
Title: Dyes and dyeing
Author: Viscount Exmouth Charles E. Pellew
Release date: February 6, 2025 [eBook #75302]
Language: English
Original publication: New York: Robert M. McBride & Company, 1913
Credits: Aaron Adrignola, A Marshall and the Online Distributed Proofreading Team at https://www.pgdp.net (This file was produced from images generously made available by The Internet Archive)
Footnote anchors are denoted by [number], and the footnotes have been placed at the end of the paragraph.
Some minor changes to the text are noted at the end of the book.


BY
CHARLES E. PELLEW
Formerly Adjunct Professor of Chemistry
at Columbia University
NEW YORK
ROBERT M. McBRIDE & COMPANY
1918
[Pg iii]
Copyright, 1913, by
McBRIDE, NAST & COMPANY
Copyright, 1918, by
ROBERT M. McBRIDE & COMPANY
New and enlarged edition
Published, January, 1918
[iv]
| PAGE | ||
| Chapter I—INTRODUCTION | 5 | |
| Dyes of the Ancients—Dyes of Our Ancestors—Animal, Vegetable and Mineral Dyes—Outfit for Practical Dyeing. | ||
| Chapter II—MODERN DYESTUFFS | 40 | |
| Discovery of the Aniline or Coal-Tar Colors—Their Properties and Uses—How Obtained—How Named—Classification of Coal-Tar Colors for Craftsmen. | ||
| Chapter III—THE DIRECT COTTON OR SALT COLORS | 53 | |
| Discovery, Properties and Uses of the Salt Colors; with Lists of Selected Dyestuffs, and Dying Directions for Cotton and Linen—Fastness to Light and Washing—After-treatment. | ||
| Chapter IV—THEORY AND PRACTICE OF COLOR DYEING | 71 | |
| Even and Shaded Dyeing with the Primary Colors—Experiments with Secondary Colors—Matching Shades. | ||
| Chapter V—THE SULPHUR COLORS | 85 | |
| Discovery—Properties and Uses of the Sulphur Colors—List of Selected Dyestuffs, and Dyeing Directions for Cotton and Linen. | ||
| Chapter VI—THE INDIGO OR VAT COLORS | 91 | |
| Natural and Synthetic Indigo—Properties and Application—Vat Dyeing, Old and Modern—Dyeing Directions—The Modern Vat Colors—Their Properties and Uses—Selected Dyestuffs—Fastness to Light and Washing—Dyeing Directions for Cotton, Linen and Silk.[v] | ||
| Chapter VII—THE BASIC COLORS | 108 | |
| History, Properties, and Application to Cotton, Wool, Silk, etc.—Disadvantages—Not Fast to Light—Dyeing Directions for Straw, Raffia, etc. | ||
| Chapter VIII—THE ACID COLORS | 123 | |
| History, Properties, Uses, and List of Selected Dyestuffs—Dyeing Directions for Wool. | ||
| Chapter IX—DYEING FEATHERS | 131 | |
| The Dye-bath—The Dyeing Method—The Finishing Process—Dry and Wet Starching—Dyeing in the Starch—Black Dyeing of Feathers—Painting Feathers. | ||
| Chapter X—LEATHER AND LEATHER DYEING | 141 | |
| History—Preparation of Leather—Oil, Mineral and Bark Tanning—Dyeing, Staining and Finishing Leather. | ||
| Chapter XI—SILK I | 156 | |
| History, Origin and Varieties of Silk—Preparing Silk for Dyeing—Piece Dyeing—Skein Dyeing—Dyeing Wild Silks. | ||
| Chapter XII—SILK II | 168 | |
| Black Dyeing of Silk—Coal-Tar Colors—Logwood—Weighting of Silk—Properties and Tests for Weighted Silk—Dyeing Silk with Colors Fast to Washing. | ||
| Chapter XIII—IMITATION AND ARTIFICIAL SILK | 181 | |
| History, Preparation and Properties of Mercerized Cotton—History, Preparation and Properties of Artificial Silk—Precautions to be Taken in Dyeing and Finishing. [vi] | ||
| Chapter XIV—TIED AND DYED WORK | 192 | |
| As Used in South America, India, Philippines and U.S.—Variations in Tying Process—How Dyed—Tied and Discharged Work. | ||
| Chapter XV—STENCILS AND STENCILLING | 211 | |
| Japanese Practice—U.S. Practice—Knives, Brushes, Paper, etc.—Colors for Leather, Silk, and Cotton—Stencilling with Aniline Black Paste. | ||
| Chapter XVI—RESIST AND DISCHARGE STENCILLING | 228 | |
| Japanese Practice—Resist Paste and the Sulphur Colors—Discharge Stencilling with Bleaching Powder and Hydrosulphite. | ||
| Chapter XVII—BATIK OR WAX RESIST | 241 | |
| Javanese Practice—Modern Practice and Apparatus—Dyeing of Batiked Goods—Use of Batik Process on Cotton, Linen, Silk, Leather, Wood, Bone, etc. | ||
| Chapter XVIII—THE INFLUENCE OF THE WAR UPON THE DYESTUFF INDUSTRY | 260 | |
| Rise of the German Dyestuff Monopoly—Ruin of the English Dyestuff Industry—Dyestuff Industry in the United States—Changed Conditions Due to the War—Lists of Best Dyestuffs. | ||
THE ILLUSTRATIONS
PLATES IN COLOR
| PLATE | |||
| I | Indigo dyed batik from Madras | Frontispiece | |
| FACING PAGE | |||
| II | Japanese towelling, showing impression of fresh damp leaves | 26 | |
| III | Same towelling after immersion in iron spring | 30 | |
| IV | (a) Example of tied and dyed work (b) Example of tied and discharged work |
} | 210 |
| V | Japanese towelling stencilled in resist and dyed by immersion in iron spring | 230 | |
ILLUSTRATIONS IN HALF-TONE
| FIG. | |||
| 1 | Shellfish used by the ancients for Tyrian purple | 12 | |
| Sir W. H. Perkin | 42 | ||
| 2 | Tied and dyed headdress from an Inca tomb in Peru | 192 | |
| 3 | Shikar chundri, from Rajputana, with knots still untied | 196 | |
| 4 | Same chundri untied and shaken out | 198 | |
| 5 | Bagobo headdress from the Island of Mindanao | 200 | |
| 6 | Sample of tied and dyed work, “tied on itself” | 202 | |
| 7 | Sample of tied and dyed work, “tied in bands” | 204 | |
| 8 | Tied and dyed work—Folding the cloth | 206 | |
| 9 | Starting to tie | 206 | |
| 10 | Centre portion tied | 206 | |
| 11 | Centre and corners tied | 208 | |
| 12 | Dyed, untied and shaken out | 208 | |
| 13 | Japanese stencil knife | 212 | |
| 14 | Japanese stencil brushes | 212 | |
| 15 | Japanese stencil, showing holes punched by hand tool | 216[viii] | |
| 16 | Japanese stencil, showing use of stops | 216 | |
| 17 | Japanese stencil, showing use of sewing instead of stops | 216 | |
| 18 | Japanese stencils, showing use of both stops and net | 218 | |
| 19 | Large and handsome Japanese stencil, showing use of net | 224 | |
| 20 | “Teapot” model of tjanting | 248 | |
| 21 | Walther glass tjanting | 248 | |
| 22 | “Wax pencil” model of tjanting | 248 | |
| 23 | Javanese tjantings | 250 | |
| 24 | American modification of Javanese tjanting | 250 | |
DIAGRAMS IN THE LETTERPRESS
| Primary Colors | 73 | |
| Mixed Colors | 79 |
[1]
When a new text-book is offered to an innocent and long-suffering public about such an ancient subject as Dyes and Dyeing, it is, perhaps, the very least that the author can do, to explain briefly his reasons for hoping that his particular book may prove of some special usefulness.
As a matter of fact this book is intended for the use of craftsmen and others who are trying to dye and stain textiles by hand and on a small scale, rather than for professional dyers or dyeing chemists who are interested in factory dyeing, conducted on a large scale. For the latter there is little or no difficulty in getting any information that they desire, either from the large and carefully written text-books or, still better, from the many excellent dyeing manuals and books of directions issued at frequent intervals by the great color houses.
But for craftsmen and their like, the amateur dyers as opposed to the regular professionals, the required information is not easy to obtain. Their leaders and teachers, as a rule, profess a scorn of the wonderful discoveries which, in the last half century, have revolutionized the art of dyeing more, perhaps, than any other branch of handicraft. And the dyeing chemists and writers have devoted themselves almost exclusively to the far larger and more important and more profitable[2] field of commercial or professional dyeing, and only here and there is one found who has given any special attention to the dyes and processes needed by those working only on a small scale.
For my own part, after teaching the principles and practice of modern dyeing to class after class of chemical students at Columbia, my attention was called to this particular branch of the subject by finding, one spring, that some friends had started a hand-weaving industry at a settlement house in which I was interested, but had not made any arrangement for a dyehouse at the same time. This was a serious omission because it is almost impossible to buy in the market raw materials for hand-woven rugs, table-covers, and the like, that are dyed just the right shade and, at the same time, are fast to both light and washing; and, unless this last is guaranteed, there is little or no excuse for charging the large prices necessary to pay for the extra expense of the hand labor.
Wishing, therefore, to help out my friends, I offered to assist as far as possible in this part of the work. That summer was spent on the St. Lawrence, where it was possible to study some of the textile work of the Frenchhabitants whose dyeing processes, designs, and looms had descended from mother to daughter since the old Colonial days; and in the autumn I fitted up a little dyehouse and started with a small but intelligent class of neighbors who were working at the looms.
Of course, it was foolish to attempt to teach them the scientific chemical formulæ used by my students[3] uptown. The processes must be short and simple—must give the desired shades on cotton, linen, wool, and silk in the course of an hour or an hour and a half at the outside, counting from the time when the class was called to order. And the colors must be absolutely fast to light, and, wherever possible, to washing also.
The work was very interesting and proved successful enough, at least as far as the dyeing went. After a few months some visiting reporter, in an article on Greenwich House and its industries, mentioned the dyeing, in a magazine, and stated that the colors resulting were not only beautiful but fast. Immediately I was bombarded with letters from all over the country, begging for information about permanent dyestuffs to be used for hand-woven textiles. Requests came from friends and acquaintances to help them in various side branches of the subject, such as feather dyeing, leather dyeing and staining, stencilling, tied and dyed work, and, above all, Batik. And it soon became a source of much interest to look up some old process of dyeing, originating perhaps in the East, perhaps among the ancient Egyptians, and to work it out with the best modern dyestuffs.
Finally, my correspondence grew so burdensome that I arranged with the well-known New York magazine, The Craftsman, for a series of articles upon “Modern Dyestuffs and Dyeing Processes for the Use of Craftsmen”; and from these articles the present book is a natural result.
It is hoped that it will prove useful, not only for[4] individuals who are trying, under considerable difficulties, to get satisfactory results, by means of long-abandoned processes, upon textile materials of many sorts and kinds, but also for teachers of art in our public as well as private schools. Much attention is being given now to training the hands of children in various drawing and decorating and weaving processes. But the modern dyestuffs give a much greater opportunity to train their eyes to a sense of color and to its beauties, as well as giving them an introduction into an art which can be used at home for most useful as well as beautiful purposes.
My hearty thanks are due to many friends, notably, to Mr. Philip Clarkson, head chemist of H. A. Metz & Co., to Dr. Ludwig, of the Cassella Color Co., and to many other expert dyeing chemists, who have most kindly helped me with advice and information about many widely varying branches of the subject. Also to many of my craftsman friends, notably Mrs. C. L. Banks, of Bridgeport, Conn., and Mrs. Charlotte Busck, of this city, who have been of the greatest assistance in working out many of the problems involved in stencilling and Batik; and to Miss Mary Grey, of Hackettstown, N. J., who has kindly allowed me to insert an illustration of some of her interesting and well-designed tied work (Fig. 7). It is my earnest hope that the information contained in this book may encourage and assist other craftsmen throughout the country to come up to the high standard of these skilled textile workers.
C. E. P.
[5]
There has been so much said and written about the beauty and value of the old-fashioned dyestuffs and dyeing processes and their superiority to the modern coloring matters, that many well-meaning people of artistic tastes have never ceased to deplore the discovery and introduction of the so-called aniline or coal-tar dyes, and to regard them as a serious detriment to the art of dyeing.
Some, indeed, have gone so far as to decry the discoveries not only of the last fifty years, but also of the last nineteen or twenty centuries. These quote with approval the great John Ruskin, founder and original leader of the whole Arts and Crafts movement in England, if not in the world, as having said, “There has been nothing discovered of the slightest interest in the tinctorial art” (the art of dyeing) “since the days of the ancient Greeks and Romans.”
To suppose for an instant that this important and highly specialized art has not advanced during nearly two thousand years is, on the face of it, absurd. A very little knowledge of dyestuffs forces recognition of the fact that many of the very best, fastest, and most beautiful of the dyes of our ancestors—such as cochineal, with which they dyed practically all of their[6] fast pinks and scarlets; logwood, with which silk as well as wool was, and is still dyed black; fustic, which was used for fast yellows on wool and cotton, and several others—were natives of America, and therefore only known to the world at large since the seventeenth century.
Indeed, as we shall see, the art of dyeing, based as it is on chemical processes, discovered one by one, but never properly explained or understood until the last sixty or seventy years, is, perhaps, the one art above all others in which not only the ancient world, but the world of comparatively a few years ago, was very distinctly inferior to that of the present day.
In drawing, sculpture, painting, architecture, ceramics, wood-carving, lacemaking, metal working, and almost every other art that can be mentioned, the craftsman of the Middle Ages, if not indeed of ancient Rome or Greece, could still hold his place against modern competitors. Even in such a modern art as book printing, the lover of books will claim, with considerable reason, that no more beautiful or more nearly perfect specimen of the printer’s art has ever been produced than the Gutenberg Bible, the first product of the European printing press.
The art of dyeing, however, has been changing and developing so much from century to century, that, even before the wonderful discoveries of the last fifty years, the effects produced by any one generation of dyers would have been totally impossible for their ancestors of a few generations before them.
It would seem hardly worth while to dwell further[7] upon this subject, were not the idea so fixed in the minds of craftsmen in general that to get permanent and artistic effects in dyeing we must go back to the colors of our ancestors, if not to those of the ancient world. To this day we hear of new industries being started in the lines of hand-made tapestries, hand-woven linens, homespun cloths, and the like, where, as a great inducement to prospective purchasers, the goods are loudly proclaimed as dyed with “pure vegetable colors”; and the first question commonly asked about a pretty piece of dyed work is, “Are you sure that it is fast? Did you use the vegetable dyes?”
As a result of this ignoring and scorning of the wonderful results of modern science in its application to this most important industry, the work of textile craftsmen all over the world is far behind the times, and comparatively far behind other lines of craftwork.
Nobody expects a modern sculptor to do his carving with the bronze tools used by the old Athenians; nor do we consider that the present day worker in metals should refrain from using the modern gas furnace, or limit his products to the few metals and alloys known in the Middle Ages, ignoring those which modern chemistry has developed. And yet, all over the world, craftsmen are still pottering with long since obsolete dyestuffs and obscure and antiquated formulæ, instead of spending their energies in getting, with the minimum expenditure of time and trouble, results of a quality never dreamed of by the most skilful dyers of half a century ago.
[8]
As a matter of fact, so far from Mr. Ruskin’s estimate of the value of ancient dyes being correct, it is actually no more than fair to say that hardly a single dyeing process, known and used more than fifty years ago, is of the slightest practical importance now to any one.
So far as we can tell, the art of dyeing is an extremely ancient one. It seems to have developed in every country and to have been practised by every race of mankind, as soon as that race ceased to rely exclusively upon the skins of fur-bearing animals for clothing and coverings. Wherever we find people using woven goods, whether vegetable, like cotton or linen, or animal, like wool or silk—or wherever, as in the case of the North American Indians, they have learned the art of dressing skins so as to make them soft, pliable, and with a comparatively smooth surface, we find at least the rudiments of the process of dyeing, in the staining of these materials to add to their beauty and interest.
Vegetable Dyes.—The earliest dyes were probably of vegetable origin, discovered by accidentally staining garments with juices of fruits or plants. Thus, for instance, in the Bible we read of “garments dyed in the blood of grapes”; and we can all call to mind fruits in common use—blackberries, huckleberries, peaches, and the like, whose juice could be used, if nothing better presented itself, to dye or stain light-colored fabrics.
[9]
In most cases, as in those just mentioned, the colors would be fugitive, and after a short time become dull and uninteresting. But in the process of time vegetable dyes were discovered, in one part and another of the world, which, in the hands of those who knew how to work with them, gave colors both fast and beautiful. And thus grew and developed the art of the professional dyer.
For instance, in many widely separated countries, such as India, Java, South and Central America, plants are found, known asindigoferae, whose juices, yellow when fresh, rapidly turn blue when exposed to the air. These juices impart a rich and permanent blue stain to objects moistened with them while they are still yellow; and this blue is the coloring matter known as indigo. The plants bearing it have been cultivated for hundreds, if not, indeed, thousands of years, and used for dyeing.
Garments and blankets found in the so-called Inca graves in Peru and Chili, dating from long before the Spanish conquest, as well as the oldest specimens of Hindoo workmanship, and even some of the textiles found in the tombs of Egypt, all show examples of this same dyestuff. It was so valuable that, in small quantities and at vast expense, it was imported by the Romans from India, as is shown by its Latin name, Indicum (Indian), from which its present name, indigo, is directly derived.
But, curiously enough, exactly the same dyestuff, but in a very impure form, and derived from an entirely different plant, theisatis tinctoria, commonly[10] known aswoad, has been discovered and used in Western Europe from time immemorial. And when Julius Cæsar, nearly two thousand years ago, led a Roman army for the first time across the channel into England, he found the native Britons adorning themselves by smearing their bodies with a dirty blue dyestuff obtained from this source.
So, little by little, the knowledge of these natural dyestuffs and their application grew and expanded. But as a matter of fact, so far at least as can be gathered from the old writers, those known and used by the ancient Greeks and Romans were few in number and of comparatively little interest.
For blues they were obliged to use the inferior color derived, as above mentioned, from the native woad, excepting when, for some special purposes, a little indigo was imported from the East at enormous expense.
Their principal yellow dyestuff was saffron, which is derived from the flowers of the common yellow crocus. This gives pleasant, warm shades of golden yellow, not fast, however, to either light or washing. This same saffron, though long since entirely abandoned as a dyestuff, is still used in small quantities for staining candy and foodstuffs, and occasionally for medicinal purposes.
The ancients are believed to have discovered the dyeing properties of the roots of madder—rubia tinctorum—(the dyer’s root), and to have used it in small quantities for producing purple and brown and, possibly, even red shades, on cotton and wool. Whether,[11] however, the art of dyeing the brilliant crimson and scarlet shades known as Turkey red was ever worked out before the Middle Ages, is extremely doubtful.
Animal Dyes.—Unquestionably the best red dyes known to the people of those early times were of animal origin, and were used for various shades of red and of purple.
Kermes.—One of these, called kermes, is very closely related to the more important and, up to a few years ago, the very generally used, cochineal, and to the lac dye.
These three dyestuffs—kermes, cochineal, and lac—come to the market in the form of little dark colored grains, which, when ground up with hot water, give a bright red solution called carmine, which contains a considerable amount of a coloring known as carminic acid. When wool or silk that has been previously mordanted—that is, impregnated with chemical agents; in this case salts of tin, aluminium, iron, or copper—is boiled in one of these solutions, it becomes scarlet, crimson, purple, or claret color, according to the mordant employed. From the appearance and form, as they come to market, of these dyestuffs, the shades thus derived are commonly known as the “grain colors.”
When these granules are soaked for some time in warm water they swell, and their true character becomes apparent. They consist of the dried bodies of small insects, known as “cocci” (berries), which are carefully cultivated on particular kinds of trees or shrubs and when full grown are brushed off and dried[12] for market. They are very small—the cochineal grains, which are the most important, running about 70,000 to the pound.
Kermes, which was the only one of the three known to the old Greeks and Romans, consists of the dried bodies of the “coccus ilicis,” a variety of the insect which lives on a species of oak, and which, it is said, is still occasionally used in Southern Europe, and in Morocco, for dyeing leather and wool.
Tyrian Purple.—The most highly prized ancient dyestuff, and one concerning which much interest has always been felt, was the so-called “Tyrian purple.” This was obtained from the juices of certain species of snails found in the waters of the Mediterranean Sea, and, indeed, in the ocean waters of many other warm climates. Two species of this class—themurex Brandaris and themurex trunculus—were used extensively by the ancients, and great mounds of their shells, such for instance as the so-called Monte Testaccio at Tarentum, are still found along the shores at places famous, in old days, for their dyeing establishments.
Other shellfish of the same general type, known aspurpura lapillus, are found quite abundantly, not only in the Mediterranean, but also on our own coast and along the shores of Central and South America. They have been used by the natives in Nicaragua and elsewhere, from time immemorial, for obtaining a similar color.
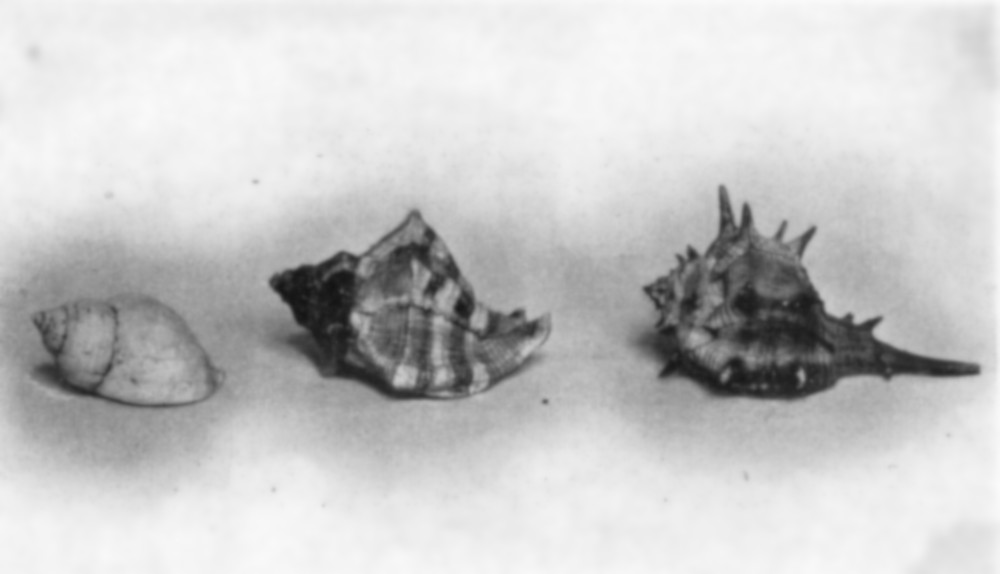
Purpura lapillus Murex trunculus Murex Brandaris
These shellfish were so much sought after in the old days that, by the time of the early Middle Ages,[13] they were almost exterminated, and the dye disappeared from commerce entirely. But, long before that, in the early days of the Roman Empire, the coloring matter was so expensive that fabulous sums were paid for cloth or yarns dyed with it, and its use was practically confined to the imperial family. In fact one of the imperial titles in the Eastern empire—purpureogenitus, “born to the purple”—was due to this fact.
Some interesting information upon the value set on this dyestuff by the ancients is afforded by the so-called Edict of Diocletian, fragments of which, engraved on stone tablets, have been found in different parts of the old Roman Empire, ranging from Egypt to Asia Minor. By this edict, issued in A.D. 301, the emperor Diocletian attempted to fix the market price of the principal articles of commerce, for the Eastern empire. According to this, the price of wool, heavily dyed with this color, was worth about $350 a pound, in gold.
The dyestuff, as we learn from the description of the process by ancient writers, was obtained from a whitish or yellowish liquid found, two or three drops at a time, in a particular vein in the body of these animals. This juice, when exposed to air and especially to sunshine, forms the purple or violet color, much in the some manner that the blue color of indigo is formed from the yellow juice of the indigo plant.
The shellfish in question, having for many centuries been left undisturbed, are now quite common in the waters of the Mediterranean, and are occasionally[14] to be found in the poorer quarters of Venice and other Italian seaports, exposed for sale as food.
A year or two ago a German color chemist, famous for his discovery of the brilliant and extremely permanent reddish violet dyestuff, known as Thio Indigo red B., made a careful investigation to see whether, by any chance, this color of his might happen to be the same as the famous old Tyrian purple.
He managed to secure some twelve thousand specimens ofmurex Brandaris, and, with an immense amount of labor, obtained from these twelve thousand specimens about twenty-one grains of pure dyestuff. This he carefully analyzed and experimented with, until finally he was able to prove that, while it was not identical with his own Thio Indigo red dyestuff—which, as the name shows, is a compound of indigo and sulphur—the Tyrian purple was a similar compound of the same indigo dyestuff, with the comparatively rare acid element, bromine. In fact it is what the chemists would call a brom-indigo; and this same famous chemist, Dr. Friedlaender, of Biebrich on the Rhine, after discovering its composition, amused himself by manufacturing some of it artificially; and, with the artificial reproduction of the ancient Tyrian purple, he dyed some skeins of silk, as an illustration to his article detailing his discovery.
Now, if there were any truth in the theory of the superlative value and beauty of these ancient dyestuffs, it is evident that this rediscovery of the true and genuine Tyrian purple would have been a matter[15] of great practical importance. On the assumption that one pound of dyestuff would color at least twenty pounds of wool, this would put the price of the dye itself, in Diocletian’s day, at a pretty high figure.
It can now be manufactured, at a profit, for not over one one-thousandth of what it cost in those days, not allowing, either, for the difference in value of money between then and now. And yet this famous dye, which was so highly esteemed and of which so much has been written, is so inferior in color and tone to several of the modern dyestuffs that it probably would not pay to put it on the market. Dr. Friedlaender’s samples were, indeed, fast to both light and washing, but their color showed dull and, to modern eyes, distinctly uninteresting shades of violet. And there are already on the market several violet, red and blue dyes of the same general class—the indigo or vat dyes—which are quite as fast to light and washing, and far superior in beauty and brilliancy of shade.
It is only proper, however, to state that Dr. Friedlaender’s investigation did not completely clear up the subject, though there is no question but that he really discovered the true Tyrian purple; and the color of the specimens dyed and exhibited by him corresponded very closely to some still surviving from antiquity.
Among the fine collections of textiles from the Egyptian tombs that are in the Metropolitan Museum of Art in New York City, are some excellent examples of Tyrian purple. These are what the Greeks[16] used to call “di-bapha,” or double dyed—i.e., dyed very deep, full shades of dark purple. While a wonderful example of the lighter, violet, shades of the same dye can be seen in a famous manuscript, known as “The Golden Gospels,” now in Mr. J. Pierpont Morgan’s collection in the same city, but which was given about 1520, by Pope Leo X to King Henry VIII. This was written, in golden characters, upon vellum dyed with Tyrian purple, and the shades of the latter correspond quite closely with the violet of the artificial brom-indigo compound.
On the other hand there is evidence to show that the ancients were also able to obtain, with the same Tyrian purple dye, perhaps from the shellfishpurpura lapillus, fast and brilliant shades of scarlet, as well as these rather dull tones of violet and purple. In the days of the Roman Empire, as above mentioned, the use of “purple” garments was denied to all but the imperial family; but later, after the rise of the Christian Church, the ecclesiastics gained sufficient power to obtain this privilege for themselves. And to this day the cardinals of the Roman Catholic Church are called “porporati” on account of the “purple” or, as we would say, scarlet, color of their characteristic robes. So, whenever we see the red robes of a high dignitary of the church we are probably looking at one of the tints of the real old Tyrian purple, although the art of actually producing it has long since been lost; and, if rediscovered, would probably be of as little practical value as Dr. Friedlaender’s remarkable investigation.
[17]
Between the days of the ancient Greeks and Romans, and the discovery of the first aniline dye in 1856, many and important additions were made to the list of available dyestuffs, some of which have continued in use, for special purposes, up to the present day.
Indian Dyes.—The opening of trade to the Far East, due to the discovery of the sea route round the Cape of Good Hope, brought to Europe the free use of some of the Indian dyestuffs. Indigo, for instance, was introduced for the first time in considerable quantities, and, after much opposition, completely took the place of the much inferior native dyestuff, woad.
For yellow, the old saffron dye was superseded by the more powerful, but still rather fugitive, turmeric, or Indian saffron. This came from the root of the curcuma tinctoria, a plant freely grown to this day in both India and China. The safflower was also imported from India; this is a kind of thistle,carthamus tinctorum, the dried heads of flowers of which were largely used for dyeing pretty shades of pink upon cotton,directly—that is, without any mordanting process. This color, too, is comparatively fugitive to light, and has almost disappeared from sight.
Of more importance were the so-called red woods, which came partly from India and partly from the east and west coasts of Africa; and of which the most important are the sandal wood, bar wood, and cam wood. The wood of each of these trees probably[18] contains the same coloring matter. The color is not very easy to extract, but when used with mordants of chromium, aluminium, or tin salts, it dyes wool various shades of red and reddish-brown. These colors are very fast to milling—in other words to the action of alkalies when the wool is finished in the manufacture of broadcloth; but they are not particularly fast to light, and for this reason, as well as because of their greater expense, they have been for the most part abandoned.
From India, too, were introduced the well-known brown dyes known as cutch (catechu) and gambier. These come to the market in the form of dark colored pastes, formed by evaporating infusions of leaves, seed pods, nuts, and sometimes the wood of various species of acacia and areca trees. They contain large amounts of a peculiar variety of the substance known as tannin or tannic acid, which is widely distributed among many plants, and which is very useful in dyeing, as will be described later. The brown coloring matter has been isolated, and is called catechin. Both cutch and gambier will dye cotton and wool rich shades of brown, which are quite fast to light when after-treated with copper or chromium salts.
Dyes from the New World.—The discovery of America, and the colonizing and opening to trade of South America and the West Indies, in the sixteenth and seventeenth centuries, still further enlarged the field for dyers.
Cochineal.—One of the first dyes introduced from there was cochineal, a “grain color,” similar to[19] kermes, already described, consisting of the dried bodies of an insect known ascoccus cacti, because it lives upon certain kind of cactus which are native to Mexico and Central America.
This dyestuff was largely used for dyeing wool and silk goods, and produced fairly fast shades of crimson or of scarlet, according to the mordant employed. But it has been replaced almost entirely now by the various acid dyes, to be described later, which are cheaper, are much easier to apply, and are of equal and, in many cases, of much greater, fastness to light.
One of the few cases where cochineal is still used on a large scale is in England, where the scarlet coats of the British regulars are dyed with this color, on a tin mordant. It is believed, however, that this is not due to any real or fancied superiority of the old dye over many of the modern colors, but simply to the terms of an old “perpetual” contract, which, a hundred and fifty years or more ago, gave the privilege of dyeing the English “redcoats” to one particular firm and their successors, on condition that they use this dye and none other. Although both dyers and government would profit by the use of modern dyes, the terms of the old contract are still rigidly adhered to for fear of losing the monopoly.
Lac Dye.—The similar dyestuff called lac dye, which had been known and used in India for hundreds of years, was introduced into Europe towards the end of the eighteenth century. It also is the body of a small insect, thecoccus laccae, which lives on the twigs of[20] the banyan tree, and other varieties of fig trees. When these twigs are broken off and dried to kill the insect, there is found present on them, along with the coloring matter, a large amount of a peculiar resinous or gummy substance, which, when extracted and purified, is known and widely used, as “shellac.”
Lac dye was used in practically the same way as cochineal, and produced, upon wool, scarlet, orange, and crimson shades, which were faster and more solid, but not as brilliant, as the cochineal. It is now used but rarely, even in the East, having been largely superseded, there, by brilliant but, unfortunately, in many cases, cheap and worthless modern dyestuffs.
Fustic.—From America, also, came the excellent yellow dyestuff, “fustic,” yielded by the tree commonly called yellow wood, Cuba wood, etc. Its true botanical name, however, ischlorophora tinctoria, and it was largely used for dyeing, either directly in the form of chips, or as a solid or liquid extract made from the wood.
It was principally used with mordants of aluminium or tin salts, for dyeing wool bright, fast shades of yellow, or, with the aid of bichromate of potash as a mordant, for obtaining mixed shades, in conjunction with indigo, cutch, madder, and logwood. It has been almost entirely replaced now by fast modern dyestuffs.
Logwood.—The most important of all these dyestuffs, and the only one still used on a large scale, is logwood, a dye extracted from the wood of quite a large tree, thehaematoxylon Campechianum (the “blood-red[21] wood from Campeachy”), which grows freely in the West Indies and Central American states.
It was discovered and used by the Spaniards early in the sixteenth century, and in Queen Elizabeth’s reign was introduced into England, much against the wishes of the older school of dyers who furiously denounced it as producing fugitive colors, and had its use prohibited by Act of Parliament. It was over a hundred years before the real value of the dyestuff was appreciated, and this law was repealed.
The operation of extracting the coloring matter from the wood itself, of which it forms only some three per cent. by weight, is a troublesome and delicate one. The logs are chipped or rasped into fine pieces, then moistened and piled in heaps and the color developed by a process of fermentation. Accordingly, extracts of logwood have been put on the market by various large firms, especially of late years, and, while the use of the wood itself by dyers has for the most part been abandoned, these extracts are widely used for dyeing blacks upon silk, in spite of there now being many excellent acid blacks.
The dyeing process, too, is rather complicated, for the goods must be carefully mordanted before dyeing, with salts of iron, chromium, or tin. For this reason wool is rarely dyed with logwood. It is, however, still used for silk dyeing, partly because it gives very full, deep, permanent shades of black, but principally because, by using one mordant after another before dyeing, it is possible to increase enormously the weight of the dyed silk, at very moderate expense.
[22]
Turkey Red.—The use of madder which, as before mentioned, was probably known to the ancients, was greatly developed during the sixteenth and seventeenth centuries, owing to the introduction from the near East of the so-called Turkey red process for obtaining, upon cotton and wool, very fast and very brilliant shades of scarlet.
The process took some three months, and consisted of an elaborate series of mordanting operations, before the dyeing proper began. The goods were first soaked in a bath of some fatty material, such as milk or, later, rancid olive oil, and then dried carefully. After this they were soaked in a bath of alum and then in limewater, or a chalk bath—and these operations were repeated over and over, with various manipulations in between.
Finally, the mordanted material was dyed by boiling it in a bath containing the finely-ground madder root, and then “brightened” by washing out, in a boiling soap bath, all the loose color and the unfixed mordant. This process was repeated until the proper shade was reached.
During the early part of the nineteenth century, various extracts of madder were made, by treating the ground root with strong sulphuric acid and other agents, which destroyed the woody tissues and other inert matter, without injuring the coloring matter. The dyeing process also was greatly simplified and shortened. Later the real active principles of the madder root were investigated, and found to be two crystalline bodies named alizarine and purpurine, respectively.[23] And finally, several years after aniline dyestuffs had been discovered and manufactured, two German chemists, Graebe and Liebermann, discovered a method for making these very identical substances out of coal tar.
Since that time the cultivation and use of madder has disappeared almost entirely. But real Turkey red is manufactured to-day, and in very large quantities—and, though freely imitated by inferior products, the modern Turkey red is just as fast to light and to washing as it ever was in the past, and possesses a brilliance and a lustre which never could have been obtained formerly. The process, however, is completed now in hours, not days, and instead of yielding a few shades of red and purple, the alizarine colors have been added to until they cover a large range of blues, purples, reds, oranges, yellows, and browns, all of them as fast as the original Eastern products, and all of them made from coal tar.
The dyes already mentioned were the ones which, after hundreds of years of experiment, proved to be of distinct value. Many of them were expensive in themselves and, in almost every case, the process of dyeing with them was a quite complicated one, worked out by generations of practical dyers, and passed down from father to son as a precious trade secret.
Besides these there were, in almost every community, certain special formulæ and recipes for obtaining, by comparatively simple methods, dyes of varying degrees of value from more or less common vegetable materials. Some of these are occasionally[24] met with to this day. Thus, in the province of Quebec, well down on the St. Lawrence, the French Canadian women still dye their homespun worsteds an orange shade of yellow, of very moderate fastness to light, by boiling them with the skins of the yellow or brown onions. And they get a pretty, but fugitive, shade of golden yellow by using the dried flowers of the goldenrod.
Some recipes from the mountain districts of North Carolina, where the sheep are raised and sheared, and the wool carded, spun, dyed, and woven into homespun, are unique, and wool dyed with them shows extremely good color. Thus, for green, we are told to “Git blackjack or black oak bark, and bile it right good, and put in a li’l piece of alum. This makes the pur’tiest green, mighty nigh, that ever was.” And for purple and black the instructions are to “git maple bark and bile it. Throw in a grain of copperas and put in your wool. Bile it just so long if you want purple, and longer if you want black. The longer you bile it the darker it gits.”
Recipes like these can be picked up in country districts all over the land to this day, and where no other coloring agents can be obtained, they may still be of some use. They are to be compared, however, to the somewhat similar recipes of the herb or “yarb” doctor, now almost extinct, who concocted various brews and teas and messes from roots and leaves, and administered them as valuable remedies.
Useful these brews undoubtedly were in their day, when it was impossible to get better medicines at[25] any price, and the available drugs, even in large cities, were few and costly and but little understood. But who of us would now prefer to treat a serious illness with herb tea when within reach of even a third-class drug store?
And so to-day, when modern dyestuffs, even if not of the very best varieties, can be bought in packages at the nearest grocery or druggist, who has time to waste upon the laborious processes and messy, uncertain formulæ of former and unscientific ages?
Tribes and nations in different parts of the world seem, at a comparatively early date, to have found out the art of coloring and staining textiles with mineral compounds. Iron springs, containing iron salts in solution, are found in many countries; and such springs are always noteworthy from the taste of the waters, and the color of the sediments left when the water stands exposed to the air.
Therefore discovery of the fact that those waters would impart a permanent and quite pleasing orange or reddish-brown color to textiles was perfectly natural.
Iron Buff.—Accordingly, in different parts of the world, people learned to dip cloths in these springs and then expose them to the air, thus dyeing them this iron rust color, commonly called by dyers “iron buff.” When iron became a common metal, it was found that any soluble salt of iron would act as a[26] dyeing solution, just as well as a natural iron spring; and hence we find use made, in widely separated countries, of iron salts for dyeing.
This iron buff is used to this day, though of course it has lost the importance it had in the past. The red sails of the fishermen in the Mediterranean show this color; and it is a useful and interesting dye for weavers of hand-made rugs, curtains, and the like, because of its pleasing tone and great permanence. On the other hand, it is very likely to rub; and it fills the fibre of the cloth with mineral matter, thereby making the material stiff and hard to sew or cut.
Preparation.—Our colonial ancestors made this color cheaply enough. They carefully saved all the scraps of iron and steel that they could find—old horseshoes, broken knife blades, etc., etc.—and placed them in a barrel half filled with vinegar and water. Little by little the iron dissolved in the acid and, when it was strong enough, the housewife would soak her homespun cloth, or other material, in the solution, warming and stirring it, and making it absorb as much of the liquor as possible. Then she would take it out, wring it thoroughly, rinse it slightly, and dip it for a minute or two in another barrel half filled with a water extract of wood ashes.

After removing from the solution and wringing again, the goods were shaken out and exposed to the air for some minutes, during which time the color would develop—in other words, would make its final change to yellow or orange, or even to brownish-red, according to the amount of iron absorbed by the fibre.
[27]
The process, nowadays, is much the same, excepting that, for the first or iron bath, it is cheaper and easier to use a solution of the green crystalline iron salt, known as copperas, or asferrous (iron)sulphate. This can be obtained at, or through, any drug store at a very low price, as it is not necessary to buy a chemically pure product. The ordinary commercial salt is as pure as the work requires; this dissolves quite readily in warm water.
The amount of copperas to be used, to dye a particular lot of material a particular shade, can only be determined by experience and experiment. It is always easy to build up a color, i.e., to deepen its shade if it is too light, by dipping the fabric over again in the same dye-bath. Indeed there is a general rule to be observed in dyeing all colors like this iron buff or the manganese brown—as well as the sulphur and indigo colors, which will be described later—that are developed, or fixed, by exposure to the air. Whenever dark shades of these colors are desired, they should be produced by successive dippings in weak baths, rather than by one or two dippings in strong baths. This avoids rubbing, as far as possible, and lessens the injury to the cloth fibre. In general, it is best to start with a dye-bath containing some three or four tablespoonfuls of copperas to one gallon of hot water.
For the second, or fixing, bath—that is, the alkali bath—it is now customary to use a solution of soda instead of the extract made from wood ashes. Either cooking soda (bicarbonate of soda) or the stronger[28] washing soda or soda crystals, known to the chemist as carbonate of soda, will be satisfactory, and instead of soda the corresponding potash salts may be used, though these are usually more expensive. It is possible, too, to use a bath of the so-called caustic soda, or caustic potash, known to the chemist as hydroxide of soda and hydroxide of potash. But these, as the name implies, must be handled with care because, when strong, they are likely to burn the hands and clothes. Careful analyses of dyed mummy cloths show that the ancient Egyptians were accustomed to use for their second or fixing bath, a solution of slaked lime, or lime water.
Khaki.—By mixing in the first bath of copperas or other iron salt an equal quantity of chrome alum, and then fixing and developing as above, a certain amount of greenish chromium oxide is deposited in the fibre along with the oxide of iron. This gives rise to the shade known as “khaki.” Sometimes shaded a little with manganese brown, this was the regular dye for the army uniforms, until the recent introduction of the extremely fast and very satisfactory vat dyes.
Uses.—Iron buff is chiefly used for cotton, linen, and other vegetable fabrics; on them it gives pleasant, warm shades of orange and reddish-brown. But on wool, and especially on silk, it is not so satisfactory, owing to its tendency to roughen and injure the fibre. Indeed, in the case of silk, it is likely to greatly diminish, or even to destroy, the lustre. On cotton and linen, however, it has great fastness to light and to washing. Indeed, every one who has tried to get rust[29] stains out of a garment or a piece of table linen knows how hard a matter it is to get rid of the color.
Another important reason for using this dye is that the coloring agents are very cheap, and are easily obtained in any quantities. It has, however, some serious disadvantages, one of which is that the color, especially in dark shades, is very liable to rub. This can best be obviated by building up the shades with successive dippings; and by thoroughly washing the finished goods in a hot soap bath. The dyed goods are pretty certain to be a little stiff, and therefore hard to sew or cut, owing to the fact that the final color is composed of iron rust. When vegetable fibres are filled with a mineral matter they are naturally stiffer and harder than they were originally.
Then there is the final objection on the part of professional dyers to this color, as well as to all the other developed colors, i.e., those colors fixed by exposure to the air. It is not easy to get a smooth, even color with them, and it is very difficult to dye to shade. For handicraft work, where these two points are of minor importance as compared with the beauty of the color, this objection is not so serious, but where it is necessary to dye large amounts of yarn or cloth to a definite shade with this, or similar, dyes, it is, as a rule, far easier to use a dyestuff which does not materially change its shade after the goods leave the dye-bath.
Iron Grey.—Soon after the discovery, in different localities, of the iron buff color, it was discovered that by the action of various vegetable extracts upon the iron salts, dark grey stains could be produced which,[30] under certain conditions, would be fairly fast to light and washing.
This color was, later, found to be due to the combination with iron of the peculiar vegetable acid called tannic acid or tannin. This is found in small quantities in the juices of twigs and leaves of many varieties of plants, and, until the introduction of the modern dyestuffs, this process offered the chief method of obtaining grey or black shades upon cotton. At present it is rarely, if ever, used for that purpose, but the compound is still the basis of most of the writing inks on the market.
To make this color, the cloth is soaked for some time in a solution of an iron salt—nitrate of iron, formed by boiling a solution of copperas for a minute or two with a few drops of nitric acid, is preferable to the untreated copperas—and then, after being wrung and slightly rinsed, it is plunged into a bath containing tannic acid. This can be made by dissolving a few tablespoonfuls of the dry tannic acid in some water, or by making a hot infusion of the leaves, twigs, or bark of any plant or tree containing it. Tea leaves contain much tannin, and so do unripe English walnuts and butternuts. Acorns, oak leaves with nut galls on them, the green twigs of alders, and hazelnut bushes, have all been used to form this color.

The grey color quickly develops and, after rinsing, the material can be dried and pressed, or dipped again to obtain a deeper shade, first into the iron and then into the tannin bath. The color is a pleasant, soft shade of grey or, if dyed deeply, a black. It is fast[31] to washing, and fairly so to light, though it may become rusty on standing; like the iron buff, it is not fast to acids.
Some interesting examples of the dyeing of cotton cloth with iron buff and iron grey are shown in Plate I. They came from the mineral springs at Arima, near Kobe in Japan, where the waters are so saturated with iron salts, that comparatively short immersion, and exposure to air, will bring out a deep orange shade. The Japanese, not content with dyeing their goods plain colors, have for many generations utilized these springs in the production of figures and designs on the cloth. Plate V is an example of stencil work, where the white patterns are made by covering parts of the cloth with a “resist paste” which protects whatever it is in contact with from the action of the coloring agent.
Plate II shows a piece of soft calico on which impressions of leaves have been made by placing fresh juicy leaves between two pieces of cloth, and beating them with wooden mallets.
Plate III shows the same piece of cloth as in Plate II, after immersion in the iron spring, and exposure to air. The tannin from the leaf juice converts some of the iron oxide into iron grey; while the white figures are made by tying the cloth with string or tape (Tied and Dyed work) before dyeing it.
So far as we can tell, these two were the only mineral colors known to the ancients. Several other mineral colors, however, were in common use by the cotton dyers in the days preceding the introduction[32] of modern dyestuffs, but it is hardly worth while to dwell here on many of them. Yellow and orange shades were obtained by impregnating cloth with lead salts, and then developing with a bath of chromate or bichromate of potash, with more or less caustic alkali added for the darker shades.
Prussian blue, too, was used as a substitute for the more expensive indigo. This was formed by using the nitrate of iron for the first bath, and then developing the color with a bath of yellow prussiate (ferrocyanide) of potash. These colors, however, are so far inferior in their application, and in fastness to light and to rubbing, to the colors now at our command, that they have disappeared entirely for textile work, though they are still widely used for pigments.
Manganese Bronze (Manganese Brown, Bistre).—There is one good mineral color, however, which came into use early in the last century and which, while hardly ever used by professional dyers, is of interest to craftsmen. This color, in its chemical composition, greatly resembles the iron buff. It is quite cheaply produced by first impregnating the cloth to be dyed with a solution of a manganese salt (manganese chloride is the cheapest), and then, by means of a second bath of alkali, forming a deposit on the fibre of pink manganese hydroxide—corresponding to the greenish ferrous hydroxide—which, on exposure to the air, absorbs oxygen and forms the final brown color.
Unfortunately the alkali used in this case must be caustic alkali—potassium hydroxide orsodium hydroxide—and not one of the mild alkalies like the[33] carbonates or bicarbonates, which will do for the iron color. And, therefore, although it is rather more expensive, and is somewhat liable to weaken the fabric, it is generally more convenient to obtain this color by a one-bath process. A purple solution of the salt known aspermanganate of potash, is prepared and the cloth dipped. After being immersed it is wrung carefully and shaken out, and the red or purplish color gradually changes into the final brown. As soon as this change has taken place the goods should be plunged into a hot soap bath and thoroughly scoured, both to remove any loosely adhering particles of color which cause rubbing, and to prevent tendering of the cloth.
The latter danger, however, is always present with this process and, therefore, full shades should not be dyed excepting on heavy, strong goods like rugs or very coarse yarns or cloth. Even then it should be done carefully and by successive dippings, with a careful washing, after the color has been developed in the air, between each bath.
This injury to the cloth which, hitherto, has been the great drawback to the permanganate process, can be avoided by dipping the goods, as soon as possible after leaving the dye-baths, into a solution containing glucose, as, for instance, two or three spoonfuls of Karo (corn syrup) or molasses in each gallon of hot water. Directly the purple-stained cloth touches this solution the color changes to brown, without affecting the strength of the materials.
This color, like the other mineral colors, is rarely,[34] if ever, to be used on silk, being altogether too likely to injure the texture and the lustre of the material.
In at least one instance, however, it has been used on animal fibres with considerable success. During the critical part of the Boer war, it was at one time necessary for England to put as many of her troops as possible—especially her mounted troops—into the field. Among others the Scots Greys, distinguished at Waterloo and made famous in many other bloody campaigns as a fine old fighting regiment, were ordered to the front. There is a tradition, dating back over two hundred years, that the horses of this regiment must all be either white or grey in color. Some heaven-sent genius at the Horse Guards—the English War Department—hinted quite forcibly to the authorities that to send out a cavalry regiment on white horses to face the Boer sharpshooters, was rather a dangerous experiment. The authorities, therefore, consulted a well-known dyeing chemist. He advised them to send down, on the troopship, some kegs of permanganate; and to instruct the officers and men to sponge each horse with a weak solution of the salt, every day at “Stables.” This was done, and, in consequence, long before reaching Cape Town, the skin and hair of every horse was thoroughly colored a soft, quiet shade of brown.
The color produced by permanganate varies, according to the strength of the solution, or rather with the number of dips in comparatively weak solutions, from a light brownish tan to a full, rich, soft, seal brown. Pleasant shades, too, can be obtained by dyeing first[35] with the iron rust dye and then covering with the permanganate. This color is discharged, not only from textiles but from the hands, by soaking in a solution ofsodium hydrosulphite (commonly used in dyeing indigo) and then washing.
Before proceeding to the practical dyeing instruction it is well to say a few words about the equipment needed for the work.
Fortunately no elaborate or expensive outfit is necessary, even for the beginner. And after one has had a little experience, it is astonishing what an amount of interesting, and even important work can be turned out with a few of the very simplest utensils. The essentials may be set down as follows:
Dye-pots.
Heating devices.
Stirring rods, or dye-sticks.
Wringers.
Drying arrangements.
Dye-pots.—For this purpose, common agateware vessels are best and most convenient. There should be varying sizes to accommodate different amounts of material to be dyed. The so-called “miner’s cups,” which are agateware cups holding a pint or more, are large enough for practical work, when single skeins are being dyed. For large pieces use the wash boilers[36] which vary in capacity from one to five gallons. It is always best, especially for amateurs, to dye in one batch enough material to complete the work on hand, whether rug, portière, or piece of tapestry. This avoids the necessity of exactly matching the shade afterward.
For three and a half to four pounds of cotton rags, such as are used in making rag carpets, three and one-half gallon pots are about the right size. This amount of material will be about enough for the filling for one rug about 6×4 feet, woven on a hand loom.
Heating Devices.—Work may be done over any flat-topped stove that burns wood or coal; gas is, of course, an advantage and so is an oil stove, as with these the heat may be regulated very exactly and much time saved. For actual work, a stove with space for four or five pots is the most convenient type to use.
There should always be one large pot set aside for heating water, another for boiling out the raw goods, and still a third for boiling out and brightening the finished materials with soap, when very fast colors are used on cotton or linen; and each of these pots should be reserved for its special purpose andnot used for dyeing. This will avoid the danger of staining the goods.
The top of a kitchen range will do for heating, but whenever possible, it is best to have a separate stove, so placed that the top of it will not be more than about twenty-four inches from the ground or floor. This enables the operator to look down into[37] the dye-pot and so avoid strain, and the consequent excessive fatigue while stirring the goods.
Stirring Rods.—While the material is being dyed, it should be kept in constant motion. When working with small amounts of material, or with goods such as straw, raffia, muslin, or silk in skeins, which are delicate and easily spoiled, it is far more satisfactory in every way to use heavy glass rods for stirring. These are rather expensive. They are about fifteen inches in length and well rounded at the ends. If carefully handled and thoroughly washed, they are always clean and smooth. Care must, of course, be exercised in their use, as sudden variations of heat and cold may cause them to crack or chip, and lifting or stirring large quantities of heavy materials—anything above five pounds—is liable to break them. In these cases, it is best to use wooden dye-sticks. Broomsticks or dowel sticks, cut into two-foot lengths, with the ends rounded carefully by whittling with a sharp penknife, are excellent substitutes. For careful work it is necessary to have several sets of wooden dye-sticks—two for each main color at least—and these must be carefully washed each time after using, or they will stain cloth that is being dyed light shades. They are bound to get soft and rotten before very long, from the action of the alkali in the dye-baths, but they are easily replaced.
Good rubber gloves are extremely useful while dyeing, to protect the hands not only from being stained and discolored by the dyes, but also from the action of the chemicals—especially while dyeing with indigo[38] and other dyes wherein the caustic alkalies are employed.
After some experience in the use of dye-sticks, however, it will be found comparatively easy to handle the materials, in and out of the dye-baths, with the sticks, without at any time taking hold of them with the hand. Nothing demonstrates more clearly the skill of the dyer than the ability to carry, immerse in the dye-bath, stir, take out, wring, and rinse the materials without getting stains on either clothes or fingers. On the other hand, the amount of slopping that can be accomplished by a careless, but enthusiastic, amateur must be lived with to be thoroughly appreciated.
Wringers.—Both before and after dyeing it is very important to have at hand a good clothes wringer, preferably with metal frame. In fact, for very careful work there should be two wringers; one to wring out the raw materials after boiling them in soap and water, or, if clean, in plain water, to insure that they are thoroughly and evenly wet; and the other to wring out the excess of dye-liquor from the goods before rinsing, or, as in some cases, before hanging up to oxidize. The rubber rolls of these wringers should be kept clean by scouring with soap and sapolio immediately after finishing the day’s work, and by carefully rinsing free from dye-liquor.
It is always well to keep on hand near the wringer a supply of clean blotting paper, or cheap filter paper, or even soft, dry cheesecloth or muslin. For by wrapping the materials that have just been dyed, in any[39] of these, and then running them backwards and forwards through the wringer, it is possible to dry them with a minimum of time and exposure. This is particularly important in the case of natural and artificial silks, either in skeins or scarfs, of ostrich feathers, and of other light and fragile materials.
Drying Arrangements.—Sufficient room should be provided for hanging up the cloth to dry. An ordinary clothes-line, conveniently fastened, is the best means of support. For special purposes, where the material handled is very delicate or where the work is done in a classroom, a simple clothes-horse made of thick glass tubing, one inch or so in diameter and supported on a wooden frame, will occupy the least possible space and give the best support.
[40]
The whole art and practice of dyeing was completely revolutionized once and forever, by the discovery in 1856 of the artificial dyestuff named mauveine, or, more commonly, mauve, a name, by the way, derived from the French name of the violet-colored mallow flower.
The discovery was made accidentally, by a young chemical student, William Henry Perkin, while experimenting in a very crude and simple way, with a view to forming artificial quinine from a curious oily body known as aniline. This aniline was originally prepared by distilling indigo in a dry retort, and it had received its name from the native Javanese word “anil,” meaning indigo. While thus prepared it was, of course, very expensive. But about this time methods were invented for obtaining this same compound in practically unlimited quantities from coal tar—that heavy, foul-smelling refuse of gas works—which, up to that time, had been not only useless but actually a source of annoyance and expense to the gas companies.
Perkin conceived the idea that, by partially burning or oxidizing it, this aniline might be changed into[41] quinine. He made the experiment and there resulted a black molasses-like mass, very far removed from the white crystals he was hoping for. But by testing this with various chemicals, he found that hot alcohol dissolved part of it, and turned it into a violet liquid which had the power to dye silk and wool the same bright color. Finding that the color was fairly fast to light, and that it could be produced without too much expense, he took out a patent and, with the aid of his father and brother, set up near Manchester, England, the first factory for artificial dyestuffs.
His discoveries were at once published, and chemists all over the world began to manufacture and experiment with the new dyestuffs. Great factories were started all over Europe. From this beginning the manufacture of coal-tar dyestuffs, and more recently all their allied compounds, has become one of the most important and most profitable of all chemical industries.
The dyes first discovered, the so-called “Basic dyes,” were of great brilliancy and strength; but they were not of any particular beauty when used individually. Compared with the vegetable colors which preceded them, and especially the same shades we are accustomed to see in nature, these dyes were hard, coarse, crude, and very inartistic. This could be remedied, however, by mixing two or three of them together, such mixture tending to soften the different colors and blend all into pleasant and delicate shades.
A more serious difficulty was the fact that those early dyestuffs were usually quite fugitive to light or,[42] at any rate, far less fast than the best of the vegetable dyes that preceded them. Besides, they did not fade true. In other words, a piece of cloth might to-day be a bright red, and after a few days of exposure to the sunlight, the exposed portions might turn a yellow, a white, or even some dark color; and, in any case, the change would entirely spoil the original color scheme.
By 1868, however, the artificial manufacture of alizarine, first by two German chemists, and then by Perkin himself, served to open up another whole class of new dyestuffs, which, when submitted to the proper tests, proved to be exceedingly fast both to light and to washing. In consequence, within a few years after this discovery, the commercial use of madder was everywhere abandoned. Chemists could now produce on cotton, linen, wool, and silk, practically the whole range of colors, brilliant and dull, hard and soft, light and dark, not only of a beauty, but of a fastness to light and to washing, never before surpassed, if indeed equalled.

| AT THE AGE OF 14 | AT THE AGE OF 22 |

AT THE TIME OF THE COAL-TAR COLOR JUBILEE—50 YEARS AFTER HIS DISCOVERY OF MAUVEINE
Since that time, not a year has gone by without scores of new dyestuffs being put on the market by some of the great color houses. Of late years special efforts have been made to simplify dyeing processes, and at the same time to insure the fastness as well as the beauty of the colors. At the present time it is possible for the veriest amateur, with practically no previous knowledge of chemistry or of dyeing, and with only intelligence enough to follow some simple directions, to get, in one bath,[43] with very little expenditure of time, an immense variety of shades that are exceedingly fast to light and to washing. A very few years ago this result could not possibly have been obtained, except by some expert dyer, and then only after long and tedious, as well as difficult, processes.
We are all familiar with the constant complaint that it is now impossible to get goods dyed or printed in good, fast colors. For instance, take the brilliant scarlet calico commonly known as Turkey red. In the days of our grandfathers a piece of cloth dyed Turkey red would stand rain and sun, washing and scouring, and thefibre would wear out before thecolor would fade. But nowadays, if you buy Turkey red cloth for the purpose of covering cushions for a piazza-lounge, you will be fortunate if the color does not begin to change after three or four days in the open air.
The reason is simple. In the old days theonly way to get that particular shade was by dyeing the cloth with ground-up madder root, through a series of operations lasting the best part of two months. Now any capable dyer would be able to dye cotton that exact shade with any of, say, twenty different colors, most of which would not require more than one or two hours to dye. Out of these twenty dyestuffs, four or five, rather more expensive than the rest, would give just as fast, just as brilliant, and just as strong color as the good old madder color. But the rest, which are distinctly cheaper and easier to apply, would furnish goods which wouldlook exactly[44] the same to the average purchaser, but which might notlast any time at all.
Naturally, the average manufacturer carefully instructs his dyer to furnish him with the “cheap and nasty” goods, not only because it costs less money, but also, unfortunately, because he reasons that “it will be good for business.” The manufacturer has the greatest sympathy with the inclination of the fastidious housewife to throw away anything that looks faded, and to buy in its place something new and fresh. Curtains or portières that hold their original shade indefinitely, he has little or no patience with. A calico dress that keeps its color so that it can be worn for a second summer, is an abomination not to be endured. And in every case, when complaint is made, it is always said to be the fault of the chemist who produced and put on the market such “horrid, fugitive dyes.”
As a matter of fact, it is simply a case of picking and choosing. There have been discovered, so far, several thousand different coal-tar dyestuffs of all sorts and kinds. Out of these, probably one hundred, or less, can be considered really fast to both light and washing. The remaining ones, most of which never were considered valuable enough to put on the market, vary in degrees of fastness, the poorest being simply stains which will “bleed” indefinitely with moderate washing, and which will turn almost any color after exposure for a few hours to sun and weather.
In the following pages, considerable pains will be taken to emphasize the names and properties of the[45] very best and fastest dyestuffs in the different classes,[1] so that the results of work done with them can be depended upon.
[1] In some classes there are no absolutely fast dyestuffs.
Perhaps the most interesting thing, in connection with the whole subject of the artificial dyestuffs, is the enormous influence that they have had upon the life of the whole human race. This influence was but slightly appreciated, even by the chemists themselves, until a few years ago. The awakening dates from the time of the fiftieth anniversary of the discovery of mauveine, when from one end of the world to the other, honors were showered upon Sir William Henry Perkin, then grown old and nearing the end of his useful and prosperous life. It was then announced, and was for the first time generally recognized as true, that no one of the great discoveries of the nineteenth century—the steam locomotive, the steamship, the telephone, the telegraph, the gas light, the electric light, and the rest—had been more important to the world at large than the discovery of the first coal-tar dye. And probably never in the history of the world have such enormous results been produced from a single discovery, during the lifetime of the discoverer himself.
The artificial dyestuffs form such a large body of complicated chemical compounds, that at first glance it would seem hopeless for any one who is not a trained[46] chemist, to attempt to get any clear or definite ideas about them. This, indeed, would be the case if any attempt were made to study them chemically, i.e., with reference to their composition, or their method of manufacture; but when it comes to the application of them to the various textile fabrics and other materials, for which dyes are valuable, we soon find that the problem is not so very difficult after all.
To be sure there are many hundreds of different dyes on the market now, great numbers of which are known under three or four different trade names, according to the trade-mark of each particular manufacturer. But besides the great manufacturers, and their accredited agents, there are numerous retail agencies all over the country, large and small, which make a business of distributing dyes made by the great concerns. Some of these are very energetic, and have pushed the sale of artificial dyestuffs in ten- and fifteen-cent packages, until in almost every village, large enough to boast of a decent country store, these dyes can be obtained.
It is common to hear these dyes sneered at and abused. They are frequently referred to, especially by those of “artistic tastes,” as harsh and crude in color, fugitive to light and washing, and, in short, generally inferior and worthless products. This is not the case. They are, in some cases, individual dyestuffs, and in other cases, mixtures, generally belonging to the class of colors next to be described, the Salt dyes; and very good, if not indeed the very best specimens of that class. These Salt dyes, until[47] the last few years, were far from fast, either to light or washing; but the more recent members of the class are much more satisfactory, and these colors, too, are found in the fifteen-cent packages.
Nor, too, can objection be fairly taken to the shades as being crude and harsh. That is all a matter of taste and skill on the part of the dyer. There is no better practice in dyeing than to take the very hardest, clearest, most brilliant red, blue, and yellow colors that can be found at the corner grocery and, following the directions on the packages, proceed to dye yarn or cheesecloth with them, at first using the individual dyes, and afterwards modifying the shade of one dye with traces of each of the other two. The softness and richness of the tones that can be thus obtained will satisfy the most critical.
The real objection to these widely distributed popular dyes is a very different one. They are not sold under their own names, and therefore it is almost impossible to identify them. To be sure, from the accompanying directions it is possible for a trained dyer to recognize at once the class to which the dyestuff belongs. But it is impossible for him, excepting after a long, tedious and often very troublesome analysis, to tell just what member or members of that particular class is contained in any given package. For this reason the dyer who has to depend on them for an important piece of work is in much the same position as a doctor would be who had to treat a difficult case with patent medicines compounded after secret formulæ.
[48]
In the following chapters, a discussion of each class of dyestuffs, and an explanation of their application and general properties will be followed by lists of three or four of the very best colors, sold by the New York agents of six of the largest and most reliable color manufacturers.
Workers wishing to obtain these dyes in comparatively large quantities, say one pound and upwards, can get them by writing directly to the addresses in the following table:
Badische— The Badische Anilin & Soda Fabrik,
128 Duane St.,
N. Y. City.
Cassella— The Cassella Co.,
184 Front St.,
N. Y. City.
Elberfeld—The Farbenfabriken of Elberfeld Co.,
117 Hudson St.,
N. Y. City.
Kalle— Kalle & Co.,
530 Canal St.,
N. Y. City.
Klipstein—A. Klipstein & Co.,
Agent for Society of Chemical Industry of Basle,
654 Greenwich St.,
N. Y. City.
Metz— Farbwerke-Hoechst Co., formerly H. A. Metz & Co.,
Agent for the Meister Lucius & Bruning Co.,
122 Hudson St.,
N. Y. City.
N. B. Further information concerning dyestuffs, apparatus, textiles, chemicals, etc., connected with this work may be obtained on writing to the author at 7 West 43rd St., New York.
[49]
It is important to remember that, in order to identify a color by name, it is necessary to know three things: first, the trade name; second, the shade, or distinguishing, letter; and third, the manufacturer or agent. The trade name sometimes bears a reference to the class, properties, or color of the dye, as “fast acid blue”; or to its chemical composition, as “methylene blue,” or “diamine red”; but in most cases it is simply an arbitrary name, given by the original discoverer when the patents were issued, or assigned later by the manufacturer or his local agents.
The letter or letters, following the name, refer generally to the shade, as for instance, B for blue, R for red, Y or G for yellow (Germangelb), and so on. Thus “methyl violet” is sold in brands running all the way from 6 B to 6 R—that is, from full purple shades that are very close to blue, to bright violet shades, very close to red. Sometimes, however, the letter refers to the composition of the dye or its class, as “fuchsine S” (Germansauer) often called acid fuchsine or acid magenta; or “alizarine blue, D,” when the D indicates a “direct” cotton color. And sometimes the letter F is used to indicate fastness to light, in which case “F F” would signify a brand of very unusual fastness, for that particular class of colors at any rate.
But not infrequently the letter is merely a mark applied for purposes of identification, whose significance[50] cannot easily be learned by those not in the business of color selling, even when it is not a secret closely guarded by the particular firm supplying the dyestuff.
For this reason, the name of the manufacturer or agent shouldalways be added to the color name and letter, if it is important to get a particular color in any case. The best of the older dyes are manufactured by all of the larger firms, of substantially the same strength and shades, although often not under the same names. The later colors, whose patents have not expired, are of course the individual property of the different manufacturers, and can be, and are, marketed by them under any name they like to give them. Accordingly it frequently happens that two different firms may sell, under the same name, two entirely different colors; it would be impossible to tell which dyestuff was intended unless the firm name were attached.
But with these three essentials correctly given—name, brand, and maker—a color can be identified and obtained true in composition and shade, even after the lapse of many years.
[51]
| Class Name. | Materials on which to be used. | How applied. | How developed. | How finished. |
I. Direct Cotton or Salt Colors: |
Cotton, linen, and artificial silk. Rarely wool and silk. |
In boiling water, with addition of salt. |
By rinsing in water. |
|
II. Sulphur Colors: |
Cotton and linen. Rarely silk. |
In hot or lukewarm water, with addition of soda, sodium sulphide, salt, and Turkey red oil. |
By exposure to air after wringing. |
By washing in a hot soap bath, and rinsing. |
III. Indigo or Vat Colors: |
Cotton and linen. Rarely silk. Also as stencil pastes on cotton and linen. |
In hot or warm water, with addition of caustic soda and sodium hydrosulphite. |
By exposure to air after wringing. |
By washing in a hot soap bath, and rinsing. |
| [52]IV. Basic Colors: | Raffia, straw, rattan, and basketry in general. Artificial silk. Leather. Rarely wool and silk. Also as stencil pastes on cotton, linen, and silk. |
In hot or warm water, with addition of a little acetic acid (vinegar). |
Raffia, etc., finished by rinsing in water. Leather by rubbing with wax when dry. Stencilled work, by steaming and passing through a weak bath of Tartar Emetic. |
|
V. Acid Colors: |
Wool, silk, and feathers.Sometimes leather. Rarely rattan and basketry. |
In hot or cold water with addition (for wool) of sulphuric acid and Glauber’s salt. For silk add soap and acid. For leather add a little acetic acid. For feathers add oxalic acid or formic acid. |
Wool needs very careful rinsing in water, to remove every trace of acid. Silk finished by a cold soap bath, followed by a weak bath of acetic acid. Leather finished with wax. Feathers finished with starch. |
Among the many changes made in the art of dyeing since the introduction of the coal-tar dyestuffs, perhaps the most important has been the gradual overcoming of the necessity for mordanting the textiles before coloring them in the dye-bath. Almost all of the old vegetable dyes were mordant dyes; that is, the color could not be fastened to the fibre, whether wool, cotton, linen, or even silk, unless the latter had been impregnated with some chemical which would act as amordant to—(i.e., would combine with and hold) the color. These mordants were, in general, the salts of some metal, aluminium, tin, chromium, and iron salts being the ones in common use; and the processes involved in properly mordanting the goods were in many cases—notably in the case of madder and the Turkey red process—far more difficult and tedious and expensive than the actual dyeing.
The first dyestuffs discovered, the true aniline dyes, which were manufactured directly from aniline and from substances strongly resembling aniline in chemical composition, were at once found to act in a different manner on textile fibres. Animal fibres like wool and silk, fur and leather, were dyed by them directly, without the use of any mordant at all. If[54] the dyestuff were dissolved in water (the addition of a little acid makes the color dissolve more readily, but is without other effect) and a wet skein of wool or silk were immersed in it, and a little heat applied, the color would leave the liquid, and fasten itself firmly on to the goods.
But with cotton and linen and other vegetable fibres, these dyes would not work so well. When these materials are warmed in such a dye-bath, the color does not adhere to the fibres, but washes off directly in a hot soap bath, if not, indeed, under a stream of clear hot water. This was noticed by Perkin very soon after his famous discovery, and, wishing to use his new color for dyeing cotton and linen as well as silk and wool, he set to work to discover how to prepare these materials; in short, how to mordant them so that they too would take firm hold of the color. As will be described later in the chapter on Basic colors, his experiments soon led to the introduction and the use of tannic acid and tartar emetic combined, in a process widely used to this day.
The next class of dyestuffs discovered were the so-called acid colors, thus named because they all exhibited distinctly acid properties—that is, they would form salts with the substances known as bases (of which last, by the way, aniline is an important member). These colors, like the earlier ones, would dye the animal fibres directly, but would not color the vegetable fibres, unless the latter were carefully mordanted with alumina, or iron oxide, or some similar[55] metallic base. And even this treatment does not give colors that are fast to washing, so these acid colors are never used on cotton or linen.
After this came the discovery of alizarine, and an important series of very fast and very valuable dyes, all of which were characteristic mordant colors. Even wool and silk, as well as every other textile, must be carefully mordanted with aluminium, chromium, or iron salts, in order to have any coloring effect produced by these dyestuffs. This is the chief reason why, in spite of their beauty and great permanence, the alizarine and other mordant colors are being less used every year. At the end of some twelve or thirteen years after the discovery of the aniline colors, therefore, it was still impossible to dye cotton with them without a more or less elaborate mordanting process. And yet the problem did not seem to be an impossible one. One of the natural dyes, the safflower, already mentioned, has the property of dyeing cotton pretty, and not very fugitive, shades of pink and rose colors, directly, without the necessity for any mordant; and if a natural dyestuff could do that why could not some artificial ones?
Some thirty years ago, a chemist (one story says that it was a laboratory boy) while experimenting with a dyestuff which was then a recent discovery—Congo red, a very brilliant but fugitive and unstable scarlet color—noticed that while filtering a hot solution of it through filter paper, the paper was stained deeply, and, which was more important, the color was not easily washed out with hot water.[56] This excited his curiosity, and after following the matter up a little, he found that not only this Congo red, but a whole series of dyestuffs formed in the same general way, had the power of dyeing cotton directly. This discovery has practically revolutionized the whole art of cotton dyeing. From these few bright and pretty, but distinctly untrustworthy dyes, which were at once named and advertised as “direct cotton colors,” have sprung great numbers of dyestuffs—several hundreds at least—of every conceivable shade, and of late years of every conceivable degree of fastness to light. All resemble the original Congo red in that they will dye cotton and linen, if not absolutely fast, at any rate very fairly fast to washing, in one bath, without the need of any mordants.
This, of course, means that the cost of dyeing cloth with these dyes is very much less than with the other classes mentioned. And, by the way, it also explains why, under the name of Turkey red, so many extremely bad colors have been sold. To dye Turkey red on cotton, using alizarine, and with the most improved and simplified methods, necessitates at least six or seven different steps, each of which requires not only time and expense, but great skill and care; and any one of them, if carelessly performed, may spoil the goods. On the other hand, a mere beginner, by using one of the early, bright, direct colors (quite cheap in itself, because the patents have expired) can, by boiling the goods for half an hour in a dye-bath with a little soap and salt in it, produce a piece of cloth dyed almost the exact shade of the old Turkey[57] red, for probably one-third, or one-quarter of the price. It will look the same on the shop counter; will probably sell just as well to the average, or even to the painstaking customer; but when exposed to air and light for a few weeks, perhaps even for a few days, will lose its brilliancy, and turn some queer, dull shade, probably of purple.
Indeed this particular substitution has been going on for some years on a large scale; and at one time promised to be of some international importance. The Turkey red dyers in Manchester, a few years ago, complained bitterly to the English Government that their market in India was falling off very seriously; and they demanded an investigation, to know what was the matter.
After careful inquiry by the local officials, word came back that there was no difference in the taste of the people for bright scarlet clothes and headgear. Just as much red was worn as ever before. But active agents of the large German color houses had been going through the country, introducing some of these cheap direct cotton scarlets and showing the natives how to use them. And in consequence, up and down India in all the little towns, even in the villages, local dyers were at work who, for a few cents, would dye up an old piece of calico bright red. When it became faded again in a few weeks, they would dye it over again for a very small sum, thus renewing the same piece whenever it was desirable to appear in bright, new clothes.
Names.—These dyes have long been made by all of[58] the great firms, although two or three have made more of a specialty of them than the rest. It was soon found that the presence of common (table) salt in the dyestuffs was valuable, as lessening the waste of dyestuff in the dye-liquor, and also increasing the fastness to washing of the dyed goods. For this reason the common name given to this class is that of “Salt Colors.” Owing, however, to the fact that Congo red, the first discovered of the whole class, was derived from the chemical known as benzidine, these salt colors are sometimes referred to, in general, as the “Congo,” or as the “benzidine” dyes. Besides this they are frequently known as “cotton colors,” or “direct cotton colors.” The different manufacturers, however, have assigned certain class names to their own dyestuffs, as follows:
Benzo (Elberfeld); Diamine (Cassella); Dianil (Metz); Mikado (Elberfeld); Naphthamine (Kalle); Oxamine (Badische); Phenamine (Badische).
Uses.—These colors are chiefly used for dyeing cotton, linen, and paper. They take particularly well on mercerized cotton, and on some varieties of artificial silk. They can also be used to dye wool and silk, and, indeed, in many cases give colors faster, both to light and to washing, on these fibres than on cotton. As a rule they will not dye animal fibres excepting at a high temperature—near the boiling point—and in an acid bath. Whereas cotton and linen are preferably dyed in an alkaline or at least a neutral bath, and, while they must be boiled in the dye-bath for at all[59] permanent results, will take the color as a stain at quite low temperatures.
For this reason these dyes are often used for dyeing even shades in one bath, upon mixed goods—that is, wool and cotton, cotton and silk, etc. The goods are first dyed in a lukewarm bath till the cotton is nearly the proper shade, and then, on heating, the wool or silk will take up the color and, before long, catch up with the cotton. It must, however, be remembered that on cotton and linen these dyes are not, as a rule, at all fast to washing, unless they have been well boiled with the goods. When dyed on silk at a boil, they are fast to hot soap and water, a fact which, sometimes, is of much importance.
Dye-bath.—The color must first be dissolved in water, care being taken not to leave any undissolved lumps or specks of color floating around in, or settled at the bottom of, the dye-bath. For this reason it is generally best, in all dyeing operations, first of all to make a decidedly strong solution of the color, by dissolving a considerable quantity of it (depending of course on the amount of goods to be dyed) in hot water, in a pitcher or saucepan. In the dyehouse this would be called a “stock solution,” and would always be made of a definite strength,—say five parts of color to one hundred of water—and kept well covered up. Sometimes in hot weather it would be treated with a little preservative like benzoate of soda, so[60] that it could be used at any time it was needed. When this color solution is added to the dye-bath, it should always be carefully strained through a piece of cheesecloth or any other fine medium that will catch the specks and undissolved lumps. Otherwise spots are liable to appear, on the finished goods, which it is almost impossible to eradicate without stripping off every trace of color from the dyed material.
Water.—The dye-bath is prepared with plain water. The amount necessary for each lot of goods can only be told by experience. For some classes of dyes, like the Acid colors and the Basic colors, to be described later, the quantity of water makes but little difference. But for dark shades with these Salt colors it is best not to have more than enough water to thoroughly soak, and comfortably cover, the wetted goods, with enough room to stir and turn them easily. The dye-bath is now set on the stove to warm up and, when dyeing light or medium shades, some soap is usually dissolved in it. This is not absolutely necessary but helps to make the color go on more evenly, and penetrate the fibres better.
Soap.—For dyeing purposes in general, any pure, carefully made soap acts satisfactorily. For silk dyeing, and especially for silk finishing, it is said that greater lustre can be gained with olive oil (Castile) soap. But when this cannot be obtained, Ivory soap or Pears’ soap or, in fact, any good brand of bath or toilet soap will do almost as well. For the washing and finishing of wool and silk the use of strong laundry soaps should be avoided if possible, because they[61] usually contain alkali, in the form of borax or of carbonate of soda, which is liable to “tender the goods.” For cotton and linen dyeing and finishing, this does not make any difference. The easiest way to add the soap to the dye-bath is to use it in one of the wire soap-shakers, which has a convenient handle, and holds half a cake or even a whole cake of soap at one time.
Even Dyeing.—The goods should be well washed, rinsed, and wrung out, so as to be sure that they are free from dirt and grease, and have been thoroughly and evenly wet. They are then placed in the dye-bath, completely under the liquid, and stirred round and round and turned over and over with the dye-sticks. The chief objects in stirring are, first, to prevent part of the goods from resting on the bottom and then getting more heat than the rest of the material, in which case, naturally, it will become darker when finished; and second, to prevent the outside portion of the goods from getting more color than the inner portions. Accordingly the goods, when placed in the dye-bath, must be well opened up and, excepting when deliberately making patterns by the method described later under the name of “Tied and Dyed Work,” they should not be tied or entangled in knots or bunches. Every part must be equally exposed, by the turning and lifting and stirring, to the action of the color solution.
If only light shades are desired, the goods are heated and turned until the proper shade has been reached—remembering always that, unless the color[62] has been boiled on, it is likely to be only a stain which will wash off easily.
Salt.—For full and indeed for medium shades, it is customary to add to the dye-bath some agent—usually table salt or, when the shade is not very dark, phosphate of soda—which will make the color less soluble in the dye-liquor and will tend to throw it on the fibre. For, after all, there is comparatively little affinity between the cotton fibre and the dyestuff (far less than between silk or wool and the Acid or Basic colors), and when a skein is warmed or even boiled in the dye-bath a large proportion of the color remains in the liquid. The bath is not “exhausted” as the dyers say. Hence, if we try to dye full shades with these colors dissolved in water only, or in soap and water, it can only be done by using large quantities of the dyestuff, most of which will be wasted in the spent dye-liquor.
For dark shades, then, where there is little danger of uneven dyeing, the goods are usually dyed for a short time with the color dissolved in hot water. And then, to deepen the shade, the goods are lifted, and common salt added in considerable quantities, three or four tablespoonfuls to the gallon, and stirred round till it is dissolved. Then the goods are put back and well boiled for half an hour or so, before the dyeing is considered complete. The presence of salt, by increasing the temperature of the boiling bath, also helps to make the dyed goods fast to washing.
Soap cannot be used in the presence of so much salt for fear of its depositing on the fibre in spots and[63] so causing trouble. For medium shades, however, where it is well to use soap in the dye-bath so as to have the color go on the fibre evenly, a little phosphate of soda is often employed instead of salt, one or two tablespoonfuls to the gallon, to diminish the waste of color, without making the soap insoluble.
For the darker shades it is particularly important to thoroughly boil the goods for half an hour or more, before taking them out of the dye-bath. Otherwise the dyestuff will not penetrate the fibre, but will simply stain the surface, and will not only be easily washed off, with very mild soaping, but, when dry, will be apt to crack and rub.
Finishing.—After the materials have been dyed as just described, they should be taken out of the dye-bath, rinsed with water to wash off the excess of dye-liquor, and then shaken out and dried.
When used in this way the best dyes of this class, such as those listed a little further on, will give, on cotton and linen, shades that are very fast to light, and fairly fast to washing. On wool and silk the shades are fast to both light and washing. For purposes of comparison it may be stated here what is generally meant by these terms.
Fastness to Light.—The test for light-fastness is usually made by partially covering a dyed skein with a piece of wood, or heavy piece of blotting paper, and exposing it to direct sunlight, back of a window with southern exposure. At intervals the skein is taken out and the color studied, and it is then easy to see whether any change has taken place in the portion of[64] the goods exposed to the light. If the goods have faded appreciably in the space of one week, the dyestuff is considerednot fast.
If the color changes after two weeks’ exposure, but not after one week, it is to be consideredfairly fast.
If it stands for two weeks but fades in four weeks it is to be calledfast.
And if it resists, without appreciable change, the action of the summer sunlight for full four weeks, it is calledvery fast.
It should be remembered, in this connection, that the comparative fastness to light depends largely (a) upon the materials to be dyed, and (b) upon the depth and shade of color used in the test. For instance, if a skein of heavy cotton yarn, and one of very fine, brilliant, artificial silk are dyed the same color, and exposed to light under the same conditions, the cotton skein will hold its color longer than the silk. The latter, being semi-transparent, allows the sunlight to pierce it through and through, while the more opaque cotton gives some distinct protection to the color that has penetrated beneath the surface. So, too, a dark shade of any given color will stand the light much better than a very light or delicate shade, for the same general reason. The color beneath the surface is protected from the direct action of the sun’s rays by the surface color.
Fastness to Washing.—The test for washing-fastness is made somewhat differently. A skein dyed a full shade with the color is twisted up with two white[65] skeins, one of wool and the other of cotton, and the three are thoroughly scoured for ten minutes in a strong bath of good quality laundry soap, heated to 140°F. This temperature is uncomfortably hot for the hands and yet is well below the boiling point. A fast color is one where, with this treatment, neither the soap liquor nor either one of the skeins becomes colored.
If the soap liquor is colored but neither one of the skeins, the dye is calledfairly fast.
If the soap bath is tinged, and one or the other of the skeins becomes colored at the same time, the dye is considerednot fast.
It must, however, be borne in mind that before making this washing-test, all excess of dye-liquor must first be removed by thorough rinsing. And it should be remembered that even the fastest of the Salt colors, as well as of the Acid and Basic colors described later, when applied directly to the fibre, without mordanting or after-treating, are never as fast to washing as those where the dyestuff is fixed or developed in an insoluble form in the fibre, by the action of the air, as are the Sulphur and Vat colors—or by the action of mordants, as with the Alizarine colors—or by after-treatment with certain special chemicals, as with the Salt colors in the process described below. All dyes can, sooner or later, be dissociated from the fibres to which they are attached. But if they are in an insoluble condition they drop off in the form of a powder, and are washed clean off, and leave sharp, clear outlines on the dyed goods.[66] If, however, they have gone on in solution they will go off in solution, and are liable tobleed, and stain light-colored fibres near them.
The earlier dyestuffs of this class were deservedly criticised as being, even when carefully applied, much given to bleeding, and also distinctly fugitive to the action of sunlight.
Of late years the quality of these dyestuffs has greatly improved, and the best of them, like those mentioned below, when carefully dyed on cotton, are fast, if not very fast to light, although for washing the very best can hardly be classed even as fairly fast, without after-treatment.
List of Selected Dyestuffs.—
| Badische— | Oxamine Fast Red, F |
| Cotton, Yellow, G I | |
| Stilbene Yellow, G K | |
| Oxamine Blue, B | |
| Cotton Black, E, extra | |
| Cassella— | Diamine Fast Red, F |
| Diamine Fast Yellow, G G | |
| Diamine Fast Blue, F F G | |
| Diamine Fast Black, F | |
| Elberfeld— | Benzo Fast Red, 8 B L |
| Benzo Fast Yellow, 4 B | |
| Brilliant Fast Black, 4 B | |
| Pluto Black, F, extra | |
| Kalle— | Naphthamine Fast Red, H |
| Naphthamine Fast Yellow, 2 G L | |
| Naphthamine Fast Blue, 4 B L | |
| Naphthamine Fast Violet, R L | |
| Naphthamine Direct Black | |
| Metz— | Dianil Fast Scarlet, 4 B S |
| Dianil Orange, G | |
| Dianil Yellow, O O | |
| Dianil Fast Blue, 3 B | |
| Dianil Fast Black, conc. |
As above mentioned, even the very best dyes belonging to this class of Salt colors, give on cotton and linen results only “fairly fast” to washing. As the modern laundress is not averse to using stronger agents than good laundry soap in her washtub, and not infrequently indulges in considerable amounts of washing soda (sodium carbonate) and even of bleaching powder, to clean quickly a dirty piece of goods, dyes that are “fairly fast” according to the regular standard, will, in practice, need some care spent on them if they are to hold their color for long periods. Against light the best ones are almost as fast as any dyes known, but none of them are a match for the Sulphur colors, or especially the Vat colors, when exposed to severe washing.
After-treatment.—The professional dyer, who is occasionally called upon to produce fast colors with these dyes, and even with the inferior members of this class, has found various methods of after-treatment, by which the colors are rendered more permanent.
A favorite process, where the dyer is enough of a chemist to carry it out, consists of making an entirely new dyestuff in the fibre, generally of an entirely different shade, and with much greater power of resistance to washing and to light, by treating the dyed goods first with a mixture of sodium nitrite and of sulphuric acid, and, after this, passing them through a solution of some organic chemical such as carbolic acid, alpha- or beta-naphthol, or others known as developers.
[68]
This process, known as “diazotizing and developing,” is considerably used in the trade, especially for various shades of black, but is too complicated and delicate for craftsmen in general.
A simpler process is to warm the dyed goods for five or ten minutes in a weak solution of the orange-colored salt, bichromate of potash, acidified with a little acetic acid—or of the not uncommon chemical, sulphate of copper, long known to chemists as blue vitriol.
When the best dyes are used, like those in the preceding list, it is not often necessary to use either of these reagents. But when, as sometimes happens, one is obliged to use dyes of this general class, bought at the country store without a chance of knowing how fast they are, it is well to know about it. For a piece of goods the size of an ordinary linen skirt, the after-treating bath would be made as follows: In two and a half gallons of hot water, dissolve two tablespoonfuls of sulphate of copper, one tablespoonful of bichromate of potash, and two teaspoonfuls of ordinary acetic acid (equivalent, say, to three or four teaspoonfuls of strong vinegar). The goods, after dyeing and rinsing, but before drying, should be soaked in this bath and heated for ten minutes until not far from the boiling point. They should then be taken out, rinsed carefully, and dried. This after-treatment does not benefit every single color of this class, but it helps greatly the fastness to light and to washing of almost all of them. The chief objection to it, besides the time and expense, is that the shade[69] of the finished goods is often considerably changed by the process.
Properties and Uses of the Salt Colors.—Generally speaking, the shades produced by the individual members of this group cover all the colors of the rainbow and include several good greys. It is hard, however, to get a full deep black on cotton or linen with these dyes, without using the “diazotizing and developing” process of after-treatment. The dyes go on the fibre in a soluble form, and unless a developing process like this is used they combine directly with the fibre, and do not form a coating or layer upon it, as do some of the “developed” dyestuffs. Accordingly, no matter how fully or how deeply we dye a piece of yarn or cloth with a black dye of this class, the finished goods will showgrey, a very dark grey, to be sure, but still grey, and not a flat, heavy, true black. The color of most of the salt blacks is greatly improved, however—as well as their fastness to light and washing—by soaking the dyed goods, after rinsing, in a solution containing four or five spoonfuls of formaldehyde to the gallon.
This same property, however, of combining directly with the fibre, makes the colors brighter and more brilliant than many of the other classes, especially in the lighter shades. Accordingly for bright, pretty shades of pinks, blues, yellows, and of mixed shades, fast to light, but not very fast to washing, very easily and simply applied, these colors are extremely valuable. For instance, in dyeing large quantities of bright colors on calico or cheesecloth, for some special occasion,[70] as a pageant or spectacle, these are the colors to use.
Another great advantage they possess is that they dye true; that is, they do not alter their color when exposed to the air, and the color of the finished goods can be fairly estimated from the color of the dye-bath.
Accordingly, the student is strongly urged to practise the art of dyeing with these colors. They are cheap and can be readily obtained, although not always of the very best quality, under the name of Diamond Dyes for cotton, ezy dyes, etc., from druggists and grocerymen all over the country.
They can be easily applied to cheesecloth, muslin, and other inexpensive materials, and if care is taken to soak and boil the goods thoroughly, to linens and heavy cottons. In case of necessity they can be used on wool and silk, but, as a rule, their use is limited to vegetable fibres. They are particularly valuable to amateur dyers and to beginners in the art, because they have great “levelling” power; that is, it is easy to dye evenly with them.
On the other hand, it is a nuisance, oftentimes, to have to boil the goods, and even then the colors are not really fast to washing. At any rate, before proceeding to the study of the more permanent but more complicated Sulphur and Vat colors, the art of dyeing even and rainbow shades and at least the beginnings of the art of combining and matching shades should be carefully and conscientiously worked out with these often despised, but really very useful and valuable, Salt colors.
[71]
Directly the student has mastered the instruction contained in the three previous chapters, and can use the dyeing apparatus and the unmixed dyestuffs so as to get reasonably fast colors on cotton and linen goods, it is time to attack the more difficult subject of dyeing to shade. This art is not an easy one, by any means, and only a few fundamental principles can be learned from a book. To make any real progress in it, constant and continuous practice is necessary; even then, unless the student is naturally gifted with an eye capable of readily detecting any changes of color, and has trained it to distinguish and identify the causes of such changes, little success in the matching of colors can be hoped for.
This does not mean, however, that unless a dyer can match shades perfectly, he cannot turn out very interesting and, indeed, beautiful results. But it does mean that he will find it difficult, if not impossible, to reproduce such results, and will be frequently handicapped in trying to utilize his dyeing skill and knowledge commercially.
The beginner thinks—not unnaturally perhaps—that[72] in order to get any considerable variety of shades it is necessary to have on hand a large and varied assortment of dyestuffs; and it is consequently a surprise to find that skilled workers keep in stock chiefly a good supply of blue, yellow, and red only. Black is convenient and useful, but not essential, excepting for special purposes. By mixing these three “primary” colors it is possible to get every conceivable shade needed. And another point, which will be emphasized below and which is also likely to be a surprise, is that practically every pretty and agreeable shade, no matter how delicate, is composed of all three of these primary colors. Blue and yellow produce green, blue and red produce violet, and yellow and red produce orange, while the addition of the third or “complementary” color to any of these combinations of two makesgrey, when all three colors are perfectly balanced, and when one color or another predominates, it is greyed and softened by the presence of small quantities of the other two.
Experiments with Single Colors
The way to study color dyeing is, first of all, to get a clear idea of the effect of different strengths of each of these three primary colors in producing both light and dark shades of a single color. This can be easily accomplished with the red, blue, and yellow of the Salt dyes described in the last chapter. Dissolve each color separately and keep them in separate dye-pots so that you can readily dye pieces of cheesecloth or other[73] cheap, easy-dyeing materials any light, medium, or dark shade, to serve as a basis for future comparisons.
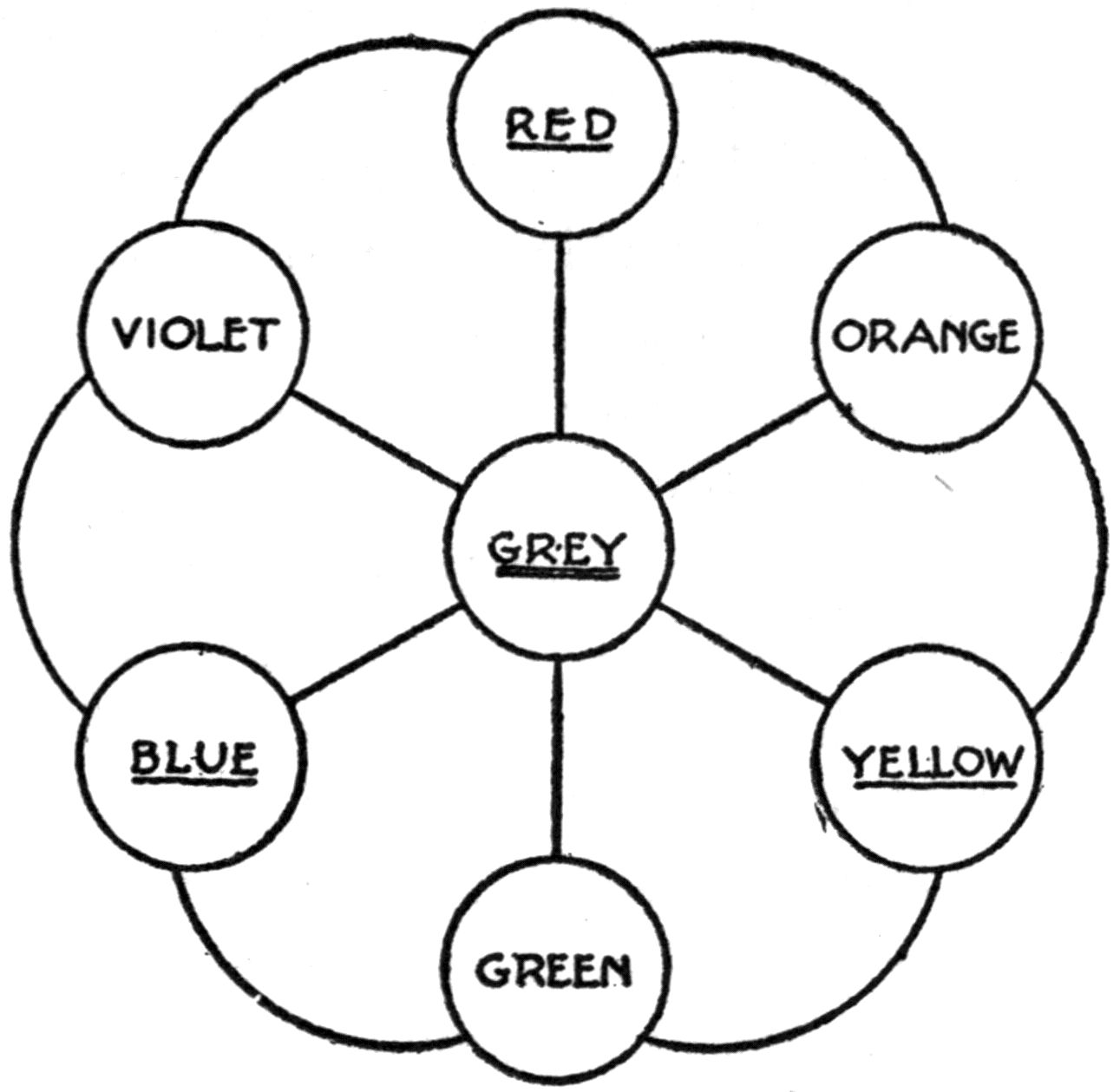
| Two color shades— | Red + Blue = | Violet | |
| Red + Yellow = | Orange | ||
| Yellow + Blue = | Green | ||
| Complementary colors— | Red + Blue + Yellow = | Grey | |
| Red + Green = | } | ||
| Blue + Orange = | } | Grey | |
| Yellow + Violet = | } | ||
Even Dyeing.—First wet the cloth or yarn thoroughly by soaking in hot water, then rinse well and wring it dry—if necessary, using a wringer. The dyestuff should already be carefully dissolved in a little boiling water. Pour some of this solution (not too much, for the shades should all be pretty light) into the dye-pot half full of lukewarm water. Then quickly and wholly immerse the wet material, stirring and working[74] about with the dye-sticks, and let the whole heat steadily until it boils. After a few minutes’ boiling take out the material and rinse in cold water until it stops bleeding. When this is carefully done, good, even, and smooth shades will result.
Shaded Effects.—Of more real interest, although an abomination to most professional dyers, are the shaded effects. Instead of trying to get even, smooth colors, the cloth is intentionally dyed unevenly to get effects of light and shade in the color, otherwise impossible. This does not mean that a skein or piece of cloth badly dyed or discolored by some accident or carelessness should be proudly exhibited as a piece of really artistic dyeing, as is done occasionally, by some workers, with painful results. It is only when the work is done carefully and thoughtfully that shaded or so-called “rainbow” effects may be obtained upon skeins, basket materials, and cloth, which are distinctly interesting and beautiful, though very different from the regular work of the professional dyers.
Many methods of obtaining unique results in this work will occur to the student, after some practical experience. Perhaps the best way to begin is to take a piece of cheesecloth, cut in the form of a scarf—say two yards or so in length—and hemmed on both ends, if it is to be kept for exhibition or future use. Before it is wet, tie it in a rather tight knot in the middle, or, if the scarf is long enough, two knots about six or eight inches from each end. For this first piece tie a very simple knot by merely folding the scarf over on itself and pulling the goods tight. Then wet[75] the cloth thoroughly and dye quickly in the boiling dye-liquor; rinse off, and untie the knots. The open part of the cloth will be found dyed the full strength, and where there were knots there will be shaded places varying from the full color down to white.
Another method is to take the wetted scarf in the middle and gradually lower the ends into the hot dye-liquor, stopping just before the middle reaches the dye. If carefully done this will give regularly shaded effects running from white or very light at the centre, to heavy, full shades at the ends. Of course, if preferred, the ends can be kept out of the dye-liquor and the middle portions immersed. This will give a scarf that is dark in the centre and light at each end—which is not so good a color arrangement, ordinarily, as the light centre and dark ends.
The same can be done with a square piece of cloth, well wetted: this will shade in an interesting manner, if held in the middle and dipped slowly and gradually. Further developments of this work, known as “Tied and Dyed Work,” are described in a following chapter.
Experiments with the Secondary Colors
After the above methods have been fairly mastered, the student should make some experiments in which two of the primary colors are mixed together, or better, superimposed one on the other to show the “secondary” shades produced by these combinations. This can be done by mixing the colors two by two, until three baths of green, violet, and orange respectively[76] are formed as before. Then try dyeing first for even colors and later for the shaded effects.
The most interesting experiments in this line are made by the so-called “double shading” method. Here the same baths of straight primary colors—red and blue and yellow—should be used as in the earlier experiments; but the goods are first dyed in one bath, and then after-dyed or “topped” in a second color.
A scarf of cheesecloth is good for a first attempt. This, well wet, is held at one end and very slowly lowered into the hot bath, until all but about six inches of the entire length is immersed in the dye. This much is left free from color. Try a blue dye color for this series of shades, fading evenly and smoothly from the deepest full blue at one end to a pure white at the other.
After rinsing with water till the bleeding is over, reverse the scarf, holding it by the opposite end, and lower it slowly and gradually into a bath of, let us say, yellow, keeping about six inches out of the dye as before. This will produce a scarf shaded from clear blue at one end to clear yellow at the other end and showing the whole range of green shades produced by mixing these two colors, along its length.
Similar tests made with red and blue, and then with red and yellow, will emphasize to the student’s mind the fact that green is formed from blue and yellow; violet from red and blue; and orange from red and yellow; and that each combination gives an infinite variety of intermediate shades, according to the comparative strength of the individual dyes.
[77]
Matching Colors
The next step is to dye some pieces evenly with green, violet, and orange, made by two of the primary colors, and then to try matching these with fresh, newly-mixed baths of the same dyes. It will be found here that success depends upon going slowly; and upon beginning with light shades and building the color up to the desired strength carefully, by means of successive dippings. Note that the color of cloth when wet is much darker than when dry. Some dyers hold the wet cloth to the bright sky and look through it, to get an idea of what the finished color will be like; but positively certain and satisfactory results are arrived at only by wetting the sample to be matched or drying the piece that is being dyed, so that both sample and piece are equally wet or dry, while their color is being compared.
The real difficulty of color dyeing is not met with until the student tries to obtain shades embodying all three of the primary colors. A very few experiments will quickly show that with most modern dyestuffs it is hard to get soft, pleasant tones with the use of only two colors. Natural colors, as we find them in the sky, water, meadow, and woodlands, are never pure; they are invariably mixed. And our eyes are so accustomed to them that shades dyed with simple or pure colors look hard, cold, and inharmonious. Mixtures of two colors are better and softer than single colors, but still rather hard. But when the secondary shade resulting from the combination of[78] two primary colors is mixed with even a small quantity of the third primary color, the result is invariably a soft and pleasing tone.
The above statements presuppose that it is possible, in practice, to obtain good dyestuffs in each class, which are absolutely pure, clean shades of blue, yellow, and red without any admixture whatever. As a matter of fact, while the artificial dyestuffs are much more pure, and hence much more hard and brilliant than the best natural colors, they still in many, if not indeed, in most cases, when carefully studied, show shades that are mixed and not pure. It is very rare to find a blue that does not incline a little to the yellow (a Blue G as it would probably be labelled) or else contain a trace of violet or red (Blue R, or RR). The reds are almost invariably either scarlets, containing a trace of yellow, or crimsons containing blue. And the yellows, also, are very apt to tend towards orange or occasionally show a trace of green.
This, of course, complicates the problem for the practical dyer greatly, and means that instead of being able to cover the whole range of shades with a red, blue, and yellow, it is frequently, if not always, necessary to have some mixed colors, giving sharp, clear shades of violet, green, and orange respectively, to obtain certain effects.
The following diagram will perhaps make this more clear. In this the three primary colors have been divided, each into two shades as indicated by the shade letters, R meaning red, B blue, and G yellow (Germangelb) shades of the colors. By combining[79] these colors as shown in the table, clean, clear shades will be given, whereas other combinations would be likely to spoil the shades.
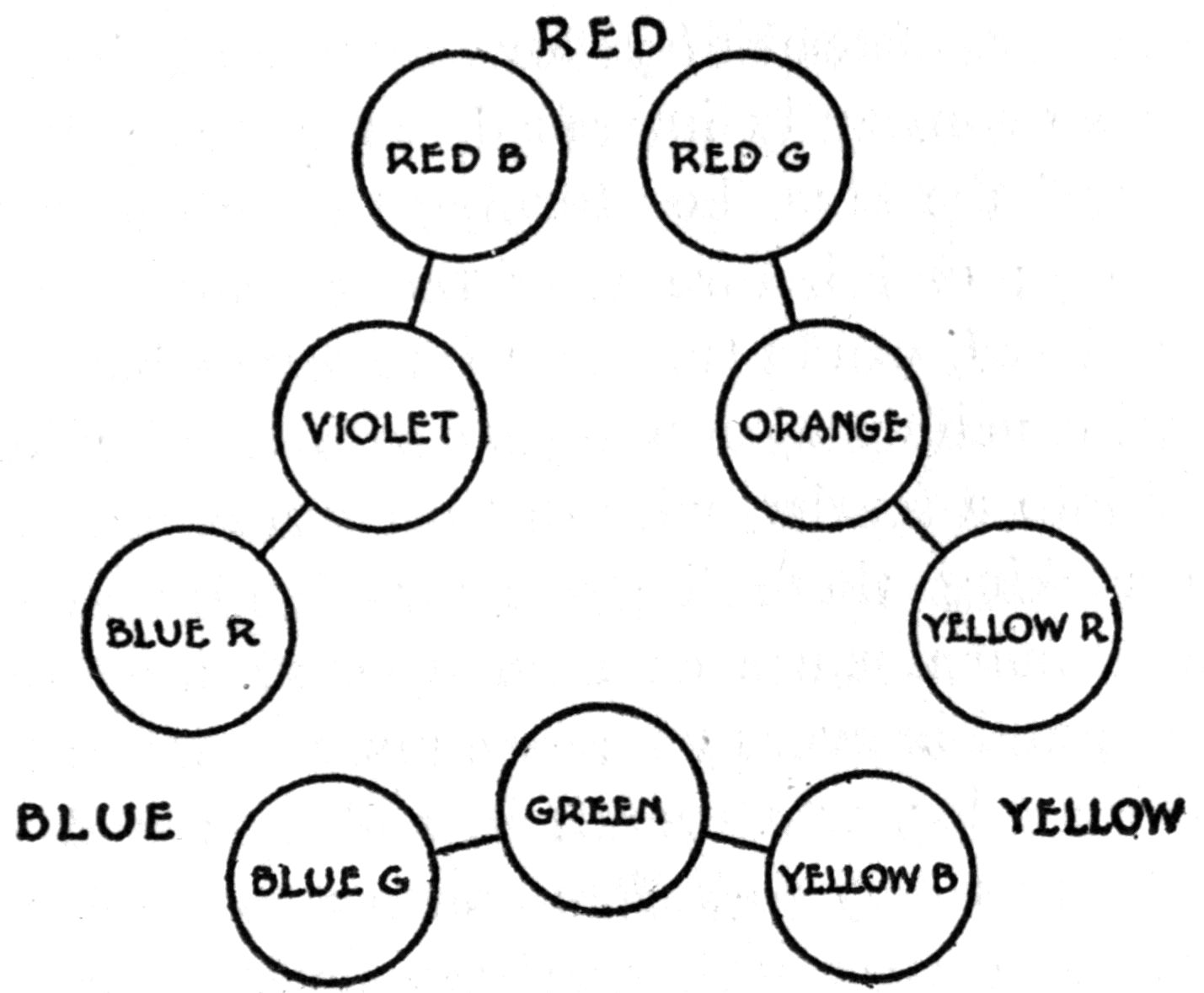
Red B + Blue R = Violet Blue R + Red B = Violet Yellow R + Red G = Orange
Red B + Orange = Red G Blue R + Green = Blue G Yellow R + Green = Yellow G
Red G + Yellow R = Orange Blue G + Violet = Blue R Yellow B + Orange = Yellow R
Red G + Violet = Red B Blue G + Yellow B = Green Yellow B + Blue G = Green
Take, for example, a special case, namely to turn a piece of crimson calico into a full rich scarlet. The crimson color contains a great deal of red, mixed with a little blue. If the piece were after-dyed, or “topped,” with yellow, even in small quantities, the result would probably be “muddy,” the yellow and[80] blue together being in such strength as to seriously diminish the strength of the red, and make it more or less brown in shade.
If, however, a reddish shade of orange were used for shading, instead of yellow, the red of the mixture would be constantly increased, while the yellow was “killing” the blue, i.e., turning it, with a little red, into grey; and before long the crimson, or bluish shade of red, would turn first into a true but softened red, with neither blue nor yellow predominating, and finally into a scarlet, with distinct traces of yellow.
In making these Three-color Shades, therefore, the component parts of each dyestuff used must be studied; and in every case care must be taken to have the third color, whatever it is, added in such minute quantities as only tosoften and not to spoil the first shade. A teaspoonful, sometimes even a few drops of a solution of one strong color, will generally be enough to soften, and take the edge off, some gallons of dye-liquor containing a hard, clear mixture of the other two. A cupful, on the other hand, or even two or three tablespoonfuls might utterly spoil the bath and turn it into “mud,” as a dyer would say.
It is worth mentioning here that, as a general thing, it is distinctly more interesting to build up shades by dipping first in one bath, and then topping with the second and the third color than it is to mix the different colors to the desired shade first and then dye the material in the single bath. On a small scale there is the same difference, although not so marked and less easily noticed, as that between even dyeing and[81] rainbow dyeing. There is often a loss in regularity and evenness, but the gain in life and light when one color shines through another which covers it more than compensates. This overlaying is not so perceptible in the even dyeing of fine, thin materials, whether yarn or cloth; but with coarse, heavy yarns and thick textiles, effects can be obtained by after-dyeing which cannot be approached when the goods are dyed in one bath.
Matching Shades.—Some people, I believe, go so far as to say that, in order to be really expert at true shade matching when using the three colors in dyeing, a dyer must have begun to learn the art in the person of his grandfather, ninety or a hundred years ago, and kept in practise ever since.
It certainly is true that heredity and early training both have a great deal to do with skill in this art, and a good color dyer will show an almost uncanny instinct, as he instantly picks out differences in shade which an untrained eye would never notice, and without any hesitation prescribes the exact remedy for the defect. Still there are plenty of good, even first-class dyers, nowadays, who have learned their art quite late in life, with the aid of a good eye and intelligent perseverance.
The chief rule to remember is this: Red, blue, and yellow, when mixed in equal strength, make a neutral grey or black. Accordingly any one color will form grey or, as we may say, willneutralize, or becomplementary to a mixture of the other two. Thus red will form grey with green; blue with orange, and[82] yellow with violet. Accordingly if there is too much red in the dye-bath, it can be killed by the addition of a little green; and vice versa. The same is true with the other complementary colors. If this simple rule be kept clearly in mind, most of the problems of matching colors and of getting pleasant and harmonious shades can be worked out easily. It is chiefly a matter of practice, and perseverance.
The student is strongly advised to attack this study in three ways:
First, mix the three primary colors together in one bath, to form definite shades—grey, brown, olive green, steel blue, etc.; then dye the cloth in the bath to see how the colors look when on the materials and dried.
Second, to dye a piece of cloth one mixed shade and by topping with other colors, to alter that shade to match some shade previously selected. For instance, dye a piece a good shade of reddish or copper brown, and then try to “kill” the red in it without materially deepening the shade, i.e., change it from a copper brown to a greyish or dirt brown of about the same depth of color.
Very pretty and instructive experiments can be made along this line of building up soft grey shades, by dyeing the cloth successively in weak baths of the three primary colors. As fast as one color predominates, it can be killed by dipping into successive baths of the other two.
Attractive scarfs and table covers can be made with a little care, by knotting the material and dyeing light[83] rainbow shades of the three colors, one after the other, changing the knots or tied portions after each bath. Properly done, this will produce remarkably interesting, opalescent effects, each color being toned and softened by the other two, although predominating in different parts of the material.
When, in the operation of rainbow dyeing, strongly contrasting colors have been used with unhappy results (such as the red, yellow, and blue tri-color effects that some students will produce) try the effects of toning, or “covering,” as it is often called, with some soft, neutral color which combines in itself all the contrasting tones, or else with a color that is complementary to the most obnoxious one, softening that one and strengthening the weaker shades. Grey, of course, can be used for this; but in general, a soft shade of brown will be found very valuable for taking the edge off of too violent contrasts. The permanganate brown (Manganese bronze), described in the first chapter, can be used with advantage for this purpose.
It is not difficult for a skilful dyer to match any desired shade by using three complementary colors, red, blue, and yellow, provided, of course, that these are pure and unmixed. It often happens, however, that after matching carefully a soft mixed shade by daylight, the colors appear entirely different when viewed by artificial light, and especially by ordinary gaslight. Daylight, as we are accustomed to it, is comparatively evenly balanced in color, is in fact a white light. But artificial light as a rule is distinctly[84] colored, and it is difficult, though now not impossible, to find a light that so closely resembles daylight that colors can be matched at night.
If the light, for instance, has a bluish tinge, like some kinds of electric light, it will kill the corresponding orange in a shade, while yellow light, such as commonly results from the use of oil, candles, or gas (less marked when incandescent mantles are used), dulls and even blackens lavender, violet, and purple shades, while having little or no effect upon yellow, orange, and green.
It is therefore advisable when matching shades that are to be used at night not to use three-color shades wherever that is possible, but to get the desired soft effects by covering directly with grey (i.e., light shades of black) on top of a single or two-color shade.
[85]
Nearly thirty years ago one of the French color houses put on the market a new dyestuff which it named “Cachou de Laval”; Cachou being the same as catechu or “cutch,” the natural brown dyestuff long known and used in the East, and Laval being the name of the town in France where one of its discoverers was born.
This dyestuff was made by heating sawdust, bran, turf, leaves, or other vegetable substances with the strongly reducing alkaline salt,sodium sulphide, in the absence of air. The product, dissolved in water, makes a dark green solution which, after standing in the air a short time, turns brown and deposits a fine brownish powder. Cotton or linen, heated in a fresh solution of this dyestuff, is colored green, but, when wrung out and exposed to air, the green color, which easily washes out, changes into a very permanent, though dull and uninteresting, shade of greyish brown.
This Cachou de Laval was not a success, commercially, because of its poor color. It existed, however, as a chemical curiosity for some twelve or fourteen years; then suddenly, within a few months or even weeks of one another, all the great color houses put out a whole series of colors—chiefly browns, blues,[86] yellows, and blacks—all formed, like this old “Cachou de Laval,” by the action of sodium sulphide or, which amounts to the same thing, of sulphur and caustic alkali, upon organic material, and all capable of dyeing cotton and linen, in one bath, colors extremely fast to washing and generally quite fast to light, after they have been “set” by exposure to the air.
While in general these are known and identified as the Sulphur colors, the different manufacturers have given special class names to their own series thus:
Immedial (Cassella), Katigene (Elberfeld), Kyrogene (Badische), Pyrogene (Klipstein), Thiogene (Metz), Thion (Kalle).
These colors are used almost exclusively for dyeing cotton and linen, when shades fast to washing are required, without first putting them through a mordanting process. The dyeing is done in one bath, with little more difficulty than in the case of the Salt colors described in the last chapter; and, while not faster to light than the best of that class, they are not nearly so liable to bleed.
On wool they are rarely, if ever, used. Wool is almost always dyed with the acid colors in an acid bath; and nowadays the range of these colors is so great and the best of them are so very satisfactory, that there is hardly ever a necessity for using colors of another class.
Neither are these Sulphur colors often used on silk, although methods have been devised for employing them in special cases. All the animal fibres, however,[87] and silk especially, are very easily “tendered,” and indeed destroyed, by heating in an alkaline solution. And so it is very easy to spoil a skein or piece of silk by dyeing it, in the usual manner, with these dyes, dissolved as they must be in the strongly alkaline sodium sulphide.
The presence in the bath of glucose (corn syrup, molasses, etc.), or of glue or gelatine, helps greatly to protect these fibres from the action of the chemicals. But even when dyed with great care, using glucose, and dyeing the goods for but a short time in a bath strong in color but weak in alkali, the results are not very satisfactory, so far as shade and lustre are concerned. They have the advantage, however, of being extremely fast to washing, more so, even, than the Salt colors. In general, however, silk should be dyed with the Acid colors for ordinary work, and with the Salt colors when fastness to washing is required. The Sulphur colors should be reserved for cotton and linen.
On mercerized cotton and artificial silk these dyestuffs take easily and well, when dyed in cold or lukewarm baths. The lustre, however, of the finished goods is apt to be less than when Salt colors or Basic colors are used.
For cotton and linen, measure out the color and dissolve it in hot water to which has been added twice its amount of sodium sulphide (crystals) and a quarter[88] or third the amount of soda ash. (In all these formulæ washing soda may be used in place of soda ash—only in quantities almost twice as large.) It is advisable, though not absolutely necessary, to add also to the dye-bath one or two tablespoonfuls of Turkey red oil—a kind of liquid soap made by treating castor oil first with sulphuric acid and then with soda. This prevents the formation of a dark scum on the surface of the dye-liquor, which is likely to cause streaks in the finished goods, hard to wash out.
Into the dye-liquor immerse the well-wetted goods, and heat them, turning them constantly, and keeping them as far as possible away from the air and under the level of the liquid. Just before the boiling point is reached take out the goods, and add salt in the proportion of, say, two spoonfuls of salt for every teaspoonful of dyestuff used. Stir till the salt is all dissolved, put the goods back, and continue to turn them as before, keeping the goods down under the liquor and not allowing it to boil.
After dyeing just below the boiling point for fifteen minutes, remove the heat, take out the goods, and—as quickly as possible—run them carefully backward and forward through the wringer (changing the folds of the goods each time) until the excess of dye liquor is entirely squeezed out. Then shake them out, hang them up for fifteen or twenty minutes in the air to oxidize and “set,” and after this wash them thoroughly in a bath of boiling soapsuds until all the loose color has been removed. Finally, rinse them free from soap, and hang up to dry.
[89]
When light shades are desired, or when the goods are tender, the dyeing can be done at lukewarm temperature, and without the addition of salt, with no detriment to the fastness of the color. In this case, however, much of the dyestuff will be wasted in the unexhausted dye-liquor.
List of Selected Dyestuffs.—
| Badische— | Kyrogene Brown, R R O |
| Kyrogene Yellow, G G, extra | |
| Kyrogene Direct Blue, 3 B, extra | |
| Kyrogene Black, T G O | |
| Cassella— | Immedial Bordeaux, G |
| Immedial Yellow Olive, 5 G | |
| Immedial Direct Blue, B | |
| Elberfeld— | Katigen Yellow, G F, extra |
| Katigen Indigo, C L G, extra | |
| Katigen Deep Black, B | |
| Kalle— | Thio Indigo Red, B |
| Thion Yellow, 3 G, extra | |
| Thion Blue, B, conc. | |
| Thion Black, G, conc. | |
| Metz— | Thiogene Brown, G R |
| Thiogene Gold Yellow, A | |
| Thiogene Green, G | |
| Thiogene Cyanine, G | |
| Thiogene Black, M A, extra strong |
These Sulphur colors are particularly strong in various shades of black, blue, and brown. Some of the yellow shades, also, are very fast and good. The class is deficient, however, in reds—the only one so far discovered being Thio Indigo Red B (Kalle), which really belongs to the Indigo or Vat colors, described in the next chapter, and which does not give very powerful shades when used as a Sulphur color. As a rule, these dyes produce shades that are softer,[90] deeper, and much less brilliant than those of the Direct Cotton or Salt colors. Being usually mixed, and not simple primary, colors, they are not very easy to dye to shade, especially as the color of the freshly dyed goods changes considerably while it is being oxidized. On the other hand, they give, without mixing, extremely pleasant tones, and are all very fast to washing and, at any rate as regards the selected colors, are fast to light.
When exposed to strong direct sunlight some even of the best of them are liable to change their shade somewhat; but even then they will be found to fade to nice, soft shades not out of harmony with the original. When very great fastness to light is necessary, it may be worth while to after-treat them as described in Chapter III, by keeping the dyed goods for twenty or thirty minutes in a hot bath (not boiling) containing small amounts of copper sulphate, bichromate of potash, and acetic acid.
[91]
History.—Most of the colors of this group have been discovered and put on the market within the last two years. Thus they form the most recent as well as, in many respects, the most interesting and, perhaps, the most important class of modern dyestuffs. On the other hand, to this same group belong not only indigo itself, which has been known and valued in the East from the earliest ages, but also that most famous of all the ancient dyestuffs, Tyrian Purple.
Indigo itself does not exist as such in nature; but it is easily formed by oxidation, or the exposure to air, of a substance—Indican—which occurs as such, or can be produced by a simple process of fermentation, in the juices of many widely distributed plants. Accordingly, even quite barbarous races in different parts of the world noticed the deep permanent blue stains formed on their bodies and clothing when they crushed, accidentally or on purpose, the leaves and stems of the variousIndigoferæ. Gradually they learned to extract the color in a solid and permanent form so that they could dye with it, instead of using the juice of the fresh plant itself—and then they took to cultivating the plants.
[92]
These plants—Indigofera Anil,I. tinctoria, and others originally found wild have been, up to the last four or five years, extensively cultivated in many tropical countries, notably in India (some of the best qualities came from the province of Bengal, and hence the common name for the natural dyestuff—Bengal Indigo), Japan, China, Java, South and Central America, and Africa. From these plants the indigo of commerce, in the form of dark blue granular lumps with a characteristic coppery lustre, was prepared by a comparatively simple process of fermentation, extraction, and oxidation.
Indigo may also be obtained, although in small quantities only, and in an impure condition, from other plants. Notably among these isIsatis tinctoria, or woad, which in early days was extensively cultivated in England and the Continent, and which, even now, is used in small quantities in some processes of indigo dyeing.
Artificial Indigo.—The exact composition of indigo was first determined some sixty years ago, and from that time on some of the greatest chemists of the world have been attempting to prepare it, artificially, from some comparatively inexpensive source, obtained from coal tar or elsewhere. As early as 1875 the problem was solved, at least from a scientific standpoint, but the process proved too expensive for commercial purposes. During the last five years, however, at least two of the great German firms have discovered methods for making, in any desired quantities and at very reasonable expense, absolutely pure[93] indigo from some of the important coal-tar derivatives. And since that time the cultivation of the indigo plant has proved so unprofitable that it has been almost entirely abandoned, and the land formerly used for this crop is being turned over to other and, at present, more useful purposes.
This synthesis—i.e., chemical formation—of indigo from coal-tar products has been justly regarded as one of the great triumphs of modern science. Right here let me impress upon my readers this fact: the real dyestuff, indigo, is absolutely the same material, whether it comes mixed with a great mass of impurities, as in the woad; or whether it contains from 5 to 25 per cent. of foreign matter of little or no value, as in the Bengal or natural indigo; or whether we get it from Metz or the Badische Company, chemically pure, either in the dry state or, thinned with water, in the form of a 20 per cent. paste. It is positively the same dye; and being absolutely without contamination of any kind, the artificial or synthetic dyestuff presents advantages in the matter of purity of shade, ease and surety of manipulation, and permanence of the color produced, which could never be obtained before its introduction.
Application of Indigo.—The principles of indigo dyeing are the same now as with the Egyptians, the only difference being in the means used to bring about the chemical changes involved. Indigo itself is a blue solid, insoluble in water, acids, and alkalies, and practically unaffected by sunlight. If, however, the element hydrogen be added to it, or,[94] as the chemist would say, if it is “reduced” by the action of any one of numerous deoxidizing or reducing agents, the indigo blue is changed to a new substance, indigo white, which is almost colorless, and which dissolves, in the presence of alkalies, to a bright yellow liquid. If cotton, wool, paper, wood, or indeed almost any solid materials (noticeably the fingers and nails, as some of my readers may find out), are immersed in the solution, they will absorb some of this indigo white, and then, on exposure to the air, the white indigo will rapidly take up oxygen, and become converted into the insoluble blue coloring matter.
Fermentation Method.—Until recently the methods used for reducing the indigo—i.e., changing the solid blue into the soluble white—were just about the same as those used by the ancients, and were based upon some kind of fermentation, usually alcoholic. It was found out at a very early date that if indigo, ground up with water to a paste and rendered alkaline by the addition of wood ashes, lime, or other simple alkali, were mixed with grape juice or any other sugary liquid, and then kept warm and allowed to ferment, the resulting fluid would contain the dyestuff dissolved in a form suitable for dyeing. The vessel in which this process was conducted was known as a vat, and the process of indigo dyeing is still called “Vat Dyeing.”
Disadvantages.—At the very best this method is slow, uncertain, and difficult to manage, especially on a small scale. In wool dyeing, to this day, a few vats are still to be found where syrup, ground madder root or, in[95] some instances, woad, wheat bran, and other materials which ferment readily in the presence of alkali, are stirred up with warm water and soda, and then allowed to stand. In two or three days they are in active fermentation, and the indigo, in the form of paste, is added and well stirred in. After much further delay, if all goes well, the indigo is finally “reduced,” and, if the amount of alkali, the temperature, the concentration of the vat, and various other factors are carefully attended to, the bath can be used for several days, or even weeks, without being made over again; fresh indigo and other ingredients being added, from time to time, as needed. Cotton, linen, wool, and even silk can be dipped in this bath, which should be light greenish yellow in color, with a blue or bluish-green scum or coating, where the indigo is oxidized on the surface. Goods immersed in this bath turn yellow, and then, when taken out, wrung free from loose liquor, and exposed to the air, the yellow color quickly changes to a permanent blue.
A serious drawback to all these various fermentation vats is that a good deal of the dyestuff is always spoilt—i.e., decomposed into colorless compounds which can never be regenerated or made useful. Indeed, the loss from this cause frequently amounts to 20% or 25% of all the dye used, and occasionally, especially in hot weather, and on a small scale, to far more.
But, apart from the actual loss in valuable dyestuff, there is a much more serious drawback to this method of indigo dyeing, namely, the waste of time[96] and energy involved. There is always a considerable delay in getting a fermentation vat fairly started, even where all the conditions are favorable; and when it is running smoothly, the reducing process is a very slow one. Furthermore, the indigo, not being dissolved in the liquid but only suspended in it, has a constant tendency to sink to the bottom in the form of a blue mud, and thus escape the chemical action of the fermentation gases entirely.
A short time ago a teacher of handicraft dyeing was expatiating, in my presence, upon the impropriety of using any of the new chemical processes for dyeing, and insisted that the only way to dye indigo was to set up a vat, and feed it, and work with it as our ancestors used to. It was suggested to her that it would be at least two or three days before cloth could be dyed in such a vat. “Eight or ten days at the earliest,” was the reply. And when it was hinted that the vat would have to be frequently stirred during all that time, she proudly answered, “Stirred regularly and thoroughly every single half-hour, night and day, during the whole period.”
“H—m,” remarked a bystander, “that’s a little worse than sitting up with a baby sick of the croup.”
Somehow the great advantage of this particular process over the modern ones, by which a proper bath can be prepared in perhaps five minutes, failed to impress itself on some of her listeners.
Modern Chemical Vats.—As soon as it was understood just what chemical action was going on in the vats, and the object of it, chemists began to find out[97] methods for reducing the indigo without the necessity of a long, tedious, and even nasty fermentation process. They first introduced the “copperas-lime” vat, where the reduction was done by the use of ferrous sulphate (green vitriol or copperas), and slaked lime was the alkali used to keep the indigo white dissolved.
Later they introduced zinc dust, a very powerful reducing agent, in place of the copperas, avoiding in this way the large amount of precipitated iron oxide which always forms in the copperas vat, and leads to the loss of dye, and muddiness and dulness of color, necessitating a special clearing bath of dilute mineral acid.
At present the most satisfactory method is to use the chemical known assodium hydrosulphite, as a reducing agent, in a bath made strongly alkaline with caustic soda. Hydrosulphite is not expensive; it acts very rapidly, leaving no sediment; it causes no loss or waste of the indigo; and it does its work perfectly. Hence, with its introduction, the dyeing of indigo has become extremely simple.
To still further shorten and simplify the process, the large manufacturers not only furnish indigo already ground up to a fine paste with water, but also supply it already reduced by hydrosulphite or some other reducing agent, so that it is almost ready to dye with as it is, and will dissolve almost instantaneously in an alkaline bath with the addition of just a little more reducing agent. Such products are the Indigo Vat III (Metz), and the Indigo Solution 20% (Badische). By using either of these, the preparation of[98] a vat large enough to dye 3 or 3½ pounds of cotton is the task of but a few moments. These special preparations, however, are more expensive than the regular 20% pastes, and the hydrosulphite vat is so easy to prepare that the saving of time is hardly worth the extra cost.
For dyeing by the Vat method the dye-pot is two-thirds filled with warm water, at about 120° F. (when the finger can hardly bear the heat), and one or two tablespoonfuls of caustic soda are added—enough to make the bath decidedly alkaline. The dyestuff, preferably first mixed up with some hot water, to thin the paste, is stirred into the liquid, and then to this is added sodium hydrosulphite, in powder, or preferably dissolved in water, until the color of the bath changes from blue, first to green, and then to greenish yellow, with a bluish-green coppery scum. If the bath is bright yellow, too much hydrosulphite has been used, and some more indigo should be added; or, if this is not desirable for fear of getting too dark shades, the bath should be exposed to the air and stirred frequently until the color is right. If the bath, on scraping aside the scum, looks blue, or even markedly green, it needs a little more hydrosulphite. If, after reduction, the bath looks yellow but turbid, it probably needs more alkali.
Into this bath the material is placed, and stirred around until thoroughly saturated—the temperature[99] being kept about 120° F. for heavy goods, to assist penetration. Light goods can be dyed equally well in a lukewarm, or even a cold bath. The goods are then taken out, wrung lightly by hand, and are carefully passed two or three times through the wringer, to get the color evenly distributed. They are then shaken out and hung up in the air to oxidize. In fifteen or twenty minutes, after the color has changed, they should be rinsed well in two or three waters, to get rid of all traces of the caustic alkali, and then boiled for several minutes in a soap bath, to wash off the loose dyestuff and prevent rubbing. This after-treatment with boiling soapsuds is of even more importance in the case of the other Vat dyes than it is with indigo, for with most of them the oxidation is not completed in the air—and so the color is developed as well as brightened by the soap bath.
It is very important, when working with these Vat colors, to remember that hot solutions of caustic alkali are very hard on the hands and that, therefore, rubber gloves are extremely useful, if not essential. Stains left on hands, clothes, and utensils, although difficult to remove by washing, are almost instantly dissolved by warm solutions of hydrosulphite with a little soda or other alkali in them.
Results.—Colors produced by synthetic indigo are clear and clean, but not brilliant. If the slightly purplish shades of natural indigo are desired, they can be obtained with special brands—Indigo R, or Indigo RR, Metz—or by mixing small quantities of Algol Red B, Elberfeld, or Thio Indigo Red B, Kalle, with the indigo[100] before reducing it. It is generally supposed that the characteristic shade, the so-called “bloom” of natural indigo, was due to the presence of small quantities of a reddish dyestuff, known as indigo red. As a matter of fact, however, the method of dyeing has more to do with this than the composition of the dyestuff.
For instance, if the indigo is very thoroughly reduced in the vat before the goods are immersed, as is generally the case in the modern hydrosulphite method, and the bath is made up with fresh reducing agent for each dyeing, the resultant color will be a very clear, rather greyish, shade of blue without any purple lustre. If, however, the dyestuff is not very perfectly reduced, as was generally the case with the old fermentation vats, and the bath, from standing in the air, has a heavy scum on the top, and is greenish rather than clear yellow in color below the surface, then the dyed fabrics will be apt to show the marked purplish tone which is so characteristic of the older indigo dyeings.
Uses.—While of less importance than it used to be before the discoveries of the last few years, the use of indigo for dyeing cotton, especially for the craftsman, is not to be neglected. It furnishes, easily and rapidly, in one bath, without either boiling, mordanting, or after-treatment, exceedingly pleasant, soft shades which are fast to both light and washing. For resist dyeing, such as Tied and Dyed work, Resist Stencil work, and Batik, it will be found particularly useful, because the fabric can be dyed in the cold.
[101]
Indigo possesses, however, certain disadvantages, especially for the professional dyer, which it shares with the other Vat dyes described below, and which prevent it, and the other Vat dyes, from being used as widely as the Salt colors or even the Sulphur colors. In the first place these dyes are all of them expensive. They cost more than most others, pound for pound of the dry color, and full shades need much larger proportions of them in the bath.
Then it is difficult to dye to shade with them, because the color, as a rule, alters so much when exposed to the air. In practice, when dyeing large quantities of goods to the same shade, it is customary to divide the materials into several lots of the same weight; and to make a strong “stock solution” of the dyestuff, properly reduced with alkali and hydrosulphite. By making up a fresh vat for each lot of goods, using exactly the same volume of water and of “stock solution,” and working each lot for the same length of time and at the same temperature, even results can be produced with much less trouble than by dyeing to shade by the eye.
Another drawback is that indigo-dyed goods, especially of the heavy full shades, are apt to “rub.” This can best be avoided by always using a well-reduced bath; by washing with boiling soap after each dip; and by building up the deep shades by successive dippings in moderately weak vats, rather than by obtaining the shade, once for all, by using a very strong, concentrated dye-liquor.
For many hundreds, and even thousands, of years,[102] indigo has been universally recognized as the most permanent and most valuable blue dyestuff for cotton and indeed for woolen goods. For the latter purpose it is now but little used, thanks to the introduction of the exceedingly fast dyestuffs of the Acid and Mordant classes. But for cotton it is still considerably used, for fast shades.
Up to a very recent date indigo was the only dyestuff, of any importance at any rate, that was used in the manner just described, and produced colors fast to light and to washing. During the past three or four years, however, the attention of the dyeing chemists has been directed to this question, and at least five of the great dye houses have introduced dyestuffs covering a great range of colors which, when dyed in the same way as indigo, not only rival but distinctly surpass that color in permanence as well as beauty.
Names.—These dyestuffs, while known generally as the Vat colors, have been given special class names by their manufacturers, as follows: Algol (Elberfeld); Ciba (Klipstein); Helindone (Metz); Indanthrene (Badische), and Thio Indigo (Kalle). The Cassella Company are just introducing the first members of their series, to be known as Hydrons.
List of Selected Dyestuffs:—
| Badische— | Indanthrene Claret, B, Extra | |
| Indanthrene Yellow, G | ||
| Indanthrene Blue, G C D | ||
| * | Indigo pure | |
| Cassella—[103] | * | Hydrone Blue, R |
| * | Hydrone Blue, G | |
| Elberfeld— | Algol Red, 5 G | |
| Algol Yellow, 3 G | ||
| Algol Blue, 3 G | ||
| Kalle— | * | Thio Indigo Red, B G |
| * | Thio Indigo Scarlet, S | |
| Thio Indigo Brown, G | ||
| * | Indigo, K G | |
| Klipstein— | Ciba Red, G | |
| Cibanone Yellow, R | ||
| Ciba Green, G | ||
| Ciba Blue, 2 B | ||
| Ciba Violet, R | ||
| Metz— | Helindone Red, 3 B | |
| Helindone Fast Scarlet, R | ||
| * | Helindone Yellow, 3 G N | |
| * | Indigo M L B, 6 B |
N.B.—The dyestuffs marked * will dye in a lukewarm or even cold bath.
Properties and Uses.—These Vat dyes are not all of equal value, but as a class they are, distinctly, the fastest of any as yet introduced; and the best of them may properly be considered as the most permanent coloring agents of any sort or kind that have ever yet appeared on the earth. They not only far surpass in this respect the best of the vegetable dyestuffs, with the possible exception of the very best qualities of Turkey red, but in resistance to chemicals and outside agencies of various sorts, are much better than the best mineral colors. This is so much the case that the modern specifications for dyed cloth for Government purposes, as for instance the khaki uniforms for soldiers in active service, which up to a year or two ago were dyed with iron buff modified[104] with oxide of chromium, have been raised, in one country after another, until they exclude every class of dyestuffs except these new Vat colors.
During the last year or two these dyes have been introduced, though with some difficulty, into commerce, and it is possible to obtain shirtings and other printed goods, dyed in permanent colors, so permanent indeed that the cloth will wear completely out before the color changes in the slightest. The extra cost of the dyestuffs, and the comparative difficulty of dyeing to shade, furnish an excuse for increasing the price of the goods. And the perhaps not unnatural disinclination of the shopkeepers to push the sale of materials which, in their opinion, are quite unnecessarily fast, has combined with the cost to delay the general adoption of these remarkably valuable coloring agents.
For craftsmen, however, where the price of the dyestuffs constitutes such a small percentage of the cost of the finished article, and where the absolute permanence of the color is of the utmost importance, these colors are most useful. They are not to be used, excepting under special circumstances, for animal fibres—wool, silk, leather, feathers, etc.—for fear of injuring the materials by the action of the caustic alkali. But on cotton and linen, both in direct or resist dyeing, and for stencil work, there are no colors to compare with them in fastness, not excepting even the very best of the Sulphur colors.
[105]
These dyes are all applied, just like indigo, in an alkaline hydrosulphite vat. The colors are applied in paste form, usually 20% strong, or at any rate equivalent in strength to a 20% paste of pure indigo. Care must be taken to thoroughly mix and stir up this paste with a glass rod, in the original package, each time it is used, so as to keep its composition uniform.
The proper amount, to be determined only by experience, is first thinned with a little hot water, and then stirred into the dye-pot, two-thirds full of hot water, about 140° F. (This is well below a boil, and yet hot enough to slightly scald the tips of the fingers.) To this is added caustic soda, in the proportion of two to three spoonfuls to each one of the color, the amount of soda being proportionately greater for light shades than where large amounts of color are used.
After this has been dissolved the dyestuff is reduced by adding slowly, with constant stirring, spoonful after spoonful of the powdered sodium hydrosulphite until the bath clears and generally the color changes. In most cases, as with indigo, the completion of the reducing change can be told by a marked alteration in the shade of the bath.
Thus, in general, the blue dyes, like indigo, turn yellow or orange when the proper amount of hydrosulphite is added. For the other colors there is no general rule. Thus Indanthrene Yellow (Badische), when reduced, is blue—while the Helindone Yellow[106] (Metz) is blood red. Helindone Scarlets (Metz), when reduced, appear green, while the Thio Indigo Red and Scarlet have about the same color, when reduced, that they have when oxidized.
The best way to tell whether the bath is in proper condition is to dip a piece of white blotting paper into it, and notice, on taking it out, whether the color is in specks or is dissolved. On standing in the air for a few minutes the color should become oxidized, and firmly fixed to the paper. As a rule these Vat colors should be reduced warm, because, in many cases at least, the reduced color does not dissolve in a bath of cold alkali. In most cases, however, after having been reduced at a temperature of about 140° F, the bath may be allowed to cool considerably, before it loses its dyeing value. This enables these colors to be used for Batik, or other processes where the temperature must be kept below 80° or 90° F. The dyestuffs which can be thus used will be found marked with an asterisk in the list of selected dyestuffs above.
The well-wetted materials are placed in the reduced dye-bath, and stirred and worked for five or ten minutes, or longer, according to the depth of shade experienced. For full shades, however, as in the case of indigo, it is much better to build up the color by successive dippings than to try to put it all on in one bath. For heavy goods the addition of a little Turkey red oil, about half a tablespoonful to the gallon, is an advantage, though not absolutely necessary. When thoroughly impregnated with the dye-liquor, the goods are taken out, wrung carefully, two[107] or three times, to remove the waste liquor as evenly as possible, and then shaken out and exposed to the air for fifteen or twenty minutes. They are then boiled in a soap bath for about twenty minutes, and then well rinsed, and dried. This hot soap bath, as before mentioned, is of great importance in most of these colors, not only for getting rid of loosely fixed dyestuff, but for oxidizing and fixing the color itself.
For dark shades it is well, as in the case of the Sulphur colors, to add salt—three or four tablespoonfuls per gallon of dye-liquor—to the bath, taking care to have it well dissolved before the goods are entered. This is always done when dyeing with Helindone Yellow 3GN, Metz.
The shades of these new Vat colors are extremely bright and clear, and, by combining these properly, any desired effects may be produced. The splendid series of reds and scarlets for the first time allow the characteristic shades of Turkey red to be obtained, in one bath, and of at least equal, if not of superior fastness to the original. One peculiarity of these colors is their extreme fastness, not only to light and washing, acids and alkalies, but also to various oxidizing agents, such as chloride of lime or bleaching powder. Accordingly goods properly dyed and finished with these dyestuffs can be entrusted with safety, so far as the color goes, to agencies which would speedily ruin fabrics dyed in any other manner.
[108]
In an earlier chapter it was mentioned that the modern dyestuffs originated with the discovery by Perkin, in 1856, of the violet coloring matter known as Mauveine. This dye was made by the oxidation of the then rare chemical, aniline. Following this discovery, other chemists, especially in France and Germany, soon obtained from the same chemical or from substances very closely resembling it, a considerable quantity of powerful and brilliant dyestuffs of the same general character.
The original Mauveine was before long superseded, first by Hofmann’s Violet, and then by a very important series of violet and purple dyes known as Methyl Violet, with shades ranging from 6 or 7B for the deep, full purples, to the 6 or 7R for the very red shades. These violet colors have never been surpassed, or even equalled by any other dyes for brilliancy and richness, although, in common with almost all the other dyes of this class, they are not fast to sunlight.
Another extremely powerful and brilliant color of this class, used considerably to this day although discovered nearly fifty years ago, is the dye often called, from its origin, Aniline Red. It was, however, named[109] by the German manufacturers, Fuchsine, from its rich, full, crimson shades, resembling the deep tints of the flower, fuchsia, while the French, who discovered and manufactured it soon after the close of Louis Napoleon’s Italian campaign, called it Magenta, after the famous victory of that name.
About this time some German chemists discovered and introduced a full, rich, brown dye, still largely used for dyeing leather (kid gloves and the like), and, naturally enough, gave it the name of Bismarck Brown. And at approximately the same date was discovered the very valuable blue dyestuff, perhaps the best of the whole class, with quite a range of full, deep shades, and with considerable fastness to light, called Methylene Blue.
General Properties.—The early colors of this group are the dyestuffs properly known as the “Aniline Colors” because of their origin; although this name has been applied, loosely, to all of the thousands of artificial dyestuffs without regard to their source of composition. To the chemist, their chemical structure and their behavior toward reagents, such as acids and alkalies, naturally suggested the name “Basic Colors.” This means that they are substances with strong affinity for all sorts of acids, with which they form more or less stable salts, while they can be liberated from these salts by the action of stronger bases, such as ammonia, or the fixed alkalies, soda and potash.
Application.—These facts were discovered by Perkin while trying to introduce his Mauveine into the dyeing industry, and he discovered the methods, used[110] to this day, for applying these dyes to the different textile materials. He found that the dyes of this class have a strong affinity for the different animal fibres, such as wool, silk, leather, etc., all of which seem to possess some acid properties of their own; but pure vegetable materials, like cotton, linen, and paper, from which all impurities such as vegetable acids, gums, etc., have been removed, have no affinity at all for even the most powerful of the Basic dyes. A cotton handkerchief, boiled for hours in a strong solution of Methyl Violet, can be washed in a few minutes clear of every particle of color, while a piece of silk or wool, soaked for an instant in the same dye-bath, will be permanently stained, deep and full.
Cotton, Linen, etc.—In order to fasten these dyes to vegetable fibre it is necessary to give the latter a distinctly acid character, and this was done by Perkin in a manner still used. He steeped the material for several hours in a hot bath of the acid vegetable compound, tannic acid or tannin, found so largely in hemlock and chestnut bark, sumac leaves, nut-galls, and the like; and then loosely fixed the tannin, thus absorbed, by a weak bath of tartar emetic. Cotton or linen fabrics, thus “mordanted,” will combine with the Basic dyes as readily and as firmly as any animal fibre, and the resulting colors, while not, as a rule, fast to light, are extremely fast to washing.
Since the introduction of the direct cotton dyes, both Salt colors and Sulphur colors, this method of dyeing, for skeins or piece goods, has been largely discontinued; but, by using a modification of this process,[111] enormous quantities of Basic colors are still employed, on cotton and linen, in the manufacture of calicoes, organdies, and other printed fabrics.
Curiously enough the Salt and the Sulphur colors, in almost every instance, possess sufficient acid properties of their own to act as very fair mordants for the Basic colors. Accordingly, it is not uncommon for dyers to “top,” with Basic colors, cotton or linen goods dyed directly. In the case of the Salt colors, this increases their fastness to washing, and with Sulphur colors it makes the shades more brilliant.
Most vegetable materials that are used in a more or less natural condition, like straw, raffia, grass, wood-shavings, jute, and the like, contain enough of this natural tannic acid to act as a mordant for the Basic colors, which may in this direction be used as direct dyes.
Wool, Silk, etc.—For animal fibres, such as wool, silk, furs, feathers, etc., the Basic colors have been almost entirely superseded, in commerce, by the class of dyestuffs known as the Acid colors. These occur in much greater abundance and variety, and can be applied with less danger of spoiling the goods by uneven results. For leather, on the other hand, the Basic colors are still largely used, especially for dark shades, or when fastness to light is not particularly desired. On bark-tanned leather, which is full of tannic acid, they take hold particularly well, and are often more convenient to work with than the Acid colors, although they do not, as a rule, give such even results.
[112]
Uses.—On a small scale it is hardly worth while for the amateur to try to use these Basic colors for dyeing either cotton or linen. The difficulty of correctly and evenly mordanting the goods is quite as great as that of applying the dyes afterward. And the Sulphur colors and Vat colors will be found quite as fast to washing as the best mordanted Basic colors, with the additional advantage of being very much faster to light, as well as easier of application.
By using some of the methods of the calico printer, it is possible to employ these dyes, with some success, for stencilling. But even for this purpose, excepting, perhaps, on silk, the modern Vat colors are more convenient, as well as being infinitely more permanent to light.
Disadvantages.—The chief drawback to the use of these dyes is that they are not fast to light. Several of them—Methylene Blue, for instance, and Methylene Heliotrope O (Metz)—are fairly fast, but the rest, especially in light shades, and on transparent or translucent fabrics, are liable, when exposed to sunlight for any length of time, to alter their shade to a very marked degree.
For dark shades this is not so noticeable, for, when goods are strongly colored, the effect of the sunlight on at least the deeper portions of the fibre is largely counteracted by the color of the goods themselves. So, too, an opaque material, like leather, will hold the same shade of color distinctly longer than silk or, especially, artificial silk, where the sunlight strikes through and through the fibre, without any[113] protection at all. But, generally speaking, these dyes will not stand strong sunlight.
Nor are the shades of these Basic dyes, as a rule, as attractive as those of other classes. The strong and brilliant, not to say coarse, shades of Methyl Violet, Malachite Green, Aniline Red, and the rest, which created such a sensation when they first appeared in the early sixties, were the particular colors which provoked John Ruskin to vehement, if not unparliamentary remarks. When unmixed they certainly do harrow the feelings of those artistically inclined, as much now as then. They are rarely seen now, for the taste of the public has been sufficiently educated to make a demand for softer shades. As before explained, nothing is easier than to soften these fierce, harsh colors to most beautiful and harmonious tints by mixing into them a mere trace of their complementaries.
Advantages.—In spite of all that can be said against them, these cheap, brilliant, and very powerful dyes are not to be despised, and should still be found in the outfit of a well-equipped dyer. For straw, raffia, chips, willow, and other materials used so largely for hats and for basket-work, these dyes are distinctly valuable, and, if supplemented by fast Acid colors for light shades, or for particularly fast effects, will be found satisfactory enough. So, too, for leather they will be found extremely useful, excepting where delicate shades, fast to light, are required.
Some kinds of artificial silk, also, especially those made from nitro-cellulose and hence possessed of acid[114] properties, dye far better with these than with any other dyes, although, as explained above, the colors will be far from permanent.
For the craftsman, the fastness to washing of these dyes is a matter of very little importance, because they are used by him so exclusively upon materials such as basketry, leather, and artificial silk, which are never exposed to rough handling in boiling soap and water.
As regards their fastness to light, the greater number of these must be classed as belonging to the fourth class, i.e., distinctly fugitive in character. On the other hand, some special ones can be selected from the group which are not only distinctly faster than the rest, but are fast enough to be well up in the third class, or can at a stretch, be placed in the second class, i.e., can be considered as satisfactory, at any rate, against any but very severe exposure.
The Fastest Basic Colors.—Among these may be placed the well-known dyestuff, Methylene Blue, perhaps the most satisfactory of the whole class. A very good color also is Methylene Heliotrope O (Metz), which, while less brilliant, is far faster than the many brands of Methyl Violet, Hofmann’s Violet, and the rest, which to most dyers are the characteristic basic violets.
For blacks, many composite dyes are on the market, made by the different color houses, known as Leather Blacks. These are fast enough, for deep shades, but not to be trusted when thinned down to form greys. The fastest individual basic black is Diazine Black,[115] (Kalle), and this should be used for the lighter shades.
The Red and Yellow colors are distinctly less satisfactory. None of them can really be considered better much than third class. Of the Reds the best is probably the color known as Safranine, different brands of which, giving as a rule the yellow shades, are manufactured by the various color houses; one brand being about as fast as another. For the bluish shades of red, probably the fastest is Diazine Red, (Kalle).
As regards Yellow, the list is even more unsatisfactory.
There is a very beautiful golden yellow, known as Auramine O, manufactured by most of the color houses, which, however, is hardly fast enough to be in the third class. This dyestuff, by the way, is injured by boiling, and therefore should never be used in a dye-bath heated to over 130° or 140° Fahrenheit. Less pleasing in shade, but somewhat faster to sunlight, are the rather orange or brownish yellows known as New Phosphine G (Cassella), and Methylene Yellow (Metz). Somewhat brighter colors, though less fast to light, are produced by Thio flavine T. None of these, however, compares in fastness to the selected colors of any other class in this book.
The various brands of the common dyestuff, Bismarck Brown, are largely used for leather, and while probably inferior in fastness to any of the colors mentioned above, are not found in commercial practice too fugitive to be pretty satisfactory. When, however,[116] materials are liable to be exposed for any length of time, two or three weeks in succession, to direct powerful sunlight, it will generally be advisable to use mixed browns made from fast Acid colors.
Upon the whole, although we are still frequently called upon to employ them, they must, from the craftsman’s standpoint, always be considered as untrustworthy. They should, therefore, never be used where dyestuffs of any other class can be made to take their place.
The application of Basic colors to leather dyeing will be discussed later. We shall now discuss their application to basketry materials, such as straw, raffia, willow, and the like, where they will be found useful.
It will at once be noticed that these dyestuffs are far more powerful than any thus far met with in these lessons. Indeed, while there will be needed, for full shades of the Vat colors, pastes from about 15% to 20% of the total weight of the dry materials, of the Sulphur colors from 7% to 10%, of the Salt colors from 4% to 6%, and of the Acid colors from 1½% to about 3%, most of these Basic colors will give very full shades with from ½% to 1% of the total weight of dry material.
These Basic colors do not dissolve readily in water, but are easily soluble in alcohol, and also in even very dilute acids. Acids form salts with the dyestuffs and these salts dissolve when the free coloring matters[117] do not. Accordingly the Basic colors should always be dissolved carefully in a separate cup or vessel, using hot water, and adding, for each spoonful of dyestuff, two or three spoonfuls of acetic acid or, if more convenient, of strong vinegar.
The color, thus dissolved, should be added to warm water in the dye-pot, preferably through a fine strainer or piece of cheesecloth, to avoid any undissolved particles which would cause spots. The well-wetted goods are immersed in this dye-bath, and turned, either in the cold or with gentle heat, until the desired shade is reached, or the bath is exhausted. The material is then taken out, rinsed once or twice in water, cold or warm, carefully dried, and, if necessary, straightened and pressed or ironed out.
Straw.—Care must be taken when dyeing these materials to have them quite free from grease and dirt, before dyeing them. If they do not wet readily and evenly, after being soaked in warm water for a couple of hours, they should be carefully washed in warm soapsuds, and thoroughly rinsed. The soap, however, should be of good quality and, especially with straw, either in the form of straw braid or made up into hats, no soda or other free alkali should be allowed in the bath, for fear of injuring the surface and destroying the gloss. This last is sometimes improved by dipping the straw, after dyeing and rinsing, into a weak bath of Castile (olive oil) soap, or of Turkey red oil (about one tablespoonful to the gallon), before it is dried.
In dyeing straw, the greatest pains must be taken[118] to dye it evenly. Braid should be tied up in loose hanks or bundles, so that the dyestuff can penetrate readily into every part; and with a loop of tape or string, by which it can be raised or lowered in the dye-bath. It should be kept in motion sufficiently to cause uniform circulation of the liquid. The dye-bath should not be too strong, especially at the beginning, and should be heated slowly to the boiling point, where it should be kept for half an hour or so, to insure penetration. It is best to add the dyestuff in small portions, from time to time, as the bath becomes exhausted, lifting the goods out of the bath each time, and stirring in the new color before putting the goods back again. If the goods once become uneven it is very hard, if not impossible, to get them level again, or to strip them fully, without spoiling the materials. The best thing to do, if this misfortune overtakes them, is to dye them some dark color, where minor irregularities will be covered up and pass unnoticed. In other words, “Dump it in the black,” as the dyers say.
Ladies’ straw hats are dyed in just the same way as the loose braid, the same care being taken to clean and wet the goods thoroughly, and to dye evenly. It is often of interest to experiment with old hats of good material, but faded, and to dye them up some pleasant new shade, and the ribbons and trimmings to match. Sometimes the remains of the old coloring will strip well by washing in hot soapsuds, and sometimes by soaking in warm water containing about one tablespoonful to the gallon ofsodium hydrosulphite—the[119] same salt that was used as a reducing agent for the Vat colors in the last chapter.
If the color comes out well, it is then easy enough, after thoroughly rinsing, to dye them any desired shade. Otherwise they can be dyed Navy Blue, with a good shade of Methylene Blue and a trace of red, or Seal Brown, using a large amount of red and a little yellow and blue; or they can be dyed black with a black dye, such as one of the so-called Leather Blacks, usually made by mixing a deep purple with a yellow, or one of the strong, powerful Basic greens with red.
In general, a well dyed piece of braid is supposed to show smooth, even coloring, good gloss, and good penetration of the dyestuff into the folds of the straw. There are, however, decided possibilities for the intelligent worker to obtain more interesting effects with but little trouble. It is very easy to use the principles, already explained, of rainbow dyeing, for straw braid, and beautiful effects can be obtained in this way, though it would need an artistic as well as an experienced milliner to fully utilize the same in making hats. But it frequently happens, when dyeing coarse braid without boiling, that the dye penetrates unevenly, from the edge towards the centre. Very pretty shaded effects can be produced in this way, the general color being uniform, and yet the straw, when looked at closely, showing tints instead of one flat, uniform shade. By dyeing the straw a solid color first, and then shading it in this manner[120] with a different color, very interesting effects can be produced.
It may be worth while to mention here that, when bought at wholesale places, it is astonishing how cheap the raw materials are. Bodies of straw, chip, etc., framing wire, white satin ribbon, artificial flowers, wing feathers, etc., from which not only pretty but even handsome and elegant head coverings can be created, and cost next to nothing at wholesale. The mechanical part of dyeing all these things can be learned in a very short time; after that the possibilities for a skilled worker, who has a good eye for color and can dye to the desired shades herself without having to hunt them far and near, are very large.
Raffia.—This is a material so widely used in the public schools, as well as by craftsmen, for weaving baskets, that it is well worth while to pay more attention to the dyeing of it. It is quite cheap, and very bulky, and takes these colors extremely well. So that it is one of the most satisfactory of all raw materials to experiment with, especially if there is a school or workshop at hand, where the dyed goods can be utilized.
The raffia should be shaken out thoroughly, and soaked in soft water over night, or at least for several hours, to thoroughly wet and soften it. If even shades are desired it can then be dyed, just like straw braid, in a warm dye-bath containing the dyestuff, previously dissolved in diluted acetic acid or vinegar.
It is much more interesting, however, to dye it rainbow shades from the start. If red, blue, and yellow[121] dyestuffs are dissolved separately, in different cups or pitchers, these solutions can be used to replenish the large dye-pots of the same colors. To keep the colors reasonably clear, and prevent them from speedily degenerating into “mud,” it is well to keep on hand one or two rinsing-pots, full of warm water, or to have a sink near at hand, where each hank or bundle of raffia should be rinsed after being taken out of one dye-pot and before going into the next.
The raffia, when thoroughly wetted out, should, for convenience’ sake, be made up into separate loosely-tied bundles, with a loop on each by which to handle it in the dye-bath without staining the hands. It is well, too, to have some oil-cloth around, for these bundles drip a good deal, and the dye-liquor will stain anything of an animal or vegetable nature with which it comes in contact. After a little experimenting with dipping these bundles first into the first dye-pot and then—rinsing each time—into the other two, it will be easy to get the general effect of any particular shade, although, when examined closely, the fibre will show the presence of all three colors.
It is interesting to notice, here, as previously with the Salt colors, how easy it is to modify and soften the harsh shades of the individual unmixed dyestuffs. And, as before, it is very interesting as well as very useful to dye some bundles even shades of some important compound color, such as brown, for instance, or olive green, or steel grey, and to notice how the color is changed on the fibre by adding a little more red, or yellow, or blue to the bath.
[122]
The “eye for color” obtained in this way is of the greatest possible advantage to a dyer, whether amateur or professional; and where, as in this case, the materials are cheap, easy to dye, and possible to utilize, every advantage should be taken of the opportunity.
Permanent Colors on Basketry.—While for most purposes the straw, raffia chips, willows, etc., dyed with Basic colors will be found satisfactory enough, it is best for craftsmen who are making a specialty of very high-grade baskets, to use some of the fast Acid colors, described and listed in the next chapter, for their reds and yellows, and for all mixed shades in which these two colors play an important part. The Acid dyes are applied in a boiling bath, with the addition of a little acetic acid, and, while not fast to washing, and not imparting their colors as readily as the Basic dyes, can be thoroughly depended upon, even in light and delicate shades, against the action of sunlight. Salt dyes can also be used, in a boiling bath with the addition of some salt, but, excepting in some special cases, are not superior to the Acid dyes, although somewhat faster to washing.
[123]
The discovery and introduction into commerce of Mauveine and the other Basic dyes, focussed the attention of chemists, all over the world, upon this new and important application of their science. And it was soon noticed that certain organic bodies, of a decidedlyacid character, had the power of dyeing wool and silk. These early dyes were so-called “nitro” compounds, formed by the action of strong nitric acid upon derivatives of coal tar, and in most cases they gave strong and brilliant, but rather fugitive, shades of yellow. The most interesting of these, perhaps, was the compound known as “picric acid,” which at one time was considerably used for dyeing silk yellow. Now it has been abandoned for that purpose but is manufactured on an enormous scale for use as an explosive.
These original acid dyes were of little importance. But in the early seventies chemists began to make use of a reaction—known as “diazotizing”—for making new organic compounds by the coupling of aniline or bodies similar to aniline, with all sorts and kinds of other compounds derived from coal tar. The number of derivatives of this sort proved enormous, and many of them had more or less valuable[124] dyeing properties. And in a very short time new dyestuffs had been discovered, good, bad, and indifferent, numbering not hundreds, but thousands.
A very few of these so-called “Azo” dyes were of the Basic class, like Bismarck Brown, mentioned in the last chapter. Others, discovered ten or fifteen years later, constituted the class of Direct Cotton colors or Salt colors. But the great bulk of these colors belonged to the so-called “Acid” class, forming salts with bases and alkalies, and being liberated from the salts by strong acids.
The number of Acid Azo colors is very large. In the catalogues of commercial coal-tar colors there are some two hundred and fifty of these dyes which have been picked out of the rest as having sufficient value to be carefully described, and to have been placed on the market by the great dye houses. Most of these are red and orange colors, with a few yellows. As a rule they are brilliant and clear, but, with a few exceptions, not particularly fast to light.
When these were introduced it was soon recognized that they were of practically no value for cotton and linen. They are as a rule much more soluble than the Basic dyes of the foregoing chapter, and hence are occasionally used as stains for wood, rattan, and other vegetable materials where considerable penetration is needed, without fastness to washing. But such use is of little importance.
Properties.—Acid dyes are almost exclusively employed for dyeing wool, silk, feathers, and other animal fibres, and for this they are extremely valuable.[125] The introduction of the Acid Azo colors so simplified and improved the dyeing of wool and silk, that every effort was made to increase the range of colors. And when it was found that the Azo colors were weak on the line of blue, purple, and green, efforts were made, which after several years proved successful, to change the various powerful Basic dyes, the Methyl Violets, Fuchsin or Aniline Red, Aniline Blue, Malachite Green, and the rest, into Acid dyes, so that they could all be used in the same dye-baths. This has resulted in a very wide range of colors indeed, for the Acid Azo colors cover fully all the shades of yellow, orange, and especially of red, from scarlets of all sorts and kinds to deep full crimsons. And then the remaining shades are covered by the acidified or sulphonated Basic colors.
These latter, by the way, though very brilliant and strong and rich, are no faster to light than the original Basic colors from which they are derived. Of late years the Acid colors have held their own, and still monopolize the commercial, as well as the special, dyeing of wool and silk excepting under unusual circumstances, when considerable fastness to washing is required.
With these dyes, as in the case of the Basic dyes, the fastness to washing is of little or no consequence to the craftsman. Nobody expects to scrub hand-dyed leather; and woollen and silk goods, unless specially prepared, are not supposed to be turned over to the tender mercies of the family laundress. However, it may be well to emphasize here the fact that these dyes[126] are as a rule “stripped” quite readily by boiling in a neutral soap bath. And when the craftsman wishes to dye wool or silk fast to washing, he must either use the Salt dyes, in a boiling bath, or must dye, with special precautions against tendering, with either the Sulphur or the Vat Dyes.
With regard to light-fastness, however, the case is different. A great many hundreds, possibly even thousands, of Acid dyes have been discovered, and scores of them, covering every shade, can be obtained in the open market. Most of these are of but little permanence, but a few products, from each of the great color houses, can be selected, whose fastness to light is extremely satisfactory. The dyes in the following list can hardly be considered as fast as the Vat dyes, previously described, but are probably faster, as a class, than any other class mentioned in this book. They would rank at the very top of the second class, and some at least would fairly enter the first class, being absolutely satisfactory against even the strongest sunlight.
A series of skeins, dyed all colors of the rainbow, including many delicate light shades, with a red, yellow, and blue dye of those mentioned below, withstood an exposure test which quite ruined a similar set of skeins dyed with the very best natural dyestuffs. And a large hand-woven rug, made of wool dyed light shades with the same dyes, was placed for two weeks on a roof in New York, half of it being covered with boards and the rest exposed to the direct action of the July sunlight, and at the end of this[127] time it was impossible to notice any difference in shade.
The colors in the following list are to be used, principally, for wool. They will all dye silk, leather, and feathers, but in the chapters dealing with those materials some additional dyes may be mentioned, which are specially suited for them.
List of Selected Dyes.—
| Badische— | Palatine Scarlet A, 3 R |
| Palatine Light Yellow, R | |
| Tartrazine (yellow) | |
| Wool Fast Blue, B L | |
| Cassella— | Brilliant Cochineal, R R |
| Acid Yellow, A T, conc. | |
| Tetracyanol, S F | |
| Elberfeld— | Azo Crimson, S |
| Fast Red, A | |
| Fast Yellow, 3 G | |
| Alizarine Blue, S A P | |
| Cashmere Black, 3 B N | |
| Kalle— | Biebrich Acid Red, 2 B |
| Wool Yellow, T A | |
| Nero cyanine Blue, B | |
| Nero cyanine Black, D | |
| Metz— | Fast Acid Red, M |
| Fast Acid Orange, G | |
| Fast Acid Yellow, 3 G | |
| Fast Acid Blue, B B |
The Acid dyes, like the Basic, are used in an acid bath, but for a different reason. With the Basic dyes acetic acid or some other weak acid is added, for the purpose of readily dissolving the color. In the case of the Acid dyes, however, the dyestuffs are almost[128] always put on the market in the form of the potassium or ammonium salts of the color acid. And the presence of some acid is always necessary, to liberate the color acid, and allow it to combine with the basic principles existing in the animal fibres.
For Wool.—The goods, well washed and soaked, are warmed gently in a bath containing, besides the dyestuff dissolved in plenty of water, a little sulphuric acid and a good deal of Glauber’s salt. Both acid and salt should be free from iron, or the shade will be dulled.
The amount of acid to be used may vary between considerable limits without affecting the results. If too much is present, there is danger of injuring the feel and the lustre of the fibre. If there is not enough acid in the bath, the color will wash right out of the wool, as soon as it is rinsed. In general it is well to start with about one tablespoonful of dilute (30%) sulphuric acid for each gallon of dye-liquor and about twice that amount of Glauber’s salt.
It is hard to tell just what is the function of the Glauber’s salt. It seems, however, to open up the pores of the wool in some way, and to make it dye more evenly and deeply. The bath is gently heated, with constant stirring of the goods, until the right shade is produced, or, if it is desired to exhaust the bath and so waste no color, until near the boiling point.
The goods when taken out of the dye-bath must be washed very thoroughly, to remove the last trace of acid, which otherwise on drying would ruin the wool.
[129]
It must be remembered that these Acid dyes hardly affect cotton in the least, and so the goods dyed in this way must be free from vegetable fibres, if level dyeings are to be obtained.
In dyeing wool skeins commercially it is, of course, of the utmost importance to have the colors perfectly level and uniform. This uniformity is obtained easily enough, when using these Acid dyes, by having the wool thoroughly wet before placing it in the dye-bath; by having it well loosened out and well stirred so that the color will penetrate evenly every part of the material; and, finally, by starting the bath at a moderate temperature, and heating it gradually, until the proper shade is obtained.
For handicraft dyeing the student is strongly advised to practise shaded and irregular effects, the so-called Rainbow dyeing, with wool in skeins, just as, in previous lessons, with raffia and with cotton. By using coarse heavy yarns, very beautiful two and three color effects can be produced, which, when used for embroidery or weaving, will prove most interesting.
Great care must always be taken, in wool dyeing, to preserve the lustre and the soft effect of the wool, and to avoid felting. This can best be done by using moderate amounts of acid, by dyeing at moderate temperature and never raising the dye-bath quite to the boil; and finally, by handling the goods as little as possible in the acid dye-bath, consistent of course with exposing every portion equally to the action of the dyestuff. Cotton skeins can be worked and rubbed,[130] and pulled, and thrown up and down in the hot dye-bath, without fear of injuring them. But wool should be handled carefully, and worked in the dye-pot quietly and gently, just sufficiently to accomplish two results. First, the wool at the bottom of the pot should be raised by a lifting and turning motion and replaced by fresh material; and second, when the wool is lowered back into the liquor it should be loosened, so as to allow the dye-liquor to penetrate the mass.
[131]
The use of feathers and, especially, of ostrich feathers for millinery has, during the past few years, increased to enormous proportions. Besides the home product, from California and the Western States, which, however, is but small, the importation of raw feathers from abroad has averaged, during the past two or three years, nearly eight millions of dollars. As yet, the dyeing of these feathers is almost entirely confined to professionals—their processes, although simple, not being generally known or published.
As before mentioned, feathers, like other animal products, can be colored with ease by either the Basic or the Acid dyestuffs. In practice, as with wool and silk, the Acid dyes are universally used, because of their greater variety, their greater fastness to light, and their better levelling properties. To use the Acid colors with success the following points must be carefully considered. First, the baths must be such as not to ruin or “burn” the feathers, i.e., they must leave intact the tiny barbules upon the barbs or “flues,” as the dyers call them, which make the feather look soft and full and not stringy.
Second, the quill must be fully dyed, and the[132] shaft, or stem of the feather, must also be colored just as well as the flues. This is a very common defect in feather dyeing. The quill, being hard and stiff and horny, is much more difficult to penetrate with the dyestuff than the soft, delicate fibres. If the feather, therefore, is dyed hurriedly or carelessly, the latter may be colored dark and full, long before the quill or the lower part of the stem has been dyed at all. This necessitates painting the stem after the finishing process, with oil colors, to match the rest of the feather.
Finally, after dyeing, the feather must be properly finished so that the flues will not look woolly on the one hand, nor stringy on the other hand, but soft and full.
The whole secret of feather dyeing lies in the proper attainment of these three requirements, success in which depends respectively upon (a) the composition of the dye-bath, (b) the method of dyeing, and (c) the finishing process.
(a) The Dye-bath.—As is universally the case when using Acid dyes on animal fibres, the bath must be distinctly acid, in order to release the free color acid from the dyestuff, which, in its commercial form, is a salt. A very little experimenting with ostrich feathers will show that the presence, not only of mineral acids like sulphuric or hydrochloric, but even of the much milder organic acids, like acetic or citric, is liable to “burn” the feather badly and convert a well barbuled flue into a bare fibre which, under no conditions, can look other than stringy. The[133] acid commonly used by the professionals is oxalic acid, but, of late years, dyeing chemists have been introducing into the dyeing industry the use of the volatile and pungent formic acid, and in the dyeing of ostrich feathers this acid has been found particularly advantageous. Excepting when a large number of feathers, strung together on a line, are to be dyed the same color, it is customary to dye feathers in an agateware pan or flat dish, and about two-thirds of a teaspoonful of formic or oxalic acid in a pint of water, is about the right proportion for one or two feathers at a time.
(b) Method of Dyeing.—
Softening the Feathers.—Before immersing the feathers in the dye-bath the greatest pains should be taken, first, to thoroughly cleanse them, and, second, to thoroughly soften them. As a rule, the feathers are bleached before dyeing and in this process they generally lose all of their original grease. But if they show signs of wetting unevenly when plunged into hot water, they should be carefully scrubbed with Castile soap and hot water, and well rinsed till the last trace of soap has been removed.
The clean feathers should then be thoroughly softened by immersing them in hot water. This is especially important as regards the quills and the stems, which may have to soak for half an hour or more before they are soft enough to take the dyestuff.
Dyeing the Feathers.—After softening, each feather is held by the tip, and laid, butt first, in the dye-bath.[134] For light shades the dye-liquor may remain cold, but for darker shades it is best to enter the feathers at a low temperature, and raise the latter very gently till the right shade is reached, or the bath is decidedly hot, although still far below the boiling point.
Above all, care must be taken to dye the quill and butt first, and to keep them in the bath very much longer than the flues and tip. The latter will dye in a minute or two, but to thoroughly stain the former may take twenty minutes or half an hour.
(c) Finishing.—When the desired shade has been reached, the feather is taken from the bath and rinsed thoroughly in warm water, to get rid of the loose color. Then it must be “starched.” This is the technical name for the drying process, and is very different from the laundryman’s idea of “starching,” although the two processes have occasionally been confused, with most disastrous results, as far as the feathers were concerned.
Dry-starching.—After the dyed feathers have been thoroughly rinsed, they should be partially dried, by wiping with a soft piece of cloth, like a handkerchief or piece of cheesecloth, and then laid flat on a piece of stiff paper and covered with a heaping tablespoonful or so of dry, finely powdered starch (on a small scale the quality known as “Electric Starch” is eminently satisfactory). The starch is thoroughly rubbed into the feather with the fingers, and then the feather, full of starch, is beaten and dusted against the edge of the table or the back of the hand until the starch has all[135] been shaken out. After one or two repetitions of this process, the feather will be found not only dry but with the barbules properly filled out. Sometimes the feather, thus treated, has a woolly look, the starching process having gone too far. In this case it should be dampened in cold water, and restarched.
Under no circumstances should any starch paste be allowed to touch or form on the flues. The starching must be done in the cold and with the unbroken starch grains.
Wet-starching.—Some dyers prefer wet-starching to the dry process just described. In this process, the feathers, after dyeing and rinsing, are worked for a minute or two in a thick milk (not paste) made by stirring one or two large tablespoonfuls of dry starch in half a pint or so of cold water, till all the lumps have been broken up. After this milk has been thoroughly rubbed into every part of the feather, the latter is taken out, dried roughly by wiping with cheesecloth, and then by wrapping between blotting paper or folded cheesecloth and running carefully through a not too tight wringer. The feather is then taken out and thoroughly dried, either by laying it on the table in the sunlight or in a warm room for some time, or, if very great care is taken, by holding and moving it over a hot-air register, or high over the stove or gas flame. Of course, if this is done carelessly and too great heat is applied, some of the starch grains will be converted into paste, and the feather probably ruined. When thoroughly dry, “bone dry,” as the dyers call it, the feather is beaten against the back of the hand,[136] or edge of the table until all the starch is shaken out.
Dyeing in the Starch.—When dyeing light shades time may be saved by dyeing and wet-starching at the same time, in the same bath. The feather, thoroughly soaked in hot water, is placed in the starch milk, to which a quarter teaspoonful or so of formic acid and a little dyestuff have been added, and then worked, in the cold, until the proper shade has been reached, the starch being taken up at the same time. Then on drying and beating, the feather will come out both dyed and finished. This has the disadvantage of leaving a little acid in the finished feather, but when using small quantities of oxalic acid, this is of little, if any importance.
Suggestions as to Feather Dyeing.—These processes should enable any intelligent craftsman to dye even the most costly and most delicate feathers without danger of spoiling them. Shade effects in one, two, or more colors can be easily obtained by the use of a little ingenuity, remembering always that the quill and the stem are very much more difficult to dye than the flues or tip. It will be remembered that comparatively few ostrich feathers are now used, singly; the plumes so abundantly in use, nowadays, being almost invariably built up by sewing two or usually three feathers together, one underneath the other, the stem being carefully shaved down so as not to make them too clumsy.
Very charming effects can be obtained by dyeing the individual feathers different but harmonious colors, and then combining them into one plume later.[137] But, usually, the plume is made first, and then dyed afterwards. It may be suggested, here, that very beautiful effects can be produced by taking large, handsome, single feathers, before they are bleached, and dyeing them a pleasant shade of red or blue or of some mixed color. The natural black of the feather, with its irregular markings, often gives very interesting results, and the expense is much less than that of a built-up feather.
After the starching process, the dried feather is usually finished by “curling,” a process simple enough in itself, but which had best be left to the professional, for fear of injury. The bleaching of feathers, also, is a process which is hardly to be attempted by the amateur, unless he is prepared to spend a good deal of his time and money in experimenting. The process, however, is well understood by dyeing chemists and can be learned without much difficulty, by a careful student with some knowledge of chemistry.
Stripping Feathers.—By soaking in warm water, containing a teaspoonful or so of ammonia water to the pint, and then carefully washing with soap and hot water, these Acid colors can be, as a rule, stripped from feathers almost entirely. This does not, to be sure, improve the original quality of the goods, but, carefully done, its bad effects are hardly, if at all, perceptible, and it enables the dyer to remedy a bad piece of dyeing, or to dye an old feather that has become faded or discolored by exposure. This, of course, does not apply toblack dyed feathers.
After white feathers have been worn for some[138] time they generally become soiled and yellow. If the stock was good to start with they can be immensely improved in appearance, if not made quite equal to new, by simply scrubbing them with a piece of Castile soap, in hot water, and then, after thorough rinsing, by dyeing them, in the starch-bath, with a very faint trace of blue or bluish violet.
Black Dyeing of Feathers.—This is the most difficult process in feather dyeing, and, as a rule, should be avoided by the amateur. It is impossible, so far, to get a thoroughly good black by the use of any artificial dyestuff, or any simple process. The best Acid blacks on the market, dyed with the greatest care, give a color to feathers that by themselves may look pretty well, but, when compared with first-class products, show dull and grey.
The only satisfactory blacks, so far, are produced by a long and tedious series of operations, depending on mordanting for, and dyeing with, logwood. As a rule, the professional black dyer—and really good ones are few and far between—allows at least five or six days for the process, the different steps of which he usually guards as a valuable secret, which indeed it is. The writer possesses one or two of these formulas, obtained, as special marks of favor, from first-class dyers, but has never had occasion to test them thoroughly, and therefore is unwilling to publish them here. Good dyeing chemists have tried again and again to shorten and simplify the process, and have had some success. But to this day no color has been found to replace logwood, and this black dyeing of[139] feathers is perhaps the only dyeing problem that has not as yet been satisfactorily solved with the aid of modern dyestuffs.
Painting Feathers.—Some dyers, instead of dyeing feathers, paint them. They dip the cleansed and carefully dried feather, for a moment, into a bath of oil paint, thinned greatly with gasolene. The feather is then taken from the bath, dried by waving in the air, and, when thoroughly dry, finished by beating and, if necessary, with a light dry-starching.
The results, for colors, are fairly satisfactory but are not so permanent as the dyeing process. In an oil paint the solid coloring matter, or pigment, is ground up finely in boiled linseed oil, an oil which has the property of drying to a firm varnish when exposed to the air. This mixture is thinned with turpentine or gasolene to the desired consistency before using.
It is evident that, in coloring feathers, if enough oil is applied to fasten the pigment very firmly to the flues, there is danger at the same time of plastering the fine barbules so that they will never get back to their proper places, and the product will be hopelessly stringy. On the other hand, if the amount of oil is so small, thanks to the abundant thinning with gasolene, that there is no fear of its sticking the barbules together, there will hardly be enough oil left to firmly fasten the pigment to the flues, on drying, and the color is apt to rub, and to wear off quickly.
Paint, thinned with gasolene, has been applied to feathers occasionally by means of stencils, some of[140] the so-called “barred” effects, looking like the feathers from a barred Plymouth Rock hen, being made in this way—the color, black paint or varnish, greatly thinned, being applied by means of an “air brush” or atomizer. Occasionally very large, wide, and handsome feathers have appeared decorated with flowers and other figures, in bright colors, applied in the same way with an air brush, sometimes with the help of stencils, but generally free-hand. These effects are often rather crude and inartistic, but there is no reason why, skilfully used, this method of decorating the backs of feathers might not produce interesting effects.
[141]
So far as can be learned, in every part of the world, the first materials used by man for clothing and coverings were the skins of animals. In its natural condition, however, the hide stripped from a dead animal has certain properties which greatly interfere with such use. When dry it is stiff and hard; when moist it rapidly decomposes, and when exposed to hot water it swells and in time dissolves. These difficulties had to be overcome before skins and furs could be properly utilized. And, accordingly, in the history of every nation and race, one of the very earliest of all developing industries was the art of leather making; that is, of converting the hard and easily decomposed rawhide into a soft, pliable, and comparatively permanent substance, well suited for the use of man.
In most uncivilized nations this conversion was accomplished by rubbing and working some oily or greasy substance into the hide, until it was thoroughly soft and flexible. Thus, in our Indian tribes, the old squaws would turn the deer skins and the pelts of various fur-bearing animals into beautifully soft and strong leather, by rubbing and working into them the[142] brains of the animals. The Esquimaux and other Northern tribes from time immemorial, too, have worked out this method with great perfection. Indeed without it they would have been unable to survive at all.
In other parts of the world it was discovered that rawhide could be made more durable by treatment with metallic salts, especially with alum, and then, by softening this product by rubbing in some oily material, a very fair leather could be produced. On the other hand, in warmer climates, as for instance among the Egyptians, the very earliest records show the use of vegetable extracts, containing the substances now known as tannins, for softening and preserving skins; and these races understood the art of dyeing, painting, gilding, and embossing the leather thus made, and used it for shoes, straps, aprons, and harness.
The Romans and Babylonians were famous for their leather industry, and the ancient Romans not only imported but manufactured it themselves, and used it freely. In the Middle Ages the greatest developments in the art were made by the Moors in Spain, whose leather, commonly called Cordovan leather, from the city which was the centre of the industry, has probably never been equalled for beauty and importance. This Cordovan leather, of which fine specimens are still to be found in museums and private collections, was made of sheepskin, tanned with bark. It was ornamented with silver foil, laid on a backing of size, and covered with a yellow varnish or lacquer, sometimes tinted with bitumen. This[143] protected both the leather and design very perfectly from injury by air or moderate moisture, and, being done on a large scale with splendid designs, was used largely for handsome wall coverings, competing favorably with tapestries manufactured in France and elsewhere for the same purposes.
In general, we may say that at the present day there are the same three classes of leather as in the days of the ancients, according to whether the hide is treated with oil or fatty materials, with alum or other metallic salts, or with the bark of trees or other vegetable substances containing the compound known as tannin.
1. Oil Tanning.—This, while of less importance than the other two methods, is still used in considerable quantities for lighter and cheaper qualities of leather. The process most commonly used is often called chamoising, or “shamoying,” because it is used principally for the production of “chamois leather” or wash leather. The hides used for this form are usually thin and light, the flesh sides of split sheepskins being the commonest, and the resultant leather is not only soft and flexible and strong, but is also unaffected by water. For this reason it is more difficult to dye than other varieties.
2. Mineral Tanning or Tawing.
Alum.—For thousands of years it has been known that if a solution of alum is rubbed or soaked into a raw hide the fibres of the leather become changed to an[144] insoluble and permanent condition, and by afterwards rubbing and rolling, and working in some greasy material, like the yolk of eggs, a useful variety of leather can be produced. The alum in this case does not form a permanent compound with the animal fibres, but can be washed out by working in warm water. Chemists have agreed, therefore, to call this temporary reaction by the name “tawing” as opposed to “tanning” where the chemical action is a permanent one. The “kid” leathers used for gloves are commonly made by this process.
Chrome.—During the last few years a new process has been introduced, based upon the use of chromium salts, which are absorbed by the hide in the form of the yellow or orange-colored salts, chromate and bichromate of sodium, and then are reduced in the fibres to a green compound by the use of hydrosulphite of sodium, or some other strong but harmless reducing agent.
This chrome leather is extremely valuable, and is freely used, especially for the “uppers” of good quality in the boot and shoe trade. This leather is very strong, and is water-proof, but possesses a serious disadvantage for the dyer, in that when it is once dry it can never be again wetted, and therefore it must be dyed fresh from the tannery wash tanks, or not at all.
3. Vegetable, or Bark Tanning.—At some very early period in the world’s history it was discovered that certain vegetable extracts, possessing in general a peculiar “puckery” taste, also possessed valuable properties in the treatment of raw hide. This process was[145] certainly well known to the Romans, for Pliny mentions, as tanning materials, the three great sources of tannin to-day, namely, gall nuts, the bark of trees, and sumach. These and many other vegetable materials, used for tanning, all contain a peculiar substance, known as “tannin” or tannic acid, which gives them their useful properties.
The tannins from different plants are not identical, although closely related to each other. They all have a strong astringent taste, and dissolve readily in water, forming weak acid solutions. They make dark-colored compounds with iron salts, and convert the hide tissue of animals into a tough, insoluble, and comparatively indestructible material which, when loosened and softened by some mechanical action, is known as leather.
Tannin.—Pure tannin can best be obtained from gall nuts—small excrescences on the leaves and twigs of certain plants caused by the puncture of some insect preparing to deposit its eggs there. The best varieties, called Aleppo galls, come from Turkey and Austria, where they are found on oak trees, and contain from 60 to 70 per cent. of tannic acid. From these it can be extracted in a very pure form, and it comes to market as an extremely light, fine, grey or light tan-colored powder, which dissolves in very little water to an almost colorless solution. Tannin in this form is largely used for dyeing, especially in the dyeing of cotton or linen goods with the Basic colors.
For tanning purposes it is customary to use the bark of various trees, oak bark being the most esteemed in Europe and, in this country, hemlock bark being the[146] most used. These contain from 12 to 15 per cent. of tannin, as a rule, with a moderate amount of brown coloring matter. Pine bark is also frequently used, and the bark of fir, spruce, and larch, while, in Russia especially, much willow bark and birch bark is used for light grades, the so-called Russia leather.
The next most valuable source of tannin is known as sumach, consisting of the finely-ground twigs and leaves of several species of that plant. The American sumach contains more tannin—18 to 25 per cent—than other varieties, but it is less valuable than the Sicilian sumach, which contains less coloring matter, and therefore can be used for tanning light shades of leather. All the materials can be used in the tannery either directly, or in the form of previously prepared extracts. From the Far East come some very important sources of tannin, used for dyeing as well as for leathermaking, in the form of dried extracts of various plants. One of these is Catechu or Cutch, now of value only for its tannin contents, but in former years used as a brown dyestuff as well. A similar product, known as Gambier, is still imported on a large scale from Singapore and other Eastern ports. It contains less tannin than Cutch, but less coloring matter as well. It is used not only for leather but for black silk dyeing with logwood.
The Tanning Process.—Without going too much into detail, the conversion of raw hide into leather by means of tannin is a very lengthy and mechanical process. The hides are first softened by soaking in water, and then are dehaired, usually by steeping in a[147] bath of slaked lime until the hair is loosened and can be scraped off with a blunt knife.
This lime must then be extracted by steeping in an acid bath, preferably containing some organic acid like lactic or acetic acid; some manufacturers, for the sake of cheapness, use dilute sulphuric acid for this purpose, with the invariable result of making the leather brittle and rotten when it is fully dried.
After the acid has been rinsed off, the hides are placed in the tan liquor, made either by dissolving one of the extracts in water, or by mixing the finely-ground bark or sumach with water and placing the hides in the mixture. The tanning process is a very slow one, especially for heavy hides, and it may take several months before the tannin penetrates to the center of the goods. When that time has come, the hides are taken out, brushed off, rinsed with cold water, drained off on horses, and then hung up in a drying shed to slowly dry.
When in the proper condition they are thoroughly rolled by hand or machinery, to break up any adhesions, and to make the leather soft and flexible. Then they are ready to be finished, are dyed to the required shade, rubbed down and polished with wax or varnish, grained by being run through rollers with engraved patterns, and otherwise prepared for the trade.
General.—It has been mentioned, in previous chapters, that animal fibres of all sorts, such as wool, silk,[148] feathers, etc., seem to possess at the same time both acid and basic properties, and therefore they combine readily with dyestuffs belonging to the Basic and also to the Acid class. This at once distinguishes animal fibres from vegetable fibres such as cotton, linen, and paper, which, being practically neutral in composition, will not combine with either Basic or Acid dyestuffs without the assistance of mordants.
This same rule applies to leather, and we are therefore able to dye leather successfully with either Acid or Basic dyestuffs, using a dye-liquor made acid with, preferably, a volatile organic acid such as acetic or formic acid.
Acids.—For Basic colors acetic acid is generally used, as being cheaper than the other, and quite as good for dissolving the dyes for the dye-bath. For Acid colors it is generally best to use formic acid, for acetic acid in many cases fails to liberate the color-acids from the dyes, and then the colors fail to “bite.”
Professional leather dyers, for the sake of economy, often use a little sulphuric acid in the dye-bath, a practice which is one of the chief causes of the short life of modern leathers.
With other animal fibres, such as wool and silk, the Acid colors take quite as readily as the Basic; but with leather, there is some little difference, according to the way in which the leather has been prepared.
For our purposes it is hardly worth while to discuss the dyeing of chrome leather or of chamois leather. The leather almost universally employed for hand work has been bark tanned, excepting where very[149] white goods are used, of rather light quality. These are generally tawed with alum, and for this reason have a greater affinity for the Acid colors than when the fibres have already been fully charged with tannic acid, which at the same time, it will be remembered, acts as an excellent mordant for the Basic colors.
Dyestuffs.—Accordingly, while Acid colors may be used, they do not act nearly so readily as the Basic colors. For this reason, except for special shades such as a clear sky-blue or a pure scarlet, which can hardly be obtained excepting by the use of Acid dyes, or where special fastness to sunlight is required, the best Basic colors, such as Methylene Blue, Methylene Heliotrope, Thioflavine T (for yellow) and Safranine (for red) are usually employed. For black, it is well to use one of the many Leather blacks, made by mixing together powerful Basic dyes. For brown, the standard leather color, used in enormous quantities for gloves and the like, is the well-known Bismarck Brown, or for more orange shades, the closely related dyestuff, Chrysoidine. And, although neither of these colors is as fast to light as the Basic dyes mentioned above, they give very satisfactory results. These colors should be dissolved in water acidified with a little acetic acid.
The greatest pains must be taken in each case to see that the color is all in solution, and that no specks of undissolved color are allowed to come in contact with the leather. The leather must be very carefully and thoroughly moistened by soaking, if necessary over night, in lukewarm water softened, if the[150] surface of the leather seems to demand it, with a few drops of ammonia water.
Dyeing Leather and Staining Leather.—As regards the application of the color; dyers generally make a distinction between leather that isdyed and leather that isstained.
Indyeing leather the moistened goods are placed in a tray or pan (agateware is most convenient for small pieces) and floated backwards and forwards in the dye-liquor, which should be deep enough to fully cover them. The liquor is usually about lukewarm on starting, and may be heated very gradually and gently to about 120° or 130°, if desired. For light shades, however, this is not at all necessary, and indeed the color, as a rule, penetrates deeper and is laid on more evenly when the bath is kept cold. The leather is kept in the dye-bath until the desired shade is reached, which should be at the end of half an hour or so.
When dyed in this manner, the dyestuff has a chance to soak into the leather, and so, when finished, the color is not so liable to be affected by rubbing or by wear. The leather should come out evenly coated on both sides, shaded effects if desired being produced later, by the staining process.
Stained Leather.—In staining leather, on the other hand, the color solution is applied directly to the surface of the damp—not wet—goods by means of a brush or soft sponge, or a little pad of cloth. Accordingly, no matter how carefully the leather has been softened and moistened beforehand, the color[151] does not penetrate far, and is found only on the particular surface where it has been applied.
For flat, even shades, the dyeing process is usually preferable, but by staining, it is possible for the craftsman to work on the surface of the leather, as an artist does on paper with water colors, and beautiful effects can be produced. Oil paint is often used for decorating leather, and when applied skilfully in thin layers, the effects are good. But staining with dyestuffs is usually preferable, as showing more of the grain of the leather, and being more transparent.
The staining of leather may either be done free-hand, or else by the filling in of set designs, marked out previously by tooling or some other method; or, as will be discussed later, by the use of stencils. In any case success chiefly depends upon the condition of the surface that is to receive the dye. The surface of the leather should be dampened, thoroughly and evenly, so that the dye will adhere, and even penetrate a little; but it must not be so wet that the colors will run.
To get this exactly right requires considerable practice. As a rule, the leather is, first, carefully and evenly soaked in water or, if it is at all greasy, in water with a little ammonia in it. When this has been thoroughly done, the leather is taken out and dried off, first on one side and then on the other, with pieces of cloth and then later with blotting paper. After this it is exposed to the air for a little time until the exact point of dryness has been reached.
[152]
The color solution should be applied with a camel’s hair brush or a small, soft pad of cotton, and any excess of liquid wiped off, or soaked up with blotting paper, and the color rubbed in with the fingers or pad, as soon as possible.
Acid Dyes for Leather.—As above mentioned, certain shades are hard to obtain without the use of Acid colors. This is particularly true in the case of blue. For the lighter and brighter shades it is necessary to use one of the Acid blues such as Cyanole FF. (Cassella), or Patent Blue (Metz). These are applied in exactly the same way as the Basic colors. Some of the Acid reds, too, will be found valuable for certain shades of scarlet, etc., that can hardly be reached with Safranine. Among the best of the fast Acid colors for leather may be mentioned:
Red.—Fast Scarlet, BXG, Badische; Biebrich Acid Red, 2B, Kalle, and Fast Acid Red, M, Metz.
Yellow.—Tartrazine, Badische; Wool Yellow, 1A, Kalle, and Fast Acid Yellow, 3G, Metz.
Blue.—Wool Fast Blue, BL, Badische; Nerocyannic Blue, B, Kalle, and Fast Acid Blue, BB, Metz.
When using these Acid dyes side by side with the Basic colors, it will be noticed that the latter, as a rule, are far more powerful, and color the leather much more rapidly than the Acid dyes. Accordingly forstaining leather the Basic dyes are the most satisfactory. On the other hand indyeing leather, where the dye-liquor is allowed to act longer on the goods, the Acid colors are more valuable, not only because[153] they are fast to light, but also because they will penetrate more deeply and more evenly.
Finishing Leather.—After coloring the leather it is necessary to finish it carefully, to get a smooth surface and to protect it from injury by rubbing or moisture. Some workers simply let the leather dry and then rub down the surface (without using any wax or oil) with the finger or the palm of the hands. Usually the grain or hair side of the leather is rubbed down with a little wax, the white or yellow wax, used largely as a finishing polish for tan shoes, being frequently employed for this purpose. It can be readily obtained from almost any good shoe store or, if desired, can be made by mixing together equal quantities of beeswax and carnauba wax in a molten condition, and thinning the mixture with a little turpentine.
A recipe used with success by many leather workers calls for a mixture of beeswax, turpentine, and neatsfoot oil. The wax is carefully melted, mixed with a small amount of turpentine, and then enough oil is stirred in to make it soft. When used upon embossed or figured leather this wax is never applied directly, but is placed inside a little bag of soft muslin, and rubbed on and into the leather with a circular motion—the palm of the hand being often used to finish the waxed surface.
Bronze Effects.—An interesting point in connection with the use of the Basic dyes, and some of the Acid dyes, too, for staining leather is that, when applied in a strong solution, as is very likely to be the case[154] when one is trying to get dark shades with an application of the brush or pad, they quite frequently, on drying, show a very marked metallic lustre. This is due to the formation of minute, bright-colored crystals, which reflect the light, thus imparting to the fabric colors which have nothing to do with the shade produced by the dyestuff itself. Thus, Cyanole FF, Cassella, when dissolved, or when dyed on leather or any other material, gives a rather greenish shade of blue. But it gives a very brilliant old gold effect, almost as bright as gold leaf, when applied in a strong solution and allowed to dry quickly.
When this effect is not desired it can be avoided by building up the dark shades by successive applications of weak solution, and by rubbing down the little crystals with, if necessary, a little moisture, whenever they appear to be forming.
In some cases, however, this bronzing property is of some value, and enables the skilful craftsman to obtain interesting and effective results with a minimum of trouble and expense. By painting on a strong solution of dyestuff, and letting it dry quickly, the bronze effect will be produced, and then by rubbing in portions, the true coloring of the dyestuff will be brought out in strong contrast to the crystal-covered surface. Unfortunately, these bronze effects are not fast to either rubbing or moisture, and even dry rubbing will break down the crystals, while rubbing with a damp cloth or a moist finger will dissolve the color off in blotches. To render this bronze effect more durable, it is possible to make a regular bronze[155] lacquer, by adding varnish or gum like orange shellac or gum benzoin to a strong alcoholic solution of a Basic dye. The bronze varnish thus produced will, when dry, stand light finishing with wax in the usual way. The addition of a little benzoic acid to the solution increases the lustre of the crystals.
[156]
So far as we can tell, silk was first discovered and manufactured in China about 1700 B.C., a date corresponding in Biblical history to the time of the patriarch Joseph. From China it was exported to the great and wealthy empire of Persia, and from there was first brought into Europe by Alexander the Great after his defeat of the Persian king. Its origin, although known and described by Aristotle, was for several hundred years a mystery. During the Roman Empire, silken garments, woven in Europe, from Chinese silk imported by way of Persia, were important and very highly prized articles of luxury.
About 555 A.D., while commerce with Persia was interrupted by warfare, two monks in the pay of the Emperor Justinian smuggled eggs of the silkworm and seeds of mulberry trees from China to Constantinople. This was the origin of the European silk industry. It spread rapidly to the various countries bordering on the Mediterranean, and by the seventeenth century was firmly established not only in Spain and Italy, but also in France.
Efforts were made to introduce it, at this time, into England, but without success. In 1622 King James I[157] started the industry, for the first time, in the colony of Virginia in this country. Since that time numerous attempts have been made to develop the American silkworm industry, but with very little success, owing to the large amount of hand labor necessary to produce the material.
At the present time the very finest raw silk in the world is produced in the south of France, and next to that come certain brands of Italian silk. The Japanese silk is more variable in quality, although steadily improving, while the Chinese silk, as a rule, is less satisfactory and more apt to be light and fluffy.
With regard to the consumption, it was estimated that in 1907 Europe used some twenty-five million pounds, and the United States fifteen million pounds of raw silk, which, at an average price of nearly $5.50 per pound, amounted to over two hundred and eighteen million dollars.
Origin and Varieties of Silk.—Silk has been defined as a “smooth, lustrous, elastic fibre of small diameter and of animal origin.” As is well known, ordinary commercial silk is secreted or “spun” by the silkworm, the caterpillar form of a moth known asBombyx Mori, the moth of the mulberry tree. These silkworms have been cultivated for thousands of years, but there exist in different parts of the world, notably in India and Japan, wild or uncultivated silkworms, derived from nearly related, but not identical, families of moths, and whose silk is collected in the forests by the natives, forming what is known in commerce as wild or tussah silk.
[158]
Of course, the silk from silkworms, cultivated and wild, is the only one yet produced on a commercial scale. But silk can also be obtained from other animals, notably from spiders and from a peculiar shellfish, the pinna, found in the waters of the Mediterranean.
Silk from the silkworm can be divided into two classes, according to whether the silkworms are the cultivated or the wild varieties. In each case the silk is produced by the caterpillar spinning a covering or shroud, the so-called cocoon, around itself to protect it when in the form of the chrysalis or pupa, awaiting its transformation into the moth.
The ordinary or cultivated silk of commerce comes from worms fed almost exclusively upon the leaves of the white mulberry tree, and cannot be produced successfully without that particular plant. The somewhat similar worms that produce the wild or tussah silks live upon the leaves of the oak, elm, ailanthus, castor oil plant, and others. While the two varieties resemble each other greatly in their chemical properties, they can always be distinguished, because cultivated silk is much more lustrous than the other, but is decidedly less strong.
Tussah Silk, Pongee, Shantung.—The tussah silks, when woven, are commonly known under the general name of pongee. Of late years this name has been applied to imitation goods possessing the characteristic dull color, and even the feel of the real article, but far less strong. These are generally made out of spun silk, derived from “Shappe,” i.e., the by-products of the[159] silk industry, spoilt cocoons, waste from the spinning machines and the dyehouses, and the like—silk, to be sure, but silk of very inferior quality. Accordingly, it is now customary to call real pongee by the name Shantung, after the Chinese province from which much of the wild silk is brought.
Shantung, or true pongee, can be readily distinguished from the imitation by examination of the threads, both warp and filling. These should be very long, and loosely spun or rather “thrown,” whereas the imitation threads are spun together tightly, from fibres of many different lengths, generally quite short.
Reeling.—All silk, whether cultivated or wild, comes originally from the cocoons, which are, as a rule, each formed out of a continuous strand or thread woven by the silkworm round and round its own body before it passes into the chrysalis state. These cocoons are collected, carefully dried to kill the quiescent animal inside, and then, in due course of time, they are placed in basins of warm water which softens the gum which binds the cocoon threads together, and the separate fine threads from several cocoons are picked up by brushing, and are combined into one which is reeled off on machines. The silk thus obtained is made up into hanks and bundles, and constitutes the raw silk of commerce.
Raw Silk.—The raw silk is very different in appearance and texture to the finished silk that we are accustomed to. It is without lustre, white, yellow, or even, in the case of some Italian silks, orange in color,[160] and quite stiff when handled. These qualities are due to the presence of from 25 to 35 per cent. of gum, which is insoluble in cold water, but is softened by hot water and dissolves readily in a hot soap bath.
Throwing.—The threads of this raw silk are far too fine and delicate to be fit for the weaving processes or even for dyeing. So they are combined into coarser and stronger threads by being “thrown,” a process equivalent to the spinning process of cotton, linen or wool. In throwing, the raw silk fibres are again softened in hot water, and are loosely spun or twisted together while still sticky. Three, four, or five threads of raw silk are usually combined to form one strand of thrown silk, varying, of course, with the quality of the original silk and the objects for which the thrown silk is to be used, when woven. For instance, silk used for filling—“tram,” as it is called in the trade—is usually thicker and softer, and less strong than the warp, or “organzine,” and therefore is usually built up, by the “throwster,” from many threads of less valuable raw silk, loosely twisted, while the organzine, used for warp, is generally of the best and strongest available material, thrown in finer strands out of fewer threads of raw silk, twisted more tightly.
It must always be remembered that the skein silk is thrown from very long continuous threads of raw silk, full of gum, whereas spun silk, which is being used more and more every year, is made from short lengths of waste and scrap silk, held together not by gum, but by tight twisting and spinning, just like cotton or linen.
[161]
Stripping or Degumming.—This thrown silk must then be prepared for the dyeing by getting rid of the gum, which not only makes the silk stiff and destroys its lustre, but which also would interfere with the smooth, even dyeing of the fibres themselves. For this purpose the silk, in skeins, is thoroughly washed, or “stripped,” by soaking in two or three successive baths of hot, strong, neutral soap solutions. In the dyehouses Castile (olive oil) soap is invariably used for this purpose, and, while made of cheap grades of olive oil, it is always, in good dyehouses, of excellent quality, for the presence of even minute amounts of free alkali in these baths is liable to greatly injure and “tender” the silk.
Boiled-off Liquor.—The soap solution from these stripping baths is not thrown away in the dyehouses, but is carefully stored as a valuable reagent. Under the name of “boiled-off liquor” it is almost exclusively used, by the dyers, for color dyeing. It is not often used in black dyeing, and therefore, in a dyehouse, the presence of a large and well-patronized black department is considered of great importance as providing the color dyer with an abundant supply of boiled-off liquor.
The stripped or degummed silk is now ready for weaving directly, the resulting white cloth being sometimes finished and sold as such, and sometimes “dyed in the piece.” In most cases, however, the stripped silk is weighted, dyed, and finished “in the skeins,” before weaving.
Piece Dyeing.—In dyeing by the piece, the stripped[162] silk is passed through a weak acid bath, usually acetic, and then woven into goods of the desired quality. These goods are then dyed in the piece by being run through the dye-bath until they are of the proper shade. The dye-bath (for colors) is made by stirring the proper quantity of Acid dyestuffs into a hot bath of boiled-off liquor (the bath in which the silk has been stripped), which is faintly acidified, or “broken,” as the technical phrase goes, by the addition of some sulphuric acid. This boiled-off liquor has the property of laying the dyes on the silk evenly and thoroughly, and is better for that purpose than any other medium. For amateur work, or where boiled-off liquor cannot be obtained, very fair results can be obtained with a strong bath of olive oil soap (Castile or Marseilles), “broken” with weak acid, generally dilute sulphuric acid.
The term “breaking” the soap bath is very significant. The acid should be added drop by drop to the frothing soap bath until the bubbles disappear and a thin iridescent film of fatty acid rises to the top of the liquid.
After the piece goods are brought to the proper shade, they are finished, usually by carefully rinsing in water to take away all traces of free acid, then by passing through a cold soap bath, often with a little olive oil emulsified in it, to increase the lustre; finally, through a bath of weak organic acid, like acetic acid, to develop the so-called “scroop” or “feel” of the silk. When silk is washed in soap, or is dipped in even a weak bath of alkali, it becomes soft and clammy[163] to the touch, and has no “life” or “snap” to it when dry. The passage through a bath of weak acid develops the characteristic stiffness of the silk fibre, and causes it to give its peculiar rustling sound when pressed.
Skein Dyeing.—When weighting or adulteration is not employed, i.e., in the so-called “pure dye” process, the dyeing of skein silk resembles the piece dyeing described. The degummed silk is immersed in a dye-bath containing the dyestuffs (Acid colors) dissolved in boiled-off liquor, broken with dilute sulphuric acid. The bath is heated nearly to the boiling point, and the silk turned in it until the desired shade is produced. It is then taken out, washed thoroughly in water to remove the last traces of acid, and then brightened by passing through a soap bath with some oil, and later through a bath of acetic acid to develop the “scroop.”
Drying.—An important part of the process is the final drying and finishing. The drying should be done slowly and carefully, and not proceed too far, or the silk will be brittle. As is well known to dyers, silk has the power of absorbing 25% or 30% of its weight of water without becoming perceptibly damp to the hand, and this moisture, when not carried too far, is of actual benefit to the material, making it stronger and more elastic. This property is often made use of by the honest (?) dyer when, in case some of the silk in a lot has been spoiled by accident or carelessness, he makes up the difference in weight by the liberal use of the watering pot.
Finishing.—This process is perhaps the most difficult[164] and technical of all, for the value of the finished product depends very largely on it, and it is almost impossible for an amateur to accomplish it. The skeins, after drying, are hung on a heavy polished wooden bar and, with a smooth wooden stick, are shaken out, straightened, pulled, twisted, and worked until the fibres are all parallel, the kinks taken out, any weak or injured portion cut out, and the whole skein has acquired the proper amount of lustre.
Sometimes, for specially brilliant fabrics, the skeins are “lustred” by machinery; this is the so-called “metallic lustring” when the silk, generally enveloped in steam so as to be both hot and damp, is pulled out between two steel arms until it has been stretched a considerable percentage of its original length. This undoubtedly lessens the strength of the fibre considerably and diminishes its elasticity, but under this strain each fibre is stretched out perfectly smooth and thus becomes much more brilliant and lustrous.
Dyeing Wild Silks.—It has been found difficult to handle satisfactorily the different sorts of wild silks in the factory. The bleaching of them has been very troublesome, although of late years the problem has been solved pretty well. And the ordinary process for dyeing silk with Acid dyes in a broken soap, or boiled-off liquor, bath is, for full deep shades at any rate, not always satisfactory. In consequence most of the genuine pongee or Shantung cloth is sold in the natural unbleached color, a pleasant shade of tan, or else in light shades.
Perhaps the best results in dyeing pongee silk full,[165] deep, even shades are obtained by mordanting the material with tannin and tartar emetic, just as cotton is mordanted before dyeing it with Basic colors, and then using in the dye-bath one or the other of the so-called “Janus” colors,—a group of colors on the border line between Basic and Acid, of which the best are Janus Yellow G, Janus Yellow R, Janus Red B, and Janus Black 1 (Metz).
This process, however, is too complicated for the unprofessional dyer to use with much success.
For all but the very full shades the craftsman is advised to use the Acid colors, as, for instance, some of the selected colors of the different houses, listed in Chapter VII, in a bath acidified with acetic acid, and without the use of soap.
For dark dull shades the Sulphur colors can be used, especially if some care is taken to reduce the alkalinity of the bath by neutralizing or nearly neutralizing the sodium sulphide with a little acid sodium sulphite. If the desired shade is so dark as to necessitate heating and dye-bath, it is also advisable to add a little gelatin.
For full shades of rather brighter quality the Vat dyes may be employed, also with precautions against the tendering action of the caustic alkali upon the fibre.
Before, however, starting in to dye a piece of pongee on the assumption that it is made from tussah silk, it is very advisable to examine it carefully, picking out the individual threads and untwisting them, and to make a few dyeing tests upon small samples.[166] For a large proportion of so-called pongee, which in color, lustre, feel, and general appearance resembles the genuine Shantung very closely, is simply made from spun or waste silk, and can be dyed like ordinary silk.
Acid Dyes, to be used on Silk.—Any of the dyestuffs mentioned in the lists on page 127, as suitable for wool, can be used successfully for silk dyeing. These colors have all been selected as unusually fast to light and, in this respect, are to be classified as “practically all of the first class,” i.e., as absolutely satisfactory against the action of sunlight.
But, for a valuable and comparatively fragile material like silk, it is quite allowable to use colors for special shades which are less fast to sunlight, if they possess other valuable qualities. Such, for instance, are the two red dyestuffs, Fast Acid Eosine G (Metz) and Fast Acid Phloxine (Metz), which belong to the group of so-called Eosine or Fluoresceïn dyestuffs most of which, while very beautiful, are extremely fugitive. These two dyes, which give shades of pink and red with yellow and blue fluorescence, respectively, are considerably more fast than the rest of their group, and will rank in the third class, if not at the foot of the second class, as regards light-fastness.
With regard to fastness to washing, it must be remembered that these Acid dyes are not fast at all, when dyed on silk in a broken soap bath. They may stand very light washing in a cold soap bath, but in boiling soapsuds will strip completely. This is important for the amateur, and indeed, for the professional[167] dyer, for whom a dyed silk, either skein or in the piece, has come out unsatisfactorily—uneven or spotted, or too dark in shade—for it is possible, if the silk is of good quality, to clean off the color completely by boiling soapsuds, without injuring the goods.
If the trouble is unevenness, while the shade is satisfactory, the color can be dissolved off in the boiling soap bath and then, on breaking the bath with a little acid, the same dye can be laid right on again, it is to be hoped this time in a satisfactory manner. The question of dyeing silk fast to washing, and also of dyeing silk black, will be dealt with in the next chapter.
[168]
BLACK DYEING OF SILK. WEIGHTING AND ADULTERATION
OF SILK. DYEING SILK WITH COLORS
FAST TO WASHING
The dyeing process described in the last chapter, while well suited for dyeing silk bright and lustrous colors, is not so well adapted to dyeing it black. To be sure, there are several good fast acid blacks, such as Silk Patent Black, 2R, Kalle, or Neutral Wool Black, B, Cassella, or Cashmere Black, 3BN, Elberfeld, or Amido Black, 4024, Metz, which, dyed in full shades in a broken bath of soap or boiled-off liquor, will give fairly good results. But the best of these are not always quite satisfactory, the resulting color generally showing a tendency to be a deep full grey rather than a perfectly true lustrous black.
Salt Colors.—Silk may also be dyed black with some of the good Salt colors—but unless the dyer takes the trouble to after-treat the goods by the troublesome process of diazotizing and developing, the results are no better, if indeed as good as those resulting from the Acid blacks mentioned above.
[169]
Sulphur Colors.—These have very often been tried on silk without much success, because for dark colors like blacks, it is necessary to boil the goods in the dye-liquor for some time and to have the latter very concentrated. Unfortunately the sodium sulphide, necessary for dissolving the sulphur dyes, is a powerful alkali, and hence readily attacks an animal fibre, like silk. It is possible, however, by the abundant use of glucose (Karo syrup, etc.) to greatly protect the silk from this tendering action. It is also possible for a dyer fairly well trained in chemistry, to very carefully neutralize the dye-bath by the cautious addition of acid sodium sulphite, until the dye-liquor is no longer alkaline and yet the dyestuff is not precipitated. This process, however, is hardly fitted for an amateur, and has not proved very successful even among the professionals.
Logwood Blacks.—Nearly all professional dyers continue to use the old vegetable dyestuff, logwood, about which some information was given in the first chapter.
To dye with this it is customary to use one of the many good logwood extracts on the market. Great care must be taken in the proper mordanting of the silk before it goes into the bath. For this purpose the silk is impregnated first with iron salts, and later with tannin, and in some processes, with salts of chromium or of tin, before entering the logwood bath. In all cases, therefore, silk dyed black with logwood contains a certain amount, say 15% to 20% of its weight, or 2-3 ounces to the pound, of foreign ingredients. When carefully done this does not injure the material[170] at all, and the “pure dyed” logwood blacks are perfectly satisfactory both for shade, lustre, and durability.
This moderate increase of weight, however, which is hardly enough to replace the weight of the gum lost in the stripping process, was far from satisfying the demands of the manufacturer for a cheaper raw material. And accordingly both dyer and dyeing chemist have exhausted all their energies and skill in trying to increase this percentage of cheap foreign matter in the finished silk, to the utmost limit of what the market will stand.
The first efforts in this direction were based upon the saving of some or, indeed, nearly all, of the gum which is wasted in the stripping or degumming process previously described. This gum, which amounts to from 20 to 35 per cent. of the raw silk, makes the silk stiff in texture and dull in color and more difficult to dye. Accordingly, in former years, it was invariably washed out of the silk with the greatest care before any attempt was made to dye it. But by modifying the dyeing, and especially the finishing process, it was found possible to produce the so-called “souples”—i.e., silks with little or no lustre, but with the characteristic “scroop” or “feel”—capable of replacing bright silk as a filling in many fabrics and yet containing almost all the natural gum left in the fibre.
The black silks were then attacked and an elaborate system of mordanting was introduced before the dyeing[171] proper began. For instance, the silk can be steeped alternately in one solution after another, first of iron salts and then of ferrocyanide of potash, thus forming Prussian blue in the fibre. Then the excess of iron can be converted by immersion in tannin solutions, such as Gambier or Cutch, into black tannate of iron, or ink, and finally, after perhaps a light bath in chromium salts, the real black color is brought out by boiling in logwood extract. The silk is then brightened by boiling with good neutral Castile soap, is shaded, if necessary, by dyeing with either an Acid or Basic dye in a weak bath, and, after drying and finishing, the finished product may easily weigh two or even three times as much as the original raw silk, and still retain its strength, lustre, and elasticity.
Tin Weighting.—The weighting of colored and bright silks did not proceed so rapidly, and it was not much more than ten years ago that, by accident, some French dyers discovered that by immersion in a strong bath of tin chloride (stannic chloride acidified with some hydrochloric acid) the silk fibre would absorb a large percentage of tin salts without necessarily losing lustre, dyeing capacity, or even strength. This at first was kept a secret, but its use gradually spread, until now it is a very poor silk dyer who cannot weight his silk 100 or 150 per cent. without spoiling its immediate commercial value.
Without going into unnecessary details, the process is somewhat as follows: The silk, after being degummed and thoroughly washed free of soap, is plunged into a bath of tin chloride and kept there for[172] some hours. It is then taken out and the loose tin salts are washed off in a tank of water (technically called a box), or in a washing machine. To further “set” the tin, the silk is then placed for a short time in a solution of phosphate of soda and again washed thoroughly. It has now gained from 15 to 25 per cent of its original weight (2½ to 4 ounces to the pound of raw silk).
If further weighting is desired, this treatment, first in tin chloride and then in phosphate of soda, can be repeated three or four up to five or even six times, increasing the weight with each immersion. Then a bath is usually given of silicate of soda, which adds a little weight, ½ to ¾ of an ounce, and, it is claimed, benefits the lustre and strength of the goods. Then, after a final washing, the silk is ready for the dye-bath.
The weighted goods are dyed, dried, and finished about the same as with the “pure dye” process, and the proud dyer can rejoice at returning to the honest manufacturer from 150 to 250 pounds of finished silk for every 100 pounds of raw silk (containing, by the way, 25 to 30 pounds of gum) which was sent in to the dyehouse! This “tin-weighing” process is also applied to black dyeing, and enables the black dyer to build up his weight with tin salts instead of limiting him to iron, chromium, ferrocyanide of potash, tannin, and logwood.
Properties of Weighted Silk.—It is scarcely necessary to point out that silk, weighted to the extreme limit, is hardly to be considered as the most durable and trustworthy of fabrics, even when dyed by the most[173] expert workmen. And when carelessly prepared heavily weighted silk is an abomination, liable to crack and wear away with the least provocation.
It may be worth reminding some of my fair readers that the old test of a silk taffeta, “so thick and stiff that it will stand of itself,” is nowadays anything but a proof of good quality. One or two manufacturers in this country a few years ago tried to revive the almost forgotten art of making and selling pure-dyed goods, and one trouble they experienced in disposing of their products, outside the high price, was the criticism that their silk felt so light and thin.
Prevalence of Weighted Silk.—At present it is almost impossible, at least in New York, to buy pure-dyed heavy silks. The writer, at any rate, has tried diligently, during the last year or two, to find for some special experiments a piece of white taffeta which was not markedly weighted. After visiting department stores and the very best dry-goods stores in the city, at all of which he was informed that no such material now existed, the best that could be obtained was one make of silk where the organzine or warp was fairly pure, the tram being well weighted. Light-weight Japanese and Chinese silks, however, undyed or dyed in the piece, can still be procured with little or no weighting.
Tests for Weighted Silk.—This silk may be identified by a very simple test. Pure-dyed silk, when dry, is easily inflammable. When touched with a lighted match it catches fire at once, “carries the flame” well, especially if in the form of thread; and, if followed[174] up with a flame, it will before long burn away completely, leaving little or no ash or residue.
On the other hand, weighted silk, especially when the added mineral matter amounts to 25% or over, is quite hard to burn. If it catches fire at all, it just flashes up for a moment and then the flame dies right out. And when persistently heated, until the organic matter is all burnt away, it still leaves a very considerable residue of ash.
When this test is to be made on unwoven or skein silk, it is enough to take two or three threads, five or six inches long, and to light them in the flame of a match. For piece goods it is best to pick out the threads carefully, with a pin or fine knife blade, separating the tram from the organzine, and then, with a match, to test each of these in turn. A very little practice will enable the most inexperienced student to make this test satisfactorily.
Of course, for an accurate determination of the percentage of weighting contained in a given sample of silk, it is necessary to resort to delicate chemical analyses. But for all ordinary purposes this simple flame test is quite sufficient.
As a rule the method previously described of dyeing silk with Acid dyes in a broken bath of soap, or better, of boiled-off liquor, will be found perfectly satisfactory. The shades are easily obtained, the colors are brilliant, and, if the right dyes are used, exceedingly[175] fast to light, and the material, if properly rinsed, suffers no deterioration.
On the other hand these colors are not, in the slightest degree, fast to washing.
The dyed goods can be cleaned with gasoline and the like, but when passed through a lukewarm bath of soap and water they bleed badly, and in boiling soapsuds the color can be completely stripped from them.
In most cases this is not a serious objection, for a person who will send a handsome hand-dyed silk scarf or piece of embroidery to the family washtub is entitled to scant sympathy if the results are disastrous. But occasionally it is important to have colors on silk which can be guaranteed against moderate or even against, severe, washing.
Fast Colors on Silk.—There are two grades of fastness known to the dyers—“fast” and “embroidery fast.”
“Fast” means simply that the silk is to be dyed fast to ordinary, careful handling so that the colors will not bleed or run in a warm or even hot soap bath, but does not guarantee them against every possible maltreatment.
The best way of doing this is by the use of the Direct Cotton or Salt dyes, described in Chapter III, which, it will be remembered, only dye wool or silk at a high temperature, at or near the boiling point and, preferably, in an acid bath, but, when once on, are very hard to dislodge. The selected ones are very fast to light and present a great range of bright, attractive[176] colors, which are nearly, if not quite, as brilliant as those produced by the Acid dyes.
They are applied in a boiling bath containing a little acetic acid, and a good deal of salt, especially for full shades. For lighter shades, the presence of salt is hardly necessary. The goods are to be finished just as with the Acid dyes, with a soap bath followed, if the scroop is desired, by a weak bath of acetic acid.
The results, when carefully done, are very good. They possess, however, one disadvantage for the amateur dyer. These colors are quite hard to strip, and so, the desired effect must be produced the first time, or not at all. It is not possible to strip an unsatisfactory shade in a hot soap bath, and dye it over and over again without injury, as in the case with Acid dyes. They are best stripped by soaking in a bath of sodium hydrosulphite, and then washing.
Embroidery Fast Colors.—While the above process gives shades fast enough against all ordinary washing, it sometimes happens that silk must be dyed fast enough to withstand exactly the same treatment that coarse cotton or linen goods are subjected to, without bleeding or staining. The salt dyes are not quite fast enough for this, particularly because, not having been converted in the dyeing process into a special insoluble condition, if they should be detached from the fibre by strong or hot soaping, they would be liable to stain the neighboring tissues and not wash off quite clear.
One of the hardest tests that colored silk is called upon to stand is when, in small quantities, it is used with a large amount of white linen or cotton goods.[177] Thus, for instance, when monograms are embroidered in red or blue silk upon white towels or napkins, and the latter are scrubbed, week after week, in the regular wash, the color must be fast, indeed, not to show some evidences of running. Hence the term “embroidery fastness” as applied to this class of dyes. Thanks, also, to the amiable practice of the modern laundress of lightening her labors by the addition of bleaching powder and other strong chemicals to the washtub, it is very important that a silk dyed “embroidery fast” should be able to withstand the action of these agents as well as of soap. Up to the last few years these colors were only obtained by the use of the Alizarine dyestuffs, the full rich scarlet so often used for this purpose being the modern form of the old, madder-dyed, Turkey red of our forefathers.
But, during the last few years, the troublesome and tedious mordanting processes necessary for the proper development of color by the Alizarine dyes, have been replaced, for craftsmen, and, indeed, by most professional dyers, by the much simpler and shorter processes of vat dyeing. As long as Indigo was the sole representative of the class, it was of very little use for silk dyeing. But since the introduction of the splendid series of new vat dyes, the Algol, Ciba, Helindone, Indanthrene, and Thio Indigo colors, which, dyed in a single bath, give a whole range of brilliant shades, wonderfully fast to light and to washing, the necessity for mordant colors has very largely disappeared.
[178]
It must always be remembered when working with silk, wool, leather, or any other animal material, that such materials are extremely sensitive to the action of alkalies, especially when hot or caustic, while they are but slightly injured, if at all, by the action of dilute acids. For this reason it is always better, whenever possible, to dye silk with the Acid dyes or the Salt dyes, in an acid or neutral bath, rather than to use dyestuffs like the Vat dyes or the Sulphur colors, which need an alkaline dye-liquor. Furthermore, the silk is likely to have a more brilliant lustre when dyed with a color which fastens to it by chemical affinity, from a solution, rather than one where the color is fixed because the oxygen of the atmosphere changes it into an insoluble powder, while in the pores of the silk. It is, however, perfectly possible to dye silk full shades with the Vat dyes and even—though this is not often advisable—with the Sulphur dyes, by using some simple precautions.
The best Vat dyes for silk are Indigo itself, and its substitution products, like Brom-Indigo, Elberfeld, or the Thio Indigo dyes, Kalle, or else the rather closely related colors like the Helindones, Metz, and the Ciba colors, Klipstein. It is of importance to use only those which are shown in the table on page 102, as dyeing in a cold, or at most, a lukewarm bath.
The dye-bath should be made with a considerable amount of dyestuff, so as to avoid the necessity of keeping the goods in it long. And the amount of[179] caustic alkali should be kept as low as possible, consistent of course with dissolving the reduced dyestuff. It has been found in practice that the presence of glue or gelatine in the bath, or even of glucose (molasses, corn syrup, Karo syrup, etc.), protects the silk, wool, and other animal fibres greatly from the action of alkalies. It should, therefore, be added in quantities of two or three large tablespoonfuls to the gallon of dye-liquor.
The wet goods should be immersed in the cold or lukewarm bath, and turned constantly for a few minutes only, before taking them out, wringing them, and hanging them up to oxidize. As soon as the color sets, which is shown generally, by the change of shade and which never takes more than, say, twenty minutes if the materials are well opened up, the goods should be brightened in a hot bath of good, neutral, olive oil soap, and then finished as previously described. It will be remembered that several, indeed most of the best Vat colors do not develop their final shade at all, until after the soaping process.
When carefully done, this process will give exceedingly fast and quite brilliant colors, without injury to the strength of the goods.
Comparative Results of Vat Dyes and Sulphur Dyes on Silk.—It is hard to get full shades with Sulphur colors because it is generally necessary to heat the dye-bath, and this, owing to the powerful alkaline properties of the sodium sulphide, is very injurious to the silk. Besides this, the sulphur dyes are much less brilliant than the Vat dyes, and have no good red or[180] orange shades in the whole class. They accordingly should not be used, excepting where no other are available, or, as will be described in a later chapter, when doing “resist stencilling” on silk.
On the other hand, such very unusual advantages do some of these new Vat dyes possess, for the dyeing of silk for special purposes, that large quantities of Helindones, Thio Indigoes, and other good specimens of this class are being sold, at comparatively very high prices, to manufacturers of fine shirtings where the patterns are made by weaving fine lines or figures of brightly dyed silk into the linen or cotton fabric. Until the introduction of these dyes in the last two or three years these shades could not have been produced fast enough for this purpose.
Sulphur dyes can also be used on silk without injuring the goods, by taking the precautions described earlier in this chapter. The shades, however, are quiet and dull, as compared to those produced by other classes of dyestuffs; and it is almost, if not quite, impossible to get a good full red and, especially, a good scarlet, by using these colors.
Silk properly dyed with Sulphur colors is extremely fast to washing. But these dyes, unlike the best Vat colors, are as a rule quite sensitive to bleaching agents, and therefore are not so well adapted for general use on “embroidery fast” silk.
[181]
Owing to the high price of pure silk and the bad wearing qualities of the highly adulterated silks, described in the last chapter, there has been for a long time a strong demand for a fabric which would combine as far as possible the strength and wearing power of the one, with the cheap price of the other, while still retaining the lustre and “scroop” and characteristic appearance of both.
The demand at present is met, and not so unsuccessfully, first by imitation silk, of which mercerized cotton is the best example, and second, by the various forms of artificial silk which during the last few years have been introduced widely in both Europe and our own country. The competition of these two classes of products is not at all to be despised. Their quality is constantly improving, their price diminishing, and their production increasing rapidly from year to year. And if the silk manufacturers continue to produce such poor material in the line of weighted silk fabrics as they have in the past, it will be but a short time before they will find the market almost entirely divided between pure-dyed silks, on the one hand, for expensive goods, and some of these new products for cheap materials.
[182]
History and Preparation.—This material was first introduced as a substitute for silk some ten or twelve years ago, although the process for making it was invented about 1840, by a celebrated English dyer, John Mercer. He discovered that when cotton, either in cloth or yarn, was subjected for a short time to the action of strong caustic alkali, and then thoroughly washed, the resulting material was much stronger than before, had shrunk very considerably, and had a much greater affinity for dyestuffs. For instance, dyes like the Basic colors, which give but a temporary stain on ordinary cotton, will dye with some degree of fastness cotton thus treated with alkali, without the use of mordants. Mercer patented his discovery and made some use of it in calico printing; as, for instance, in the making of “crinkled” goods. But the process was nearly forgotten until, in 1889, it was discovered that, by proper treatment, cotton could by this means be made so lustrous as to compare not unfavorably with silk.
To make the cotton lustrous, the goods, after dipping into the strong alkali, are kept firmly stretched, and their strong tendency to shrink resisted, until the alkali has been thoroughly rinsed off and the last traces neutralized with a little acid. If this is done carefully, when finally dried the cotton fibres will be found drawn out smooth and lustrous, while still retaining their new qualities of strength and increased dyeing power. To get good results in this process the materials treated,[183] whether in yarn or cloth, must be made of the very best and longest stapled cotton, preferably Egyptian, and when well done the results are extremely satisfactory. The lustre is not as good as the very best silk, but it is quite well marked, and for replacing the cheap grades of heavily weighted silks, as, for instance, for underwear, linings, etc., the mercerized goods are of very great value, owing to their strength and durability, as well as their cheapness.
Dyeing of Mercerized Cotton.—Cotton, thus treated, is dyed in the same way that ordinary cotton is, with the exception that it takes the dyes more rapidly, and, as a rule, gives deeper and more brilliant shades with the same amount of coloring matter.
For special purposes it may be best to use the Sulphur or the Vat dyes, but in general this material is best dyed with the Salt dyes, which are not only easy to apply, but are fast to light, very brilliant, and on these goods, at any rate, very fairly fast to washing. As before mentioned, the fastness to both light and washing may be considerably improved by after treatment of the dyed goods, i.e., by passing them through a hot bath containing a tablespoonful each of copper sulphate, potassium bichromate, and acetic acid to the gallon of water.
This, however, will rarely be found necessary, provided the selected colors are used, and the color has been applied at the boil in a bath containing considerable salt.
[184]
History.—The famous old French chemist, Réaumur, in the year 1734, suggested, after a study of the silk worm, and of the method by which it “spins” the natural thread, that it might be possible to make a jelly-like substance which could be drawn out into a fine thread and, coagulating, form an artificial silk.
This suggestion was first acted on, in a practical way, in the year 1855, when Andermars obtained some curious results by dipping a needle or fine metal rod into a thin viscous solution known as collodion, and then drawing it out rapidly, made fine, smooth threads as the material solidified. This collodion, which for many years has been in common use in minor surgery to paint on wounds and cuts, because it leaves a film of artificial skin, and in more recent times has been much used in photography, is a solution of gun cotton or nitro-cellulose in a mixture of alcohol and ether. In 1885 Count Hilary de Chardonnet made improvements in this last process, and produced successfully the first real artificial silk threads on a commercial scale.
Chardonnet Silk.—He also used a thick collodion solution, but instead ofdrawing it out hepressed it out through fine holes by using very great pressure. As fast as the gummy thread exuded it was picked up, carried along into a drying room, where the alcohol and ether could escape (to be condensed later and used over again), and then the solid fibre was passed into a solution of some suitable reducing agent, such as[185] ammonium or sodium sulphydrate, which converts the inflammable gun cotton into its original condition of cellulose. These resulting threads, being smooth and uniform when properly made, have very great lustre. Indeed, they are often far more brilliant than the very best and finest natural silk, and can be dyed and woven into beautiful fabrics.
This discovery of Chardonnet’s was at once utilized, and large and flourishing factories of Chardonnet silk sprang up all over Europe. The first large factory, which is still doing a very profitable business, was at Besançon, in France, and later a large factory was established at Frankfort, Germany.
Pauly Silk.—The success of this process aroused the interest of other chemists, and before long several rival processes came into existence, also based on the use of a viscous solution of a cellulose compound. One company, making the so-called Pauly silk, utilized the solvent action of an ammoniacal copper solution upon cellulose for their starting point.
Elberfeld Silk, Glanzstoff.—The Farbenfabriken von Elberfeld, famous manufacturers of dyestuffs, took up the manufacture of silk from a solution of a compound of cellulose with acetic acid; and the Elberfeld silk, or, as it is widely known in Germany, Glanzstoff, is every year becoming a more and more important factor in the silk business.
Viscose Silk.—A still different process, which during the past two or three years has been successfully introduced into the United States, depends upon the curious substance called Viscose, a thick, sticky solution of[186] cellulose made by first treating wood pulp, cotton or other vegetable fibre with strong caustic soda and then dissolving the resulting product in carbon disulphide.
This Viscose was first introduced for many different purposes. The solvent, carbon disulphide, is very volatile, and flies off readily, leaving the cellulose behind in the form of a stiff jelly which, on drying, becomes solid and strong. So Viscose was used for water-proofing paper, etc., for making solid articles like piano keys and billiard balls, and even for making opaque patterns in calico printing. But its most valuable application is for artificial silk. It is pressed out through fine holes, and the thread resulting quickly solidifies as the solvent evaporates, and can be dried carefully and worked up on reels or bobbins, to be dyed later.
Properties.—Artificial silk, as a rule, is a little stiffer than natural silk, but has an exceedingly fine lustre. It cannot be spun in as fine threads as fine, natural silk, but, on the other hand, can be produced in thick, smooth threads which, stained as a rule black or dark colors, quite replace horsehair for furniture coverings, etc. Similar products are made, too, by coating cotton with a layer of artificial silk.
Another curious use of this artificial silk process is when it is formed into still larger threads, very lustrous and quite stiff, and used for plumes and aigrettes. They can be dyed any color, have excellent lustre, and are extremely useful for millinery.
Precautions Necessary in Dyeing.—One great drawback is common to all these different varieties of artificial[187] silk. They are quite strong, although not particularly elastic, when dry, but when wet lose their strength very markedly. Indeed, at one time it was found extremely troublesome to dye them, as the silk skein dyers, accustomed to work and wring and stretch their silk, with impunity, in and out of the hot dye-baths, would try the same treatment with this new product, and in consequence ruin every skein. When thoroughly wet through in a hot bath the thread will soften until a skein may hardly bear its own weight. Accordingly, the dyeing is always done as quickly as possible, and generally at a lukewarm or only moderately high temperature. The skeins should be handled as little as possible in the dye-bath, and, when taken out to wring, should be rinsed slightly to get rid of extra color, acid, etc., and then carefully dried, not by twisting on two sticks, as is customary with other materials, but by wrapping in cheese cloth or blotting paper and then running the skeins backward and forward through the clothes wringer.
Tests for Artificial Silk.—It has been ascertained that all varieties of artificial silk now on the market are made from some form of cellulose. Efforts have been made to take thick jellies made from gelatine or similar animal compounds, and make threads from them, coagulating them later by treatment with formaldehyde or similar chemicals.
These experiments have, however, not as yet proved successful. Accordingly, any test that will distinguish between a vegetable and an animal fibre will show whether a brilliant thread or piece of textiles[188] contains natural silk or not. The simplest of tests is, of course, to burn a little with a match or at a flame and see if there results the characteristic “burnt feather smell” of charring animal tissues. This odor accompanies the natural silk. The chemist would probably make the same test more accurately by heating a wad of the material in the bottom of a small test tube and noticing whether ammonia was being evolved, and whether the distillate was alkaline in reaction. The ammonia and alkali resulting from the nitrogenous organic matter is a certain indication of animal matter.
To distinguish between mercerized cotton and artificial silk, it is generally enough to soak the samples for a short time, say a quarter of an hour, in boiling water and test their strength. Mercerized cotton properly made would be just as strong afterward as before, while the artificial silk would be soft and weak, if it would not, indeed, break down completely. Besides this, it must be remembered that the mercerized cotton, in spite of its lustre, is made up of threads tightly spun together from a large number of short fibres, none of which are over two inches or so in length, while the artificial silks are made up, like the natural silk, of long, continuous fibres twisted together to form the yarn.
In general, these artificial silks, manufactured as they are from wood pulp and other vegetable materials, are to be dyed with the Salt, Sulphur, or Vat dyes, care always being taken to expose them to the action of hot dye-liquors as short a time as possible.[189] The Salt dyes are less apt to interfere with the brilliant lustre, but the Sulphur and Vat dyes have the great advantage of dyeing in a cold or lukewarm bath, without any loss in fastness.
The Chardonnet silk has a special affinity for the Basic dyes, and in the trade is usually dyed both light and dark shades with these coloring matters, without previous mordanting, in a slightly acid bath. This practice, however, while simple and easy, is not to be recommended. For the Basic dyes, with but few exceptions, fugitive under all circumstances, are particularly sensitive to light, when dyed in light shades, upon such a brilliant and almost transparent medium as this is. On the other hand, articles made of artificial silk, being easily injured by rain, are not so liable to be exposed to the open weather as some other less delicate materials.
The Viscose and Elberfeld silks (Glanzstoff) have less affinity for the Basic dyes, and dye more readily with the Salt and Sulphur colors than the Chardonnet silk, made from gun cotton. But it is perfectly possible to dye the latter also with fast colors of the Salt, Sulphur, or Vat classes, providing large amounts of dyestuff are used to bring up the shade. Indeed it is poor economy to be sparing of the coloring matter, when working with any kind of artificial silk. For speed is essential, and the dyer who lets his material remain long in the dye-bath is liable to get into difficulties.
The artificial silk, after dyeing, should be finished much like natural silk, by rinsing and then passing[190] through a bath containing some olive oil, emulsified in a weak bath of soda ash. This increases the lustre. It should also be dried at a fairly low temperature and, while drying, kept stretched out by hanging a wooden or glass rod in the loop of the hanging skein, or some similar device, taking care to avoid strain great enough to pull apart the weakened fibre.
When dyeing this material great care should also be taken in tying up the individual skeins and in handling them. Turn them in the dye-bath as little as possible consistent with even dyeing. The threads, unless very tightly spun, are constantly liable to come untwisted, and the knots to untie, causing much annoyance.
In conclusion, when carefully made and dyed these artificial silks furnish beautiful, brilliant, lustrous fibres, which can be used to great effect in many kinds of handicraft work. They can easily be procured with more lustre than the very best natural silk, but even when dry are deficient in elasticity, and to some extent in strength, and when wet are very fragile. The price is kept at a rather high figure, as a rule only from 25 to 50 cents a pound less than that of good natural silk. But every year the production is increasing, new factories are springing up in every country, and as there is no limit to the production excepting the demand, it is probable that in a few years, thanks to competition, the price will be dropped very considerably and the whole silk business will be revolutionized. At present it is estimated that the production of the artificial silk is not far from one-fifth[191] that of natural silk, and this fraction is getting larger every month.
Indeed, the rise of this particular industry may fairly be considered as one of the most interesting, most useful, and most valuable contributions of the manufacturing chemist during the last quarter century.
[192]
Hitherto, in this book, the student has been instructed in the general art of dyeing and coloring the various fabrics, both in the yarn and in piece, without any attention to the subject of coloring them in patterns or designs. The remaining chapters will be devoted to various methods, suitable for craftsmen, by which the dyestuffs can be applied so as to give more or less definite patterns to the objects to be colored.
This art, in its general principles, was worked out in various parts of the world at very early periods in their civilization. In a great many cases colored designs in textiles were formed, in the process of weaving, by incorporating yarns of different colors in certain portions of the fabric.
But along with this, at a very early stage in the textile industry, there was developed the art of making patterns, regular or irregular, by the action of dyestuffs upon previously woven goods. In general there are three methods for doing this which, it is claimed, were known to the ancient Egyptians just as well as they are to the modern calico printer. These three methods are known as Direct Coloring, Discharge, and Resist dyeing.
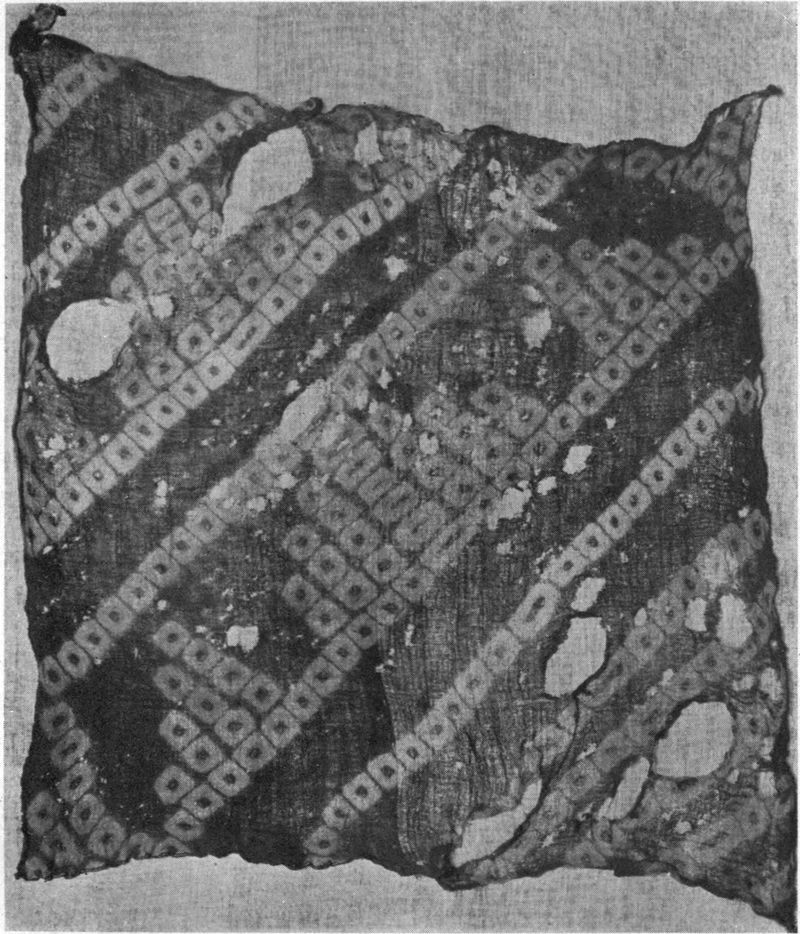
[193]
Direct Coloring.—This means the application of the dyestuff or coloring matter to different special portions of the textile or fabric, so as to give a colored design, upon a lighter background. The dye may be applied by dipping special portions of the fabric into it, in which case the pattern is apt to be a very loose and irregular one. Or, if the material will take the dye readily enough, as for instance in the staining of leather, it may be applied with a brush, or a small pad.
More formal and intricate designs can be made by applying the color in the form of a paste, through the help of stencils, as worked out by the Japanese so beautifully, or by means of wooden or metallic blocks, as in the block printing in the East, which in Europe and America has developed into the art of calico printing, by rolls run by machinery.
Discharge.—This process is the exact reverse of the preceding one, in that the cloth or other material is dyed first, and later the color is either entirely removed or, it may be, very decidedly altered in shade, in certain special parts, by the application of some chemical.
The earliest examples of this are where cloths stained with Iron buff, have had patterns made in them by washing out certain portions with acid. Just as some of the earliest forms of “direct coloring” are shown in the dark patterns of leaves, formed by the same Iron buff dye, upon cloth against which moist fresh leaves have been crushed.
The discharge process is not as commonly used by craftsmen as the other two methods, because it has[194] not always been easy to find or to use a chemical that will properly destroy or change any particular color, without at the same time, if fast dyes are used, destroying or at least injuring the fabric. The professional dyer, working in conjunction with the chemist, carefully weighing the reagents, and using steam chests and drying chambers with definite and carefully regulated temperatures, can fully discharge even the fastest dyes without danger. But this is difficult, if not impossible for the craftsman, and while the process will be discussed and described under the subject of stencilling, it will be found, comparatively, of but little practical importance.
Resist.—The third and last method for getting colored patterns is one which has been used in different ways, by the most widely scattered nations, and which, to this day, furnishes one of the most interesting and important processes at the disposal of the craftsman, as opposed to the professional dyer.
It consists of applying to certain portions of the fabric, before dyeing, some agent which, acting either chemically or mechanically, will “resist” the action of the dyestuff at the places where it is applied. These parts accordingly will remain in their original color, or at any rate will be but slightly colored, while other portions, not so protected, will be dyed full shades. This, in many respects, is the most advantageous way of obtaining patterns for the craftsman, because no action has taken place tending to injure the strength or durability of either material or dyestuff, and as the color is applied in a regular dye-bath there is generally[195] an opportunity to apply the dyestuffs in the most approved manner.
Variations in Resist Work.—The resist method has been discovered in many parts of the world, and has been carried out in many ways. In Java, for instance, a beautiful art was developed known as Batik, to be described later, in more detail. These people used, as a resisting medium, molten beeswax, which could be poured or painted on to the cloth wherever desired, and, according to whether it was applied hot or only just warm enough to be liquid, would protect the material covered, either wholly or partially, against the action of dyestuffs in a cold bath.
Less elaborate, but still very interesting processes are reported from many other quarters. As will be described in the next chapter the Japanese have long used a resist paste, to make white patterns against dark backgrounds with their stencils. In some of the Pacific Islands natives have learnt to make patterns by pressing pieces of cloth tightly between shells, as for instance the two halves of a clam shell, and then dyeing or staining around them. Other tribes learnt the trick of tying or sewing flat thin pieces of wood together, tightly compressing the cloth between them and thus preventing the dyestuff from reaching those parts of the goods when dyed later.
But the most common process, and one which is not only the simplest and easiest to carry out, but also offers to the skilful dyer an almost unlimited range of interesting and effective results, in color and design, is the so-called “Tied and Dyed Work.”
[196]
In this process, Tied and Dyed Work, the pattern is made by tying string or cord, more or less tightly, around certain selected portions of the material. When the goods, thus treated, are subsequently dyed, these tied portions will be kept from the action of the dyestuff, and after the operation is finished and the strings cut or untied, they will be lighter in color than the adjacent parts of the fabric.
This process has been known and widely used in many different parts of the world. Some interesting examples of it are found among the textiles from the so-called Inca graves, in Peru and Bolivia, dating from before the Spanish conquest in the sixteenth century (see Fig. 2). Some extremely interesting specimens of tied work can be seen in the Philippine collection in the New York Museum of Natural History, brought from the Bagobo tribe in Mindanao (see Fig. 5). While perhaps the most extraordinary development of this process can be found in the so-called chundries or chunaries, imported from Central Hindustan, and sold by traders in Eastern goods and textiles at very moderate prices.

Chundries.—These are chiefly manufactured in the native State of Kotah, in Rajputana, and have been produced there from time immemorial, for use as clothing and hangings. Those that are imported to this country (see Figs. 3 and 4) are generally made of extremely thin, flimsy muslin, most elaborately decorated in three or four colors, with patterns made[197] up of an infinite number of small round or rectangular rings of white or light colors, against a darker background. They can be obtained in the same condition that they left the dyer’s hands, folded tightly together, colored red or brown or black from the final dye-bath, and covered over with hundreds of little hard knots or lumps. These, on examination, prove to be the tied places, each tied by hand, by winding round and round the base of the projecting loop of cloth, a very fine thread, closely laid and knotted extremely firm and tight.
When unwound, which must be done with much care on account of the thin, fragile nature of the cloth, the knotted portions often show most beautiful and interesting designs—done in different colors, put on before tying, and protected from the final bath by the close tight layer of thread. Among the most interesting of them are the so-called “Shikar” chundries, where the design, repeated over and over again, illustrates some hunting scene, as, for instance, a tiger hunt, with the animal springing at a man armed with a sword, and a horse or elephant with howdah. When fully opened one of these chundries makes a strip of cloth some five or six yards long, and in Rajputana is used as the full-dress costume of a young lady of fashion, being folded round and round the body and over the head in most graceful and charming lines.
On studying one of these chundries one is struck by the immense amount of labor expended in the tying process. The knots which form the pattern make, frequently, as many as twenty-five or thirty to the[198] running inch, and each one is tied so tightly around the cloth, folded so as to form four thicknesses, and drawn or pressed out into loops, that it completely protects the part it covers from the dyestuff, only the tip of the loop remaining exposed. Hence, when it is untied, there results a small circular or rectangular ring not over three-quarters of an inch in diameter. To obtain a surface around which the string can be thus tightly tied, the folded cloth is evidently pressed out from the back by a thin pin or spike (the effect can be produced by tying a thin piece of cloth tightly around a wooden toothpick) around which the thread can be tightly drawn and knotted, and which usually is left in during the dyeing process and taken out afterwards.
The patterns are so elaborate, and yet are repeated over and over again, on the same chundries, with such regularity, that it is probable that some simple apparatus is used to press out the cloth in exactly the proper places. This could be done by using a little frame with holes in it, into which pins of wood or ivory could be set, like the markers in a cribbage board, for instance, forming definite figures on which piece after piece of cloth could be placed and pressed out into shape.

The most interesting thing, after all, about these extraordinarily elaborate pieces of handicraft work is the fact that this vast amount of time and labor is expended upon such poor materials. The muslin of which they are made is so thin and poor that considerable pains must be taken in opening them, to prevent[199] their tearing from the strain of pulling off the knots of fine thread. Then, too, the colors as a rule not only are fugitive to sunlight, but are easily affected by washing. Two minutes scrubbing in hot soapsuds will almost completely efface the pattern and color from some of the most elaborate and beautiful of them all. And this is not, as is claimed frequently by modern writers upon Eastern handicrafts, due to the introduction of cheap and fugitive “aniline” dyestuffs. The dyes, used for generations by the Rajput craftsmen, for their most elaborate chundries, were principally tumeric, safflower, and other inferior vegetable colors, applied so loosely as to be merely stains rather than dyes—and it would be hard to get modern dyestuffs which, applied with any care, would be as fugitive as those commonly used for the very best examples of these beautiful textiles.
Tied Work in the Philippines.—Of different quality is the work of the Bagobo tribe in Mindanao, interesting specimens of which are to be seen in the Philippine collection of the New York Museum of Natural History. As shown in Fig. 5, a headdress belonging to Miss Laura Benedict, the work is not unlike that done by the ancient Peruvians, and the patterns, although often exceedingly complex, are invariably geometrical, and do not approach in variety or in interest those from India. The coloring, too, is far simpler—practically all the examples showing light patterns on a dull purplish background. But the dyeing is most carefully and thoroughly made—taking about thirty days to complete, dyeing each night and washing[200] thoroughly each morning during all that time, until the final product is exceedingly permanent to both light and washing.
Miss Benedict, who was the first white person to enter the Bagobo country and study and report on their handicrafts, states that the patterns are made in a curious manner. The pattern is first outlined upon the cloth by a series of basting stitches, the intersection of two stitches being the mark for the centre of one of the tied places. Then the operator, seated, puts over her big toe a ring attached to a line some three feet long, on the end of which is a simple hook made from a bent and sharpened piece of copper or brass wire. Holding the cloth in one hand, she then fastens the hook into one of the marked places, pulls the part out with her foot, and ties up the loop thus formed, rapidly and tightly, with waxed thread. This she winds round and round the loop, beginning with the bottom first, and knots it tight, using the free hand, assisted, except with very expert workers, with the thumb and forefinger of the other.
Specimens of textiles thus tied, and not yet dyed or opened, and also of the toe-ring, line, and hook used in the process, can be seen at the Museum, along with a great variety of beautiful specimens of the finished work.

It is rare that, in our present surroundings, any craftsman can spare the time and patience to copy the elaborate patterns made in these ways by the Eastern dyers. But equally beautiful and interesting results can be produced with very little expenditure of[201] time and labor, by the skilful dyer, who knows something of the fundamental principles of design and can use his dyes so as to get soft and beautiful as well as permanent color effects. It is impossible, in a work like this, to do more than suggest some of the many ways in which this process can be used. The rest depends entirely upon practice—and more can be learned about its possibilities in a couple of hours’ work with muslin or cheesecloth, and a ball of twine or tape, in connection with a dye-pot of a good Sulphur dye, than by weeks of listening or reading about it.
Tied on Itself.—Interesting effects may often be produced on long pieces of cloth, scarfs, and the like, by folding them over and tying them into knots at one or two selected places, before dyeing. Fig. 6 shows an example of this, (a) Tied and ready for dyeing; (b) Dyed and opened out. This when worked out in different colors, dyeing first, with some light color, then tying and dyeing with another color, or else coloring the tied and dyed piece with a second light bath of another color, gives very pleasant results as applied to draperies—as, for instance, simple costumes for pageants and out-of-doors plays. It is, however, almost, if not quite, impossible to obtain definite designs in this way, and it is hardly possible to duplicate results. But occasionally the process is useful.
Tied with String or Tape.—Far more important is the process generally meant by the term “tied and dyed work,” where the pattern is made by tying either[202] thread, string, cord, or even tape, more or less tightly around special portions of the cloth. These portions are usually drawn out, or pressed out, or folded, so as to form a sort of loop around which the string can be tied. But occasionally the whole cloth, laid flat and with but little folding, is tied tightly across, so that the reserved part forms, when untied, a more or less straight band.
Tied in Bands.—It is often desirable to separate one part of a design from another by means of a broad line or band of white or light color. This can be readily done by tying a piece of strong twine or tape, tightly, right across the goods at the desired place before dyeing it. Quite elaborate and interesting effects can be produced in this way by first folding the cloth lengthways, and then tying a width of several inches with a broad piece of tape. If it is not tied too tight some of the color will work up and down the folds, under the tape, and give, when finished, curious wavy effects. (See Fig. 7.)
Tied in Small Loops.—This banding, though interesting and useful, differs from the sharp little round or diamond-shaped rings forming the patterns in the Rajput or Bagobo textiles. These are produced by pressing or pulling out the cloth into loops or bunches which are then tied tightly round and round with string or thread, the middle of the loop being usually left exposed to the dyestuff, so as to form a colored centre.
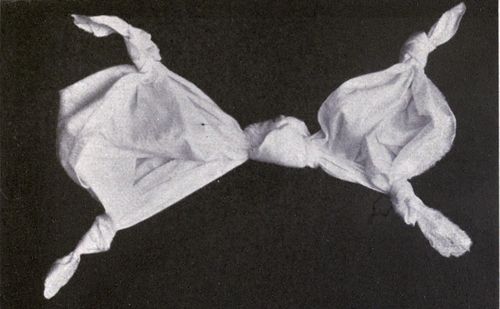
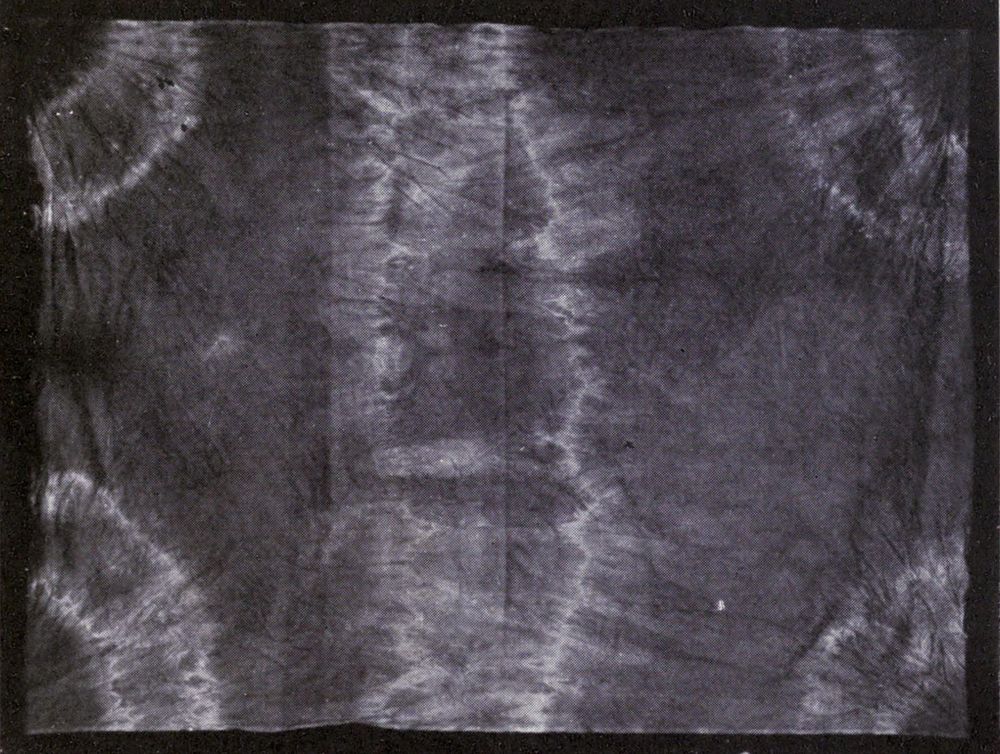
Very small loops can be made, as mentioned above, by pressing out the cloth with a wooden pin (or toothpick)[203] and tying tightly around this, leaving in the pin until after the dyeing is completed.
Skilful workers can tie quite small loops by placing a bead, or dried pea, or piece of gravel in the cloth and tying the cloth tightly around this. It is best, always, to have something of the sort, pin or bead, to act as a centre, or else the knot, after tying, is very apt to slip off, and spoil the pattern.
The design for this sort of work should be carefully planned beforehand, and marked out on the cloth with pencil or chalk. For, with small loops like this, the interest is more in the pattern formed by them than in the changes and contrasts in color between the different tied parts and the rest of the cloth.
A very interesting specimen of work done in this way by Miss Mary Grey is shown in Fig. 7.
Tied in Large Knots and Loops.—It is hard for a Western craftsman to obtain sharp, well-defined knots by this method, of a diameter of less than half an inch or so. Usually, indeed, it is too much of a bother and nuisance to try any knots covering less than an inch and a half. From this size, up to fifteen and twenty inches in diameter, will be found the vast majority of all American work. The reason is very simple. The trouble of tying a knot covering five inches is very little more than that for a half-inch knot, indeed far less for most people, while the large knot produces an immediate effect not equalled by a dozen of the latter. Furthermore, with large knots, big bold designs can be produced, which,[204] with pleasant and skilfully selected colors, give results far more striking and effective than can be shown by the small knots, no matter how carefully carried out. On the other hand, intricate and carefully planned designs can be worked out with small knots, which cannot be attempted with the large ones.
For designs with large knots, beside the cloth, which should be soft and free from dressing, and a ball of soft thick twine or better, of cheap cotton binding tape, half to three-quarters of an inch wide, it is well to have a supply of large glass beads, of marbles of different sizes, and, if these are not easy to get, of pebbles, beans, hazelnuts, and the like. These are not always to be used, but in most cases it makes a more interesting contrast to have the centre of the tied spot come out dark, with the lighter parts, more or less shaded, around it. That means that the centre must be exposed to the dyestuff by being stretched out over a marble or pebble, while the parts around it are tied up. And the tying, too, is greatly facilitated by having a hard centre to work against.
By tying around one marble first, and then putting in another and tying round that, a series of concentric rings will be formed, the black rings showing where the cloth, covering the marble, has been exposed, and the light-colored part showing where it has been covered by the tape or string.

FIG. 7—SAMPLE OF TIED AND DYED WORK, “TIED IN BANDS,” WITH INCIDENTAL KNOTS. BY MISS MARY GREY
As before, the design, if at all elaborate, should be marked out beforehand on the open cloth, and the parts tied in accordingly. Much experience is required to know just how tight to tie the tape so as[205] to get a desired effect with each particular kind of cloth, and each class of dyestuffs. In general, with small knots the string should be tied very tight, or otherwise no effect is produced at all. The larger the tied parts, however, the more pains should be taken to have the cloth folded before tying, so that some of the color may work down through the folds past the tape, and thus produce shaded effects, which may be of great beauty (see Plate IV, Fig. a). Of course, in this, much depends on the cloth; a thick heavy calico tying with difficulty, but not letting the dyestuff soak through; while soft open materials like scrim or cheesecloth, for instance, must be tied much tighter, or the color will work through so much as to spoil the design.
The student is advised to practise, from the start, tying his tape with a slip loop, or at any rate a bow knot, and not with a fast square knot each time, so as to save trouble and bother when untying later. A skilful craftsman will tie quite a large piece of cloth, in an interesting and fairly complicated design, in a few minutes. But after dyeing, while the cloth is still wet, and the tape or string has shrunk, and the knots have tightened, it is often more trouble to untie, or cut it open, than it was to make it, and there is always the danger of cutting holes in it. A little pains in laying down one end of the tape, before starting to tie, so that, when the whole loop is tied up, the other end will come out alongside of the first so that it can be joined to it by a bow knot, will save any amount of time and vexation.
[206]
Sewed and Dyed Work.—Besides protecting the cloth from the action of the dyestuff by tying string or tape around it, the same effect can be produced by sewing up certain parts of it, before dyeing, and then, after the rest has been colored, and the loose dye-liquor washed off, the sewed-up parts can be opened and pressed into shape.
This modification of the process, so far as I can learn, is not practised by the Rajputs with their chundries, but in the Benedict collection can be seen some most extraordinary and elaborate pieces of dyed work made just in this way. The Japanese, also, have been in the habit of using this method, and sometimes they produce curious zigzag lines by taking coarse stitches across the cloth, alternately, first to one side and then to the other side of the centre line, and then drawing the thread tight. The needle is often used for borders—for straight lines can easily be made in soft materials (and such only should be used for tied work) by hemming the cloth with strong thread, and then drawing it up close and tight before putting it in the dye-bath. The development of this branch of the process, however, belongs properly to the fair sex.
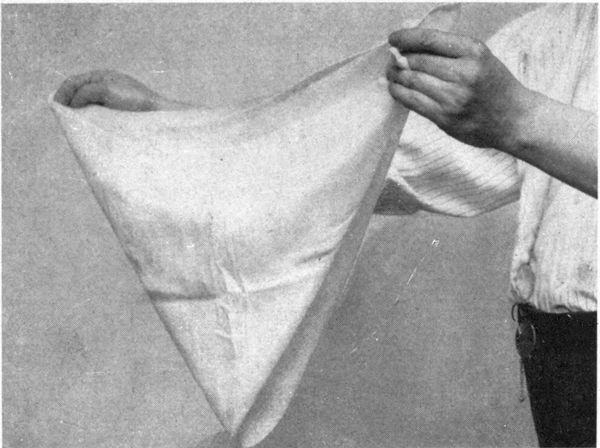
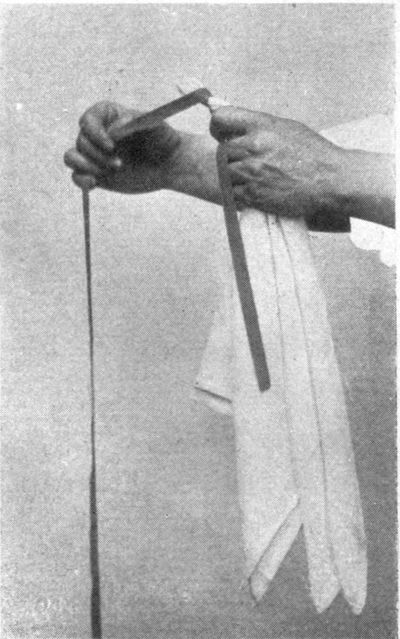
|
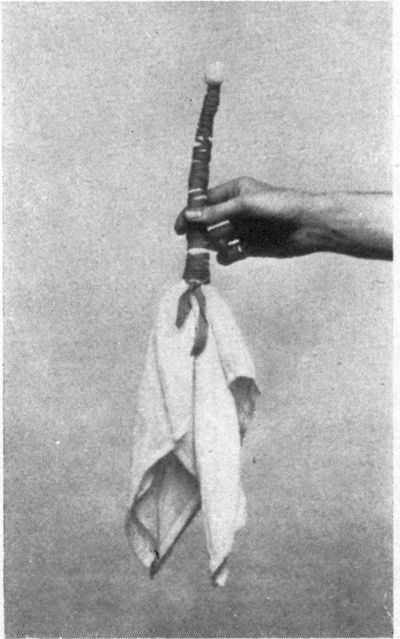
|
TIED AND DYED WORK
Dyeing Process.—Now for the dyeing process. Of course, for practise, the craftsman will use cotton as his raw material, in the form of muslin, cheesecloth, scrim, or best of all, light grades of mercerized cotton, and hence will use the various cotton dyestuffs. The Salt colors are hardly advisable, because though fast to light they are not all fast to washing unless well boiled on, and that means that, unless tied extremely[207] fast and tight, the color would be bound to penetrate, and wipe out the design. The Sulphur colors and the Vat colors are the best for the purpose—for they can be dyed cold or lukewarm, without injuring the fastness of the dye, and give colors fast both to light and to washing. In general, it is easier to get even shades with the Sulphur colors, and their shades are soft and pleasing, but while fast, they are not as fast as the Vat dyes, and it is impossible to get a decent scarlet with them. The skilful dyer will, of course, select his class to suit the shade he is trying to get and also to meet the requirements about fastness. But, in general, he will use the Salt colors for covering and shading the patterns produced with either the Sulphur or the Vat dyes. When using the oxidation dyes, like the Sulphur or Vat colors, plenty of time must be given for the dyestuffs to oxidize and set before they are untied. But, on the other hand, directly they are once untied it is important to wash off the loose dye-liquor from the cloth, and especially from the tied-in portions, as soon as possible after untying, otherwise some dye-liquors that may have soaked in without having had a chance to oxidize, will, when exposed to air, suddenly fix themselves and obscure or ruin the pattern.
After attaining some skill in this process the craftsman is urged to try it on more important materials like silk. Most beautiful effects can be, and are being produced by this means, on soft delicate scarfs made of Chinese or Indian silks. The Acid colors are, of course, used for this, and as they take so readily on silk, the possibilities of shading and over-shading different[208] portions of the design, or of adding a touch of color here and there where it seems desirable, offer infinite possibilities to an artistic workman. The combinations of color that can be produced are infinite, and the curious blending of regularity and irregularity, in the designs and figures, renders it a most attractive process to practise with.
One great attraction about it is the sense of suspense, and the impossibility of telling just what effect is being produced, until the knots are all untied, and the cloth washed off and opened out.
Another attraction is the feeling of working all the time in an unexplored or very partially explored country. There is the constant chance of obtaining at any moment effects never thought of before. The experimenter is always trying some new little trick in tying, or in folding, or in dyeing, the results of which can never be foreseen accurately, and which are always interesting and often very beautiful.
Tied and Discharged Work.—One day, in our laboratory, some experiments were made which resulted in a modification of this process which, so far as we know, was entirely new, and which presents very interesting possibilities, to say the least. We made the experiment of dyeing the cloth first, and then tying it up, and putting it in a bleaching solution, so as to discharge the color everywhere excepting where it was protected by the tying. The experiment was successful, resulting (see Plate IV, Fig. b), in a series of dark patterns on a light background. All kinds of modifications of this can be made. For instance, the[209] cloth can be dyed with a mixture of two or three dyes, some of which are fast and the other or others can be discharged by the chemical used. The pattern thus will be the full mixed color, say brown, against a background of red or yellow or blue as the case may be.
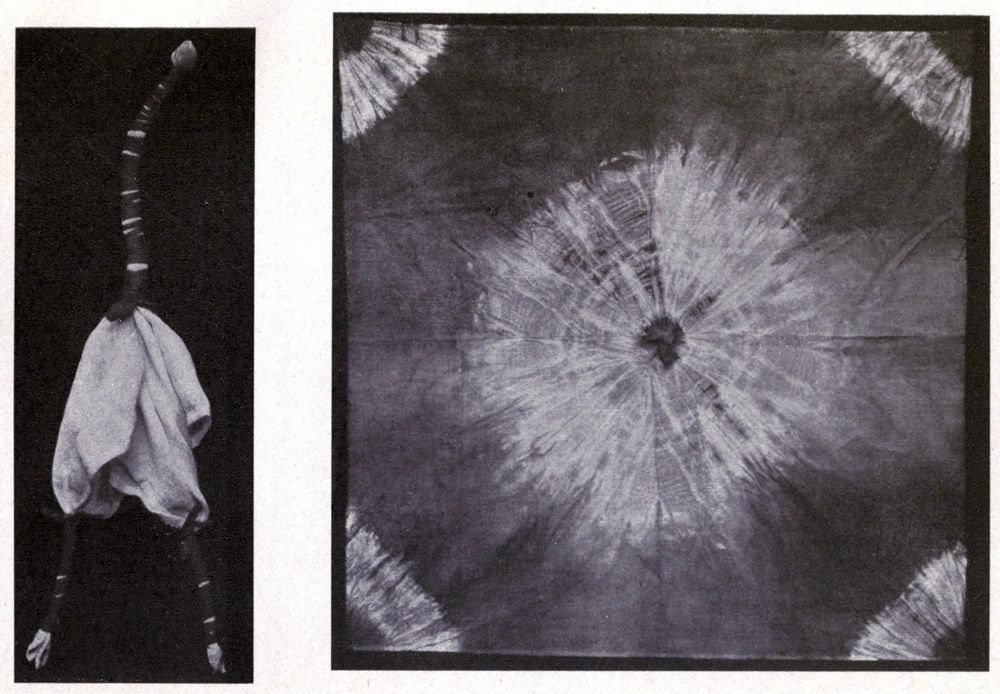
| FIG. 11—CENTRE AND CORNERS TIED | FIG. 12—DYED, UNTIED AND SHAKEN OUT |
The important thing about this modification is to select the proper bleaching agent to act on the particular colors, and the particular kind of material, used. Our first experiments were with bleaching powder (chloride of lime), dissolved in water, say two tablespoonfuls to the gallon, with, if necessary, a few drops of acetic acid or weak sulphuric acid stirred into it. This powerful bleaching agent is very apt to attack the cloth, and only heavy materials, such as scrim or heavy calico should be used with it. But although so strong, it does not act at all readily on a large number of the dyestuffs, including many of the Vat colors. Some of these, like the Indanthrene colors, are not affected at all, Indigo is changed from blue to a brilliant shade of yellow. And Thio Indigo Red B produces curious shades of purple, settling, where exposed to the full action of the bleaching agent, to orange.
Later we repeated the experiments, using hydrosulphite of soda, say two tablespoonfuls to the gallon of warm water, as a discharge, with much better success. The cloth was not injured, even when delicate materials like silk and light poplins were used. And the great majority of colors, including nearly all the best Salt, Sulphur, and Acid dyes, reduced rapidly and well. The Vat dyes will reduce, and, in the presence[210] of caustic soda, will dissolve out of the exposed cloth almost entirely, but it is hard to reduce them to white in this way. In every case the color, after reduction, must be washed at once in warm soap and water, or else, on exposure to the air, the color may come back to some extent, owing to oxidation.
A weak bath of hydrosulphite of soda, also, should always be on hand, in the former bleaching process; for, when bleaching powder (chloride of lime) or other chlorine compounds, such as Javelle water or Labarraque’s solution, are used for destroying the color, their further action can be stopped, and also the offensive smell removed, by dipping the bleached material into a so-called antichlor, like this hydrosulphite.
This subject of discharge is dealt with more at length in a future chapter.

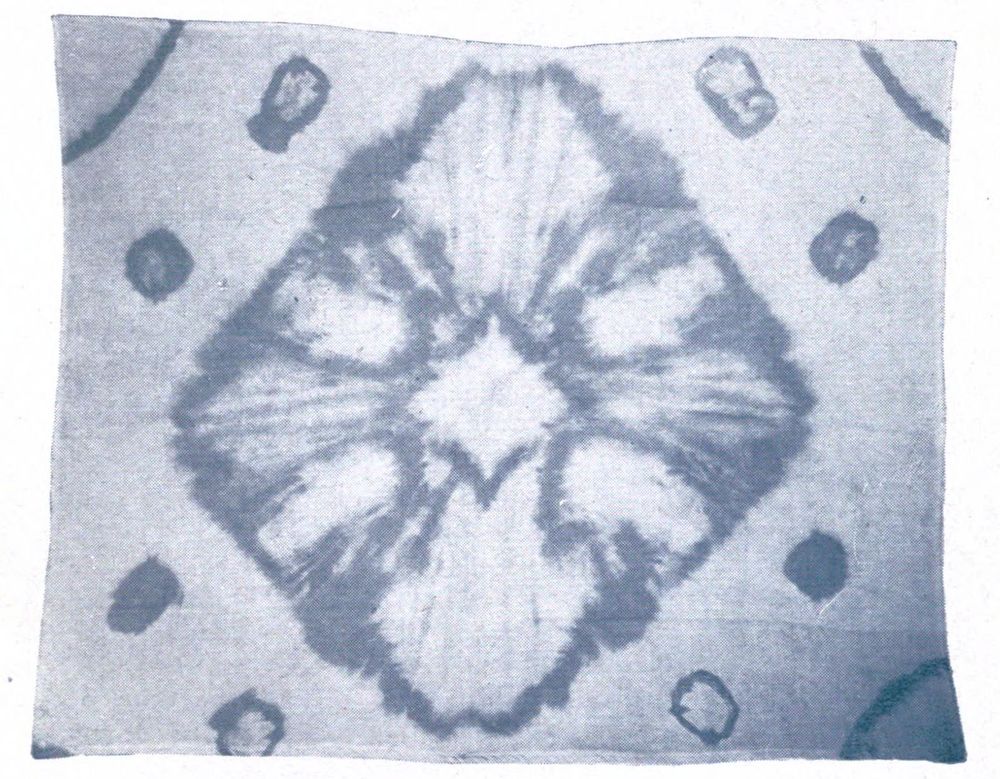
[211]
History.—During the last few years a great deal of attention has been paid to the manufacture and use of stencils for decorating textiles, not only by craft workers of different kinds, but also by art teachers in private and public schools.
The art is not a modern one, even in this country, for I have seen and worked with a series of very interesting stencils cut in brass, which were owned in Philadelphia by the famous old physician, Dr. Benjamin Rush, over a hundred years ago, and were used in his family for marking linen, as well as for decorating homespuns and paper.
The real home of the art, however, is Japan, where, for over three hundred years, stencils have been in common use, largely replacing the wood blocks used in other countries, for decorating the common cotton goods, towels, head coverings, and the like of the lower classes, and also for ornamenting, where embroidery was not desired, the beautiful silks and satins of the wealthy.
Ever since Japan has been opened to the world travelers have been telling wonderful stories of[212] the great skill of the natives in this beautiful art. According to some writers, as soon as a child is born it is given a nickname, and with it, as a sort of totem, a design—a flower, for instance, for a girl—a tree or an animal for a boy—and the like. This design, worked out carefully, after due criticism from all the family elders, is drawn on brown paper and then carefully cut out with a sharp knife by some member or friend of the family. And this stencil is then sent to the local dyer to be used in dyeing the infant’s clothes. This same design, or a modification of it, is attached to the person through life, as his or her own private pattern, and whenever new clothes are needed they are dyed after this same pattern.
Japanese Stencils.—Paper.—It is a common fact that the very first thing noticeable about Japanese stencils, whether brought from some dyehouse in the interior, or whether made more or less mechanically, for the American market, to be sold to students or craftsmen, is the quality of the paper. It is thin, hardly heavier than ordinary writing paper, but exceedingly tough and strong, and cuts very easily, without tearing. It can occasionally be obtained from importers in sheets, and even better qualities can be secured, from among a mass of old stencils, by finding some which have been only partially cut or used up, and carefully cutting out from them the unused portions where these are large enough for the purpose.
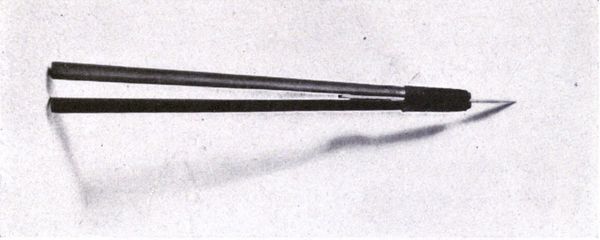

Knives.—In cutting stencil designs our American practice is to use a sharp penknife, or a Sloyd knife, or, as happens occasionally with some of my friends[213] with amiable professional husbands, a surgeon’s scalpel. None of these, however, compare for neatness, accuracy, and ease and comfort of manipulation, with the very simple but extremely effective little Japanese knives shown in Fig. 13. The knife blade, of very highly tempered steel, is two or three inches long and fits between two flattened plates of wood, tied together tightly at the bottom but springing apart a little toward the top, as a handle. This little spring of the handle is most satisfactory. And as the blade, which is triangular and sharply pointed, is worn away gradually by the constant grinding and sharpening it must receive, the steel can be pushed forward from between the two halves of the handle, until the proper length is reached.
Cutting.—The Japanese draw their designs on paper with India ink, and then, with incredible swiftness and accuracy, the lines are cut, by pushing the knife blade, held with the back downwards, away from the workman, and through the paper which is placed flat on a piece of wood or small tray, with depressions in it half an inch or so deep, to avoid the danger and bother of running the knife point into the wood.
American Practice.—Our way differs somewhat. The design is usually drawn on a separate piece of white paper, and filled in—in black—with India ink. This is then placed underneath the stencil paper which, especially if it has been oiled or paraffined, is translucent enough to show the pattern through, so that the outline can be drawn with a sharp pencil. The outline[214] can also be made by tracing the design down on the stencil paper with the help of a piece of carbon copying paper. This is laid between the design and the stencil paper and then the outline of the design is carefully traced with a sharp-pointed pencil. From these outlines it is easy, with a sharp stencil knife, to cut out the design, although it is customary with us to cut toward the body with the point of the knife down, upon a piece of blotting paper or soft wood so as not to dull it too rapidly.
Ties and Stops.—When stencilling is taught in America great pains are taken to show how the pattern must be planned and cut out, so as to have plenty of “ties” or “stops” in the right places, so as to hold the stencil together. For instance, in making a stencil of a large capital O, the student should be warned that, if the paper was cut all the way around, it would leave a big hole; for the central piece, which would form the centre of the finished letter, would drop out, and could not be kept in place. Accordingly, the stencil would have to be cut carefully, leaving at least two “bridges” or little “tie pieces” of paper, one probably at the top, and the other at the bottom of the O, these being the narrowest points, which would hold the centre in place, and thus complete the figure. Indeed, if these little “steps” or “bridges” of paper should be left out, or become torn or broken, the stencil would be useless. But a situation like this has little or no terror for the Japanese, at any rate when working for their home trade. Their stencils cut for the American market while always interesting, and often[215] charming, are cut, as ours are, from one piece of paper, with stops in the exposed places. But the stencils that have been used, or cut for use, over there, show a very different state of affairs. All of the large, handsome ones, and a large proportion of the smaller, less artistic, and less valuable ones are made, with almost inconceivable skill and patience, in duplicate. And the two parts are afterwards pasted together with absolute accuracy, but with a layer of fine hair, supposedly human hair, between them. These hairs, laid irregularly but evenly, make a sort of network which ties together all portions of the stencil, no matter how disconnected with the rest, or, as we would say, “in the air,” it might be.
So, too, they are in the habit of sewing in, with the finest of hair or of single threads of fine silk, loose pieces or broken pieces, and thus holding them in shape.
It is interesting to study some of them closely and see how neatly this tying is done and how little the time of these unknown workmen must be valued at. For apart from the large picture stencils which, of course, would be worth taking a great deal of pains with, some of the simplest and most ordinary of their native stencils are not only cut but tied in, with extraordinary skill. One of these, valued here at but a few cents, consisted of a background of small figures in shape and size very much like a capital O of the type of this page. The stencil measures some eighteen by ten inches, and there must be between fifteen hundred and two thousand of these O figures on it.[216] Some few of these are now imperfect, but with the exception of a dozen or two, every single one of all these has had the centre cut out, and then sewed into place again, from the sides, so as to be in the exact centre, without a single “stop” or “tie” on the whole paper.
Brushes.—With stencils so very delicately made, it is evident that our crude American style of rubbing in the color, with heavy hands and stiff bristle brushes, would not be much of a success! About one good rub with a brush like that, and every hair in sight would be torn and broken, and what was a minute before a work of art would be a torn mass of brown paper.
Whether any of our American craftsmen have light enough hands to use, successfully, a fine Japanese stencil is doubtful. Personally, I could no more stencil six inches with any of them without ruining it or making a mess of the cloth than I could in a year cut, without tearing, six square inches of any one of a score of cheap and ordinary Japanese stencils which I own, either presented to me or sold at a very low price, as being really too insignificant in value to amount to anything.
But at any rate, the Japanese do not use a stiff bristle brush. Their brushes, in general, are of two sorts, as shown in Fig. 14. One is a sort of pad, often quite large, five or six inches in diameter, made of rabbit’s fur, tightly bound together with cord or wire, and with a bundle of small sticks spreading out to enclose the pad, and drawn together and tied above, at the upper end, in a sort of pyramid.

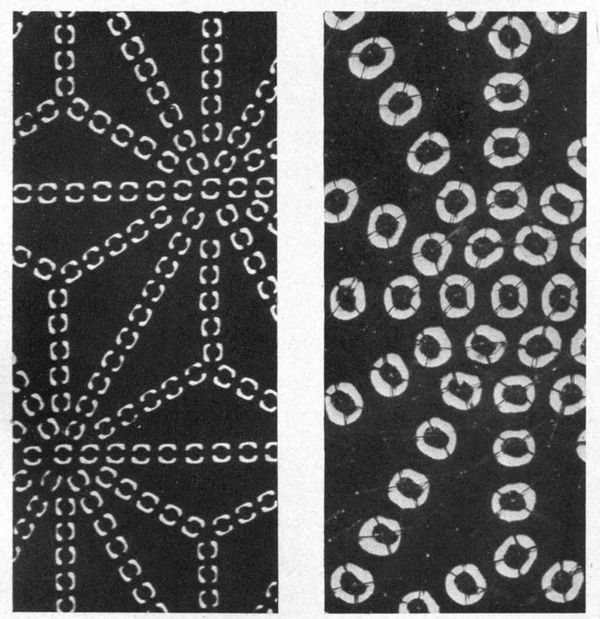
| FIG. 16—JAPANESE STENCIL, EXACT SIZE, SHOWING USE OF STOPS | FIG. 17—JAPANESE STENCIL, EXACT SIZE, SHOWING USE OF SEWING INSTEAD OF STOPS |
The other variety is a true brush, of a more ordinary shape, like a flat paint brush, but also made of the very softest and finest, most velvety hairs imaginable, laid extremely close together, and compressed tightly between the two halves of the handle. These can be obtained occasionally from the dealers at reasonable prices, and are delightful to work with. Only, being meant for the soft, light touches of their native workmen, they do not last long when rubbed down on the cloth as is our practise. Their life is considerably increased by pouring some molten beeswax into the back of both goods and brushes with a batik pot, or Tjanting, which prevents the fine hairs from pulling out until the brush is all worn to pieces.
The Care of Stencils.—A word may here be said about taking care of stencils, after they have been cut or purchased. They should always be used on one side, and carefully wiped off with a damp cloth, directly after using. They should always be kept flat, never folded. And, when using them, it must always be remembered that the ties or bridges are the weak spots, and that breaking or tearing them, as a rule, will spoil the stencil. It is, of course, possible to mend them by sewing, or sometimes by patching with tape. But this is always troublesome, and with well paraffined stencils is rarely satisfactory.
The Different Methods of Using Stencils.—In this country, so far as can be ascertained, the common way in which stencils have been used is by brushing through them, on to the cloth, oil paints thinned with turpentine or gasoline. As previously explained, in the chapter on[218] feather dyeing, this is not very satisfactory. For when paint is sufficiently thick to adhere well to the cloth, it is apt to look stiff and shiny. And when it is applied so thin that the structure of the cloth shows through, it is, as a rule, not fast to washing or even to rubbing. Various varnishes are on the market which help considerably to make the paint fast, but even then the results are not nearly so durable as when the proper dyestuffs are used.
The Japanese practice is exclusively with dyes, and they have worked out processes which are perfectly satisfactory, so that their simple, cheap, stencilled towels can stand washing indefinitely without loss of color. And by the use of modern dyestuffs there is no insuperable obstacle to our doing just as well as they.
The use of stencils gives an excellent opportunity to illustrate the three general methods of coloring fabrics, which, as mentioned in the last chapter, consist of:
Direct application of color.
Resist, and
Discharge.
The last two of these will be reserved for the next chapter.
Direct Application of Color.—In this intricate work it will generally be found almost a necessity to apply colors through a stencil in the form of a paste, for when the coloring liquid is thin it is very apt to run under the edges of the paper and spoil the design.[219] It is best to thicken it with a little “gum dragon,” a carefully prepared paste of gum tragacanth, to which the coloring matter, and any reagents that are needed, can be added. The nature of the reagents and the class of dyestuffs used depends, of course, upon the kind of material to be stencilled.
(a) Leather.—While not very often used, students interested in leather work will find a carefully designed and neatly cut stencil a most useful medium for obtaining interesting and beautiful effects. The leather, whether bark- or alum-tanned, should be carefully dampened, and then stencilled with a paste containing Basic colors dissolved with a drop of acetic acid. On drying, the leather should be finished as usual. The Acid colors are not nearly so satisfactory for stencilling, although, as already mentioned, they are often advantageous for dyeing, rather than staining, leather fast colors.
(b) Silk.—Silk may easily be stencilled provided the pattern is not expected to be fast to washing.
1.Acid Colors.—These dyes, mixed with a few drops of formic or acetic acid, will color it well, but to make the dyestuff penetrate it is advisable to steam the goods. This can be done with a teakettle provided with a wing tip for the spout, made of tin, or by heating a flatiron or iron plate very hot, and pressing the stencilled goods back down against it, with a damp cloth in between. The hot steam thus produced, passing through the goods, melts the paste and drives the color down into the fibres and sets it there, so that,[220] later, the stencilled goods will stand light rinsing in lukewarm soap and water without running.
2.Salt Colors.—Faster results can be obtained, on silk, with a paste containing salt dyes, with a drop or two of acetic acid, provided the silk is thoroughly steamed afterwards.
3.Basic Colors.—Basic dyes may be used on silk as on wool, leather, or any other animal fibres for direct application, the dyestuff dissolved with a drop of acetic acid, being added to the paste, and then brushed in and, preferably, lightly steamed to sink the paste down into the fibres. These dyes, however, with but few exceptions, are not fast to light, and applied in this way are not fast, either, to washing. By adding some reagents to the paste, however, a Basic stencil paste can be formed which gives colors on silk which will stand active scrubbing excellently.
The Basic Stencil Paste is prepared by mixing with the paste a solution containing the Basic color, dissolved in acetic acid, and also containing a considerable quantity of tannic acid. As long as there is free acetic acid present in this mixture the color remains in solution, but directly the acid is driven off, an insoluble compound remains, formed by the combination of the tannic acid with the color base. This happens on steaming, and the insolubility of the product is still further increased by passing it through a weak bath or wetting it with a weak solution (half a teaspoonful to the quart) of tartar emetic.
Accordingly, to use this stencil paste on silk or, indeed, on cotton, the slightly dampened goods are[221] stencilled with the paste, thinned if desired with water and a little acetic acid. Then directly they are dry enough so as not to run they are well steamed, then the gum rinsed off with a little warm water, and the goods moistened with the tartar emetic. After this they can be washed with soap with little or no danger of running.
(c) Wool.—Wool is rarely stencilled, although stencil patterns can be produced very well on it by using acid colors with a little oxalate of ammonia (about the same amount as the dyestuff), dissolved in a drop or two of water, and thickened with a little gum tragacanth. When this paste is applied with a brush, and then dried, the result is not fast at all, merely a distinct stain; but if steamed at once the oxalate of ammonia decomposes, leaving oxalic acid, which, combining with the color and melting down with it in the fibres, makes the dyestuff adhere quite firmly.
(d) Cotton and Linen.—It is much more difficult to stencil satisfactorily on vegetable goods, such as cotton and linen, than on the animal fibres above mentioned, because they are expected to stand very much more severe treatment. The fastness to washing needed for a handsome silk scarf is far less than for a cotton shirtwaist, or linen table-cover, and unless the results on the latter are at least as fast as the average calico print, the result is considered a failure.
There are three classes of dyes which can be used in this connection, the Basic dyes, the Sulphur dyes, and the Indigo or Vat dyes. The Basic stencil pastes have just been described, in connection with silk stencilling,[222] and when carefully used they will give very fair results on cotton, and even on linen, provided it is free from dressing, and is not too coarse and thick. It is hardly worth while trying to fasten Basic dyes, by hand stencilling, upon such materials as heavy, coarse Russian crash, for instance, such as friends and students have frequently brought in to experiment with. But for light, thin materials, and especially for mercerized goods, poplins and the like, it is possible, with a little practice, to get effects that are fast to ordinary washing.
On the other hand, this method of stencilling has certain disadvantages. It is rather complicated, needing the use of a fixing bath of tartar emetic, a very active poison, by the way, although more uncomfortable than actually dangerous when taken by mistake in one dose, because of the severe vomiting it produces almost immediately. And then, too, the results at best are not really fast to light, and in the case of light pinks and yellows are distinctly fugitive.
Vat Color Stencil Pastes.—Many experiments have been made in our laboratory to work out a satisfactory stencil paste, so that Indigo and other Vat dyes could be applied, simply and easily, with no more difficulty than the usual one of brushing the paste in carefully, and then steaming as soon as possible. In these stencil pastes the Indigo and the other Vat dyes are reduced with the aid of caustic alkali and hydrosulphite before being mixed with the paste, and some special precautions are taken to prevent, as far as possible, the oxidation of the dyestuff before it gets well into the[223] fibre. But, as the ordinary hydrosulphite is apt to decompose on standing, especially when it is wet, it is always best, just before using, to mix well with the paste a little fresh reducing agent, dissolved in a drop of hot water. The reducing agent that should be used for this purpose is not the ordinary hydrosulphite of soda, used for vat dyeing, but a compound of sodium hydrosulphite, “Stencil Salt,” which has the property of keeping better than the other, and also of not acting as a reducing agent until it is heated. This, then, is stirred into the Vat color stencil paste, just before using, and then, when the goods are steamed, the heat and moisture combined will enable it to reduce the color, which will be carried into the fibres in a reduced and dissolved condition. After steaming well for five minutes the color should be developed by a bath in hot soapsuds, after which the goods should be rinsed and dried. With care this process will give very satisfactory results, perfectly fast to both light and washing, after the first loose color has been washed off.
The indigo stencil paste, as prepared, will keep well reduced for quite a long time, and it is frequently quite unnecessary to add any fresh reducing agent to it. If, when taken from the tube or bottle, it looks yellow or yellowish green, it can be applied at once to the cloth, and, if steamed just as soon as possible, it will generally penetrate quite satisfactorily. With the other colors of the series, however, it is hard to tell by the color whether they are reduced or not, and hence the fresh reducing agent, Stencil Salt, should always be added to them. The cloth for stencilling[224] with these pastes, as with the Basic pastes, should not be too thick or heavy, and must be washed quite free from dressing, or the result will not be satisfactory. It should also be slightly dampened, if only by holding over boiling water for a moment or two, so as to help the color to penetrate.
Sulphur Stencil Paste.—We have also found very satisfactory results from pastes made with one of the Sulphur colors, dissolved in a little sodium sulphide and sodium carbonate, and stiffened with a little gum. The presence of a reducing agent helps to keep the color reduced; and, when quickly applied and rapidly steamed, the colors will sink into the fibre and adhere firmly.
The chief drawback with these pastes is the lack of a good red.
Black Stencil Paste.—So far as can be learned, the Japanese use for their stencilling an Indigo paste made on the same general principles as the one just described. Besides this, which is a very favorite color of theirs, they use a red and also a very full black dye, both of which are fast to washing and to light.
What the composition of these last pastes may be it is hard to tell. In our laboratory we have made careful experiments on the subject of stencilling black, and have worked out a method that we consider satisfactory by the use of a modification of the well-known Aniline Black process.

Aniline Black.—It was noticed, early in the history of dyestuffs, that if aniline was mixed with strong oxidizing agents, and carefully heated, it would undergo[225] a series of color changes resulting, finally, in black. This color, so-called “Aniline Black,” was at one time manufactured and used for a black pigment; but it was soon recognized that its real value would only be developed when it could be formed, in the fibre itself, by the oxidation of aniline or some compound of aniline upon the fibres. After many years of experimenting this problem was solved, and for fifteen or twenty years the blacks most used on cotton and linen by the calico printers, as well as by the dyers, have been one or another of the forms of Aniline Black.
The principle on which these processes are based is as follows: The aniline, usually in the form of aniline salt (aniline hydrochloride), is mixed with an oxidizing agent like chlorate of soda, and also with a small amount of a third substance which, on steaming, acts as a carrier of oxygen between the aniline and the chlorate. This substance, often called a catalytic agent, because at the end of the operation it remains unchanged, although it has accomplished a large amount of work, may be one of a number of compounds as, for instance, a salt of the metal vanadium, prussiate of potash, a salt of copper, etc., each one having special advantages and disadvantages of its own.
Now, almost any printing paste properly composed so as to give a good clear Aniline Black on steaming, (the formulæ can be obtained from any good book on calico printing, or from any competent dyeing chemist), will generally work fairly well as a stencil[226] paste—as long as it is fresh. But even when kept from the air as far as possible, in a tight tube, it decomposes on standing and becomes very unsatisfactory. Besides this, there is always a difficulty with these regular pastes on account of the irregular and uncertain steaming process that can be used by the average craftsman. In a calico print works, the temperature of the steam chest, the proportion of steam in it, and the length of passage of the cloth through it, are all accurately determined, and kept at the exact points necessary for the best results with any given formula. But with irregular steaming, unless very great care is taken with the formula, there is always a danger of “tendering” and burning the fibre, if too much oxidizing agent is present, or of not developing a full black, but a dark green color, if the oxidizing agent is not active enough.
We have, after a great deal of experimenting, worked out a formula which, with reasonable care in steaming, will give a good full black, absolutely fast to light and washing, upon cotton, linen, and silk, without any tendering of the cloth. And, by dividing up the component parts into two separate pastes, which are kept in separate tubes or bottles, and are mixed together only when about to be used, we have gone far to solve the important problem of keeping.
The use of this Black stencil paste is very simple. It comes in two tubes or bottles marked A and B.
When the cloth, free from dressing and slightly dampened, is all ready, equal amounts are taken from each of the two tubes, and mixed together in a watch-glass[227] or small glass or porcelain dish with, if necessary, a drop of water to soften them if they have dried up at all. This mixed paste is then brushed on to, and into, the cloth, and, as soon as dry, is steamed as before described. The black color will develop almost immediately, and, after a few minutes’ steaming, will be found fast to hard washing as well as to light.
[228]
Travelers in Japan inform us that, with their customary ingenuity, the natives there have developed the use of stencils to a point which quite matches the best achievements of our modern calico printers, even though backed by good dyeing chemists. When a young lady there wishes a new dress, she will draw, perhaps with the help of her best young man, and certainly with the advice and criticism of her family, her favorite design on a piece of brown paper, cut it out in stencil form, and send it to the local dyer, with the proper amount of calico or silk or what not, to be properly applied.
Now, in most cases the dyer is instructed to put the pattern on the cloth in colors, blue, black, red, yellow, or mixed shades, and this he does, much as my readers were taught to do in the last chapter, by painting on a stencil paste, to be fixed later by steaming.
The Japanese dyer, by the way, has a great advantage over the American craftsman in his steaming apparatus. No matter how small his place, or how poor his equipment, he always is provided with a neat and satisfactory steam chest, consisting of a copper pot[229] set in a brick or stone fireplace, to hold the boiling water, and above it, a close-fitting box with sides made of lacquered paper, double jacketed to avoid condensation in cold weather, which can be kept full of dry steam for hours at a time, and in which the stencilled goods can be steamed thoroughly and well without fear of spoiling them.
Sometimes, however, the color is to be applied in another way; the cloth itself is to be colored blue or red or black, and the pattern is to be light, either pure white or some light color on a dark background.
The Japanese dyer, from time immemorial, has known how to do this properly, by means of a “Resist.” He prepares a resist paste which he carefully applies to the cloth through the stencil. This is allowed to dry, the cloth is then dyed, and, after the color is properly fixed, it is all thoroughly scrubbed, and the paste, washing off, leaves the cloth, underneath, in its original color.
Resist Stencil Paste.—This process of resist, ancient as it is, is used in Japan to this day, and many, indeed most, of the stencilled towels and piece goods that come from there are done in this way. It has the advantages, especially for the craftsman, over the Direct Color process, in that the color, being applied in a dye-bath, can be fixed readily and uniformly, without the bother and uncertainty of a steaming process. Through a friend, a well-known dyeing chemist, who has travelled in Japan, I learned the composition of the Japanese Resist Paste. They mix rice flour, wheat bran, and a little quicklime (the[230] calcium oxide of the chemist) with water and boil it to make a paste. This they strain, and then they stir in some powdered carbonate of lime (powdered chalk), which thickens and gives some body to the mixture. The paste thus formed is applied, as a rule, not with a brush but with a flat wooden instrument or spatula, with which the paste is laid on as with a trowel, and further, to get the dead white effects so commonly noticed, the paste is put on the back of the cloth as well as on the front.
My friend also explained to me how the Japanese were able to get irregular shaded effects with their stencil work, and at the same time to furnish such beautiful and intricate hand-made work, at such absurdly low prices. These goods are made of very thin porous materials, and the dyer applies with his trowel the thick resist paste, through the stencil, to one piece after another, laying each one, as fast as it is stencilled, carefully on top of the previous one, until a pile has been formed of ten or more separate pieces. This pile is pressed very tightly together, and then the dyestuff, as, for instance, Indigo in solution and thoroughly reduced, is poured on to this mass of goods, soaking through from one to the other, but always kept out of the white parts by the double coating of thick paste.
After a few minutes these pieces are carefully taken off, one by one, exposed to the air until oxidized, and then thoroughly washed until the paste and loose color have all disappeared. For an example of Japanese resist stencil work, dyed in an iron spring, see Plate III.

Resist Stencilling with Sulphur Dyes.—Without lavishly[231] copying the Japanese practice it is possible to get very interesting results by using suitable dyestuffs with a simpler paste.
The most useful dyes for this purpose are the Sulphur dyes, which, as the student will remember, can be applied in the cold, with very short exposure to the dye-liquor, and are fixed firmly by exposure to the air, giving results fast to light and extremely fast to washing. A paste made from wheat flour, thickened a little with an inert powder, like powdered chalk or zinc oxide, will work fairly well, acting as a purely mechanical protection to the fibre. But much better results can be obtained by adding to the paste as much as it will absorb of the easily soluble chemical, zinc sulphate, which acts chemically in resisting the action of these particular dyestuffs.
The Sulphur colors, as before explained, are kept in solution in the dye-bath, by the presence of sodium sulphide, and when this is absent or is destroyed by any cause, the dyestuff is precipitated as an insoluble, inert powder. Now, when zinc sulphate comes in contact with sodium sulphide it at once decomposes the latter, forming a white precipitate, zinc sulphide, which has no action at all on either dyestuff or cloth. Accordingly a paste containing zinc sulphate has far greater efficiency as a resist than any mixture that acts purely mechanically.
Resist stencil pastes can be obtained, in tubes, at moderate prices, but can also be readily prepared by making not too stiff a paste, with wheat flour thoroughly boiled with a saturated solution of zinc sulphate[232] instead of with water, and then stirring into this paste some powdered chalk or zinc oxide, until of the proper consistency for stencilling.
To use this paste, the cloth, as usual, should be washed free from dressing, and after being smoothed with a hot iron, should be slightly dampened. The paste is then brushed through the stencil on to, and into, the cloth, which is then allowed to dry. The dye-bath should then be prepared of Sulphur dyes carefully dissolved, in a separate cup or saucepan, in a hot solution of sodium sulphide and sodium carbonate (soda), and added to cold water in the dye-bath.
A few drops of “Turkey red oil” added to the dye-bath helps to prevent a thick scum from forming on top of the liquor, while the addition of a tablespoonful of salt dissolved in a little hot water helps the rapidity and depth of the dyeing.
Plenty of color should be used excepting for very light shades, for the dyeing should be done just as quickly as possible. For silk some syrup should be added.
The stencilled cloth is then quickly moistened in cold water, placed in the dye-bath, kept there two or three minutes, below the level of the liquid; it is then taken out, the liquor drained off, and after a minute or two, wrung off; the cloth is then shaken out, and exposed to the air, for some ten minutes, to set the color. After this it is well washed in a boiling soap bath, and, as the paste washes out, the stencilled pattern will show light against the dark background.
The whiteness of the pattern depends, of course,[233] upon the skill with which the paste has been applied, and the care taken to prevent it from washing off before or during the dyeing process. It is difficult, though not absolutely impossible, to get as sharp and clear-cut results as those of the Japanese, for instance. But, on the other hand, with a dark background it is often, indeed generally, more pleasing to have the white patterns softened and not standing out too vividly.
In our laboratory we have had considerable success with this process. And some of our friends and students have used it with very good results upon articles of clothing, which, made of linen, calico, etc., must be fast to severe washing as well as to light.
Of course, it is perfectly easy to alter the color of the background, as in other classes of resist work, such as Tied and Dyed work, for instance, or Batik, by either starting off with colored cloth which is protected all through by the resist paste, or else by covering the stencilled and dyed goods, afterwards, with some shade which will soften and harmonize both pattern and background. For this covering shade, which need not be very fast to washing, but must be distributed uniformly over the whole cloth, the student will find the Salt colors very useful.
Discharge Stencilling.—Though it is not certain whether this process is known to, and used by, the Japanese, it is not a difficult matter, with modern dyes and modern chemicals, to get interesting results with it. There are two distinct and separate ways open to the dyer for discharging, i.e., destroying his dyestuffs,[234] whether they are dyed on cloth, or whether, as is not infrequently the case with amateurs, they are present as a stain on his hands and fingers. In each case, however, care must be taken, as may easily be imagined, to use such chemicals as will spare the materials, whether cotton and linen, or nails and skin, while attacking the coloring matter.
(a)Discharge by Oxidation. Chlorine Compounds, Bleaching Powder, etc.—In the first place, chemists have long known that certain chemicals, more particularly the powerful gaseous element known as chlorine and certain of its compounds, have the power of permanently destroying coloring matters by oxidizing or burning them.
At first this was done by using chlorine itself, or a water solution of chlorine. Later, however, it was found that on passing chlorine into some caustic alkali, like quicklime, or caustic soda, or caustic potash, these would absorb immense quantities of chlorine which would be again given out, as desired, on the addition of acid, or even, though very slowly and gradually, by the action of the carbonic acid gas in the air.
The lime compound, which contains more chlorine than the others, and has the great advantage of being dry, has long been known as chloride of lime or as bleaching powder, and has been, and is, commonly used from one end of the world to the other as a quick, ready, cheap source of chlorine either for bleaching or for disinfection. The potash and soda compounds, known respectively as Labarraque’s solution and Javelle[235] water, are less active and powerful than bleaching powder, but have the same general properties.
Over a hundred years ago, very soon after the discovery of the bleaching properties of these compounds, chemists began to use them, not only for decolorizing and whitening raw cotton and linen cloth, but also for discharging the color in patterns from dyed goods. The process was not a difficult one, and is used to this day to some extent in the calico printing mills. The cloth is first dyed to shade, fixed, and dried. The pattern is then printed on with a paste containing some solid organic acid, like citric acid or tartaric acid, dissolved in it. After drying, the printed cloth is passed through a bath of bleaching powder in water, possibly with a little weak alkali added, to be sure that no free chlorine is present; and wherever the bleaching powder meets the acid the cloth is decolorized, but the rest of the cloth comes out of the bath without being much, if at all, altered in color. Of course, on coming out of this bath the cloth must be thoroughly washed to get rid of any traces of chloride of lime, which otherwise, on exposure to the air, would play havoc with the rest of the colors.
This process worked very well with the old vegetable dyes, and, every now and then, some craftsman, of an experimental turn of mind, revives it for stencil work. The dyed cloth is stencilled with a paste made of wheat flour boiled with a saturated solution of citric acid, it is dried, and then passed through a bath of bleaching powder in water, say two or three tablespoonfuls to the gallon. It is generally best to stir[236] in a few drops of a soda solution to the bath, till all smell of chlorine has gone, or else the background may be affected. The stencilled cloth is dipped in this bath, and kept there for a few minutes, until the bleaching process is well under way, and then taken out, and washed in hot soap and water, and rinsed well.
Advantages and Disadvantages of Bleaching Powder Discharge.—The chief advantage of this process is that it is very cheap and the materials can be bought at almost any grocery. The disadvantages are, however, important. As long as it is confined to easily discharged, comparatively fugitive, colors, it will destroy the color all right in the stencilled parts, although the bleaching powder bath is apt to attack the color in the body of the cloth, and the outlines of the pattern are apt to be soft and irregular because of the escaping chlorine, where the citric acid is acting.
When, however, very fast dyes are being used, as for instance, the Vat colors or, indeed, a great many of the best dyes in all the classes, the action of chlorine is very slow, and slight, and, in order to really destroy the color both the acid and the bleaching powder will often have to be so strong that the chlorine set free will destroy the fibre as well. For the term “fastness to light” implies, as a rule, fastness also to oxidation in general, and dyes like the best modern ones which will let the cloth rot away from under them, when long exposed to the weather without changing color, are very apt also to keep their color, even when the cloth isburnt away from under them by the action of chlorine.
[237]
Accordingly, this process is distinctly one that needs careful experimentation before it is tried on any important piece of work. There are plenty of dyestuffs among the Salt colors, and also among the Sulphur colors, which discharge well with chlorine. And the calico printer, working, as he generally does to this day, with comparatively fugitive dyes, and weighing accurately both acid and bleaching powder, can generally get good results with it. But there is always the disadvantage, that the least excess of chlorine will attack and tender the cloth, and the better the dyestuff, as a rule, the stronger the oxidizing agent must be to discharge it.
(b)Discharge by Reduction, Hydrosulphite, etc.—The wary craftsman will find the process much less dangerous to the cloth, and not much more difficult, if instead of trying tooxidize the dyestuff, he attempts to discharge it byreducing it; or, in other words, if instead of trying to burn it out, he tries to take the oxygen away from it.
It so happens that in a vast majority of cases a dyestuff becomes decolorized by reducing it, just as well as by oxidizing it. There is, however, a difference. When the color is oxidized, it is burnt up and destroyed forever. When it is reduced, however, it is, in many cases, only decolorized and not destroyed; and on standing in the air it is apt to take up oxygen again, and to regain some, at least, of the original color. On the other hand, while any oxidation process is liable to attack the cloth as well as the color, the reducing agents now in use have no effect upon the materials,[238] even when powerful enough to act on the very fastest dyestuffs.
As before mentioned, the most satisfactory reducing agent at present known to dyers is hydrosulphite of soda, and this can be incorporated in a paste, and used for discharge stencilling. It is, however, as a rule, more satisfactory to use the more expensive, but more permanent hydrosulphite compound, described, in the last chapter, as acting only when heated.
The reducing stencil paste can be easily made by mixing with some “gum dragon” or flour paste, as much as it will hold of a saturated solution of the “Stencil Salt.”
The student should experiment with the different dyes and classes of dyes before attempting a serious piece of work; but in general, all the Salt colors and the Acid colors will discharge readily with this paste, and remain colorless. The Vat colors and the Sulphur colors can also be reduced to colorless compounds, but it is not always easy to wash them out of the cloth after the reduction, and, if they remain in it, they are apt to regain their color, on standing in the air.
The dyed cloth, carefully washed and pressed and dampened, is stencilled with the above paste and allowed to dry. When dry it is steamed, as described in the last chapter, and it will be noticed that when a certain temperature is reached the color will be discharged. As soon as possible afterwards the cloth is to be washed in a hot soap bath to remove the reduced color compound (which, as a rule, has little[239] affinity for the cloth) and to get rid of the paste. Then the cloth is dried and finished.
When trying this process with the Vat dyes it is best to soak the cloth directly after steaming, and before soaping, in a warm bath containing a little free caustic soda (remember this is apt to burn the fingers) because the reduced colors of this class are not, as a rule, soluble in water, and are apt to oxidize again in a soap bath.
Results.—In following up these various experiments in our laboratory we have not used this process in much as the Resist stencilling, but there is no reason why it should not give just as good results. Indeed, the craftsman will probably find, after a little practice, that it is easier to get clear white patterns with this than with the other. It has the disadvantage of requiring the rather bothersome steaming process, which reduces its value for many purposes. Still it will often be found that simply ironing the dried stencilled cloth with a hot flatiron, with a damp cloth between, will cause the reduction to take place quite satisfactorily.
The chief advantage of this process over the other is that, as the dyeing is done before and not after the stencilling, it is possible to get the exact shade of background required. While, in the resist stencilling every minute, almost indeed every second that the stencilled goods are left in the dye-bath, is liable to obscure the pattern. And it is hard to get first-class results, as regards fastness to rubbing and washing, and it[240] is impossible to match shades, when working so hurriedly.
Then, too, this discharge process permits the use of almost every color on the list, while the resist process practically confines the craftsman to the use of the Sulphur dyes only.
Those who are interested in this line of work are advised to try these two processes upon silk, where very beautiful and interesting effects can be produced with but little difficulty. The resist process, using Sulphur colors, gives quiet soft tones on silk, fast to the hardest kind of washing. But brighter shades, equally fast to light, and fairly fast to washing, can be made with the discharge process by using Salt colors.
For ordinary work the Acid dyes, of course, would be used, and these, too, as a rule, discharge readily.
[241]
The last and perhaps the most interesting and most important process to which we shall call our reader’s attention is one which, after being practised in the East for many centuries, has been brought quite recently to the attention of European and American craftsmen.
The term “Batik” is a Javanese word, signifying painting in wax, and the process, somewhat modified, is known to professional dyers and calico-printers by the name of “wax resist.” When in the hands of a trained draughtsman the process has a charm and character of its own, which will warrant the interest now manifested in it, wherever it has been introduced.
History.—Batik was first introduced by the Dutch discoverers of Java, who, in 1648, sent home descriptions, with drawings, of the wonderfully beautiful textiles worn by the people, especially by the chiefs of that country. The art was known and practised in the East long before that time, for in Madras goods were made, by a combination of block printing and Batik, at least as early as the fifteenth century. And in the interior of Java there are some famous old ruins in which are found stone statues of Buddha, supposed[242] to be at least 1,200 or 1,300 years old, clothed in garments the same as those used at the present day; and showing, from their decorations, that they were ornamented by Batik in the same general style of patterns that are still popular there.
During the last few years very careful studies have been made, especially by the Dutch Government, upon this Javanese process, and they have endeavored to introduce it into Europe. It was amusing to notice that in one of the reports issued by the Dutch Government on this subject it was stated that none of the modern dyestuffs could be utilized for this purpose, and that the only colors that could be recommended as fast to light were the old vegetable dyestuffs, applied in the complicated and troublesome methods of past ages. This curiously unscientific attitude has seriously interfered with the success of the process in Western lands, and is only now being abandoned.
Javanese Practice.—Detailed information about the history, technique, and designs of the Javanese process has been set down in a monumental work: “Die Batikkunst in Niederlandisch Indien,” published in Harlem under the auspices of the Dutch Government in 1899. Perhaps of more interest to the non-scientific reader is a short but well-written account of “Battack Printing in Java,” read before the Manchester Literary and Philosophical Society in 1906 by an English chemist, John Allan, who spent several months among the natives, studying the process at first-hand.
According to these authorities the Javanese and, indeed, most of the natives of Malaysia, wear garments[243] simple enough in style and cut, but elaborately decorated with great variety of both color and design. The principal garment, common to both men and women, is the sarong, in shape not unlike a large and elongated bath towel, which, according to the desire and sex of the owner, may be made to serve as trousers or skirt, overcoat or blanket, and is the universal bathing costume. It is made of calico, rarely homespun, almost always imported from Lancashire or Holland, and as the natives, both men and women, are exceedingly fond of bathing, the colors must be fast enough to stand constant exposure to water as well as to the fierce tropical sun.
They also wear head-dresses made from squares of calico, dyed with square centres of plain color and elaborately decorated at the sides; and slendangs, a kind of girdle or shawl, usually made of silk and less elaborately decorated. The costume is completed, for full-dress occasions, by a thin shirt or chemise and a light jacket.
For producing the designs on the sarongs, the process of wax resist is almost always employed by the natives. Unfortunately of late years the Javanese market has been flooded with an immense quantity of cheap and, generally, neatly printed goods made in Manchester and in Holland in rough imitation of the native styles. So it is not an easy matter, nowadays, even in Java, to get genuine specimens of Batik work. These can always be recognized, however, on careful examination by the peculiar and characteristic odor and “feel” of the wax left behind in the cloth, and,[244] better, by the fine irregular “crackle” formed in the dye-pot.
Variations in the Process.—Although there are different methods, the Batik process, as usually meant, is a means of dyeing in which, before immersing the goods in the dye-pot, the patterns are carefully drawn in molten beeswax, applied from a little copper cup with a fine spout called a tjanting. Frequently, however, to save time, the Javanese apply the wax by means of a metal die or block, made by inserting thin strips of sheet brass in a wooden frame, so that the edges of the brass form the desired pattern. These blocks, provided with a handle covered with cloth, are first dipped into the molten wax, and then the excess is removed by pressing against a pad, which is kept warm by being near the fire of the melting pot. The pattern is thus stamped onto the cloth instead of being poured onto it, through a small spout, out of a cup.
This Batik process is sometimes used by native craftsmen in other parts of the Far East. Plate I, for instance, shows a specimen of East Indian work, part of a long piece of stout cotton bought, years ago, at Liberty’s in London, with an elaborate design made with molten wax, applied by brush or tjanting. Even in the plate the characteristic ‘crackle’ shows plainly.
Wax.—In Java, the wax used for pouring is a mixture of paraffin and beeswax, or an impure wax imported from Japan for this purpose. For stamping the patterns it is necessary to use a stiffer wax made from rosin and paraffin, sometimes mixed with varnish gums.
[245]
Dyes.—The principal colors used are indigo and a beautiful golden-brown dye made from the bark of the mango tree. The combination of these gives a black, so that the fine old sarongs usually contain white, blue, brown, and black. Indigo is dyed first, and, before dyeing, all the cloth, excepting that which is to come out blue or black, is carefully covered with the wax. After the indigo bath (the Javanese use a fermentation vat) the color is set by oxidation. The old wax is then all washed off with boiling soap and water, and after drying, the wax is again applied to all parts, whether white or blue, which are not to receive the brown dye. The latter is made from a strong, syrupy extract of bark, and is used without mordanting, the color being set by exposure to air. As the dyes must be used cold, to avoid melting and obliterating the pattern, the goods are usually dipped in each dye-bath and exposed, several times, before reaching the desired shade. After the final dyeing, the wax is removed by a hot bath of wood ashes or soap, and the garment is pressed out ready to wear.
When a red color is desired, the natives use a variation of the old Turkey red process, dyeing with madder or munjeet upon cloth mordanted with alum and oil. The wax in this case acts as a resist against the alum mordant, which is applied cold, and thus prevents the dyestuff, which is applied at the boil, from coloring the cloth in the protected portions.
Cloth.—The cloth used for this Batik process is strong common calico, but, before beginning to wax it, they give it a careful treatment, to improve[246] both its texture and its ground color. For a period of several days they alternately soak it in castor oil, wring it out, boil out the oil with soda lye, and expose it to the blazing sun; until finally it becomes soft and smooth, and has a pleasant tan color which goes excellently with the brown, blue, and black dyes.
The peculiarity of all these Batik goods, whether from the East or made at home or in Europe, is the characteristic “crackle” effect, due to the breaking of the wax upon the cloth in the process of dyeing, thereby admitting the color to the protected cloth in fine lines and streaks. This distinguishes the wax resist work from the previously described paste resist, which if desired will leave a smooth, clean, white background, or if applied more lightly will give backgrounds shaded more uniformly and without so many irregular lines of color.
This crackle effect, so generally admired in the West, is often by the Javanese considered a defect, and a sign of poor workmanship. It can be largely, if not wholly, avoided by adding a large proportion of rosin to the wax, by batiking the cloth on both sides, and by dyeing the goods with as little crumpling as possible.
The application of the artificial dyestuffs to this ancient process has simplified it greatly, and has brought it within the scope of craftsmen in general.
[247]
Apparatus—Brushes.—You will soon find that for a good deal of the work, such as covering large surfaces with wax, or filling in large and bold designs, a small-sized paint brush is all that is necessary. The wax is melted in a cup or casserole, and painted on the cloth wherever the design calls for it. It will be found, however, no easy matter to get sharp and clear outlines in this way, and intricate or delicate patterns cannot be worked out by the brush only. When the wax is hot, it is hard to prevent it from spreading and running too far over the cloth, and, on the other hand, it cools so rapidly on the brush that, unless applied at once, it is hard to spread it at all, and the wax is liable not to stick to the cloth.
Much is saved both in time and in accuracy and clearness of outline, by using the brush in combination with the tjanting, drawing the outlines with the latter, and filling in with the brush.
When large surfaces have been covered with the wax, and the characteristic “crackle” effect is desired, it is often well to cool the goods, by placing them in the ice box or out of doors for a few minutes, and then to crumple them in the hands, before dyeing them. The composition of the wax, also, has much to do with this part of the work, as will be explained later.
The brush can also be employed for painting molten wax on to the goods through a stencil, in resist stencil work. This, however, is not satisfactory, even with metallic stencils, and fails completely with paper stencils, because the wax, on cooling, fastens[248] stencil and cloth together so that they cannot be separated without injury.
It is much better practice, where a stencil design is to be worked with wax resist, to make an outline of the design on the goods with a sharp pencil, and then, removing the stencil, to fill in the pattern with tjanting and brush. This same practice of drawing the outline on the goods with pencil, or tracing paper, or by transferring from a charcoal drawing, by rubbing, is always to be recommended: except for those craftsmen who are such thoroughly trained draughtsmen that they can draw their designs free-hand, with the tjanting, without danger of slip or mistake. A pencil or crayon line, if not quite true, can be erased without spoiling the whole design, but it is quite a troublesome matter to correct a mistake made in molten wax.
Tjantings.—The real interest in this Batik process lies in the use of some form of pouring instrument by which the molten wax can be applied to the material in a fine stream, with much the same freedom that a drawing can be made with soft pencil or crayon. This practice has been developed in Java to its fullest extent, and a fine sarong, containing two or three yards of calico, will be completely covered, from one end to the other, with wonderfully intricate and elaborate designs in two or three colors, all produced, perfectly free-hand, by curious little tjantings, in the light fingers of the little Javanese women.
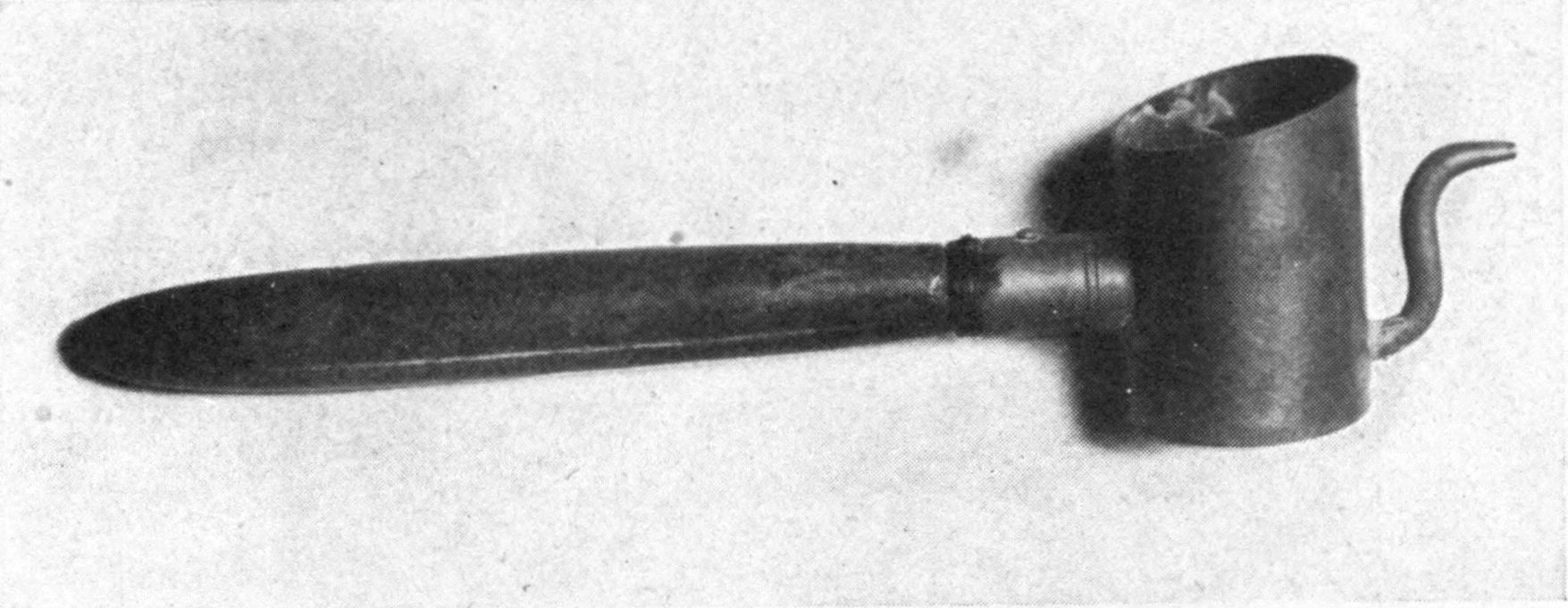
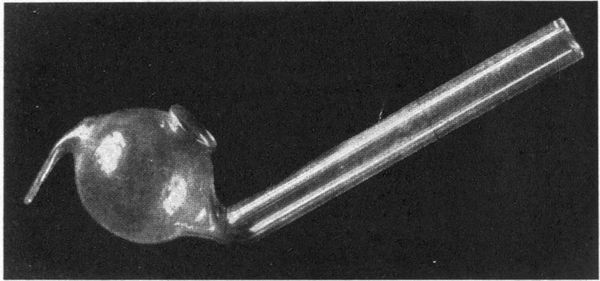
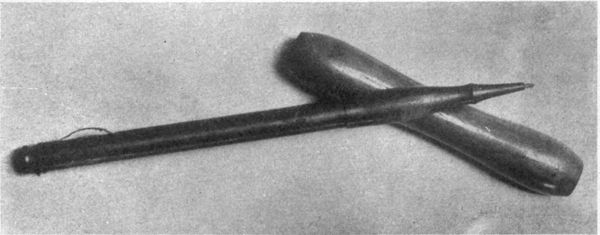
Teapots.—When we began experimenting with Batik, in our laboratory, we had no model of the Javanese tools to work with, and, from the drawings, we[249] could not see how they could be used without constant dripping. So we designed a little brass apparatus, which we and our friends nicknamed a “teapot,” which, with some modification, is shown in Fig. 20, in which the molten wax remains in the cup until it is poured out. This, with practice, works fairly well, and some very interesting work has been done with it.
It is hard, however, to draw with it on a horizontal surface, such as we are accustomed to work on. And to follow Javanese practice, and have the cloth hanging over a frame, and to press it out with the left hand while the wax is applied with the instrument in the right, is oftentimes a nuisance.
The Walther Glass Tjanting.—It is evident that we were not alone in our fear of the Javanese models with spouts at the bottom, because, in Germany, Dr. C. Walther of Crefeld has designed and introduced a glass tjanting, shown in Fig. 21, which also delivers only on tipping the instrument forward. This we have experimented with but without much success. For we have found it difficult and awkward to draw with it on a horizontal surface, and also, being made of glass, while it is cheaper than the metal models, it is at the same time more fragile.
Wax Pencil.—An entirely new idea has recently been applied to the art of Batik by the introduction of a (patented) “wax pencil” (see Fig. 22), made on the principle of the early stylographic pens.
This tool is made of heavy brass, with a removable wooden holder, and the wax, in cylinders, is shoved into it from the top after removing a cap. To melt[250] the wax the wooden holder is slipped off, and the pencil is heated over a flame or on a hot electric plate, while the liquid wax is prevented from flowing out by a “needle valve” held in place by a small spiral spring. To use the tool, the holder is slipped over the pencil, taking care not to burn the fingers in so doing, and the pattern is traced in just the same way that it would be in ink, pressure on the projecting needle, by raising the valve, permitting a greater or lesser flow of wax.
These instruments are certainly more convenient to draw with than any of the forms previously mentioned, and, on hard smooth surfaces, such as leather, wood, bone, metal, etc., are satisfactory enough. But it is no easy matter to make them so that they will work well. For the valve which regulates the flow of wax works with a spiral spring. Now, if this spring is, or becomes, lax, the wax drips incessantly. If on the other hand it is too stiff, it is quite troublesome to press down the pin, at the tip, just hard enough to deliver a fine stream, without opening it too wide.
In our experience these instruments, which are quite expensive, do not work well with cloth and, especially, with rough weaves of cloth, like crash, scrim, pongee, coarse calico, and the like. For the pin is liable to catch and jump on the threads, and then it delivers the wax very unevenly.
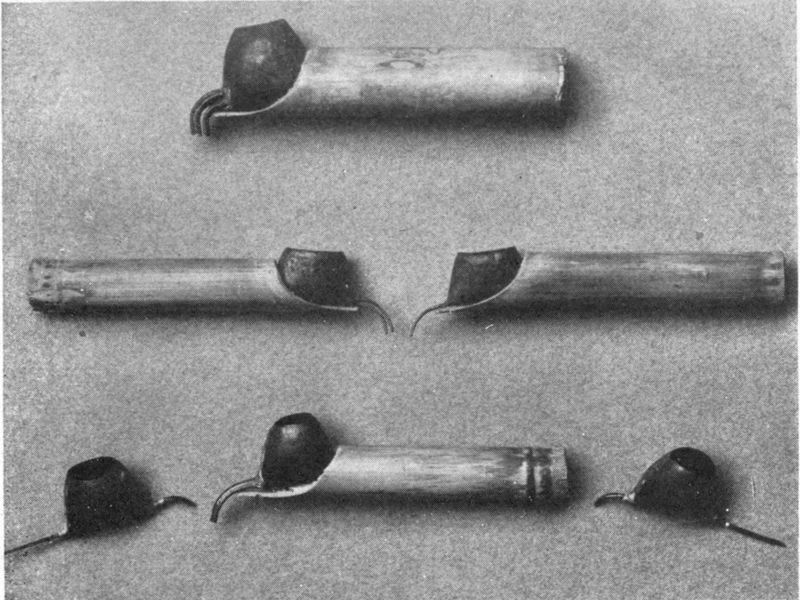
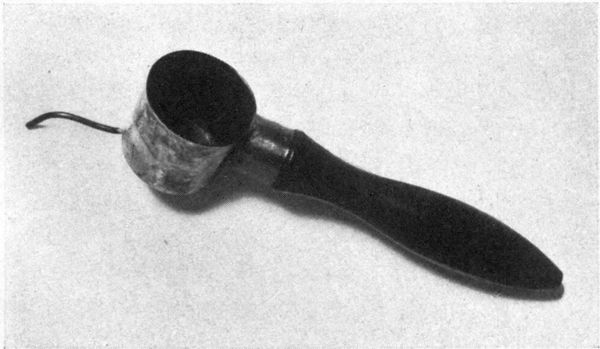
The Javanese Tjanting.—After much experimenting one of our friends finally brought us, from Holland, a real tjanting copied directly from the Javanese, and five minutes’ practice with it satisfied us that it was superior to any of the “improved” models that we had[251] been working with. Since then we have seen, and studied, several different styles of Javanese tjantings, and have learned how they must be used to get the best results.
The secret of these instruments is two-fold. First, the size of the delivery tube, and second, the temperature of the wax.
The genuine Javanese tjanting (see Fig. 23) is a little bit of a tool, holding only about 15 or 20 cubic centimeters of wax, made of very thin hammered copper, and fastened into a little bamboo handle, some four or five inches long. At the lowest part of the cup, which is drawn out at that point into a spout, is fastened the delivery tube, which is ofexceedingly small calibre, what chemists would call, in fact, a “capillary” tube. It will be noticed that the wooden handle extends forward, under the bowl, making it impossible to heat the bowl itself, or melt the wax in it, by a direct flame.
The wax is melted in a separate pot or large cup, and kept at a high temperature throughout; and the operator scoops out the wax from this pot with the bowl of the tjanting, wipes off the drip with a rag, and then proceeds to draw on the cloth. In Java, or wherever the cloth is kept upright, by hanging from a frame, the drip from the outside of the cup and the end of the handle is not so important, for it will fall in front of the cloth. When, however, the cloth is laid flat, for drawing, it is of the utmost importance to avoid all unnecessary dripping, and so it is probably advisable to ladle the wax from the pot into the tjanting,[252] with a small casserole or ladle, rather than to dip it out directly.
Now, if the size of the delivery hole is right, and the wax is neither too hot nor too cold, it will form a little globule on the end of the tube, and stay there; and when this drop is wiped off and the tube at once applied to the cloth or other material, the wax will flow out in a fine thin stream, as long as it is drawn along in contact with the cloth, and when lifted up it will stop flowing until again applied to it. If the wax is too hot, so that it runs too freely, it is easy to cool it to the proper temperature by blowing on it. If it is too cool, so that it begins to chill in the tube, and to flow slowly and unevenly, it must be warmed by being again dipped into the hot wax for a new supply. Great pains must be taken to have the wax free from dust or grit, or else the delivery tube will be constantly stopping up. A fine but stiff bristle or a very thin whisk of broom corn should be always on hand for cleaning the tube. And after using the tjanting pains must be taken to clean out all the wax thoroughly before laying it aside, so that the tube will be clear for the melted wax, when it is next filled. The whole tjanting, tube and cup, should be gently warmed before filling, for fear of the wax chilling in the capillary tube before it can be applied. But the arrangement of the handle is such as to call for the use of an outside melting pot for the wax, while the small size of the cup is evidently so that the melted wax can be all poured out before it has time to chill.
Modification of the Javanese Tjanting.—We have with[253] much trouble had some tjantings made here (see Fig. 24), following closely the Javanese principles, which have proved extremely satisfactory. The delivery tube is equally fine, and the general action is the same. But the cup has been made somewhat larger, and very considerably heavier, so that it will hold more wax, and will hold the heat better. While, for economy’s sake, instead of hammered copper, the cup is made of spun brass, and the wooden handle is attached to its side, and not to the bottom of it. This enables the worker to heat the cup directly over the alcohol lamp, without danger of scorching the handle. Of course, when this is done carelessly, it is liable to char some of the wax in and near the tube, and so to cause stoppages. And also, it is hard to draw a series of fine lines of exactly the same thickness, unless the wax in the tjanting is always of practically the same temperature.
But there is no difficulty in filling these modified tjantings, just as the Javanese do, by scooping up the melted wax from a pot, or by pouring the melted wax into them from a casserole or ladle. While, in case the wax gets chilled in the tjanting, it is very convenient to be able to warm the cup quickly over a low clean flame, or by setting it upon the corner of a hot plate.
Composition of the Wax.—As a general rule we have found that ordinary unrefined beeswax, carefully melted and strained, or poured off, free from dust and sediment, is fairly satisfactory. It is, however, pretty expensive, and so can be replaced, without disadvantage,[254] by the cheaper mineral wax, known in a crude state asOzocerite, and in its refined form, which alone should be employed, asCeresine. To make the wax more brittle, and thus to improve the “crackle,” it is well to add more or less paraffin. And it is well, too, to add considerable rosin, to make the wax adhere better to the goods, and not be so liable to rub or peel off. On the Continent, it is customary to use Japanese vegetable wax instead of beeswax, but we have not found this to be advantageous.
Where economy is desirable, or where it is hard to replace supplies, it is well to save the once used wax and use it over again, by extracting the wax from the goods, after dyeing, with boiling water, and then, when this cools, collecting the wax as a cake floating on the top.
Dyeing Batiked Goods.—In Holland and, to some extent, elsewhere on the Continent, where this process has been introduced, great stress has been laid upon the importance of using the old vegetable colors of the Javanese, along with their tjantings. It is hardly necessary to tell my readers that this practice is both unscientific and, in a true sense, uncraftsmanlike. The object of any intelligent craftsworker should be to produce beautiful and interesting and characteristic results in the most durable and effective manner possible, with the minimum expenditure of energy upon the mechanical, as opposed to the artistic, details. Why, after carefully batiking a good design on a piece of silk or calico, must the craftsman spend hour after hour of valuable time in some tedious, complicated,[255] and expensive dyeing process, simply because “That is the way they do things in Java,” especially when, by using modern dyestuffs, he can get results quite as beautiful and far more permanent, in a few minutes’ time, and with far less danger of spoiling his work. Even the clever and skilful little Javanese could learn something from modern dyeing chemists.
The class of dyestuffs to use depends, of course, on the kind of materials that are being worked on. One of the great charms of this process is that it can be applied to all sorts and kinds of textiles and, indeed, of a host of things never included under that name.
Batik can be applied to cotton, linen, wool, silk, and other woven goods. It can also, if desired, be used upon basketry. And charming effects can be produced, by its aid, upon leather, pasteboard, parchment, vellum, and other bookbinding materials, as well as upon wood, bone, or indeed anything that possesses a smooth surface, and will hold a dyestuff.
On copper, brass, and other metals it can also be used, not, indeed, for dyeing, but for etching, with acids and other chemicals, with great success.
(a)Calico and Linen.—There is no doubt that for vegetable fabrics in general Batik is very well fitted, especially since the introduction of modern dyes, which are applied in a cold bath and are set by oxidation. The Sulphur dyes work extremely well, in cold or lukewarm baths, especially if used in a strong dye-bath. But they, it will be remembered, are not very bright colors, and are very short on the red side. For[256] soft, quiet colorings, however, extremely fast to washing, and quite fast to light, which can be applied easily and readily, they will be found very useful.
But the fastest colors known, both for light and for washing, are the modern Vat colors, many of which, once reduced, will dye in a lukewarm or even a cold dye-bath. While indigo, the type of these colors, and still most useful, gives a soft rather greyish shade of blue, more effective by itself than when mixed, there can be found among the Helindones, Thio indigoes, and the rest, a full palette of dyes which, properly mixed, will furnish any shade that may be desired.
The dyeing directions for batiked goods are the same as for ordinary calico. The materials, well wetted, are immersed, drained, wrung, and oxidized as usual. The wax is usually removed in one or more boiling soap baths, which help as well to set the color and to remove unattached dyestuff.
(b)Silk.—Silk, as in other processes, can be dyed in several different ways, according to the fastness to light and washing desired.
The easiest way, especially when trying to match shades, is to dye, with the Acid dyes, in a soap bath acidified with a little sulphuric, or, preferably, with acetic acid. These shades, however, while brilliant and fast to light, are not at all fast to washing, and so the wax must be removed later, with benzine or gasoline, and not with a hot soap bath.
The sulphur dyes, with a little glucose in the bath, and plenty of dyestuff, will give extremely fast colors[257] on silk, but in most cases these shades will be too dull for proper effect. They can be greatly improved in color, though with some sacrifice of fastness, by topping them, without removing the wax, in a cold bath of Basic dyes, dissolved with a little acetic acid.
For extremely fast colors the Vat dyes can be used. Easier to apply, especially for rather light shades, are some of the Salt colors which, though they do not take as well on silk, in the cold, as they do on cotton will, nevertheless, color it well, with prolonged immersion, in a strong bath, in the presence of formic acid, and once on, will stand a very considerable amount of washing.
(c)Wool.—In case it is necessary to apply this process to wool, the latter will probably be dyed in the cold with Acid dyes, in the presence of some sodium sulphate (Glauber’s salt) and dilute sulphuric acid.
To make this color faster to washing, steaming, and the like, it is best, after dyeing, drying, and removing the wax with benzine, to boil the dyed goods for half an hour or more in a bath containing a little Glauber’s salt and dilute sulphuric acid, but no dyestuff.
(d)Leather.—As a rule, the Batiked leather should be dyed with Acid colors, acidified with acetic or formic acid, though they can be shaded afterwards, if desired, by staining with Basic colors.
After dyeing, the wax can be removed by benzine or, softened carefully by the cautious approach of a hot iron, can be incorporated with the polishing wax, used for rubbing down and finishing the surface.
[258]
(e)Wood.—Batiked wood can be stained by soaking it in, or by brushing it with, a solution of an acid color, acidified with a little acetic acid. These dyes are more soluble than most of the other classes, and hence soak into and penetrate the fibres better. They may bleed, however, if exposed to warm water.
The Basic colors or even the Salt colors can be used, but, while they are apt to adhere more firmly, they do not soak in as well.
The wax is either used for polishing, or is removed by benzine.
(f)Baskets.—Basketry can be decorated by Batik, although it is but rarely done. The baskets would be dyed with Basic colors and acetic acid, excepting where yellows and reds were needed, fast to light, in which case the Acid colors would be used.
(g)Bone.—Very pretty effects can be produced with Batik upon polished surface of bone or ivory. These are dyed carefully with Acid colors in a bath containing acetic acid.
This process is a combination of dyeing and etching, for the acid attacks the exposed surfaces, removing the polish and opening the way for the action of the dyestuff later.
Batik Used for Etching.—The talents of Batik are numerous, for the usefulness of the Batik tjanting and brush are not confined to the dyer, but can be readily availed of by any metal or wood worker who happens to be a skilled draughtsman as well. Wax is a good resist, not only against dyes and the weak chemicals used in connection with them, but also against many[259] of the most powerful reagents known to the chemist, such as sulphuric acid, for instance, or strong caustic alkali.
Accordingly, if a piece of smooth wood is carefully batiked and then, instead of being painted with dyestuff in solution, it has some strong sulphuric acid, or a concentrated solution of caustic potash poured and spread upon it, in a few minutes, after the reagent is washed off and the wax removed with gasoline or otherwise, the exposed surfaces of the wood will be found softened and corroded, so that on scrubbing with a stiff brush, they can be readily rubbed away, and the waxed portions will stand out in relief.
Metal work, like copper or brass plates and dishes, can be etched readily in the same way, the pattern of the relief being drawn in wax, and the metal exposed for a greater or less time to the action of dilute nitric acid.
Without going further into details it is hoped that enough has been stated here to impress on the student the possibilities of this beautiful process in a large number of different directions.
[260]
In Chapter II of this book it has been explained how the dyeing industry of the whole world was changed by the discovery and commercial preparation of the first aniline dyestuff, mauveine, in 1856, by the English chemist Perkin. Under his leadership the supremacy in this new industry was kept in England; but when he retired from the field the manufacture of dyestuffs was soon concentrated in Germany. For over forty years before the beginning of the Great War, the Germans had almost complete and absolute control over the whole color business, including many allied industries like the manufacture of organic chemicals, drugs, perfumes, flavoring matters and the like, derived originally from coal tar. In Germany were four or five great and splendidly equipped factories, and some ten or fifteen others of less importance, all thoroughly organized and working together most harmoniously under what would, in the United States, be called a most perfect specimen of a Trust. Opposed to them all over the world there could be found but a handful of comparatively small and unimportant firms in Switzerland, France, England and the United[261] States—producing altogether not over about ten per cent of the output of their German competitors.
Compared to other industries the output of dyestuffs needed for the whole world’s consumption is not a very large one—some sixty or seventy million dollars a year all told; and it was freely boasted, and more or less accepted by the rest of the world, that “the dyestuff industry is a one-nation industry, and that nation is Germany!”
Rise of the German Dyestuff Monopoly.—The story of how this came about was once told the writer by Sir William Perkin, when he was in New York, in 1896, at the time of the “Coal Tar Color Jubilee,” the fiftieth anniversary of his famous discovery.
He said that in the early days, when he was running his plant near Manchester, the most dangerous competitors he had to face were the French. He described them as excellent chemists and keen, but fair-fighting business men; and the Germans, in those days, were far inferior to them in every way—in ability, in originality, and, above all, in honesty.
He went so far as to say that, for years before he left the business, he and other English chemists had entirely abandoned attempts to patent their discoveries in Berlin. He had found, by sad experience, that whenever he sent over an application for a patent on a new dyestuff, or new chemical compound of importance, the German Patent Office would at once call in, for consultation, the leading German chemists who were interested in that line of work. He would get request after request for more and more detailed information[262] about every part of the process; and then, when they had got from him every bit of information that they could, they would grant the patent to some one of his German competitors, who, in many cases at least, had never even dreamed of the thing, until Perkin had sent his application to Berlin. In fact, he said the English and French chemists considered them as rank, bare-faced pirates, and none too successful pirates at that.
Two Germans however, in 1869, did work out the composition of alizarine, the dyestuff of madder, and published their discovery in the chemical journals. But while they discovered and patented one method for preparing this Alizarine from coal tar on a commercial scale, Perkin in England, and some dyestuff chemists in France discovered other methods equally good or perhaps better for producing the same identical color at less expense. So they still kept well ahead of the Germans even in that.
Soon after this, in 1870, the Franco-Prussian war broke out. At once the French and German factories closed, at any rate for any foreign trade, and as the cultivation of madder had by that time been abandoned, Perkin found that all the Turkey red for the whole Eastern market must be dyed with his Manchester alizarine. Orders came pouring in, and in order to keep up with the demand, it would be necessary for him to greatly increase the size of his plant, and to put back into it all his savings of the past fourteen or fifteen very profitable years.
This, he told me, he was unwilling to do. But, just[263] at that moment, he was approached by a firm of Manchester business men, who had been supplying his works with some of the raw materials from coal tar (crudes and intermediates as they call them now), with an offer to buy his works and his interest in the business. He was perfectly frank and open with them, showed them his books, his profits for the past few years, his present orders and the rest, and after a little bargaining he sold out to them for a very fair price, which he immediately invested in the best of securities and on which he lived in comfort for the rest of his long and extremely happy life.
Ruin of the English Dyestuff Industry.—As soon as they had gained possession of his factory, the Manchester people began to pass word around among their friends, that they were going to show the whole world how to run a chemical industry. Perkin, they agreed, was indeed a clever fellow in his way, and undoubtedly a good chemist, but he was nobusiness man. They were going to run those works on good, practical, common-sense business lines, and they and their few friends whom they allowed to join them, boasted loudly and deeply of their expected profits. Their motto was the well-established one “Manufacture cheap and sell dear”—and they proceeded to follow it implicitly.
They went over all the details of the business with the greatest care, and soon found what seemed to them a willful piece of extravagance. Perkin himself, and three or four other chemists, were drawing salaries, not for the actual making of the dyestuffs but forexperimental[264] purposes, and they had quite an expensive laboratory used for that purpose alone!
Of course this was at once eliminated—and great was their satisfaction when they found that they had thereby cut down the price of making their dyes two or three cents a pound.
Then it came to the “selling dear” part of it. Perkin told me that the last few years that he ran his factory, he kept the price of his dyestuffs at a reasonable figure, so that, indeed, he would get a good profit from them, but that, on the other hand, it would be no easy matter for competitors to break into his field with success. His alizarine, in particular, he had kept at a price just below what it would pay to grow madder in opposition to it, and he had not raised the price to any great extent since the war had given him a monopoly. These Manchester people, however, fully recognized that they were the only manufacturers of alizarine, anywhere, and were over-flooded with orders—so they instantly jumped up the price of their alizarine to four or five times its former figures.
Barely had they completed their “business” reorganization of the plant when the war came to an end, and the Germans marched back to their own country, with “five milliards” of French money, full of self-confidence (to use a very mild term) and looking around for new fields to conquer in peace, now that they had won all that they could at that time by war. Instantly every German with any knowledge of the textile or dyestuff industries turned his eyes at once in that direction. “What! Alizarine at five dollars a[265] pound instead of a dollar; why, any fool can make a profit on colors at that price!” And immediately, in different parts of the country, factory after factory was started, each one centered around some first-class chemist, of national if not international reputation, with instructions to gather around himself a staff of the most brilliant and best trained organic chemists he could find, to be used first of all in experimental and investigating work as well as for the mere preparation of dyestuffs.
As a result, in a very short time, these new German firms were supplying alizarine and other dyestuffs to the Manchester Turkey red manufacturers at lower prices than they could be made for in Perkin’s old factory in the immediate neighborhood; and, before the end of the year, those clever business men were complaining bitterly to Perkin that he had cheated them in the sale of his works, and were wanting him to give them their money back, which, as the old gentleman told me with a chuckle, he very positively and decidedly refused to do.
From that time until the beginning of the Great War the great English textile industry, with its enormous trade all over the world, was obliged to buy practically all its dyestuffs from Germany.
Dyestuff Industry in the United States.—The manufacture of dyestuffs in this country was a little better than in England, because of the tariff protection granted it by the Government for many years. Four or five factories of very moderate size kept up a rather precarious existence, because their chief raw materials,[266] the so-called “intermediates,” organic chemicals made from coal tar and from which the principal products, dyes, drugs, perfumes and the like are made in turn, all had to be imported from Europe, and, in most cases, from their German rivals who naturally kept a tight rein upon the quantity and quality of their output.
In 1913 even this industry was destroyed by the abolition of the duties on dyestuffs in the new tariff, thanks to the pressure for free raw materials brought by the great textile industries, probably at the instigation of the foreign color houses.
Changed Conditions Due to the War.—Since 1914 this whole situation has been radically and completely changed all over the world. Appreciating the great danger to their textile trades from the lack of dyestuffs, and also the vast military importance of a large and highly developed coal tar products industry, for the manufacture of high explosives, smokeless powder and the like, nation after nation has given government assistance not only in the line of money, but also with patent legislation and new tariff. England with its British Dye Works, Ltd., France with the St. Denis Works, now greatly enlarged and strengthened, Italy, Japan, all have made arrangements for supplying their trade with home-made dyestuffs, of excellent quality, not only during but after the temporary disturbance due to the actual fighting.
In the United States there soon were made many more or less independent and spasmodic efforts to supply at least the principal and most generally used[267] colors, notably the Basic dyes, Methylene Blue, Methyl Violet and the like, so much used in calico printing, silk and wool dyeing, leather and other lines, and the simpler Sulphur colors, like Sulphur Black, Blues, and Browns. These were selling, before the end of 1914, at comparatively huge prices, and until the peace will probably still command from five to ten times their usual values.
But out of these scores of generally quite small and isolated factories, there have sprung, by the fourth year of hostilities, a few large, well equipped and fully financed organizations which will be able, within a very short time, indeed probably before these lines appear in print, to fully provide this country with the main standard dyes, quite as good in every respect as the same dyes made by the best German color houses. And, unless very adverse tariff legislation should be introduced, they should be in a position, after the close of the war, to hold their trade against any foreign competition. It will, of course, take several years before they can supply in this country the very finest special dyestuffs, of which but small quantities are ever needed or used, and which in most cases are fully protected by patents, as well as by secret methods of manufacture. But, with the exception of the vat colors, of which artificial Indigo and the closely allied Brom-indigo are at present the only ones made in this country, the dyeing trade will be, in a short time, well supplied with excellent standard colors “made in America.”
The three important American dyestuff houses[268] already started with the addresses of their New York offices are as follows:—
American—American Aniline Products. Inc.,
80 Fifth Avenue.
Marden—Marden, Orth and Hastings,
61 Broadway.
National—National Aniline and Chemical Co.,
244 Madison Avenue.
And also, soon to engage in the manufacture of dyestuffs on a large scale:—
The Dupont de Nemours Chemical Co.,
of Wilmington, Del.
Lists of the Best Dyestuffs, in the Different Classes, Made Thus Far by the American Manufacturers
At the present moment, November, 1917, but few of the home-made colors are as fast to light as the specially selected dyes of the great German houses, listed on pages 66, 89, 103 and 127. Those in the following lists are the best made at present, in the United States, and will be steadily improved upon as time goes on.
Direct Cotton or Salt Dyes.—
| American— | Benzo Fast Yellow, A |
| Direct Sky Blue | |
| Marden— | Stilbene Yellow |
| Direct Blue | |
| Direct Brown | |
| National— | Delta Red, 2 B |
| Niagara Fast Yellow, F | |
| Niagara Blue, 2 B | |
| Erie Black, G X OO |
Sulphur Colors.—
| Marden— | Sulphur Black |
| Sulphur Brown | |
| National— | Sulphur Brown, W F |
| Sulphur Yellow, B W | |
| Sulphur Direct Navy Blue | |
| Sulphur Black, F conc. |
Vat Colors.—
| Dibrom Indigo, powder and paste | |
| Synthetic Indigo, 20% paste |
Made by the Dow Chemical Company of Midland, Michigan. New York Agents, Geisenheimer & Co., 134 Cedar Street.
Synthetic Indigo and Sodium hydrosulphite can also be obtained from Klipstein, 634 Greenwich Street, New York.
Basic Colors.—
Many of these such as Methylene Blue, Methyl Violet, Phosphine, Bismarck Brown and others, including leather Black, are being made by American, Marden and National, as well as by many firms that so far have not gone into the general color business. One of the most important of these last, who, besides the above, make a brilliant basic Green, called by them Methylene Green, is the Meth-O-Lene Co., Inc., 81-83 Fulton Street, New York.
Auramine, at present, can best be obtained from Klipstein. Nigrosine soluble in water, in jet black and bluish shades, is made by Marden, Meth-O-Lene and other firms, and is largely used for dyeing leather fast brilliant shades of black.
[270]
Acid Colors.—
| American— | Fast Yellow, H Ex. |
| Brilliant Blue, conc. | |
| Cloth Red, H | |
| Acid Black, L conc. | |
| Marden— | Metanril Yellow |
| Orange, No. 2 | |
| Fast Acid Red | |
| Croceine Scarlet | |
| National— | Azo Yellow, A S W |
| Scarlet, B R | |
| Acid Black, 4 A B |
Also Tartrazine, a fast acid yellow much used for wool, not for silk.
Further information concerning dyestuffs, apparatus, textiles, chemicals, etc., connected with this work, may be obtained on writing to the author at 7 West 43rd Street, New York.
There is no mention either of the Plate illustrations or of the Figure illustrations in the index; these may be found in a list of the illustrations in the front of the book.
Illustrations in this eBook have been positioned between paragraphs and outside quotations. In versions of this eBook that support hyperlinks, the page references in the List of Illustrations lead to the corresponding illustrations.
Illustrations without captions have had a description added, this is denoted with parentheses.
The index was not checked for proper alphabetization or correct page references.
Obvious typographical errors and punctuation errors have been corrected after careful comparison with other occurrences within the text and consultation of external sources.
Some hyphens in words have been silently removed, some added, when a predominant preference was found in the original book.
Except for those changes noted below, all misspellings in the text, and inconsistent or archaic usage, have been retained.
Pg 101: removed duplicate ‘to’ in ‘are apt to to “rub.”’.