A GUIDE
TO THE
DETECTION OF POISONS,
EXAMINATION OF TEA, STAINS, ETC.,
AS APPLIED TO
CHEMICAL JURISPRUDENCE.
TRANSLATED WITH ADDITIONS FROM THE FRENCH OF
A. NAQUET,
Professor to the Faculty of Medicine of Paris.
BY
J. P. BATTERSHALL, Nat. Sc. D., F.C.S.
SECOND EDITION, REVISED, WITH ADDITIONS.
NEW YORK: D. VAN NOSTRAND, Publisher, 23 Murray Street and 27 Warren Street.
1884.
Copyright.
D. VAN NOSTRAND.
1876.
The importance of exact chemical analysis in a great variety of cases which come before the courts is now fully recognized, and the translation of this excellent little book on Legal Chemistry, by one of the most distinguished French Chemists, will be appreciated by a large class of American readers who are not able to consult the original. While it is to be regretted that the author has not presented a much more complete work, there is an advantage in the compact form of this treatise which compensates, in some degree, for its brevity.
The translator has greatly increased the value of the book by a few additions and his copious index, and especially by the lists of works and memoirs which he has appended; and while he could have further increased its value by additions from other authors, we recognize the weight of the considerations which induced him to present it in the form given to it by the author. Some chapters will have very little value in this country at this day, but the translator could not, with propriety, omit anything contained in the original.
C. F. Chandler.
PREFACE TO THE SECOND EDITION.
The principal change to note in this edition of the Legal Chemistry is the addition of a chapter on Tea and its Adulteration. The general interest at present evinced concerning this species of sophistication appeared to call for a simple and concise method of examination which would include the requisite tests without entering upon an exhaustive treatment of the subject. The translator's practical experience in the testing of tea at the United States Laboratory of this city has enabled him to make a few suggestions in this regard which, he trusts, may be of use to those interested in food-analysis. Numerous additions have also been made to the bibliographical appendix.
J. P. B.
| PAGE | |
|---|---|
| Introduction | 5 |
| Methods of Destruction of the Organic Substances | |
| By means of Nitric Acid | 8 |
| " " Sulphuric Acid | 9 |
| " " Nitrate of Potassa | 10 |
| " " Potassa and Nitrate of Lime | 12 |
| " " Potassa and Nitric Acid | 12 |
| " " Chlorate of Potassa | 13 |
| " " Chlorine | 13 |
| " " Aqua Regia | 14 |
| Dialysis | 15 |
| Detection of Poisons, the presence of which is suspected. | |
| Detection of Arsenic | 17 |
| Method used prior to Marsh's test | 17 |
| Marsh's test | 21 |
| Raspail's test | 29 |
| Reinsch's test | 30 |
| Detection of Antimony | 30 |
| Flandin and Danger's apparatus | 32 |
| Naquet's apparatus | 34 |
| Detection of Mercury | 36 |
| Smithson's pile | 36 |
| Flandin and Danger's apparatus | 37 |
| Detection of Phosphorus | 39 |
| Orfila's method | 39 |
| Mistcherlich's method | 40 |
| Dusart's method, as modified by Blondlot | 40 |
| Fresenius and Neubauer's method | 42 |
| Detection of Phosphorus by means of bisulphide of carbon | 43 |
| Detection of Phosphorous Acid | 45 |
| Estimation of Phosphorus | 45 |
| Detection of Acids | 46 |
| Hydrochloric Acid | 46 |
| Nitric " | 47 |
| Sulphuric Acid | 47 |
| Phosphoric " | 48 |
| Oxalic " | 49 |
| Acetic " | 49 |
| Hydrocyanic " | 50 |
| Detection of alkalies and alkaline earths | 52 |
| Detection of chlorine, bromine and iodine | 54 |
| Chlorine and Bleaching Chlorides | 54 |
| Bromine | 55 |
| Iodine | 56 |
| Detection of Metals | 56 |
| Detection of alkaloids and some ill-defined organic substances | 65 |
| Stas's method | 65 |
| " " as modified by Otto | 69 |
| " " " " Uslar and Erdman | 70 |
| Rodgers and Girdwood's method | 71 |
| Prollius's method | 72 |
| Graham and Hofman's method | 73 |
| Application of Dialysis in the detection of Alkaloids | 74 |
| Identification of the Alkaloid | 74 |
| Identification of Digitaline, Picrotoxine and Colchicine | 80 |
| Method to be employed when no clew to the nature of the Poison present can be obtained | 85 |
| Indicative tests | 86 |
| Determinative tests | 94 |
| Miscellaneous Examinations | 96 |
| Determination of the nature and color of the hair and beard | 96 |
| Determination of the color of the hair and beard | 96 |
| Determination of the nature of the hair | 99 |
| Examination of Fire-arms | 100 |
| The gun is provided with a flint-lock and was charged with ordinary powder | 100 |
| The gun is not provided with a flint-lock | 103 |
| Detection of human remains in the ashes of a fire-place | 104 |
| Examination of writings | 105 |
| Examination of writings, in cases where a sympathetic ink has been used | 110 |
| Falsification of coins and alloys | 112 |
| Examination of alimentary and pharmaceutical substances | 114 |
| Flour and Bread | 114 |
| Fixed Oils | 128 |
| a Olive Oil intended for table use | 128 |
| b Olive Oil intended for manufacturing purposes | 130 |
| c Hempseed Oil | 130 |
| Tea | 130 |
| Milk | 137 |
| Wine | 142 |
| Vinegar | 147 |
| Sulphate of Quinine | 148 |
| Examination of blood stains | 150 |
| Examination of spermatic stains | 158 |
| Appendix | 163 |
| Books of Toxicology, etc. | 163 |
| Memoirs on Toxicology, etc. | 168 |
| Index | 187 |
LEGAL CHEMISTRY.
The term Legal Chemistry is applied to that branch of the science which has for its office the solution of problems proposed in the interest of Justice. These most frequently relate to cases of poisoning. When the subject of the symptoms or anatomical lesions produced by the reception of a poison is under consideration, the services of a medical expert are resorted to; but when the presence or absence of a poison in the organs of a body, in the egesta of an invalid or elsewhere is to be demonstrated, recourse is had to the legal chemist. Investigations of this character require great practice in manipulation, and, however well the methods of analysis may be described in the works on the subject, there would be great danger of committing errors were the examination executed by an inexperienced person. The detection of poisons, although perhaps the most important, is not the only subject that may come within the province of the legal chemist; indeed, it would be somewhat difficult to define, a priori, the multitude of questions that might arise. In addition to cases of supposed poisoning, the following researches are most often required:
1. The examination of fire-arms.
2. The analysis of ashes, in cases where the destruction of a human body is suspected.[6]
3. The detection of alteration of writings, and of falsification of coins and precious alloys.
4. The analysis of alimentary substances.
5. The examination of stains produced by blood and by the spermatic fluid.
Each of these researches justly demands a more extended consideration than the limits of this work would permit. The several subjects will be treated as briefly as possible, and at the same time, so as to convey an exact idea of the methods employed, leaving to the expert the selection of the particular one adapted to the case under investigation. We will first mention the methods used in the search for toxical substances. The poisons employed for criminal purposes are sometimes met with in a free state, either in the stomach or intestines of the deceased person, or in the bottles discovered in the room of the criminal or the victim. Under these circumstances, it is only necessary to establish their identity by means of their chemical properties, as directed in the general treatises on chemistry, or by their botanical, or zoological character, in case a vegetable or animal poison, such as cantharides, has been administered. Examinations of this class are extremely simple, the analysis of the substances found, confined to a few characteristic reactions, being a matter of no great difficulty. We will not here dwell longer upon this subject, inasmuch as the analytical methods used are identical with those employed in more complicated cases, with the sole difference that, instead of performing minute and laborious operations in order to extract the poisons from the organs in which they are contained, with a view of their subsequent identification, we proceed at once to establish their identity. The directions given in regard to complicated investigations apply, therefore, equally well to cases of a more simple nature. The detection of a poison[7] mixed with the organic substances encountered in the stomach, or absorbed by, and intimately united with the tissues of the various organs is more difficult. If, however, other information than chemical can be obtained, indicating the poison supposed to be present, and the presence or absence of this one poison is the only thing to be determined, positive methods exist which admit of a speedy solution of the question. When, on the other hand, the chemical expert has not the advantage of extraneous information, but is simply asked,—whether the case be one of poisoning?—nothing being specified as to the nature of the poison used, the difficulty of his task is greatly increased. Up to the present time, the works on Toxicology have, it is true, given excellent special tests for the detection of particular poisons; but none have contained a reliable general method, which the chemical expert could use with the certainty of omitting nothing. Impressed with this need, we proposed, in 1859, in an inaugural dissertation then presented to the Faculty of Medicine, a general method, which, after some slight modifications, is now reproduced. The special methods which allow of the detection of various individual poisons will, however, first be indicated. In cases where the poison is mixed with organic matter, the latter must be removed as the first step in the investigation, as otherwise the reactions characteristic of the poison searched for would be obscured. When the poison itself is an organic substance, this separation is effected by processes modified according to the circumstances. If the detection or isolation of a metallic poison is to be accomplished, the most simple method consists in the destruction of the organic substances. The various methods for effecting this decomposition will now be described.[8]
In order to destroy the organic matters by this process, a quantity of nitric acid equal to one and a half times the weight of the substances taken is heated in a porcelain evaporating dish, the amount of acid being increased to four or six times that of the organic substances if these comprise the brains or liver. As soon as the acid becomes warm, the suspected organs, which have previously been cut into pieces, are added in successive portions: the organs become rapidly disintegrated, brownish-red vapors being evolved. When all is brought into solution, the evaporation is completed and the carbonaceous residue obtained separated from the dish and treated either with water, or with water acidulated with nitric acid, according to the nature of the poison supposed to be present.
Several objections to this method exist, the most serious of which is based upon the fact that the carbonaceous residue, containing, as it may, nitric acid, readily takes fire and may[9] therefore be consumed, or projected from the vessel. This objection is a grave one, and is not always entirely removed by the continual stirring of the materials. According to M. Filhol, the addition of 10 to 15 drops of sulphuric acid to the nitric acid taken obviates the difficulty; not having personally tested the question we cannot pronounce upon it. If it be the case, this process is an advantageous one, as it is not limited in its application, but can be used in the separation of all mineral poisons.
The organic matter to be decomposed is heated with about one-fifth of its weight of concentrated sulphuric acid, the complete solution of the materials being thus accomplished. The excess of acid is next removed by heating until a spongy carbonaceous mass remains. The further treatment of this residue depends upon the nature of the poison supposed to be present. If the sulphate of the suspected poison is a soluble and stable compound, the residue is directly treated with water; if, on the contrary, there is reason to think that the sulphate has suffered decomposition, the mass is taken up with dilute nitric acid; if, finally, the presence of arsenic is suspected, the residue is moistened with nitric acid, in order to convert this body into arsenic acid. The acid is afterwards removed by evaporation, the well pulverized residue boiled with distilled water, and the solution then filtered.
This method, when applied in the detection of arsenic, is objectionable in that the carbonaceous residue, in contact with sulphuric acid, almost invariably contains sulphurous acid, detected by means of permanganate of potassa. This acid, being reduced in the presence of hydrogen, would cause the formation of insoluble sulphide of arsenic, and in this way[10] prevent the detection of small amounts of arsenic by the use of Marsh's apparatus. M. Gaultier de Claubry, indeed, states that he has not been able to detect the presence of sulphurous acid in the carbonaceous residue; but one affirmative result would, in this case, outweigh twenty negative experiments. A further objection to this process consists in the fact that the materials to be destroyed almost always contain chlorides, which, in presence of sulphuric acid and an arsenical compound, might determine the formation of chloride of arsenic, a volatile body, and therefore one easily lost. This difficulty is doubtless of a less serious nature than the preceding, as the operation can be performed in a closed vessel provided with a receiver which admits of the condensation of the evolved vapors; but even then the process would be prolonged. The above method is still again objectionable on account of its too limited application, it being serviceable almost exclusively in cases where the poisoning has been caused by arsenic, for, if applied in other instances, a subsequent treatment would be necessary in order to redissolve the metal separated from its decomposed sulphate.
This method was formerly executed as follows: Nitrate of potassa was fused in a crucible, and the substances to be destroyed added in small portions to the fused mass. The organic matter soon acquired a pure white color; owing, however, to the imperfect admixture of the organic matter with the salt used for its decomposition, it was necessary to take a large excess of the latter.
The following process, suggested by M. Orfila, remedies this inconvenience: The organs are placed in an evaporating dish, together with one tenth of their weight of caustic potassa,[11] and a quantity of water varying with the weight of the substances taken. An amount of nitrate of potassa equal to twice the weight of the organic matter is next added, and the mixture evaporated to dryness. The residue is then thrown by fragments into a Hessian crucible heated to redness, the portions first taken being allowed to become perfectly white before more is added.
Whichever process has been employed, the fused mass is decanted into a porcelain crucible, which has previously been heated in order to avoid danger of breakage. The portion remaining in the vessel is taken up by boiling with a small quantity of distilled water, and the solution so obtained likewise added to the crucible. The mass is then heated with sulphuric acid until all nitrous fumes are expelled, as these could give rise to an explosion, when, in the search for arsenic, the substance is introduced into Marsh's apparatus. As soon as the nitric acid is completely expelled, the liquid is allowed to cool; the greater portion of the sulphate of potassa formed now separating out in crystals. The fluid is next filtered and the crystalline salt remaining on the filter, washed, at first with a little distilled water, then with absolute alcohol, which is subsequently removed from the filtrate by boiling. This method is scarcely applicable otherwise than in the detection of arsenic, as in other instances the presence of a large amount of sulphate of potassa would be liable to affect the nicety of the reactions afterwards used. Its application, even in the search for arsenic, is not to be strongly recommended; on the contrary, the separation of the potassa salt by filtration is indispensable, as otherwise a double salt of zinc and potassium, which might be formed, being deposited upon the zinc used in Marsh's apparatus, would prevent the disengagement of hydrogen, and every chemist[12] is too well aware of the difficulty of thoroughly washing a precipitate, not to fear the possible loss of arsenic by this operation.
In this method the organic materials are heated with water and 10 to 15 per cent. of caustic potassa. As soon as disintegration is completed, nitrate of lime is added, and the mixture evaporated to dryness. A glowing coal is then placed upon the carbonaceous residue obtained: the mass, undergoing combustion, leaves a perfectly white residue. This residue dissolves in hydrochloric acid to a clear fluid which is then examined for poisons.
The above process possesses the undeniable advantage of completely destroying the organic substances, at the same time avoiding the introduction of sulphate of potassa, the presence of which impairs the usefulness of the preceding method; but it necessitates the presence of numerous foreign bodies in the substance to be analysed, and this should be avoided. The absolute purity of reagents is not always to be attained, and the results of an analysis are the more certain, in proportion as they are less numerous and more easily purified.
It has been proposed, instead of using nitrate of lime, to dissolve the organic matter in potassa and then saturate the fluid with nitric acid. This method is evidently more complicated than the simple treatment with nitrate of potassa, and possesses, moreover, no advantages over the latter process.[13]
The organic materials are treated with an equal weight of pure hydrochloric acid, and water added, so as to form a clear pulp. This being accomplished, two grammes of chlorate of potassa are added to the mixture at intervals of about five minutes. The fluid is next filtered, and the insoluble residue remaining on the filter washed until the wash-water ceases to exhibit an acid reaction. The filtrate is then evaporated, an aqueous solution of sulphurous acid added, until the odor of this reagent remains distinctly perceptible, and the excess of the acid removed by boiling the solution for about an hour. The fluid is now adapted to further examination for arsenic, or other metallic poisons.
This method is one of the best in use, both chlorate of potassa and hydrochloric acid being reagents easily procured in a state of great purity; their use, however, is liable to the objection that they convert silver and lead into insoluble chlorides.
M. Jacquelain suggests, in the search for arsenic, the decomposition of the organic matters by means of a current of chlorine, and recommends the following process: The organic substances are bruised in a mortar and then macerated with water. The fluid so obtained, in which the organic matter is held suspended, is next placed in a flask into which a current of chlorine is passed until all the organic matter is deposited in colorless flakes on the bottom of the vessel. The flask is then well closed and allowed to stand for 24 hours, when the odor of the gas should still be perceptible. The fluid is now filtered, the filtrate concentrated by heating in a vessel which[14] permits of the preservation of the volatile chloride of arsenic possibly present, and then examined for poisons.
This process fails to possess the degree of generality desirable, and presents the disadvantage of requiring considerable time for its execution.
This method is exceedingly simple: Aqua regia (a mixture of two parts of hydrochloric and one part of nitric acids) is placed in a tubular retort provided with a receiver, and the organic materials, which have previously been cut into small pieces, added; the reaction commences immediately; if it is not sufficiently active, it is accelerated by a gentle heat: lively effervescence now occurs, and the destruction of all non-oleaginous substances is soon accomplished. The latter substances alone are not immediately decomposed by aqua regia, which attacks them only after prolonged action. As soon as the operation is concluded, the apparatus is removed from the fire and taken apart. The fluid condensed in the receiver is added to that remaining in the retort, and the whole thoroughly cooled in an open dish. The fatty matters now form a solid crust upon the surface of the fluid, which is removed and washed with distilled water, and, the washings being added to the rest of the solution, the latter is directly examined for metallic poisons. It is recommended by Gaultier de Claubry, in cases where the detection of arsenic is desired, to saturate and afterwards boil the suspected fluid with sulphuric acid, in order to remove the nitric and hydrochloric acids present.[15]
The application of the dialytic method was first proposed by Graham. By its use we are enabled to distinguish between two large classes of bodies, viz., colloids and crystalloids. Albumen, gelatine, and analogous substances are typical of colloid bodies; crystalloid substances, on the other hand, are those that are capable of crystallization, either directly or in their compounds, or, in case they are fluids, would possess this property when brought to the solid state. Graham discovered that when an aqueous solution containing a mixture of colloid and crystalloid substances is placed in a vessel having for its bottom a piece of parchment or animal membrane, and this is immersed in a larger vessel filled with water, all of the crystalloids contained in the first vessel transverse the porous membrane and are to be found in the larger vessel, the colloid bodies being retained above the membrane. The organic matter to be eliminated in toxicological researches being colloids, and the poisons usually employed being crystalloids, the value of dialysis as a method of separation is evident. The process is executed as follows:

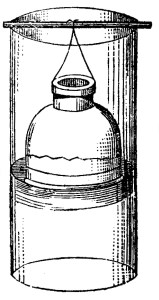
A wooden,—or better, a gutta-percha—cylinder (Fig. 1), 5 cubic centimetres in height and from 20 to 25 c. c. in diameter, is employed. A piece of moistened parchment is securely attached to one of the openings of the cylinder,[16] which, upon drying, shrinks and completely closes the aperture. If its continuity becomes impaired, the pores of the membrane should be covered with the white of an egg which is subsequently coagulated by the application of heat. The organs previously cut into small pieces, or the materials found in the alimentary canal, etc., after having been allowed to digest for 24 hours in water at 32°[A]—or, in dilute acids, if the presence of an alkaloid is suspected,—are then placed in the upper vessel, which is termed the dialyser. The whole should form a layer not over 2 cubic centimetres in height. The dialyser is next placed in the larger vessel filled with distilled water. In about 24 hours three-quarters of the crystalloid substances present will have passed into the lower vessel. The solution is then evaporated over a water-bath, and submitted to analysis. The portion remaining in the dialyser is decomposed by one of the methods previously described, in order to effect the detection of any poisonous substances possibly present. Instead of the above apparatus, the one represented in Fig. 2 can be employed. The fluid under examination is placed in a bell-shaped jar, open at the top and closed below with a piece of parchment, which is then suspended in the centre of a larger vessel containing water. In other respects the operation is performed in the same manner as with the apparatus represented in Fig. 1.
It is frequently required, in chemical jurisprudence, to institute a search for arsenic in the remains of a deceased person, whose death is supposed to have been caused by the reception of a poison. Under these circumstances the poison is mixed with a mass of substances which would obscure its characteristic properties, and it becomes necessary, in order to accomplish its identification, to isolate it, and then, by decisive reactions, determine its character. Three methods exist which permit of this result; they are:
1st. The method used prior to Marsh's test.
2nd. Marsh's test.
3rd. A method more recent than Marsh's, proposed by M. Raspail.
The materials supposed to contain arsenic are boiled in water which has been rendered strongly alkaline by the addition of pure potassa. The fluid is then filtered, an excess[18] of hydrochloric acid added, and a current of sulphuretted hydrogen conducted through it. If arsenic be present in the suspected fluid, it is soon precipitated as a yellow sulphide. In dilute solutions the formation of the precipitate fails to take place immediately, and only a yellow coloration of the fluid is perceptible; upon slightly boiling the solution, however, the precipitation of the sulphide is soon induced. The precipitate is collected on a filter, well washed with boiling water, and then removed, if present in a quantity sufficient to admit of this operation. It is next dissolved in ammonia,[B] and the solution so obtained subsequently evaporated to dryness on a watch-glass. The residue of sulphide of arsenic is placed in a tube closed at one end containing nitrate of potassa in a state of fusion: it is decomposed by this treatment into a mixture of sulphate and arsenate of potassa, the reaction being completed in about fifteen minutes. The mixture is now dissolved in water, and lime water added to the solution: a precipitate of arsenate of lime is formed, which is separated from the fluid by filtration, dried, mixed with charcoal, and introduced into a second tube. A few pieces of charcoal are then placed in the tube adjoining the mixture and exposed to a red heat, the part of the tube containing the arsenical compound being also heated. By this operation the arsenic acid is reduced to arsenic, which is deposited upon the cold portion of the tube in the form of a metallic mirror. This mirror is then identified by subsequent reactions. The method just described is no longer in use, although the precipitation of the arsenic by sulphuretted hydrogen is still often resorted to in its separation from the other metals with which it may be mixed. The destruction of the organic sub[19]stances is, however, accomplished by means of chlorate of potassa and hydrochloric acid. To insure the complete precipitation of the arsenic, it is advisable to conduct sulphuretted hydrogen through the solution, at a temperature of 70° for twelve hours, and then allow the fluid to remain in a[20] moderately warm place, until the odor of the gas is no longer perceptible, the vessel being simply covered with a piece of paper. The precipitate is next freed from the other metals possibly present, as directed in the general method of analysis, collected on a filter, and dissolved in ammonia. The ammoniacal solution is evaporated on a watch crystal, as previously described, and the residuary sulphide reduced to metallic arsenic. This reduction is effected by a process somewhat different from the one previously mentioned: the residue is fused, in a current of carbonic acid gas, with a mixture of carbonate of soda and cyanide of potassium. The apparatus employed is represented in Fig. 3: a, is an apparatus producing a constant supply of carbonic acid. Upon opening Mohr's clamp, g, the gas passes into the flask h, which contains sulphuric acid; it is then conducted, by means of the tube i, into the reduction tube k, which has an interior diameter of 8 mm. This tube is represented, in half size, in Fig 4.
The reduction is performed as follows: The sulphide of arsenic is ground in a small mortar, previously warmed, together with 12 parts of a mixture consisting of 3 parts of carbonate of soda and 1 part of cyanide of potassium, both salts being perfectly dry. The powder thus obtained is placed upon a piece of paper rolled in the form of a gutter, and introduced into the reduction tube. The latter is then turned half round its axis, so as to cause the mixture to fall in de without soiling the other parts of the tube. The paper is now withdrawn and the apparatus mounted. Upon opening the clamp g, and strongly heating the mixture by either the flame of a gas or an alcohol[21] lamp, a mirror-like ring of metallic arsenic is deposited at h, if this poison be present in the substances under examination. When the coating is too minute to permit of perfect identification, it should be driven by heat to a thinner part of the tube; in this way it is rendered easily visible, being condensed upon a smaller space.
The above process possesses the advantage of not allowing arsenic to be confounded with any other body; it also permits of a quantitative estimation of the poison present. For this purpose, it is only necessary to previously weigh the watch-crystal, upon which the ammoniacal solution of sulphide of arsenic was evaporated, and to determine its increased weight after the evaporation; the difference of the two weighings multiplied by 0.8049, gives the corresponding weight of arsenious acid, and by 0.6098, the weight of the corresponding amount of metallic arsenic.
Marsh's test is based upon the reduction of arsenious and arsenic acids by nascent hydrogen, and the subsequent transformation of these bodies into water and arsenetted hydrogen, a compound from which the arsenic can be readily isolated. When pure hydrogen is generated in a flask having two openings, one of which is provided with a perforated cork through which a safety-tube passes, the other with a tube bent at a right angle and drawn out to a small point at the free extremity, the evolved gas, if ignited, burns with a pale non-luminous flame. The air should be completely expelled from the apparatus before igniting the gas. Upon bringing a cold porcelain saucer in contact with the point of the flame, only water is formed. If, however, a small quantity of a solution containing[22] arsenious or arsenic acids is introduced into the apparatus by means of the safety-tube, arsenetted hydrogen is produced. This gas burns with a bright flame, yielding fumes of arsenious acid. In case a large amount of the poison is present, it can be recognized by the appearance of the flame, and by inclining a glass tube towards it upon which a portion of the arsenious acid becomes deposited. These indications are, however, not distinguishable in presence of only a small amount of arsenic, and the following distinctive properties of the gas should be verified:
1st. At an elevated temperature it is decomposed into its two constituent elements.
2nd. The combustibility of the constituents differs: the arsenic being less combustible than the hydrogen, begins to burn only after the complete consumption of the latter body has taken place. For this reason the flame (Fig. 5) is composed of a dark portion O and a luminous portion I, which surrounds the first. The maximum temperature exists in O at the point of union of the two parts of the flame. Owing to an insufficient supply of oxygen, the complete combustion of the arsenic in this part of the flame is impossible, and if it be intersected by the cold surface A B, that body is deposited as a brown spot, possessing a metallic lustre. The metallic deposit originates, therefore, from the decomposition of the arsenetted hydrogen by heat and from its incomplete combustion. If the spot is not large, it fails to exhibit a metallic lustre; an experienced chemist, however, will be able to identify it by the aid of proper tests. Spots are sometimes obtained when the substance[23] under examination does not contain the least trace of arsenic. These may be caused by antimony or by a portion of the zinc salt in the generating flask being carried over by the gaseous current. This difficulty is remedied by giving the apparatus the form represented in Fig. 6. A is the flask in which the gas is generated. The delivery-tube I connects with a second tube H, filled with asbestus or cotton; this is united by means of a cork with a third tube C, made of Bohemian glass. The latter tube is quite long, and terminates in a jet at its free end, enclosed in tin-foil;[C] it passes through the sheet-iron furnace R, supported upon G. The screen D protects the portion D E of the tube C from the heat. The gas disengaged is ignited at E and the porcelain dish P is held by the hand in contact with the flame. The apparatus being mounted, zinc, water and some sulphuric acid are placed in the[24] generating flask,[D] and the solution containing arsenious acid added: the evolution of gas commences immediately. The tube H serves to retain any liquids that may be held suspended. The gas then passes through the part C D of the tube C, which is heated by placing a few live coals upon the furnace R. The greater portion of the arsenetted hydrogen is decomposed here, and is deposited on the cold part of the tube, in a mirror-like ring. The small quantity of gas that escapes decomposition, if ignited at E, produces a metallic spot on the dish P. In order to determine that the spots are due to the presence of arsenic, and not produced by antimony, the following tests should be applied:
1. The color of the spots is distinctive: arsenical spots are brown and exhibit a metallic lustre, whereas those originating from antimony possess a black color, especially near their border. This difference is, however, not perceptible when the deposits have a large surface.
2. If the mirror be arsenical, it is readily volatilized from one part of the tube to another, when the latter is heated, and a current of hydrogen, or carbonic acid gas made to pass through it. Spots that are due to the presence of antimony are much less volatile.
3. If the tube is held in an inclined position so that a current of air traverses it, and the part containing the arsenical mirror heated, the arsenic oxidizes and arsenious acid is sublimed and deposited higher up in the tube in the form of a ring, which exhibits octahedral crystals when examined with a magnifying glass. This ring should be further tested as follows:
a. If it is dissolved in a drop of hydrochloric acid and a so[25]lution of sulphuretted hydrogen added, a yellow precipitate of sulphide of arsenic is formed. This compound is soluble in ammonia and in alkaline sulphides, but insoluble in hydrochloric acid.
b. If the ring is dissolved in pure water and an ammoniacal solution of sulphate of copper added, a beautiful green precipitate ("Scheele's green"), consisting of arsenite of copper, is produced.
4. When produced by arsenic the spots are soluble in nitric acid, and upon evaporating the solution so obtained to dryness, a residue of arsenic acid, which is easily soluble in water, remains. If an ammoniacal solution of nitrate of silver is added to the aqueous solution of the residue, a brick-red precipitate is produced. Spots consisting of antimony give, when treated with nitric acid, a residue of an intermediate oxide, insoluble in water.
5. Upon treating the spots with a drop of solution of sulphide of ammonium, the sulphide of the metal present is formed: if sulphide of arsenic is produced its properties, as enumerated above, can be recognized. It may be added that the sulphide of antimony formed is soluble in hydrochloric acid, and possesses an orange red color, whereas sulphide of arsenic is yellow.
6. When spots originating from arsenic are treated with a solution of hypochlorite of soda (prepared by passing chlorine into solution of carbonate of soda), they are immediately dissolved; if, on the other hand, they are produced by antimony, they remain unaltered by this treatment.
Such are the properties exhibited by soluble compounds of arsenic when treated by Marsh's process; the following precautions are, however, necessary when this test is made use of in medico-legal examinations.[26]
1. If small white gritty particles, resembling arsenious acid, are discovered in the stomach or intestines, they are directly introduced into Marsh's apparatus. When this is not the case, the destruction of the organic matter is indispensable even though, instead of the organs themselves, the contents of the alimentary canal are taken. In the latter instance, the solids are separated from the fluids present by filtration, the solution evaporated to dryness and the residue united with the solid portion; the organic matter is then destroyed by one of the methods previously described. In the special case of arsenic, the separation of the poison from the accompanying organic materials can be accomplished by a process not yet mentioned which may prove to be of service. The suspected substances are distilled with common salt and concentrated sulphuric acid. By this operation the arsenic is converted into a volatile chloride which distils over. The poison is isolated by treating this compound with water, by which it is decomposed into hydrochloric and arsenious acids. We must give preference, however, to the method by means of chlorate of potassa and hydrochloric acid.
2. The solution having been obtained in a condition suitable for examination, the air is completely expelled from the apparatus by allowing the gas to evolve for some time, and the suspected fluid then introduced into the generating flask. Danger of explosion would be incurred were the gas ignited when mixed with air.[E][27]
3. It is indispensable, in applying this test, to have a second apparatus in which only the reagents necessary to generate hydrogen are placed: in this way, if no spots are now produced by the use of the second apparatus, it is certain that those obtained when the first apparatus is employed do not originate from impurities present in the reagents used.
It has come under the author's observation, however, that a sheet of zinc sometimes contains arsenic in one part and not in another; in fact, the shavings of this metal, as purchased for laboratory use, are often taken from lots previously collected, and may therefore have been prepared from several different sheets. If this be the case, it is supposable that the zinc used in the second apparatus may be free from arsenic, whereas the metal with which the suspected solution is brought in contact may contain this poison; serious danger would then exist of finding indications of the presence of arsenic in materials that did not originally contain a trace of the metal. In order to obviate this important objection, which might possibly place a human life in jeopardy, we propose the following modifications: Pure mercury is distilled and its absolute purity established. As the metal is a fluid and is therefore homogeneous, it is evident if one portion be found pure, the entire mass is so. Sodium is then fused under oil of naphtha, in order to cause the complete admixture of its particles, and the purity of the fused metal in regard to arsenic tested. An amalgam is next prepared by uniting the mercury and sodium. This is eminently adapted to toxicological investigations: in order to generate a supply of very pure hydrogen, it is only necessary to place the amalgam in water kept slightly acid by the addition of a few drops[28] of sulphuric acid, by means of which the disengagement of gas is rendered more energetic.[F]
It should be borne in mind that the solution introduced into Marsh's apparatus must not contain organic substances, and that, in case their destruction has been accomplished by means of nitric acid all traces of this compound are to be removed. The sulphuric acid used should also be completely freed from nitrous vapors. According to M. Blondeau, nascent hydrogen in the presence of nitrous compounds converts the acids of arsenic not into arsenetted hydrogen (As H3), but into the solid arsenide of hydrogen (As4 H2). This latter compound, upon which pure nascent hydrogen has no effect, is transformed into gaseous arsenetted hydrogen by the simultaneous action of nascent hydrogen and organic substances. These facts are of the greatest importance, for they might possibly cause a loss of arsenic when it is present, as well as determine its discovery when it is absent.
The first case is supposable: should traces of nitric acid remain in the solution, the arsenic would be transformed into solid arsenide of hydrogen and its detection rendered impossible. The second case may also occur: if the zinc placed in the apparatus contains arsenic, and the sulphuric acid used contains nitrous compounds, the evolved gas will fail to exhibit any evidence of the presence of arsenic, owing to the formation of the solid arsenide of hydrogen. Upon adding the suspected solution, which, perchance, may still contain organic substances, this arsenide is converted into arsenetted hydrogen, and the presence of arsenic will be detected, although the solution under examination was originally free from this metal.[29]
M. Raspail suggests the following method for detecting arsenic: The surface of a brass plate is rasped by filing. In this condition the plate may be regarded as an innumerable quantity of voltaic elements, formed by the juxtaposition of the molecules of zinc and copper. The suspected materials are boiled with caustic potassa, the solution filtered, a drop of the filtrate placed upon the brass plate, and a drop of chlorine water added. If the plate is then allowed to stand for a moment and the substance under examination contains arsenic, a mirror-like spot is soon deposited upon its surface. In order to avoid confounding this deposit with those produced by other metals, the substitution of granulated brass for the plate is in some cases advisable. The granulated metal is dipped successively in the suspected solution and in chlorine water. The granules retain a small quantity of the solutions and, owing to the action of the chlorine water, become covered with metallic spots, if arsenic be present. They are then dried, placed in a tube closed at one end, and exposed to the heat of an alcohol lamp. In case the spots are arsenical, the metal volatilizes and condenses in a ring upon the cold part of the tube, which is submitted to the tests previously described.
This method can hardly be of great service, inasmuch as[30] it extracts the poison from but a very small portion of the solution containing it: we have not, however, personally tested its merits.[G]
Strictly speaking the salts of antimony are more therapeutic than poisonous in their action. In fact they usually act as emetics and, under certain circumstances, may be taken in large doses without incurring serious results. There are instances, however, in which their action is truly toxical, and it becomes necessary to effect their detection in the organs[31] of a body. It should be remarked that these salts, if absorbed, remain by a kind of predilection in the liver and spleen. A special examination of these organs should therefore be instituted, particularly if the fluids of the alimentary canal are not at hand, which is frequently the case when some time has elapsed before the investigation is undertaken.
The remarks made in the preceding article concerning the distinctive properties of arsenic and antimony need not be repeated here. The search for antimony is likewise executed by aid of Marsh's apparatus. We will confine ourselves to a description of a modification to this apparatus proposed by MM. Flandin and Danger, and employed in the separation of antimony and arsenic, when a mixture of these metals is under examination. Another process, by means of which we arrive at the same result with greater certainty and by the use of a less expensive apparatus, will then be mentioned. We will, however, first indicate the preferable method of destruction of the organic substances.
Were the decomposition performed by means of sulphuric acid, sulphate of antimony, a slightly soluble salt and one not well adapted to the subsequent treatment with nascent hydrogen, would be formed. In order to obtain the metal in a soluble state, the formation of a double tartrate of antimony and soda is desirable. This may be accomplished in the following manner:
1. A cold mixture of nitrate of soda, sulphuric acid, and the suspected materials is prepared in the proportion of 25 grammes of the nitrate to 39 grammes of the acid, and 100 grammes of the substance under examination. This mixture is heated and evaporated to dryness, and the decomposition of the organic matter completed in the usual manner. The carbonaceous residue obtained is pulverized, and then boiled with a solution[32] of tartaric acid. By this treatment the antimonate of soda present is converted into a double tartrate of antimony and soda, which is easily soluble in water. The solution is filtered and then introduced into Marsh's apparatus.
2. Another method consists in heating the substances under examination with one half of their weight of hydrochloric acid for six hours on a sand-bath, avoiding boiling. The temperature is then increased until the liquid is in a state of ebullition, and 15 to 20 grammes of chlorate of potassa, for every 100 grammes of the suspected matter taken, added in successive portions, so that a quarter of an hour is required for the operation. The liquid is next filtered, and the resinous matter remaining on the filter well washed with distilled water; the washings being added to the principal solution. A strip of polished tin is then immersed in the liquid: in presence of a large amount of antimony the tin becomes covered with a black incrustation: if but a minute quantity of the metal is contained, only a few blackish spots are perceptible. After the tin has remained immersed for 24 hours, it is withdrawn and placed in a flask together with an amount of hydrochloric acid sufficient for its solution in the cold. If, after several hours, blackish particles are still observed floating in the liquid, they can be dissolved in a few drops of aqua regia. The solution may then be directly introduced into Marsh's apparatus.
This apparatus consists of a wide necked jar A (Fig. 7) for the generation of the gas, the mouth of which is closed with a cork having two openings. The safety tube S, which is funnel-shaped at its upper extremity and has its lower end drawn out to a point, passes through one of these apertures;[33] the other opening contains the small delivery tube B, open at both ends, and terminating in a point at its upper extremity: it is also provided with lateral openings, in order to prevent the solution being carried up to the flame. The second part of the apparatus is the condenser C, 0.03 metre in diameter, and 0.25 metre in length. This terminates at its lower extremity with a cone, and connects at the side with the tube T, slanting slightly downwards. In the interior of the condenser, the cooler E is contained, the lower end of which is nearly in contact with the sides of the opening O. The combustion tube D, 0.01 metre in diameter, is connected by means of a cork with the tube T; it is bent at right angles, and encloses the tube B, in such a manner as to allow the evolved gas to burn in its interior. The dish F is placed beneath the opening O. If the gas which burns in the combustion tube contains[34] arsenetted hydrogen, water and arsenious acid are produced. A portion of this acid is retained in the tube D, the remainder is carried over, with the aqueous vapor, into C, where it condenses, and finally falls into the dish F. Both portions are subsequently examined by means of reactions necessary to establish the presence of the acid. If the ignited gas contains antimonetted hydrogen, water and an intermediate oxide of antimony are formed. The latter compound is entirely retained in the tube D separated from the greater part of the arsenious acid, if this body be present, and can be brought into solution by means of a mixture of hydrochloric and tartaric acids. A fluid is then obtained which can be introduced into Marsh's apparatus, or otherwise examined for antimony.
Although the separation of arsenic from antimony is the chief object in making use of the apparatus proposed by Flandin and Danger, it is evident that this result is not fully accomplished, since a small portion of arsenious acid remains[35] in the tube D (Fig. 7), together with the intermediate oxide of antimony. The following method secures the complete separation of these metals: An amalgam of sodium and mercury is introduced into the flask A, (Fig. 8), which is provided with two openings. The tube B, terminating in a funnel at its upper extremity, passes through one of these orifices. The other aperture contains a cork enclosing the small tube C, which is bent at a right angle and communicates, by means of a cork, with the larger tube D filled with cotton or asbestus. A set of Liebig's bulbs, E, containing a solution of nitrate of silver, is attached to the other extremity of this tube. The apparatus being mounted, the solution under examination is slightly acidulated and introduced by means of the tube B into the flask A: the disengagement of gas begins immediately. If arsenic and antimony are contained in the solution, arsenetted hydrogen and antimonetted hydrogen are evolved. Both gases are decomposed in passing through the solution of nitrate of silver contained in the Liebig bulbs: the arsenetted hydrogen causes a precipitation of metallic silver, all the arsenic remaining in solution as arsenious acid; the antimonetted hydrogen is decomposed into insoluble antimonate of silver. After the operation has continued for several hours, the apparatus is taken apart, the nitrate of silver solution thrown on a filter, and the precipitate thoroughly washed. An excess of hydrochloric acid is then added to the filtrate, and the precipitate formed separated from the solution by filtration, and well washed. The wash-water is added to the solution, and the whole then examined for arsenic by means of Marsh's test.
The precipitate formed in the nitrate of silver solution, which contains antimonate of silver, is well dried, mixed with a mixture of carbonate and nitrate of soda, and calcined in a[36] porcelain crucible for about three-quarters of an hour. The crucible is then removed from the fire, and the cooled mass treated with hydrochloric acid until a drop of the filtered fluid ceases to give a residue when evaporated upon a watch-glass to dryness. A current of sulphurous acid is now conducted through the filtered solution until the odor of this gas remains persistent. The excess of acid is then removed by boiling, and the solution placed in Marsh's apparatus and tested for antimony.
If a mercurial salt exists in a considerable quantity in the substances extracted from the alimentary canal, or ejected either by stools or vomiting, it can be isolated by treating these materials with water, filtering the liquid, and evaporating the filtrate to dryness. The residual mass is taken up with alcohol, and the solution again filtered and evaporated. Upon dissolving the residue obtained by this operation in ether and filtering and evaporating the solution, a residue is obtained which when dissolved in water forms a fluid wherein the presence of mercury can be detected by means of the ordinary tests.
When, however, only a minute quantity of mercury is present, and this has been absorbed, its detection is more difficult. It will be necessary under these circumstances to make use of either Smithson's pile or Flandin and Danger's apparatus.
Smithson's pile consists of a small plate of copper around which a piece of thin gold foil is wrapped. This is immersed in the solution to be tested for mercury, which has previously[37] been slightly acidulated: if mercury be present, the plate acquires a white color which disappears upon exposure to the flame of a spirit-lamp. A similar reaction occurs in presence of tin, as this metal would likewise be deposited upon the plate, and, upon heating, would penetrate the metal and restore to it its natural color. The danger of mistake arising from this fact is obviated by introducing the copper plate into a tube closed at one end and bent at a right angle. The open extremity of the tube is drawn out to a fine point and immersed in water contained in a second tube also closed at one end. Upon heating the plate in the flame of an alcohol lamp, the white color disappears if produced by mercury, and at the same time this metal condenses in the narrow extremity of the tube. The metallic globules formed can be recognized either by the naked eye or with the aid of a lens, or by rubbing them with a piece of gold foil when the latter will acquire a white coating.
When Smithson's pile is employed, the organic substances are most advantageously decomposed by means of chlorine. It is advisable to operate with as small a quantity of fluid as possible, for, owing to the volatility of bichloride of mercury, a portion of this salt may be lost by the evaporation of aqueous, alcoholic, and even etherial solutions, and the detection of minute quantities rendered impossible.
This apparatus consists of a stand S, (Fig. 9) supporting a balloon A, which serves as the reservoir of the suspected solution, and a funnel B, into which the neck of the balloon is dipped. The funnel B is bent at a right angle and is drawn out at its lower end under which the dish C is placed for the reception of the escaping fluids. A fine wire of pure gold,[38] forming the negative electrode of a Bunsen's battery, passes through the lower extremity of the funnel. The end of this wire nearly comes in contact with a second wire, inserted in the upper part of the funnel, and connected with the positive pole of the battery. If the balloon filled with the solution is inverted and immersed in the funnel B, its neck will be submerged at first; soon, however, it becomes uncovered, owing to the depression of the level of the fluid caused by the escape of the latter through the tapering extremity of the funnel: a bubble of air then passes in the balloon and expels a drop of the solution. This process is repeated at short intervals, causing a continuous flow of the fluid, the rapidity of which is easily regulated by elevating or lowering the balloon, thus raising or depressing the level of the liquid. The apparatus having been mounted in this manner and the battery set in action, the disengagement of gas commences. Should mercury be contained in the solution under examination, this metal will be deposited upon the negative wire. When the operation is completed this wire is detached from the apparatus, washed with ether, and dried. It is then introduced into a small tube provided with a bulb, and the mercury volatilized by means of the blow pipe flame: the metal condenses in the bulb of the tube in globules which are readily recognized. They can also be dissolved in nitric acid, and the presence of a mercurial salt in the solution confirmed by further tests.[39]
The solution to be examined in the preceding apparatus, is prepared as follows:
The suspected organic matter is treated with cold sulphuric acid of 66° B. until liquefied, and hypochlorite of lime, and distilled water then added: if necessary, the evolution of chlorine can be accelerated by a further addition of sulphuric acid. As soon as the liquid becomes clear, it is filtered, concentrated and examined as described above. The solution contains the mercury in the state of bichloride, a salt soluble in water and well adapted to the above test.
The substitution of a large balloon, having a capacity of about 2 litres, in place of the small vessel of Flandin and Danger's apparatus, is to be recommended as doing away with the necessity of evaporation; an operation which invariably causes a loss of substance. The apparatus, modified in this manner, is the most delicate in use for the detection of mercury.
The solid substances found in the alimentary canal are mechanically separated from the fluids present by means of a linen cloth. They are then examined by aid of a magnifying glass, and any fragments of phosphorus found separated and preserved under water. If none are discovered, the presence of phosphorescent vapors may possibly be detected by examining the materials in the dark. In any case, a portion of the suspected materials should be treated with nitrate of silver: in presence of phosphorus the materials acquire, first, a reddish-brown, then, a black color. The remaining[40] portion is spread upon a shovel and heated: a white flame, burning at various points of the mass, and originating from the combustion of phosphorus, is observed, if this body be contained in the substances under examination. This method is evidently far from perfect.
Mistcherlich's method is based upon the luminosity of the vapors of phosphorus. The suspected materials are moistened with dilute sulphuric acid, and heated, in a flask communicating with a glass worm which passes through a glass cooler into a receiver. If the apparatus is placed in the dark, and the materials contain phosphorus, luminous vapors will be observed in the flask and receiver. When the quantity of the poison present is considerable, the phosphorous acid formed can be collected and its properties tested.
Dusart's process takes advantage of the facility with which hydrogen combines with phosphorus. The substances under examination are placed between two asbestus stoppers in a tube, one end of which tapers to a point, and a current of pure hydrogen conducted over them. In presence of phosphorus the evolved gas will burn with a green flame, and, upon bringing this in contact with a porcelain plate, red spots will be deposited upon the latter. Blondlot prefers to introduce the suspected materials into the flask in which the hydrogen is generated. He employs the apparatus represented in Fig. 10: a is a flask for evolving hydrogen; b is a U tube, filled with fragments of pumice stone which are saturated with a concentrated solution of potassa; c is a Mohr clamp; d a screw-clamp; e a platinum jet. This jet is necessary in[41] order to avoid a yellow coloration of the flame by the soda contained in the glass. Pure hydrogen is at first evolved, in order to ascertain that the flame is colorless and red spots are not produced when it is intersected by a cold plate. The purity of the reagents used having thus been confirmed, the[42] clamp d is closed until the acid is forced back into f; and the materials to be examined are then added to the fluid. Upon opening the clamp the liquid passes from f into a, and the evolution of gas recommences. The gas is then ignited: the flame possesses the characteristic properties mentioned above, if the suspected substances contain phosphorus.
According to this method, the materials are brought into a flask provided with a doubly-perforated stopper, and water, acidulated with sulphuric acid, added. The flask is then heated over a water-bath, and a current of carbonic acid conducted through the mixture for at least two hours. The gas, on leaving the flask, passes into a solution of nitrate of silver. Should no precipitate form in this solution, the absence of free phosphorus is established, for, were this body present, a portion would be volatilized, and a black precipitate, consisting of phosphide of silver, together with phosphoric acid, produced. The formation of a black precipitate is, however, not necessarily a proof of the presence of phosphorus. In order to conclusively determine the character of the precipitate, it is collected on a filter and examined by the method of Dusart and Blondlot.
This process has given result in cases where none were obtained by Mistcherlich's method. It possesses, moreover, an advantage over the latter process, in not being influenced by the presence of foreign bodies; whereas, in Mistcherlich's method, some time must elapse before the luminosity of the vapors becomes apparent if ether or alcohol is contained in the solutions, and this phenomenon totally fails to appear in presence of oil of turpentine.[43]
In a report read before the Academy of Sciences in 1856, presented by an examining commission, of which MM. Dumas, Pelouze and Claude Bernard were the reporters, the following results were contained: Phosphorus may remain, in the free state, in the organs fifteen days after death, and even then its isolation can easily be accomplished. For this purpose the stomach or intestines, and the articles of food contained therein, are cut into pieces and treated with bisulphide of carbon. Upon filtering the liquid, a solution is obtained containing all the phosphorus present, which exhibits the following properties: 1st, When ignited, it burns with a very luminous flame; 2nd, if allowed to spontaneously evaporate (the combustion of the phosphorus being prevented by the organic matter present [Naquet]) an inflammable residue is obtained, which, if dissolved in boiling monohydrated nitric acid, gives a solution that, after saturation with ammonia, produces a precipitate soluble in acids in solutions of barium salts. If the solution is mixed with perchloride of iron, and the sesquioxide of this metal subsequently eliminated by the addition of ammonia, it no longer causes a precipitation in barium solutions. The fluid acquires a yellow coloration when boiled with a solution of molybdate of ammonia.
According to our personal experience, the apparatus employed by Flandin and Danger for the detection of arsenic, can also be made use of in the examination of the bisulphide of carbon solution. To this end, the fluid supposed to contain phosphorus is mixed with perfectly pure alcohol, and the mixture placed in a small spirit-lamp provided with a very loose asbestus wick. The lamp is then ignited and the flame introduced in the combustion tube D (Fig. 11).[44]
By the combustion of the mixture, sulphurous, carbonic, phosphorous acids and water are formed. The water condenses in c, and, falling into the dish F, carries with it the sulphurous and phosphorous acids. The acid liquid collected in this way is evaporated to dryness, some nitric acid added, and the solution again evaporated. The remaining mass is then dissolved in water to which some ammonia is added, and the solution tested for phosphoric acid. This method is an advantageous one as the phosphoric acid formed must originate from phosphorus in the free state, and not from any phosphates which, owing to the presence of organic matter, might be contained in the bisulphide of carbon solution. It would, however, lead the analyst into error if the person, supposed to have been poisoned had eaten cerebral substances or eggs previous to death, as these contain glycero-phosphoric acid; it is there[45]fore advisable to compare the results given by this process with those obtained by the use of other methods.
Provided free phosphorus has not been detected, it is necessary to search for phosphorous acid. To this end, the residue remaining in the flask, in either Mistcherlich's or Fresenius and Neubauer's method, is introduced into the apparatus of Dusard and Blondlot. If the phosphorus reaction appears, it is sufficient; otherwise, its production may have been hindered by the presence of organic matter. In case, therefore, the flame is colorless, the evolved gas is conducted into a neutral solution of nitrate of silver. If the materials contain phosphorous acid, a precipitate of phosphide of silver is formed which should be collected and washed. The precipitate, which is now free from organic matter, is then examined for phosphorous acid by means of the apparatus of Dusard and Blondlot.
The best process for determining quantitatively the amount of phosphorus present is the one recommended by Fresenius and Neubauer. The gaseous current is continued until a fresh nitrate of silver solution is no longer precipitated. The solution is filtered, the precipitate washed and then dissolved in nitric acid. The silver is next precipitated by addition of hydrochloric acid, the fluid again filtered, and the precipitate well washed. The washings are added to the filtrate, and the liquid concentrated in a porcelain capsule. A solution of sulphate of magnesia, containing ammonia, is next added to the fluid, and the phosphoric acid determined as pyrophosphate[46] of magnesia: the precipitate formed, is washed, heated to redness, in order to convert it into the pyrophosphate, and then weighed.
The search for acids is to be instituted exclusively in the alimentary canal and its contents. Were acids contained in the other organs, their presence would be due to the blood in which they had previously been absorbed, and, as in this case they would be partially neutralized by the bases contained in the blood, a conclusive decision in regard to their original existence in the suspected materials would be impossible, the salts of the acids usually searched for being normal constituents of the blood. In order to detect the presence of acids, the alimentary canal and contents are first boiled with water which is renewed until the solution ceases to exhibit an acid reaction when tested with litmus paper. The fluid is then filtered, alcohol added to the filtrate, in order to precipitate organic substances, the liquid again filtered, and the solution tested separately for the various acids as directed below.
The solution is placed in a retort provided with a receiver and distilled until the residual fluid assumes a pasty consistence: the operation is then discontinued. If hydrochloric acid be present in the materials under examination, the distillate will have an acid reaction, and, upon addition of solution of nitrate of silver, a white precipitate, which is easily soluble in ammonia but insoluble in nitric acid and in short possesses all the properties of chloride of silver, will be formed.[47]
The distillate, obtained as in the preceding process, is neutralized by the addition of potassa or soda, and evaporated to dryness. The residue is mixed with copper filings, and introduced into a glass tube closed at one end and provided at the other with a cork through which a delivery-tube passes. Sulphuric acid is then added to the mixture, the cork inserted, the tube heated, and the evolved vapors conducted into a solution of protosulphate of iron. The latter solution acquires a brown coloration which, upon addition of sulphuric acid, changes to a violet, if nitric acid be present. Upon conducting the disengaged gas into a solution of narcotine, the latter acquires a beautiful red color.
Another portion of the residue should deflagrate when saturated with an alkali and projected upon live coals.
In order to detect this acid, the solution obtained by treating the organs with water is not distilled but is concentrated to one-sixth of its original volume, and then agitated with ether for about ten minutes. By this treatment the ether takes up the free sulphuric acid, but not the acid sulphates present. After ten minutes contact, the ether is decanted and allowed to spontaneously evaporate. Upon treating the residue, which contains the free sulphuric acid and fatty substances, with water, a solution containing only the sulphuric acid is obtained. Nitrate of baryta is then added to a portion of the fluid: in presence of sulphuric acid, a white precipitate, insoluble in acids, is produced. If this is heated on charcoal before the blow-pipe, a mass is formed, which, when moistened[48] with hydrochloric acid and placed upon a clean silver coin, produces a black spot on the metal. Another portion of the solution is mixed with copper and the mixture evaporated in a tube closed at one end: sulphurous acid is evolved towards the end of the operation. This gas is detected by allowing it to pass over paper saturated with a mixture of iodic acid and starch; a blue coloration is produced which, owing to the transformation of the iodine set free into hydriodic acid, subsequently disappears. (We have never been able to effect the disengagement of sulphurous acid spoken of above when an exceedingly dilute sulphuric acid was used, even upon evaporating the mixture to dryness, notwithstanding Orfila's statement that the reaction occurs very readily.)
The aqueous solution is evaporated to dryness, the residue taken up with alcohol of 44° B., the fluid again evaporated, and the second residue dissolved in water. Upon adding acetate of lead to the solution, a white precipitate is produced if phosphoric acid be present. The precipitate is washed, suspended in water and a current of sulphuretted hydrogen passed through the mixture. If the fluid is then filtered, and the excess of sulphuretted hydrogen expelled from the filtrate by boiling, a liquid possessing the distinctive properties of a solution of phosphoric acid will be obtained. This should then be submitted to the following tests: Some pulverized charcoal is added to a portion of the solution, the mixture evaporated to dryness, and the residue obtained introduced into a Hessian crucible heated to redness: in presence of a considerable amount of the acid, free phosphorous is liberated and burns with a bright flame in the upper part of the crucible. In[49] case this reaction fails to occur, other portions of the fluid are treated with a solution of a baryta salt, which causes a white precipitate, soluble in nitric acid; with an ammoniated solution of sulphate of magnesia, which throws down a crystalline white precipitate; and by boiling with molybdate of ammonia, acidulated with nitric acid, which produces a yellow precipitation, or at least a yellow coloration of the solution.
The solution is subjected to the same treatment as in the search for phosphoric acid, with the exception that, instead of adding acetate of lead to the fluid obtained by taking up the residue left from the alcohol with water, it is divided into two portions which are examined separately. A solution of a lime salt is added to one portion: if oxalic acid be present, a precipitate, which is insoluble in acetic acid or in chloride of ammonium, and effervesces when slightly calcined and treated with hydrochloric acid, is formed. Nitrate of silver is added to the remaining portion of the solution: the formation of a precipitate, which detonates when dried and heated in a glass tube closed at one end, is further evidence of the presence of the acid.
The solution obtained by treating the alimentary canal with water is distilled, as in testing for nitric and hydrochloric acids, and the following properties verified in the distillate: 1st. It has an acid reaction, and possesses the odor of vinegar; 2nd, unless previously neutralized with a base, it fails to redden the per-salts of iron; 3rd, if the distillate is added to a solution of[50] the per-salts mentioned and sulphuretted hydrogen conducted through the fluid, a black precipitate is formed; 4th, upon boiling the still acid fluid with a small quantity of starch, the property of the latter to become colored in presence of free iodine is not changed; 5th, if heated with an excess of litharge, a basic salt which restores the blue color to reddened litmus paper is produced.
The detection of hydrocyanic acid requires special precautions. The substances to be examined are mixed with water, if solids are present, and introduced into a retort provided with a delivery-tube which dips in a solution of nitrate of silver. The retort is then heated over a water-bath. If the evolved vapors produce a precipitate in the silver solution, the heating is continued until a fresh portion of the latter is no longer affected. The operation is now interrupted, hydrochloric acid added to the retort, and heat again applied. Should a second precipitation of cyanide of silver occur, the presence of a cyanide in the suspected materials is indicated; whereas the formation of a precipitate by the simple action of heat would point to the presence of free hydrocyanic acid or cyanide of ammonium.[H] In case the latter compound is present, ammonia will be contained in the distillate.
In order to identify the cyanogen, a portion of the precipitate is collected upon a small filter, washed, dried, and then allowed to fall into a rather long tube, closed at one end, in the bottom[51] of which some iodine has previously been placed. A column of carbonate of soda is then introduced above the precipitate for the purpose of retaining the excess of iodine probably taken. Upon heating the lower end of the tube, white fumes of iodide of cyanogen, which condense in needles upon the cold portion of the tube, are produced. These are easily recognized by aid of a magnifying glass. They are colorless and are readily volatilized by heat. Some ammonia is next added to a solution of protosulphate of iron, the precipitate formed thoroughly washed, and exposed to the air until it acquires a greenish hue. The iodide of cyanogen is then withdrawn from the tube and mixed with potassa-lye and the precipitate mentioned above. The mixture is evaporated to dryness, the residue obtained treated with water and the filtered solution then acidulated with hydrochloric acid. If a solution of a persalt of iron is now added to the fluid, a blue precipitate is formed. The addition of salts of copper produces a reddish precipitation.
The remainder of the precipitate formed in the nitrate of silver solution is heated with sulphur and then boiled with an aqueous solution of chloride of sodium: if cyanogen is contained in the precipitate, a solution of sulphocyanate of soda will be formed, and upon adding sesquichloride of iron an intense red coloration produced.
It is evident that the presence of another acid in the solution examined for hydrocyanic acid would render the detection of cyanides impossible, but in all cases hydrocyanic acid can be separated without arriving at a decision in regard to its original state of combination. Nitric, hydrochloric, and several other acids would not be distilled at the temperature of the water-bath; an examination for these by the methods already described can therefore be instituted simultaneously with the search for hydrocyanic acid.[52]
The separation of these bodies in the caustic state is a matter of difficulty owing to the great tendency they possess to become converted into carbonates; the carbonates of lime, baryta and strontia, moreover, being non-poisonous in their effects, will not be employed with criminal intent, and the carbonates of soda and potassa are extensively used as pharmaceutical preparations. Notwithstanding the small chances of success, the isolation of the compounds under consideration in the caustic state is to be attempted.
To this intent, the organs to be analysed, together with their contents, are placed in a glass retort provided with a receiver, water added, and the mixture boiled. The distillate will contain the ammonia present. When, however, putrefaction has begun, the detection of this compound does not necessarily indicate its original presence in the suspected materials. If, after an hour's boiling, the fluid in the retort possess an alkaline reaction, it is to be examined for soda, potassa, strontia, baryta and lime. The undistilled solution is filtered, the filtrate evaporated to dryness, and the residual mass treated with alcohol. By this treatment, potassa and soda go in solution, lime, baryta and strontia[I]—as well as the alkaline carbonates—remaining undissolved. The potassa and soda are separated from the other salts present by filtering and evaporating the alcoholic solution to dryness and then calcining the residue in a silver crucible. The mass, which should still be alkaline, is then dissolved in dilute sulphuric acid. If the solution is turbid, traces of baryta or strontia may still be present and should be[53] removed by filtration. Some hydrochloric acid and solution of bichloride of platinum are then added to a portion of the filtered liquid: in presence of potassa a yellow precipitate is formed.
Another portion is treated with tartaric acid: a white granular precipitate is produced. Hydrofluosilicic acid is added to a third portion of the solution: the formation of a gelatinous precipitate is a further indication of the presence of potassa. If the preceding tests have given negative results, and a white precipitate is formed by the addition of antimonate of potassa to another portion of the solution, soda is present. In both cases, it is necessary to confirm the results by means of the spectroscope.
The above reactions are distinctive only in the absence of metals precipitated by sulphuretted hydrogen, sulphide of ammonium or carbonate of soda, and small portions of the solution should be tested with these reagents.
In order to detect baryta, strontia and lime, the residue, insoluble in alcohol is dissolved in dilute nitric acid, and an excess of carbonate of ammonia added to the solution: the three bases, if present, are precipitated as carbonates. The precipitate formed is separated from the solution by filtration, dissolved on the filter in dilute hydrochloric acid, and the solution then filtered and divided into two parts: sulphuric acid is added to one, the fluid filtered from the precipitate of sulphate of baryta formed, and the filtrate treated with ammonia and oxalate of ammonia. If lime be present,—although its sulphate is not easily soluble—sufficient will be contained in the filtrate to give a white precipitate of oxalate of lime.
The remaining portion of the solution is evaporated to dryness, and the residue treated with absolute alcohol. Chloride of strontium goes into solution, chloride of barium remaining undissolved. If upon evaporating the alcoholic solution a[54] residue is obtained which, when dissolved in water, produces turbidity in a solution of sulphate of lime, strontia is present.
The residue, insoluble in alcohol, is dissolved in water. If a precipitate is produced by the addition of sulphuric acid or hydrofluosilicic acid to the solution, baryta is present. The latter reaction distinguishes baryta from strontia, which is not precipitated by hydrofluosilicic acid. Should the tests mentioned above fail to give affirmative results, and poisoning by means of baryta and strontia be nevertheless suspected, these compounds may possibly have remained in the materials contained in the alimentary canal, in the state of insoluble sulphates. To effect their detection under these circumstances the organic substances must be decomposed by means of sulphuric acid. The carbonaceous residue is calcined in a crucible at an elevated temperature, and the remaining mass treated with water. In this way, a solution of sulphides of barium and strontium is obtained, which is then tested as directed above.
The detection of chlorine is very difficult owing to the great tendency it possesses to become converted into chlorides or hydrochloric acid, and it is only when found in a free state that its discovery is of importance.
In case the gas exists uncombined in the alimentary canal, its odor will be perceptible, and, upon boiling the suspected materials with water, vapors will be evolved which impart a blue color to paper saturated with a mixture of iodide of potassium and starch paste. If the addition of sulphuric acid is necessary in order to produce the above reactions, there is[55] reason to suspect the presence of "chloride of lime" or "Eau de Javelle."[J]
In case bromine exists in a free state at the time the autopsy is made, its presence will be detected by the reddish color and unpleasant odor it possesses. Its isolation is accomplished by treating the materials with bisulphide of carbon which, upon dissolving the bromine, acquires a red color. If potassa is then added to the solution, it combines with the bromine and, upon evaporating the decanted fluid, calcining the residue, and treating it with water, a solution of bromide of potassium is obtained. Upon adding chlorine-water and ether to a portion of the fluid, and shaking the mixture, the bromine is liberated and is dissolved by the ether. The etherial solution of bromine, which possesses a reddish-yellow color, does not mingle with, but floats upon the surface of the colorless aqueous solution.
If nitrate of silver is added to another portion of the aqueous solution of bromide of potassium, a precipitate of bromide of silver, soluble in ammonia, is formed.
In case the bromine has been converted into a bromide, it is necessary to boil the alimentary canal and the articles of food contained therein with water. The fluid is next filtered and agitated with chlorine-water and ether. The liberated bromine is dissolved by the ether, which acquires a reddish-yellow color. Upon decanting the solution, and treating it with potassa, bromide of potassium is formed, and can be detected as directed above.[56]
The detection of iodine is accomplished by a process almost identical with the above. The isolation of the iodine having been effected, it remains to be ascertained that it imparts a blue color to starch paste, and a violet color to bisulphide of carbon.
Under this head we will indicate the systematic course of analysis to be pursued, supposing a mixture of several metals including arsenic and antimony, to be under examination.
The organic substances are first destroyed by means of chlorate of potassa and hydrochloric acid. When this is accomplished, the excess of chlorine is removed by boiling and the liquid filtered. The portion remaining on the filter is preserved: it contains all the silver and a large portion of the lead, if these metals are present. We will designate the residue as A, the filtrate as B.
The residue is calcined with a little carbonate of soda and cuttings of pure Swedish filtering paper, the chlorides present being reduced to the metallic state by this treatment. The residue is next taken up with water acidulated with nitric acid, and the solution filtered. An insoluble residue, that may remain, is washed with hot water until the wash-water ceases to precipitate solution of nitrate of silver, and dried. It is then dissolved in boiling nitric acid, the solution diluted with water, and filtered.[K][57]
Sulphuric acid is added to the filtrate: if no precipitate forms, the absence of lead, in the residue A, is indicated. If, on the contrary, a precipitate is produced, it is collected upon a filter and washed. In order to make sure that the precipitate consists of sulphate of lead, it is treated with a solution of tartrate of ammonia: it should dissolve, forming a solution in which sulphuretted hydrogen produces a black precipitate.
The fluid which has failed to be precipitated by the addition of sulphuric acid, or the filtrate separated from the precipitate formed, can contain only silver. Upon adding hydrochloric acid, this metal is thrown down as a caseous white precipitate, which is soluble in ammonia, but insoluble in boiling nitric acid, and blackens upon protracted exposure to light. The formation of a precipitate possessing these properties, leaves no doubt as to the presence of silver.
Remark.—In the operations described above, as well as in those following, the difficulty in separating minute precipitates from the filter is often experienced. When the precipitate is to be dissolved in reagents that do not affect the paper, such as ammonia, tartrate of ammonia, and dilute acids, it can be brought in solution directly on the filter. In cases, however, where reagents which attack the paper are employed, the precipitate should be separated. This is accomplished by mixing a small quantity of pure silica, obtained by the decomposition of fluoride of silicium by water, with the solution, before filtering. The precipitate becomes intimately mixed with[58] the silica, and can then be readily removed from the paper. The presence of silica does not interfere, it being insoluble in the reagents commonly made use of.
A current of sulphuretted hydrogen is conducted for twelve hours through the solution, which is kept at a temperature of 70°. by means of a water-bath. The flask containing the liquid is then closed with a piece of paper, and allowed to remain in a moderately warm place until the odor of the gas is no longer perceptible. The solution is next filtered with the precaution mentioned in the preceding remark, and the precipitate (a) thoroughly washed. The water used in this operation is united to the filtrate, and the fluid (b) examined as directed further on.
In order to free the precipitate from the organic substances possibly present, at the same time avoiding a loss of any metal, it is dried, moistened with nitric acid, and the mass heated on a water-bath. Some Swedish filtering paper is next added, the mixture well impregnated with sulphuric acid, and then maintained for several hours at a temperature of about 170°. until a small portion (afterwards returned) gives a colorless solution when treated with water. The residue is now heated with a mixture of one part of hydrochloric acid and eight parts of water, the liquid filtered, the matter remaining undissolved washed with dilute hydrochloric acid, and the washings united with the filtrate.
The residue I. and the solution II. are separately examined as directed below.[59]
This may contain lead, mercury, tin, bismuth and antimony. It is heated for a considerable time with aqua regia, the solution filtered, and the second residue, should one remain, washed with dilute hydrochloric acid. If the second residue is fused with cyanide of potassium, the compounds present are reduced to the metallic state. The liberated metals are treated with nitric acid, which dissolves lead, but leaves tin as insoluble metastannic acid. The nitrate of lead is then filtered from the metastannic acid, and both metals are identified as described in the treatment of residue A.
The solution, obtained by the action of aqua regia on residue I, is treated with sulphuretted hydrogen. The tin and antimony are separated from the lead, mercury and bismuth by treating the precipitate produced with sulphide of ammonium, which dissolves only the sulphides of the first two metals. The solution in sulphide of ammonium is afterwards examined for these metals, as directed under the head of solution IV., the search for arsenic, however, being here omitted.
Upon treating the residue insoluble in sulphide of ammonium with nitric acid, lead, copper and bismuth go into solution, mercury remaining undissolved. The liquid is filtered, and the undissolved mercury submitted to the special examination previously described.
Sulphuric acid is added to the solution and the precipitate of sulphate of lead formed, separated, washed, and examined as directed while treating of residue A.
Finally, the solution separated from the lead is tested for bismuth and copper, as in examination of precipitate III.[60]
The solution is concentrated by heating on a water-bath, a small quantity of carbonate of soda cautiously added to a portion, and notice taken if a precipitate forms. The part taken is then acidulated with a little hydrochloric acid, returned to the principal solution, and sulphuretted hydrogen conducted through the fluid, as in the examination of solution B. In case a precipitate fails to form, all metals are absent; if, on the contrary, a precipitate (c) is produced, it is examined as directed below.
If the solution merely became turbid, or the precipitate formed was of a pure white color, it consists probably of sulphur. It is, however, indispensable, even in this case, to collect the precipitate and examine it for arsenic. Provided it is of a pure yellow color, it is treated with ammonia. In case it is entirely dissolved by this treatment, and the addition of carbonate of ammonia failed to produce a precipitate in solution II., it is certain that arsenic, and no other metal, is present. Under these circumstances, the ammoniacal solution is examined as directed in the article on the detection of arsenic. If, on the other hand, the precipitate is not yellow, or being yellow, is but imperfectly soluble in ammonia, and a precipitate was formed by the addition of carbonate of ammonia to solution II., it is necessary to likewise search for tin, antimony, mercury, copper, bismuth and cadmium. In this case, the precipitate is placed in a small flask, allowed to digest for several hours with ammonia and sulphide of ammonium in a moderately warm place, and the solution filtered.[61]
The remaining residue (III.) is washed, labelled, and preserved for subsequent examination; the filtrate (IV.) is treated as directed below.
The solution, to which the water used in washing the residue has been added, is evaporated to dryness, the residue obtained taken up with pure fuming nitric acid, and the liquid again evaporated. The second residue is next saturated with a solution of carbonate of soda. A mixture of 1 part of carbonate and 2 of nitrate of soda is then added, the mixture evaporated to dryness, and the residual mass heated to fusion. The fused mass, when cold, is treated with cold water, and any remaining residue washed with a mixture of equal parts of alcohol and water. The filtered fluids are now evaporated in order to remove the alcohol, sulphuric acid is then added, and the mixture heated until white fumes of the acid begin to evolve. In this way the complete expulsion of the nitric acid present is rendered certain. When cold, the residue is treated with water and the solution introduced into Marsh's apparatus, or, in case a quantitative estimation of the arsenic is desired, it is treated with sulphuretted hydrogen and the weight of the precipitate formed determined, as directed under the detection of arsenic.
Should a residue insoluble in water remain, it may contain tin, antimony and traces of copper. Upon dissolving it in aqua regia and placing a sheet of pure zinc in the solution, these metals are thrown down in the metallic state. The precipitate is collected, the zinc present completely removed by treatment with dilute hydrochloric acid, and the residue boiled with concentrated hydrochloric acid which dissolves the tin present.[62] The fluid is filtered and the filtrate tested for this metal by adding solution of chloride of gold, which, in its presence, produces a purple precipitate, and, by treating it with sulphurated hydrogen, which forms a brown precipitate, soluble in sulphide of ammonium.
If the residue, insoluble in concentrated hydrochloric acid, is thoroughly washed and then treated with nitric acid, the copper present goes in solution. The fluid is filtered, and ammonia added to the filtrate: in presence of copper, the solution acquires a blue color, and gives a reddish precipitate upon addition of ferrocyanide of potassium.
Antimony, if present, remains by the treatment with nitric acid as an insoluble intermediate oxide. This is dissolved in hydrochloric acid, in which it is now soluble, and the solution introduced into Marsh's apparatus.
This precipitate may contain the sulphides of mercury, copper, cadmium and bismuth. Upon treating it with nitric acid, all but the sulphide of mercury are dissolved. In case no residue remains, the absence of mercury is indicated; if, on the other hand, a residue is left, it is well washed, dissolved in aqua regia, and the solution examined, either by means of Smithson's pile, or in the apparatus of Flandin and Danger. (Vide Detection of Mercury.)
Whether a residue remains or not, an excess of ammonia is next added to the filtered solution in nitric acid: the formation of a permanent precipitate denotes the presence of bismuth. In this case, the fluid is filtered, and the alkaline filtrate further tested for copper and cadmium. For this purpose, cyanide of potassium is added, and sulphuretted hydrogen con[63]ducted through the filtrate: if cadmium be present, a yellow precipitate is produced, copper not being thrown down in presence of an alkaline cyanide. The precipitate of sulphide of cadmium is separated from the solution by filtration, and the filtrate saturated with hydrochloric acid. Copper, if present, is now precipitated as sulphide: its separation is completed by conducting sulphuretted hydrogen through the fluid.
The precipitate is collected, washed, dissolved in nitric acid, and its identity established as previously directed. If the metal be present in sufficient quantity, it should be obtained in a metallic state upon a plate of iron; it is then coherent, possesses its natural color, and can conveniently be exhibited to the Jury.
This solution may contain: cobalt, nickel, iron, manganese, chromium, zinc and aluminium. Of these, only zinc and chromium are poisonous; the search for these two metals is therefore all that is necessary in criminal cases. The solution is treated with a slight excess of ammonia, sulphide of ammonium added, and the fluid, after being allowed to stand for several hours, filtered. The precipitate may consist of sulphide of zinc and hydrated oxide of chromium, as well as of traces of sulphide of iron and phosphate of lime. If the suspected materials contained a chromate, this salt, in presence of hydrochloric acid and sulphuretted hydrogen, would be converted into sesquichloride of chromium a compound which is precipitated by sulphide of ammonium as a hydrated oxide.
The precipitate is washed with water, to which a little sulphide of ammonium is added, then dried, and fused with four times its weight of a mixture of equal parts of carbonate and nitrate of potassa. After the mass has remained in a[64] state of fusion for a quarter of an hour, it is treated with boiling water, mixed with a little alcohol, in order to decompose the manganate that would be present were manganese contained in the materials under examination. The alcohol is then expelled by boiling the fluid, and the solution filtered. The filtrate may contain phosphate of potassa, originating from the phosphate of lime present, and chromate of potassa, resulting from the oxidation of the sesquioxide of chromium. In presence of the latter compound, the following reactions will occur in the solution: 1st., Upon acidulation with acetic acid and addition of solution of acetate of lead, a yellow precipitate, soluble in potassa, is formed; 2nd., if hydrochloric acid is added and sulphuretted hydrogen conducted into the solution, the latter acquires a green color, and, upon adding ammonia, a bluish-grey precipitate of chromic hydrate is produced; 3rd., if nitrate of silver is added to the solution, a brick-red precipitate is formed.
The precipitate remaining on the filter, may consist of zinc, mixed with the oxides of iron, nickel, cobalt, aluminium and manganese. It is dissolved in boiling hydrochloric acid, acetate of soda added, and the fluid boiled until no further precipitation occurs. The iron is now completely separated. The solution is then filtered, the precipitate washed, and an excess of potassa added to the filtrate; if the solution contains cobalt, nickel or manganese—which is improbable—a permanent precipitate is formed. This is separated from the fluid by filtration: its further examination is, however, unnecessary, as the metals of which it consists are not poisonous. The filtrate may contain aluminium and zinc. The latter metal is detected by acidulating the filtrate with acetic acid, and adding a solution of sulphuretted hydrogen: in presence of zinc a white precipitate of its sulphide is formed.[65]
In case organic substances are present, the precipitation of chromium by sulphide of ammonium may possibly have been hindered, and the metal have passed into the filtrate. When, therefore, chromium is not detected in the precipitate, the filtrate should also be examined. For this purpose, the fluid is evaporated to dryness, and the residue obtained fused with a mixture of nitrate and carbonate of soda. The fused mass is then taken up with water, the solution acidulated with acetic acid, and a solution of acetate of lead added: if chromium be present, a yellow precipitate, soluble in potassa, is produced.
A general method for effecting the detection of alkaloids was first proposed by Stas. Since the publication of this method, modifications to it have been recommended by Otto, and by L. Uslar and J. Erdman. Other processes have been suggested by Rodgers and Girwood, by E. Prollius, and by Graham and Hofman. The latter will doubtless become general in their application; but up to the present time they have been employed exclusively in the detection of strychnine. Dialysis has also been recently applied in the separation of alkaloids.
This method is based upon the facts: (a), that the acid salts of the alkaloids, especially those containing an excess of tar[66]taric or oxalic acids, are decomposed by caustic alkalies and by the bicarbonates of soda and potassa; (b), that the alkaloids, when liberated in this manner, are combined with a certain amount of water which determines their solution in ether, although, in a desiccated state they may be insoluble in this menstruum; (c), that they may be extracted from their aqueous solutions by agitation with ether.
Stas's original method is as follows: The suspected substances, if organs are contained, are cut into fine shreds, then mixed with absolute alcohol, 0.5 to 2. grammes of tartaric or oxalic acid added and the whole introduced into a flask and heated at a temperature of 60° to 75°. When quite cold, the mixture is filtered, and the undissolved portion remaining on the filter washed with absolute alcohol, the washings being added to the filtrate. The alcoholic solution is evaporated, either by placing it under a bell-jar connected with an air-pump, or by passing a current of air, having a temperature not exceeding 35° over it, until reduced to a quarter of its original volume: the complete expulsion of the alcohol being then rendered certain. If insoluble matter separates during this operation, the concentrated fluid is passed through a moistened filter, the water used in washing the residue being united to the filtrate which is then evaporated to dryness by aid of the air-pump or by placing the fluid in a bell-jar over concentrated sulphuric acid. When the evaporation is completed, the residue is treated with absolute alcohol, the alcohol allowed to evaporate at the ordinary temperature of the air, and the second residue dissolved in the smallest possible amount of water. The fluid thus obtained is placed in a test-tube, and a concentrated solution of bicarbonate of soda added so long as effervescence takes place. Ether is then added, the mixture thoroughly shaken, and after it has remained at rest for[67] some time, a small portion of the supernatant ether removed and evaporated on a watch-glass: the residue obtained will consist of the alkaloid present. Two cases are now possible: the alkaloid is a solid, or it is a liquid and is volatile.
The further treatment of the solution is modified according to these circumstances.
If, upon the evaporation of the ether, oily streaks were left on the watch-glass, a volatile alkaloid is probably present.
In this case, a solution of caustic potassa is added to the test-tube, the mixture shaken, the supernatant ether decanted[M] into a flask and the remaining solution again washed with ether until the last portion fails to leave a residue upon evaporation. The etherial fluids are then united, and two cubic centimetres of water, acidulated with one-fifth of its weight of sulphuric acid, added. This acid retains the alkaloid, which is now in the state of a pure acid-sulphate soluble in water, the animal matters present remaining dissolved in the ether. The ether, in which some sulphate of conia may be contained—although the greater portion of this compound would remain in the aqueous solution—is then decanted. The remaining aqueous solution of the pure sulphate of the alkaloid is placed in a test-tube, a solution of caustic potassa and some ether added, and the mixture well shaken. The ether is next decanted and allowed to spontaneously evaporate in a[68] dry place at a very low temperature, and the ammonia possibly present is then removed by placing the vessel containing the residue over sulphuric acid. The residue now obtained consists of the alkaloid present in a state of purity, and can be directly identified by means of the reactions described further on.
It sometimes occurs that ether fails to take up all of the alkaloid present in the fluid treated with bicarbonate of soda. Under these circumstances the fluid should be mixed with caustic potassa, the mixture shaken, and the ether decanted; this operation being repeated several times, until the entire amount of the alkaloid is removed; the ethereal fluids are then united in a capsule, and allowed to spontaneously evaporate. The result of the evaporation may be solid; more frequently, however, a milky liquid remains which restores the blue color to reddened litmus paper; if so, the presence of a vegetable alkaloid is certain. In order to purify the residue, a few drops of water, slightly acidulated with sulphuric acid, are added to the capsule, and the latter turned, so as to bring the fluid in contact with the substance at all points; in this manner a colorless and limpid fluid is obtained, the fatty substances adhering to the dish. The liquid is decanted into a second capsule, the remaining residue washed with a little acidulated water, and the washings likewise added to the principal solution. The fluid is now evaporated either in vacuo, or over sulphuric acid, to about three-fourths of its original volume a concentrated solution of neutral carbonate of potassa added, and the mixture treated with absolute alcohol, which dissolves the liberated alkaloid, and separates[69] it from the sulphate of potassa formed and the excess of carbonate of potassa. The alcoholic solution is decanted and allowed to evaporate in vacuo or in the air: the alkaloid now crystallizes out in a state suitable for further examination.
In Stas's method, the loss of morphine is possible, for, if ether is not added immediately after the addition of carbonate of soda, this alkaloid crystallizes and is then no longer soluble in that menstruum; and, if the ethereal solution is not quickly decanted, the portion dissolved will likewise separate out in small crystals. In both cases, morphine will remain in the aqueous solution from which the other alkaloids have been extracted by the ether. M. Otto recommends the addition of chloride of ammonium and a little soda-lye, in order to dissolve the alkaloid. Upon allowing the solution so obtained to stand for some time exposed to the air, crystals of morphine are deposited.
According to the same authority, it is advisable to omit the distinction drawn by Stas between volatile and fixed alkaloids, and submit both to the treatment recommended for those that are volatile.
Otto also recommends the agitation of the fluid containing the oxalates or tartrates of the alkaloids with ether, previously to their separation by means of bicarbonate of soda. By this treatment the elimination of the coloring matter present—as well as of colchicine, digitaline, picrotoxine, traces of atropine, and various impurities—is accomplished. As soon as the ether ceases to become colored and to leave a residue upon evaporation, alkali is added, and the operation concluded as usual. In this way the alkaloid is obtained, almost[70] directly, in a pure condition. This last modification appears to us to be a very happy one, inasmuch as it greatly facilitates the purification of the alkaloid present.
1st. The materials to be examined are brought to the consistence of a thin paste, and digested for about two hours with water, to which some hydrochloric acid has been added, at a temperature of 60° to 80°. The mixture is then filtered through a moistened linen cloth, and the residue washed with warm acidulated water; the washings being added to the solution.
2nd. Some pure quartz sand—or, preferably, silica prepared by the decomposition of fluoride of silicium—is added to the filtrate, the fluid supersaturated with ammonia, and evaporated to dryness over a water-bath: the addition of silica renders the residue friable.
3rd. The residue is boiled repeatedly with amylic alcohol, which extracts all the alkaloid present as well as the fatty and coloring matters, and the extracts filtered through filter paper that has been moistened with amylic alcohol.
4th. The filtered fluid is thoroughly agitated with ten or twelve times its volume of almost boiling water acidulated with hydrochloric acid: the hydrochlorate of the alkaloid present goes into the aqueous solution, the fatty and coloring substances remaining dissolved in the oily supernatant layer. The latter is separated by means of a pipette, and the acid aqueous solution shaken with fresh quantities of amylic alcohol until completely decolorized.
5th. The aqueous solution is then concentrated, ammonia[71] added, and the mixture well shaken with warm amylic alcohol, in which the alkaloid dissolves. As soon as the solution forms a supernatant layer upon the surface of the fluid, it is drawn off with a pipette and evaporated on a water-bath. In this manner, the alkaloid is usually obtained in a sufficient state of purity to admit of its immediate identification; if, however, a small portion turns brown when treated with concentrated sulphuric acid, the process of purification must be repeated. Under these circumstances it is re-dissolved in dilute hydrochloric acid, the solution repeatedly shaken with amylic alcohol, in order to extract the impurities present, and the alkaloid then extracted with ammonia and amylic alcohol, as previously directed.
The method of von Uslar and Erdman differs from that of Stas merely in the substitution of amylic alcohol for ether, and of hydrochloric acid for oxalic or tartaric acid. It offers no advantages over Stas's method if the alkaloids present are soluble in ether but is even less advantageous in this case, inasmuch as its execution requires a longer time. In cases where the detection of morphine, or an unknown alkaloid, is desired, the use of amylic alcohol instead of ether is, it is true, preferable; still, with the exercise of care, ether can also be employed, and, as this process greatly facilitates examinations when no clew to the poison present exists and all alkaloids may possibly be absent, we prefer it to the one just described.
This method—which as yet has only been employed in the detection of strychnine—is based upon the solubility of this alkaloid in chloroform. The substances under examination[72] are digested with dilute hydrochloric acid, and the mixture filtered. The filtrate is then evaporated to dryness on the water-bath, the residue taken up with pure alcohol, the alcoholic solution evaporated, the second residue treated with water, and the solution so obtained filtered. The filtrate is next supersaturated with ammonia, and well shaken with chloroform, which, upon being separated by means of a pipette and evaporated, leaves the alkaloid in an impure state. Concentrated sulphuric acid is then poured upon the alkaloid: the latter is not affected by this treatment, whereas the foreign organic substances present are carbonized. After the lapse of several hours, the mixture is treated with water, the fluid filtered, and the strychnine extracted from the filtrate by means of ammonia and chloroform, as already described. The operation is repeated until the residue obtained by evaporating the chloroform is no longer affected by the treatment with sulphuric acid.
The suspected substances are boiled with aqueous alcohol, mixed with tartaric acid, and evaporated at a gentle heat. The remaining aqueous solution is then passed through a moistened filter, ammonia added to the filtrate, and the mixture shaken with chloroform. The chloroform is separated, the last trace of the original solution removed by washing with water, three parts of alcohol added, and the fluid evaporated. If strychnine be present, it will now separate out in crystals. This method is applicable only in presence of a considerable quantity of strychnine, and is less serviceable than the one preceding.[73]
This method, which is applied to the detection of strychnine in beer, is founded upon the fact that an aqueous solution of a strychnine salt yields the alkaloid to animal charcoal, from which it can be subsequently extracted by boiling with alcohol. The beer to be examined is shaken with 30 grammes of animal charcoal, and the mixture then allowed to stand twenty-four hours, with occasional shaking. The solution is next filtered, the animal charcoal washed with water, and boiled for half-an-hour with four times its weight of 90 per cent. alcohol. The apparatus represented in Fig. 12 is employed, in order to avoid a loss of substance in this operation.
The alcohol is filtered hot, evaporated, and the residue obtained treated with a small quantity of solution of potassa,[74] and then agitated with ether. Upon spontaneous evaporation, the ethereal solution leaves the strychnine present in a comparatively pure state.
Macadam proposes to use this process for the detection of strychnine in animal bodies. For this purpose, the suspected materials are heated with a solution of oxalic acid, as in Stas's method, and the strychnine detected in the filtered solution in the manner just described. This method is scarcely to be recommended: the use of animal charcoal is doubtless serviceable in the examination of beer, as it effects the separation of a small amount of strychnine from a large quantity of fluid, but its application to other researches is much less to be advised.
In order to apply the dialytic method to the separation of alkaloids, the suspected substances are heated with hydrochloric acid, and the solution introduced into the dialyzer. The hydrochlorates of the alkaloids, being crystalline bodies, transverse the membrane, and are contained, for the greater part, after twenty-four hours, in the outer solution. The fluid is then concentrated, and the alkaloids either directly precipitated, or purified by one of the preceding methods.
The alkaloid having been isolated by one of the preceding methods, it remains to establish its identity. Owing to the small number of reactions characteristic of organic compounds, this is a matter of considerable difficulty. There are two cases possible: the alkaloid may either be volatile or fixed.[75]
In this case it may consist of nicotine, conine or aniline: less known alkaloids (piccoline, etc.) may also be present. We will confine ourselves to the consideration of the three first mentioned.
The alkaloid is divided into several portions which are placed on watch-glasses and submitted to the following tests:
a. A drop is treated with nitric acid: this may, or may not, impart a red tint to the alkaloid; if it does, another drop is treated with dry hydrochloric acid gas: if it assumes a deep violet color, it probably consists of conine.
b. In case a red color was not produced by the
addition of nitric acid, another drop is treated with
chloride of lime. If it acquires a violet tint, and
two other drops, when heated, one with arsenic acid,
the other with nitrate of mercury, become red, the
body present consists of aniline.
or an homologous base.
c. Should the above tests fail to give positive results, and the substance, when treated with chlorine, assumes a blood-red color, and with hydrochloric acid does not change in the cold but turns to a deep violet color upon boiling, it probably consists of nicotine.
A very minute quantity is dissolved in the smallest possible amount of hydrochloric acid, and an excess of ammonia[76] added. Three cases are now possible: (a) A precipitate, insoluble in an excess of the precipitant, is immediately formed; (b) a precipitate is formed, which, at first dissolves, but is subsequently deposited from the fluid; (c) no precipitate is produced, or, in case one forms, it dissolves in an excess of the precipitant and fails to separate out upon allowing the fluid to stand.
A small quantity of an aqueous solution of carbonic acid is poured over the alkaloid in the water-glass, and notice taken whether it dissolves or not: in either case the mixture is evaporated on a water-bath to dryness, in order to avoid a loss of substance.
CARBONIC ACID FAILS TO DISSOLVE THE ALKALOID.
After the evaporation is completed, ether is added to the watch-glass: the alkaloid may, or may not, be dissolved. The ether is then evaporated at the ordinary temperature of the air.
Ether fails to dissolve the alkaloid.
It probably consists of berberine.
In this case, it will possess a yellow color, and its hydrochlorate will give a reddish-brown precipitate upon addition of sulphide of ammonia.
Ether dissolves the alkaloid.—A small portion is treated with nitric acid. If an intense green coloration is produced, the remaining portion is dissolved in ether, and an ethereal solution of oxalic acid added. If the precipitate[77] now formed does not dissolve upon the addition of a little water, there is reason to suppose the presence of aricine.
Provided the addition of nitric acid did not produce a coloration, the mixture of the alkaloid and this acid is treated with a small quantity of sulphuric acid: if the fluid now acquires a red color, the substance probably consists of narcotine.
Should both nitric and sulphuric acids fail to cause a reaction, the alkaloid is dissolved in ether, precipitated by an ethereal solution of oxalic acid, and the precipitate treated with a little water. If it dissolves, it probably consists of papaverine.
CARBONIC ACID DISSOLVES THE ALKALOID.
The substance is treated with ether, notice being taken if it dissolves, which is evaporated at the ordinary temperature of the air so as to prevent a loss of minute portions of the alkaloid.
Ether dissolves the alkaloid.—If nitric acid gives first a scarlet, then a yellow color, sulphuric acid a yellow, changing to red and violet, and hydrochloric acid a violet color, the alkaloid present is probably veratrine.
If the above colorations are not produced, chlorine water is added to another portion of the substance, then ammonia; the formation of a green color, changing to violet and turning red upon a renewed addition of chlorine water, denotes the presence of quinine.
In case all of these tests give but negative
results, and the alkaloid is soluble in concentrated
sulphuric acid, a solution being formed which[78]
assumes a reddish-violet tint when stirred with
a glass rod previously dipped in bromine water,
the presence of delphine.
is indicated.
Ether fails to dissolve the alkaloid.—If the substance is capable of being sublimed,[N] it consists of cinchonine.
The substance is treated with cold absolute alcohol and its solubility in this menstruum noted. If it readily dissolves, it probably consists of brucine.
The presence of this alkaloid is confirmed by applying the following tests: (1) Nitric acid imparts a blood-red color to the substance; (2) if treated with sulphuric acid, it acquires a reddish tint which subsequently changes to yellow and green; (3) chlorine at first fails to cause a coloration, but after some time a yellow color which afterwards changes to a red is produced; (4) upon treating the substance with bromine, it immediately assumes a violet tinge.
In case the alkaloid is only slightly soluble in alcohol, there is reason to infer the presence of strychnine.
The following confirmatory tests should be applied: (1) If the substance is treated with a mixture of sulphuric acid and an oxidizing body, such as bichromate of potassa, binoxide of manganese, or peroxide of lead it acquires a[79] violet color, which changes into red and finally passes into a clear yellow; (2) the addition of bichloride of platinum produces a precipitation of the hydrochlorate.
Should, however, the substance be only slightly
soluble in alcohol, and the above reactions
fail to take place, the presence of solanine.
is indicated. In presence of this alkaloid the
following reactions will occur: (1) Upon treating
the substance with concentrated sulphuric acid,
it assumes a rose tint, which changes after some
time has elapsed first to a deep violet, then to
a brown color; (2) a solution of a salt of the
alkaloid reduces gold and silver salts; (3) the
addition of oxalic acid produces a precipitate in
the aqueous and even acid solution of its
salts.
The solubility of the alkaloid in ether is ascertained. If it be soluble, it may consist of aconitine, atropine or codeine; if insoluble, of emetine or morphine.
The alkaloid is soluble in ether.—If bichloride of platinum fails to precipitate the hydrochlorate from a neutral solution of the alkaloid, and sulphuric acid causes it to assume a yellow color which subsequently changes to a reddish-violet, it probably consists of aconitine.
In case bichloride of platinum causes a pre[80]cipitate and sulphuric acid fails to produce the yellow coloration referred to above, the presence of either atropine or codeine is indicated. In order to decide which of these bases is present, the substance is dissolved in pure chloric acid and the solution allowed to spontaneously evaporate. If the alkaloid is deposited during this operation, it probably consists of atropine.
If this is not the case, there is reason to infer the presence of codeine.
The alkaloid is insoluble in ether.—If it dissolves in acetone it probably consists of emetine.
If acetone fails to dissolve it, the presence of morphine.
is indicated.
The following confirmatory tests should be applied: (1) Upon treating the substance with nitric acid, it acquires a blood-red color; (2) the addition of a solution of a persalt of iron produces an evanescent blue coloration; (3) chloride of gold is colored blue, when treated with the alkaloid; (4) the substance reduces iodic acid: this reduction is detected by adding to the acid a little starch-paste, which turns blue upon the liberation of the iodine; (5) permanganate of potassa, if heated with the substance, is reduced and acquires a green color.
It has already been remarked that in exhausting the first acid solution with ether—previous to the neutralization, ac[81]cording to Otto's method—colchicine, a weak alkaloid, digitaline, an indefinite mixture, picrotoxine (which appears to possess the properties of an acid), and traces of atropine, pass into solution.
The ether is evaporated on a water-bath to dryness, the residuary mass treated with slightly warmed water and the solution filtered from the undissolved resinous matter. The aqueous solution is next rendered feebly alkaline by addition of soda lye, and then well agitated with ether, until this fluid ceases to leave a residue upon evaporation. The ethereal solution is now decanted, and the water present removed by means of chloride of calcium. If it is evaporated, a residue containing the colchicine, digitaline and traces of atropine (mixed possibly with a minute quantity of picrotoxine, which is here left out of consideration) is obtained.
a. The alkaline solution, from which the ether has been removed, is acidulated with hydrochloric acid and again shaken with ether. The picrotoxine present is now dissolved, and upon dehydrating (by means of fused chloride of sodium) and evaporating the ethereal solution can be obtained in crystals. The crystals of picrotoxine are easily recognized by their forming in feathery tufts as well as by their length and silky brilliancy. Should crystals fail to form in a short time, it is advisable to take up the residue, left by the evaporations of the ether, with slightly warmed alcohol, and to allow the latter to spontaneously evaporate on a watch-glass, or, if the quantity of substance is exceedingly minute, on the slide of a microscope. After determining the form of the crystals, it should be ascertained that they possess an intense bitter taste and exhibit the other characteristic properties of picrotoxine. The following reaction is distinctive: If the crystals are dissolved in an aqueous solution of soda and a few drops[82] of "Fehling's solution"[O] added, a reddish precipitate of cuprous oxide is formed.
b. Provided picrotoxine has not been found, the ethereal solution obtained by agitating the alkaline fluid with ether is to be examined for colchicine and digitaline. To this end, the residue obtained upon evaporating the solution to dryness is taken up with water, and the filtered fluid tested as follows: 1. It is ascertained if a drop of the solution possesses the bitter taste of digitaline. 2. Another drop is treated with solution of tannin; if either alkaloid be present, a precipitate is formed. 3. Two drops of the solution are next tested: one with tincture of iodine, the other with chloride of gold. These reagents precipitate colchicine, but do not affect solutions of digitaline or picrotoxine. Unfortunately traces of atropine, possibly present, would cause the same reaction; the test therefore fails to be conclusive. 4. Several portions of the solution are evaporated on watch crystals. Concentrated nitric acid is added to one portion: if colchicine be present, an evanescent violet coloration is produced, which changes to a light yellow upon addition of water, and to a pure yellow or reddish-orange color, if the mixture is saturated with a slight excess of caustic alkali. 5. Another portion of the residue is dissolved in a few drops of concentrated sulphuric acid, and the solution stirred with a glass rod moistened with bromine water: in presence of digitaline a violet-red color is produced. This coloration is more distinct when a small quantity of the alkaloid and an excess of sulphuric acid are present. 6. If a large amount of substance is at hand, the residue can be boiled with hydrochloric acid, and the green or brownish color and characteristic odor of digitaline pro[83]duced, in case this body be present: this, however, is not a very delicate test. 7. Finally; it is advisable when the presence of digitaline is suspected to ascertain its physiological action. For this purpose, a minute quantity of the substance is placed upon the heart of a frog: in presence of the alkaloid, the pulsations are immediately retarded, or even arrested.
Although by means of the tests given above the existence of a special alkaloid, or of one of the ill-defined substances just mentioned, may be justly regarded as probable, its presence has not yet with certainty been demonstrated. This is especially true in cases where the compound possesses but few characteristic properties. When possible, the suspected substance should be obtained in a crystaline form, and then compared by aid of the microscope—if the small quantity present permits of no other examination—with crystals of the pure alkaloid, prepared under the same conditions.
In case 20 or even 10 centigrammes of substance are at hand, it is best to convert the alkaloid into its hydrochlorate, and evaporate the solution of this salt to dryness. The residue, after being weighed, is dissolved in water, and a solution of sulphate of silver added. The precipitate of chloride of silver formed is collected and carefully weighed, in order to calculate the weight of the chlorine contained in the hydrochlorate and consequently the molecular weight of the alkaloid. The filtrate from the chloride of silver, which contains the alkaloid in the state of sulphate, is treated with hydrochloric acid, to remove the excess of silver present and the fluid then filtered. The filtrate is next shaken with potassa and ether. Upon decanting and evaporating the ethereal solution, a residue consisting of the alkaloid present is obtained, which is then purified by crystallization from alcohol. An elementary analysis of the alkaloid[84] is now executed. Certainty as to the presence of an individual alkaloid is attainable only when the execution of this confirmatory test is possible. The reactions previously described can be performed with fifteen centigrammes of substance, and this amount is sometimes contained in a cadaver. If but one or two centigrammes are at hand, it is still possible to detect the presence of an alkaloid; a conclusion, however, as to which cannot be arrived at, especially if the substance found is a liquid or an amorphous body, and one that presents few distinctive properties.[85]
If poisoning has been caused by the administration of a mixture of numerous substances and these greatly differ in their properties, it is impossible to demonstrate in an incontestible manner the presence of each individual poison. This contingency fortunately but seldom arises; the criminal usually has recourse to one or two poisons, the detection of which is possible. It must not be imagined, however, that the presence of a poison in an organ can at once be detected with certainty by the mere application of a few tests; because, in searching for a substance which is absent, we may unwittingly destroy the one present, or, at least, transform it into combinations which would not allow of a definite conclusion as to its original condition.
In order to follow a systematic method in researches of this nature, it is advisable to divide the materials under examination into three parts: one portion is preserved, in order to ascertain its physiological effects on animals, the chemical analysis having failed to give positive results. The other portions are submitted to analysis, but with slightly different objects[86] in view; one is subjected to a series of tests which are adapted, under all circumstances, to place the chemist on the track of the poison present, and which, in some cases, may even give conclusive and definite results. Should these tests furnish only indications of the nature of the poison, the remaining portion serves, with the assistance of this information, to establish beyond doubt the identity of the substance.
Two cases may present themselves: the materials to be examined possess either an alkaline (or neutral) or an acid reaction. As the methods to be pursued in either of these cases differ somewhat, they will be treated separately.
The materials are mixed with water, placed in a retort provided with a delivery-tube which dips in a solution of nitrate of silver, and heated over a water-bath: if a cyanide be present, hydrocyanic acid will be disengaged, and a white precipitate of cyanide of silver formed: this is examined as previously directed (vide p. 50).
In case a precipitate is not produced by the above treatment, more water is added to the retort, and the mixture boiled for about an hour, care being taken to collect the evolved vapors in a well-cooled receiver. The portion remaining in the retort is thrown on a filter and the filtrate obtained united with the distillate. The residue remaining on the filter is next washed with boiling absolute alcohol, the washings being added to the aqueous solution. In this way, the suspected substances are divided into soluble and insolu[87]ble portions, which are examined separately, as directed below.
If the addition of alcohol caused a precipitation of animal matters, these are separated by filtering the solution. The filtrate is then placed under a bell-jar over concentrated sulphuric acid until its volume is considerably reduced. The solution may contain organic and inorganic bases and acids. In order to detect all bodies that are present, the following course is pursued:
(1). A current of sulphuretted hydrogen is conducted through the solution: the precipitation of some metals, usually thrown down by this gas, may fail to take place in this instance, owing to the presence of organic substances; however, some metals are precipitated, even in presence of organic compounds, and organic acids are but seldom present. In case a precipitate is formed, it is mixed with pure silica, collected on a filter, and treated with nitric acid. If the precipitate fails to dissolve, it is treated with aqua regia. In either case, the solution obtained is examined for metals by the ordinary methods.
(2). The solution in which sulphuretted hydrogen failed to produce a precipitate, or the filtrate separated from the precipitate formed, is divided into two parts: one portion is treated with ether and a solution of potassa; the other with ether and a solution of soda. Both mixtures are then well agitated, and notice taken if the ether dissolves any thing: if so, the operation is repeated several times until all soluble substances are removed. The ethereal solutions are next decanted and united, and then submitted to the examination for alkaloids as directed pp. 65-84.[88]
(3). If—the above treatment giving either positive or negative results—a precipitate insoluble in ether is formed by the addition of potassa or soda, it is collected on a filter, washed, and dissolved in an acid. The solution is then tested for mineral bases.
(4). In case no definite result has been obtained by the preceding operations, one of the portions (for instance, the one to which potassa was added) is tested for the acids possibly present in the state of salts. The solution is divided into two parts (A and B) which are examined separately:
Portion A.—This is evaporated to dryness and the residue divided into four parts which are then tested for hydrofluoric, nitric, oxalic, and acetic and formic acids.
a. Hydrofluoric acid.—A portion of the residue is heated in a platinum crucible with sulphuric acid, and the crucible covered with the convex face of a watch-crystal coated with wax in which lines have been traced with a pointed piece of wood. If, after gently heating the crucible for some time and removing the watch-crystal, the lines traced in the wax are found to be etched in the glass, the substance under examination contains a fluoride.
b. Nitric acid.—If this acid be present, and a second portion of the residue is heated with sulphuric acid and copper, reddish-fumes are evolved. Upon conducting the vapors into a solution of sulphate of iron or narcotine, the reactions already mentioned in treating of nitric acid take place.
c. Oxalic acid.—The third portion of the residue is heated with sulphuric acid, and the evolved gas carefully collected. It should then be confirmed by an elementary analysis that the gas consists of equal volumes of carbonic oxide and carbonic acid. This test is not conclusive; it is also necessary to ascertain if the precipitate produced by the addition of a[89] baryta solution (vide: under portion B.) produces the same reaction, inasmuch as other organic bodies could give rise to carbonic oxide and carbonic acid, and the danger of both admitting the presence of oxalic acid, when it is absent, and omitting its detection, in case it is present, would be incurred.
d. Acetic and Formic acids.—The fourth portion of the residue is distilled with dilute sulphuric acid. After determining that a small portion, previously neutralized with a base, acquires a red color, upon addition of a solution of a persalt of iron, the distillate is divided into two parts. One portion is treated with bichloride of mercury: if formic acid be present, metallic mercury is formed, with evolution of carbonic acid which produces turbidity in lime-water. The remaining portion of the fluid is digested, in the cold, with an excess of litharge: in presence of acetic acid, a soluble basic salt of lead, possessing an alkaline reaction, is produced.
Portion B.—The second portion of the solution is supersaturated with nitric acid, and this neutralized by addition of a slight excess of ammonia. The ammonia is then expelled by boiling the fluid, and a solution of nitrate of baryta added. If a precipitate forms, it is collected and subsequently examined for sulphuric, phosphoric, oxalic and boric acids as directed below. The filtrate is preserved and tested for hydrochloric, hydrobromic and hydriodic acids.
a. Oxalic acid.—A portion of the precipitate produced by the addition of nitrate of baryta is submitted to the test mentioned under the treatment of portion A.
b. Sulphuric acid.—If an insoluble residue remains upon treating the remainder of the precipitate with dilute hydrochloric acid, it consists of sulphate of baryta and indicates the presence of sulphuric acid.[90]
c. Phosphoric acid.—An excess of solution of alum and ammonia is added to the portion of the precipitate dissolved in hydrochloric acid. If phosphoric acid be present, insoluble phosphate of alumina is precipitated. This is brought upon a filter: the filtrate being preserved and subsequently examined for boric acid. Upon boiling the precipitate with solution of silicate of potassa, silicate of alumina is thrown down, and phosphate of potassa remains in solution. Chloride of ammonia is now added to the liquid—in order to eliminate the excess of silica from the silicate—and the solution filtered. The filtrate is then tested for phosphates, by means of molybdate of ammonia (vide: detection of phosphoric acid, p. 48).
d. Boric acid.—The filtrate from the precipitate of phosphate of alumina is evaporated to dryness, the residue mixed with sulphuric acid and alcohol, and the latter ignited. If the substance contains boric acid, the alcohol will burn with a green flame.
The filtrate, separated from the precipitate produced by the addition of nitrate of baryta, may contain hydrochloric, hydrobromic and hydriodic acids. In order to detect these compounds, some nitrate of silver is added to the solution, and the precipitate that may form carefully washed and decomposed by fusion with potassa. The mass is then dissolved in water, and the solution submitted to the following tests:
e. Hydriodic acid.—Some starch paste and nitric acid—containing nitrous acid in solution—are added to a portion of the solution: in presence of an iodide, the fluid immediately acquires a blue color.
f. Hydrobromic acid.—In case iodine has not been detected, chlorine water and ether are added to a second portion of the fluid, and the mixture well agitated. If bromine be present, the ether will assume a brown color. In case[91] iodine is also contained in the fluid, and the detection of bromine is desired, it is necessary to acidulate the solution with hydrochloric acid, and then shake it with chloride of lime and bisulphide of carbon. The bisulphide of carbon dissolves the iodine, acquiring a violet color, which disappears upon a renewed addition of chloride of lime; whereas, in presence of bromine an orange coloration remains, even after the disappearance of the iodine reaction.
g. Hydrochloric acid.—Since the substance under examination will already contain hydrochloric acid, it is unnecessary, in most cases, to institute a search for this compound. Nevertheless, it may be well to take a quantity of the solution, corresponding to a known weight of the original substance, and precipitate the acid by adding nitrate of silver. The precipitate formed is dried and weighed. It is then heated in a current of chlorine, in order to completely convert it into chloride of silver, and its weight again determined. Only in case the amount of chloride found is very large, is it to be inferred that the poisoning has been caused by hydrochloric acid.
h. Hydrosulphuric acid.—(Sulphuretted hydrogen). If the precipitate produced by nitrate of silver possesses a black color, it may consist of a sulphide. Upon treating a portion with solution of hyposulphite of soda, all but the sulphide of silver is dissolved. In case a residue remains, it is calcined with nitrate of soda, and the sulphate formed detected by adding a soluble barium salt to its solution.
Sulphates, chlorides, carbonates and phosphates are most frequently met with in the preceding examination, and it should be carefully noticed which of these salts exist in the greatest abundance. If acids of comparatively rare occurrence (such as the oxalic and tartaric) are found, their approximate amount[92] is also to be noted. These facts, together with the original acidity of the materials and the absence of other toxical bodies, would lead to the conclusion that the poisoning was caused by the reception of an acid, as well as to the identification of the special acid used. In subsequently effecting the detection of the poison by the determinative tests, the danger of destroying other poisons possibly contained in the substance will be obviated, as the question of the absence or presence of these latter will have been previously decided.
(5). The examination for acids concluded, the various fluids which have accumulated, and from which the acids present have been separated, are united and the whole evaporated to dryness. The organic substances, present in the residue obtained, are destroyed by means of nitric acid, and the residual mass examined for soda. If this substance has not been introduced into the portion of fluid examined, and is discovered in a quantity largely in excess of the amount normally contained in the organism, it is probable that poisoning has been caused by its administration, and that an acid has also been given, either in order to mask the poison, or to act as an antidote. In this case, it is necessary to carefully search for acetic acid, as this is the substance usually employed as an antidote for alkalies.
(6.) Whatever results have been obtained by the preceding examinations, the portion of the fluid which has been treated with soda (vide p. 87) is evaporated to dryness. The organic matters possibly present are destroyed by means of nitric acid, or aqua regia, and the residue taken up with water. The solution so obtained is then examined for metals (including potassa, which salt has not been introduced into this portion of the fluid in any of the preceding operations) by the usual methods.[93]
(7). The soluble portion of the suspected materials having been thoroughly tested, the undissolved substances remaining on the filter are next examined.
(1). The organic matter present is first destroyed by treatment with aqua regia. The fluid is then evaporated to dryness, and the residue heated until the nitric acid is entirely expelled; the escaping vapors being collected in a cold receiver. The residue is next taken up with water, the solution filtered, and sulphuric acid added. Should a precipitate of sulphate of lime, sulphate of baryta or sulphate of strontia form, it is separated from the fluid and further examined. The filtered solution is then introduced into Marsh's apparatus, sodium amalgam being employed for generating the hydrogen, and tested for arsenic and antimony by means of the reactions previously given.
(2). Whether one of the above poisons be discovered or not, the still acid fluid is removed from the flask, a current of chlorine conducted through it for several hours and the solution then examined for mercury by Flandin and Danger's method. In case mercury is found it could scarcely have originated from the metal in Marsh's apparatus, as this would not be attacked by cold dilute sulphuric acid: however, to remove all doubts, the test should be repeated with a portion of the substances reserved for the examination by the determinative tests.
(3). Whatever have been the results of the above examinations, it is still to be ascertained if the fluid, which has been successively treated by Marsh's and Flandin and Danger's methods, does not contain other metals. This is accomplished by means of the ordinary reactions.[94]
The examination is conducted in precisely the same manner as in the preceding case, excepting that the materials are first acidulated with oxalic or tartaric acids. Particular attention should be given to the search for soda, potassa, lime, baryta and strontia, and the determinative tests subsequently applied according to the indications obtained.
In many instances the tests we have termed indicative become determinative in their character. This is the case when the isolation of an alkaloid or a metal (unless mercury be found under the circumstances already mentioned) is accomplished; the results obtained are then conclusive. If, on the other hand,—not being able to separate either an alkaloid or a metal—upon saturating the originally acid fluid with potassa, or soda, the salts of these bases have been found in abundance, there is reason to infer that the poisoning has been caused by an acid; or, if, after the neutralization of the originally alkaline solution with an acid, potassa or soda are discovered in a large quantity, poisoning by an alkali is indicated.
In case the fluid is neutral, but more or less colored and odoriferous, and iodides or bromides are detected, we may justly suspect that the poisoning has been caused by the reception of iodine or bromine.
According to the indications furnished, iodine, bromine, one, or all of the acids, the caustic alkalies, etc., are then detected by means of the methods to be employed in cases where the expert has a clew to the poison present. In this manner,[95] the presence of potassa and soda, and of bromine and iodine, even in mixtures, is easily ascertained. It only remains to mention the course to be pursued when suspicion exists that poisoning has been caused by the administration of a mixture of several acids. The suspected materials are boiled with water, and alcohol added to the solution in order to coagulate the animal matters. The solution is next filtered, the filtrate placed in a retort provided with a receiver and distilled until the residual portion acquires a pasty consistency. In this way, the acids present are separated into two classes: (a) those that are sufficiently volatile to have passed into the receiver, such as, acetic, nitric, hydrochloric and sulphuric acids (the latter acid will only be partially volatilized); and (b) those that remain in the retort. The former are detected by examining the distillate as previously directed.
The residue remaining in the retort is treated with absolute alcohol, the fluid filtered, and a solution of acetate of lead added to the filtrate: sulphuric, phosphoric and oxalic acids, if present, are precipitated. The precipitate is suspended in water and decomposed by means of sulphuretted hydrogen. The acids contained are now set free, and are detected by applying the tests already mentioned.
If there be reason to suspect the presence of both sulphuric and oxalic acids, the distillation is discontinued after a short time. The two acids are dissolved by shaking the moderately concentrated fluid remaining in the retort with ether, and, upon evaporating the solution, will be obtained in a state suitable for examination. Oxalic acid is then detected by means of sulphate of lime; sulphuric by means of oxalate of baryta.
The above examinations would fail to effect the detection of phosphorus, and it is necessary to examine a separate portion of the original substance for this body.[96]
A criminal, in order to conceal his identity, may change the color of the hair and beard by artificial means; either to a darker shade, in case they were naturally of a light color, or, to a lighter hue, if they were originally dark, and the chemical expert may be called upon to detect this artificial coloration, and restore the original color of the hair.
It may also happen, that portions of hair still adhere to the clots of blood sometimes found on an instrument which has been employed in the commission of a crime, and consequently the question may arise as to the nature of the hair, whether it be human or animal.
The mode of examination necessary when the hair has been blackened is different from that used when it has been decolorized.[97]
As various methods of dyeing hair black are in use, the means of restoring the original color differ. The following are the methods most usually employed in dyeing:
1º. The hair is well rubbed with a pomade, in which finely pulverized charcoal is incorporated. This preparation, which is sold under the name of "mélaïnocome," possesses the disadvantage of soiling the fingers and clothing, even for several days after its application.
2º. The hair is moistened with a dilute solution of ammonia, and a perfectly neutral solution of a bismuth salt (chloride or nitrate) is then applied. It is subsequently washed, and allowed to remain in contact with a solution of sulphuretted hydrogen.
3º. The same operation is performed, a lead compound being substituted for the bismuth salt.
4º. A mixture of litharge, chalk, and slacked lime is applied, and the head covered with a warm cloth. The hair is afterwards washed, first with dilute vinegar, then with the yolk of an egg.
5º. The hair is first cleansed with the yolk of an egg, and then moistened with a solution of plumbate of lime; or,
6º. It is moistened with a solution of nitrate of silver, to which a quantity of ammonia sufficient to dissolve the precipitate first formed has been added.
The first method merely causes a mechanical admixture of a coloring matter with the hair. In the four succeeding processes, a black metallic sulphide is produced; either by the subsequent application of a solution of sulphuretted hydrogen, or by the action of the sulphur normally present in the hair.[98]
In the last method, the formation of sulphide of silver doubtless occurs; but the principal change that takes place is probably due to the action of light, which, as is well known, decomposes the salts of silver.
In order to restore the original color to hair which has been treated with "mélaïnocome," it is only necessary to dissolve in ether the fatty matters present, and then remove the charcoal by washing with water.
In case the hair has been dyed by means of a bismuth or lead salt (as in methods 2, 3, 4 and 5), it is immersed for several hours in dilute hydrochloric acid: the metal present dissolves, as chloride, and the original color of the hair is rendered apparent. It then remains to detect the metal dissolved in the acid solution, in order to establish, beyond doubt, the fact that a dye has been employed. This is accomplished by means of the methods used for the detection of metals in cases of supposed poisoning.
If, finally, an ammoniacal solution of nitrate of silver has been employed to cause the coloration, the hair is immersed, for some time, in a dilute solution of cyanide of potassium, and the fluid subsequently examined for silver. In case a portion of the salt has been converted into the sulphide, it will be difficult to restore the original color, as the removal of this compound is not easily effected.
Black hair can be bleached by means of chlorine-water, the various shades of the blonde being produced by the more or less prolonged action of the reagent. In this case, the odor of chlorine is completely removed only with great difficulty, and the hair is rarely uniformly decolorized.[99] The expert may therefore be able to observe indication that will greatly assist him in arriving at a definite conclusion. The hair should be carefully examined up to the roots: if several days have elapsed since the decolorization has been performed, the lower portion of the hair will have grown and will exhibit its natural color. No method has yet been proposed that restores the original color to bleached hair. It is very possible, however, that this end would be attained by allowing nascent hydrogen to act upon the decolorized hair. For this purpose, it would be necessary to immerse it in water containing some sodium amalgam, and slightly acidulated with acetic acid.
In examinations of this character use is made of the microscope. The hair to be examined is suspended in syrup, oil, or glycerine and placed between two thin glass plates. Human hair is sometimes cylindrical; sometimes flattened. It consists either of a central canal, or of a longitudinal series of oblong cavities which contain oily coloring matter, and possesses the same diameter throughout its entire length. The brown hair of the beard and whiskers, medium-sized chestnut hair, the hair of a young blonde girl, and the downy hair of a young man possess respectively a diameter of 0.03 to 0.15; 0.08 to 0.09; 0.06; and 0.015 to 0.022 millimetres. These exhibit on the surface slightly projecting scales, which are irregularly sinuous at the border, separated from each other by a space of about 0.01 m.m., and are transparent, whatever may be their color.
The hair of ruminants is short and stiff, and is characterized by containing cavities filled with air. Wool, however,[100] forms an exception, as it consists of entire hairs, homogeneous in appearance and possessing imbricated scales, which bestow upon it the property of being felted.
The hair of the horse, ox and cow never exceeds 12 m.m. in length, and is tapering, its diameter gradually diminishing from the base. It is perfectly opaque, and does not appear to possess a central canal; has a reddish color, and frequently exhibits lateral swellings, from which small filaments occasionally become detached, in the same manner as a twig separates itself from the parent branch.
The examination of fire-arms is sometimes useful in determining the date at which a weapon has been discharged or reloaded. The methods used in examinations of this nature vary, as the weapon under inspection is one provided with a flint or an ordinary percussion lock. The value of the tests employed is also affected by the kind of powder used; i. e., whether common gunpowder, gun-cotton or white gunpowder (prepared by mixing yellow prussiate of potassa, chlorate of potassa and sugar) has been taken.
In case the weapon has been wiped or exposed to moisture subsequent to its seizure, it is impossible to form any conclusion as to the date of its discharge, etc. It is therefore advisable, upon receiving the weapon, to carefully wrap the[101] lock in a woollen cloth, and to close the barrel. The exterior of the gun is at first submitted to a careful examination, and notice taken of the approximate thickness of any existing rust spots. The fire-pan and adjacent portion of the barrel are also examined by aid of a magnifying glass, especial attention being given to the detection of traces of a moist and pulverulent incrustation of a greyish or blackish color, formed by the combustion of the gunpowder, and of crystals of sulphate of iron. If the weapon is loaded, the wad is withdrawn and the color of its cylindrical portion and of the powder, as well as the size of the ball or shot, noted.
This preliminary examination ended, the barrel and fire-pan are separately washed with distilled water, and the washings passed through filter paper which has previously been well washed, first with pure hydrochloric acid, then with distilled water. The filtrate is next divided into three portions, and these separately examined for: (1) sulphuric acid, by addition of chloride of barium; (2) for iron, by oxidizing the salts contained in the fluid with a few drops of nitric acid and adding a solution of ferrocyanide of potassium, the presence of iron being indicated by the formation of a blue coloration, or a blue precipitate; and (3) for sulphides, by means of a solution of subacetate of lead.
If a bluish-black incrustation is discovered on the fire-pan or on the neighboring portions of the barrel, and both rust and crystals of sulphate of iron are absent, and the washings, which were originally of a light-yellow color, assume a chocolate-brown coloration upon the addition of solution of subacetate of lead, the gun has been discharged within two hours at the longest.
If the incrustation possesses a lighter color and traces of iron have been detected in the washings, but neither rust nor[102] crystals have been discovered on the barrel or fire-pan, the weapon has been discharged more than two, but less than twenty-four hours.
In case minute crystals of sulphate of iron and spots of rust are found, and the washings contain iron in a considerable quantity, the weapon has been discharged at least twenty-four hours, at the longest ten days.
If the quantity of rust found is considerable, but iron is no longer to be detected, the discharge of the gun occurred ten days, at the longest fifty days, previously.
If the weapon has been reloaded immediately after its discharge without having been previously washed, the portions of the wadding which have come in contact with the barrel will possess a greyish-black color during the first four days, the color gradually becoming lighter, until, at the fifteenth day, it turns grey and remains so permanently. In this case, the washings will contain sulphuric acid. The objection has been advanced to the last test that sulphuric acid might be discovered, even if the gun had not been discharged, if the paper of which the wadding was made contained plaster. M. Boutigny states, however, that this objection is untenable, if the wadding has not been moistened by the water introduced into the barrel.
In case the gun has been washed and dried before being reloaded, the cylindrical portion of the wadding possesses an ochre-yellow color up to the first or second day, assumes a decided red hue on the days following, and acquires a clear rusty color on the sixth day. During the fifth day the powder also possesses a reddish appearance, owing to an admixture of rust. Sulphuric acid is not present in the washings.
If the weapon has been reloaded immediately after being washed, the wadding possesses a greenish-yellow appearance for the first[103] few hours, and subsequently acquires a reddish color, as in the preceding case.
If, finally, the barrel has been washed with turbid lime-water, rust is still to be found and the wadding possesses the color mentioned above. The following colorations are also to be observed in case the gun has not been washed, or has been dried near a fire:
| BARREL DRIED NEAR A FIRE. | UNWASHED BARREL. | |
|---|---|---|
| After 1 day | slight reddish yellow color | greenish yellow color. |
| — 2 or 3 days | a little darker " | reddish-brown " |
| — 4 days | a redder " | reddish-brown " |
| — 5 or more days | a rusty-red " | rusty-red " |
At present weapons having flint-locks have almost entirely gone out of use and have been superseded by the ordinary percussion gun; these latter, in turn, are being gradually replaced by breech-loaders, charged with or without a metallic cartridge. The indications obtained in the preceding examinations by means of the fire-pan, will therefore disappear; the results given by the inspection of the barrel may possibly hold good. In regard to breech-loaders, all the useful indications furnished by the coloration of the wadding and powder fail to occur; the latter being enclosed either in a paper cylinder or in a copper socket.
The fact that gun cotton and white gunpowder are occasionally made use of, adds to the difficulty of obtaining reliable results by the mere inspection of a weapon. White gunpowder does not oxidize the gun, fails to give rise to any salt of iron, and possesses a white color; gun-cotton produces distinctive indications varying with its purity. Owing to these facts, it is evident that the method proposed by M. Boutigny is of no real[104] value, save in the rare instances where a gun provided with a fire-pan, and charged with ordinary powder, is under examination, and the question of the lapse of time since the discharge of a weapon must remain undetermined so far as scientific tests are concerned.
This class of examinations is particularly necessary when the crime of infanticide is suspected. As the complete incineration of a cadaver is a long and difficult operation, it frequently occurs that bones—partially or completely carbonized, but retaining their original form—are discovered by the careful examination of the ashes of the fire-place in which the combustion was accomplished.
When this is not the case and complete incineration and disaggregation have occurred, recourse must be had to the indications furnished by a chemical analysis. These indications are reliable, however, only when the certainty exists that bones of animals have not been consumed in the same fire-place; otherwise, the results obtained are entirely worthless, the reactions given by ashes of animal bones being identical with those produced by the ashes of a human body. Two tests are employed to detect the presence of bones in the residue left by the combustion of animal matter.
1. A portion of the ashes is placed in a silver crucible, heated with potassa, and the mass afterwards treated with cold water. If animal matter is contained in the consumed materials, cyanide of potassium will be present in the aqueous solution. In order to detect this salt, the fluid is acidulated with hydrochloric acid, and a solution of persulphate of iron added: the formation of a blue precipitate indicates the presence of the cyanide.[105]
2. The ashes are next examined for phosphate of lime. As wood, coal, and the other substances usually employed for heating purposes contain none or little of this salt, its detection in a notable quantity would lead to the inference that bones have been consumed. The ashes are allowed to digest for twenty-four hours with one-quarter of their weight of sulphuric acid. Water is next added to the pasty mixture, and the fluid filtered. If phosphate of lime be present, it is converted by this treatment into a soluble acid phosphate, which passes into the filtrate. Upon adding ammonia to the filtrate, a precipitate of neutral phosphate of lime is formed, neutral phosphate of ammonia remaining in solution. The fluid is again filtered, the filtrate acidulated with nitric acid, and then boiled with a solution of molybdate of ammonia likewise acidulated with nitric acid: in presence of a phosphate, a yellow precipitate, or at least a yellow coloration of the fluid, will be produced. It has been stated that the disengagement of sulphuretted hydrogen, upon treating the ashes with sulphuric acid, is an indication that the combustion of a human body has occurred; this reaction is, however, valueless, inasmuch as coal and certain vegetable ashes likewise evolve the gas when subjected to the same treatment.
Contracts, checks, etc., are frequently altered with criminal intent, either by erasing the portion of the writing over the signature and substituting other matter, or by changing certain words, in order to modify the signification of a sentence.
Writings are altered either by erasure or by washing. Erasure, although more easily executed, is seldom employed,[106] as it renders the paper thin in places, and in this way leaves effects apparent even to the naked eye, and, although the original thickness can be restored by application of sandarac or alum, these substances possess properties differing from those exhibited by paper, and may, moreover, be completely removed, thus exposing the thinning of the paper.
In case washing by means of chlorine has been resorted to, the sizing—which renders the paper non-bibulous, and which is only with difficulty replaced—may have been removed. Formerly paper was sized by immersion in a solution of gelatine; at present, however, a soap of resin, or wax, and alumina (a little starch being added) is more commonly used. In the latter case, the sizing is less easily removed by the action of water than when the gelatine preparation is employed; the detection of its attempted restoration is also a matter of less difficulty, as gelatine would be employed for this purpose, and this body possesses properties different from those exhibited by the substances normally contained in paper: iodine, for instance, which imparts a yellow color to gelatine, turns starch violet-blue. In order to detect the alteration of a writing, the following examinations are made:
1º. The paper is carefully examined in all of its parts, and in various positions, by aid of a lens. In this way, either thinned points, caused by erasure, or remaining traces of words, may possibly be discovered.
2º. The paper is next placed upon a perfectly clean piece of glass, and completely and uniformly moistened with water. The glass is then removed, and the transparency of the paper examined by aid of a lens. When uniform transparency is exhibited, and certain portions are neither more transparent nor more opaque than the rest of the paper, it is probable that erasure has not been attempted. If, on the other hand,[107] opaque points are observed, it is almost certain that letters have been erased, and sandarac, which is not affected by water, subsequently applied. In case transparent points are detected, there is reason to suspect that words have been removed, and the spots either left intact or afterwards coated with a substance soluble in water, such as alum.
3º. The paper is dried and the above operation repeated with alcohol of 87 per cent. Indications may now be observed which failed to occur in the treatment with water; as well as these latter confirmed. As alcohol dissolves sandarac, the points that formerly appeared opaque may now become transparent.
4º. The paper is again dried, then placed under a sheet of very thin silk-paper, and a warm iron passed over it. This operation frequently causes the reappearance of words that have been partially obliterated. It is also advisable—as suggested by M. Lassaigne—to expose the paper to the action of iodine vapors. If alteration has not been attempted, the paper will acquire an uniform color; yellow, if sized with gelatine; violet blue, if sized with the mixture of soap, resin and starch. When, on the contrary, a subsequent sizing of gelatine has been applied in order to mask the alteration—the paper having been originally sized with the above mixture—it will assume in some portions a yellow, in others a violet-blue color.
5º. It is ascertained whether the paper possesses an acid reaction. If so, its acidity may result from the presence of hydrochloric acid, in case the paper was washed with chlorine, or of other acids. Alum, used to disguise erasure, would also cause an acid reaction. The mere detection of acidity is, in itself, of little importance, as, in the manufacture of paper, the pulp is bleached by means of chlorine, and this reagent may not have been entirely removed by washing. If, however, the paper is[108] acid only in certain spots, and these points produce a red coloration upon blue litmus paper, having the form of letters, the indication is of value. In order to ascertain if this be the case, it is advisable, before wetting the paper, to slightly press it upon a sheet of moist litmus paper: the acid spots will then leave a reddish trace upon the latter.
6º. The manuscript under examination is again spread upon a glass-plate, and a solution of tannin (or preferably, a solution of ferrocyanide of potassium containing one per cent. of the salt, and acidulated with acetic acid) applied by means of a brush. If the original writing was executed with ordinary ink (which has as its base tannate of iron), and the washing has been but imperfectly performed, it is quite possible that a blue coloration will be produced by the action of the ferrocyanide. It is, however, often necessary to apply the above reagents several times before the original writing becomes apparent; indeed, in some cases months have elapsed before the reaction has occurred.
In case the alteration or destruction of the document is feared in the above test, it is well to previously provide the court with a certified copy, and then proceed with the examination.
7º. If the paper possesses a friable appearance, it has possibly been washed with sulphuric acid. This property may however originate from other causes, and the presence of the acid should be confirmed by washing the document with distilled water, and adding a solution of chloride of barium to the washings. The precipitate should form in a considerable quantity, as a slight cloudiness could be due to sulphates contained in the water used in the preparation of the pulp.
If much sulphuric acid be present, it may be so concentrated by heating as to cause the carbonization of the paper.[109]
8º. It is also well, should washing with sulphuric acid be suspected, to ascertain, by aid of a lens, if the filaments on the surface of the manuscript possess an inflated appearance. This would be caused by the escape of carbonic acid, originating from the action of sulphuric acid upon the carbonates contained in the water used in the manufacture of the paper.
9º. Old ink is more difficult to remove than new, and it is therefore sometimes possible to cause the reappearance of old writings, over which words have been subsequently written. For this purpose, a solution containing 50 per cent. of oxalic acid is applied with a fine brush over the suspected points. As soon as the ink disappears, the acid is immediately removed by washing with water, and the paper dried. Upon now repeating the operation, the presence of a former writing may be detected after the complete disappearance of the words last written.
10º. According to M. Lassaigne, when the same ink has not been used throughout a document, washing with dilute hydrochloric acid will demonstrate the fact. This acid, while causing the gradual obliteration of characters written with ordinary ink—the shade of the paper not being altered—produces a red color, if ink containing log-wood has been employed, and a green coloration, in case the ink used contained Prussian blue.
The expert may possibly be called upon to give evidence as to the existence of a "trompe-l'oeil;" as was the case in the trial of M. de Preigne, which took place at Montpelier in 1852. A "trompe-l'oeil" consists of two sheets of paper, glued together at the edges, but having the upper sheet shorter than the other which therefore extends below it. This species of fraud is executed by writing unimportant matter on the[110] uppermost sheet, and then obtaining the desired signature, care being taken that it is written on the portion of the paper projecting below. The signature having been procured, it is only necessary to detach the two sheets in order to obtain a blank paper containing the signature, over which whatever is desired can be inserted. The trial referred to above, was in reference to a receipt for 3,000 francs. The expert, upon placing pieces of moistened paper upon the suspected document, noticed that they adhered to certain points, and that these formed a border around the paper but passing above the signature. The fraudulency of the act was thus established, and so recognized by the court, although the accused was acquitted by the jury.
Numerous means have been proposed, in order to render the falsification of documents a matter of difficulty. The most reliable of these is the use of "Grimpe's safety-paper," containing microscopic figures, the reproduction of which is impossible. Unfortunately, up to the present, the government has adopted methods less sure.
Sympathetic inks are those which, although invisible at the time of writing, become apparent by the application of certain agents. They are of two classes: those which are rendered visible by the mere application of heat, such as chloride of cobalt, or the juice of onions; and those which are brought out only by the action of a reagent. The inks of the second class most frequently used are solutions of acetates of lead, and other metals which give a colored sulphide when treated with sulphuretted hydrogen. Characters written with a solution of ferrocyanide of potassium acquire a blue color, if[111] washed with a solution of perchloride of iron. It is scarcely necessary to add that the latter solution can be used as the ink, and the ferrocyanide as the developer.
When the presence of characters written with a sympathetic ink is suspected, the document is examined as follows:
1. The paper is at first warmed: if the ink used is of the first class, the characters will now become legible; otherwise the examination is continued as below.
2. The paper is exposed to the action of steam, in order to moisten the ink present (care being taken to avoid dissolving the characters), and a current of sulphuretted hydrogen allowed to act upon it. If the ink used consists of a lead, bismuth, or gold salt, a black coloration will ensue; if salts of cadmium or arsenic were employed, the characters will acquire a yellow color; if, finally, a salt of antimony was used, a red coloration will be produced.
3. If no coloration was caused by the action of sulphuretted hydrogen, it is probably that either a solution of ferrocyanide of potassium or a persalt of iron has been resorted to. Each of these solutions is separately applied on a small portion of paper by means of a brush, and notice taken if the characters become visible. The solution that produced the change is then applied over the entire sheet.
4. In case only negative results were obtained in the preceding operations, it must not yet be concluded that a sympathetic ink has not been used, although we are left without further recourse to chemical tests. Numerous organic compounds may have been resorted to, the detection of which is almost impossible; moreover, if a mistake was made in regard to the preparation supposed to have been used, the reagents employed for its detection may render the discovery of another ink absolutely impossible. It is therefore often neces[112]sary to apply mechanical tests. For this purpose, the paper is spread upon a glass plate, uniformly moistened with water, and a second plate placed over it: if the characters were written with a pulverulent substance suspended in water or mucilage, they may often be observed upon examining the transparency of the paper. In case the substance used is both colorless and soluble, the detection of the written characters will be more difficult; still, indelible traces may possibly have been left by the pen. If, however, the ink employed is a colorless and transparent organic compound of rare occurrence, and was applied with a fine pencil-brush which failed to affect the paper, it must be acknowledged that little or nothing can be definitely determined as to its presence or absence.
In all civilized countries a fixed standard for coins and precious alloys is established by law, in order to prevent the perpetration of frauds which would be of serious injury to the public welfare. The substitution of coins consisting of an alloy inferior in value to the standard fixed by law, is too advantageous a fraud not to be often attempted.
Coins are most frequently altered by clipping; by stuffing, that is, by boring the coin and inserting an alloy of small value; by doubling, which operation consists in covering its face with two thin laminæ taken from a genuine coin; and by applying a coating of gold or silver by means of electro-plating.
In order to ascertain if a coin has been counterfeited, its weight should at first be determined. If it has been clipped, or consists of an alloy possessing a density less than that of silver or gold, the fact is immediately demonstrated by its decreased gravity.[113]
The coin is further tested by throwing it down upon a hard substance: gold and silver give a ringing sound, whereas the majority of other metals produce a dull sound.
The result obtained by this latter test often fails to be reliable. A skilful counterfeiter may prepare an alloy equally sonorous and heavy as silver or gold; in fact, M. Duloz exhibited to the author an alloy, prepared by him, possessing the density, sonorousness and lustre of silver; the composition of which, for obvious reasons, has not been published.
In instances of this nature the fusibility of the coin should be determined, and the result obtained compared with the melting point of the legal alloy, or, this failing, a chemical analysis executed. In order to perform the latter test, the coin under examination is boiled with nitric acid: all metals are dissolved, with exception of gold and platinum, which remain unaltered, and tin and antimony, which are converted respectively into metastannic and antimonic acids. The fluid is filtered, the insoluble residue well washed, and then boiled with hydrochloric acid, which dissolves the metastannic and antimonic acids. The solution is again filtered, and the second residue dissolved in aqua regia. The metals dissolved in the several filtrates are then detected, either by the processes previously given for the detection of metallic poisons, or by the more complete methods contained in works on chemical analysis. This qualitative test is, however, insufficient, in case the falsification consisted in merely diminishing the proportions of the valuable metals contained in the alloy, without changing its qualitative composition: it is then necessary to execute a quantitative estimation of the metals present. As this operation requires considerable practice and the methods employed are to be found in all treatises on quantitative analysis, we will not reproduce them here.[114]
We will next enumerate the methods employed in the detection of the principal adulterations to which flour, bread, oils of seeds, milk, wines, vinegar and the sulphate of quinine are subjected. These researches, united with those preceding, fail to embrace all the diverse examinations which the chemical expert may be expected to execute; but we do not claim to foresee all the contingencies that may arise, and will describe the steps to be pursued in instances which are anticipated, at the same time indicating general methods applicable to cases not here included.
The adulterations to which flour and bread are exposed usually consist in adding damaged or an inferior grade of flour to wheaten flour, or in disguising the presence of a poor quality of flour by the addition of mineral substances, such as: plaster, chalk, lime, alum, and sulphate of copper.
Good flour has a white color, possessing a slightly yellow tinge, but is entirely free from red, grey or black specks. It is soft to the touch and adheres to the fingers, acquiring, when compressed in the hand, a soft cushion-like form. If mixed with water, it forms an elastic, homogeneous, but slightly coherent dough, which can be extended out in thin layers.
Flour of an inferior quality possess a dull white color, and does not assume the cushion-like condition mentioned above, when pressed in the hand, but escapes between the fingers: the dough formed is of a poorer quality.
Flour which has been damaged by moisture has a dull or[115] reddish-white hue, and possesses a mouldy, or even a noxious, odor, as well as a bitter and nauseous taste which produces a marked acid sensation in the throat. Occasionally the presence of moisture causes the growth of fungi, the introduction of which in the digestive organs would cause serious results.
The constituents of pure flour are:
In the process of bread-making, the gluten undergoes fermentation by the action of the leaven and liberates carbonic acid, which causes the dough to become porous and swell up, or, as it is termed, to rise. Bread contains the same substances as flour, but gluten and starch are present in a state that does not admit of their separation by mechanical means, and glucose, if present at all, exists in a smaller quantity: the proportion of dextrine and water is, on the other hand, considerably increased. The bread of the Paris city bakeries contains 40 per cent. of water—the crumb, which forms 5/6 of the weight of the bread, containing 45 per cent.; the crust, which constitutes the remaining 1/6, containing 15 per cent. In army bread 43 per cent. of water are contained—the crumb, which constitutes 4/5 of the weight of the bread, holding[116] 50 per cent.; the crust which forms the remaining 1/5, containing 15 per cent.
The addition of common salt naturally increases the proportion of ash left upon calcining bread.
Water is contained in stale bread in the same quantity as in fresh bread; but exists in a modified molecular condition: upon heating stale bread, it acquires the properties of fresh bread.
The following substances are used in the adulteration of wheaten flour:[P]
In order to detect these substances, the gluten, the starch, and the ash are separately examined.
In order to separate the gluten, two parts of the flour to be examined and one part of water are mixed into a paste,[117] and this is placed in a fine linen sack, in which it is kneaded under a stream of water so long as the washings have a turbid appearance: these are preserved. The gluten obtained from good wheaten flour possesses a light-yellow color; emits a stale odor; and spreads out, when placed in a saucer. In case the flour has been too strongly heated in the grinding, or otherwise badly prepared, the gluten is granulous, difficult to collect in the hand, and somewhat resembles flint-stone in appearance.
Gluten prepared from a mixture of equal parts of wheat and rye is adhesive, blackish, without homogeneousness, spreads out more readily than pure wheaten gluten, separates easily and adheres somewhat to the fingers.
Gluten obtained from a mixture of wheat and barley is non-adhesive, of a dirty reddish-brown color, and appears to be formed of intertwined vermicular filaments.
Gluten formed from a mixture of equal parts of wheat and oats has a blackish-yellow color and exhibits, at the surface, numerous small white specks.
The gluten from a mixture of wheat and corn has a yellowish color, is non-adhesive, but firm, and does not readily spread.
Gluten prepared from a mixture of wheat and leguminous flour is neither cohesive nor elastic, and, if the proportion of the latter present be considerable, can be separated and passed through a sieve, like starch.
The gluten obtained from a mixture of equal parts of wheat and buckwheat flour is very homogeneous, and is as easily prepared as the gluten from pure wheaten flour. It possesses when moist a dark-grey color; which changes to a deep black upon drying. The proportion of gluten in flour is exceedingly variable: good flour contains from 10 to 11 per[118] cent. of dry gluten; poor flour from 8 to 9 per cent. of moist gluten, equal to about one-third of its weight of the dry compound.
The washings of the flour are allowed to stand for some time in a conical-shaped vessel. As soon as the amylaceous matter has entirely settled to the bottom of the vessel, the greater portion of the water is decanted, and the residual mass brought upon a small filter and allowed to dry. The residue is then examined for potato and rice starch.
Potato starch. The grains of potato starch are much larger than those of wheaten starch. If a portion of the residue mentioned above is crushed in an agate mortar, the granules of potato starch present are ruptured, and their contents liberated; the wheaten starch remaining unaltered. The mass is then taken up with water, and the fluid filtered. If potato starch be present, the filtrate will acquire a blue color upon addition of an aqueous solution of iodine; otherwise, a yellow or violet-rose coloration is produced. It is necessary to avoid crushing the residue for too long a time, as the granules of wheaten starch would also become ruptured by prolonged comminution.
Besides the difference presented by potato starch in the size of the granules in comparison to those of wheaten starch, the former swell to ten or fifteen times the volume of the latter, when treated with a solution of potassa: wheaten starch granules are not affected by the treatment, if the solution used does not contain more than 2 per cent. of the salt. The results obtained by the above operation should be confirmed by a microscopic examination.[119]
A portion of the residue is moistened with solution of iodine, then carefully dried, and placed on the slide of a microscope. The mass is next moistened with a solution containing 2 per cent. of potassa, and examined. The addition of iodine causes the potato starch granules to acquire a blue color, and renders their shape and volume more easily perceptible; thus allowing the two varieties of starch to be readily distinguished. Fig. 13 represents the relative size of the granules as observed under the microscope.[Q]
The presence of potato starch in bread is also detected by crushing a small portion of the sample under examination on the glass, and then adding a few drops of the alkaline solution.
[120] Rice and Corn.—If rice or corn meal have been mixed with the flour, angular and translucent fragments (Fig. 14) are observed in the microscopic examination. Corn meal acquires a yellow color, if treated with dilute potassa solution.
Linseed and rye meals.—If linseed meal is moistened with an aqueous solution containing 14 per cent. of potassa and examined under the microscope, numerous minute characteristic granules, smaller than the grains of potato-starch, are observed. These possess a vitreous appearance, sometimes a reddish color, and usually form in squares or very regular rectangles. The test is equally applicable to bread. The detection of linseed and rye meals is simultaneously effected by exhausting the suspected flour with ether, then filtering the solution and allowing it to evaporate. If the flour contains rye, the oil left by the evaporation, when heated with a solution of mercury in concentrated nitric acid, is converted into a solid substance having a fine red color; but it remains unaltered, if entirely due to linseed. In case the oil becomes solidified, the mercury salt present should be removed by washing with water, the residue taken up with boiling alcohol of 36° B. and the solution filtered: upon evaporating the alcoholic filtrate, a residue is obtained consisting of the linseed oil present.
Buckwheat.—Flour adulterated with buckwheat is less soft to the touch, does not pack as easily, and passes more readily through a sieve than pure wheaten flour. It presents, here and there, blackish particles, due to the perisperm of the grain, and has a dirty-white color. As previously remarked, the gluten obtained from a mixture of buckwheat and wheaten flour possesses a grey or even a black color. The starch furnished by buckwheat flour exhibits polyhedral agglomerations, analogous to those presented by corn.
Darnel.—The use of darnel in the adulteration of wheaten flour may give rise to serious sanitary results. To effect its[121] detection, the flour to be examined is digested with alcohol of 35° B.: if the flour be pure, the alcohol remains limpid: it acquires a straw-yellow tint, due to traces of bran present, but—although a peculiar resin may be dissolved—the solution does not possess a disagreeable taste. When, on the contrary, darnel is present, the alcohol assumes a green tint, which gradually deepens, and possesses a bitter and nauseous taste; the residue, left by the evaporation of the tincture to dryness, has a greenish-yellow color, and a still more disagreeable flavor than the alcoholic solution.
Legumens.—Leguminous meals cannot be added otherwise than in small proportions to wheaten flour, owing to the rapidity with which they change the properties of the latter, and communicate to it their characteristic odor—noticeable upon treating the flour with a little boiling water. Their presence is also easily detected by the distinctive properties of the vegetable itself, and by the appearance of the amylaceous residue in the microscopic examination. In order to decide as to the presence of legumens, the washings containing the starchy matter of the flour, after the particles of gluten present have been separated by passing the fluid through a silk sieve, are divided into two portions. One portion is allowed to undergo fermentation, at a temperature of 18° to 20°: in case leguminous substances are not present, lactic fermentation occurs and the odor of sour milk is alone perceptible; if, on the other hand, legumens are contained in the fluid, rancid fermentation takes place, and an odor is emitted resembling that of decayed cheese. The remaining portion of the washings, after being decanted from the residue of amylaceous matter, is filtered and evaporated until a yellowish translucent pellicle appears upon its surface. The fluid is then again filtered from the coagulated albumen common to all flours, and the legumin[122]ous substances present coagulated by the addition, drop by drop, of acetic acid.
The leguminous deposit produced appears white and flaky; when examined under the microscope, it presents lamilla emarginated at the border; it is odorless and tasteless; when dried, it assumes a horny appearance; it is insoluble, both in water and alcohol, and does not become gelatinous when treated with boiling water; it is readily soluble in potassa and other alkaline solutions, from which it is precipitated upon addition of nitric, hydrochloric, acetic, oxalic, and citric acids; upon protracted boiling in water, it loses its property of being soluble in ammonia. The above tests having been applied, the residue containing the starch is next examined. For this purpose, a small portion is moistened with a little water, a few drops of iodine solution added, and the mixture placed on the side of the microscope: the bluish grains contained in the polyhedral and cellular envelope (Fig. 15) are easily recognized. The mixture on the glass may also be treated with an aqueous solution of potassa (containing 10 per cent. of the salt), or with dilute hydrochloric acid: these reagents dissolve the starch present, leaving the reticulated tissue intact. Should this examination fail to give a definite result, the remaining portion of the amylaceous residue is subjected to a sort of levigation, and the part most slowly deposited separated. In this portion the reticulated tissues of the leguminous substances present are contained, and, as they are comparatively free from foreign matters, their identification is a matter of comparative ease. In case the presence of reticulated tissue is indicated, it is still necessary to apply confirmatory chemical tests.
Meals prepared from beans, horse-beans, and lentils, contain a tannin which imparts a green or black color to salts of iron. The coloration is rendered very sensitive if a rather considerable quantity of the flour to be examined is passed through a silk sieve, and the remaining bran treated with a solution of sulphate of iron (ferrico-ferrous sulphate): the reaction immediately occurs, even if the sample contains but 10 per cent. of bean meal. The meals of horse-beans and of vetches acquire a red color, when exposed to the successive action of nitric acid and of ammonia vapors. In order to apply this test, the suspected flour is placed upon the edge of a capsule containing nitric acid, the latter heated, and, as a yellow coloration appears, the acid removed and replaced by ammonia. The capsule is then set aside: if the flour is adulterated with either of the above vegetables, reddish spots, which are easily perceptible by aid of a magnifying glass, are soon produced.
In case bread is to be examined, it is exhausted with water, the fluid passed through a sieve, the upper layer decanted, then evaporated, and the residue taken up with alcohol. The tincture so obtained is evaporated, and the second residuum treated with nitric acid and ammonia, as directed above. When meals prepared from beans, vetches, or lentils are heated on a water-bath with hydrochloric acid, diluted with three to four times its volume of water, a cellular tissue, possessing the color of wine-dregs, remains behind; flours of wheat, peas, and kidney-beans leave a colorless residue, when subjected to the same treatment.
Finally; the grains of the starch (fecula) of legumens possess a volume about equal to that of potato granules, and exhibit either a longitudinal furrow in the direction of their longer axis, or a double furrow arranged in a star-like form.[124]
Leguminous substances, and more particularly mineral salts, are detected by the examination of the ash left upon the incineration of the flour.
Detection of Legumens.—Pure wheaten flour furnishes an ash consisting of about 2 per cent. of its weight; whereas meals of legumens leave from 3 to 4 per cent. of their weight in ash. This difference is, however, too slight to furnish conclusive results; the analysis of the ash is also necessary. The ash of wheaten flour is non-deliquescent, dry, semi-fused, and chiefly consists of phosphates of potassa, soda, magnesia and lime, of sulphates, and of silica. The solution obtained by treating the ash with water has an alkaline reaction. The phosphates of the alkalies, present in the ash of wheat, exist in the state of pyrophosphates, and, as chlorides are absent, the addition of nitrate of silver to the aqueous solution of the ash produces a white precipitate, consisting entirely of pyrophosphate of silver, which is not affected by exposure to the light.
The ash of leguminous meals is deliquescent and soluble in water, forming a strongly alkaline solution, which contains both chlorides and neutral phosphates. The latter give a clear yellow precipitate with nitrate of silver. Upon adding a solution of this salt to the aqueous solution of the ash, a pale yellow precipitate, which turns violet if exposed to the light, is therefore produced.
Detection of mineral substances.—The principal mineral substances, that are fraudulently added to flour, are ground calcined bones, sand, lime, plaster, alum, and sulphate of copper. The two last named salts are almost invariably added in small quantities; alum renders the flour white, even when used in the proportion of one per cent.; sulphate of copper is added[125] to impart a good appearance to bread made from a damaged flour.
a. Ground bones (carbonate and phosphate of lime).—The washings of the gluten are placed in a conical vessel, and, after some time has elapsed, the clear supernatant fluid is removed by means of a syphon, a conical shaped deposit remaining on the bottom of the vessel: two hours later, the fresh layer of fluid that has formed is removed with a pipette. As soon as the residue becomes nearly solid, it is detached from the vessel, placed upon a fragment of plaster, and allowed to dry. The bones, being heavier than the amylaceous substances, are to be found in the apex of the cone formed by the residue. This is detached, and incinerated: in case the ash obtained contains phosphate and carbonate of lime, the addition of hydrochloric acid will cause effervescence, and, upon adding ammonia to the acid solution, a white precipitate will be formed. If the solution is then filtered and oxalate of ammonia added to the filtrate, a precipitate will be produced which, when heated to redness, leaves a residue of caustic lime possessing an alkaline reaction.
b. Sand.—As this substance possesses a much greater specific gravity than the usual constituents of flour, it is only necessary, in order to accomplish its separation, to repeatedly stir the flour with water, and remove the deposit at first formed, which, if consisting of sand, will be insoluble in acids, and will grate, when placed between the teeth.
c. Carbonates of lime and magnesia; vegetable ashes.—Carbonic acid is always evolved, upon treating flour with hydrochloric acid. If the base present be calcium, upon adding oxalate of ammonia to the filtered solution—which has previously been neutralized with ammonia—a white precipitate, possessing the properties mentioned above, will be formed; in case[126] the base is magnesia, the addition of oxalate of ammonia will fail to cause a precipitate, but upon adding solution of phosphate of ammonia to the fluid a granular precipitate of phosphate of ammonia and magnesia is produced; if, finally, the flour contains vegetable ashes—i. e. carbonates of the alkalies—bichloride of platinum will produce in the acid solution a yellow precipitate: the addition of vegetable ashes, moreover, would render the ash of the flour deliquescent and very strongly alkaline.
d. Lime.—In presence of lime, carbonic acid produces a white precipitate, when conducted into the filtered aqueous extract of the flour.
[127]e. Plaster.—The flour is boiled with water acidulated with hydrochloric acid, the fluid filtered, and lime detected in the filtrate by means of ammonia and oxalate of ammonia. The presence of sulphuric acid is indicated by the formation of a precipitate insoluble in acids, upon addition of solution of chloride of barium. Upon calcining the flour without access of air, sulphate of lime is converted into the corresponding sulphide: the residue of the calcination, when treated with hydrochloric acid, evolves sulphuretted hydrogen, and the lime present in the filtered acid solution is likewise precipitated by the addition of ammonia and oxalate of ammonia.
f. Alum.—A portion of the flour to be examined is treated with water, the fluid filtered, and the filtrate divided in two portions: in one, sulphuric acid is detected by means of chloride of barium; in the other, alumina by adding a solution of potassa, which gives with its salts a white gelatinous precipitate, soluble in an excess of the reagent.[R][128]
g. Sulphate of copper.—About 200 grammes of the bread under examination are incinerated; the ash treated with nitric acid; the mixture evaporated until it acquires a sticky consistence, and the mass then taken up with water. The aqueous solution is next filtered; an excess of ammonia and several drops of solution of carbonate of ammonia added; the fluid again filtered, the filtrate slightly acidulated with nitric acid, and divided into two parts. It is then ascertained if sulphuretted hydrogen produces in one portion of the solution a brown precipitate of sulphide of copper, and if solution of ferrocyanide of potassium produces in the other a reddish-brown precipitate of ferrocyanide of copper.[S]
Olive oil designed for table use is frequently adulterated with the oils of poppy, sesamé, cotton-seed, pea-nuts, and other nuts; olive oil, intended for manufacturing purposes, is often mixed with colza and nut oils.
The tests used are of a rather unsatisfactory character. In all instances, when the chemist is called upon to pronounce as to the adulteration of an oil, it is necessary to execute comparative experiments with the pure oil, and with admixtures arbitrarily prepared: it is only when this is done that the indications obtained are of value.
a. The density of the oil is determined by means of a hydrometer (oleometer) provided with a scale giving the densities from 0.8 to 0.94, for the temperature of 15.° Pure olive oil possesses a specific gravity of 0.917; poppy oil one of 0.925; a mixture of the two, an intermediate density. Since the fixed oils are not definite chemical compounds, this test is seldom conclusive.
b. Two or three cubic centimetres of concentrated nitric acid, containing nitric peroxide in solution (or a solution of mercury in strong nitric acid), are added to the oil to be examined, as well as to a sample of pure olive oil. The two samples are then allowed to stand in a room where the temperature does not exceed 10.° The oleine of the olive oil is converted into solid elaidine, and the mixture after some time becomes sufficiently thick to remain in the vessel upon inversion. If the sample under examination is free from adulteration, it will solidify at the same time as the pure oil; whereas, the presence of one per cent. of poppy oil, or of other drying oils, suffices to retard the solidification for forty minutes.[129]
c. Fifteen grammes of the oil are mixed in a glass vessel with the same amount of strong sulphuric acid, the temperature of the two liquids being previously observed. The mixture is stirred with a thermometer, and the maximum temperature noted: pure olive oil produces an elevation of temperature of 37.°7; pure poppy oil, an elevation of 70.°5; and a mixture of the two an elevation of temperature intermediate between 37.°7 and 70.°5.
d. One volume of nitric acid of sp. gr. 1.33 is agitated with 5 grammes of the oil, and notice taken of the coloration produced after the lapse of five minutes. If the olive oil is pure, it acquires a pale green color; in case it is mixed with sesamé or nut oil, a deep-red color appears: poppy oil also communicates a reddish coloration, but one less deep than the preceding.
If an acid of sp. gr. 1.22 is taken, it is still less difficult to distinguish between sesamé, nut and poppy oils; the latter assumes, in this case, a pale yellowish-red color.
Pea-nut oil fails to exhibit a coloration; but can be recognized by its conversion into a white solid, when mixed with 1/5 of its volume of a solution of caustic soda of sp. gr. 1.34.
The chief adulterations are colza and nut oils. The latter is detected by means of the reaction with nitric acid, as described above. Colza oil is recognized by mixing 5 volumes of the sample to be examined, with 1 volume of sulphuric acid of sp. gr. 1.655: if colza or nut oils are present, a brown coloration ensues; under the same circumstances, pure olive[130] oil assumes a pale greenish hue. In case the sample acquires a brown color when treated with sulphuric acid, and a red coloration is produced by the addition of nitric acid, it contains nut oil; if sulphuric acid produces a brown coloration, and nitric acid fails to change it, the presence of oil of colza is indicated.
This oil is frequently adulterated with linseed oil. The reactions exhibited by these oils are nearly identical, and the detection of the admixture is extremely difficult. It is advisable to mix the suspected oil with sulphuric acid, notice being taken of the elevation of temperature produced, and to treat it with nitric acid and with dilute potassa solution, subjecting, at the same time, an artificial mixture of the two pure oils to the same treatment, and comparing the results obtained.
Among alimentary substances probably no article is subjected to more adulteration than tea. The sophistications practised may be conveniently divided into three classes:
1. Additions made for the purpose of giving increased bulk and weight, which include foreign leaves and exhausted tea-leaves, and also certain mineral substances, such as metallic iron, sand, brick-dust, etc.
2. Substances added in order to produce an artificial appearance of strength in the tea decoction, catechu, or other bodies rich in tannin, and iron salts being chiefly resorted to for this purpose.
3. The imparting of a bright and shining appearance to the tea by means of various coloring mixtures or "facings,"[131] which adulteration, while sometimes practised upon black tea, is much more common with the green variety. This sophistication involves the use of steatite (soap-stone), sulphate of lime, China clay, Prussian blue, indigo, turmeric, and graphite; chromate of lead and copper salts being but very rarely employed. The compound most frequently used consists of a mixture of soap-stone (or gypsum) with Prussian blue, to which a little turmeric is sometimes added.
Genuine tea is the prepared leaf of Thea sinensis. It contains: moisture, 6% to 10%; theine, 0.4% to 4.0%; tannin, (green) 20%, (black) 10%; ash, 5% to 6%; soluble extractive matters, 32% to 50%; and insoluble leaf, 47% to 54%.
The presence of foreign leaves, and, in some instances, of mineral adulterants, in tea is best detected by means of a microscopic examination of the suspected sample. The genuine tea-leaf is characterized by its peculiar serrations and venations. Its border exhibits serrations which stop a little short of the stalk, while the venations extend from the central rib, nearly parallel to one another, but turn just before reaching the border of the leaf (see Fig. 16). The Chinese are said to employ ash, plum, camellia, velonia, and dog-rose leaves for admixture with tea, and the product is stated to be often subjected in England to the addition of the leaves of willow, sloe, beech, hawthorn, elm, box-poplar, horse-chestnut, and fancy oak (see Figs. 17, 18, and 19). For scenting purposes chulan flowers, rose, jasmine, and orange leaves are frequently em[132]ployed. In the microscopic examination the sample should be moistened with hot water, spread out upon a glass plate, and then submitted to a careful inspection, especial attention being given to the general outline of the leaf and its serrations and venations. Most foreign leaves will, in this way, be identified by their botanical character. The presence of exhausted tea-leaves may also often be detected by their soft and disintegrated appearance. If a considerable quantity of the tea be placed in a long glass cylinder and agitated with water, the coloring and other abnormal bodies present frequently become detached, and either rise to the surface of the liquid as a sort of scum or fall to the bottom as a deposit. In this way Prussian blue, indigo, soap-stone,[133] gypsum, sand, and turmeric can sometimes be separated and subsequently recognized by their characteristic microscopic appearance. The separated substances should also be chemically tested. Prussian blue is detected by heating with a solution of caustic soda, filtering, and acidulating the filtrate with acid, and then adding chloride of iron, when, in its presence, a blue color will be produced. Indigo is best discovered by its appearance under the microscope; it is not decolorized by caustic alkali, but it dissolves in sulphuric acid to a blue liquid. Soap-stone, gypsum, sand, metallic iron, etc., are identified by means of the usual chemical tests. A compound, very aptly termed "Lie-tea," is often met with. It forms little pellets consisting of tea-dust mixed with foreign leaves, sand, etc., and held together by means of gum or starch. This, when treated with boiling water, falls to powder. In the presence of catechu the tea infusion usually becomes muddy upon cooling; in case iron salts have been employed to deepen the color of the liquor, they can be detected by treating the ground tea-leaves with acetic acid and testing the solution with ferrocyanide of potassium. Tea should not turn black upon immersion in hydrosulphuric acid water, nor should it impart a blue color to ammonia solution. The infusion should be amber-colored, and not become reddened by the addition of an acid.
TEA ASSAY.
In the following tea assay proper the estimation of theine is not included. The processes suggested for this determination are rather unsatisfactory; and there appears, moreover, to exist no direct relation between the quality of tea and the proportion of theine contained. The tests here[134] mentioned, in connection with those already given, will, it is believed, usually suffice to indicate to the analyst the presence of spent leaves, inorganic coloring matters, and other mineral adulterations.
Tannin.—A good process for the estimation of tannin in tea has been published by Allen (Chem. News, vol. xxix. p. 169 et seq.) A standard solution of lead acetate is prepared by dissolving 5 grammes of the salt in distilled water and diluting the liquid to 1,000 c.c. As an indicator, 5 milligrammes of potassic ferricyanide are dissolved in 5 c.c. of water, and an equal volume of strong ammonia-water added. The exact strength of the lead solution is to be determined by means of a solution of pure tannin of known strength. Two grammes of the tea to be tested are powdered, boiled with water, and, after filtering and thorough washing, the decoction is made up to a volume of 250 c.c.; 10 c.c. of the lead solution are now diluted with 90 c.c. of boiling water, and the tea infusion is gradually added from a burette until a few drops of the liquid, when filtered and added to a little of the indicator placed upon a porcelain slab, causes a pink coloration to appear; 125, divided by the number of c.c. of tea infusion found to be necessary to produce the pink color, will give directly the percentage of tannin in the sample examined. As previously stated, green tea contains 20% of tannin, and black tea 10%. In spent tea, however, only about 2% of tannin is present; and, although any tea deficient in this constituent could be fortified by the addition of catechu, its determination often affords indications of value.
The Ash—a. Total Ash.—5 grammes of the sample are placed in a platinum vessel and heated over a Bunsen burner until complete incineration has been accomplished.[135] The vessel is allowed to cool in a desiccator, and is then weighed as quickly as possible. In genuine tea the total ash should not be much below 5% or much above 6%, and it should not be magnetic; in "faced" teas the proportion of total ash is often 10% or 15%; in "lie-tea" it may reach 30%, and in spent leaves it may fall as low as 3%, the ash in this case being abnormally rich in lime salts and poor in potash salts. Tea-dust sometimes contains 10% of total ash without necessarily being considered bad in quality. In the proposed United States tea-adulteration law (1884) a maximum of 8% of total ash is allowed for tea-leaf.
b. Ash insoluble in water.—The total ash obtained in a is washed into a beaker and boiled with water for a considerable time. It is then brought upon a filter and the insoluble residue washed, dried, ignited, and weighed. In unadulterated tea it will not exceed 3% of the sample taken.
c. Ash soluble in water.—This proportion is obtained by deducting ash insoluble in water from the total ash. Genuine tea contains from 3% to 3.5% of soluble ash, or at least 50% of the total ash, whereas in spent or exhausted tea the amount is often but 0.5%.
d. Ash insoluble in acid.—The ash insoluble in water is boiled with dilute hydrochloric acid and the residue separated by filtration, washed, ignited, and weighed. In pure tea the remaining ash ranges between 0.3% and 0.8%; in "faced" teas, or in teas adulterated by the addition of sand, etc., it may reach the proportion of 2% to 5%. Fragments of silica and brick-dust are occasionally to be found in the ash insoluble in acid.
The Extract.—Two grammes of the carefully-sampled tea are boiled with water until all soluble matter is dissolved,[136] water being added from time to time to prevent the solution becoming too concentrated. The solution is poured upon a tared filter, and the remaining insoluble leaf repeatedly washed with hot water until the filtered liquid becomes colorless. The filtrate is now diluted to a volume of 200 c.c., and of this 50 c.c. are taken and evaporated in a weighed dish over the steam-bath until the weight of the extract remains constant; its weight is then determined. Genuine tea affords from 32% to 50% of extract, according to its age and quality; in spent tea the proportion of extract will be greatly reduced.
Insoluble Leaf.—The insoluble leaf obtained in the preceding operation, together with the weighed filter, is placed in an air-bath and dried for at least eight hours at a temperature of 110° C.; its weight is then determined. In unadulterated tea the amount of insoluble leaf ranges between 47% and 54%; in exhausted tea it may reach a proportion of 75%.
It should be noted that in the foregoing estimations the tea is taken in its ordinary air-dried condition. If it be desired to reduce the results obtained to a dry basis, an allowance for the moisture present in the sample (an average of 8%), or a direct determination of the same, must be made.
The following tabulation gives the constituents of genuine tea so far as the ash, extract, and insoluble leaf are involved:
The table below may prove useful as indicating the requirements to be exacted when the chemist is asked to give an opinion concerning the presence of facing admixtures or of exhausted or foreign leaves in a sample of tea:
Note.—The British Society of Public Analysts adopt:
The chief constituents of milk are water, butter, caseine, lactose (milk-sugar), traces of albumen and mineral salts. Butter is present in the form of minute globules, held in suspension; the caseine, for the greater part, is in solution, only a small portion being present in an insoluble suspended condition. In milk only a few days old, the colostrum (the milk secreted during the first few days after parturition) consists largely of rather voluminous cellular conglomerations, containing a sufficient quantity of albumen to coagulate upon heating.
The normal density of milk is 1.030, water being 1.000; the density rising to 1.036, if the fluid has been skimmed.
Good milk contains, on an average, 3.7 per cent. of[138] butter; 5.7 per cent. of lactose, and leaves upon evaporation 12 to 14 per cent. of solid matters.[T] The most common adulteration of milk consists in the addition of water. This fraud is detected by means of an areometer (lactodensimeter) which gives directly the specific gravity of the fluid under examination. Should the density be much below 1.030, it is certain that water has been added. It does not, however, necessarily follow if it is about 1.030 that the milk is pure, since the gravity of the fluid, which would be increased upon skimming, could be subsequently reduced to 1.030 by the addition of water. The lactodensimeter, therefore, although useful in the detection of a simple admixture, fails to give reliable results if the fraud perpetrated is a double one; and a determination of the proportion of butter present is also usually necessary. Numerous methods have been proposed to accomplish this estimation. The most preferable of these, owing to the rapidity with which the operation is executed, is the use of the lactoscope (galactoscope). This instrument consists of a tube provided with a glass plate fitted at one end, and with a movable glass plate at the other extremity. A few drops of the milk to be tested are placed between the two plates, and the tube lengthened, by screwing out the movable plate, until the fluid no longer transmits the light of a candle placed at a distance of one metre. As the opacity of milk is[139] due to the butter present, it is evident that the proportion of this substance contained in the sample can be estimated by the relative distance which the plates have been separated.
The lactoscope possesses, however, but a limited degree of precision. M. Marchand substitutes to its use the following tests: A test-tube is graduated in three equal divisions, the upper one being subdivided into hundredths extending above, in order to determine accurately the correct volume of the fluid, expanded, as it is, by the temperature of 40°, at which the examination is executed. The first division of the tube is filled with milk, a drop, or two of strong potassa lye added, and the mixture well shaken: the second portion is then filled with ether, and the third with alcohol. The mixture is next again thoroughly agitated, and then exposed to a temperature of 40° in a water-bath. After standing for several hours, a layer of fatty matter becomes sufficiently separated to allow of measurement: but, as it contains some ether and as a small amount of butter may still be retained in the lower aqueous fluid, a correction of the results obtained is necessary. M. Marchand has compiled a table, which facilitates this correction (vide: Journ. de Pharm., Novembre 1854, and Bulletin de l'Académie de Médecine, Paris, 1854, xix., p. 1101).
Previously to the introduction of Marchand's apparatus, use was made of the lactometer, which consists simply of a graduated glass tube, in which the suspected milk is allowed to remain for 24 hours, at a temperature of 15°. After the lapse of this time, the cream present completely separates as a supernatant layer, the thickness of which indicates the quality of the sample taken.
M. Lacomte recommends the addition of glacial acetic acid, in order to cause the more rapid separation of the cream.
The estimation of the butter being accomplished, it is fre[140]quently needful to determine the amount of lactose present. For this purpose, recourse is had to Barreswil's method, based upon the reduction of cupro-potassic tartrate by milk-sugar in the presence of alkalies. A solution is prepared containing 40 grammes of pure crystallized sulphate of copper, 600 or 700 grammes of caustic soda lye of sp. gr. 1.12, and 160 grammes of neutral tartrate of potassa. The sulphate of copper and tartrate of potassa are previously dissolved separately in a little water, the three solutions united, and water added until the fluid acquires a volume of 1154.4 cubic centimetres. In order to standardize this test solution, a known weight of pure lactose is dissolved in water and the fluid added, drop by drop, from a graduated burette, to a small flask containing 10 cubic centimetres of the copper solution, diluted with 40 cubic centimetres of distilled water, and heated to boiling. At first a yellow precipitate forms, which gradually turns red, and is deposited on the bottom of the flask, leaving the solution colorless. As soon as the test solution is completely decolorized, the addition of the lactose solution is discontinued, and the weight of lactose corresponding to 10 cubic centimetres of the test fluid calculated from the quantity used. The standard of the test solution having been determined, the above operation is repeated, the milk under examination being substituted for the solution of pure lactose. The quantity of milk necessary to decolorize 10 cubic centimetres of the copper solution will evidently contain the same amount of lactose as the quantity of solution used in the preliminary test, and the actual amount of lactose present is very easily calculated. When an estimation of the solid matter contained in the milk is required, a known weight is evaporated to dryness over a water-bath, and the residue weighed. In performing this evaporation, the addition of a known amount of sand, or ground glass, is advisable.[141] The amount of ash present is determined by incinerating the residue left by the evaporation.
Foreign substances are sometimes added to milk, for the purpose of disguising the presence of an abnormal quantity of water, the principal of which are: chalk, bicarbonate of soda, emulsion of almonds, gum tragacanth, gum arabic, starch, flour, decoction of barley or rice, sugar, and cerebral substances. These bodies are detected as follows:
Chalk.—If chalk is contained in the milk, it readily subsides upon allowing the sample to remain at rest for some time in a flask, forming a deposit which effervesces when heated with hydrochloric acid, and dissolves to a solution, in which the characteristic properties of a lime salt can be recognized.
Bicarbonate of soda.—In presence of this compound the milk possesses a strongly alkaline reaction, furnishes a serum having a sharp and bitter taste, and leaves a residue of the salt upon evaporation.
Emulsion of almonds.—The milk has a specific gravity of at least, 1.033. If it is passed through a gauze, small opaque lumps are separated. When examined under the microscope, numerous minute globules, having a diameter of 1/400 of a millimetre, are observed, and, upon adding a few centigrammes of amygdaline to one or two grammes of the milk, the characteristic odor of bitter almonds is produced.
Gum tragacanth.—When shaken in a glass flask and allowed to rest, the milk deposits on the sides small transparent lumps, which usually present a slightly elongated or angular form.
Gum arabic.—The addition of alcohol produces an abundant white opaque precipitate.
Starch, flour, decoction of barley, rice, etc.—Upon boiling the[142] suspected milk, and adding tincture of iodine, the amylaceous substances present produce a blue coloration in the fluid.
Sugar.—If yeast is added, and the mixture allowed to stand for some time at a temperature of 30°, alcoholic fermentation ensues; under these circumstances, lactose does not undergo fermentation.
Cerebral substances.—Adulteration by these substances is probably of much less frequent occurrence than was formerly supposed. The admixture is detected by evaporating the milk to dryness, dissolving the residue in ether, evaporating the etherial solution, and fusing the second residue, which consists of fatty matters, with nitrate of potassa in a platinum crucible. The mass is then taken up with water, and chloride of barium added to the solution. If cerebral substances were contained in the milk, ether will dissolve the fatty matters present, the phosphorus of which is converted into a soluble phosphate by the calcination with nitrate of potassa and is thrown down as a white precipitate, upon the addition of a solution of chloride of barium. This test may be confirmed by a microscopic examination of the milk, when the peculiar appearance of cerebral matter will be detected.[U]
The most common adulteration to which wines are subjected is the addition of water: wines having a rich color are frequently mixed by the dealer with lighter wines, and the fraud consummated by adding water. The detection of this adulteration is somewhat difficult, as water is a normal constituent of wine. In Paris the following method is usually employed: As soon as the wine is confiscated, it is ascer[143]tained what kinds of wine are manufactured by the inculpated dealer, and a statement obtained from him, giving the proportions of alcohol, etc., contained in the various brands. A wine is then prepared, according to the information received, an estimation of the alcohol contained in the prepared sample made, and the results compared with those furnished by a similar examination of the suspected wine. In case the proportion of alcohol is less in the suspected wine than in the prepared sample, it is evident that a fraudulent adulteration has been committed. If, however, the quantity of alcohol is the same in both wines, it does not necessarily follow that the wine has escaped admixture, since this body may have been added after the adulteration with water. In addition to the estimation of alcohol, it is also necessary to determine the amount of cream of tartar (bitartrate of potassa) present, as the proportion of this salt would be sensibly decreased by the addition of alcohol and water to the wine. This fraud could, however, be disguised by subsequently adding the proper amount of cream of tartar.
It is also well to ascertain if two equal quantities of the prepared sample and the wine under examination require the same amount of solution of hypochlorite of lime for decolorization. In case the suspected wine has been adulterated, the quantity of hypochlorite solution used will be less than the amount necessary to decolorize the prepared wine. Foreign coloring matter may be added by the adulterator, but this fraud is easily detected by adding potassa to the sample: if its coloration is natural, a green tint is produced; whereas, if foreign matter has been introduced, the wine assumes various other colors upon the addition of the alkali.[V] [144]
The indications furnished by the above test are rendered valueless, if the wine has been artificially colored by the addition of the coloring matter of grape-skins; but the execution of this fraud would require some knowledge of chemistry, and fortunately adulterators, as a class, are deficient in this branch of science.
Another method for detecting the addition of water is based upon the fact that fermented liquors do not contain air in solution, but only carbonic acid; whereas, water dissolves oxygen and nitrogen. It is executed as follows:
The wine to be tested is placed in a flask, the delivery-tube of which is also filled, and heated; the evolved gas being collected in a tube filled with mercury. In case the wine is pure, the disengaged gas will be completely absorbed by potassa; if, on the other hand, water has been added, an unabsorbed residue, consisting of oxygen and nitrogen, will remain.
This test is useless in case water, through which a current of carbonic acid gas has been passed for a considerable time, has been employed. Under these circumstances, however, the presence of the gas would probably be detected by the taste[145] of the wine, as well as by the estimation just mentioned, since the sample would invariably contain a larger proportion of the gas than the standard with which it is compared; indeed, it would be almost impossible to prepare a solution which contained exactly the proportion of carbonic acid ordinarily present in wine.
It remains to mention the methods employed in determining the amount of alcohol and cream of tartar contained in wine.
The alcometrical method usually employed is based upon the difference in density possessed by pure alcohol and by mixtures of alcohol and water. Gay-Lussac has proposed an areometer (alcoholmeter), provided with a scale which directly indicates the proportion of alcohol contained in a mixture. As the indications furnished by this instrument vary with the temperature, and the scale is constructed on the basis of a temperature of 15°, a correction of the results obtained is necessary if the determination is made at other temperatures. Gay-Lussac has compiled a table which indicates at once the required correction; the following formula can also be used: x = c ± 0.4 t, where x is the quantity of alcohol present in the sample; c the degree indicated by the alcoholmeter, and t the number of degrees differing from the temperature of 15°: the second member of the formula is subtracted from, or added to the first, as the temperature at which the estimation is made is greater or less than 15°.[W]
In case the wine to be examined contains substances other than water and alcohol, which would affect its density, it is[146] necessary, before making use of the alcoholmeter, to distil the sample and subsequently examine the distillate, which will consist of a simple mixture of water and alcohol. Usually the distillation is discontinued as soon as one-third of the sample has passed over, and a quantity of distilled water, sufficient to render the volume of the mixture equal to the original volume of the wine, added to the distillate: the fluid remaining in the flask will be entirely free from alcohol. The addition of water to the distillate is not indispensable, but otherwise it is necessary to divide the degrees indicated by the alcoholmeter by 3, in order to reduce the result to the original volume of the wine taken.
M. Salleron offers for sale a small apparatus (Fig. 20) used in examinations of this character, consisting of a flask, closed with a gutta-percha cork, containing a tube which connects with a worm passing through a cooler. The flask is supported by an iron stand, and heated with a gas or spirit lamp.
In order to estimate the cream of tartar, the wine is evaporated to the consistency of an extract, alcohol of 82° B. added, and the residue obtained calcined in a crucible. The amount of salt present in the fused mass is then determined by the alkalimetric method, as directed in all works on quantitative analysis. The carbonate obtained from 1 gr. of cream of tartar exactly saturates 9.75 cubic centimetres of a solution[147] containing 100 grammes of sulphuric acid of 66° B., and 1800 grammes of distilled water.
The detection of toxical substances, often contained in wine, is accomplished by the methods described under the head of detection of poisons.
Vinegar is frequently adulterated with water, and occasionally sulphuric acid is added to artificially increase its acidity.
The ordinary reagents—such as chloride of barium, or nitrate of silver—are not adapted to the direct detection of sulphuric acid, or of other mineral acids, as sulphates and chlorides, which are as readily precipitated as the free acids, may also be present.
The following method, proposed by M. Payen, is usually employed:
Five centigrammes of starch (fecula) are added to a decilitre of table vinegar, the mixture boiled for 12 or 15 minutes, and, after the fluid has become completely cooled, a few drops of iodine solution added: dilute acetic acid does not affect starch, and, in case the vinegar is pure, a blue coloration is produced; if, on the other hand, even a minute quantity of a mineral acid be present, the starch is converted into dextrine, and the addition of iodine fails to cause a blue coloration.
The water present is indirectly estimated by determining the amount of acetic acid contained in the vinegar. This can be accomplished in different ways: either the quantity of a standard solution of an alkali, necessary to exactly neutralize a measured quantity of the vinegar, is ascertained, or[148] the vinegar is supersaturated with solution of baryta, the excess of the salt eliminated by conducting carbonic acid through the fluid, the precipitate removed by filtration, and the baryta salt in the filtrate precipitated by the addition of sulphuric acid. The second precipitate is then collected on a filter, washed, weighed, and the amount of acetic acid present calculated: this is done by multiplying its weight by 0.515.
Owing to the high price of this salt, it is frequently adulterated. The substances used for this purpose are: crystalline sulphate of lime, boric acid, mannite, sugar, starch, salicine, stearic acid, and the sulphates of cinchonine and quinidine. These bodies are detected as follows:
a. Upon slightly warming 2 grammes of sulphate of quinine with 120 grammes of alcohol of 21° B., the pure salt completely dissolves; if, however, starch, magnesia, mineral salts, or various other foreign substances are present, they are left as insoluble residues.
b. Those mineral substances that are soluble in alcohol are detected by calcining the suspected sample: pure sulphate of quinine is completely consumed; whereas, the mineral substances present remain behind as a residue.
c. In presence of salicine, the salt acquires a deep red color, when treated with concentrated sulphuric acid.
d. Stearic acid remains undissolved upon treating sulphate of quinine with acidulated water.
e. To detect sugar and mannite, the sample is dissolved in acidulated water, and an excess of hydrate of baryta added: a precipitate, consisting of quinine and sulphate of baryta, is[149] produced. Carbonic acid is then passed through the fluid, in order to precipitate the excess of baryta as insoluble carbonate, the fluid saturated with ammonia, to throw down the quinine which may have been re-dissolved by the carbonic acid, and the mixture filtered. If the salt be pure, no residue will be obtained upon evaporating the filtrate; a residue of sugar or mannite is formed, if these substances are present.
f. Sulphate of quinine invariably contains 2 or 3 per cent. of cinchonine, originating, not from a fraudulent admixture, but from an incomplete purification of the salt. One of the best methods for detecting the respective quantities of quinine and cinchonine, present in a sample of the sulphate, is the following: Several grammes of ammonia and ether (which has previously been washed with water) are added to one or two grammes of the salt under examination, the mixture thoroughly agitated, and then allowed to remain at rest. The supernatant etherial solution contains all of the quinine; the cinchonine, which is almost completely insoluble, both in water and ether, remaining suspended between the layers of the two fluids. The ether is next removed by means of a stop-cock funnel, evaporated to dryness, and the weight of the residue obtained determined. The operation is then repeated, the ether being replaced by chloroform in which both quinine and cinchonine are soluble. The residue, formed by the evaporation of the second solution, will be heavier than the first residue: the difference between the two weighings gives the weight of the cinchonine present.
g. The detection of the presence of sulphate of quinidine is based upon the difference in the solubilities of the oxalates of quinine and quinidine. Oxalate of quinidine is sufficiently soluble in cold water not to be precipitated by double decomposition when solutions of oxalate of ammonia and sul[150]phate of quinidine are mixed. Under the same circumstances, quinine is almost completely thrown down. The test is applied as follows:
The suspected salt is dissolved in water, a slight excess of oxalate of ammonia added, and the precipitate formed separated by filtration. If the salt be pure, the filtrate is scarcely rendered turbid by the addition of ammonia; when, however, sulphate of quinidine is present, it will be entirely contained in the filtrate, in which ammonia will produce an abundant precipitate.
This branch of legal chemistry formerly gave but very unreliable results. It is scarcely ten years since the reactions that are now regarded as only secondary and confirmative in their character, and far from conclusive, were the only ones in use: these are the tests based upon the presence of iron and albumen in the blood. Since then, great progress has been made in the methods employed. It must not be understood, however, that the question under consideration always admits of an easy and decisive solution: the stains are sometimes too greatly altered to be identified; but in cases where the distinctive reactions of blood can be produced, the real nature of the stains under examination can, at present, be determined with certainty.

The tests more recently introduced consist in the production of small characteristic crystals, termed haemin crystals, and in the use of the spectroscope. Crystals of haemin (first discovered by Teichman) are formed when dry blood is dissolved in concentrated acetic acid, and the solution evaporated to dryness: they are of a brownish-red color. Brücke first suggested an analytical method, based upon this property of[151] blood, which is equally characteristic and sensitive: It is only necessary to dissolve a minute portion of the matter to be examined (dried blood, or the residue left by the evaporation of the fluid obtained by treating the stain, or the dried blood, with cold water) in glacial acetic acid and evaporate the solution to dryness in order to obtain crystals of haemin, which can be readily recognized by means of a microscope having a magnifying power of 300 diameters. If the crystals originate from fresh blood, they appear as represented in Fig. 21; crystals from old blood are represented in Fig. 22.
The former possess a reddish-brown, the latter a lighter color.
The various methods now employed to produce haemin crystals were proposed by Hoppe-Seyler, by Brücke and by Erdman. Whichever process is used, the suspected stains are at first carefully separated from the material upon which they are deposited. If they are present on linen, or other fabrics, the stained portions, which always remain somewhat stiff, are cut off: they will present a reddish-brown color, in case the cloth is not dyed: if the stains are on wood, they are removed by means of a sharp knife; if on stone or iron, they are detached by scraping.
In case Hoppe-Seyler's method is used, the stains, separated as directed above, are macerated with a little cold water (warm water would coagulate the albumen present, and consequently[152] prevent solution taking place): the stains become soft, striae and brown or reddish clouds are observed, especially when the dried blood is fresh, and, at the same time, the objects upon which the stains were deposited are decolorized. Upon allowing the fluid obtained in this way to spontaneously evaporate on a watch-glass, a reddish brown or brownish residue is left, from which the crystals of haemin are prepared in the following manner: An almost imperceptible amount of common salt is added to the residue, then, six to eight drops of concentrated acetic acid, and the mass thoroughly mixed by stirring with a small glass rod. The mixture is at first heated over a small gas flame, then evaporated to dryness by the heat of a water-bath. If the stains were produced by blood, a microscopic examination of the residue will reveal the presence of haemin crystals. This method presents an objection: if the stained objects have been washed with warm water previously to the examination, the albumen will be coagulated, and the blood rendered insoluble; in this case, cold water will fail to dissolve anything, and the residue will not produce crystals when treated with acetic acid.
In order to remedy this difficulty Brücke operates directly upon the stained woven or ligneous fibre, or the matter removed from the stone or iron: The materials are boiled in a test-tube with glacial acetic acid, the fluid decanted or filtered, a trace of common salt added, and the liquid then evaporated on a watch-glass at a temperature between 40 and 80°. If the stains really originated from blood, haemin crystals will now be easily perceptible upon examining the residue obtained under the microscope.
The stained fabric, the matter removed from the stone or iron, or the residue left by the solution with which the stains have been treated, is placed on the glass, a trace of chloride[153] of sodium added, and the whole covered with a thin glass plate. A drop of acetic acid is then placed at the edge of the plates—between which it is soon introduced by capillary attraction—and the mixture allowed to rest in the cold for a few moments. The mass is next brought into solution by slightly heating, and is then evaporated by holding the plate at a considerable distance above a gas burner. The fluid is examined from time to time under the microscope: when it is sufficiently concentrated, crystals, presenting the appearance represented in Figs. 21 or 22, will be observed. These are especially well-defined, if an insoluble substance is also present between the plates—which prevents their adhering. The fluid collects by capillary attraction at the points of contact of the plates as a more or less colored layer, in which the crystals are deposited.
Should the above test fail to present distinctive indications at first, one or two fresh drops of acetic acid are introduced between the plates, and the examination is repeated. The result is not to be regarded as negative, until several trials have proved fruitless, as the stained portions are but slowly soluble, and crystallization may have been prevented by the too rapid evaporation of the acetic solution.
Haemin crystals, once seen, can hardly be confounded with other substances; still, it is well to identify them by confirming their insolubility in water, alcohol, and cold acetic acid, as well as their instantaneous solubility in soda lye.
The addition of common salt is ordinarily superfluous, as it is normally contained in the blood; but it is possible, if the stains were washed with warm water, that, in addition to the coagulation of the albumen, the solution of the salt may have taken place, in which case crystals will fail to form. The addition of salt is to remedy this possible contingency; albeit, the delicacy of the test is not affected, even if crystals of chlo[154]ride of sodium are produced, as these are easily soluble in water, and are readily distinguished from those of haemin by aid of the microscope.
The indications furnished by means of the spectroscope are less reliable than those given by the production of haemin crystals; moreover, the spectroscopic examination requires favorable weather for its execution. Still, the test should be employed in all possible instances. The course pursued is the following:
The aqueous fluid, with which the stains have been treated, is placed in a watch glass, and evaporated in vacuo over sulphuric acid; the last remaining portion of the fluid being united in the bottom of the glass by causing it to collect in a single drop. When the evaporation of fluid is completed, the watch-glass is placed before the narrowed slit of a spectroscope, and a ray of diffused light (or better, light reflected from a heliostat) made to pass through the part of the glass containing the residue. If the stains originate from blood, the absorption lines of haemoglobin, consisting of two large dark bands, to the right of the sodium line (Frauenhofer's line D), will be observed in the spectrum. In case both of the above tests fail to give positive results, it is almost certain that the stains examined were not caused by blood. If, on the contrary, the reactions were produced, scarcely any doubt exists as to the presence of blood. Under these circumstances it is advisable to confirm the results by means of the tests that have been previously spoken of as being formerly exclusively employed; these are the following:
a. 1/2 to 1 c. c. of ozonized oil of turpentine, i. e. turpentine which has been exposed to the air sufficiently long to acquire the property of decolorizing water that is slightly tinted with indigo—is introduced in a test-tube, and an equal volume of[155] tincture of guaiacum added (the latter tincture is prepared by treating an inner portion of the resin with alcohol, until its brownish color is changed to a brownish-yellow).
If upon adding some of the substance under examination to the above mixture a clear blue coloration ensues, and the insoluble matter thrown down possesses a deep blue color, the presence of coloring matter of the blood is indicated. The mixture also imparts a blue color to moistened spots from which the blood stains have been as completely extracted as possible. Unfortunately sulphate of iron gives the same reaction.[X]
b. Upon heating the fluid obtained by treating the stains with cold water in a test-tube, its brown or reddish color disappears, and greyish-white flakes of coagulated albumen are thrown down. The precipitate acquires a brick-red color, when treated with an acid solution of nitrate of mercury containing nitrous acid. The albumen is also coagulated by the addition of nitric acid: it assumes a more or less yellow color, if heated with a slight excess of the acid. Chlorine-water, especially upon heating, likewise precipitates albumen in the form of white flakes.
c. If the fluid is acidulated with a few drops of acetic acid, and a drop of ferrocyanide of potassium added, a white precipitate, or, at least, turbidity is produced.
d. The flakes of albumen, separated by heating, dissolve in caustic alkalies to a solution, from which they are re-precipitated by nitric acid, or chlorine water.
e. Upon treating blood stains with chlorine-water, a solution which contains chloride of iron, and acquires a red coloration by the addition of sulphocyanide of potassium, is formed.[156]
f. Should the stains have failed to be affected by cold water (which, as has already been remarked, is the case when they have been previously washed with hot water), they are treated with weak soda lye. Nitric acid, hydrochloric acid, and chlorine water will produce in the solution so obtained a white precipitate, which exhibits the general properties of albumen previously described. In case the stains are deposited upon linen, it is necessary to replace the soda by ammonia, in order to avoid dissolving the fabric.
g. Solutions of the alkalies, which dissolve the albumen, leave the coloring matters intact, and consequently do not decolorize the fabric. If the latter is afterwards subjected to the action of hydrochloric acid, the coloring matter is dissolved, forming a solution that leaves upon evaporation to dryness a residue containing iron, which gives a blue coloration with ferrocyanide of potassium, and a red coloration with sulphocyanide of potassium.
h. The coloring matter of blood dissolves in boiling alcohol, to which sulphuric acid has been added, to a brown dichroic fluid (appearing green by transmitted light, and red by reflected light). A mixture of rust and blood exhibits the same phenomenon.
i. If substances containing blood are heated in a dry tube, an odor resembling that of burnt horn is emitted. In case the stained fabric is a substance that would produce this odor, (such as wool, silk, or hair), the test naturally loses all value.
j. If the fluid obtained by treating the stains either with water or alkali is evaporated with a little carbonate of potassa, and the residue heated, at first at 100°, then to redness, in a glass tube to which a fresh quantity of carbonate of potassa has been added, cyanide of potassium is formed. When cold, the tube is cut above the part containing the fused mixture,[157] the mass heated with iron-filings and water, the fluid filtered, and the filtrate then acidulated with hydrochloric acid: ferrocyanide of potassium will be present in the fluid, and upon adding a drop of solution of perchloride of iron a green, or blue, color will be produced, and a precipitate of Prussian blue gradually thrown down.
If the stained cloth is non-nitrogenous (per ex.: hemp, linen, or cotton), instead of treating it with water, it may be heated until pulverulent, mixed with carbonate of potassa, the mixture calcined, and the operation then completed as just described. This test having given affirmative results, the operations should be repeated with an unstained portion of the cloth, to remove all doubt that the indications obtained do not really originate from the fabric.
In the present state of science, it is impossible to discriminate chemically between human and animal blood. M. Barruel, it is true, is able, not only to accomplish this, but also to distinguish the blood of the various species of animals by its odor! But this test has a somewhat hypothetical value for scientific purposes. In regard to the crystals of haemin, they do not present sufficient difference to allow the blood of different animals to be distinguished. We have not yet treated of the globules. It often occurs that these minute organs are so altered as to be no longer recognized in the microscopic examination; when, however, the stains are tolerably recent, they may be detected by examining the moistened stained cloth, directly under the microscope: a discrimination between animal and human blood is then possible: corpuscules of human blood possess the greater size: those of the sheep, for instance, have only one-half the diameter of the former. It is, however, but seldom that this distinction can be made use of.[Y] [158]
In cases where attempt at violence, rape or pederasty is suspected, the expert may be required to determine the nature of stains found on clothing, sheets, etc. The fact that the stains were produced by semen, may often be regarded, per se, as criminating evidence. This class of investigation possesses, therefore, considerable importance.
External appearance of the stains.—Dry spermatic stains are thin, and exhibit a greyish or, occasionally, a citron-yellow color, if present on white cloth. In case the fabric is colored, they appear whitish and, if on linen, present a glossy aspect. They are translucid, when observed by transmitted light. If the fabric, upon which the stains are deposited, is of a heavy texture, they are visible only on one side: under all circumstances, their circumference is irregular and undulated. These indications, however, are not conclusive, but vary according to whether the stains were produced by the thick semen of a vigorous man, or the aqueous seminal fluid of an aged and diseased person, or by semen more or less mixed with the prostatic fluid. Upon moistening spermatic stains, the distinctive stale odor of fresh semen is sometimes emitted, but this characteristic is usually obscured by the presence of foreign substances.
Semen stains are soluble in water, forming a gummy fluid, in which chlorine, alcohol, bichloride of mercury, acetate and subacetate of lead produce a white precipitate, but which fails to be coagulated by heating. Plumbate of potassa does not impart a fawn-color to these stains, at a temperature above 20°, as is the case with those produced by albuminous substances.[159]
Persulphate of iron imparts to spermatic stains a pale yellow color,
Sulphate of copper, a bluish grey color,
Cupro-potassic tartrate, a bluish grey color,
Nitrate of silver, a pale grey color,
Nitric acid, a pale yellow color.
The above reactions, separate or united, are insufficient; they are not very delicate, and are likewise produced by stains originating from the other varieties of mucus: the indications furnished by a microscopic examination of the stains are alone conclusive.
Microscopic examination.—Semen contains as its principal and fecundating constituent, peculiar vibratory filaments, (spermatozoa), held suspended in a viscous fluid. These filaments, when preserved in a warm and moist place, retain their activity for a considerable time: it is even possible that they may exhibit vitality in the organs, into which they have been voluntarily or forcibly ejaculated, for ten, or even twenty-four hours. When exposed to cold air, the spermatozoa quickly expire; still, they preserve their form for some time, and, as this is very characteristic, it is then easy to identify them; moreover, since they originate exclusively in the testicles, their detection may be considered as certain evidence of the presence of semen. In stains produced by aged persons, and by persons enfeebled by excesses, the spermatozoa fail to be presented; in case they are discovered, this fact evidently does not affect the certainty of the spermatic origin of the stains. The contrary conclusion is never absolutely certain: still, if the use of the microscope fails to establish the presence of spermatozoa, it is almost certain that the stains were not produced by semen.
Of the various methods for obtaining from the stains a[160] preparation adapted to the microscopic examination, the one proposed by M. Charles Robin is the most simple and reliable.
A strip, 1 c. c. in size (comprising the entire stain, if this be small, containing its inner portion, if it be large), is cut from the fabric under examination, care being taken that the two extremities of the sample extend beyond the stained portion.
One end of the cloth is then immersed in a capsule, or watch-glass, containing pure water: the stains become moistened by capillary attraction, and, in a space of time varying from twenty minutes to two hours, acquire the appearance of fresh semen. As soon as the stained portion becomes swollen and softened, the surface of the cloth is gently scraped with a spatula, and the substance removed placed on the slide of the microscope. The particles are next slightly detached, a drop of water added, if necessary, and the whole covered with a small plate of very thin glass. The preparation is then examined by a microscope, having a magnifying power of from 500 to 600 diameters. In this way, the presence of either entire or broken spermatozoa is readily detected. Their existence is rendered still more apparent, if the mucus present is dissolved by adding a drop of acetic acid to the preparation.
Entire spermatozoa consist of long slender filaments, having a length of 0.04041 to 0.04512 millimetre; the anterior extremity presents an oval enlargement, either round or pyriform, exhibiting a double outline, when magnified to 500 diameters. This enlarged end is termed the "head;" the entire remaining portion being regarded as the "tail." In case the spermatozoa are broken, they are severed either near the head or in the middle of the tail, and a mass of detached fragments will be observed in the microscopic examination. The spermatozoa are not the only corpuscules revealed by the microscope; other substances, entirely different in character, are often observed.[161] Although the detection of these bodies is, in itself, of no value, it will be well to enumerate and characterize them; they are:
a. Oily globules.
b. Leucocytes, or spherical and finely granulous globules of mucus.
c. Corpuscules, originating from the seminal vesicles, termed sympexions. These are rounded or ovoid, possess an irregular outline, and are usually mixed with the spermatozoa and globules of mucus.
d. Crystals of phosphate of magnesia, varying greatly in size; the largest are from 0.mm. 001 to 0.mm. 002 in length. The crystals formed upon cooling the semen, present the form of an oblique prism, with a rhomboidal base. Occasionally they are elongated and flattened; they then assume the form of a rhomboid.
e. Epithelial cells; originating from the mucous follicles of the urethra.
f. Irregular grains of dust; soluble in acetic and hydrochloric acids, with gaseous evolution.
g. Brownish-red grains of rust; only slightly soluble in acetic acid, but easily soluble in hydrochloric acid.
h. Filaments of the strained fabric; detected by their texture, and general appearance.
i. Grains of starch, in case the cloth has been stiffened. These are almost invariably swollen, and are frequently broken and deformed.
If the examination is to be secretly executed, and the cloth cannot well be cut, it is rolled in a cone, in such a way that the external side contains the stained portion. The lower extremity of the cone (which should be free from stains) is dipped in a watch-glass containing water, so as to avoid directly wetting the stains. The cone soon becomes moistened[162] by absorption, and the operation is then completed in the same manner as when the fabric has been cut; which is always preferable, when possible.
The examination of spermatic stains consists, then, in moistening the stains with water, separating them as completely as possible from the stained cloth, and determining the presence of the spermatozoa by means of the microscope.
All other tests are valueless; even their execution for confirmatory purposes is not advisable; inasmuch as they fail to possess a distinctive character, and the reagents employed in their production may destroy the fabric, and thus prevent the formation of the only conclusive reaction—the detection of the spermatozoa.
In case the stains are deposited upon a woman's chemise, they are usually present on both the front and back portions, and are sometimes to be found on the sleeves. When a man's shirt is under examination, especial attention should be given to the anterior portions. The pantaloons are also often stained; usually in the interior, but sometimes also on the exterior, just above the thighs. In reporting the decision to the court, as to the nature of the stains, their precise position should invariably be stated, as, by this means, the circumstances attending the commission of the crime may be, at least partially, elucidated.
THE END. [163]
The following list of the literature of toxicology, and its allied branches, will, it is hoped, be of service to those readers who are desirous of obtaining further information on the subjects treated in this work.—Trans.
The following are the most important works relating to poisons and food-adulteration that have been issued since the publication of the first edition of this book:
Within the last few years the subject of food-adulteration has been so prominently brought before the public that, in many instances, the various State Boards of Health have commissioned their chemists to furnish reports on this subject. These may be found in the annual publications of the same, notably in the volumes issued by the Massachusetts, Michigan, New Jersey, and New York State Boards of Health. It may also be mentioned in this connection that the Sanitary Engineer of New York, the Analyst of London, the Zeitschrift für Untersuchung von Lebensmitteln, Eichstatt, and the Zeitschrift gegen Verfälschung der Lebensmittel, Leipzig, are journals devoted to the consideration of adulterations and the more recent methods employed for their detection.
J. P. B.
A.
B.
C.
D.
E.
F.
G.
H.
I.
L.
M.
N.
O.
P.
Q.
R.
S.
T.
U.
V.
W.
Z.
9/5 C° + 32 = F°.
—Trans.| Fat | 2.5 | per cent. |
| Solids, not fat | 9. | " " |
| Total | 11.5 | " " |
| Water | 88.5 | " " |
| Pure wine | no precipitate | greenish hue | |
| Elderberry | violet | " | |
| Beet-sugar | red | " | |
| Logwood red | violet-red | " | |
| Privet | violet-blue | " | |
| Turmeric | light-blue | " |
BECKER & SONS,
MANUFACTURERS OF
Balances and Weights of Precision,
FOR
Chemists, Assayers, Jewelers, Druggists,
And in general for every use where accuracy is required,
No. 6 Murray St., New York.
Every Balance and Set of Weights leaving this establishment is guaranteed to be accurately adjusted, as represented in our Price List.
☞Our Illustrated Price List mailed on application.
CATALOGUE
OF THE
Scientific, Military, and Naval
PUBLICATIONS
OF
D. VAN NOSTRAND,
23 Murray Street and 27 Warren Street, New York.
ABBOT, Maj. HENRY L.—Siege Artillery against Richmond.
Illustrated. 8vo, cloth $3 50
ADAMS, J. W.—Sewers and Drains for Populous Districts.
Embracing Rules and Formulas for the dimensions and construction of works of Sanitary Engineers. Second edition. 8vo, cloth 2 50
ALDRICH, M. ALMY.—History of the United States Marine Corps.
From Official Reports and other Documents. Compiled by Capt. Richard S. Collum. 8vo, cloth 2 50
ALEXANDER, J. H.—Universal Dictionary of Weights and Measures, Ancient and Modern, reduced to the Standards of the United States of America.
New edition, enlarged. 8vo, cloth 3 50
ANDERSON, Gen. ROBERT.—Evolutions of Field Batteries of Artillery.
Translated from the French, and arranged for the Army and Militia of the United States. Published by order of the War Department. 33 plates. 24mo, cloth 1 00
ANDREWS, Maj.-Gen. C. C.—Campaign of Mobile.
Including the Co-operation of General Wilson's Cavalry in Alabama. With five maps and views. 8vo, cloth 2 50
—— Hints to Company Officers on their Military Duties.
18mo, cloth 50
ARNOLD, Maj. A. K.—Cavalry Service.
Notes on Horses for Cavalry Service, embodying the Quality, Purchase, Care, and Diseases most frequently encountered, with Lessons for Bitting the Horse and Bending the Neck. Illustrated. 18mo, cloth 75
ARNOLD, Maj. FRANK S.—The Discipline and Drill of Militia.
Crown 8vo, limp cloth 2 00
ATWOOD, Geo.—Practical Blow-Pipe Assaying.
12mo, cloth, illustrated 2 00
AUCHINCLOSS, W. S.—Link and Valve Motions Simplified.
Illustrated with 37 wood-cuts and 21 lithographic plates, together with a Travel Scale and numerous useful tables. 8vo, cloth 3 00
AXON, W. E. A.—The Mechanic's Friend.
A Collection of Receipts and Practical Suggestions Relating to Aquaria—Bronzing—Cements—Drawing—Dyes—Electricity—Gilding—Glass-working—Glues—Horology—Lacquers—Locomotives—Magnetism—Metal-working—Modelling—Photography—Pyrotechny—Railways—Solders—Steam-Engine—Telegraphy—Taxidermy—Varnishes—Waterproofing, and Miscellaneous Tools, Instruments, Machines, and Processes connected with the Chemical and Mechanic Arts. With numerous diagrams and wood-cuts. Fancy cloth 1 50
BACON, F. W.—A Treatise on the Richards Steam-Engine Indicator, with directions for its use.
By Charles T. Porter. Revised, with notes and large additions as developed by American practice; with an appendix containing useful formulæ and rules for engineers. Illustrated. Fourth edition. 12mo, cloth 1 00
BARBA, J.—The Use of Steel for Constructive Purposes;
Method of Working, Applying, and Testing Plates and Brass. With a Preface by A. L. Holley, C.E. 12mo, cloth 1 50
BARNARD, Maj.-Gen. J. G.—The "C. S. A." and the Battle of Bull Run.
8vo, cloth 1 25
—— The Peninsular Campaign and its Antecedents,
As developed by the Report of Maj.-Gen. Geo. B. McClellan and other published Documents. 8vo, cloth 1 00
12mo, paper 30
—— Notes on Sea-Coast Defence.
Consisting of Sea-Coast Fortification; the Fifteen-Inch Gun; and Casemate Embrasure. With an engraved plate of the Fifteen-Inch Gun. 8vo, cloth 2 00
BARNARD, Maj.-Gen. J. G., and BARRY, Maj.-Gen. W. F.—Report of the Engineer and Artillery Operations of the Army of the Potomac,
From its Organization to the Close of the Peninsular Campaign. Illustrated by 18 maps, plans, etc. 8vo, cloth 2 50
BARNES, Lieut.-Com. JOHN S.—Submarine Warfare, Defensive and Offensive.
Comprising a full and complete History of the invention of the Torpedo, its employment in War, and results of its use. Descriptions of the various forms of Torpedoes, Submarine Batteries, and Torpedo Boats actually used in War. With 20 lithographic plates and many wood-cuts, 8vo, cloth 5 00
BARRE DUPARCQ, EDWARD DE LA.—Elements of Military Art and History.
Translated by Col. Geo. W. Cullum, U.S.E. 8vo, cloth 3 50
BARRETT, Capt. EDWARD.—Dead Reckoning; or, Day's Work.
8vo, flexible cloth 1 25
—— Gunnery Instructions.
12mo, cloth 1 25
BEILSTEIN, F.-An Introduction to Qualitative Chemical Analysis.
Translated by I. J. Osbun. 12mo, cloth 75
BENET, Gen. S. V.—Electro-Ballistic Machines,
And the Schultz Chronoscope. Second edition. Illustrated. 4to, cloth 3 00
—— Military Law and Courts-Martial.
A Treatise on Military Law and the Practice of Courts-Martial. Sixth edition, revised and enlarged. 8vo, law sheep 4 00
BENTON, Col. J. G.—Ordnance and Gunnery.
A Course of Instruction in Ordnance and Gunnery. Compiled for the use of the Cadets of the U. S. Military Academy. Illustrated. Fourth edition, revised and enlarged. 8vo, cloth 5 00
BERRIMAN, Maj. M. W—The Militiaman's Manual and Sword-Play without a Master.
Rapier and Broad-Sword Exercises, copiously explained and illustrated; Small-Arm Light Infantry Drill of the United States Army; Infantry Manual of Percussion Musket; Company Drill of the United States Cavalry. Fourth edition. 12mo, cloth 1 00
BLAKE, W. P.—Report upon the Precious Metals;
Being Statistical Notices of the principal Gold and Silver producing regions of the World, represented at the Paris Universal Exposition. 8vo, cloth 2 00
—— Ceramic Art.
A Report on Pottery, Porcelain, Tiles, Terra-Cotta, and Brick. 8vo, cloth 2 00
BOW, R. H.—A Treatise on Bracing,
With its application to Bridges and other Structures of Wood or Iron. 156 illustrations. 8vo, cloth 1 50
BOWSER, Prof. E. A.—An Elementary Treatise on Analytic Geometry.
Embracing Plain Geometry, and an Introduction to Geometry of three Dimensions. 12mo, cloth 1 75
—— An Elementary Treatise on the Differential and Integral Calculus.
With numerous examples. 12mo, cloth 2 25
BOYNTON, Maj. EDWARD C.—History of West Point,
And its Military Importance during the American Revolution; and the Origin and Progress of the U. S. Military Academy. With 36 maps and engravings. Second edition. 8vo, fancy cloth 3 50
BRANDT, J. D.—Gunnery Catechism.
As applied to the service of the Naval Ordnance. Adapted to the latest Official Regulations, and approved by the Bureau of Ordnance, Navy Department. Revised edition. Illustrated. 18mo, cloth 1 50
BREWERTON, G. D.—The Automaton Battery; or, Artillerist's Practical Instructor.
For all Mounted Artillery Man[oe]uvres in the Field. In box 1 00
When sent by mail 1 30
—— The Automaton Regiment; or, Infantry Soldier's Practical Instructor.
For all Regimental Movements in the Field. In box 1 00
When sent by mail 1 33
—— The Automaton Company; or, Infantry Soldier's Practical Instructor.
For all Company Movements in the Field. In box 1 25
When sent by mail 1 94
BRINKERHOFF, Capt. R.—The Volunteer Quartermaster.
12mo, cloth 1 00
BUCKNER, Lieut. W. P.—Calculated Tables of Ranges for Navy and Army Guns.
8vo, cloth 1 50
BURGH, N. P.—Modern Marine Engineering,
Applied to Paddle and Screw Propulsion. Consisting of 36 colored plates, 259 practical wood-cut illustrations, and 403 pages of descriptive matter, the whole being an exposition of the present practice of James Watt & Co., J. & G. Rennie, R. Napier & Sons, and other celebrated firms. Thick 4to vol., cloth 10 00
Half morocco 15 00
BURT, W. A.—Key to the Solar Compass, and Surveyor's Companion.
Comprising all the rules necessary for use in the field; also description of the Linear Surveys and Public Land System of the United States, Notes on the Barometer, suggestions for an outfit for a survey of four months, etc. Fifth edition. Pocket-book form, tuck 2 50
BUTLER, Capt. JOHN S.—Projectiles and Rifled Cannon.
A Critical Discussion of the Principal Systems of Rifling and Projectiles, with practical suggestions for their improvement, as embraced in a report to the Chief of Ordnance, U. S. Army. 4to, 36 plates, cloth 6 00
CAIN, Prof. WM.—A Practical Treatise on Voussoir and Solid and Braced Arches.
16mo, cloth extra 1 75
CALDWELL, Prof. GEO. C., and BRENEMAN, Prof. A. A.—Manual of Introductory Chemical Practice.
For the use of Students in Colleges and Normal and High Schools. Third edition revised and corrected. 8vo, cloth, illustrated. New and enlarged edition 1 50
CAMPIN, FRANCIS.—On the Construction of Iron Roofs.
8vo, with plates, cloth 2 00
CASEY, Brig.-Gen. SILAS—U. S. Infantry Tactics.
Vol. I.—School of the Soldier; School of the Company; Instruction for Skirmishers. Vol. II.—School of the Battalion, Vol. III.—Evolutions of a Brigade; Evolutions of a Corps d'Armée. Lithographed plates. 3 vols. 24mo, cloth 1 50
CHAUVENET, Prof. W.—New Method of Correcting Lunar Distances, and Improved Method of Finding the Error and Rate of a Chronometer, by Equal Altitudes.
8vo, cloth 2 00
CHURCH, JOHN A.—Notes of a Metallurgical Journey in Europe.
8vo, cloth 2 00
CLARK, D. KINNEAR, C.E.—Fuel,
Its Combustion and Economy; consisting of Abridgments of Treatise on the Combustion of Coal and the Prevention of Smoke, by C. W. Williams; and the Economy of Fuel, by T. S. Prideaux. With extensive additions on recent practice in the Combustion and Economy of Fuel: Coal, Coke, Wood, Peat, Petroleum, etc. 12mo, cloth 1 50
—— A Manual of Rules, Tables, and Data for Mechanical Engineers.
Based on the most recent investigations. Illustrated with numerous diagrams. 1,012 pages. 8vo, cloth 7 50
Half morocco 10 00
CLARK, Lt. LEWIS, U. S. N.—Theoretical Navigation and Nautical Astronomy.
Illustrated with 41 wood-cuts. 8vo, cloth 1 50
CLARKE, T. C.—Description of the Iron Railway Bridge over the Mississippi River at Quincy, Illinois.
Illustrated with 21 lithographed plans. 4to, cloth 7 50
CLEVENGER, S. R.—A Treatise on the Method of Government Surveying,
As prescribed by the U. S. Congress and Commissioner of the General Land Office, with complete Mathematical, Astronomical, and Practical Instructions for the use of the United States Surveyors in the field. 16mo, morocco 2 50
COFFIN, Prof. J. H. C.—Navigation and Nautical Astronomy.
Prepared for the use of the U. S. Naval Academy. Sixth edition. 52 wood-cut illustrations. 12mo, cloth 3 50
COLBURN, ZERAH.—The Gas-Works of London.
12mo, boards 60
COLLINS, JAS. E.—The Private Book of Useful Alloys and Memoranda for Goldsmiths, Jewellers, etc.
18mo, cloth 50
COOKE, Brig.-Gen. PHILIP. ST. GEORGE.—New Cavalry Tactics.
16mo, morocco 2 00
—— Cavalry Practice.
Regulations for the movements of the Cavalry of the Army. 12mo. 1 00
CORNWALL, Prof. H. B.—Manual of Blow-Pipe Analysis, Qualitative and Quantitative.
With a Complete System of Descriptive Mineralogy. 8vo, cloth, with many illustrations 2 50
CRAIG, B. F.—Weights and Measures.
An account of the Decimal System, with Tables of Conversion for Commercial and Scientific Uses. Square 32mo, limp cloth 50
CRAIG, Prof. THOS.—Elements of the Mathematical Theory of Fluid Motion.
16mo, cloth 1 25
CRAIGHILL, WM. P.—The Army Officer's Companion.
Principally designed for Staff Officers in the Field. Partly translated from the French of M. de Rouvre, Lieut.-Col. of the French Staff Corps, with additions from Standard American, French, and English authorities. 18mo, full roan 1 50
CULLUM, Col. GEORGE W.—Military Bridges.
Systems of Military Bridges in use by the U. S. Army; those adopted by the Great European Powers; and such as are employed in British India. With Directions for the Preservation, Destruction, and Re-establishment of Bridges. With 7 folding plates. 8vo, cloth 3 50
DAVIS, C. B., and RAE, F. B.—Hand-Book of Electrical Diagrams and Connections.
Illustrated with 32 full-page illustrations. Second edition. Oblong 8vo, cloth extra 2 00
DIEDRICH, JOHN.—The Theory of Strains.
A Compendium for the Calculation and Construction of Bridges, Roofs, and Cranes. Illustrated by numerous plates and diagrams. 8vo, cloth 5 00
DIXON, D. B.—The Machinist's and Steam-Engineer's Practical Calculator.
A Compilation of Useful Rules, and Problems Arithmetically Solved, together with General Information applicable to Shop-Tools, Mill-Gearing, Pulleys and Shafts, Steam-Boilers and Engines. Embracing Valuable Tables, and Instruction in Screw-cutting, Valve and Link Motion, etc. 16mo, full morocco, pocket form 2 00
DODD, GEO.—Dictionary of Manufactures, Mining, Machinery, and the Industrial Arts.
12mo, cloth 1 50
DOUGLASS, Prof. S. H., and PRESCOTT, Prof. A. B.—Qualitative Chemical Analysis.
A Guide in the Practical Study of Chemistry, and in the Work of Analysis. Fourth edition. 8vo, cloth 3 50
DUANE, Gen. J. C.—Manual for Engineering Troops.
Consisting of—Part I. Ponton Drill; II. Practical Operations of a Siege; III. School of the Sap; IV. Military Mining; V. Construction of Batteries. With 16 plates and numerous wood-cut illustrations. 12mo, half morocco 1 50
DUBOIS, A. J.—The New Method of Graphical Statics.
With 60 illustrations. 8vo, cloth 1 50
DUFOUR, Gen. G. H.—The Principles of Strategy and Grand Tactics.
Translated from the French, by William P. Craighill, U. S. Engineers, from the last French edition. Illustrated. 12mo, cloth 1 50
DURYEA, Col. A.—Standing Orders of the Seventh Regiment National Guards.
New edition. 16mo, cloth 50
EASSIE, P. B.—Wood and its Uses.
A Hand-Book for the use of Contractors, Builders, Architects, Engineers, and Timber Merchants. Upwards of 250 illustrations. 8vo, cloth 1 50
EDDY, Prof. H. T.—Researches in Graphical Statics.
Embracing New Constructions in Graphical Statics, a New General Method in Graphical Statics, and the Theory of Internal Stress in Graphical Statics. 8vo, cloth 1 50
ELIOT, Prof. C. W., and STORER, Prof. F. H.—A Compendious Manual of Qualitative Chemical Analysis.
Revised with the co-operation of the authors. By Prof. William R. Nichols. Illustrated. 12mo, cloth 1 50
ELLIOT, Maj. GEO. H., U. S. E—European Light-House Systems.
Being a Report of a Tour of Inspection made in 1873. 51 engravings and 21 wood-cuts. 8vo, cloth 5 00
ENGINEERING FACTS AND FIGURES.
An Annual Register of Progress in Mechanical Engineering and Construction for the years 1863-64-65-66-67-68. Fully illustrated. 6 vols. 18mo, cloth (each volume sold separately), per vol 2 50
FANNING, J. T.—A Practical Treatise on Water-Supply Engineering.
Relating to the Hydrology, Hydrodynamics, and Practical Construction of Water-Works in North America. Third edition. With numerous tables and 180 illustrations. 650 pages. 8vo, cloth 5 00
FISKE, Lieut. BRADLEY A., U. S. N.—Electricity in Theory and Practice; or, The Elements of Electrical Engineering.
8vo, cloth 2 50
FOSTER, Gen. J. G., U. S. A.—Submarine Blasting in Boston Harbor, Massachusetts.
Removal of Tower and Corwin Rocks. Illustrated with seven plates. 4to, cloth 3 50
FOYE, Prof. J. C.—Chemical Problems.
With brief Statements of the Principles involved. Second edition, revised and enlarged. 16mo, boards 50
FRANCIS, JAS. B., C. E.—Lowell Hydraulic Experiments:
Being a selection from Experiments on Hydraulic Motors, on the Flow of Water over Weirs, in Open Canals of Uniform Rectangular Section, and through submerged Orifices and diverging Tubes. Made at Lowell, Massachusetts. Fourth edition, revised and enlarged, with many new experiments, and illustrated with twenty-three copperplate engravings. 4to, cloth 15 00
FREE-HAND DRAWING.
A Guide to Ornamental Figure and Landscape Drawing. By an Art Student. 18mo, boards 0 50
FRY, Brig.-Gen. JAMES B.—Army Sacrifices; or, Briefs from Official Pigeon-Holes.
Sketches based on Official Reports, grouped together for the purpose of illustrating the Services of the Regular Army of the United States on the Indian Frontier. 16mo. 1 25
—— History of Brevet Rank.
The History and Legal Effects of Brevets in the Armies of Great Britain and the United States, from the origin in 1692 until the present time. Crown 8vo, extra cloth 3 00
GILLMORE, Gen. Q. A.—Treatise on Limes, Hydraulic Cements, and Mortars.
Papers on Practical Engineering, U. S. Engineer Department, No. 9, containing Reports of numerous Experiments conducted in New York City during the years 1858 to 1861, inclusive. With numerous illustrations. 8vo, cloth 4 00
—— Practical Treatise on the Construction of Roads, Streets, and Pavements.
With 70 illustrations. 12mo, cloth 2 00
—— Report on Strength of the Building-Stones in the United States, etc.
8vo, illustrated, cloth 1 50
—— Coignet Beton and other Artificial Stone.
9 plates, views, etc. 8vo, cloth 2 50
—— Fort Sumter.
Official Report of Operations against the Defences of Charleston Harbor, 1863. Comprising the descent upon Morris Island, the Demolition of Fort Sumter, and the siege and reduction of Forts Wagner and Gregg. With 76 lithographic plates, views, maps, etc. 8vo, cloth 7 50
Half Russia 12 00
—— Supplementary Report on Fort Sumter.
Supplementary Report to the Engineer and Artillery Operations against the Defences of Charleston Harbor in 1863. With 7 lithographed maps and views. 8vo, cloth 3 50
—— Siege and Reduction of Fort Pulaski, Georgia.
Illustrated by maps and views. 8vo, cloth 2 50
GOODEVE, T. M.—A Text-Book on the Steam-Engine.
143 illustrations. 12mo, cloth 2 00
GORDON, J. E. H.—Four Lectures on Static Induction.
12mo, cloth 80
GRAFTON, Capt. HENRY D.—A Treatise on the Camp and March.
With which is connected the Construction of Field-Works and Military Bridges. 12mo, cloth 75
GREENER, WM., R. C. E.—A Treatise on Rifles, Cannon, and Sporting Arms.
8vo, cloth 4 00
Full calf. 6 00
GRUNER, M. L.—The Manufacture of Steel.
Translated from the French, by Lenox Smith, with an appendix on the Bessemer process in the United States, by the translator. Illustrated. 8vo, cloth 3 50
GUIDE TO WEST POINT and the U. S. Military Academy.
With maps and engravings. 18mo, flexible cloth 1 00
HALF-HOURS WITH MODERN SCIENTISTS.—Lectures and Essays,
By Professors Huxley, Barker, Stirling, Cope, Tyndall, Wallace, Roscoe, Huggins, Lockyer, Young, Mayer, and Reed. Being the University Series bound up. With a general introduction by Noah Porter, President of Yale College. 2 vols. 12mo, cloth, illustrated 2 50
HAMERSLY, LEWIS B.—The Records of Living Officers of the U. S. Navy and Marine Corps.
Compiled from Official Sources. Third edition. Cloth, 8vo. 2 50
HAMILTON, W. G.—Useful Information for Railway Men.
Sixth edition, revised and enlarged. 562 pages, pocket form. Morocco, gilt. 2 00
HARRISON, Col. WALTER.—Pickett's Men.
A Fragment of War History. With portrait of Gen. Pickett. 12mo, cloth 1 25
HARRISON, W. B.—The Mechanic's Tool Book,
With Practical Rules and Suggestions for Use of Machinists, Iron-Workers, and others. Illustrated with 44 engravings. 12mo, cloth 1 50
HARWOOD, A. A.—Naval Courts-Martial.
Law and Practice of United States Naval Courts-Martial. Adopted as a Text-Book at the U. S. Naval Academy. 8vo, law-sheep 3 00
HASKINS, C. H.—The Galvanometer and its Uses.
A Manual for Electricians and Students. Second edition. 12mo, morocco 1 50
HAUPT, Brig.-Gen. HERMAN.—Military Bridges.
For the Passage of Infantry, Artillery, and Baggage-Trains; with suggestions of many new expedients and constructions for crossing streams and chasms. Including also designs for Trestle and Truss Bridges for Military Railroads, adapted specially to the wants of the Service of the United States. Illustrated by 69 lithographic engravings. 8vo, cloth 6 50
HEAD, Capt. GEORGE E.—A New System of Fortifications.
Illustrated. 4to, paper. 50
HEAVY ARTILLERY TACTICS: 1863.
Instructions for Heavy Artillery; prepared by a Board of Officers, for the use of the Army of the United States. With service of a gun mounted on an iron carriage, and 39 plates. 12mo, cloth 1 00
HENRICI, OLAUS.—Skeleton Structures, especially in their application to the Building of Steel and Iron Bridges.
With folding plates and diagrams. 8vo, cloth 1 50
HENRY, GUY V.—Military Record of Civilian Appointments in the United States Army.
2 vols. 8vo, cloth 10 00
HETH, Capt. HENRY.—System of Target Practice.
For the Use of Troops when armed with the Musket, Rifle-Musket, Rifle, or Carbine. Prepared principally from the French. 18mo, cloth 50
HEWSON, WM.—Principles and Practice of Embanking Lands from River Floods, as applied to the Levees of the Mississippi.
8vo, cloth 2 00
HOLLEY, ALEXANDER L.—A Treatise on Ordnance and Armor.
Embracing descriptions, discussions, and professional opinions concerning the materials, fabrication, requirements, capabilities, and endurance of European and American Guns, for Naval, Sea-Coast, and Iron-Clad Warfare, and their Rifling, Projectiles, and Breech-Loading; also, results of experiments against armor, from official records, with an appendix referring to Gun-Cotton, Hooped Guns, etc., etc. 948 pages, 493 engravings, and 147 Tables of Results, etc. 8vo, half roan 7 50
Half Russia 10 00
—— Railway Practice.
American and European Railway Practice in the economical Generation of Steam, including the Materials and Construction of Coal-burning Boilers, Combustion, the Variable Blast, Vaporization, Circulation, Superheating, Supplying and Heating Feed-water, etc., and the Adaptation of Wood and Coke-burning Engines to Coal-burning; and in Permanent Way, including Road-bed, Sleepers, Rails, Joint-fastenings, Street Railways, etc., etc. With 77 lithographed plates. Folio, cloth 12 00
HOTCHKISS, JED., and ALLAN, WILLIAM.—The Battle-Fields of Virginia.
Chancellorsville, embracing the Operations of the Army of Northern Virginia, from the First Battle of Fredericksburg to the Death of Lt.-Gen. T. J. Jackson. Illustrated with five maps and portrait of Stonewall Jackson. 8vo, cloth 3 50
HOWARD, C. R.—Earthwork Mensuration on the Basis of the Prismoidal Formulæ.
Containing simple and labor-saving method of obtaining Prismoidal Contents directly from End Areas. Illustrated by Examples, and accompanied by Plain Rules for Practical Uses. Illustrated. 8vo, cloth 1 50
HUNTER, Capt. R. F.—Manual for Quartermasters and Commissaries.
Containing Instructions in the Preparation of Vouchers, Abstracts, Returns, etc. 12mo, cloth 1 00
Flexible morocco 1 50
INDUCTION-COILS.—How Made and How Used.
63 illustrations. 16mo, boards. 50
INSTRUCTIONS FOR FIELD ARTILLERY.
Prepared by a Board of Artillery Officers. To which is added the "Evolutions of Batteries." Translated from the French by Brig.-Gen. R. Anderson, U. S. A. 122 plates. 12mo, cloth 1 00
ISHERWOOD, B. F.—Engineering Precedents for Steam Machinery.
Arranged in the most practical and useful manner for Engineers. With illustrations. Two volumes in one. 8vo, cloth 2 50
IVES, Lieut. R. A.—Military Law.
A Treatise on Military Law, and the Jurisdiction, Constitution, and Procedure of Military Courts. With a Summary of the Rules of Evidence as applicable to such Courts. 400 pages. 8vo, law-sheep 4 00
JANNETTAZ, EDWARD—A Guide to the Determination of Rocks:
Being an Introduction to Lithology. Translated from the French by G. W. Plympton, Professor of Physical Science at Brooklyn Polytechnic Institute. 12mo, cloth 1 50
JEFFERS, Capt. W. N., U. S. N.—Nautical Surveying.
Illustrated with 9 copperplates and 31 wood-cut illustrations. 8vo, cloth 5 00
JOMINI, Gen. BARON DE.—Campaign of Waterloo.
The Political and Military History of the Campaign of Waterloo. Translated from the French by Gen. S. V. Benét. Third edition. 12mo, cloth 1 25
—— Treatise on Grand Military Operations.
Illustrated by a Critical and Military History of the Wars of Frederick the Great. With a Summary of the Most Important Principles of the Art of War. Illustrated by maps and plans. Translated from the French by Col. S. B. Holabird, U. S. A. 2 vols. 8vo and Atlas. cloth 15 00
Half calf or morocco 21 00
Half Russia 22 50
JONES, H. CHAPMAN.—Text-Book of Experimental Organic Chemistry for Students.
18mo, cloth 1 00
JOYNSON, F. H.—The Metals used in Construction: Iron, Steel, Bessemer Metal, etc., etc.
Illustrated. 12mo, cloth 75
—— Designing and Construction of Machine Gearing.
Illustrated. 8vo, cloth 2 00
KANSAS CITY BRIDGE, THE.
With an account of the Regimen of the Missouri River, and a description of the methods used for Founding in that River. By O. Chanute, Chief-Engineer, and George Morrison, Assistant-Engineer. Illustrated with five lithographic views and twelve plates of plans. 4to, cloth 6 00
KELTON, Gen. J. C.—New Bayonet Exercise.
A New Manual of the Bayonet, for the Army and Militia of the United States. With 40 beautifully engraved plates. Fifth edition. Revised. 12mo, cloth 2 00
KING, W. H.—Lessons and Practical Notes on Steam,
The Steam-Engine, Propellers, etc., etc., for young Marine Engineers, Students, and others. Revised by Chief-Engineer J. W. King, U. S. Navy. Nineteenth edition, enlarged. 8vo, cloth 2 00
KIRKWOOD, JAS. P.—Report on the Filtration of River Waters for the supply of Cities,
As practised in Europe, made to the Board of Water Commissioners of the City of St. Louis. Illustrated by 30 double-plate engravings. 4to, cloth 15 00
LARRABEE, C. S.—Cipher and Secret Letter and Telegraphic Code, with Hogg's Improvements.
The most perfect secret code ever invented or discovered. Impossible to read without the key. 18mo, cloth 1 00
LAZELLE, Capt. H. M., U. S. A.—One Law in Nature.
A New Corpuscular Theory, comprehending Unity of Force, Identity of Matter and its Multiple Atom Constitution; applied to the Physical Affections, or Modes of Energy. 12mo. 1 50
LECOMTE, FERDINAND.—The War in the United States.
A Report to the Swiss Military Department. Translated from the French by a Staff Officer. 12mo, cloth 75
LE GAL, Col. EUGENE.—School of the Guides.
Designed for the use of the Militia of the United States. 16mo, cloth 60
LENDY, Capt.—Maxims and Instructions on the Art of War.
A Practical Military Guide for the use of Soldiers of all Arms and of all Countries. Translated from the French. 18mo, cloth 75
LEVY, Com. U. P.—Manual of Internal Rules and Regulations for Men-of-War.
Third edition, revised and enlarged. 18mo, flexible cloth 30
LIEBER, FRANCIS, LL.D.—Instructions for Armies.
Instructions for the Government of Armies of the United States in the Field. 12mo, paper. 25
LIPPITT.—Special Operations of War. 12mo, cloth 1 00
—— Field Service in War. 12mo, cloth 1 00
—— Tactical Use of the Three Arms. 12mo, cloth 1 00
—— Intrenchments. 12mo, cloth 1 25
LOCK, C. G., WIGNER, G. W., and HARLAND, R. H.—Sugar Growing and Refining.
Treatise on the Culture of Sugar-Yielding Plants, and the Manufacture and Refining of Cane, Beet, and other sugars. 8vo, cloth, illustrated 12 00
LOCKWOOD, THOS. D.—Electricity, Magnetism, and Electro-Telegraphy.
A Practical Guide for Students, Operators, and Inspectors. 8vo, cloth
LORING, A. E.—A Hand-Book on the Electro-Magnetic Telegraph.
Paper boards. 0 50
cloth 75
morocco 1 00
LUCE, Capt. S. B.—Seamanship.
For the use of the United States Naval Academy. Fourth edition. Crown 8vo, revised and improved, illustrated by 89 full-page copperplate engravings, half roan 7 50
☞Text-Book at the U. S. Naval Academy, Annapolis.
—— Naval Light Artillery.
By Lieut. W. H. Parker, U. S. N. Third edition, revised by Capt. S. B. Luce, Assistant Instructor of Gunnery and Tactics at the U. S. Naval Academy. 22 plates. 8vo, cloth 3 00
MacCORD, Prof. C. W.—A Practical Treatise on the Slide-Valve by Eccentrics,
Examining by methods the action of the Eccentric upon the Slide-Valve, and explaining the practical processes of laying out the movements, adapting the valve for its various duties in the steam-engine. Second edition. Illustrated. 4to, cloth 2 50
McCLELLAN, Gen. GEO. B.—Report of the Army of the Potomac,
Of its operations while under his command. With maps and plans. 8vo, cloth 1 00
Paper. 50
McCULLOCH, Prof. R. S.—Elementary Treatise on the Mechanical Theory of Heat, and its application to Air and Steam Engines.
8vo, cloth 3 50
MANUAL OF BOAT EXERCISE.
At the U. S. Naval Academy, designed for the practical instruction of the Senior Class in Naval Tactics. 18mo, flexible cloth 50
MENDELL, G. H.—Military Surveying.
A Treatise on Military Surveying, Theoretical and Practical, including a description of Surveying Instruments. With 70 wood-cut illustrations. 12mo, cloth 1 50
MERRILL, Col. WM. E., U. S. A.—Iron Truss Bridges for Railroads.
The method of calculating strains in Trusses, with a careful comparison of the most prominent Trusses, in reference to economy in combination, etc., etc. Illustrated. 4to, cloth 5 00
MICHAELIS, Capt. O. E.—The Le Boulenge Chronograph.
With three lithographed folding plates of illustrations. 4to, illustrated, cloth 3 00
MICHIE, Prof. P. S.—Elements of Wave Motion relating to Sound and Light.
Text-Book for the U. S. Military Academy. 8vo, cloth, illustrated 5 00
MINIFIE, WM—Mechanical Drawing.
A Text-Book of Geometrical Drawing for the use of Mechanics and Schools, in which the Definitions and Rules of Geometry are familiarly explained; the Practical Problems are arranged, from the most simple to the more complex, and in their description technicalities are avoided as much as possible. With illustrations for Drawing Plans, Sections, and Elevations of Railways and Machinery; an Introduction to Isometrical Drawing, and an Essay on Linear Perspective and Shadows. Illustrated with over 200 diagrams engraved on steel. Ninth edition. With an Appendix on the Theory and Application of Colors. 8vo, cloth 4 00
"It is the best work on drawing that we have ever seen, and is especially a text-book of Geometrical Drawing for the use of Mechanics and Schools. No young Mechanic, such as a Machinist, Engineer, Cabinet-maker, Millwright, or Carpenter, should be without it."—Scientific American.
—— Geometrical Drawing.
Abridged from the octavo edition, for the use of schools. Illustrated with 48 steel plates. Fifth edition. 12mo, cloth 2 00
MODERN METEOROLOGY.
A Series of Six Lectures, delivered under the auspices of the Meteorological Society in 1878. Illustrated. 12mo, cloth 1 50
MONROE, Col. J.—Light Infantry Company and Skirmish Drill.
Bayonet Fencing; with a Supplement on the Handling and Service of Light Infantry. 32mo, cloth 75
MOORE, FRANK—The Rebellion Record.
Containing a full and concise Diary of Events from December, 1860, to the close of the War of the Rebellion, with Official Reports of State Officers and Narratives of all the Battles and Skirmishes that occurred. Complete in twelve volumes royal 8vo. Illustrated with 158 steel engraved Portraits of distinguished Generals and Prominent Men, together with numerous Maps and Plans of Battles. Price in cloth 60 00
Library sheep 72 00
Half calf, antique 78 00
Half morocco 78 00
Half Russia 84 00
—— Portrait Gallery of the War, Civil, Military, and Naval.
A Biographical Record. Illustrated with 60 fine portraits on steel. 1 vol. 8vo, cloth 6 00
Half calf. 7 50
MORRIS, E.—Easy Rules for the Measurement of Earthworks, by Means of the Prismoidal Formula.
78 illustrations. 8vo, cloth 1 50
MORRIS, Gen. WM. H.—Field Tactics for Infantry.
Illustrated. 18mo, cloth 75
—— Infantry Tactics.
2 vols. 24mo. 2 00
2 vols. in one, cloth 1 50