
Title: The Cambridge natural history, Vol. 08 (of 10)
Editor: S. F. Harmer
Author: Hans Gadow
Editor: Sir A. E. Shipley
Release date: June 21, 2024 [eBook #73885]
Language: English
Original publication: London: Macmillan and Co, 1909
Credits: Keith Edkins, Peter Becker and the Online Distributed Proofreading Team at https://www.pgdp.net (This file was produced from images generously made available by The Internet Archive)
THE
CAMBRIDGE NATURAL HISTORY
EDITED BY
S. F. HARMER, Sc.D., F.R.S., Fellow of King's College, Cambridge; Superintendent of the University Museum of Zoology
AND
A. E. SHIPLEY, M.A., Fellow of Christ's College, Cambridge; University Lecturer on the Morphology of Invertebrates
VOLUME VIII
MACMILLAN AND CO., Limited
LONDON . BOMBAY . CALCUTTA
MELBOURNE
THE MACMILLAN COMPANY
NEW YORK . BOSTON . CHICAGO
ATLANTA . SAN FRANCISCO
THE MACMILLAN CO. OF CANADA, Ltd.
TORONTO
AMPHIBIA AND REPTILES
By Hans Gadow, M.A. (Cantab.), Ph.D. (Jena), F.R.S., Strickland Curator and Lecturer on Advanced Morphology of Vertebrata in the University of Cambridge.
MACMILLAN AND CO., LIMITED
ST. MARTIN'S STREET, LONDON
1909
First Edition 1901
Reprinted 1909
Linnaeus had but a poor opinion of the Amphibia and their describers, or he would not have called the former "pessima tetraque animalia," nor would he have dismissed the latter with the terse remark: "Amphibiologi omnium paucissimi sunt nullique veri." That was, however, nearly 150 years ago; and at the present time there are fewer difficulties in writing a book on Amphibia and Reptiles. Those who care for the study of Amphibia and Reptiles–the Herpetologists, to give them their scientific title–have never been numerous; but most of them have been serious students. One reason for the fact that this branch of Natural History is not very popular, is a prejudice against creatures some of which are clammy and cold to the touch, and some of which may be poisonous. People who delight in keeping Newts or Frogs, Tortoises or Snakes, are, as a rule, considered eccentric. But in reality these cold-blooded creatures are of fascinating interest provided they are studied properly. The structure of animals is intimately connected with their life-habits; and this correlation is perhaps more apparent in Amphibia and Reptiles than in any other class. The anatomist who studies internal and external structure is as much struck with the almost endless variety in details as he who takes the trouble to observe the living animal in its native haunts, or at least under conditions not too unnatural. He will agree with V. von Scheffel's Toad "that those above seem to have no {vi}notion of the beauties of the swamp"–brilliantly coloured Newts engaged in amorous play, concert-giving Frogs, and metamorphosing Tadpoles. The motto assigned to the Reptiles seems singularly appropriate when we consider that poisonous snakes have been developed from harmless forms, and that many kinds of reptiles have lost limbs, teeth, and eyesight in the process of evolution.
The present work is intended to appeal to two kinds of readers–to the field-naturalist, who, while interested in life-histories, habits, and geographical distribution, beauty or strangeness of forms, is indifferent to the homologies of the metasternum or similar questions;–and to the morphologist, who in his turn is liable to forget that his specimens were once alive.
A great portion of the book is anatomical and systematic. It was necessary to treat anatomy, especially that of the skeleton, somewhat fully, since it has long been recognised that it is impossible to base a scientific classification upon external characters. The reader familiar with Vertebrate anatomy has a right to expect that questions of special morphological interest will be dwelt upon at length. Those who have no anatomical foundation must be referred to one of the now numerous introductory manuals on the subject.
The account of the Amphibia is more complete than that of the Reptilia. It was possible to diagnose practically all the recent genera; and this has been especially done in the Anura, in order to show how in an otherwise very homogeneous group almost any part of the body, internal or external, can be modified in kaleidoscopic variety. The same could not be done with the Reptilia. Their principal groups,–called sub-classes in the present work, in order to emphasise their taxonomic importance in comparison with the main groups of Birds and Mammals,–differ so much from each other that it was decided to refrain {vii}from attempting a general account of them. Moreover, the number of species of recent lizards and snakes is so bewildering, the genera of many families being but tedious variations of the same theme, that only those forms have been described which are the most important, the most striking, or which the traveller is most likely to come across. The student who wishes to go farther into systematic details must consult the seven volumes of the Catalogue of Reptiles in the British Museum (London, 1889-1896). Mr. G. A. Boulenger, the author of this magnificent series, has rendered the systematic treatment of recent Amphibia and Reptiles an easy task. During many years of the most friendly intercourse I have profited on countless occasions by his ever-ready advice. Although he has kindly read the proofs of the part dealing with the Amphibia it would be unfair to associate him with any of its shortcomings or with contestable opinions, for which I alone am responsible.
Cope's large work on the Crocodilians, Lizards, and Snakes of North America (Rep. U.S. Nat. Mus. for 1898 (1900)) has unfortunately appeared too late to be used in the present work.
The drawings on wood were, with few exceptions, made by Miss M. E. Durham, mostly from living specimens–a procedure which has to a great extent determined the selection of the illustrations.
Since both the metric and the English systems of measurements have been employed, it may be well to state for the convenience of the reader that the length of a line of the text is four inches or approximately ten centimeters.
I have frequently and freely quoted accounts of previous authors instead of paraphrasing them. Especial thanks are due to Messrs. Longmans, Green, and Co., and to Messrs. Murray, {viii}for their courteous permission to make several long quotations from Sir J. E. Tennent's Ceylon, and from H. W. Bates' Naturalist on the River Amazons.
Lastly, a remark about my Editors. Instead of being a source of annoyance they have rendered me the greatest help.
H. GADOW.
Cambridge, December 19, 1900.
| PAGE | |
| Preface | v |
| Scheme of the Classification adopted in this book | xi |
| PART I. AMPHIBIA | |
| CHAPTER I | |
| Characters and Definition—Position of the Class Amphibia in the Phylum Vertebrata—Historical Account of the Classification of Amphibia | 3 |
| CHAPTER II | |
| Skeleton of Urodela and Anura—Skin—Colour-changing Mechanism—Poison-glands—Spinal Nerves—Respiratory Organs—Suppression of Lungs—Urino-genital Organs—Fecundation—Nursing Habits—Development and Metamorphosis | 11 |
| CHAPTER III | |
| Neoteny—Regeneration—Temperature—Geographical Distribution | 63 |
| CHAPTER IV | |
| Stegocephali or Labyrinthodonts—Lissamphibia—Apoda | 78 |
| CHAPTER V | |
| Lissamphibia (continued)—Urodela | 94 |
| CHAPTER VI | |
| Lissamphibia (continued)—Anura | 138 |
| {x}
PART II. REPTILIA |
|
| CHAPTER VII | |
| PAGE | |
| Definition and Characters—Position of the Class Reptilia in the Phylum Vertebrata—Classification—Skull and Vertebrae | 277 |
| CHAPTER VIII | |
| Proreptilia—Prosauria—Theromorpha | 285 |
| CHAPTER IX | |
| Chelonia—Athecae—Thecophora | 312 |
| CHAPTER X | |
| Dinosauria—Crocodilia | 412 |
| CHAPTER XI | |
| Plesiosauria—Ichthyosauria—Pterosauria—Pythonomorpha | 473 |
| CHAPTER XII | |
| Sauria—Autosauri or Lacertilia—Lizards | 491 |
| CHAPTER XIII | |
| Sauria (continued)—Ophidia—Snakes | 581 |
| INDEX | 651 |
| CLASS AMPHIBIA. | ||||
| Sub-Class. | Order. | Sub-Order. | Family. | Sub-Family. |
| STEGOCEPHALI (p. 78) | Stegocephali Lepospondyli (p. 80) |
Branchiosauri (p. 80). Aistopodes (p. 81). |
||
|
Stegocephali Temnospondyli (p. 81) Stegocephali Stereospondyli (p. 83) |
||||
| LISSAMPHIBIA (p. 84) | Apoda (p. 84) | Coeciliidae (p.89). | ||
| Urodela (p. 94) | Amphiumidae (p. 97). | |||
| Salamandridae (p. 102) |
Desmognathinae (p. 102). Plethodontinae (p. 103). Amblystomatinae (p. 109). Salamandrinae (p. 115). |
|||
|
Proteidae (p. 132). Sirenidae (p. 136). |
||||
| Anura (p. 138) | Aglossa (p. 143). | |||
| Phaneroglossa (p. 152) |
Discoglossidae (p. 152). Pelobatidae (p. 160). Bufonidae (p. 166). |
|||
| Hylidae (p. 185) |
Amphignathodontinae Hylinae (p. 189). |
|||
| Cystignathidae (p. 209) |
Hemiphractinae (p. 210). Cystignathinae (p. 211). Dendrophryniscinae |
|||
| Engystomatidae (p. 225) |
Engystomatinae (p. 225). Dyscophinae (p. 235). Genyophryninae (p. 236). |
|||
| Ranidae (p. 237) |
Ceratobatrachinae (p. 237). Raninae (p. 238). Dendrobatinae (p. 272). |
|||
|
CLASS REPTILIA (p. 277). |
||||
| PROREPTILIA (p. 285).
Eryops (p. 286). Cricotus (p. 287). |
||||
| Sub-Class. | Order. | Sub-Order. | Family. | Sub-Family. |
| PROSAURIA (p. 288) | Microsauri (p. 288). | |||
| Prosauri (p. 290). |
Protorosauri (p. 290). Rhynchocephali (p. 292). |
|||
| THEROMORPHA (p. 300) |
Pareiasauri (p. 304). Theriodontia (p. 306). Anomodontia (p. 309). Placodontia (p. 311). |
|||
| CHELONIA (p. 312) | Atheca (p. 333) | Sphargidae (p. 333). | ||
| Thecophora (p. 338) | Cryptodira (p. 338) |
Chelydridae (p. 338). Dermatemydidae (p. 341). Cinosternidae (p. 342). Platysternidae (p. 345). Testudinidae (p. 345). Chelonidae (p. 378). |
||
| Pleurodira (p. 388) |
Pelomedusidae (p. 390). Chelydidae (p. 399). Carettochelydidae (p. 404). |
|||
| Trionychoidea (p. 404) | Trionychidae (p. 404). | |||
| DINOSAURIA (p. 412) |
Sauropoda (p. 418). Theropoda (p. 420). |
|||
| Orthopoda (p. 424) |
Stegosauri (p. 425). Ornithopoda (p. 426) |
|||
| Ceratopsia (p. 430). | ||||
| CROCODILIA (p. 431) |
Pseudosuchia (p. 432). Parasuchia (p. 433). |
|||
| Eusuchia (p. 434) |
Teleosauridae (p. 450). Metriorhynchidae (p. 451). Macrorhynchidae (p. 451). Gavialidae (p. 451). Atoposauridae (p. 453). Goniopholidae (p. 453). Crocodilidae (p. 454). |
|||
| PLESIOSAURIA (p. 473) | Nothosauri (p. 476) |
Mesosauridae (p. 476). Nothosauridae (p. 477). |
||
| Plesiosauri (p. 477) |
Pliosauridae (p. 477). Plesiosauridae (p. 478). Elasmosauridae (p. 478). |
|||
| ICHTHYOSAURIA (p. 478) | Ichthyosauri (p. 483). | |||
| PTEROSAURIA (p. 484) | Pterosauri (p. 486) |
Pterodactyli (p. 486). Pteranodontes (p. 487). |
||
| PYTHONOMORPHA (p. 487) |
Dolichosauri (p. 489). Mosasauri (p. 489). |
|||
|
SAURIA (p. 491) |
Lacertilia (p. 491) | Geckones (p. 502) | Geckonidae (p. 507) |
Geckoninae (p. 507). Eublepharinae (p. 512). Uroplatinae (p. 512). |
| Lacertae (p. 513) |
Agamidae (p. 515). Iguanidae (p. 528). Xenosauridae (p. 536). Zonuridae (p. 536). Anguidae (p. 537). Helodermatidae (p. 540). Lanthanotidae (p. 541). Varanidae (p. 542). Xantusiidae (p. 547). Tejidae (p. 547). Lacertidae (p. 549). Gerrhosauridae (p. 559). Scincidae (p. 559). Anelytropidae (p. 564). Dibamidae (p. 564). Aniellidae (p. 564). Amphisbaenidae (p. 565). Pygopodidae (p. 567). |
|||
| Chamaeleontes (p. 567) | Chamaeleontidae (p. 573). | |||
| Ophidia (p. 581) |
Typhlopidae (p. 593). Glauconiidae (p. 594). Ilysiidae (p. 594). Uropeltidae (p. 595). |
|||
| Boidae (p. 596) |
Pythoninae (p. 598). Boinae (p. 601). |
|||
| Xenopeltidae (p. 605). | ||||
| Colubridae (p. 606) Aglypha (p. 606) |
Acrochordinae (p. 606). Colubrinae (p. 607). Rhachiodontinae (p. 622). |
|||
| Colubridae Opisthoglypha (p. 623) |
Dipsadomorphinae (p. 623). Elachistodontinae (p. 625). Homalopsinae (p. 625). |
|||
| Colubridae Proteroglypha (p. 625) |
Elapinae (p. 626). Hydrophinae (p. 635). |
|||
| Amblycephalidae (p. 637). | ||||
| Viperidae (p. 637) |
Viperinae (p. 638). Crotalinae (p. 644). |
|||
| CLASS AMPHIBIA. | ||||||||
| Sub-Class. | Order. | Sub-Order. | Family. | Sub-Family. | ||||
| STEGOCEPHALI (p. 78) |  |
Stegocephali Lepospondyli (p. 80) |
 |
Branchiosauri (p. 80). Aistopodes (p. 81). |
||||
|
Stegocephali Temnospondyli (p. 81) Stegocephali Stereospondyli (p. 83) |
||||||||
| LISSAMPHIBIA (p. 84) |  |
Apoda (p. 84) | Coeciliidae (p.89). | |||||
| Urodela (p. 94) |  |
Amphiumidae (p. 97). | ||||||
| Salamandridae (p. 102) |  |
Desmognathinae (p. 102). Plethodontinae (p. 103). Amblystomatinae (p. 109). Salamandrinae (p. 115). |
||||||
|
Proteidae (p. 132). Sirenidae (p. 136). |
||||||||
| Anura (p. 138) |  |
Aglossa (p. 143). | ||||||
| Phaneroglossa (p. 152) |  |
Discoglossidae (p. 152). Pelobatidae (p. 160). Bufonidae (p. 166). |
||||||
| Hylidae (p. 185) |  |
Amphignathodontinae Hylinae (p. 189). |
||||||
| Cystignathidae (p. 209) |  |
Hemiphractinae (p. 210). Cystignathinae (p. 211). Dendrophryniscinae |
||||||
| Engystomatidae (p. 225) |  |
Engystomatinae (p. 225). Dyscophinae (p. 235). Genyophryninae (p. 236). |
||||||
| Ranidae (p. 237) |  |
Ceratobatrachinae (p. 237). Raninae (p. 238). Dendrobatinae (p. 272). |
||||||
|
CLASS REPTILIA (p. 277). |
||||||||
| PROREPTILIA (p. 285).
Eryops (p. 286). Cricotus (p. 287). |
||||||||
| Sub-Class. | Order. | Sub-Order. | Family. | Sub-Family. | ||||
| PROSAURIA (p. 288) |  |
Microsauri (p. 288). | ||||||
| Prosauri (p. 290). |  |
Protorosauri (p. 290). Rhynchocephali (p. 292). |
||||||
| THEROMORPHA (p. 300) |  |
Pareiasauri (p. 304). Theriodontia (p. 306). Anomodontia (p. 309). Placodontia (p. 311). |
||||||
| CHELONIA (p. 312) |  |
Atheca (p. 333) | Sphargidae (p. 333). | |||||
| Thecophora (p. 338) |  |
Cryptodira (p. 338) |  |
Chelydridae (p. 338). Dermatemydidae (p. 341). Cinosternidae (p. 342). Platysternidae (p. 345). Testudinidae (p. 345). Chelonidae (p. 378). |
||||
| Pleurodira (p. 388) |  |
Pelomedusidae (p. 390). Chelydidae (p. 399). Carettochelydidae (p. 404). |
||||||
| Trionychoidea (p. 404) | Trionychidae (p. 404). | |||||||
| DINOSAURIA (p. 412) |  |
Sauropoda (p. 418). Theropoda (p. 420). |
||||||
| Orthopoda (p. 424) |  |
Stegosauri (p. 425). Ornithopoda (p. 426) |
||||||
| Ceratopsia (p. 430). | ||||||||
| CROCODILIA (p. 431) |  |
Pseudosuchia (p. 432). Parasuchia (p. 433). |
||||||
| Eusuchia (p. 434) |  |
Teleosauridae (p. 450). Metriorhynchidae (p. 451). Macrorhynchidae (p. 451). Gavialidae (p. 451). Atoposauridae (p. 453). Goniopholidae (p. 453). Crocodilidae (p. 454). |
||||||
| PLESIOSAURIA (p. 473) |  |
Nothosauri (p. 476) |  |
Mesosauridae (p. 476). Nothosauridae (p. 477). |
||||
| Plesiosauri (p. 477) |  |
Pliosauridae (p. 477). Plesiosauridae (p. 478). Elasmosauridae (p. 478). |
||||||
| ICHTHYOSAURIA (p. 478) | Ichthyosauri (p. 483). | |||||||
| PTEROSAURIA (p. 484) | Pterosauri (p. 486) |  |
Pterodactyli (p. 486). Pteranodontes (p. 487). |
|||||
| PYTHONOMORPHA (p. 487) |  |
Dolichosauri (p. 489). Mosasauri (p. 489). |
||||||
|
SAURIA (p. 491) |
 |
Lacertilia (p. 491) |  |
Geckones (p. 502) | Geckonidae (p. 507) |  |
Geckoninae (p. 507). Eublepharinae (p. 512). Uroplatinae (p. 512). |
|
| Lacertae (p. 513) |  |
Agamidae (p. 515). Iguanidae (p. 528). Xenosauridae (p. 536). Zonuridae (p. 536). Anguidae (p. 537). Helodermatidae (p. 540). Lanthanotidae (p. 541). Varanidae (p. 542). Xantusiidae (p. 547). Tejidae (p. 547). Lacertidae (p. 549). Gerrhosauridae (p. 559). Scincidae (p. 559). Anelytropidae (p. 564). Dibamidae (p. 564). Aniellidae (p. 564). Amphisbaenidae (p. 565). Pygopodidae (p. 567). |
||||||
| Chamaeleontes (p. 567) |  |
Chamaeleontidae (p. 573). | ||||||
| Ophidia (p. 581) |  |
Typhlopidae (p. 593). Glauconiidae (p. 594). Ilysiidae (p. 594). Uropeltidae (p. 595). |
||||||
| Boidae (p. 596) |  |
Pythoninae (p. 598). Boinae (p. 601). |
||||||
| Xenopeltidae (p. 605). | ||||||||
| Colubridae (p. 606) Aglypha (p. 606) |
 |
Acrochordinae (p. 606). Colubrinae (p. 607). Rhachiodontinae (p. 622). |
||||||
| Colubridae Opisthoglypha (p. 623) |
 |
Dipsadomorphinae (p. 623). Elachistodontinae (p. 625). Homalopsinae (p. 625). |
||||||
| Colubridae Proteroglypha (p. 625) |
 |
Elapinae (p. 626). Hydrophinae (p. 635). |
||||||
| Amblycephalidae (p. 637). | ||||||||
| Viperidae (p. 637) |  |
Viperinae (p. 638). Crotalinae (p. 644). |
||||||
AMPHIBIA
"'s scheint, dass die hier oben keine
Ahnung haben von dem Sumpf und
Seiner Pracht."
The "plattgedrückte Kröte,"
Scheffel's Trompeter von Säkkingen.
AMPHIBIA
CHARACTERS AND DEFINITION–POSITION OF THE CLASS AMPHIBIA IN THE PHYLUM VERTEBRATA–HISTORICAL ACCOUNT OF THE CLASSIFICATION OF AMPHIBIA
A bird is known by its feathers, a Beast by its hairs, a Fish by its fins, but there is no such obvious feature which characterises the Amphibia and the Reptiles. In fact, they are neither fish, flesh, nor fowl. This ill-defined position is indicated by the want of vernacular names for these two classes, a deficiency which applies not only to the English language. All the creatures in question are backboned, creeping animals. Those which are covered with horny scales, and which from their birth breathe by lungs only, as Crocodiles, Tortoises, Lizards, and Snakes, are the Reptiles. The rest, for instance, Newts or Efts, Frogs and Toads, are the Amphibia. Their skin is mostly smooth and clammy and devoid of scales; the young are different from the adult in so far as they breathe by gills and live in the water, before they are transformed into entirely lung-breathing, terrestrial creatures. But there are many exceptions. Proteus and Siren the mud-eel, always retain their gills; while not a few frogs undergo their metamorphosis within the egg, and never breathe by gills. If we add the tropical limbless, burrowing Coecilians, and last, not least, the Labyrinthodonts and other fossil forms, the proper definition of the class Amphibia,–in other words, the reasons for grouping them together into one class, separated from the other backboned animals,–requires the examination of many other characters.
So far as numbers of living species are concerned, the Amphibia are the least numerous of the Vertebrata. There are about 40 limbless, burrowing Apoda; 100 Urodela or tailed two- or four-footed newts, and about 900 Anura, or tailless, four-footed frogs and toads; in all some 1000 different species. Few, indeed, in comparison with the 2700 Mammals, 3500 Reptiles, nearly 8000 Fishes, and almost 10,000 Birds. But we shall see that the Amphibia have not only "had their day," having flourished in bygone ages when they divided the world, so far as Vertebrata were concerned, between themselves and the Fishes, but that they never attained a dominant position. Intermediate between the aquatic Fishes and the gradually rising terrestrial Reptiles they had to fight, so to speak, with a double front during the struggle of evolution, until by now most of them have become extinct. The rest persist literally in nooks and corners of the teeming world, and only the Frogs and Toads, the more recent branch of the Amphibian tree, have spread over the whole globe, exhibiting almost endless variations of the same narrow, much specialised plan. The greatest charm of the Anura lies in their marvellous adaptation to prevailing circumstances; and the nursing habits of some kinds read almost like fairy-tales.
Characters of the Amphibia.[1]
1. The vertebrae are (a) acentrous, (b) pseudocentrous, or (c) notocentrous.
2. The skull articulates with the atlas by two condyles which are formed by the lateral occipitals. For exceptions see p. 78.
3. There is an auditory columellar apparatus, fitting into the fenestra ovalis.
4. The limbs are of the tetrapodous, pentadactyle type.
5. The red blood-corpuscles are nucleated, biconvex, and oval.
6. The heart is (a) divided into two atria and one ventricle, and (b) it has a conus provided with valves.
7. The aortic arches are strictly symmetrical.
8. Gills are present at least during some early stages of development.
9. The kidneys are provided with persistent nephrostomes.
10. Lateral sense-organs are present at least during the larval stage.
11. The vagus is the last cranial nerve.
12. The median fins, where present, are not supported by spinal skeletal rays.
13. Sternal ribs and a costal or true sternum are absent.
14. There is no paired or unpaired medio-ventral, copulatory apparatus.
15. Development takes place without amnion and allantois.
None of these characters is absolutely diagnostic, except 1 (c), and this applies only to the Anura and most of the Stegocephali.
Numbers 1 (b), 1 (c), 2, 3, 4 and 12 separate the Amphibia from the Fishes.
Numbers 1, 6 (b), 7, 8, 9, 11, 13, 15 separate them from the Reptiles, Birds, and Mammals.
Number 2 separates them from the Fishes, Reptiles, and Birds.
Number 5 separates them from the Mammals.
Number 6 (a) separates them from the Fishes (excl. Dipnoi), Birds and Mammals.
We can, therefore, very easily define all the Amphibia, both recent and extinct, by a combination of the characters enumerated above. For instance, by the combination of numbers 2, 3 or 4 with either 7, 8, 9, 11, 13 or 15.
Amphicondylous Anamnia would be an absolutely correct and all-sufficient diagnosis, but it would be of little use in the determination of adult specimens; and the tetrapodous character is of no avail for Apoda. Amphicondylous animals without an intracranial hypoglossal nerve is a more practical diagnosis.
In the case of living Urodela and Anura the absence of any scales in the skin affords a more popular character; it is unfortunately not applicable to the Apoda, many of which possess dermal scales, although these are hidden in the imbricating transverse rings of the epidermis; and the frequent occurrence of typical scales of both ecto- and meso-dermal composition in many of the Stegocephali forces us to discard the scales, or rather their absence, as a diagnostic character of the class Amphibia. The same applies to the mostly soft, moist, or clammy, and very glandular nature of the skin.
The position of the class Amphibia in the Phylum Vertebrata.–There is no doubt that the Amphibia have sprung from fish-like ancestors, and that they in turn have given rise to the Reptilia. The Amphibia consequently hold a very important intermediate position. It was perhaps not a fortunate innovation when Huxley brigaded them with the Fishes as Ichthyopsida, thereby separating them more from the Sauropsida (= Reptilia and Aves), than is justifiable,–perhaps more than he himself intended. The connecting-link, in any case, is formed by the Stegocephali; all the recent Orders, the Apoda, Urodela, and Anura, are far too specialised to have any claims to the direct ancestral connections. The line leading from Stegocephali to fossil Reptiles, notably to such Proreptilia as Eryops and Cricotus, and even to the Lepospondylous Prosauria, is extremely gradual, and the steps are almost imperceptible. Naturally, {6}assuming evolution to be true, there must have lived countless creatures which were a "rudis indigestaque moles," neither Amphibia nor Reptilia, in the present intensified sense of the systematist. The same consideration applies equally to the line which leads downwards to the Fishes. But the great gulf within the Vertebrata lies between Fishes and Amphibia, between absolutely aquatic creatures with internal gills and "fins," and terrestrial, tetrapodous creatures, with lungs and fingers and toes. On the side of the fishes only the Dipnoi and the Crossopterygii come into consideration.
The piscine descent of the Amphibia is still proclaimed by the following features.–(1) The possession by the heart of a long conus arteriosus, provided with, in many cases, numerous valves, or at least (Anura) one series at the base, another at the beginning of the truncus where the arterial arches branch off; (2) the strictly symmetrical arrangement of these arches; (3) the trilocular heart is still like that of the Lung-fishes or Dipnoi; (4) the occurrence of as many as four or even five branchial skeletal arches in the larval stage; (5) the glottis is supported by cartilages which themselves are derivatives of posterior visceral arches; (6) the development of the vertebrae (Stegocephali and Urodela) from four pairs of arcualia, and the formation of the intervertebral joints by a split across the intervertebral ring of cartilage: this feature is unknown in Reptilia, but it occurs also in Lepidosteus, most probably also in Polypterus; (7) the hypoglossal still retains the character of a post-cranial or cervical spinal nerve; (8) the presence of lateral sense-organs; (9) the possession of external gills is of somewhat doubtful phylogenetic value, although such gills occur amongst fishes only in Dipnoi and Crossopterygii. It is not unlikely that in the Amphibia these organs owe their origin to entirely larval requirements, while the suctorial mouth of the larvae of the Anura and many fishes has certainly no ancestral meaning, but is a case of convergent development.
The usual diagnoses of the Amphibia contain the statement that they, or most of them, undergo a metamorphosis, or pass through a larval stage. The same applies to various fishes; while, on the other hand, the larval (not ancestral) stage has become permanent in the Proteidae and Sirenidae; and lastly, we cannot well speak of larvae in the viviparous Salamandra atra.
The evolution of an adequate classification of the Amphibia has been a long process. Even their recognition as a class, separate from, and of equal rank with that of, the Reptilia, was by no means generally accepted until comparatively recent times. A historical sketch of the laborious, often painful, striving for light, in France and Germany, then in England, and lastly in America, is not without interest.
The term Amphibia was invented by Linnaeus for the third class of animals in his famous "Systema Naturae." It comprises a very queer assembly, which, even in the 13th edition (1767), stands as follows:–
1. Reptiles pedati, with the four "genera" Testudo, Draco, Lacerta, and Rana. Lacerta includes Crocodiles, Lizards, and Newts!
2. Serpentes apodes.
3. Nantes pinnati. Elasmobranchs, Sturgeons, Lampreys, and various other fishes.
Laurenti, 1768, in a dissertation entitled "Specimen medicum, exhibens Synopsin Reptilium ...," uses Brisson's term, Reptiles, and divides them into:–
Reptilia salientia, these are the Anura.
Gradientia, namely the Urodela and Lizards.
Serpentia, the Snakes and the Apoda.
Brongniart, 1800, "Essay d'une classification naturelle des Reptiles,"[2] distinguishes:–
Chelonii, Saurii, Ophidii, Batrachii; the last for the Frogs, Toads, and Newts.
Latreille, 1804, "Nouveau Dict. Hist. Nat." xxiv.,[3] accepts the four Orders of Brongniart's "Reptiles," but clearly separates the fourth Order, "Batrachii," from the rest by the following, now time-honoured, diagnosis: Doigts des pattes n'ayant pas d'ongles; des branchies, du moins pendant un temps; des métamorphoses. But there is not one word about "Amphibia" in opposition to "Reptilia."
Duméril, 1806, "Zoologie analytique" (p. 90), and "Élémens de l'histoire naturelle," 1807, divides the "Reptiles batraciens," or "Batracii," into Ecaudati and Caudati; he also introduces the terms "Anoures" and "Urodèles" as their equivalents; but since these terms appear in the French form purists do not admit their having any claim to recognition!
Oppel, 1811, "Die Ordnungen, Familien und Gattungen der Reptilien," establishes the term Apoda for the Coeciliae, and recognises their affinity to the Ecaudata and Caudata by removing them from the Snakes.
De Blainville, 1816, "Prodrome d'une nouvelle distribution du règne animal"[4]–
Amphibiens squamifères. [The Reptilia.]
Amphi"iens nudipellifères s. Ichthyoides. [The Amphibia.]
Merrem, 1820, "Tentamen systematis Amphibiorum."
Pholidota. [The Reptilia.]
Batrachia: APODA.
Batrachia: SALIENTIA.
Batrachia: GRADIENTIA { Mutabilia
[with metamorphosis, e.g.
Batrachia: GRADIENTIA { Newts.]
Batrachia: GRADIENTIA { Amphipneusta
[Perennibranchiate Urodeles.]
F. S. Leuckart, 1821, "Einiges ueber die fischartigen Amphibien."[5]
Monopnoa. [The Reptilia.]
Dipnoa. [The Amphibia] { with
temporary gills: Ecaudata + Caudata pt.
Dipnoa. [The Amphibia] { with permanent gills: "Proteidae,"
Menopoma
Dipnoa. [The Amphibia] { and Amphiuma.
Latreille, 1825, "Familles naturelles du règne animal." The Vertebrata are divided into Haematherma and Haemacryma. These terms for warm and cold-blooded creatures were later on amended by Owen to Haematotherma and Haematocrya. The latter are divided by Latreille as follows:–
Reptilia. Still including the Coeciliae amongst the Snakes.
Amphibia { Caducibranchiata.
Amphibia { Perennibranchiata.
Pisces.
Wagler, 1830, "Systema Amphibiorum."
Testudines, Crocodili, Lacertae, Serpentes, Angues, Coeciliae, Ranae, Ichthyodi.
Ranae I. AGLOSSA.
Ra"ae II. PHANEROGLOSSA: 1. Cauda nulla. [The Anura.]
Ra"ae II. PHANER" GLOSSA: 2. Cauda distincta. [The Salamandridae.]
Ichthyodi I. ABRANCHIALES. Menopoma [Cryptobranchus] and Amphiuma.
Icht"yodi II. BRANCHIALES. [The Perennibranchiate Urodela.]
J. Müller, 1831, "Beiträge zur Anatomie ... der Amphibien."[6]
Gymnophiona, Derotremata, Proteidae, Salamandrina, Batrachia.
J. Bell, 1836, Todd's "Cyclopaedia of Anatomy and Physiology," Art. "Amphibia."
Amphipneusta, the Perennibranchiate Urodeles; Anoura, Urodela;
Abranchia, Menopoma and Amphiuma; Apoda.
Stannius, 1856, "Handbuch der Zootomie: Anatomie der Wirbelthiere." (2nd ed.)
Amphibia Monopnoa. The Reptilia.
Amphibia Dipnoa. 1. Urodela.
PERENNIBRANCHIATA.
Amphibia Dipnoa. 1. Urodela. DEROTREMATA: Amphiuma and
Menopoma.
Amphibia Dipnoa. 1. Urodela. MYCTODERA.[7]
{9}Amphibia Dipni. 2. Batrachia. AGLOSSA.
Amphibia Dipnoa. 1. Urodela. PHANEROGLOSSA: Systomata =
Engystomatidae.
Amphibia Dipnoa. 1. Urodela. Bufoninae. Without manubrium sterni.
Amphibia Dipnoa. 1. Urodela. Raninae. With manubrium.
Amphibia Dipnoa. 1. Urodela. Hyloidea. With adhesive finger-discs.
Amphibia Dipnoa. 1. Urodela. GYMNOPHIONA.
Gegenbaur, 1859, "Grundzüge der vergleichenden Anatomie."
Amphibia as a separate class, equivalent to that of the Reptilia, are divided into the four Orders: PERENNIBRANCHIATA, SALAMANDRINA, BATRACHIA, and GYMNOPHIONA. In the second edition of the "Grundzüge" (1870) they are divided into URODELA, ANURA, and GYMNOPHIONA.
Huxley, 1864, "The Elements of Comparative Anatomy."
Mammals.
Sauroids, subsequently changed into Sauropsida
= Reptilia + Aves.
Ichthyoids, bseque"tly
chan"ed into Ichthyopsida = Amphibia +
Pisces.
Haeckel, 1866, "Generelle Morphologie."
Amphibia. A. Phractamphibia s. Ganocephala =
Labyrinthodonta + Peromela [Apoda].
Amphibia. B. Lissamphibia s. Sozobranchia =
Sozura [Urodela] + Anura.
Cope, 1869.[8]
Stegocephali, Gymnophidia, Urodela, Proteidea, Trachystomata, Anura.
Huxley, 1871, "A Manual of the Anatomy of Vertebrated Animals."
Amphibia I. Saurobatrachia [v.d. Hoeven's term] s.
Urodela
Amphibia 1.
Proteidea.
Amphibia 2.
Salamandridae.
II. Labyrinthodonta.
III. Gymnophiona.
IV. Batrachia s.
Anura.
Boulenger, 1882, "Catalogue of the Batrachia Gradientia s. Caudata and Batrachia Apoda," divides the Caudata simply into: SALAMANDRIDAE, AMPHIUMIDAE, PROTEIDAE, and SIRENIDAE.
1882, "Cat. Batrachia Salientia s. Ecaudata," see p. 140.
Cope, 1890, "Synopsis of the Families of Vertebrata."[9]
Class Batrachia.
IISub-Class I. Stegocephali.
Order 1. Ganocephali: Trimerorhachis,
Archegosaurus.
Order 2. Rhachitomi:
Eryops.
Order 3. Embolomeri:
Cricotus.
Order 4. Microsauri:
Branchiosaurus, Hylonomus, etc.
{10} ISub-Class II. Urodela.
Order 1. Proteidae: Proteus.
Order 2.
Pseudosauria. [All the rest of the Urodela + Coeciliidae.]
Order 3.
Trachystomata: Sirenidae.
Sub-Class III. Salientia.
P. and F. Sarasin, 1890, "Zur Entwicklungsgeschichte der Ceylonesischen Blindwühle, Ichthyophis glutinosa."[10]
Sub-Class I. Archaeobatrachi s. Stegocephali.
Sub-ClassII. Neobatrachi.
Order 1. URODELA.
Order 1. a.
Salamandroidea. [The Urodela.]
Order 1. b.
Coeciloidea = Amphiumidae + Coeciliidae.
Order 2.
ANURA.
The classification adopted in this volume is as follows:–
Class Amphibia.
Sub-Class I. Phractamphibia.
Order III.
Stegocephali Lepospondyli.
Sub-order 1. Branchiosauri.
Sub-order 2. Aistopodes.
Order III.
Stegocephali Temnospondyli.
Order III. Stegocephali Stereospondyli.
Sub-Class II. Lissamphibia.
Order III.
Apoda.
Order III.
Urodela.
Order III. Anura.
Sub-order 1. Aglossa.
Sub-order 2. Phaneroglossa.
SKELETON OF URODELA AND ANURA–SKIN–COLOUR-CHANGING MECHANISM–POISON-GLANDS–SPINAL NERVES–RESPIRATORY ORGANS–SUPPRESSION OF LUNGS–URINO-GENITAL ORGANS–FECUNDATION–NURSING HABITS–DEVELOPMENT AND METAMORPHOSIS
Skeleton of the Urodela
The vertebral column.–The number of vertebrae is smallest in the terrestrial, greatest in the entirely aquatic forms, and is exceptionally large in the eel-shaped Amphiuma. In the following table the sacral vertebra is included in those of the trunk.
| Trunk. | Tail. | |
| Siren lacertina | 22 | 35 + |
| Necturus maculatus | 19 | 29 |
| Proteus anguinus | 30 | 28 + |
| Cryptobranchus alleghaniensis | 20 or 21 | 24 + |
| C. scheuchzeri | 21 | |
| C. japonicus | 22 | 22 to 26 |
| Amphiuma means | 63 | 35 + |
| Amblystoma tigrinum | 17 or 16 | 32 + |
| Salamandra maculosa | 17 | 27 |
| Triton cristatus | 17 | 36 |
| Triton taeniatus | 14 or 15 | 36 + |
| Triton palmatus | 14 | 23 to 25 |
| Salamandrina perspicillata | 15 | 32 to 42 |
| Spelerpes fuscus | 16 | 23 |
The vertebrae of the Urodela and those of the Apoda differ from those of all the other Tetrapoda[11] by possessing no special centra or bodies. That part which should correspond with the centrum is formed either by the meeting and subsequent complete co-ossification of the two chief dorsal and ventral pairs of arcualia {12}(tail-vertebrae), or entirely by the pair of chief dorsal arcualia. There is consequently no neuro-central suture. Moreover, the central region of each vertebra is strongly pinched in laterally, widening towards the ends. Another feature of the vertebral column of the Urodela is the possession of a considerable amount of intervertebral cartilage, by which the successive vertebrae are held together. This cartilage does not ossify, and it either remains continuous, serving in its entirety and owing to its flexibility as a joint, or it becomes more or less imperfectly separated into a cup and ball portion, the cup belonging to the posterior end of the vertebra. Such joints are called opisthocoelous, and occur in the Desmognathinae and Salamandrinae. In the adult the cup and ball frequently calcify, and the chorda dorsalis or notochord is completely destroyed. Those vertebrae between which the intervertebral cartilage remains unbroken, are called amphicoelous, since in them, most obviously in macerated or dried skeletons, the vertebrae appear hollowed out at either end. In such amphicoelous vertebrae a considerable amount of the chorda always remains, running in an unbroken string through the whole length of the vertebral column. Towards adult life the chorda becomes constricted, and is ultimately squeezed out or destroyed, in the middle of the vertebra, by the invasion of cartilage from the chief arcualia. This intravertebrally situated cartilage has been described erroneously as chordal cartilage.
The development of the vertebrae proceeds as follows. First appear a pair of basidorsalia and a pair of basiventralia (Fig. 1, 1, B.D, B.V), blocks of cartilage, imbedded in and resting upon the thin sheath of the chorda dorsalis. Next appears a pair of interdorsal blocks, immediately behind the basidorsals; and somewhat later appears a pair of interventral blocks. These four pairs of cartilages or "arcualia" each meet, above or below the chorda, and form semi-rings, which again by extending upwards or downwards fuse into complete rings, in such a way that the interdorsal and interventral elements form the intervertebral mass spoken of above. The basidorsals fuse with the basiventrals, and form the body of the vertebra, the fusion being effected chiefly by the calcification and ossification of the lateral connecting portion of the skeletogenous layer. The basidorsalia form the neural arches with their unpaired short spinous or neural, and the paired anterior and posterior zygapophysial processes. Concerning the {13}basiventralia we have to distinguish between the trunk and the tail. In the latter they produce a pair of ventral outgrowths or haemapophyses, which ultimately enclose the caudal blood-vessels. In the trunk the basiventral blocks of cartilage are suppressed; they appear in the early larvae, but disappear during or even before metamorphosis.
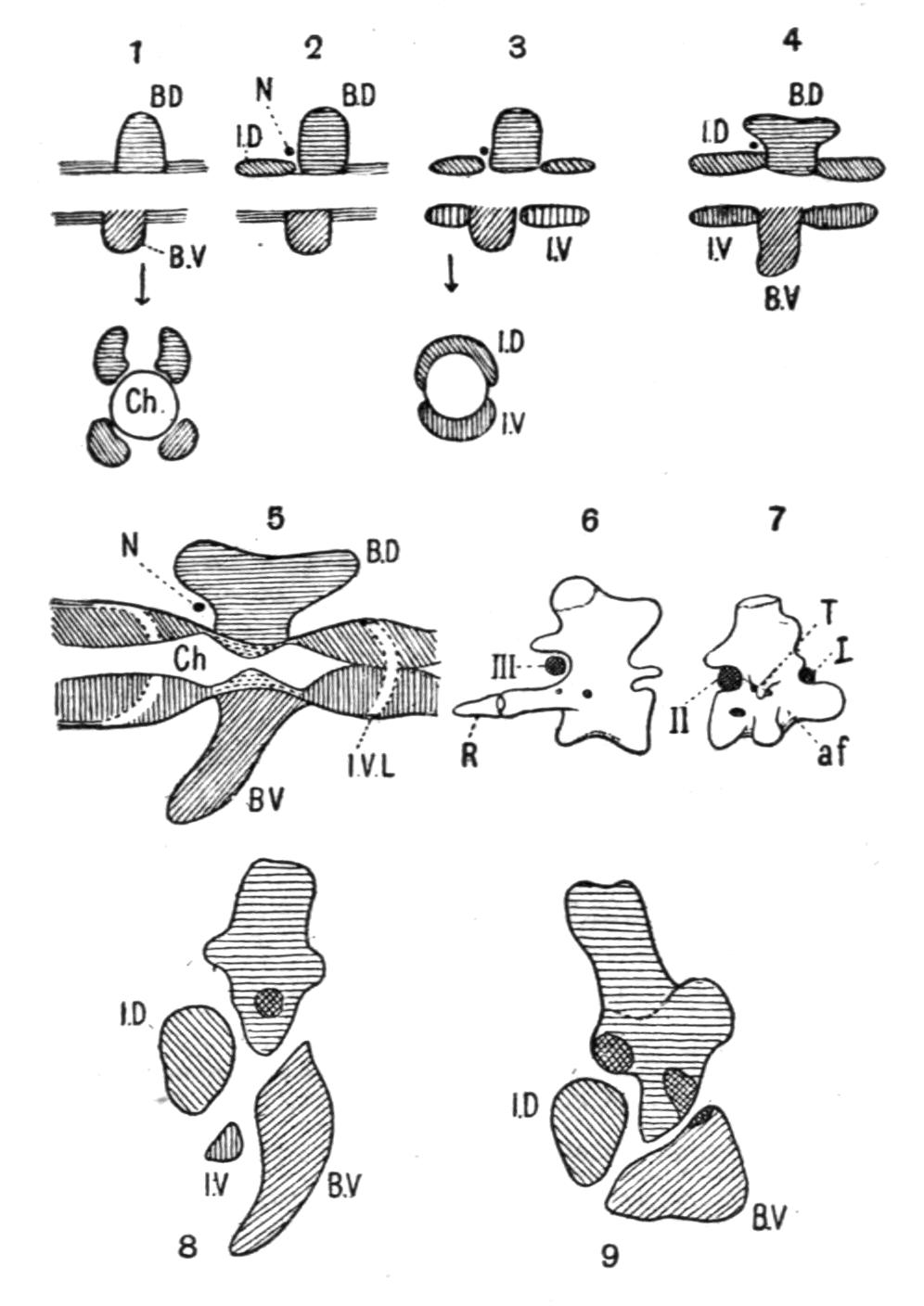
Fig. 1.–1-5, Five successive stages of the development of a caudal vertebra of a newt; 6-7, the second and the first cervical vertebra of Cryptobranchus; 8-9, side view of the constituent cartilaginous blocks of a caudal vertebra (8) and a trunk-vertebra (9) of Archegosaurus as typical examples of Temnospondylous quadripartite and tripartite vertebrae. The cross-hatched parts indicate the articular facets for the ribs. The anterior end of all the vertebrae looks towards the right side. af, In 7, articulating facet for the occipital condyle; B.D, basidorsal piece or neural arch; B.V, basiventral piece or ventral arch; Ch, chorda dorsalis, or notochord; I.D, interdorsal piece; I.V, interventral piece; I.V.L, intervertebral ligament; N, spinal nerve–these are numbered I, II, III in 6 and 7; R, rib; T, in 7, rib-like tubercle on the first vertebra.
Towards the end of the tail the vertebrae diminish in size, and their constituent cartilages assume a more and more indifferent shape, until they become confluent into a continuous rod of cartilage, resembling in this respect the Dipnoi and Holocephali. A periodical revival of this rod, at least of its connective tissue, appears in the tail-filament of the male Triton palmatus during the breeding-season.
The first vertebra, called the atlas, because it carries the head, is remarkable for the possession of an odontoid process. The latter is formed by a pair of cartilages and represents part of a vertebra, the dorsal portion of which seems to have been added to the occipital part of the cranium.
All the trunk-vertebrae, with the exception of the atlas, carry ribs, at least vestiges thereof. Owing to the early disappearance of the basiventral cartilages the capitular portions of the ribs are much reduced, and are mostly represented by strands of connective tissue only. The ribs develop therefore occasionally at some distance from the vertebral column, and that portion of the rib which in the metamorphosed young newt looks like the capitulum is to a great extent really its tuberculum. Witness the position of the vertebral artery, which still indicates the true foramen transversarium. The homologies of these parts are still more obscured by the fact that a new process grows out from the rib, by which the latter gains a new support upon a knob of the neural arch. Thus an additional foramen is formed, sometimes confounded with the true transverse canal. The meaning which underlies all these modifications is the broadening of the body, the ribs shifting their originally more ventral support towards the dorsal side. The whole process is intensified in the Anura; it is an initial stage of the notocentrous type of vertebrae. The transverse ossified processes of the adult are often much longer than the vestiges of the ribs themselves, and are somewhat complicated structures. They are composed first of the rib-bearing cartilaginous outgrowths of the neural arches; secondly, of a broad string of connective tissue which extends from the ventro-lateral corner of the perichordal skeletogenous layer to the ribs.
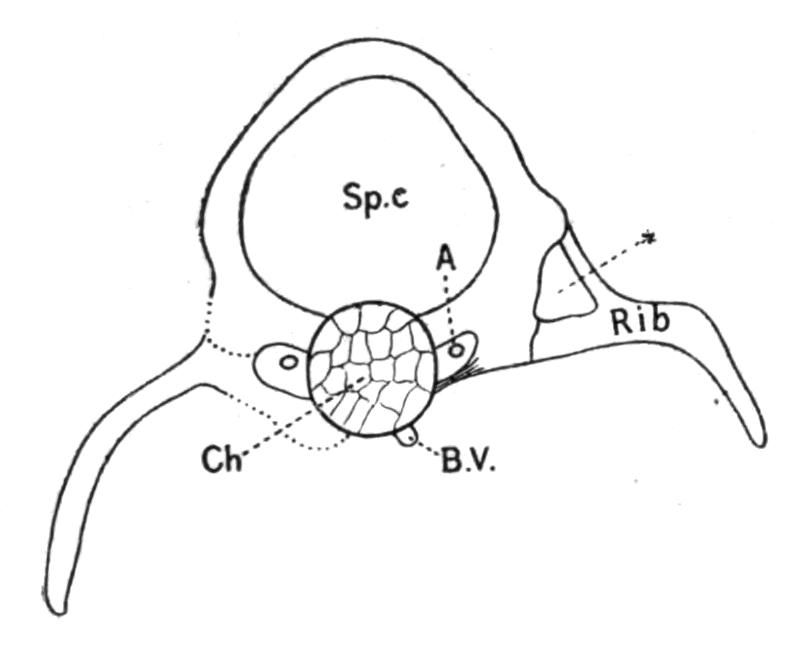
Fig. 2.–Transverse section through a trunk-vertebra of a larva of Salamandra maculosa, enlarged. The right side shows the actually existing state, while on the left side the rib and its attachments are restored to their probable original condition. A, Vertebral artery within the true transverse canal; B.V, remnant of the basi-ventral cartilage; Ch, chorda dorsalis; Sp.c, spinal canal; *, the false transverse canal.
The shoulder-girdle is extremely simple. It remains almost entirely cartilaginous, and the three constituent elements are not separated by sutures. Ossification is restricted to the base of the shaft of the scapula, and may extend thence over the glenoid cavity. The coracoids are broad, loosely overlap each other, and are "tenon and mortised" into the triangular or lozenge-shaped {15}cartilaginous sternum, which latter has no connection with the ribs. The precoracoid is a large, flat process, directed forwards, not meeting its fellow; it is absent in Siren.
The humerus articulates with both radius and ulna, and these two bones of the forearm remain separate. The elements which compose the wrist and hand exhibit an almost ideally simple arrangement, slightly varied by the frequent fusion of two or more neighbouring carpalia into one, and by the reduction of the number of fingers. Most frequently the intermedium and the ulnar carpal element fuse together, and there is more often one centrale instead of two. The wrist and hand of the Urodela represent, however, no longer the entirely primitive pentadactyle type, owing to the loss of one finger together with its metacarpal and carpal element. Comparison with the Anura makes it probable that the Urodela have lost the pollex, their four fingers being consequently the 2nd, 3rd, 4th, and 5th. Siren has four or three fingers; Proteus has only three fingers and three large compound carpal cartilages. In Amphiuma, with either three or two fingers, the ulnare, intermedium, and carpale are fused together, the radiale with the neighbouring carpale. The number of phalanges in the four-fingered species is generally 2, 3, 3, 2 respectively.
The pelvic girdle.–The ilium stands vertically to the vertebral axis, slanting slightly forwards and downwards. It is attached by means of a rib to only one vertebra, and this ilio-sacral connection is acetabular in its position, i.e. it lies in the same transverse plane with the acetabulum, in other words vertically above it. The ventral portion of the pelvis is formed by one large continuous mass, the united pubo-ischia, the anterior or pubic portion of which extends forwards in the shape of a broad triangle (Necturus) or as a slender, stalked, Y-shaped cartilage, the epipubis, which is often movably jointed at its base. The lateral portion of the pubic cartilage is always perforated by the nervus obturatorius. Ossification is restricted to the ischium and to the middle of the shaft of the ilium. The acetabular fossa for the femur is closed. The tibia and fibula remain separate. The foot is still more primitive than the anterior extremity, as the majority of Urodela possess the full complement of five toes, with 2, 2, 3, 3, 2 phalanges respectively. Concrescence of the tarsalia applies most frequently to the fourth and fifth distal {16}and to the two centralia; exceptional, for instance, in Cryptobranchus japonicus, are as many as three centralia, but this is an individual, even a one-sided variation, as shown for instance by a specimen in the Cambridge Museum. Loss of the fifth toe occurs sporadically in genera of different groups, namely, in Salamandrella, Batrachyperus, Salamandrina, Necturus, Manculus, Batrachoseps. In Amphiuma the number is reduced to three or two; in Proteus to two; and in Siren the hind limbs, with their girdle, are altogether absent. Lastly, in some species of Spelerpes and Batrachoseps both fore and hind limbs have become so small as to be practically without function, parallel cases being found among various Scincidae and other Lizards.
The hyoid apparatus is still very primitive in many, especially in larval, Urodela. Besides the hyoid there are as many as four pairs of branchial arches, which, however, decrease in size and completeness, so that the last two have lost their connection with the median copular piece, and become attached in various ways to the second branchial arch. This is the arrangement apparently in all larvae, but four pairs of branchials persist in the adult Siren, Amphiuma, and Cryptobranchus alleghaniensis. The whole branchial apparatus is reduced to three pairs of arches in Necturus and Proteus, to two in the adult Cryptobranchus japonicus and in the Salamandridae. Of considerable interest is the vestige of a fifth pair of arches in the larvae of Triton and Salamandra, in the shape of a pair of tiny cartilages, which lie in front and on each side of the opening of the trachea, and give rise to the formation of the laryngeal cartilages, better developed in the higher Vertebrata.
The following are noteworthy characters of the skull of Urodela. The articulation of the skull with the vertebral column is not always effected entirely by the two condyles of the lateral occipital bones, but the median basal cartilage often possesses a pair of facets for the odontoid-like process of the first vertebra; such additional facets are perhaps best developed in Cryptobranchus and in the Salamandrinae.
The middle portion of the primitive cranium, from the exit of the optic nerve to the ethmoid cartilage, is formed by a pair of separate bones, the orbito-sphenoids. The parietal and frontal bones remain separate. One or more periotic bones exist, besides the prootic, in the aquatic families.
A pair of prefrontal bones is present in most Salamandridae, e.g. Salamandra, Triton, Amblystoma, especially in the larva, and in Cryptobranchus; these bones are absent in Amphiuma, Necturus, Proteus, and Siren.
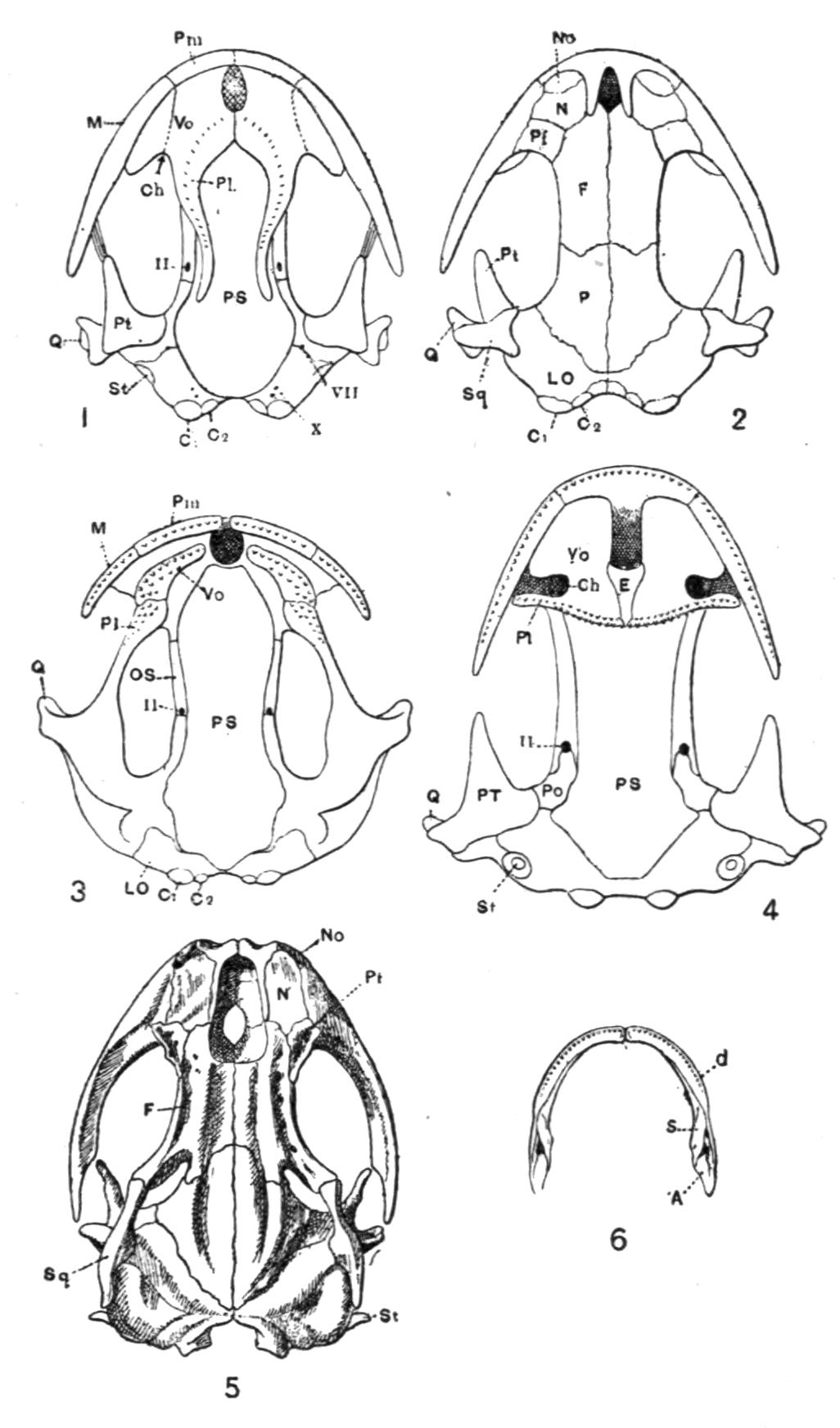
Fig. 3.–Skulls of various Urodela. 1, Salamandra maculosa, ventral view, and 2, dorsal view; 3, Axolotl stage of Amblystoma; 4, adult stage of Amblystoma; 5, Salamandrina perspicillata (after Wiedersheim); 6, Salamandra maculosa, dorsal view of the lower jaw. A, Articulare; C1, C2, outer and inner occipital condyles; Ch, choana or posterior nasal opening; d, dentary; E, ethmoid; F, frontal; LO, lateral occipital; M, maxillary; N, nasal; No, nostril; OS, orbito-sphenoid; P, parietal; Pf, prefrontal; Pl, palatine; Pm, premaxillary; Po, prootic; PS, parasphenoid; Pt, pterygoid; Q, quadrate; S, angulo-splenial; Sq, squamosal; St, stapes; Vo, vomer; II, VII, X, exits of the optic, facial, and glosso-vagus nerves.
The lacrymalia are still separate in some Amblystomatinae, e.g. Ranidens and Hynobius. A pair of nasalia are generally present, but are absent in Necturus, Proteus, and Siren. The parasphenoid is furnished with teeth in the Plethodontinae and Desmognathinae.
Separate palatine bones exist in Necturus and Proteus, and in the larva of Amblystoma, but in the adult form they fuse with the vomers, producing the vomero-palatines characteristic of the majority of Urodela.
The pterygoid bones are most fully developed, so as to reach the vomero-palatines, in the Amblystomatinae, in Necturus, and in Proteus; they are reduced, so as to leave a gap, in Cryptobranchus, and still more in the Salamandrinae; they are absent in Amphiuma and in Siren.
The quadrates are directed forwards in Necturus, Proteus, and Siren, while in the other Urodela they extend transversely and almost horizontally. The hyomandibular remnant, the so-called operculum, is small, and forms a plate which fits into the fenestra ovalis, extending as a ligamentous process upon the quadrate.
The quadrato-jugal elements are reduced to ligaments. In many Salamandrinae the large orbito-temporal space is divided into an orbital and a temporal fossa by an arch which is formed by the meeting of two corresponding processes from the squamosal and frontal bones respectively. This bridge is rarely bony (Salamandrina, Triton), mostly ligamentous;–apparently a reminiscence of the Stegocephalous condition. The two premaxillary bones are liable to fuse into one, for instance in Cryptobranchus, generally in adult Tritons. They are most reduced, and are toothless, in Siren.
The two maxillary bones are absent only in Necturus, Proteus, Typhlomolge, and Siren. Their posterior end is frequently free, loosely connected by ligaments with the pterygoid in Cryptobranchus; or with the distal portion of the quadrate, and in this case either just touching it (Tylototriton), or forming a broad junction (Pachytriton).
Each half of the lower jaw consists of a dentary, articular and angulo-splenial. The splenial remains as a separate element in Siren; in others only during the larval period. There are no mento-Meckelian elements.
Skeleton of the Anura
The vertebral column.–The distinctive peculiarities of the vertebrae of the Anura are that they are notocentrous, and that about a dozen of them are modified and fused into an os coccygeum. The whole column is the most specialised found in the Vertebrata; and various stages are rapidly hurried through and obscured caenogenetically during the embryonic development. Paired cartilages appear on the dorsal side of the thin chordal sheath, and whilst tending to enclose the spinal cord in a {19}canal, their bases grow head- and tail-wards into what will ultimately become the intervertebral region. This extension of cartilage leads to a fusion with that of the next following pair of arches, so that the axial column at this early stage consists of a right and left longitudinal ridge of cartilage which sends off dorsal processes, neural arches, in metameric succession. Next, the intervertebral cartilage increases in such a way as to constrict the chorda either laterally (Rana) or obliquely from above downwards and inwards (Bufo, Hyla). We recognise in this cartilage the interdorsalia. Ventral arcualia are late and much obscured. There is scarcely any cartilage which could represent the interventralia, the intervertebral cartilage being almost entirely made up of the interdorsalia. These fuse together and form a disc or nodule, which later fuses either with the vertebra in front, and in this case fits into a cup carried by the vertebra next behind (procoelous vertebrae), or the knob is added to the front end of the vertebra, fitting into a cup formed by the tail end of the vertebra next in front (opisthocoelous vertebrae). Much later than the two longitudinal dorsal bands there appears on the ventral side an unpaired band in which appear metamerically repeated swellings of cartilage, likewise unpaired. These swellings become confluent, in a way similar to that which produced the dorsal bands, and form the unpaired ventral band of cartilage, the hypochordal cartilage of some authors. The swellings in this band, equivalent to the basiventralia, become semilunar in a transverse view, their horns tending upwards towards the basidorsal cartilages, but there is no actual meeting. Both dorsal and ventral elements are, however, joined together and form the chief portion of the vertebrae, owing to the rapidly proceeding calcification and later ossification of the all-surrounding "membrana reuniens" or skeletogenous layer so far as that is not cartilaginous.
Procoelous vertebrae exist in the overwhelming majority of Anura; opisthocoelous are those of the Aglossa, the Discoglossidae, and of some Pelobatidae. The systematic value of this pro- or opistho-coelous character has been much exaggerated. We have seen that the centra of the vertebrae of the Anura are formed entirely by the interdorsal elements, hence the term "notocentrous," and these centra sometimes remain in adult specimens of Pelobates as separately ossified and calcified pieces, {20}not fused with the rest of the vertebrae. This important discovery has been made by Boulenger, but Stannius had previously mentioned a specimen of Pelobates in which the second and fourth vertebrae are biconvex, the third, sixth, and eighth biconcave. Moreover, since the sacral vertebra, generally the ninth, in all the Anura is invariably biconvex, the eighth being biconcave in the procoelous families, opisthocoelous like the remaining seven vertebrae in the other families, it is not difficult to imagine that in the Anura the production of pro- or opistho-coelous vertebrae depends simply upon the centra or articulating knobs happening to fuse either with the hind or the front end of the vertebrae. This must of course ultimately be determined by a mechanical problem of motion.
A second type of the vertebrae amongst the Anura is the epichordal type, an exaggeration in degree of the notocentrous tendencies of the more usual perichordal arrangement. It shows, namely, the almost complete suppression of all the ventral cartilaginous elements, so that the chorda remains for a long time on the ventral surface of the axial column in the shape of a flattened longitudinal band. These two types are not unconnected. The suppression of the ventral elements applies most typically to the trunk region, while hypochordal cartilage exists in the anterior cervical vertebrae, and above all in the coccyx. Typically epichordal are the vertebrae of Pipa, Xenopus, Bombinator, Pelobates, Discoglossus and Alytes. It is significant that the epichordal often coincide with opisthocoelous vertebrae, and still more suggestive is the fact that Bombinator is eminently aquatic, Pipa and Xenopus entirely so, having lost the tympanum, at least externally. The epichordal feature is not necessarily indicative of relationship. It has probably been developed independently in various groups, in correlation with a resumption of aquatic life. Various genera of Pelobatidae and most likely some Cystignathidae, e.g. Pseudis, will not improbably connect the two types and their several correlated features, for instance, the frequent reduction of the tympanic cavity.
The os coccygeum has retained rather primitive features in so far as much dorsal and ventral cartilage is developed; but this has almost entirely lost its metameric arrangement, and the posterior half of the coccyx is formed chiefly by the ventral mass of cartilage, while the dorsal elements are more or less reduced. {21}Only two vertebrae, generally the tenth and eleventh of the whole column, are clearly visible, each being composed of a pair of dorsal and a pair of ventral cartilaginous blocks. The sacral vertebra articulates with the coccyx by one or two convexities, but in the Aglossa, in some Pelobatidae, and a few others, the coccyx is fused with the sacral vertebra. Beyond the first and second component vertebrae of the embryonic coccyx, the cartilage is continued in the shape of two dorsal, and one ventral, bands, which soon fuse with each other. Dorsally this cartilage surrounds the spinal cord; the latter degenerates towards the end of the tadpole-stage, leaving, however, the empty spinal canal. The chorda, completely surrounded by cartilage, persists into the post-larval stage, but is destroyed long before the creature attains maturity. Ultimately the whole coccyx ossifies.
The tail proper, namely that portion which is absorbed during the metamorphosis, remains throughout its existence in an apparently primitive condition. The chorda dorsalis and the spinal cord extend through its whole length, surrounded by continuous connective tissue without any cartilage; in fact it represents a piece of typical vertebral column before the appearance of cartilage. The reduction of this swimming organ begins at the hind end.
The vertebral column of the adult.–The first vertebra (we will call it the atlas since it carries the skull) is not, as in the Urodela, provided with an odontoid process. It articulates by two cups with the condyles of the occiput. In some Anura it co-ossifies, rather incompletely, with the second vertebra, regularly in the fossil Palaeobatrachus, often in Ceratophrys, Breviceps, and occasionally in Pelobates, Bufo, Rana, and Xenopus. This is, however, no justification for looking upon the first vertebra as a complex of two vertebrae, although the atlas is frequently very thick and broad, and even carries, in the Aglossa, considerable lateral wings or diapophyses. Those of the trunk-vertebrae are often very long, acting thereby as substitutes for ribs which are absent, except on the second, third, and fourth vertebrae of the Discoglossidae, and on the second and third of the Aglossa. In the adult Aglossa these ribs fuse with the processes which carry them.
The diapophyses of the sacral vertebra carry no ribs, the ilia being attached to them directly. They are either cylindrical {22}as in the Ranidae and Cystignathidae, or they are more or less dilated as in all the other families, most strongly in the Pelobatidae and the Aglossa. In some members of the large sub-family of the Cystignathidae the otherwise cylindrical diapophyses are slightly dilated.
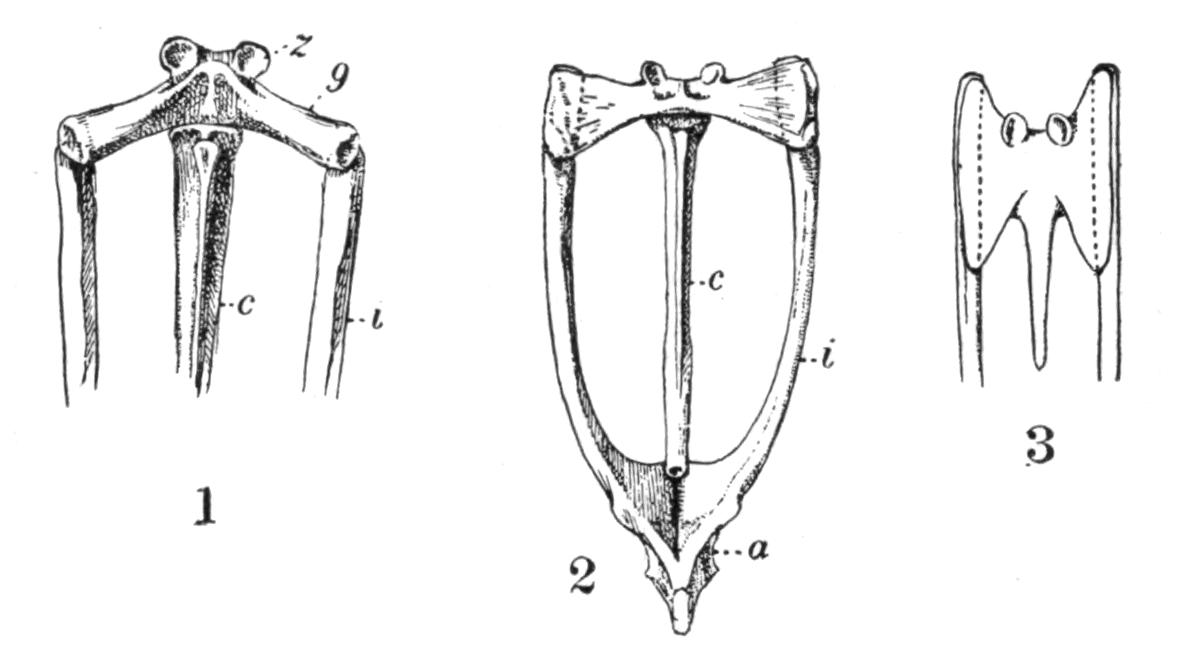
Fig. 4.–Dorsal view of the sacral or ninth vertebra (9), with the attachment of the ilium, of (1) Rana temporaria, (2) Bufo vulgaris, showing the whole coccyx and pelvis, (3) Pelobates fuscus, as examples of cylindrical and of dilated sacral diapophyses. (About nat. size.) a, Acetabulum; c, coccyx; i, ilium; z, anterior zygapophyses.
The sacrum is formed by the ninth vertebra, but there are a few interesting exceptions. Pelobates, Pipa, and Hymenochirus possess two sacral vertebrae; and, neglecting individual abnormalities, these three genera form the only exception amongst recent Amphibia. In the three genera the coccyx is fused with the second sacral vertebra, and such a fusion occurs elsewhere normally only in Bombinator with its single sacral vertebra. The morphologically oldest condition is normally represented by Pelobates, the sacral vertebrae being the tenth and ninth. One case has been recorded by Boulenger of Bombinator pachypus "with eleven segments," the last carrying the ilium. Individual lop-sided abnormalities have been described in Bombinator and Alytes, where the right ilium articulated with the tenth, the left ilium with the ninth vertebra. This shifting forwards of the ilium to the extent of one metamere has been continued further in Pipa, in which the sacrum is formed by the ninth and eighth vertebrae, their diapophyses fusing on either side into extra broad wing-like expansions. In old specimens of Palaeobatrachus fritschi the seventh vertebra is in a transitional condition, the ilium being carried by the ninth and eighth, and slightly also by the diapophyses of the seventh vertebra; and in P. diluvianus the {23}diapophyses of all these vertebrae are united into one broad plate to which the ilia are attached. Lastly, in Hymenochirus the first sacral is the sixth vertebra, and this creature has thereby reduced the pre-sacral vertebrae to the smallest number known.
This shifting forwards of the iliac attachment implies the conversion of original trunk into sacral vertebrae, and the original sacral vertebra itself becomes ultimately added to the urostyle. The second sacral, the tenth of Pelobates, the ninth of Pipa, and the tenth on the right side of the abnormal Bombinator, are still in a transitional stage of conversion. In Discoglossidae the tenth is already a typical post-sacral vertebra, and is added to the coccyx, but it still retains distinct, though short, diapophyses. In the majority of the Anura the tenth vertebra has lost these processes, and its once separate nature is visible in young specimens only. In Bombinator even the eleventh vertebra is free during the larval stage. In fact the whole coccyx is the result of the fusion of about twelve or more vertebrae, which from behind forwards have lost their individuality. We conclude that originally, in the early Anura, there was no coccyx, and that the ilium was attached much farther back; and this condition, and the gradual shifting forwards, supply an intelligible cause of the formation of an os coccygeum. The fact that the sacral vertebrae of the Anura possess no traces of ribs as carriers of the ilia, is also very suggestive. The ilia have shifted into a region, the vertebrae of which had already lost their ribs. By reconstructing the vertebral column of the Anura, by dissolving the coccyx into about a dozen vertebrae, so that originally, say the twenty-first vertebra carried the ilia, we bridge over the enormous gap which exists between the Anura and Urodela. That whole portion of the axial continuation behind the coccyx, more or less coinciding with the position of the vent, is the transitional tail.
The disappearance of both notochord and spinal cord, and the conversion of the cartilaginous elements into a continuous rod in the case of the os coccygeum, find an analogy in the hinder portion of the tail of Dipnoi and Crossopterygii, and in the tail-end of most Urodela, portions which are not homologous with the os coccygeum. The term urostyle should be restricted to such and similar modifications of the tail-end, and this latter happens to be lost by the Anura during metamorphosis.
Strictly speaking, or rather in anatomical parlance, the Vertebrate tail begins with the first post-sacral vertebra. In the Anura that portion of the whole tail has retained most cartilage, and has become the coccygeum, which is required as a "backbone" for the often enormous belly. This requirement is an outcome of the great shortening of the trunk proper (if the trunk be defined as ending with the pelvic region), and this shortening of the trunk is again intimately connected with the jumping mechanism, enlargement of the hind-limbs, elongation of the ilia, and throwing the fulcral attachment forwards as much as possible. The pre-acetabular ilio-sacral connection is carried to the extreme in the Anura.
The shoulder-girdle and "sternum" are more complete than in the Urodela, there being also a pair of clavicles, fused with the precoracoidal bars. The whole apparatus presents two types. In the arciferous type the coracoids and precoracoids retain a great amount of cartilage in their distal portions, and these cartilages (the epicoracoids of some authors) overlap each other movably on one another, the right usually lying ventrally upon the left. The epicoracoidal cartilage of each side, by connecting the distal end of the coracoid with the precoracoid of the same side, forms an arc, hence "arciferous." In the firmisternal type the epicoracoidal cartilages are much reduced, and, instead of overlapping, meet in the middle line and often fuse with each other, forming thereby a firm median bar, which connects the ventral ends of the precoracoids with those of the coracoids. This type is morphologically the higher and more recent, and passes in the larval stage through the arciferous condition. It is restricted to the Ranidae, Engystomatinae, and Aglossa. Although these two types afford an excellent distinctive character for the main divisions of the Anura, they are to a certain extent connected by intermediate forms in such a way, that, for instance, in Bufo and among Cystignathidae in Ceratophrys, the two opposite epicoracoidal cartilages begin to unite at the anterior end.
In many Engystomatinae the precoracoids together with the clavicles are much reduced, sometimes to thin ligaments, being in this case mostly curved back and lying closely against the coracoids; or they may be lost completely. Very rarely the precoracoidal bars are actually much stronger than the coracoids, {25}and the median symphysial bar of cartilage is lost; this is the case in Hemisus.
The scapula is always large and curved into transverse, dorsally broadening blades, the dorsal greater portion of which, the so-called supra-scapula, does not ossify but calcifies.
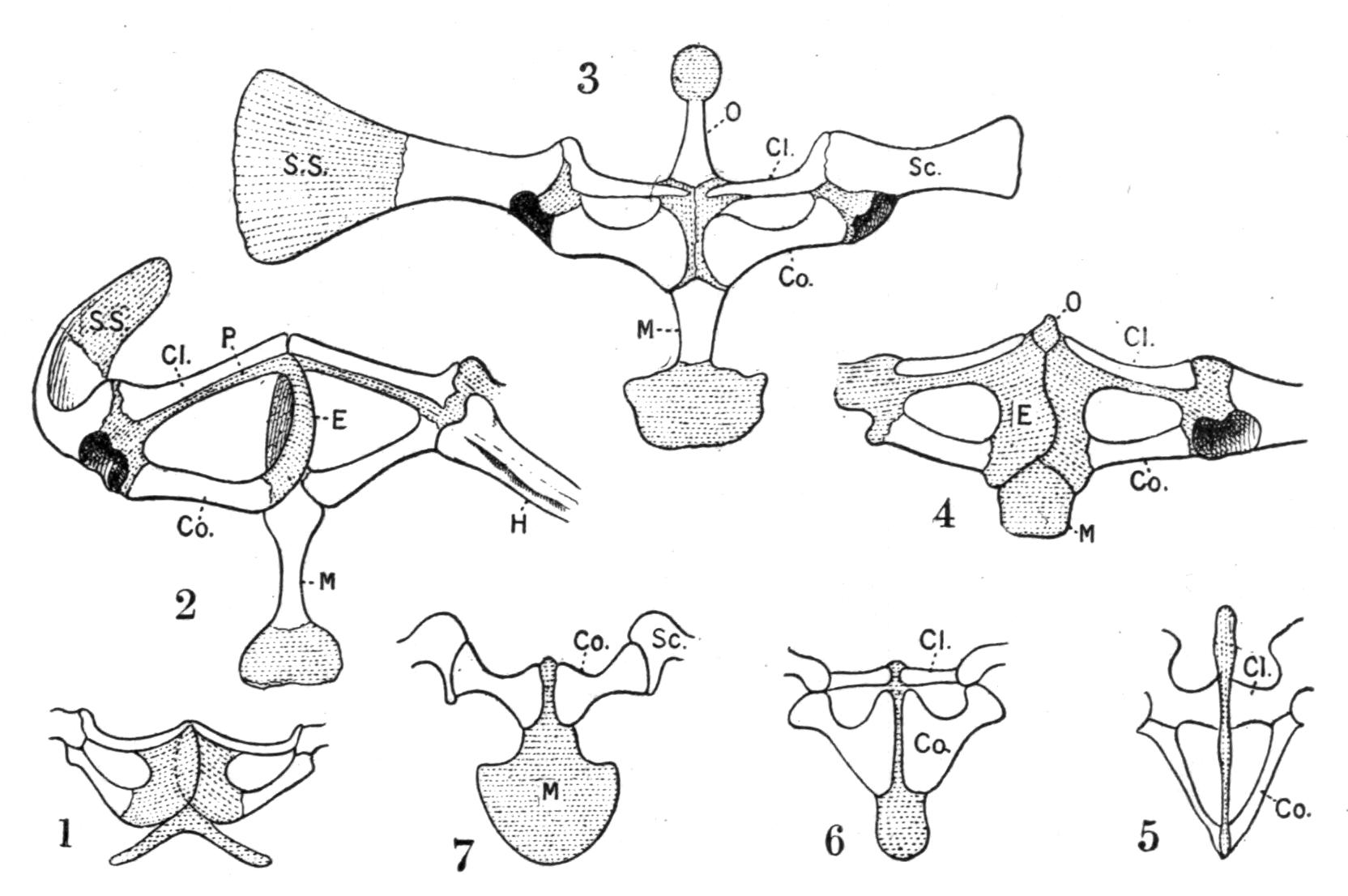
Fig. 5.–Ventral views of the shoulder-girdles of various Anura. (Slightly enlarged.) 1, Bombinator igneus, and 2, Bufo vulgaris, as examples of the arciferous type; 3, adult, 4, metamorphosing Rana temporaria showing change from the arciferous into the firmisternal type; 5, Hemisus guttatum; 6, Breviceps gibbosus; 7, Cacopus systoma. (5, 6, 7, after Boulenger.) Cartilaginous parts are dotted; ossified parts are left white. Cl, Clavicle; Co, coracoid; E, epicoracoidal cartilage; H, humerus; M, metasternum; O, omosternum; P, precoracoid; Sc, scapula; S.S, supra-scapula.
It is very doubtful if the Anura possess a true sternum, if by sternum we understand a medio-ventral apparatus which owes its origin to the ventral portions of ribs. The so-called sternal apparatus of the Anura consists of two pieces. One, anterior, variously named episternum, presternum, or omosternum, rests upon the united precoracoids and extends headwards, being either styliform or broadened out. Sometimes it is partly ossified, with a distinct suture at its base; this is the case especially in the Firmisternia; in many Arcifera the omosternum remains cartilaginous and is continuous, without a sutural break, with the cartilage of the precoracoids, indicating thereby its genetic relation to the shoulder-girdle. Hence omosternum is the {26}preferable name. It is frequently much reduced, even absent, for instance in most Bufonidae and in the Engystomatinae. The posterior so-called sternal part may be termed metasternum. It forms the posterior counterpart of the omosternum. It is attached behind to the epicoracoidal cartilages, or fusing with them forms their posterior continuation. It appears mostly in the shape of a style, which is frequently ossified, and broadens out behind into a cartilaginous, partly calcified blade. In the Discoglossidae only it diverges backwards into two horns, assuming a striking resemblance to the typical xiphisternum of the Amniota. In young Anura the metasternal cartilage is intimately connected with the pericardium, an indication of its being derived not from ribs but from the shoulder-girdle.
The glenoid cavity is always formed by the coracoids and by the scapula, but the precoracoid often takes part in its formation, for instance in Bufonidae, Hylidae, and Discoglossidae.
In the fore-limb the humerus has a crest, stronger in the males than in the females; it assumes extraordinary strength in some Cystignathidae, notably in the male Leptodactylus. Radius and ulna are fused into one bone. The carpalia are originally nine in number: radiale, ulnare, two centralia, and five carpalia distalia, the fifth of which is reduced to a tiny nodule or to a ligamentous vestige. The primitive condition still prevails in the Discoglossidae. In most of the other Anura the fourth and third distal carpalia, in any case very small, fuse with the enlarged ulnar centrale; the radial centrale comes, in the Bufonidae and Pelobatidae, into contact with the radius, so that the forearm articulates with three elements as in the Urodela, but with this difference, that the intermedium of the Urodela has been lost by the Anura. There are five metacarpalia and five fingers, but the elements of the first or thumb are nearly vestigial, so that the pollux is reduced to one or two nodules, scarcely visible externally. The normal number of the phalanges of the second to fifth fingers is 2, 2, 3, 3. The distal phalanges are generally straight, either pointed or expanded or with Y or T-shaped ends; but in the Hylidae, in Hylambates amongst the Ranidae, and in Ceratohyla, one of the Hemiphractinae, the terminal phalanges are produced into curved claws which support the adhesive finger-discs. There are, however, many genera of different families, which possess finger-discs and have no claw-shaped {27}phalanges. The Hylidae, and many of the climbing members of the Ranidae with adhesive discs, possess an extra skeletal piece intercalated between the last and last but one phalanges of the fingers and toes. This piece, a mere interarticular cartilage in Hyla, is in the following Raninae developed into an additional phalanx, so that their numbers are 3, 3, 4, 4 in the hand and 3, 3, 4, 5, 4 in the foot: Cassina, Hylambates, Rappia, Megalixalus, Rhacophorus, Chiromantis, Ixalus, and Nyctixalus. All the other Ranidae are without this additional phalanx, irrespective of the presence or absence or size of digital expansions.[12]
The pelvic girdle looks like a pair of tongs (see Fig. 4, p. 22). The ilium is enormously elongated and is movably attached to the sacral diapophyses. This connection is always pre-acetabular in position. The ilium and ischium co-ossify completely, and make up nearly the whole of the pelvis; the pubis is very small, and remains cartilaginous unless it calcifies. It rarely possesses a centre of ossification, for instance in Pelobates, where the osseous nodule is excluded from the acetabulum, recalling certain Labyrinthodonta, whose ossa pubis likewise do not reach that cavity. The latter is open or perforated in young Anura and remains so in the Discoglossidae, but in the others it becomes closed up as in the Urodela. The ventral halves of the pelvis, besides forming a symphysis, closely approach each other, just leaving room for the passage of the rectum and the urino-genital ducts.
The hind-limbs are in all cases longer than the fore-limbs. The femur is slender, the tibia and fibula are fused into one bone. The tarsus is much modified by the great elongation of the two proximal tarsalia (there being no intermedium) into an astragalus and a calcaneum, both of which fuse together distally and proximally, or completely as in Pelodytes; in the latter case the limb assumes a unique appearance, since it consists of three successive and apparently single bars of nearly equal length. The other tarsal elements, especially the more lateral ones, are practically reduced to pads. The Anura have thereby acquired two well-marked joints, one cruro-tarsal, the other tarso-metatarsal; this shows a high stage of specialisation in comparison with the Urodelous and Stegocephalous type of still undefined joints.
The Anura possesses five well-developed toes with normally 2, 2, 3, 4, and 3 phalanges, and the rudiments of a sixth digit, the so-called prehallux, which consists of from two to four pieces, including the one which represents its metatarsal. This prehallux, as a vestige of a once better developed digit, is exactly like the elements on the radial side of the wrist, which, we are certain, are the remnants of a once complete finger, namely the pollex. The only weighty difficulty against its interpretation as a prehallux lies in the fact that hitherto no six-toed Stegocephali have been found; but the fact that there are no Stegocephali known with more than four fingers could be used as an argument against there being a pollex-vestige in recent Anura with just as little reason.
The skull of the Anura differs from that of the other recent Amphibia in the following features:–
The orbital region of the primitive cranium remains cartilaginous, but further forward the cranial cavity is closed by the unpaired sphenethmoid, which forms a ring round the anterior portion of the brain-cavity, hence called "os en ceinture" by some anatomists. The frontals and parietals fuse into one pair of fronto-parietal bones, and these again can fuse together in the middle line; as in Aglossa and Pelobates. The palatal portion of the palato-quadrate cartilage is complete, reaching forwards to the sides of the ethmoid region. The curved arch, formed by this cartilage, is covered by the following bones: (1) the quadrato-jugal, reduced to a thin splint which connects the quadrate and squamosal with the posterior end of the maxilla; (2) the pterygoid, always strong, extending from the distal inner corner of the quadrate to the maxilla, sometimes also to the palatine, and with a broad, median process to the parasphenoid, this process covering ventrally most of the otic region; (3) the palatines, which vary considerably in shape and size; they are placed transversely and meet in the middle line; in Bombinator and Pelodytes they are absent.
The quadrates are directed transversely and backwards, in conformity with the wide gape of the mouth. The squamosal is always well developed, covering the whole of the quadrate on its outer side; it has a forwardly directed process which ends freely in Rana, meets a corresponding process of the maxilla and forms a bony arch with it in Discoglossus, Pelobates, and others, or {29}is scarcely developed at all, for instance in Bufo. In Pelobates cultripes the squamosal is very wide and forms a junction with the fronto-parietals, thus producing a broad bridge across the temporal fossa.
The nasal bones are large and meet in the middle line. Frequently they leave a space between them and the diverging anterior portion of the fronto-parietals, through which gap appears part of the dorsal surface of the ethmoid cartilage. A fontanelle between the frontals occurs in most Hylidae, many Cystignathidae, some few Bufonidae, in Pelodytes amongst the Pelobatidae, and in the Discoglossidae.
The tympanic cavity is bordered in front, above, and below by the squamosal and quadrate, behind by the musculus depressor mandibulae, internally by the otic capsule, and by the cartilage of the cranium between this and the lateral occipital bone. The cavity communicates, however, by the wide and short Eustachian tube with the mouth, the passage being bordered anteriorly by the pterygoid, posteriorly by soft parts. Partly imbedded in these soft tissues is the styloid process or stylohyal, which is attached to the cranium, mostly behind the otic region, and is continued downwards into the anterior horn of the hyoid. The whole partly cartilaginous, ligamentous, and osseous string is, in fact, the entire ventral half of the hyoid arch, while the dorsal half or hyomandibular portion of this, the second visceral arch, is modified into the columellar or auditory chain. The inner end of this chain, the stapes, is inserted into and around the fenestra ovalis of the otic capsule, while the outer end is somewhat T-shaped, and is loosely attached to or near the upper rim of the tympanic ring and to the middle of the tympanic disc. In many Anura this terminal bar can be seen from the outside. The middle portion of the columellar chain is ossified, the rest remains cartilaginous. But the whole chain exhibits various modifications in different genera, especially in the number and the extent of the processes sent out by the outer cartilaginous portion; these are attached in various ways to the tympanum and its rims. The tympanic disc is carried by a cartilaginous ring, which rests against a special process sent out by the quadrate, and is probably itself a differentiation of this element.
In some very aquatic genera, but also in Pelobates, the {30}tympanic cavity is much reduced, for instance in Bombinator, Liopelma. In Batrachophrynus not only the cavity, but also the Eustachian tubes are suppressed. In the Aglossa only the two tubes are united into one short but wide median canal, opening at the level of the pterygoids on the roof of the mouth.
The lower jaw is remarkable for the possession of mento-Meckelian cartilages, absent only in the Aglossa and Discoglossidae. At first they are much longer than the rest of the jaw; during the larval life they indeed form the functional jaw, and they are now covered with horny sheaths instead of teeth. Owing to the absence of teeth on them, these mento-Meckelian cartilages are later not invested by bone, although in many Anura they ultimately ossify, either retaining their separate nature or fusing partly with the dentary bones. The bulk of the lower jaw, the Meckelian cartilage, becomes invested by the dentary, a small articulare, and an inner angulare, while a splenial element is absent. The dentary itself is mostly reduced to a small dentigerous splint, while the angulare forms by far the greater part of the bony jaw.
Teeth are more restricted in their occurrence than in the Urodela. On the jaws they always stand in one row. With the exception of the Hemiphractinae, Amphignathodontinae, Ceratobatrachinae, and Genyophryninae, no recent Anura carry teeth on the lower jaw, and even in these genera they are mostly much reduced in size and firmness, having all the appearance of vanishing structures. The premaxillae and maxillae are frequently furnished with teeth, except in the Dendrobatinae, Genyophryninae, Engystomatinae, Dendrophryniscinae, Bufonidae, Pipa, and Hymenochirus. The vomers mostly carry a series of teeth on their posterior border; when these teeth are absent, as in many species of Bufo, a kind of substitute sometimes occurs on the palatines in the shape of a row of tuberosities. The palatines carry teeth in Hemiphractinae. The parasphenoids are rarely toothed, e.g. Triprion, Diaglena, Amphodus, and occasionally in Pelobates.
A few Anura possess peculiar substitutes for teeth in the anterior portion of the lower jaw, namely, a pair of conical bony processes, sometimes rather long, but always covered by the dense gums, or investment of the jaws; e.g. Lepidobatrachus, several Rana, e.g. R. adspersa, R. khasiana, R. kuhli, and Cryptotis brevis.
Cranial dermal ossifications are developed in some species of Bufo, still more in the Hemiphractinae, and above all in Pelobates cultripes and in the Cystignathoid genus Calyptocephalus.
The hyoid apparatus of the Anura is complicated. It is originally composed of the hyoidean and four branchial arches, with one median, copular piece. The branchial arches form in the early life of the tadpole the elaborate framework of the filtering apparatus mentioned on p. 44. During metamorphosis the whole filter disappears, owing to resorption of the greater part of the branchial arches; only their median portions remain, and fuse with the enlarged copular piece and the hyoidean arches into a broad shield-shaped cartilage (corpus linguae), whence several lateral processes sprout out, the posterior pair of which are generally called thyrohyals or thyroid horns. The true hyoid horns give up their larval lean-to articulation with the quadrate, become greatly elongated, and gain a new attachment on the otic region of the cranium. The transformation of the whole apparatus has been studied minutely by Ridewood, in Pelodytes punctatus.[13]
Skin
The epidermis of the young larvae of Amphibia is furnished with cilia, which later on are suppressed by the development of a thin hyaline layer or cuticula, but clusters of such cilia remain, at least during the larval life and during the periodical aquatic life of the adult, in the epidermal sense-organs. In the frog, currents are set up by the ciliary action at an earlier stage, and are maintained to a later stage than in the newt. In the latter the tail loses its ciliation, whereas in the frog it remains active almost up to the time of the metamorphosis. In tadpoles of 3-10 mm. nearly the whole surface is ciliated (Assheton).[14] The cilia work from head to tail, causing the little animal, when perfectly quiet, to move forwards slowly in the water. Beneath the cuticula, in the Perennibranchiata and the larvae of the other Urodela, lies a somewhat thicker layer of vertically striated cells, the so-called pseudo-cuticula, which disappears with the transformation of the upper layers of the Malpighian cells into the stratum corneum. The latter is very thin, consists of one or two layers of flattened cells, and is shed periodically by all {32}Amphibia in one piece. In the Urodela it generally breaks loose around the mouth, and the animal slips out of the delicate, transparent, colourless "shirt," which during this process of ecdysis or moulting becomes inverted. In the Anura it mostly breaks along the middle line of the back, the creature struggles out of it, pokes it into its mouth, and swallows it. Urodela also eat this skin. As a rule the first ecdysis takes place towards the end of the metamorphosis, preparatory to terrestrial life. So long as the animal grows rapidly, the skin has to be shed frequently, since this corneous layer is practically dead and unyielding. Adult terrestrial Urodela do not seem to moult often, mostly only when they take to the water in the breeding season. Anura, on the other hand, moult often on land, at least every few months. The surface of the new skin is then quite moist and slimy, but it soon dries and hardens.
The Malpighian stratum consists of several layers, thickest in the Perennibranchiata; in them it contains mucous cells throughout life, in others such slime-cells are restricted to larval life. Later, regular slime-glands are developed, which open on the surface. They are very numerous, and more evenly distributed, over most parts of the body, than the specific or poison-glands, which are restricted to certain parts, often forming large clusters, especially on the sides of the body. They reach their greatest development in the "parotoid glands" of the Anura. Both kinds of glands are furnished with smooth muscle-fibres, which are said to arise from the basal membrane underlying and forming part of the Malpighian layer; these muscle-cells extend later downwards into the corium. For the action of the poison, see p. 37.
The stratum corneum is mostly thin, but on many parts of the body, especially in Anura, the epidermal cells proliferate and form hard spikes or other rugosities, generally stained dark brown. With these may be grouped the nuptial excrescences so frequent in the Anura, especially on the rudiment of the thumb, and on the under surface of the joints of the fingers and toes. In many Anura, less frequently in the Urodela, the tips of the fingers and toes are encased in thicker horny sheaths, producing claws or nails. They are best developed among newts in Onychodactylus, among the Anura in Xenopus and Hymenochirus. The horny covering of the metatarsal tubercles reaches its greatest size in {33}the digging spur or spade of Pelobates. In most of these cases the cutis is elevated into more or less wart-like papillae, covered, of course, by the proliferated and cornified epidermis. In the female of Rana temporaria nearly the whole surface of the body becomes covered with rosy papillae during the breeding season. Similar nuptial excrescences are common, and are most noteworthy in the male of the Indian Rana liebigi.
The epidermis also contains sense-organs. They attain their highest development in the larvae; later on they undergo a retrogressive change. Each of these sense-organs is a little cup-shaped papilla, visible to the naked eye. It is composed of elongated cells which form a mantle around some central cells, each of which ends in a stiff cilium perforating a thin, hyaline membrane which lines the bottom of the cup, and is perhaps the representation of the cuticula. These ciliated cells are connected with sensory fibres, the nerve entering at the bottom of the whole organ. The cilia are in direct contact with the water, but the outer rim of the whole apparatus is protected by a short tube of hyaline cuticula-like secretion. These sense-organs are, in the larvae, scattered over the head, especially near the mouth and around the eyes, whence they extend backwards on to the tail, mostly in three pairs of longitudinal rows, one near the vertebral column, the others lateral. They are supplied by the lateral branch of the vagus nerve. They disappear during the metamorphosis, at least in the Anura, with the exception of Xenopus, in which they form conspicuous white objects. The white colour is caused by the tubes becoming choked with the débris of cells or coagulating mucous matter, so that it is doubtful if these organs, which moreover have sunk deeper into the skin, are still functional. In the terrestrial Urodela these organs undergo a periodical process of retrogression and rejuvenescence. During the life on land they shrink and withdraw from the surface, and their nerves likewise diminish, but in the breeding season, when the newts take again to aquatic life, they revive, are rebuilt and become prominent on the surface. They are an inheritance from the fishes, in which such lateral line organs are universally present.
The cutis of most Amphibia is very rich in lymph-spaces, which, especially in the Anura, assume enormous proportions, since the so-called subcutaneous connective tissue forms {34}comparatively few vertical septa by which the upper and denser layers, the corium proper, are connected with the underlying muscles. The spaces are filled with lymph, and into some of them the abnormally expanded vocal sacs extend, notably in Paludicola, Leptodactylus, and other Cystignathidae, and in Rhinoderma.
The cutis frequently forms papillae and prominent folds, sometimes regular longitudinal keels on the sides of the back; but dermal, more or less calcified or ossified scales are restricted to the Stegocephali and to the Apoda, q.v., pp. 79, 87. We conclude that the Urodela and Anura have entirely lost these organs. Dermal ossifications, besides those which now form an integral part of the skeleton, like many of the cranial membrane-bones, are rare, and are restricted to the Anura. They are least infrequent on the head, where the skin is more or less involved in the ossification of the underlying membrane-bones, for instance in Triprion, Calyptocephalus, Hemiphractus and Pelobates. The thick ossifications in the skin of the back of several species of Ceratophrys are very exceptional. In Brachycephalus ephippium these dermal bones enter into connection with the vertebrae; small plates fuse with the dorsal processes of the first to third vertebrae, while one large and thick plate fuses with the rest of the dorsal vertebrae. Simple calcareous deposits in the cutis are less uncommon, for instance, in old specimens of Bufo vulgaris. We are scarcely justified in looking upon these various calcifications and even ossifications as reminiscences of Stegocephalous conditions.
The skin contains pigment. This is either diffuse or granular. Diffuse pigment, mostly dark brown or yellow, occurs frequently in the epidermis, even in the stratum corneum. The granular pigment is stored up in cells, the chromatophores, which send out amœboid processes, and are restricted to the cutis, mostly to its upper stratum, where they make their first appearance. Contraction of the chromatophores withdraws the pigment from the surface, expansion distributes it more or less equally. The usual colours of the pigment are black, brown, yellow, and red. Green and blue are merely subjective colours, due to interference. A peculiar kind of colouring matter is the white pigment, which probably consists of guanine, and is likewise deposited within cells; cf. the description of the white spots in the skin of Hyla coerulea.
Most Amphibia are capable of changing colour, the Urodela, however, far less than the Anura, some of which exhibit an extraordinary range and adaptability in their changes.
The mechanism by which the change of colour is produced in frogs has been recently studied by Biedermann.[15] If we examine the green skin of the common Tree-frog, Hyla arborea, under a low power and direct light, we see a mosaic of green, polygonal areas, separated by dark lines and interrupted by the openings of the skin-glands. Seen from below the skin appears black. Under a stronger power the black layer is seen to be composed of anastomosing and ramified black pigment-cells. Where the light shines through, the skin appears yellow. The epidermis itself is quite colourless. The mosaic layer is composed of polygonal interference-cells, each of which consists of a basal half which is granular and colourless, while the upper half is made up of yellow drops. Sometimes the tree-frog appears blackish, and if then the black pigment-cells are induced to contract, for instance, by warming the frog, it appears silver-grey; in this case the pigment in the yellow drops is no longer diffuse, but is concentrated into a round lump lodged between the interstices of the granular portions; the black pigment-cells are likewise balled together. These black chromatophores send out numerous fine branches, which occasionally stretch between and round the polygonal cells. When each of these is quite surrounded and covered by the black processes, the frog appears black. On the other hand, when the black pigment-cells withdraw their processes, shrink up, and, so to speak, retire, then the light which passes through the yellow drops is, by interference, broken into green.
Stoppage of the circulation of the blood in the skin causes the black chromatophores to contract. Carbon dioxide paralyses them and causes them to dilate. This is direct influence without the action of nerves. But stimulation of the central nerve-centres makes the skin turn pale. Low temperature causes expansion, high temperature contraction, of the chromatophores. Hence hibernating frogs are much darker than they are in the summer. Frogs kept in dry moss, or such as have escaped into the room and dry up, turn pale, regardless of light or darkness, probably owing to a central, reflex, nerve-stimulus.
Tree-frogs turn green as the result of the contact with leaves. {36}Dark frogs will turn green when put into an absolutely dark vessel in which there are leaves. This is reflex action, and blinded specimens do the same. The principal centres of the nerves which control the chromatophores, lie in the corpora bigemina and in the optic thalami of the brain. When these centres are destroyed, the frog no longer changes colour when put upon leaves, but if a nerve, for instance the sciatic, be stimulated, the corresponding portion of the body, in this case the leg, turns green. Rough surfaces cause a sensation which makes the frog turn dark. Rana seems to depend chiefly upon temperature and the amount of moisture in the air, so far as its changes of colour are concerned. Biedermann concludes that the "chromatic function of frogs in general depends chiefly upon the sensory impressions received by the skin, while that of fishes depends upon the eye."
All this sounds very well, but the observations and experiments are such as are usual in physiological laboratories, and the frogs, when observed in their native haunts, or even when kept under proper conditions, do not always behave as the physiologist thinks they should. There is no doubt that in many cases the changes of colour are not voluntary, but reflex actions. It is quite conceivable that the sensation of sitting on a rough surface starts a whole train of processes: roughness means bark, bark is brown, change into brown; but one and the same tree-frog does not always assume the colour of the bark when it rests, or even sleeps upon, such a piece. He will, if it suits him, remain grass-green upon a yellow stone, or on a white window-frame. I purposely describe such conditions, changes, coincidences, and discrepancies in various species, notably in Hyla arborea, H. coerulea, Rana temporaria, Bufo viridis, to show that in many cases the creature knows what it is about, and that the eye plays a very important part in the decision of what colour is to be produced. The sensory impression received through the skin of the belly is the same, no matter if the board be painted white, black, or green, and how does it then come to pass that the frog adjusts its colour to a nicety to the general hue or tone of its surroundings?
Boulenger[16] has given us a summary of the action of the poison of Amphibia:
It is well known to all who have handled freshly-caught newts, and certain toads, especially Bombinator, that their secretion acts as a sternutatory, and causes irritation of the nose and eyes, the effects produced on us by Bombinator being comparable to the early stages of a cold in the head. Many collectors of Batrachians have learned, to their discomfiture, how the introduction of examples of certain species into the bag containing the sport of their excursion may cause the death of the other prisoners; for although the poison has no effect on the skin of individuals of the same species, different species, however closely allied, may poison each other by mere contact. But when inoculated the poison acts even on the same individual.
Miss Ormerod, to personally test the effect, pressed part of the back and tail of a live Crested Newt between the teeth. "The first effect was a bitter astringent feeling in the mouth, with irritation of the upper part of the throat, numbing of the teeth more immediately holding the animal, and in about a minute from the first touch of the newt a strong flow of saliva. This was accompanied by much foam and violent spasmodic action, approaching convulsions, but entirely confined to the mouth itself. The experiment was immediately followed by headache lasting for some hours, general discomfort of the system, and half an hour after by slight shivering fits."
Numerous experiments have shown that the poison of toads, salamanders, and newts is capable, when injected, of killing mammals, birds, reptiles, and even fishes, provided, of course, that the dose be proportionate to the size of the animal. Small birds and lizards succumb as a rule in a few minutes; guinea-pigs, rabbits, and dogs in less than an hour.
This poison of Amphibia is not septic, but acts upon the heart and the central nervous system. That of the common toad has been compared, in its effects, to that of Digitalis and Erythrophlaeum. Some authorities hold that the poison is an acid, others regard it as an alkaloid.
Phisalix[17] has come to the conclusion that toads and salamanders are possessed of two kinds of glands, different both anatomically and physiologically. These are, first the mucous glands, spread over the greater part of the body, with an alkaloid secretion, which acts as a narcotic; secondly, specific glands, as {38}the parotoids and larger dorsal glands, the secretion of which is acid, and acts as a convulsive.
The Indians of Colombia are said to employ the secretion of Dendrobates tinctorius for poisoning their arrows. The poison is obtained by exposing the frog to a fire, and after being scraped off the back is sufficient for poisoning fifty arrows. It acts on the central nervous system, and is used especially for shooting monkeys. Concerning the use of this poison for "dyeing" parrots, see p. 272.
The milky secretion of toads protects them against many enemies, although not always against the grass-snake. A dog which has once been induced to bite a toad, suffers so severely that it will not easily repeat the experiment. The handling of tree-frogs also irritates both nose and eyes. The hind limbs of the Water-frog, Rana esculenta, have a very bitter, acrid taste. In short, most, if not all, Amphibia are more or less poisonous, and it is significant that many of the most poisonous, e.g. Salamandra maculosa, Bombinator, Dendrobates, exhibit that very conspicuous combination of yellow or orange upon a dark ground, which is so widespread a sign of poison. Other instances of such warning colours, protective in a defensive sense, are the Wasps and Heloderma, the only poisonous lizard.
Nerves
Spinal nerves.–Each spinal nerve issues originally immediately behind the neural arch of the vertebral segment to which it belongs. This intra-vertebral position is ultimately modified into a more inter-vertebral one, owing to the predominant share of the neural arches, basidorsalia, in the composition of the whole vertebra. Consequently the nerves issue behind their corresponding vertebra.
The first spinal nerve, or N. suboccipitalis, is exceptional in several respects. It develops a dorsal and a ventral root like a typical spinal nerve, but the dorsal root soon degenerates in all Amphibia, while in the Phaneroglossal Anura the whole nerve disappears. The first spinal nerve reduced to its ventral half persists therefore only in the Apoda, Urodela, and the Aglossal Anura. It issues originally between the occiput and the atlas, but in the adult it is partly imbedded in the anterior portion of the atlas. Its own vertebra is lost, having probably been added to the cranium.
In the Urodela the first spinal nerve either remains separate, or it joins the second spinal, forming with it and with a branch from the third nerve the cervical plexus, which supplies the muscles of the cervical region. The third, fourth, and fifth nerves, and sometimes also the sixth, form the brachial plexus.
In the Aglossal Anura N. spinalis I. mostly sends a fine thread to the second spinal nerve, the rest supplies chiefly the M. levator scapulae, in Pipa the abdominal muscles also. In all the other Anura this N. spinalis I. is lost; occasional vestiges have been reported in Bufo vulgaris and Rana catesbiana, and remnants of it may possibly be found in Pelobatidae and Discoglossidae. The first actually persisting nerve of the Phaneroglossa is consequently N. spinalis II.
The brachial plexus is composed as follows:–Pipa, N. spinalis II. and III.; Xenopus and Phaneroglossa, N. spinalis III. and IV., with a small branch from the second; the next following three nerves, numbers V., VI., and VII., behave like ordinary trunk nerves.
The pelvic plexus of the Phaneroglossa is formed in Rana by the VIII. + IX. + X. + XIth nerves, the tenth issuing between the sacral vertebra and the coccyx. In Bufo and Hyla the plexus is composed of five nerves, the seventh spinal sending a branch to it. Occasionally the twelfth nerve contributes a small branch to the posterior portion of the plexus. This and the eleventh nerve leave the coccyx by separate holes, thereby indicating its composition. The rest of the spinal cord gives off no more recognisable nerves, owing to its reduction during the later stages of metamorphosis; its terminal filament passes out of the posterior end of the coccygeal canal.
Concerning the cranial nerves it is necessary to draw attention to one point only. The last nerve which leaves the cranium of the Amphibia is the vagus or tenth cranial nerve. There is consequently no eleventh, and no twelfth or hypoglossal, pair of cranial nerves. Their homologues would be the first and second spinal nerves, but the whole tongue of the Amphibia, with its muscles, is supplied by the glossopharyngeal, or ninth cranial pair, and is morphologically not homologous with the tongue of the Amniota.
Respiratory Organs
A very important and characteristic feature of the Amphibia is the development of two sets of respiratory organs: Gills and Lungs. It is as well to give definitions of these organs. Lungs are hollow evaginations from the ventral wall of the pharynx, and their thin, vascularised walls enable the blood to exchange, by osmosis, carbon dioxide for oxygen from the air which enters the lungs by the mouth or the nostrils, and the windpipe. The latter is unpaired, the lungs themselves are paired. Gills are highly vascularised, more or less ramified excrescences, covered by a thin epithelium of ecto- or endo-dermal origin, which permits of the exchange of carbon dioxide for oxygen from the air which is suspended in the surrounding water. It is obvious that this definition applies to all sorts of well-vascularised organs whose thin surface comes into contact with the water. Various recesses of the pharyngeal cavity, the dorsal and ventral folds of the tail-fin, nay, even any part of the skin of the body can, and does occasionally, assume additional respiratory functions. The proper definition of gills, in Vertebrates, requires, therefore, the restriction that they must be developed upon and carried by visceral arches.
The general statement that the Amphibia breathe by lungs, and, at least during some stage of their life, also by gills, requires various restrictions. As a rule the majority of Amphibia first develop gills, later on also lungs, whereupon, during the metamorphosis, the gills are gradually suppressed, so that the perfect animal breathes by lungs only (see p. 61). But a number of Urodela retain their gills throughout life, although the lungs are also functional. These are the Perennibranchiata, not a natural group, but a heterogenous assembly, Proteidae and Sirenidae. Some species of Amblystoma remain individually Perennibranchiate (cf. Axolotl, p. 112). On the other hand, in some Anura the gills are almost or entirely suppressed, or restricted to the embryonic period only. Lastly, a considerable number of Salamandridae have lost their lungs; they breathe by gills until their metamorphosis, but have in the adult state to resort to respiration by the skin (cf. p. 46).
The general plan of the development of the branchial respiratory apparatus is as follows:–The six visceral arches, {41}namely, the mandibular, the hyoidean, and the four branchial arches, correspond, long before they are cartilaginous, with four main arterial arches of the truncus arteriosus. The first, the arteria hyo-mandibularis, belongs to the hyoidean and mandibular segments, the second to the first branchial, the third to the second branchial, while the fourth soon splits in two for the third and fourth or last branchial arch. On the dorsal side these branchial arterial arches combine to form the radix of the dorsal aorta. These arches, especially the three branchials, appear in newts, less clearly in frogs, as transverse ridges on the sides of the future neck. Between the arches the pharynx gradually bulges out in the shape of five lateral gill-pouches; the first between the mandibular and the hyoidean arch, the second between the hyoidean and the first branchial arch, etc. These pouches soon break through to the outside and become gill-clefts, except the first pouch in Urodela. Before the breaking through of the clefts there appears upon the outside of the middle of the rim of each arch a little knob, which soon ramifies and forms an external gill. The knob owes its origin to the development of a blood-vessel which buds from the arterial arch, ramifies and breaks up into capillaries, and returns a little further dorsalwards into the arch. A secondary loop to the outside of the primary arterial arch is thus formed; and whilst this outer loop sprouts out further, driving before it the likewise proliferating skin, and thus producing the gill, the middle portion of the primary arch remains in the Urodela as a short cut, but in the Anura it partly obliterates, and henceforth acts as the internal efferent vessel of the gill. When, during metamorphosis, the gills disappear, their intrinsic afferent and efferent vessels vanish likewise, and the short cut completes the circuit. In order to do this they have, in the Anura, to form new connections with the trunks of the afferent vessels.
The arterial arches themselves are modified as follows:–The first pair become the carotids, the second form the right and left aortic arches, while the third and fourth unite and are transformed into the pulmonary arteries and "ductus Botalli," the last arterial arch having previously sent a branch into the developing lungs. In the Anura the third arch obliterates.
The gills and clefts present various modifications. The Urodela possess three pairs of gills, one each upon the dorsal {42}half of the three branchial arches, just near the upper corners of the clefts; and the skin of the body is continued upon the stem of each gill, pigmented like the rest of the surface of the body. Such a gill is more or less like a blade, standing vertically, and is composed of a stem of connective tissue, thick at the base, and, as a rule, carrying two series of fine lamellae, which, however, do not form two opposite series, but hang downwards, being, so to speak, folded down, so that the upper surface of the stem is bare, and carries the lamellae on its under side. In the Axolotl some of these lamellae are further subdivided. In Necturus they are enormously increased in numbers, but are rather short, and they stand no longer in two rows, but are crowded into one. Those of Proteus form two rows of dendritic filaments; those of Siren are likewise much ramified.
The larvae of the Urodela have four clefts. In the adult Siren these are reduced to three, the first, namely, that between the hyoid and the first branchial arch, being closed up. In Necturus, Proteus, and Typhlomolge the clefts are further reduced to two, owing to the closing up of the first and last, only those between the first, second, and third arches remaining. Amphiuma, and usually Cryptobranchus alleghaniensis, possess only one pair of clefts, while in C. japonicus and in the Salamandridae all the clefts are abolished.
The gills of the Urodela are always uncovered, although a short operculum is formed from the posterior margin of the hyoidean arch; the halves of this fold meet below the throat, and persist in various terrestrial and aquatic species as the "gular fold." It reaches its greatest size just before metamorphosis, but scarcely ever produces a proper outer gill-chamber, and it does not cover the gills owing to their rather pronounced dorsal position. It is perhaps best developed in Typhlomolge, and even there its dorsal portion is continued upon the first of the three broad vertical and short-fringed blades which form the gills.
A description of the gills of the Apoda will be found in the systematic part.
In the Anura the gills are complicated, owing to the development of the so-called internal gills. First appear, exactly in the same way as in the Urodela, the external gills, one upon each of the first three branchial arches. In the larva of Rana esculenta, 5 mm. in length, a little protuberance appears upon the first, {43}and then upon the second arch. In the 6 mm. larva the first gill shows four knobs, the second two, the third one knob. They are always delicate and thin, although sometimes pigmented, long, and much-ramified structures. The first pair is always the largest; well developed and persisting a long time in Rana temporaria; smaller in R. esculenta and Bufo vulgaris; very short, scarcely forked, in B. viridis and Hyla arborea. They are relatively largest in Alytes, while still in the egg. Numerous descriptions of these gills will be found in the systematic part.
Great changes take place about the time when the fourth or last branchial arch and the pulmonary arteries are developed. This occurs in R. esculenta when the larva is about 9 mm. long. The sprouting of the gills extends gradually downwards along the arches upon their ventral halves, and these new gill-filaments or loops transform themselves into numerous dendritic bundles, resting in several thickset rows upon the hinder margin of the first to the third arch, one row only on the fourth arch, which carries no external gill. These "internal gills" look like red bolsters or thick and short-tasselled bunches. Whilst they are developing the dorsal, older gills become arrested in their growth and disappear, and at the same time a right and left opercular fold grows out from the head and covers these new gills, shutting them up in an outer branchial chamber, just like that of Teleostei and other Tectobranch fishes. This is the reason why these new gills have been called internal, and the mistaken notion has sprung up that they are comparable with the true internal gills of fishes. In reality Amphibia have only external gills. They are always covered by ectoderm, are restricted to the outside of the branchial arches, and are developed before the formation of the clefts. These gills are in many cases directly continuous with the more dorsally and more superficially placed earlier external gills; but although nearly every one who has studied their development has observed this agreement, the old error still prevails. They are morphologically as little internal as the true internal gills of Elasmobranch embryos are external gills, because these have become so elongated that they protrude out of the gill-clefts.
The fact that the Amphibia possess only external gills throws important light upon their phylogeny. Not only do the Apoda, Urodela, and Anura agree much more with each other than {44}would be the case if the Anura possessed both internal and external gills, but the Amphibia reveal themselves also in this point as connected with the Crossopterygii and the Dipnoi, some of which fishes also possess external gills. It is of course quite possible that the Amphibia have developed these organs independently, but we understand now that the latter are accessory, and not the primitive respiratory organs; they are developed in adaptation to embryonic conditions and to prolonged larval, occasionally perennibranchiate, aquatic life (cf. the chapter on Neoteny, p. 63).
There is no valid reason for supposing that the Stegocephali had true internal gills. We know their branchial skeleton, and we can discern even gill-rakers on the arches. Such gill-rakers occur also, although but feebly developed, in Urodela. The whole branchial framework of the Urodela and Apoda undergoes simple reductions during metamorphosis (see p. 86), but in the Anura these arches are in early tadpole life transformed into a most complicated basket-work which acts as a straining apparatus or filter, to prevent any particle of food or other foreign matter from finding its way into the delicate gills, the current of water passing from the mouth through the filter, past the gills and out of the clefts. During metamorphosis this whole elaborate apparatus is again transformed, almost beyond recognition, into the hyoidean apparatus for the support of the generally very movable and much-specialised tongue. The fact that the hyoid apparatus of the Aglossa, especially that of Xenopus, is constructed upon the same lines, is a strong indication that these creatures have arrived at their tongueless condition through the loss of this organ, and this is intelligible in correlation with their absolutely aquatic life.
The opercular folds assume great dimensions in all tadpoles. They cover the whole gill-region, thereby producing on either side an outer gill-chamber. The posterior margins of the folds gradually become continuous with the rest of the surface of the body. Each gill-chamber opens at first by one lateral canal, usually called the spiracle. This condition prevails in the tadpoles of the Aglossa. In the Discoglossidae the two canals gradually converge and combine into one median opening on the middle of the belly. In all the other Anura the right opening becomes closed, or rather its canal passes over to and joins that of the {45}left side, both opening by one short tube laterally on the left side, at a variable distance between the eye and the vent. Hence the elegant terms of Amphi-, Medio-, and Laevo-gyrinidae (γυρῖνος being the Greek for tadpole).
The external gills lead to a further consideration. Protopterus possesses a vestigial external gill on the shoulder-girdle. Lepidosiren has them on the gill-arches, resembling piscine internal gills, and Polypterus has a large biserially fringed external gill (in some cases not disappearing until the fish is adult), which starts from the mandibular arch, at the level of the spiracle or first visceral cleft, and overlaps the operculum externally. The axis of this peculiar organ is possibly based upon the homologues of the spiracular cartilages, which themselves are the branchiostegal rays of the dorsal half of the quadrato-mandibular arch. The branchiostegal rays of the hyoidean arch, at least their material, have given rise to the elaborate opercular apparatus; and, in conformity herewith, the hyomandibular itself is not known to carry a gill. Quite possibly the large external gill of Polypterus is not serially homologous with other external gills–it may not be a true gill at all, it has perhaps quite a different function–but it seems to throw light upon a mysterious pair of organs which are common in larval and young Urodela, in the larval Aglossa and in the Apoda. These are the "balancers."
In Triton taeniatus, before hatching, there appears a little protuberance behind and below the eye; it rests upon the angle of the mandibular arch, and is separated from the first transverse, externally visible ridge of the first branchial arch by the beginnings of the hyoidean arch. A few days later the arteria hyomandibularis sends a vessel into this knob, forms a vascular coil, and leaves it as a vein which, instead of returning into the arterial arch, passes into the veins of the body. Its epithelium is not covered with flat, but with cubical cells; and sensory cells have not been found in it. These organs attain some size, and are shaped like rods, with thickened ends; they are movable, and are used by the larvae as "balancers," keeping the head from sinking into the slime at the bottom. But they may have other functions besides, and it is not unlikely that they develop into sensory organs like feelers. They occur in many Salamandridae, and are not reduced until, or even after, the metamorphosis, and during this time they shift their place with relation to the eye and the mouth.
The same kind of organs occur in Amblystoma.[18] They appear, previous to the breaking open of the gill-clefts, as protrusions of epiblast, long before any of the external gills on the branchial arches. When the clefts have broken open, the quadrate sends out laterally a tiny crescent-shaped process a little above the jaw-joint, and this process extends to the base of the balancer, but not into it, and a bundle of muscle-cells grows into the balancer. It is easy to recognise the same organ in the extremely long thread-like structures of the larva of Xenopus. In the Apoda they are likewise present, but are retained permanently as highly specialised, probably tentacular organs (cf. p. 86, Apoda).
One of the most unexpected features is the suppression of the lungs in various kinds of Salamandridae. The lungs are either reduced to useless vestiges or they are quite absent. This occurs in aquatic and terrestrial, American and European forms, and it is noteworthy that the reduction of the lungs does not apply to all the species of the various genera, nor is it restricted to one sub-family.
The following list is due to the researches of H. H. Wilder,[19] L. Camerano,[20] E. Lönnberg,[21] and G. S. Hopkins[22]:–All the Desmognathinae and Plethodontinae; Amblystomatinae, Amblystoma opacum; Salamandrinae, Salamandrina perspicillata. In Triton and other Salamandrinae the length of the lungs varies; in some they extend more, in others less, than half way down the distance between head and pelvis. Hopkins remarks: "Two questions are naturally suggested by this apparently aberrant condition of the respiratory organs. First, what structures or organs have taken on the function of the lungs and branchiae; and secondly, is there any modification in the form or structure of the heart which in any way may be correlated with the above-mentioned peculiarities of the lungless forms?" Wilder concluded that respiration was probably carried on by the skin, and perhaps, to some extent, by the mucosa of the intestine. Camerano thinks that, at least in the European forms, respiration is effected by the bucco-pharyngeal cavity, and that the skin affords no efficient aid. The left auricle in the lungless forms is much {47}smaller in comparison than the right, and there is no pulmonary vein. The auricular septum has a large aperture, the communication between the auricles being larger than even in Necturus (which breathes essentially by gills). The sinus venosus, instead of opening into the right auricle only, opens more freely into the left than into the right, and the latter communicates more directly with the ventricle than the left, instead of about equally. In short, the heart of these creatures appears almost bilocular, instead of being trilocular, at least functionally.
The lungs of the Urodela are always simple, extremely thin-walled bags. They are highly developed in the Anura, the walls being modified into numerous air-cells, whereby the respiratory surface is considerably increased. The lungs are filled with air by the pumping motion of the throat while the mouth is closed, the nostrils being provided with muscular valves. A muscular apparatus assists the filling of the lungs in the Anura.[23]
Fig. 6.–Internal view of the mouth of A, Rana esculenta, B, Bufo calamita (cf. Fig. 52, p. 269). Ch, Choana, or inner nasal opening; E, opening of the Eustachian tube; S, slit leading into the vocal sac; T, tongue; Vo, patches of teeth on the vomers.
Most, if not all, Anura and some Urodela have a voice produced by the larynx, which, especially in the Anura, is provided with a complicated cartilaginous and muscular apparatus and with vocal cords. The voice of the Urodela is at the best a feeble squeak. The females of the Anura are either mute or they produce a mere grunt, but that of many males is very loud, and, moreover, in many species it is intensified by vocal sacs which act as resonators. These sacs are diverticula of the lining of the mouth-cavity, and bulge out the outer skin and the muscles, chiefly the mylo-hyoid, of the throat. The nostrils and the mouth are firmly closed during the croaking. "The sacs are called internal when they are covered by the unmodified gular integument, however much this may be distended; external when their membrane projects through slits at {48}the sides of the throat, as in Rana esculenta (Fig. 52, p. 269), or when the skin is thinned and converted into a bladder-like pouch, as in Hyla arborea."[24] These sacs exhibit many modifications. They may be unpaired and median, and open by two slits into the mouth, on either side below the tongue; in Bufo one of the slits or openings, either the right or the left, is obliterated. They may be paired and symmetrical, and open one on each side of the head, below and near the posterior angle of the jaws. These modifications differ in closely allied species. They reach their greatest complication in Rhinoderma and in some of the Cystignathidae by extending far back beneath the skin into the wide lymphatic spaces. In Rhinoderma they are put to the unique use of nurseries for the young (see p. 228). Leptodactylus typhonius has a very distinct pair of outer vocal sacs and a well-marked unpaired sac which extends into the belly and communicates with each outer sac. Several species of Paludicola, e.g. P. fuscomaculata and P. signifera, have a similar arrangement, in addition to an unpaired gular sac which can be inflated independently of the rest (see Fig. 45, p. 220).
Urino-Genital Organs
The kidneys and the male generative glands are still intimately connected with each other. The general plan is as follows:–
The kidneys consist of a large number of glomeruli, produced by the coiled segmental tubes, each of which is composed of a nephrostome or funnel opening into the body-cavity, a Malpighian body and an efferent canal. The latter combine to form the segmental duct which opens into the cloaca. The testes, composed of a large number of sperm-producing glands, are drained by transverse canals which combine into a longitudinal canal, and this again sends off numerous efferent canals which open into the efferent canals of the kidney, so that the segmental duct (Leydig's duct of many authors) conveys both sperma and urine.
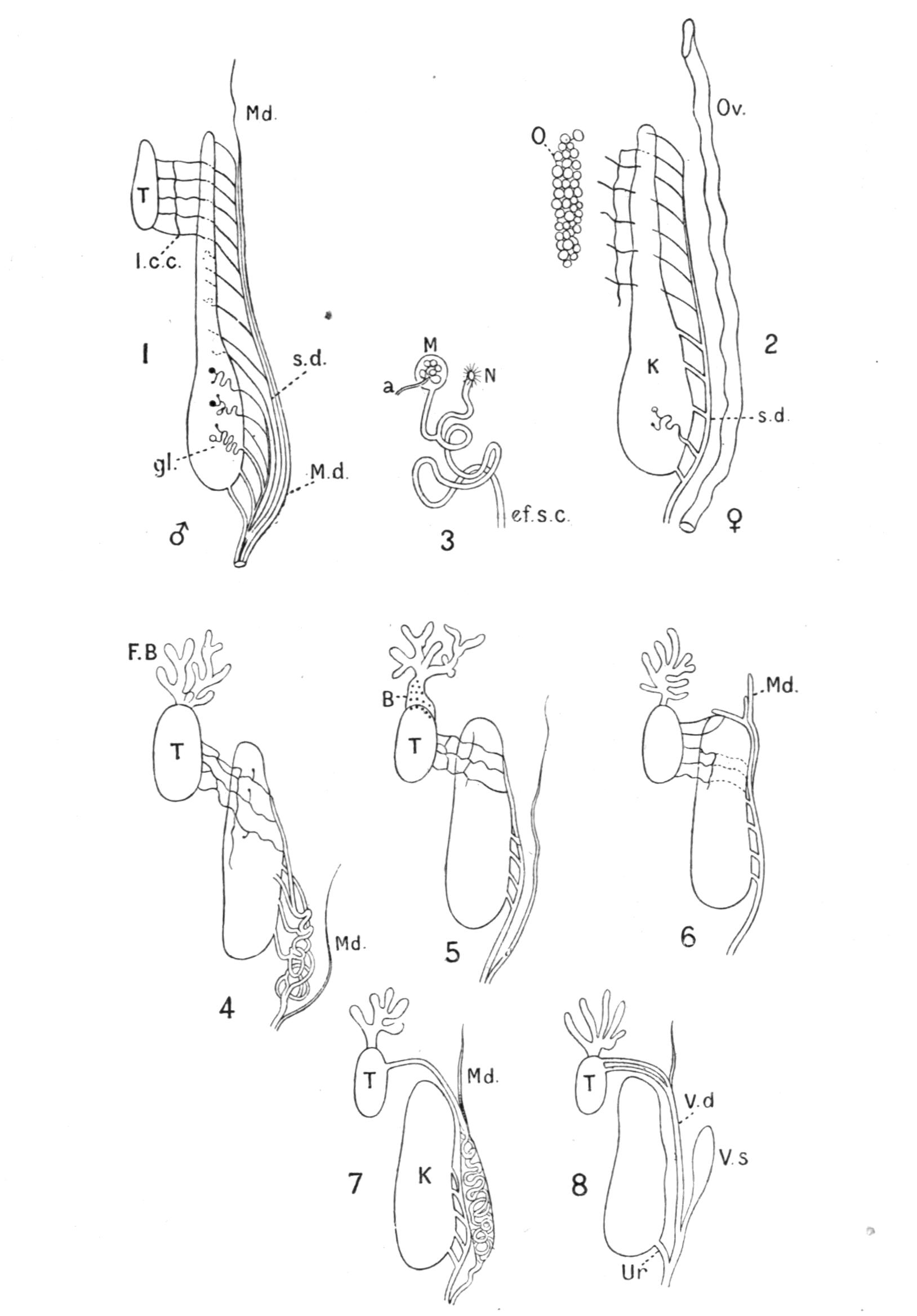
Fig. 7.–Diagrammatic representation of modifications of the urino-genital ducts. 1, 2, Male and female Newt; 3, a tubule of the kidney; 4, male Rana; 5, male Bufo; 6, male Bombinator; 7, male Discoglossus; 8, male Alytes. a, Artery entering, and producing a coil in, the Malpighian body, M; B, Bidder's organ; ef.s.c, efferent segmental canal; F.B, fat-body; gl, glomerulus; K, kidney; l.c.c, longitudinal collecting canal; M, Malpighian body; Md, Müllerian duct; N, nephrostome; O, ovary; Ov, oviduct; s.d, segmental duct; T, testis; Ur, ureter; V.d, vas deferens; V.s, vesicula seminalis.
In the female the network of transverse and longitudinal canals, which originally connect the generative glands with the kidney's efferent canals, is deduced in so far as the connection is interrupted and the vestiges of the transverse canals are no longer functional. The eggs fall into the body-cavity and are caught up by the ostium or inner abdominal opening of a special duct, the oviduct (Müllerian duct of many authors). Vestiges, more or less complete, of these oviducts persist in the males of most Amphibia.
This general scheme presents some modifications in the various groups of Amphibia.
The Apoda retain the most primitive conditions. The kidneys are still long and narrow, and the glomeruli are, at least in the anterior part of the organ, still strictly segmental, agreeing in number and position, each with a vertebral segment; later, the number of the glomeruli is greatly increased, and the former agreement becomes quite disturbed. The generative glands still retain their segmental arrangement, but they are restricted to a much shorter region than the kidneys. In the male Apoda a considerable portion of the cloaca can be everted by special muscles, and acts as an intromittent organ. Both sexes possess a ventral urinary bladder.
In the Urodela both kidneys and testes are much concentrated, the testes especially have lost all outward appearance of segmentation, and their efferent canals, connecting them with the longitudinal collecting canal, are much reduced in numbers. The greater portion of the kidneys, at least their anterior half, has all the appearance of a degenerating organ and is on the way to losing its urinary function, although it still possesses Malpighian bodies and complete ducts; the main function of the latter is now the conveyance of the sperma. In the Perennibranchiata, and in some others, e.g. Spelerpes variegatus, the longitudinal collecting canal, between testis and kidney, is sometimes suppressed, a very simple, but pseudo-primitive arrangement. A urinary bladder is present. The cloaca is not eversible.
In most Anura, e.g. Rana and Bufo (Fig. 7; 4, 5), the same scheme is adhered to. The efferent canals of the testis form a network, with a longitudinal canal, and open into the efferent canals of the kidney, in the substance of which they are more or less deeply imbedded. The ducts which lead out of the kidney to compose Leydig's duct, are frequently dilated, or the latter duct is much elongated, convoluted or varicated, and this whole portion is enclosed in a sheath of connective tissue, giving an {51}appearance as if the single duct itself were dilated in the greater part of its length; hence the occasional name of vesicula seminalis. Such means of storing the sperma enable the latter to be ejected suddenly in great quantities.
In Bombinator (6) some of the most anterior seminal canals do not perforate the kidney, but run over it superficially and open directly into a branch of Leydig's duct. This branch, no doubt equivalent to a number of segmental canals which have lost their uriniferous function, is curved round the upper end of the permanent kidney, while its forward continuation, ending blindly, is the remnant of its former headward extension. This arrangement of Bombinator is carried further in Discoglossus (7). The testis conveys its sperma through a wide duct directly into Leydig's canal, without interfering with the kidney, and all the testicular efferent network is lost. The anterior end of Leydig's duct still extends headwards; its middle portion acts solely as a vas deferens, while the lower portion still behaves like a typical segmental duct, conveying both sperma and urine. Lastly, in Alytes (8) the functional division of the old segmental duct has been carried to an extreme. The kidney is drained by one canal only, now a true ureter, and this is of course produced by a consolidation of the multiple exclusively uriniferous canals of the lower half of the kidney. The whole of the segmental duct is now in the service of the testis, and near its junction with the ureter it forms a large diverticulum or true vesicula seminalis.
Remnants of oviducts, or Müllerian ducts, are common in the male Anura; they are best developed in Bufo, much reduced, and individually absent, in Rana. In Bombinator each duct is restricted to its upper or abdominal portion, and is attached to the vestigial headward extension of Leydig's duct. Lastly in Discoglossus and in Alytes all traces of oviducts seem to have vanished, at least in the adult males.
It is interesting to note that in the arrangement of the urino-genital ducts the Discoglossidae are the most advanced of all Amphibia, instead of showing the most primitive conditions. This is rather unexpected, but is paralleled by the epichordal type of the vertebral column.
The oviducts of the Apoda and Urodela remain more or less straight; in the viviparous species they form uterus-like dilatations. In the Anura they become greatly elongated during the {52}breeding season and form many convolutions. As a rule each oviduct opens separately into the cloaca, but in Hyla they have one unpaired opening, while in Bufo and Alytes the lower parts of both oviducts are themselves confluent.
All Amphibia possess Fat-bodies. They consist of richly vascularised lymphatic tissue, the meshes of which are filled with lymph-cells, globules of fat and oil. In the Apoda these bodies lie laterally to the generative glands, and along the posterior half of the kidneys. In the Urodela they accompany the anterior half of the kidney. In the Anura they are lobate, and are placed upon the anterior end of the testes or ovaries. Their exact function is still doubtful, but it is intimately connected with that of the generative glands. The old notion, that they are simply stores of fat for the nourishment of the animal during hibernation, is quite untenable. The fat-bodies do not decrease during this period, on the contrary they attain their fullest size in the spring at the time of the rapidly awaking activity of the reproductive organs, and they enable considerable quantities of sperma and of eggs to be produced and ripened without detriment to, or utter exhaustion of, the animals, which often spawn before they have had time or opportunity to feed. After the spawning season the fat-bodies have dwindled down to inconspicuous dimensions.
Lastly, there is in some Anura, hitherto observed in Bufo only, a mysterious organ, intercalated between the fat-body and the testis or ovary. This is "Bidder's organ" and it seems to be a rudimentary ovary, or rather that upper, anterior portion of the whole organ which undergoes retrogressive metamorphosis. It disappears in old female toads, but in the males it sometimes assumes a size equal to, or surpassing that of the testes. The males are in this respect hermaphrodite, and cases are known in which parts of the generative glands have developed testes and egg-bearing ovaries.
The spermatozoa of the Apoda and Urodela have an undulating membrane along the tail, while the head-end is either pointed or truncated. Those of Spelerpes fuscus and of Ichthyophis glutinosa measure about 0.7 mm. in total length, those of the other Urodela being much smaller. A peculiarity of the Urodela is that their spermatozoa are massed together in or upon spermatophores, an arrangement which undoubtedly facilitates the internal {53}fecundation of the female without actual copulation. The female takes up such a deposited spermatophore with the cloacal lips, squeezes the sperma out of the capsule which remains behind, and either conveys the former into a special receptaculum seminis, e.g. in Salamandra atra and in Triton, or the spermatozoa wriggle their way, thanks to the undulating tail, directly up the oviducts to the ova.
The spermatophores are composed of a colourless, soft, gelatinous mass, which is probably produced by the cloacal gland. The shell of jelly is in fact a cast of the cloacal cavity, reproducing all its ridges, furrows and folds, while a toad-stool-shaped papilla of the cloaca makes the inside lumen of the cast, e.g. in Triton. Those of Salamandra maculosa are much simpler, consisting, in conformity with the absence of a cloacal papilla, merely of a cone with a globular mass of sperma on the top. Those of Amblystoma are similar.
The spermatozoa of the Anura show considerable differences in the various genera, of which, however, only the European forms have been properly examined. The "head" is wound like a corkscrew in Discoglossus, Pelobates, and Pelodytes; spindle-shaped, more or less curved, in Rana temporaria and R. agilis, Hyla, Bufo and Bombinator, in the latter with an irregular membrane on one side; cylindrical in Rana esculenta and R. arvalis. The tail is mostly long and filiform, but in Bufo vulgaris and Discoglossus it is provided with an undulating membrane. Their size is generally very small, only about 0.1 mm., excepting those of Discoglossus which reach the astonishing length of 3 mm. These differences in shape, especially that of the head, explain why species of the same genus, e.g. Rana temporaria and R. arvalis, cannot fertilise each other.
Fig. 8.–A bell-shaped spermatophore of Triton alpestris. × 3. (After Zeller.)[25]
The eggs differ much in size, colour, and numbers. They are holoblastic, with unequal cleavage, but those species which possess an unusual amount of food-yolk, for instance Rhacophorus schlegeli and the Apoda, approach the meroblastic type of segmentation. As a rule, the greater the amount of yolk, the smaller is the number of eggs produced. But the number which is laid {54}during one season is not only difficult to calculate, but it varies individually, old females laying more than young specimens. Moreover, some kinds, e.g. the Discoglossidae, spawn several times in one year. Alytes, Rhinoderma, Hylodes, Rhacophorus, Pipa, in fact those kinds which are remarkable for special nursing habits, lay only a few dozen eggs at a time. Hyla arborea produces up to 1000, Rana temporaria about 3000, Bufo vulgaris averages 5000, Bufo viridis and Rana esculenta up to 10,000 and more. T. H. Morgan[26] has observed a Bufo lentiginosus which laid 28,000 eggs within ten hours! The number of eggs produced by the Apoda and Urodela is comparatively moderate, in the average a few dozen, Amblystoma alone laying about 1000.
The eggs possess a gelatinous mantle of variable thickness and consistency. In Amphiuma they are strung together like the beads of a rosary, and the envelope hardens into a kind of shell. Many Newts and some Anura fasten their eggs singly on to plants and other objects in the water, with or without threads of stiffening mucus. In many Anura, e.g. Bufonidae, they pass out as closely-set strings of beads, one string out of each oviduct; in others, e.g. Ranidae, they are disconnected, and form large, lumpy masses, especially when the gelatinous mantle swells up in the water. The use of this mantle seems to be chiefly the protection of the growing embryo, which in many species, when hatched out of the egg proper, drops into and remains for some time in the softened jelly. Possibly the latter affords some nutriment to the early larva.
Concerning the mode of fecundation it is to be remarked that copulation proper takes place only in the Apoda. For the Urodela Boulenger[27] has given the following summary. In no case does actual copulation take place. The male deposits the spermatophores which it is the office of the female to secure:–
II. No amplexus, but a lengthy courtship in the water; the male is more brilliantly coloured than the female, and ornamented with dorsal and caudal crests, or other appendages: Triton, cf. also systematic part.
II. Amplexus takes place; there are no marked sexual differences in colour and no ornamental dermal appendages.
A. Amplexus of short duration, partly on land, but deposition of the sperma in the water. No accessory sexual characters: Terrestrial Salamanders, namely Salamandra, Chioglossa, Salamandrina. Spelerpes breeds in damp caves without water.
B. Amplexus of lengthy duration and in the water.
a. The male, distinguished by a greater development of the fore-limbs, which are armed with temporary excrescences, clasps the female in the axillary region with the fore-limbs: Triton waltli.
b. The male, distinguished by a greater development of the hind-limbs and a prehensile tail, clasps the female in the lumbar and caudal regions: The Euproctus-group of newts: Triton asper, T. rusconii, and T. montanus.
The act of fecundation of most of the other kinds of Urodela, notably Cryptobranchus, Amphiuma, Proteus, has not yet been observed.
Embracing of the two sexes is the universal rule with the Anura, the male creeping on to the back of the female and clasping her firmly with the arms and hands either in the inguinal region, higher up, or under the armpits. See the numerous statements in the systematic part. This often extremely forcible, pressing embrace seems to be necessary, although the females can deposit the eggs without the help of the male, but in such cases the expulsion takes place at irregular intervals instead of at one time. When the eggs appear at last, and this happens in many species many hours, or even some days, after the beginning of the embrace, the male voids the contents of its seminal vesicles over them. Fertilisation is consequently external, with the possible exception of Pipa, q.v. p. 152.
Deposition of the eggs and nursing habits.–The majority of the Amphibia are oviparous, but some Apoda and Urodela are viviparous. It is unnecessary to call the latter condition ovo-viviparous, since this is really a distinction without a difference.
Viviparous forms:–amongst Urodela; Salamandra maculosa, the young burst the egg-membrane in the act of being born, and are provided with long gills; S. atra, the young undergo their whole development and metamorphosis within the uterus (see p. 119); Spelerpes fuscus, the young are likewise born in the perfect condition: amongst Apoda; Typhlonectes compressicauda and Dermophis thomensis.
The oviparous Apoda, at least Ichthyophis and Hypogeophis, and a few of the Urodela, as Desmognathus and Amphiuma, take care of their eggs by coiling themselves around them in a hole underground.
Nursing habits are very common amongst the Anura. {56}Boulenger[28] has summarised the various conditions concerning the deposition and care that is taken of the eggs, in the following list, in which more recent discoveries have been interpolated.
I. The ovum is small, and the larva leaves it in a comparatively early embryonic condition.
A. The eggs are laid in the water:–
a. Without further care or preparations: probably the majority of Anura; all European forms, except Alytes.
b. The eggs are laid in a specially walled-in part of the pond: Hyla faber.
B. The eggs are deposited out of the water:–
a. In holes, or under grass, near the banks of pools. The larvae are liberated and washed into the water by the next heavy rain: Leptodactylus ocellatus, L. mystacinus, Paludicola gracilis, Pseudophryne australis and P. bibroni.
b. On leaves above the water, the larvae dropping down when leaving the egg: Chiromantis rufescens, Phyllomedusa iheringi, Ph. hypochondrialis.
II. The yolk is very large and the young undergoes the whole or part of the metamorphosis within the egg; at any rate the larva does not assume an independent existence until after the loss of the gills.
A. The eggs are deposited in damp situations, or on leaves. The young escape as:–
a. Tadpoles: Arthroleptis seychellensis, Rhacophorus schlegeli, Rh. maculatus.
b. Perfect, air-breathing frogs: Rana opisthodon, Hylodes martinicensis, Hyla nebulosa.
B. The eggs are carried by a parent.
a. By the male:–
α. Round the legs; the young leaves the egg in the tadpole stage: Alytes.
β. In the enlarged vocal sacs; the young leave in the perfect state: Rhinoderma.
b. By the female:–
α. Attached to the belly: Rhacophorus reticulatus.
β. Attached to the back; the young complete their metamorphosis within the egg: Pipa.
γ. In a dorsal pouch which the young leave as tadpoles: Nototrema marsupiatum;–or in the perfect state: Nototrema testudineum, N. cornutum, N. oviferum, N. fissipes, and Hyla goeldii.
The development and metamorphosis of many species have been described in the systematic part. The following is a short general account of some of the more important features. Metamorphosis in the Apoda and Urodela is restricted chiefly to the reduction of the gills, the closing of the clefts, and the loss of the {57}gill-chamber and the finny margins of the tail; but the change from the tadpole to the final Anurous animal implies an almost entire reorganisation.
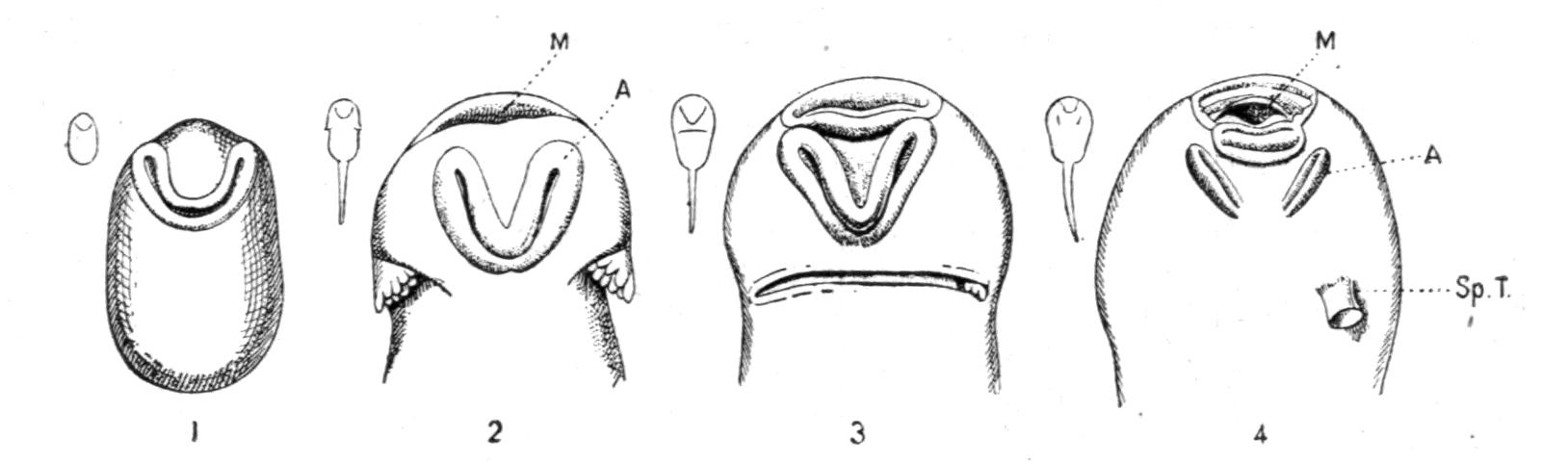
Fig. 9.–Four stages of the development of the adhesive apparatus (A) of Bufo vulgaris; M, Mouth; Sp.T. spiracular tube. In 3 the gills are almost completely hidden by the united right and left opercular folds. The small outlined figures indicate the shape and natural size of the tadpoles. (After Thiele.)
In the earliest condition the embryo consists of a large head and body, while the tail is still absent. Behind the beginnings of the future mouth appears a transverse crescentic fold, with the convexity looking backwards, which develops into the paired or unpaired adhesive apparatus. This consists of large complex glands, developed in the Malpighian layer, originally covered by the cuticula, which soon disappears, whereupon the sticky secretion enables the larva to attach itself to the gelatinous mantle of the egg, later on to weeds or other objects in the water. The name of suckers, often applied to this apparatus, conveys a wrong idea, there being neither muscles nor any suctorial function. The shape of this organ undergoes many changes during the early life of the individual, and differs much in the various genera, affording thereby diagnostic characters.[29] At first a crescent, it divides into a right and a left oval or disc, which either remain asunder and behind the mouth (Rana, Bufo), or they move forwards to the corners of the mouth (Hyla) or further back, and unite again more or less completely, as in Discoglossus and Bombinator. It is mostly of short duration, and disappears by the time that the larva, by the proper development of the gills and the tail and the functional mouth, changes into the tadpole. But in a few species these discs transform themselves into an elaborate ventral disc. Such an organ persists throughout the greater part of the tadpole-stage in certain Oriental species of Rana, all of which, when adult, possess fully webbed toes and {58}strongly dilated discs on the fingers and toes, e.g. Rana whiteheadi, R. natatrix, and R. cavitympanum of Borneo, R. jerboa of Java (this larva was originally described and figured as that of Rhacophorus reinwardti), and R. afghana of the Himalayan system. These tadpoles, at least those of R. jerboa, are further remarkable for having the "spiracular" opening very far back on the left side, nearer to the base of the tail than to the snout, so as to be well out of the way when the creature has attached itself by the adhesive disc.
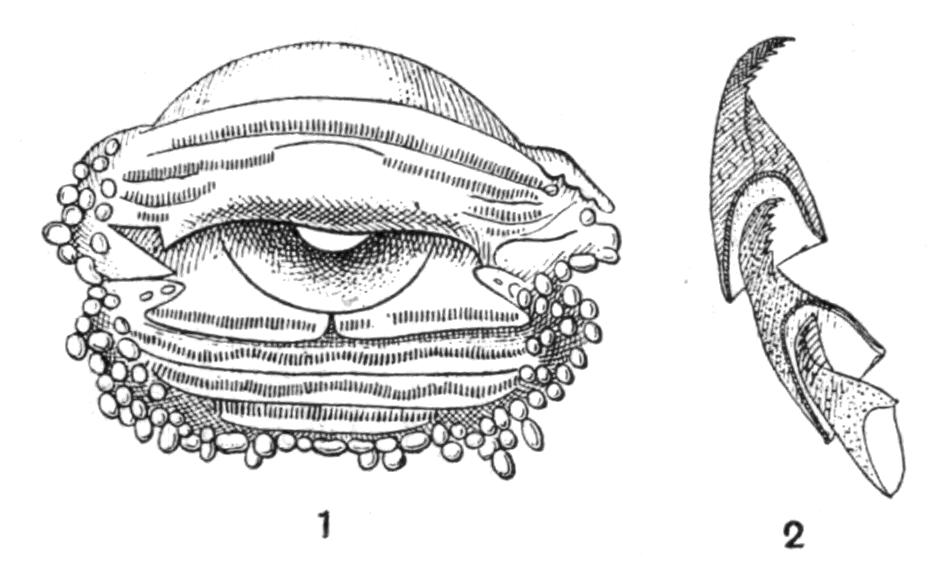
Fig. 10.–1, Front view of the mouth of a tadpole of Rana temporaria, showing the transverse rows of tiny horny teeth; 2, three successive horny teeth, much magnified. (After Gutzeit.)
The mouth of the tadpoles of Anura is furnished with horny armaments, substitutes for teeth. Their development and that of the mouth in general has been well described by Gutzeit.[30] In the young larvae of Rana temporaria, one or two days after hatching, a shallow groove appears above the conspicuous pair of adhesive organs. The groove becomes rhombic in outline, and when the mouth has been formed in its centre, the jaws appear in the median corners of the rhombus. The epidermis then rises like a circular wall around the jaws, and divides into an upper and lower lip; furrows appear on them, and between these various papillae and comb-like transverse plates of teeth. The papillae are possibly tactile organs, but although nerves enter them, nerve-endings of a sensory nature have not yet been discovered. On the fourth day the jaws become black, by the tenth day horny teeth have appeared upon all the plates of the mouth-armature, and on the seventeenth day the mouth-apparatus has reached the configuration typical of the tadpole, which is now about 14 mm. long. The number of horny teeth in R. temporaria amounts to about 640. These teeth are not cuticular products, but cornified cells; they are very small, and consist each of one horny cell, which is shaped like a nightcap, the apex of which is curved back and serrated. The little teeth are shed continuously, {59}the renewal taking place by successive cells growing into the bases of the older series. The shape and size differ much in the various genera and species. The comb-like plates, composed of those teeth which surround the lips, seem to be used chiefly for the fixing or hooking of the food, while those which compose the horny beak proper, the armature of the jaws, are used like the radulae of snails. These beaks are likewise composed of a great number of individual teeth, closely packed together in several rows, but the teeth themselves are simple and not serrated.
In Hyla arborea there are in all about 560 teeth. The development of the mouth does not begin before the eleventh day; the horny teeth break through, and the jaws get black edges, on the eighteenth. In Pelobates fuscus the number of horny teeth is increased to about 1100. In Borborocoetes taeniatus the horny teeth form series of five bells, which fit into each other like the joints of a rattlesnake's tail.
One of the most extraordinary kinds of tadpoles is that of Megalophrys montana.[31] Mr. Annandale (Skeat Expedition) found it at Bukit Besar, Malay Peninsula, from 2000 to 3000 feet above the level of the sea. The tadpoles (Fig. 11) were found in the beginning of the month of May 1899 in sandy streams and in pools of rain-water; they floated in a vertical position, the peculiar membranous funnel-shaped expansion of the lips acting as surface-floats. The inside of the funnel is beset with radiating series of little horny teeth, and the whole apparatus is possibly used for scraping the under-surface of the leaves of water-plants in search of food. Total length of the tadpoles 1 inch.[32]
The gills, the formation of the operculum, and the modifications of the branchial arterial arches have been described fully on p. 43; those of the hyo-branchial skeleton on p. 31. Fusion of the opercular fold with the skin of the neck, across the branchial region, causes the head to become confluent with the trunk (cf. Fig. 9, 3, p. 57). The body becomes oval, more or less globular, and the alimentary canal is greatly elongated and stowed away in the shape of a neat, very regular spiral, shining through {60}the ventral wall of the body; the anus opens at the end of a somewhat protruding tube, either in the median line, just in front of the ventral fin (Discoglossidae, Pelobates, Bufo), or it assumes an asymmetrical position by turning to the right side (Hyla, Rana).
Although both pairs of limbs begin to bud simultaneously, or the fore-limbs even earlier, the hind-limbs are hurried on, and appear first, long before the fore-limbs. The latter lie ready beneath the skin of the gill-chamber, and the right always breaks through the skin, while the left does the same in the Mediogyrinidae, while in the Laevogyrinidae it is generally pushed through the left-sided spiracular opening, immediately behind the outer gills. According to Barfurth the right limb appears, in about 80 per cent. of Rana esculenta, from two to eight hours before the left.
Meanwhile the lungs are being developed, and the tadpole occasionally rises to the surface to breathe air. The gills, which, as has been explained elsewhere, are less ancestral than they are larval organs, degenerate, and all the organs are modified for the coming terrestrial life. The fins of the tail are absorbed, the horny armature of the mouth and lips is shed in pieces and makes room for the true teeth, the eyes receive lids, and the whole cranium, especially the apparatus of the jaws, undergoes the final modifications–widening and lengthening of the mouth, arresting of the mento-Meckelian cartilages, elongation of the Meckelian cartilages or lower jaw proper, shifting backwards of the suspensorium, and lengthening of its orbital process to form the pterygo-palatine bridge.
The tadpole ceases to feed, the whole intestinal canal is voided of its contents, and by "histolysis" is thoroughly rebuilt, becoming wider and shrinking to about one-sixth of its original length,–undoing thereby the spiral–preparatory for the coarser food, which consists of insects, worms, and other strictly animal, living matter. Hitherto the tadpoles have lived on "mud," confervae, Diatoms, rotting vegetable and animal matter. The anal tube collapses, becomes ultimately absorbed, and a new vent is formed at and below the root of the tail.
Barfurth[33] has made interesting observations and experiments with regard to the absorption of the tail and other organs which disappear during the metamorphosis. This is retarded by low temperature; it is accelerated by rest and freedom from mechanical disturbances, as, for instance, concussion of the water. Hunger shortens or hurries on the last stages of metamorphosis, the absorption of the tail taking place in four instead of five days. Amputation of the tail has no retarding influence; it is followed at once by regeneration, although the tadpole may be on the verge of reducing the tail. Whilst hungering the whole organism draws upon its available store of material, naturally first upon those parts which sooner or later are to become superfluous. This applies eminently to the tail, which represents a considerable amount of "edible" matter, and also to that portion of the skin which still covers the fore-limbs. The elements of the cutis are resorbed, thereby thinning the skin; and consequently the limbs break through earlier in fasting than in well-fed {62}specimens. Nature herself seems to apply hunger as an accelerator. Mlle. von Chauvin found that the larvae of Urodela normally fast during the transformation, and according to Barfurth the larvae of Rana temporaria eat less after their hind-limbs are fully developed. This is, however, also preparatory for the reorganisation of the gut, which has to be more or less empty during the shortening process.
The loss of the tail is not due to a sudden dropping off of this organ–a crude but by no means uncommon belief–but is brought about by a very gradual process of resorbtion. When the fore-limbs begin to break through the skin, the tip of the tail shrinks and becomes black, owing to an increase, or rather concentration, of the pigment cells. The reduction proceeds from the tip forwards until on about the fifth day there remains only a short, conical, black stump. From the beginning of this process of reduction the tail is scarcely used for locomotion, the tadpole rowing with its legs, or it crawls and hops about, although the tail may still be 20 mm. long. The cells of the epidermis atrophy, shrink, and peel off, while those of the cutis, blood-vessels, nerves, muscles, and chorda dorsalis become disintegrated, often undergoing fatty degeneration. The leucocytes eat up the débris and other dissolved tissue, and carry it away through the lymphatic vessels, to be used as new building material in the rest of the animal.
Barfurth asks very properly, Why do these tissues degenerate and die? Because the vasomotor nerve-fibres cease to regulate the circulation. And why does this trophic influence of the central nervous system stop? Because the function of the tail becomes superfluous through the appearance of the fore-limbs. The tail is doomed, and degenerates like any other organ without a function. The whole process is, of course, a recapitulation of ancestral, phylogenetic evolution.
NEOTENY–REGENERATION–TEMPERATURE–GEOGRAPHICAL DISTRIBUTION
Neoteny.–It has long been known that the larvae of the Spotted Salamander occasionally attain the size of 80 mm. or about 3 inches, whilst the majority undergo metamorphosis when they are only 40 mm. long. Again, larvae of Triton have been found, in the months of April and May, 80 to 90 mm. long, still with functional gills, but with the sexual organs fully developed. De Filippi[34] found in one locality in Lombardy, besides a few normal fully metamorphosed specimens of only 30 mm. in length, more than forty specimens, which, although they had attained full size, about 55 mm., and were sexually mature, still retained their gills. According to him such gill-breathing, otherwise mature specimens, occur constantly in a small lake in the Val Formazzo, on the Italian slope of the Alps, in the province of Ossola. Later Duméril[35] astonished the world by his account of the metamorphosis of the Mexican gill-breathing Axolotl into an entirely lung-breathing and terrestrial creature, hitherto called Amblystoma, and supposed to be not only a different species, but to belong to a different family from the Axolotl, which was known as Siredon axolotl s. pisciforme, and naturally classed with the Perennibranchiata.
This discovery led to a series of observations and experiments, chiefly conducted by Marie von Chauvin, instigated thereto by Koelliker and by Camerano.[36] It was then found that many, if not most of the European Amphibia, both Urodela and Anura, {64}occasionally postpone their metamorphosis, and also that such Urodela sometimes become adult for all practical purposes, but retain their gills.
This retardation, the retention of larval characters beyond the normal period, was called Neotenie by Kollmann[37] (νέος, young; τείνω, extend, stretch). He distinguished further between:–I. Partial Neoteny, namely, simple retardation of the metamorphosis beyond the normal period, for instance, the wintering of tadpoles of Pelobates fuscus, Bombinator pachypus, Pelodytes punctatus, Alytes obstetricans, Hyla arborea, Rana esculenta, R. temporaria, Bufo vulgaris, and B. viridis: II. Total Neoteny, where the animal retains its gills, but becomes sexually mature; hitherto observed in Urodela only, e.g. Triton vulgaris, T. alpestris, T. cristatus, T. boscai, T. waltli and Amblystoma. Intermediate stages between these two categories are not uncommon.
A satisfactory explanation of the meaning of neoteny is beset with difficulties. Some authorities look upon the phenomenon simply as the result of adaptation to the surroundings, which make it advantageous for the creature to retain its larval features. Others think that the surroundings somehow or other retard or prevent the assumption of the adult characters. Undoubtedly there are many cases in which larvae have been reared in water-holes with steep walls, so that they could not change from aquatic to terrestrial life, and it stands to reason that abnormally forced and prolonged use of the gills and of the tail may stimulate these organs into further growth at the expense of the limbs and other organs which are intended for terrestrial life. But not unfrequently typical neotenic and overgrown specimens occur side by side with others which have completed their metamorphosis, and the same is true of larvae of newts which were reared, for experimental purposes, under exactly the same conditions–for instance, in a high-walled glass vessel.
Weismann tried to explain neoteny as cases of reversion to atavistic ancestral conditions, but this idea is based upon an assumption which is probably wrong. His idea necessitates the supposition that all the Amphibia were originally gill-breathing, aquatic, and limbless animals, and that every feature seen in a larva must necessarily indicate an ancestral phylogenetic stage. It is, on the contrary, much more probable that {65}the external gills of the Urodela have been developed in adaptation to their embryonic and larval, essentially aquatic, life. Consequently the possession of such gills would be a secondary, and not, strictly speaking, an atavistic feature. Normal loss of these gills, exclusively pulmonary respiration, and preponderating terrestrial life characterise the final adult Amphibian. These cases of neoteny are therefore instances of more or less complete retardation, or of the retention, of partially larval conditions.
The whole problem is, however, by no means simple. Salamandra atra has become viviparous, and the whole metamorphosis takes place within the uterus; in fact, the young have an embryonic, but no larval period, if by the latter we understand the free swimming and still imperfect stage. Similarly, various Anura–for instance, Hylodes martinicensis–pass rapidly through their metamorphosis, and have suppressed the stage of free swimming tadpoles. On the other hand, in many newts, the duration of the larval period is much prolonged, and moreover is very subject to individual variation. In the Axolotl this larval period is continued until and after sexual maturity is reached. The extreme condition would then be represented by the Perennibranchiate genera. It may seem reasonable to look upon these as the youngest members of the Urodela, and the loss of the maxillae in the Sirenidae and Proteidae supports this idea. But it so happens that the majority of the most neotenic genera are more primitive in the composition of the skull and the vertebral column than the typically terrestrial and rapidly metamorphosing genera. Witness the amphicoelous vertebrae, the completeness of the pterygoids, the separate nature of the palatine bones, and the separate splenials, as mentioned in detail in the description of their skull.
We have therefore to conclude, first, that the various Perennibranchiate genera do not form a natural group, but are a heterogeneous assembly; secondly, that they have become Perennibranchiate at a phylogenetically old stage–in fact, that they are the oldest, and not the newest, members of the present Urodela. At the same time, it would be erroneous to suppose that the first Urodela were aquatic creatures, provided with a finny tail, with small, ill-developed lungs, and with epidermal sense organs. All these features are, on the contrary, directly correlated with aquatic life, and are larval acquisitions, not ancestral {66}reminiscences. It would be equally wrong to allude to the absence of lungs in many newts as a piscine and therefore ancestral feature. The development of the typical pentadactyloid limb, the connexion of the pelvic girdle with the vertebral column, the development of the lungs, and absolute suppression of internal gills point without doubt to terrestrial creatures. What then, may we ask, were the first Amphibia like? and how about the external gills? They were undoubtedly akin to the less specialised Lepospondylous Stegocephali, in particular the gill-less Microsauri, and the various stages may perhaps be reconstructed as follows:–
(1) Terrestrial, with two pairs of pentadactyloid limbs; breathing by lungs only; with a fully developed apparatus of five pairs of gill-arches, which during the embryonic life perhaps still carried internal gills; with or without several pairs of gill-clefts. Reduction of the dermal armour and of the cutaneous scutes had taken place.
(2) Additional respiratory organs were developed by the embryo, in the shape of external gills; these were at first restricted to embryonic life (as in the existing Apoda), but were gradually used also during the aquatic life of the larva. These external gills, together with the lungs, have superseded the internal gills, of which there are now no traces either in Urodela or in Anura.
(3a) Some Urodeles, retaking to aquatic life, retained and further enlarged the external gills into more or less permanent organs (cf. also Siren, p. 136).
(3b) The majority of Urodela hurried through the larval, aquatic stage, and some–e.g. Salamandra atra–became absolutely terrestrial. The possession of unusually long external gills by this species and by the Apoda indicate that these organs are essentially embryonic, not larval, features.
Regeneration.–Most Amphibia possess the faculty of regenerating mutilated or lost limbs. This takes place the more certainly and quickly the younger the animal. The amputation necessary to study these phenomena need not be experimental. Axolotls and other Urodelous larvae frequently maim each other fearfully, by biting off the gills or one or more limbs. The gills do not even require amputation. If the larvae are kept in stagnant water the gills often shrivel up or slough off and grow again. {67}The same applies to the larvae of viviparous species, e.g. Salamandra atra, which, when cut out of the uterus and put into water, soon cast off their long, tender gills and produce a stronger set. In an Axolotl,[38] two years old, a hand was cut off. After four weeks there was a conical stump; after the sixth week this stump had two points; in the eleventh week three or four fingers were discernible, and a week later the complete hand. Frequently these creatures reproduce five instead of the normal four fingers. But the more proximal the cut, the more liable is the new limb to reproduce supernumerary fingers, or even extra hands and feet. Complete regeneration of the limb, cut off in the middle of the humerus, took place within five months.
Triton taeniatus, adult, reproduces cut fingers within five or six weeks, and if the hand be cut above the carpus, new finger-stumps appear in about one month. Götte has observed that an adult Proteus did not completely reproduce its whole leg until after eighteen months; and, according to Spallanzani, more than one year elapses before the limb, bones, and cartilages of Triton regain their normal strength.
The Anura are likewise capable of regenerating their limbs, the more readily the younger the specimens. For instance, in a tadpole of Rana temporaria, in which the fore-limbs were still hidden, the hind-limb, cut at the middle of the thigh, reproduced nineteen days later a knee, followed by a short two-toed stump. Ultimately the whole limb became completed. The tail of tadpoles regenerates very quickly and completely, even if it be cut off shortly before the final metamorphosis, when the tail would in any case be reduced. Metamorphosed Anura have almost entirely lost this faculty, but not absolutely. I myself have kept two specimens of Rana temporaria, which, when already adult, had each lost a hand at the wrist. First there was only the clean-cut stump with a scar, but within a year this changed into a four-cornered stump, and two of the protuberances developed a little further, reaching a length of about 4 mm. These specimens lived for four years without further changes.
Temperature.–Amphibia, like Fishes and Reptiles, are, as a rule, classed as cold-blooded animals, in opposition to the warm-blooded Birds and Mammals. This distinction is one of degree only. The terms poikilothermous and homothermous (ποίκιλος, {68}variable; ὅμος, equable) are based upon a sounder principle, but are likewise liable to exceptions. Those creatures which, like Birds and Mammals, possess a specific temperature of their own under normal conditions, that of hibernation being excepted, are homothermous. Cold-blooded creatures have no specific temperature; they more or less assume that of their surroundings. Frogs and newts, for instance, when living in the water, naturally assume its temperature, which is, of course, many degrees lower in a cold spring than in a shallow pond warmed by the sun on a hot summer's day. The same applies to the changes from day to night. Dark-coloured tortoises basking in the sun are sometimes so hot that they are disagreeable to touch, since they possess but little mechanism for regulating their heat. The same individual cools down during a chilly night by perhaps 40° C. Anura are, however, very susceptible to heat; most of them die when their temperature rises to about 40° C. Under such conditions they die quickly when in the water, but in the air their moist skin counteracts the heat, lowering it by evaporation; otherwise it would be impossible for a tree-frog to sit in the glaring sun in a temperature of 120° F. Toads and others with drier skins seek the shade, hide under stones, or bury themselves in the coolest spots available, and many Amphibia and Reptiles aestivate in a torpid condition during the dry and hot season. Many of them can endure a surprising amount of cold, and during hibernation their temperature may sink to freezing-point. This power of endurance does not apply to all alike; tropical species can stand less than those which live in temperate and cold regions. In spite of many assertions to the contrary, it may safely be stated that none of our European frogs, toads, and newts survive being frozen hard. They may be cooled down to nearly -1° C., and they may be partially frozen into the ice. Circulation of the blood is suspended in such cooled-down frogs; their limbs may become so hard that they break like a piece of wood, but the citadel of life, the heart, must not sink much below freezing-point, and must itself not be frozen, if the animal is to have a chance of recovering. The protoplasm resists a long time, and so long as some of it is left unfrozen the rest will recover. Hibernating frogs are lost if they are reached by prolonged frost during exceptionally severe winters. Every frog will be killed in an artificial pond with a clean concrete bottom, {69}but if there is sufficient mud, with decaying vegetable matter, the creatures survive, simply because they are not absolutely frozen. A severe winter not infrequently kills off all the younger creatures, while the older and more experienced hide themselves more carefully and live to propagate the race.
Geographical Distribution.
There is a very ably written chapter on the geographical distribution of the Amphibia by Boulenger in the Catalogue of Batrachia Gradientia, pp. 104-118. He came to the important conclusion that the geographical distribution of the Amphibia agrees in general with that of the freshwater fishes. Günther's division into a Northern, Equatorial, and Southern zone is modified only in so far as the last two are combined into one, "Tasmania and Patagonia not differing in any point regarding their Frog Fauna from Australia and South America respectively."
Boulenger recognises–
II. The Northern zone–(1) Palaearctic, (2) North American, region.
II. The Equatorial Southern zone.
A. Firmisternia division = Cyprinoid division of Günther.
1. Indian region.
2. African region.
B. Arcifera division = Acyprinoid division of Günther.
1. Tropical American region.
2. Australian region.
In the chapter on geographical distribution in Bronn's Thierreich, Vögel, Systematischer Theil, p. 296 (1893), and in my Classification of Vertebrata (1898), due attention had been paid to the Amphibia as well as to the other classes of Vertebrata. It will be seen in the following pages that my arrangement is well applicable to the Amphibia so far as fundamental principles are concerned.
It cannot be sufficiently emphasised that any attempt to form the various faunas of the different classes of animals into one scheme must necessarily be a petitio principii. The time-honoured six zoo-geographical regions established by Sclater and Wallace represent fairly well the main continental divisions: North America, South America, Africa, Australia, and the large northern continental mass of the Old World, with India as a tropical appendix. There is no correlation and no subordination {70}in this scheme. Huxley's division (1868) into Notogaea and Arctogaea (see p. 74) is of fundamental importance. The next improvement was the combination of the Palaearctic and Nearctic "regions" into one, an advance originally due to Professor Newton, carried out by Heilprin (1887) as the Holarctic region. I have, in 1893, substituted for it the more appropriate term Periarctic, meaning the whole mass of land which lies around the indifferent Arctic zone. The want of further co-ordination and subordination required the combination of the African and Oriental or Indian countries into a Palaeotropical region (1893); the Ethiopian or African and the Indian or Oriental regions of Sclater and Wallace thereby assuming their proper subordinate rank of subregions.
The two primary divisions Notogaea and Arctogaea are fundamental. The four secondary divisions, namely the Australian and Neotropical, Periarctic and Palaeotropical regions, also stand the test of application to the various classes and main groups of Vertebrata; but naturally, under the present configuration of the world, the Palaeotropical region is nothing but the Southern continuation of the Eastern half of the Periarctic mass of land. This is especially obvious so far as India is concerned. There is, however, that broad belt of desert, sand, and salt-steppes, which extends from North-West Africa to Manchuria, and this belt is one of the most important physical features of the Old World. It is complicated by the system of mountain-chains which, broadly speaking, centre at the Pamirs, and radiate westwards through the Caucasus and Alps into Spain, eastwards through the Himalayas into China, and north-eastwards to Kamtschatka; interrupted by Bering's Sea, it is continued as the backbone of both Americas to Patagonia.
The tertiary divisions, the subregions, have no real existence. They depend upon the class, or even order, of animals, which we happen to study. The faunistic distribution of the Urodela is not that of the Anura, and both follow separate lines of dispersal, different from those of the various orders of Reptiles, Birds, and Mammals. This must be so. There is no doubt that the distribution of land and water was totally different in the Coal Age from what it is now. The face of the globe at the Jurassic Age can scarcely be compared with the aspect which the world has assumed in the Miocene period.
This leads to another consideration, often neglected. We {71}know that the various classes, orders, families, etc., of animals have appeared successively upon the stage. A group which arose in the Coal Age followed lines of dispersal different from one which was not evolved until Jurassic times, and post-cretaceous creatures could not avail themselves of what assisted their ancestors, and vice versâ. The Amphibia are bound absolutely to the land and to fresh water; transportation across salt water is not excluded, but must be accidental, and is not a case of regular "spreading." Speaking generally, the older a group, the more likely is it to be widely distributed. If it appears scattered, this may be due to extinction in intermediate countries or to submergence of former land-connexions.
There is great danger of arguing in a circle. It is one of the most difficult tasks to decide in cases of great resemblance of groups of animals between their being due to direct affinity or to heterogeneous convergence, or parallel development. It is the morphologist who is ultimately responsible for the establishment of faunistic regions, not the systematist, least of all he who accepts an elaborate classification, and then mechanically, mathematically, by lists of genera and species, maps out the world. Let us take an example. The Neotropical region and Madagascar, but not Africa, are supposed to be faunistically related to each other. In both namely occur Boa and Corallus amongst snakes, Dendrobatinae amongst Ranidae, and of the Insectivora Solenodon in Cuba, Centetes in Madagascar. More cases can no doubt be found which would strengthen this resemblance, perhaps in support of the startling view that Madagascar and South America have received part of their fauna from the famous Antarctica. But the value of the Insectivores has been disposed of by their recognition as an extremely ancient group, or as a case of convergence, and the two genera are no longer put into the same family as Centetidae. The Dendrobatinae (Mantella in Madagascar, the others in South America) are decidedly not a natural group, but an instance of very recent convergence (cf. p. 272). About the members of the ancient Boidae we do not feel quite so sure.
It is therefore advisable to eliminate for zoogeographical purposes groups about which there can be any reasonable doubt, otherwise we may argue that certain genera must constitute a very old family, because they are now restricted to widely {72}separated countries, or on the strength of their distribution we may conclude that the genera in question cannot be related to each other, and do not belong to the same sub-family or family as the case may be. Such groups are the Engystomatinae and the genus Spelerpes; amongst reptiles the Eublepharidae, Helodermatidae, Anelytropidae, Ilysiidae, Amblycephalidae.
It is customary to represent the various regions and sub-regions as if they had boundaries as fixed as political frontiers. Such limitations are quite arbitrary, and what is of more importance, they differ in reality according to the class or order of animals with which we happen to deal. Moreover, there has been, and is probably still going on, an exchange or overlapping of faunas. Such debatable grounds are Central America and the highlands of North-western South America. The famous Wallace's line, between Borneo and Celebes, Java and Lombok, is absolutely inapplicable to the Anura. From their point of view the Austro-Malayan countries, Papuasia and Polynesia do not form a sub-region of the Australian, but rather of the Palaeotropical region. Concerning the Urodela, the division into Palae- and Ne-arctic sub-regions is unjustifiable since Eastern Asia has emphatically American affinities (cf. also p. 96). The Sahara and the rest of Northern Africa are intimately connected with Arabia, Persia, Afghanistan, and Northern India, just as equatorial Africa and Madagascar possess strong faunistic relationship with Southern India and the Malay islands.
Limiting factors of distribution.–Common salt is poison to the Amphibia; even a solution of 1 per cent prevents the development of their larvae. Consequently seas, salt lakes, and plains encrusted with saline deposits act as most efficient boundaries to normal "spreading." But undoubtedly many individuals have made long and successful voyages across the seas on floating trees. Solutions of lime are likewise detrimental to many species, and it is a general fact that limestone-terrain is poor in Amphibian life, unless, of course, sufficient accumulation of humus counteracts or prevents the calcareous impregnation of the springs and pools in meadows. Salamandra maculosa is, for instance, absent in Central Germany on the Muschelkalk, but it occurs in abundance in neighbouring districts of red sandstone or granite; nor can the larvae be reared successfully in very "hard" water. On the other hand, Proteus lives in the {73}subterranean waters of Carniola, where the whole country is nothing but limestone.
Cold is another powerful limiting factor. The absolute northern limit of Amphibian life coincides rather closely with the somewhat erratic line of 0° Centigrade of annual mean temperature, a little to the north of which line the ground remains permanently frozen below the surface. The surface-crust, which thaws during the summer, engenders an abundance of insects as food-supply, but its freezing down to the icy bottom makes hibernation impossible. There are, of course, some exceptions, for instance the occurrence of Urodela in the Schilka river and in the district of Lake Baikal.
Ranges of mountains are far less effective barriers than is generally supposed. In many cases the fauna is the same on either slope, and they act rather as equalising or dispersing factors, especially when they extend from north to south. Witness the Andes, owing to which Ecuador and Peru bear a great resemblance to the Central American fauna, and differ from the tropical parts of South America. The existence of an Amblystoma in Siam is another instance.
The more specialised a family the more intimately is it connected with the physical features of the country. Typically arboreal frogs are dependent on the presence of trees. Some have undoubtedly spread into treeless countries and have changed into prairie-frogs, e.g. Acris. They come out, so to speak, as something different at the other end, and it is unlikely that these modified descendants redevelop exactly the same features as their ancestors before the migration. Baldwin Spencer[39] met with only six species of frogs in Central Australia, Limnodynastes, Chiroleptes, Heleioporus, and Hyla. They are in the main identical with certain forms found in the dry inland parts of New South Wales and Queensland. They are to be regarded as immigrants from the latter regions, which have been able in the majority of cases to adapt themselves to unfavourable climatic conditions by means of a marked development of the burrowing habit, to which in certain cases has been added a capacity for absorbing and holding water.
Faunistic divisions of the Amphibia.
NOTOGÆA.–South World.
* indicates Amphibia which are peculiar to the respective regions or sub-regions.
Characterised by the Cystignathidae* and by the predominance of Arcifera, which form nearly 90 per cent of the Anurous population.
I. Australian region.–Absence of Apoda and Urodela. All the Anura are arciferous, with the exception of one species of Rana in the Cape York peninsula. The fauna of the Australian continent and of Tasmania consists chiefly of Cystignathidae and Hylidae (Hyla and Hylella) and several small genera of Bufonidae (Pseudophryne,* Notaden,* and Myobatrachus*).
It is customary, and from the study of other Vertebrata quite justifiable, to divide the Australian region into several sub-regions, but the Amphibia lend no support to this. The only Amphibian in the Sandwich Islands is a Bufo, closely related to North American species. The only Amphibian in New Zealand is Liopelma,* one of the Discoglossidae which are otherwise confined to Europe, North-east Asia, and North-west America, and, to judge from their low organisation, had formerly a much wider distribution. New Caledonia possesses no Amphibia. The Fiji Islands are inhabited by one or two species of Cornufer, a genus of Ranidae. The same genus is typical of the Austro-Malayan and Papuasian islands, the fauna of which consists of Rana and Cornufer, Ceratobatrachus, several genera of Engystomatinae, Hylidae, and Pelobatidae.
II. Neotropical region.–Characterised by Apoda, Aglossa (Pipa), abundance of Cystignathidae (Hemiphractinae,* Cystignathinae, and Dendrophryniscinae*), Hylidae (Hylinae and Amphignathodontinae*), numerous Bufonidae and Engystomatinae; Dendrobatinae*; the Raninae are represented by a few peculiar genera, mostly restricted to the Andesian province; the genus Rana occurs there in a few species only.
Absence of Discoglossidae, Pelobatidae and Dyscophinae.
Several species of Urodela, of the genus Spelerpes, extend from Central America into the Andesian province, one occurs in Hayti, and Plethodon platense in Argentina.
This region is by far the richest in the number of families, {75}genera and species; the total number of the latter being, according to Boulenger, about four-ninths of the known species. The region comprises South America, Central America, and the West Indian islands. Central America is naturally debatable ground; one species of Hylodes and one Engystoma, besides about twenty Hylidae, extend into North America proper, while possibly the Raninae have entered the Neotropical region from the north. Bufo is too cosmopolitan to assist our conclusions. The occurrence of four species of Hylella in South America, one in Australia, and one in New Guinea indicate that this is not a natural genus.
From the point of the Amphibia the whole region can be divided into two sub-regions only: (1) The West Indian islands with Central America and the north-western Andesian province; (2) the rest of South America.
ARCTOGAEA.–North World.
Characterised by the absence of Cystignathidae.
I. Periarctic Region.–Characterised by the Urodela, these being almost peculiar to the region (cf. p. 96). Absence of Apoda. Presence of Discoglossidae, Pelobatidae, Bufonidae, Raninae. Few Hylinae occur.
The whole region can be subdivided into three sub-regions.
1. Western Palaearctic.–Prevalence of Salamandrinae (Salamandra,* Chioglossa,* Salamandrina,* Triton); Proteidae (Proteus anguinus*); Spelerpes fuscus.*–Discoglossus, Bombinator, Alytes,* Bufo, Hyla arborea, Pelobates,* Pelodytes,* Rana.
2. Eastern Palaearctic.–Amphiumidae (Cryptobranchus); Salamandrinae (Triton, Pachytriton,* Tylototriton*); Amblystomatinae.–Bombinator, Bufo, Hyla arborea, Rana, Rhacophorus.
3. Nearctic.–Amphiumidae (Cryptobranchus, Amphiuma*); Proteidae (Typhlomolge,* Necturus*); Sirenidae*; Amblystomatinae; most Plethodontinae; Desmognathinae.*–Discoglossidae, Pelobatidae (Scaphiopus*); Bufo; Hylidae (Hyla, Acris, Chorophilus); Rana.
II. Palaeotropical region.–Characterised by the presence of Apoda and by the great prevalence of Firmisternal Anura, which amount to nearly 90 per cent of the total population. {76}Absence of Urodela (except Amblystoma persimile*), of Cystignathidae, and practically of the Hylidae, only two of which occur in the Himalayan district. But this great chain of mountains should not be included within the region, while the outlying spurs in Upper Burma (with Amblystoma) are debatable ground. The subdivision of this widely extended region is beset with difficulties, chiefly on account of Madagascar and Papuasia. The fauna of Madagascar is very remarkable. All its Amphibia are Firmisternal, a mixture of African and Indian forms. The island agrees with Africa, in opposition to the Oriental countries, in no special point; all the Raninae, except Megalixalus, Rappia, and two rather common species of Rana, belong to different genera. Madagascar differs from Africa by the absence of Apoda, of Aglossa, and Bufonidae. On the other hand, it agrees with India or with the Malay islands, in opposition to Africa, by the possession of Dyscophinae, of the Ranine genus Rhacophorus, and the Engystomatine genus Calophrynus.
Africa and India agree with each other, and differ from Madagascar by the possession of Apoda, the genera Bufo and Nectophryne, and by the close resemblance of several genera of Raninae.
India, the Malay islands, and Papuasia with Melanesia possess Pelobatidae (Leptobrachium,* Batrachopsis,* Asterophrys*), and thereby differ considerably from Africa and Madagascar. Batrachylodes* of the Solomon Islands has unmistakable affinities with Phrynoderma* of Karen, between Burma and Siam; Oreobatrachus* of Borneo much resembles Phrynobatrachus* of West Africa; and Cornufer, typical of the Malay and Melanesian islands, occurs also in West Africa. All these Raninae indicate that the Austro-Malayan and Melanesian islands belong to the Palaeotropical region. Ceratobatrachus,* type of a sub-family, is peculiar to Melanesia.
There are consequently several possible modes of subdivision, all with a different result, according to the group of Amphibia, which we may select as of leading importance, e.g. Apoda or Pelobatidae, or Dyscophinae and Rhacophorus. The Engystomatinae and Raninae are to be eliminated, since they occur in all the countries in question. We have either to leave the whole region undivided–and it is a significant fact that the {77}Indian countries possess not one sub-family of their own–or we must break it up into four provinces, not sub-regions:–
1. Ethiopian, or continental African, with Aglossa and Apoda, no Pelobatidae, no Dyscophinae, few Bufonidae, and many Raninae.
2. Indian and Malayan, with Apoda, no Aglossa, but with Pelobatidae, Dyscophinae, many Bufonidae and Raninae, amongst which Rhacophorus.
3. Malagasy, without either Apoda or Aglossa; with Firmisternal Anura only, chiefly Dyscophinae, and Rhacophorus and other Raninae.
4. Papuasian, without Apoda, Aglossa, Dyscophinae, and Bufonidae, but with Pelobatidae and Ranidae.
DISTRIBUTION OF FAMILIES AND SUB-FAMILIES OF THE AMPHIBIA.
| Australian. | Neotropical. | Ne-arctic. | Palae-arctic. | Ethiopian. | Malagasy. | Indian. | Papuasian. | New Zealand. | ||
| West. | East. | |||||||||
| Amphiumidae | + | + | ||||||||
| Salamandridae | + | + | + | + | 1 | |||||
| Proteidae | + | + | ||||||||
| Sirenidae | + | |||||||||
| Apoda | + | + | + | |||||||
| Aglossa | + | + | ||||||||
| Discoglossidae | + | + | + | |||||||
| Pelobatidae | + | + | + | + | ||||||
| Bufonidae | + | + | + | + | + | + | + | + | ||
| Hylinae | + | + | + | 1 | 1 | |||||
| Amphignathodontinae | + | |||||||||
| Hemiphractinae | + | |||||||||
| Cystignathinae | + | + | 1 | |||||||
| Dendrophryniscinae | + | |||||||||
| Genyophryninae | ||||||||||
| Engystomatinae | + | 1 | + | + | + | |||||
| Dyscophinae | + | + | + | |||||||
| Dendrobatinae | + | × | ||||||||
| Raninae | + | + | + | + | + | + | + | + | ||
| Ceratobatrachinae |
+ | |||||||||
| 1 signifies the occurrence of only one species of an elsewhere
numerous group. × Mantella, cf. p. 71 and p. 272. |
||||||||||
STEGOCEPHALI OR LABYRINTHODONTS–LISSAMPHIBIA–APODA
Sub-Class I. STEGOCEPHALI OR PHRACTAMPHIBIA
With a considerable amount of dermal armour, especially on the head.
The earliest known terrestrial four-footed creatures occur in the Carboniferous strata of Europe and North America. They and their immediate allies, which extend through the Permian into the Upper Trias, are now comprised under the name of Stegocephali, so called because the whole of the dorsal side of the cranium is covered, or roofed over, by dermal bones (στέγος, roof; κεφαλή, head). That these creatures, of which naturally only the skeletal parts are known, were not fishes, is shown by the typically pentadactyloid limbs; but to recognise them as Amphibia, and as distinct from Reptiles, is difficult, especially if the incipient Reptilia, which have sprung from some members of this Stegocephalous stock, are taken into account. However, they possess either two occipital condyles, or none, and their vertebrae are either pseudocentrous or notocentrous, but not gastrocentrous. Moreover, the whole skeletal organisation is still so ideally generalised, that it is easy to derive directly from it the arrangement prevailing in the Apoda and Urodela.
The vertebral column always comprises a well-developed, sometimes a very long tail. The vertebrae exhibit three types, two of which are fundamentally distinct, while the third is a further development of the second.
1. Lepospondylous and pseudocentrous.–The vertebra consists of a thin shell of bone surrounding the chorda dorsalis, and is composed of two pairs of arcualia, which meet each other, {79}forming a suture, along the lateral side of the vertebra, both partaking in the formation of a transverse process which carries the rib.
2a. Temnospondylous.–The vertebra is composed of three pairs of units, which remain in a separate, unfused state. Two of them are dorsal arcualia, one of which tends to form the centrum of the vertebra, which then carries the neural arch.
2b. Stereospondylous.–The three component units fuse by co-ossification into a solid, amphicoelous vertebra.
The ribs are one- or two-headed, rather strong, but short, rarely reaching half-way round the body. They occur on all the vertebrae of the trunk and on most of those of the tail. One pair of ribs connects one vertebra, the sacral, with the pelvis, of which the ilium and ischium are generally ossified, rarely also a portion of the pubic region.
The shoulder-girdle is very primitive, greatly resembling that of the Crossopterygian fishes. It consists of the following bones:–a median, rhombic, or T-shaped interclavicle, a pair of clavicles, of cleithra, of coracoids, and of scapulae. The limbs show the typical pentadactyle plan, but even in these earliest Tetrapoda the hand possesses only four fingers, with 2, 2, 3, 2 phalanges respectively. The foot has five toes, with 2, 3, 4, 4, 3, or 2, 2, 3, 4, 3 phalanges.
Many Stegocephali were possessed of a dermal armour, covering either the whole body or only the under parts. Hence the term Phractamphibia (φρακτός, armoured). The armour consists of a great number of small cutaneous scales, partly calcified, or perhaps ossified, and arranged in many more or less transverse rows. We can only surmise that these scales were covered by corresponding epidermal sheaths. The skull is ideally complete in the number of separate bones which appear on its surface. Besides the outer nares and the orbits there is always an unpaired, small, interparietal foramen. The whole temporal region is completely roofed over. The following bones are present:–nasals, frontals, parietals, supra- and latero-occipitals; lacrymals (unless fused with the jugals?), prefrontals, postfrontals, postorbitals, squamosals, and epi-(or opisth-)otics; premaxillaries, maxillaries, jugals, quadrato-jugals, and supra-temporals; quadrates, pterygoids, palatines, vomers, and an unpaired parasphenoid.–The lower jaw is composed of a pair of dentaries, {80}articulars, angulars, and splenials. The dentaries and apparently sometimes the splenials, the palatines, maxillae, and vomers carry teeth. The eyes possess a ring of sclerotic bones.
Order I. STEGOCEPHALI LEPOSPONDYLI.
Vertebrae pseudocentrous.
Sub-Order 1. Branchiosauri.–The young had several pairs of gill-arches, which, to judge from their size and from the fact that they are beset with numerous nodules, denticles, or irregular little processes like gill-rakers–seem to have been exposed to the surface and to have carried gills. In the adult the arches and gills seem to be absent.
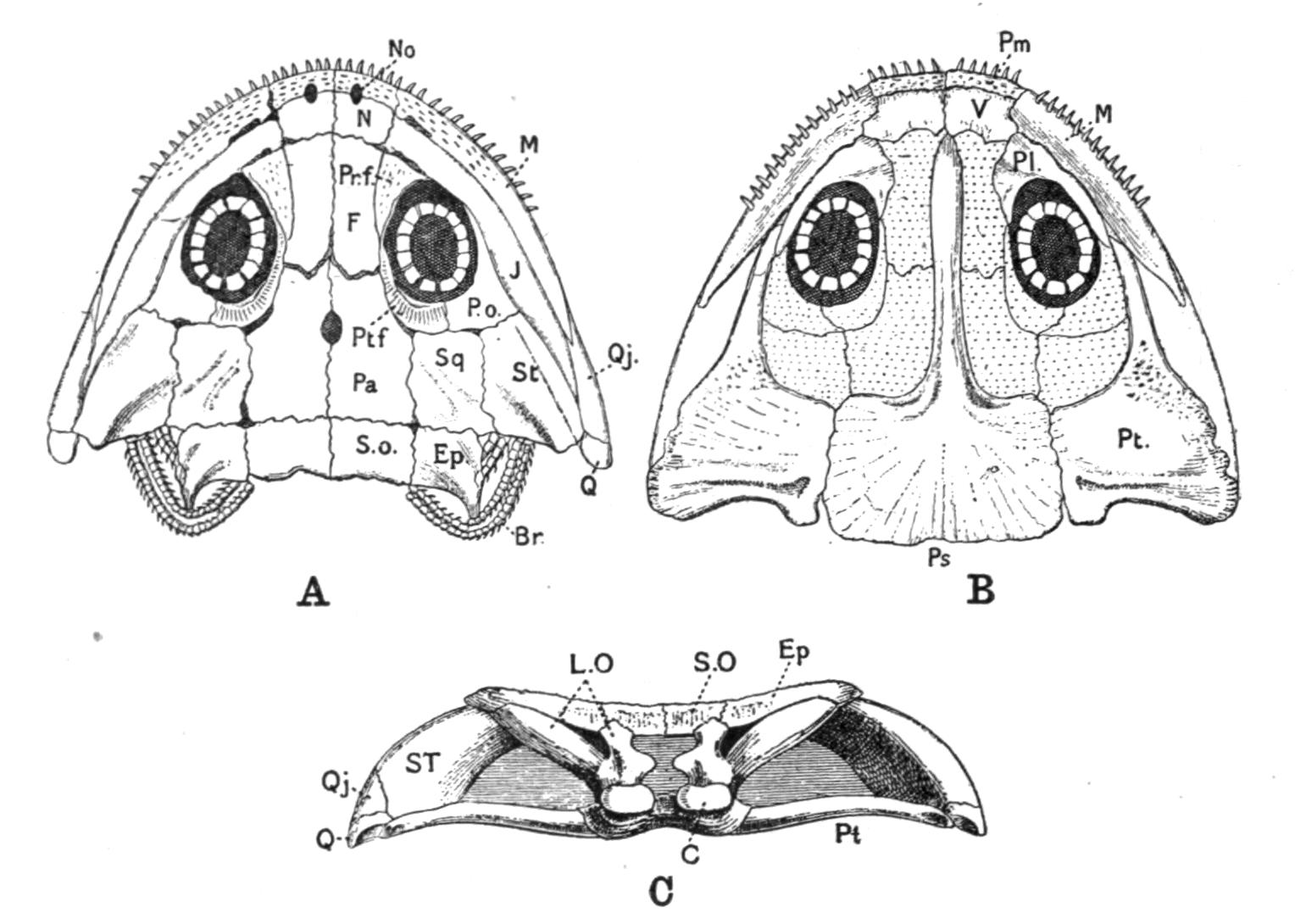
Fig. 12.–A, Dorsal and B, ventral views of the cranium of Branchiosaurus salamandroides, × about 4. (After Fritsch.) C, Posterior view of the cranium of Trematosaurus, × about ½. (After Fraas.) Br, Branchial arches; C, condyle; Ep, epiotic; F, frontal; J, jugal; L.O, lateral occipital; M, maxillary; N, nasal; No, nostril; Pa, parietal; Pl, palatine; Pm, premaxillary; P.o, postorbital; Pr.f, prefrontal; Ps, parasphenoid; Pt, pterygoid; Ptf, postfrontal; Q, quadrate; Qj, quadrato-jugal; S.o, supraoccipital; Sq, squamosal; St, supratemporal; V, vomer.
One of the commonest genera is Branchiosaurus, including Protriton. B. salamandroides of the Lower Red Sandstone of Europe is known in every stage, from larvae of 16 mm. to the full grown animal of 64 mm. in length. The whole body was {81}covered with little cutaneous scales. Pelosaurus and perhaps Melanerpeton are allied genera.
The following genera are small newt-like creatures of the Carboniferous age of Europe and North America. In Keraterpeton of Bohemia, Ireland, and Ohio, the dermal scales were restricted to the under parts; and the ribs were rather long, reaching half way round the body. Gills have not been observed. K. crassum, a European species, reached more than one foot in length, two-thirds of which fall to the tail. The ventral side is covered with a most elaborate armour, which consists of about eighty chevron-shaped rows of little scale-shaped nodules. The epiotic bones end in strange processes, carrying a pair of spikes, giving the skull a "horned" appearance, hence the generic name. Urocordylus is an allied genus.
Sub-Order 2. Aistopodes.–Body snake-like and without any limbs, hence the name ἄιστος, unseen; ribs long, and reaching half way round the body; from Carboniferous strata in Ireland and Bohemia, with allied, or perhaps identical forms in Ohio. Dolichosoma longissimum possessed more than 150 vertebrae, and was about a yard long. The epiotics end in obtuse projections, recalling those of Keraterpeton. These marvellous creatures had strange appendages, extending from behind the sides of the head, which were possibly the supports of external gills; since the upper end of one of the visceral arches, probably the hyoidean arch, is attached to the labyrinthic region, and from this arch starts a bony rod which carries long skeletal filaments. The body seems to have been naked.
Ophiderpeton had a compound ventral shield, while the skin of the back contained granular scutes. Although the Aistopodes have, not without reason, been looked upon as greatly resembling the Coeciliae or Apoda in organisation, especially in that of the vertebral column, the total absence of any other fossils which might bridge over the enormous gulf between the Coal Age and recent times, makes the attempt to derive the Apoda from these creatures very hazardous.
Order II. STEGOCEPHALI TEMNOSPONDYLI.
Mostly with rather long ribs and with chiefly ventral armour.
Chelydosaurus from the Lower Red Sandstone of Bohemia was 3 feet long, and possessed a beautiful, complicated, ventral armour, consisting of about sixty chevron-shaped rows, about three times as numerous as the vertebrae in the corresponding region. Sphenosaurus from the same strata and localities must have been 2 yards long. The trunk-vertebrae of both these genera were composed of four pairs of arcualia. Trimerorhachis from the Permian of Texas is very imperfectly known, but its trunk-vertebrae, as the name implies, consist of three pairs of separate arcualia, one of which, the interdorsal pair, tends to form a kind of centrum.
Dissorophus multicinctus, also from the Permian of Texas, has been described by Cope[40] as a "Batrachian Armadillo," and considered allied to Trimerorhachis. Ten vertebrae are known, of an aggregate length of 93 mm.; the length of the creature was perhaps one yard. The neural spines are elevated, and the apex of each extends in an arch on each side to the ribs. These spinous branches touch each other, forming a carapace. Above, and corresponding to each of them, is a similar dermal and osseous element, which extends from side to side without interruption in the median line, forming a dermal layer of transverse bands which correspond to the skeletal carapace beneath it. This creature remotely approaches the genus Zatachys, Cope, where a dermosteous scute is co-ossified with the apex of the neural spine. The systematic position of this genus is at least doubtful.
Archegosaurus decheni from the Lower Red of Germany, known by many well-preserved specimens, reached a length of 4 or 5 feet. The trunk vertebrae are tri-partite, those of the tail quadri-partite, like those of the trunk of Chelydosaurus. Young specimens show traces of gill-arches. The thoroughly terrestrial walking limbs have four fingers and four toes; the arrangement of the tarsalia, most of which are ossified, lend support to the view that the morphological axis went through femur, fibula, intermedium, the centralia, the second distal tarsale, and the second toe. The dentine and enamel of the teeth are much folded, and this feature, which applies to most members of this Order, to a lesser degree also to others, has caused them to be comprised under the name of Labyrinthodonta. The upper {83}surface of the head shows very characteristically arranged grooves, which probably contained slime-canals and possibly sensory organs.
Actinodon and Euchirosaurus are closely allied forms, chiefly from the Lower Red Sandstone of France; Gondwanosaurus occurs in the Permian of India.
Order III. STEGOCEPHALI STEREOSPONDYLI.
These are the most highly developed members of the typical Labyrinthodonta, characterised by their much-folded teeth, and by their solid, bi-concave vertebrae. Loxomma occurs in the Upper Carboniferous of England and in the Lower Red of Bohemia: Trematosaurus, Capitosaurus, and Metopias from the New Red or Lower Trias to the Keuper of Germany. Mastodonsaurus from the Trias of England and Germany is the most gigantic Amphibian known, with a skull of nearly 1 yard in length.
Labyrinthodon from the Keuper of Warwickshire is one of the latest members of the group. Labyrinthodont creatures have also been described from the Trias of South Africa, e.g. Rhytidosteus; those from North America are insufficiently preserved.
Many of these and allied genera have left their footprints in slabs of Sandstone, both Lower and New Red, in Europe, Africa, and America. But although their spoors are common enough, only a few can with certainty be referred to Stegocephali, e.g. Saurichnites salamandroides of the Lower Red of Germany. The spoors of Chirotherium, common in the New Red of Germany and England, for instance in Cheshire, belong to unknown owners; both the large hind feet (which measure nearly half a foot in length) and the much smaller fore feet, had five digits, the first of which stood off like a thumb. Five-fingered Stegocephali are unknown.
There is an almost complete absence of fossil Amphibia from the Upper Trias to the Oligocene. The Stegocephali as such seem to have died out with the Trias. The recent Amphibia, of course, must have had ancestors in the Mesozoic age. There is one little skeleton, from the Wealden of Belgium, which belonged to a newt-like creature, called Hylaeobatrachus croyi. Scarce fragments, described as Megalotriton, are known from the Oligocene of France, and Triton itself seems to be indicated by {84}remnants in the Lower Miocene of France and Germany. But fairly complete specimens of large creatures, much resembling Cryptobranchus, have been found in the Upper Miocene of Oeningen, Canton Solothurn, Switzerland. The first known specimen, now at Haarlem, indicating a total length of 3 feet or more, was described and figured in the year 1726 by Scheuchzer, in a learned dissertation entitled "Homo diluvii testis."
Betrübtes Beingerüst von einem alten Sünder
Erweiche Herz und Sinn der neuen Bosheitskinder.
Which may be rendered as follows:–
Oh, sad remains of bone, frame of poor Man of sin
Soften the heart and mind of sinful recent kin.
This was the motto attached to the illustration, and it remained a warning to mankind until Cuvier declared the skeleton to be that of some large newt. Tschudi named it Andrias scheuchzeri, but it is scarcely generically distinct from Cryptobranchus, being almost intermediate between C. alleghaniensis and C. japonicus, see p. 97.
Sub-Class II. LISSAMPHIBIA.
Amphibia without dermal armour.
Order I. APODA or LIMBLESS AMPHIBIA.
The Amphibia Apoda, Coeciliae or Gymnophiona, are a small group of worm-shaped, burrowing creatures, restricted to the Neotropical and Palaeotropical regions, excluding Madagascar. They have no limbs and no girdles. The tail is extremely short; the vertebrae are pseudo-centrous, and most of them carry rather long ribs, none of which, however, meet to form a sternum. The whole snake-like body is covered with a smooth and slimy skin which forms numerous transverse folds or rings.
The most remarkable feature of the skull is its solid compactness, which stands in direct correlation with the burrowing habits of these creatures. The whole dorsal surface of the cranium is practically roofed in by bone, so that, in this respect, it greatly resembles that of the Stegocephali; but this resemblance is produced chiefly by a broadening of those bones which exist also in the other Lissamphibia, while supratemporals and supra-occipitals are absent.
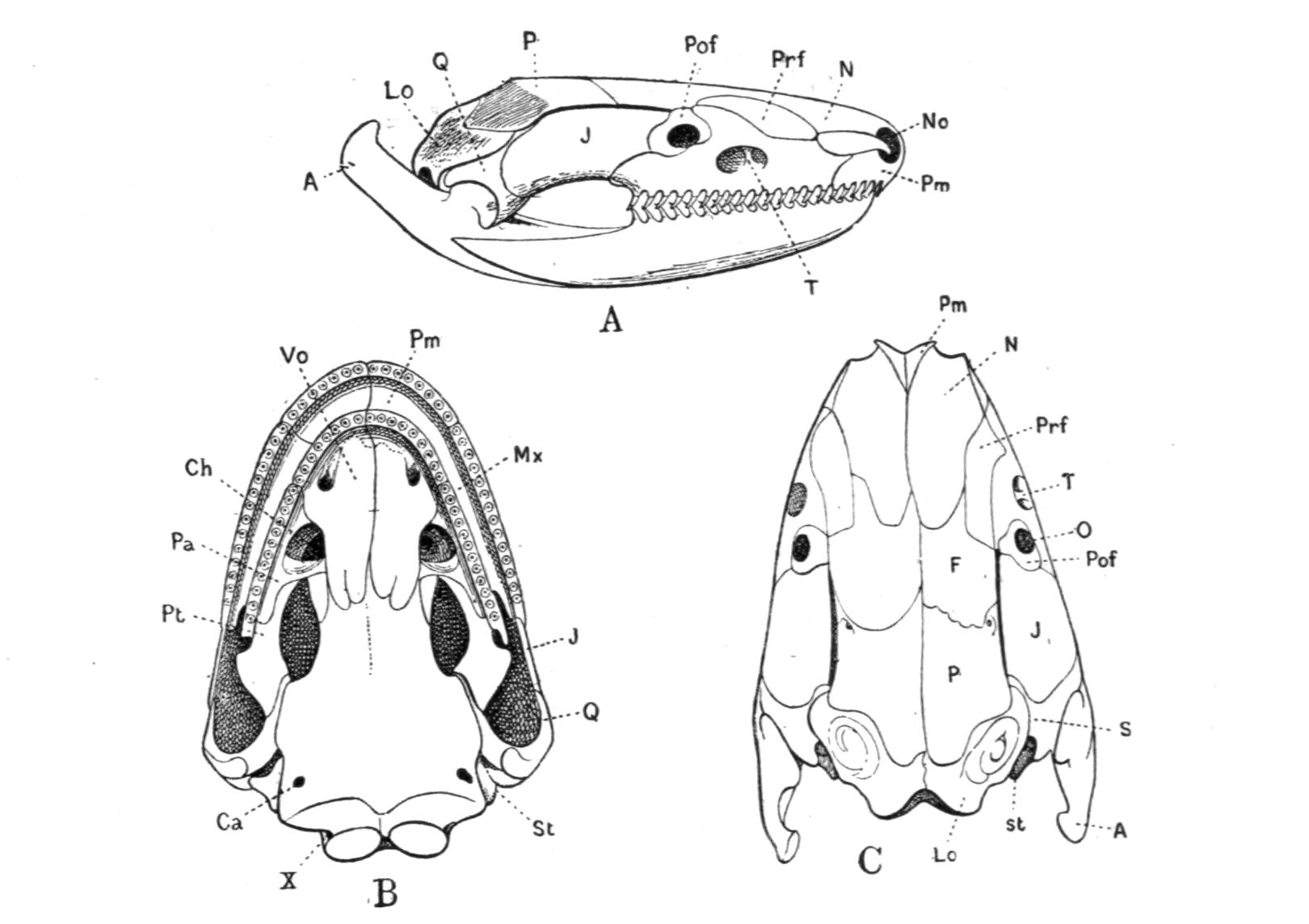
Fig. 13.–Skull of Ichthyophis glutinosa. × 3. (After Sarasin.) A, Lateral, B, ventral, C, dorsal view. A, Posterior process of the os articulare; Ca, carotid foramen; Ch, choana or posterior nasal opening; F, frontal; J, jugal; Lo, lateral occipital; Mx, maxillary; N, nasal; No, nostril; O, orbit; P, parietal; Pa, palatine; Pm, premaxillary; Pof, postfrontal; Prf, prefrontal; Pt, pterygoid; Q, quadrate; S, squamosal; St, stapes; T, tentacular groove; Vo, vomer; X, exit of vagus nerve.
There is, however, a pair of bones which represent either the postorbitals or the postfrontals, perhaps both, of the Stegocephali. The quadrato-jugal arch is enormously developed, and by reaching the parietal, frontal, and postorbito-frontal bones (which latter occur only in Ichthyophis and Uraeotyphlus) and the maxilla, extends over the whole of the orbito-temporal fossa. The squamosal is completely fused with the quadrato-jugal. The stapes has the typical stirrup-shape, is even perforated by an artery, and articulates distally with the shaft of the quadrate (as in the snakes). The maxilla is very large and broad. Owing to its broad junction with the quadrato-jugal arch, the prefrontal and frontal, the orbital fossa is reduced to a very small hole, or the maxilla completely covers the eye. Somewhere between the latter and the nares the maxilla is perforated by the tentacular groove. The periotic bones are represented by the prootics and epiotics; they fuse with the lateral occipitals and with the parasphenoid. The whole {86}orbito-ethmoidal region of the primordial skull is also turned into one mass of bone.
The angular element of the lower jaw forms a thick and large process which projects upwards and backwards from the mandibular joint. The former possession of a splenial bone is indicated by the occurrence of a second series of teeth in the mandibles of Ichthyophis and Uraeotyphlus. Other genera have vestiges of this second row, or it may be completely lost.
The hyoid and branchial apparatus is more primitive than in any other recent Amphibia. In the larva the hyoid and the first and second branchial arches are connected with each other by a median copular piece. The third branchial arches are free from the rest, but are fused in the middle line, the fourth are loosely attached to the previous pair. In the adult both fuse into one transverse, curved bar, and the second pair of branchials lose their connexion with the basal longitudinal piece and likewise form a transverse bar.
The vertebrae are built upon the pseudocentrous type, are amphicoelous, and the chorda is intravertebrally destroyed by cartilage, as in the majority of the Urodela. The number of vertebrae is great, amounting in some species to between 200 and 300, of which a few belong to the tail. The first vertebra is devoid of an odontoid process. The ribs are proximally bifurcated as in the Urodela.
The eyes are practically useless, being either more or less concealed under the skin, or they are covered by the maxillary bones. All Coecilians possess a peculiar tentacular sensory apparatus, which consists of a conical flap-shaped or globular soft tentacle, which is lodged in a special groove or canal of the maxilla, between the eye and the nose, whence it is frequently protruded while the animal is crawling about. These tentacles in the young Siphonops lie, according to the Sarasins, quite close to the eyes, but are later transferred nearer to the nose. The organ consists of a peculiarly rolled up and pointed fold which arises from the bottom of the sac or pit, where it receives a nerve. It is protruded by becoming turgid with blood, and is retracted by a strong muscle. Into the lumen of the sac are poured secretions from the large orbital (Harderian) gland, to keep the apparatus clean. Hence arose the mistaken {87}notion of its being a poison-organ. The whole structure is possibly an offshoot of the naso-lacrymal duct.
The skin is most remarkable. In the ripe embryo the epidermis passes smoothly over the surface. Beneath follow two layers of soft cutaneous connective tissue, bound together by transverse or vertical lamellae, so that ring-shaped compartments are formed, and in these are embedded slime-glands. In the adult each compartment is modified into an anterior glandular belt and a posterior space, from the bottom of which grow several scales. The number of cutaneous rings agrees originally with that of the vertebrae; but later, and especially in the hinder portion of the trunk, each ring breaks up into two or more secondary segments, and these no longer agree with those of the skeleton. Each scale is beset with numerous smaller scales which consist of hardened cell-secretions infiltrated with calcareous matter. The whole scale is consequently an entirely mesodermal product of the deeper layers of the cutis. The usual statement that the skin forms imbricating lamellae, on the inner side of which appear the scales, is wrong. The "lamellae" can be lifted up only after the general epidermal sheath has been broken artificially in the constrictions between the rings. No scales exist in the Indian genus Gegenophis and in the American Siphonops, Typhlonectes, and Chthonerpeton, a secondary loss which does not indicate relationship. The scales develop late in embryonic life, and they are reasonably looked upon as inheritances from the Stegocephali. The glands either produce slime, whose function seems to be the keeping clean of the surface of the body, or they are squirt-glands. The latter kind are also numerous and are filled with a fluid which is squeezed out by muscular contraction, and seems to be poisonous, as it causes sneezing to those who handle or dissect fresh specimens.
The Coecilians live in moist ground and lead a burrowing life. Their developmental history has only recently been studied, and in but a few species, see Ichthyophis, p. 91, and Hypogeophis, p. 92. The female is fertilised internally, copulation taking place by means of eversion of the cloacal walls in the shape of a tube. The spermatozoa possess an undulating membrane; the eggs undergo meroblastic division and the embryos have three pairs of long external gills. Some are viviparous.
The snake-like, limbless shape of the body (Fig. 15) is, as in {88}snakes, correlated with an asymmetrical development of the lungs; the left is reduced, while the right is drawn out into a long cylindrical sac. The liver is likewise very long, and partly constricted into a great number of lobes. Owing to the great reduction of the ribs progression is effected in an almost earthworm-like fashion by the peristaltic motion of the skin, assisted by its numerous ring-shaped constrictions.
The systematic position of the Coeciliae has been, and is still, a controversial matter. The Sarasins took up Cope's suggestion, that their nearest allies are the Urodela, especially Amphiuma, and they went so far as to look upon Amphiuma as a neotenic form of the "Coecilioidea," which they divided into Amphiumidae and Coeciliidae; the Coecilioidea and Salamandroidea forming the two sub-orders of the Urodela. They based this startling conclusion chiefly upon remarkable resemblances between Amphiuma and Ichthyophis, namely, (1) the mode of laying the eggs on land and coiling themselves around them; (2) the existence of remnants of a tentacular apparatus in Amphiuma; (3) Cope's statement that Amphiuma alone among the Urodela possesses an ethmoid like the Coeciliae. This latter point is, however, erroneous; it has since been shown by Davison[41] that Amphiuma possesses no ethmoid bone, but that, instead of it, descending plates of the frontals join below the premaxilla and function as a nasal septum, with a canal for the olfactory nerves.
We look upon the Apoda with more reason as creatures which of all the Lissamphibia have retained most Stegocephalous characters and at the same time form a highly specialised group equivalent to the Urodela and the Anura. The following are Stegocephalous inheritances peculiar to the Apoda in opposition to the other recent Amphibia: retention of cutaneous scales with calcareous incrustations, greatly resembling the scales of the Carboniferous Microsauri; occasional retention of post-frontal and lateral nasal or lacrymal bones, and of a second row of teeth in the mandible. To these may be added the presence of epiotic bones, and the primitive character of the branchial arches. The loss of all these characters would turn the present Apoda into limbless Urodela, but this assumption does not justify their inclusion in this Order. The possible homology of the tentacular apparatus has been discussed elsewhere, p. 45.
Fossil Apoda are not known; their subterranean life does not favour preservation.
Only family, Coeciliidae. About forty species are known. These have been placed in seventeen genera, mostly on comparatively slight grounds, and several of these genera are probably unnatural, the distinctive characters having undoubtedly been developed independently in various countries. We have to remember that the recent species are the remainder of a formerly much more numerous group; it is also likely that more will be discovered in the tropical forests of South America and Sumatra.
Boulenger[42] has distinguished them as follows:–
I. Cycloid scales embedded in the skin.
A. Eyes distinct, or concealed under the skin.
a. Two series of teeth in the lower jaw.
α. Quadrato-jugal (squamosal) and parietal bones in contact.
Tentacle between eye and nostril.
Ichthyophis, 2 species, India and Malay islands, p. 90.
Tent"cle below and behind nostril.
Hypogeophis, 3 species, East Africa and Seychelles, p. 92.
Tent"cle below and in front of eye.
Dermophis, 5 species, America and Africa, p. 93.
Tent"cle below the nostril.
Coecilia, 6 species, America.
β. Quadrato-jugal separated from parietal.
Tentacle close to the eye.
Rhinatrema, 2 species, America.
Tent"cle below and behind nostril.
Geotrypetes, 1 species, West Africa.
Tent"cle below nostril.
Uraeotyphlus, 3 species, West Africa and India.
b. One series of teeth in the lower jaw.
Tentacle in front of the eye.
Cryptopsophis, 1 species, Seychelles.
B. Eyes below the cranial bones. Quadrato-jugal in contact with parietal.
Tentacle near the nostril.
Gymnophis, 4 species, South America.
Herpele, 2 species, Panama and Gaboon.
II. Without scales.
A. Eyes distinct, or concealed under the skin.
a. Two series of teeth in the lower jaw.
α. Quadrato-jugal in contact with parietal.
Tentacle behind nostril; end of body laterally compressed.
Typhlonectes, 3 species, America, p. 93.
β. Quadrato-jugal separated from parietal.
Tentacle between eye and nostril.
Chthonerpeton, 2 species, America.
b. One series of teeth.
α. Quadrato-jugal and parietal in contact; tentacle in front of the eye.
Siphonops, 4 species, America.
β. Quadrato-jugal separated from parietal.
Bdellophis, 1 species, East Africa.
B. Eyes below the cranial bones.
a. Two series of teeth. Quadrato-jugal and parietal in contact; tentacle behind and below nostril.
Gegenophis, 1 species, India.
b. One series of teeth. Quadrato-jugal separated from parietal.
Scolecomorphus, 1 species, East Africa.
Boulengerula, 1 species, East Africa.
Ichthyophis glutinosa extends from the slopes of the Himalayas to Ceylon, the Malay islands, and into Siam. A second species, I. monochrous, occurs in Malabar, Malacca, Borneo, and Java. I. glutinosa reaches about one foot in length, with a greatest thickness of a little more than half an inch. The general colour is dark brown or bluish black, with a yellow band along each side of the body.
This species has been studied extensively by the Sarasins.[43] It breeds in Ceylon after the spring monsoon. The ovarian egg is oval, measuring 9 by 6 mm. The yolk is yellow; the blastoderm lies towards one of the poles. The strong vitelline membrane becomes surrounded in the oviduct by a dense albuminous membrane, which forms twisted chalazae, just like those of birds' eggs, and by these two cords the eggs are strung together. Around all this lies another mantle of albumen. The female digs a hole close to the surface in moist ground near {91}running water, and there lays about two dozen eggs. The egg-strings become glued together, entangled into a bunch, and the female coils herself round the bunch and remains in that position, probably to protect the eggs against other burrowing creatures, as blind snakes (Typhlops and Rhinophis) and certain limbless lizards, with which the ground literally swarms. During this kind of incubation the eggs assume a round shape, and grow to twice their original size, and the mature embryo weighs four times as much as the newly laid egg.
Fig. 15.–Ichthyophis glutinosa × 1. (After P. and F. Sarasin.) 1, A nearly ripe embryo, with gills, tail-fin, and still with a considerable amount of yolk; 2, female guarding her eggs, coiled up in a hole underground; 3, a bunch of newly laid eggs; 4, a single egg, enlarged, schematised to show the twisted albuminous strings or chalazae within the outer membrane, which surrounds the white of the egg.
The external gills are delicately fringed and red, and they move up and down in the fluid of the egg. The body of the embryo is at first white, but becomes pigmented with dark grey. A strong line of lateral sense-organs is formed, and a ring of them lies around the eye and others on other parts of the head. The short tail develops a fin. Of the three pairs of gills the third is the shortest, and is generally turned dorsalwards. In embryos of 4 cm. in length the longest gill measures as much as 2 cm. Yolk is still present in embryos which have reached the surprising length of 7 cm. Then the gills begin to shrink a little, and at this time one pair of gill-clefts breaks through at the base of the third external gill.
When the larvae are hatched the gills are lost. The young larva takes to the water in a gill-less state, and moves about like an eel. At the bottom of the gill-hole on each side two arches are visible, and there are at this stage neither inner nor {92}outer gills. The larvae frequently come up to the surface to breathe. The eyes are large and clearly visible, but the tentacles are still undeveloped. The epidermal sense-organs are numerous, and appear as white spots in the grey skin; about fifty extend from the gill-opening to the tip of the tail.
Ichthyophis seems to live a long time in the larval state. At last the gill-clefts close, the tail-fin disappears, and the tentacles come to the surface. The whole skin assumes a totally new structure, and the fish-like larva turns into a burrowing, subterranean creature so terrestrial that it gets drowned when made to remain in the water.
Hypogeophis.–According to A. Brauer[44] three species of Coecilians are found in the Seychelles: Cryptopsophis multiplicatus, which is rare, Hypogeophis rostratus and H. alternans. They live in moist ground, near the coast in swamps, higher up in humus, under rotten trees and rocks, down to the depth of one foot. In the island of Silhouette, Brauer found them in brooks, at least during the dry season, from May to September. The natives call them "vers de terre." They seem to propagate during the greater part of the year, provided there is sufficient moisture. The female coils round the eggs, which vary from half a dozen to thirty in number, those of H. rostratus measuring 7-8 mm., those of H. alternans only 4-5 mm.
The embryos undergo their whole development in the egg. Four pairs of gill-clefts break through, the first between the hyoid and the first branchial arch, the fourth between the third and fourth branchial arches. There appears also a spiracular cleft between the quadrate and the hyoid arch; this cleft is, however, only developed dorsally, and persists for a shorter time. The external gills appear at the same time as the clefts, upon the first three branchial arches; the third gill is the latest, and remains in a vestigial condition covered up by the two others. The gills, of which the second is the longest, are not (as stated by the Sarasins) direct prolongations of the gill-arches, but they begin as button-like growths upon the arches. They begin to disappear with the absorption of the yolk, getting actually smaller. In embryos of 6 cm. they are 6 mm. long, while in embryos of 6.5 cm. they are reduced to 4.5 mm. in length. The {93}first to disappear is the third gill, of course by being resorbed; and the clefts are closed before the creature leaves the egg. Hypogeophis not leading an aquatic larval life possesses no tail-fin in the embryonic state, the gill-holes are closed, and the epidermal sensory organs disappear long before the time of hatching.
Vestiges of gills appear also on the hyoid and on the mandibular arch, but on the latter they are of very short duration. Those of the hyoid gradually fuse with the first of the branchial gills, and these also concentrate with their bases so that they ultimately seem to spring from one common stem. Brauer remarks that the distinction between internal and external gills seems to be one of degree only; the hyoidean and mandibular gills namely start from the hinder margin of the arches, just like the internal gills of Torpedo according to Ziegler, while the other gills start from the sides of the branchial arches. He also found a pair of little swellings behind the last gill-cleft, and an unpaired swelling (corresponding with a double one in Ichthyophis) in front of the vent. Not unreasonably he sees in these swellings the last, very transitional vestiges of the paired limbs.
Typhlonectes compressicauda of Guiana and Venezuela is one of the largest Coecilians, reaching a length of 18 inches, with a body-diameter of ¾ inch. The general colour, as in most of these creatures, is olive brown to black. A sort of adhesive disc surrounding the vent occurs in this genus. Peters, who described this species, found in one female six embryos of comparatively enormous size, one of them being 157 mm. (more than 6 inches) long, and 12 mm. thick, and devoid of a tail-fin. Instead of lateral gill-openings there is a "bag" on each side 55 mm. long, upon which is distributed a blood-vessel. The Sarasins have examined the same specimen: The gills are not a bag, but consist of two flat, unbroken membranes which are closely connected with each other. In fact the outer gills of all Amphibia may be said to begin in the shape of small bags, whence sprout secondarily the gill-fringes; but in Typhlonectes they form these flaps instead of growing into the usual three gills. The embryos have no epidermal sense-organs, but plenty of skin-glands. Probably when born they take at once to terrestrial life, the flaps are possibly shed at birth, and there remains a little cicatrix.
Dermophis thomensis of West Africa (its other relations live in East Africa, South and Central America) is also viviparous.
LISSAMPHIBIA (CONTINUED)–URODELA
Order II. URODELA or TAILED AMPHIBIA.
The recent tailed Amphibia, Salamanders and Newts in the wider sense, have been grouped into four families which can be conveniently diagnosed by the following characters:–
Both the upper and lower jaws are furnished with teeth. Fore- and hind-limbs are always present.
Maxillary bones present.
Eyes free and devoid of lids .......... Amphiumidae, p. 97.
Eyes with movable lids[45] .......... Salamandridae, p. 102.
Maxillary bones absent.
Eyes without lids. Perennibranchiate .......... Proteidae, p. 132.
Both jaws are toothless. The hind-limbs, the maxillary bones and eyelids are absent. Perennibranchiate .......... Sirenidae, p. 136.
These four families are closely allied to each other, especially the Amphiumidae and the Salamandridae.
The geographical distribution of the Urodela is essentially Periarctic, except that about one dozen species each of Amblystoma and of Spelerpes extend southwards into Central America, and in the case of the latter genus even into the Andesian parts of South America. Plethodon platense inhabits Argentina.
The Urodela afford good reasons for dividing the Periarctic region into three co-ordinate sub-regions, namely, Nearctic, Eastern and Western Palaearctic. The difference between the European and the Eastern Asiatic fauna is well marked; the two are–at least with our present knowledge–separated by a wide stretch of country very poor in Urodele forms; while, lastly {95}there are not a few resemblances between this Eastern Asiatic and the American fauna. The Urodela thus lend no support to the usual division of the Periarctic into a Palaearctic and a Nearctic sub-region. Nor is it possible to divide the Palaearctic into a Eurasian and a Mediterranean province. We have in this case to distinguish between an American, an Asiatic, and a European fauna. The Asiatic or Eastern Palaearctic sub-region assumes the central position, at least from a merely geographical point of view. It would be unjustifiable to assume a spreading from this centre into Europe, and, on the other hand, into America. The centre existed more probably in the Arctic circle, now devoid of Urodela.
Fig. 16.–Map showing the distribution of the Urodela. "Ichthyodea" = Amphiumidae + Proteidae + Sirenidae.
So far as mere numbers of species are concerned the huge Asiatic or Eastern Palaearctic region is the poorest, but it is also the least explored, and China will probably yield a good many new forms. We know at present only 15 species, nearly all from the eastern half. These 15 species represent no less than 11 genera, 8 of which (= 73 per cent) are peculiar to the sub-region. Next comes the Western Palaearctic or European sub-region with about 21 recent species of 5 genera, 4 of which are peculiar. America is by far the richest, with no less than 66 species (36 eastern, about 16 western, and the rest Central American, etc.), belonging to 19 genera, 17 of which (= 90 per cent) are peculiar to the New World. But this richness in species is due mainly to the abundance of the two genera Amblystoma and Spelerpes, just as Europe is characterised by its many Tritons.
One of the most striking features of the Asiatic sub-region is {96}its difference from the European. They have very little in common. Pachytriton, Tylototriton, and two species of Triton (T. pyrrhogaster and T. sinensis) are the only Salamandrinae, while all the rest are Lechriodont (see p. 102), like the American Urodela, excepting the two American Tritons, T. torosus and T. viridescens.
Geographical Distribution of the Urodela
| Western Palaearctic. | Eastern Palaearctic. | American. | ||||
| Sirenidae |  |
... ... |
1 Siren 1 Pseudobranchus |
|||
| Proteidae | 1 Proteus | 1 Necturus | ||||
| Amphiumidae |  |
... (1 Andrias, Miocene) |
... 1 Cryptobranchus |
1 Amphiuma 1 Cryptobranchus |
||
| Salamandridae |  |
Desmognathinae |  |
... ... ... ... |
1 Thorius 1 Haptoglossa 3 Desmognathus 1 Typhlotriton |
|
| Pleithodontinae |  |
... ... ... ... ... ... |
21 Spelerpes 2 Manculus 7 Plethodon 3 Batrachoseps 1 Typhlomolge 2 Autodax |
|||
| Amblystomatinae |  |
1 Amblystoma ... 1 Batrachyperus 1 Ranidens 1 Geomolge 1 Onychodactylus 2 Salamandrella 3 Hynobius |
16 Amblystoma 1 Dicamptodon |
|||
| Salamandrinae |  |
... ... 14 Triton 1 Salamandrina 1 Chioglossa 3 Salamandra |
1 Pachytriton 1 Tylototriton 2 Triton |
2 Triton |
||
| 21 species, 6 genera | 15 species, 11 genera | 66 species, 18 genera | ||||
The occurrence of an Amblystoma, A. persimile, in the mountains of Siam and Burmah, is most suggestive, and others will in all probability be found. It must also be borne in {97}mind that the differences between the genera of Amblystomatinae are in reality very slight; and the same applies to the sub-families themselves. The presence or absence of teeth on the parasphenoid, the possession of amphi- or opistho-coelous vertebrae, do not mean much, and certainly do not forbid the notion that all the recent Urodela are the offspring of one common generalised stock which inhabited the northern portion of the globe. Nothing is gained by hiding the solitary European species of the essentially American genus Spelerpes under the name of Geotriton. It is a Spelerpes in all characteristic points. Speaking broadly, each of the three principal sub-families of Salamandridae is characteristic of a sub-region; the Salamandrinae of the Western Palaearctic, the Plethodontinae of the American, while the Amblystomatinae are chiefly Asiatic, at least so far as diversity of genera is concerned.
Fam. 1. Amphiumidae.–Without gills in the perfect state. The gill-clefts are in a vanishing stage, being either reduced to one pair of small holes or being altogether absent. The maxillary bones are present. Teeth occur in both jaws; those of the vomers form transverse rows. The vertebrae are amphicoelous. The fore-limbs and hind-limbs are present, but small. The small eyes are devoid of lids.
This family is now represented by two genera, with only three species, found in the United States and in Eastern Asia.
Cryptobranchus.–The limbs are functional, with four fingers and five toes. The outer digits and the sides of the limbs are bordered with folds of skin. The head and body are stout and depressed; the tail is short, laterally compressed, and provided with a fin. The skin is very glandular and slimy, and forms a thick, irregularly-shaped fold along the side of the body.
C. (Menopoma) alleghaniensis.–The gill-clefts are normally reduced to one pair, individually to the left cleft, the right closing up. There are, however, four branchial arches and vessels. The general colour is brown or grey above, sometimes with darker patches, lighter below. The "Hellbender" reaches a length of nearly 18 inches (about 46 cm.), is entirely aquatic, and is apparently restricted to the rivers and streams of the mountainous districts of the Eastern United States. It is very voracious, living on worms and on fish, being much disliked by the fishermen, as it takes the angler's bait, and destroys great quantities of the valuable food-fish Coregonus albus. Although rather common and easily kept, its larvae still remain unknown.
C. japonicus s. maximus.–The Giant Salamander of Japan differs from its American relation in one essential point only, namely, by the absence of gill-openings and of the modifications of the branchial apparatus connected therewith. It has but three branchial vessels, and the skeletal arches are reduced to two. It lives in Japan and in China, from 600 to 4500 feet above the level of the sea, in small streams of mountain-meadows. It feeds upon fishes, Amphibia, worms, and insects. It is easily fished with the hook and is eaten by the Japanese.
The first living specimen was brought to Europe in 1829 by Th. von Siebold, its discoverer. It grew within a few years from 1 foot to 3 feet in length, and died in 1881, at least fifty-two years old. Another specimen lived in the Hamburg aquarium for fourteen years, during which time it is said to have grown 36 cm. (more than 14 inches), having attained a length of nearly 4½ feet, or 134 cm. The largest specimen known measures 159 cm = 5 feet 3 inches.
The life-history of this species is still imperfectly known. Japanese picture-books contain drawings of the adult and of larvae, the latter showing three pairs of fringed external gills. Young specimens of 16 cm. length have already lost the gills, but still retain a cleft on either side of the neck, in the shape of a horizontal slit, and this is soon after closed up by the skin.
The best account has recently been given by Sasaki.[46] According to him the Giant Salamander leads a solitary life, concealed in dark places, under rocks in swift-flowing, thickly shaded small brooks of clear and cold water.
The animal may be easily captured with a fish-hook, baited with a fish, frog, or several earth-worms, and tied to a string a few feet in length. This is thrust by the aid of a small bamboo-stick into the salamander's retreat. The string is not tied to the stick, but the point of the loaded hook is forced into one end of it, far enough to keep it in place while this end of the rod is pushed under the rock. When the bait has been thus brought near the salamander, any bite will be instantly felt through the {100}rod. The latter is then withdrawn as quietly as possible, the hook and bait being left. As soon as a jerk of the string is noticed, a pull is made, which generally ends in the capture of the unfortunate animal. If the first pull should fail, the bait is replaced as before, and a second opportunity is offered, which the unwary creature accepts as readily as the first. The fisherman, having obtained one bite, is sure of ultimate success, as the salamander does not learn by experience to refuse the proffered morsel. When captured, it emits a peculiar slimy secretion, having an odour much like that of the leaves of the Japan pepper (Xanthoxylon peperitum). This secretion hardens into a gelatinous mass after a short exposure to the air.
Temminck and Schlegel state that the act of inspiration is ordinarily performed once every 6-10 minutes. This is true for specimens kept in tubs; but Sasaki is inclined to think that they perform this act less frequently in their native brooks. The eyes are so small that they are obviously of little importance; the salamanders capture their prey not by pursuing, but by waiting for its near approach, whereupon they seize it with their teeth by a swift lateral movement of the head. The eggs are said to be laid in August and September, and they form a string resembling a rosary. Each egg floats in a clear fluid, inclosed in a bead-shaped gelatinous envelope, and this is connected with the next by means of a comparatively small string. The egg measures about 6 mm. by 4 mm., and is yellow everywhere except at the upper pole, where it is whitish. All attempts to make Cryptobranchus breed in captivity have failed hitherto, owing no doubt to the difficulty of obtaining the cool temperature of its mountain streams. Sasaki's smallest specimens measured 19 to 20 cm. These had three pairs of very short branchial processes, from 3 to 5 mm. in length, attached just inside the branchial orifice. Each process was somewhat flattened and tapering, most of them still with branchlets. In another specimen, 20.5 cm. in length, the gills had almost wholly disappeared, but the branchial slits were still visible. One of 24.5 cm. length showed no trace of gills, and the branchial orifice was completely closed, but still marked by a light streak.
Amphiuma means s. tridactyla.–The limbs are very much reduced, and end in two or three little fingers or toes. Just in front of the fore-limbs lies the pair of small gill-clefts, each guarded by two flaps of the skin. There are four branchial arches. The general colour of this eel-shaped creature is black, lighter below. The head is covered with numerous pores, arranged in several rows, which unite in the region of the neck, so that only two rows extend along the sides of the body. It reaches a length of three feet, and lives in swamps or muddy waters, for instance in the ditches of rice-fields, burrowing occasionally in the mud, feeding on crayfishes, molluscs, small fishes, etc. It is confined to the south-eastern States of North America, from Carolina to Mississippi. According to Davison,[47] copulation takes place in May. The rather hard-shelled eggs are deposited in the following August or September, and are connected by a twisted cord. The female lies about them in a coil. The embryos, which are hatched in the month of November or December, have well-developed external gills. By the following February they have {102}reached a length of from 68 to 90 mm. (about 3 inches), living in damp localities under rocks or rooted stumps, and have already lost their gills. The legs are said to be relatively longer than they are in the adult.
Fam. 2. Salamandridae (Salamanders and Newts).–Without gills in the perfect state. Maxillaries are present. Both jaws are furnished with teeth. The eyes are protected by movable lids, except in Typhlotriton. Fore- and hind-limbs present, although sometimes very much reduced.
To this family belong by far the greater number of tailed Amphibia. They have been, for the sake of convenience, grouped into four sub-families, the determining characters of which are all internal and of comparatively slight importance. Little better is the division into Mecodonta, with the teeth of the palate in two longitudinal rows diverging behind and inserted upon the inner margins of the two palatine processes, which are much prolonged posteriorly, and Lechriodonta, in which the series of palatal teeth are restricted to the posterior portion of the vomers and form either transverse or posteriorly converging rows.
III. Series of palatal teeth transverse, restricted to the posterior portion of the vomers. Parasphenoid beset with dentigerous plates.
III. Series of palatal teeth transverse or posteriorly converging, restricted to the posterior portion of the vomers. Parasphenoid toothless. Vertebrae amphicoelous: Amblystomatinae, p. 109.
III. Series of palatal teeth in two longitudinal series, diverging behind, inserted on the inner margin of the long palatine processes. Parasphenoid toothless. Vertebrae amphicoelous: Salamandrinae, p. 115.
Sub-Fam. 1. Desmognathinae.–Comprising only three genera, with five species, in North America. Five toes.
Desmognathus.–The tongue is attached along the median line, free behind, oval in shape. Three species in the eastern half of the United States. D. fuscus is one of the lungless Urodela, for which condition see p. 46. The skin is nearly smooth; parotoids prominent, gular fold strongly marked. General colour above, brown suffused with pink and grey, sometimes with a dark lateral band; under parts mottled brown. The vomerine teeth are frequently absent. Total length, about 4 to 5 inches. They live, carefully concealed in the daytime, under {103}stones in or on the edge of the banks of little mountain streams. The eggs are laid in two long strings, and are wrapped round the body of the female like a rosary, the female having resorted to a hollow in the mud, below a stone or other suitable place. The outer envelope of each egg tapers out into a short stalk, and the several stalks all converge, or are glued together into one common knot, "much like a bunch of toy balloons held in the hand of a street vendor." The egg is said to be meroblastic. The larvae seem to remain in the egg until they are nearly adult, and they emerge at midsummer, with the gills already much reduced. The complete metamorphosis takes place in the autumn of the same year. These little newts can, according to Wilder,[48] be collected all the year round, in Massachusetts from March to December, except during the time of deep snow. They are nocturnal and are easily kept.
Thorius pennatulus, from Orizaba, Mexico, the only species, is noteworthy for its extremely large nostrils, and for the tongue, which is supported by a central pedicle, free all round, and ending in a thick knob, which can probably be protruded. The limbs are weak, and the digits are also much reduced. Total length, under 2 inches, or 50 mm.
Typhlotriton spelaeus, of the Rock House Cave in Missouri, is blind, the eyes becoming concealed by the skin during metamorphosis, when the gills are lost.
Sub-Fam. 2. Plethodontinae.–The five genera of this almost entirely American sub-family (only one species of which, Spelerpes fuscus, occurs in Europe) can be distinguished as follows:–
I. The tongue is attached by its central pedicle only, is free all round, ends in a soft knob and can be shot out to a considerable distance.
II. The tongue is attached along the middle line and cannot be protruded out of the mouth.
Spelerpes.–Except in a few species the limbs are well developed and possess 4 fingers and 5 toes, which are either free or webbed. But in the Colombian S. parvipes, still more in S. lineolus of Orizaba and S. uniformis of Costa Rica the limbs and digits are reduced to mere vestiges, and are practically without function, the body, with the extremely long tail, having assumed a wormlike shape. The young of many, if not all, species have a pair of short balancers below each nostril; in the adult these organs are reduced to little swellings or lost completely. Several species are lungless, see p. 46.
The geographical distribution of this genus, of which some twenty species are known, is very remarkable. The majority live in Mexico and in the United States, a few are found in Colombia and Northern Peru (S. altamazonicus and Plethodon platense being the only Urodeles hitherto recorded from south of the equator), one in Hayti (S. infuscatus), two (S. subpalmatus and S. uniformis) in Costa Rica, and S. fuscus in Europe.
S. bilineatus is a little newt under 4 inches in length–60-95 mm.–found in the Atlantic States. It is brownish-yellow above, with a black lateral line extending from the eye to nearly the end of the tail. The under parts are bright yellow. It lives on land, in damp places, concealed during the daytime under stones or old trees, whence it emerges after a rain or in the dusk of evening.
According to H. H. Wilder,[49] "the eggs are deposited in May and June in a single layer upon the lower side of submerged stones, each batch containing 30 to 50 eggs. The stones which are suitable for this purpose must be in the form of an arch, allowing the water to flow beneath. They are generally in the more rapidly flowing portions of the brook, but the depth of water must be such that the eggs are at all times entirely submerged. They are attached to the stone by gelatinous threads, proceeding from the outer envelope, and although they are generally contiguous, they {105}are each attached separately." The eggs are holoblastic. The larvae hatch early and continue for a long time in the larval state, probably two or three years.
S. porphyriticus s. salmoneus.–Yellowish-brown or purplish-grey above with tiny darker dots and markings. The sides of the body are salmon-coloured, with a tinge of yellow. The under parts are whitish, turning into salmon-pink on the tail. This beautiful newt reaches about 6 inches in length and has a very moist, slimy skin, which, combined with the lively motions of the creature, make it as slippery as an eel. It is found in the Alleghany range, from New York to Alabama.
Specimens which I am keeping prefer the wettest part of the cage, where they lie concealed in the moss and mud, leaving their hiding-places at night in search of insects. One of them escaped into the greenhouse and was discovered after nine months, having established its permanent home in a cleft between mossy stones: when the sweepings of a butterfly-net are emptied near its hiding-place it peeps out and with a flash of its long, forked, white-coloured tongue it secures its prey. Occasionally it goes into a tank, when it swims with rapid, undulating motions, the limbs being laid back and remaining inactive; it sometimes rises to the surface to emit and to take in air, but, although mostly resting half in the water, upon a rotten stump, it often lies for hours at the bottom without stirring. When kept in dry surroundings, the skin soon dries and wrinkles, and the animals show every sign of suffocation and general discomfort. The respiration of this lungless species by means of rapid movements of the throat is very limited, most of the necessary oxidisation of the blood being effected through the skin.
S. fuscus.–This, the only European species, is thoroughly terrestrial. It is found in the mountains bordering the Gulf of Genoa, and in Sardinia. Its total length remains under four inches. The smooth, very delicate and easily broken skin is brown above, light below, and speckled with lighter and darker markings. Below each nostril is a slight swelling, the remnant of the cirri or balancers common to the young of many species. It lives in shady surroundings, under stones, in old trees and in limestone-caves, glued to the walls with spread-out toes, belly and tail, quietly waiting for insects and spiders which it catches by flashing out the long tongue.
According to J. Berg,[50] it keeps well in cool, moist and well-ventilated places. It lives on flies, small beetles, and maggots; ants are also taken at once, probably owing to their lively movements, but a few minutes later the newts roll about in spasms and soon die. Towards the end of March one of Berg's specimens gave birth to four young, which were 36 mm., or nearly 1½ inches long, and differed from the adult only by their exceptionally large nostrils, thereby resembling the Mexican Thorius. The little ones shot out their tongues about 10 mm., feeding on Aphides.
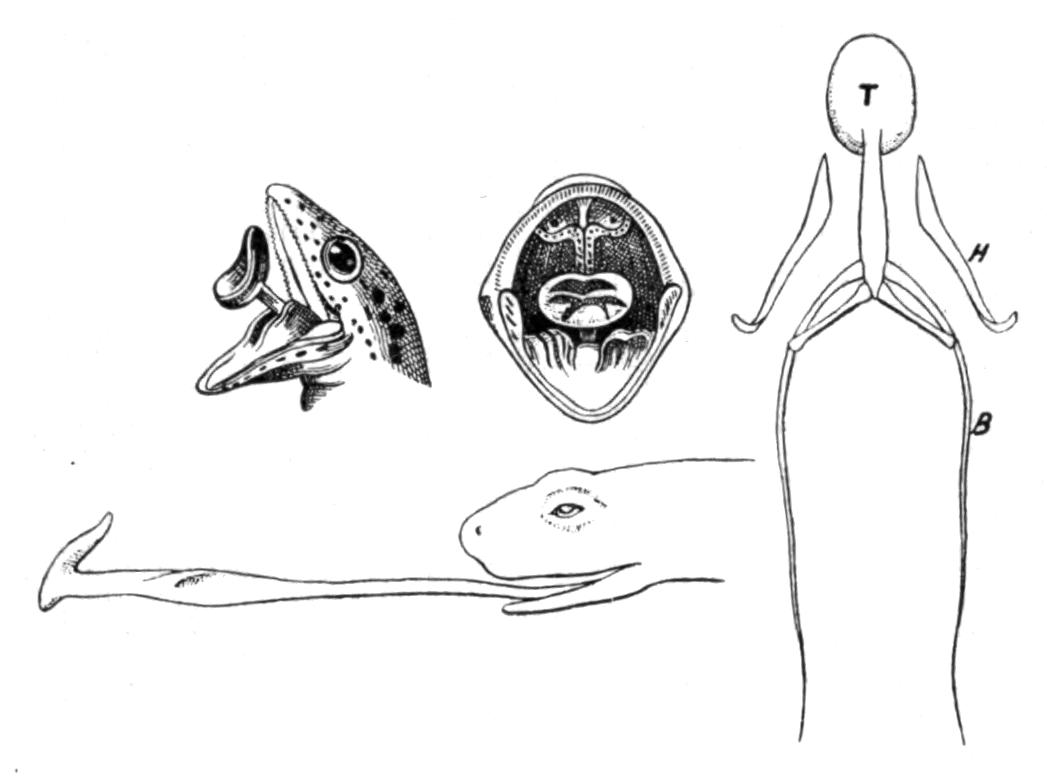
Fig. 20.–Spelerpes fuscus, showing the position and shape of the partly and fully protruded tongue. The figure on the right side shows the tongue and the skeleton of the hyoid apparatus. B, the threadlike, elongated, first branchial arch; H, hyoid, in reality attached by its outer end to the vicinity of the quadrate; T, tongue. About × 2. (After Berg and Wiedersheim.)
Manculus.–The two species of this genus live in Carolina and Florida. M. quadridigitatus is a very slender, graceful little animal, about 3 inches in length, the long and thin tail being considerably larger than the rest of the body. Yellowish, minutely speckled with brown above and on the sides, greyish-white below. Life entirely terrestrial.
Plethodon.–About seven species in North America. This genus has given its name to that of the subfamily, which might with more reason be called Spelerpinae.
P. glutinosus is slaty or bluish-black, with small whitish specks, especially on the sides of the trunk, where they are large and often confluent. The skin is smooth and shiny. Total length about 5 inches, half of which belong to the tail. Holbrook considered this as one of the commonest of the North American newts, and mostly widely distributed, from Ohio to the Gulf of Mexico. It usually lives concealed under stones, but prefers fallen trees, probably on account of the insects upon which it {107}preys. When taken in the hand it gives off a great quantity of slime.
P. erythronotus extends into Canada and is much smaller. Brown or grey above, mostly with a broad, reddish-brown band over the head, back, and tail. The under parts are white, with grey and brown specks.
Autodax s. Anaides.–The large tongue is attached along the median line. The jaws are furnished with few, but surprisingly large, knife-shaped teeth, about ten in the upper and fewer in the lower jaw. The small teeth of the vomers form a chevron-shaped series behind the choanae, those of the parasphenoid stand in one elongated patch. The tail is round; number of toes, five. Three species in Western North America, from California to Oregon.
A. lugubris.–The eyes are very large and prominent. The upper jaw shows a peculiar recess on either side for the reception of the large lower teeth. The skin is smooth, devoid of parotoid glands, but has a strong gular fold. The upper parts are dark brown or lead-coloured, with whitish dots on the sides; under parts white. Total length some 6 inches, about half of which belongs to the tail. The fingers and toes are very rich in subcutaneous venous sinuses.
The habits of these creatures are in many respects peculiar. Van Denburgh[51] says of A. iecanus "that it usually moves quite slowly, moving one foot at a time, but is capable of motion surprisingly rapid for a salamander. When moving rapidly, it aids the action of its legs by a sinuous movement of its whole body and tail. The latter is prehensile. Several individuals, when held with their heads down, coiled their tails around my finger, and, when the original hold was released, sustained themselves for some time by this means alone. One even raised itself high enough to secure a foothold. This animal's tail is also of use in another way. When caught, it will often remain motionless, but if touched, will either run a short distance with great speed, or quickly raising its tail and striking it forcibly against the surface on which it rests, and accompanying this with a quick motion of its hind-limbs, will jump from four to six inches, rising as high as two or three."
Ritter and Miller[52] have made extensive observations on the life-history of A. lugubris. When wishing to pass from the hand to {108}the table, the creature will frequently execute a well co-ordinated spring and alight on its feet some distance away, instead of falling over the edge in the typical salamander-fashion. This species is nocturnal and entirely terrestrial, and seems to be indifferent even to proximity to water. Rotten stumps and logs are the habitations preferred, and wherever these occur in the region about San Francisco Bay, even though at the places remotest from water, specimens are sure to be found.
The eggs are laid in a hollow under ground, and the female seems to remain curled around them until they are hatched, which takes place in two or three weeks. The specimen observed by Ritter and Miller laid 19 eggs. Each was contained in a gelatinous capsule 6 mm. in diameter, and was firmly anchored to a clump of earth by a narrow peduncle about 8 mm. long. The embryos developed very large gills, each being composed of three broad membranous lobes, the latter being thin and delicate, much expanded, highly vascular and widely confluent at their bases, so that the gills of each side really form one three-lobed mass. Their dorsal surfaces are applied to the inner surface of the egg-capsule. The amount of food-yolk is considerable. The whole larval life is passed through within the egg. Before the young is hatched the gills wither and cease to be functional, and the gill-slits close up. The tail is round, and shows no indication of a fin at any time during the larval period. Newly hatched individuals appeared much distressed when put into water, and were quite unable to swim. They immediately sank to the bottom and remained there until they were removed. The integumentary sense-organs, so well developed in the aquatic larvae of Urodeles, are entirely wanting. When hatched the young creature is about 32 mm. long; its general colour is blackish-grey, finely sprinkled with bluish-silver. During the second year this garb is changed to the dusky brown of the adult, and the fine silver speckling is replaced by much larger and less numerous yellow spots.
Although one of the most terrestrial of Urodeles, this species is lungless, but the skin remains delicately smooth and moist throughout life. According to the observers quoted, the pharynx plays an important part in respiration. From 120 to 180 or even more vibrations are made by the throat in a minute, and in some cases these movements are grouped into series of about {109}20 to 25 extremely rapid vibrations, with periods between each two series.
Subfam. 3. Amblystomatinae.–Composed of seven closely allied genera, the distinguishing characters of which are the grouping of the palatal teeth and the number of the toes, which varies between 4 and 5. The geographical range of the subfamily extends over the whole of North America and Mexico and over the whole of Northern Asia, from Kamtchatka and Japan westwards to the Ural, and southwards into China. The occurrence of one species, Amblystoma persimile, in the mountains of Siam, makes it highly probable that other species and genera exist in the hitherto unexplored intervening countries.
Boulenger gives the following synopsis:–
III. The series of palatal teeth converge backwards, forming a V-shaped figure.
With 5 toes: Hynobius, 3 species in Japan.
With 4 toes: Salamandrella, 2 species Lake Baikal, Ussuri and Schilka rivers, and Kamtchatka, p. 109.
III. The series of palatal teeth form an uninterrupted, doubly arched V-shaped figure.
The 4 fingers and 5 toes are furnished with black, horny claws: Onychodactylus japonicus.
III. The series of palatal teeth form two arches, convex forwards, separated by a wide interspace.
The two series are short, confined to the space between the choanae.
With 5 toes: Ranidens sibiricus, Eastern Siberia and N.E. China.
With 4 toes: Batrachyperus sinensis, Moupin in China.
The series are long and converge backwards, 5 toes: Dicamptodon ensatus, California.
IV. The palatal teeth are arranged in a nearly straight, transverse line, or they form an angle which points slightly forwards; they are not separated by a wide median space. With 5 toes: Amblystoma. Some 16 species in North and Central America, one in Siam, p. 110.
Salamandrella keyserlingi.–The mode of propagation of this newt-like species has been observed by Shitkow near Jekaterinburg in the Ural mountains. The eggs were laid at the end of April and were deposited in bags, which were attached to a plant, with one end about an inch below the surface of the water. The bag measured 15 cm. in length and 2 cm. in width and contained 50 to 60 eggs. The larvae were hatched in 14 days in a sunny aquarium; in another with a northern {110}aspect the hatching took 23 days. The larvae were 10 mm. long, and remarkable for the length (1 mm.) of their balancers.
Amblystoma opacum.–The general shape is very much like that of the European Spotted Salamander. The head is short and broad, the snout is rounded. The eyes are very prominent, with a black pupil and a dark-grey iris. The neck has a well-marked gular fold. The tail is thick and almost round. The hind-limbs are considerably larger than the fore-limbs. The general colour of the shiny, moist skin is a purplish-black with light grey, transverse, partly confluent bars, giving the creature a pretty appearance; the under parts are paler, bluish-grey. Total length between 3 and 4 inches, or 9 cm.
This beautiful species inhabits many of the United States east of the Rocky Mountains, from New Jersey to Florida and Texas. In the perfect state it is thoroughly terrestrial and easily kept. My specimens prefer the holes of rotten and moist, moss-covered stumps, or holes beneath stones, which they leave, at night only, in search of earthworms and insects.
A. talpoideum is closely allied, somewhat stouter and almost uniform brownish-back. According to Holbrook, "it chooses light soil in which it will bury itself in a few seconds like a mole, and there continue its course concealed from view; but its track can often be followed by the elevation produced on the surface of the soil, similar to that seen in fields infested by moles."
A. punctatum is bluish-black, with a row of roundish yellow spots on each side of the body and tail and upon the limbs.
E. A. Andrews[53] has made observations upon the breeding of this species. Near Baltimore the eggs are very abundant in March and even in February, in small pools in the woods, but the adults are then rarely seen. Even when small pools, but 4 feet wide and 9 inches deep, were thoroughly raked out {111}before and after the eggs appeared, no adults were found, so that it is to be inferred that the laying takes place in the night and that the adults leave the water every day to conceal themselves under stones. One female was found moving away from a bunch of eggs early in the morning. This specimen was kept isolated, and laid many eggs, and as these developed into normal larvae, the existence of internal fertilisation was proved. Previously to the laying of the eggs white spermatophores were found in the small pools, on the dead twigs and leaves covering the bottom.
A. jeffersonianum.–This very slender and slippery species, reaching a length of 6 inches, is remarkable for its long fingers and toes, and its rather compressed tail. The general colour is brown above, dirty whitish below, generally with numerous, small, light blue and pale brown spots on the sides of the neck, body, limbs, and tail. There are several colour-varieties, one of them with white specks. It is a very active and surprisingly good climber, easily escaping out of high-walled bell-glasses, hiding in the daytime in dark and moist localities. Its range extends from Indiana and Virginia to Quebec.
A. persimile.–This species is remarkable on account of its geographical distribution. It is the only non-American species, inhabiting the higher mountains of Siam and Upper Burmah. There is no doubt about its belonging to the genus Amblystoma, although it had originally been described as a Plethodon. It closely resembles A. jeffersonianum in most of its characters, notably in the arrangement of the palatal teeth, general proportions, slender toes, and even in the presence of whitish spots, which are scattered over the sides of its blackish, smooth skin.
A. tigrinum.–This, the commonest species, is conspicuous for its large, depressed head, which is as broad as it is long, its width being enhanced by the unusually large parotoid glands. The mouth is very wide. The large, prominent eyes are golden, and reticulated with brown. The gular fold is strong. The limbs are stout, the fingers and toes short. The trunk is strongly constricted by twelve intercostal grooves. The tail, which is as long as the rest of the body, is somewhat compressed laterally, but bears no trace of a fin. The general colour is more or less dark brown or bluish black, marked with numerous yellow spots and large blotches; the under surface inclines to {112}grey. The length of the adult male is about half a foot; the females, as usual being larger, sometimes reach the length of 9 inches. The range is from New York to California and to Central Mexico.
The larva of this species is the famous Axolotl. It is provided with three pairs of delicate and much-branched external gills, a flat, long tail with a broad ventral and dorsal fin, the latter extending along the back almost to the neck. The limbs, although comparatively slender, are fully developed, and the head is much more pointed than it is in the perfect form. The larvae usually reach 8 or 9 inches in length; exceptional specimens have been recorded of one foot in length, and have been described as Triton ingens.
These larvae were found by the Spanish conquerors to occur in great numbers in the lakes near Mexico City, and were called Axolotl by the natives, a word signifying "play in the water." They were, and are still, eaten, either roasted or boiled, with vinegar or cayenne pepper.
For many years these creatures were looked upon as a species of the Perennibranchiata, under the generic name of Siredon (S. axolotl, s. pisciformis, s. mexicanus, etc.), although Cuvier suspected that they were but the larvae of an otherwise unknown terrestrial Urodele. The mystery was not cleared up until the year 1865, when some Axolotls which had been kept for a year in the Jardin des Plantes at Paris, suddenly began {113}to pair, and laid eggs which within six months developed into full-sized Axolotls. This certainly looked as if these creatures were not larvae, but a true Perennibranchiate species. But to the general surprise several of these young Axolotls gradually lost their gills, the clefts closed up, the fins of the back and tail disappeared, the head became broader, the creatures left the water permanently, and in fact turned into the already well-known terrestrial Amblystoma tigrinum. The other brothers and sisters of the same brood remained aquatic Axolotls, which thereby revealed themselves after all as the larval and not as the perfect stage of this remarkable species.
At the suggestion of Kölliker and Weismann, Frl. Marie von Chauvin[54] undertook, at the University of Freiburg, long and carefully conducted experiments, showing (1) that little Axolotls can comparatively easily be caused to develop further into the perfect Amblystoma if they are induced to breathe air more frequently than usual; shallow vessels, perhaps also insufficiently aerated water, will produce the desired result; (2) that the commencing metamorphosis can again be checked, the shrinking gills then undergoing fresh development; (3) that they can be forced to remain Axolotls; (4) that the cutting off of the gills has no influence upon their possible metamorphosis, the gills being easily and quickly renewed. The same lady found also that Amblystoma, the perfect form, lives in the water during the pairing time and behaves in the same way as the Axolotls.
The latest observations have been made by Metzdorff.[55] Axolotls, at least those which are kept in captivity in Europe, are ready for propagation several times in the year, either in the spring, from April to June, or in December. The male deposits spermatophores, which in the following night are taken up by the female into the cloaca. On the following day, preferably in the afternoon, she grasps a suitable leaf, for instance that of Vallisneria, with the hind-limbs, and presses it against the vent. The eggs are expelled by strong wriggling movements of the body, and are formed into three or four packets of six to ten eggs each, so that about thirty eggs are laid at one sitting. {114}Then she takes a rest before proceeding again; the whole process, in which the male takes no further interest, lasting about two days. The most suitable temperature is one of 18-20° C., or about 68° F. The water must be well aerated. Sterile eggs turn white on the second day. The little larvae are hatched in about a fortnight. Eggs which are kept in a higher temperature, from 22-24° C., develop more quickly, but the resulting young are smaller; they show already on the fifth day head, tail, and the beginning of the gills. According to Bedriaga, they live at first upon Infusoria and Daphnia; when they are 20-25 mm. long they eat Tubifex rivulorum; later on they take scraped meat and are liable, when hungry, to nibble off each other's gills, but these are easily reproduced. When 20-25 cm. long, at the age of about six months, they are able to breed. The chief point of interest is the fact that this species of Amblystoma frequently remains throughout life in the larval state, except that it develops generative organs. The natural causes of this retention are not completely known. According to Shufeldt, who observed them under natural conditions near Fort Wingate in New Mexico, plenty of food, the drying up of the swamps, and the increasing temperature of the diminishing water, hurries on the metamorphosis, while deeper water retards it. Weismann[56] suggested that the specimens in the Mexican lakes which remained Axolotls were prevented from becoming perfect Amblystomas on account of these lakes, after the disappearance of the surrounding forests, having receded from their former boundaries, which are now covered with a saline, uninhabitable crust. This may be an explanation, although Axolotls do not live in brackish water. But Weismann went farther, and with his well-known dialectic powers has succeeded in spreading the belief not only that the Axolotl is a case of reversion to an ancestral stage, but that the present Amblystoma, instead of being the progressive, perfect form, is likewise a case of reversion. A reversion from a reversion! The whole line of evolution would then be as follows: Amblystoma; its young, owing to adverse circumstances, revert to the stage of the Perennibranchiate ancestors of all Urodela; if some of these Axolotls lose their gills and fins, they revert thereby into the original Amblystoma. {115}Surely a roundabout way of explaining a curious but after all rather simple process of Neoteny; cf. p. 63.
Observations on the metamorphosis of Siredon lichenoides into Amblystoma mavortium have been made by Marsh, who also gives figures of the larval and adult forms.[57]
Sub-Fam. 4. Salamandrinae.–The six genera of this subfamily fall into two natural groups: I, True Salamanders, with the palatal teeth arranged in a pair of S-shaped figures, and without a fronto-squamosal arch. II, Tritons, with the palatal teeth in the shape of a Λ, i.e. the right and left series meet at an angle; the fronto-squamosal arch is present, either bony, or at least ligamentous. Triton cristatus is, however, exceptional, in that the two palatal series often do not meet and that the arch is absent. The number of fingers is universally four, that of the toes is five except in Salamandrina, which has only four.
The geographical distribution of the sub-family, entirely Periarctic, may be said to be the reverse of that of the Amblystomatinae. Of the twenty-five species namely, only two are American, four are Eastern Asiatic, and of the remaining nineteen, two are Algerian, while the rest live in Europe or in Asia Minor. It is in fact an essentially Palaearctic group.
The six genera can be distinguished as follows:–
II. The palatal teeth are arranged in two S-shaped curves. True Salamanders.
II. The palatal teeth are arranged in a Λ shape. True Tritons.
Salamandra.–Without fronto-squamosal arch. Five toes. Tail round. Three species in Europe and Western Asia.
S. maculosa.–The Spotted or Fire Salamander. General habit stout. Usual length about 5 to 6 inches; the females are mostly larger than the males; specimens of more than 8 inches in {116}length are giants. Head as broad as it is long, snout rounded. Limbs and digits stout and short. The skin is smooth, shiny and full of pores, with a strong gular fold. The parotoid glands are large and covered with large pores. A series of distinct swellings, or cutaneous glands, each with a distinct opening, extends along either side of the back, and a shorter series along the flanks. The general colour of the Spotted or Fire-salamander is black, with irregular, large yellow patches on the back and limbs. These markings vary extremely, so much so that scarcely two specimens, collected at random, are alike. In some the yellow patches form two more or less regular bands, in others they are partly confluent; again the yellow may be preponderant on the back or much restricted. Occasionally the chrome-yellow is replaced by orange. The under surface is as a rule bluish grey-black. This combination of shiny yellow and black is a good instance of warning colours. The creature is poisonous, cf. p. 38. When left in peace, or handled gently, it is perfectly harmless, but when treated with violence, or submitted to severe pain, a milky white fluid exudes from the glands and is, under violent contractions of the muscular skin and body, sometimes squirted out in fine jets to the distance of a foot. Burning pain and subsequent inflammation result if this poison gets into the eye. The same applies to the mucous lining of the mouth and throat. A few drops of this poison introduced into the blood or into the stomach of a small animal are sufficient to cause its death. Cold-blooded animals are as susceptible as warm-blooded creatures.
I once put two American bull-frogs into the same outdoor enclosure with a large number of salamanders. Next morning the huge frogs were found dead, each having swallowed a salamander, which they were not acquainted with and had taken without suspicion.
The Fire-salamander has a wide range, namely the whole of Central, Southern, and Western Europe with the exception of the British Isles. It extends southwards into Corsica and Algeria, eastwards through Asia Minor into Syria. Where it does occur it is rather common, provided the terrain is mountainous or hilly and covered with vegetation. There it lives under moss or rotten leaves, in the roots of old trees, in the cracks and clefts of the ground, of rocks or of ruins of buildings; {117}in default of anything better under heaps of stones, or in the holes dug by mice or moles. One chief necessity for its happiness is moisture.
The salamander does not occur everywhere, but is rather local. On certain kinds of limestone it is rare or absent; granitic terrain and red sandstone seem to suit it best, for instance the Hartz Mountains, Thuringia, and Heidelberg are favourite localities. But even there we may spend days and weeks and never come across a single specimen. We may turn stones, rake up the moss and leaves, pry into cracks, and we unearth perhaps a few sorry-looking, listless, dull and dry, half-emaciated creatures. The same place after a thunderstorm will be literally swarming with sleek, lively salamanders, in search of earthworms and all kinds of insects, especially at dusk or during the night. They disappear in the autumn, in October, to hibernate in the ground, out of the reach of frost, and they reappear again in April. Later on they congregate at little springs, always at running water, to reach which they have often to make long migrations. This is the only time when these thoroughly terrestrial creatures approach water, in which they easily get drowned.
Although this species is so common its mode of reproduction has been satisfactorily discovered only quite recently. There are some puzzling facts which it took a long time to observe correctly and to interpret. The larvae are born in April, May, or June, while there are no eggs in the oviducts, but in July these are full of fertilised eggs before copulation takes place. This seems contradictory. The explanation is as follows. In July there is an amplexus of the sexes, short, and often on land–a sort of preliminary exciting performance. Both sexes then descend into the water, but generally remain on land with the fore part of the body. The male deposits a spermatophore and the female takes part of this into its cloaca. In the case of a virgin female the eggs are fertilised in the oviduct and ripen until the autumn, but the larvae nearly ready for birth remain within the uterus until the following May, i.e. about ten months. The mother then crawls half into the water, mostly at night, and gives birth to from a few to fifty young, fifteen being perhaps the average. The young are surrounded by the egg-membrane, which either bursts before or shortly after expulsion. This species is consequently viviparous in the proper sense. If she produces a few young only, say from {118}two to five, these are much larger and stronger than those of a large litter. Occasionally a few addled or only partly developed eggs are also expelled.
In the case of old females which have produced offspring before, the whole process is more complicated. The sperma, taken up in July, remains in the receptaculum of the cloaca until the May or June following, i.e. until the previous larvae have passed out of the uterus and are born. Then the spermatozoa ascend to the upper ends of the oviducts, where they meet and fertilise the new eggs. After these have descended into and filled the uterus, and are already developing into embryos, copulation takes place again in July, preparatory for next year's eggs.
The new-born salamanders have three pairs of long external gills, a long tail furnished with a broad dorsal and ventral fin, and four limbs, although these are small. The total length is about 25 mm. or 1 inch. The general colour is blackish with a pretty metallic golden and greenish lustre. The little creatures are very active, and at once eat living or dead animal matter. In captivity they are liable to nibble each other's gills and tails. During the first six or eight weeks they assume a row of dark spots on the sides; these spots enlarge, and the whole skin becomes darker. Yellow spots appear next, first above the eyes and on the thighs, later upon the back; the ground-colour at the same time becomes black, until at the beginning of the fourth month they look like the parents.
The metamorphosis is very gradual. The tail-fin diminishes first, but the gills grow until shortly before the little creatures leave the water. Darkness, cold, and insufficient food retard the metamorphosis, sometimes until October. It is easy to rear them artificially provided they are well fed, kept in a light place, and in clean, well aerated water. If prevented from leaving the latter, for instance when kept in a glass vessel with vertical walls, or if hindered by a piece of gauze from rising to the surface and taking in air, they can be kept as larvae well into the winter.
Very young, perfect little salamanders, of from 1 to 2 inches in length, are excessively rare; even specimens of 3 inches are far from common. They probably spend the first two or three years of their life in careful seclusion.
A few adults can be easily kept for many years in shady {119}places provided with moss, rotten stumps and stones, to afford them suitable moist and cool hiding-places, and they readily take earthworms, larvae of beetles, snails, woodlice, etc. But any attempt to keep them in large numbers ends in failure. They congregate together in clumps, all making for the same cavity or recess, as if that were the only one in existence (very likely they are right in so far as that place is probably the best), and they get rapidly enlarging sores, chiefly on the elbows and knees. These are soon infested with fungoid growths, and this disease spreads like an epidemic and soon carries them off.
S. atra.–The Alpine Salamander differs from the Spotted Salamander by its uniform black colour and smaller size, which averages between 7 and 5 inches. It is restricted to the Alps of Europe, from Savoy to Carinthia, at from 2000 to as much as 9000 feet elevation, living with predilection near waterfalls, the spray of which keeps the neighbourhood moist, or in mossy walls, in the shade of forests near brooks, or under flat stones on northern slopes. The most interesting feature of this species is that it produces only two young at a time. These are nourished at the expense of the partially developed eggs in the uterus, and they undergo their whole metamorphosis before they are born. By far the best and most complete account of this mode of propagation has been given by G. Schwalbe.[58] The length of the ripe embryos is about 45 mm.; they lie mostly bent up, with their heads and tails turned towards the head of the mother. The gills are beautiful, delicate red organs, the first pair being generally directed forwards and ventralwards, the second upwards, the third backwards; they are longest when the creature is about 32 mm. long, while there is still much yolk present. At this stage the gills are so long as to envelop nearly the whole embryo. There is rarely a second embryo in the same uterus, and an extra foetus is generally smaller, frequently a monstrosity not fit to live; it is probable that it is not used as food, but that it is expelled at parturition. The embryo passes through three stages, (1) still enclosed within its follicle and living on its own yolk, (2) free within the vitelline mass which is the product of the other eggs, (3) there is no more vitelline mass, but the embryo is possessed of gills 10-12 mm. in length, and is still growing. During the {120}second stage the yolk is directly swallowed by the mouth. The walls of the maternal uterus are rather red. The exchange of nutritive fluid takes place through the long external gills, which thereby function in the same way as the chorionic villi of the Mammalian egg. Each gill contains a ventral artery and a dorsal vein, each of which looks like the midrib of a pinnate leaf; there is also a fine nerve and a weak bundle of striped muscular fibres. Each gill-filament receives a capillary artery which extends to the epithelium of the tip, where it turns into a capillary vein. The epithelium of these filaments, which are full of blood, is ciliated, the resulting current being directed from the base towards the tip. In older larvae this ciliation becomes restricted to the tips. The body of the gills is furnished with flat epithelium, these non-ciliated portions alone are closely appressed to the uterine wall, and it is here that the exchange of gas takes place between mother and larva. The nutrition takes place through the gills, as they are bathed by the yolk-mass.
Schwalbe also explains the whole question of the reduction of the number of embryos. He says rightly that in S. maculosa, which gives birth to many young, there are in the oviduct many eggs which have only partly developed into embryos, and these, perhaps from want of room and nourishment, degenerate into the irregularly shaped whitish-yellow bodies which are occasionally found packed in between the developing embryos. Consequently all those eggs had been fertilised near the ovaries. S. atra exhibits a further stage in so far as most of the eggs, fertilised above in the oviduct, degenerate, and only two or three become fully developed. These few embryos live on the degenerating eggs, which together produce the vitelline material spoken of above. The two full-grown and metamorphosed embryos, each measuring about 50 mm. in length, are equivalent to the numerous new-born larvae of S. maculosa, especially if the smaller size of the adult Alpine Salamander is taken into consideration.
Mlle. von Chauvin[59] has experimented with the unborn larvae of this Salamander. She cut out 23 larvae and put them into water. One of them, already 43 mm. long, took earthworms on the next day, and the beautiful long, red gills became pale and shrunk, and on the third day were cast off close to the {121}body. New gills sprouted out on the same day, first in the shape of three tiny knobs on either side. After three weeks they had become round globes, which gradually sprouted out into several branches, far shorter and more clumsy than the original gills. During the whole time the larva was lying quietly at the bottom, in the darkest corner, but showed a good appetite. The fin of the tail disappeared and was supplanted by a stronger one. In the sixth week the skin was shed in flakes, and this process took fifteen days. This larva lived in the water for fourteen weeks and grew to 6 cm. in length! When the new gills gradually shrank, the compressed and finny tail assumed a round shape, the skin became darker and shinier, and after the larva had again shed its skin, there appeared the dark rugose skin of the typical S. atra. The gills were reduced to useless appendages–not cast off–and the creature crawled out of the water. A fortnight later the gill-clefts were closed. A second larva behaved similarly, first casting off the feathery gills, substituting a new and stronger set, which, however, fourteen days after excision from the uterus, shrank again, and on the nineteenth day the gill-clefts were closed. The lady also observed that nearly ripe larvae, when cut out, rushed about in the water and ate, just like the new-born larvae of the Spotted Salamander.
A third species, S. caucasica, is found in the Caucasus. It rather resembles the Spotted Salamander in coloration, but has a larger tail and lacks the lateral warts. The male is remarkable for the possession of a soft permanent knob or hook at the top of the root of the tail. This pommel possibly prevents the slipping off during the amorous amplexus, provided the sexes then entwine like certain Tritons.
Chioglossa lusitanica.–The only species of this genus is restricted to the north-western third of the Iberian peninsula. This graceful, slenderly-proportioned and beautiful Salamander is apparently very rare and local, having hitherto been found at a few places, namely, near Coimbra, Oporto and Coruña. It lives under moss, and runs and climbs with an agility surprising in a Urodele. The tongue is long, ending in a fork, and is supported by a median pedicle so that the tip can be quickly protruded to the distance of more than an inch. The whole length of the animal is about 5 to 6 inches, two-thirds of which belong to {122}the long tail, which is compressed at the end. The skin is smooth and shiny, with a gular fold and large parotoids. The general colour is a rich dark brown, with a pair of broad reddish-golden bands along the back and tail, the bands being separated by an almost black vertebral line.
The few specimens which I have been lucky enough to observe made little holes or passages in the moist moss of their cage, peeping out with their heads in wait for little insects, which they caught with flash-like quickness. They seem to be crepuscular.
Salamandrina perspicillata.–This genus, represented by one species, a native of Liguria and Northern Italy, possibly extending into Dalmatia, is the only Salamander which has but four toes. The skin is not shiny and smooth, but is finely granular and dry, forms no gular fold, and is devoid of parotoid glands. The tail is more than half the length of the animal, which measures from 3 to 4 inches. The general colour is black-brown with a broad V-shaped orange-yellow mark extending from eye to eye over the occiput. A faint irregular yellowish line extends along the middle of the back and tail. The throat is black, with a diffused white patch in the middle; the belly is white, with black dots; the anal region, the inner sides of the legs and the under side of the tail are carmine-red.
This slender and pretty Salamander is diurnal, and feigns death when discovered. Only the female goes into the water, in March, to glue the eggs on to submerged rocks or water-plants. The young finish their metamorphosis by the month of June, and reach full size during the winter, the climate of their home being sufficiently genial to make hibernation scarcely necessary.
Triton s. Molge.–The tail is strongly compressed and frequently has a permanent fin. The fronto-squamosal arch is variable, it being either bony as in the South European, Eastern and American species, or reduced to a ligament, or lastly absent as in T. cristatus. The males of all the English Newts, of T. vittatus and of T. marmoratus, develop a high cutaneous crest on the back and tail during the breeding season, and this crest acts not only as a swimming organ and ornament, but also as a sensory organ.
The whole genus comprises some eighteen species, twelve of which are European, although some of these extend into Western {123}Asia; T. pyrrhogaster and T. sinensis are found in N.E. China, the former also in Japan; T. poireti and T. hagenmuelleri live in Algeria, and only two, T. torosus and T. viridescens, are North American. Some of the species have a limited range; thus T. montanus is confined to Corsica, T. rusconii to Sardinia, T. boscai to the north-west of the Iberian peninsula and T. asper to the Pyrenees.
Newts all prefer moisture without heat. During the pairing season they take to the water, mostly to stagnant pools, which sometimes implies long migrations. During this period, which is in some cases rather prolonged, they become thoroughly aquatic and undergo some important changes. The tail-fins are much enlarged; in the males of some species a high cutaneous fold grows out on the back, devoid of muscles, but rich in sense-organs. The whole skin, instead of being dry, possesses numerous mucous glands and, what is of more importance, specialised sensory apparatuses which are arranged chiefly along the lateral lines of the body and part of the tail.
After the breeding season Newts become terrestrial, hiding in cracks, trees, or in the sandy soil. Some species aestivate during the hot and dry season. They hibernate either in the ground, or occasionally in ponds. T. vulgaris is difficult to keep in the water beyond the pairing season, while this is easily done with T. alpestris and T. cristatus; T. waltli can live in the water for years. The food consists of all kinds of insects, centipedes, worms, snails, etc., which are searched for chiefly at night. It is astonishing to see a little Triton getting hold of and gradually swallowing a wriggling earthworm almost as thick and as long as itself. When two newts seize the same worm, as these voracious and jealous creatures often do, each gets hold of one end, and swallowing as much as it can, twists and rolls round in a direction opposite to that of its rival, until the worm breaks, or until the jaws of the two newts meet and the stronger of the two draws it out of the weaker one and swallows the whole worm. They do not drink, but soak themselves in the water.
The skin is shed periodically, and rather often by the rapidly growing young; by the adult, during the life in the water, rarely during the sojourn on dry land. The skin breaks round the mouth; assisted by the fingers and by contortions of the {124}body, it is then slipped backwards over the trunk and tail, whereupon the newt seizes the skin with the mouth, draws the shirt off entirely, and–swallows it. Such freshly shed skins are very delicate and pretty objects when suspended in water or some preserving fluid. The shed skin, consisting only of the outermost layer of the epidermis, is entire, but turned inside out, with fingers and toes complete, the only holes being those for the mouth, eyes, and vent.
None of the Tritons are viviparous. The eggs, which are glued singly or in small numbers on to stones or water-plants, are hatched in about a fortnight, sooner or later according to the species and the prevailing temperature. The larvae are always provided with three pairs of branched external gills; the fore-limbs appear much earlier than the hind-limbs. Most, perhaps all, larvae develop two pairs of thread-like protuberances on the sides of the upper jaw, by means of which they attach or anchor themselves on to water-plants shortly after they are hatched. Thus moored they remain motionless in a slanting position, now and then wriggling their tails and shifting their place, or sinking to the bottom. The metamorphosis is finished during the first summer, and the little newts, often partially transparent, leave the water to hide under stones. Not unfrequently the metamorphosis is retarded and not finished by the autumn. The larvae of T. cristatus, especially when reared in ponds with abrupt or overhanging banks, so that they cannot leave the water, retain considerable remnants of the gills, still more frequently the clefts, although breathing chiefly by the lungs. Such individuals reach a length of 3 inches, and are larvae so far as the finny tail and the gills are concerned. They hibernate in this condition, and in exceptional cases reach sexual maturity;–at least the females, which develop ripe eggs; the males are not known to produce spermatozoa.
Much has been written on the amorous games of newts, but it is only recently that the mode of fecundation has been actually observed. Gasco[60] placed the newts in glass vessels suspended from the ceiling of his laboratory. The antics of the enamoured male around the female, rubbing the latter with its head, or lashing it gently with the tail, and playing around it in its often beautiful nuptial dress, are meant to excite the {125}female. The male then at intervals emits spermatophores, which sink to the bottom, and the female takes them up into its cloaca. For further information see p. 54.
Triton cristatus.–The Crested Newt has a slightly tubercular skin with distinct pores on the head, on the parotoid region and on a line along the side of the trunk. There is a strong gular fold. The general colour above is dark or black-brown with an olive tinge, interspersed with darker spots; the sides of the body bear irregular white spots. The under parts are yellow, almost always with large black spots. The iris is golden yellow.–The nuptial dress of the male is very striking. A high, serrated crest occurs on the head and body; the upper surface of the head is marbled with black and white; the under parts are orange-yellow with black spots, and the sides of the tail are adorned with a bluish-white band.–The female, always devoid of a crest, generally exhibits a yellow line along the middle of the back.–The average length of fully adult specimens is about 5-6 inches or 13-15 cm.; the females are as usual larger than the males; 144 and 162 mm. for an English male and female respectively are exceptional records.
Propagation takes place in April. The newly hatched larvae are yellowish-green, with two black dorsal bands, and with a whitish edge to the tail-fin. By the middle of July they are about 5 cm. long, and the white-margined tail now ends in a {126}thread 1 cm. in length. The general colour above is light olive-brown, dotted with black; the flanks and belly have a golden shimmer.
The Crested Newt has a wide distribution, extending from England and Scotland through Central Europe into Transcaucasia; the northern limits are Scotland and Southern Sweden. Although found in Greece and Lombardy, it does not occur in the Iberian peninsula nor in the South of France, where it is represented by the next following species.
Triton marmoratus.–The Marbled Newt is of the same size as the Crested Newt. Its ground colour is grass-green above, brown below, with numerous large and small irregularly shaped marbling patches, spots and dots of black. The crest of the neck and trunk is entire, not serrated, adorned with dark vertical bands, and separated from the high dorsal fin of the tail by a deep indenture or gap. The female has an orange line, slightly sunk in, instead of the crest. This newt is confined to France and the Iberian peninsula. In the North of Portugal and in Galicia it is frequently seen in little streams and ponds during the months of March and April. The rest of the year it spends on land. In France occur hybrids of this species and T. cristatus. They have been described as T. blasii.
T. alpestris.–The Alpine Newt is easily distinguished by the rich orange colour of its under parts, which are unspotted, excepting a few dark specks across the throat, below the gular fold. Specimens with many ventro-lateral black spots are exceedingly rare. All the upper parts are dark, but vary individually. The prettiest specimens are dark purplish grey, with black marblings; others incline more towards brown ground-tones, the blackish markings then appearing more prominent. The sides are often stippled with tiny whitish dots. The iris is golden yellow.–The nuptial male has a low, not serrated crest, which extends uninterruptedly from the nape into the dorsal fin of the tail. The crest is pale yellow, with black vertical bands and spots. The ground-colour of the upper parts inclines to blue, especially on the sides. The lower fin of the tail assumes an irregular band of bluish-white confluent patches.
This newt is rather small, females rarely exceeding 100 mm. or 4 inches in length. Its home is chiefly the hilly and mountainous parts of Central Europe, from Holland to Lombardy, {127}Austria-Hungary, and Greece. Although it ascends the Alps to between 6000 and 7000 feet, it is also found in the Netherlands, but not in the North German plain.
T. vulgaris (s. taeniatus, s. punctatus).–The Common or Spotted Newt usually reaches 3 inches (7-8 cm.) in length. Boulenger's record-specimen measured 104 mm. It is characterised by the yellow, partly orange under surface, which is always spotted with black. The upper parts are olive-green or brown, inclining to white on the flanks; the black spots of the back, sides, and especially of the tail, are arranged in more or less distinct lines, giving a somewhat banded appearance to some females.–The breeding dress of the male shows a non-serrated, but "festooned" high and very wavy crest, which extends from the neck without interruption into the likewise wavy tail-fin. The tail is adorned with a lateral, glittering blue stripe, interrupted by vertical dark spots. The larvae are marked by a series of yellow dots, which extend over the lateral line and the tail, which latter temporarily possesses a terminal filament like that of the larvae of T. cristatus.
The distribution of the Spotted Newt is the same as that of T. cristatus, namely Europe with the exception of the Iberian Peninsula, and Western Asia.
T. palmatus s. helveticus.–This is the smallest of all the European newts, rarely reaching more than 3 inches in length. It is distinguished by several specific characters. The tail ends in a thread which is in some males 10 mm. in length, but is only just indicated in the female. The breeding male develops a cutaneous fold along each side of the back, and a low, entire, vertebral crest; the toes are fully webbed. The under parts are pale yellow, inclining to orange towards the middle of the belly, and with a few blackish dots. The lower caudal crest has its edge blue in the male, orange in the female. The general colour of the smooth skin is olive-brown above, with numerous dark spots, which are arranged in more longitudinal streaks on the head.
The Webbed Newt is a native of Western middle Europe, ranging from Great Britain and Northern Spain to Switzerland and Western Germany.
Closely allied to the last species are T. boscai of Spain and Portugal, T. italicus, T. montadoni of Moldavia, and the {128}beautiful T. vittatus of Asia Minor. From China and Japan are known T. pyrrhogaster and T. sinensis.
The North American species are T. torosus and T. viridescens. The former, of Western North America, is one of the largest newts, reaching a length of more than six inches. The head is much depressed and broad, and has very prominent parotoid and other glands. The limbs are strong, especially in the male. The skin of the upper parts is very granular, uniform dark brown, without a crest. The tail, which is larger than the head and body, is strongly compressed, with a low dorsal and ventral fin. The under parts and the lower edge of the tail are uniform yellow or orange red. The iris is green. A specimen in my keeping spends most of its time in the cracks of rotten stumps or on the top of moss in the darkest shade. It lives on earthworms but despises insects. Like most of the other newts it becomes lively at dusk.
Fig. 24.–Triton viridescens. 1, Egg just after deposition, with the outer membrane opened, × 6; 2, a spermatophore just discharged showing its gelatinous base with a projecting spike which bears a tuft of spermatozoa, × 2. (After Jordan.)
T. viridescens is common throughout the Northern and Eastern parts of the United States. Large females are about 11 cm. long, the males 1 cm. less. The general colour above is brown, with a tinge of green; on each side of the trunk, with a row of bright vermilion spots; the under parts are orange, studded with small black dots. Half-grown specimens are brownish red, with the same lateral red spots as the adult. According to Jordan,[61] this voracious species lives chiefly on the larvae of insects, on small molluscs such as Cyclas and Planorbis, on earthworms and on small Crustacea. It is eminently aquatic in the adult stage. The eggs are laid from April to June, the period lasting for one individual four to six weeks, or even longer. One female laid 108 eggs in all from 20th April to 30th May. After having selected a suitable plant, for instance an Anacharis or a bunch of Fontinalis leaflets, she bestrides the plant and gathers in the surrounding shoots with her hind-limbs, {129}pressing the leaves closely around the cloaca. She next turns on her side, or occasionally on her back; with fore-limbs outstretched and rigid, with hind-limbs and leaves completely hiding the cloaca, she remains perfectly motionless for six to eight minutes. Then she slowly leaves the "nest," which now holds an egg well protected by a tangle of shoots glued together by the gelatinous secretion poured out of the cloaca. Jordan concludes, from the fact that he never found spermatozoa in the oviducts, that the eggs are fertilised just before they are expelled, when passing the receptaculum seminis.
The metamorphosed young pass their life on land under stones and logs as the so-called red variety, which is merely a stage in the life-history of the species. It seems to take them several years to reach maturity, and to become again typically aquatic. Young, red individuals which I have myself kept, have behaved for more than a year like the young of other newts, spending their time under moss and bark without going into the water.
The change from the red-spotted stage has been exhaustively studied by Gage.[62] He remarks that this species is very common near Ithaca, in an upland forest and along the head-waters of the Susquehannah. The transformation takes place either in the autumn or in the spring, either while the newt is still on land, or after entering the water.
Of two which were kept in a jar with moist wood, one was especially brilliant, but within two weeks it assumed, in the middle of September, the characteristic coloration of the viridescent form. The two specimens were in the jar until the following July, when they were placed where they could enter the water. This they did with great readiness, and they remained submerged for a considerable time at first. The time under water increased in length, until within two or three days the pharyngeal respiration under water was fully established. On the other hand, viridescent specimens never reassume the red garb when kept out of the water.
Red specimens entering the water in the spring, changed into the greenish form within a few weeks, and established the pharyngeal respiration, losing the ciliated oral epithelium. Branchiate larvae and the adult aquatic forms have non-ciliated {130}epithelium, and the cilia are re-established when a green specimen is forced again to live on land. Ciliation always exists in the red stage, and in the green stage before the newt has taken to the water. The cilia sweep towards the stomach.
The three following South European species belong to the Euproctus group, so called on account of the mostly conical, backward directed, and vividly coloured vent.
T. asper s. pyrenaeus.–The Pyrenean newt has hitherto been found only in the Pyrenees, for instance in Lac Bleu and Lac d'Oncet, which latter lies about 7000 feet above the level of the sea. According to Bedriaga,[63] it prefers lakes which are supplied during the whole summer with water from glaciers. It is very sluggish, only moving to breathe and when in search of food, which consists of worms and insects. The general colour is greenish brown, dark above; the under side of the head and body are bright orange red in the female, yellow in the male; dark spots separate this bright colour from the flanks. The tail has a narrow ventral stripe of bright red and yellow. The cloaca of the female is bright red, that of the male dull grey. The total length amounts to about 4 inches or 10 cm.
The pairing time is the end of June, or later in cold seasons. The male gets hold of the female by forming a noose with its tail round her; it lies underneath, the cloacae being pressed together so that the spermatozoa can be taken in directly. The larvae have large yellow-green spots on the back and sides, and a bright red ventral tail-fin; when metamorphosed the greenish spots become more confluent on the back, producing a broad spinal band. Larvae which live in deep water are dark, while those in sunny places are light-coloured and spotted with yellow.
T. montanus in Corsica and T. rusconii in Sardinia are allied forms, but the males are distinguished by a spur-like process or dilatation at the end of the fibula.
T. waltli, the Iberian Newt, is olive-brown above, yellowish with blackish markings below. The tail has a yellow or orange ventral line. There is no crest. A remarkable peculiarity of this species (which it shares only with Tylototriton andersoni of the Loo-Choo Islands) is its ribs, which are very long, sharply pointed, and frequently perforate the skin. Before {131}perforation the point of the rib lies in a lymphatic space. This surprising feature has by many authorities been considered as abnormal or pathological. Certainly young, and even many adult, individuals are found in which the skin is not perforated, but when these are handled the wriggling motions of this strong newt force the points of the ribs through the skin, and they remain sticking out to the extent of several millimetres. The wounds heal up, the skin forming a neatly finished-off hole through which the spike projects, not as a formidable, but as a sufficiently awkward, protective weapon.
Large females reach a length of 10 inches. The larvae metamorphose, as a rule, when they are between 2 and 3 inches long, but those which have been bred in tanks often reach double this length. These newts are frequent inhabitants of the rain-water cisterns common in the South of Portugal and Spain, into which they tumble without ever being able to get out again. This species spends most of its time in the water, {132}preferring ponds, among the vegetation of which they can be watched lying motionless, with their limbs hanging down and with the head close to the surface; but they are lively during the night. When their ponds dry up they leave them, crawling into the most unexpected places, to aestivate under rocks, or even in the walls of old buildings, where they are found by accident only. The range extends from Central Spain and Portugal into Morocco.
Tylototriton verrucosus lives in the Eastern Himalayas and in the mountains of Yunnan. The skin is tubercular, with large parotoids; above uniform black-brown, pale below; the tail has a ventral yellow or orange line. Total length about 6 inches. T. andersoni of the Loo-Choo Islands is remarkable for the pointed ribs which perforate the skin.
Pachytriton brevipes, discovered in Kiansi, Southern China, has a smooth skin, olive-brown above, with many black dots; the under parts are yellowish, dotted with black. Total length about 7 inches.
Fam. 3. Proteidae.–The three pairs of fringed external gills persist throughout life. Both fore- and hind-limbs are present. The eyes are devoid of lids. The maxillaries are absent. Teeth are present on the premaxillaries, on the vomers, and on the mandible. The vertebrae are amphicoelous.
This family consists of only three genera, with one species in each.
Necturus maculatus s. Menobranchus lateralis.–The eyes are functional, being covered by the thin transparent skin. The limbs, although short, are well developed, and have four fingers and four toes. The whole animal, which reaches the length of one foot, is quite smooth and slimy, brown with irregular dark, blackish spots and patches, which frequently form a dark lateral band extending from the mouth to the tail. The latter, which measures about one-third of the whole length, is strongly compressed, carries a thick dorsal and ventral fin, and is rounded off at the end. The skin of the throat forms a strongly-marked transverse fold. The thick stalks of the gills are brown, while the numerous and delicate fringes are dark red in life; beneath and behind them are two gill-clefts. N. maculatus is found in the eastern half of the United States, chiefly the eastern part of the basin of the Mississippi and the Canadian lakes.
These creatures are rather dull; they remain mostly at the bottom of the water, more or less concealed in the weeds or between rocks during the daytime. Mine, which are kept in a roomy, light-coloured tank, lie motionless, with their gills spread out transversely. Every now and then the gills contract suddenly and become pale, whereupon they are filled again with blood. Very rarely they rise to the surface, but tiny air-bubbles are let out more frequently, especially when the animals are disturbed. Then the gills collapse, are laid flat against the neck, and the creature darts about with quick, eel-like motions. At night they leave their hiding-places, swim about or creep along the ground with slow, undulating movements, the limbs being scarcely used, in search of food, which in their wild state consists of rather large Crustacea, small fishes, worms, insects and frogs. They are most voracious, and absolutely indifferent to cold. The spawning takes place in the months of April and May.
Proteus anguinus.–The fore- and hind-limbs are fully developed, but possess only three fingers and two toes. The eyes are completely hidden beneath the opaque skin. This peculiar creature is restricted to the subterranean waters of Carniola, Carinthia, and Dalmatia. The vast caves of Adelsberg not far from Trieste are especially celebrated for the occurrence of the "Olm," the German name of this animal. The river Poik, a moderate mountain-stream, but a large, fierce torrent during the rainy season, disappears into the limestone-hills, and rushes through enormous stalactite-grottoes, most of which have been only partially explored, until several miles farther on it reappears on the surface. There, deep down below the surface, in absolute darkness, in an almost constant temperature of about 50° F. is the home of Proteus.
Their total length is scarcely one foot. The whole body is white, occasionally suffused with a slight fleshy, rosy tinge, while the three pairs of gill-bunches are carmine-red. They are easily kept in captivity, and live for many years, provided three conditions are strictly adhered to, viz. fresh and clean water, an equable low temperature of about 50° F. = 10° C. and darkness. The question of food is not so very important, since specimens are known to have existed for years, although they refused to take any nourishment. How far darkness is an {134}absolute necessity is not known. Anyhow, the white skin is almost as susceptible to light as is a photographic plate. If light is not absolutely excluded the white skin becomes in time cloudy, with grey patches, and if kept exposed to stronger light, the whole animal turns ultimately jet-black. Mr. Bles has succeeded in producing several totally black specimens, having kept them for several months in a white basin under ordinary conditions of light. No experiments have yet been made to find out if the black pigment deposited is lost again in darkness. Those which are kept in a tank in an absolutely dark cellar of the Cambridge Museum, with permanent water-supply, are doing very well. When approached with a candle they become restless or remain partly hidden in all sorts of seemingly most uncomfortable attitudes, squeezed in between the sharp-edged tiles and drain-pipes with which their lodgings are furnished. But the introduction of a wriggling worm, a little crustacean or other live bait draws them from their hiding-places, and, guided by the motions of the prey in the water, possibly also by the sense of smell, they snap it up and devour it.
If the water is not sufficiently well aerated, they rise to the surface, emit a bubble of air, and take a new supply into their lungs. As a rule they remain motionless under water, but the gills contract spasmodically and become paler, whereupon they fill again with blood and darken; the contrast between the pure white body and the carmine-red feathery gills is very beautiful.
Until recently the mode of propagation was quite unknown. Several Proteus, kept by E. Zeller, laid, in the middle of April, {135}a number of eggs which were then fastened singly on to the under side of projecting stones in the water. The pale yellow yolk measured 4 mm. in diameter and was surrounded by a cover of 1 mm. in thickness, besides an outer gelatinous mantle, so that the whole egg measured about 11 mm. The larvae were hatched after 90 days; they were 22 mm. long, and already much like the adult, except that the fin was not restricted to the tail, but extended over the last quarter of the trunk, and that their eyes were still visible. The fore-limbs were already typical in shape, but the hind-limbs were still toe-less little stumps.[64]
Typhlomolge rathbuni.–It is of the greatest interest that a subterranean Perennibranchiate newt, in many respects closely resembling Proteus, has recently been discovered in Texas. There can be no doubt that similar conditions of life have produced these two forms from Necturus- and Spelerpes-like ancestors,[65] one in Europe, the other in North America, absolutely independently of each other. The limbs of Typhlomolge are long and very slender, the four fingers and five toes are thin, free and pointed. The head is large, the mouth square. The eyes are completely hidden and the whole animal is colourless and white. The tail is furnished with a dorsal and a ventral fin. The very deep gular fold is nothing but the pair of united but large opercular flaps. The three pairs of gills are remarkable for their blade-like stalks, while the gill-lamellae proper are short and restricted to the tapering ends. Total length about 75 mm., of which the head measures 15, the tail 32 mm.
This peculiar creature inhabits subterranean caves in Texas, to judge from the fact that all the specimens hitherto known have come up with the water of an artesian well 188 feet deep, near San Marcos. According to Blackford,[66] "the legs are used for locomotion and the animals creep along the bottom of the aquarium with a peculiar movement, swinging the legs in irregular circles at each step. They climb easily over the rocks piled in the aquarium, and hide in the crevices between them. All efforts to induce them to eat have been futile, as has also been the case with blind cave-fish in captivity, and they are {136}either capable of long fasts or live on infusoria in the water." It seems more reasonable to suppose that these newts live upon Crustacea, four kinds of which, all new to science, also came up with the water.
Fam. 4. Sirenidae.–The three pairs of fringed external gills persist throughout life. The body is eel-like. Hind-limbs are altogether absent, while the fore-limbs are short and have three or four fingers. The maxillary bones are absent. With the exception of small teeth on the vomer the mouth is toothless, but the jaws are furnished with horny sheaths. The eyes are devoid of lids, but shine through the skin.
The Sirenidae are the most degraded members of the Urodela and are represented by two closely-allied genera, each with one species, in the south-eastern parts of the United States. Their most interesting feature, which bears upon the question of neoteny, is their retrograde metamorphosis as described by Cope.[67] The gills atrophy in the young and are subsequently redeveloped. Cope therefrom concludes rightly that the ultimate or persistent gills of Siren are signs of maturity and not a larval character. In young specimens of Siren of 5 to 6 inches in length the gills are functionless; in one of 3 inches they were found to be entirely vestigial and "subepidermal," i.e. covered by a common dermal investment. Unfortunately really young larvae are still unknown. Old Sirens can live without gills, as has been shown by aquarium-specimens. In the adult Pseudobranchus all the gills are normally covered up by an investment of the skin so as to be quite without function and movability.
Siren lacertina, the "mud-eel," is distinguished by the {137}possession of three pairs of gill-clefts and by its four fingers. It reaches a length of 70 cm., or about 2½ feet, of which about one-third is taken up by the tail, which is strongly compressed and finned. The skin is smooth, mostly blackish, lighter below, sometimes with whitish specks all over the body. This creature is frequently found in ditches and ponds, where it burrows in the mud. When swimming the limbs are folded back. They are said sometimes to leave the water and to crawl about on the moist ground.
Pseudobranchus striatus has only one pair of gill-clefts and only three fingers. The slightly granular skin is dusky brown above, with a broad yellow band on either side and with a paler, narrower stripe below. Total length about 7 inches.
LISSAMPHIBIA (CONTINUED)–ANURA
Order III. ANURA or TAILLESS AMPHIBIA.
The recent tailless Amphibia, or Frogs and Toads in the widest sense, contain such a great number of species (about 900), with such a diversity of characters, that it is necessary, if only for the sake of mere convenience, to group them into a considerable number of families and sub-families. The characters available for this purpose are few.
1. The possession of a tongue characterises the Phaneroglossa, the absence of a tongue the Aglossa.
2. The character of the shoulder-girdle.–Overlapping of the two halves of the shoulder-girdle on the ventral side characterises the Arcifera, while in the Firmisternia the two ventral halves meet in the middle line and form a firm, median bar. See, for details, p. 24.
3. The shape of the transverse processes or diapophyses of the sacral vertebra which carries the iliac or hip-bones. These processes are either dilated or cylindrical.
4. The presence or absence of teeth in the upper and lower jaws. This is indicated by a formula in which 0 means absence of teeth; max. means presence of teeth in the upper jaw; mand. means presence of teeth in the lower jaw.
5. The terminal joints or phalanges of the fingers and toes are sometimes claw-shaped. See p. 26.
6. The shape of the centra of the vertebrae.–Opisthocoelous, if the posterior end is cup-shaped or concave, procoelous if the anterior end is concave and the posterior is convex. See p. 19.
By means of these characters we can arrange the Anura in the following key:–
| II. Aglossa. Sacral diapophyses dilated. |  |
AGLOSSA, p. 143. |
| Vertebrae opisthocoelous, with ribs. | ||
| II. Phaneroglossa. | ||
| A. Arcifera. | ||
| a. Sacral diapophyses dilated. | ||
| α. Terminal phalanges not claw-shaped. | ||
| Opisthocoelous, with ribs, max./0 | DISCOGLOSSIDAE, p. 152. | |
| Procoelous, without ribs, 0/0 | BUFONIDAE, p. 166. | |
| Precocious, or opisthocoelous, without ribs, max./0 |
 |
PELOBATIDAE, p. 160. |
| β. Terminal phalanges claw-shaped– |  |
max./mand. Amphignathodontinae, p. 188. |
| HYLIDAE | max./0 Hylinae, p. 189. | |
| b. Sacral diapophyses cylindrical– |  |
max./mand. Hemiphractinae, p. 210. max./0 Cystignathinae, p. 211. 0/0 Dendrophryniscinae, p. 227. |
| CYSTIGNATHIDAE | ||
| B. Firmisternia. | ||
| a. Sacral diapophyses dilated– |  |
max./0 Dyscophinae, p. 235. 0/mand. Genyophryninae, p. 236. 0/0 Engystomatinae, p. 225. |
| ENGYSTOMATIDAE | ||
| b. Sacral diapophyses cylindrical– |  |
max./mand. Ceratobatrachinae, p. 237. max./0 Raninae, p. 238. 0/0 Dendrobatinae, p. 272. |
| RANIDAE | ||
Concerning the evolution of the classification of the Anura, it is interesting to follow the changes of the value attached to the various anatomical characters by systematists. At first the presence or absence of teeth and of adhesive discs on the fingers and toes were considered to be of prime importance for the division of the Phaneroglossa.
Duméril et Bibron, 1841. "Erpétologie générale."
II. Phrynaglosses = Aglossa of Wagler: Pipa and Xenopus.
II. Phanéroglosses. 1. With teeth. a. Without discs: Raniformes.
II. Phanéroglosses. 1. With teeth. b. With discs:out Hylaeformes.
II. Phanéroglosses. 2. Toothless. Bufoniformes.
Stannius, 1856 (see p. 8), separated the Engystomatidae as "Systomata," and used the presence or absence of the "manubrium sterni" (omosternum) as a character of distinction between his Bufoninae and Raninae.
Günther, 1858, "Catalogue of the Batrachia Salientia." No progress was made by his scheme, which relied upon the tongue and digits.
Aglossa with Myobatrachus.
Opisthoglossa. a. Oxydactyla. b. Platydactyla.
Proteroglossa: Rhinophrynidae.
Cope, 1864. "On the limits and relations of the Raniformes."[68] He introduces the shoulder-girdle and the sacral diapophyses, and drops the discs as too adaptive and misleading. He distinguishes between Raniformes and Arciferi.
Cope, 1865. "Sketch of the primary groups of the Batrachia Salientia."[69]
Aglossa.
Bufoniformia (Bufonidae).
Arcifera (Discoglossidae, Scaphiopodidae, and Hylidae).
Raniformia.
In 1867 Cope separates the genus Hemisus as Gastrechmia on account of its peculiar pectoral arch.[70]
In 1875, "Check-list of North American Batrachia and Reptilia," Cope elaborates his system:
Class Batrachia. Order Anura.
1. Raniformia.
2. Firmisternia. [Dendrobatinae and Engystomatidae.]
3. Gastrechmia: Hemisus.
4. Bufoniformia. [Bufonidae.]
5. Aglossa. Pipa.
6. Odontaglossa. Xenopus.
7. Arcifera. [Cystignathidae, Hylidae, Pelobatidae and Discoglossidae.]
Cope consequently considered the characters of the pectoral arch as equivalent to those of the dentition.
Boulenger, 1882, "Catalogue of the Batrachia Gradientia s. Ecaudata," recognises that the pectoral arch is of greater systematic value than the dentition. The latter is used, together with the shape of the sacral diapophyses, for the separation into families.
| I. Phaneroglossa. | A. Firmisternia. |  |
1. Ranidae. |
| 2. Dendrobatidae. | |||
| 3. Engystomatidae. | |||
| 4. Dyscophidae. | |||
| B. Arcifera. |  |
5. Cystignathidae. | |
| 6. Dendrophryniscidae. | |||
| 7. Bufonidae. | |||
| 8. Hylidae. | |||
| 9. Pelobatidae. | |||
| 10. Discoglossidae. | |||
| 11. Hemiphractidae. | |||
| 12. Amphignathodontidae. | |||
| II. Aglossa. |  |
13. Dactylethridae. | |
| 14. Pipidae. | |||
This emendation of the Arcifera and Firmisternia was accepted by Cope in his synopsis of the families of Vertebrata (Amer. Natural. xxiii., 1890), except that he still retained his suborder Gastrechmia.
Since the publication of Boulenger's great work a number of forms have been discovered which, from the characters of their dentition, have necessitated the establishment of certain new families, namely, Ceratobatrachidae and Genyophrynidae; and Boulenger was the first to recognise that the taxonomic value of the mere presence or absence of teeth in the jaws had been overestimated. I therefore propose using it as a character distinctive of the sub-families only, thereby reducing the number of families, relying first (leaving the Aglossa aside) upon the firmisternal or arciferous condition of the pectoral arch, secondly upon the dilated or cylindrical shape of the sacral diapophyses, thirdly upon the dentition. Blindly consistent application of these principles would reduce the Phaneroglossa to four families only, namely Ranidae, Engystomatidae, Cystignathidae and a fourth family comprising all the Arcifera with dilated sacral diapophyses. This would obviously be wrong. We have therefore to resort to other additional characters or rather peculiarities. The opisthocoelous character of the vertebrae and the possession of distinct ribs, together with the disc-shaped tongue, separate the Discoglossidae and justify their retention as a family. The Hylidae are marked off by the claw-shaped terminal phalanges, but the remaining forms, comprising the Bufonidae and Pelobatidae, cannot be separated except by their dentition, and I plead guilty of inconsistency in retaining them as separate families.
After all, our classification may not represent the natural system, and it may be nothing but a convenient key.
When we have eliminated the characters of the vertebrae, the dentition, the claw-shaped phalanges and the adhesive discs, it may well be asked what characters remain. The firmisternal is a further, higher modification of the older, more primitive arciferous condition. The difference between the dilated and cylindrical shape of the sacral diapophyses is in not a few cases very slight, and there are various, most suggestive exceptions. The presence or absence, size and shape of the omosternum and metasternum are of very limited taxonomic value, not always applicable to all {142}the members of the same family. The fact is, that the Anura are a very recent and a most adaptive, plastic group. The earliest known fossils are scarcely older than the Middle Eocene.
Almost every one of the greater families has produced terrestrial, arboreal, aquatic, and burrowing forms. Their habits have modified, and are still shaping their various organs, first of course those by which the animals come first and most directly into contact with their surroundings (e.g. adhesive discs, dentition, general shape of the body, length of limbs, wartiness of the skin, tympanic disc). These are the so-called adaptive characters, sometimes decried as merely physiological; as if habits, use, and requirements did not likewise influence and ultimately model every other organ (e.g. tympanic cavity, Eustachian tubes, vertebrae, ribs, coccyx, pectoral arch, etc.). There are true Toads, Bufonidae, which are as smooth, wartless, slender-bodied and long-legged as the most typical of "Frogs"; true Ranidae, like Rhacophorus, which by their green colour, large adhesive discs and arboreal habits may well put many of the Hylidæ to shame. Ceratohyla has developed the claw-shaped terminal phalanges which are otherwise typical of, and peculiar to, the Hylidae, but this genus reveals itself by various details as a close relation of the other Hemiphractinae; and these fall in with the Cystignathidae on the strength of their cylindrical, not dilated, sacral diapophyses.
In sketching the phylogenetic tree of the families of the Anura we have to proceed with great caution.
There is not much doubt about the Aglossa. They have retained some of the most primitive characters, but have by now been so much modified and specialised that they are to be looked upon as an early side-branch.
Among the Phaneroglossa the Discoglossidae are with certainty the oldest, but are now scarce in genera and species, and much specialised. The Pelobatidae connect them with the Bufonidae. The Cystignathidae form a rather ill-defined assembly which points downwards to the Pelobatidae, upwards to the Hylidae. There is no divergence of opinion about the Ranidae being the highest of all the Anura, and amongst them the Raninae the most typical, the Dendrobatinae the most specialised. If we assume that moderately dilated sacral diapophyses represent a more primitive stage than cylindrical processes, we shall {143}naturally look to the Engystomatidae as the connecting link between the Ranidae and the Arcifera, through Bufonoid creatures still with teeth in both jaws. If, on the other hand, we take the dilatation to be a further development from more or less cylindrical processes, then the Ranidae can be considered as having sprung from Cystignathoid creatures, which have consolidated their pectoral arch into the firmisternal condition; and in this case the Firmisternia would not be a natural group, the Engystomatidae pointing, to the Bufonoid stock. This would, to a great extent, mean a reversion to Cope's idea.
Sub-Order 1. Aglossa.–The two diagnostic peculiarities of the few members of this group are: first, the absence of a tongue; secondly, the union of the Eustachian tubes into one median pharyngeal opening in the posterior portion of the palate.
The pharyngeal opening and the tubes themselves are wide, the tympanic cavities are present, but the tympanic discs are not distinct from the rest of the skin. The fronto-parietal bones are fused into one mass, a rare feature in the Anura. The nasals are large. Pipa and Hymenochirus have no teeth, Xenopus has teeth on the upper jaw. The vertebrae are opisthocoelous and typically epichordal in their development; the second, third, and fourth carry long ribs, which in old specimens fuse with the supporting diapophyses. The sacral diapophyses are enormously dilated, and the sacrum is fused with the os coccygeum. The serial number of the sacral vertebrae exhibits a most interesting gradation. In Xenopus the ilium is carried by the diapophyses of the 9th, in Pipa the 9th and 8th, in Hymenochirus the 7th and 6th. In these cases the two diapophyses of each side are fused together into a single broad blade, and their original duplicity is indicated only by the {144}holes for the spinal nerves. Hymenochirus has consequently only 5 presacral vertebrae, the vertebral column being shortened to the greatest extent known amongst Vertebrata. For further information see p. 22. The ilia are much broadened vertically, and are firmly attached to the sacrum. The shoulder-girdle is sometimes described as of the arciferous type, but this is quite unjustifiable. The epicoracoid cartilages do not overlap each other, but meet, and partly fuse in the middle line. The three genera exhibit some differences. In Pipa and Hymenochirus the bony portions of the coracoids are much expanded dorsally, and there is a considerable amount of epicoracoid cartilage, that of the precoracoid bars extending backwards as a broad-based and blunt omosternum. Xenopus is devoid of an omosternum, and the configuration of the whole apparatus is more slender. The metasternum of Xenopus and Hymenochirus broadens out laterally. Hymenochirus greatly resembles Breviceps, a genus of Engystomatinae, in the relative position and size of the various parts of the shoulder-girdle and sternum.
The tibio-fibula of Hymenochirus has a wing-like expansion of thin bone on each side, forming a deep groove on the outer aspect. The astragalus and calcaneum are united by a similar bony expansion with wing-like projections.
The lungs are remarkable for the prominent development of trabecular projections and niches, so that their free lumen is much restricted; they have thereby reached a much higher stage than in any other Amphibia or even many Autosauri. The persistence of an arteria sacralis s. caudalis, a vessel absolutely absent in the adult Rana, is a primitive feature, and the same applies to the presence of a true first spinal or suboccipital nerve.
The skin of the back and belly is supplied by two great branches from the arteria anonyma, one arising proximally, the other distally from the subclavian; herewith is correlated the almost complete absence of the arteria cutanea magna, which as a branch of the ductus pulmo-cutaneus plays such a prominent rôle in the other Anura. Only in Pipa, but not in Xenopus, is the great cutaneous vein represented by a very small branch. Both these genera possess a much more complicated "diaphragm" than the other Anura, chiefly owing to a special muscle which arises {145}from the anterior end of the ilia and spreads out fan-like to the oesophagus and to the bases of the lungs.[71] This diaphragmatic arrangement is correlated with the great development of the lungs, and is not a primitive but an advanced feature. It is reasonable to suppose that this has caused the reduction of the usual arteria pulmo-cutanea, and that the other two cutaneous arteries have been developed secondarily. The Aglossa are generally considered as the lowest Anura, and only Cope looked upon Pipa and Xenopus as two convergent terminal branches. Beddard came to the conclusion that both are closely related to each other, chiefly on account of their peculiar diaphragmatic arrangement. The whole question has entered upon a new stage since the recent discovery of Hymenochirus, which is in many ways intermediate between the two other genera. Moreover, the mid-Tertiary Palaeobatrachus of Europe is undoubtedly related to them, and we conclude now that all these four genera belong to one group with a distribution formerly much wider than Africa and part of South America. But this does not necessarily mean that the Aglossa are in all respects the most primitive group of living Anura. On the contrary, they possess few decidedly primitive characters, namely, the long typical ribs, the presence of the first spinal nerve, the unimportant persistence of the arteria sacralis, and lastly, the possession in the tadpoles of a right and left opercular "spiracle." The absence of the tongue cannot possibly be an archaic feature, considering its universal presence in all the other Amphibia, including the Apoda, and the suggestive circumstance that this organ is least developed in the entirely aquatic members of the Urodela. In fact, thoroughly aquatic creatures, which seize and swallow their prey under water, require no elaborate tongue; and since we know that the Anura must owe their typical formation to terrestrial life, it follows that those which have again taken to the water and are tongueless, have lost this organ. As I have shown elsewhere,[72] the epichordal development of the vertebrae is likewise a secondary feature, far from primitive; and the tendency of the shortening of the vertebral column, which has reached its extreme in Hymenochirus, points to the same conclusion. The apparatus of the shoulder-girdle and sternum is in the last transitional stage from the former arciferous to the typically consolidated firmisternal {146}type. In fact there is little left which is primitive, but much that is very specialised and highly developed in the Aglossa, mostly in adaptation to their absolutely aquatic life, to which they must however have taken very early. They are in a position somewhat analogous to the Ratitae among Birds, which are likewise an old group, although many of their most striking features have been acquired secondarily.
Xenopus s. Dactylethra. The upper jaw is furnished with teeth. The ilia are attached to the ninth vertebra. The pupil is round. The terminal phalanges are pointed. The fingers are free, the toes broadly webbed, and the first three are covered with sharply pointed, horny, black-brown nails, a feature which is alluded to by the alternative generic names. A cutaneous tentacle projects from below the eye and naturally invites comparison with the tentacle of the Apoda and of Urodela. The skin is smooth, rich in mucous glands, besides certain tube-like apparatuses, possibly sensory, which are scattered over the body, especially on the head, and form a conspicuous series of white dots along the dorso-lateral line, from the eye to the vent. The general colour of the upper parts is olive brown, mottled darker, while the under parts are whitish. The female has three cutaneous flaps closing the vent. The male develops black nuptial brushes along the inner side of the fingers. There are several species, all African (Ethiopian).
X. laevis, ranging from the Cape to Abyssinia, is distinguished by the absence of a metatarsal spur. The tentacle is very short. Size about 3 inches. X. muelleri of Zanzibar and Benguella, is smaller. The tentacle is conspicuous, as long as the diameter of the eye. The inner metatarsal tubercle carries a sharp claw. X. calcaratus of tropical West Africa is only 2 inches long, and has strong metatarsal claws, short tentacles and very minute eyes.
The habits and oviposition of the "Clawed Toad" have been described by Leslie.[73] The Boers call it "Plathander," i.e. flat hand. Entirely aquatic, it rests floating in the water, with the nostrils exposed, and leaves the water only if it has to change the locality on account of drought or scarcity of food. The pairing takes place, at least at Port Elizabeth, in the early spring, i.e. in the month of August. The only sound which is emitted is heard during this time, a very slight and dull tick-tick, audible at only a few feet distance. The male grasps the female by the loins; the eggs are extruded singly, measuring only 1.5 mm. in diameter, but swell to double that size. They are attached singly to stones or water-plants.
Latterly these creatures have frequently been brought over to England. They stand confinement very well, even in a little aquarium with sufficient water-weeds to keep the water fresh; and they do not require special heat. They greedily snap up worms, strips of liver, or meat, and poke the food in with their hands. A few kept by Boulenger in a glass jar have lived for the last eleven years in the ordinary temperature of a room in London. Curiously enough they are often in amorous embrace, regardless of the season, but they have never shown any signs of spawning.
Some of those in the Zoological Gardens in London laid eggs on Saturday the 27th of May, and on the morning of the following Monday the larvae were already hatched. They have been described by Beddard.[74] The larvae are provided with an unpaired circular, ventral sucker. The tentacles begin to sprout out on the sixth day after hatching, at first not in connexion with the cranial cartilage, but soon a cartilaginous rod runs into the tentacle from the ethmoid "just above the joint with the under jaw.". Boulenger has most reasonably compared these organs with the "balancers" of Triton and Amitystoma (cf. p. 46 for the possible homologies of the balancers). The tentacles soon reach a great length and give the tadpole a curious appearance. In tadpoles of X. calcaratus, 65 mm. long, the tentacles are 30 mm. long, and are inserted just at the angle of the mouth. By the time that these tadpoles show their fore-limbs, the feelers are reduced to 4 mm. in length, and their relative position has been shifted to a little above the angle of the gape, and whilst the latter gradually extends further and further back, the feelers come to lie, or rather remain, below and a little in front of the eyes.
The tadpoles have no traces of horny teeth. External gills project as low conical or lamellar processes from the first three branchial arches, but so-called internal gills are not developed.
Amongst a number of Clawed Toads imported in the spring one female became swollen with eggs, but as they did not show signs of wanting to breed, a pair was put into the tropical tank {149}in the Cambridge Botanic Gardens, a transfer which had the desired effect. Eggs were laid, and more during the following nights; they hatched out within thirty hours. The whole brood was lost, before any of them were older than a few days, since they were attacked, beyond the possibility of a cure, by a Saprolegnia or some similar pest.
Hymenochirus, represented by one species, H. boettgeri, has been discovered in the Ituri, German East Africa, and in the French Congo, and has no doubt a much wider distribution. It is scarcely 1½ inch long, and is easily recognised by the toothless mouth, the half-webbed fingers (hence the generic name), the incompletely webbed toes, the third of which is longer than the fourth, and the absence of sensory muciferous canals in the skin. The three inner toes are, as in Xenopus, furnished with small black claws. The skin is rough, beset with small granular tubercles. The general colour above and below is olive-brown. The vent is, as in Xenopus, produced into a spout or semi-canal, but is devoid of dorsal flaps of skin.
Pipa.–This Neotropical member of the Aglossa is quite toothless, but the jaws of the adult have horny substitutes. The only species is P. americana, the famous Surinam Toad, chiefly known from the Guianas, but undoubtedly extending much further, having recently been reported from the neighbourhood of Pará.
The general shape of this creature is very peculiar. The head is much depressed and triangular; the eyes are very small; the skin forms several short, irregularly-shaped flaps and tentacles on the upper lips and in front of the eye, and at the angle of the mouth. The tympanum is invisible. The pupil is round. The fingers are very slender and free, ending in star-shaped tips; the toes are broadly webbed. The whole skin is covered with small tubercles and is dark brown above, while the under parts of the very flat and depressed body are whitish, sometimes with a dark brown stripe along the middle line. In the female the skin of the back forms growths for the reception of the eggs, and in these the young undergo their whole metamorphosis.
The most characteristic feature of the skin,[75] which has exactly the same structure in both sexes, is the papillae, which are spread over the whole surface, except on the webs of the toes, on the cornea and on the star-shaped points of the fingers. Each papilla carries a little horny spike, and a poison-gland frequently opens near its base. Larger poison-glands exist on the dorsal and ventral side in four rows, and smaller glands open upon the sides of the body, but there are no parotoid complexes. Slime-glands occur all over the surface. The epidermis consists of the usual layers, namely the Malpighian, the stratum corneum, and the part which is shed periodically. The latter is completely horny, appearing to be structureless like a cuticle, but it is in reality composed of polygonal cells with flattened nuclei; each little spike is one modified horny cell. The whole outermost layer contains black-brown pigment. The upper portion of the cutis is devoid of pigment, then follows a layer of clusters of ramified dark pigment-cells, and lastly the rest of the cutis.
Each of the four fingers ends in a four-armed star, the tips of which again carry four or five sensory papillae. The cartilage of the terminal phalanges is correspondingly star-shaped.
According to Klinckowstroem these toads, which are entirely aquatic, are easily collected at the end of the long dry period, when they are all confined to the half-dried-up pools. But they do not spawn there. This happens after the rains have inundated the forest, and then it is very difficult to get the females with eggs on their backs. Each of the eggs, when once they have been glued on to the back, sinks into an invagination of the skin. The initial stages are probably the same as those caused by the eggs on the belly of Rhacophorus reticulatus (see p. 248). Later, each egg is quite concealed in a cavity with a lid. These cavities are simply pouches of the skin, and are not formed by enlarged glands as has been suggested by some anatomists. Each cavity consists of the epidermal pouch and the lid. How the latter is produced is not known. According to the authors quoted above, the lid looks like a shiny or sticky layer which has hardened into horn-like consistency. It lies exactly like a lid upon the rim of the pouch itself, and is certainly not in structural or organic continuity with the epidermis. Most probably it is produced by the remnant of the egg-shell itself, which, after the larva is hatched, is cast up to and remains on the top of the cup.
Bartlett[76] has described the spawning of specimens in the Zoological Gardens in London.
"About the 28th of April 1896 the males became very lively, and were constantly heard uttering their most remarkable metallic, ticking call-notes. On examination we then observed two of the males clasping tightly round the lower part of the bodies of the females, the hind parts of the males extending beyond those of the females. On the following morning the keeper arrived in time to witness the mode in which the eggs were deposited. The oviduct of the female protruded from her body more than an inch in length, and the bladder-like protrusion being retroverted, passed under the belly of the male on to her own back. The male appeared to press tightly upon this protruded bag and to squeeze it from side to side, apparently pressing the eggs forward one by one on to the back of the female. By this movement the eggs were spread with nearly uniform smoothness over the whole surface of the back of the female to which they became firmly adherent. On the operation being completed, the males left their places on the females, and the enlarged and projected oviduct gradually disappeared from one of the females. In the other specimen, the oviduct appears not to have discharged the whole of the eggs."
Boulenger, who examined this second specimen, which died, confirmed this egg-bound condition. He remarks further: "The ovipositor formed by the cloaca (not by the prolapsed uterus), was still protruding and much inflamed. It may be deduced from the observation made by the keeper, that fecundation must take place before the extrusion of the eggs, and it is probable that the ovipositor serves in the first instance to collect the spermatozoa which would penetrate into the oviducts, the eggs being laid in the impregnated condition, as in tailed Batrachians."
Sub-Order 2. Phaneroglossa–Fam. 1. Discoglossidae.–The tongue has the shape of a round disc, adherent by nearly the whole of its base, and it cannot be protruded. The vertebrae are opisthocoelous, and in the aquatic genera are of the most exaggerated epichordal type; the diapophyses of the second to the fourth vertebrae carry short, free ribs, and those of the sacral vertebra are dilated. The metasternum behind is forked. The {153}upper jaw and the vomers are provided with teeth. The males have no vocal sac. The tadpoles are distinguished by having the opercular spiracle placed in the middle of the thoracic region (see general anatomical part, p. 44).
The few members of this family have a peculiar distribution. Liopelma is confined to New Zealand, where it is the solitary representative of the Amphibia. Ascaphus is found in North America. The other genera, Discoglossus, Bombinator, and Alytes, are typical of the Palaearctic sub-region, and are, with the exception of Bombinator, confined to the Western Provinces (cf. Map, Fig. 32, on p. 161).
Discoglossus.–The tympanum is frequently more or less concealed by the skin. The pupil is round or triangular. The omosternum is small. The vertebrae are of the epichordal type.
D. pictus, the only species, has a smooth and shiny skin, provided with numerous small mucous glands. The palms of the hands are provided with three tubercles, of which the innermost is the largest, and is carried by the vestige of the thumb. The coloration of this species is very variable. The ground-colour of the upper parts is a rich olive brown with darker, light-edged patches, which are either separate or confluent in various ways, forming broad, longitudinal bands, or a few larger asymmetrical patches, separated in some individuals by a broad and conspicuous light brown or yellowish vertebral stripe. An irregular reddish band frequently extends from the eyes backwards along the sides. The under parts are mostly yellowish white. This variability is purely individual, the most differently marked and variously coloured specimens being found in the same locality and even amongst the members of one and the same brood. The male develops various nuptial excrescences, consisting of minute, dark, horny spines, notably on the inner palmar pad, on the inner side of the first and second finger, on the chin and throat, and smaller and more scattered spicules on the belly and legs.
This pretty and extremely active little creature, which measures between 2 and 3 inches in length, is confined to the south-western corner of the Palaearctic sub-region, being found in Algiers and Morocco, Sicily, Sardinia, Corsica, and the southern and western parts of the Iberian Peninsula. Curiously {154}enough it is absent in the Balearic Isles. Rather aquatic in its habits, frequenting pools and streams, it is also often found on land.
The male has a feeble voice, which sounds like "ha-a, ha-a-a," or "wa-wa-wa," uttered in rapid succession. The pairing season lasts a long time, in Algeria from January to October, but a much shorter time in the north of Portugal, where it extends over the spring and summer months. Boulenger has made extensive observations on many specimens kept in captivity. The embrace, which never lasts long, is lumbar. The eggs are small, 1 to 1.5 mm. in diameter, dark brown above and greyish below, each surrounded by a gelatinous capsule of 3-7 mm. in diameter. The eggs are laid singly, and a set amounts to from 300 to 1000, the whole mass sinking to the bottom of the pool. Each female lays several times during the season. The eggs are developed very rapidly, the larvae escaping sometimes after thirty-six hours, but usually from the second to the fourth day. The external gills are lost on the seventh day, when the tadpoles are 11 mm. long; the hind-limbs appear on the tenth, and after four weeks the tadpoles reach their greatest length, namely from 25-30 mm. The fore-limbs appear on the thirtieth day, and a few days later the most precocious specimens leave the water and hop about. Others, however, of the same brood took from two to three months in metamorphosing.
This species lives on insects and worms, and can swallow its prey under water.
Bombinator.–The tympanum is absent and the Eustachian tubes are very minute. The pupil is triangular. The omosternum is absent. The vertebrae are absolutely epichordal. The fingers are free, the toes are webbed. The upper parts are uniformly dark, and are covered with small porous warts. The general shape of the head and body is depressed or flattened downwards. The habits are eminently aquatic. This genus consists of three species, two of which are European, the third Chinese.
B. igneus.–The under parts are conspicuously coloured bluish black with large irregular red or orange-red patches; the upper parts are more or less dark grey or olive black. The iris is golden, speckled with brown. The male has a pair of internal vocal sacs by which the throat can be inflated; nuptial excrescences are developed on the inner side of the fore-arm and the {155}first two fingers. Total length from 1½ to 2 inches, the males being generally smaller than the females. This "Fire-bellied toad," the "Unke" of the Germans, is essentially a native of lakes, ponds, and other standing waters of the plains.
It ranges through the whole of North Germany, Bohemia, and Hungary into Russia, eastwards as far as the Volga. The latter river, the Danube, and the Weser form, roughly speaking, its boundaries; northwards it extends into Denmark and the southern extremity of Sweden.
B. pachypus.–The under parts are yellow instead of red. The male is devoid of vocal sacs, but has nuptial excrescences on the under surface of most of the toes, in addition to those on the fore-arm and fingers. The "Yellow-bellied Toad" is the representative of the red-bellied species in Southern and Western Europe, preferring, although not exclusively, the hilly and mountainous districts. It ranges from France and Belgium through South-Western Germany, continental Italy, and the whole of Austria and Turkey in Europe. Where both species meet, for instance in the hilly districts between the Weser and the Rhine, in Thuringia and in Austria, the predilection of the yellow-bellied {156}species for the hills, and that of the other for the plains, is well marked.
While B. igneus prefers standing waters with plenty of vegetation, B. pachypus is often found in the smallest occasional puddles produced by recent rain, for instance in the ruts of roads. Both species have otherwise much in common. They are essentially aquatic. They hang in the water, with their legs extended, nose and eyes just above the surface, and bask or lie in wait for passing insects, the fire-bellied kind preferring to conceal itself in the vegetation of the margins of ponds. During the pairing season, in Germany in the month of May, they are very lively and perform peculiar concerts, one male beginning with a slowly repeated note like "hoonk, hoonk," or "ooh, ooh," in which all the other males soon join, so that, when there are many, an almost continuous music is produced. This sound is not at all loud, a little mournful and very deceptive. It appears to be a long way off, certainly at the other end of the pond, until by careful watching you see the little creature almost at your very feet. But on the slightest disturbance the performance ceases, they dive below and hide at the bottom. The yellow-bellied kind, when surprised in a shallow puddle, skims over the mud, disturbs it, and allows it to settle upon its flat body, so that nothing but the little glittering eyes will betray its concealment. When these toads are surprised on land, or roughly touched, they assume a most peculiar attitude, as shown in Fig. 31. The head is partly thrown back, the limbs are turned upwards with their under surfaces outwards, and the whole body is curved up so that as much as possible of the bright yellow or red markings of the under parts is exposed to view. The creature remains in this strained position until all danger seems passed. In reality this is an exhibition of warning colours, to show the enemy what a dangerous animal he would have to deal with. The secretion of the skin is very poisonous, and the fire-toads are thereby well protected. I know of no creature which will eat or even harm them. I have kept numbers in a large vivarium, together with various snakes, water-tortoises, and crocodiles, but for years the little fire-bellies remained unmolested, although they shared a pond in which no other frog or newt could live without being eaten. Hungry water-tortoises stalk them under water, touch the intended prey with the nose in order to {157}get the right scent, and then they withdraw from the Bombinator, which has remained motionless, well knowing that quick movements, or a show of escape, would most likely induce the tortoise to a hasty snap, with consequences to be regretted by both.
After they have been handled frequently, they do not readily perform, but simply lie still, or hop away. Miss Durham experienced considerable difficulty in inducing her tame specimens to assume and to keep up the correct warning attitude. The statement that they "turn over on the back" is a fable, graphically fixed in various illustrated works.
It has been said that these two species are diurnal and thoroughly aquatic. They are certainly active in the daytime, sing in full sunshine, and spend most of their time in the water, but they display much more liveliness towards the evening and during the night, especially when there is a moon. My fire-toads live by no means always in the water, but conceal themselves in the daytime under stones, while they are regularly all astir at night in search of worms and all kinds of small insects.
The spawning takes place several times during the spring and summer. The amplexus is lumbar, and the eggs are extruded singly. They sink to the bottom, or are attached to water-plants. The oviposition takes a long time, perhaps the whole night, and several dozen eggs, not hundreds as in the allied genera, make a set. The egg, with its swollen gelatinous capsule, is large for so small a creature, namely 7-8 mm. in diameter. The embryos escape after a week, and the tadpoles reach two inches in total length. Those of B. igneus have a triangular mouth, but in B. pachypus this is elliptical, as in Alytes and Discoglossus. Metamorphosis is completed in the same autumn; the little toad is then about 15 mm. long, and differs from the adult by the absence of the conspicuous coloration of the under parts. In reasonable conformity herewith it does not take up the warning attitude. The colour appears gradually during the second year, but full growth is generally not reached until the third year. They do not hibernate in the water, but hide on land out of the reach of frost.
Alytes.–The tympanum is distinct, the pupil vertical, the omosternum is absent. The only two species live in South-Western Europe. The male attaches the eggs to its hind limbs, and nurses them until they are hatched.
A. obstetricans, the "Midwife-toad," has the general appearance of a smooth toad. The upper parts are rather smooth, sometimes almost shiny, in spite of the numerous more or less prominent warts, of which those of the lateral lines, and those above the ear, are generally most marked. The colour of the upper parts varies a great deal according to the prevalence of greenish and reddish spots upon the grey or brown ground-colour. The red is sometimes, especially in the breeding males, rather conspicuous on the parotoid region and on the upper sides of the body. The under parts are whitish grey. The iris is pale golden, with black veins. The male has no vocal sac, and is as a rule smaller than the female, the latter reaching a length of two inches.
This species occurs in the whole of the Iberian Peninsula and in France, extending into Switzerland and beyond the Rhine valley into Thuringia. Altitude above the sea does not seem to have any influence upon its range, which reaches from sea-level to the tops of subalpine mountains. I have found great quantities of its tadpoles in Portugal on the Serra d'Estrella, nearly 6000 feet high, and they are recorded from 6500 feet in the Pyrenees. They seem to be ubiquitous in Spain and Portugal, not that they are often found or seen, but they are heard everywhere; besides, tadpoles are sure to be in the clear cold lakes on the tops of the mountain-ranges, in the dirty puddles caused by the village fountains, and in the sun-heated swampy ditches on the roadside with scarcely enough water to hold the wriggling mass. Wherever there is water within easy reach, on the lonely mountains, in fertile valleys, in the gardens of the busy towns, you hear during the whole night, from March to August, the double call-note of the male, sounding like a little bell; but to see the performer is quite a different matter. He sits in front of his hole, dug out by himself or appropriated from a mouse, in a crack of the bottom of a wall, under stones, or in a similar place into which he withdraws for the day.
The pairing and the peculiar mode of taking care of the eggs by the male, which habit has given it the specific name obstetricans, the midwife, have been most carefully observed by A. de l'Isle du Dréneuf, near Nantes. A condensed account has been given by Boulenger. Several males collect around a female on land, not in the water, and the successful one grasps {159}her round the waist. For nearly half an hour the male lubricates the cloacal region of the female by more than one thousand strokes of his toes, whereupon the female extends the hind-limbs, forming with the bent hind-limbs of the male a receptacle for the eggs, which are then expelled with a sudden noise. The eggs are yellow and large, up to 5 mm. in diameter, and are fastened together in two rosary-like strings, several dozen making one set. During the expulsion of the eggs the male shifts its body forwards, clasps his fore-limbs round the female's head, and fecundates the eggs. After a rest he pushes first one hind-limb and then the other through the convoluted mass of eggs, which then have the appearance of being wound round the hind-limbs in a figure of 8. Then the sexes separate and the male withdraws with its precious load into its hole, which it, however, leaves during the following nights, in search of food, taking this opportunity to moisten the eggs in the dew, occasionally even immersing them in the water. After at least three weeks, when the larvae are nearly ready, he betakes himself to the nearest water, and the larvae burst the thereby softened gelatinous cover of the eggs. Not infrequently the same male ventures upon a second pairing, and adds another load to the one which already hampers its movements. The eggs being large, owing to the great amount of yellow food-yolk, the embryos are enabled to be hatched in a more advanced stage than in most other Anura. The larva develops only one pair of external gills within the egg. These appear first in the shape of oval bags upon the third branchial arch, which sprout out secondary branches, soon in their turn to be resorbed and replaced by the so-called internal gills before hatching.
Fischer-Sigwart[77] gives the following account of the growth of this species. The male took to the water, with its load of twenty to thirty eggs, on the 6th of June. The larvae escaped out at once, 16-17 mm. long, the body measuring 5 mm. On the 14th they had reached 32 mm. in length, whereupon they grew very slowly, although they were well fed, in a temperature of about 50° F. This same brood did not metamorphose until May of the next year. The growth took place as follows:–The hind-limbs appeared on the 8th of September, when the tadpoles were 50 mm. long; by the middle of the next May they {160}had reached their greatest length, 76 mm., the hind-limbs being 18 mm. long, whilst the fore-legs were just indicated. On the 21st of May the hind-limbs were 27 mm. long, and the whole creature was practically metamorphosed, except for the tail. The latter was resorbed on the 13th of July, and the little toads, 25 mm. in length, were actually smaller, certainly far less bulky and heavy, than the tadpoles, which had required one year and a quarter for their metamorphosis.
The early broods probably finish their development by the autumn of the same year, but those which are born later, in July and August, certainly hibernate in the water. I have found very small tadpoles, scarcely 15 mm. long, on the Cantabrian mountains as late as the end of September, and rather large ones in the spring at the time of first pairing; the fact that this takes place during the whole summer explains the occurrence of tadpoles in all stages of development almost the whole year round.
A. cisternasi has only two palmar tubercles, the middle or third one of A. obstetricans being absent; the outer finger is short and thick. Instead of a very long and wide fronto-parietal fontanelle, the fronto-parietal bones diverge only in front so that there are two fontanelles, a small one in the parietal and a large triangular one in the frontal region. The limbs are relatively shorter and stouter in conformity with the habits of this species, which prefers to burrow in sandy localities. Otherwise it leads the same kind of life as A. obstetricans, and the male carries the eggs. It has hitherto been found in Central Spain and in the middle provinces of Portugal.
Liopelma is intermediate between Alytes and Bombinator, agreeing with the latter, in conformity with its essentially aquatic life, in the absence of a tympanum, while the Eustachian tubes are entirely suppressed. The tongue is disc-shaped, but is slightly free behind. The pupil is triangular. The male is devoid of a vocal sac. L. hochstetteri is the sole representative of the Amphibia in New Zealand, where it is apparently rare. The upper parts are covered with smooth tubercles, and are dark brown with blackish spots; the under parts are whitish. Total length only 1½ inch.
Fam. 2. Pelobatidae.–The upper jaw and, as a rule, the vomers are provided with teeth. The tongue is oval, slightly {161}nicked, and free behind, so that it can be thrown out, except in Asterophrys turpicola of New Guinea, which has a large but entirely adherent tongue. The vertebrae are procoelous, except in Asterophrys and the Malay genus Megalophrys, where they are opisthocoelous. The sacral diapophyses are strongly dilated. The omosternum is small and cartilaginous. The metasternum has a bony style, and ends in a cartilaginous, rounded or heart-shaped disc, but in Scaphiopus it forms an entirely cartilaginous plate. The tympanic disc is mostly hidden or indistinct, and is quite absent in Pelobates. The Eustachian tubes are very small in Pelobates, and exceedingly minute in Scaphiopus stagnalis of New Mexico. The pupil is vertical. This family contains seven genera with about twenty species, with a rather scattered distribution.
A. Toes extensively webbed, sacrum and coccyx confluent.
a. Metasternum a cartilaginous plate. America .......... Scaphiopus, p. 164.
b. Metasternum with a bony style. Europe .......... Pelobates, p. 162.
B. Toes nearly free. Metasternum with a bony style.
a. Vertebrae procoelous.
α. Sacral vertebra articulating by one condyle with the coccyx.
Europe .......... Pelodytes, p. 165.
New Guinea .......... Batrachopsis.
β. Sacral vertebra with two condyles.
India and Malaya .......... Leptobrachium, p. 166.
b. Vertebrae opisthocoelous.
Ceylon and Malayan Islands .......... Megalophrys, p. 60 (Fig. 11).
New Guinea .......... Asterophrys.
Pelobates ("Spade-foot").–The tympanum is absent; the toes are webbed. The inner tarsal tubercle is large, and is transformed into a shovel which is covered with a hard, sharp-edged, horny sheath. The skin of the upper surface of the head is partly co-ossified with the underlying cranial bones, giving them a pitted appearance. The general shape is toad-like.
P. fuscus.–The smooth skin is brown above, with darker marblings, while the under parts are whitish, but the coloration varies greatly, from pale to dark brown or olive-grey with more or less prominent irregular dark, sometimes confluent, patches. Some specimens are adorned with numerous red spots. The tarsal spur is yellow or light brown. The iris is metallic red or golden. The male has a long oval gland on the upper surface of the upper arm, and although possessed of a voice, has no vocal sacs. The total length of full-grown females is nearly 3 inches, that of males half an inch less.
The "Spade-footed Toad," which occurs throughout the whole of Central Europe, extends from Belgium and the middle of France to North-Western Persia, and from the southern end of Sweden to Northern Italy. It prefers sandy localities, in order to dig its deep hole, in which it sits concealed during the daytime. Owing to the looseness of the sand, the hole is filled up so that no trace of its inhabitant is left. The digging is done by means of the spades, and in suitable localities the animal soon vanishes, sinking backwards out of sight. Except in the breeding season, or at night, it is therefore found only accidentally. The sand-loving habits do not, however, prevent it from enjoying moist localities. Several which I have kept for years dig themselves into the wettest moss in preference to the drier parts of their habitation. Being thoroughly nocturnal, they hunt after nightfall, the food consisting of all sorts of insects and of worms. When captured they utter a startling shrill cry, and their skin becomes covered with a dermal secretion which smells like garlic, a peculiarity which has given them in Germany the name of "Knoblauchskröte," "garlic-toad." Although they become very tame, so that they no longer smell when handled, they can be made ill-tempered by being pinched or otherwise teased, whereupon they take up a defiant attitude, and with open mouth continue to cry for several minutes. Some such scenes occur now and then, without {163}my interference, with the specimens which share their abode with several species of Amblystoma and Spelerpes; there are heard now and then sudden loud yells, like the squeak of a cat or the yapping of a little dog.
In the spring the Spade-footed Toads take to the water for about a week, and the male's call-note is an ever-repeated clucking sound, which can also be produced under water, with the mouth shut, the air being shifted backwards and forwards through the larynx. The male grasps his mate below the waist; the eggs are combined into one thick string, which is about 18 inches long, and is wound round and between the leaves and stalks of water-plants. The eggs measure 2-2.5 mm., and are very numerous, a large string containing several thousands. The larvae are hatched on the fifth or sixth day in a very unripe condition. They are only 4 mm. long, quite black, and still devoid of gills and tail. They attach themselves to the empty gelatinous egg-membranes, which they possibly live upon. On the following day the tail begins to grow; two days later fringed external gills sprout out and serve for about ten days, when they in turn give way to new, inner gills. The little tadpoles then leave their moorings and become independent. The hind-limbs appear in the ninth week, the fore-limbs in the twelfth. At the age of three months they begin to leave the water. The most remarkable feature is the enormous size of the full-grown tadpole, the body of which is as large as a pigeon's egg; the usual total length, including the tail, amounts to about 4 inches or 100 mm., but occasionally regular monsters are found. This was the case some thirty years ago, when the Berlin Museum received a number of tadpoles, the largest of which measured nearly 7 inches. They were found in the month of December near Berlin, in a deep clay-pit with high, steep walls, so that the tadpoles were prevented from leaving the water. Similarly hemmed-in broods probably hibernate in the water under the ice, and such instances have been recorded. Normally they metamorphose into the much smaller toad within the same year.
P. cultripes.–This is the Spade-foot of the whole of Spain and Portugal and of the southern and western parts of France. It is similar in habits to P. fuscus, from which it differs but slightly. The tarsal spur is black, and there is a parieto-squamosal bridge which completely roofs over the temporal fossa {164}and closes the orbit behind.–Boulenger has discovered the rare, individual occurrence of minute teeth on the parasphenoid and on the pterygoids of this species. These teeth are unquestionably the last reminiscences of a condition almost entirely superseded in the recent Anura.
P. syriacus from Asia Minor and Syria agrees with P. cultripes in the cranial configuration, but has the yellow or brown spur of P. fuscus.
Scaphiopus.–The Spade-foot of North America and Mexico differs slightly from those of Europe, chiefly by the presence of a more or less hidden tympanum and of a subgular vocal sac, and by the sternum, which forms an entirely cartilaginous plate without a special style. The close relationship of these two genera is further indicated by the occurrence of peculiar large glandular complexes in some of the species, pectoral in S. solitarius, tibial in S. multiplicatus of Mexico. At the same time this genus approaches Pelodytes.–About eight species are known, two of which inhabit the United States, the others Mexico.
S. solitarius is the commonest species of the Southern States. It is brown above, with darker patches; its total length is about 2 inches. According to Holbrook it excavates small holes half a foot deep, in which it resides, seizing upon such unwary insects as may enter its dwelling. It never leaves the hole except in the evening or after long-continued rains. It appears early in March, and soon pairs; as an instance of hardiness Holbrook mentions that he has met it whilst there was still snow on the ground. When teased they assume a humble attitude, bending the head downwards with their eyes shut, as illustrated by Boulenger.[78]
Pelodytes is, like the rest of the genera, devoid of the tarsal digging spur. The tympanic disc is rather indistinct; the male has a subgular sac. The general appearance of the slender body with long hind-limbs and toes is frog-like. Two species only are known, one in South-Western Europe, the other in the Caucasus.
P. punctatus.–The "Mud-diver" has the upper parts covered with small warts, and is about 1½ inch in length. Its coloration is variable, and changes much. One day it may appear greenish brown, the next day pale grey; in the daytime perhaps with many bright green spots, and in the evening spotless and unicoloured. The under parts are mostly white, sometimes with a fleshy tinge. The male has a voice like "kerr-kerr" or "creck-creck," uttered during the breeding season, which lasts from the end of February until May, according to the temperature and the more Southern or Northern locality. Occasionally they breed a second time in the summer or autumn. The male develops nuptial excrescences, chiefly three rough patches on the inner side of the fore-limbs or on the inner side of the first two fingers, while the belly and thighs are covered with small granules. In the mode of copulation, the laying of the small and numerous eggs, the hatching of the larvae in a tail- and gill-less condition, this genus closely resembles Pelobates; but the tadpoles never reach a colossal size, the usual length being 2 inches, and even this is comparatively large for so small a species. It inhabits the greater part of France, most of Portugal, and the southern half of Spain, avoiding, however, the central plateaux and the mountain-ranges. Its habits are essentially nocturnal, {166}living in the immediate vicinity of the water, into which it hops with a long jump in order to hide in the mud. Easily kept, it breeds regularly in captivity, according to circumstances at almost any time of the year.
P. caucasicus has been discovered in the Caucasus at an altitude of 7000 feet. The remaining genera of this family contain only a few species each, and are restricted to South-Western Asia, the Malay and Papuan Islands. The commonest is Leptobrachium, which ranges from the Himalayas to Borneo and Java. Pupil vertical. Vomerine teeth sometimes absent. Tongue roundish, very slightly nicked behind. Tympanum indistinct. Omosternum small, cartilaginous. Male with internal vocal sacs. Tarsus with a roundish tubercle. Some of the species, e.g. L. carinense from the Karen Hills, attain to a large size, namely, 6 inches; they seem to live on rats and mice, and one specimen contained a young squirrel.
Fam. 3. Bufonidae (Toads).–The formula:–no teeth in the upper and lower jaws, vertebrae procoelous and without ribs, sacral diapophyses dilated,–is sufficiently diagnostic of this cosmopolitan family. The generally entertained notion that toads have a rather thick-set, short-limbed, warty appearance, does not apply to all the members of the family. The majority are quite terrestrial, many are burrowing, the Javanese Nectes is aquatic, the Afro-Indian Nectophryne is arboreal, while the Australian Myobatrachus and the Mexican Rhinophrynus eat termites and are correspondingly modified; lastly, Bufo jerboa is a slender, long-legged creature.
Teeth are almost entirely absent, except in Notaden, which has teeth on the vomers. The omosternum is mostly absent, except in Engystomops and in some species of Bufo, while in Notaden it is merely vestigial. The metasternum shows more variety. The tympanum is usually distinct, but varies even within the same genus, being hidden beneath the skin or being entirely absent. The terminal phalanges are modified according to the habits of the species, but they are never claw-shaped.
The Bufonidae are connected in various directions. The Neotropical Engystomops greatly resembles the likewise Neotropical Cystignathoid Paludicola, and the Australian Pseudophryne closely approaches the Australian Cystignathoid Crinia. It is therefore all the more remarkable that a similar approach, in another direction, namely, towards the Firmisternal family of the {167}Engystomatidae, is indicated by the Mexican Rhinophrys and the Australian Myobatrachus. However, since there are no true Engystomatidae in Australia, although several genera occur in Papuasia, these cases may be instances of convergence without necessarily implying relationship. An unmistakable line of connexion leads, according to Boulenger, to the Pelobatidae, the link being the Himalayan Cophophryne, with very strongly dilated sacral diapophyses, with a single condylar articulation of the coccyx with the sacral vertebra (as in some Indo-Malayan Pelobatidae), while this articulation is bicondylar in all the other Bufonidae.
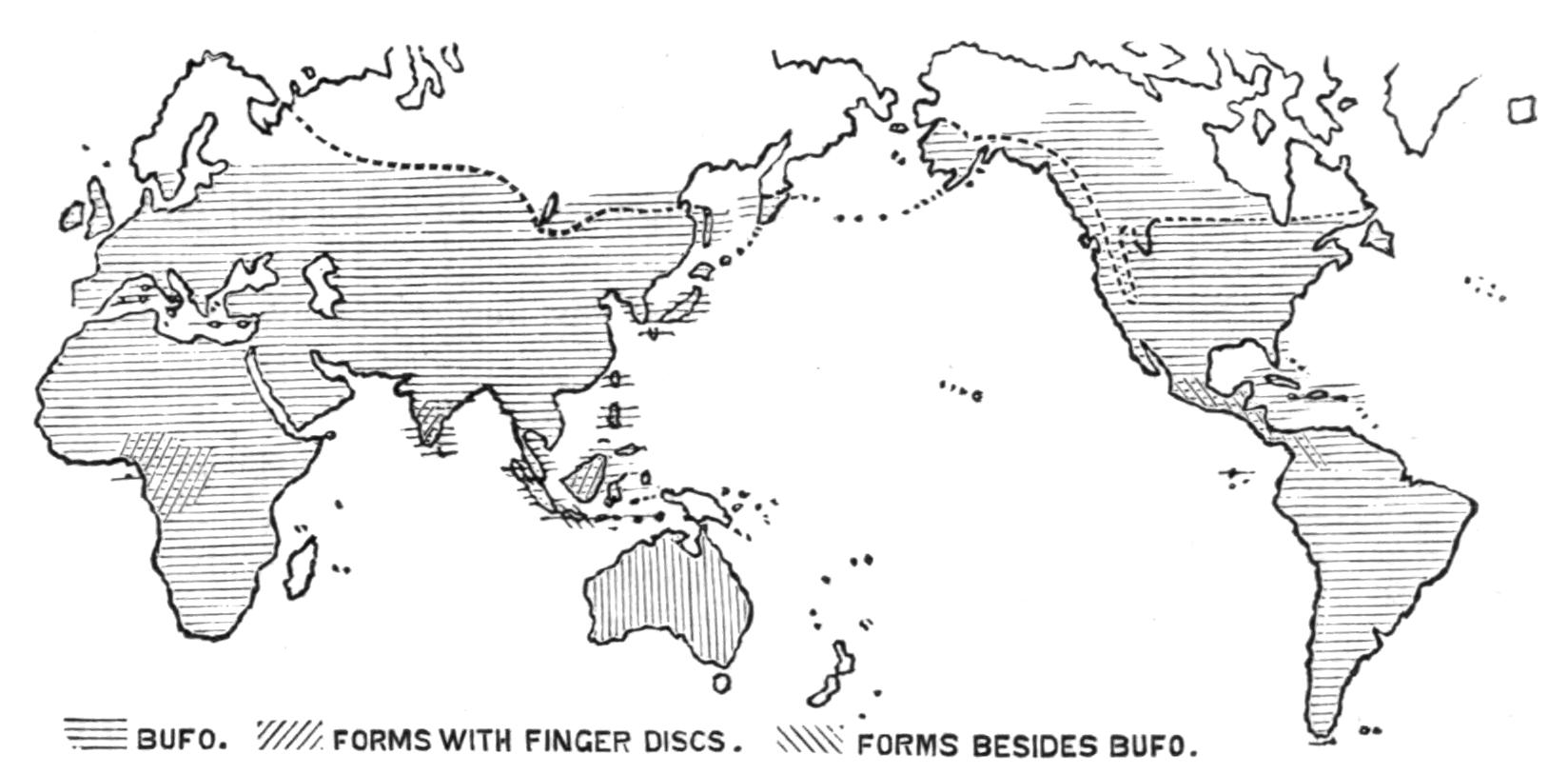
Fig. 34.–Map showing distribution of Bufonidae. The vertical lines indicate the occurrence of Bufonidae, but not of Bufo.
The whole family is divided into nine genera with more than a hundred species, of which only about fifteen do not belong to the genus Bufo. The distribution of the family is well-nigh cosmopolitan, with the remarkable exception of Madagascar, Papuasia, and the small islands of the Pacific; Bufo has been wrongly said to inhabit the Sandwich Islands. The greatest number of species, chiefly Bufo, occur in the Neotropical region, the greatest number of genera in Central America, where Bufo is rare, and in Australia, where it is absent.
A. Pupils contracted to a horizontal slit. Typically arciferous.
a. Australian. Tympanum invisible. Fingers and toes not dilated.
1. With vomerine teeth. Both the omo- and meta-sternum are rudimentary. East Australia: .......... Notaden bennetti.
2. Without vomerine teeth. Omosternum absent. Metasternum cartilaginous: .......... Pseudophryne, p. 168.
b. Not Australian.
1. Omosternum narrow and cartilaginous. Metasternum with a bony style ending in a cartilaginous disc. Fingers and toes slightly swollen. Neotropical: .......... Engystomops, p. 168.
2. Omosternum absent. Metasternum cartilaginous.
α. Fingers and toes webbed; terminal phalanges T-shaped and with adhesive broadened tips. Africa and India: .......... Nectophryne, p. 169.
β. Fingers free, toes webbed; terminal phalanges simple, not dilated. Tympanum distinct. Java: .......... Nectes, p. 169.
3. Metasternum cartilaginous, sometimes ossified along the middle. Fingers free; toes more or less webbed; tips simple or dilated into very small discs: .......... Bufo, p. 169.
B. Pupil a vertical slit. The epicoracoid cartilages are narrow and scarcely overlap. Omosternum absent except in Cophophryne. Vomerine teeth absent. Sacral diapophyses strongly dilated. The terminal phalanges are simple and the tips are pointed.
a. Australian. Tympanum distinct. The metasternum is calcified along the middle: .......... Myobatrachus, p. 184.
b. Mexican. Tympanum absent. Metasternum rudimentary: .......... Rhinophrynus, p. 185.
c. Himalayan. Tympanum absent. Metasternum with a slender bony style: .......... Cophophryne sikkimensis.
Engystomops is interesting because it closely resembles the Cystignathoid genus Paludicola, and thereby seems to connect these two families. It differs from Paludicola chiefly by the absence of teeth, by the moderately dilated sacral diapophyses and by the slightly swollen tips of the fingers and toes, the end-phalanges of which are, in one species, E. petersi, T- or anchor-shaped The tympanic disc is either distinct or hidden. The males have a large subgular vocal sac. The generic name refers to the small head with a prominent snout. Three species are known from Central America and Ecuador.
Pseudophryne appears to be another link with the Cystignathidae by its resemblance to the Australian genus Crinia, from which it differs by the absence of teeth and by the absence of an omosternum. The sacral diapophyses are but moderately dilated. The males have a flat oval gland on the hinder side of the thighs, and they are provided with a subgular vocal sac. The 3 or 4 species of this genus which live in Australia, both East and West, are not unlike Bombinator in their general shape, short limbs and coloration. The skin of P. australis and P. bibroni is covered with small smooth warts and is blackish brown, while the under parts are blackish with large yellow {169}patches. Total length little more than one inch. Concerning the breeding habits, see p. 223.
Nectophryne.–The sacral diapophyses are strongly dilated. N. afra, without a tympanum, but with fully-webbed digits and several broad, cushion-like or lamellar pads on the fingers and toes, inhabits the Cameroons, N. tuberculosa of Malabar, and N. guentheri and N. hosei of Borneo, have a visible tympanum and the fingers are webbed at the base only. These slender and long-legged species are most probably arboreal, as indicated by the broadened, but truncated, tips of their fingers and toes. N. hosei is about 4 inches long, N. misera is a little creature of only ¾ inch in length. Nectes, hitherto known by one species, N. subasper of Java, is a swimmer and exceeds 6 inches in length. The tympanum is very distinct; the small nostrils look upwards. The toes are long and webbed to the tips; the hind-limbs are very long. The sacral diapophyses are strongly dilated. The skin of the upper parts is very rugose, covered with round warts, and dark brown; the under parts are granular and uniformly light brown.
Bufo.–The great number of species, more than 100, renders a strict definition of this genus difficult. The tongue is pear-shaped, thicker in front, entire, not cut out, but free behind, so that it can be projected. The fingers are free, the toes more or less webbed although never completely so. The terminal phalanges are obtuse and sometimes carry tiny discs. The omosternum is absent or merely vestigial. The metasternum is a rather large cartilaginous plate with a waist, which is sometimes incompletely calcified. The sacral diapophyses are moderately dilated. The tympanum is distinct or hidden. The skin of the upper parts is always rich in specific poison-glands, a concentration of which forms in many species very conspicuous, thickened parotoid glands. The surface of the skin may be smooth, moist and slimy, or rough and warty, sometimes covered with tiny, sharp, horny spikes and quite dry.
The genus is cosmopolitan, with the exception of the whole Australian region and Madagascar, from which we may perhaps conclude that its original centre was not in Notogaea, in spite of the diversity of species in the Neotropical region, which now contains about half of all the species known. Next to Central America the Indian region is richest in species of Bufo.
B. vulgaris.–The Common Toad of the Palaearctic region. The skin of the upper parts is much wrinkled and beset with numerous round warts or poison-glands, the openings of which can be seen with the naked eye, especially on the large parotoid complexes. The outermost layer of the epiderm, in fact all that portion which is periodically shed, is elevated into numerous little cornified spines. The extent of their development varies much; southern specimens, especially those from Portugal, being perhaps the roughest. Others appear quite smooth to the touch, and this is the case with many English specimens. The skin of the under parts is more granular and devoid of specific glands. The general colour of the upper parts is olive grey to dark brown, more or less mottled; the under parts are whitish, often with a brown, yellow or reddish tinge.
The coloration of this species varies considerably and is moreover very changeable. These changes depend chiefly upon the surroundings and the locality, in which certain styles of coloration seem to be the fashion, not necessarily to the absolute exclusion of others. Some specimens are of a rich brown colour, with or without dark brown spots and patches, and these are sometimes confluent, forming irregular, longitudinal bands. The ground-colour of other individuals is olive grey, with or without darker patches, and these paler tones prevail in toads which live on light-coloured soil, for instance on chalk. I recently found one between two dark-coloured slates, and this creature was so black that it gave the impression of having soiled itself with coal-dust. One and the same specimen will appear paler or darker according to its mood and the leading tones of its immediate surroundings, but it cannot change its dominant ground-colour. A third colour-variety occurs more frequently in the mountainous districts of Southern Europe. I have obtained the most handsome specimens in the Serra Gerez, in North Portugal. Their ground-colour is pale brownish-yellow, with many large and small, rich brown patches, or if the latter colour predominates, these patches and spots are separated from each other by creamy seams, with the occasional effect of dark brown, yellow-ringed eyes. Eastern Asiatic specimens often have a fine yellow vertebral line and the under parts are inclined to be marked with dark spots.
The iris is red or coppery, mottled with black. The male {171}has no vocal sacs, and, besides being smaller than the female, is distinguished by slight nuptial excrescences in the shape of little horny brushes on the inside of the inner palmar tubercle and the three inner fingers. The full size of this toad varies extremely. Taking the standard of everyday experience in England and Central Europe, one would call any female beyond 3½ inches in length, and any male of more than 2½ inches, unusually large. But occasionally they grow to a much larger size, especially in the mountains of Southern Europe, provided there is a rich vegetation of meadows and deciduous trees so as to insure a variety of plentiful food. Although Fatio[79] mentions a toad 153 mm. = 6 inches long, and Boulenger succeeded in getting a toad from Paris which measured 132 mm., i.e. almost 5¼ inches, one of my specimens from the Serra Gerez seems to hold the record with a total length from snout to vent of 135 mm. or more than 5¼ inches. Jersey is also famous for its large toads, possibly on account of the many large greenhouses. These large specimens do not constitute a special race. The monsters among them are without exception females, often but not always sterile, as I have often found large masses of eggs in them. Food is the chief cause. At least I have observed that the more voracious of some Spanish and Portuguese specimens, which were already 3½ inches long, and therefore entitled to respect, continued to grow rather rapidly, adding about half an inch within a year. Again, if the growth of a promising toad is arrested for a season–not necessarily by starvation, but by uncongenial surroundings, sameness, and unvaried nature of food–they consolidate so to say, or settle down, and no amount of future good feeding will turn them into exceptionally big specimens. There are no data to tell how old such monsters really are. At least ten years are required by the Southerners to reach four inches. The usual length of life attained by a toad is likewise unknown. Boulenger kept one in a box provided with a sod, a pan of water and plenty of varied food, but twelve years of close captivity did not make any appreciable difference in its appearance.
A number of large Spanish and Portuguese specimens in my greenhouse were at first very shy, and tried every possible means of escape or sullen hiding, but gradually they condescended to take food when lifted on to the slate-covered stage upon which their food was spread. After a few weeks they had learned this so thoroughly that, towards the usual hour of feeding, they climbed most laboriously on to the slates, lying in wait between the flower-pots, and coming forward when we entered the house. The rest of the day and night they spent on the ground, under stones or plants, each in its individual lair. The biggest of all, and several others, became so tame that they took food whilst sitting on the hand, and then they looked up for more. The food must be alive and show movement. Mealworms, snails, beetles and other small creatures are first carefully inspected with bent-down head, and are sometimes followed for a few inches; then comes an audible snap, a flash of the rosy tongue and the prey has disappeared. Large earthworms are nipped up with the jaws and laboriously poked in with the hands, the fingers being so placed as to clean the worm of adherent soil and other impurities. Very large worms are shaken, twisted, pressed against the ground and gulped down with convulsive movements, but not unfrequently the tip-end remains for some minutes sticking out of the tightly shut mouth. Several are taken at one sitting, until the toad is gorged. One of the biggest took full-grown mice, which {173}were not "fascinated by the fiery eyes" but were stalked into a corner and then pounced upon immediately when they moved. The shells of snails can for half a day be felt through the body; they then dissolve or are disgorged. The dung, which is passed in large, long masses, is often full of fine earthy matter, the contents of the earthworm's intestines, and sometimes it contains the chitinous remains of certain beetles which are supposed to be excessively rare. I know of no instance of slugs being eaten.
The regular hunting-time begins with the evening and is continued throughout bright nights, the toads crawling and hopping about. They are expert climbers of rocks, and succeed in reaching apparently inaccessible places by shoving themselves up between vertical walls, and taking advantage of any roughnesses for foothold. Every few weeks they shed their skins. Without any preliminary symptoms or loss of appetite or liveliness, the body makes a few twisting motions, the back is now and then curved, and the skin splits down the middle line. Owing to the more forcible contortions of the body it slides down to the right and left of the back, whereupon the toad gets hold of the peeling-off skin with fingers and toes, scraping the head and sides, and conveys the thin, transparent, slightly tinged skin into the mouth, slips out of it backwards and swallows it. The new surface is then quite wet and shiny, but it soon dries and hardens.
Many toads, for instance the Common Toad and the Pantherine Toad, assume a peculiar attitude when surprised. Instead of blowing themselves up by filling their lungs with air, they raise themselves upon their four limbs as high as possible, but turning the back towards the enemy in a slanting position, either to the right or to the left side, apparently in order to present as much surface as possible, in other words to look their biggest.
Some of my specimens hibernated regularly for a few months, burying themselves completely in loose, dry soil, under leaves, or,–a favourite place,–in a heap of cocoa-nut fibre. Others, and this applies also to English specimens transferred from the garden into the greenhouse, are lively all the year round, but even they withdraw for an occasional sleep of a few weeks at any time of the year.
The whole family of large toads came to a sad end after four years, when they were put into new temporary quarters, a {174}slate-bottomed terrarium. Being kept during my absence in wringing wet moss, which became fouled by their own excretions, they contracted a mysterious disease from which they never recovered. They are rather averse to wet surroundings, and except during the short pairing season they live in cool, shady places, preferably with just a little dampness. Occasionally they take a soaking bath. One specimen, living in the garden, repaired during the hot and dry summer nights to a standpipe in the garden, enjoying the occasional drips of water.
Considering the amount of snails and other noxious creatures destroyed by them during their regular nocturnal hunts, toads are eminently useful creatures. Nevertheless, they suffer much through the stupid superstition of people who ought to know better. It is difficult to find a gentle, absolutely harmless and useful creature that is more maligned than the European toad. It brings ill-luck to the house, the "slimy toad" spits venom, sucks the cows' udders and after that destroys their power of giving milk; it poisons the milk in the cellar, and a certain builder's horse, which was grazing in the grounds of the Cambridge Museums, and died there from a large concrement obstructing its bowels, was solemnly declared to have swallowed one of my toads. Silly superstitions, owing to faulty, or rather entire want of, observation! The toad is not slimy, but dry; it is often found in buildings, where it keeps down the woodlice; it cannot suck, nor does it drink at all; it does not spit venom, but becomes covered with milky white and very strong poison when in acute agony, for instance when trodden upon; and unless the big skin-glands be forcibly squeezed, there will be no squirting. Therefore, leave it alone, or put down food on its evening beat, and it will soon come to know and to recognise its friends.
The Common Toad can exist without food for a long time, provided the locality is cool and damp, but it wastes away almost to skin and bones. In order to disprove the persistently cropping up fable and sensational newspaper-accounts of toads having been discovered immured in buildings, where they were supposed to have lived for many years, Frank Buckland put a dozen specimens into separate holes bored in a block of porous limestone, covered them up tightly with a glass plate and buried the block a yard deep in the soil. A second dozen were treated similarly, but were put into a block of dense sandstone. After a {175}year and two weeks all the toads enclosed in the latter block were of course found dead and decomposed, but most of those in the porous block were still alive, with their eyes open, and did not succumb to starvation until eighteen months of confinement. These poor creatures could of course not move about, and were practically undergoing enforced continuous hibernation. Otherwise they would soon have wasted away and have died within six months. Those which tumble into deep and dry wells remain rather small, but generally manage to keep alive for years on the spiders, woodlice, earwigs and other insects which likewise tumble in.
Toads hibernate far from the water in dry holes or clefts, retiring in the middle of October in Central Europe, and they do not reappear before March. Soon after, and this depends naturally upon the season, they congregate in ponds or pools, and the males, which far outnumber the females, for whom they fight, make a peculiar little noise, something like the whining bleat of a lamb, uttering this sound day and night. The male having, after much wrestling with competitors, secured a female, which is often several times bigger than himself, clasps her tightly, by pressing his fists into the armpits, and the pair swim or crawl about in this position sometimes for a week before the spawning takes place. The number of eggs laid at one sitting is enormous, varying from 2000 to 7000. They are very small, only 1.5-2.0 mm. in diameter, and are expelled in two double rows or strings, one coming out of each oviduct. These strings consist of a soft gelatinous mass, in which the double rows of entirely black eggs are imbedded, and they measure in the swollen condition about 6 mm. or ¼ inch in diameter, and from 10 to 15 feet in length. The strings are wound round and between water-plants by the parents, which move about during the laying and fertilising process. According to the coldness or warmth of the season the larvae are hatched in about a fortnight, and for the next few days they hang on to the dissolving gelatinous mass of the egg-strings. They then leave the slime and fasten themselves by means of their suckers to the under side of grasses and water-plants or sticks, with their tails hanging downwards, still in a rudimentary condition, but henceforth progressing rapidly.
Fischer-Sigwart[80] found the time of development as follows:–The {176}eggs were laid on the 6th of March; the larvae left the jelly on the 16th, being 4 mm. long. On the 2nd of April they measured 13 mm.; on the 25th, 20 mm. On the 7th of May the hind-limbs appeared. On the 18th of May the tadpoles had reached their greatest length, namely 24 mm., and this is a rather small size for so large a species. The fore-limbs broke through on the 28th, and the metamorphosis was completed eighty-five days after the eggs were laid, the creatures leaving the water on the 30th of May. The tadpoles showed a preference for rotten pieces of Agaricus, which were floating in the water. The little baby-toads are surprisingly small, scarcely 15 mm. long, and live in the grass, under stones, in cracks of the ground, and hop about in much better style than their heavier and more clumsy-looking parents. Where many broods have been hatched they can be met with in myriads, the ground literally swarming with them, and as they are naturally stirred up by a sudden warm rain, perhaps after a drought, people will occasionally state it as an observed and well-ascertained fact that "it has rained toads."
What becomes of all these hopeful little creatures? Although it takes them fully five years to reach maturity, one would expect that the whole country would be swarming with toads; but since this is not the case, there being not more toads now than there were before, it follows that their enormous fecundity is only just sufficient to keep the race going. Adult toads seem to have scarcely any enemies except the Grass Snake, which takes them in default of anything better. But how about the reduction where there are no snakes? We know nothing about epidemics which might carry them off, but elderly toads are liable to a horrible disease produced by various kinds of flies, notably by Lucilia bufonivora and Calliphora silvatica, the maggots of which somehow or other eat their way from the nostrils into the brain and into the eyes. Those which reach the brain at first produce effects similar to those of Coenurus cerebralis, the hydatid or bladder-worm of sheep. The toad inclines its head towards one side, and cannot crawl straight, but walks in a circle. By eating away the brain they gradually destroy the host's life. But if none enter the brain, and a few only find their way into the eye, they only impair or destroy its sight. Such toads show signs of pain, poking at or stroking the affected eye, which becomes {177}inflamed, and ultimately remains enlarged, with the iris partially or entirely destroyed by the maggot, which does not develop further, but dies in the eye-chamber, this being really an unsuitable place for it. The eyesight is of course affected, and is mostly, but not in all cases, lost. Such half-blind individuals–the disease affecting sometimes one eye only–recover their health, and except for a little awkwardness, behave like normal specimens. This applies to Bufo vulgaris as well as to B. calamita. Australian Anura are cursed with a fly of their own, called Batrachomyia.[81]
B. vulgaris inhabits almost the whole of the Palaearctic region;–the whole of Europe, with the exception of Ireland, the Balearic Islands, Sardinia and Corsica. Northwards it extends to Trondhjem, and thence along a line drawn across Russia and Siberia to the Amoor. Its southern limit in Asia is indicated by a line drawn from the Caucasus through the Himalayas into China. In Asia Minor and in Persia it is absent. South of the Mediterranean it occurs only in Morocco and Algeria.
B. melanostictus is the common toad of the whole Indian region and of the Malay Archipelago. The epidermis of the fingers and toes is thicker and more cornified than usual, and is stained black brown, hence its specific name. The male has a subgular vocal sac. In other respects the Indian species much resembles the more spinous or rough-skinned and brown varieties of the European species. According to S. S. Flower this toad is very common in the Straits Settlements, hiding by day under stones or logs, or in holes, coming out shortly before sunset, and remaining abroad till dawn; it may be met with on the roads and in the grass, hopping or crawling about in search of ants, bees, and similar food. It utters a rather feeble, plaintive cry when handled for the first time. It can change its colour from light yellowish to dark brown. The spawn, which resembles that of B. vulgaris, may be seen in March and April in ponds, in long strings twined about the water-weeds. The tadpoles are very like those of the common English toad in form, size, colour, and structure of mouth. The largest adult found in Penang measured 115 mm. (about 4 inches) from snout to vent.
B. lentiginosus s. americanus is the common toad of North America, from Mexico to the Great Bear Lake. It is worth noting that this species resembles in its coloration the Eastern races of B. vulgaris, in so far as they generally have a light vertebral line, and frequently dark spots on the under surface. The upper parts are brown and olive, with darker spots, two of which form a chevron behind the eyes. But the tympanum is large, and the male has a subgular vocal sac; the inner metatarsal tubercle is very large, and is used as a kind of digging spur. During the pairing time they take to the pools in great numbers, uttering their music, which consists of a prolonged trill, continued by different individuals, both day and night. Holbrook knew an individual which was kept for a long time, and became perfectly tame. During the summer months it retired to a corner of the room into a habitation which it had prepared for itself in a small quantity of earth placed there for its convenience. Towards the evening it wandered about in search of food. Some water having been squeezed from a sponge upon its head one hot day in July, it returned the next day to the spot, and seemed well pleased with the repetition, nor did it fail during the extreme heat of the summer to repair to it frequently in search of its shower-bath.
Several varieties of this widely distributed species, whose average length is 2½ inches, have been described. The prettiest was called B. quercinus by Holbrook–according to whom it is mostly found in sandy places covered with a small species of oak–which springs up abundantly where pine-forests have been destroyed. It is called the "oak-frog," as it spends most of its time in concealment under fallen oak-leaves, or partially buried in the sand.
B. marinus s. agua is the giant among toads, and is one of the commonest species of the Neotropical region, ranging from the Antilles and Mexico to Argentina. It frequently reaches a length of 6 inches, with a width of 4 inches when squatting down in its favourite attitude. The upper parts are rough, owing to the prominent warty glands, of which the parotoid complex is enormous. The general colour above is dark brown, with sooty dark patches; below whitish, often with blackish patches. This creature appears at dusk, often in large numbers, especially during the rainy season, hopping about, not crawling, with surprising {179}activity. The voice of the male, strengthened by a subgular sac, is said to be a kind of loud snoring bark. The pairing time begins, according to Hensel,[82] with the winter rainy season, especially June, and lasts several months, until October, but it is interrupted by the cold, which in the hills of South-Eastern Brazil covers the ponds with ice. Then the tremulous bass voice of the males is heard no longer; they have all withdrawn beneath stones and trees in the neighbourhood of the water. The eggs are laid in strings. The larvae are at first quite black and very small, and the young baby-toads are only 1 cm. in length. They differ considerably from the adult until they are more than 1 inch long; the upper parts are yellowish brown, with darker ocellated patches, each with a light seam, most conspicuous along the sides of the head and back. The under parts are grey, finely stippled with yellow.
Budgett[83] remarks that B. marinus feeds on all kinds of insects. "One half-grown specimen sitting by a man's foot picked off fifty-two mosquitoes in the space of one minute, picking them up with the tongue as they settled. The call of this very common toad consists of three bell-like notes; the middle one being the highest. The enormous parotoid glands are discharged like squirts when the creature is roughly handled. When wet weather comes on it hops out from its hiding-place to sit in a puddle, with its head out."
In many species of Bufo the crown of the head forms more or less prominent ridges, especially strong in the region between the eyes; for instance, in B. melanostictus and B. lentiginosus. The skin overlying these ridges is liable to be involved in the cranial ossification, and this reaches its greatest extent in the two Cuban species B. empusus and B. peltocephalus. It is a curious coincidence, to say the least, that such dermal ossifications should be best developed in Neotropical species, in those very countries which amongst the Cystignathidae have produced the abnormal genera Triprion, Calyptocephalus, and Pternohyla. The most peculiar and odd-looking species is Bufo ceratophrys, a native of Ecuador, which has the upper eyelid produced into a horn-like appendage, the two sharply-pointed cones standing out transversely, reminding us of several species of the Cystignathoid genus Ceratophrys; there is also a series of four small pointed {180}appendages on each side of the body. Protective concealment is possibly the reason of these queer outgrowths.
B. viridis s. variabilis, the Green or Variable Toad, reaches a length of about 3 inches, and is the prettiest toad of Europe. The skin is distinctly smooth, the numerous porous, large and small warts being flattened. Parotoid glands are well developed, and a similar pair of glands sometimes occurs on the inner side of the calf, especially in Central Asiatic and in Algerian specimens. The coloration is very variable and changeable. The ground-colour of the upper parts is creamy, with large and small, partly confluent and irregularly shaped spots and patches of green, here and there interspersed with vermilion-red specks, especially along the sides of the back. The under parts are whitish, sometimes spotted with black. The iris is brass-coloured, greenish-yellow, with fine dark dots. The male does not differ from the female in size, but has an internal subgular vocal sac, a conspicuous callosity on the inner side of the first finger, and nuptial brushes on the first three fingers and on the inner palmar tubercle.
The changing of colour affects mainly the intensity of the green; the same individual which now looks almost uniformly dull, almost grey, with dusky olive patches, will, if put into grass and sprinkled with water, within a few minutes appear in a tastefully combined garb of grass-green on a creamy ground. Some Southern and Eastern specimens have a creamy stripe along the vertebral line, thereby closely resembling B. calamita, from which, however, they can always be distinguished by the little pads below the joints of the toes; these pads being single in B. viridis, and double in B. calamita and in B. vulgaris.
The Green Toad spends most of the day in holes, although it is not averse to daylight, and it roams about chiefly in the evening. It can jump well, much better and oftener than the Brown Toad. The food consists strictly of insects of all kinds, and most individuals prefer slow starvation to eating an earthworm. Although continuing to live four or five years in captivity, they do not readily become tame; they are indeed no longer wild, and when handled they no longer emit their peculiar insipid smell, but on being approached they still crouch deeply into the grass, or withdraw into their holes, just as they did when recently caught. The voice is heard during the pairing season, and sounds like the slow creaking of a door, or a combination of a spinning top and {181}rattle. In Germany, during the months of April and May, they take to the ponds, or, improvident like the common frog, to a roadside ditch. The male sits upon the female and grasps her below the arms, his hands on her breast, and in this position they remain for days. The eggs are laid in two strings, twisted around water-plants, and are very numerous. Héron-Royer has calculated them at 10,000 or more in one set. The embryos are hatched, like those of the Common Toad, before the appearance of the external gills and of the tail. In this imperfect condition they remain in the jelly of the egg-strings for a few days, while their external gills sprout out like unbranched little stumps, only to disappear again. In about eight weeks the tadpoles, which reach a length little more than 1½ inch or 40 mm., have metamorphosed and leave the water as baby-toads scarcely half an inch in length.
This species has a very wide range, namely, the whole of Middle Europe excepting the British Isles, France and the Iberian Peninsula; the region between the Elbe and Rhine being its western limit; southwards it extends over all the Mediterranean islands and the north coast of Africa, eastwards through the whole of Russia, Western and Central Asia, not entering India, but spreading along the Himalayas into China. Stoliczka mentions its having been found in the Himalayas at an altitude of 15,000 feet, the highest record of any Amphibian, at least in such latitudes.
B. calamita.–The Natterjack is practically the representative of the Green Toad in Western Europe, but both species occur together in Denmark, Southern Sweden, and nearly the whole of Germany. Its southern limit is Gibraltar. In the British Isles it occurs in South-Western Ireland, in Co. Kerry, and in England and Wales, being however local, and preferring sandy localities, where it is found in considerable numbers. This predilection is shown by its frequency on the sandy dunes of most of the islands off the German and Dutch coast, where it may be seen running about in glaring sunshine.
Besides in the coloration, it differs from B. viridis in the following points. The little subarticular pads of the toe-joints are paired, not single, and the hind-limbs are decidedly shorter, so much so that this species cannot hop. But it runs well, like a mouse, generally in jerks, stopping every few seconds, and owing {182}to this habit it is called the "running toad" by the field-labourers of Cambridgeshire. The skin is smooth, but less so than in B. viridis, owing to the slightly more prominent warts; the parotoids are small; a similar pair of glands lies on the upper surface of the fore-arm and another on the calf. The tympanum is rather indistinct. The ground-colour of the upper parts is light brownish yellow, with a green tinge and scattered green spots; most specimens have a narrow yellow stripe along the vertebral line and over the head. The under parts are white, more or less speckled with black. The iris is greenish yellow and speckled. The male, which is of the same size as the female,–very large specimens reaching 3 inches in length,–has a large subgular vocal sac, and develops nuptial brushes on the first three fingers, but the first lacks the thickened pad of B. viridis.
The yellow vertebral line is sometimes absent in specimens from the south of France and the Iberian Peninsula; and since these southerners are as a rule more handsomely marked, the green being more pronounced and arranged in larger patches, interspersed with red spots, they much resemble B. viridis. Boulenger, who has paid especial attention to this vertebral streak, which is a not uncommon design in various species of different families, has made the interesting observation that the streak has never been found in Danish and German specimens of B. viridis, where B. calamita occurs also, while it is not uncommon in B. viridis of Italy, South-Eastern Europe, Asia, and North Africa, where B. calamita is not found. Lastly, he remarks that in Eastern Asia, where neither B. viridis nor B. calamita with such a line occurs, the same character is assumed by some specimens of B. vulgaris. The only conclusion we can draw from these facts is, that for some unknown reason the streak is a desirable, but not necessary, possession, but that it is not kept by two species in the same country, B. viridis dropping it entirely where the typically streaked species, B. calamita, also occurs. The breeding season does not begin in England and Middle Europe until the end of April, in cold springs not before May, but it lasts for several months. The males, congregating in pools in great numbers, make a loud noise, each individual uttering a rattling note which lasts a few seconds, the repetition distending its bluish throat into the shape of a {183}globe as large as its head. As the note is taken up by all the other males, a continuous chorus is established, which on warm and still nights can be heard nearly a mile off. Single croaks are uttered at any time of the day. The embrace, the male digging its fists into the armpits of the female, often takes place on land, near the edge of the water, to which they resort in the night for spawning. The egg-strings are slung around water-plants, unless the water is a mere puddle, and are much shorter than those of B. viridis, measuring only 5 to 6 feet, and containing altogether 3000 to 4000 eggs. The larvae, when hatched, are very small, imperfect, and blackish; the external gills last a very short time. The young tadpoles live on mud, subsisting on diatoms and low Algae; they are the smallest tadpoles of all the European kinds, scarcely reaching more than one inch in length, and they metamorphose quickly, the baby-toads leaving the water and running about in less than six weeks, when they are only 10 mm., scarcely three-eighths of an inch, in length. By the end of their second summer they are still only three-quarters of an inch long, and they do not reach maturity until the fourth or fifth year, with a size of 1½ to 2 inches; still smaller young males become mature several years before they are full grown.
Natterjacks stand captivity well and become very tame. When discovered, they first do their best to run away, instead of hiding or squatting down, and when caught they become covered with a slightly foamy lather, the exudation of their glands, which has a peculiar smell, reminding some people of gunpowder, others of india-rubber. They are not very particular as to food, all sorts of insects and earthworms being taken. Natterjacks are great climbers and diggers. Many of mine have established themselves in the peat with which the walls of the greenhouse are covered, where they have dug out, or enlarged, holes in which they pass the daytime, just peeping out with their bright eyes; others sit high up, always in dry places, and bask. In the evening they descend, hunting about on the ground, and occasionally they go into the water, whereupon they become quite flaccid and soft. When taken up and held between two fingers, being slightly pressed under the armpits, both sexes utter little jerky notes, as–by the way–most toads and frogs do under similar conditions.
In Cambridgeshire they frequent certain clay-pits surrounded {184}by high and steep walls of sand, the breeding places of large colonies of sand-martins. During the months of May and June they are found in the shallow water, running about on the mud, sometimes swimming, in which they are not very proficient, and rarely diving. But they spend most of the time on land. Early in October they climb up and enter the holes of the sand-martins, or they dig large, deep burrows for hibernation, and the old males are the first to disappear.
B. mauritanica s. pantherina.–The "Pantherine Toad" is one of the few African species, and is one of the prettiest of all toads. The skin is almost smooth, although provided with porous glands. The parotoids are large, but flat; large glandular complexes on the legs or arms are absent. The tympanum is very distinct. The upper parts are adorned with a delicate pattern of dark-edged, rich brown or olive patches upon a light, buff-coloured ground; the under parts are uniform white; the male has a subgular vocal sac. The total length is 3 to 4 inches. This beautiful species is one of the gentlest, and it becomes tame enough to lap up food whilst sitting on one's hand. It lives entirely upon insects, prefers shade and dusky light, and utters a sound like "kooh-rr." It is a native of North-Western Africa, Algiers, and Morocco. In the rest of Africa, from Egypt to the Cape, Senegambia to Abyssinia, it is represented by B. regularis. This species has often little spiny tubercles upon the warts, and occasionally a light vertebral line; the colour of the upper parts either closely resembles that of the previous species, or it is uniform light brown, while the under parts are whitish, or variegated with brownish patches. West African specimens are the smallest, only 2 inches long; those of the Cape are the largest, reaching 5 to 6 inches.
The next two genera approach the Engystomatinae, and thereby lead from the arciferous towards the firmisternal type. The epicoracoid cartilages are narrow, and they scarcely overlap, so that by a further step in this direction they could easily fuse into the firmisternal condition. Another bond between these two genera and the Engystomatinae is their habits, they being ant-eaters of an extremely stout appearance, with exclusively short limbs and very small heads.
Myobatrachus gouldi, living in Australia, has a smooth skin, brown above, lighter beneath, and is about 2 inches long.
Rhinophrynus dorsalis of Mexico is remarkable for its tongue, which is elongated, subtriangular and free in front, so that it can be protruded directly–not by reversion as in other toads–and can be used for licking up the termites which seem to be its principal food. The body of this ugly creature is almost egg-shaped, and the head is merged into this mass, only the narrow truncated snout protruding. The limbs are very short and stout. The toes are more than half webbed, and there is a large oval, shovel-like metatarsal tubercle, covered with horn and used for digging. The general colour is brown, with a yellow stripe along the spine and with irregular spots and patches on the flanks and limbs. Total length 2 to 2½ inches.
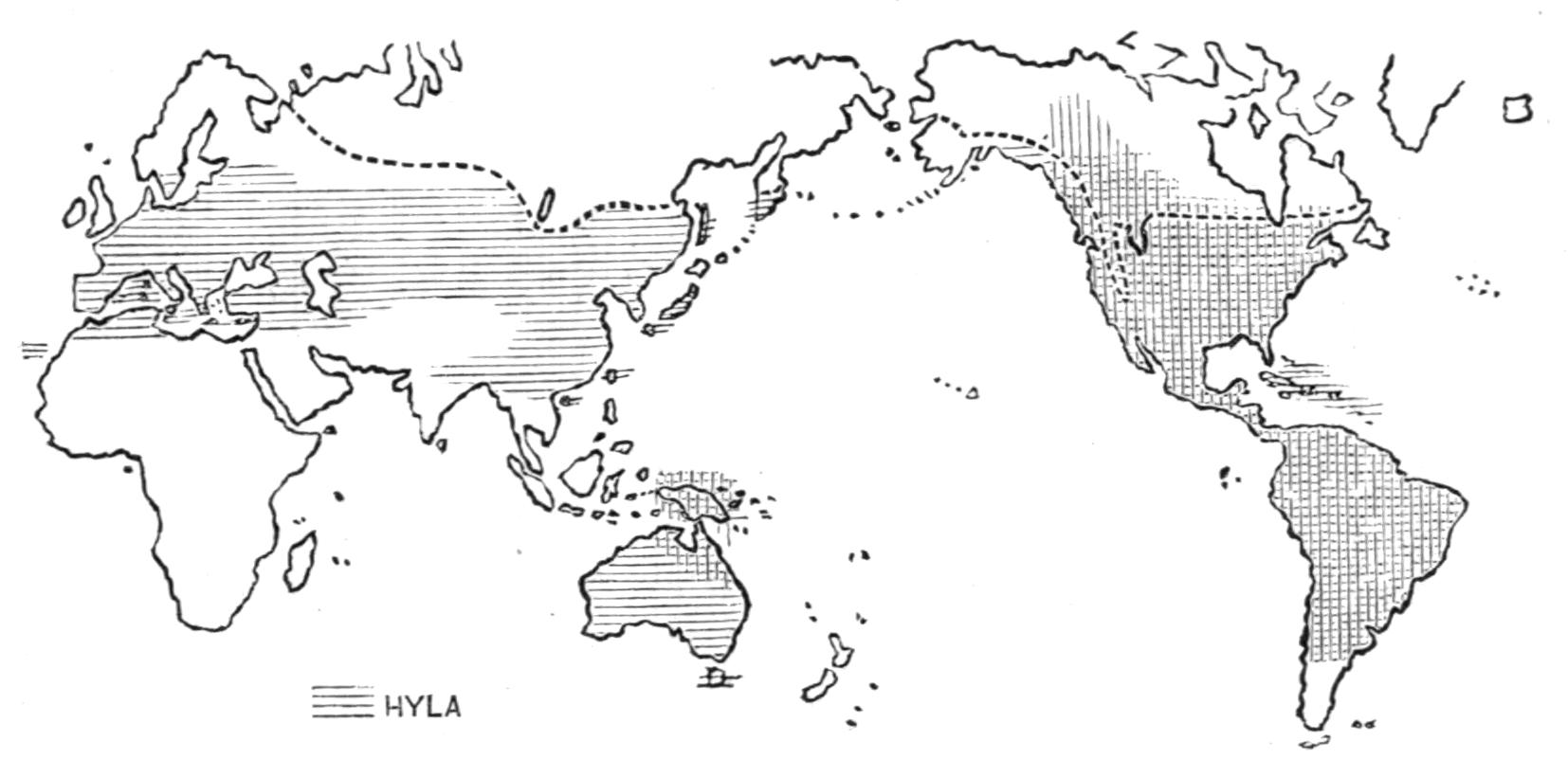
Fig. 36.–Map showing distribution of Hylidae. The vertically shaded countries are inhabited by Hyla and by other genera of Hylidae; the horizontally shaded countries only by Hyla.
Fam. 4. Hylidae (Tree-frogs).–The upper jaw–in Amphignathodon the lower jaw also–and the vomers carry teeth; Triprion and Diaglena alone have teeth on the parasphenoid also, and the latter genus is further distinguished by possessing palatine teeth. The vertebrae are procoelous and have no ribs; the sacral diapophyses are dilated. The omo- and meta-sternum are cartilaginous, the latter forming a plate with scarcely any basal or style-shaped constriction. The terminal phalanges are invariably claw-shaped and swollen at the base, and carry a flattened, roundish, adhesive cushion. The tympanic disc is variable in appearance, being either free, or more or less hidden by the skin. The tongue is also variable in its shape and in the extent to which it can be protruded.
Most, if not all, Hylidae are climbers, and many lead an arboreal life, but it does not follow that all the "Tree-frogs" are green.
Their distribution is very remarkable. To say that this family is cosmopolitan with the exception of the African region, is literally true, but very misleading. There are in all about 150 species, and of these 100 are Notogaean; one-half of the whole number, or 75, being Neotropical; 23 are Central American, 7 Antillean, and about 18 are found in North America. One species, Hyla arborea, extends over nearly the whole Palaearctic sub-region, and two closely allied forms occur in Northern India and Southern China. Consequently, with this exception of three closely allied species, the Hylidae are either American or Australian. We conclude that their original home was Notogaea, and that they have spread northwards through Central and into North America. The enormous moist and steamy forests of South America naturally suggest themselves as a paradise for tree-frogs, and it is in this country, especially in the Andesian and the adjoining Central American sub-regions, that the greatest diversity of generic and specific forms has been produced. It is all the more remarkable that similar forest-regions, like those of Borneo and other Malay islands, are absolutely devoid of Hylidae (while there are about a dozen species in Papuasia), whose place has however been taken for all practical purposes by correspondingly modified Ranidae, notably the genus Rhacophorus. Lastly, the fact that tropical evergreen forests of Africa and Madagascar possess no Hylidae, but are inhabited by several kinds of tree-climbing Rhacophorus, points with certainty to the conclusion that the origin of this large and flourishing family of Hylidae was not in Arctogaea.
The versatility and the wide distribution of the Hylidae has naturally produced cases of convergent analogy, and the various species of one "genus" may be in reality a heterogeneous assembly. Such an instance is probably the genus Hylella, of which four species live in the Andesian and Central American provinces, while the two others occur in New Guinea and Australia.
The two North American genera Chorophilus and Acris, and the Brazilian Thoropa, connect the Hylidae with the Cystignathidae, in so far as their finger-discs are very small, or even {187}absent, and their sacral diapophyses are only slightly dilated. On the other hand, it has to be emphasised that the possession of adhesive discs on the fingers and toes does not necessarily constitute a member of the Hylidae. That requires the further combination of an arciferous sternum, with dilated sacral diapophyses and teeth in the upper jaw. Finger-discs are easily developed, and still more easily lost. Those of the typical Hylidae are constructed as follows. The terminal phalanx is elongated, claw-shaped, swollen at its base. Between it and the penultimate phalanx lies an interphalangeal cartilaginous disc which projects ventrally below the end-phalanx, thus assisting the formation of the ventral pad, and the turning upwards of the whole disc-like phalanx like the claw of a cat. This peculiar motion can be well observed in Tree-frogs which are at rest upon a horizontal leaf, or, better still, upon a rough stone, when the creatures take good care to adjust their discs into a safe and easy position. The pad or disc itself is furnished with unstriped, smooth muscular fibres, the contraction of which produces one or more longitudinal furrows on the under side. When the disc is in action or adhering, being flattened to a smooth surface, the end-phalanx sinks into the cushion; when not in action, the cushion swells and the phalanx appears as a slight dorsal ridge. The disc is rich in lymph-spaces, and its surface contains mucilaginous glands.
Various suggestions have been made to explain the function of these discs. Suction, adhesion, and glueing-on have been resorted to. Suction, through production of a vacuum, is quite imaginary and does not exist. The question has been thoroughly studied by Schuberg.[84] Adhesion is due to the molecular attraction of two closely appressed bodies. The less air remains between them the stronger it is. Consequently it can be increased by the interference of a thin layer of fluid, which as everyday observation shows, possesses both adhesion and cohesion. The more sticky the fluid, the more effective it is, as shown experimentally by Schuberg, who moistened the under surface of a glass plate, and pressed it against a little disc of glass from which was suspended a weight. A disc of 16 square millimetres, approximately equal to the aggregate surface of the 18 discs of a European tree-frog of 4 grammes in weight, carried with water-adhesion {188}no less than 14 grammes, with glycerine-solution 20 grammes,–more than sufficient to suspend the frog. The sticky secretion of its glands greatly enhances the adhesive power. Tree-frogs, when hopping on to a vertical plane of clean glass, slide down a little, probably until the secretion stiffens, or dries into greater consistency. After a few days I find the glass-walls of their recently cleaned cage quite dirty, covered everywhere with their finger-marks. On the other hand, wet leaves or moist glass-walls afford no hold. The adhesion of these frogs is assisted in most cases by their soft and moist bellies, just as a dead frog will stick to a pane of glass.
All Hylidae have a voice, often very loud, and enhanced by vocal sacs, which are either internal, swelling out the throat, or external, paired or unpaired.
The various Hylidae resort to all kinds of modes of rearing their broods. Most of them lay many eggs, up to one thousand, in the water, not coherent in strings but in clumps; others lay only a few, attach them to various parts of the body, or, as in the genus Nototrema, the female receives them in a dorsal pouch. These modifications will be described in connexion with the different species.
Sub-Fam. 1. Amphignathodontinae.–Both upper and lower jaw with teeth.
Amphignathodon, of which only one species is known, A. guentheri of Ecuador, agrees with Nototrema in all important characters except that it possesses teeth in the lower jaw in addition to those in the upper. There are further differences, but they are of degree only. The sacral diapophyses are more strongly dilated and the omosternum is absent. The tympanum is distinct. The pupil is horizontal; the roundish tongue is slightly free behind. The terminal phalanges are claw-shaped and carry large discs. The female has a dorsal pouch opening backwards. The skin of the head is involved in the ossification of the cranial bones. The skin of the back is smooth, slightly tubercular, non-granular below. The middle of the upper eyelid carries a small, pointed, cutaneous appendage, and even this little character occurs also in some species of Nototrema, e.g. in N. longipes and in N. cornutum. The heel carries a triangular little flap. The upper parts are olive in spirit-specimens, probably green in life; the borders of the dorsal pouch {189}are black. The sides of the body are adorned with a black, white-edged streak, the limbs are whitish, with black cross-bars. The total length of the female type-specimen is 3 inches.
Sub-Fam. 2. Hylinae.–Lower jaw toothless.
The Hylinae are divided by Boulenger into 13 genera, which can be recognised by the following key, without reference to their natural affinities:–
A. The contracted pupil forms a horizontal slit.
a. Tips of the fingers and toes with large discs,
α. With vomerine teeth.
Female without a dorsal pouch .......... Hyla, p. 189.
Female with a dorsal pouch .......... Nototrema, p. 202.
β. Without vomerine teeth .......... Hylella, p. 203.
γ. With parasphenoid teeth and peculiar helmet-shaped head.
b. Tips with very small discs. Tongue free behind.
Tympanum distinct. North America and Peru .......... Chorophilus, p. 208.
Tympanum indistinct. North America .......... Acris gryllus, p. 207.
c. Tips simply swollen, not dilated into discs. Brazil .......... Thoropa miliaris, p. 209.
B. The contracted pupil forms a vertical slit. Tropical America.
a. Tips with large discs.
α. Tongue extensively free behind.
Inner finger and toe opposable .......... Phyllomedusa, p. 203.
Inner finger and toe not opposable .......... Agalychnis, p. 206.
β. Tongue scarcely free behind. Ecuador .......... Nyctimantis rugiceps, p. 206.
b. Tips without discs. Without parasphenoid teeth, but head peculiar in shape. Mexico .......... Pternohyla fodiens, p. 207.
C. Pupil rhomboid. Without parasphenoid teeth. Large discs. Head helmet-shaped. Brazil .......... Corythomantis greeningi, p. 207.
Hyla.–The pupil is horizontal. The tympanum is distinct or hidden. The tongue is entire or slightly nicked in its hinder margin, which is more or less free behind. The fingers and toes are provided with typical adhesive discs.
This is the largest genus of all Amphibia, containing about 150 species, and its distribution coincides with that of the whole family. Many of the species are very closely allied to each {190}other, differing only in small points, for instance in the extent of the webs to the fingers and toes, the configuration of the vomerine teeth, the size and appearance of the tympanic disc, and the relative length of the hind-limbs. In some of the West Indian, and in one Brazilian species, H. nigromaculata, the upper surface of the head is rough, owing to the cutis being involved in the cranial ossification. Bony or perhaps only calcareous deposits in other parts of the skin are rare, but are notably developed in H. dasynotus of Brazil, in which they extend from the head to the sacrum, rendering the skin immovable.
Many are capable of changing colour to a great extent, and it is a popular error to suppose that all tree-frogs are green, although this colour is perhaps the most common in the arboreal kinds.
H. arborea.–The tongue is rather round, slightly nicked behind, and can be protruded but little. The tympanum is distinct, but small. The upper parts are grass-green, quite smooth and shiny owing to the skin being covered with a film of moisture; the under parts are yellowish-white and granular, flesh-coloured or rosy on the thighs. Total length of large females 2 inches. This, the Tree-frog of Europe, has an enormous range, namely, from Morocco, France, and the south of Sweden, across the whole of Europe and Asia Minor to Japan and Southern China.
Several varieties have been described: the typical or European form is ornamented with a narrow black stripe, which, beginning at the nose, extends backwards along the side of the body to the groin, where it generally forms a hook turned upwards. This black colour forms the ventral boundary of the green, and is itself narrowly seamed with white on its upper border.
In the south of France, the Iberian Peninsula, Morocco, and the Canary Islands the black lateral stripe is often absent; this is the var. meridionalis. In Spain and Portugal both forms are found in the same localities.
In the Asiatic, chiefly in the eastern specimens, the lateral stripes tend to break up into irregular spots, vanishing altogether towards the groins; this var. savignyi s. japonica occurs also on most of the Mediterranean islands.
H. arborea can change colour to a great extent, mostly in adaptation to its immediate surroundings, but ill health and moulting may also influence it. The change is slow. The usual colour is green, brightest on bright, sunny hot days, dull when the sky is overcast, or when it is windy and showery. Day and night have no influence upon the colour-changes. The hue of the green agrees mostly with that of the foliage on which the frog happens to take its rest, for instance a field of Indian corn, birch-trees, or oak-trees. I once received a consignment from Saxony. When the box with moss was unpacked, they were of the dullest greenish-grey; they were put into a wired-off corner of the yard and were given the freshly cut branches of a lime-tree to sit upon. On the following morning I at first looked for most of the frogs in vain. The leaves had withered and all those frogs which sat upon the dark brown branches had put on a light brown garb, mottled with darker patches.
Another specimen, one of several which were at liberty in a greenhouse, took to resting on the frame of the window-pane, in a corner where putty, glass, and discoloured white paint met; in the morning it was always of a mottled leaden colour, but during the nocturnal hunting it was green. In the winter, the window-corner being of course cold, the frog remained stationary for several months, but kept the leaden grey colour, until one day in the early spring it was mottled with green, and soon after it joined its green mates.
Liebe observed a half grown tree-frog which he kept in Gera {192}during the winter in a glass with water-cress. While the temperature was near freezing the frog sat in the water, very lethargic, breathing perhaps once every quarter of an hour. Its colour was light green. When the water-cress was cut and removed, the frog darkened and became at last quite a discoloured grey. When the water-cress was put back, the creature reassumed the light yellowish-green colour, remaining in its lethargic condition until it became lively in the spring sunshine.
The European tree-frog spends most of its time in the summer, after the pairing is over, in trees, often in the very crowns; but the neighbourhood of even a small patch of Indian corn has still greater attractions. There are all sorts of green insects to be caught, there are fair chances of coming across the common Cabbage White, a butterfly which the tree-frog loves, and last not least the large luscious leaves afford a firm foothold, and the axillae between stalk and broad-based leaves are just the places for the frog to slip into, where nobody can find it. During the day they mostly sit still, on the keen look-out for passing insects, which, when they settle within reach, are jumped at; otherwise they have first to be stalked. The jump is quite fearless, regardless of the height above ground; there is the leaf upon which the prey sits, and even if this leaf be missed, there are others, and one of them is sure to be struck by some of the discs of either fingers or toes. If the fall is broken by the toes, and the new leaf or branch is very elastic and bends down, then there are some frantic antics to be gone through until the frog has settled itself again. Then the large blue-bottle, or the butterfly, is devoured at leisure, wings and all being poked in with the assistance of the little hands. But the real hunting-time is the night.
During a shower the frog shifts its position to the under side of the leaf, or into a less slippery position, and during continuous wet it descends into the grass, or it takes to the water. Its greatest enemy is the Grass Snake, which prefers it to anything else, not minding the poisonous secretion of the skin, which is sharp enough to produce sneezing or even temporary blindness when incautiously brought into the human eye.
The male has an internal vocal sac, which, when inflated, bulges out the whole throat into a globe, much larger than the head. The voice is a sharp and rapidly-repeated note, something {193}like "epp-epp-epp," or "creck, creck, creck," with more or less of an a sound. It is uttered at any time of the day, more frequently at dusk, and of course chiefly during the pairing season. This tree-frog suffers from the reputation of being a good weather-prophet, and it is for this reason often kept in confinement, the orthodox abode being a muslin-covered glass jar, with a hole to put flies through, water and plants at the bottom, and a little ladder to sit upon. The prophesying is of the usual popular unreliable nature, although the little creature, provided it is a male, often sounds its voice on the approach of a shower, or when there is a thunderstorm in the air. During continuous fine weather it sits on the top of the ladder, or is glued on near the rim of the glass, while on wet and dull days it is less active, and may keep nearer the ground or in the water. There is a German rhyme which well expresses the prophet's reliability by its ambiguity:–
Wenn die Laubfrösche knarren,
Magst du auf Regen harren.
When the tree-frogs croak, you may wait for rain. Sometimes it does come true.
Tree-frogs are not very intelligent, although they have a keen sense of locality; but they are nice pets, being easily kept, and have a pretty appearance. There is a record of one which lived for twenty-two years in confinement.
The pairing begins soon after the frogs reappear from their hibernation in the ground; in Germany in the month of May. The congregating males make a great noise and take to the water before the females, which join them when ready to spawn. The male grasps his mate near the shoulders, and the pair swim about together, sometimes for days, until the eggs are expelled. These are laid in small clumps of 800 to 1000, which soon swell up and remain at the bottom of the pond. The larvae are hatched in ten days; two days later the adhesive sucker below the throat appears, and after another two days a pair of thread-like external gills are developed. The tadpoles, which reach a length of 2 inches, owing to the long tail, which is nearly three times as long as the body, metamorphose in about twelve weeks, and the baby tree-frogs, scarcely half an inch in length, hide in the grass for the next two years, until they are about half grown, not reaching maturity until the fourth year.
Since many pairs congregate in the same pool, and each produces up to one thousand eggs, most of which are hatched, the neighbouring meadows sometimes literally swarm with tiny tree-frogs. Nevertheless the adults are comparatively rare and are very local.
H. carolinensis s. lateralis of the South-Eastern States of North America greatly resembles H. arborea in general appearance, size, and habits. But the head is more pointed, and the vivid green of the upper parts is separated from the yellowish white under surface by a conspicuous, pure white line, giving the little creature a very smart and neat appearance. According to Holbrook, it ascends trees, but most commonly lies upon broad-leaved water-plants, like Nymphaea, and in fields of Indian corn. Motionless during the daytime, they emerge in the morning and evening from their hiding-places, and become very brisk and noisy, often repeating their single note, which is not unlike that of a small bell. When one begins, hundreds take it up from all parts of the corn-field.
Among other tree-frogs of the South-Eastern States may be mentioned H. squirella, 1½ inch in length, which is very changeable in colour, generally olive above with darker spots and bars on the limbs, and with a white upper lip. It lives in trees, sheltering in the bark. H. femoralis of the same size, without the white lip, lives high up in the trees of the dense forests of Georgia and Carolina.
H. versicolor is one of the most delicately coloured species of Eastern North America, extending northwards into Canada. It is about 2 inches long. Its colour passes within a short time from dark brown or olive grey to pale delicate grey, almost white, occasionally retaining a few large darker patches on the back, and delicate cross-bars on the limbs. A small portion of the sides and the posterior part of the belly are bright yellow. The skin is granular, owing to the presence of small warts which produce an acrid secretion. It is said to be found in trees, or about old stone fences overgrown with lichens, the colour of which it resembles to perfection. It becomes very noisy towards the evening, in cloudy weather or before rain, the voice consisting of a liquid note, terminating abruptly, like "l-l-l-l-luk." My own captives fully bear out this statement of Holbrook's. Settled motionless during the day upon a piece of bark in a shady {195}corner, but occasionally uttering the quaint and rather faint note, they become very lively in the evening, catching insects by long jumps, or investigating the hollows of decaying mossy stumps. Their general colour is then spotless, almost silvery grey. In the day-time they are sometimes suffused with delicate green.
The propagation has been studied by Miss M. H. Hinckley.[85] They pair in shallow pools, in Massachusetts, in May. On the 10th of that month eggs were attached singly, and in groups, on the grasses resting upon the surface of the water; first drab-coloured, they became lighter in a few hours. Some larvae escaped from the gelatinous envelopes on the following day, the others on the third day; they clung to the grasses by means of their prominent suckers. The head and body were cream-coloured, with olive dots, and averaged ¼ inch in length. Gills appeared on the fourth day, to disappear again during the four following days, first those of the right, then those of the left, side; the suckers became less conspicuous, and the general colour turned into deep olive-green, with fine golden dots on the upper and lower surfaces. The eyes were of a brilliant flame-colour. On the eleventh day the suckers or "holders" had disappeared, and the hind-limbs were indicated by small white buds. By June 5th, i.e. the twenty-seventh day, the toes developed the terminal discs; the mottling of gold had given way to a uniform olive or pea-green. Movements of the future arms beneath the skin appeared on the 28th of June, at the age of seven weeks. The arms, mostly the right one first, were thrust out on the 2nd of July; the fins of the tail were absorbed rapidly, and towards the end of the seventh week the nearly transformed creatures began to leave the water. The young frogs changed colour rapidly, in adaptation to their surroundings, but the four specimens which survived were never all found to be of the same colour during the next three months. They first lived upon Aphides, later upon flies, and they were alert nocturnally. About the beginning of October they left the fronds of their fernery and nestled away in the damp earth, which they left only when the temperature rose above 60° F.
H. vasta of Hayti is the giant of the tree-frogs, reaching a length of 5 inches. In order to support its great weight the {196}adhesive discs of the fingers and toes are of a surprising size, about as large as a threepenny piece. The skin is covered with small warts, and forms a peculiar fold on the hinder surface of the fore-arm and on the tarsus, and small flaps near the vent. The colour is grey above, blackish on the head, with a brown band between the eyes; the under parts are flesh-coloured, the throat with black spots.
H. maxima, of the forests of British Guiana, is scarcely less gigantic, and is distinguished by a projecting rudiment of the pollex, while the adhesive discs are smaller than the tympanum. The skin forms folds on the arms and tarsus, like those of H. vasta, in addition to a triangular flap at the heel. The general colour is reddish-brown above, sometimes with a dark vertebral line, the under parts are whitish and covered with large granules; the throat of the male, which has an inner vocal sac, is brown.
H. faber of Brazil is closely allied to the last species, but the skin of its upper parts is quite smooth. There is a small tarsal fold, and one extending from the upper eyelid to the shoulder. It is light brown above, with darker marks which form a conspicuous vertebral line, transverse bars on the hind-limbs, and a few irregular, scattered, vermicular or linear marks on the head and body. The adult, when put into a strong light, will rapidly turn pale; at night the longitudinal stripe on the back and the bars on the hind-limbs become very distinct; the under parts are white, and exhibit a beautiful orange tinge. This is the famous "Ferreiro" or "smith." As will be seen from the following graphic account by Dr. Goeldi[86] of Para, this species doubly deserves its name of faber, not only in virtue of its voice, but also because of the marvellous nest-building habits recently discovered.
"The Ferreiro is common in the Province Rio de Janeiro, more frequently still in the mountain regions of the Serra dos Orgãos than in the hot lowland. Its voice is one of the most characteristic sounds to be heard in tropical South America. Fancy the noise of a mallet, slowly and regularly beaten upon a copper plate, and you will have a pretty good idea of the concert, given generally by several individuals at the same time and with slight variations in tone and intensity. When you approach the {197}spot where the Tree-frog sits, the sound ceases. But keep quiet, and it will be resumed after a few moments. You will discover the frog on a grass-stem, on a leaf of a low branch, or in the mud. Seize it quickly, for it is a most wonderful jumper, and it will utter a loud and shrill, most startling cry, somewhat similar to that of a wounded cat."
The "Smith" makes very regular pools, in the shallow water of ponds, or nurseries for the tadpoles surrounded by a circular wall of mud. Dr. Goeldi has watched the building process during a moonlit night: "We soon saw a mass of mud rising to the surface carried by a Tree-frog, of which no more than the two hands emerged. Diving again, after a moment's time, the frog brought up a second mass of mud, near the first. This was repeated many times, the result being the gradual erection of a circular wall. From time to time the builder's head and front part of body appeared suddenly with a load of mud on some opposite point. But what astonished us in the highest degree was the manner in which it used its hands for smoothing the inside of the mud wall, as would a mason with his trowel. When the height of the wall reached about 4 inches, the frog was obliged to get out of the water. The parapet of the wall receives the same careful smoothing, but the outside is neglected. The levelling of the bottom is obtained by the action of the lower surface (belly and throat principally) together with that of the hands."
The male takes no active share in the construction of the nest, but will suddenly climb up the wall of his home, and then upon the back of his busy mate. The building operation may take one or two nights, and is performed in the most absolute silence; the croakers around are all males clamouring for a mate.
The eggs are laid during one of the following nights, and are hatched some four or five days later, the parents keeping hidden in the neighbourhood of the nursery. Heavy rains may destroy the walls, and thus prematurely release the tadpoles.
It is only owing to such keen observers and lovers of nature's fascinating ways that the breeding habits of some Brazilian Hylidae have become known.
H. nebulosa s. luteola also living in Brazil, is yellow above, with brown dots; the sides of the belly and thighs have {198}transverse bluish bars, the under parts are whitish. Its size is under 2 inches. Goeldi has often found it in the sheaths of decaying banana-leaves. It glues the lumps of eggs on to the edges and to the inside of the withered leaves, where even during the hot hours of the day sufficient coolness and moisture are preserved. These lumps are enveloped in a frothy substance, in which the nearly metamorphosed tadpoles can be watched wriggling. If these are put into water, all will die in a few hours.
H. polytaenia deposits its eggs in free lumpy masses on water-plants. It is a small creature, little more than 1 inch in length, light olive above, with numerous brown parallel longitudinal bands on the body and limbs. A dark, white-edged band extends from the nose along the side of the body. The heel has a short flap of skin. The male has an internal vocal sac.
H. goeldii is a most interesting form, leading to the allied genus Nototrema. Boulenger[87] has described a female which was captured by Goeldi in January on the Serra dos Orgãos. It is about 1½ inch long. The whole surface of the back is occupied by a layer of twenty-six pale yellow eggs which are 4 mm. in diameter. The skin of the back is expanded into a feebly reverted fold, which borders and supports the mass of eggs on the sides, thus suggesting an incipient stage of a dorsal brood-pouch. Owing to the great amount of yolk, the young are probably able to remain upon the mother until they are nearly metamorphosed.
H. coerulea s. cyanea is one of the largest Australian green tree-frogs, ranging from the South to the very North of Australia. The discs are as large as the fully-exposed tympanum. There is no projecting rudiment of the pollex, but a slight cutaneous fold borders the inner side of the tarsus. The skin is smooth and shiny, always a little moist, and studded with numerous rather large pores on the nape and shoulders; this somewhat thickened region forms a prominent fold which begins behind the eyes. The belly and the under parts of the thighs are granular as in most Hylidae. The male has an internal vocal {199}sac; and during the breeding season, which seems to occur during our autumn and winter, develops brown rugosities on the inner side of the first finger. The tongue is round, slightly notched behind and free enough to be protruded a little.
Fig. 39.–Hyla coerulea. Australian Tree-frog (from photographs). Length of the large specimen 4.2 inches. The upper right specimen with vocal sac inflated.
The alternative specific names are most unfortunately chosen, as they apply only to spirit-specimens. During life this tree-frog exhibits a considerable amount of colour-changes. The normal colour is bright green above, white below. A conspicuous feature of this species is the frequent occurrence of white specks or spots, which are probably due to the deposition of guanine, a peculiar white colouring matter. The spots appear in any part of the green skin, and are quite irregular in their distribution. Sometimes they remain for weeks in the same place, or they disappear after a few days and others appear. They are in no way connected with the shedding of the skin, nor do they indicate ill-health. H. coerulea lives well in confinement, and becomes tame enough to take food from one's fingers, even when sitting upon the hand. Some of mine took to living during the daytime in a small box, preferring a crowded condition in companionship with {200}Natterjacks. Others squeeze themselves into the most uncomfortable cracks, while others again prefer the broad leaves of Philodendron. A favourite place for two or three at a time is the funnel-shaped spaces formed by Bromelia-plants. Those specimens which are hidden in the box or in the hollows of rotten stumps are, almost without exception, dull, very dark brownish olive, while those on the Bromelias assume exactly the sombre dull green of its leaves. Lastly, those which sit in the light, exposed places, no matter if upon a leaf, on a white stone, or upon a board, are emerald-green, especially beautiful on hot, sunny days;–and they are not always averse to the full glare of the sun. When squatting upon a flat surface, such as a broad leaf, they tuck the fore-paws under the head like a cat, and with half open eyelids, the pupil contracted to a tiny slit, so that the golden iris is exposed, they remain motionless during the day. They take food when offered, but at night they roam about, either hopping on the ground, or making enormous leaps from leaf to leaf, sometimes deliberately stalking some choice insect, and patiently climbing up a stem, hand over hand. At night their whole aspect is changed. The colour is saturated green, the eyes are transformed into round, projecting shiny black beads, and the head is erect. The ludicrously dreamy, complacent look has given way to wide-awake alertness. They take all kinds of living food. When they find an earthworm, they first look at it, bending the head sharply down, lift themselves upon the fore-limbs and then pounce upon it, nipping the prey with the jaws, and then poking it down deliberately with the hands. Cockroaches are simply lapped up, and disappear in the twinkle of an eye. Mealworms, wood-lice, butterflies and moths, flies and spiders are taken. The stomach of a specimen in the Dresden Museum, from the Aru Islands, contained some four or five young freshwater Crustaceans of the genus Sesarma. They fortunately do not molest smaller frogs of their own kind and of other species. Like many Amphibia they like a change of diet, and ultimately refuse their food if it is unvaried. To my surprise my largest specimen, which measures a little more than 4 inches, takes snails, Helix virgata, half-a-dozen at a time, and on the following day, not during the night, vomits the sucked-out shells in a lump, like the pellets of birds of prey. During this rather painful-looking procedure the whole tongue and about half an {201}inch of the everted gullet are protruded out of the mouth, and are then slowly withdrawn. After having roamed about all night, they return to their respective resting-places, where each individual is sure to be found in exactly the same spot, day after day. They do not mind being looked at, but if taken up and put back they avoid that place for perhaps a week, taking shelter somewhere else.
Both sexes have a voice, but that of the female is only a grunting noise, while the male inflates its gular sac and sends forth a sharp cracking sound, which can turn into a regular bellowing like the gruff barking of an angry dog. They bellow at any time of the year, frequently on the approach of a shower or during a thunderstorm. Certain noises will also induce them to bark. The rattling produced by the syringing of the greenhouse, sawing of wood, hammering, the raking of the gravel, or even the scraping of boots on the gravel-path is liable to start one of the males, and the others are sure to chime in.
According to Fletcher, H. coerulea and H. aurea lay their eggs in round white frothy patches, which float in the water, chiefly during the months of August and September; but when the spring months are very dry, the pairing is delayed until the following January. Several other Australian species of Hyla, e.g. H. ewingi, spawn at any time of the year if the conditions are favourable. They attach their eggs to submerged blades of grass or to twigs.
H. aurea is one of the commonest and most beautiful species, occurring throughout Australia and Tasmania, excepting of course in the large deserts. It has the appearance and restlessness of a water-frog, is not unlike Rana esculenta, and grows to about three inches in length. The tympanum is very distinct, but rather small. The fingers are without a pollex-rudiment, the tarsus has a fold along its inner edge. The adhesive discs are decidedly small. The male has two internal vocal sacs, which bulge out sideways. The skin is smooth and shiny. The under parts are white; the upper parts are, speaking generally, a mixture of blue and olive, with blue or brown spots, but spirit-specimens give no idea of the beauty which this changeable species can assume. Sometimes the same individual is saturated blue and green, with several longitudinal stripes of burnished copper along the back; a few minutes later the stripes glitter {202}like gold, and in other moods the whole upper surface is mottled blue, green, and brown. My specimens often went into the water and did not climb. The food is said to consist chiefly of other small frogs in preference to insects.
Nototrema differs from Hyla in so far as the female has a pouch on the back for the reception of the eggs. This bag is formed by an infolding of the skin; it opens backwards in front of the vent, it has a sphincter and is permanent, although it distends to larger dimensions when in use. An initial stage of such a pouch is possessed by Hyla goeldii (Fig. 38). The pupil is horizontal, the tongue can be protruded but little; the tympanum is free, and the adhesive discs of the fingers and toes are well developed. These "marsupial frogs," of which about half-a-dozen species are known, live chiefly in the tropical forest-region of South America, notably from Peru to Venezuela.
N. marsupiatum is green with darker blue-green spots, or with longitudinal patches which are each surrounded by a whitish or yellow seam of little dots. The limbs have cross-bars. Total length about 2½ to 3 inches. The eggs of this species are comparatively small and numerous. The very small tadpoles have no external gills, and escape from the pouch to finish their metamorphosis in the water.
N. testudineum, about 3 inches in length, is of a uniform lead-colour, but is lighter beneath. The skin of the back is studded with stellate calcareous deposits, a peculiarity which is alluded to in the specific name.
N. oviferum is brown above, with darker patches on the sides of the body and with cross-bars on the limbs. The last two species and N. fissipes of Brazil, near Pernambuco, carry their young in the pouch until the metamorphosis is completed. This long nursing-period necessitates a great amount of food-yolk in the eggs, and this enlargement in turn implies a considerable reduction in their number. The female's load consists of about fifteen eggs only, but these are of a great size, namely one-eighth of the length of the mother's body.
N. pygmaeum, in Venezuela, is a tiny creature. The female, just one inch in length, carries only from four to seven eggs. It looks then "as if it carried a sac filled with a few gigantic balls." This species is further worthy of note on account of the opening of the brood-pouch, which is a longitudinal slit, whence a kind {203}of thin and slightly elevated ridge or fold of the skin extends on to the neck. The suggestion, that this seam is burst open, in order to set the full-grown young free, instead of their passing through the existing opening, is scarcely credible.
These Neotropical tree-frogs seem to be rare, and females with embryos are of course still more uncommon, so that the best account of their structure is still that given by Weinland[88] of N. oviferum. How the eggs get into the pouch has not yet been observed, but it is most likely with the help of the male, immediately after fertilisation. The pouch forms two blind sacs which extend forwards over the sides of the back. The eggs are large, 1 cm. in diameter, and the enclosed embryos, or rather tadpoles, had a length of 15 mm., with a large amount of yolk still contained in the spirally wound intestine. The first two gill-arches carried each a double thread, which expanded into a funnel-shaped membrane, not unlike the flower of a Convolvulus, and furnished with a capillary network; the stalk contained muscular fibres. These most peculiar structures are of course the much modified external gills. Those of N. testudineum and N. cornutum are likewise bell-shaped.
Hylella differs from Hyla chiefly by the absence of vomerine teeth, and consists of about half-a-dozen small species, about one inch in length. The fact that two species live in Queensland and New Guinea, while the others are natives of tropical America, suggests that this genus is not a natural but an artificial assembly, an instance of convergent evolution.
Phyllomedusa, composed of about one dozen species of tree-frogs, is characterised by the vertically contracted pupil, large adhesive discs, and the opposable nature of the inner finger and of the hallux, the last joints of which are like thumbs. The sacral diapophyses are strongly dilated. The range of the genus extends from tropical Central America to Buenos Aires. Most of the species are about 2 inches in length, blue-green to violet above, with white purple-edged patches on the sides of the body; the under parts uniform white, or with purple or brown patches. The male has a subgular vocal sac. Some have more or less distinct parotoid glands. Ph. dacnicolor of Mexico is uniform green above, whitish below, and attains a size of more than 3 inches. In Ph. bicolor of Brazil, the skin of the upper {204}parts is studded with calcareous deposits, and the parotoids are large. It is blue-green above, purplish white below, the sides of the body and limbs with white purple-edged spots.
Ph. hypochondrialis has been found breeding freely in the Paraguayan Chaco by Budgett,[89] from whose account the following notes have been extracted. This brilliantly coloured frog is green above, which colour may become brown-grey or bluish at will; below, white and granular. The flanks are scarlet, with black transverse bars, and the plantar surfaces are deep purplish black. Total length about 1½ inch.
The "Wollunnkukk," as it is called by the Indians, from the call of both male and female at pairing time, is extremely slow in its movements, and is active only at night. At this time, if it is seen by the aid of a lantern as it slowly climbs over the low bushes and grass, it is very conspicuous. In the daytime, however, nothing is seen but the upper surface of the body as it lies on the green leaf of a plant. It has a remarkable power of changing its colour to harmonise with its surroundings, and can effect a change from the brightest green to light chocolate in a few minutes. The skin is also directly sensitive to light; for if the frog is exposed to the sun while in a tuft of grass in such a way that shadows of blades of grass fall across it, on removal it will be found that dark shadows of the grasses remain on the skin, while the general colour has been raised to a lighter shade. Its food consists largely of young locusts. The ovaries on each side are divided into five distinct clusters. The rectum has a large saccular diverticulum, which is very heavily pigmented.
In the breeding season–December to February–this beautiful frog collects in considerable numbers in the neighbourhood of pools. During the night-time they call incessantly to one another, and produce a sound as of a dozen men breaking stones, well imitated by the native name.
The eggs are enclosed in batches in leaves near the margin of the water. Budgett has been able to watch the whole process of oviposition and fertilisation. He found, at 11 P.M., a female carrying a male upon her back, wandering about in search of a suitable leaf. At last the female, climbing up the stem of a plant near the water's edge, reached out and caught hold of the {205}tip of an overhanging leaf, and climbed into it. Both male and female held the edges of the leaf together, near the tip, with their hind-legs, while the female poured her eggs into the funnel thus formed, the male fertilising them as they passed. The jelly in which the eggs were laid was of sufficient firmness to hold the edges of the leaf together. Then moving up a little further, more eggs were laid in the same manner, the edges of the leaf being fastened together by the hind-legs, and so on up the leaf until it was full. As a rule, two briar-leaves were filled in this way, each containing about 100 eggs. The time occupied in filling one leaf was three-quarters of an hour.
Development proceeds rapidly. Within six days the embryo increases from the 2 mm. of the egg-diameter to 9 or 10 mm. When it leaves the leaf it is a transparent glass-like tadpole, whose only conspicuous parts are the eyes. These are very large and of a bright metallic green colour, so that when swimming in the water all that is seen is a pair of jewel-like eyes. The newly-hatched tadpole has also a bright metallic spot between the nostrils somewhat in front of the pineal spot. This is the point which touches the surface of the water when the tadpole is in its favourite position. Whether it is a protective coloration, or some mechanical arrangement for holding the surface, Budgett could not make out.
The egg contains a great amount of yolk; the rest of the jelly-like contents of the egg becomes fluid, so that towards the end of embryonic life the larva comes to lie quite freely within a membranous capsule. The external gills appear on the third day, and reach their greatest size on the fifth, when these bright red filamentous organs extend beyond the vent. By the time the tadpoles are ready to be hatched these gills have quite disappeared, there is a median spiracle, and the lungs are shining through the transparent body-wall. Five weeks later, i.e. six weeks after the eggs were laid, the tadpole is 8 cm. long, glossy green above, rosy and silvery below, and the hind-limbs protrude. The young frog at the close of its metamorphosis is two-thirds the length of the adult, and at this time acquires the red flanks barred with black.
The first account of the breeding of Phyllomedusa was given by v. Ihering[90] concerning Ph. iheringi of Southern Brazil.
"Phyllomedusa does not lay its eggs in the water, although the larva develops in that element, but in the open air in masses 50 millim. long by 15-20 broad, between leaves hanging over the water. Willows are frequently used for that purpose. The egg-mass contains rather large white ova, wrapped up between two or three leaves in such a way as to be completely enveloped save an inferior opening. My attempts at rearing the eggs failed owing to the leaves drying up; but I am assured that the tailed larvae may be seen wriggling in the gelatinous mass. As at a later period the latter is found empty, we must infer that the larvae drop into the water below. The eggs are found only on plants hanging over stagnant water."
Fig. 40.–A branch with eggs of Phyllomedusa iheringi, × 1, enveloped in the leaves. (After v. Ihering.)
"The adult animal is a stupid creature, and will let itself be taken without attempting to escape. Their moderately loud voice resembles somewhat the sound produced by running the finger nail over the teeth of a comb. Only during the breeding season, in the month of January in Rio Grande do Sul, do these frogs make their appearance; at other times not one is to be seen, probably because they establish themselves high up in the trees."
Agalychnis, with two species in Central America, is practically like Hyla; but the pupil is vertical, and the tongue is extensively free behind.
Nyctimantis differs from either by its round tongue, which is not nicked behind, and is almost completely adherent, much resembling that of the Discoglossidae. The sacral diapophyses are but slightly dilated. The only species, N. rugiceps, lives in Ecuador, and grows to nearly three inches in length. The head is large and rough owing to the skin being involved in the cranial ossification. It is further peculiar in its coloration, the under parts being chestnut-brown instead of whitish. The upper parts are olive-grey or brown.
The following four genera, each represented by one or two species {207}only, much resemble each other in the curious shape of the head, which forms a flat projecting snout, used probably for digging in rotten wood in search of insects. There is a peculiar degradation in the extent of dentition of the palatal region. Diaglena and Triprion are the only Hylidae which possess a longitudinal row of parasphenoid teeth. Diaglena petasata of Mexico and D. jordani of Ecuador have, moreover, a transverse row of teeth on the palatine bones in addition to those on the vomer.
Triprion petasatus of Yucatan has parasphenoid and vomerine teeth. The head is a bony casque, with strong superciliary ridges, the skin being extensively ossified. The mouth forms a flat snout, owing to the long projection of the upper over the lower jaw. The skin of the back is smooth brown with darker spots; the under parts are uniform whitish. The male has a subgular vocal sac. Like Diaglena and Corythomantis they possess adhesive discs on the fingers and toes, and climb trees. The total length of this curious creature is 2 inches.
Corythomantis greeningi of Brazil has a similar head. The vomers alone carry teeth, besides of course the maxillae. The pupil is rhomboid. The tongue, as in the two previous genera, is roundish, scarcely free. General colour above olive, with darker freckles; the sides are studded with whitish tubercles; the under parts are whitish. The male is devoid of vocal sacs. Total length 3 inches.
Pternohyla fodiens of Mexico approaches the previous three genera by the curious shape of the head and prominent upper jaw, although these features are not so exaggerated. The dentition agrees with that of Corythomantis and other normal tree-frogs. The fingers and toes are not provided with discs, in conformity with the burrowing, not climbing, habits of this creature. The next following three genera connect the Hylidae with the Cystignathidae. The sacral vertebrae are but slightly dilated.
Acris.–The adhesive discs are very small, the tympanum is indistinct. A. gryllus, the only species, inhabits the greater part of Eastern and Central North America, extending northwards into Canada. It attains a length of 1½ inch. The coloration is very changeable, in adaptation to the surroundings. As {208}a rule it is brown, with a more or less reddish or grey ground-tone, ornamented with dark brown or blackish irregular, longitudinal patches,one of which is bordered with light green,and there is often a light vertebral streak. The legs are cross-barred, the under parts are whitish brown and yellowish. The male has a subgular vocal sac, and its most remarkable feature is the voice, which closely resembles the noise of a cricket or of certain grasshoppers. Holbrook describes it as a merry little frog, constantly chirping like a cricket, even in confinement. It frequents the borders of pools, and is often found on the leaves of aquatic plants, rarely on the branches of such low shrubs as overhang or dip into the water. When disturbed it takes long jumps, and hides at the bottom of the pond. Insects are secured by leaps. It can easily be domesticated, and takes food readily from the hand. Sprinkling them with water never fails to make them more lively and noisy. Appearing in April in great numbers, they are said to vanish early in the autumn for hibernation. The tadpoles are metamorphosed by the end of August.
Chorophilus.–The fingers and toes are provided with very small adhesive discs. The sacral diapophyses are very slightly dilated. About seven species occur in North America, chiefly in the Southern States, one, Ch. cuzcanus, in Peru. Ch. ocularis is the smallest of the frog-kind known, and lives in South Carolina, frequenting damp places, the vicinity of stagnant pools, water-plants or low shrubs, for instance the "myrtle," Myrica cerifera. I once had two of these tiny creatures less than three-quarters of an inch in length. They were very active, and took surprisingly long leaps, jumping distances of 2 feet, but could not be kept through the winter, although they took minute insects readily enough. The head is narrow, long and pointed; the upper parts are of a rich chestnut-brown with a bronzy gloss. The upper jaw is white; a black band extends along the sides of the head and body. The under parts are yellowish white.
Ch. ornatus is another inhabitant of the South-Eastern States; its name refers to the dark brown patches on the back and sides, bordered with golden yellow, upon a reddish-brown ground-tone, while the under parts are silvery white with fine grey spots. This frog, a little more than one inch in length, lives on land in dry places, preferably in corn-fields, has no voice, and, except during the pairing season, carefully avoids the water.
Thoropa.–The fingers and toes are free, the tips simply swollen and not dilated into discs. Closely allied to Chorophilus. Th. miliaris, of Brazil, the only species, has very long toes. The head is broad and flat. The upper, nearly smooth surface of the body is flesh-coloured, with brown marblings; the limbs are cross-barred; the under parts whitish, granular on the belly. The male is devoid of vocal sacs. The total length may be 2 inches. Hensel has published the following notes of this species, under the name of Hylodes abbreviatus. The tadpoles are quite flat, their bellies forming a kind of sucking disc, so that these creatures, even before the appearance of the hind-limbs, can quickly wriggle up vertical walls of stones, provided these are covered with a little water. In correlation with this habit, the root of the tail is not compressed laterally, but is as broad as it is high, and the usual vertical fin is restricted to its distal third. On the proximal portion of the tail the ventral fin is flattened and broadened out so as to form almost the continuation of the peculiar disc-like belly. The anal opening is not a projecting tube, but is a flattened transverse slit.
Fam. 5. Cystignathidae.–This is one of the largest families, and also one of the least satisfactory. Its numerous members, more than 150, exhibit such a versatility in adaptation to circumstances (there are aquatic, terrestrial, arboreal, and burrowing species), with a corresponding development or loss of anatomical characters which we should like to rely upon as taxonomic marks, that the numerous genera not only run into each other, but also get entangled with those of other families. In fact the whole family is ill defined. It can be characterised as follows:–The shoulder-girdle is arciferous; the sacral diapophyses are cylindrical or but slightly dilated; the metasternum has either a bony style or it forms a cartilaginous plate; the terminal phalanges, although they sometimes carry adhesive discs, are never claw-shaped.
The last statement is, of course, intended to separate the Cystignathidae from the Hylidae, of which, however, the three genera Thoropa, Chorophilus, and Acris stand on debatable ground (cf. p. 186, Hylidae), while, on the other hand, most of the Australian genera, notably Chiroleptes, have unmistakably dilated sacral diapophyses. The difference from the Pelobatidae can in this case be one of degree only.
The Cystignathidae may be said to represent the Ranidae in Notogaea. Some of them can be distinguished from the true, typical frogs solely by the arciferous type of the shoulder-girdle and sternum. There is in both families the same adaptive versatility, the same amplitude in the formation of the fingertips, the occasional slight dilatation of the sacral diapophyses, the same range in the configuration of the omo- and meta-sternum. In fact, young Ranidae, before the firmisternal character is assumed, are indistinguishable from Cystignathidae, and the latter would turn into Ranidae if they could be induced to consolidate their sternal apparatus.
The geographical distribution of the Cystignathidae is suggestive of their being an old family, most of whose members have reached a high stage of morphological development. The overwhelming majority inhabit the Neotropical region, a few forms extending into tropical Central America and into the Antilles; the rest, some twenty species only, are confined to the Continent of Australia and to Tasmania.
The family name is rather a misnomer. It is taken from the genus Cystignathus, which is, or rather was, characterised by the peculiarly broadened lower jaw, hollowed out by the vocal sacs; but this generic name had to give way to that of Leptodactylus, in obedience to the often senseless rule of priority. The family is composed of three subfamilies.
Sub-Fam. 1. Hemiphractinae.–Teeth are carried by both jaws, the vomers and the palatine bones; or by the palatines and parasphenoids in Amphodus. The vertebrae are opisthocoelous, devoid of ribs, and the sacral diapophyses are not dilated. The shoulder-girdle and sternum are strictly arciferous. The omosternum is very much reduced; the metasternum forms a cartilaginous plate. The tongue is slightly free behind. The tympanum is distinct. Three genera, with eight species, all inhabitants of South America.
Hemiphractus.–The head is large; the upper surface of all the cranial bones appears pitted, owing to most of the covering skin being involved in the ossification. The temporal fossa is bridged over or roofed in by the fronto-parietal and the squamosals, so that the orbit is completely encircled by bone, as in Pelobates cultripes. The terminal phalanges are simple and are not dilated into discs. The teeth of the lower jaw are {211}very small and numerous. The tongue is round and very small. H. scutatus, the only species, living in Ecuador and Colombia, is a frog-like creature, with a large helmet-shaped head. Total length 2½ inches.
Ceratohyla has the same kind of helmet-shaped head, and the orbit is likewise enclosed by bone, but the terminal phalanges are claw-shaped and carry regular adhesive discs. This genus, the five species of which live in Ecuador, bears undoubted resemblances to the Hylidae. In C. proboscidea the upper eyelid is produced into a little upright fold, as in Amphignathodon and some species of Nototrema and Ceratophrys among Cystignathidae. The snout is produced into a long, compressed, bifid appendage, and the heel carries a triangular flap. In C. bubalus the partly ossified helmet sends out a pair of diverging processes, formed by the squamosals, extending backwards and sideways from the concave and ridged interorbital spaces. The tip of the snout and the tips of the divergent horns form an equilateral triangle, and the whole head bears a striking resemblance to some of the fossil Reptiles from the Elgin Sandstone, e.g. Triceratops. Total length 3 inches.
Amphodus wucheri.–The only species of this genus has been found near Bahia. It has teeth on the palatine bones and five series of small teeth on the parasphenoid, but none on the vomers. The teeth of the mandible number about eleven on each side and decrease in size towards the symphysis. The tympanum is distinct; the heart-shaped tongue is free behind. The cranial bones are only slightly pitted. The skin is smooth above, chocolate-brown, spotted with yellow, and with a yellow band on the sides of the body beginning with the upper eyelid and ending in a broad patch above the vent. The under parts are yellowish white.
Sub-Fam. 2. Cystignathinae.–The upper jaw alone is provided with teeth. Vertebrae procoelous. The twenty-seven genera of this sub-family have been arranged in the following key, merely for convenient determination.
I. American genera.
A. The metasternum forms a cartilaginous plate without a narrow handle. The pupil contracts into a horizontal slit.
a. The terminal phalanges are bifurcated, Y-shaped, and provided with large discs; the tympanum is distinct; the omosternum is absent .......... Centrolene geckoideum, Ecuador.
b. The terminal phalanges are T-shaped and carry discs. The omosternum is cartilaginous.
α. Discs divided by a dorsal groove.
With vomerine teeth .......... Elosia, 3 species in Brazil.
Without vom"r teeth .......... Syrrhopus,[91] 9 species, South America.
β. Discs undivided.
With vomerine teeth .......... Hylodes, p. 214.
Without vom"r teeth .......... Hylopsis.
c. Terminal phalanges simple, pointed, or with very small discs. First finger opposed to the others .......... Pseudis, p. 213.
d. Terminal phalanges simple, without discs.
α. Tympanum hidden. A large, flat gland on each side of the body .......... Cyclorhamphus fuliginosus, Brazil.
β. Tympanum distinct. Head rough, entirely bony. .......... Calyptocephalus, p. 215.
γ. Tympanum hidden or absent. Tongue roundish, not nicked, free behind. Toes webbed. .......... Telmatobius, 6 species in Western South America.
δ. Tongue heart-shaped, free. Toes webbed. .......... Ceratophrys, p. 215.
ε. Tongue round, free behind. Toes webbed. With two tooth-like projections in the lower jaw. .......... Lepidobatrachus, p. 218.
ζ. Tongue entire, or slightly nicked, free behind. Toes free. .......... Borborocoetes, 11 species in Western South America.
η Tongue entirely adherent Tympanum distinct. .......... Zachaenus parvulus, Brazil.
B. Metasternum with a bony style.
a. Pupil horizontal
α. Terminal phalanges T-shaped, with discs. Tympanum distinct. .......... Plectromantis, 2 species in Western South America.
β. Terminal phalanges simple; tips not dilated into regular discs.
b. Pupil vertical. Terminal phalanges simple and not dilated. Chili.
α. Tongue slightly nicked .......... Limnomedusa macroglossa.
β. Tongue entire, but free behind. Digits very long. .......... Hylorhina silvatica.
II. Australian genera. The terminal phalanges are simple and not dilated. The omosternum and metasternum are cartilaginous, the latter forming a plate, semi-ossified only in Heleioporus.
A. Pupil contracted into a horizontal slit.
a. Omosternum rudimentary. Vomerine teeth present.
α. Tympanum distinct .......... Phanerotis fletcheri.
β. Tympanum hidden .......... Cryptotis brevis.
b. Omosternum present. Vomerine teeth vestigial. .......... Crinia, 4 species.
c. First finger opposed to the others .......... Chiroleptes, p. 221.
B. Pupil contracted into a vertical slit.
a. Omosternum rudimentary. Vomerine teeth absent. .......... Hyperolia marmorata.
b. Omosternum fully developed. Vomerine teeth present.
Pseudis, widely distributed over South America, consists of four species which have the appearance of long-legged frogs. The fingers, of which the first is opposed to the others, are free; the long toes are fully webbed. The tympanum is exposed.
P. paradoxa is absolutely aquatic, floating in pools, and is extremely shy. In life it is most beautifully coloured with bronze, bright green, and black markings above; underneath it is shiny yellow, with brown spots on the body and stripes on the thighs. Within a few minutes after death all the brilliant colours of the smooth skin of the back turn into dull uniform brown, with indistinct darker spots. Total length of the adult from 2 to 2½ inches. The specific name refers to the peculiar shape and monstrous size of the larva or tadpole.
One of the larvae described and figured by Parker measures 10⅓ inches in length, the head and body taking up 3⅓ inches. The spiracle lies on the left side and the hind legs are ½ inch long, just breaking through the skin. The vent is median. The huge tail is very thick and muscular, and is furnished with a high, irregularly shaped dorsal and ventral fin, the whole organ measuring 4 inches dorso-ventrally. Another larva, or rather tadpole, in the national collection is older, and although still very large, namely, 7 inches long, has fully developed hind-limbs 3 inches long; the fore-limbs are less than half that size, the left protrudes through the spiracle, while the right has broken through the skin. The dorsal and ventral fins of the tail have much shrunk; the whole organ, 5 inches long, is gradually tapering to a point like the tail of ordinary tadpoles. By the time that the {214}tadpole is nearly ready to leave the water, its whole bulk is reduced to less than one-fifth that of the largest tadpole. It measures from snout to vent only 1½ inch (in the 7-inch tadpole this distance is fully 2 inches), and the tail, devoid of fins, is reduced to 2 inches in length. Instead of the solitary left spiracle there are now two, one on the ventral side and a little in front of the base of each arm, the border of each hole being continued by a peculiar semilunar fold.
Fig. 42.–Hylodes martinicensis. 1, an egg with embryo about seven days old; 2, another, twelve days old; 3, the young Frog just hatched; all × ¾; 4, adult male × 1. (After Peters.)
Hylodes.–The numerous species, nearly fifty, of this tropical American genus exhibit several anatomical differences. The tympanum is sometimes indistinct or hidden, in which case the Eustachian tubes are generally very narrow. The fingers are free, and carry discs, like the toes, which are sometimes slightly webbed. The males have a subgular vocal sac, producing a loud, or whistling, voice. The general appearance is that of land- and tree-frogs; the size is small, mostly between 1 and 2 inches.
H. martinicensis is about 1½ inch in length. The ground-colour is pale yellow-grey, with a large brown patch on the nape, which colour is continued over the back in the shape of more or less coherent or dissolved patches. A dark brown stripe runs along the middle of the sides. The limbs are barred with brown, the under parts are whitish. This species, known by the vernacular name of "coqui" inhabits many of the West Indian islands, e.g. Barbadoes, Martinique, Porto Rico, and Hayti. It has become famous, as it was the first instance known of a frog which undergoes its whole metamorphosis within the egg. The pairing takes place on land, in the months of May and June, when the female lays about twenty eggs, which are enveloped in a foamy mass and glued on to a broad leaf, or hidden in the axillae of Iridaceous plants. The mother seems to remain in the neighbourhood watching the eggs, which are {215}large, measuring 4-5 mm. in diameter. Dr. Gundlach, a resident in Porto Rico, was one day, in the month of May, attracted by sounds like those of a young bird, and found three males and one female of this species sitting between two large leaves of an orange-tree. He put them all into a glass vessel and soon saw a pair in embrace. The female laid about twenty-five pale straw-coloured eggs. The embryo develops neither gills nor gill-openings, but a large well-vascularised tail, by means of which, being immersed in the watery fluid contained within the egg, it seems to breathe. After twenty-one days the tadpole, having used up all the available yolk and fluid, and most of its own tail, bursts the egg-shell and hops away as a little frog of 5 mm. in length, but still with a stumpy white tail, which is quite absorbed within the same day.
This species has several times made its appearance in the tropical houses of Kew Gardens. It seems to have bred and vanished again.[92]
Calyptocephalus is remarkable for the dermal ossification of the cranium, which has assumed the greatest possible extent. It affords a curious parallelism to Triprion and other Hylidae, which are likewise Central American forms. Only two species are known; C. gayi of Chili, and C. testudiniceps of Panama. They are large, thoroughly aquatic creatures, 5 to 6 inches in length, with huge heads. The tadpoles grow to an enormous size. One specimen of C. gayi in the National Collection is more than 6 inches in length, the tail taking up more than half of the total: the spiracle lies on the left side, the vent on the right, and the hind-limbs are still half enveloped in a kind of fold of the skin.
Ceratophrys is a genus of some ten toad-like species, living in South America, from Guiana to Argentina. The generic name alludes to the peculiar modification of the eyelid, which in most species is developed into a triangular, upright, but flexible appendage. The head, in conformity with the huge mouth, is very large. The tympanum is rather indistinct, sometimes quite hidden. Several of the species have a large dorsal shield, which is produced by a thick ossification of the cutis, but is not fused with any of the vertebral processes. The male has a vocal sac. C. dorsata s. boiei of equatorial Brazil is a monster toad, reaching {216}a length of 6 inches. The upper eyelid is transformed into a triangular horn, whence a cutaneous ridge extends all along the side of the back, meeting that of the other side above the vent. There is no osseous shield on the back. The tympanum is hidden. Ground-colours, orange or green, with sharply marked dark brown or blackish patches.
C. cornuta, in Northern Brazil, lacks the dorsal shield, but has horned eyelids and a visible tympanum. Its coloration renders it one of the most beautiful toad-like creatures known. The ground-colours are green, black and brown, with an orange-yellow stripe over the head and back. All these colours are most pleasingly blended and arranged in marbled patches or stripes radiating from various centres, as, for instance, from the eyes towards the circumference of the mouth, the slit of which they pass, the same line of the pattern being continued upon the lower jaw. The whole surface makes the impression of a gay but exquisitely harmonious carpet. The under parts are yellow, inclining to white towards the middle.
C. ornata has a dorsal shield. The tympanum is just visible, and the eyelids form only low but sharp-edged projections. This is likewise a beautiful toad, living chiefly in Uruguay, Northern Argentina, and Paraguay, where it is universally known as the "escuerzo," one of the Spanish words signifying a toad. Its size rarely surpasses 4½ inches. The ground-colours are greenish and yellow, with large dark green patches on the back, decreasing in size on the flanks.
Each of these insular patches is surrounded by a narrow line of white and yellow dots, interspersed here and there with lines of rusty brown or red. The object of this elaborate carpet-like pattern is concealment. These toads–and this applies to all the species,–bury themselves half in the ground, preferably in the grass, where they are well-nigh invisible. If there is not enough green vegetation, they throw, with their feet, little lumps of earth upon their backs, the skin of which becomes at the same time more crinkled and assumes duller tones. There the creature lies, perfectly concealed, betrayed only by the metallic glittering eyes, waiting for some unfortunate creature to pass into the trap represented by the enormous mouth, which opens and shuts with lightning-rapidity and with an audible snap. They seem to live chiefly on frogs, and sometimes they turn cannibals. Two specimens were brought over to me from Buenos Aires by a friend, in a well-closed basket with moist soil at the bottom, but only one was visible on arrival. The other was inside the {218}larger one, and could still be felt through the soft body. This same cannibal took large-sized frogs greedily, one or two for a meal, swallowing them whole and then sinking back into its lair, which it scarcely ever left, except for an occasional soaking bath in its water-pan, especially before shedding its skin. It lived for many months in the same enclosure with a pantherine toad, Bufo mauritanica, of equal bulk, until one morning I found the Moroccan half swallowed and almost lifeless in the mouth of the American, whence it was rescued with difficulty. It came round after a few hours, but never fully recovered, lingering on for weeks; the skin was changed to a lead-colour so far as it had been swallowed and partly dissolved by the gastric juices, and soon began to develop festering ulcers.
These "horned toads" make a squeaking noise when teazed, not at all loud or strong in proportion to their size. Ill-tempered individuals jump at their aggressor and can inflict rather painful nips. They hibernate during the dry season in the ground.
Lepidobatrachus.–Large teeth in the upper jaw, and two large tooth-like projections in the lower jaw near the symphysis. Vomer toothless. Sacral diapophyses not dilated. Tongue round, and free behind. Tympanum distinct. Great development of the membrane-bones on the head, and a weaker ossification in the skin of the back, recalling that in Ceratophrys. The eyes are closely set together, and the nostrils take up the most elevated portion of the head. Pupil horizontal. The two species of this genus were discovered by Budgett[93] in the Paraguayan Chaco. L. asper lives continually in muddy pools, floating with just the eyes and nostrils above the surface. If disturbed it slowly sinks to the bottom, leaving no ripple. It feeds largely on Bufo granulosus. Total length from about 3 inches. The skin of the upper parts is tubercular, tough, and of a dull leaden colour; the tips of the toes are horny. L. laevis is smooth and slimy, "with the organs of the lateral line showing clearly upon it," a feature elsewhere known to exist in Xenopus and Leptobrachium only.
Leptodactylus = Cystignathus.–Some twenty species inhabit tropical America, from Central Mexico to Buenos Aires. The fingers and toes are not webbed and end mostly in points; only a few species, e.g. L. hylaeodactylus, having small adhesive discs. {219}The legs are long and the general appearance is very much like that of an ordinary frog.
One of the commonest and prettiest Brazilian species is L. ocellatus, which is characterised by a number of longitudinal glandular folds on the back and flanks. The colour of the upper parts is olive-brown, that of the prominent folds is yellowish white, interspersed with black spots. The under parts are yellowish white, with blackish marblings on the throat. The males have a sharp black spur on the inner carpal edge and one on the rudiment of the thumb. Total length about 4 inches.
According to Hensel[94] the spawning takes place in Rio Grande do Sul after hibernation. The voice of the male is then very loud, resembling the sound made by a carpenter chopping a beam. They repair to ponds and produce a cup-shaped puddle, about 1 foot in width, by raising a wall of mud, which separates the inner water from that of the pond. The tadpoles remain in this nursery until the spring-rains demolish it and set the young ones free. Drought causes the drying up of these water-pans and subsequent destruction of the brood.
L. mystacinus is another Brazilian species, about 2 inches in length. Its specific name refers to the dark brown stripe which runs from the tip of the mouth through the eye to the tympanum. This species is thoroughly terrestrial, and never enters the water. It digs a cavity, the size of an ordinary tea-cup, under stones or rotten trunks, always in the neighbourhood of ponds and just so high above the water that the latter can rise up to the nest in the rainy reason. The straw-coloured eggs are laid in this cavity, and are enveloped in a foamy, sticky mass, like the well-beaten white of an egg. The young tadpoles seem to live on this froth until the rains set them free. When, however, the rains delay and a drought kills the broods of other less circumspect species, these tadpoles, still provided with gills and long tails, remain in their moist nest or withdraw further beneath the rotten stumps, huddled together in large numbers until the next rainy season.
Similar nursing habits have been recorded of L. albilabris, which inhabits Mexico, Cuba, and several other West Indian islands. The same applies to L. typhonius. Gundlach found eggs {220}of this "Sapo" in Puerto Rico on the 4th of November; on the 25th the young showed the first signs of hind-limbs, on the 3rd of December of fore-limbs, and on the 7th of the same month they began to climb out of the water.
Paludicola is a semi-aquatic genus with some eighteen species, ranging from Mexico to Patagonia and across the Andes into Chili. Some of them have a peculiar gland on the lumbar region, or large, flat warts on the back, sometimes arranged in longitudinal folds. The toes are slightly webbed, or free, according to the more or less pronounced aquatic habits.
P. fuscomaculata, an inhabitant of Southern Brazil, Paraguay, and Uruguay, is a short-limbed frog, with spreading slender toes and a small head. There are shovel-shaped, black, horny tubercles on the metatarsus. The general colour is olive above, with darker markings and confluent white-edged spots; the limbs are cross-barred; the lumbar glands are black, with a white margin in front. The male has a vocal sac. Budgett[95] gives the following account of its habits:–
The peculiar cry, which is so constantly heard in the neighbourhood of shallow pools in the Paraguayan Chaco, and resembles that of a kitten, is produced by the alternate inflation of throat and abdomen. When fully inflated, the frog appears to be the size of a golf-ball, but, if startled, instantaneously shrinks to one-fifth of that size, so that it seems to have vanished. It has also the power of ventriloquising. The food consists largely of water-beetles. In the spawning time it was found at night floating on the surface of pools in the distended condition, and crying to the females in a most mournful way. On coming to the surface it fills its lungs with a few gasps, greatly distending the walls of the abdomen, and then drives the air into the vocal sacs, causing them to become distended as the body collapses, and giving rise to a kitten-like cry.
The eggs are chiefly laid in January, and are found embedded {221}in a frothy mass floating upon the surface of the water. The eggs measure only 1 mm. and are without pigment, and with extremely little yolk. The larvae become free-swimming within from eighteen to twenty-four hours after the first segmentation. When ready for hatching they wriggle their way through the froth to the water below, and hang into it from the floating froth.
P. biligonigera s. notata, in Brazil, lacks the lumbar gland, the place of which is marked by a black spot. The upper parts are olive, with darker marblings and a dark lateral stripe. The male has a black throat and two external vocal sacs. Hensel found the eggs, in Rio Grande do Sul, in September, forming a frothy mass of the size of a fist, floating between grass upon the water near the margin.
The following three genera may serve as Australian examples, especially since we are indebted to Baldwin Spencer for interesting observations made on their habits in Central Australia.[96]
Chiroleptes, of which six species are known, is easily recognised by the first finger, which is opposed to the others. The sacral diapophyses are slightly dilated. The general shape is that of a thick-headed, rather stout land-frog or of a tree-frog. The tympanum is distinct, and the toes are only half webbed, or even less, except in Ch. platycephalus, in which the toes are entirely webbed and the tympanum is indistinct. This species is about 2 inches long, uniformly olive-green above, with a few tubercles on the otherwise smooth skin. Other species rather resemble the European Natterjack in coloration.
Spencer's account is as follows:–"In Central Australia Ch. platycephalus seems to prefer the hard clay pans rather than sandy creeks, as the sand-beds of the latter are too loose for the formation of the burrow. We came across the animal first when encamped by the side of a very shallow clay pan, the floor of which was deeply cracked with the sun's heat. Around the edge were withered shrubs of Chenopodium nitrariaceum, and it was at the base of these that the black fellows looked for the burrow. In the hard-baked clay were imprints made by the frog as it burrowed, and about a foot underground we came across the animal, puffed out into a spherical shape, and just filling up a cavity, the walls of which were moist but not wet. The ground {222}was so hard that it had to be chipped away. When one side of the burrow was opened, the frog remained perfectly still; its lower eyelid was drawn up over the eye and was very opaque, giving rise to the belief amongst the blacks that the animal is blind. In the sunlight, after a short time, it opened its eyes.
"On squeezing the body, water was forced out of the cloaca; this was accumulated principally in the urinary bladder. On cutting the body open it was seen that there was a certain amount of water in the subcutaneous spaces, but that the greater portion, which caused the great swelling-out of the body, was contained in the body-cavity itself; and it was also observed that the lungs were considerably distended and lengthened, their apices lying right in the pelvic region. They contained air and not water, but their outer faces were bathed with the water in the body-cavity." The larvae and tadpoles probably develop with extreme rapidity, soon to aestivate as very small frogs.
Heleioporus has a calcified metasternal plate and slightly dilated sacral vertebrae. The two species have a toad-like appearance, owing to their stout bodies, short limbs and conspicuous parotoid glands. H. albopunctatus is mottled whitish red and brown above; it extends from Western into Central Australia. H. pictus is olive, with darker marblings, and is distinguished by a light vertebral line. It is likewise found in Central Australia, and it extends into Victoria and New South Wales. Spencer found it in swarms after heavy rains, the specimens being much swollen and distended with caterpillars and beetles. They looked as if they were simply gorging themselves with food preparatory to returning again to their long aestivating condition.
Limnodynastes is one of the commonest genera in Australia. The six species have the habits and appearance of stout frogs or smooth toads. L. dorsalis seems to range through the whole of Australia, from east to west, and looks like the European Pelobates. The skin is smooth, but with an elongated white gland extending from beneath the eye to the shoulder, and another glandular complex on the thigh. The upper parts are mottled olive-brown, often with a light vertebral line. The under parts are whitish, with brown spots. The male has a vocal sac. One of the specimens in the National Collection contained a half-grown Heleioporus albopunctatus in its stomach.
Concerning the pairing and the other habits of the Anura of New South Wales we have some valuable notes by J. J. Fletcher.[97] He observes that Australian frogs spawn whenever they are ready, and when the very irregular conditions of moisture will allow it, but that they are not all ready at the same time, i.e. they have no fixed period of the year. Limnodynastes, Hyla aurea, and H. coerulea deposit their spawn in the water, in more or less irregular floating patches, which look white and frothy. The period extends from July to May, and is at its height in August and September; but if there is a spring-drought vigorous spawning may be looked for about the middle of January, when heavy showers are likely to occur. Crinia and several Hyla, e.g. H. ewingi, spawn at any time of the year. The eggs form small submerged bunches, enclosed in a transparent jelly, attached to the blades of grass or twigs of dead branches in the water.
Pseudophryne, a genus closely resembling Crinia, but on account of the absence of teeth in the lower jaw relegated to the Bufonidae, spawns during the Australian summer and autumn. The numerous ova of P. australis and P. bibroni are laid separately, not in the water, but under stones, or in the débris of reed- and grass-tussocks, on the edge of a pool.
The larvae of Pseudophryne and others have often to depend upon the next following rain, sometimes waiting for months to be released from the eggs, wherein they have so far developed. But the tadpoles, once hatched, probably do not bury themselves; they either metamorphose or die.
The males of Mixophyes and Hyla, grasp the females in the axillary region; those of Limnodynastes, Hyperolia, Crinia, and Pseudophryne throw their arms round the inguinal or lumbar region.
For some three months during the winter, commencing about May, the frogs, like lizards and snakes, resort to shelter under logs and stones, beneath which they are then to be met with in a more or less sleepy condition. During the hot and very dry periods many bury themselves in the drying-up mud, which becomes very hard, and does not release them until the next rains. They croak during showery times of the year. There is no evidence that any Australian species live in the high Eucalyptus-trees.
Hylopsis platycephalus, of South America, is of importance as forming a link with the Dendrophryniscinae, owing to the very small size of the teeth in the upper jaw. There are no vomerine teeth. The fingers and toes are webbed, and furnished with discs. The very small omosternum and the metasternum are cartilaginous. The pupil is horizontal. Total length, about or under 1½ inch.
Sub-Fam. 3. Dendrophryniscinae.–The two Neotropical genera of this sub-family are characterised by the entire absence of teeth. The toothless condition of the upper jaw is really the sole character which separates them from the Cystignathinae, taken as a whole. The suppression of the tympanum and of the Eustachian tubes in Batrachophrynus, and the fully webbed toes of B. macrostomus indicate complete adaptation to aquatic life. The absence of the omosternum in Dendrophryniscus, the absence of vomerine teeth, the dilated phalangeal tips, the entire and quite adherent tongue, are all features which likewise occur in some of the Cystignathinae, and therefore cannot be urged against their affinity. Lastly, the recently discovered South American genus Hylopsis is, as pointed out by Werner,[98] an intermediate link, owing to the extremely small, scarcely visible teeth in the upper jaw.
Dendrophryniscus brevipollicatus has been found in the neighbourhood of Rio Janeiro. The head is depressed and triangular. The tongue is entire, but free behind. The tympanum is suppressed. The omosternum is absent; the metasternum forms a long bony style. The sacral diapophyses are cylindrical. The terminal phalanges are simple, but carry dilated tips. The first finger is rudimentary. The skin is nearly smooth, reddish brown above, whitish below; the limbs are cross-barred.
Batrachophrynus inhabits the mountains of Peru. The head is much depressed and small, with the eyes directed upwards, as is usual in essentially aquatic species. The tongue is large, circular, and entirely adherent. The tympanum and the Eustachian tubes are suppressed. The omosternum is cartilaginous, and the metasternum forms a cartilaginous plate. The sacral diapophyses are cylindrical. The terminal phalanges are simple, and carry no discs. The four fingers are short; the toes are webbed. The male has no vocal sac. B. brachydactylus has a {225}smooth skin, olive-brown above with darker spots. B. macrostomus, 2 inches in length, is distinguished by its larger size, and by its completely webbed toes.
Fam. 6. Engystomatidae (Narrow-mouthed Toads).–Firmisternia with dilated sacral diapophyses.
Sub-Fam. 1. Engystomatinae.–Without teeth in the upper jaw.–Although there are only about 60 species known, these have been grouped into more than two dozen genera, many of which are represented by one or two species only. The range of this sub-family is peculiar, namely, Neotropical and Palaeotropical. Scaphiophryne and Rhombophryne are peculiar to Madagascar; Calophrynus occurs in the same island and in the Indian region; Xenobatrachus, Sphenophryne, Liophryne, Mantophryne, Callulops and Xenorhina live in New Guinea. Breviceps, Cacosternum and Hemisus are confined to Africa, while of Phrynomantis two species live in Africa, and the third in the Malay island of Amboina. Such freaks of distribution indicate either that many of these genera are not established upon very valid characters, or that their respective species are instances of convergent evolution, and do not form natural genetic groups.
Many of the members of this sub-family live upon ants and termites, and it is a well-known fact, not restricted to the Anura, that this kind of fare has a peculiar, modifying influence upon the structure of the mouth, teeth, tongue, limbs, and various other organs. In the present case the tongue is not much affected; it is, with few exceptions, more or less oval, not nicked, but free behind; in the Indian Glyphoglossus and in Rhombophryne of Madagascar only is it modified into a rather long and grooved, almost double, apparatus.
A very common feature is the small size of the mouth and the formation of a snout, which projects beyond the upper rim of the mouth and beyond the nostrils. Such a prominent and pointed snout is well developed in Rhinoderma, Phryniscus, Calophrynus, Stereocyclops, Hypopachus and Engystoma. The mouth is very narrow in Cacopus, Glyphoglossus, Breviceps, Rhombophryne, and Hemisus, all creatures which seem to be confirmed eaters of ants and termites. However, it must not be supposed that the mouth of all the genera is narrow, although this character, rather marked in Engystoma, is now embodied in the name of the family. A peculiar development of the palatal region {226}is possibly correlated with this food. The palate is mostly toothless, but its skin is frequently raised into a transverse fold, between or behind the vomers, and into a second fold in front of the oesophagus; these folds are sometimes rather hard and serrated or denticulated. The palatine bones carry true teeth in Rhombophryne, and sometimes in Callula; in Xenobatrachus the teeth are reduced to two large pairs. The tympanum is usually hidden.
The shape of the body is generally very stout. The limbs are short, notably so in Glyphoglossus, Breviceps, Rhombophryne, Hemisus, Stereocyclops and Cacopus. Others, for instance most species of Microhyla, Phryniscus, Callula, and Sphenophryne, are of a very slender build; and their limbs, instead of being short and well adapted to digging, are long and may even be provided with typical adhesive discs, supported by T-shaped phalanges, especially in the two genera last named, and in Scaphiophryne and Phrynomantis. However, none of the forms provided with discs are known to be arboreal.
Exceptional diversity is shown in the shoulder-girdle and sternum. The omosternum occurs only in Rhinoderma and Hemisus. The metasternum is a cartilaginous plate, very large in Cacopus, distinctly small in Breviceps, and almost absent in Hemisus. The precoracoids and clavicles show all stages from a well-developed condition (Breviceps, Rhombophryne, Hemisus, Rhinoderma, Phryniscus and Brachycephalus) to complete absence. The circumstance that these bars are very weak in Melanobatrachus, Calophrynus, Scaphiophryne and Hypopachus, i.e. in Palæo- and Neo-tropical genera, indicates a widespread tendency towards complete suppression, a feature independently aimed at both in America (Engystoma) and in the Old World.
Until we know something about the habits of the members of this much diversified sub-family, it is idle to connect the various modifications with each other, and thus, by correlation, to find out their meaning. Those forms which possess well-developed discs on their fingers and toes are said not to be arboreal. What is the true meaning of the prominent snout which is not restricted to the digging forms? Most of the good diggers have well-developed precoracoid bars, and the coracoids are distinctly strengthened, but in Glyphoglossus and in Cacopus the precoracoids are entirely {227}absent, and this loss is compensated for by exceptionally strong coracoids.
On the whole, those genera are to be considered as the most primitive which have undergone the fewest losses. Those with a complete shoulder-girdle, with an omo- and meta-sternum and with simple phalanges, are necessarily the older forms. One step farther back in another direction, the possession of teeth on the palate, and on the upper jaw, leads to those genera which have been separated off as Dyscophinae, while teeth in the lower jaw constitute the Genyophryninae. Lastly, the firmisternal type has necessarily been evolved from the arciferous condition, and there the two Bufonid genera Myobatrachus and Rhinophrynus, the former Australian, the latter Mexican, with their narrow and scarcely overlapping epicoracoid cartilages, seem to form a connecting link, although their ant-eating habits, with concomitant modifications in structure, may be nothing but cases of convergent evolution.
Key to the genera:–
I. American. A. with omosternum .......... Rhinoderma, p. 228.
B. without omosternum.
a. Pupil horizontal. Precoracoids present.
Sacrals strongly dilated. .......... Oreophrynella.
Sac"als moderately "dla .......... Phryniseus, p. 230.
Sac"als feebly moel"dla .......... Brachycephalus, p. 231.
b. Pupil vertical.
α. Precoracoids feeble. .......... Hypopachus.
β. Precor"coids absent. .......... Engystoma, p. 231.
c. Pupil round. Precoracoids present .......... Stereocyclops, p. 231.
II. Palaeotropical. a. Pupil horizontal.
α. Precoracoids present.
With palatal teeth. Madagascar. .......... Rhombophryne.
Palate with dermal papillae. Africa. .......... Breviceps, p. 232.
With palatal dermal folds. Madagascar. .......... Scaphiophryne.
With serrated palatal folds. Madagascar and India. .......... Calophrynus.
Palate smooth. New Guinea. .......... Sphenophryne and Liophryne.
β. Precoracoids absent.
Malacca .......... Phrynella, p. 233.
New Guinea .......... Mantophryne.
Africa .......... Cacosternum.
b. Pupil vertical.
α. Precoracoids present. India. .......... Melanobatrachus.
Africa. Hemisus, p. 232.
β. Precoracoids absent.
Tongue oval. India. .......... Cacopus.
Tongue elliptical. India. .......... Microhyla.
Tongue divided by a longitudinal furrow. India. .......... Glyphoglossus, p. 233.
Fingers and toes with discs. Africa and Amboina. .......... Phrynomantis.
New Guinea. .......... Callulops.
c. Pupil round. Precoracoids absent. Tongue round. India. Callula, p. 234.
Tongue long, oval, with a deep groove. New Guinea. .......... Xenorhina.
Note.–Xenobatrachus ophiodon, New Guinea. Palatine bones, each with two large curved teeth. Otherwise imperfectly known.
Rhinoderma.–Omosternum and precoracoids present. Palate without teeth. Tympanum indistinct. Terminal phalanges simple, and not dilated. Tongue heart-shaped, and free behind. Pupil horizontal. Habitat, Chili.
Rh. darwini, the only species, was discovered by Darwin, during the voyage of the Beagle. Its total length is only 3 cm., or little more than one inch. The shape is grotesque, as the skin is prolonged, beyond the very small triangular mouth, into a false nose, i.e. a nose-shaped projection, while the nostrils remain at their original place. The skin is smooth above, granular on the under parts, and forms a triangular flap or spur-shaped appendage on the heel. A glandular fold extends along the sides of the body. The general colour is brown above, black below, with large white patches, the latter colour being sometimes predominant on the throat and chest. The male has a pair of internal vocal sacs, and the use of these as nurseries for the young has made this species famous.
Espada[99] has given an elaborate account of this species, which lives on the ground in shady woods. Its voice sounds like a little bell, and before taking its short jumps, it erects itself vertically upon the hind-limbs. The gular sac of the male opens by two slits, one on each side of the tongue. Generally this sac does not extend beyond the middle of the chest, but during the breeding time the eggs are put into it, whereupon it becomes greatly distended, so much so indeed that it reaches back as far as the groins; {229}dorsalwards around the flanks, almost to the vertebral diapophyses; ventrally and forwards it reaches the chin. The walls of the sac are of the same structure as the buccal lining, of which they are in fact continuations. They adhere, at intervals, to the cutis and to the pectoral and abdominal muscles.
The effect of the distension of the sac upon neighbouring organs is twofold. First, the viscera are pressed back within the abdomen; this disturbance is temporary and does not apply to all specimens; the feeding in no way impeded. Secondly, a permanent change is produced in the direction of the precoracoid bars, in such a way that each bar is curved tailwards and rests with its ventral half upon the coracoid; owing to this forcible bending the clavicles do not meet each other. There is, of course, not so much space gained by this slight rearrangement of the shoulder-girdle as Espada implies, but we have here, perhaps, an illustration of direct correlation between two originally independent organs, namely, shoulder-girdle and vocal sacs. Repeated distension of the throat-bag during every breeding season, while the whole organisation of the male is in a highly excitable condition, has pressed the clavicular bars back, or rather has staved them in, and this at first pathological and abnormal condition has at last become a fixed feature. It is to be regretted that we know next to nothing about the habits, especially the mode of breeding, of the other genera which likewise have reflected or very feeble precoracoids and clavicles. Their weakness or even complete absence must have a reason, or rather must have had a cause.
The pairing and oviposition, and the manner in which the eggs are conveyed into the gular sac, have not yet been observed. Espada examined five males with young, the number of which varied from five to fifteen. In one male with eleven embryos the most developed tadpoles measured 13.5 mm. from the snout to the end of the tail, and they were lying within the chest of the father, the less advanced in the farther recesses of the bag. Three of the tadpoles had already completely-formed fore- and hind-limbs, while the arms were still hidden. The least developed were still globular, a proof that the eggs are conveyed into the bag. Another male with fifteen embryos looked as if it had gorged itself with the almost fully-formed tadpoles, which measured 14 mm. They were quite irregularly distributed, and nowhere {230}attached to the walls of the bag. None of them had horny jaw-armaments, and not even the smallest specimens showed any traces of gills, resembling in this latter character those in the female brood-pouch of Nototrema. The intestine of the tadpoles is short and thick, coiled up spirally and filled with yolk, certainly not with vegetable or other foreign matter. Consequently the entire development from the egg to the complete stump-tailed little creature is undergone within the pouch; and this, after the young have escaped, probably shrinks back to its original size and acts as a gular vocal sac.
Phryniscus.–About ten species of this tropical American genus are known; they extend from Costa Rica to Buenos Aires. They differ not inconsiderably in various details. The tongue is elliptical, entire, and free behind. The palate is smooth. The tympanic disc is absent. Fingers and toes more or less webbed, sometimes with swollen tips, without, however, forming adhesive discs. In a few species the first toe is quite indistinct. The male has a subgular vocal sac. The mouth is small, and there is a short snout. The general appearance varies much. Ph. nigricans of Uruguay, etc., is stout and has very short hind-limbs; the skin of the upper parts is black, spotted with white, and covered with warts. Most of the other species are slender, with larger hind-limbs and a perfectly smooth skin, the coloration of which ranges from dull uniform brown, or black with crimson markings, to bright green with purple spots. The under parts are, as a rule, conspicuously coloured, a rare feature in Anura, the favourite colours being orange, yellow, or even crimson, with or without black patches.
Phryniscus nigricans has been observed in Paraguay by Budgett,[100] who gives the following account. This is a brilliantly coloured frog of toad-like appearance, and about 33 mm. in length. The ground-colour is black, with yellow spots or patches on the upper parts, the under parts are black, with scarlet blotches, the palms of the hands and soles of the feet are scarlet. At the breeding season both sexes utter a call-note which consists of two clear musical "rings," followed by a long descending "trill," like that of our British Greenfinch. This frog, which at ordinary times is the slowest and boldest of frogs, is now active and excessively shy. Swimming rapidly between the blades of grass, it climbs a {231}tuft, and dilating its throat, repeats its call; but if in the least disturbed, it is suddenly gone. The eggs are laid in quite temporary pools in grassy ground, and form separate globules of jelly, which float on the surface of the water, and are heavily pigmented. The development is excessively rapid. The segmentation beginning at 10 A.M., they were hatched and wriggling about by 7 A.M. the following day. They are probably washed down into deeper pools by the retreating waters, and for this purpose the manner in which the eggs are laid, namely, in separate globules of jelly, seems especially suited.
Brachycephalus ephippium in Brazil, the only species, is remarkable for the development of a broad dorsal shield of bone, which is fused with the processes of the second to seventh vertebrae, an ossification which strongly resembles that of several species of the likewise Brazilian Ceratophrys, a genus of the Cystignathinae.
Stereocyclops is remarkable for the peculiar formation and protection of the eyeballs. The anterior portion of the sclerotic is ossified into a ring, which surrounds the transparent cornea. Another peculiarity lies in the metasternum, which is so much broadened out that its cartilage is in wide contact with the posterior edge of the coracoids. The epidermis is everywhere "thickened by a chitin-like deposit." The only species, S. incrassatus, found near Rio Janeiro, is an altogether aberrant creature. Its general appearance recalls that of Pipa. The gape is large, with a slightly projecting muzzle; the limbs are so short that the upper arms and the thighs scarcely stand out from the broadened and flattened body, which is leathery brown, with a narrow white median line extending dorsally from the nose to the vent.
Engystoma, with about five species in the Southern States, Central and South America, is the type-genus of the whole family, chiefly on account of priority of name. It is fairly characteristic in so far as the mouth forms a narrow, somewhat projecting snout; the precoracoids, the clavicles, and the omosternum are absent, the palate is devoid of teeth, the lining of the mouth forms a dermal ridge across the palate and another in front of the oesophagus, the tympanum is hidden, the sacral diapophyses are moderately dilated, and the tongue is elliptical and free behind. The pupil is vertical. The fingers and toes are free, ending in slightly dilated or blunt tips; the terminal phalanges are simple {232}and the hind-limbs are short. The male has a subgular vocal sac.
The most northern species is E. carolinense, living in the Southern United States, concealed under the bark of fallen trees or in old fences. The skin is smooth, but forms a fold across the head, behind the eyes. The general colour is brown, with light, whitish dots on the under parts. Total length 1 inch.
Breviceps is a South African genus with three species. The coracoids are very strong and directed backwards, but so broadened that they form a long and strong symphysis, touching in front that of the precoracoids, which stand transversely and are well developed. The metasternum is cartilaginous and decidedly small. The sacral vertebra has much dilated diapophyses and is co-ossified with the coccyx. The general appearance is extremely stout and short, the head being almost drawn into the nearly globular body, and ending in a short snout with a small mouth-opening. The tongue is long and oval, not nicked, but slightly free behind. B. mossambicus is about 2 inches long, and looks like an overstuffed round bag, out of which the short arms and legs project from the elbows and knee-joints only. The tarsus is provided with a strong horny, spade-like tubercle, which enables the creature to dig into the ground, and into the nests of termites, which seem to be its chief food. Peters found this species in enormous numbers, during the tropical rains, coming out of the ground, whither they withdraw again completely for the dry season. The skin is smooth, reddish brown above, with darker patches; the under parts are dull white, with a large black patch on the throat.
Hemisus is another African genus, with two species, H. guttatum in Natal, and H. sudanense in East and West Africa. This genus is so exceptional in its shoulder-girdle, that Cope separated it from all the other Anura as a special sub-order Gastrechmia. The precoracoids are extremely strong, and form a broad symphysis from which springs the long cartilaginous omosternum; the coracoids are slender, very long, and converge backwards to a narrow symphysis, and there is no metasternum. The two symphyses are connected by a narrow cartilaginous median bar, probably produced by the much modified epicoracoid cartilages. However, except for the reverse development shown by the omo- and meta-sternum, it is easy to connect this apparently quite anomalous shoulder-girdle of Hemisus with that of Breviceps. {233}(cf. Fig. 5, 5 and 6, p. 25). The sacral diapophyses are slightly dilated; the fingers and toes are free and end in points. The tongue is triangular, broader in front. The lining of the mouth forms a transverse ridge across the palate, and another in front of the oesophagus. The male has a subgular sac. The general shape is stout, the head small and ending in a pointed snout. Colour brown above, with whitish spots. Total length about 2 inches.
Glyphoglossus has a peculiar tongue. It is elongated, notched behind and in front, divided into two lateral halves by a deep groove; moreover, the tongue is not only extensively free behind, but also slightly so in front. The skin of the palate forms a transverse serrated ridge. The precoracoids and the omosternum are absent; the metasternum is a well-developed cartilaginous plate. The sacral diapophyses are moderately dilated; the terminal phalanges are simple. G. molossus, the only species, is olive-brown above, marbled on the sides; the under parts are uniformly whitish. This creature, about 2 inches in length, looks like a roundish bag, with a ridiculous, short face. The type-specimen, still the only one known, was taken by Dr Theobald under the following circumstances:–"I had halted one day within the tidal portion of the Irawaddy delta, to enable my boatmen to prepare their dinner. One of my servants, having cooked his rice, poured out the hot water as usual on the ground, and some of it went down a hole that happened to be near the spot. No sooner, however, had the hot water disappeared than out scrambled in great haste a fine Glyphoglossus, only, alas! to be transferred to a collecting jar."
Phrynella.–The tongue is heart-shaped, free behind. The palate is smooth and toothless. The fingers and toes end in small discs, supported by T-shaped phalanges; the fingers are free, the toes extensively webbed. Precoracoids absent; metasternum cartilaginous. Pupil horizontal. Malay Peninsula.
Ph. pollicaris is dark olive brown above; an oblique yellow line runs from the eye to the angle of the mouth; a pale yellow mark, across the forehead, through the eyes, and down the sides of the body. A dark-centred yellow patch on the anal region. The limbs are banded yellow and brown. The under parts are brown, with paler specks, dark on the throat. Iris red brown. The whole coloration changes considerably.
"They inhabit the hills of Perak from 3000 feet upwards, {234}and live in holes in trees, which are so situated as to contain more or less rain-water. They have a loud flute-like, musical note, which they utter at irregular intervals, principally during the night. The form and size of the hole in which they are seem to have a great deal to do with the loudness of the note, as specimens when extracted from their holes have far more feeble vocal powers than they had when in them. These frogs blow themselves out with air, and look more like bladders than anything else. When inflated they float on the surface of the water, and will remain motionless for a long time, with legs and arms stretched out."[101]
Callula.–The tongue is round, entire, and free behind. The palatine bones form an acute, sometimes toothed ridge across the palate; two dermal serrated ridges in front of the oesophagus. Fingers free, sometimes with dilated tips, supported by T-shaped phalanges. Precoracoids and omosternum absent; metasternum cartilaginous. Pupil round. About seven species in the Indian region.
C. pulchra.–The following account has been extracted from Mr. S. S. Flower's observations:[102]–
This pretty creature inhabits most of the warm portions of the continental Indian region, from India and Ceylon to South China and Malacca. The back is a rich dark brown, divided from the yellow of the head by a narrow black line which extends from eye to eye and forwards to each nostril. A conspicuous yellow band runs from the eyes to the hind-limbs. The sides of the body and the limbs are mottled yellow and brown. The under parts are dirty buff; the throat of the male is black. The intensity of colouring varies individually and from time to time, the contrast between the brown and yellow being occasionally very brilliant. Total length up to 3 inches, the male being the smaller sex.
"I have been told by both English and natives that this frog was unknown in Singapore until some nine or ten years ago, when it was introduced by a half-caste (why, it is not known), and that it rapidly spread about the island. It is now well known as the 'Bullfrog' by the English in Singapore, and detested for the noise it makes at night. The voice of these rotund animals can be heard every night after heavy rain; it is {235}a deep guttural croak, 'wau-auhhhh,' very strident and prolonged. The males croak while floating on the surface of the water, the single vocal sac under the mouth inflated like a globe, and the arms and legs extended. They can hop well on land and are good swimmers. The skin is excessively slimy; the secretion comes off profusely, and dries on the hand into a sort of white gum, with a faint aromatic smell. This gum dissolves in hot water and coagulates in cold. The general appearance of these frogs is very stout, their girth being about twice the length from snout to vent. The tongue, which is oblong in spirit specimens, in life is very elastic, assuming, when extended, a vermiform shape and reaching about 4 cm. in length. They appear after sunset, crawling on old wood and feeding on white ants."
Sub-Fam. 2. Dyscophinae.–With teeth in the upper jaw.
This small group of nine genera, with scarcely more than one dozen species, all with one exception living in Madagascar, has been separated by Boulenger from the Engystomatinae merely on account of the presence of teeth on the upper jaw and on the vomerine margin of the palatine bones. He himself remarks that Calluella may be considered a toothed Hypopachus, and Plethodontohyla a toothed Callula. These are obvious cases of convergent analogy. Except for the teeth, the Indian Calluella would be merged into the American Hypopachus, and this would present an instance of the most puzzling geographical distribution. In the case of the other two genera, one Indian and Malayan, the other Malagasy, no such suspicion would arise, since there are many other instances of such a coincidence of distribution. There is the same divergence or unsettled condition in the modification of various parts in the Dyscophinae as in the Engystomatinae. The precoracoid bars are weak and curved backwards, and closely pressed against the strong coracoids, in Dyscophus, Calluella and Platypelis, while these elements are reduced to unossified bars, and the clavicular portions completely lost, in Plethodontohyla and in Phrynocara. The omosternum is absent and the metasternum is small in all except Dyscophus, in which both these parts are exceptionally well developed and large, although remaining unossified. The palate of Dyscophus and Calluella is provided with curious, serrated dermal folds like those which are so common in the Engystomatinae; and well-developed discs on the fingers and toes, supported by T-shaped phalanges, are {236}possessed by Platypelis, Cophyla and others. The sacral diapophyses are dilated. The pupil is either horizontal or vertical. Those which are provided with discs to the fingers and toes are climbers, and mostly slender and long-legged, sometimes of very small size, for instance Cophyla, the body of which is scarcely one inch in length.
The genera can be determined by means of the following key:–[103]
A. Pupil vertical. Palatine teeth in long transverse series.
a. Precoracoids ossified. Tips of fingers and toes not dilated.
Sternum very large. Madagascar .......... Dyscophus.
Sternum small. Burmah .......... Calluella.
b. Precoracoids not ossified. Tips dilated .......... Plethodontohyla.
B. Pupil horizontal.
a. Palatine teeth in long transverse series.
α. Precoracoids ossified. Tips dilated.
Fingers and toes free. Precoracoids entirely ossified .......... Mantipus.
Fingers and toes webbed at the base. Precoracoids semi-ossified .......... Platyhyla.
β. Precoracoids not ossified. Tips not dilated .......... Phrynocara.
b. Palatine teeth in one or two small groups.
Precoracoids ossified. Tips dilated.
Two small groups of palatine teeth .......... Platypelis.
One single group in the middle of the palate .......... Cophyla.
No teeth on the palate .......... Anodontohyla.
Dyscophus antongili.–Madagascar. General appearance stout, with short legs and a wide mouth. Total length about 3 inches. The skin is mostly smooth, and forms a broad glandular fold which extends from the eye to the groin. The upper parts are beautiful magenta red, with a purplish streak beneath the lateral folds; the under parts are yellowish white, with minute grey specks. Red or pink colours, and the lateral folds, occur also in most of the other members of this family, for instance in the Indian genus Calluella.
Sub-Fam. 3. Genyophryninae.–With very small teeth on the anterior portion of the lower jaw.
Genyophryne thomsoni.–Pupil horizontal. Tongue oblong and entire. With teeth on the palatine bones, and a serrated transverse dermal ridge in front of the oesophagus. Sternum cartilaginous. Precoracoids absent. Sacral diapophyses moderately {237}dilated. Tympanum hidden. Head large and much depressed. Heel with a triangular dermal flap. The smooth skin is pink brown above, with blackish marks; a light line extends on each side from the eye along the back. Under parts black. About 32 mm. in length. Sudest Island, between New Guinea and the Louisiade Archipelago.
Fam. 7. Ranidae.–Frogs, in the true sense, are all well diagnosed as Firmisternia, with cylindrical sacral diapophyses. According to the presence or absence of teeth in the jaws they can be subdivided as follows:–
Sub-Fam. 1. Ceratobatrachinae, with teeth in the upper and in the lower jaws. The sole representative is the genus Ceratobatrachus.
Sub-Fam. 2. Raninae, with teeth in the upper, but none in the lower jaw. These are the Ranidae of Boulenger in the Catalogue of Batrachia Salientia.
Sub-Fam. 3. Dendrobatinae, without teeth in the upper and lower jaws.
Sub-Fam. 1. Ceratobatrachinae.–Teeth present in both jaws. Those of the lower jaw, between 20 and 30 in number in Ceratobatrachus, the only genus, are nearly all inserted upon the articular bone; only 2 or 3 are carried by the dentary element, which, although large, enters into the formation of the upper border of the jaw at the anterior end only. In the small extent of the share of the dentary in the formation of the edge of the lower jaw, and in its anterior "toothlike" process, Rana adspersa of Africa bears unmistakable resemblance to this genus. The tongue is deeply notched, and free behind. Pupil horizontal. Vomers furnished with teeth. Tympanum distinct and large. Precoracoids present. Omosternum and presternum with a bony style. Sacral diapophyses cylindrical. Fingers and toes free, with swollen tips. Outer metatarsals united. Male with two internal vocal sacs.
G. guentheri, Solomon Islands, the only species, has an enormous mouth and a triangular head not much smaller than the rest of the body. The skull is furnished with prominent ridges and a small curved spine at the angle of the jaws. The hind-limbs are rather short. The skin of the upper parts shows linear ridges, variously arranged; that of the belly is granular. A triangular dermal flap on the tip of the muzzle, one on the {238}upper edge of the eyelids, others on the heel and above the vent. The colour and markings are very variable, the ground-colour is yellowish to pink, brown, grey or olive, with darker and lighter markings. Total length of the males 3 inches, of females 3½ inches.–Guppy, the discoverer of this peculiar creature, remarks that "horned Frogs are very numerous in these islands, and so closely do they imitate their surroundings in colour and pattern, that on one occasion I captured one by accidentally placing my hand on it when clasping a tree."
Sub-Fam. 2. Raninae.–The vertebrae are procoelous and devoid of ribs. The precoracoids are always present and ossified from the clavicles, and are parallel with the much stronger and ossified coracoids. The omosternum usually possesses a bony style, but in the Indian genera Nannobatrachus and Nannophrys and in Phyllodromus of Ecuador it remains cartilaginous, and in Colosthetus of Colombia it is absent. The metasternum also possesses a bony style, but it remains cartilaginous in the Indian genera Oxyglossus, Nannophrys, Nannobatrachus and Phyllodromus, in the last two genera rather reduced and slender, while in the Ecuadorian and Colombian genera Hylixalus, Prostherapis and Colosthetus, it is reduced to a membranous piece. In quite a number of genera the normal number of phalanges is increased by one owing to the intercalation of an extra phalanx between the terminal and the otherwise penultimate phalanx.[104] This is the case in all the species of Cassina, Hylambates, Rappia, Megalixalus, Rhacophorus, Chiromantis, Ixalus and Nyctixalus, but it is doubtful if all these genera are thereby more nearly related to each other than to the rest of the Raninae. The structure of the tips of the fingers and toes exhibits more variety. The terminal phalanges are mostly simple, with slight swellings at the ends, or they are Y- or T-shaped in conformity with more or less developed adhesive discs; in the African genus Hylambates only they are claw-shaped, as in the Hylidae.
Gampsosteonyx batesi, recently described by Boulenger from the Gaboon, shows a unique modification of the terminal phalanges of the second to the fifth toes. They are transformed into sharp and curved claws, like those of a cat, but instead {239}of horny sheaths, it is the bone itself which is thus sharpened and perforates the skin, an anomaly reminding us of the ribs of Triton waltli. Total length of the type-specimens, about 3 inches.
Adhesive discs are common, and are best developed in Rhacophorus, Ixalus, Rappia, and Megalixalus. In the Neotropical genera, excepting Colosthetus, the discs are very peculiar, being provided on the upper side with leathery scales which are separated by a fissure. The fourth and fifth metatarsals either diverge and are connected by a distinct web, or they lie close together with only a groove between them, or lastly they appear externally united.
The tympanic disc is very variable, large, small or quite hidden. Vomerine teeth are present or absent. The pupil contracts into a horizontal slit except in some Palaeotropical genera. The tongue is universally free behind, mostly deeply notched, and can be well protruded; only in the Indian Oxyglossus and in the Neotropical genera, excepting Hylixalus, its posterior margin is entire.–There are terrestrial, arboreal, and aquatic members in this large sub-family. The geographical distribution of the Raninae, which comprise about twenty genera with at least some 270 species, is almost entirely Arctogaean. None, with the exception of three species in the Papuan subregion, occur in the Australian region; and only four genera, with one or two species each, inhabit the tropical Andesian district, the {240}remainder of South America being without any Raninae. All the species of the whole Periarctic region belong to the genus Rana except in Eastern Asia, where the closely allied genus Rhacophorus occurs also. The entire sub-family of Raninae is, in its fulness and diversity of development, essentially Palaeotropical.
Many of the genera, even in the present more liberal sense as interpreted by Boulenger, are based upon unimportant characters, and in reality run into each other. This is for instance the case with Rana and Rhacophorus.
The following tabular arrangement is merely a key for determination and does not necessarily express relationships. The presence or absence of vomerine teeth is a character easily ascertained, but it separates closely allied genera, for instance, Rhacophorus from Ixalus and Micrixalus from Rana.
The genera with extra, interpolated phalanges are marked *.
Key for the Determination of the genera of Raninae.
I. Pupil vertical.
A. With vomerine teeth.
a. Omosternum very slender and cartilaginous. Small discs. India and Ceylon, 3 species .......... Nannobatrachus.
b. Omosternum with a bony style.
α. Outer metatarsals webbed. Small discs. South India, 2 species .......... Nyctibatrachus.
β. Outer metatarsals close together. Africa.
Fingers and toes with interpolated phalanges.
Without terminal discs. 2 species .......... Cassina.*
With discs supported by claw-shaped phalanges, 10 species .......... Hylambates.*
Fingers and toes without interpolated phalanges; without discs.
B. Without vomerine teeth. Discs well developed. Outer metatarsals united. Tropical Africa and Madagascar, 7 species .......... Megalixalus.*
II. Pupil horizontal.
A. With vomerine teeth.
a. Outer metatarsals webbed together.
Fingers free, toes webbed .......... Rana, p. 249.
Fingers and toes more or less webbed. Always with discs .......... Rhacophorus,* p. 245.
Two fingers opposed to the others. Africa .......... Chiromantis,* p. 244.
b. Outer metatarsals united, or separated by a groove only.
Omo- and meta-sternum with a bony style .......... Cornufer, p. 243.
Omo- and meta-sternum slender and cartilaginous.
Ceylon, 2 species .......... Nannophrys.
Mozambique .......... Phrynopsis boulengeri.
B. Without vomerine teeth.
a. Palaeotropical.
α. Tongue narrow and entire. No discs. Outer metatarsals webbed. India, 3 species .......... Oxyglossus.
β. Tongue oval, feebly nicked. Large discs. Solomon Islands .......... Batrachylodes vertebralis.
Karin Hills .......... Phrynoderma asperum.
γ. Tongue deeply notched. Outer metatarsals united by a web.
Discs none or very small.
Africa, 3 species .......... Phrynobatrachus.
Borneo .......... Oreobatrachus baluensis.
With regular discs.
Number of phalanges normal. India, 5 species .......... Micrixalus.
With an extra, interpolated phalanx. India, 18 species .......... Ixalus.*
Two fingers opposed to the others. Karin Hills .......... Chirixalus doriae.*
δ. Tongue heart-shaped. Outer metatarsals united.
Fingers and toes free, tips blunt. Africa, 8 species .......... Arthroleptis, p. 242.
Fingers and toes more or less webbed, with regular discs. Africa and Madagascar, 23 species .......... Rappia.*
b. Neotropical. Metasternum small, cartilaginous or membranous. With discs.
1. With a pair of dermal scales on the discs. Omosternum with a bony style.
Tongue heart-shaped. Ecuador, 2 species .......... Hylixalus.
Toes free. 5 species .......... Phyllobates, p. 242.
Tongue entire. Ecuador and Colombia, 3 species .......... Prostherapis.
Omosternum cartilaginous. Ecuador .......... Phyllodromus pulchellus.
2. Discs without scales. Omosternum absent. Colombia .......... Colosthetus latinasus.
Phyllobates.[105]–This is one of the few Neotropical genera, and like nearly all of these has peculiar adhesive discs on the fingers and toes, each disc bearing on its upper surface two dermal scales. The tympanum is distinct. Vomerine teeth are absent. The general appearance of the five species is that of tree-frogs. One species, Ph. bicolor, yellowish above, dark brown beneath, lives in Cuba. The others inhabit Central America and Venezuela. They seem to have peculiar nursing habits. Ph. trinitatis of Venezuela and Trinidad carries its tadpoles on its back, on to which the young fix themselves by means of their suckers. Nothing is known about their breeding habits, for instance whether the young are hatched on the back, or, as seems more likely, if the parents (the specimen described by Boulenger[106] is a male) only give their offspring a temporary lift in order to convey them from a drying-up pool to a healthier place. It is remarkable that several species of Dendrobatinae, which inhabit the same countries, have precisely the same habits.[107]
Arthroleptis.–Slender and long-limbed little frogs, about one inch in length. The fingers and toes are free, very slender, and end in slightly dilated tips, the supporting phalanges being simple. The tympanum is variable. The skin is smooth or finely granulated. The colours are inconspicuous, brown or grey tones usually prevailing. About ten species are known, mostly {243}from Continental Africa, a few from Madagascar and the islands in the Indian Ocean.
A. seychellensis.–Brauer[108] has discovered the mode of nursing of this frog. He found a specimen of A. seychellensis which carried nine tadpoles on its back, in the month of August, in the Seychelles, about 1500 feet above sea-level, upon an old tree-fern. The little ones were already provided with long tails, the hind-limbs were partly free, the fore-limbs still covered by the skin, and they held on by their bellies; not, like the young of Phyllobates, by their "suckers." Another specimen carried young which were still further developed. He also found an old frog, near which was lying a little heap of eggs, not enveloped in a common mass of jelly. The old frog escaped, but the eggs were taken care of in a vessel with moist sand at the bottom. By the following morning the eggs were hatched and the tadpoles were clinging by their bellies on to the walls of the glass. Brauer concludes that the young, when hatched, creep on to the parent's back, he or she waiting near the heap of eggs until the latter are ready. Curiously enough, he did not find out the sex of the nurse, nor are we told if the young are taken to the nearest water to finish their metamorphosis, or if they remain upon the parent's back until they hop off as baby-frogs. The yolk is very large. When the four limbs are already developed, the gill-cavity possesses no gills and no outer opening; and since the lungs are only just beginning to sprout, the tadpole must needs breathe by means of its skin. The jaws have no horny coverings. The adults live on the ground between moist leaves, and eat chiefly termites.
Cornufer, with about twelve species, is an essentially Austro-Malayan and Polynesian genus, but one species, C. johnstoni, has been found in the Cameroons. The fingers and toes are free, and their T-shaped phalanges support adhesive discs. The tympanum is distinct. The general shape is frog-like, usually with slender and very long hind-limbs and toes, the discs of the latter being much smaller than those of the fingers. The coloration is dull, mostly brown, more or less marbled, whitish below. The {244}upper eyelid of some species, e.g. of G. unicolor of New Guinea, has a small tubercle, hence the generic name. The skin of the back is glandular and granular, forming slight folds on the back and on the sides of the head in some species. The male has one or two internal vocal sacs.
C. corrugatus is one of the most widely distributed species, inhabiting the Philippines, New Guinea, and Duke of York Island. The granular skin forms longitudinal folds on the back, one of which reaches from the eye to the shoulder. Brownish above with darker markings, below yellowish, with or without brown spots on the throat.–Three species inhabit the Fiji Islands.
Of C. solomonis of the Solomon Islands little is known about the propagation, although the large size of the egg, which measures 5 mm. in diameter, suggests that the young undergo most or the whole of their metamorphosis within the egg.
Chiromantis is distinguished by the peculiar arrangement of the fingers, the first and second being opposed to the others; their terminal phalanges are obtuse and support small knobs or discs. The general shape is that of a frog with long and slender hind-limbs. The tympanum is distinct.
Ch. xerampelina, the type-species, was discovered by Peters at Mozambique; it is a middle-sized frog, about 2 inches in length, brown above with reddish spots on the sides; the male is devoid of vocal sacs.
Ch. petersi, a native of East Africa, differs from the preceding by the possession of an internal vocal sac. Ch. rufescens = guineensis shows very little of the typical grasping arrangement of the fingers; the two inner ones are separated from the two outer fingers by a wide gap, but they all lie in the same plane, are much webbed and possess large discs, so that by the latter two characters a link is formed with Rhacophorus, to which the present genus is closely allied. Total length about 2½ inches.
Buchholz[109] has observed the peculiar breeding habits of this rather large, brown, and slender tree-frog in the Cameroons. In the month of June he found on the leaves of a low tree, standing in the water, a white foamy mass, like the froth of a broken egg, containing a number of newly hatched larvae and quite transparent eggs. Within three or four days this mass became {245}fluid, and the larvae, provided with external gills and a long tail, swam about in the slime. In the natural course of events the larvae are probably washed down into the water by the rain. He found that the female deposits the eggs in the foamy mass at night, during the months of June and July, on various kinds of trees, either between the roots or in a cavity formed by gluing together several leaves, sometimes 10 feet and more above the water, or near the margin. On one occasion the mother was seen sitting upon the foamy mass, clasping the same with its four limbs.
Rhacophorus.–This large genus, containing more than forty species, has a curious distribution. At least one dozen species are found in Madagascar, eight or nine in Ceylon, the rest in Southern India, the Himalayas, the Malay Islands and Philippines, extending northwards through China and Southern Japan. Therefore this genus, with the three species of the African Chiromantis, extends over the whole of the Palaeotropical region. The generic name has reference to the possession by many species of little dermal flaps, especially at the inner side of the heel, and it has nothing to do with the parachute-like use of the hands and feet of certain species, to be mentioned presently.
The terminal phalanges are generally bifurcated, rarely obtuse, and support well-developed adhesive discs. The fingers and toes are webbed to a variable extent. The two outer metatarsals are likewise connected by a web. The tympanum is distinct. The general appearance is that of tree-frogs, and many of them are green. The males have one or two internal vocal sacs. Not all the species have dermal appendages. Rh. maximus, for instance, the largest of all, living in the Himalayan forests, has none. A heel-flap occurs in some half-dozen Indian species; and Rh. madagascariensis has these flaps on the heels and on the elbows. Some have queer little lappets above the vent, or on the edges of the arms and legs; in others the bend of the arm is fringed. The small size of these appendages, in comparison with the webs and discs, makes them practically useless so far as increase of surface is concerned, and they have most likely some other, although unknown meaning, especially the flaps over the vent. Lastly, in the majority of species the fingers are not more than half-webbed, or even less, and in a few only, the webs reach down to the discs.
Several species of this genus are remarkable for two reasons. First, the great enlargement of the fully-webbed hands and feet, which are then used as parachutes; secondly, the mode of propagation.
Greatly exaggerated notions are, however, entertained about the parachutes, ever since Wallace's description[110] of the first "flying frog." The creature was brought to him in Borneo by a Chinese workman. "He assured me that he had seen it come down, in a slanting direction, from a high tree, as if it flew.... The body was about four inches long, while the webs of each hind-foot, when fully expanded, covered a surface of four square inches, and the webs of all the feet together about twelve square inches."
The species in question is Rh. pardalis, an inhabitant of Borneo and of the Philippine Islands. Specimens from Wallace's Collection are in the National Collection and the largest specimen {247}shows the following measurements. Total length 6.5 cm. or 2½ inches, not 4 inches.
| Area covered by one fully-expanded hand | 3.4 square cm. |
| Area"covered by"one fully-e"panded foot | 6.0 sq"are c" |
| --- | |
| 9.4 square cm. |
i.e. for the four limbs 18.8 square cm. = about 3 square inches, and not 78 square cm. or 12 square inches. By some unfortunate oversight Wallace must have mixed up the total expanded area with that of the four hands and feet! In Brehm's Thierleben the 78 square cm. have increased to 81 cm., and the artist has in the somewhat larger species Rh. reinwardti improved upon this, and has produced a truly startling picture by a further exaggeration based upon the figure given by Wallace.
Rh. reinwardti lives in the forests of the mountains of Java and Sumatra. It reaches 3 inches in length, and is grass-green above, yellow below. Younger specimens are further adorned with large blue patches on the webs of the hands and feet and behind the armpits. Besides the flap on the heel and the curious cutaneous fringe on the forearm, suggestive of an incipient flying-membrane, the skin forms a projecting fringe on the inner side of the fifth toe and a transverse flap above the vent.
Of Rh. leucomystax, Annandale, who accompanied the Skeat Expedition to Malacca, gives the following account:–"This frog, which is called by the Malays of Lower Siam either 'Berkata Pisang' (banana-frog) or 'Berkata Rhumah' (house-frog), lays its eggs either on leaves of branches overhanging the water, or on the mud surrounding buffalo-wallows. The ova are enclosed in a round mass of yellow froth, which afterwards becomes steel-grey, about as large as a cricket-ball. Should they be placed judiciously in a position sheltered from the sun, the tadpoles may either hatch, and reach a considerable degree of development, before the mass is washed into the water, or the froth may be melted almost as soon as it is formed and the eggs be carried into a pool by a shower of rain. Very often, however, the whole mass is dried up by the heat of the sun before the rain comes. During the breeding season, which seems to occur as often as the land is flooded under the trees, for I have never seen the eggs of this frog on the bank of a river, the {248}males croak loudly, producing a sound which can hardly be distinguished from the chattering of the large black and yellow squirrel, Sciurus bicolor."
These arboreal frogs have a peculiar mode of nursing the young and taking care of the eggs. Rh. maculatus of Ceylon, Malacca, etc., and Rh. schlegeli of Japan, lay their eggs in a foamy mass, the size of a fist, on the margins of ponds, and the whole process has recently been described by Ikeda.[111] He observed the Japanese Rh. schlegeli depositing the eggs in soft, muddy ground covered with grass, and in wet, muddy banks of paddy-fields, ponds, and similar localities near Tokyo. Sometimes they are deposited between the leaves of trees, near the ground. The breeding season extends from the middle of April to the middle of May. Towards the evening the female, bearing the much smaller male on her back, retires underground for the deposition of the eggs. The spots chosen are 10-15 cm. above the surface of the water; the female digs a spherical hole 6-9 cm. wide. Sitting thus concealed underground, the frogs assume a dark colour and the spawning takes place during the night, whereupon the parents leave the nest. The eggs are enveloped in a white mass of jelly full of air-bubbles, the whole frothy lump looking like the well-beaten white of a hen's egg, with the yellowish eggs scattered through it, and measuring some 6 cm. in diameter. The air-bubbles are 2-3 mm. large. The froth is originally very elastic and sticky, but it gradually sinks down, becomes liquid and ultimately runs out of the hole. It is produced in the following peculiar manner. During and after the deposition of the eggs the female puts her feet upon the sticky jelly, part of which adheres and is then pulled out as a thin, transparent membrane stretching between both feet. The latter are then thrust backwards, the membrane is folded downwards and becomes a vesicle of 5 to 10 mm. in width. By repeated working of the limbs the successively formed bubbles are trodden and kneaded into froth, which ultimately surrounds and at the same time separates the eggs.
The female of Rh. reticulatus of Ceylon attaches the eggs, about twenty in number, to the under surface of her belly, on the skin of which they leave little cellular impressions. What becomes of the tadpoles is not known.
Rh. leucomystax is found in the Malay Archipelago, Farther India, and the Philippine Islands.
S. S. Flower[112] found the tadpoles about Singapore, from January to April, in small ponds and in rain-water butts. The spiracle lies on the left side, directed backwards and upwards, nearer the anus than the end of the snout. The anus opens on the right side. Exceptionally large tadpoles measured 46 mm. in total length, the recently transformed young only 14-18 mm.
"A cheerful little frog of most graceful build. It comes out from its hiding-places shortly before sunset, and remains abroad all night. The males are easily found as they sit on shrubs or trees, or on the edges of the rain-water butts under the verandahs of the houses, and from time to time utter a single, rather musical, short croak. In March and April they can be found both by day and night in embrace, in the ponds. This species changes both its colour and markings very rapidly and frequently, but dark bands across the legs can always be more or less distinguished; the lower parts are some shade or other of buff, but the principal variations of the upper part are as follows: pale bronze, either uniform or with four longitudinal dark-brown or black lines; uniform, almost orange, bright bronze; chocolate, with darker mottling; pale brownish green or olive, with irregular dark spots; yellowish green, mottled with darker or brown." The females are considerably larger than the males; the largest male caught was 48 mm. from snout to vent, and the largest female 68 mm.
Rana.–The following combination of characters should be a sufficient diagnosis: pupil horizontal; tongue deeply notched and free behind; vomers with teeth; fingers free, toes webbed, fourth and fifth metatarsals diverging and webbed together.
In conformity with the great number of species and the wide distribution of this genus some of the organs vary considerably, indeed so much so that many of these modifications have been deemed sufficient to be of generic importance. Fortunately the species are so numerous that these characters mostly form an uninterrupted series from one extreme to the other.
The terminal phalanges are mostly simple and pointed; sometimes transversely dilated or T-shaped, according to the presence of more or less developed discs. Such discs are, for {250}instance, present in the Malay species R. erythraea and R. chalconota and in the Indian R. corrugata. The tympanum occurs in every stage from a conspicuous, free disc to being quite hidden by the skin. The vomerine teeth either form a pair of tiny, mostly transverse rows, between the choanae, or they are arranged in two oblique series which extend beyond the hinder edges of the choanae.
The vocal sacs vary greatly. Many species, e.g. R. agilis, have none at all. Most species have a pair of internal sacs, and in comparatively few, about a dozen, these sacs have become external, a feature which indicates no relationship of the species thus distinguished, for instance the European R. esculenta, the Japanese R. rugosa, the Indian R. hexadactyla, R. cyanophlyctis and R. Chloronota, the Bornean R. glandulosa, the African R. oxyrhynchus and R. mascareniensis, the Mexican R. montezumae. In R. esculenta, and perhaps in a few others, even the female has some traces of these otherwise male organs, indicated by slit-like folds of the outer skin below the angles of the lower jaw.
Nuptial excrescences on the inner metacarpal tubercle and on the inner fingers of the male are common; they reach their greatest development in the Himalayan R. liebigi, the male of which is "remarkable for the extreme thickness of its arms, the inner sides of which are studded with small conical black spines, each supported on a rounded base produced by a swelling of the skin. A large patch of similar spines exists on each side of the breast."[113]
Specific glandular complexes in the skin are mostly restricted to a pair of lateral or dorso-lateral folds; they are often absent, but a few species, e.g. R. glandulosa of Borneo, R. temporalis of Ceylon, R. elegans and R. albolabris of West Africa, have a pair of large flat glands at the base or inner side of the arms.
All the species of Rana, except those in the Solomon Islands, spawn in the water, where the development of the tadpoles takes its course. Those of some Indian species, notably R. alticola and R. afghana of the Himalayas, and R. curtipes of Malabar, are very peculiar, being provided on either side of the shoulders with a large oval parotoid-like gland, well defined and crowded with pores; R. alticola possesses in addition an unpaired, sharply {251}marked glandular complex on the top of the root of the tail, or rather upon the future coccyx. These complexes gradually disappear with age.
The genus Rana, with about 140 species and subspecies, is distributed over the whole of Arctogaea so far as this is available for Amphibian life, while there are only a few stragglers in Notogaea, namely, a few species in Ecuador and in the Peruvian or Upper Amazon district. None exist in the rest of the Neotropical region, including the Antilles, and practically none in Australia; but R. arfaki and R. papua inhabit New Guinea and the northern corner of Australia, R. kreffti the Solomon Islands. A few species are restricted to Madagascar, and a few others live there and on the continent of Africa.
So far as number of species is concerned, the home of the genus Rana is the Palaeotropical region; about one dozen (some of them with a very wide range) live in the Palaearctic sub-region, scarcely more in the Nearctic sub-region, and a few in Central America.
R. temporaria (the common European Brown Frog or Grass-frog).–The tympanum is distinct, two-thirds the diameter of the eye in size. The first finger is slightly longer than the second, which is shorter and weaker than the others, whilst the fourth is the longest. All the fingers are quite free. When the hind-limbs are laid forwards along the body, the ankle-joint reaches to a point between the eye and the tip of the snout. The five toes, which are about half webbed, increase in length from the first to the fourth, while the fifth is about equal to the third. The sole of the foot has a small, blunt, inner metatarsal tubercle; the outer one is scarcely visible. The skin is smooth, always moist, owing to the minute mucous glands; but a series of larger glands forms a pair of folds along the upper sides of the back; beginning behind the eyes they converge slightly beyond the shoulders, diverge a little in the sacral region, and converge again towards the vent. Another, much feebler, Λ-shaped ridge lies between the shoulders.
The male has two internal vocal sacs, which, when in use, bulge out the skin of the throat beneath the angles of the mouth like a pair of globes. It is further distinguished from the female by the stronger muscles of the arms and by a pair of swollen pads on the inner side of the first finger. During the pairing {252}season these pads are enlarged into cushions covered with black horny rugosities.
The iris is golden, with dark specks. The coloration is, generally speaking, brown above, with black-brown irregular spots, especially on the sides of the body, and with cross-bands on the legs. The under parts of the male are white or pale yellow, with a bluish tinge on the throat, while the female is more yellow instead of white, inclining to orange. In both sexes the under parts are mostly spotted with darker colours. A large dark-brown patch, extending from behind the eye over the tympanum towards the shoulder, is always present and has given this frog its specific name. Otherwise the coloration varies considerably; more or less according to the locality and nature of the surroundings, and to individual variation and temporary change of colour.
Some specimens are almost spotless above and of a rich brown, or almost yellow colour, the spots being restricted to the sides below the lateral folds. Others have very few spots, but these are then arranged in two interrupted streaks on the back. The under parts, especially the flanks, may be lemon yellow instead of whitish, and the darker markings may be almost absent. Boulenger has figured a beautiful specimen, almost orange red, with red spots and vermiculations on the yellow under surface. I have found similar red specimens of unusually striking appearance between Berlin and Spandau in a forest-glade, through which run little streams with banks of red ferruginous soil. Specimens which live in woods with rich black soil are often very dark, all the brown and reddish tints being absent. The variations are, however, really endless, and it is difficult to find two individuals exactly alike, even amongst a great number collected in the same locality. Moreover, they change colour. Warmth makes them paler, cold causes the chromatophores to expand and the whole frog appears darker. During the breeding season the males assume a delicate bluish hue, especially on the throat, but this film quickly fades away when they are taken out of the water. It is caused by the swelling of the cutaneous lymph-spaces which extend their ramifications into the epidermal layer, and it is not a question of pigmentation or of chromatophores, but a case of interference-colours, blue being frequently the result of the light passing through a cloudy, colourless, but not {253}quite transparent and thin stratum, in this case the turgid epidermis.
The habits of the Grass-frog are essentially terrestrial. It spends most of its time on land, preferably in damp places, but local fashion permits of a great deal of freedom, as these frogs are sometimes found not only in very wet, naturally irrigated places, but also in the water itself. However, the Grass-frog when pursued rarely takes to the water for safety. It trusts to flight, first by a few long and fast jumps, and then to concealment by squatting down between grass, under leaves; it rarely creeps into a hole, even if there be one near. The jumps soon become shorter and shorter after a few dozen repetitions. It swims well, but cannot climb. The food, which consists chiefly of insects, snails, and worms, must be moving to excite interest; then the frog, whose favourite position is half squatting, half supported by the arms, erects itself, and, facing the insect, turns round upon its haunches, adjusts its position anew by a shifting of the legs, and betrays its mental agitation by a few rapid movements of the throat. All this time the prey is watched intently until it moves; then there follows a jump, a flap of the tongue and the insect is seen no more. As a rule these frogs do not crawl, they jump or hop, even whilst stalking, and this takes place at any time of the day; in fact they are very diurnal, although they become more active towards the evening. When caught they are at first very wild and, like all true frogs, very impetuous, committing acts of astonishing stupidity without any apparent sense or appreciation of distance or height. The captive will not only jump off the table, whilst a toad stops at the edge and looks carefully down, but without hesitation he jumps out of the window, regardless of the height above the ground. This is due to sheer fright; he loses his head. When at large in his native surroundings, nothing will induce him, although hotly pursued, to commit suicide by jumping down a precipice. But all this wildness calms down wonderfully soon. The captive no longer dashes his head against the glass, he does not struggle or twist when taken up; on the contrary, he makes himself at home, watches your coming with intense expectation, and without hesitation accepts the proffered mealworm, maggot, butterfly or earthworm; in short, he shows what a jolly and intelligent fellow he really is.
The Grass-frog has many more obvious enemies than perhaps any other Amphibian, and it is not even slightly protected by any appreciable poisonous secretion. Nevertheless it is extremely common. A whole host of birds eat it–for instance, buzzards, harriers, and above all storks. Foxes, polecats, and stoats are not averse to it, and the Grass-snake derives its main sustenance from it. In fact the enemies of the little frog are legion, one of the worst being Man. In France, Italy, and other parts of the Continent, the skinned fleshy hind-limbs are turned into a by no means disagreeable ragoût, or into dainty morsels when fried in butter and encrusted with bread-crumbs. This frog, together with its cousin the Water-frog, also suffers from the distinction of being one of the chief martyrs to science. Perhaps the story is true that Galvani was led to his investigations into animal magnetism and electricity by observing that the legs of a number of skinned frogs, strung up by his wife upon the bronze railings of the balcony, jumped whenever the scissors, which cut off the feet, touched the other metal. Frogs have suffered ever since. Easily procured and of a convenient size, they are used in every biological laboratory, and the young student is supposed to be initiated into the mysteries of Vertebrate structure by the careful dissection and study of this, the worst of all the so-called types. Next to Man there is no animal which has been studied so minutely, and has had so many primers and text-books written on it, as this frog. In spite of all this it is very little understood, thanks to its rather aberrant and far from generalised structure.
However, the frog, by reason of its fertility, holds its own. Early in the year, sometimes while there is still ice and snow, the frogs leave their hibernating places (mostly holes in the ground, under moss, or in the mud), and they begin to pair in standing or slowly flowing, mostly shallow, waters.
They are not always very careful in the selection of the spawning locality, many of them lay their eggs in a ditch, or even in the shallowest puddle, which is sure to dry up, and thus to cause the destruction of the whole brood. This carelessness is all the more surprising when there are large pools or lakes in the immediate vicinity, perhaps only one hundred yards to the other side of the road. The Natterjack is, by the way, equally careless, while other toads and the tree-frogs are very circumspect.
Fig. 49.–Rana temporaria. Eight successive stages in the development from the egg to the almost complete Frog, × 1.
Both sexes can croak, and this sound is frequently produced under water; but there are no regular concerts, although many collect in the same pond or spring, which is perhaps the only suitable place for miles around. The male puts its arms around the chest of the female, behind her arms, and the embrace is so firm that nothing will induce him to loosen his hold. The process becomes an involuntary reflex-action, a cramp which may last for days, or even for weeks, if sudden cold weather sets in, until the female is ready to expel the eggs, an act which is quick and soon over. The usual time of spawning in Middle Europe is the month of March, earlier in warm, later in cold seasons; in southern countries, February or even January, but in Norway not until May. Although the males of this species are not more numerous than the females, and therefore should be able to mate without much trouble, their ardour is so great that they occasionally get hold not only of the wrong kind of frogs, but of toads or even fishes, and, if taken off by force, they fasten on to anything else, a log or on to your own fingers. The eggs measure 2-3 mm. in diameter, are black with a whitish spot on the lower pole, number from 1000 to 2000, and sink at first to the bottom. Their gelatinous cover soon swells to a large globe more than 10 cm. in width, and the whole mass, as large as a man's head, floats on the surface, often stained with mud and other impurities. During the cold weather which often prevails in the spring, the dark brown larvae are slow in their development; and provided with rather large branched external gills and a well-developed tail, they wriggle about in the dissolving slime for three or four weeks. Fischer Sigwart[114] has timed and measured them as follows.–The eggs were laid on the 10th of {256}March. On the 15th the larvae were 4 mm. long and began to leave the eggs. On the 19th they measured, body 4, tail 9, total 13 mm.; on the 5th of April 10, 16, and 26 mm. respectively. On the 13th of May they were 40 mm. long and the hind-limbs appeared; the fore-legs burst through on the 25th, when the tadpoles had reached their greatest length, namely 45 mm., the body measuring 15 mm. On the 31st of May they left the water, still provided with a rather long tail of 20 mm., the total length being reduced to 35 mm. The larvae of this set developed unusually fast, perhaps owing to artificial conditions. The whole development is, however, mostly finished in three months, so that the little stump-tailed baby-frogs swarm about well before midsummer, and have time enough to grow to the size of 20 mm. or ¾ inch before they begin to hibernate in October.
In higher localities and in northern countries the tadpoles are sometimes obliged to winter in the unfinished condition.
In spite of the unusually hot summer of 1899 I found plenty of tadpoles on the 10th of September in the tarns of the hills of North Wales, 1500 feet above the level of the sea; while thousands of little frogs, with and without stumpy tails, were hopping about in the surrounding bogs. The water of these tarns is always very cool. Cold and rainy weather set in by the middle of the month, and on the 26th the tadpoles, all rather small, measuring only 35 mm., with the four limbs developed, but still with a broad fin on the tail, had all settled down under stones at the bottom of the now very cold water, prepared for hibernation. A few were taken home and kept in a glass vessel with water, cool, but less so than that of their native tarns. Within two days they lost the fins on their tails; before the end of a week they left the water, and crawled on to the moss, and the tails were reduced to little stumps. By the 10th of October the metamorphosis was complete, the little frogs measured only 13 mm. in length and showed no desire to hibernate in the genial atmosphere of the greenhouse.
This species has a very wide distribution. It ranges from the west of Ireland to the islands of Saghalin and Yezzo, being found everywhere in the enormous stretch of intervening countries, practically the whole of Central and Northern Europe and the middle belt of Asia. Its most northern extent is the whole of Sweden and Norway. I have found it to the east of the {257}Dovrefjeld, at an elevation of 4000 feet, well-nigh the snow-line. In conformity herewith it ascends the Italian Alps up to 10,000 feet. The southern limit in Europe is the Cantabrian range and the hilly province of Galicia. In the rest of the peninsula, in Italy and Lombardy, Greece and Turkey, and on the Mediterranean islands it is absent.
R. arvalis is often confounded with R. temporaria, as it differs from the latter only by the following characters. The snout is rather more pointed and is narrower; the inner metatarsal tubercle is large, compressed, and hard; the dorso-lateral glandular folds are more prominent and the belly is white and immaculate; lastly, it scarcely reaches 3 inches in length, a size which is not rarely surpassed by the other species. There are also some differences in habits. R. arvalis prefers moist, boggy, open localities, and does not ascend beyond 2000 feet in Central Europe. It pairs as a rule later in the spring and the eggs are smaller, only 1½-2 mm. in diameter; they do not swell up so much, and the whole mass does not float but remains at the bottom of the shallow water. The coloration much resembles that of R. temporaria, and is likewise subject to much variation, except that the pale vertebral stripe is perhaps more common. This species is distributed over the whole of Central Europe, Russia, and Western Siberia, south of the 60th degree of latitude, living side by side with R. temporaria. Between the rivers Elbe and Rhine it becomes decidedly rare, and the latter river is practically its western boundary, while the Bavarian Alps and the Danube form its southern limits.
R. agilis is still more frequently confounded with both the two former species. It differs from either by the absence of the two internal vocal sacs of the male, and by the decidedly longer hind-limbs, the tibio-tarsal joint reaching often a little beyond the tip of the snout. The inner metatarsal tubercle is as prominent as in R. arvalis. Total length up to 3 inches. The prevailing colour of the upper parts is rather yellow or pink-brown with few and small blackish spots; a Λ-shaped dark mark on the neck is often present, and the large dark patch on the temporal regions is always conspicuous. The under parts are white, inclining to lemon yellow on the flanks and thighs. The iris is golden yellow in its upper half, dark brown in the lower half.
This species has a much smaller range than the first two:–from France through Middle and Southern Germany, Switzerland, and Lombardy to Hungary and Greece. The specific name refers to the quick and long leaps of this pretty, or rather delicately coloured frog, which prefers woods and wooded glens to large open places. Their voice differs much from the croak of the common Brown Frog, and agrees with that of R. arvalis, which is transcribed by Boulenger, who has kept them alive, as a rapidly uttered "co-co-co," or "cor-cor-cor." According to the same authority, the pairing takes place as in R. temporaria, but is of much shorter duration, the females usually resorting to the water only at night and when quite ready to spawn. Specimens in embrace are therefore seldom found in the daytime. The eggs resemble those of R. temporaria in size, but they do not swell up so much and they do not float.
These three species of European brown frogs, difficult enough to distinguish, have of late been increased by three more, thanks to the sagacity of Boulenger. These latter inhabit South Europe, and the males all lack the internal vocal sacs.
R. iberica has a very small range, namely the north-western portion of the Iberian peninsula, from the Tagus northwards into Galicia, but south of the main extension of the Cantabrian chain. The rest of the Peninsula south of these mountains has no brown frogs, the only species of Rana being R. esculenta. R. iberica is rather local, being restricted to those hilly and mountainous districts which are well watered. A favourite haunt is the numerous streams in the wooded parts of the Serra Gerez, the red, disintegrated granite of which suits this little, extremely active, and reddish frog to perfection. The prevailing ground-colour varies according to the district, from pale to dark reddish or orange brown, with red specks and larger, dark brown spots, which in some specimens begin with the Λ-shaped mark between the shoulders. Dark spots on the flanks are very variable; the hind-limbs show the usual darker cross-bars, and the temporal region has the conspicuous dark patch. The ground-colour of the under parts is whitish, suffused with a pink tinge, and the throat is much speckled with brown; the toes are pink. The size of this pretty frog amounts to 2 inches. The breeding time is the month of March. When caught and squeezed they emit a slight "co-co-co."
R. graeca inhabits Italy and the Balkan peninsula from Rosina to Morea, together with R. agilis, from which it is very difficult to distinguish except that it is a little smaller, remaining below 2½ inches, and is generally more uniformly pale grey brown to yellowish and pinkish brown above, with scarcely any, or only a few, small dark specks on the back and limbs. The temporal patch is likewise paler than in the other species. The flanks are spotless, their colour gradually passing into the light buff of the under parts, which are more or less marbled with grey. The iris is golden, speckled with dark brown.
R. latastei of Lombardy and Northern Italy down to Florence is the last of these closely allied frogs. Its affinities lie with R. iberica and R. agilis. The latter and R. latastei, although living side by side in the same locality, for instance near Turin, are said not to interbreed. The voice is a rapidly uttered "keck-keck-keck;" the length remains below 2½ inches. The ground colour is greyish or reddish brown with a dark brown Λ-shaped mark between the shoulders, and a few red, orange, or blackish spots on the back. The flanks are without definite dark spots. The under parts are whitish, with a strong pink tinge, especially along the middle of the throat and on the chest, the paler portions being mottled with pale grey brown.
Perhaps the least unsatisfactory way of distinguishing between R. agilis, R. graeca, and R. latastei (R. iberica need not be confounded with them on account of its distribution) is the size of the tympanum, and its distance from the eye. The tympanum is smallest in R. graeca, its diameter being about half that of the eye and from ¾ to the whole of its width distant from the eye. In R. latastei the tympanum is a little larger, and about ½ to ⅔ its own width distant from the eye. R. agilis has the largest tympanum, measuring about ¾ of the diameter of the eye, and the distance between the two organs amounts to only ⅓ of the size of the tympanum.
Brown land-frogs of the R. temporaria group are found in most countries of nearly the whole Periarctic and Oriental regions, and by the time their races and varieties have been studied as minutely as those of Europe are now being scrutinised, the number of species will indeed be great.
R. silvatica is the chief representative in North America. It closely resembles R. agilis, but is smaller, only 2 inches in length, {260}and possesses a pair of internal vocal sacs. Its specific name refers to its predilection for forests of oak, among the dried leaves of which it conceals itself so successfully that it is discovered with difficulty. R. japonica of Eastern Asia is almost indistinguishable from this American species and from the European R. agilis.
R. opisthodon of the Solomon Islands has the vomerine teeth in two oblique series entirely behind the level of the choanae. The general shape of this large frog is stout, the type specimen of the male measuring 78, that of the female 125 mm. = 5 inches. The upper surface of the female is covered with small, flat warts, that of the male is much smoother. The upper parts are dark brown, while the under surface is brownish white. The male has two internal vocal sacs.
This species is interesting as affording another instance of shortened development, the whole metamorphosis being gone through within the egg. Mr. Guppy, its discoverer, has supplied the following notes: "During a descent from one of the peaks of Faro Island I stopped at a stream some 400 feet above the sea, where my native boys collected from the moist crevices of the rocks close to the water a number of transparent gelatinous balls, rather smaller than a marble. Each of these balls contained a young frog, about 4 inches in length, apparently fully developed, with very long hind-legs and short fore-legs, no tail, and bearing on the sides of the body small tufts of what seemed to be branchiae. On my rupturing the ball or egg in which the little animal was doubled up the tiny frog took a marvellous leap into its existence, and disappeared before I could catch it. On reaching the ship an hour after, I found that some of the eggs which I had put in a tin had been ruptured on the way by the jolting, and the liberated frogs were leaping about with great activity. On placing some of them in an open-mouthed bottle, 8 inches long, I had to put the cover on, as they kept leaping out."
Boulenger[115] has figured and further described the eggs and young. The egg measures 6-10 mm. in diameter, and is a transparent capsule in which the young frog is coiled up in the same way as figured by Peters in Hylodes martinicensis; but none of the specimens, which are in an advanced stage of development, show anything of a tail. There are no gills, but on each side {261}of the abdomen are several regular transverse folds, the function of which is probably that of breathing organs, like the tail of Hylodes. The tip of the snout is furnished with a small conical protuberance projecting slightly through the delicate envelope of the egg, and evidently used to perforate that envelope.
R. guppyi, likewise an inhabitant of the Solomon Islands, is a giant among frogs. It was discovered by Mr. Guppy on the Shortland Islands. The type-specimen measures 165 mm. = 6½ inches in length! The skin of the upper parts is covered with minute warts, and forms a strong fold above the distinct, but small, tympanum. General colour dark olive brown above, dirty white below.
R. tigrina is a common species of Eastern Asia, including the Malay Islands. On account of the strength of its voice, and its size, which is said to reach 7 inches, it is called the "Indian Bullfrog." Mainly aquatic, it has a strong cutaneous fringe along the outer side of the fifth toe. The skin of the back is thrown into longitudinal folds, and a strong fold marks the upper border of the tympanum. The general colour above is olive brown, with dark spots, often with a light vertebral line; the under parts are white. The male has a pair of large external vocal sacs.
R. gracilis has the same distribution, but it remains much smaller, and the toes are only half, instead of fully, webbed.
R. catesbiana is now the settled name of the "Bullfrog" of North America, the much more appropriate name of mugiens having been sacrificed to the fetish of priority. The tympanum is extraordinarily large, at least equal to the size of the eye, largest in the male. The first finger does not extend beyond the second; the toes are connected by a broad web down to the ends, and there is a small inner, but no outer, metatarsal tubercle. The upper parts are olive brown, clouded with dark brown or blackish spots; the under parts are yellowish white, often marbled with brown, especially on the throat. The iris is reddish, with an outer yellow ring. The male possesses two internal vocal sacs. Total length of adult specimens about 5 inches, but there are giants on record 7 inches in length, while the stretched hind-limbs measure another 9 or 10 inches. Its home extends over the whole of the United States, East of the {262}Rocky Mountains, southwards into Mexico, northwards into Canada.
According to Holbrook the Bullfrogs are solitary in their habits, only collecting together in the breeding season, when hundreds may be seen in the same small pond; and then the croak uttered by the males is so loud as to resemble the distant roaring of a bull, and can be heard on still evenings at a distance of half a mile. The voice is a hoarse bass "brwoom," playfully translated into "more rum." "They cannot be said to abound, but are found commonly enough sitting half immersed in water, or on the banks of ponds, waiting for their prey. If alarmed they hop suddenly into the water, but do not conceal themselves at once, frequently skimming along the surface for several yards before they dive below." They are the most aquatic of all the North American frogs, and Holbrook has known specimens to live in wells for years, where they could not rest a moment on solid ground above the water.
The Bullfrog is voracious, and takes almost anything that lives or gets into his own pond–Mollusca, Crustacea, fishes and, above all, frogs. There is no doubt that they drag down and swallow a good many ducklings and the young of other water-fowl, but certainly not the half-grown birds which have a way of disappearing from the farms wherever there are negroes and other farm-hands about. In turn the bullfrog has sufficient enemies to keep its numbers down, in fishes, birds, otters, and snakes, and, in the South, alligators. Although easily kept and growing comparatively tame, they are dull, having to be kept in solitary confinement on account of their greediness, which knows no limits. Two of our specimens each swallowed a full-grown Salamandra maculosa, and died within the same night, probably not understanding the meaning of the conspicuous black and yellow warning colours of the European.
E. clamata s. fontinalis, likewise an inhabitant of Eastern North America, may be called a smaller edition of the Bullfrog, its usual full-grown size being about 3½ inches. The tympanum is conspicuously large, but the toes are webbed to a lesser extent, and the skin forms a glandular fold which extends from the shoulder in a curve to the flank. This species is partial to the neighbourhood of running streams; it is said to be {263}exceedingly timid, and to utter a short cry when disturbed and making its enormous leaps.
Another North American relation is R. halecina s. palustris, frequenting the neighbourhood of ponds and rivers, very lively and capable of jumping 8 to 10 feet. The tympanum is smaller than the eye, but there is the same glandular lateral fold as in R. clamata. The vocal sacs are internal and decidedly small.
R. esculenta.–The common Water-frog of nearly the whole Palaearctic region is closely allied to the American Water-frogs described above, and, like most of them, has the vomerine teeth in two small oblique rows between the choanae and extending a little beyond their posterior border. But the males have a pair of external vocal sacs. The tympanum is distinct, about two-thirds the size of the eye. The first finger is slightly longer than the second. The toes are entirely webbed. Besides the usual subarticular phalangeal tubercles, the sole of the foot is provided with two metatarsal tubercles, the outer of which is very small, while the inner is much larger, although varying in size from a soft oval to a long, curved, shovel-shaped structure. The skin is smooth, except for a pair of prominent glandular folds which extend from behind the eye along the dorso-lateral line. The coloration varies considerably. The upper parts are mostly greenish brown, with black brown spots on the back, and larger patches on the limbs. Most specimens have three lighter stripes along the back, the middle one mostly green, the two lateral bronzy brown and coinciding with the glandular folds. The tympanum is brown, and there is occasionally a dark temporal patch. The posterior aspect of the thighs is invariably {264}spotted with black and white or yellow, in opposition to the R. temporaria group, where these parts are never spotted.
The total length of this species varies much. Specimens 2½ inches in length are certainly mature, those of 4 inches are unusually large, and Boulenger has received a giant from Damascus, which measured 125 mm., or nearly 5 inches. The females are larger than the males.
The variations in colour are not only local but also individual, moreover the colours are changeable. The ground-tint ranges from dull brown through olive to bright green, the dark spots being more or less pronounced and numerous; the light vertebral line is olive-yellowish, bright green, or altogether absent.
Those which inhabit waters with plentiful vegetation, like water-lilies and other luxuriant plants, are generally prettier and more vividly coloured than those which live in swamps and ponds with dark mud, or where the prevailing vegetation has a sombre aspect. Cold and dull, warm and sunny days also influence the water-frogs, and those which have been kept in a dark tank look very different from the bright assembly which had been put in some weeks before.
Various attempts have been made at subdividing R. esculenta of Linnaeus into sub-species, and Boulenger has now, after the attentive study of an enormous material, arranged them in four principal and recognisable races. The chief differences are the relative length of the femur to the tibia and the size of the metatarsal tubercles.
1. Var. ridibunda, Pallas.–The right and left heels overlap each other when the thighs are stretched out at right angles to the vertebral column, and the tibia is closely folded up against the thighs. When stretched forwards, the heel reaches the eye or even the tip of the snout. The inner metatarsal tubercle is feebly developed, very small and blunt; the outer tubercle is absent.
That part of the thighs which is concealed by the legs when the animal is at rest is whitish or pale greenish, marbled with dark olive, or bronze, or of the latter colour with or without small light spots. No trace of yellow is ever to be detected on that region, nor at the axillae or on the groin. The vocal sacs are strongly pigmented with black, when inflated they are pale grey. The iris is a mixture of black and gold.
This form or race has the widest distribution, namely, all over Europe with the exception of England, the northern half of France, the Rhine countries, Denmark, and Italy. Southwards it extends from France through Spain and Portugal into the Sahara, eastwards into Turkestan. It attains a larger size than the others, but only in certain localities in various countries, where circumstances favour its development. Eastern countries produce the largest of all; those of the Volga are said to be very large. German physiological laboratories prefer those from the Danube, from Bohemia, and from the lakes and broad expansions of the Spree, to specimens from other localities.
2. Var. typica (esculenta, Linnaeus).–The heels just meet, but do not overlap. The inner metatarsal tubercle is strong, compressed, and prominent. A small outer tubercle is present. The heel reaches to the eye or a little further; the hinder surface of the thighs is "marbled with black, usually with more or less bright yellow pigment" in the living specimens; the vocal sacs are white or feebly pigmented. This race inclines to rather more green than the others, the males especially are often dark grass-green, with scarcely any markings. The vertebral stripe is then yellowish, and the lateral stripes almost golden. The range extends over the whole of Central Europe and the kingdom of Italy. Its northern limit is the southern end of Sweden. In the greater portion of Germany, Poland, and Austria it overlaps the var. ridibunda, with which it does not seem to pair, owing to a difference in the time of spawning; the var. typica being about a fortnight later, and beginning to spawn when the other has finished.
3. Var. lessonae, Camerano.–Except that the inner tubercle is stronger, while the outer one is near the vanishing point, and that the fourth toe is proportionally longer, this variety is really not distinguishable from the typical form, and Boulenger himself confesses that the distinction is arbitrary. The var. lessonae seems to have a rather sporadic distribution. It has been found in Piedmont and other parts of Italy, in Hungary and Transylvania, near Vienna, Halle, Upper Bavaria, on the Rhine, near Brussels, Paris, and what is of especial interest to us, in a few places in the eastern counties of England.
According to Boulenger's "Notes on the Edible Frog in England,"[116] the individuals of R. esculenta which live in Foulmire {266}Fen in Cambridgeshire, near Stow Bedon, and between Thetford and Scoulton in Norfolk, and are generally supposed to have been introduced from France, belong to the Italian form of var. lessonae. "It used to be found in Cambridgeshire, in Foulmire Fen, where it was discovered in 1844; and Bell[117] assures us that his father, who was a native of Cambridgeshire, had noticed the presence of these frogs many years before at Whaddon and Foulmire, where they were known from their loud croak as 'Whaddon organs' and 'Dutch nightingales.' The species was afterwards rediscovered in Norfolk, between Thetford and Scoulton, where it is now still very abundant, and from inquiries made by Lord Walsingham, must have existed for the last seventy (80) years at least. These frogs belong to the var. lessonae, and differ widely (by the much stronger inner metatarsal tubercle) from those found in a few other places in Norfolk, which are undoubtedly the descendants of a number imported from France and Belgium in 1837, 1841, and 1842, and turned loose in the Fens at Foulden and in the neighbourhood.... Within the last ten years large numbers of all the three forms have been imported from Brussels, Berlin, and Italy, and liberated in various localities in West Surrey and Hampshire. Berlin specimens of the var. ridibunda have also been introduced in Bedfordshire, and Italian ones in Oxfordshire."[118]
Leaving aside the question whether the so-called var. lessonae is merely sporadically developed out of the typical form, the inquiry of the possible origin of the English specimens of the var. lessonae is of special interest. Have they been introduced, as has been suggested, from Lombardy, or are they the last lingering descendants of native English frogs? The suggestion as to their Italian origin has naturally lost in value since similar specimens have been found in Belgium and near Paris; but we must remember that there existed considerable intercourse between East Anglia and the monks of Lombardy, who, to mention only one instance, came regularly to the old Priory of Chesterton, near Cambridge, in order to collect their rents. If the frogs were introduced by them for culinary purposes into various suitable localities their descendants would remain as local as they, and as the undoubtedly introduced French typical specimens actually are. On the other {267}hand, if we assume the lessonae specimens to be the last living descendants of English natives, it is inconceivable why they should now be restricted to that eastern corner while there are hundreds of other suitable places in England and Wales which, if on the Continent, would be perfect paradises for Water-frogs. The same vegetation, the same insects, the same climate, and–an enormous advantage to the frogs–no storks.
These English specimens are "olive-brown or bronzy-brown above, with black spots, strongly marked on the flanks, where a light longitudinal area remains unspotted; glandular folds lighter; the sides of the head and the ground colour of the flanks are sometimes green; tympanum chestnut-brown; a pale yellow or pale green vertebral line, frequently edged with black; the dark cross-bands on the limbs usually very irregular, sometimes absent; lower surfaces more or less profusely spotted with blackish; iris golden. Length of a male from Stow Bedon, 64 mm. or 2½ inches; of a female, 78 mm. or 3 inches."[119]
4. Var. chinensis, Osb.–Distinguished by short glandular folds along the back, in addition to the long dorso-lateral pair. The metatarsal tubercle is large and shovel-shaped. Distribution from Corea and Japan to Siam.
All these Water-frogs are decidedly aquatic. They make short excursions on land when their homes are dried up, but as a rule they remain in the lake, pond, river, morass, or ditch in which they were born. Their favourite resorts are the broad floating leaves of water-plants, for instance water-lilies, or a prominent stone, a tussock of grass, or the banks of their homes, where they sit motionless, basking for hours in a half-erect, alert position, watching for insects and other small fry, which are secured by a jump, and then lapped up. Sunshine is sure to bring them out, and on our approach they make straight for the water, either by one tremendous leap or with quick bounds, but without the slightest hesitation or stopping on the way. With folded arms they take a header, swim, with the arms still folded, for some distance under water, and conceal themselves in the mud, between stones, or in the vegetation. We perhaps have not seen them at all, whilst their watchful eyes and keen ears have noticed our approach, and the pond might appear uninhabited if we had not heard the plumping noise. If we {268}keep quite still, and they have not been disturbed previously, one after another will wriggle out of the mud, rise slowly to the surface under cover of the plants, and, without causing a ripple, rise just enough for the prominent eyes and the nose to clear the surface. Then one scrambles partly on to a leaf, but the sight of the huge human figure strikes him as uncanny, as it certainly does not belong to the scenery, and he doubles back, the broadly-webbed feet making a little splash. But another appears, jumps on to a leaf in the middle, or at the farther end of the pond, settles down, and utters a little croak, somewhat like "ooaar," and soon the whole company appear one after another, each taking up its favourite position. After all, their observing powers cannot be very great. If we ourselves keep still we may wield a rod and fish for them. There is no need of a hook, a piece of red cloth tied to the end of the line and skimmed over the water causes a lively commotion. The new bait is noticed at once, and arouses their curiosity; several jump at it, and the one which swallows the bait can be lifted out before it has time to let go. However, this is after all poor sport; the game is too eager. When a boy I have often caught them with a noose of slender wire at the end of a long hazel rod. They do not mind the rod at all, their attention being fixed on the person; they allow the noose to be slipped over their heads, and a sudden jerk secures the captive. In this way they can be singled out individually. Old frogs are more wary and experienced than the younger members; they take up safer positions, and by their sudden plunges give the alarm.
The males are great musicians, singing for sheer enjoyment not only during the pairing time, but throughout the months of June and July. Warm moonlit nights are the favourite times {269}for the concert, which takes place in the water, beginning at sunset, and continuing until the early dawn. A few individuals here and there utter a single note, "gwarr, oo-arr," or "coarx," but these are only preliminaries. The precentor–the country-folk in North Germany firmly believe that in each pond one old male holds the dignified position of choir-master–begins with a sharp-sounding "brekeke," and this is the signal for all the others to chime in with the same notes, varied with all sorts of other sounds, bass, tenor, and alto, each performer filling its resounding vocal sacs to bursting size, and these bags then look as if they acted as floats. When there are several hundred of these sociable creatures, the din is continuous, and may be heard more than a mile off. There can be too much of this, just as there can be too many nightingales; and a well-stocked pond in the neighbourhood may become a perfect nuisance. There are accounts of servants having been employed in the Middle Ages for the sole purpose of keeping the noise down by beating the pond, throwing stones into the water, or otherwise disturbing the frogs. Sometimes more vigorous and lasting measures seem to have been taken; the monks exorcised them in order not to be disturbed in their vigils. Near the former monastery of Chorin, in the province of Brandenburg, the frogs have still the reputation of keeping very quiet on account of some powerful abbot who threatened them with awful consequences if they did not forego their concerts.
The length of life which these frogs can attain is quite unknown. They do not reach maturity until the fourth or fifth year, but this is long before they stop growing, and it is no exaggeration to say that few, if any, frogs die of old age, since they have so many enemies. The stork is their king in the fable, and his daily visits to his realm strike dire distress amongst his subjects, which soon learn to know his conspicuous white and black garb, and seek imperfect safety at the bottom of shallow ponds and ditches, not too deep for the long-legged and long-billed despot. Numbers are taken by birds of prey; snakes and tortoises hunt them up in the water, and they are good bait for pike and other voracious fishes. The specific {270}name esculenta needs no comment, and this species is as much a martyr to science as the brown Grass-frog. The destroyers of tadpoles and young frogs are unlimited. In their turn the frogs themselves, especially the old ones, are very rapacious, and eat any living creature they can master,–insects, worms and snails, other frogs, especially the brown kind, and the young brood of fishes.
Recently caught Water-frogs are wild beyond description, much more so than the Grass-frog, but even they calm down after some time, learn to know their keeper, and allow him to handle them without trying to commit suicide by jumping on to, into, and down anything. However, they do not thrive well in captivity, and it is rare that they can be induced to breed, unless their enforced new home affords them ample freedom, and plenty of water and fresh air.
The Water-frogs appear in Germany rather late in the year, not before the middle of April, first the younger, then the adult members. In Southern Europe they show themselves earlier, and still further south they do not hibernate at all. The breeding season begins in Germany towards the end of May and continues well into June, the var. ridibunda beginning mostly a fortnight earlier. The male clasps the female under the arms, throwing its own round her breast, the nuptial grey excrescences on his inner fingers pressing against her skin, the palms being turned outwards. The embrace does not last long, rarely extending over a few days. The eggs, to the astonishing number of 5000 to 10,000 in full-grown specimens, are expelled in several masses, which sink down and remain at the bottom. The eggs measure only 1.5 mm. and are yellowish-grey above, pale yellow below; their gelatinous cover swells to 7-8 mm. in width. The embryo escapes on the fifth or sixth day as a very small larva, in which, however, the mouth, eyes, and beginnings of the external gills are already discernible. At the age of two weeks the gills have shrunk away, the left-sided "spiracle" is completed, and the well-tailed tadpoles, olive brown above, yellowish white below, still hang with their suckers on to plants and stones, or lie at the bottom, nibbling away at any rotting animal matter or scraping off the green algae.
It may here be mentioned that small tadpoles of any kind can with advantage be used as cleaners of delicate and small {271}skeletons. The object is put into a vessel, and the tadpoles will soon nibble and rasp away all the edible portions, leaving the skeletal framework beautifully cleaned. But they require attention lest they rasp away the cartilage.
The tadpole stage lasts three to four months; but cold, absence of sunshine, and scarcity of food delay the metamorphosis well into the end of summer, or force them to hibernate in the unfinished condition. They are very gregarious, and when the tadpoles of several families combine, they make imposing shows. By the time that their hind-limbs begin to sprout, they frequently combine into large shoals, and instead of always feeding they swim about in their tens of thousands, all moving in the same direction, and making almost regular evolutions. Mill-ponds with steep banks are good places for watching these peculiar habits. The tadpoles reach a considerable size, the total length averaging 2½ inches, or some 60 mm. the tail taking up ⅔ of the whole length. Specimens which measure more than 3 inches are rare. The baby-frogs hop on land while still provided with a stumpy tail; when this is resorbed the little creature is scarcely half-an-inch long, and for the rest of the available season leads a rather more terrestrial life than ever after.
Ex Africa semper aliquid novi! Quite recently Boulenger has received a consignment of Anura from the French Congo, amongst which were several new, remarkable genera, notably Trichobatrachus and Gampsosteonyx. Both are true Ranidae. Pupil vertical, with vomerine teeth. Omosternum with a bony style. The outer metatarsals are bound together. In Trichobatrachus robustus the toes are webbed, and both sexes have the flanks and corresponding portions of the thighs covered with numerous darkly pigmented, filamentous, cutaneous excrescences; these are several millimeters in length, giving the flanks and thighs a "hairy" appearance. Mr. F. F. Laidlaw has examined these structures. Their most remarkable feature is the presence in them of a great number of ordinary flask-shaped cutaneous glands, whilst such glands are scarce on the surrounding skin. They differ in no way from those seen in sections of the skin of the Common Frog. The fibrous connective tissue is dense and vascular; the pigment-cells are most plentiful at the base. Contrary to expectation no nerve-endings were found in these filaments.
Gampsosteonyx has free toes. The terminal joints of the digits stand out beyond the skin, and end in sharp, bony claws, like those of a cat.
Sub-Fam. 3. Dendrobatinae.–About one dozen arboreal little frogs have been separated from the Raninae proper on account of the entire absence of teeth. This mere loss of teeth, and the geographical distribution suggest that these frogs do not form a natural group, but have been developed independently from other Ranidae, the Neotropical Dendrobates from some likewise Neotropical genus like Prostherapis, the Malagasy Mantella from an African form like Megalixalus.
The sacral diapophyses are cylindrical. The omo- and meta-sternum are well developed. The fingers and toes are free, their terminal phalanges are T-shaped and carry regular, round, adhesive discs. The tympanum is distinct, although sometimes, in Dendrobates, very small. The pupil is horizontal.
Dendrobates.–The tongue is elongate, entire and free behind. The omosternum has a weak, semi-ossified style, but the metasternum remains cartilaginous. The males have a subgular vocal sac. Seven closely-allied species inhabit tropical America.
D. tinctorius.–This pretty little species, scarcely 1½ inch in length, is quite smooth, varies much in coloration, and forms local races to a certain extent. Some are quite black, others are grey above, black on the sides and under parts; or they are grey with large black patches. A fourth variety is black above with several white or pink longitudinal stripes, while the under parts are grey, spotted with black. In others, again, the ground-colour is black, with white stripes and spots above, marbled below. But this enumeration does not exhaust the list, since living specimens are sometimes much more conspicuously coloured, some being black with large patches of saturated yellow on the head and back, while the limbs are orange red and black. This species has a wide range, from Panama to Ecuador and to the mouth of the Amazon. It owes its specific name to the peculiar use made by man of the strongly poisonous secretion of the tiny glands of the otherwise smooth skin. Other species are doubtless employed in the same way. The poison is mainly used for "dyeing" the green Amazon-parrots. This is done as follows:–The green and blue feathers on the head and neck, or other parts, according to the fancy of the {273}operator, are plucked out, and these places are rubbed with the poison, often simply with the living frog, certainly not with its blood, as is sometimes asserted. This operation may be repeated when the new, young feathers begin to bud. The result is that these appear yellow instead of green, and since the Brazilians, and to a certain extent the Portuguese, are rather partial to these artificially-produced freaks or "contrafeitos" as they call them, the industry is kept up. That the poison is also used for arrows has been mentioned on p. 38.
D. trivittatus, chiefly in Northern Brazil, has the first finger slightly longer than the second. It likewise varies considerably in its coloration, being either quite black, or spotted with white and brown, or with a whitish forehead and several white patches on the back and hind-limbs. D. typographus of Central America is vermilion red, with small dark marks on the back; the legs are black.
The various species of Dendrobates take remarkable care of their young. D. braccatus lives in Brazil in "varzeas," i.e. moist but waterless places, and carries its tadpoles on its back, to which they are attached by a peculiar secretion. The same is said to be true of D. trivittatus, which sits down in a drying-up puddle, lets the little tadpoles, when they are only 6-7 mm. long, fasten themselves on, and conveys them to a safer locality, where the water is calculated not to evaporate before the metamorphosis is completed.
Mantella.–Both omo- and meta-sternum possess a bony style. The tongue is free and distinctly mitred or cut out behind. The skin is very granular. Several species, in Madagascar, were formerly put into the same genus as the American forms, until Boulenger established the genus Mantella for them. The coloration is strikingly pretty. M. madagascariensis is a rare instance of difference in colour between the two sexes. The male is bluish black, with light blue spots on the belly, while the thighs and the inner sides of the legs are beautifully red. The female is deep black, with a light green spot at the base and in front of the limbs; the rest is coloured like the male.
Cardioglossa gracilis, quite recently discovered at the Gaboon, has likewise to be added to the Dendrobatinae, on account of the absence of teeth. It is a small, slender, arboreal frog, bearing an unmistakable resemblance to the other genera by its general appearance and conspicuous, contrasting coloration of black and white.
REPTILIA
"Cada uno es como Dios le hizo,
y aun peor muchas vezes."
"We are all as God made us
and many even worse."
Sancho Panza,
Don Quixote.
REPTILIA
DEFINITION AND CHARACTERS–POSITION OF THE CLASS REPTILIA IN THE PHYLUM VERTEBRATA–CLASSIFICATION–SKULL AND VERTEBRAE.
The recent Reptiles comprise, broadly speaking, the Crocodiles, Tortoises, Lizards, and Snakes. They are the only Vertebrates which are cold-blooded, breathe by lungs, and have a median occipital condyle. Another equally sufficient diagnosis is the following:–Tetrapoda, with a median occipital condyle, with nucleated red blood-corpuscles, and with complete right and left functional aortic arches. A still shorter diagnosis is:–Monocondylia with a scaly skin.
If our diagnosis is to include the fossil Reptiles we have not only to discard the characters drawn from the soft parts as unavailable, but we are forced to treat the condition of the occipital condyle with caution, since there exist, or must have existed, transitional stages between Reptiles and Amphibia and Mammals; and the winged class Pterosauria does not permit us to use the wings as a differential character for the Birds. In fact, while the Reptilia are sufficiently separated from the Amphibia by their absolutely gastrocentrous vertebrae, it is difficult to distinguish them as a class from the Birds; hence the term Sauropsida, which is intended to indicate the close relationship of the Reptiles to the Birds in opposition to the Mammalia, and to the Ichthyopsida or Amphibia and Fishes. However, the Reptilia take up a very central position in the evolution of the main classes of the Vertebrata. On the one hand, there is not the slightest doubt that they are evolved from some branch of {278}the Stegocephali, whilst on the other hand the Reptiles, probably through some branch of the Theromorpha, have given rise to the Mammals; some other Reptilian branch, at present unknown, has blossomed out into the Birds.
Principal Characters of the Reptilia.
1. The vertebrae are gastrocentrous.
2. The skull articulates with the atlas by one condyle, which is formed mainly by the basioccipital.
3. The mandible consists of many pieces and articulates with the cranium through the quadrate bones.
4. There is an auditory columellar apparatus fitting into the fenestra ovalis.
5. The limbs are of the tetrapodous, pentadactyle type.
6. There is an intracranial hypoglossal nerve.
7. The ribs form a true sternum.
8. The ilio-sacral connexion is post-acetabular.
9. The skin is covered (a) with scales, but (b) neither with feathers nor with hairs; and there is a great paucity of glands.
10. Reptiles are poikilothermous.
11. The red blood-corpuscles are nucleated, biconvex, and oval.
12. The heart is divided into two atria and an imperfectly divided ventricle. It has no conus, but semilunar valves exist at the base of the tripartite aortic trunk.
13. The right and left aortic arch are complete and remain functional.
14. Respiration is effected by lungs; and gills are entirely absent, even during embryonic life.
15. Lateral sense-organs are absent.
16. The kidneys have no nephrostomes. Each kidney has one separate ureter.
17. There is always a typical cloaca.
18. The eggs are meroblastic.
19. Fertilisation is internal, and is effected, with the single exception of Sphenodon, by means of male copulatory organs.
20. An amnion and an allantois are formed during development.
The evolution of the classification of the Reptiles has to a certain extent been already treated on pp. 7-9. For a long time only Chelonia or Tortoises, Ophidia or Snakes, and Saurii were recognised as their principal divisions. Then the Crocodiles were separated from the Lizards; later the Coeciliae were removed from the Snakes and referred to the Amphibia, {279}and ultimately Sphenodon was recognised as deserving a separate position, equal in rank to the other groups. Stannius showed that the Crocodiles and Tortoises are relatively near allies in opposition to the likewise closely allied Lizards and Snakes (Sphenodon was then unknown), and he expressed this by the term Monimostylica, or creatures with fixed quadrate bones, for the former, and Streptostylica, creatures with movable quadrates, for the latter combination. The fossil Reptiles were hardly allowed proper places in the system. In various zoological text-books they were, or are even now, treated as inconvenient, outlying, or supernumerary members. A long time elapsed before, thanks to the labours of H. von Meyer, Owen, Huxley, Marsh, Cope, Zittel, and Seeley, it was recognised that the extinct groups form the preponderant mass of Reptiles, and that it is the recent groups which, in spite of the bewildering number of species of Lizards and Snakes, are the comparatively few and much-reduced members of a once flourishing class. With the exception of the Lizards and Snakes, which are on the ascending branch, the modern Sphenodon, the Crocodiles and the Tortoises are a mere fraction, comprising a few survivals of richly-developed groups, while all the others, the overwhelming majority, have died out.
The classification adopted in this volume is as follows:–
Class Reptilia.
Sub-Class IIIII. Proreptilia.
Sub-"ClasIII II. Prosauria. Orders: Microsauri, Prosauri.
Sub-"ClasII III. Theromorpha. Orders: Pareiasauri, Theriodontia, Anomodontia, Placodontia.
Sub-"ClasII IV. Chelonia. Orders: Athecae, Thecophora.
Sub-"ClasIII V. Dinosauria. Orders: Sauropoda, Theropoda, Orthopoda, Ceratopsia.
Sub-"ClasII VI. Crocodilia. Orders: Pseudosuchia, Parasuchia, Eusuchia.
Sub-"ClasI VII. Plesiosauria. Orders: Nothosauri, Plesiosauri.
Sub-"Clas VIII. Ichthyosauria.
Sub-"ClasII IX. Pterosauria.
Sub-"ClasIII X. Pythonomorpha. Orders: Dolichosauri, Mosasauri.
Sub-"ClasII XI. Sauria. Orders: Lacertilia, Ophidia.
The eleven principal groups are here called "sub-classes" to emphasise the undeniable fact that these Reptilian groups are of much greater morphological value than those which are most generally called "Orders" in the Mammalia, that class which we consider as the standard or model of classificatory units.
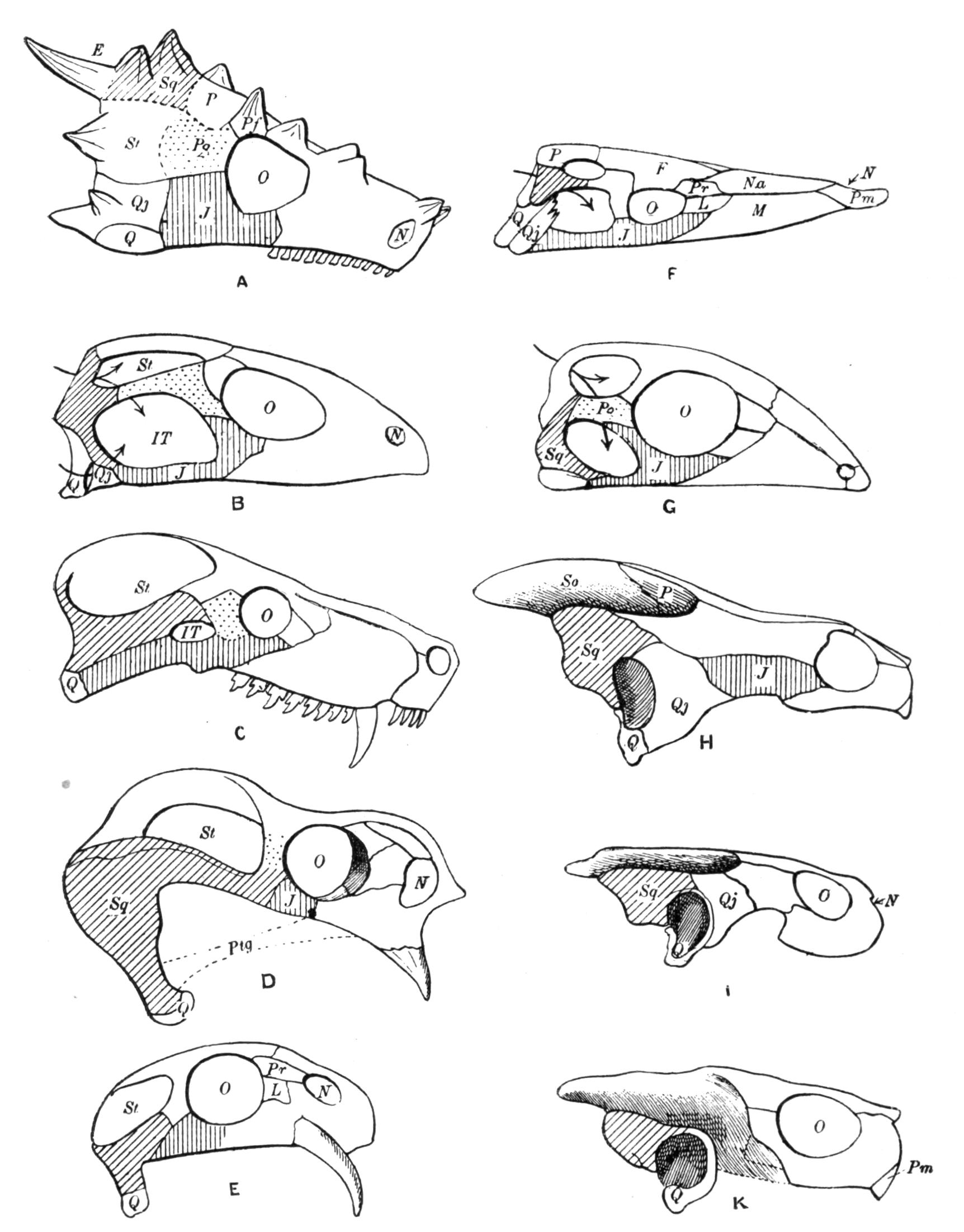
Fig. 54.–Diagrams of skulls, showing especially the composition of the bony arches of the orbito-temporal region.
A, C, D, E, Theromorpha. A, Elginia, p. 305; C, Cynognathus, p. 306; D, Gordonia, p. 310; E, Dicynodon, p. 310.
B, G, Prosauria. B, Sphenodon, p. 294; G, Palaeohatteria, p. 291.
F, Crocodilia, p. 434.
H, I, K, Chelonia, p. 316. H, Chelydra, p. 338; I, Chrysemys, p. 346; K, Cistudo, p. 361.
E, Epiotic; F, frontal; IT, infratemporal fossa; J, jugal, shaded vertically; L, lacrymal; M, maxillary; N, nasal groove; Na, nasal bone; O, orbit; P, parietal; Po, postorbital, dotted; Pf, post-frontal; Pm, premaxillary; Pr, prefrontal; Ptg, pterygoid; Q, quadrate; Qj, quadrato-jugal; So, supra-occipital; Sq, squamosal, shaded obliquely; St (in B-E), supratemporal fossa; St (in A), Supratemporal bone.
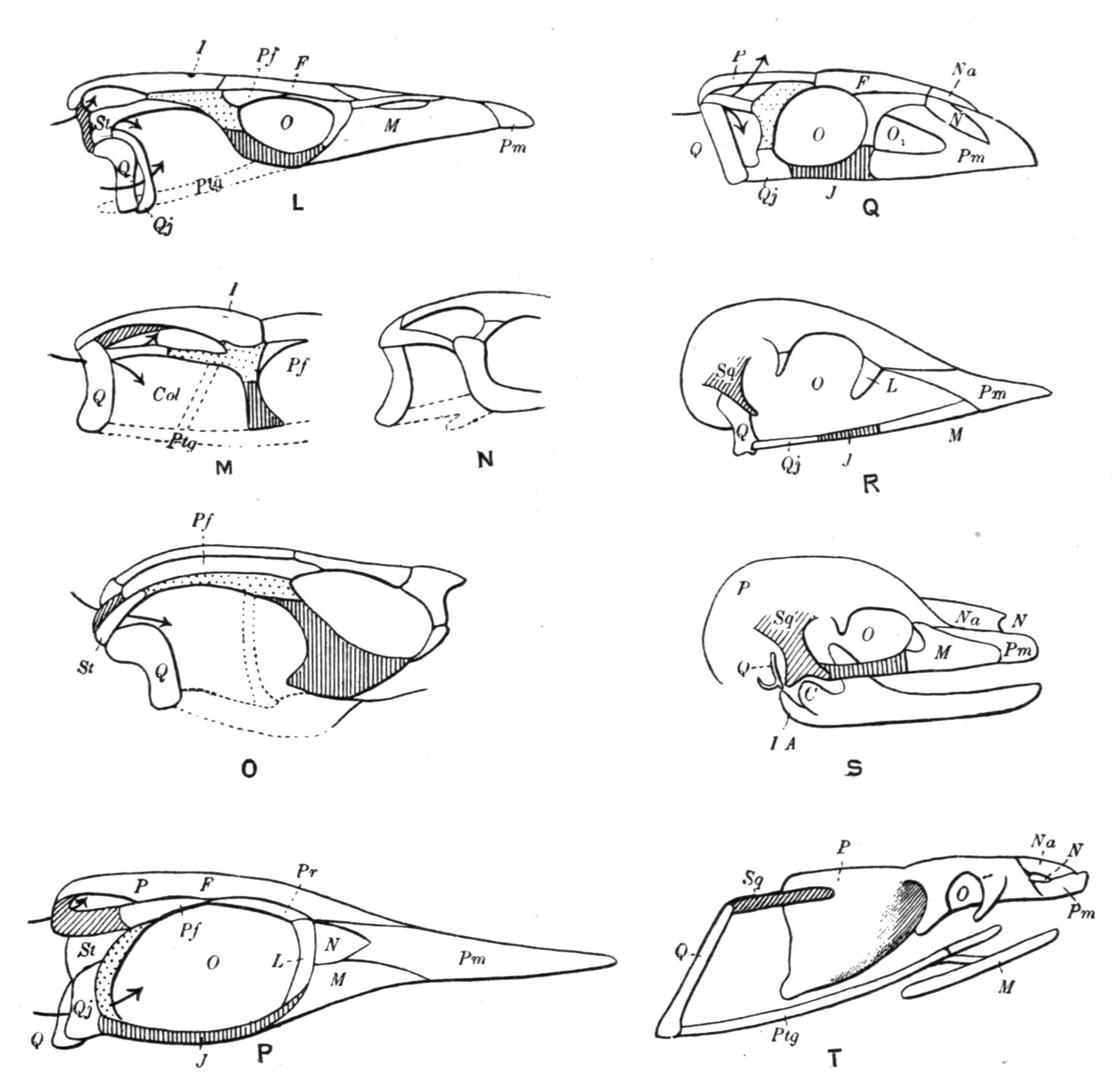
Fig. 55.–Diagrams of skulls, showing especially the composition of the bony arches of the orbito-temporal region.
L, Pythonomorpha. Clidastes, p. 490.
M, N, O, Lacertilia, p. 496. M, Varanus, p. 543; N, Uromastix, p. 524; O, Lacerta, p. 550.
P, Ichthyosauria, p. 479. Ichthyosaurus, p. 483.
Q, Pterosauria, p. 484. Dimorphodon, p. 486.
R, Aves, generalised, for comparison.
S, Mammalia, generalised, for comparison.
T, Ophidia, p. 581.
C, Condyle of mandible; Col, columella cranii; F, frontal; I, interparietal or pineal foramen; I.A, Inner angle of mandible; J, jugal, shaded vertically; L, lacrymal; M, maxillary; N, nasal groove; Na, nasal bone; O, orbit; O1, preorbital fossa; P, parietal; Pf, postfrontal; Pm, premaxillary; Pr, prefrontal; Ptg, pterygoid; Q, quadrate; Qj, quadrato-jugal; Sq, squamosal, shaded obliquely; St, supratemporal bone.
The families cannot well be changed, and terms like super-families and super-orders are sometimes resorted to by those who do not like to look stern facts in the face.
The sequence of the groups, although arranged as much as possible in ascending order, is of necessity as unnatural as that of the maps in an atlas. We cannot yet construct a satisfactory phyletic tree of the Reptiles. The Proreptilia connect them with the Amphibia. Next follow the Prosauria with Sphenodon among the Prosauri as the key to most other groups. Then follow the Theromorpha, and it is probable that from various branches of these have arisen the Chelonia, Dinosauria, Crocodilia, and Plesiosauria. The descent of the Ichthyosauria is very problematic. The same applies to the Pterosauria and to the Pythonomorpha, but it is possible that they, together with the Sauria, are connected with the Prosauria.
With all reserve these hypothetical affinities may be expressed by the following diagram:–
The eleven sub-classes of the Reptilia present so many important differences that it is not advisable to give here a further general account of their structure. The diagrammatic figures A to T on pp. 280, 281, representing various types of skulls, are intended to explain their chief modifications, all referable to Proreptilian and to certain Theromorphous conditions. One of the most important features is that the mandible, which is always composed of many pieces (cf. Fig. 142, p. 550), is invariably carried by the quadrate bone. Diagrams of the generalised skulls of a Bird and a Mammal have been added for comparison.
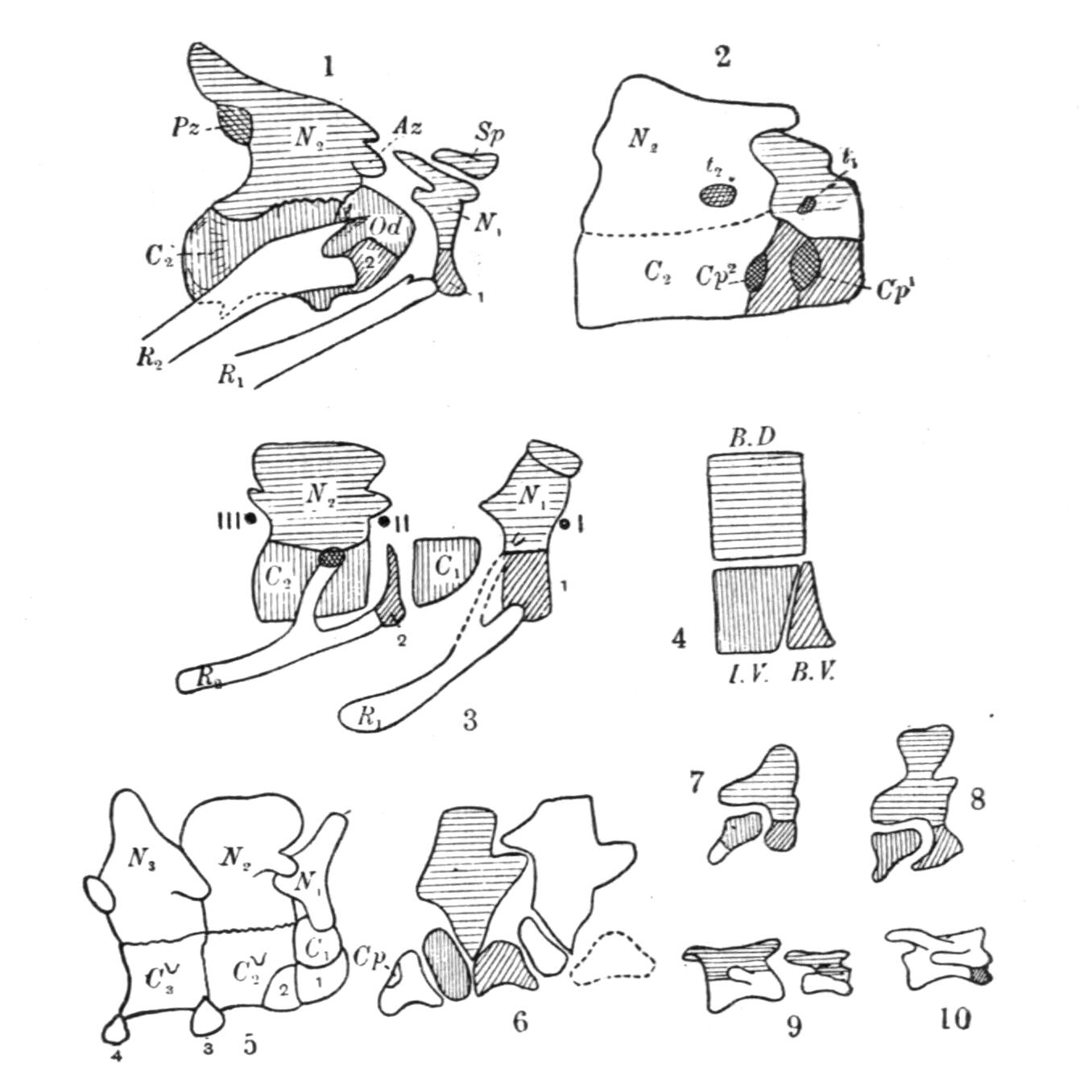
Fig. 56.–Composition of vertebrae of Reptiles, illustrated by the first and second cervical vertebrae. (1) Atlas (first cervical) and axis (second) vertebra of Crocodilus. (2) Atlas and axis of Metriorhynchus, a Jurassic Crocodile. (3) Analysis of the first two cervical vertebrae of a Crocodile; 2, second basiventral complex or "intercentrum" continued upwards into the meniscus or intervertebral pad. (4) Diagram of the fundamental composition of a Reptilian vertebra; compare this and (6) with Fig. 1 (8 and 9) on p. 13. (5) The first three cervical vertebrae of Sphenodon. (6) Trunk-vertebrae of Eryops, a Permian Proreptile; typically temnospondylous; cp, articular facet of the capitulum of a rib. (7) The complete atlas of an adult Trionyx hurum; the second basiventral (intercentrum) is attached to the posterior end of the first centrum, which, not being fused with the second centrum, is not yet an odontoid process. (8) The complete atlas of an adult Trionyx gangeticus; still typically temnospondylous. (9) The first and second cervical vertebrae of an adult Platemys. (10) The complete atlas of a Chelys fimbriata. Az, Anterior zygapophysis; B.D, basidorsal; B.V, basiventral; C1, C2, C3, first, second, and third centra, formed by the interventralia; Cp1, Cp2, articular facets of the capitular portions of the first and second ribs; I.V, interventral; N1, N2, N3, first, second, and third neural arch, formed by basidorsalia (B.D); Od, odontoid process = first centrum; Pz, posterior zygapophysis; R1, R2, ribs; Sp, detached spinous process of the first neural arch; t1, t2, tubercular attachments of the first and second ribs; 1, 2, 3, 4, "intercentra" = basiventrals; I, II, III, position of the exit of the first, second, and third spinal nerves.
As mentioned on p. 278 the vertebrae of the Reptilia and those of all other Amniota are gastrocentrous; that is to say the centra or bodies of the vertebrae are formed by the pairs of interventralia, while the basiventralia are reduced, persisting either as so-called intercentra or wedge-bones, or as intervertebral pads, or disappearing altogether. At the earlier stages of development the gastrocentrous vertebrae behave in the {284}same way as that described on p. 12 (Fig. 1), except that the interdorsal elements are suppressed from the beginning. If the remaining three pairs of constituent elements of each vertebra (the basidorsalia, forming the neural arch; the interventralia, forming the body or centrum; and the basiventralia) remain separate, the vertebrae are called temnospondylous (τέμνω, I cut, σπόνδυλος, a vertebra). If the neural arches and the centra are suturally united or are fused with each other, the vertebrae are called stereospondylous (στερεός, solid). In many Amniota the atlas or first vertebra remains in a relatively primitive, embryonic condition, and is temnospondylous but for the usual modification that its centrum becomes attached to that of the second vertebra, and forms the odontoid process of the latter. The composition of gastrocentrous vertebrae (cf. p. 282) is best illustrated by the first and second cervical vertebrae of the Crocodile (Fig. 56, 3, p. 283).
Concerning geographical distribution, even a cursory study shows that the sub-classes have come into existence at very different geological periods, and have each followed their own lines of dispersal.
PROREPTILIA–PROSAURIA–THEROMORPHA
Sub-Class I.–PROREPTILIA.
Permian Temnospondylous Reptiles with well-developed limbs and girdles of the terrestrial type.
The two genera Eryops and Cricotus of the North-American Permian formation had until recently[120] been relegated to the Stegocephali. By grouping them and their nearest allies together as Proreptilia it is intended to indicate that they are the lowest known Reptiles and that they probably link this class to the Amphibia. The superficial resemblance of their tri- or bi-partite vertebrae, and their occurrence in the Lower Permian, have caused the error of classing them with the Stegocephali, but the composition of their typically gastrocentrous vertebrae leaves no doubt as to their affinities. After all, we feel certain that Reptiles have arisen from Stegocephalous Amphibia, and it is in the Lower Permian, exactly where these debatable creatures lived side by side with Stegocephali, undoubtedly likewise temnospondylous, that the change from Amphibia into Reptiles seems to have taken place. Both are referable to Amphibia with quadripartite vertebrae. The condition of the occipital condyles determines nothing. This greatly exaggerated character has lost in importance since we have known the condylar modifications of the Theromorpha; moreover, Cricotus itself seems to have possessed a single condyle. We should even expect the Proreptilia to present many Stegocephalous inheritances, for instance the condition of the skull roofed in by dermal bones, a ventral dermal armour, a very complete pectoral arch still without a sternum, and only one sacral vertebra.
Until more genera are better known than they are now, it is premature to divide the present sub-class into orders.
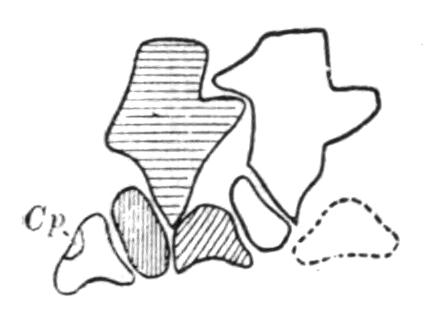
Fig. 57.–Trunk vertebrae of Eryops (cf. Fig. 56, 4, p. 283). Cp, Articular facet of the capitulum of a rib.
Eryops, with several species in Texas and New Mexico. E. megacephalus is the most abundant and the largest species, its broad and flattened skull measuring more than 18 inches in length and 12 in width. With the exception of the nostrils and the small orbits, the skull is entirely encased in bone, with a rough, pitted surface, but without any distinguishable sutures. The absence of mucous canals, so common in the Stegocephali, is worthy of note. The quadrates extend obliquely outwards and backwards, so that the joint with the mandible lies in a plane behind the occiput. The mandibles are devoid of a projecting angular process. The teeth are numerous, small, and pointed. The vertebrae are typically temnospondylous, consisting each of three pairs of separately ossified pieces, which, although closely packed together, are not suturally connected. The neural arches possess high spinous processes, they articulate by short and broad zygapophyses and are, with their triangular bases, wedged in between the two ventral pieces, the posterior of which (the united interventralia) is in broader contact with the neural arch and lies behind it; the anterior piece (the united basiventrals) appear as typical, but large, intercentra, and bear on their posterior, dorsal margin the facets for the ribs. The latter are short, but are broad at their proximal ends, which are not bifurcated; they extend their articulation from the "intercentra" upon the short lateral processes of the neural arches. The tail is short and ends in a pointed coccyx, owing to fusion of the last vertebrae.
The pubes and ischia are heavy, the former flattened and broadened out. The limbs are of an almost ideal pentadactyloid type; strongly developed for terrestrial locomotion. The ulna possesses a large olecranon. The carpus consists of ten separate pieces, ulnare, intermedium, radiale, two centralia and five distal carpalia. The latter support only four metacarpals and fingers, the second finger being completely abolished, an explanation suggested by Cope and corroborated by Emery.[121]
Cricotus, with several species in Texas and Illinois. C. heteroclitus was perhaps 10 feet long and probably aquatic. The skull has a long, narrow, depressed snout, the margins overhanging those of the lower jaw; its surface is encased in dermal bones, most of which still show sutures, so that for instance postfrontals, postorbitals, supratemporals and squamosals can be distinguished; all these are in contact with the long parietals and with the quadrato-jugal arch, covering the temporal region; but the supratemporals have a free projecting border, like the squamosals of the crocodiles. According to Cope's description the basioccipital is connected with the first vertebra by an undivided discoid "intercentrum," probably the true centrum, while the first basiventral mass, which would be, if independent, the first true intercentrum, is more probably connected with the first neural arch, thus constituting the ring of the atlas.
The vertebrae are still temnospondylous, but no longer tripartite. The neural arch is fused with the interventralia into one mass, which carries the capitula and tubercula of the ribs, while the united basiventrals still remain as separate intercentral wedges. In the tail these wedges carry chevron-bones, and are enlarged into thick almost complete discs, or rather rings, while the whole vertebral column is still perforated, as also in Eryops, by the chorda dorsalis. The tail is long. The digits are devoid of claws.
Remains of dermal armour exist on the throat in the shape of several large gular plates, while the whole belly is covered with many closely packed bony scales, which are arranged in chevron-shaped transverse rows.
Probably several other genera of American Permian and also of European Permian strata will, when better known and critically examined, have to be referred to the Proreptilia. Thus for instance the European Melosaurus may have affinities with Eryops, while Diplovertebron of Bohemia seems to be allied to Cricotus. The difficulty of division will lie with those Lower Permian Amphibia which, like Archegosaurus, Euchirosaurus, Actinodon, possess tripartite vertebrae, which at first sight are strikingly like those of Eryops. But the tail-vertebrae permit of no mistake, and since these are quadripartite in Archegosaurus, Chelydosaurus, and Sphenosaurus, these genera are safely to be classed with the Amphibia, unless, indeed, for mere argument's {288}sake, it be assumed that the intercentral discs of Diplovertebron and Cricotus are formed by the fusion of Amphibian interdorsals with interventrals. Anyhow, simply to state that the tripartite vertebrae of Eryops are the same as those of Actinodon, would be as convincing as saying that the English and French flags are essentially the same, both containing the same colours, but one is white, red, and blue, the other blue, white, and red. Tripartite Amphibian vertebrae are composed of basidorsals + basiventrals + interdorsals, those of Reptiles are made up of basidorsals + basiventrals + interventrals. (Cf. Fig. 56, p. 283, and Fig. 1, p. 13.)
Sub-Class II.–PROSAURIA.
Mostly extinct Reptiles, with deeply amphicoelous but stereospondylous vertebrae, with movable chevron-bones in the tail and frequently with intercentra in the trunk. Sphenodon, the only recent genus, has no copulatory organs.
Order I. MICROSAURI.
Extinct, small Reptiles, mostly Carboniferous and Permian, with dermal armour on the dorsal and ventral side and with bifurcated ribs.
We retain this term of Dawson's for those small, newt-shaped, chiefly Permian reptiles, which are allied to Hylonomus, after elimination of contemporary forms like Keraterpeton and Urocordylus, which belong to the Branchiosaurian order of the Stegocephali. Until recently[122] all these creatures had been classed with the Stegocephali. The Microsauri in the present restricted sense reveal themselves, however, as reptiles by the movable chevron-bones in their tail, their broad neurocentral sutures, the possession of two sacral vertebrae (Petrobates), the bifurcated ribs which always articulate with the centra (most clearly shown in Orthocosta), and the possession of five fingers and toes.
Considering the age of these little creatures and their low position in the reptilian scale–in fact, they stand almost as low {289}as the Proreptilia–it is not to be wondered at that they still retain a number of amphibian features. The skull is encased in dermal bones as in the Stegocephali, and the dermal armour of the trunk and tail is composed of many bony, sculptured scales, which cover back, sides, and under surface. The middle rows on the back are the largest, while the scales on the belly are arranged in transverse rows, which imbricate and converge obliquely headwards. Special gular plates seem to be absent. The skull has an interparietal foramen. The jaws and the palate are furnished with small, simple teeth, and there is a large parasphenoid bone, an eminently amphibian character. The occipital condylar articulation is supposed to be double. The centra of the vertebrae are deeply amphicoelous, elongated, and constricted in the middle, just like those of the Aistopoda and Branchiosauri. The dorsal spinous processes are strongly developed, and with the zygapophyses are very reptilian. Transverse processes are absent or very short, the tubercular portions of the ribs articulating with the centra, the capitula mostly intervertebrally, in any case close to the anterior end of the centra. The tail-vertebrae possess very typical, movable chevrons, placed intervertebrally, and bear an extraordinary resemblance to those of Geckos. The ribs are long and slender, but there is no sternum. The fore- and hind-limbs are pentadactyle, in opposition to the invariably four-fingered Stegocephali. The shoulder-girdle consists of scapulae, coracoids, clavicles, cleithra, and a T-shaped interclavicle. The pelvis also resembles that of certain Stegocephali by the separately ossified, somewhat disc-shaped, flat ischia and pubes, which seem to have been joined together by cartilage into one broad mass.
Hylonomus, Dawson's type of Microsauri, was found in the Coal-measures of Nova Scotia, within decayed tree-stumps. Closely allied, if not identical, but much better known is Hyloplesion, e.g. H. longicostatum of the uppermost Permian of Nyrschan in Bohemia. Total length under 4 inches; eyes with bony sclerotic rings; neck short. The truly Permian genera Dawsonia, Melanerpeton, Orthocosta, and Seeleya are allied forms, the last scarcely one inch in length, but well preserved. Petrobates of the Triassic Lower Red Sandstone of Saxony has an arrangement of the ventral dermal armour closely resembling abdominal ribs.
Order II. PROSAURI
Mostly extinct, chiefly Permian and Triassic, terrestrial, unarmoured reptiles with deeply biconcave vertebrae, numerous intercentra and chevron-bones, fixed quadrates, complete pentadactyle limbs and shoulder-girdle, entepicondylar foramina, acrodont teeth, and many small abdominal ossifications.
The Prosauri differ from the Microsauri, with which they are closely allied, by the more advanced solidification of the vertebrae, the reduction of the tubercular portions of the ribs, the presence of an entepicondylar foramen in the humerus, and the loss of the dermal ossifications on the upper surface.
Their ancestors are the Microsauri, whilst they themselves seem to be very near the root whence have sprung most, if not all, other main branches of the reptiles, notably Crocodilia, Dinosauria, and Sauria. In fact the Prosauri, although apparently few in number, seem to represent the central stem of the reptilian tree. Only one of them is still surviving, the famous Sphenodon, now represented by a single species in New Zealand.
Sub-Order 1. Protorosauri.–The ventral half of the pelvis seems to have formed one broad, continuous mass of cartilage in which the pubic bones are represented by a pair of oval, rather disc-shaped ossifications, while the ischia are more elongated. The pelvis consequently still bears a great resemblance to that of the Microsauri, and thereby also to the Stegocephalous condition, but the ilium seems to be attached to more than two vertebrae. The vertebrae are deeply biconcave, perhaps even with a persistent continuous chorda. The neural arches bear high, laterally compressed spines, but no diapophysial or lateral processes, the ribs being placed mostly intervertebrally and having lost their tubercular portions. The ribs are continued to about the sixth caudal vertebra. Intercentral wedges exist in an unbroken series between all the vertebrae from the atlas to the tail, where they are represented by movable chevrons. A costal sternum seems to be absent, unless it was quite cartilaginous. The shoulder-girdle is complete, consisting of a long interclavicle, clavicles, disc-shaped coracoids, and scapulae; but there are no cleithra, and no indication of precoracoids or even notches in the coracoids. The fore- and hind-limbs are complete and primitive, with five digits. The abdomen is protected by numerous oat-shaped little {291}ossifications, which are arranged in many transverse or rather chevron-shaped rows, still greatly resembling the condition prevailing in the Microsauri, except that they have sunk deeper into the skin, being no longer directly covered by the scales. The skull, being no longer completely encased by bones, and possessing now wide supra- and infra-temporal fossae, appears at first sight much like that of a generalised lizard, except that it possesses three very conspicuous and distinct arcades in the temporal region: namely, the orbito-squamosal bridge across the temporal fossa, formed by the postorbital and squamosal; the arch formed by the squamosal with the postero-lateral buttress of the parietal; and the infratemporal arch or jugal bridge. The jugal itself is long, connecting the quadrato-jugal with the maxillary and lacrymal, and sending up an ascending process to the postorbital bone, thus taking a considerable share in the formation of the orbit. The quadrato-jugal is small, apparently fused with the quadrate, which itself is firmly overlaid by the squamosal. The quadrates are further fixed by being buttressed by the pterygoids, which rest upon short basisphenoid processes and extend far forwards, meeting the vomers and separating the palatines. The premaxillae are short, the nares small and terminal, the nasal bones are large. There is a small interparietal foramen. The teeth are acrodont and pointed, forming unbroken series on the premaxillaries, maxillaries, palatines and dentaries, and there are scattered little teeth on the vomers.
Palaeohatteria longicaudata from the Lower Red Sandstone of Saxony. Total length about 18 inches, with six cervical, twenty trunk, three or four sacral, and about fifty caudal vertebrae. The teeth are ankylosed with the supporting bones. The five fingers have 2, 3, 4, 5, 3 phalanges respectively. For the skull see Fig. 54, G, p. 280. Telerpeton elginense from the Triassic sandstone of Scotland, and perhaps Saurosternon of the South African Karroo sandstone seem to be allied.
Protorosaurus (πρῶτος = first, ὤρα = spring, or dawn, not Proterosaurus) apparently several species, e.g. P. lincki in the Upper Permian (marl-slate and magnesian limestone) of Thuringia and Durham. About 4 or 5 feet long, and in its general appearance rather like a Monitor-lizard, with about eight cervical vertebrae, most of which carry slender backwardly-pointing ribs, sixteen long-ribbed trunk-vertebrae, followed by three or four {292}sacrals and more than thirty caudals, some of which have bifurcated spinous processes.
Sub-Order 2. Rhynchocephali.–The ventral pelvic bones resemble those of lizards and enclose a wide pubo-ischiadic foramen. There are only two sacral vertebrae. The abdominal ribs are closely packed, each transverse set consisting of only three rod-shaped pieces instead of many small oat-shaped nodules. The intercentra are sometimes suppressed in the trunk-region.
Rhynchosaurus from the Upper Trias of Warwickshire and Shropshire, and Hyperodapedon of the same age, found at Elgin, in Warwickshire, and also in Central India, are rather large, H. gordoni measuring 6 feet in length. Both have a short, broad, and stout cranium, and curved down, toothless premaxillae, hence the name Rhynchocephali; the nares are confluent; the teeth are numerous and small, and are liable to be worn down so that the animals ultimately bite with the edges of the jaws, to which the teeth are ankylosed. The premaxillaries of Rhynchosaurus are curved downwards over a slightly upcurved, likewise toothless process of the mandibles, which form a strong symphysis. All the teeth are very small, absent, or minute on the mandibles, forming one series on the maxillae, several rows on the vomers and especially on the palatines, which latter remain separated from each other. Hyperodapedon seems to have lost the intercentra; its vertebrae are solid, those of the neck are opisthocoelous. The interparietal foramen is likewise abolished. The hook-shaped end of the curved-down premaxillae fits into a bifurcation of the mandibles in front of their stout symphysis. The teeth are similar to those of the other genus. Whilst these, the earliest known genera of Rhynchocephali, are already in various ways rather specialised, e.g. the hooked beak and the loss of the intercentra, the two following fossil genera, although of much later date, namely Upper Triassic, are more closely allied to the recent Sphenodon.
Homoeosaurus pulchellus and other species in Germany are only 6 to 8 inches long. The vertebral column consists of twenty-three presacral and many caudal vertebrae. The first five cervicals are devoid of ribs. Intercentra are restricted to the neck and the anterior portion of the tail. The mandibles are not fused together. The nares are divided by a bony septum. Each premaxillary has one rather broad tooth.
The teeth of the maxillaries and mandibles are triangular, much worn down in front. The ribs are devoid of uncinate processes. Closely allied but larger is Sauranodon of France, which has lost the upper teeth and uses the sharp margins of the jaws instead.
Pleurosaurus of Germany and France, about 5 feet in length, is remarkable for the shortness of its still pentadactyle extremities, for its short neck, and very long tail;–an interesting parallel to what has happened in many genera of recent lizards.
Sphenodon s. Hatteria is the sole surviving member of the whole group of Prosauria, and is represented by one species only, S. punctatum, in New Zealand. As the last living witness of bygone ages this primitive, almost ideally generalised type of reptiles, this "living fossil," deserves a detailed description.
Total length of very large male specimens up to two feet and a half; in general appearance like many a stoutly built lizard. The general colour of the skin is dark olive-green with small white or yellowish specks on the sides. A series of slightly erectile spines of yellowish colour extends from the top of the head to the end of the tail, but is interrupted on the neck; they are cutaneous, covered with a thin sheath of horn. The under surface is covered with numerous scales, arranged in transverse rows; the rest of the body is rather granular. The tail is thick, slightly compressed laterally. The eye is large, dark brown, with a vertical pupil.
Those who are satisfied with superficial resemblances still group this creature with the lizards, but it reveals itself as a primitive reptile or Prosaurian by the following characters, every one of which distinguishes it from the lizards:–The temporal region is bridged by three bony arcades. The large vomers, palatines, and pterygoids form a broad bony roof to the mouth; the large quadrates are firmly fixed by the pterygoids, squamosals, lateral occipital bones, and by the jugal bridge. The vertebrae possess an unbroken series of intercentral wedge-bones. There is an elaborate system of abdominal ribs. The humerus has an entepicondylar foramen, and there is also, in contradistinction to the fossil Rhynchocephalia, an ectepicondylar foramen for the passage of the radial nerve. The carpus still has the primitive number of ten bones, all of which remain separate, including the intermedium. Of soft parts are to be mentioned above all the entire absence of external copulatory organs, Sphenodon being the {295}only recent reptile which is devoid of them; a most primitive condition, sufficient by itself to separate this creature from all the other living reptiles.
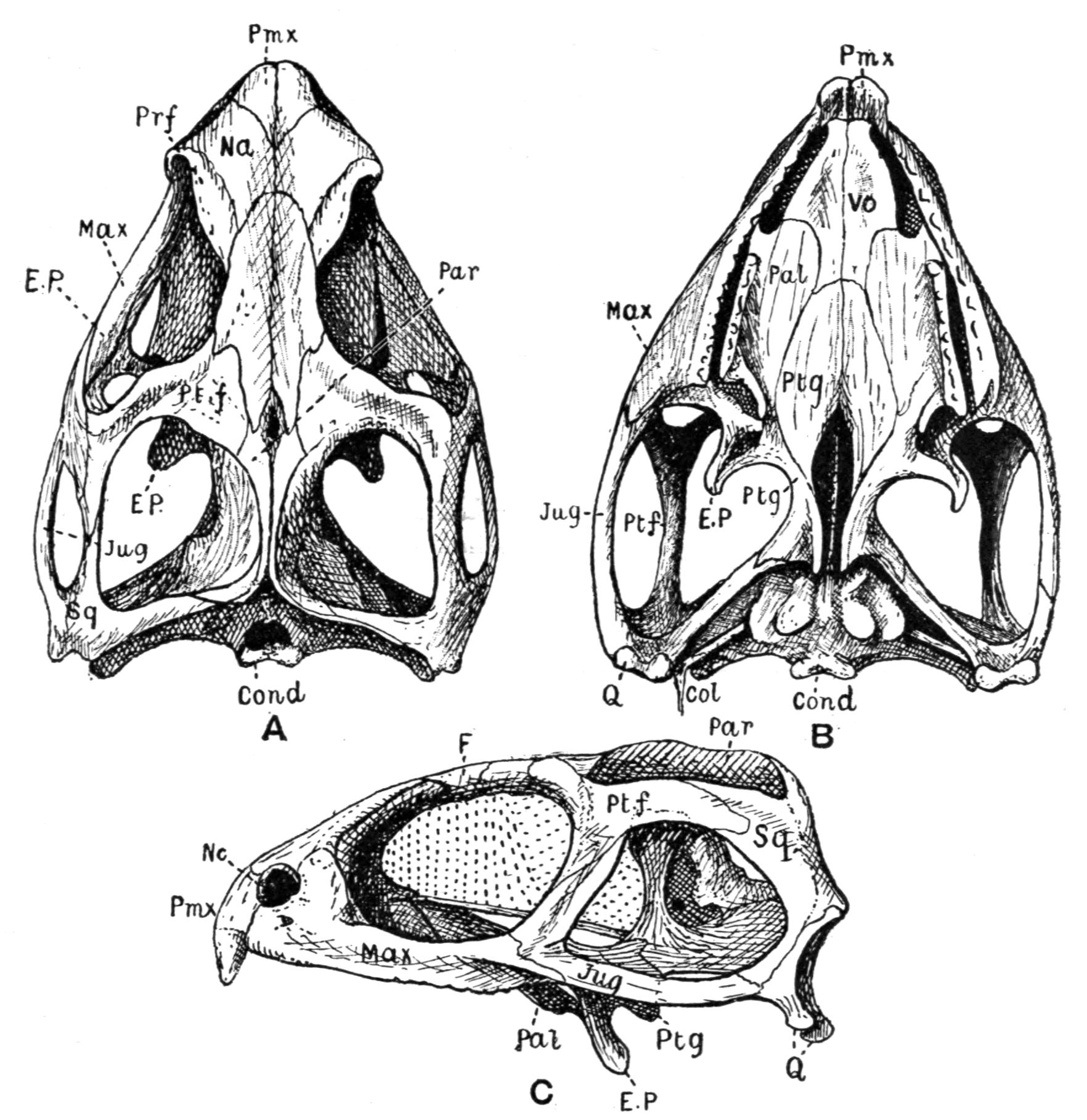
Fig. 59.–A, Dorsal; B, ventral; C, left-sided view of the skull of Sphenodon. × 3⁄2. Col, columella auris; Cond, occipital condyle; E.P, ectopterygoid; F, frontal; Jug, jugal; Max, maxillary; Na, nasal; No, anterior nasal opening; Pal, palatine; Par, parietal; Pmx, premaxillary; Prf, prefrontal; Pt.f, postfrontal and postorbital; Ptg, pterygoid or endopterygoid; Q, quadrate and quadrato-jugal; Sq, squamosal; Vo, vomer. See also Fig. 54, B, p. 280.
The supratemporal bridge is formed by the squamosal and postorbital (Fig. 59, C, Pt.f), the latter being continued forwards and fused with the postfrontal (A, Pt.f). The postorbital joins the ascending branch of the jugal, both together forming the hinder border of the orbit; this is bordered below chiefly by {296}the maxillary, which is long, while the anterior process of the jugal is much reduced. There is no pre-orbital fossa. The nares are terminal and lateral, well separated by the premaxillaries. The posterior temporal bridge is formed by the squamosal and parietal, the bridge extending laterally over the quadrate and enclosing a wide space between itself and the buttress-like expansion of the lateral occipital bone. The space enclosed between this occipital buttress, the quadrate, and the pterygoid support of the latter is likewise very large; it is of course the cavity of the middle ear, and as such is crossed by the columellar chain of the ear. The infratemporal bridge or jugal arch is formed by the jugal, which joins the descending process of the squamosal, and by the quadrato-jugal, which is small and fused with the quadrate. The latter is consequently very firmly fixed.
The teeth are acrodont, ankylosed in one series with the supporting bones, triangular and much worn down in older specimens. Originally there seem to be several in the premaxilla, but the adult bite with the somewhat curved-down portions of the premaxillaries themselves, or with what remains of the fused bases of the original teeth, which then, together with the bone, look like one pair of large chisel-shaped incisors. The lateral edges of the palatines likewise carry teeth, those of the mandibles fit into the long slit-like space between the palatine and the maxillary teeth. Young specimens have a few small teeth on the vomers, which are large, and separate the long choanae from each other. The pterygoids form an anterior symphysis, posteriorly they rest upon short processes of the basisphenoid and send short flanges to the quadrates.
The vertebral column is very primitive. The atlas is still typically temnospondylous. The first intercentrum or fused pair of basiventrals is broad and thick, and forms the ventral half of the atlas-ring, which articulates with the first centrum and with the second intercentrum. The irregularly shaped neural arches remain separate from each other and from the centrum; they carry on the dorsal side a pair of disconnected supradorsals, the so-called pro-atlas. The second intercentrum is fused with the first and second centrum. The second to ninth intercentra have low median ridges or knobs, and are as a rule more firmly attached to the cranial ends of the centra. Those of the trunk are small. From the third or fourth caudal vertebra {297}backwards they appear as chevrons, articulating more with the vertebra in front than with the one behind. The bases of the right and left chevrons are frequently fused across so that the caudal canal is completely surrounded by bone, a feature common in Dinosaurs. Every intercentrum, be it a pair of chevrons, or an unpaired nodule, or crescent, extends dorsalwards into a fibro-cartilaginous ring which surrounds the chorda. The centra of the vertebrae are deeply amphicoelous, the cavity being filled throughout life by the chorda; but the middle of the centra is solid. Most of the caudal vertebrae are transversely divided into two parts, the posterior of which carries the greater share of the arches; they resemble in this respect those of lizards, and the lost tail is likewise reproduced. The first three ribs are represented by bands of connective tissue. The first is attached to the side of the first intercentrum; the second arises from the second intercentrum, and forms a small tubercle on the side of the second centrum; the third behaves similarly. The vertebral arteries and lateral strands of the sympathetic nerve-chain pass through these double basal attachments of the reduced ribs. The other ribs are osseous; they possess short capitula which retain their partly intercentral attachment, while the short tubercula are carried by low processes of the centra, not of the neural arches. Already in the thoracic region both capitulum and tuberculum merge into one facet, at first dumb-bell shaped, further towards the tail oval, gradually shifted backwards and dorsalwards upon the middle of the centrum, until the facet reaches and ultimately lies right across the neuro-central suture. The first few caudal vertebrae also possess ribs, which are however very short and fuse with the diapophyses, immediately below which lies the neuro-central suture.
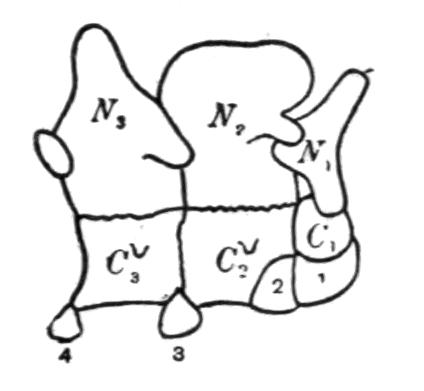
Fig. 60.–The first three cervical vertebrae of Sphenodon. 1, 2, 3, 4, Intercentra; C1-C3, centra; N1-N3 neural arches.
The whole column consists of twenty-five presacral, two sacral, and about thirty caudal vertebrae. Some of the thoracic ribs have cartilaginous uncinate processes. Three or four pairs of ribs join a typical sternum, into the antero-lateral portion of which are let in the coracoids. The sternum is raised into a low median crest which fuses with the posterior branch of the {298}T-shaped interclavicle, while the lateral branches of the latter fuse with the clavicles. The coracoids are broad and entire, still without fenestrae or notches indicative of precoracoids. The parasternum is very elaborate; it extends from the sternum to the pubic bones, and consists of about twenty-four transverse rows, each of which is composed of a median and two lateral splint-bones. They are irregularly shaped, partly with imbricating hooks, and are firmly attached to, in fact still connected with, the deeper portions of the cutaneous scales of the belly. The three pairs of pelvic bones are fused together at the acetabulum. Pubes and ischia each form one symphysis, and these are connected with each other by partly ossified cartilage and ligaments, so that the originally heart-shaped space is divided into a pair of ovals. The lateral processes of the pubes are thick, but very short. The ischia have postero-lateral processes. There is also a mostly cartilaginous, unpaired hypo-ischium.
The fore- and hind-limbs are still primitive in structure; both pentadactyle. The carpus consists of ten, sometimes eleven pieces, according to the single or double nature of the central element. The proximal series is formed by the radiale, intermedian, and ulnare, with a pisiform. The ulna and radius remain separate. The humerus has the usual ectepicondylar in addition to the entepicondylar foramen common to all the Prosauri and Theromorpha. The hind-limbs are typically plantigrade.
The tail is capable of regeneration, as in many lizards.
The development of this reptile has recently been studied and described by Howes,[123] who quotes the literature bearing upon the whole subject.
A good account of the occurrence and habits of the "Tuatera" has been given by Newman.[124] The Maoris call it "ruatara," "tuatete," or "tuatara," the latter meaning "having spines." Formerly common on the main islands of New Zealand, they are now apparently restricted to some of the islets in the Bay of Plenty, North Island. Bush-fires, wild pigs, dogs and cats, reptile-eating Maori tribes, and the advance of civilisation, have swept them away except on some of the small uninhabited islands, difficult of access, where they dig burrows, into which they retreat at the slightest sign of danger. They sleep during the {299}greater part of the day, are very fond of lying in the water, and they can remain below for hours without breathing. They live strictly upon animals, but these are only taken when alive and moving about. The kind of food seems to vary according to the custom or fancy of the individuals. Sir W. L. Buller observed that some of his captives stubbornly refused to eat until one day, rather accidentally, minnows were offered. Others eat insects and worms; those which live near the seashore not improbably eat also crustaceans. From November to January they lay about ten eggs–white, hard-shelled, long and oval–about 28 mm. long, in holes in the sand, where they can be warmed by the sun. They are as a rule lazy in their movements. The usual pace is a slow crawl, the belly and tail trailing on the ground, but when chasing prey they lift the whole trunk off the ground. After running, or rather "wobbling" three or four yards, they grow weary and stop. They cannot jump the smallest obstacle.
Von Haast[125] has carefully examined their habitations on the Chicken Islands. The Tuatara excavates its own hole, and this is shared sociably by various kinds of Petrels. The entrance to the chamber is generally 4 or 5 inches in diameter, and the passage leading into the inner chamber is 2 to 3 feet long, first descending and then ascending again. The chamber itself is one foot and a half long, by one foot wide and 6 inches high, lined with grass and leaves. The petrel lives usually on the left side, the Tuatara on the right side of the inner chamber. Whilst very tolerant of the bird with its egg and young, it does not allow another of its own kind to live in the same hole, which it is ready to defend by lying in such a manner that the head is placed where the passage widens out into the chamber. On putting one's hand or a stick into the burrow the Tuatara bites at them furiously. They can run very fast, and defend themselves with great pluck against dog or man by biting or scratching. As soon as the sun has set they leave their holes to seek food. During the night, and especially during the pairing season, they croak or grunt.
The eggs, having been deposited during the Southern summer, from November to January or February, in holes on a sunny and sandy spot, contain nearly ripe embryos in the following August. They are, however, not hatched until about thirteen {300}months old. In the meantime they seem to undergo a kind of aestivation. The nasal chambers become blocked with proliferating epithelium, which is resorbed shortly before hatching.
I have kept half-a-dozen specimens in a green-house for several years, and have come to the conclusion that they are dull, not companionable creatures, in spite of their imposing, rather noble appearance when, with their heads erect, they calmly look about with their large, quiet eyes. Each dug its own hole in the hard ground underneath and between large stones. At dusk they sat in front of the holes or walked leisurely to the pan with the earthworms which formed their principal food. Meat they did not touch, but they killed and chewed up lizards and blind-worms. Sometimes they soaked themselves for many hours in the shallow, warm water. The skin is shed in flakes. I never found them basking in the sun, and the pineal eye, still so well developed in these strange creatures, caused them no distress when bright light was thrown upon it. They grew tame enough not to run away when found roaming about at night, but they did not like being handled, and they inflicted the most painful bites when taken up carelessly. The biggest, a male, was rather quarrelsome, grunted much, and worried the others.
Sub-Class III.–THEROMORPHA.
The Theromorpha comprise a great number of extraordinary, extinct reptiles, which as a group had a wide range in space and time. The earliest known occur in the Lower Red Sandstone of Thuringia and Bohemia, and in the middle Permian strata of Russia. The majority have been found in strata transitional between the Permian and the Triassic age, notably in the Karroo sandstone of South Africa and in corresponding levels of North America. Closely allied to them are those of the Triassic sandstone of Elgin in Scotland, and of India. They seem to have died out with the Muschelkalk or Middle Trias.
The various genera exhibit such a diversity of structure, shape, and size, and many are still so imperfectly known, that {301}any diagnosis is liable to be faulty, even assuming that they are a homogeneous group. To avoid confusion, we characterise the Theromorpha as Reptiles with a firmly fixed quadrate, a single temporal arch, an interparietal foramen, and a pelvis in which the pubes and ischia form one stout, ventral symphysis.
The dentition is most abnormal, and permits the division of the Theromorpha into two or three main groups. In the Pareiasauri the teeth of the upper and lower jaws form rather even series of nearly equal size; smaller teeth are carried by the palatal bones. In the Theriodontia the teeth are differentiated in a truly Mammalian fashion into incisors, prominent canines, and multicuspid or tubercular molars. Each tooth, and this applies to all Theromorpha, is implanted in a separate alveolus; Tritylodon only seems to have double-rooted molars. The lower canines cross in front of the upper, just as in Mammals. In Placodus, which probably belongs to this assembly, the teeth are few in numbers, very broad and flat, especially those of the palate. In Dicynodon and Gordonia the teeth are restricted to a pair of conical, sometimes very large, tusk-like upper canines, and in Oudenodon the whole mouth is toothless.
The configuration of the skull shows two main types. In the Pareiasauri it is completely roofed in by dermal bones, the only holes on the surface being the nostrils, orbits, and the interparietal foramen.
The most striking feature of the second type of skull is the tendency to form an almost Mammalian zygomatic arch by the junction of the much elongated squamosal with the jugal bone, both abutting against a downward process of the postfrontal bone. The skull shows a pair of wide supratemporal foramina bordered by the parietals, squamosals, and postfrontals. The composition of the temporal arch varies considerably in detail, and in Cynognathus crateronotus at least there is a small hole within the arch, between the squamosal and jugal, probably the last remnant of the otherwise absent infratemporal foramen. Except in the roofed-in skulls of Pareiasaurus and Elginia there is no separate quadrato-jugal element. The quadrate is firmly fixed by the overlapping squamosal, and the whole pedicle for the support of the mandible is rather elongated, and either stands vertically or slants forwards. The mandible itself is compound. The pterygoids extend backwards so as to approach or reach the {302}distal portion of the quadrate; separate ectopterygoids do not seem to be developed. The shoulder-girdle consists on either side of a large scapula, which is mostly directed obliquely backwards, and is fused with the coracoid; a precoracoid is present or at least indicated by a notch or foramen; it is usually fused with the other bones. At least some genera possess a T-shaped interclavicle and clavicles; Pareiasaurus possesses also a pair of cleithra.
The pelvis is in every respect constructed upon the Mammalian plan. The three constituent parts meet at the acetabulum, and the ventral bones, pubes and ischia, form one broad symphysis, leaving two, sometimes very small, obturator-foramina. The ilium is attached to one to five sacral vertebrae, and since the whole pelvis slants obliquely downwards and backwards, this sacral attachment is distinctly pre-acetabular, perhaps most markedly so in Dicynodon. The limbs are mostly stout, humerus and femur with strong crests; the feet are thoroughly plantigrade, with five fingers and toes. The details of the carpus and tarsus are not well enough known to permit of generalisation, but there is a tendency to form a heel, and to develop the cruro-tarsal joint into the chief joint of the hind feet. The vertebrae are amphicoelous, sometimes with rather thin-walled centra, so that in these cases the chorda was continuous. Intercentral wedges, or basiventral elements, are frequent in the cervical and caudal regions. Most of the ribs, especially those of the neck, have a tuberculum attached to the neural arch, and a distinct capitulum which articulates either with the centrum or with the intercentrum, or lastly, if the latter is absent, between two centra. The axis and atlas vertebrae are united.
The occipital condyle exhibits every stage between the single median knob (Pareiasaurus) formed almost entirely by the basioccipital bone, a triple condyle (Dicynodon) to which both lateral and the basioccipital bones contribute, and a kidney-shaped or double condyle (Cynognathus) from which the middle or basioccipital portion is more or less withdrawn.
Dermal bony armour reached an extraordinary development on the head of Pareisaurus and Elginia; whether other parts of the body were protected is doubtful, but the flattened tops of the neural spines of Pareiasaurus suggest that they carried bony scutes. Abdominal protective ossifications are unknown. {303}Many of the Theromorpha[126] reached a considerable size, massive skulls of one foot in length being not uncommon. The tail was comparatively short.
The many resemblances of these strange creatures to Mammals have naturally suggested that the Mammalia have sprung from some such Theromorpha or "beast-shaped" animals. The resemblances are chiefly the dentition, the zygomatic arch, the pelvis, the cruro-tarsal joint, the scapula which is sometimes possessed of a spine, and the occasionally double occipital condyle. The general shape of the skull of Cynognathus is indeed strikingly like that of a Carnivorous Mammal, and the shape of the whole body suggests rather a Mammal than a reptile; and when we have to deal with the fragmentary skulls of Tritylodon (cf. p. 309) it is, indeed, difficult to decide to which of the two classes such a creature belongs. But the Theromorpha possess a number of important characters by which they reveal themselves at once as reptiles: (1) the large and fixed quadrate bone, which is still the sole support of the lower jaw; (2) the compound mandible, which is composed of at least an articular, dentary, angular, supra-angular, and splenial element; (3) the interparietal foramen; (4) the possession of prefrontal and postfrontal bones, sometimes also postorbital, supratemporal, and quadrato-jugal bones. Of course, any of these ancestral bones may be lost, and the interparietal hole may be closed as in tortoises and crocodiles. We can also imagine that the quadrate may be relieved of its jaw-bearing function and become loosened, but this is not easy, considering the strong development of the squamoso-quadrate pedicle. Those Theromorpha in which the quadrate itself is small, whilst the squamosal reaches down, or at least approaches the mandible, as in Dicynodon and Gordonia, are so hopelessly pledged, or specialised in other directions, that it is impossible to connect them ancestrally with Mammals.
However, it is beyond reasonable question that the Mammals have sprung from some reptilian stock (the attempts to derive {304}them from Amphibia, without the intervention of Reptiles, are as gratuitous as they have proved futile), and the Theromorpha undoubtedly comprise creatures which of all animals approach nearest to Mammals, and coincide with them in most important features. But we have not yet found a single Theromorph which can claim to be a direct ancestor of Mammals. Since the latter occur already in the Trias, we have to look for their reptilian forefathers at least in the Lower Permian, and this naturally excludes all the known forms. The filling up of this gap is but a question of time.
The ancestry of the Theromorpha themselves is also shrouded in mystery. Attempts have been made to connect them with the Permian Protorosaurus, Palaeohatteria, and Eryops. On the other hand, some retain various Stegocephalous reminiscences (e.g. the roofed-in condition of the skull by membrane-bones, amongst which, besides others, supratemporals and postorbitals can be recognised; occurrence of cleithra in Pareiasaurus; distinct epiotic bones in Elginia). Although they have died out as a group, they have perhaps given rise to several side-branches, one of which (leaving aside the question of Mammalian origin) seems to have flourished as the Dinosauria.
We divide the Theromorpha into four orders, which are, however, liable to run into each other, and it is reasonably to be hoped that many forms may be discovered which will connect not only these provisional orders with each other, but also with other sub-classes.
Order I. PAREIASAURI
Cranium completely roofed in by membrane-bones. The only foramina are the nostrils, orbits, and the interparietal foramen. The teeth are comparatively small, and stand in even series in both jaws.
Pareiasaurus, several species from the Karroo sandstone of South Africa. P. baini was an extremely clumsy brute, of most uncouth appearance, standing between 2 and 3 feet high, and measuring with the short tail nearly 8 feet in length. The skull is very massive, 18 inches long and slightly broader, with a rugose, deeply pitted surface. The teeth are thickly enamelled, serrated at the margin, with many pointed cusps; those {305}of the vomer, palatines, and pterygoids are recurved and arranged in several longitudinal rows. There is a small incisive foramen in the premaxilla; the choanae lie within the pterygoids. The palate has a pair of large lateral vacuities. Between the squamosal and quadrate is a small foramen, as in Belodon and Sphenodon. The nares are terminal, bordered behind by the nasals, and divided by the premaxillaries. The occipital condyle is a single knob, but the lateral occipital bones also partake in its formation. The shoulder-girdle is strong. The scapula slants backwards, is broad, and possesses a longitudinal spine, an almost exclusively Mammalian character. The scapula, coracoid and precoracoid are fused together, and are united ventrally with those of the other side. There is a T-shaped interclavicle, a pair of clavicles, and a pair of slender, long cleithra, which extend along the upper anterior margin of the scapulae. The humerus possesses enormous crests. The broad ilium is attached to two, or perhaps three, sacral ribs. The acetabulum is closed. The pubes and ischia are united into one broad mass of bone, and the obturator-foramina seem to be just large enough to permit of the passage of the nerve. Both fore- and hind-limbs are plantigrade and five-toed. The tibia articulates with one large bone, which is supposed to represent the united astragalus and calcaneum, the latter being without an indication of a prominent heel, although there is a tendency to develop the crurotarsal into the chief joint. The number of vertebrae amounts to eighteen presacrals, eight to ten of which are cervicals. There are two or three sacral and about twenty-four mostly shortened caudal vertebrae. The latter possess intercentral wedges and chevron-bones; wedges occur also between the cervical and some thoracic vertebrae. Some of the posterior cervical ribs are very peculiar–straight, broadened out, turned backwards, partly overlapped by one another, and 18 inches long, recalling the first two ribs of the crocodiles. Sternum and abdominal ribs are unknown.
Elginia mirabilis.–The skull (Fig. 54, A, p. 280)–nothing else is known–indicates one of the most remarkable reptiles hitherto found on this side of the Atlantic. It was discovered in the Red Sandstone of Elgin (Lower Trias). The skull reminds us in its general shape and by its spikes and horns of the little American Iguanoid lizard, Phrynosoma. The length of the cranium is about 6 inches, the distance between the tips of the two largest {306}horns measures 9 inches. The teeth are small and resemble those of an Iguana in their shape and finely serrated edges, indicating herbivorous habits, but there are also several rows of smaller teeth on the palate, the configuration of which is not unlike that of Sphenodon. The top and sides of the skull, except the interparietal foramen, the orbits, and nostrils, are completely encased by rugose, pitted, dermal bones, most of them with strange, horn-like spikes. In the encasement of the temporal region can be discerned a postfrontal, parietal and squamosal, a conically projecting epiotic, a postorbital and supratemporal, a jugal and a quadrato-jugal, which latter almost completely covers the quadrate bone. The interparietal foramen lies far forwards, almost on a level with the orbits. The nostrils are terminal, surrounded by the short nasals, the maxillaries and the premaxillaries, which latter divide them.
Order II. THERIODONTIA.
The cranium is not roofed in, but shows a pair of large supratemporal fossae, bordered below by the zygoma, which is formed mainly by the squamoso-jugal bridge, and is shut off from the orbit by the postfrontal joining the bridge. The teeth are differentiated into incisors, canines, and molars (Fig. 54, C, p. 280). The lower canines close in front of the upper.
Cynognathus, Karroo formation of South Africa. C. crateronotus has a skull about 16 inches long, looking like that of a ferocious Carnivore; there are four incisors, huge canines, and nine molars, the latter with serrated edges and anterior and posterior cusps. The wide supratemporal fossa is bordered and closed behind by the broad lateral extension of the parietal, which joins a similar extension of the squamosal bone. The latter is very long, extending to the postfrontal and to a bone which, bordering the orbit posteriorly, is either an upward branch of the jugal, or a postorbital bone; the latter interpretation is made probable by the occurrence of a suture with the jugal in C. platyceps. The jugal bone is very long, beginning at the quadrate, running along the squamosal, and forming the lower border of the orbit.
The number of vertebrae is large, there being as many as twenty-nine presacrals, six of which belong to the cervical region. {307}The atlas is fused with the axis; most of the thoracic ribs articulate partly upon the intercentra. The lumbar ribs are very peculiar; they are much expanded horizontally, and overlap each other, forming thereby intercostal foramina. The broad ilium is attached to three or four sacral ribs. The acetabulum is closed. The ventral side of the pelvis shows a broad symphysis and has a pair of obturator-foramina. The scapula is large, directed backwards, and shows a distinct, very Mammalian spine; it is fused with the coracoid and precoracoid.
The occipital condyle of C. platyceps is kidney-shaped, with the concavity directed upwards; in C. berryi it is separated into two distinct knobs, the middle, basioccipital portion being apparently wanting. The mandible possesses a long coronoid process which ascends obliquely into the temporal fossa.
Aelurosaurus, Lycosaurus, Galesaurus, and many others, likewise of the Karroo formation. In the first genus the splenial bones help to form the symphysis of the lower jaw; teeth are also found on the palate, in opposition to Lycosaurus. This has a skull 6 inches in length; the dental formula on either side is i. 4/3, c. 1/1, m. 5/5; the molars are slender, conical, and recurved. Galesaurus seems to have been rather small, the low, triangular skull measuring only 2 to 3 inches in length, with four or five sharply pointed incisors, prominent canines and four or five small multicuspid or deeply serrated little molars.
Endothiodon, with several species from the Karroo formation, is of uncertain systematic position, only imperfect skulls being known. The animals must have been large and bulky, the skulls being very massive and at least one foot in length. The premaxillaries and the maxillaries are toothless, their alveolar borders forming cutting, prominent edges. The same applies to the very strong lower jaw; but there is a pair of tooth-like stout projections in the upper and lower jaws in the place of canine teeth. True, enamelled, small, apparently conical or low and perhaps blunt teeth occur on either side in one or three longitudinal series upon the palate, and in corresponding positions on the inner sides of the two halves of the lower jaw. It is doubtful if the upper teeth are carried by the palatines or by the broadened inner flanges of the maxillaries. The choanae seem to lie between the pterygoids and the palatines, incompletely roofed in by ventral extensions of the latter towards the middle line.
Direct affinity of Endothiodon (ἐνδοθί, within) with Placodus is unlikely; the same applies to the Dicynodontia, although the restriction of the teeth to the palate seems to point as much to the former genus as do the toothless cutting edges of the jaws to the forms like Oudenodon.
Other Theriodont reptiles have been described from the upper Permian of Russia, for instance Deuterosaurus and Brithopus, but the determination rests upon insufficient fragments. North America has yielded many strange Theromorphous fossils, some of which may belong to the Theriodont order, while others seem to be intermediate between this and the other orders. Diadectes of Texas, for instance, seems to be a Theriodont creature; while in Empedias molaris, with a skull about 8 inches in length, the teeth form an uninterrupted series without distinct canine tusks, and the incisors are distinguished from the molars only by the transversely broadened shape of the latter. Very small teeth are arranged along the median line of the vomer and united palatine bones. In Clepsydrops, Dimetrodon, and Naosaurus of Texas the teeth are differentiated into incisors, canines, and molars, although not so regularly as in the typical Theriodont forms described above, one or more pairs of teeth being enlarged into canine-like tusks. In the latter two genera the spinous processes of the thoracic vertebrae are enormously elongated, standing up vertically to a height of 2 feet, while the centra of the vertebrae measure only one inch in diameter. In Naosaurus claviger these upright spines carry on either side half a dozen transverse projections. Stereorhachis of the Permian of France is typically Theriodont in the structure of its shoulder-girdle, humerus, and pelvis, but the dentition is composed of 3/3 incisors, no canines, and 6/10 pointed molars.
The following genera have been placed by Seeley in the family Gomphognathidae. Microgomphodon, with broader and less prominently multicuspid teeth than those of the typical Theriodonts, seems to lead to Gomphognathus, which has the following dentition: i. 3/3, c. 1/1, m. 12/12, with a long diastema between the canines and molars, some of which latter are nearly as broad as they are long, and have comparatively low tubercles on the crowns. The skull is remarkably like that of a Carnivorous Mammal. There are incisive foramina behind the premaxilla. The maxillaries and palatines form a united palatal roof, and behind them open the {309}choanae. The occipital condyle is kidney-shaped. The mandible is most extraordinary, approaching that of the Mammalian, especially the Marsupial type, except that it is still composed of several pieces. The articular facet for the mandible is borne by an outward or lateral projection, while the bulk of the posterior half of the jaw projects inwards like a broad flange, undoubtedly recalling the so-called inner inverted angle of the Marsupial jaw. The coronoid process is large and extends far into the temporal fossa. Nearly the whole skeleton of Microgomphodon is known; the lumbar ribs are broadened and overlap as in Cynognathus, and the mandible is typically compound, so that there is no doubt about the affinities of this genus with the Theriodontia. It throws light upon Gomphognathus and the three likewise South African genera Diademodon, Trirachiodon and Tritylodon, which are all known from imperfect skulls only. Their teeth are restricted to the jaws, the molars have flat, multitubercular crowns and bear an extraordinary resemblance to those of Mammals. Some of the molars of Tritylodon are said even to possess two roots, but this point, absolutely unique in Reptiles, but common in Mammals, is not certain. The few upper incisors of Tritylodon are rather large, chisel-shaped, and extend like those of the Rodent-type back into the maxillaries; canines are absent, leaving a diastema. Trirachiodon has prominent canines, the five upper molars are multitubercular, rather flat, and much broader transversely than in the longitudinal direction. Still, even these creatures, with skulls of the size of that of a small fox, possessed distinct prefrontal and postfrontal bones, and are, at least in this respect, typical Reptiles.
Order III. ANOMODONTIA.
The cranium is not roofed in. The pedicle for the suspension of the lower jaw is much elongated, slants slightly forwards, and is composed of the long quadrate, which is laterally overgrown by the squamosal bone. The teeth are restricted to a pair of strong, tusk-like canines, or they are altogether absent. The margins of the upper and especially those of the lower jaw are trenchant, and were possibly furnished with a thick horny armature like those of tortoises.
Dicynodon, with many species from the Karroo formation of South Africa, reached formidable dimensions. The thick, curved skull is in size and outline not unlike that of a large lion, hence D. leoniceps, D. tigriceps, etc. The zygomatic arch is almost mammalian, except that the posterior boundary of the orbit is formed by a distinct postfrontal bone. The nostrils are lateral. The canine tusks (Fig. 54, E, p. 280) are very large. The choanae open behind the rhomboid vomer and between the separated palatine bones, which are posteriorly confluent with the medially united pterygoids. The latter send out flat extensions, along the lateral side of the palatines; these extensions reach the maxillaries and probably represent the ectopterygoids. The occipital condyle is distinctly triple, being equally composed of the basi- and latero-occipital bones.
The three bones of the shoulder-girdle meet at the glenoid fossa; the scapula has the indication of a spine. The pelvis is stout, attached to four or five vertebrae, converting the latter into a very Mammalian-like sacrum, the position of which lies distinctly in front of the acetabulum. The latter is closed, composed by the three pelvic bones. The pubes and ischia are fused together, leaving only a very small obturator-foramen. The limbs are plantigrade and pentadactyle, very stout; the humerus and femur have enormous crests.
Oudenodon, of which several species have been described, is so much like Dicynodon, except for the complete absence of teeth, that it has been suggested that these skulls belong to females of this genus. This view is strengthened by the fact that tusk-like canines exist, or are absent in some of the species which have been described as Cistecephalus, a genus closely allied to Dicynodon. The latter, which, like Oudenodon and Cistecephalus, occurred in Africa, extended also into India, D. orientalis having been found in the Panchet formation of Bengal, of transitional age between the Permian and Triassic epochs. Oudenodon rugosus, on the other hand, has been described from the Ural.
Gordonia and Geikia, of the New Red Sandstone of Elgin, are known from their skulls only, but these are so well preserved that there is no doubt about their close relationship to the typical South African Dicynodontia. The skull of Gordonia is about 7 inches long and 4 inches high. The canines (Fig. 54, D, p. 280) are reduced to short, but thick, conical tusks. The most {311}remarkable feature is the very elongated squamoso-jugal arch, which arises moreover from the dorsal end of the long squamoso-quadrate pedicle. The two wide and long temporal fossae are dorsally divided by narrow parietal crests. There is a distinct interparietal bone, and the usual interparietal foramen. The choanae are united and lie within the palatines, which themselves are united; the large lateral palatal foramina are otherwise enclosed by the pterygoids, quadrates, and laterally by the squamoso-jugal arch.
Order IV. PLACODONTIA.
These are the latest and last members of the Theromorpha, unfortunately known from skulls only, from the Muschelkalk or Middle Trias of Germany and Russia. The skull of Placodus gigas is about one foot long, rather high and triangular owing to the lateral expansion of the temporal arches, which diverge posteriorly. The squamoso-jugal arch is very broad, and most of the posterior border of the orbit is formed by the large postorbital bone. The maxillary bone seems to extend back to beyond the level of the orbits. The choanae lie behind the premaxillaries. The palatines and pterygoids are fused in the middle line, forming a broad bony palate, which, owing to the broad, posteriorly extended wings of the pterygoids, much resembles that of the crocodiles. The teeth are very remarkable. There are two or three stout, conical, or chisel-like teeth in each premaxillary bone, and three to five broad and flat maxillary teeth; three pairs of huge, broad, and quite flat teeth are crowded together and fill up the whole vomerine and palatine portion of the palate. These crushing teeth indicate that Placodus probably lived upon hard-shelled molluscs, and this would be in conformity with its occurrence in the Muschelkalk, which is a strictly marine deposit and full of shells. Another closely allied genus is Cyamodus, one species of which is known from Russia. The teeth are fewer in number and not so large as those of Placodus.
CHELONIA–ATHECAE–THECOPHORA
Sub-Class IV.–CHELONIA.
There is no mistaking a tortoise. The shell and the horn-covered toothless jaws separate them from all other four-footed creatures.
They may be described as terrestrial or aquatic, pentadactyle reptiles, with walking limbs or with paddles; ribs with capitular portions only, two sacral vertebrae, humerus with entepicondylar foramen, pubes and ischia forming symphyses, quadrate bones fixed, jaws without teeth, but with cutting horny sheaths. Trunk encased in a bony shell, composed of numerous dorsal and ventral dermal bones, forming a carapace and a plastron, which may or may not be covered with horny shields. Copulatory organ unpaired, cloacal opening more longitudinal than round, never transverse. Oviparous.
It is customary to distinguish the marine, paddle-limbed kinds as Turtles, the others as Land- and Water-tortoises.
Tortoises occur already in the Trias. They reached their greatest development towards the end of the Mesozoic and in the earlier Tertiary periods. They are now comparatively reduced in the number of families and genera, although they are still represented by about 200 species. The sub-class as a whole is cosmopolitan, but does not occur in the colder regions.
Their origin is quite unknown. Of recent groups only the Crocodilia and the Rhynchocephalia come into consideration. Combination of these groups with the Chelonia leads to some unknown forms whence also the Theromorpha have arisen. Palaeontology does not help us, all the leading, main groups of Chelonia having been in existence in the earlier Mesozoic ages, {313}and Palaeozoic Chelonia are still unknown. We can, however, to a certain extent, reconstruct an ideal primordial Chelonian by assigning to it all the ancestral characters actually observed in recent and fossil kinds, and by reducing to simpler conditions those features which we know to be more or less exaggerated specialisations. It is reasonable to assume that originally each metamere, except those of the anterior half of the neck and the posterior half of the tail, carried a transverse series of dermal plates, covered with horny shields, while the trunk, according to the greater bulk of the body, increased in size, converging towards the root of the neck and tail. By concentration, reduction of the number, and increase in the size of some of the remaining plates and shields, the skull assumed its characteristic box-like shape, the neck and tail becoming at the same time free. Chelonia are without doubt descendants of terrestrial, or at least semi-aquatic reptiles, and the marine paddled forms subsequently developed from terrestrial kinds.
Classification of Chelonia.–After many vicissitudes it was recognised that the Chelonia cannot naturally be divided according to the modification of their feet. The Trionychoidea were clearly separated from the rest by Stannius in 1854. Cope, in 1870, was the first to emphasise the important character of the mode in which the neck is either bent sidewards (Pleurodira) or withdrawn in an S-shaped curve in a vertical plane (Cryptodira); and he also separated Sphargis as Athecae from all the other Chelonians, for which Dollo in 1886 proposed the term Thecophora. The division of the latter into recognisable families, based upon reliable, chiefly internal, skeletal, characters, has been effected by Boulenger;[127] and his classification has been adopted in the present volume, after intercalation of the more important fossil forms. The relationships between these various families may perhaps be indicated as follows:–
| Chelonia |  |
Athecae . . . . | Sphargidae | |||
| Thecophora |  |
Pleurodira | 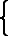 |
Pelomedusidae | ||
| Chelydidae–Carettochelydidae | ||||||
| Cryptodira |  |
Chelydridae–Dermatemydidae–Cinosternidae | ||||
| Platysternidae | ||||||
| Testudinidae–Chelonidae | ||||||
| Trionychoidea | Trionychidae | |||||
The guiding taxonomic characters are fully mentioned at the head of the different families, and are mostly internal. The following "key," adapted from Boulenger, and based upon external characters, is preferable for practical purposes.
For the position and names of the horny shields see Fig. 61 on p. 315.
Shell covered with horny shields.
Digits distinct, with 5 or 4 claws.
Pectoral shields separated from the marginals by inframarginals.
Tail long and crested. Plastron small and cruciform. North America .......... Chelydridae, p. 338.
Tail long, covered with rings of shields. Plastron large. Indo-China .......... Platysternidae, p. 345.
Tail short. North and Central America .......... Dermatemydidae, p. 341. Cinosternidae, p. 342.
Pectoral shields in contact with the marginals.
Limbs paddle-shaped, with one or two claws .......... Chelonidae, p. 378.
Shell without horny shields, covered with soft, leathery skin.
Digits distinct, broadly webbed, but with only three claws .......... Trionychoidea, p. 404.
Limbs paddle-shaped.
The vertebrae are, sometimes in the various regions of the same individual, amphi-, opistho- or pro-coelous, or even biconvex. Traces of the chorda remain longest in the middle of the centra. Intercentra occur regularly on the first two or three cervicals, and then again in the tail as paired or unpaired nodules, or as short chevrons. The latter occasionally fuse with the caudal end of their centra. Intercentral discs of fibrous cartilage occur regularly in the neck and tail. The ribs develop originally in the same transverse level with these discs, and frequently the anterior thoracic vertebrae retain this intercentral or intervertebral position throughout life. Farther back they often show a gradual change from the intercentral to a more central and ultimately {315}remarkable to a purely neural attachment. In all the Chelonia the ribs are devoid of the tubercular portion.
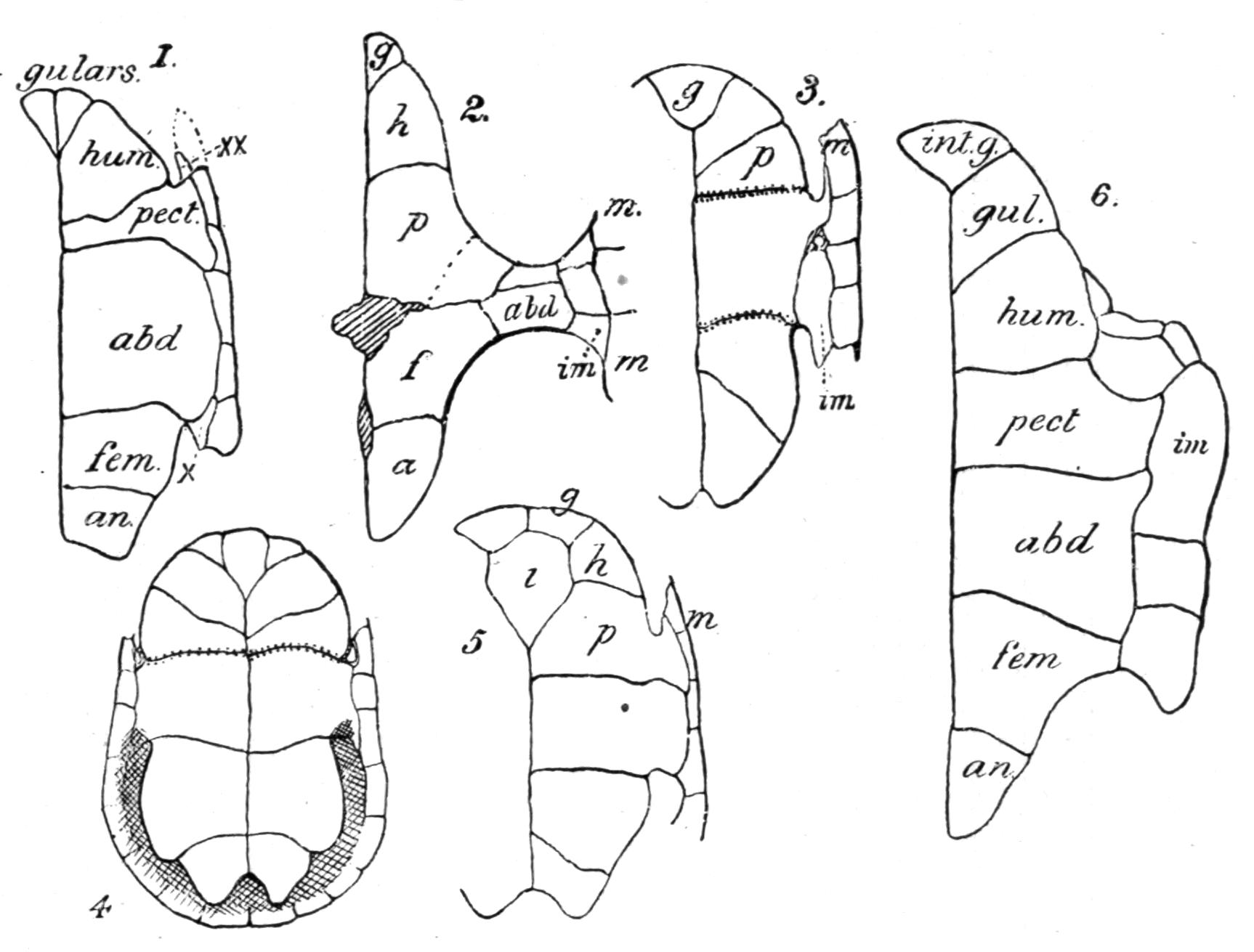
Fig. 61.–Various plastra and their horny shields. 1, Testudo ibera; 2, Macroclemmys temmincki; 3, Cinosternum odoratum; 4, Sternothaerus nigricans; 5, Chelodina longicollis; 6, Chelone mydas. a or an, Anal shield; abd, abdominal shield; f or fem, femoral; g or gul, gular, unpaired in Fig. 3; h or hum, humeral shield; i or. int.g, intergular; im, infra-marginals; m, marginals; p or pect, pectoral; x, in Fig. 1, inguinal shield constituting, with the axillary xx, the last trace of infra-marginals.
The cervical vertebrae have no ribs, except mere traces in the shape of small nodules. On the tail the ribs are often large, and, when fused with their neural supports, look like transverse processes; the whole arrangement exactly resembles that of Crocodilia. The first pair of thoracic ribs, those borne by the ninth vertebra, are peculiar. They arise from the anterior portion of the centrum, are much reduced, sometimes to mere threads of bone, and lean against the anterior rim of the second pair of ribs, in many cases without reaching the carapace. The next following ribs, those of the tenth to the sixteenth vertebra, are intimately involved in the formation of the first to seventh costal plates. The ribs of the two sacral vertebrae sometimes remain quite distinct throughout life, just touching the upper {316}ends of the iliac bones; but since these find a much more effective support in the shell, the distal ends of the sacral vertebrae fuse with the eighth, or so-called last, pair of costal plates.
The neural arch of the ninth vertebra rests upon its centrum; but the neural arches of the other trunk-vertebrae, although long, rest upon two centra; retaining, like the ribs, their original intercentral position; and in most cases the neuro-central sutures remain throughout life. The atlas and the last cervical vertebra deserve special attention. In many tortoises, e.g. Trionyx, Clemmys, Testudo, the three constituent parts of the atlas, namely, the neural arch, the centrum, and the intercentrum or first pair of united basiventralia, do not ankylose, but remain loosely connected; and the first centrum, instead of forming an odontoid process, remains movably attached to the second centrum, although it sometimes carries, and fuses with, the second intercentral piece. In other tortoises, e.g. Platemys and Chelys, however, all the parts of the atlas co-ossify and form a complete, solid vertebra which articulates by a concavo-convex joint with the centrum of the second vertebra. The normal number of cervical vertebrae is eight in all Chelonians. The first spinal nerve issues between occiput and atlas, all the others behind the neural arches of their vertebrae. The last, or eighth cervical, owing to the retractility of the neck, forms elaborate joints; its centre fits with a knob into a cup of the ninth, and its post-zygapophyses form broad, curved articulating concave facets for the reception of the anterior zygapophyses of the fixed ninth vertebra. In the Trionychidae the zygapophyses are most elaborate, and they alone articulate with the ninth vertebra, while the centra do not join, but remain, or rather become, separated by partial resorption. In the Chelonidae, in conformity with the non-retractile and short neck, all the cervical joints are much reduced.
Fig. 62.–1, The complete atlas of an adult Trionyx hurum. The second basiventral (white) is attached to the posterior end of the first centrum, which, not being fused with the second centrum, is not yet an odontoid process. 2, The complete atlas of an adult Trionyx gangeticus, still typically temnospondylous. 3, The first and second cervical vertebrae of an adult Platemys. 4, The complete atlas of a Chelys fimbriata.
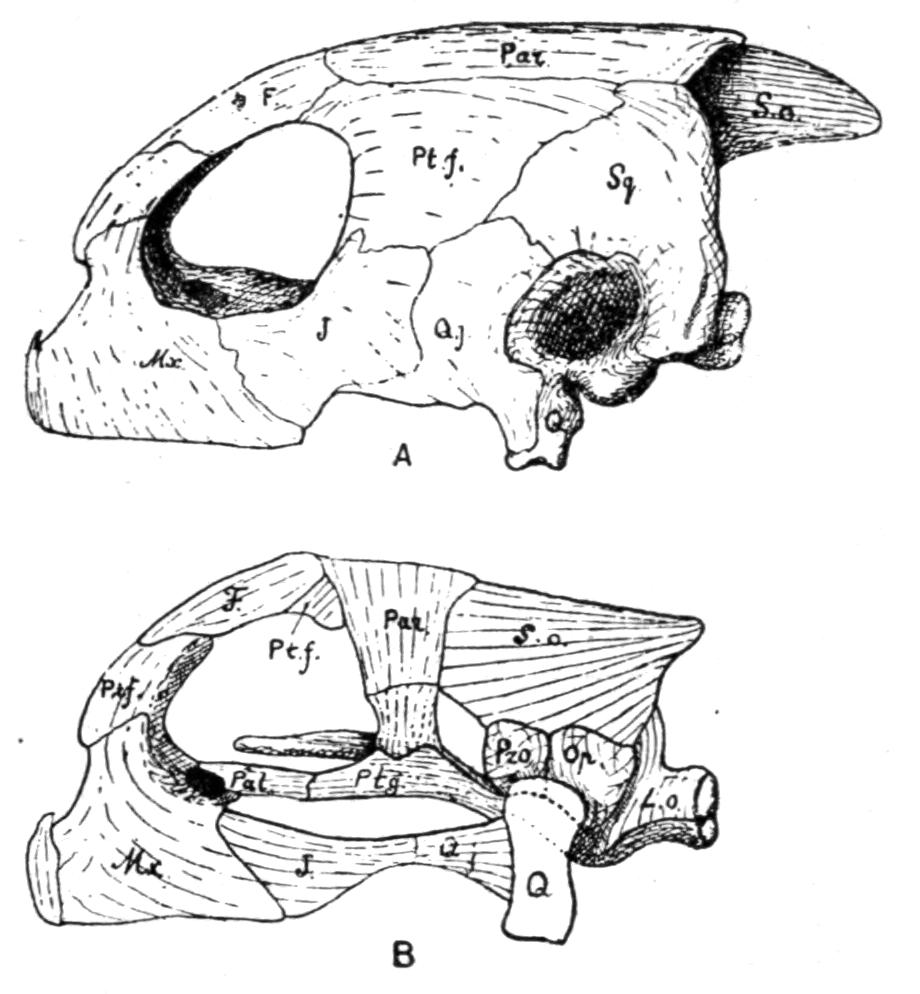
Fig. 63.–Skull of Chelone mydas. A, from the left side; in B, the postfrontal and squamosal bones have been removed, and the broad expansions of the jugal, quadrato-jugal, parietal, and quadrate bones have been reduced in order to reduce the skull to more primitive conditions. F, Frontal; J, jugal; L.o, lateral occipital; Mx, maxillary; Op, opisthotic; Pal, palatine; Par, parietal; Prf, prefrontal; Pro, pro-otic; Pt.f, postfrontal; Ptg, pterygoid; Q, quadrate; Qj, quadrato-jugal; S.o, supra-occipital; Sq, squamosal.
The skull (cf. Fig. 54, H, I, K, p. 280) agrees fundamentally with that of Sphenodon and of the Crocodilia, but it is characterised by several special features. There are no ectopterygoids or ossa transversa; no lacrymal bones, no interparietal or pineal foramen; the vomer is unpaired and the nasal bones are mostly absent, unless they are fused with the prefrontals. The premaxillae are very small. The single vomer forms a septum between the choanae; and these are, except in Sphargis, ventrally roofed over by wings sent out by the palatines. The latter form a continuous bony roof to the mouth with the pterygoids, and these diverge posteriorly, being connected suturally with the quadrates, lateral and basi-occipital bones, and with the unpaired basi-sphenoid, which appears between the basi-occipital and the diverging pterygoids, but is in most cases to a great extent overlapped by the latter. The occipital condyle is distinctly triple; the basi-occipital sometimes helps to border the foramen magnum. The supra-occipital sends out a long vertical blade, directed backwards and generally projecting far over the neck, for the attachment of the powerful cranio-cervical muscles. The quadrate is very peculiar. Firmly attached, and hemmed in on nearly all sides by the neighbouring bones, it stands nearly vertically and forms a broad articulating surface for the mandible. Its posterior side shows either a transverse, horizontal groove, in which lies the columella auris, or the groove is transformed into a more or less closed canal. Moreover, the hinder lateral margin of the quadrate forms most of the tympanic frame; its margins being curved backwards, leaving in the Cryptodira, however, a {318}wide notch behind; in the Pleurodira this part of the quadrate is transformed into a trumpet, the wide rim of which, forming a complete ring, carries the tympanic membrane. The tympanic cavity thus formed often leads into a deep recess which extends beneath the squamosal towards the opisthotic and bears some resemblance to the intricate tympanic recesses which pervade that region of the Crocodilian skull.
Dorsally the quadrate is broadly overlaid by the squamosal, which frequently forms an arch with the parietal. Anteriorly the quadrate is connected through a variably sized quadrato-jugal with the jugal; and this, by joining the maxilla and postfrontal, helps normally to form the posterior rim of the orbit. All the bones which border the temporal fossa vary much in extent in the different groups of Chelonia. The extremes are represented by Cistudo and Geoemyda, in which the bony infratemporal arch is absent, owing to the loss of the quadrato-jugal; and on the other hand by the Chelonidae and by Sphargis, in which the whole temporal region is covered over by an additional "false cranial" roof. This roof is produced chiefly by lateral wing-like expansions of the parietal and postfrontal bones, which meet the likewise much expanded jugal, quadrato-jugal, and squamosal bones. In the lower diagram of Fig. 63 (Chelone mydas) the squamosal has been removed, and the other bones have been reduced to their normal, or rather primitive condition, for comparison with the external view of the complete skull of the same animal. The lower diagram shows also the connexion of the pterygoid with a descending process of the parietal; this column, paired of course, usually contains a separate bone, the epipterygoid, the portion between Ptg and Par.
The hyoidean apparatus is well developed, and sometimes assumes large dimensions, especially in Chelys. The two pairs of "horns" are the first and second branchial arches, whilst the hyoid arches are reduced to a pair of small, frequently only cartilaginous, nodules attached near the anterior corners of the basis linguae, which generally fuses with the os entoglossum in the tip of the tongue.
The pectoral arch consists of a pair of long coracoids sloping obliquely backwards, the distal cartilages of which scarcely touch each other in the middle line, and the scapulae. The upper end of the scapula frequently touches the inside of the {319}first costal plate, protected by a cartilaginous pad. Near the glenoid cavity arises a long process (PC in Fig. 65), placed transversely and approaching its fellow. The distal end is connected with that of the coracoid by a fibro-cartilaginous band. The homology of this scapular process is not quite clear. The band just mentioned favours the idea that the process represents the precoracoid, but its being an outgrowth from the scapula suggests that it is merely the much enlarged acromion. It certainly does not represent the clavicle, which forms part of the plastron: and this is not in contact with the shoulder-girdle at all.
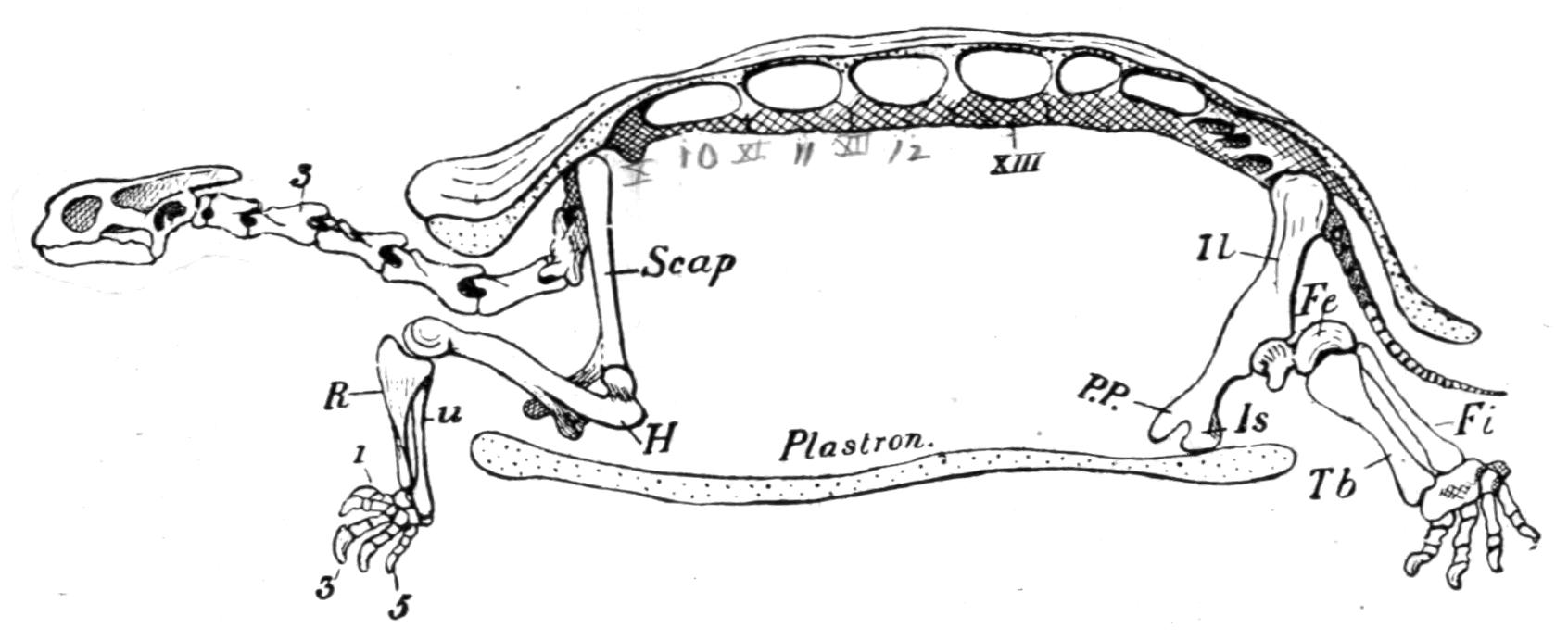
Fig. 64.–Diagram of the skeleton of Testudo elephantopus, after removal of the left half of the carapace. The plastron is roughly indicated by a section through the middle line. Fe, Femur, foreshortened; Fi, fibula; H, humerus; Il, ilium; Is, ischium; P.P., pubis; R, radius; Scap, scapula; Tb, tibia; u, ulna; 3, third cervical vertebra; 1, 3, 5, first, third, and fifth fingers; XIII, thirteenth (fifth thoracic) vertebra.
The pelvis is strong. Ilium, pubis, and ischium meet at the acetabulum. The dorsal end of the ilium is generally broadened, and is attached to one or both sacral vertebrae, but it is also in contact with the superimposed last costal plate. This additional connexion often becomes predominant and the sacral vertebrae are partly or completely relieved of the iliac support, fusing in this case more or less with the costal plates. The pubes have strong lateral processes, directed obliquely forwards and downwards. The pubes and the ischia, which latter are much smaller, form broad symphyses, and these are connected with each other by a longitudinal cartilaginous band (Chelone, Trionyx); or the connecting bridge is broad and quite ossified (Testudo), forming in the latter case two roundish obturator-foramina. Cartilage frequently remains at the anterior end of {320}the pubic symphysis, and a smaller, longer, and narrow piece of cartilage extends sometimes backwards from the ischiadic symphysis, as the so-called hypo-ischium. In the Pleurodira the ends of the ilia, and those of the lateral processes of the pubes, are much broadened and firmly ankylosed with the posterior costal plates and with the xiphiplastron respectively.
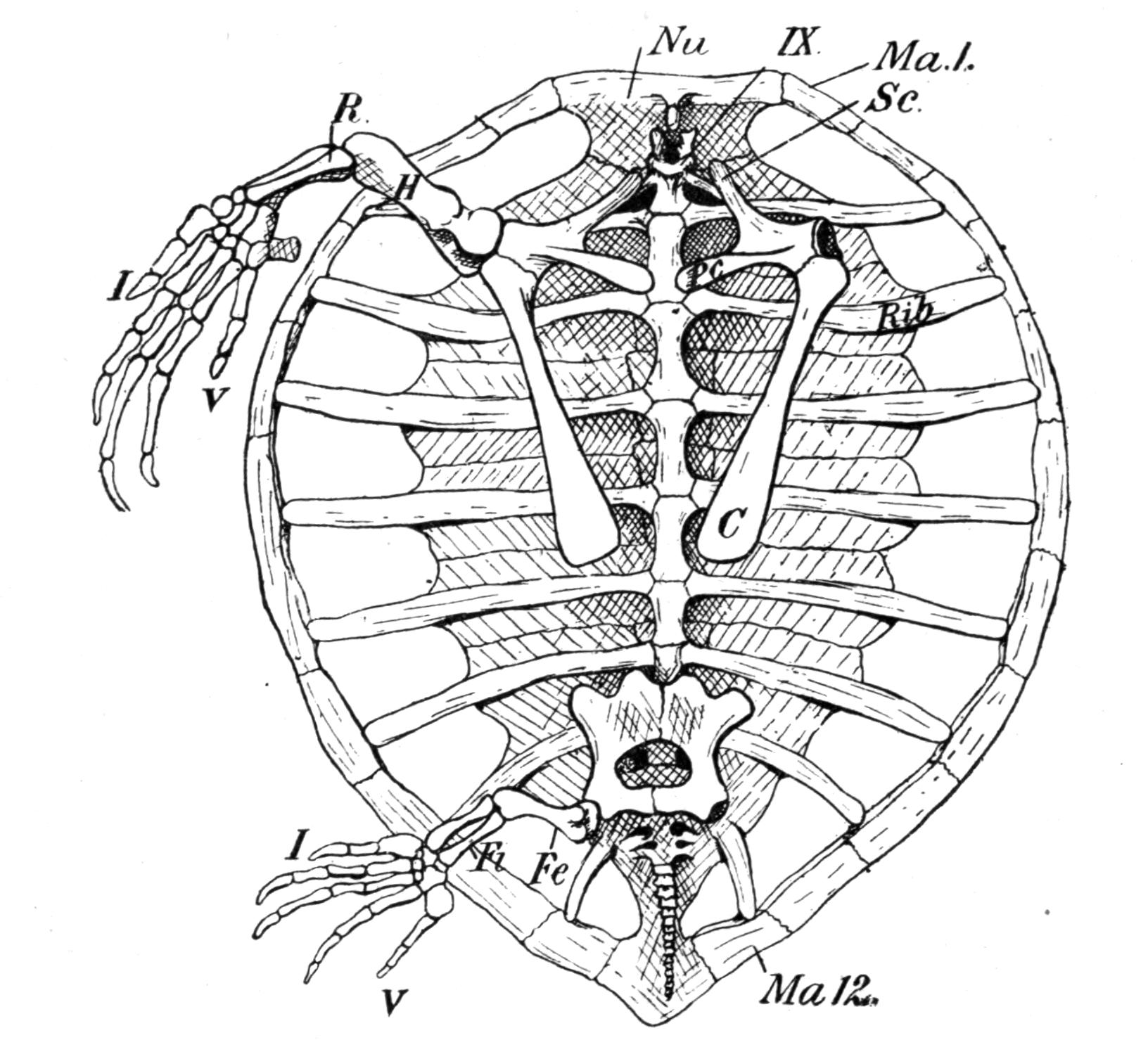
Fig. 65.–Ventral view of the bony shell of Chelone mydas, the Green Turtle, after removal of the plastron (Fig. 66). The costal plates are marked by cross lines to distinguish them from the ribs. C, coracoid; Fe, femur; Fi, fibula; H, humerus; Ma.1-Ma.12, marginal plates, some of which are fused together; Nu, nuchal plate; PC, "precoracoid"; R, radius; Sc, scapula; I, V, first and fifth digits; IX, Ninth vertebra or first thoracic.
The limbs are typically pentadactyle and complete, and are most primitive in water-tortoises, e.g. Chelydra and Emys, in which the carpus consists of the typical ten separate elements, including the pisiform. In Testudo the centrale is fused with the intermedium, and the first three distal carpals are also fused together. In the marine turtles the limbs are transformed into paddles, but all the bones retain their independence; the pisiform {321}and the first metacarpal are enlarged and flattened, thereby giving additional width to the paddle. The tarsus remains less primitive; the centrale and the proximal elements have a tendency to fuse together, most completely in land-tortoises; the fifth distal carpal is enlarged, and stands out hook-like from the rest. The number of the phalanges of the fingers and toes varies slightly. It is noteworthy that none of the Chelonia possess more than three phalanges. The three middle fingers and toes have mostly three phalanges; the pollex and hallux have always two; the number of phalanges of the fifth finger varies from three to one, of the fifth toe from two to none. The greatest reduction occurs in Testudo and its allied genera of typical land-tortoises, Homopus, Pyxis, and Cinixys, the formula for the fingers being 2, 2, 2, 2, 2 or 1, and 2, 2, 2, 2, 0 for the toes. In Pelomedusa all the fingers possess two phalanges only, owing to fusion of the first and second phalanges with each other.
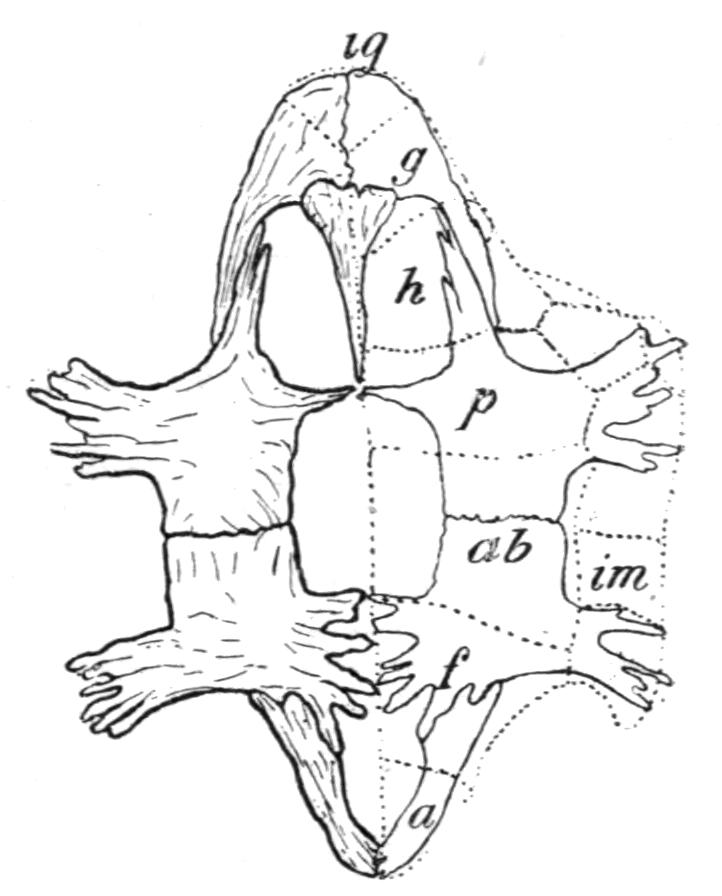
Fig. 66.–The bones composing the plastron of Chelone mydas. On the right side the position of the covering horny shields[128] is indicated by dotted lines. a, Anal horny shield; ab, abdominal; f, femoral; g, gular; h, humeral; ig, intergular; im, infra-marginals; p, pectoral.
The shell, which is the most characteristic feature of the Chelonia, consists of the dorsal "carapace" and the ventral "plastron." Each is composed of a considerable number of bony plates which arise as ossifications of nearly the whole thickness of the cutis, only a thin layer of subcutaneous connective tissue remaining soft and lining the inside of the shell. We restrict ourselves to a description of the shell of the Thecophora, leaving the discussion of the peculiar shell of Sphargis to p. 336 f. Very young tortoises are still soft, and the plates which are beginning to ossify are not yet suturally united. The plastron (Figs. 66 and 67) consists of the paired epi-, hyo-, hypo-, and xiphi-plastral plates, and the unpaired endo-plastral plate.
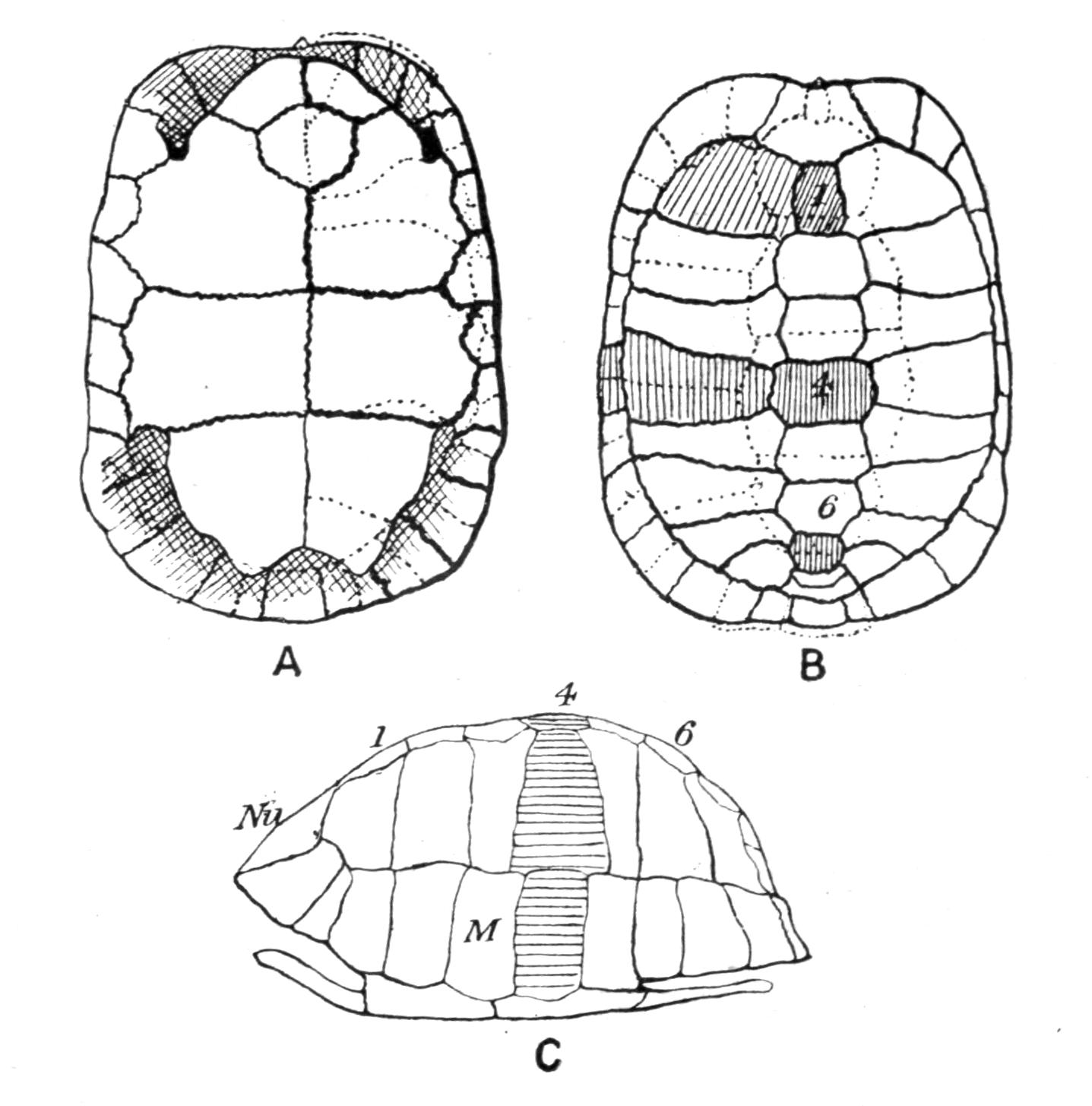
Fig. 67.–Bony shell of Testudo ibera. A, Ventral; B, dorsal; C, left-side view. In B, and on the right half of A, the position of the horny shields is indicated by dotted lines. The underlying bony plates are marked by strong lines. In B the 1st neural and costal plates, the 4th neural, costal, and 6th marginal plates, and the 7th neural plate are shaded. 1, 4, 6, First, fourth, and sixth neural plate; M, in C, fifth left marginal plate; Nu, nuchal plate.
The latter is homologous with the interclavicle, the epi-plastra are homologous with the clavicles of other Reptiles, while the other pieces are genetically derived from, and are further modifications of, the so-called abdominal ribs of the Crocodilia and Prosauria, These plastral plates are never in direct contact with the shoulder-girdle or with any other parts of the internal skeleton. In the young of all tortoises, and in the adult of the Chelonidae and Trionychidae, the several plastral plates enclose large, irregularly-shaped fontanelles. These are more or less filled up in the other groups; and in the Testudinidae especially the whole plastron forms one continuous mass. The navel is situated between the hyo- and hypo-plastrals. Both these pairs are broader than the others, and are connected with the carapace by {323}means of several marginals. The connecting region is called the bridge. In several tortoises, e.g. Emys, the connexion with the marginals is formed by ligaments only and remains movable. In others, transverse, more or less perfect hinges are formed across the plastron. A rather imperfect joint between the hypo- and xiphi-plastrals develops with age in Testudo ibera. In Cistudo and Cyclemys a very effective hinge lies below the hyo- and hypo-plastrals, just in front of the bridge; and the anterior and posterior lobes of the plastron can be closed against the inner rim of the box, fitting tightly in Cistudo. In Pyxis the front lobe only is movable.
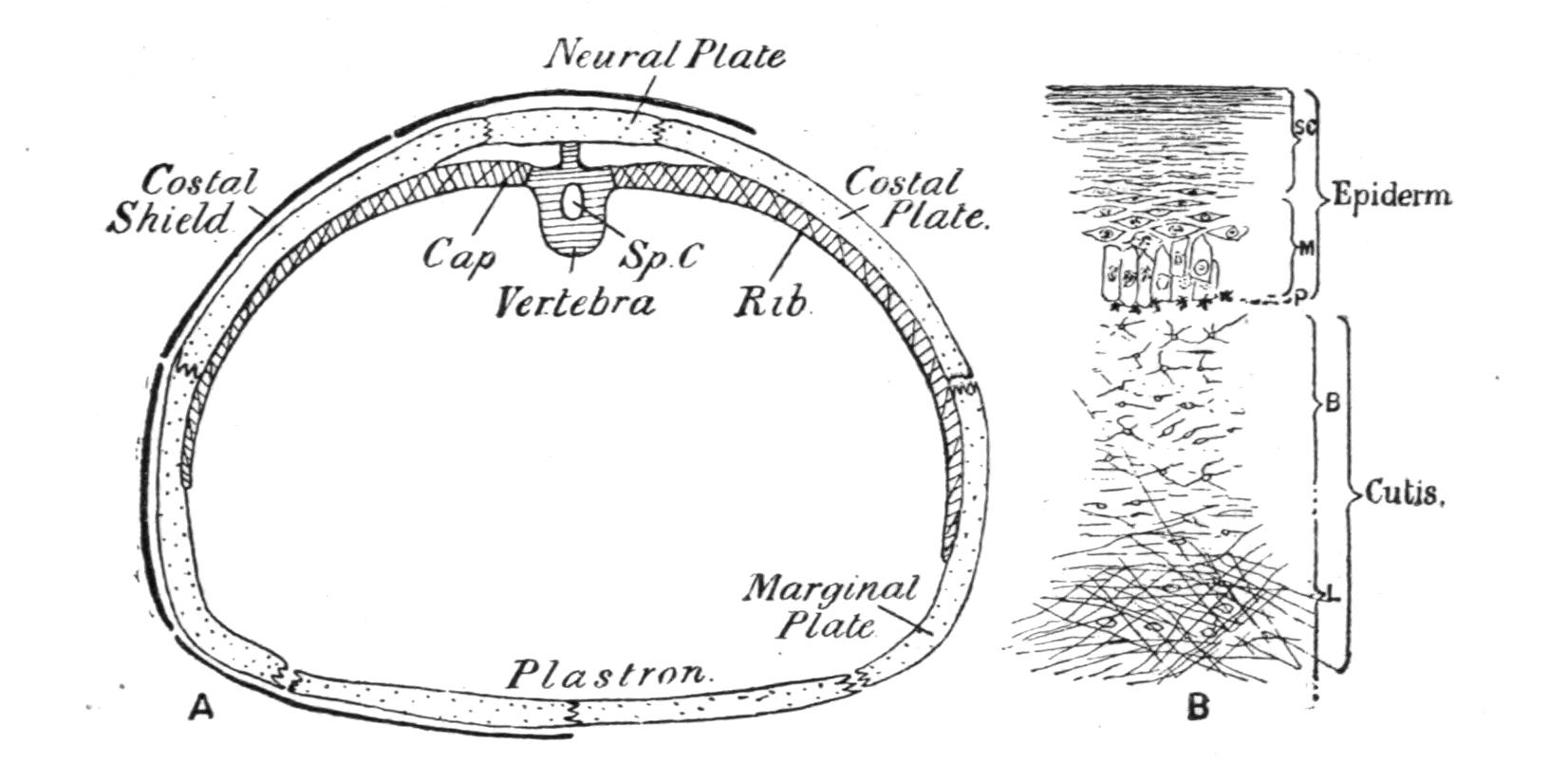
Fig. 68.–A, Diagrammatic transverse section through the shell of Testudo. On the right side the horny shields have been removed, on the left are shown the neural, costal, marginal, and pectoral shields. The bony dermal plates are dotted. Cap, Capitular portion of rib; Sp.C, position of spinal cord. B, Vertical section through part of the shell, magnified and diagrammatic. B, Bony layer of the cutis; L, leathery layer of the cutis; M, cells of the Malpighian layer; P, star-shaped pigment-cells; SC, stratum corneum, composing the horny shields.
The carapace is composed of one median series, a right and left lateral series of costal plates, and a series of marginals which surround the whole. The median series consists of one large nuchal plate, normally eight neurals and one to three supracaudal plates. The characteristic feature of the neural plates is that they are firmly fused with the broadened neural spinous processes of the underlying vertebrae. The nuchal plate lies in front of the first thoracic or ninth vertebra; it overlies the last cervical vertebrae, with the eighth of which it is connected by ligament only; but the posterior corner of the plate often fuses with the spine of the ninth vertebra. In the Chelydridae, and still {324}more in the Trionychidae, the nuchal sends out a pair of long rib-like processes, which either extend to below some of the neighbouring marginals, or their ends overlap those of the ribs of the second thoracic vertebra (e.g. Trionyx), or, lastly, they are in turn overlapped by the first costal plates (e.g. Cyclanorbis). Such rib-like processes are also present, well developed in the young, shorter in the adult, in the Dermatemydidae and Cinosternidae. It is possible that the nuchal plate represents the fused neural of the eighth and the costal plates of the ninth vertebrae. An indication of the compound nature of the nuchal may be found in the fact that two nuchals have been described in Chelydropsis carinata, a Miocene relation of Chelydra. Somewhat similar modifications have taken place in the post-sacral region. The one to three supracaudal plates are, namely, neurals which have lost their connexion with, or perhaps have never been fused with, the spinous processes of the movable tail-vertebrae. The number of neural plates is mostly eight, but there are sometimes individually nine or ten, the gradual suppression taking place first in the sacral region. When such a plate is suppressed the neighbouring costal plates usually close up and meet in the median line. In Cistudo, for instance, there are only seven normal neurals, the eighth pair of costals meet, and the original eighth neural is transformed into a supracaudal. In Cinosternum the sixth to eighth costals meet, separating the one supracaudal widely from the remaining five neurals. The meeting of the last pair of costals, with co-ordinate reduction of the neurals to seven, is almost universal in the Pleurodira; and this tendency is carried out to an extreme in the Brazilian Platemys and in the Australian Chelodina and its allies, in which all the costals meet in the middle line, and the neurals are completely suppressed. Every stage intermediate between complete neurals (Sternothaerus) and interrupted, vestigial, and vanished neurals, is still represented by some genus. This process takes place independently, both in America and in Australia, and is one of the most recently introduced modifications.
The costal plates arise, like the neurals, independently in the cutis, but they soon come into contact with the underlying cartilage of the ribs, which are long enough to reach the marginals. The ribs flatten, become surrounded by the growing membrane-bone of the plates, and the cartilage of the ribs, {325}instead of ossifying, undergoes a process of calcification. Ultimately this is more or less absorbed, its place is taken by the dermal bone, which forms so to speak a cast of the rib, preserving in many cases the shape of the vanished rib, only the capitular portions of which remain unaffected. The number of costal plates is very constant, namely eight on each side, but some fossils have nine or ten, and there are still individual variations in recent forms, indicative of that number. In a large Chrysemys concinna I find the last pair of costals clearly composed of at least two pairs, and this same specimen has nine distinct neural plates.
The marginal plates are originally paired, almost always eleven pairs, very rarely ten or twelve; an unpaired posterior plate, the pygal, is always present, and is probably the result of fusion. In the Chelonidae large fenestrae remain between the costal and marginal plates, only covered by leathery unossified cutis, and of course by the horny shields. In the Indian fresh-water genus Batagur similar windows are gradually filled up with age, and the horny shields become extremely thin and almost confluent. On the other hand, in Testudo polyphemus, the bony shell, always very thin, becomes still thinner with age and finally fenestrated by absorption.
Great reduction has taken place in the carapace of the Trionychidae. The American species of Trionyx have only seven pairs of costal plates; in Cyclanorbis the neurals are reduced to two. The whole dorsal shell is much smaller than the body, and marginal plates are absent or merely vestigial. It is doubtful if the ossifications in the posterior half of the marginal flap of some genera are homologous with true marginals.
Externally the whole shell is covered, except in the Trionychidae, in Sphargis and Carettochelys with horny, epidermal shields. These are phylogenetically older than the dermal plates, and they do not correspond with them either in numbers or in position, although there exists a general resemblance in their arrangement. On the plastron we distinguish an unpaired or paired gular, and a pair of gular, humeral, pectoral, abdominal, femoral, and anal shields (Fig. 66). Sometimes there are also intergulars, paired in Macroclemmys and Chelys, unpaired in Chelone; in many of the Pleurodira an unpaired intergular lies behind the gulars.
The carapace of most Chelonians is covered with five neural, four pairs of costal and twelve pairs of marginal shields, the last of which often forms an unpaired pygal. In front of the first neural lies the nuchal shield, very variable in size, often absent. The Chelydridae, Dermatemydidae, Platysternidae, and Cinosternidae possess moreover several inframarginals, intercalated on the bridge between the marginal and some of the plastral shields. In many of the other families these inframarginals are restricted to the anterior and posterior corners of the bridge, as the so-called axillaries and inguinals, mostly small and variable. Lastly, Macroclemmys has several small supramarginals.
There are consequently eleven longitudinal rows of shields in all; by elimination of the supra- and infra-marginals they are reduced to seven rows. It is absolutely certain that the number of transverse rows also was originally much greater than it is now. The mode of reduction of the number of the neural and costal shields has been studied in Thalassochelys caretta (cf. p. 388.) The accompanying illustration (Fig. 69) shows some of the main stages actually observed in the reduction of these shields. The chief point is that certain shields are squeezed out, or suppressed by their enlarging neighbours. The ultimate result is the formation of fewer, but larger shields.
Each shield grows individually as follows. Every year, or rather during every periodically recurring period of growth, the area of the Malpighian layer belonging to each shield increases peripherally in size, and at the same time produces a new layer of horn. The original little shield, with which the tortoise is born, remains for years, often throughout life, as the so-called "areola;" it increases in thickness owing to the new layer of horn added from below, and peripherally the increase in size is indicated by the overlapping concentric rings. Each ring represents a year's growth, at least in tortoises which live in temperate zones, where hibernation means a complete suspension of growth. It is not known if the same applies to tropical species, which grow either throughout the year, or which undergo one or more periods of rest. The areola does not remain central; the growth is uneven. With age the oldest layers of the areola are frequently rubbed off, and the areola then appears enlarged.
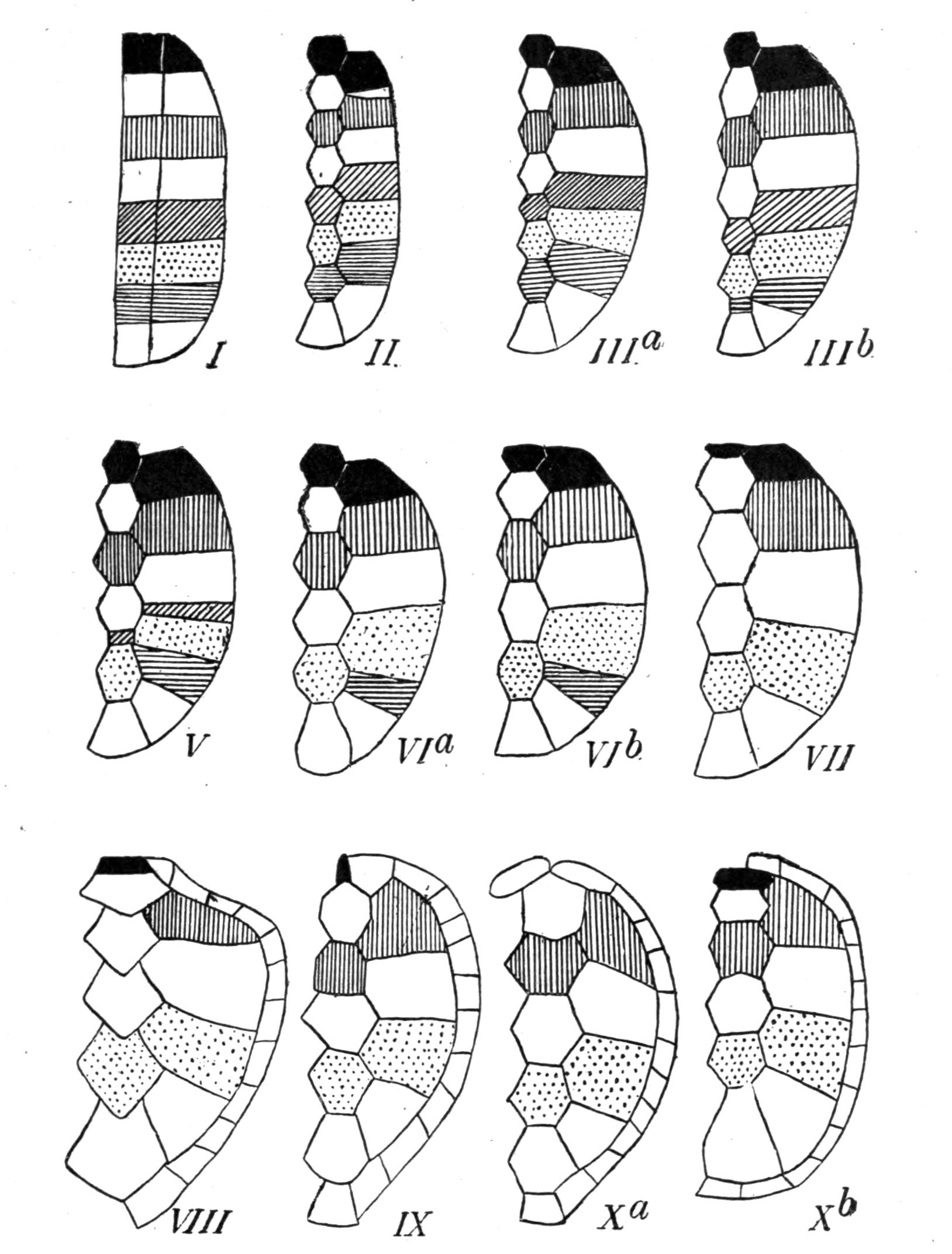
Fig. 69.–Diagrams illustrating the progressive reduction of the horny shields in various Chelonians. The shields, the fate of which it is desired to follow, are indicated by distinctive shading. I. Hypothetical, primitive stage. Eight neural (including the nuchal) and eight costal shields. Both neurals and costals lie in the same transverse planes. II.-VII. Successive stages in the reduction and suppression of various shields, observed in specimens of Thalassochelys, the normal condition of which is represented by VII. VIII. Six neurals and only four costals. The normal condition of Chelone. IX. The nuchal shield has become very small and the resulting gap has been filled up by an enlargement of the first pair of marginals. This is the normal condition of most Cryptodirous tortoises. X. The first marginals meet in front and the nuchal is either suppressed (Xa[Xa in diag]), e.g. in several species of Testudo, or it is surrounded by the marginals (Xb), e.g. in Sternothaerus. (From Willey's Zool. Results, 1899.)
For the first dozen years or so the annual rings can be easily followed, but when the creature approaches maturity each shield adds very little to its growth, and the rings become very fine, crowded and irregular. Only by careful counting and comparison of the rings on the costals, marginals, and plastrals, can a reliable average be arrived at. In some tortoises, e.g. Chrysemys, the whole outer layer of the shields peels off periodically; only a thin smooth layer like mica or tracing-paper remains, of course without any indication of rings. The pigment is formed in the Malpighian layer, but it frequently diffuses into the horny shields themselves, notably in Chelone imbricata, which yields the beautiful "tortoise-shell." The colour of the pigment is either black, yellow, or red, with resulting combinations. The green colour, often so beautiful in baby-specimens of Chrysemys, is optical, produced, according to Agassiz, by a network of black pigment, spread over a layer of yellow oil.
Horny scales, sometimes forming spines, and covering a nodule of dermal ossification, are also common on other parts of the skin, especially on the limbs of land-tortoises, and also on the tail of Chelydra. Sometimes the end of the tail is protected by a claw-like nail, for instance in Pyxis. In some of the gigantic land-tortoises, and in Chelone mydas, this nail assumes large dimensions, and several of the terminal caudal vertebrae are fused together into a regular urostyle. In some subfossil specimens of Mauritian tortoises, these ankylosed complexes are 12 cm. long and more than 5 cm. broad!
Before leaving the description of the shell, it is worth while to draw attention to the enormous correlative changes in other organs produced by this case. Nearly the whole organism has been altered. The hard, firm carapace has partly rendered the supporting functions of the vertebral column unnecessary or impossible. In many tortoises, especially in the large land-tortoises, the vertebrae and the capitular portions of the ribs are reduced to mere bony outlines; the reduction to thin paper-like bony lamellae proceeds with age. The iliac bones find a better support in the costal plates; the contact with the sacral ribs is given up, and these ribs fuse partly with the costal plates, or they are absorbed. The whole mass of muscles of the trunk is completely lost in the region of the shell, but traces of them exist in young specimens. Neck, limbs, and tail can in most cases be withdrawn and hidden in the shell. When this is not possible it is due to secondary changes. The neck is withdrawn either by being tucked away {329}sideways (Pleurodira[129]), or by being bent in an S-shaped curve in a vertical plane. In a left-sided profile-view of the animal, the head represents the tail of the S. The neck is withdrawn by long muscles, which are inserted into the ventral side of the middle of the neck, and extend in the shape of vertical ribbons far back into the shell, arising from the centra of some of the middle or even more posterior thoracic vertebrae.
Lastly, a few remarks on the partial regeneration, or the mending of injuries to the shell. If part of the horny covering is badly bruised, torn off, or rubbed through, or if part of the shell is crushed, the underlying portion of the bony plate becomes necrotic, and the horny covering also dies so far as its Malpighian layer is destroyed. Soon, however, the uninjured Malpighian cells, around the margin of the wound, multiply, grow into and beneath the injured portion of the bone, and form a new horny layer, casting off the necrotic portion. After several months the deficiency is patched up; new bone has grown in the deeper remaining strata of the cutis, and the outside is covered by a continuous horny layer, without, however, reproducing the original concentric moulding of the shields. In badly crushed shells sometimes almost one-third of the whole shell is thus cast off and mended within one or two years. The regeneration of the forcibly stripped-off shields of Chelone imbricata is described on p. 386. Bitten-off tails and limbs, rather frequent occurrences in water-tortoises, are of course not reproduced, but the wounds are healed and covered again with scaly skin.
Sense-organs.–The EYE is by far the best developed sense-organ. It is comparatively small. The pupil is round. The iris is mostly dark in terrestrial forms, while in water-tortoises it is often brightly coloured, for instance pale yellow in Chelodina, greenish and mottled with black, pale grey, brown, etc., in various species of Chrysemys. Cistudo presents a curious sexual dimorphism; the males have red, the females brown, eyes. The sclerotic wall contains a ring of numerous small ossified plates. There is no trace of a pecten. The eye is protected externally by the two lids and the nictitating membrane. In some water-tortoises, notably in Chelodina, the lower lid is transparent. Lacrymal and Harderian glands are present.
The SENSE OF HEARING is apparently not very acute, although tortoises and turtles are frightened by noise, and can distinguish sounds; otherwise they would have no voice, which is very tiny and piping in most tortoises during the pairing season. In most water-tortoises the tympanic membrane is thin and quite exposed; in land-tortoises it is often thick and covered by the ordinary skin; lastly, in Chelone the tympanic cavity is filled with a plug of the much-thickened skin, possibly in adaptation to the water-pressure when these creatures dive to considerable depths. The ossicular chain is mostly reduced to a long, bony, columellar rod.
The SENSE OF SMELL is well developed. All Chelonians carefully smell their food, in the air as well as under water. The individual predilection shown by many species for different kinds of animal and vegetable food,–since they are, for instance, able to distinguish between the various sorts of cabbage, cauliflower, sprouts, etc.,–proves that they possess a considerable amount of smell and taste.
Tortoises have a fine sense of touch; even the slightest tap on the shell is noticed, and the skin of the soft parts is extremely sensitive. Tickling of the sides of the tail, or of the hinder surface of a thigh, produces ridiculous scratching actions of the same or of the opposite foot.
The digestive apparatus is simple. Only a few peculiarities need be mentioned. The tongue is mostly broad and soft; it cannot be protruded. The oesophagus of the Chelonidae is covered with many conical projections pointing towards the stomach. The latter is simple, except in Sphargis. The intestine is devoid of a caecum, but the difference between the small intestine and the rectum is very marked and often abrupt. The cloaca is very roomy. It contains the large copulatory organ, which is unpaired, grooved on its dorsal side, and is altogether constructed like that of the Crocodilia. The large bladder opens ventrally into the urodaeum, a recess of the cloaca; near its base open the urinary and genital ducts. Many water-tortoises possess also a pair of lateral thin-walled sacs, the so-called anal sacs, dorso-lateral diverticula of the walls of the urodaeum. These sacs, which have highly vascularised walls, are incessantly filled and emptied with water through the vent, and act as important respiratory organs. When such a water-tortoise, for instance an Emys or a Clemmys, is suddenly taken out of the water, it squirts out a {331}stream of this water, which is not, as is generally supposed, the urine from the bladder.
The mode of respiration is interesting. The lungs are very complicated, highly-developed, spongy structures. They are attached by their whole dorsal surface to the inner lining of the shell. As they cannot expand through their own initiative, and since the shell has made costal and abdominal expansion impossible, the tortoise has to resort to other means of producing the necessary vacuum. This is done partly by the neck and the limbs, which act like pistons in being drawn in and out; partly by the greatly developed hyoidean apparatus, by which, when the neck is stretched out, the throat is alternately inflated and emptied, the air being swallowed, or pumped into the lungs. Additional respiration, besides that of the anal sacs mentioned above, is effected in various aquatic tortoises by slightly vascularised recesses of the pharyngeal region. Most Chelonians can exist for a very long time without breathing; sulky individuals remain for hours or days under water. Cistudo can shut itself up for an equally long time. Nevertheless this and other land-tortoises easily get drowned.
All Chelonians lay white eggs, round or oval, according to their kind, but the shape of the eggs of one set sometimes varies within the greatest limits. The shell varies from a parchment-like, flexible, scarcely calcareous cover to a hard, well-polished case. As a rule the eggs, imbedded in the ground, are hatched after a few months, but in some of the northern kinds, e.g. Emys orbicularis, the hatching is deferred until the next spring, the embryo's development being arrested during the winter. How such eggs, buried a few inches only below the surface, withstand the often very severe North German and Russian winter is a mystery. Whilst the plastron is generally flat, it is more or less concave in the males of many species, notably in Testudo, Cistudo, and Emys.
The general conclusions which can be drawn from the present geographical distribution of the Chelonia are as few and unsatisfactory as those applying to the Crocodilia, since all the main groups of Chelonians, and many more extinct families, occurred together in bygone ages in the same countries, for instance in Europe.
The marine forms are naturally cosmopolitan, but the Testudinidae are likewise cosmopolitan, except in the Australian region. The Chelydridae, now restricted to North and Central America, occurred formerly also in Europe. The Pleurodira, in Mesozoic times plentiful in Europe, India, and North America, are now restricted to South America, Australia, and Africa; the Pelomedusidae to Africa, Madagascar, and South America; the Chelydidae to South America and Australia. In the latter country all the Chelonians belong to the Chelydidae. The Trionychoidea, occurring since the Cretaceous epoch in North America, in Early and Mid-Tertiary times in Europe, are now restricted to North America, Asia, and Africa. The country richest in Chelonians is America; North and Central America together possessing representatives of all the families except the Pleurodira, and these we know to have died out there. The Dermatemydidae, {333}Cinosternidae, and Chelydridae are now restricted to the Nearctic sub-region (including Central America). Poorest in genera and species, all of them Chelydidae, is the Australian region, where no fossils of other families have yet been discovered. Europe, with its few Testudinidae, does not come into consideration; Asia has at least Testudinidae and Trionychidae, and in addition the solitary Platysternum in Indo-China, representative of a family whose affinities with the Chelydridae again proclaim the validity of the Periarctic region.
Order I. ATHECAE.
The vertebrae and ribs are not fused with, but are free from, the carapace, which consists of numerous small polygonal plates and is covered with leathery skin without any epidermal shields. The limbs are transformed into paddles. The neck is not retractile. Marine.
Fam. Sphargidae.–Sphargis s. Dermatochelys coriacea, the Leathery Turtle or Luth, is the only recent species and is the largest of all recent Chelonians. The biggest specimen in the national collection is about six feet and a half long, from the nose to the end of the shell, which latter is about four feet long; such a specimen may weigh half a ton. Agassiz, however, says that he has seen some "weighing over a ton." The general colour is dark brown, either uniform or with yellow spots. The Leathery Turtle has a wide distribution, ranging over all the intertropical seas, but it is rare everywhere; least so perhaps in the Western Atlantic from Florida to Brazil and in the Indian Ocean.
According to Agassiz it breeds regularly every year in the spring on the Bahamas, on the Tortugas, and on the coast of Brazil, depositing its many eggs on the sandy shore like other turtles. Accidentally it visits the northern coast up to Long Island, and specimens, perhaps carried with the Gulf Stream, have been caught on the coasts of Europe, for instance off Dorsetshire. One was caught near Nantes in 1729, and is said to have made a terrible noise when being killed. This is perhaps the reason why Merrem in 1820 invented the generic name Sphargis, supposed to be derived from σφαραγέω (I make a noise). It has also been recorded from the Mediterranean. It seems to be entirely carnivorous, living upon Molluscs, Crustacea, and fish. The flesh is supposed to be unwholesome. It is a very curious fact that of this rare species only large specimens, besides a very few baby-turtles, are known or preserved in collections, while individuals of intermediate size, say from four inches to three feet in length, have never been recorded. If it were not for the fact that they are still known to breed, it would look as if the {335}species were dying out. Perhaps they are very shy, leading a pelagic life, diving at the least sign of danger, and coming near the land only for the sake of breeding.
The structure of Sphargis is so peculiar in many respects that it deserves a somewhat full account. The neuro-central sutures persist on all the vertebrae. The eight cervicals are short. All the ten trunk-vertebrae carry ribs, and these, with the exception of the last, articulate between the centra and with the neural arches; the first and tenth ribs are short, the others are long and flattened, but not broad, with wide spaces between them. The tail is short, although it consists of about twenty vertebrae; these are devoid of chevrons.
The skull superficially resembles that of Chelone, chiefly owing to the completely roofed-in temporal region. The supraoccipital crest is rather short, covered completely by the parietals, the posterior margin of which is rounded off instead of forming, as in the Chelonidae, a long projecting triangular crest with the supra-occipital. The parietals are in broad contact with the postfrontals, posteriorly they are just reached by the squamosals. The quadrato-jugal is small, separated from the postfrontal by the meeting of the squamosal with the jugal. The quadrate is notched behind, and it separates the opisthotic from the squamosal. The basisphenoid is large and broad, extending far forwards so as to separate the pterygoids widely from each other except in their anterior portions, which, instead of sending a lateral arm to the jugal and maxillary, as in Chelone, are widely separated from these bones by the palatines. The choanae lie on either side of the anterior half of the vomer, and are not roofed over by ventral vomero-palatine wings.
The limbs and their girdles are essentially like those of the Chelonidae, but are not derivable from them. The most remarkable feature is the shell. The dorsal and ventral halves are directly continuous, forming one unbroken case all round, which is composed of many hundreds of little bony plates, irregularly polygonal, fitting closely into each other with their sutural edges, and giving the shell a beautiful mosaic appearance. On the dorsal side are a median row and three pairs of lateral rows of larger plates, and these form seven longitudinal blunt ridges which all converge towards the triangularly pointed tail-end of the shell. The ridges are not so much produced by thickened {336}or spine-like edges of the plates, but by the right and left halves of the plates being actually bent at an angle. This is most conspicuous at the sides of the shell where it passes into the ventral portion. The latter has two pairs of lateral and one median ridge. The whole shell has consequently twelve ridges. The mosaic plates are deeply imbedded in the cutis, being externally as well as internally covered or lined with dense leathery skin. The epiderm is thin, and shows no indications of horny scales. In young specimens the whole shell is soft and very imperfectly ossified, later on it is quite rigid, although comparatively thin. It is nowhere in contact with the internal skeleton, except by a nuchal bone, which by a descending process articulates with the neural arch of the eighth cervical vertebra.
The affinities of the Sphargidae and their position in the system are still debatable. Whilst some authorities, e.g. Cope, Dollo, and Boulenger look upon Sphargis as the sole remnant of a primitive group in opposition to all the other recent Chelonia, Baur considered it the most specialised descendant of the Chelonidae. Dames agreed with him. Van Bemmelen has modified this view in so far as he regards Sphargis as the most specialised Chelonian, but considers the differences between it and the Chelonidae great enough to conclude that both Sphargidae and Chelonidae represent two independent, partly parallel, branches which have arisen from two different groups of terrestrial tortoises. Case,[130] from the study of Protostega and other fossil forms, tends towards Baur's view. He believes that Sphargis is the culminating form of a branch which through Psephophorus and with Eosphargis has sprung from some creature like Lytoloma, which at the same time is the starting-point of another branch which culminates in the genera Thalassochelys and Chelone, while lastly a third branch contains Protostega, Protosphargis, and Pseudosphargis. In other words, he considers them all Chelonidae. If he is right we have of course no business to separate Sphargis with its fossil allies from the rest of the Chelonia as "Athecae."
However, Case has not proved his point. It is easy enough to understand that the characters of the cranium and plastron of Sphargis are in a condition which by partial reduction can be derived from that of typical Chelonidae. The structure of the {337}cervical vertebrae, the absence of the marginal plates and the peculiar articulation of the nuchal with the last cervical vertebra can be explained as convergent analogies, just like the paddles of Carettochelys. But the shell of Sphargis is fundamentally different from and not homologous with that of the others. Cope was therefore quite justified in distinguishing the Sphargidae as "Athecae" in opposition to the others which Dollo later on, by contrast, named "Thecophora." Unfortunate names, since both groups are undeniably in possession of a θήκη or shell. Both authors meant, however, by Theca the epidermal shields, but even this distinction is rendered invalid by Carettochelys.
The most reasonable explanation has been suggested by Hay.[131] The mosaic polygonal components of the shell of Sphargis are, so to speak, an earlier generation of osteodermal plates than the later generation of longer and broader bony plates which in the Thecophora come into contact, and fuse with, the neural arches and ribs. The osteoderms of Sphargis belong to the same category as the dermal ossifications in the scutes of Crocodilia, whilst the plates of the carapace and plastron of the Thecophora belong to the category of the abdominal ribs. Sphargis has the first kind in its peculiar shell, the second kind in the deeper lying plastron and in its nuchal plate. But it has lost, or perhaps had never developed, the horny shields. The only difficulty is, however, the presence of a plastron and of a typical neural plate in Sphargis. This difficulty is not very serious. The plastron is a very old institution. It occurs together with the more superficial osteoderms in Caiman, and the nuchal plate may be the oldest of all dorsals. We can scarcely imagine that the direct ancestors of Sphargis had developed both kinds of shells, and that comparatively recently the inner shell of the carapace was lost, leaving only the nuchal plate. Fossils do not support such an assumption. Undoubted ancestral forms of Sphargis are very rare. Psephophorus of the Oligocene and Miocene of Europe had a continuous mosaic shell much resembling that of Sphargis; Eosphargis is represented by a well-preserved skull from the London clay. Then follows a wide gap until we come to Psephoderma of the Rhaetic, or Upper Trias of Bavaria; the large fragment of whose dorsal shell is composed of about 200 mosaic pieces. If this fragment really formed part of the shell {338}of a Chelonian, its age would speak greatly in favour of the Athecae being a very primitive and independent group.
Order II. THECOPHORA.
Thoracic vertebrae and ribs united with a series of median or neural and a paired series of lateral or costal plates. Parietals prolonged downwards, meeting the pterygoids directly or by interposition of an epipterygoid.
Sub-Order 1. Cryptodira.–The carapace is covered with horny shields. The neck, if retractile, bends in an S-shaped curve in a vertical plane. The pelvis is not fused with the shell.
Fam. 1. Chelydridae.–The plastron is small and cross-shaped (Fig. 61, 2, p. 315); the bridge is very narrow, and the displaced abdominal shields are widely separated from the marginals by a few irregularly shaped inframarginals. The tail is long. The limbs, neck, and head are so stout that they cannot be completely withdrawn into the shell. Snout with a powerful hooked beak. American; only two genera, each with one species.
The temporal region is roofed very incompletely and only anteriorly by the expanded parietals and postfrontals, which form a long suture. The plastron consists of nine bony plates, a small entoplastron being present; there are lacunae in the middle line, the plates meeting imperfectly, and the horny abdominal shields are likewise separated by soft skin. The carapace has a nuchal with long rib-like processes which underlie the marginals; the neural plates form a continuous series. There are twenty-three marginal plates. The pubic and ischiadic symphyses remain separate, enclosing one large heart-shaped foramen. The five fingers and toes are webbed and are protected by claws except the outer toe, the nail of which is usually suppressed.
Chelydra serpentina, the Snapping Turtle, attains a large size, namely, a shell-length of more than one foot, and a total length from the nose to the tip of the tail of more than three feet. Its range extends from the Canadian lakes east of the Rocky Mountains, through the United States and Central America. The carapace of young specimens has three very marked series of keels, which gradually disappear with age, until in very old individuals the shell becomes quite smooth. The skin is very warty, especially on the neck, and there is a pair of minute {339}barbels on the chin. The tail carries three series of originally triangular horny crests, which with age are transformed into blunt knobs. The general colour of this rather ugly creature is olive, mottled with dark brown above and with yellowish below.
According to Holbrook the Snapping Turtle is found in stagnant pools, or in streams where the waters are of sluggish motion. Generally they prefer deep water, and live at the bottom of rivers; at times, however, they approach the surface, above which they elevate the tip of their pointed snout, all other parts being concealed; and in this way they float slowly with the current, but if disturbed they descend speedily to the bottom. They are extremely voracious, feeding on fish, reptiles, or any animal substance that falls in their way. They take the hook readily, whatever may be the bait, though most attracted by pieces of fish; in this way many are caught for the market. It is, however, necessary to have strong hooks and tackle, otherwise they would be broken, for the animal puts forth great strength in his struggles to escape, both with his firm jaws and by bringing his anterior extremities across the line. When caught they always give out an odour of musk, which in very old animals is sometimes disagreeably strong.
Occasionally the Snapping Turtle leaves the water, and is seen on the banks of rivers or in meadows, even at a distance from its accustomed element. On land his motions are awkward; he walks slowly, with his head, neck, and long tail extended, elevating himself on his legs like the Alligator, which at that time he greatly resembles in his motions; like the Alligator also, after having walked a short distance, he falls down to rest for a few moments, and then proceeds on his journey. In captivity they prefer dark places, and are exceedingly ferocious; they will seize upon and bite severely anything that is offered them, and their grasp upon the object with their strong jaws is most tenacious.
The Snapping Turtles, or "Snappers," are feared on account of the ferocious bites which they inflict, and they are hated because of the destruction of valuable fish and water-fowl. They in turn atone for this damage by being eaten, especially the younger half-grown individuals, the flesh of the older ones being too much tainted with the odour of musk. The round eggs, which are laid to the number of twenty to thirty in the summer {340}(in the Northern States about June), are likewise good to eat. The first act of the young creature on leaving the shell is said to be snapping and biting. In captivity they are often very sulky, and refuse food stubbornly for many months, perhaps for a whole year, and apparently without much harm to themselves, since they lie quietly in the distant corner of the tank, now and then slowly rising to the surface to breathe. Fresh-water algae grow on the shell and in the mud which settles on it, and since this happens also in the wild state, they are rendered as inconspicuous as old rotten logs. In order to attract fishes they protrude a pair of worm-like, pale pink filaments from the tip of the tongue.
Macroclemmys temmincki, the "Alligator Turtle."–In size and general appearance much like the other Snapping Turtle, but the dorsal shields have each a strong and prominent keel, and these three series increase in size with age. The costal shields are separated from the marginals by an additional series of about four supramarginals, well shown in the illustration. The shields of {341}the cross-shaped plastron are subject to much individual variation, small shields being frequently intercalated, or rather retained, between the usual ones, especially between the pectorals and abdominals, in the gular region, and on the narrow bridge, where the inframarginals number one to three or even more. This species inhabits, broadly speaking, the whole basin of the Mississippi and Missouri rivers.
This beast is as vicious as the other Snapping Turtle. According to Agassiz it does not withdraw its head and limbs on the approach of danger, but resorts to more active defence. It raises itself upon the legs and tail, highest behind, opens the mouth widely, and throwing out the head quickly as far as the long neck will allow, snaps the jaws forcibly upon the assailant, at the same time throwing the body forward so powerfully as often to come down to the ground when it has missed its object.
It lives mostly in the water, but makes considerable journeys overland. Both in the water and on dry land the limbs move nearly perpendicularly, and the body is raised high. On dry land a considerable part of the weight of the body is borne by the long, strong tail.
"They are as ferocious as the wildest beast of prey, but the slowness of their motions, their inability to repeat the attack immediately, their awkwardness in attempting to recover their balance when they have missed their object, their haggard look, and the hideous appearance of their gaping mouth, constitute at such times a picture as ludicrous as it is fearful and revolting. Their strength is truly wonderful. I have seen a large specimen bite off a piece of a plank more than an inch thick. They take hold of a stick with such tenacity that they may be carried for a considerable distance suspended to it free above the ground. Fishes and young ducks are their ordinary prey. They lay from twenty to forty or more round eggs only about the size of a small walnut in holes which they dig in sloping banks not far from the water" (Agassiz).
Fam. 2. Dermatemydidae.–The pectoral shields are widely separated from the marginals by inframarginals, the gular shields are very small or absent, and the tail is extremely short. Only two or three genera, with three or four species in Central America.
The plastron is composed of nine plates. In Dermatemys mawi it is large, firmly joined to the carapace, covered with {342}eleven or more shields, and there are four inframarginals; in Staurotypus salvini of Mexico the plastron is cruciform, with the anterior lobe movable, covered with seven or more shields, according to the fusion of the anal shields and the presence or absence of the gulars; there are only two inframarginals. The pubic and ischiadic symphyses remain separate; the temporal fossa remains widely open, the postfrontals scarcely touching the parietals. There are 23 marginal shields in Staurotypus, 25 in Dermatemys, including the unpaired nuchal. The nuchal plate has a pair of rib-like processes like those of the Chelydridae, but some of the posterior costal plates, sometimes only one pair, meet in the middle line, overlying or suppressing the corresponding neural plates. The shell of these aquatic tortoises is rather flat, more or less keeled, especially in young specimens, and in the fully adult condition is about one foot in length.
Fam. 3. Cinosternidae, represented by the single genus Cinosternum, with about ten species in North and Central America, and one in Guiana. Closely allied to the two previous families, with which it agrees by the separation of the pubic and ischiadic symphyses, the presence of an ento-plastral plate, the possession of inframarginal shields (Fig. 61, 3, p. 315), the widely open temporal fossae, and the rib-like pair of processes to the nuchal plate. It agrees with the Dermatemydidae in the interruption of the neural plates by the meeting of several pairs of the costal plates. There are 23 marginal shields; five or four shields, according to the presence or absence of the gular on the plastron, and in some species these plastral shields become, with age, more and more separated from each other by soft skin (see Fig. 75). The shape and size of the plastron differ considerably in the various species; in most of them, e.g. in C. pennsylvanicum and C. leucostomum, but not in C. odoratum, the anterior and posterior lobes are movable, with transverse soft hinges, so that the animal can completely close its shell. The skin of the legs and neck is so baggy and loose that these parts slip in, the skin rolling off, when the creature withdraws into its shell. They lay only a few–from three to five–elliptical eggs, which have a shining, glazed, and thick, but very brittle shell.
Cinosternum odoratum, the Mud-Turtle, or Stinkpot Terrapin, so called on account of the disagreeable smell which exudes from the inguinal glands. The head is disproportionately large, with the snout rather compressed laterally, and pointed underneath, with several short barbels. The neck is long and slender. The carapace of the young is keeled, each of the neural shields being raised in the middle line; but in full-grown specimens the shell becomes quite smooth and rounded. The horny shields of the plastron are relatively largest in the young, but they soon leave ever-increasing spaces between them, which are then filled with soft skin only, which thinly covers the underlying bone. The {344}fore- and hind-limbs, especially the latter, are extensively webbed, and are provided with five short claws. The general colour of the shell is horny brown, either uniform or with darker spots or streaks. The neck and limbs are mottled brown. The only ornamental colouring is a pair of clear yellow broad lines on each side of the head, and a similar streak on each side of the lower jaw. On the chin and upper throat are two pairs of small tentacles. The tail of the male is of about the length of the hind-limbs, while that of the female is so short that its tip scarcely reaches beyond the hinder margin of the carapace. Length of the shell of full-grown specimens between four and five inches. Very young specimens have a rather droll appearance, owing to the long and slender neck with the large head, and the humpy back.
This species is common in the eastern half of North America, from Canada to Texas. It is mainly aquatic, and is one of the dullest and shyest species. My own specimens spend most of their time in the water, invariably in the darkest corners, preferably under a stone or a log, and they do not leave their hiding places until dark, in search of worms, meat, and all sorts of animal food. For months I could never induce them to take food from a stick, or even to eat in my presence, and it was not until after many weeks that one of them at last protruded its head far enough to exhibit the yellow stripes. When taken out of the water they draw in their heads, just allowing the vicious little eyes to be visible, and opening the sharp-edged mouth widely to bite deliberately and furiously at the unwary finger. Some spent the winter in the water, in the greenhouses, feeding as usual, others crept on land, hiding under moss, half buried in the soil, where they slept for several months, but with interruptions in order to soak and to drink. When spring is well advanced they prefer the water for their regular sojourn. Some which had been sent over from New York arrived in a deplorably dried-up condition, the skin being quite flabby and shrivelled, but after a few hours' soaking they came round, and increased considerably in weight, the limbs and neck becoming turgid.
C. pennsylvanicum of Eastern North America has a larger, more oval plastron. The head is not so strikingly large as in the other species and, like the neck, is brown with yellowish spots, and often has streaks on the sides. The tail of the male ends in a {345}nail-like horny point. The lobes of the plastron are well hinged in the adult.
C. leucostomum of Central America is larger, with a shell-length of six inches. The plastron is not at all cruciform, but has a broad bridge, and fills the box, moreover it has an anterior and a posterior hinge, so that the box can be completely closed. Hence the vernacular name of the Box-Terrapin.
Fam. 4. Platysternidae, represented by the single species Platysternum megacephalum in Burma, Siam, and Southern China.
The pectoral shields are widely separated from the marginals by inframarginals, the plastron is large, oblong, not cruciform, and the tail is long.
The plastron consists of nine plates, and is covered with six pairs of shields, the most anterior of which are the broad gulars. The nuchal plate has no rib-like processes. The neurals form a continuous series, and there are twenty-three marginal scutes. The temporal fossae are completely roofed over, owing to the long sutures formed by the parietals with the postfrontals, moreover the postfrontals expand laterally so much that they posteriorly come into broad contact with the quadrato-jugals and squamosals, anteriorly with the maxillaries, so that the jugals are completely surrounded by bones, and are shut off from the orbits and from the temporal fossae. This is a unique arrangement, found nowhere else in Tortoises. The pubic and ischiadic symphyses are connected with each other by ligaments only.
The general appearance of this water-tortoise is rather curious, since the carapace is much depressed, looking, especially in younger specimens, as if it had been crushed in. The head, provided with very strong hooked jaws, is strikingly heavy and large, and is covered above with one single large shield. The tail is longer than the shell, which, in full-grown specimens, reaches about six inches in length; it is, throughout its length, covered with rings of squarish shields. A large specimen measures 14 inches in total length, of which only five fall to the shell.
Fam. 5. Testudinidae.–The shell is always covered with well-developed horny shields. Those which form the plastral bridge are in direct contact with the marginals. The plastron is composed of nine bones. The digits have four or five claws. The neck is completely retractile. The skull is devoid of parieto-squamosal arches.
This large family is cosmopolitan, with the exception of the Australian and the adjoining Austro-Malayan countries. It contains genera which form a continuous gradation between absolutely terrestrial and thoroughly aquatic tortoises; and many are truly amphibious. As a general rule the typically terrestrial kinds have a more curved or arched shell, the digits are short, the eggs are more oval or round, and they are chiefly herbivorous; the essentially aquatic kinds have a flatter or depressed shell, webbed feet, with longer, often slender claws, the eggs are more cylindrical, and they live on animal diet. About 20 genera, with more than 110 species, are recognised by Boulenger, but their essential characters are nearly all internal, and therefore of no avail for the determination of live or entire specimens.
Chrysemys.–One of the most typical and widely distributed genera of American Terrapins or water-tortoises. The carapace is flat; the plastron is quite immovable, with a strongly developed bridge. Feet well webbed. Tail short. Skull with a broad, complete, lateral, temporal arch. About one dozen species, mostly in the eastern half of the United States, but the whole genus ranges from Canada to Argentina.
Most of the young Chrysemys are very pretty, the ground-colour of the upper shields being green, variegated with yellowish-brown or blackish markings, which often form exquisitely delicate patterns, either concentrical (Ch. concinna, Ch. rubriventris), or more longitudinal (Ch. elegans), or apparently quite irregular. The ground-colour of the plastron is yellow, but the various species are best distinguished, at least in very young individuals, by the arrangement of the dark brown spots and patches. There are, for instance, several pairs of bold lateral and several median patches in Ch. rubriventris; five pairs of ocellated spots in Ch. elegans; only small median patches, where four plastral shields meet, in Ch. concinna; while the plastron of Ch. picta is uniformly yellow.
These water-tortoises are very lively and shy, most so perhaps Ch. picta, which is very quick and active. The food varies, often according to individual fancy. Most of them eat fish. Ch. picta is partial to insects, but it also takes worms. Some of my specimens refused meat for a long time, but ultimately they became so fond of it and of worms, that they came out of the pond to take the food from the fingers; those in the Zoological {347}Gardens of London have developed a taste for biscuits. One of my largest Ch. concinna fasted deliberately for eight months, refusing worms, insects, meat, and frogs, only occasionally sniffing at the food, until it was tempted with whitebait, which it took greedily. It refused, however, smelts and pieces of soles, but after another month it condescended to take meat regularly. Very young individuals live chiefly on flies, which they watch for near the surface of the water; and they are fond of smooth caterpillars, maggots, the larvae of humble-bees, and similar soft creatures. They all spend most of their time in the water, preferably floating near the surface, hidden between weeds; and they are fond of basking. Some of them spend the night in the water, lying motionless on the bottom, with heads and limbs turned in. Others prefer hiding under moss. Those species, which, like Ch. concinna and Ch. picta, are common in the North, are of course perfectly hardy. For the winter they dig themselves holes in the banks near the water, and they do not come out again until the spring is well advanced. The eggs are hard-shelled, mostly long and oval, and they are hatched before the end of the summer. The larger species of Terrapin are eaten.
Ch. picta (Fig. 76), the "Painted Terrapin," of the Eastern United States, e.g. of New York and Long Island, is easily recognised by the much depressed shell, which is absolutely smooth, and without a trace of a keel. The colour above is dark olive-brown or blackish, with broad yellow bands across the anterior ends of the neural and costal shields. Three or four of these transverse bands are very conspicuous. The marginals are red, with more or less concentric black and yellow markings. The pretty red colour, with some black stripes, extends over the bridge, but the plastron itself is uniformly yellow. The soft parts are likewise prettily marked, the ground-colour is black-brown, with delicate bright yellow and red stripes on the sides of the neck, limbs, and tail. The stripes are originally yellow, but they develop an orange or red line in the middle, so that each red stripe is ultimately narrowly edged with yellow; or the yellow and red stripes alternate, for instance on the tail, which is short, narrow, and pointed. The head is further adorned with a pair of conspicuous bright yellow patches behind the eyes, and a smaller pair on the occiput. The black and yellow stripes run across the gape of the mouth, some of the lines even looking as if they had been {348}painted across. The nuchal shield is elongated and very narrow, its anterior edge and that of the neighbouring marginals are finely serrated. Very young individuals are at once recognised by the prominent longitudinal median stripe of bright orange extending over the nuchal and neural shields; the yellow transverse bands are still absent; they appear when the longitudinal line vanishes.
The "Painted Terrapin" is one of the few species of which, thanks to L. Agassiz,[132] complete data of growth from the new born to old age are known. During the first six or seven years the rate of growth is so uniform that numerous specimens collected at the same time are readily arranged in sets of the same age, simply by the differences they show in their size. The successive lines of growth on the shields indicate the number of years. After the seventh year the age is much more difficult to distinguish in those tortoises, which, like Ch. picta, have a perfectly smooth epidermis. This smoothness is due to the fact that the shields undergo a process of moulting. An upper, quite {349}transparent layer of each shield peels off completely like a piece of mica. I have been able to confirm Agassiz' statement on Ch. concinna in their third and fourth springs, and on a number of adult Ch. picta. The latter were not allowed to hibernate, being kept in a warm tank; they peeled completely during the late autumn, and then the red and yellow colours underlying the newly formed shields appeared very vividly; others moult at midsummer.
Growth of Ch. picta, after Agassiz.
| Year. | Length of carapace. |
Breadth of carapace. |
Height of box. |
Length of tail. |
| millim. | millim. | millim. | millim. | |
| Third | 042 | 039.5 | 017 | 017.5 |
| Fourth | 051 | 049 | 021.5 | 020.5 |
| Fifth | 054 | 051 | 023.5 | 021.5 |
| Sixth | 059 | 056 | 025 | 023.5 |
| Seventh | 066 | 060 | 026.5 | 026 |
| Eighth (♂) | 072.5 | 061 | 028 | 027.5 |
| Ninth (♂) | 074 | 062 | 028 | 027.5 |
| Tenth (♂) | 077 | 064 | 030 | 028 |
| Eleventh (♂) | 080 | 067 | 030 | 028.5 |
| Fourteenth (♂) | 092 | 074.5 | 033 | 028.5 |
| Twenty-fifth (♀) | 121 | 092 | 043 | 034 |
| Old ♀ | 129 | 096 | 047 | 037 |
| Very old ♀ | 163 | 113 | 059 | 053 |
The size of the eggs varies considerably, from 26 by 17 to 30 by 16 millimeters; sometimes they are perfectly round, 17 mm. in diameter.
Ch. concinna.–The specific character by which this Terrapin may be easily recognised is a pair of orange-red broad streaks, which extend from above the eye to the sides of the neck. The general colour is olive-brown above, variegated with yellowish dark-edged lines, which, together with numerous rugosities, radiate from the middle field of each shield. The plastron is yellow, often with blackish symmetrical patches, and sometimes these become confluent and preponderant. Very young specimens are extremely pretty, the ground-colour of the carapace being green, each shield with darker, somewhat concentric markings, most conspicuous and regular on the upper surface of the marginals, where the marks of the adjoining shields form one pattern-system across the dividing lines. The plastron is either uniform yellow or has a few pairs of blackish spots {350}which stand so closely together that they form almost median patches.
The carapace is rough. The horny shields become very thin with age. The anterior margin of the small nuchal and the neighbouring marginals is faintly serrated. The posterior marginals form slight notches or indentations between their edges. The plastron is almost square behind. The edges of the jaws are nearly smooth, without hook and receiving-notch. The tail is short.
This species inhabits the South-Eastern States of North America, from Missouri and North Carolina to the Gulf of Mexico. Very large female specimens have a shell sixteen inches in length. The eggs measure from 33 by 25 to 39 by 25 mm. or about 1½ inch in the long diameter.
Emys.–The plastron is movably united to the carapace by ligament, and in the adult has a slightly flexible hinge across the middle, between the hyo- and hypo-plastral plates and the pectoral and abdominal shields. The plastron is large, but does not quite close the box. Besides the small nuchal there are twelve pairs {351}of marginal shields. The head is covered with smooth skin; the temporal arch is complete. The limbs are extensively webbed. The tail of the very young is nearly as long as the shell, but it becomes relatively shorter with age, being reduced in the males to about two-thirds, in the females to half the length of the shell. Only two species are found in Europe, the other, E. blandingi, in Canada and north-eastern U.S.A.
E. orbicularis s. europaea s. lutaria, the European Pond-tortoise.–The shape and coloration of the shell change likewise much with age. In the very young the shell is round, and the shields are rough and slightly keeled, uniform dark brown above, black below, with a yellow spot on each marginal and plastral shield. When half grown the dorsal shields become quite smooth, and are striated or spotted, with yellow upon a dark ground. The head, limbs, and tail are dark, with yellow or light brown spots and small dots. In very old specimens all these yellow marks disappear on the shell, which then becomes uniform brown or almost black. The coloration is subject to much local and individual variation, and there are two main types, the spotted and the radiate. It is difficult to say which of the two is the prettier. One male which I caught in the Alemtejo was very beautiful. The shell was almost black with a greenish shine when in the water, and had many bright yellow and whitish spots. In the radiate type the yellow is sometimes preponderant, so that each shield becomes a study of delicately painted yellow, brown, and blackish lines radiating from the centre. This variety seems to prevail in the south of Spain, decidedly so in the Marismas, also in Northern Italy, whence most of the European markets are supplied. The largest shell in the British Museum is 19 cm. = 7½ inches long. Fischer Sigwart received one from Naples which was about 9 inches long, and this seems to have been kept as a pet, since its shell had been gilt. Specimens about 5 inches in length may be considered as fully adult. There are very few reliable observations on the growth of individuals. One of F. Sigwart's grew in eleven years only about 2.5 cm. = 1 inch, when its shell was 13.4 cm. = 5¼ inches long–total weight of the tortoise 491 grammes, about 1 lb. One of my own grew from 11 to 13.2 cm. shell-length, and 8.3 to 10.6 cm. in width within eight years, but this was one of the specimens which, living in a greenhouse, {352}did not hibernate. This European pond-tortoise is now restricted to Southern and Middle Europe, extending eastwards towards St. Petersburg and into Asia Minor, southwards into Algeria. Formerly it had a much wider range, having been found in post-glacial deposits in Southern Sweden, Denmark, the Netherlands, and in East Anglia. Specimens have been found in the peat of the fens of Norfolk and Cambridgeshire, contemporary with bones of the Beaver, Roe-deer, and Pelican. The same applies to North Germany, where its gradual disappearance from the western and central parts is obvious. Except in Central France it is now practically unknown to the west of the Elbe river. The country between the Elbe and Oder is now debatable ground, Emys being exceedingly rare. Some fifty years ago this seems to have been different, to judge from the fact that farmers were rather fond of keeping a tortoise in the water-troughs of the cattle to keep the water free from worms and other impurities. Hence arose a silly superstitious custom. It was considered equally conducive to the health of the pigs to keep a tortoise in the foul tub into which all the dish-water and kitchen-refuse–as potato-peels, sour milk, etc.,–were collected before the mess was given to the pigs.
A specimen is still occasionally caught in the Havel and Spree rivers. I myself have heard of one or two in the backwaters of the Oder near Frankfurt, but they are vanishing, and it is difficult to say exactly why. The universal lowering of the water-level owing to better drainage cannot quite account for it, since there are thousands of suitable ponds, swamps, and backwaters left. In Poland and in Eastern Prussia the tortoise is still common.
This creature lives on a strictly animal diet. Worms, insects, frogs, fishes form its main sustenance. Fishes are regularly stalked. The tortoise watches its opportunity, slowly it half crawls, half swims along the bottom, rises imperceptibly by a few gentle movements of the widely spread-out webbed feet, then opens its sharp cutting jaws wide, and makes a grab at the belly of the fish. Frogs are most easily stalked when they sit upon a floating leaf. The tortoise rises from below, and often waits with the nostrils and eyes just above the water and close to the frog. After a while it sinks, and rises again, this time actually touching the toes of the non-suspecting frog, smelling at them and deliberately biting with a sideward turn of the head.
Fig. 79.–Emys orbicularis, European Pond-tortoise (left), and Clemmys leprosa, Iberian Water-tortoise (right). × ½.
What the jaws have got hold of is not allowed to escape again. The tortoise holds on and tears the prey to pieces with the sharp-clawed fingers. This takes a long time, only the scraped-off flesh and the intestines being eaten. The skeleton remains and sinks to the bottom, while in the case of a fish, the air-bladder floats away on the surface, and remains there as one of the surest signs of the existence of tortoises in that locality. The bones are cleaned with wonderful neatness. Some of my grass-snakes shared this fate, their backbones, with the hundreds of pairs of ribs, being picked or rather scraped clean, scarcely less well than if they had been prepared for a museum.
As a rule the prey must be in motion to be seized, unless the tortoise has watched it before, and even then the latter prefers to smell it before biting. In captivity they soon learn to eat meat, and they become very tame, but in their native haunts they are extremely shy and cautious. Fond of basking upon a stone or on the banks, with the four limbs sprawling, or with the hind-limbs stretched backwards, and with the webs spread out so as to offer as large a surface as possible to the rays of the sun, they lie motionless for hours and appear fast asleep. But the slightest noise, or any other sign of our approach, is sufficient to send them plumping into the water, and to make them scuttle along with unsuspected agility. Nothing but the audible plump of the flat body and the widening rings of the disturbed water indicate their presence. After a long time of waiting we give it up, and turn away. That very instant we see a little ripple, caused by the withdrawing of the tortoise, which had come to the surface and had been watching us, with only the nose and eyes peeping out of the water, the rest being concealed between the floating vegetation. Apparently they cannot see us well with their eyes still under water, owing to the difference of refraction, otherwise they would not peep out and then at once turn back. It is certainly not for the want of air, since they can remain below for many hours without breathing.
Although they generally feed in the water, they come on land when tame and hungry enough to take the offered food. Sometimes they make long migrations, perhaps because their old home is dried up or does not yield food enough. They hibernate during the cold season, buried in the mud, and they do not appear until {355}the spring is well advanced. During the pairing season, on warm spring nights, they emit short piping sounds, and when they have found each other, the couple swim about together. The white, hard-shelled, long, oval eggs, averaging 25 to 15 mm., and about ten in number, are laid on land. This is a very laborious and curious business. The female having selected a suitable spot, not loose sand, but rather hard soil free from grass and other dense vegetation, prepares the ground by moistening it from the bladder and the anal water-sacs. Then it stiffens the tail and bores a hole with it, moving the tail but not the body. The hind-limbs then scoop out the hole, the broad feet moving alternately and heaping up the soil on the side, until the hole is about five inches deep, that is as far as the hind legs will reach. The eggs are laid at the bottom in one layer, divided and distributed by the feet. Lastly, the soil is put in again, and the tortoise, by repeatedly raising its body and falling down, stamps the soil firm and flat, roughens the surface a little with its claws, and leaves the nest to its fate. Nothing but an accident leads to its discovery. The young are hatched, according to locality and the kind of season, either in the same autumn or not until the next spring. Eggs laid in a garden at Kieff, in Russia, were hatched eleven months later. This implies hibernation of the embryo within the egg, and this is probably the usual course of events, resembling the conditions of the development of Sphenodon (cf. p. 299). The pretty little creatures, scarcely larger than a shilling-piece, are exceedingly difficult to rear. They require a tank with green vegetation, stones to bask on and to hide under, and also dry ground and moss for a change. They eat flies, tiny worms, tadpoles, etc., greedily enough, but for some occult reasons they do less well than many another kind of water-tortoise. Miss Durham has, however, succeeded in rearing one, which is now in its fourth year; the shell is 2 inches long, and each shield shows three annual rings around the areola. This specimen spent the winters in an unheated room under moss, not in the water.
E. blandingi, the North American species, has a more elongated and decidedly higher carapace than its smaller European relation. The carapace is dull black with many pale yellowish spots; the plastron is yellow, with a large dark patch on the outer and hinder corner of each shield. The head is dark brown above, bright {356}yellow below and on the throat, a contrast which gives this tortoise a striking appearance. This species is extremely voracious, becomes easily tame, and spends a great part of the day on land, hiding under grass to avoid great heat, and withdrawing into the water for the night.
Clemmys.–The plastron is immovably united with the carapace, and is devoid of any transverse hinge. The skull has a complete bony temporal arch. This genus, consisting of eight species, is otherwise very much like Emys, and is truly Periarctic.
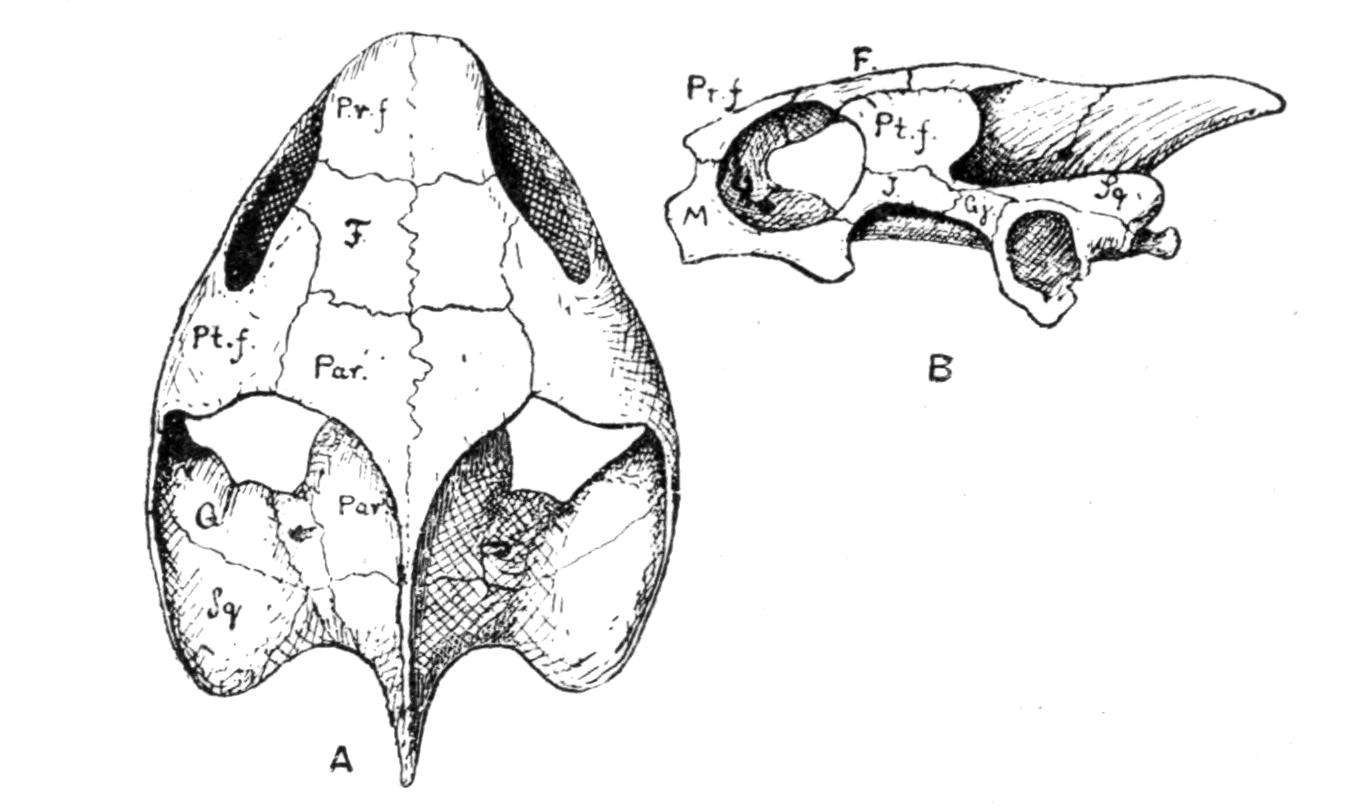
Fig. 80.–Skull of Clemmys leprosa. × 3⁄2. A, dorsal view; B, from the left side; F, frontal; J, jugal; M, maxillary; Par, parietal; Pr.f, prefrontal; Pt.f, postfrontal; Q, quadrate; Qj, quadrato-jugal; Sq, squamosal.
C. leprosa s. sigris (Fig. 79).–The upper jaw has a median notch for the reception of the upturned point of the lower jaw; the cutting edges of the powerful beak are smooth. The shell is flat and long-oval, nowhere serrated. The plastron does not quite fill the box. In the young the shell is nearly round, and the horny shields form three series of keels, of which the lateral pair disappear early; the shields are olive-brown, each with an orange spot or streak; the plastron is dark brown, with a yellowish margin. The adult looks very different. The shell has become much more oval, with the greatest width behind the bridge. The long shields are smooth, and in elderly specimens are without any trace of the original connective rings of growth. The general colour of the shell is uniform pale olive-grey, inclining to yellow on the plastron. The ground-colour of the soft parts is olive-grey, but the sides of the head are adorned with orange-red or yellow marks, the patch between the eye and ear and three or {357}four stripes on the neck being especially conspicuous. The limbs have pale yellowish streaks. All these markings are, however, subject to much individual variation. While, for instance, the half-grown creatures are distinctly agreeably coloured, often with a rich brown, nicely sculptured shell, and with conspicuous orange and yellow marks on the skin, the very old ones become rather ugly, the prevailing colour varying more and more into dull uniform pale olive-grey.
The "Iberian Water-tortoise" is typical of the Iberian Peninsula, and extends through Morocco and Algeria far into North-Western Africa. Unknown to the north of the Cantabrian range, decidedly scarcer than its cousin Emys in the northern half of the Peninsula, it becomes common in the south. In the Alemtejo, in the lower parts of Andalucia and in Morocco, there is scarcely a pool, stream, or river in which it is not found, feeding on any living thing it can master, although fishes and frogs are its principal prey. When the streams and watercourses run dry, during the hot and dry season, the tortoises crowd together into the remaining pools, which soon become stagnant and filthy. But even these havens of refuge are not of lasting avail. They are soon cleared of anything edible, and the stinking water becomes dirtier and hotter day by day. Ultimately the tortoises leave the pool to hide under ledges of rocks, where they aestivate for months. This life in the muddy, slimy pools renders these tortoises peculiarly liable to the attacks of a certain fresh-water alga, which enters through the cracks in the horny shields and then flourishes in the Malpighian layer, and even in the underlying bone itself. This becomes gangrenous in patches, and the whole shell assumes a leprous appearance, hence the specific name of leprosa. Everything combines in favour of this destructive little alga. The tortoise, covered with mud, basks in the hot sun, the horny shields become brittle and crack, often peeling off in thin flakes. But those happy individuals which inhabit permanent rivers, or pools which do not dry up, are, and remain, as clean as other water-tortoises.
C. leprosa has a most disagreeable, offensive smell, something like concentrated essence of fish, due to the secretion of a pair of large glands situated beneath the skin of the inguinal region, and opening behind the bridge. Freshly caught specimens stink horribly, but when they have become accustomed to being {358}handled, they no longer void these glands. They always withdraw into the water for the night, and the cold season is spent in the mud. Their time of propagation is still somewhat doubtful. Very young tortoises are met with in the Peninsula in March, when they are already in the rivers. Those which I imported in the summer and autumn invariably dug their nests and laid their long, oval eggs (28 to 33 mm. long) in the month of November, pairing having taken place some two or three months previously. The mode of making the nest is exactly the same as that described for Emys. As most of my specimens were kept in a greenhouse with a permanent current of warm water through their tanks, they never hibernated, nor did they pass through a torpid time in the summer, but they showed an irresistible love for the hot-water pipes, huddling together by the dozen, so that the pipes had to be screened off to prevent the creatures from getting burnt. Until this precaution was taken, they heated themselves so much that the shields and even the bones of the plastron were injured. The artificial warm temperature and the complete suppression of seasonal rest had no bad influence, most of the tortoises living with undiminished appetite for more than twelve years, but the sexual period became disturbed, pairing occurring ultimately at all times of the year. The eagerness of the males, however, had a peculiar evil secondary influence upon the females. The male tries to fasten on to its mate by biting into the collar-like fold of the neck into which the head is withdrawn, and this repeated irritation produces sores and swellings, which latter in their turn prevent the female from wiping the eyes with the back of the fore-limbs, a habit common to most, if not all, tortoises. Ultimately the eyes fester, and the tortoise, becoming practically blind, falls off its feed, leaves the water, which makes matters worse, and is very difficult to cure.
In other respects they are very hardy, and they stand acclimatisation in England perfectly. Some, thriving in a deep concreted pond, passed through the very severe winters of several years ago, hiding in the mud below the ice, and appeared in the spring in perfect health. They can also successfully pass the winter under moss and a heap of loose garden-rubbish.
C. caspica is closely allied to C. leprosa, which it represents in the Balkan Peninsula and in Asia Minor. It differs from the south-western species chiefly by having the cutting edges of the {359}upper jaw finely denticulated, and by its prettier coloration, each shield being ornamented with yellowish streaks which form a kind of ∞ on the costals, and a ring on the marginals. The plastron is black in the young, with yellow and black patches in the adult. The head and sides of the neck are striped with yellow lines, narrowly edged with black, and the rest of the soft parts is marbled dark olive and yellow. A few other species occur in China, Japan, and North America.
Clemmys insculpta, one of the American species, ranging from Maine to Pennsylvania and New Jersey, is easily recognised by the peculiar reddish-brown and brick-dust colour of the soft parts. The strongly keeled, posteriorly emarginate carapace is reddish brown, with radiating yellow lines. Each shield is delicately sculptured. The plastron, which is notched behind, is yellow, with a large black patch on the outer corner of each shield. Length of a full-grown specimen 8 inches. They frequent the rivers and ponds, but are also very fond of leaving the water, sometimes remaining for months in dry places.
Malacoclemmys of North America, with three species only, is closely allied to Clemmys, from which it differs chiefly by the very broad alveolar surface of the upper jaw, and by the more forward position of the entoplastron, this being placed anteriorly to the humero-pectoral suture. We mention this genus since one of its species, M. terrapin, is so extensively eaten in the Eastern United States. The shell is oval, slightly emarginate behind, obtusely carinated along the middle line. The upper parts of the shell are brown or greenish, with dark concentric lines; the marginals are yellow below, each with a ring of dark grey, and forming a peculiarly up-turned rim. The plastron is yellowish, either with concentric stripes and dusky lines or uniform yellow. But it is the colour of the soft parts which gives this otherwise dull-looking creature its delicately pretty appearance. The skin is, namely, greenish white with countless small black dots. The males remain much smaller than the females, and have the concentric stripes more pronounced. This species, the choicest of the edible Terrapins, frequents the salt marshes of the east coast of North America, from Rhode Island to the Gulf of Mexico, being most abundant around Charleston.
The following is a condensed account of an article which appeared in the New York Sun, 18th September 1898, the data {360}of which were supplied by the manager of the terrapin-farm at Beaulieu, Georgia. The continued hunting and the unfailing demand for them are making them very scarce, so that enterprising men have established terrapin-farms or "crawls" for the keeping and breeding of terrapins. The "crawls" in question are near the river. The larger is 310 by 60 feet, and is divided into three compartments for three sizes. The smaller "crawl" is for the babies, and is 100 by 8 feet. Through both "crawls" runs a ditch connected with the river and making a circuit of the farm. The bottom of the "crawls" is on a level with the low tide, and is covered with a layer of mud about six inches deep. Into this the terrapins burrow in the winter. The average population of terrapins is about 40,000, one half "bulls" and the other half "heifers." The latter are much better eating, and grow to a much larger size, namely, eight inches on the plastron, while the "bulls" rarely grow over five inches long. When a female reaches six to eight inches it is called a "count." Those between five-and-a-half to six inches long are known as "two-for-threes," while those from five to five-and-a-half inches are known as "halves." They are fed exclusively on shrimps and crabs on account of the flavour, although they will eat almost anything. The 40,000 consume on an average twenty bushels of crustaceans a day. They are quite indifferent to cold. The manager saw some placed in a block of ice and frozen fast to it; after four or five days they were chopped out, thawed, and were soon as lively as ever. The statement that it takes these terrapins only seven years to attain full commercial growth is surprising, and is probably an underestimate. At the end of the large "crawl" is a board to enable the females to creep into a sand-pit, where they lay the eggs from April to June, eight to twelve forming a set. It is necessary to get the babies away from their parents as soon as they hatch, else they will be eaten. The young must not be exposed to the cold. The old ones have a large amount of curiosity. The best way of catching them is for two men to go out in a boat with a net. They row carefully along until they come to a likely spot. Then one man raps several times sharply on the boat with a stick, and if there are any terrapin about they will come to the surface just as fast as they can get there to see what is going on, and the other man scoops them up with a little net. Another {361}way, used in the salt marshes, is for the negroes to go tramping through the mud and water. If they pass any terrapin these will rise out of the mud to see what the disturbance is. The captives are then fattened in the "crawl." When the men go in to feed them they whistle, and terrapin from all over the "crawl," thousands of them, come swimming through the water, piling over each other in their efforts to get close to the man with the shrimps and crabs.
Cistudo.–The plastron, without forming a bridge, is connected with the carapace by ligaments, and is divided into two movable lobes, the transverse hinge being so perfect that the box can be completely closed after head, legs, and tail have been withdrawn. The nuchal shield is very small; the first four neurals are large and broad, the fifth much broader than long. There are twelve pairs of marginal shields. The carapace is high and arched. The digits are almost completely free. The tail is very short. The skull is without a bony temporal arch, the quadrato-jugal and the jugal being absent. Only two species, in North America.
C. carolina of the Eastern United States is a very interesting species. Closely allied by its internal structure to the water-tortoises, it has become absolutely terrestrial; and the shape of the head, the convex shell which is coloured black and yellow or orange-brown, and the short webless fingers are all terrestrial features. But the rather long toes, provided with long and sharp claws, the broad and flat feet, enlarged by a broad fold of skin on the outer margin, the long oval eggs, the smooth covering of the head, and the preponderant animal diet, still proclaim the aquatic relationship of this tortoise. It is in fact a genus which has changed habits and features from aquatic to terrestrial life. The head is covered with a smooth skin, and the upper beak, especially in old specimens, is strongly and broadly hooked. The eyes of the males are red, those of the females are brown. The plastron of the males is concave, that of the females is flat. Large females reach a length of nearly six inches. The young are nearly round, with high, arched back and prominent keels. The keels of the middle line remain a long time, but they gradually flatten down with age, being prominent only at their posterior ends. Each dorsal shield is originally nicely sculptured, with a well-marked areola and concentric rings. Very old individuals {362}become much flatter on the top of the shell, but the sides remain steep, so that the whole shell roughly resembles a somewhat oblong box with the corners rounded off, and the whole upper surface rubbed down quite smooth. The variations of colour are almost endless, and they occur in the same localities. I have a number of all ages from Long Island, near New York. The half-grown are beautifully reddish or orange-brown with dark patches, median keels prominent, plastron uniform black-brown. In others the dark-brown prevails over the lighter markings, which are yellower and more spotted or dotted than patched. Some of the oldest, with quite smooth shells, are black, with small, round, light yellow spots. Others are vermiculated or striped with yellow and black. The soft parts vary to the same extent, some showing on the neck a beautiful intricate pattern of yellow, reddish and brown, while in others these colours are arranged more or less in longitudinal stripes.
These "Box-tortoises" are often caught in the States and kept as pets in the gardens, and their owners mark them by cutting their initials into the plastron. These marks heal up and widen in time like letters cut into the bark of a tree. One of my specimens, certainly a very old one to judge from his hooked beak, perfectly smooth and flat shell, and from the condition of the marginals, which have the edges rubbed down quite smooth and rounded off, has two initials and the date 1837 on its plastron. Of course there is no proof that the date had been cut in that year, more than sixty-three years ago, but it was done a long time ago. The scars on those parts of the shell which touch the ground are almost effaced, and the letters and figures have become somewhat distorted owing to the usual unequal, not concentrical, peripheral growth. Moreover, this tortoise must have been already adult, although not quite fully grown, since the marks are large and were evidently put in such a size and position as to fit the available space. I may mention that this record tortoise was, when I got it, not kept in confinement, but had been picked up at large.
These Box-tortoises become very tame. Although fond of drinking quantities of water in long and slowly repeated draughts, they do not go into the water, and if they fall in accidentally they are liable to get drowned. They enjoy a mixed diet, but animal food predominates, consisting chiefly of snails, the shells of {363}which are passed, slugs, earthworms, maggots, and soft caterpillars. Their fondness for slugs is all the more remarkable since scarcely any other Vertebrate eats these slimy, sticky molluscs; but a Box-tortoise will make a meal of two or more fat specimens of the black slug Arion, and it will eat dozens of small slugs. It first deliberately smells the prey, turns the head sidewards and gives a bite, whereupon first the intestines and then the rest are eaten. The slime is later on scraped off with the fore-limbs, or the head is rubbed against the grass. The favourite time of feeding is towards dusk or in the early dewy morning, and they are especially lively during a soft, warm rain. They also relish various kinds of fungi and fruit, for instance half-rotten bananas. Close observation of their habits gives us indications as to how the change from carnivorous to herbivorous habits may have taken place. Accidentally many a blade of grass is bitten off and swallowed together with the molluscs, also bits of rotten wood and moss, and their excrements are often full of such more or less digested matter. They are not very fond of basking, although they love warmth, creeping into the grass, where they make a shallow form by moving the shell backwards and forwards. During the cooler nights they frequently retire into a hole or under a log of wood. They require to hibernate. If kept in a warm house they become restless in the autumn, refuse food, drink and feed again after some weeks, but are liable to die during the winter. If they can find a cool place they bury themselves and sleep for several months. If left out of doors they dig into the ground, creep into a hole, at the bottom of which they half bury themselves, or they hide under a heap of garden-rubbish well out of the reach of frost. Warm April days bring them out, and the first requirement is a drink.
When walking about in search of food they assume a curious attitude, with the shell well above the ground, the long neck stretched out and raised high. Their temper varies individually. Some become tame readily and lose all shyness, and creep up to their friend to take food from his fingers. Others are decidedly shy and sulky, withdrawing with a hiss into the shell, which in some specimens shuts almost hermetically all round, and they do not come out until all imaginary danger is past. One of my males sulked thus for several months, at least we never saw anything of it except the closed shell, but it did not starve itself. {364}Propagation takes place in the summer, the long oval hard-shelled eggs being laid in June and July.
The typical Land-tortoises are easily recognised by their feet. The digits are short, have not more than two joints, and are without any trace of webs; the metacarpals are scarcely longer than broad. The hind-feet are club-footed. The skin on the anterior side of the fore-limbs is covered with strong horny scales, frequently with dermal ossifications. The plastron is united suturally by a broad bridge with the usually strongly arched carapace. The skull has complete postorbital and temporal arches. The top of the head is covered with shields. The tail is short. There are only a few recent genera, modifications of the central and typical genus Testudo. The latter is cosmopolitan in the warmer temperate and tropical regions, except in the Australian and Austro-Malayan countries.
Cinyxis (Fig. 82) with a few species in Tropical Africa from the Gambia and from Abyssinia to the Equator is remarkable for the unique modification of its carapace, the posterior portion of which is movable, the hinge passing between the seventh and eighth marginal and the fourth and fifth costal plates, externally behind the seventh marginal and the second costal shields. In the middle of the back the hinge is imperfect, the parts being merely flexible enough to permit the posterior half of the box to be closed. The head is covered with shields.
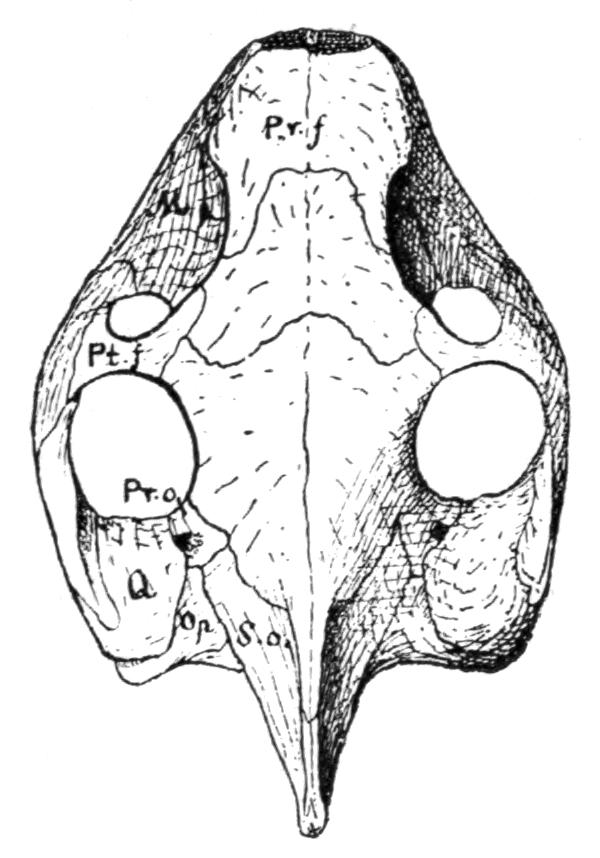
Fig. 81.–Skull of Testudo nigrita s. elephantopus, from the Galapagos Islands. × ½. M, maxillary; Op, Opisthotic; Pr.f, prefrontal; Pr.o, prootic; Pt.f, postfrontal; Q, quadrate; S.o, supra-occipital.
C. belliana, of Northern Tropical Africa, has a small nuchal shield, and the margin of the carapace is smooth. Length of shell up to seven or eight inches. C. homeana, of West Africa, has likewise a small nuchal shield, but the posterior portion of the carapace descends vertically, and the marginals are strongly reverted and serrated. C. erosa (Fig. 82), also from West Africa, has no nuchal shield; the marginals are reverted and serrated, but the posterior part of the carapace is sloping, and the anterior {365}portion of the plastron is strongly forked in front, and projects beyond the anterior border of the carapace. This peculiar creature reaches a length of nine inches. When withdrawn within the shell, which is closed behind and depressed in front, with the jagged edges of the plastron and the anterior marginals protecting the drawn-in head, it has a very quaint appearance. It lives entirely on fruit and other vegetable matter, and is said to prefer to lie in the water, while C. belliana is supposed to be entirely terrestrial.
Pyxis arachnoides, of Madagascar, a small land-tortoise, only four inches in length, has an immovable carapace, but the front lobe of the plastron is hinged.
Testudo.–The plastron is immovable, except that in old individuals of some species, e.g. T. ibera, the hinder lobe develops a transverse flexible hinge. They have existed since the Oligocene of North America and Europe; and are now represented by nearly forty species in all the tropical and warmer temperate countries excepting the Austro-Malayan and Australian region. Typically terrestrial, herbivorous and frugivorous, although occasionally varying their diet with worms, molluscs, and insects. The eggs are hard-shelled, mostly less oval than those of the aquatic and semi-aquatic tortoises. The males generally remain smaller than the females, have a slightly longer tail, and have a concave instead of a flat plastron. Most land-tortoises hibernate in the ground during the cool and cold seasons, or they aestivate during the hot and dry months of tropical countries, but this is not an invariable rule.
T. graeca, the common "Greek Tortoise." The shell is very convex, without keels, and has a smooth, not serrated margin. {366}The nuchal shield is narrow. The fifth or last neural shield is much broader than the others. The supracaudal is usually divided in the median line, so that this is really the last pair of marginals. The plastron is notched behind; the axillary and inguinal shields are small. The scales on the anterior surface of the fore-limbs are small, and form from half-a-dozen to ten longitudinal rows. The hinder surface of the thigh is quite smooth. The tip of the tail ends in a conical, horny spur. The coloration of the shell varies somewhat, but the ground-colour is yellow, each shield with a dark brown centre and irregular patches or confluent spots towards the margin. The plastron has an irregular, broad black border. The soft parts are grey-yellowish. Some specimens are rather pale, almost lemon yellow with little black; others incline towards orange with more or less black. The middle fields of the shields of young specimens are granular, although this area is rubbed smooth with age; but the rest shows clearly marked concentric lines of growth. The eyes are dark, with a brown or bluish tinge, sometimes inclining to dark grey in very old specimens.
Full-grown females have a shell six inches in length. This species inhabits the northern half of the Balkan Peninsula, parts of Asia Minor and Syria, Italy, and most of the islands of the Mediterranean, from the Grecian Archipelago to the Balearic Islands.
T. ibera is closely allied to T. graeca, from which it differs chiefly in the following points. The last pair of marginal shields are fused into an unpaired supracaudal, the median line of division being almost obliterated. The fifth neural shield is not broader, and generally a little narrower than the others. The posterior lobe of the plastron develops with age a transverse ligamentous hinge, and is thus rendered slightly movable, especially in the females. The posterior margin of the carapace is slightly expanded in old specimens. The scales of the fore-limb are large and imbricating, and form only four or five longitudinal rows. On the middle of the exposed posterior surface of the thighs the skin carries a strong, conical, horny tubercle. The coloration is much like that of T. graeca, except that the yellow of the young inclines to pale olive. Some specimens are uniform brownish. This species reaches a much larger size than T. graeca, old females often measuring eight inches, {367}rarely more than nine inches in length. Its home is Morocco and Asia Minor, extending into Persia. It also occurs in certain parts of Southern Andalucia, where it breeds regularly, for instance, in the sandy pine-forests of the Marismas, near the mouth of the Guadalquivir. Whether it has been introduced from Morocco, or is indigenous, is an open question. Its specific name refers to its Iberian home.
T. marginata is worth mentioning, since it is the Greek tortoise, although not that of the European markets, which are supplied by the other two species. T. marginata is restricted to Greece proper, where it is the only land-tortoise. It is less closely allied to T. graeca than to T. ibera, of which it may be called an exaggerated form. The posterior margin of the carapace is much expanded or flanged, and serrated. The supracaudal is undivided, the posterior lobe of the plastron is movable, but the large conical spur on the thighs is absent. The dorsal shields of adult specimens are black with a small yellowish patch; the ventral shields are yellowish, each with a large black triangular patch. The British Museum possesses a shell 28 cm. = 11 inches in length.
The habits of these Moorish and Greek tortoises are very much alike, and since they enjoy the distinction of frequently being kept as pets in gardens, where they are allowed to look after themselves, a great many incidental and odd observations have been made on them. They are essentially vegetable feeders, but their taste varies individually and with the season, also according to the vegetation of the country they happen to come from. Most of them enjoy juicy plants, for instance, lettuce and cabbage; the flowers of the dandelion attract them not merely by their bright colour; clover is also a favourite food, and an enclosure of grass-land with clover in it is soon cleared of the latter; grass is also taken, in default of anything better. Some of my specimens gradually bite large holes into gourds and pumpkins; and in Morocco I found them in the autumn feeding entirely on the terribly astringent green fruits of the dwarf palm Chamaerops humilis. The larger specimens bolted the fruit with the stones, passing the latter. In close captivity they often learn to take and to like bread soaked in milk or water. They drink slowly and at length, but scarcely ever when they have succulent food. There is one thing which they do {368}not eat, namely, "black beetles," although they are warranted to do so by the men who hawk them in the streets. Worms, slugs, etc. are often mentioned as part of their occasional diet, but I am not aware that any of the hundreds which I have watched have taken such creatures, in spite of every opportunity. Their habits are very regular. They learn to know the geography of their domain thoroughly, and the spot selected for sleeping will be resorted to over and over again, be it underneath some broad leaves, under a bushy fir-tree, between a cluster of wallflowers, or between some tussocks, or even in an almost bare corner, the attractions of which are not at all obvious. Although their mental capacities cannot possibly be called brilliant, they soon learn to distinguish between different persons, and they will come up to be fed; but their memory for localities is surprising. Here is only one instance. A tortoise which had been put into an outhouse for hibernation was six months later taken to its usual large enclosure, and in the afternoon it tucked itself away on the top of a mound under precisely the same low bush where it used to sleep during the previous autumn. It could not see that spot from where it had been put down, and it did not meander about during the day, but after having enjoyed the warm sun it made straight for its favourite place. Dr. Girtanner of St. Gallen in Switzerland testifies to their appreciation of music. When the town-band began to play on the square adjoining his garden, all his tortoises crept as fast as possible towards the fence and remained there motionless with heads and necks erect. When the piece was finished they moved about, but when the next number began they were again spellbound. This he has observed, not on one but on many occasions. That they can hear, although their ears are not visible, but covered by the ordinary skin, is obvious enough from the fact that during the pairing season they emit feeble piping sounds.
They are extremely fond of basking in the hot sun, sometimes allowing themselves to be almost baked in it, but then again at other times they seem to be anxious to seek the shade. They rise late and go to bed early, being absolutely diurnal. In the summer they leave their quarters when the sun is well up, making for a sunny spot to graze. Then they lie still and bask, unless a shower causes them to retreat under shelter. {369}After some hours' rest they feed again, and in the afternoon, long before sunset, they go to bed. Some winters in England are of course much more severe than any which these tortoises experience in their native countries. Still they manage to survive them, provided they find a place which they can burrow into, deep enough to be out of the reach of frost; and if there is a heap of mould, rotting weeds, and leaves, they are probably safe. Sometimes they are restless, coming out again in unusually mild winters without, however, taking food. If they appear too early in the spring, they run the risk of terrible colds on prolonged wet and cold days, but in the autumn they are hardier, and can stand several degrees of dry frost.
The pairing season begins in May, but lasts far into the summer. In Morocco I found them pairing as late as the month of September. The preliminaries extend over many days. The male becomes unusually active, makes a piping sound, runs after the female, draws in its head, and knocks with its shell against that of the female. This is repeated many times, until the female is excited enough to raise itself upon its hind-limbs. The eggs, only two to four in number, are laid several weeks later, and are buried in the ground. They are roundish-oval, hard-shelled, and vary according to the size of the female. Those of T. graeca measure on the average 30 by 24 mm.; those of a large specimen of T. ibera 32 to 36 by 30 mm. The newly-hatched little creatures are still quite flexible, and apparently soon bury themselves before beginning their active life in the ensuing spring.
The age which these tortoises can reach is quite unknown, but there are reliable data of individuals having been kept for many years. Rumpf[133] kept two T. graeca in his garden at Frankfort-on-the-Main, and let them hibernate in a box with hay in the cellar. One lived 33, the other 23 years. The most famous specimen of T. ibera is "Gilbert White's Tortoise,"[134] which had been kept for more than 40 years before it came into his possession. It used to bury itself in November and to come out in April. It died in 1794, having reached an age of fifty-four plus an unknown number of years, since there is no record of its size when it came to England. The same applies to every other specimen which has been, and is being, observed as a pet. My {370}largest Morocco female, which has a shell 7 inches long, shows at least 25 concentric rings of growth on the shields; the last half-dozen rings are very narrow, while some of those of the central area have been rubbed down. This creature is not improbably 30 years old. A small female, which is only 5¼ inches long, has already 14 rings on its still perfect shields. Lastly, a little one, only 4 inches long, shows 7 rings. They grow fastest when they are about 6 to 7 inches long, and they then seem to be at their prime. White's tortoise, now enshrined in the National Collection, was unusually large, the shell measuring 25 cm., or nearly 10 inches; around the much-enlarged, rubbed-down areola of each shield are about 30 very narrow rings.
T. horsfieldi is easily recognised by its possessing only four claws on the fore- and hind-limbs. It is closely allied to the species last mentioned, which it seems to represent in the sandy districts of Transcaspia and the Kirghiz Steppes to Afghanistan.
T. elegans, the "Starred Tortoise" of the southern half of India and Ceylon, is easily recognised by the very convex carapace without a nuchal shield, and by the beautiful markings of the other shields, each of which has a yellow areola, whence radiate yellow streaks upon a black ground. Moreover, the dorsal shields often form humps. It reaches the length of one foot. Old specimens lose the beautiful yellow radiation, owing to a considerable amount of peeling off of the horny layers.
The habits have been carefully watched by Captain Thomas Hutton,[135] who gives the following account. The tortoises live in the grassy jungle at the base of the hills, but owing to their colour being so blended with the rocky nature of the ground, they are with difficulty distinguished. Moreover, they remain concealed beneath shrubs or grass during the heat of the day. In the rainy season they are most active, wandering about all day, feeding and pairing. At the approach of the cold weather they select a sheltered spot and conceal themselves by thrusting their shell into some thick tuft of grass, remaining there in a sort of lethargic, but not torpid, inactivity until the hot season, at which time they remain concealed only during the heat of the day, coming out about sunset to feed.
During the hot season Hutton's captives often soaked themselves {371}in water, and they drank a great deal. Copulation lasted about ten minutes; the females received the males from the end of June to the middle of October. On the 11th of November a female dug a pit at the root of a tuft of grass, having previously watered the spot, then digging with the hind-limbs alternately, and continuing to water the soil. In two hours she had made a hole six inches deep and four wide; she then laid four pure white eggs, each about 1¾ inches or 45 mm. long, and filled the hole again with the prepared mud, pressing it well in with the feet and with the weight of the body. The whole operation took four hours. From December to the beginning of February these tortoises were listless, they then took water and some lucerne, but did not come out again until the middle of April, well in the hot season. Both males and females wrestled in a curious way. One confronted the other, with the head and fore-limbs drawn into the shell, and with the hind-limbs planted firmly on the ground, and in this manner shoving against each other in any narrow space. Sometimes, if one succeeded in placing its shell beneath the other, he tilted his adversary over on his back, from which position he had great difficulty in recovering himself.
T. polyphemus, the "Gopher Tortoise" of the south-eastern States of North America, is one of the few American species. It is characterised by the shape of the front lobe of the plastron, which is bent upwards, and extends beyond the carapace. The nuchal shield is present, not narrow; the supracaudal is undivided. The shell is much depressed, and flattened along the vertebral region, with rounded margins. The fore-limbs are armed with very strong claws. The general colour is very dark brown above, inclining to black; brownish yellow below, with blackish patches. The length of the shell is about one foot, or even eighteen inches.
The Gopher is interesting for its habits, which are described by Agassiz, Schnee, and others. Its domicile consists of an excavation, the mouth of which is just sufficient to admit the animal, the burrow running in an oblique direction to the depth of about four feet. The whole passage is sometimes more than two yards long. It expands from the entrance, and ends in a roomy space, sometimes with a few branches of fir trees which have been dragged in either for food or as a lining. The burrow {372}is inhabited by one pair only. When the dew is on the grass, or after rain, they emerge in search of food, which consists of grass, succulent vegetables, fruit, etc. They also eat the gum that exudes from trees, especially the resin of the pine. The eggs are laid in June, not in their domicile, but in a separate cavity near the entrance; a set consists of five eggs, almost round, and very large, namely, 40 mm., or more than one inch and a half in diameter. To capture the Gopher a deep hole is dug at the mouth of their home, into which they fall as they emerge for food. In Southern Texas and neighbouring parts of Mexico they are represented by a smaller and lighter coloured species.
T. tabulata, widely spread over Tropical South America, whence it is often brought over as a curiosity, reaches a large size, specimens nearly two feet in length being not uncommon. The shell is flat on the top, and is very elongated, without a nuchal, but with an undivided supracaudal shield. The carapace is very dark brown or black, each shield with a yellow or orange centre; the plastron is brown and yellow, the dark colour being mostly confined to the middle portion. The ground-colour of the skin of the limbs is blackish, but the scales are orange or red. The head is yellow and black. This species inhabits the forests, and lives chiefly on the fruits of trees; in captivity they are said to take bread soaked in milk or water, lemons, apples, bananas, cabbage, gourds, and also meat, at least the males.
Gigantic Land-tortoises differ from the others in no essential points except their large size. The term gigantic is, however, applied to many of them by courtesy only, since they do not exceed the dimensions of large Turtles. A truly gigantic species, T. atlas, has left its remains in the Sivalik Hills of late Miocene or early Pliocene date. The skull is between seven and eight inches long, and is well preserved, but the correctness of the dimensions of the specimen, as it now stands, restored in the National Collection, is open to doubt. The shell was probably not more than six feet long. Miocene and Pliocene Europe was also inhabited by large tortoises, with shells about four feet long, e.g. T. perpigniana, whose bony plates are one inch thick; others have been found in North America. Such large tortoises are now restricted to two widely separated regions of the world, namely the Galapagos Islands (which have received their name from these creatures, galápago being one of the Spanish terms for {373}tortoise), and the islands in the Western Indian Ocean, namely the Mascarenes (Bourbon, Mauritius, and Rodriguez), the Comoros, Aldabra, the Amirantes, and the Seychelles. When they became extinct in Madagascar is not known, but T. grandidieri was a very large species of apparently very recent date. Of the other islands the Comoros only were inhabited by man, the others were devoid of any but small and harmless Mammals. It was on these peaceful islands that large tortoises lived in incredible numbers, and, like the Dodo of Mauritius and the Solitaire of Rodriguez, grew to a size far beyond that of their less favourably placed continental relations. The same applies to the tortoises of the Galapagos Islands. Plenty of food, a congenial equable climate, and absence of enemies enabled them to enjoy existence to the fullest extent. There was nothing for them to do but to thrive, to feed, to propagate, to grow, and to vary. At least there was nothing to check variation within reasonable limits. Scattered over the many islands, they were prevented from inter-breeding, and thus it has come to pass that not only every group of islands, but in the case of the Galapagos almost every island, has or had its own particular kind, be these called varieties, races, forms, or species.
There are four features of special interest. First, these tortoises grow to a large size, and there are no small species on any of these islands. Secondly, they vary much individually. Thirdly, each island or group of islands has developed its own kind. Lastly, there is the widely spread tendency to reduce the thickness of the bony plates of the carapace, in spite of its size. In some cases, notably T. vosmaeri of Rodriguez, the bony shell is reduced to apparently the utmost limit compatible with mechanical safety. The horny shields are, or were, however, well developed, sometimes much more so than in other recent land-tortoises. Whatever were the original reasons for the development of a strong shell in tortoises, they cannot have prevailed in these islands.
Where did all these tortoises come from, and how did they get to these oceanic islands? Accidental transport or migration are out of the question. Land-tortoises are drowned within a few hours. Moreover, there are none of their kind on the continents of Africa, Asia, and South America, although they had a much wider distribution in past geological ages. Consequently we have to assume that they are descendants of tortoises once populating the land which, except the islands, lies now below {374}the western Indian ocean. The existence of this, "Lemuria" or "Gondwana," came to an end in Mid-Tertiary times. The large tortoises on the remaining continents died out–in any case they are gone, while those which lived on, or retreated to, what became the present islands, survived and flourished.
The tortoises were not left in peace with the advent of man, who found that they were good to eat. They were first exterminated on the Mascarene Islands. In 1759 four small vessels were specially appointed for the service of bringing tortoises from Rodriguez to Mauritius; one vessel carried a cargo of 6000; and altogether more than 30,000 were imported into Mauritius within the space of eighteen months. Dr. Günther very properly remarks that many of these tortoises must have been small-sized specimens, and that many of them were probably used for provisioning passing Government vessels. Anyhow an inter-insular traffic was carried on, and there are records of superfluous tortoises having been turned loose, at the end of the voyage, in distant islands, even in Java. Importation and exchange of choice specimens, by way of presents, seems also to have taken place. All this makes it now actually impossible to trace the original habitat of the few surviving specimens with anything like certainty. At the beginning of this century the large tortoises had been nearly cleared off most of the islands, and at the present time only the south island of Aldabra enjoys the reputation of still possessing some really indigenous tortoises. The few survivors on the other islands are said to have been introduced. The small stock at Aldabra is now under Government protection. Representatives of various species will linger on for a little time to come, when they are kept as pets on some tropical islands, but those which have been brought to Europe are of course doomed.
We can mention only a few of the large tortoises which have become famous, not to say historical. A fascinating résumé of the whole complicated question has been given by Dr. Günther.[136]
Testudo gigantea s. elephantina s. hololissa s. ponderosa, originally confined to the North Island of Aldabra, where this {375}kind has been completely exterminated, is now still to be found in the Seychelles in considerable numbers, introduced there by planters, and kept in a state of semi-domestication. A very large specimen was received by the Hon. Walter Rothschild, at Tring, in 1893. In 1897 its shell measured 40¼ inches in length, 52¼ over the curve, and 50 inches across the curve transversely; it weighed 358 lbs. The measurements taken in previous years are unfortunately not free from mistakes. "Whenever the temperature was over 60° F. this tortoise had a free run of 350 acres of grass park, and when the temperature showed permanently below 58°, it was kept in an orchid house from September to June. When at liberty in the park it lived entirely on grass, but in the hothouse it fed on carrots, cabbage, lettuce, and several other vegetables"; it was also very fond of rotten fruit. To this species belongs the large tortoise which has been living at St. Helena for more than the last hundred years.
T. daudini is the species of the South Island of Aldabra. Voeltzkow, in 1895, succeeded in carrying off seven specimens. He gives the following description:–The island is an atoll, cut through in three places, with a greatest length of about twenty miles. The chief hindrance in the search for the tortoises is the impenetrability of the island. The soil consists entirely of sharp water-worn corals, with their points uppermost, while the whole is covered with such thick masses of low scrub, that a way has to be cut with an axe, so that an extended search over a large area is out of the question. To land on the outside is dangerous, on account of the heavy surf; while landing from the inside of the atoll is much hindered by the dense thickets of mangrove trees. As drinking water, and that very bad, is only found in one place, rainwater has to be collected from the natural hollows, and carried along in tanks. Thousands of mosquitoes prevent one remaining over night in those places which the tortoises frequent. Then at last, when one has discovered, by a stroke of luck, one of these creatures, in the thick scrub, where they hide during the heat of the day, the real hard work begins, namely, the conveyance of the beast. Six reached Europe alive, two of them were sent to Frankfort, and the four others to Hamburg. Mr. Rothschild received a male of T. daudini, which, until its recent death, was the largest living tortoise known. The length {376}of its shell was 55 inches, or 67½ inches over the curve; total weight 560 lbs. This specimen had a chequered career. Although its original home must have been the Aldabra atoll, it had been known for many years on Egmont Island, one of the Chagos Islands. According to tradition, it had been there some 150 years, but the first settlement on that island was formed from Mauritius only at the beginning of this century. The owner of the tortoise, M. Antelme, took it to Mauritius, whence it came to England. On the Egmont Island it used to bury itself for six months in the ground without eating anything.
T. sumeirei.–This kind is supposed to have been the species peculiar to the Seychelles. In 1766 five large tortoises were brought from the Seychelles to Mauritius by Chevalier Marion de Tresne. Of these only three were alive in 1898, two in {377}Mauritius and one in London; the latter specimen soon died in the Zoological Gardens. One of the two survivors, the last of their race, is famous. It was kept at Port Louis, and when Mauritius became a British possession in 1810, the tortoise was especially mentioned and taken over. It still lives there in the grounds of the barracks of the garrison. According to the proverbial oldest inhabitants it had in 1810 already reached its present size, namely, a shell-length of about 40 inches with a greatest circumference of 259 cm. = 8 feet 6 inches. Total weight 160 kilo = about 358 lbs. When walking it stands 63.5 cm. = 25.4 inches high, with the plastron about 15 cm. or 6 inches above the ground, and it can then carry with ease two full-grown men on its back. This old male is now nearly blind, but is otherwise of regular habits and in good health. Although it has been known for nearly 150 years it had to wait for its scientific name until the year 1892.
Another famous individual is the Colombo tortoise. It is supposed to have come to Colombo from the Seychelles in 1798. It died in 1897. To judge from photographs, this specimen, a male, may possibly belong to T. sumeirei, in spite of the very flat shell, which is 53½ inches in length.
Leaving aside the remains of sub-fossil tortoises, e.g. the thin-shelled T. vosmaeri of Rodriguez, and several kinds which have been dug out in the Mare-aux-songes of Mauritius, one of which had a markedly forked and prolonged anterior plastral lobe, rather resembling that of the Pliocene Sivalik T. atlas, we now turn to the tortoises of the Galapagos Islands. They existed in enormous numbers towards the end of the seventeenth century, when Dampier visited those islands. Hundreds were exported and scattered early in the nineteenth century. When the islands became a penal settlement of Ecuador, the introduction of convicts and pigs proved detrimental to them, but Darwin found them still present in 1835 on most of the islands. His classical account of these old giants is to be found in the Voyage of the Beagle. They lived on the succulent cactus plants, leaves of trees, berries, and a kind of Usnea, a lichen pendant from the trees. They collected regularly at certain pools and springs, leading to which were regular well-trodden paths, formed by the coming and going of the tortoises. He calculated that they could walk a distance of about four miles in one day. During {378}the time of propagation the males emit a hoarse bark, which can be heard a hundred yards off. The round eggs measure about 5 cm. or 2 inches in diameter, and are laid in the month of October, about one dozen making a set.
Nearly every island had apparently its own kind. They are all remarkable for their small head and the length of their neck, which is decidedly longer and more slender than that of the Eastern tortoises. The most peculiar looking are or were T. ephippium and T. abingdoni, the shell of which is extremely thin, with large lacunae in the osseous plates. The profile of the shell is somewhat saddle-shaped, with the horny shields partly concave and turned upwards at the sides. The general colour of these and the other Galapagos tortoises is black. T. ephippium still survives on Duncan Island. Of T. elephantopus s. vicina Baur collected twenty-one specimens in 1893 on Albemarle Island. Some of them are still comparatively young, only 16 inches long. A large one was killed, and, being hard up for water, Baur and his companions drank the five cups full of fluid contained in the pericardial sac; they found it most refreshing, and tasting somewhat like the white of an egg. One monster is said to have measured 56 inches over the curve, with a skull 7.12 inches in length. Mr. Rothschild received one of this kind alive–a much-travelled specimen. It came to England from Sydney, whether it had been brought in 1880 from Rotuma Island, north of the Fiji group. There it had probably been left with others by Captain Porter, who, on his voyage from the Galapagos in 1813, distributed several young tortoises from his stock among the chiefs, and permitted a great many to escape into the bushes and among the grass. The shell of this specimen measured 49½ inches in length, 56 over the curve.
Fam. 6. Chelonidae (Turtles).–The limbs are paddle-shaped, and the shell is covered with horny shields. Only two recent genera, with three species, widely distributed in the seas.
The neck is short and incompletely retractile. The temporal region of the skull is completely roofed over above and laterally by the parietals, postfrontals, squamosals, quadrato-jugals and jugals. All these bones are much expanded, and form the additional or false roof. The parietals are especially large, and are in broad contact with the squamosals. Nasals are absent. The nares are bordered by the small premaxillaries, the maxillaries, {379}and the prefrontals. The choanae are enclosed by the palatines, which are separated by the vomer, and are posteriorly in broad contact with the pterygoids. The latter are connected with descending processes of the parietals by epipterygoids. The foramen magnum is bounded not only by the supra-occipital and the lateral occipitals, but also by the basi-occipital. For the skeleton see Fig. 65, p. 320. The pubic and ischiadic symphyses are connected by a narrow cartilaginous band. The pubis has a large, broad, lateral process, but the ischium is devoid of such a process. The paddles of the fore- and hind-limbs are produced by an elongation of the metacarpal and metatarsal bones and of most of the phalanges, and these have no condyles; most of the carpal and tarsal elements are flattened, and additional width is given to the hands by the much enlarged pisiform bone. The number of phalanges of the five fingers is 2, 3, 3, 2, 2; that of the five toes, 2, 3, 3, 3, 2.
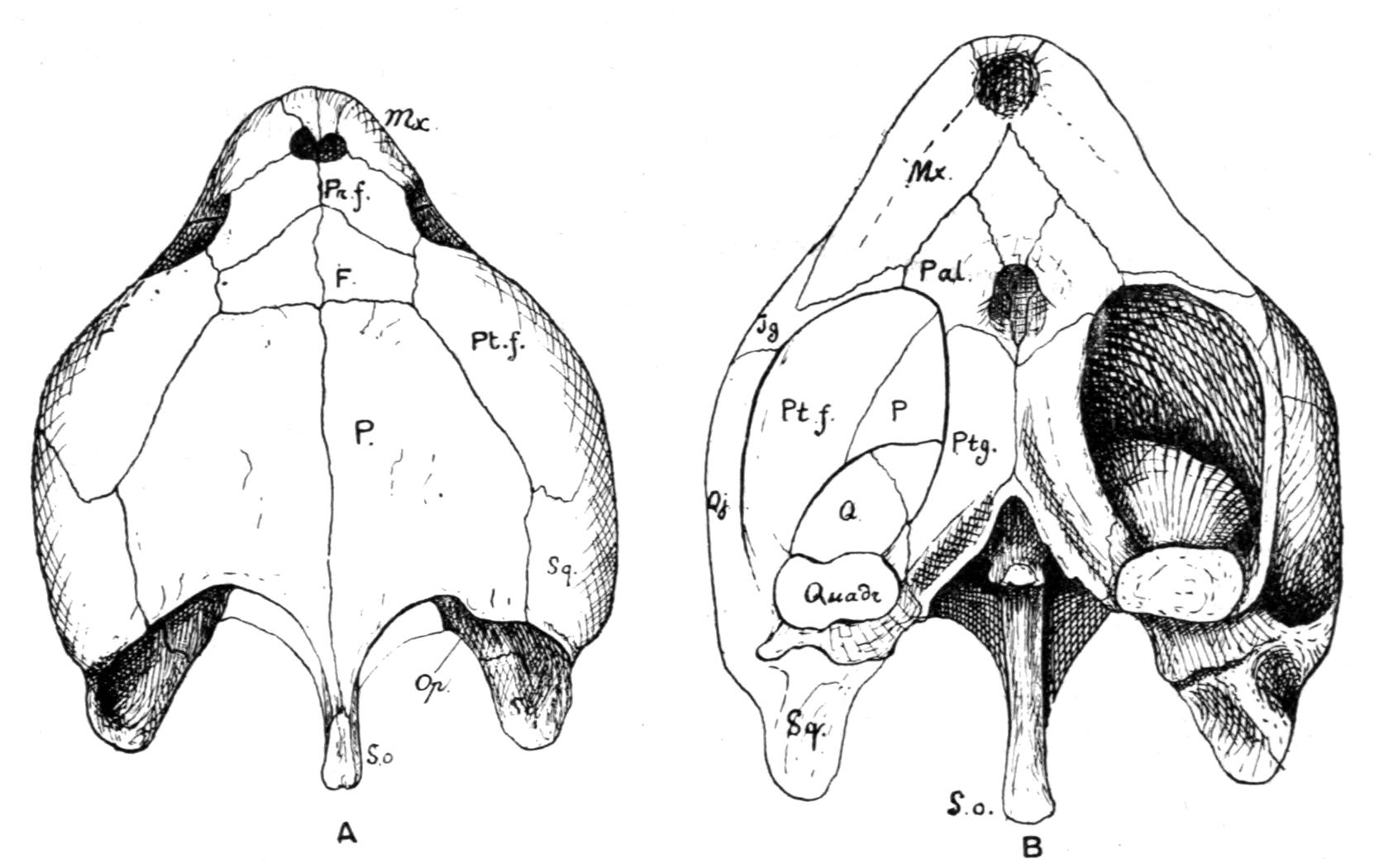
Fig. 84.–Skull of Thalassochelys caretta; cf. also Fig. 63, p. 317. A, Dorsal view; B, ventral view; F, frontal; Jg, jugal; Mx, maxillary; Op, opisthotic; P, parietal; Pal, palatine; Pr.f, prefrontal; Pt.f, postfrontal; Ptg, pterygoid; Q, quadrate; Quadr, articular surface of quadrate; Qj, quadrato-jugal; S.o, supra-occipital; Sq, squamosal.
The carapace is heart-shaped and very flat. The nuchal plate has no rib-like processes. The eight neurals form a continuous series, and the short tail is covered by two or three pygal plates besides the unpaired last marginal. The number of all the {380}marginals is 23, sometimes 25 individually. The plastron (Fig. 66, p. 321) is composed of the usual nine plates, which, however, remain entirely free from the marginals, and are only loosely connected with each other, enclosing a very large unossified space. The horny shields covering the plastron number 13, and there is a series of about 5 inframarginals (Fig. 61, 6, p. 315). There are normally 12 pairs of marginal shields, a nuchal, 5 neural, and 5 or 7 costal shields. Whilst the number of these dorsal shields is pretty constant in Chelone, it is subject to an astonishing amount of individual variation in Thalassochelys.
The Chelonidae are a highly specialised offshoot of the Cryptodira adapted to marine life. Fundamentally they agree most with the Testudinidae, paradoxical as this may appear at first sight. There is nothing primitive about them except the complete series of inframarginal shields. Fossil forerunners of marine turtle-like creatures appear in the Upper Jurassic deposits of Europe and North America. The numerous genera have been grouped together as Thalassemydidae and Chelonemydidae. They are more or less intermediate between Chelonidae and Emys-like Testudinidae, the carapace being not too much flattened and broadened out, the fontanelles between the ribs are mostly small, the plastral bones are still broad, enclose a smaller ossified space, and there is still a bony bridge in most cases. The paddle-shape of the limbs is less pronounced, and sometimes only indicated. In some forms, especially Lytoloma, from the Upper Cretaceous and Eocene of North America and Europe, the anterior portion of the skull is much longer than in the Chelonidae, the vomer and the premaxillaries are elongated, and the anterior portion of the roof of the mouth, with the corresponding parts of the lower jaw, seems to have carried crushing pads. Some of the best-known Upper Jurassic genera are Eurysternum and Idiochelys; Plesiochelys from the Purbeck and Wealden; Allopleuron hofmanni from the Upper Cretaceous of Belgium approaches Chelone by the large fontanelles between the small marginal and the short costal plates. True Chelonidae are very rare and imperfect in the Mid-Tertiary strata, but both recent genera seem to have existed since Pliocene times.
The few recent Chelonidae are entirely marine, going on land only in order to deposit their eggs in the sands of unfrequented shores. Their distribution, in conformity with their oceanic life, {381}is almost cosmopolitan within the warmer zones, but not a few find their way far into the temperate seas. They are all eagerly hunted by man either for food or for the sake of the tortoiseshell.
Chelone.–With only four pairs of costal shields. Carapace with large persisting fontanelles between the costal and marginal plates. Two species.
Ch. mydas (the "Green or Edible Turtle"), has when adult a nearly smooth shell, all the shields being juxtaposed, fitting closely into each other, and becoming quite smooth with age. The neural shields of younger specimens have a feeble keel. The twenty-five shields which surround the carapace form a smooth, or but indistinctly serrated rim. The head is covered with one pair of prefrontal shields, the others are small. The horny beaks of the upper and lower jaws have denticulated outer edges, those of the upper jaw having two pairs of strong denticulated ridges. The limbs have generally only one claw, namely on the first digit. This claw, although sometimes curved and thick, and more than an inch in length, is blunt. The general colour is olive or brown above, with yellowish spots or blotches; the under parts are pale yellowish. This species attains a large size, with a length of shell of nearly four feet, but the usual length of full-grown specimens is three feet, and these weigh, when in good condition, more than three hundredweight. Their home is in the Atlantic, Indian, and Pacific Oceans, but there are certain regions in which they are more common than in others. Famous centres are the Island of Ascension, the West Indies, and the coast of Mosquito, at least for commercial purposes. As they require sandy, easily accessible beaches for the deposition of their eggs, they congregate in certain parts of the world more than in others, and being strictly vegetable feeders, they are naturally bound to the coasts, although they are sometimes met with far out at sea. Their chief food consists of algae, and of Zostera marina, the edible "Dulce," which grows plentifully in the lagoons of the coast of Florida. When they have eaten their fill, they are said to chop off more of these plants, and roll them, together with the adherent mud, into balls of the size of a head, and these balls, receding with the tide, are followed by the Turtles.
Whilst in the water they are caught in various ways, with {382}nets or harpoons. In some parts of the world the natives follow them in a boat, and when they espy a turtle crawling along the bottom, a man, attached to a rope, dives in, clasps it, and is brought up by his companions together with his prey. Turtles are fond of basking asleep, floating on the surface, and they are then harpooned from a stealthily approaching boat. The most original mode of catching them is that used by the natives of Torres Straits, Madagascar, and Cuba. The turtle-fishers go out in the boat to a spot frequented by grazing turtles; a long string is tied to the tail of a fish, Echeneis, a member of the Mackerel family, and the Echeneis, anxious to get away to protective shelter, makes for a turtle, and attaches itself to the turtle's plastron by means of the large sucking apparatus on the top of its head and neck-region. The men are guided by the string, and the turtle is gently coaxed up towards the surface or followed into shallow water, where it is either harpooned or dived for. It is curious that this use of the Echeneis exists in such widely separated parts of the world, the natives of which cannot have any knowledge of each other. These modes of catching turtles are sportsman-like, but the greatest and most wanton destruction is practised at their breeding places. In conformity with the wide distribution of these creatures, the time of breeding is not the same everywhere. In the West Indian region, and in the Straits of Malacca, it falls within the period of April to June; on the coast of West Africa it occurs from September to January. The females come to their breeding places from afar, reconnoitre the beach carefully, are extremely wary and shy, taking alarm at the slightest disturbance, and at last crawl on land. Well out of the reach of the tide the female scoops out a hole in the sand, deposits about one hundred or more of its round, rather parchment-shelled eggs, covers the nest carefully, obliterating all traces of the dug-out sand, and makes again for the sea by another route. At least they are said to make a sort of circuitous route so that nobody can tell the position of the nest, which may be anywhere beneath the broad trail left by the heavy creature on its way from and back to the sea. The nest is discovered by probing the sand with sticks. The time of incubation is not known, but according to Agassiz, lasts at least seven weeks.
Fig. 85.–Three turned Turtles, a Seal, and Albatrosses, Laysan Islands, north-west of the Sandwich Islands. From a photograph belonging to the Hon. W. Rothschild.
The "turning" of turtles is a cruel and wanton operation, since frequently many more are turned over and left to perish than are taken away. Men lying in ambush watch the beast, or they approach the lonely sandy shore by boat, and rush the helpless creatures when these are surprised in sufficient numbers. It takes several men to lift a full-grown specimen. It is therefore necessary to secure them by turning them over with poles or by their flippers, lest they should crawl away. On board ship they are either put into tanks or tied with ropes on deck, covered with a moistened cloth; and occasionally a piece of bread, soaked in sea-water, is thrust into the parched mouth. In London they are kept in large tanks, often in considerable numbers, but since they take no food in captivity, or rather because it is difficult to supply them with the right sort, they are not kept long. After the head has been cut off, the body is suspended for a day or two, in order to drain it of the blood. It is not only the meat and the fat which are used for the making of the famous soup, but also the thick and dense layer of subcutaneous tissue which lines the inside of the shell.
Tennent describes a revolting spectacle exhibited in the markets of Jaffna, in Ceylon. The flesh of the turtles is sold piecemeal by the Tamil fishermen, while the animals are still alive. At certain seasons, says the same authority, the flesh of turtle on the south-west coast of Ceylon is usually avoided as poisonous, but some lamentable instances are recorded of neglect of this, and consequent sickness, followed by coma and death. In the Gulf of Manaar specimens are frequently found between four and five feet in length; and on one occasion, in riding along the seashore north of Putlam, he saw a man in charge of some sheep, resting under the shade of a turtle shell, which he had erected on sticks to protect him from the sun. In connexion with this curious sight, Tennent quotes Aelian's statements, copied by him from Megasthenes' Indica Frag. lix. 31, that in the Indian ocean turtles occur which measure fifteen ells, so that not a few people may find ample shelter beneath a single shell.
Ch. imbricata ("Hawksbill Turtle").–The number of shields covering the carapace is the same as in Ch. mydas, but they strongly imbricate, or overlap each other from before backwards, until the animal is very old, when the shields become juxtaposed.
In young specimens, under one foot in length, each of the neural and costal shields is strongly keeled, the three rows of keels converging towards the posterior end of the shell. The neural series of keels is almost continuous, and remains longest, even in half-grown specimens. The twelve pairs of marginal shields form at first a strongly serrated sharp edge; the serrations disappear gradually on the front portion, but remain on the posterior half of the shell. The horny covers of the jaws form a hooked beak, with sharp but smooth or feebly denticulated margins. The fore- and hind-flippers have two claws. The young are pale brown above, blackish below; the shell of the adult is beautifully marbled with yellow on a rich dark-brown ground; the plastron is yellow. The shields and scales of the head and limbs are dark brown, with yellow margins. The top of the head is covered by a large unpaired frontal and a pair of prefrontal or interorbital shields. This Turtle does not reach the size of the green or edible kind; the largest shell on record is in the National Collection, and measures 85 cm. = 34 inches in length. They range over all the tropical and subtropical seas. They are apparently strictly {386}carnivorous, living upon fish and molluscs, the shells of which they crunch. Although not eaten, they are much persecuted on account of their shells, the horny shields of which are the "tortoiseshell" of commerce. A large specimen yields up to 8 lbs. Few of the shields are, however, thick enough to be manufactured into the larger articles which art and fashion delight in, but if heated in oil, or boiled, they can be welded together under pressure, and be given any desired shape. In genuine articles of Oriental manufacture these welds can generally be detected, or their compound nature is indicated by the beautiful pattern, which is too regular in the imitations now common. Even the shavings and leavings can be welded and moulded into large pieces. The stripping of the shields has been described by Sir E. Tennent. "If taken from the animal after death and decomposition, the colour of the shell becomes clouded and milky, and hence the cruel expedient is resorted to of seizing the turtles as they repair to the shore to deposit their eggs, and suspending them over fires till heat makes the plates on the dorsal shields start from the bone of the carapace, after which the creature is permitted to escape to the water. At Celebes, where the finest tortoise-shell is exported to China, the natives kill the turtles by blows on the head, and immerse the shell in boiling water to detach the shields. Dry heat is only resorted to by the unskilful, who frequently destroy the tortoise-shell in the operation." The cruel process described above is resorted to "for economy's sake," the Singhalese believing that such maltreated turtles regenerate the shields, to be caught and shipped again. Since none of them are actually re-caught in the mutilated condition, this is looked upon as a proof of the correctness of the treatment. It is more likely that they die.
New shields can be reproduced only if the underlying Malpighian layer of cells (cf. Fig. 68, B, p. 323) is not killed by the roasting. However, Dr. Charles Hose, with his long experience in Borneo, is positive that numerous individuals are there caught which have imperfectly mended shells, the shields of which do not imbricate, are thin, and almost worthless.
It is commonly believed that the same individuals return again and again to the same spot for laying. This is very likely the case. Tennent mentions that in the year 1826 a Hawksbill was taken near Hambangtotte, which bore a ring attached to one of its fins, that had been placed there by a Dutch {387}officer thirty years before, with a view of establishing the fact of these recurring visits to the same beach. The same homing instinct has been observed in some females of the Green Turtle, which, having been brought from the Tortugas Keys to Key West off the south end of Florida, escaped, and were, a few days later, re-caught at the Tortugas. On the other hand, experiments made with turtles at Ascension are said to have had no result.
Thalassochelys, with five pairs of costal shields. The carapace is completely ossified in the adult, leaving no fontanelles between the ribs and the marginals.
Th. caretta (the "Loggerhead Turtle").–The shields of the carapace imbricate only in young specimens, in the adult they become smooth and juxtaposed. The margin is serrated posteriorly. The carapace of the young has three strong keels. The intergular shield is very small or absent. The marginals, including the nuchal, usually number 23, rarely 25. The large head is armed with hooked jaws, the crushing surface of the horny upper beak has a median prominent ridge. The top of the head has a pair of shields in front of the unpaired frontal. The flippers of the young have claws on the first and second digits; in the adult usually only that of the first digit remains. The general colour of the shell is uniform brown above, yellowish below. Very young specimens are uniform dark brown or blackish above and below.
Large individuals have a shell about three feet and a half in length. The Loggerhead is carnivorous, and is commercially of no value. Its habits seem to be the same as those of the other Turtles, but it has a much wider distribution. Besides all the tropical and intertropical seas, it inhabits the Mediterranean, and is an accidental visitor to the western coasts of Europe, especially Portugal and the Bay of Biscay. It has been caught several times on the coast of Belgium, and an old female containing 1150 eggs was captured in 1894 on the Dutch coast. In 1861 one was caught near Penman, on the coast of Banffshire, and a second in the completely land-locked Loch Lomond.[137] It has been more frequently recorded from the coast of Devon and Cornwall.
The most interesting feature of the Loggerhead is the {388}astonishing variability in the number of the horny shields of the carapace. The normal number of shields of the carapace, leaving out the marginals and counting the nuchal as the first neural, is 6 neurals and 5 pairs of costals, in all 16. The greatest number of dorsal shields observed is 8 neurals and 8 pairs of costals, in all 24. Many of the intermediate combinations have been observed, there being, for instance, specimens with 8 neurals and 16, 14, 13, 12, or 11 costals, the latter not being always in pairs, but unequal on the right and left sides; or there are 7 neurals with 20 to 16 costals, or 6 neurals with 20, 19, 18, 17, or 16 costals. The interesting fact in connexion with these variations is, moreover, that some of the shields are much smaller than the others, sometimes mere vestiges in all stages of gradual suppression, and that the abnormalities are much more common in babies and small specimens than in adults. The importance of these "orthogenetic" variations has been discussed on p. 326.
Sub-Order 2. Pleurodira.–Neck bending laterally and tucked away in the niche formed between the anterior portion of the carapace and plastron. Pelvis ankylosed to the shell, the broadened tops of the ilia to the carapace, the distal ends of the pubes and ischia to the plastron.
Freshwater tortoises, almost entirely carnivorous, inhabiting South America, Australia, Africa, and Madagascar. Fossil forms are known from the Jurassic epoch onwards.
Owing to the strong connexion of the iliac bones with the costal plates the sacrum has become practically abolished, the sacral ribs being reduced to one pair (the posterior of the original two pairs) or being absent. The centra of the cervical vertebrae articulate by cup and ball joints. The formation of the temporal region of the skull varies considerably in the three families, some genera lacking the complete zygomatic arch, while others have a narrow parieto-squamosal arch bridging over the temporal fossa, or the latter is completely roofed over by the laterally expanded parietal, which meets the jugal and quadrato-jugal. The quadrate is always trumpet-shaped; the rim of the tympanum is complete, but the posterior part of the trumpet remains open. The basisphenoid, pterygoids, and palatines form a broad and flat roof to the mouth. The vomer is large, and separates the palatines in the Chelydidae; it is very much {389}reduced or absent in the Pelomedusidae, in which the palatines meet. All the Chelydidae, except Chelys, have nasal bones which remain distinct from the prefrontals. The choanae lie in front of the palatines, divided by the vomer when this is present, but they are not roofed in ventrally.
The ilia are solidly ankylosed in the adult with the neighbouring costal plates, mostly with the last two pairs, sometimes also with the pygal plate. The lateral processes of the pubes fuse with the xiphiplastra. The ischia are also attached to the same plastral elements.
The carapace is flat and completely ossified. The nuchal plate is always conspicuous, much larger than the neurals, and these are often reduced by being encroached upon by the eight pairs of costal plates, which then meet in the dorsal line. In Sternothaerus all the eight neurals are present and form a continuous row. In most of the other genera they are reduced to seven, the last being squeezed out. In Rhinemys they are reduced to the second, third and fourth and an isolated fifth, and in Hydraspis they are all gone. The pygal plate is always, even in Sternothaerus, separated from the last neural by the eighth pair of costals. The marginals number 23, but in Carettochelys only 21.
The carapace is covered with horny shields, except in Carettochelys. The nuchal is absent in the Pelomedusidae and in a few Chelydidae (Elseya and a few species of Emydura). In Hydromedusa the nuchal is shut in by the anterior marginals, simulating a sixth neural. The plastron is composed of the usual nine elements, but the Pelomedusidae possess an additional pair, the meso-plastra, inserted between the hyo- and hypo-plastra. The bridge is strong, connected with the carapace by suture. In Sternothaerus the front lobe of the plastron is movable. The intergular shield is always present; it is terminal, forming part of the front margin, except in Chelodina, where this shield, although large, is shut in behind the gulars (cf. Fig. 61, 4 and 5, p. 315).
Although the Pleurodira are a peculiarly specialised group, one of the oldest Chelonian fossils known seems to belong to them. Proganochelys, represented by a complete shell, nearly 2 feet long, has been found in the Upper Keuper Sandstone of Würtemberg. Plesiochelys, of the Upper Jurassic of Switzerland, has eight neural and three supracaudal plates, but is without the {390}ischiadic plastral ankylosis. Pleurosternum, of the English and Continental Purbeck beds, has meso-plastral plates like the recent Pelomedusidae. Rhinochelys, of the Cambridge Greensand, has a broad parieto-postfrontal roof, and large nasal bones. Forms like Podocnemis, now restricted to South America, occur in the Eocene of Europe. One of the most aberrant Chelonians is Miolania, from the Plistocene of Queensland and from Lord Howe's Island, remarkable for its huge size and the thick armour on the head and tail; the head especially carries large paired projections, one pair of which extends horizontally like powerful horns, recalling the queer Theromorphous Elginia.
We divide the recent Pleurodira into three families, of which that of Carettochelys stands apart by its paddle-shaped limbs and the absence of horny shields. The Pelomedusidae and Chelydidae are closely allied. The former are not Australian, and are externally distinguished by the absence of a nuchal shield.
Fam. 1. Pelomedusidae.–Neck completely retractile within the shell. Carapace without a nuchal shield. The plastron is composed of eleven plates, there being besides the unpaired endo-plastron a pair of meso-plastra, situated between the hyo- and hypo-plastra; but these meso-plastra meet in the middle line in Sternothaerus only, while in Podocnemis and Pelomedusa they are restricted to small pieces on the bridge, widely separated from each other by the usual hyo- and hypo-plastral suture. A nuchal shield is absent; there are twenty-four marginal and thirteen plastral shields, inclusive of the conspicuous intergular. The temporal fossa is widely open, except in Podocnemis, where it is partly roofed in by the meeting of the much-expanded quadrato-jugal with the parietal. The palatine bones are in median contact, not separated by the vomer. Nasal bones being absent, the large prefrontals meet in the middle line. The second cervical vertebra is biconvex.
This family is now represented by only three genera, with about fifteen species in Africa, Madagascar, and South America.
Sternothaerus.–Skull without a bony supratemporal roof. Meso-plastra large, extending right across the plastron. Anterior lobe of the plastron movable, the hinge passing between the hyo- and meso-plastral plates, and between the pectoral and abdominal shields. Fore- and hind-limbs with five short digits and claws. Several species in tropical and southern Africa, and {391}in Madagascar. S. derbianus in West Africa, from the Gambia to Angola, is the largest species, with a shell nearly one foot in length.
Pelomedusa.–Skull with a slender parieto-squamosal arch. Meso-plastra small and lateral. Plastron without a hinge. Fore- and hind-limbs with five very short digits and five claws. Top of the head with one pair of shields between the eyes, and with a large interparietal and a pair of parietals behind.
P. galeata, the only species, occurs in Madagascar and nearly the whole of Africa south of the Sahara, from the Cape to Abyssinia, and in the Sinaitic peninsula. The shell, less than one foot in length, is much depressed and is obtusely keeled; brown above with black spots; brownish-yellow below. The short and broad head is coloured like the rest, without ornamentation. In Somaliland this species sleeps hidden on land during the dry seasons, from July to the end of September, and from January to March, and appears at once after the rains have set in.
Podocnemis.–With a supratemporal roof formed by the junction of the parietal with the quadrato-jugal. Meso-plastra small and lateral. Fore- and hind-limbs broadly webbed, with five and four claws respectively. The fore-arms and the outer edges of the hind-feet with several conspicuous shields, hence the generic name. Head with an interparietal, two parietals, and a narrow unpaired shield between the eyes. The tail is very short. The carapace is flat and broad, strongly serrated on the posterior margin. Chin with one or two short barbels. Several species in South America, chiefly in the basin of the Amazon, and one in Madagascar.
P. expansa.–Very common in Tropical South America, east of the Andes. The female, which is much larger than the male, has a shell nearly three feet in length. Olive-brown above with darker patches; yellowish below. With a few yellow spots above and behind the eyes, and on the parietal region. The "Arrau" turtle is of great commercial importance on account of the eggs, which are periodically collected in enormous quantities, chiefly for the oil. This is either eaten, like the eggs themselves, or used for burning in lamps, or as an addition to tar. The turtles are likewise eaten by man and beast. Thousands of the little creatures are snapped up by Jabiru storks, alligators, and fishes; the adults fall an easy prey {392}to the prowling jaguar, which turns them over on to their backs and neatly cleans out the flesh with its sharp and powerful claws.
Fertilisation takes place in the water, the eggs are deposited on land, in sand-banks, the female digging a hole about two feet deep and covering up the numerous soft-shelled eggs with sand. The time of deposition is the early hours of the morning, but the season depends upon the beginning of the principal rains, since the young are hatched shortly before the torrential rains. This season differs considerably in the various countries. The hatching takes about forty days; the eggs are consequently laid in the Amazon countries during the months of September to November, in the Orinoco district in March. This species lives in the pools of the inundated forests, and when these are dried up, the animals retire into the rivers themselves. Their food consists mainly of the fruit dropping down from the trees.
Bates, in his delightful book, The Naturalist on the River Amazon, gives the following lively and exhaustive account of his experience with these turtles:–
"I accompanied Cardozo in many wanderings on the Solimoes, during which we visited the 'praias' (sand islands), the turtle pools in the forests, and the by-streams and lakes of the great desert river. His object was mainly to superintend the business of digging up turtle eggs on the sandbanks, having been elected commandant for the year by the municipal council of Ega, of the 'praia real' of Shimuni, the one lying nearest to Ega. There are four of these royal praias within the Ega district, a distance of 150 miles from the town, all of which are visited annually by the Ega people for the purpose of collecting eggs and extracting oil from their yolks. Each has its commander, whose business is to make arrangements for securing to every inhabitant an equal chance in the egg harvest, by placing sentinels to protect the turtles whilst laying, and so forth. The pregnant turtles descend from the interior pools to the main river in July and August, before the outlets dry up, and there seek in countless swarms their favourite sand-islands; for it is only a few praias that are selected by them out of the great number existing. The young animals remain in the pools throughout the dry season. These breeding places of turtles then lie 20 to 30 or more feet above the level of the river, {393}and are accessible only by cutting roads through the dense forest....
"We found the two sentinels lodged in a corner of the praia, where it commences at the foot of the towering forest-wall of the island, having built for themselves a little rancho with poles and palm-leaves. Great precautions are obliged to be taken to avoid disturbing the sensitive turtles, who, previous to crawling ashore to lay, assemble in great shoals off the sand-bank. The men, during this time, take care not to show themselves, and warn off any fisherman who wishes to pass near the place....
"I rose from my hammock by daylight, shivering with cold; a praia, on account of the great radiation of heat in the night from the sand, being towards the dawn the coldest place that can be found in this climate. Cardozo and the men were already up watching the turtles. The sentinels had erected for this purpose a stage about fifty feet high, on a tall tree near their station, the ascent to which was by a roughly made ladder of woody lianas. They are enabled, by observing the turtles from their watch-tower, to ascertain the date of successive deposits of eggs, and thus guide the commandant in fixing the time for the general invitation to the Ega people.
"The turtles lay their eggs by night, leaving the water, when nothing disturbs them, in vast crowds, and crawling to the central and highest part of the praia. These places are, of course, the last to go under water when, in unusually wet seasons, the river rises before the eggs are hatched by the heat of the sand.... The hours between midnight and dawn are the busiest. The turtles excavate with their broad webbed paws deep holes in the fine sand; the first-comer, in each case, making a pit about three feet deep, laying its eggs (about 120 in number), and covering them with sand; the next making its deposit at the top of that of its predecessor, and so on until every pit is full. The whole body of turtles frequenting a praia does not finish laying in less than fourteen or fifteen days, even when there is no interruption. When all have done, the area (called by the Brazilians 'taboleiro') over which they have excavated is distinguishable from the rest of the praia only by signs of the sand having been a little disturbed.
"I mounted the sentinel's stage just in time to see the turtles retreating to the water on the opposite side of the sand-bank, {394}after having laid their eggs. The sight was well worth the trouble of ascending the shaky ladder. They were about a mile off, but the surface of the sands was blackened with the multitudes which were waddling towards the river; the margin of the praia was rather steep, and they all seemed to tumble head first down the declivity into the water.... Placards were posted up on the church doors at Ega, announcing that the excavation on Shimuni would commence on the 17th of October, and on Catuá, sixty miles below Shimuni, on the 25th. By the morning of the 17th some 400 persons were assembled on the borders of the sand-bank, each family having erected a rude temporary shed of poles and palm-leaves to protect themselves from the sun and rain. Large copper kettles to prepare the oil, and hundreds of red earthenware jars, were scattered about on the sand.
"The excavation of the taboleiro, collecting the eggs, and purifying the oil, occupied four days. All was done on a system established by the old Portuguese governors, probably more than a century ago. The commandant first took down the names of all the masters of households, with the number of persons each intended to employ in digging; he then exacted a payment of 140 reis (about 4d.) a head towards defraying the expense of sentinels. The whole were then allowed to go to the taboleiro. They ranged themselves round the circle, each person armed with a paddle, to be used as a spade, and then all began simultaneously to dig on a signal being given–the roll of drums–by order of the commandant. It was an animating sight to behold the wide circle of rival diggers throwing up clouds of sand in their energetic labours, and working gradually towards the centre of the ring. A little rest was taken during the great heat of mid-day, and in the evening the eggs were carried to the huts in baskets. By the end of the second day the taboleiro was exhausted; large mounds of eggs, some of them four to five feet in height, were then seen by the side of each hut, the produce of the labour of the family.
"In the hurry of digging, some of the deeper nests are passed over; to find these out, the people go about provided with a long steel or wooden probe, the presence of the eggs being discoverable by the ease with which the spit enters the sand. When no more eggs are to be found, the mashing process begins. {395}The egg, it may be here mentioned, has a flexible or leathery shell; it is quite round, and somewhat larger than a hen's egg. The whole heap is thrown into an empty canoe and mashed with wooden prongs; but sometimes naked Indians and children jump into the mass and tread it down, besmearing themselves with yolk, and making about as filthy a scene as can well be imagined. This being finished, water is poured into the canoe, and the fatty mass is then left for a few hours to be heated by the sun, on which the oil separates and rises to the surface. The floating oil is afterwards skimmed off with long spoons, made by tying large mussel-shells to the end of rods, and purified over the fire in copper kettles.
"The destruction of turtle eggs every year by these proceedings is enormous. At least 6000 jars, holding each three gallons of the oil, are exported annually from the Upper Amazons and the Madeira to Para, where it is used for lighting, frying fish, and other purposes. It may be fairly estimated that 2000 more jarfuls are consumed by the inhabitants of the villages on the river. Now, it takes twelve basketfuls of eggs, or about 6000, by the wasteful process followed, to make one jar of oil. The total number of eggs annually destroyed amounts, therefore, to 48 millions. As each turtle lays about 120, it follows that the yearly offspring of 400,000 turtles is thus annihilated. A vast number, nevertheless, remain undetected; and these would probably be sufficient to keep the turtle population of these rivers up to the mark, if the people did not follow the wasteful practice of lying in wait for the newly-hatched young, and collecting them by thousands for eating; their tender flesh, and the remains of yolk in their entrails, being considered a great delicacy. The chief natural enemies of the turtle are vultures and alligators, which devour the newly-hatched young as they descend in shoals to the water. These must have destroyed an immensely greater number before the European settlers began to appropriate the eggs than they do now. It is almost doubtful if this natural persecution did not act as effectively in checking the increase of the turtle as the artificial destruction now does. If we are to believe the tradition of the Indians, however, it had not this result; for they say that formerly the waters teemed as thickly with turtles as the air does now with mosquitoes. The universal {396}opinion of the settlers on the Upper Amazon is, that the turtle has very greatly decreased in numbers, and is still annually decreasing.
"The principal object of another expedition was to search certain pools in the forest for young turtle. We started from the praia at sunrise on the 7th of October in two canoes, containing twenty-three persons, nineteen of whom were Indians. The pool covered an area of about four or five acres, and was closely hemmed in by the forest, which, in picturesque variety and grouping of trees and foliage, exceeded almost everything I had yet witnessed. The margins for some distance were swampy, and covered with large tufts of fine grass. The pool was nowhere more than five feet deep, one foot of which was not water, but extremely fine and soft mud.
"Cardozo and I spent an hour paddling about. The Indians seemed to think that netting the animals, as Cardozo proposed doing, was not lawful sport, and wished first to have an hour or two's old-fashioned practice with their weapons. I was astonished at the skill which they displayed in shooting turtles from little stages made of poles and cross pieces of wood. They did not wait for their coming to the surface to breathe, but watched for the slight movements in the water which revealed their presence underneath. These little tracts on the water are called the siriré; the instant one was perceived an arrow flew from the bow of the nearest man, and never failed to pierce the shell of the submerged animal. When the turtle was very distant, of course the aim had to be taken at a considerable elevation, but the marksmen preferred a longish range, because the arrow then fell more perpendicularly on the shell, and entered it more deeply.
"The arrow used in turtle-shooting has a strong lancet-shaped steel point fitted into a peg, which enters the tip of the shaft. The peg is secured to the shaft by twine, being some thirty or forty yards in length, and neatly wound round the body of the arrow. When the missile enters the shell the peg drops out, and the pierced animal descends with it towards the bottom, leaving the shaft floating on the surface. This being done the sportsman paddles in his canoe to the place, and gently draws the animal by the twine, humouring it by giving it the rein when it plunges, until it is brought again near the surface, when {397}he strikes it with a second arrow. With the increased hold given by the two cords he has then no difficulty in landing his game.
"By mid-day the men had shot about a score of nearly full-grown turtles. Cardozo then gave orders to spread the net.... Three boat loads, or about eighty, were secured in about twenty minutes. They were then taken ashore and each one secured by the men tying the legs with thongs of bast.
"When the canoes had been twice filled we desisted after a very hard day's work. Nearly all the animals were young ones, chiefly, according to the statement of Pedro, from three to ten years of age; they varied from 6 to 18 inches in length, and were very fat. Cardozo and I lived almost exclusively on them for several months afterwards. Roasted in the shell they form a most appetising dish. These younger turtles never migrate with their elders on the sinking of the waters, but remain in the tepid pools, fattening on fallen fruits, and, according to the natives, on the fine nutritious mud. We captured a few full-grown mother turtles, which were known at once by the horny skin of their breast plates being worn, telling of their having crawled on the sand to lay eggs the previous year. They had evidently made a mistake in not leaving the pool at the proper time, for they were full of eggs, which, we were told, they would, before the season was over, scatter in despair over the swamp. We also found several male turtles, or capitaris, as they are called by the natives. These are immensely less numerous than the females, and are distinguishable by their much smaller size, more circular shape, and the greater length and thickness of their tails. Their flesh is considered unwholesome, especially to sick people having external signs of inflammation."
The most recent account of these water tortoises is that published by Dr. Goeldi from the MS. of João Martins da Silva Continho, a former resident at Manáos on the Middle Amazon. The "Tartaruga" (the Portuguese name for turtles) live from January to July in the inundated, quiet backwaters of the forest-region, feeding upon the various seeds of palms as these ripen and drop successively; rarely, and only when hard up, they are carnivorous. The creatures hide under water below the trees, when they are espied by the Indians, who dive down to a depth of twenty and more feet to catch them in their arms. The {398}civilised Indians use a steel-pointed lance of hard wood, about 10 feet in length. A string connects the point with the shaft around which it is wound. When stuck into the tortoise the shaft and point part; the string is either tied to the boat or to a little float of light wood. In other districts an arrow with a string is employed.
In August, when the water subsides, the tortoises return to the rivers, and the entrance of the lagoon is closed with nets. A number of boats with long poles drive them with much noise towards the entrance. On their way to the rivers the tortoises always go up-stream, and this is called the "arribaçaõ das tartarugas," the ascent of the turtles. The fishermen post themselves at shallow spots or on sand-banks, and wait for the creatures which come up to find a place for landing and laying. The arrows employed are called sararaca, i.e. a thing which can be disjointed; they are about 4 feet long, and consist of a gomo or internodium of wood 9 inches long with a one- or two-barbed steel point, and the shaft into which the gomo fits loosely. The gomo is, moreover, connected with the shaft by a string made of palm-fibres about 30 feet in length, partly wound round the shaft, which ultimately acts as a float.
The laying takes place from the end of September into October. Some of the parents seem to reconnoitre on land for a few days. As a rule only females do this, and the natives say that they are led by a "mestra." The laying takes place early in the morning. The number of females is so great that they often block the way of the boats, and make a great noise by knocking against their neighbours' shells. Each digs a hole about 18 inches or 2 feet deep, and lays from 80 to 200 eggs. Sometimes the laying individual is entirely buried by its neighbours which are scraping their own holes.
In some districts the eggs are wanted for "manteiga" (Portuguese for butter); and the turning over, or viraçaõ of the tortoises takes place later. In other districts they are caught before the eggs are laid, and this barbaric and destructive custom was formerly forbidden by the people themselves. Although the provincial assembly tried to reinstitute the old reasonable customs, the inspectors are often got over by bribery.
There are two ways of extracting the oil from the eggs. To get the thick oil used, mixed with tar, for shipbuilding, caulking, {399}etc., the eggs are heaped up for five days and then worked. The fluid oil for lighting is made from fresh eggs, which are put into a boat and then trampled out with the feet. The oil is drawn off into large earthen jars and put on the fire. Then it is rapidly cooled. The best oil, used for frying fish, is that which is gained from the roasted tortoises themselves. Fresh eggs are either fried or taken with sugar, or mixed with manioca-flour and water. The young, which are hatched in January, are likewise eaten fried, or they are preserved in the fat of the parents.
An average tortoise yields 5 lbs. of fat, costing on the spot two milreis. The whole full-grown animal, of one yard in length, costs the same, and its meat is sufficient to sustain a family of six people for three days. To make 24 lbs. of oil requires 3000 eggs. Two or three tortoises would yield the same amount from their fat. Consequently the destruction of the eggs causes an enormous waste, and is after all the least economical procedure. In the year 1719, 192,000 lbs. were exported from the Alto Amazonas, representing 24,000,000 eggs. In 1700 there were still plenty of tortoises 50 leagues above the mouth of the Para river. Now there is no assembly of more than fifteen tortoises to be found anywhere within 300 leagues from Para to the mouth of the Rio Negro. On the Rio Madeira, from the mouth to the first cataract, 186 leagues distance, there are now only two regular nesting localities. The upper Solimoes and the Rio Yapura are still rich. Near Ega are regular tortoise-ponds, called "curral," which yield sufficient support to their owners; the animals are fed with manioca-flour and leguminous plants.
Fam. 2. Chelydidae.–The neck bends under the margin of the carapace, but remains partly exposed. The nuchal shield is absent except in two Northern Australian species. There are twelve pairs of marginal shields. The plastron is composed of nine plates, and is covered with thirteen shields, one of which is the conspicuous intergular. The temporal region of the skull shows great diversity. It is quite open in Chelodina, covered in by broad expansions of the parietal bones in Platemys, Emydura, and Elseya, or bridged over by a parieto-squamosal arch, which is very slender in Rhinemys, strong in Chelys and Hydraspis. The palatine bones are separated by the vomer; the nasals are variable, mostly present, but the prefrontals are always small, and separated {400}by the frontals. The fifth and eighth cervical vertebrae are biconvex.
This family, still represented by nearly thirty species, which are divided into eight genera, is restricted to Notogaea, namely, South America and Australia.
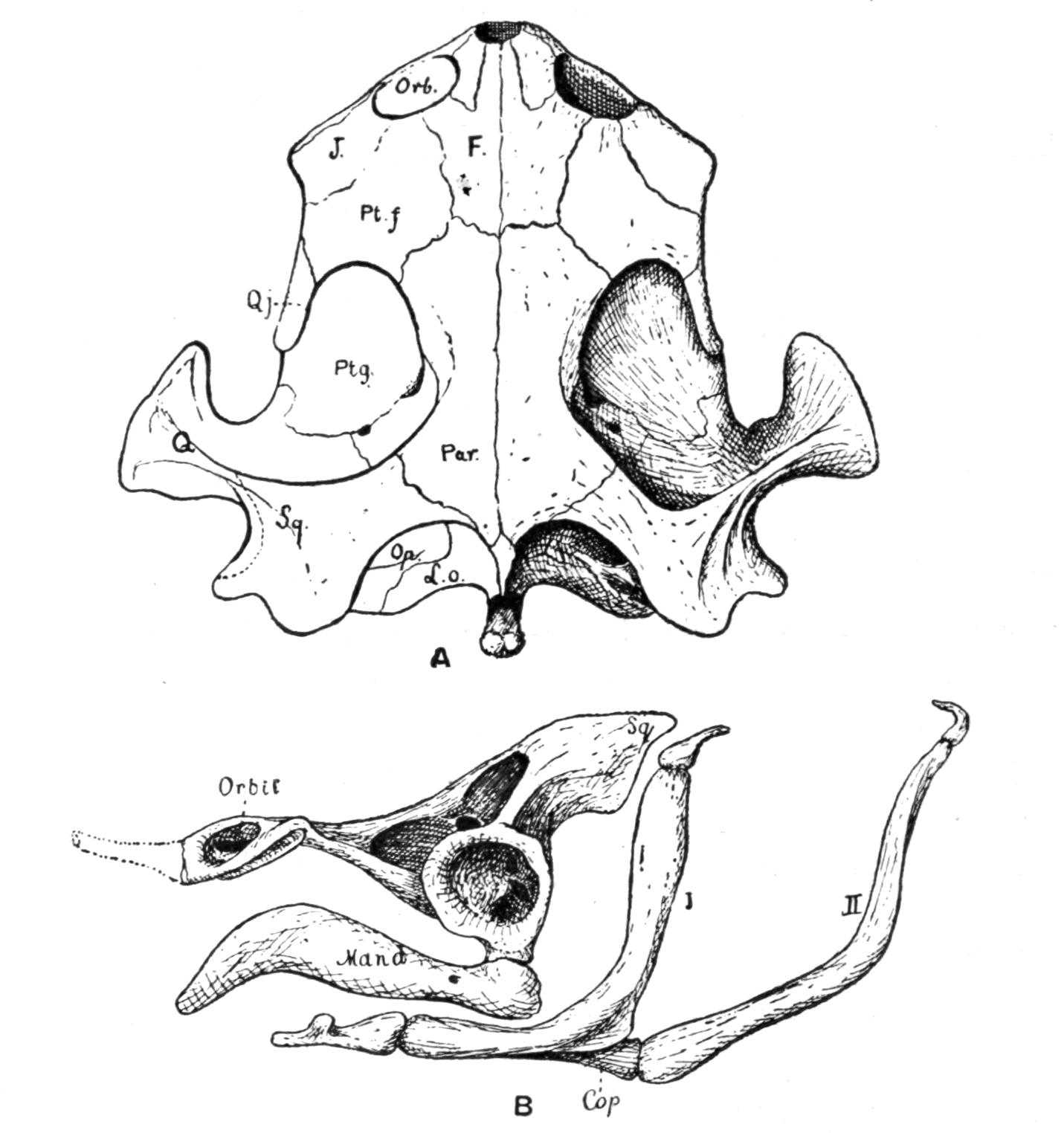
Fig. 87.–Skull of Chelys fimbriata. × 1. A, Dorsal view of skull; B, side view of skull and hyoid apparatus. Cop, copular piece; F, frontal; J, jugal; L.o, lateral occipital; Mand, mandible; Op, opisthotic; Orb, orbit; Par, parietal; Pt.f, postfrontal; Ptg, pterygoid; Q, quadrate; Qj, Quadrato-jugal; Sq, squamosal; I, II, First and second branchial arch.
Chelys fimbriata, the "Matamata," the only species of this genus, inhabits the rivers of Guiana and Northern Brazil. Besides the nuchal, there are seven neural plates; the last pair of costals form a median suture. Nasal bones are absent. The jaws are very weak. The Matamata has a very peculiar appearance. The nose is produced into a long, soft tube, at the end of which open the tiny nostrils. The eyes are very small, and the orbits are placed very near the anterior end of the skull, while the parietal {401}region is broad and much elongated (Fig. 87, p. 400). The quadrates are drawn out into trumpet-shaped tubes. The hyoid apparatus is very large, with enormous anterior and posterior horns. The head and neck are as long as or even longer than the carapace, which is covered with thick, lumpy shields. The skin of the thick neck, of the sides and under parts of the head, is produced into many soft arborescent excrescences or fimbriae, those of the chin and throat and the large ear-flaps being movable at will, and probably used to attract fishes and other prey. The tail is very short. The fore- and hind-limbs are webbed, the former with five, the latter with four claws. Old specimens, which reach a total length of three feet, are uniformly dark brown, and look like a log covered with rough bark. The young are far less ugly, with black and yellow spots on the shell, and with dark stripes along the neck.
Very little is known about the habits of this peculiar creature. It is said to lie submerged in the water, waiting for fishes, frogs, or tadpoles, which are attracted by the playing motions of its cutaneous excrescences. The jaws being so weak, and being covered with a partly soft lip-like skin, it is probable that they are not used for seizing the prey, but that the latter is engulfed into the mouth with the inrush of water into the throat. {402}That this can be widened enormously is indicated by the greatly developed hyoid apparatus.
Chelodina.–The neck is long and slender, the head small and smooth. The nuchal is terminal; the intergular is large. The neural plates are completely suppressed, all the eight pairs of costal plates meeting in the middle line. The shell is very flat. Anterior and posterior limbs entirely webbed, and with only four claws. The tail is very short. Three species in Australia, one in New Guinea.
Ch. longicollis reaches a shell-length of ten inches. It inhabits Southern Australia. The illustrations make a detailed description unnecessary. The colour of the dorsal shield is uniformly dark rich brown, while the shields of the under surface are yellow, with broad dark brown lines along the sutures. These "long-necked Chelodines" have a striking appearance, when they swim or creep about, with the neck either stretched out straight or bent horizontally in an S-shape. The whole creature looks neat and elegant; the iris is pale yellow, and gives the eye a very intelligent expression. They keep well in captivity, provided they are given the choice of land and water. My own prefer to spend most of the day on land, preferably under the ledge of a stone, or perched upon the stone itself if the latter is in the shade, and not too much exposed to view. There they lie motionless, with the neck neatly tucked under the shell, either to the right or to the left. Although the eyelids may be closed, they can see well enough, owing to the transparent condition of the lower lid. They feed in the water upon soft animals, as for instance worms, smooth caterpillars, cockroaches or little frogs; and they also take meat readily, provided this is moved about. The food is invariably taken with a quick sideward jerk of the neck and head.
My specimens soon became so tame that they left the water, and ran up to me with the necks stretched to their full length, then snatching the bit of food, and retiring into the pond to swallow it. When left to themselves they are rather nocturnal in their feeding habits. Now and then they tuck themselves away for weeks without feeding, for instance when they go through a regular term of {404}aestivation in the summer. The last winter they spent buried in the moss, but occasionally, especially on bright and sunny days, they went into the water for a few hours, chiefly to drink, but sometimes also to take a little food.
Hydromedusa, a South American genus, has a neck even longer than that of Chelodina, which it much resembles externally. But the nuchal shield, large and broad transversely, is situated behind the anterior marginals, looking therefore like a sixth neural shield. The neural plates form a continuous row, only the last pair of costal plates meeting in the middle line. H. tectifera occurs in Southern Brazil, and in the La Plata. The shell is dark brown above; yellowish, with dark spots, below; the head and neck are olive-coloured, adorned with a broad white, black-edged band on either side. Fore- and hind-limbs broadly webbed, and with four claws. Total length of the shell about eight inches.
Fam. 3. Carettochelydidae.–The shell is covered with soft skin instead of horny shields. The limbs are transformed into paddles, with elongated digits, and have only two claws. The neck is short, and not retractile. In other respects the skeleton, notably the plastron, pelvis, and skull, conform with the Pleurodirous type. Only one species, Carettochelys insculpta, still imperfectly known, from the Fly River, New Guinea. Length of the shell of the only complete specimen about 18 inches. This peculiar creature seems to stand in the same relation to the typical Pleurodira, as do the Chelonidae to the Testudinidae, except for the complete reduction of the horny shields upon the shell, recalling in this respect Sphargis and Trionyx.
Sub-Order 3. Trionychoidea.–The shell is very flat, oval, or almost round, and is covered with soft, leathery skin instead of with horny shields. The limbs are broadly webbed, and only the three inner digits are provided with claws. Carnivorous, found in the rivers of Asia, Africa, and North America.
The head and neck are completely retractile, bending by a sigmoid curve in a vertical plane like that of the Cryptodira. The jaws are concealed by soft, lip-like flaps, and the nose forms a soft short proboscis. The ear is hidden. The skull, Fig. 91, is flat, with three long posterior processes, formed by the supra-occipital above, and the squamosals on either side. The whole temporal region forms a wide, shallow fossa, without any {405}indication of being arched or bridged over. The premaxilla is extremely small, unpaired, not even reaching the nasal cavity or the vomer. The maxillaries are correspondingly enlarged, surrounding the choanae, which are separated by the narrow vomer. The palatines form a median suture, and are joined behind by the long basisphenoid, which separates the long pterygoids from each other. The quadrate is trumpet-shaped, with a posterior notch for the stapes. The zygomatic arch is complete, and is formed by the quadrato-jugal and the jugal; the latter joins the maxillary and postfrontal, mostly reaching the orbit; in some cases it also just meets the parietal, thereby adding to the strength of the postorbital arch. The prefrontals are large; nasals are absent. The mandible is remarkable for the great development of the coronoid process.
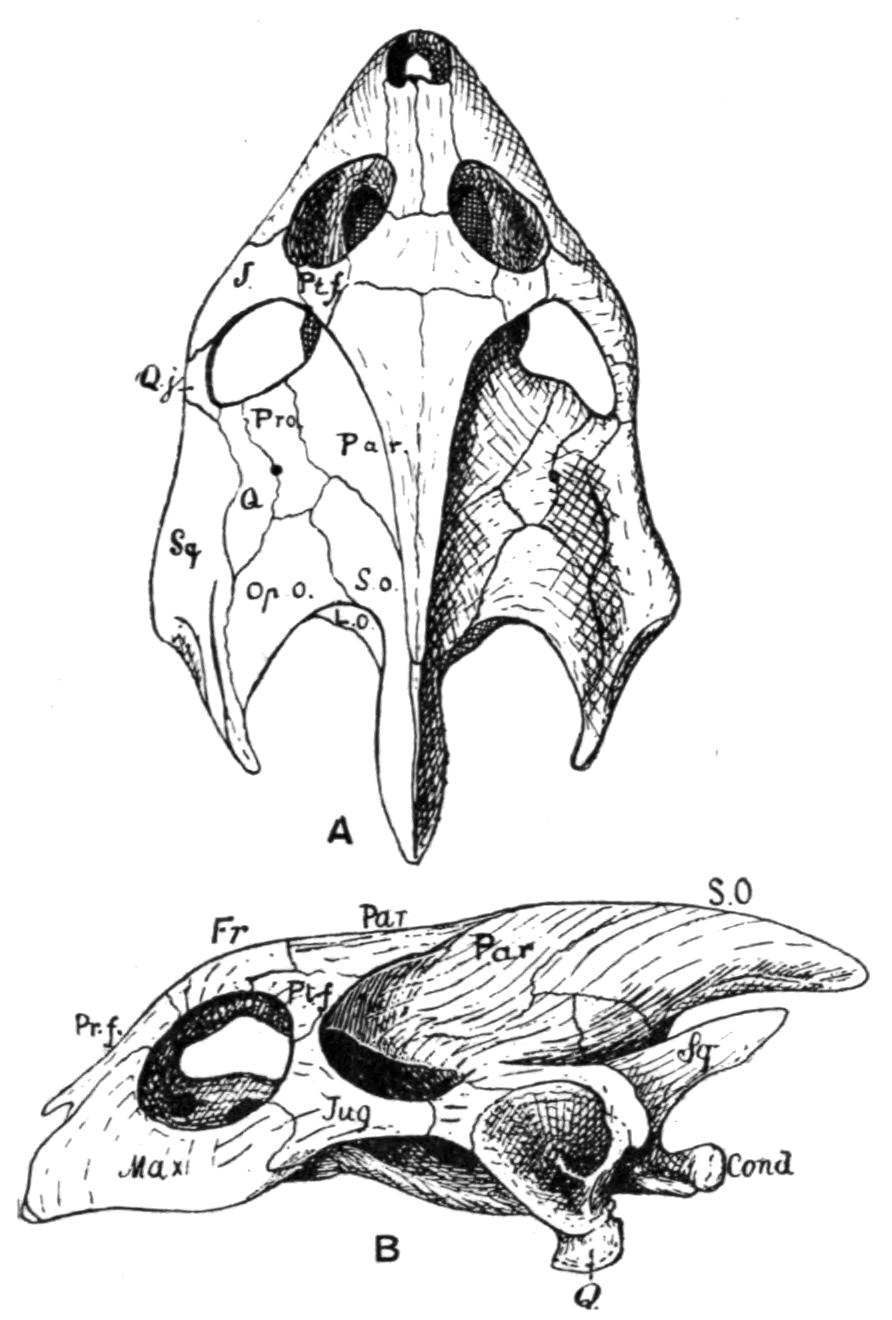
Fig. 91.–Skull of Trionyx hurum. A, From above; B, from the left side; Cond, occipital condyle; Fr, frontal; J, Jug, jugal; L.o, lateral occipital; Max, maxillary; Op.o, opisthotic; Par, parietal; Pr.f, prefrontal; Pro, prootic; Pt.f, postfrontal; Q, quadrate; Qj, quadrato-jugal; S.o, supra-occipital; Sq, squamosal.
The pubic and ischiadic bones enclose a large heart-shaped foramen, and are free from the plastron; the ilia are attached only to the sacral ribs. The carapace is peculiar in so far as it is very incomplete peripherally, the ribs extending considerably beyond the costal plates, nor are they joined by marginal plates, which are absent, unless they are represented by a few small ossifications imbedded in the posterior marginal flap of the disc (Emyda of India). The rim of the disc is always formed by a horizontal, cutaneous, very flexible flap. All the dorsal plates have a rough upper surface, vermiculated or rugose, as usual with such dermal bones, which have lost most of or all their horny covering, and have sunk more deeply into the skin. The {406}nuchal plate has usually a pair of rib-like processes. The neurals form a continuous series, except in the African Cyclanorbis, in which they are much reduced in size, and separated by the costal plates.
The plastron is imperfect, all its constituent nine elements being only loosely connected with each other, and there remains a wide median vacuity between the lateral elements. Most of these plastral bones are reduced to splints, which, instead of meeting by regular sutures, loosely interdigitate with their jagged edges. In the young all these ventral elements are deeply imbedded in the soft, leathery skin, and they do not at all resemble in appearance those of the dorsal side. With age they develop upon their ventral surface stronger and denser ossifications, which ultimately broaden out, sometimes beyond the original underlying bone, and assume the characteristic vermiculated surface-appearance. This is undoubtedly a process of exostosis, a step towards revival of that armour which had been much reduced ancestrally. To appreciate this condition, it is at least suggestive that these mud-tortoises, when kept in the usual hard-bottomed tanks, invariably become sore, the skin wearing through where the imbedded plastral bones touch the ground. Thus what is crammed into the short life of a captive individual, is in the natural course of events spread over many generations, whereby it has ceased to be pathological, and has become a comparatively new, tertiary, but regular feature.
It is not open to much doubt that the characteristic features of the Trionychoidea are not primitive but secondary. This is indicated by the whole structure and behaviour of the carapace and plastron. The softening of the whole shell, the loss of the horny shields, the reduction of the claws, are the direct and almost unavoidable results of life in muddy waters.
Geologically they do not seem to be very old. They appear, already referable to the genus Trionyx, in the Upper Cretaceous strata of North America. In the Lower and Middle Tertiary strata many species existed in North America and in Europe, and it is of great importance that in these species the costal plates were much broader, and the marginal plates better developed, than in the recent forms. Now their half-dozen genera, with about twenty-four species, are confined to North America, the tropical and warmer parts of Asia, and the Malay {407}Islands, and to Africa from the Nile to the Senegal and to the Congo.
The habits of Trionychoidea have found few observers. According to L. Agassiz,[138] they live in the muddy bottom of shallow waters, burying themselves in the soft mud, with only the head, or a small part of it, exposed. They breathe without moving the body, by raising up the long neck and carrying the leathery snout above water. When moving through the water they strike horizontally with both pairs of limbs, alternating, however, the right and left; but when they start suddenly, the front limbs are seen moving together towards the tip of the snout, and then striking simultaneously backward with great power. As the shield does not project forward, the fore-limbs usually move beyond the shield, and as its outer edge is sharp, and the feet are broad, their webs reach above as well as below the plane of that edge, so that the water is driven partly over and partly under it. When they move along the bottom, the limbs still move horizontally, the webs striking against the water, and the inner toes, those with the claws, against the bottom. They also bury themselves horizontally, becoming covered by only a thin layer of mud. They readily resort to the shell for protection. The neck and head are withdrawn entirely, the loose skin rolling off from the greater part of the neck; and the skin of the legs also slips off, as far as the elbows and knees. In confinement they exhibit great quickness; their movements are abrupt and unsteady, except when they swim rapidly in one direction. They then dart their long and slender neck quickly forwards or sideways and upwards, as snakes do, and bite in the same way, striking suddenly. Their temper is bad or even ferocious, and large specimens are quite dangerous.
Their food consists of all sorts of aquatic animals, fish, frogs, and molluscs, for instance Anodonta and Paludina. According to the different diet, many species develop a peculiar kind of dimorphism, a reasonable explanation of which has been given by Boulenger. In the young the horny coverings of the jaws are sharp, with cutting edges, and in those specimens which keep to a diet of fish and other soft creatures, the jaws remain in the same condition. But in those which take to living upon molluscs, the hard shells of which they have to crush, the horny edges are {408}worn down; and broad, thick, horny, crushing pads are developed in their stead, the supporting parts of the jaws becoming more massive. The masticatory muscles are likewise enlarged, and a tubercle grows upon the lower border of the jugal bone, whence arises part of the masseter muscle.
The eggs are round, thick-shelled, but very brittle; they are laid in the sand above the level of the water, and this is the chief occasion on which these tortoises creep on land.
Trionyx.–The plastron has no special cutaneous valves for the concealment of the hind-limbs. This is the principal genus, with the greatest number of species and the widest distribution, the latter coinciding with that of the whole family. The upper surface of the shell of young specimens frequently forms numerous longitudinal ridges or series of little horny tubercles which disappear with age.
T. ferox, the commonest "Soft-shelled Turtle" of the United States. Olive above with scattered, small, round, black spots; young with conical, spine-like tubercles, especially on the nuchal border and on the posterior portion of the shell, which has a pale, black-edged border. A light, black-edged streak passes through the eye and joins its fellow on the snout. The limbs are olive brown, spotted and marbled with black. The under parts of the shell are white. Very large specimens have a shell 18 inches in length and 16 inches wide. Holbrook gives the following account of its habits:–
"A voracious, carnivorous creature. They reside most constantly in the water, swim with rapidity, and choose for their retreat holes under the banks of rivers, or under rocks; and not unfrequently the trunk of some huge forest tree, fallen into the stream, affords them shelter. Sometimes they leave the water and conceal themselves in the mud: I have frequently seen them thus buried to the depth of 2 or 3 inches, leaving only a small breathing hole for the long neck and narrow head, which is occasionally thrust out, but most commonly it is retracted so that one would pass near without observing their habitation; and if seen, it might easily be mistaken for the residence of some large insect. At other times they may be seen in numbers on rocks in shallow water, basking in the sun, apparently asleep. They bite severely when provoked, darting forward with great velocity the long neck and head, and not unfrequently spring upward at the same time and make a loud hiss.
In the month of May the females seek sandy places along the banks of the waters they inhabit to lay their eggs, generally about sixty in number; and it is remarkable that, though their motions are slow and difficult on dry land, yet at this season they sometimes mount hillocks several feet high. The flesh affords the most delicate food, surpassing that even of the Green Turtle. The geographical distribution is interesting. It inhabits the Savannah as well as all those rivers that empty into the northern borders of the Gulf of Mexico; it ascends up the broad Mississippi, and is found in all its tributaries, even to the very foot of the Rocky Mountains; it abounds in the chain of great northern lakes both above and below the Falls of Niagara, and is common in the Mohawk, a tributary of the {410}Hudson river; but it is not found in any other Atlantic stream between that and the Savannah river, a distance of nearly 800 miles."
T. triunguis, the only African species, ranging from the Senegal and Congo into the Nile-system, but occurring also in Syria, is perhaps the largest of all Trionychidae, reaching a shell-length of almost 3 feet. The adults are olive-brownish above, the throat and under parts of the shell with round, white spots separated by a dark network. The young have whitish specks and spots.
T. gangeticus and T. hurum are the principal Indian species. The former is the larger of the two, with a shell of more than 2 feet in length; olive above, the young with fine black vermiculations; head with a black longitudinal streak from between the eyes to the nape, intersected by two or three chevron-shaped black streaks; under parts yellowish. T. hurum is olive brown above {411}and below, in younger specimens with conspicuous, large, yellow spots on the sides of the head. The young are ornamented with two or three pairs of large round spots on the back, and the same applies to the beautiful young of the Burmese, T. formosa.
The three genera, Cycloderma and Cyclanorbis of Tropical Africa, and Emyda of India, have a pair of cutaneous femoral valves or flaps on the plastron, beneath which the hind-limbs are withdrawn.
DINOSAURIA–CROCODILIA
Sub-Class V.–DINOSAURIA.
Mesozoic, long-tailed, toothed reptiles, with distal ischiadic symphysis, terrestrial limbs, large fixed quadrate bones and bifurcated ribs.
The Dinosaurs begin and end with the Mesozoic epoch, and have a world-wide distribution. The name, "terrible Reptiles," refers to the gigantic proportions which many of them attained, not a few of them surpassing in size and shape the fantastic pictures of the dragons of our fables. Although these creatures came to an end millions of years before the first man-like beings appeared, it is reasonable to suppose that the widely-spread myths of dragons are based upon the accidentally disclosed skeletons of these monsters.
The skull is built after a plan which may be derived from a combination of the Crocodilian and Rhynchocephalian skulls, but the detail varies considerably in the many and much diversified members of this large sub-class. There is as a rule a pre-orbital foramen, which is smallest in the Ornithopoda. The orbit is completely encircled by bones, and the temporal fossa is divided by a squamoso-postfrontal or post-orbital bridge into a smaller supra-, and a much wider infra-temporal portion, the latter being bordered below by the jugal and quadrato-jugal, and this is firmly connected with the quadrate by an ascending process. The quadrate is long, more or less vertical in position, slanting either forwards or backwards, and firmly fixed above by the squamosal, perhaps also by a supra-temporal bone. The orbit is bordered by the jugal, lacrymal, pre- and post-frontals. The interparietal foramen seems to be {413}abolished. Teeth, mostly alveolar and laterally compressed, are restricted to the dentary, maxillary, and premaxillary bones. In the Orthopoda the latter carry no teeth, or these are restricted to the lateral portion, leaving a wide diastema. This toothless part plays upon a peculiar crescent-shaped bone, the so-called predentary, which rests loosely upon the anterior ends of the mandibular rami, which latter do not as a rule form an osseous symphysis. The Ceratopsia possess in addition a similar upper toothless piece, the prerostral, a kind of pre-premaxilla. The morphological value of these extra pieces is quite obscure; they were in all probability provided with thick, horny pads. The bones of the roof of the mouth recall in their arrangement that prevailing in the Rhynchocephalia and the Parasuchia. There are two pairs of large vacuities; one between the maxillae, ectopterygoids and palatines; the other between the latter, the maxillae and the usually small or slender vomers. The pterygoids are perhaps the largest bones, and form a rather long symphysis; laterally and behind they abut against the quadrate, anteriorly against the ectopterygoids and the palatines, which latter they sometimes separate. A peculiar feature of some skulls, e.g. Ceratosaurus and Triceratops is the great size of the groove in which the large hypophysis of the brain is lodged.
The vertebrae are very variable, amphicoelous, opisthocoelous, nearly plain, with a slight concavity behind, or occasionally procoelous in the anterior region of the tail. Besides the usual pre- and post-zygapophyses many Sauropoda and Theropoda possess on the posterior trunk-vertebrae additional joints, effected by a vertical wedge, the hyposphene, which extends backwards from between the post-zygapophyses and fits into a notch between and below the anterior zygapophyses of the next following vertebra. These additional articulations are analogous to the zygosphenes and zygantra of snakes and iguanas, except that in these Sauria the wedges are formed on the opposite, namely the anterior ends of the vertebrae. The vertebrae of the neck and trunk are devoid of intercentra, but those of the tail carry long chevron-bones. The number of sacral vertebrae is generally increased to four or five. The ribs have well-developed capitula and tubercula, and the former have the tendency to shift from the centra or from their parapophysial processes on to the usually much elongated diapophyses of the neural arches. This {414}arrangement, recalling the Crocodilian condition, results in an increased capacity of the dorsal portion of the body-cavity. Intervertebral articulation of the ribs does not occur except sometimes in the sacral region. Abdominal ribs are rare, but they occur in some of the Theropoda, e.g. in Compsognathus.
The sternum seems to have been mainly cartilaginous, with a pair of irregular, disc-shaped ossifications. How the coracoids were attached is unknown; they are small, generally with a foramen, but the scapulae are always very strong and slant backwards. Clavicles and interclavicles seem to be absent.
The fore-limbs are as a rule powerful, although often much shorter than the hind-limbs, which are then enormously developed, and in many genera of two of the main groups show a tendency towards a semi-erect gait. Some of the Dinosaurs, e.g. Iguanodon and Brontozoum, were absolutely bipedal. Others seem to have hopped like Kangaroos. In correlation with this more or less erect mode of progression the iliac bones are very strong, much elongated horizontally, and attached to more than three, often to five or even more, vertebrae. The pubic bones show two main types. Each consists either of a single strong shaft, which is connected distally with its fellow; or (Orthopoda) this main shaft sends out, below its point of contact with the ischium, a long process, the so-called post-pubis, which is directed downwards and backwards. In the latter case it runs parallel and in close contact with the ischium. Such bifurcated pubic bones never meet in the middle line. The ischia, on the other hand, are always connected with each other, not so much by fusion as by syndesmosis.
The hind-limbs exhibit all stages from a simple, plantigrade and five-toed state to a decidedly digitigrade, four, and even three-toed arrangement. Many genera exhibit the tendency to form an intertarsal joint, a feature elsewhere known in birds only, where it is typical and universal. The astragalus sends up an ascending process which tends to fuse with the anterior aspect of the distal end of the tibia, and the calcaneum is sometimes more or less firmly attached to the fibula. In Compsognathus even the distal tarsalia have begun to fuse with the metatarsalia, so that this reptile at least has a typical intertarsal joint. The femur is remarkable for the frequent possession of a "fourth" trochanter on the middle of the inner aspect of the shaft, undoubtedly {415}for the insertion of the long caudi-femoral or long adductor muscle.
Many Dinosaurs possess hollow instead of solid bones. The vertebrae have large cavities in the Sauropoda, notably in Brontosaurus; in many Theropoda, e.g. Coelurus, Anchisaurus, Compsognathus, the limb-bones and the vertebrae are hollow, the latter being reduced to thin-walled shells with a few inner partitions, the bones being at the same time much swollen and enlarged. In the Ornithopoda the vertebrae are solid, but the limb-bones are hollow. The reason of this hollowing out is not easily found. Undoubtedly it results in a saving of material and weight, whilst at the same time, without loss of strength, the surfaces for the attachment of the necessarily powerful muscles are increased. But Compsognathus is a small, Brontozoum a gigantic, creature. On the other hand, the bones of the huge Stegosauri are solid. Most probably these cavities were, as in birds, filled with air-sacs ultimately in communication with the lungs; and it is by no means a baseless suggestion of Haeckel's that the Dinosaurs were warm-blooded. Their mode of propagation can only be guessed at from the circumstance that a rather well-preserved specimen of Compsognathus contains in its abdomen what may possibly be an embryo. There is nothing against the assumption that the Dinosaurs were viviparous; on the contrary, it seems more natural than that, for instance, an Atlantosaurus of more than 100 feet in length and many tons in weight, should have laid eggs.
Some of the herbivorous Dinosaurs, namely, the Stegosauri and the Ceratopsia, had a dermal armour of variable extent; the plates were loosely imbedded in the skin, and reached their greatest size along the middle of the back and tail, and these crested plates were probably covered with horny scutes, obviously weapons of defence. The Ceratopsia were armed with a pair of huge pointed horns on the head, and a smaller one on the nose (see Fig. 102, p. 430). It is difficult to guess the use of the weapons of these terrestrial monsters, unless they were employed against the equally large carnivorous Dinosaurs or in the combats for the possession of their charming mates.
About the ancestry of the Dinosaurs we know nothing except that their affinities lie with the Crocodilia; but it is impossible to derive either from the other. The oldest forms, in the {416}present state of our knowledge–those which have left their three-toed spoors in the Trias of Connecticut–were already much specialised by having attained to an upright bipedal gait, while the Sauropoda, which except for their gigantic size are the most generalised, are of comparatively recent date, none of them being known from strata older than the Upper Jurassic. Twenty years ago, until the discoveries of numerous kinds in the United States, our knowledge of the whole group was very limited. There is a widely spread notion that the birds have sprung from some Dinosaurian stock. Huxley was the first to show clearly that birds were an offshoot of the reptiles, and he said of the Dinosaurs, especially his Ornithoscelida (Iguanodon, Scelidosaurus, Megalosaurus, Compsognathus, and others), that they "present a large series of modifications intermediate in structure between existing reptiles and Aves." Baur proved to his own satisfaction that we have to look for the ancestors of the Ratitae among the herbivorous Dinosaurs, especially the Ornithopodous forms, whilst the Carinatae are descendants of the Ratitae. However, even he had to give up this absolutely unwarrantable view.
It is easy to select a considerable number of characters amongst the various Dinosaurs which also occur in birds, and some of these have until a recent date been considered as peculiar to birds. For instance, the double, bifurcated pubic bones of the Orthopoda; the increased number of vertebrae to which the horizontally elongated ilia are attached, especially in the forms with an upright gait, and the bipedal feature itself; the possession of an ascending process of the astragalus and its fusion with the tibia in Compsognathus and Ceratosaurus among the Theropoda, and in Ornithomimus; the attachment of the distal tarsalia to the metatarsalia, e.g. in Compsognathus,–in fact, the formation of an intertarsal joint, a feature otherwise characteristic of, and peculiar to, birds; the frequent reduction of the fifth metatarsal bone; the backward position of the hallux and the proximal reduction of its metatarsal in Compsognathus; the elongation and partial fusion of the functional metatarsals in the latter genus and in Ceratosaurus; the regular increase of the phalangeal numbers of the first four toes from two to five in many of the Ornithopoda;–in short, the great resemblance between the feet of some of the Dinosaurs and those of the birds. However striking these arguments are, they are instances of {417}convergent analogies. The upright walk, which has been assumed and improved upon independently by members of both Theropoda and Orthopoda, has produced the same, or nearly the same modifications in them as in the birds.
It is easy to show that these features are mere coincidences. The oldest bird known is Archaeopteryx from the Upper Oolite of Bavaria. Consequently all those Dinosaurs, which are of the same and of later date, have to be excluded from the supposed ancestry, and they happen to be those in which (as in Ceratosaurus, Compsognathus, Ornithomimus, Iguanodon) the resemblances are greatest. There remains only Anchisaurus of the Upper Trias, more or less contemporary with the Brontozoum, which left its three-toed footprints (Archaeopteryx has four well-developed toes) with Zanclodon. Moreover, the most bird-like foot is either that of the Theropoda, which, like Anchisaurus and Zanclodon, differ from birds by the formation of the pelvis, or of some of the latest Ornithopoda. What, then, is the good of selecting a number of bird-like features from members of Dinosaurs which we are bound to class in different groups, and which existed, some in the lower, others in the middle, or even in the latest Mesozoic periods?
Lastly, the advocates of the Dinosaurian ancestry of birds cannot have fully appreciated the enormous differences between the wing of Archaeopteryx and the fore-limb of any Dinosaur with the most avian resemblances in the hind-limbs. The fore-limbs of these reptiles are modified in a direction diametrically opposed to that from which a bird-like wing could be developed. The skull presents another difficulty,and here again Compsognathus, a contemporary of Archaeopteryx, comes perhaps nearest to that of a generalised bird's skull. The ancestors of the birds must have combined the following characters:–Of not later than Mid-Oolitic age, with bifurcated pubic bones, four functional toes, elongated metatarsals, complete clavicles, premaxillary teeth, and free, not firmly fixed quadrate bones. But such creatures are not Dinosaurs.
We divide the enormous number of Dinosaurs according to the formation of the pelvis, that of the hind-limbs, and the dentition, into four orders.
Order I. SAUROPODA.
Pubes simple, with symphysis. Premaxillae with teeth. Plantigrade.
The teeth are mostly spatulate, laterally compressed, with sharp edges, but without serrations. Skull with a pair of large pre-orbital fossae. The centra of the vertebrae of the trunk have large lateral cavities. The fore- and hind-limbs are pentadactyle, plantigrade, and hoofed, of the typical walking type; the bones of the limbs are stout and solid; the femur is devoid of an inner distal or fourth trochanter. The carpal and tarsal bones are free. Herbivorous. The Sauropoda comprise some of the most gigantic terrestrial creatures which have ever existed, compared with some of which the bulk of an elephant appears almost insignificant. Their range in time extends from the Lower Oolite into the Cretaceous, with a perhaps world-wide distribution, namely, Western Europe, North America, Patagonia, Madagascar, and India. Although they are, except for their size, the least specialised of all Dinosaurs, none of the Sauropoda hitherto discovered are old enough to claim to be the ancestors of the other Dinosaurs.
Brontosaurus excelsus of the Upper Jurassic of Wyoming was a giant at least 60 feet long and about 10 feet high. The head is extremely small in proportion, not so broad as the fourth of the thirteen vertebrae of the long and flexible neck. The trunk is comparatively short, the tail longer than the neck, and provided with numerous chevron-bones. Most of the vertebrae are hollow, especially the five co-ossified sacrals. The spinal canal of the sacral region is very wide, indicating a strong sacral swelling in conformity with the huge posterior limbs. The pubic bones are stronger than the ischia. The long axis of the {419}former stands almost vertically like that of elephants, and the knee is scarcely bent in the erect position. The shoulder-girdle consists of long scapulae, broad at the base and small, almost square and perforated coracoids, which latter fit into a pair of partly ossified plates representing the sternum.
Atlantosaurus immanis of the Upper Jurassic of Wyoming and Colorado, is supposed to have been 115 feet long, perhaps the biggest and bulkiest of all animals, the femur measuring more than 6 feet in length and 2 in width at the upper end.
Morosaurus grandis, of the Upper Jurassic of Wyoming, with allied forms in the Purbeck and Wealden of England, reached a length of 30 feet; in general appearance resembling Brontosaurus, but the sacrum consists of four vertebrae only, and the ischia are bent backwards in their distal halves, so that their symphysis is formed by the shafts instead of by their ends.
Ornithopsis and Cetiosaurus, likewise huge creatures, from the English Wealden and from the Great Oolite respectively, are rather imperfectly known, although several species of each, under many generic synonyms, have been described.
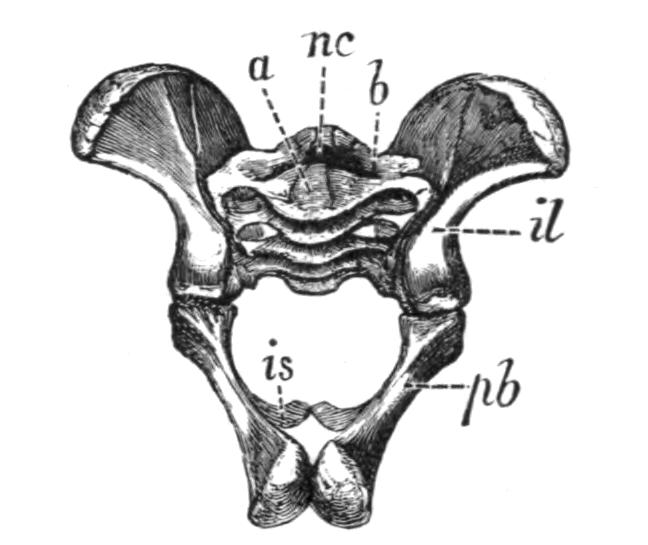
Fig. 96.–Front view of the pelvis of Morosaurus grandis. × 1⁄30. (After Marsh.) a, First sacral vertebra; b, "transverse process" (rib) of first sacral; il, ilium; is, ischium; nc, neural canal; pb, pubis.
Diplodocus longus, of the Upper Jurassic of Colorado and Wyoming, is almost completely known. More than 40 feet long, it had a head in its general outlines not unlike that of a horse, the skull being about two feet long. The outer nasal openings are confluent, elongated, and lie far back on the top of the skull. There is a pair of large antorbital, and a pair of smaller lacrymal fossae. The teeth, long and slender, are restricted to the anterior portion of the mouth, with many successors, which, decreasing in size, lie on the inner or lingual side of the functional tooth, like the cartridges in a repeating rifle. The {420}functional teeth themselves are implanted in sockets. The generic name refers to the peculiar chevron-bones, each half of which diverges into an anterior and a posterior branch.
It is difficult to understand how these huge, long-necked Sauropoda lived and moved about. The long neck suggests at first sight predacious habits, but the teeth, rather feeble in Diplodocus, and distinctly of the plant-cutting type in other genera, put this out of the question. The high position of the unpaired nasal opening, and the shortened nasal bones of Diplodocus, are features indicative of aquatic habits, but the short-toed, plantigrade limbs are absolutely adapted to terrestrial life, and we cannot well assume that such enormous brutes as Atlantosaurus could possibly have ventured into swampy ground.
Order II. THEROPODA.
Pubes simple, with symphysis. Premaxillae with teeth. Digitigrade. Carnivorous.
The teeth are pointed, recurved, laterally compressed and serrated. The nasal openings are large, lateral, and nearly terminal. The vertebrae and the large bones of the limbs are hollow. The fore-limbs are considerably shorter than the hind-limbs, which are distinctly digitigrade, many of the species having a pronouncedly upright gait. The proximal tarsalia show a tendency to fuse with the tibia, and the astragalus has sometimes an ascending process, by which the fusion with the tibia is strengthened. The first and fifth metatarsals are often reduced, while the three middle bones are elongated and sometimes even fused with each other, so that the whole foot assumes a striking resemblance to that of birds. The terminal phalanges are protected by curved claws. Owing to the shortness of the fore-limbs, and the often considerable length of the hind-limbs, which are strongly bent at the knee and the ankle-joint, these animals must have progressed somewhat like clumsy kangaroos.
The Theropoda, of which a great number of genera are now known, from the size of a slender cat to that of an elephant, lived from the Upper Trias to the Upper Oolite, both in Europe and in North America.
Brontozoum giganteum, one of the oldest forms, is known {421}from its foot-spoors only, which, together with other three-toed spoors in the sandstone of the Connecticut valley, were originally described and figured by Hitchcock as Ornithichnites (ἴχνος = track, or spoor). Some of these imprints are more than a foot in length, the right and left spoors following alternately at a distance of from four to six feet. In some cases the long trailing tail has left a furrow behind, and the large tracks are accompanied or crossed by much smaller, and even by quite tiny tracks, otherwise similar, and undoubtedly made by the young.
Anchisaurus, from the same locality, was still Sauropodous, in so far as the metatarsals are still free, with two, three, four, and five phalanges on the first four toes, but the fifth metatarsal is reduced, carrying a vestige of only one phalanx, and the proximal tarsal bones are fused with the tibia and fibula respectively. Total length some seven feet, of which about four belong to the tail.
Zanclodon, from the Keuper of Würtemberg, about ten feet long, with pentadactyle hands and feet. Ischia stronger than the pubic bones, which are distally much broadened. The femur is nearly three feet long, and possesses a fourth trochanter. The astragalus has an ascending process, and is fused with the tibia. The toes are short, strong, and clawed. The shoulder-girdle and fore-limb are strong, the latter well adapted to grasping. The teeth are much compressed laterally, with sharp, finely serrated edges. Several allied genera have been described from the Upper Trias of France and England: others from corresponding strata of India and South Africa.
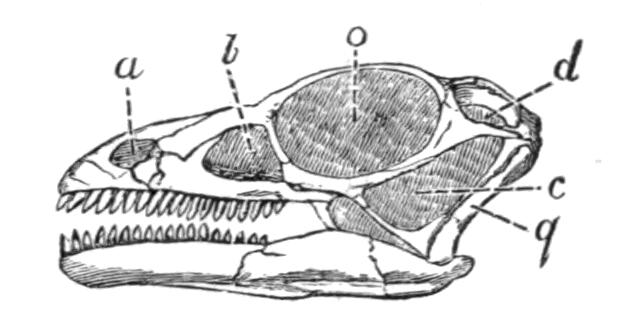
Fig. 98.–Skull of Anchisaurus coelurus. × ¼. (After Marsh.) a, Nasal fossa; b, antorbital, c, infra-temporal, d, supra-temporal, and o, orbital fossa; q, quadrate bone.
Megalosaurus, from the Trias to the Wealden in England and France, with other species in Colorado and India, reached a considerable size, larger than that of any other Theropoda, the scapula of M. bucklandi being nearly three feet long, and the femur still longer. The hind-limbs are twice as long as the fore-limbs. The cervical vertebrae are short, the neck being much shorter than the tail. Hands with five fingers, feet with four toes. Pubic bones long and slender, with a broad symphysis. With well-developed abdominal ribs, resembling those of crocodiles.
Allosaurus, from the Upper Jurassic of North America, with only three toes. Ischia and pubes united into one symphysis. Anterior extremities very short. Sacrum consisting of four vertebrae. Total length of some of the larger species about twenty feet.
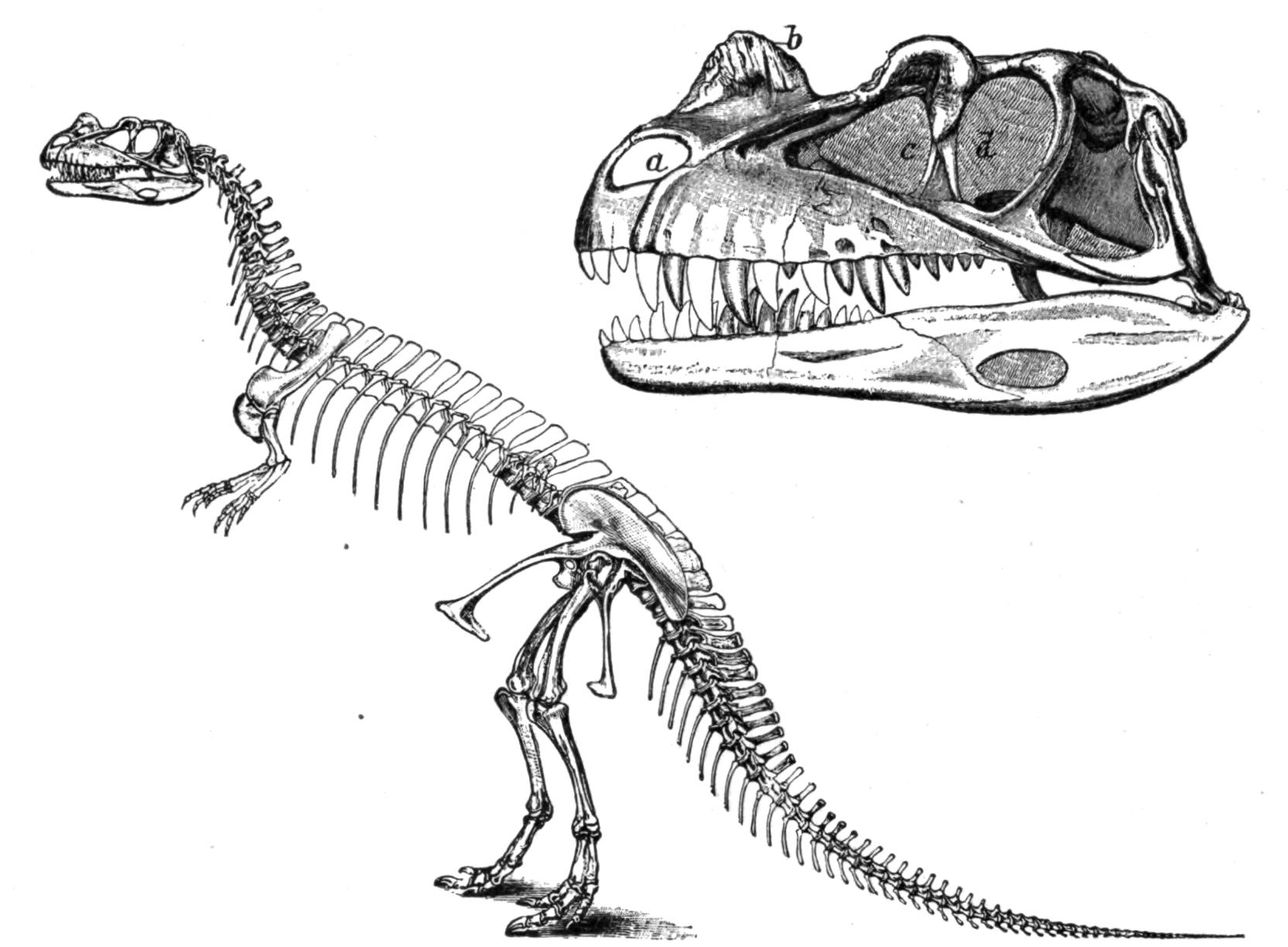
Fig. 99.–Skeleton (× 1⁄40) and skull of Ceratosaurus nasicornis. (After Marsh.) a, Nasal cavity; b, bony horn-supporting excrescence; c, pre-orbital fossa; d, orbital fossa.
Ceratosaurus nasicornis, from the Upper Jurassic of Colorado, is about seventeen feet long. The generic and specific names refer to the nasal bones, which are raised into an unpaired longitudinal crest. This, by its rough surface, suggests that it was covered by a horny sheath, or carried a horn. The large skull, about two feet in length, is armed with strong, slightly curved, laterally compressed, sharp teeth, unequal in size. The pre-orbital foramen is large, bordered above by the prefrontals, which are raised into prominent knobs. The supratemporal foramina are extremely small, the lateral foramina very large. The quadrate slants backwards. The sacrum consists of five vertebrae. The caudal vertebrae carry long and slender chevron-bones. The pubes and ischia are long and slender, each forming a separate symphysis at their broadened ends. The three {423}metatarsals are elongated and fused with each other. There seems to have been some dermal armour in the shape of osseous plates, which extended in one series from the occiput over the neck.
Coelurus gracilis, of the Upper Jurassic of Wyoming, and closely allied forms in the Wealden of England, are remarkable for the pneumaticity of the centra and processes of their vertebrae, the bony parts of which are restricted to thin, hollowed-out shells, so that the whole skeleton must have been very light. Computed length of these imperfectly preserved creatures about five feet.
Hallopus victor, of the Upper Jurassic of Colorado. Anterior extremities very short, with only four fingers; posterior limbs very long and slender, especially the tibia; the much elongated metatarsals are separate, the first absent, the fifth much reduced, so that the foot is tridactyle; the calcaneum projects like a heel. The ilium is attached to two sacral vertebrae only; the pubes are slender, forming a narrow symphysis, while that of the ischia is broad. Most of the bones of this creature, which probably progressed by hops, are hollow. Total length about three feet, the length of the hind-limbs being about nine inches.
Compsognathus longipes, of the Upper Jurassic of Bavaria, is one of the smallest of all the Dinosaurs. It is most remarkable on account of its almost bird-like feet. The fibula is much thinner and somewhat shorter than the tibia; the latter is closely attached to, although not fused with the proximal tarsal bones, while the distal tarsals are fused with the united and much elongated second, third, and fourth metatarsals; the fifth is reduced to a short bone near the intertarsal joint; while the first is represented by its distal portion only, which is stowed away on the hinder aspect of the middle of the second metatarsal, and carries two phalanges. The three middle toes consist of three, four, and four phalanges respectively. Whilst the whole hind-limb is typically avian, the pelvis is quite different; the pubic bones are simple, slender, and directed forwards, forming a symphysis with their whole distal halves, and broadening out distally into a horizontal process directed towards the symphysis, which is likewise formed by the fusion of the inner surfaces of the thin and rather flat ischia. The fore-limbs are only half the size of the hind-limbs. The neck consists of about ten vertebrae, mostly with long and {424}pointed ribs. Tail long with well-developed chevrons. The skull is long and pointed, composed of thin bones, which have lost most of the sutures; with large lateral, temporal, and pre-orbital, but without supratemporal, foramina. Premaxillae, maxillae, and mandible with numerous slender and rather long, conical, alveolar teeth.
Order III. ORTHOPODA
Each pubic bone consists of an anterior or pre-pubic and a posterior or post-pubic branch, neither of which forms a symphysis. Premaxillae without teeth. With a premandibular predentary piece. Herbivorous.
The so-called pre-pubis is homologous with the pubis of most recent reptiles, and with the pectineal process of birds, while the "post-pubis" is homologous with the processus lateralis of Chelonians and Saurians, and with the "pubis" of birds. The right and left halves of the pubis remain widely asunder ventrally. In many cases the post-pubis, always directed obliquely backwards, lies closely against the shaft of the ischium, which always forms a distal syndesmosis, or a symphysis, with its fellow. The fore-limbs are usually very short, provided with five or four short and strong fingers. The hind-limbs are long and strong, mostly with three, sometimes with four functional short toes, either plantigrade (Stegosauri) or digitigrade (Ornithopoda). Femur with an inner distal, or fourth, trochanter. The dentition is of the herbivorous type, restricted to the dentaries of the mandible and to the maxillary bones, leaving the whole or the greater part of the premaxillaries free. The additional "predentary" piece of the mandible is possibly a calcified, but originally horny, pad. The teeth are greatly compressed laterally, and finely serrated, but are much ground down by use; several rows of successional teeth lie on the inner or lingual side. The skull is strongly built, with large anterior nasal openings; pre-orbital foramina very small or absent; orbits completely encircled by bones; supratemporal foramina small, lateral foramina large. Quadrate large, vertical or slanting slightly forwards. The vertebrae are solid, not hollow; sacrum consisting of four, five, or more vertebrae; ribs bifurcated, the capitula carried either by the centra, or moved up to the diapophyses of the neural arches; chevron-bones {425}numerous, and frequently long, especially on the anterior half of the long and heavy tail.
Orthopoda occur from the Lias to the Upper Cretaceous, both in Europe and in North America. The name Orthopoda, invented by Cope in 1866, is appropriate for obvious reasons; it comprises the Stegosauri and Ornithopoda of Marsh (1881). The latter term is not very fortunately chosen, considering that the whole hind-limb of the Theropodous Compsognathus is far more ornithic than that of any three-toed Ornithopoda, in which the tarsalia rarely fuse with the tibia and never with the metatarsals. To apply the term Ornithopoda to the whole order is quite unjustifiable, unless it is meant to apply to the strikingly bird-like configuration of the pelvis.
Sub-Order 1. Stegosauri.–The fore- and hind-feet are plantigrade, or nearly so, the metapodials being but little elongated, with more than three functional digits. The bones of the limbs are solid. The ribs of the trunk are bifurcated, and are carried by the diapophyses of the neural arches. The body, especially the back, is protected by dermal bony plates, which are not connected with the internal skeleton.
Scelidosaurus harrisoni. One nearly complete skeleton, about 11 feet in length, from the Lias of Lyme Regis. About twenty-four pre-sacral vertebrae, of which six or seven belong to the neck, four sacral and about forty caudal vertebrae. Four fingers, four toes, with 2, 3, 4, 5 phalanges, the fifth metapodials being quite vestigial; the hallux and pollex are very short, so that the foot at least is functionally tridactyle. The tarsal bones remain separate. The head is very small. Two rows of ridged bony plates extend from the neck over the back, and converge into one row upon the long tail; smaller plates, arranged in many rows, seem to have protected the sides and under parts. Hylaeosaurus and Polacanthus of the English Wealden are allied forms.
Stegosaurus, with several species from the Upper Jurassic of Colorado and Wyoming, and others, e.g. S. armatus (= Omosaurus), from the Kimmeridge Clay of Wiltshire in England. The head is relatively very small, and the brain is surpassed several times in thickness by the huge sacral swelling of the spinal cord. Teeth numerous and small. All the cervical and trunk-vertebrae carry bifurcated ribs, those of the trunk being carried entirely by the very high neural arches. The fore-limbs are only about half {426}the length of that of the hind-limbs, so that these creatures, which were undoubtedly quadrupedal, must have had a very peculiar gait, standing with the head, neck, and shoulders much lower than the arched back and pelvic region. The ulna has a strong olecranon; the hand has four functional fingers. The pre-acetabular portion of the ilium is much elongated; the pre-pubic branch stands horizontally, while the post-pubis is closely adpressed to the ischium. The astragalus is fused with the tibia, the calcaneum with the fibula. The foot has only three short toes, protected, like the fingers, by hoofs. The dorsal dermal armature consists of very high, crest-like plates. S. ungulatus of North America has a computed length of 28 feet, with the hind-limbs about 7 feet long. This creature was nearly 10 feet high, when measured from the ground to the tips of the dermal crests on the middle of the back. These bony, laterally compressed plates are themselves nearly 3 feet high, and are replaced, on the hinder portion of the tail, by several pairs of pointed spikes about 2 feet in length.
Sub-Order 2. Ornithopoda.–The hind-limbs are distinctly digitigrade, usually with only three functional toes, protected by claws. The long bones are hollow. Femur with a long fourth trochanter. Without dermal armour-plates.
Camptosaurus.–Several species, up to 10 feet in length, from {427}the Upper Jurassic and the Wealden of North America and England. Five fingers, with 2, 3, 3, 3, 2 phalanges and four toes, with 2, 3, 4, 5 phalanges, but the hallux is much shortened and does not touch the hard ground; astragalus and calcaneum separate.
Laosaurus of Colorado is a smaller form, intermediate in structure between the former genus and Hypsilophodon foxi from the Wealden of the Isle of Wight. A small creature, less than 5 feet in length. Four fingers, with 2, 3, 4, 2 phalanges; fifth metacarpal vestigial. Four toes with 2, 3, 4, 5 phalanges and long claws. Astragalus and calcaneum separate. Post-pubis very slender. Each premaxillary with five pointed alveolar teeth, leaving a wide median diastema; maxillaries with eleven, dentaries with ten laterally compressed blade-like teeth.
Iguanodon from the Wealden of England, Belgium, and Germany. Apparently two species, I. mantelli, about 16 feet, I. bernissartensis nearly 30 feet long. The premaxilla is quite toothless; the teeth of the maxillae and mandibles stand in close series, implanted in alveolae; they are spatulate, laterally compressed, with finely serrated edges, and slightly curved, the lower outwards, the upper inwards, and bear a general resemblance to those of Iguana, hence the generic name. There is only one functional set of teeth, and these are much worn down by use, but in such a way that, owing to the different curvature of the opposed teeth, the worn-down crowns form cutting, and at the same time crushing, almost triturating surfaces, indicating that these animals lived upon herbs. The gait of these creatures was upright, as shown by their spoors; the long almost vertical ischia, which form a padded symphysis, only slightly raised above the ground, suggest that this symphysis was used as a true sitting support, the animal resting upon it, the hind-limbs and the long tail. The latter, to judge from the long chevrons and the high neural spinous processes, must have been furnished with strong muscles. The whole tail was undoubtedly used as a balance during the upright position. Many of the tendons of the dorsal spinal muscles on the back and upper half of the tail are ossified. The post-pubic branches are very slender, distally much reduced, and, except at the obturator-foramen, separated from the ischia; the pre-pubes are very strong and broad. The femur has a fourth trochanter, a feature which {428}induced the unfortunate late Paul Albrecht to declare that Iguanodon was a reptilian Duck! The tarsal bones are separate. The metatarsals and toes are reduced to three, with 3, 4, 5 phalanges respectively, the first being a mere styliform vestige. The anterior limbs are likewise very powerful, but are much shorter; the hands are adapted for grasping, possibly for defence and offence, as indicated by the pollex, which, although short, is transformed into a formidable spur-like weapon, firmly fixed at a right angle to the other four fingers, the phalanges of which number 3, 3, 3, 4; the second and third fingers were protected by hoof-like nails, the fifth finger is feeble, and stands somewhat apart. The whole vertebral column consists of more than eighty vertebrae, of which ten are cervical, eighteen thoracic and lumbar, while five or six are fused into the sacrum. The cervical vertebrae are opisthocoelous, and carry short ribs, except the atlas, which possesses two separate supra-dorsal pieces, which fill the gap between it and the occiput.
Many specimens of I. bernissartensis, which is now completely known, including even the hyoid bones, were discovered in 1878, in the Belgian colliery of Bernissart, between Mons and Tournai, close to the French frontier. The bones were in a fault or crack, filled with clay of Wealden age, about one thousand feet below the present sea-level, and there about thirty Iguanodons, all {429}apparently adult, had become embedded. Five of them are now mounted in one of the public galleries of the Brussels Museum, of which these perfect monsters form one of the chief attractions. Having proved to be such a valuable find, they were claimed by the Government, on the ground that Iguanodons were not included in the license of the Coal Mining Company. The fact that not only I. bernissartensis, but also a few specimens of I. mantelli, already known from England, where the large form likewise occurs, were found in the same place, makes the specific differences somewhat doubtful; they are perhaps sexual.
Claosaurus of the uppermost Cretaceous strata of Wyoming, is one of the latest of Dinosaurs. It is nearly allied to Iguanodon, but has only three functional fingers, the fifth being absent, whilst the pollex is very short.
Hadrosaurus s. Diclonius of the same level as the preceding genus in North America, apparently also in the Middle and Upper Chalk of England and Belgium, has a most peculiar spoon-shaped bill, the premaxilla and the predental bone being spatulate and quite toothless. The teeth in the upper and lower jaws are numerous and small, and whilst one set of teeth is being ground down, the several successional series are already functional. H. mirabilis has in all about 2000 teeth; the total length of the skeleton is 38 feet, of which nearly 4 feet are taken up by the skull; in other respects this genus is allied to Iguanodon.
Ornithomimus, of the Upper Cretaceous of Colorado, is known only from its fore- and hind-limbs. The fore-limbs are short, with three fingers. The hind-limbs are very long and strikingly bird-like. The metatarsals, of which only the second, third, and fourth are developed, are much elongated; the proximal half of the third is pushed back between the second and fourth, and imperfectly fused with them, exactly as in young birds. The astragalus has a long ascending process, and is fused with the tibia. The fibula is very slender, distally much reduced; the calcaneum is represented by a tiny nodule; the terminal phalanges end in pointed claws. O. grandis must have reached a considerable size, to judge from its middle metatarsal, which is 60 cm. or 2 feet long. Until more is known of these extraordinary creatures, nothing definite can be said about their affinities. They may perhaps belong to the Theropoda.
Order IV. CERATOPSIA.
Pubic bones simple, forming a symphysis, post-pubic branches being absent. The mandible carries a toothless "pre-dental," and the fused premaxillaries carry a similar, toothless, "rostral" bone.
The teeth of the upper and lower jaws are alveolar, and have two roots. The fore-limbs are little shorter than the hind-limbs; pentadactyle and plantigrade, with broad hoofs. Femur without a fourth trochanter. Limb-bones solid. The skull is large, and remarkable for a pair of long frontal bony cores, which probably carried large, pointed horns; the parietal bones form a huge, horizontally broadened out crest, which extends backwards over the neck. Upon this cranial neck-shield follow small dermal bony plates. These miraculous creatures flourished during the Cretaceous epoch in Europe and in North America. Some, for instance, the American Triceratops flabellatus, reached a huge size, its skull alone measuring more than 5 feet in length, while that of T. prorsus is, including the neck-shield, about 7 feet long. The total length of this monster, the back of which stands about 8 feet high, is more than 20 feet. Other genera seem to have a well-developed dermal armour, e.g. Nodosaurus of the Middle Cretaceous period of Wyoming.
The Ceratopsia combine characters of the Sauropoda and of the Stegosaurian Orthopoda; in their pelvis they agree with the former, in the development of dermal armour and a predental bone they agree with the latter, while they differ from either by the possession of a rostral element.
Sub-Class VI.–CROCODILIA.
If we had to deal only with the recent Crocodilia the following would be an all sufficient diagnosis:–Four footed, long-tailed reptiles, with fixed quadrate bones, with teeth separately implanted in alveolae and restricted to the upper and lower jaws.
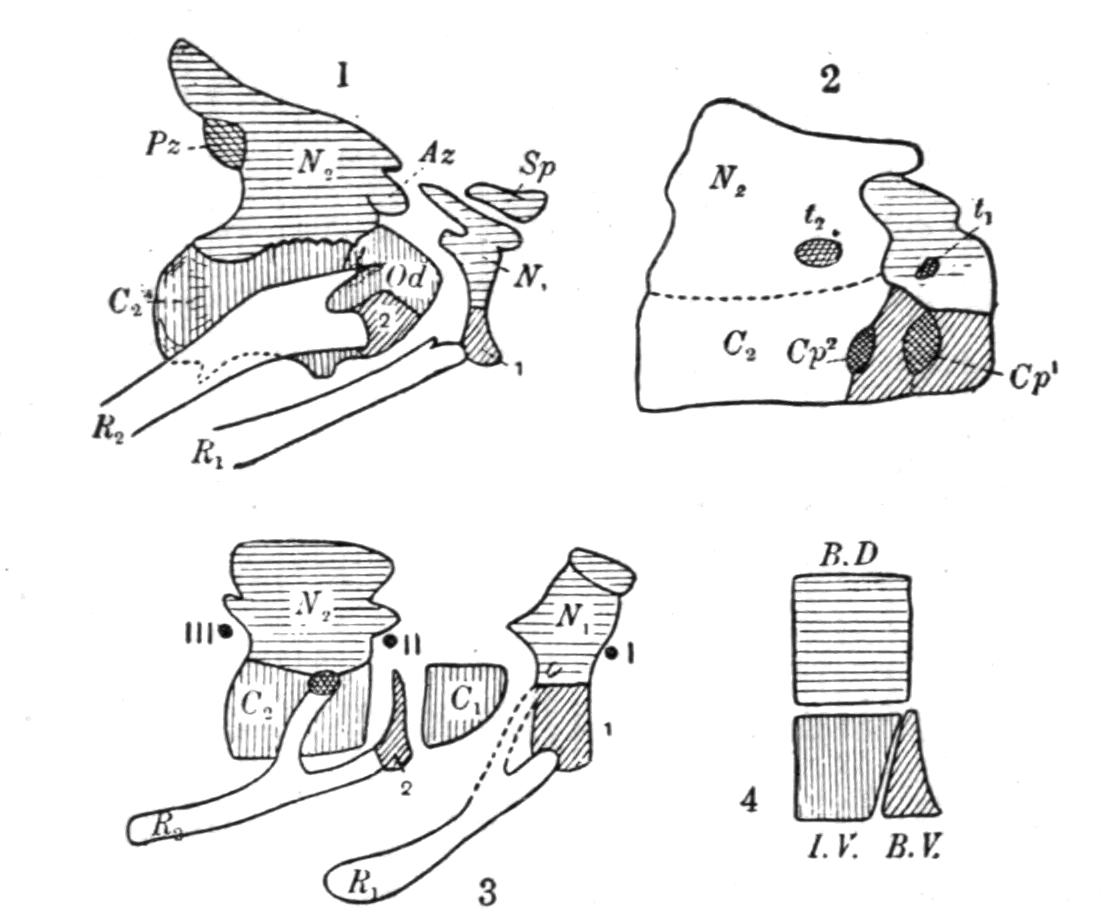
Fig. 103.–1, Atlas and axis of Crocodilus. 2, Atlas and axis of Metriorhynchus, a Jurassic Crocodile, see p. 439. 3, Analysis of the first two cervical vertebrae of a Crocodile. 4, Diagram of the fundamental composition of a Reptilian or other Amniotic, typically gastrocentrous vertebra. Az, Anterior zygapophysis; B.D, basidorsal; B.V, basiventral; C1, C2, first and second centra, formed by the interventralia; Cp1, Cp2, articular facets of the capitular portions of the first and second ribs; I.V, interventral; N1, N2, first and second neural arch, formed by the basidorsalia (B.D in 4); Od, odontoid process = first centrum; Pz, posterior zygapophysis; R1, R2, ribs; Sp, detached spinous process of the first neural arch; t1, t2, facets of the tubercular portions of the first and second ribs; 1, 2, intercentra = basiventralia; 2 (in 3), second basiventral "complex or intercentrum," continued upwards as a meniscus or intervertebral pad; I, II, III, position of the exit of the first, second, and third spinal nerves.
To define Crocodilia in general and to distinguish them from various extinct groups we have to resort to additional characters. The vertebrae are solid; the ribs of the neck and thorax possess a distinct capitulum and tuberculum; there is a series of loose, compound abdominal ribs; the humerus is devoid of an entepicondylar foramen; the iliac bones are broadened out and attached to two sacral vertebrae; the pubic bones are simple, not bifurcated, and neither they nor the ischia are ventrally united. The skull always has a strong, bony, quadrato-jugal arch. The possession of a longitudinal cloacal opening and of {432}an anterior or ventral single copulatory organ can of course be asserted of recent forms only.
In spite of these many characters common to all Crocodilia, it is very difficult to separate the latter from the Dinosauria, the only absolute difference lying in the ventral pelvic bones. It is therefore most suggestive that the fore-limbs of the Mesozoic Crocodilia are so much shorter and weaker than their hind-limbs, a discrepancy which is not lessened before the Tertiary epoch. The Mesozoic Crocodilia were almost entirely marine; the strongly-developed ankle-joint (indicated already by such early forms as Aetosaurus and Mystriosaurus) must have been inherited from some terrestrial group with digitigrade tendencies and shortened hind-limbs. All this points to some Theropodous Dinosaurian stock of which the Crocodilia may well form an aquatic, further-developed branch. Loss of the pubic and ischiadic ventral symphysis is not a serious modification. So far as modern reptiles are concerned only the Chelonia and Sphenodon are related to the Crocodilia, whilst Monitors and other lizards resemble them only superficially. We divide them into three Orders.
Order I. PSEUDOSUCHIA.
The few members of this peculiar group of reptiles are all restricted to the Keuper or variegated marls, although they seem to have had a wide distribution, some having been found in Germany, others in New Mexico. They perhaps form an early side-branch of the generalised Crocodilian stock, which died out with the Jurassic age.
The skull is distinctly short and pointed. The premaxillaries are very small and are dorsally separated from each other by the large nasals, which also keep the maxillae widely asunder. The nostrils are latero-terminal, bordered chiefly by the nasals, below by the premaxillae and part of the maxillae. The orbit is bordered below by the strong jugals, in front by the prefrontal, above by a supra-orbital and a small postfrontal, behind by a postorbital, which, firmly connected with the jugal and squamosal, shuts off a supratemporal foramen. There is also a lateral temporal fossa, and a large hole enclosed by the lacrymal and the maxillary bones. The teeth are restricted to the anterior {433}half of the jaws. The neck, back, and tail are covered by two rows of large and broad, closely-jointed bony plates; smaller plates protect the sides and the ventral surface. The vertebrae are still unknown.
Aëtosaurus ferratus of the Upper Keuper near Stuttgart is the best known. One of the greatest treasures of the Stuttgart Museum is a slab of sandstone, about 2 square yards in size, upon which lie huddled together twenty-four individuals of various sizes, the largest measuring 86 cm. or 2 feet 10 inches. They are in a beautiful state of preservation, and many of them are in the most life-like attitudes, just as if a mass of sand had fallen upon them and crushed them down, and as if they were struggling to get out.
Erpetosuchus and Ornithosuchus of the Elgin sandstone seem to be allied forms.
Order II. PARASUCHIA.
As the name implies, a collateral branch of the true Crocodilia. They are, like the Pseudosuchia, restricted to the Keuper formation. The vertebrae are mostly biconcave, sometimes with nearly plain, scarcely concave, central joints. The premaxillae are very long and powerful. The nostrils lie far back, rather near the orbits, on the top of the snout, within the anterior half of each nasal and almost above the choanae. The latter are situated in front of the palatine bones and are divided by a backwardly directed process of the vomer, which is plainly visible on the roof of the mouth. The palatines and pterygoids leave a wide median space between them. The pterygoids are narrow and have three processes, the antero-lateral of which joins the palatines and the maxillary bones (there being no separate ectopterygoid), the inner joins the basi-occipital, and the postero-lateral the quadrate.
The orbit is surrounded by the frontal, prefrontal, lacrymal, postorbital and postfrontal, while the strong jugal is excluded. The temporal region shows a lateral and a dorsal foramen; the latter opens backwards and above the occiput, being bordered in front by the parietal, laterally by the squamoso-occipital bridge.
The vertebrae are amphicoelous. The first and second {434}vertebrae are devoid of ribs; the cervicals and first thoracics carry separate capitular and tubercular processes for the attachment of the ribs, while the ribs of the rest of the trunk are carried entirely by the long diapophyses, as in the modern Crocodiles. The dermal armour consists of two rows of broad, dorsal, and several rows of smaller, lateral, bony plates.
Belodon is by far the best-known genus, with several species in South Germany and North America, some of which reached a length of 10 feet, without ventral armour. The closely allied Stagonolepis of the Elgin sandstone in Scotland had dorsal and ventral armour. Other genera in the Triassic formations of India and North America.
Order III. EUSUCHIA.
Crocodilia in the stricter sense. The premaxillae are short and always enclose the nostrils. The choanae lie behind the palatines, in recent forms even within the pterygoids. They occur from the Liassic or Lower Jurassic period to the present time.
The direct ancestors of the Eusuchia are still unknown. They cannot have been developed from the Pseudosuchia, nor do we know intermediate stages which connect them with the Parasuchia. The nostrils, situated within the premaxillaries, always lie in front of the nasals, although these sometimes extend forwards and form a bony internasal septum fusing with the usual cartilaginous septum. The choanae, instead of opening immediately behind the vomer, are carried far back, owing to the formation of a secondary bony palate. In the Jurassic Crocodiles this roof is formed by the meeting of the palatine bones in the medio-ventral line, and the choanae open immediately behind. From Cretaceous times onwards this roofing is continued by the pterygoids, which likewise form a median suture; and the united choanae (which may, or may not, be divided by a thin bony septum) are pushed towards the posterior end of the pterygoids. Since the Jurassic times there exists also a tendency to enclose the Eustachian passages (the remnants of the first gill-clefts) by bone. In the earlier members they were still wide slits or open grooves on the ventral side of the basi-occipital bone. Since the Cretaceous epoch they have been transformed into bony canals and open through one median hole, situated between the basi-occipital and the {435}basisphenoid, immediately behind the posterior symphysis of the dorsal portion of the pterygoids, which latter almost completely cover the basisphenoid. The vomer is not visible (except in Caiman niger), being covered by the ventral junction of the palatines and maxillaries. The broad, lateral wings of the pterygoids are connected by separate bones, the ectopterygoids = transpalatines = transverse bones, with the maxillaries, and in recent forms also with the jugals. Thus an extensive, very firm bony palate is produced; and the large palatal foramina, between the palatines, maxillaries, ectopterygoids and pterygoids, are closed by the same dense mucous membrane which cover the whole roof of the mouth.
The opisthotic and epi-otic bones fuse early with the lateral and with the supra-occipital bones; only the pro-otic remains longer as a separate element, perforated anteriorly by a large hole for the exit of the third branch of the trigeminal nerve. The basisphenoid is scarcely visible, being covered by the pterygoids. The presphenoid is large, continued forwards and upwards into the usually cartilaginous interorbital septum. Near the anterior and upper margin of the presphenoid is a large notch on either side for the passage of the optic nerve, the three eye-muscle nerves and the first branch of the trigeminal nerve. There are no separate orbito-sphenoids, their place being taken by membrane or cartilage in continuation with the interorbital septum, but the alisphenoids are large, abutting upwards against the frontals. Each prefrontal sends down a vertical process which joins the palatine of its side.
The configuration of the snout varies much. There are two parallel lines of development since the Jurassic epoch, namely, long-snouted creatures, of which two still survive as Gavialis and Tomistoma, and more broad and short-snouted members like the rest of the Crocodiles and Alligators. In opposition to the Parasuchia the elongation of the snout is effected by the maxillaries. The length of the nasals varies much, mostly in conformity with that of the maxillaries. As a rule they reach the premaxillaries but not always the nasal groove. In Gavialis they are short, far separated from the premaxillaries by the maxillaries, which meet in the dorso-median line. The orbit is bordered by the frontals, which at an early age fuse into an unpaired piece, and by the prefrontal, lacrymal, jugal, and postfrontal. {436}At a deeper level the orbit is partly divided from the lateral temporal fossa by a strong column which is formed by the meeting of a downward process of the postfrontal with an inner process of the jugal, and an ascending process of the ectopterygoid (cf. Fig. 108, p. 458). This arrangement adds considerably to the strength of the skull. The lateral temporal fossa is bordered in front by the column just described; below by the jugal and the quadrato-jugal, which is firmly wedged in between the jugal and quadrate; behind by the quadrate; above by the postfrontal, which forms a strong superficial bridge with the squamosal. This rests upon and often fuses with the quadrate and an intervening transverse wing-like extension of the lateral occipital bone. By this squamoso-postfrontal bridge part of the original temporal fossa is divided into the lateral one just described, and a dorsal fossa. The latter is bordered by the postfrontal, squamosal, and united parietals. This dorsal temporal fossa is consequently not homologous with that of the Parasuchia, a vestige of which is however present in many, especially in young skulls of Crocodiles, in the shape of a narrow passage which extends backwards from the dorsal fossa, bridged over by the junction of the parietal with the squamosal, and bordered below by the occipitals.
The size of the upper temporal fossae stands in an inverse ratio to that of the lateral fossae. In the older Eusuchia the upper were the larger of the two. The temporo-mandibular muscle which lifts or shuts the lower jaw arises from the walls of the upper fossa, passes beneath the jugal arch, and is inserted into the supra-angular portion of the lower jaw. In the more recent Crocodiles this muscle is more and more superseded by the pterygo-mandibular muscle, which, arising chiefly from the dorsal surface of the much broadened-out pterygoid bone, fills the widened space between the latter and the quadrate, and is inserted into the outer surface of the os angulare of the lower jaw. This muscle, owing to its general disposition, is capable of much more powerful development and leverage than the temporo-maxillary muscle, which latter, being more reduced, allows the dorsal fossae to be more and more closed up by the surrounding bones.
The fossae are still comparatively large in the long-snouted genera Gavialis and Tomistoma, which live entirely upon fish and scarcely chew their food, whilst these holes almost completely {437}disappear in some of the Alligators, namely in the broad- and short-snouted members, which, having a varied diet, taken from every available group of the animal kingdom, chew their prey.
The quadrate extends obliquely backwards, and is immovably wedged in and partly fused with the quadrato-jugal, the squamosal, and the lateral occipital wings. Between the latter and the quadrate remains a slit-like canal, well visible from behind, through which passes the continuation into the mandible of the columellar or ossicular chain of the auditory apparatus. Intricate passages, used as additional enlargements of the space of the middle ear, pervade the proximal portions of the quadrate and the roof of the cranium beneath the parietal bridges mentioned above, the two sides communicating with each other. The supra-occipital bone is visible from behind; its top is covered and partly fused with a continuation of the parietals, which are, like the frontals, fused into an unpaired mass. The occipital condyle is formed entirely by the basi-occipital bone, so far as the articulating facet is concerned, but it is supported on either side by a lamella from the lateral occipitals.
The two halves of the lower jaw form a symphysis of very variable length. Each half is composed of six bones. (1) The articulare, perforated in its upper, posterior, inner corner by a canal for the reception of the siphonium, a narrow tube of connective tissue, which connects the cavities of the middle ear with the large empty space enclosed within the lower jaw; (2) the angulare; (3) the dentary, which alone carries the teeth; (4) the splenial, a long splint-like bone on the surface of the inner or median side of the jaw, of variable length; (5) the operculare, the counterpart of the splenial on the outer side; (6) the supra-angulare, which forms the dorsal border of the lower jaw between the dentary and the angulare.
The teeth, which are more or less conical or compressed laterally, are deeply implanted in separate sockets. They are often shed throughout life, the successors lying on the median side, and with their caps partly fitting into the wide, open roots of the teeth to be expelled. The number of teeth in the premaxilla is universally five on either side in recent forms, but in a few species, e.g. Crocodilus niloticus and C. porosus, the second pair is lost with maturity and is not replaced. In the broad-snouted {438}kinds, especially in the Alligators, most of the upper teeth overlap laterally those of the lower jaw. In most species of Crocodilus the overlapping is less marked and the teeth partly interlock, but the fourth mandibular tooth, generally the strongest and longest, is received into a lateral notch at the junction of the premaxillary and maxillary. Frequently those of the longer lower teeth which fit into pits of the upper jaw, gradually transform the pits into holes by continued pressure upon the bone, and in old specimens the tip of the lower tooth may even perforate and stand out above the skin of the snout.
The vertebrae are solid, but remnants of the notochord persist for a long time in the middle of the centra. These are still amphicoelous in the Jurassic Eusuchia, and there were probably considerable intervertebral portions of the notochord. From the Lower Chalk onwards the vertebrae are procoelous, with the exception of the first caudal vertebra, which has a knob at either end, so that naturally the posterior of the two sacral vertebrae is opisthocoelous. This peculiar formation of the first caudal is probably correlated with the flexibility of the tail.
Cartilaginous intercentral rings, pads or menisci, occur regularly throughout the vertebral column, unless they are abolished by fusion of adjoining vertebrae. It is most instructive to follow the attachment of the ribs in one and the same individual. The position of the capitulum, vertically below the tuberculum in the neck, changes in the thorax into one in which the capitulum lies anterior to the tuberculum and in the same horizontal plane with it. Moreover, whilst on the cervical vertebrae the capitulum is carried by the centrum (enclosing with the tuberculum a typical transverse canal for the vertebral artery, etc.), further back it moves its point of attachment upwards, lying right upon the neuro-central suture on the tenth and eleventh vertebrae. From the twelfth vertebra backwards both capitulum and tuberculum are carried by the transverse process or diapophysis of the neural arch. The ribs of the five or six lumbar vertebrae are merely vestigial or absent. The ribs of the two sacral vertebrae are very stout, fusing in the adult with both centrum and neural arch. Some of the anterior caudal vertebrae also carry ribs, attached across the neuro-central suture; long before maturity they fuse with their vertebrae, and then look like transverse processes. Most of the caudal vertebrae carry also a {439}pair of chevron-bones, and these are continuous with the intercentral rings of cartilage.
The atlas and the epistropheus or axis are of supreme interest. Crocodiles are, in fact, the only animals in which these two vertebrae retain all their constituent hard parts in an almost undisturbed primitive condition (Fig. 103, 1-4). The basal piece of the atlas-ring, the first basiventral or intercentrum, carries a pair of long ribs attached by their capitular portions. A small knob near the dorsal edge of the rib occurs in many specimens, and is the last remnant of the tubercular portion. The latter was still complete in Jurassic Crocodiles, for instance in Metriorhynchus (Fig. 103, 2, t1). The first centrum joins that of the second vertebra as its so-called odontoid process, not directly, however, but by the intercalation of the complete second basiventral, represented by a cartilaginous disc, and by a large unpaired pyramidal piece (Fig. 103, 3, 2). This, serially homologous with the ventral half of the atlas-ring, is the second basiventral intercentrum, wedged in from below between the odontoid process and the second centrum, with which it soon fuses. Moreover, it carries the capitulum of the second rib (2, Cp2), the tuberculum of which is articulated with a facet of the second neural arch in Jurassic Eusuchia (t2). In recent Crocodiles this tubercular portion is much reduced, and, curiously enough, is attached to a knob which belongs to the odontoid piece or first centrum. This shifting explains the apparently anomalous condition that "the atlas of the Crocodiles carries two pairs of ribs, the second vertebra none." To complete the account of the atlas we have to mention the separate unpaired piece which lies upon the two neural arches. It is the detached neural spine, and not the remnant of a "pro-atlas."
The first and second ribs (R1 and R2), at least in the recent forms, are very long and are quite movable. Those of the next five cervical vertebrae are firmly fixed, short, and adze-shaped. The eighth and ninth are again long, and make the transition to the thoracic ribs, which are mostly eight in number, some with uncinate processes. Then follow several shorter or floating ribs, mostly two or three pairs. The next following three presacral vertebrae carry no ribs. The two sacral and the caudal ribs have already been mentioned.
As a rule the vertebral column of recent Crocodiles, Alligators, {440}and Gavials is composed of twenty-six precaudal vertebrae (namely, nine cervical, fifteen thoracic and lumbar, two sacral), and about thirty-four to forty or more caudal vertebrae. Individual variations, including lop-sided attachment of the iliac bones, are by no means uncommon.
The sternum remains cartilaginous. It consists of an anterior rhomboid portion, which carries the coracoids and two pairs of ribs, and a posterior longer and narrower portion formed by the median fusion of the next following five or six ribs. Posteriorly the sternum bifurcates, each half carrying two or three ribs, of which the last sometimes loses its proximal connexion, and thus appears as a xiphisternal process. Ventrally, upon the anterior part of the sternum lies the longitudinal, originally paired, episternum. The shoulder-girdle consists of the coracoids and the scapulae, which fuse with each other into one bony piece on each side. A pre-coracoid is indicated in fossil forms by a notch in the coracoid.
The space between the posterior end of the sternum and the pubic bones is occupied by the so-called abdominal sternum, composed of seven pairs of ossifications, resting upon the ventral side of the rectus abdominis muscle. Each pair consists of two closely apposed pieces, while the right and left remain separate in the median line. The last pair is much stronger than the rest, is more deeply imbedded in the rectus muscle, and is loosely connected with the anterior margin of the two "pubic" bones.
The limbs are built upon the typical terrestrial pentadactyle type, but were in the Jurassic species undoubtedly more adapted to swimming locomotion. The fore-limbs were conspicuously shorter and smaller than the hind-limbs, and it is only since Tertiary times that the difference has decreased to a great extent. Ulna and radius remain separate. The proximal row of carpal bones consists now of the ulnare and radiale, both strong and distinctly elongated. On the outer side, between ulna and ulnare, lies a pisiform bone. Upon the radiale follows a compound bone, often imperfectly ossified towards the median side, and consisting of the first distal carpal, the centrale, and the intermedium. The third, fourth, and fifth carpals are fused into one mass. The second distal carpal remains separate. All five fingers are present and well developed. The number of phalanges of the pollex is two, of {441}the others three, four, four and three respectively. During the embryonic development the number of phalanges of the fourth and fifth finger increases temporarily, to as many as seven on the fourth, to five or six on the fifth finger. Before the young animal is hatched the numbers are reduced again, chiefly by fusion of adjoining phalanges. This hyperphalangeal condition, typical of Plesiosauri, Ichthyosauri, Cetacea, and several other absolutely aquatic animals, naturally suggests the descent of the present Crocodiles from more essentially aquatic ancestors, but hitherto no trace of supernumerary phalanges has been found in any Jurassic Eusuchia, nor in the Parasuchia and Pseudosuchia.
The composition of the pelvis is difficult to understand. It consists in the adult stage of three separate bones, of which two only partake in the formation of the acetabulum. The broad ilium sends out two processes; the posterior and stronger articulates with the ischium, which sends out a short and stout process towards the anterior process of the ilium, enclosing a foramen. This process contains a separate centre of ossification, possibly homologous with the true pubis, while each club-shaped bone, loosely attached to it and directed forwards, generally called the pubis of the Crocodiles, would then be equivalent to an epipubis. Neither the "pubes" nor the ischia form a ventral median symphysis.
The femur is devoid of a prominent inner trochanter. Tibia and fibula are of almost equal strength. The tarsal elements are, in the adult, reduced by fusion to five bones. The fibulare is transformed into a typically projecting, heel-shaped calcaneum, while the intermedium is fused with the tibiale into a broad astragalus. The first, second, and third distal tarsalia are much reduced towards the inner side, and form one wedge-shaped, partly cartilaginous mass. The fourth tarsale lies between the fibulare and the fourth metatarsal, while the fifth tarsale is hook-shaped and loosely attached to the outer side of the fourth. It has lost its metatarsal and the rest of the fifth finger. Embryos are hyperphalangeal, the fourth toe developing six phalanges, and there are traces of the fifth toe. The numbers are ultimately reduced to 2, 3, 4, 4, 0 on the five toes. The fourth toe remains without a claw.
Skin.–The epidermal horny layer is not shed periodically nor in pieces; the wear and tear is made good imperceptibly. The {442}scales, which cover the whole body, have a hard, horny, waterproof covering, but between them the skin is soft. Each scale of the sides, belly, and tail, and especially those of the lower jaw, shows a little dot or pit. At this spot the epidermis is not cornified or thickened, and a nerve with sensory corpuscles ends beneath the bottom of the pit. Sometimes these pits are filled with débris of cells, and on the lower jaw, especially on the chin, these organs, instead of forming pits, are raised into little wartlike prominences.
The scutes or dermal portions of the scales consist of thickened, cutaneous connective tissue, and are more or less extensively ossified, thus forming a proper dermal armour. In most recent Crocodilia the armour is restricted to the back, with occasional osseous plates on the throat, as in Osteolaemus; regular although thin ossifications in the ventral scutes occur in the Caimans only. The Crocodile and Alligator skins of commerce consist entirely of the tanned cutis, minus the epidermis and the horny coverings of the scutes. In some fossil genera the ventral armour was extensively developed, especially in Teleosaurus, in some genera to the exclusion of dorsal ossifications. The armour of the recent forms consists, so far as the large scutes are concerned, of a considerable number of scutes, which are arranged in transverse rows, each row corresponding with one skeletal segment of the trunk proper. Mostly there is a detached cluster of scutes on the back of the neck. On the trunk some of the scutes are larger and more crested than others, and form in their totality a variable number of longitudinal rows. The median pair is generally the most conspicuous on the back. Some of the more lateral rows of keeled scutes converge more and more towards the tail, the inner rows drop out imperceptibly, and two lateral rows combine on the middle of the tail into an unpaired series of vertical blades. These are no longer bony, but show more strongly developed horny sheaths; they are very flexible, and transform the tail into an effective propelling organ.
Most of the larger scutes and the upper surface of the bones of the skull have a peculiar gnawed-out, almost honeycombed appearance, as is usual wherever most of the cutis itself is transformed into bone or co-ossifies with underlying bone, while the uppermost layers and the horny layer of the epidermis are much reduced and thinned out.
All the recent Crocodilia possess two pairs of skin-glands, both secreting musk. One pair is situated on the throat, on the inner side of the right and left half of the lower jaw. The opening of the gland, visible from below (see the figure of Crocodilus niloticus, p. 461), is slit-like, and leads into a pocket, which in large specimens is of the size of a walnut; the bag is filled with a smeary pale brownish substance, a concentrated essence of musk, much prized by natives. The secretion is most active during the rutting time, when the glands are partly everted. My young Crocodiles and Alligators often turned them inside out, like the finger of a glove, when they were taken up and held by force. The other pair lies within the lips of the cloacal slit, and is not visible from the outside. The use of these strongly scented organs, which are possessed by both sexes, is obviously hedonic. The sexes are probably able to follow and find each other, thanks to the streak of scented water left behind each individual.
The tongue is flat and thick, attached by its whole under-surface, so that it can be elevated but not protruded. It fills the whole space between the two halves of the lower jaw behind their symphysis. The dorsal surface shows numerous irregular polygonal fields, in the middle of most of which opens the duct of a large mucous gland. Tactile and gustatory corpuscles are scattered over the surface in the shape of tiny wartlike elevations. The hinder margin of the tongue is raised into a transverse fold, which, by meeting a similar fold from the palate, the velum palatinum, can shut off the mouth completely from the deep and wide cavity of the throat, which leads of course into the gullet. Dorsally the choanae open into this cavity; and since the narial passages are transformed into long tubes, completely surrounded by bone, Crocodiles can lie submerged in the water, with only the nostrils exposed and with the mouth open, and breathe without water entering the windpipe. The opening of the latter, the glottis, is a longitudinal slit, protected by the laryngeal cartilages, opened and closed by muscles. There is also a pair of membranous folds within the glottis, which serve as vocal cords. Ventrally below the larynx lies the cartilaginous, broad, shield-shaped hyoid; on the sides are attached the short hyoid horns. The trachea is long, consists of about sixty or more complete cartilaginous rings, and divides into two short bronchi, likewise protected by complete rings. The trachea is depressed; its transverse diameter decreases {444}from the glottis backwards. The lungs have attained a high degree of efficiency. Each lung is an oval sac, and is transformed into a complicated system of tubes, at the end of which are the countless honeycomb-like respiratory cells, the whole lung being spongy. The main bronchus is continued straight down to the posterior end of the lung, and sends off during its course regular secondary bronchi, and these send off tertiary bronchi. The whole arrangement is very regular, the tubes coming off like rows of organ-pipes. Each lung hangs freely in the thoracic cavity. Besides its ventral attachment by its arteries, veins, and the bronchus, it is connected by loose tissue with the liver and the pericardial septum. Each half of the thoracic cavity is partitioned off from the abdominal cavity by a strong transverse mesenteric lamella. The partition between the lungs and the stomach is at first simple, it then divides, to enclose the liver; the anterior partition passing between liver and lung to the inner surface of the sternum; the posterior lamella between the liver and the stomach. Both meet on the ventral surface of the liver, and are continued into or attached to the peculiar "diaphragmatic" muscle. This is covered by the internal rectus muscle of the abdomen, arising from the last pair of abdominal ribs near the pubic bones; it is innervated by a branch of the last precrural nerve, and extends as a broad but thin muscular sheath (always within and unconnected with the abdominal wall) to the ventral posterior vein of the liver; thence it is continued as an aponeurosis, together with the peritoneal lamella mentioned above, to the inner surface of the sternum. Contraction of this singular muscle indirectly widens the pulmonary cavity, and thereby directly aids inspiration. It acts consequently like the diaphragm or midriff of Mammals, although it is morphologically an entirely different muscle.
The stomach is smaller than one might expect from the fact that large Crocodiles can eat up nearly a whole man; but a great deal of their prey is stowed away preliminarily in the wide gullet until the rapid, powerful digestion, which dissolves every bone, makes room in the stomach. This consists of a wide, somewhat globular gizzard, rather muscular, with a pair of tendinous centres like those of birds, and a much smaller pyloric, globular, more glandular compartment. It leads into the duodenum, which is coiled up into a double loop, and receives at its end the {445}hepatic and pancreatic ducts. The small intestine is narrow, and is stowed away in a few irregular coils; the rectum is wide; a caecum is absent.
The cloaca is peculiar. The coprodaeum and urodaeum, cf. p. 498, are confluent, and form a wide, oval bag, closed in front and behind by strong sphincters, and it acts normally as a urinary receptacle. In the dorsal wall open the two ureters; a little towards the sides, and ventrally, open the two oviducts, on the right and left, near the base of the clitoris. Then follows a transverse, soft, muscular fold, which shuts off this cavity from the proctodaeum or outermost chamber. In the latter is stowed away the rather large copulatory organ. It arises out of the medio-ventral wall of the cloaca, and has a deep, longitudinal groove on its morphologically dorsal side for the conduction of the sperma, the vasa deferentia opening near its basal end. On either side of the root of this organ, in both sexes alike, opens a peritoneal canal, wide enough in large specimens to pass a goose-quill. The outer opening of the cloaca forms a longitudinal slit; within it, dorso-laterally, are the openings of the two anal musk-glands.
The kidneys are much lobed. The testes are long and oval; the ovaries are much elongated and flat; and the eggs contained therein in great numbers are extremely small, except those which ripen during the time of propagation.
The vascular system has attained the highest state of development of all reptiles. The heart is practically quadrilocular, the partition between the right and left ventricle being complete; but there is still a small communication, the foramen Panizzae, which lies in the middle of the wall common to both aortae, where they leave their respective ventricles. The left aortic arch conveys all the arterialised blood out of the left ventricle, and supplies head, neck, trunk, and tail. The right aortic arch, coming from the right ventricle, supplies venous blood, mixed with what little arterial blood it receives through the foramen Panizzae, to most of the viscera. On a level with the stomach both descending aortic arches are still connected with each other; the left aorta supplies most of the gut; the right, the trunk and the kidneys.
The outer ear lies in a recess, dorsally overhung by the lateral edge of the bony squamoso-postfrontal bridge; and this {446}carries a flap of skin, provided with muscles, to close the ear tightly. The tympanic membrane is visible at the bottom of the recess; shining through it is part of that cartilage which is homologous with the malleus of the auditory ossicular chain; the outward extension of the latter on its way to the mandible, behind the joint, passes as a partly cartilaginous string through the slit-like hole which is visible at the back of the skull, between the quadrate and the latero-occipital wing.
The eyes have, besides the lower and upper lid, a third, the nictitating membrane, which can be drawn over the front of the eyeball. In the upper lid lies a cup-shaped bony plate of variable size. The pupil contracts into a vertical slit. The iris is greenish.
The recent geographical distribution of the various kinds of Crocodilia loses its mystery when we recollect that during the Tertiary period Alligators, Crocodiles, and long-snouted Gavials existed in Europe. The solitary species of Alligator in China is the last living reminder of their former Periarctic distribution. The group, taken as a whole, is otherwise now intertropical, Crocodiles alone inhabiting the Palaeo-tropical region, together with long-snouted forms in the Oriental sub-region, while Alligators and Caimans, with a few Crocodiles, live in America.
They are all rapacious, doing much damage by their predatory habits, and are fierce and sulky in temper. But the danger to man differs much in different countries. While Crocodiles are dreaded in some localities, they are in others considered almost harmless, and men swim through the haunted waters without hesitation. It seems as if certain old and wily individuals turn into man-eaters, just like tigers and lions.
Their home is the water, in which they pass the night, their time of hunting. The prey is either patiently watched or stalked, and nothing falls amiss. Water-birds are seized by the beast, which rises imperceptibly from below. Some species are said to make use of their powerful tails for hitting the victim and even jerking it into the mouth. The strength of their jaws is enormous, and they do not let go what they have seized, unless, in the case of a man, he has the presence of mind and the opportunity to dig his fingers into the monster's eyes whilst being dragged down.
In the morning they crawl on to sandbanks, or on to logs of wood, which they closely resemble, in order to bask, mostly in such a position that on the slightest alarm they can plunge into the water. For this reason they frequently make a half circle before they settle down to rest, with the heads turned towards the river. There they bask all day long, apparently fast asleep, often with gaping mouths. But their sense of hearing and of sight is sharp, and they learn from experience, old individuals being by far the most wary. Commercially the skins are now of considerable value. The flesh is white, and is tolerable eating but for the combination of fishy and musky odour, which, although faint, is not to everybody's liking.
All the species have a voice, a kind of loud, short bark or croak, heard at night and when angered. The female lays several dozen or even three score white, oval, hard-shelled eggs in the sand, well out of the reach of moisture; and some species construct an elaborate kind of nest. The mother watches it, takes care of and fights for her offspring, numbers of which fall an easy prey to large storks, fishes, and to the stronger members of their own kind.
In the cooler countries they hibernate in the ground; and in hot countries, which are subject to drought, some kinds aestivate in the hardened mud; or they migrate. When during a prolonged drought on the island of Marajó, at the mouth of the Amazon, the swamps and lakes were dried up, the Alligators migrated towards the nearest rivers, and many perished in the attempt. On one farm were found 8500 dead, and at the end of Lake Arary more than 4000. Such occurrences in bygone times may perhaps explain the masses of bones found here and there in a fossil state.
The age to which Crocodiles can live is quite beyond calculation. They are capable of propagation long before they are anything like half-grown, maybe at an age of little more than ten years; then they continue to grow perhaps for more than one hundred years, until they die.
It is customary to divide the Eusuchia, most of which are extinct, into a longirostral and a brevirostral section. In the former the snout is much elongated and narrow, and the nasal bones, although they are sometimes very long, do not reach the nasal groove. The mandibular symphysis is very long, and is formed not only by the dentary but also by the splenial bones. In the brevirostral section the snout is shorter, sometimes broad and rounded off, and the nasal bones are supposed to reach the nasal groove, or at least to approach it very nearly; the mandibular symphysis is formed by the dentaries only. But these distinctions are quite arbitrary, and there exist all kinds of intermediate forms. For instance, in Goniopholis and Diplocynodon, which are both undoubtedly near allies of the recent Crocodiles and Alligators, the nasal bones are considerably removed from the nasal groove; and in Crocodilus cataphractus they are separated even from the premaxilla by the medio-dorsal suture of the maxillaries. Again, in Goniopholis the mandibular symphysis is so long that it comprises part of the splenial bones. Both typically long- and short-snouted forms occur already in the Upper Oolite, but in the Lower Jurassic age only long-snouted kinds seem to have existed. The latter cannot easily be connected with Belodon, one of the Parasuchia, on account of the position of the nostrils; the mere shortening of the long premaxillaries of Belodon would not transfer its distinctly paired nostrils to the anterior end of the premaxilla. To account for the position of the nasal groove in the Eusuchia, we have to go back to a primitive condition, such as that of the Pseudosuchian Aëtosaurus, and this consideration shows that the Parasuchia and Eusuchia are collateral branches.
The Eusuchia have been split into many families. Zittel, for instance, divides them into ten, some of them on insufficient grounds, since there are too many intermediate forms; and more, sometimes quite unexpected, modifications are still being found. Several of the accepted families represent collateral or convergent lines of development.
Fig. 105.–Group of Crocodiles. A long-snouted Gharial or Gavial (Gavialis gangeticus) on the top of Crocodilus acutus; a Nile Crocodile (C. vulgaris) in the foreground; C. palustris, a "Mugger," in the right upper corner. Observe the peculiar floating attitude of the young specimen.
There is the same tendency to transfer the choanae further back, owing to the formation of a solid secondary roofing in of the mouth, to transform the amphicoelous into procoelous vertebrae, to reduce the supratemporal foramina, and to obtain a better development of the dorsal armour, whilst that on the ventral side is gradually reduced. Lastly, there is a tendency towards a shortening and broadening of the snout, a condition which has reached its culmination in the Alligators, while the Gavials are survivals of another branch. The notches in the premaxilla, for the reception of some of the lower teeth, have also been acquired independently. Although the recent Crocodilia cannot now, as has been pointed out by Boulenger, be separated into different families, no valid diagnoses being possible owing to the existence of Tomistoma, their phylogeny shows them to belong to at least two heterogeneous groups.
Key to the Genera of recent Crocodilia.
I. Snout very long and slender. The mandibular symphysis extends at least to the fifteenth tooth, and is partly formed by the splenial bones.
a. Nasal bones very small, and widely separated from the premaxillaries .......... Gavialis gangeticus, p. 451.
b. Nasal bones long, in contact with the premaxillaries .......... Tomistoma schlegeli, p. 453.
II. Snout not slender, but triangular or rounded off. The mandibular symphysis does not reach beyond the eighth tooth, and does not reach the splenial bones.
Fam. 1. Teleosauridae, in the Lias and Oolite of Europe; marine.–Snout very long and slender. Nasals widely separated from the premaxillae by the maxillaries. Choanae at the posterior end of the palatines. In front of the eye a small sub-lacrymal foramen. Supratemporal foramina large. Vertebrae amphicoelous. Anterior limbs scarcely half as long as the posterior pair. The dermal armour consists of two rows of broad scutes on the back, while the belly is protected by a shield of numerous bony scutes, which are connected with each other by sutures. Teeth numerous and rather slender. General appearance like that of Gavials.
Teleosaurus of the Middle and Upper Oolite in England and France. Snout very slender. Nasals narrow and short. The under side is protected by a beautifully finished armour, consisting of a square breast-shield of four rows of bony scutes, and a larger, long, oval shield on the belly, with about six longitudinal and seventeen transverse rows of scutes.
Mystriosaurus, of the Upper Lias in France and Germany, reached a length of 15 feet, and is characterised by an additional series of keeled but smaller caudal plates running parallel with the middle pairs, which are neatly sutured together.
Fam. 2. Metriorhynchidae, in the Upper Oolite of Europe; marine.–Nasals broad posteriorly, sometimes extending with a pointed wedge very near the premaxillae. Without sub-lacrymal foramina. Eyes with a ring of ossifications in the sclerotic. Dermal armour unknown. Vertebrae and choanae like those of the previous family. Metriorhynchus and Geosaurus.
Fam. 3. Macrorhynchidae, in the freshwater deposits of the Purbeck, Wealden, and Greensand of Europe. Snout long and slender. The nasals are narrow, and so elongated that they meet a similar long extension of the premaxillaries. Choanae between the palatines and pterygoids. Vertebrae amphicoelous. Dermal armour consisting of two imbricating dorsal and eight ventral rows, e.g. Pholidosaurus of the English Wealden.
Fam. 4. Gavialidae.–Snout long and slender. The choanae are situated entirely within the pterygoids. Vertebrae procoelous. Members of this family make their first appearance in the littoral marine deposits of the Upper Chalk of Europe and North America; others are common in tertiary, marine, and freshwater deposits, whilst only two genera and species occur now in the Oriental sub-region.
Thoracosaurus in the Upper Chalk of New Jersey and France and Belgium is intermediate between Gavialis and Tomistoma. The prefrontal bones are very small, while the lacrymals are very long and surround the nasals posteriorly. The nasals themselves are slender, and reach the posterior likewise long and narrow prolongations of the premaxillaries.
Gavialis.–The snout is extremely long and slender. The mandibular symphysis is so long that it comprises a great portion of the splenial bones, and extends backwards almost to the level of the last teeth and to the palatal foramina. The nasal {452}bones are very short, and are separated from the premaxillaries by the long suture of the maxillaries. About twenty-eight upper and twenty-five lower teeth on each side.
G. gangeticus, the only recent species, is essentially Indian, inhabiting chiefly the basins of the Ganges, Brahmaputra, and Indus; it occurs also in the Mahanadi of Orissa and in Arakan, but does not live in the Irrawaddy, nor in the Narbada, Kistna, and farther south. In spite of its great size, which reaches 20 feet or even more, it is harmless, and lives entirely upon fish; hence its Hindustani name, gharial, meaning fish-eater, of which the generic name is a corruption.
The nuchal and dorsal scutes form a continuous shield, but there are two small postoccipital scutes. General colour, dark olive-brown above; the young are paler, with dark markings. The male is remarkable for several peculiarities. The nose is very much swollen, and can be inflated like a bag when the nostrils are closed. In connexion herewith, probably produced by the recoil of the air in the long narial passages towards the choanae or posterior nares, there is a pair of hollow globular swellings, in large specimens of the size of a goose's egg. The shell of these globes is formed by the dorsal wings of the palatine bones above the floor of the choanae, and they extend forwards to the right and left of the ethmoid almost to the vertical downward process of the prefrontals.
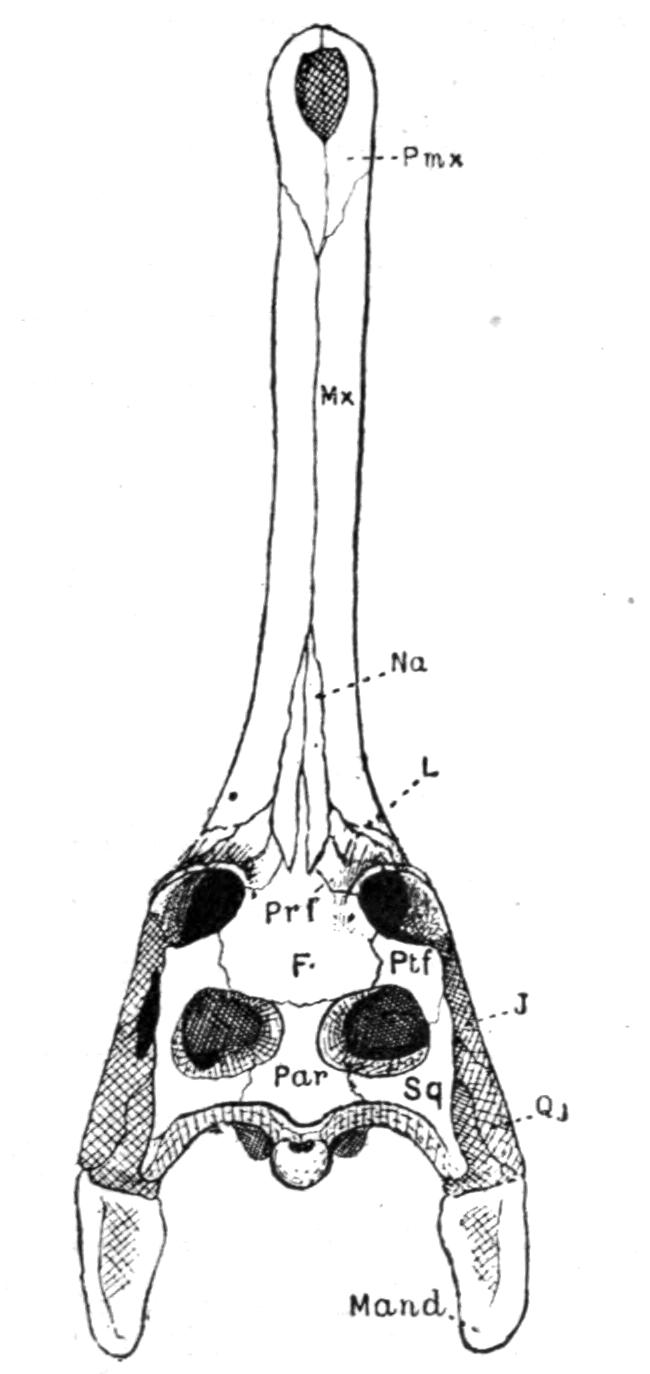
Fig. 106.–Skull of Gavialis gangeticus (the Gharial). × ⅛. F, frontal; J, jugal; L, lacrymal; Mand, mandible; Mx, maxillary; Na, nasal; Par, parietal; Pm.c, premaxillary; Prf, prefrontal; Ptf, postfrontal; Qj, quadrato-jugal; Sq, squamosal.
Although the Gharial is common enough, we know next to nothing about its habits, and in zoological gardens it is rather rare. A. Anderson[139] has, however, made the following observations. Forty eggs were dug out of the sand, where they were {453}lying in two tiers, twenty below and twenty above, with a foot of sand between. The young ran with amazing rapidity the moment they were hatched. Some of them actually bit his fingers before he had time to remove the shell from their bodies! The length of these new-born creatures was 15 to 16 inches, 9 of which belonged to the long and slender tail.
Several fossil species have been described from the Pliocene deposits of the Sivalik Hills of India; and in the same district occurred the closely allied Rhamphosuchus crassidens, which reached the gigantic length of about 50 feet!
Tomistoma.–The general configuration of the skull and snout is that of Gavialis, but the nasal bones are long and reach the premaxillaries, although not the nasal groove, thereby separating the maxillaries. The first and fourth mandibular teeth fit into notches of the upper jaw, while most of the others fit into pits between the teeth of the upper jaw. About twenty upper and eighteen lower teeth on each side.
T. schlegeli, the only species, reaches a length of 15 feet; it inhabits the rivers and swamps of Borneo, Malacca, and Sumatra. Fossil specimens of Tomistoma have been found in the Miocene of Malta and Sardinia. Gavialosuchus of the Miocene of Hungary is closely allied.
Fam. 5. Atoposauridae.–The few members of this family, Atoposaurus, Alligatorium, and Alligatorellus, lived in the Upper Oolitic period of France, and were small, about one foot in length. The vertebrae are amphicoelous. The nasal groove is divided by a prolongation of the nasal bones. The head is short, and in its general shape rather like that of a lizard.
Fam. 6. Goniopholidae, in the Purbeck and Wealden of Europe and the corresponding level of North America. The vertebrae are amphicoelous. The choanae are still elongated but are situated between the palatines and pterygoids. The premaxillaries are rather large, and each sends a broad triangular process between the nasal and maxillary. The nasals are broad and are well separated from the nasal groove. The splenials help to form the mandibular symphysis.
Goniopholis.–The general configuration of the skull is rather like that of Crocodilus vulgaris. There is a pair of deep notches in the upper jaw for the reception of the lower canine teeth. G. simus and G. crassidens in England and continental Europe, and {454}others in Colorado, were large-sized Crocodiles, some with a skull 2 feet in length. The dermal armour consisted of a pair of dorsal rows, a thoracic and an abdominal shield, composed as in the Teleosauridae of six to eight longitudinal sutured rows.
Fam. 7. Crocodilidae.–Beginning in the Upper Cretaceous period of Europe and North America, many forms of Crocodiles, Alligators, and Caimans existed in the Tertiary period in America, Europe, and India; persisting in Europe until the Plistocene. The vertebrae are procoelous. The choanae are completely surrounded by the pterygoids. The nasals reach the nasal groove, except in Crocodilus cataphractus. The orbits are larger than the small supratemporal fossae, and always continuous superficially with the lateral temporal fossae, the postfronto-jugal bridge not reaching the surface. The dorsal armour consists of more than one pair of longitudinal rows, while the ventral armour is much reduced in thickness or absent.
Diplocynodon.–Common in the Oligocene and Miocene of Europe, e.g. D. hastingsiae. The skull resembles that of the Alligators, but has a pair of lateral notches in the premaxilla for the reception of the third, and sometimes also of the fourth mandibular tooth. The ventral armour is still rather strong.
Crocodilus.–The fourth mandibular tooth fits, as a rule, into a notch in the upper jaw. The other teeth are more or less interlocked with those of the other jaw. The fifth upper tooth is the largest. The nasal bones form the posterior border of the nasal groove, but do not extend into it as a septum. The bony scutes of the dorsal shield are keeled, and stand closely together, being rarely united by suture; and they form from four to six principal rows.
Crocodiles have occurred since the Upper Chalk in Europe; many species existed in the Tertiary epoch in Europe and North America, decreasing in numbers in the Pliocene and disappearing with the beginning of the Plistocene. About ten recent species are known, and these have now a somewhat scattered distribution; namely, three species in Africa, one of them extending into Syria; three in tropical America and the West Indian Islands; the rest in the Malay, Indian, and North Australian countries.
C. palustris.–The "Mugger" of India. The premaxillo-maxillary suture is transverse, as in the Alligators. The adults {455}retain the five teeth in each half of the premaxilla. The mandibular symphysis is short, extending only to the level of the fourth or fifth tooth. The snout is stout, rather broad; the top of the head is rough but without any ridges. The upper and lower jaw each contain nineteen teeth on either side. The nuchal scutes, six in number, are packed closely together, the four biggest forming a square. Four smaller scutes are arranged in a curved line on the occiput. The dorsal shield is composed of four, sometimes of six rows of larger scutes, of which the central pair is the broadest. The fingers are webbed at the base; the outer toes are broadly webbed, and the outer edge of the hind-limbs is turned into a serrated fringe. The general colour of the upper parts is dark olive-brown; the young are pale, with black spots. The length of twelve feet is considered a fair average size for a large specimen.
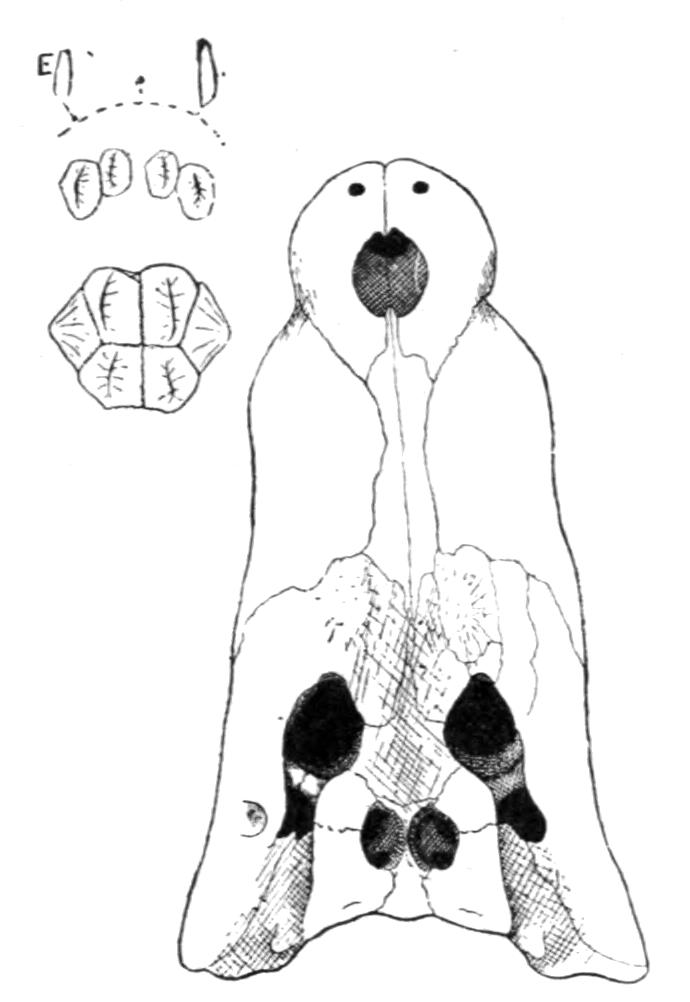
Fig. 107.–Dorsal view of the skull of Crocodilus palustris. × ⅛. The arrangement of the nuchal scutes is shown in the upper left-hand corner; E, position of the ear-flap.
This, the "Marsh Crocodile," has a wide distribution. It inhabits the rivers, ponds, tanks, and marshes of India and Ceylon, extending eastwards through Burma and Malacca into most of the Malay islands, westwards into Beluchistan. This species is frequently venerated by the Hindoos, and is kept in a kind of domesticated condition, attended by fakirs. One of the most famous crocodile ponds, the so-called "mugger-peer," lies in an oasis of the sandy stretches to the north-west of Karachi. A. L. Adams has described a visit to this pond.[140]
"The greater pond is about 300 yards in circumference, and contains many little grassy islands, on which the majority of the Crocodiles were then basking; some were asleep on its slimy sides, others half submerged in the muddy water, while now and then a huge monster would raise himself upon his diminutive legs, and waddling for a few paces, fall flat on his belly. Young ones, {456}from a foot in length and upwards, ran nimbly along the margin of the pond, disappearing suddenly in the turbid waters as soon as we approached. The largest crocodile lives in a long narrow tank separated from the others. The fakirs, and natives who worship in the neighbouring temples, have painted his forehead red; they venerate the old monster, making a salaam to his majesty whenever he shows himself above water. A handsome young Beloochee, whose occupation it was to feed the animals, informed us that this specimen was upwards of 200 years old, and that by way of a 'tit-bit' he was in the habit of devouring the young crocodiles. During our visit this enormous brute was asleep on the bank of his dwelling-place, and seemed quite indifferent to our presence, although we came within a foot of him, and even attempted to arouse him by rubbing his nose with a leg of goat's flesh, which, however, a young one greedily seized. Our attendant tried in vain to excite their ferocity, but beyond a feeble attempt to snap their trenchant teeth, the animals showed no disposition to attack us.
"A pony was wading about in the pond and feeding on the grassy hillocks, but the crocodiles took no notice of him.
"The crocodiles dig deep in the sand, under the neighbouring date-trees, and there deposit their eggs. Quantities of deciduous teeth, of various sizes, were strewn along the slimy sides of the pond.
"Strangers are expected to stand treat, not only by the fakirs and natives, who gain a livelihood by hanging about the pond and showing the monsters, but even the crocodiles themselves seem to anticipate a feast, and on the arrival of a party come out in unusual numbers. Accordingly, we had a goat slaughtered, during which operation the brutes seemed to rouse themselves, as if preparing for a rush. Then our guide, taking piece after piece of the flesh, dashed it on the bank, uttering a low growling sound, at which the whole tank became in motion, and crocodiles, of whose existence we had been before ignorant, splashed through the shallow water, struggling which would seize the prize. The shore was literally covered with scaly monsters, snapping their jaws at one another."
Sir J. Emerson Tennent[141] has had many opportunities of studying the habits of the Marsh Crocodile. According to him {457}it is essentially cowardly in its instincts, and hastens to conceal itself on the approach of man. One of these creatures, which was overtaken in the jungle by a gentleman riding on horseback, fled to a shallow pool, and thrusting its head into the mud till it covered up its eyes, remained motionless, in profound confidence of perfect concealment.
"There is a popular belief that the crocodile is exceedingly sensitive to tickling, and that it will relax its hold of a man if he can only contrive to reach and rub with his hand the softer parts of its under side. An incident of some reality in this piece of folk-lore came under my own observation. One morning ... we came suddenly upon a crocodile asleep under some bushes of the buffalo-thorn, several hundred yards from the water. The terror of the poor wretch was extreme when it awoke and found itself discovered and completely surrounded. It was a hideous creature, upwards of 10 feet long.... It started to its feet and turned round in a circle, hissing and clanking its bony jaws, with its ugly green eye intently fixed upon us. On being struck with a stick, it lay perfectly quiet and apparently dead. Presently it looked cunningly round, and made a rush towards the water, but on a second blow it lay again motionless and feigning death. We tried to rouse it, but without effect; pulled its tail, slapped its back, struck its hard scales, and teased it in every way, but all in vain; nothing would induce it to move till, accidentally, my son, then a boy of twelve years old, tickled it gently under the arm, and in an instant it drew the limb close to its side and turned to avoid a repetition of the experiment. Again it was touched under the other arm, and the same emotion was exhibited, the great monster twisting about like an infant to avoid being tickled."
In the dry season, when the tanks become exhausted, the Marsh Crocodiles have occasionally been encountered in the jungle, wandering in search of water. During a severe drought, in 1844, they deserted a tank near Kornegalle, and traversed the town during the night, on their way to another reservoir in the suburb; two or three fell into the wells; others, in their trepidation, laid eggs in the street, and some were found entangled in garden fences and killed.
Generally, however, during the extreme drought, when unable to procure their ordinary food from the drying up of the {458}watercourses, they bury themselves in the mud and remain in a state of torpor till released by the recurrence of rains.
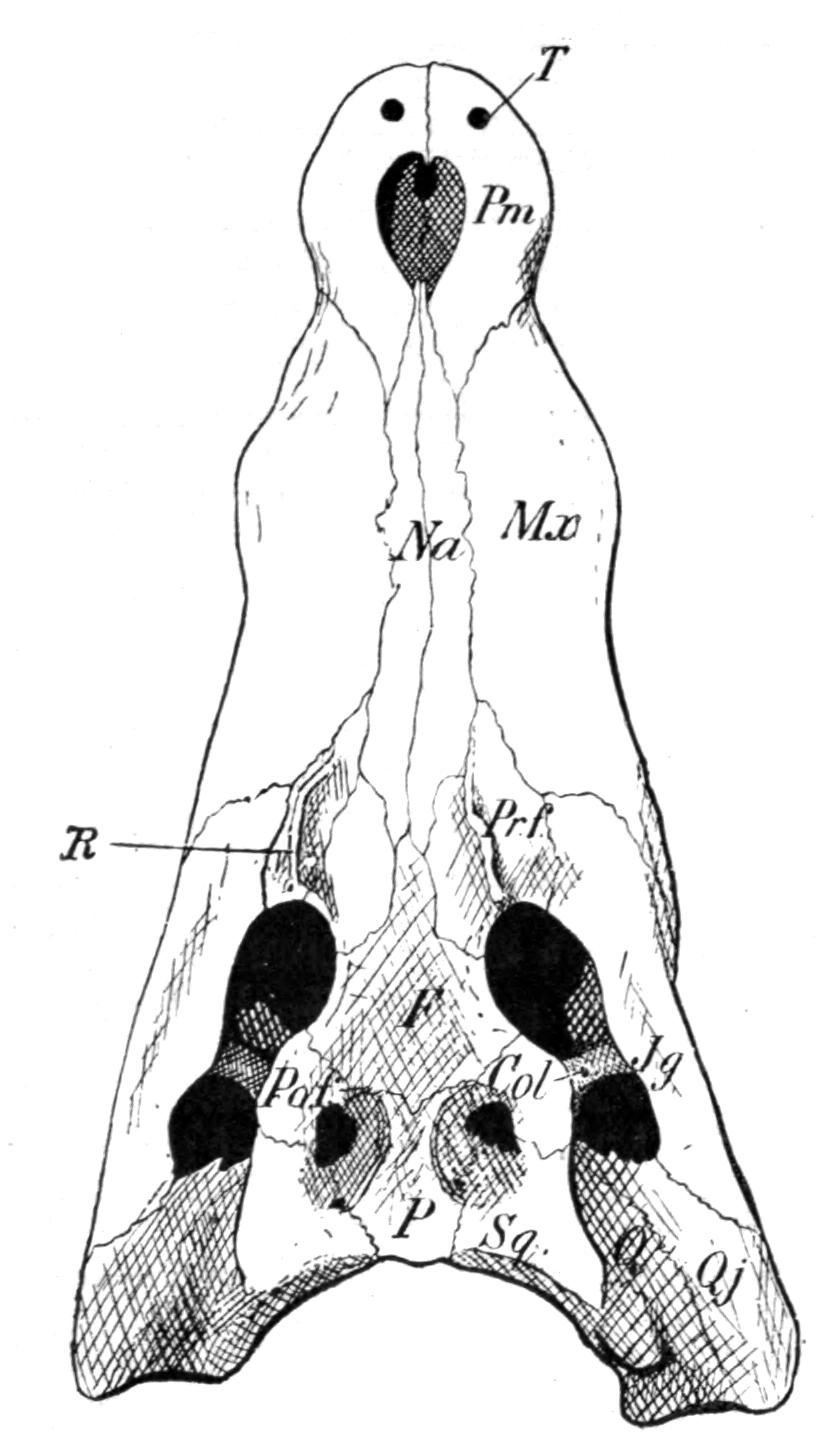
Fig. 108.–Dorsal view of the skull of Crocodilus porosus. × about ⅙. Col, buttress connecting the postfrontal with the jugal and ectopterygoid; F, frontal; Jg, jugal; Mx, maxillary; Na, nasal; P, parietal; Pm, premaxilla; Pof, postfrontal; Pr.f, prefrontal; Q, quadrate; Qj, quadrato-jugal; R, the characteristic ridge on the prefrontal bone; Sq, squamosal; T, perforations in the premaxilla caused by a pair of lower incisor teeth.
C. porosus s. biporcatus.–The premaxillo-maxillary suture on the palate does not form a transverse line, but is W-shaped, and extends backwards as in the rest of the species of Crocodiles to be described. This Indian species is easily recognised by the prominent longitudinal ridge which extends in front of each eye, over the prefrontal bones, and by the absence of sub-occipital scutes. The nuchal scutes consist of four large ones, which form a square, and one or two smaller scutes on each side. The dorsal shield contains four to eight principal longitudinal rows. The digits, webs, and the serrated fringe of the legs are like those of C. palustris. The head and snout, however, are distinctly longer, and more slender in proportion, and the adult has only four teeth in each premaxilla. The general colour is dark olive-brown. Young specimens, as usual, are much paler and are spotted with black.
This species attains a much larger size than the Marsh Crocodile. Specimens of 15 to 20 feet in length are not uncommon, and there is a record of one monster of 33 feet. Consequently this is, both in bulk and length, undoubtedly the largest species of recent reptiles. It is essentially an inhabitant of tidal waters or estuaries, frequently entering salt water and going out to sea. Herewith corresponds its wide distribution, namely, the whole coast of the Gulf of Bengal, extending to {459}Southern China, and across the Malay Archipelago to the northern coasts of Australia. Eastwards it ranges to the Solomon Islands and even to Fiji. Curiously enough, it does not seem to occur on the west coast of India.
According to Tennent it is ready to assail man when pressed by hunger, and the same authority mentions the following serio-comic incident. A man was fishing, seated on the branch of a tree overhanging the water, and to shelter himself from the drizzling rain he covered his head and shoulders with a bag folded into a shape common with the natives. While in this attitude, a leopard sprang upon him from the jungle, but missing its aim, seized the bag and not the man, and fell with it into the river. Here a crocodile, which had been eyeing the angler in despair, seized the leopard as it fell, and sank with it to the bottom.
I have had some personal experience in the bringing up of the young of this species. Two dozen of them had come from Ceylon when quite young, only one foot long. At first they were very shy, and huddled together in their tank, but they took food greedily–strips of fish and, later on, sheep's heart. When frightened they emitted peculiar, high-pitched, half-croaking sounds. Some of them snapped at the finger when touched; others were of a more gentle disposition; the shy ones were undoubtedly the most vicious. Within one year they grew to 18 or 20 inches, and added much to their bulk. Then they were transferred to a deeper and larger tank in a greenhouse, in which they could roam about at liberty. In the daytime they dozed on the margins of their pond, mostly in such a position that, at the slightest alarm, they could plunge back into the water. The strongest specimen left the tank entirely, and took up its favourite place for basking on the stump of a tree, to reach which it had to climb up a rough wall of stones. After three years, several had grown to the length of three and a half, and even four feet, and had by this time become formidable pets. Although handled frequently, they never became tame, the only change in their behaviour being that, instead of rushing off in a fright, and hiding for half an hour at the bottom of the tank, they became more vicious and confident, making for and snapping at the hand which fed them. The nights were spent regularly in the water, either floating {460}with just the nostrils exposed, or in search of food, frogs being their favourite prey, while their main sustenance consisted of "lights," with an occasional mouse, or a piece of solid meat by way of an entrée. Small pieces were bolted. The tough "lights," namely lungs with the windpipe and blood-vessels, were causes of great quarrels. Two or three would get hold of a lump of this kind, tearing at it, and twisting and rolling over in opposite directions. The supply of warm water came through a stout pipe of red india-rubber, and this was an irresistible attraction to the crocodiles. On many a morning the tube was found twisted into a knot, one of the creatures having spent hours in chewing it and in trying to wrench it off. In order to aid digestion they swallowed pebbles. The most favourable temperature of the water was 85° F.; if below 75° F. they refused to eat, but a continued exposure to 60° F. did not hurt them. When the temperature rose above 95° F. they left the water, although means had to be taken to prevent them from lying on the hot-water pipes.
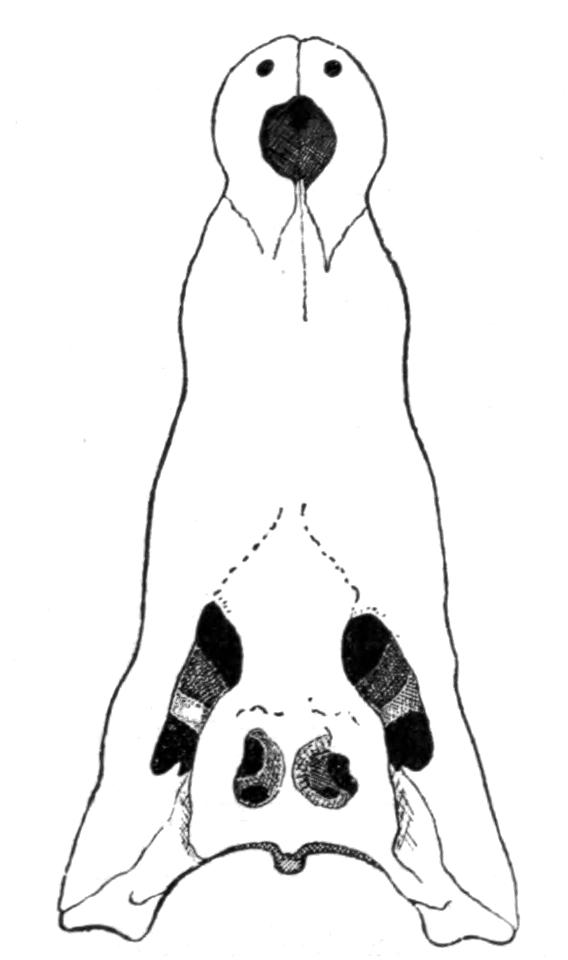
Fig. 109.–Dorsal view of the skull of a very old specimen of Crocodilus niloticus, in which most of the bony sutures are obliterated, × about ⅒.
C. niloticus s. vulgaris.–The premaxillo-maxillary suture on the palate is W-shaped. The nasal bones form only a small part of the posterior border of the nasal groove. There are eighteen or nineteen upper and fifteen lower teeth on each side. In old specimens some of the anterior mandibular teeth perforate the premaxillae, as indicated in Fig. 109, and they even pierce through the integument so as to be visible from above. The nuchals are composed of four large scutes, with a smaller one on each side and sometimes one behind, and there is a row of smaller pieces across the occiput. The dorsal shield contains six to eight principal longitudinal rows. The fingers are webbed at the base; the outer toes are very broadly webbed; and there is a serrated fringe on the outer side of the leg. The general colour of the adult is dark olive-brown; the young are paler, with black spots and vermiculations. The under parts are yellowish white.
The Nile Crocodile is essentially African, ranging from the Senegal to the Cape and to Egypt. It is also very common in Madagascar. Nothing is known about its occurrence in Arabia, but a few specimens of rather small size seem still to exist in Syria, in the Wadi Zerka, an eastern tributary of the Jordan.
Even in historical times the Crocodile must have been very common in lower Egypt, to judge from the number of mummies preserved by the old Egyptians. Now it is practically exterminated, and there are scarcely any left below Wadi Halfa.
Fig. 110.–Ventral view of a young Crocodilus niloticus, showing the arrangement of the bony scutes and the two openings of the musk-glands on the lower jaw. The upper right-hand figure shows on a larger scale the disposition of the nuchal scutes and the first row of dorsal scutes.
Such a conspicuous and dangerous creature has naturally always enjoyed notoriety. It is well described in one of the oldest writings of the world, the Book of Job. "Canst thou draw out leviathan with an hook? or his tongue with a cord which thou lettest down?... His scales are his pride, shut up together as with a close seal. One is so near to another, that no air can come between them. They are joined one to another, they stick together, that they cannot be sundered.... Lay thine hand upon him, remember the battle, do no more." Bows and arrows, spears and clubs, are of little avail against such a {462}monster; the dragging out of a hooked, full-grown specimen requires many men and is a formidable task. Of course firearms have changed all this, and its invulnerability to bullets is nonsense. It is true that a bullet sent into the head is generally ineffective, since it is a hundred to one that the bullet does not hit the small brain, and even if it does, the creature sinks to the bottom and is lost to view until decomposition sets in and the gases developing in the body cause it to float.
Herodotus has quaint stories about these crocodiles and their worship. Amongst other stories he mentions that the bird Trochilus, supposed to be the Pluvianus aegyptius, a kind of Plover, slips into the gaping mouth to pick off the leeches which infest the reptile's gums. "In Egypt it is called Champsa, but the Ionians call them κοκοδρίλοι on account of the resemblance to the lizards which live on their garden walls." This is in fact the origin of the name crocodile, κόρδυλος being the ancient Greek for lizard and newt. With reduplication κορκόρδυλος and by metathesis ultimately κροκόδειλος. The Arabic name is ledschun.
The story about the Plover seems to be true. These birds are sometimes seen sitting upon basking crocodiles, and since the latter are in the habit of resting, perhaps half asleep, with the mouth wide open, it is possible that these agile birds do pick their teeth, and that they, being also very watchful, by their own cry of warning and by fluttering off on the approach of danger, give the alarm to the crocodiles and thus benefit them in more than one way.
But the equally old story about the Ichneumon or Mongoose is an idle invention. Mongooses are partial to eggs, but they certainly prefer those of hens and other birds to those of the crocodile, which are far too hard and strong to be broken by such a little animal. Moreover, as we shall see presently, the eggs are far too well concealed.
The best account of the habits of these crocodiles is the one given recently by Voeltzkow,[142] who has spent a long time in Madagascar to collect material for the study of their development.
He says that C. niloticus is not only the most common reptile, but perhaps the most common vertebrate in Madagascar. {463}It occurs in every pool and river in great numbers, especially upon the sandbanks of the Betsiboka River, where one may see more than one hundred within one hour's paddling down stream. The largest specimen measured by Voeltzkow was 13 feet long; the largest in the National Collection is a little less than 15 feet.
The crocodiles are caught in various ways. The simplest apparatus consists of two pointed sticks, which are fastened cross-wise within the bait to which is attached a rope, and this is made fast on the bank of the river or lake. The animal, when it has once swallowed this spiked bait, keeps its jaws firmly closed, so that it can be dragged out of the water. Another method is more reliable. A long and strong rope is made into an easily slipping noose, with an opening of about 18 inches. The bait is attached to the upper part of the noose, while the lower portion is kept open by a springy branch, the whole thing being so balanced that it will float upright. When a crocodile seizes the bait, which it does with a side jerk of the head, the branch falls out of the noose and the latter closes around the upper or lower jaw.
These crocodiles dig long subterranean passages of 30 to 40 feet in length; the passage opens in the bank below the level of the water, and gradually ascending ends in a somewhat wider compartment, which allows the creature to turn round. Two or three air-holes are pierced through the ceiling of the burrow, in which bones and other remains of food are often found, so that the natives' belief, that the crocodiles retire into these chambers in order to devour their prey in undisturbed secrecy, appears very probable. When suddenly disturbed or frightened they take to these lairs, and since their position is clearly marked by the air-holes, the natives block the passage and then dig the animal out from above.
Eggs are laid, in Madagascar, from the end of August to the end of September; the number of one set varies from twenty to thirty. They are deposited in a nest. This is in the ground, mostly in white sand, and consists of a hollow 18 inches to 2 feet deep. The walls are rather vertical, but near the bottom they are undermined, and here the eggs are placed. The centre of the pit being somewhat higher, the eggs roll by themselves into the undermined peripheral region. The laying takes place {464}during the night, mostly a little before daybreak. After one half of the eggs has been laid, they are covered up with sand, whereupon the other half is deposited. Then the hole is completely filled up and no visible traces are left behind; but the mother sleeps upon the nest and thus leads to its discovery. The position of the nest is so chosen that it cannot be reached by moisture from below; the eggs are most susceptible to moisture, a very slight amount of which causes them to turn bad.
The shape of the eggs of one and the same clutch varies much, some being elliptical, others cylindrical with rounded off ends. Their size varies from 5.5 to 9 cm. in length, and 4 to 5 cm. in width. The shell is white and glossy, thick and hard, either roughly granular or smooth. They are hatched in about twelve weeks.
Voeltzkow feels certain that the mother returns to the nest at the proper time in order to dig the young ones out and to conduct them to the water. To test this story he had a nest surrounded with a fence; the mother returned several times and partly destroyed the fence, which was then replaced by a stronger one. One day, when the young had been hatched, the nest was found to be filled with sand, the shells and one dead little crocodile being at the bottom of the hole. The mother had dug a deep ditch below the fence, but had not succeeded in reaching the nest, although she had received and conducted her offspring away. As a rule, when the young are hatched, the sand and the shells are found to be scraped out of the nest. The mother is probably warned by the hiccough-like sound which the young emit while still within the unbroken shell. Voeltzkow heard them piping from the other end of his room, the eggs being covered with a layer of sand two feet high. The sounds were heard when he walked past the nest, or knocked against the box. Possibly the young hear the mother when she retires to the nest to sleep on it, and give her warning to remove the eggs out of the groove. However, they do not break the shell until several days later.
The hatching is not caused by the rainy season, since it took place a fortnight before the first showers. The "egg-tooth" of the newly hatched young is 0.5 to 0.75 mm. high, bicuspid, and acts like a borer or auger. It is still visible on the tip of the upper jaw, in front of the nose, when the creature is two weeks old. The {465}newly hatched crocodile is of an astonishing size, so that it is rather puzzling to understand how it was stowed away in the egg. For instance, an egg of 8 cm. length and 5 cm. width, sends forth a crocodile 28 cm. or 11 inches in length. Even at this early age they snap at the finger.
The egg is covered by a hard shell, within which is a thicker outer and a thinner inner membrane. The "white" is jelly-like, sometimes of a greenish tinge, and is so consistent that it will not flow. The yolk is round, and so large that it nearly reaches the shell-membrane in the short diameter. The yolk itself is surrounded by a very thin but strong membrane.
The embryo begins to develop long before the egg is laid. When laid the germ is about 4 mm. long and shows about twelve somites. The cephalic bend begins at the end of the second week, the tail grows longer and the embryo becomes curled up. At the end of the third week it measures 10 mm. in a straight line from brain to vent. The limbs begin to bud in the fourth week. With the sixth week the final shape begins to reveal itself, and is completed at the age of eight weeks; but a third month is necessary to ripen the embryo.
C. cataphractus is the Common Crocodile of West Africa, from the Senegal to the Congo. In opposition to C. niloticus it does not enter brackish water. It is easily recognised by the very slender snout, which rather resembles that of the Gavial; but the mandibular symphysis, although extending to the level of the eighth tooth, does not reach the splenial bones. The premaxillo-maxillary suture on the palate is not transverse, but extends backwards. In conformity with the length of the snout the maxillaries meet in the dorso-medial line behind the nasal opening, thus excluding the nasals from the latter. The nuchal scutes consist of two large pairs, almost in contact with the dorsals, six of which form the principal longitudinal rows. The gular and ventral scutes ossify in the adult, hence the specific name. The fingers and toes are slightly webbed. General colour above, dark olive-brown; yellowish below. The young are olive with large black spots.
The natives of the Lower Congo catch the crocodiles with two pointed sticks tied together cross-wise, surrounded with entrails by way of a bait. The whole is fastened to a pole or a strong rope and thrown into the river; and a narrow line, with a float {466}attached to the cross-sticks, indicates the whereabouts of the crocodile when it has taken the bait and has sunk to the bottom.
C. johnstoni, of Northern Australia and Northern Queensland, and C. intermedius, of the Orinoko, are allied to C. cataphractus, at least so far as the configuration of the bones of the slender and long snout is concerned. The former is small, scarcely reaching the length of 7 feet, while the South American species grows to 13 feet.
C. americanus s. acutus.–This species, which inhabits the West Indian Islands, being there the only representative of the order, occurs also in Florida, and extends through the warmer parts of Central America into Venezuela, Colombia, and Ecuador. Its characteristic feature is a median ridge or swelling on the snout. The length and relative width of the latter varies considerably. The maxillaries sometimes meet dorsally, or they remain separated by the narrow nasals, which in this case reach the posterior corner of the nasal groove. The nuchal scutes vary likewise; there being often a smaller pair on the side of and another behind the four principal scutes, which, as usual, form a square. A transverse row of little suboccipital scutes is also common. Largest size about 12 feet long.
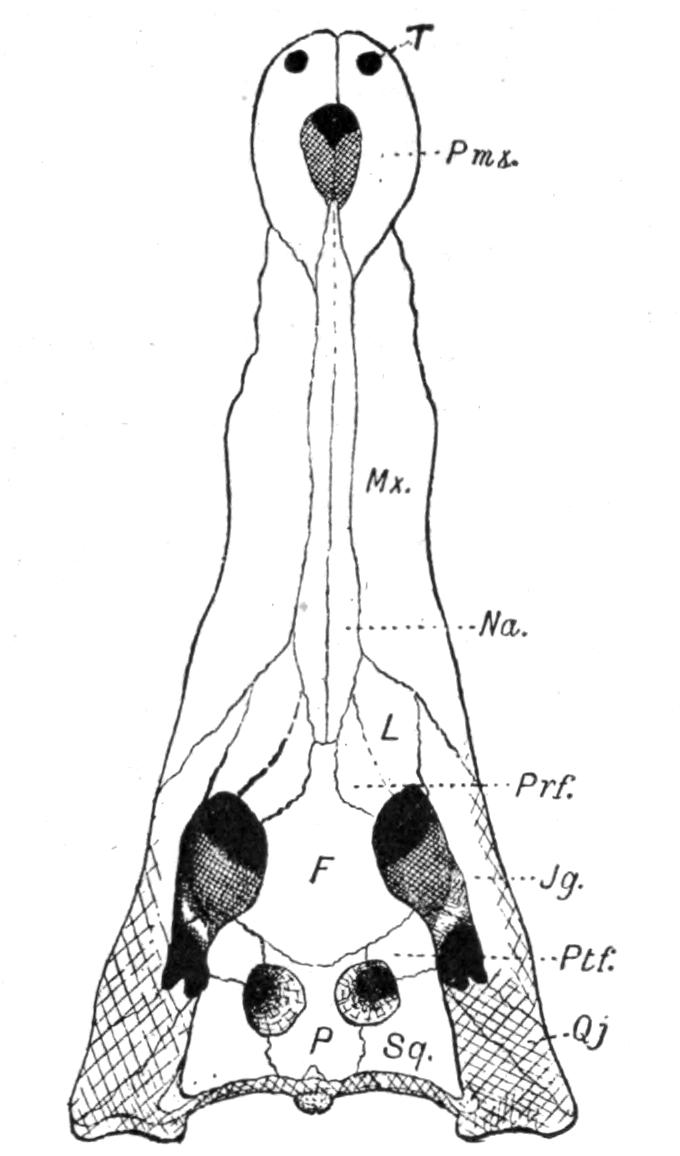
Fig. 111.–Dorsal view of the skull of Crocodilus americanus. × ⅙. F, Frontal; Jg, jugal; L, lacrymal; Mx, maxillary; Na, nasal; P, parietal; Pmx, premaxillary; Prf, prefrontal; Ptf, postfrontal; Qj, quadrato-jugal; Sq, squamosal; T, tooth-perforation.
Osteolaemus tetraspis s. frontatus.–The only species of this genus inhabits the rivers of the west coast of Africa, from Sierra Leone to the Ogowai. It differs from Crocodilus chiefly by the bony septum of the nasal groove, produced by forward extension of the nasal bones. The snout is rather short and stout; the upper surface very rugose and deeply pitted, but without ridges. The gular and ventral scutes are ossified, hence the generic name. Total length about 5 feet.
Alligator.–The fourth mandibular tooth fits into a pit in {467}the upper jaw, and this pit is in some adult specimens transformed into a hole, the tip of the tooth appearing on the upper surface through the perforation. Most of the other teeth of the lower jaw are overlapped by those of the upper jaw. The number of teeth on either side amounts to seventeen to twenty in the upper and eighteen to twenty in the lower jaw. The nasal bones form not only the posterior border of the nasal groove, but they divide the latter by a median bony septum. The dorsal shield is formed by six or eight longitudinal series of keeled bony scutes, which, although standing close together, do not articulate with each other. Ossification of the gular and ventral scutes is absent or very slight.
Alligators occur in the fluviatile deposits of the age of the Upper Chalk in Europe, where they did not die out until the Pliocene age; they are now restricted to two species, one in the Southern States of North America, the other in China.
A. mississippiensis.–The much-depressed and broadly rounded snout bears some resemblance to that of a pike, hence the now discarded specific name of lucius. The neck is protected by two pairs of large scutes, which form a square, interrupted in the middle line, with a pair of small scutes in front and another behind. Of the eighteen transverse dorsal rows of scutes eight are broad and prominent. The fingers are about half webbed, the outer toes about two-thirds webbed. The general colour is greenish black or dark brown above, yellowish below. Young specimens have yellowish cross-bands on a darker brown ground.
The Alligator's northern limit is the mouth of the river Neuss in North Carolina, 35° N. lat. From this point they abound near the mouths of all the creeks and rivers as far south as the Rio Grande, ascending the Mississippi to the entrance of the Red River in 33° 50' N. lat.
The habits and the embryology of the American Alligator have been described by S. F. Clarke,[143] who gives the following vivid and minute account:–
"Usually one finds them in the waters of the smaller streams and ponds, lying with only the tip of the nose and the eyes exposed, or lying on an exposed place on the bank where the grass and other plants are beaten down, and the black, rich mud of the river bank is smoothed by the repeated movements of the {468}alligators in climbing up and down. There they bask in the sunlight until disturbed by the hunter or the desire for food. When aroused they make for the bottom, and I have never waited long enough to see one return unless he were vigorously stimulated with a long pole. They frequently dig a cave for themselves in the bottom of the pond or stream, or in the bank beneath the water. Oftentimes one can start them out of the cave by using a pole, but if very obstinate, the hunters dig them out with spades.
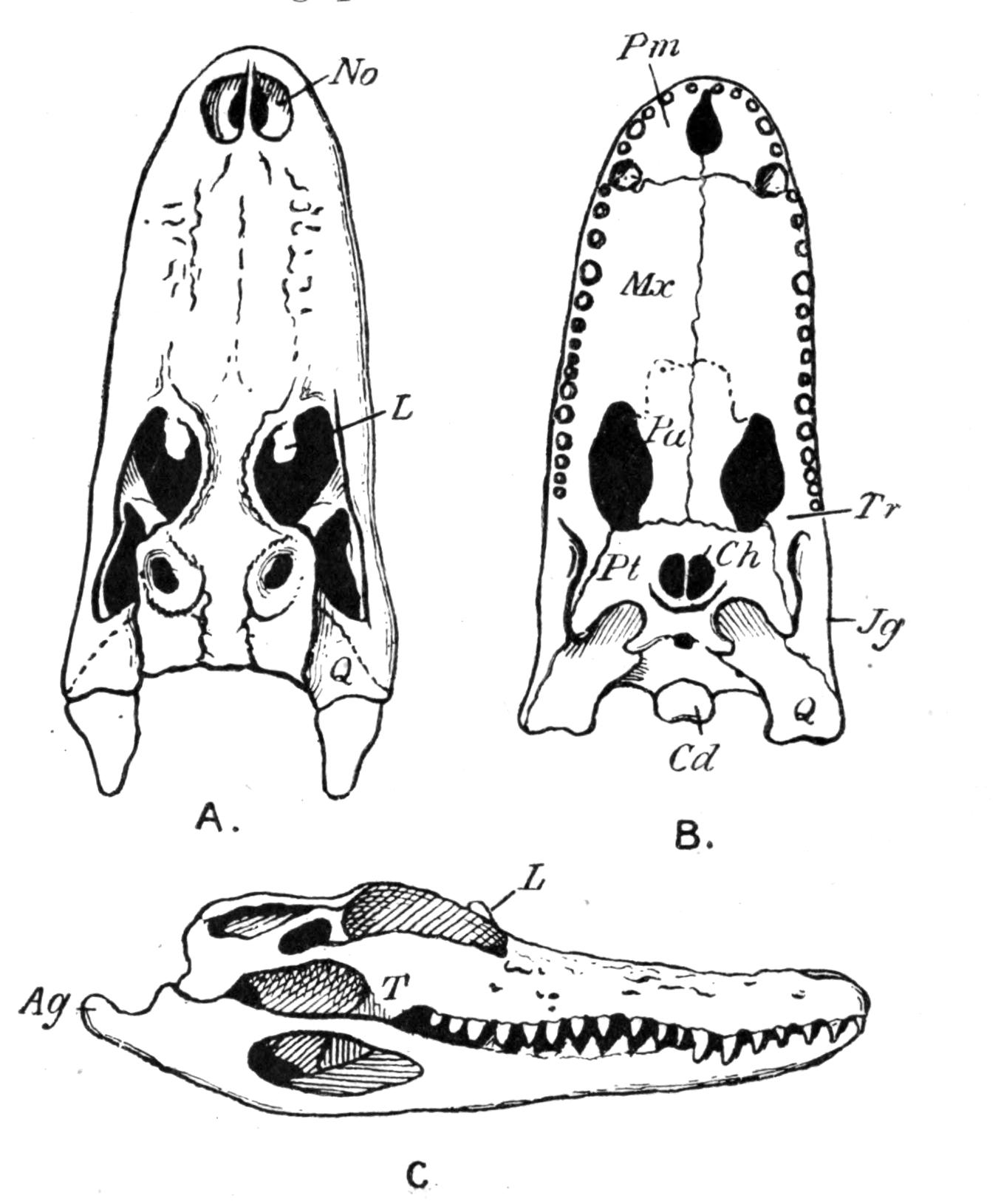
Fig. 112.–Skull of Alligator mississippiensis. A, Dorsal; B, ventral; C, lateral view. Ag, Angular bone of mandible; Cd, occipital condyle; Ch, choanae or posterior narial openings–the median small hole behind them indicates the position of the opening of the Eustachian tubes; Jg, jugal; L, lacrymal; Mx, maxillary; No, nostrils; Pa, palatine; Pm, premaxillary; Pt, pterygoid; Q, quadrate; T, Tr, transverse bone or ectopterygoid.
"As the water decreases in the streams and ponds with the summer heat, the alligators travel to the larger bodies of water. {469}During the breeding season, from the end of May to the beginning of July, the males are very active, wandering about to various ponds and rivers in search of the females. Fierce battles are said to take place during this time between the excited males; and the mutilated specimens that one sees are weighty evidence for the truth of this assertion.... It is in the breeding season also that their bellowing is mostly heard, and more in the night than during the day. I have frequently heard them, while lying in the swamps at night, when they were in ponds fully a mile distant.
"The largest specimen I saw measured 12 feet in length; and none of the many hunters and other natives of Florida I have met have seen any longer than 13 feet. All the hunters agree that it is only the males that acquire the great size; no one had ever seen a female that measured over 8 feet, and the majority are not over seven.
"The male has a heavier, more powerful head, and during the breeding season especially is more brilliantly coloured. The more brilliant colour occurs in patches and streaks on the sides of the head and body; it is generally a light yellow, or even whitish, and on one large male I saw a fairly bright red spot over each eye.
"The alligators are rapidly diminishing in numbers under the stimulus of the high prices offered to the hunters for their hides. Both Whites and Indians make increasing war upon them. Several thousand skins were brought into the little station of Fort Pierce in 1890. The pioneers and settlers always destroy the nests and eggs, because the alligators eat their pigs; and the cleaned eggs and young alligators are sold by hundreds in the curio shops farther north. As their numbers diminish in Florida it is noticed that the Moccasin snakes increase. In Louisiana also the alligators are disappearing; and there the musk-rats are at the same time increasing, and are doing much damage by burrowing in the levees along the Mississippi. While the alligator can make a very stout fight, I have never seen one offer fight if there was any chance of retreat. They never offered to molest us, even when we waded through the ponds where they were.
"The nest of the alligator is very large, and is built by the female. A great quantity of dead leaves and twigs, together with {470}much of the finely divided humus underlying them, is scraped together into a low mound about 3 feet high; this varies considerably in its other dimensions, being in some instances 8 feet in diameter at the base. The nests are built on the bank of a stream or pool, and the female digs a cave under water in the bank close to the nest. Careful examination, of the largest nest found showed a root of a neighbouring palmetto-tree, nearly an inch in diameter, running through it at about a foot above the ground; there were also roots of a grape vine growing near, which extended nearly through the nest. This furnishes strong support to the statement of many of the hunters, that the nests are used for more than one season. I could get no evidence whatever that the nests are used more than once a year.
"The eggs are laid near the top of the nest, within 8 inches of the surface, are four or five layers deep, and have no regular arrangement or uniform position of their axes in relation to the nest. The number of eggs to a nest varies from twenty to thirty, and averages twenty-eight; the maximum found was forty-seven.
The eggs are white, elliptical, and vary in length from 50 to 90 mm. or 2 to 3½ inches, and in the shorter diameter from 28 to 45 mm. Generally there is only slight variation in the eggs of one nest, but occasionally a nest is found in which most of the eggs are about the average size, while from two to five are very much smaller.
"The shell is much rougher than that of a hen's egg, and much thicker. The shell membrane consists of an outer and an inner layer, in both of which the fibres are arranged spirally about the egg, but at right angles to one another.
"The white of the egg has the consistency of a very thick jelly, is very clear and transparent, and is so firm that the whole egg, when perfectly fresh, may be turned out of the shell and shell membrane, and transferred from one hand to the other without breaking, and with but slight change of form. The white lies mostly at either end of the shell, but extends also in a thin layer between the yolk and the sides of the shell. The yolk holds a median position in the egg, is spherical, of a very light pale yellow, and so large that it almost touches the shell membrane about the midline."
According to Holbrook the young as soon as they are disengaged from the shell seek the water and shift for themselves, {471}the parents taking no care of them, though they may remain for some weeks in the same locality. In the spring and early summer months, and during the time of incubation, and especially on cloudy days or in the evening, alligators make a great noise; their croak is not unlike that of the bullfrog, but louder and less prolonged. On the approach of winter they select holes in the ground, where they remain torpid until spring. In this state of hibernation many are dug out by the negroes, who esteem the tail as an article of food.
A. sinensis.–The first intimation of the existence of a Crocodilian in the Yang-tse-kiang was made by Swinhoe in 1870, but it was not until nine years later that Fauvel[144] described the creature as A. sinensis. The same gentleman gave also an exhaustive account of the former records of this species in Chinese literature. According to Boulenger its nearest ally is A. mississippiensis, but it approaches the Caimans by the presence of ossifications in the ventral shields, which ossifications are, however, wide apart from each other. There are three pairs of large nuchal scutes in contact in the median line, besides smaller scutes in front of the nuchals and behind the occiput. The dorsal shield contains six rows of larger scutes. The fingers are not webbed. The general colour is greenish black above, speckled with yellow; greyish below. Total length only about six feet.
Caiman.–The five species of this genus, confined to Central America or to the East Andesian parts of South America, resemble the Alligators in most features, but differ from them in the following points. The nasals, although bordering the nasal groove, do not form a bony nasal septum. The supratemporal fossae are very small; or closed up, as in C. trigonatus and C. palpebrosus of Guiana. The ventral armour is composed of overlapping bony scutes, each of which is formed of two parts united by a suture.
C. sclerops has the widest distribution, from Southern Mexico to the northern half of Argentina. The upper eyelid is rugose, although only incompletely ossified, and is often more or less produced into a small horn. C. niger has flat upper eyelids.
According to Bates, Caimans exist in myriads in the waters of the Upper Amazons. One species, C. trigonatus, the Jacaré-tinga {472}of the natives, reaches only six feet in length and has a slender muzzle and a black-banded tail. Another species, C. niger, the Jacaré-nassu or large Caiman, attains an enormous bulk and a length of 20 feet. They migrate annually, retreating to the flooded forests in the wet season and descending to the main rivers in the dry season.
PLESIOSAURIA–ICHTHYOSAURIA–PTEROSAURIA–PYTHONOMORPHA
Sub-Class VII.–PLESIOSAURIA.
Mesozoic aquatic reptiles, with two pairs of pentadactyle limbs, firmly fixed quadrate bones, single temporal arches, numerous alveolar teeth, and ribs which articulate only with the centra of the biconcave vertebrae.
The Plesiosauria comprise the Mesosauri, Nothosauri, and Plesiosauri in an ascending order of development, which concerns especially the changes from a semi-terrestrial to an absolutely aquatic life;–elongation of the neck with corresponding shortening of the tail, and the gradual transformation of the limbs into hyperphalangeal paddles.
The skull varies considerably in length. Seen from above it shows the nostrils, orbits, very large supratemporal foramina, and the interparietal hole. The nostrils lie rather far back, in front of the orbits, between the elongated premaxillaries, short nasals, and the usually large maxillaries. The orbits are rather small, bordered behind by the postfrontals and postorbitals, which two bones fuse together in the Plesiosauri. The temporal bridge is long, and is formed by the junction of the two bones just mentioned with the squamosal mass, which overlaps the greater portion of the quadrate, and perhaps contains the quadrato-jugal. The dorsal branch of the squamosal joins a corresponding diverging branch of the parietal, and completely shuts off the posterior region of the supratemporal foramen. The interparietal hole is small and placed far back. The palate possesses a row of teeth on the pterygoids in Lariosaurus. The choanae open separately between the vomers and maxillaries. The pterygoids are very long; posteriorly they join the quadrates, anteriorly they extend {474}right up to the vomers, separating the palatines from each other thereby. Palatal vacuities are absent in Nothosaurus; small and oval, between the palatines, pterygoids, ectopterygoids and maxillaries in Lariosaurus; still smaller in the Plesiosauri.
The vertebrae are mostly biconcave, in the Triassic genera still perforated by the chorda, while in many Plesiosauri the centra are solid, with almost plane articulating surfaces. The neural arches are usually firmly sutured, or quite fused with the centra. Intercentra are absent, except as chevrons in the tail. Although the cervical and some of the thoracic ribs of the Triassic genera have typical capitula and tubercula, they articulate exclusively upon the centra, and not upon the neural arches also. The number of cervical vertebrae amounts to nine in Mesosaurus; in Lariosaurus it is increased to about twenty; and in some Plesiosauri to between thirty and forty. The cervical ribs are very short, but they increase gradually towards the thorax, which is well protected by long and strong ribs, which decrease again very gradually, being still long in the lumbar region. There is, properly speaking, no sacrum, because the one to four sacral ribs remain quite separate. The tail is still long in Lariosaurus, consisting of about forty much shortened vertebrae; considerably shorter than the neck in most of the Plesiosauri. A sternum is absent, but the belly is protected by many strong abdominal ribs, crowded together, and consisting each of a median and two pairs of lateral pieces.
The shoulder-girdle is very strong, composed of scapulae, very strong coracoids, clavicles, and an interclavicle. The precoracoids are indicated by a process and a notch in the Triassic genera; in the later forms they are abolished. The coracoids always meet in the median line, and often produce a strong symphysis. The scapulae possess a very prominent and large acromial process, upon which rest the dorsal or lateral ends of the clavicles. In some Plesiosauri the shoulder-girdle has undergone an absolutely unique modification. The correct interpretation has been given by C. W. Andrews after the examination of exquisitely preserved specimens of Cryptoclidus from the Oxford clay of the Middle Oolite, near Peterborough. The dorsal portion or main shaft of the scapula is reduced to what now looks like a dorso-lateral process, while the broad acromial process is much elongated, and lies in Plesiosaurus upon the {475}ventral surface of the clavicle; the latter and the irregularly T-shaped interclavicle being, however, still visible from below. In Cryptoclidus the two acromial processes meet each other and form a long ventral symphysis, which meets that of the much-enlarged coracoids, the latter enclosing with the scapulae a pair of roundish foramina. The clavicles are not visible from below; they rest upon the dorsal surface of the scapular symphysis, and the interclavicle seems to be suppressed. Young Cryptoclidus (Fig. 113, B) and various species of Plesiosaurus show intermediate conditions.
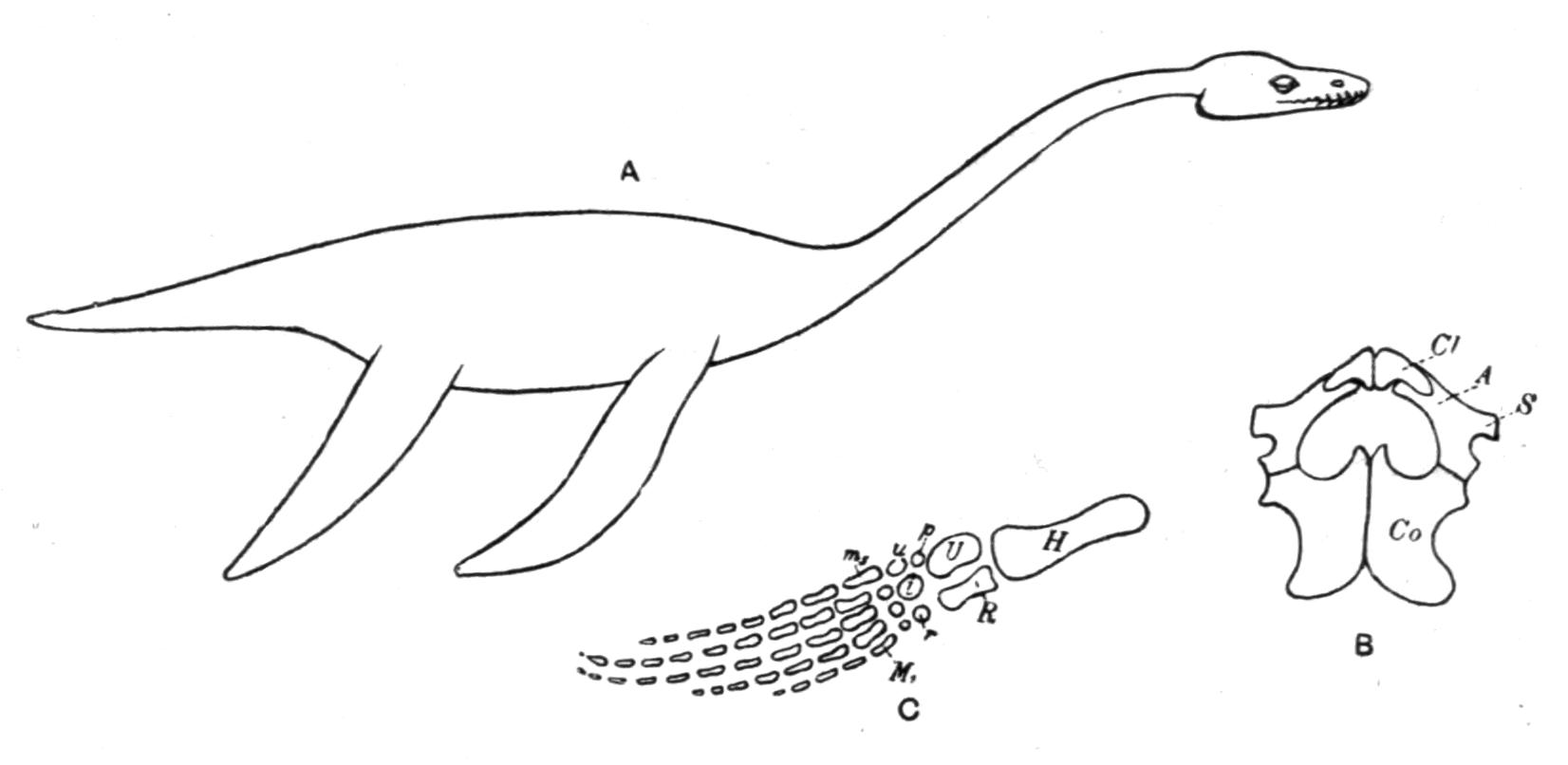
Fig. 113.–A, Restored outlines of a Plesiosaurus, × 1⁄50; B, dorsal view of the pectoral arch of an immature Cryptoclidus, from the middle Oolite; C, fore-limb of a Plesiosaurus, from the Lias. A, Acromial process of scapula; Cl, clavicle; Co, coracoid; H, humerus; i, carpale intermedium; M1 to m5 first to fifth metacarpals; p, pisiform bone; R, radius; r, radial carpal; S, scapula; U, ulna; u, ulnar carpal.
This unique arrangement is correlated with the enormous development of the fore-limbs, although nothing of the kind has taken place in the Ichthyosauri, which have similar large paddles. The limbs exhibit considerable differences in the various groups of Plesiosauria, but they are all pentadactyle. In the oldest, the Mesosauri and Nothosauri, the limbs are still of the terrestrial type, although fitted for swimming; the chief bones are still slender and elongated, and none of the five fingers and toes have more than five phalanges, the usual number of which seems to be 2, 3, 4, 5, 3 for the first to fifth digits respectively. In the Plesiosauri the limbs are transformed into long hyperphalangeal paddles, unfit for progression on land, rather like those of the Ichthyosauria, with much {476}shortened radius and ulna, tibia and fibula; but the phalanges, which increase to about ten, are always longer than broad, and there is no indication of an increase of the number of fingers, or of additional, lateral, phalanx-like nodules. The pelvis is very strong; the broad pubes and ischia meet in the middle line, and they either enclose one wide undivided foramen, or the two symphysial portions meet, and there are then two obturator-foramina. The pubes are generally much larger, especially broader, than the ischia; and although partaking in the formation of the acetabulum, they do not articulate with the ilia, at least not in Plesiosauri. The ilia are always small; in Plesiosauri attached to only one or two sacral ribs; to three or four in the Triassic genera.
Ichthyosauri and Plesiosauri were combined as "Enaliosauria" by Conybeare. Owen recognised their fundamental differences, and separated them as "Ichthyopterygia" and "Sauropterygia," according to the structure of the limbs. We now know that the paddles of the Ichthyosauri bear but a superficial resemblance to the fins of fishes, and are fundamentally referable to the pentadactyle type, as are the paddles of the Plesiosauri, although the latter retain more of the typical features of reptilian limbs. It was soon recognised that the Nothosauri are allied to the Plesiosauri, but the Mesosauri (until then vaguely grouped with the Rhynchocephalia, or linked with Protorosauri as Proganosauria) have only recently[145] received their proper place in the system as members of the Plesiosauria, which we divide into two main groups.
Order I. NOTHOSAURI.[146]
The limbs are of the terrestrial type; the five digits have the usual number of phalanges, which do not exceed five. The bones of the limbs are slender; the humerus has an entepicondylar foramen.
Fam. 1. Mesosauridae.–The neck contains about ten vertebrae. The vertebrae are deeply biconcave, perforated by the chorda dorsalis. Sacral vertebrae four in number. Clavicles strong; interclavicle very small. Mesosaurus, the only genus, with one species, M. tenuidens, about one foot in length, was found in {477}South Africa, probably in Triassic sandstone. Very similar specimens are known from São Paolo in Brazil.
Fam. 2. Nothosauridae.–With sixteen to twenty-one cervical and three to five sacral vertebrae. The vertebrae are biconcave. The clavicles are strong; the interclavicle is much reduced. Coracoids with distinct acromial processes.
Nothosaurus mirabilis, of the Muschelkalk of Germany. Total length about ten feet. Length of head about one foot. The teeth are very irregular. About five slender, long teeth are implanted in each side of the premaxilla, with wide spaces between them, similar to those of the symphysial portion of the lower jaw. Those of the maxillaries are numerous and small, except two large pairs in the anterior portion, on a level between the orbits and nostrils. The upper and lower teeth overlap, or cross each other. The palate of the long and slender skull is quite bony, without anterior palatal or infra-orbital vacuities.
Lariosaurus balsami, about one foot in length, from the fresh-water deposits of the Upper Trias in Lombardy. Neck with about twenty, tail with about forty vertebrae. Head comparatively shorter; more triangular than in Nothosaurus; palate with small infra-orbital vacuities. The number of the phalanges of the fingers and toes is apparently 2, 3, 4, 4, 3 and 2, 3, 4, 5, 4.
Anarosaurus pumilio, of the Muschelkalk, near Magdeburg, and Neusticosaurus and Simosaurus of the same geological age, are allied forms.
Order II. PLESIOSAURI.
The limbs are transformed into hyperphalangeal paddles. The clavicles are small, and are overlapped ventrally by the strongly developed acromial processes of the scapulae. The vertebrae are slightly biconcave or plane. The neck consists of at least twenty vertebrae; those of the thoracic region have long transverse processes; the sacral vertebrae are mostly reduced to two or one. Very large, massive animals.
Fam. 1. Pliosauridae.–About twenty cervical vertebrae, with proximally bifurcated ribs. The scapulae do not meet ventrally; they enclose with the coracoids a single large foramen, and are fused with the clavicles. Pliosaurus, the principal genus, contains several species of gigantic size; for instance, P. grandis, of the Kimmeridge clay, Upper Oolite, of England, has a skull {478}nearly 5 feet long and 2 feet broad, armed with many enormous conical teeth, some of which reach one foot in length, inclusive of the long collar and root-portion. The neck is rather short, owing to the much condensed, disc-shaped centra of the vertebrae. Total length of this species about 30 feet. Other species in England and continental Europe as far as Russia.
Fam. 2. Plesiosauridae.–The neck is very long, and consists of from twenty-eight to forty vertebrae. The scapulae do not meet ventrally, but the symphysial portion of the coracoids meets the clavicles and the interclavicle, the pectoral arch thus enclosing two foramina. Chief genus Plesiosaurus, with many species. The head is comparatively small, the neck very long, the tail short, although consisting of from thirty to forty vertebrae. The third digit (Fig. 113, C) is the longest, and possesses nine or ten phalanges. The abdominal ribs are very strong, and reach from the pectoral to the pelvic girdle. Range from the Lower Trias to the Lower Oolite, chiefly European. P. dolichodirus and P. conybeari, the latter reaching a total length of more than 15 feet, from the Lower Lias, especially at Lyme Regis.
Fam. 3. Elasmosauridae.–The neck is extremely long, possessing from thirty-five to seventy-two vertebrae, with single-headed, not bifurcated, ribs. The scapulae meet ventrally, and enclose with the very broad coracoids two foramina. The tail is short. The pisiform bone articulates with the humerus. Otherwise much resembling the Plesiosauridae. Principal genus Cimoliasaurus, with many synonyms, and many species from the Middle Oolite to the Upper Chalk; cosmopolitan distribution, e.g. C. cantabrigiensis, of the Greensand and Upper Chalk; C. trochantericus, of the Kimmeridge clay; C. haasti in New Zealand; C. australis, C. chilensis; others in North America. Cryptoclidus of the Middle and Upper Oolite of Europe. Elasmosaurus, of the Upper Cretaceous formation in Kansas, with a computed total length of 45 feet, of which 22 belong to the neck, with its seventy-two vertebrae.
Sub-Class VIII.–ICHTHYOSAURIA.
Marine, whale-shaped reptiles, with the anterior and posterior limbs transformed into hyperphalangeal paddles. Restricted to the Mesozoic age from the Trias to the Upper Chalk.
The skull is long, owing to the elongated slender snout, which {479}is formed mainly by the premaxillary bones. The nostrils lie far back, in front of the orbits, and are bordered by the long nasals, the premaxillaries, a small part of the maxillaries, and posteriorly by the large lacrymal bones. The eyes are large, and are strengthened by a sclerotic ring composed of many closely overlapping bones. The orbits are very large, and are directed sideways so as to be scarcely visible from above. They are formed above by the long prefrontals, which join the postfrontals; behind by the long postorbitals; below by the long and slender jugals; in front by the lacrymals and prefrontals. The postorbito-temporal region of the skull is short but high, and, with the exception of the supratemporal foramen, is entirely closed in by bones, namely, the quadrato-jugals, supratemporals, and squamosals. The latter, with the parietals and large postfrontals, surround the supratemporal foramina. The parietals and the small frontals enclose the parietal foramen. The whole temporal arch consequently recalls much that of the Pareiasauri and Stegocephali, chiefly owing to the presence of conspicuous supratemporal and postorbital bones, which, together with the quadrato-jugal, close in the whole side without any indication of a lateral or infratemporal foramen. The postorbital completely separates the jugal from the quadrato-jugal, and this almost hides the quadrate. The occipital condyle is single. The lateral occipitals and the supra-occipital bones retain their sutures. The pro-otic and opisthotic bones remain separate. The latter lie between the basi- and lateral occipitals, the squamosal, quadrate, and pterygoid. The pterygoids, which posteriorly touch the quadrato-jugals, basi-occipitals, opisthotics, and basisphenoid, are very long and remain widely separated from each other; in the space between them appears the long ensiform presphenoid. Anteriorly they are connected through the ectopterygoids with the maxillae, and touch the palatines. These are likewise narrow and slender, but touch each other in the middle line, and contain the well-separated, slit-like choanae, laterally to which lie the elongated, rather narrow, palatal vacuities. The vomers are mostly not visible; when they appear on the surface they are long and narrow, and enclose the choanae between them and the palatines.
The teeth are pointed, conical and thickly covered with enamel, which in transverse sections forms vertical ridges, recalling {480}the folds of the Labyrinthodonts. The teeth have open roots, and are not implanted in separate alveoli, but lie in long grooves of the premaxillaries, maxillaries, and dentals.
The vertebrae are numerous, up to 150, two-thirds of which belong to the tail. The centra are deeply biconcave and short, not co-ossified with the neural arches, which have therefore often broken loose. The atlas much resembles the other cervical vertebrae in so far as its centrum is concave in front and scarcely ankylosed with that of the second. Its basiventrals, equivalent to the ventral half of the atlas-ring of other reptiles, thus become an unpaired intercentral wedge, between the first centrum and the basis of the cranium; the neural arches rest upon the centrum, but remain separate from each other, or at least diverge dorsally. The atlas carries no ribs. Intercentra occur also between the second and third vertebrae; they reappear in the tail as chevron-bones. All the other vertebrae carry ribs, which gradually increase in length towards the trunk and decrease again equally gradually on the tail. In the neck and trunk they have separate capitula and tubercula, which articulate upon short knobs of the centra; towards the tail these shift farther and farther towards the ventral side, and ultimately unite. Although the ribs of the trunk are so long, there is no trace of a sternum, but there are many "abdominal ribs" crowded together, each consisting of a middle and a pair of lateral pieces.
The shoulder-girdle is very complete, but the pieces remain separate, or at least do not co-ossify; it consists of a T-shaped interclavicle, clavicles, broad coracoids touching each other in the middle line, and short scapulae. The existence of small separate precoracoids is doubtful. The pelvis is much reduced; the small ilium is quite unconnected with any vertebrae; the small pubes and ischia form no symphyses. The fore- and hind-limbs are very similar to each other; the posterior are, however, much smaller. Both are transformed into highly specialised paddles. It is of the greatest importance, as an indication that the Ichthyosauri are descendants of a terrestrial stock, and have been modified into what they are owing to having taken to marine life, that in the oldest members known, the paddle-like structure of the limbs was less advanced than in the later species. In Mixosaurus of the Muschelkalk of Europe the ulna and radius are still distinctly longer than broad, and they enclose a space {481}between them. They articulate with three carpal bones, the ulnare, intermedium, and radiale, while a small pisiform bone lies on the outer side, between the ulnare and the outer distal carpal bone. In Ichthyosaurus, from the Liassic period onwards, the ulna and radius are much shortened, broader than long, and touch each other without any intervening space; the pisiform element is enlarged. Lastly, in Ophthalmosaurus of the Middle Oolite (but not in contemporary species of Ichthyosaurus) the ulna and radius are still more reduced, and the pisiform has moved up to the humerus, so that the latter articulates with three bones.
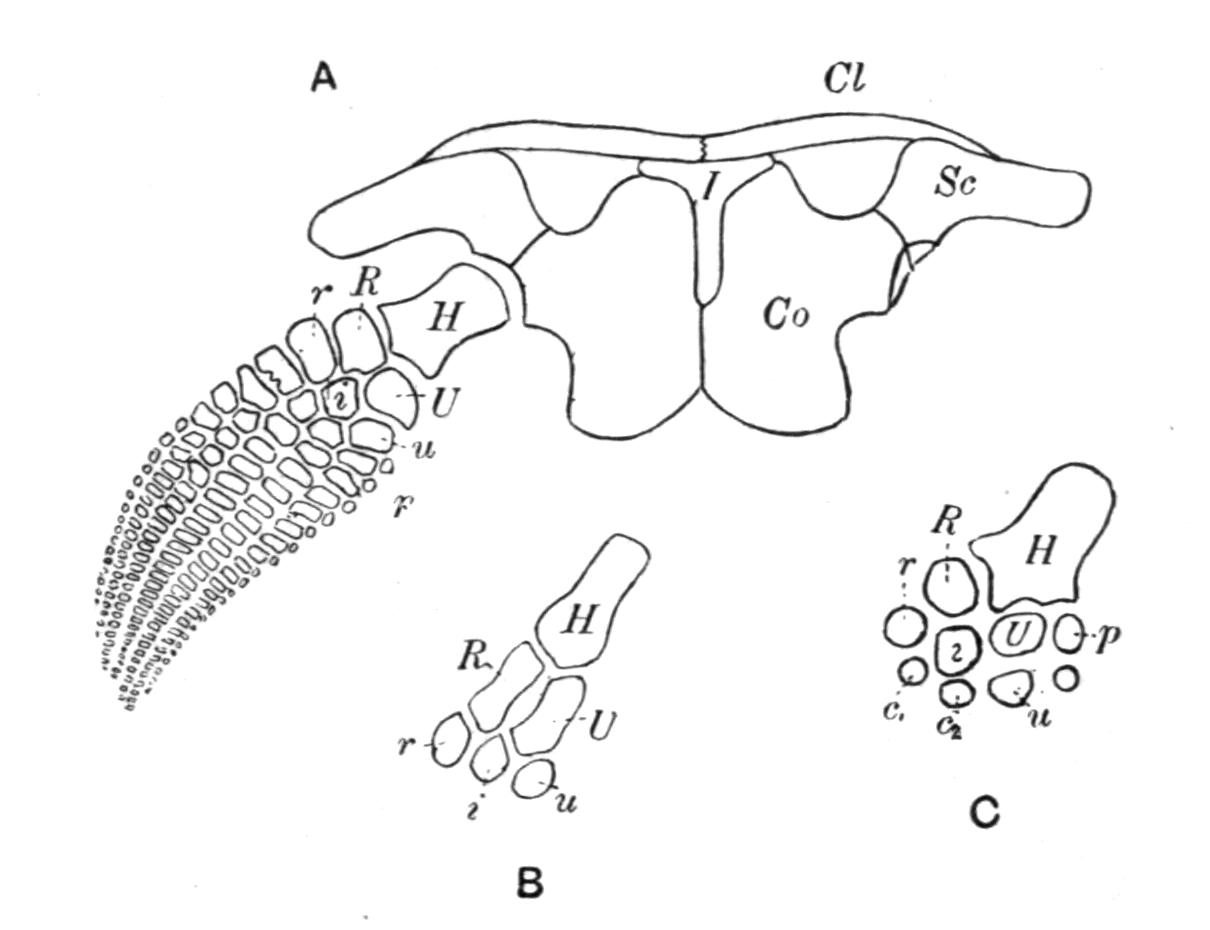
Fig. 114.–A, Ventral view of the shoulder-girdle and right fore-limb of an Ichthyosaurus, from the Lias; B, part of the fore-limb of a Mixosaurus, from the Trias; C, part of the fore-limb of an Ophthalmosaurus, from the Chalk. c1, c2, first and second centrale carpi; Cl, clavicle; Co, coracoid; H, humerus; I, interclavicle; i, intermedium carpi; p, pisiform; R, radius; r, radial carpal; Sc, scapula; U, ulna; u, ulnar carpal.
Other important features of these paddles are not only the much-increased number of phalanges (sometimes up to twenty or more), but also the increase of digits to six or more, produced apparently by a splitting of the third finger into two series, and by the development of additional rows of phalanx-like bones on the outer and inner margins of the paddle. This increase of fingers exists, for instance, in Ichthyosaurus communis, but not in I. tenuirostris. Owing to this peculiar development of paddles the constituent bones are extremely numerous, and from the radius and ulna downwards they are all closely packed, and have assumed a polygonal, often hexagonal, shape, dwindling to more or {482}less flattened nodules towards the ends of the digits. These carpal and phalangeal bones are common objects in amateurs' collections; they fit together by the short angular facets, while the two flat and broader surfaces are those of the dorsal and ventral sides.
The Ichthyosaurs lived upon fishes and cuttlefish, as is indicated by their dentition and the shape of the snout, and proved by the coprolites, most of which are full of fragments of bones and ganoid scales of fishes, and of the beaks and shells of cuttlefish; the larger of these true coprolites (literally "petrified dung"), in coprolite-beds, contain also an abundance of other fossils, such as Ammonites, Terebratulae, molluscs and fish-remains; they are several inches long, and many of them show on the outside ring-like impressions, undoubtedly caused by a spiral valve of the intestinal canal. In conformity with their absolutely aquatic life the Ichthyosaurs were viviparous. Several well-preserved adult specimens have been found, which contain the skeletons of one or more rather large young within the body, in exactly the position in which such foetal creatures would lie, namely, with the head in the pelvic region of the mother, while the rest of the body stretches along the vertebral column towards the chest. The suggestion that these young Ichthyosaurs have been swallowed by their cannibal elders is too idle to require serious refutation.
Until within a few years Ichthyosaurs were always restored with a smooth and even back, but several well-preserved specimens have come to light in Würtemberg which show the complete contour of the animals, with a long, somewhat jagged fin on the middle of the back. Since then not a few specimens in various collections have on closer examination revealed the same feature, except, of course, those in which the outlines of the fin had been chiselled away in order to "improve" the look of the slab. The fins were undoubtedly of the "adipose" kind;–raised folds of the skin. The latter is now known to have been covered, at least at the bases of the dorsal fins, with hard little scales, probably osteoderms.
Many specimens are beautifully preserved, others present a very peculiar appearance. They look, namely, like long rolls of clay, and nobody but an expert would suspect an Ichthyosaurus within such a log. The explanation is simple. The dead {483}creature was rolled about by the waves of the surf on the Liassic muddy beach until it was wrapped in a mantle of clay and then imbedded on the shore.
The distribution of Ichthyosaurs in time and space is wide. The earliest are found in the Middle Trias; in the Lias they are very common, fairly frequent in the Oolites, dying out with the Cretaceous epoch. They have left no descendants, being far too specialised, and their origin is quite unknown. Mixosaurus, the oldest genus, occurred in Europe, and has also been found in the Triassic strata of Spitsbergen. Ichthyosaurus, the chief genus, is known from the Liassic, Oolite, and Cretaceous strata of Europe, a famous place being Lyme Regis; and also from the Cretaceous strata of Queensland and New Zealand. The Jurassic of Wyoming has yielded Baptanodon.
Order ICHTHYOSAURI.
The few genera are easily recognised.
Mixosaurus, Triassic, with radius and ulna still elongated, a longitudinal space occurring between them. Both jaws with numerous uniform teeth.
Ichthyosaurus, with much shortened radius and ulna; both jaws with uniform series of teeth. Many species are known, some with four to five, others with several additional and incomplete rows of fingers and toes. I. trigonodon of the Lias in Würtemberg seems to have reached the size of 30 feet, the vertebrae showing a diameter of 9 inches, while the skull is 6 feet long. I. communis and I. tenuirostris are common in the English Lias. The long-snouted I. campylodon, with large, spaced teeth, occurs in the Gault of Cambridge, Dover, and France; and {484}there are many others. Ophthalmosaurus, of the Upper Oolitic and Cretaceous formations of England, had very small vestigial teeth.
Baptanodon, of the Upper Jurassic epoch of Wyoming, was toothless, and was one of the six-toed forms.
Sub-Class IX.–PTEROSAURIA.
Mesozoic reptiles with fixed quadrate bones and with the anterior limbs transformed into wings, the enormously elongated ulnar finger carrying a patagium.
The skull bears a superficial resemblance to that of Birds. It articulates with the neck by a single condyle, at nearly a right angle. The interparietal foramen is absent, but there are five pairs of foramina on the surface of the skull, namely, the nostrils, orbits, supra- and infra-temporal and pre-orbital foramina. Most of the constituent bones of the cranium fuse with each other, and the composition of the various arches is therefore difficult to make out with certainty. The premaxillaries are fused together, and extend dorsally backwards between the nasals, which themselves diverge towards the prefrontals. The nostrils are bordered chiefly by the maxillaries, nasals, and prefrontals. The orbits are very large, mostly shut off in front from the pre-orbital foramina by a bridge, which is formed by descending processes of the prefrontals and ascending processes of the jugal. Above and behind, the orbits are bordered by the frontals, postfrontals, and possibly the quadrato-jugals. The whole temporal region is shortened from before backwards, but heightened dorso-ventrally, and the whole temporal fossa is divided into a supra- and infra-temporal portion by the junction of the postfrontal with the squamosal, the latter joining the parietal, thus closing the supratemporal fossa behind. This is conspicuous only in the older forms, e.g. Dimorphodon, but is very small in Pterodactylus, and quite abolished in Pteranodon. The infratemporal fossa is a narrow slit, slanting obliquely upwards and backwards, between the quadrate and the quadrato-jugal. A foramen of this kind occurs elsewhere only in the Rhynchocephalia. The quadrate is long, firmly fixed, and slants so far forwards that the mandibular joint lies on a level below the middle of the orbit. The pterygoids articulate with strong and long processes of the basisphenoid, touch the quadrate posteriorly, enclose an interpterygoid vacuity, {485}and extend forwards as slender bones to the vomer, separating the palatines. The choanae are enclosed by the vomer, palatines, and maxillaries, and they lie in dorsal recesses above the level of the roof of the mouth. The teeth are alveolar, pointed, of variable size, and restricted to the jaws; in the Pteranodonts they are absent.
The brain is known from the natural cast of Scaphognathus, and shows some remarkably bird-like features, especially the width of the hemispheres, which touch the well-developed cerebellum, while the optic lobes lie on the sides of the cerebellum, with a pair of appendices, the so-called flocculi, elsewhere known in birds only.
The caudal vertebrae are still amphicoelous, while the presacral vertebrae are procoelous. Abdominal ribs are few in number and are very thin. The true ribs possess capitula and tubercula; those of the neck are very short and directed backwards; in the thoracic region they are long, and some are attached to a broad sternum with a keel and a median anterior process, on the sides of which latter articulate the coracoids. Precoracoids and clavicles are absent. The scapulae are long, sabre-shaped, and turned back as in birds; in Pteranodon they show the unique modification of articulating with special processes of the neural arches of several ankylosed thoracic vertebrae.
The hand possesses only four fingers; the four phalanges of the ulnar finger are very much elongated for the support of the patagium; the other fingers remain short and are provided with little claws. The ilia are expanded horizontally, and are firmly attached to from three to six vertebrae, which mostly fuse together into a sacrum. The ventral half of the pelvis consists of a pair of broad bones, which contain a small obturator-foramen; they form a ventral symphysis, and are usually fused with the ilium. These bones represent the conjoint ischia and pubes, while the so-called pubes, a pair of flat and club-shaped bones, are excluded {486}from the acetabulum. The whole arrangement resembles that of the Crocodilian pelvis. The hind-limbs are bird-like in so far as the fibulae are reduced to splints, and attached to the proximal halves of the long and slender tibiae. The feet contain five separate toes with rather long metatarsals and short claws. Many of the bones are hollow.
The Pterosauria have no relationship with the birds, in spite of the number of apparently striking resemblances (e.g. choanae, pre-orbital foramina, brain, scapula, fibula, cervical vertebrae), which are, however, coincidences, cases of convergence, in conformity with the aerial life. The totally different plan of the wings is sufficient to show this. On the other hand, the real affinities of this group of flying reptiles are unknown. They turn up "fully fledged" in the Lower Lias, and they reach their highest specialisation in the Upper Cretaceous epoch, with which they have died out. In fact we do not know any forms through which to connect them with other extinct reptiles. The skull shows some Rhynchocephalian features; the pelvis, Crocodilian features; and this combination points back a long way.
Order PTEROSAURI.
Sub-Order 1. Pterodactyli, with alveolar teeth in the upper and lower jaws. Imperfect remains, impressions of phalanges of the long patagial finger, are known from the Rhaetic of Würtemberg. The oldest well-known genus is Dimorphodon, Lower Lias of Lyme Regis. D. macronyx.–Total length between 3 and 4 feet, of which the large light skull takes up about 9 inches, and the long thin tail about 2 feet. The patagial finger is about 20 inches, the whole wing about 28 inches long. Rhamphorhynchus longicaudatus of the Upper Oolite of Germany is remarkable for the long slender teeth, which are directed forwards and separated by wide spaces from each other. The nine or ten cervical vertebrae are elongated. R. phyllurus of the same geological age has left impressions of the flying membranes. They extend from the whole length of the wing and the sides of the trunk to the thigh as far as the knee, and from the inside of the hind-limbs to the tail. The end of the tail carries a spatulate membrane. Allied is Ornithocheirus, with many species in the English Wealden and Greensand. {487}Pterodactylus, with many species from the Upper Oolite, chiefly of Germany.–The tail is very short, consisting of a few vertebrae only. The seven neck-vertebrae are so much elongated that the neck is as long as the trunk with the tail. P. longirostris measures about 1 foot in total length, while P. spectabilis is one of the smallest, only of the size of a lark. The wings, however, measure 10 inches from tip to tip. The largest is P. giganteus, with a "spread" of more than 5 feet.
Sub-Order 2. Pteranodontes.–The beak is long, pointed, toothless, and laterally compressed; mandibular symphysis very long. Pteranodon longiceps.–The skull has a long parieto-supraoccipital crest, which extends far back. The supratemporal foramina are abolished. The pre-orbital and orbital foramina are confluent. The scapulae are attached to several thoracic vertebrae. The skull of this gigantic species has a length of two feet and a half, and the spread of the wings measures nearly 20 feet. This, and several much smaller species, are from the Middle Cretaceous formation of Kansas.
Sub-Class X.–PYTHONOMORPHA.
Very long-necked and long-bodied marine Cretaceous reptiles, with movable quadrates, single lateral temporal arches and procoelous vertebrae; with paddle-shaped, pentadactyle limbs; and with the teeth ankylosed to the jaws.
The skull possesses many of the essential features of the typical lizards. The premaxillaries, frontals, and parietals are fused into unpaired bones. There is an interparietal foramen. {488}The nostrils are dorsal, bordered by the premaxillae, nasals, prefrontals, and maxillaries. The quadrato-jugal arch is incomplete, and the orbit is posteriorly confluent with the infratemporal fossa, but a supratemporal space is shut off by the single arch, which is composed of the postfrontal, squamosal, and supratemporal. The latter is interposed between, and connects the squamosal and quadrate with the latero-posterior branch of the parietal. There is a space between this parieto-squamosal arcade and the epi-otic, which is fused with the lateral wing of the lateral occipital bone. The foramen magnum is bordered by the two supra-occipital, lateral occipital, and the unpaired basioccipital bones; the condyle is triple. The quadrate is movable, articulating with the squamosal and laterally expanded epi-otic. There is no bony connexion of the quadrate with the jugal, which is restricted to its anterior half, and attached to the maxillary and lacrymal. The quadrato-jugal is absent as a separate bone; it is probably fused with the anterior surface of the quadrate, as indicated by a perforation of the quadrate, resembling in this respect the Rhynchocephalia. The vomers are long, and separate the elongated choanae from each other. The palatines separate the vomers from the pterygoids, which enclose a long median vacuity and are not connected with the quadrates. The teeth are conical, and stand near the inner margin of the jaws upon little prominences, with which they fuse. Some genera have teeth upon the pterygoids also.
The vertebrae are very numerous and are mostly procoelous. They are noteworthy for the possession of an additional anterior and a posterior pair of articulating processes on the neural arches, homologous with the zygosphenes and zygantra of Snakes and Iguanidae (see p. 582). Intercentra are absent, except in the tail. The ribs have no tubercula, and articulate with the centra of the vertebrae to which they belong.
The pectoral arch is strong. The scapulae are short and broad; the coracoids, fused with the precoracoids, except for a notch, are flat and broad, and meet ventrally; posteriorly they articulate upon the anterior margin of the flat sternum, to the lateral margin of which are attached several ribs. Clavicles and interclavicle seem to be absent. Abdominal ribs are likewise absent. The pelvic girdle is feeble; the ilia, ischia, and pubes are loosely connected with each other, the pairs of ventral elements {489}meeting also in the middle line. The ilia are loosely attached to two vertebrae in the Dolichosauri; in the Mosasauri they have lost this connexion. Both anterior and posterior limbs are transformed into pentadactyle paddles, with much shortened and broadened bones of the arms and legs. The digits are to a certain extent hyperphalangeal, since several of them possess five phalanges.
The Pythonomorpha are undoubtedly allied to the Sauria, but they are certainly not their ancestors, since typical Autosauri occur in the Lower Chalk; nor are the Snakes their descendants, in spite of many convergent resemblances. We consider them to be the marine collateral branch of the Sauria, which rapidly developed highly specialised, often very large forms, restricted to the Cretaceous epoch, with a wide, cosmopolitan distribution.
Order I. DOLICHOSAURI.
This older group is characterised by the sutural symphysial connexion of the two mandibles and by the possession of two sacral vertebrae. The body is snake-like. Pleurodont. Dolichosaurus longicollis of the Lower Chalk of Kent and Sussex; total length about 3 feet, with about seventeen cervical vertebrae and pleurodont teeth. Acteosaurus of Istria; anterior extremities distinctly shorter than the posterior pair; tail long. Vertebrae, like those of Dolichosaurus, with zygosphenes. Plioplatecarpus of the Upper Chalk of Holland has a slender interclavicle; the vertebrae are without zygosphenes, but those of the cervical region possess a downwardly directed long hypapophysial process with a separately ossified epiphysis.
Order II. MOSASAURI.
The two halves of the lower jaw are connected by ligament and are therefore movable as in Snakes. There are no sacral vertebrae, the pelvis having lost its connexion with the vertebral column. The formation of the limbs into paddles is more pronounced than in the Dolichosauri.
Mosasaurus, the chief genus, so called from Mosa, the Latin name of the river Maas, with several species from the Upper Cretaceous strata of the Netherlands, England, and North {490}America. M. camperi, from Belgium, with a skull about 4 feet in length, armed with many large, curved, acrodont teeth. The vertebral column consists of about one hundred caudal and thirty-four precaudal vertebrae, of which seven are cervical, without zygosphenes. The total length of the type-specimen is estimated at 25 feet.
Platecarpus of North America and New Zealand, and various other North American genera, also contained species of large size.
Liodon.–Premaxilla without teeth, the others nearly smooth instead of being ridged. With a very wide distribution in the Chalk of Europe, North America, and New Zealand. L. haumuriensis of New Zealand seems to have been the giant amongst these monstrous marine creatures; its total length has been computed from imperfect fragments at 100 feet.
Clidastes, of the Upper Cretaceous of North America and Europe, although not so massive, comprises the most elongated forms. The cervical vertebrae possess long median hypapophyses with separate epiphyses; most of the vertebrae are much elongated and have well-developed zygosphenes. C. tortor had a skull nearly two feet and a half long.
SAURIA–AUTOSAURI OR LACERTILIA–LIZARDS
Sub-Class XI.–SAURIA.
Reptiles with movable quadrate bones, with a transverse, external, cloacal opening, near the posterior lateral corners of which open the eversible, paired (right and left) copulatory organs.
The Sauria, which comprise the Autosauri or Lacertilia in the wider sense and the Ophidia or Snakes, are the most recently developed groups of Reptiles. No fossils are known from strata earlier than those of the Cretaceous epoch. Their origin has probably to be looked for among the Prosauria, of which Sphenodon, cf. p. 294, is the only surviving member. The Sauria have attained their great development within the Tertiary period. They, both Autosauri and Ophidia, are now the two dominant Reptilian groups, and they have, so to speak, a future before them, being apparently still on the increase in numbers and species, but certainly not in size.
Order I. AUTOSAURI or LACERTILIA–LIZARDS.
Saurians which have the right and left halves of the mandibles connected by a sutural symphysis.
The overwhelming majority possess well-developed limbs, movable eyelids and cutaneous scales, covered by the mostly thin and horny epidermis. But there are many kinds of Autosauri, especially those belonging to the degraded, burrowing families, which have lost not only one or both pairs of limbs, but even the limb-girdles, while the eyes have become concealed beneath the skin, and in some cases the scales have been lost, or reduced {492}to mere vestiges. Moreover in some of these burrowing and limbless forms the quadrate bones have become more or less immovable.
We divide the Autosauri into three sub-orders:–I. Geckones, p. 502; II. Lacertae, p. 513; III. Chamaeleontes, p. 567, with about 270, 1500, and 50 species respectively.
The Autosauri are of great interest, since they exhibit a great, almost endless variety in shape, size, and structure in direct adaptation to their surroundings. Most of these modifications are restricted to the external organs, or rather to those which come into direct contact with the outer world, namely the skin, the limbs, the tail, or the tongue. The majority of the Autosauri are terrestrial, but there are also semi-aquatic forms. There are climbing, swiftly running, and even flying forms, while others lead a subterranean life like earthworms. Most of them live on animal food, varying from tiny insects and worms to Birds and Mammals, while others live upon vegetable diet. According to this diet, the teeth and the whole digestive tract are modified. The intestine is relatively short in the carnivorous, long in the herbivorous species. But swiftness, the apparatus necessary for climbing, running, and digging, the mechanism of the tongue, the armament and the muscles of the jaws (hence modifications of the cranial arches, etc.), stand also in correlation with the kind of food and with the way in which it has to be procured.
A very interesting study of the influence of the climate and the nature of the country upon Reptiles has been made by Boettger[147] with especial reference to the Transcaspian desert-region. The winter is there short, but very severe, and there is a considerable amount of snowfall, while the summer is intolerably hot. The spring arrives suddenly. Lilies and tulips, which have been asleep for nine or ten months, sprout towards the end of February, and a carpet of flowers covers the ground for a short time. Then everything shrivels up during the rainless and fierce heat of the summer, and the autumnal storms of dust and sand kill off the last remnants of vegetation. There are no trees, and even prickly shrubs are rare. Instead of broad leaves the plants have grass-like blades or needles. The little shrubs do not form coherent patches, but they are scattered {493}about, and around the roots of each shrub the wind accumulates little mounds of sand and dust, a place of retreat for rodents, lizards, snakes, and even for the female tortoises. G. Kadde's "law of the steppe" is in full force;–there is little change of forms in a wide district, but all these forms are peculiar, and they congregate socially in great numbers. Most characteristic are those kinds of Geckos which, like Teratoscincus, cf. p. 507, have become inhabitants of sand instead of climbers of rocks and trees; various kinds of Phrynocephalus, cf. p. 521, and Varanus griseus; the four desert-species of Lacertidae are brownish-grey or sandy yellow, with conspicuous stripes or spots. Of snakes are to be mentioned Eryx jaculus, digging in the sand, and about ten other non-poisonous snakes. Tropidonotus is, of course, restricted to permanently watery places, where they can get frogs and fishes. Of poisonous snakes there is the Cobra and Echis arenicola. Of Amphibia only Bufo viridis and Rana esculenta var. ridibunda exist in suitable places, but there are neither Tree-frogs nor Newts.
Characteristic features of these inhabitants of the desert are the following:–
1. Velocity. The Lizards are slender. The Sand-snake, Tephrometopon, is whip-like; even the Cobra has a relatively narrower and longer tail than the Indian specimens, although the number of the vertebrae and of the scales is the same. All the desert-snakes are remarkable for the great number of their ventral shields, two hundred and more.
2. Hard, scaly covering, for instance in Agama, Echis, Gymnodactylus, Teratoscincus; the latter with its fish-like scales is exceptional among Geckos, resembling the likewise deserticolous Geckolepis and Homopholis of Africa.
3. Capacity for digging in the sand in order to escape great cold, or burning heat. All the Lizards and the Tortoise, Testudo horsfieldi, have strong claws. The snakes Typhlops and Eryx dig with their specially modified snouts, and their tails are very short and blunt. The Sand-viper, Echis, has the scales of the back arranged in very oblique rows, so that it can heap sand upon its body by wriggling, shaking, and up-and-down motions of the body. The Agamoid Phrynocephalus does this by means of lateral folds of the skin.
4. Arrangements for running on sand. The lizard Eremias {494}has very large crural shields; Scapteira has the digits broadened out into shovels; others, e.g. Phrynocephalus and Teratoscincus, have long lateral fringes on the digits, a very rare arrangement among Geckos, occurring elsewhere among them only in Ptenopus and Stenodactylus, which are likewise inhabitants of the desert.
5. Protection against the everlasting, ubiquitous sand. In the digging species the nostrils are directed upwards instead of forwards; in most of the snakes they are protected by complicated valves, or they are reduced to small pin-holes. The eyes of Typhlops are overhung by the head-shields. In Agama and Phrynocephalus the margins of the lids are broadened into plates and are furnished with peculiar scales. In Teratoscincus the upper lid is enlarged. The lizard Mabuia has the lower lid much enlarged, with a transparent window in it, so that the eye can be closed without impeding sight, an arrangement carried to the extreme in Ablepharus, cf. p. 560. The ear-opening is either small, or protected by fringes of scales, or it is abolished, e.g. in Phrynocephalus.
6. Coloration. Pure green is quite absent, even in Bufo viridis and in Rana esculenta, since there is no green in that country, at least not of long duration. White, with grey and black spots, occurs only in the nocturnal Geckos. Yellow, brownish, reddish colours are common, in adaptation to the sand. The advantages of the carmine-red, and of the blue spots of Phrynocephalus, and the yellow or bright red under surface of its tail, are unknown. Striation is of frequent occurrence among the lizards and snakes, probably in adaptation to the dry grass heaped up around the scattered shrubs.
Concerning the various organic systems of the Autosauri only some of the more important features may here be mentioned.
Skeleton.–The vertebrae are procoelous, with the exception of most of the Geckones, in which they are amphicoelous. So-called intercentra, in the shape of unpaired nodules or wedges, persist between most of the cervical vertebrae. In the tail these wedges, the remnants of the basiventralia, are generally present, frequently in the shape of chevron-bones. Sometimes they fuse with the centra of the vertebrae; occasionally the axial or central portion of these basiventrals persists as a sort of fibrous disc, which may calcify separately, and is interposed between the caudal end of the centrum and the articulating {495}knob. The caudal vertebrae of the Geckones and of most Lacertae are liable to break across, like those of Sphenodon. They are enabled to do this owing to a transverse split, which makes its appearance with the ossification of the vertebral bodies and extends later into and across the neural arch and the various lateral processes. The split is ultimately referable to a transverse septum of cartilage, wrongly called chordal cartilage, which develops in the shell of the body of the vertebra, destroys the chorda, and extends peripherally. The cells of this septum retain throughout life their juvenile quasi-embryonic character. When the tail is broken off–and this always happens at such a septum–the cells of the remaining half reproduce a new tail. The latter is, however, in reality a sham tail, since neither new centra nor arches, but only a non-segmented rod or tube of fibro-cartilage is produced by this process of regeneration. Reproduction of centra is precluded by the previous normal reduction of the chorda, around which alone proper bony centra could be formed. The regenerated tail is, however, invested with new muscles, and with skin, but the scales often differ considerably from those of the normal organ. Boulenger[148] has found that the new or aberrant scaling is in some cases a reversion to an ancestral form. This is, for instance, the case in Pseudopus, and in the Tejoid genus Gymnophthalmus; to a certain extent also in Geckos and Skinks. On the other hand, Lacertidae, Gerrhosauridae, and also Anguidae reproduce a caudal scaling true to their type. Injured or broken-off tails are often reproduced double, or even trifid; sometimes an additional little tail grows out from an injured spot, anywhere on the side of the old remaining but mended tail.
The ribs of the trunk articulate by their capitula only, while the reduced tubercula are attached to their vertebrae by ligaments. In the tail the capitular portion is much reduced, while the tuberculum is much stronger and lies behind, no longer above, the capitulum, fusing sometimes directly with the centrum. The ribs of the poststernal region of Geckos and Chameleons are very long, and meet each other in the middle line, forming thin cartilaginous hoops.
The limbs are of the typical pentadactyloid type. The distal tarsalia are often fused with the metatarsals, so that the chief {496}bending of the foot is effected by truly intertarsal joints. The greatest modification occurs in the foot of the Chameleons, in which the proximal tarsalia are reduced in number, and form a globe for the articulation with the tibia and fibula.
The shoulder-girdle and sternum much resemble that of Sphenodon in their completeness. The coracoids articulate with the sternum; the precoracoids and the basal parts of the scapulae often send out several processes towards those of the other side, so that several fenestrae are formed. The clavicles are complete, but are absent in the Chameleons. The interclavicle is mostly T-shaped. A presternum is absent, but the sternum proper is well developed, often forming a rhomboid plate, usually cartilaginous, often diverging backwards into xiphisternal processes.
The pelvis is attached to two vertebrae by means of several ribs. The ischium and pubis form symphyses. The pubis carries a well-developed lateral process, and the obturator-nerve pierces the shaft of the pubis. Epipubic and hypo-ischial cartilages are of frequent occurrence.
The hyoid apparatus consists of a median, styliform rod, which extends forwards into the tongue; it is often bifid behind. The unpaired piece carries two pairs of horns. The posterior of these, the first pair of branchial arches, extends backwards along the gullet, and is very long if the tongue is very slender and protractile. The anterior pair, the hyoid arches, consists of two pieces on either side, one short and directed forwards, the other long, connected with the former at a sharp angle and continued upwards to the sides of the skull, often in direct continuity with the columellar chain of the ear.
The modifications of the skull concern chiefly the composition of the temporal arches, see Figs. 55, M, N, O, p. 281. The quadrate bone is movable, but it has become fixed in various degraded families, where the skull shows a great reduction and concentration; the postorbital and temporal arches, the interorbital septum, and with it the columellae cranii are lost. The columella cranii of the Chameleons, which is generally stated to be absent, is really present, although in a much reduced state, and is partly imbedded in the interorbital septum. The occipital condyle has become bifid in Amphisbaenidae.
Burrowing and living in sand are often correlated with partial or complete reduction or loss of the limbs and their {497}girdles. This loss of limbs is as a rule correlated with an elongation of the trunk, not always at the expense of the tail, which in such cases is much shortened. The vestiges of the hind-limbs come to lie as near the vent as possible. This reduction of the limbs occurred in several families which are not directly related to each other. Moreover, it does not occur in all the members of the family, not always in those of the same genus, and there is a considerable amount of individual variation. In most cases of reduction the fore-limbs disappear before, or are smaller than, the hind-limbs. In the Amphisbaenidae (cf. Chirotes, p. 566), and in the Tejidae the reverse takes place. In extreme cases the reduction is so complete that even the pectoral girdle has disappeared, leaving scarcely any trace, e.g. in Dibamus, p. 564.
The skin is normally covered with scales, which are formed by the cutis and have a horny epidermal coating. The latter, thin and transparent, is shed periodically, peeling off in flakes, except in Anguis and perhaps other snake-shaped creatures, which shed the skin in one piece. In the Amphisbaenidae the scales have practically disappeared. When well developed the scales are prominent, and imbricate or overlap with their free posterior edges; but in many cases the scales are not "scale-like" at all, only like little tubercles, which give the skin a granular appearance. Frequently, for instance in the Scincidae and Anguidae, all the scales contain "osteoderms," or ossified portions of the cutis, and encase the whole body and tail. In other families, e.g. Lacertidae, such osteoderms are restricted to the scales or shields on the head, where they come into contact and fuse with the underlying cranial bones, and moreover roof in the supratemporal fossa.
The skin of the Autosauri is entirely devoid of glands. The femoral and pre-anal pores of many families, occurring especially in the males, are probably not glands. They are arranged in rows on the under surface of the thighs and in front of the anal opening. Each of these organs perforates a scale and leads into a tubular invagination, which is lined with epidermal cells, the proliferation of which produces a horny yellowish débris, and this fills the tube and appears above the surface in the shape of a little cone. The use of this "excretion" is unknown; it is possibly hedonic.
Most Autosauri are capable of changing colour. In most of them this faculty is restricted to the assumption of paler or darker tints owing to the shifting of the colouring matter contained in the chromatophores. In others new, often vivid colours are the result. The mechanism is described in detail in the Chameleon on pp. 570 and 574.
Pigment is deposited either directly in the upper strata of the cutis, just below the Malpighian layer, or it is contained in chromatophores. The latter are imbedded in the deeper layers of the cutis, and send out movable contractile processes, in which their pigmented protoplasm is conveyed towards or away from the surface. The only colours available are black, red, yellow, and white, with their combinations of grey and brown. The white pigment consists of guanin-salts. Blue and green are structural colours, not due to pigment. The same can no longer be said of the Ophidia, since Boulenger has observed accidentally that green Tree-snakes (e.g. Dryophis) give the alcohol in which they are kept the colour of green Chartreuse.
Digestive organs.–The tongue is very variably developed, and affords good taxonomic characters. It is always furnished with many tactile, or with gustatory, corpuscles. When the tongue is very long and narrow it is generally forked, and in these cases, for instance in the Varanidae, is almost entirely used as a sensory organ. In others, especially where it is broad, it assists in catching the food, and in the Chameleons it has attained a most elaborate development (see p. 569).
Salivary glands are restricted to labial glands. In Heloderma those of the lower jaw are transformed into poison-glands, an analogy to what prevails in the poisonous snakes. The intestinal canal is longest in the herbivorous forms; the rectum sometimes possesses a short blind sac or caecum.
The cloaca of the Sauria is somewhat modified; instead of the Coprodaeum, Urodaeum, and Proctodaeum forming three successive chambers, the urodaeum is practically reduced to its dorsal half, forming a dorsal recess between the two other chambers. The Coprodaeum is constricted into several successive chambers, and is always well shut off from the urodaeum by a strong sphincter. The urodaeum receives the urinary excretions, which are mostly chalky white and are rather consistent instead of being fluid. The right and left oviducts also open into it. The vasa {499}deferentia open into the dorso-lateral portions of the walls of the urodaeum, but the sperma is conducted by folds of the lining of this chamber towards the bases of the copulatory organs, which, although arising from the lateral and posterior corners of the cloaca, where uro- and procto-daeum meet, are stowed away outside the cloaca. These organs are always paired. The proctodaeum or outermost cloacal chamber is shallow. Its inner opening is round and is furnished with a sphincter, but it is surrounded and covered by lips of the outer skin, which form a transverse slit. This is due to the peculiar arrangement of the copulatory organs.
Each organ consists of a tube of erectile tissue, and can be everted like the finger of a glove. To the apex of the tube is attached a long retractor muscle, which arises from the ventro-lateral surfaces of the caudal vertebrae and extends a considerable distance back. When at rest and withdrawn the organs form slight conical, longitudinal swellings on either side of the root of the tail, an external feature by which male specimens can generally be distinguished. Only one organ is inserted at one time.
The majority of Autosauri lay eggs, surrounded by a white or yellowish shell, which is either hard, for instance in Geckos, or parchment-like, e.g. in Chameleons, in Lacerta viridis and L. agilis, and in L. vivipara. Eggs with a thin and soft shell sometimes exhibit the paradoxical feature of increasing in size after they have been laid. This is explained by the growth of the embryo, which stretches the shell and does not merely live upon the white and yellow contents of the egg itself, but also takes in air and moisture. Many Lizards do not lay their eggs until they contain ripe embryos, which burst the shell shortly after deposition. Some, for instance Lacerta vivipara, Anguis fragilis, and Chamaeleo pumilus, are practically viviparous. The embryos, especially those which are enclosed in hard-shelled eggs, are provided with a sharp, calcareous "egg-tooth" on the top of the snout.
The lungs are thin-walled sacs, sometimes provided with lateral ex-sacculations, and these reach their greatest development in the Chameleons. The breathing is effected by the motion of the ribs. Inflatable sacs on the throat, or on the sides of the neck, for ornamental or sexual purposes, occur in various families. The lungs of much-elongated, snake-shaped Lizards are generally {500}asymmetrical; the right being reduced in Amphisbaenidae; the left in other cases.
Several Autosauri, for instance the Geckos, Psammodromus, and various other Lacertidae have a weak voice.
The Fat-bodies are mysterious organs which are situated beneath the skin, and extend from the inguinal region forwards along the ventral sides of the belly. They are often of considerable dimensions; largest in the spring, in both sexes, at the time of propagation. Their colour is greyish-white or yellow, owing to the great accumulation of fat in the meshes of the connective tissue which composes the frame-work of these organs. An artery enters them, breaks up into capillaries, and these combine to form an efferent vein. After the time of propagation these organs are reduced to grey or reddish flaps, consisting mainly of very vascular connective tissue. G. W. Butler[149] has written a long paper on their morphology. The same author[150] has investigated the "sub-divisions of the body-cavity in Lizards, Crocodiles, and Birds," with reference to peritoneal diaphragmatic structures.
The geographical distribution of the Autosauri teaches few, but important lessons. We have to restrict ourselves to the principal families, leaving out those which are small and have a limited distribution; also those which, like the few Anelytropidae in Africa and in Mexico, are not natural groups.
The Geckones, which are probably the oldest of modern Autosauri, are practically cosmopolitan, being absent only in the cold and in the cooler temperate regions. They are common even in Oceanic Islands, for instance in New Zealand and in the Sandwich Islands. Although not at all aquatic, they are particularly fit to be transported accidentally on or in the trunks of floating trees, to which they cling firmly, and they can exist without food for months. I once received a little South American Gecko in perfect health from a grocer, who found it in a well-closed wooden box containing canned meat, two months after delivery of the box in Cambridge.
The Scincidae, likewise an old family, are equally cosmopolitan, but although many exist in the islands of the Pacific a few only occur in New Zealand. Many of the genera have a very wide distribution; for instance, Lygosoma, with its one hundred and sixty or more species, occurs in the Australian and {501}Palaeotropical regions, and also in North and Central America, not extending, however, into South America. Mabuia, with more than sixty species, occurs in the Palaeotropical and the Neotropical regions. Whether these and other widely-distributed genera are all natural is another question.
The Agamidae, Varanidae, Lacertidae, and the Chamaeleontes are restricted to the Old World. The Agamidae and Varanidae have the widest distribution, occurring in the whole of the Old World with the notable exception of Madagascar and New Zealand. The Lacertidae are Palaearctic and Palaeotropical, being however absent in Madagascar, and, broadly speaking, not extending eastwards beyond Wallace's line. It is a most suggestive fact that most of those families of Reptiles, and even of other Vertebrates which have a wide distribution and are apparently debarred from transgressing Wallace's line, are also absent from Madagascar.
The Chameleons are essentially African, with their centre of greatest abundance and development in Madagascar, only one or two species occurring in Socotra, Southern Arabia, and in Ceylon and Southern India. Since they also exist, Ch. sechellensis, on various islands in the Indian Ocean, for instance in Mauritius and the Seychelles, the Chameleons are perhaps an indication of the former existence of a direct land-connexion between Southern India and Southern Africa.
The Iguanidae are essentially American, with the remarkable exceptions of Chalarodon and Hoplurus in Madagascar, and Brachylophus in the Fiji and Friendly Islands. This peculiar distribution finds some analogies in that of Dendrobatinae (p. 272), certain Boinae (p. 601), and Centetes and Solenodon among Insectivora. An Iguana (I. europaea) has, however, been described from the Eocene of France and England. The supposed relationship of the Iguanidae with the Agamidae makes the problem only more puzzling, since Agamidae are absent in Madagascar. If we have recourse to the Zonuridae, which are confined to Africa and Madagascar, and are supposed to be intermediate between Anguidae and Iguanidae, then we may have ultimately to conclude that the Malagasy Iguanoid genera and the American Iguanidae are a case of convergent evolution.
The Amphisbaenidae are distributed over America, including the West Indies, Africa exclusive of Madagascar, and the {502}Mediterranean countries. This is very puzzling, considering that these subterranean, helpless creatures positively cannot travel. Boulenger regards them "as a degraded type of the Tejidae, with which they are to some extent connected by Chalcides and its allies," i.e. genera with reduced limbs, cf. p. 562.
However, this supposed relationship with a strictly American family does not explain the occurrence of Amphisbaenidae in Africa. Either they are not a natural group, or they had, as already degraded, limbless creatures, a much wider range; and this would imply their being a very old family, perhaps as old as we suppose the Coecilians to be.
Anguidae occur in North and South America, in Europe and the Mediterranean parts of North Africa, and in Trans-Gangetic India. Their older relations, the Zonuridae, inhabit Africa and Madagascar.
Madagascar is consequently devoid of Agamidae, Varanidae, Lacertidae, Anguidae, and Amphisbaenidae, while it possesses, besides the cosmopolitan Scincidae and Geckones, only Chameleons, Gerrhosauridae, and Zonuridae,–all three essentially African families,–and a few Iguanidae. This means that the Autosaurian fauna of Madagascar is intimately related to that of Africa, and that it possesses only old families so far as Sauria are concerned. But since this great island was separated from its continent not earlier than in Mid-Tertiary times, it follows that most of these "old" families are comparatively recent.
Australia possesses only Agamidae and Varanidae besides the ubiquitous Geckos and Skinks. Besides the latter two families it has nothing in common either with Madagascar (an analogy with the Anura) or with America. The Autosauri consequently do not support the idea of a Notogaea, cf. p. 74. This again indicates the comparatively recent age of Autosaurian families. The marked difference which exists between the Old and the New World points to the same conclusion. On the other hand, the Autosauri support the idea that the Palaeotropical region is but the tropical and therefore richer continuation of the now impoverished Palaearctic sub-region.
Sub-Order 1. Geckones.–The typical Geckos are characterised as follows. Four-footed Autosauri with amphicoelous vertebrae; skull without bony temporal arches; clavicles dilated and with a perforation near the ventral end; parietal bones {503}separate; eyes (with few exceptions) without movable lids; pleurodont; tongue fleshy and broad, slightly nicked anteriorly, and capable of protrusion.
This definition does not apply to a few forms. In the Eublepharinae the vertebrae have advanced to the procoelous condition, and the parietals are fused together, while the eyes are provided with typical, movable lids. In the Uroplatinae the clavicles are not dilated, and the nasals are fused into one bone. The Geckos seem to be not only a very independent but also a very old branch of Saurians. Although fossil representatives are unknown, the resemblance of their vertebrae to those of the Palaeozoic Microsauri is at least remarkable. They are now practically cosmopolitan within the warmer zones, being found in abundance in all intertropical countries and islands, even in New Zealand. About two hundred and seventy species are known, which have been subdivided into about fifty genera. The generic differences are trivial with few exceptions, and refer mostly to the structure of the digits.
The more important features of the vertebral column are the absence of axial joints and the persistence and life-long growth of the chorda dorsalis. Each vertebral centrum consists of a cartilaginous tube, more or less calcified or ossified, with a narrow waist and a cartilaginous septum in the middle. In the tail this septum, which is only slightly invaded by ossification, coincides exactly with the line of transverse division of the vertebrae into an anterior and a posterior half. This is the level where the tail breaks off and whence it is renewed. Between every two successive centra lies an intercentrum, broadest ventrally, crescent- {504}or wedge-shaped. Dorsally it is continued as fibro-cartilage, and the whole ring acts as an articular pad instead of the joint. Chevron-bones are common in the tail.
The ribs are bifurcated, but the tubercular portion is frequently reduced. The post-thoracic ribs are usually very slender, and so long that they meet each other in the middle line, in this case bearing an extraordinary resemblance to the so-called "abdominal ribs" of other reptiles.
The bony frame of the skull is slender. There is a complete absence of bony arches spanning over the temporal fossae, or bordering the orbit, which is open posteriorly. The upper jaw, owing to the slender and flexible nature of the respective bones, is movable upon the rest of the skull; in this respect not unlike the upper jaw of a duck. The dentition is pleurodont and the teeth are minute. The eyes of the typical Geckos are peculiar. They are covered with an absolutely transparent skin, shaped like a watch-glass, beneath which the eye moves freely, while the true upper and lower lids are reduced to tiny folds. The covering "watch-glass" is probably a modification of the nictitating membrane. In the Eublepharinae, however, and in the few species of the Geckonine genera Aelurosaurus of Borneo and Australia, and Ptenopus of South Africa, the upper and lower lids are present and movable. The pupil contracts mostly into a vertical slit, except in the few diurnal kinds, e.g. Phelsuma, of the islands in the Indian Ocean, and the African Lygodactylus.
Another peculiarity of at least many Geckos is the extraordinary development of the endo-lymphatic sacs of the ear, which, being filled with the chalk-like otoconia or otolithic crystals, perforate the skull, and are stowed away in the shape of a pair of large bags behind the ears, or on the sides of the neck.
The skin exhibits considerable variety. It is mostly soft above, with little granular tubercles, sometimes containing small dermal ossifications or calcifications. The latter are most developed on the head, where they occasionally fuse with the underlying bones. A few species of Tarentola possess supra-orbital bones, independent remnants of such osteoderms. The ventral surface is generally covered with small imbricating scales, but in some genera, e.g. Homopholis, such scales occur also on the dorsal surface, reaching their highest development in Teratoscincus (p. 507). In a few forms, notably in Ptychozoon {505}(p. 512), the skin of the sides of the body and tail is produced into a series of lobes and flaps, the object of which seems to assist adhesion. Many, perhaps the majority of Geckos, have adhesive digits, by means of which some kinds are enabled to climb absolutely smooth and vertical surfaces, for instance a window-pane; or, what is more startling, they run along the smooth, white-washed ceiling, back downwards. The apparatus is complicated in its minute detail, but is very simple in principle. The adhesion is effected neither by sticky matter, nor in the way described in the Anura (p. 187), but by small and numerous vacua. The under surface of each digit is furnished with many transverse lamellae. The pressing down of the foot upon a smooth surface causes the lamellae to spread asunder and to drive out the air; partial retraction lets them return to their original position by virtue of their elasticity; and little vacua are produced. Each lamella is further beset with tiny hair-like excrescences, which secure adpression to even the slightest irregularity of surface and at the same time enhance the elasticity of the pads. The arrangement of the lamellae and pads differs much in the various genera. For instance, the lamellae are either broad and entire, or they are divided into two parallel rows, with or without lateral hairy fringes; or the under surface of the digits is granular, but strongly fringed; or the lamellae are restricted to the dilated tips of the digits, etc. The fingers and toes are mostly furnished with sharp, curved claws, and these are in many cases retractile between some of the lamellae, or into a special sheath. Those Geckos which live on sandy, barren ground are as a rule devoid of adhesive pads, the digits being narrow. The typically padded, adhesive digits cause a peculiar sensation when a Gecko hangs on to one's finger, and this feeling has perhaps given rise to the erroneous notion of stickiness.
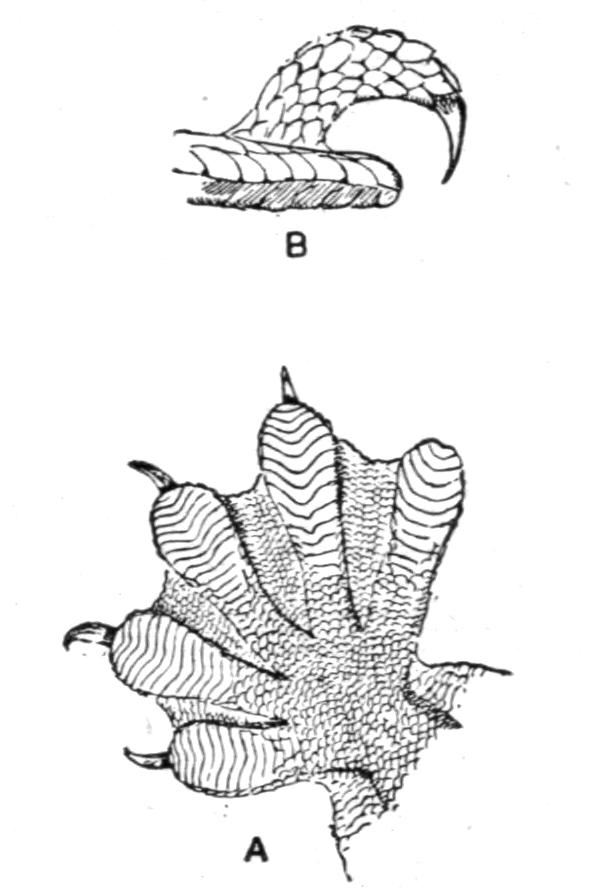
Fig. 119.–Ptychozoon homalocephalum. A, Ventral view of the right hand. × 2. B, Side view of a finger to show the peculiar arrangement of the claw-bearing joint.
The tail exhibits many kinds of shape and size. Mostly {506}cylindrical and tapering to a point, it is leaf-like in Gymnodactylus platurus of Australia; provided with many lobes, and used as a parachute in the Malay Ptychozoon. In Nephrurus asper of Eastern Australia the tail is quite short, much shorter than the limbs, much swollen at the base, and very thin towards the end, which carries a round knob. The tail of all Geckos is very brittle and can be quickly regenerated, except the long rat-like tail of the Persian Agamura. In many other desert-forms the tail is long, slender, and laterally compressed, acting in such cases like that of desert-forms among the Lizards.
Many Geckos have a voice, mostly rather feeble, and sounding like a soft "click" or "chick" produced by our tongue. Repetition of this sound resembles in some species the word "gecko." They lay eggs, rather globular, or but slightly oval, hard-shelled, and white, mostly two in number. Naultinus elegans of New Zealand is said to be viviparous. The males are generally larger than the females, and they are further distinguished by the possession of femoral or pre-anal pores.
All Geckos feed upon animals, chiefly upon insects, but the larger forms take anything they can master. With few exceptions they are nocturnal, which, however, does not prevent them from occasionally baking themselves in the sun. They are capable of changing colour, but since their ground-colour is almost universally grey, yellow, or brown, the range of the colour-changes is restricted to the adoption of darker or lighter hues. The skin is shed in flakes and eaten.
Geckos are absolutely harmless; they cannot even inflict painful bites. However, in many countries they are feared as much or even more than the most poisonous snakes. In the south of Spain and Portugal, for instance, where Geckos are plentiful in and outside the houses, and are consequently objects of daily observation, the "osga" is considered a dreadfully poisonous creature. They become very tame, or rather confiding in their regular habits, provided they are not molested. If caught–and they have many enemies among other lizards and snakes–the only safety of these defenceless and mostly small creatures lies in their tail, which, being extremely brittle, is left in the claws or jaws of the pursuer. The remaining stump soon produces a new tail, in shape and size like the old one, but with a different and simpler scaling. I knew of several specimens of {507}the Portuguese Platydactylus facetanus, which, having lost their tails in the act of being caught, were kept in a box for six weeks without food. On their arrival in England they had each grown a new stump nearly half an inch long!
Fam. Geckonidae. Sub-Fam. 1. Geckoninae.–Vertebrae amphicoelous; parietal bones separate; clavicles dilated and perforated. Hereto belong the overwhelming majority of Geckos, only a few of which can be mentioned.
Teratoscincus scincus.–This most peculiar creature, about six inches in length, inhabits the steppes of Turkestan and neighbouring desert-regions of Persia. It is a thorough desert-form. The digits are devoid of adhesive lamellae, but are granular inferiorly and strongly fringed laterally, an arrangement which is rare among Geckos, practically restricted to it with Ptenopus and Stenodactylus, which are likewise deserticolous. This is a beautiful illustration of adaptation to the surroundings. A Gecko, instead of climbing rocks and trees, has lost the climbing apparatus, or has transformed parts of it for running upon loose sand. The body is covered with imbricating, rather large and smooth scales. The tail is round at the base, compressed in its posterior half, covered below and on the sides with scales like those of the body, but on the upper side with a series of large, transverse, nail-like plates. By rubbing these plates upon each other, this Gecko produces a shrill, cricket-like noise, sitting at night in front of his house, perhaps in order to attract grasshoppers. The noise is made by both sexes.
Ptenopus, a Gecko of Damara Land, likewise adapted to desert-life, produces a similar chirping noise by its throat.
Phyllodactylus is a genus of world-wide distribution, occurring in tropical America, Africa, Madagascar, and Australia, extending to the Norfolk Islands and to Lord Howe's Island. One species, Ph. europaeus, occurs on the islands in the Western Mediterranean. The digits are furnished with transverse lamellae, the greater number of which are broken up into small scales forming three longitudinal series. The ends of the digits are dilated, with two large plates inferiorly, separated by a longitudinal groove into which the claw is retracted. The upper parts of the body are covered with juxtaposed scales intermixed with larger tubercles. The abdominal scales are small and imbricating. The cylindrical, tapering tail is slightly prehensile, covered with {508}small scales arranged in verticils. This species is devoid of femoral or anal pores. General colour above grey-brown, with darker and lighter markings; a dark streak on the side of the head, passing through the eye. Under parts whitish. Total length up to 3 inches. The eggs are almost round, measuring 8.7 by 7 mm.
Hemidactylus, likewise a widely distributed genus, with many species. The digits are dilated, inferiorly with two rows of lamellae; the clawed joints are slender, bent at an angle, and rising from within the extremity of the dilated portion. H. turcicus, between 3 and 4 inches long.–The upper parts of the body are covered with minute granules, mixed with larger tubercles. The abdominal scales are small and slightly imbricating. The male has several pre-anal pores. The tail is covered above with minute scales and tubercles, below with a series of large transversely dilated plates. The general colour is white below, brown above, with darker spots, and with white specks on many of the tubercles. This species extends from {509}Southern Portugal and Spain to Karachi. Like Phyllodactylus and various other kinds of Geckos, the body is semi-transparent; so much so indeed that the white eggs shimmer through the body in certain lights.
Tarentola mauritanica s. Platydactylus facetanus.–The digits are strongly dilated, with undivided lamellae below, and a flat, nail-like scute on their upper surface near the tip. Only the third and fourth digits are clawed. Femoral or pre-anal pores are absent. The upper parts are covered with scales and granules, and bear several longitudinal rows of strongly keeled, large tubercles; the under parts have hexagonal scales. General colour above greyish-brown, with darker or lighter markings; with a dark streak through the eye. Total length of large males about 6 inches. This species is one of the commonest Geckos in the Southern Mediterranean countries. In Portugal it extends northwards to the Douro. It has been introduced by ships into the ports of Cette, Toulon, and Marseilles. It is easily kept in captivity, like most Geckos indeed, provided they are supplied with a variety of insect-food, water in the shape of drops, and suitable places to hide in. A female, which I had received from Algiers in a little tin box, with a lump of meat (presumably its food!), laid two eggs six weeks after its arrival. This was towards the end of April. Towards the end of June in the same year it again laid two eggs, measuring 13 × 10 mm. Another specimen laid in June in two successive years. These and other Geckos live very well in a greenhouse, or in a large glass cage. They change colour most adaptively. They hunt preferably at night for insects, which are stalked and then suddenly rushed at. Drops of water are taken by a lapping motion of the tongue. For sleeping-places they selected bits of hollow bamboo, but these had to be vacated when some tree-frogs crept into them for the daytime, and the Geckos took to some curved pieces of bark, on the under side of which they slept, with their backs downwards. This is, by the way, a favourite position of rest of most Geckos. But Stenodactylus guttatus of Egypt lies flat on its belly, tucks the fore-feet under and inwards like a cat, rests the head upon them, and stretches the hind-limbs out backwards. The little Geckos are rather intelligent. They take no notice of a finger put against the other side of the glass to which they happen to cling; but {510}when the hand is put inside their cage and approaches them too near, they dart off suddenly. When driven into a corner they wriggle and wag their tails, or even raise the latter, perhaps as an invitation to grasp it, in which case it would of course break off. When caught, they emit feeble sounds, and attempt to bite with the mouth widely open. During the moulting, which takes place at least twice a year, in the spring and in the autumn, the skin peels off in flakes; if, as happens sometimes, the skin upon the lamellae is not stripped off neatly, these refuse to act, and the creature cannot climb until all the old skin has been rubbed off.
In their native haunts they are very regular in their habits. Favourite resorts of theirs are old olive trees or oak trees, the rough and cracked bark of which affords excellent places for hiding in. Hollow trees are of course preferred. Not a single specimen is seen during the early hours of the morning or in the forenoon; but when the sun has become broiling hot, and our own shadow passes over the stem of a tree, we become aware of flitting little shadows which jerk over its surface. These are Geckos which had been basking, motionless; very dark grey, almost blackish, just like the colour of the grey bark upon which the last wet season's moss has been scorched to a black cinder. It is difficult to espy a Gecko whilst it is glued on to such a tree. Only the little beady eyes betray it, watching you carefully. Nothing appears more easy than to catch that motionless thing. You put out your hand and it is gone; like a flash it has moved a foot higher up, or down, to the right or to the left, just where you least expected it to go, and there it clings on motionless as before. It does not seem to run; it glides along, dodging over to the other side of the stem and back again. There is system in its motions, since, taking a last leisurely look around, it gently disappears in a rent or hole. Towards the evening, or when the shadows become longer, the Geckos become lively. One after another appears on the surface, upon the tree, or at the entrance of the cave, and they all move about in their peculiar rushing jerks. Spiders, flies, mosquitoes, moths, form the principal diet, and the hunting goes on well into the night. Where a gecko has been seen once it is sure to reappear the next day at the same hour. Those which take up their abode inside a house become almost domesticated. They are strange sights when hunting for flies, {511}running up and down the papered walls; but we fairly gasp when they come to the upper corner, calmly bend over, and with the next jerk slide along the white-washed ceiling. We are accustomed to flies performing such feats, but at animals five inches long, supple and fat, we are inclined to draw the line. However, that is the way of Geckos, and–be it confessed–the more we ponder over the mechanism of their fingers and toes, the less we comprehend how such little vacua can support or suspend such heavy creatures from a dry and often porous surface.
Gecko.–The digits are strongly dilated with undivided lamellae. All, except the pollex and hallux, have a very short compressed terminal phalanx with a retractile claw. Males with femoral or pre-anal pores. This Eastern genus includes some of the largest of all Geckos.
G. stentor of the Malay countries reaches a length of 15 inches. G. verticillatus s. verus s. guttatus ranges from Eastern Bengal to China and through the Indian archipelago. It grows to about one foot in length. The head is large; the back is covered with small granules and about a dozen rows of large tubercles. The tail, when intact, and the belly are covered with scales, those of the tail being arranged in transverse rows, several of which make up distinct rings. The upper parts of the body are grey or yellowish with red spots and vermiculations. According to Theobald[151] it lays about eight hard-shelled white eggs as big as a musket-ball, cementing them to trees, rocks, or secluded buildings. The cry is "touk-tay," several times repeated, and ending in a long-drawn out, diminuendo, guttural rumble. This animal does not confine itself to insects, but eats young rats also. Dr. Mason has seen it devour smaller species of house-lizards, and Theobald has seen it seize a bat flying round the room, and devour it.
Tennent[152] tells the following story about one of these creatures: "In an officer's quarter in the fort of Colombo, a Gecko had been taught to come daily to the dinner-table, and always made its appearance along with the dessert. The family were absent for some months, during which the house underwent extensive repairs, the roof having been raised, the walls stuccoed, and the {512}ceilings whitened. It was naturally surmised that so long a suspension of its accustomed habits would have led to the disappearance of the little lizard; but on the return of its old friends, it made its entrance as usual at their first dinner the instant the cloth was removed."
Ptychozoon.–The digits have the same structure as described in the genus Gecko, but they are entirely webbed. The extraordinary feature of Ptychozoon is the membranous expansions on the sides of the head, body, limbs, and tail, which are said to act as parachutes. P. homalocephalum, the only species, inhabits the Malay Islands and the Malay Peninsula. It reaches a length of 8 inches. A specimen obtained by F. H. Bauer in Java, in the month of November, laid two eggs a few days after its capture. One young was hatched in the middle of the following May, and two days later another came out of the second egg. The characteristic folds of the skin were already clearly discernible.
Sub-Fam. 2. Eublepharinae.–Differing from the true Geckos by their procoelous vertebrae and the fusion of the two parietal bones into one. The eyelids are not reduced, but remain functional. This sub-family is undoubtedly a heterogeneous assembly, as indicated by the very scattered distribution of its few species (about seven), in India, West Africa, and Central America.
Sub-Fam. 3. Uroplatinae, composed of a few species of the genus Uroplates in Madagascar. The distinctive characters of {513}these otherwise typical Geckos are the fusion of the nasal bones into one, the small size of the interclavicle, and the non-dilated shape of the clavicles.
Neither the Eublepharinae nor the Uroplatinae are more nearly related to other Autosauri than are the other Geckos. They are modifications within the sub-order of the Geckones.
Sub-Order 2. Lacertae.–Autosauri with procoelous, solid vertebrae, and with the ventral portions of the clavicles not dilated.
Cope,[153] discarding outer appearances as deceptive in the classification of the Lacertae, laid stress upon internal characters, notably the presence or absence of osteoderms, the formation of the skull, and the structure of the tongue. Boulenger[154] has followed and improved upon Cope's arrangement, and has elaborated the classification, which, being used by himself in the three volumes of the Catalogue of Lizards in the British Museum, has also been followed in the present work, with slight alterations in the order of treatment of the families. For our present purpose we diagnose the families as follows, giving preference to such characters as are most easily ascertained:–
Synopsis of the Families of Lacertae.
| Fam. 1. Agamidae. | Acrodont. Tongue broad and thick. No osteoderms. Old World, p. 515. |
| Fam. 2. Iguanidae. | Pleurodont. Tongue short and thick. No osteoderms. America, Madagascar, Fiji Islands, p. 528. |
| Fam. 3. Xenosauridae. | Pleurodont, solid teeth. Anterior part of tongue retractile. No osteoderms on the body. Mexico, p. 536. |
| Fam. 4. Zonuridae. | Pleurodont. Tongue short, not retractile. With osteoderms at least upon the skull, where they roof in the supratemporal fossae. African sub-region, p. 536. |
| Fam. 5. Anguidae. | Pleurodont, solid teeth. Anterior part of tongue emarginate, retractile into the posterior portion. Osteoderms on body and head, roofing over the supratemporal fossae. Limbs mostly reduced. America, Europe, India, p. 537. |
| Fam. 6. Helodermatidae. | Pleurodont, lower teeth grooved, with poison-glands. Tongue bifid. Osteoderms tiny. Postfronto-squamosal arch absent, p. 540. |
| {514}
Fam. 7. Lanthanotidae. |
Pleurodont. Tongue short and bifid. Postfronto-squamosal arch absent. No osteoderms. Borneo, p. 541. |
| Fam. 8. Varanidae. | Pleurodont. Tongue very long, bifid, smooth, very protractile. No osteoderms. Postorbital and temporal arches incomplete. Old World, p. 542. |
| Fam. 9. Xantusiidae. | Pleurodont. Tongue very short and scaly. No osteoderms. Supratemporal fossa roofed over by the cranial bones. No movable eyelids. Central America and Cuba, p. 547. |
| Fam. 10. Tejidae. | Teeth solid, almost acrodont. Tongue long, deeply bifid, with papillae. No osteoderms. Limbs sometimes reduced. America, p. 547. |
| Fam. 11. Lacertidae. | Pleurodont. Tongue long, bifid, with papillae or folds. With osteoderms on the head. Supratemporal fossae roofed over by the cranial bones. Old World, p. 549. |
| Fam. 12. Gerrhosauridae. | Pleurodont. Tongue long, with papillae, but feebly nicked. With osteoderms on the head and body, roofing over the supratemporal fossae. African sub-region, p. 559. |
| Fam. 13. Scincidae. | Pleurodont. Tongue scaly, feebly nicked. Osteoderms on the head and body. Limbs often reduced. Cosmopolitan, p. 559. |
The following five "families" are much degraded in conformity with their usually subterranean life, see p. 496:–
| Fam. 14. Anelytropidae. | Without limbs. Body covered with scales. Mexico and Africa, p. 564. |
| Fam. 15. Dibamidae. | Vermiform, limbless body covered with scales, without osteoderms. Australasia and Nicobar Islands, p. 564. |
| Fam. 16. Aniellidae. | Without limbs; body covered with scales, without osteoderms. California, p. 564. |
| Fam. 17. Amphisbaenidae. | The body is covered with soft skin, forming numerous rings with mere vestiges of scales. Without limbs, except Chirotes with four-clawed fore-limbs, p. 565. |
| Fam. 18. Pygopodidae. | Snake-shaped, with scales. Fore-limbs absent, hind-limbs appearing as a pair of scaly flaps. Australia, p. 567. |
These eighteen "families" of the Lacertae fall into four main groups. We naturally assume that the presence of osteoderms and of complete cranial arches indicate more archaic conditions than their absence, just as we conclude that limbless forms have been evolved from creatures with fully developed limbs. We arrange the four groups with their families as follows:–
| Group I. |
Zonuridae and Anguidae assume a central position, with Iguanidae and Agamidae as two parallel families of highest development. Aniellidae as the most degraded forms. Helodermatidae and Lanthanotidae as rather primitive and solitary survivals. |
| Group II. | Xantusiidae–Tejidae–Amphisbaenidae. |
| Group III. | Scincidae–Gerrhosauridae–Lacertidae.–Here also Anelytropidae and perhaps also Dibamidae as degraded Scincoids. |
| Group IV. | Varanidae, which are in many respects the most highly developed of all. |
| Pygopodidae are of obscure relationship. |
Fam. 1. Agamidae.–Acrodont, Old-World Lizards, with a broad and short tongue. The teeth are usually differentiated into incisors, canines, and molars. The orbit is closed posteriorly; the temporal fossa is bridged over by an arch which is formed chiefly by the squamosal and the well-developed jugal; the postorbital mostly remaining small, and the postfrontal and supratemporal bones being either absent or not present as separate elements. The limbs are well developed. The eye, provided with complete eyelids, is distinctly small and has a round pupil. The skin is devoid of osteoderms, although large and numerous spines are often present, especially on the head and on the tail. The Agamidae, of which about two hundred species, arranged {516}in about thirty genera, are known, exhibit a great diversity of mostly flat-bodied, terrestrial and more laterally compressed, arboreal forms. The majority are insectivorous, a few Agamas have a mixed diet, while Uromastix and some others are chiefly, if not entirely, frugivorous and herbivorous. They are an exclusively Old-World family, avoiding the cooler parts of the Palaearctic sub-region, and also, a very curious fact, Madagascar. The majority live in Australia and in the Indian and Malay countries, comparatively few in Africa, chiefly the genus Agama.
Draco ("Flying Dragon").–The body is much depressed and the sides extend as a pair of large wing-like membranes, which are supported by five or six of the much-elongated posterior ribs, and can be folded up like a fan. On the throat are three pointed appendages, a short one on either side and a long one in the middle. The tail is very long and slender, but not brittle. About twenty species of this extraordinary genus inhabit the various Indo-Malayan countries; one, D. dussumieri, occurs in Madras. D. volans of the Malay Peninsula, Sumatra, Java, and Borneo is about 10 inches long, 5 of which are taken up by the tail. The {517}male has a small nuchal crest. The upper parts of this pretty creature have a metallic sheen, with small dark spots and undulating cross-bands upon the rich brown ground-colour. The wings are orange with black markings. The gular sac of the male is orange, that of the female is blue.
The "Flying Dragons" use their wings as parachutes, but their sailing powers are said to be very moderate. Certainly they do not fly by moving the wings, but when at rest upon a branch, amidst the luxurious vegetation and in the immediate neighbourhood of gorgeously coloured flowers, which partly conceal them by their likeness, they greatly resemble butterflies, especially since they have the habit of opening and folding their pretty wings.
Ceratophora.–This exclusively Ceylonese genus is remarkable for a flexible, erect, and pointed appendage which arises from the top of the snout; it is best developed in the males, vestigial or absent in the females. Gular appendages are absent. The trunk is crestless, slightly compressed, and covered with partly keeled scales. The tail is slender and very long, about two-thirds of the total length of the animal. The general colour is olive-brown, with irregular darker markings and with light streaks on the head and thighs. C. stoddarti and C. tennenti are about 10 inches long, the former without, the latter with, little scales upon the rostral appendage.
Lyriocephalus, with L. scutatus (Fig. 124) of Ceylon as the only species, is remarkable for its Chameleon-like appearance. A splendid case of convergent evolution, but most improbably of mimicry. The tympanum is quite hidden. The head is raised into a pair of sharp bony edges. On the top of the nose is a thick globular lump, recalling the genus Ceratophora, and also various Malagasy Chameleons. The back and sides are covered with very small granular scales, intermixed with several rows of enlarged scales as in Chameleo pumilus, and there is a serrated crest along the back from neck to tail. The under parts are covered with large keeled scales with sharp points directed backwards, especially on the tail. The whole body is laterally compressed. The pollex and the fifth toe are strongly opposed to the other digits. The general colour is greenish above, whitish below. Total length about one foot.
Calotes, with many species in India and in the Malay Islands, is distinguished by a crest on the neck and back. Many of the males have a gular sac. The tail is extremely long. These lizards are remarkable for their changes of colour.
C. versicolor ranges from Afghanistan through the whole of India to Southern China, and attains a length of 14 inches, 11 of which are taken up by the tail. It possesses no gular sac, but has a well-developed crest. The whole body and tail are covered with strongly keeled scales. When the lizard is irritated, or swallowing its food, the head and neck become brilliant red, whilst the usually brownish tint of the body is converted into pale yellow. Mr. Annandale has favoured me with the following observations on C. emma:–"In the Malay Peninsula the Europeans misname this lizard Chameleon. The colour-changes appear to be brought about by emotions, although the lizard is often darker towards {519}evening than it is at mid-day. The males are very pugnacious, and change colour as they fight. At the time of courtship a curious performance is gone through by the male, the female remaining concealed in the foliage hard by. He chooses some convenient station, such as a banana leaf or the top of a fence, and advances slowly towards the female. His colour is then pale yellowish flesh-colour, with a conspicuous dark spot on each of the gular pouches, which are extended to their utmost. He stands upright, raising the fore part of the body as high as possible, and nodding his head solemnly up and down. As he does so, the mouth is rapidly and repeatedly opened and shut, but no sound is emitted. When he is driven away, caught or killed, the dark spot disappears entirely from the neck. If one male is captured, another takes his place within a few hours."
C. ophiomachus of Southern India and Ceylon reaches 2 feet in length, has a fold of skin in front of each shoulder, and is generally known as the "blood-sucker" on account of the red colour displayed during excitement on the head and neck.
C. mystaceus, chiefly in Burma and Siam, but also in the Nicobar Islands and in Ceylon, has a small gular sac and an oblique fold in front of each shoulder. The specific name seems to refer to the yellowish lips. Mason[155] gives the following vivid account of it:–
"This is a very common species in gardens in Toung-ngoo. A pair made their home in the mango trees near my study window. The female blundered into the house a few days ago, but I found her a very unattractive animal of a uniform earth-brown colour. The male, however, is sometimes a beauty. He may be often seen jerking his head up and down, with the head, pouch, and whole front of the body a glowing ultramarine blue, contrasting beautifully with the reddish-brown of the hinder part of the body and tail. From the nose to the shoulders, below the eye, is a broad white band, which is interrupted by three reddish-brown patches, in line with the white band, before reaching the uniform reddish brown of the hinder part of the body. Occasionally the white band below the eye assumes a brownish colour, and the animal appears to have a brown band down each side. He does not always, however, appear in this gay dress. While I am writing, I see him coming down the trunk of one of the trees {520}in a very faded garment. His skin suggests a bright calico after it has been washed, whose colours succumb to soap. The blue is there, but it is no longer the bright blue of yesterday. It has changed to a dull light indigo colour. He runs across the grass to the foot of another tree, and stops on the bare ground at its base, where for a minute or more he bites with great energy at a struggling grasshopper, and while thus exercising himself the blue fades out from his body altogether, and his whole body takes the colour of the brown earth on which he stands. After tarrying a minute or two he ran up the other tree, and the dull light blue colour seemed to return to him."
Agama with many species in Africa and Asia; some in South-Eastern Europe. The body is somewhat depressed. There is a fold across the throat and a pit on either side; the presence of a gular sac is variable. A dorsal crest is absent or but feebly developed. The males have pre-anal pores.
A. sanguinolenta.–The body is covered with strongly keeled and pointed scales. On the sides of the head are a few spine-like scales. The ear-opening is partly concealed by a fringe of spinous scales. The males have a gular pouch. This is a typical inhabitant of the deserts and steppes of Turkestan. Zander[156] has observed the habits and many changes of colour of this lizard. The usual garb is earthy brown above, with somewhat darker and rather indistinct markings. The under parts are whitish. Sometimes the creature changes to dirty white, at other times into blackish or grey brown. Bluish-red stripes may appear on the sides of the body; blue lines begin to show on the throat, and ultimately the whole belly, originally white, may become ultramarine blue. When the general tone happens to be sulphurous yellow, blue often appears on the tail and limbs. Brick red appears on four longitudinal rows of patches on the sides of the body. Sometimes the whole animal assumes a vinous tinge, or it is at first greenish before turning into blue. The change begins on the tail and limbs, extends over the head, and at length reaches the back. Red appears in both sexes, more frequently in the female; blue almost entirely in the male. Sunlight and warmth only intensify the colours. Adaptive coloration, besides the usual sandy garb, has not been observed. The lizards live on soil which is baked as hard as bricks, or in {521}cavities of old walls, provided there is vegetation. They require vegetable food, besides insects, grazing on grass, and having a fondness also for Mesembryanthemum cardiforme. Very large males reach a total length of one foot. They are pugnacious, especially during the time of breeding. The male inflates its gular sac into the size of a walnut, stands up upon its four limbs, with its head slightly lowered and turned to one side. Then it darts upon the foe which it has been eyeing for some time.
A. stellio is the commonest Agama in Egypt, Asia Minor, and in some of the Grecian Islands, where the Greeks still call it korkordilos, just as they did in the time of old Herodotus. The Arabic name is hardun. This lizard is easily recognised by the irregular folds on the neck, which are beset with spinous horny scales. It grows to a length of 15 inches. The general colour is brown, with dark patches on the back. When basking they become almost black; in the breeding season the male assumes red tints on the head and neck.
Phrynocephalus.–This is a typical Agamoid of the steppes and deserts of Asia. The head is short and thick, the ear is {522}hidden. The body is depressed, devoid of a dorsal crest; on the throat is a transverse fold but no sac.
A. Zander[157] has made interesting observations upon the habits of several species.
Ph. helioscopus lives on hard stretches of soil, which are absolutely bare of vegetation, the soil being baked as hard as a paved road. The lizards live on any insects they can get hold of, chiefly, however, upon mining ants. When chased they run with short jerks, carrying the tail high or rolled up.
Ph. interscapularis occurs, in Transcaspia, on the shifting, loose sand. It runs so fast that one scarcely sees anything but its shadow. The tail is rolled upwards. With short jerks it suddenly changes its direction, stops behind a few blades of grass, or in the open, makes a few shaking, wavy movements, and covers itself lightly with sand. Shortly after that the top of the head appears, the grains of sand rolling off between the strong supraciliary ridges, and the little creature, only about 3 inches long, peeps out of its temporary hiding-place.
Ph. mystaceus, which inhabits Transcaspia and parts of Southern Russia, often faces its aggressor, raising itself upon its fore-limbs, curling and uncurling its tail in its excitement, and holding its mouth widely open. The creature, which attains a length of 9 inches, inclusive of the long tail, then assumes a markedly changed aspect. The flaps of skin at the corners of the mouth swell up into a half-moon-shaped transverse plate, the hinder surface of which is covered by the outer skin, while the front is a continuation of the rosy lining of the mouth, which thereby appears hugely enlarged. When biting it hangs firmly on to the finger. This frightening attitude is interesting, since it occurs in a much more developed condition in the following genus.
Chlamydosaurus kingi.–This peculiar Agamoid, which inhabits Queensland and Northern and North-Western Australia, is easily recognised by the large frill-shaped dermal expansion on either side of the neck. The two halves are confluent on the throat. The whole frill can be erected, and is worked by the much-elongated arches or horns of the hyoid apparatus, which extend into the flaps of skin, somewhat like the ribs of an umbrella. The specially modified hyoidean muscles spread out {523}and fold the frill. When this curious creature is pursued it folds the frill and runs in a semi-erect position upon its hind-limbs, with its fore-limbs hanging down. However, it cannot keep up this peculiar gait for long, and it then suddenly turns to bay, frequently at the root of a tree, which it can climb with ease. When standing at bay it spreads out the shield to its full extent, in the middle of which appears the widely opened mouth, which is red inside and armed with powerful teeth. Altogether this lizard presents a formidable aspect, and is an enemy not to be despised, considering that it is strongly built and grows to nearly 3 feet in length. For a further account of the habits and of the mechanism of the frill see De Vis.[158]
Physignathus.–This is a water-loving genus, inhabiting well-watered districts with luxuriant vegetation in Australia, Papuasia, Siam, and Cochin China. The body and the very long tail are laterally compressed and furnished with a low, serrated crest. Ph. lesueuri of Queensland reaches a length of about 18 inches. The general colour is dark olive above, with darker and lighter {524}cross-bands, and with a broad black band reaching from the eye to the shoulder. The under parts are pale olive, with small black dots. The throat, although devoid of a special sac, is frequently bulged out by the hyoid apparatus, as shown in Fig. 127, taken from a specimen in the Zoological Gardens in London.
Uromastix is a typical desert-form, inhabiting the dry and sandy tracts of North Africa, Arabia, Syria, Persia, and North-Western India. The genus is easily recognised by the short and thick tail, which is covered with whorls of large spinous scales, while the much-depressed body and head are almost smooth, being covered with very small scales. The tympanum of the ear is quite exposed. The incisors are large, uniting in the adult into one or two pairs of large cutting teeth, separated from the molars by a toothless space. There is a transverse fold on the throat. Pre-anal and femoral pores are well developed.
These "Spiny-tailed Lizards" live chiefly upon vegetable food, leaves, grass and fruit, but they vary this diet with insects, at least in captivity, where they become rather partial to meal-worms. They are absolutely terrestrial and diurnal, preferring sandy places, where they bask or rather roast themselves in the sun; for the night, at the approach of rain, or on dull and chilly days, they retire into their burrows, which they dig in {525}the sand or in the hard ground, unless they hide in the cracks of rocks. They have a regular mania for digging with their strong limbs and short, curved claws. Although they love a great amount of heat, and become stiff when cooled down to about 16° C. = 60 F., they can stand several degrees of dry frost without injury. During the cold season they hibernate. The spiny tail is used for defence. The lizard lies as a rule in such a position in its hole that the tail blocks the narrow passage; when touched with the hand it deals out jerky side-blows with the tail. The bite is deliberate and very painful.
U. hardwicki is a native of North-Western India and Beluchistan, occurring especially in Sindh and Rajputana, for instance near Delhi and Agra. This species is of a delicate sandy colour, with dark dots or vermiculations, interspersed, occasionally, with pale blue specks. The under parts are whitish on the tail with a greenish hue. A distinctive and obvious mark is a large blackish patch on the anterior side of the thigh. Total length up to one foot.
I have several times received consignments of the Indian Spiny-tailed Lizard through the kindness of friends, but I must confess that they are far less easily kept than one is led to believe from certain exaggerated accounts. They are lovely, most interesting, and surprisingly tame creatures. I received one lot in the month of June. They made burrows in the dry soil, basked in the sun and on the grassy sods of their roomy cage, and showed great curiosity. When approached, they at first scrambled off or sank down flat, shut their eyes and feigned death. They then opened their tiny yellow eyes a little, while others peeped out of their retreats to see if all was safe, or attracted by some noise. Soon they became so tame that they crawled over my hand. But the difficulty consisted in feeding them. They greedily lapped up drops of water. Their dung consisted of the indigestible parts of some species of Equisetum or Mare's tail, mixed with fragments of beetles and ants. Lettuce, cabbage, cauliflower, grass, the flowers of red and white clover, Mare's tail, wheat, rice, and Indian corn were offered, but they only took a few blades of grass and the hard Indian corn, besides meal-worms. This is all the more astonishing since other specimens are known to partake freely of herbaceous food. None of them survived the late autumn, and most of them succumbed to a disease {526}known as intussusception of the gut. They certainly could not complain of the want of heat, since the bottom of their cage was kept permanently warm by a lamp, and in the autumn they invariably slept in the warmest part of the soil, avoiding the cool regions which would have given them a chance of hibernating.
Another consignment arrived in the month of February. None of them ate anything or survived the early summer.
U. acanthinurus and U. spinipes are common in Algeria, Tunis, and Egypt, where they prefer sandy and rocky localities. Their Arabic name is Dab. In Algeria they are sometimes called "lézards des palmiers," perhaps because they eat dates, besides berries, grass, and various flowers. Very large specimens attain a length of 18 inches. Like the other species of Uromastix they have no voice. The African species can change colour to a great extent. At a low temperature they are mostly grey or brownish black above, dirty white below. When it is warmer they change to lighter shades of brown or even to orange yellow and to green, with black or brown specks and vermiculations. {527}A young specimen of U. acanthinurus has been observed to grow within twelve months from 90 to 150 mm. in length.
Moloch.–The mouth of this peculiar-looking creature is very small; the lateral teeth of the upper-jaw are implanted horizontally and directed inwards. The body is much depressed, and, like the short tail and head, is covered with small scales or tubercles intermixed with large spines. M. horridus, the only species, inhabits the sandy districts of Western and Southern Australia. Nothing is known about its habits except that it seems to live upon ants. Its extremely rough skin is, according to an accidental observation by Dr. Willey, highly hygroscopic. He happened to put a live specimen into a shallow dish with water, when, to his surprise, the water was sucked up as by blotting-paper.
Liolepis.–The body is depressed, without a crest, and is covered with minute granular scales. The tail is long, and has small keeled scales. There is a strong transverse gular fold, and a fold along the side of the body. The tympanum is distinct. Femoral, but no pre-anal, pores are present.
L. belli, the only species, about 18 inches long when full grown, is a native of South-Eastern Asia. The general colour is brownish, with pale black-edged spots along the back; the sides are marked with black and orange vertical bars; the under parts are orange, variegated with blue. Annandale remarks that this is perhaps the commonest lizard on the barren stretches of sand in Lower Siam, especially near the coast. It is exceedingly active and timid. Though its colour is brilliant, the green and {528}grey eye-like spots which ornament its back, and the orange and purple stripes on its sides, are not conspicuous amidst the natural surroundings, the former harmonising with the shadows cast upon the sand by the scanty vegetation which it supports, and the latter being more or less concealed by the folds into which the skin that covers the ribs naturally falls. When the male is roughly handled and is unable to use its powerful jaws, it flattens its body in such a way that the stripes become most conspicuous. The female is unable to do this with such effect, as her ribs do not seem to be so mobile and her colours are less bright. Liolepis lives in holes in the ground, which often go down vertically for more than 2 feet before there is a bend in their course. Each burrow generally contains a pair of these lizards, which, according to the natives, are strictly monogamous.
Fam. 2. Iguanidae.–Pleurodont lizards with a short and thick, non-protractile tongue; almost entirely American, with the remarkable exception of two genera, Hoplurus and Chalarodon in Madagascar, and one, Brachylophus, in the Fiji Islands. Most of the Iguanidae are insectivorous, but some of the most striking forms are herbivorous, e.g. Iguana, Amblyrhynchus, and Basiliscus. In their general structure the Iguanidae closely resemble the Agamidae, from which they differ chiefly by the pleurodont dentition. The orbit is surrounded by bone, and the temporal fossa is bridged over by an arch which is formed by the junction of the squamosal chiefly with the postorbital, the jugal taking as a rule less share in the arch. Dermal ossifications are absent on the body.
There are about three hundred different species, which have been grouped into about fifty genera, representing arboreal, terrestrial, burrowing, semi-aquatic forms, and even one semi-marine species. Their external appearance varies consequently within wide limits.
Anolis is distinguished by the partial dilatation on the middle phalanges, which carry a series of transverse adhesive lamellae. In its general shape Anolis resembles slenderly built and long-tailed Lacertidae, which it may be said to represent in tropical and sub-tropical America, inclusive of the West Indian Islands. The males have a large gular appendage, which can be distended by the hyoid bones. Anolis is an expert climber, living in trees, or rushing about on fences or walls of houses in search of insects; {529}most species can change colour to a great extent. More than a hundred species are known, of which we mention only one, very common in the Southern United States.
A. carolinensis of the South-Eastern United States and of Cuba is beautiful golden green on the whole upper surface; the gular sac becomes vermilion when stretched; when flaccid, it is white with occasional red lines and spots. The under parts are white. In cold weather and in confinement this little lizard, which is scarcely 6 inches in length, appears dark brown, sometimes with a white line along the back. The changes of colour are very sudden. They are thoroughly arboreal, leaping from leaf to leaf like Tree-frogs.
In Anolis, Polychrus, Hoplurus, Chalarodon, Liosaurus, and a few others, the posterior ribs are much elongated and imbedded in the abdominal muscles, often reaching the medioventral line, a feature elsewhere known in the Geckos only.
Polychrus.–The body is laterally compressed, covered with small scales, but devoid of crests. The digits are likewise compressed, with keeled lamellae on the under surface and with four large scales at the base of each claw. Both sexes have femoral pores. The male possesses a small gular sac. A few species in Tropical America.
P. marmoratus in South America, where it is often called the Chameleon on account of its power of changing colour. The tail is nearly three times as long as the head and body, and is covered with keeled scales. The general colour of this arboreal creature, {530}which reaches a length of 18 inches, is green, but the hues are very variable, and within a short time the creature can change into dull brown, with or without blackish spots and bands, or with whitish spots and black lines on the head and other parts of the body.
Basiliscus is remarkable for the high and erectile crests which are developed on the back and tail of the males. The toes are bordered on the outer side with small lobes. The limbs are long, the hind-limbs when stretched forwards reaching the tip of the snout. Several species in Central America and the adjoining countries to the south.
B. americanus reaches the considerable length of nearly 3 feet. The male has a crest on the top of the head, and this is produced backwards into a leathery lobe. The back is adorned with a very high crest; the folds and dark-coloured marks in which give, in the accompanying illustration, the impression that the crest is supported by spines. The long tail carries a similar crest. The general colour of the "Basilisc" is green and brown with dark cross-bars on the back. The crest of the male is said to be red. These creatures are very common amidst the {531}luxuriant vegetation on the banks of the rivers of the Tierra Caliente of Mexico and in Guatemala. They lie upon the branches of trees, preferring those which overhang the water, into which they plunge at the slightest alarm. The high crests, being restricted to the male sex, are not essential to their swimming; they propel themselves by rapid strokes of the fore-limbs, letting the long rudder-like tail drag behind. The eggs, measuring 20 by 13 mm., are laid in April or May, and are hidden in a hole at the base of a tree. About one dozen make a set, and they are said to be hatched within a very short time. Owing to their being strictly herbivorous, these pretty and striking-looking creatures do not endure captivity in Europe, unless indeed their particular food can be procured.
Iguana.–The body and tail are laterally compressed and are covered with very small scales, while those on the top of the head are large. The neck and back carry a high crest, which is composed of separate, laterally compressed, soft spines. A similar but lower crest borders the anterior edge of the large gular sac, which is not dilatable. The lateral teeth are remarkable for their finely serrated or denticulated anterior and posterior edges. Both sexes have long rows of femoral pores. Only two species in Tropical America, absolutely herbivorous. Their delicate flesh is much esteemed as food.
I. tuberculata (Fig. 132), of South and Central America and the West Indies, reaches a length of 5 to 6 feet. The general colour of the upper parts is a mixture of green and blackish, frequently speckled with white or yellow, and there is usually a pale band in front of each arm. The flanks are marked with dark, light-edged bars. The under parts are pale greenish or whitish. The Iguanas live in the trees, and when there is danger they jump into the water whatever the height of the tree, coming down with violence. In going up some of the narrow, unfrequented creeks in the Mosquito country, according to Napier Bell,[159] the voyager often encounters quite a shower of falling Iguanas, and runs some risk of getting his neck broken. Large specimens, 6 feet long, weigh perhaps 30 lbs. They burrow deep horizontal holes in the sloping side of a bank. About two dozen eggs, nearly 2 inches long, are laid in a hole, where they are hatched in the month of May.
Iguanas are often brought to the markets, either lashed lengthwise to a branch of the tree on which the specimen happened to be surprised, or tied up with the long tendons of their own toes.
Metopoceros cornutus of Hayti is closely allied to Iguana, but the male has three conical horn-like scales on its head. The general colour of the whole animal, which grows to more than one yard in length, is dull black.
The following two genera, each containing one species only, are restricted to the Galapagos Islands. Darwin[160] gives a long and vividly written account of their habits.
Conolophus subcristatus.–Fully grown specimens are a yard long. Their shape is stout, the head and fore part of the body appearing especially heavy. The head is covered, or rather paved, with large cobble-stone-like scales. On the neck is a low crest of recurved spines, while the median line of the back appears simply serrated. All the teeth are trilobate. A gular sac is absent. The coloration is striking. The head is lemon-yellow; {533}the back is red, merging into dark brown on the flanks. The belly is dark yellow with a tinge of reddish brown.
This lizard was found by Darwin on some of the Galapagos Islands. On James' Island it was so common that the party found it difficult to pitch their tent, on account of the ground being undermined by the many burrows of the reptiles. They feed during the daytime upon the succulent cactus and the leaves of various trees. The perfectly harmless creatures are, or were, eaten by the inhabitants.
Amblyrhynchus cristatus is closely allied to Conolophus, of which it may be said to be an aquatic modification. The top of the blunt head is covered with low, conical, broad-based scales. Over the neck, back, and tail extends a continuous crest of low, recurved, spiny scales. All the teeth are trilobate. The body and even more so the tail are laterally compressed. The general colour is dark brown above, paler and inclining to whitish below. Younger specimens have pale grey spots and blackish cross-bands on the back and sides. Total length up to 4 feet. The remarkable feature of this Iguanoid is its semi-marine life. It inhabits the rocky and sandy strips of coast of most of the Galapagos Islands, feeding upon certain kinds of algae, which it has to dive for, since these plants grow below tide-marks.
Phrynosoma ("Horned Toads").–The body of these little creatures is much flattened and broadened, devoid of a dorsal crest, but covered with larger and smaller, strongly keeled scales. The head is bordered posteriorly by conspicuous osseous spines. The under parts are covered with small, very regular scales. Both sexes have a long row of pores on the under surface of the thighs. The general colour of the upper parts is a mixture of yellow, grey, brown, and black, the larger spiny scales causing the animal to look as if it were sprinkled with the dried husks of seeds, for instance those of Buckwheat. The object is concealment, by close adaptation to the arid, sandy localities which are the home of "Horned Toads." About one dozen species inhabit the western half of the United States and Central America. All the species are viviparous, almost the only instance among Iguanidae.
Ph. cornutum has five spikes on each side of the head: one postorbital, three temporal, and one occipital, the latter being by far the largest. The sides of the lower jaw project in the shape {534}of prominent ledges, and are protected by a series of small spines. The ventral scales are keeled. The under parts are yellowish, frequently with a few brown spots. This species, which grows to a length of 5 inches, ranges from Illinois through Kansas and Texas to Northern Mexico.
Ph. coronatum, an inhabitant of California, has an additional smaller spine between the two large occipitals. The scales of the belly are quite smooth.
These peculiar-looking and interesting creatures recall some of the extinct Dinosaurs in the curious configuration of their head: small miniatures indeed. In order to be kept in good health, and to be observed properly, they require, above all, warmth, sunshine to bask in, sand to burrow in, and proper food. The latter consists of all kinds of small insects, the necessary variety of which is best procured by making sweepings with a butterfly-net in a meadow. They take green-flies, house-flies, ants, smooth caterpillars, small moths, meal-worms, wood-lice, etc. The food is snapped up very quickly by a flash of the tongue, which can be turned out, almost like that of a frog, but only to the extent of half an inch. Water in the shape of dew, or drops, is absolutely necessary. When in good condition, they defaecate regularly every alternate day.
They love to bask in the broiling sun, heating themselves well through; and in the afternoon, long before sunset, when the sand is warmed up to 40° C., or fever-heat, they prepare to go to bed. For this they select a dry and soft spot, and within a few minutes manage to dig themselves in flat, literally sinking into the sand by pushing themselves forwards, and by shovelling the sand upon their backs with peculiar motions of the fringed sides of their flat bodies. Sometimes the spines of the head remain sticking out, looking like dry thorns scattered over the sand. To prevent the latter from getting into the nostrils, these are provided with closely-fitting valves. Thus they remain concealed during the night, and not until the sun is well up do they leave their hiding-place, first peeping out, and then raising their head and neck, letting the sand roll off between the spines. Still half concealed, the back covered with little pebbles, seeds, or bits of dry leaves, they wait for a long time before they feel lively enough to sally forth. Although mostly slow and deliberate in their movements, stalking about with arched back, and raised upon the fore-limbs, they can run fast enough for a few yards before they stop again and nod in a ridiculous way. When they see themselves observed, they shut their eyes and slowly sink {536}down. On cool and dull days they do not appear at all, and during part of the cooler season they require artificial heat until they are ready to hibernate. Unless they are allowed to hibernate, they will keep on feeding through the winter, but in that case are sure to die in the following spring.
Fam. 3. Xenosauridae, with Xenosaurus grandis in Southern Mexico as the sole species, seems to connect the Iguanidae with the Anguidae. According to Boulenger, its affinity to the former is shown by the T-shaped interclavicle, the absence of symmetrical bony shields on the head and of osteodermal plates on the body. Affinity to the Anguidae is indicated first by the short tongue, which has a narrow, feebly incised, retractile anterior part, covered with flat papillae; secondly, by the teeth, which, instead of being hollow at the base, are solid; lastly, by the palatine bones, which are widely separated.
X. grandis, scarcely one foot in length. The body is depressed, covered above with minute granules and tubercles, below with smooth scales. A distinct fold of skin extends from the axilla to the groin, recalling the more strongly developed lateral fold of some of the Anguidae.
Fam. 4. Zonuridae.–This family, comprising four genera with about one dozen species in South and Tropical Africa, and in Madagascar, likewise seems to connect Iguanidae and Anguidae. It is distinguished from the former by dermal ossifications, which roof over the supratemporal fossa; from the latter by the tongue, the hollow teeth, and, in Zonurus at least, by the occurrence of dermal ossifications on the trunk and tail. The tongue is short, villose, scarcely protractile, entire, or but feebly nicked at the tip. The Zonuridae may therefore be defined as pleurodont African lizards with a short tongue, and with a bony roof to the supratemporal fossae.
Zonurus.–The whole head, back, and tail are covered with bony scales, the horny covering of which forms very sharp spikes, especially on the tail. The body is depressed. The ear-opening is large. South Africa, in dry and rocky localities; one species, Z. tropidosternum, in Madagascar.
Z. giganteus s. derbianus, with strong spikes on the occiput, neck, and tail. General colour yellowish brown. Total length about 15 inches.
Chamaesaura of South Africa closely approaches the {537}Anguidae by its snake-shaped body, extremely long tail, and vestigial limbs. In Ch. aenea both pairs of limbs are still present and pentadactyle, but are very small; in Ch. anguina the limbs are reduced to little styliform stumps; and in Ch. macrolepis they are altogether absent. The scales of the body and tail are strongly keeled and imbricating, but are devoid of dermal ossifications. Total length up to 2 feet.
Fam. 5. Anguidae.–Pleurodont lizards with osteoderms, and with the tongue composed of two distinct portions, of which the anterior is thin, emarginate, extensible, and retractile into the posterior thicker portion. The supratemporal fossa is roofed in by dermal bones. The whole body is protected by bony plates underlying the imbricating scales. The teeth vary much in shape, but they are always solid, the new teeth not growing into the base of the old ones, but between them. The limbs are in a very unstable condition, there being in the family a general tendency to reduce and lose the limbs. The shoulder- and pelvic-girdle however remain, although sometimes merely vestigial. The tail is long, very brittle, and easily reproduced. All the Anguidae are strictly terrestrial, and live on animal diet. {538}Some Anguis, at least, are viviparous. The distribution of the seven genera, with some forty species, is very scattered. The majority, chiefly Gerrhonotus, inhabit Central America, a few occur farther north and south–two, Anguis fragilis and Pseudopus pallasi, in Europe, and one in the Himalayas and in Burmah.
Gerrhonotus has a pair of deep longitudinal folds, each of which extends from the region of the neck along the side of the body towards the tail. The four limbs are well developed and pentadactyle. The teeth are conical. Many species, mostly in Central America. G. coeruleus has the widest range, extending from Costa Rica to Vancouver. It is also one of the largest species, reaching a length of more than one foot. The tail is nearly twice as long as the rest of the body. General colour above brown with blackish bars and spots, especially on the more yellowish flanks; under parts whitish with a greenish tinge, often with brown spots arranged in longitudinal rows.
Ophisaurus s. Pseudopus is closely allied to the previous genus, being possessed of the same kind of deep lateral folds; the limbs are, however, reduced to a pair of tiny spikes, half concealed at the sides of the anal cleft. The teeth are conical, and in the adult have somewhat flattened crowns. The body and tail are very long and snake-like, but the head is that of a typical Lizard.
O. apus s. Pseudopus pallasi, the Glass-Snake of the Balkan Peninsula, South Russia, Asia Minor, and Morocco, grows to more than one yard in length, of which about two-thirds belong to the tail. The general colour is brown above, paler below. Young specimens are olive-grey with dark brown cross-bands on the back. O. gracilis inhabits the Eastern Himalayas and Burmah, the others live in North America.
The "Glass-Snake" inhabits bushy localities, where it can hide under the fallen leaves and in the sand; it cannot climb, and avoids the water. Its movements resemble those of a snake, but are far less graceful, owing to the stiff armour in which the whole body is encased. The food consists chiefly of snails, the shells of which are crushed, and of mice, but nothing comes amiss which can be mastered, namely insects, worms, small lizards, young birds, and vipers. The prey, when caught, is rapidly twisted round and round, or shaken until it is giddy or stunned, whereupon the Glass-Snake proceeds to chew it with its powerful jaws, and then to swallow it in pieces. {539}Although it can bite so well, it never does so when caught, but resorts to the much more disagreeable defence of twisting itself around one's hand and arm, and besmearing them with its disgustingly stinking excrements. Those who have observed Glass-snakes praise their tameness, and the intelligent way in which they hunt about in search of their food. They lay eggs under moss and leaves, and the young seem to require many years to grow up.
Anguis, with only one species, A. fragilis, the "Slow-worm" or "Blind-worm," is devoid of a lateral fold. Limbs are entirely absent. The whole body is covered with smooth roundish scales, with a substratum of dermal ossifications. The teeth are curved backwards, fang-shaped, and have a very faint longitudinal groove on their anterior surface. The ear-opening is very minute, more or less hidden by surrounding scales. The eyes are perfectly well developed, provided with movable lids, and it does not speak well for the power of observation of most people that this creature should generally be known as the "Blind-worm." The whole skin is shiny, metallic, quite smooth, brown above, blackish below. But the coloration is subject to much individual variation. Old specimens are sometimes adorned with blue specks. The very young are exquisitely beautiful, the upper surface being silvery white, with a median and two more lateral lines of deep black; the under parts are black. The iris is yellowish red. Very large specimens measure more than one {540}foot in length, more than half of which belongs to the tail. One in the British Museum is 425 mm. = 17 inches long.
The Slow-worm is viviparous, i.e. the young are fully developed, and burst the transparent, soft, yellowish eggs immediately after these are laid. This takes place in the months of August or September, about one dozen making a litter. The little creatures are at first about one inch and a half long, and as thin as an ordinary match. They eat the smallest of spiders and delicate insects; later on earth-worms, which they bite into and then suck out before devouring them. When six weeks old and well fed they are about 3 inches long, but it is at least four or five years before they are mature. The little ones carefully avoid the hot sunshine, and the adults are likewise rather partial to the shade, although strictly diurnal. Their chief food consists of earth-worms and slugs. For the night they retire under moss, leaves, stones, or into the ground. In the autumn the Slow-worms dig passages or burrows, which often serve as the winter-quarters of many specimens, as if there were no other place available, or rather as if the spot selected were by far the best with regard to safety, dryness, and warmth.
Fam. 6. Helodermatidae.–Pleurodont, poisonous lizards of North America. The teeth are fang-like, recurved, with slightly swollen bases, rather loosely attached to the inner edge of the jaws. Each tooth has a groove on its anterior and posterior surface, and a series of labial glands which secrete the poison open near the bases of the teeth of the lower jaw. The skull has strong postorbital but no postfronto-squamosal arches. The pre- and post-frontals are in contact, separating the frontal from the orbit; the premaxillaries are fused into one; the nasals and frontals remain separate. The limbs are short, but strong and well developed. The tongue is villose, with an anterior smooth portion, which is bifid and protractile, resembling the tongue of the Anguidae and of Aniella. The skin of the upper surface is granular, with many irregular bony tubercles, which give it an ugly warty look. The under parts are covered with flat scales.[161]
Heloderma, the only genus, with H. horridum in Mexico and H. suspectum in New Mexico and Arizona, reaches about 2 feet {541}in length. The animal, stout, depressed, thick-tailed, looks rather repulsive when it squats down in its usual lethargic way. The whole skin is blackish brown and yellow or orange, these two "warning" colours being distributed unevenly, except on the thick, peculiarly-shaped tail, where they are arranged in alternate rings. The specific differences are rather imaginary. The New Mexican form is supposed to be more orange and yellow than black, with a somewhat smoother skin and with shorter toes and tail.
The "Gila Monster" inhabits dry localities, spends most of the daytime in concealment between the roots of trees, and crawls about in the evening in search of worms, centipedes, frogs, and the eggs of large lizards. Frogs are probably paralysed or killed by the bite which, although not so dangerous as that of poisonous snakes, is effective enough to produce severe symptoms even on man, and a few cases of death of people who had been bitten are on record. In captivity they are very partial to eggs, which they break and then lap up. During the dry and hot season they aestivate.
Fam. 7. Lanthanotidae.–Lanthanotus borneensis, of which only two specimens are known, one in the Vienna Museum, the {542}other in the Sarawak Museum, was described by Steindachner as the type of a distinct family, near the Helodermatidae. Boulenger,[162] after examination of the Sarawak specimen by means of a sciagraph, has come to the conclusion "that the affinity of Lanthanotus to the Helodermatidae is fully confirmed." The teeth of Lanthanotus show, however, no traces of grooves; poison-glands are probably absent, and there are no osteoderms. The skin is covered with wart-like tubercles, each with a horny, peeled scale. The eyes are very small, the ears are concealed. The general colour is reddish brown above, yellowish, with brownish bands, below. Total length about one foot, a little more than half of which belongs to the roundish tail.
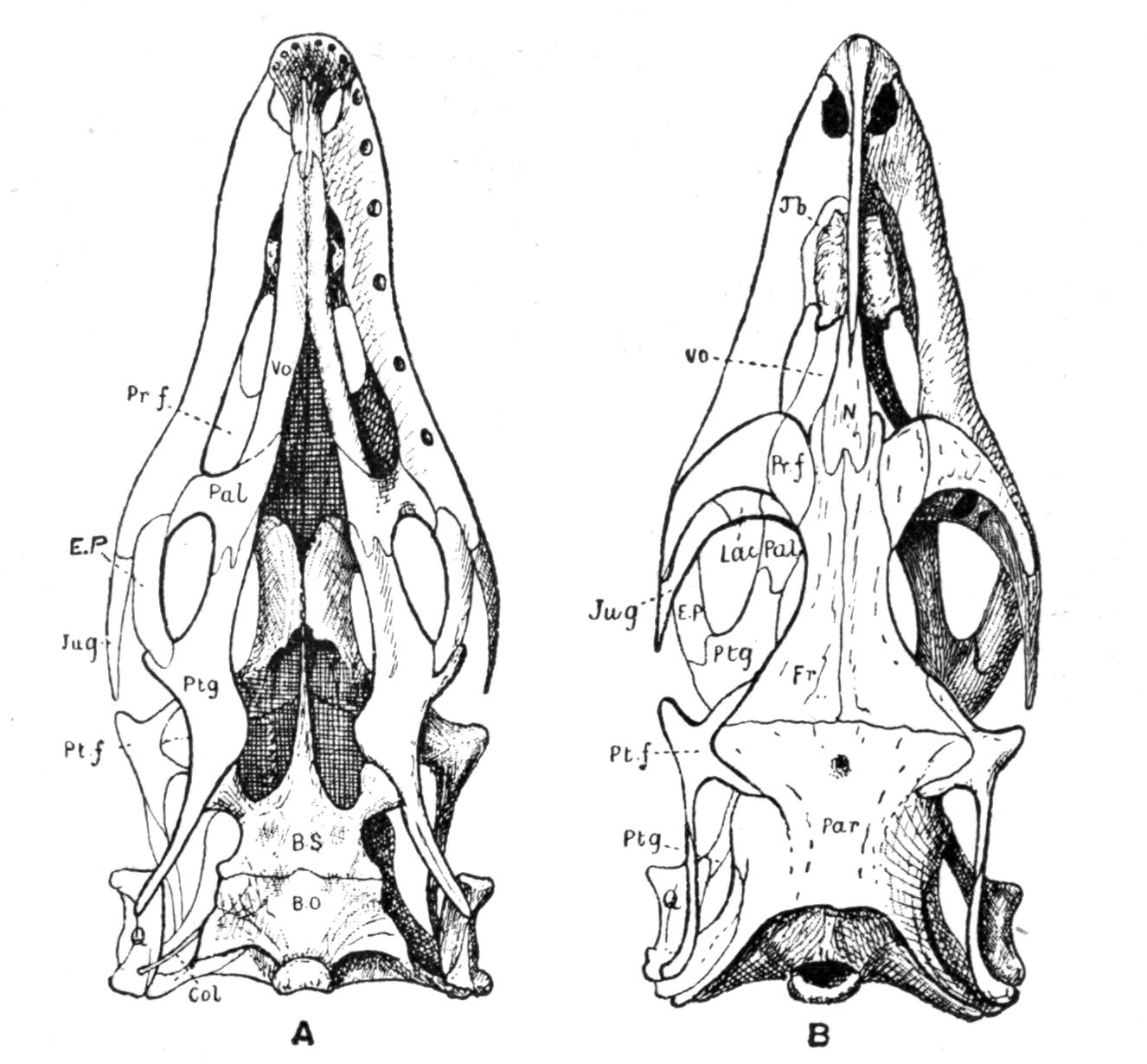
Fig. 138.–A, Ventral, B, dorsal view of the skull of Varanus griseus. × 1. B.O, Basi-occipital; B.S, basisphenoid; Col, columella auris or stapedial rod; E.P, ectopterygoid; Fr, frontal; Jug, jugal; Lac, lacrymal; N, nasals; Pal, palatine; Par, parietals; Pr.f, prefrontal; Pt.f, postfrontal, fused with postorbital; Ptg, pterygoid (endopterygoid); Q, quadrate; Tb, turbinal; Vo, vomer.
Fam. 8. Varanidae.–Pleurodont Old-World Lizards, with a long, deeply bifid and protractile smooth tongue. They reach a large size, and the neck is relatively much longer than that of other lizards. The limbs are well developed. The skin is {543}covered with very small juxtaposed scales and tubercles above, while the ventral scales are squarish and arranged in transverse rows. Osteoderms are entirely absent. The tail is very long, often laterally compressed. The teeth are large and pointed, dilated at the base. The premaxilla is unpaired and dorsally extends backwards to the likewise unpaired nasal. There is a pair of small supra-orbital bones, easily lost during maceration. The orbit is open behind, the jugal being short and not meeting the postfrontal; the postorbital forms a slender arch with the supratemporal. The vomers are long and diverge posteriorly. The palatines, pterygoids, and ectopterygoids enclose on either side an oval infra-orbital foramen. The Varanidae contain only one genus, Varanus, with nearly thirty species in Africa, Southern Asia, and Australia, but not in Madagascar.
Varanus.–The name of "Monitor" bestowed upon these creatures has a curious origin, owing to a ridiculous etymological mistake. The Arabic term for Lizard is "Ouaran"; this has been wrongly taken to mean warning lizard, hence the Latin Monitor, one of the many synonyms of this genus, e.g. Hydrosaurus and Psammosaurus. Many of the "Monitors" are semi-aquatic, others inhabit dry, sandy districts, while others are at home in well-wooded localities. They are all rapacious, taking whatever animals they can master according to their size, which in some species amounts to 6 or 7 feet.
V. niloticus inhabits the whole of Africa, except the north-western part. It reaches a length of more than 5 feet. The colour of the adult is brownish or greenish grey above, with darker reticulations and yellowish ocellated spots on the back and limbs. The under parts are yellowish with blackish cross-bands. The ground-colour of the young is black above with yellow lines on the head and neck, and with yellow spots on the back and limbs; the tail has black and yellow bars.
V. salvator ranges from Nepal to Ceylon, Cape York, and {544}Southern China, inclusive of the Malay Islands and the Philippines. This is the largest species, specimens of 7 feet in length being on record. The general colour is dark brown or blackish above, with yellow spots or ocelli. The snout and chin have transverse black lines on a lighter ground. A black band, bordered with yellow, extends from the eye along the side of the neck. The under parts are yellow.
Mr. Annandale has favoured me with the following observations:–"Varanus salvator is common in Lower Siam, where it is equally at home on land, in water, and among the branches of trees. The eggs are laid in hollow tree-trunks. When in the water the lizard swims beneath the surface, the legs being closely applied to the sides, and the tail functioning both as oar and as rudder. Their food is very varied. In the states of Patalung and Singora, in which the Siamese practise a form of tree-burial, these great lizards are accused, probably with justice, of eating the flesh of the corpses in the aërial coffins. I have disturbed a large Monitor devouring the body of one of its own species, which had evidently been dead for some days. Another, which was chased by some men, dropped from its mouth a small flying squirrel (Sciuropterus); a third, which I dissected, had lately swallowed a small tortoise, the hard shell of which had been broken into innumerable fragments. The stomachs of several others contained nothing but dung-beetles, for which Varanus may often be seen hunting, turning over the dung of elephants and buffaloes with its fore-feet. The Malay name of these lizards is Biawak."
According to Mason and Theobald[163] all the Varanidae and their eggs are highly esteemed for food, and are sought for in hollow trees with the aid of dogs. If not wanted at once, the wretched creature has its fore-feet bent over its back, a few of its toes are broken and the sinews drawn out and tied into a knot, rendering the animal helpless. The Karens, who are extravagantly fond of the flesh, steal up the tree with a noose at the end of a bamboo, and often noose them while leaping for the water, or catch them in a boat which is brought under the tree. The head, the natives say, is venomous, and they discard it altogether, but the flesh of the other parts, which smells most odiously, is deemed preferable to that of fowls.
Sir J. G. Tennent[164] gives the following account of V. salvator:–
"The 'Kabara-goya' of the Singhalese is partial to marshy ground, and when disturbed upon land will take refuge in the nearest water. From the somewhat eruptive appearance of the yellow blotches on its scales, a closely allied species, similarly spotted, obtained the name of Monitor exanthematicus, and it is curious that the native appellation of this one, Kabara, is suggestive of the same idea. The Singhalese, on a strictly homoeopathic principle, believe that its fat, externally applied, is a cure for cutaneous disorders, but that taken inwardly it is poisonous. The skilfulness of the Singhalese in their preparation of poisons and their addiction to using them are unfortunately notorious traits in the character of the rural population. Amongst these preparations the one which above all others excites the utmost dread, from the number of murders attributed to its agency, is the potent kabara-tel, a term which Europeans sometimes corrupt into cobra-tel, implying that the venom is obtained from the hooded-snake; whereas it professes to be extracted from the Kabara-goya.
"In the preparation of this mysterious compound, the unfortunate Kabara-goya is forced to take a painfully prominent part. The receipt, as written down by a Kandyan, was sent to me from Kornegalle by Mr. Morris, the civil officer of that district; and in dramatic arrangement it far outdoes the cauldron of Macbeth's witches. The ingredients are extracted from venomous snakes by making incisions in the head of these reptiles and suspending them over a basin to collect the poison as it flows. To this, arsenic and other drugs are added, and the whole is boiled in a human skull, with the aid of three Kabara-goyas, which are tied on three sides of the fire, with their heads directed towards it, and tormented by whips to make them hiss so that the fire may blaze. The froth from their lips is then added to the boiling mixture, and so soon as an oily scum rises to the surface, the kabara-tel is complete. Before commencing the operation of preparing the poison, a cock has to be sacrificed to the demons.
"This ugly lizard is itself regarded with such aversion by the Singhalese that if one enter a house or walk over the roof, it is regarded as an omen of ill-fortune, sickness, or death; and in {546}order to avert the evil, a priest is employed to go through a rhythmical incantation."
Captain Robinson, renowned as a hunter of tigers on foot in the old days of muzzle-loading rifles, has told me the following unique use to which these large lizards are put by ingenious thieves in India. In order to be able to get over a wall too high for climbing without assistance, the thief provides himself with a strong lizard, ties a rope round its waist and lets the animal go, when it at once scales the mud wall by its strong and sharp claws, and jumps down on the other side. The weight of the lizard, which, moreover, holds vigorously on to the ground, and the friction of the rope on the top of the wall, are sufficient to help the man over!
It is a sight, never failing in its attraction to the visitors of the Zoological Gardens in London, to see one of the big Monitors fed with an egg. The lizard knows the treat well that is in store for it. It raises itself up high in expectation, then examines the egg with the long tongue, takes it up gingerly and swallows it entire, crushing it by the contraction of the muscles of its gullet. On one occasion it was given a rotten egg which burst in its mouth, and the lizard refused for a long time to take another.
V. gouldi is common in Australia and in New Guinea. It reaches a length of about 4 feet. Its colour is brown above {547}with yellow spots on the back and limbs, and with yellow rings on the tail. Two yellow streaks separated by a black band extend from the temples along the side of the neck. The under parts are yellowish, sometimes with black spots.
Fam. 9. Xantusiidae.–Three Californian, or West-Indian genera, with less than half-a-dozen species. Pleurodont with a short tongue and with the supratemporal fossa roofed over by bone. The tongue is scarcely extensible, with oblique overlapping folds which converge towards the median line, and with scale-like imbricate papillae towards the tip. The skull possesses complete postorbital and postfronto-squamosal arches, the latter meeting the parietals and roofing over the supratemporal fossa. The palatines are in contact with each other, and there are no infra-orbital fossae. There are no osteoderms; the body is covered above with small granular scales, below with larger scales. The eyes are devoid of movable lids. The tympanum is exposed. Femoral pores are present. Limbs and tail well developed. Xantusia and Lepidophyma.
Fam. 10. Tejidae.–American Lizards with a long and bifid tongue. The greater portion of the tongue is covered with scale-like papillae; the anterior forked and pointed ends are smooth. The teeth are solid and implanted almost upon the edge of the jaw, being therefore intermediate between the acrodont and pleurodont condition. The shape of the posterior teeth shows several modifications; they are conical or tricuspid, or molar-like in the adult Tejus; in Dracaena they are transformed into large, oval crushers. The palatines are in contact anteriorly. The infra-orbital fossae are surrounded by the palatine, pterygoid, and ectopterygoid bones, the maxillary being excluded from the fossa, as in Varanus (see Fig. 138, p. 542). The skull has no supra-temporal arch. Osteoderms are absent; the body is covered with small scales, or the skin is simply granular above; the under surface is covered with larger scales, generally arranged in transverse rows.
This large family, which comprises nearly forty genera with more than a hundred species, exhibits great diversity of form. Some are inhabitants of forests and are arboreal, while others are strictly terrestrial, preferring hot and sandy plains, or they dwell below the surface and are transformed into almost limbless and blind-worm-shaped creatures. The range of the family extends over the whole of the South American continent, over the West {548}Indian Islands, and through Central America into the warmer parts of the United States.
Tupinambis ("Teju").–The skin of the back is smooth, covered with small scales; with large scales on the top of the head. The skin on the neck is generally thrown into two irregular transverse folds. The long and narrow tongue is capable of being telescoped into a sheath at its base. The lateral teeth are compressed and tricuspid in the young, but the later generations of teeth have obtuse crowns in the adult. T. teguixin is the largest member of the whole family; it reaches a length of a yard, most of which, however, belongs to the tail. The general colour is bluish black, with pale or whitish-yellow spots on the back, flanks, and tail, combining into more or less transversely arranged bands. The limbs are black, with many and tiny yellow dots. The ground-colour of the under parts is reddish yellow, with irregular black bars. This species inhabits the greater part of South America, east of the Andes, from Uruguay to the West Indies. T. nigropunctatus is confined to the Continent, and lacks the dark cross-bands on the belly, which is uniformly yellowish or speckled with black.
The "Tejus" frequent forests and plantations, and are carnivorous. Their strength and swiftness enable them to catch all kinds of animals, from insects and worms to frogs, snakes, mice, and birds. As they take chickens and eggs from the farms they are considered noxious, and they are frequently hunted down with dogs for the sake of their flesh, which is regarded as good to eat. They defend themselves with lashing strokes of their long tail and with their powerful jaws. They retire into burrows, and they deposit their hard-shelled eggs in the ground. In captivity they can easily be kept on meat.
Dracaena guianensis of the Guianas and the basin of the Amazon has the lateral teeth transformed into regular large molars, with broad and rounded crowns. The tail is strongly compressed, with a double, denticulated keel. It seems to be semi-aquatic, and, to judge from the teeth, herbivorous.
Ameiva and Cnemidophorus, with many species chiefly in tropical America, have laterally compressed bi- or tri-cuspid teeth. The skin forms a double fold on the neck, and is covered on the upper surface of the body with very small scales; those on the ventral surface are large, and arranged in regular rows. Most of the species are small, under one foot in length, and are extremely pretty, very active, timid, and mainly insectivorous.
C. sexlineatus is one of the few species of Cnemidophorus which inhabits the southern half of North America. Like all its relations it has the appearance of an ordinary lizard (Lacerta). The head is dark brown. A purple or brownish band extends over the back and tail, bordered on either side with three golden-yellow longitudinal lines. The flanks are brown, the under parts bluish white. The iris is golden, and the inner margins of the lids are bordered with a narrow band of bright yellow. This species is a very fast runner, and frequents dry and sandy places. Its total length amounts to about 10 inches.
Fam. 11. Lacertidae.–Pleurodont Old-World Lizards, without osteoderms on the body, and with the supratemporal regions roofed over by osteoderms.
The limbs are always well developed, and have five fingers and five toes, always provided with sharp claws. The skin covering the head forms large shields, mixed with small scales; most of which, especially the shields, contain dermal ossifications. These frequently fuse with the underlying bones of the top of the skull.
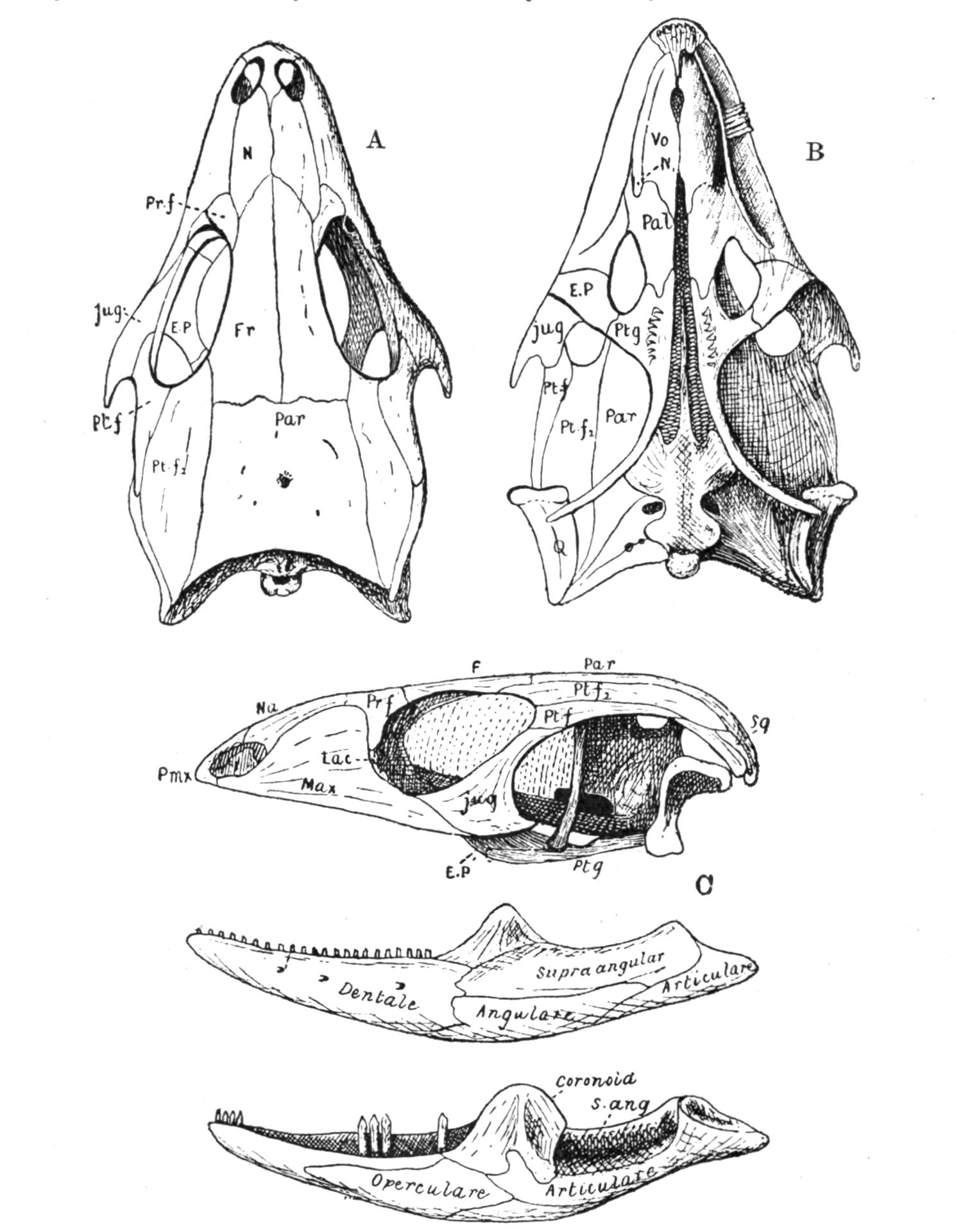
Fig. 142.–Skull and lower jaw of Lacerta viridis. A, Dorsal view; B, ventral view; C, from the left side; D, right half of the lower jaw, from the inner side, with some of the pleurodont teeth. E.P, Ectopterygoid; F, Fr, frontal; jug, jugal; Lac, lacrymal; Max, maxillary; N, Na, nasal; N1, in B, inner narial opening; Pal, palatine; Par, parietal; Pmx, premaxillary; Pr.f, prefrontal; Pt.f, postorbital; Pt.f2, postfrontal; Ptg, pterygoid; Q, quadrate; S.ang, supra-angular; Sq, squamosal; Vo, vomer.
The latter is always well marked off from the neck. The postorbital arch is complete. The temporal region is completely roofed over by bones dorsally, chiefly owing to the size of the postfrontal (Fig. 142, pt.f2) which fills the space between the parietal and the squamoso-postorbital bridge, thus abolishing the supra-temporal fossa. The squamosal is very small, placed {551}between the postfrontal (pt.f2), the lateral occipital and the supratemporal. The large jugal and the quadrate are not connected with each other. The columella cranii is well developed. The infra-orbital fossae are surrounded by the palatines, pterygoids, ectopterygoids, and maxillaries. The palatines and pterygoids remain separated in the middle line. The pterygoids frequently carry little teeth. The other teeth are typically pleurodont, hollow, slightly curved, and bi- or tri-cuspid.
The skin covering the body, the legs, and the tail is devoid of osteoderms. The scales on the dorsal surface vary much in size, from large, strongly keeled scales to tiny granulations. Those of the ventral surface are large, broader than long, and are frequently arranged in regular transverse and longitudinal rows. The tail, generally long and pointed, is very brittle. All the sense-organs are well developed. The tympanum is exposed. The tongue is deeply bifurcated, narrow, flat, and covered with scale-like papillae.
Various Lacertidae, especially some of those genera which live and dig in the sand, have a transparent disc in the middle of the lower eyelid, so that they can see while the eye itself is protected. This is for instance the case in some specimens of the Indian and African Eremias. In the Indian genus Cabrita the transparent disc is very large, and in Ophiops, which inhabits sandy stretches from North Africa to India, the lower eyelid is fused with the rim of the much-reduced upper lid, and forms a large transparent window.
The Lacertidae or True Lizards comprise nearly twenty genera, with about one hundred species, and are typical of the Old World, being found in Europe, Asia, and Africa, but not in Madagascar nor in the Australian region. They are most abundant in Africa. Their northern limit coincides fairly closely with the limit of the permanently frozen under-ground. This is indicated in the map (Fig. 143) by the dotted line. All the Lacertidae live upon animal food, chiefly insects, and after them worms and snails; but the larger lizards take what they can master, frequently other lizards, and even younger members of their own kind. Many of them love sugar, which they lick, and all require water. They are all terrestrial, preferring, according to their kind, such localities as yield them their particular food.
Sunshine and warmth make a marvellous change in the same individual, which on dull, rainy, or cold days lies in its hole, or shows only sluggish movements. Their sense of locality is great, or rather each individual inhabits one place, of which it knows every nook and corner, cranny, tree, and bush. It has its favourite hole to sleep in, a stone, the branch of a tree, or a wall to bask upon, and when disturbed or chased it makes with unerring swiftness for a safe spot to retire into. The same lizard, when once driven away from its own locality, seems to lose all its presence of mind, flounders about, and is comparatively easily caught. Most lizards are extremely curious, although shy, and this state of their mind can be made use of by those who want to catch them without injury, and above all without getting the animal minus the brittle tail. This safe way of catching lizards consists in taking a thin rod with a running noose of thread at the end, in drawing the latter over the lizard's head, and then raising it. The little creature does not mind the rod in the least; on the contrary, it watches it carefully, and often makes for the thread. The boys in Southern Italy have improved upon and simplified this mode of catching lizards by bending the end of a wisp of grass into a noose, and covering the latter over with a thin film of saliva. The shiny film, like a soap-bubble, is sure to excite the curiosity of the creature. The late Professor Eimer[165] refers to this practice {553}as carried out by the children of two thousand years ago, and he sagaciously explains that the beautiful statue of the so-called Apollo Sauroctonos represents a boy who is in the act of noosing the little lizard on the tree.
Lacerta.–A row of enlarged scales forms a distinct collar across the ventral half of the neck, in front of the chest. The scales on the back are much smaller than those on the tail, which is long, round, and pointed. The digits have smooth, tubercular lamellae on the under surface. Femoral pores are well marked. This genus, with about twenty species, ranges through Europe, Northern and Western Asia, and Africa north of the Equator.
L. vivipara, the Common English Lizard, has a very wide range, through Northern and Central Europe and Siberia to the Amoor country and the Island of Saghalien. It occurs throughout Great Britain, even in Ireland, where it is the only species of reptile, occurring, for instance, in the County of Meath and in the south-eastern counties, e.g. Waterford. It does not occur south of the Pyrenees or south of the Alps. The supra-ocular and the supraciliary scales are in contact with each other, not being separated by a series of little granules. Normally there is a single postnasal and a single anterior loreal shield. The ventral scales are arranged in six or eight longitudinal series, of which the second series on each side from the median ventral line is the largest. The coloration of this species is subject to much variation. The general colour of the adult is brown or reddish above, with small darker and lighter spots; many specimens have a blackish vertebral streak and a dark lateral band edged with yellow. The under parts are orange to red in the male, with conspicuous black spots; yellow or pale orange in the female, either without or with scanty black spots. The newly-born specimens are almost black. The males are slightly smaller than the females; males of a total length of 6 inches, and females 7 inches long, may be considered rather large specimens.
This lizard is, as the specific name implies, viviparous, i.e. the six to twelve young burst the eggs immediately after they have been laid; sometimes the mother has to retard the laying, in which case the young are born free. The female does not make a nest, but simply deposits her offspring on the ground and leaves the young to their fate. For the first few days the little ones, which scarcely measure three-quarters of an inch in {554}length, remain almost motionless between leaves or in cracks of the ground, and they do not take any food. They grow, however, quickly, living upon the remains of the yolk which has slipped into their body. Their first food consists of Aphides and similar tiny insects.
The Common Lizard prefers moist localities and is very hardy. It extends northwards to Archangel, and in the Alps it ascends to nearly 10,000 feet above the level of the sea. However, on the approach of the cold season, in the month of October, it withdraws into its winter quarters, frequently in company with many of its own kind.
L. agilis, the Sand-Lizard, has nearly the same wide range as L. vivipara, except that it does not go so far north and does not extend eastwards beyond Central Siberia. It is absent in Ireland and Scotland, while in England it is restricted to the southern half.
The characters which distinguish the Sand-Lizard from L. vivipara are few, although the majority of the specimens of either kind are very distinct in their coloration, and L. agilis is strictly oviparous, depositing its eggs in the ground, under leaves, in heaps of weeds and similar places. The Sand-Lizard has usually a single postnasal and two superposed anterior loreals, the three shields forming a triangle. The temples are covered with flat scales, two or three of which are enlarged and in contact with the parietals, but there is no tympanic scale.
The coloration is subject to much variation, local as well as individual. As a rule the Sand-Lizard gives the impression of being striped longitudinally, the striation being caused by rows of dark and white spots and patches along the sides of the back, flanks, and tail. In the male a more or less pronounced green, in the female brown and grey are the prevailing ground-colours. A typically coloured male during the breeding season is grass-green on the sides and suffused with green on the yellow under parts; the sides are dotted with black, with whitish eye-spots. The under parts are spotted with black. The adult female is brown or grey above, with large dark brown, white-centred spots, which are arranged in three rows on each side. The under parts are cream-coloured, with or without black specks. The young are grey-brown above with white, black-edged spots; the under parts are whitish. Total length of the adult up to 8 inches. {555}The male is a little smaller than the female but has a relatively longer tail, a little less than half the total length.
The Sand-Lizard is easily kept in captivity, and lives for years if allowed a variety of food and proper places to hibernate in. It pairs in the spring, in England in May or June; the white, parchment-like eggs, numbering five to eight, are hatched in the following July or August.
L. viridis, the Green Lizard, inhabits Southern and Middle Europe and South-Western Asia. The general colour of this beautiful lizard is emerald-green above, changing into greenish yellow on the flanks and into yellow on the belly. The throat, especially in the males during the breeding season, is blue. The upper parts are frequently speckled with black. The young are brown or green above with one or two yellowish lateral stripes, which persist in some adult females. There are usually two superposed postnasal shields. The semilunar collar on the neck is well pronounced, and there is usually a distinct gular fold. The tail is often very long, especially in the males, sometimes nearly three-quarters of the total length, which in very large males reaches 16 or 17 inches. The females do not quite reach this length.
The Green or Emerald-Lizard prefers rocky localities, from the sea-level, as for instance in Jersey, up to a height of several thousand feet. It is extremely swift and can climb trees, which it sometimes resorts to when chased. When hard pressed it takes tremendous leaps down to the ground, marvellously enough without injury to body or tail, which latter is otherwise very brittle. They pair in the spring or early summer after much fighting between the males; the eggs, to the number of about ten, are whitish and are deposited a month later. The young are hatched after another four weeks.
This beautiful lizard does not keep well in captivity, although it becomes very tame; it eats meal-worms, snails, earth-worms, and insects, especially butterflies, but it sickens after the first winter even if it has been allowed to hibernate.
In Portugal and Spain L. viridis is represented by a slightly different kind, L. schreiberi, the chief interest of which lies in the fact that it approaches L. ocellata in several respects. The occipital shield is large and is usually broader than the interparietal. The dorsal scales are smaller, and there are eight {556}well-developed rows of ventral scales. Instead of being uniformly green, the upper parts are usually spotted and vermiculated with black; sometimes, especially in the females, the black spots have a white ocellus in the centre. The under parts are yellowish, with or without black spots. The throat is blue. The young look very different. They are olive-brown above with large yellow, or bluish-white, black-edged ocelli on the side of the head and body.
Other forms, perhaps of sub-specific rank, approaching L. ocellata, occur in the Balkan Peninsula, where, for instance in Dalmatia, the typical L. viridis attains its most beautiful development.
L. ocellata, the Eyed Lizard, inhabits Spain and Portugal, extending northwards into the South of France and into the Riviera, southwards into Morocco and Algeria; these southern forms (L. pater and L. tangitana) approach L. viridis. The Eyed Lizard is green or dark olive above, with black or yellowish dots, which are sometimes combined into a kind of network pattern. The under parts are uniformly greenish yellow. The sides of the body are adorned with about two dozen blue, black-edged spots or "eyes." The intensity of the blue and the depth of the green ground-colour vary much according to sex, time of the year, and state of health. Males during the breeding season are most beautiful and brilliant. The occipital shield is broad; there are two superposed nasal but no tympanic shields. The supraoculars are separated from the supraciliaries by a series of granules. The collar is well marked, but not the gular fold. {557}The dorsal scales are minute and granular; the ventral shields are arranged in eight or ten longitudinal rows.
The "Eyed Lizard" reaches a considerable size, especially the males, which develop a very strong and thick head, and are much more robust and powerful than the more slender females. Old males reach a length of 2 feet, two-thirds of which length belong to the tail; but the latter varies much, even if it has never been broken and renewed.
The Eyed Lizard keeps extremely well in captivity, and in this respect is unlike the Green Lizard. A case has been recorded of its living thirteen years. This species is very intelligent. Although at first ferociously wild and biting furiously, these lizards soon become tame and take food regularly. One of my own, a half-grown male from Northern Spain, about one foot in length, made its home in a little niche of the greenhouse-wall, whence it emerged regularly to take the offered food from my hand. It soon knew the whole place thoroughly, making use of the creepers whilst scaling up to its retreat, jumping over certain gaps, descending to the ground at certain spots, basking on certain stones, invariably in the same methodical way. In the month of October it retires into the ground on the coolest side of the greenhouse, and although the latter is well warmed, the lizard remains invisible until the next February or March, when on some fine day it is rediscovered basking upon exactly the same stone where it had been seen five months before. The only drawback in connexion with keeping this kind of lizard in company with other creatures is their voraciousness; since large, fully adult specimens attack and eat any other small lizard, slow-worm, or snake they can find. They also take mice. The eggs are often deposited in hollow trees.
L. muralis, the Wall-Lizard, is very common in Southern Europe, Asia Minor, and Northern Africa. Northward it extends into Belgium and into South Germany. In the Iberian Peninsula it ascends up to 5000 or 6000 feet above the level of the sea. This graceful little creature, with an average length of 6 to 8 inches, is easily recognised by the series of granules between the supraocular and supraciliary scales and usually by having only six rows of ventral scales. The great variety in coloration has given rise to the establishment of many races, varieties, and sub-species. In the typical forms the upper parts are brown or {558}greyish, with blackish spots or streaks, sometimes with a bronzy greenish sheen. The under parts are white, yellow, pink, or red, either uniform or, especially in the males, with large black spots. The lateral rows of ventral shields are frequently blue. The colour-varieties are almost endless. One of the most noteworthy is that described as var. coerulea by Eimer; this, confined to the Faraglione Rocks near Cápri, is blackish above, like the rock, and sapphire-blue below. Similarly coloured specimens, var. lilfordi, occur on some of the rocky islets of the Balearic Isles.
The Wall-Lizard deserves its name, since in the Mediterranean countries there is scarcely a wall on which these active lizards do not bask or run up and down, often head downwards, in search of insects. They are oviparous. The hibernation is short and not very deep, since these lizards can sometimes be seen basking on sunny winter days before their regular appearance in the early spring.
Psammodromus, with a few species in South-Western Europe, notably in the Iberian Peninsula and in North-Western Africa, has no distinct semilunar collar, but has a short fold in front of each arm. The back is covered with large, rhombic, strongly keeled and imbricating scales. The lateral scales pass gradually into the ventrals, which are smooth and arranged in six longitudinal rows.
P. hispanicus is bronzy brown above, with small black and white specks, and with one or two longitudinal streaks on each side. The under parts are white. Total length about 5 inches. Although also found inland, this species prefers sandy dunes, studded with prickly and scanty vegetation. It runs very fast and digs itself rapidly into the sand when pursued. When caught it either utters a faint cry like "tsi-tsi," or it feigns death. The pairing takes place in June; half-a-dozen eggs are laid about eighteen days later, deeply imbedded in the warm sand, and they are hatched in eight weeks. The eggs are said to grow[166] after they have been laid from 13 by 7 mm. to 17-20 by 10-11 mm. The newly hatched little creatures measure about 2 inches in length, more than half of which belongs to the tail.
P. (Tropidosaura) algirus has the same range as P. hispanicus, but grows to 10 inches in length, and is much more {559}beautifully coloured. The upper parts are bronzy brown with one or two golden, dark-edged, lateral streaks; the under parts are whitish; the male has one or more blue-eyed spots above each shoulder.
Acanthodactylus is distinguished by the laterally fringed digits. This genus ranges throughout Northern Africa to the Punjab. One species, A. vulgaris, extends into Spain and Portugal. The dorsal scales are small and almost smooth, but those on the tail are strongly keeled; the ventrals are much broader than long, and are arranged in eight to ten rows. The fringes on the digits are but feebly developed in the shape of lateral denticulations. The adults are grey-brown with faint longitudinal stripes, and with more conspicuous black and pale spots; in the breeding season larger blue-eyed spots appear on the sides near the limbs. The tail is often pink, especially on the under surface. Total length about 7 inches.
Fam. 12. Gerrhosauridae.–Pleurodont African Lacertidae with osteoderms on the head and body.
This family is intermediate between the Lacertidae and the Scincidae. The tongue is constructed like that of the Lacertidae, but is only feebly nicked anteriorly. Dermal ossifications roof over the temporal region, and femoral pores are present. On the other hand, the osteoderms, which cover the whole body, are in their structure and arrangement typically Scincoid. The tail is long and fragile. A lateral fold is usually present. The limbs are sometimes reduced to useless stumps. The few genera and species of this family are strictly confined to the African sub-region, being found in the whole of Africa south of the Sahara, and in Madagascar.
Gerrhosaurus, with a strongly developed lateral fold and complete limbs, occurs in Africa. G. flavigularis, of South Africa, has a total length about one foot.
Tetradactylus, of South Africa, has also a strong lateral fold, but the limbs are either very short and pentadactyle (T. seps), or tetradactyle, or they are minute pointed stumps, as in T. africanus.
Fam. 13. Scincidae.–Pleurodont lizards with strongly developed osteoderms on head and body, with very feebly nicked, scaly tongue, with complete cranial arches, and with separated premaxillaries.
The temporal region is covered over, as in the Lacertidae, with strongly developed, bony, dermal ossifications. Similar osteoderms underlie the scales which cover the body and tail. The tongue is relatively short, not forked behind, and but very feebly nicked at the tip; it is covered with scale-like papillae. Femoral pores are absent.
All the Skinks prefer dry, sandy ground, in which they not only burrow, but move quickly about, either for protection or in search of their animal food. In connexion with this sand-loving and at least temporary subterranean life stands the frequent reduction of the limbs. Every stage from the fully developed and functional pentadactyle limb to complete absence of limbs is represented. There are species within the same genus with five, four, three, or two fingers or toes. There are Skinks without fore-limbs, but with vestigial hind-limbs, and vice versa. The interesting point is that these reductions do not indicate relationship within the family, but have happened independently. They are impressive illustrations of convergent retrogressive evolution.
Ablepharus, widely distributed in the Old World, has the lower eyelid transformed into a transparent cover, which is fused with the rim of the reduced upper lid, exactly as in the Lacertine genus Ophiops.
All the Scincidae seem to be viviparous, some of them, e.g. Trachysaurus, in the strict sense of the word, since the hard or parchment-like egg-shell has been dispensed with.
The family contains about four hundred species, which have been arranged in nearly thirty genera, many of them on fanciful grounds. The family is cosmopolitan, but reaches its greatest diversity in numbers and forms in the tropical parts of the Old World, especially in the Australian region, inclusive of the islands of the Pacific. America, notably South America, has the smallest number.
Trachysaurus, with one species, T. rugosus, inhabits the whole of the Australian continent. It is easily recognised by the large and rough scales, and the short and broad stump-like tail. It is dark brown above with yellowish irregular markings; the under parts are yellowish, marked with brown. Embryos of this species have yellow transverse bands on the back, but these often fade away before birth. The creature is strictly {561}viviparous, the egg-membrane being very thin, and the two or three embryos are ripened in uterus-like dilatations of the oviducts. The period of gestation is about three months, and the birth takes place, in South Australia, about April. According to Fischer[167] this species, which is often in the market, is easily kept. It requires warmth, sand and stones for basking, and water, in which it soaks itself preparatory to the shedding of the skin, which takes place half-a-dozen times in the year, and is a slow process, requiring eight to ten days. The food consists chiefly of worms, lizards, and snakes, but meat, cabbage, and lettuce are also taken. The total length is about one foot.
Cyclodus s. Tiliqua, of Australia, Tasmania, and the Malay Islands, has stout lateral teeth with spherical crowns. The imbricating, cycloid scales of the body and the rather short but pointed tail are quite smooth and shiny. C. gigas, of New Guinea and the Moluccas, reaches a length of nearly 2 feet. The general colour is brownish yellow, with broad, dark bands across the body and tail.
Scincus, of North Africa, Arabia, Persia, and Sindh, has pentadactyle limbs, with laterally serrated digits. The eyelids are well developed, but the ear is hidden under scaly flaps. S. officinalis, of the Sahara and of Egypt, grows to about 8 inches in length. The snout is peculiarly shaped, cuneiform. The eyes are very small. The scales of the body are perfectly smooth; the sides of the belly are somewhat angular. The {562}whole shape of the creature, the scales, and the digits are adapted to burrowing and moving quickly through the loose sand. The general colour is yellowish or brownish above, each scale with small brown and whitish spots; the under parts are uniform whitish. The young are quite beautiful, being uniform pale salmon-coloured above, silvery white below. When a little older, yellow spots appear on the flanks and grey bands across the back. These Skinks live in the absolutely dry reddish-yellow sand of the desert, in which they may almost be said to swim about, so swift and easy are their movements. They live on insects, while in their turn they are eaten by snakes, and above all by the Varanus lizards.
Of Mabuia with about forty species, in the whole of Africa, Southern Asia, and in Tropical America, we mention only M. (Euprepes) vittata, on account of its partly semi-aquatic life, a very rare condition among Scincidae. This creature, about 7 inches long when full grown, frequents damp localities in Tunis and Algeria, where the French call it "Poisson de sable." It often sits on the floating leaves of Nymphaea alba, and dives into the water in order to escape. Its proper element is, however, the sand, and for the night it retires under stones. The general colour is olive brown with a lighter vertebral band and two narrow whitish lines on each side, sometimes edged with black. The under parts are yellowish or greenish white.
Chalcides s. Seps s. Gongylus, of the Mediterranean countries {563}also occurs in South-Western Asia. The lower eyelid has a transparent disc. The body is much elongated, and is covered with smooth shiny scales. The limbs are very short, or reduced to mere vestiges.
Ch. ocellatus, of the Southern Mediterranean countries, occurring also in Malta and Sardinia, reaches about 10 inches in length. The snout is conical, the ear-opening a small slit or hole. The limbs have five fingers and toes. The under parts are uniform silvery white, but the colour of the upper parts is very variable, mostly olive brown with black spots and irregular cross-bars, or with dark and light spots; sometimes uniform bronzy brown with a light upper and a black lateral band. This Skink seems to have no fixed abode, but digs itself into the sand wherever it wants to hide. The skin is not shed in flakes, but, as in most Skinks, it peels off by a process of gradual desquamation. Fischer's specimens paired towards the end of December. The gestation lasted 56 days, when nine young were born, which measured about 75 mm. or 3 inches; when three weeks old they had increased to nearly double this length.
Ch. lineatus, of Spain and Portugal, and of the South of France, like Ch. tridactylus of Italy and North-West Africa, has only three fingers and toes. The fore-limbs are only about one quarter of an inch in length in large specimens of 10 inches total length; the hind-limbs are a little longer. The general colour is bronzy olive or brown above, in the former species with nine or eleven darker longitudinal streaks; uniform, and with an even number of streaks in the latter species. Ch. bedriagae, of Spain and Portugal, has mostly five fingers and toes, and the limbs are relatively longer in this smaller species; but it is a question if these and other species of this genus are not to a great extent simply individual variations, since the reduction of the limbs and toes seems to be a very recent feature. Ch. guentheri, of Palestine, otherwise in every respect like Ch. tridactylus, but reaching a length of more than 14 inches, has the limbs reduced to tiny conical stumps without a trace of separate digits.
I have caught Seps accidentally under stones or pieces of bark in sandy districts. On the western coast of Galicia and Portugal, close to the sea, they frequent the gorse-bushes, on which they can be seen basking, provided they are approached {564}stealthily. They disappear on the slightest alarm, almost swimming, as it were, with great agility through the prickly cover, and then hiding and wriggling through the loose sand between the roots.
The following five "families" are composed of degraded forms of various descent. Most of them lead a burrowing, subterranean life, in adaptation to which the body has become snake-shaped or worm-like. The fore-limbs are entirely absent, except in Chirotes; the hind-limbs are absent, or reduced to small flaps; the girdles are reduced correspondingly. The skull is devoid of postorbital, postfronto-squamosal, supratemporal, and jugal arches. The quadrate bone is mostly immovable. The eyes and ears are concealed, except in the Pygopodidae.
Fam. 14. Anelytropidae.–An artificial assembly of a few degraded Scincoids. The worm-shaped, limbless body is devoid of osteoderms. The tongue is short, slightly nicked anteriorly, and covered with imbricating papillae. Columellae cranii are present. Anelytropsis papillosus in Mexico. Typhlosaurus and Feylinia in South and West Africa.
Fam. 15. Dibamidae, consisting of the genus Dibamus, with D. novae-guineae, in New Guinea, the Moluccas, Celebes, and the Nicobar Islands. The tongue is arrow-shaped, undivided in front, covered with curved papillae. Columellae cranii are absent. The vermiform body is covered with cycloid imbricating scales without osteoderms. The limbs and even their arches are absent, but in the males the hind-limbs are represented by a pair of flaps. Total length of the animal about 6 inches.
Fam. 16. Aniellidae.–The genus Aniella comprises a few small worm- or snake-shaped species in California, which seem to be degraded forms of Anguidae. The eyes and ears are concealed, limbs are entirely absent, the body and tail are covered with soft, imbricating, more or less hexagonal scales. The tongue is villose, smooth, and bifid anteriorly. The teeth are relatively large, few in numbers, recurved, with short swollen bases. The skull, by reduction, approaches the Ophidian type; there is no columella cranii, the postorbital arch is ligamentous, the premaxillary is single, the nasals and frontals remain separate, the pre- and post-orbitals are in contact with each other, excluding the frontal from the orbit.
A. pulchra.–Silvery, the scales edged with brown; back and {565}tail with a narrow, brown, median line. Total length, 7 to 8 inches.
Fam. 17. Amphisbaenidae.–Worm-shaped lizards with the soft skin forming numerous rings, each of which is divided into many little squares, the vestiges of scales which are otherwise restricted to the head. The eyes and ears are concealed. Limbs are absent except in Chirotes, which has short four-clawed fore-limbs. The pectoral arch, and still more so the pelvic arch, are reduced to minute vestiges. The tail is very short. The skull is small, compact, and strongly ossified, in adaptation to the burrowing life, and is devoid of postorbital and postfronto-squamosal arches and of columellae. The teeth are either acrodont or pleurodont. The tongue is slightly elongated, covered with scale-like papillae, and bifurcates into two long and narrow smooth points.
The Amphisbaenas lead an entirely subterranean, burrowing life, like earth-worms. They are frequently found in ants' nests or in manure-heaps. Their progression is very worm-like, their annulated soft skin enabling them to make almost peristaltic motions and to move backwards as well as forwards. They crawl in a straight line, with slight vertical waves, not, like other limbless lizards or snakes, by lateral undulations. The food consists of worms and small insects. About one dozen genera with more than sixty species are known, most of which inhabit the warmer parts of America, the West Indies, and Africa. Four inhabit Mediterranean countries.
If the tongue and the dentition be taken as indications of relationship, the Amphisbaenidae may perhaps be considered as degraded descendants of Iguanidae, a family which contains various limbless, burrowing, worm-shaped forms. But it is also possible that the Amphisbaenidae are not a natural group. This consideration applies with most force to the genera Amphisbaena and Anops, the various species of which occur in America and in Africa.
Chirotes canaliculatus, the only species of the genus, is the only Amphisbaenid which still possesses fore-limbs. These are short, stout, placed close behind the head, and are provided with four-clawed digits. This species occurs in Mexico and California, is brownish or flesh-coloured, and reaches a length of about 8 inches.
Amphisbaena, with nearly thirty species, in Tropical America and Africa. On account of the short rounded-off head and the almost equally blunt tail these creatures are called by the natives "cobras de dous cabezas," i.e. snakes with two heads, or they are known as "maes das formigas," i.e. mothers of ants, because of their predilection for taking up their quarters in the nests of ants or termites. The scientific name refers of course to their capability of moving forwards and backwards (ἀμφίς, at both ends, and βαίνω, walk).
A. fuliginosa, one of the commonest species in South America and in the West Indies, is chequered black and white. The skin of the body has about two hundred rings, the tail about thirty. Total length between one and two feet. A more or less distinct fold extends along each side of the body from the neck to the tail, at the level where the dorsal scales originally joined the ventral scales.
Blanus is the only genus of the Mediterranean province. B. cinereus, of Portugal, Spain south of the Cantabrian range, Morocco, and Algeria, reaches a length of 10 inches, but such large specimens are rather rare. The general colour of the living animal is pink with a brownish tinge and with minute grey specks. The lateral lines or folds are well marked, and a stronger transverse fold is placed behind the head. The body shows from one hundred and ten to one hundred and twenty-five rings, the tail from twenty to twenty-two; each body-ring contains about thirty little squares or remnants of scales. There are a few pre-anal pores.
I have sometimes found this species in Portugal whilst digging for earth-worms in manure-heaps and similar moist places, where they lead the same life as the worms except that they live upon them and upon insects. When kept dry they become very thin and shrunken, but when put back into moist soil they again become turgid and supple within a short time. Those which I have kept in glass jars filled with rich mould throve very well, living upon the tiny insects and worms which infest such compost soil; they dug long tortuous channels, in which they moved forwards and sometimes backwards, but they never came to the surface.
Fam. 18. Pygopodidae.–Pleurodont, snake-shaped lizards, without fore-limbs, but with the hind-limbs appearing as a pair of scaly flaps.
The shoulder-girdle is much reduced. The hind-limbs, although very small and hidden within the scaly, almost fin-like flaps, still possess five toes. The ischium appears externally as a small spur on either side of the anal cleft. The eyes are devoid of movable lids, remaining open and unprotected; the pupil is vertical. The ear is either concealed or exposed. The tongue is fleshy, slightly forked and extensible. The body is covered with roundish imbricating scales. The tail is very long and brittle. The few genera of this undoubtedly natural family of unknown relationship contain in all about ten species, restricted entirely to Australia, Tasmania, and perhaps New Guinea. Next to nothing is known about their habits, except that some of them eat other lizards.
Pygopus lepidopus is distributed over the whole of Australia. It reaches a total length of about 2 feet, 16 inches of which belong to the tail. General colour coppery grey above, sometimes with several longitudinal series of dark spots.
Lialis burtoni of nearly the same size and equally wide distribution has the hind-limbs reduced to extremely small, scarcely visible, narrow appendages.
Sub-Order 3. Chamaeleontes.–Acrodont Old-World Saurians with a laterally compressed body, prehensile tail, and well-developed limbs with the digits arranged in opposing, grasping, bundles of two and three respectively.
The Chameleons are an essentially African family. About half of the fifty species known inhabit Madagascar, the others {568}the African continent. One, the common Chameleon, is North African, extending into Andalucia; two others occur in South Arabia and Socotra, and only one in Southern India and Ceylon.
This sub-order is well distinguished from all other Saurians by several, mostly unique, characters. The tongue is club-shaped and extremely projectile, to a length equal to that of the body. The head is usually described as forming a casque, with prominent crests and tubercles. There is no tympanum and no tympanic cavity. The parietal bones, united into one, extend backwards far beyond the occiput, and the tip of this projection is met by a much-elongated supratemporal bone, which, partly fused with the squamosal, helps to enclose a huge supratemporal fossa. The latter is widely open behind. The postfronto-squamosal arch and the postorbital arch are strong. The jugal is widely separated from the quadrate; the latter stands vertically and is not reached by the pterygoid. There is no columella cranii. The pre- and post-frontals often join to form a supra-orbital roof. The nasals are very small and are excluded from the nares, which are bordered entirely by the enlarged prefrontals and by the maxillaries. The premaxillaries are small and carry no teeth. The latter are acrodont, compressed and tricuspid, and are restricted to the maxillaries and mandibles.
The limbs are peculiar. Not only are they relatively long and very slender, but two digits are permanently opposed to the other three. On the hand the first three fingers form an inner bundle opposed to the outer, or fourth and fifth fingers. On the foot the inner bundle is formed by the first and second, the outer by the other toes. The shoulder-girdle is of the ordinary Saurian type, but there are no clavicles and no interclavicle. The costal sternum is well developed; the ribs posterior to those which meet the sternum are very thin and elongated: they meet and fuse with their fellows in the medio-ventral line. These hoops are not connected with their neighbours in front or behind. The tail is prehensile by being rolled downwards; it is not brittle and is incapable of being renewed. The skin is not covered with scales, but with {569}granules. The eyes are very remarkable. The eyeballs themselves are large, but the eyelids are united into one fold with a small central opening. However, when the Chameleon is asleep the margins of this opening sometimes become more slit-like. The right and left eye can be, and are incessantly, moved separately from each other, and the creature squints terribly. Each eyeball, together with the pin-hole eyelid, is rolled up and down, backwards and forwards, independently of the other eye. This is a unique feature, but it also occurs in people who squint badly. The question "What, and how, do these creatures see?" is therefore quite idle, especially since in reptiles binocular vision does not exist at all and, consequently, cannot be disturbed by squinting.
The tongue has attained an extraordinary development. The tongue proper (Fig. 152) is club-shaped, and is covered with a sticky secretion. The base or root of the tongue is very narrow, composed of extremely elastic fibres, and is supported by a much-elongated copular piece of the hyoid. The elastic part of the tongue is, so to speak, telescoped over the style-shaped copula, and the whole apparatus is kept in a contracted state like a spring in a tube.
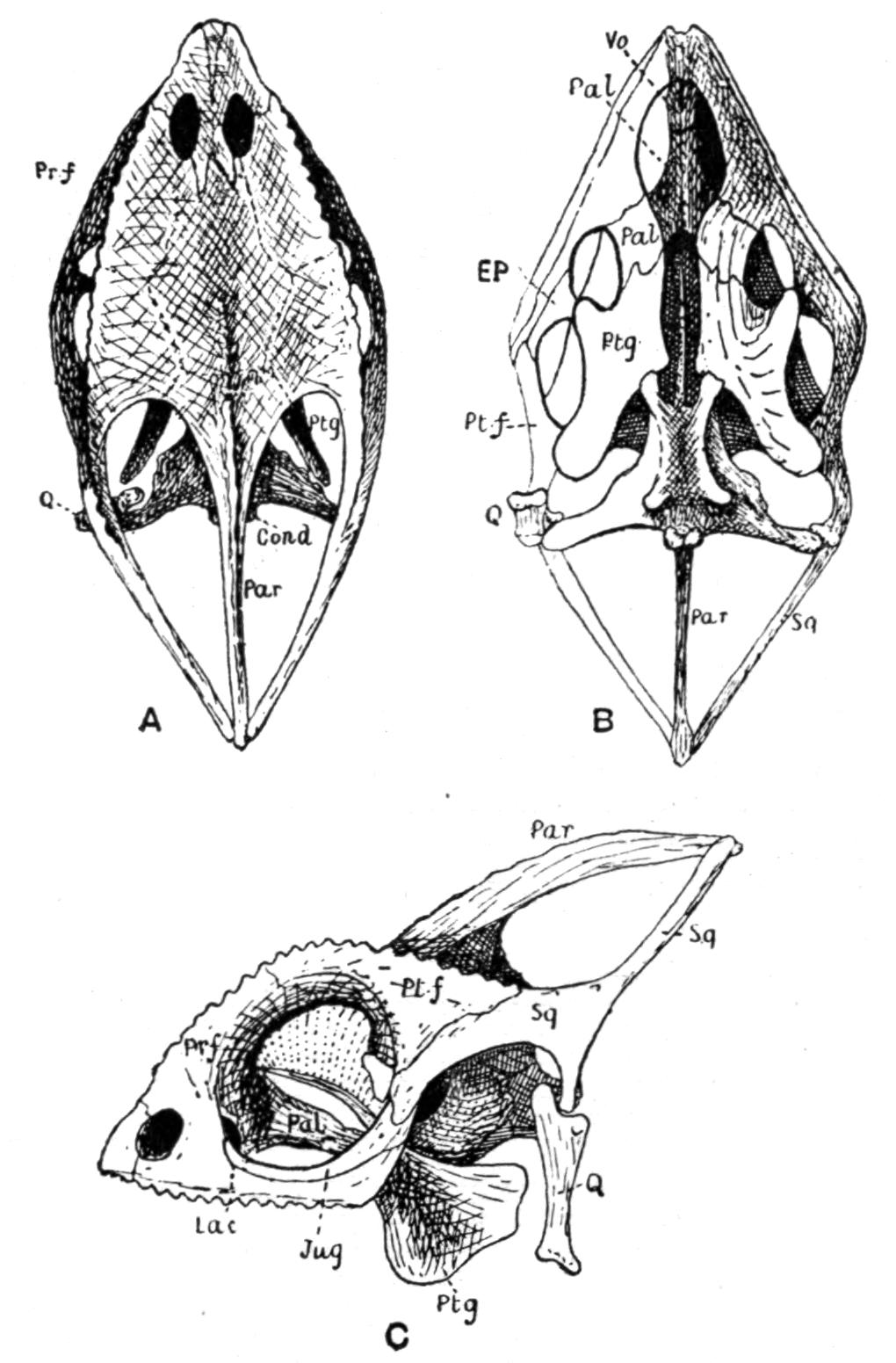
Fig. 149.–A, Dorsal, B, ventral, and C, lateral view of the skull of Chamaeleon vulgaris. × 1. Cond, occipital condyle; EP, ectopterygoid; Jug, jugal; Lac, lacrymal; Pal, palatine; Par, parietal; Prf, prefrontal; Pt.f, postfrontal; Ptg, pterygoid; Q, quadrate; Sq, squamosal; Vo, vomer.
A pair of wide, very elastic blood-vessels and special elastic bands extend from the base into the thick end of the tongue. {570}By rapidly filling the apparatus with blood, and by the action of certain hyoid muscles, the spring is, so to speak, released, and the momentum gained by the thick and heavy club-shaped tongue proper projects it far out of the mouth. The sticky end of the club shapes itself into an upper and a lower flap, which partly envelop the prey, and the elastic bands of the far-stretched stalk withdraw the whole. The detailed working of this ingenious shooting apparatus is not easy to follow. An ordinary full-grown Chameleon can shoot a fly at the distance of 7 or 8 inches. The whole performance is very quick, lasting less than one second. When the desired object is very near, only 2 or 3 inches off, the Chameleon has a certain difficulty in shooting its prey. The tongue is at first put out slowly, tentatively, the following jerk is feeble, and it seems as if the apparatus refuses to work unless it is allowed to shoot out with full force.
Another remarkable and quite proverbial feature of Chameleons is their changing of colour. This is by no means restricted to Chameleons, which indeed are rivalled in this respect by various other lizards, for instance by the Indian Agamoid Calotes and by the American Ameiva.
The microscopical structure and mechanism of the colour-changing apparatus is, in Chamaeleon vulgaris, as follows:–
The epidermis is colourless, and the Malpighian layer is not particularly modified except that in it are imbedded some iridescent cells, with very minute wavy striation on their surface. The cutis contains in its leathery tissue a great number of small and closely packed cells, filled with strongly refractive granules, chiefly guanine-crystals. These cause the white colour by diffuse reflection of direct light. The cells nearer the surface are charged with oil-drops and appear yellow. Large chromatophores are imbedded in the white granular mass, most of them with blackish-brown, others with reddish pigment, the granules of which are shifted up and down, towards and away from the surface of the cutis, in ramified branches of the chromatophores. When these branches are contracted the pigment is conveyed back into the bulbous basal portion of the chromatophores and the skin appears yellow or white. When all the pigment is shifted towards the surface of the cutis, the animal looks dark, sometimes black. In intermediate conditions the light is changed into green by diffraction through the yellowish upper strata and by the finely {571}striated iridescent cells of the Malpighian layer. Those parts into which the chromatophores do not send pigment appear as yellow spots. The chromatophores are to a great extent under control of the will of the Chameleon, but external stimuli, as heat and cold and other reflex actions, also play a great part in their movements.
For further information on this subject see Brücke,[168] P. Bert,[169] Pouchet,[170] Thilenius,[171] and lastly Keller,[172] who has written a very long but rather confused account.
The process of moulting is curious. When the Chameleon is in good health the whole process is accomplished within a few hours. The skin to be cast off becomes loose and assumes a blistered appearance. Sometimes the creature looks as if it were wrapped up in white, semi-transparent tissue paper. By rubbing against stones, or between the twigs of trees, the skin comes off in large flakes, first on the lips, then on the contorted body, and last on the under surface of the hands and feet. During a rapid and successful moult the changes of colour go on as usual in the new skin. Sometimes large flakes of the old skin remain adherent for days, especially on the top of the head. The moulting takes place several times in one year. One of my Ch. vulgaris moulted in January and September, and then not until June of the next following year. A Ch. pumilus moulted in the months of May, October, and March.
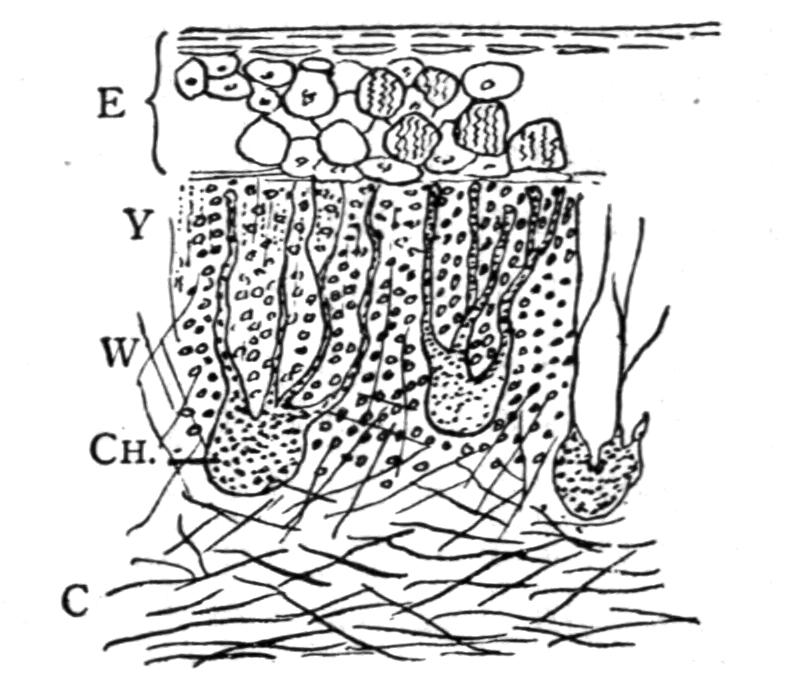
Fig. 150.–Diagrammatic section through the skin of a Chameleon. Highly magnified. C, deeper portion of the cutis; Ch, three chromatophores, in various stages of contraction, filled with black, brown, or reddish pigment; E, epidermis; W, white layer of granules; Y, yellow layer of cells.
When they know themselves to be discovered, Chameleons make themselves as thin as possible by compressing the body or rather the belly. This is done by means of the peculiarly elongated abdominal ribs described above. The whole body is then put into such a position that, by presenting only its narrow edge to the enemy, it has become as little visible as {572}possible. At the same time the Chameleon turns round upon its twig, so that the latter comes to stand between the observer and its own body, which may thereby be completely hidden. When angry, the creature either presents its broadest surface, swaying to the right and left, or it blows itself up and hisses. The lungs are very capacious, and, instead of being bag-shaped, end in several narrow blind sacs which extend far down into the body-cavity, so that not only the chest but the whole body can be blown up.
The usual mode of propagation is by means of eggs, but a few species allied to Ch. pumilus are viviparous. The time of incubation and of gestation is long. For instance, the pairing of Ch. vulgaris takes place in the month of August. The eggs are laid in the last week of October, about fifty to sixty days later. Sometimes, however, the eggs are retained much longer, since I have received specimens with ripening eggs in July which did not lay until the end of October. The eggs are deposited in the ground and are not hatched until the following February or March, i.e. about 130 days later. The new-born little creatures are snowy white, and cannot change or rather assume colour until after the second week.
All Chameleons are insectivorous and require enormous quantities of food, which must be alive to be taken. Most of them prefer Orthoptera, e.g. Locusts and Grasshoppers, and Lepidoptera. They also eat flies, meal-worms, and cockroaches, but their tastes differ not only individually but also temporarily. They require change of diet. One individual will take cockroaches greedily, whilst another of the same kind will rather starve itself than touch one. The same applies to meal-worms. It is a great but common mistake to suppose that Chameleons do not require water. On the contrary they drink regularly and often, generally by licking up drops of water or by scooping them up with their lips, shoving the snout along the edges of wet leaves. It is not too much to say that most Chameleons are short-lived in captivity on account of the want of water. Those which are sold by the dealers are generally in a parched condition. Sprinkling the twigs or leaves of their cage with water works a wonderful change in them; the dull, apathetic-looking creatures drink and drink, revive, assume brighter colours, and will soon take food, which they have until then refused {573}obstinately. Once I have even seen a Chameleon, when put into the greenhouse, make straight for a tank and actually drink in gulps.
After they have fattened themselves in the autumn, Chameleons, at least those of North Africa, withdraw to hibernate in the ground. But nothing is known about how, when, and where they do this, nor is it known if tropical species aestivate during the dry season.
Chameleons are notoriously difficult to keep successfully, whereby we do not mean the keeping for three to six months. This is easy enough, since it takes them several months to die of starvation. The difficulty is to keep them through the winter. To enable them to do this, it is absolutely necessary to fatten them up during the summer and autumn. Otherwise, although kept in a warm place, they are liable to lose their appetite in the autumn, when they become restless, probably with the desire to hibernate. Those few individuals which get over this critical period, say during the month of October, and do not refuse food, are probably safe. But those are doomed which refuse to eat meal-worms or cockroaches or such food as can be procured easily during the winter.
The origin of the Chameleons is unknown. They form only one family, Chamaeleontidae, with between fifty and sixty species, which, with a few exceptions, belong to the genus Chamaeleon.
Ch. vulgaris is the Common Chameleon of North Africa, Syria, and Asia Minor. It occurs also in a few parts of Southern Andalucia, for instance near Jerez, and near Nerja, to the east of Malaga, where it has possibly been introduced. A series of conical, slightly enlarged granules forms a little crest on the median line of the throat. A whitish line, which does not change colour, extends from the chin to the vent. The rest of the skin, with the exception of a median dorsal series of slightly enlarged tubercles on part of the back, is composed of small granules. A small but distinct lobe of leathery skin extends along either side of the occiput towards the posterior end of the median parietal crest. Dead or spirit-specimens are usually pale yellow; living ones are greenish, usually with differently coloured patches on the sides. Exceptionally large males reach a total length of about 9 inches, females reach the length of perhaps a foot, but about half of the total length belongs to the tail.
It is impossible to say what is the colour of this Chameleon, since the same specimen may within a few days appear in half-a-dozen different garbs, not counting minor combinations of colour. After it has been watched for several months, when all its possibilities seem to be exhausted, it will probably surprise us by a totally new combination. Not every specimen changes alike: some keep the same appearance for a long time, others change often; some are partial to specks, others to large patches. In the group of Chameleons shown in Fig. 152 several of the more usual arrangements of colour have been indicated by stippling and various kinds of cross-hatching.
A represents the usual coloration at night. The whole animal, which has just been stirred up from its sleep in the dark, is cream-coloured, with irregular patches of yellow on the head, the back, the sides of the body, the legs, and the tail.
B has the usual coloration: grey-green, with innumerable small darker specks, with two series of pale brown patches on the sides of the body, and with one patch on the region of the ear.
Fig. 152.–Showing changes of colour in Chameleons. A to D, Chamaeleon vulgaris (see p. 574). Chamaeleon pumilus in the right upper corner.
C is the same specimen in an excited frame of mind; it is represented in the act of shooting a fly. The light brown patches have changed to maroon brown; and many round golden yellow spots have appeared on the green parts.
D shows a specimen, coloured like C, within a few seconds after it has been put into an angry mood, in the present case by having its tail squeezed. The whole body is blown out, the thick tongue causes the throat to bulge out, and all the yellow spots have become blackish green.
Many small spots scattered over the body are usually a sign of anger. One of the specimens described above was, when fast asleep in a dark room, dirty white, with about two dozen large and small round spots of a rich yellow on each side of its body. Then a lighted lamp was brought into the room without in any way disturbing the animal. Within sixteen minutes the yellow spots had vanished completely; the whole body and tail had become suffused with greenish yellow, which gradually turned to pale yellowish green, and those parts which in Fig. B are pale brown, were just distinguishable as pale yellowish-white regions. The Chameleon was found to be fast asleep, and it kept this coloration during the rest of the evening. Other specimens behaved on similar occasions in the same way, but the greatest interest is attached to the fact that frequently only that side of the body "greened up" which happened to be exposed to the light, whilst the opposite side remained whitish. These changes are not absolutely unconscious; they are, after all, under the control of the creature. In order to test the possibility of direct action of the light, I have taken the precaution of throwing the light of a candle only upon the body, whilst the head was kept in darkness. No changes of colour took place whilst the animal was asleep, but when a little light was allowed to sweep across the closed eye, this soon began to twitch, and although the creature did not open the eye, the usual changes of colour began to take place. When the light was removed, the animal soon re-assumed its whitish appearance. Artificially coloured light, for instance green, red, or blue glass or paper, has apparently no influence upon the changes of colour. The Chameleons behave as they would behave under ordinary conditions. Direct and hot sunshine however causes them to darken, sometimes to turn uniform dull black, except for the white median ventral {577}line. Occasionally I found one of the specimens described above deep maroon brown, with dozens of round orange spots. Blue and red do not seem to be within the range of Ch. vulgaris, but the combinations of green, yellow, brown, black, and white, with their various shades, are almost endless. Sometimes the Chameleons do not turn pale during the night, but remain more or less dull green, with or without brownish patches. Adaptation to their immediate surroundings takes place to a very moderate degree only, but as a rule they are brightest, especially in their green tints, when they are allowed to sit amongst green foliage. The introduction of a branch with fresh leaves generally has a brightening effect upon those which have previously been confined in a cage with dry twigs only. Cold does not necessarily make them pale, but they appear duller, and the changes take place more slowly. After all, Linnaeus has summed up the little we really know about the causes of these changes, in the following terse sentence: "Vivus varios colores assumit secundum animi passiones, calorem et frigus."
Chameleons are not very amiable. When taken up they blow themselves out or they bite painfully, and it is a long time before they are tame enough not to go through various antics of anger when one approaches them. When taken in the hand they produce a peculiar faint grunting noise, which, however, can be better felt than heard. They quarrel much amongst each other; and the males, during the pairing season, are particularly ill-tempered. Each individual selects its own particular branch to sleep on, if possible a horizontal one, upon which it crouches down lengthwise, with the head and belly resting upon the branch. The tail generally makes a turn round another branch, and the four legs, grasping some supporting branch, are put into any, sometimes into an almost incredibly, awkward position. Although they climb about a good deal during the daytime, they generally resort to their accustomed sleeping branch, and they defend this vigorously against would-be intruders.
Chameleons are most deliberate in their movements, sometimes provokingly slow. Each arm and foot leaves the firmly grasped branch with great hesitation, and makes with equal deliberation for some other foothold. It does not matter if the thigh appears almost twisted out of its joint. The creature will {578}remain in the most uncomfortable position, forgetting, one might think, to put one or more of its limbs down, but keeping them instead in the air.
It is most interesting to watch them stalking their prey. Suppose we have introduced some butterflies into their roomy cage, which is furnished with living plants and with plenty of twigs. The Chameleons, hitherto quite motionless, perhaps basking with flattened-out bodies so as to catch as many of the sun's rays as possible, become at once lively. One of them makes for a butterfly which has settled in the farther upper corner of the cage. With unusually fast motions the Chameleon stilts along and across the branches and all seems to go well, until he discovers that the end of the branch is still 8 inches from the prey, and he knows perfectly well that 7 inches are the utmost limit to a shot with his tongue. He pauses to think, perhaps with two limbs in the air, but stability is secured by a judicious turn of the tail. After he has solved the puzzle, he retraces his steps to the base of the branch, climbs up the main stem, creeps along the next branch above, and when arrived at the 7 inch distance, he shoots the butterfly with unerring aim. The capacity of the mouth and throat is astonishing. A full-grown Chameleon will catch, chew, and swallow the largest moth, for instance a Sphinx ligustri. When large, the prey is chewed, but the wings and legs are swallowed with the rest. Occasionally these parts are bitten off, especially the prickly long legs of large locusts.
In water Chameleons are quite helpless. Sometimes they inflate themselves, but they always topple over on to the side, and the movements of their limbs are absolutely without any definite purpose.
When the eggs are ripe, and this happens with the Common Chameleon about the end of October, the female refuses to take food, and becomes restless. One of my specimens searched about probing the ground for about a week before she dug a hole in some more solid soil. This took two days. In the evening I found her sitting in the hole to the middle of her body. On the following morning she was still there, but busy filling the hole with soil and covering it with dry leaves. A few eggs were lying about outside, two of which at least I saw her taking up by the hand and putting them on the {579}nest, which was found to contain some thirty soft-shelled eggs closely packed upon each other. During the whole process she was very snappy, and hissed much when approached. After that she crept into the twigs as usual, but refused to eat, vomited at once the artificially introduced food, became restless on the sixth day, crawling about at the bottom of the cage, and died on the following day. This is the usual fate, almost without exception, of females after they have deposited their eggs in captivity. The great number of eggs and their deposition naturally exhausts them, and they probably want to hibernate at once. The eggs, which are yellowish, long-oval, about half an inch long and covered with a parchment-like shell, are very difficult to rear, chiefly on account of the difficulty of regulating the moisture. They shrink up when too dry, and they are very liable to become mouldy. According to Fischer[173] the eggs can be hatched in a large flower-pot with a layer of horse-droppings at the bottom, then a layer of 6 inches of slightly moist soil, then the eggs, then another 6 inches of loose soil, with a glass plate covering the top, securing at the same time ventilation. In this way he succeeded in hatching several sets of eggs after 125 and 133 days respectively.
Ch. calcaratus, the Indian Chameleon, is found in the southern half of the Peninsula and in Ceylon, but it is far from common. It much resembles Ch. vulgaris, but the male is distinguished by a tarsal process or "spur," covered with skin, on the inner side of the foot.
Ch. pumilus, the Dwarf Chameleon of South Africa, reaches a total length of 5 to 6 inches. It has a well-marked, serrated gular crest, which extends from the chin to the end of the neck. The chest and belly are without a toothed line, but a strongly serrated series extends from the occiput over the back and tail (see the right upper corner of Fig. 152 on p. 575). A row of enlarged tubercles or scales extends along the sides of the body. The general colour is green, with a large and long patch of brick-red on the sides; small dots and spots of intense red are scattered over various parts of the body. The changes of colour are rather limited. At night the Dwarf Chameleon does not turn pale, but generally keeps its colour. When they are very well the green is quite saturated, and the large red patch on the side is {580}interrupted by several blue spots. When they are angry or unhappy the red turns into dirty brown, and the green becomes quite dull. Sometimes the whole animal turns dull black.
This pretty little species is relatively hardy, being, as a native of South Africa, accustomed to cold nights. It does well in an ordinary temperate greenhouse, where it will live for several years, provided it has an ample supply of flies and meal-worms. It is viviparous, the young being probably born in the month of March or April.
Ch. bifidus, of Madagascar, shows an extraordinary difference between the sexes. The male reaches the great length of 16 inches, and develops two long rostral processes, which extend forwards beyond the snout; these processes are formed of dense connective tissue, which ossifies in the adult, and they are covered with scaly skin.
Ch. parsoni, likewise of Madagascar, is the giant amongst Chameleons, reaching a total length of 2 feet. The male has two large rostral processes which diverge upwards and outwards.
Brookesia, with several species in Madagascar, may be mentioned on account of its stunted appearance. The tail is much shorter than the body and scarcely prehensile; the scales on the soles are spinous. Total length only about 3 inches.
Rhampholeon, of tropical continental Africa, with several species, is likewise remarkable for the stunted and dwarfed appearance, and for the peculiar claws, each of which is furnished with a second cusp which is directed downwards. The tail is much shorter than the body. The total length of Rh. spectrum of the Camaroons is about 3 inches.
SAURIA, continued–OPHIDIA–SNAKES
Order II. OPHIDIA–SNAKES.
Saurians which have the right and left halves of the lower jaw connected by an elastic band.
The Snakes are the most highly specialised branch of the Sauria, from which they do not differ in any fundamental characters. The chief modifications consist in the absence of the limbs and limb-girdles (a feature intimately correlated with the much-elongated body), and in the swallowing apparatus. The reduction of the limbs and the elongation of the body also occurs in many Lacertilia; in several of the older families of Snakes (e.g. Typhlopidae and Boidae) vestiges of the hind-limbs and even of the pelvis are still in existence. Even the peculiar suspensorial apparatus of the lower jaw approaches that of the Lacertilia in the burrowing Ilysiidae and in Xenopeltis.
In the majority of the Snakes the quadrate is very loosely suspended from the squamosal (by some authorities homologised with the supratemporal bone of other reptiles), and this again is loosely attached to the lateral parietal region of the skull, placed horizontally, and elongated so far backwards that the vertically placed quadrate lies in a plane behind the skull. In most Snakes the elongated pterygoids are loosely attached to the inner side of the distal end of the quadrates, and they also often touch the mandibles. The whole palatal apparatus is movably attached to the skull, except in some burrowing families. The right and left pterygoids and palatines are widely separated from each other. The pterygoids and maxillaries, connected by the ectopterygoids, are absent, owing to reduction, in the {582}Typhlopidae and Glauconiidae only. The premaxilla is unpaired and small, and is rarely furnished with teeth. The latter are always sharp and recurved, and are lodged in sockets upon the edge of the supporting bone, with which they become firmly ankylosed. There is a perpetual succession of teeth. In the majority of Snakes teeth are carried by the maxillaries, palatines, pterygoids, and dentaries, rarely by the premaxillaries. The palatal teeth are restricted to the palatines in Oligodon, Dasypeltis, and Atractaspis only.
Peculiar modifications prevail in the poisonous Snakes. Those maxillary teeth which are at their base in connexion with the openings of poison-glands (modified upper labial glands), either have a furrow on the anterior side (Proteroglypha if the anterior teeth are grooved, e.g. the Cobras; Opisthoglypha if some of the posterior teeth are grooved), or the groove is converted into a canal, as in the Solenoglypha or Viperidae. The special modification of the maxillaries of the vipers with their long poison-fangs is described on pp. 587 and 637.
The orbit is generally closed behind by the postfrontal. Quadrato-jugal, postfronto-squamosal, and other arches are absent, so that the temporal fossa is quite open (see Fig. 156, p. 597, and Fig. 155, p. 596). The occipital condyle is distinctly triple. The mandibles are composed of several bones, but the coronoid is absent in the Xenopeltidae, Colubridae, Amblycephalidae, and Viperidae; it is large in the Boidae, reduced to a nodule in the Ilysiidae.
The parietals are always fused into a large unpaired bone, which generally forms a sharp crest and partly overlaps the occipitals; there is no interparietal or pineal foramen.
The vertebral column consists of many, often nearly three hundred vertebrae, and these skeletal segments correspond in number with those of the ventral and transverse scales of the skin. The vertebrae are procoelous; in addition to the anterior and posterior zygapophyses they have a pair of accessory articulations on the neural arches, dorsally to the zygapophyses;–the "zygantrum" carried by the posterior end of the neural arches, its articular surfaces looking upwards; and the "zygosphene" carried by the anterior end and looking downwards. Such accessory articulations occur also in a few Lizards, e.g. Iguanidae. The vertebrae of many Snakes have unpaired vertical, blade-like {583}haemapophyses on their centra for the more effective attachment of the muscles. All the vertebrae, except the atlas, carry ribs. These articulate by their capitular portions only, and are very movable in a head- and tail-ward direction. The ribs being long, and fitting with their ventral ends into the connective tissue of the sides of the ventral transverse scales, are the principal agents in pushing the body forwards, the posterior edges of these scales being sharp and imbricating.
The skin is covered with scales, absolutely devoid of osteoderms. When the scales are enlarged they are called shields. The keel, a common feature, is caused by a slight ridge of the cutaneous part of the scale. The whole skin is covered with a thin layer of horny epidermis, which is shed frequently, at least several times in one year; the shedding begins at the lips, and the whole outer skin is turned inside out from head to tail, retaining every minute detail of the cutaneous scales; even the watch-glass-like covering of the eyes is preserved.
The eyes are peculiar in so far as they possess no lids. The latter are still present in a vestigial condition in the embryo, but their place is taken by what is probably a modification of the nictitating membrane, which is drawn over the eye and covered with a single transparent scale of the horny skin, like a watch-glass. The eyes themselves are quite movable. The "tears," which of course cannot appear on the outside, are drained off into the nasal cavities by the naso-lacrymal ducts.
The ear is likewise peculiar. There is a long columellar rod with a fibrous or cartilaginous pad at the outer end, which plays against the middle of the shaft of the quadrate, an arrangement which, we must assume, produces a thundering noise in the internal ear, since every motion of the quadrate during the act of swallowing conveys the vibrations directly to the fenestra ovalis. The tympanic cavity, the Eustachian tubes, and the tympanum are abolished, and no external traces of the ear are visible. However, in spite of all this, Snakes can hear very well.
The nose is well developed, and many Snakes, for instance the Grass-Snake, are guided to their prey as much by the sense of smell as by the eyes and ears. The tongue is slender, very protractile and bifid, always moist, and furnished with many sensory corpuscles. It acts entirely as an additional sense-organ, hence the incessant play of the tongue of a snake which wants {584}to investigate anything. In spite of the protractility of the tongue, the hyoid apparatus is very small; the hyoid arches themselves are reduced to mere vestiges near the base of the first and only branchial arches, which are thread-like and extend backwards down the throat.
The trachea is very long, and opens far forwards in the mouth; it can be slightly protruded between the two halves of the lower jaw so as not to be blocked during the act of swallowing. This is a laborious process. The snake, having got hold of its prey with its teeth, generally shifts it into the most convenient position, in order to swallow the head first. One half of the mandible is then pushed forwards, then the other half; the recurved teeth afford the necessary hold, and the snake, little by little, draws its mouth-cavity, and later on itself, over the prey. In fact, it literally gets outside it. Sometimes with a large victim this process may last for hours; the whole mouth and head become painfully distended and the veins swollen almost to bursting. The snake pushes the prey against a stone or other obstacle, rests awhile quite exhausted, and begins afresh. At last the bulk of the prey has passed the mouth, the skin of the neck is stretched to the utmost, the scales being separated by wide interstices, the ribs work spasmodically, the victim is pressed into the shape of a sausage, and the deed is done. In order to assist deglutition there is a great amount of salivation, but the often-heard story that Snakes cover their prey with saliva before they swallow it, is a fable, or based upon faulty observation, snakes sometimes being forced to disgorge the half-swallowed prey, which, in such a case, is covered with slime. One of my tame snakes had swallowed a frog on my table when a friend entered the room. The snake was frightened, jumped on to the ground, striking it with its full belly, and thereby hurting the frog, which squeaked loudly, whereupon the snake reversed its mechanism and the frog hopped away, none the worse for its terrible experience.
In correlation with the elongated narrow space of the body-cavity the lungs are not equally developed, the right being much smaller than the left. The latter is a very thin-walled, hollow bag, and the posterior half or third scarcely contains any of the honey-comb-like respiratory "cells," but acts merely as a reservoir of air.
The cloacal arrangement is essentially the same as that of the Lacertilia, but Snakes possess no urinary bladder. The copulatory organs are stowed away beneath the skin in recesses of the posterior lateral corners of the shallow cloacal vestibulum. Each organ is generally bifurcated at the free end, and furnished with little spike-shaped, but scarcely horny, excrescences. On each side of the outer cloacal chamber, in both sexes, lies a roundish gland with an offensive, strongly-scented secretion; that of various Boas smells disagreeably sweet and musky. The majority of Snakes lay eggs, but most of the Viperidae and the thoroughly aquatic kinds, besides a few terrestrial forms, are viviparous. The egg-shells are like parchment, with very little or no calcareous deposit, so that they are always soft; many embryos are, however, provided with a little "egg-tooth" on the tip of the snout.
Snakes are intelligent creatures; some become quite affectionate in captivity, but most of them are of a morose disposition, and do not care for company.
The geographical distribution of Snakes has been dealt with in detail in connexion with the various families. Unfortunately very few fossils are known. One of the oldest is Palaeophis, of the London clay (Lower Eocene). Remains of Elapine and of innocuous Colubrine snakes have been found in the Lower Miocene of Germany; Crotaline forms are known from the Miocene of Turkey and North America. All the Plistocene {586}remains belong to recent genera. There are indications that the Ophidia are a relatively young branch of Reptilia, essentially of Tertiary date, but the foundations of the distribution of most of the older families were laid in Miocene times. The older families, notably those which still possess vestiges of hind-limbs or of the pelvis, are circumtropical, e.g. Typhlopidae, Boidae. The few survivors of the Glauconiidae are likewise circumtropical, with the exception of Australia. The Ilysiidae occur in South-Eastern Asia and in tropical South America; their offshoot the Uropeltidae are restricted to India and Ceylon. The Colubridae and even many of their sub-families are cosmopolitan. It is quite possible that the Opisthoglypha and Proteroglypha are not natural groups, but that their respective conditions have been developed on various occasions and in different countries. The same applies more strongly to the Viperidae, a further development of the Opisthoglyphous type. To judge from their distribution, the Crotaline snakes were possibly developed in the Palaearctic sub-region; they spread all over America, but they were debarred from entering either Australia or Africa. The Viperidae, on the other hand, are restricted entirely to the Palaeotropical region and to the Palaearctic sub-region. The fact that no separating belt of water existed for them between Europe and Africa, indicates their being the most recently developed of poisonous snakes. Madagascar is the only large country which, besides snakeless New Zealand, enjoys a total absence of poisonous snakes of any kind, while the Oriental is the only sub-region which suffers from the presence of numerous species of every sub-family of poisonous Elapine, Crotaline, and Viperine snakes.
Snake-Poison.–Many Snakes, belonging to different families, are poisonous, and unfortunately there is no external character, easily ascertained, by which every poisonous snake can be distinguished from a harmless kind. If the head is very broad, this is probably due to the pair of poison-glands on the sides of the head; but many harmless snakes can flatten and broaden their heads in a suspicious way, and, what is much worse, many of the most poisonous snakes, for instance the Cobras, have a head as smooth and as sleek-looking as the Grass- or Ring-Snake, the most harmless of species. It so happens that, with a few exceptions, for instance among the Crotalines and Vipers, no {587}badly poisonous snake has loreal shields, i.e. a pair of shields intercalated between the nasals and the preoculars, but this character is obviously no good for any practical purposes. Therefore, unless you know a snake well enough when you see it, leave it alone, because a mistake may be fatal.
The poison is secreted in modified upper labial glands, or in a pair of large glands which are the homologues of the parotid salivary glands of other animals.[174] A duct passes from the gland forwards along the side of the upper jaw. Just in front of the fang it doubles on itself, so as to open by a small papilla on the anterior wall of the sheath of mucous membrane which embraces the base of the tooth like a pocket. As mentioned before (p. 582), the poison is conveyed either along a furrow on the anterior side of the tooth, or the growing substance of the tooth partly converts the furrow into a canal which opens only near the end of the tooth. This is a perfectly devilish contrivance, ensuring the conveyance of the poison into the very deepest part of the wound. The Elapinae have relatively short fangs, while those of the Vipers, and especially those of the Crotaline snakes, are much longer, sometimes measuring nearly an inch in length. The most formidable apparatus is that of the Viperidae, since in them the maxillaries, each provided with only one acting fang, and without any other teeth behind, can be erected. The mechanism is explained in Fig. 154 and Fig. 179 (p. 647). The apparatus of the upper jaw is so constructed that the pushing forwards of the horizontal pterygoid bar will, by acting on the ectopterygoid, rotate and erect the short maxillary. The pulling forwards is effected by contraction of the spheno-pterygoid muscle, which arises far forwards from the basal orbito-sphenoid region, and is inserted on to the inner dorsal surface of the pterygoid. The principal closing muscles of the mouth are the temporo-masseteric muscles (Fig. 179, T.a. and T.p.) and the inner and outer pterygoid muscles, which latter arise from the outer surface of the pterygoid bone, or from the maxillary, and are inserted on to the articular region of the mandible.
A strong ligament arises from the squamoso-quadrate junction, and spreads fan-shaped upon the connective tissue {588}wall of the poison-gland; the anterior and posterior ends of the gland are held by another strong band, which stretches from the maxilla to the mandibular joint. The whole is so arranged that the acts of opening the jaws (by the digastric muscles) and the erection of the fang-bearing maxillaries are enough to mechanically squeeze the contents out of the poison-gland. A portion of the anterior temporal muscle is attached to the capsule of the poison-gland.
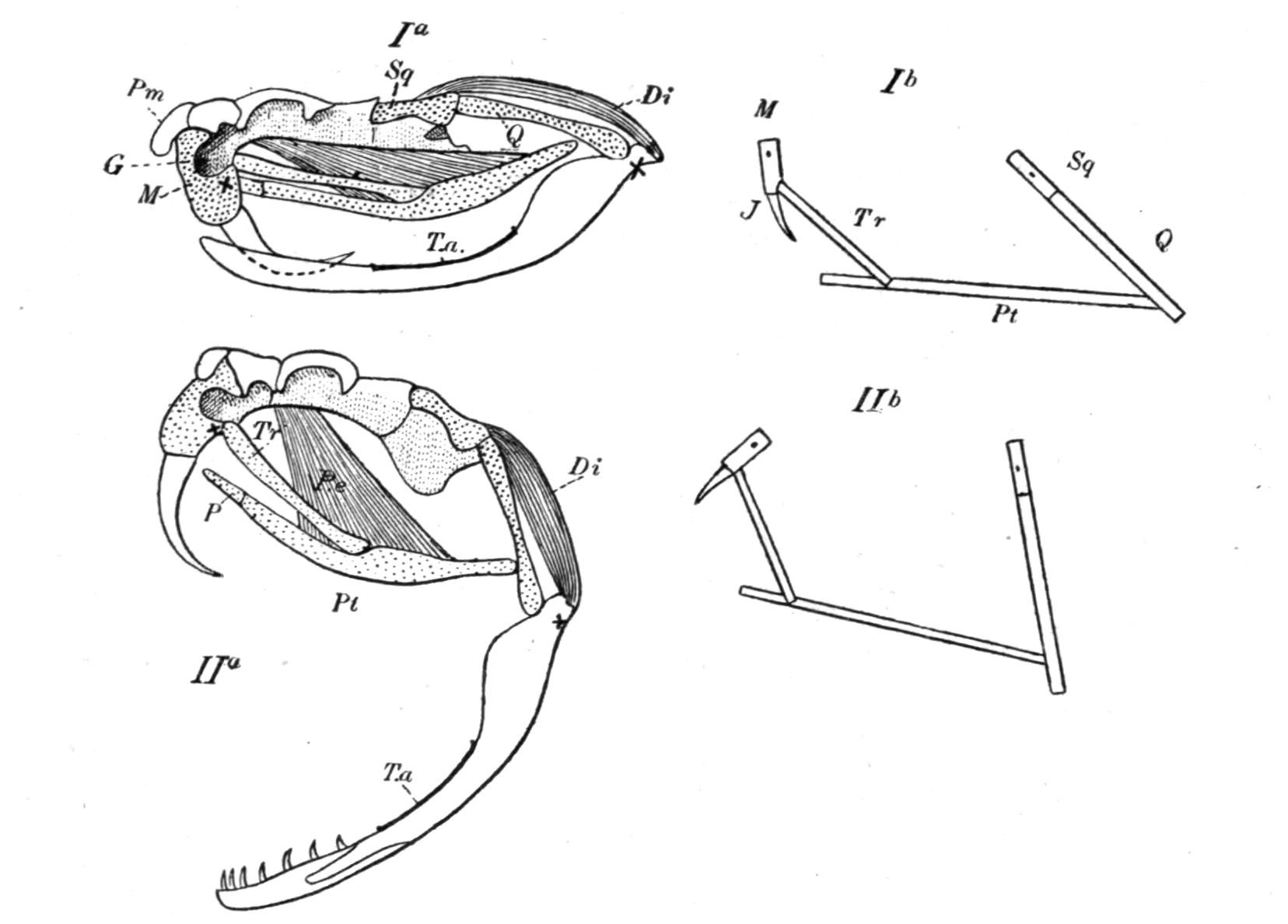
Fig. 154.–Explanation of the biting mechanism of a rattlesnake. Ia and Ib, position of the apparatus when the mouth is shut. IIa and IIb, position of the apparatus when the mouth is opened widely; the spheno-pterygoid muscle (P.e) is contracted, the pterygoid (Pt) is pulled forwards, the transverse bone or ectopterygoid (Tr) pushes the maxillary (M), rotates it and thereby causes the poison-fang (J) to assume an erect position. Di, Digastric muscle, contraction of which lowers, or opens, the lower jaw; G, the groove or pit characteristic of the Crotaline snakes; J, poison-fang; M, maxillary; P, palatine; P.e, spheno-pterygoid muscle; Pm, premaxillary; Pt, pterygoid; Q, quadrate; Sq, squamosal; T.a, insertion of the anterior temporal muscle, by contraction of which the mouth is shut; cf. Fig. 179 (p. 647); Tr, transversum or ectopterygoid; X, origin and insertion of a muscle and a strong ligament, contraction of which draws the maxillary and its tooth back into the position of rest and assists in shutting the mouth.
An excellent account of the nature and of the effect of the venom of Snakes has been written by Charles J. Martin.[175] The following condensed account has been abstracted from it:–
"The poison is a clear, pale yellow, or straw-coloured fluid, {589}which reacts acid, and contains about 30 per cent of solids, but this varies much according to the state of concentration. Most venoms are tasteless, but Cobra poison is said to be disagreeably bitter. Dried venom keeps indefinitely, and dissolves readily in water. It keeps also in glycerine. It contains albuminous bodies in solution. The venom is, in fact, a pure solution of two or more poisonous proteids, which are the active agents, with a small quantity of an organic acid or colouring matter. The venom is destroyed by reagents which precipitate proteids in an insoluble form, or which destroy them, e.g. silver nitrate or permanganate of potash. Hypochlorites have the same effect. Carbolic acid and caustic potash destroy it only after a day or two.
"The venom is generally introduced into the subcutaneous tissue, whence it reaches the general circulation by absorption through the lymph and blood-vessels. When introduced directly into a vein, the effects are instantaneous. It is absorbed by the conjunctiva, but, excepting Cobra poison, not by the mouth or alimentary canal, provided there be no hollow teeth or no abrasions. The venom of the various kinds of Snakes acts differently.
"The symptoms of Cobra poison. Burning pain, followed by sleepiness, and weakness in the legs after half an hour. Then profuse salivation, paralysis of the tongue and larynx, and inability to speak. Vomiting. Incapability of movement. The patient seems to be conscious, but is unable to express himself. The breathing becomes difficult. The heart's action is quickened. The pupil remains contracted and reacts to light. At length breathing ceases, with or without convulsions, and the heart slowly stops. Should the patient survive, he returns rapidly to complete health.
"The symptoms of Rattle-snake poison. The painful wound is speedily discoloured and swollen. Constitutional symptoms appear as a rule in less than fifteen minutes: prostration, staggering, cold sweats, vomiting, feeble and quick pulse, dilatation of the pupil, and slight mental disturbance. In this state the patient may die in about twelve hours. If he recovers from the depression, the local symptoms begin to play a much more important part than in Cobra poisoning: great swelling and discoloration extending up the limb and trunk, rise of {590}temperature and repeated syncope, and laboured respiration. Death may occur in this stage. The local haemorrhagic extravasation frequently suppurates, or becomes gangrenous, and from this the patient may die even weeks afterwards. Recovery is sudden, and within a few hours the patient becomes bright and intelligent.
"Symptoms of bite from the European Viper. Local burning pain; the bitten limb soon swells and is discoloured. Great prostration, vomiting, and cold, clammy perspiration follow within one to three hours. The pulse is very feeble, with slight difficulty in breathing, and restlessness. In severe cases the pulse may become imperceptible, the extremities may become cold, and the patient may pass into coma. In from twelve to twenty-four hours these severe constitutional symptoms usually pass off, but in the meantime the swelling and discoloration have spread enormously. Within a few days recovery usually occurs somewhat suddenly, but death may occur from the severe depression, or from the secondary effects of suppuration.
"Symptoms of bite from the Daboia or Vipera russelli. These resemble the effects of Rattle-snake poison, but sanious discharges from the rectum, etc., are an additional and prominent feature. The recovering patient suffers from haemorrhagic extravasations in various organs, besides from the lungs, nose, mouth, and bowels. Kidney haemorrhage and albuminuria is a constant symptom. The pupil is always dilated and insensitive to light.
"Symptoms of bite of Australian Elapine snakes. Pain and local swelling. The first constitutional symptoms appear in fifteen minutes to two hours. First faintness, and an irresistible desire to sleep. Then alarming prostration and vomiting. The pulse is extremely feeble and thread-like, and uncountable. The limbs are cold, and the skin is blanched. Respiration becomes shallow with the increasing coma. Sensation is blunted. The pupil is widely dilated, and insensible to light. There is sometimes passing of blood. If the patient survives the coma, recovery is complete and as a rule rapid, without secondary symptoms. The Australian venom and that of all viperine snakes, perhaps also that of the Cobra, if introduced rapidly into the circulation, occasions extensive intravascular clotting. If the venom is slowly absorbed, the blood loses its coagulability, {591}owing to the breaking down of the red blood-corpuscles, most so with vipers, less with Australian snakes, least so with the Cobra. The Cobra venom is supposed to extinguish the functions of the various nerve-centres of the cerebro-spinal system, the paralysation extending from below upwards, and it has a special affinity for the respiratory centre. The toxicity or relative strength of the Cobra venom has been calculated to be sixteen times that of the European Viper. Snakes can poison each other, even those of the same kind.
"Treatment.–Apply a ligature above, not on the top of the situation of the bite; twist the string tightly with a stick. Then make a free incision into the wound. Sucking out is dangerous! Then bandage the limb downwards, progressing towards the wound; repeat this several times. Direct application into the widened wound of calcium hypochlorite, i.e. bleaching powder, is very good, or of a 1 per cent solution of permanganate of potash, or Condy's fluid. Amputation of the finger is the best remedy of all if a large snake has bitten it. Do not keep the ligature longer than half an hour. Then let the circulation return, and apply the ligature again. In any case, do not keep the ligature on for more than one hour for fear of gangrene.
"Internal remedies.–The administration of enormous doses of alcohol is to be condemned strongly; small stimulating doses are good, but stimulation can be more effectively produced by ammonia or strychnia. Hypodermic injection of strychnine, in some cases as much as one to two grains (but not into a vein!) has in some cases had good results; but injection of ammonia, instead of doing any good, has disastrous sloughing results. There is only one fairly reliable treatment, that by serum therapeutics, the injection of considerable quantities of serum of animals which have been partially immunised by repeated doses of snake-venom. Unfortunately this treatment will not often be available."
Several well-known Mammals and Birds are immune by nature against snake-venom, but most of them avoid being bitten. Some birds induce the snake to strike and bite frequently into their spread-out wings. Such more or less immune creatures are the Mongoose, the Hedgehog, and the Pig, the Secretary bird, the Honey Buzzard, the Stork and probably other snake-eaters.
Classification of Ophidia.–Duméril and Bibron[176] divided Snakes according to their teeth into Opotérodonts, Aglyphodonts, Solenoglypha, Proteroglypha, and Opisthoglypha.
J. E. Gray[177] divided Snakes into two sub-orders: Viperina and Colubrinia. Günther[178] distinguished between Ophidii colubriformes, O. colubriformes venenosi (Elapidae and Hydrophidae) and O. viperiformes. Cope[179] laid stress upon the modifications of the squamosal, ectopterygoid, and ectopterygoid bones, and also upon the condition of the vestigial limbs. He divided the snakes into Scolecophidia (Typhlopidae), Catodonta, Tortricina, Asinea (the harmless snakes without limb-vestiges), Proteroglypha, and Solenoglypha.
Boulenger[180] has accepted Cope's principles, and, mainly by combining the Asinea with the Proteroglypha as Colubridae, has produced a logically conceived system, by far the best hitherto proposed. It has been followed in the present work. Boulenger's phylogenetic system stands as follows:–
I. No ectopterygoid; pterygoid not extending to quadrate or to mandible; no supratemporal (squamosal); prefrontal forming a suture with nasal; coronoid present; vestiges of pelvis.
Maxillary vertical, loosely attached, toothed; mandible edentulous; a single pelvic bone. .......... Typhlopidae, p. 593.
Maxillary bordering mouth, forming a suture with premaxillary, prefrontal, and frontal, toothless; lower jaw toothed; pubis and ischium present, latter forming a symphysis. .......... Glauconiidae, p. 594.
II. Ectopterygoid present; both jaws toothed.
A. Coronoid present; prefrontal in contact with nasal.
1. Vestiges of hind-limbs; supratemporal (squamosal) present.
Squamosal large, suspending quadrate. .......... Boidae, p. 596.
Squamosal small, intercalated in the cranial wall. .......... Ilysiidae, p. 594.
2. No vestiges of limbs; squamosal absent. .......... Uropeltidae, p. 595.
B. Coronoid absent; squamosal present.
1. Maxillary horizontal; pterygoid reaching quadrate or mandible.
Prefrontal bone in contact with nasal. .......... Xenopeltidae, p. 605.
Prefrontal not in contact with nasal. .......... Colubridae, p. 606.
2. Maxillary horizontal; pterygoid not reaching quadrate or mandible. .......... Amblycephalidae, p. 637.
3. Maxillary vertically erectile, perpendicularly to ectopterygoid; pterygoid reaching quadrate or mandible. .......... Viperidae, p. 637.
For ordinary practical purposes this synopsis is useless, being based entirely upon anatomical characters, not all easily ascertained. The following characterisation of families may therefore be preferred:–
Eyes vestigial; no teeth in the lower jaw; without enlarged ventral scales. .......... Typhlopidae.
Eyes vestigial; teeth restricted to the lower jaw; without enlarged ventral scales. .......... Glauconiidae.
Eyes very small; head not distinct; ventral scales scarcely enlarged; tail extremely short, ending obtusely and covered with peculiar scales. .......... Uropeltidae.
With vestiges of the hind-limbs appearing as claw-like spurs on each side of the vent; ventral scales transversely enlarged; eyes functional, free.
Ventral scales scarcely enlarged. .......... Ilysiidae.
Ventral scales transversely enlarged. .......... Boidae.
With a pair of poison-fangs in the front part of the mouth, carried by the otherwise toothless, much shortened, and vertically erectile maxillaries; ventral scales transversely enlarged; eyes free. .......... Viperidae.
All the remaining Snakes combine the following characters: the maxillaries are typical, not separately movable, horizontal, with a series of teeth.[181] The mandible is toothed but has no coronoid bone. There are no vestiges of limbs or of their girdles. The eyes are free.
Dentary movably attached to the tip of the articular bone of the mandible; skin beautifully iridescent. .......... Xenopeltidae.
Without a mental groove; the ends of the pterygoids are free, not reaching the quadrates. .......... Amblycephalidae.
With a median longitudinal groove between the shields of the chin; the squamosal is horizontally elongated, movable; the pterygoid reaches the quadrate. .......... Colubridae.
Fam. 1. Typhlopidae.–Burrowing snakes which have the whole body covered with uniform cycloid scales, and with the teeth restricted to the small and transversely placed maxillary bones. The pterygoids do not extend backwards to the quadrates, and there are no endopterygoids. The quadrates slant obliquely forwards, and are attached directly to the pro-otics, {594}owing to the absence of squamosal bones. The prefrontals are in lateral contact with the nasals. There are vestiges of the pelvis, reduced to a single bone on each side. The eyes are hidden by shields of the skin.
The Typhlopidae, mainly composed of the genus Typhlops, with about one hundred species, are undoubtedly the last living descendants of formerly cosmopolitan, rather archaic, snakes, which in adaptation to their burrowing life and insectivorous diet have undergone degradation. They are still widely distributed in all tropical and sub-tropical countries, some on the solitary Christmas Island, but not in New Zealand. One species, T. vermicularis, inhabits the Balkan Peninsula and South-West Asia. It is brown above, yellowish below, and reaches a length of about 10 inches. The tail is extremely short and ends in a horny spine. T. braminus is widely distributed in Southern Asia, the Malay Islands, the islands in the Indian Ocean and in Southern Africa.
Fam. 2. Glauconiidae.–In most respects resembling the Typhlopidae, but the maxillaries retain their normal position and are toothless, teeth being restricted to the lower jaw, which is stout and short. The pelvic girdle and the hind-limbs show the least reduction found in any recent Snakes; in the pelvis the ilia, pubes, and ischia can still be distinguished, the last even retaining their symphysis; there are also vestiges of femurs. About thirty species, nearly all belonging to the genus Glauconia, are found in South-Western Asia, Africa, and the warmer parts of America, including the West Indies.
Fam. 3. Ilysiidae.–The scales of the cylindrical body are smooth and small, those on the ventral side are scarcely larger. The tail is extremely short and blunt. The head is very small, not distinct from the neck. The gape of the mouth is very narrow. Teeth are carried by the mandibles, the pterygoids, palatines, maxillaries, and one or two or more by the premaxillae. The endopterygoids are short. An important cranial feature is the short quadrates, which stand rather vertically and are connected with the cranium by the squamosals; these are very small and are firmly wedged in between the upper ends of the quadrates and the pro-otic, lateral, and supra-occipital bones; now forming part of the cranial wall. Vestiges of the pelvis and hind-limbs are very incomplete, and terminate in claw-like spurs, {595}protruding between the scales on either side of the vent. The eyes are very small, and are either free or covered by transparent shields. The few, scarcely half-a-dozen, species are found in South America (Ilysia) and in Ceylon, the Malay Islands, and Indo-China.
Ilysia (Tortrix) scytale, the Coral-Snake of Tropical South America, is a beautiful coral-red with black rings. On account of its beauty, perfectly harmless nature, and for "cooling purposes," this snake, which grows to nearly a yard in length, is sometimes worn as a necklace by native ladies. All the Ilysiidae lead a partly burrowing life, live chiefly upon worms, insects, and little Typhlopidae, and are viviparous.
Fam. 4. Uropeltidae.–Burrowing snakes of Ceylon and Southern India, with a short and rigid cylindrical body and a very short tail, which ends in a large peculiar shield, often obliquely truncated. The scales of the body are smooth, and are little larger on the belly; the coloration is mostly very beautiful. The eyes are very small.
The Uropeltidae are somewhat intermediate between the Ilysiidae, Glauconiidae, and Boidae. The pterygoids do not reach the quadrates; but ectopterygoids are present; the quadrates are very small and directly attached to the skull, squamosals being absent. Teeth are carried by the mandibles and by the maxillaries, which are normal in their position. There are no vestiges of hind-limbs or of the pelvis. The Uropeltidae, of which about forty species are known, are viviparous, burrow in the ground, and frequent damp localities, preferring mountain-forests. The use of the characteristic tail-shield is not clear; perhaps it assists these rather rigid creatures in digging, by being pressed against the ground.
Uropeltis.–The tail is obliquely truncated, ending in a roundish, flat shield.
U. grandis s. philippinus.–The latter name seems to have misled W. Marshall[182] into including the Philippine Islands in the range of the family, a mistake which is sure to be propagated. The species, the only one of the genus, is confined to Ceylon; it is blackish above, yellow below, frequently with small yellow spots above and brown spots on the under surface. It grows to about 18 inches in length.
Rhinophis.–The tail-shield is convex and the snout is pointed. Rh. sanguineus of Southern India is black above with a bluish gloss, sometimes with small pale specks; the belly and several of the lateral series of scales are bright red, spotted with black. The tail-shield is black and red.
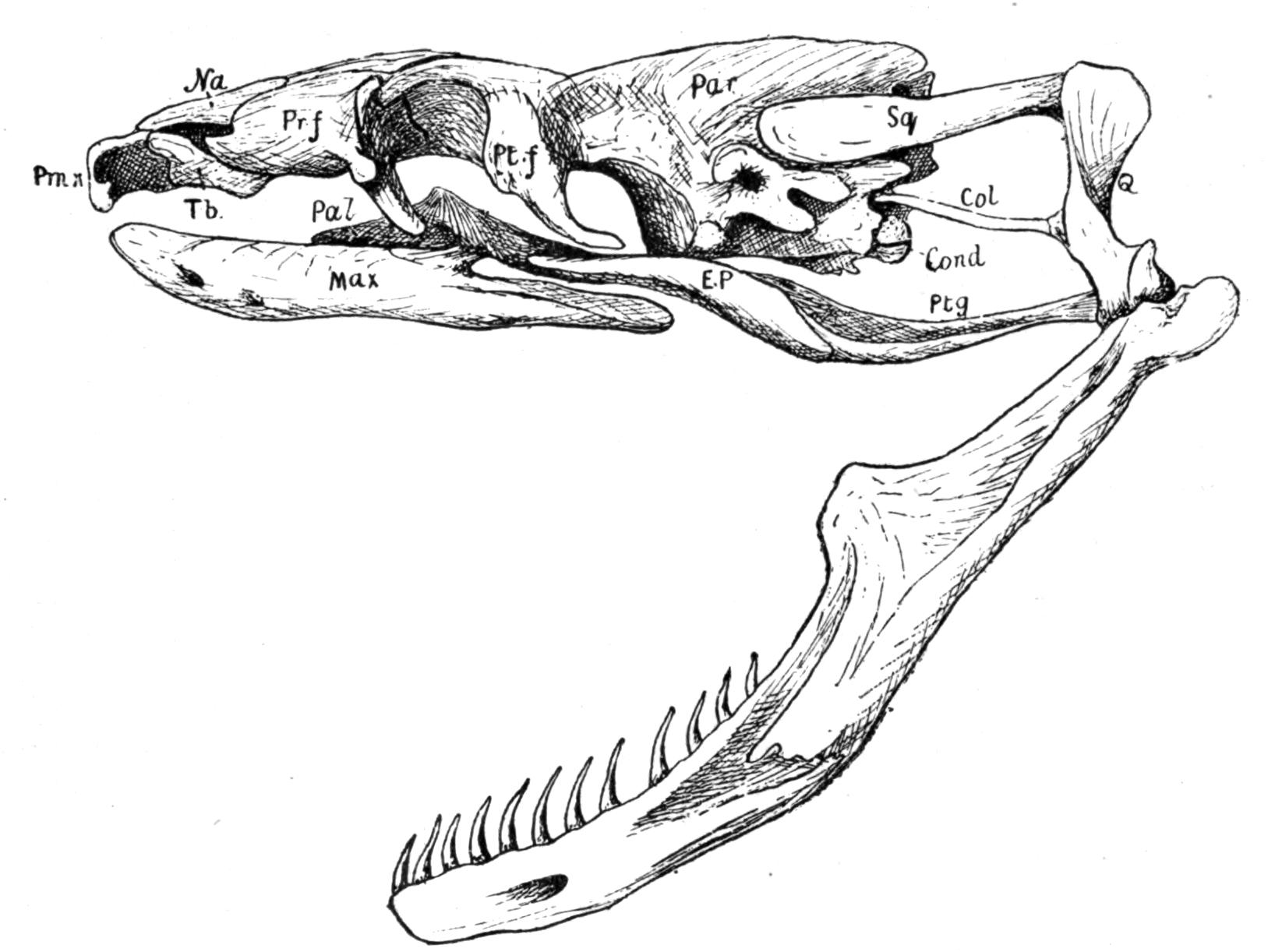
Fig. 155.–Skull of Eunectes murinus. × 1. The teeth on the maxillary, palatine, and pterygoid have been omitted. Col, Columella auris; Cond, occipital condyle; E.P. and E.Ptg, ectopterygoid or transverse bone; F, frontal; Mand, mandible; Max, maxillary; Na, nasal; Pal, palatine; Par, parietal; Pmx, premaxillary; Pr.f, prefrontal; Pt.f, postfrontal; Ptg, pterygoid; Q, quadrate; Sq, squamosal; Tb, turbinal.
Fam. 5. Boidae.–Typical Snakes, usually large, and with vestiges of pelvis and hind-limbs, appearing externally as claw-like spurs on each side of the vent. The scales of the upper surface are usually small and smooth, while those of the ventral surface form one broad series on the belly, and one or two rows on the tail. The quadrate is carried by the horizontally elongated squamosal, which rests loosely upon the lateral occipital region. The prefrontal is in contact with the nasal. Teeth are carried by the mandibles, the pterygoids, palatines, maxillaries, and, in the Pythoninae, by the premaxillaries also. For further details see Figs. 155, 156.
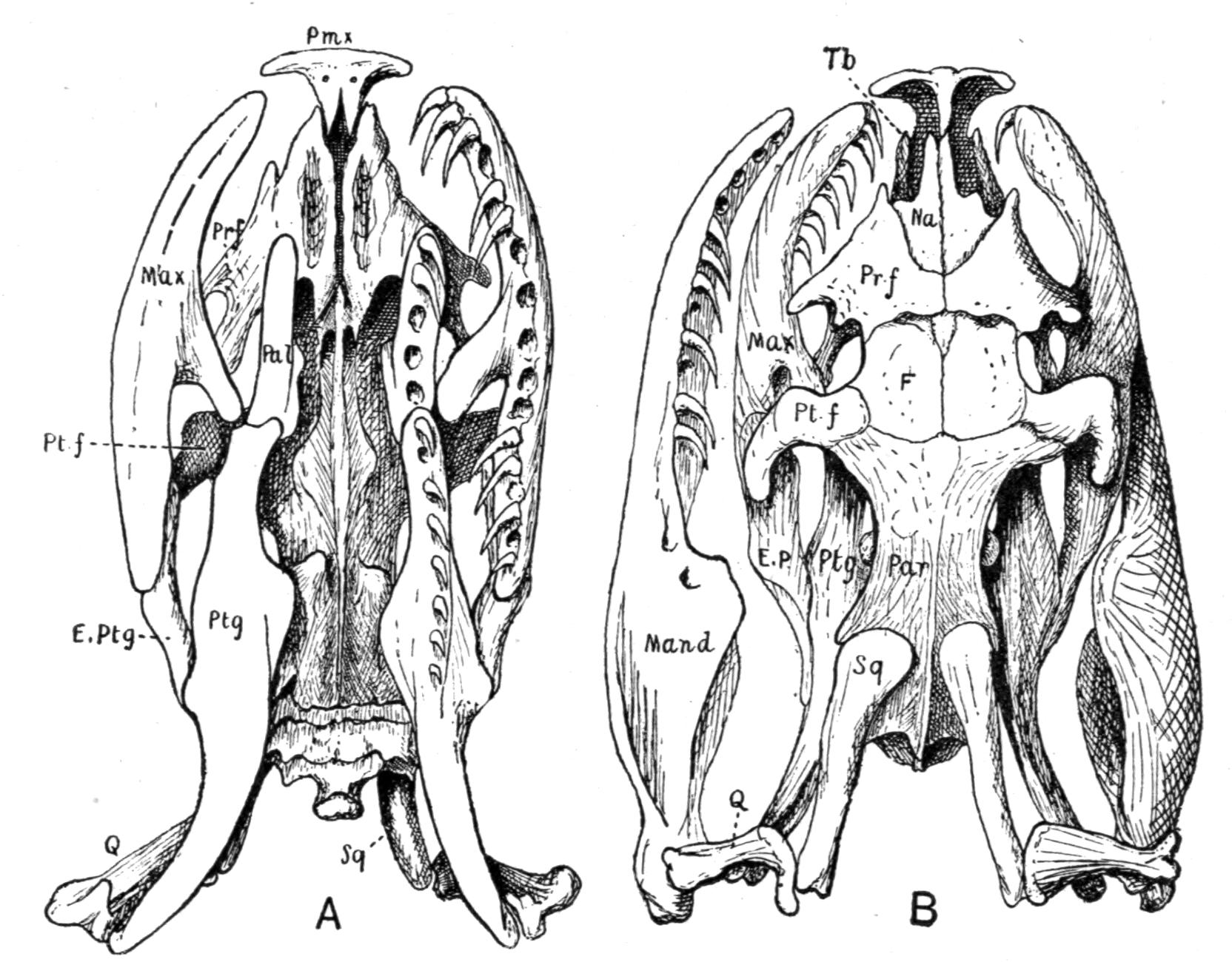
Fig. 156.–A, Ventral, B, dorsal, view of the skull of Eunectes murinus. Lettering as in Fig. 155. × 1.
The Boidae comprise between sixty and seventy species, which have been grouped into many genera, on unimportant characters, referring to the scales and shields of the head. It is doubtful if they are natural groups, a consideration which detracts much from their value in the study of geographical distribution. Even the two sub-families are not free from this reproach. The range of the family is world-wide, Boidae occurring in all tropical and sub-tropical countries, including islands, except New Zealand. A few species live in South-Eastern Europe (Eryx) and in North-Western America. They mostly prefer wooded districts, especially forests; climbing trees, assisted by the short and partly prehensile tail. Others are semi-aquatic, and a few live in sandy localities. They are all rapacious, and by preference feed on warm-blooded creatures, which they constrict by coils of the body in order to hold, kill, and crush the victim before swallowing it. Exaggerated notions are entertained about their swallowing capacity. It is obvious that a large snake, 20 feet long, half a foot thick, and weighing several hundred pounds, can crush a tiger, a stag, or even a {598}cow; but common sense tells us where to draw the line when it comes to the swallowing of the prey. Small game, although of a bulk apparently far too big for the snake, is so crushed and mangled that it is turned into the shape of a sausage preparatory to the long process of swallowing. The Boidae lay eggs, and some species incubate them, or rather the female coils herself round them for the sake of protection. No appreciable amount of extra warmth is developed. Unfortunately the observations of one of the best cases on record[183] were conducted so imperfectly that they are of little value.
Sub-Fam. 1. Pythoninae.–With a pair of supra-orbital bones, intercalated between the prefrontal, frontal, and postfrontal bones. The sub-caudal scales are mostly in two rows. The premaxilla often carries a few small teeth.
The Pythoninae, comprising about twenty species, are restricted to the Palaeotropical and Australian regions, with the sole exception of Loxocemus bicolor in Southern Mexico.
Python, the principal genus, has teeth on the premaxilla. The rostral, each of the anterior upper labials, and some of the lower labial shields, contain a deep, probably sensory, pit. The maxillary and mandibular teeth are long, but decrease from before backwards. The head is distinct from the neck, and is covered with symmetrical shields or with small scales. The scales of the body are small and smooth. The tail is short and prehensile; below with two rows of scales. The pupil of the eye is vertical. The range of the genus extends over the whole of the Palaeotropical and Australian regions, excepting Madagascar and New Zealand.
P. spilotes, the "Carpet Snake" of Australia and New Guinea, is mostly beautifully marked, but is subject to much variation in colour. The more typical specimens are black above, each scale with a yellowish dot, with yellow spots or combinations of dots, more or less arranged in rows. The under parts are yellow. It reaches a length of about two yards, and spends a great part of its time in trees.
P. reticulatus is the commonest species in Indo-China and in the Malay Islands. Four upper labial shields of each side are pitted. The specific name refers to the bold, dark, lozenge-shaped markings upon the lighter yellowish or brown ground. A black {599}line extends over the head from the nose to the neck, and another on each side from the eye to the angle of the mouth. The under parts are mostly yellowish, with small brown spots on the sides.
This is one of the largest species of Python, some specimens being known which measured about 30 feet in length.
As a sample of folk-lore connected with this monstrous snake the following Burmese fable has been recorded by Mason:–[184]
"According to a Karen legend all the poisonous serpents derive their virulence from the Python, which, though innocuous now, was originally the only one that was venomous. In those days he was perfectly white, but having seduced away a man's wife, Aunt Eu (Eve), he made her, while she was in his den, weave figures on his skin in the forms which are now seen. At that time, if he bit the footstep of a man in the road, such was the virulence of his poison that the man died, how far soever that man might have passed from the bitten track. The Python had not, however, an ocular demonstration of the fact, so he said to the Crow: 'Crow, go and see whether people die or not when I bite the foot-track.' The Crow went to the neighbourhood of a Karen cabin, and found the people, as is their custom at funerals, laughing, singing, dancing, jumping, and beating drums. He therefore returned to the Python, and told him that so far from {600}his efforts producing death, on the contrary they produced joy. The Python was so angry when he heard this that he ascended a tree and spit up all his venom, but other creeping things came and swallowed it, and people die of their malignancy to this day. The tree, therefore, from which the Python spat up his venom became deadly, and its juice is used to this day for the purpose of poisoning arrows. The Python made the other creatures promise not to bite without provocation. The Cobra said: 'If there be transgression so as to dazzle my eyes, to make my tears fall seven times in one day, I will bite.' So said the Tiger (whose bite the Karens esteem as virulent as a serpent's) and others, and they were allowed to retain their poison. But the Water Snake and Frog said they would bite with or without cause as they liked; so the Python drove them into the water, where their poison melted away and their bite became harmless."
P. molurus is the species of India and Ceylon, ranging, however, also into Indo-China. Boulenger quotes W. Elliot[185] {601}as the authority for the statement that this species grows to the length of 30 feet. Only two pairs of upper labials are pitted. The general colour above is greyish or yellowish brown with a dorsal series of large reddish-brown, black-edged patches, and on the sides of the body with a series of smaller spots with light centres. On the head is a lance-shaped marking; a brown stripe passes from the eye backwards. The under parts are yellowish.
P. sebae and P. regius are African species. The former has two pairs of upper labials pitted, the latter four pairs. P. sebae is generally pale brown above with dark brown, black-edged cross-bars, which are usually connected by a sinuous dark stripe along each side of the back. The upper surface of the tail has a light stripe between two black stripes. The belly is spotted and dotted with dark brown. P. sebae ranges over the whole of Tropical and Southern Africa, perhaps with the exception of Eastern Africa. P. regius of West Africa is beautifully marked, and may be recognised by the dark brown, black-edged band along the back, sending down triangular or Y-shaped processes on the sides, which are pale brown. This dorsal band encloses a light streak on the neck and another on the tail. The belly is yellowish.
These African Pythons grow to a length of about 15 feet, but specimens so large as this are not often met with. The negroes of certain parts of the coast of Guinea are said to worship them and to keep them in special temples, where they are regularly attended to. Their food consists chiefly of small Mammals, notably rats, and of Birds. A couple of these snakes paired in the Zoological Gardens of London in the month of June. The female laid nearly one hundred eggs in the following January, and incubated them until April, when the embryos were found to be still unripe.
Sub-Fam. 2. Boinae.–Without supra-orbital bones. The premaxilla is toothless. The subcaudal scales form mostly a single row.
The Boinae comprise between forty and fifty species. Most of them are American, but the genus Eryx inhabits North Africa, Greece, and South-Western Asia; the genus Enygrus inhabits New Guinea and many of the Pacific Islands, for instance New Britain (Neu Pommern), the Solomon, Loyalty and Fiji Islands, {602}and the New Hebrides. Casarea dussumieri is found on Round Island near Mauritius; and two species of Boa and one of Corallus represent the Boidae in Madagascar, while all the others live in Central and South America.
Boa.–The maxillary and mandibular teeth gradually decrease in size. The scales of the upper parts of the body and tail are smooth and very small. The rostral shield is enlarged. The nostrils are placed between two or three nasals, and these are separated from those of the other side by small scales. The tail is short and prehensile. The pupil is vertical.
B. constrictor, of South America, has the head covered with small scales, one of the pre-oculars being enlarged. The eye is separated from the labials by several series of tiny scales. The general colour is a delicate "pale brown above, with fifteen to twenty dark brown cross-bars widening on each side, and, if connected by a dark dorso-lateral streak, enclosing large elongate oval spots.... On each side is a series of large dark brown spots with light centres, most of which alternate with the cross-bars. On the tail the markings become much larger, brick-red, edged with black, and separated by narrow, yellowish interspaces." Under parts yellowish with black dots. Boa constrictor, a name applied in popular parlance to many species, reaches a length of more than 10 feet; the largest specimen in the British Museum measures exactly 11 feet. A few other species inhabit Central America and the West Indies. B. dumerili and B. madagascariensis, both of Madagascar, cannot be separated from the genus Boa.
A. D. Bartlett[186] has described the following incident:–
"In the evening of 5th October 1892 two pigeons were put into the cage in which two fine specimens of Boa constrictor had been living on friendly terms since the beginning of the year. The larger snake seized one of the pigeons and the keeper left the house. The next morning only one of the snakes, the larger specimen, was visible, and from its enormously extended body it was evident that it had swallowed its companion, which was about 9 feet in length. It had no longer the power of curling itself round, but remained extended nearly to its full length in a straight line, and appeared to be at least three times its normal circumference. It was almost painful to see the distended skin, {603}which had separated the scales all over the middle of the body. By 2nd November, twenty-eight days later, the snake had not only digested its companion but had regained its appetite as well as its normal size, and it immediately swallowed a pigeon put into its den."
This peculiar case is not one of ordinary cannibalism. It is rather an unintentional accident. When two snakes happen to get hold of the same animal (in the present case a pigeon) and begin to swallow it, the action of swallowing becomes almost mechanical, the snakes continuing to push their jaws over the prey–which in the case of a bird or mammal they cannot taste, nor can they see it–so long as they feel something in the mouth. After the original prey has been mastered, it is the turn of the opposite snake's head, and if the weaker snake does not give way it is swallowed by its stronger mate. Grass-Snakes will swallow several frogs if these are tied together in a string, and other snakes do the same with mice. There are instances on record in which a Python swallowed its blanket, which, being absolutely indigestible, caused its death.
Casarea, the "Round-Island Snake," differs from Boa chiefly by the rough and strongly keeled scales, and by the relatively much longer tail.
Eunectes murinus, the "Anaconda," is an aquatic Boa. It differs from this genus mainly by the inner of the three nasal shields being in contact with that of the other side (see Fig. 159), and by the absence of the little scales between the eye and the labials; the snout is, moreover, covered with shields instead of small scales. The pupil of the eye is normally vertical, but it had contracted into a round pinhole in the dead but still fresh {604}specimen from which the figure was drawn. The general colour is dark olive-brown, with large oval black spots arranged in two more or less alternating rows along the back, and with smaller black, white-eyed spots along the sides. The under parts are whitish, spotted with black. The upper parts of this and of many other dark-coloured species of Boidae are often shiny, with an iridescent lustre.
The Anaconda combines an arboreal with an aquatic life, a kind of existence eminently in harmony with the well-watered, dense forests of Tropical South America, which are the home of this, the largest of all modern Snakes. It is said to attain a length of as much as 33 feet. There is no inherent impossibility in such statements, but the giant specimens seem to have a knack of keeping out of the naturalist's way.
The Anaconda feeds chiefly upon Birds and Mammals, which it catches either on land, mostly during the night-time, or in the water. For the latter purpose it lies submerged in the rivers or floats about leisurely, only the head being above the surface, and anything suitable is attacked. In other localities the snake, if so inclined, establishes itself upon the branches of a tree which overhangs the water, or the track of the game. These aquatic Snakes seem to be viviparous.
Eryx has the head not distinct from the neck and covered entirely with small scales. Those of the body are likewise small, and are either smooth or keeled. The tail is very short. The anterior maxillary and mandibular teeth are longer than the posterior teeth. These snakes, most of which are less than 3 feet in length, inhabit the sandy districts of North Africa, Arabia, and South-Western Asia, extending into Central Asia. One species, E. jaculus, extends into Greece and the Ionian Islands. Like the other species it is an ugly creature, pale grey or yellowish above, with darker patches and spots. The under parts are whitish. The scales are smooth on the front half of the body, becoming keeled further back and on the tail. Total length under 2 feet. The pupil is vertical.
According to Zander[187] and Werner[188] this snake lives in sandy localities, digging itself into the sand, or covering the body lightly with sand and leaving only the eyes and nostrils free. The whole body is very flabby, and presses itself into any irregularity of the {605}ground over which the snake creeps. Some specimens live on lizards, others prefer mice. The prey is caught by the head, and further secured by several turns of the body of the captor, whose tail is then turned forwards, round the head of the victim, so as to form a kind of knot.
Not less striking than their agility is their jealousy, which is so strong that a snake will occasionally leave the mouse which it has just strangled in order to seize another snake's mouse. Sometimes several snakes fight for the same mouse, coiled together into one inextricable lump so that the mouse itself is quite invisible. The snakes poke their heads about in search of the hidden prey, and every attempt of one of the snakes to free itself, causes the others to squeeze it firmer and firmer, thinking apparently that the motion was caused by the lost prey.
Occasionally one of Werner's captives caught several mice in succession. With these it crawled into a corner, dropped the mice, and then proceeded quietly to swallow one after another. After a fortnight the whole repast was digested, and the snake was ready for more.
Fam. 6. Xenopeltidae.–The single species, Xenopeltis unicolor, of South-Eastern Asia, including the Malay Islands, has been raised to the dignity of family-rank on account of the following combination of characters. The prefrontal bones are still in contact with the nasals as in the previous families, but the coronoid bones of the mandibles are absent as in the remaining families. The whole suspensorial apparatus and the lower jaw itself are peculiar. The dentary bone is movably attached to the end of the much-elongated articular bone, the movability being enhanced by the absence of the coronoid element.[189] The quadrate is short and thick, and is carried by the short and broad squamosal, which lies flat against the skull, resembling in this respect that of some of the Ilysiidae. Boulenger rightly considers Xenopeltis to be in various ways intermediate between this family, the Boidae and the Colubridae. The head is small and not distinct from the neck. The eyes are small and have a vertical pupil. The body is cylindrical, covered above with {606}smooth black or brown and highly iridescent scales, hence the generic name. The ventral scales are white and transversely enlarged as in the majority of snakes. The tail is short, but not stunted, measuring about 4 inches in full-grown specimens of a total length of 3 feet.
Fam. 7. Colubridae.–This family comprises those snakes (about nine-tenths of all recent species) which combine the following characters:–ectopterygoids are present: the squamosals are loosely attached to the skull, and carry the quadrates, which are not reached by the pterygoids: the prefrontals are not in contact with the nasals: the maxillaries are horizontal and form the greater portion of the upper jaws: the mandibles lack the coronoid process or element: both jaws are toothed.
The best arrangement of this enormous cosmopolitan family with terrestrial, arboreal, and aquatic forms, is that by Boulenger, who, adopting Duméril's terms, has divided them into three parallel series.
A. Aglypha.–All the teeth are solid and not grooved.
B. Opisthoglypha.–One or more of the posterior maxillary teeth are grooved.
C. Proteroglypha.–The anterior maxillary teeth are grooved or "perforated."
The Aglypha are harmless, non-poisonous. Most of the Opisthoglypha are poisonous, although few of them are dangerously so. The Proteroglypha, which comprise the "Cobras" and their allies, are deadly poisonous.
Series A. AGLYPHA.
Sub-Fam. 1. Acrochordinae.–The postfrontal bones, besides bordering the orbits posteriorly, are extended forwards so as to form the upper border of the orbits, separating the latter from the frontals. The few genera and species of this sub-family are mostly aquatic, inhabiting rivers, or estuaries with brackish water, and they have been known to swim far out into the sea. The body is covered with small, frequently granular scales; in the typically aquatic forms the body is slightly compressed laterally, and the ventral scales are scarcely larger than the others. Most of these ugly snakes inhabit the rivers of coasts of South-Eastern Asia and Papuasia; one, Stoliczkaia, is found in the Khasia Hills {607}of North-Eastern India; another, Nothopsis, lives far from its supposed allies, on the Isthmus of Darien, Central America.
Acrochordus javanicus has no ventral shields. The head is flat, covered with small granules, with the eyes and nostrils on the upper surface. The general colour is dull olive-brown, lighter and spotted beneath. The food consists of fishes. Total length up to 4 feet.
Chersydrus granulatus ranges from the coast of Madras to New Guinea. The body and tail are compressed, and form a ventral fold, covered with tiny scales like the rest of the body. General colour grey above, yellow below.
Sub-Fam. 2. Colubrinae.–The postfrontal bones are restricted to the posterior border of the orbits. The maxillary and dentary bones carry teeth on their whole length. The scales are usually imbricating. This sub-family contains the overwhelming majority of snakes, about 1000 species, all of them harmless so far as poison is concerned. None of them reach a great size, species of 6 or 7 feet in length being rare, e.g. Zamenis mucosus, but a few species of the Indian genus Zaocys s. Coryphodon grow to 10 feet. Most of the Colubrine snakes are oviparous, but some, e.g. Coronella, are viviparous. Some are aquatic, or semi-aquatic, others are absolutely arboreal, others again prefer dry, sandy, or rocky localities, according to their food. The distribution of the sub-family is cosmopolitan, finding its natural limits only in the permanently frozen under-ground, a condition which makes hibernation impossible. Most of them love warmth and like to bask, although many are not fond of the broiling sun. In the temperate regions they hibernate. As a rule they are intelligent and some of them become even affectionate.
Tropidonotus.–The teeth form closely set series on the whole length of the maxillaries, palatines, pterygoids, and the greater portion of the dentaries. The premaxilla is toothless. The teeth of the maxillaries gradually increase in length, the posterior teeth being the longest. The pupil is round. There is a pair of internasal shields. The scales covering the body have each an apical, sensory pit, are mostly keeled, and are arranged in longitudinal series. The ventral shields are broad; the sub-caudals form two rows. This genus, with more than seventy species, has a wide range, practically over the whole world with the exception of New Zealand and the southern half of Australia.
T. natrix, the common Grass-Snake, has a divided, or double, anal shield. The strongly keeled scales of the body form nineteen rows. There are normally seven upper labials, the third and fourth of which border the eye. The usual colour of the Grass-Snake is olive-grey or brown above, with black spots and narrow cross-bands. The labials are white or yellowish, with black sutures. The belly is checkered black and white, more or less suffused with grey. There are several colour-varieties. The typical or northern form has a white, yellow, or orange collar, bordered behind by a black collar; the pale collar is sometimes faint or absent. The second variety, rather common in Spain and Portugal, although not the only form in the Peninsula, has no collar whatever, and these specimens are sometimes almost uniformly grey-green above. The third variety, common in South-Eastern Europe and in Asia Minor, has a well-marked collar and a yellowish streak along each side of the back. But there are also almost black specimens.
The usual length of an adult female Grass-Snake is about 3 feet, but very exceptional cases of more than 6 feet are on record; the males are smaller and more slenderly built. The range extends over the whole of Middle Europe, Algeria, West and Central Asia. It does not, however, occur in Ireland or Scotland. Its northern limit is the southern part of Sweden.
The Grass-Snake prefers moist, grassy localities, with the neighbourhood of water, chiefly on account of the food, which consists entirely of fishes and Amphibia, notably of frogs; tree-frogs are preferred to anything else; toads are occasionally eaten, but mice are never taken.
The Grass-Snake can climb trees or rather shrubs and is an accomplished swimmer, often spending much of its time in water for fishing purposes. The fish is caught by the belly and then generally swallowed on land. The Grass-Snakes appear in the spring and disappear in the autumn to hibernate in the ground. They pair, in England, in the month of May or June, usually on warm and sunny mornings. The eggs are laid from July to the end of August, mostly in rich vegetable soil, in heaps of weeds or in manure-heaps. Young snakes lay fewer eggs than old specimens, which sometimes produce more than three dozen at a time. The eggs are soft, whitish yellow, about one inch long, and soon stick together, so that the whole clump {609}can be taken up at once. As a rule the new-laid eggs do not contain any visible sign of the embryo, but it often happens that the snake has to delay oviposition, and then the embryos are more or less advanced. This is especially the case with recently caught specimens. The young are hatched in the late summer or in the autumn, and seem to live at first upon soft insects and worms. Curiously enough they are easily drowned when they fall into the water, even in a shallow tank. My tame snakes have often laid eggs between the stones in the greenhouse; the young throve well upon unknown food, but most of them met their fate in the water. When they are a few weeks old they are strong enough to take baby-frogs.
The Grass-Snake becomes very tame, learns to distinguish between different people, allows itself to be handled without hissing or without voiding the obnoxiously smelling contents of its cloaca and anal glands, will in time take the offered food from the hand, and will even crawl up the arm or sleeve and coil itself up contentedly. One of the finest specimens, quite green, without a trace of a collar, and with brownish-red eyes, I caught in the Guadiana, where it had been fishing in midstream. It swam towards the bank, dived, and hid itself at the bottom between rocks. This snake, a female, became very tame. It never hibernated, shed its skin regularly every few months, and grew within nine years from 35 inches to 42 inches in length.
The Grass-Snake is perfectly harmless: although hissing, and striking out furiously with its head, it never bites, not even when it is severely handled. Its only defence consists of the awful contents of the cloaca and the anal glands, the secretion of which smells of concentrated essence of garlic mixed with other indescribable odours. The wildest specimens I have ever met with inhabited a swamp with a little stream to the north of Oporto close to the coast. To my utter surprise some of them actually made for me, swimming along rapidly with the head erect, about 6 inches above the water, and darting forwards with widely opened jaws, but they did not bite. These and other kinds of allied snakes require to drink much and often. Occasionally they drink milk when this is offered them, but that they suck the udders of cows or the breasts of women is an idle fable.
T. viperinus.–The scales are strongly keeled and form twenty-one to twenty-three longitudinal rows. The third and fourth labials border the eye. The anal shield is divided. The eyes and nostrils are directed upwards instead of sidewards, in adaptation to the essentially aquatic habits of this species, which lives upon fishes and Amphibia. The general colour is grey to reddish brown, with a black zigzag band along the back and a lateral series of black, yellow-eyed spots. The belly is yellow or red, checkered with black.
The Viperine Snake bears a general resemblance to the common viper. It inhabits France, Italy, Spain and Portugal, and Morocco. Very large specimens attain a length of nearly 3 feet, but the ordinary size of adults is 2 feet. This snake spends most of its time in the water, but it is often found on land, basking on the top of a low wall or on a low shrub. It is exceedingly common in Spain and Portugal, where it inhabits almost every ditch, any standing water or slow river. In the Alemtejo, when during the rainless and hot summer the small {611}rivers have nearly dried up, these snakes collect in great quantities in the remaining stagnant and muddy pools, and as the stock of suitable fish gets exhausted, are often reduced to a deplorably emaciated condition. By the month of August they have become so thoroughly aquatic that they cannot be kept alive in dry surroundings for twenty-four hours. Those which I collected generally died, apparently from some kind of cutaneous suffocation, during the night following their capture. Taken under other conditions they are very easily kept and tamed.
I once caught a Viperine Snake in a ditch whilst it was swallowing an eel of nearly its own length. Both were separated, and then put into a small bag together with other creatures, and no more attention was paid to them for several hours. When I opened the bag again, the snake, undisturbed by my incessant walking about, was again busily engaged in trying to get outside that same eel!
T. sirtalis (Fig. 160) is one of the almost endless varieties of what is now known by the name T. ordinatus, of North and Central America.
T. tesselatus is closely allied to T. viperinus, which it represents in South Germany, Italy, South-Eastern Europe, and Asia; but the scales form only nineteen rows, and the fourth, or fourth and fifth labials, border the eye. The usual colour is olive-grey with dark little spots, and with a dark chevron-shaped band behind the occiput. The lower parts are yellow or red checkered with black, hence the specific name.
Zamenis.–The maxillary teeth are not closely packed; they increase slightly in size backwards, and the last two are often a little larger and separated from the rest by a diastema. The mandibular teeth rather decrease in size from before backwards, inversely with the upper teeth. The scales are smooth with apical pits; the sub-caudals form two rows. The eye is large, and has a round pupil. The range of this genus, with about thirty species, extends over the whole of the Periarctic region.
Z. (Ptyas) mucosus (Fig. 161), the Rat Snake of India, extending from Transcaspia to Java, is a very common species, often seen in menageries. Its general colour is brown above, often with black cross-bands on the hinder part of the body and tail. The under parts are yellowish. The fourth and fifth labials border the eye. {612}The scales on the body form only seventeen rows. Another feature of this species is the prominent ridge of the back-bone, not only in half-starved but in well-conditioned specimens. The Rat Snake grows to a length of more than 7 feet, and is as ill-tempered as most species of this genus.
Z. gemonensis s. viridiflavus inhabits France, Italy, the Balkan Peninsula, and Asia Minor. Its coloration is very variable. In general it is either green above and yellow below, hence the appropriate name viridiflavus, or the ground-colour of the back is greyish or olive-yellow with brownish spots, which form more or less longitudinal rows on the trunk, but gradually pass into blackish continuous lines on the tail; the under parts are yellow or greenish white, often with many very small, dark specks. The scales form seventeen or nineteen rows; the anal shield is divided. There are two small postocular scales and one subocular; of the eight labials, the fourth and fifth border the eye. This species is very lively, attacks {613}and bites furiously, climbs well, and when suspended from branches can protrude half of its length in a horizontal direction. It eats any kind of Reptile, Bird, or Mammal it can master; small animals are swallowed directly, rats and moles are first killed by constrictions. Large specimens reach perhaps 6 feet in length.
Z. hippocrepis is the representative species in the Iberian Peninsula and in North-Western Africa. It is rarely more than 4 feet long, and is very pretty, the ground-colour being reddish or olive-yellow with a row of large, dark brown, yellow-edged spots along the back. Two rows of smaller spots adorn the sides; where the dark spots are large, the pale ground-colour is restricted to forming rings around the spots, producing a pretty appearance. The under parts are yellow or orange, with black spots. On the head is a dark, pale-edged patch in the shape of a horse-shoe, a feature alluded to by the specific name. Structural characters are the possession of a row of little subocular scales, which completely separate the eye from the labials, the double anal shield, and the small and smooth scales on the body, which form generally as many as twenty-seven rows.
Z. constrictor.–The American Black Snake. The scales are smooth, and arranged in seventeen rows; the anal shield is divided. The general colour above is uniform bluish-black; below slaty, tinged with blue; the chin and throat are silvery white, sometimes with a black spot. Large specimens attain a total length of 6 feet.
Holbrook gives the following exhaustive account of this species, about which many sensational stories are current even in would-be scientific periodicals:–
The "Black Snake" is one of the commonest of North American species. It is extremely active, climbing with facility, and running with great rapidity, whence it is not uncommonly called the "Racer." It frequents shady places, covered with thick shrubs, on the margins of water. It feeds on mice, toads, or small birds; and, as it is an excellent climber, is frequently seen on trees in search of birds' nests. It is a bold and daring serpent, enters barns and out-houses without fear, and has been known to destroy young chickens. Its specific name constrictor would imply that it suffocates or crushes its prey, but this according to Holbrook is at least doubtful. In the {614}breeding season it is extremely irascible, and will frequently attack persons passing at a distance of several steps; the tail then quivers with rage, making a quick vibrating motion, which in forests and among dead leaves sounds not unlike the Rattle-Snake; it now elevates the head one or two feet from the ground, and darts upon its adversary; luckily its bite is harmless, and not more painful than the scratch of a pin.
"It will even descend from trees to attack its enemy if teazed, yet it does not twine itself around the legs, as is commonly supposed.
"The same power of charming its prey has been attributed to the Black as to the Rattle-Snake, and with still less appearance of reason; for this is a nimble animal, and can pursue its prey, while the Rattle-Snake must lie in wait for his. It is remarkable that the birds most commonly found 'charmed' are the Cat-bird (Turdus carolinensis) or red-winged Black-bird (Icterus phoeniceus). These birds choose thick and shady places on the margins of streams for their residence, and generally build their nests on such shrubs as the alder; the latter bird not unfrequently takes the precaution to select such bushes as are on small islands, or such as have their roots surrounded by water, and thus their home is more secure. Now the Black Snake chooses precisely the same localities, knowing probably the haunts of its prey. The serpent begins the war by besieging the nest; the old bird, aware of its intention, attacks it with fluttering and uncertain motions, accompanied by a plaintive cry of distress, and is then said to be 'charmed.' The snake is at last either driven off, or it captures the young and not unfrequently the old bird too.
"Sometimes the old bird, by her cries, calls in the assistance of her neighbours to drive away the aggressor. I have seen more than a dozen birds thus engaged with a large Black Snake that had probably just committed some depredation, but was now quietly stretched on a rock, basking in the sun; and it was not a little singular that birds of very different genera, and those seldom seen together, all united in this warfare against a common enemy, and finally compelled him to seek shelter among some low, thick shrubs, by the violence of their assault."
Zaocys, with about half-a-dozen species in South-Eastern Asia, is closely allied to Zamenis. Z. carinatus, of the Malay {615}Islands, grows to 10 feet in length; it is consequently one of the largest harmless Colubrine snakes. The scales form only sixteen to eighteen rows. The sub-caudals are double. The general colour above is dark olive, passing into greenish brown farther back. The under parts are yellowish; black and yellow posteriorly. The fifth and sixth labials border the eye.
Coluber.–The maxillary teeth are of equal size, but the anterior mandibular teeth are the longest. The head is distinct from the neck. The nasals are distinct; not fused with the loreals. The eye is rather large, with a round pupil. The scales, smooth or keeled, have apical pits; the ventrals are rounded or angulate laterally; the sub-caudals are double. They all lay eggs and constrict their food. Nearly fifty species in the Periarctic region.
C. (Elaphis) quatuorlineatus s. quaterradiatus inhabits Italy and South-Eastern Europe. It occurs also in the Southern Tyrol. The scales of this large snake, which grows to nearly 6 feet in length, are arranged in twenty-five rows, and are feebly {616}keeled. The anal is divided. Adult specimens have a yellowish-brown ground-colour with a pair of black streaks on each side of the back. A black line extends from the eye to the angle of the mouth; the under parts are yellow, mostly closely spotted with brown. This snake is good-tempered, and keeps well in captivity. They live on sparrows, mice, lizards, etc., and are very fond of eggs. Large specimens can swallow several fowls' eggs in succession; the crushed remains of the shells are later disgorged. This handsome snake climbs extremely well in search of birds and their eggs, and it is not afraid of the water. The prey is caught either with the teeth or by a rapid twist of the tail; in any case, the prey is always strangulated by the constriction of coils thrown round it. A sparrow thus secured is literally passed through the moving coils along the snake's body into a position convenient for swallowing. Hungry snakes catch and secure several birds or mice before eating them. My own specimens became almost affectionately tame, never attempted to bite, and took food from the hand.
C. leopardinus is smaller, but is one of the handsomest snakes of Southern Italy, South-Eastern Europe, and Asia Minor. It is closely allied to the previously described species. The ground-colour is pale brown with a dorsal series of dark brown or reddish, black-edged, transverse spots, and a lateral alternating series of smaller black spots, or with two dark brown, black-edged stripes bordering a yellowish vertebral stripe; usually with a forked black mark on the occiput and nape. The under parts are white, checkered with black, sometimes with the latter colour prevailing.
C. flavescens s. aesculapii is the Aesculap-Snake, for which the almost unknown name of longissimus has now been unearthed in deference to the fetish of priority. This snake is of an extremely graceful and slender build, with a very long tail. Its home is the South of France, Italy, and South-Eastern Europe. It occurs sporadically in the Tyrol, for instance near Bozen, in Austria, at Baden near Vienna, in Germany only in the Taunus, especially at Schlangenbad, which has received its name from the frequent occurrence of this snake. This sporadic distribution favours the idea that these snakes were introduced by the Romans as inmates of the temples erected to Aesculapius at such watering-places. Specific characters are the smooth and shiny scales, {617}which are arranged in twenty-one to twenty-three rows, the distinctly angulate ventrals and the double anal and sub-caudals; the fourth and fifth of the upper labials border the eye, which has a round pupil. The coloration is very variable, as a rule olive-brown above with a dark streak behind the eye; the upper lips and a triangular patch on the temples are yellow; the under parts are uniform pale yellow. Some specimens are pale golden brown; others are very dark, almost black; while some have four darker stripes along the body, and lastly whitish specks occur on the upper surface. Large Aesculap snakes grow to a length of 5 feet. Their food consists chiefly of mice. They become very tame, although many of them at first bite furiously. Their climbing capacities are astonishing, the snakes being able to scale high and vertical walls provided there is the slightest "foothold." Some of my specimens escaped in the room and were at last found near the ceiling, resting on the rods of the curtains, up the folds of which they had managed to wriggle. Boulenger kept one for many years in a glass cage, where the snake entwined himself round the branches of a stick and allowed us to take him with the stick out of its socket and to inspect him. Being kept in an inhabited room, the snake did not exactly hibernate, creeping into the moss at the bottom of the cage; but it refused to feed, and remained in a rather drowsy condition coiled up on its favourite stand. During the pairing season they frequently resort to the water, at Schlangenbad at least; the few eggs are deposited under dry moss or in dry, decayed wood, and are hatched in about six weeks.
C. (Rhinechis) scalaris has the smooth scales disposed in twenty-seven rows. The snout is strongly projecting, and has a V-shaped dark mark on the top; a black streak runs through the eye, and another black spot lies below the eye. Young specimens are pale brown with a series of dark H-shaped marks on the back, suggesting a ladder, hence the specific name. In the adult these marks are replaced by a pair of brown stripes running along the back; the under parts are always uniform yellow. Total length about 3 feet. This snake is restricted to the Iberian Peninsula and to the South of France. Most specimens are very ill-tempered. The young live upon locusts and small lizards, the old eat mice and small birds. In captivity they also take dead animals–a rare habit with snakes.
Dendrophis with about ten species inhabits South-Eastern Asia and Australia. They are typical Tree-Snakes. The scales are keeled, and form only thirteen or fifteen rows; those of the vertebral row are enlarged; the ventrals have a pair of suture-like lateral keels and a notch on each side, arrangements which are of great assistance in climbing, these snakes being able to slide up the branches of trees in almost straight lines instead of having to twist and undulate their way up.
D. punctulatus, of Northern and Eastern Australia, is olive-brown above, uniform or with black edges and yellow outer borders to the scales. The upper lips, the throat and anterior ventrals, are yellowish. Total length up to 6 feet.
Leptophis is a Neotropical genus of Tree-Snakes. The body and the extremely long, whip-like tail are very slender. The head is very distinct from the neck; the eye is large, with a round pupil. The scales form thirteen or fifteen rows; the ventrals are sometimes angulate laterally; the sub-caudals are double. L. (Ahaetulla) liocerus is a beautiful snake, green above {619}with a golden lustre, while the under parts are yellow or white. The total length of this species amounts to 6 feet, the tail then being nearly 2 feet long. These graceful Tree-Snakes live upon small reptiles and birds and their eggs. When shaken out of a tree or frightened off they let themselves fall down from considerable heights, coiling body and tail like a watch-spring, and alighting on the ground upon the spiral, which breaks the fall.
Coronella.–The teeth are nearly all of equal size and form continuous series. The scales are smooth and have apical pits; the sub-caudals are double. The head is scarcely distinct from the neck. The pupil is round. This genus, with nearly twenty species, is widely distributed except in the Australian region, the northern half of Asia, and South America. We can mention only the two European species, one of which occurs in England.
C. austriaca s. laevis, the Smooth Snake. The scales are arranged in nineteen rows. Mostly the third and fourth labials border the eye. The anal shield is divided. The general colour is brown or reddish above, often with one or two lighter stripes, with small dark brown or red spots; two dark brown or red stripes on the nape, usually confluent with a large dark patch on the occiput; a dark streak extends from the nostril through the eye to the angle of the mouth. The under parts are red, orange, brown, grey or blackish, either uniform or speckled with black and white. The coloration is, however, subject to much variation, and some specimens strikingly resemble some of the Common Viper, which is also very variable in its coloration. The resemblance is enhanced when the Smooth Snake broadens its head by widening the jaws, as it is in the habit of doing. Two such similarly coloured specimens are represented in Fig. 165. On closer inspection the differences are great enough, the harmless snake having smooth scales, and the top of the head being covered with large shields; while the Viper has keeled scales, the top of the head being covered mostly with scales, a vertical (not round) pupil, and, moreover, when attacked, usually coils itself into a spiral disc with the head standing out in the middle, ready to strike. However, these two species are sometimes mistaken for each other.–The Smooth Snake prefers lizards as food to anything else, but it also takes mice. The prey is hunted chiefly in the late afternoon and in the evening, and is constricted by the coils of the snake. When caught or even when handled after months of captivity, the Smooth Snake bites deliberately and firmly, selecting a suitable spot, for instance a finger, opens the mouth widely and almost chews the spot. The bite is of course quite harmless, and scarcely draws blood, few of these snakes attaining a length of more than 2 feet. They are viviparous, bringing forth about half-a-dozen young at a time. The range of the Smooth Snake extends over the greater part of temperate Europe, from England and the Iberian Peninsula to Berlin, and south-eastwards to Asia Minor. In England it occurs in a few counties only, for instance in Hampshire and in Dorsetshire.
C. girondica, of the South of France, Italy, the Iberian Peninsula and North-Western Africa, much resembles the English Smooth Snake, from which it differs in a few points only. The scales are arranged in twenty-one, rarely in nineteen, rows; usually the fourth and fifth labials border the eye; and the rostral {622}shield, covering the end of the snout, is much broader than high. The coloration is variable, but there is always a pair of elongated blackish spots or a U-shaped mark on the nape.
Sub-Fam. 3. Rhachiodontinae.–With only a few teeth on the posterior part of the maxillaries, on the palatines and dentaries. Some of the vertebrae in the region of the lower neck have strongly developed hypapophyses, which are directed forwards and pierce the oesophagus. They are used for filing through or breaking the birds' eggs which seem to be the chief food of these snakes.
Dasypeltis scabra, the only species, inhabits Tropical and South Africa; although it reaches scarcely more than two feet and a half in length, such a specimen is able to swallow an ordinary fowl's egg. Pigeons' eggs are swallowed by snakes little more than one foot in length, which seems at first sight quite impossible. The swallowed egg distends the skin to its utmost capacity; it then slides down further, the snake makes some slight contortions and the swelling collapses; after a while the broken and sucked-out shell is vomited out as a crumpled up {623}mass. Miss Durham has illustrated this curious process in a series of drawings.[190]
Series B. OPISTHOGLYPHA.
One, or a few, of the posterior maxillary teeth have a groove or furrow in front, which conducts the secretion of the enlarged upper labial glands. Apparently all these snakes are more or less poisonous, paralysing their prey before or during the act of deglutition. So far as man is concerned they are rather harmless, since the poison is not very strong, not available in large quantities, and above all because the small poison-teeth stand so far back that the snakes cannot easily inflict wounds with them.
The Opisthoglypha are of considerable morphological interest, since they connect the Colubridae with the Viperidae, the characteristic poisonous apparatus of which seems to have been derived from that of the Opisthoglypha by the reduction or shortening of the anterior portion of the maxillaries and the harmless teeth, so that the posterior or poison-fangs come to the front.
The Opisthoglypha comprise about three hundred species and are cosmopolitan, including Madagascar but excepting New Zealand. They contain truly terrestrial, arboreal, and thoroughly aquatic forms.
Sub-Fam. 1. Dipsadomorphinae.–The nostrils are lateral and the dentition is well developed. Long-tailed, terrestrial, and arboreal forms. Most of the arboreal species are green above, often with white or yellow longitudinal bands, while the under parts are white or yellow. They feed chiefly upon lizards, birds and their eggs.
Dipsadomorphus s. Dipsas (part).–Typical, very long-bodied and long-tailed Tree-Snakes, with a vertical pupil. The median or vertebral row of smooth scales is enlarged; the broad ventral scales are bent at an obtuse angle on the sides, the resulting ridge assisting in climbing. The sub-caudals are arranged in two rows. Ten to fourteen maxillary teeth are followed by two or three enlarged, grooved fangs.
D. trigonatus, of India, grows to one yard in length. Yellowish olive or pale grey above, with a white, black-edged {624}zigzag band along the back, or with a series of white, black-edged spots.
D. cyaneus, of Northern India, Assam, etc., is a beautiful Tree-Snake, green above, with the skin between the scales black, uniform greenish yellow below. Total length up to 4 or 5 feet.
Dipsas e.g. D. bucephala.–Maxillaries with eleven or more teeth. Pterygoids toothless. Body strongly compressed, with thirteen rows of smooth scales; the vertebral row enlarged; sub-caudals double; tail very long. Tropical South America.
Leptognathus with many species in Central and South America, like Dipsas, but with teeth on the pterygoids.
Coelopeltis.–Terrestrial and diurnal, with a round pupil. The row of small maxillary teeth is followed by one or two much larger, grooved fangs situated at a level below the posterior border of the eye. The first half-a-dozen mandibular teeth are much larger than the rest. The scales of the adult are more or less distinctly grooved longitudinally, hence the generic name, and are arranged in seventeen or nineteen rows. The sub-caudals form two rows; the ventrals are rounded off laterally. Two species in the Mediterranean countries and in South-Western Asia.
C. monspessulana s. lacertina is one of the largest snakes in Europe, reaching a length of 6 feet, of which the tail takes up 18 inches. Olive-brown or yellowish or reddish above, frequently with small, dark, light-edged spots. The sides are often blackish, with whitish specks. The under parts are yellowish white, with or without brownish markings. Some specimens are very green, with a dull blackish neck. One of the specific names of this terrestrial snake is the latinised form of Montpellier; the other refers to the shape of the head, which is not unlike that of a lizard, partly owing to the concave forehead. This species inhabits rather dry localities studded with shrubs, where it hunts for lizards, birds, and mice. It is sure to attract notice by its loud hissing when it is disturbed. When driven into a corner it strikes out furiously, but does not, as a rule, bite. I have caught some which after a few days became quite gentle. Small animals become torpid a few minutes after they have been bitten.
Macroprotodon cucullatus occurs in Andalucia, the Balearic Islands, and in North Africa. The dentition is peculiar. The {625}fourth and fifth maxillary teeth are enlarged, followed by an interspace, then follow several small teeth, and lastly the two enlarged, grooved teeth. The sixth mandibular tooth is very long, separated by a space from the much smaller posterior teeth. The general colour of this sand-loving snake is pale brown or grey above with small spots or streaks on the trunk, and with a large black patch behind the head extending over the sides of the neck, hence the specific name. The under parts are bright red or yellowish, sometimes spotted with black. Total length under 2 feet.
Sub-Fam. 2. Elachistodontinae.–With only a few teeth on the posterior part of the maxillary and dentary bones, and on the palatines and pterygoids. Some of the vertebrae in the thoracic region have much-developed unpaired hypapophyses, which are directed forwards and pierce the dorsal wall of the gullet. In this respect Elachistodon westermanni, of Bengal, the only species, bears a striking resemblance to the South African Aglyphodont Dasypeltis (see p. 622), and it is probable that this apparently very rare Indian snake also swallows eggs. It is brown above, with a yellowish vertebral stripe; yellowish below.
Sub-Fam. 3. Homalopsinae.–The nostrils of these absolutely aquatic and viviparous snakes are valvular, and are situated on the upper surface of the snout. The eyes are small with vertical pupils. The two dozen species, mostly very ugly, inhabit the rivers and estuaries of the East Indies from Bengal to North Australia. Some species have very small and narrow ventral scales, recalling the Hydrophinae, or the burrowing snakes, none of which use their ventral scales for locomotory purposes.
Homalopsis buccata, Cerberus rhynchops and Hypsirhina, e.g. H. plumbea, have well-developed ventral scales; the other scales of the first two genera are keeled, those of the third are smooth. In Hipistes the whole head is covered with very small scales; all the scales of the body are smooth except the very narrow ventrals, which have double keels. H. hydrinus, of Siam and the Malay Peninsula, has a compressed body, and in its general appearance much resembles the Hydrophinae. It lives, like its allies, upon fishes, and it swims far out into the sea.
Series C. PROTEROGLYPHA.
The anterior maxillary teeth are deeply grooved, or so folded {626}as to appear hollow or perforated. Behind these enlarged poison-fangs the maxilla carries a series of smaller, solid teeth; hence the term "proteroglyphous," which means that the anterior teeth are grooved, in opposition to "opisthoglyphous." Both series have been developed independently.
The Proteroglypha are all extremely poisonous, mostly viviparous, and widely distributed over the whole of the Australian, Palaeotropical and Neotropical regions, with the exception of Madagascar and New Zealand; they extend northwards into the warmer parts of North America, and they also range over a great portion of the Palaearctic sub-region, being found in North Africa and South-Western Asia. They form two natural sub-families: Elapinae, with cylindrical tails, and Hydrophinae or Sea-Snakes, with laterally compressed tails.
Sub-Fam. 1. Elapinae.–The tail is cylindrical. The Elapinae comprise nearly 150 species, which have been grouped into a great number of, mostly somewhat imaginary, genera. In Australia they constitute the great majority of Snakes, there being besides the deadly Elapinae only a few Pythons and Typhlopidae, and very few Colubrinae.
Naja.–The pair of large and grooved poison-fangs are separated by an interspace from one to three small, faintly grooved teeth near the posterior end of the maxillaries. The scales are smooth and without pits, and are arranged in fifteen to twenty-five oblique rows on the trunk, although more occur in the region of the neck; the vertebral row is not enlarged. The head is but slightly distinct from the neck. Each nostril lies between two nasals and the internasal. The sub-caudals form two rows. The pupil is round. The neck-region can be expanded {627}into a hood by the spreading and moving headwards of the ribs. Several species in Southern Asia and in Africa.
N. tripudians (the "Cobra").–The coloration varies much. The typical form is yellowish to dark brown with a black and white spectacle-mark on the dorsal side of the hood, and with a large black and white spot on each side of the corresponding under surface. Other specimens are uniform pale brown to blackish grey, without any markings on the hood. The Cobra is widely distributed, from Transcaspia to China and to the Malay Islands; in the Himalayas it ascends to about 8000 feet above the level of the sea. Very large specimens are said to attain more than 6 feet in length, but a cobra of 5 feet, inclusive of the tail of 9 inches, is considered large. The Cobra prefers places which afford it a convenient hole to retire into; for instance, deserted hills of termites, ruins, heaps of stones and stacks of wood, and it has the disagreeable habit, like the harmless Rat-snake, Zamenis mucosus, of making itself at home in inhabited houses, probably attracted by the rats. Its chief food consists of small Vertebrates;–frogs, lizards, rats, occasionally fishes and {628}small birds. It drinks much, and hunts chiefly in the late afternoon and in the evening, although it possesses a round pupil. It avoids hot sunshine. Many observations show that the cobras live in pairs, otherwise they do not take much notice of each other or of other kinds of snakes. The female lays about a dozen soft-shelled eggs as large as those of pigeons.
This cobra is used by Indian conjurers. The "dance" is the habit of these snakes of erecting themselves, when agitated, upon the hinder third or quarter of their length, whilst they spread out the hood and sway the head and neck to the right and left, always in an attitude ready for striking. They are docile and by nature not vicious. Most of the performing cobras have their teeth drawn, and they then know well that they cannot bite. They only strike at the hand, just as uninjured specimens soon avoid biting into the iron rod with which they are lifted up in menageries. The drawing of the teeth is an operation which has to be repeated, since reserve-teeth soon take the place of the lost pair.
I cannot refrain from relating an abstract of a ridiculous episode which happened in the Munich Aquarium in the year 1882. One of six specimens of the African species Naja haje was missing. The police closed the establishment, which during the following eight days was turned inside out without any other effect than that two other, harmless, snakes were discovered. Twice the building was fumigated with sulphur, until the Cobra was at last found suffocated, fifteen days after the beginning of the search. This snake caused the owner of the Aquarium a loss of nearly £1500. But the cruel joke was, that during the commotion the man who had collected and sold the six snakes declared upon oath that their teeth had been so well drawn and the germs of possible reserve-teeth had been so thoroughly destroyed that the snakes were rendered absolutely harmless. But he was not believed, in spite of a commission of professors and doctors appointed, who experimented upon the remaining five Cobras with sulphur and did not find any poison-fangs, "although the mouth was probed and poked into as far down as the larynx."
Cobras have quite a number of enemies. Peafowl and Jungle-cocks are said to be partial to young snakes; pigs eat them greedily, and are to a certain extent immune against {629}their bite. The same applies, according to the most recent observations, to the famous Mongoos. Sir E. Tennent, in his Natural History of Ceylon, quoted several times in the present book, makes the following remarks about the immunity of this little creature:–
"I have found universally that the natives of Ceylon attach no credit to the European story of the Mongoos (Herpestes griseus) resorting to some plant, which no one has yet succeeded in identifying, as an antidote against the bite of the venomous serpents on which it preys. There is no doubt that, in its conflicts with the cobra and other poisonous snakes, which it attacks with as little hesitation as the harmless ones, it may be seen occasionally to retreat, and even to retire into the jungle, and, it is added, to eat some vegetable.... A number of plants, such as the Ophioxylon serpentinum and Ophiorhiza mungos, the Aristolochia indica, the Mimosa octandria, and others, have each been asserted to be the Ichneumon's specific.... If the Ichneumon were inspired by that courage which would result from the consciousness of security, it would be so indifferent to the bite of the serpent, that we might conclude that, both in its approaches and its assault, it would be utterly careless as to the precise mode of attack. Such, however, is far from being the case; and next to its audacity, nothing can be more surprising than the adroitness with which it escapes the spring of the snake under a due sense of danger, and the cunning with which it makes its arrangements to leap upon the back and fasten its teeth in the head of the cobra. It is this display of instinctive ingenuity that Lucan celebrates where he paints the Ichneumon diverting the attention of the Asp by the motion of his bushy tail, and then seizing it in the midst of its confusion. See Pharsalia, lib. iv. verses 729-734."
There is a widespread belief in the efficacy of "Snake-stones," which are generally pieces of charred bone, well polished, occasionally pieces of chalk or some similar porous substance, which, if pressed upon the bleeding wound, are supposed to absorb the poison. Snake-charmers profess to prepare such "stones," and to preserve the composition as a secret. The manufacture is a lucrative trade. The Boers bought them, imported from India, at high prices. Mr. Selous saw one, or heard of one, that was kept as an heirloom. Snake-stones are {630}also made, and used, in Mexico, of charred hartshorn; they are called "piedras ponsonas."
The use of the Snake-stone, called "Pamboo-Kaloo," has probably been communicated to the Singhalese by the itinerant snake-charmers who resort to the island from the coast of Coromandel.
Although Sir E. Tennent describes several instances of the successful application of snake-stones as well authenticated, he has never himself been an eye-witness. Although two cases have been fully described, they do not at all exclude the possibility, nay the probability, that the Tamils imposed upon the Europeans in order to sell the snake-stones.
"No doubt the snake-stones, owing to their porous nature, adhered to the bleeding wound, became saturated with blood, and then fell off. Very likely, in case of a poisonous bite, some of the venom would be sucked up too, but we do not know if those snakes were still in the possession of their poison-fangs. Properly conducted experiments with snake-stones have proved as little efficacious as the application of dry cup.
"Theoretically snake-stones as quick absorbent agents of the blood with the poison are good; they will certainly prevent some of the poison from entering the system, but that would, at best, be a partial cure only.
"In March 1854 a friend of mine was riding, with some other civil officers of the Government, along a jungle path in the vicinity of Bintenne, when he saw one of two Tamils, who were approaching the party, suddenly dart into the forest and return, holding in both hands a Cobra de capello, which he had seized by the head and tail. He called to his companion for assistance to place it in their covered basket, but in doing this, he handled it so inexpertly that it seized him by the finger, and retained its hold for a few seconds, as if unable to retract its fangs. The blood flowed, and intense pain appeared to follow almost immediately; but with all expedition the friend of the sufferer undid his waist-cloth, and took from it two snake-stones, each of the size of a small almond, intensely black and highly polished, though of an extremely light substance. These he applied, one to each wound inflicted by the teeth of the serpent, to which they attached themselves closely; the blood that oozed from the bites being rapidly imbibed by the porous texture of {631}the article applied. The stones adhered tenaciously for three or four minutes, the wounded man's companion in the meanwhile rubbing his arm downwards from the shoulders towards the fingers. At length the snake-stones dropped off of their own accord; the suffering of the man appeared to subside; he twisted his fingers till the joints cracked, and went on his way without concern. Whilst this had been going on, another Indian of the party, who had come up, took from his bag a small piece of white wood, which resembled a root, and passed it gently near the head of the cobra, which the latter immediately inclined close to the ground; he then lifted the snake without hesitation, and coiled it into a circle at the bottom of his basket. The root by which he professed to be enabled to perform this operation with safety he called the "Naya-thalic kalanga" (the root of the snake-plant), protected by which he professed his ability to approach any reptile with impunity."
The following narrative, communicated to Sir E. Tennent by H. E. Reyne, of the Department of Public Works, Colombo, seems to exclude the possibility of deception:–
"A snake-charmer came to my bungalow in 1854, requesting me to allow him to show me his snakes dancing. As I had frequently seen them, I told him I would give him a rupee if he would accompany me to the jungle and catch a cobra that I knew frequented the place. He was willing, and as I was anxious to test the truth of the charm, I counted his tame snakes, and put a watch over them until I returned with him. Before going I examined the man, and satisfied myself he had no snake about his person. When we arrived at the spot, he played on a small pipe, and after persevering for some time, out came a large cobra from an ant-hill, which I knew it occupied. On seeing the man it tried to escape, but he caught it by the tail and kept swinging it round until we reached the bungalow. He then made it dance, but before long it bit him above the knee. He immediately bandaged the leg above the bite, and applied a snake-stone to the wound to extract the poison. He was in great pain for a few minutes, but after that it gradually went away, the stone falling off just before he was relieved. When he recovered he held a cloth up, which the snake flew at, and caught its fangs in it; while in that position, the man passed his hand up its back, and having seized it by the throat, {632}he extracted the fangs in my presence and gave them to me. He then squeezed out the poison on to a leaf. It was a clear oily substance, and when rubbed on the hand produced a fine lather. I carefully watched the whole operation, which was also witnessed by my clerk and two or three other persons."
N. haje is the common hooded cobra of Africa, the "Aspis," so called on account of its shield or hood–the "Spy-Slange" of the Boers. As a rule the spectacle-marks on the neck are absent or indistinct, the general colour varies much, either brown above, yellowish beneath, with or without brown spots; or dark brown above with yellowish spots, dark brown beneath; or blackish above and beneath. The name Spy-Slange, meaning Spitting Snake, refers to the habit which this and other African Cobras have of letting the poison drop from the mouth like saliva when they are excited. This is not a particularly economical habit, nor is it of the slightest use to the snake.
N. (Ophiophagus s. Hamadryas) bungarus s. elaps is the "Hamadryad" or "Snake-eating Cobra" or "King Cobra." It has a well dilatable hood; the very variable coloration is yellowish to black, with or without an olive gloss. Many specimens have more or less distinct dark cross-bands or rings around the body, while others are olive above with black-edged scales, and others again are very dark above and beneath. The distinctive, specific character is the small number of scales, these forming only fifteen rows on the middle of the body, nineteen or twenty-one on the dilatable neck. There is a pair of large occipital shields behind the parietals.
This snake reaches the length, enormous for a poisonous snake, of 12 feet or more. Its size and very poisonous nature make it the curse of the jungle. It ranges from India to South China, and to the Philippines. The food seems to consist entirely of other snakes.
Sepedon haemachates is another hooded snake in South Africa, where it is known as the "Ringhals," i.e. banded neck. It differs from Naja by the absence of small teeth on the maxillaries behind the fangs, and by the strongly keeled scales, which form nineteen rows. The general colour is black above variegated with yellow or pale brown; the under parts are also black, often with one or two whitish bands across the lower portion of the neck.
The Rev. G. Fisk[191] mentions the case of two young "Ringhals," of 10 and 9 inches in length, having been attacked and partly devoured by a mouse, supposed to be Dendromys melanotis, which was put with the snakes in a band-box. On the habits of the Ringhals see Symonds.[192]
Bungarus.–The scales are smooth, and form thirteen to seventeen rows. The spine is very prominent, and the median row of scales which covers the ridge is much enlarged. There is no dilatable hood. In other respects Bungarus is closely allied to Naja; about half-a-dozen species, in South-Eastern Asia.
B. fasciatus reaches a length of 5 feet. The general colour is bright yellow, alternating with blackish rings.
B. coeruleus s. candidus is the dreaded "Krait," occurring in the whole of the Indian sub-region. It is dark brown or bluish black with narrow cross-bars or white specks, or it is alternately barred brown and yellow; the under parts are uniform white. Total length rarely 4 feet.
The "Krait" seems to cause more deaths in India than any other snake, since it is very common, especially in Bengal and in {634}Southern India, and often creeps into the houses. It lives chiefly on rats, lizards, and snakes.
Callophis.–With only thirteen rows of smooth scales. The head is small, not distinct from the neck. The small eye has a round pupil. The short tail has two ventral rows of scales. The whole body is cylindrical. Several small species, one or two feet in length, in South-Eastern Asia. C. macclellandi in India and Indo-China is reddish brown above, yellow below, with regular, equidistant, black, light-edged cross-bands or rings. Total length up to 2 feet.
Doliophis differs from Callophis mainly by the enormously developed poison-glands which, instead of being restricted to the head, extend along the anterior third of the body, gradually thickening, and terminating in front of the heart with club-shaped ends. Owing to the extension of these glands, which can be felt through the skin as thickenings at the end of the first third of the body, the heart has been shifted farther back than in any other snake. Several species in Indo-China and in the Malay Islands, D. intestinalis with many colour-variations.
Australia suffers from an abundance of Elapine snakes, of which we will mention only the three commonest.
Pseudechis e.g. Ps. porphyriaceus, the "Black Snake" of Australia, has seventeen rows of smooth scales on the body, a few more on the neck, which however is not, or is only slightly, dilated. A few of the sub-caudal scales are undivided, the rest are paired. The head is distinct from the neck; the pupil is round. Total length up to 5 or 6 feet. The general colour above is black, with the outer row of scales red at the base; the ventral scales are red with black edges. The females are generally more brown than black, and are therefore sometimes known as "Brown Adders." They live on small mammals, birds, lizards and other snakes.
Notechis scutatus s. Hoplocephalus curtus, the "Tiger Snake," has rather small eyes with round pupils. The head is distinct from the cylindrical body, which is covered with fifteen to nineteen rows of smooth scales. The sub-caudals are single. The head of this variably coloured snake is mostly black, the body olive brown with dark cross-bands; towards the tail the coloration becomes more uniformly blackish. The under parts are pale {635}yellow. The range of this very common snake extends over Tasmania and Australia.
Acanthophis antarcticus, the "Death Adder," is easily recognised by the peculiar tail, the end of which is laterally compressed, beset with a few rows of enlarged imbricating scales, and terminates in a thin horny spine. The head is distinct from the neck, and flat; the eye has a vertical pupil. The short and thick body is covered with twenty-one or twenty-three rows of keeled scales. The anterior caudals are single, the posterior double. The colours of the upper parts are a mixture of brown, reddish and yellow, with dark cross-bands. The belly is pale yellow, often spotted with brown or black. The end of the tail is yellow, reddish brown or black. The total length of this stout and ugly viviparous creature remains under 3 feet. It is widely distributed from South Australia to the Moluccas. The use of the peculiar tail very probably consists in attracting or fixing the attention of small animals; the snake, lying coiled up on a dry and sandy spot, slightly raising and vibrating the tip of the tail.
Elaps is an entirely American genus, with many species, most of which are extremely prettily coloured, red and black in alternate rings being a favourite pattern. The maxillaries carry no teeth behind the poison-fangs. The scales of the body are smooth and form fifteen rows. The tail is short. The small eye has mostly a vertical pupil. The head is very small, not distinct from the neck. The squamosal and quadrate bones are short, and the gape of the mouth is so limited that these beautiful snakes, although possessing strong poison, are practically harmless to man. One of the prettiest is E. corallinus of the forests of Tropical South America and the Lesser Antilles. The whole body, above and below, is adorned with about twenty deep black rings, which are edged with yellow and again separated by red rings equalling in width the black ones. Sometimes the red rings are dotted with black, and the black dots may form additional rings between the red and the yellow. Total length under 3 feet.
Sub-Fam. 2. Hydrophinae (Sea-Snakes).–The tail is strongly compressed, sometimes the body also. All the scales are small, and there are often no enlarged ventrals. The eyes are small, with round pupils. All these snakes are very poisonous and live in the sea, often at considerable distances from the land, {636}with the exception of one species of Distira, D. semperi, which is confined to the land-locked freshwater Lake Taal at Luzon in the Philippines. They live on fish, and range from the Persian Gulf to Central America. In conformity with their absolutely aquatic life they are viviparous, and they die when kept out of the water for any length of time. About fifty species are known.
Enhydrina valakadien s. bengalensis has scales with a small tubercle or keel, which is stronger in the males; the ventrals are very small, forming a scarcely enlarged series. The maxillaries carry two or more small grooved teeth in addition to the poison-fangs. The back is olive or dark grey, with black transverse bands, which are most distinct in the young. The under parts are white. This species ranges from Persia to the Malay Islands.
Hydrophis e.g. H. obscura.–The body is long; the head and neck are very slender, the body becoming much thicker farther back. The small teeth behind the poison-fangs are not grooved. The ventral scales are very small, the others are keeled, strongly so {637}in the males. The general coloration of this Sea-Snake, which reaches about one yard in length, is dark olive-green above with yellowish cross-bars, which form complete rings round the slender part of the body. Other specimens are pale olive, with dark cross-bands. This species occurs in the Bay of Bengal and the Malay Archipelago.
As a rule Sea-Snakes are not found in mid-ocean. After leaving Ceylon, the steamer meets them again in the Straits of Malacca. Those which occur near the south coast of Japan, e.g. Distira cyanocincta, are found there only in the summer, and are probably carried there by the south-west monsoon.
According to Semper the gravid female visits the shores of low islands, there to give birth to its young between the rocks, and she remains with her offspring for some time. Semper once found a large female, probably Platurus fasciatus s. colubrinus, coiled up amongst rocks, and between the folds were at least twenty young, each already about 2 feet long.
Boulenger[193] has written an interesting popular account of Sea-Snakes.
Fam. 8. Amblycephalidae.–Some thirty species of Neotropical and Oriental Snakes have been separated from the Colubridae on account of the pterygoids, which are widely separated from the quadrates, the posterior ends of the pterygoids not reaching beyond the level of the occipital condyle. This condition can be ascertained when the mouth is opened widely. The prefrontals are not in contact with the nasals. The squamosals are reduced to pad-like vestiges. Externally the Amblycephalidae are easily distinguished from the Colubridae by the absence of a longitudinal median mental groove. The head is thick, very distinct from the neck, and gives these harmless snakes a "poisonous" appearance. The pupil is vertical.
Amblycephalus, e.g. A. monticola.–Maxillaries short, with only five or six teeth. Sub-caudals in two rows. Body compressed, covered with fifteen rows of scales. South-Eastern Asia.
Fam. 9. Viperidae.–The maxillaries are very short, movably attached to the prefrontals and ectopterygoids, so that they can be erected together with the large poison-fangs, which (besides reserve-teeth) are the only maxillary teeth. The prefrontals are not in contact with the nasals. The squamosals are very loosely {638}attached. For further details see Fig. 180. The poison-fangs are perforated, having a wide hole on the anterior side at the base, in connexion with the large poison-gland; the hole leads into a canal, which opens gradually as a semi-canal on the anterior surface of the distal third or quarter of the tooth. As usual in poisonous snakes, several reserve-teeth are stowed away behind the acting fang. When the latter is broken off or has served its time it is cast off at the base, and the next reserve tooth takes its place. The supply of reserve-teeth is indefinite, half-finished teeth down to mere germs constantly growing.
All the Viperidae are very poisonous, and all, except the African Atractaspis, are viviparous. They include terrestrial, arboreal, semi-aquatic, and burrowing types. The family is cosmopolitan, excepting Madagascar and the whole of the Australian region; it is divided into Vipers and Pit-Vipers.
Sub-Fam. 1. Viperinae (Vipers).–There is no sensory external pit between the eye and the nose, and the maxillary is not hollowed out above. The Vipers are absolutely restricted to the Old World, ranging over the whole of Europe, Africa, and Asia, with the exception of Madagascar; their northern extension is limited only by the permanently frozen condition of the underground. Nine genera with about forty species are known.
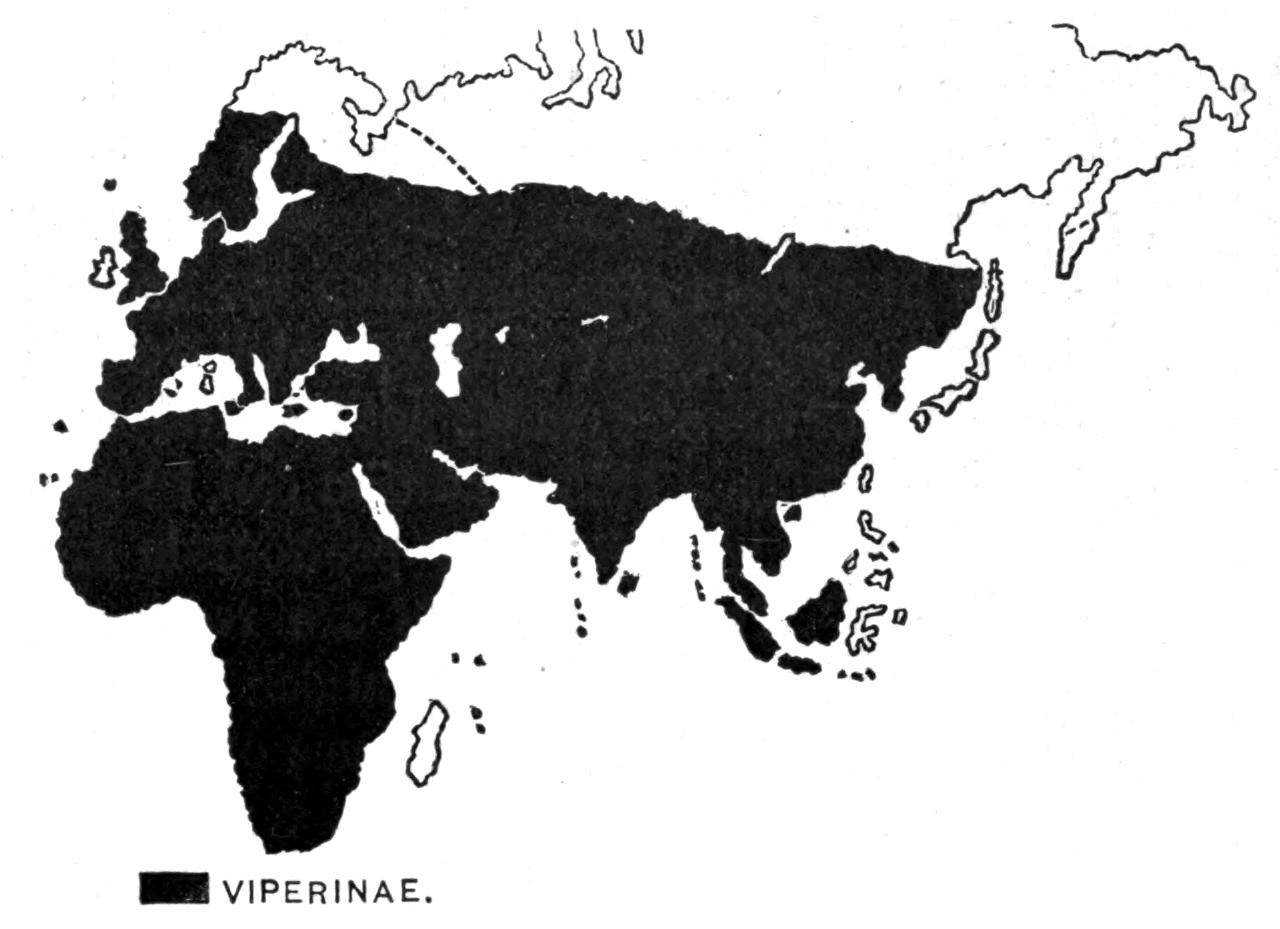
Fig. 171.–Map showing the distribution of the Sub-Family Viperinae. Corsica and Sardinia should be black in the map.
Causus with a few species in Africa and Azemiops feae in Upper Burmah are the only vipers which have the head covered {639}with large symmetrical shields, while in the other genera the head-shields are broken up into scales or small shields. Causus rhombeatus is very common in Africa, from the Gambia to the Cape. It reaches a length of a little more than 2 feet. Pale olive-brown above, usually with a dorsal series of large rhombic or V-shaped dark brown, sometimes white-edged spots, and with a dark arrow-shaped mark on the occiput; under parts yellowish white or grey.
Bitis s. Echidna.–Very much like Vipera, but the nasal shields are separated from the rostral by small scales, and the postfrontal bone is very large. Several species in Africa.
The head is very distinct from the neck, chiefly owing to the large poison-glands and to its being, like the body, much depressed. The small eye has a vertical pupil, and is separated from the labials by a series of small scales. The scales are keeled, and form many, from twenty-nine to forty-one, rows; the tail is very short, with two rows of scales below.
In B. arietans, the "Puff Adder," the nostrils are directed upwards. This ugly brute is yellowish to orange brown above with regular, chevron-shaped dark bars or other markings, helping {640}to conceal the creature when it is lying on sandy and stony ground; the under parts are yellowish white. The Puff Adder reaches a length of 4, or very rarely 5 feet, ranging all over Africa, except the north coast, and extending into Southern Arabia. It is very slow, and trusts to not being discovered when lying in the dry grass; when approached it inflates the body and hisses loudly with a puffing sound, watches the enemy with raised and characteristically bent head and neck; but it bites only when actually touched or attacked. The effect of the bite is very dangerous. Its prey consists chiefly of small mammals, which are hunted during the night.
B. (Echidna) nasicornis, of Tropical West Africa, has two or three enlarged scales above the supranasals; they stand upon erectile tissue so as to form horn-like elevations. This "Nose-horned Viper" grows to a length of 4 feet, and is rather prettily marked; the ground-colour is purplish or reddish brown, with a vertebral series of large, pale, dark-edged spots and oblique crosses. The young are at birth as much as one foot in length, and are very tastefully coloured.
Cerastes and Echis prefer to burrow in sand. The lateral scales are smaller than the dorsals, and arranged obliquely with serrated keels, so that the snakes can cover themselves with sand by lateral shovelling motions of the sides of the body.
Cerastes cornutus, the "Horned Viper" of North-Eastern Africa, from Algeria to Arabia, extending also into Palestine, has the sides of the ventral scales bent angularly, with an obtuse keel on either side. Above each eye stands a large horny, spiky scale. The upper parts are pale yellowish brown, mostly with dark spots arranged in several longitudinal rows. The under parts are white. This, or perhaps C. vipera, which has no horns, is supposed to be the species which has become famous through the suicide of Cleopatra.
About twenty years ago a number of "Horned Vipers" were brought to the Zoological Gardens of London, and attracted attention by their unusually long horns. It was found that some wily Egyptian snake-catcher had tried to manufacture a new species by taking specimens of the hornless C. vipera and inserting a pair of hedgehog's spines, pushing them upwards through the mouth.
The "Horned Viper" attains a length of two feet and a half. {641}In the daytime it is invisible, being buried in the sand with only the eyes, nostrils, and the "horns" appearing above the surface.
Vipera.–The head is distinct from the neck, and is covered with small scales and a few larger shields. The eye is separated from the labials by scales; the nasals are in contact with the rostral shield or separated by one naso-rostral shield. The scales on the body are strongly keeled; they are in two rows on the short tail. This genus with about ten species ranges over Europe, Asia, and the greater part of Africa.
Fig. 173.–Cerastes cornutus, the "Horned Viper" (right), and Vipera ammodytes, the "European Nose-horned Viper" (left). × 1.
V. berus, the Common European Viper (see Fig. 165, p. 620). The snout is not turned up at the end; between the small head-scales there is generally a pair of well-developed parietal and frontal shields. The scales of the trunk form twenty-one rows. The coloration is very variable, there being grey, brown, red, or black specimens in the same country, and the much-spoken-of black zigzag line along the back is so often indistinct that it is a character not to be relied upon. Usually the grey, yellowish, olive, brown or red ground-colour is set off by a dark zigzag band along the spine, and by a series of lateral spots; an oblique or St. Andrew's cross or two diverging bold streaks of dark brown or black are usually present on the back of the head, and there is a dark streak behind the eye. The under parts are grey, brown, or black, uniform or speckled; the end of the tail is usually yellow or red.
According to Boulenger, who is making a special study of the individual variations of Vipers (concerning colour, scaling, number of vertebrae, etc.), some specimens are entirely black in the males through extension of the black markings, in the females through darkening of the ground-colour. Males are usually distinguishable from females by darker, deep black markings and lighter ground-colour. The females are mostly larger than the males. The largest specimen in the British Museum measures 700 mm. = 28 inches, but a viper 2 feet long may be considered a very large specimen. The Common Viper has a wide range, from Wales to Saghalien Island, and from Caithness to the north of Spain. It ascends the Alps to a considerable altitude, up to 6000 feet. J. Blum[194] has published an elaborate statistical account of the Viper in Germany, unfortunately confining himself strictly to the political frontiers. According to the map attached to his work, the Viper is common all over Germany with the exception of South-Western and parts of Middle Germany. It is absent in Alsace, the Bavarian Palatinate, Rhenish Prussia, Hesse, the northern half of Baden, Würtemberg, and Franconia, countries which, speaking broadly, have a warm subsoil, composed of Red Sandstone and Basaltic formation. As a rule the Viper prefers heaths, moors, and mixed woods with sunny slopes. Brambles, clumps of nettles, hedges, the edges of little copses, heaps of stones, are favourite places of retreat, affording shelter, holes, and the vicinity of mice, which form its chief sustenance. At harvest-time it is often found in cornfields, and it frequently hides in the sheaves. Vipers are fond of basking on certain spots, on the top of a stone, the stump of a tree, or a patch of sand: a shower of rain or even passing clouds drive them back into their holes. They are eminently nocturnal, when they regularly "beat" their district, biting and paralysing their prey before swallowing it. A fire kindled at night is sure to draw vipers near; the same applies to other vipers, for instance Cerastes, which appears in perplexing {643}numbers at the camp-fire. They cannot climb, and they avoid going into water. The pairing takes place as a rule from March to May, a number of individuals, mostly males, collecting around the females, and forming entangled lumps of snakes; parturition takes place in the following July and August. In exceptionally warm winters they have been known to pair in December, having left their winter-quarters. They hibernate for about six months, more or less according to the climate, congregating in great numbers, sometimes in dozens. With very rare exceptions Vipers do not take food in captivity, but prefer starving themselves to death. The bite is as a rule not fatal. The seriousness of the case depends of course upon many circumstances, as for instance the state of concentration of the venom, the position and depth of the bite, and last but not least upon the general condition of health of the victim. General depression aggravated by nervousness, weakness of the bitten limb, occasional breaking out of the wound, are of frequent and protracted occurrence. (See also p. 590.)
V. aspis is a more southern and western European Viper, occurring from France to the Tyrol, and in Italy. The snout is slightly turned up at the end, and still more so in V. latastei of Spain and Portugal. In V. ammodytes, of South-Eastern Europe, the raised portion is produced into a soft, scaly appendage (see the lower figure on p. 641). Vipers are sometimes unpleasantly common in certain localities. This was for instance the case at the drill-ground near Metz, and the military authorities paid a price for each viper delivered to them. The supply of the latter increased to an alarming extent until the German authorities discovered that a regular trade had been established across the frontier, and that the French Lorrainers were importing vipers briskly.
V. russelli, the "Daboia" or Russell's Viper, is one of the scourges of India, Ceylon, Burma, and Siam. The scales form about thirty rows on the body. The upper surface of the head is covered with small, imbricating, usually keeled scales. The general colour is pale brown above with three longitudinal series of black, light-edged rings, which sometimes encircle reddish spots. The under parts are yellowish white, uniform, or with small crescentic black spots. Total length up to about 5 feet. The poisoning symptoms are described on p. 590.
Sub-Fam. 2. Crotalinae ("Pit-Vipers").–With a deep cavity or pit between the eye and the nose, lodged in the hollowed-out maxillary bone. This pit is lined with a modified continuation of the epidermis, and is amply supplied with branches from the trigeminal nerve. It is undoubtedly sensory, but we do not know its function. A good anatomical account of this organ has been given by West.[195] Some of the Pit-Vipers have a rattle at the end of the tail; these are the Rattle-Snakes. The rattle is composed of a number of horny bells which fit into each other. The oldest or terminal bell is in reality the horny covering of the tip of the tail, and with each moult or shedding of the skin the youngest bell becomes loose, but is held by the new covering which has been developed in the meantime. There is thus produced an ever-increasing number of loosely-jointed bells, but now and then most or all the bells break off, probably when they are worn out, and a new set is gradually developed. Rattles with a dozen bells are, for instance, very rare. They naturally increase in bulk with the age of the snake, but the number of joints is no indication of the snake's age.
Pit-Vipers have a very wide distribution. They are divided into four genera with about sixty species. Rattle-Snakes are {645}restricted to America, but other Pit-Vipers occur in North and South America and in the southern half of Asia.
Ancistrodon.–Without a rattle. The upper surface of the head is covered with nine large shields, but the internasals and prefrontals are sometimes broken up into scales. The scales of the body have apical, sensory pits. About ten species, some in Central and North America, others in the Caspian district (A. halys), in the Himalayas (A. himalayanus), in Ceylon, Java, etc.
A. piscivorus s. Trigonocephalus cenchris (part), the "Water-Viper," inhabits North America from Carolina and Indiana to Florida and Texas. The general colour is reddish to dark brown, with darker cross-bands or with C-shaped markings; a dark, light-edged band extends from the eye to the angle of the mouth. The under parts are yellowish, spotted with black, or the latter is the prevailing colour. Total length up to 5 feet. The Water-Viper is semi-aquatic and lives chiefly on fishes, but it also eats other snakes and various Amphibia, Birds, and Mammals. This snake is very good-humoured in captivity, and becomes {646}easily tame. A gentleman in Berlin, rather too much addicted to making pets of poisonous snakes, had a pair which propagated regularly. When I was a boy he invited me to feed the young Water-Vipers with fishes cut into strips, and I enjoyed this immensely until he warned me not to touch the mother, which might bite strangers.
A. contortrix s. Trigonocephalus cenchris (part), the "Moccasin-Snake" or "Copper-head," is one of the few poisonous snakes which possess a loreal shield, i.e. a shield intercalated between the pre-oculars and the nasals; below it lies the pit. The general colour is yellowish to pink or pale brown, with dark brown or red cross-bars or triangular marks. The under surface is yellowish or reddish, speckled with grey or brown, and with a lateral series of large blackish spots. Total length of full-grown specimens about one yard. The Moccasin-Snake ranges from Massachusetts and Kansas to Northern Florida and Texas. It prefers swampy localities or meadows with high grass, where it hunts for small Mammals and Birds.
Lachesis.–Without a rattle. The upper surface of the head is covered with very small shields or with scales. About forty {647}species in South-Eastern Asia and in Central and South America.
L. (Bothrops s. Craspedocephalus) lanceolatus inhabits nearly the whole of South America, extending into Mexico and the Lower Antilles, e.g. Martinique, Guadaloupe, and Santa Lucia, where it is known as the "Fer-de-Lance," and is the curse of the sugar-plantations on account of its being so very common and so deadly poisonous. The Mongoose was introduced as a possible antagonist, but the little Indian Mammal wisely left the dangerous reptile alone, and has in some places established himself as another pest–as a destroyer of poultry. The Fer-de-Lance grows to a length of 6 feet, establishes itself everywhere–in swamps, plantations, forests, in the plains and in the hills–and is very prolific, producing, according to its size, dozens of young which are 10 inches long, very active and snappy.
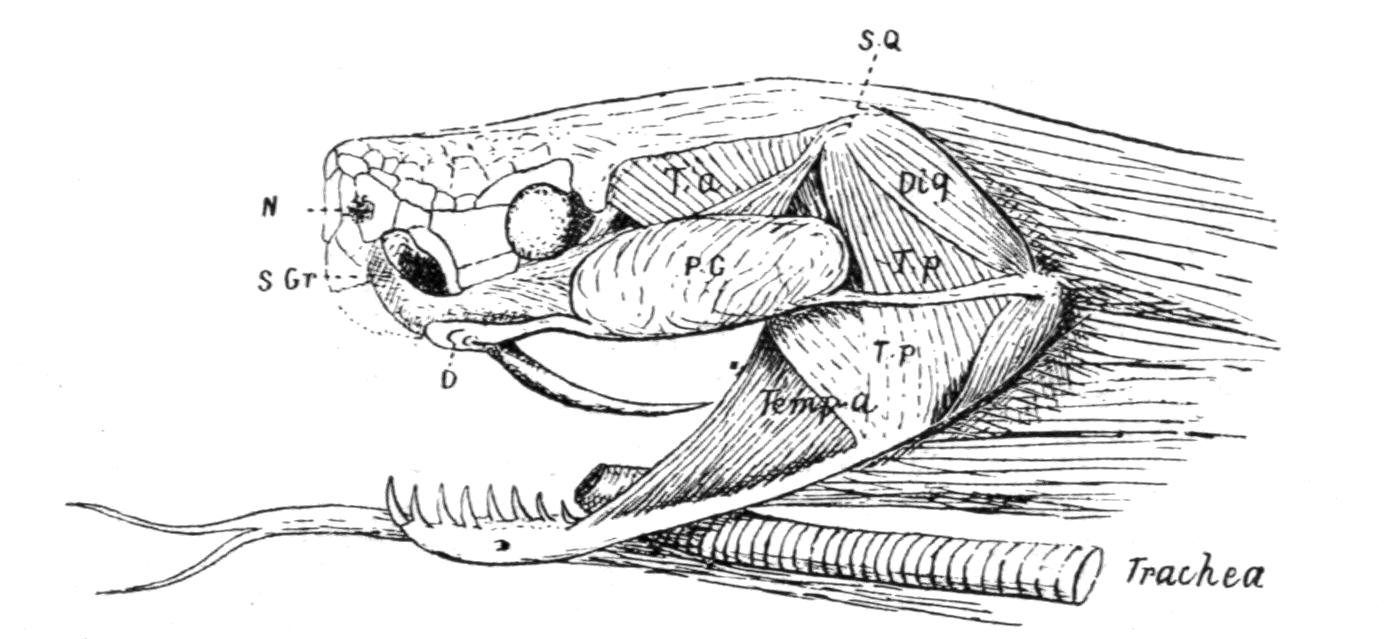
Fig. 179.–Head of Lachesis lanceolatus after removal of the skin. × 1. D, Duct, bent upon itself, from the poison-gland into the tooth; Dig, digastric muscle or opener of the jaw; N, nostril; P.G, poison-gland; S.Gr, sensory groove or pit; S.Q, point of junction of the squamosal and quadrate; T.a, Temp.a, anterior, and T.p, posterior, temporal muscle.
L. (Trimeresurus) gramineus s. viridis, to mention one Asiatic species, grows to less than 3 feet in length, is bright green above, sometimes with faint blackish bars; green, yellow, or whitish below, and with a light streak along the outer row of scales. The end of the tail is usually bright red. This beautiful snake has a prehensile tail and is arboreal. Its range extends over the whole of India, to Hong-Kong and to Timor, and even into the Andaman and Nicobar Islands.
Sistrurus.–With a rattle. The upper surface of the head is covered with nine large shields. A few species in North America east of the Rocky Mountains, e.g. S. miliarius.
Crotalus.–With a rattle. The upper surface of the head is covered with small scales. Range from Southern Canada and British Columbia to Northern Argentina, but not in the West Indian Islands. About ten, mostly closely-allied species.
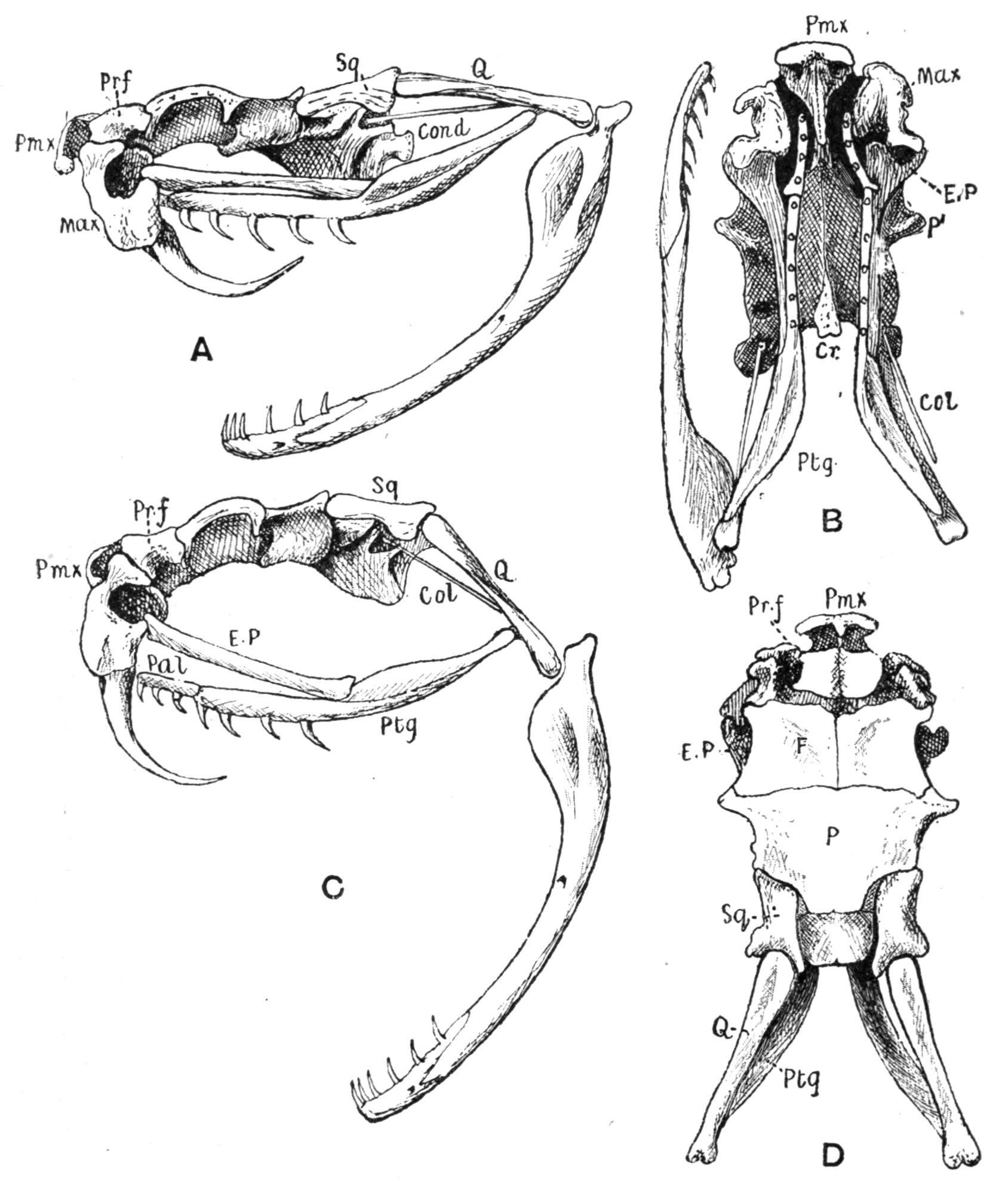
Fig. 180.–Skull of a Rattle-Snake, Crotalus durissus. × 1. A, Lateral view, jaws slightly opened; B, ventral view; C, lateral view, the jaws opened fully in the position of striking; D, dorsal view. Compare this with the diagrammatic figures on p. 588, where the mechanism has been explained. Col, Columella auris; Cond, condyle; Cr (in B), sphenoidal crest for the attachment of the powerfully developed ventral cranio-cervical muscles; E.P, ectopterygoid or transverse bone; F, frontal; Max, maxillary; P, parietal; P1, post-orbital process; Pal, palatine; Pmx, pre-maxillary; Pr.f, prefrontal; Ptg, endopterygoid; Q, quadrate; Sq, squamosal.
The effect of the poison of Rattle-Snakes has been discussed on p. 589.
C. horridus is the common Rattle-Snake of the United States; C. confluentus is the species in Western and C. durissus the common species in South-Eastern North America. Very large Rattle-Snakes, C. durissus, attain a length of 8 feet, others not often more than five. They prey chiefly upon small Mammals, hunting for them at night. In the daytime they are also about, mainly in order to bask. Although they occasionally take to the water in pursuit of their prey, they dislike being wetted by rain, withdrawing then into their holes, appropriating as a rule those of ground-squirrels, rats, and Prairie-dogs. The often-repeated story about Rattle-Snakes living in neighbourly friendship in the holes of Prairie-dogs, together with the little Prairie-owls, is an exaggeration. We do not know how many of the original inmates are eaten. Pairing takes place in the spring. During the cold months they hibernate under ground, often in considerable numbers.
Rattle-Snakes have few enemies besides man and pigs. The latter kill and eat them wherever they can. The rattle is decidedly useful to the snake as an instrument of warning off any approaching possible enemy, since no snake likes to bite unless in self-defence or in order to kill its prey. The noise of the rattle is very loud in dry weather, much duller on clammy days; it is a shrill sound like that of a rattling alarm-clock, and a well-conditioned snake in a room can make conversation well-nigh impossible, and can keep on rattling for half an hour or longer. The rattle is kept in such rapid lateral vibrations that it shows only a blurred image, the rattle standing with its broader sides vertically, not horizontally. They endure captivity for many years, and become tame enough not to hiss and to rattle whenever they are approached.
C. horridus is grey-brown above, usually with a rusty vertebral stripe and with V- or M-shaped blackish cross-bands; the under surface is yellowish; the end of the tail is blackish. The supra-ocular shields are smooth and much narrower than the scaly space between them, and there is only one pair of internasals.
C. durissus s. adamanteus differs from the previous species chiefly by possessing two pairs of internasals; and the dark {650}markings on the body form a handsome pattern of rhombs with lighter centres and yellowish edges. This is the largest species of Rattle-Snake, reaching a length of 8 feet.
C. confluentus has broader, transversely striated, supra-ocular shields. The specific name refers to the continuous series of large brown or red rhomboidal spots on the back.
C. terrificus ranges from Arizona to Argentina, and is the only species of Rattle-Snake in South America. It differs from the others by having a pair of prefrontal shields behind the pair of internasals.
Every reference is to the page: words in italics are names of genera or species; figures in italics indicate that the reference relates to systematic position; figures in thick type refer to an illustration; f. = and in following page or pages; n. = note.
Abdominal armour, of Cricotus, 287;
of Microsauri, 289;
of Prosauri, 290
Abdominal ribs, of Rhynchocephali, 292, 298;
of Dinosauria, 414;
of Megalosaurus, 421
Aberrant scaling of Lacertilia, 495
Ablepharus, 560;
eyelids, 494
Acanthodactylus vulgaris, 559
Acanthophis antarcticus, 635
Acentrous vertebrae, i.e. those without a centrum or body, 4
A. gryllus, 207 f.
Acrochordinae, 606
Acrochordus javanicus, 607
Acteosaurus, 489
Adams, visit to the Mugger-peer, 455 f.
Adaptive characters of Anura, 142
Adhesive apparatus, of tadpoles, 57, 57;
of Tree-frogs, 187;
of Thoropa, 209;
of finger-discs of Raninae, 239;
Aelurosaurus, 307
Aestivation, of Crocodiles, 457;
Aetosaurus, 432;
Agama, 520;
A. sanguinolenta, 520;
Agamura, tail, 506
Agassiz, on habits of Alligator Turtle, 341;
of Trionyx, 407
Age of Chelonia, how to estimate, 326;
great age attained by Tortoises, 369, 376, 377;
see also Growth, rate of
distribution, 143
Ahaetulla s. Leptophis, 618, 619
Aistopodes, 81
Aldabra, gigantic tortoises of, 373 f., 375
Algae, destructive to shell of tortoises, 357
Allantois, an embryonic outgrowth from the posterior part of the gut, acting as a respiratory organ, 278
A. mississippiensis, 467 f.;
skull, 468;
nesting, 469;
A. sinensis, 471
Alligator Turtle, 340
Allopleuron hofmanni, 380
Allosaurus, 422
Alpine, Newt, 126;
Salamander, 119
Altitude, high, in which Anura have been found, 181
Alytes, 157 f.;
A. cisternasi, 160;
A. obstetricans, 158;
urino-genital organs, 49
Amblycephalidae, 592, 593, 637
Amblycephalus monticola, 637
Amblyrhynchus, 528;
A. cristatus, 533
Amblystoma, skull, 17, 94, 96, 109, 110 f., 112;
A. jeffersonianum, 111;
A. mavortium, 115;
A. opacum, 110;
A. persimile, 111;
A. punctatum, 110;
A. talpoideum, 110;
A. tigrinum, 111 f.;
metamorphosis of, 112 f.
Ameiva, 549
Amnion, a membrane round the embryo, 278
Amphibia, 3 f.;
definition, 5;
systematic position, 5;
numbers of species, 4
Amphicondylous, i.e. the occipital part of the skull articulates with the neck by a right and a left knob, 4
Amphignathodon, 185;
A. guentheri, 188
Amphisbaena, 566;
A. fuliginosa, 566
A. means s. tridactyla, 100, 101
Amphodus, 210;
A. wucheri, 211
Anaides = Autodax (q.v.), 107
Anal sacs of Chelonia, used as additional respiratory organs, 330
Anarosaurus pumilio, 477
skull of A. coelurus, 421
Ancistrodon, 645;
A. halys, 645;
A. himalayanus, 645;
Anderson, on nest of Gavialis, 452
Andrews, on Amblystoma, 110
Andrias scheuchzeri, 84
Anelytropsis papillosus, 564
Aniella pulchra, 564
Annandale, on habits of Calotes, 518;
of Liolepis, 527;
of Rhacophorus, 247;
of Varanus salvator, 544
Anodonta, as food of Trionyx, 407
Anodontohyla, 236
Anolis, 528;
A. carolinensis, 529
Anomodontia, 309
Anura, 7;
characters, 138;
phylogenetic tree of, 142
Anus, asymmetrical position of, 60
Apoda, 84 f.;
affinities, 88;
distribution, 89;
eyes, 86;
skin, 87;
spermatozoa, 87;
tentacular apparatus, 88;
vertebrae, 86;
visceral arches, 86
Archaeopteryx, 417
Archegosaurus, vertebrae, 13, 82, 287
Arcifera, of Cope, 140;
of Boulenger, 140
Arciferous, type of shoulder-girdle, 24, 25
Arion, slug, eaten by tortoises, 363
Arrau-turtle (Podocnemis), 391 f.
Arteria cutanea magna, 144;
A. sacralis of Anura, 144
Ascaphus, 153
Asterophrys, 161
Athecae, 333;
definition of name, 337
Atlas and Axis, i.e. first and second cervical vertebrae; of Cryptobranchus, 13;
of Crocodilia, 283;
atlas fused with axis, 307
Atoposauridae, 453
Atractaspis, 638;
dentition, 593 n.
Atria, the thin-walled receptive parts (auricles) of the heart
Auditory columellar apparatus, of Amphibia, 24;
of Anura, 29
Australian, Anura, spawning time and habits of, 201;
Lacertilia, 502
A. lugubris, 107;
A. iecanus, 107
Autosauri, 491 f.
Axis;
see Atlas
Azemiops feae, 638
Balancers of Amphibia, 45
Barfurth, on absorption of Tadpole's tail, 61
Bartlett, on Boa constrictor, 602;
on Pipa, 152
Bates, on habits of Podocnemis, 392 f.
Batrachomyia, fly infesting Bufonidae, 177
Batrachophrynus, 224;
B. macrostomus, 225;
B. brachydactylus, 224
Batrachopsis, 161
Batrachylodes, 241
Batrachyperus, 96;
B. sinensis, 109
Baur, on Sphargis, 336
Bdellophis, 90
Bedriaga, on Axolotl, 114;
synopsis of Urodelous Larvae, 59 n.
Bell, J., on classification, 8
Bell, Napier, on habits of Iguana, 531
Bemmelen, on Sphargis, 336
Berg, on Spelerpes fuscus, 106
Bert, quoted, 571 n.
Biedermann, on change of colour in Hyla, 35
Birds not related to Dinosaurs, 416 f.
B. nasicornis, 640
Black Snake, of Australia, 634;
of North America, 613
Blainville, de, on classification, 7
Blanus cinereus, 566
Blood, shape of red corpuscles, 4;
temperature, 67 f.
Blood-sucker = Calotes ophiomachus, 519
Blum, quoted, 642 n.
Boa, 602;
B. constrictor, 602;
B. dumerili, 602;
B. madagascariensis, 602
Boettger, on influence of climate and country upon reptiles, 492 f.
Boinae, 601 f.
habits, 156 f.;
tadpoles, 157;
abnormal vertebrae, 22;
shoulder-girdle, 25;
urino-genital organs, 49;
B. pachypus, 155
Bothrops, 647
{653}Boulenger, classification of Amphibia Caudata, 9;
on vertebrae of Pelobates, 20;
on vertebrae of Bombinator, 22;
number of phalanges in Anura, 27;
on poison of Amphibia, 36;
on vocal sacs, 48;
on modes of fecundation and nursing habits, 54, 56;
synopsis of Tadpoles, 59 n.;
on tadpoles of Rana opisthodon, 260;
on classification of Anura, 140, 141;
on Pipa, 152;
on Scaphiopus solitarius, 165;
on Alligator sinensis, 471;
on Lanthanotus, 542;
on aberrant scaling, 495;
on Heloderma, 540 n.;
on classification of Snakes, 592;
on Sea-Snakes, 637;
on Sphargis, 336
Boulengerula, 90
Brachial plexus, of Anura, 39
B. ephippium, 231
Brachylophus, distribution, 501, 528
Brain, of Scaphognathus, 485;
small size of, in Dinosaurs, 425
Branchial arches, of Urodela, 16;
of Anura, 42
Branchiosauri, 80
Branchiosaurus, skull, 80;
B. salamandroides, 80
Brauer, on development of Apoda, 92;
on nursing habits of Arthroleptis, 243
Breeding of Axolotl, 113
Breviceps, shoulder-girdle, 26, 225, 226, 227, 232;
B. mossambicus, 232
Brithopus, 308
Brongniart, on classification, 7
B. excelsus, 418
B. giganteum, 420
Brood-pouches, of Anura, 151, 248;
of Hyla goeldii, 198;
of Nototrema, 202;
of Rhinoderma, 228
Brookesia, 580
Brown Adder, 634
Brown Frog, Common, 251 f., 255
Brücke, quoted, 571
Buchholz, on Chiromantis, 244 f.
Budgett, on breeding habits of Phyllomedusa, 204;
on Paludicola, 220;
on Lepidobatrachus, 218;
quick development of Phryniscus, 231;
on Bufo marinus, 179
Bufo, sacral vertebra, 22;
shoulder-girdle, 26;
urine-genital organs, 49;
development of adhesive apparatus, 57;
B. agua, 178;
B. americanus, 178;
B. calamita, 181 f.;
B. ceratophrys, 179;
B. empusus and B. peltocephalus, dermal ossifications, 179;
B. jerboa, 166;
map of distribution, 167, 168, 169 f.;
B. marinus, 178;
B. mauritanica s. pantherina, 184;
B. quercinus, 178;
B. variabilis = viridis, 180;
large-sized specimens, 171;
immured in buildings, 174;
diseases, 176;
distribution, 177
distribution, 167;
affinities, 166
Bufoniformes, 139
Bullfrog, of America, Rana catesbiana, 261;
of India, Callula pulchra, 234;
Rana tigrina, 261
Bungarus coeruleus s. candidus, 633;
B. fasciatus, 633
Butler, on fat-bodies, 500
Cabrita, 551
Cacopus, shoulder-girdle, 25, 225, 226, 228
vomer, 435;
O. palpebrosus, 471;
C. sclerops, 471;
Calcareous deposits in the skin of Amphibia, 31, 34
Calliphora silvatica, fly infesting Bufo, 176
Callophis macclellandi, 634
C. pulchra, habits of, 234 f.
Calotes, 517;
C. emma, 518;
C. mystaceus, 519;
C. ophiomachus, 519;
C. versicolor, 518
Calyptocephalus, 179, 212, 215
Camptosaurus, 426
Capitosaurus, 83
Carapace, 321 f., 319, 320, 322, 323;
posterior portion movable in Cinyxis, 364, 365;
carapace of tortoises, evolution of, 337;
composition of, 324 f.;
reduction of component elements, 325;
reduction in thickness, 373;
correlative changes, 328;
of Sphargis, 335 f.;
of Chelone, 379;
of Testudo, 322;
of Pleurodira, 389;
reduction in Trionychidae, 325;
fenestration, 325;
with hinge in Cinyxis, 364, 365
Cardioglossa, 274
C. insculpta, 404;
absence of horny shields, 325
Carpus (see also Limbs), of Eryops, 286;
of Sphenodon, 294;
of Eusuchia, 440
Casarea, 603
Case, on Sphargis, 336
Cassina, 240
Causus, 638;
C. rhombeatus, 639
Centrolene geckoideum, 211
{654}Ceratobatrachidae, 141
Ceratobatrachinae, 139, 237 f.
Ceratobatrachus guentheri, 237
Ceratohyla, 211
Ceratophora, 517;
C. stoddarti, 517;
C. tennenti, 517
C. cornuta, 216;
C. dorsata, 215;
Ceratopsia, 430
Cerberus rhynchops, 625
Cetiosaurus, 419
Chalarodon, 528;
geographical distribution of; 501
Chalcides, 562;
Ch. bedriagae, 563;
Ch. guentheri, 563;
Ch. lineatus, 563;
Ch. ocellatus, 563;
Ch. tridactylus, 563
Chamaeleon, 573;
Ch. bifidus, 580;
Ch. calcaratus, 579;
Ch. parsoni, 580;
Chamaeleontes, 567 f.;
distribution, 568;
tongue, 569 f.;
colour-changing mechanism, 570, 571, 573 f.;
eggs, 572
Chamaeleontidae, 573 f.
Chamaerops humilis, dates of, eaten by Testudo, 367
Chameleon, misnamed Calotes, 518;
misnamed Polychrus, 529
Chauvin, Marie von, on Axolotl, 113;
on Salamandra atra, 120
Chelodina, suppression of neural plates, 324;
skull, 399;
Chelone, skull, 317;
skeleton, 320;
plastron, 321;
shields, 327;
intergular shields, 325;
Ch. mydas, 381 f.;
various modes of fishing, etc., 382, 383;
Chelonemydidae, 380
Chelonia, 312;
number of species, 312;
affinities of, 312;
classification, 313;
key to living families, 314;
plastron, names of the horny shields, 315; 321, 325;
skull, 280, 317, 356, 364, 379, 400, 405;
skeleton of Testudo, 319;
of Chelone, 320;
pectoral arch, 318;
pelvis, 319;
plastron, bones of, 321;
limbs, 320;
evolution of, 337;
evolution of the horny shields, 326 f., 327;
regeneration, 329;
sense-organs, 329;
digestive apparatus, 330;
respiration, 331;
growth of Chrysemys, 349
affinities of, 380
Chelydra, 328;
Ch. serpentina, 338
distribution of, 332
Chelydropsis, nuchal plates, 324
Chelys fimbriata, 400;
intergular shields, 325
Chersydrus granulatus, 607
Ch. lusitanica, 121
Chirixalus, 241
Ch. platycephalus, 221
Ch. petersi, 244;
Ch. xerampelina, 244
Chirotes, 564;
Ch. canaliculatus, 566
Chirotherium, 83
Chlamydosaurus kingi, 522, 523
Choanae, or inner nasal openings, 47
Chorda dorsalis, the axial rod between the gut and the spinal cord, around which the vertebrae are formed, 12
Ch. ornatus, 208
Chromatophores, 35
Chrysemys, costal plates of, 325;
colour of iris, 329;
Ch. elegans, 346;
Ch. rubriventris, 346
Cimoliasaurus, 478;
C. australis, 478;
C. cantabrigiensis, 478;
C. chilensis, 478;
C. haasti, 478
distribution, 332
Cinosternum, 342 f.;
arrangement of neural plates, 324;
Cinyxis belliana, 365;
C. homeana, 364
Cistecephalus, 310
Cistudo, arrangement of neural plates, 324;
colour of iris, 329
Claosaurus, 429
Clarke, on habits and development of Alligator, 467
Classification of Amphibia, historical account, 7 f.
Clawed Toad (Xenopus), 146 f.
Claws or nails of Amphibia, 32
Cleithra = the pair of additional clavicles;
of Stegocephali, 79;
Clemmys, 356 f.;
C. caspica, 358;
C. insculpta, 359;
skull, 356
Clepsydrops, 308
Clidastes tortor, 490
Cloaca, of Chelonia, 330;
of Crocodiles, 445;
of Lacertilia, 498
Cnemidophorus, 549;
C. sexlineatus, 549
{655}Coccyx, s. Os coccygeum, of Anura, 20, 21, 22
Coecilia, 89
Coeciliidae, 89 f.;
distribution of, 89
Coelopeltis, 624;
C. monspessulana s. lacertina, 624
Coelurus, 415;
C. gracilis, 423
Colombo, gigantic tortoise of, 377
Coloration, warning colours of Amphibia, 38, 156;
protective, of Amphibia, 191, 238, 252;
of deserticolous reptiles, 494
Colour, changes of, in Anura, 35;
in Geckos, 509;
in Lacertilia, 498;
mechanism of changing, in Chameleons, 570, 571
Coluber, 615 f.;
C. aesculapii = flavescens = longissimus, 616 f.;
C. leopardinus, 616;
C. (Rhinechis) scalaris, 617
Colubrinae, 607 f.
Columella cranii, 496, 550, 551
Columellar auditory chain, of Amphibia, 4;
of Anura, 29;
of Crocodiles, 446;
of Lizards, 496
Comoro Islands, Tortoises of, 373
Condyle, occipital, of Theromorpha, 302;
exaggerated importance of its character, 285
Conolophus subcristatus, 532
Conus arteriosus, continuation of the heart beyond the ventricles so far as it contains valves, 6
Cope, on classification of Amphibia, 9;
on Siren, 136;
on hand-skeleton of Eryops, 286;
on Sphargis, 336;
classification of Lacertae, 513;
classification of Snakes, 592
Cophyla, 236
Copulatory organs, of Lacertilia, 499;
absent in Sphenodon, 294;
of Chelonia, 330;
of Snakes, 585
Coqui, 214
Coronella, 619;
C. austriaca s. laevis, 619, 620;
C. girondica, 621
C. corrugatus, 244;
C. johnstoni, 243;
C. solomonis, 244;
C. unicolor, 244
C. greeningi, 207
Costal plates of Chelonia, 324 f., 322, 323
Craspedocephalus, 647
C. heteroclitus, 287
Crinia, 213;
spawning, 223
Crocodilia, 431 f.;
skeleton, 434 f.;
atlas and axis, 283, 431, 439;
affinities, 432;
teeth, 437;
skin, 442;
dermal armour, 442;
skin glands, 443;
tongue, 443;
respiratory organs, 444;
"diaphragm," 444;
digestive organs, 444;
cloaca, 445;
heart, 445;
ear, 445;
eye, 446;
geographical distribution, 446, 446;
voice, 447;
habits, 447;
propagation, 447;
classification, 448
Crocodilidae, 454
teeth, 437;
skin glands, 443;
C. americanus, 466;
skull, 466;
C. biporcatus = porosus, 458;
rate of growth, 459;
C. cataphractus, 465;
C. intermedius, 466;
C. johnstoni, 466;
C. niloticus = vulgaris, 460 f., 449, 461;
habits, 462 f.;
skull, 455;
C. porosus, 458;
skull, 458;
Crotalinae, 644
Crotalus, 648;
rattle of, 644;
C. horridus, 649;
C. terrificus, 650
fossil, 84;
C. alleghaniensis, 97;
Cryptoclidus, shoulder-girdle, 474, 475, 478
Cryptopsophis, 89;
C. multiplicatus, 92
Cryptotis, 213
Cutis, of Amphibia, 33 f.
Cyamodus, 311
Cyclanorbis, 411;
nuchal plate, 324
Cycloderma, 411
Cyclodus s. Tiliqua, 561;
Cyclorhamphus, 212
C. berryi, 307;
C. crateronotus, 306;
C. platyceps, 307
distribution, 161
Cystignathus = Leptodactylus, 210, 218
Daboia, 643
Dactylethra;
see Xenopus, 146 f.
Darwin, on Conolophus, 532 n.;
on tortoises of Galapagos Islands, 377
dentition, 593 n.
Davison, on breeding of Amphiuma, 101
Dawsonia, 289
Death Adder, 635
Denburgh, van, on Autodax, 107
Dendrobates, 272;
D. braccatus, 273;
D. trivittatus, 273;
D. typographus, 273;
various uses of its poison, 38
{656}Dendrobatinae, 139, 237, 272 f.;
distribution, 239
Dendrophis, 618;
Dendrophryniscus brevipollicatus, 224
Dentition, of snakes, 582, 592, 593;
see also Teeth
Dermal armour, of Cricotus, 287;
of Microsauri, 289;
of Prosauri, 290;
of Theromorpha, 302;
of Dinosauria, 415;
of Pseudosuchia, 433;
of Parasuchia, 434;
of Crocodiles, 442
Dermal ossification in Anura, 179, 190, 210
Dermatemydidae, 313, 314, 341;
distribution of, 332
Dermatochelys coriacea, 333 f., 334
D. thomensis, 93
Deserticolous reptiles, 493 f.
Desmognathinae, 102
Deuterosaurus, 308
Development, of Anura, 56 f., 57;
of horny teeth, 58;
of Apoda, 92;
of Crocodilus, 465;
of Alligator, 467
Diadectes, 308
Diademodon, 309
D. jordani, 207;
D. petasata, 207
Diaphragm, of Anura, 144;
of crocodiles, 444
Diapophyses (the lateral or "transverse" processes of the neural arches) of Anura, 138, 141
Dibamus novae-guineae, 564
Dicamptodon, 96;
D. ensatus, 109
Diclonius = Hadrosaurus, 429
Dicynodon, 301, 302, 303, 310;
skull, 280;
D. leoniceps, 310;
D. orientalis, 310;
D. tigriceps, 310
Digestive apparatus, of Chelonia, 330;
of crocodiles, 444;
of Lacertilia, 498
Digits = Fingers and Toes.
Number of digits in Urodela, 15, 16;
in Anura, 26;
terminal phalanges, 26;
number of joints, 27;
adhesive discs, 27;
variability in numbers, 563;
digits of Eryops, 286;
of Crocodiles, 441;
of Plesiosauri, 475;
of Geckos, 505
Dimetrodon, 308
Dimorphodon macronyx, 486
Dinosauria, 412;
affinities of, 415;
analogies with Birds, 416
Diplocynodon, 448;
D. hastingsiae, 454
Diplodocus longus, 419 f.;
skull, 419
Dipsadomorphinae, 623 f.
Dipsadomorphus, 623;
D. cyaneus, 624;
D. trigonatus, 623
Dipsas bucephala, 624
Discoglossus, urino-genital organs, 49, 153;
D. pictus, 153 f.
Dissorophus multicinctus, 82
Distira cyanocincta, 637;
D. semperi, 636
Distribution, geographical;
see Maps
Dolichosauri, 489
Dolichosaurus longicollis, 489
Dolichosoma longissimum, 81
Doliophis intestinalis, 634
Dollo, on Sphargis, 336
Dracaena, 547;
D. guianensis, 549
Draco, 516;
D. dussumieri, 516;
and Bibron, on classification of Snakes, 592
Dwarf Chameleon, 579
Dyscophus, 236;
D. antongili, 236
Ear, of Chelonia, 330;
of Crocodiles, 445 f.;
of Snakes, 583
Ear-opening of deserticolous reptiles, 494
Echeneis remora, used for turtle fishing, 382
Echis, 640;
E. arenicola, deserticolous, 493
Edalorhina, 212
Eggs of Amphibia, 53;
mode of deposition in Amphibia, 54-56;
of Ichthyophis, 91;
and spermatophore of Triton viridescens, 128;
nursing and taking care of, 55;
by Pipa, 151;
by Alytes, 159;
by Rhacophorus reticulatus, 248;
by Amphignathodon, 188;
by Leptodactylus mystacinus, 219;
by Rhinoderma, 228;
by Rhacophorus, 248;
by Desmognathus fuscus, 103, 103;
number of: in Bufo vulgaris, 175;
in Bufo viridis, 181;
in Hyla arborea, 193;
in Rana esculenta, 270
Eggs of Reptilia: Sphenodon, 299;
Chelonia, 331;
Testudo graeca, 369;
T. ibera, 369;
T. elegans, 371;
T. polyphemus, 372;
Emys orbicularis, 355;
Clemmys leprosa, 358;
Chelone mydas, 382;
Thalassochelys caretta, 387;
Podocnemis expansa, 393 f., 398;
Trionyx, 408;
mode of laying by Emys, 355;
by Podocnemis, 393;
used commercially, 394 f.;
enormous destruction of, 395, 399;
Alligator, 470;
eggs and nest of Gavialis, 452;
Lacertilia, 499;
increasing in size after deposition, 499;
Tarentola, 509;
Lacerta viridis, 555;
Chameleons, 572
{657}Egg-sac, of Salamandrella, 110
Egg-tooth, of Lacertilia, 499
Eimer, on habits of Lacerta, 552;
on L. coerulea, 558
Elachistodon westermanni, 625
Elachistodontinae, 625
Elaphis s. Coluber, 615 f.
Elapinae, 626
Elaps corallinus, 635
Elasmosauridae, 478
Elasmosaurus, 478
Elosia, 212
Emerald Lizard, 555
Emery, on hand-skeleton of Eryops, 286
Empedias molaris, 308
Emyda, 411
Emys, 350 f.;
E. blandingi, 355;
E. europaea = orbicularis, 351 f., 353
Enaliosauri, 476
Endothiodon, 307
E. carolinense, 232
Enhydrina valakadien s. bengalensis, 636, 636
Enygrus, 601
Epichordal type of vertebrae, 20, 145
Epidermis, of Amphibia, 31 f.;
sense-organs in, 33
Equisetum, eaten by Uromastix, 525
Eremias, 551;
deserticolous, 493
Erpetosuchus, 433
trunk-vertebrae, 286, 288, 304;
E. megacephalus, 286
Eryx, 604;
E. jaculus, 604;
deserticolous, 493
Escuerzo = Ceratophrys, 216
Espada, on Rhinoderma, 228
Eublepharinae, 512
Euprepes vittata, 562
Euproctus = Triton, 130
Eurysternum, 380
Eustachian tubes, of Anura, 29;
of Pelobatidae, 161;
of Aglossa, 143
Eusuchia, 434
Eye, of Apoda, 86;
of Chelonia, 329;
of deserticolous reptiles, 494;
of Chameleons, 569;
of Snakes, 583
transparent in Chelodina, 329;
lower, transparent in Lacertidae, 551;
in Scincidae, 560
Fasting, of Chrysemys, 347
Fat-bodies, of Amphibia, 49, 52;
of Lacertilia, 500
Fecundation, various modes of, in Amphibia, 54;
in Apoda, 87
Fer-de-Lance, 647
Ferreiro = Hyla faber, 196 f.
Feylinia, 564
Fingers, number of, in Urodela, 15;
number of joints in Anura, 26, 27;
terminal modifications of, in Anura, 26;
mechanism of adhesive discs in Hylidae, 187
Fire Salamander, 115
Firmisternal shoulder-girdle, 24, 25
Firmisternia, 140
Fischer-Sigwart, on growth of Alytes, 159 f.;
on growth of Bufo, 175;
on gestation of Chalcides, 563
Fletcher, on spawning of Australian frogs, 201, 223
Flower, S. S., on habits of Rhacophorus, 249;
Phrynella pollicaris, 233;
Callula pulchra, 234
Flying Dragon, 516
Flying Frog, Rhacophorus, 245 f., 246
Foot, tridactyle, in Hallopus, 423;
bird-like in Compsognathus, 423
Fore-limb, of Urodela, 15;
of Anura, 26;
of Proreptilia, 286;
of Microsauri, 289;
of Theromorpha, 302;
of Chelonia, 320;
of Dinosauria, 414, 423, 425, 427;
of Crocodilia, 440;
of Plesiosauria, 475;
of Ichthyosauria, 481;
of Pterosauria, 485;
of Pythonomorpha, 489;
of Lacertilia, 497
Frog, see Rana.
Grassfrog, 251;
Gage, on Triton viridescens, 129
Galapagos Islands, tortoises of, 372, 377 f.
Galesaurus, 307
Gampsosteonyx, 271;
Gasco, on spawning of newts, 124
Gastrocentrous vertebrae, defined, 282
Gaupp, on frogs' respiration, 47 n.
Gavialidae, 451 f.
G. gangeticus, 452;
Gavialosuchus, 453
Gecko, 511;
G. stentor, 511;
G. verus = guttatus = verticillatus, 511
Geckolepis, deserticolous, 493
Geckones, 502 f.;
voice, 506;
reproduction of tail, 506;
Geckonidae, 507 f.
Geckoninae, 507 f.
Gegenbaur, on classification, 9
{658}Geikia, 310
Genital organs, of Amphibia, 48 f., 49
Genyophryne, 236;
G. thomsoni, 236
Genyophrynidae, 141
Geographical distribution, principles of, 69 f.;
regions and sub-regions, 74 f. (for details see also Maps);
of Apoda, 89;
of Anura, 143, 161, 167, 185, 239;
of Chelonia, 331 f., 332, 333;
of Crocodilia, 446;
of Lacertilia, 500 f., 515, 529, 543, 552, 565, 568;
of Snakes, 585
Geomolge, 96
Geosaurus, 451
Geotriton, 97
Geotrypetes, 89
Gerrhonotus, 538;
G. coeruleus, 538
Gerrhosaurus flavigularis, 559
Gharial, 452;
see also Gavialis
Gigantic Tortoises, 372 f.
Gila Monster, 541
Gills, definition, 40;
retention of, 40;
external and internal, 43 f.;
operculum of, 44;
of Nototrema, 203
Gill-clefts, 42;
of Urodela, 42;
of Anura, 42
Girtanner, on musical appreciation of tortoises, 368
Glass-Snake, 538
Glauconia, 594
Glyphoglossus, 225, 226, 228, 233;
G. molossus, 233
Goeldi, on Hyla faber, 197;
on habits of Podocnemis expansa, 397 f.
Gondwanosaurus, 83
Gongylus, 562
Goniopholidae, 453
G. crassidens, 453;
G. simus, 453
skull, 280
Grass-Snake, 608 f.
Greek Tortoise, 365 f.
Green Lizard, 555
Green Toad, 180
Green, or Edible, Turtle, 381 f.
Groenberg, on Pipa, 149
Growth, rate of, in Testudo ibera, 370;
Chrysemys picta, 349;
Gular shields of Chelonia, 315
Gundlach, on Leptodactylus, 219
Günther, 140;
on gigantic Tortoises, 374;
on classification of Snakes, 592
Gutzeit, on horny teeth of Tadpoles, 58
Gymnodactylus, tail, 506, 512, 512;
deserticolous, 493
Gymnophiona, 84 f.
Gymnophis, 90
Gymnophthalmus, aberrant scaling, 495
Haast, on habits of Sphenodon, 299
Hadrosaurus mirabilis, 429
Haeckel, on classification, 9
Hallopus victor, 423
Hamadryad, 632
Hand-skeleton, excalation of second finger in Eryops, 286
Haptoglossa, 96
Hardun = Agama stellio, 521
Hatteria–see Sphenodon, 293 f.
Hawksbill-Turtle, 384 f.
Hay, on Sphargis, 337
Hearing of Chelonia, 330
Heart, modification of, in lungless Amphibia, 47
Hedonic glands (ἡδονή, lust), 443
H. albopunctatus, 222;
H. pictus, 222
Helix virgata, eaten by Hyla coerulea, 200
Heloderma horridum, 540;
Hemidactylus turcicus, 508, 508
Hemiphractus, 210
shoulder-girdle, 25;
H. guttatum, 232;
H. sudanense, 232
Hensel, on Bufo marinus, 179;
on tadpoles of Thoropa, 209;
on nest-building of Leptodactylus, 219
Herodotus, on Crocodiles, 462
Herpele, 90
Herpestes griseus (Mongoos), 629
Hibernation, temperature of blood during, 68;
of Tortoises, 347, 349, 354, 358, 360, 363, 365, 369, 376;
of Crocodiles, 447
Hinckley, on tadpoles of Hyla versicolor, 195
Hind-limbs, of Urodela, 15;
of Anura, 27;
of Prosauria, 289;
of Chelonia, 321;
of Dinosauria, 414, 423, 425, 427, 429;
of Crocodilia, 440;
of Plesiosauria, 476;
of Ichthyosauria, 480;
of Pterosauria, 486;
of Lacertilia, 497;
Hipistes hydrinus, 625
Holbrook, on the Black Snake, 613;
on habits of Alligator, 470 f.
Holoblastic eggs;
the whole mass of the egg undergoes the process of cleavage, 53
Homalopsinae, 625
Homalopsis buccata, 625
Homing of turtles, instances of, 386
{659}Homoeosaurus pulchellus, 292
Homopholis, deserticolous, 493
Homothermous, defined, 68
Hoplocephalus curtus, 634
Hoplurus, 528;
geographical distribution, 501
Horned Toad = Ceratophrys, 215 f., 216, 217
Horny nail, on tail of Chelonia, 328
Horny scales, of Chelonia, 328
Horny shields, of Chelonia, 314, 315, 322, 323, 326 f., 327;
their growth, 326
Horny teeth, of Anura, 58
Hose, on reproduction of tortoise-shell, 386
Howes, on development of Sphenodon, 298
Humerus of Sphenodon, 294
Hutton, on Starred Tortoise, 370 f.
Huxley, on classification, 9
Hydraspis, 389;
skull, 399
H. tectifera, 404
Hydrophinae, 635
Hydrosaurus, 543
Hyla, 189 f.;
var. meridionalis, 191;
var. savignyi = japonica, 191;
H. aurea, 201 f.;
spawning, 201;
H. carolinensis s. lateralis, 194;
spawning, 223;
H. dasynotus and H. nigromaculata, dermal ossifications of, 190;
H. ewingi, 201;
spawning, 223;
H. faber, peculiar nursing habits, 196 f.;
H. femoralis, 194;
female with eggs, 198;
H. maxima, 196;
H. nebulosa s. luteola, 197;
nest-building, 198;
H. polytaenia, 198;
H. squirella, 194;
H. vasta, 195;
H. versicolor, 194 f.
Hylaeformes, 139
Hylaeobatrachus croyi, 83
Hylaeosaurus, 425
mechanism of climbing, 187;
map of distribution, 185;
distribution, 186
Hylodes, 212;
H. martinicensis, 214 f., 214;
H. abbreviatus = Thoropa miliaris, 209
Hyloplesion longicostatum, 289
Hylopsis, 212;
H. platycephalus, 224
Hylorhina, 212
Hyoid apparatus, of Urodela, 16;
of Anura, 31;
of Chelonia, 318;
of Chelys, 400;
of Lacertilia, 496
Hyperodapedon gordoni, 292
Hyperolia, 213;
spawning, 223
Hyperphalangeal limbs, of Eusuchia, 441;
of Ichthyosauri, 480
H. alternans, 92;
H. rostratus, 92
Hypsilophodon foxi, 427
Hypsirhina plumbea, 625
Iberian Water-tortoise, 357 f.
Ichthyodea, distribution of, 95
Ichthyophis, skull, 85, 88, 89 f., 91;
I. monochrous, 90
Ichthyopterygia, 476
Ichthyosauri, 483 f.
Ichthyosauria, 478 f.;
vertebrae, 480;
limbs, 481;
shoulder-girdle, 481
Ichthyosaurus, 483;
I. communis, 483;
I. campylodon, 483;
I. quadriscissus, 483;
I. tenuirostris, 483;
I. trigonodon, 483
Idiochelys, 380
I. tuberculata, 531
I. mantelli, 427
Ihering, on breeding habits of Phyllomedusa, 205 f., 206
Ikeda, on nursing habits of Rhacophorus, 248
Ilysia, 595
Inframarginal shields, 326, 315
Intergular shields of Chelonia, 325, 315
Iris, colour of, in Chelonia, 329
Jaw, lower, of Salamandra, 17;
of Urodela, 18;
of Anura, 30
Keller, quoted, 571 n.
K. crassum, 81
Kidneys of Amphibia, 48 f., 49
Klinckowstroem, on Pipa, 149
Kollmann, on Neoteny, 64
Krait, 633
Labial glands of Heloderma, 498
Labyrinthodon, 83
Labyrinthodonta, 82
Lacerta, 553;
L. agilis, 554;
L. muralis, 557;
L. pater, 556;
L. schreiberi, 555;
L. tangitana, 556;
L. viridis, 555;
skull, 550;
L. vivipara, 553
Lacertae, 513 f.
skull, 550;
distribution, 552
{660}Lacertilia, 491 f.;
skeleton, 494 f.;
skin, 497;
change of colour, 498
Lachesis gramineus, 647;
Land-tortoises, 364 f.
Lanthanotus borneensis, 541
Laosaurus, 427
L. balsami, 477
Larvae, of Ichthyophis, 91;
of Hypogeophis, 92;
of Amblystoma, 112;
of Triton waltli, 131
Latreille, on classification, 7, 8
Laurenti, on classification, 7
Leathery Turtle = Sphargis, 333 f., 334
Lechriodonta, distribution of, 95;
defined, 102
Lepidophyma, 547
Lepospondylous, defined, 79
Leptobrachium, 161;
L. carinense, 166
L. albilabris, 219;
L. mystacinus, 219;
L. ocellatus, 219;
L. typhonius, 219 f.
Leptognathus, 624
Leptophis, 618;
Leslie, on Xenopus, 146
Leuckart, on classification, 8
Leydig's duct, defined, 48, 49
Lialis burtoni, 567
Eryops, 286;
Microsauri, 289;
Prosauri, 291;
Sphenodon, 298;
Theromorpha, 302;
Pareiasauri, 305;
Sphargis, 335;
Chelonidae, 379;
Compsognathus, 423;
Stegosauri, 426 f.;
Eusuchia, 440;
Lariosaurus, 477;
Ichthyosauria, 480;
Lacertilia, 495;
Geckones, 505;
Chameleons, 568;
reduction of, in Lacertilia, 497;
spawning, 223
Limnomedusa, 212
Linnaeus, on classification, 7
Liodon haumuriensis, 490
Liolepis belli, 527
Liopelma, 153;
L. hochstetteri, 160
Liosaurus, 529
Lissamphibia, 84 f.
Lizard, Common English, 553;
Emerald, 555;
Green, 555;
Sand, 554;
Wall, 557
Lizards, 491 f.
Locality, sense of, in Tortoises, 368, 387
Loggerhead Turtle, 387;
individual varieties of shields, 327, 388
Longevity, of Testudo daudini, 376;
T. graeca, 369;
T. ibera, 369;
T. sumeirei, 377
Loxocemus bicolor, 598
Loxomma, 83
Lucilia bufonivora, fly infesting Bufo, 176
Lungs, definition, 40;
suppression of, 46;
of Aglossa, 144;
of Lacertilia, 499
Luth, or Leathery Turtle, 333 f., 334
Lycosaurus, 307
Lygosoma, distribution, 501
Lymph-spaces, in the cutis of Anura, 34
Lyriocephalus scutatus, 517, 518
Mabuia, 562;
distribution, 501;
eyelids, 494;
M. vittata, 562
Macroclemmys, 326;
Macroprotodon cucullatus, 624
Macrorhynchidae, 451
Madagascar, Lacertilia of, 502
Malacoclemmys terrapin, 359 f.;
commercial breeding-farms, 360
Malpighian, body, 49;
stratum, 32
Mammalian affinities of Theromorpha, 303, 309
M. quadridigitatus, 106
Mandible, composition of, in Crocodiles, 437;
very Mammalian in Gomphognathus, 309
Mantella, 274
Maps showing geographical distribution, of Coeciliidae, 89;
Urodela, 95;
Aglossa, 143;
Cystignathidae, Discoglossidae, Pelobatidae, 161;
Bufonidae, 167;
Hylidae, 185;
Ranidae, 239;
Chelydridae, 332;
Cinosternidae, 332;
Dermatemydidae, 332;
Pelomedusidae, 332;
Platysternidae, 332;
Trionychidae, 333;
Crocodilia, 446;
Geckonidae, 503;
Agamidae, 515;
Anguidae, 529;
Iguanidae, 529;
Zonuridae, 529;
Varanidae, 543;
Lacertidae, 552;
Amphisbaenidae, 565;
Chamaeleontes, 568;
Snakes, dangerously poisonous, 585;
Elapinae, 626;
Crotalinae, 644;
Viperinae, 638
Marbled Newt, 126
Marginal plates of Chelonia, 325, 322, 323
Marginal shields, 326
Marsh, on Axolotl, 115
Marsh Crocodile, 455
Marshall, on distribution of Uropeltidae, 595
Mascarene Islands, tortoises of, 373 f.
Mason, on habits of Calotes, 519;
on Python legends, 599;
on Varanus, 544
Mastodonsaurus, 83
Matamata = Chelys fimbriata, 400, 401
Mauritius, gigantic tortoises, 373 f., 376
{661}Mecodonta, distribution of, 95;
defined, 102
Megalophrys, 161;
tadpole, 60
Megalosaurus, 416;
M. bucklandi, 421
Megalotriton, 83
Melosaurus, 287
Menobranchus lateralis, 132
Menopoma, 97
Mento-Meckelian cartilages, 30
Meroblastic eggs; part of the egg only undergoes the process of cleavage, 53
Merrem, on classification, 8
Mesosauridae, 476
Mesosaurus, 476;
M. tenuidens, 476
Metamorphosis of Tadpoles, 56 f.
taxonomic value, 141;
definition, 26
Metatarsalia of Theropoda, 420
Metopias, 83
Metopoceros cornutus, 532
Metriorhynchidae, 451
Metriorhynchus, atlas and axis, 283, 431, 451
Metzdorff, on Axolotl, 113
Micrixalus, 241
Microhyla, 228
Microsauri, 288
Midwife-toad, 158
Mimosa (plant), 629
Miolania, 390
Mixophyes, 213;
spawning, 213
Mixosaurus, limbs, 480, 481, 483
Molge–see Triton, 122
Mongoos and Cobra, 629
Monitor, 543
Morosaurus grandis, 419;
pelvis, 419
Mosasauri, 489 f.
Mosasaurus, 489;
M. camperi, 490
Moult of Geckos, 510;
of Chameleons, 571;
of Snakes, 583
Mud-diver, 165
Mud-turtle, 342
Mugger, 454
Müller, J., on classification, 8
Musical appreciation of Tortoises, 368
Nails or claws of Amphibia, 32
Naja, 626;
N. bungarus s. elaps, 632;
Naosaurus claviger, 308
Natterjack, 181
Naultinus elegans, 506
Neck, mode of withdrawing in Chelonia, 328 f.
N. subasper, 169
N. afra, 169;
N. tuberculosa, 169;
N. guentheri, 169;
N. hosei, 169;
N. misera, 169
Necturus, pelvis, 15, 96, 132;
N. maculatus, 132
Neoteny, 63 f.;
defined, 64
Nephrurus asper, tail, 506
Nerves, spinal, of Amphibia, 38;
cranial, 39
Nest, of Crocodilus, 463;
of Gavialis, 452
Neural plates, of Chelonia, 323 f., 322, 323;
suppression of plates, 324;
in Pleurodira, 389;
of Dermatemys, 342
Neusticosaurus, 477
Newt, Common, 127;
Marbled, 126;
Spotted, 127
Newton, E. T., on fossil Reptiles, 303 n.
Nile Crocodile, 461
Nodosaurus, 430
Nose-horned Viper, 640
N. bennetti, 167
Notechis scutatus, 634
Nothosauri, 476 f.
Nothosauridae, 477
Nothosaurus, 474;
N. mirabilis, 477
Notocentrous vertebrae, defined, 19
Notochord = Chorda dorsalis, q.v.
Nototrema, 189;
N. cornutum, 203;
N. marsupiatum, 202;
N. oviferum, 202;
peculiar gills of embryos, 203;
N. pygmaeum, 202;
N. testudineum, 202
Nuchal plate of Chelonia, 323 f.;
of Pleurodira, 389
Nuchal shield of Chelonia, 326, 327;
Nuptial excrescences of Anura, 33
Nursing, habits, of Arthroleptis seychellensis, 243;
of Chiromantis rufescens, 244;
of Rhacophorus, 248;
of Rhinoderma, 228 f.;
of Pipa, 151;
of Hyla faber, 196 f.;
of H. nebulosa, 198;
of H. goeldii, 198;
of Nototrema, 203;
of Phyllomedusa, 204 f.;
of Leptodactylus, 219 f.;
of eggs by Desmognathus, 103, 103;
by Autodax, 108
Nyctibatrachus, 240
Nyctimantis rugiceps, 189, 206
Nyctixalus, 238
Occipital condyle, of Reptilia, 278;
exaggerated importance of, 285;
of Theromorpha, 302;
of Pareiasauri, 305;
of Cynognathus, 307;
of Crateronotus, 307;
of Dicynodon, 310;
of Eusuchia, 437;
of Amphisbaenidae, 496
Odontaglossa, 140
Oligodon, dentition, 593 n.
{662}Omosaurus = Stegosaurus, 425
Omosternum of Anura, 25;
taxonomic value, 141
Onychodactylus, 96;
O. japonicus, 109
Operculum of gills, 44
Ophiderpeton, 81
Ophiophagus, 632
Ophiops, 551
Ophioxylon (plant), 629
Ophisaurus, 538;
O. apus, 538;
O. gracilis, 538
Ophthalmosaurus, limbs, 481, 481, 484
Opisthocoelous, definition, 12
Opisthoglossa, 140
Opisthoglypha, 592, 606 f., 623 f.
Oppel, on classification, 7
Oreobatrachus, 241
Oreophrynella, 227
Ornithocheirus, 486
Ornithomimus, 417;
O. grandis, 429
Ornithopsis, 419
Ornithoscelida, 416
Ornithosuchus, 433
Orthopoda, 424
Ossifications, dermal, in Anura, 31, 34, 179, 190, 210, 211
Osteoderms = ossifications in the skin, of Sphargis, 337;
of Caiman, 337;
Osteolaemus, 450;
O. tetraspis, 466
Ouaran, 543
Oudenodon, 301;
O. rugosus, 310
Ovary, 49
Owen, on fossil Reptiles, 303 n.
Oxydactyla, 140
P. brevipes, 132
rate of growth, 349
Palaeobatrachus, vertebral column, 22, 145
Palaeohatteria longicaudata, 291;
Paludicola, resembles Engystomops, 166, 212, 220;
P. fuscomaculata, 220;
P. biligonigera, 221
Paludina, as food of Trionyx, 407
Parasternum = the sum total of the Abdominal ribs, q.v.;
of Sphenodon, 298;
of Crocodilia, 440;
of Ichthyosauria, 480
Parasuchia, 433
Pareiasaurus baini, 304
Parrots, feathers dyed with poison of Dendrobates, 272
Pelobates, variation of vertebrae, 19;
P. fuscus, 162;
P. syriacus, 164
distribution, 161
P. punctatus, 165;
P. caucasicus, 166
Pelomedusa galeata, 391
Pelosaurus, 81
Pelvic, plexus of Anura, 39
Pelvis, of Urodela, 15;
of Eryops, 286;
of Microsauri, 289;
of Sphenodon, 298;
of Theromorpha, 302;
of Pareiasauri, 305;
of Cynognathus, 307;
of Dicynodon, 310;
of Dinosauria, 414;
of Eusuchia, 441;
of Plesiosauria, 476;
of Ichthyosauria, 480;
of Pterosauria, 485;
of Pythonomorpha, 489;
of Lacertilia, 496
not a natural group, 65
Petrels living with Sphenodon, 299
Phalanges, number of, in Urodela, 15;
in Stegocephali, 79;
in Palaeohatteria, 291;
in Chelone, 379;
in Scelidosaurus, 425;
in Camptosaurus, 427;
in Laosaurus, 427;
in Iguanodon, 428;
in Eusuchia, 441;
in Plesiosauria, 475;
in Lariosaurus, 477;
shape in Anura, 138;
peculiar in Pipa, 151
Phaneroglossa, 152
Phanéroglosses, 139
Phanerotis, 213
Phisalix, on poison of Amphibia, 37
Pholidosaurus, 451
Phractamphibia, 78 f.
Phrynaglosses, 139
Phrynella, 227;
Ph. pollicaris, 233
Ph. nigricans, 230
Phrynobatrachus, 241
Phrynocephalus, 521;
deserticolous, 493;
coloration, 494;
Ph. helioscopus, 522;
Ph. interscapularis, 522;
Ph. mystaceus, 522
Phrynoderma, 241
Phrynopsis, 241
Phyllobates, 242;
Ph. bicolor, 242;
Ph. trinitatis, 242
Phyllodactylus, 507;
Ph. europaeus, 507
Ph. bicolor, 203;
Ph. dacnicolor, 203;
{663}Ph. hypochondrialis, breeding habits and development, 204;
Ph. iheringi, 205;
breeding habits, 206
Phylogeny, of Amphibia, 66;
of Anura, 142 f.;
of Reptilia, 282;
of Lacertilia, 515;
of Ophidia, 592
Physignathus lesueuri, 523, 524
Pigment in the skin, 34
Pit-Vipers, 644
Placodontia, 311
Placodus, 301;
P. gigas, 311
Plastron, of Chelonia, 315, 321, 321;
provided with hinges, 323;
sexual characters of, 331;
movable in Emys, 350;
of Chelonidae, 321, 321, 322, 380;
of Pelomedusidae, 390;
of Chelydidae, 399;
of Trionychoidea, 406
Platecarpus, 490
Platemys, suppression of neural plates, 324;
skull, 399
Plathander = Xenopus, 146 f.
Platurus fasciatus s. colubrinus, 637
Platydactyla, 140
Platydactylus facetanus, 509, 508
Platyhyla, 236
Platysternum megacephalum, 345
Plectromantis, 212
Plesiosauri, 477 f.
Plesiosauria, 473 f.;
vertebrae, 474
Plesiosauridae, 478
P. conybeari, 478;
P. dolichodirus, 478
P. erythronotus, 107;
P. glutinosus, 106
Pleurosaurus, 294
Pleurosternum, 390
Plioplatecarpus, 489
Pliosauridae, 477
Pliosaurus grandis, 477
Plover, Egyptian, and Crocodile, 462
P. expansa, 391 f.;
Bates, on habits of, 392 f.
Poikilothermous, defined, 67
peculiar use of, 272
Poison-apparatus, of Heloderma, 540;
of Snakes, 586 f.
Polacanthus, 425
Polychrus marmoratus, 529
Polyodontophis, 605 n.
Portschinsky, on parasitic flies, 177 n.
Postpubis, of Dinosaurs, 414, 424, 426
Pouchet, quoted, 571
Predentary bone, of Dinosaur, 424
Prehallux, of Anura, 28
Prepubis, of Dinosaurs, 414, 424, 426
Proganochelys, 389
Proganosauria, 476
Proreptilia, 285
Prosauri, 290
Prosauria, 288
Prostherapis, 242
Protection of Amphibia by poison, 38
Proteroglossa, 140
Proteroglypha, 625
Proteus, 96;
Protorosaurus lincki, 291
Protosphargis, 336
Protostega, 336
Psammodromus hispanicus, 558;
P. algirus, 558
Psammosaurus, 543
Psephoderma, 337
Pseudechis porphyriaceus, 634
P. paradoxa, 213 f.
Pseudobranchus, 96;
P. striatus, 137
Pseudocentrous, defined, 79
spawning, 223;
P. australis, 168;
P. bibroni, 168
Pseudopus, aberrant scaling, 495;
P. pallasi, 538
Pseudosphargis, 336
Pseudosuchia, 432
Ptenopus, 507;
deserticolous, 494
Pteranodon longiceps, 487
Pteranodontes, 487
P. fodiens, 207
Pterodactyli, 486
Pterodactylus longirostris, 487;
P. spectabilis, 487
Pterosauri, 486
Pterosauria, 484 f.
Ptyas = Zamenis, 611
Ptychozoon, tail, 506;
adhesive apparatus, 505
Pubis, of Dinosaurs, 414, 424, 426
Pygopus lepidopus, 567
Python, 598;
P. regius = P. sebae, 601;
P. reticulatus, 598;
Pythoninae, 598 f.
Pythonomorpha, 487 f.
Pyxis arachnoides, 365
Radde's "law of the steppe," 493
sacral vertebrae, 22;
shoulder-girdle, 25;
urino-genital organs, 49;
Tadpoles' horny teeth, 58;
vocal sacs, 250;
nuptial excrescences, 250;
large glandular complexes, 250;
distribution, 251;
species with finger-discs, 250;
{664}R. afghana, 250;
R. agilis, 257;
R. albolabris, 250;
R. alticola, 250;
R. arvalis, 257;
R. catesbiana, 261;
R. chalconota, 250;
R. chloronota, 250;
R. corrugata, 250;
R. curtipes, 250;
R. cyanophlyctis, 250;
R. elegans, 250;
R. erythraea, 250;
R. esculenta, 263;
mechanism of tongue, 268;
vocal sacs, 269;
var. chinensis, 267;
var. lessonae, 265;
var. ridibunda, 264;
var. typica, 265;
R. fontinalis, 262;
R. glandulosa, 250;
R. gracilis, 261;
R. graeca, 259;
R. guppyi, 261;
R. halecina, 263;
R. hexadactyla, 250;
R. iberica, 258;
R. latastei, 259;
R. liebigi, 250;
R. mascareniensis, 250;
R. montezumae, 250;
R. mugiens, 261;
R. opisthodon, 260;
R. oxyrhynchus, 250;
R. rugosa, 250;
R. silvatica, 259;
R. temporalis, 250;
R. tigrina, 261
Ranidens, 96;
R. sibiricus, 109
distribution, 239
Rappia, 241
Rattle of Rattle-Snake, 644
Rattle-Snake, 648 f., 648, 650
Reduction of limbs, in Urodela, 16;
in Lacertilia, 497
Regeneration, in Amphibia, 66 f.;
of tail in Sphenodon, 298;
of shell in Chelonia, 329;
of horny shields in Chelonia, 329, 386;
of tail in Lacertilia, 495;
of tail in Geckos, 506
Regions, geographical, 74 f.
Reproduction of Tortoise-shell, 386
Reptilia, defined, 277;
principal characters of, 278;
classification of, 279;
diagram of affinities of principal groups, 282;
affinities to Mammalia, 303, 309
Respiration, mode of, in Chelonia, 331;
assisted by anal sacs, 330
Respiratory organs, of Amphibia, 40
Rhachiodontinae, 622
Rhacophorus, 151, 186, 238, 241, 244, 246;
Rh. leucomystax, 247;
tadpoles, 249;
Rh. maculatus, nesting, 248;
Rh. madagascariensis, 245;
Rh. maximus, 245;
Rh. reinwardti, 247;
Rh. reticulatus, 248;
Rh. schlegeli, nesting, 248
Rhampholeon spectrum, 580
Rhamphorhynchus longicaudatus, 486;
Rh. phyllurus, 486;
Rh. muensteri, 487
Rhamphosuchus crassidens, 453
Rhinatrema, 89
Rhinochelys, 390
Rh. darwini, 228 f.
Rhinophis, 91;
Rh. sanguineus, 596
Rhinophrys, 167
Rhynchocephali, 292
Rhynchosaurus, 292
Rhytidosteus, 83
Ribs, of Urodela, 14;
of Anura, 21;
of Microsauri, 288;
of Sphenodon, 297;
of Theromorpha, 302;
of Cynognathus, 307;
of Microgomphodon, 309;
of Dinosauria, 413;
of Crocodilia, 438;
of Parasuchia, 434;
of Eusuchia, 439;
of Lacertilia, 495;
of Geckones, 504;
much elongated in certain Iguanidae, 529;
meeting ventrally in Chameleons, 568
Ridewood, on hyoid apparatus of Anura, 31
Ritter and Miller, on Autodax, 107
Robinson, on peculiar use of Varanus, 546
Rodriguez, gigantic tortoises, 374
Rostral bone of Ceratopsia, 430
Round Island snake, 603
Sacral vertebrae of Anura, 21, 22
Salamandra, 115 f.;
trunk-vertebra, 14;
skull, 17;
lower jaw, 17;
S. atra, 119 f.;
S. caucasica, 121;
S. maculosa, 115 f.
S. keyserlingi, 109;
S. schrenki, egg-sac, 110
S. perspicillata, 122;
skull, 17
Sarasin, P. and F., 10;
on Coeciliae, 88;
on Ichthyophis, 90
Sauria, 491 f.
Saurichnites salamandroides, 83
Sauropoda, 418
Sauropterygia, 476
Saurosternum, 291
Scales of Apoda, 87
Scaling, aberrant, 495
S. solitarius, 165
Scapteira, deserticolous, 494
Scapula, attached to thoracic vertebrae, 487
Scelidosaurus, 416;
S. harrisoni, 425
Scheuchzer, on Homo diluvii testis, 84
Schlegel, on Cryptobranchus, 100
Schuberg, on mechanism of finger-discs of Hylidae, 187
Schwalbe, on Salamandra atra, 120
{665}Scincus officinalis, 561
Sciurus bicolor, squirrel, 248
Scolecomorphus, 90
Sea Snakes, 635
Seeley, on fossil Reptiles, 303 n.
Segmental duct, 49
Sense-organs, of Chelonia, 329 f.;
of Crocodiles, 445 f.
Seps, 562
Seychelles, gigantic tortoises of, 373
Shell of Chelonia, 321 f., 319, 320, 321, 322, 323, 327;
partial regeneration of, 329;
correlated changes, 328
Shields, horny, of Chelonia, 322, 323, 325 f., 327;
evolution of, 326 f.;
individual variation in, 326, 327;
periodical peeling of, 328
Shoulder-girdle, of Urodela, 14;
of Aglossa, 144;
of Microsauri, 289;
of Protorosauri, 290;
of Theromorpha, 302;
of Pareiasauri, 305;
of Dicynodon, 310;
of Dinosauria, 414;
of Eusuchia, 440;
of Plesiosauria, 474;
of Cryptoclidus, 475;
of Pterosauria, 485;
of Pteranodon, 487;
of Pythonomorpha, 488;
of Lacertilia, 496
Shufeldt, on Axolotl, 114;
on Heloderma, 540 n.
Simosaurus, 477
Siredon (Axolotl), 112
Siren, 96;
Sistrurus miliarius, 647
Skeleton, figured, of Testudo, 319;
of Chelone, 320;
of Brontosaurus, 418;
of Ceratosaurus, 422;
of Stegosaurus, 426;
of Iguanodon, 428;
of Triceratops, 430;
of Pterodactylus, 485
Skin, of larval Amphibia, 31;
shedding of, 32;
glands, 32;
pigment, 34;
change of colour, 35;
poison, 36;
of Apoda, 87;
of Pipa, 149;
forms receptacles for eggs, 151, 248;
of Eusuchia, 441 f.
Skin-glands, of Crocodiles, 443;
of Lacertilia, 497;
of Geckones, 504;
of Snakes, 583
of Amblystoma, 17;
of Salamandrina, 17;
of Salamandra, 17;
of Anura, 28 f.;
–of Proreptilia: Cricotus, 287;
Eryops, 286:
–of Microsauri, 289:
–of Protorosauri, 280;
–of Rhynchocephali (Sphenodon), 280, 295, 295:
–of Theromorpha, 280, 301, 303;
Theriodontia, 306;
Mammalian resemblances, 308 f.;
Lycosaurus 307;
Endothiodon, 307;
Gomphognathus, 308;
Oudenodon, 310;
Placodus, 311:
–of Mammalia, generalised, 281:
Sphargis, 335;
Thalassochelys, 379;
Clemmys, 356;
Testudo, 364;
Pelomedusidae, 390;
Anchisaurus, 421;
Ceratosaurus, 422;
Diplodocus, 419:
–of Crocodilia, 280;
Pseudosuchia, 432;
Parasuchia, 433;
Eusuchia, 434 f.;
Gavialis, 452;
Crocodilus americanus, 466;
C. niloticus, 460;
C. palustris, 455;
C. porosus, 458;
Alligator, 468:
–of Plesiosauria, 473;
Nothosaurus, 477:
–of Ichthyosauria, 479;
Ichthyosaurus, 281:
–of Pterosauria, 484;
Dimorphodon, 281:
–of Pythonomorpha, 488;
Clidastes, 281:
–of Lacertilia, 281;
Geckones, 504;
Uromastix, 281;
Iguanidae, 528;
Anguidae, 537;
Helodermatidae, 540;
Varanus, 281;
Tejidae, 547;
Lacerta, 281;
Scincidae, 559;
Amphisbaenidae, 565;
–of Ophidia, 281, 596, 597, 588;
Crotalus, 588
Slugs eaten by tortoises, 363
Smell, sense of, of Chelonia, 330
Smith, the, = Hyla faber, peculiar nursing habits, 196 f.
Snakes, 581 f.;
skull, 581 f., 281, 588, 596, 597 f.;
vertebrae, 582;
general anatomical structure, 583 f.;
geographical distribution, 585 f., 585;
classification, 592 f.
Snake-charming, 631
Snake-poison, 586 f.
Snake-stones, 629 f.
Snapping Turtle, 338 f.
Soft-shelled Turtle, 408
Sound produced by rubbing of scales of Teratoscincus, 507
Spade-foot, 162
Spelerpes, 94, 96, 97, 103, 104, 106;
S. altamazonicus, 104;
S. bilineatus, 104;
tongue, 106;
S. infuscatus, 104;
S. lineolus, 104;
{666}S. parvipes, 104;
S. porphyriticus, 105;
S. salmoneus, 105;
S. subpalmatus, 104;
S. uniformis, 104
Spencer, on habits of Chiroleptes, 221 f.
Spermatozoa of Amphibia, 52 f.
affinities, 336;
morphology of shell, 337
Sphargis coriacea, 333 f., 334;
absence of horny shields, 325
Sphenodon, 288, 290, 305, 306, 432;
skull, 295;
cervical vertebrae, 297;
habits, 298 f.
Spiny-tailed Lizard, 524 f.
Spiracle, development, 45
Spotted Newt, 127
Spy-Slange, 632
St. Helena, gigantic tortoises introduced, 375
Stagonolepis, 434
on vertebrae of Pelobates, 20
Staurotypus salvini, 342
Stegocephali, 78 f.;
St. Lepospondyli, 80 f.;
St. Temnospondyli, 81 f.;
St. Stereospondyli, 83 f.;
vertebrae, 78 f.;
shoulder-girdle, 79;
dermal armour, 79
Stegosauri, 425
Stegosaurus armatus, 425;
Stenodactylus, deserticolous, 494;
sleeping attitude, 509
S. incrassatus, 231
Stereorhachis, 308
Stereospondylous vertebrae, defined, 284
S. derbianus, 391;
shields of, 327
Sternum, of Urodela, 15;
of Anura, 25;
of Sphenodon, 297 f.;
Protorosauri, 290;
Dinosauria, 414;
Eusuchia, 440
Stewart, quoted, on Heloderma, 540 n.
Stinkpot Terrapin, 342
Suboccipital (first spinal nerve) of Anura, 144
Subregions, geographical, 74 f.
Syrrhopus, 212
Systomata, 139
Tadpoles, horny teeth of, 58 f.;
absorption of tail, 61 f.;
of Bombinator, 157;
of Alytes, 159;
of Hyla arborea, 193;
of H. versicolor, 195;
of Bufo viridis, 181;
of B. calamita, 183;
of B. vulgaris, 176;
of Thoropa miliaris, 209;
of Pseudis paradoxa, 213;
of Hylodes martinicensis, 214;
of Rhinoderma darwini, 229;
of Arthroleptis seychellensis, 243;
of Rana temporaria, 255;
of R. opisthodon, 260;
of R. esculenta, 270
its absorption, 61;
of Chelonia, 328;
of Geckos, various shapes, 506;
reproduction of, 506
Tarentola mauritanica, 508, 509 f.
Tarsus (see also Limbs), of Chelonia, 319, 320, 321;
of Dinosauria, 416, 418, 420, 421, 423, 426;
of Theropoda, 420;
of Compsognathus, 423;
of Iguanodon, 428
Teeth, of Anura, 30, 138, 139;
substitutes for, 30, 58, 218, 237;
of Apoda, 86;
of Rhynchosaurus, 292;
of Homoeosaurus, 292;
of Rhynchocephali, 292;
of Sphenodon, 296;
of Theromorpha, 301;
of Lycosaurus, 307;
of Galesaurus, 307;
of Endothiodon, 307;
of Empedias, 308;
of Stereorhachis, 308;
of Gomphognathus, 308;
of Tritylodon, 309;
Mammalian resemblances, 309;
of Anomodontia, 309;
of Gordonia, 280;
of Placodus, 311;
of Orthopoda, 424 f.;
of Eusuchia, 437;
of Ichthyosauri, 479;
of Snakes, 582
Teleosauridae, 450
Teleosaurus, 451
Telerpeton elginense, 291
Temnospondylous vertebrae, defined, 284
Temperature of blood, 67 f.;
of water for Crocodiles, 460
Tennent, on immunity of Cobras, 629 f.;
on turtles at Ceylon, 384, 386;
on habits of Crocodilus palustris, 456 f.;
on habits of C. porosus, 459;
on peculiar use of Varanus, 545;
on habits of Gecko, 511
Tentacular apparatus of Apoda, 45, 86, 88
Tephrometopon, 493
Teratoscincus, deserticolous, 493;
eye, 494;
T. scincus, 507
Terrapin, 359 f.
Testis, 49
distribution, 332
Testudo, 365;
shields of, 327;
T. elegans, 370 f.;
T. elephantina, 374;
T. elephantopus, 378;
T. ephippium, 378;
T. gigantea, 374;
T. graeca, 365 f.;
habits, 367;
eggs, 369;
great age, 369;
T. grandidieri, 373;
T. horsfieldi, 370;
T. ibera, 366;
age attained, 369;
rate of growth, 370;
T. marginata, 367;
T. perpigniana, 372;
{667}T. polyphemus, 371 f.;
T. sumeirei, 376;
Tetradactylus, 559;
T. africanus, 559;
T. seps, 559
Tetrapoda, Credner's name for "four-footed" creatures in opposition to the fishes, which have fins, 4, 11
Thalassemydidae, 380
Thalassochelys caretta, individual variation of shields, 326, 327, 387;
skull, 379
Thecophora, definition of term, 337, 338
Theobald, on Varanus, 544
Theriodontia, 306
their affinity to Mammals, 303 f., 309
Theropoda, 420
Thilenius, quoted, 571 n.
Thoracosaurus, 451
Th. pennatulus, 103
Th. miliaris, 209
Tiger Snake, 634
Tiliqua s. Cyclodus, 561
Toad, see Bufo, 169;
Toes, number of, in Urodela, 16;
in Anura, 28;
of Geckos, structure, 505, 505
T. schlegeli, 453
Tongue, of Amphibia, nerve-supply, 39;
shape of, in Anura, 47;
of Spelerpes, 106;
absent in Aglossa, 145;
of Rana esculenta, 268;
of Crocodiles, 443;
of Lacertilia, 498;
of Chameleons, 569 f.
Tortoise, Greek, 365 f.;
habits, 367 f.;
Moroccan, 366;
habits, 367 f.;
Starred, 370 f.;
Gopher, 371 f.;
Gigantic Land-Tortoises, 372 f.
Tortoises = Chelonia, 312 f.
Tortoise-shell of commerce, 386
Trachea, of Crocodiles, 443
Tree-frogs, 185 f.;
change of colour, 35
Triceratops, 413;
T. flabellatus, 430
Trichobatrachus, 240;
T. robustus, 271
Trigonocephalus cenchris, 645, 645, 646, 646
Trimerorhachis, 82
Trionychidae, 313;
distribution, 333
Trionychoidea, 313, 314, 404 f.;
habits, 407
Trionyx, nuchal plate, 324;
skull, 405;
plastron, 406;
number of costal plates, 325;
T. hurum, 410;
T. triunguis, 410
T. petasatus, 207
Trirhachiodon, 309
Triton, 122 f., 96, 115, 125, 128, 131;
fossil, 83;
spermatophores, 53;
T. blasii, 126;
T. hagenmuelleri, 123;
T. helveticus, 127;
T. italicus, 127;
T. montadoni, 127;
T. palmatus, 127;
T. poireti, 123;
T. punctatus = vulgaris, 127;
T. pyrenaeus, 130;
T. taeniatus = vulgaris, 127;
egg, 128;
Tropidonotus, 607;
T. natrix, 608 f.;
T. ordinatus, 611;
T. tesselatus, 611
Tropidosaura, 558
Tuatera, 293
Tupinambis, 548;
T. teguixin, 548;
Turtles, 378 f.;
skeleton, 320;
plastron, 321;
on Laysan Islands, 383;
Green or Edible, 381 f.;
T. andersoni, 130;
T. verrucosus, 132
Tympanic cavity, reduction of, in Anura, 30;
in Ophidia, 583
Tympanum of Aglossa, 143
Typhlomolge, 96;
T. rathbuni, 135
T. compressicauda, 93
Typhlops, 91;
T. braminus, 594;
T. vermicularis, 594
Typhlosaurus, 564
T. spelaeus, 103
Urino-genital organs, 48 f., 49
Urodaeum of Chelonia, 330
Urodela, 94 f.;
geographical distribution, 96
Uromastix, 524;
U. hardwicki, 525
Uropeltis, 595;
U. grandis, 595
Uroplates, 512
Uroplatinae, 512
Urostyle, of Anura, 23;
of Chelonia, 328
skull, 542;
distribution, 543
Varanus, 543;
V. gouldi, 546;
V. griseus, skull, 542;
V. niloticus, 543;
Vertebrae, procoelous, definition, 19, 138;
{668}acentrous, i.e. without a centre or body, 4;
amphicoelous, defined, 12;
of Urodela, 11;
gastrocentrous, defined, 282;
lepospondylous, 5;
defined, 78;
notocentrous, 4;
defined, 19;
opisthocoelous, defined, 12, 138;
stereospondylous, defined, 79, 284;
temnospondylous, 13;
development of–in Urodela, 12, 13;
in Anura, 19;
of trunk of Salamandra, 14;
epichordal, 20;
sacral, of Anura, 22;
shifting forwards of sacral attachment of ilium, 23;
of Reptilia, composition of, 283, 288;
trunk-vertebrae of Eryops, 283, 286, 286;
of Cricotus, 287;
of Microsauri, 289;
atlas and axis of Sphenodon, 283;
of Theromorpha, 302;
of Pareiasauri, 305;
atlas fused with axis in Cynognathus, 307;
of Dimetrodon, with peculiar processes, 308;
of Chelonia, 314 f.;
atlas of Trionyx, 283;
of Chelys, 283;
of Dinosauria, 413;
hollow in Dinosaurs, 415, 420;
of Eusuchia, 438 f.;
atlas and axis of Crocodilus, 283;
of Metriorhynchus, 283;
of Pterosauria, 485;
of Ichthyosauria, 480;
of Pythonomorpha, 488;
of Lacertilia, 494;
of Geckones, 503;
of Snakes, 582
Vertebral column, instance of greatest shortening, 144;
of Stegocephali, 78;
Palaeobatrachus, 22;
Bombinator, 22;
of Apoda, 86;
number of vertebrae of Protorosaurus, 291;
of Palaeohatteria, 291;
of Homoeosaurus, 292;
of Sphenodon, 297;
of Cynognathus, 306;
of Iguanodon, 428;
of Eusuchia, 440;
of Plesiosauria, 474;
of Elasmosauridae, 478
Viper, Common, 641 f., 620, 642
Vipera, 641;
V. aspis, 643;
V. latastei, 643;
V. russelli, 643
Viperinae, 638
Viperine Snake, 610
Vis, de, on Chlamydosaurus, 523
Viviparous, Chameleon, 572;
Lacertilia, 499;
Geckos, 506
Vocal sacs, 47 f.;
of Paludicola, 220;
of Rhinoderma, used as brood-pouches, 228
Voeltzkow, on nesting of Crocodiles, 462 f.;
on Testudo daudini, 375
Voice, 47
Wagler, 8
Wallace, on Rhacophorus, 246 f.
Wall-Lizard, 557
Warning, attitudes, of Bombinator, 157;
of Heloderma, 541
Werner, on Eryx, 604
White's aged Tortoise, 369
Wilder, on Desmognathus, 103
Xantusia, 547
Xenobatrachus, 225;
X. ophiodon, 228
Xenopeltis unicolor, 605
Xenopus, 143;
distribution, 143, 144, 146 f.;
X. calcaratus, 146;
X. muelleri, 146
Xenorhina, 228
Xenosaurus grandis, 536
Zachaenus, 212
Zamenis constrictor, 613;
Z. gemonensis s. viridiflavus, 612;
Z. hippocrepis, 613;
Zander, on habits of Agama, 520;
of Phrynocephalus, 522;
of Eryx, 604
Zatachys, 82
Zeller, on spermatophores, 53;
on Proteus, 134
END OF VOL. VIII
Printed by R. & R. Clark, Limited, Edinburgh.
THE CAMBRIDGE NATURAL HISTORY
EDITED BY
S. F. Harmer, Sc.D., F.R.S. and A. E. Shipley, M.A., F.R.S.
In Ten Volumes. Fully Illustrated. Medium 8vo. 17s. net each.
PROTOZOA, COELENTERATES, ECHINODERMS, etc.
VOLUME I.
Protozoa, by Marcus Hartog, M.A., D.Sc.; Porifera (Sponges), by Igerna B. J. Sollas, B.Sc.; Coelenterata and Ctenophora, by S. J. Hickson, M.A., F.R.S.; Echinodermata, by E. W. MacBride, M.A, F.R.S.
NATURE.–"Taken in conjunction with the earlier published volumes, the work seems to fulfil the purpose of providing an intelligible and adequate survey of the entire animal kingdom without giving undue prominence to particular groups.... The illustrations are excellent."
FIELD.–"The book can be in the strongest manner recommended to those for whose benefit it has been written. We know of no work from which a more truly scientific account of the Protozoa, Echinodermata, and other lower forms of animal life could be gained."
OUTLOOK.–"There is much valuable matter in these well-planned sections which will render the volume, like the others which have preceded it, a necessary book of reference in every well-equipped library."
WORMS, ROTIFERS, AND POLYZOA
VOLUME II.
Flatworms and Mesozoa, by F. W. Gamble, D.Sc.; Nemertines, by Miss L. Sheldon; Threadworms and Sagitta, by A. E. Shipley, M.A., F.R.S.; Rotifers, by Marcus Hartog, M.A., D.Sc.; Polychaet Worms, by W. Blaxland Benham, D.Sc., M.A.; Earthworms and Leeches, by F. E. Beddard, M.A., F.R.S.; Gephyrea and Phoronis, by A. E. Shipley, M.A., F.R.S.; Polyzoa, by S. F. Harmer, Sc.D., F.R.S.
CAMBRIDGE REVIEW.–"Several of the groups treated of in this volume are unknown, by sight even, to the general reader, and possess no popular name whatsoever; and as only a few insignificant details are known of the habits of the animals composing them, their treatment in the volume before us has necessarily been to a large extent anatomical. This circumstance renders the book of especial value to students, more particularly as in some cases the articles on the groups in question are the first comprehensive ones dealing with their respective subjects.... Most of the articles are of a very high order of merit–taken as a whole, it may be said that they are by far the best which have as yet been published.... We may say with confidence that the same amount of information, within the same compass, is to be had in no other zoological work."
NATURAL SCIENCE.–"This second volume of the Cambridge Natural History is certain to prove a most welcome addition to English Zoological literature. It deals with a series of animal groups, all deeply interesting to the specialist in morphology; some important from their economic relations to other living things, others in their life-histories rivalling the marvels of fairy-tales. And the style in which they are here treated is also interesting; history and the early observations of the older writers lend their charm; accounts of habits and mode of occurrence, of life, in a word, from the cradle to the grave, are given in ample detail, accompanied by full references to modern and current literature. The whole is admirably illustrated."
MOLLUSCS AND BRACHIOPODS
VOLUME III.
Molluscs, by the Rev. A. H. Cooke, M.A.; Brachiopods (Recent), by A. E. Shipley, M.A., F.R.S.; Brachiopods (Fossil), by F. R. C. Reed, M.A.
TIMES.–"There are very many, not only among educated people who take an interest in science, but even among specialists, who will welcome a work of reasonable compass and handy form containing a trustworthy treatment of the various departments of Natural History by men who are familiar with, and competent to deal with, the latest results of scientific research. Altogether, to judge from this first volume, the Cambridge Natural History promises to fulfil all the expectations that its prospectus holds out."
FIELD.–"We know of no book available to the general reader which affords such a vast fund of information on the structure and habits of molluscs."
KNOWLEDGE.–"If succeeding volumes are like this one, the Cambridge Natural History will rank as one of the finest works on natural history ever published."
ATHENÆUM.–"The series certainly ought not to be restricted in its circulation to lecturers and students only; and, if the forthcoming volumes reach the standard of the one here under notice, the success of the enterprise should be assured."
VOLUME IV.
Crustacea, by Geoffrey Smith, M.A.; Trilobites, etc., by H. Woods, M.A.; Limulus, Linguatulida, and Tardigrada, by A. E. Shipley, M.A., F.R.S.; Spiders, Mites, Scorpions, etc., by C. Warburton, M.A.; Pycnogonids, by D'Arcy W. Thompson, C.B., M.A.
[In the Press.
PERIPATUS, MYRIAPODS, AND INSECTS–Part I.
VOLUME V.
Peripatus, by Adam Sedgwick, M.A., F.R.S.; Myriapods, by F. G. Sinclair, M.A.; Insects, Part I., Introduction, Aptera, Orthoptera, Neuroptera, and a portion of Hymenoptera (Sessiliventres and Parasitica), by David Sharp, M.A., M.B., F.R.S.
FIELD.–"Although written for the student and the specialist, the book is not the less adapted to all intelligent readers who wish to make themselves thoroughly acquainted with the habits, structure, and the modern classification of the animals of which it treats. To such it cannot be recommended too strongly."
SCIENCE GOSSIP.–"Every library, school, and college in the country should possess this work, which is of the highest educational value."
Prof. RAPHAEL MELDOLA, F.R.S., F.C.S., in his Presidential Address to the Entomological Society of London, said:–"The authors of this volume are certainly to be congratulated upon having furnished such a valuable contribution to our literature. When its successor appears, and I will venture to express the hope that this will be at no very distant period, we shall be in possession of a treatise on the natural history of insects which, from the point of view of the general reader, will compare most favourably with any similar work that has been published in the English language."
ENTOMOLOGISTS MONTHLY MAGAZINE.–"We venture to think the work will be found indispensable to all who seek to extend their general knowledge beyond the narrowing influence of exclusive attention to certain orders or groups, and that it will take a high position in 'The Cambridge Natural History' series."
INSECTS–Part II.
VOLUME VI.
Hymenoptera continued (Tubulifera and Aculeata), Coleoptera, Strepsiptera, Lepidoptera, Diptera, Aphaniptera, Thysanoptera, Hemiptera, Anoplura, by David Sharp, M.A., M.B., F.R.S.
SATURDAY REVIEW.–"Dr. Sharp's treatment is altogether worthy of the series and of his own high scientific reputation. But in a work of this sort it is not only necessary that information should be accurate, but also that it shall be presented to the eye, so far as illustrations and printing are concerned, in such a way as to render its matter as easily intelligible as possible, and readily usable for purposes of reference. Under both these heads we have nothing but commendation for Mr. Sharp's treatise. The illustrations are indeed beautiful, and the use of the heavy type for the headings of the various sections and leading paragraphs materially helps the reader in the progress of his study. Certainly this is a book that should be in every entomologist's library."
DAILY NEWS.–"It would be hard to say too much in praise of this most admirable volume. It is too often the case that scientific books are written in a dull and uninteresting style. The reader will find nothing of that kind to complain of here. The descriptions are clear, the illustrations are excellent; while, as in the previous volumes of the series, printing and paper are all that could be desired."
FISHES, ASCIDIANS, etc.
VOLUME VII.
Hemichordata, by S. F. Harmer, Sc.D., F.R.S.; Ascidians and Amphioxus, by W. A. Herdman, D.Sc., F.R.S.; Fishes (exclusive of the Systematic Account of Teleostei), by T. W. Bbidge[**Bridge??], Sc.D., F.R.S.; Fishes (Systematic Account of Teleostei), by G. A. Boulenger, F.R.S.
ATHENÆUM.–"All who take a serious interest in the advance of ichthyology will find this a fascinating book."
NATURE NOTES.–"It is a thoroughly scholarly work for students, amply sustaining the reputation of an ancient university as being in the van of scientific progress."
AMPHIBIA AND REPTILES
VOLUME VIII.
By Hans Gadow, M.A., F.R.S.
FIELD.–"The work is worthy of the series in which it appears, and we cannot give it higher praise."
SCIENCE GOSSIP.–"More than maintains the high scientific reputation of this series. The herpetologists, or students of the Amphibia and Reptiles, have now a standard work of the highest class."
LANCET.–"An account of both Amphibia and Reptiles which should satisfy the expert, and at the same time entertain the reader who is merely interested in the tit-bits of natural history.... A book full of accurate information and pleasant reading."
MORNING POST.–"A delightful as well as a serviceable book.... Herein perhaps lies the great charm and merit of Dr. Gadow's book, that, while satisfying all the inquiries of the student, it is also in great part written for the ordinary intelligence, and the naturalist in the crowd may, while necessarily gliding over distressing technicalities, find in its pages many hours of profitable and entertaining study of the habits of the classes under notice."
NATURE.–"In concluding the review we would express the opinion that by this handsome volume a very important addition to science has been made; that the beautiful illustrations, together with the clear and charming accounts of the life-histories which it contains, will do much to popularise the study of a rather neglected section of zoology; and that lovers of Reptiles, of which there are more than one generally thinks, will feel that the new knowledge imparted to them emanates from one who is thoroughly in sympathy with their enthusiasm."
BIRDS
VOLUME IX.
By A. H. Evans, M.A.
IBIS.–"Mr. Evans has produced a book full of concentrated essence of information on birds, especially as regards their outer structure and habits, and one that we can cordially recommend as a work of reference to all students of ornithology."
NATURE NOTES.–"We venture to predict that, of the ten volumes of which this excellent series is planned to consist, none will secure a wider popularity than Mr. Evans's treatise on birds. Strange as it may appear, among the many books on birds that have appeared of late years, we do not recall any that covers the same ground.... We are grateful to the author for the mine of valuable information which he has crowded between his two covers."
SCIENCE GOSSIP.–"General readers will find this work most useful in obtaining a proper understanding of birds, and will be assisted by the effective diagram of a hawk in the introduction, showing the recognised names of every part of the exterior appearance. The expressions used in naming the various portions are fully explained on the adjoining page. As we have already said, the illustrations are admirable. The book is a useful addition to any library, as it treats of nearly every known kind of bird throughout the world."
SATURDAY REVIEW.–"The expert and the novice alike must be at once delighted by the accuracy and the beauty of the illustrations.... It is astonishing to note the mass of information the author has been able to bring together.... With a little practice any observant person would soon learn by the help of this volume to track down any bird very nearly to its ultimate place in classification."
MAMMALIA
VOLUME X.
By Frank Evers Beddard, M.A., F.R.S.
NATURE.–"Cannot fail to be of very high value to all students of the Mammalia, especially from the standpoints of morphology and palæontology."
ATHENÆUM.–"Mr. Beddard has produced a volume equal in interest and value to the others in the Cambridge series."
LAND AND WATER.–"A notable book, the result of long study, patient labour, sound reasoning, and careful selection, for which we are deeply indebted to the author."
DAILY NEWS.–"A volume which, for the interest of its contents and for its style and method of treatment, is not only worthy of its predecessors, but may be regarded as one of the most successful of a brilliant series."
KNOWLEDGE.–"In this volume Mr. Beddard has undoubtedly made an important contribution to the history of mammals, his text-book being the only one which can be said to be up to date and to contain notices of the many important types–both recent and fossil–discovered during the last few years."
FIELD.–"Its utility to the working zoological student can hardly be overrated. It is exceedingly well illustrated."
LONDON: MACMILLAN AND CO., Ltd.
References to explanations of the terms used below will be found in the index.
Bull. Soc. Philom. ii. p. 81.
Tableaux méthodiques, p. 61.
Bull. Soc. Philom. p. 113.
Isis, 1821.
Treviranus' Zeitschr. f. Physiol. 1831, p. 190.
δέρη, neck; μύω, close.
Proc. Ac. Philad. p. 209.
Americ. Natural. xxiii. p. 849.
Sarasins' Ergebnisse ... Ceylon, 1887-1890.
Credner's term for all Vertebrates higher than fishes.
Boulenger, P.Z.S. 1888, p 204.
P.Z.S. 1897, p. 577.
Q.J.M.S. xxxviii. 1896, p. 465.
Arch. ges. Physiol. li. 1892, p. 455.
Nat. Sci. i. 1892, p. 185.
C. R. Ac. Sci. cix. 1889, pp. 405, 482.
Orr, Quart. J. Micr. Sci. xxix. 1889, p. 316.
"Lungenlose Salamandriden," Anat. Anz. 1894, p. 676; 1896, p. 182.
"Nuove ricerche anatomo-fisiologiche intorno ai Salamandridi normalmente apneumoni." Torino, 1894.
Zool. Anz. 1896, p. 33; 1899, p. 545.
Amer. Natural, xxx. 1886, p. 829.
For the mechanism of the frog's respiration, see Gaupp, Arch. Anat. 1896, p. 239.
Boulenger, The Tailless Batrachians of Europe, Ray Soc. 1896.
Zeitschr. wiss. Zool. xlix. 1889, p. 583.
Amer. Natural, xxv. 1891, p. 753.
Zool. Jahrb. Syst. vi. 1892, p. 447.
Ann. Nat. Hist. (5), xvii. 1886, p. 463.
J. Thiele, Zeitschr. wiss. Zool. xlvi. 1888, p. 67.
Zeitschr. wiss. Zool. xlix. 1889, p. 43.
M. Weber, Ann. Jard. Botan. Buitenzorg, Suppl. ii. 1898, p. 5.
For "A Synopsis of the Tadpoles of European Batrachians," see Boulenger, P. Z. S. 1891, pp. 593-627, pls. xlv.-xlvii.; also Bedriaga, "Tableaux synoptiques pour servir à la détermination des larves des Batraciens Urodèles," C. R. Ass. Franç. Sci. ii. 1891, pp. 540-546.
Arch. mikr. Anat. xxix. 1887, p. 1.
Arch. per zool. e per l'anat. comp., Genova, 1861, p. 206.
Ann. sci. nat. (5), vii. 1876.
Mem. Acc. Torino, xxxv. 1883, and Atti Acc. Torino, xvii. 1883, p. 84. See also Woltersdorff, Zool. Garten, 1896, p. 327.
Verh. Ges. Basel, vii. 1882, p. 387.
Barfurth, Arch. Entwickmech. I. 1895, p. 117.
The Horn Scientific Expedition, 1897, p. 155.
Amer. Natural. xxix. 1895, p. 998.
J. Morphol. xi. 1895, p. 375.
P. Z. S. 1895, p. 401.
P. and F. Sarasin, "Zur Entwicklungsgeschichte der ceylonesischen Blindwühle, Ichthyophis glutinosa." Ergebnisse naturwiss. Forschungen auf Ceylon, 1887-1890, vol. ii.
"Beiträge zur Kenntniss der Entwicklungsgeschichte und der Anatomie der Gymnophionen," Zool. Jahrb. Anat. x. 1897, p. 389, and xii. 1899, p. 477.
The existence of such a form as Typhlotriton, in the adult of which the eyes become closed up, makes such short diagnoses of the families defective, although there is no doubt about the Desmognathine affinities of this genus. See p. 103.
J. Coll. Japan. i. 1887, p. 269.
J. Morphol. xi. 1895, p. 375.
Amer. Natural. xxxiii. March 1899, p. 231.
Amer. Natural. March, 1899, p. 235.
Zool. Garten, 1896, p. 88.
P. Calif. Ac. (2) v. 1895, p. 776.
Amer. Natural. xxxiii. 1899, p. 691.
Amer. Natural. xxxi. 1897, p. 635.
Zeitschr. wiss. Zool. xxvii. 1877, p. 522; xli. 1891, p. 365; Zool. Anz. 1882, p. 513.
Zoolog. Garten. 1896, p. 114.
Zeitschr. wiss. Zool. xxv. 1875, p. 297. See also Hahn, Rev. Quest. Sci. (2), i. 1892, p. 178.
Amer. Journ. Sci. (2), xlvi. Nov. 1868, p. 364.
Zeitschr. Biol. xxxiv. 1896, pp. 340-396.
Zeitschr. wiss. Zool. xxix. 1877, pp. 324 f., pl. xxii.
Ann. Mus. Genova, xvi. 1880, p. 83.
Journ. Morphol. viii. 1893, p. 269.
Amer. Natural. 1891, p. 1084.
P.Z.S. 1895, p. 150.
See also M. von Chauvin, Zeitschr. wiss. Zool. xxxviii. 1883, p. 671.
E. T. Emerson, Proc. Boston Soc. xxxii. 1905, p. 43.
Nature, lx. 1899, p. 389.
Amer. Natural. xix. 1885, p. 1226.
Proc. Ac. Philad. 1864, p. 181.
The Natural History Review, No. xvii. 1865, p. 97.
Journ. Ac. Nat. Hist. Philad. vi. p. 189.
Beddard, P.Z.S. 1895, p. 841.
Phil. Trans. B. 136, 1896, p. 1.
P.Z.S. 1890, p. 69.
P.Z.S. 1894, p. 101.
Groenberg und Klinckowstroem, "Zur Anatomie der Pipa americana," Zool. Jahrb. Anat. vii. 1894, p. 609.
P.Z.S. 1896, p. 595.
Zool. Garten, 1885, p. 299.
P.Z.S. 1899, p. 790.
Faune Vertebr. Suisse, iii. 1872, p. 587.
Zool. Garten, 1885, p. 299.
For further information, cf. Portschinsky, "Biologie des mouches coprophages et nécrophages, 2me partie. Étude sur la Lucilia bufonivora, parasite des batraciens anoures."–Horae Soc. ent. Ross. xxxii. pp. 225-279 (in Russian). German summary in Zool. Centralbl. v. 1898, pp. 855-859.
Arch. Naturg. xliv. 1868, p. 141.
Quart. J. Micr. Sci. xlii. 1899, p. 3.
Arbeiten Instit. Würzburg, x. 1895, p. 57.
Proc. Bost. Soc. Nat. Hist. xxi. 1883, p. 104.
P.Z.S. 1895, p. 89 (with a sketch of a pond, with nests, in Dr. Goeldi's garden).
P.Z.S. 1895, p. 209.
Arch. Anat. und Phys. 1854, p. 449. Also Boulenger, P.Z.S. 1898, p. 107.
Quart. J. Micr. Sci. xlii. 1899, p. 313.
Ann. Mag. Nat. Hist. (5) xvii. 1886, p. 461.
=Phyllobates (part) Bibron; cf. Boulenger, P.Z.S. 1888, p. 207.
See Günther, Nature, lii. 1895, p. 643.
Quart. Micr. Sci. xlii. 1899, p. 329.
Arch. Naturg. xxxiii. 1867, p. 124.
Quart. J. Micr. Sci. xlii. 1899, p. 309.
Report on the Work of the Horn Scientific Expedition to Central Australia, pt. ii. "Zoology," 1896, p. 164.
Proc. Linn. Soc. N.S.W. (2), iv. 1898, p. 357.
Zool. Anz. xvii. 1894, p. 156.
An. Soc. Espan. i. 1822. See also Howes, P.Z.S. 1888, p. 231.
Quart. J. Micr. Sci. xlii. 1899, p. 307.
S. S. Flower, P.Z.S. 1896, p. 910.
Ibid. p. 909.
Boulenger, Ann. Nat. Hist. (6), iv. 1889, p. 247.
See Boulenger, P.Z.S. 1888, p. 204.
Boulenger has shown (P.Z.S. 1888) that Bibron's species of Phyllobates, hitherto grouped amongst the Cystignathidae, are Ranoids, closely allied to Hylixalus and Prostherapis. The other species now form the Cystignathoid genus Syrrhopus, Cope (cf. p. 212).
P.Z.S. 1895, p. 209.
Zool. Jahrb. Syst. xii. 1898, p. 89.
Monatsber. Berl. Ac. 1875, p. 204; 1876, p. 714.
Malay Archipelago, 2nd ed. i. 1869, p. 38.
Annotat. Zool. Jap. i. 1897, p. 113.
P.Z.S. 1896, p. 906.
Boulenger, Cat. Batrach. Salientia, p. 22.
Zool. Gart. 1885, p. 299.
Trans. Zool. Soc. xii. 1884, p. 51.
P.Z.S. 1884, p. 573.
British Reptiles, 2nd ed. 1849, p. 110.
Boulenger, "Tailless Batrach. of Europe," pt. ii. p. 287, Ray Society, 1897.
Boulenger, op. cit. p. 278.
Phil. Trans. clxxxvii. 1896, B. p. 23.
Anat. Anz. xix. 1897, p. 201.
Phil. Trans. clxxxvii. 1896, B. p. 23.
Trans. Zool. Soc. xv.
Trans. N. Zealand Inst. x. 1878, p. 222.
Trans. N. Zealand Inst. xiv. 1881, p. 276; cf. also Reischek, op. cit. xiv. p. 274.
Cope, the inventor of this most appropriate name, soon changed it, unnecessarily, into Theromora (μωρός = sluggish), perhaps in order not to emphasise too much their possible Mammalian affinities; while others rashly called them Sauro-Mammalia. For detailed illustrations of Theromorpha reference should be made to Owen, British Fossil Reptiles, 4to, London, 1849-55, and to numerous papers by Seeley, Phil. Trans. 178 (1887), 186 (1895), and by E. T. Newton in Phil. Trans. 184 (1893), 185 (1894).
Cat. Chelonians, Brit. Mus. 1889.
It should be noted that the horny pieces of the carapace are termed "shields" and the bony pieces "plates."
πλευρόν, side; δειρή, neck.
Journ. Morph. xv. 1897, p. 21.
Amer. Natural. xxxii. 1898, p. 929.
Contributions to the Nat. Hist. of the U.S.A., Boston, 1857.
Zool. Garten. 189, p. 260.
Natural History of Selborne.
J. Asiat. Soc. Bengal, vi. 1837, p. 689.
Presidential Address. Proc. Linn. Soc. 1898. See also Günther, Gigantic Land-Tortoises, Brit. Mus. London, 1877; Gadow, Trans. Zool. Soc. xiii. 1894, p. 313; Rothschild, Novit. Zool., several notes.
Notes Leyden Mus. xvi. 1895, p. 211.
Contributions to the Natural History of the U.S.A., vol. i. 1857, p. 333.
P.Z.S. 1875, p. 2.
Wanderings of a Naturalist in India, Edinburgh, 1867.
Sketches of the Natural History of Ceylon, London, 1861.
Sitzber. Ak. Berlin. 1891, p. 115; 1893, p. 347.
J. Morphol. v. 1891, p. 181.
J. China Asiat. Soc. xiii. 1879, pp. 1-36, with Figs.
Boulenger, Trans. Zool. Soc. xiv. 1898 (read Nov. 1893).
νόθος = spurious.
Zool. Gart. 1889, p. 1.
P.Z.S. 1888, p. 351, and 1891, p. 466.
P.Z.S. 1889, p. 602.
P.Z.S. 1889, p. 452.
F. Mason's Burma, London, 1882.
Sketches of the Nat. Hist. of Ceylon, London, 1861.
P. Ac. Philad. 1864, p. 224, and P. Amer. Ass. xix. 1871, p. 236.
Ann. Nat. Hist. (5) xiv. 1884, p. 117.
Burma, its People and Productions, London, 1882.
Zool. Garten. 1895, p. 232.
Zool. Garten. 1895, p. 257.
P. Linn. Soc. N.S.W. viii. 1883, p. 300.
Tangweera, London, 1899.
Voyage of the Beagle, London, 1845, chap. xvii.
For further anatomical details see Shufeldt, P.Z.S. 1890, p. 148; Boulenger, P.Z.S. 1891, p. 109; and Stewart, P.Z.S. 1891, p. 119.
P.Z.S. 1899, p. 596.
Burma, its People and Productions, London, 1882.
Sketches of the Nat. Hist. of Ceylon, London, 1861.
Organic Evolution. Translation, London, 1890.
Fischer, Zool. Garten. 1884, p. 38.
Zool. Gart. 1882, p. 206.
Denk. Ak. Wien. iv. 1852.
C. R. Ass. Franc. lxxx. 1876, No. 21.
J. de l'anat. physiol. viii. 1872, p. 401.
Morphol. Arbeit. vii. 1897, p. 515.
Arch. Physiol. lxi. 1895, p. 123.
Fischer, Zool. Gart. 1882, p. 4.
For a detailed anatomical account, see West, J. Linn. Soc. xxv. 1895, p. 419; xxvi. 1898, p. 517; and xxviii. 1900.
Clifford Allbutt's System of Medicine, vol. ii. London, 1896, p. 809.
Erpétologic générale, Suites à Buffon, vol. vii. Paris, 1852.
Catalogue of Snakes, British Museum, London, 1849.
Reptiles of British India, Ray Society, 1864.
P. Ac. Philad. 1864, p. 230.
Catalogue of Snakes, British Museum, London, 1893-1896.
Except Oligodon, Dasypeltis and Atractaspis (see. p. 582), in which palatal teeth are restricted to the palatines.
Atlas der Thierverbreitung, pt. v. Gotha, 1887.
W. A. Forbes, P.Z.S. 1881, p. 960.
Burma, its People and Productions, London, 1882.
Rep. Brit. Ass. 1870. Trans. p. 115.
P.Z.S. 1894, p. 669.
Zool. Gart. 1895, p. 330.
Ibid. 1896, p. 85.
The same arrangement occurs in the Colubrine genus Polyodontophis, with about ten species in South-Eastern Asia, Madagascar, the Comoro Islands, and in Central America.
P.Z.S. 1896, p. 715.
P.Z.S. 1887, p. 340.
P.Z.S. 1887, p. 489.
Natural Science, i. 1892, p. 44.
Verbreitung der Kreuzotter in Deutschland. Frankfurt a. M. 1888.
J. Linn. Soc. xxviii.