 5. Tryxalides. (Fig. 165, Tryxalis nasuta; Fig. 173, Mecostethus grossus.)
4′. Planes of the vertex and front of the head connected in a rounded manner. Face looking forwards. Tribe 6. Oedipodides. (Fig. 177, Pachytylus; Fig. 185, Methone.)
3′. Prosternum with an elevated lamina in front, either irregularly swollen or mucronate.
4. Foveoles of the vertex superior, contiguous, forming the apex of the vertex. Face looking much downwards. Tribe 7. Pyrgomorphides. (Fig. 183, Pyrgomorpha grylloides.)
4′. Foveoles of the vertex, either superior (but not forming the apex of the vertex), or lateral, or inferior, or quite obsolete.
5. Foveoles superior, open behind. Prosternum irregularly swollen, rarely mucronate. Tribe 8. Pamphagides. (Fig. 184, Xiphocera asina.)
5′. Foveoles lateral or inferior, closed behind or (usually) entirely obsolete. Prosternum distinctly mucronate or tuberculate. Tribe 9. Acridiides. (Fig. 84, Acridium peregrinum; Fig. 176, Caloptenus spretus.)
ORTHOPTERA CONTINUED—LOCUSTIDAE, GREEN GRASSHOPPERS, KATYDIDS
Fam. VII. Locustidae—Green Grasshoppers.
Orthoptera, with very long delicate antennae composed of many more than thirty joints; hind legs longer than the others, thicker at the base. Tarsi with four joints. Front tibiae usually provided with tympanal organs placed below the knee; stridulating apparatus of males, when present, situate on the basal part of the tegmina. Females usually with an elongate exserted ovipositor, formed by the apposition of six pieces. Wingless forms numerous.
An unfortunate confusion has long existed as to the term Locustidae, and has resulted in the application of the name to a group of Insects that contains none of the locusts of ordinary language. Some entomologists therefore use the term Phasgonuridea for this family, but the great majority prefer the term Locustidae.
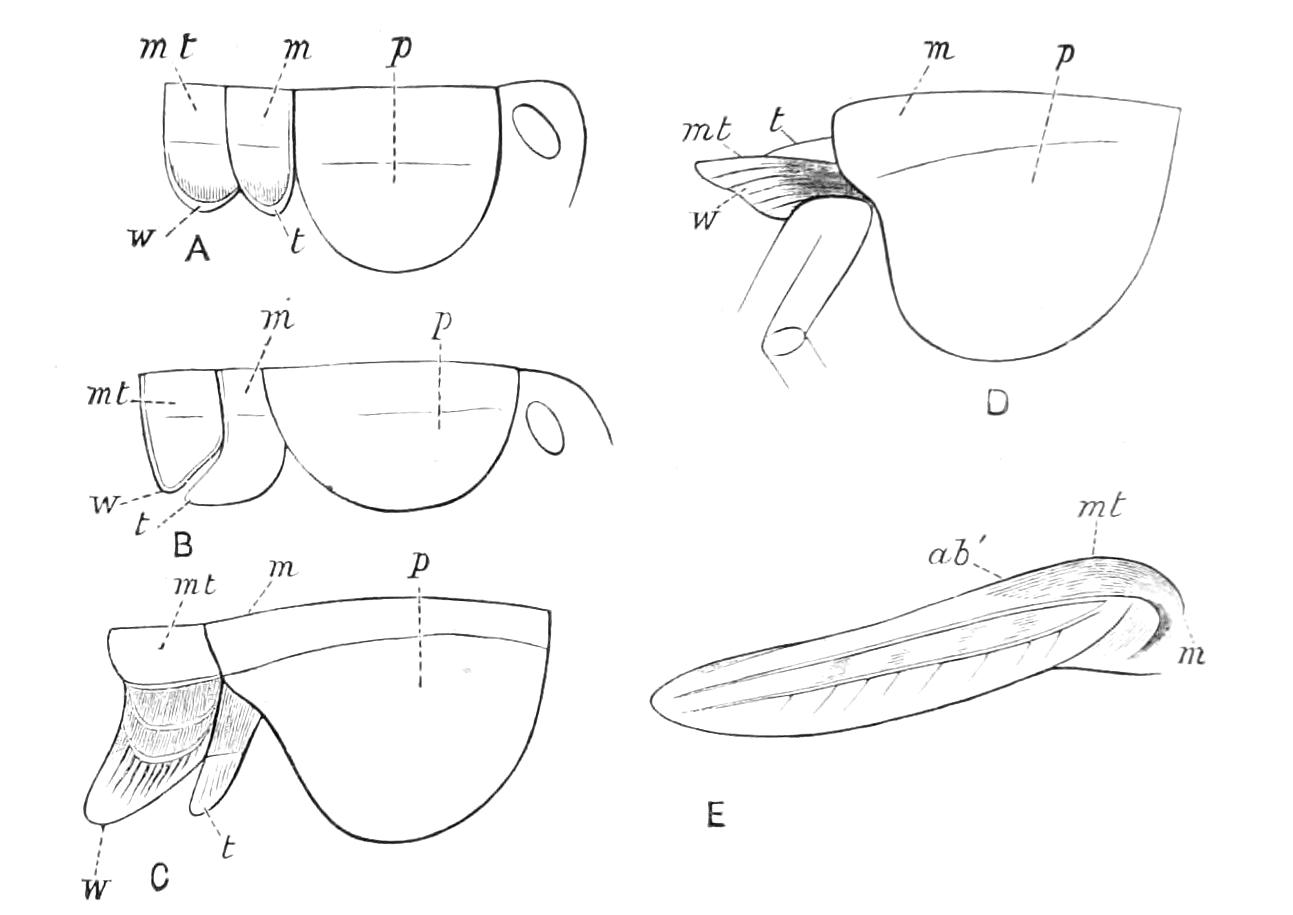
Fig. 188.—Development of wings in Platycleis grisea: A, B, C, D, E, consecutive stages; p, prothorax; m, mesothorax; mt, metathorax; t, tegmen; w, wing; ab′, position of first abdominal segment. In C, D, and E, m points to the part by which the m, shown in A and B, is concealed; in D and E only the positions of mt are indicated. (After Graber.)
The Locustidae are, as a rule, more fragile Insects than the Acridiidae, from which they can be readily distinguished by the characters we have mentioned in our definition. According to Dufour, there are no air vesicles connected with the tracheal system in this family; possibly to this it may be due that none of the family undertake the long flights and migratory wanderings that have made some of the Acridiidae so notorious. Very little is known as to the life histories of the members of this extensive family of Orthoptera. Graber, however, has given some particulars as to the development of Platycleis grisea, and of one or two other species. He recognises five instars, but his first is probably really the second, as he did not observe the Insect in its youngest condition. Although his figures are very poor, we reproduce them, as they give some idea of the mode of growth of the wings, and of the correlative changes in the thoracic segments. It will be seen that in the first three of these instars the alar organs appear merely as prolongations of the sides of the posterior two thoracic rings, and that in D a great change has occurred in the position of these segments, so that the alar organs are free processes, the two posterior thoracic rings being insignificant in size in comparison with the now greatly developed prothorax. In E the tegmen is shown fully developed, the positions of some {313}of the rings covered by it being indicated by the letters m, mt, ab′. These changes are very similar to those we have described in Acridiidae, the chief difference being the greater development of the dependent wing-pads previous to the fourth instar.
The ocelli in Locustidae are much more imperfect than they are in Acridiidae, and are frequently rudimentary or nearly totally absent, or there may be but one instead of three. They are, however, present in a fairly well-developed state in some species, and this is the case with the one whose face we portray in Fig. 189, where the anterior of the three ocelli is quite conspicuous, the other two being placed one on each side of the curious frontal cone near its base. The peculiar head ornament shown in this figure exists in both sexes, and something similar occurs in a large number of Conocephalides. We have not the slightest idea of its import. Individuals of one or more species of this curious South American genus are occasionally met with alive in gardens near London. They are, no doubt, imported as eggs, for they are sometimes met with in the juvenile state, but in what way they are introduced is not known.
The ovipositor frequently attains a great length in these Insects, so as to exceed that of the body. It is used in different ways, some of the family depositing their eggs in the earth, perhaps in vegetable matter under the surface; but other species place the ova in twigs or stems of plants, arranging them in a very neat and compact manner in two series, as depicted by Riley[245] in the case of Microcentrum retinerve (Fig. 190). These eggs are laid in the autumn, and in the following spring become more swollen before hatching. The Insect undergoes a moult during the process of emerging from the egg. By the time the emergence is completed the Microcentrum has expanded so much {314}in size that it is a matter of astonishment how it can ever have been packed in the egg; the young commence jumping and eating leaves in a few minutes. Including the ecdysis made on leaving the egg, they cast their skins five times. The post-embryonic development occupies a period of about ten weeks. The larvae eat their cast skins. When the final moult occurs the tegmina and wings are at first quite soft and colourless, but within an hour they assume their green colour. These Insects, as remarked by Riley, make interesting pets. The people of the Amazon valley are in the habit of keeping a species in cages, and our British Locusta viridissima does very well in confinement. One of the most curious habits of these Locustidae is a constant licking of the front paws. Riley says that M. retinerve bestows as much attention on its long graceful antennæ as many a maiden does upon her abundant tresses, the antennæ being drawn between the jaws and smoothed by the palpi. This American naturalist also tells us that he reared three successive broods in confinement, and that the Insects gradually deteriorated, so that the eggs of the third generation failed to hatch.
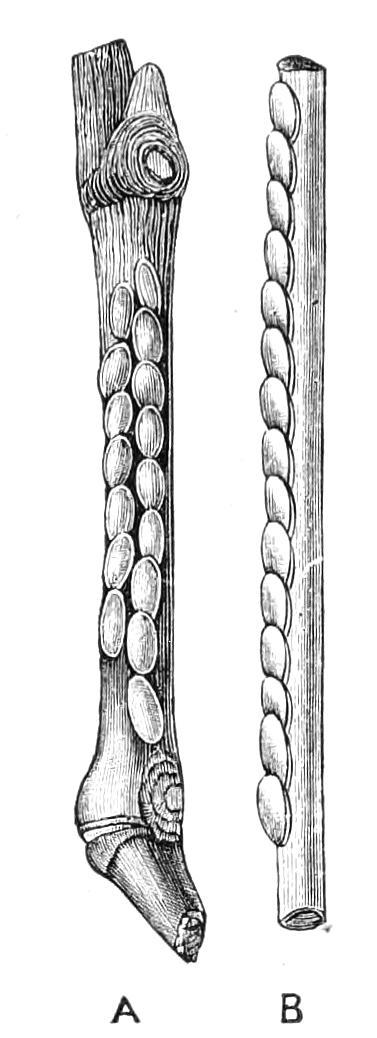
Fig. 190.—Eggs of Katydid (Microcentrum retinerve): A, the two series at deposition; B, side view of a single series. (After Riley.)
The ovipositor, which is one of the most characteristic features of the Locustidae, is not present in the newly-hatched Locustid (Fig. 191, A), the organ being then represented only by two papillae placed on the penultimate segment. The structure and development of the ovipositor in Locusta viridissima have been described by Dewitz.[246] Fig. 191, A, shows the young Insect taken from the egg just as it is about to emerge. The abdomen consists of ten segments, the terminal one bearing at its extremity two processes, the cerci, a′. These persist throughout the life of the Insect, and take no part in the formation of the ovipositor. The tenth segment subsequently divides into two (a, a'′, Fig. 191, C), giving rise to the appearance of eleven abdominal segments, and of the ovipositor springing from the antepenultimate.
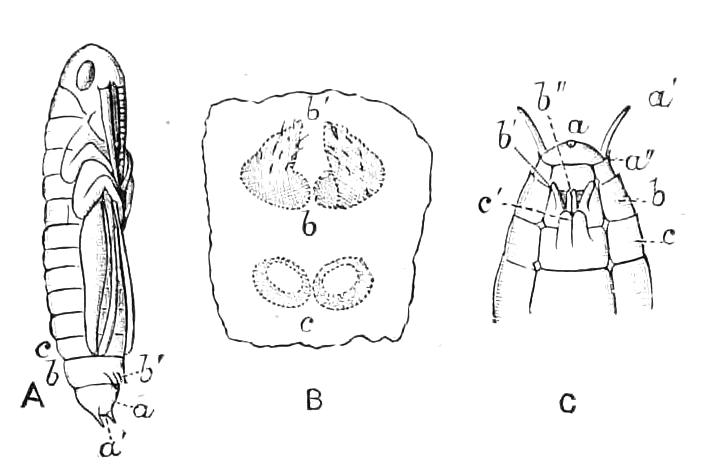
Fig. 191.—Development of ovipositor of Locusta viridissima: a, terminal segment; a′, cerci; a″, secondary division of terminal segment; b, penultimate (ninth) segment; b′, primary papillae of this segment; b″, secondary divisions thereof; c, eighth segment; c′, its papillae. (After Dewitz.) A, embryo ready for emergence; B, portion of integument of the ventral plates of eighth and ninth segments; C, the appendages in a condition somewhat more advanced than they are in A.
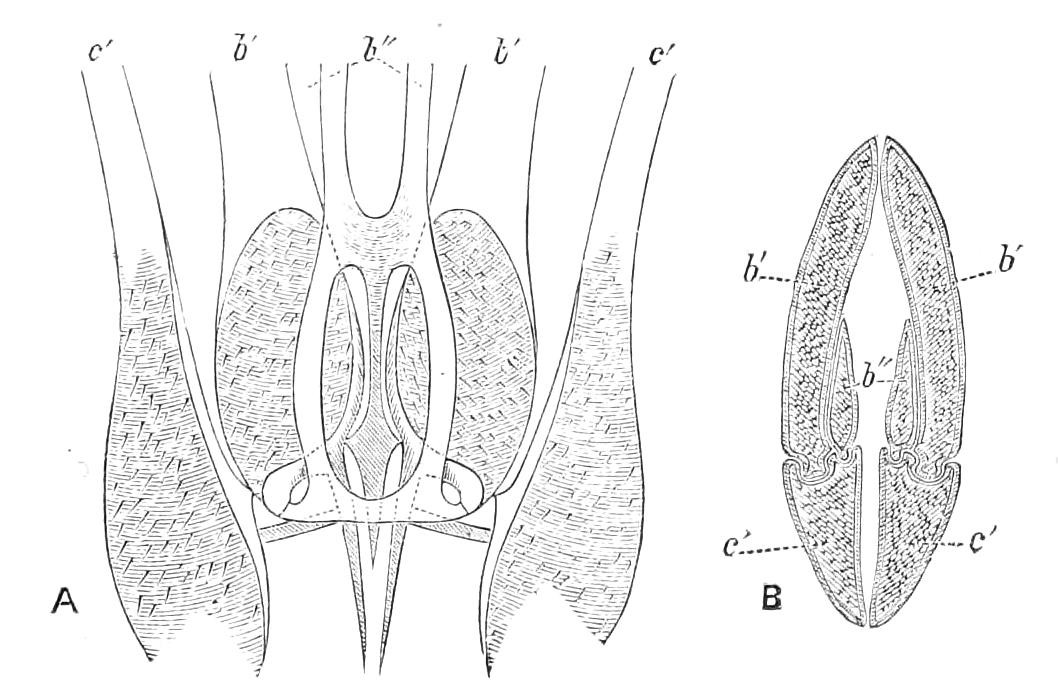
Fig. 192.—Structure of ovipositor of Locusta viridissima: A, arrangement of parts at base, c′ being separated and turned outwards; B, transverse section. The parts of the appendage bear the same lettering as in Fig. 191. (After Dewitz.)
Near to one another, on the middle of the ventral aspect of the true ninth abdominal segment, are seen the two papillae (b′), which at first are the only visible indications of the future ovipositor. If, however, the integument be taken off and carefully examined, it will be found that there exist on the eighth abdominal plate two spots, where there is a slight thickening and prominence of the integument (Fig. 191, B, c). From these two spots the two lower rods of the ovipositor are produced; these two, together with the two growths from the ninth segment, form the four external rods of the ovipositor. Inside these there exist in the completed structure two other rods (Fig. 192, B, b'′). These are produced by a growth from the inner parts of the two papillae of the ninth segment. The relations of the six rods in their early condition are shown in Fig. 191, C, where the two primary papillae b′ of the ninth segment are seen with their secondary offshoots b″; c′ being the papillae of the eighth segment. The subsequent relations of the pieces are shown in Fig. 192; A exhibiting the base of the organ with the lower rods turned on one side to show the others, the shaded parts indicating {316}muscular attachments; B is a transverse section of the organ. In these figures the different parts of the appendages bear the same lettering as they do in Fig. 191. It will be seen that in the completed structures the parts c′ have become very intimately connected with the parts b′ and b″, which belong to another segment.
The Locustidae resemble the Acridiidae in the possession of specialised ears and sound-producing organs; neither of these is, however, situate in the same part of the body as in Acridiidae. The ears of Locustidae are placed on the front legs, below the knee; a tympanum (Fig. 193, A), or a crack giving entrance to a cavity in which the tympanum is placed (Fig. 193, B), being seen on each side of each of the anterior pair of limbs. In this family, as in the Acridiidae, three kinds of ear are recognised according to the condition of the tympanum, which is either exposed (Fig. 193, A) or closed by an overgrowth of the integument (Fig. 193, B), or in a condition to a certain extent different from either of these. The existence of ears placed on the legs is a curious fact, but it is beyond doubt in the Locustidae, and there is good reason for believing that analogous organs exist in this situation in other Insects that have special means of sound-production, such as the ants and the Termites.
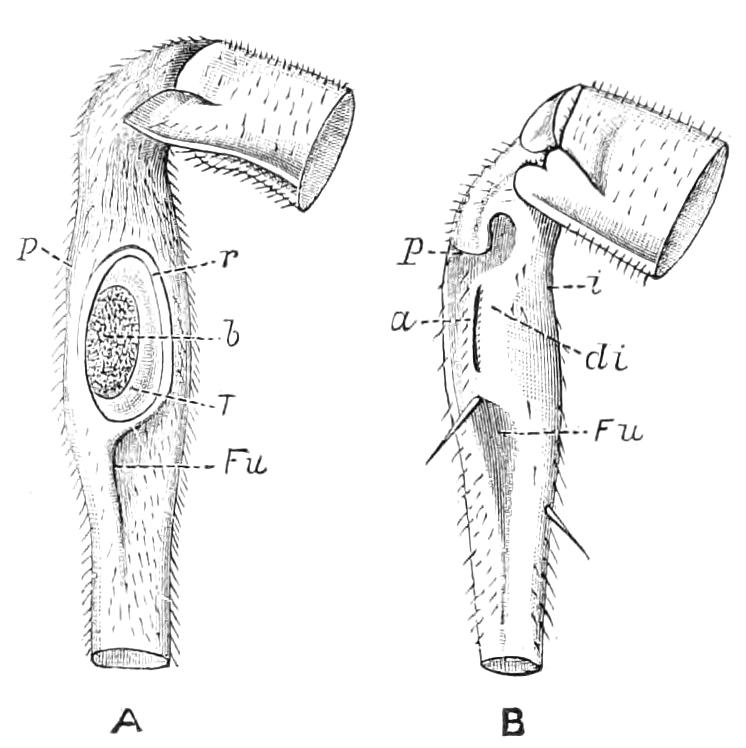
Fig. 193.—Ears of Locustidae: A, portion of front leg of Odontura serricauda, adult; p, prominence of integument; r, rim of ear; T, tympanum; b, thickened area thereof; Fu, remains of groove in which the structure was developed. B, portion of front leg of Thamnotrizon apterus; i, inner margin; a, slit-like external aperture of ear; di, overlapping cover of the ear. (After Graber.)
The structure of these organs in the Locustidae has been investigated by Graber,[247] and their acoustic functions placed beyond doubt, though to what special kind of sounds they may be sensitive is not ascertained, this point being surrounded by even greater difficulties than those we have discussed in the case of the Acridiidae. In the Locustidae there is a special structure of a remarkable nature in connexion with the ears. In Acridiidae {317}a stigma is placed close to the ear, and supplies the internal structures of the organ with air. There are no stigmata on the legs of Insects, consequently admission of air to the acoustic apparatus in Locustidae is effected by means of a gaping orifice at the back of the prothorax, just over the base of the front leg (Fig. 101); this communicates with its fellow of the other side, and from them there extend processes along the femora into the tibiae, where they undergo dilatation, so as to form vesicular cavities, one of which is in proximity to each drum of the ear. These leg-tracheae are not connected with the ordinary tracheal system; the prothoracic stigma exists in close proximity to the acoustic orifice we have described, but is much smaller than it. It is not yet clear why the acoustic apparatus should require a supply of air apart from that which could be afforded by the ordinary tracheal system. This special arrangement—to which there is hardly a parallel in Insect anatomy—has still to be accounted for; we do not know whether the necessity for it may be connected with the respiratory system or the acoustic organ.
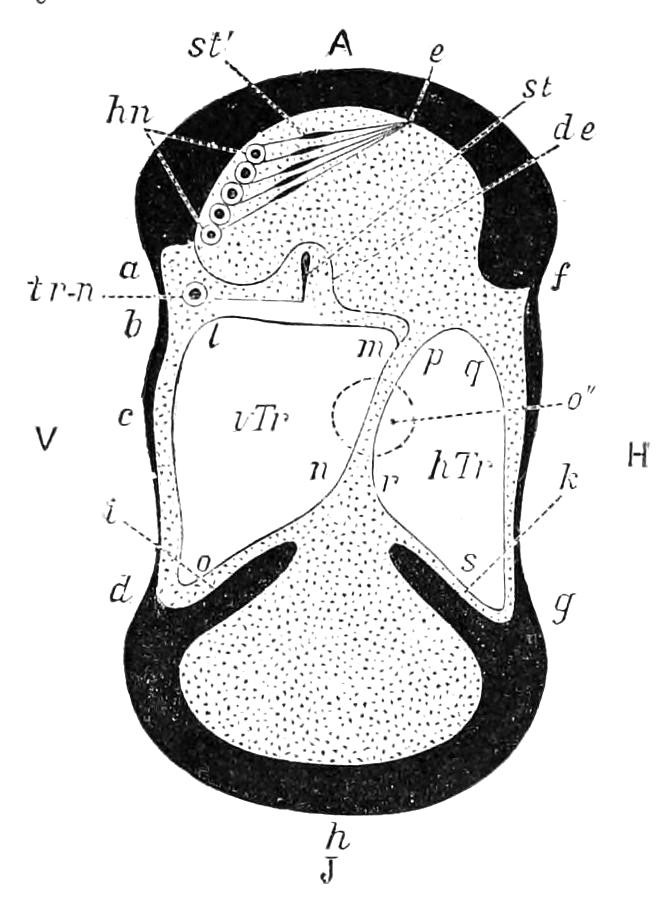
Fig. 194.—Diagram of arrangement of parts of the ear as seen in transverse section of the tibia of a Locustid. A, J, V, H, outer, inner, anterior, posterior aspects of leg; a, d, thin part of integument forming anterior tympanum; b, c, thicker portion of same; f, g, posterior tympanum; a, f, and d, h, g, thick portions of integument; i, k, internal protuberances of same; l, m, n, o, walls of the anterior tracheal vesicle, vTr; p, q, s, r, walls of the posterior tracheal vesicle, hTr; o″, projection of tympanal orifice of prothorax; tr-n, tracheal nerve-end organ, crista acustica; st, rod; de, curtain-membrane; hn, e, supra-tympanal, nerve-end organ; hn, ganglion cells; st′, rods; e, point of integumental fixation of nerve endings. (After Graber.)
The chief features of the acoustic apparatus of the legs of Locustidae will be gathered from the accompanying diagrammatic transverse section through the tibia. In this figure the deep black parts indicate the outer wall of the tibia and its prolongations, the white spaces indicate the parts filled with air, while the dotted portions are occupied by blood or some of the body organs;[248] the {318}circular space o″ is not part of the actual structure, but represents the area of the external acoustic orifice of the prothorax; it is not, however, so large as it should be.
Although the tibial ears of Locustidae are very perfect organs, there is great difficulty in deciding on the exact nature of their functions. They would appear to be admirably adapted to determine the precise locality from which a sound proceeds, especially in those cases—and they are the highest forms—in which the tympanum is placed in a cavity the external orifice of which is a slit (Fig. 193, B); for the legs can be moved in the freest manner in every direction, so as to bring the drum into the most direct line of the vibrations. But as to what kinds of vibrations may be perceived, and the manner in which they may be transmitted to the nerves, there is but little evidence. On reference to the diagram it will be noticed that the tympanum, the tympanal vesicles, and the nervous apparatus are not in close connexion, so that even the mode by which the impulses are transmitted is obscure.
The musical organs of the Locustidae are different from those of the Acridiidae, and are invariably situate on the basal part of the tegmina. They are found, in the great majority of cases, only in the male; in the tribes Ephippigerides and Callimenides they exist in each sex. One of the wings bears a file on its inner surface, while the other—on the right side of the body—is provided with a sharp edge placed on a prominent part of its inner margin. By slightly tilting the tegmina and vibrating them rapidly, the edge passes under the file, and a musical sound is produced. These structures are limited to the small anal area of the wing, and when the tegmina are very greatly reduced in size, it is this part that still remains. There is much variety in the details of the structure. The nervures of this part of the tegmina are different in the male from what they are in the female, and, moreover, the two wing-covers of the male differ from one another. It is apparently the vibrations of the right tegmen that produce the sound, and this part usually bears a space of a glassy nature, which probably improves the character of the sound produced. Our chief British songster of this group, Locusta viridissima, is only provided with phonetic organs (Fig. 195) of a somewhat imperfect character, but in the genus Mecopoda there is great perfection of the structures. The anal areas of the two tegmina are in this case {319}very different; that of the left one, which bears the file, being similar in texture to the rest of the wing-cover, while the corresponding part of the other tegmen is rigid and transparent, and greatly distorted, so as to create a cavity which, no doubt, improves the sound; the scraper too is very perfectly formed. The difference between this form of musical organ and that of L. viridissima is curious, inasmuch as in the better instrument the important modifications are confined to one tegmen, while in the other form both tegmina are largely changed. The difference appears to be that in Locusta the left tegmen, as well as the right one, acts as a sounding-board, while in Mecopoda it does not do so, but when the wings are closed quite covers and conceals the musical instrument.
The Locustidae, notwithstanding the fact that their alar organs are generally more ample than those of the Acridiidae, seem to be, as a rule, of more sedentary habits, and more nocturnal in their activity. The musical powers of the different species are very varied. Locusta viridissima produces a shrill and monotonous but not disagreeable, sound, and is capable of sustaining it for a quarter of an hour without any intermission, except a break for the sake of starting again immediately with greater force, like a performer on a flute. It occasionally chirps in the day, but the act is then very brief. Bates informs us that one of these singing grasshoppers, called Tananá by the natives of the Amazon valley, is much admired for its singing, and is kept in little cages. The Amazonian naturalist thought the music of this species superior to that of any other Orthopterous Insect he had heard. The name of this grasshopper is Thliboscelus camellifolius. It is very similar in appearance to Cyrtophyllus crepitans, the Insect we have represented in Fig. 187.
Fig. 195.—Inner face of base of tegmina of Locusta viridissima: A, the two wing-covers separated; B, in natural position with mesonotum connecting them, showing file and edge scraping it; a, the stridulating file; b, the rudimentary file on other tegmen.
The most notorious of the musical Locustids are the Katydids {320}of North America. There are several species of them—they belong, indeed, to more than one genus,—but it seems that sounds somewhat resembling the words Katy-did are perceptible in most of their performances. These sounds are frequently repeated with slight variations—Katy-did, O-she-did, Katy-did-she-did. Riley describes the music of the Katydid we represent in Fig. 196 as follows:[249] "The first notes from this Katydid are heard about the middle of July, and the species is in full song by the first of August. The wing-covers are partially opened by a sudden jerk, and the notes produced by the gradual closing of the same. The song consists of a series of from twenty-five to thirty raspings, as of a stiff quill drawn across a coarse file. There are about five of these raspings or trills per second, all alike, and with equal intervals, except the last two or three, which, with the closing of the wing-covers, run into each other. The whole strongly recalls the slow turning of a child's wooden rattle, ending by a sudden jerk of the same; and this prolonged rattling, which is peculiar to the male, is invariably and instantly answered by a single sharp 'chirp' or 'tschick' from one or more females, who produce the sound by a sudden upward jerk of the wings."
Pertinacity is one of the most curious features of the performance of musical Locustids. One would say they desire to distinguish themselves as much as possible. Harris says that Cyrtophyllus concavus mounts on the uppermost twigs of trees and there performs its Katy-did-she-did in rivalry with others. He says even the female in this species gives forth a feeble noise. Scudder says that some of the Katydids sing both by day and night, but their day song differs from that of the night. "On a summer's day it is curious to observe these little creatures suddenly {321}changing from the day to the night song at the mere passing of a cloud, and returning to the old note when the sky is clear. By imitating the two songs in the daytime the grasshoppers can be made to respond to either at will; at night they have but one note."
Although but little is known as to the habits of Locustidae, it is ascertained that they are less exclusively herbivorous in their food habits than the Acridiidae are; many seem to prefer a mixed diet. Locusta viridissima will eat various leaves and fruits, besides small quantities of flesh. It has been recorded that a specimen in confinement mastered a humble-bee, extracted with its mandibles the honey-bag, and ate this dainty, leaving the other parts of the bee untouched. Many of the Locustidae are believed to be entirely carnivorous. Brunner considers a minority to be exclusively phytophagous. The species very rarely increase to large numbers; this, however, occurs sometimes with Orphania denticauda and Barbitistes yersini in Europe, and Anabrus purpurascens in North America. We have already mentioned that the eggs of some species are deposited in parts of plants, and of others in the earth. The British Meconema varium deposits its eggs in the galls of Cynips in the autumn; these eggs do not hatch till the following spring. Xiphidium ensiferum has somewhat similar habits in North America, the gall selected for the reception of the eggs being the scales formed by a species of Cecidomyia on the leaves of willows. It has been ascertained that the development of the embryo in the last-named species is commenced in the autumn, but is suspended during the winter, being only completed in the following spring, eight or nine months afterwards. We owe to Wheeler[250] a memoir on the embryology of this Insect.
Some of the species have the peculiar habit of dwelling in caves. This is especially the case with the members of the tribe Stenopelmatides (Fig. 197), which frequently possess enormously long antennae and legs, and are destitute of alar organs and ears. The species with this habit, though found in the most widely separated parts of the world, have a great general resemblance, so that one would almost suppose the specimens found in the caves of Austria, in the Mammoth cave of Kentucky, and in the rock-cavities of New Zealand to be one {322}species, although they are now referred by entomologists to different genera.
Fig. 198.—Leaf-like tegmen of Pterochroza ocellata: a, a, a, marks like those made by Insects on leaves.
The Locustidae display in the greatest possible perfection that resemblance of the tegmina to leaves which we mentioned when speaking of the general characters of the Orthoptera. The wing-covers are very leaf-like in colour and appearance in many Locustidae, but it is in the tribe Pseudophyllides and in the South American genus Pterochroza (Fig. 198) that the phenomenon is most remarkable. The tegmina in the species of this genus look exactly like leaves in certain stages of ripeness or decay. In the tegmina of some of the species not only are the colours of faded leaves exactly reproduced, but spots are present like those on leaves due to cryptogamic growths. Perhaps the most remarkable feature of these resemblances is the one pointed out by Brunner von Wattenwyl,[251] viz. that the tracks and spots formed on leaves by the mining of Insects in their tissues are also represented in the leaf-like wing-covers of the Pterochroza; transparent spots (a, a, Fig. 198) being present, just as they are in many leaves that have been attacked by Insects. Brunner was so much impressed by these facts that he came to the conclusion that they cannot be accounted for on the grounds of mere utility, {323}and proposed the term Hypertely to express the idea that in these cases the bounds of the useful are transcended. We will mention here another peculiar case of resemblance described by Brunner as occurring in a Locustid. Two specimens of a little Phaneropterid were brought from the Soudan by the Antinori expedition, and have been described by Brunner under the name of Myrmecophana fallax. The Insect is said to bear an extraordinary resemblance to an ant. The most peculiar feature in the resemblance is shown in Fig. 199, A, B. The most characteristic point in the external form of an ant is the stalked abdomen, this structure being at the same time quite foreign to the Orthoptera. In the other parts of the body and in the colour generally, the Myrmecophana resembles an ant, but the abdomen of the Orthopteron is not stalked; it has, however, the appearance of being so, in consequence of certain parts being of a white colour, as shown in our figure. If abstraction be made of the white parts, the form of the stalked abdomen of the ant is nicely reproduced. The specimens brought from the Soudan were wingless and destitute of ovipositor, and may be immature, but Brunner suggests that they may prove to be really mature, the ovipositor, tegmina, and wings being permanently absent. The existence of a long ovipositor would certainly detract greatly from the ant-like appearance of the Orthopteron.
It is certain that the plant-like appearance of some of the Locustidae renders them inconspicuous to the human eye in the situations they frequent. It is a matter of common observation that though the noise of their chirpings may be heard to such an extent as to make it certain that many individuals must be in the immediate neighbourhood, yet at the same time it may be most difficult to detect even a single individual. M. Boutan noticed this phenomenon in the case of Ephippigera rugosicollis, and tells us that the human eye can, with a little practice, acquire the art of detecting these concealed creatures. This consists {324}apparently in making use, not of a general inspection, but of a scrutiny of the outlines of the leaves and twigs of a tree. By this means, when the eye is accustomed to the task, the Insects can be detected with comparative ease; much in the same way, M. Boutan says, as a figure, placed in an engraving in such a way as to elude the eye, is appreciated with ease after the eye has once perceived it.
Some of the Locustidae are provided with means of defence of a positive nature. The Algerian Eugaster guyoni ejects two jets of a caustic orange-coloured fluid from two pores situate on the sides of the mesosternum, and covered by the anterior coxae. This species is carnivorous as well as herbivorous, and produces a sound more like humming than stridulation.[252]
We have previously pointed out that some of the Acridiidae resemble the stick-Insects rather than the members of their own group; and similar cases occur amongst the Locustidae. Such a resemblance has, however, only been found in a few species of the tribe Prochilides. We figure one of these, Phasmodes ranatriformis, a native of South-West Australia. The very elongate linear form and the total absence of alar organs give this Insect a considerable resemblance to the stick-Insects or apterous Phasmidae. Prochilus australis is allied to this curious Locustid, but the alar organs are present in both sexes, and the Insect bears a great resemblance to the winged Phasmidae. This is due not only to the general form and colour, but also to the fact that the tegmina are very narrow, which {325}causes them to look like the coloured slip on the anterior parts of the wings of some of the Phasmidae (cf. p. 266). Another case of a Locustid with elongate, slender form is found in the extraordinary Peringueyella jocosa of South Africa, a member of the tribe Sagides. It has minute organs of flight, and reproduces, to a considerable extent, the form and appearance of Proscopides or of some Tryxalides.[253]
We follow Brunner in placing among the Locustidae the large Insect we represent in Fig. 201. It is remarkable on account of its tegmina and wings; these have their extremities much prolonged and curled; moreover, the flat interior area and the abruptly {326}deflexed exterior area make them look more like the wings of Gryllidae. This species has no ocelli, and is said to be destitute of ears. The inflated condition of the anterior and middle tibiae suggest that it possesses auditory structures, though there appears to be no external opening for them. This Insect is found in India, where it is said to be common on the banks of sandy rivers, living there in burrows of the depth of three feet. Very little is known, however, as to this curious Insect. It has recently been reported[254] as being injurious to tobacco and other crops on high ground in Durbungha by cutting off their roots. The local name for the Insect is bherwa. We should think it somewhat doubtful whether this refers really to S. monstrosus.
In number of species the Locustidae are perhaps scarcely inferior to the Acridiidae, and in variety of form they surpass this latter family. Many of the most gigantic forms are apterous, and these very often have a repellant aspect. The genus Anostostoma is remarkable for its large head. Allied to it is Deinacrida heteracantha, the "Weta-punga" of the New Zealand natives, an Insect formerly abundant in the forests north of Auckland, but of late years become extremely rare. The head and body of this Insect may measure more than 2½ inches in length, and when the antennae and legs are stretched out the total length may be 14 or 15 inches. Although bulky and absolutely wingless, yet, as Buller informs us,[255] it climbs with agility, and is sometimes found on the topmost branches of lofty trees. When disturbed it produces a clicking, accompanied by a slow movement {327}of its hind legs. A second species, D. thoracica, lives in decayed wood, and a third, D. megacephala, is remarkable from the very large size of the head and mandibles in the male sex. The fact that a clicking noise is produced by the Weta-punga is of some interest, for the genus Deinacrida is among the Locustidae that possess ears, but are said to be destitute of sound-producing organs.
Amongst the most remarkable of the Locustidae are the two species of which Brongniart has recently formed the genus Eumegalodon and the tribe Eumegalodonidae, which is not included in Brunner's table of the tribes of Locustidae. The ovipositor is large and sabre-shaped; the male is unknown. The genus Megalodon is placed by Brunner in the tribe Conocephalides; it also consists of extremely remarkable Insects.
The Locustidae appear to be of slow growth, and the autumns of Britain are usually not warm enough for them. Hence we have but nine British species, and of this number only three or four are known to occur north of the Thames. The only one that attracts attention is Locusta viridissima, which in some districts of the south of England occurs in considerable numbers, and attests its presence by its peculiar music. It is called the green grasshopper.
The geological record is rather obscure in the matter of Locustidae. Scudder considers that a fair number of Tertiary forms are known, and says that they represent several of the existing tribes and genera. One or two have been found in Mesozoic rocks.
Table of the Tribes of Locustidae
1. Tarsi more or less depressed.
2. Front tibiae furnished with auditory cavities.
3. Antennae less distant from the summit of the occiput than from the labrum; inserted between the eyes.[256]
4. First two joints of the tarsi laterally smooth. (Posterior tibiae furnished on each side with an apical spine.) Tribe 1. Phaneropterides. (Fig. 196, Microcentrum; Fig. 199, Myrmecophana. Fig. 101, Poecilimon affinis.)
4′. First two joints of the tarsi laterally, longitudinally sulcate.
5. Foramina of the anterior tibiae normally open. (Fig. 193, A.)
6. Posterior tibiae furnished on each side with apical spines.
7. Prosternum unarmed. Tribe 2. Meconemides.
7′. Prosternum bispinose or bituberculate. Tribe 3. Mecopodides.
6′. Posterior tibiae with no apical spines. (Head prognathous.) Tribe 4. Prochilides. (Fig. 200, Phasmodes.)
5′. Foramina of the anterior tibiae forming a chink, or protected by a scale. (Fig. 193, B.)
6. Anterior tibiae with no apical spines.
7. Margins of the scrobes[257] of the antennae prominent. Tribe 5. Pseudophyllides. (Fig. 187, Cyrtophyllus crepitans; Fig. 198, Pterochroza ocellata.)
7′. Margins of the scrobes of the antennae not prominent.
8. Posterior tibiae furnished above on each side with apical spines, or with a single spine on the side.
9. Posterior tibiae either furnished with apical spines on each side, or only on the inner side. Tribe 6. Conocephalides. (Fig. 189, Copiophora cornuta.)
9′. Posterior tibiae furnished above with an apical spine placed only on the outer side. Tribe 7. Tympanophorides.
8′. Posterior tibiae without apical spines. Tribe 8. Sagides.
6′. Anterior tibiae furnished with an apical spine on the inner side.[258]
7. The first joint of the posterior tarsi destitute of a free sole-lobe. Tribe 9. Locustides.
7′. The first joint of the posterior tarsi furnished with a free sole-lobe. Tribe 10. Decticides.
3′. Antennae more distant from the summit of the occiput than from the labrum, inserted either beneath the eyes or on their inferior border. Tegmina and wings greatly abbreviate, scale-like; when tegmina are present they are furnished in each sex with a tympanum.
4. Third joint of the posterior tarsi shorter than the second. Both anterior and posterior tibiae furnished on each side with a spine. Tribe 11. Callimenides.
4′. Third joint of posterior tarsi longer than the second joint. Anterior tibiae with no apical spine on the inner side, and posterior tibiae with no apical spine on the outer side.
5. Antennae inserted at the edge of the eyes. Pronotum unarmed. Tegmina present in each sex. Anterior tibiae furnished on the outer side with an apical spine. Posterior tibiae furnished beneath with four apical spines. Tribe 12. Ephippigerides.
5′. Antennae inserted distinctly below the eyes. Pronotum spinous. Elytra in the females wanting. Anterior tibiae without apical spine on either side. Posterior tibiae beneath with two apical spines or with none. Tribe 13. Hetrodides.
2′. Anterior tibiae without auditory cavities. Tegmina with no tympanum. Tribe 14. Gryllacrides. (Fig. 201, Schizodactylus monstrosus.)
1′. Tarsi distinctly compressed (most of the species apterous.) Tribe 15. Stenopelmatides. (Fig. 202, Anostostoma australasiae; Fig. 197, Dolichopoda palpata.)
ORTHOPTERA CONTINUED—GRYLLIDAE, CRICKETS
Fam. VIII. Gryllidae—Crickets.
Antennae very slender, generally long and setaceous; hind legs long, saltatorial. Tegmina with the outer portion deflexed on to the side of the body, and with the inner part lying flat on the body. Tarsi usually three-jointed (rarely two- or four-jointed). Female with a long ovipositor (except in Gryllotalpides). Apterous forms numerous.
The Gryllidae are closely connected with the Locustidae, the musical and auditory organs being in both similarly situate, and the female in both possessing, in most of the tribes, an elongate exserted ovipositor. The two families differ in the number of joints of the tarsi, in the form of the tegmina, and in the fact that in Gryllidae the portion of the wing modified for musical purposes consists of a larger portion of the organ—according to de Saussure, the discoidal as well as the anal area.
The family would be a very natural one if we were to exclude from it the mole-crickets which have fossorial front legs and no ovipositor, and the Tridactylides, which also are {331}destitute of ovipositor, and have short antennae, consisting of about ten joints.
The head is generally very large; ocelli are present, though usually imperfect; the extremity of the body bears a pair of remarkably long cerci. The hind tibiae are usually armed with very strong spines; the first joint of the hind tarsus is elongate, and terminates in two spines, between which the small second joint is often almost completely concealed; the feet are not provided beneath with pads, but only bear remote setae.
The alar organs are difficult of comprehension, and different opinions prevail as to their morphology. The tegmina are extremely different to the hind wings, and never attain large dimensions, neither do they exhibit any leaf-like or ornamental structures. In the genus Pteroplistus they are formed somewhat like the elytra of Coleoptera, and close over the back of the Insect in a fashion very like that found in beetles. According to Brunner the larger part of the tegmen—which, as we have said, reposes flat on the back of the Insect—represents merely the anal area, and all the other parts must be sought in the smaller, deflexed portion of the wing-cover. De Saussure's opinion, to a somewhat different effect, we have already mentioned. The tegmina of the male are extremely different from those of the female, so that it is a matter of much difficulty to decide what nervures correspond.[259]
Fig. 205.—Tegmina (sinistral) of the house-cricket. A, male, inner aspect; B, female, outer aspect: a, inner margin; b, outer margin; c, nervure bearing stridulating file.
The wing-covers of the male differ from those of the Locustidae, inasmuch as the pair are of similar formation, each bearing a stridulating file on its lower aspect. This file projects somewhat inwards, so that its position is marked on the outer aspect of the wing-cover by a depression. Usually the right tegmen overlaps the other, an arrangement contrary to that which prevails in other Orthoptera. The wings are ample and delicate; they possess numerous nervures that are not much forked and have a {332}simple, somewhat fan-like arrangement; the little transverse nervules exhibit only slight variety. These wings are frequently rolled up at the apex, and project beyond the body like an additional pair of cerci (Fig. 204). The abdomen is chiefly remarkable for the large development of the pleura, the stigmata being consequently very conspicuous. The cerci are not jointed, though they are flexible and, often, very long; they bear a variety of sense-organs (Fig. 67). The saltatorial powers of the crickets are frequently considerable.
Graber has observed the post-embryonic development of the field-cricket, Gryllus campestris, though unfortunately not from the very commencement, so that we do not know whether there are five, six, or seven ecdyses; the number is probably either six or seven. The manner in which the alar organs are developed is similar to that we have described and figured in the Locustidae. In the earlier instars there is a slight prolongation of each side of the meso- and meta-notum, but about the middle of the development a considerable change occurs—the rudimentary organs then become free appendages and assume a different position.
The Gryllidae possess a pair of tympana on each front leg, but these organs contrast with those of the Locustidae in that the pair on each leg usually differ from one another, the one on the outer or posterior aspect being larger than that on the inner or front face of the leg.
The ears of the Gryllidae have not been so well investigated as those of the Locustidae, but are apparently of a much less perfect nature. No orifice for the admission of air other than that of the prothoracic stigma has been detected, except in Gryllotalpa. On the other hand, it is said[260] that in addition to the tibial organs another pair of tympana exists, and is seated on the second abdominal segment in a position analogous to that occupied by the ear on the first segment of Acridiidae.
The musical powers of the crickets are remarkable, and are familiar to all in Europe, as the performance of the house-cricket gives a fair idea of them. Some of the Insects of the family are able to make a very piercing noise, the note of Brachytrypes megacephalus having been heard, it is said, at a distance of a mile from where it was being produced. The mode of {333}production is the same as in the Locustidae, rapid vibration of the tegmina causing the edge of one of them to act on the file of the other.
The mole-cricket, Gryllotalpa vulgaris—the Werre of the Germans, Courtilière of the French—is placed with a few allies in a special group, Gryllotalpides, characterised by the dilated front legs, which are admirably adapted for working underground. Like the mole, this Insect has a subterranean existence. It travels in burrows of its own formation, and it also forms beneath the surface a habitation for its eggs and family. Its habits have been alluded to by Gilbert White,[261] who tells us that "a gardener at a house where I was on a visit, happening to be mowing, on the 6th of May, by the side of a canal, his scythe struck too deep, pared off a large piece of turf, and laid open to view a curious scene of domestic economy: there were many caverns and winding passages leading to a kind of chamber, neatly smoothed and rounded, and about the size of a moderate snuff-box. Within this secret nursery were deposited near a hundred eggs of a dirty yellow colour, and enveloped in a tough skin, but too lately excluded to contain any rudiments of young, being full of a viscous substance. The eggs lay but shallow, and within the influence of the sun, just under a little heap of fresh moved mould like that which is raised by ants."
The front legs are remarkable structures (Fig. 206), being beautifully adapted for burrowing; the tibiae and tarsi are arranged so as to act as shears when it may be necessary to sever a root. The shear-like action of the tarsus and tibia is very remarkable; the first and second joints of the former are furnished with hard processes, which, when the tarsus is moved, pass over the edges of the tibial teeth in such a way as to be more effective than a pair of shears. In consequence of its habit of cutting roots, {334}the mole-cricket causes some damage where it is abundant. It is now a rare Insect in England, and is almost confined to the southern counties, but in the gardens of Central and Southern Europe it is very abundant. Its French name courtilière is supposed to be a corruption of the Latin curtilla. Its fondness for the neighbourhood of water is well known. De Saussure says that in order to secure specimens it is only necessary to throw water on the paths between the flower-beds of gardens and to cover the wetted places with pieces of board; in the morning some of these Insects are almost sure to be found under the boards disporting themselves in the mud. The Gryllotalpae swim admirably by aid of their broad front legs.
Ears exist in the mole-cricket, and are situate on the front leg below the knee, as in other Gryllidae, although it seems strange that a leg so profoundly modified for digging and excavating as is that of the mole-cricket should be provided with an ear. In Gryllotalpa the ear is concealed and protected by being placed in a deep slit or fold of the surface, and this depression is all that can be seen by examination of the exterior (Fig. 206, e). In the allied genus Scapteriscus the tympanal membrane is, however, destitute of special protection, being completely exposed on the surface of the leg.
Although the tegmina or upper wings in Gryllotalpa are of small size, yet the true wings are much more ample; they are of delicate texture and traversed by many nearly straight radii, so that they close up in the most complete manner, and form the two long delicate, flexible processes that in the state of repose may be seen projecting not only beyond the tegmina, but actually surpassing the extremity of the body hanging down behind it, and looking like a second pair of cerci.
The mole-cricket is believed to be chiefly carnivorous in its diet, though, like many other Orthoptera, it can accommodate its appetite to parts of the vegetable as well as of the animal kingdom. The Insect is capable of emitting a sound consisting of a dull jarring note, somewhat like that of the goat-sucker. For this purpose the tegmina of the males are provided with an apparatus of the nature we have already described, but which is very much smaller and less elaborate than it is in the true crickets.
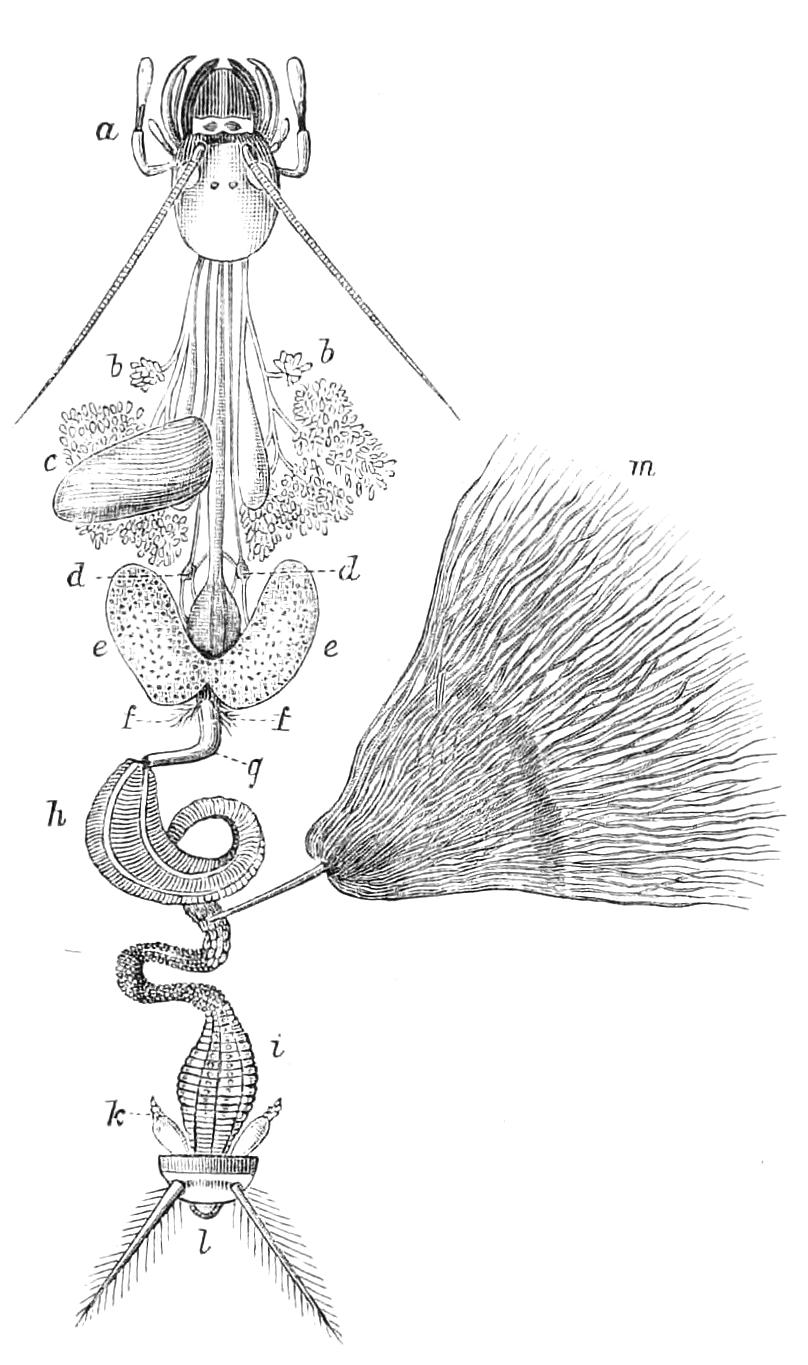
Fig. 207.—Alimentary canal and appendages of the mole-cricket: a, head; b, salivary glands and receptacle; c, lateral pouch; d, stomato-gastric nerves; e, anterior lobes of stomach; f, peculiar organ; g, neck of stomach; h, plicate portion of same; i, rectum; k, lobulate gland; l, extremity of body; m, Malpighian tubes. (After Dufour.)
The alimentary canal and digestive system of Gryllotalpa present peculiarities worthy of notice. Salivary glands and reservoirs are present; the oesophagus is elongate, and has on one side a peculiar large pouch (Fig. 207, c); beyond this is the gizzard, which is embraced by two lobes of the stomach. This latter organ is, beyond the lobes, continued backwards as a neck, which subsequently becomes larger and rugose-plicate. On the neck of the stomach there is a pair of branching organs, which Dufour considered to be peculiar to the mole-cricket, and compared to a spleen or pancreas. The single tube into which the Malpighian tubules open is seated near the commencement of the small intestine. These tubules are very fine, and are about one hundred in number. The arrangement by which the Malpighian tubules open into a common duct instead of into the intestine itself appears to be characteristic of the Gryllidae, but is said to occur also in Ephippigera, a genus of Locustidae. According to Leydig[262] and Schindler the Malpighian tubules are of two kinds, differing in colour, and, according to Leydig, in contents and histological structure. Near the posterior extremity of the rectum there is a lobulated gland having a reservoir connected with it; this is the chief source of the foetid secretion the mole-cricket emits when seized. The nervous chain consists of three thoracic and four abdominal ganglia; these latter do not extend to the extremity of the body; {336}the three anterior of the four ganglia are but small, the terminal one being much larger.
The number of eggs deposited by a female mole-cricket is large, varying, it is said, from 200 to 400. The mother watches over them carefully, and when they are hatched, which occurs in a period of from three to four weeks after their deposition, she supplies the young with food till their first moult; after this occurs they disperse, and begin to form burrows for themselves.
It has been said that the young are devoured by their parents, and some writers have gone so far as to say that 90 per cent of the progeny are thus disposed of. M. Decaux, who has paid considerable attention to the economy of the mole-cricket,[263] acquits the mother of such an offence, but admits that the male commits it. The number of eggs in one nest is said to be about 300.
The embryonic development of the mole-cricket has been studied by Dohrn[264] and Korotneff,[265] and is considered by the former to be of great interest. The tracheae connected with each stigma remain isolated, while, according to Korotneff, the development of the alimentary canal is not completed when the young mole-cricket is hatched. Perhaps it may be this condition of the digestive organs that necessitates the unusual care the mother bestows on her young.
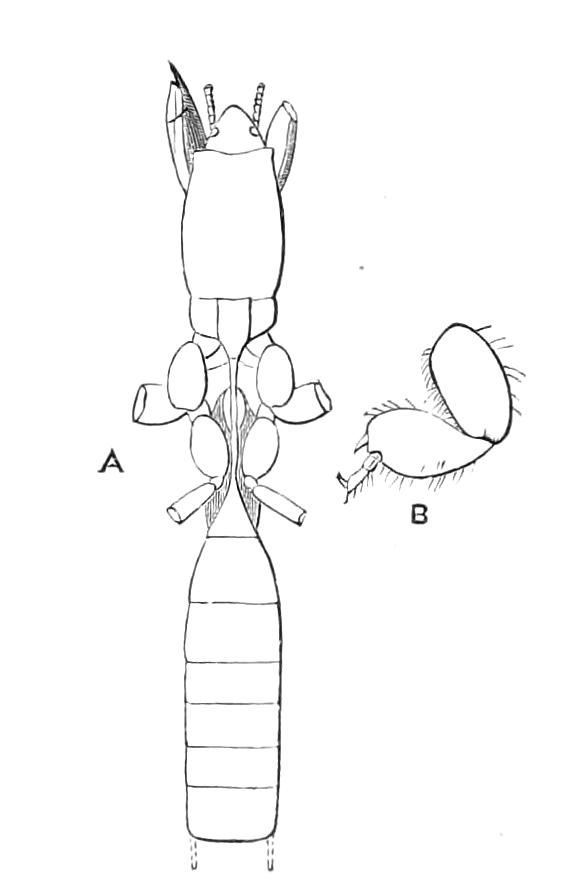
Fig. 208.—Cylindrodes kochi. Australia. A, outline of the Insect with five of the legs and the extremity of the body mutilated; B, middle leg. (After de Saussure.)
The genus Cylindrodes (Fig. 208, C. kochi) comprises some curious and rare Insects of elongate, slender form. They are natives of Australia, where the first species known of the genus was found in Melville Island by Major Campbell, from whom we learn that these Insects burrow in the stems of plants, and are so destructive that he was unable to keep a single plant in his greenhouse on account of the ravages of Cylindrodes campbellii. The form of these Insects is beautifully adapted to {337}their habits, the body being contracted in the middle in such a way as to permit the middle and hind legs to be packed against it, so that the cylindrical form is not interfered with by these appendages while the excavating anterior legs are at work in front of the Insect. The abdomen has nine segments; the terminal one, said to be remarkably long and destitute of cerci, is not shown in our figure.
The genus Tridactylus is considered by de Saussure to form, with its ally Rhipipteryx, a division of Gryllotalpinae, but they are treated, perhaps more correctly, by Brunner as a separate tribe. T. variegatus (Fig. 209) is a small Insect, abundant in sandy places on the banks of rivers in Southern Europe,—extending on the Rhone as far north as Geneva,—and is remarkable for its great power of leaping, and for the rapidity with which it can burrow in the sand. This anomalous Insect has only ten joints to the antennae. Its alar organs are imperfect, and not like those of other Gryllidae in either form or neuration. The hind legs are of peculiar structure, the tibiae terminating in two processes between which is situate a rudimentary tarsus. Near the extremity of the tibia there are some plates, forming two series, that can be adpressed to the tibia, or extended as shown in our figure. The body is terminated by four rather short, very mobile processes; the upper pair of these are each two-jointed, and are thought by de Saussure and Haase[266] to be cerci; the inferior pair, being articulated processes of the anal segment, their presence in addition to cerci is remarkable. It is difficult to distinguish the sexes of this Insect.
The exotic genus Rhipipteryx is allied to Tridactylus. It is widely distributed in South America, but the little Insects that {338}compose it are rare in collections, their saltatorial powers no doubt making it difficult to catch them; little is known as to their habits. In the undescribed Amazonian species we figure (Fig. 210), the wings, instead of being mere rudiments, as in Tridactylus, are elongate and project beyond the body; they are of a blue-black colour, and arranged so as to look as if they were the abdomen of the Insect; they, moreover, have a transverse pallid mark, giving rise to an appearance of division. It is difficult to form any surmise as to the nature of so curious a modification of the wings.
The Tridactylides have no tympana on the legs, and their affinity with the Gryllidae is very doubtful. Dufour thought T. variegatus to be more allied to the Acridiidae. He based this opinion chiefly on some points of the internal anatomy, but pointed out that Tridactylus differs from the Acridiidae in having no air-sacs in the body.
Not many of the Gryllidae are so peculiar as the forms we have mentioned. The family consists in larger part of Insects more or less similar to the common cricket, though exhibiting a great variety of external form. The common cricket of our houses, Gryllus (Acheta) domesticus (Fig. 204), has a very wide distribution in the Old World, and is also found in North America. It is believed to have had its natural distribution extended by commerce, though really nothing is known as to its original habitat. The shrill chirping of this little Insect is frequently heard at night in houses, even in the most densely inhabited parts of great cities. Neither the female nor the young are musical, yet the chirping may be heard at all seasons of the year, as young and adults coexist independent of season. The predilection of Gryllus domesticus for the habitations of man is very curious. The Insect is occasionally found out of doors in the neighbourhood of dwelling-houses in hot weather, but it does not appear that this species leads anywhere a truly wild life. It is fond of heat; though it rarely multiplies in dwelling-houses to any great extent, it is sometimes found in profusion in {339}bake-houses. Usually the wings in the cricket are elongate, and project backwards from under the tegmina like an additional pair of cerci; a variety, however, occurs in which these tails are absent, owing to abbreviation of the wings.
There is no beauty in the appearance of any of the Gryllidae, though many of them are very bizarre in shape. Very few of them venture to leave the surface of the earth to climb on plants. The species of Oecanthus, however, do so, and may be found sitting in flowers. They have a more Locustoid appearance than other Gryllidae. One of the most curious forms of the family is Platyblemmus, a genus of several species found in the Mediterranean region, the male of which has the head prolonged into a curious process (Fig. 211); this varies greatly in development in the males of the same species. It would seem that this organ is of a similar nature to the extraordinary structures we have figured in Locustidae (Fig. 189) and Mantidae (Fig. 136), though it appears impossible to treat the cephalic appendages of Platyblemmus as ornamental objects; their import is at present quite obscure.
A curious form of variation occurs in this family, and is called micropterism by de Saussure; we have already mentioned its occurrence in the house-cricket. The hind wings, which are usually ample, and frequently have their extremities rolled up and protruding like cerci, are sometimes much smaller in size, and not visible till the tegmina are expanded. De Saussure at one time supposed these micropterous individuals to be distinct species; it is now, however, known that intermediate examples can be found by examining a great many specimens. Some species are always micropterous.
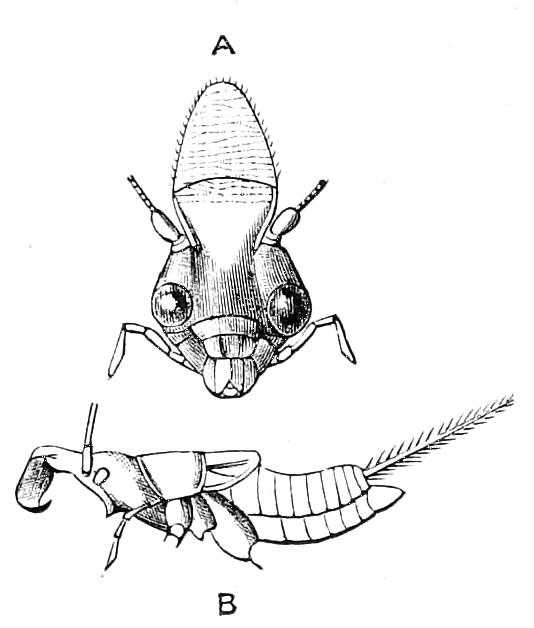
Fig. 211.—Platyblemmus lusitanicus, male. A, front of head; B, profile of Insect with most of the appendages removed.
In Britain we have only four representatives of the Gryllidae, viz. the mole-cricket, the house-cricket, and two field-crickets, one of which, Nemobius sylvestris, is considerably smaller than the house-cricket, while the other, Gryllus campestris, the true field-cricket, is a larger Insect. Its habits have been described in an interesting manner in Gilbert White's 88th letter. {340}This Insect, like so many others, is apparently becoming rare in this country.
A single fossil from the Lias has been described as belonging to the Gryllidae, but in the Tertiary strata a variety of members of the family have been discovered both in Europe and North America.
The classification of Gryllidae is due to de Saussure,[267] and is said by Brunner to be very natural. In the following synopsis of the tribes of crickets we give de Saussure's arrangement, except that we follow Brunner in treating Tridactylides as a distinct tribe:—
1. Antennae ten-jointed; posterior tarsi aborted. Tribe 1. Tridactylides. (Fig. 209, Tridactylus variegatus; Fig. 210, Rhipipteryx sp.)
1′. Antennae many jointed; posterior tarsi normal.
2. Tarsi compressed, the second joint minute.
3. Anterior legs fossorial; anterior tibiae at the apex with two to four divisions. Pronotum elongate, ovate, rounded behind. Female without ovipositor. Tribe 2. Gryllotalpides. (Fig. 206, front legs of Gryllotalpa; Fig. 208, Cylindrodes kochi.)
3′. Anterior legs formed for walking. Ovipositor of the female visible (either elongate or rudimentary).
4. Posterior tibiae biseriately serrate. Tribe 3. Myrmecophilides.
4′. Posterior tibiae biseriately spinose. Ovipositor straight.
5. Antennae short, thickish, almost thread-like. Facial scutellum exserted between antennae. Posterior tibiae dilated. Gen. Myrmecophila.[268]
5′. Antennae elongate, setaceous. Facial scutellum transverse, visible below the antennae. Tibiae slender.
6. Posterior tibiae armed with two strong spines, not serrate between the spines. Tribe 4. Gryllides. (Fig. 204, Gryllus domesticus; Fig. 211, Platyblemmus lusitanicus.)
6′. Posterior tibiae slender, armed with slender spines, and serrate between them. Tribe 5. Oecanthides.
2′. Second joint of the tarsi depressed, heart-shaped.
3. Posterior tibiae not serrate, but biseriately spinose.
4. The spines on each side three and mobile; apical spurs on the inner side only two in number. Ovipositor short, curved. Tribe 6. Trigonidiides.
4′. The spines numerous, fixed. Ovipositor elongate, straight. Gen. Stenogryllus.
3′. Posterior tibiae serrate and spinose on each side, the apical spurs, as usual, three on each side. Ovipositor straight or curved. Tribe 7. Eneopterides.
NEUROPTERA—MALLOPHAGA—EMBIIDAE
Order III. Neuroptera.
Imago with biting mouth; with two pairs of wings, the anterior as well as the posterior membranous, usually with extensive neuration, consisting of elongate nervures and either of short cross-nervules forming numerous cells or of a complex minute mesh-work. (One division, Mallophaga, consists entirely of wingless forms; in Termitidae some of the individuals of each generation become winged, but others do not: except in these cases adult wingless forms are few.) The metamorphosis differs in the several divisions.
The Neuroptera form a heterogeneous, though comparatively small, Order of Insects, including termites, stone-flies, dragon-flies, may-flies, caddis-flies, lace-wings, scorpion-flies, ant-lions, etc. Bird-lice are also included in Neuroptera, though they have no trace of wings.
We treat the Order as composed of eleven distinct families, {342}and, as a matter of convenience, arrange them in five divisions:—
1. Mallophaga.—Permanently wingless Insects, living on the bodies of birds or mammals. (Development very imperfectly known.) Fam. 1. Mallophaga.
2. Pseudoneuroptera.—Insects with wings in adult life (in some cases wings are never acquired). The wings are developed in a visible manner outside the body. There is no definite pupa. Live entirely on land. Fam. 2. Embiidae; 3. Termitidae; 4. Psocidae.
3. Neuroptera amphibiotica.—Wings developed as in division 2. Three ocelli usually exist. Life aquatic in the early stages. Fam. 5. Perlidae; 6. Odonata; 7. Ephemeridae.
4. Neuroptera planipennia.—Wings developed internally; not visible in early stages, but becoming suddenly evident when the pupal form is assumed. Mandibles present in the adult Insect. Life in early stages aquatic or terrestrial. Fam. 8. Sialidae; 9. Panorpidae; 10. Hemerobiidae.
5. Trichoptera.—Development as in division 4. Mandibles absent in the adult Insect. Life aquatic in the early stages. Fam. 11. Phryganeidae.
The families we have enumerated in the preceding scheme are now generally adopted by entomologists. Great difference of opinion exists, however, as to the groups of greater value than the family, and for a long time past various schemes have been in vogue. Though it is necessary to allude to the more important of these systems, we can do so only in the briefest manner.
Some of the families of Neuroptera are similar in many points of structure and development to Insects of other Orders; thus Termitidae are somewhat allied to Blattidae, Perlidae to Phasmidae in Orthoptera, while the Phryganeidae or Trichoptera make a considerable approach to Lepidoptera. Some naturalists—among whom we may mention Burmeister and Grassi—unite our Aptera, Orthoptera, and most of our Neuroptera into a single Order called Orthoptera. Others treat our Neuroptera as consisting of eight or nine distinct Orders; these, together with the names proposed for them, we have already alluded to in our chapter on classification, pp. 171-177.
Erichson, impressed by the variety existing in Neuroptera, separated some of the groups into a sub-Order called Pseudoneuroptera; this sub-Order comprised our Termitidae, Psocidae, Ephemeridae, and Libellulidae. This division is still adopted in several treatises; the Pseudoneuroptera are indeed by some naturalists retained as an Order distinct from both Orthoptera {343}and Neuroptera. Gerstaecker subsequently made use of a system somewhat different from that of Erichson, uniting the Perlidae, Ephemeridae, and Odonata into a group called Orthoptera amphibiotica, from which the Termitidae and Psocidae were excluded. The divisions we have here adopted differ but little from those of Gerstaecker, though we have arranged them in a very different manner. It is probable that not one-tenth part of the Neuroptera existing in the world have yet been examined by entomologists, and of those that are extant in collections, the life-histories and development are very imperfectly known. We have, therefore, not considered it wise to adopt a system that would involve great changes of nomenclature, while there can be little hope of its permanency.
Fossils.—When considering the subject of fossil Insects we briefly alluded to the discussions that have occurred as to whether the fossils of the palaeozoic period should be referred to existing Orders. Since the pages we allude to were printed, M. Brongniart's very important work[269] on the Insects of that epoch has appeared. He considers that these ancient fossils may be classified with the existing Orders of Insects, though they cannot be placed in existing families; and he assigns the palaeozoic fossil Insects at present known, to the Orders Neuroptera and Orthoptera, and to the homopterous division of Hemiptera. The greater part of the species he looks on as Neuroptera, and places in six families—Megasecopterides, Protephemerides, Platypterides, Stenodictyopterides, Protodonates, and Protoperlides. Of these he considers the ancient Protephemerides, Protodonates, and Protoperlides as the precursors, which, we presume, we may interpret as the actual ancestors, of our existing Ephemeridae, Odonata, and Perlidae.
Some of the fossils restored and described by the French entomologist are of great interest. We shall notice the Protephemerides, Protodonates, and Protoperlides in connexion with the families to which they are specially allied, and shall now only allude to the quite extinct families of Neuroptera, the Megasecopterides, Platypterides, and Stenodictyopterides.
It is a peculiarity of these ancient Insects that they were much larger creatures than the corresponding forms that now exist. This may be due, to some extent, to the fact that tiny, {344}fragile forms have not been preserved in the rocks, or have not attracted the attention of collectors; but as some of the palaeozoic Insects were absolutely the largest known—surpassing considerably in size any Insects at present existing—it is probable that, even if small forms existed at the remote epoch we are alluding to, the average size of the individual was greater than it is at present. The Megasecopterides of the carboniferous epoch were Insects of large size, with long, narrow wings, a small prothorax, and large meso- and meta-thorax, these two segments being equal in size; the abdomen was elongate and moderately voluminous, and was terminated by a pair of very elongate, slender filaments like those of the may-flies. The family includes several genera and species found at Commentry. One of these forms, Corydaloides scudderi, is of great interest, as it is believed by Brongniart that the imago possessed tracheal gills situated on the sides of the abdomen, analogous with those that exist at present in the immature condition of certain Ephemeridae. They are of interest in connexion with the gills found at the present time in the imagos of Pteronarcys (see p. 401). Although these fossils are of such enormous antiquity, the tracheae can, M. Brongniart says, be still perceived in these processes.
The Platypterides include also a considerable number of Insects of large size, with four large equal wings, frequently spotted or variegate. Some of these Insects were provided with expansions or lobes on the sides of the prothorax (Fig. 213); these are looked on as analogous to the expansions of meso- and meta-thorax, which are supposed by some writers to have been the rudiments from which wings were developed. These prothoracic wing-rudiments, if such they be, are said to have a system of nervures similar to what we find in true wings. The genus Lithomantis includes a Scotch fossil, and has already been mentioned by us on p. 259.
The third family of extinct carboniferous Neuroptera is the Stenodictyopterides, in which Brongniart places the Dictyoneura of {345}Goldenberg, the North American Haplophlebium, and several genera from Commentry. Some of them were very large Insects, with robust bodies, and possessed wing-like expansions on the prothorax, and lateral gill-like appendages on the sides of the abdomen.
It is worthy of note that though so large a number of carboniferous Neuroptera have now been discovered, no larvae or immature forms have been found.
We now pass to the consideration of the divisions of Neuroptera still living.
Fam. I. Mallophaga—Bird-Lice or Biting Lice.
Small Insects, wingless, with large head; thorax usually of two, rarely of one or three segments; prothorax always distinct; hind body consisting of eight to ten segments, in addition to the posterior two thoracic segments which usually are but little or not at all separated from it. The metamorphosis is very slight. The creatures live on the skins of birds or mammals, finding nourishment in the epidermal products.
The whole of the Insects of this family live a parasitic, or rather epizoic, life. They all creep about those parts that are near to the skin, the feathers of birds or the hair of mammals; they rarely come quite to the surface, so that they are not detected on a superficial examination. It is curious that under these circumstances they should exhibit so great a variety of form and of anatomical characters as they do.
They are very depressed, that is, flat, Insects, with a large head, which exhibits a great variety of shape; frequently it is provided in front of the antennae with some peculiar tubercles called trabeculae, which in some cases are mobile. The antennae are never large, frequently very small; they consist of from three to five joints, and are sometimes concealed in a cavity on the under side of the head.
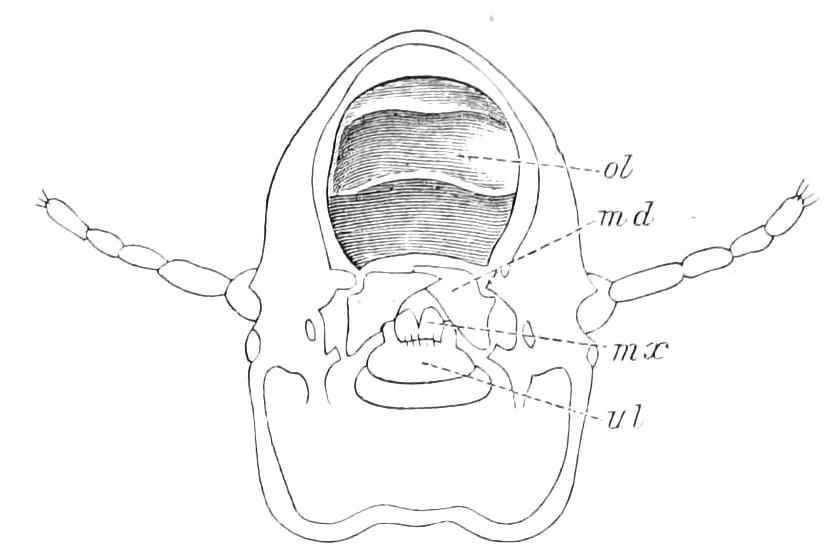
Fig. 215.—Under-surface of head of Lipeurus heterographus. (After Grosse.) ol, Labium; md, mandible; mx, maxilla; ul, labium.
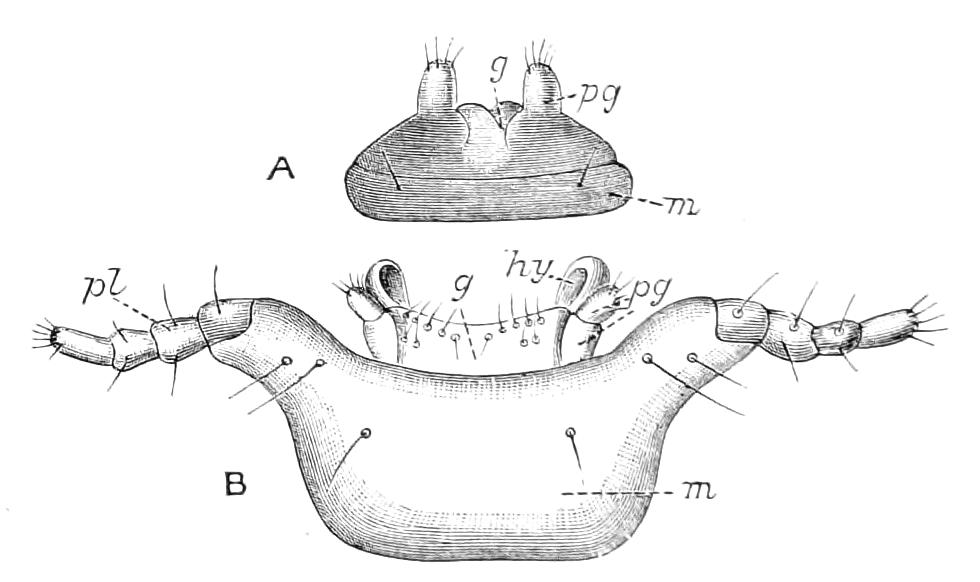
Fig. 216.—Under lip of Nirmus, A; and of Tetrophthalmus chilensis, B. (After Grosse.) m, Mentum; g, ligula; pl, palp; pg, paraglossa; hy, lingua.
The eyes are very rudimentary, and consist of only a small number of isolated facets placed behind the antennae; sometimes they are completely absent. The mouth parts are situated entirely on the under-surface of the head and in a cavity. The upper lip is frequently of remarkable form, as if it were a scraping instrument (ol, Fig. 215). The mandibles are sharply toothed and apparently act as cutting instruments. The maxillae have been described in the principal work on the family[270] as possessing in some cases well-developed palpi. According to Grosse[271] this is erroneous; the maxillae, he says, are always destitute of palpi, and are of peculiar form, being each merely a lobe of somewhat conical shape, furnished on one aspect with hooks or setae. The under lip is peculiar, and apparently of very different form in the two chief groups of Mallophaga. The large mentum bears, in Liotheides (Fig. 216, B), on each side a four-jointed palpus, the pair of palps being very widely separated; the ligula is broad and undivided; on each side there is a paraglossa bearing an oval process, and above this is a projection of the hypopharynx. In Philopterides (Fig. 216, A) the palpi are absent, and the parts of the lower lip are—with the exception of the paraglossae—but little differentiated. The lingua (hypo-pharynx) in Mallophaga is largely developed, {347}and bears near the front a chitinous sclerite corresponding with another placed in the epipharynx.
The prothorax in Mallophaga is a distinct division of the body even when the meso- and meta-thorax appear to be part of the abdomen. The mesothorax is frequently very small; it and the metathorax are sometimes intimately connected. In other cases (Laemobothrium) the metathorax appears to differ from the following abdominal segment only by having the third pair of legs attached to it. In Trinoton (Fig. 214) the three thoracic segments are well developed and distinct. The abdominal segments visible, vary in number from eight to ten; there is sometimes a difference according to sex, the male having one segment taken into the interior in connexion with the reproductive organs. The legs have short, broad coxae and small tarsi of one or two joints; very rarely three joints are present; there are either one or two claws; the legs with one claw being adapted for clinging to or clutching hairs. The front pair of legs is used not for locomotion so much as for grasping the food and bringing it within the range of the mouth. No trace of wings has been detected in any species.
The nervous system has been examined by Giebel in Lipeurus bacillus; there is a supra- and an infra-oesophageal ganglion, and three thoracic, but no abdominal ganglia. The supra-oesophageal is remarkably small, in fact not larger than the infra-oesophageal; it consists evidently of two conjoined halves. The alimentary canal has a slender, elongate oesophagus, dilated behind into a crop; this is frequently received between two cornua formed by the anterior part of the stomach, which, except for these, is simply tubular in form, though somewhat narrower at the posterior extremity. In some forms—Philopterides—the crop is of a very peculiar nature (Fig. 218), forming an abrupt paunch separated from the stomach by the {348}posterior portion of the oesophagus. There are only four Malpighian tubes; in some species the basal half of each tube is much dilated. The two divisions of the intestine are short and are separated by the intervention of a glandular girdle. Salivary glands exist; Giebel figures what we may consider to be an enormous salivary reservoir as existing in Menopon leucostomum.
The testes and ovaries are of a simple nature. The former consist of two or three capsules, each having a terminal thread; the vasa deferentia are tortuous and of variable length; they lead into the anterior part of the ejaculatory duct, where also opens the elongate duct proceeding from the bicapsular vesicula seminalis; these structures have been figured by Grosse[272] as well as by Giebel. The ovaries consist of three to five short egg-tubes on each side; the two oviducts combine to form a short common duct with which there is connected a receptaculum seminis.
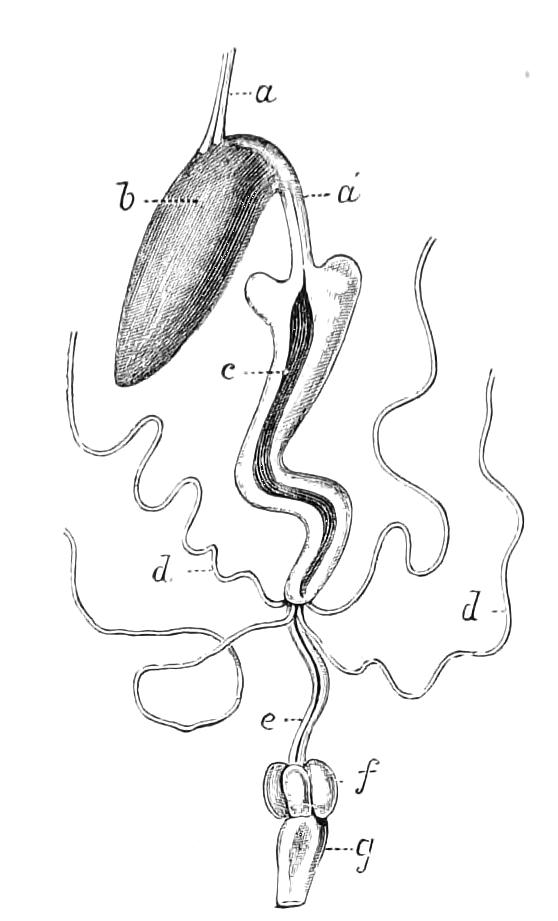
Fig. 218.—Alimentary canal of Docophorus fuscicollis. (After Giebel.) a, Oesophagus; b, paunch; a′, posterior division of oesophagus; c, chylific ventricle or stomach; d, Malpighian tubes; e, small intestine; f, glandular girdle; g, rectum.
The eggs of some Mallophaga have been figured by Melnikow;[273] they possess at one extremity a cover with a multiple micropyle-apparatus, and at the opposite pole are provided with seta-like appendages. They are very like the eggs of the true lice, and are said in some cases to be suspended by threads to the hairs or feathers after the fashion of the eggs of Pediculi.
Little is known as to the development; the young are extremely like the adult, and are thought to moult frequently; the duration of life is quite unknown.
It has been stated by some writers that the mouth is truly of the sucking kind, and that the Mallophaga feed on the blood of their hosts. This is, however, erroneous; they eat the delicate portions of the feathers of birds, and of mammals perhaps the young hair. Their fertility is but small, and it is believed that {349}in a state of nature they are very rarely an annoyance to their hosts. The majority of the known species live on birds; the forms that frequent mammals are less varied and have been less studied; most of them have only one claw to the feet (Fig. 220), while the greater portion of the avicolous species have two claws.
Most of the forms have the anterior legs small, and they are usually drawn towards the mouth, owing, it is believed, to their being used after the manner of hands to bring the food to the mouth; hence in some of our figures (219, 220) the body looks as if it had only four legs.
Very diverse statements have been made as to whether allied forms of Mallophaga are found only on allied birds. It would appear that this is the case only to a limited extent, as certain species are found on quite a variety of birds; moreover, some birds harbour several species of bird-lice, even five genera having been found, it is said, on one species of bird. Docophorus icterodes has been recorded as occurring on many kinds of ducks and geese; the swan, however, harbours a distinct species, Docophorus cygni, and this is said to have also been found on the bean-goose.
At least five species, belonging to three distinct genera, have been found on the common fowl. The parasite most frequently met with on this valuable creature is Menopon pallidum (Fig. {350}221), which is said to have been figured by Redi two hundred years ago under the name of Pulex capi. This species multiplies to a considerable extent; it is of very active habits, and passes readily from one bird to another, so that it is found on other species besides the domestic fowl. It is even said that horses kept near hen-roosts have been seriously troubled by Menopon pallidum, but it is suggested by Osborn that these attacks may perhaps have been really due to itch-mites. There is, however, no doubt that this species may infest poultry, especially if sickly, to an enormous extent. The dust-baths in which poultry are so fond of indulging are considered to be of great use in keeping down the numbers of this Insect.
A table of the birds and mammals on which Mallophaga have been found, together with the names of the latter, has been given by Giebel.[274] The classification of the group, so far as the principal divisions are concerned, by no means accords with the kind of animals that serve as hosts, for the only two genera peculiar to quadrupeds (Trichodectes, Fig. 220; and Gyropus) belong to the two chief divisions of Mallophaga. The genus Menopon includes numerous species found on birds, and three or four others peculiar to mammals.
Two very natural divisions, Philopterides and Liotheides, were adopted by Giebel and Nitzsch, but unfortunately the chief character they made use of for diagnosing the two groups—the presence or absence of maxillary palpi—was illusory. Apparently the labial palps will serve the purpose of distinguishing the two divisions, they being present in the Liotheides and absent in the Philopterides. A table of the characters of the avicolous genera of these two groups is given by Grosse.[275]
The Liotheides are more active Insects, and leave their host after its death to seek another. But the Philopterides do not do so, and die in about three days after the death of their host. Possibly Mallophaga may be transferred from one bird to another {351}by means of the parasitic two-winged flies that infest birds. The writer has recorded[276] a case in which a specimen of one of these bird-flies captured on the wing was found to have some Mallophaga attached to it.
We should perhaps point out that these Mallophaga, though called bird-lice, have nothing to do with the true lice which are so frequently found with them, and that live by sucking the blood of their hosts. It would in fact be better to drop the name of bird-lice altogether, and call the Mallophaga biting lice. Trichodectes latus, according to this method, would be known as the biting louse of the dog, the true or sucking louse of which animal is Haematopinus piliferus, and belongs to the anoplurous division of Hemiptera.
Fam. II. Embiidae.
Elongate feeble Insects; with small prothorax, elongate meso- and meta-thorax, which may either bear wing or be without them. In the former case these organs are not caducous, are delicately membranous, and all of one consistence, with three or four indefinite longitudinal nervures and a few cross-veinlets. The development is incompletely known. The individuals do not form organised societies.
The Embiidae are one of the smallest families of Insects; not more than twenty species are known from all parts of the world, and it is probable that only a few hundred actually exist. They are small and feeble Insects of unattractive appearance, and shrivel so much after death as to render it difficult to ascertain their characters. They require a warm climate. Hence {352}it is not a matter for surprise that little should be known about them.
The simple antennae are formed of numerous joints, probably varying in number from about fifteen to twenty-four. The mouth is mandibulate. Chatin states[277] that the pieces homologous with those of a maxilla can be detected in the mandible of Embia. The labium is divided. The legs are inserted at the sides of the body, the coxae are widely separated (Fig. 223), the hind pair being, however, more approximate than the others. The abdomen is simple and cylindrical, consisting of ten segments, the last of which bears a pair of biarticulate cerci. In the male sex there is a slight asymmetry of these cerci and of the terminal segment. The thorax is remarkable on account of the equal development of the meso- and meta-thorax and their elongation in comparison to the prothorax. When they bear wings there is no modification or combination of the segments for the purposes of flight, the condition of these parts being, even then, that of wingless Insects; so that the Embiidae that have wings may be described as apterous-like Insects provided with two pairs of inefficient wings. The wings are inserted on a small space at the front part of each of the segments to which they are attached. The legs have three-jointed tarsi, and are destitute of a terminal appendage between the claws.
Fig. 224.—Anterior wing of Oligotoma saundersii: A, the wing; B, outline of the wing, showing nervures. (After Wood-Mason.) 1, Costal; 2, subcostal; 3, radial; 4, discoidal; 5, anal nervure.
The wings in Embiidae are very peculiar; they are extremely {353}flimsy, and the nervures are ill-developed; stripes of a darker brownish colour alternate with pallid spaces. We figure the anterior wing of Oligotoma saundersii, after Wood-Mason; but should remark that the neuration is really less definite than is shown in these figures; the lower one represents Wood-Mason's interpretation of the nervures. He considers[278] that the brown bands "mark the original courses of veins which have long since disappeared." A similar view is taken by Redtenbacher,[279] but at present it rests on no positive evidence.
One of the most curious features of the external structure is the complex condition of the thoracic sternal sclerites. These are shown in Fig. 223, representing the under-surface of an Embia of uncertain species recently brought by Mr. Bateson from Andalusia.
According to Grassi[280] there are ten pairs of stigmata, two thoracic and eight abdominal; these are connected by longitudinal and transverse tracheae into a single system. The ganglia of the ventral chain are, one suboesophageal, three thoracic, and seven abdominal; these are segmentally placed, except that there is no ganglion in the fifth abdominal segment. There is a stomato-gastric system but no "sympathetic." Salivary glands are present. The stomodaeal portions of the alimentary canal are remarkably capacious; the stomach is elongate and slender, without diverticula; the Malpighian tubes are elongate and slender; they vary in number with the age of the individual, attaining that of twenty in the adult. The ovaries are arranged somewhat after the fashion of those of Japyx, there being in each five short egg-tubes, opening at equal intervals into a straight duct. The testes are remarkably large; each one consists of five masses of lobules, and has a large vesicula seminalis, into the posterior part of which there open the ducts of two accessory glands. The large joint of the front tarsus includes glands whose secretion escapes by orifices at the tips of certain setae interspersed between the short spines that are placed on the sole.
Species of this genus occur in the Mediterranean region, but their characters have not yet been examined. Our information {354}as to these is chiefly to be found in Grassi's work. The two species studied by him were wingless. They live under stones, where they spin webs by means of the front feet, whose first joint is, as we have said, enlarged and contains glands; the Insect uses the webs as a means of support in progression, acting on them by means of papillae and a comb-like structure placed on the four posterior feet.
Grassi informs us that these Insects are not uncommon under stones in Catania; they require moisture as well as warmth, but not too much; sometimes there is only one individual found under a stone, at others eight or ten. In the winter and spring their galleries are found on the surface of the earth, but in the hot months of summer they secure the requisite amount of moisture by sinking their galleries to the depth of ten or fifteen centimetres. Their food consists chiefly of vegetable matter. They may be reared with ease in glass vessels. Other species of the family attain wings; the details of the process are not well known. Oligotoma michaeli (Fig. 222) was discovered in a hothouse in London among some orchid roots brought from India, and was found in more than one stage of development; the young greatly resemble the adult, except in the absence of wings. A nymph-form is described by M‘Lachlan[281] as possessing wings of intermediate length, and Hagen has suggested that this supposed nymph is really an adult female with short wings. If this latter view be correct, nothing is known as to the mode of development of wings in the family. It is still uncertain whether female Embiidae ever possess wings. Wood-Mason and Grassi have shown that there are wingless females in some species, and we know that there are winged males in others, but what the usual relation of the sexes may be in this respect is quite uncertain. These Insects have been detected in various parts of the world. In the Sandwich Islands Oligotoma insularis was discovered by the Rev. T. Blackburn in the wood and thatch forming the roofs of natives' houses. A species has been found in Prussian amber, and Grassi thinks that Embia solieri—one of the Mediterranean species—is not to be distinguished with certainty from the Insect found in amber.
Embiidae still remains one of the most enigmatic of the families of Insects. Although Grassi's recent observations are {355}of great value from an anatomical point of view, they rather add to, than diminish, the difficulties we encounter in endeavouring to understand the lives of these obscure creatures. That Embiidae form webs has long been known, and it was thought by some that the webs, like those of spiders, might be of assistance in procuring food. We may, however, infer from Grassi's observations that this is not the case, but that the silken tunnels or galleries—as he calls them—serve chiefly as a means of locomotion and protection, the feet of the Insects being highly modified in conformity with this mode of life. Grassi seems to be of opinion that the galleries are also useful in preserving a proper degree of humidity round the Insects. We have already alluded to the mystery that surrounds the mode of growth of their wings. Nearly all that is known as to the Embiidae is contained in Grassi's paper, or is referred to in Hagen's monograph of the family.[282]
Considerable difference of opinion has prevailed as to the allies of these obscure Insects. It would seem that they are most nearly allied to Termitidae and Psocidae. Grassi, however, considers these affinities only remote, and suggests that Embiidae should form a separate Order, to be placed in a super-Order Orthoptera, which would include our Aptera, the two families mentioned above, Mallophaga, Embiidae, and the ordinary Orthoptera. Brauer places the family in his Orthoptera genuina.
NEUROPTERA CONTINUED—TERMITIDAE, TERMITES OR WHITE ANTS
Fam. III. Termitidae—White Ants, Termites.
Each species is social, and consists of winged and wingless individuals. The four wings are, in repose, laid flat on the back, so that the upper one only is seen except just at the bases; they are membranous and very elongate, so that they extend far beyond the apex of the abdomen; the hind pair is remarkably similar in size, form, and consistence to the front pair: near the base of each wing there is a suture, or line of weakness, along which the wings can be broken off, the stumps in that case remaining as short horny flaps reposing on the back. Ligula channelled but not divided into two parts. The wingless individuals are very numerous, and have the head and thirteen body segments distinct; the body {357}is terminated by a pair of short cerci. The metamorphosis is slight and gradual, and in some individuals is dispensed with.
The term White Ants has been so long in use for the Termitidae that it appears almost hopeless to replace it in popular use by another word. It has, however, always given rise to a great deal of confusion by leading people to suppose that white ants differ chiefly from ordinary ants by their colour. This is a most erroneous idea. There are scarcely any two divisions of Insects more different than the white ants and the ordinary ants. The two groups have little in common except that both have a social life, and that a very interesting analogy exists between the forms of the workers and soldiers of these two dissimilar Orders of Insects, giving rise to numerous analogies of habits. The word Termites—pronounced as two syllables—is a less objectionable name for these Insects than white ants.
The integument in Termites is delicate, and the chitinous plates are never very hard; frequently they are so slightly developed that the creature appears to consist of a single membranous sac with creases in it, the head alone being very distinct. The head is exserted, frequently of large size, sometimes as large as all the rest of the body together. Termites may be quite blind, or possess facetted and simple eyes, the latter when present being two in number and always accompanied by facetted eyes. The antennae are simple, consisting of from nine to thirty-one joints, which differ but little from one another; the number in each individual increases as the development progresses. The parts of the mouth are large, the ligula consists of one piece (Fig. 226, A), but often has the appearance of being formed by two united pieces; on its extremity are seated two pairs of lobes.
Fig. 226.—Termes bellicosus. Labium, A, maxilla, B, of winged adult; lower face of each. (After Hagen.)
The head is articulated to the thorax by means of two very large cervical sclerites on each side, placed at right angles to one another, and visible on the under-surface. The prothorax is well developed and distinct from the parts behind it. The {358}pronotum, of variable form and size, is very distinct in the perfect Insects; with it are connected the largely developed pleura. The episternum is very peculiar, consisting of an elongate chitinous slip on each side hanging downwards, the two not quite meeting in the middle; they thus form the margin of the very large anterior orifice, and are in contiguity with the cervical sclerites; behind them are the very large epimera. The prosternum appears to be usually entirely membranous; in some cases the sclerite in it is small and delicate, and apparently differs according to the species. The meso- and meta-thorax are sub-equal in size; the mesosternum forms a peculiar, large, adpressed fold. The metasternum is membranous, but is terminated behind by a sclerite apparently of variable form. The hind body is voluminous, simple in form, consisting of ten segments and bearing at the extremity two short distant cerci of a variable number of joints. The terminal ventral sclerites differ greatly in form according to the species and sometimes according to the sex; there are sometimes, if not always, present near the extremity two peculiar minute biarticulate styles, called appendices anales. The coxae are all large, free, and exserted; at the base of each is a transverse trochantin. The femora are articulated with the trochanters, not with the coxae; both femora and tibiae are slender, the tarsi small, four-jointed; the terminal joint elongate.
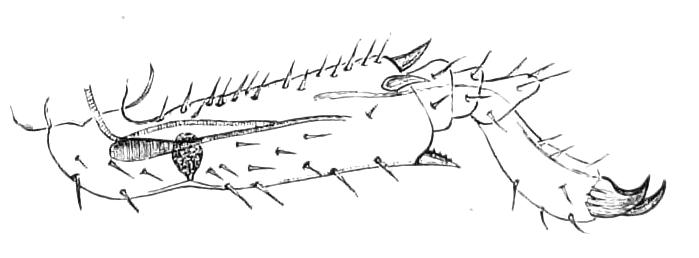
Fig. 227.—Front tibia and tarsus of Calotermes rugosus larva, showing auditory organ. × 90. (After F. Müller.)
It is now well established that Termites have a means of communication by sounds. The individuals have a peculiar way of jerking themselves, as has been frequently noticed by observers of the Insects; these convulsive movements may possibly be connected with the production of sound, which may perhaps be evoked by contact between the back of the head and the pronotum; the exact mode by which the sounds are produced is not, however, known. The existence of an auditory organ in the front tibia has been demonstrated by Fritz Müller,[283] and we reproduce (Fig. 227) one of his figures. The structure seems to {359}be in plan and position similar to the ear of Locustidae, though much less perfect.
Fig. 228.—Wings of Termites: A, Termes lucifugus; B, Hodotermes brunneicornis; C, Culotermes nodulosus. (After Redtenbacher: B and C diagrammatic.) III, V, VII, homologous areas and nervures according to Redtenbacher. 1, Costal; 2, subcostal; 3, median; 4, submedian nervures according to Hagen.
The wings of Termitidae are not like those of any other Insects; their neuration is very simple, but nevertheless the wings of the different forms exhibit great differences in the extent to which they are made up of the various fields. This is shown in Fig. 228, where the homologous nervures are numbered according to the systems of both Hagen and Redtenbacher. The area, VII, that forms the larger part of the wing in C, corresponds to the small portion at the base of the wing in B. The most remarkable feature of the wing is, however, its division into two parts by a suture or line of weakness near the base, as shown in Fig. 225. The wings are used only for a single flight, and are then shed by detachment at this suture; the small basal portion of each of the four wings is horny and remains attached to the Insect, serving as a protection to the dorsal surface of the thorax.
The nature of the suture that enables the Termites to cast their wings with such ease after swarming is not yet understood. There are no true transverse veinlets or nervules in Termites. Redtenbacher suggests[284] that the transverse division of the wing at its base, as shown in Fig. 225, along which the separation of the wing occurs at its falling off, may have arisen from a coalescence of the subcostal vein with the eighth concave vein of such a wing as that of Blattidae. The same authority also informs us that the only point of resemblance between the wings of Termitidae and those of Psocidae is that both have an unusually small number of concave veins.
The information that exists as to the internal anatomy of {360}Termites is imperfect, and refers, moreover, to different species; it would appear that considerable diversity exists in many respects, but on this point it would be premature to generalise. What we know as to the respiratory system is chiefly due to F. Müller.[285] The number of spiracles is ten; Hagen says three thoracic and seven abdominal, Müller two thoracic and eight abdominal. In fertile queens there usually exist only six abdominal stigmata. There is good reason for supposing that the respiratory system undergoes much change correlative with the development of the individual; it has been suggested that the supply of tracheae to the sexual organs is deficient where there is arrest of development of the latter.
The alimentary canal is only of moderate length. Salivary glands exist, as also do salivary reservoirs; these latter are large, in some species remarkably so. The oesophagus is slender, but abruptly enlarged behind to form a large crop; a proventriculus is apparently either present or absent; the chylific ventricle, or stomach, is slender and simple. The Malpighian tubules are very long; their number is probably from four to eight in the adult, and in the earlier stages less. Behind the tubes the alimentary canal forms a large paunch, and after this there is a small intestine and rectum. The paunch is a peculiar structure, and probably of great importance in the economy of Termites.
These creatures emit minute quantities of a secretion that is corrosive, and can act on metal and even glass;[286] its nature and source are not understood. Hagen describes peculiar structures in the rectum to which he is inclined[287] to ascribe the origin of this substance, but this is very uncertain.
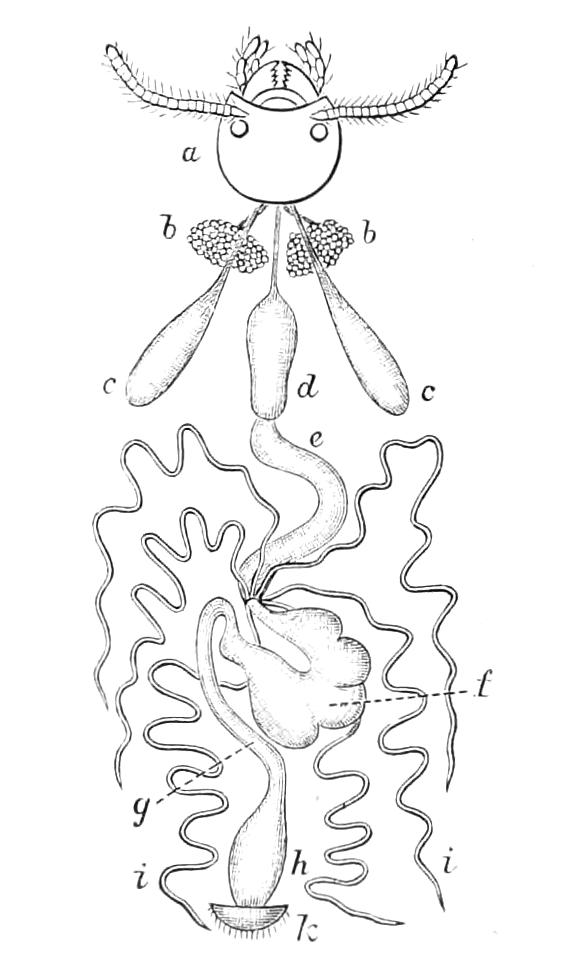
Fig. 229.—Head and alimentary canal of Termes lucifugus (nymph). a, head; b, salivary glands; c, salivary receptacles; d, crop; e, stomach; f, intestinal paunch; g, small, h, large intestine; i, Malpighian tubes; k, extremity of body. (After Dufour.)
The brain is small; the infra-oesophageal ganglion is placed {361}immediately under the supra-oesophageal; there are three thoracic and six abdominal ganglia. The nervous system apparently differs but little in the various forms, or in the different stages of life, except that in the fertile females the abdominal ganglia become so much enlarged that they even exceed the brain in size.
The testes are unusually simple; each consists of eight capsules opening into the vas deferens; the two vasa converge and are continued as a short ejaculatory duct; at the point of convergence there is a pair of curled vesiculae seminales.
The ovarian system is also simple; there is a variable number of elongate egg-tubes, each of which opens separately into the oviduct; the two ducts unite to form a short uterus, on which there is placed first a spermatheca, and near the extremity a convolute tubular sebific gland. The number of egg-tubes is subject to extraordinary variation, according to the species, and according to the age of the fertilised individual.
Social Life.—Termites live in communities that consist sometimes of enormous numbers of individuals. The adult forms found in a community are (1) workers; (2) soldiers; (3) winged males and females; (4) some of these winged forms that have lost their wings. Some species have no worker caste. The individuals of the third category are only present for a few days and then leave the nest in swarms. In addition to the adult individuals there are also present various forms of young. The individuals that have lost their wings are usually limited to a single pair, king and queen; there may be more than one king and queen, but this is not usual. The king and queen may be recognised by the stumps of their cast wings, which exist in the form of small triangular pieces folded on the back of the thorax (Fig. 235). The continuance of the community is effected entirely by the royal pair; they are the centres of activity of the community, which is thrown into disorder when anything happens to them. Usually the pair are physically incapable of leaving the nest, especially the queen, and frequently they are enclosed in a cell which they cannot leave. In consequence of the disorganisation that arises in the community in the absence of a royal pair, Termites keep certain individuals in such a state of advancement that they can rapidly be developed into royalties should occasion require it. These reserve individuals are called complementary by Grassi; when {362}they become royalties they are usually immature as regards the condition of the anterior parts of the body, and are then called by Grassi and others neoteinic, as is more fully explained on p. 380.
Swarms.—As a result of the Termite economy large numbers of superfluous individuals are frequently produced; these, in the winged state, leave the community, forming swarms which are sometimes of enormous extent, and are eagerly preyed on by a variety of animals including even man. Hagen has given particulars[288] of a swarm of T. flavipes in Massachusetts, where the Insects formed a dark cloud; they were accompanied by no less than fifteen species of birds, some of which so gorged themselves that they could not close their beaks.
There is but little metamorphosis in Termitidae. Young Termites are very soft; they have a thin skin, a disproportionately large head, and are of a peculiar white colour as if filled with milk. This condition of milkiness they retain, notwithstanding the changes of form that may occur during their growth, until they are adult. The wings first appear in the form of prolongations of the meso- and meta-nota, which increase in size, the increment probably taking place at the moults. The number of joints of the antennae increases during the development; it is effected by growth of the third joint and subsequent division thereof; hence the joints immediately beyond the second are younger than the others, and are usually shorter and altogether more imperfect. The life-histories of Termites have been by no means completely followed; a fact we can well understand when we recollect that these creatures live in communities concealed from observation, and that an isolated individual cannot thrive; besides this the growth is, for Insects, unusually slow.
Natural History.—The progress of knowledge as to Termites has shown that profound differences exist in the economy of different species, so that no fair general idea of their lives can be gathered from one species. We will therefore briefly sketch the economy, so far as it has been ascertained, in three species, viz. Calotermes flavicollis, Termes lucifugus, and T. bellicosus.
Fig. 230.—Some individuals of Calotermes flavicollis: A, nymph with partially grown wing-pads; B, adult soldier; C, adult winged individual. (After Grassi.)
Calotermes flavicollis inhabits the neighbourhood of the Mediterranean Sea; it is a representative of a large series of species in which the peculiarities of Termite life are exhibited in a comparatively simple manner. There is no special caste of workers, consequently such work as is done is carried on by the other members of the community, viz. soldiers, and the young and adolescent. The habits of this species have recently been studied in detail in Sicily by Grassi and Sandias.[289] The Insects dwell in the branches and stems of decaying or even dead trees, where they nourish themselves on those parts of the wood in which the process of decay is not far advanced; they live in the interior of the stems, so that frequently no sign of them can be seen outside, even though they may be heard at work by applying the ear to a branch. They form no special habitation, the interior of the branch being sufficient protection, but they excavate or increase the natural cavities to suit their purposes. It is said that they line the galleries with proctodaeal cement; this is doubtful, but they form barricades and partitions where necessary, by cementing together the proctodaeal products with matter from the salivary glands or regurgitated from the anterior parts of the alimentary canal. The numbers of a community only increase slowly and remain always small; rarely do they reach 1000, and usually remain very much below this. The king and queen move about, and their family increases but slowly. After fifteen months of their union they may be surrounded by fifteen or twenty young; in another twelve months the number may have increased to fifty, and by the time it has reached some five hundred or upwards the increase ceases. This is due to the fact that the fertility of the queen is at first progressive, but ceases to be so. A queen three or four years old produces at the time of maximum production four to six eggs a day. When the community is small—during its first two years—the winged individuals that depart from it are about eight or ten annually, but the numbers of the swarm augment with the increase of the {364}population. The growth of the individuals is slow; it appears that more than a year elapses between the hatching of the egg and the development of the winged Insect. The soldier may complete its development in less than a year; the duration of its life is not known; that of the kings and queens must be four or five years, probably more. After the winged Insects leave the colony they associate themselves in pairs, each of which should, if all goes well, start a new colony.
The economy of Termes lucifugus, the only European Termite besides Calotermes flavicollis, has been studied by several observers, the most important of whom are Lespès[290] and Grassi and Sandias. This species is much more advanced in social life than Calotermes is, and possesses both workers and soldiers (Fig. 231, 2, 3); the individuals are much smaller than those of Calotermes. Burrows are made in wood of various kinds, furniture being sometimes attacked. Besides making excavations this species builds galleries, so that it can move from one object to another without being exposed; it being a rule—subject to certain exceptions—that Termites will not expose themselves in the outer air. This is probably due not only to the necessity for protection against enemies, but also to the fact that they cannot bear a dry atmosphere; if exposed to a drying air they speedily succumb. Occasionally specimens may be seen at large; Grassi considers these to be merely explorers. Owing to the extent of the colonies it is difficult to estimate with accuracy the number of individuals composing a community, but it is doubtless a great many thousands. Grassi finds the economy of this species in Sicily to be different from anything that has been recorded as occurring in other species; there is never a true royal pair. He says that during a period of six years he has examined thousands of nests without ever finding such a pair. In place thereof there are a considerable number of complementary queens—that is, females that have not gone through the full development to perfect Insects, but have been arrested in various stages of development. In Fig. 231, Nos. 4 and 5 show two of these abnormal royalties; No. 4 is comparatively juvenile in form, while No. 5 is an individual that has been substituted in an orphaned nest, and is nearer to the natural condition of perfect development. We have no information as to whether any {365}development goes on in these individuals after the state of royalty is assumed, or whether the differences between these neoteinic queens are due to the state of development they may happen to be in when adopted as royalties. Kings are not usually present in these Sicilian nests; twice only has Grassi found a king, but he thinks that had he been able to search in the months of August and September he would then have found kings. It would appear therefore that the complementary kings die, or are killed after they have fertilised the females. Parthenogenesis is not thought to occur, as Grassi has found the spermathecae of the complementary queens to contain spermatozoa.
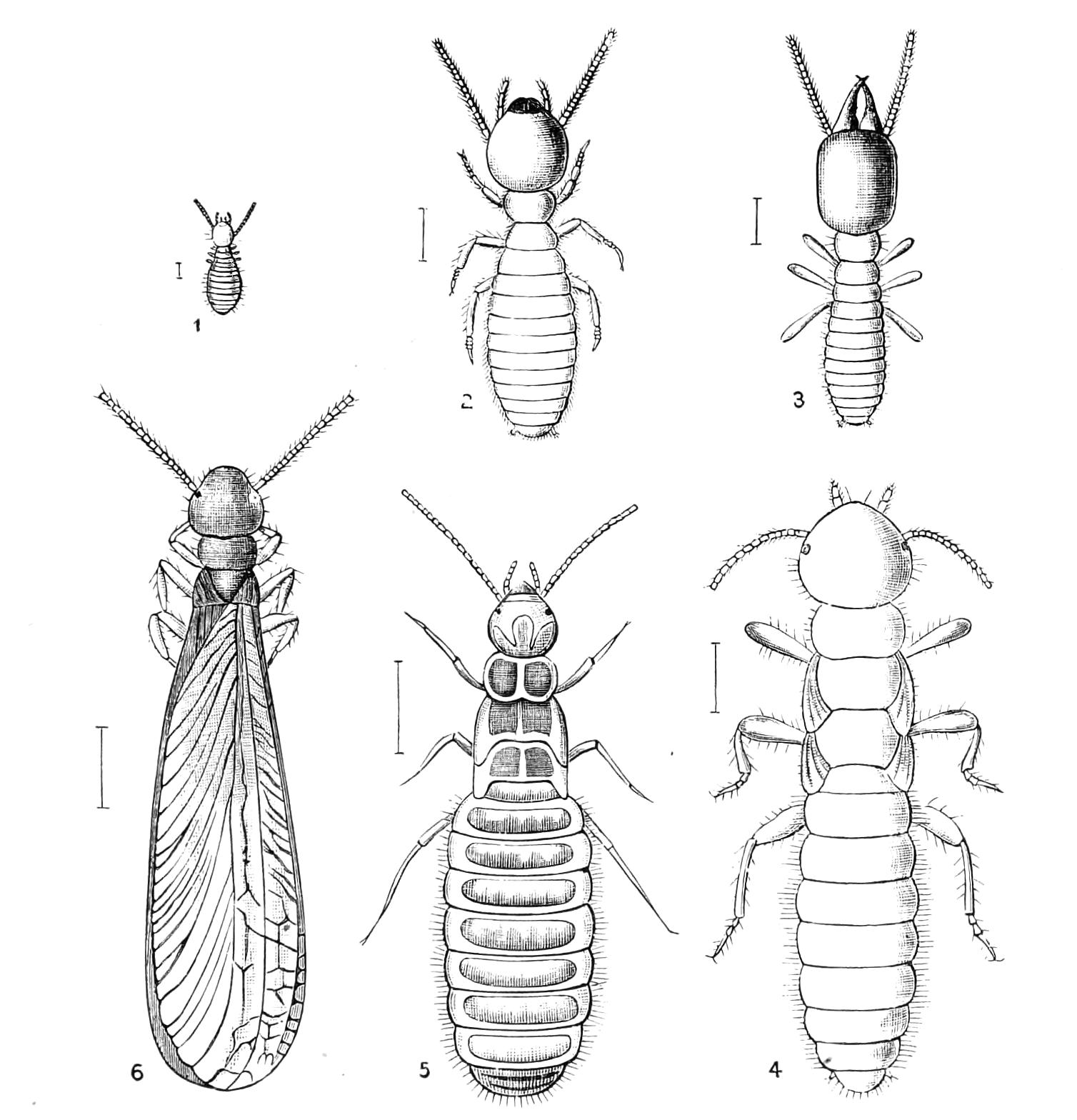
Fig. 231.—Some of the forms of Termes lucifugus. 1, Young larva; 2, adult worker; 3, soldier; 4, young complementary queen; 5, older substitution queen; 6, perfect winged Insect. (After Grassi.)
The period of development apparently occupies from eighteen to twenty-three months. At intervals swarms of a great number of winged individuals leave the nest, and are usually promptly eaten up by various animals. After swarming, the wings are thrown off, and sometimes two specimens or three may be seen running off together; this has been supposed to be preliminary to pairing, but Grassi says this is not the case, but that the object is to obtain their favourite food, as we shall mention subsequently.
Although these are the usual habits of Termes lucifugus at present in Sicily, it must not be concluded that they are invariable; we have in fact evidence to the contrary. Grassi has himself been able to procure in confinement a colony—or rather the commencement of one—accompanied by a true royal pair; while Perris has recorded[291] that in the Landes he frequently found a royal pair of T. lucifugus under chips; they were accompanied in nearly every case by a few eggs. And Professor Perez has recently placed a winged pair of this species in a box with some wood, with the result that after some months a young colony has been founded. It appears probable therefore that this species at times establishes new colonies by means of royal pairs derived from winged individuals, but after their establishment maintains such colonies as long as possible by means of complementary queens. It is far from improbable that distinctions as to the use of true and complementary royalties may be to some extent due to climatic conditions. In some localities T. lucifugus has multiplied to such an extent as to be very injurious, while in others where it is found it has never been known to do so.
The Termitidae of Africa are the most remarkable that have yet been discovered, and it is probably on that continent that the results of the Termitid economy have reached their climax. Our knowledge of the Termites of tropical Africa is chiefly due to Smeathman, who has described the habits of several species, among them T. bellicosus. It is more than a century since Smeathman travelled in Africa and read an account of the Termites to the Royal Society.[292] His information was the first of any importance about Termitidae that was given to the world; it is, as may be well understood, deficient in many details, but is nevertheless of great value. Though his statements have been doubted they are truthful, and have been confirmed by Savage.[293]
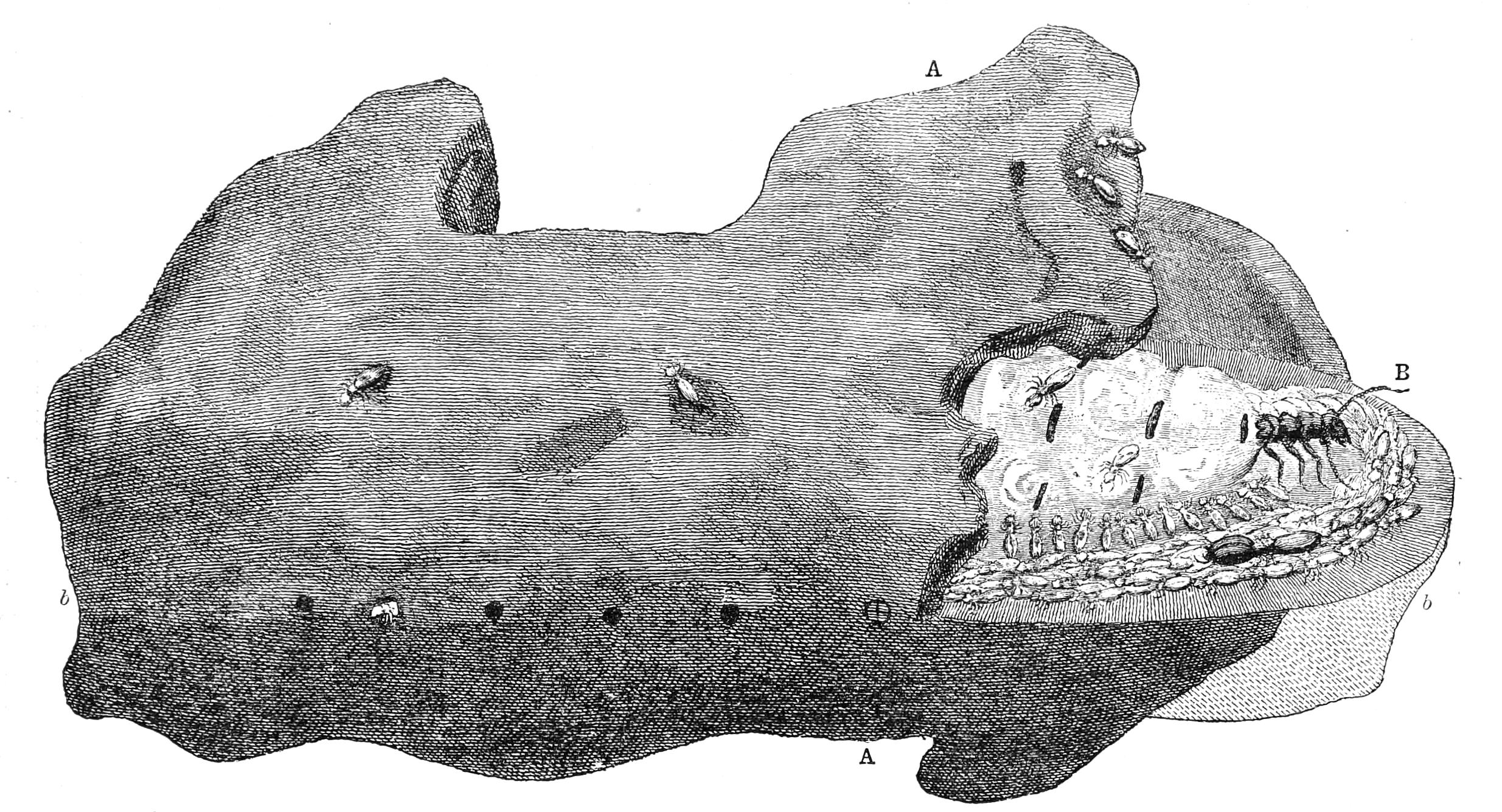
Fig. 232.—Royal cell of Termes bellicosus, partially broken open to show the queen and her attendants. (After Smeathman.) B, Antenna of the queen; b, b, line of entrances to the cell; A, A, an entrance, in this line, closed by the Termites. × ⅞.
T. bellicosus forms buildings comparable to human dwellings; some of them being twenty feet in height and of great solidity. In some parts of West Africa these nests were, in Smeathman's time, so numerous that they had the appearance of villages. Each nest was the centre of a community of countless numbers of individuals; subterranean passages extended from them in various directions. The variety of forms in one of these communities has not been well ascertained, but it would seem that the division of labour is carried to a great extent. The soldiers are fifteen times the size of the workers. The community is dependent on one royal couple. It is the opinion of the natives that if that couple perish so also does the community; and if this be correct we may conclude that this species has not a perfect system of replacing royal couples. The queen attains an almost incredible size and fertility. Smeathman noticed the great and gradual growth of the abdomen, and says it enlarges "to such an enormous size that an old queen will have it increased so as to be fifteen hundred or two thousand times the bulk of the rest of her body, and twenty or thirty thousand times the bulk of a labourer, as I have found by carefully weighing and computing the different states." He also describes the rate at which the eggs are produced, saying that there is a constant peristaltic movement[294] of the abdomen, "so that one part or other alternately is rising and sinking in perpetual succession, and the matrix seems never at rest, but is always protruding eggs to the amount (as I have frequently counted in old queens) of sixty in a minute, or eighty thousand and upward in one day of twenty-four hours."
This observer, after giving an account of the great swarms of perfect winged Insects that are produced by this species, and after describing the avidity with which they are devoured by the Hymenopterous ants and other creatures, adds: "I have discoursed with several gentlemen upon the taste of the white ants; and on comparing notes we have always agreed that they are most {369}delicious and delicate eating. One gentleman compared them to sugared marrow, another to sugared cream and a paste of sweet almonds."
From the preceding brief sketch of some Termitidae we may gather the chief points of importance in which they differ from other Insects, viz. (1) the existence in the community of individuals—workers and soldiers—which do not resemble their parents; (2) the limitation of the reproductive power to a single pair, or to a small number of individuals in each community, and the prolongation of the terminal period of life. There are other social Insects besides Termitidae: indeed, the majority of social Insects—ants, bees, and wasps—belong to the Order Hymenoptera, and it is interesting to note that analogous phenomena occur in them, but nevertheless with such great differences that the social life of Termites must be considered as totally distinct from that of the true ants and other social Hymenoptera.
Development.—Social Insects are very different to others not only in the fact of their living in society, but in respect of peculiarities in the mode of reproduction, and in the variety of habits displayed by members of a community. The greatest confusion has arisen in reference to Termitidae in consequence of the phenomena of their lives having been assumed to be similar to those of Hymenoptera; but the two cases are very different, Hymenoptera passing the early parts of their lives as helpless maggots, and then undergoing a sudden metamorphosis to a totally changed condition of structure, intelligence, and instinct.
The development of what we may look on as the normal form of Termitidae—that is, the winged Insects male and female—is on the whole similar to that we have sketched in Orthoptera; the development in earwigs being perhaps the most similar. The individuals of Termitidae are, however, in the majority of cases if not in all, born without eyes; the wing-rudiments develop from the thoracic terga as shorter or longer lobes according to the degree of maturity; as in the earwigs the number of joints in the antennae increases as development advances. All the young are, when hatched, alike, the differences of caste appearing in the course of the subsequent development; the most important of these differences are those that result in the production of two special classes—only met with in social Insects—viz. worker and soldier. Of these the workers are individuals whose {370}development is arrested, the sexual organs not going on to their full development, while other organs, such as the eyes, also remain undeveloped; the alimentary canal and its adjuncts occupy nearly the whole of the abdominal cavity. The adult worker greatly resembles—except in size—the young. Grassi considers that the worker is not a case of simple arrest of development, but that some deviation accompanies the arrest.
The soldier also suffers an arrest of development in certain respects similar to the worker; but the soldier differs in the important fact that the arrest of the development of certain parts is correlative with an extraordinary development of the head, which ultimately differs greatly from those of either the worker or of the sexual males and females.
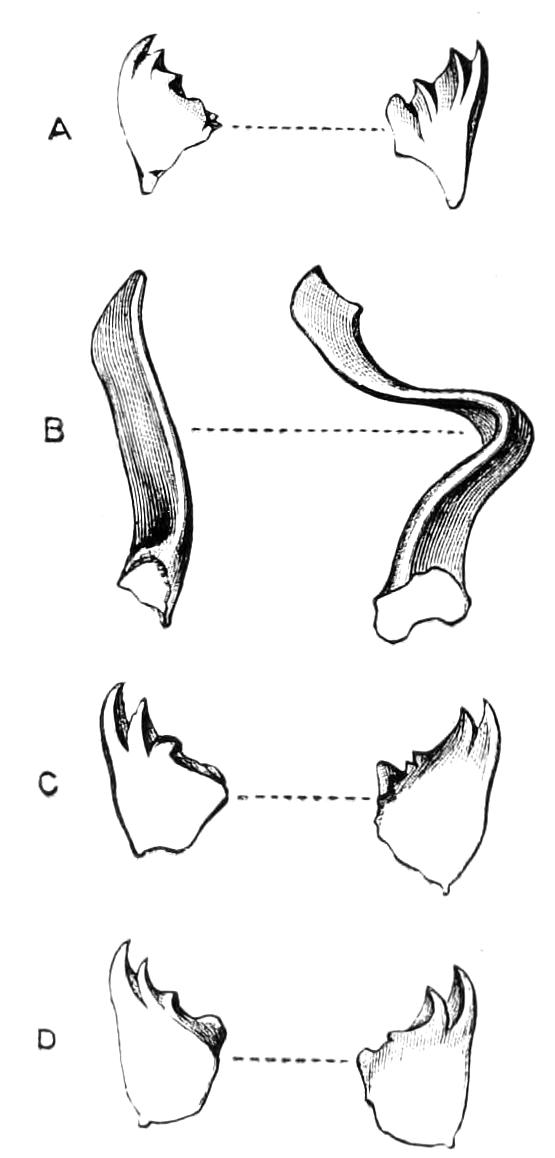
Fig. 233.—The pairs of mandibles of different adult individuals of Termes sp. from Singapore. A, Of worker; B, of soldier; C, of winged male; D, of winged female.
Soldier.—All the parts of the head of the soldier undergo a greater or less change of form; even the pieces at its base, which connect it by means of the cervical sclerites with the prothorax, are altered. The parts that undergo the greatest modification are the mandibles (Fig. 233, B); these become much enlarged in size and so much changed in form that in a great many species no resemblance to the original shape of these organs can be traced. It is a curious fact that the specific characters are better expressed in these superinduced modifications than they are in any other part of the organisation (except, perhaps, the wings). The soldiers are not alike in any two species of Termitidae so far as we know, and it seems impossible to ascribe the differences that exist between the soldiers of different species of Termitidae to special adaption for the work they have to perform. Such a suggestion is justifiable only in the case of the Nasuti (Fig. 234, 1), where the front of the head is prolonged into a point: a duct opens at the extremity of this point, from which is exuded a fluid that serves as a cement for {371}constructing the nest, and is perhaps also used to disable enemies. Hence the prolongation and form of the head of these Nasuti may be fairly described as adaptation to useful ends. As regards the great variety exhibited by other soldiers—and their variety is much greater than it is in the Nasuti—it seems at present impossible to treat it as being cases of special adaptations for useful purposes. On the whole it would be more correct to say that the soldiers are very dissimilar in spite of their having to perform similar work, than to state that they are dissimilar in conformity with the different tasks they carry on.
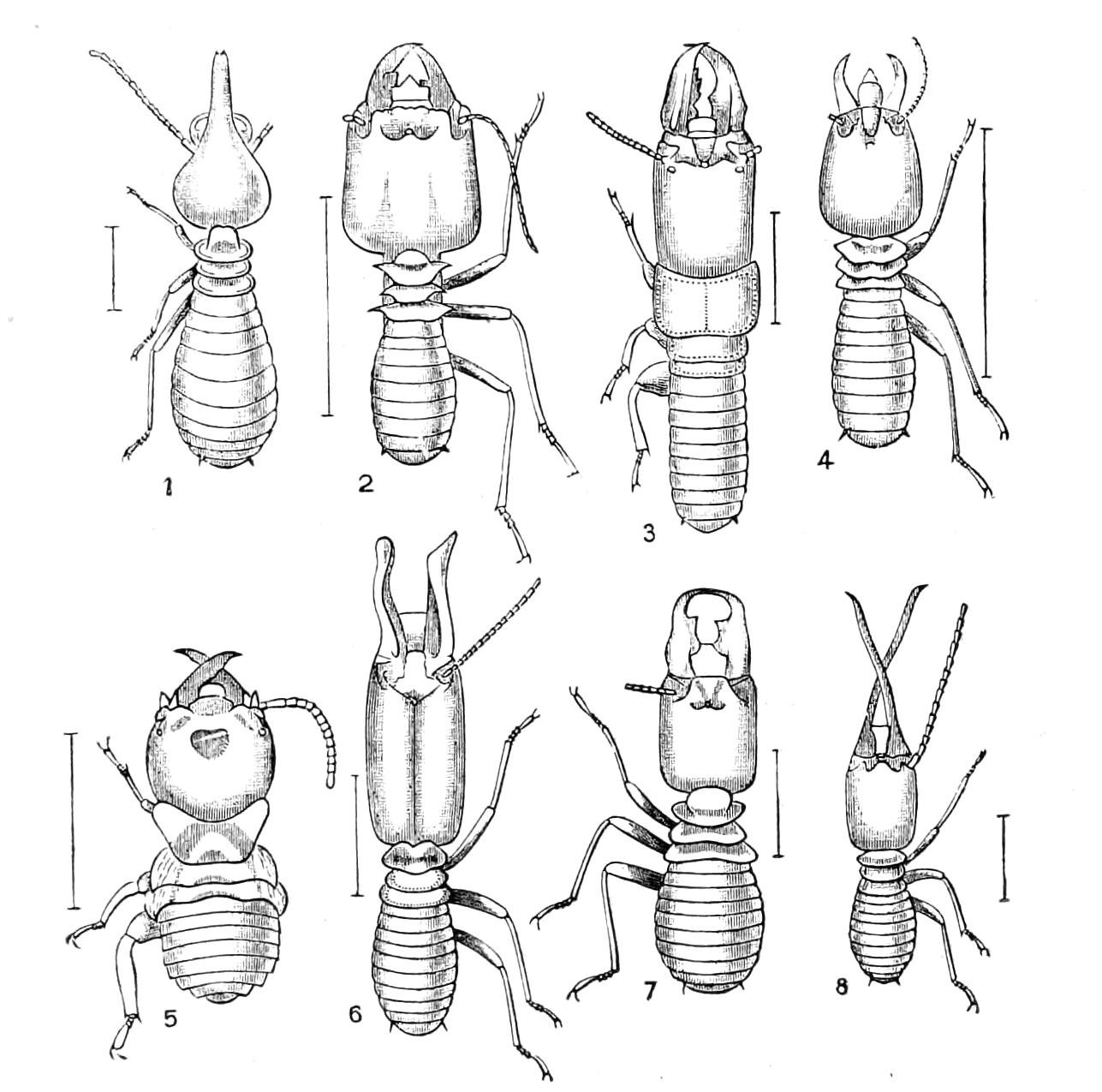
Fig. 234.—Soldiers of different species of Termites. (After Hagen.) 1, Termes armiger; 2, T. dirus; 3, Calotermes flavicollis; 4, T. bellicosus; 5, T. occidentis; 6, T. cingulatus (?); 7, Hodotermes quadricollis (?); 8, T. debilis (?), Brazil.
The Termite soldier is a phenomenon to which it is difficult to find a parallel among Insects. The soldier in the true ants is usually not definitely distinguished from the worker, but it is possible that in the leaf-cutting ants, the so-called soldier may prove to be more similar in its nature to the Termite soldier. The soldiers of any one species of Termite are apparently {372}extremely similar to one another, and there are no intermediates between them and the other forms, except in the stages of differentiation. But we must recollect that but little is yet known of the full history of any Termite community, and it is possible that soldiers which in the stage of differentiation promise to be unsatisfactory may be killed and eaten,—indeed there is some evidence to this effect. There is too in certain cases some difference—larger or smaller size being the most important—between the soldiers of one species, which may possibly be due to the different stage of development at which their differentiation commenced.
It would at present appear that, notwithstanding the remarkable difference in structure of the soldiers and workers of the white ants, there is not a corresponding difference of instinct. It is true that soldiers do more of certain things than workers do, and less of others, but this appears to be due solely to their possession of such very different structures; and we are repeatedly informed by Grassi that all the individuals in a community take part, so far as they are able, in any work that is going on, and we find also in the works of other writers accounts of soldiers performing acts that one would not have expected from them. The soldiers are not such effective combatants as the workers are. Dudley and Beaumont indeed describe the soldiers as merely looking on while the workers fight.[295] So that we are entitled to conclude that the actions of the soldiers, in so far as they differ from those of the rest of the community, do so because of the different organisation and structures of these individuals. We shall, when speaking of food, point out that the condition of the soldier in relation to food and hunger is probably different from that of the other forms.
Various Forms of a Community.—The soldiers and workers are not the only anomalous forms found in Termitid communities; indeed on examining a large nest a variety of forms may be found that is almost bewildering. Tables have been drawn up by Grassi and others showing that as many as fifteen kinds may be found, and most of them may under certain circumstances coexist. Such tables do not represent the results of actual examination in any one case, and they by no means adequately represent the number that, according to the most recent observations of Grassi, may be present; but we give one taken {373}from Grassi, as it conveys some idea of the numerous forms that exist in certain communities. In this table the arrangement, according to A, B, C, D, E, represents successive stages of the development:—
Forms of Termes lucifugus. (After Grassi.) Zool. Anz. xii. 1889, p. 360.
On inspecting this table it will be perceived that the variety of forms is due to three circumstances—(1) the existence of castes that are not present in ordinary Insects; (2) the coexistence of young, of adolescents, and of adults; and (3) the habit the Termites have of tampering with forms in their intermediate stages, the result of which may be the substitution of neoteinic individuals in place of winged forms.
This latter procedure is far from being completely understood, but to it are probably due the various abnormal forms, such as soldiers with rudiments of wings, that have from time to time been discovered in Termite communities, and have given rise to much perplexity.
In connexion with this subject we may call attention to the necessity, when examining Termite nests, of taking cognisance of the fact that more than one species may be present. Bates found different Termites living together in the Amazons Valley, and Mr. Haviland has found as many as five species of Termitidae and three of true ants in a single mound in South Africa. In this latter case observation showed that, though in such close proximity, there was but little further intimacy between the species. There are, however, true inquiline, or guest, Termites, {374}of the genus Eutermes, found in various parts of the world living in the nests of other Termitidae.
Origin of the Forms.—The interest attaching to the various forms that exist in Termites, more particularly to the worker and soldier, is evident when we recollect that these never, so far as we know, produce young. In the social Hymenoptera it has been ascertained that the so-called neuters (which in these Insects are always females) can, and occasionally do, produce young, but in the case of the Termites it has never been suggested that the sexual organs of the workers and soldiers, whether male or female, ever become fruitful; moreover, the phenomena of the production of young by the white ants are of such a nature as to render it in the highest degree improbable that either workers or soldiers ever take any direct part in it. Now the soldier is extremely different from the sexual individuals that produce the young, and seeing that its peculiarities are not, in the ordinary sense of the word, hereditary, it must be of great interest to ascertain how they arise.
Before stating the little information we possess on this subject, it is necessary to reiterate what we have already said to the effect that the soldiers and workers are not special to either sex, and that all the young are born alike. It would be very natural to interpret the phenomena by supposing the workers to be females arrested in their development—as is the case in social Hymenoptera—and by supposing the soldiers to be males with arrested and diverted development.
The observations already made show that this is not the case. It has been thoroughly well ascertained by Lespès and Fritz Müller that in various species of Calotermes the soldiers are both males and females. Lespès and Grassi have shown that the workers of Termes lucifugus are of male and female sex, and that this is also true of the soldiers. Although the view of the duality of the sexes of these forms was received at first with incredulity, it appears to be beyond doubt correct. Grassi adds that in all the individuals of the workers and soldiers of Termes lucifugus the sexual organs, either male or female, are present, and that they are in the same stage of development whatever the age of the individual. This statement of Grassi's is of importance because it seems to render improbable the view that the difference of form of the soldier and worker arises from the arrest of the {375}development of their sexual organs at different periods. The fact that sex has nothing whatever to do with the determination of the form of workers and soldiers may be considered to be well established.
The statement that the young are all born alike is much more difficult to substantiate. Bates said that the various forms could be detected in the new-born. His statement was made, however, merely from inspection of the nests of species about which nothing was previously known, and as it is then very difficult to decide that a specimen is newly hatched, it is probable that all he meant was that the distinction of workers, soldiers, and sexual forms existed in very small individuals—a statement that is no doubt correct. Other observers agree that the young are in appearance all alike when hatched, and Grassi reiterates his statement to this effect. Hence it would appear that the difference of form we are discussing arises from some treatment subsequent to hatching. It may be suggested, notwithstanding the fact that the young are apparently alike when hatched, that they are not really so, but that there are recondite differences which are in the course of development rendered conspicuous. This conclusion cannot at present be said with certainty to be out of the question, but it is rendered highly improbable by the fact ascertained by Grassi that a specimen that is already far advanced on the road to being an ordinary winged individual can be diverted from its evident destination and made into a soldier, the wings that were partially developed in such a case being afterwards more or less completely absorbed. This, as well as other facts observed by Grassi, render it probable that the young are truly, as well as apparently, born in a state undifferentiated except as regards sex. Fig. 230 (p. 363) is designed to illustrate Grassi's view as to this modification; the individual A is already far advanced in the direction of the winged form C, but can nevertheless be diverted by the Termites to form the adult soldier B.
According to the facts we have stated, neither heredity nor sex nor arrest of development are the causes of the distinctions between worker and soldier, though some arrest of development is common to both; we are therefore obliged to attribute the distinction between them to other influences. Grassi has no hesitation in attributing the anatomical distinctions that arise between the soldiers, workers, and winged forms to alimentation. {376}Food, or the mode of feeding, or both combined, are, according to the Italian naturalist, the source of all the distinctions, except those of sex, that we see in the forms of any one species of Termite.
Feeding.—Such knowledge as we possess of the food-habits of Termitidae is chiefly due to Grassi; it is of the very greatest importance, as giving a clue to much that was previously obscure in the Natural History of these extraordinary creatures.
In the abodes of the Termites, notwithstanding the enormous numbers of individuals, cleanliness prevails; the mode by which it is attained appears to be that of eating all refuse matter. Hence the alimentary canal in Termitidae contains material of various conditions of nutritiveness. These Insects eat their cast skins and the dead bodies of individuals of the community; even the material that has passed through the alimentary canal is eaten again, until, as we may presume, it has no further nutritive power. The matter is then used for the construction of their habitations or galleries, or is carried to some unfrequented part of the nest, or is voided by the workers outside of the nests; the pellets of frass, i.e. alimentary rejectamenta, formed by the workers frequently betraying their presence in buildings when none of the Insects themselves are to be seen. The aliments of Calotermes flavicollis are stated by Grassi and Sandias to be as follows: (1) wood; (2) material passed from the posterior part of the alimentary canal or regurgitated from the anterior part; (3) the matter shed during the moults; (4) the bodies of other individuals; (5) the secretion of their own salivary glands or that of their fellows; (6) water. Of these the favourite food is the matter passed from the posterior part of the alimentary canal. We will speak of this as proctodaeal food. When a Calotermes wishes food it strokes the posterior part of another individual with the antennae and palpi, and the creature thus solicited yields, if it can, some proctodaeal food, which is then devoured. Yielding the proctodaeal food is apparently a reflex action, as it can be induced by friction and slight pressure of the abdomen with a small brush. The material yielded by the anterior part of the alimentary canal may be called stomodaeal product. It makes its appearance in the mouth in the form of a microscopic globule that goes on increasing in size till about one millimetre in diameter, when it is {377}either used for building or as food for another individual. The mode of eating the ecdysial products has also been described by Grassi and Sandias. When an individual is sick or disabled it is frequently eaten alive. It would appear that the soldiers are great agents in this latter event, and it should be noticed that owing to their great heads and mandibles they can obtain food by other means only with difficulty. Since they are scarcely able to gnaw wood, or to obtain the proctodaeal and stomodaeal foods, their condition may be considered to be that of permanent hunger, only to be allayed by carnivorous proceedings. When thrown into a condition of excitement the soldiers sometimes exhibit a sort of Calotermiticidal mania, destroying with a few strokes five or six of their fellows. It is, however, only proper to say that these strokes are made at random, the creature having no eyes. The carnivorous propensities of Calotermes are apparently limited to cannibalism, as they slaughter other white ants (Termes lucifugus) but never eat them.
The salivary food is white and of alkaline nature; when excreted it makes its appearance on the upper lip. It is used either by other individuals or by the specimen that produced it; in the latter case it is transferred to the lower lip and swallowed by several visible efforts of deglutition. The aliments we have mentioned are made use of to a greater or less extent by all the individuals except the very young; these are nourished only by saliva: they commence taking proctodaeal and stomodaeal food before they can eat triturated wood.
Royal Pairs.—The restriction of the reproductive powers of a community to a single pair (or to a very restricted number of individuals) occurs in all the forms of social Insects, and in all of them it is concomitant with a prolongation of the reproductive period far exceeding what is natural in Insects. We are not in a position at present to say to what extent the lives of the fertile females of Termitidae are prolonged, there being great difficulties in the way of observing these Insects for long periods owing to their mode of life; living, as they do, concealed from view, light and disturbance appear to be prejudicial to them. We have every reason to believe, however, that the prolongation extends as a rule over several years, and that it is much greater than that of the other individuals of the community, although the lives of even these latter are longer than is usual in Insects; but this {378}point is not yet satisfactorily ascertained. As regards the males there is reason to think that considerable variety as to longevity prevails. But the belief is that the royal males of Termitidae also form an exception to other Insects in the prolongation of the terminal periods of their lives. In Hymenoptera, male individuals are profusely produced, but their lives are short, and their sole duty is the continuation of the species by a single act. We have seen that Grassi is of opinion that a similar condition of affairs exists at present with Termes lucifugus in Sicily, but with this exception it has always been considered that the life of the king Termite is, roughly speaking, contemporaneous with that of the queen; it is said that in certain species the king increases in bulk, though not to an extent that can be at all compared with the queen.
It must be admitted that the duration of life of the king has not been sufficiently established, for the coexistence of a king with a queen in the royal cell is not inconsistent with the life of the king being short, and with his replacement by another. Much that is imaginary exists in the literature respecting Termites, and it is possible that the life of the king may prove to be not so prolonged as has been assumed.
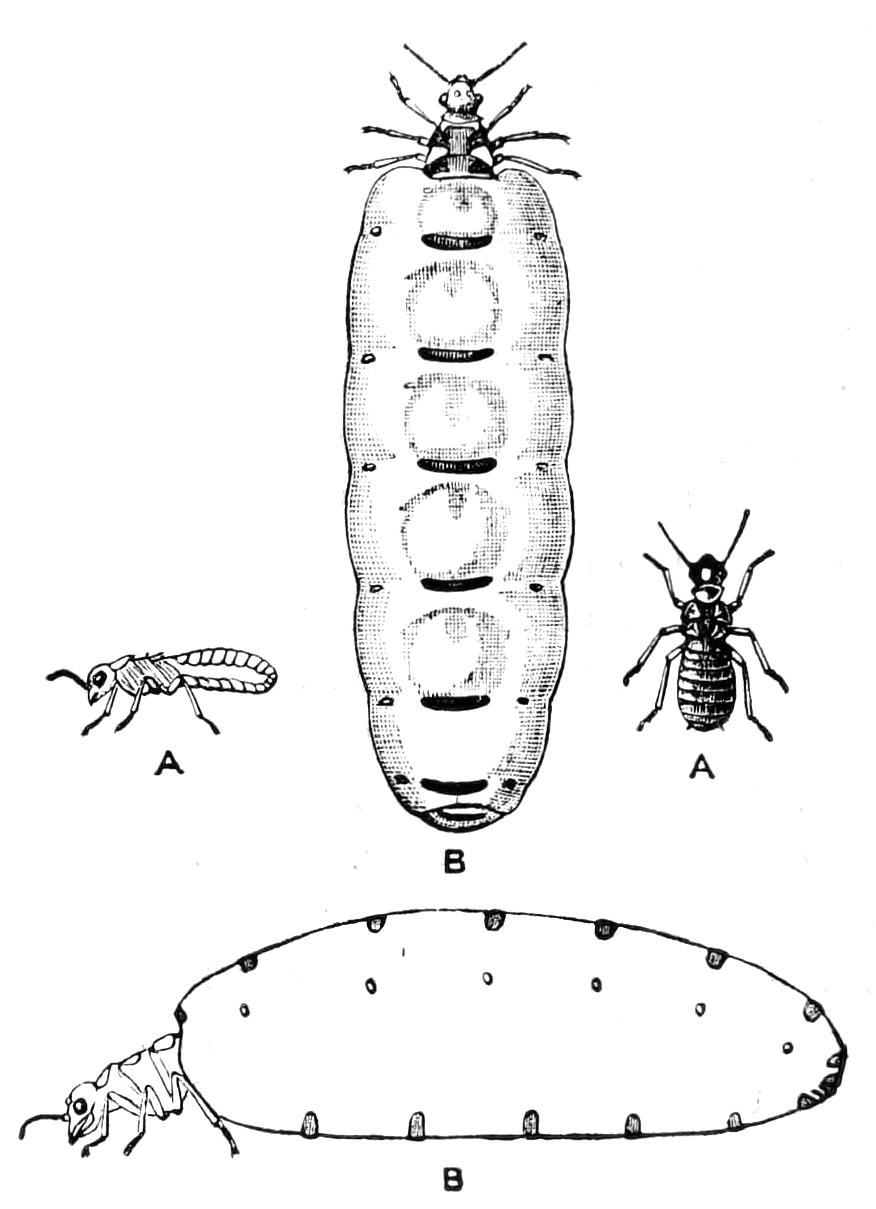
Fig. 235.—Royal pair of Termes sp. from Singapore, taken out of royal cell. A, A, King, lateral and dorsal views; B, B, queen, dorsal and lateral views. Natural sizes.
Returning to the subject of the limitation of the reproduction of the community to a single pair, we may remark that a priori one would suppose such a limitation to be excessively unfavourable to the continuation of the species; and as it nevertheless is the fact that this feature is almost, if not quite, without exception {379}in Insect societies, we may conclude that it is for some reason absolutely essential to Insect social life. It is true that there are in Termitidae certain partial exceptions, and these are so interesting that we may briefly note them. When a royal cell is opened it usually contains but a single female and male, and when a community in which royal cells are not used is inspected it is usually found that here also there are present only a single fertile female and a single king. Occasionally, however, it happens that numerous females are present, and it has been noticed that in such cases they are not fully matured females, but are imperfect, the condition of the wings and the form of the anterior parts of the body being that of adolescent, not adult Insects. It will be recollected that the activity of a community of Termites centres round the great fertility of the female; without her the whole community is, as Grassi graphically puts it, orphaned; and the observations of the Italian naturalist make it clear that these imperfect royalties are substitution queens, derived from specimens that have not undergone the natural development, but have been brought into use to meet the calamity of orphanage of the community. The Termites apparently have the power of either checking or stimulating the reproductive organs apart from other organs of the body, and they appear to keep a certain number of individuals in such a condition that in case of anything going wrong with the queen, the reserves may be brought as soon as possible into a state of reproductive activity. The individuals that are in such a condition that they can become pseudo-royalties are called complementary or reserve royalties, and when actually brought into use they become substitution royalties. It is not at present quite clear why the substitution royalties should be in such excess of numbers as we have stated they were in the case we have figured (Fig. 236), but it may be due to the fact that when the power of the community is at a certain capacity for supporting young a single substitution royalty would not supply the requisite producing power, and consequently the community adopts a greater number of the substitution forms. Termites are utterly regardless of the individual lives of the members of the community, and when the reproductive powers of the company of substitution royalties become too great, then their number is reduced by the effective method of killing and eating them.
According to Grassi's observations, the communities of Termes {380}lucifugus are now kept up in Sicily almost entirely by substitution royalties; the inference being that the age of each community has gone beyond the capacity for life of any single royal queen.
The substitution royalties are, as we have said, called neoteinic (νεος, youthful, τείνω, to belong to), because, though they carry on the functions of adult Insects, they retain the juvenile condition in certain respects, and ultimately die without having completed the normal development. The phenomenon is not quite peculiar to Insects, but occurs in some other animals having a well-marked metamorphosis, notably in the Mexican Axolotl.[296]
Fig. 236.—Pair of neoteinic royalties, taken from the royal chamber of Termes sp. at Singapore by Mr. G. D. Haviland. The queen was one of thirteen, all in a nearly similar state. A, king; B, C, queen.
A point of great importance in connexion with the neoteinic royalties is that they are not obtained from the instar immediately preceding the adult state, but are made from Insects in an earlier stage of development. The condition immediately preceding the adult state is that of a nymph with long wing-pads; such specimens are not made into neoteinic royalties, but nymphs of an earlier stage, or even larvae, are preferred. It is apparently by an interference with one of these earlier stages of development that the "nymphs of the second form," which have for long been an enigma to zoologists, are produced.
Post-metamorphic Growth.—The increase of the fertility of the royal female is accompanied by remarkable phenomena of growth. Post-metamorphic growth is a phenomenon almost unknown in Insect life, except in these Termitidae; distension not infrequently occurs to a certain extent in other Insects, and {381}is usually due to the growth of eggs inside the body, or to the repletion of other parts. But in Termitidae there exists post-metamorphic growth of an extensive and complex nature; this growth does not affect the sclerites (i.e. the hard chitinous parts of the exo-skeleton), which remain of the size they were when the post-metamorphic growth commenced, and are consequently mere islands in the distended abdomen (Fig. 236, B, C). The growth is chiefly due to a great increase in number and size of the egg-tubes, but there is believed to be a correlative increase of various other parts of the abdominal as distinguished from the anterior regions of the body. A sketch of the distinctions existing between a female of a species at the time of completion of the metamorphosis and at the period of maximum fertility does not appear to have been yet made.
New Communities.—The progress of knowledge in respect of Termitidae is bringing to light a quite unexpected diversity of habits and constitution. Hence it is premature to generalise on important matters, but we may refer to certain points that have been ascertained in connexion with the formation of new communities. The duration of particular communities and the modes in which new ones are founded are still very obscure. It was formerly considered that swarming took place in order to increase the number of communities, and likewise for promoting crossing between the individuals of different communities. Grassi, however, finds as the result of his prolonged observations on Termes lucifugus that the swarms have no further result than that the individuals composing them are eaten up. And Fritz Müller states[297] that in the case of the great majority of forms known to him the founding of a colony by means of a pair from a swarm would be just about as practicable as to establish a new colony of human beings by placing a couple of newly-born babes on an uninhabited island. It was also thought that pairs, after swarming, re-entered the nests and became royal couples. It does not, however, appear that any one is able to produce evidence of such an occurrence. The account given by Smeathman of the election of a royal couple of Termes bellicosus is imperfect, as, indeed, has already been pointed out by Hagen. It suggests, however, that a winged pair after leaving the nest do again enter it to become king {382}and queen. The huge edifices of this species described by Smeathman are clearly the result of many years of labour, and at present substitution royalties are not known to occur in them, so that it is not improbable Smeathman may prove to be correct even on this point, and that in the case of some species mature individuals may re-enter the nest after swarming and may become royal couples. On the whole, however, it appears probable that communities of long standing are kept up by the substitution royalty system, and that new communities when established are usually founded by a pair from a swarm, which at first are not in that completely helpless condition to which they come when they afterwards reach the state of so-called royalty. Grassi's observations as to the sources of food remove in fact one of the difficulties that existed previously in regard to the founding of new colonies, for we now know that a couple may possibly bear with them a sufficient supply of proctodaeal and stomodaeal aliment to last them till workers are hatched to feed them, and till soldiers are developed and the community gradually assumes a complex condition. Professor Perez has recently obtained[298] the early stages of a community from a winged pair after they had been placed in captivity, unattended by workers. Müller's observation, previously quoted, is no doubt correct in relation to the complete helplessness of royal pairs after they have been such for some time; but that helplessness is itself only gradually acquired by the royal pair, who at first are able to shift for themselves, and produce a few workers without any assistance.
Anomalous Forms.—Müller has described a Calotermes under the name of C. rugosus, which is interesting on account of the peculiar form of the young larva, and of the changes by which it subsequently becomes similar in form to other species of the genus. We represent the development of this larva in Fig. 237. We may call attention to the fact that this figure illustrates the large size of the paunch, which is so extraordinary in some of the states of the Termitidae.
It will be recollected that the genus Calotermes is destitute of workers. There is another genus, Anoplotermes, in which the reverse condition prevails, and the soldier is absent; this is the only case yet known in which such a state of affairs exists. {383}The species is called A. pacificus by Fritz Müller; it differs from other Termitidae in possessing a proventriculus destitute of triturating ridges. The nests of this species are utilised by a little Eutermes (E. inquilinus Müller) for its own advantage; whether by first destroying the Anoplotermes or whether by merely taking possession of the nests abandoned by their owners is not known. It is a most remarkable fact that the Eutermes resembles the Anoplotermes so extremely that the two can scarcely be distinguished, though anatomically they are quite different. The resemblance is indeed so great that it deceived Von Jhering into supposing that the two genera were alternate generations of a single species, one generation possessing soldiers, the other being without them. Subsequently, by anatomical investigation, he recognised[299] the error into which he had fallen—an error that, under such peculiar circumstances, was quite pardonable.
Fig. 237.—Changes in external form of the young larva of Calotermes rugosus. A, Newly hatched with nine joints in antennae, × 8; B, older larva with ten joints, × 8; C, next stage with eleven joints, × 8; D, larva with twelve joints; the position of the parts of the alimentary canal are shown—v, crop; m, stomach; b, paunch; e, intestine; r, dorsal vessel, × 16⁄3 (After Fritz Müller.)
Hagen has suggested[300] that Hodotermes japonicus never produces winged forms. Very little, however, is actually known as to this species.
Marching and Harvesting Termites.—Smeathman alluded to a remarkable Termes seen by him in Africa, giving it the name of T. viarum. Nothing further is known of this Insect, which, according to Smeathman's account, may possibly be the most remarkable of the family. T. viarum is said to be larger than T. bellicosus, and was discovered issuing in large numbers from a hole in the ground and marching in columns consisting of workers directed by soldiers of enormous size, some of whom {384}climbed up plants and gave audible signals to the army, which immediately responded with a hissing noise and by increasing their pace with the utmost hurry; they continued marching by the spot where Smeathman observed them for upwards of an hour. He was not able to find their nests, and no specimens have been preserved; both soldiers and workers possessed eyes. Marching in this way by daylight is contrary to the nature of ordinary Termites, and some doubt has existed as to the correctness of Smeathman's observation, which has in fact remained for upwards of a century without confirmation.
Fig. 238.—Eyed, grass-cutting Termite, Hodotermes havilandi, A, soldier; B, worker. South Africa. In life the head is carried horizontally, so the piece of grass sticks up like a flag-pole.
Mr. G. D. Haviland has, however, this year discovered in Natal a Termite which shows that there are species in Africa of the kind described by Smeathman, the workers and soldiers being possessed of facetted eyes. Mr. Haviland states that the workers of this species issue from holes in the ground during the heat of the day and cut grass both dead and green. They carry it, in lengths of about two inches, to the mouths of the holes, often leaving it there and going at once to fetch more. Under acacia bushes they carry acacia leaflets as well as grass. In the middle of the day more grass accumulates at the entrance to the holes than can be taken in, but as the heat of the day diminishes the workers cease to forage and take in the accumulation. When the grass is all in they sometimes close the mouth of the hole with moistened pellets of earth brought in their mouths. The soldiers remain in the holes; when disturbed they jerk themselves like soldiers of other species to frighten away the intruder; when they bite, their grip is very tenacious. The holes are about ⅓ of an inch in diameter, and there are usually several of them a few yards apart; around each {385}of them is a patch over which the grass has been cut quite short. Mr. Haviland followed these holes by digging for a distance of 20 feet and to a depth of 5½ feet; they remain uniform in size except that near the entrance there may be one or two chambers in which the grass is temporarily stored, but these do not hold more than would be collected in an hour or two. As the burrow descends it is occasionally joined by another, and at the point of junction there is usually a considerable widening. Sometimes they run straight for 6 or 7 feet, sometimes they curve abruptly, sometimes they are nearly horizontal, but near the mouth may be almost vertical in direction. These Termites are very local, but the specimens are numerous when found. Mr. Haviland dug for these Insects at two places on the Tugela river, one of them being at Colenso. It is much to be regretted that he was unable to reach the nest. We figure a soldier selected from specimens sent by Mr. Haviland to the Cambridge University Museum. This Insect is apparently much smaller than Smeathman's T. viarum. Other species of Termitidae have been described[301] as forming underground tunnels in Africa, but none of the species have yet been satisfactorily identified.
It was stated by Smeathman that some species of Termites had chambers in their habitations in which grew a kind of fungus used by the Insects for food; Mr. Haviland is able to confirm Smeathman in this particular; he having found fungus-chambers in the nests of more than one species both in Singapore and South Africa (Fig. 240).
Habitations.—In nothing do Termites differ more than in the habitations they form. Sometimes, as we have mentioned in the case of Calotermes, there is no real structure formed; only a few barriers being erected in burrows or natural hollows in wood. In other cases very extensive structures are formed, so that the work of the Termites becomes a conspicuous feature in the landscape. This is of course only the case in regions that are not much interfered with by man; the great dwellings spoken of by Smeathman and others soon disappear from the neighbourhood of settlements, but in parts of Africa and in Australia large dwellings are still formed by these creatures. In the latter part of the world there exists a very remarkable one, formed by an {386}undetermined species called by the officers and crew of her Majesty's ship Penguin the "compass ant." The outline of one of the structures formed by this Termite we represent in Fig. 239. Mr. J. J. Walker, to whom we are indebted for the sketch from which this figure is taken, has also favoured us with the following extract from his diary, of date 4th August 1890: "The most interesting feature in the scenery (about forty miles inland from Port Darwin) was the constant succession of huge mounds raised by the Termites, of which I had seen some comparatively small examples in my rambles near Port Darwin; but these exceeded in dimensions all I had ever seen. The most frequent as well as the largest kind was usually of a reddish or ferruginous colour outside, and generally almost cylindrical in shape with obtusely-pointed top, but nearly always more or less weather-worn, with great irregular buttresses and deep ruts down the sides; many of them look like ruined towers in miniature. Their usual height was from 8 to 10 feet, but many were much higher, and some attained an (estimated) elevation of at least 20 feet. Another kind, seen only in one or two places along the line, was of a much more singular character; they averaged only 4 to 5 feet high, were built of a dark-gray mud, and in shape were like thin flat wedges set upright (see Fig. 239), reminding one of tombstones in a churchyard. But the most remarkable feature about these mounds was that they had all the same orientation, viz. with the long faces of the wedge pointing nearly north and south. Why this is so I am quite at a loss to imagine, and I much regret that I had no opportunity of closely examining these most singular structures. A third kind of mound, usually not exceeding 2 feet in height, was of a simple, acute, conical figure, and generally of a gray colour somewhat paler than the last."
Fig. 239.—Termitarium of compass or meridian Termite of North Australia. A, face extending south and north; B, cross-section.
The material used for the construction of the dwellings is either earth, wood, or the excrement of the Termites. The huge edifices mentioned by Smeathman are composed of earth cemented {387}together so as to look like stone or brick, and the buildings appear to be almost as strong as if they were actually constructed with these materials. In many cases the substance used is comminuted wood that has passed one or more times through the alimentary canal of the Insects, and may therefore be called excrement. Whether the stone-like material is made from earth that has passed through the alimentary canal or from grains gathered for the purpose has not been well ascertained. In any case the material is cemented together by means of the secretions of glands. Dudley and Beaumont have described the process of construction, in a species observed by them, saying that earth is brought and placed in position by the mandibles, and cemented by liquid from the abdomen.[302] Von Jhering says[303] that some species form the exterior walls of their dwellings of stone-like material, but make use of woody matter for the construction of the interior. Smeathman has described the nest of Termes bellicosus. The whole of the very strong external wall consists of clay-like material, cemented by the secretions of the Termites to a very firm consistence. The royal cell is built of the same material as the framework of the nest; whilst the nurseries in which the young are chiefly found are built of woody material, and are always covered with a kind of mould—the mycelium of a fungus—and plentifully sprinkled with small white bodies, which, under the microscope, are found to be filled with a number of oblong, spore-like cells.
Fig. 240.—Fragment of Termitarium of Termes angustatus, S. Africa, showing fungus chambers and orifices of communication.
These nurseries rest on the clay-like framework of the nest, but are not attached thereto; they in no way support it, or one another, indeed they have the appearance of being constantly added to on their upper margins and constantly eaten away on their under parts. Fig. 240 represents the appearance of the upper boundary of a nursery taken from a nest of Termes angustatus. The small white bodies, mentioned above, have disappeared: the mycelium of the fungus, though not shown in the {388}figure, is still visible on the specimen from which it was drawn, and gives rise to a whitish, glaucous appearance.
In various parts of the world nests formed on trees by Termites are to be seen; these tree nests are, it would appear, in some cases only parts of a community, and are connected with the main body by galleries. In other cases nests are formed in various positions of advantage; Messrs. Hubbard and Hagen have given us an account[304] of some of these—probably the work of Eutermes ripperti—as seen in Jamaica. They describe the nests as spherical or conical masses, looking externally as if composed of loamy earth; they are placed on trees, fences, or walls; they vary in size from that of a man's fist to that of a hogshead; they appear to be composed of finely comminuted wood fastened together by saliva. These nests are formed on the same principle as those of the wasps that make nests hanging to trees and bushes, as they consist of an external protecting envelope covering a comb-like mass in the interior. At the bottom of the nest there is a covered gallery leading to the earth, where the main nest appears to be situate; galleries also are constructed so as to lead to the tops of trees and other places, in such a manner that the Termite can still keep up its peculiarity of working and travelling in tunnels and yet roam over a large area; the activity of these Termites continues day and night. In each nest there is a queen, who lays eggs that are removed by the worker Termites to the bottom of the nest. The young are fed on a prepared food, consisting apparently of comminuted vegetable matter, of which considerable masses are laid in store. Some of the nests are rich in containing many pounds' weight of this material, while others are apparently quite destitute of it. There is a soldier form and at least two kinds of workers. Some species of true ant frequently shares the nest of these white ants, but on what terms the two kinds of Insects live together is not stated.
Termite Ravages.—In countries whose climate is favourable to their constitutions certain kinds of Termites become of great importance to our own species. Owing to their taste for woody matter and to their habit of working in concealment, it is no uncommon thing for it to be discovered that Termites have obtained access to a building and have practically destroyed the wooden materials used in its construction; all the interior of the {389}wood being eaten away and only a thin outer shell left intact. A Termite, T. tenuis, was introduced—in what manner is not certainly known[305]—to the Island of St. Helena, and committed such extensive ravages there that Jamestown, the capital, was practically destroyed and new buildings had to be erected. Other such cases are on record. Destructive species can sometimes be destroyed by placing in the nests a portion of arsenicated food. This is eaten by some individuals, who perish in consequence; and their dead bodies being consumed by their comrades, the colony becomes checked if not exterminated.
The number of described species of Termitidae does not much exceed 100, but this is certainly only a small portion of those existing, the total of which may probably reach 1000 species.
Termitidae are classed by some naturalists with the Orthoptera, and they have a great deal in common with some of the cursorial division of that Order, more particularly Forficulidae and Blattidae; but they differ from Orthoptera in the nature and form of the wings. They are also classed by some, with a few other forms, as a separate Order of Pseudo-Neuroptera called Corrodentia, but this is not a very satisfactory course, as the Termitidae do not agree closely with the forms associated with them, while the aggregate so formed is far from being very distinct from other forms of Neuroptera. On the whole the best plan appears to be to treat the Termitidae as forming a distinct family of the Order Neuroptera, or to make it a distinct Order, as proposed by Grassi. Packard now associates Termites in an Order with the biting-lice, and calls it Platyptera.
Fossil Termites.—Termitidae were very abundant in Tertiary times, and the genera appear to have been then much the same as at present. In Mesozoic strata the remains of true Termitidae apparently exist in the Lias in Europe, but farther back than this the family has not been satisfactorily traced. It was formerly supposed that Termitidae existed in the Carboniferous strata, but this appears to be very doubtful; and the fossil remains of that epoch, which were presumed to be those of Termites, are now referred by Scudder and others to the Neuropteroid division of the Order Palaeodictyoptera, an Order which is formed entirely of Palaeozoic fossil remains.
NEUROPTERA CONTINUED—PSOCIDAE (BOOK-LICE AND DEATH-WATCHES)—THE FIRST FAMILY OF AMPHIBIOUS NEUROPTERA (PERLIDAE, STONE-FLIES).
Fam. IV. Psocidae—Book-Lice, Death-Watches.
Minute Insects with slender, thread-like, or hair-like antennae; four delicate membranous wings, the front pair of which are the larger; their neuration is not abundant and is irregular, so that the cells are also irregularly arranged; the transverse nervules are only one or two in number.[306] Prothorax very small, in the winged forms quite concealed between the head and the large mesothorax; this latter closely connected with, or fused with, the metathorax. Species quite wingless, or with wings unfitted for flight, exist; in them the prothorax is not so extremely small, while the mesothorax is smaller than in the winged forms. Tarsi of two or three segments. Metamorphosis slight, marked chiefly by the development of wings and ocelli.
The Psocidae are without exception small and soft-bodied Insects, and are only known to those who are not entomologists by the wingless forms that run about in uninhabited or quiet apartments, and are called dust-lice or book-lice. They are perhaps more similar to Termitidae than to any other Insects, but the two families differ much in the structure of their wings, and are totally dissimilar in the nature of their lives.
Fig. 242.—Transverse horizontal section of head of Psocus: f, fork or pick; t, lingua; mx, left maxilla; c, cardo; p, stipes; m.m, muscles; m.s, socket of mandible.
Fig. 243.—A, Front of head of Psocus heteromorphus; cl, post-clypeus; g, epicranium: B, transverse horizontal section of post-clypeus of Psocus: cl, post-clypeus; c.m, clypeal muscles; g, epicranium; t, tendons; l.m, labial muscle in section; oe, oesophagus; oe.b, oesophageal bone. (After Burgess and Bertkau.)
The antennae consist of eleven to twenty-five joints, or even more, about thirteen being the usual number; the basal two are thicker than the others, and are destitute of setae or pubescence such as the others possess. The maxillae and labium are remarkable. The former possesses a peculiar hard pick or elongate rod; this is considered by many naturalists to be the inner lobe, but Burgess thinks it more probably an independent organ,[307] as it has no articulation of any kind with the outer lobe. The latter is remarkably thick and fleshy; the palpus is 5-jointed. Other authorities consider the pick to be certainly the inner lobe; if it be not, the latter is quite wanting. Hagen agrees with Burgess in stating that the pick slides in the outer lobe as in a sheath. The labium has a large mentum and a ligula divided anteriorly into two lobes; at each outer angle in front there is a globular projection, which is doubtless the labial palpus; reposing on the labium there is a large free lingua. The labrum is large, attached to a distinct clypeus, behind which there is a remarkable post-clypeus, which is usually prominent as if inflated; to its inner face are attached several muscles which converge to be inserted on a plate placed below the anterior part of the oesophagus, and called by Burgess the {392}oesophageal bone; under or within the lingua there is a pair of lingual glands. Judging from Grosse's study of the mouth of Mallophaga, we may conclude that the oesophageal bone will prove to be a sclerite of the hypopharynx. The eyes of the winged forms are frequently remarkably convex, and there are also three ocelli, triangularly placed on the vertex. The head is free and very mobile. The coxae are rather small, exserted, contiguous; the sterna small. The abdomen has usually ten segments, though sometimes only nine can be detected.
The thorax in Psocidae usually looks as if it consisted of only two segments. This is due to two opposite conditions: (1) that in the winged forms the prothorax is reduced to a plate concealed in the fissure between the head and the mesothorax bearing the first pair of wings; (2) that in the wingless forms (Fig. 247), though the prothorax is distinct, the meso- and metathorax are fused into one segment.
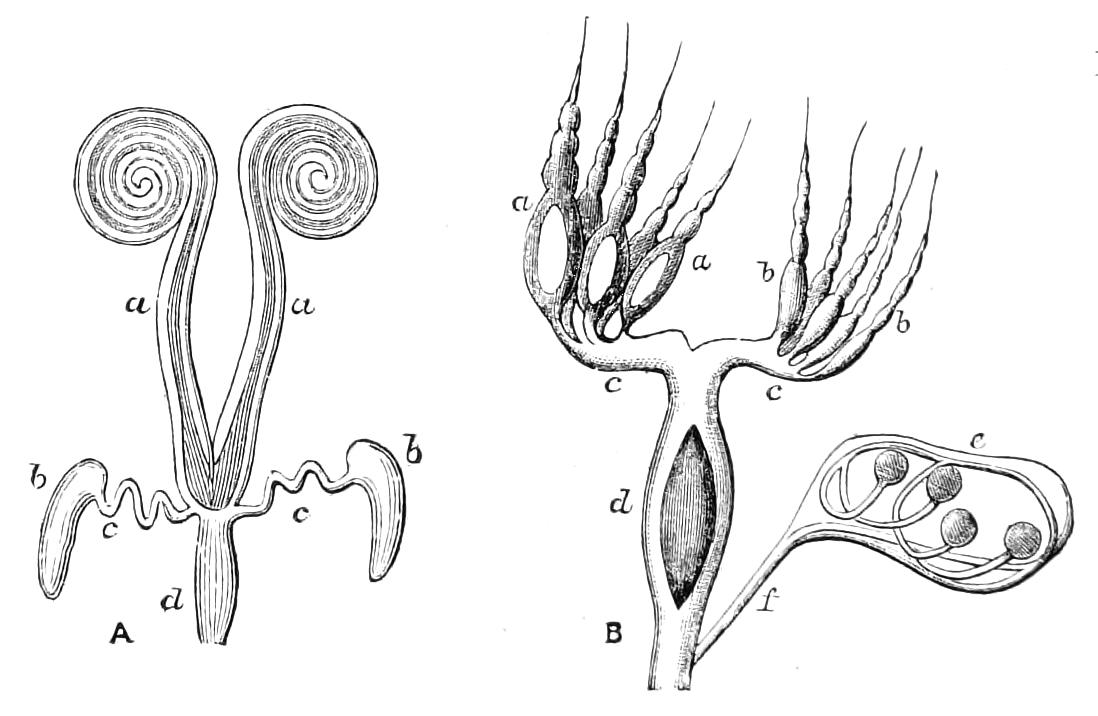
Fig. 244.—Reproductive organs of Clothilla pulsatoria. A, Male; a, vesiculae seminales; b, testes; c, vasa deferentia; d, ejaculatory duct. B, Female; a, b, egg-tubes; c, oviduct; d, uterus, containing egg; e, accessory gland (the enveloping sac in section); f, its duct. (After Nitzsch.)
The internal anatomy is only very incompletely known. Nitzsch[308] has, however, described the alimentary canal and the reproductive organs of Clothilla pulsatoria. The former is remarkably simple: no proventriculus or crop was found; the stomach is very elongate, and consists of a sac-like anterior portion and an elongate, tubular posterior part. There are four Malpighian tubes. The posterior part of the canal is remarkably short, the small intestine being scarcely as long as the rectum. The ovaries (Fig. 244, B) consist of five egg-tubes on each side; connected with the oviduct there is a peculiar accessory gland consisting of a sac containing other small sacs each {393}with an elongate efferent duct; the number of the secondary sacs varies from one to four according to the individual. The testis (Fig. 244, A, b) is a simple capsule; connected with the base of the ejaculatory duct there is a pair of elongate accessory glands or vesiculae seminales.
The life-history has never been satisfactorily sketched. The young greatly resemble the old, but have no ocelli or wings, and sometimes the tarsi are of two joints, while in the adult they have three. The antennae have also in these cases a less number of joints in the young stage. The food is animal or vegetable refuse substances; many live on fungoid matter of various kinds, mouldy chaff being, it is said, a favourite pabulum; the mould on palings is a source of food to many; others live on the rust-fungi of leaves, and many frequent the bark of trees. They are able to spin webs, probably by the aid of the lingual glands; the eggs are deposited, in some cases, on leaves and covered with a web. Hagen says that a peculiar organ, possibly a gland—he calls it a hose[309]—exists at the base of the tarsal claws. In our climate most of the species pass the winter in the egg-state. There may be two generations in a year, perhaps more.
The nomenclature of the wing-veins of Psocidae has given rise to much discussion.[310] The system shown in the accompanying figure is probably the most convenient; the subcostal vein (2) is always obscure, and sometimes can only be detected by very minute examination. Some interesting information as to the minute structure and mode of formation of the wings and their nervures has been given by Hagen.[311]
Fig. 245.—Anterior wing of Elipsocus brevistylus. (After Reuter.) 1, Costal vein; 2, subcostal; 3, radial; 4, cubitus; 4a, branches of cubitus; 5, sector of the radius; 5a, forks thereof.
In the young the wings first appear as buds, or outgrowths of the sides of the meso- and meta-thorax; afterwards the prothorax decreases, while the other two thoracic segments and the wing-rudiments attached to them increase. The wings from their very origin appear to be different from those of the Orthoptera, and the changes that take place in the thoracic {394}segments in the course of the development, differ from those that occur in Orthoptera.
There are several peculiarities connected with the wings. Frequently they exist, though of no use for flight; some Psocidae that have perfectly-formed wings are so reluctant to use them that, M‘Lachlan says, they will allow themselves to be crushed without seeking to escape by flight. At certain periods, however, some Psocidae float on the wing in considerable numbers, especially in a moist still atmosphere, and then drift about like the winged Aphididae, which are frequently found with them. There is evidence that individuals, or generations, of some of the winged species occur with only rudimentary wings; although this has been denied by Kolbe, there can be no doubt about it. The form figured above (Fig. 246) was described by Bertkau[312] as a distinct genus, but was afterwards recognised by him[313] to be a short-winged form of Mesopsocus unipunctatus. It is probable that the adult female of this species has the wings always micropterous, while the male has these organs of the full size. In other species the condition of the rudimentary wings seems to be quite constant. The facts concerning the wings of Psocidae are so peculiar that Kolbe came to the conclusion that the organs exist not because they are of use for flight, so much as because it is the nature of an Insect to develop wings.[314]
Some of the species of Psocidae have never any trace of wings. These apterous forms are mostly included in the division Atropinae, and are usually very minute; it has been again and again erroneously stated that they are the young state of winged forms. Hagen kept a large colony of Atropos divinatoria for some years in confinement, so that he saw numerous generations as well as many specimens. He found the apterous condition quite constant.
The association of ocelli with wings is nearly constant in Psocidae. The genus Clothilla—allied to Atropos—possesses very rudimentary wings but no ocelli. Hagen, however, found[315] that in a certain locality no less than 12 per cent of the individuals of this species were provided with ocelli,—a most extraordinary variation.
In some of these apterous forms there is found on each side of the prothorax a tubercular prominence which, according to Hagen, can be considered only as the rudiment of a wing that never develops. Though no existing Insect is known to possess rudimentary wings on the prothorax, we have previously mentioned (p. 344) that in the Carboniferous epoch appendages of the nature alluded to were not very rare.
A genus of living forms—Hyperetes—in which the three thoracic segments are well developed, but in which there are no alar appendages or rudiments, is considered by Hagen to be more primitive than the Psocidae found in amber to which we shall subsequently allude.
The number of described species of Psocidae does not reach two hundred; we have, however, thirty species or more in Britain.[316] Nietner observed about the same number in the immediate vicinity of his house in Ceylon. The isolated and remote Hawaiian group of islands is remarkably rich in Psocidae. Two thousand is a moderate estimate of the number of existing species. The largest forms yet discovered belong to the Brazilian genus Thyrsophorus; they attain, however, a breadth of only about one inch with the wings fully expanded. The Cuban genus Embidopsocus is said to be of great interest from its approximation to Embiidae. It is at present very inadequately known.
One (or more) very minute Insects of this family—Clothilla pulsatoria according to Hagen, Atropos[317] divinatoria according to some other authors—is widely known under the name of the death-watch, owing to its being believed to make a peculiar {396}ticking noise, supposed to be prophetic of the decease of some individual—a human being we fancy, not a death-watch. It is difficult to believe that so minute and soft an Insect can produce a sound audible to human ears, and many entomologists are of opinion that the sound in question is really produced by a beetle—of the genus Anobium—which lives in wood, and that as the beetle may be concealed in a hole, while the Clothilla is seen running about, the sound is naturally, though erroneously, attributed to the latter. But the rapping of the Anobium is well known, is produced while the Insect is at large, and is said to be a different noise from that of the Psocid; evidence too has been given as to the production of the sound in a workbox when the Psocid was certainly present, and the most careful search failed to reveal any beetle.
The Rev. W. Derham, who two hundred years ago was Rector of Upminster, in Essex, and was well known as a distinguished writer and philosopher, gave an account of the ticking of death-watches to the Royal Society.[318] This gentleman was a most accurate and minute observer; he was well acquainted with the ticking of the greater death-watch—Anobium—which he describes very accurately, as well as the acts accompanying it, the details he mentions being exactly such as occur at the present time. He not only heard the ticking of the Psocid or lesser death-watch, but repeatedly witnessed it. He says: "I am now so used to, and skilful in the matter as to be able to see, and show them, beating almost when I please, by having a paper with some of them in it conveniently placed and imitating their pulsation, which they will readily answer." He also states that he could only hear them beating when it was done on paper, and that this death-watch will tick for some hours together without intermission, with intervals between each beat, so that it much resembles the ticking of a watch. The act of ticking was {397}accompanied by rapping the front of the head on the paper, but Mr. Derham could not be sure that the sound was produced in that manner, because each stroke was also accompanied by a peculiar shudder, or recoil. After a prolonged ticking he observed that another individual of the other sex made its appearance. The species figured by Mr. Derham more resembles a Hyperetes than it does either of our two known book-lice, Atropos and Clothilla.
Numerous species of Psocidae are preserved in amber; Hagen[319] has made a careful study, based on a considerable number of specimens, of about thirteen such species. They belong to no less than nine genera and five sub-families. Sphaeropsocus is the most remarkable; this Insect has a well-developed prothorax, as is the case in the wingless Psocids, and a pair of large wings or tegmina meeting by a straight suture along the back, as is usual in beetles, though quite unknown in existing Psocidae. Another species, Amphientomum paradoxum, has the body and appendages covered with scales like a butterfly or moth; other species, found in gum-copal or still living, have scales on various parts of the body, but not to so great an extent as this amber species. The genus Amphientomum is still represented in Ceylon and elsewhere by living forms; Packard has figured some of the scales;[320] they appear to be extremely similar to those of Lepidoptera or Thysanura. The facts connected with this fauna of amber Psocidae would seem to show that the family was formerly more extensive and important than it is at present; we should therefore expect to find numerous fossil forms in strata of date {398}anterior to that of the amber; but this is not the case, all that is known as to fossil Psocidae being that Scudder has recently ascribed traces of an Insect found in the Tertiary rocks of Utah to this family as a distinct genus.
Fam. V. Perlidae.
Insects of moderate or large size, furnished with four membranous wings; these are usually complexly reticulate; the hind pair are much the larger, and have a large anal area of more simple venation, which becomes plicate when folded. The coxae are small, the legs widely separated. The larvae are aquatic in habits; the metamorphosis is slight.
The Perlidae form a small family of Insects unattractive in their general appearance. The life-history of each individual consists of two abruptly contrasted portions; the earlier stage being entirely aquatic, the later aerial. Hence the Perlidae come into the amphibious division of Neuroptera. The definition we have given above would, except as regards the texture of the front wings and the aquatic habits of the larvae, apply to many Insects of the Order Orthoptera. The Phryganeidae, another {399}family of Neuroptera, have aquatic larvae and wings somewhat similar in form to those of the Perlidae, but the members of the two families cannot be confounded, as the Phryganeidae have hairy front wings and large and contiguous coxae.
The antennae of the Perlidae are long, very flexible, and composed of a very large number of joints. The parts of the mouth vary a good deal. The mandibles and maxillae are usually rather small, and all the parts of the mouth are of feeble consistence or even membranous; the maxillary palpi are, however, well developed and exserted from the mouth, five-jointed. The labium is short and but little conspicuous. The mandibles in some forms are almost membranous, but in other genera they are firmer and are toothed. The labium is composed of a very large mentum, beyond which is a large piece, usually undivided, bearing the four terminal lobes; the three-jointed palpus is seated on the side of the large middle sclerite, which is no doubt of composite nature. Considerable variety as to the lower lip prevails. The head is broad and flat; there is an indistinctly-indicated clypeus, three—more rarely two—ocelli, and on each side an eye neither very large nor perfect. The prothorax is free, and has a flat, margined notum. The meso- and the meta-thorax are large, equal segments. The pro-, meso-, and meta-sternum are large pieces; between the first and second, and between the second and third there is an intervening membrane. The metasternum is much prolonged backwards, and has on each side a peculiar slit; similar orifices exist on the other sterna (Fig. 254, o). Newport, who has examined them in Pteronarcys, says that they are blind invaginations of the integument; he calls them the sternal or furcal orifices.[321] According to this naturalist these very peculiar openings pass into the body "as strong bone-like tubes, diverging from the axis to the periphery of the body in the immediate vicinity of some of the principal tracheae, but that they do not in any way communicate with them, as they terminate abruptly as caecal structures." He thinks them analogous with the endo-skeleton of other Insects; a view which cannot be considered sufficiently established. Laboulbène states[322] that when Perla parisina is seized and placed on its back, it does not move, but emits a liquid at the base of the articulation of the legs. {400}This suggests that it may come from these sternal orifices. The abdomen consists of ten dorsal plates, the first being short, and of nine ventral; the dorsal plates are much more ample transversely than the ventral. Frequently the hind body is terminated by two long, many-jointed cerci, looking like antennae. The coxae are small, not prominent, and are directed outwards. The legs are slender, the tibiae often grooved. The tarsi are three-jointed, terminating in two claws and a more or less distinct pad. In the genus Isopteryx an auditory organ has been described as existing in the legs, in a position similar to that of the analogous structures in Termitidae and Blattidae. The wings when closed repose flat on the back, and fold and overlap so that only one is seen (Fig. 251); in this state the costal portion of each front wing is turned downwards, so as to protect to some extent, the sides of the body.
The early stages are known, but have not been described minutely, and there appears to be very little information as to the youngest life. All the species are, when immature, aquatic in their habits; the larvae greatly resemble the perfect Insects in form, though differing in not possessing wings and in the ocelli being merely opaque spaces. They have rather large compound eyes; the future wings are represented by lobe-like prolongations—varying in length according to age—of the meso- and meta-notum. In the Nemourae the cerci are absent in the imago though present in the young. The larvae of Perlidae are carnivorous {401}and are able to swim well, the legs being provided with abundant swimming hairs; they, however, as a rule, prefer to walk at the bottom of the pool, or on rocks or boulders in the water they live in.
One of the most peculiar features of the Perlidae is their respiratory system. Unfortunately the greatest differences of opinion have prevailed on various matters in connexion with this subject, and there are several points about which it is not possible at present to express a decided opinion.
The larvae have no stigmata; it appears to be generally agreed that there is in them no means of admitting air to the tracheal system by means of orifices. Some breathe entirely through the integument, the process being aided by the accumulation of tracheae at the spots where the breathing orifices should be, and where the integument is more delicate. Others, however, possess gills in the form of protruded bunches of filaments, connected with tracheae in the manner shown in Fig. 253. These filamentous branchiae occur in numerous species of the family, and are situate on various parts of the body, but many species are destitute of them in genera, other members of which possess the filaments. In some Nemourae instead of bunches of filaments there are tubular projections on the prothoracic segment; and in Dictyopteryx signata similar structures occur even in the cephalic region, Hagen stating[323] that there exists a pair on the submentum and another on the membrane between the head and the thorax. In the imago state, stigmata are present in the normal fashion, there being two thoracic and six abdominal pairs. In several species the filaments persist in the imago, so that in these cases we meet with the curious condition of the coexistence of branchiae with a well-developed and functionally active system of spiracles; this is the more curious because the creatures usually have then nothing to do with the water, it having been ascertained that in these cases the species live out of the water as other terrestrial and aerial Insects do. These instances of persistence {402}of branchiae during the aerial life have been the source of some perplexity; the condition was shown to exist in Pteronarcys by Newport, and has since been demonstrated in various other forms. Newport believed that the imago of Pteronarcys breathes by means of the gills, although it lives out of the water and possesses spiracles; and he informs us that Mr. Barnston observed the Insect when on the wing "constantly dipping on the surface of the water." Hence Newport concluded that Pteronarcys in the winged state is "an amphibious animal." That a winged Insect should live in the air and yet breathe by means of gills would be truly extraordinary, and there can be little doubt that Newport's idea was erroneous. Hagen[324] was able to examine living imagos of the species in question. He found that they avoided the water, and though he placed some individuals therein, yet they did not use the gills. He also informs us that the branchiae have, during life, a shrivelled appearance, indicating that they are not functionally active, but are merely useless organs carried over to the imago from the previous instar, in which they were truly the means of obtaining air. Hagen also ascertained that the spiracles of the imago are in a normal state, being adapted for breathing, even as far back as the seventh abdominal segment.
Fig. 254.—Under side of body of Pteronarcys regalis, imago. (After Newport.) g, Tracheal gills; o, sternal orifices.
Great difference of opinion has prevailed as to the relations of the branchiae to the stigmata, it having been contended that the falling off of some of the branchiae left the stigmatic orifices. The facts appear to be only consistent with the conclusion that the two are totally independent organs. This subject has been investigated by Palmén,[325] who finds that in Perlidae—contrary to what occurs in may-flies—the species are either entirely destitute of gills, or these organs are persistent throughout life. It is not to be inferred from this that the gills in the {403}perennibranchiate Perlidae are as conspicuous as they are in the exceptional Pteronarcys: for it appears that at the final moult the gills usually become very much contracted and concealed by the new integument; in some cases they merely appear as slight prominences in the neighbourhood of the stigmata.
Pictet, Dufour, Newport, and Imhof[326] have studied the internal anatomy. The alimentary canal is remarkable for the enormous oesophagus; there is no distinction between this and the crop. A proventriculus is quite absent, and there are no chitinous folds in the position it usually occupies. The true stomach is small, and only commences in the fourth abdominal segment. It has a prolonged lobe on each side in front, and in addition to this eight sacs; thus there are formed ten diverticula, fastened to the posterior part of the oesophagus by ligaments. The terminal portion of the stomach is small, and apparently only distinguished from the short intestine by the point of insertion of the Malpighian tubes; these vary in number from about twenty to sixty. There are two pairs of large salivary glands. In Pteronarcys the caecal diverticula of the stomach are wanting. In some Perlidae the terminal parts of the gut are more complex than in Perla maxima; Newport figures both an ilium and colon very strongly differentiated, and states that these parts differ much in Perla and Pteronarcys. According to him the stomach is embraced by a network of tracheae, and Imhof tells us that he found the stomach to contain only air.
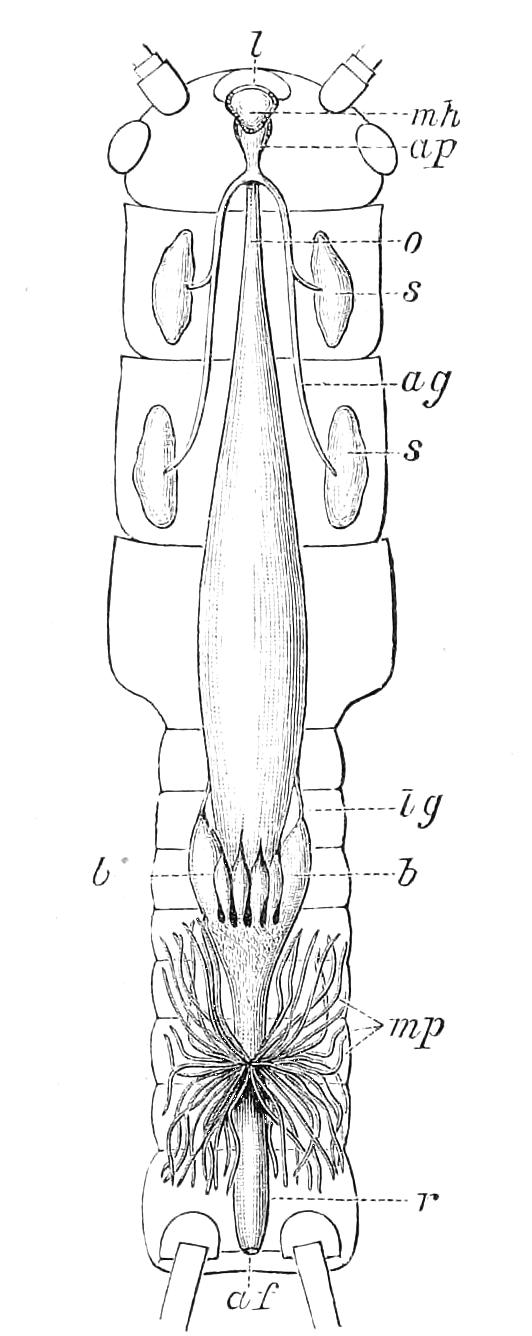
Fig. 255.—Alimentary canal and outline of body of Perla maxima. (After Imhof.) l, Upper lip; mh, buccal cavity; ap, common termination of salivary ducts; o, oesophagus; s, salivary glands; ag, duct of salivary gland; b, anterior diverticula of stomach; lg, their ligaments of attachment; mp, Malpighian tubes; r, rectum; af, anal orifice.
The brain is small, but, according to Imhof, consists of four amalgamated divisions; the infra-oesophageal ganglion is small, {404}and placed very near the brain. There are three thoracic and six abdominal ganglia on the ventral chain. The nerves to the wings are connected with the longitudinal commissures of the ventral chain by peculiar, obliquely-placed, short commissures. The reproductive glands are peculiar, inasmuch as in each sex the pair of principal glands is connected together in the middle. The testes thus form an arch consisting of a large number of sub-spherical or pear-shaped follicles; the vasa deferentia are short in Perla maxima, and there are no vesiculae seminales; the ejaculatory duct is divided into three parts by constrictions. In Pteronarcys and in Perla bicaudata, according to Newport and Dufour, the vasa deferentia are very long and tortuous, and there are elongate vesiculae seminales. The arrangement of the extremely numerous egg-tubes is analogous to that of the follicles of the testes, so that, as Dufour says, there is but a single ovary; connected with the short, unpaired portion of the oviduct, there is a large receptaculum seminis, and near the terminal orifice of the duct there is in P. maxima an eight-lobed accessory gland.
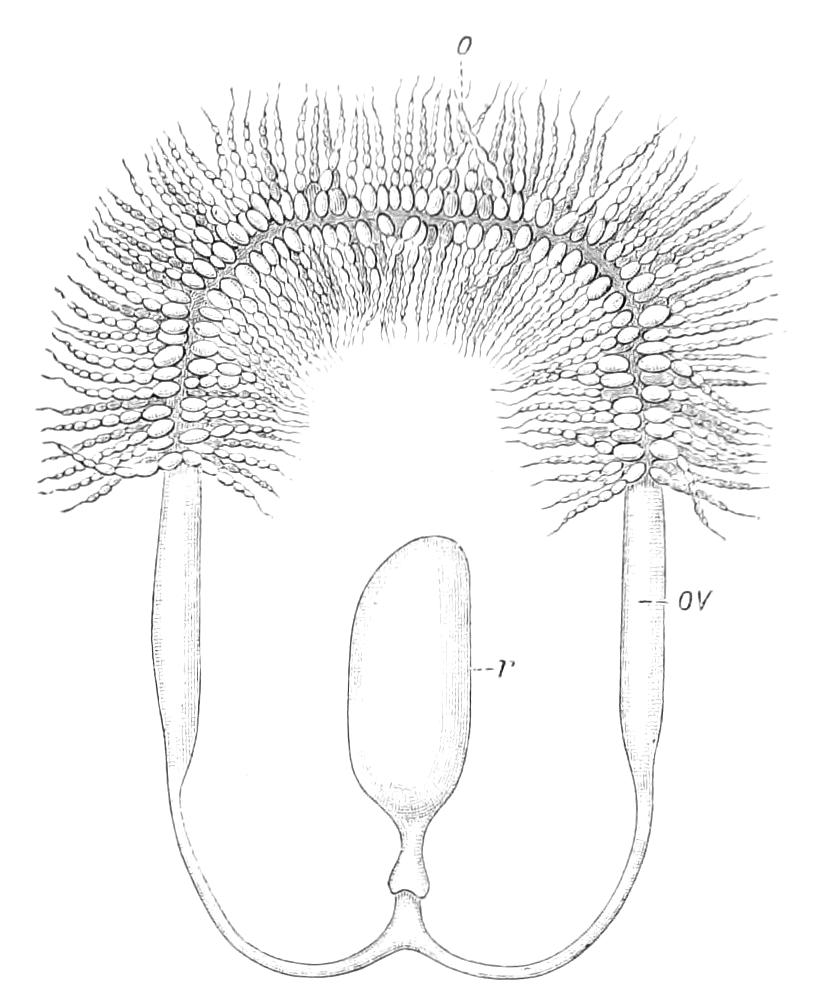
Fig. 256.—The pair of united ovaries of Perla maxima: o, egg-tubes; ov, oviduct; r, receptaculum seminis concealing the orifice of the duct and an accessory gland.
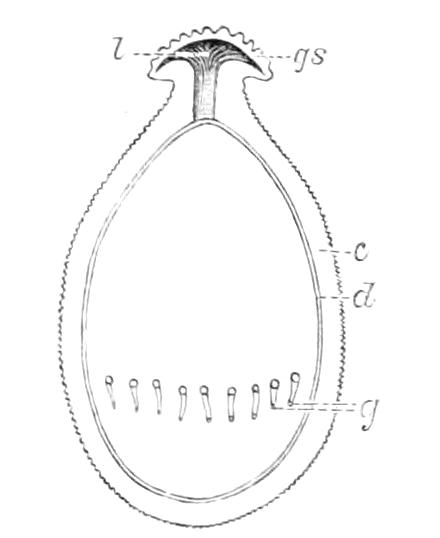
Fig. 257.—Egg of Perla maxima. (After Imhof.) c, chorion; d, oolemn; gs, glass-like covering of micropyle apparatus; l, cavity under same; g, canals penetrating chorion.
The eggs are produced by Perlidae in enormous numbers: they are rather small, but peculiar in form, and possess at one extremity a micropyle apparatus, covered by a glassy substance through which Imhof could find no orifice. On the other hand, the chorion on another part of the egg is perforated by several canals.
The Perlidae being of aquatic habits in their early stages, and, notwithstanding their ample wings, very poor adepts in the art of flying, are rarely found at any considerable distance from their native element. They are specially fond of running water, and delight in the neighbourhood of waterfalls, or other spots where the current is broken by obstacles so that a foaming water results. It is probable that the larvae which breathe by means of gills find an advantage in living in strongly-aerated water. Mountain streams and torrents are therefore specially affected by them; but Pictet informs us that they do not like the waters descending from glaciers. The food of the larvae is believed to be chiefly young may-flies, or other small, soft creatures, and it may possibly be owing to the absence of these that the Perlidae do not affect the glacier streams. Although Perlidae are remarkable for their capacity for enduring cold, it is possible that they may require warmth of the water at some period of their development, and this the glacier-streams cannot offer to them. They are among the earliest Insects to appear in the spring in Europe. Mr. Barnston says that on the Albany river in Canada the nymph of Capnia vernalis comes up frequently in the cracks of the ice and casts its skin there; "it frequently comes up when the thermometer stands at freezing." Of Nemoura glacialis, which inhabits similar localities, he says that "it appears in the spring (end of March or beginning of April) when the ice becomes honeycombed, and even before then, at the same time as Capnia vernalis. It pairs in the crevices of decaying ice. The male has long antennae, and his wings are generally rumpled as if glued together." Newport entertained the idea that those Perlidae that live at low temperatures are of lower organisation than the other forms of the family.
It is a remarkable fact that several Perlidae frequently have—like Nemoura glacialis—the wings of the male much reduced in size; this being the contrary of the rule that usually prevails among Insects to the effect that, when there is a difference in the powers of flight, or even in the size of the wings, it is the male that is superior. Mr. J. J. Lister met with a very interesting Perlid at Loch Tanna in Arran at the beginning of April 1892. In this Insect, which is, according to Mr. M‘Lachlan, a form of Isogenus nubecula, the wings of the female (Fig. 258, B) are reduced to a size much less than those of ordinary {406}Perlidae, while those of the male (Fig. 258, A) are mere useless rudiments. Morton has pointed out that in Scotland more than one species of Taeniopteryx occasionally produces micropterous males, and he associates this phenomenon with the early time of their appearance "almost in winter."[327] In Nemoura trifasciata this reduction of the wings takes another but equally curious form; the hind wings of the male being long enough to cover the body, while the anterior pair are reduced to mere rudiments.
Fig. 258.—Isogenus nubecula, Loch Tanna. A, Male; A', wings of male more magnified; B, wings of female.
The phenomena of micropterism in Perlidae are well worthy of more detailed investigation. Mr. Morton informs the writer that the male of Perla maxima (Fig. 251) in North Britain has the wings so short that they cannot be of any use as organs of flight. In Central Europe the wings are ample, as shown in our figure. In Perla cephalotes the male is short-winged in both Britain and Central Europe; of the male of Dictyopteryx microcephala only the micropterous form is known to exist. In Isogenus nubecula (Fig. 258) it appears that the wings of the female are always more ample than those of the male of the same locality, and that local micropterism affects the two sexes unequally. Within the Arctic circle this Insect is usually of the Scotch form, though the male there occasionally has more ample wings.
It has been observed that in some Perlidae the eggs, after they have been extruded, are carried about by the female; for what reason is not at all known. They are said to be enclosed in a membranous capsule at the apex of the abdomen. The number of eggs deposited is sometimes very large, amounting to five or six thousand, and they are often of very minute size.
About twenty-four species of Perlidae occur in Britain.[328] The {407}species from all parts of the world existing in collections probably scarcely exceed two hundred. The insignificance of this number is no doubt chiefly due to the fact that these unattractive Insects are rarely captured by collectors, and are so fragile that unless good care is taken of them, specimens soon go to destruction after being dried. Perlidae are known to occur in most parts of the world, so that the number of species really existing may reach two or three thousand. They are known to anglers as stone-flies and creepers and are a favourite bait for trout.
The family in its character comes near to the Orthoptera, especially to the more simple forms of Phasmidae, but the two groups differ in the texture of the front wings and in the structure of the mouth-parts, as well as in the different proportions of the mesothorax and metathorax. According to Pictet, in the Australian genus Eusthenia the trophi (Fig. 259) approach nearer to those of the Orthoptera, so that it appears possible that a more intimate connexion will be found to exist as more forms are discovered. Of the groups we include in Neuroptera, Perlidae are in structure most allied to Sialidae, but the development in the two groups exhibits very important distinctions. Brauer treats the Perlidae as forming a distinct Order called Plecoptera, a name applied to the family by Burmeister many years ago.
Several species of Perlidae, considered to belong to existing genera, have been found in amber. A fossil from the Eocene deposits in the Isle of Wight and another from the Miocene of Continental Europe are referred to the family. Brauer has recently described[329] some fossils from the Jurassic formation in East Siberia as forming three genera, now extinct, of Perlidae.
Brongniart informs us[330] that several fossils have been found {408}in the Carboniferous strata of Commentry that justify us in asserting that allies of Perlidae then existed. He considers these Carboniferous Insects to have belonged to a separate family, Protoperlides. The fragments are, however, so small that we must await further information before forming a definite opinion as to these Protoperlides.
AMPHIBIOUS NEUROPTERA CONTINUED—ODONATA, DRAGON-FLIES
Fam. VI. Odonata—Dragon-flies.
(LIBELLULIDAE OF SOME AUTHORS)
Elongate Insects with very mobile head and large eyes, with small and inconspicuous antennae ending in a bristle; with four elongate wings sub-equal in size and similar in texture, of papyraceous consistency and having many veinlets, so that there exists a large number of small cells. All the legs placed more anteriorly than the wings. The earlier stages of the life are aquatic; there is great change in the appearance of the individual at the final ecdysis, but there is no pupal instar.
The dragon-flies form a very natural and distinct group of Insects. All the species are recognised with ease as belonging to the family. They are invariably provided with wings in the perfect state, and many of them are amongst the most active of Insects. Their anatomy is, in several respects, very remarkable.
The head is large and is concave behind; it is attached to the thorax in such a way that it rotates on two cervical sclerites that project forwards, and in some cases almost meet in a point in front; hence it possesses extreme mobility, the power of rotation being very great.
The eyes are always large; in some cases they are even enormous, and occupy the larger part of the area of the head: the upper facets of the eye are in many cases larger than the lower, and in a few forms the line of division is sharply marked transversely. There are three ocelli, which, when the size of the compound eyes is not too great, are placed in the usual {410}manner as a triangle on the vertex; but in the forms where the compound eyes are very large the portion of the head between is, as it were, puffed out so as to form a projection just in front of where the eyes meet, and one ocellus is then placed on each side of this projection, an antenna being inserted quite close to it; the third ocellus is placed in front of the projection we have mentioned, by which it is often much concealed; this anterior ocellus is in some cases of unusually large size, and oval or transverse in form.
The parts of the mouth are very peculiar, especially the lower lip: we will briefly allude to its characters in the highly modified forms, premising that in the smaller and less active species it is less remarkable. The Libellulidae are carnivorous, their prey being living Insects which are captured by the dragon-fly on the wing; it is believed that the mouth is largely instrumental in the capture, though the flight of these Insects is so excessively rapid that it is difficult, if not impossible, to verify the action of the mouthpieces by actual observation.
Fig. 261.—A, Maxilla of Libellula quadrimaculata; B, labium of Aeschna grandis. p, p′, Palpus; a, terminal spine of palpus; c, cardo; t, stipes; s, squama; le, outer lobe of maxilla, partly covered by, li, inner lobe; m, mentum; r, intervening lobe. (After Gerstaecker.)
For the purpose of securing the prey a mouth that can change its capacity to a considerable extent and with rapidity is a desideratum, and these qualities are present in the mouths of those Libellulidae that capture their prey while hawking. The upper lip is very mobile, is pendent, and closes the mouth above, while the lower lip entirely closes the under part by means of two mobile plates; these in some forms (Libellula) meet together in the mesial line, while in others a third plate separates them in the middle (Fig. 261, B, li). These plates are, according to Gerstaecker's view,[331] portions of the much changed labial palpi, the part that separates them in Aeschna being the inner lobes of the labial maxillae; in Libellula, where the dilated and valve-like joints of the palpi meet in the middle line, the labial lobes remain small and are overlapped by the dilated portions of the palpi. The maxillae proper (Fig. 261, A) are less peculiar, their chief character being that the inner and outer lobes are not separated, and that the palpus is of only one joint. Some entomologists take, however, another view of this structure, looking on the palp-like outer part (p of our figure) as the true outer lobe of the maxillae, the palpus proper being in that case considered to be entirely absent. The mandibles are very powerful, and armed with largely developed teeth. In the interior of the mouth there is a large, free, semi-membranous lingua, the posterior part of its delicate inferior lamina being connected with the mentum; the upper lamina of the lingua is stronger and is pilose. The antennae of the dragon-flies are always small, and consist of two stouter joints at the {412}base, and a terminal part which is very slender and pointed, and formed of four or five joints.
The prothorax is always small; the pronotum is distinct, though in some forms it is quite concealed in the concavity of the back of the head; the sternum is small; the anatomy of the pleura and basal pieces of the legs is obscure.
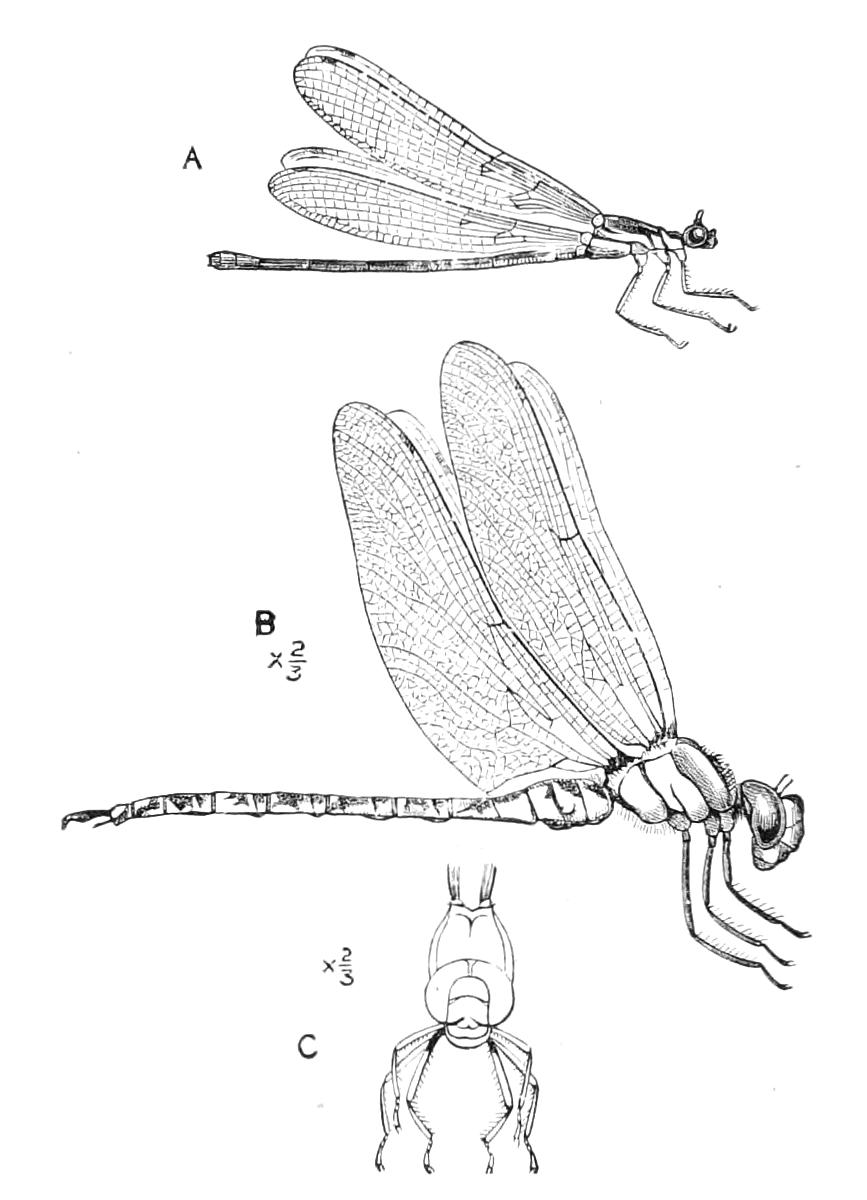
Fig. 262.—A, Agrion pulchellum, natural size; B, Aeschna cyanea, profile; C, same from front to show position of legs. ⅔ natural size.
The meso- and meta-thorax are very intimately combined, and their relations are such that the former is placed much above the latter. This peculiarity is carried to its greatest extent in some of the Agrioninae (Fig. 262, A), where not only are the wings placed at a considerable distance behind the three pairs of legs, but also the front pair of wings is placed almost directly above the hind pair. In the Anisopterides these peculiarities are much less marked (Fig. 262, B), nevertheless even in them the three pairs of legs are placed quite in front of the wings. This peculiar structure of the wing-bearing segments is accompanied by an unusual development of the pleura, which, indeed, actually form the larger part, if not nearly the whole, of the front region of the dorsal aspect of these two segments. We shall not enter into more minute particulars as to the structure of the thorax, for difference of opinion prevails as to the interpretation of the parts.[332] The abdomen is remarkable for its elongation; it is never broad, and in some genera—Mecistogaster, e.g.—it attains a length and slenderness which are not {413}reached by any other Insects. It consists of ten segments and a pair of terminal calliper-like or flap-like processes of very various sizes and forms.
The wings of the dragon-flies are usually transparent and provided with a multitude of small meshes. The hind wings are about as large as the front pair, or even a little larger; the main nervures have a sub-parallel course, and are placed in greater part on the anterior region of each wing. The relations of the more constant nervures and the cells of which they are parts form a complex subject, and are amongst the most important of the characters used in classifying these Insects. The wings are always elongate in comparison to their breadth and have no folds; they are held partially extended, or are placed so as to project backwards, or backwards and outwards. They exhibit another peculiarity, inasmuch as the front or costal margin is slightly uneven before or near the middle, giving rise to an appearance such as might result from the breaking and subsequent mending of the marginal rib at the spot in question, which is called the nodus. In some forms a peculiar character exists in the shape of a small opaque space called the membranule, lying close to the body of the Insect in the anal area of the wing, as shown in Fig. 260.
The legs are slender and are chiefly remarkable for the beautiful series of hair-like spines with which they are armed, and which in some forms (e.g. Platycnemis, Fig. 264) are of considerable length. We believe that the legs are of great importance in capturing the prey, they being held somewhat in the position shown in Fig. 262, C. The tarsi are three-jointed. In the male of Libellago caligata the legs exhibit a remarkable condition, the tibiae being dilated, and on the upper side of a vivid red colour, while below they are white. This coloration and form are each unusual in the family. The male of Platycnemis pennipes, a British species (Fig. 264), shows a similar dilatation of the tibiae, but to a less extent and without any great difference in the colour of the two faces of the dilatation. This dilatation reaches its maximum in Psilocnemis dilatipes M‘Lach. The position of the legs in relation to the other parts of the body is peculiar to the dragon-flies; the legs seem to be unfit for walking, the Insects never using them for that purpose.
Several peculiarities in the internal anatomy deserve notice. The alimentary canal in Libellula is about as long as the body, the oesophagus and chylific stomach being elongate, while the intestine is short and divided into only two parts; there is no definite proventriculus. The Malpighian tubules are shorter than usual; they are about forty in number. The male has no vesiculae seminales; the vasa deferentia are elongate, and the ejaculatory duct is very short, being in fact merely a common sinus formed by the terminations of the vasa deferentia. The opening of this duct is situated on the penultimate ventral plate; the organs of intromission are, however, placed much anterior to this, on the under side of the second segment. The mode in which the fertilising fluid is transferred from the ninth to the second segment is not well understood, but it is known that the abdomen is flexed by the Insect so as to bring the ninth ventral plate into contact with the second. The three thoracic ganglia of the nervous chain are all contiguous, though not completely amalgamated; the abdominal ganglia are seven in number, and are all separated, the terminal one being larger than the others. Dufour, after repeated dissections, was unable to find any salivary glands, but Olga Poletajewa[333] states that they exist.
The Odonata must be ranked among the most highly-organised Insects so far as external structure and powers of locomotion are concerned; the peculiar modifications of the thoracic segments and the relative positions of the wings and legs mark a great departure from the normal type of Insect structure. Their prey consists of living Insects, which they capture on the wing by their own superior powers of flight. They destroy a great many Insects, their appetite for food being, as in the cases of the Mantidae and of the tiger-beetles, apparently almost insatiable. They are admirably constructed for the purposes of their predatory lives; they fly with great swiftness and change the direction of their flight with admirable facility. They are, however, dependent on sunshine, and conceal themselves in dull and cloudy weather. The larger Insects of the family belong to the division Anisopterides (Fig. 260, Anax formosus) and some of these may, in our own country, usually be seen, in the bright sunshine of the summer and autumn, engaged in hawking in their favourite haunts. Places where other Insects {415}abound are naturally those most frequented; the glades of woods, country lanes and hedge-sides, the borders of streams and the margins of sheets of water are the places they most affect. They inspire the rustics with some feeling of fear, and hence have received the name of "horse-stingers," and in North America are called "devil's darning-needles." The aversion to dragon-flies may perhaps be due to their appearance, which is certainly, in the case of some of our species of Aeschna, Cordulegaster, and Gomphus, very remarkable, consisting of a dark ground-colour with bars and spots of vivid green or yellow, giving, it must be admitted, a peculiar, even savage appearance to the Insects. Whatever the reason may be, they are, it is certain, held in much fear, and it is difficult to induce a country lad to touch one even when it is captured and held by another person. The idea of dragon-flies being dangerous to anything but their Insect victims is, however, entirely erroneous; they may be captured and handled without their inflicting any injury. It is probable that the life of the imago may endure for several weeks if not months. It is known that Sympycna fusca—a common European though not British dragon-fly—hibernates in the imago state.
In the case of the large dragon-flies we have mentioned, each individual appears to have a domain, as it were, of its own. Westwood tells us that he has seen what he believed to be the same individual hawking daily for several weeks together over a small pond. The writer observed a specimen of Cordulegaster annulatus to frequent a particular bush, to which it returned—frequently to the same leaf—after an excursion in search of food. The way in which these Insects actually seize their prey has not yet been made clear; it is certain that they capture flying Insects, and it seems most probable, as we have already said, that this is done by means of the legs. These, as we have said, are inserted so as to be very near to the mouth; they are directed forwards, and are held bent at right angles so as to form a sort of net, and are armed with a beautiful system of fine spines; it is probable that if the dragon-fly pursue an Insect on the wing and strike it with the trap, formed by its six legs (Fig. 262, C), then these immediately come together under the mouth, so that the victim, directly it is captured by the leg-trap of its pursuer, finds itself in the jaws of its destroyer. It is perhaps impossible to {416}verify this by actual observation, as the act of capture and transfer is so very brief and is performed in the midst of a rapid dash of flight, but it seems more probable that the prey is first struck by the legs than that the mouth is the primary instrument of capture. The excessive mobility of the head permits the victim to be instantly secured by the mouth, and the captured fly is turned about by this and the front pair of legs, and is nipped rapidly so that the wings and drier parts fall off; the more juicy parts of the prey are speedily squeezed into a little ball, which is then swallowed, or perhaps we should rather say that the mouth closes on it, and submits it to further pressure for the extraction of the juices. We have already noted that many of these large and active dragon-flies, particularly in the Libellulinae and Aeschninae, have their eyes distinctly divided into two parts, the facets in the lower part of the eye being different from those of the upper part. Exner considers[334] that the upper division is for the perception of movement, the lower for the perception of the form of resting objects. Plateau thinks[335] that the dragon-flies perceive only movement, not form.
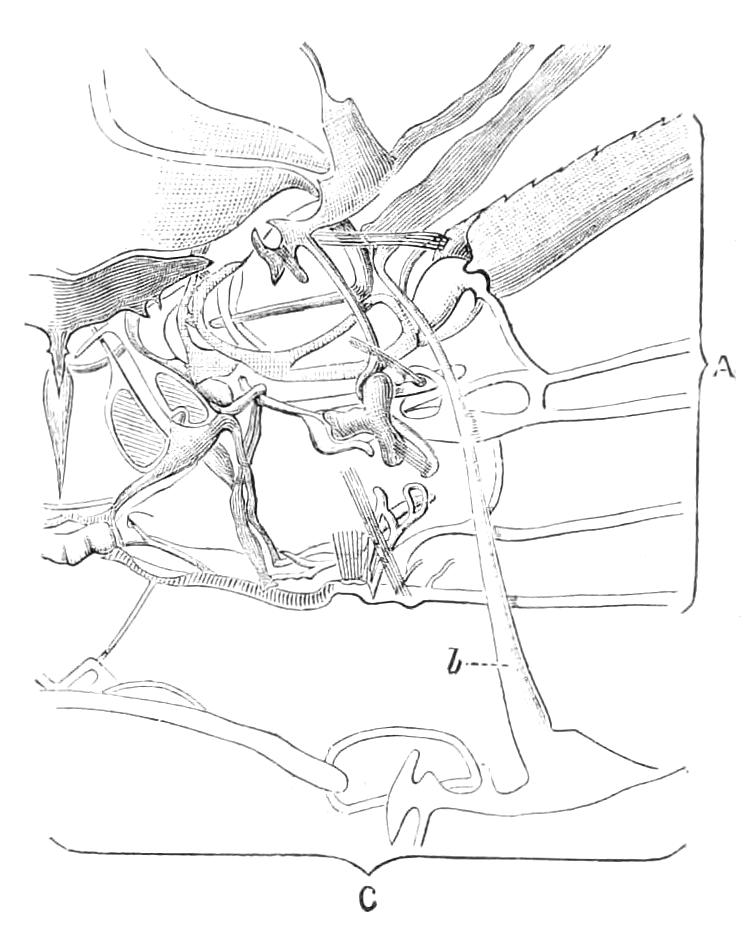
Fig. 263.—Inner view of a portion of the left side of body of Libellula depressa, showing a part of the mechanism of flight, viz. some of the chitinous ridges at base of the upper wing, and some of the insertions of the tendons of muscles. A, line of section through base of upper wing, the wing being supposed to be directed backwards; C, upper portion of mechanism of the lower wing; b, lever extending between the pieces connected with the two wings. (After von Lendenfeld.)
The splendid acts of flight of the Anisopterid Odonata are accomplished by the aid of a complex arrangement of chitinous pieces at the bases of the wings (Fig. 263). In Insects with considerable powers of flight the hind wings are usually subordinate in functional importance to the anterior, to which they are attached by a series of hooks, or some other simple mechanism, on the wings. {417}In the Odonata the two wings of each pair are quite free, but they are perhaps brought into correlative action by means of a lever of unusual length existing amongst the chitinous pieces in the body wall at the base of the wings (Fig. 263, b). The wing muscles are large; according to von Lendenfeld[336] there are three elevator, five depressor, and one adductor muscles to each wing: he describes the wing movements as the results of the correlative action of numerous muscles and ligaments, and of a great number of chitinous pieces connected in a jointed manner.
Amans[337] has suggested that the mechanism of flight of the dragon-fly would form a suitable model for a flying-machine, to be propelled by electricity.
The Zygopterides—the second of the two divisions of the Odonata—are Insects different in many respects from the large and robust Anisopterides. The division comprises the delicate Insects called "demoiselles," damsel-flies, by the French (Fig. 262, A, and Fig. 264). Great power of flight is not possessed by these more fragile Insects; they flit about in the most gentle and airy manner from stem to stem of the aquatic plants and grasses that flourish in the localities they love. To this group belong the fairy-like Insects of the genus Calepteryx, in which various parts of the body and wings are suffused with exquisite {418}metallic tints, while sometimes the two sexes of one species have differently coloured wings. The smallest and most delicate dragon-flies that are known are found in the tropics; some of the genera allied to Agrion consist of Insects of extraordinary fragility and delicacy.
Although the mature Odonata are so pre-eminently endowed for an aerial and active life, yet in the earlier stages of their existence they are very different; they are then, without exception, of aquatic habits; though carnivorous also in this period of their existence, they are sluggish in movement, lurking in concealment and capturing their prey by means of a peculiar conformation of the mouth, that we shall subsequently describe. Their life-histories are only very imperfectly known.
The eggs are deposited either in the water or in the stem of some aquatic plant, the female Insect occasionally undergoing submersion in order to accomplish the act. The young on hatching are destitute of any traces of wings (Fig. 265), and the structure of the thoracic segments is totally different from what it is in the adult, the rectal respiratory system (Fig. 265, x) to which we shall subsequently allude, being, however, already present. The wings are said to make their first appearance only at the third or fourth moult. At this time the pleura of the second and third thoracic segments have grown in a peculiar manner so as to form a lateral plate (Fig. 266, B, shows this plate at a later stage), and the wing-pads appear as small projections from the membranes at the upper margins of these pleural plates (Fig. 266, A, B). The plates increase in size during the subsequent stadia, and meet over the bases of the wing-pads, which also become much longer than they were at first. The number of moults that occur during growth has not been observed in the case of any species, but they are believed to be numerous. There is no pupa, nor is there any well-marked quiescent stage preceding the assumption of the winged form at the last ecdysis, although at the latter part of its life the nymph appears to be more inactive than usual. When full grown, the nymph is more like the future perfect Insect than it was at first, and presents the appearance shown in Figs. 266 and 270. At this stage it crawls out of the water and clings to some support such as the stem or leaf of an aquatic plant; a few minutes after doing so the skin of the back of the thoracic region splits, and the imago emerges from the nymphal skin.
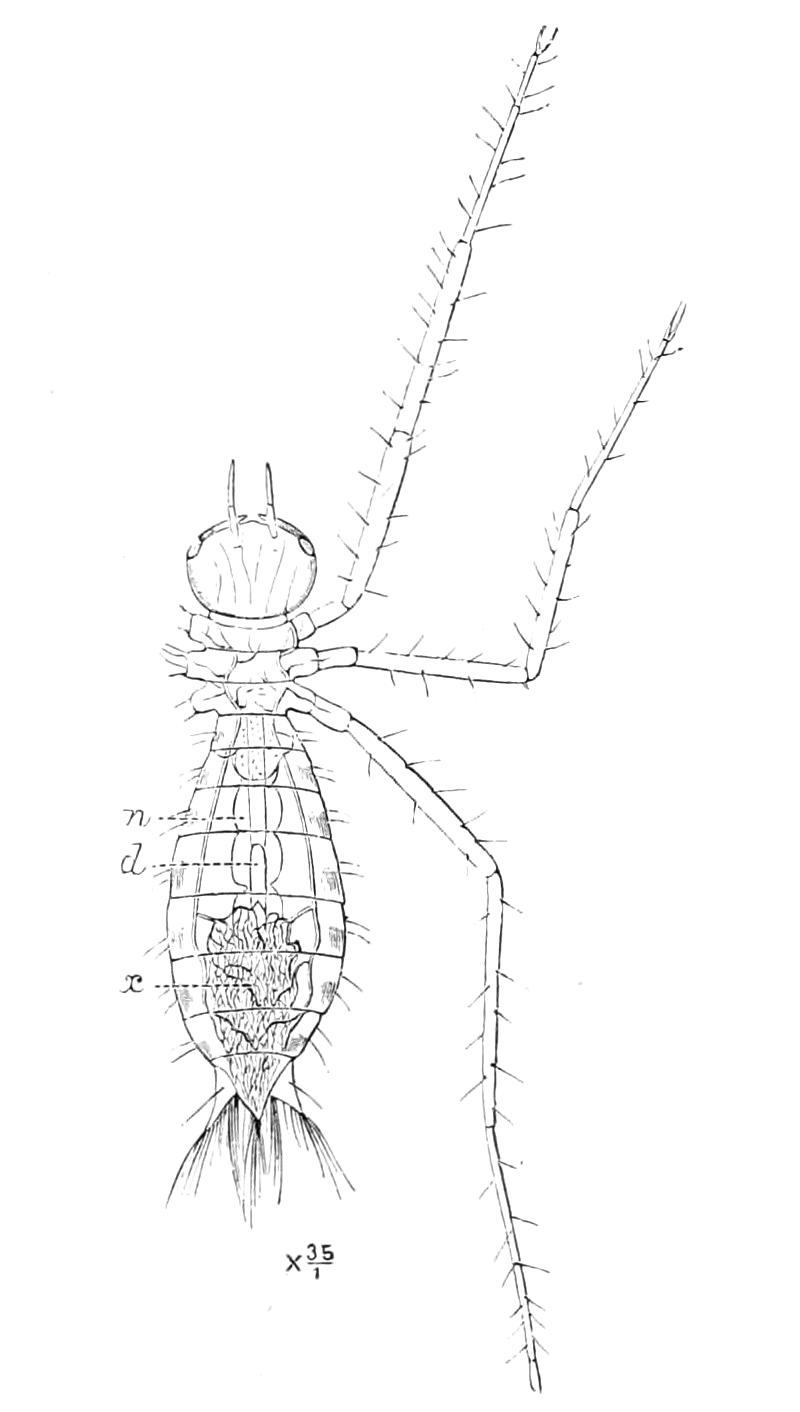
Fig. 265.—Larva of Diplax just hatched. n, a ganglion of the ventral chain; d, dorsal vessel; x, tracheal network round rectum. (After Packard, P. Boston Soc. xi. 1868, p. 365.)
The nymphs never have the body so elongate as the perfect Insect, the difference in this respect being frequently great, and the nymphs of the subfamily Libellulinae being very broad (Fig. 266, nymph of Ictinus sp.); consequently the creature on emergence from the nymph-skin is very much shorter than it will soon become. Extension begins to take place almost immediately; it has been thought by some that this is accomplished by swallowing air; this is, however, uncertain. At first the wings have only the length of the wing-pads of the nymph, and their apical portion is an unformed mass. The colour of the perfect Insect is not present when the emergence takes place. The wing grows quickly until the full length is attained. In the genus Agrion {420}the expansion of the wings is accompanied by frequent elevations and depressions of the body, and occupies an hour or so; the elongation of the abdomen is not so soon completed, and its brilliant colours do not appear for several hours.
The mouth of the nymph bears a remarkable structure called the mask (Fig. 268). It is apparently formed by a backward growth of the bases of the labium and lingua, a hinge being formed between the two at the most posterior point of their growth. The prolonged portions of these parts are free: usually the mask is folded under the head, but it can be unfolded and thrust forward, remaining then attached to the head by means of the more anterior parts of the lingua and by the maxillae, the whole of the elongate apparatus being, when used, extended from this anterior part of its attachment. The front parts of the labium form a prehensile apparatus armed with sharp teeth, so that the structures make altogether a very effectual trap, that can be extended in order to secure the prey.
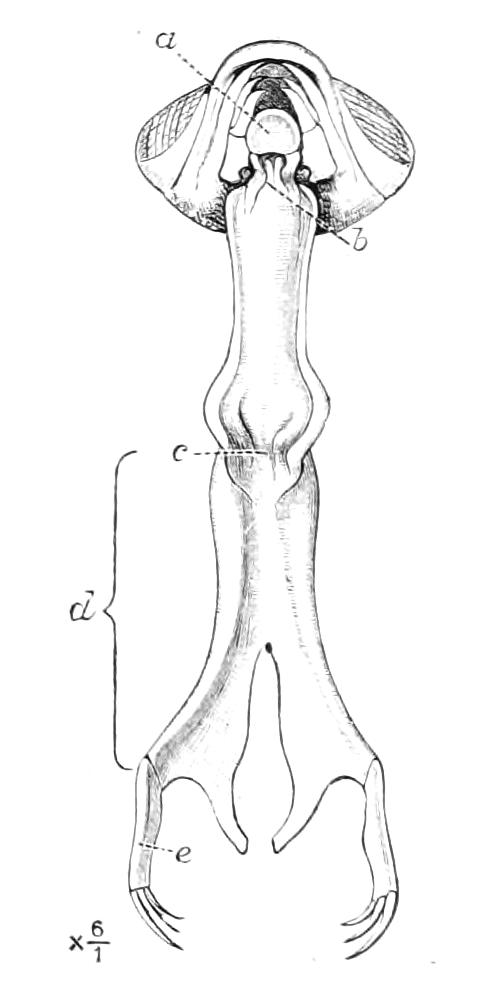
Fig. 268.—Under side of head of Calepteryx virgo, nymph, with mask unfolded. a, Lingua; b, line from which the mask swings; c, line of doubling up; d, lower lip proper; e, articulated lateral processes thereof.
The fact that the dragon-fly passes suddenly, in the middle of its existence, from an aquatic to an aerial life, makes the condition of its respiratory organs a subject for inquiry of more than usual interest. Réaumur was of opinion that the nymph was, in spite of its aquatic existence, provided with an extensive system of stigmata or orifices for breathing air; this was, however, denied by Dufour, and his opinion seemed to be supported by the fact that other means of obtaining air were discovered to exist in these nymphs. The inquiries connected with the respiration {421}of Odonata are still very incomplete, but some interesting points have been ascertained, the most important of which is perhaps the existence in some forms of a respiratory system in connexion with the posterior part of the alimentary canal (Fig. 269). In the nymphs of Anisopterides the system consists of four main tracheal trunks, traversing the length of the body, and by their ramifications and inosculations forming an extensive apparatus. Connected with the four main trunks we have described, there is a shorter pair confined to the abdomen, where it supplies a large number of branches to the walls of the stomach. The dorsal pair of the main tubes give numerous subsidiary branches to the outside of the rectum, and the ventral pair furnish a smaller number. The walls of the gut are penetrated by the branches, which inside the rectum form numerous loops; these, covered by a membrane, project into the interior in the form of multitudinous papillae (Æschninae). In the Libellulinae the papillae are replaced by more flattened processes or lamellae. The structures attain a remarkable development, there being in Aeschna cyanea upwards of 24,000 papillae.
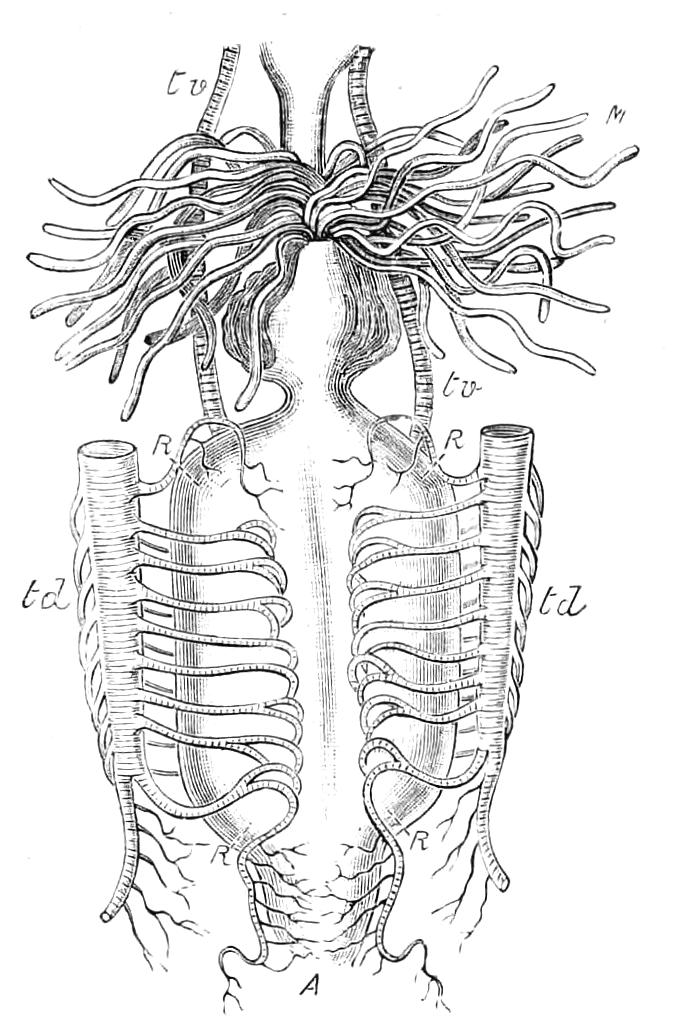
Fig. 269.—Portion of tracheal system of nymph of Aeschna cyanea. R, R, R, R, rectum; A, anus; td, dorsal; tv, ventral, tracheal tubes; M, Malpighian tubes. (After Oustalet.)
These rectal gills obtain air from water admitted into the rectum for the purpose; the extremity of the body being armed with projections of variable form, that can be separated to allow ingress and egress of the fluid, or brought into close apposition so as to close the orifice. The water so taken in can, by some species, be ejected with force, and is used occasionally as a means of locomotion. These rectal branchiae can absorb free air, as well as air dissolved in water. If the fluid in which the creatures are placed has been previously boiled, so as to expel the air from it, the nymphs then thrust the {422}extremity of the body out of the water and so obtain a supply of air.
Oustalet and Palmén state that at the last ecdysis the lamellae, or the papillae, do not disappear, but remain quite empty, and are consequently functionless, while on the tracheal trunks there are developed air vesicles to fit the creature for its aerial career. Hagen says that in Epitheca the whole structure of the gills is shed at the final moult.
The subject of the rectal branchiae of Libellula has been discussed and illustrated by Chun,[338] who states that Leydig has made known that in Phryganea grandis a structure is found connecting the rectal branchiae of Libellula with the rectal glands of some other Insects. We have not been able to find a confirmation of this in the writings of Leydig or elsewhere.
In the nymphs of the Zygopterides the highly-developed rectal branchiae found in the Aeschninae and Libellulinae do not exist, and the respiration seems to be of a complex character. In one division of the Zygopterides—Calepteryginae—rectal gills of an imperfect character are said by Hagen and others to exist.[339] The nymphs of the Zygopterides are provided with three mobile processes at the extremity of the body (Fig. 270); these serve the purposes of locomotion. They are believed to possess also a respiratory function, but this must be of an accessory nature, for the nymphs live after the removal of the processes, and indeed reproduce them; the skin of these processes is harder than is usual in Insect gills. In the nymph of Euphaea—a genus of Calepteryginae found in tropical Asia—there are also external {423}abdominal gills, and in this case respiration may, according to Hagen,[340] take place in four different manners: (1) by ten pairs of stigmata; (2) by lateral branchiae well furnished with tracheae; (3) by caudal branchiae; (4) by rectal branchiae. It is further said that in this Insect the lateral branchiae persist in the imago.
Although the means of respiration of the nymphs have been fairly well ascertained, yet the mode in which the nymph is prepared for the sudden change from the aquatic to aerial life is still obscure, the condition of the stigmata not being thoroughly elucidated. It appears probable, however, that the young nymph has no stigmata; that these organs appear in the course of its development, being at first quite impervious, but becoming—at any rate in the case of the larger and more important pair—open previous to the final ecdysis. We have mentioned the contradictory opinions of Réaumur and Dufour, and will now add the views of some modern investigators. Oustalet says[341] that there are two pairs of spiracles in the nymphs; the first pair is quite visible to the naked eye, and is situate between pro- and meso-notum; it is in the nymph closed by a membrane. The other pair of spiracles is placed above the posterior pair of legs, is small and completely closed. He does not state what stage of growth was attained by the nymphs he examined. Palmén was of opinion that not only thoracic but abdominal spiracles exist in the nymph,[342] and that they are completely closed so that no air enters them; he says that the spiracles have tracheae connected with them, that at each moult the part closing the spiracles is shed with some of the tracheal exuviae attached to it. The breathing orifices are therefore for a short time at each ecdysis open, being subsequently again closed by some exudation or secretion. This view of Palmén's has been thought improbable by Hagen and Dewitz, who operated by placing nymphs in alcohol or warm water and observing the escape of bubbles from the spots where the supposed breathing orifices are situate. Both these observers found much difference in the results obtained in the cases of young and of old nymphs. Hagen concludes that the first pair of thoracic spiracles are functionally active, and that abdominal stigmata exist though {424}functionless; he appears to be of opinion that when the first thoracic stigma is closed this is the result of the abutting against it of a closed trachea. Dewitz found[343] that in the adult nymph of Aeschna the thoracic stigma is well developed, while the other stigmata—to what number and in what position is not stated—are very small. In a half-grown Aeschnid nymph he found the thoracic stigma to be present in an undeveloped form. On placing a full-grown nymph in alcohol, gas escaped from the stigma in question, but in immature nymphs no escape of gas occurred although they were subjected to a severe test. A specimen that, when submitted to the above-mentioned immersion, emitted gas, subsequently moulted, and thereafter air escaped from the spiracle previously impervious. The observations of Hagen and Dewitz are perhaps not so adverse to the views of Palmén as has been supposed, so that it would not be a matter for surprise if Palmén's views on this point should be shown to be quite correct.
The number of species of Odonata or Libellulidae that have been described is somewhat less than two thousand, but constant additions are made to the number, and when the smaller and more fragile forms from the tropics are collected and worked out it will probably be found that the number of existing species is somewhere between five and ten thousand. They are distributed all over the world, but are most numerous in species in the warmer regions, and their predominance in any one locality is very much regulated by the existence of waters suitable for the early stages of their lives.
A good work on the British Odonata is still a desideratum.[344] In Britain about forty-six species are believed to be native. They are said to be of late years less numerous than they used to be. Notwithstanding their great powers of flight, dragon-flies are destroyed by birds of various kinds; several hawks are said to be very fond of them, and Merops persicus to line its nest with their wings. The number of Insects killed by dragon-flies in places where they are abundant must be enormous; the nymphs, too, {425}are very destructive in the waters they inhabit, so that dragon-flies have no doubt been no mean factor in maintaining that important and delicate balance of life which it is so difficult for us to appreciate. The nymphs are no doubt cannibals, and this may perhaps be an advantage to the species, as the eggs are sometimes deposited in large numbers in a limited body of water, where all must perish if the nymphs did not, after exhausting other food, attack one another. Martin, speaking of the Odonata of the Département de l'Indre in France, says:[345] "The eggs, larvae, and nymphs are the prey of several fishes, snakes, newts, Coleoptera, aquatic Hemiptera, and of some diving birds. Sometimes the destruction is on a considerable scale, and one may notice the dragon-flies of some piece of water to diminish gradually in numbers, while the animals that prey on them increase, so that a species may for a time entirely disappear in a particular spot, owing to the attacks of some enemy that has been specially prosperous, and also eager in their pursuit. De Selys found that from a pond filled with carp, roach, perch, and eels, several of the dragon-fly denizens disappeared directly the bream was introduced." On the other hand, there can be little doubt that the nymphs are sometimes injurious to fish; it has been recorded that in a piscicultural establishment in Hungary 50,000 young fishes were put into a pond in spring; in the following autumn only fifty-four fish could be found, but there were present an enormous quantity of dragon-fly nymphs.
Odonata are among the few kinds of Insects that are known to form swarms and migrate. Swarms of this kind have been frequently observed in Europe and in North America; they usually consist of species of the genus Libellula, but species of various other genera also swarm, and sometimes a swarm may consist of more than one species. L. quadrimaculata is the species that perhaps most frequently forms these swarms in Europe; a large migration of this species is said to occur every year in the Charente inférieure from north to south.[346] It is needless to say that the instincts and stimuli connected with these migrations are not understood.
The nymphs are capable, under certain circumstances, of accommodating themselves to very peculiar conditions of life. The Sandwich Islands are extremely poor in stagnant waters, and {426}yet there exist in this remote archipelago several highly peculiar species of Agrioninae. Mr. R. C. L. Perkins has recently discovered that the nymphs of some of these are capable of maintaining their existence and completing their development in the small collections of water that accumulate in the leaves of some lilies growing on dry land. These nymphs (Fig. 271) have a shorter mask than occurs, we believe, in any other Odonata, and one would suppose that they must frequently wait long for a meal, as they must be dependent on stray Insects becoming immersed in these tiny reservoirs. The cannibal habits of the Odonata probably stand these lily-dwellers in good stead; Mr. Perkins found that there were sometimes two or three nymphs of different sizes together, and we may suspect that it sometimes goes hard with the smaller fry. The extension in the length of the body of one of these lily-frequenting Agrions when it leaves the water for its aerial existence is truly extraordinary.
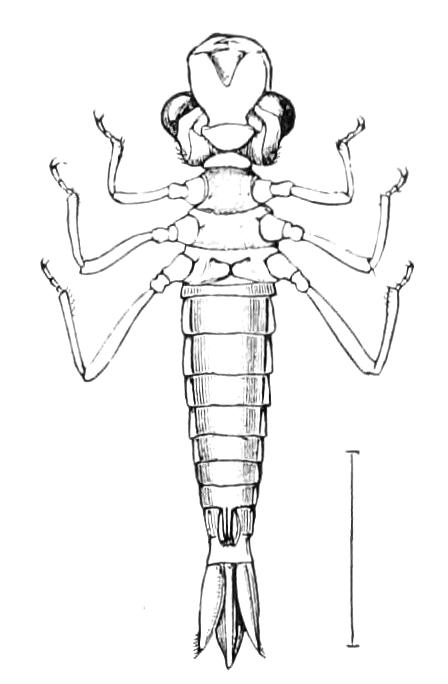
Fig. 271.—Under side of Agrionid nymph, with short mask, living in water in lilies. Hawaiian Islands. × 3.
The Odonata have no close relations with any other group of Insects. They were associated by Latreille with the Ephemeridae, in a family called Subulicornia. The members of the two groups have, in fact, a certain resemblance in some of the features of their lives, especially in the sudden change, without intermediate condition, from aquatic to aerial life; but in all important points of structure, and in their dispositions, dragon-flies and may-flies are totally dissimilar, and there is no intermediate group to connect them. We have already, said that the Odonata consist of two very distinct divisions—Anisopterides and Zygopterides. The former group comprises the subfamilies Gomphinae, Cordulegasterinae, Aeschninae, Corduliinae, and Libellulinae,—Insects having the hinder wings slightly larger than the anterior pair; while the Zygopterides consist of only two subfamilies—Calepteryginae and Agrioninae; they have the wings of the two pairs equal in size, or the hinder a little the smaller. The two groups Gomphinae and Calepteryginae are each, in several respects, of lower development than the others, and authorities are divided {427}in opinion as to which of the two should be considered the more primitive. It is therefore of much interest to find that there exists an Insect that shares the characters of the two primitive subfamilies in a striking manner. This Insect, Palaeophlebia superstes (Fig. 272), has recently been discovered in Japan, and is perhaps the most interesting dragon-fly yet obtained. De Selys Longchamps refers it to the subfamily Calepteryginae, on account of the nature of its wings; were the Insect, however, deprived of these organs, no one would think of referring Palaeophlebia to the group in question, for it has the form, colour, and appearance of a Gomphine Odonate. Moreover, the two sexes differ in an important character,—the form of the head and eyes. In this respect the female resembles a Gomphine of inferior development; while the male, by the shape and large size of the ocular organs, may be considered to combine the characters of Gomphinae and Calepteryginae. The Insect is very remarkable in colour, the large eyes being red in the dead examples. We do not, however, know what may be their colour during life, as only one pair of the species is known, and there is no record as to the life-history and habits. De Selys considers the nearest ally of this Insect to be Heterophlebia dislocata, a fossil dragon-fly found in the Lower Lias of England.
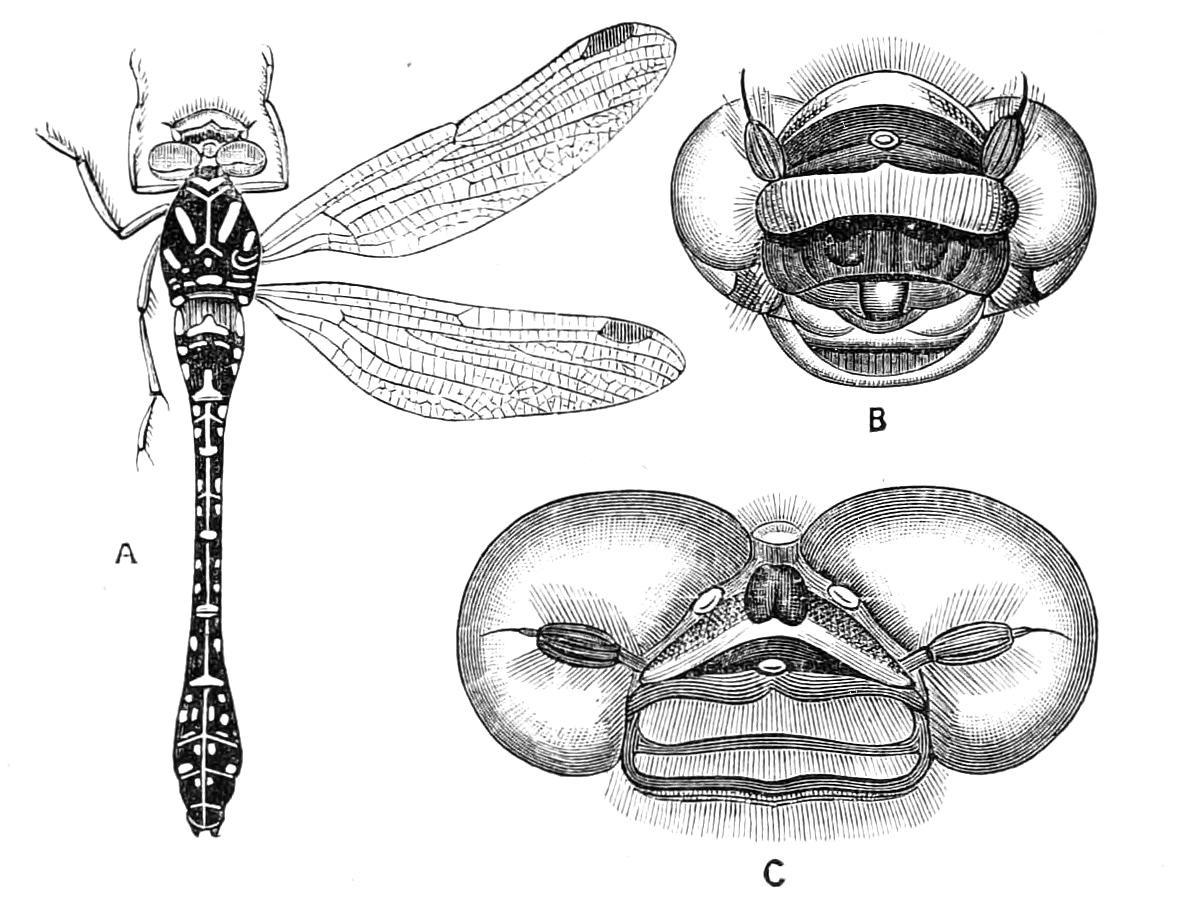
Fig. 272.—Palaeophlebia superstes. A, The Insect with wings of one side and with two legs removed; B, front view of head of female; C, of male. (After De Selys.)
Numerous fossil dragon-flies are known; the group is well represented in the Tertiary strata, and specimens have been found in amber. In strata of the Secondary age these Insects {428}have been found as far back as the Lower Lias; their remains are said to exist in considerable variety in the strata of that epoch, and some of them to testify to the existence at that period of dragon-flies as highly specialised as those now living. According to Hagen[347] Platephemera antiqua and Gerephemera simplex, two Devonian fossils, may be considered as dragon-flies; the evidence as to this appears inadequate, and Brongniart refers the latter Insect to the family Platypterides, and considers Platephemera to be more allied to the may-flies.
One of the most remarkable of the numerous discoveries lately made in fossil entomology is the finding of remains of huge Insects, evidently allied to dragon-flies, in the Carboniferous strata at Commentry. Brongniart calls these Insects Protodonates,[348] and looks on them as the precursors of our Odonata. Meganeura monyi was the largest of these Insects, and measured over two feet across the expanded wings. If M. Brongniart be correct in his restoration of this giant of the Insect world, it much resembled our existing dragon-flies, but had a simple structure of the thoracic segments, and a simpler system of wing-nervures. On p. 276 we figured Titanophasma fayoli, considered by Scudder and Brongniart as allied to the family Phasmidae, and we pointed out that this supposed alliance must at best have been very remote. This view is now taken by M. Brongniart himself,[349] he having removed the Insect from the Protophasmides to locate it in the Protodonates near Meganeura. There appears to be some doubt whether the wings supposed to belong to this specimen were really such, or belonged rather to some other species.
AMPHIBIOUS NEUROPTERA CONTINUED—EPHEMERIDAE, MAY-FLIES
Fam. VII. Ephemeridae—May-flies.
Delicate Insects with atrophied mouth and small, short antennae; with four membranous wings having much minute cross-veining; the hinder pair very much smaller than the other pair, sometimes entirely absent: the body terminated by three or two very elongate slender tails. The earlier stages are passed through in water, and the individual then differs greatly in appearance from the winged Insect; the passage between the two forms is sudden; the creature in its first winged state is a subimago, which by shedding a delicate skin reveals the final form of the individual.
The may-flies are well known—in literature—as the types of a brief and ineffective life. This supposed brevity relates solely to their existence in the winged form. In the earlier stages the may-fly is so unlike its subsequent self that it is not recognised as a may-fly by the uninitiated. The total life of the individual is really quite as long as that of most {430}other Insects. The earlier stages and life-histories of these Insects are of great importance. The perfect Insects are so delicate and fragile that they shrivel much in drying, and are very difficult to preserve in a condition suitable for study.
The mouth of the imago is atrophied, the trophi scarcely existing as separate parts. Packard says that in Palingenia bilineata he could discover no certain traces of any of the mouth-parts, but in Leptophlebia cupida he found, as he thought, the rudiments of the maxillae and labium, though not of the mandibles. The antennae are always short, and consist of one or two thick basal joints succeeded by a delicate needle-like segment, which, though comparatively long, is not divided. The ocular organs are remarkable for their large size and complex development; they are always larger in the male than they are in the female. The compound eyes of the former sex are in certain species, e.g. Cloëon (Fig. 274), quite divided, so that each eye becomes a pair of organs of a different character; one part forms a pillar facetted at its summit, while the other part remains as a true eye placed on the side of the head; in front of these compound eyes there are three ocelli. Thus the Insect comes to have three different kinds of eyes, together seven in number.
The prothorax is small, the pronotum being, however, quite distinct. The mesothorax is very large; its notum forms by far the larger part of the upper surface of the thoracic region, the metathorax being small and different in structure, resembling in appearance a part of the abdomen, so that the hind wings look as if they were attached to a first abdominal segment. The mesosternum is also disproportionately large in comparison with the homologous piece preceding it, and with that following it. The pleural pieces are large, but their structure and disposition are only very imperfectly understood. The coxae are small and are widely separated, the anterior being, however, more elongate and approximate than the others. The other parts of the legs are slender; the number of joints in the tarsi varies from five to one. {431}The legs throughout the family exhibit a considerable variety of structure, and the front pair in the males of some species are remarkably long. The abdomen is usually slender, and consists of ten segments; the terminal one bears three, or two, very long flexible appendages. The first dorsal plate of the abdomen is either wanting or is concealed to a considerable extent by the metanotum. The wings are peculiar; the anterior pair vary a great deal in their width, but are never very long in proportion to the width; the hind pair are always disproportionately small, and sometimes are quite wanting. The venation consists of a few, or of a moderate number, of delicate longitudinal veins that do not pursue a tortuous course, but frequently are gracefully curved, and form a system of approximately similar curves, most of the veins being of considerable length; close to the anterior margin of the wing there are two or three sub-parallel veins. Frequently there are very numerous fine, short cross-veinlets, but these vary greatly and may be entirely wanting.
The earlier stages of the life of Ephemeridae are, it is believed, in the case of all the species, aquatic. May-flies, indeed, during the period of their post-embryonic development are more modified for an aquatic life than any other Insects, and are provided with a complex apparatus of tracheal gills. The eggs are committed to the waters without any care or foresight on the part of the parent flies, thus the embryonic development is also aquatic; little, however, is known of it. According to Joly[350] the process in Palingenia virgo is slow. The larva on emerging from the egg has no respiratory system, neither could Joly detect any circulation or any nervous system. The creature on emergence is very like Campodea in form, possessing long antennae and tails—caudal setae. Owing to the organisation being inferior, the creature in its earlier stages is called a larvule; in its later stages {432}it is usually spoken of as a nymph, but the term larva is also frequently applied to it. Soon the gills begin to appear in the form of small tubular caeca placed in the posterior and upper angles of the abdominal rings; in fifteen days the gills begin to assume their characteristic form, are penetrated by tracheae, and the circulation can be seen. The amount of growth accomplished after hatching between March and September is but small.
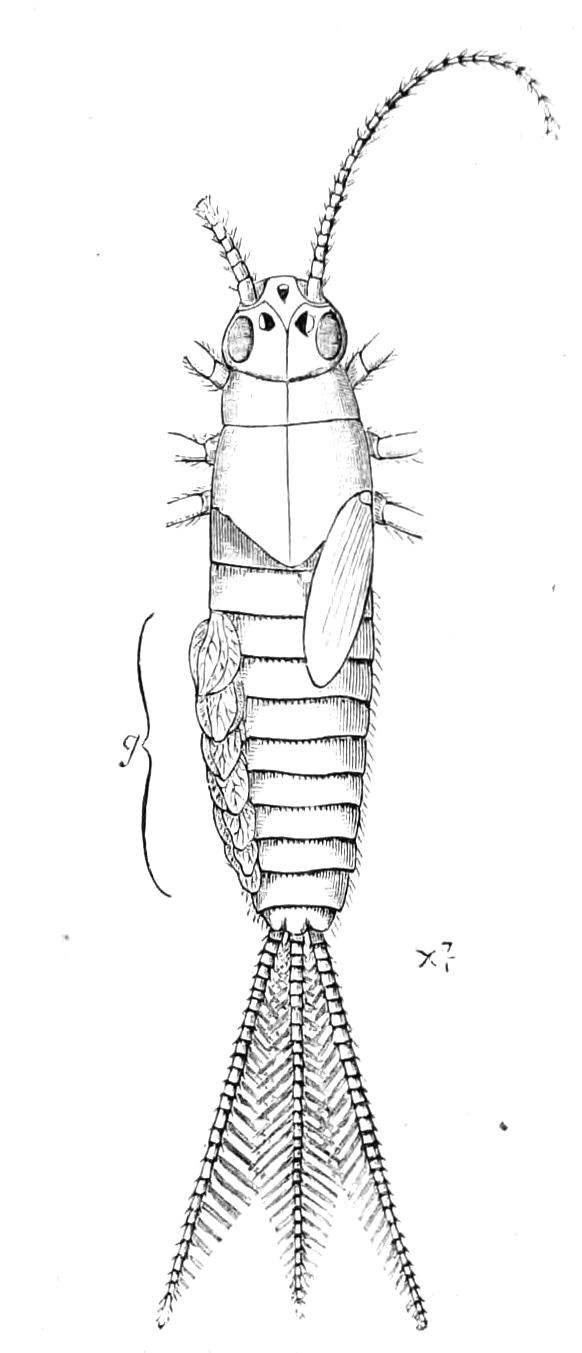
Fig. 276.—Nymph of Cloëon dipterum.[351] Wing-sheath of left side, gills of right side, removed; g, tracheal gills. (After Vayssière.)
The metamorphosis of Cloëon has been described by Sir John Lubbock; he informs us that the young creature undergoes a constant and progressive development, going through a series of more than twenty moults, each accompanied by a slight change of form or structure. His observations were made on captured {433}specimens, so that it is not certain that what he calls[352] the first stage is really such. He found no tracheae in the earliest stages; the small first rudiments of the gills became visible in the third stage, when there were no tracheae; the fourth instar possessed tracheae, and they could be seen in the gills. The wing rudiments could first be detected in the ninth and tenth stages. The changes of skin during the winter months are separated by longer intervals than those occurring at other periods of the year.
The nymphs differ greatly in the structure and arrangement of their tracheal gills, and display much variety in their general form and habits; some of them are very curious creatures. Pictet[353] divides them in accordance with their habits into four groups: (1) Fossorial larvae: these live in the banks of streams and excavate burrows for shelter; they are of cylindrical form, possess robust legs, abundant gills at the sides of the body, and frequently processes projecting forwards from the head: examples, Ephemera (Fig. 278) and Palingenia. (2) Flat larvae: these live attached to rocks, but run with rapidity when disturbed; they prefer rapid streams, have the breathing organs attached to the sides of the body and not reposing on the back; they are exclusively carnivorous, while the fossorial forms are believed to obtain their nutriment by eating mud: example, Baëtis. (3) Swimming larvae: elongate delicate creatures, with feeble legs, and with strongly ciliated caudal setae: example, Cloëon (Fig. 276). (4) Climbing larvae: these live in slowly-moving waters, especially such as have much slimy mud in suspension, and they have a habit of covering themselves with this mud sometimes to such an extent as to become concealed by it: example, Potamanthus.
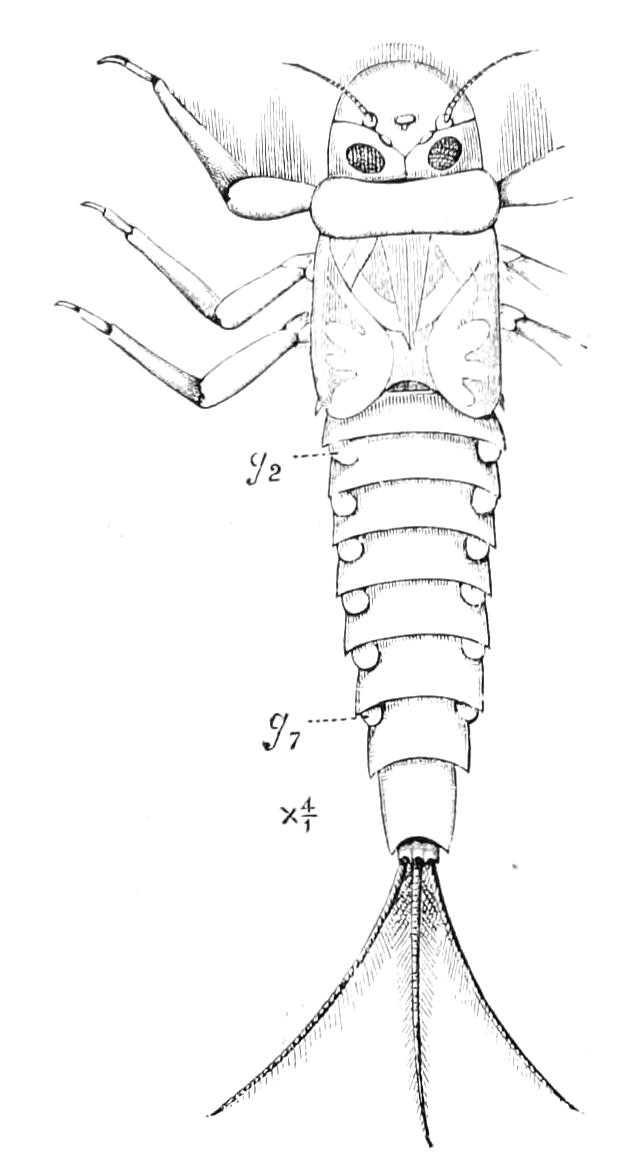
Fig. 279.—Nymph of Oligoneuria garumnica, France. g2 and g7, two of the dorsal tracheal gills. (After Vayssière.)
The anatomy of the nymphs has been treated by Vayssière,[354] who arranges them in five groups in accordance with the conditions of the tracheal gills: (1) The gills are of large size, are exposed and furnished at the sides with respiratory fringes: example, Ephemera (Fig. 278). (2) The branchiae are blade-like, not fringed, and are exposed at the sides of the body: example, Cloëon (Fig. 276). (3) The respiratory tubes are placed on the under surface of plates whose upper surface is not respiratory: example, Oligoneuria garumnica (Fig. 279). (4) The anterior gill is modified to form a plate that covers the others: example, Tricorythus (Fig. 282, B). (5) The gills are concealed in a respiratory chamber: example, Prosopistoma (Fig. 280). The last of these nymphs is more completely adapted for an aquatic life than any other Insect at present known; it was for long supposed to be a Crustacean, but it has now been shown to be the early stage of a may-fly, the sub-imago having been reared from the nymph. The carapace by which the larger part of the body is covered is formed by the union of the pro- and meso-thorax with the sheaths of the anterior wings, which have an unusually extensive development; under the carapace there is a respiratory chamber, the floor and sides of which are formed by the posterior wing-sheaths, and by a large plate composed of the united nota of the metathorax and the first six abdominal segments. In this chamber there are placed five pairs of tracheal gills; entrance of water to the chamber is effected by two laterally-placed orifices, and exit by a single dorsal aperture. These nymphs use the body as a sucker, and so adhere strongly to stones under water. When detached they swim rapidly by means of their caudal setae; the form of these latter organs is different from that {435}of other Ephemerid nymphs. This point and other details of the anatomy of this creature have been described in detail by Vayssière.[355] These nymphs have a very highly developed tracheal system; they live in rapid watercourses attached to stones at a depth of three to six inches or more under the water. Species of Prosopistoma occur in Europe, Madagascar, and West Africa.
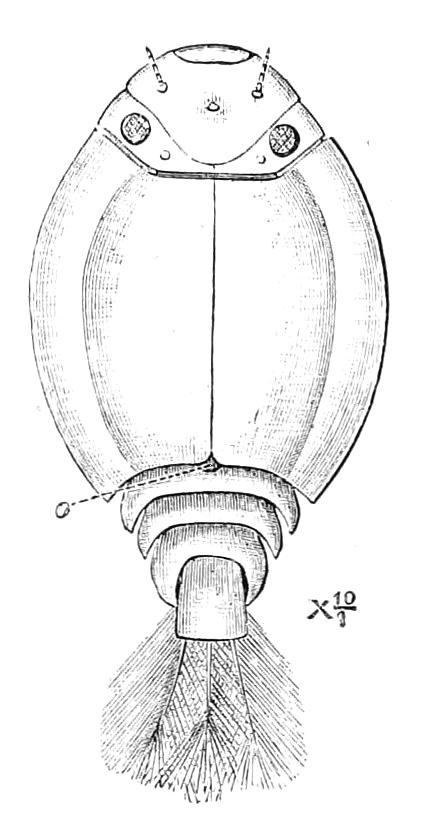
Fig. 280.—Prosopistoma punctifrons, nymph. France. (After Vayssière.) o, Orifice of exit from respiratory chamber.
According to Eaton,[356] in the nymphs of some Ephemeridae the rectum serves, to a certain extent, as a respiratory agent; he considers that water is admitted to it and expelled after the manner we have described in Odonata, p. 421.
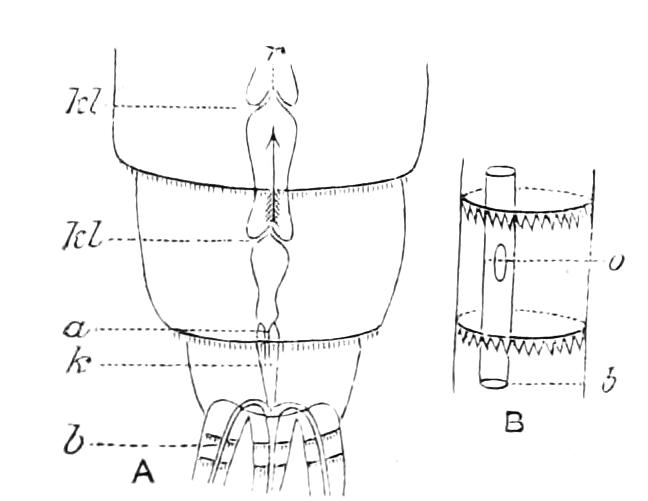
Fig. 281.—A, Last three abdominal segments and bases of the three caudal processes of Cloëon dipterum: r, dorsal vessel; kl, ostia thereof; k, special terminal chamber of the dorsal vessel with its entrance a; b, blood-vessel of the left caudal process; B, twenty-sixth joint of the left caudal process from below; b, a portion of the blood-vessel; o, orifice in the latter. (After Zimmermann.)
The internal anatomy of the nymphs of Ephemeridae shows some points of extreme interest. The long caudal setae are respiratory organs of a kind that is almost if not quite without parallel in the other divisions of Insecta. The dorsal vessel for the circulation of the blood is elongate, and its chambers are arranged one to each segment of the body. It drives the blood forwards in the usual manner, but the posterior chamber possesses three blood-vessels, one of which is prolonged into each caudal seta. This terminal chamber is so arranged as to drive the blood backwards into the vessels of the setae; on the under surface of the vessels there are oval orifices by which the blood escapes into the cavity of the seta so as to be submitted to the action of the surrounding medium for some of the purposes of respiration. This structure has been described by {436}Zimmermann,[357] who agrees with Creutzberg[358] that the organ by which the blood is propelled into the setae is a terminal chamber of the dorsal vessel; Verlooren,[359] who first observed this accessory system of circulation, thought the contractile chamber was quite separate from the heart. The nature of the connexion between this terminal chamber that drives the blood backwards and the other chambers that propel the fluid forwards appears still to want elucidation.
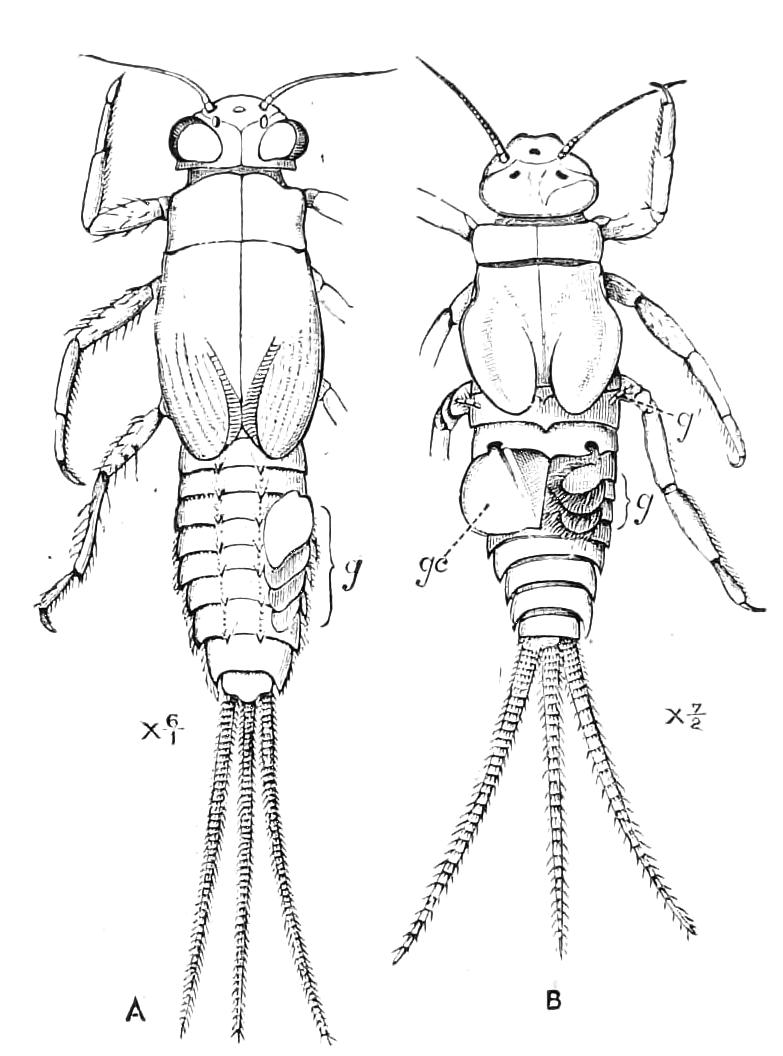
Fig. 282.—A, Nymph of Ephemerella ignita with gills of left side removed; g, gills: B, nymph of Tricorythus sp. with gill cover of right side removed; g.c, gill cover; g, g′, gills. (After Vayssière.)
The nymphs of the Ephemeridae being creatures adapted for existence in water, the details of their transformation into creatures having an entirely aerial existence cannot but be of much interest. In the nymphs the tracheal system is well developed, but differs from that of air-breathing Insects in the total absence of any spiracles. Palmén has investigated this subject,[360] and finds that the main longitudinal tracheal trunks of the body of the nymph are not connected with the skin of the body by tracheae, but are attached thereto by ten pairs of slender strings extending between the chitinous integument and the tracheal trunks. When the skin is shed these strings—or rather a chitinous axis in each one—are drawn out of the body, and bring with them the chitinous linings of the tracheae. Thus notwithstanding the absence of spiracles, the body wall is at each moult pierced by openings that extend to the tracheae. After the ordinary moults these orifices close immediately, but at the change to the winged state they remain open and form the spiracles. At the same time the tracheal gills are {437}completely shed, and the creature is thus transformed from a water-breather to an Insect breathing air as usual. In addition to this change there are others of great importance, such as the development of the great eyes and the complete atrophy of the mouth-parts. The precise manner of these changes is not known; they occur, however, within the nymph skin. The sudden emergence of the winged Insect from the nymph is one of the most remarkable facts in the life-history of the may-fly; it has been observed by Sir John Lubbock,[361] who describes it as almost instantaneous. The nymph floats on the water, the skin of the back opens, and the winged Insect flies out, upwards and away; "from the moment when the skin first cracks not ten seconds are over before the Insect has flown away." The creature that thus escapes has not, however, quite completed its transformation. It is still enveloped in a skin that compresses and embarrasses it; this it therefore rapidly gets rid of, and thus becomes the imago, or final instar of the life-cycle. The instar in which the creature exists winged and active, though covered with a skin, is called the sub-imago. The parts of the body in the sub-imago are as a whole smaller than they are in the imago, and the colour is more dingy; the appendages—wings, legs, and caudal setae—are generally considerably shorter than they are in the imago, but attain their full length during the process of extraction. The creatures being, according to Riley, very impatient and eager to take to the wing, the completion of the shedding of the skin of the sub-imago is sometimes performed while the Insect is flying in the air.
The food of young Ephemeridae is apparently of a varied and mixed nature. Eaton says[362] that though sometimes the stronger larvae devour the weaker, yet the diet is even in these cases partly vegetable. The alimentary canal frequently contains much mud; very small organisms, such as diatoms and confervae, are thought to form a large part of the bill of fare of Ephemerid nymphs. Although {438}the mouth is atrophied in the imago, yet it is highly developed in the nymphs. This is especially notable in the case of the lingua or hypopharynx (Fig. 283); indeed Vayssière[363] seems to incline to the opinion that this part of the mouth may be looked on in these Insects as a pair of appendages of a head-segment (see p. 96 ante), like the labium or maxillae.
The life-history has not been fully ascertained in the case of any species of may-fly; it is known, however, that the development of the nymph sometimes occupies a considerable period, and it is thought that in the case of some species this extends to as much as three years. It is rare to find the post-embryonic development of an Insect occupying so long a period, so that we are justified in saying that brief as may be the life of the may-fly itself, the period of preparation for it is longer than usual. Réaumur says, speaking of the winged fly, that its life is so short that some species never see the sun. Their emergence from the nymph-skin taking place at sunset, the duties of the generation have been, so far as these individuals are concerned, completed before the morning, and they die before sunrise. He thinks, indeed, that individuals living thus long are to be looked on as Methuselahs among their fellows, most of whom, he says, live only an hour or half an hour.[364] It is by no means clear to which species these remarks of Réaumur refer; they are doubtless correct in certain cases, but in others the life of the adult is not so very short, and in some species may, in all probability, extend over three or four days; indeed, if the weather undergo an unfavourable change so as to keep them motionless, the life of the flies may be prolonged for a fortnight.
The life of the imago of the may-fly is as remarkable as it is brief; in order to comprehend it we must refer to certain peculiarities of the anatomy with which the vital phenomena are connected. The more important of these are the large eyes of the males, the structure of the alimentary canal, and that of the reproductive organs. We have already remarked that the parts of the mouth in the imago are atrophied, yet the canal itself not only exists but is even of greater capacity than usual; it appears to have much the same general arrangement of parts as it had in the nymph. Its coats are, however, of great tenuity, and according {439}to Palmén[365] the divisions of the canal are separated by changes in the direction of certain portions anterior to, and of others posterior to, its central and greater part—the stomach—in such a manner that the portions with diverted positions act as valves. The stomach, in fact, forms in the interior of the body a delicate capacious sac; when movement tends to increase the capacity of the body cavity then air enters into the stomachic sac by the mouth orifice, but when muscular contractions result in pressure on the sac they close the orifices of its extremities by the valve-like structures we have mentioned above; the result is, that as complex movements of the body are made the stomach becomes more and more distended by air. It was known even to the old naturalists that the dancing may-fly is a sort of balloon, but they were not acquainted with the exact mode of inflation. Palmén says that in addition to the valve-like arrangements we have described, the entry to the canal is controlled by a circular muscle, with which are connected radiating muscles attached to the walls of the head. Palmén's views are adopted, and to a certain extent confirmed, by Fritze,[366] who has examined the alimentary canal of the may-fly, and considers that though the normal parts of the canal exist, the function is changed in the imago, in which the canal serves as a sort of balloon, and aids the function of the reproductive organs. The change in the canal takes place in an anticipatory manner during the nymph and sub-imago stages.
The sexual organs of Ephemeridae are remarkable for their simplicity; they are destitute of the accessory glands and diverticula that, in some form or other, are present in most other Insects. Still more remarkable is the fact that the ducts by which they communicate with the exterior continue as a pair to the extremity of the body, and do not, as in other Insects, unite into a common duct. Thus in the female there is neither bursa copulatrix, receptaculum seminis, nor uterine portion of oviduct, and there is no trace of an ovipositor; the terminations of the ducts are placed at the hind margin of the seventh ventral plate, just in front of which they are connected by a fold of the integument. The ovary consists of a very large number of small egg-tubes seated on one side of a sac, which forms their calyx, and one of whose extremities is continued backwards as one of the {440}pair of oviducts. The male has neither vesiculae seminales, accessory glands, nor ductus ejaculatorius. The testes are elongate sacs, whose extremities are prolonged backwards forming the vasa deferentia; these open separately at the extremity of the body, each on a separate intromittent projection of more or less complex character, the two organs being, however, connected by means of the ninth ventral plate, of which they are, according to Palmén, appendages. We should remark that this authority considers Heptagenia to form, to some extent, an exception as regards the structures of the female; while Polymitarcys is in the male sex strongly aberrant, as the two vasa deferentia, instead of being approximately straight, are bent inwards at right angles near their extremities so as to meet, and form in the middle a common cavity, which then again becomes double to pass into the pair of intromittent organs.
According to the views of Exner and others, the compound eyes of Insects are chiefly organs for the perception of movement; if this view be correct, movements such as those made during the dances of may-flies may, by the number of the separate eyes, by their curved surfaces and innumerable facets, be multiplied and correlated in a manner of which our own sense of sight allows us to form no conception. We can see on a summer's evening how beautifully and gracefully a crowd of may-flies dance, and we may well believe that to the marvellous ocular organs of the flies themselves (Fig. 274) these movements form a veritable ballet. We have pointed out that by this dancing the peculiarly formed alimentary canal becomes distended, and may now add that Palmén and Fritze believe that the unique structure of the reproductive organs is also correlated with the other anatomical peculiarities, the contents of the sexual glands being driven along the simple and direct ducts by the expansion of the balloon-like stomach. During these dances the momentary conjugation of the sexes occurs, and immediately thereafter the female, according to Eaton, resorts to the waters appropriate for the deposition of her eggs. As regards this, Eaton says:[367] "Some short-lived species discharge the contents of their ovaries completely en masse, and the pair of fusiform or subcylindrical egg-clusters laid upon the water rapidly disintegrate, so as to let the eggs sink broadcast upon the river-bed. The less perishable species extrude their eggs {441}gradually, part at a time, and deposit them in one or other of the following manners: either the mother alights upon the water at intervals to wash off the eggs that have issued from the mouths of the oviducts during her flight, or else she creeps down into the water to lay her eggs upon the under-side of stones, disposing them in rounded patches, in a single layer evenly spread, and in mutual contiguity." The eggs are very numerous, and it is thought may sometimes remain in the water as much as six or seven months before they hatch.
The number of individuals produced by some kinds of may-flies is remarkable. Swarms consisting of millions of individuals are occasionally witnessed. D'Albertis observed Palingenia papuana in countless myriads on the Fly River in New Guinea: "For miles the surface of the river, from side to side, was white with them as they hung over it on gauzy wings; at certain moments, obeying some mysterious signal, they would rise in the air, and then sink down anew like a fall of snow." He further states that the two sexes were in very disproportionate numbers, and estimates that there was but a single female to every five or six thousand males.
Ephemeridae in the perfect state are a favourite food of fishes, and it is said that on some waters it is useless for the fly-fisher to try any other lure when these flies are swarming. Most of the "duns" and "spinners" of the angler are Ephemeridae; so are several of the "drakes," our large E. danica and E. vulgata being known as the green drake and the gray drake. Ronalds says[368] that the term "dun" refers to the pseud-imago condition, "spinner" to the perfect Insect. E. danica and E. vulgata are perhaps not distinguished by fishers; Eaton says that the former is abundant in rapid, cool streams, while E. vulgata prefers warmer and more tranquil rivers.
These sensitive creatures are unable to resist the attractions of artificial lights. Réaumur noticed this fact many years ago, and since the introduction of the electric light, notes may frequently be seen in journals recording that myriads of these Insects have been lured by it to destruction. Their dances may frequently be observed to take place in peculiar states of light and shade, in twilight, or where the sinking sun has its light rendered broken by bushes or trees; possibly the broken lights {442}are enhanced in effect by the ocular structures of the Insects. It has recently been ascertained that a species of Teleganodes is itself luminous. Mr. Lewis,[369] who observed this Insect in Ceylon, states that in life the whole of the abdomen was luminous, not brightly so, but sufficient to serve as a guide for capturing the Insect on a dark night. It has also been recorded that the male of Caenis dimidiata gives a faint blue light at night.
Nearly 300 species of Ephemeridae are known, but this may be only a fragment of what actually exist, very little being known of may-flies of other parts of the world than Europe and North America. One of the more curious forms of the family is Oniscigaster wakefieldi; the body of the imago is unusually rotund and furnished with lateral processes. In Britain we have about forty species of may-fly. The family is treated as a distinct Order by Brauer and Packard, and is called Plectoptera by the latter.
That Insects so fragile, so highly organised, with a host of powerful enemies, but themselves destitute of means of attack or defence, should contrive to exist at all is remarkable; and it appears still more unlikely that such delicate Insects as Ephemeridae should leave implanted in the rocks their traces in such a manner that they can be recognised; nevertheless, such is the case,—indeed, the may-fly palaeontological record is both rich and remarkable. Several forms are preserved in amber. In the Tertiary bed of the old lake at Florissant, Scudder has been able to distinguish the remains of no less than six species; while in the Jurassic layers of the Secondary epoch, in more than one locality, the remains of several other species have been detected and described. Still more remarkable is the fact that in the Devonian and Carboniferous layers of the {443}Palaeozoic period, remains are found that appear to be akin to our existing Ephemeridae. Palingenia feistmantelii from the Carboniferous of Bohemia is actually referred to a still existing genus; it is said to have been of gigantic size for a may-fly.
The families Megasecopterides, Platypterides, and Stenodictyopterides of the Carboniferous epoch (see p. 343) are all more or less closely allied to the Ephemeridae, and in addition to these Brongniart has established the family Protephemerides for some Insects that he considers to have been the precursors in the Carboniferous epoch of our existing may-flies. These ancient Insects differed in having the wings of another form from those of existing Ephemeridae, and in having the hind wings equal in size to the front pair. Besides this, these Insects had, as shown in Fig. 285, prothoracic dorsal appendages; some had also projections from the abdominal segments, considered by Brongniart to be of the nature of gills. Some doubt must exist as to this point, for we find in the imago of one of our existing Ephemeridae, Oniscigaster wakefieldi, Fig. 284, abdominal processes that are not gills.
It is remarkable that may-flies, which now form a comparatively unimportant part of the Insect tribe, should in far distant times have been represented by so great a variety of allied forms. Our fragile, short-lived may-flies appear to be, as Scudder says, the lingering fragments of an expiring group.
NEUROPTERA PLANIPENNIA—SIALIDAE, ALDER-FLIES, SNAKE-FLIES—PANORPIDAE, SCORPION-FLIES—HEMEROBIIDAE, ANT-LIONS, LACEWINGS, ETC.
Fam. VIII. Sialidae—Alder-flies and Snake-flies.
Four wings of moderate size, meeting in repose over the back at an angle; the hinder of the two pairs slightly the smaller; the anal area small or nearly absent, not plicate. Nervures moderately numerous, transverse veinlets moderately numerous, forming irregularly disposed cells. The metamorphosis is great; there is a quiescent pupa. The larva has the mandibles formed for biting, armed with strong teeth.
The Sialidae, though but a small family of only some six or eight genera, comprise a considerable variety of forms and two sub-families—Sialides and Raphidiides. The former group has larvae with aquatic habits possessed of branchiae but no spiracles.
Sialis lutaria is one of the commoner British Insects frequenting the vegetation about the banks of tranquil streams; it is well known to anglers, being used by them for a bait. According to Ronalds it is called the alder or orl-fly, and in Wales the humpback.
It is very unattractive in appearance, being of a blackish colour, with wings of a yellow-brown tinge, and makes but a poor show when flying. The female deposits patches of elongate eggs, placed on end and packed together in a very clever manner (Fig. 287). These patches of eggs, of a stone-gray colour, are common objects on rushes or stems of grass near water, and it is stated that there may be no less than 2000 or 3000 eggs in one of them. Our figure gives some idea of the mode in which the eggs are arranged, and the curious narrow process that exists at the end of each. The eggs are said to be sometimes placed at a considerable distance from water, so that when the tiny larvae are hatched they must begin their lives by finding the way to a suitable pool or stream. The larvae (Fig. 288) are objects of very great interest owing to each of segments 1 to 7 of the hind body being furnished on each side with a jointed filament, while the last segment ends in a still longer, but unjointed process. These filaments are branchiae by means of which the Insect obtains air, being, as we have said, destitute of spiracles. It is an active creature and waves its filaments in a very graceful manner; this process no doubt aids the branchiae in their respiratory work. These larvae are well able to exist out of water if they have a sufficiently damp environment. They live on animal matter, but their life-history has not been followed in much detail and it is not known {446}how many moults they make. The young larva has the head disproportionately large and the branchial filaments longer. When the growth is completed the larva returns to land, seeks a suitable situation in the soil, and after an interval changes to a pupa, in which the characters of the perfect Insect are plainly visible. Subsequently, without becoming again active, it changes to the perfect Insect, and enjoys, for a few days only, an aerial life.
The anatomy of the larva has been treated by Dufour.[370] The supra-oesophageal ganglion is remarkably small; nothing is said as to the existence of an infra-oesophageal ganglion; there are three thoracic and eight abdominal ganglia; the first pair of these latter are nearer together than the others, and this is also the case with the last three. The alimentary canal in the adult is provided with a large paunch attached to the crop by a narrow neck,[371] but Dufour could find no trace of this in the larva. The structure of the branchiae has also been described by the indefatigable French entomotomist. A tracheal tube sends a branch into one of the appendages (Fig. 289); the branch gives off numerous smaller tracheae, which at their extremities break up into branchlets close to the integument. The tracheal tube that receives each main branchial trachea, sends off from near the point of entry of the latter another trachea, that distributes its branchlets on the alimentary canal. The margins of each appendage are set with swimming hairs, so that the branchiae act as organs of locomotion as well as of respiration, and by their activity in the former capacity increase the efficiency of their primary function.
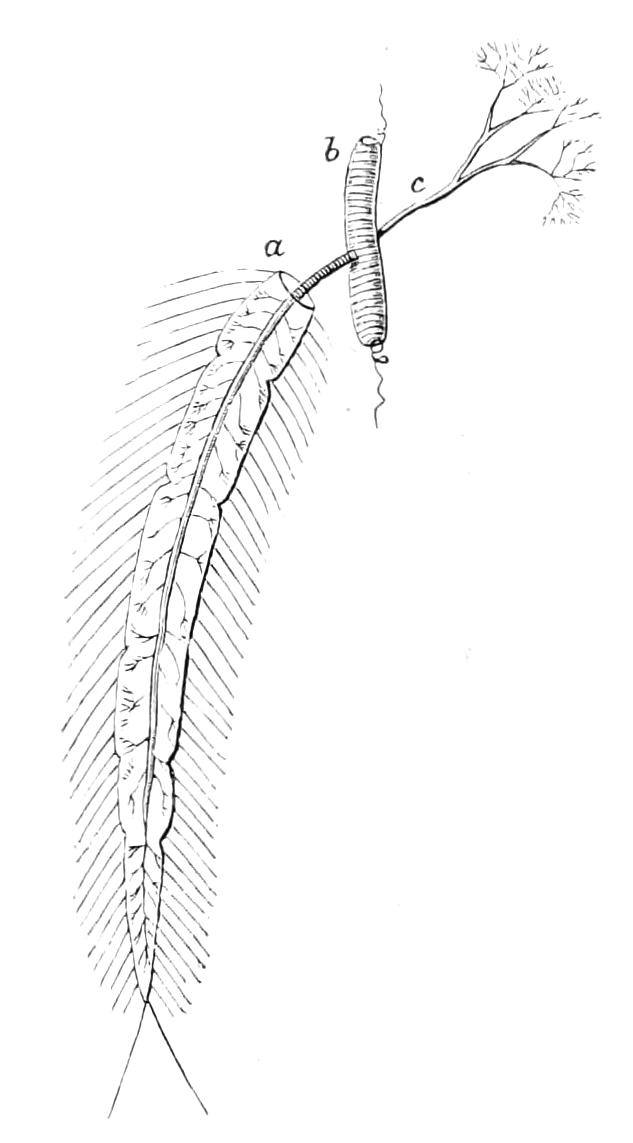
Fig. 289.—Structure of tracheal gill of Sialis lutaria. (After Dufour.) a, Base of the gill; b, tracheal trunk with which it is connected; c, trachea given off to alimentary canal.
The genus Sialis occurs in a few species only, throughout the {447}whole of the Palaearctic and Nearctic regions, and reappears in Chili,[372] though absent in all the intervening area. Several other genera of Insects exhibit the same peculiarity of distribution.
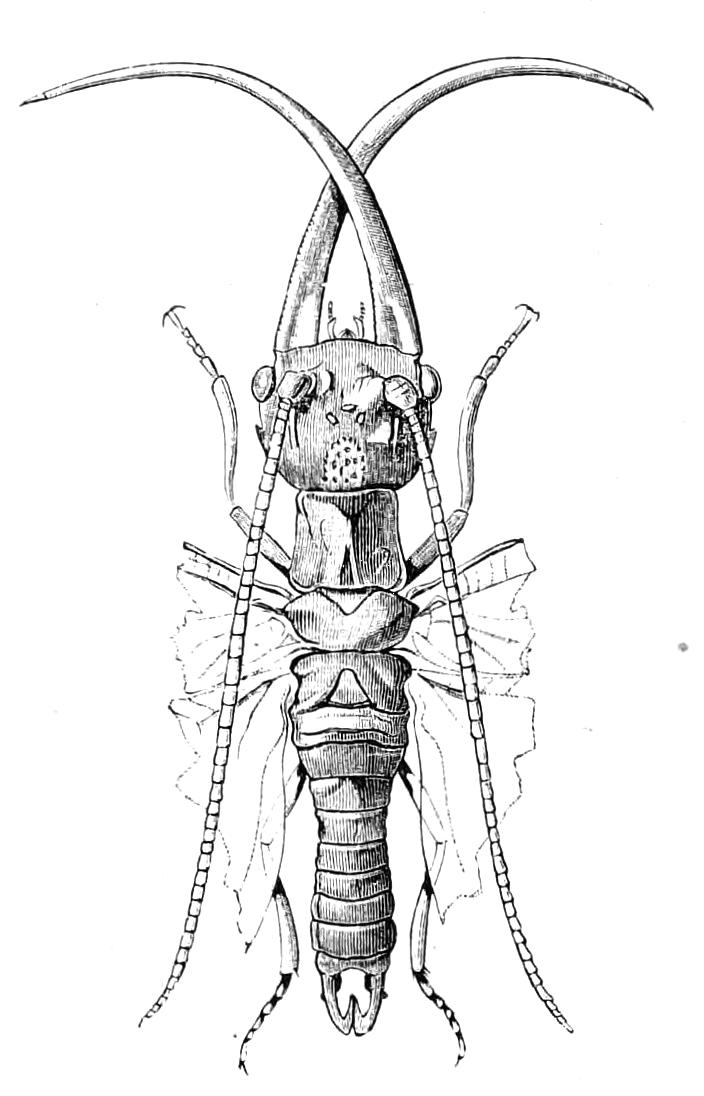
Fig. 290.—Corydalis crassicornis, male, with greater portions of the wings removed. Texas. (After M‘Lachlan.)
The genera Corydalis and Chauliodes form a group distinct from Sialis, and are totally different in appearance, being gigantic Insects, sometimes with the mandibles of the male enormously elongated (Fig. 290). The species of Corydalis are called in North America Hellgrammites; Riley has described and figured the metamorphosis of C. cornutus,[373] the life-history being very similar to that of our little Sialis. A mass consisting of two or three thousand eggs is formed by the female, and the young larva has long filaments at the sides of the body like Sialis. These in the later larval life are comparatively shorter, but the Insect is then provided with another set of gills in the form of spongy masses on the under-side of the body. Riley, however, considers that these organs serve the purpose of attachment rather than of respiration. The larvae are known to the Mississippi fishermen as crawlers, and are greatly esteemed as bait.
The Raphidiides or snake-flies form the second tribe of Sialidae. There are only two genera, Raphidia and Inocellia, peculiar to the Palaearctic and Nearctic regions. The perfect Insects are chiefly remarkable for the elongation of the prothorax and back of the head to form a long neck, and for the existence in the female of an elongate exserted ovipositor. {448}The species are rather numerous, and have been recently monographed by Albarda.[374] The three or four British species of the genus are all rare Insects, and occur only in wooded regions.
The Raphidiides, like the Sialides, have a carnivorous larva, which, however, is terrestrial in habits, feeding, it would appear, chiefly on Insects that harbour in old timber. The snake-fly larvae (Fig. 292) are very ingenious in their manner of escaping, which is done by an extremely rapid wriggling backwards. They are capable of undergoing very prolonged fasts, and then alter in form a good deal, becoming shorter and more shrivelled; Fig. 292 is taken from a specimen that had been fasting for several weeks. They are excessively voracious, and hunt after the fashion of beasts of prey; their habits have been described by Stein,[375] who states that he kept a larva from August to the end of May of the following year without food; it then died in a shrivelled-up state. The larva of the snake-fly changes to a pupa that is remarkably intermediate in form between the perfect Insect and the larva; the eyes, legs, wing-pads, and ovipositor being but little different from those of the imago, while the general form is that of the larva, and the peculiar elongation of the neck of the imago is absent. This pupa differs from that of Sialis in the important particular that before undergoing its final ecdysis it regains its activity and is able to run about.
The internal anatomy of Raphidia has been treated by Loew,[376] and is of a very remarkable character; we can here only mention that the salivary glands consist of a pair of extremely elongate tubes, that there is a very definite paunch attached as an appendage to one side of the crop, and that the most peculiar character consists of the fact that, according to Loew, four of the six Malpighian tubes have not a free extremity, being attached {449}at each end so as to form elongate loops; the mesenteron is very complex in character.
A considerable number of fossil remains from both Tertiary and Mesozoic strata are referred to Sialidae; and a larval form from the red sandstone of Connecticut has been considered by Scudder to be a Sialid, and named Mormolucoides articulatus, but the correctness of this determination is very doubtful (Fig. 293). These fossils are, however, of special interest as being the most ancient Insect larvae yet brought to light. A still older fossil, from the Carboniferous strata of Illinois called Miamia bronsoni, is considered by Scudder to have several points of resemblance to Sialidae.
Fam. IX. Panorpidae—Scorpion-flies.
Head prolonged to form a deflexed beak, provided with palpi near its apex; wings elongate and narrow, shining and destitute of hair, with numerous, slightly divergent veins and moderately numerous transverse veinlets (in one genus the wings are absent). Larvae provided with legs, and usually with numerous prolegs like the saw-flies: habits carnivorous.
The majority of the members of this family are very readily distinguished by the beak-like front of the head, this being chiefly due to enlargement of parts of the head itself, and in a less degree to prolongation of the mouth-parts. The upper (or front) face of the beak is formed entirely by the clypeus, the labrum being scarcely {450}visible, though it may be detected at the sides of the tip of the beak; the sutures between the various parts of the head are nearly or quite obliterated, but it is probable that the sides of the beak are formed by the genae and by the stipites of the maxillae, and its under-surface chiefly by the submentum: the mentum itself is but small, the ligula is small, bifid at the extremity, and each branch bears a two-jointed palpus, the basal article being of very peculiar structure in Panorpa. The mandibles are but small, and are placed at the apex of the beak; they have each the form of an oblong plate armed with two very sharp teeth, and they cross freely. The maxillae are the only parts of the mouth-pieces that are very elongated; each cardo is articulated at the base of the head, and the stipes extends all the length of the side of the beak; each maxilla bears a five-jointed palpus and two small but very densely ciliated lobes. The antennae are long, very slender, and flexible, and are many-jointed; they are inserted between the eyes in large foramina; there are three ocelli, or none, and the compound eyes are moderately large. The prothorax is small, its notum is quite small or moderate in size, and the prothoracic stigma is placed behind it; the side-pieces are small, and there is no chitinous prosternum except a small longitudinal strip placed in the membrane between the coxae; these latter are of only moderate size, and are free and dependent. The meso- and meta-thorax are large, their side-pieces are of considerable dimensions and bear large, dependent coxae and supporting-pieces (Fig. 58); there is a stigma placed between the meso- and meta-thorax at the hind margin of the upper part of the meso-trochantin; both meso- and meta-notum are transversely divided. The abdomen is elongate, slender, conico-cylindrical, consisting of nine segments; the basal segment is membranous and concealed; the terminal appendages are of variable nature according to the species and sex. The legs are elongate and slender, the tarsi five-jointed. The internal anatomy of Panorpa communis has been examined by Dufour[377] and Loew.[378] They agree in describing the alimentary canal as being of peculiar structure: there is a short, slender oesophagus leading to an organ in which there is seated a remarkable arrangement of elongate hairs; this structure might be looked on as the proventriculus, but Loew considers it to be rather a {451}division of the true stomach. The particulars given by these two anatomists as to some other parts of the internal anatomy are very discrepant.
The Panorpidae form a small family of only nine or ten genera, two or three of these being exotic and only imperfectly known; the three genera found in Europe are composed of very curious Insects. The scorpion-flies—Panorpa proper—are very common Insects, and have received their vernacular name from the fact that the males have the terminal segments elongate and slender and very mobile, and carry them curved up somewhat after the fashion of the scorpions (Fig. 294). It is said that Aristotle was acquainted with these Insects, and considered them to be really winged scorpions.
A second European genus, Boreus, is still more peculiar; it is destitute of wings, and has the appearance of a minute wingless grasshopper; it is found from late autumn to early spring in moss and under stones, and is said to be sometimes found disporting itself on the surface of the snow: the female of this Insect has an exserted ovipositor. The writer has found this little creature in Scotland among moss in November, and under stones early in March (Fig. 295). The third European genus, Bittacus, does not occur in our islands, but is common on many parts of the Continent; the perfect Insect has a great resemblance to a Tipula, or "daddy-long-legs" fly, and attaches itself to the stems of grasses, and preys on flies; according to Brauer it has the peculiar habit of using the hind pair of legs as hands (Fig. 296), instead of the front pair, as is usual in Insects. This remarkable genus is widely distributed, and species of it are found even in the Antipodes. A species inhabiting caves has been mentioned by M‘Lachlan.[379]
The early stages of the Panorpidae were for long unknown, but have recently been discovered by Brauer: he obtained eggs of Panorpa by confining a number of the perfect flies in a vessel containing some damp earth on which was placed a piece of meat; when the young larvae were hatched they buried themselves in the earth and nourished themselves with the meat or its juices.
These larvae (Fig. 297) bear a great resemblance to those of the Hymenopterous family Tenthredinidae; they have biting mandibles and palp-bearing maxillae, and show no approach to the peculiar mouth structure found in the Hemerobiidae; there are three pairs of feet placed on the three thoracic segments, and there is also a pair of less perfect feet on each of the first eight abdominal segments, those behind being the larger. The upper surface of the body bears spines, which, however, disappear after the first change of skin, with the exception of the larger processes on the posterior segment, which persist throughout the life of the larva. The larvae are active for about one month; after this they become quiescent, but do not change to the pupa state for several weeks; when this happens they change in form and cannot creep, although their limbs are not enclosed in any pupa case. Brauer also discovered larvae of Panorpa communis at large in numbers in an old tree stump that was quite covered with moss, and contained many ants in the mouldering wood. The ants appeared to be on friendly terms with the Panorpa larvae. The earlier stages of {453}Boreus and Bittacus were also observed by Brauer; they are essentially similar to those of Panorpa, but the larva in Boreus is not provided with abdominal prolegs. The Panorpidae have been separated from the other Neuroptera by certain naturalists as a distinct Order, called Panorpatae by Brauer, Mecaptera by Packard; but in their structure as well as in their metamorphoses they are not so distinct from the Phryganeidae and the Hemerobiidae as to justify this step.
Fossil forms of Bittacus and of Panorpa have been found in amber and in the Tertiary strata, and Scudder has described some forms from Florissant in which there are no cross-veinlets in the wings. Some remains from the English Lias have been referred to Panorpidae by Westwood under the name Orthophlebia, but it is by no means certain that they really belong to the family.
Fam. X. Hemerobiidae—Ant-lions, Lacewing-flies, etc.
Head vertical; maxillae free, with five-jointed palpi; labial palpi three-jointed. Wings subequal in size, with much reticulation, without anal area. Tarsi five-jointed. Metamorphosis great; the larvae with mandibles and maxillae coadapted to form spear-like organs that are suctorial in function. Pupa, similar in general form to the imago, enclosed in a cocoon.
The Hemerobiidae are an extremely varied assemblage of Neuroptera; the perfect Insects of the various sub-families are very different in appearance, but the family as a whole is naturally defined by the very peculiar structure of the mouth-organs of the larvae. These Insects have, in fact, a suctorial {454}mouth in their early life, and one of the ordinary biting type in adult life.
This is a very unusual condition, being the reverse of what we find in Lepidoptera and some other of the large Orders, where the mouth is mandibulate in the young and suctorial in the adult. The suctorial condition is in Hemerobiidae chiefly due to modification of the mandibles; but this is never the case in the Insects that have a suctorial mouth in the imaginal instar. Nearly all the Hemerobiidae are terrestrial Insects in all their stages; a small number of them are, to a certain extent, amphibious in the larval life, while one or two genera possess truly aquatic larvae. The metamorphosis is, so far as the changes of external form are concerned, quite complete. There are no wingless forms in the adult stage.
The classification given by Hagen[380] and generally adopted recognises seven sub-families. These we shall mention seriatim.
Sub-Fam. 1. Myrmeleonides or Ant-lions.—Antennae short, clubbed, the apical space of the wing with regular, oblong cellules.
The ant-lions in their perfect state are usually unattractive Insects, and many are nocturnal in their habits; the species of the genus Palpares and allies (Fig. 299) are, however, of more handsome appearance, and attain a large expanse of wing. No member of the sub-family is an inhabitant of Britain, though species of the typical genus Myrmeleon are common in Central and Northern Europe. The {455}remarkable habits of their larvae attracted the attention of naturalists so long ago as two hundred years. We owe to Réaumur an accurate and interesting account of M. formicarius, the species found in the neighbourhood of Paris. The larvae are predaceous, and secure their prey by means of pitfalls they excavate in the earth, and at the bottom of which they bury themselves, leaving only their elongate jaws projecting out of the sand at the bottom of the pit. They move only backwards, and in forming their pit use their broad body as a plough, and throw out the sand by placing it on the head and then sending it to a distance with a sudden jerk. When about to construct its trap the larva does not commence at the centre, but makes first a circular groove of the full circumference of the future pit. Burying its abdomen in the surface of the earth, the Insect collects on to its head, by means of the front leg, the sand from the side which is nearest to the centre, and then jerks the sand to a distance. By making a second circuit within the first one, and then another, the soil is gradually removed, and a conical pit is formed, at the bottom of which the ant-lion lurks, burying its body but leaving its formidable mandibles widely extended and projecting from the sand. In this position the young ant-lion waits patiently till some wandering Insect trespasses on its domains. An ant or fly coming over the edge of the pitfall finds the sand of the sloping sides yielding beneath its body, and in its effort to secure itself probably dislodges some more of the sand, which, descending to the bottom of the pit, brings the lurking lion into activity. Availing himself of his power of throwing sand with his head, the ant-lion jerks some in the neighbourhood of the trespasser, and continues to do so until the victim is brought to the bottom of the pit and into the very jaws of its destroyer; then there is no further hope of escape; the mandibles close, empale their prey, and do not relax their hold till the body of the victim is exhausted of its juices. The position chosen is in a place that will keep dry, as the larva cannot carry on its operations when the sand is wet or damp, hence the soil at the base of a high wall or a rock frequently harbours these Insects.
The parts of the mouth of the Myrmeleon are perfectly adapted for enabling it to empty the victim without for a moment relaxing its hold. There is no mouth-orifice of the usual character, and the contents of the victim are brought into the buccal cavity by means of a groove extending along the under side of each mandible; in this groove the elongate and slender lobe that replaces the maxilla—there being no maxillary palpi—plays backwards and forwards, probably raking or dragging backwards to the buccal cavity at each movement a small quantity of the contents of the empaled victim. The small lower lip is peculiar, consisting in greater part of the two lobes that support the labial palpi. The pharynx is provided with a complex set of muscles, and, together with the buccal cavity, functions as an instrument of suction. After the prey has been sucked dry the carcass is jerked away to a distance. When the ant-lion larva is full grown it forms a globular cocoon by fastening together grains of sand with fine silk from a slender spinneret placed at the posterior extremity of the body; in this cocoon it changes to an imago of very elongate form, and does not emerge until its metamorphosis is quite completed, the skin of the pupa being, when the Insect emerges, left behind in the cocoon. The names by which the European ant-lion has been known are very numerous. It was called Formicajo and Formicario by Vallisneri about two hundred years ago; Réaumur called it Formica-leo, and this was adopted by some modern authors as a generic name for some other of the ant-lions. The French people call these Insects Fourmilions, of which ant-lion is our English equivalent. The Latinised form of the term ant-lion, Formicaleo, is not now applied to the common ant-lion as a generic term, it having been proposed to replace it by Myrmecoleon, Myrmeleo, or Myrmeleon; this latter name at present seems likely to become generally adopted. There are several species of the genus found in Europe, and their trivial names have been confounded by various authors in such a way as to make it quite uncertain, without reference to a synonymic list, what species is intended by any particular writer. The species found in the neighbourhood of Paris, and to which it may be presumed Réaumur's history refers, is now called {457}Myrmeleon formicarium by Hagen and others; M‘Lachlan renamed it M. europaeus, but now considers it to be the M. nostras of Fourcroy. The popular name appears to be due to the fact that ants—Formica in Latin, Fourmi in French—form a large part of the victims; while lion—the other part of the name—is doubtless due to its prowess as a destroyer of animal life, though, as Réaumur long ago remarked, it is a mistake to apply the term lion to an Insect that captures its prey by strategy and by snares rather than by rapidity and strength. The imago of Myrmeleon is of shy disposition, and is rarely seen even in localities where the larva is abundant. It is of nocturnal habits, and is considered by Dufour to be carnivorous.
Considerable difference of opinion has existed as to the structure of the mouth and of the alimentary canal in these larvae. Réaumur was of opinion that there exists no posterior orifice to the alimentary canal, but Dufour ridiculed this idea, and stated positively that such an orifice undoubtedly exists. It is also usually said that the mouth is closed by a membrane. Meinert has recently examined these points,[381] and he states that the mouth is not closed by any membrane, but is merely compressed. He finds that there is no posterior exit from the stomach; that there is a compact mass without any cavity between the stomach and the point where the Malpighian tubes connect with the small intestine. The portions of aliment that are not assimilated by the larva collect in the stomach and are expelled as a mass, but only after the Insect has become an imago. This peculiar excrementitious mass consists externally of uric acid, and from its form and appearance has been mistaken for an egg by several naturalists. The posterior portions of the alimentary canal are, according to Meinert, of a remarkable nature. The small intestine is elongate, slender, and is coiled. There are eight very long and slender Malpighian tubes; a pair of these have free extremities, but the other six in the posterior part of their course are surrounded by a common membrane, and, following the course of the intestine, form ultimately a dilated body seated on a coecum. These six Malpighian tubes are considered to be partially, if not entirely, organs for the secretion of silk for forming the cocoon, the coecum being a reservoir. The canal terminates as a slender tube, which acts as a spinneret and is surrounded by a sheath. A complex set of muscles {458}completes this remarkable spinning apparatus. The alimentary canal of the imago has been described and figured by Dufour[382]; it is very different from that of the larva.
The ant-lion is capable of sustaining prolonged fasts. Dufour kept specimens for six months without any food. These Insects are said to give off a peculiar ant-like odour, due, it is thought, to their ant-eating habits. Although no species inhabits Great Britain, yet one is found in Southern Sweden. Introduced specimens get on very well in confinement in our country,[383] and would probably flourish at large for some years if they were liberated.
Although the number of known species and genera of Myrmeleonides is considerable—that of the species being now upwards of 300—the members of the small genus Myrmeleon are the only forms that are known to make pits of the kind we have described. Other larvae[384] are known similar in general form to the common ant-lion, but they walk forwards in the normal manner, and apparently hunt their prey by lurking in a hidden place and, when a chance occurs, rushing on the victim with rapidity. Brauer has observed this habit in the case of Dendroleon pantherinus in the Prater at Vienna.
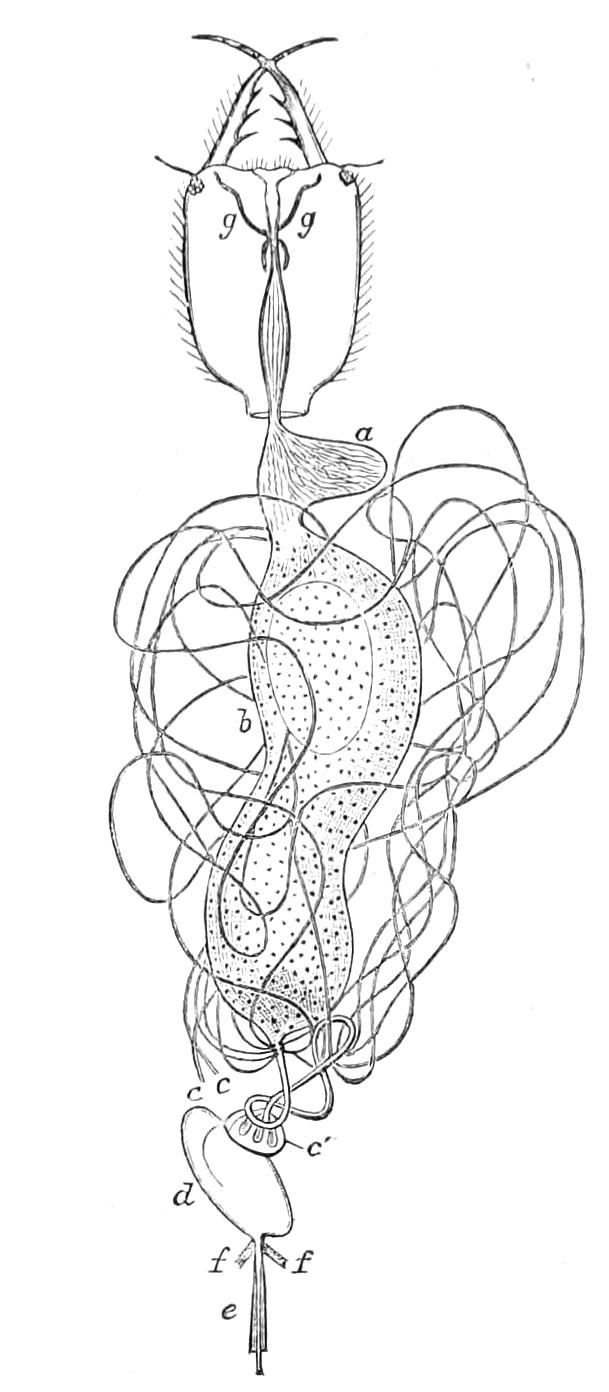
Fig. 301.—Upper aspect of head and alimentary canal of Myrmeleon: a, crop; b, stomach; c, free extremities of two Malpighian tubes; c′, terminal common portion of other six tubes; d, coecum; e, spinneret; f, f, muscles for protruding its sheath; g, g, maxillary glands. (After Meinert.)
The most remarkable forms of Myrmeleonides are contained in the genus Palpares. We figure Tomateres citrinus (Fig. 299), an allied genus found in Eastern Africa as far south as Natal. These Insects have conspicuous blotches and marks on their wings. The species of Myrmeleon are similar in form, but are smaller, more feeble, and less ornate in appearance.
Pitfalls, formed in all probability by ant-lions, have been noticed in the Galapagos islands and in Patagonia, though none of the Insects forming them have been found.
Sub-Fam. 2. Ascalaphides.—Antennae elongate, with a knob at the tip; the apical area of the wing with irregular cellules.
The sub-family Ascalaphides is not represented by any species in Britain, though Ascalaphus longicornis occurs as far north as Paris. In the mountainous regions of Central and Southern Europe some species of the group form a conspicuous part of the Insect fauna, owing to their bold and active flight; they are predaceous in their habits, and fly about in a hawking fashion somewhat like that of dragon-flies. Some of the larger of the numerous exotic species of the group are very like dragon-flies, but can be distinguished by a glance at the elongate antennae with a knob at the end. The sub-family consists of two groups—Holophthalmi and Schizophthalmi. M‘Lachlan says[385]: "The eyes in the Schizophthalmous division are really double, the upper portion overlapping the under; if the upper portion be separated the lower division looks like a small spherical ordinary eye." There appears, however, to be considerable differences in the genera in this respect.
Fig. 303.—A, Eggs of Ascalaphus macaronius. B, Sketch of position of the young larvae of Helicomitus insimulans (?); C, outline of natural size. (After Westwood.)
When the weather is wet or cold the Ascalaphi repose on the stems of grass, with their wings placed in a roof-like manner, with the head downwards, and are then very successful in concealing themselves by the positions they assume, and by sidling round the stems to escape from enemies. Some information as to their metamorphosis has been obtained, though knowledge of this point is far from complete even as regards our European species of the typical genus Ascalaphus. For a long time it was supposed that a larva mentioned by Bonnet in his writings was that of Ascalaphus, but Brauer[386] is of opinion that such is not the case, and as he has described the metamorphoses of A. macaronius he is no doubt correct. The eggs (Fig. 303, A), forty or fifty in number, are laid in two parallel rows on the stems of grass. The larvae (Fig. 304, larva of Helicomitus ?) are in general appearance somewhat like those of Myrmeleon; they are carnivorous in their habits, like the ant-lions, and have similar extraordinarily developed mandibles. Efforts to rear the young larvae failed, but they were kept alive for some time by supplying them with Aphidides found on Centaurea jacea. The cocoon is globular, and the change from the nymph state to the imago is made in the cocoon, the structure of the mandibles of the pupa being peculiar, and specially adapted to the purpose of opening the cocoon.[387] The larvae of Ascalaphides, although so like the ant-lions in appearance, do not form pitfalls for the capture of their prey, but lurk under leaves on the ground, or under stones; they do not move backwards, but progress forwards in an ordinary manner; the habit of backward movement that we noticed in Myrmeleon being probably correlative with the habit of forming pitfalls. Hagen states[388] that the larvae of Ascalaphides and Myrmeleonides, in addition to their peculiarities of form and mandibular structure, are distinguished from those of other Hemerobiidae by the hind legs having the tibia and tarsus united {461}without articulation. Westwood[389] has recently given an account of the young larvae of a Ceylonese Ascalaphid of doubtful species, but possibly Helicomitus insimulans; these were observed by Mr. Staniforth Green to have the very peculiar habit of sitting together in a long row on the stem of a plant, with the jaws widely extended and the body of each one covered by the head of the individual next it (Fig. 303, B). The little creatures waited patiently in this position until a fly walked between the mandibles of one of them, then these formidable weapons immediately closed, and did not relax their hold until the fly was sucked dry. If Westwood is correct, the young larva of this species differs much from the adult one, the back of the head being broad and the setigerous processes of the body very much more developed. Nearly thirty genera of Ascalaphides are known.[390] In the genus Haplogenius we find an exception to the usual rule that the wings in repose are held in a roof-like manner, it having been noticed by Bates that in the species in question the wings are held expanded as in the dragon-flies.
Guilding has described[391] a very peculiar mode of oviposition on the part of Ulula macleayana in the island of St. Vincent; the eggs are said to be deposited by the female in circles on the extremity of a twig, and nearer the base of this there is placed a kind of barrier to repel intruders. "The female may be seen expelling from her ovary these natural barriers with as much care as her real eggs." Guilding's description was accompanied by drawings of the eggs, barriers and larvae, but unfortunately these were never published, and no further information has been obtained on the subject. Hagen[392] suggests that the barriers may {462}be somewhat similar to the long stalks on which the eggs of Chrysopa (Fig. 314) are placed.
Sub-Fam. 3. Nemopterides.—Head more or less produced and beak-like. Hind wings of peculiar form, being elongate and somewhat strap-like.
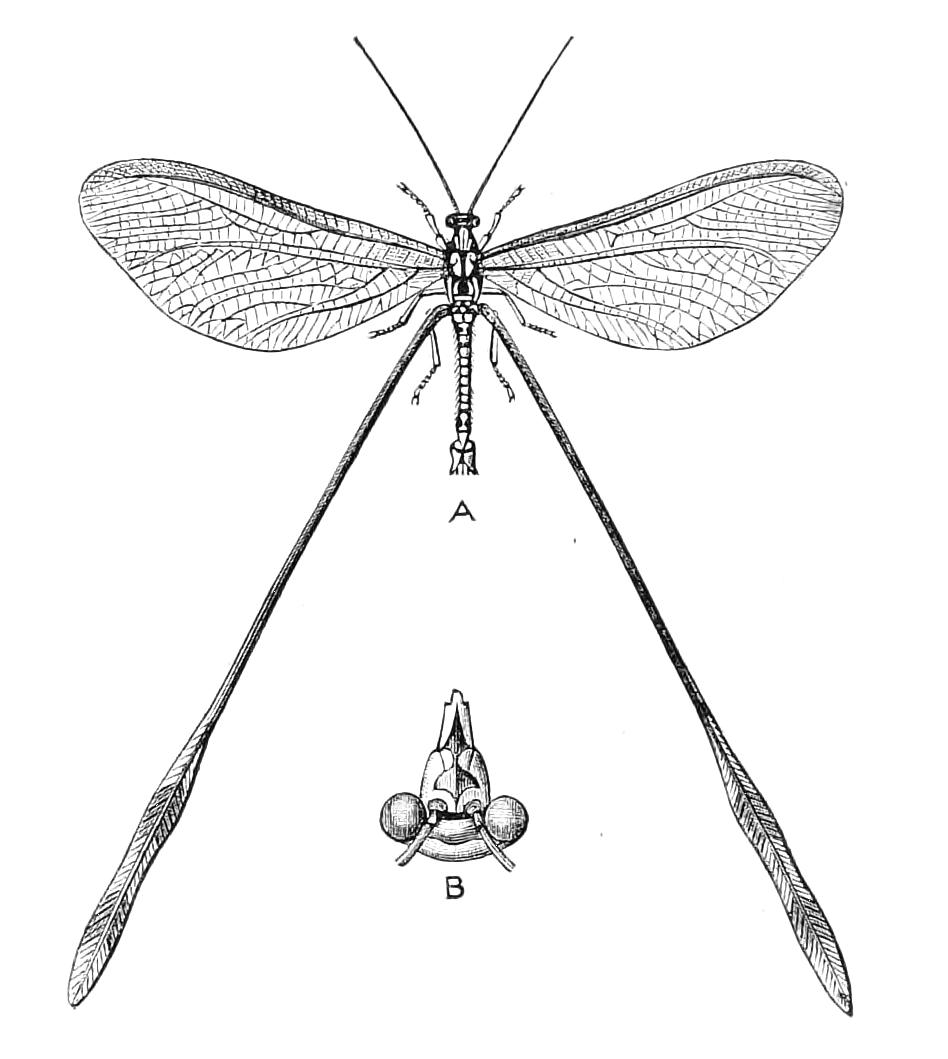
Fig. 305.—Nemoptera ledereri. Asia Minor. (After Selys.) A, The imago; B, its head seen from in front and magnified.
The Nemopterides are a small group of delicate, graceful Insects. About thirty species are known. Knowledge of the group is still very imperfect. A larva has been found of a most remarkable nature that probably belongs to it; it was described under the name of Necrophilus arenarius, and considered to be a fully-developed Insect. This larva occurs in the tombs and pyramids of Egypt where sand has accumulated. The perfect Insects of the genus Nemoptera are, however, found in open places amongst bushes, and flit about in a very graceful manner. Several species are found in Southern Europe and the Mediterranean region {463}(Fig. 305, N. ledereri), but none come so far north as Central Europe. Formerly the genus Nemoptera was considered to be allied to Panorpa on account of the beak-like front of the head. The parts of the mouth are, however, different from those of Panorpa, and it seems more probable that if the Nemopterides have to be merged in any of the divisions of Hemerobiidae, they will be placed in Chrysopides or Osmylides. The species of the sub-family were for a long time believed to be peculiar to the continental regions of the Old World, but a species has recently been discovered in Northern Chili.[393]
Sub-Fam. 4. Mantispides.—Prothorax elongate; the raptorial front legs inserted at its anterior part.
The members of this small group are readily recognised by the peculiar structure of the front legs; these organs resembling those of the Orthopterous family Mantidae, so that the earlier systematic entomologists, deceived by this resemblance, placed the Mantispides in the Order referred to.
The Mantispides possess four membranous wings, either sub-equal in size or the posterior pair smaller than the front pair and not folded; the veins of these wings are rather numerous, as are also the cells they form; there is considerable difference amongst the species in this latter respect, owing to the transverse veinlets differing in their abundance. The antennae are short, not in the least thickened at the tip. The head is not produced into a beak. The anterior legs, placed quite at the front part of the thorax, have the coxae very long; the femur is somewhat incrassate, and is armed on one side with spines; the tibia is shaped and articulated so as to fold closely on to the spines, and to thus constitute a formidable and perfect prehensile organ, the tarsus being merely a small appendage.
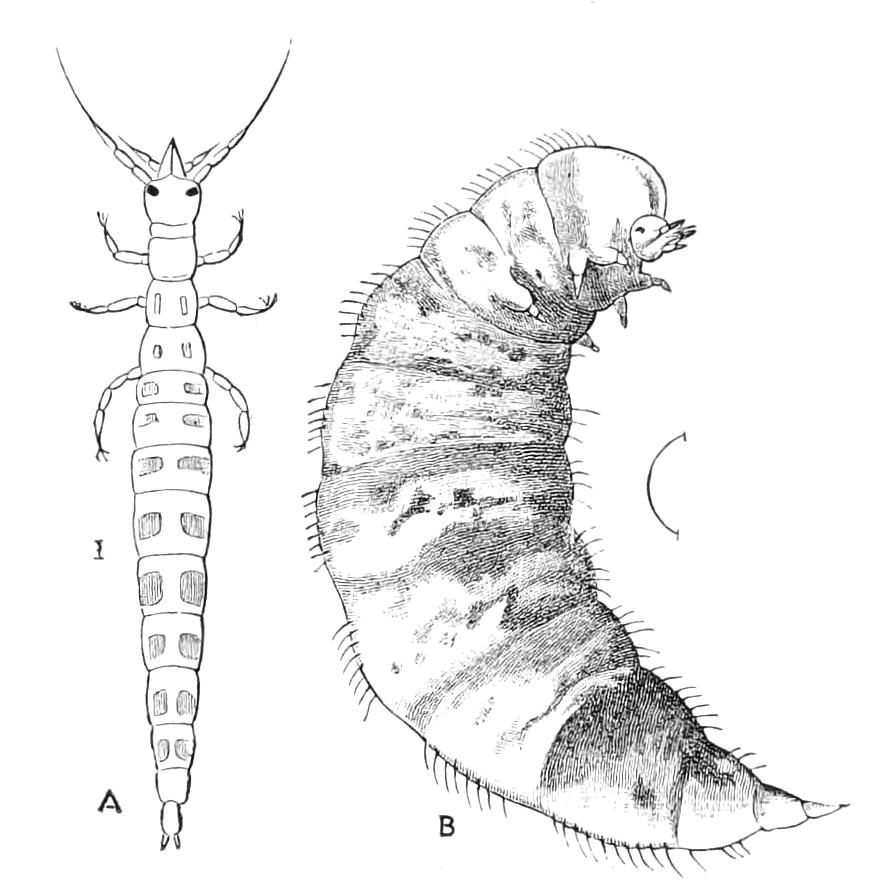
Fig. 308.—Mantispa styriaca. A, Larva newly hatched, or first form; B, mature larva. (After Brauer.)
Only a few species of Mantispa are found in Southern Europe; but the group has representatives in most of the warmer regions of the world, and will probably prove to be rather numerous in species. The front legs are used for the capture of prey in the same way as the somewhat similar front legs of the Mantidae. The transformations have been observed by Brauer[394] in the case of one of the European species, M. styriaca. The eggs are numerous but very small, and are deposited in such a manner that each is borne by a long slender stalk, as in the lacewing flies. The larvae are hatched in autumn; they then hibernate and go for about seven months before they take any food. In the spring, when the spiders of the genus Lycosa have formed their bags of eggs, the minute Mantispa larvae (Fig. 308, A) find them out, tear a hole in the bag, and enter among the eggs; here they wait until the eggs have attained a fitting stage of development before they commence to feed. Brauer found that they ate the spiders when these were quite young, and then changed their skin for the second time, the first moult having taken place when they were hatched from the egg. At this second moult the larva undergoes a considerable change of form; it becomes unfit for locomotion, and the head loses the comparatively large size and high development it previously possessed. The Mantispa larva—only one of which flourishes in one egg-bag of a spider—undergoes this change in the midst of a mass of dead young spiders it has gathered together in a peculiar manner. It undergoes no further change of skin, and is full fed in a few days; after which it spins a cocoon in the interior of the egg-bag of the spider, and changes to a nymph inside its larva-skin. {465}Finally the nymph breaks through the barriers—larva-skin, cocoon, and egg-bag of the spider—by which it is enclosed, and after creeping about for a little, appears in its final form as a perfect Mantispa. Thus in this Insect hypermetamorphosis occurs; the larval life consisting of two different instars, one of which is specially adapted for obtaining access to the creature it is to prey on. It should be noticed that though this Insect is so destructive to the young spiders, the mother spider shows no hostility to it, but allows the destroying larva to enter her bag of eggs without any opposition; she appears, indeed, to be so unconscious of the havoc that is going on amongst her young that in one case she continued to watch over and protect the egg-bag in which the destruction was taking place during the whole of the period of the larval development and half the period of pupation of the Mantispa.
The larval history of a second species of the Mantispides, Symphrasis varia, is partially known;[395] this Insect lives parasitically in the nests of a South American wasp, and each larva when full fed spins a cocoon in one of the cells of the Hymenopteron.
Sub-Fam. 5. Hemerobiides.—Wings in repose forming an angular roof over the body; the antennae moniliform or pectinate, not clavate.
The Hemerobiides consist of several minor groups about whose number and characters systematists are not very well agreed, and about some of which very little is known. We merely mention the latter, giving details as to some of the better known only.
1. The Dilarina are a small group found chiefly in the Old World, where, however, they have a wide distribution. North and South America have each one species. They are distinguished by their antennae, which, in the male, are pectinate somewhat like those of many Lepidoptera, this character being of extremely rare occurrence in the Neuroptera; the abdomen of the female terminates in a long ovipositor. The metamorphoses are not known.
2. Nymphidina: Australian Insects resembling Myrmeleonides, but having antennae without club. Metamorphoses not known.
3. Osmylina: a group of delicate and elegant Insects of small or moderate size, distinguished by the possession of three simple eyes placed on the middle of the head just above the antennae. A species of this group, Osmylus chrysops (maculatus of some authors), is an inhabitant of Britain (Fig. 212); its larva is to some extent amphibious. The metamorphoses have been observed by Dufour, Brauer, and Hagen;[396] it lurks under stones in or close to the water, or in moss, or on the stems of aquatic plants, and pierces and empties small Insects with its sucking-spears, which are very elongate. The young are hatched from the egg in the autumn and hibernate before becoming full grown; when this moment arrives the larva spins a round cocoon of silk mixed with sand. The pupa, or nymph, in general appearance somewhat resembles the perfect Insect, except that it is shorter and has the short wing-pads clinging close to the body. Dufour denied the existence of abdominal spiracles in either larva or imago, but, according to Hagen, they are certainly present in both. It would appear that in the larva the alimentary canal is not open beyond the chylific ventricle, and that its terminal section is modified to form a spinning apparatus.
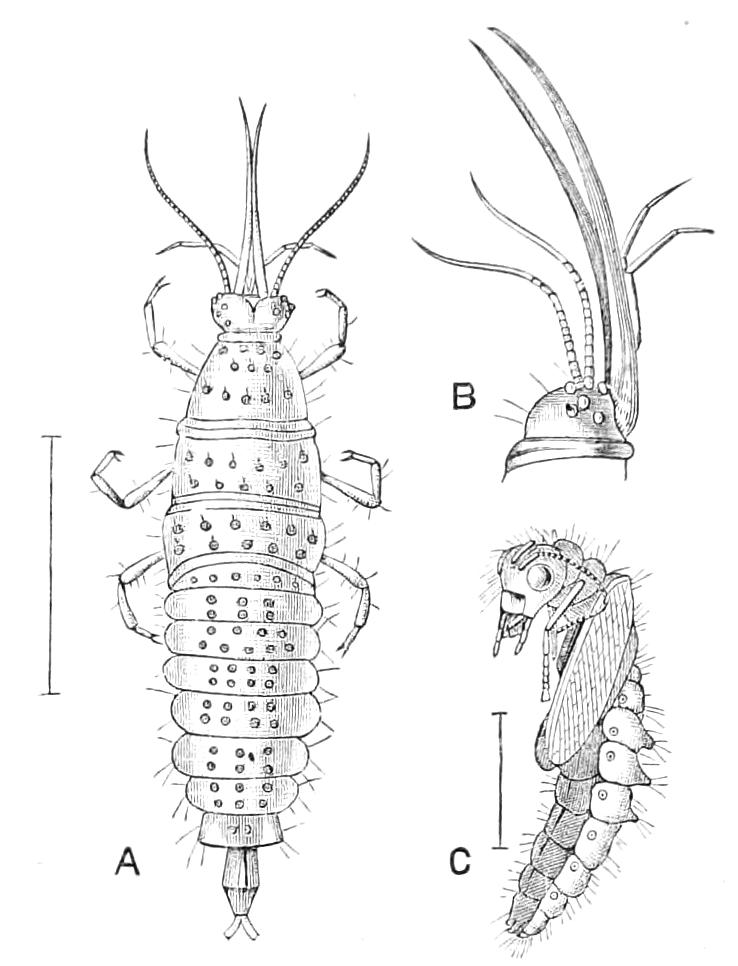
Fig. 309.—Osmylus chrysops. A, Larva; B, side view of head of larva (after Brauer); C, pupa (after Hagen).
Osmylus and its allies, including Sisyra, are now frequently treated as a separate sub-family, Osmylides, equivalent to the Chrysopides. In it is placed a very anomalous Insect—Psectra dispar—of great rarity. The male has only two wings, the posterior pair being the merest rudiments, though the female has the four wings normally developed. Individuals of the male have been found[397] in widely separated localities in the Palaearctic region—Somersetshire being one of them—and also in North America.
The genus Sisyra forms for some Neuropterists the type of a separate group called Sisyrina, though by others it is placed, as we have said, with the Osmylina, though it is destitute of ocelli. The larvae of at least one species of this genus are aquatic, and have been found in abundance living in Spongilla (Ephydatia) fluviatilis, a fresh-water sponge; when discovered their nature was not at first recognised, as they possess on each ventral segment a pair of articulated appendages, looking like legs, but which are considered to be more of the nature of gills. The sucking-spears of this Insect are so long and slender as to look like hairs; whether the little animal draws its nutriment from the sponge, or merely uses this latter as a place of shelter, is not ascertained.
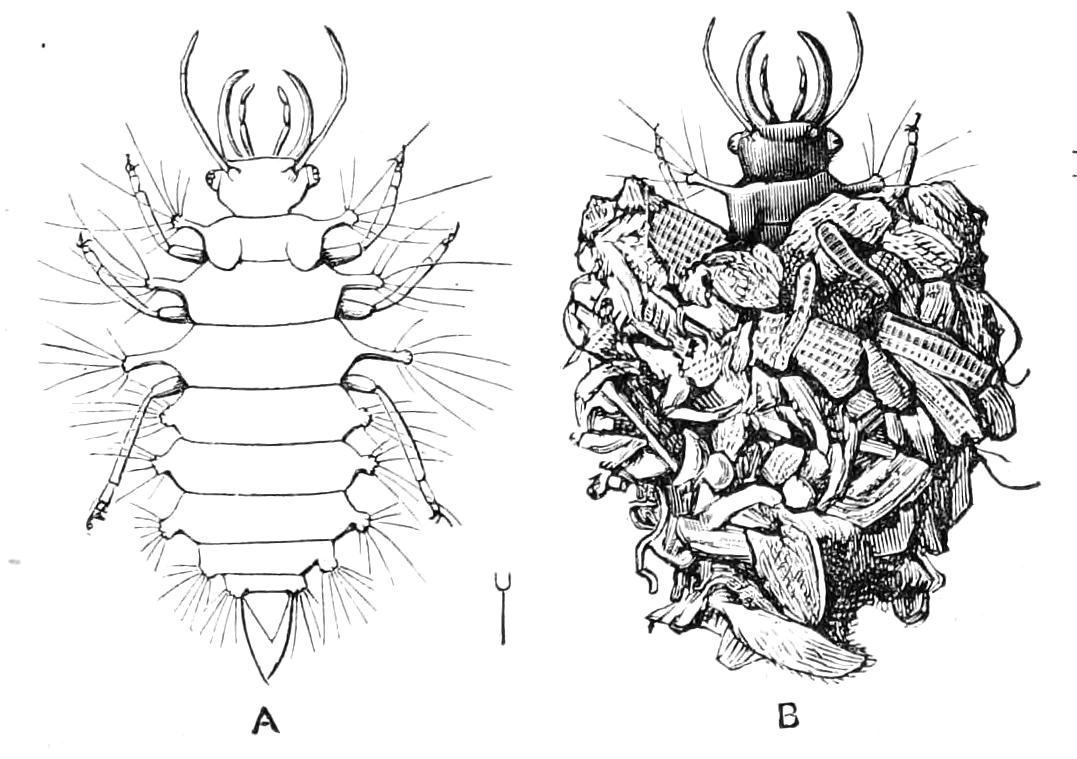
Fig. 311.—Larva of Hemerobius sp. from Kent. A, The larva bare; B, the same, partially concealed by the remains of its victims, etc.; a portion of the covering has been removed in order to show the head.
4. Hemerobiina: a somewhat numerous group of small or more rarely moderate-sized Insects, with moniliform antennae, no ocelli, a complex and comparatively regular system of wing-nervures; the veinlets are especially numerous at the margins, owing to the mode of forking of the nervures there (Fig. 298, Drepanepteryx phalaenoides). The larvae of most of the species of which the habits are known {468}live on Aphides, which they suck dry, and at least one species, in all probability several, has the habit of covering itself with the skins of the victims it has sucked; to these remains it adds other small debris, and the whole mass completely covers and conceals the Insect (Fig. 311, B). The larva is furnished at the sides with projections which serve as pedicels to elongate divergent hairs, and these help to keep the mass in place on the back of the Insect; some fine threads are distributed through this curious mantle and serve to keep it from disintegration, but whether they are fragments of spiders' webs or are spun by the Insect itself is not quite clear.
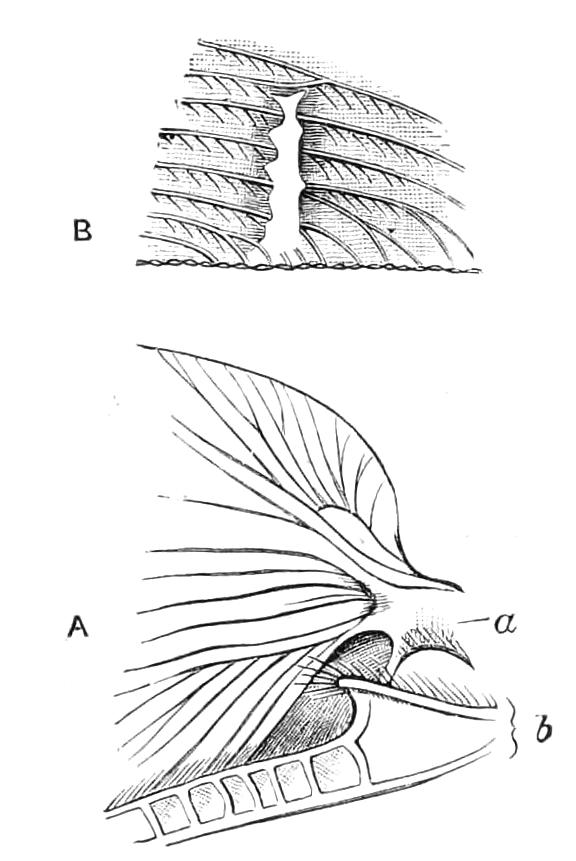
Fig. 312.—Portions of wings of Drepanepteryx phalaenoides. A, Under-face of basal parts of the two wings; a, base of front wing; b, of hind wing. B, Portion of front wing, showing the apparent interruption of nervures.
The genus Drepanepteryx consists of several species, and appears to be best represented in the Antipodes; we have, however, one species in Britain—D. phalaenoides (Fig. 298)—an extremely interesting member of our fauna. This Insect has, like several of its congeners, a moth-like appearance, and it has a peculiar structure for bringing the hind and fore wings into correlation, the costa at the base of the hind wing being interrupted and prominent, furnished with setae (Fig. 312, A), and playing in a cavity on the under-surface of the front wing. This character is of great interest in connexion with analogous structures of a more perfect nature existing in various moths. M‘Lachlan has described and figured[398] a more primitive, though analogous, condition of the wings in Megalomus hirtus, also a species of British Hemerobiina. Another very curious feature of D. phalaenoides is shown in Fig. 312, B, there being a narrow space on the hind part of the front wing from which the colour is absent, while the nervures appear to be interrupted; they are, however, really present, though transparent; the nature of this peculiar mark is quite unknown, but is of considerable interest in connexion with the small transparent spaces that exist on the wings of some butterflies.
Sub-Fam. 6. Chrysopides, Lacewing-flies.—Fragile Insects with elongate, setaceous antennae.
Fig. 315.—Larva of Chrysopa sp. Cambridge. A, The Insect magnified; B, foot more magnified; C, terminal apparatus of the claws, highly magnified.
The lacewing-flies—also called stink-flies and golden-eyes—are excessively delicate Insects, of which we possess about 15 species in Britain. Their antennae are more slender and less distinctly jointed than they are in Hemerobiides, and the Chrysopides are more elongate Insects. The peculiar metallic colour of their eyes is frequently very conspicuous, the eyes looking, indeed, as if they were composed of shining metal; this fades very much after death. Although not very frequently noticed, the Chrysopides are really common Insects, and are of considerable importance owing to their keeping "greenfly" in check.
The eggs are very remarkable objects (Fig. 314), each one being supported at the top of a stalk many times as long as itself; in some species (C. aspersa) the eggs are laid in groups, those of each group being supported on a common stalk. The larvae (Fig. 315) are of a very voracious disposition, and destroy large quantities of plant-lice by piercing them with sucking-spears, the bodies of the victims being afterwards quickly exhausted of their contents by the action of the apparatus connected with the spears. The larvae of two or three species of Chrysopa cover themselves with the skins of their victims after the manner of the larvae of Hemerobius; but most of the larvae of Chrysopa are unclothed, and hunt their victims after the fashion of the larvae of Coccinellidae, to which these Chrysopa larvae bear a considerable general resemblance. These larvae have a remarkable structure at the extremity of their feet, but its use is quite unknown (Fig. 315, B, C). Some larvae of the genus make use of various substances as a means of disguise or protection. Dewitz noticed[399] that some specimens he denuded of their clothing and placed in a glass, seized small pieces of paper with their mandibles and, bending the head, placed the morsels on their backs; here the pieces remained in consequence of the existence of hooked hairs on the skin. Green algae or cryptogams are much used for clothing, and Dewitz supposes that the Insect spins them together with webs to facilitate their retention. According to Constant and Lucas[400] the larvae of Chrysopa attack and kill the larvae {471}of Lepidoptera and Phytophagous Hymenoptera. The curious form we figure (Fig. 316) has been hatched from eggs found by Brauer on Pinus abies in Austria. The eggs were of the stalked kind we have described; the young escaped from them in the autumn, twelve days after deposition, but did not take any food till the following spring.
The Chrysopides are widely distributed over the earth's surface. They form an important part of the fauna of the Hawaiian islands.
Sub-Fam. 7. Coniopterygides.—Minute Insects with very few transverse nervules in the wings; having the body and wings covered by a powdery efflorescence.
These little Insects are the smallest of the Order Neuroptera, and have the appearance of winged Coccidae; their claim to be considered members of the Neuroptera was formerly doubted, but their natural history is quite concordant with that of the Hemerobiid groups, near which they are now always placed. Löw has made us acquainted with the habits and structure of an Austrian species, Coniopteryx lutea Wallg., but for which he has proposed the new generic name Aleuropteryx; the larvae are found on Pinus mughus at Vienna feeding on Aspidiotus abietis, which they pierce with sucking-spears, after the fashion of the Hemerobiides; when full fed they spin a cocoon formed of a double layer of silk, in which metamorphosis takes place in a manner similar to that of other Hemerobiidae. The better-known genus Coniopteryx differs from Aleuropteryx in having the sucking-spears short and nearly concealed by the front of the head, which is somewhat prolonged.
Fig. 317.—Coniopteryx psociformis. Cambridge. (After Curtis.) A, The insect with wings expanded, magnified; B, with wings closed, natural size.
We may conclude this sketch of the Hemerobiid groups by remarking that fossil remains of specimens of most of them have been detected in the Tertiary strata, and that in the Secondary strata these groups are represented by only a small number of fossils, which are referred specially to Hemerobiina, Nymphidina, and Chrysopides.
Fig. 318.—A, Larva of Coniopteryx tineiformis (?). (After Curtis.) B, Head and prothorax of larva of Coniopteryx sp.; C, upper surface of head of larva of Coniopteryx (after Löw), much magnified.
NEUROPTERA CONTINUED—TRICHOPTERA, THE PHRYGANEIDAE OR CADDIS-FLIES
Fam. XI. Phryganeidae—Caddis-flies.
(TRICHOPTERA OF MANY AUTHORS)
Wings more or less clothed with hair, nervures dividing at very acute angles, very few transverse nervules; hind pair larger than the front, with an anal area which is frequently large and in repose plicately folded. Antennae thread-like, porrect, of many indistinct joints. Mandibles absent or obsolete. Coxae elongate and free but contiguous. Metamorphosis great; larvae caterpillar-like, usually inhabiting cases of their own construction. Pupa resembling the perfect Insect in general form, becoming active previous to the last ecdysis. Wingless forms of the imago excessively rare.
The caddis-flies are Insects of moth-like appearance, found in the neighbourhood of water; their larvae live in this element, where they may sometimes be found in abundance. Phryganeidae are not very attractive Insects, and there are few of large size; Hence they have been much neglected by entomologists, and very little is known about the exotic forms of the family. The habitations constructed by the larvae are, many of them, of a {474}curious nature, and usually attract more attention than do the creatures they serve to protect.
The Phryganeidae form the division or series Trichoptera; the two terms are therefore synonymous; those entomologists who consider these Insects to form a distinct Order use the latter appellation for it.
The perfect Insect, though the wings are usually ample, has but feeble powers of flight, and rarely ventures far from the water it was reared in; it has a moth-like appearance, and the wings in repose meet, at an angle, in a roof-like manner over the back (Fig. 326, E). The head is small, with the front inflexed; it has two large compound eyes, and usually three ocelli; the antennae are slender, thread-like, and occasionally attain a great length. The parts of the mouth are very peculiar, the labrum and the palpi—especially the maxillary palps—being well developed, while the lobes of the maxillae and labium are amalgamated and therefore indistinct. The labrum is more or less elongate, and is more mobile than is usual in mandibulate Insects; it is held closely applied to the maxillae. These latter are small, have usually only a single small free lobe; they are united to one another and to the labium by membrane in such a manner as to form a channel along the middle of the mouth, the labrum forming the roof of this channel. The palpi are in some cases (Sericostomatides) of a remarkable nature; their joints vary in number from three to five, and differ sometimes in the sexes of the same species. The lower lip appears as a plate supporting the labial palpi, which are three-jointed and do not exhibit any peculiarities of structure comparable with those we have mentioned as so frequently existing in the maxillary palps. Difference of opinion exists as to the mandibles, some entomologists declaring them to be entirely absent, while others state that a small tubercular process that may be seen in some species on each side of the labrum is their representative. The prothorax is very small, the notum is the largest piece but is quite short, the side-pieces are very small, and the sternum appears to consist only of membrane. The mesothorax is much the largest segment of the body; its sternum {475}is large, but is nearly perpendicular in direction, and is much concealed by the elongate, free front coxae, which repose against it. The metathorax is intermediate in size between the pro- and meso-thorax; its side-pieces are rather large, but the sternum is membranous, with a heart-shaped piece of more chitinous consistence in the middle, entirely covered by the middle coxae. The side-pieces both of the meso- and meta-thorax are large, and are closely connected; the middle and posterior coxae are very large, elongate, and prominent, and the middle pair slope backwards, so that their tips are in contact with the tips of the hind pair. The abdomen is cylindric and rather slender; it looks as if formed of eight segments in addition to the terminal segment; this latter in the male usually bears remarkably modified appendages. The first ventral plate is sometimes, if not always, entirely membranous; indeed the texture of the segments is in general very delicate, so that they shrivel up to an extent that renders their comprehension from dried specimens very difficult. The legs are always elongate, the coxae attaining in some forms a remarkable length, and the tibiae and tarsi are armed with many spines; the tarsi are five-jointed, slender, frequently very elongate, terminated by two large claws and an apparatus, placed between them, consisting of a pair of hair-like processes with a membranous lobe.
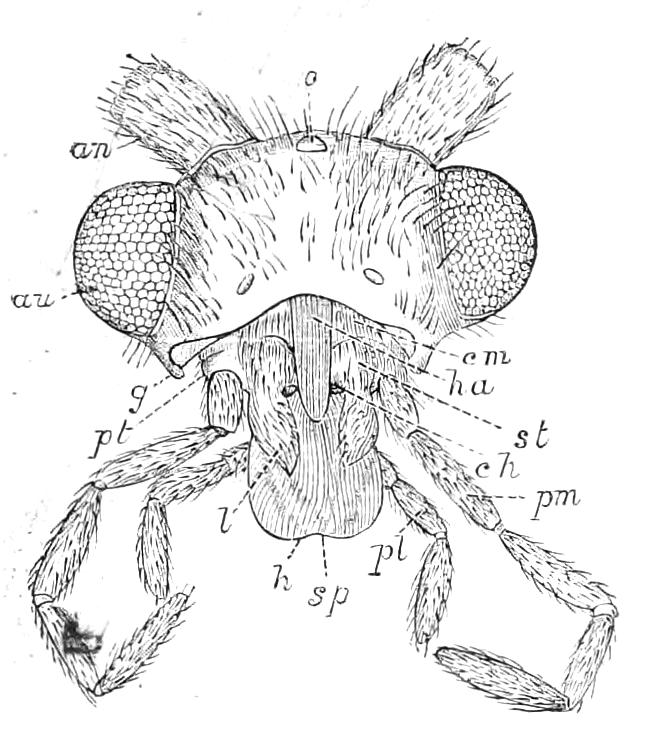
Fig. 321.—Front view of head of Anabolia furcata after removal of labrum. o, Ocellus; an, base of antenna; au, eye; cm, cardo; st, stipes; l, external lobe; pt, support of palpus; pm, palpus of maxilla; g, condyle of articulation of the absent mandible; ha, channel of haustellum; h, haustellum; sp, apex of channel of haustellum (not explained by Lucas); ch, chitinous point of external lobe of second maxilla; pl, labial palp. (After Lucas.)
The structure of the mouth-parts of the Phryganeidae has given rise to much difference of interpretation; it has recently been investigated by R. Lucas[401] in connexion with Anabolia furcata (Fig. 321). He agrees with other observers that mandibles are present in the pupa, but states that no rudiment of them exists in the imago. He calls the peculiar structure formed by the combination of the maxillae and {476}labium a haustellum. He looks on the Trichoptera as possessing a mouth intermediate between the biting and sucking types of Insect-mouths. He considers that the Phryganeidae take food of a solid, as well as of a liquid, nature by means of the haustellum, but the solid matter must be in the form of small particles, and then is probably sucked up by the help of saliva added to it. Lucas says also that in the larvae certain parts of the salivary glands serve the function of spinning organs, and it is from these that the salivary glands of the imago are formed; those salivary glands of the larva that are not spinning glands disappearing entirely.
Fig. 322.—Anabolia nervosa. A, Larva extracted from its case; B, one of the dorsal spaces of the abdominal segments more strongly magnified.
The eggs are deposited in a singular manner; they are extruded in a mass surrounded by jelly; there may be as many as one hundred eggs in such a mass. This is sometimes carried about by the female after its extrusion from the interior of the body, but is finally confided to a suitable place in stream, spring, or pool. It is said that the female occasionally descends into the water to affix the egg-mass to some object therein, but this requires confirmation, and it is more probable that the egg-mass is merely dropped in a suitable situation. As soon as the larvae are hatched they begin to provide themselves with cases; they select small pieces of such material as may be at hand in the water, and connect them together by means of silk spun from the mouth. Particulars as to these tubes we will defer till we have considered the larvae themselves. These have the general appearance of caterpillars of moths; in order to move about they must put their head and the three pairs of legs at the front of the body out of their tube or case, and they then look very like case-bearing caterpillars. The part of the body that usually remains under cover is different in texture and colour, and frequently bears outstanding processes, or filaments, containing tracheae for the purpose of extracting air from the water. Some peculiar spaces of a {477}different texture may be seen on certain larvae (Fig. 322, B); these may possibly be also connected with respiration. On each side of the extremity of the body there is a rather large hook by which the creature attaches its dwelling to its body, and there are also frequently present three large bosses on the anterior abdominal segment, which are supposed to assist towards the same end. The hold it thus obtains is so firm that it cannot be dragged out by pulling from the front; fishermen have, however, discovered a way of extracting it by a strategic operation: the cases are, as a rule, partially open behind, and by putting a blunt object in and annoying the larva it is induced to relax the hold of its hooks and advance forwards in the case, or even to leave it altogether. The firm hold of the larva is maintained in spite of the fact that the body does not fill the case. It is necessary that water should pass freely into and out of the case, and that there should be some space for the respiratory filaments to move in. The mouth of the case is open, and the posterior extremity is arranged by the larva in such manner as to allow a passage for the water; various ingenious devices are adopted by different species of larvae with the object of protecting the hind end of the body, and at the same time of permitting water to pass through the case.
The mode of changing the skin, or the frequency with which this occurs in the larval state of the caddis flies has not been recorded. The duration of life in this stage is usually considerable, extending over several months: indeed in our climate many species pass the winter in this stage, completing the metamorphosis in the following spring or summer; and as one generation each year appears to be the rule, it may be assumed that the larval condition in such cases lasts from seven to ten months. During this stage the Insects are chiefly vegetable feeders, some being said to feed on minute algae; animal diet is not, however, entirely avoided, and it is said by Pictet that not only do some of the Phryganeidae eat other Insects, but that they also sometimes devour their companions.
At the end of the larval period of existence the creature closes its case by a light web spun at each end, taking care not to prevent the ingress and egress of the water; it sometimes adds a stone or piece of stick, and having thus protected itself, changes to a nymph. During the first part of this metamorphosis the creature is completely helpless, for there is so great a difference between the external structures of the larva and nymph as to make the latter a new being, so far as these organs are concerned. The changes take place in the interior of the larval skin, and as they are completed this latter is shed piecemeal. The resulting pupa or nymph greatly resembles the perfect Insect, differing consequently very much from the larva. Pictet, who paid special attention to the nymph condition of these Insects, concludes, however, that many of the organs of the nymph are actually formed within the corresponding parts of the larva, and has given a figure that, if trustworthy, shows that the legs of the nymph, notwithstanding the great difference between them as they exist in the larva and in the perfect Insect, are actually formed within the legs of the larva; each nymphal leg being rolled up in the skin of the corresponding larval leg, in a spiral, compressed manner, and the only articulations that can be detected in the leg being those of the tarsus. The head of the nymph is armed in front with two curious projections that are, in fact, enormously developed mandibles (Fig. 323, B); they serve as cutting implements to enable the nymph to effect its escape from its prison; they are cast off with the nymph-skin, the perfect Insect being thus destitute of these organs. The abdomen of the nymph differs from that of the perfect Insect in possessing external respiratory filaments; the nymphs of some species have also the middle legs provided with swimming-hairs, that do not exist in the imago.
As a rule the larvae bring the respiratory filaments into contact with the water by moving the abdomen, but Fritz Müller found[402] that those of a Macronema move the gills themselves—after the manner of Ephemeridae—with much rapidity. Many kinds of larvae of Phryganeids possess at the posterior extremity of the body exsertile pouches in the form of finger-like, or even branched, processes into which tracheae do not enter. Müller observed that in the Macronema alluded to these pouches were generally not {479}exserted; when, however, the larva ceased to move the tracheal gills, then these pouches were protruded. He is inclined to consider them blood-gills. Similar structures are found in Eristalis and some other Dipterous larvae that have to breathe under difficulties.
The imagines of certain species possess filaments—or something of the sort—on the abdomen. Palmén, who has examined these organs in Hydropsyche, thinks that they are the remains of gills that existed in the larva and pupa, and that they are functionless in the imaginal instar. M‘Lachlan thinks that in Diplectrona, where the filaments are elongate, they may be functionally active even in the imago.[403]
The skin of the nymph is at first very soft, but it soon hardens, and when about fifteen or twenty days have elapsed the nymph opens its case by means of the mandibular processes, and swims through the water with its back downwards till it reaches some solid object by which it can ascend to the air; the nymph skin then swells and splits, and the thorax of the imago protrudes; this is soon followed by the disengagement of the head and other parts, and the imago having thus escaped, the nymph skin remains a complete model of the external structure of the nymph, and contains a considerable number of tracheae. This sketch of the metamorphosis of a caddis-fly does not apply in all its details to all the forms of caddis-flies, there being exceptions, as we shall mention hereafter.
Dewitz has described[404] the first appearance and development of the wings in larvae of Phryganeidae. Each one appears at first in the form of a small thickening of the hypodermis, accompanied outwardly by a minute depression of the chitin (Fig. 324, A). He compares the structure in the earliest stage to the entothoracic projections into the interior of the body. The rudiment grows as the larva increases in size, the chitinous portion being duly shed at the ecdyses. When the rudiment is larger and more complex, a mesoderm layer appears in it (Fig. 324, B); this is derived from a nerve-sheath near the rudiment. During the resting state of the larva—after its case has been closed, but before the pupal form has appeared—the wing assumes the form and position shown in C, Fig. 324. Dewitz's description of the process leaves much to be desired, and it is doubtful whether in {480}C the position of the wing on the exterior of the body is due to the stripping off of the chitinous integument, or to a process of eversion, or to both.
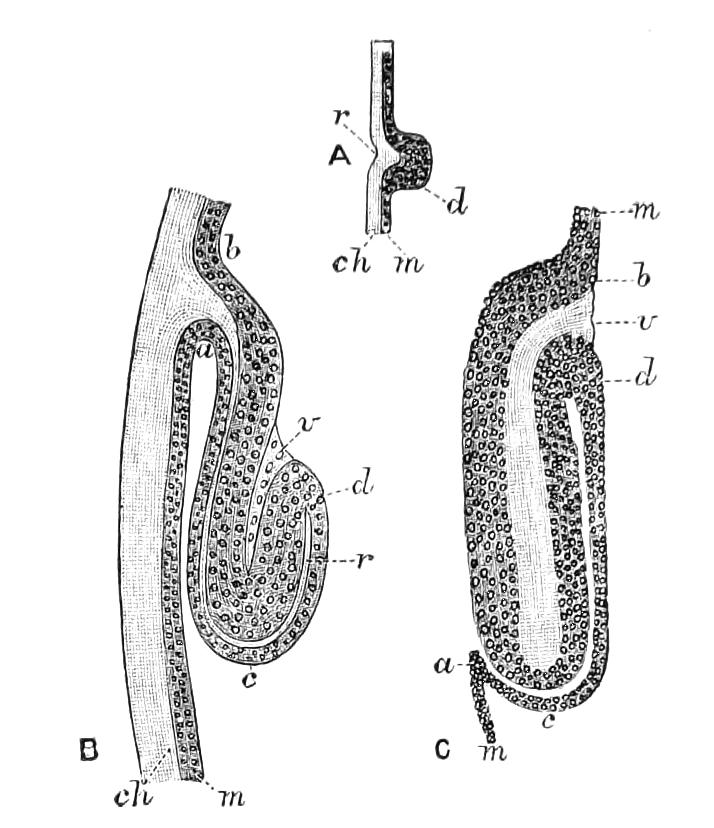
Fig. 324.—Development of wings of Phryganeidae. (After Dewitz.) A, Portion of body-wall of young larva of Trichostegia; ch, chitin, forming at r a projection into the hypodermis m; r and d forming thus the first rudiment of the wing. B, The parts in a largely grown larva; a, c, d, b, the much grown hypodermis separated into two parts by r, the penetrating extension of the chitin; v, mesoderm. C, Wing-pad of another Phryganeid freed from its case at its change to the pupa; b, d, outer layer of the hypodermis, m, of the body-wall; v, inner layer without nuclei.
Fig. 325.—Cases of British Trichoptera. A, Of Odontocerum albicorne; A1, its termination; B, quadrangular case of Crunoecia irrorata; B1, mouth of case.
There are about 500 species of this family of Insects known as inhabiting the European region, and about 150 of this number occur in Britain. These are arranged by M‘Lachlan[405]—whose zealous and persevering work at this neglected family of Insects is beyond praise—in eight sub-families, on a system in which the structure of the maxillary palpi plays a principal part; they are called Phryganeides, Limnophilides, Sericostomatides, Leptocerides, Oestropsides, Hydropsychides, Rhyacophilides, Hydroptilides. The first three of these form the division "Inaequipalpia."
Phryganeides.—This group includes the largest forms of the family, and appears to be almost confined to the temperate regions of the northern hemisphere, though a few species are already known from the corresponding districts of the southern hemisphere. This feature in their geographical {481}distribution is, however, by no means peculiar to them, for a similar discontinuity of distribution exists in numerous other groups of Insects, and even in other divisions of the Phryganeidae.
The Phryganeides almost without exception inhabit still waters, and it is more specially to them that the brief sketch of metamorphosis given in the preceding pages will be found to apply. The larva always has the respiratory filaments simple and thread-like, though elongate, and lives in a case that it carries about; this case is open at both ends, and the larva is said to occasionally cut off the end having the least diameter and increase the other end, thus accommodating the habitation to its own growth.
Limnophilides.—These Insects have only three, instead of four, joints in the maxillary palpi, but in most other respects agree with the Phryganeides. There is, however, greater variety in the habits of the larvae, though all live in free cases. In the genus Enoicyla (Fig. 326) we meet with the anomaly of a Trichopterous Insect that lives amongst moss and dead leaves, far away, it may be, from water. The cases of the Limnophilides are constructed of a great variety of materials, and are often decorated with shells containing living inmates.
In the genus Apatania the phenomenon of parthenogenesis is thought to occur, there being at least two species in which no male specimen has ever been discovered, though M‘Lachlan has made special efforts to discover the sex of A. muliebris. It should, however, be stated that these species have not been extensively investigated; A. arctica has been detected in the Arctic regions, and A. muliebris has occurred in several localities in Europe, in Britain chiefly near Arundel in a lake of intensely cold water.
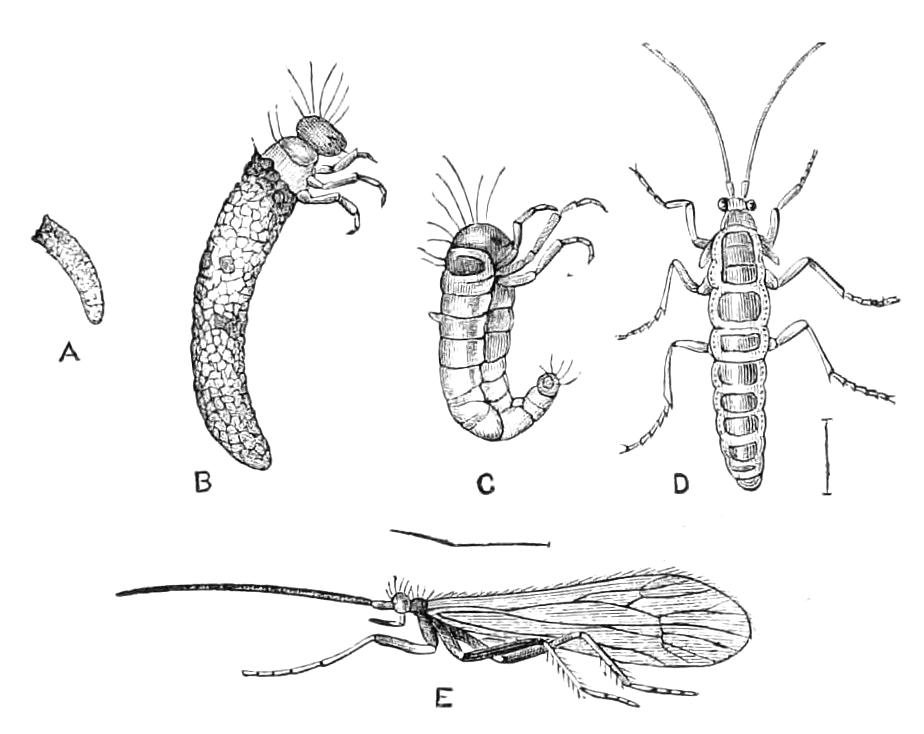
Fig. 326.—Metamorphoses of Enoicyla pusilla. (After Ritsema.) A, Case of full-grown larva; B, larva and case magnified; C, larva extracted; D, wingless adult female; E, male.
Sericostomatides, like the Limnophilides, is a group rich in species; the larvae are chiefly found in streams. They form portable cases out of sand and stones (Fig. 325, B, case of Crunoecia irrorata) in preference to vegetable matter. It is here that the genus Helicopsyche, which for long was an enigma to naturalists, is now placed. This genus consists of Insects whose larvae form spiral cases, similar to small snail shells, of sand or minute stones. These objects occur in various parts of the world. Fritz Müller[406] has informed us that the larva inhabiting one of them, when it withdraws entirely within its abode to repose, takes the precaution of anchoring its snail-like habitation, fixing it to a rock or stone by spinning some temporary silken threads. The respiratory filaments in this group are filiform.
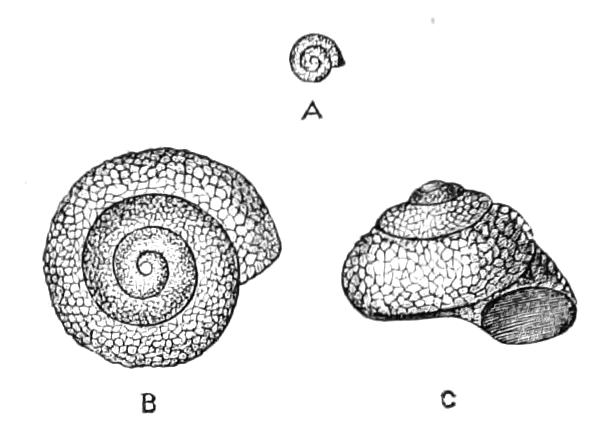
Fig. 327.—Cases of Helicopsyche shuttleworthi. (After von Siebold.) A, Natural size; B, C, magnified.
Leptocerides.—The first group of the division Aequipalpia; so that there are five-jointed maxillary palpi in both sexes; these organs are frequently developed in a remarkable manner. The antennae are usually extremely long and slender. The case of the larva is portable (Fig. 325, A, case of Odontocerum); the respiratory filaments are not very conspicuous; they form short tufts placed on various parts of the abdomen. Müller[406] has called attention to a species whose larva lives in Brazil between the leaves of Bromeliae on trees.
The Oestropsides is a small group, and has recently been reduced by M‘Lachlan to the rank of an inferior division.
Hydropsychides.—An extensive group, in which the larvae are believed to be chiefly of carnivorous habits. They vary, according to species, as to the nature of the respiratory filaments, and live in fixed abodes; these are less tubular than is the rule with the portable cases, and are formed from pieces of sand and stone spun together and fixed to larger stones under water. Sometimes several larvae live together in loosely compacted structures of this kind, and only form true cases when about to undergo their metamorphosis. Müller describes[406] a Brazilian species of Rhyacophylax as forming a case in which the mouth-end has a {483}large funnel-shaped verandah, covered by a beautiful silken net. This larva lives in the rapids of various rivulets, and the entrance to the verandah is invariably directed towards the upper part of the rivulet, so as to intercept any edible material brought down by the water. Several of these larvae, moreover, build their cases so that they form a transverse row on the upper side of a stone; as many as thirty cases may be placed in one of these rows, and sometimes several rows are placed parallel with one another. This same larva has the habit of coming out of its case when necessary, and suspending itself in the water—as some caterpillars do in the air—by means of a silken thread. Other members of the Hydropsychides form tubes or covered ways of silk, earth and mud attached to stones, and in which they can move freely about. Some of the Hydropsychidae have been ascertained with certainty to be carnivorous in the larval state. A species of the genus Hydropsyche has been found by Howard[407] to help itself in the task of procuring food by spreading a net in the water in connexion with the mouth of its case. This net is woven in wide meshes with extremely strong silk, and supported at the sides and top by bits of twigs and small portions of the stems of water-plants. Small larvae brought down by the current are arrested by this net for the advantage of the larva that lurks in the tube. The breathing organs of the larvae of Hydropsychides are apparently of a varied character, and would well repay a careful study. Mr. Morton informs the writer that some of our British species of Philopotamus and Tinodes have no gills either in the larval or pupal state, and probably respire by means of modified tracts in the integument. In some of the allied genera, e.g. Polycentropus, the larvae are destitute of gills, but the pupae possess them.
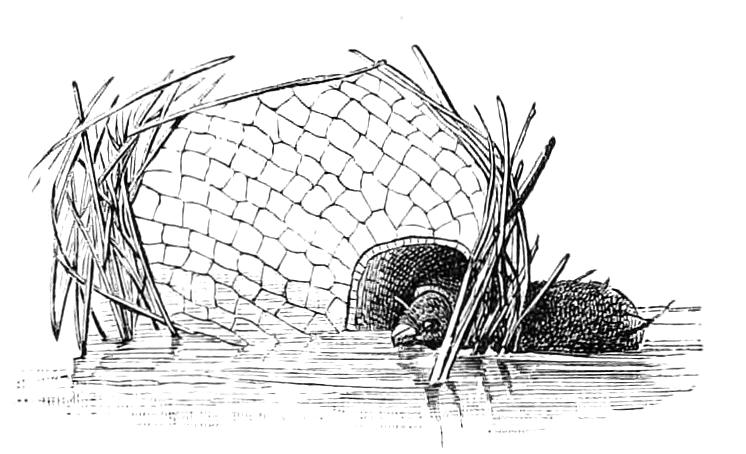
Fig. 328.—Case, with head of larva and snare of North American Hydropsychid. (After Riley and Howard.)
The Rhyacophilides is another group in which the larval habitations are fixed. Some of these larvae have no respiratory filaments, breathing only by means of the stigmata, but others have tufts of filaments. These Insects have a peculiarity in their {484}metamorphosis inasmuch as the larva, instead of lying free, constructs a cocoon in its case or other habitation in which to change to a nymph. In the larvae that do not make use of a portable case the abdominal hooks are not essential, and are replaced by other organs differing much in structure, being sometimes apparently of a sensitive nature, in other forms possibly respiratory. Müller tells us of a carnivorous larva of this group in which the anterior legs are armed with powerful forceps for predatory purposes.
The Hydroptilides comprise the most minute of the Phryganeidae, and their species will probably prove to be very numerous in well-watered tropical regions, though few have yet been described from there. The perfect Insects (Fig. 320) bear an extreme resemblance to small moths of the group Tineidae. The larvae (Fig. 329) are destitute of respiratory filaments, and construct portable cases of a variety of forms, some resembling seeds. Müller has given particulars of a curious nature as to the cases of some Brazilian Hydroptilides; one species moors its dwelling to a stone by means of a long silken cable, by this artifice combining safety with the power of ranging over a considerable extent of water. In Diaulus there is only a narrow slit at each end of the case, but one side of it is provided with two chimneys to permit the flow of water for respiratory purposes.
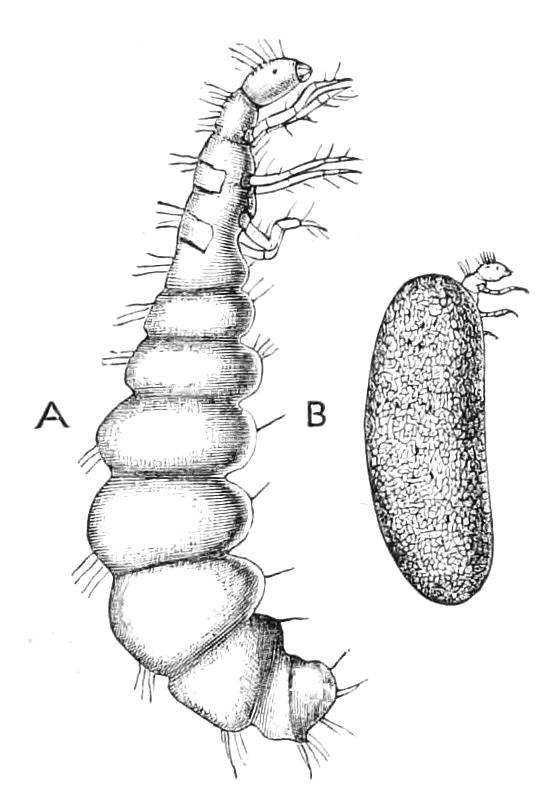
Fig. 329.—Hydroptila maclachlani. B, Case with larva magnified; A, larva more magnified. (After Klapálek.)
The larva of Oxyethira (Fig. 330) is a curious form, possessing comparatively long legs, and a head and thorax slender in comparison with the distended hind body. The cases are fastened, for the purposes of pupation, to a leaf of a water-lily.
Some very curious anomalies as regards the development of the wings exist in the Phryganeidae; Anomalopteryx, for instance, has the wings quite short and useless for flight in the male, while in the other sex they are ample; in Enoicyla—the curious Insect figured on p. 481, in which the larvae are of terrestrial habits—we find the females with only rudiments {485}of wings, while in Thamastes the posterior wings are absent in both sexes. These anomalies are at present quite inexplicable; and we may here mention that we are in complete ignorance as to the functional importance of many of the peculiarities of the Phryganeidae. We do not know why the mouth is reduced from the normal state, the maxillary palpi being, on the other hand, extraordinarily developed; we do not know the importance of the numerous spines and of the spurs on the legs, nor of the hairs on the wings, although these are amongst the most characteristic of the special features of this group of Insects.
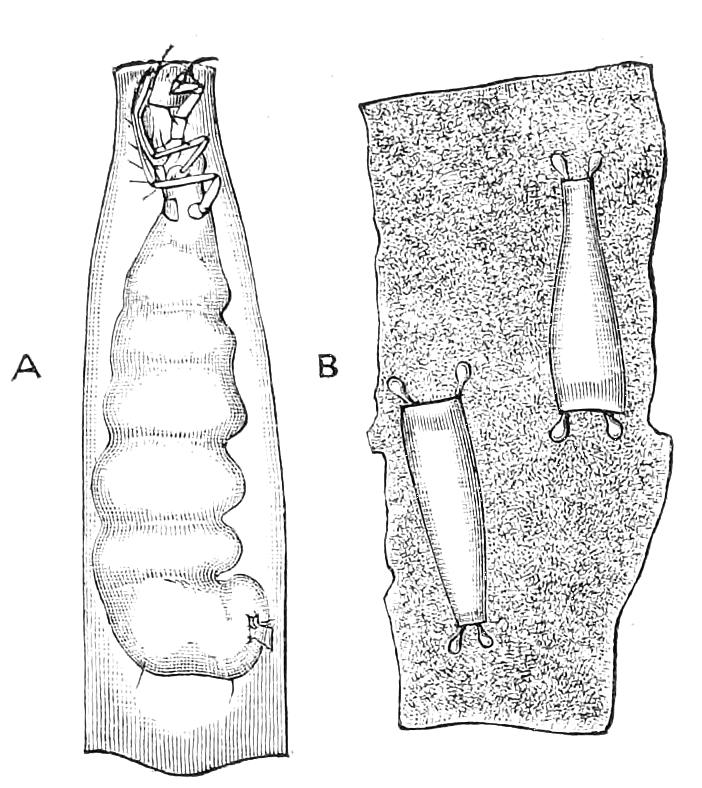
Fig. 330.—Oxyethira costalis. A, Larva in case; B, cases fastened to leaf for pupation. (After Klapálek.)
Fossils.—Abundant remains of Phryganeidae belonging to the Tertiary epoch have been discovered. They are common in amber, and it is a remarkable fact that a larval case has been found in amber. This seems almost inexplicable, except on the assumption that such larvae were of arboreal habits, a condition that, at the present time, must be excessively rare, though the terrestrial habits of Enoicyla warrant us in believing it may occur. In the Tertiary Lake Basin at Colorado the remains of Phryganeidae in the imago state are extremely abundant, so that it is curious that but few such remains have been found in Europe. In Auvergne the so-called indusial limestone, which is two or three yards thick over a wide area, is considered to be composed chiefly of the cases of larvae of this family.
In the Mesozoic epoch some wings found in the lower Purbeck strata are considered to be those of Phryganeidae; similar wings have been found in the Lias, but this is the only evidence of the existence of the family at that period except a tube, supposed to be a larval case, detected in the Cretaceous of Bohemia. Earlier than this nothing has been discovered that can be connected with the family, so that at present the palaeontological evidence appears unfavourable to the view held by some that the Phryganeidae may be considered forms allied to the early {486}conditions of the Lepidoptera. It should be noted that the head in Phryganeidae is the most important part from a systematic point of view, and that fossils have been chiefly identified from the wings; this is a much more doubtful character, as the wings of the Phryganeidae have a simple system of neuration, and in shape have nothing very characteristic.
Extinct Order Palaeodictyoptera.
This seems to be the fittest place to notice the existence of some fossil remains from the Palaeozoic rocks that cannot be fitly, or certainly, assigned to any of our existing Orders, and to which the above name has consequently been given. These remains consist chiefly of wings in a more or less imperfect state of preservation, and it is therefore quite doubtful whether the course of assigning them to a separate Order supposed to be extinct be correct. This is all the more doubtful when we recollect that an Insect fossil, Eugereon bockingi, having the head with mouth-parts of a Hemipterous or Dipterous nature, has been found, the wings attached to it being such as, had they been found separate, would have been considered to be Neuropterous, or at any rate allied thereto. About forty-two forms of Palaeodictyoptera are assigned by Scudder to a section called Neuropteroidea, and may therefore be considered to have a special resemblance to our Neuroptera. These Neuropteroidea comprise numerous genera and no less than six families. Scudder's view is at the best tentative, and is not very favourably received by some entomologists. Brauer has, indeed, objected altogether to the formation of this Order Palaeodictyoptera, and Brongniart has published a list of the Palaeozoic Insects in which a system of arrangement different to that of Scudder is adopted. In his most recent work[408] Brongniart assigns some of these Neuropteroidea to the families Platypterides and Protodonates, which we have previously discussed. The whole subject of these Palaeozoic Insect remains is still in its infancy, and it would not be proper to accept any view as final that has yet been stated, nor would it be fair to dismiss the subject as unimportant because of the great divergence of opinion amongst the authorities who have investigated it.
HYMENOPTERA—HYMENOPTERA SESSILIVENTRES—CEPHIDAE—ORYSSIDAE—SIRICIDAE—TENTHREDINIDAE OR SAWFLIES
Order IV. Hymenoptera.
Wings four, membranous, without scales, usually transparent, never very large, the posterior pair smaller than the anterior; the cells formed by the nervures irregular in size and form, never very numerous (less than twenty on the front, than fifteen on the hind, wing). Mandibles conspicuous even when the other parts of the mouth form a proboscis. The side-pieces of the prothorax are disconnected from the pronotum and overlap the prosternum, usually entirely concealing it. The females are furnished at the extremity of the body with either saw, sting, or ovipositor; these parts may either be withdrawn into the body or be permanently protruded. The metamorphosis is great and abrupt, the chief changes being revealed in the pupa disclosed at the last moult of the larva; this moult is frequently delayed till long after growth has been completed. In the pupa the parts of the perfect Insect are seen nearly free, each covered in a very delicate skin.
The term Hymenoptera includes ants, bees, wasps, sawflies, and the tribes of innumerable Ichneumon-flies. The Order is of enormous extent, consisting even at present of tens of thousands of described and named species, and yet these are but few in comparison with those that remain unknown. It has good claims to be considered the "highest" Order of Insects. Sir John Lubbock says: "If we judge animals by their intelligence as evinced in their actions, it is not the gorilla and the chimpanzee, but the bee, and above all the ant, which approach nearest to {488}man."[409] The mechanical perfection of the structures of the individuals, and the rapid and efficient manner in which their functions are discharged, are very remarkable. In many species of Hymenoptera the individuals have the habit of living together in great societies, in which the efforts of the members are combined for the support of the whole society and for the benefit of a younger generation. To fit them for this social life the bodies of the larger number of the individuals are more or less changed in structure, so that they become workers. These workers are in all cases imperfect females; besides carrying on the ordinary work of the society, they tend and feed the young. The duty of reproduction is restricted to a single female, called a queen, or to a small number of such individuals in each society. The males occupy an unimportant position in the society, and are usually much shorter-lived than the workers and queens. The social Hymenoptera do not form a single zoological group, but are of three different kinds—wasps, bees, and ants. There are numerous non-social, or solitary, wasps and bees.
In the Order Hymenoptera—especially in the higher forms—the males and females are often different in appearance and structure. In the ants, one of the social groups, the workers, or imperfect females, are quite wingless. There are numerous other groups in which species, not social, are found, having the females wingless while the males have wings. In a few species there is an apterous condition of the male, perhaps usually only as a {489}dimorphic form. In the parasitic division there are species that are apterous in both sexes. The structure of the outer skeleton, or external part of the body, exhibits some peculiarities, the chief of which is the detachment of the side-pieces of the prothorax and their great development. Not less remarkable is the abstraction of a segment from the abdomen to become, as it were, part of the thorax; while between the first and second true segments of the abdomen there exists a joint, or articulation, of the utmost mechanical perfection, enabling the operations of stinging and piercing to be executed with an accuracy that cannot be surpassed.
As a result of the detachment of the thoracic side-pieces, the front legs and the structures connected with them are disjoined from the notum, as shown in Fig. 332, and act in connexion with the head, while the dorsal portion of the segment remains attached to the great thoracic mass. The head is quite free from the thorax and very mobile; the upper organs of the mouth—the labrum and the mandibles—are not subject to modifications equal to those exhibited by the maxillae and lower lip, which parts in the bees are prolonged to form a suctorial apparatus that may exceed in length the whole body of the Insect. The mandibles remain cutting or crushing implements even when the maxillae and lower lip are modified to form a suctorial apparatus of the kind we have mentioned; so that in the higher forms—ants, bees, and wasps—the mouth-pieces are completely differentiated for two sets of functions, one industrial, the other nutritive.
Behind the head there is a large consolidated mass representing the thorax of other Insects, but made up, as we have already indicated, in an unusual manner, and which therefore may be called by a special name, the alitrunk (Fig. 333). The pronotum forms the anterior part of the alitrunk, with which it is usually very closely connected, being indeed frequently immovably soldered {490}thereto. It exhibits, however, considerable variety, and is seen in its simplest and least soldered state in Cephus. In the higher bees the pronotum takes on a form not seen in any other Insects, being one of the most beautiful sclerites to be found in the class (Fig. 334, pronotum of Xylocopa). We have already remarked that in Hymenoptera the lower portions of the prothoracic segment are detached from the upper, so that the pronotum is not supported beneath by a sternum as usual. In the bees in question the pronotum makes up for the removal of the corresponding side-pieces and sternum, by becoming itself a complete ring, its sides being prolonged and meeting in the middle line of the under surface of the body. At the same time a large lobe is developed laterally on each side, overlying and protecting the first breathing orifice. The intermediate stages of this remarkable modification may be observed by dissecting a small series of genera of bees.
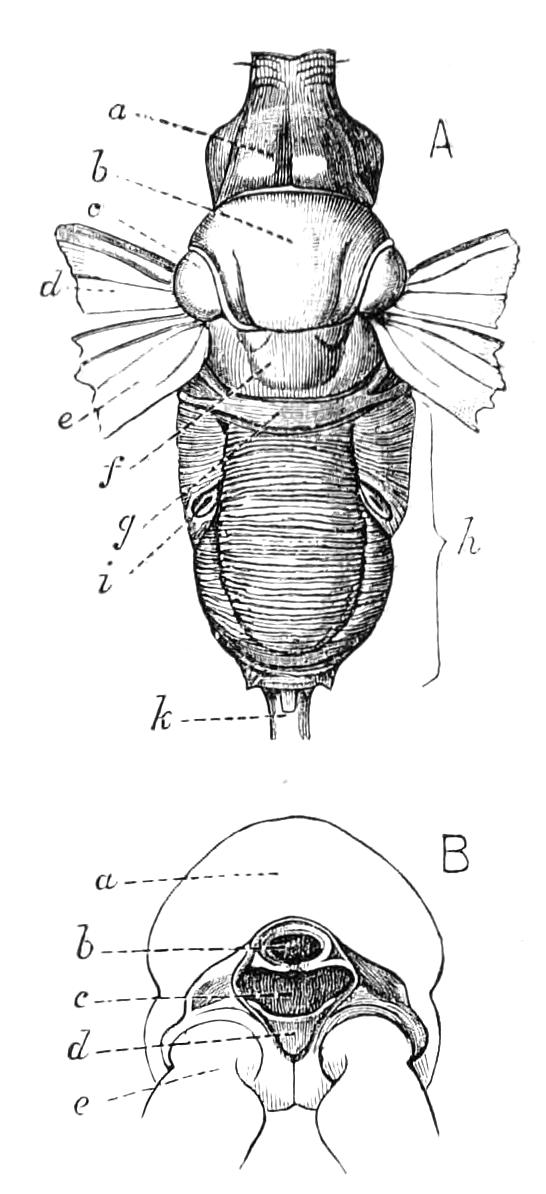
Fig. 333.—Alitrunk of Sphex chrysis. A, Dorsal aspect: a, pronotum; b, mesonotum; c, tegula; d, base of anterior, e, of posterior, wing; f, g, divisions of metanotum; h, median (true first abdominal) segment; i, its spiracle; k, second abdominal segment, usually called the petiole or first abdominal segment. B, Posterior aspect of the median segment: a, upper part; b, superior, c, inferior abdominal foramen; d, ventral plate of median segment; e, coxa.
Although the prosternum of a Hymenopterous Insect is not usually visible owing to its being overwrapped by the side-pieces, it is really, as shown in Fig. 335, B, of complicated form. In Cimbex and some other sawflies the side-pieces are not so large as usual, but the prosternum is larger and is exposed. The prothoracic spiracle is rarely visible externally, but its position is remarkably constant, and is usually indicated by a peculiar lobe or angle of the pronotum projecting backwards just below {491}the insertion of the front wing. This stigmatic lobe is frequently fringed with short hairs.
The mesothorax is the largest of the three divisions of the thorax proper; its notum is large, and usually divided into two parts by a transverse suture. The side-pieces are so placed that the epimeron is rather behind than below the episternum. The mesosternum forms the larger part of the under-surface of the alitrunk. A very large phragma projects from the mesothorax into the interior of the body. The mesothoracic spiracle is usually not visible; its existence was unknown to the older entomotomists, who were in consequence led to consider the spiracle of the median segment as belonging to the thorax. The mesothoracic spiracle is, however, easily seen in Cimbex, placed in the suture between the mesothoracic epimeron and the metathoracic episternum, a little below the insertion of the front wing; close to this spot the mesophragma, just spoken of, comes, in Cimbex, to the surface. The mesothoracic spiracle is generally conspicuous in the worker ant. The parts of the metathorax are usually small, but so much variety prevails in this respect that no general description can be given.
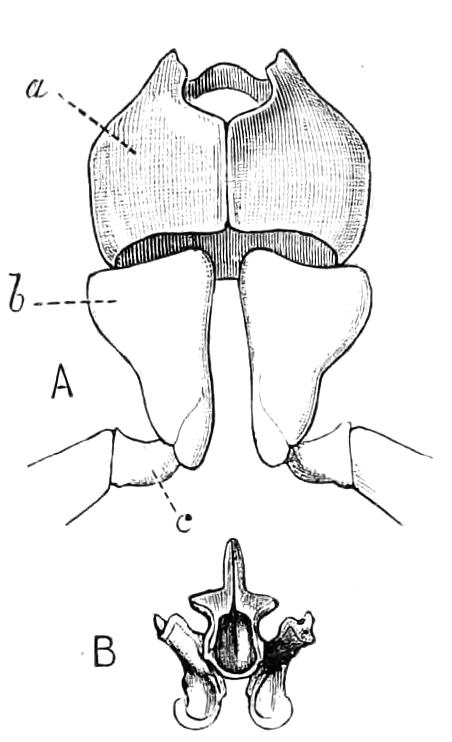
Fig. 335.—Articulation of front legs of the hornet (Vespa crabro, ♀). A: a, side-piece of prothorax overlying the prosternum; b, coxa; c, trochanter. B, prosternum proper, as seen from front when extracted.
The structure of the posterior part of the alitrunk has given rise to an anatomical discussion that has extended over three-quarters of a century,[410] with the result that it is now clear that the posterior part of what appears to be thorax in Hymenoptera is composed of an abdominal segment. This part has been called "Latreille's segment," the "median segment," and the "propodeum." The latter term was proposed by Newman, under the form of propodeon,[411] and appears to be on the whole the most {492}suitable term for this part, which is of great importance in systematic entomology, owing to the extreme variety of characters it affords. Although it is clear that the propodeum is, in large part, an abdominal segment, yet its morphology is still uncertain; what parts are pleural, what tergal, and what may be chitinised spiracular area, or portions of the metathorax, being undetermined. The ventral portion of the propodeum affords a strong contrast to the dorsal part, being so small that it has frequently been described as absent; it is, however, not difficult to detect it in the position shown at d, Fig. 333, B.
Although the true first segment of the abdomen is detached from its normal position and added to the thorax, yet the term abdomen is conventionally restricted to the part that commences with the true second segment, which, in counting the number of abdominal segments, is reckoned as being the first. There are two modes of articulation of the Hymenopterous abdomen with the alitrunk; in one (Fig. 336, A) the base of the abdomen remains of the calibre usual in Insects, while in the other (Fig. 336, B) it is greatly contracted, so that the two parts are connected only by a slender stalk, the petiole. The petiole, besides articulating in a very perfect manner with the propodeum by means of certain prominences and notches, is also connected therewith by means of a slender ligament placed on its dorsal aspect and called the funiculus, shown in Fig. 333, A, just at the extremity of the pointing line k. This mode of articulation gives great freedom of motion, so that in some Petiolata (Ampulex) the abdomen can be doubled under the body and the sting brought to the head. It is worthy of note that even in the Sessiliventres—as the sub-Order with broad-based abdomen is called—some amount of movement exists at the corresponding spot; while, as shown in Fig. 336, A, between a and b, there exists an exposed membrane, the homologue of the funiculus.
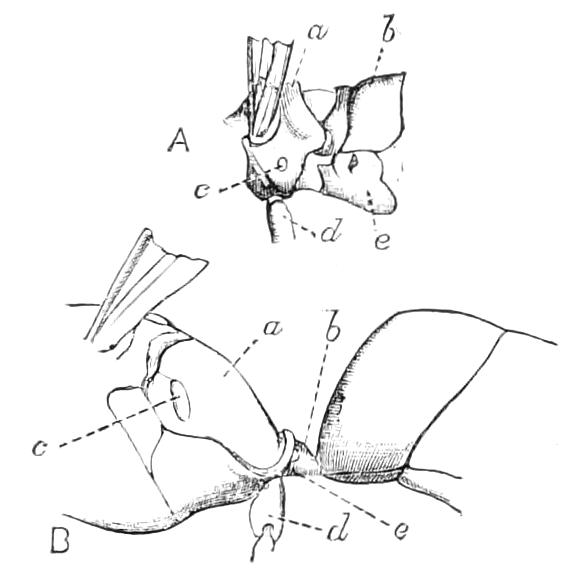
Fig. 336.—Articulation of abdomen and alitrunk of, A, Cimbex, B, Vespa. a, Propodeum or median segment; b, dorsal plate of first (second true) abdominal segment or petiole; c, spiracle of the propodeum; d, hind coxa; e, ventral plate of first (second true) abdominal segment.
The number of abdominal segments that can be seen in the {493}perfect Insect varies greatly. In Tenthredinidae nine can be distinguished, while in some of the Chrysididae it is difficult to detect more than three behind the petiole. These distinctions are, however, superficial or secondary, being due to changes in the later life in connexion with the stings or borers; in the larvae that have been examined thirteen segments behind the head have usually been detected.
Nothing is more remarkable in the Hymenoptera than the great differences that exist in the form of the petiole. This may be very short, as in the bees, so that the abdomen when not deflexed does not appear to be separated from the thorax (Fig. 331, C); in this condition it is said to be sessile, a term which it would be well to replace by that of pseudosessile. In many of the solitary wasps the petiole is very long. In ants it is replaced by one or two curiously-shaped small segments called nodes (Fig. 60, B, 2, 3), and in many ants these are provided with structures for the production of sound. The abdomen is formed by a system of double imbrications; each dorsal plate overlaps each ventral plate, and the hind margin of each segment embraces the front part of the one following; thus this part of the body has not only great mobility, but is also capable of much distension and extension. The pleura are apparently absent, but each one has really become a part of the dorsal plate of the segment to which it belongs. This is shown to be the case by Cimbex, where the division between pleuron and dorsal plate exists on each segment except the basal one. Owing to this arrangement, the abdominal stigmata in Hymenoptera appear to be placed on the dorsal plates.
The organs for mechanical purposes existing at the extremity of the body in Hymenoptera exhibit a great diversity of form; they are saws, borers, piercers, or stings. Notwithstanding their great differences they are all, in their origin, essentially similar, and consist of six parts developed from limb-like prolongations on the penultimate and antepenultimate segments of the larva, as described by Packard and Dewitz.[412] These processes have by some been thought to be not essentially different from abdominal legs, and Cholodkovsky has recently advocated this opinion.[413]
The legs of bees exhibit modifications for industrial purposes. In the stinging Hymenoptera the trochanters are usually of a single piece, and these Insects are called monotrochous; but in most of the other forms the trochanters are more or less distinctly divided into two parts (Fig. 345, b). The usual number of joints in the tarsus is five, but is subject to diminution in many cases. In the bees and ants the first joint is altered in form; in the bees to act as an instrument for gathering or carrying pollen; in the ants to act, as it were, as a second tibia. Between the claws there is a very perfect pad, already described and figured on p. 106.
The wings are remarkable for the beautiful manner in which the hinder one is united to the anterior, so that the two act in flight as a single organ. The hind wing is furnished with a series of hooks, and the hind margin of the front wing is curled over so that the hooks catch on to it. In some of the parasitic forms the wings are almost destitute of nervures, and have no hooks. The powers of flight in these cases are probably but small, the wings merely serving to float the Insect in the air. In some Hymenoptera, especially in Pompilides and Xylocopa, the wings may be deeply pigmented or even metallic; and in some forms of Tenthredinidae, Ichneumonidae, and Braconidae the pigmentation assumes the form of definite patterns.
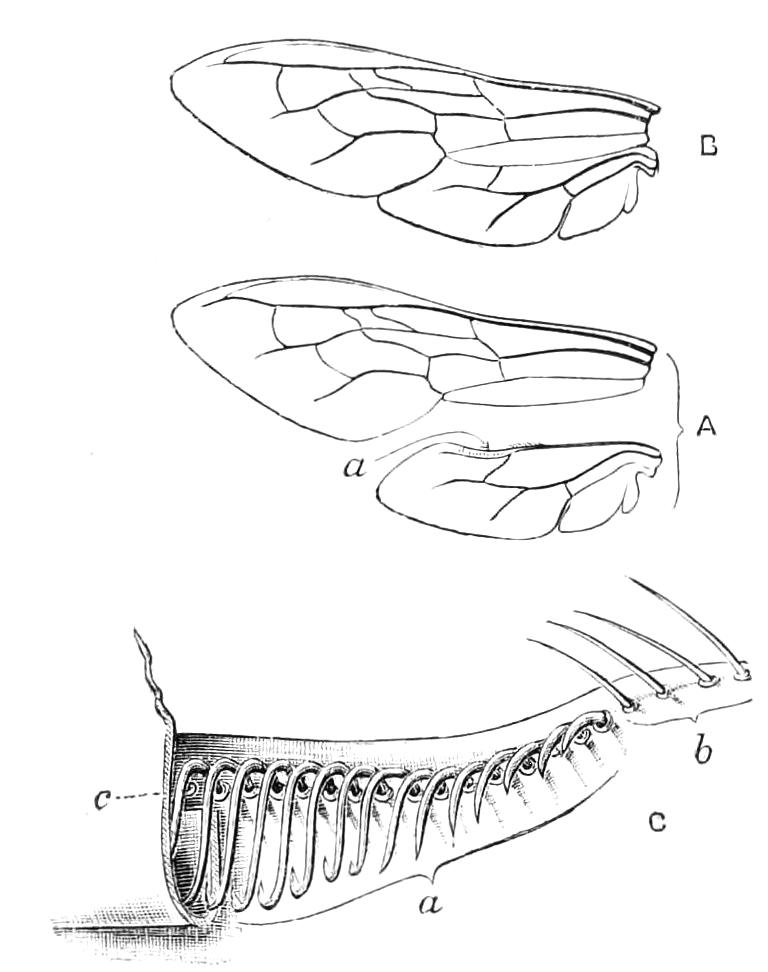
Fig. 337.—Wings of a carpenter bee. A, The pair of wings separated; a, position of the hooks: B, the same wings when united by the hooks. C, Portions of the two wings: a, the series of hooks; b, marginal hairs; c, portion of edge of front wing, of which the other part has been broken away in order to show the hooks.
The studies of the internal anatomy of Hymenoptera are at present by no means numerous or extensive. The alimentary canal (Fig. 69) possesses a crop, gizzard, and chylific stomach in addition to the oesophagus and intestine. The social Hymenoptera have the power of disgorging matter from the alimentary canal for the purpose of supplying food for their young.
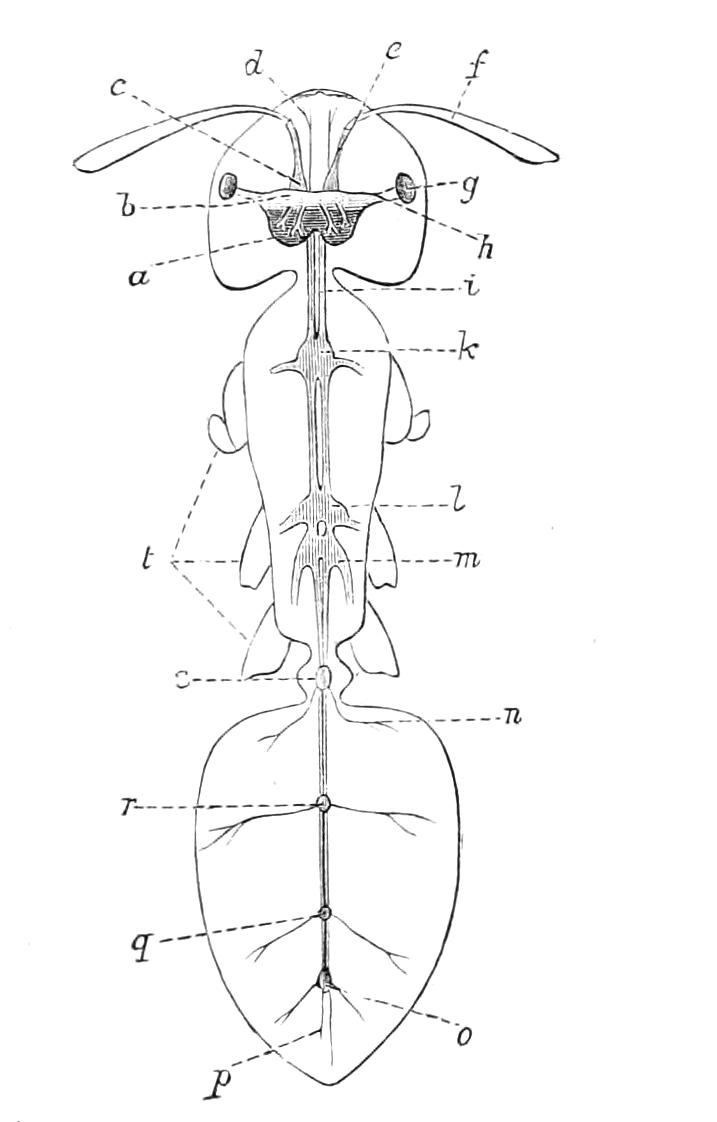
Fig. 338.—Central nervous system (supra-oesophageal ganglion and ventral chain) of a worker ant, Camponotus ligniperdus. (After Forel.) a, Cerebral hemisphere; b, primordial cerebral lobe or pedunculate body (depressed so as to show other parts); c, olfactory lobe (raised from natural position); d, nerve to labrum; e, antennary nerve; f, scape of antenna; g, eye; h, optic nerve; i, longitudinal commissures connecting the hidden sub-oesophageal ganglion with k, the prothoracic ganglion; l, mesothoracic, m, metathoracic ganglion; s, ganglion of the petiole; n, nerve from petiole to other part of abdomen; r, q, o, 2nd, 3rd, 4th abdominal ganglia; p, terminal nerve to cloaca; t, bases of legs.
The crop—which is situated in the anterior part of the abdomen—is the reservoir that contains this matter. The mode of disgorgement is believed to be pressure exerted on the crop by contraction of the abdomen. Salivary glands are present in remarkable variety. The tracheal system possesses, in the higher winged forms, large saccular dilatations situated at the side of the abdomen. The nervous system is of peculiar interest on account of the high intelligence of many of the members of this Order; and on this point of the anatomy, Brandt[414] has made rather extensive investigations, having examined it in the adult of seventy-eight species, and in the larva of twenty-two. In the adult there are two cephalic—the supra- and the sub-oesophageal—two or three thoracic, and from three to seven abdominal ganglia. The bees, wasps, and some other of the Aculeata have only two thoracic ganglia, while some ants have three. The supra-oesophageal ganglion is very large. The most remarkable fact revealed by Brandt's investigations is the great difference that exists between the sexes and the worker caste in the same species. The {496}pedunculate bodies of the supra-oesophageal ganglion are considered to be in their development correlative with that of the intelligence or instinct. In the workers of the social Hymenoptera these bodies are very large, while in the males and females they are small. The workers and females of Bombus have six abdominal ganglia, while the males have only five; and the worker of the honey-bee has five abdominal ganglia, while the male and the queen-bee have but four. In the leaf-cutting bee (Megachile) the male has four abdominal ganglia and the female five, and in the wasps the workers have five, the males and females six. The nervous system in the larvae shows but little difference between the ganglia, which are thirteen in number, eight being abdominal. In the embryo of the bee Kowalewsky has observed seventeen ganglia. The changes that take place from the embryonic to the imago condition are therefore directed to the reduction in number of the ganglia, which is accomplished by the fusion of some of them. In the adult Hymenopterous Insect it would appear that the first abdominal ganglion is always joined with the last thoracic.
Sub-Orders.—The distinction in the form of the abdominal articulation, previously alluded to (p. 492, Fig. 336, A, B), divides the Hymenoptera into two great sub-Orders, the members of which are very different in their habits and life-histories. The Sessiliventres are plant-eaters; their larvae (Fig. 343, A) are provided with legs, and are able to procure their vegetable food for themselves. The larvae of the Petiolata are maggot-like and helpless, and are dependent for food on supplies afforded them by their parents or companions. It is said by Dewitz that although the larvae of the Petiolata appear to be legless, there are thoracic legs within the body. The metamorphosis, so far as it is known, and the early life-history of the Sessiliventres are very similar to those of butterflies and moths, except that the pupa is soft and has no hard external skin. A few of these plant-eating Sessiliventres become carnivorous in the perfect state—a change of habit that is most unusual in Insects, though the reverse occurrence is common. The larvae of the Petiolata exhibit, in the cases that have been examined, the peculiarity that the alimentary canal has not any outlet posteriorly until the termination of the larval stage of existence is approaching. In some cases there is no anal orifice; in others this orifice exists, {497}but there is no communication between the stomach and the posterior intestine.
Packard informs us[415] that in Bombus the larva, after it is full fed, passes into the pupa state (Fig. 331, A, B) by a series of transformations accompanied by moultings of the skin. Packard's statements have been confirmed by others, but details have not been fully given, so that the number of the moults, their intervals and other particulars, are still unknown. We have remarked that the pupal instar is very like the perfect instar, except that it is colourless and soft, and that each of the members is wrapped in a very delicate skin; the colour appears gradually. This metamorphosis exhibits important differences from that of the Lepidoptera. Packard calls the Insect, during the stages of transformation from the full-fed larva to the pupa, the semi-pupa; the later stages of the pupa, when the colouring has appeared, he terms the subimago. Altogether he considers there is a series of at least ten moultings of the skin. His ideas were apparently derived from examination of a series of specimens after death rather than from observation of the development in living individuals. The parasitic forms of Hymenoptera have apparently extraordinary metamorphoses of very varied kinds.
Parthenogenesis.—One of the most remarkable facts connected with this Order is the prevalence of parthenogenesis in a considerable number of widely separated species. In many of these Hymenoptera it is not a mere occasional occurrence, but plays an important part in the continuity of the species; indeed, it is believed that in some members of the Order the reproduction is entirely parthenogenetic. We shall give particulars as to some of these cases in subsequent chapters, and will here make some remarks on the different forms of parthenogenesis existing in the Order. The three forms of parthenogenesis mentioned on p. 141 all occur in Hymenoptera. In the gall-making Cynipidae parthenogenesis is frequently accompanied with alternation of generations, a generation consisting of the two sexes being followed by another consisting entirely of females, which in its turn gives origin to a bisexual generation. In this case deuterotokous parthenogenesis is established as a part of the normal economy of the species. This same form of parthenogenesis also occurs in other species of Cynipidae unaccompanied by alternation {498}of generations. Thus in Rhodites rosae the generations resemble one another, and the male is very rare, but is still occasionally produced,[416] and the same condition exists in other Cynipidae. According to the observations of Adler, we may assume that the male, in the latter cases, is useless, the continuation of the species being effected by virgin females although males exist. Deuterotokous parthenogenesis also occurs in the sawflies, but as a comparatively rare phenomenon.[417]
Thelyotokous parthenogenesis is common in sawflies, and it also occurs in some Cynipidae. There are several species of this latter family in which no males have ever been found.[418] The phenomena in Rhodites rosae we have mentioned, give rise to the idea that in that species deuterotokous parthenogenesis occurs as an exception, the species being usually thelyotokous. A most remarkable case of thelyotokous parthenogenesis is said to exist in the case of the parasitic ant Tomognathus. This species is said to be monomorphic, only the female existing, and reproducing by uninterrupted parthenogenesis.
Arrhenotokous parthenogenesis—i.e. parthenogenesis in which the progeny is entirely of the male sex—occurs in several species of sawflies. We find it also occurring in the case of the social Hymenoptera; the workers of ants, bees, and wasps occasionally produce eggs parthenogenetically, and the progeny in these cases is always of the male sex. In the honey-bee the queen sometimes produces eggs before she has been fertilised, and the parthenogenetic young are then always of the male sex.
Some species of Hymenoptera exhibit two forms of parthenogenesis. In Nematus curtispina the parthenogenetic generation is generally of the male sex, but a female is occasionally produced;[419] while in Hemichroa rufa parthenogenesis may result in either deuterotokous or thelyotokous progeny. No case is yet known of a species exhibiting the three forms of parthenogenesis. From this review we may conclude that parthenogenesis does not favour the formation of one sex more than another; but it is clear that it decidedly favours the production of a brood that is {499}entirely of one sex, but which sex that is differs according to other circumstances.
Production of Sex.—It is believed that a very peculiar form of parthenogenesis exists in the honey-bee, and it is confidently stated that the drones, or males, of that species are always produced from unfertilised eggs. These views are commonly called the Dzierzon theory, and are widely accepted. They assume that the eggs are male till fertilised, and then become female. After the queen-bee is fertilised most of the spermatozoa soon find their way into a small chamber, the spermatheca, near the posterior orifice of the body; it is believed that each egg may be fertilised as it passes the door of this chamber, and that the eggs that produce females (i.e. workers or queens) are so fertilised, but that the eggs that produce drones are not fertilised. Hence it is supposed that the sex is determined by this act of fertilisation, and Cheshire has described what he calls an apparatus for differentiating the sexes. It is also confidently stated that no male honey-bee ever has a father.
The facts we have stated as to the sexes resulting from parthenogenetic reproduction in Hymenoptera generally, are extremely opposed to the Dzierzon theory, in so far as this relates to the production of sex. There have always been entomologists[420] who have considered this view unsatisfactory, and the observations of several recent French naturalists[421] are unfavourable to the idea that the sex of an egg is determined by its fertilisation.
There can be no doubt that the queen honey-bee frequently produces males parthenogenetically, and the error of the views we are alluding to consists in taking the parthenogenesis to be the cause of the sex of the individual. It must be recollected that the laying of an unfertilised egg by a fertilised female may be different physiologically from the laying of an egg by an unfertilised female; for, though both have as result an unfertilised egg, it is possible that the fertilisation of the female may initiate processes that modify the sex of the eggs produced by the ovaries, so that though these may produce previous to fertilisation only male eggs, yet after fertilisation they may produce eggs of the opposite sex or of both sexes. In other {500}words, the act of fertilisation may initiate a different condition of nutrition of the ovaries, and this may determine the sex of the eggs produced.
Polymorphism, or Castes.—The question of the causes of the modified individuals forming the various castes of the social Hymenoptera has been much discussed. These individuals are many of them very different in size and structure from either of their parents, and are also different in their habits and instincts. This difficult subject is far from being completely elucidated. In the case of the honey-bee it is well established that an egg of the female sex can, after deposition, be made either into a queen or a worker-bee by the mode of nutrition—using that word in the largest sense. On the other hand, Dewitz thought that in the case of the ant Formica rufa, the caste—whether worker or winged female—is already determined in the Insect before leaving the egg.[422] Weismann and others associate the caste with some hypothetic rudiments they consider to exist at the very earliest stage of the embryonic, or oogenetic process.
Herbert Spencer says:[423] "Among these social Insects the sex is determined by degree of nutrition while the egg is being formed," and "after an egg, predetermined as a female, has been laid, the character of the produced Insect as a perfect female or imperfect female is determined by the nutrition of the larva. That is, one set of differences in structure and instincts is determined by nutrition before the egg is laid, and a further set of differences in structures and instincts is determined by nutrition after the egg is laid."
Spencer's generalisation is not inconsistent with the facts hitherto brought to light, though it is possible that the progress of knowledge may show some variety as to the periods of the development at which the commencements of the modifications occur.
Fig. 339 represents the chief castes, or adult forms, existing in a community of one of the most highly developed of the species of social Hymenoptera, the leaf-cutting ant, Atta cephalotes. We shall, when dealing with Formicidae, enter into some details as to these and other cases of polymorphism.
Fig. 339.—Adult forms of Atta (Oecodoma) cephalotes, taken from a nest in Trinidad by Mr. J. H. Hart, 25th June 1895. A, male; B, winged female; C-F, various forms unwinged; C, so-called soldier; D, large worker; E, smaller worker; F, smallest worker or nurse. All equally magnified (one and half times).
Our object at present is to bring to the eye of the reader the great diversity of outer form that is believed, rightly or wrongly, to result from the mode of treatment of the young. And we will also take this opportunity of more fully illustrating the remark we made on p. 85 as to the profound distinctions that exist between ants and white ants, or Termites, notwithstanding the remarkable analogies that we shall find to exist in many of their social arrangements.
The analogies we allude to, coupled with the fact that there is a certain general resemblance in outer form between the workers of Termites and ants, and even between the extraordinary castes called soldiers in the two groups, have given rise to the idea that there is a zoological relationship between the social forms of Neuroptera and Hymenoptera. The two are, however, zoologically amongst the most different of Insects. The external skeleton in Termites is remarkable for its imperfect development, the sclerites being small and isolated, while the segmental differentiation of the body is low (Fig. 225, etc.), so that there is no difficulty in counting the segments. In ants the reverse is the case as regards both these facts, the various segments being most unequal, so that their homologies have only been detected after prolonged studies, while the chitinisation and articulation of the various parts is so complete that the ant may be described as cased in armour, fitting together so exactly that it is difficult anywhere to introduce the point of a needle into its chinks. The wings of the two kinds of Insects are also extremely different. The differences between the modes of growth and development of the two sets of Insects are as profound as the distinctions in their anatomy. Termitidae belong to the division of Insects in which the wings are developed outside the body; Hymenoptera to the division in which they are developed inside the body. In Termites the growth of the individual is slow, and the final form is reached gradually. In the ants the growth is carried on with great rapidity, and during it the Insect is a helpless maggot absolutely dependent on the attentions of its seniors, while the difference in form and structure between the ant-larva and the ant are enormous. Both anatomy and ontogeny are profoundly different in ants and Termites. To these distinctions must be added, as of much importance, the fact that in Hymenoptera only the female sex {503}is modified for the division of labour, while in Termites both sexes undergo this change. Hence it is impossible to suppose that the remarkable analogies that exist between the societies of ants and those of Termites are due to any common origin. It is probably to some similar physiological susceptibilities in the ancestors, at an extremely remote epoch, of both groups that we must look for an explanation of the interesting resemblances in the social lives of ants and Termites.
The Hymenoptera are no doubt one of the largest Orders of Insects, the species of the parasitic tribes being apparently innumerable. No doubt 250,000 species of the Order exist, and possibly the number may prove to be very much larger. Up to the present time 25,000 or 30,000 have been discovered. No remains of Insects of this Order, of older age than the Lias, have been brought to light; it is indeed doubtful whether the fossils considered to be Hymenopterous of the period referred to are really such.
The Order, as already mentioned, consists of two very distinct sub-Orders, viz.:—
1. Hymenoptera Sessiliventres.—Insects with the abdomen broad at the base, its first segment not completely amalgamated with the thorax.
2. Hymenoptera Petioliventres or Petiolata.—The abdomen connected with what appears to be the thorax by a slender joint, the posterior part of the apparent thorax consisting of an abdominal segment.
Hymenoptera Sessiliventres.—This group has been variously called Hymenoptera phytophaga, H. securifera, H. sessiliventres, H. serrifera, H. symphyta. We prefer an old term, taken from a character that enables us to recognise at a glance which group a species belongs to. The division or sub-Order may be formally defined as follows:—
Abdomen nearly continuous in outline with the thorax, the two parts having a broad connexion instead of a small highly mobile articulation. Anal lobe of hind wings usually of considerable size. Trochanters ditrochous (transversely divided into two, Fig. 345). Extremity of body of female furnished with saws or boring instruments, usually concealed, in some cases visible in part. Larvae with complex mouth-parts; three pairs of thoracic legs (imperfect in Cephidae and {504}Siricidae), and frequently with numerous abdominal legs, which are destitute of hooks. Food vegetable.
The Insects of this sub-Order never exhibit the highly specialised habits and activity of the better known petiolate Hymenoptera. Though the food in the larval stages is always vegetable, there is considerable variety in the larvae and their habits; some feed in galls, some in the twigs of plants, some in the hard wood of trees and shrubs. The majority, however, live on the leaves of plants. Those that live in wood (Fig. 342, C) resemble in appearance Coleopterous larvae that have similar habits, and those that live on leaves (Fig. 343, A) resemble Lepidopterous larvae that do likewise. There are four families included in the sub-Order, viz. Cephidae, Oryssidae, Siricidae, Tenthredinidae.
The British Sessiliventres—under the name Phytophagous Hymenoptera—have recently been monographed by Mr. Peter Cameron in a series of vols. published by the Ray Society.[424] These contain many figures and many details relating to natural history, in addition to the descriptions of genera and species.
Fam. I. Cephidae—Stem Sawflies.
Slender Insects, with weak integument; free, more or less elongate pronotum; one spine on the front tibia. Larvae living in the stems of plants or in the tender shoots of trees and shrubs.
The obscure little Insects composing this family have slender antennae of peculiar form, composed of eighteen to thirty joints, two of which are short and stout; then come several long joints, with more or less power of movement, the terminal portion consisting of an elongate club of many joints with little power of movement. The pronotum is longer than is usual in the Hymenoptera, and instead of being very closely connected with the mesonotum, it is free and mobile, although its base overwraps the front of the mesonotum. The median plate (i.e. the dorsal plate connecting the thorax and abdomen) is divided to the base along the middle, the divisions being separated by a membranous piece broader behind; the anal lobe of the posterior {505}wings is small but distinct. The female bears a saw at the extremity of the body, but it is covered by two flaps; these form a short, terminal projection. Although too much neglected, the Cephidae are really of great interest as being of more imperfect or primitive structure than any of the other families of Hymenoptera. The larval history has been traced in several species. C. pygmaeus is sometimes very injurious to corn crops on the continent of Europe, and even in our own country its effects in this respect are considered to be occasionally serious. The egg is laid in the stem of the corn plant; the larva soon hatches and eats its way upwards in the stem. It is a soft grub, apparently footless, but really possessing six small projections in place of thoracic legs. It occupies all the summer in feeding, and when full fed and about to prepare for its metamorphosis, it weakens the stem by a sort of girdling process below the ear; it then descends in the stem to near the root, where it constructs a transparent cocoon, in which it passes the winter as a larva, changing to a chrysalis in the month of May, and completing its development by appearing as a perfect Insect shortly thereafter. The girdling operation is very injurious, and causes the corn stem, when ripe or nearly so, to break in two under the influence of a strong wind, so that the ears fall to the ground.
The history of C. integer has been given by Riley. This Insect attacks the young shoots of willows in North America. Riley states[425] that by a wonderful instinct the female, after she has consigned her egg to the twig, girdles the latter, preventing it from growing any further, and from crushing the egg by so doing. The larva after hatching eats downwards, sometimes destroying a length of two feet of the twig; when full grown it fills the bottom of the burrow with frass, and then previous to making its cocoon eats a passage through the side of the shoot about a quarter of an inch above the spot where the cocoon will be placed, thus making it easy for the perfect Insect to effect its {506}escape; it leaves the bark, however, untouched, and is thus protected in its retreat. A delicate transparent cocoon is then spun in which the larva passes the winter, changing to a pupa in the following March, and emerging as a perfect Insect about six weeks thereafter.
Somewhat less than 100 species of this family are at present known; the great majority are found in the Mediterranean region, but there are several in North America. As a single species is known from Mexico and another from Japan, it is probable that the family may prove to have a wider geographical extension than at present appears to be the case.
Fam. II. Oryssidae.
The median plate behind the metanotum entire, not divided in the middle; antennae inserted below the eyes immediately above the mandibles, under a sharp edge.
This family consists of the genus Oryssus, and includes only about twenty species, but is nevertheless very widely distributed over the world. They are very rare Insects, and little is known as to their habits; one species, O. abietinus, was formerly found in England. Should any one be so fortunate as to meet with it, he can scarcely fail to recognise it on noticing the peculiar situation of the base of the antennae. In this respect the Chrysididae somewhat resemble Oryssus, but in that group of Hymenoptera the hind body or abdomen is remarkably mobile, so that the Insects can coil themselves up by bending at this joint; whereas in Oryssus the hind body is very closely amalgamated with the thorax—more so, in fact, than in any other Hymenopterous Insect—and has no power of independent movement.
Oryssus abietinus very closely resembles C. sayi (Fig. 341); it has indeed been recently suggested by Mr. Harrington that the two supposed species may really be identical.
Fam. III. Siricidae or Uroceridae.
Pronotum closely connected with the mesonotum, perpendicular in front; the anterior lobe of the latter not separated by the lateral lobes from the posterior lobe: the median plate (behind the metathorax) is divided longitudinally along the middle. The female is provided at the extremity of the body with an elongate, cylindrical boring instrument. The larvae live in the wood of trees.
Fig. 342.—Tremex columba. North America. A, Imago, female: B, pupa, female, ventral aspect: C, larva; a, imperfect legs: D, parasitic larva of Thalessa. (B and D after Riley.)
The Insects of this family are usually of large size and of bright conspicuous colours; these, however, frequently differ greatly in the sexes of the same species, and may be very variable even in one sex. The antennae are filiform and usually elongate; the head is usually contiguous with the thorax, but in one division, Xyphidriides, it is exserted and separated from the thorax by a well-marked neck. The pronotum is attached to the mesonotum, and possesses very little, if any, freedom of movement; it varies in its size, being sometimes conspicuous {508}from above; in the Xyphidriides it is smaller, and in the middle is entirely vertical in its direction. The mesonotum is moderate in size, and its divisions are delimited by broad vague depressions. The prosternum appears to be entirely membranous, but the prosternal plates (pleura) are large, and meet together accurately in the middle, so as to protect the greater part of the under-surface of the neck. The abdomen is cylindrical or somewhat flattened above; it has seven dorsal plates in addition to the spine-bearing terminal segment. The trochanters are double, the outer division being, however, short; the anterior tibia has only one spur; the anal lobe of the posterior wings is large. The "borer" or ovipositor of the female is a remarkable organ; it is held projecting directly backwards from the extremity of the body, and has the appearance of being a powerful sting. The apparatus is much longer than it appears, for it proceeds not from the apex of the body, but from the under-surface far forwards, so that the part exposed is only about one-half of the total length; it consists of a pair of elongate sheaths, which are easily separable though they wrap together, and enclose a slender tube. This tube is rigid and quite straight; though appearing solid, it is really composed of two very perfectly adjusted laminae and a third arched piece or roof. The two lower laminae are called the spiculae; they are serrated or grooved in a peculiar manner near the tip, and although so closely adjusted to the borer or upper piece of the tube as to appear to form one solid whole with it, they are said to be capable of separate motion. In addition to these parts, the termination of the abdomen bears above a shorter piece that projects in a parallel plane, and forms a sort of thick spine above the ventral pieces we have described; this process is very strong, and has in the middle of its under-face in Sirex gigas a membranous cavity, replaced in S. juvencus, according to Westwood, by a pair of minute pilose styles. The Insect, by means of this powerful apparatus, is enabled to deposit her eggs in the solid wood of trees, in which the larva sometimes penetrates to the depth of eight inches.
Sirex gigas is one of the most remarkable of our British Insects, but is little known except to entomologists, being usually rare. On the continent of Europe it is, however, an abundant Insect, especially in the neighbourhood of forests of fir-trees, and is a cause of considerable terror. As the Insect is not {509}capable of inflicting much injury to the person, it is probable that the peculiar ovipositor is believed to be a sting. The eggs are laid—it is said to the number of 100—in the solid wood of fir-trees, but not in perfectly healthy wood; the reason for this, it is thought, being that in a healthy tree the great affluence of sap caused by the burrows and presence of the Insect would be injurious to the latter. The Sirex will, however, attack a perfectly healthy tree immediately after it has been felled. The larva, small at first, enlarges its burrows as itself grows larger, and thus the wood of a tree may be rendered completely useless for trade purposes, although there may be very little outward indication of unsoundness. The larva (Fig. 342, C, larva of Tremex) is a pallid, maggot-like creature, with six projections representing thoracic legs; there are no other legs behind these, but some slight protuberances take their place; the terminal segment is enlarged, and bears a hard spine. There is a difference of opinion as to the duration of the life of the larva, Kollar saying that in seven weeks after the deposition of the egg the maggot is full fed, while others consider that it takes two years to attain this condition; the latter statement is more probably correct, it being the rule that the life of wood-feeding larvae is more than usually prolonged. After becoming full fed, the Insect may still pass a prolonged period in the wood before emerging as a perfect Insect. As a result of this it not infrequently happens that the Insect emerges from wood that has been carried to a distance, and used for buildings or for furniture. A case is recorded in which large numbers of a species of Sirex emerged in a house in this country some years after it was built, to the great terror of the inhabitants. The wood in this case was supposed to have been brought from Canada.
Fabre has studied[426] the habits of the larva of Sirex augur, and finds that it forms tortuous galleries in the direction of the longitudinal axis of the tree or limb, and undergoes its metamorphosis in the interior, leaving to the perfect Insect the task of finding its way out; this the creature does, not by retracing its path along the gallery formed by the larva, but by driving a fresh one at right angles to the previous course, thus selecting the shortest way to freedom. By what perception or sense it selects the road to the exterior is quite unknown. Fabre is not {510}able to suggest any sort of perception that might enable the larva to pursue the right course, and considers it must be accomplished by means of some sensibility we do not possess. Fabre's observation is the opposite of what has been recorded in the case of S. gigas, where the larva is said to prepare the way for the exit of the perfect Insect.
Individuals of Sirex are often found in dried and solid wood, encased by metal. When the Insect finds itself so confined, it gnaws its way through the metal, if this be lead, and escapes. The perseverance displayed by the Insect in these circumstances seems to indicate a knowledge of the direction in which liberty is to be found.
About 100 species of Siricidae are known. They form two sub-families:—
1. Siricides: back of head nearly or quite contiguous with the pronotum.
2. Xyphidriides: back of head separated from the pronotum by an elongate neck.
We are reputed to possess in Britain two species of each of these sub-families, but it is doubtful whether more than one Siricid is truly native. Sirex gigas is frequently brought over in timber, and certainly breeds at times freely in Britain. Mr. Leech has recorded the occurrence of the larvae in abundance in fir-trees in the neighbourhood of Dublin. Sirex juvencus is more rarely met with. Xyphidria camelus is doubtless a native, though now apparently rare. It used to occur about old willows, near London, in the New Forest, and, I believe, also in the neighbourhood of Cambridge.
Fam. IV. Tenthredinidae—Sawflies.
Hymenoptera Sessiliventres, having the pronotum small, accurately adapted to the mesonotum; the anterior lobe of the latter is widely separated from the posterior; there are two spurs on the anterior tibiae. The larvae usually live on leaves after the manner of caterpillars, but a few inhabit galls.
The sawflies are an important family of Insects, their species being numerous, while some of them are, in the larval state, very destructive to vegetables and fruit. Being quiet creatures, rarely seen on the wing, they are, though common Insects in this {511}country, but little known, and few persons recognise a sawfly as such. They are usually of small or moderate size, and the numerous species have a great family resemblance. This remark requires some qualification in the case of the Cimbicides, they being Insects of larger size—usually surpassing the honey-bee—of more robust structure, and with greater powers of flight.
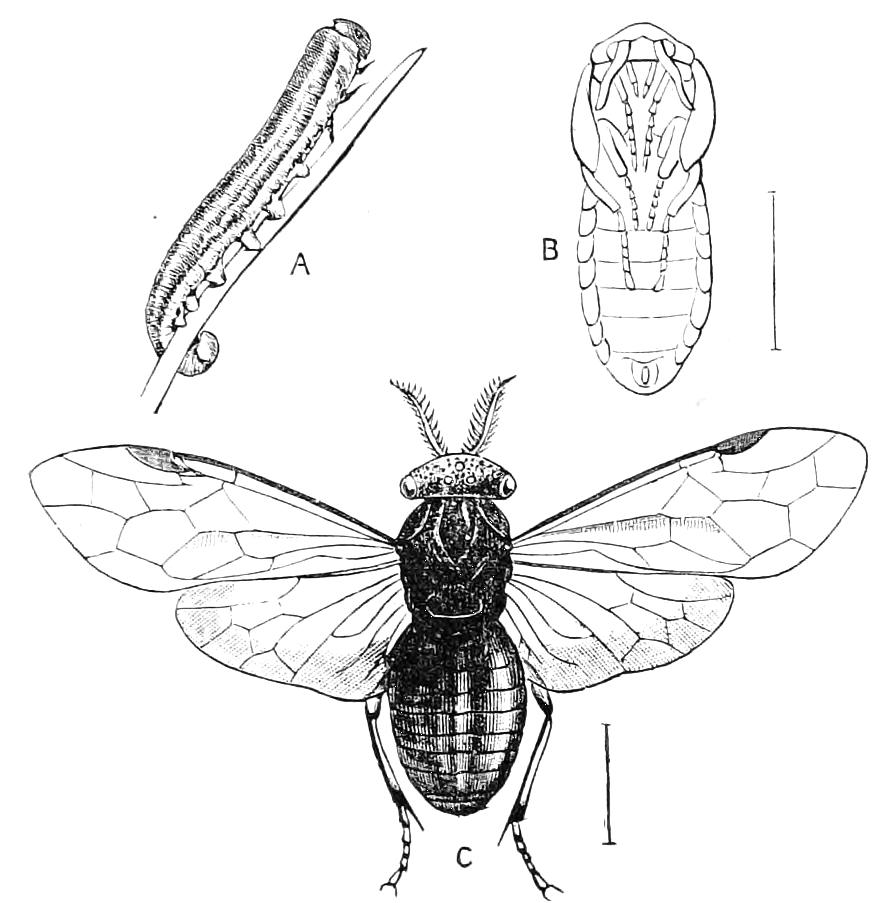
Fig. 343.—Lophyrus pini. Britain. A, Larva; B, ventral aspect of pupa; C, imago, male. (After Vollenhoven.)
The antennae are remarkably variable in form and structure. Cameron considers that nine should be taken as the normal number of their joints; but there are only three in Hylotoma, while in Lyda there may be forty or more. The head is usually held closely applied to the thorax, but is really borne on a neck capable of much elongation (Fig. 332). The pronotum forms a part of the alitrunk, but is not soldered thereto. Usually the prosternum is more or less completely concealed by the side-pieces, but in Cimbicides it is larger and conspicuous, the side-pieces being in this group smaller than usual. The dorsal pieces of the mesothorax have their relative proportions different to what we find them in the other families of Sessiliventres, and even in most of the other Hymenoptera. There is first an antero-median lobe of triangular shape projecting, like a wedge, far backwards, into the great lateral lobes. These latter form the larger part of the area of the mesonotum; they meet together in the middle line, and behind are separated by a deep depression from the posterior lobe, or scutellum of the mesothorax, which is frequently divided into two parts, the anterior being the so-called scutum. The pieces of the metanotum are short and obscure, owing to the great unevenness of their parts; on each side of the middle there is a small membranous space of pallid colour. The cenchri, as these spaces are called, are, in Lyda, {512}delicate, membranous, depressed spaces, in front of each of which there stands up a flap of membrane. The function of the cenchri is quite unknown. The median plate is fastened to the hind margin of the metanotum, and looks quite like one of the dorsal plates of the following abdominal segments, from which, however, it is separated by a more or less conspicuous membrane. In the majority of the Tenthredinidae the median plate is divided along the middle, but in the Cimbicides this is not the case. The mesosternum is very large, and the metasternum small, so that the middle and hinder pairs of coxae are placed close together. The abdomen consists of nine segments, there being eight dorsal plates in addition to the median plate, and seven ventral plates besides the terminal armature. There is a pair of short cerci, each of a single segment. The trochanters are divided; each tibia bears two spurs at the extremity, and the tarsi are 5-jointed.
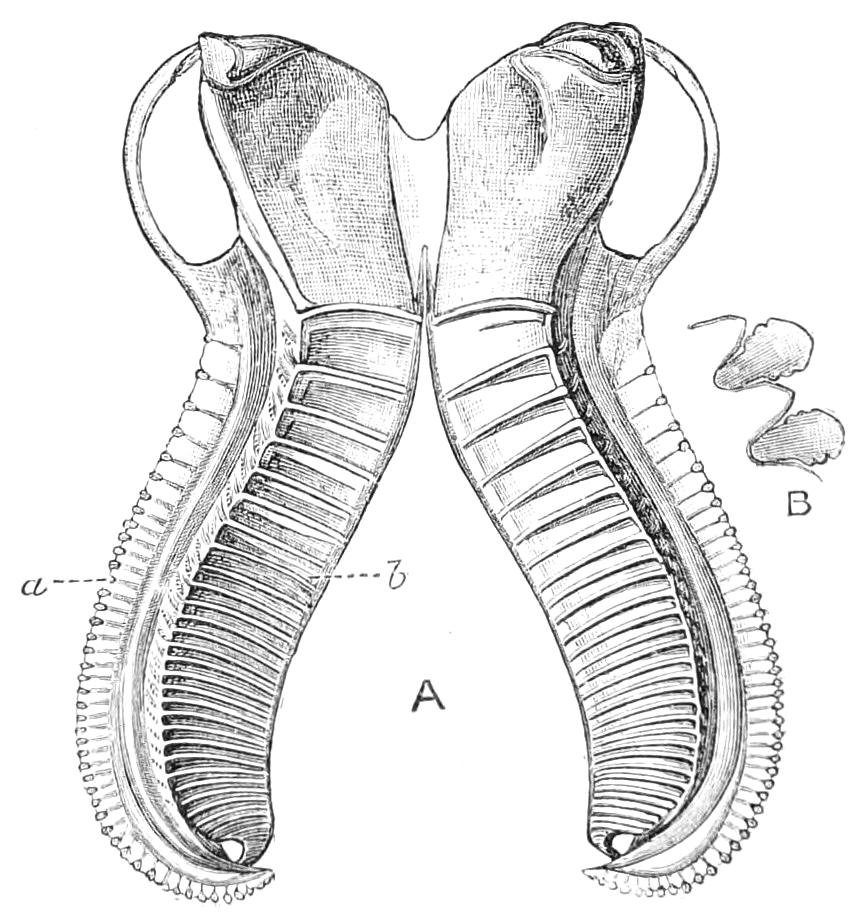
Fig. 344.—Saws of Cimbex sylvarum. A, The pair spread out and placed in a horizontal position; a, the lower margin of the saw proper; b, the upper margin of the support: B, two teeth of the saw more highly magnified.
The most characteristic and interesting of the structures with which the Insects of this family are provided is the apparatus from which the name of sawfly is derived. As long as two centuries ago these instruments excited the admiration of Vallisnieri and of Réaumur, who described them at length; and it is truly astonishing that any part of a living being should be changed into tools so mechanically perfect as these saws are (Fig. 344). They serve the purpose of assisting the female in depositing the eggs in a suitable situation, the place selected being frequently the tender stems of shrubs or other plants, or the interior of leaves. These organs are therefore of course possessed only by the female. They are placed on the {513}lower aspect of the hinder extremity of the body, where they are enclosed and protected by a pair of sheaths, from which they can be made to protrude by a little pressure exercised on the parts immediately in front of them. Each female possesses a pair of these saws; they consist of thin laminae of very hard consistence, and are not only toothed at their edge, but in many cases each tooth is itself serrate; at the same time the outer face of the saw is sculptured or plicate in a remarkable manner, so that the saw in this way acts as a file or rasp. The Insect having selected a suitable place, uses the saws by placing the extremity of the abdomen against a twig or leaf, protruding the blades, which, moving with an alternate motion, one being thrust forward while the other is retracted, act on the plant so as to make an incision. Each saw is directed in its movement by the support, the pair of supports being united at the base by membrane as shown in Fig. 344. In the case of some species,—Hylotoma rosae, the common sawfly of our rose-bushes, for instance—there is no difficulty in observing the operation; indeed old Réaumur, when speaking of the placid disposition of the sawflies, suggests that it was given them so that we may easily observe their charming operations. We cannot but regret that in these days we are unable to take so complacent a view of the arrangements of nature. There is much variety in the details of the structure of these saws; so much indeed that it is possible to identify most of the species by means of the saw alone. According to certain observers, the eggs are laid by some kinds on, not in, the leaves, so that we may conclude that in these cases the saws are not used by their possessors. An incision having been made, an egg is placed in it, and also a drop of some liquid matter. The egg is at first small, but soon increases till it becomes twice or three times its former size, and the development of the embryo commences.
The larvae of the Tenthredinidae exhibit great variety, and are indeed in this respect more interesting than the perfect Insects. The usual rule is that the larvae much resembles those of Lepidopterous Insects, and feed exposed on plants in the same way as Lepidopterous larvae do. But the exceptions are numerous; sometimes the larva is covered with slime, and thus protected from various enemies. In other cases it is very depressed, a broad creature, of irregular outline, living closely {514}attached to the leaf, somewhat after the fashion of a huge scale-Insect. Some larvae mine between the layers of a leaf, others roll up leaves; a few live in the stems of plants, and one or two inside fruits. Even this does not complete the list of their habits, for a few species of Nematus live in galls caused by the deposition of the egg. A species of Lyda forms for itself a case out of bits of leaves, and carries this habitation about with it after the fashion of the Phryganeidae. The number of legs in these larvae is unusually great, varying from eighteen to twenty-two—that is, three pairs of thoracic legs and eight of abdominal or pro-legs. This character offers a ready means of distinguishing, in the majority of cases, these larvae from those of the Lepidoptera in which the number of legs varies, but is only from ten to sixteen; moreover, the pro-legs in sawflies are destitute of the circles of hooklets that exist in Lepidoptera. This mode of identifying the immature stages of the Tenthredinidae is not, however, always satisfactory, as there are some of these larvae that have no pro-legs at all, but only the three thoracic pairs. Another point of distinction exists, inasmuch as the larvae of the sawflies have only one ocellus on each side of the head, whereas in the Lepidopterous caterpillars the rule is that there are several of these little eyes on each side. In addition to this, we should mention that the Lepidopterous larva never has any pro-legs on the fifth body-segment, whereas in the sawflies when pro-legs are present there is always a pair on the segment in question.
These larvae are of various colours, but the patterns and markings they exhibit are not quite like those of the Lepidoptera, though it would be difficult to make any correct general statement as to the nature of the differences. The variety of their postures is very remarkable; and in respect of these also Tenthredinidae differ considerably from Lepidoptera. Some of them hold the posterior part of the body erect, clasping the leaf by their anterior legs; others keep the posterior part of the body curled up (Fig. 343, A), and some combine these methods by curving the posterior part of the body and holding it away from the food. These attitudes, like the general form, are characteristic for each species. The Nematus larvae that inhabit galls possess all the characteristics of those that feed externally. As a rule the skin of the larva is naked and free from hair, but it is often minutely tuberculate, and in a few species it is armed {515}with remarkable forked spines. These spines may exist during part of the larval life, and completely disappear at one of the moults. The creatures are as a rule very sluggish, and move about much less than Lepidopterous larvae; many of them, when alarmed, have the power of exuding a disagreeable liquid, either from the mouth or from pores in the skin; in the latter case it may be sent as a sort of spray to some little distance from the body. This operation is said to be very efficacious as a means of protecting the larvae from the attacks of parasitic flies that are desirous of laying eggs in their bodies. One peculiarity as to their colour has attracted the attention of Réaumur and subsequent naturalists, namely, that in the case of many species a great change takes place in the colour during the life of the larva, and more especially at the period of the last moult. The change to the pupal state usually takes place in a cocoon, and some species have the peculiar habit of forming a double cocoon, the outer one being hard and coarse, while the inner is beautifully delicate. The cocoon is sometimes formed in the earth, and in that case it may be to a large extent composed of earthy matter. The Insect frequently remains a long time in its cocoon before emerging as a perfect Insect; however long this time may be, it is nearly all of it passed in the larval state; when the Insect does change to a pupa it speedily thereafter emerges as a perfect Insect. In the pupa the parts of the imago may be seen enveloped in a very delicate, transparent skin.
In Brazil Dielocerus ellisii, a sawfly allied to Hylotoma, constructs a nest in which the cocoons of many specimens are crowded together, being packed side by side like the cells in the comb of the bee, while the whole mass is protected by a thick outer wall. It is not known in what manner this communal work is carried out, but it is interesting to note that the cocoons assume to a considerable extent the hexagonal form of the cells in the comb of the bee. Some doubt was expressed as to the interpretation put on this structure by Curtis, but his observations have been confirmed by Smith and Peckholt.
Several species of sawflies are known to be very injurious to crops. One of these—the sawfly of the turnip, Athalia spinarum (centifoliae Panz.)—sometimes commits excessive depredations on the turnip crops in this country as well as on the continent of Europe; its life-history and anatomy were described by Newport {516}in an essay published by the Entomological Society in 1838. The eggs, it appears, are laid singly at the edges of the leaves in the month of May, as many as 200 or 300 being deposited by one female; as the parent flies are usually gregarious, appearing in large numbers in fields of turnips, it is not difficult to form an idea of the serious nature of their depredations. The egg grows very considerably; the development of the embryo is rapid, occupying, even in unfavourable weather, only seven or eight days, while in quite congenial circumstances it is probable that the eggs may hatch about the fourth day after their deposition. The young grub immediately begins to feed, and in about five days changes its skin for the first time; it repeats this operation twice at similar or slightly longer intervals, the third moult thus occurring when the larva is three or four weeks old; it is then that the larva begins to be most destructive. Sunshine and warm weather are very favourable to it, and under their influence it grows so rapidly that in a few days a field may be almost completely stripped of its foliage. This larva is of a sooty black colour, and will live on other Cruciferous plants quite as well as on the turnip. When full grown it buries itself to a slight depth under the surface of the earth, and forms an oval cocoon of a firm texture, and with many particles of earth closely adherent to it. The perfect fly emerges towards the end of July, and a second brood will be produced in the same season if circumstances are favourable; in that case the resulting larvae enter the ground for the formation of their cocoons in September or October, and pass the winter in their cocoons, but still in the larval state; changing to pupae in the following spring, and appearing as perfect Insects in May. From this account it appears not improbable that the offspring of a single female existing in the April of one year may amount by the following May—three generations having been passed through in the interval—to as many as 27,000,000 larvae. Fortunately the creatures are, as Frauenfeld observed, destroyed in very large numbers by a parasitic fungus and by a Nematode (Filaria).
We have, earlier in the chapter, alluded to the fact that the phenomena of parthenogenesis prevail somewhat extensively among sawflies. It is the rule in the family that males are very much less numerous than females, and there are some species of which no males have been discovered. This would not be of {517}itself certain evidence of the occurrence of parthenogenesis, but this has been placed beyond doubt by taking females bred in confinement, obtaining unfertilised eggs from them, and rearing the larvae produced from the eggs. This has been done by numerous observers with curious results. In many cases the parthenogenetic progeny, or a portion of it, dies without attaining full maturity. This may or may not be due to constitutional weakness arising from the parthenogenetic state. Cameron, who has made extensive observations on this subject, thinks that the parthenogenesis does involve constitutional weakness, fewer of the parthenogenetic young reaching maturity. This he suggests may be compensated for—when the parthenogenetic progeny is all of the female sex—by the fact that all those that grow up are producers of eggs. In many cases the parthenogenetic young of Tenthredinidae are of the male sex, and sometimes the abnormal progeny is of both sexes. In the case of one species—the common currant sawfly, Nematus ribesii—the parthenogenetic progeny is nearly, but not quite, always, entirely of the male sex; this has been ascertained again and again, and it is impossible in these cases to suggest any advantage to the species to compensate for constitutional parthenogenetic weakness. On the whole, it appears most probable that the parthenogenesis, and the special sex produced by it, whether male or female, are due to physiological conditions of which we know little, and that the species continue in spite of the parthenogenesis, rather than profit by it. It is worthy of remark that one of the species in which parthenogenesis with production of males occurs—Nematus ribesii—is perhaps the most abundant of sawflies.
Although many kinds of Insects display the greatest solicitude and ingenuity in providing proper receptacles for their eggs, and in storing food for the young that will be produced, there are extremely few that display any further interest in their descendants; probably, indeed, the majority of Insects die before the eggs are hatched, one generation never seeing the individuals of another. It is therefore interesting to find that a fairly well authenticated case of maternal attachment, such as we have previously alluded to as occurring in earwigs, has been recorded in Perga lewisii, an Australian sawfly of the sub-family Cimbicides. The mother, having deposited about eighty eggs on the leaf of a Eucalyptus, remains with them until they hatch, {518}after which she sits over her brood with outstretched legs, and with admirable perseverance protects them, so far as she is able, from the attacks of parasites and other enemies; she quite refuses to be driven away from her charges. Mr. Lewis, to whom we are indebted for this account,[427] states that the sawfly does not recognise her own special brood, but will give equal attention to another brood if she be transferred thereto; and he adds that many of the batches of larvae were destitute of any maternal guardian.
There are about 2000 species of sawflies known. A large majority of them are found in the European and North American regions; still, a good many are known to live in South America, and Perga—one of the genera of the family containing many species of large size—is peculiar to the Australian region. Although the family includes so many species, very few anomalies of structure have been detected in it; one species, Pompholyx dimorpha Freymuth, is described as being apterous in the female, and as having the thorax curiously modified in its form. There are no very small Insects in the family, and none over the middle size. Nearly 400 species have been detected in Britain; this number could certainly be increased by persevering researches. The palaeontological record has hitherto given only a very meagre evidence about sawflies. Several species have been preserved in amber, and three or four are known from Tertiary strata in Europe and North America.
HYMENOPTERA PETIOLATA–PARASITIC HYMENOPTERA–CYNIPIDAE OR GALL-FLIES–PROCTOTRYPIDAE–CHALCIDIDAE–ICHNEUMONIDAE–BRACONIDAE–STEPHANIDAE–MEGALYRIDAE–EVANIIDAE–PELECINIDAE–TRIGONALIDAE.
We now pass to the consideration of the Hymenoptera of the sub-Order Petiolata, or Apocrita, as they are styled by Brauer. We should make use of the term Petioliventres, for it contrasts naturally by its termination with Sessiliventres, were it not that the word is so uncouth that we think it better to adopt the shorter and more euphonious expression, Petiolata.
The members of this sub-Order, without exception, have the hind body connected with the thorax by means of a deep constriction, so that the base of the abdomen (Fig. 336, B, b) is very narrow; the articulation between the two parts is effected by means of a complex joint allowing great play, and facilitating the operations of boring and stinging, processes that are of extreme importance in the economy of the great majority of the species. The petiole is sometimes extremely short, but it may be so long that it appears like a stalk, at whose extremity is borne the remaining part of the abdomen (Fig. 369). When the petiole is very short the abdomen reposes close to the back of the thorax (Fig. 331, C), and in this case the abdomen is usually described as sessile; while, when it is evidently stalked, it is said to be petiolate. These terms are, however, unsuitable, as the words sessile and petiolate should be reserved for the conditions characteristic of the two sub-Orders. We shall therefore use the terms pseudo-sessile and pedicellate for the two conditions of the Petiolata.
The Hymenoptera Petiolata comprises an enormous majority {520}of the Order. Although it includes many of the most interesting and important of Insects, its classification is but little advanced, for a great many of the forms are still rare or unknown. Three series may be adopted for the purposes of nomenclature.
1. Parasitica.—Trochanters of two pieces, female with an ovipositor.
2. Tubulifera.—Trochanters undivided; abdomen consisting of only three, four, or five visible segments.
3. Aculeata.—Trochanters undivided; abdomen consisting of six or seven visible segments; female furnished with a retractile sting.
In the absence of any clear distinction between sting and ovipositor, these groups are merely conventional. The character furnished by the trochanters is unfortunately subject to some exceptions, there being a few parasitic forms in which the trochanters are not divided, and a few aculeates in which the reverse is more or less distinctly the case; moreover, the division, when it exists, is in some cases obscure, and the two pieces are of unequal size. Ratzeburg calls the upper division, which is frequently much larger than the other, the trochanter, and the lower division the apophysis. There is much reason for believing that the apophysis is really merely a secondary division of the femur. The Tubulifera are a comparatively small group, and will probably be merged in one of the other two, when the anatomy and morphology of the abdomen have been more thoroughly elucidated.
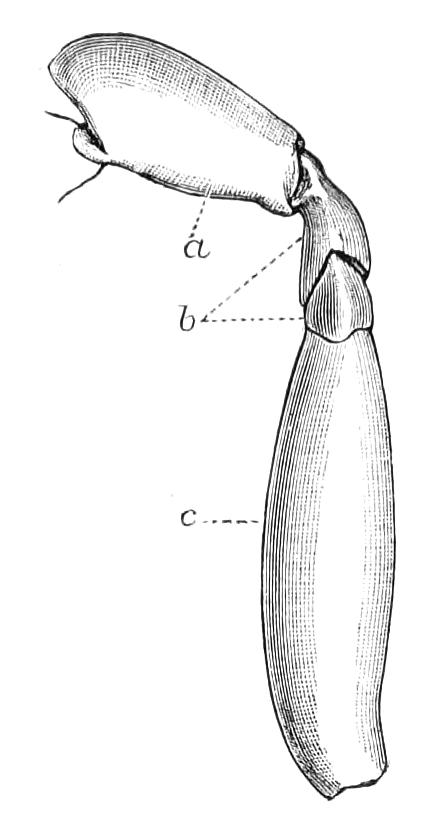
Fig. 345.—Divided (ditrochous) trochanter of an Ichneumon: a, coxa; b, the two divisions of the trochanter; c, femur. (For monotrochous trochanter see Fig. 335, A, c.)
Hymenoptera Parasitica or Terebrantia.
This is one of the most extensive divisions of the class Insecta. There can be little doubt that it contains 200,000 species, and possibly the number may be very much greater than this. It is, however, one of the most neglected of the great groups of Insects, though it is perhaps of greater economic importance to mankind than any other.
Insects derive their sustenance primarily from the vegetable kingdom. So great and rapid are the powers of assimilation of the Insect, so prodigious its capacity for multiplication, that the Mammal would not be able to compete with it were it not that the great horde of six-legged creatures has divided itself into two armies, one of which destroys the other. The parasitic Hymenoptera are chiefly occupied in destroying the tribes of vegetarian Insects; the parasites do this by the simple and efficient device of dwelling in the bodies of their hosts and appropriating the nutriment the latter take in. The parasites do not, as a rule, eat the structures of their host,—many of them, indeed, have no organs that would enable them to do this,—but they absorb the vegetable juices that, in a more or less altered state, form the lymph or so-called blood of the host. The host could perhaps starve out his enemies by a judicious system of abstention from food; instead, however, of doing this, he adopts the suicidal policy of persistent eating, and as the result of his exertions, furnishes sufficient food to his parasites, and then dies himself, indirectly starved. Ratzeburg considers that the traditional view that the larvae of parasitic Hymenoptera live by eating the fat-body of their host is erroneous. They imbibe, he considers, the liquid that fills the body of the parasitised Insect.[428]
The wide prevalence of Insect parasitism is appreciated only by entomologists. The destructive winter moth—Cheimatobia brumata—is known to be subject to the attacks of sixty-three species of Hymenopterous parasites. So abundant are these latter that late in the autumn it is not infrequently the case that the majority of caterpillars contain these destroyers. Although Lepidoptera are very favourite objects with parasitic Hymenoptera, yet other Insects are also pertinaciously attacked; there is quite a host of Insect creatures that obtain their sustenance by living inside the tiny Aphididae, or "green-flies," that so much annoy the gardener. A still larger number of parasites attack eggs of Insects, one or more individuals finding sufficient sustenance for growth and development inside another Insect's egg. As Insects have attacked Insects, so have parasites attacked parasites, and the phenomena called hyperparasitism have been developed. These cases of secondary parasitism, in which another {522}species attacks a primary parasite, are extremely numerous. It is also pretty certain that tertiary parasitism occurs, and Riley is of opinion that even quaternary destruction is not outside the range of probability.
The physiological problems connected with Insect parasitism are of great interest to the entomologist; the modes of nutrition and respiration of these encaged creatures could not fail to be most instructive were we fully acquainted with them. It is obvious that when an Insect-egg is laid inside another Insect's egg, and the parasite has to undergo the whole of its growth therein, it is in the strangest condition as regards nutrition. It is unnecessary for the intruded egg to have yolk of its own; moreover, the embryonic mode of nutrition may be continued during what would, with other Insects, be the larval period. And it seems to be the case that both these conditions are actually met with in the lives of egg-parasites. The embryology and post-embryonic development of parasitic Hymenoptera have already been ascertained to be of the most extraordinary nature. Great variety, however, will no doubt be found to exist, as will be readily understood if we tabulate the conditions of the early life of various parasitic Hymenoptera.
1. The egg may be laid outside a larva, and the embryonic and larval developments may both be passed on the exterior.
2. The egg may be laid and the embryonic development passed through, outside the host, but the parasite on hatching may enter the host, so that the post-embryonic development is passed in the lymph of the host.
3. The egg may be laid inside the host, both embryonic and post-embryonic developments being gone through in the fluids of the host.
4. The egg may be laid inside another egg, the embryonic and post-embryonic developments being passed therein.
We shall find that all these conditions exist in the Insects we are about to consider.
We shall treat the series as composed of ten families; but we must remind the student that this great subject is still in a very unadvanced state; the combined efforts of generations of naturalists will be required to perfect it. Of the ten families five are comparatively insignificant in number of species. Many of the Cynipidae are not parasitic in habits, but live in galls. {523}After what we have said as to the mode of nutrition of parasites it will be understood that the physiological conditions of life may not be so different in a gall-dweller and a parasite as would at first be supposed; and it is perhaps not a matter for much surprise that good characters cannot be found to separate the gallicolous from the parasitic forms.
Fam. I. Cynipidae—Gall-flies.
Wings with very few cells, with no dark patch (stigma) on the anterior margin; pronotum fixed to the mesonotum, and at each side extending back to the point of insertion of the front wing. Antennae not elbowed but straight, composed of a moderate number (12-15) of joints. Early stages passed either in galls or as parasites in the bodies of other Insects.
The Cynipidae are always small, frequently minute, Insects; usually black or pitchy in colour. The simple structure of the antennae and the number of their joints are of importance as an aid in identifying a Cynipid. The mesonotum is usually remarkably convex, and has, behind, a prominent scutellum, which more or less overhangs the small metanotum and the median segment; these are perpendicular in their direction; the sculpture of these posterior parts of the alitrunk is usually deep and remarkable. The abdomen has usually only a short petiole, so as to be pseudo-sessile; but there are some genera in which this part is rather long. The abdomen is generally so very much changed in outer form that its structure is not easily understood. The visible portion is frequently in larger part made up of the greatly enlarged dorsal plate of the second or third segment, or of both. These large plates are really chiefly composed of free flaps, and on lifting them up the large ventral plates are disclosed, although these appeared previously to be nearly or quite absent. In the female {524}there is a very slender ovipositor, of which only a small part protrudes, although the organ is really elongate; it is drawn into the abdomen by means of a peculiar series of structures, the modified terminal segments to which it is attached being folded over into the interior of the body in such a way that the posterior part becomes situated anteriorly. In conformity with this arrangement, the ovipositor is bent double on itself, the anterior and the middle portions of the borer being carried into the body, leaving only a small part projecting beyond the extremity. The Cynipid ovipositor is an instrument of much delicacy, and is capable of a great deal of movement; it is usually serrate just at the tip, and although it looks so very different from the cutting apparatus of the sawflies (Fig. 344), it seems that it is really composed of pieces similar in their origin to those of the Tenthredinidae.
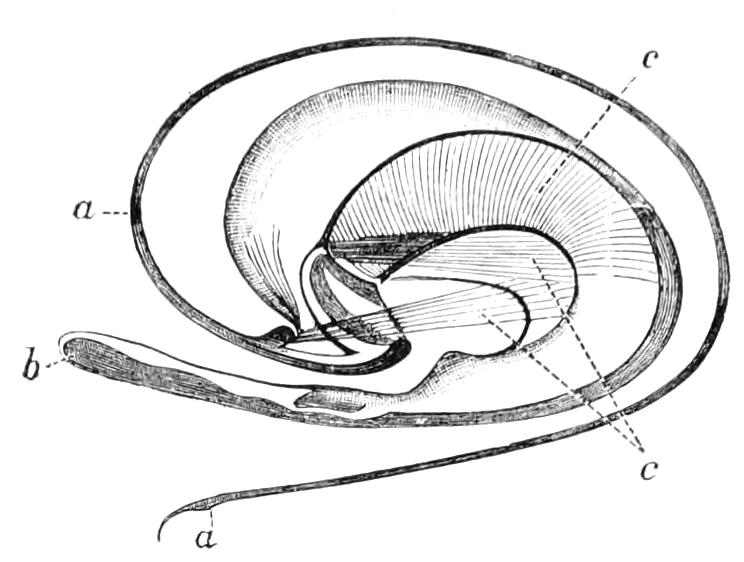
Fig. 347.—Ovipositor of Neuroterus laeviusculus. (After Adler.) a, a, The ovipositor partially coiled; b, extremity of posterior plate; c, c, muscles.
The wings frequently bear fine hairs; the paucity of nervures and the absence of the "stigma" are of importance in the definition of the family. The most important of the cells is one called the radial cell, situate just beyond the middle of the front part of the wing.
We cannot enter into a consideration of the classification of the family, as authorities are not agreed on the subject.[429] As regards their habits Cynipidae are, however, of three different kinds: (1) the true gall-flies, or Psenides, which lay an egg or eggs in the tissues of a growing plant, in the interior of which the larva lives after it is hatched; this mode of life may or may not, according to the species, be accompanied by formation of a peculiar growth called a gall: (2) Inquilines,[430] or guest-flies; {525}these lay their eggs in the galls formed by the gall-makers subsequent to the growth of the galls, of which they obtain the benefit: (3) Parasites; these live, like most Ichneumon-flies, in the interior of the bodies of other living Insects; they prey on a considerable variety of Insects, but chiefly, it is believed, on Aphididae, or on Dipterous larvae. These parasitic flies belong to the sub-family Figitides.
A great deal of discussion has occurred relative to the nature and origin of galls, and many points still remain obscure. Considerable light has been thrown on the subject by the direct observations of modern naturalists. Previous to Malpighi, who wrote on the subject two hundred years ago, it was supposed that galls were entirely vegetable productions, and that the maggots found in them were due to spontaneous generation, it having been an article of belief in the Middle Ages that maggots in general arose from the various organic substances in which they were found, by means of the hypothetical process called, as we have said, spontaneous generation. Malpighi was aware of the unsatisfactory nature of such a belief, and having found by observation that galls arose from the punctures of Insects, he came to the further conclusion that the growth of the gall was due to the injection by the Insect into the plant of a fluid he termed Ichor, which had, he considered, the effect of producing a swelling in the plant, something in the same way as the sting of a bee or wasp produces a swelling in an animal. Réaumur also made observations on the gall-Insects, and came to the conclusion that the latter part of Malpighi's views was erroneous, and that the swelling was not due to any fluid, but simply to irritation caused by the prick; this irritation being kept up by the egg that was deposited and by the subsequent development of the larva. Observations since the time of Réaumur have shown that the matter is not quite so simple as he supposed, for though in the case of some galls the development of the gall commences immediately after the introduction of the egg, yet in other cases, as in the Cynipidae, it does not occur till some time thereafter, being delayed even until after the hatching of the egg and the commencement of the development of the larva. Galls are originated {526}by a great variety of Insects, as well as by mites, on many plants; and it must not be concluded that a gall has been formed by Hymenoptera even when these Insects are reared from one. Extremely curious galls are formed by scale-Insects of the sub-family Brachyscelides on Eucalyptus trees in Australia; they are much inhabited by parasitic Hymenoptera, and Froggatt has obtained 100 specimens of a small black Chalcid from a single dead Brachyscelid.[431] The exact manner in which many of these galls originate is not yet sufficiently ascertained; but the subject of the galls resulting from the actions of Cynipidae has received special attention, and we are now able to form a conception of their nature. They are produced by the meristematic or dividing tissue of plants, and frequently in the cambium zone, which is caused to develop to an unusual extent, and in a more or less abnormal manner, by the presence of the Insect. The exact way in which a Cynipid affects the plant is perhaps not conclusively settled, and may be found to differ in the cases of different Cynipidae, but the view advocated by Adler and others, and recently stated by Riley,[432] seems satisfactory; it is to the effect that the activity of the larva probably affects the meristem, by means of a secretion exuded by the larva. The mere presence of the egg does not suffice to give rise to the gall, for the egg may be deposited months before the gall begins to form. It is for the same reason improbable that a fluid injected by the parent fly determines the gall's growth. It is true that the parent fly does exude a liquid during the act of oviposition, but this is believed to be merely of a lubricant nature, and not to influence the development. It is said that the gall begins to form in some cases before the larva is actually hatched, but the eggs of some Hymenoptera exhibit remarkable phenomena of growth, so that the egg, even during development of the embryo in it, may in these cases, exert an influence on the meristem. It is to reactions between the physiological processes of the meristem and the growing Insect that the gall and its form are due.
The investigations of several recent naturalists lend support to the view that only the meristematic cells of the plant can give rise to a gall. Riley says that the rate of growth of the gall is dependent on the activity of the meristem, galls on {527}catkins developing the most quickly; those forming on young leaves also grow with rapidity, while galls formed on bark or roots may take months to attain their full size.
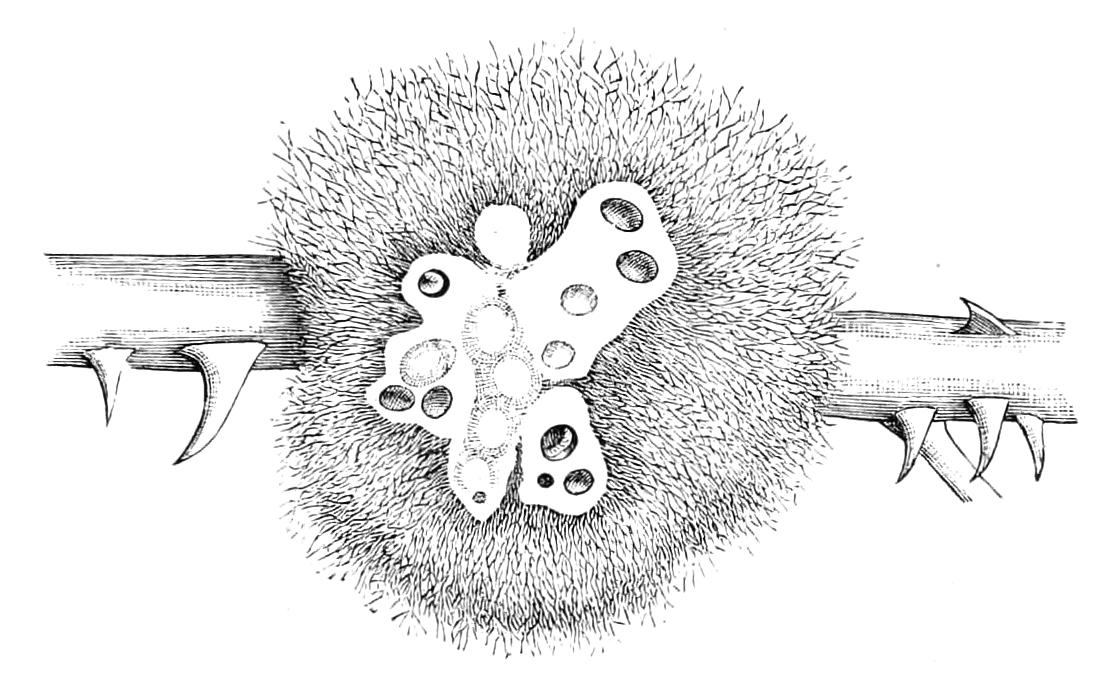
Fig. 348.—Bedeguar on rose, cut across to show the cells of the larvae; in some of the cells larvae are seen.
It is a curious fact that Cynipid galls are formed chiefly on oaks, this kind of tree supplying a surprising number and variety of galls. The plants that furnish Cynipid galls in Europe are not numerous. A list of them is given by Cameron.[433] Several species, of the genus Rhodites, attack rose-bushes. One of the best known of our British galls is the bedeguar, found in various parts of the country on both wild and cultivated rose-bushes (Fig. 348), and caused by Rhodites rosae (Fig. 349). This gall has the appearance of arising from a twig or stem, but it is really a leaf gall. Pazlavsky[434] has described the mode of formation of the bedeguar. The female Rhodites in the spring selects a rose-bud—not a flower-bud—that should produce a twig and leaves, and pricks this bud in a systematic manner in three places. The three spots of the bud pricked by the Insect are the three undeveloped leaves that correspond to a complete cycle in the phyllotaxis of the plant. The three rudiments do not develop into leaves, but by a changed mode of growth give rise to the bedeguar. Usually this gall, as shown in our figure, is of large size, and contains numerous cells; but abortive specimens are not infrequently met with; sometimes a small one is seated on a rose-leaf, and it is thought that these are due to a failure on the part of the Insect to complete the pricking operation. {528}Cynipidae will not go through their gall-making operations except under natural conditions. Giraud[435] attempted to obtain oviposition, on gathered twigs of oak, from flies in confinement; but, although he experimented with thousands of specimens, they on no occasion laid their eggs in the fresh shoots placed at their disposal, but discharged their eggs in little heaps, without attention to the twigs. The same observer has also called attention to the fact that after being deposited in a bud the eggs of certain species of Cynips will remain dormant without producing, so far as can be seen, any effect on the tree for a period of fully ten months, but when the bud begins to develop and the egg hatches then the gall grows.
The exact mode in which the egg is brought to the requisite spot in the plant is still uncertain. The path traversed by the ovipositor in the plant is sometimes of considerable length, and far from straight; in some cases before it actually pierces the tissues, the organ is thrust between scales or through fissures, so that the terebra, or boring part of the ovipositor, when it reaches the minute seam of cambium, is variously curved and flexed. Now as the canal in its interior is of extreme tenuity, and frequently of great length, it must be a very difficult matter for the egg to reach the tissue where it should develop. The eggs of Cynipidae are very remarkable bodies; they are very ductile, and consist of a head, and of a stalk that in some cases is five or six times as long as the head, and is itself somewhat enlarged at the opposite end. Some other Hymenoptera have also stalked eggs of a similar kind (Fig. 357, A, egg of Leucospis). It has been thought that this remarkable shape permits of the contents of the egg being transferred for a time to the narrower parts, and thus allows the broader portion of the egg to be temporarily compressed, and the whole structure to be passed through a very narrow canal or orifice. It is, however, very doubtful whether {529}the egg really passes along the canal of the borer. Hartig thought that it did so, and Riley supports this view to a limited extent. Adler, however, is of a different opinion, and considers that the egg travels in larger part outside the terebra. It should be remembered that the ovipositor is really composed of several appendages that are developed from the outside of the body; thus the external orifice of the body is morphologically at the base of the borer, the several parts of which are in longitudinal apposition. Hence there is nothing that would render the view of the egg leaving the ovipositor at the base improbable, and Adler supposes that it actually does so, the thin end being retained between the divisions of the terebra. Riley is of opinion that the act of oviposition in these Insects follows no uniform system. He has observed that in the case of Callirhytis clavula, ovipositing in the buds of Quercus alba, the eggs are inserted by the egg-stalk into the substance of the leaf, and that the egg-fluids are at first gathered in the posterior end, which is not inserted. "The fluids are then gradually absorbed from this exposed portion into the inserted portion of the egg, and by the time the young leaves have formed the exposed [parts of the] shells are empty, the thread-like stalk has disappeared, and the egg-contents are all contained within the leaf tissue." He has also observed that in Biorhiza nigra the pedicel, or stalk, only is inserted in the embryonic leaf-tissue, and that the enlarged portion or egg-body is at first external. The same naturalist also records that in the case of a small inquiline species, Ceroptres politus, the pedicel of the egg is very short, and in this case the egg is thrust down into the puncture made by the borer, so that the egg is entirely covered.
Some Cynipidae bore a large number of the channels for their eggs before depositing any of the latter, and it would appear that it is the rule that the boring of the channel is an act separate from that of actual oviposition. Adler distinguishes three stages: (1) boring of the canal; (2) the passage of the egg from the base of the ovipositor, where the egg-stalk is pinched between the two spiculae and the egg is pushed along the ovipositor; (3) after the point of the ovipositor is withdrawn, the egg-body enters the pierced canal, and is pushed forward by the ovipositor until it reaches the bottom.[436]
About fifty years ago Hartig reared large numbers of certain species of gall-flies from their galls, obtaining from 28,000 galls of Cynips disticha about 10,000 flies, and from galls of C. folii 3000 or 4000 examples of this species; he found that all the individuals were females. His observations were subsequently abundantly confirmed by other naturalists, among whom we may mention Frederick Smith in our own country, who made in vain repeated attempts to obtain males of the species of the genus Cynips. On one occasion he collected in the South of England 4410 galls of C. kollari (at that time called C. lignicola), and from these he obtained 1562 flies, all of which were females. A second effort was attended with similar results. Hartig, writing in 1843, after many years' experience, stated that though he was acquainted with twenty-eight species of the genus Cynips, he had not seen a male of any one of them. During the course of these futile attempts it was, however, seen that a possible source of fallacy existed in the fact that the Insects were reared from collected galls; and these being similar to one another, it was possible that the males might inhabit some different gall. Adler endeavoured to put the questions thus raised to the test by means of rearing females from galls, and then getting these females to produce, parthenogenetically, galls on small oaks planted in pots, and thus completely under control. He was quite successful in carrying out his project, and in doing so he made a most extraordinary discovery, viz. that the galls produced by these parthenogenetic females on his potted oaks, were quite different from the galls from which the flies themselves were reared, and were, in fact, galls that gave rise to a fly that had been previously considered a distinct species; and of this form both sexes were produced. Adler's observations have been confirmed by other naturalists, and thus the occurrence of alternation of generations, one of the two generations being parthenogenetic, has been thoroughly established in Cynipidae. We may mention one case as illustrative. A gall-fly called Chilaspis lowii is produced from galls on oak-leaves at Vienna at the end of April, both sexes occurring. The female thereafter lays eggs on the ribs of the leaves of the same kind of oak, and thus produces a different gall from that which nourished herself. These galls fall off with the leaves in the autumn, and in July or August of the following year a gall-fly is produced from them. It is a different creature from the {531}mother, and was previously known to entomologists under the name of Chilaspis nitida. Only females of it occur, and these parthenogenetic individuals lay their eggs in the young buds of the oak that are already present in the autumn, and in the following spring, when the buds open and the leaves develop, those that have had an egg laid in them produce a gall from which Chilaspis lowii emerges in April or May. In this case therefore the cycle of the two generations extends over two years, the generation that takes the greater part of the time for its production consisting only of females. Adler's observations showed that, though in some species this alternation of generations was accompanied by parthenogenesis in one part of the cycle, yet in other species this was not the case. He found, for instance, that some gall-flies of the genus Aphilothrix produced a series of generations the individuals of which were similar to one another, and were all females and parthenogenetic. In some species of the old genus Cynips no males are even yet known to occur. A very curious observation was made by the American, Walsh, viz. that of galls gathered by him quite similar to one another, some produced speedily a number of both sexes of Cynips spongifica, while much later on in the season the remainder of the galls gave rise to females only of an Insect called Cynips aciculata. It is believed that the galls gathered by Walsh[437] were really all one species; so that parts of the same generation emerge at different times and in two distinct forms, one of them parthenogenetic, the other consisting of two sexes. It has, however, been suggested that Cynips spongifica and C. aciculata may be two distinct species, producing quite similar galls.
Turning now to the questions connected with inquiline or guest-flies, we may commence with drawing attention to the great practical difficulties that surround the investigation of this subject. If we open a number of specimens of any kind of gall it is probable that several kinds of larvae will be found. In Fig. 350 we represent four kinds of larvae that were taken out of a few bedeguar galls gathered on one day in a lane near Cambridge. It is pretty certain that No. 1 in this figure represents the larva of Rhodites rosae, and that Nos. 2 and 3 are larvae of inquilines, possibly of Synergus, or of a parasite; while No. 4, which was engaged in feeding on No. 3 in the position {532}shown, is possibly a Chalcid of the genus Monodontomerus, or may be Callimome bedeguaris. It is clear that, as we cannot ascertain what is inside a gall without opening it, and thereby killing the tenants, it is a most difficult matter to identify the larvae; the only safe method is that of observation of the act of oviposition; this may be supplemented by rearing the flies from galls, so as to ascertain what variety of flies are associated with each kind of gall. This last point has been well attended to; but the number of cases in which oviposition of inquiline gall-flies in the galls formed by the Psenides has been ascertained by direct observation is still very small; they are, however, sufficient to show that the inquilines deposit their eggs only after the galls are formed.
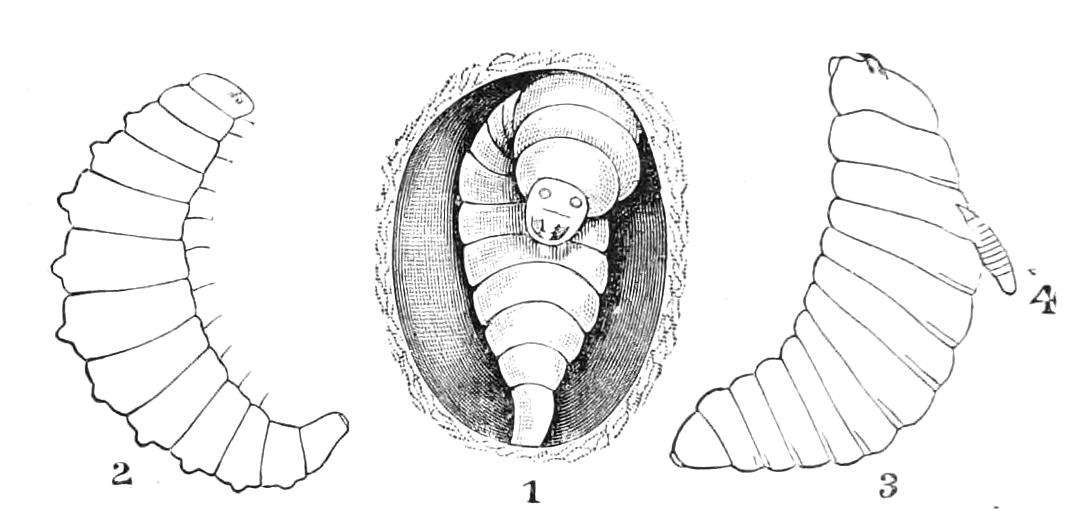
Fig. 350.—Larvae inhabiting bedeguar gall at Cambridge. 1, Rhodites rosae in cell; 2 and 3, larvae of inquilines; 4, larva of a parasitic Hymenopteron.
Bassett recorded the first case of the kind in connexion with a North American species, Cynips (Ceroptres) quercus-arbos Fitch. He says: "On the first of June galls on Quercus ilicifolia had reached their full size, but were still tender, quite like the young shoots of which they formed part. Examining them on that day, I discovered on them two gall-flies, which I succeeded in taking. They were females, and the ovipositor of each was inserted into the gall so deeply that they could not readily free themselves, and they were removed by force."
The great resemblance of the inquiline gall-fly to the fly that makes the gall both dwell in, has been several times noticed by Osten Sacken, who says "one of the most curious circumstances connected with the history of two North American blackberry galls is, that besides the Diastrophus, which apparently is the genuine originator of the gall, they produce another gall-fly, no doubt an inquiline, belonging to the genus Aulax, and showing the most striking resemblance in size, colouring, and sculpture to the Diastrophus, their companion. The one is the very counterpart {533}of the other, hardly showing any differences, except the strictly generic characters! This seems to be one of those curious instances, so frequent in entomology, of the resemblance between parasites and their hosts! By rearing a considerable number of galls of D. nebulosus I obtained this species as well as its parasite almost in equal numbers. By cutting some of the galls open I ascertained that a single specimen of the gall frequently contained both species, thus setting aside a possible doubt whether these Insects are not produced by two different, although closely similar galls."[438]
The substance of which galls are composed, or rather, perhaps, a juice they afford, is apparently a most suitable pabulum for the support of Insect life, and is eagerly sought after by a variety of Insects; hence by collecting galls in large quantities many species of Insects may be reared from them; indeed by this means as many as thirty different kinds of Insects, and belonging to all, or nearly all, the Orders, have been obtained from a single species of gall. Some galls are sought by birds, which open them and extract their tenants, even in cases where it might be supposed that the nauseous flavour of the galls would forbid such proceedings.
Not more than 500 species of Psenides and Inquiline Cynipidae are known from all parts of the world; and of described Parasitic Cynipidae there are only about 150 species. The British forms have recently been treated by Cameron in the work we have already several times referred to.[439]
A few Cynipidae have been found in amber; and remains of members of the family, as well as some galls, are said by Scudder to have been found in the Tertiary strata at Florissant.
Fam. II. Proctotrypidae, or Oxyura.
Small Hymenoptera, with few, or even no, nervures in the wings: the pronotum closely adherent to the mesothorax, and at the sides reaching backwards to the points of insertion of the wings. The abdomen is pointed, and the pointed apex is frequently deflexed; the ovipositor is not coiled, but is retractile, and when extruded is of tubular form, and {534}apparently a continuation of the tip of the body. The earlier stages are passed in the bodies, or in the eggs, of other Arthropods.
The Proctotrypidae is one of the most difficult groups of Hymenoptera to define; some of its members exhibit a great resemblance to Aculeate Hymenoptera. This is the case with the Insect we figure (Fig. 351). It, however, is an undoubted Proctotrypid, but there are other forms that approach very closely in appearance to the Aculeata, or stinging Hymenoptera; so that until a better comprehension is reached as to the distinction between a sting and an ovipositor the separation between Proctotrypidae and Aculeata must be considered somewhat arbitrary.
There is extreme variety in the family; the wings differ considerably in shape and neuration; they are not infrequently altogether absent in one or both sexes. The chief distinction of the family from other parasitic Hymenoptera is the tubular form of the ovipositor; which part appears to be a continuation of the tip of the body. This latter is more definitely acuminate than usual, and has given rise to the term Oxyura, by which name the Proctotrypidae are distinguished in many books. From the Chalcididae they are distinguished also by the angles of the pronotum attaining the tegulae. In this character they agree with the Cynipidae, but the ovipositor and abdomen are very different in form in these two groups, and the Proctotrypidae very frequently have a pigmented spot or stigma on the front wings which is absent in Cynipidae. As if to add to the difficulties the systematist meets with in dealing with this family, some of its members have the trochanters undivided, as in the case of the stinging Hymenoptera. The larvae of all that are known lead a completely parasitic life in the bodies or eggs of other Insects or of Spiders. Sometimes half a dozen specimens may find the means of subsistence during the whole of their development in a single Insect's egg. Usually Proctotrypids pupate in {535}the position in which they have fed up, enclosed each one in a more or less distinct cocoon. In Fig. 352 we represent a very remarkable case of Proctotrypid pupation; a larva of some beetle has nourished many specimens of a species of the genus Proctotrypes, and the pupae thereof project from the body of the host, a pair of the parasites issuing from each segmental division in a remarkably symmetrical manner.
Comparatively little is known as to the habits of the members of this family, but such information as has been obtained leads to the conclusion that great variety will be found to exist in this respect. We have already mentioned that numerous species have been ascertained to feed inside the eggs of Insects or of Spiders; others have been reared from larvae or from galls of the minute Dipterous midges of the family Cecidomyiidae; others have been obtained from Cynipid galls, a few from ants' nests and from green-fly; some species are known to attack Coleoptera. The distinguished Irish entomologist, Haliday, has written an account of the proceedings of a species of Bethylus,[440] from which it has been supposed that this Insect carries off living caterpillars, and stores them in a suitable receptacle as food for its progeny, thus anticipating, as it were, the habits of the fossorial division of the Aculeata, in which group this instinct has, as we shall subsequently relate, attained an astonishing degree of perfection. Haliday's observation was unfortunately incomplete and has not been subsequently confirmed. The Bethylides are remarkable for their great approach in structure to the Aculeates, so much so that entomologists are not agreed as to whether certain Insects are Proctotrypids or Aculeates. Pristocera, with a very wide distribution, may be mentioned as illustrative of these doubtful forms; but other genera of the Bethylides are in many respects very similar to the Aculeates, and it is not matter for surprise that Haliday should have considered the Bethylides to be a tribe of the stinging Hymenoptera. {536}The genus Scleroderma consists of small Insects much resembling ants, and, as well as some of its allies, is of great interest from the remarkable phenomena of polymorphism presented by certain species. The males in this genus are winged, the females completely apterous; yet at times winged females are produced—as exceptional individuals in a brood of wingless specimens—the females in these cases being not only winged, but possessed of ocelli like the females of other winged Hymenoptera. Particulars of a case of this kind have been given by Sir Sidney Saunders,[441] and Ashmead also mentions[442] the exceptional occurrence of these winged females. Westwood[443] was of opinion that there are three forms of the female sex. This subject is of importance in connexion with the production of the various castes in ants. Although the presence of wings in these Insects is always accompanied by the existence of ocelli (which, it will be remembered, are normally absent from the wingless individuals), yet the converse is not always the case, for a form of the female of Cephalonomia formiciformis, without any wings, yet having ocelli, as well as eyes, well developed, is figured by Westwood.[444]
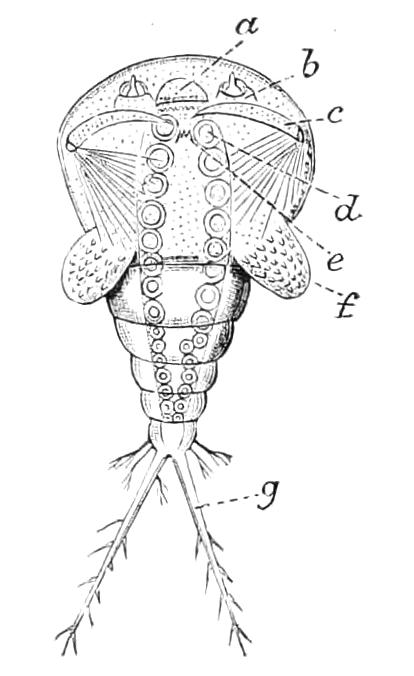
Fig. 353.—Cyclops form of larva of Platygaster sp. (After Ganin.) a, Mouth; b, antenna; c, claw-limb; d, lower lip (the pointing line is a little too short); e, doubtful "zapfenförmig" organ; f, wing-like lobe; g, branch of the tail.
The development of some of the Proctotrypids has been partially described by Ganin and others, and is of an extraordinary character. Ganin's observations[445] were most complete in the case of a species of Platygaster, which he found in the larva of a very minute Dipteron of the genus Cecidomyia. The Platygaster larva changes its form very much in the course of its life, resembling at first a minute Crustacean rather than an Insect-larva; it has a very large rounded anterior portion, while behind it terminates in two, or more, tail-like processes. By a {537}very peculiar kind of metamorphosis this Cyclops-like larva changes into an almost unsegmented, oviform larva, destitute of appendages; by a second change this creature assumes a third condition, in which it is similar to the ordinary form of parasitic Hymenopterous larvae. Sometimes several of the Platygaster larvae are found in a single host, but only one of them reaches this third stage. Afterwards the third larval instar passes into the pupal stage, which lasts five or six days, and then the perfect Insect appears. It is worthy of remark that the internal organs undergo quite as remarkable a change as the outer form does. The metamorphoses of some other Proctotrypidae have been examined by Ganin, and appear to be of an equally interesting character.[446]
There is reason to suppose that these Platygaster parasites are of great economic importance as well as of scientific interest, for Platygaster herrickii is one of the enemies of the larva of the destructive Hessian fly, Cecidomyia destructor.
The Proctotrypidae are no doubt extremely numerous in species, but as yet they have been very little studied; a good work on the British species is much required. A valuable contribution has recently been made to the study of the family by Ashmead, in the book we have already referred to. This volume includes much information on the natural history of these Insects, and the outline figures give some idea of the great variety of external form.
Many entomologists include the Mymarides in Proctotrypidae, but Ashmead considers that they should be treated as a separate family. Alaptus excisus Westw. (Fig. 354) has been frequently said to be the smallest known Insect, the {538}measurement given for it by Westwood[447] being a length of ⅙ of a millimetre—about 1⁄150 of an inch. Mr. Enock has recently examined Westwood's type in the Museum at Oxford, and from his information we may conclude that this Insect is probably the same as Alaptus fusculus Hal., and that the measurement mentioned by Westwood is erroneous, the Insect being really about half a millimetre long. The Mymarides are, however, very minute, some of them not exceeding one-third of a millimetre in length. Whether any of them are smaller than the beetles of the family Trichopterygidae, some of which are only one-fourth of a millimetre long, may be doubted.
The Mymarides are recognisable by their very minute size, and by their peculiar wings. These are slender, destitute of nervures, fringed with long, delicate hairs, and stalked at the base. Probably Mymarides may all prove to be dwellers in eggs of other Insects. The group is remarkable from the fact that it contains some of the very few Hymenoptera with aquatic habits. Two species were discovered in their winged condition in the water of a pond near London by Sir John Lubbock[448]; one of them—Polynema natans Lubbock—probably, according to Mr. Enock, the same as Caraphractus cinctus Hal., uses its wings freely for swimming under water, while the other—Prestwichia aquatica—performs this operation by the aid of its legs. This latter Insect seems to be very anomalous, and its position quite doubtful. The embryogeny of Polynema is very peculiar, and takes place in the egg of a dragon-fly—Calepteryx virgo—under water. According to Ganin,[449] in the earliest stages the developments of the embryos of the Calepteryx and of the Polynema progress simultaneously, but that of the dragon-fly does not proceed beyond the formation of the ventral plate. The Polynema appears to leave its own egg at an extremely early stage of the embryonic development. It would appear, in fact, that there is no definite distinction between embryonic and larval stages. The information given by Ganin leads to the conclusion that a complete study of this remarkable mode of development is necessary before forming any general ideas as to the nature of Insect embryogeny and metamorphosis.
Fam. III. Chalcididae.
Pronotum with some freedom of movement, its angles not extending to the insertion of the front wings. Antennae elbowed, consisting of from seven to thirteen joints. Wings without a system of cells; with a single definite nervure proceeding from the base near the front margin, or costa; afterwards passing to the costa, and giving off a very short vein more or less thickened at its termination. The species are, with few exceptions, of parasitic habits.
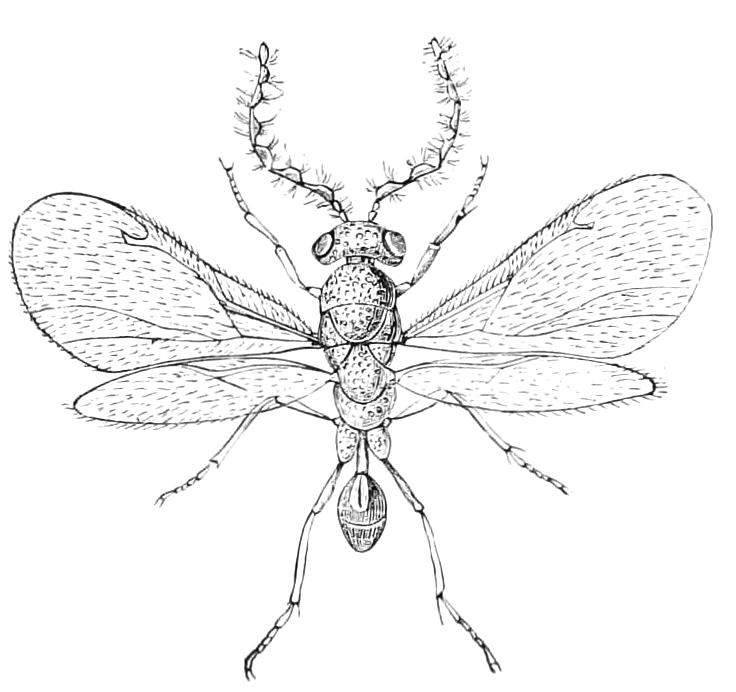
Fig. 355.—Eurytoma abrotani, male. Britain. Hyper-parasite through Microgaster of Liparis dispar, and according to Cameron, parasite of Rhodites rosae and other gall-flies in Britain, × 10. (After Ratzeburg.)
The Insects of this family—the Pteromalini of Ratzeburg—are frequently of brilliant colours and of remarkable form; the species are very numerous, some 4000 or more having already been described. Of this number nearly 3000 are European, and as there is good reason for supposing that Chalcididae are quite as numerous in the Tropics and in the New World as they are in Europe, the family will probably prove to be one of the largest in the class. About twenty sub-families have already been proposed for the classification of the group; they are based chiefly on the number of joints in the tarsi, and the details of the antennae and of the ovipositor. This latter exhibits great variety in external appearance, due chiefly to the modification in form of the basal, or of the following ventral abdominal plates, one or more of which may be prolonged and altered in form or direction, giving rise in this way to considerable diversity in the shape of the abdomen. Correlative with this is a great variety in the mode of parasitism of the larva. Many live in galls, feeding on the larvae of the makers of the galls or on those of the inquilines; others attack caterpillars, others pupae only; some flourish at the expense of bees or other Hymenoptera, or of Coccidae {540}and Aphididae, and some deposit their eggs in the egg-cases of Blattidae. The details of the life-history are well known in only a few cases.
The career of Leucospis gigas has been investigated by Fabre, and exhibits a very remarkable form of hypermetamorphosis.[450] This Insect is of comparatively large size and of vivid colours, wasp-like, black contrasting with yellow, as in the case of the wasps; and like these it has the wings folded or doubled. The female bears a long ovipositor, which by a peculiar modification is packed in a groove on the back of the Insect. This species lives in Southern Europe at the expense of Chalicodoma muraria, a mason-bee that forms cells of a hard cement for its nest, the cells being placed together in masses of considerable size; each cell contains, or rather should contain, a larva of the bee, and is closed by masonry, in the construction of which the bee displays much ability. It is the mission of the Leucospis to penetrate the masonry by means of its ovipositor, and to deposit an egg in the cell of the bee. The period chosen for this predatory attack is the end of July or the beginning of August, at which time the bee-larva is in the torpid and powerless condition that precedes its assumption of the pupal state. The Leucospis, walking about leisurely and circumspectly on the masonry of the nest, tests it repeatedly by touching with the tips of the antennae, for it is most important that a proper spot should be selected. The bee's cell is placed in a mass of solid masonry, a considerable part—but a part only—of whose area is occupied by the group of cells; every cell is closed by hard mortar, making an uneven surface, and the face of the masonry is rendered more even by a layer of hardened clay outside the rougher material; it is the task of the Leucospis to detect a suitable spot, in the apparently uniform external covering, and there to effect the penetration so as to introduce an egg into a cell. By what sensations the fly may be guided is unknown. After a spot has been selected and the ovipositor brought into play, the masonry is ultimately pierced {541}by patient work; sometimes a quarter of an hour is sufficient for the purpose, but in other cases three hours of uninterrupted effort are required before the end is attained. Fabre expended much time in watching this operation, and after the Insect had completed it, he marked with a pencil the exact spot of the masonry that was penetrated, and the date on which it was done, and he states that he afterwards found that without any exception a proper spot had been selected, and a cell consequently penetrated. Admirable as the instinct of the parasite appears from this point of view, it is nevertheless accompanied by a remarkable deficiency in two other respects. The first is that though the spot selected by the Leucospis invariably gives entrance to a cell, yet in the majority of the cases the selected cell is not a suitable one; a large number of the cells of the Chalicodoma are not occupied by living larvae on the point of pupation—though in that case only can the egg of the Leucospis hatch and successfully develop—but by dead and shrivelled larvae, or by mouldy or dried-up food. And yet, in each case of penetration, Fabre believes that an egg is deposited, even though it may be impossible that it can undergo a successful development. Strange as this may appear, it is nevertheless rendered less improbable by the second deficiency in the instinct of the parasite. The Insect has no power of recognising a cell that has been previously pierced either by itself or by another of its species. One bee larva can only supply nourishment for a single larva of the parasite, and yet it is a common occurrence for a cell to be revisited, pierced again and another egg introduced; indeed Fabre, by means of the cells he had marked, was able to assure himself that it is no uncommon thing for this to be done four times; four eggs, in fact, are sometimes deposited in a cell that cannot by any possibility supply food for more than one larva. The egg of the Leucospis is a curious object (Fig. 357, A), very elongate oval, with one end drawn out and bent so as to form a hook; it is not placed at random in the cell of the bee, but is suspended on the delicate cocoon with which the Chalicodoma larva is surrounded at the period of pupation.
Fabre allowed sufficient time to elapse for the hatching of the larvae from the eggs, and then opened some cells where Leucospis eggs had been deposited, in order to obtain the larvae; when doing this he was surprised that he never found more than one Leucospis larva in a cell. Even in cells where he had observed more than one act of oviposition, and which he had marked at the time, only one larva existed. This induced him to think that it was possible that no egg was deposited by the Leucospis at the second penetration. He accordingly examined cells soon after the eggs were laid, and thus discovered some that contained more than one egg,—indeed in one cell he observed no less than five eggs suspended from the cocoon of the Chalicodoma; he was also able to demonstrate that eggs were actually deposited in some cells that contained no means of support for the larva. How then could these two facts be reconciled—four or five eggs deposited in a cell, only one larva present afterwards? It is of course impossible to observe the operations of a larva shrouded in the obscurity of a cell formed of masonry, so he transferred some bee larvae with their destructive companions to glass tubes, in which he was able to note what took place. He found that the egg deposited by the Leucospis hatches and produces a very peculiar larva, having little resemblance to the Leucospis larva that he had found eating the Chalicodoma larva. The primary larva (Fig. 357, B) of the Leucospis is an arched worm, moderately deeply segmented, a millimetre or a little more in length, with a remarkably large and abruptly-defined head. The body bears erect setae, the most remarkable of which are a pair on the ventral aspect of each of the segments, each of these ventral setae being borne on a small conical prominence. These prominences and setae serve as ambulatory organs, and are supplemented in their function by a protuberance at the posterior extremity. The little creature has considerable powers of locomotion; it moves, after the fashion of many other larvae, by contracting and arching the body so as to bring the posterior part nearer to the anterior; then fixing the hinder part, the anterior is extended and fixed, the posterior being again brought nearer to the front. The Leucospis larva when hatched does not at once attack the bee larva which is to be its future food, but every few hours makes excursions over its surface, and even explores the walls of the cell; returning, however, always to the cocoon for repose. The object of these {543}excursions is, Fabre believes, to ascertain if another Leucospis egg has been laid in the cell, and in that case to destroy it. For the food, as we have said, being only enough for one larva, and the mother Leucospis frequently laying more than one egg in a cell, it is necessary that all the eggs except one should be destroyed. Fabre did not actually observe the act of destruction, but he found repeatedly in his glass tubes that the supernumerary eggs were destroyed, being, in fact, wounded by the mandibles of the first-hatched larva. After several days of this wandering life the tiny destroyer undergoes a first moult, changing its skin and appearing as a very different creature (Fig. 357, C); it is now completely destitute of any means of locomotion, very deeply segmented, curved at one extremity, with a very small head, bearing extremely minute, scarcely perceptible, mandibles. The sole object of its existence in this state is to extract the contents of the Chalicodoma larva, and appropriate this material to the purposes of its own organisation. This it accomplishes not by wounding, tearing, or destroying the larva, for that apparently would not answer the purpose; the contents must be conveyed while still in their vital state to itself; and this it effects by applying its mouth to the extremely delicate skin of the victim, the contents of whose body then gradually pass to the destroyer, without any visible destruction of the continuity of the integument. Thus the Leucospis larva gradually grows, while the bee larva shrinks and shrivels, without, however, actually suffering death. The process of emptying the bee larva apparently does not occupy the Leucospis more than two or three weeks, being completed by about the middle of the month of August; afterwards the larva remains in the cell by the side of the shrivelled skin of its victim for ten or eleven months, at the end of which time it assumes the pupal condition, and very shortly thereafter appears as a perfect Insect.
Monodontomerus cupreus is another member of the Chalcididae that lives parasitically at the expense of bees of the genus Chalicodoma. Its habits have been sketched by Fabre,[451] and exhibit considerable difference from those of Leucospis. It is much less in size, and can accommodate itself to a greater variety of food; it will, in fact, eat not only the larva of Chalicodoma, but also that of another bee, of the genus Stelis, that is frequently found {544}shut up in the cell of the Chalicodoma, at whose expense the Stelis also lives parasitically. The Monodontomerus bores a hole through the masonry of the bee and deposits its eggs in the cell after the fashion of the Leucospis; one bee larva is, however, sufficient food for several individuals of the young of this smaller parasite. There is no hypermetamorphosis, the early larval condition resembling the later. This Insect attacks not only Chalicodoma and Stelis, as already mentioned, but also other bees; and a single larva of some of the larger kinds will afford sufficient food for fifty young of the Monodontomerus. They feed on the bee larva, as the Leucospis does, without wounding it. This fly has the power of recognising what is suitable provender for its young by the use of the antennae, even when the conditions are so changed that it is clear the sense of sight has nothing to do with the recognition. Fabre relates that he had extracted a number of the bee larvae from their cells of masonry, and that as they were lying on his table enclosed in their cocoons, the Monodontomerus recognised the latter as containing the desired provender for its young by examining them with its antennae; after which, without hesitation, the Monodontomerus pierced the cocoon with its ovipositor and deposited the eggs in a suitable position. This observation, together with those made on Leucospis, seem to indicate that it is neither by sight nor smell that these Insects discover the desired object, but by some sense we do not understand, though its seat is clearly in the antennae of the Insect.
Newport discovered a Monodontomerus, which he described as M. nitidus,[452] in the cells of the bee Anthophora retusa, and demonstrated that the alimentary canal, as is usual in Petiolate Hymenoptera, is closed behind until the Insect is about to enter the pupal state, when it becomes perforated and faecal matters are for the first time passed from it. "These matters were composed of the refuse of digestion and of epithelial cells accumulated during the period of feeding, and retained in the digestive sac until the period of its perforation. In this way the food and abode of the Insects are maintained pure and uncontaminated, and the digestive apparatus is completed, and the refuse of nutrition ejected only when the whole of the food has been consumed."
In the cells of the same bee Newport discovered another curious parasitic Chalcid, Anthophorabia retusa.[453] The male has short wings, and the compound eye is replaced by an ocellus on each side of the head, the female having fully developed wings and eyes. A variation may occur in the metamorphosis of this Insect, inasmuch as when the growth is completed during the month of August, the Insect changes to a pupa, the imago appears ten or twelve days thereafter, and the perfect Insect then hibernates for seven or eight months; but should the completion of growth be deferred till after the end of August, hibernation takes place in the larval condition. A large and brilliant Chalcid Eucharis myrmeciae, has been described by Cameron as preying on the formidable Australian ants of the genus Myrmecia.
The development of Smicra clavipes has been partially described by Henneguy.[454] This Insect lives in the interior of the aquatic larva of Stratiomys strigosa, a Dipterous Insect. As many as fifty eggs of the parasite are found in one larva, but a large number of embryos die during development, so that he has never found more than two or three well-grown larvae in one Stratiomys larva. It has been ascertained that the eggs of many of these parasitic Insects are deficient in yolk, and the ovum of Smicra is said to obtain the nutritive materials necessary for the development of the embryo from the blood of its host by endosmosis. For a long time after the assumption of the larval condition, the larva appears to nourish itself only at the expense of the blood of its host. The segmentation of the ovum is total, and a single embryonic membrane appears at an early period, before the formation of the embryo, by a process very different from that giving origin to the amnion of the majority of Insects.
A very interesting sketch of the development of Encyrtus fuscicollis has been given by Bugnion.[455] This small parasite passes its earlier stages in the interior of the larva of Hyponomeuta cognatella or other Lepidoptera. The female Encyrtus deposits her eggs in the interior of a caterpillar, in the form of a series of 50 to 100 or more eggs enclosed in a sac; the origin {546}of the sac is obscure, but the embryonic development and the early part of the larval life are passed in the sac, which contains a supply of nutritive matter. The larvae of the Encyrtus are at first entirely confined to this sac, but when they have consumed all the nutritive matter in it, they leave it and pass the remainder of their larval and pupal existence in the body-cavity of the caterpillar. They live at first on the lymph (blood) of the Insect, and apparently do it no harm; nevertheless the strength of the caterpillar is so much enfeebled that it fails to undergo the transformation to a pupa; the parasites then devour its interior, and use the empty skin as a nidus for their own pupation; they form cocoons which divide the area into compartments. Usually the individuals disclosed from one Hyponomeuta are all of one sex, which may be either male or female. Unfortunately the most interesting points of this development, viz. the history of the common sac for the larvae, the nature of the eggs, the earlier embryonic stages, and the nutriment in the sac, are still without elucidation. The account given by Bugnion raises a great desire for information on these points.
We have in a previous page described the remarkable mode of oviposition of Mantis. Captain Xambeu[456] has made a very curious observation to the effect that a minute Chalcid, Podagrion (Palmon) pachymerus, shelters itself under the wings of the Mantis so as to be in a position to oviposit in the eggs of the latter when it shall be forming its peculiar ootheca.
The genus Isosoma consists of Insects that differ in habits from their congeners, being phytophagous instead of parasitic. I. tritici and I. hordei live in the stalks of corn, and in North America, where they are known to the agriculturist as joint-worms, are frequently very injurious to crops. They are sometimes obtained in large numbers without any males appearing, and a wingless as well as a winged form of the female occurs. Owing to the fact that the allies of these Insects are parasitic, it has been frequently maintained that this may also prove to be the case with Isosoma, but the observations of Riley[457] and others leave no doubt that the Insects of this genus are really plant-feeders.
Riley has called attention[458] to some facts in connection with I. tritici and I. grande, that make it clear that these two supposed species are really alternate generations, and that both generations are probably in larger part, if not entirely, parthenogenetic. Some species of the genus Megastigmus are known to be of phytophagous habits.[459]
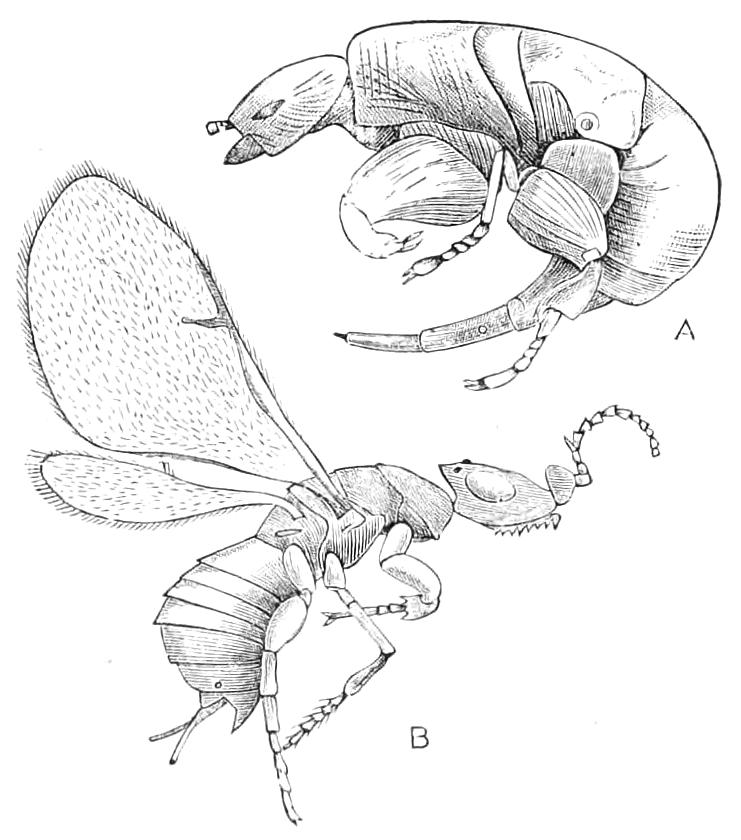
Fig. 358.—Blastophaga grossorum. A, Male, × 22; B, female, × 15. (After Mayer, Mitt. Stat. Neapel, iii. 1882.)
The most interesting of all the forms of Chalcididae are perhaps those called fig-Insects. A considerable number of species are now known, and amongst them we meet with the unusual phenomenon of species with wingless males, the females possessing the organs of flight normally developed. The wingless males exhibit the strangest forms, and bear no resemblance whatever to their more legitimately formed partners (Fig. 358, A, B). Many of the fig-Insects belong to a special group called Agaonides. Others belong to the group Torymides, which contains likewise many Chalcididae of an ordinary kind; possibly some of these may be parasitic on the Agaonides. Some of these Torymid fig-Insects have winged males, as is normal in the family, but in other cases winged and wingless forms of the male of one species may be present.
The most notorious of these fig-Insects is the one known as Blastophaga grossorum (Fig. 358), this being the chief agent in the custom known as caprification of the cultivated fig-tree. This process has been practised from time immemorial, and is at the present day still carried on in Italy and the Grecian archipelago. The Greek writers who describe it say that the wild fig-tree, though it does not ripen its own fruit, is absolutely essential for the perfection of the fruit of the cultivated fig. In accordance with this view, branches from the wild fig {548}are still gathered at certain seasons and suspended amongst the branches of the cultivated fig-trees. The young fig is a very remarkable vegetable production, consisting of a hollow, fleshy receptacle, in which are placed the extremely numerous and minute flowers, the only admission to which is by a small orifice at the blunt end of the young fig; this orifice is lined with projecting scales, that more or less completely fill it up or close it; nevertheless inside this fruit the Blastophaga grossorum develops in large numbers. The males are, as we have seen, wingless creatures, and do not leave the fruit in which they were bred, but the females make their way out of the wild fig, and some of them, it is believed, enter the young fruit of the cultivated trees and lay their eggs, or attempt to do so, therein; and it has been supposed by various writers that these proceedings are essential to the satisfactory development of the edible fruit. It is a curious fact that the Blastophaga develops very freely in the wild fig—so much so, indeed, as to be a means of preventing it from coming to maturity; but yet the Insect cannot complete its development in the cultivated fruit. This is due to the fact that the fly must lay its egg in a particular part of the fig-ovule, so that when the egg hatches the larva may have a proper supply of food. In the cultivated fig the structure of the flower differs somewhat from that of the caprificus, as the wild fig is called, and so the egg, if deposited at all, does not reach a proper nidus for its development. Hence the Blastophaga can never live exclusively on the cultivated fig, and if it be really necessary for the development of the latter, must be brought thereto by means of the caprifig. Whether the Blastophaga be really of use, as has been for so long supposed, is, however, a matter for doubt. The reasons for this are (1) that those who think caprification beneficial do not agree as to the mode in which they suppose it to be so; (2) that there is but little reason for believing that when introduced amongst the cultivated figs the Blastophaga occupies itself to any great extent therewith; and (3) that in some parts of the world caprification is not performed, but the cultivated fig nevertheless ripens its fruit there. Hence many writers on the subject—Solms-Laubach,[460] Mayer,[461] and Saunders[462]—entertain considerable doubt as to whether caprification is at present anything {549}more than an old custom destitute of practical utility. On the other hand, Riley states[463] "that the perfect Smyrna fig, the most esteemed of the edible species, can be produced only by the intervention of the Blastophaga psenes [grossorum]."
Although the questions connected with the effect the Blastophaga is supposed to produce on the fruit are of a botanical rather than a entomological nature, we may briefly say that two views have been held: (1) that, as in the fruit of the cultivated fig, only female flowers are produced, the Blastophaga is necessary for their fertilisation and the subsequent development of the fruit; (2) that the Insects stimulate the fig by biting parts thereof or by burrowing in it, and so give rise to the processes that have as their result the edible fruit. There seems to be little doubt that the Insect agency is necessary to the fertilisation of some species of figs. Cunningham, who has recently carried out an elaborate investigation as to the fertilisation of Ficus roxburghii,[464] concludes that in this fig, and probably also in other kinds, the perfect development is dependent on the access of the fig-Insects to the interior of the receptacular cavity. Should access fail to occur, both male and female flowers abort, without the formation of pollen grains by the former or seeds by the latter. The access of the Blastophaga is thus as necessary for the perfect evolution of the normal male and female flowers as it is for that of the modified ♀ or gall-flowers, with their contained ova and Insect-embryos. Whether the successful fertilisation of the flowers is really essential to the production of the edible fig is not a question for our discussion.
Fig-Insects are apparently more numerous in South America than they are in any other part of the world; and Fritz-Müller has discovered[465] a number of species there of a very extraordinary character, several of them possessing two forms of the male, one winged like the female, the other wingless and so different in character that they were considered to belong to a different genus. The wingless male of a species found in Madagascar, Kradibia cowani, has the peculiarity of possessing only four legs, the middle pair being represented merely by minute two-jointed rudiments. Some of these Insects live in galls on the figs. The fig-Insects {550}were formerly considered to belong to the Proctotrypidae or to the Cynipidae (gall-makers), but there can be no doubt, notwithstanding they differ so much in their habits from the parasitic Chalcididae, that they probably belong to the same family. If treated as different from Chalcididae, they should be separated as a distinct family rather than united with the Cynipidae.[466]
It is impossible for us to do more than allude to the extraordinary shapes exhibited by some Chalcididae. The genus Thoracantha is specially remarkable in this respect. T. latreillei is said to resemble a beetle of the family Mordellidae, and has the wings concealed by false wing-cases—really projections from the thorax—so that from above the Insect bears no resemblance to the other Insects of the Order it really belongs to.
Howard has called attention to some peculiarities in the pupation of Chalcididae.[467] Like the Cynipidae, they do not make a silken cocoon, but some of them that change to pupae inside the victims on which they were nourished have the power of forming oval cells in which to undergo their transformation, and they thus cause a peculiar inflation of the skin of their deceased victim, which after death still continues to serve as a protection to the destroyers. The statement made by Haliday, and repeated subsequently in various works, to the effect that Coryna spins a cocoon under the Aphis in which it has lived, is an error, the cocoon being really formed by Praon, a Braconid that is a parasite of the Aphis, and on which the Chalcid Pachycrepis (Coryna) lives as a hyperparasite. The pupae of some species differ from those of other Hymenoptera, in that the integument is hard, and the limbs are soldered to the body as in Lepidoptera. These forms pupate external to the victim.
Fritz Müller has recorded that the pupa of an unnamed species of Chalcid that attacks a Brazilian ant (Azteca instabilis Forel) is suspended on the wall of the cell the ant lives in by its posterior extremity, just like the chrysalis of a butterfly.
Notwithstanding the small size of Chalcididae, their remains have been detected in the tertiary strata of both Europe and North America.
Fam. IV. Ichneumonidae (Ichneumon-flies).
Wings with a well-developed series of nervures and cells; the space on the front wing separating the second posterior cell from the cubital cells is divided into two cells by a transverse veinlet. The abdomen is attached to the lower or posterior part of the median segment. Larvae parasitic in habits.
The Ichneumonidae form a family of enormous extent, containing nearly 6000 described species. The study of the family is but little advanced, owing to their parasitic habits and to this bewildering multiplicity in their specific forms. Most of the species, in the larval state, live inside the larvae of Lepidoptera, and they thus keep the myriads of caterpillars within bounds, the number of these destroyed by Ichneumons being prodigious. Some of the family are, however, external parasites, and some are known to attack Spiders and Insects of other Orders than Lepidoptera. Their antennae are not elbowed and are many-jointed, the joints being closely compacted, especially towards the extremity. This character readily distinguishes Ichneumonidae from the families we have previously considered. The ocelli are well developed even in the apterous forms, and are placed in a triangular position on the vertex. The pronotum is small in front; and extends backwards at the sides to the points of insertion of the front wings; it is fixed to the mesonotum. The wings (Fig. 367, A) have a more complex neuration than those of most of the other parasitic Hymenoptera, but are occasionally absent in one or both sexes of a species. The metathorax is very small, and the middle and hind legs are placed close together. The propodeum is very large, and is frequently covered with a highly-developed sculpture.
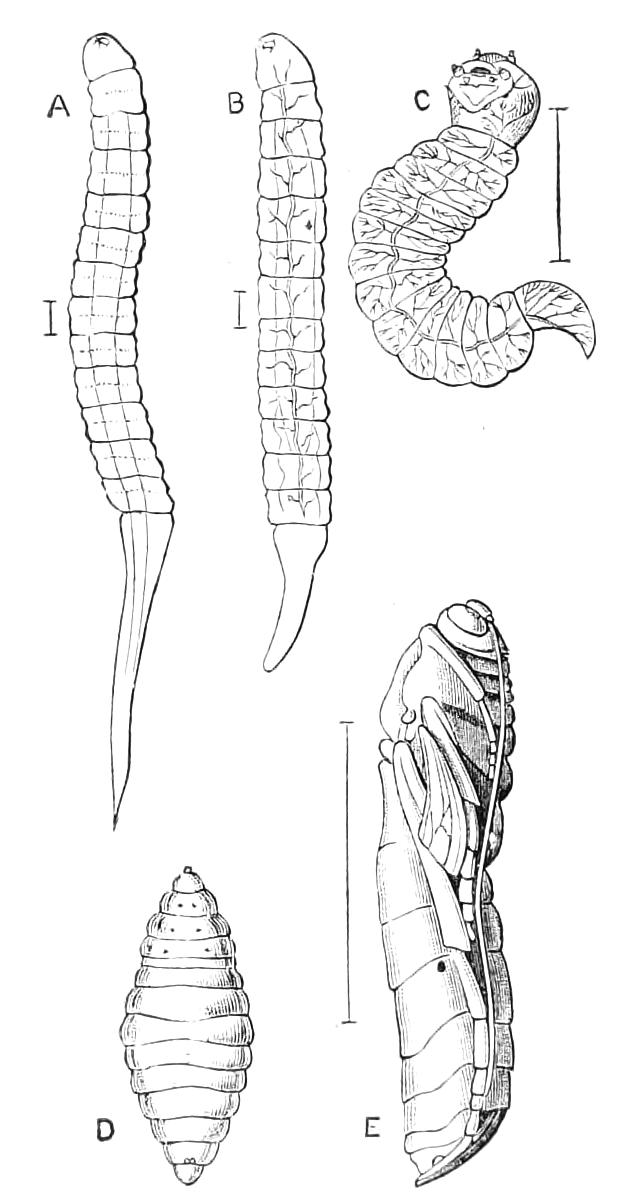
Fig. 361.—Anomalon circumflexum, larval development. (After Ratzeburg.) A, First instar; B, second instar; C, the larva in the third or encysted stage extracted from its cyst; D, the mature larva; E, pupa.
The hind body springs from the lower part of the propodeum; it is usually of slender form, and its segmentation is very conspicuous. The females bear an ovipositor, which differs greatly in length according to the species, and is known in the case of one species to attain a length six times that of the whole of the rest of the body.[468] The egg is deposited by some species on the skin, by others within the body of the victim; it varies much in form and colour, some eggs being stalked and of peculiar shape. The larvae issuing from the eggs are legless maggots with a delicate integument of pallid white or creamy colour. If the eggs are laid on the surface of the body, the resulting larvae (except in the cases of the external parasites) soon bore into the interior of their victim, and disappear therein. The changes that take place in the lifetime of the larvae have been studied in only a few cases; but if we can judge from Ratzeburg's history[469] of the changes that take place in Anomalon, they are of great interest. From observation of the differences existing amongst a great number of larvae of A. circumflexum he distinguished four stages. It is of course impossible to follow directly the growth of one individual, because it is concealed in the interior of the caterpillar in which it lives, and to open this involves the death of both caterpillar and Ichneumon-larva. The life history must therefore be constructed from a great number of separate observations; and it is not ascertained that the four instars described by Ratzeburg represent the number of moults of the larva that actually take place. He, however, entertained no doubt that all the forms he observed {553}were stages in the development of one species. In the earliest stage, when only one millimetre in length and about as thick as a horse-hair, the larva is free in the interior of the caterpillar's body, and has a small head armed only with a pair of mandibles. There are, in addition to the head, thirteen segments, and the last of these is an elongate tail forming nearly one-half the length of the creature. No trace of tracheae can be discovered. In the second stage the larva is still free, an elongate tracheal tube exists, the tail has diminished to half the length, the head has become much larger, and rudimentary antennae of one joint are visible; possibly stigmata are present at this stage, though they cannot afterwards be detected. In the third stage (Fig. 361, C) the larva is encysted, the head is large, the parts of the mouth are all developed, the tracheal system is extensive, and the caudal termination of the body is quite short; notwithstanding the extensive development of the tracheal system, no stigmata can be found. In the fourth stage the larva is still encysted, the tail has disappeared, the head and mouth parts are reduced in size and development, and the creature has now the appearance of a normal larva. The changes to pupa and perfect Insect take place within the body of the victim, in some cases, if not usually, after it has undergone its metamorphosis into a chrysalis. Very little information is extant as to the duration of the various stages, but it appears to be the rule that only one generation appears annually, though in some cases there are pretty certainly two.
It is very difficult to observe the act of oviposition; the Ichneumon-flies usually decline to notice caterpillars with which they are placed in confinement. Ratzeburg thinks they will only attack caterpillars that are in a deficient state of health or vitality. Occasionally we may by a happy chance observe the act in Insects at large, and from the records of observers it may be deduced with tolerable certainty that the sense of sight takes no part in the operation. Ratzeburg relates that he saw a Pimpla alight on a leaf of Rhus and thrust its ovipositor through the leaf. On looking to the under-side of the leaf he found that a cocoon of Bombyx neustria was concealed there in such a position that it could not have been seen by the Ichneumon.
Among the most remarkable of the Ichneumon-flies are the Insects of the genera Rhyssa and Thalessa. These fine Insects have an ovipositor three or four inches in length, and are parasitic on species of the family Siricidae, which, as we have previously described, live in solid wood. In order therefore to deposit the egg in a suitable place, the wood must be pierced by the Ichneumon. The ovipositor is not only of extreme length, but is also furnished with serrations on its apical part, so that it forms a very effective boring apparatus. It is brought into use by being bent on itself over the back of the Insect (Fig. 362), so as to bring the tip vertically down on to the wood, through which it is then forced by a series of efforts; the sheaths do not enter the wood. The egg is laid anywhere in the burrow of the Sirex; the young larva seeks its prey, and lives on it as an external parasite (Fig. 342, D). Erne, however, states[470] that the young larva of Rhyssa persuasoria enters its victim, and remains within the latter till its death occurs. This happens when the young Rhyssa is two or three lines in length, and it then makes its exit from the interior of the body and gradually eats it up. Should the larva it has attacked be of large size, it of itself affords sufficient food for the completion of the growth of the Rhyssa. Should the Rhyssa, however, have attacked a small larva, this does not furnish it with sufficient food, and it consequently dies without seeking another larva. Erne says, indeed, that it will not eat another if offered to it, so that in order to rear the Rhyssa in captivity, the victim it has first attacked must always be given to it. The same observer states that the Rhyssa larva is sometimes transported by the Sirex deep into the wood, so that when it has completed its metamorphoses the Ichneumon-fly may find itself buried in solid wood to a depth of about two inches. In that case it excavates the wood with its mandibles, and should it fail to gain the exterior after {555}three days of work, it dies. In the case of Thalessa it is stated that it sometimes bores into wood where there are no larvae, but Riley thinks this erroneous; it is, on the other hand, certain that the Insect after penetrating the wood is frequently unable to withdraw the ovipositor, and consequently dies.
Packard has recorded,[471] without mentioning the species, the oviposition of an Ichneumon of which the egg is deposited externally. It was placed on the head of the caterpillar, and speedily hatched; the young larva at once bored through the prothoracic segment of the victim, the head of the latter then became swollen, and covered the opening into the prothorax, made by the parasite.
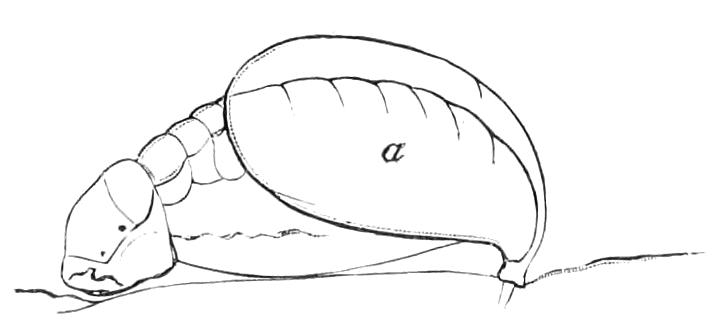
Fig. 363.—Young larva of Paniscus in position of feeding on the skin of Mamestra. (After Newport.) a, The egg-shell.
The history of an Ichneumon larva that feeds as an external parasite has been sketched by De Geer and Newport. The observations of the latter[472] refer to Paniscus virgatus; he found small, shining, black bodies attached to the skin of the larva of a moth, Mamestra pisi; these were the eggs of the Ichneumon. They are furnished with a short peduncle, which is implanted in the skin of the victim; the egg, according to De Geer, being retained more firmly by the peduncle subsequently swelling, so as to form two knobs. The hatching takes place by the egg-shell splitting longitudinally, while from the split protrudes the little head of the destroying larva. This becomes fixed to the caterpillar, from which the nutriment is to be drawn; the Paniscus larva does not, however, leave the egg-shell, but, on the contrary, becomes adherent to it, so that the parasite is in this manner fixed by the two ends to its victim. In fifteen days the parasite was full-grown, and had become half an inch in length. At first no tracheae were to be seen, but these were detected after the second day. Moulting took place three times, and in a peculiar manner, very different from that described by Ratzeburg as occurring in the internal parasites (which, he states, change their very delicate skin by detaching it in almost imperceptible fragments). In the external parasite the {556}skin remains entire, and is shuffled down to the extremity of the body, but cannot be completely detached owing to the anchoring of the posterior part of the body to the caterpillar; the cast skins thus remain as envelopes to the posterior part of the body. Newport states that if the mouth of the parasite be detached, it usually cannot again seize hold of the victim, and consequently perishes. It is a curious fact that more eggs than one caterpillar can support are habitually placed on it, and some of the resulting larvae of necessity perish during the period of growth. Poulton, who has recently made some additional observations on the development of Paniscus,[473] says that if three larvae are close together, it is the middle one that perishes, and suggests that this is due to some simple physical condition. From Newport's account it may be gathered that the Mamestra retains sufficient vitality to form its cocoon, and that the Paniscus larvae likewise construct their own cocoons within that of the Mamestra. In the case of Paniscus cephalotes feeding on Dicranura vinula, Poulton relates that the latter died after the twelfth day of attack. The parasites, having relaxed their hold on the victim just previous to this event, then thrust their heads into the dead body, and devoured the larva, leaving only a dried and empty integument. These larvae span a loose sort of web in which to undergo their metamorphosis. In a natural state, however, they form cocoons inside the cocoon of the Dicranura. The period passed in the pupal condition was about four weeks. This parasite only attacks the Lepidopterous larva during the last stage of its existence as a larva, but the eggs may be laid on the victim in an earlier stage; and in such case De Geer has stated, and Poulton has confirmed the observation, that though the larva sheds its skin it does not get rid of the eggs.
The little Ichneumons of the genus Pezomachus are quite destitute of wings and somewhat resemble ants; they are common Insects in Britain. Only the female sex is known, and it is believed that the winged Ichneumons assigned to the genus Hemiteles—of which no females are known—are the males of Pezomachus. Repeated efforts have been made to place this beyond doubt, but they have usually failed, for when a brood of these parasites is reared, the individuals generally prove to be {557}either all Hemiteles or all Pezomachus. It is to be hoped that this interesting case will be fully elucidated.
Although the Ichneumonidae are perhaps the most purely carnivorous of all the great families of Hymenoptera, there is nevertheless reason for supposing that some of them can be nourished with vegetable substances during a part at any rate of the larval existence, Giraud and Cameron[474] having recorded observations that lead to the conclusion that some species of the genus Pimpla may inhabit galls and live on the substance, or juices thereof.
Over 1200 species of Ichneumonidae are known to inhabit Britain, and there can be no doubt that this number will be increased as a result of further observation. Unfortunately no general work has yet been published on this department of our fauna, and the literature is very scattered.[475] The species of North America have not received so much investigation as those of Europe, and the Ichneumon fauna of the tropics remains almost uninvestigated. Six sub-families are recognised: Agriotypides, Ichneumonides, Cryptides, Tryphonides, Pimplides, Ophionides. Of these the first is the most remarkable, as it consists of an Insect having aquatic habits. It has for long been known that the unique species Agriotypus armatus, a rare Insect in our islands, is in the habit of going under water and remaining there for a considerable period, and it has now been satisfactorily ascertained that it does this for the purpose of laying its eggs in the larvae of Trichoptera.[476] The resultant larva lives inside the cases of species of Silo, Goëra, etc. It undergoes a sort of hypermetamorphosis, as its shape before assuming the pupal condition {558}is very different to what it was previously. It changes to a pupa inside the case of the Trichopteron in a cocoon attached to the walls of the case. Previous to making this, however, the Agriotypus forms a curious, elongate, string-like process attached to the anterior extremity of its cocoon. The use of this is unknown. Full information as to the life-history of this aquatic Hymenopterous larva, especially as to its respiratory functions, would be of great interest. The affinities of this remarkable Insect are still doubtful. It may probably prove to be between Proctotrypidae and Ichneumonidae.
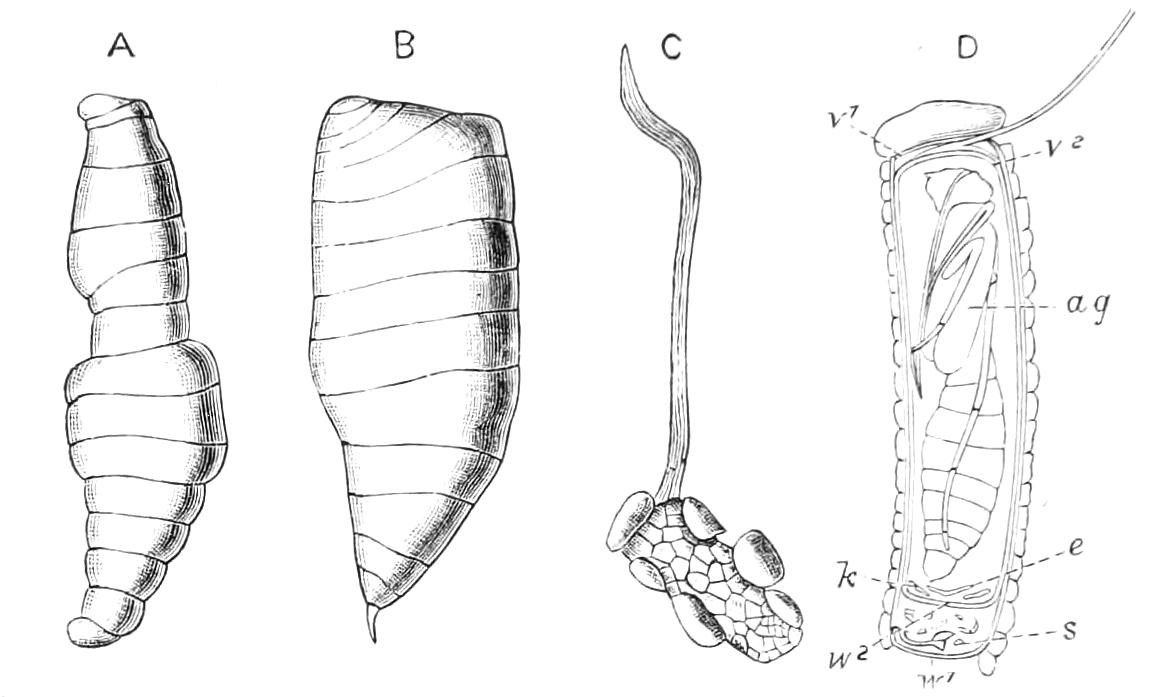
Fig. 365.—Metamorphosis of Agriotypus. (After Klapálek.) A, Larva; B, sub-nymph; C, case of the Silo with the string of attachment formed by Agriotypus; D, section of the case: v1, operculum of case; v2, cocoon; ag, pupa of Agriotypus; e, exuvia of Agriotypus; w2, wall of cocoon; s, remains of Silo; w1, closure of case.
Remains of Insects that may be referred with more or less certainty to Ichneumonidae have been found in some abundance in various tertiary strata both in Europe and North America, but nothing indicative of the existence of the family has yet been found in the older rocks.
Fam. V. Braconidae—Supplementary Ichneumon-flies.
Antennae with many (nearly always more than fifteen) joints, not geniculate. Wings with a moderate number of cells, which on the anal part of the front wing are more or less imperfect, the anal (i.e. the second posterior) cell being separated from the cubital cells by a large space in which there is no cross-nervure. Abdomen with but little mobility between the segments; the suture between the second and third usually {559}absent, or obsolete. Larvae living parasitically in—possibly exceptionally outside—the bodies of larvae or pupae of Insects.
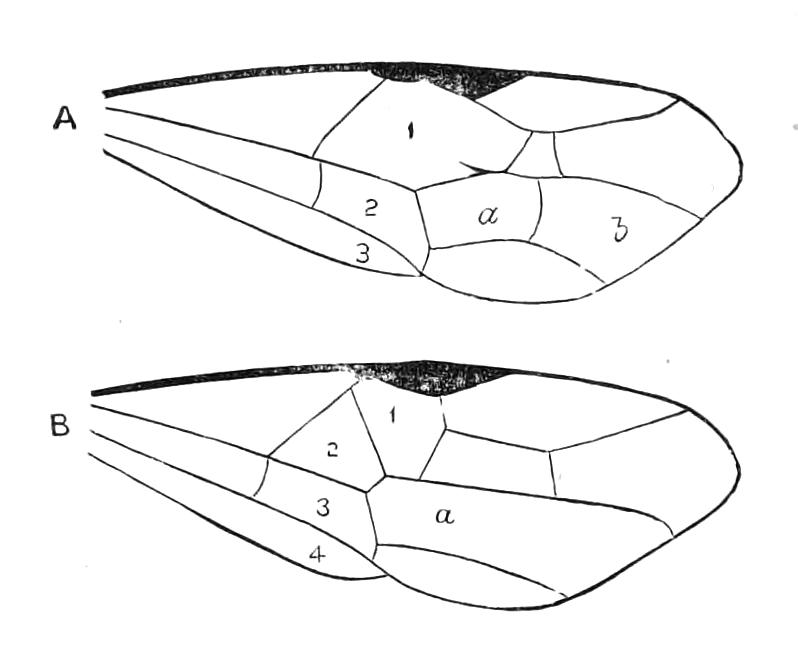
Fig. 367.—Diagram of wing of Ichneumonid (A) and of Braconid (B). 1, 2, 3, 4, series of cells extending across the wing; a, b, divided cell of the Ichneumonid wing, corresponding with a, the undivided cell of the Braconid wing.
The Braconidae are the Ichneumones, or Ichneumonides, adsciti of the older Hymenopterists. They are extremely similar to the Ichneumonidae, but the hind body has a much less degree of mobility of its segments, and there are some constant distinctions in the wings. Although there is a great deal of difference in the various forms of each of the two families, yet there are two points of distinction easily appreciated; the series of cells running across the wing (Fig. 367) being only three in the Ichneumonides (Fig. 367, A), but four in the Braconids (Fig. 367, B); besides this the space a of the Braconid wing is divided into two (a, b) in the Ichneumonid wing. A glance at these characters enables us at once to separate correctly the thousands of species of the two families.
The habits of the Braconidae are similar to those of Ichneumonidae, it being believed that all are parasites. Usually they attack larvae, but they are bred in great numbers from pupae, and even from imagos of other Insects. Elasmosoma is one of the few parasites known to attack ants. As many as 1200 specimens of Microgaster have been reared from a single Lepidopterous larva. Although such parasitism raises a feeling of repulsion, yet there is reason for supposing that there may be {560}little or no cruelty or acute suffering connected with this mode of life. The victim attacked is not eaten, the parasites in the interior taking in the lymph of the caterpillar either by the mouth or by endosmosis, but not biting their host. The latter displays no sign of sickness, but eats voraciously, so that it serves merely as a sort of intermediary between the juices of the plant and the larvae inside itself. It is only when the metamorphosis is at hand that the host sickens, but this does not always happen: parasitised larvae frequently change to pupae, and they may occasionally even become perfect Insects. Cases are known in which imagos have appeared with some of the small parasites embedded in some of the outer parts of their bodies. These cases are, however, very rare; in the enormous majority of instances the host is destroyed either when it is in the larval stage or before the pupa has advanced to any great extent on its metamorphosis to an imago. Particulars as to various species will be found in the valuable work of Ratzeburg we have already referred to.[477] Reference may also be made to Goureau's account of Microgaster globatus,[478] this latter including some suggestions by Dr. Boisduval on some of the difficult physiological questions involved in the lives of these parasites.
The metamorphosis of Microgaster fulvipes has been studied by Ratzeburg, and an epitome of his observations is given by Marshall.[479] The larva goes through a series of changes somewhat similar to those we have already sketched in Anomalon circumflexum. Usually these Insects after emerging from the body of their host spin a mass of cocoons more or less loosely connected together. A most curious case has, however, been recorded by Marshall[479] of a stalked cocoon (Fig. 368) being formed as an exceptional act by Apanteles formosus. Mr. Marshall has recently received other specimens of this cocoon as well as the Insects reared therefrom in France, and inclines to the opinion that the stalked cocoon may be the usual form, and is sometimes departed from by the Insect for unknown reasons.
This family is of enormous extent; we have several hundred species of it in Britain,[480] and there are no doubt many thousands of undescribed exotic forms. To Apanteles glomeratus we are indebted for keeping our cabbages and kindred vegetables from destruction by the caterpillars of the white butterflies. The larvae of the various species of Pieris, as well as those of other Lepidoptera, are attacked by this little Insect, the masses of whose cocoons may frequently be found in numbers in and near cabbage gardens. The tropical species of Braconidae are greatly neglected, but many large and remarkable forms—some of brilliant colours—have been brought from there, so that we are justified in believing that Insects of this family will prove to be very numerous. There are but few apterous Braconidae. Both sexes of Chasmodon apterus are destitute of wings; the females of one species of Spathius, and also those of Pambolus and Chasmodon are apterous; in a small number of species of various genera the wings are so minute as to be incapable of serving as organs of flight. In the genus Alloea the wings of the male are shorter than those of the female.
Fam. VI. Stephanidae.
Antennae composed of many (thirty to seventy) joints; hind body attached to the lower and posterior part of the median dorsal {562}plate. Wings with a distinct costal cellule; head globose, posterior femora frequently toothed.
This is a doubtful family, consisting of a few anomalous Insects. Schletterer assigns to it only two genera, Stephanus and Stenophasmus;[481] both have a wide distribution over the world, though we have no species in Britain. Nothing is known of their habits, and they are apparently all very scarce Insects. The definition is compiled from those of Cameron and Schletterer. There seems very little to distinguish these Insects from Braconidae.
Fam. VII. Megalyridae.
Hymenoptera with short broad hind body, which is not separated by a pedicel from the thorax. The female has a very long bristle-like ovipositor. Antennae with fourteen joints.
This family is constituted by the Australian genus Megalyra,[482] one of the most interesting of the numerous extraordinary Insect-forms found in that region; the species appear to be very rare and not numerous. Apparently nothing is known as to their habits. It is quite possible that these Insects will prove to be anomalous Braconidae.
Fam. VIII. Evaniidae.
Petiole of the abdomen attached to the upper part of the median dorsal plate; antennae not elbowed, of thirteen or fourteen joints. Wings with a moderate number of nervures. Larva of parasitic habits.
This family is composed of only three genera—Evania, Gasteruption, and Aulacus, each possessing a considerable number of species; they agree in the characters mentioned above, and may be readily recognised by the peculiar insertion of the hind body. This character occurs outside the limits of the Evaniidae only in one or two genera of Chalcididae and Braconidae; it is to this latter family that the Evaniidae must be considered most closely allied.
The species of the genus Evania are believed to live at {563}the expense of cockroaches (Blattidae), and to deposit their eggs in the egg-capsules of those Insects. The species of Gasteruption live, in the larval state, on the larvae of other Hymenoptera, more especially of such as form nests in wood. Very little is known as to the habits of the species of Aulacus, but it is believed that they are parasitic on members of the Hymenopterous families, Siricidae and Oryssidae. Only the most meagre details as to the life history of any of the Evaniidae have been recorded. The species of Evania are met with most freely where cockroaches abound, and are said, hence, to be frequently observed on board ship. Two or three species of each of the two genera Evania and Gasteruption occur in Britain. The latter genus is more widely known under the name of Foenus.[483]
Fam. IX. Pelecinidae.
Sexes very different; the female without exserted ovipositor, but with extremely long abdomen. Articulation between the femur and trochanter oblique and elongate, but without division of the trochanter.
This family at present comprises, according to Schletterer,[484] only the three genera Pelecinus, Ophionellus, and Monomachus. The systematic position of the Insects is very doubtful, and their habits are but little known. Pelecinus polyturator (Fig. 370) appears, however, in the female sex, to be a common Insect over a large part of the warmer regions {564}of the New World; it is in all probability parasitic in its habits, the elongate ovipositor of the female Ichneumon being in this Insect replaced by an extraordinary linear extension of the abdomen itself. Doubleday has recorded that he saw twenty or thirty specimens of this species that had perished with their elongated hind bodies inserted into the stem of a tree, from which they could not extricate themselves. On the other hand, Patton thinks they are parasitic on locusts.[485]
The male in Pelecinus has the proportions of the parts of the body normal, there being no elongation of the abdomen; it thus differs very much in appearance from the female. There seems to be very little to distinguish Pelecinus from Proctotrypidae. The undivided trochanters have led to these Insects being placed, by some, among the Aculeate Hymenoptera. This character, as we have already shown, occurs also in Proctotrypidae.
Fam. X. Trigonalidae.
Abdomen ovate, not separated by a pedicel from the thorax. Antennae twenty-five-jointed. Trochanters imperfectly two-jointed. Both the anterior and posterior wings provided with a well-developed neuration. Abdomen composed of only five apparent segments. Larva (in some cases) parasitic on Aculeate Hymenoptera.
This family is chiefly constituted by the very rare Insects contained in the genus Trigonalys, of which we have one species in Britain. Although, so far as appearance goes, they have little in common with the parasitic Hymenoptera, and look quite like members of the Aculeata, yet the late F. Smith found a species in the cells of Polistes lanio, thereby showing it to be of parasitic habits. Although some Aculeate Hymenoptera are also of parasitic habits, yet the characters of Trigonalys perhaps agree, on the whole, better with the Hymenoptera parasitica. The British species is very {565}rare. The South American genus, Nomadina, looks still more like a bee, and the trochanters are even more imperfectly divided than they are in some of the Aculeate group, Nyssonides, the outer portion being merely a small piece imperfectly separated from the base of the femur.
Note.—The citation of Saint Augustine on p. 85 is made in the words used by Wasmann in Der Trichterwickler, eine naturwissenschaftliche Studie über den Thierinstinkt, 1884.
The authenticity of the passage we have adopted as the motto for this volume is somewhat doubtful. It is explained in an "admonitio ad lectorem" of the soliloquy, that this work is probably a compilation by a later writer, from two, or more, works of Saint Augustine. Father Wasmann has been so kind as to inform the writer that the idea of the passage quoted occurs frequently in the undoubted works of the Saint, as, for instance, de Civitate Dei, lib. xi. cap. 22; Serm. ccxiii. in traditione symboli II. cap. i.; contra Faustum, lib. xxi. cap. v. etc. The passage quoted is, however, the only one in which "angeli" and "vermiculi" are associated.
Every reference is to the page: words in italics are names of genera or species; figures in italics indicate that the reference relates to systematic position; figures in thick type refer to an illustration; f. = and in following page or pages.
Abdomen, 109;
of Hymenoptera, 492 f.
Abdominal appendages, 188, 189, 190
Achorutes murorum, 194
Acini, 126
Acoustic orifice, 317
Acridiides, 310
Acridium peregrinum, 298;
growth, 156;
at sea, 297
—see also Schistocerca
Acrophyllides, 278
Aculeata, 520
Aculeates and Proctotrypids, 535, 564
Adler, on alternation of generations, 530;
on galls, 526 f.;
on useless males, 498
Aeschna cyanea, 412;
A. grandis, labium, 411;
Agaonides, 547
Agrion nymph, 426
Agrion pulchellum, 412
Agriotypides, 557
Agriotypus armatus, 557
Air sacs, 128, 282, 283, 294, 495
Alaptus excisus, 537;
A. fusculus, 538
Alar organs, of earwigs, 206;
of Blattidae, 225;
of Mantidae, 245;
of Phasmidae, 269;
of Acridiidae, 281;
development of, in earwigs, 212;
in Mantidae, 248
—see also Tegmina, Wings, Elytra
Alary muscles, 134
Albarda on Raphidiides, 448
Alder flies, 444
Aleuropteryx, 471
Alimentary canal, 123-127, 403, 446;
of may-flies, 438 f.;
of Panorpa, 450;
Alloea, 561
Alternation of generations, 497, 530
Amber, Myriapods in, 74, 76, 77;
Insects, 179;
Aptera, 196;
Blattidae, 239;
Mantidae, 258;
Phasmidae, 276;
Psocidae, 397;
Perlidae, 407;
Phryganeidae, 485;
Tenthredinidae, 518;
Cynipidae, 533
Ambua, 40
Ameles, 245
Amnios, 291
Amorphoscelis annulicornis, 251
Amphibiotica, 342
Amphientomum paradoxum, 397
Ampulex, abdomen, 492
Ampulla, 290
Amylispes, 76
Anabolia furcata, mouth-parts, 475;
A. nervosa, larva, 476
Anabrus purpurascens, 321
Anaplecta azteca, folded wing, 227
Anaplectinae, 240
Anatomy—see External Structure and Internal Anatomy
Anderson, Dr. J., on Gongylus, 254
Anechura scabriuscula, 208
Anisolabis maritima, 205;
A. moesto, 205;
A. tasmanica, 216
Anisomorpha pardalina, 274
Anisomorphides, 278
nymphs, 421
Anomalon, metamorphosis, 552
Anomalopteryx, 484
Anostostoma australasiae, 326
Anoura, 190
{568}Ant, brain, 119;
nervous system, 495;
castes, 500
Ante-clypeus, 93
Antennae, 97;
growth of, 212
Anthophora retusa, parasites of, 544, 545
Anthophorabia retusa, 545
Apanteles glomeratus, 561
Apatania, 481;
A. arctica, A. muliebris, 481
Apex, 112
Aphilothrix, 531
Apocrita, 519
Apodeme, 103
Apterous Insects, 205, 216, 217, 220, 234, 235, 252, 261, 262, 264, 269, 272, 274, 277, 299, 302, 303, 307, 321, 322, 323, 324, 325, 326, 329, 518, 556, 561
—see also Wingless Insects
Aq. Phasmids, 272
Arachnides antennistes, 77
Archidesmidae, 76
Archidesmus, 76
Archijulidae, 76
Arthromeres, 87
Arumatia ferula, anatomy, 262
Ascalaphides, 459 f.
Ascalaphus coccajus, 459;
A. longicornis, 459;
A. macaronius, 460;
eggs, 460
Aschipasma catadromus, 263, 266
Aschipasmides, 278
Ashmead, on Mymarides, 537;
on Proctotrypids, 537;
on Scleroderma, 536
Astroma, 300
Asymmetry, 216
Athalia (centifoliae) spinarum, 515
Atropinae, 394 f.
Atropos divinatoria, 394 f., 396
Atta (Oecodoma) cephalotes, 501
Attitude, 248, 250, 256, 268, 514
Attraction of light, 230
Auditory organ, 400;
of Calotermes, 358
—see also Ear
Aulacus, 562
Aulax, 532
Avicenna, 41
Axes of body, 113
Bacillides, 278
Bacillus patellifer, 263
Bacteria, 276
Bacteriides, 277
Bacunculides, 277
Baëtis, 433
Ballostoma, 196
Ballowitz on spermatozoa, 140
Barber, Mrs., on S. African locust, 294
Barbitistes yersini, 321
Barnston on Perlidae, 402, 405
Barriers with eggs, 461
Base, 112
Basement membrane, 162
Bassett on oviposition of inquilines, 532
Bataillon, on metamorphosis, 131, 168;
on reversed circulation, 135
Bates, on singing grasshopper, 319;
on Termites, 375
Bateson, on forceps of earwig, 209;
on antennae of same, 212
Batrachotettix whiti, 305
Bees killed by Locusta, 321
Belt on domestic cockroaches, 231
Bermuda, 33
Bertkau, on Psocus, 391;
on micropterous Psocidae, 394
Bethylus habits, 535
Bherwa, 326
Bird eaten by Mantis, 250
B. tipularius, 452
Blabera, 235;
wings, 237;
B. gigantea, 222
Blaberides, 241
Black beetle, 221
Blanjulidae, 44
Blanjulus, 44
Blastoderm, 147
Blastophaga grossorum, 547 f.
Blatta, 240
parasites of, 563
Blattinae, 240
Blood, 132
Blood-gills, 479
Blowfly, egg, 145;
metamorphosis, 163
Bolivar on eyes of Machilis, 185
Bombus, dorsal vessel of, 133;
metamorphosis, 497;
B. lucorum, 488
Bombyliidae, 291
Bonnet and Finot on Eugaster, 324
Book-lice, 390 f.
Boreus hiemalis, 451;
larva, 453
Boutan on concealment of leaf-like Insects, 323
Brachyscelides, 526
Brachystola magna, 308
Brachytrypes megacephalus, 332
Bracon palpebrator, 559
Braconidae, 558 f.
{569}Bradford Cave, Myriapods in, 34
of ant, 119;
of Perlidae, 404
—see also Gills
Brandt on nervous system, 119, 495
Brauer, on classification, 175;
on median segment, 491;
on hypermetamorphosis, 160;
on Menorhyncha, etc., 161;
on Ascalaphus larva, 460;
on development of Mantispa, 464;
on Palaeodictyoptera, 486;
on Panorpa larva, 452;
on tegmina of Phyllium, 270
Breitenbach on Proscopiides, 299
Bridgman on British Ichneumonidae, 557
Brindley on growth of cockroach, 229
British, Myriapods, 36;
Orthoptera, 201;
earwigs, 215;
grasshoppers and locusts, 308;
crickets, 339;
Psocidae, 395;
Perlidae, 406;
Odonata, 424;
Chrysopides, 469;
Trichoptera, 480;
Phytophagous Hymenoptera, 504;
Siricidae, 510;
Cynipidae, 533;
Ichneumonidae, 557;
Braconidae, 561
Brongniart, on fossil Insects, 428;
on fossil Neuroptera, 343;
on Neuropteroidea, 486;
on post-embryonic development of locust, 287;
on young Mantis, 247 f.
Brongniart and Becquerel on chlorophyll in Phyllium, 268
Bruner on variation of Orthoptera, 304
Brunner, on Hypertely, 322;
on classification of Orthoptera, 202;
of Blattidae, 240;
of Mantidae, 259;
of Phasmidae, 277;
of Acridiidae, 309;
of Locustidae, 328;
on variation of Oedipoda, 304
Bryodema tuberculata, 281
Bugnion on histolysis, 166;
on Encyrtus, 545
Buller on Weta-punga, 326
Burchell on Mantis, 249
Burgess on Psocus, 391 f.
Burmeister on Mantidae, 250
Bursa copulatrix, 139
Caddis-flies, 473 f.
Caecum, 125
Caenis dimidiata, 442
Calcares, 104
its eggs' parasite, 538
Callimome bedeguaris, 532
Caloptenus spretus, 288 f., 289, 298, 303;
development, 289
Calotermes flavicollis, 362, 363, 371, 376;
C. nodulosus, 359;
Calvert on Odonata, 412
Calvisia atrosignata, 266, 273
Cameron, on ant-parasite, 545;
on gall-producing plants, 527;
on parthenogenesis, 498, 499, 517;
on Pimpla larva, 557
Campodea, 61;
Campodeidae, 183
Camponotus, nerves, 495
Cantharidae, 291
Capnia vernalis, 405
Caprification, 547 f.
Capsule of eggs, 201
—see also Egg-capsule
Caraphractus cinctus, 538
Carboniferous, Myriapods, 75, 76;
Insects, 196, 238 f., 259, 276, 408, 428, 442 f., 449
Cardiophorus larva, 90
Cardo, 95
Carnivorous and vegetarian, 250
Carpenter bee wings, 494
Carruthers on locust swarm, 292
Case, Hymenopterous, 514
Cases, caddis-fly, 476 f., 480, 481, 482, 483, 484, 485
Caudal branchiae, 423
Locustidae, 321;
Cecidomyia, parasites of, 536, 537
Cenchri, 511
Cephalocoema lineata, 299
Cephalonomia formiciformis, 536
Cephidae, 504 f.
Cephus integer, 505;
C. pygmaeus, 505
Cerci, 110, 183, 216, 257, 337, 400;
Cermatia, 35
Cermatiidae, 46
Ceroys saevissima, 264
Cervical sclerites, 99, 99, 409
Chalcididae, 539
Chalicodoma muraria, nest, parasites, 540 f.
Changing colour, 288, 253, 267, 268
Chasmodon apterus, 561
Chatin on labrum, 93;
on mandibles, 95
Chauliodes, 447
Cheeks, 94
Cheimatobia brumata, parasites, 521
Chelidura dilatata, 205
Cheshire on fertilisation of bee, 499
Chilaspis lowii, 530;
C. nitida, 531
Chilopoda, 30, 33, 44, 47, 52, 74, 75;
{570}Chitin, 162
Chitinogenous cells, 162
Chlorophyll in tegmina, 269
Choeradodis cancellata, 252
Cholodkovsky, on head, 87;
on styles of cockroach, 224;
on embryology of Phyllodromia, 237;
on morphology of sting, 493
Chordeuma, 31
Chordeumidae, 44
Chordotonal organs, 121
Chorion, 144
Chorisoneura, 240
Chromosomes, 146
Chrysopa eggs, 469;
larva, 469;
C. aspersa, 470;
C. flava, 469;
C. pallida larva, 470
Chun on rectal gills, 422
Chyle, 133
Cimbex abdomen, 493;
abdominal articulation, 492;
dorsal vessel, 134;
C. sylvarum, saws, 512
Cinura, 182
Circulation, 132 f.;
in caudal setae, 435
Cladomorphides, 278
Cladonotus humbertianus, 301
Classification, 171 f.;
of Blattidae, 240;
of Mautidae, 259;
of Phasmidae, 277;
of Acridiidae, 309;
of Locustidae, 328;
of Gryllidae, 340
Clitumnides, 278
Cloëon, eyes, 430;
C. dimidiatum, larvule, 432;
C. dipterum, nymph, 432;
respiration of nymph, 435
Clothilla, 395;
anatomy, 392
Cockroaches, 220
Cocoons of sawfly, 515
Coeloblast, 149
Coleoptera, 173
Collophore, 193
Colour, 200
Commissures, 116
Common cocoons, 515
Compass Termite, 386
Complementary Termites, 361
(=facetted eyes) in Myriapods, 36
Concealment by movement and position, 268;
by selection of place, 308
Coniopterygides, 471
Coniopteryx lutea, 471;
C. psociformis, 471;
C. tineiformis, 472
Copiophora cornuta, 313
Cordulegaster, 415;
C. annulatus, 415
Cordulegasterinae, 426
Corduliinae, 426
Correlative variation, 536
Corrosion by Termites, 360
Corydalis, 447;
C. crassicornis, 447
Corydaloides scudderi, 344
Corydia, 221;
C. petiveriana, 233
Corydiides, 241
Coryna, 550
Corynothrix borealis, 191
Costa, 108
Cotes on Indian locusts, 298
Cotylosoma dipneusticum, 272
Craspedosoma, 76
Crawlers, 447
Creepers, 407
Cretaceous Myriapods, 75;
Insects, 485
Creutzberg on circulation, 436
Crioceris asparagi, legs of larvae, 106
Crunoecia irrorata, case of, 480
Cryptides, 557
Cryptocerus, abdomen of, 109
Crystalline cone, 98
Cuculligera flexuosa, 304
Cunningham on fig fertilisation, 549
Cursoria (Orthoptera), 201
Cuvier, 77
Cyclops form, 536
Cylindrodes campbellii, 336;
C. kochi, 336
Cynipidae, 523
Cynips aciculata, 531;
C. disticha, 530;
C. folii, 530;
C. kollari, 530;
C. lignicola, 530;
C. spongifica, 531
Cyphocrania aestuans, 266
Cyprus, 32
Cyrtophyllus concavus, 320;
C. crepitans, 311
Dahl and Ockler on feet, 105
D'Albertis on may-flies, 441
Damsel-flies, 417
Dancing may-flies, 439 f.
Dasyleptus lucasii, 196
Death-watch, 395 f.
Decaux on cannibalism of mole-cricket, 336
Decoys, 257
Decticides, 329
De Geer on earwigs, 214
Degeeriidae, 190
Deinacrida heteracantha, 326
Demoiselles, 417
Dendroleon pantherinus, 458
Denny on Mantis in England, 258
Derham on death-watches, 396, 397
Dermatoptera, 202
{571}Derocalymma, 235
Deroplatys sarawaca, 243
De Saussure, on Orthoptera, 202;
on wings of Blattidae, 226 f.;
on classification of Gryllidae, 340;
on Hemimerus, 217;
on nomenclature of Blattidae, 240;
on oceans as barriers to migration, 297
Deuto-cerebron, 118
Development, of alar organs of Platycleis, 312;
of crickets, 332
—see also Embryology and Metamorphosis
Dewitz on caste, 500;
on ovipositor of Locusta, 314;
on morphology of sting, 493;
on internal legs, 496;
on development of wings of Phryganeidae, 479, 480;
on dragon-fly nymphs, 423;
on Chrysopa larva, 470
Diaphana fieberi, 226
Diapheromera femorata, 263, 264, 265, 267
Diastrophus, 532
Diaulus, 484
Dicranota, larva, glands of, 142
Dictyopteryx microcephala, 406;
D. signata, 401
Dielocerus ellisii, 515
Digestion, 127
Dilarina, 465
Dilke, Sir Charles, on Orchis-like Mantis, 254
Dimorphic cocoons, 560;
Diplectrona, 479
Diploglossata, 217
Diploptera silpha folded wing, 227
Diptera, 173
Disgorgement, 495
Distant on S. African locust, 298
Divided eyes, 409
Docophorus fuscicollis anatomy, 348;
D. icterodes, D. cygni, 349
Dog, biting-louse of, 349
Dohrn on tracheal system of Gryllotalpa, 132;
on embryology of Gryllotalpa, 336
Dolichopoda palpata, 322
reversed action, 435
Dorsum, 100
Dragon-flies, 409
Drakes, 441
Drepanepteryx phalaenoides, 453, 468;
wings, 468
Drones, 499
Drummers, 237
Dubois on decapitated Mantis, 250
Duchamp on egg-capsule of cockroach, 228
Ductus ejaculatorius, 140
Dudley and Beaumont on Termites, 372, 387
Dufour, on alimentary canal, 124;
on tracheal system, 129;
on air sacs of Acridiidae, 283;
on testes, 140;
on phonation, 286;
on Tridactylus, 338;
on Mantidae, 246;
on earwigs, 210;
on anatomy of cockroach, 228;
on anatomy of Gryllotalpa, 335;
on anatomy of Termites, 360;
on anatomy of Panorpa, 450;
on larva of Sialis, 446;
on Myrmeleon larva, 458
Duns, 441
Dust-lice, 390 f.
Dwellings of Termites, 385 f.
Dytiscus, mesothorax, 101;
Dzierzon theory, 499
of Locustidae, 316 f., 316, 317;
of crickets, 332;
Earliest Insect, 238
Earwig, 202 f., 211, 213, 214;
wing, 206;
the name, 214
Eaton, on nymph, 157;
nature of, 169
Ectobia, 236;
E. lapponica, egg-capsule, 229
Ectobiides, 240
Ectoblast, 149
Ectoderm, 148;
Ectognathi, 189
Ectotrophi, 189
of Peripatus, 19;
of Ascalaphus, 460;
growing, 513;
of parasites, 552;
of egg-parasites, 545;
of Corydalis, 447;
of Cynipidae, 528;
of Limacodes, 153;
of Mallophaga, 348;
of Microcentrum, 314;
of Phasmidae, 265, 270 f., 270;
of Perla, 404;
of Sialis, 445;
of Trichoptera, 476
of Phyllium, histology, 271
—see also Ovaries
Eileticus, 76
Eisig on chitinous excretion, 130, 163
Ejection of fluid, 264, 324, 399, 515
Elasmosoma, 559
Elater larva, 29
Elipsocus brevistylus, 393
Elytra, 108
Embidopsocus, 395
of Peripatus, 19 f.;
of Myriapods, 63 f.;
of parasites, 522;
of earwig, 216;
of Blattidae, 237;
of Encyrtus, 546;
{572}of Gryllotalpa, 336;
of Polynema, 538;
of Smicra, 545;
of Proctotrypidae, 536 f.
Emergence from egg, 263, 264, 290, 291, 313
Empodium, 105
Empusides, 259
Encyrtus fuscicollis development, 545
Endoblast, 149
Endoderm, 148;
Endolabium, 97
Endo-skeleton, 399
Eneopterides, 340
Enock on Alaptus and Caraphractus, 538
Enoicyla pusilla, 481
Entognathi, 189
Entomology, 86
Entotrophi, 189
Eocene, 407
Ephemera, 434;
wing, 431;
E. vulgata, 441;
nymph, 433
Ephippigera Malpighian tubes, 335;
E. rugosicollis, 323
Epidemes, 107
Epilamprides, 240
Epistome, 92
Epithelium of stomach, 126
E. turcica, 253
Eremobiens, 304
Erianthus, 301
Erichson on Neuroptera, 342
Erne on Rhyssa, 554
Etoblattina manebachensis, 238, 239
Eucharis myrmeciae, 545
Euchroma, head and neck, 99
Eucorybas, 37
Eugaster guyoni, 324
Eugereon bockingi, 486
Eumegalodon blanchardi, 327
Eumegalodonidae, 327
Euorthoptera, 216
Euphaea, 422
Euphoberia, 76
Euprepocnemis plorans, 303
Eurycantha australis, 274
Eurytoma abrotani, 539
Eusthenia spectabilis, 407
Eutermes, 374;
E. ripperti, 388
Euthyrhapha, 226
Evania, 562
Evaniidae, 562
Exner on sight, 416
Exodus, locust of the book of, 298
Exsertile blood-sacs, 132
External parasite, 555
External structure, 87;
diagram, 88;
of earwigs, 203 f.;
of cockroaches, 221;
of Mantidae, 242 f.;
of Phasmidae, 260 f.;
of Acridiidae, 280 f.;
of Odonata, 409 f.;
of Ephemeridae, 430 f.;
of Panorpa, 450;
of Phryganeidae, 474;
of Hymenoptera, 489 f.;
of Tenthredinidae, 511
Eyes, 97
—see also Compound Eyes and Ocelli
Fabre on Leucospis, 540;
on Monodontomerus, 543;
on Sirex, 509
Facetted eyes—see Compound Eyes
Family, 177
Fat-body, 136
Feeding, by Termites, 376;
young, 495
Fenestra, 221
Fenestrate membrane, of eye, 98;
of pericardium, 134
Fertilisation, 499;
of fig, 549
Field-cricket, 332
Fields of wings, 206
Fig-Insects, 547 f.
Figitides, 525
Finot on Japyx, 196
Fire-brats, 186
Fischer on instars, 158
Fish destroyed, 425
Fletcher on parthenogenesis, 498
Flight, 416
Floral simulators, 254 f.
Flying-machine, model for, 417
Foenus, 563
Foetus of Hemimerus, 218
Forbes on Blattid, 235
Forel on nervous system of ant, 495
Forficula auricularia, 202 f., 204, 209, 211;
F. gigantea, 210
Formica-leo, 456
Formicajo, 456
Formicario, 456
Fossil, Insects, 178, 472, 485, 486;
Acridiidae, 308;
Blattidae, 238;
cricket, 340;
dragon-flies, 427;
earwigs, 216;
Locustidae, 328;
Mantidae, 258;
Phasmidae, 276;
Panorpidae, 453;
Perlidae, 407;
Sialidae, 449;
Termites, 389;
Thysanura, 196;
Myriapods, 72 f.;
Palaeozoic Neuroptera, 343
Founding communities, 381
Fourmilions, 456
Fowl, biting-louse of, 350
{573}Fritze on Ephemerid alimentary canal, 439
Frons, 94
Front wings absent, 260 f.
Fungus chambers, 387
Fungus-growing Termites, 385, 387
Funiculus, 492
Furca, 103
Galapagos Islands, 459
Galea, 95
Gall-flies, 523 f.
Galls, 514 f.;
Ganglia, 116
Ganin, on metamorphosis, 162;
Gasteruption, 562
Gena, 94
marine, 30;
phosphorescent, 34
Geoscapheusides, 241
Gerephemera simplex, 428
Gerstaecker, on Neuroptera, 343;
on mouth of Odonata, 411
Giebel on Mallophaga, 347
Gigantic Insects, 276, 306, 428
Gilbert White, on mole-cricket, 333;
on field-cricket, 339
Gills, 132, 400, 421, 432 f., 478;
filamentous, 476;
spongy, 447;
prothoracic, 443;
of pupa, 483;
blood-gills, 479
Giraud on Cynipid oviposition, 528
Glacier water, 405
Glande sébifique, 139
conglobate, 229;
maxillary, 458;
mushroom, 228
—see also Salivary Glands
Glandulae odoriferae, 31, 36, 54
Golden-eyes, 469
Göldi on eggs of Phasmidae, 265
Gomphinae, 426
Gomphocerus, 308
Gomphus, 415
Gonapophysis, 110
Gongylus gongylodes, 254 f., 255
Gosch on median segment, 491
Goureau on Microgaster, 560
Graber, on dorsal vessel, 134;
on blood cells, 137;
on ears, 286;
on ears of Locustidae, 316, 317;
on chordotonal organs, 121;
on blood, 133;
on phonation of Stenobothrus, 284;
on Platycleis, 312
Grassi, on Myriapoda, 47;
on Campodea, 163;
on Embia, 353;
on Termitidae, 361 f.
Grassi and Rovelli on Thysanura, 182
Green grasshoppers, 311
Green, Mr. Staniforth, on Helicomitus larva, 461
Gromphadorhina portentosa, 235
Grosse on Mallophaga, 346
Growth of wings, 393;
of Mantidae, 248
Gryllacrides, 329
Gryllides, 340
Gryllotalpa, 332;
dorsal vessel, 134;
Malpighian tubes, 127;
tracheal system, 132
Gryllotalpides, 340
Gryllus, head, 93;
Guilding on Ulula, 461
Gyri cerebrales, 119
Gyropus, 350
Haase on abdominal appendages, 189, 192
Hagen, on segments, 88;
on wing-rudiments, 395;
on respiration of immature dragon-fly, 423 f.;
on larvae of Ascalaphides, 460;
on amber Psocidae, 397;
on Platephemera, 428;
on Perlidae, 401;
on Psocidae, 393 f.;
on Termites, 360 f.
Haldmanella, 308
Halesus guttatipennis, 473
Haliday on Bethylus, 535
Halobates, 83
Halteres, 108
Hansen on Hemimerus, 217
Haplogenius, 461
Haplophlebium, 345
Haplopus grayi, egg, 265
Harpagides, 259
Harpalus caliginosus, head, 92
Harpax ocellata, 253;
H. variegatus, 244
Harrington on Oryssus, 507
Harris on Katydids' music, 320
Hart on forms of Atta, 501
Hartig on gall-flies, 530
Harvesting Termites, 383
Harvey on metamorphosis, 168
Hatchett Jackson on ecdysis, 162;
on oviduct of Lepidoptera, 139
Haustellata, 94
Haustellum, 476
Haviland on Termites, 368, 373, 384
Hawaiian Islands, 354, 395, 425, 471
Heart, 133
Heat, 131
Helicomitus insimulans, 460, 461
Helicopsyche shuttleworthi, cases of, 482
{574}Hellgrammites, 447
Helorus anomalipes, 534
Hemerobiidae, 453 f.
Hemerobiides, 465 f.
Hemerobius larva, 467
Hemichroa rufa, 498
Hemimerus hanseni, 217;
foetus of, 218;
H. talpoides, 218
Hemimetabola, 158
Hemiptera, 173
Hemiteles, 556
Henking on embryology, 146
Henneguy on egg-capsule of Phyllium, 271;
on embryology of Smicra, 545
Heptagenia, 440;
H. longicauda, 437
Hessian-fly, parasites, 537
Heterogamia, 222;
H. aegyptiaca, 220;
egg-capsule, 229
Heterometabola, 158
Heteromorpha, 158
Heterophlebia dislocata, 427
Heteropteryx grayi, 262
Hetrodides, 329
Hexapoda, 86
Heymons on earwig embryology, 216
Hind body, 109
Hind wings absent, 429
Histoblasts, 167
Histogenesis, 165
Hodotermes japonicus, 383;
H. havilandi, 384;
H. mossambicus, 356;
H. brunneicornis, 359;
H. quadricollis, 371
Hoffbauer on elytra, 108
Holocampsa, misprint—see Holocompsa
Holometabola, 158
Holophthalmi, 459
Homomorpha, 158
Hooks for wings, 494
Hoplolopha, 303
Hose, 393
Howard, on pupation of Chalcididae, 550;
on Hydropsyche, 483
Hubbard and Hagen on Termites, 388
Humboldt, 31
Humpback, 445
Huxley, on head, 87;
on cervical sclerites, 99
Hydropsyche, 479
Hydropsychides, 482;
larva, 483
Hydroptila angustella, 474;
H. maclachlani, larva, 484
Hydroptilides, 484
Hylotoma rosae, 513
Hymenoptera phytophaga, 503 f.
Hymenopus bicornis, 253
Hypermetamorphosis, 158, 159, 465, 540, 552, 557
Hyperparasitism, 521
Hypertely, 323
Hypnorna amoena, 234
Hypocephalus, 99
Hypochrysa, 470
Hypoglottis, 96
Hyponomeuta cognatella, parasite of, 545
Hypopharynx, 96
—see also Lingua
Ichneumones adsciti, 559
uninjurious, 264;
supplementary, 558
Ichneumonides, 557
Ictinus, 419
folds, 165
Imago, 157
Imbrications, 493
Imhof on Perla, 403 f.
Inaequipalpia, 480
Indusial limestone, 485
Infra-oesophageal ganglion, 117
Inner margin of wing, 108
Inocellia, 447
Inquilines, 373, 524, 531, 533
Insecta, definition, 86
Instinct of Leucospis, 541
Integument, 162
Internal anatomy, 186 f.;
of Acridiidae, 282 f.;
of earwigs, 210;
of Gryllotalpa, 335;
of Hymenoptera, 494;
of Libellula, 414;
of Mantidae, 246;
of Odonata, 414;
of Stilopyga orientalis, 228;
of Phasmidae, 262;
of Raphidia, 448;
of Sialis larva, 446;
of Thysanura, 187 f.
Involucrum alarum, 206
Iris oratoria, 248
Isopteryx, 400
Isosoma, 546
Isotoma, 190
Jamaica, 388
Japygidae, 184
Japyx, abdomen of, 109;
Jhering, Von, on Termites, 387
Joint, 105
Joint-worms, 546
Joly, on Ephemeridae, 431;
on anatomy of Phyllium, 262
Julopsis, 74
J. nemorensis, 43;
breeding, 37;
heart, 50;
eye, 69
Jurine on pieces at base of wing, 102
Kampecaris, 76
Karabidion, 274
Klapálek, on Trichopterous larvae, 484 f.;
on Agriotypus, 557
Knee, 104
Koch, 42
Koestler on stomatogastric nerves, 120
Kolbe, on entothorax, 103;
on wings of Psocidae, 394
Kollar on Sirex, 509
Korotneff on embryology of Gryllotalpa, 336
Korschelt on egg-tubes, 138
Korschelt and Heider on regenerative tissue, 167
Kowalevsky, on phagocytes, 166;
on regenerative tissue, 167;
on bee embryo, 496
Kradibia cowani, 549
Krancher on stigmata, 111
Krawkow on chitin, 162
Kulagin, on embryology, 537;
of Encyrtus, 545
Künckel d'Herculais, on histoblasts, 167;
on emergence of Stauronotus, 290
Labia minor, 214
Labidura riparia, 210, 211, 214, 215
Labium, 95;
of O. larva, 420
Laboulbène, on Anurida maritima, 194;
on Perla, 399
Lachesilla, 395
Lacinia, 95
Laemobothrium, 347
Lamarck, 77
Lamina, subgenitalis, 224;
supra-analis, 224
Landois on stigmata, 111
Languette, 96
Lankester, 40
Larva, 157;
(resting-larva), 164;
oldest, 449
Latreille, 30
Latreille's segment, 491
Lead, eating, 510
Leaf-Insects, 260
Legs, 104;
internal, 496;
four only, 549;
Lendenfeld, on dragon-flies, 416, 417;
on muscles of dragon-fly, 115
Lens, 98
Lepidoptera, 173
L. saccharina, 186;
L. niveo-fasciata, 195
Lepismidae, 185
Leptocerides, 482
Leptophlebia cupida, 430
Lespès on Calotermes, 364
Leuckart on micropyle apparatus, 145
Leucocytes, 137
Leucospis gigas, 540;
larva, egg, 542;
habits, 540 f.
Lewis, Geo., on luminous may-fly, 442
Lewis on Perga, 518
on Malpighian tubes of Gryllotalpa, 335;
on glands, 142
Lias, 216, 239, 340, 427, 428, 453, 485, 503
Libellago caligata, 413
Libellula quadrimaculata, 411, 425
Libellulidae, 409
Lichens, resemblance to, 253
Liénard on oesophageal ring, 118
Light, attraction of, 441
Ligula, 96
Lilies and dragon-flies, 426
Limacodes egg, 153
Limnophilides, 481
Lingua, 95, 96, 391, 411, 420, 437
Linnaeus quoted, 84
Lipeurus heterographus, 346;
L. bacillus, 347;
L. ternatus, 349
Lipura burmeisteri, 190;
L. maritima, 194
Lipuridae, 190
Liquid emitted, 264, 324, 399, 515
Lissonota setosa, 551
Lithobius, 32, 36-39, 41, 45, 58;
breeding, 38;
Lithomantis, 259;
L. carbonaria, 344
Locusta, ovipositor, development and structure, 315;
L. viridissima, 318, 319, 321, 324, 327
Locustides, 329
Locusts, 291 f.;
of the Bible, 298;
in England, 299;
eggs, 292
Loew on anatomy of Panorpa, 450;
of Raphidia, 448
Lonchodes duivenbodi, egg, 265;
Lonchodides, 277
of cockroach, 229
Lopaphus cocophagus, 264
Lophyrus pini, 511
Löw, F., on snow Insects, 194
{576}Lowne, on embryonic segments, 151;
on integument, 162;
on stigmata, 111;
on respiration, 130
Lubbock, Sir John, on Pauropus, 62;
on aquatic Hymenoptera, 538;
on auditory organs, 121;
on sense organs, 123;
on respiration, 130;
on stadia, 165;
on Collembola, 192;
on Insect intelligence, 487
Lucas on mouth-parts of Trichoptera, 475
Luminous may-flies, 412
Lycaenidae, eggs, 144
Lyonnet on muscles, 115
Lysiopetalidae, 76
Machilidae, 184
Machilis maritima, 185;
M. polypoda, 184
Macronema, 478
Malacopoda, 77
Malpighi on galls, 525
Malpighian tubes, 114, 124, 127, 187, 353, 360, 392, 403, 414, 421, 448, 457, 458;
of Gryllotalpa, 335;
of Ephippigera, 335;
of Mantis, 246;
of Myriapods, 48
Malta, Myriapods at, 35
Mandibulata, 94
Manticora, 304
Mantides, 259
Mantis, immature tegmina, 248;
parasite, 546;
Mantispa areolaris, 463;
M. styriaca larva, 464
Mantispides, 463 f.
Mantoida luteola, 251
Marchal on Malpighian tubes, 127
Marine Myriapods, 30
Marshall, on Apanteles cocoons, 560;
on Braconidae, 561
Mask, 420
Mastax guttatus, 301
of Odonata, 411;
absent, 190
May-flies, 429;
number of, 442
Mayer, on Apterygogenea, 196;
Mazon Creek, Myriapods at, 75
M‘Coy on variation of ocelli, 267
M‘Lachlan, on Ascalaphides, 459;
on Oligotoma, 354;
on Psocidae, 395;
on Trichoptera, 480 f.
Mechanism of flight, 416
Mecistogaster, 412
Meconema varium, 321
Meconemides, 328
Mecopoda, 319
Mecopodides, 328
Mecostethus grossus, 285, 299, 308
Median plate, 504, 506, 507, 512
Megachile, nervous system, 496
Megaloblatta rufipes, 235
Megalomus hirtus, 468
Megalyra, 562
Megalyridae, 562
Meganeura monyi, 428
Megasecopterides, 344
Megastigmus, 547
Meinert, on earwigs, 210, 211, 212;
on Myrmeleon larva, 457;
on stink-glands, 210
Melittobia, 545
Melliss on Termite of St. Helena, 389
Melnikow on eggs of Mallophaga, 348
Membranule, 413
Menognatha, 161
Menopon leucostomum, 348;
M. pallidum, 350
Menorhyncha, 161
Mesonotum, 88
Mesopsocus unipunctatus, 394
Mesothoracic spiracle, 491
Mesothorax, 101
Metagnatha, 161
of Hymenoptera, 497;
of nervous system, 495 f.
Metanotum, 88
Metapodeon, 491
Methone, 200;
Miall, on imaginal discs, 165, 167;
on unicellular glands, 142
Miall and Denny, on pericardial tissue, 135;
on epithelium of stomach, 126;
on spermatheca of cockroach, 228;
on stigmata, 111;
on stomato-gastric nerves, 120
Miamia bronsoni, 449
Microcentrum retinerve, 313, 314, 320
Microgaster, 559;
M. fulvipes, 560;
M. globatus, 560
Micropterism, 339, 394, 405 f., 484
Micropyle, 145;
apparatus, 404
Millepieds, 41
Molanna angustata, mandibles of pupa, 477
Mole-cricket, 333;
leg, 333
Moniez on Anurida maritima, 194
Monodontomerus, 532;
M. cupreus, 543;
M. nitidus, 544
{577}Monomachus, 563
Monomorphic ant, 498
Monotrochous trochanters, 494, 520, 564, 565
Mordella eye, 98
Mormolucoides articulatus, 449
Morton, on gills of Trichoptera, 483;
on Perlidae, 406
Moult, 156
Moulting, 437;
of external parasite, 556
Mouth-parts, of dragon-fly, 411;
of dragon-fly nymph, 420;
atrophied, 430
Müller, Fritz, on caddis-flies, 482 f.;
on fig-Insects, 549;
on Termites, 358, 360, 374, 381, 382
Müller, J., on anatomy of Phasmidae, 262
Murray, on Phyllium scythe, 263; on
post-embryonic development of Orthoptera, 265
Musca, metamorphosis, 163, 167
Muscles, 115
Music, of Locusta, 318;
of Tananá, 319;
of Katydids, 319
—see also Phonation
Mylacridae, 239
Myoblast, 149
definition, 29;
as food, 31;
habits, distribution, and breeding, 29-40;
locomotion, 40;
names for, 41;
affinities, 78
Myrmecoleon, 456
Myrmecophana fallax, 323
Myrmecophilides, 340
Myrmeleo, 456
Myrmeleon, 456;
M. europaeus, 457;
M. nostras, 457;
M. pallidipennis, 456
Myrmeleonides, 454 f.
Nasuti, 370
Necrophilus arenarius, 462
Necroscides, 278
Needham on locusts at sea, 297
Nematus, 514;
N. curtispina, 498
Nemobius sylvestris, 339
Nemoptera ledereri, 462;
N. larva, 462
Nemopterides, 462
Nemoura, 401;
N. glacialis, 405
Nervous system, 116
of Psocidae, 393;
of Embiidae, 352;
of Termitidae, 359
N. amphibiotica, 342;
N. planipennia, 342
Neuropteroidea, 486
Neuroterus lenticularis, 523
Neuters, 137
Newman on abdomen, 491
Newport on Anthophorabia, 545;
on Monodontomerus, 544;
on Paniscus, 555;
on Pteronarcys, 399 f.;
on turnip sawfly, 515
Nicolet on Smynthuridae, 191
Nietner on Psocidae, 395
Nirmus, 346 f.
Nitzsch, on Mallophaga, 346 f.;
on Psocidae, 392
Nocticola simoni, 232
Nodes, 493
Nodus, 413
Nomadina, 565
Notophilidae, 45
Notophilus, 45
Number of species, of Insects, 83, 171, 178;
of Cephidae, 506;
of Chalcididae, 539;
of gall-flies, 533;
of Hymenoptera, 503;
of Parasitica, 520;
of Ichneumonidae, 551;
of Odonata, 424;
of Orthoptera, 201;
of earwigs, 215;
of cockroaches, 236;
of Mantidae, 258;
of Phasmidae, 272;
of migratory locusts, 297;
of Perlidae, 407;
of Psocidae, 395;
of sawflies, 518
Nurseries of Termites, 387
Nusbaum on embryology, 149, 152
Nyctiborides, 240
Nymph, 157;
of dragon-fly, 418, 419, 420, 422, 426;
of Ephemeridae, 432 f., 432, 433, 434, 435, 436
Nyssonides, 565
Oak-galls, 527
Occiput, 94
Ocelli, 97, 282, 313, 400, 409, 430;
Odonata, 409 f.
Odontocerum albicorne, case of, 480
Odontura serricauda, 316
Oecanthides, 340
Oecanthus, 339
Oecodoma—see Atta
Oenocytes, 137
Oesophageal "bone," 391
Oesophageal nervous ring, 118, 121
Oestropsides, 482
Oligonephria, 175
Oligoneuria garumnica, nymph, 434
O. saundersi, 352;
O. insularis, 354
Ommatidium, 98
Oniscigaster wakefieldi, 442
Ontogeny, 153
Oolemm, 144
Oolitic, 239
Ophionellus, 563
Ophionides, 557
Opisthocosmia cervipyga, 215
{578}Orders, 172
Orientation, 112
Origin of wings, 206
Orl-fly, 445
Ormerod, Miss, on importation of locusts, 299
Ornament, 200, 215, 233 f., 243, 244, 282, 302, 313, 339
Orphania denticauda, 321
Orthodera ministralis, 249
Orthophlebia, 453
Oryssidae, 506
Oryssus abietinus, 506;
O. sayi, 506
Osborn on Menopon, 350
Osmylides, 466
Osmylina, 466
Osmylus chrysops, 341;
larva, 466;
O. maculatus, 466
Osten Sacken on similar gall-flies, 532
Oudemans on Thysanura, 182
Outer margin of wing, 108
of earwigs, 211;
of Perla, 404;
of Thysanura, 188
Oviposition, 229, 246, 265, 290, 291, 440;
of Agriotypus, 557;
of Cynipidae, 527 f., Adler on, 529;
of Encyrtus, 545;
of Ichneumon, 555;
of inquiline gall-flies, 532;
of Meconema, 321;
of Pelecinus, 564;
of Pimpla, 553;
of Podagrion, 546;
of sawflies, 513;
of Sirex, 509;
of Xiphidium, 321
Cynipid, 524;
of Locusta, development, 314, 315
Oxyethira, 484;
O. costalis, larva, 485
Pachycrepis, 550
Pachytylus cinerascens, 293, 297, 298, 299, 308;
P. marmoratus, 298;
P. migratorioides, 298;
P. migratorius, 298, 299, 308;
Packard, on cave-Myriapods, 34;
on air sacs of locusts, 283, 294;
on classification, 173;
on development of Diplax, 419;
on may-flies, 430;
on metamorphosis of Bombus, 497;
on scales, 397;
on spiral fibre, 129
Pad, 105
Paedogenesis, 142
Pagenstecher on development of Mantis, 247
Palaeacrididae, 309
Palaeoblattariae, 239
Palaeoblattina douvillei, 238 f.
Palaeocampa, 73
Palaeodictyoptera, 486
Palaeomantidae, 259
Palaeontology, 178
Palaeophlebia superstes, 427
Palaeozoic, Myriapods, 76;
Palingenia bilineata, 430;
P. feistmantelii, 443;
P. papuana, 441;
P. virgo, 431
Palmén, on dragon-fly nymphs, 423;
on Ephemeridae inflation, 439;
on gills of Perlidae, 402;
on rectal gills, 422;
on tracheal system of immature Ephemeridae, 436
Palmon, 546
Palmula, 105
Palophus centaurus, 275
Palpares, 454
Palpiger, 95
Palpus, 95;
of Pieris brassicae, 122
Pambolus, 561
Panchlora viridis, 229
Panchlorides, 241
Panesthiides, 241
Paniscus virgatus larva, 555 f.
leg, 104;
P. communis, 449;
larva, 452
Pantel on phonation of Cuculligera, 304
Papiriidae, 191
Paraderm, 164
external, 555
Parasitism, 521 f., 535, 559, 560
Parthenogenesis, 141, 481, 497, 516 f. 530 f., 547;
utility, 517
Passalidae, mandibles, 95
Patagonia, 459
structure, 62
Pauropus, 47
Pazlavsky on bedeguar, 527
Pedicellate, 519
Pedunculate body, 495
Pelecinidae, 563
Pelecinus polyturator, 563
Pelopaeus spinolae foot, 105, 106
Perga lewisii, 517
Periblast, 149
Pericardial septum, 134;
sinus, 134;
tissue, 135
Peringueyella jocosa, 325
affinities, 4;
external features, 5;
{579}head, 6;
tail, 6;
colour, 6;
jaws, 7;
legs, 8;
habits, food, 9;
alimentary canal, 11;
body-wall, 13;
muscles, vascular system, 15;
reproductive organs, 18;
species, 23;
Periplaneta americana, 236;
P. australasiae, 221, 236, 239
Periplanetides, 241
Perisphaeriides, 241
Perla, anatomy, 403 f.;
nymph, 400;
P. cephalotes, 406;
P. parisina, 399
Perlidae, 398 f.
Petasia, 303
Petiolate, 519
Peyrou on atmosphere in bodies, 131
Peytoureau on styles of cockroach, 224
Pezomachus, 556
Phasgonuridea, 311
Phasma, 276
Phasmidae, 201, 407, 260-278, 277
Phasmides, 278
Phasmodes ranatriformis, 324
Philopotamus, 483
Phonation, 200, 257, 302, 306;
of Locustidae, 318, 319, 320, 324, 327;
of Gryllidae, 331 f;
of Gryllotalpa, 334;
of Brachytrypes, 332
Phosphorescent Myriapods, 34;
may-flies, 442
Phryganea grandis, 422;
P. pilosa, pupa, 477
Phryganeides, 480
P. crurifolium, 269 f.;
egg-capsule, structure, 271;
egg, 270;
P. siccifolium, egg, 265
Phyllodromia germanica, 229, 236;
egg-capsule, 229
Phyllodromiides, 240
Phymateus, 303
Phytophagous Parasitica, 522, 546, 547, 557
Pick, of death-watch, 391
Pictet on nymphs of Ephemeridae, 433
Pieris, palpus, 122;
instars, 156;
parasites, 561
Pigment, of iris, 98;
retinal, 98
Pillared eyes, 430
Pimplides, 557
Pitfalls of ant-lions, 455, 459
Planipennia, 342
Plantula, 105
Plateau, on marine Myriapods, 30;
on digestion, 127;
on sight, 416
Platephemera antiqua, 428
Platyblemmus lusitanicus, 339
Platycleis grisea, 312
Platycnemis, 413;
Platycrania edulis, egg, 265
Platygaster, embryology, 536
Platyptera, 174
Plectopterinae, 241
Pleura, abdominal, 493
Plica of earwig, 209
Pneumora scutellaris, 302
Pocock on W. Indian Myriapods, 33
Podacanthus wilkinsoni, 272
Podagrion, parasitism of, 546
Podeon, 491
Podura, 194;
P. aquatica, 194
Poduridae, 190
Poecilimon affinis, 200
Poisers, 108
Poletajewa, Olga, on dorsal vessel, 133;
on Odonata, 414
Polistes lanio, parasite of, 564
Polycentropus, 483
Polymitarcys, 440
Polynema natans, 538
Polynephria, 175
Polyxenus, 33, 37, 48, 55, 72;
transverse section, 56;
sense-organ, 51
Pompholyx dimorpha, 518
Pompilides, 494
Post-clypeus, 93
Post-embryonic development, 154
Potamanthus, 433
Potts on Mantis, 249
Poulton on Paniscus, 556
Praon, 550
{580}Pratt, on imaginal discs, 167
Praying Insects, 242
Prestwichia aquatica, 538
Primary larva, 542
Primary segmentation, 150
Prisopus, 272
Prochilides, 328
Prochilus australis, 324
in Musca, 124
Production of sex, 499
Pro-legs, 514
Pronotal wing-rudiments, 344, 395
of Xylocopa, 490
Pronymph, 164
Propodeon, 491
Prosopistoma punctifrons, 435
Prostemmatic organ, 195
of Vespa crabro, 491
Protephemerides, 443
Prothoracic dorsal appendages, 443
Prothorax, 102
Protoblast, 149
Proto-cerebron, 118
Protodonates, 428
Protoperlidae, 408
Protosyngnatha, 75
Prototracheata, ix, 4
Proventriculus, 114, 124, 125, 450
Psalis americana, 215
Psectra dispar, 466
Pseudoglomeris fornicata, 235
Pseudoneuroptera, 342
Pseudonychium, 105
Pseudophyllides, 328
Pseudo-sessile, 493
Psilocnemis dilatipes, 413
Psocidae, 390 f.
Psocus fasciatus, 390;
P. heteromorphus, 391
Pteromalini, 539
Pteronarcys frigida, 398;
P. regalis, 402
Pteroplistus, 331
Pulvillus, 105
Pupation, of Chalcididae, 550;
of Encyrtus, 546;
Pupipara, 143
Pygidicrana hugeli, 202
Pygidium, 205
Pylorus, 127
Pyramids of Egypt, 462
Pyrgomantis singularis, 252
Pyrgomorpha grylloides, 303
Radial cell, 524
Raphidia, 447;
R. notata, 447;
larva, 448
Raptorial legs, 242 f., 257, 463, 484
Ratzeburg, on Anomalon, 553;
on trochanter, 520
Ravages of Termites, 388
Réaumur, on ant-lions, 455;
on circulation of silkworm, 135;
on galls, 525;
on spheroidal condition, 164
Receptaculum seminis, 139, 404
Rectal, gills, 421 f.;
respiration, 435
Rectum, 125
Redtenbacher, on migratory locust, 297;
on wing, of Oligotoma, 353;
of Termes, 359
Reduviid egg, 145
Reflex action, 250
Reproduction of lost parts, 213, 265, 266
Reproductive organs of Ephemeridae, 439
Resemblance, of eggs to seeds, 265, 270, 271;
of one part to another, 208, 266;
of parasite to host, 532;
histological, 271;
of Trichoptera to moths, 484;
to bark, 251;
to inorganic things, 253, 304, 307;
to leaves, 255, 267, 268, 322 f., 323;
to lichens, 253;
to other creatures, 235;
to other Insects, 197, 215, 235, 251, 274, 300, 301, 323, 324, 504, 513, 550;
Respiration [and respiratory organs], 128-132, 431;
by integument, 483;
by setae, 435;
of nymphs of Odonata, 420 f.;
of Perlidae, 401 f.
Respiratory chamber, 434
Retinula, 98
Reuter on ventral tube, 192
Rhabdom, 98
Rhizotrogus egg-tubes, 138
Rhodites rosae, 498, 527, 528, 531;
larva, 532;
parasite, 539
Rhyacophilides, 483
Rhyacophylax, 482
Rhynchota, 175
Rhyparobia maderae, 237
Rhyssa persuasoria, 554
Riley, on caprification, 549;
on Cephus, 505;
on development of Caloptenus, 288, 289;
on galls, 526 f.;
on Katydids, 320;
on locust swarms, 293;
on Microcentrum, 313;
{581}on ovipositing of locust, 290;
on subimago, 437;
on Thalessa, 554
Ritsema on Enoicyla, 481
Ronalds on anglers' flies, 441
Roux on Necrophilus, 462
Royal pairs, 377
Rühl on earwig, 213
Sacs—see Air Sacs
Sagides, 328
Salivary glands, 124, 126, 187, 210, 228, 246, 283, 335, 348, 353, 403, 414, 495;
of Peripatus, 11;
Salivary receptacle, or reservoir, 126, 228, 246, 335, 348, 360
Saltatoria (Orthoptera), 201
Sandwich Islands—see Hawaiian Islands
Saunders, Sir Sydney, on Scleroderma, 536;
on caprification, 548
Saussure, H. de—see De Saussure
Savage on Termites, 368
Sawflies—see Tenthredinidae
Scapteriscus, 334
Scelimena, 301
Schindler on Malpighian tubes, 246;
of Gryllotalpa, 335
Schistocerca peregrina, 298;
development, 287;
Schizodactylus monstrosus, 325
Schizophthalmi, 459
Schizotarsia, 35, 46, 57, 58, 70, 75;
structure, 59
Schletterer on parasitic Hymenoptera, 562, 563
Sclerite, 91
Scleroderma, 536
Scolia, ovaries, 138
Scolopendra, 30, 31, 32, 41, 78
Scolopendridae, 31, 33, 39, 45, 75;
spermatophores, 39
Scorpion-flies, 449 f.
Scudder, on grasshopper music, 287;
on Katydids' music, 320;
on locusts at sea, 297;
on reproduction of lost limbs, 265;
on fossil Insects, 486;
on fossil earwigs, 216;
on fossil may-flies, 443;
on fossil Sialidae, 449;
on Tertiary Insects, 179
sense organ, 51
Scutigeridae, 35, 36, 40, 46, 50
larva, 542
Securifera, 503
of ovum of Smicra, 545
number of, 87
Selys, De, on dragon-flies, 425, 427
Semi-pupa, 497
Serosa, 148
Serrifera, 503
Sessile abdomen, 493
Sexes, 137
Sexual organs, external, 141
Shaw on Orthoptera, 201
Sialides, 444
Sialis lutaria, 444;
eggs, 445;
larva, 445;
tracheal gill, 446
Silk, 127
Silo, parasite of, 558
Silurian Insect, 238
Silver fish, 186
—see also Ocelli
Sirex, habits of its parasite, 554;
S. augur, 509;
S. juvencus, 508
Siricidae, 507;
parasites of, 563
Siricides, 510
Sisyra 467;
S. fuscata larva, 467
Sisyrina, 467
Sitaris humeralis, early stages, 159
Sloane, Sir Hans, on locusts at sea, 297
Smallest Insect, 537
Smeathman on Termites, 366 f., 381, 383, 387
Smicra clavipes embryology, 545
Smith, F., on Cynips, 530;
on Trigonalys, 564
Smynthuridae, 191
Smynthurus variegatus, 191;
S. fuscus, 192
Snow-Insects, 194
Social, Insects, 85, 361, 369;
Somites, 87
Soothsayers, 242
Sound production, 358
—see also Phonation
Spathius, 561
Species, number of—see Number
Spencer, Herbert, on caste and sex, 500
Spermatophores, 39
Spermatozoa, 140
Sphaeropsocus kunowii, 397
Sphaerotheriidae, 43
Sphaerotherium, 43
Sphex chrysis, 490
Spinneret, 458
Spinners, 441
number of, 186;
of dragon-fly nymph, 423;
absent, 436
—see also Stigmata
Spiral fibre, 128
Spongilla fluviatilis, larva in, 467
Spontaneous generation, 525
Spring of Collembola, 191
Spurs, 104
Stalked, cocoons, 560;
eggs, 469
Stein on Raphidia larva, 448
Stelis, parasitic, 544;
parasitised, 543
Stem sawflies, 504
Stenobothrus, 308;
sound-instruments, 284
Stenodictyopterides, 344
Stenophasmus ruficeps, 561
Stenophylla cornigera, 257, 258
Stephanidae, 561
Stephanus, 562
St. Helena, 389
Stick-Insects, 260
position, 493;
on head, 193;
S. repugnatoria, 36
—see also Spiracles
Stilopyga orientalis, 223, 228, 231, 236
Sting, 493;
and ovipositor, 534
Stink-flies, 469
Stink-glands, 31, 125, 210, 264, 335
Stipes, 95
Stoll on spectres, etc., 254
Stomato-gastric nerves, 120, 121
Stone-flies, 407
Stratiomys strigosa parasite, 545
Stridulation, 304
—see also Phonation
Stummer-Traunfels on Thysanura and Collembola, 189
St. Vincent, island of, 461
Sub-Order, 177
Subulicornia, 426
Sucking spears, 466, 467, 470, 471
Super-Orders, 177
Supplementary Ichneumon-flies, 558
Supra-oesophageal ganglion, 117
Sutures, 92
of may-flies, 441;
of Termites, 362
Sympathetic nervous system, 120;
absent, 353
Symphrasis varia, 465
structure, 61
Symphyta, 503
Sympycna fusca, 415
Synaptera, 175
Synergus, 531
Syngnatha, 44
Tananá, 319
Tarachodes lucubrans, 249
Taschenberg on parthenogenesis, 141
Tausendfüsse, 41
Teeth, 95
Tegmina, 108;
leaf-like, of Pterochroza, 322;
of crickets, 331;
of Phyllium, 269;
of Schizodactylus, 325
Teleganodes, 442
Telson, 205
Temples, 94
Templeton on Lepisma, 195
Tendons, 116
Tenthredo sp., 489;
testes, 140
Tentorium, 99
Tepper on fossorial Blattid, 241
Terebrantia, 520
Termes sp., 378;
T. lucifugus, 359, 360, 364, 365, 373, 374;
T. mossambicus, 356;
trophi, 357;
cell of, 367;
T. occidentis, 371;
T. armiger, 371;
T. tenuis, 389;
T. cingulatus, 371;
T. dirus, 371;
T. debilis, 371;
T. viarum, 383
Termites, 357 f.;
distinctions from ants, 502;
wings, 359;
anatomy, 360
Termitidae, 356;
number of species, 389
Tertiary, 196, 216, 239, 276, 309, 340, 398, 427, 442, 449, 453, 472, 485, 533, 551, 558
Testes, 18, 49, 140, 404, 440;
of Psocidae, 392;
of Stilopyga orientalis, 228
Tetrophthalmus chilensis, 346
Tettix bipunctatus, 300
Thalessa larva, 507;
oviposition, 554
Thamastes, 485
Thamnotrizon apterus, 316
Thecla egg, 145
Thermobia furnorum, 186
Thliboscelus camellifolius, 319
Thoracantha latreillei, 550
Thorax porcellana wing, 227
Thyrsophorus, 395
Thysanoptera, 173
{583}Thysanura, 182 f.;
distinctions from Symphyla, 61, 77, 79
Tillus elongatus larva, 90
Tinodes, 483
Tomognathus, 498
Tongue, 96
—see also Lingua
Torymides, 547
Toxodera, 253;
T. denticulata 254
Trabeculae, 345
Tracheae, 128;
—see also Branchiae
Tremex columba, 507
Trias, 449
Triassic, 239
Trichijulus, 76
Trichodectes, 350;
T. latus, 349
Trichostegia, 480
Tridactylides, 340
Tridactylus variegatus, 337
Trigonalidae, 564
Trigonalys maculifrons, 564
Trigonidiides, 340
Trimen on Trachypetra bufo, 304
Trinidad, 501
Trito-cerebron, 118
Trochanter, 88, 104, 491, 494, 520
Trochantin, 104;
of cockroach, 222
Tryphonides, 557
Tryxalis nasuta, 279
Tubulifera, 520
Tympanophorides, 328
Tympanum, 285 f.
Tyndall on grasshopper music, 286
Ulloa, 33
Uroceridae, 507
Useless wings, 199, 394, 484, 561
Vagus nervous system, 120
Van Rees on metamorphosis, 162, 164
Variation, 536;
of colour, 252, 288, 304, 308;
in desert Insects, 305;
Vatides, 259
Vas deferens, 18, 140, 187, 392
Vayssière, on nymphs of Ephemeridae, 434;
on lingua, 438
Veins, 206
of Perlidae, 404
Ventral plate, 148;
Verhoeff, 38
Verloef [misprint for Verhoeff]
Verlooren on circulation, 436
Vertex, 94
Vespa crabro prosternum, 491
Vestibule, 112
Viallanes, on head, 87;
on metamorphosis, 162
Visceral nervous system, 120
Viviparous Insects, 217, 229, 143, 218
Voetgangers, 295 f.
Vom Rath on sense organs, 122
Vosseler on stink-glands, 210
Walker, J. J., on Australian Termites, 386
Walking-leaves, 267
Walking on perpendicular and smooth surfaces, 106
Walsh on galls, 531
Wasmann on St. Augustine's works, 565
Wattenwyl, Brunner von—see Brunner
Weismann, on caste, 500;
on imaginal discs, 167
Westwood, on Forficula, 204;
on Helicomitus larva, 460, 461;
on Lachesilla, 395;
on Scleroderma, 536
Weta-punga, 326
Wheeler, on Malpighian tubes, 127;
on embryology of Orthoptera, 199;
on embryology of Xiphidium, 321;
on vitellophags, 147, 152, 168;
on segmentation, 150
White ants, 356
—see Termites
Wielowiejski on blood-tissue, 133, 137
Will on brain of Aphididae, 118
Wingless: caddis-fly, 481;
earwigs, 205;
Insects, 345, 352, 356, 451, 488, 536, 547;
wingless Psocidae, 394 f.
—see also Apterous
Wings, 107;
origin and function, 394;
development of, in locust, 288;
of dragon-fly, 413;
of earwigs, 206;
of Ephemera, 431;
growth of, 418;
of Ichneumon and Bracon, 559;
wing-hooks, 494;
veins, 107
—see also Tegmina and Alar Organs
Wistinghausen on tracheae, 129
Wood-Mason on Cotylosoma, 272;
on mandibles, 95;
on Oligotoma, 352;
on phonation of Mantidae, 258
Woodworth on embryology, 146, 153
Wyandotte Caves, Myriapods in, 34
{584}Xambeu on Palmon, 546
Xerophyllum simile, 301
Xiphidium ensiferum, 321
Xiphocera asina, 303
Xylocopa, 494;
metamorphosis, 170;
pronotum, 490
Yolala, 298
Zimmermann on caudal respiration, 435
Zittel, figure from, 276
Zygaena embryo, 151
nymphs, 422
END OF VOL. V
Printed by R. & R. Clark, Limited, Edinburgh.
THE CAMBRIDGE NATURAL HISTORY.
EDITED BY
S. F. HARMER, M.A.,
FELLOW OF KING'S COLLEGE, CAMBRIDGE, SUPERINTENDENT OF
THE
UNIVERSITY MUSEUM OF ZOOLOGY;
AND
A. E. SHIPLEY, M.A.,
FELLOW OF CHRIST'S COLLEGE, CAMBRIDGE, UNIVERSITY LECTURER
ON THE
MORPHOLOGY ON INVERTEBRATES.
Vol. III. MOLLUSCS AND BRACHIOPODS. By the Rev. A. H. Cooke, M.A., Fellow and Tutor of King's College, Cambridge; A. E. Shipley, M.A., Fellow of Christ's College, Cambridge; and F. R. C. Reed, M.A., Trinity College, Cambridge. Illustrated. Medium 8vo. 17s. net.
EDINBURGH REVIEW.—"Much of it we have read with pleasure, and much of it with profit, deriving satisfaction in the one case from the process, in the other from the accomplished fact."
TIMES.—"There are very many, not only among educated people who take an interest in science, but even among specialists, who will welcome a work of reasonable compass and handy form containing a trustworthy treatment of the various departments of Natural History by men who are familiar with, and competent to deal with, the latest results of scientific research.... Altogether, to judge from this first volume, The Cambridge Natural History promises to fulfil all the expectations that its prospectus holds out."
DAILY CHRONICLE.—"There is no doubt that if the succeeding volumes are carried out upon the same plan as is that which we have just received, the Cambridge Natural History will be an indispensable work; for it appeals to a far wider class than works upon Natural History generally do.... It can be read with profit by the zoologist, and there is a vast amount of matter which is interesting to those who like the tit-bits of science, but do not care so much for the more serious aspects of the subject."
DAILY NEWS.—"Promises to be, in its own department of science, the most important work of the day."
FIELD.—"We know of no book available to the general reader which affords such a vast fund of information on the structure and habits of molluscs."
KNOWLEDGE.—"If succeeding volumes are like this one, the Cambridge Natural History will rank as one of the finest works on Natural History ever published."
WESTMINSTER REVIEW.—"Should find a place in the library of every naturalist. The numerous illustrations are clear and accurate."
ATHENÆUM.—"The series certainly ought not to be restricted in its circulation to lecturers and students only; and if the forthcoming volumes reach the standard of the one here noticed, the success of the enterprise should be assured."
PALL MALL GAZETTE.—"Mr. Cooke has selected a beautifully lucid and simple method of arrangement, which permits of easy reference; and he has the advantage of excellent type and first-rate woodcut illustrations."
MACMILLAN AND CO., LONDON.
BOOKS FOR
STUDENTS OF NATURAL HISTORY
NATURAL HISTORY OF SELBORNE AND OBSERVATIONS ON NATURE. By Gilbert White. With the Text and New Letters of the Buckland Edition. Introduction by John Burroughs. Illustrated by Clifton Johnson. In Two Volumes. Crown 8vo. 10s. 6d.
NATURAL HISTORY AND ANTIQUITIES OF SELBORNE. By Gilbert White. With Notes by Frank Buckland, a Chapter on Antiquities by Lord Selborne, and New Letters illustrated by P. H. Delamotte. New and Cheaper Edition. Crown 8vo. 6s.
FORTY YEARS IN A MOORLAND PARISH. Reminiscences and Researches in Danby-in-Cleveland. By Rev. J. C. Atkinson, D.C.L., Canon of York and Incumbent of the Parish, Author of "A History of Cleveland," "A Glossary of the Cleveland Dialect." With Maps and Illustrations. Extra Crown 8vo. 8s. 6d. net. Illustrated Edition. 12s. net.
A HANDBOOK OF BRITISH LEPIDOPTERA. By Edward Meyrick, B.A., F.Z.S., F.E.S., Assistant Master at Marlborough. Illustrated. Extra Crown 8vo. 10s. 6d. net.
AN INTRODUCTION TO THE STUDY OF SEAWEEDS. By George Murray, F.R.S.E., F.L.S., Keeper of Botany in the Natural History Department of the British Museum. With 8 Coloured Plates and 88 other Illustrations. Crown 8vo. 7s. 6d.
HANDBOOK OF FIELD AND GENERAL ORNITHOLOGY. A Manual of the Structure and Classification of Birds. With Instructions for Collecting and Preserving Specimens. By Elliott Coues. Profusely Illustrated. 8vo. 10s. net.
STRUCTURE AND LIFE OF BIRDS. By F. W. Headley, Assistant Master in Haileybury College. Illustrated. 8vo. 7s. 6d.
TALES OF THE BIRDS. By W. Warde Fowler, M.A. With Illustrations by Bryan Hook. Second Edition. Crown 8vo. 3s. 6d.
A YEAR WITH THE BIRDS. By W. Warde Fowler, M.A. With Illustrations by Bryan Hook. Third Edition, enlarged. Crown 8vo. 3s. 6d.
SUMMER STUDIES OF BIRDS AND BOOKS. By W. Warde Fowler, M.A. Crown 8vo. 6s.
BIRDCRAFT. A Field Book of Two Hundred Song, Game, and Water Birds. By Mabel Osgood Wright, Author of "The Friendship of Nature." With full-page Plates, containing 128 Birds in the Natural Colours. Extra Crown 8vo. 12s. 6d. net.
THE NATURAL HISTORY OF AQUATIC INSECTS. By L. C. Miall, F.R.S., Professor of Biology in the Yorkshire College, Leeds. Illustrated. Crown 8vo. 6s.
ROMANCE OF THE INSECT WORLD. By L. N. Badenoch. With Illustrations by Margaret J. D. Badenoch and others. Crown 8vo. 6s.
THE DEPTHS OF THE SEA. An Account of the General Results of the Dredging Cruises of H.M.SS. Porcupine and Lightning during the Summers of 1868, 1869, and 1870, under the Scientific Direction of Dr. Carpenter, F.R.S.; J. Gwyn Jeffreys, F.R.S.; and Dr. Wyville Thomson, F.R.S. By Sir C. Wyville Thomson, F.R.S. With numerous Illustrations and Maps. Second Edition. Medium 8vo. 31s. 6d.
THE VOYAGE OF THE CHALLENGER. THE ATLANTIC. A Preliminary Account of the General Results of the Exploring Voyage of H.M.S. Challenger during the Year 1873 and the Early Part of the Year 1876. With Portrait, Plates, and Maps. In two Vols. Published by Authority of the Lords Commissioners of the Admiralty. Medium 8vo. 45s.
OBSERVATIONS ON THE GEOLOGY AND ZOOLOGY OF ABYSSINIA, made during the Progress of the British Expedition to that Country in 1867-1868. By W. T. Blanford, F.R.S. With Illustrations and Geological Map. 8vo. 21s.
WORKS BY A. R. WALLACE, F.R.S.
DARWINISM: An Exposition of the Theory of Natural Selection, with Some of its Applications. With Maps and Illustrations. Extra Crown 8vo. 9s.
NATURAL SELECTION AND TROPICAL NATURE. Essays on Descriptive and Theoretical Biology. Extra Crown 8vo. 6s.
THE MALAY ARCHIPELAGO: The Land of the Orang Utan and the Bird of Paradise. A Narrative of Travel. With Studies of Man and Nature. With Maps and Illustrations. Fourth Edition. Extra Crown 8vo. 6s.
ISLAND LIFE: or the Phenomena and Causes of Insular Faunas and Floras. Including a Revision and Attempted Solution of the Problem of Geological Climates. With Illustrations and Maps. Second Edition. Extra Crown 8vo. 6s.
THE GEOGRAPHICAL DISTRIBUTION OF ANIMALS; with a Study of the Relations of Living and Extinct Faunas as Elucidating the Past Changes of the Earth's Surface. With Maps and Illustrations. In Two Vols. Medium 8vo. 42s.
* * * * *
AN INTRODUCTION TO THE OSTEOLOGY OF THE MAMMALIA: being the substance of the course of lectures delivered at the Royal College of Surgeons of England in 1870. By Sir W. H. Fowler, F.R.S., F.R.C.S., Director of the Natural History Museum. Illustrated. Third Edition. Revised with the assistance of Hans Gadow, Ph.D. Crown 8vo. 10s. 6d.
WILD BEASTS AND THEIR WAYS. Reminiscences of Europe, Asia, Africa, and America, from 1845-1888. By Sir Samuel W. Baker, Pacha, F.R.S. With Illustrations. New and Cheaper Edition. Extra Crown 8vo. 12s. 6d.
WANDERINGS IN SOUTH AMERICA, THE NORTH-EAST OF THE UNITED STATES, AND THE ANTILLES, IN THE YEARS 1812, 1816, 1820, and 1824. With Original Instructions for the perfect Preservation of Birds, etc., for Cabinets of Natural History. By Charles Waterton, Esq. Edited by the Rev. J. G. Wood. With 100 Illustrations. Crown 8vo. 6s.
FROM A NEW ENGLAND HILLSIDE. Notes from Underledge. By William Potts. Pott 8vo. 3s.
THE FRIENDSHIP OF NATURE. A New England Chronicle of Birds and Flowers. By Mabel Osgood Wright. 16mo. 3s.
MACMILLAN AND CO., LONDON.
WORKS ON
BIOLOGY AND ZOOLOGY
MATERIALS FOR THE STUDY OF VARIATION. Treated with especial regard to Discontinuity in the Origin of Species. By William Bateson, M.A., Fellow of St. John's College, Cambridge. 8vo. 21s. net.
AMPHIOXUS AND THE ANCESTRY OF THE VERTEBRATES. By Arthur Willey, B.Sc., Tutor in Biology, Columbia College; Balfour Student of the University of Cambridge. With a preface by Henry Fairfield Osborn. 8vo. 10s. 6d. net.
THE MYOLOGY OF THE RAVEN (Corvus corax Sinuatus). A Guide to the Study of the Muscular System in Birds. By R. W. Shufeldt, of the Smithsonian Institute, Washington, U.S.A. With Illustrations. 8vo. 13s. net.
ORGANIC EVOLUTION AS THE RESULT OF THE INHERITANCE OF ACQUIRED CHARACTERS ACCORDING TO THE LAWS OF ORGANIC GROWTH. By Dr. G. H. Theodor Eimer, Professor of Zoology and Comparative Anatomy in Tübingen. Translated by J. T. Cunningham, M.A., F.R.S.E., late Fellow of University College, Oxford. 8vo. 12s. 6d.
FROM THE GREEKS TO DARWIN. An Outline of the Development of the Evolution Idea. By Henry Fairfield Osborn, Sc.D., Da Costa Professor of Biology in Columbia College, Curator in the American Museum of Natural History. 8vo. 9s. net.
LESSONS IN ELEMENTARY BIOLOGY. By T. Jeffrey Parker, B.Sc., F.R.S., Professor of Biology in the University of Otago, New Zealand. Illustrated. Second Edition. Crown 8vo. 10s. 6d.
KEY TO NORTH AMERICAN BIRDS. Containing a concise account of every species of living and fossil bird at present known from the continent north of the Mexican and United States boundary, inclusive of Greenland. Second Edition, with which are incorporated General Ornithology—an outline of the structure and classification of birds, and Field Ornithology—a manual of collecting, preparing and preserving birds. By Elliott Coues, M.A., M.D., Ph.D., Member of the National Academy of Sciences. Profusely illustrated. Super Royal 8vo. 42s.
MONOGRAPH OF THE BRITISH CICADÆ OR TETTIGIIDÆ (Froghoppers and Grassflies). By George Bowdler Buckton, F.R.S., Corr. Memb. Acad. Nat. Hist. of Philadelphia, Memb. de la Soc. Ent. de France. Illustrated by more than 400 Coloured Drawings. Two Vols. Demy 8vo. 33s. 6d. each net. Also in Eight Parts. 8s. each net.
THE FOSSIL INSECTS OF NORTH AMERICA. With Notes on some European Species. By Samuel H. Scudder. Vol. I. The Pretertiary Insects. With 34 Plates. Vol. II. The Tertiary Insects. With a Map and 28 Plates. 4to. Paper Boards. 90s. net.
MACMILLAN AND CO., LONDON.
L. Guilding, "Mollusca caribbaeana: an Account of a New Genus of Mollusca," Zool. Journ. vol. ii. 1826, p. 443, pl. 14; reprinted in Isis, vol. xxi. 1828, p. 158, pl. ii.
H. N. Moseley, "On the Structure and Development of Peripatus capensis," Phil. Trans. clxiv. pls. lxxii.-lxxv. pp. 757-782; and Proc. R. S. xxii. pp. 344-350, 1874.
A. Sedgwick, "A Monograph of the Genus Peripatus," Quart. Journ. of Mic. Science, vol. xxviii., and in Studies from the Morphological Laboratory of the University of Cambridge, vol. iv.
A. Sedgwick, "A Monograph of the Development of Peripatus capensis," Studies from the Morphological Laboratory of the University of Cambridge, vol. iv.
F. M. Balfour, "The Anatomy and Development of Peripatus capensis," edited by Professor H. N. Moseley and A. Sedgwick, Quart. Journ. Mic. Sci. xxiii. pp. 213-259, pls. xiii.-xx. 1883.
See Whitman, Journal of Morphology, vol. i.
There are now, I am told by Professor Jeffrey Bell, specimens from Natal (I believe undescribed) at the British Museum with twenty-three and twenty-four pairs of legs.
This name was first applied by Blanchard to a species from Cayenne. The description, however, is very imperfect, and it is by no means clear that the Cayenne species is identical with the species here named Edwardsii.
The existence of this species is very doubtful. The description of it was taken from a single specimen. The evidence that this specimen was actually found in Sumatra is not conclusive.
Not to be confused with the larva of Elater lineatus, also known as "wire-worm."
See L. Jenyns' Observations in Nat. Hist. London, 1846, p. 296.
"A Revision of the Lysiopetalidae, a family of the Chilognath Myriapoda, with a notice of the genus Cambala," by A. S. Packard, junior, Proc. Amer. Phil. Soc. xxi. 1884, p. 187.
C. L. Koch, System der Myriapoden. Regensburg, 1847.
C. L. Koch, Die Myriapoden. Halle, 1863.
Latzel, Die Myriapoden der Œsterreichisch-Ungarischen Monarchie. Wien, 1880.
Tres muscae consumunt cadaver equi, aeque cito ac leo. Syst. Nat., ed. xii. ref. I. pt. 2, p. 990.
Ann. Sci. Nat. (7) iv. 1887, p. 111.
Stettin. Ent. Zeit. l. 1889, p. 165.
The wings, by many morphologists, are not included in the category of "appendages"; they apparently, however, differ but little in their nature from legs, both being outgrowths of the integument; the wings are, however, always post-embryonic in actual appearance, even when their rudiments can be detected in the larva. No insect is hatched from the egg in the wing-bearing form.
Ann. Sci. Nat. I. 1824, p. 97, etc.
See also Fig. 47 (p. 88).
In entomological language the piece between each two joints of an appendage is itself called a joint, though segment is doubtless a better term.
Arch. f. Naturgeschichte, lvi. 1890, p. 221.
Zeitschr. wiss. Zool. liv. 1892, p. 579.
Mem. Acc. Lincei Rom. (4) iv. 1888, p. 554.
Zeitschr. wiss. Zool. xvii. 1867, p. 187.
Zool. Anz. iii. 1880, p. 584.
The Cockroach, 1886, p. 151.
Anatomy of the Blowfly, 1893, p. 362.
Lyonnet, Traité anatomique de la Chenille qui ronge le bois de Saule. La Haye, 1762. On p. 188 he says that he found 1647 muscles, without counting those of the head and internal organs of the body. He puts the number found in the human body at 529.
SB. Ak. Wien, Abth. 1, lxxxiii. 1881, pp. 289-376.
Mem. Acc. Torino (2), xliii. 1893, p. 229.
Bull. Soc. Philom. Paris (7), xi. 1887, p. 119, etc., and C. R. civ. 1887, p. 444.
Zool. Jahrbuch. Anat. iii. 1888, p. 276.
Kolbe, Einführung, 1893, p. 411.
Arch. de Biol. i. 1880, p. 381.
Ann. Sci. Nat. Zool. (7) ii. 1887, and iv. 1887.
Zool. Anz. iv. 1881, p. 452.
Zeitschr. wiss. Zool. xlvi. 1888, pl. xxxi.
On the Senses, Instincts, and Intelligence of Animals, with special reference to Insects. Vol. LXV. International Scientific Series, 1888.
Bijd. Dierkunde, 16, 1888, p. 192.
For a review of their number see Wheeler, Psyche, vi. 1893, pp. 457, etc.
Ann. Soc. Ent. France, lxi. 1892, Bull. p. cclvi.
Mem. Ac. Belgique (2), xli. 1875, and Bull. Ac. Belgique (2), xliv. 1877, p. 710.
American Naturalist, xx. 1886, pp. 438 and 558.
Zeitschr. wiss. Zool., xlix. 1890, p. 565.
See Miall and Denny, Cockroach, p. 158.
Eisig, Mon. Capitelliden, 1887, p. 781.
Tr. Linn. Soc. London Zool. xxiii. 1860, p. 29.
Blowfly, etc. p. 376.
C. R. Ac. Sci., cxv. 1892, p. 61, and Bull. Sci. France Belgique, xxv. 1893, p. 18.
Compt. rend. Ac. Paris, cii. 1886, p. 1339.
See Newport, Phil. Trans. 1837, and Lubbock Linn. Trans. xxiii. 1860, p. 29, etc.
Zeitschr. wiss. Zool. xxvi. 1876, p. 137.
Zeitschr. wiss. Zool. xliii. 1886, p. 512.
Zool. Anz. ix. 1886, p. 13.
Cockroach, p. 140.
Bull. Sci. France Belgique, xxv. 1893, p. 22.
Biol. Centralbl. xi. 1891, p. 212.
Acta. Ac. German. xxxiii. 1867, No. 2.
Linnaea entomologica, xii. 1858, p. 313.
Zeitschr. wiss. Zool. 1886, xliii. p. 539.
Tr. Linn. Soc. London, 2nd ser.; Zool. v. 1890, p. 173.
Zeitschr. wiss. Zool. l. 1890, p. 317.
Abh. Ges. Halle, xvii. 1892, p. 365.
Tr. Ent. Soc. London, 1893, p. 241.
Arch. Anat. Phys. 1855 and 1859.
Acta. Ac. German. xxxiii. 1867, No. 2, p. 81.
Müller's Arch. Anat. Phys. 1855, p. 90.
Zeitschr. wiss. Zool. xxvi. 1876, p. 115.
Scudder, Butterflies of New England, i. 1889, p. 99.
J. Morphol. viii. 1893, p. 81; see also Graber's table on p. 149.
Denk. Ak. Wien, lv. 1888, p. 109, etc.
Morph. Jahrb. xiv. 1888, p. 347.
In Miall and Denny, Cockroach, p. 188.
Morph. Jahrb. xiv. 1888, p. 345.
J. Morphol. viii. 1893, p. 1.
J. Morphol. viii. 1893, pp. 64, 65, and 81.
Trans. Linn. Soc., 2nd Series, "Zool." 1888, iii. p. 12.
Orthoptera europaea, 1853, p. 37.
Ann. Sci. Nat. Zool. Ser. iv. vol. vii. 1857, pl. 17.
Nature Series, 1874.
See Proctotrupidae subsequently.
Verh. Zool.-bot. Ges. Wien, xix. 1869, p. 839.
"Syst. Zool. Stud." SB. Ak. Wien, Abth. 1, xci. 1885, p. 291.
Zeitschr. wiss. Zool. xiv. 1864, p. 187.
Viallanes, Ann. Sci. Nat., Series 6, "Zool." xiv. 1882.
Unfortunately in the Russian language.
Zool. Jahrb. Abth. Anat. iii. 1888, p. 1.
Zeitschr. Biol., xxix. 1892, p. 177.
Trans. Linn. Soc. London, "Zoology," 2nd series, v. 1890, p. 174.
Trans. Linn. Soc. xxv. 1866, p. 491.
Zeitschr. wiss. Zool. xli. 1885, p. 712.
"Fauna und Flora d. Golfes von Neapel," Die Capitelliden, 1887, p. 781.
Trans. Linn. Soc. xxiv. 1863, p. 65.
Trans. Linn. Soc. "Zool." v. 1892, p. 267.
Ann. Sci. Nat., Series 6, "Zool." xiv. 1882, p. 150.
Zool. Anz. viii. 1885, p. 125.
Mitt. Schweiz. ent. Ges. viii. 1893, p. 403.
Recherches Org. des Volucelles, 1875, p. 143.
Zeitschr. wiss. Zool. xlv. 1887, p. 587.
Lehrbuch Entwicklungsgeschichte, Spec. Theil. 1890, p. 875.
Zeitschr. wiss. Zool. xiv. 1864, p. 187.
Arch. f. Naturges. lix. 1893, 1, p. 168.
Wheeler, in J. Morphol. viii. 1893, p. 81.
Syst. Nat. Ed. 12, ref. i. pars ii. p. 536 (by error, 356).
It must not be supposed that all wingless Insects fall within the limits of this Order.
American Naturalist, xx. 1886, p. 808.
"Syst. Zool. Studien." S.B. Ak. Wien, xci. 1885, Abth. I. p. 374.
The term natural is here employed in the empirical sense described by Brunner von Wattenwyl, Nouv. Syst. Blattaires, 1865, p. vii.
Lord Walsingham, Proc. Ent. Soc. London, 1889, p. lxxx.
We may mention that fossil Insects are chiefly determined from their wing-remains, which are often surprisingly perfect. This is one of the reasons that have induced us to prefer a classification of Insects in which the nature of the wings is considered of great value. It would be impossible to refer fossil Insects to groups that are established on account of the metamorphosis or of the internal structure of their components, for there is not yet any evidence on either of these points in the fossil remains preserved for us by the rocks.
Bull. U.S. Geol. Survey, No. 31, 1886, p. 109.
Mem. Acc. Lincei Roma (4), iv. 1888, p. 543, etc., and other preceding memoirs mentioned therein.
Bijdr. Dierkunde, xvi. 1888, pp. 147-227.
Natural. Sicil., ix. 1889, pp. 25, etc.
Ann. Soc. ent. France, 1892, p. 34.
Morph. Jahrb. xv. 1889, p. 363.
SB. Ak. Wien, c. 1891, Abth. I. p. 216.
Ent. Tidskr. i. 1880, p. 159.
Morphol. Jahrb. xv. 1888, p. 361.
Verh. zool.-bot. Ges. Wien, viii. 1858, p. 564.
Ann. Soc. ent. France, 4th ser. iv. 1864, p. 705.
Rev. biol. Nord France, ii. 1890, p. 347.
J. Morphol. viii. 1893, p. 64.
Ent. Mo. Mag. xxv. 1889, and xxvi. 1890.
Ann. Mus. Genova, xxxiii. (1892).
Orthoptera Europaea, 1853, pl. vi. f. 4, p. 434.
Morph. Bedeut. Seg. Orthopt. 1876, p. 14; and Prod. Orthopt. Europ. 1882, p. 3.
Proc. Zool. Soc. London, 1892, p. 586.
Naturhistorisk Tidsskrift, 3rd ser. ii. 1863, p. 475.
Arch. mikr. Anat. xxxvi. 1890, p. 565.
Ann. Sci. Nat. xiii. 1828, p. 337.
Naturhistorisk Tidsskrift, 3rd ser. ii. 1863, p. 475.
Some writers are of opinion that there are only two thoracic spiracles in Insects, considering the third as belonging really to the abdomen. Looking on the point as at present chiefly one of nomenclature, we make use of the more usual mode of expression.
As on last page, and also op. cit. v. 1868, p. 278.
Bull. Ent. Ital. xii. 1880, p. 46.
It may be worth while to repeat that "joint" means a piece, and is the equivalent of "link" in a chain.
Materials for the Study of Variation, 1894, p. 413.
Naturhist. Tidsskrift, 3rd ser. ii. 1863, p. 474.
Mt. Schweiz. ent. Ges. vii. 1887, p. 310.
Mem. hist. Insectes, iii. 1773, p. 548.
SB. Ges. naturf. Fr. Berlin, 1893, p. 127.
Ent. Tidskr. 1894, p. 65.
This enigmatic structure is similar in position to the aural orifice of Locustidae (see Fig. 101); but it is closed by a transparent membrane, whereas the ear orifice of Locustidae is, as we shall subsequently see, quite open.
Rev. biol. Nord France, vii. 1894, p. 111.
Ann. Nat. Hist. Decr. 6th, ser. x. 1892, p. 433.
Prod. Orth. europ. 1882, p. 27, and Rev. Syst. Orthopt. 1892, p. 15. Unfortunately de Saussure adopts a different nomenclature; we have preferred Brunner's as being more simple.
Ann. Sci. Nat. Zool. ser. 5, x. 1868, p. 161.
Nouv. Syst. Blattaires, 1865, p. 265.
The Cockroach, p. 170.
Cf. Duchamp, Rev. Sci. Nat. Montpellier, vii. [?1879], p. 423.
Huxley, Manual Anat. Invert. Animals, 1877, p. 416.
Riley, Insect Life, iii. 1891, p. 443, and iv. 1891, p. 119.
Essais entomologiques, St. Petersburg, 1821.
Beiträge zur näheren Kenntniss von Periplaneta orientalis, Elberfeld, 1853.
Nouv. Syst. Blattares, 1865, p. 16, etc.
Naturalist in Nicaragua, 1874, p. 110.
See Bolivar, Ann. Soc. ent. France, 1892, p. 29.
P. ent. Soc. London, 1881, p. 1.
Biol. Centr. Amer. Orthopt. 1893, p. 57.
Schäff, Zool. Anz. xvi. 1893, p. 17.
Westwood, Modern Class. Insects, i. 1839, p. 418.
Zeitschr. wiss. Zool. xlviii. 1889, p. 89; and Mem. Ac. St. Petersb. xxxviii. No. 5, 1891.
Ann. Hofmus. Wien., i. 1886, p. 104.
Zittel, Handb. Palaeont. I Abth. ii. 1885, p. 753.
Biol. Centr.-Amer. Orthoptera, 1893.
Although the genus Chorisoneura has unarmed femora, it must be placed in this division.
The "black beetle," Stilopyga orientalis, belongs to this tribe, as does also Periplaneta americana.
Tr. R. Soc. S. Austral. xvii. 1893, p. 68.
Zeitschr. wiss. Zool. xxx. 1878, p. 609, pl. xxxviii. fig. 7.
Arch. f. Naturgesch. xxx. Band 1, 1864, p. 7.
Biol. Centr. Amer. Orthopt. 1894, p. 160.
Our figures do not exhibit this attitude; if portrayed in their natural position in a drawing the front legs would be to a large extent obscured.
The name of the species is not given (Tr. N. Z. Inst. xvi. 1883, p. 114), but it is probably Orthodera ministralis Fab., an Australian Insect perhaps taken to New Zealand by miners. Cf. Wood-Mason, Cat. Mantodea, i. 1889, p. 20.
Berlin. ent. Zeitschr. viii. 1864, p. 234.
Ann. Soc. Linn. Lyon, xi. 1893, p. 205.
Proc. ent. Soc. London, 1867, p. cv.
Cat. Mantodea, i. 1889, p. 4.
Ann. Soc. ent. France, 1835, p. 457.
P. ent. Soc. London, 1877, p. xxix.
Afbeeldingen der Spoken en wandelende Bladen, etc., Amsterdam, 1813.
P. Asiat. Soc. Bengal, 1877, p. 193.
Tr. ent. Soc. London, 1878, p. 263.
Ann. Nat. Hist. 3rd ser. xix. 1867, p. 144.
Bull. Soc. Philomat. (8) ii. 1890, p. 154.
Insectes fossiles des temps primaires, 1894, p. 353.
Acta Ac. German. xii. 1825, pp. 555-672, pls. l.-liv.
Mem. Ac. Sci. Toulouse, series 7, iii. pp. 1-30.
Edinburgh Philosoph. Journ. January 1856.
Zool. Jahrb. Syst. i. 1886, p. 724.
P. Boston Soc. xii. 1869, p. 99.
Prod. Zool. Victoria, Decade vii. 1882, p. 34.
See de Borre, CR. Soc. ent. Belgique, xxvii. 1883, p. cxliii.
See Murray, Edinburgh New Philosophical Journal, January 1856.
CR. Ac. Paris, cxviii. 1894, No. 24, p. 1299.
SB. Ak. Wien, xci. 1885, p. 361. The nomenclature applied to the nervures by these authors is not the same as that of Brunner; according to their view the wing of Phyllium, female, differs more from the wing of Blatta than it does according to a comparison made with the nomenclature we adopt.
Bull. Soc. Philomathique (8), ii. p. 18.
Laboulbène, Bull. Soc. ent. France, 1857, p. cxxxvi., and Henneguy as above.
Ann. Nat. Hist. (5) i. 1878, p. 101.
The antennae in the specimen represented were no doubt mutilated, though Westwood did not say so.
CR. Ac. Paris, xcviii. 1884, p. 832.
In his recent Insectes fossiles des temps primaires, pp. 373 and 396, M. Brongniart has himself removed this Insect to Protodonates. We shall again mention it when discussing that group.
Bactridium, though placed in this tribe, has only short antennae, of 20 joints.
Bostra and Clonistria, belonging to Bacunculides, have the median segment almost as long as the metanotum.
The American genera Pterinoxylus, Haplopus, and Candaules, as well as the African Palophus, possess winged females.
The African and Australian genera Orobia and Paraorobia, although they have a short median segment, are placed in the tribe Phasmides of this division.
This character is evidently erroneous as regards the males of the genus Phyllium.—D. S.
Ann. Hofmus. Wien, i. 1886, p. 175.
Newport, Tr. Linn. Soc. xx. 1851, p. 419.
Mem. Ac. Sci. Étrang. vii. 1834, p. 274.
First Ann. Rep. U.S. Ent. Comm. 1878, p. 271.
Rep. U.S. Ent. Comm. ii. 1880, p. 223.
Ann. Sci. Nat. (7) iv. Zool. 1887.
Verh. zool-bot. Ges. Wien, xxi. 1871, p. 1097.
Verh. zool.-bot. Ges. Wien, xxiv. 1874, p. 286.
Denk. Ak. Wien, xxxvi. 1875; Arch. mikr. Anat. xx. and xxi., 1882.
Mem. Ac. Sci. Étrang. vii. 1834, p. 306.
Bull. Soc. Philomath. (8) v. 1893, p. 5.
First Ann. Rep. U.S. Ent. Comm. 1878, p. 279.
Rep. Ins. Missouri, ix. 1877, p. 86.
Bull. Soc. ent. France (6), x. 1890, p. xxxvii., and CR. Ac. Paris, ex. 1890, p. 657.
Carruthers in Nature, xli. 1889, p. 153.
Blue-book, C, 4960, 1887; and P. ent. Soc. London, 1881, p. xxxviii.
Rep. Entomologist, 1885, p. 229.
Tr. S. Afr. Phil. Soc. i. 1880, p. 193. The species is thought to be Pachytylus sulcicollis Stål.
CR. Soc. ent. Belgique, xxi. 1878, p. 5.
Addit. ad Prodromum Oedipodiorum, 1888, p. 12.
See Redtenbacher, Über Wanderheuschrecken, in Jahresber. Realschule Budweis, 1893.
J. Bombay N. H. Soc. viii. 1893, p. 120.
P. ent. Soc. London, 1893, p. xxi.
Rep. injurious Insects, xvii. 1893, p. 47.
Ent. Nachricht. viii. 1882, p. 160.
Monograph by Bolivar, Ann. Soc. Esp. xiii. 1884, p. 1, etc.
Monograph, de Saussure, Spicilegia entomologica Genavensia, pt. 2, Geneva, 1887.
Monograph, de Saussure, Mem. Soc. Phys. Genève, xxviii. 1884, No. 9; and xxx. 1888, No. 1.
Prod. Eur. Orthopt. 1882, p. 160.
Science, xxi. p. 133.
An. Soc. Espan. xv. 1886, p. 273.
Nature, iv. 1871, p. 333.
Bull. Soc. Rouen, 1885, and Insectes fossiles, etc. 1894, p. 439.
A few species of Proscopiides and Oedipodides, though placed in the next division, are destitute of any claw-pad.
This applies specially to the males.—D. S.
Ann. Rep. Insects Missouri, vi. 1874, p. 155.
Zeitschr. wiss. Zool. xxv. 1875, pp. 174-200, pl. xii.
Arch. f. mikr. Anat. xx. 1882, and xxi. See also von Adelung, Zeitschr. wiss. Zool. liv. 1892, p. 316.
The small space above lm left free from dots is, we presume, due to an omission on the part of Graber's artist, but we have not thought it right to interfere with his diagram.
Ann. Rep. Insects Missouri, vi. 1874, p. 159.
Wheeler, J. Morphol. viii. 1893.
Verh. zool.-bot. Ges. Wien, xxxiii. 1883, p. 248.
Bonnet and Finot, Rev. Sci. Nat. (3) iv. p. 345. The word we have translated as humming is "bruissement."
De Saussure, Ann. Soc. ent. France, 1888, p. 151, pl. v. fig. 1.
Indian Mus. Notes, ii. 1893, p. 172.
Zoologist, 1867, p. 489.
This diagnosis is an attempt to express in something approaching an exact manner the distinction of the flattened from the arched or convex head.
Scrobes are the depressions in which the antennae are inserted.
There are unfortunately a few exceptions in the case of this character.
See Pungur, Termes. Füzetek, 1877, p. 223.
Brunner, Verh. zool.-bot. Ges. Wien, xxiv. 1874, p. 288.
Natural History of Selborne, Letter xc.
Müller's Arch. 1859, p. 159.
Bull. Soc. ent. France, 1893, p. cccxli.
Zeitschr. wiss. Zool. xxiii. 1876, p. 122.
Ibid. xli. 1885, p. 570.
Morph. Jahrb. xv. 1889, p. 400.
Mem. Soc. phys. Genève, xxv. 1877, and Biol. Centr. Amer. Orthoptera, 1894, p. 198.
The genus Myrmecophila, being exceptional in several respects, is treated separately.
Insectes fossiles des temps primaires, 1893, vol. i. and atlas.
Giebel and Nitzsch, Insecta epizoica, folio, 1874.
Zeitschr. wiss. Zool. xlii. 1885, p. 537.
Zeitschr. wiss. Zool. xlii. 1885, pl. xviii. f. 15.
Arch. f. Naturg. xxxv. i. 1869, p. 154, pls. x. xi.
Op. cit. pp. vii.-xiv. For classification, etc., see also Piaget, Les Pédiculines. Leyden, 1880.
Zeitschr. wiss. Zool. xlii. 1885, p. 532.
P. ent. Soc. London, 1890, p. xxx.
Bull. Soc. Philom. (7) ix. p. 33.
P. Zool. Soc. London, 1883, p. 628.
Ann. Hofmus. Wien, i. 1886, p. 171.
Atti Acc. Gioenia, vii. 1893.
J. Linn. Soc. Zool. xiii. 1878, pl. xxi. f. 2.
Canadian Entomologist, xvii. 1885, throughout.
Jena. Zeitschr. Naturw. ix. 1875, pl. xii. See also Stokes in Science, xxii. 1893, p. 273.
Ann. Hofmus. Wien, i. 1886, p. 183.
Jena. Zeitschr. Naturw. ix. 1875, p. 257.
Bidie, in Nature, xxvi. 1882, p. 549.
Linnaea Entomologica, xii. 1858, p. 305.
P. Boston Soc. xx. 1878, p. 118.
Atti Acc. Gioen. vi. and vii. 1893 and 1894.
Ann. Sci. Nat. Zool. (4) v. 1856, p. 227.
Ann. Soc. ent. France (5), vi. 1876, p. 201.
Phil. Trans. lxxi. 1781, pp. 139-192.
Ann. Nat. Hist. (2) v. 1850, p. 92.
Dr. G. D. Haviland informs the writer that he thinks it probable this so-called peristaltic movement is merely the result of alarm; he has not, however, had any opportunity of observing T. bellicosus.
Tr. N. York Ac. viii. 1889, pp. 85-114; and ix. 1890, pp. 157-180.
Camerano, Bull. Soc. ent. Ital. xvii. 1885, p. 89; and Kollmann, Verh. Ges. Basel, vii. 1883, p. 391.
Jena. Zeitschr. Naturw. vii. 1873, p. 458.
CR. Ac. Paris, cxix. 1894, p. 804.
Congr. internat. Zool. ii. 1892, pt. i. p. 249.
P. Boston Soc. xi. 1868, p. 399.
Kolbe, Ent. Nachr. xiii. 1887, p. 70.
Trans. N. York Ac. viii. 1889, p. 91.
Congr. internat. Zool. ii. 1892, p. 249.
P. Boston Soc. xix. 1878, p. 267; and xx. 1881, p. 121.
According to Melliss, it is thought that the Insect may have been carried to the island in a captured slave-ship. Melliss, St. Helena, 1875, p. 171.
In some exotic species there is a dense network on a part of the anterior wing.
P. Boston Soc. xix. 1878, p. 292.
Germar, Mag. Entomol. iv. 1821, p. 276, pl. ii.
Psyche, iii. 1881, p. 196.
Kolbe, Stettin, ent. Zeit. xli. 1880, p. 179.
Op. cit. p. 209, etc.
Arch. f. Naturg. xlix. i. 1883, p. 99.
Verh. Ver. Rheinland, xxxix. 1882, Corr.-bl. p. 128.
Berlin ent. Zeit. xxviii. 1884, p. 36.
Stettin. ent. Zeit. xliv. 1883, pp. 299, 305.
For the British species, see M‘Lachlan, Ent. Month. Mag. iii. 1867, p. 177.
The genera Atropos and Clothilla were named after the two fates Atropos and Clotho. Westwood attempted some years ago to complete the trio by establishing a genus Lachesilla. This proved a failure, the genus being a misconception. As the name Lachesis is in use in various branches of zoology, the desired circle of Psocid fates is likely to remain always incomplete.
Phil. Trans. xxii. 1701, pp. 832-834; and xxiv. 1704, pp. 1586-1594, Plate 291, Figs. 4, 5 (pp. 1565 to 1604 occur twice in this volume).
Stettin. ent. Zeit. xliii. 1882, p. 265.
P. Boston Soc. xiii. 1871, p. 407.
Tr. Linn. Soc. xx. 1851, p. 433.
Bull. Soc. ent. France (4), viii. 1868, p. xxxvii.
Zool. Anz. iii. 1880, p. 304.
Stettin. ent. Zeit. xxxviii. 1877, p. 487.
Morphologie des Tracheensystems, Helsingfors, 1877, p. 21.
Beitr. Anat. Perla maxima. Inaug.-Diss. Aarau, 1881.
Entom. Month. Mag. xxix. 1893, p. 249.
No satisfactory systematic work of a general character on British Perlidae exists. References to the scattered descriptions and notes will be found in the Catalogue of British Neuroptera published by Entom. Soc. London, 1870.
Mem. Ac. Pétersb. (7) xxxvi. No. 15, 1889.
Insectes fossiles, etc., p. 407, 1893.
Festschrift Ges. naturf. Freunde Berlin, 1873.
Reference may be made to Calvert's recent paper introductory to the study of Odonata, in Tr. Amer. ent. Soc. xx. 1893, pp. 159-161.
Horae Soc. ent. Ross. xvi. 1881, p. 3.
Physiol. facett. Aug. 1891, p. 115.
Bull. Ac. Belgique (3), xvi. 1888, No. 11, p. 31.
SB. Ak. Wien, lxxxiii. 1881, pp. 289-376, pls. i.-vii.
Rev. Sci. Nat. Montpellier (3), ii. p. 470.
Abh. Senckenb. Ges. x. 1875, p. 13, pl. iii.
Zool. Anz. iii. 1880, p. 160.
CR. Soc. ent. Belgique, xxiii. 1880, p. lxvii.
Ann. Sci. Nat. (5) xi. Zool. 1869, p. 377.
Zur Morphologie des Tracheensystems, Helsingfors, 1877, p. 38.
Zool. Anz. xiii. 1890, p. 500.
The following works convey the best information: Evans's British Libellulinae or Dragon-flies, illustrated in a series of lithographic drawings, 1845. Hagen, "A Synopsis of the British Dragon-flies," in Entomologists' Annual, 1857. M‘Lachlan, Catalogue of the British Neuroptera, published by the Entomological Society of London in 1870; and "The British Dragon-flies annotated," Entom. Month. Mag. xx. 1884, pp. 251-256.
Rev. d'Entomol. v. 1886, p. 232.
Riveau, Feuille Nat. xii. 1882, p. 123.
Bull. Mus. Harvard, viii. 1880-81, p. 276.
Insectes fossiles, p. 394.
Insectes fossiles, p. 396.
Mem. Ac. Sci. Toulouse (7), iii. 1871, p. 379.
In reference to a doubt as to the name of this nymph cf. Eaton, Tr. Linn. Soc. Zool. (2) iii. p. 20.
Tr. Linn. Soc. xxiv. 1863, p. 62, and xxv. 1866, p. 477.
Hist. Nat. Neuropt. Ephémérines, 1843, p. 24.
Ann. Sci. Nat. Zool. (6) xiii. 1882, pp. 1-137, pls. 2-11.
Ann. Sci. Nat. Zool. (7) ix. 1890, pp. 19-87, pls. 2-5.
Ann. Nat. Hist. (3) xviii. 1866, p. 145.
Zeitschr. wiss. Zool. xxxiv. 1880, p. 404.
Ann. Nat. Hist. (5) xv. 1885, p. 494.
Mem. Cour. Ac. Belg. 4to, xix. 1847, p. 1.
Zur Morphologie des Tracheensystems, Helsingfors, 1877, pp. 1-20.
Tr. Linn. Soc. xxv. 1866, p. 483.
Ann. Nat. Hist. (3) xviii. 1866, p. 145.
Ann. Sci. Nat. Zool. (6) xiii. 1882, p. 113.
Réaumur, Mem. vi. 1742, p. 457.
Über vaarige Ausführsgänge, etc., Helsingfors, 1884, p. 53.
Ber. Ges. Freiburg, iv. p. 5; cf. J. R. Micr. Soc. 1889, p. 206.
Tr. Linn. Soc. 2nd ser. Zool. iii. 1883, p. 11.
Fly-Fisher's Entomology, 4th ed. 1849, p. 49.
P. ent. Soc. London, 1882, p. xiii.
Ann. Sci. Nat. series 3, ix. Zool. 1848, p. 91, pl. 1.
Newport, Tr. Linn. Soc. xx. 1851, pl. 21, fig. 13. Loew, however, who also describes and figures the anatomy of S. lutaria, states that there is no paunch. Linnaea entomologica, iii. 1848, p. 354.
M‘Lachlan, Ent. Month. Mag. vii. 1870, p. 145.
Rep. Ins. Missouri, ix. 1877, p. 125.
Tijdschr. Ent. vol. xxxiv. 1891.
Arch. f. Naturg. iv. i. 1838, p. 315.
Linnaea entomologica, iii. p. 1848, 346, pl. i.
Mem. Ac. Sci. étrang. vii. 1841, p. 582.
Linnaea entom. iii. 1848, p. 363.
Ent. Month. Mag. 1894, p. 39.
Stettin. ent. Zeit. xxvii. 1866, p. 369; this author has also sketched a classification of the larvae in P. Boston Soc. xv. 1873, p. 243.
Ov. Danske Selsk. 1889, p. 43.
Ann. Sci. étrang. vii. 1834, pl. 12.
M‘Lachlan, Ent. Month. Mag. ii. 1865, p. 73.
Redtenbacher, Denk. Ak. Wien, xlviii. 1884, p. 335.
J. Linn. Soc. Zool. xi. 1873, p. 227.
Verh. zool.-bot. Ges. Wien, iv. 1854, p. 471.
Westwood, l.c. p. 12.
P. Boston Soc. xv. 1873, p. 244.
Tr. Entom. Soc. London, 1888, p. 1, pls. 1, 2.
Cf. M‘Lachlan, J. Linn. Soc. Zool. ii. 1873, p. 219.
Tr. Linn. Soc. xiv. 1825, p. 140, and xv. 1827, p. 509.
P. Boston Soc. xv. 1873, p. 245.
M‘Lachlan, Tr. Ent. Soc. London, 1885, p. 375.
Verh. zool.-bot. Ges. Wien, xix. 1869, p. 831.
Brauer, Zool. Anz. x. 1887, pp. 212 and 218.
Linnaea entomologica, vii. 1852, p. 368, with plates.
See Albarda in Tijdschr. Ent. xvii. 1874, p. xvi.
Tr. ent. Soc. London, 1868, p. 189.
Biol. Centralbl. iv. 1885, p. 722.
Bull. Soc. ent. France (6), i. 1881, pp. xxi. and xxxi.
Arch. Naturges. lix. 1893, Band I. p 285.
Ent. Nachr. xiv. 1888, p. 274.
Trichoptera europ. 1878, p. 356, note.
Berl. ent. Zeitschr. xxv. 1881, p. 54.
Monograph of the British Trichoptera in Tr. ent. Soc. London, third series, vol. v. 1865; and Monographic Revision of the European Trichoptera, 1874-1880.
Zeitschr. wiss. Zool. xxxv. 1881, Pl. IV. fig. 6.
Rep. of the Entomologist, 1886, p. 510, Washington.
Insectes fossiles des temps primaires, 1893, p. 38.
P. ent. Soc. London, 1866, p. lxv.
For a history of this complex question, see Gosch, Naturhist. Tidskr. (Rk. 3) vol. xiii. 1881; and also Brauer, Sitzb. Ak. Wien, lxxxv. 1882.
Introd. hist. Insects, 1841, p. 143. The names proposed by Newman may be adopted when it is specially requisite to use terms that are morphologically correct. According to his nomenclature the true whole abdomen of petiolate Hymenoptera consists of three anatomical parts: 1, the petiole or podeon; 2, the propodeon or part in front of the petiole; 3, the metapodeon or part behind the petiole.
Zeitschr. wiss. Zool. xxv. 1874, p. 184.
Ann. Mag. Nat. Hist. (6) x. 1892, p. 442.
CR. Ac. Paris, lxxxiii. 1876, p. 613, and Ann. Mag. Nat. Hist. (4) xviii. 1876, p. 504; also Horae Soc. Ross. xv. 1880, pp. 20 and 31.
P. Boston Soc. x. 1866, p. 279.
Adler, Deutsche ent. Zeitschr. xxi. 1877, p. 209.
Cameron, Brit. Phyt. Hym. Ray Society, i. 1882, p. 29, and ii. 1885, p. 218.
Cameron, op. cit. iv. 1893, p. 9.
Brit. Phyt. Hym. i. p. 27. Fletcher's record, referred to by Cameron, mentions N. miliaris, but this name was probably erroneous.
See Perez and Cameron, Tr. Nat. Hist. Soc. Glasgow, n.s. ii. 1889, p. 194.
Fabre, Marchal, Nicolas.
Zeitschr. wiss. Zool. xxx. Supp. 1878, p. 103.
Rejoinder to Professor Weismann, p. 11. Reprint from Contemporary Review, December 1893.
Mon. Brit. Phyt. Hym. 4 vols. 1882 to 1893.
Insect Life, i. 1888, p. 8.
Souvenirs entomologiques: quatrième série, 1891, p. 308.
Tr. ent. Soc. London, i. 1836, p. 232.
Ichneumonen der Forstinsecten, i. 1844, p. 86.
See Cameron, Brit. Phyt. Hym. iii. Ray Soc. 1890, p. 152.
The term inquiline is applied in entomology to a great variety of conditions covered by the Latin word "inquilinus" (incolinus), signifying a tenant or dweller in another's property. The term parasite is used in a still wider and vaguer sense, being in fact applied to a large number of cases, in many of which we do not at present understand the exact relations between the two parties concerned. This subject is no doubt destined to become a most interesting department of entomology. See Riley, P. ent. Soc. Washington, ii. 1893, p. 397; and Wasmann, Zusammengesetzten Nester, etc., 1891.
P. Linn. Soc. N. S. Wales (2), vii. 1892, p. 357.
Science (n.s.), i. 1895, p. 457.
Ray Soc. vol. iv. 1893, p. 24.
Term. Füzetek, v. 1882, p. 198, and Biol. Centralbl. ii. 1882, p. 617.
Ann. Soc. ent. France (4), vi. 1866, p. 198.
Adler and Straton, Alternating Generations, 1894, p. 119.
P. entom. Soc. Philadelphia, ii. 1864, pp. 447, etc.
P. ent. Soc. Philad. ii. 1863, p. 34.
Brit. Phyt. Hym. vols. iii. and iv. Ray Soc. 1891 and 1893.
Entom. Mag. ii. 1835, p. 219.
Tr. ent. Soc. London, 1881, p. 109.
Bull. U. S. Museum, No. 45, 1893, p. 28.
Tr. ent. Soc. London, 1881, p. 117.
Tr. ent. Soc. London, 1881, pt. vi. f. 3; pp. 120, 126.
Zeitschr. wiss. Zool. xix. 1869; Ganin's observations are described by Lubbock, Origin and Metamorphoses of Insects, 1874, p. 34.
See also Kulagin, Zool. Anz. xiii. 1890, p. 418; xv. 1892, p. 85; and Congr. internat. Zool. ii. 1892, pt. i. p. 258.
Tr. Linn. Soc. (2) Zool. i. 1878, p. 587.
Tr. Linn. Soc. xxiv. 1863, p. 135.
Zeitschr. wiss. Zool. xix. 1869, p. 417.
Souvenirs entomologiques. Troisième série, 1886, p. 155.
Souvenirs entomologiques. Troisième série, 1886, p. 179.
Tr. Linn. Soc. xxi. 1855, p. 67.
According to Ashmead, P. ent. Soc. Washington, ii. 1893, p. 228, this genus should take the name of Melittobia.
Ann. Nat. Hist. (6) x. 1892, p. 271.
Rec. Zool. Suisse, v. 1891, pp. 435-534. Cf. Koulaguine, Congr. internat. Zool. ii. 1892, pt. i. p. 265.
Bull. Soc. Ent. France (5) vii. 1877, p. lxix.; also André, Feuille Natural. vii. 1877, p. 136, and Riley and Howard, Insect Life, iv. 1892, p. 242.
Insect Life, i. 1888, p. 121.
Report of the Entomologist, Dep. Agriculture, Washington, 1886, p. 542.
Wachtl, Wien. ent. Zeit. xii. 1893, p. 24, and Howard, P.U.S. Nat. Mus. xiv. 1892, p. 586.
Abh. Ges. Göttingen, xxviii. 1882.
Mitt. Stat. Neapel, iii. 1882, p. 55.
Tr. ent. Soc. London, 1883. p. 389.
P. biol. Soc. Washington, vii. 1892, p. 99.
Ann. Botan. Garden, Calcutta, i. 1889, Appendix L.
P. ent. Soc. London, 1886, p. x.
For a systematic memoir refer to Mayr, Verh. zool.-bot. Ges. Wien, xxxv. 1885, p. 147, etc.
Insect Life, iv. 1891, p. 193.
Tosquinet, Ann. Soc. ent. Belgique, xxxviii. 1894, p. 694.
Ichneum. Forst. Ins. 1844, p. 81.
Mitt. schweizer. ent. Ges. iv. 1876, p. 518.
Fifth Rep. U. S. Ent. Comm. 1890, p. 15.
Tr. Linn. Soc. xxi. 1852, p. 71.
Tr. ent. Soc. London, 1886, p. 162, and 1887, p. 303.
Ent. Month. Mag. xiii. 1877, p. 200.
A catalogue, with references, of the British Ichneumonidae was published by the Entomological Society of London in 1872. Since then many additional species have been detected and recorded, by Mr. Bridgman and others, in the Transactions of the same Society.
Klapálek, Ent. Month. Mag. xxv. 1889, p. 339, and Arch. Landesdurchforschung Böhmen, viii. No. 6, 1893, p. 53.
Ichneum. Forst. Ins. 1844.
Ann. Soc. ent. France (2), iii. 1845, p. 355.
Tr. ent. Soc. London, 1885, pp. 224, 219.
A monograph of the British Braconidae was commenced by the Rev. T. A. Marshall in 1885, and is still in progress, in the Transactions of the Entomological Society of London; cf. op. cit. 1885, 1887, 1889, 1891, 1894.
Berlin entom. Zeitschr. xxxiii. 1889, p. 197.
Ibid.
Monograph, Schletterer, Verh. zool.-bot. Ges. Wien, xxxv. 1885, p. 267, etc.; xxxvi. 1886, p. 1, etc.; and Ann. Hofmus. Wien, iv. pp. 107, etc.
Berlin. entom. Zeitschr. xxxiii. 1889, p. 197.
Amer. Nat. xxviii. 1894, p. 895.