The Project Gutenberg EBook of The Works of Francis Maitland Balfour,
Volume III (of 4), by Francis Maitland Balfour
This eBook is for the use of anyone anywhere at no cost and with
almost no restrictions whatsoever. You may copy it, give it away or
re-use it under the terms of the Project Gutenberg License included
with this eBook or online at www.gutenberg.org
Title: The Works of Francis Maitland Balfour, Volume III (of 4)
Author: Francis Maitland Balfour
Editor: Sir Michael Foster
Adam Sedgwick
Release Date: February 26, 2014 [EBook #45019]
Language: English
Character set encoding: ISO-8859-1
*** START OF THIS PROJECT GUTENBERG EBOOK WORKS OF FRANCIS MAITLAND BALFOUR, VOL III ***
Produced by Bryan Ness, Carol Brown, and the Online
Distributed Proofreading Team at http://www.pgdp.net (This
file was produced from images generously made available
by The Internet Archive/Canadian Libraries)
THE WORKS
OF
FRANCIS MAITLAND BALFOUR.
VOL. III.
Memorial Edition.
Cambridge:
PRINTED BY C. J. CLAY, M.A. AND SON,
AT THE UNIVERSITY PRESS.
Memorial Edition.
THE WORKS
OF
FRANCIS MAITLAND BALFOUR,
M.A., LL.D., F.R.S.,
FELLOW OF TRINITY COLLEGE,
AND PROFESSOR OF ANIMAL MORPHOLOGY IN THE UNIVERSITY OF
CAMBRIDGE.
EDITED BY
M. FOSTER, F.R.S.,
PROFESSOR OF PHYSIOLOGY IN THE UNIVERSITY OF CAMBRIDGE;
AND
ADAM SEDGWICK, M.A.,
FELLOW AND LECTURER OF TRINITY COLLEGE, CAMBRIDGE.
VOL. III.
A TREATISE ON COMPARATIVE EMBRYOLOGY.
Vol. II. Vertebrata.
London:
MACMILLAN AND CO.
1885
[The Right of Translation is reserved.]
PREFACE TO VOLUME II.
The present volume completes my treatise on Comparative Embryology.
The first eleven chapters deal with the developmental history of the
Chordata. These are followed by three comparative chapters completing
the section of the work devoted to Systematic Embryology. The
remainder of the treatise, from Chapter XIV. onwards, is devoted to
Organogeny. For the reasons stated in the introduction to this part
the organogeny of the Chordata has been treated with much greater
fulness than that of the other groups of Metazoa.
My own investigations have covered the ground of the present volume
much more completely than they did that of the first volume; a not
inconsiderable proportion of the facts recorded having been directly
verified by me.
The very great labour of completing this volume has been much
lightened by the assistance I have received from my friends and
pupils. Had it not been for their co-operation a large number of the
disputed points, which I have been able to investigate during the
preparation of the work, must have been left untouched.
My special thanks are due to Mr Sedgwick, who has not only devoted a
very large amount of time and labour to correcting the proofs, but has
made for me an index of this volume, and has assisted me in many other
ways.
Dr Allen Thomson and Professor Kleinenberg of Messina have undertaken
the ungrateful task of looking through my proof-sheets, and have made
suggestions which have proved most valuable. To Professors Parker,
Turner, and Bridge, I am also greatly indebted for their suggestions
with reference to special chapters of the work.
CONTENTS OF VOLUME II.
Chapter I. Cephalochorda. Pp. 1-8.
Segmentation and formation of the layers, pp. 1-3.
Central nervous system, pp. 3,
4.
Mesoblast, p. 5.
General history of larva, pp. 6-8.
Chapter II. Urochorda. Pp. 9-39.
Solitaria, pp. 9-23. Development of embryo, pp. 9-15. Growth and
structure of free larva, pp. 15-19. Retrogressive metamorphosis, pp.
19-23. Sedentaria, p. 23. Natantia, pp. 23-28. Doliolidæ, pp.
28, 29. Salpidæ, pp. 29-34. Appendicularia, p. 34. Metagenesis,
pp. 34-38.
Chapter III. Elasmobranchii. Pp. 40-67.
Segmentation and formation of the layers, pp. 40-47. Epiblast, p. 47.
Mesoblast, pp. 47-51. Hypoblast and notochord, pp. 51-54. General
features of the embryo at successive stages, pp. 55-62. The yolk-sack,
pp. 62-66.
Chapter IV. Teleostei. Pp. 68-82.
Segmentation and formation of the layers, pp. 68-73. General history
of the layers, pp. 73-75. General development of the embryo, pp.
76-81.
Chapter V. Cyclostomata. Pp. 83-101.
Segmentation and formation of the layers, pp. 83-86. Mesoblast and
notochord, pp. 86, 87. General history of the development, pp. 87-97.
Metamorphosis, pp. 97-100. Myxine, p. 100.
Chapter VI. Ganoidei. Pp. 102-119.
Acipenser, pp. 102-110. Segmentation and formation of the layers,
pp. 102-104. General development of the embryo and larva, pp. 104-110.
Lepidosteus, pp. 111-119. Segmentation, pp. 111, 112. General
development of embryo and larva, pp. 112-119. General observations on
the embryology of Ganoids, p. 119.
Chapter VII. Amphibia. Pp. 120-144.
Oviposition and impregnation, pp. 120, 121. Formation of the layers,
pp. 121-124. Epiblast, pp. 125-127. Mesoblast and notochord, pp. 128,
129. Hypoblast, pp. 129-131. General growth of the embryo, pp.
131-143. Anura, pp. 131-141. Urodela, pp. 141-143. Gymnophiona, p.
143.
Chapter VIII. Aves. Pp. 145-201.
Segmentation and formation of the layers, pp. 145-166. General history of
of the germinal layers, pp. 166-169. General development of the
embryo, pp. 169-180. Fœtal membranes, pp. 185-199. Amnion, pp.
185-191. Allantois, pp. 191-193. Yolk-sack, pp. 193-199.
Chapter IX. Reptilia. Pp. 202-213.
Lacertilia, pp. 202-209. Segmentation and formation of the layers,
pp. 202-207. General development of the embryo, p. 208. Embryonic
membranes and yolk-sack, pp. 208-210. Ophidia, p. 210. Chelonia,
pp. 210-212.
Chapter X. Mammalia. Pp. 214-274.
Segmentation and formation of the layers, pp. 214-227. General growth
of the embryo, pp. 227-232. Embryonic membranes and yolk-sack, pp.
232-239. Comparative history of the Mammalian fœtal membranes, pp.
239-257. Comparative histology of the placenta, pp. 257-259. Evolution
of the placenta, pp. 259-261. Development of the Guinea-pig, pp.
262-265. The human embryo, pp. 265-270.
Chapter XI. Comparison of the formation of the Germinal Layers and of
the early stages in the development of Vertebrates. Pp. 275-310.
Formation of the gastrula, pp. 275-292. The formation of the mesoblast
and of the notochord, pp. 292-300. The epiblast, pp. 300-304.
Formation of the central nervous system, pp. 301-304. Formation of the
organs of special sense, p. 304. Summary of organs derived from the
three germinal layers, pp. 304-306. Growth in length of the Vertebrate
embryo, pp. 306-309. The evolution of the allantois and amnion, pp.
309, 310.
Chapter XII. Observations on the ancestral form of the Chordata. Pp.
311-330.
General considerations, pp. 311-316. The medullary canal, pp. 316,
317. The origin and nature of the mouth, pp. 317-321. The cranial
flexure, pp. 321, 322. The postanal gut and neurenteric canal, pp.
322-325. The body-cavity and mesoblastic somites, p. 325. The
notochord, pp. 325, 326. Gill clefts, pp. 326, 327. Phylogeny of the
Chordata, pp. 327-329.
Chapter XIII. General Conclusions. Pp. 331-388.
I. Mode of origin and homologies of the germinal layers, pp. 331-360.
Formation of the primary germinal layers, pp. 332, 333. Invagination,
pp. 333-335. Delamination, pp. 335-338. Phylogenetic significance of
delamination and invagination, pp. 338-345. Homologies of the germinal
layers, pp. 345, 346. The origin of the mesoblast, pp. 346-360.
II. Larval forms: their nature, origin, and affinities. Preliminary
considerations, pp. 360-362. Types of larvæ, pp. 363-384. Phylogenetic
conclusions, pp. 384, 385. General conclusions and summary, pp. 385,
386.
PART II. ORGANOGENY;
Introduction. Pp. 391, 392.
Chapter XIV. The Epidermis and its Derivatives. Pp. 393-399.
Protective epidermic structures, pp. 393-397. Dermal skeletal
structures, p. 397. Glands, pp. 397, 398.
Chapter XV. The Nervous System. Pp. 400-469.
The origin of the nervous system, pp. 400-405. Nervous system of the
Invertebrata, pp. 405-414. Central nervous system of the Vertebrata,
pp. 415-447. Spinal chord, pp. 415-419. General development of the
brain, pp. 419-423. Hind-brain, pp. 424-427. Mid-brain, pp. 427, 428.
General development of fore-brain, pp. 428-430. Thalamencephalon, pp.
430-435. Pituitary body, pp. 435-437. Cerebral Hemispheres, pp.
437-444. Olfactory lobes, pp. 444, 445. General conclusions as to the
central nervous system of the Vertebrata, pp. 445-447. Development of
the cranial and spinal nerves, pp. 448-466. Spinal nerves, pp.
448-455. Cranial nerves, pp. 455-466. Sympathetic nervous system,
pp. 466-468.
Chapter XVI. Organs of Vision. Pp. 470-511.
Cœlenterata, pp. 471, 472. Mollusca, pp. 472-479. Chætopoda, p. 479.
Chætognatha, p. 479. Arthropoda, pp. 479-483. Vertebrata general, pp.
483-490. Retina, pp. 490-492. Optic nerve, pp. 492, 493. Choroid
fissure, p. 493. Lens, pp. 494, 495. Vitreous humour, pp. 494, 495.
Cornea, pp. 495-497. Aqueous humour, p. 497. Comparative development
of Vertebrate eye, pp. 497-506. Ammocœte eye, pp. 498, 499. Optic
vesicles, p. 499. Lens, p. 499. Cornea, p. 500. Optic nerve and
choroid fissure, pp. 500-505. Iris and ciliary processes, p. 506.
Accessory organs connected with the eye, p. 506. Eyelids, p. 506.
Lacrymal glands, p. 506. Lacrymal duct, pp. 506, 507. Eye of the
Tunicata, pp. 507-509. Accessory eyes in the Vertebrata, pp. 509,
510.
Chapter XVII. Auditory organ, Olfactory organ, and Sense organs of the
Lateral line. Pp. 512-541.
Auditory organs, pp. 512-531. General structure of auditory organs,
pp. 512, 513. Auditory organs of the Cœlenterata, pp. 513-515.
Auditory organs of the Mollusca, pp. 515, 516. Auditory organs of the
Crustacea, p. 516. Auditory organs of the Vertebrata, pp. 516-530.
Auditory vesicle, pp. 517-524. Organ of Corti, pp. 524-527. Accessory
structures connected with the organ of hearing of terrestrial
vertebrata, pp. 527-530. Auditory organ of the Tunicata, pp. 530, 531.
Bibliography of Auditory organs, p. 531.
Olfactory organs, pp. 531-538. Bibliography of Olfactory organs, p.
538. Sense organs of the lateral line, pp. 538-540. Bibliography of
sense organs of lateral line, pp. 540, 541.
Chapter XVIII. The Notochord, the Vertebral Column, the Ribs, and the
Sternum. Pp. 542-563.
Introductory remarks on the origin of the skeleton, pp. 542-544.
Bibliography of the origin of the skeleton, pp. 544, 545. The
notochord and its cartilaginous sheath, pp. 545-549. The vertebral
arches and the vertebral bodies, pp. 549-559. Cyclostomata, p. 549.
Elasmobranchii, pp. 549-553. Ganoidei, p. 553. Teleostei, p. 553.
Amphibia, pp. 553-556. Reptilia, pp. 556, 557. Aves, pp. 557, 558.
Mammalia, pp. 558, 559. Bibliography of the notochord and vertebral
column, p. 560. Ribs, pp. 560-562. Sternum, pp. 562, 563.
Bibliography of the ribs and sternum, p. 563.
Chapter XIX. The Skull. Pp. 564-598.
Preliminary remarks, pp. 564, 565. The cartilaginous cranium, pp.
565-571. The parachordals and notochord, pp. 566, 567. The trabeculæ,
pp. 567-570. The sense capsules, pp. 570, 571. The branchial skeleton,
pp. 572-591. General structure of, pp. 572-575. Mandibular and hyoid
arches, pp. 575-591. Elasmobranchii, pp. 576-579. Teleostei, pp.
579-581. Amphibia, pp. 581-588. Sauropsida, pp. 588, 589. Mammalia,
pp. 589-591. Membrane bones and ossifications of the cranium, pp.
592-597. Membrane bones, pp. 592-595. Ossifications of the
cartilaginous cranium, pp. 595-597. Labial cartilages, p. 597.
Bibliography of the skull, p. 598.
Chapter XX. Pectoral and Pelvic Girdles and the Skeleton of the Limbs.
Pp. 599-622.
The Pectoral girdle, pp. 599-606. Pisces, pp. 599-601. Amphibia and
Amniota, pp. 601, 602. Lacertilia, p. 603. Chelonia, p. 603. Aves,
pp. 603, 604. Mammalia, p. 604. Amphibia, p. 605. Bibliography of
Pectoral girdle, pp. 605, 606.
The Pelvic girdle, pp. 606-608. Pisces, pp. 606, 607. Amphibia and
Amniota, pp. 606, 607. Amphibia, p. 607. Lacertilia, p. 607.
Mammalia, p. 608. Bibliography of Pelvic girdle, p. 608. Comparison
of pectoral and pelvic girdles, pp. 608, 609.
Limbs, pp. 609-622. The piscine fin, pp. 609-618. The cheiropterygium,
pp. 618-622. Bibliography of limbs, p. 622.
Chapter XXI. The Body Cavity, the Vascular System and the Vascular
Glands. Pp. 623-666.
The body cavity, pp. 623-632. General, pp. 623, 624. Chordata, pp.
624-632. Abdominal pores, pp. 626, 627. Pericardial cavities, pleural
cavities and diaphragm, pp. 627-632. Bibliography of body cavity, p.
632.
The vascular system, pp. 632-663. General, pp. 632, 633. The heart,
pp. 633-643. Bibliography of the heart, p. 643. Arterial system, pp.
643-651. Bibliography of the arterial system, p. 651. Venous system,
pp. 651-663. Bibliography of the venous system, p. 663. Lymphatic
system and spleen, p. 664. Bibliography of spleen, p. 664. Suprarenal
bodies, pp. 664-666. Bibliography of suprarenal bodies, p. 666.
Chapter XXII. The Muscular System. Pp. 667-679.
Evolution of muscle-cells, pp. 667, 668. Voluntary muscular system of
the Chordata, pp. 668-679. Muscular fibres, pp. 668, 669. Muscular
system of the trunk and limbs, pp. 673-676. The somites and muscular
system of the head, pp. 676-679. Bibliography of muscular system, p.
679.
Chapter XXIII. Excretory organs. Pp. 680-740.
Platyelminthes, pp. 680, 681. Mollusca, pp. 681, 682. Polyzoa, pp.
682, 683. Branchiopoda, p. 683. Chætopoda, pp. 683-686. Gephyrea, pp.
686, 687. Discophora, pp. 687, 688. Arthropoda, pp. 688, 689.
Nematoda, p. 689. Excretory organs and generative ducts of the
Craniata, pp. 689-737. General, pp. 689, 690. Elasmobranchii, pp.
690-699. Cyclostomata, pp. 700, 701. Teleostei, pp. 701-704. Ganoidei,
pp. 704-707. Dipnoi, p. 707. Amphibia, pp. 707-713. Amniota, pp.
713-727. General conclusions and summary, pp. 728-737. Pronephros, pp.
728, 729. Mesonephros, pp. 729-732. Genital ducts, pp. 732-736.
Metanephros, pp. 736, 737. Comparison of the excretory organs of the
Chordata and Invertebrata, pp. 737, 738. Bibliography of Excretory
organs, pp. 738-740.
Chapter XXIV. Generative Organs and Genital Ducts. Pp. 741-753.
Generative organs, pp. 741-748. Porifera, p. 741. Cœlenterata, pp.
741-743. Chætopoda and Gephyrea, p. 743. Chætognatha, pp. 743-745.
Polyzoa, p. 745 Nematoda, p. 745. Insecta, p. 745. Crustacea, pp. 745,
746. Chordata, pp. 746-748. Bibliography of generative organs, p. 748.
Genital ducts, pp. 748-753.
Chapter XXV. The Alimentary Canal and its appendages in the Chordata.
Pp. 754-780.
Mesenteron, pp. 754-774. Subnotochordal rod, pp. 754-756. Splanchnic
mesoblast and mesentery, pp. 756-758. Respiratory division of the
Mesenteron, pp. 758-766. Thyroid body, pp. 759-762. Thymus gland, pp.
762, 763. Swimming bladder and lungs, pp. 763-766, The middle
division of the Mesenteron, pp. 766-771. Cloaca, pp. 766, 767.
Intestine, pp. 767, 768. Liver, pp. 769, 770. Pancreas, pp. 770, 771.
Postanal section of the Mesenteron, pp. 771-774.
The stomodæum, pp. 774-778. Comparative development of oral cavity,
pp. 774-776. Teeth, pp. 776-778.
The proctodæum, pp. 778-780. Bibliography of alimentary canal, p. 780.
[Pg 1]
EMBRYOLOGY.
CHAPTER I.
CEPHALOCHORDA.
The developmental history of the Chordata has been studied far more
completely than that of any of the groups so far considered; and the
results which have been arrived at are of striking interest and
importance. Three main subdivisions of this group can be recognized:
(1) the Cephalochorda containing the single genus Amphioxus; (2) the
Urochorda or Tunicata; and (3) the Vertebrata[1].
The members of the
second and probably of the first of these groups have undergone
degeneration, but at the same time the members of the first group
especially undergo a less modified development than that of other
Chordata.
Cephalochorda.
Our knowledge of the development of Amphioxus is mainly due to
Kowalevsky (Nos. 1 and 2). The ripe eggs appear to be dehisced into
the branchial or atrial cavity, and to be transported thence through
the branchial clefts into the pharynx, and so through the mouth to the
exterior. (Kowalevsky, No. 1, and Marshall, No. 5.)
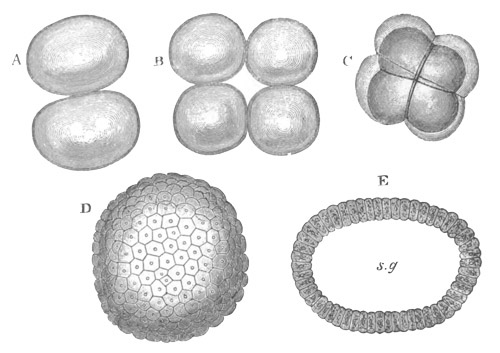
Fig. 1. The Segmentation of Amphioxus. (Copied from Kowalevsky.)
B. Stage with four equal segments.
C. Stage after the four segments have become divided by an
equatorial furrow into eight equal segments.
D. Stage in which a single layer of cells encloses a central
segmentation cavity.
E. Somewhat older stage in optical section.
sg. segmentation cavity.
[Pg 2]
When laid the egg is about 0.105 mm. in diameter. It is invested by a
delicate membrane, and is somewhat opaque owing to the presence of
yolk granules, which are however uniformly distributed through it, and
proportionately less numerous than in the ova of most Chordata.
Impregnation is external and the segmentation is nearly regular (fig.
1). A small segmentation cavity is visible at the stage with four
segments, and increases during the remainder of the segmentation; till
at the close (fig. 1 E) the embryo consists of a blastosphere formed
of a single layer of cells enclosing a large segmentation cavity. One
side of the blastosphere next becomes invaginated, and during the
process the embryo becomes ciliated, and commences to rotate. The
cells forming the invaginated layer become gradually more columnar
than the remaining cells, and constitute the hypoblast; and a
structural distinction between the epiblast and hypoblast is thus
established. In the course of the invagination the segmentation
[Pg 3] cavity
becomes gradually obliterated, and the embryo first assumes a
cup-shaped form with a wide blastopore, but soon becomes elongated,
while the communication of the archenteron, or cavity of invagination,
with the exterior is reduced to a small blastopore (fig. 2 A), placed
at the pole of the long axis which the subsequent development shews to
be the hinder end of the embryo. The blastopore is often known in
other Chordata as the anus of Rusconi. Before the invagination is
completed the larva throws off the egg-membrane, and commences to lead
a free existence.
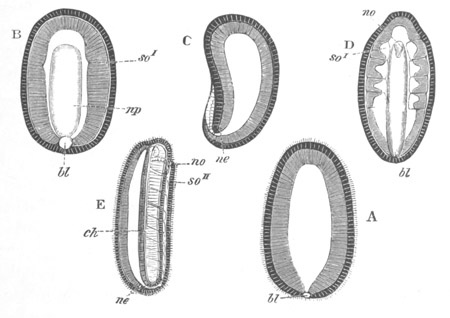
Fig. 2. Embryos of Amphioxus. (After Kowalevsky.)
The parts in black with white lines are epiblastic; the shaded parts
are hypoblastic.
A. Gastrula stage in optical section.
B. Slightly later stage after the neural plate np has become
differentiated, seen as a transparent object from the dorsal side.
C. Lateral view of a slightly older larva in optical section.
D. Dorsal view of an older larva with the neural canal completely
closed except for a small pore (no) in front.
E. Older larva seen as a transparent object from the side.
bl. blastopore (which becomes in D the neurenteric canal); ne.
neurenteric canal; np. neural or medullary plate; no. anterior
opening of neural canal; ch. notochord; soI, soII. first
and second mesoblastic somites.
Up to this stage the larva, although it has acquired a cylindrical
elongated form, has only the structure of a simple two-layered
gastrula; but the changes which next take place[Pg 4] give rise on the one
hand to the formation of the central nervous system, and on the other
to the formation of the notochord and mesoblastic somites[2].
The
former structure is developed from the epiblast and the two latter
from the hypoblast.
The formation of the central nervous system commences with the
flattening of the dorsal surface of the embryo. The flattened area
forms a plate (fig. 2 B and fig. 3 A, np), extending backwards to
the blastopore, which has in the meantime passed round to the dorsal
surface. The sides of the plate become raised as two folds, which are
most prominent posteriorly, and meet behind the blastopore, but shade
off in front. The two folds next unite dorsally, so as to convert the
previous groove into a canal[3]—the
neural or medullary canal. They
unite first of all over the blastopore, and their line of junction
extends from this point forwards (fig. 2 C, D, E). There is in this
way formed a tube on the floor of which the blastopore opens behind,
and which is itself open in front. Finally the medullary canal is
formed for the whole length of the embryo. The anterior opening
persists however for some time. The communication between the neural
and alimentary tracts becomes interrupted when the caudal fin appears
and the anus is formed. The neural canal then extends round the end of
the notochord to the ventral side, but subsequently retreats to the
dorsal side and terminates in a slight dilatation.
In the formation of the medullary canal there are two points deserving
notice—viz. (1) the connection with the blastopore; (2) the relation
of the walls of the canal to the adjoining epiblast. With reference to
the first of these points it is clear that the fact of the blastopore
opening on the floor of the neural canal causes a free communication
to exist between the archenteron or gastrula cavity and the neural
canal; and that, so long as the anterior pore of the neural canal
remains open, the archenteron communicates indirectly with the
exterior (vide fig. 2 E). It must not however be supposed (as has
been done by some embryologists) that the pore at the front end of the
neural canal represents the blastopore carried forwards. It is
[Pg 5] even
probable that what Kowalevsky describes as the carrying of the
blastopore to the dorsal side is really the commencement of the
formation of the neural canal, the walls of which are continuous with
the lips of the blastopore. This interpretation receives support from
the fact that at a later stage, when the neural and alimentary canals
become separated, the neural canal extends round the posterior end of
the notochord to the ventral side. The embryonic communication between
the neural and alimentary canals is common to most Chordata; and the
tube connecting them will be called the neurenteric canal. It is
always formed in fundamentally the same manner as in Amphioxus. With
reference to the second point it is to be noted that Amphioxus is
exceptional amongst the Chordata in the fact that, before the closure
of the neural groove, the layer of cells which will form the neural
tube becomes completely separated from the adjoining epiblast (fig. 3
A), and forms a structure which may be spoken of as the medullary
plate; and that in the closure of the neural canal the lateral
epiblast forms a complete layer above this plate before the plate
itself is folded over into a closed canal. This peculiarity will be
easily understood from an examination of fig. 3 A, B and C.
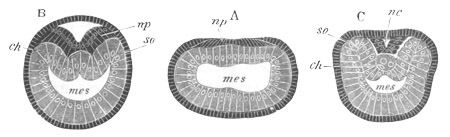
Fig. 3. Sections of an Amphioxus embryo at three stages.
(After Kowalevsky.)
A. Section at gastrula stage.
B. Section of an embryo slightly younger than that represented in
fig. 2 D.
C. Section through the anterior part of an embryo at the stage
represented in fig. 2 E.
np. neural plate; nc. neural canal; mes. archenteron in A and
B, and mesenteron in C; ch. notochord; so. mesoblastic somite.
The formation of the mesoblastic somites commences, at about the same
time as that of the neural canal, as a pair of hollow outgrowths of
the walls of the archenteron. These[Pg 6] outgrowths, which are shewn in
surface view in fig. 2 B and D, so, and in section in fig. 3 B and
C, so, arise near the front end of the body and gradually extend
backwards as wing-like diverticula of the archenteric cavity. As they
grow backwards their dorsal part becomes divided by transverse
constrictions into cubical bodies (fig. 2 D and E), which, with the
exception of the foremost, soon cease to open into what may now be
called the mesenteron, and form the mesoblastic somites. Each
mesoblastic somite, after its separation from the mesenteron, is
constituted of two layers, an inner one—the splanchnic—and an
outer—the somatic, and a cavity between the two which was originally
continuous with the cavity of the mesenteron. Eventually the dorsal
parts of the outgrowths become separated from the ventral, and form
the muscle-plates, while their cavities atrophy. The cavity of the
ventral part, which is not divided into separate sections by the above
described constrictions, remains as the true body cavity. The ventral
part of the inner layer of the mesoblastic outgrowths gives rise to
the muscular and connective tissue layers of the alimentary tract, and
the dorsal part to a section of the voluntary muscular system. The
ventral part of the outer layer gives rise to the somatic mesoblast,
and the dorsal to a section of the voluntary muscular system. The
anterior mesoblastic somite long retains its communication with the
mesenteron, and was described by Max Schultze, and also at first by
Kowalevsky, as a glandular organ. While the mesoblastic somites are
becoming formed the dorsal wall of the mesenteron develops a median
longitudinal fold (fig. 3 B, ch), which is gradually separated off
from before backwards as a rod (fig. 3 C, ch), underlying the
central nervous system. This rod is the notochord. After the
separation of those parts the remainder of the hypoblast forms the
wall of the mesenteron.
With the formation of the central nervous system, the mesoblastic
somites, the notochord, and the alimentary tract the main systems of
organs are established, and it merely remains briefly to describe the
general changes of form which accompany the growth of the larva into
the adult. By the time the larva is but twenty-four hours old there
are formed about seventeen mesoblastic somites. The body, during the
period in which[Pg 7] these are being formed, remains cylindrical, but
shortly afterwards it becomes pointed at both ends, and the caudal fin
appears. The fine cilia covering the larva also become replaced by
long cilia, one to each cell. The mesenteron is still completely
closed, but on the right side of the body, at the level of the front
end of the mesenteron, the hypoblast and epiblast now grow together,
and a perforation becomes formed through their point of contact, which
becomes the mouth. The anus is probably formed about the same time if
not somewhat earlier[4].
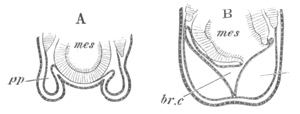
Fig. 4. Sections through two advanced embryos of Amphioxus to
shew the formation of the peribranchial cavity. (After
Kowalevsky.)
In A are seen two folds of the body wall with a prolongation of the
body cavity. In B the two folds have coalesced ventrally, forming a
cavity into which a branchial cleft is seen to open.
mes. mesenteron; br.c. branchial cavity; pp. body cavity.
Of the subsequent changes the two most important are (1) the formation
of the gill slits or clefts; (2) the formation of the peribranchial or
atrial cavity.
The formation of the gill slits is, according to Kowalevsky’s
description, so peculiar that one is almost tempted to suppose that
his observations were made on pathological specimens. The following is
his account of the process. Shortly after the formation of the mouth
there appears on the ventral line a coalescence between the epiblast
and hypoblast. Here an opening is formed, and a visceral cleft is thus
established, which passes to the left side, viz. the side opposite the
mouth. A second and apparently a third slit are formed in the same
way. The stages immediately following were not observed, but in the
next stage twelve slits were present, no longer however on the left
side, but in the median ventral line. There now appears on the side
opposite the mouth, and the same therefore as that originally occupied
by the first three clefts, a series of fresh clefts, which in their
[Pg 8]growth push the original clefts over to the same side as the mouth.
Each of the fresh clefts becomes divided into two, which form the
permanent clefts of their side.
The gill slits at first open freely to the exterior, but during their
formation two lateral folds of the body wall, containing a
prolongation of the body cavity, make their appearance (fig. 4 A), and
grow downwards over the gill clefts, and finally meet and coalesce
along the ventral line, leaving a widish cavity between themselves and
the body wall. Into this cavity, which is lined by epiblast, the gill
clefts open (fig. 4 B, br.c). This cavity—which forms a true
peribranchial cavity—is completely closed in front, but owing to the
folds not uniting completely behind it remains in communication with
the exterior by an opening known as the atrial or abdominal pore.
The vascular system of Amphioxus appears at about the same time as the
first visceral clefts.
Bibliography.
(1) A. Kowalevsky. “Entwicklungsgeschichte des Amphioxus lanceolatus.”
Mém. Acad. Impér. des Sciences de St Pétersbourg, Series VII. Tom.
XI. 1867.
(2) A. Kowalevsky. “Weitere Studien über die Entwicklungsgeschichte
des Amphioxus lanceolatus.” Archiv f. mikr. Anat., Vol. XIII. 1877.
(3) Leuckart u. Pagenstecher. “Untersuchungen über niedere Seethiere.”
Müller’s Archiv, 1858.
(4) Max Schultze. “Beobachtung junger Exemplare von Amphioxus.” Zeit.
f. wiss. Zool., Bd. III. 1851.
(5) A. M. Marshall. “On the mode of Oviposition of Amphioxus.” Jour.
of Anat. and Phys., Vol. X. 1876.
[Pg 9]
CHAPTER II.
UROCHORDA[5].
In the Solitaria, except Cynthia, the eggs are generally laid, and
impregnation is effected sometimes before and sometimes after the eggs
have left the atrial cavity. In Cynthia and most Caducichordata
development takes place within the body of the parent, and in the
Salpidæ a vascular connection is established between the parent and
the single fœtus, forming a structure physiologically comparable with
the Mammalian placenta.
Solitaria. The development of the Solitary Ascidians has been more
fully studied than that of the other groups, and appears moreover to
be the least modified. It has been to a great extent elucidated by the
splendid researches of Kowalevsky (Nos. 18 and 20), whose statements
have been in the main followed in the account below. Their truth seems
to me to be established, in spite of the scepticism they have met with
in some quarters, by the closeness of their correspondence with the
developmental phenomena in Amphioxus.
[Pg 10]The type most fully investigated by Kowalevsky is Ascidia (Phallusia)
mammillata; and the following description must be taken as more
especially applying to this type.
The segmentation is complete and regular. A small segmentation cavity
appears fairly early, and is surrounded, according to Kowalevsky, by a
single layer of cells, though on this point Kupffer (No. 27) and Giard
(No. 11) are at variance with him.
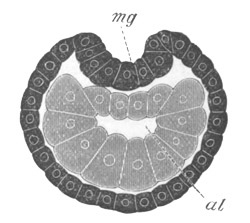
Fig. 5. Transverse section
through the front end of an embryo
of Phallusia mammillata. (After Kowalevsky.)
The embryo is slightly younger than that represented in fig. 8 III.
mg. medullary groove; al. alimentary tract.
The segmentation is followed by an invagination of nearly the same
character as in Amphioxus. The blastosphere resulting from the
segmentation first becomes flattened on one side, and the cells on the
flatter side become more columnar (fig. 8 I.). Very shortly a
cup-shaped form is assumed, the concavity of which is lined by the
more columnar cells. The mouth of the cup or blastopore next becomes
narrowed; while at the same time the embryo becomes oval. The
blastopore is situated not quite at a pole of the oval but in a
position which subsequent development shews to be on the dorsal side
close to the posterior end of the embryo. The long axis of the oval
corresponds with the long axis of the embryo. At this stage the embryo
consists of two layers; a columnar hypoblast lining the central cavity
or archenteron, and a thinner epiblastic layer. The dorsal side of the
embryo next becomes flattened (fig. 8 II.), and the epiblast covering
it is shortly afterwards marked by an axial groove continued forwards
from the blastopore to near the front end of the body (fig. 5, mg).
This is the medullary groove, and it soon becomes converted into a
closed canal—the medullary or neural canal—below the external skin
(fig. 6, n.c). The closure is effected by the folds on each side of
the furrow meeting and coalescing dorsally. The original medullary
folds fall into one another behind the blastopore so that the
blastopore is situated on the[Pg 11] floor of the groove, and, on the
conversion of the groove into a canal, the blastopore connects the
canal with the archenteric cavity, and forms a short neurenteric
canal. The closure of the medullary canal commences at the blastopore
and is thence continued forwards, the anterior end of the canal
remaining open. The above processes are represented in longitudinal
section in fig. 8 III, n. When the neural canal is completed for its
whole length, it still communicates by a terminal pore with the
exterior. In the relation of the medullary canal to the blastopore, as
well as in the closure of the medullary groove from behind forwards,
the Solitary Ascidians agree closely with Amphioxus.
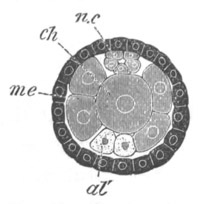
Fig. 6. Transverse optical
section of the tail of an embryo
of Phallusia mammillata. (After Kowalevsky.)
The section is from an embryo of the same age as fig. 8 IV.
ch. notochord; n.c. neural canal; me. mesoblast; al.
hypoblast of tail.
The cells of the dorsal wall of the archenteron immediately adjoining
the front and sides of the blastopore have in the meantime assumed a
somewhat different character from the remaining cells of the
archenteron, and give rise to a body which, when viewed from the
dorsal surface, has somewhat the form of a horseshoe. This body was
first observed by Metschnikoff. On the elongation of the embryo and
the narrowing of the blastopore the cells forming this body arrange
themselves as a broad linear cord, two cells wide, underlying about
the posterior half of the neural canal (fig. 7, ch). They form the
rudiment of the notochord, which, as in Amphioxus, is derived from the
dorsal wall of the archenteron. They are seen in longitudinal section
in fig. 8 II. and III. ch.
With the formation of the notochord the body of the embryo becomes
divided into two distinct regions—a posterior region where the
notochord is present, and an anterior region into which it is not
prolonged. These two regions correspond with the tail and the trunk of
the embryo at a slightly later stage. The section of the archenteric
cavity in the trunk dilates and constitutes the permanent mesenteron
(figs. 7, al, and 8 III. and IV. dd). It soon becomes shut off
from the slit-like posterior[Pg 12] part of the archenteron. The nervous
system in this part also dilates and forms what may be called the
cephalic swelling (fig. 8 IV.), and the pore at its anterior extremity
gradually narrows and finally disappears. In the region of the tail we
have seen that the dorsal wall of the archenteron becomes converted
into the notochord, which immediately underlies the posterior part of
the medullary canal, and soon becomes an elongated cord formed of a
single or double row of flattened cells. The lateral walls of the
archenteron (fig. 7, me) in the tail become converted into elongated
cells arranged longitudinally, which form powerful lateral muscles
(fig. 8 IV. m). After the formation of the notochord and of the
lateral muscles there remains of the archenteron in the tail only the
ventral wall, which according to Kowalevsky forms a simple cord of
cells (fig. 6, al). It is however not always present, or else has
escaped the attention of other observers. It is stated by Kowalevsky
to be eventually transformed into blood corpuscles. The neurenteric
canal leads at first into the narrow space between the above
structures, which is the remnant of the posterior part of the lumen of
the archenteron. Soon both the neurenteric canal and the caudal
remnant of the archenteron become obliterated.
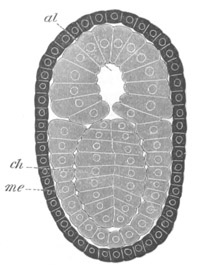
Fig. 7. Optical section of an embryo of Phallusia
mammillata. (After Kowalevsky.)
The embryo is of the same age as fig. 8 III, but is seen in
longitudinal horizontal section.
al. alimentary tract in anterior
part of body; ch. notochord; me. mesoblast.
During the above changes the tail becomes considerably elongated and,
owing to the larva being still in the egg-shell, is bent over to the
ventral side of the trunk.
The larva at this stage is represented in a side view in fig. 8 IV.
The epidermis is formed throughout of a single layer of cells. In the
trunk the mesenteron is shewn at dd and the dilated part of the
nervous system, no longer communicating with the exterior, at n. In
the tail the notochord is shewn at ch, the muscles at m, and the
solid remnant of the ventral wall[Pg 14] of the archenteron at dd´. The
delicate continuation of the neural canal in the tail is seen above
the notochord at n. An optical section of the tail is shewn in fig.
6. It is worthy of notice that the notochord and muscles are formed in
the same manner as in Amphioxus, except that the process is somewhat
simplified. The mode of disappearance of the archenteric cavity in the
tail, by the employment of the whole of its walls in the formation of
various organs, is so peculiar, that I feel some hesitation in
accepting Kowalevsky’s statements on this head[6].
[Pg 13]
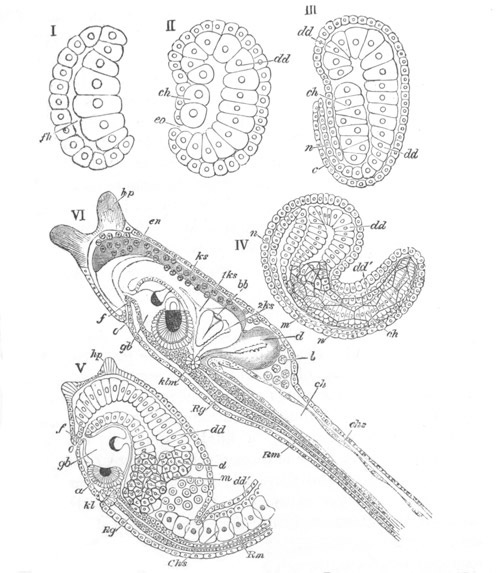
Fig. 8. Various stages in the development of Phallusia mammillata.
(From Huxley; after Kowalevsky.)
The embryos are represented in longitudinal vertical section.
I. Commencing gastrula stage. fh. segmentation cavity.
II. Late gastrula stage with flattened dorsal surface. eo.
blastopore; ch. notochord; dd. hypoblast.
III. A more advanced embryo with a partially-formed neural tube.
ch. and dd. as before; n. neural tube; c. epiblast.
IV. Older embryo in which the formation of the neural tube is
completed. dd. hypoblast enclosing persistent section of
alimentary tract; dd´. hypoblast in the tail; m. muscles.
V. Larva just hatched. The end of the tail is not represented. a.
eye; gb. dilated extremity of neural tube with otolith projecting
into it; Rg. anterior swelling of the spinal division of the
neural tube; f. anterior pore of neural tube; Rm. posterior part
of neural tube; o. mouth; Chs. notochord; kl. atrial
invagination; dd. branchial region of alimentary tract; d.
commencement of œsophagus and stomach; dd´. hypoblast in the
tail; m. muscles; hp. papilla for attachment.
VI. Body and anterior part of the tail of a two days’ larva. klm.
atrial aperture; en. endostyle; ks. branchial sack; 1ks. 2ks.
branchial slits; bb. branchial vessel between them; ch. axial
portion of notochord; chs. peripheral layer of cells. Other
reference letters as before.
The larva continues to grow in length, and the tail becomes further
curled round the ventral side of the body within the egg-membrane.
Before the tail has nearly reached its full length the test becomes
formed as a cuticular deposit of the epiblast cells (O. Hertwig, No.
13, Semper, No. 37). It appears first in the tail and gradually
extends till it forms a complete investment round both tail and trunk,
and is at first totally devoid of cells. Shortly after the
establishment of the test there grow out from the anterior end of the
body three peculiar papillæ, developed as simple thickenings of the
epidermis. At a later stage, after the hatching of the larva, these
papillæ develop glands at their extremities, secreting a kind of
glutinous fluid[7].
After these papillæ have become formed cells first
make their appearance in the test; and there is simultaneously formed
a fresh inner cuticular layer of the test, to which at first the cells
are confined, though subsequently they are found in the outer layer
also. On the appearance of cells in the test the latter must be
regarded as a form, though a very abnormal one, of connective tissue.
When the tail of the larva has reached a very considerable length the
egg-membrane bursts, and the larva becomes free. The hatching takes
place in Asc. canina about 48-60 hours after impregnation. The free
larva (fig. 8 V.) has a swollen trunk, and a very long tail, which
soon becomes[Pg 15] straightened out. It has a striking resemblance to a
tadpole (vide fig. 10).
In the free larval condition the Ascidians have in many respects a
higher organization than in the adult state. It is accordingly
convenient to divide the subsequent development into two periods, the
first embracing the stages from the condition represented in fig. 8 V.
up to the full development of the free larva, and the second the
period from the full development of the larva to the attainment of the
fixed adult condition.
Growth and Structure of the free larva.
The nervous system. The nervous system was left as a closed tube
consisting of a dilated anterior division, and a narrow posterior one.
The former may be spoken of as the brain, and the latter as the spinal
cord; although the homologies of these two parts are quite uncertain.
The anterior part of the spinal cord lying within the trunk dilates
somewhat (fig. 8 V. and VI. Rg) and there may thus be distinguished
a trunk and a caudal section of the spinal cord.
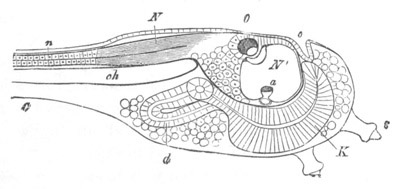
Fig. 9. Larva of Ascidia mentula. (From Gegenbaur; after Kupffer.)
Only the anterior part of the tail is represented.
N´. anterior swelling of neural tube; N. anterior swelling of
spinal portion of neural tube; n. hinder part of neural tube;
ch. notochord; K. branchial region of alimentary tract; d.
œsophageal and gastric region of alimentary tract; O. eye; a.
otolith; o. mouth; s. papilla for attachment.
The original single vesicle of the brain becomes divided by the time
the larva is hatched into two sections (fig. 9)—(1) an anterior
vesicle with, for the most part, thin walls, in which[Pg 16] unpaired
auditory and optic organs make their appearance, and (2) a posterior
nearly solid cephalic ganglion, through which there passes a narrow
continuation of the central canal of the nervous system. This ganglion
consists of a dorsal section formed of distinct cells, and a ventral
section formed of a punctated material with nuclei. The auditory
organ[8]
consists of a ‘crista acustica’ (fig. 9), in the form of a
slight prominence of columnar cells on the ventral side of the
anterior cerebral vesicle; to the summit of which a spherical otolith
is attached by fine hairs. In the crista is a cavity containing clear
fluid. The dorsal half of the otolith is pigmented: the ventral half
is without pigment. The crista is developed in situ, but the otolith
is formed from a single cell on the dorsal side of the cerebral
vesicle, which forms a projection into the cavity of the vesicle, and
then travels (in a manner not clearly made out) round the right side
of the vesicle till it comes to the crista; to which it is at first
attached by a narrow pedicle. The fully developed eye (figs. 8 VI. and
9, O) consists of a cup-shaped retina, which forms a prominence
slightly on the right side of the posterior part of the dorsal wall of
the anterior cerebral vesicle, and of refractive media. The retina is
formed of columnar cells, the inner ends of which are imbedded in
pigment. The refractive media of the eye are directed towards the
cavity of the cerebral vesicle, and consist of a biconvex lens and a
meniscus. Half the lens is imbedded in the cavity of the retina and
surrounded by the pigment, and the other half is turned toward a
concavo-convex meniscus which corresponds in position with the cornea.
The development of the meniscus and lens is unknown, but the retina is
formed (fig. 8 V. a) as an outgrowth of the wall of the brain. At
the inner ends of the cells of this outgrowth a deposit of pigment
appears.
The trunk section of the spinal cord (fig. 9, N) is separated by a
sharp constriction from the brain. It is formed of a superficial layer
of longitudinal nervous fibres, and a central core of ganglion cells.
The layer of fibres diminishes in thickness towards the tail, and
finally ceases to be visible. Kupffer detected three pairs of nerves
passing off from the spinal cord to[Pg 17] the muscles of the tail. The
foremost of these arises at the boundary between the trunk and the
tail, and the two others at regular intervals behind this point.
The mesoblast and muscular system. It has already been stated that the
lateral walls of the archenteron in the tail give rise to muscular
cells. These cells lie about three abreast, and appear not to increase
in number; so that with the growth of the tail they grow enormously in
length, and eventually become imperfectly striated. The mesoblast
cells at the hinder end of the trunk, close to its junction with the
tail, do not become converted into muscle cells, but give rise to
blood corpuscles; and the axial remnant of the archenteron undergoes a
similar fate. According to Kowalevsky the heart is formed during
larval life as an elongated closed sack on the right side of the
endostyle.
The notochord. The notochord was left as a rod formed of a single row
of cells, or in As. canina and some other forms of two rows, extending
from just within the border of the trunk to the end of the tail.
According to Kowalevsky, Kupffer, Giard, etc. the notochord undergoes
a further development which finds its only complete parallel amongst
Chordata in the doubtful case of Amphioxus.
There appear between the cells peculiar, highly refractive discs (fig.
8 V. Chs). These become larger and larger, and finally, after
pushing the remnants of the cells with their nuclei to the sides,
coalesce together to form a continuous axis of hyaline substance. The
remnants of the cells with their nuclei form a sheath round the
hyaline axis (fig. 8 VI. ch.). Whether the axis is to be regarded as
formed of an intercellular substance, or of a differentiation of parts
of the cells is still doubtful. Kupffer inclines to the latter view:
the analogy of the notochord of higher types appears to me to tell in
favour of the former one.
The alimentary tract. The anterior part of the primitive archenteron
alone retains a lumen, and from this part the whole of the permanent
alimentary tract (mesenteron) becomes developed. The anterior part of
it grows upwards, and before hatching an involution of the epiblast on
the dorsal side, just in front of the anterior extremity of the
nervous system, meets and opens into this upgrowth, and gives rise to
the permanent mouth (fig. 8 V. o).
[Pg 18]
Kowalevsky states that a pore is formed at the front end of the
nervous tube leading into the mouth (fig. 8 V. and VI. f) which
eventually gives rise to the ciliated sack, which lies in the adult at
the junction between the mouth and the branchial sack. Kupffer however
was unable to find this opening; but Kowalevsky’s observations are
confirmed by those of Salensky on Salpa.
From the hinder end of the alimentary sack an outgrowth directed
dorsalwards makes its appearance (figs. 8 V. and 9, d), from which
the œsophagus, stomach and intestine become developed. It at first
ends blindly. The remainder of the primitive alimentary sack gives
rise to the branchial sack of the adult. Just after the larva has
become hatched, the outgrowth to form the stomach and œsophagus, etc.
bends ventralwards and to the right, and then turns again in a dorsal
and left direction till it comes close to the dorsal surface, somewhat
to the left of and close to the hinder end of the trunk. The first
ventral loop of this part gives rise to the œsophagus, which opens
into the stomach; from this again the dorsally directed intestine
passes off.
On the ventral wall of the branchial sack there is formed a narrow
fold with thickened walls, which forms the endostyle. It ends
anteriorly at the stomodæum and posteriorly at the point where the
solid remnant of the archenteron in the tail was primitively
continuous with the branchial sack. The whole of the alimentary wall
is formed of a single layer of hypoblast cells.
A most important organ connected with the alimentary system still
remains to be dealt with, viz. the atrial or peribranchial cavity. The
first rudiments of it appear at about the time of hatching, in the
form of a pair of dorsal epiblastic involutions (fig. 8 V. kl), at
the level of the junction between the brain and the spinal cord. These
involutions grow inwards, and meet corresponding outgrowths of the
branchial sack, with which they fuse. At the junction between them is
formed an elongated ciliated slit, leading from the branchial sack
into the atrial cavity of each side. The slits so formed are the first
pair of branchial clefts. Behind the first pair of branchial clefts a
second pair is formed during larval life by a second outgrowth of the
branchial sack meeting the epiblastic atrial involutions (fig. 8 VI.
1ks and 2ks). The intestine at first ends blindly close[Pg 19] to the
left atrial involution, but the anus becomes eventually formed by an
opening being established between the left atrial involution and the
intestine.
During the above described processes the test remains quite intact,
and is not perforated at the oral or the atrial openings.
The retrogressive metamorphosis of the larva.
The development of the adult from the larva is, as has already been
stated, in the main a retrogressive metamorphosis. The stages in this
metamorphosis are diagrammatically shewn in figs. 10 and 11. It
commences with the attachment of the larva (fig. 10 A) which takes
place by one of the three papillæ. Simultaneously with the attachment
the larval tail undergoes a complete atrophy (fig. 10 B), so that
nothing is left of it but a mass of fatty cells situated close to the
point of the previous insertion of the tail in the trunk.
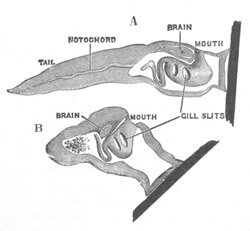
Fig. 10. Diagram shewing the mode of
attachment and subsequent retrogressive
metamorphosis of a larval Ascidian. (From Lankester.)
The nervous system also undergoes a very rapid retrogressive
metamorphosis; and the only part of it which persists would seem to be
the dilated portion of the spinal cord in the trunk (Kupffer, No. 28).
The three papillæ, including that serving for attachment, early
disappear, and the larva becomes fixed by a growth of the test to
foreign objects.
An opening appears in the test some time after the larva is fixed,
leading into the mouth, which then becomes functional. The branchial
sack at the same time undergoes important changes. In the larva it is
provided with only two ciliated slits, which open into the, at this
stage, paired atrial cavity (fig. 10).
[Pg 20]The openings of the atrial cavity at first are shut off from
communication with the exterior by the test, but not long after the
larva becomes fixed, two perforations are formed in the test, which
lead into the openings of the two atrial cavities. At the same time
the atrial cavities dilate so as gradually to embrace the whole
branchial sack to which their inner walls attach themselves. Shortly
after this the branchial clefts rapidly increase in number[9].
The increase of the branchial clefts is somewhat complicated. Between
the two primitive clefts two new ones appear, and then a third appears
behind the last cleft. In the interval between each branchial cleft is
placed a vascular branchial vessel (fig. 8 VI. bb). Soon a great
number of clefts become added in a row on each side of the branchial
sack. These clefts are small ciliated openings placed transversely
with reference to the long axis of the branchial sack, but only
occupying a small part of the breadth of each side. The intervals
dorsal and ventral to them are soon filled by series of fresh rows of
slits, separated from each other by longitudinal bars. Each side of
the branchial sack becomes in this way perforated by a number of small
openings arranged in rows, and separated by transverse and
longitudinal bars. The whole structure forms the commencement of the
branchial basketwork of the adult; the arrangement of which differs
considerably in structure and origin from the simple system of
branchial clefts of normal vertebrate types. At the junction of the
transverse and longitudinal bars papillæ are formed projecting into
the lumen of the branchial sack.
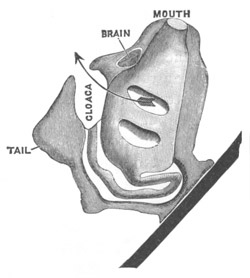
Fig. 11. Diagram of a very young
Ascidian. (From Lankester.)
After the above changes are far advanced towards completion, the
openings of the two atrial sacks gradually approximate in the dorsal
line, and finally coalesce to form the single atrial opening of the
adult. The two atrial cavities at the same time coalesce dorsally to
form a single cavity, which is continuous[Pg 21] round the branchial sack,
except along the ventral line where the endostyle is present. The
atrial cavity, from its mode of origin as a pair of epiblastic
involutions[10],
is clearly a structure of the same nature as the
branchial or atrial cavity of Amphioxus; and has nothing whatever to
do with the true body cavity.
It has already been stated that the anus opens into the original left
atrial cavity; when the two cavities coalesce the anus opens into the
atrial cavity in the median dorsal line.
Two of the most obscure points in the development are the origin of
the mesoblast in the trunk, and of the body cavity. Of the former
subject we know next to nothing, though it seems that the cells
resulting from the atrophy of the tail are employed in the nutrition
of the mesoblastic structures of the trunk.
The body cavity in the adult is well developed in the region of the
intestine, where it forms a wide cavity lined by an epithelioid
mesoblastic layer. In the region of the branchial sack it is reduced
to the vascular channels in the walls of the sack.
Kowalevsky believes the body cavity to be the original segmentation
cavity, but this view can hardly be regarded as admissible in the
present state of our knowledge. In some other Ascidian types a few
more facts about the mesoblast will be alluded to.
With the above changes the retrogressive metamorphosis is completed;
and it only remains to notice the change in position undergone in the
attainment of the adult state. The region by which the larva is
attached grows into a long process (fig. 10 B), and at the same time
the part carrying the mouth is bent upwards so as to be removed nearly
as far as possible from the point of attachment. By this means the
condition in the[Pg 22] adult (fig. 11) is gradually brought about; the
original dorsal surface with the oral and atrial openings becoming the
termination of the long axis of the body, and the nervous system being
placed between the two openings.
The genus Molgula presents a remarkable exception amongst the simple
Ascidians in that, in some if not all the species belonging to it,
development takes place (Lacaze Duthiers 29 and 30, Kupffer 28) quite
directly and without larval metamorphosis.
The ova are laid either singly or adhering together, and are very
opaque. The segmentation (Lacaze Duthiers) commences by the formation
of four equal spheres, after which a number of small clear spheres are
formed which envelope the large spheres. The latter give rise to a
closed enteric sack, and probably also to a mass of cells situated on
the ventral side, which appear to be mesoblastic. The epiblast is
constituted of a single layer of cells which completely envelopes the
enteric sack and the mesoblast.
While the ovum is still within the chorion five peculiar processes of
epiblast grow out; four of which usually lie in the same sectional
plane of the embryo. They are contractile and contain prolongations of
the body cavity. Their relative size is very variable.
The nervous system is formed on the dorsal side of the embryo before
the above projections make their appearance, but, though it seems
probable that it originates in the same manner as in the more normal
forms, its development has not been worked out. As soon as it is
formed it consists of a nervous ganglion similar to that usually found
in the adult. The history of the mass of mesoblast cells has been
inadequately followed, but it continuously disappears as the heart,
excretory organs, muscles, etc. become formed. So far as can be
determined from Kupffer’s descriptions the body cavity is primitively
parenchymatous—an indication of an abbreviated development—and does
not arise as a definite split in the mesoblast.
The primitive enteric cavity becomes converted into the branchial
sack, and from its dorsal and posterior corner the œsophagus, stomach
and intestine grow out as in the normal forms. The mouth is formed by
the invagination of a disc-like thickening of the epidermis in front
of the nervous system on the dorsal side of the body; and the atrial
cavity arises behind the nervous system by a similar process at a
slightly later period. The gill clefts opening into the atrial cavity
are formed as in the type of simple Ascidians described by Krohn.
The embryo becomes hatched not long after the formation of the oral
and atrial openings, and the five epiblastic processes undergo
atrophy. They are not employed in the attachment of the adult.
The larva when hatched agrees in most important points with the adult;
and is without the characteristic provisional larval organs of
ordinary forms; neither organs of special sense nor a tail becoming
developed. It has been suggested by Kupffer that the ventrally
situated mesoblastic mass[Pg 23] is the same structure as the mass of
elements which results in ordinary types from the degeneration of the
tail. If this suggestion is true it is difficult to believe that this
mass has any other than a nutritive function.
The larva of Ascidia ampulloides described by P. van Beneden is
regarded by Kupffer as intermediate between the Molgula larva and the
normal type, in that the larval tail and notochord and a pigment spot
are first developed, while after the atrophy of these organs peculiar
processes like those of Molgula make their appearance.
Sedentaria. The development of the fixed composite Ascidians is, so
far as we know, in the main similar to that of the simple Ascidians.
The larvæ of Botryllus sometimes attain, while still in the free
state, a higher stage of development with reference to the number of
gill slits, etc. than that reached by the simple Ascidians, and in
some instances (Botryllus auratus Metschnikoff) eight conical
processes are found springing in a ring-like fashion around the trunk.
The presence of these processes has led to somewhat remarkable views
about the morphology of the group; in that they were regarded by
Kölliker, Sars, etc. as separate individuals, and it was supposed that
the product of each ovum was not a single individual, but a whole
system of individuals with a common cloaca.
The researches of Metschnikoff (No. 32), Krohn (No. 25), and Giard
(No. 12), etc. demonstrate that this paradoxical view is untenable,
and that each ovum only gives rise to a single embryo, while the
stellate systems are subsequently formed by budding.
Natantia. Our knowledge of the development of Pyrosoma is mainly due
to Huxley (No. 16) and Kowalevsky (No. 22). In each individual of a
colony of Pyrosoma only a single egg comes to maturity at one time.
This egg is contained in a capsule formed of a structureless wall
lined by a flattened epithelioid layer. From this capsule a duct
passes to the atrial cavity, which, though called the oviduct,
functions as an afferent duct for the spermatozoa.
The segmentation is meroblastic, and the germinal disc adjoins the
opening of the oviduct. The segmentation is very similar to that which
occurs in Teleostei, and at its close the germinal disc has the form
of a cap of cells, without a trace of stratification or of a
segmentation cavity, resting upon the surface of the yolk, which forms
the main mass of the ovum.
After segmentation the blastoderm, as we may call the layer of cells
derived from the germinal disc, rapidly spreads over the surface of
the yolk, and becomes divided into two layers, the epiblast and the
hypoblast. At the same time it exhibits a distinction into a central
clearer and a peripheral more opaque[Pg 24] region. At one end of the
blastoderm, which for convenience sake may be spoken of as the
posterior end, a disc of epiblast appears, which is the first rudiment
of the nervous system, and on each side of the middle of the
blastoderm there arises an epiblastic involution. The epiblastic
involutions give rise to the atrial cavity.
These involutions rapidly grow in length, and soon form longish tubes,
opening at the surface by pores situated not far from the posterior
end of the blastoderm.
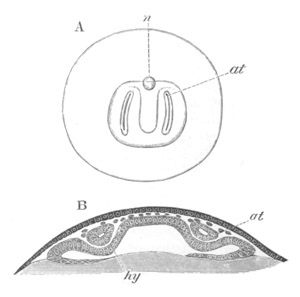
Fig. 12.
A. Surface view of the ovum of Pyrosoma
not far advanced in development. The embryonic
structures are developed from a disc-like
blastoderm.
B. Transverse section through the middle
part of the same blastoderm.
at. atrial cavity; hy. hypoblast; n. nervous disc in the
region of the future Cyathozooid.
The blastoderm at this stage, as seen on the surface of the yolk, is
shewn in fig. 12 A. It is somewhat broader than long. The nervous
system is shewn at n, and at points to an atrial tube. A
transverse section, through about the middle of this blastoderm, is
represented in fig. 12 B. The epiblast is seen above. On each side is
the section of an atrial tube (at). Below is the hypoblast which is
separated from the yolk especially in the middle line; at each side it
is beginning to grow in below, on the surface of the yolk. The space
below the hypoblast is the alimentary cavity, the ventral wall of
which is formed by the cells growing in at the sides. Between the
epiblast and hypoblast are placed scattered mesoblast cells, the
origin of which has not been clearly made out.
In a later stage the openings of the two atrial tubes gradually travel
backwards, and at the same time approximate, till finally[Pg 25] they meet
and coalesce at the posterior end of the blastoderm behind the nervous
disc (fig. 13, cl). The tubes themselves at the same time become
slightly constricted not far from their hinder extremities, and so
divided into a posterior region nearly coterminous with the nervous
system (fig. 13), and an anterior region. These two regions have very
different histories in the subsequent development.
The nervous disc has during these changes become marked by a median
furrow (fig. 13, ng), which is soon converted into a canal by the
same process as in the simple Ascidians. The closure of the groove
commences posteriorly and travels forwards. These processes are
clearly of the same nature as those which take place in Chordata
generally in the formation of the central nervous system.
In the region of the germinal disc which contains the anterior part of
the atrial tubes, the alimentary cavity becomes, by the growth of the
layer of cells described in the last stage, a complete canal, on the
outer wall of which the endostyle is formed as a median fold. The
whole anterior part of the blastoderm becomes at the same time
gradually constricted off from the yolk.
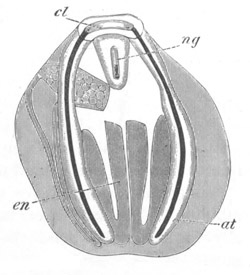
Fig. 13. Blastoderm of Pyrosoma
shortly before its division
into Cyathozooid and Ascidiozooids. (After Kowalevsky.)
cl. cloacal (atrial) opening; en. endostyle; at. atrial
cavity; ng. nervous groove.
The heart and pericardial cavity are seen to the left.
The fate of the anterior and posterior parts of the blastoderm is very
different. The anterior part becomes segmented into four zooids or
individuals, called by Huxley Ascidiozooids, which give rise to a
fresh colony of Pyrosoma. The posterior part forms a rudimentary
zooid, called by Huxley Cyathozooid, which eventually atrophies. These
five zooids are formed by a process of embryonic fission. This fission
commences by the appearance of four transverse constrictions in the
anterior part of the blastoderm; by which[Pg 26] the whole blastoderm becomes
imperfectly divided into five regions, fig. 14 A.
The hindermost constriction (uppermost in my figure) lies just in
front of the pericardial cavity; and separates the Cyathozooid from
the four ascidiozooids. The three other constrictions mark off the
four Ascidiozooids. The Cyathozooid remains for its whole length
attached to the blastoderm, which has now nearly enveloped the yolk.
It contains the whole of the nervous system (ng), which is covered
behind by the opening of the atrial tubes (cl). The alimentary tract
in the Cyathozooid forms a tube with very delicate walls. The
pericardial cavity is completely contained within the Cyathozooid, and
the heart itself (ht) has become formed by an involution of the
walls of the cavity.
The Ascidiozooids are now completely separated from the yolk. They
have individually the same structure as the undivided rudiment from
which they originated; so that the organs they possess are simply two
atrial tubes, an alimentary tract with an endostyle, and
undifferentiated mesoblast cells.
In the following stages the Ascidiozooids grow with great rapidity.
They soon cease to lie in a straight line, and eventually form a ring
round the Cyathozooid and attached yolk sack.
While these changes are being accomplished in the external form of the
colony, both the Cyathozooids and the Ascidiozooids progress
considerably in development. In the Cyathozooid the atrial spaces
gradually atrophy, with the exception of the external opening, which
becomes larger and more conspicuous. The heart at the same time comes
into full activity and drives the blood through the whole colony. The
yolk becomes more and more enveloped by the Cyathozooid, and is
rapidly absorbed; while the nutriment derived from it is transported
to the Ascidiozooids by means of the vascular connection. The nervous
system retains its previous condition; and round the Cyathozooid is
formed the test into which cells migrate, and arrange themselves in
very conspicuous hexagonal areas. The delicate alimentary tract of the
Cyathozooid is still continuous with that of the first Ascidiozooid.
After the Cyathozooid has reached the development just described it
commences to atrophy.
[Pg 27]
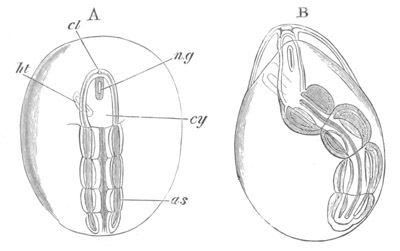
Fig. 14. Two stages in the development of Pyrosoma in which the
Cyathozooid and four Ascidiozooids are already distinctly formed.
(After Kowalevsky.)
cy. cyathozooid; as. ascidiozooid; ng. nervous groove; ht.
heart of cyathozooid; cl. cloacal opening.
The changes in the Ascidiozooids are even more considerable than those
in the Cyathozooid. A nervous system appears as a fresh formation
close to the end of each Ascidiozooid turned towards the Cyathozooid.
It forms a tube of which the open front end eventually develops into
the ciliated pit of the mouth, and the remainder into the actual
nervous ganglion. Between the nervous system and the endostyle an
involution appears, which gives rise to the mouth. On each side of the
primitive alimentary cavity of each Ascidiozooid branchial slits make
their appearance, leading into the atrial tubes; so that the primitive
alimentary tract becomes converted into the branchial sacks of the
Ascidiozooids. The remainder of the alimentary tract of each zooid is
formed as a bud from the hind end of the branchial sack in the usual
way. The alimentary tracts of the four Ascidiozooids are at first in
free communication by tubes opening from the hinder extremity of one
zooid into the dorsal side of the branchial sack of the next zooid. At
the hinder end of each Ascidiozooid is developed a mass of fatty cells
known as the elæoblast, which probably represents a rudiment of the
larval tail of simple Ascidians. (Cf. pp. 30-32.)
The further changes consist in the gradual atrophy of the Cyathozooid,
which becomes more and more enclosed within the four Ascidiozooids.
These latter become completely enveloped[Pg 28] in a common test, and form a
ring round the remains of the yolk and of the Cyathozooid, the heart
of which continues however to beat vigorously. The cloacal opening of
the Cyathozooid persists through all these changes, and, after the
Cyathozooid itself has become completely enveloped in the
Ascidiozooids and finally absorbed, deepens to form the common cloacal
cavity of the Pyrosoma colony.
The main parts of the Ascidiozooids were already formed during the
last stage. The zooids long remain connected together, and united by a
vascular tube with the Cyathozooid, and these connections are not
severed till the latter completely atrophies. Finally, after the
absorption of the Cyathozooid, the Ascidiozooids form a rudimentary
colony of four individuals enveloped in a common test. The two atrial
tubes of each zooid remain separate in front but unite posteriorly. An
anus is formed leading from the rectum into the common posterior part
of the atrial cavity; and an opening is established between the
posterior end of the atrial cavity of each Ascidiozooid and the common
axial cloacal cavity of the whole colony. The atrial cavities in
Pyrosoma are clearly lined by epiblast, just as in simple Ascidians.
When the young colony is ready to become free, it escapes from the
atrial cavity of the parent, and increases in size by budding.
Doliolidæ. The sexually developed embryos of Doliolum have been
observed by Krohn (No. 23), Gegenbaur (No. 10), and Keferstein and
Ehlers (No. 17); but the details of the development have been very
imperfectly investigated.
The youngest embryo observed was enveloped in a large oval transparent
covering, the exact nature of which is not clear. It is perhaps a
larval rudiment of the test which would seem to be absent in the
adult. Within this covering is the larva, the main organs of which are
already developed; and which primarily differs from the adult in the
possession of a larval tail similar to that of simple Ascidians.
In the body both oral and atrial openings are present, the latter on
the dorsal surface; and the alimentary tract is fully established. The
endostyle is already formed on the ventral wall of the branchial sack,
but the branchial slits are not present. Nine muscular rings are
already visible. The tail, though not so developed as in the simple
Ascidians, contains an axial notochord of the usual structure, and
lateral muscles. It is inserted on the ventral side, and by its slow
movements the larva progresses.
[Pg 29]In succeeding stages the tail gradually atrophies, and the gill slits,
four in number, develop; at the same time a process or stolon,
destined to give rise by budding to a second non-sexual generation,
makes its appearance on the dorsal side in the seventh intermuscular
space. This stolon is comparable with that which appears in the embryo
of Salpa. When the tail completely atrophies the larva leaves its
transparent covering, and becomes an asexual Doliolum with a dorsal
stolon.
Salpidæ. As is well known the chains of Salpa alone are sexual, and
from each individual of the chain only a single embryo is produced.
The ovum from which this embryo takes its origin is visible long
before the separate Salps of the chain have become completely
developed. It is enveloped in a capsule continuous with a duct, which
opens into the atrial cavity, and is usually spoken of as the oviduct.
The capsule with the ovum is enveloped in a maternal blood sinus.
Embryonic development commences after the chain has become broken up,
and the spermatozoa derived from another individual would seem to be
introduced to the ovum through the oviduct.
At the commencement of embryonic development the oviduct and
ovicapsule undergo peculiar changes; and in part at least give rise to
a structure subservient to the nutrition of the embryo, known as the
placenta. These changes commence with the shortening of the oviduct,
and the disappearance of a distinction between oviduct and ovicapsule.
The cells lining the innermost end of the capsule, i.e. that at the
side of the ovum turned away from the atrial cavity, become at the
same time very columnar. The part of the oviduct between the ovum and
the atrial cavity dilates into a sack, communicating on the one hand
with the atrial cavity, and on the other by a very narrow opening with
the chamber in which the egg is contained. This sack next becomes a
prominence in the atrial cavity, and eventually constitutes a
brood-pouch. The prominence it forms is covered by the lining of the
atrial cavity, immediately within which is the true wall of the sack.
The external opening of the sack becomes gradually narrowed, and
finally disappears. In the meantime the chamber in which the embryo is
at first placed acquires a larger and larger opening into the sack;
till finally the two chambers unite, and a single brood-pouch
containing the embryo is thus produced. The inner wall of the chamber
is formed by the columnar cells already spoken of. They form the
rudiment of the placenta. The double wall of the outer part of the
brood-pouch becomes stretched by the growth of the embryo; the inner
of its two layers then atrophies. The outer layer subsequently gives
way, and becomes rolled back so as to lie at the inner end of the
embryo, leaving the latter projecting freely into the atrial cavity.
While these changes are taking place the placenta becomes fully
developed. The first rudiment of it consists, according to Salensky,
of the thickened cells of the ovicapsule only, though this view is
dissented from by Brooks, Todaro, etc. Its cells soon divide to form a
largish mass, which becomes attached to a part of the epiblast of the
embryo.
[Pg 30]On the formation of the body cavity of the embryo a central axial
portion of the placenta becomes separated from a peripheral layer; and
a channel is left between them which leads from a maternal blood sinus
into the embryonic body cavity. The peripheral layer of the placenta
is formed of cells continuous with the epiblast of the embryo; while
the axial portion is constituted of a disc of cells adjoining the
embryo, with a column of fibres attached to the maternal side. The
fibres of this column are believed by Salensky to be products of the
original rudiment of the placenta. The placenta now assumes a more
spherical form, and its cavity becomes shut off from the embryonic
body cavity. The fibrous column breaks up into a number of strands
perforating the lumen of the organ, and the cells of the wall become
stalked bodies projecting into the lumen.
When the larva is nearly ready to become free the placenta atrophies.
The placenta functions in the nutrition of the embryo in the following
way. It projects from its first formation into a maternal blood sinus,
and, on the appearance of a cavity in it continuous with the body
cavity of the embryo, the blood of the mother fully intermingles with
that of the embryo. At a later period the communication with the body
cavity of the embryo is shut off, but the cavity of the placenta is
supplied with a continuous stream of maternal blood, which is only
separated from the fœtal blood by a thin partition.
It is now necessary to turn to the embryonic development about which
it is unfortunately not as yet possible to give a completely
satisfactory account. The statements of the different investigators
contradict each other on most fundamental points. I have followed in
the main Salensky (No. 34), but have also called attention to some
points where his observations diverge most from those of other
writers, or where they seem unsatisfactory.
The development commences at about the period when the brood-pouch is
becoming formed; and the ovum passes entirely into the brood-pouch
before the segmentation is completed. The segmentation is regular, and
the existence of a segmentation cavity is denied by Salensky, though
affirmed by Kowalevsky and Todaro[11].
At a certain stage in the segmentation the cells of the ovum become
divided into two layers, an epiblast investing the whole of the ovum
with the exception of a small area adjoining the placenta, where the
inner layer or hypoblast, which forms the main mass of the ovum,
projects at the surface. The epiblast soon covers the whole of the
hypoblast, so that there would seem (according to Salensky’s
observations) to be a kind of epibolic invagination: a conclusion
supported by Todaro’s figures.
At a later stage, on one side of the free apex of the embryo, a
mesoblastic layer makes its appearance between the epiblast and
hypoblast. This layer is derived by Salensky, as it appears to me on
insufficient grounds, from the epiblast. Nearly at the same time there
arises not far[Pg 31] from the same point of the embryo, but on the opposite
side, a solid thickening of epiblast which forms the rudiment of the
nervous system. The nervous system is placed close to the front end of
the body; and nearly at the opposite pole, and therefore at the hind
end, there appears immediately below the epiblast a mass of cells
forming a provisional organ known as the elæoblast. Todaro regards
this organ as mesoblastic in origin, and Salensky as hypoblastic. The
organ is situated in the position which would be occupied by the
larval tail were it developed. It may probably be regarded (Salensky)
as a disappearing rudiment of the tail, and be compared in this
respect with the more or less similar mass of cells described by
Kupffer in Molgula, and with the elæoblast in Pyrosoma.
After the differentiation of these organs a cavity makes its
appearance between the epiblast and hypoblast, which is regarded by
Salensky as the body cavity. It appears to be equivalent to the
segmentation cavity of Todaro. According to Todaro’s statements, it is
replaced by a second cavity, which appears between the splanchnic and
somatic layers of mesoblast, and constitutes the true body cavity. The
embryo now begins to elongate, and at the same time a cavity makes its
appearance in the centre of the hypoblast cells. This cavity is the
rudiment of the branchial and alimentary cavities: on its dorsal wall
is a median projection, the rudiment of the so-called gill of Salpa.
At two points this cavity comes into close contact with the external
skin. At one of these, situated immediately ventral to the nervous
system, the mouth becomes formed at a later period. At the other,
placed on the dorsal surface between the nervous system and the
elæoblast, is formed the cloacal aperture.
By the stage under consideration the more important systems of organs
are established, and the remaining embryonic history may be very
briefly narrated.
The embryo at this stage is no longer covered by the walls of the
brood-pouch but projects freely into the atrial cavity, and is only
attached to its parent by means of the placenta. The epiblast cells
soon give rise to a deposit which forms the mantle. The deposit
appears however to be formed not only on the outer side of the
epiblast but also on the inner side; so that the epiblast becomes
cemented to the subjacent parts, branchial sack, etc., by an
intercellular layer, which would seem to fill up the primitive body
cavity with the exception of the vascular channels (Salensky).
The nervous system, after its separation from the epiblast, acquires a
central cavity, and subsequently becomes divided into three lobes,
each with an internal protuberance. At its anterior extremity it opens
into the branchial sack; and from this part is developed the ciliated
pit of the adult. The nervous ganglion at a later period becomes
solid, and a median eye is subsequently formed as an outgrowth from
it.
According to Todaro there are further formed two small auditory (?
olfactory) sacks on the ventral surface of the brain, each of them
placed in communication with the branchial cavity by a narrow canal.
[Pg 32]The mesoblast gives rise to the muscles of the branchial sack, to the
heart, and to the pericardium. The two latter are situated on the
ventral side of the posterior extremity of the branchial cavity.
Branchial sack and alimentary tract. The first development of the
enteric cavity has already been described. The true alimentary tract
is formed as a bud from the hinder end of the primitive cavity. The
remainder of the primitive cavity gives rise to the branchial sack.
The so-called gill has at first the form of a lamella attached
dorsally to the walls of the branchial sack; but its attachment
becomes severed except at the two ends, and it then forms a band
stretching obliquely across the branchial cavity, which subsequently
becomes hollow and filled with blood corpuscles. The whole structure
is probably homologous with the peculiar fold, usually prolonged into
numerous processes, which normally projects from the dorsal wall of
the Ascidian branchial sack.
On the completion of the gill the branchial sack becomes divided into
a region dorsal to the gill, and a region ventral to it. Into the
former the single atrial invagination opens. No gill slits are formed
comparable with those in simple Ascidians, and the only representative
of these structures is the simple communication which becomes
established between the dorsal division of the branchial sack and the
atrial opening. The whole branchial sack of Salpa, including both the
dorsal and ventral divisions, corresponds with the branchial sack of
simple Ascidians. On its ventral side the endostyle is formed in the
normal way. The mouth arises at the point already indicated near the
front end of the nervous system[12].
[Pg 33]Development of the chain of sexual Salps. My description of the
embryonic development of Salpa would not be complete without some
reference to the development of the stolon of the Solitary generation
of Salps by the segmentation of which a chain of sexual Salps
originates.
The asexual Salp, the embryonic development of which has just been
described, may be compared to the Cyathozooid of Pyrosoma, from which
it mainly differs in being fully developed. While still in an
embryonic condition it gives rise to a process or stolon, which
becomes divided into a number of zooids by transverse constrictions,
in the same manner that part of the germ of the ovum of Pyrosoma is
divided by transverse constrictions into four Ascidiozooids.
The stolon arises as a projection on the right side of the body of the
embryo close to the heart. It is formed (Salensky, No. 35) of an
outgrowth of the body wall, into which there grow the following
structures:
(1) A central hollow process from the end of the respiratory sack.
(2) A right and left lateral prolongation of the pericardial cavity.
(3) A solid process of cells on the ventral side derived from the
same mass of the cells as the elæoblast.
(4) A ventral and a dorsal blood sinus.
[Pg 34]Besides these parts there appears on the dorsal side a hollow tube,
the origin of which is unknown, which gives rise to the nervous
system.
The hollow process of the respiratory sack is purely provisional, and
disappears without giving rise to any permanent structure. The right
and left prolongations of the pericardial cavity become solid and
eventually give origin to the mesoblast. The ventral process of cells
is the most important structure in the stolon in that it gives rise
both to the alimentary and respiratory sacks, and to the generative
organs of the sexual Salps. The stolon containing the organs just
enumerated becomes divided by transverse constrictions into a number
of rings. These rings do not long remain complete, but become
interrupted dorsally and ventrally. The imperfect rings so formed soon
overlap, and each of them eventually gives rise to a sexual Salp.
Although the stolon arises while the asexual Salp is still in an
embryonic condition, it does not become fully developed till long
after the asexual Salp has attained maturity.
Appendicularia. Our only knowledge of the development of
Appendicularia is derived from Fol’s memoir on the group (No. 8). He
simply states that it develops, as far as he was able to follow, like
other Ascidians; and that the extremely minute size of the egg
prevented him from pursuing the subject. He also states that the pair
of pores leading from the branchial cavity to the exterior is
developed from epiblastic involutions meeting outgrowths of the wall
of the branchial sack.
Metagenesis.
One of the most remarkable phenomena in connection with the life
history of many Ascidians is the occurrence of an alternation of
sexual and gemmiparous generations. This alternation appears to have
originated from a complication of the process of reproduction by
budding, which is so common in this group. The mode in which this very
probably took place will be best understood by tracing a series of
transitional cases between simple budding and complete alternations of
generations.
In the simpler cases, which occur in some Composita Sedentaria, the
process of budding commences with an outgrowth of the body wall into
the common test, containing a prolongation of part of the alimentary
tract[13].
[Pg 35]Between the epiblastic and hypoblastic layers of the bud so formed, a
mesoblastic and sometimes a generative outgrowth of the parent also
appears.
The systems of organs of the bud are developed from the corresponding
layers to those in the embryo[14].
The bud eventually becomes
detached, and in its turn gives rise to fresh buds. Both the bud and
its parent reproduce sexually as well as by budding: the new colonies
being derived from sexually produced embryos.
The next stage of complication is that found in Botryllus (Krohn, Nos.
25 and 26). The larva produced sexually gives rise to a bud from the
right side of the body close to the heart. On the bud becoming
detached the parent dies away without developing sexual organs. The
bud of the second generation gives rise to two buds, a right one and a
left one, and like the larva dies without reaching sexual maturity.
The buds of the third generation each produce two buds and then suffer
the same fate as their parent.
The buds of the third generation arrange themselves with their cloacal
extremities in contact, and in the fourth generation a common cloaca
is formed, and so a true radial system of zooids is established; the
zooids of which are not however sexual.
The buds of the fourth generation in their turn produce two or three
buds and then die away.
Fresh systems become formed by a continuation of the process of
budding, but the zooids of the secondary systems so[Pg 36] formed are sexual.
The ova come to maturity before the spermatozoa, so that cross
fertilization takes place.
In Botryllus we have clearly a rudimentary form of alternations of
generations, in that the sexually produced larva is asexual, and,
after a series of asexual generations, produced gemmiparously, there
appear sexual generations, which however continue to reproduce
themselves by budding.
The type of alternations of generations observable in Botryllus
becomes, as pointed out by Huxley, still more marked in Pyrosoma.
The true product of the ovum is here (vide p. 25) a rudimentary
individual called by Huxley the Cyathozooid. This gives rise, while
still an embryo, by a process equivalent to budding to four fully
developed zooids (Ascidiozooids) similar to the parent form, and
itself dies away. The four Ascidiozooids form a fresh colony, and
reproduce (1) sexually, whereby fresh colonies are formed, and (2) by
ordinary budding, whereby the size of the colony is increased. All the
individuals of the colony are sexual.
The alternation of generations in Pyrosoma widely differs from that in
Botryllus in the fact of the Cyathozooid differing so markedly in its
anatomical characters from the ordinary zooids.
In Salpa the process is slightly different[15].
The sexual forms are
now incapable of budding, and, although at first a series of sexual
individuals are united together in the form of a chain, so as to form
a colony like Pyrosoma or Botryllus, yet they are so loosely connected
that they separate in the adult state. As in Botryllus, the ova are
ripe before the spermatozoa. Each sexual individual gives rise to a
single offspring, which, while still in the embryonic condition, buds
out a ‘stolon’ from its right ventral side. This stolon is divided
into a series of lateral buds after the solitary asexual Salp has
begun to lead an independent existence. The solitary asexual Salp
clearly corresponds with the Cyathozooid of Pyrosoma, though it has
not, like the Cyathozooid, undergone a retrogressive metamorphosis.
By far the most complicated form of alternation of generations[Pg 37] known
amongst the Ascidians is that in Doliolum. The discovery of this
metamorphosis was made by Gegenbaur (No. 10). The sexual form of
Doliolum is somewhat cask-shaped, with ring-like muscular bands, and
the oral and atrial apertures placed at opposite ends of the cask. The
number of gill slits varies according to the species. The ovum gives
rise, as already described, to a tailed embryo which subsequently
develops into a cask-shaped asexual form. On attaining its full size
it loses its branchial sack and alimentary tract. While still in the
embryonic condition, a stolon grows out from its dorsal side in the
seventh intermuscular space. The stolon, like that in Salpa, contains
a prolongation of the branchial sack[16].
On this stolon there develop two entirely different types of buds, (1)
lateral buds, (2) dorsal median buds.
The lateral buds are developed in regular order on the two sides of
the stolon, and the most advanced buds are those furthest removed from
the base. They give rise to forms with a very different organization
to that of the parent. They are compared by Gegenbaur to a spoon, the
bowl of which is formed by the branchial sack, and the handle by the
stalk attaching the bud to the stolon. The oral opening into the
branchial sack is directed upwards: an atrial opening is remarkably
enough not present. The branchial sack is perforated by numerous
openings. It leads into an alimentary tract which opens directly to
the exterior by an anus opposite the mouth.
The stalks attaching the more mature buds to the stolon are provided
with ventrally directed scales, which completely hide the stolon in a
view from the ventral surface.
These buds have, even after their detachment, no trace of generative
organs, and shew no signs of reproducing themselves by budding. Their
eventual fate is unknown.
The median dorsal buds have no such regular arrangement as the lateral
buds, but arise in irregular bunches, those furthest removed from the
base of the stolon being however the oldest. These buds are almost
exactly similar to the original sexual form; they do not acquire
sexual organs, but are provided with[Pg 38] a stolon attached on the ventral
side, in the sixth intermuscular space.
This stolon is simply the stalk by which each median bud was
primitively attached to the stolon of the first asexual form.
From the stolon of the median buds of the second generation buds are
developed which grow into the sexual forms.
The generations of Doliolum may be tabulated in the following way.
| Sexual generation, |
| | |
| 1st asexual form with dorsal stolon, |
| | |
| ____________________|____________________ |
spoon-like forms developed as
lateral
buds (eventual history unknown). |
| 2nd asexual forms developed as
median
buds with vental stolon, |
| | |
| sexual generation. |
Bibliography.
(6) P. J. van Beneden. “Recherches s. l'Embryogénie, l'Anat. et la
Physiol. des Ascidies simples.” Mém. Acad. Roy. de Belgique,
Tom. XX.
(7) W. K. Brooks. “On the development of Salpa.” Bull. of the Museum
of Comp. Anat. at Harvard College, Cambridge, Mass.
(8) H. Fol. Etudes sur les Appendiculaires du détroit de Messine.
Genève et Bâle, 1872.
(9) Ganin. “Neue Thatsachen a. d. Entwicklungsgeschichte d. Ascidien.”
Zeit. f. wiss. Zool., Vol. XX. 1870.
(10) C. Gegenbaur. “Ueber den Entwicklungscyclus von Doliolum nebst
Bemerkungen über die Larven dieser Thiere.” Zeit. f. wiss. Zool.,
Bd. VII. 1856.
(11) A. Giard. “Etudes critiques des travaux d'embryogénie relatifs à
la parenté des Vertebrés et des Tuniciers.” Archiv Zool.
expériment., Vol. I. 1872.
(12) A. Giard. “Recherches sur les Synascidies.” Archiv Zool.
expér., Vol. I. 1872.
(13) O. Hertwig. “Untersuchungen üb. d. Bau u. d. Entwicklung des
Cellulose-Mantels d. Tunicaten.” Jenaische Zeitschrift, Bd. VII.
1873.
(14) Th. H. Huxley. “Remarks upon Appendicularia and Doliolum.” Phil.
Trans., 1851.
(15) Th. H. Huxley. “Observations on the anatomy and physiology of
Salpa and Pyrosoma.” Phil. Trans., 1851.
(16) Th. H. Huxley. “Anatomy and development of Pyrosoma.” Linnean
Trans., 1860, Vol. XXIII.
(17) Keferstein u. Ehlers. Zoologische Beiträge, 1861. Doliolum.
(18) A. Kowalevsky. “Entwicklungsgeschichte d. einfachen Ascidien.”
Mém. Acad. Pétersbourg, VII. série, T. X. 1866.
(19) A. Kowalevsky. “Beitrag z. Entwick. d. Tunicaten.” Nachrichten
d. königl. Gesell. zu Göttingen. 1868.
(20) A. Kowalevsky. “Weitere Studien üb. d. Entwicklung d. einfachen
Ascidien.” Archiv f. mikr. Anat., Vol. VII. 1871.
[Pg 39](21) A. Kowalevsky. “Ueber Knospung d. Ascidien.” Archiv f. mikr.
Anat., Vol. X. 1874.
(22) A. Kowalevsky. “Ueber die Entwicklungsgeschichte d. Pyrosoma.”
Archiv f. mikr. Anat., Vol. XI. 1875.
(23) A. Krohn. “Ueber die Gattung Doliolum u. ihre Arten.” Archiv f.
Naturgeschichte, Bd. XVIII. 1852.
(24) A. Krohn. “Ueber die Entwicklung d. Ascidien.” Müller’s Archiv,
1852.
(25) A. Krohn. “Ueber die Fortpflanzungsverhältnisse d. Botrylliden.”
Archiv f. Naturgeschichte, Vol. XXXV. 1869.
(26) A. Krohn. “Ueber die früheste Bildung d. Botryllenstöcke.”
Archiv f. Naturgeschichte, Vol. XXXV. 1869.
(27) C. Kupffer. “Die Stammverwandschaft zwischen Ascidien u.
Wirbelthieren.” Archiv f. mikr. Anat., Vol. VI. 1870.
(28) C. Kupffer. “Zur Entwicklung d. einfachen Ascidien.” Archiv f.
mikr. Anat., Vol. VIII. 1872.
(29) H. Lacaze Duthiers. “Recherches sur l'organisation et
l'Embryogénie des Ascidies (Molgula tubulosa).” Comptes rendus, May
30, 1870, p. 1154.
(30) H. Lacaze Duthiers. “Les Ascidies simples des Côtes de France"”
(Development of Molgula). Archiv Zool. expér., Vol. III. 1874.
(31) R. Leuckart. “Salpa u. Verwandte.” Zoologische Untersuchungen,
Heft II.
(32) E. Metschnikoff. “Observations sur le développement de quelques
animaux (Botryllus and Simple Ascidians).” Bull. d. l'Acad.
Pétersbourg, Vol. XIII. 1869.
(33) H. Milne-Edwards. “Observations s. l. Ascidies composées des
côtes de la Manche.” Mémoires d. l'Institut, T. XVIII. 1842.
(34) W. Salensky. “Ueber d. embryonale Entwicklungsgeschichte der
Salpen.” Zeit. f. wiss. Zool., B. XXVII. 1877.
(35) W. Salensky. “Ueber die Knospung d. Salpen.” Morphol. Jahrbuch,
Bd. III. 1877.
(36) W. Salensky. “Ueber die Entwicklung d. Hoden u. über den
Generationswechsel d. Salpen.” Zeit. f. wiss. Zool., Bd. XXX. Suppl.
1878.
(37) C. Semper. “Ueber die Entstehung d. geschichteten
Cellulose-Epidermis d. Ascidien.” Arbeit. a. d. zool.-zoot. Instit.
Würzburg, Vol. II. 1875.
(38) Fr. Todaro. Sopra lo sviluppo e l'anatomia delle Salpe. Roma,
1875.
(39) Fr. Todaro. “Sui primi fenomeni dello sviluppo delle Salpe.”
Reale Accademia dei Lincei, Vol. IV. 1880.
I. Caducichordata.
A. Simplicia
Solitaria ex. Ascidia.
Socialia ex. Clavellina.
B. Composita
Sedentaria ex. Botryllus.
Natantia ex. Pyrosoma.
C. Conserta
Salpidæ.
Doliolidæ.
II. Perennichordata.
Ex. Appendicularia.
[Pg 40]
CHAPTER III.
ELASMOBRANCHII.
The impregnation of the ovum is effected in the oviduct. In most forms
the whole of the subsequent development, till the time when the embryo
is capable of leading a free existence, takes place in the uterus; but
in other cases the egg becomes enveloped, during its passage down the
oviduct, first in a layer of fluid albumen, and finally in a dense
horny layer, which usually takes the form of a quadrilateral capsule
with characters varying according to the species. After the formation
of this capsule the egg is laid, and the whole of the development,
with the exception of the very first stages, takes place externally.
In many of the viviparous forms (Mustelus, Galeus, Carcharias,
Sphyrna) the egg is enclosed, during the early stages of development
at any rate, in a very delicate shell homologous with that of the
oviparous forms; there is usually also a scanty albuminous layer. Both
of these are stated by Gerbe (No. 42) to be absent in Squalus spinax.
The following are examples of viviparous genera: Hexanchus, Notidanus,
Acanthias, Scymnus, Galeus, Squalus, Mustelus, Carcharias, Sphyrna,
Squatina, Torpedo; and the following of oviparous genera: Scyllium,
Pristiurus, Cestracion, Raja[17].
The ovum at the time of impregnation has the form of a large spherical
mass, similar to the yolk of a bird’s egg, but without a vitelline
membrane[18].
The greater part of it is formed of peculiar oval
spherules of food-yolk, held together by a protoplasmic network. The
protoplasm is especially concentrated in a small lens-shaped area,
known as the germinal disc, which is not separated by a sharp line
from the remainder of[Pg 41] the ovum. Yolk spherules are present in this
disc as elsewhere, but are much smaller and of a different character.
The segmentation has the normal meroblastic character (fig. 15) and is
confined to the germinal disc. Before it commences the germinal disc
exhibits amœboid movements. During the segmentation nuclei make their
appearance spontaneously (?) in the yolk adjoining the germinal disc
(fig. 15, nx´), and around them portions of the yolk with its
protoplasmic network become segmented off. Cells are thus formed which
are added to those resulting from the segmentation proper. Even after
the segmentation numerous nuclei are present in the granular matter
below the blastoderm (fig. 16 A, n´); and around these cells are
being continually formed, which enter the blastoderm, and are more
especially destined to give rise to the hypoblast. The special
destination of many of these cells is spoken of in detail below.
Fig. 15. Section through germinal disc of a Pristiurus embryo during
the segmentation.
n. nucleus; nx. nucleus modified prior to division; nx´.
modified nucleus in the yolk; f. furrow appearing in the yolk
adjacent to the germinal disc.
At the close of segmentation the blastoderm forms a somewhat
lens-shaped disc, thicker at one end than at the other; the thicker
end being the embryonic end. It is divided into two strata—an upper
one, the epiblast—formed of a single row of columnar cells; and a
lower one, the primitive hypoblast, consisting of the remaining cells
of the blastoderm, and forming a mass several strata deep. These cells
will be spoken of as the[Pg 42] lower layer cells, to distinguish them from
the true hypoblast which is one of their products.
Fig. 16. Two longitudinal sections of the blastoderm of a Pristiurus
embryo during stages prior to the formation of the medullary groove.
ep. epiblast; ll. lower layer cells or primitive hypoblast; m.
mesoblast; hy. hypoblast; sc. segmentation cavity; es. embryo
swelling; n´. nuclei of yolk; er. embryonic rim. c. lower
layer cells at the non-embryonic end of the blastoderm.
A cavity very soon appears in the lower layer cells, near the
non-embryonic end of the blastoderm, but the cells afterwards
disappear from the floor of this cavity, which then lies between the
yolk and the lower layer cells (fig. 16 A, sc). This cavity is the
segmentation cavity equivalent to that present in Amphioxus, Amphibia,
etc. The chief peculiarity about it is the relatively late period at
which it makes its appearance, and the fact that its roof is formed
both by the epiblast and by the lower layer cells. Owing to the large
size of the segmentation cavity the blastoderm forms a thin layer
above the cavity and a thickened ridge round its edge.

Fig. 17. Longitudinal section through the blastoderm of a Pristiurus
embryo of the same age as fig. 28 B.
ep. epiblast; er. embryonic rim; m. mesoblast; al.
mesenteron.
The epiblast in the next stage is inflected for a small arc at the
embryonic end of the blastoderm, where it becomes continuous with the
lower layer cells; at the same time some of the lower layer cells of
the embryonic end of the blastoderm assume[Pg 43] a columnar form, and
constitute the true hypoblast. The portion of the blastoderm, where
epiblast and hypoblast are continuous, forms a projecting structure
which will be called the embryonic rim (fig. 16 B, er).
This rim is a very important structure, since it represents the dorsal
portion of the lip of the blastopore of Amphioxus. The space between
it and the yolk represents the commencing mesenteron, of which the
hypoblast on the under side of the lip is the dorsal wall. The ventral
wall of the mesenteron is at first formed solely of yolk held together
by a protoplasmic network with numerous nuclei. The cavity under the
lip becomes rapidly larger (fig. 17, al), owing to the continuous
conversion of lower layer cells into columnar hypoblast along an axial
line passing from the middle of the embryonic rim towards the centre
of the blastoderm. The continuous differentiation of the hypoblast
towards the centre of the blastoderm corresponds with the invagination
in Amphioxus. During the formation of the embryonic rim the blastoderm
grows considerably larger, but, with the exception of the formation of
the embryonic rim, retains its primitive constitution.
The segmentation cavity undergoes however important changes. There is
formed below it a floor of lower layer cells, derived partly from an
ingrowth from the two sides, but mainly from the formation of cells
around the nuclei of the yolk (fig. 16). Shortly after the floor of
cells has appeared, the whole segmentation cavity becomes obliterated
(fig. 17).
The disappearance of the segmentation cavity corresponds in point of
time with the formation of the hypoblast by the pseudo-invagination
above described; and is probably due to this pseudo-invagination, in
the same way that the disappearance of the segmentation cavity in
Amphioxus is due to the true invagination of the hypoblast.
When the embryonic rim first appears there are no external indications
of the embryo as distinguished from the blastoderm, but when it has
attained to some importance the position of the embryo becomes marked
out by the appearance of a shield-like area extending inwards from the
edge of the embryonic rim, and formed of two folds with a groove
between them (fig. 28 B, mg), which is deepest at the edge of the
blastoderm, and[Pg 44] shallows out as it extends inwards. This groove is the
medullary groove; and its termination at the edge of the blastoderm is
placed at the hind end of the embryo.
At about the time of its appearance the mesoblast becomes first
definitely established.
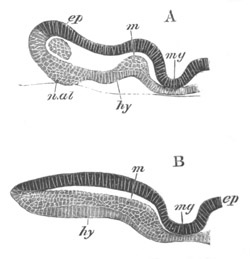
Fig. 18. Two transverse sections of an embryo of the same age as
fig. 17.
A. Anterior section.
B. Posterior section.
mg. medullary groove; ep. epiblast; hy. hypoblast; n.al.
cells formed round the nuclei of the yolk which have entered the
hypoblast; m. mesoblast.
The sections shew the origin of the mesoblast.
At the edge of the embryonic rim the epiblast and lower layer cells
are continuous. Immediately underneath the medullary groove, as is
best seen in transverse section (fig. 18), the whole of the lower
layer cells become converted into hypoblast, and along this line the
columnar hypoblast is in contact with the epiblast above. At the sides
however this is not the case; but at the junction of the epiblast and
lower layer cells the latter remain undifferentiated. A short way from
the edge the lower layer cells become divided into two distinct
layers, a lower one continuous with the hypoblast in the middle line,
and an upper one between this and the epiblast (fig. 18 B). The upper
layer is the commencement of the mesoblast (m). The mesoblast thus
arises as two independent lateral plates, one on each side of the
medullary groove, which are continuous behind with the undifferentiated
lower layer cells at the edge of the embryonic rim. The mesoblast
plates are at first very short, and do not extend to the front end of
the embryo. They soon however grow forwards as two lateral ridges,
attached to the hypoblast, one on each side of the medullary groove
(fig. 18 A, m). These ridges become separate from the hypoblast, and
form two plates, thinner in front than behind; but still continuous at
the edge of the blastoderm with the undifferentiated cells of the lip
of the blastopore, and laterally with the lower layer[Pg 45] cells of the
non-embryonic part of the blastoderm. It results from the above mode
of development of the mesoblast, that it may be described as arising
in the form of a pair of solid outgrowths of the wall of the
alimentary tract; which differ from the mesoblastic outgrowths of the
wall of the archenteron in Amphioxus in not containing a prolongation
of the alimentary cavity.
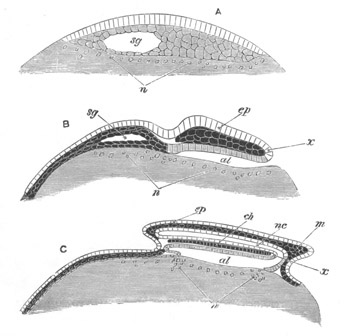
Fig. 19. Diagrammatic longitudinal sections of an Elasmobranch
embryo.
Epiblast without shading. Mesoblast black with clear outlines to
the cells. Lower layer cells and hypoblast with simple shading.
ep. epiblast; m. mesoblast; al. alimentary cavity; sg.
segmentation cavity; nc. neural canal; ch. notochord; x. point
where epiblast and hypoblast become continuous at the posterior end
of the embryo; n. nuclei of yolk.
A. Section of young blastoderm, with segmentation cavity enclosed in
the lower layer cells.
B. Older blastoderm with embryo in which hypoblast and mesoblast are
distinctly formed, and in which the alimentary slit has appeared.
The segmentation cavity is still represented as being present,
though by this stage it has in reality disappeared.
C. Older blastoderm with embryo in which the neural canal has become
formed, and is continuous posteriorly with the alimentary canal. The
notochord, though shaded like mesoblast, belongs properly to the
hypoblast.
A general idea of the structure of the blastoderm at this stage may be
gathered from the diagram representing a longitudinal[Pg 46] section through
the embryo (fig. 19 B). In this figure the epiblast is represented in
white and is seen to be continuous at the lip of the blastopore (x)
with the shaded hypoblast. Between the epiblast and hypoblast is seen
one of the lateral plates of mesoblast, represented by black cells
with clear outlines. The non-embryonic lower layer cells of the
blastoderm are represented in the same manner as the mesoblast of the
body. The alimentary cavity is shewn at al, and below it is seen the
yolk with nuclei (n). The segmentation cavity is represented as
still persisting, though by this stage it would have disappeared.
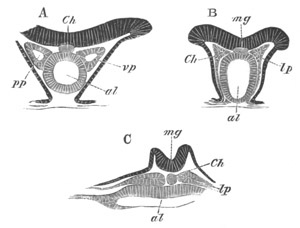
Fig. 20. Three sections through a Pristiurus embryo somewhat younger
than fig. 28 C.
A. Section through the cephalic plate.
B. Section through the posterior part of the cephalic plate.
C. Section through the trunk.
ch. notochord; mg. medullary groove; al. alimentary tract;
lp. lateral plate of mesoblast; pp. body cavity.
As to the growth of the blastoderm it may be noted that it has greatly
extended itself over the yolk. Its edge in the meantime forms a marked
ridge, which is due not so much to a thickening as to an arching of
the epiblast. This ridge is continuous with the embryonic rim, which
gradually concentrates itself into two prominences, one on each side
of the tail of the embryo, mainly formed of masses of undifferentiated
lower layer cells. These prominences will be called the caudal
swellings.
[Pg 47]By this stage the three layers of the body, the epiblast, mesoblast,
and hypoblast, have become definitely established. The further history
of these layers may now be briefly traced.
Epiblast. While the greater part of the epiblast becomes converted
into the external epidermis, from which involutions give rise to the
olfactory and auditory pits, the lens of the eye, the mouth cavity,
and anus, the part of it lining the medullary groove becomes converted
into the central nervous system and optic cup. The medullary groove is
at first continued to the front end of the medullary plate; but the
anterior part of this plate soon enlarges, and the whole plate assumes
a spatula form (fig. 28 C, h, and fig. 20 A and B). The enlarged
part becomes converted into the brain, and may be called the cephalic
plate.
The posterior part of the canal deepens much more rapidly than the
rest (fig. 20 C), and the medullary folds unite dorsally and convert
the posterior end of the medullary groove into a closed canal, while
the groove is still widely open elsewhere. The medullary canal does
not end blindly behind, but simply forms a tube not closed at either
extremity. The importance of this fact will appear later.
Shortly after the medullary folds have met behind the whole canal
becomes closed in. This occurs in the usual way by the junction and
coalescence of the medullary folds. In the course of the closing of
the medullary groove the edges of the cephalic plate, which have at
first a ventral curvature, become bent up in the normal manner, and
enclose the dilated cephalic portion of the medullary canal. The
closing of the medullary canal takes place earlier in the head and
neck than in the back.
An anterior pore at the front end of the canal, like that in Amphioxus
and the Ascidians, is not found. The further differentiation of the
central nervous system is described in a special chapter: it may
however here be stated that the walls of the medullary canal give rise
not only to the central nervous system but to the peripheral also.
Mesoblast. The mesoblast was left as two lateral plates continuous
behind with the undifferentiated cells of the caudal swellings.
The cells composing them become arranged in two layers (fig. 20 C,
lp), a splanchnic layer adjoining the hypoblast, and a[Pg 48] somatic layer
adjoining the epiblast. Between these two layers there is soon
developed in the region of the head a well-marked cavity (fig. 20 A,
pp) which is subsequently continued into the region of the trunk,
and forms the primitive body cavity, equivalent to the cavity
originating as an outgrowth of the archenteron in Amphioxus. The body
cavities of the two sides are at first quite independent.
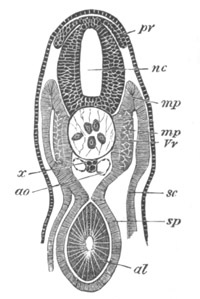
Fig. 21. Transverse section through the trunk of an embryo slightly
older than fig. 28 E.
nc. neural canal; pr. posterior root of spinal nerve; x.
subnotochordal rod; ao. aorta; sc. somatic mesoblast; sp.
splanchnic mesoblast; mp. muscle-plate; mp´. portion of
muscle-plate converted into muscle; Vv. portion of the vertebral
plate which will give rise to the vertebral bodies; al. alimentary
tract.
Coincidentally with the appearance of differentiation into somatic and
splanchnic layers the mesoblast plates become in the region of the
trunk partially split by a series of transverse lines of division into
mesoblastic somites. Only the dorsal parts of the plates become split
in this way, their ventral parts remaining quite intact. As a result
of this each plate becomes divided into a dorsal portion adjoining the
medullary canal, which is divided into somites, and may be called the
vertebral plate, and a ventral portion not so divided, which may be
called the lateral plate. These two parts are at this stage quite
continuous with each other; and the body cavity originally extends
uninterruptedly to the summit of the vertebral plates (fig. 21).
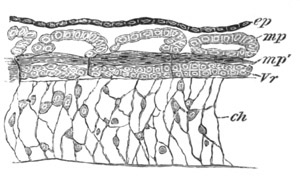
Fig. 22. Horizontal section through
the trunk of an embryo of Scyllium
considerably younger than 28 F.
The section is taken at the level of the notochord, and shews the
separation of the cells to form the vertebral bodies from the
muscle-plates.
ch. notochord; ep. epiblast; Vr. rudiment of vertebral body;
mp. muscle-plate; mp´. portion of muscle-plate already
differentiated into longitudinal muscles.
The next change results in the complete separation of the vertebral
portion of the plate from the lateral[Pg 49] portion; thereby the upper
segmented part of the body cavity becomes isolated, and separated from
the lower and unsegmented part. As a consequence of this change the
vertebral plate comes to consist of a series of rectangular bodies,
the mesoblastic somites, each composed of two layers, a somatic and a
splanchnic, between which is the cavity originally continuous with the
body cavity (fig. 23, mp). The splanchnic layer of the plates buds
off cells to form the rudiments of the vertebral bodies which are at
first segmented in the same planes as the mesoblastic somites (fig.
22, Vr). The plates themselves remain as the muscle-plates (mp),
and give rise to the whole of the voluntary muscular system of the
body. Between the vertebral and lateral plates there is left a
connecting isthmus, with a narrow prolongation of the body cavity
(fig. 23 B, st), which gives rise (as described in a special
chapter) to the segmental tubes and to other parts of the excretory
system.
In the meantime the lateral plates of the two sides unite ventrally
throughout the intestinal and cardiac regions of the body, and the two
primitively isolated cavities contained in them coalesce. In the tail
however the plates do not unite ventrally till somewhat later, and
their contained cavities remain distinct till eventually obliterated.
At first the pericardial cavity is quite continuous with the body
cavity; but it eventually becomes separated from the body cavity by
the attachment of the liver to the abdominal wall, and by a horizontal
septum in which run the two ductus Cuvieri (fig. 23 A, sv). Two
perforations in this septum (fig. 23 A) leave the cavities in
permanent communication.
The parts derived from the two layers of the mesoblast (not including
special organs or the vascular system) are as follows:—
From the somatic layer are formed
(1) A considerable part of the voluntary muscular system of the
body.
(2) The dermis.
(3) A large part of the intermuscular connective tissue.
(4) Part of the peritoneal epithelium.
From the splanchnic layer are formed
(1) A great part of the voluntary muscular system.
[Pg 50]
(2) Part of the intermuscular connective tissue.
(3) The axial skeleton and surrounding connective tissue.
(4) The muscular and connective-tissue wall of the alimentary tract.
(5) Part of the peritoneal epithelium.
Fig. 23. Sections through the trunk of a scyllium embryo slightly
younger than 28 F.
Figure A shews the separation of the body cavity from the
pericardial cavity by a horizontal septum in which runs the ductus
Cuvieri; on the left side is seen the narrow passage which remains
connecting the two cavities. Fig. B through a posterior part of the
trunk shews the origin of the segmental tubes and of the primitive
ova.
sp.c. spinal canal; W. white matter of spinal cord; pr.
commissure connecting the posterior nerve-roots; ch. notochord;
x. subnotochordal rod; ao. aorta; sv. sinus venosus; cav.
cardinal vein; ht. heart; pp. body cavity; pc. pericardial
cavity; œs. solid œsophagus; l. liver; mp. muscle-plate;
mp´. inner layer of muscle-plate; Vr. rudiment of vertebral
body; st. segmental tube; sd. segmental duct; sp.v. spiral
valve; v. subintestinal vein.
In the region of the head the mesoblast does not at first become
divided into somites; but on the formation of the gill clefts a
division takes place, which is apparently equivalent to the
segmentation of the mesoblast in the trunk. This division causes the
body cavity of the head to be divided up into a series[Pg 51] of separate
segments, one of which is shewn in fig. 24, pp. The walls of the
segments eventually give rise to the main muscles of the branchial
clefts, and probably also to the muscles of the mandibular arch, of
the eye, and of other parts. The cephalic sections of the body cavity
will be spoken of as head cavities.
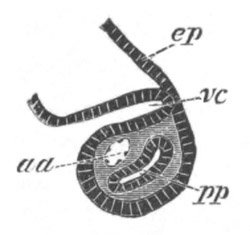
Fig. 24. Horizontal section through the last visceral arch but one
of an embryo of Pristiurus.
ep. epiblast; vc. pouch of hypoblast which will form the walls
of a visceral cleft; pp. segment of body-cavity in visceral arch;
aa. aortic arch.
In addition to the parts already mentioned the mesoblast gives rise to
the whole of the vascular system, and to the generative system. The
heart is formed from part of the splanchnic mesoblast, and the
generative system from a portion of the mesoblast of the dorsal part
of the body cavity.
The hypoblast. Very shortly after the formation of the mesoblastic
plates as lateral differentiations of the lower layer cells, an axial
differentiation of the hypoblast appears, which gives rise to the
notochord very much in the same way as in Amphioxus.
At first the hypoblast along the axial line forms a single layer in
contact with the epiblast. Along this line a rod-like thickening of
the hypoblast very soon appears (fig. 25, B and C, Ch´) at the head
end of the embryo, and gradually extends backwards. This is the
rudiment of the notochord; it remains attached for some time to the
hypoblast, and becomes separated from it first at the head end of the
embryo (fig. 25 A, ch): the separation is then carried backwards.
A series of sections taken through an embryo shortly after the first
differentiation of the notochord presents the following characters.
In the hindermost sections the hypoblast retains a perfectly normal
structure and uniform thickness throughout. In the next few sections
(fig. 25 C, Ch´) a slight thickening is to be observed in it,
immediately below the medullary groove. The layer, which elsewhere is
composed of a single row of cells, here becomes two cells deep, but no
sign of a division into two layers is exhibited.
In the next few sections the thickening of the hypoblast becomes much
more pronounced; we have, in fact, a ridge projecting from the
hypoblast towards the epiblast (fig. 25 B, Ch´). This ridge is
pressed firmly against[Pg 52] the epiblast, and causes in it a slight
indentation. The hypoblast in the region of the ridge is formed of two
layers of cells, the ridge being entirely due to the uppermost of the
two.
Fig. 25. Three sections of a Pristiurus
embryo slightly older than fig. 28 B.
The sections shew the development of the notochord.
Ch. notochord; Ch´. developing notochord; mg. medullary
groove; lp. lateral plate of mesoblast; ep. epiblast; hy.
hypoblast.
In sections in front of this a cylindrical rod, which can at once be
recognized as the notochord, and is continuous with the ridge just
described, begins to be split off from the hypoblast (fig. 25 A,
Ch). It is difficult to say at what point the separation of this rod
from the hypoblast is completed, since all intermediate gradations
between complete separation and complete attachment are to be seen.
Shortly after the separation takes place, a fairly thick bridge is
found connecting the two lateral halves of the hypoblast, but this
bridge is anteriorly excessively delicate and thin, and in some cases
is barely visible except with high powers. In some sections I have
observed possible indications of the process like that described by
Calberla for Petronyzon, by which the lateral parts of the hypoblast
grow in underneath the axial part, and so isolate it bodily as the
notochord.
It is not absolutely clear whether the notochord is to be regarded as
an axial differentiation of the hypoblast, or as an axial
differentiation of the lower layer cells.
The facts of development both in Amphioxus and Elasmobranchii tend
towards the former view; but the nearly simultaneous differentiation
of the notochord and the mesoblastic plates lends some support to the
supposition that the notochord may be merely a median plate of
mesoblast developed slightly later than the two lateral plates.
The alimentary canal or mesenteron was left as a space between the
hypoblast and the yolk, ending blindly in front, but[Pg 53] opening behind by
a widish aperture, the blastopore or anus of Rusconi (vide fig. 19
B).
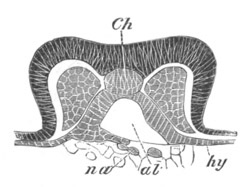
Fig. 26. Section through
the anterior part of a Pristiurus
embryo to shew the
formation of the alimentary
tract.
Ch. notochord; hy. hypoblast; al. alimentary tract; na.
cells passing in from the yolk to form the ventral wall of the
alimentary tract.
The conversion of this irregular cavity into a closed canal commences
first of all at the anterior extremity. In this conversion two
distinct processes are concerned. One of these is a process of folding
off of the embryo from the blastoderm. The other is a simple growth of
cells independent of any fold. To the first of these processes the
depth and narrowness of the alimentary cavity is due; the second is
concerned in forming its ventral wall. The process of the folding off
of the embryo from the blastoderm resembles exactly the similar
process in the embryo bird. The fold is a perfectly continuous one
round the front end of the embryo, but may be conveniently spoken of
as composed of a head-fold and two lateral folds.
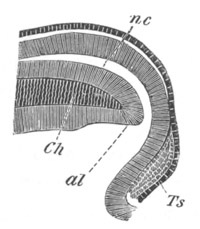
Fig. 27. Longitudinal
vertical section of an
embryo slightly younger
than that in fig. 26 D.
The section shews the communication which exists between the neural
and alimentary canals.
nc. neural canal; al. alimentary tract; Ch. notochord; Ts.
tail swelling.
Of far greater interest than the nature of these folds is the
formation of the ventral wall of the alimentary canal. This originates
in a growth of cells from the two sides to the middle line (fig. 26).
The cells for it are not however mainly derived from pre-existing
hypoblast cells, but are formed de novo around the nuclei of the
yolk which have already been spoken of (fig. 26, na). The ventral
wall of the mesenteron is in fact, to a large extent at any rate,
formed as a differentiation of the primitive yolk floor.
The folding off and closing of the alimentary canal in the anterior
part of the body proceeds rapidly, and not only is a considerable
tract of the alimentary canal formed, but a great part of the head is
completely folded off from the yolk before the medullary groove is
closed.
[Pg 54]The posterior part of the alimentary canal retains for a longer time
its primitive condition. Finally however it also becomes closed in, by
the lips of the blastopore at the hind end of the embryo meeting and
uniting. The peculiarity of the closing in of the posterior part of
the alimentary canal consists in the fact that a similar continuity to
that in Amphioxus obtains between the neural and alimentary canals.
This is due to the medullary folds being continuous at the end of the
tail with the lips of the blastopore, which close in the hind end of
the alimentary canal; so that, when the medullary folds unite to form
a canal, this canal becomes continuous with the alimentary canal,
which is closed in at the same time. In other words, the medullary
folds assist in enveloping the blastopore which does not therefore
become absolutely closed, but opens into the floor of the neural
canal. It will afterwards be shewn that it is only the posterior part
of the blastopore that becomes closed during the above process, and
that the anterior and ventral part long remains open. The general
arrangement of the parts, at the time when the hind end of the
mesenteron is first closed, is shewn in fig. 27. The same points may
be seen in the diagrammatic longitudinal section fig. 19 C.
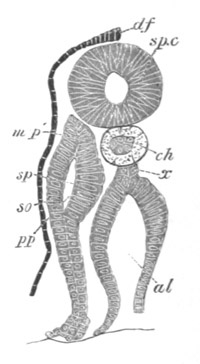
Fig. 27*. Transverse section through the tail region
of a Pristiurus embryo of the same age as fig. 28 E.
df. dorsal fin; sp.c. spinal cord; pp. body cavity; sp.
splanchnic layer of mesoblast; so. somatic layer of mesoblast; mp.
commencing differentiation of muscles; ch. notochord; x.
subnotochordal rod arising as an outgrowth of the dorsal wall of the
alimentary tract; al. alimentary tract.
The middle portion of the alimentary tract is the last to be closed in
since it remains till late in embryonic life as the umbilical or
vitelline canal, connecting the yolk-sack with the alimentary cavity.
The umbilical canal falls into the alimentary tract immediately behind
the entrance of the hepatic duct.
At a fairly early stage of development a rod is constricted off from
the dorsal wall of the alimentary canal (figs. 27* and 23 x), which
is known as the subnotochordal rod. It is placed immediately below the
notochord, and disappears during embryonic life.
[Pg 55] General features of the Elasmobranch embryo at successive stages.
Shortly after the three germinal layers become definitely established,
the rudiment of the embryo, as visible from the surface, consists of
an oblong plate, which extends inwards from the periphery of the
blastoderm, and is bounded on its inner side by a head-fold and two
lateral folds (fig. 28 B). This plate is the medullary plate; along
its axial line is a shallow groove—the medullary groove (mg). The
rudiment of the embryo rapidly increases in length, and takes a
spatula-like form (fig. 28 C). The front part of it, turned away from
the edge of the blastoderm, soon becomes dilated into a broad
plate,—the cephalic plate (h)—while the tail end at the edge of
the blastoderm is also enlarged, being formed of a pair of
swellings—the tail swellings (ts)—derived from the lateral parts
of the original embryonic rim. By this stage a certain number of
mesoblastic somites have become formed but are not shewn in my figure.
They are the foremost somites of the trunk, and those behind them
continue to be added, like the segments in Chætopods, between the last
formed somite and the end of the body. The increase in length of the
body mainly takes place by growth in the region between the last
mesoblastic somite and the end of the tail. The anterior part of the
body is now completely folded off from the blastoderm, and the
medullary groove of the earlier stage has become converted into a
closed canal.
By the next stage (fig. 28 D) the embryo has become so much folded off
from the yolk both in front and behind that the separate parts of it
begin to be easily recognizable.
The embryo is attached to the yolk by a distinct stalk or cord, which
in the succeeding stages gradually narrows and elongates, and is known
as the umbilical cord (so. s.). The medullary canal has now become
completely closed. The anterior region constitutes the brain; and in
this part slight constrictions, not perceptible in views of the embryo
as a transparent object, mark off three vesicles. These vesicles are
known as the fore, mid, and hind brain. From the fore-brain there is
an outgrowth on each side, the first rudiment of the optic vesicles
(op). The tail swellings are still conspicuous.
[Pg 56]
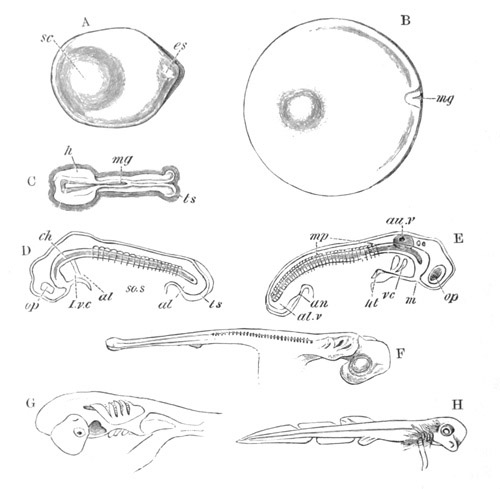
Fig. 28. Views of Elasmobranch Embryos.
A-F. Pristiurus. G. and H. Scyllium.
A. A blastoderm before the formation of the medullary plate. sc.
segmentation cavity; es. embryonic swelling.
B. A somewhat older blastoderm in which the medullary groove has
been established. mg. medullary groove.
C. An embryo from the dorsal surface, as an opaque object, after the
medullary groove has become posteriorly converted into a tube. mg.
medullary groove: the reference line points very nearly to the
junction between the open medullary groove with the medullary tube;
h. cephalic plate; ts. tail swelling.
D. Side view of a somewhat older embryo as a transparent object.
ch. notochord; op. optic vesicle; 1.v.c. 1st visceral cleft;
al. alimentary tract; so.s. stalk connecting the yolk-sack with
the embryo.
E. Side view of an older embryo as a transparent object. mp.
muscle-plates; au.v. auditory vesicle; vc. visceral cleft; ht.
heart; m. mouth invagination; an. anal diverticulum; al.v.
posterior vesicle of postanal gut.
F. G. H. Older embryos as opaque objects.
The tissues of the body have now become fairly transparent, and there
may be seen at the sides of the body seventeen mesoblastic somites.
The notochord, which was formed long[Pg 57] before the stage represented in
figure 28 D, is now also distinctly visible. It extends from almost
the extreme posterior to the anterior end of the embryo, and lies
between the ventral wall of the spinal canal and the dorsal wall of
the intestine. Round its posterior end the neural and alimentary
tracts become continuous with each other. Anteriorly the termination
of the notochord cannot be seen, it can only be traced into a mass of
mesoblast at the base of the brain, which there separates the epiblast
from the hypoblast. The alimentary canal (al) is completely closed
anteriorly and posteriorly, though still widely open to the yolk-sack
in the middle part of its course. In the region of the head it
exhibits on each side a slight bulging outwards, the rudiment of the
first visceral cleft. This is represented in the figure by two lines
(1. v.c.).
The embryo represented in fig. 28 E is far larger than the one just
described, but it has not been convenient to represent this increase
of size in the figure. Accompanying this increase in size, the folding
off from the yolk has considerably progressed, and the stalk which
unites the embryo with the yolk is proportionately narrower and longer
than before.
The brain is now very distinctly divided into the three lobes, the
rudiments of which appeared during the last stage. From the foremost
of these the optic vesicles now present themselves as well-marked
lateral outgrowths, towards which there has appeared an involution
from the external skin (op) to form the lens.
A fresh organ of sense, the auditory sack, now for the first time
becomes visible as a shallow pit in the external skin on each side of
the hind-brain (au.v). The epiblast which is involuted to form this
pit becomes much thickened, and thereby the opacity, indicated in the
figure, is produced.
The mesoblastic somites have greatly increased in number by the
formation of fresh somites in the tail. Thirty-eight of them were
present in the embryo figured. The mesoblast at the base of the brain
is more bulky, and there is still a mass of unsegmented mesoblast
which forms the tail swellings. The first rudiment of the heart (ht)
becomes visible during this stage as a cavity between the mesoblast of
the splanchnopleure and the hypoblast.
[Pg 58]The fore and hind guts are now longer than they were. An invagination
from the exterior to form the mouth has appeared (m) on the ventral
side of the head close to the base of the thalamencephalon. The upper
end of this eventually becomes constricted off as the pituitary body,
and an indication of the future position of the anus is afforded by a
slight diverticulum of the hind gut towards the exterior, some little
distance from the posterior end of the embryo (an). The portion of
the alimentary canal behind this point, though at this stage large,
and even dilated into a vesicle at its posterior end (al.v), becomes
eventually completely atrophied. It is known as the postanal gut. In
the region of the throat the rudiment of a second visceral cleft has
appeared behind the first; neither of them is as yet open to the
exterior.
In a somewhat older embryo the first spontaneous movements take place,
and consist in somewhat rapid excursions of the embryo from side to
side, produced by a serpentine motion of the body.
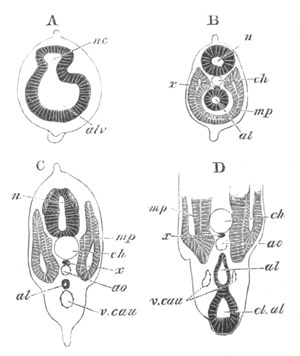
Fig. 28*. Four sections through the
post-anal part of the tail of an embryo of
the same age as fig. 28 F.
A is the posterior section.
nc. neural canal; al. postanal gut; alv. caudal vesicle of
postanal gut; x. subnotochord rod; mp. muscle-plate; ch.
notochord; cl.al. cloaca; ao. aorta; v.cau. caudal vein.
A ventral flexure of the præoral part of the head, known as the
cranial flexure, which commenced in earlier stages (fig. 28 D and E),
has now become very evident, and the mid-brain[19]
begins to project
in the same manner as in the embryo fowl on the[Pg 59] third day, and will
soon form the anterior termination of the long axis of the embryo. The
fore-brain has increased in size and distinctness, and the anterior
part of it may now be looked on as the unpaired rudiment of the
cerebral hemispheres.
Further changes have taken place in the organs of sense, especially in
the eye, in which the involution for the lens has made considerable
progress. The number of the muscle-plates has again increased, but
there is still a region of unsegmented mesoblast in the tail. The
thickened portions of mesoblast, which caused the tail swellings, are
still to be seen, and would seem to act as the reserve from which is
drawn the matter for the rapid growth of the tail, which occurs soon
after this. The mass of the mesoblast at the base of the brain has
again increased. No fresh features of interest are to be seen in the
notochord. The heart is very much more conspicuous than before, and
its commencing flexure is very apparent. It now beats actively. The
postanal gut is much longer than during the last stage; and the point
where the anus will appear is very easily detected by a bulging out of
the gut towards the external skin. The alimentary vesicle at the end
of the postanal gut, first observable during the last stage, is now a
more conspicuous organ. There are three visceral clefts, none of which
are as yet open to the exterior.
Figure 28 F represents a considerably older embryo viewed as an opaque
object, and fig. 29 A is a view of the head as a transparent object.
The stalk connecting it with the yolk is now, comparatively speaking,
quite narrow, and is of sufficient length to permit the embryo to
execute considerable movements.
The tail has grown immensely, but is still dilated terminally. The
terminal dilatation is mainly due to the alimentary vesicle (fig. 28*
alv), but the postanal section of the alimentary tract in front of
this is now a solid cord of cells. Both the alimentary vesicle and
this cord very soon disappear. Their relations are shewn in section in
fig. 28*.
The two pairs of limbs have appeared as differentiations of a
continuous but not very conspicuous epiblastic thickening, which is
probably the rudiment of a lateral fin. The anterior pair is situated
just at the front end of the umbilical stalk; and the[Pg 60] posterior pair,
which is the later developed and less conspicuous of the two, is
situated some little distance behind the stalk.
Fig. 29. Views of the head of Elasmobranch
embryos at two stages as transparent
objects.
A. Pristiurus embryo of the same stage as fig. 28 F.
B. Somewhat older Scyllium embryo.
III. third nerve; V. fifth nerve; VII. seventh nerve; au.n.
auditory nerve; gl. glossopharyngeal nerve; Vg. vagus nerve;
fb. fore-brain; pn. pineal gland; mb. mid-brain; hb.
hind-brain; iv.v. fourth ventricle; cb. cerebellum; ol.
olfactory pit; op. eye; au.V. auditory vesicle; m. mesoblast
at base of brain; ch. notochord; ht. heart; Vc. visceral
clefts; eg. external gills; pp. sections of body cavity in the
head.
The cranial flexure has greatly increased, and the angle between the
long axis of the front part of the head and of the body is less than a
right angle. The conspicuous mid-brain (29 A, mb) forms the anterior
termination of the long axis of the body. The thin roof of the fourth
ventricle (hb) may be noticed in the figure behind the mid-brain.
The auditory sack (au.V) is nearly closed, and its opening is not
shewn in the figure. In the eye (op) the lens is completely formed.
The olfactory pit (ol) is seen a little in front of the eye.
Owing to the opacity of the embryo, the muscle-plates are only
indistinctly indicated in fig. 28 F, and no other features of the
mesoblast are to be seen.
The mouth is now a deep pit, the hind borders of which are almost
completely formed by a thickening in front of the first branchial or
visceral cleft, which may be called the first branchial arch or
mandibular arch.
Four branchial clefts are now visible, all of which are open to the
exterior, but in the embryo, viewed as a transparent[Pg 61] object, two more,
not open to the exterior, are visible behind the last of these.
Between each of these and behind the last one there is a thickening of
the mesoblast which gives rise to a branchial arch. The arch between
the first and second cleft is known as the hyoid arch.
Fig. 29 B is a representation of the head of a slightly older embryo
in which papillæ may be seen in the front wall of the second, third,
and fourth branchial clefts; these papillæ are the commencements of
filiform processes which grow out from the gill-clefts and form
external gills. The peculiar ventral curvature of the anterior end of
the notochord (ch) both in this and in the preceding figure deserves
notice.
A peculiar feature in the anatomy makes its appearance at this period,
viz. the replacement of the original hollow œsophagus by a solid cord
of cells (fig. 23 A, œs) in which a lumen does not reappear till
very much later. I have found that in some Teleostei (the Salmon) long
after they are hatched a similar solidity in the œsophagus is
present. It appears not impossible that this feature in the œsophagus
may be connected with the fact that in the ancestors of the present
types the œsophagus was perforated by gill slits; and that in the
process of embryonic abbreviation the stage with the perforated
œsophagus became replaced by a stage with a cord of indifferent cells
(the œsophagus being in the embryo quite functionless) out of which
the non-perforated œsophagus was directly formed. In the higher types
the process of development appears to have become quite direct.
By this stage all the parts of the embryo have become established, and
in the succeeding stages the features characteristic of the genus and
species are gradually acquired.
Two embryos of Scyllium are represented in fig. 28 G and H, the head
and anterior part of the trunk being represented in fig. G, and the
whole embryo at a much later stage in fig. H.
In both of these, and especially in the second, an apparent diminution
of the cranial flexure is very marked. This diminution is due to the
increase in the size of the cerebral hemispheres, which grow upwards
and forwards, and press the original fore-brain against the mid-brain
behind.
In fig. G the rudiments of the nasal sacks are clearly visible as
small open pits.
[Pg 62]The first cleft is no longer similar to the rest, but by the closure
of the lower part has commenced to be metamorphosed into the spiracle.
Accompanying the change in position of the first cleft, the mandibular
arch has begun to bend round so as to enclose the front as well as the
sides of the mouth. By this change in the mandibular arch the mouth
becomes narrowed in an antero-posterior direction.
In fig. H are seen the long filiform external gills which now project
out from all the visceral clefts, including the spiracle. They are
attached to the front wall of the spiracle, to both walls of the next
four clefts, and to the front wall of the last cleft. They have very
possibly become specially developed to facilitate respiration within
the egg; and they disappear before the close of larval life.
When the young of Scyllium and other Sharks are hatched they have all
the external characters of the adult. In Raja and Torpedo the early
stages, up to the acquirement of a shark-like form, are similar to
those in the Selachoidei, but during the later embryonic stages the
body gradually flattens out, and assumes the adult form, which is thus
clearly shewn to be a secondary acquirement.
An embryonic gill cleft behind the last present in the adult is found
(Wyman, No. 54) in the embryo of Raja batis.
The unpaired fins are developed in Elasmobranchs as a fold of skin on
the dorsal side, which is continued round the end of the tail along
the ventral side to the anus. Local developments of this give rise to
the dorsal and anal fins. The caudal fin is at first symmetrical, but
a special lower lobe grows out and gives to it a heterocercal
character.
Enclosure of the yolk-sack and its relation to the embryo.
The blastoderm at the stage represented in fig. 28 A and B forms a
small and nearly circular patch on the surface of the yolk, composed
of epiblast and lower layer cells. While the body of the embryo is
gradually being moulded this patch grows till it envelopes the yolk;
the growth is not uniform, but[Pg 63] is less rapid in the immediate
neighbourhood of the embryonic part of the blastoderm than elsewhere.
As a consequence of this, that part of the edge, to which the embryo
is attached, forms a bay in the otherwise regular outline of the edge
of the blastoderm, and by the time that about two-thirds of the yolk
is enclosed this bay is very conspicuous. It is shewn in fig. 30 A,
where bl points to the blastoderm, and yk to the part of the yolk
not yet covered by the blastoderm. The embryo at this time is only
connected with the yolk-sack by a narrow umbilical cord; but, as shewn
in the figure, is still attached to the edge of the blastoderm.
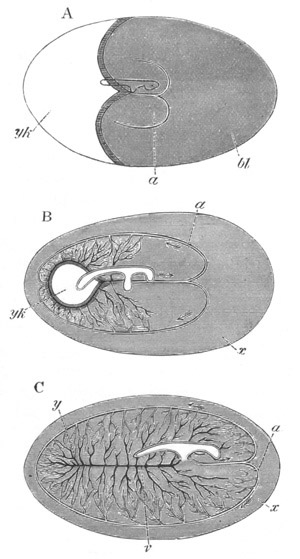
Fig. 30. Three views of the vitellus
of an Elasmobranch, shewing the embryo,
the blastoderm, and the vessels of the
yolk-sack.
The shaded part (bl) is the blastoderm; the white part the
uncovered yolk.
A. Young stage with the embryo still attached at the edge of the
blastoderm.
B. Older stage with the yolk not quite enclosed by the blastoderm.
C. Stage after the complete enclosure of the yolk.
yk. yolk; bl. blastoderm; v. venous trunks of yolk-sack; a.
arterial trunks of yolk-sack; y. point of closure of the yolk
blastopore; x. portion of the blastoderm outside the arterial
sinus terminalis.
Shortly subsequent to this the bay in the blastoderm, at the head of
which the embryo is attached, becomes obliterated by its two sides
coming together and coalescing. The embryo then ceases to be attached
at the edge of the blastoderm. But a linear streak formed by the
coalesced edges of the blastoderm is left connecting the embryo with
the[Pg 64] edge of the blastoderm. This streak is probably analogous to
(though not genetically related with) the primitive streak in the
Amniota.
This stage is represented in fig. 30 B. In this figure there is only a
small patch of yolk (yk) not yet enclosed, which is situated at some
little distance behind the embryo. Throughout all this period the edge
of the blastoderm has remained thickened: a feature which persists
till the complete investment of the yolk, which takes place shortly
after the stage last described. In this thickened edge a circular vein
arises which brings back the blood from the yolk-sack to the embryo.
The opening in the blastoderm, exposing the portion of the yolk not
yet covered, may be conveniently called the yolk blastopore. It is
interesting to notice that, owing to the large size of the yolk in
Elasmobranchs, the posterior part of the primitive blastopore becomes
encircled by the medullary folds and tail swellings, and is so closed
long before the anterior and more ventral part, which is represented
by the uncovered portion of the yolk. It is also worth remarking that,
owing to the embryo becoming removed from the edge of the blastoderm,
the final closure of the yolk blastopore takes place at some little
distance from the embryo.
The blastoderm enclosing the yolk is formed of an external layer of
epiblast, a layer of mesoblast below in which the blood-vessels are
developed, and within this a layer of hypoblast, which is especially
well marked and ciliated (Leydig, No. 46) in the umbilical stalk,
where it lines the canal leading from the yolk-sack to the intestine.
In the region of the yolk-sack proper the blastoderm is so thin that
it is not easy to be quite sure that a layer of hypoblast is
throughout distinct. Both the hypoblast and mesoblast of the yolk-sack
are formed by a differentiation of the primitive lower layer cells.
Nutriment from the yolk-sack is brought to the embryo partly through
the umbilical canal and so into the intestine, and partly by means of
blood-vessels in the mesoblast of the sack. The blood-vessels arise
before the blastoderm has completely covered the yolk.
Fig. 30 A represents the earliest stage of the circulation of the
yolk-sack. At this stage there is visible a single arterial[Pg 65] trunk
(a) passing forwards from the embryo and dividing into two branches.
No venous trunk could be detected with the simple microscope, but
probably venous channels were present in the thickened edge of the
blastoderm.
In fig. 30 B the circulation is greatly advanced. The blastoderm has
now nearly completely enveloped the yolk, and there remains only a
small circular space (yk) not enclosed by it. The arterial trunk is
present as before, and divides in front of the embryo into two
branches which turn backwards and form a nearly complete ring round
the embryo. In general appearance this ring resembles the sinus
terminalis of the area vasculosa of the Bird, but in reality bears
quite a different relation to the circulation. It gives off branches
on its inner side only.
A venous system of returning vessels is now fully developed, and its
relations are very remarkable. There is a main venous ring in the
thickened edge of the blastoderm, which is connected with the embryo
by a single stem running along the seam where the edges of the
blastoderm have coalesced. Since the venous trunks are only developed
behind the embryo, it is only the posterior part of the arterial ring
that gives off branches.
The succeeding stage (fig. 30 C) is also one of considerable interest.
The arterial ring has greatly extended, and now embraces nearly half
the yolk, and sends off trunks on its inner side along its whole
circumference. More important changes have taken place in the venous
system. The blastoderm has now completely enveloped the yolk, and the
venous ring is therefore reduced to a point. The small veins which
originally started from it may be observed diverging in a brush-like
fashion from the termination of the unpaired trunk, which originally
connected the venous ring with the heart.
At a still later stage the arterial ring embraces the whole yolk, and,
as a result of this, vanishes in its turn, as did the venous ring
before it. There is then present a single arterial and a single venous
trunk. The arterial trunk is a branch of the dorsal aorta, and the
venous trunk originally falls into the heart together with the
subintestinal or splanchnic vein. On the formation of the liver the
proximal end of the subintestinal vein becomes the portal vein, and it
is joined just as it enters[Pg 66] the liver by the venous trunk from the
yolk-sack. The venous trunk leaves the body on the right side, and the
arterial on the left.
The yolk-sack persists during the whole of embryonic life, and in the
majority of Elasmobranch embryos there arises within the body walls an
outgrowth from the umbilical canal into which a large amount of the
yolk passes. This outgrowth forms an internal yolk-sack. In Mustelus
vulgaris the internal yolk-sack is very small, and in Mustelus lævis
it is absent. The latter species, which is one of those in which
development takes place within the uterus, presents a remarkable
peculiarity in that the vascular surface of the yolk-sack becomes
raised into a number of folds, which fit into corresponding
depressions in the vascular walls of the uterus. The yolk-sack becomes
in this way firmly attached to the walls of the uterus, and the two
together constitute a kind of placenta. A similar placenta is found in
Carcharias.
After the embryo is hatched or born, as the case may be, the yolk-sack
becomes rapidly absorbed.
Bibliography.
(40) F. M. Balfour. “A preliminary account of the development of the
Elasmobranch Fishes.” Quart. J. of Micr. Science, Vol. XIV. 1876.
(41) F. M. Balfour. “A Monograph on the development of Elasmobranch
Fishes.” London, 1878. Reprinted from the Journal of Anat. and
Physiol. for 1876, 1877, and 1878.
(42) Z. Gerbe. Recherches sur la segmentation de la cicatrule et la
formation des produits adventifs de l'œuf des Plagiostomes et
particulièrement des Raies. Vide also Journal de l'Anatomie et de
la Physiologie, 1872.
(43) W. His. “Ueb. d. Bildung v. Haifischenembryonen.” Zeit. für
Anat. u. Entwick., Vol. II. 1877.
(44) A. Kowalevsky. “Development of Acanthias vulgaris and Mustelus
lævis.” (Russian.) Transactions of the Kiew Society of
Naturalists, Vol. I. 1870.
(45) R. Leuckart. “Ueber die allmählige Bildung d. Körpergestalt bei
d. Rochen.” Zeit. f. wiss. Zool., Bd. II., p. 258.
(46) Fr. Leydig. Rochen u. Haie. Leipzig, 1852.
(47) A. W. Malm. “Bidrag till kännedom om utvecklingen af Rajæ.”
Kongl. vetenskaps akademiens förhandlingar. Stockholm, 1876.
(48) Joh. Müller. Glatter Haie des Aristoteles und über die
Verschiedenheiten unter den Haifischen und Rochen in der Entwicklung
des Eies. Berlin, 1840.
(49) S. L. Schenk. “Die Eier von Raja quadrimaculata innerhalb der
Eileiter.” Sitz. der k. Akad. Wien, Vol. LXXIII. 1873.
[Pg 67](50) Alex. Schultz. “Zur Entwicklungsgeschichte des Selachiereies.”
Archiv für micro. Anat., Vol. XI.? 1875.
(51) Alex. Schultz. “Beitrag zur Entwicklungsgeschichte d.
Knorpelfische.” Archiv für micro. Anat., Vol. XIII. 1877.
(52) C. Semper. “Die Stammesverwandschaft d. Wirbelthiere u.
Wirbellosen.” Arbeit. a. d. zool.-zoot. Instit. Würzburg, Vol. II.
1875.
(53) C. Semper. “Das Urogenitalsystem d. Plagiostomen, etc.” Arbeit.
a. d. zool.-zoot. Instit. Würzburg, Vol. II. 1875.
(54) Wyman. “Observations on the Development of Raja batis.” Memoirs
of the American Academy of Arts and Sciences, Vol. IX. 1864.
[Pg 68]
CHAPTER IV.
TELEOSTEI.
The majority of the Teleostei deposit their eggs before impregnation,
but some forms are viviparous, e.g. Blennius viviparus. Not a few
carry their eggs about; but this operation is with a few exceptions
performed by the male. In Syngnathus the eggs are carried in a
brood-pouch of the male situated behind the anus. Amongst the
Siluroids the male sometimes carries the eggs in the throat above the
gill clefts. Ostegeniosus militaris, Arius falcarius, and Arius fissus
have this peculiar habit.
The ovum when laid is usually invested in the zona radiata only,
though a vitelline membrane is sometimes present in addition, e.g.
in the Herring. It is in most cases formed of a central yolk mass,
which may either be composed of a single large vitelline sphere, or of
distinct yolk spherules. The yolk mass is usually invested by a
granular protoplasmic layer, which is especially thickened at one pole
to form the germinal disc.
In the Herring’s ovum the germinal disc is formed, as in many
Crustacea, at impregnation; the protoplasm which was previously
diffused through the egg becoming aggregated at the germinal pole and
round the periphery.
Impregnation is external, and on its occurrence a contraction of the
vitellus takes place, so that a space is formed between the vitellus
and the zona radiata, which becomes filled with fluid.
The peculiarities in the development of the Teleostean ovum can best
be understood by regarding it as an Elasmobranch[Pg 69] ovum very much
reduced in size. It seems in fact very probable that the Teleostei are
in reality derived from a type of Fish with a much larger ovum. The
occurrence of a meroblastic segmentation, in spite of the ovum being
usually smaller than that of Amphibia and Acipenser, etc., in which
the segmentation is complete, as well as the solid origin of many of
the organs, receives its most plausible explanation on this
hypothesis.
The proportion of the germinal disc to the whole ovum varies
considerably. In very small eggs, such as those of the Herring, the
disc may form as much as a fifth of the whole.
The segmentation, which is preceded by active movements of the
germinal disc, is meroblastic. There is nothing very special to note
with reference to its general features, but while in large ova like
those of the Salmon the first furrows only penetrate for a certain
depth through the germinal disc, in small ova like those of the
Herring they extend through the whole thickness of the disc. During
the segmentation a great increase in the bulk of the blastoderm takes
place.
In hardened specimens a small cavity amongst the segmentation spheres
may be present at any early stage; but it is probably an artificial
product, and in any case has nothing to do with the true segmentation
cavity, which does not appear till near the close of segmentation. The
peripheral layer of granular matter, continuous with the germinal
disc, does not undergo division, but it becomes during the
segmentation specially thickened and then spreads itself under the
edge of the blastoderm; and, while remaining thicker in this region,
gradually grows inwards so as to form a continuous sub-blastodermic
layer. In this layer nuclei appear, which are equivalent to those in
the Elasmobranch ovum. A considerable number of these nuclei often
become visible simultaneously (van Beneden, No. 60) and they are
usually believed to arise spontaneously, though this is still
doubtful[20].
Around these nuclei portions of protoplasm are segmented
off, and cells are thus formed, which enter the blastoderm, and have
nearly the same destination as the homologous cells of the
Elasmobranch ovum.
[Pg 70]During the later stages of segmentation one end of the blastoderm
becomes thickened and forms the embryonic swelling; and a cavity
appears between the blastoderm and the yolk which is excentrically
situated near the non-embryonic part of the blastoderm. This cavity is
the true segmentation cavity. Both the cavity and the embryonic
swelling are seen in section in fig. 31 A and B.
In Leuciscus rutilus Bambeke describes a cavity as appearing in the
middle of the blastoderm during the later stages of segmentation. From
his figures it might be supposed that this cavity was equivalent to
the segmentation cavity of Elasmobranchs in its earliest condition,
but Bambeke states that it disappears and that it has no connection
with the true segmentation cavity. Bambeke and other investigators
have failed to recognize the homology of the segmentation cavity in
Teleostei with that in Elasmobranchii, Amphibia, etc.
With the appearance of the segmentation cavity the portion of the
blastoderm which forms its roof becomes thinned out, so that the whole
blastoderm consists of (1) a thickened edge especially prominent at
one point where it forms the embryonic swelling, and (2) a thinner
central portion. The changes which now take place result in the
differentiation of the embryonic layers, and in the rapid extension of
the blastoderm round the yolk, accompanied by a diminution in its
thickness.
Fig. 31. Longitudinal sections through the blastoderm of the
Trout at an early stage of development.
A. at the close of the segmentation; B. after the differentiation of
the germinal layers.
ep´. epidermic layer of the epiblast;
sc. segmentation cavity.
The first differentiation of the layers consists in a single row of
cells on the surface of the blastoderm becoming distinctly[Pg 71] marked off
as a special layer (fig. 31 A); which however does not constitute the
whole epiblast but only a small part of it, which will be spoken of as
the epidermic layer. The complete differentiation of the epiblast is
effected by the cells of the thickened edge of the blastoderm becoming
divided into two strata (fig. 31 B). The upper stratum constitutes the
epiblast. It is divided into two layers, viz., the external epidermic
layer already mentioned, and an internal layer known as the nervous
layer, formed of several rows of vertically arranged cells. According
to the unanimous testimony of investigators the roof of the
segmentation cavity is formed of epiblast cells only. The lower
stratum in the thickened rim of the blastoderm is several rows of
cells deep, and corresponds with the lower layer cells or primitive
hypoblast in Elasmobranchii. It is continuous at the edge of the
blastoderm with the nervous layer of the epiblast.
In smaller Teleostean eggs there is formed, before the blastoderm
becomes differentiated into epiblast and lower layer cells, a complete
stratum of cells around the nuclei in the granular layer underneath
the blastoderm. This layer is the hypoblast; and in these forms the
lower layer cells of the blastoderm are stated to become converted
into mesoblast only. In the larger Teleostean eggs, such as those of
the Salmonidæ, the hypoblast, as in Elasmobranchs, appears to be only
partially formed from the nuclei of the granular layer. In these forms
however, as in the smaller Teleostean ova and in Elasmobranchii, the
cells derived from the granular stratum give rise to a more or less
complete cellular floor for the segmentation cavity. The segmentation
cavity thus becomes enclosed between an hypoblastic floor and an
epiblastic roof several cells deep. It becomes obliterated shortly
after the appearance of the medullary plate.
At about the time when the three layers become established the
embryonic swelling takes a somewhat shield-like form (fig. 33 A).
Posteriorly it terminates in a caudal prominence (ts) homologous
with the pair of caudal swellings in Elasmobranchs. The homologue of
the medullary groove very soon appears as a shallow groove along the
axial line of the shield. After these changes there takes place in the
embryonic layers a series of differentiations leading to the
establishment of the[Pg 72] definite organs. These changes are much more
difficult to follow in the Teleostei than in the Elasmobranchii, owing
partly to the similarity of the cells of the various layers, and
partly to the primitive solidity of all the organs.
The first changes in the epiblast give rise to the central nervous
system. The epiblast, consisting of the nervous and epidermic strata
already indicated, becomes thickened along the axis of the embryo and
forms a keel projecting towards the yolk below: so great is the size
of this keel in the front part of the embryo that it influences the
form of the whole body and causes the outline of the surface adjoining
the yolk to form a strong ridge moulded on the keel of the epiblast
(fig. 32 A and B). Along the dorsal line of the epiblast keel is
placed the shallow medullary groove; and according to Calberla (No.
61) the keel is formed by the folding together of the two sides of the
primitively uniform epiblastic layer. The keel becomes gradually
constricted off from the external epiblast and then forms a solid
cord below it. Subsequently there appears in this cord a median
slit-like canal, which forms the permanent central canal of the
cerebrospinal cord. The peculiarity in the formation of the central
nervous system of Teleostei consists in the fact that it is not formed
by the folding over of the sides of the medullary groove into a canal,
but by the separation, below the medullary groove, of a solid cord of
epiblast in which the central canal is subsequently formed. Various
views have been put forward to explain the apparently startling
difference between Teleostei, with which Lepidosteus and Petromyzon
agree, and other vertebrate forms. The explanations of Götte and
Calberla appear to me to contain between them the truth in this
matter. The groove above in part represents the medullary groove; but
the closure of the groove is represented by the folding together of
the lateral parts of the epiblast plate to form the medullary keel.
According to Götte this is the whole explanation, but Calberla states
for Syngnathus and Salmo that the epidermic layer of the epiblast is
carried down into the keel as a double layer just as if it had been
really folded in. This ingrowth of the epidermic layer is shewn in
fig. 32 A where it is just commencing to pass into the keel; and at a
later stage in fig. 32 B where the keel has reached its greatest
depth.
[Pg 73]Götte maintains that Calberla’s statements are not to be trusted, and
I have myself been unable to confirm them for Teleostei or
Lepidosteus; but if they could be accepted the difference in the
formation of the medullary canal in Teleostei and in other Vertebrata
would become altogether unimportant and consist simply in the fact
that the ordinary open medullary groove is in Teleostei obliterated in
its inner part by the two sides of the groove coming together. Both
layers of epiblast would thus have a share in the formation of the
central nervous system; the epidermic layer giving rise to the lining
epithelial cells of the central canal, and the nervous layer to the
true nervous tissue.
The separation of the solid nervous system from the epiblast takes
place relatively very late; and, before it has been completed, the
first traces of the auditory pits, of the optic vesicles, and of the
olfactory pits are visible. The auditory pit arises as a solid
thickening of the nervous layer of the epiblast at its point of
junction with the medullary keel; and the optic vesicles spring as
solid outgrowths from part of the keel itself. The olfactory pits are
barely indicated as thickenings of the nervous layer of the epiblast.
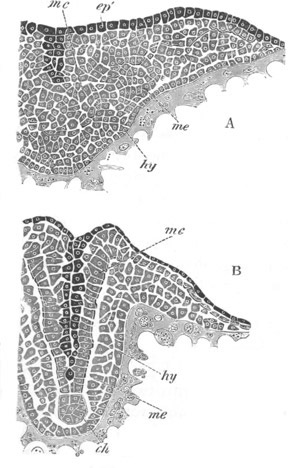
Fig. 32. Two transverse sections of
Syngnathus. (After Calberla.)
A. Younger stage before the definite establishment of the notochord.
B. Older stage.
The epidermic layer of the epiblast is represented in black.
ep. epidermic layer of epiblast; mc. neural cord; hy.
hypoblast; me. mesoblast; ch. notochord.
At this early stage all the organs of special sense are attached to a
layer continuous with or forming part of the central nervous system;
and this fact has led Götte (No. 63) to speak of a special-sense
plate, belonging to the central nervous system and not to the skin,
from which[Pg 74] all the organs of special sense are developed; and to
conclude that a serial homology exists between these organs in their
development. A comparison between Teleostei and other forms shews that
this view cannot be upheld; even in Teleostei the auditory and
olfactory rudiments arise rather from the epiblast at the sides of the
brain than from the brain itself, while the optic vesicles spring from
the first directly from the medullary keel, and are therefore
connected with the central nervous system rather than with the
external epiblast. In a slightly later stage the different connections
of the two sets of sense organs is conclusively shewn by the fact
that, on the separation of the central nervous system from the
epiblast, the optic vesicles remain attached to the former, while the
auditory and olfactory vesicles are continuous with the latter.
After its separation from the central nervous system the remainder of
the epiblast gives rise to the skin, etc., and most probably the
epidermic stratum develops into the outer layer of the epidermis and
the nervous stratum into the mucous layer. The parts of the organs of
special sense, which arise from the epiblast, are developed from the
nervous layer. In the Trout (Oellacher, No. 72) both layers are
continued over the yolk-sack; but in Clupeus and Gasterosteus only the
epidermic has this extension. According to Götte the distinction
between the two layers becomes lost in the later embryonic stages.
Although it is thoroughly established that the mesoblast originates
from the lower of the two layers of the thickened embryonic rim, it is
nevertheless not quite certain whether it is a continuous layer
between the epiblast and hypoblast, or whether it forms two lateral
masses as in Elasmobranchs. The majority of observers take the former
view, while Calberla is inclined to adopt the latter. In the median
line of the embryo underneath the medullary groove there are
undoubtedly from the first certain cells which eventually give rise to
the notochord; and it is these cells the nature of which is in doubt.
They are certainly at first very indistinctly separated from the
mesoblast on the two sides, and Calberla also finds that there is no
sharp line separating them from the secondary hypoblast (fig. 32 A).
Whatever may be the origin of the notochord the mesoblast very soon
forms two lateral plates, one on each side of the body, and between
them is placed the notochord (fig. 32 B). The general fate of the two
mesoblast plates is the same as in Elasmobranchs. They are at first
quite solid and exhibit relatively[Pg 75] late a division into splanchnic and
somatic layers, between which is placed the primitive body cavity. The
dorsal part of the plates becomes transversely segmented in the region
of the trunk; and thus gives rise to the mesoblastic somites, from
which the muscle plates and the perichordal parts of the vertebral
column are developed. The ventral or outer part remains unsegmented.
The cavity of the ventral section becomes the permanent body cavity.
It is continued forward into the head (Oellacher), and part of it
becomes separated off from the remainder as the pericardial cavity.
The hypoblast forms a continuous layer below the mesoblast, and, in
harmony with the generally confined character of the development of
the organs in Teleostei, there is no space left between it and the
yolk to represent the primitive alimentary cavity. The details of the
formation of the true alimentary tube have not been made out; it is
not however formed by a folding in of the lateral parts of the
hypoblast, but arises as a solid or nearly solid cord in the axial
line, between the notochord and the yolk, in which a lumen is
gradually established.
In the just hatched larva of an undetermined freshwater fish with a
very small yolk-sack I found that the yolk extended along the ventral
side of the embryo from almost the mouth to the end of the gut. The
gut had, except in the hinder part, the form of a solid cord resting
in a concavity of the yolk. In the hinder part of the gut a lumen was
present, and below this part the amount of yolk was small and the yolk
nuclei numerous. Near the limit of its posterior extension the yolk
broke up into a mass of cells, and the gut ended behind by falling
into this mass. These incomplete observations appear to shew that the
solid gut owes its origin in a large measure to nuclei derived from
the yolk.
When the yolk has become completely enveloped a postanal section of
gut undoubtedly becomes formed; and although, owing to the solid
condition of the central nervous system, a communication between the
neural and alimentary canals cannot at first take place, yet the
terminal vesicle of the postanal gut of Elasmobranchii is represented
by a large vesicle, originally discovered by Kupffer (No. 68), which
can easily be seen in the embryos of most Teleostei, but the relations
of which have not been satisfactorily worked out (vide fig. 34,
hyv). As the tail end of the embryo becomes separated off from the
yolk the postanal vesicle atrophies.
[Pg 76]General development of the Embryo. Attention has already been called
to the fact that the embryo first appears as a thickening of the edge
of the blastoderm which soon assumes a somewhat shield-like form (fig.
33 A). The hinder end of the embryo, which is placed at the edge of
the blastoderm, is somewhat prominent, and forms the caudal swelling
(ts). The axis of the embryo is marked by a shallow groove.
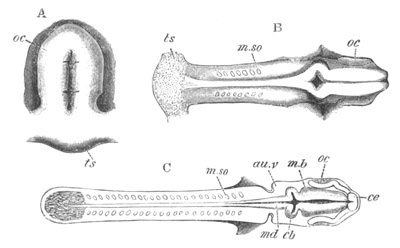
Fig. 33. Three stages in the development of the Salmon. (After
His.)
ts. tail swelling; au.v. auditory vesicle; oc. optic vesicle;
ce. cerebral rudiment; m.b. mid-brain; cb. cerebellum; md.
medulla oblongata; m.so. mesoblastic somite.
The body now rapidly elongates, and at the same time becomes
considerably narrower, while the groove along the axis becomes
shallower and disappears. The anterior, and at first proportionately a
very large part, soon becomes distinguished as the cephalic region
(fig. 33 B). The medullary cord in this region becomes very early
divided into three indistinctly separated lobes, representing the
fore, the mid, and the hind brains: of these the anterior is the
smallest. With it are connected the optic vesicles (oc)—solid at
first—which are pushed back into the region of the mid-brain.
The trunk grows in the usual way by the addition of fresh somites
behind.
After the yolk has become completely enveloped by the blastoderm the
tail becomes folded off, and the same process takes place at the front
end of the embryo. The free tail end of[Pg 77] the embryo continues to grow,
remaining however closely applied to the yolk-sack, round which it
curls itself to an extent varying with the species (vide fig. 34).
The general growth of the embryo during the later stages presents a
few special features of interest. The head is remarkable for the small
apparent amount of the cranial flexure. This is probably due to the
late development of the cerebral hemispheres. The flexure of the floor
of the brain is however quite as considerable in the Teleostei as in
other types. The gill clefts develop from before backwards. The first
cleft is the hyomandibular, and behind this there are the hyobranchial
and four branchial clefts. Simultaneously with the clefts there are
developed the branchial arches. The postoral arches formed are the
mandibular, hyoid and five branchial arches. In the case of the Salmon
all of these appear before hatching.
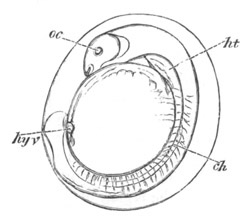
Fig. 34. View of an advanced
embryo of a Herring in the
egg. (After
Kupffer.)
oc. eye; ht. heart; hyv. postanal vesicle; ch. notochord.
The first cleft closes up very early (about the time of hatching in
the Salmon); and about the same time there springs a membranous fold
from the hyoid arch, which gradually grows backwards over the arches
following, and gives rise to the operculum. There appear in the Salmon
shortly before hatching double rows of papillæ on the four anterior
arches behind the hyoid. They are the rudiments of the branchiæ. They
reach a considerable length before they are covered in by the
opercular membrane. In Cobitis (Götte, No. 64) they appear in young
larvæ as filiform processes equivalent to the external gills of
Elasmobranchs. The extremities of these processes atrophy; while the
basal portions became the permanent gill lamellæ. The general relation
of the clefts, after the closure of the hyomandibular, is shewn in
fig. 35.
The air-bladder is formed as a dorsal outgrowth of the alimentary
tract very slightly in front of the liver. It grows in between the two
limbs of the mesentery, in which it extends itself backwards. It
appears in the Salmon,[Pg 78] Carp, and other types to originate rather on
the right side of the median dorsal line, but whether this fact has
any special significance is rather doubtful. In the Salmon and Trout
it is formed considerably later than the liver, but the two are stated
by Von Baer to arise in the Carp nearly at the same time. The absence
of a pneumatic duct in the Physoclisti is due to a post-larval
atrophy. The region of the stomach is reduced almost to nothing in the
larva.
The œsophagus becomes solid, like that of Elasmobranchs, and remains
so for a considerable period after hatching.
The liver, in the earliest stage in which I have met with it in the
Trout (27 days after impregnation), is a solid ventral diverticulum of
the intestine, which in the region of the liver is itself without a
lumen.
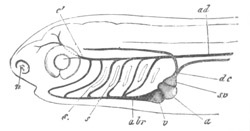
Fig. 35. Diagrammatic view of the
head of an embryo Teleostean, with the
primitive vascular trunks. (From Gegenbaur.)
a. auricle; v. ventricle; abr. branchial artery; c´.
carotid; ad. aorta; s. branchial clefts; sv. sinus venosus;
dc. ductus Cuvieri; n. nasal pit.
The excretory system commences with the formation of a segmental duct,
formed by a constriction of the parietal wall of the peritoneal
cavity. The anterior end remains open to the body cavity, and forms a
pronephros (head kidney). On the inner side of and opposite this
opening a glomerulus is developed, and the part of the body cavity
containing both the glomerulus and the opening of the pronephros
becomes shut off from the remainder of the body cavity, and forms a
completely closed Malpighian capsule.
The mesonephros (Wolffian body) is late in developing.
The unpaired fins arise as simple folds of the skin along the dorsal
and ventral edges, continuous with each other round the end of the
tail. The ventral fold ends anteriorly at the anus.
The dorsal and anal fins are developed from this fold by local
hypertrophy. The caudal fin[21],
however, undergoes a more complicated
metamorphosis. It is at first symmetrical or nearly so on the dorsal
and ventral sides of the hinder end of the notochord. This symmetry is
not long retained, but very soon the ventral part of the fin with its
fin rays becomes much more developed than the dorsal part, and at the
same time the posterior part of the notochord bends up towards the
dorsal side.
[Pg 79]In some few cases, e.g. Gadus, Salmo, owing to the simultaneous
appearance of a number of fin rays on the dorsal and ventral side of
the notochord the external symmetry of the tail is not interfered with
in the above processes. In most instances this is far from being the
case.
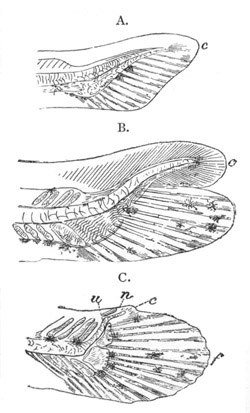
Fig. 36. Three stages in the development
of the tail of the Flounder (Pleuronectes). (After Agassiz.)
A. Stage in which the permanent caudal fin has commenced to be
visible as an enlargement of the ventral side of the embryonic
caudal fin.
B. Ganoid-like stage in which there is a true external heterocercal
tail.
C. Stage in which the embryonic caudal fin has almost completely
atrophied.
c. embryonic caudal fin; f. permanent caudal fin; n.
notochord; u. urostyle.
In the Flounder, which may serve as a type, the primitive symmetry is
very soon destroyed by the appearance of fin rays on the ventral side.
The region where they are present soon forms a lobe; and an externally
heterocercal tail is produced (fig. 36 A). The ventral lobe with its
rays continues to grow more prominent and causes the tail fin to
become bilobed (fig. 36 B); there being a dorsal embryonic lobe
without fin rays (c), which contains the notochord, and a ventral
lobe with fin rays, which will form the permanent caudal fin. In this
condition the tail fin resembles the usual Elasmobranch form or still
more that of some Ganoids, e.g. the Sturgeon. The ventral lobe
continues to develop; and soon projects beyond the dorsal, which
gradually atrophies together with the notochord contained in it, and
finally disappears, leaving hardly a trace on the dorsal side of the
tail (fig. 36 C, c). In the meantime the fin rays of the ventral
lobe gradually become parallel to the axis of the body; and this lobe,
together with a few accessory dorsal and ventral fin rays supported[Pg 80] by
neural and hæmal processes, forms the permanent tail fin, which though
internally unsymmetrical, assumes an externally symmetrical form. The
upturned end of the notochord which was originally continued into the
primitive dorsal lobe becomes ensheathed in a bone without a division
into separate vertebræ. This bone forms the urostyle (u). The hæmal
processes belonging to it are represented by two cartilaginous masses,
which subsequently ossify, forming the hypural bones, and supporting
the primary fin rays of the tail (fig. 36 C). The ultimate changes of
the notochord and urostyle vary very considerably in the different
types of Teleostei. Teleostei may fairly be described as passing
through an Elasmobranch stage or a stage like that of most
pre-jurassic Ganoids or the Sturgeon as far as concerns their caudal
fin.
The anterior paired fins arise before the posterior; and there do not
appear to be any such indications as in Elasmobranchii of the paired
fins arising as parts of a continuous lateral fin.
Most osseous fishes pass through more or less considerable
post-embryonic changes, the most remarkable of which are those
undergone by the Pleuronectidæ[22].
These fishes, which in the adult
state have the eyes unsymmetrically placed on one side of the head,
leave the egg like normal Teleostei. In the majority of cases as they
become older the eye on the side, which in the adult is without an
eye, travels a little forward and then gradually rotates over the
dorsal side of the head, till finally it comes to lie on the same side
as the other eye. During this process the rotating eye always remains
at the surface and continues functional; and on the two eyes coming to
the same side of the head the side of the body without an organ of
vision loses its pigment cells, and becomes colourless.
The dorsal fin, after the rotation of the eye, grows forward beyond
the level of the eyes. In the genus Plagusia (Steenstrup, Agassiz, No.
56) the dorsal fin grows forward before the rotation of the eye (the
right eye in this form), and causes some modifications in the process.
The eye in travelling round gradually sinks into the tissues of the
head, at the base of the fin above the frontal bone; and in this
process the original large opening of the orbit becomes much reduced.
Soon a fresh opening on the opposite and left side of the dorsal fin
is formed; so that the orbit has two external openings, one on the
left and one on the right side. The original one on the right soon
atrophies, and the eye passes through the tissues at the base of the
dorsal fin completely to the left side.
The rotating eye may be either the right or the left according to the
species.
[Pg 81]The most remarkable feature in which the young of a large number of
Teleostei differ from the adults is the possession of provisional
spines, very often formed as osseous spinous projections the spaces
between which become filled up in the adult. These processes are
probably, as suggested by Günther, secondary developments acquired,
like the Zoœa spines of larval Crustaceans, for purposes of defence.
The yolk-sack varies greatly in size in the different types of
Teleostei.
According as it is enclosed within the body-wall, or forms a distinct
ventral appendage, it is spoken of by Von Baer as an internal or
external yolk-sack. By Von Baer the yolk-sack is stated to remain in
communication with the intestine immediately behind the liver, while
Lereboullet states that there is a vitelline pedicle opening between
the stomach and the liver which persists till the absorption of the
yolk-sack. My own observations do not fully confirm either of these
statements for the Salmon and Trout. So far as I have been able to
make out, all communication between the yolk-sack and the alimentary
tract is completely obliterated very early. In the Trout the
communication between the two is shut off before hatching, and in the
just-hatched Salmon I can find no trace of any vitelline pedicle. The
absorption of the yolk would seem therefore to be effected entirely by
blood-vessels.
The yolk-sack persists long after hatching, and is gradually absorbed.
There is during the stages either just before hatching or shortly
subsequent to hatching (Cyprinus) a rich vascular development in the
mesoblast of the yolk-sack. The blood is at first contained in lacunar
spaces, but subsequently it becomes confined to definite channels. As
to its exact relations to the vascular system of the embryo more
observations seem to be required.
The following account is given by Rathke (No. 72*) and Lereboullet
(No. 71). At first a subintestinal vein (vide chapter on
Circulation) falls into the lacunæ of the yolk-sack, and the blood
from these is brought back direct to the heart. At a later period,
when the liver is developed, the subintestinal vessel breaks up into
capillaries in the liver, thence passes into the yolk-sack, and from
this to the heart. An artery arising from the aorta penetrates the
liver, and there breaks up into capillaries continuous with those of
the yolk-sack. This vessel is perhaps the equivalent of the artery
which supplies the yolk-sack in Elasmobranchii, but it seems possible
that there is some error in the above description.
Bibliography.
(55) Al. Agassiz. “On the young Stages of some Osseous Fishes. I.
Development of the Tail.” Proceedings of the American Academy of Arts
and Sciences, Vol. XIII. Presented Oct. 11, 1877.
[Pg 82](56) Al. Agassiz. “II. Development of the Flounders.” Proceedings of
the American Acad. of Arts and Sciences, Vol. XIV. Presented June,
1878.
(57) K. E. v. Baer. Untersuchungen über die Entwicklungsgeschichte
der Fische. Leipzig, 1835.
(58) Ch. van Bambeke. “Premiers effets de la fécondation sur les œufs
de Poissons: sur l'origine et la signification du feuillet muqueux ou
glandulaire chez les Poissons Osseux.” Comptes Rendus des Séances de
l'Académie des Sciences, Tome LXXIV. 1872.
(59) Ch. van Bambeke. “Recherches sur l'Embryologie des Poissons
Osseux.” Mém. couronnés et Mém. de savants étrangers de l'Académie
roy. Belgique, Vol. XL. 1875.
(60) E. v. Beneden. “A contribution to the history of the Embryonic
development of the Teleosteans.” Quart. J. of Micr. Sci., Vol.
XVIII. 1878.
(61) E. Calberla. “Zur Entwicklung des Medullarrohres u. d. Chorda
dorsalis d. Teleostier u. d. Petromyzonten.” Morphologisches
Jahrbuch, Vol. III. 1877.
(62) A. Götte. “Beiträge zur Entwicklungsgeschichte der Wirbelthiere.”
Archiv f. mikr. Anat., Vol. IX. 1873.
(63) A. Götte. “Ueber d. Entwicklung d. Central-Nervensystems der
Teleostier.” Archiv f. mikr. Anat., Vol. XV. 1878.
(64) A. Götte. “Entwick. d. Teleostierkeime.” Zoologischer Anzeiger,
No. 3. 1878.
(65) W. His.. “Untersuchungen über die Entwicklung von Knochenfischen,
etc.” Zeit. f. Anat. u. Entwicklungsgeschichte, Vol. I. 1876.
(66) W. His. “Untersuchungen über die Bildung des Knochenfischembryo
(Salmen).” Archiv f. Anat. u. Physiol., 1878.
(67) E. Klein. “Observations on the early Development of the Common
Trout.” Quart. J. of Micr. Science, Vol. XVI. 1876.
(68) C. Kupffer. “Beobachtungen über die Entwicklung der
Knochenfische.” Archiv f. mikr. Anat., Bd. IV. 1868.
(69) C. Kupffer. Ueber Laichen u. Entwicklung des Ostsee-Herings.
Berlin, 1878.
(70) M. Lereboullet. “Recherches sur le développement du brochet de la
perche et de l'écrevisse.” Annales des Sciences Nat., Vol. I.,
Series IV. 1854.
(71) M. Lereboullet. “Recherches d'Embryologie comparée sur le
développement de la Truite.” An. Sci. Nat., quatrième série, Vol.
XVI. 1861.
(72) T. Oellacher. “Beiträge zur Entwicklungsgeschichte der
Knochenfische nach Beobachtungen am Bachforellenei.” Zeit. f. wiss.
Zool., Vol. XXII., 1872, and Vol. XXIII., 1873.
(72*) H. Rathke. Abh. z. Bildung u. Entwick. d. Menschen u. Thiere.
Leipzig, 1832-3. Part II. Blennius.
(73) Reineck. “Ueber die Schichtung des Forellenkeims.” Archiv f.
mikr. Anat., Bd. V. 1869.
(74) S. Stricker. “Untersuchungen über die Entwicklung der
Bachforelle.” Sitzungsberichte der Wiener k. Akad. d. Wiss., 1865.
Vol. LI. Abth. 2.
(75) Carl Vogt. “Embryologie des Salmones.” Histoire Naturelle des
Poissons de l'Europe Centrale. L. Agassiz. 1842.
(76) C. Weil. “Beiträge zur Kenntniss der Knochenfische.”
Sitzungsber. der Wiener kais. Akad. der Wiss., Bd. LXVI. 1872.
[Pg 83]
CHAPTER V.
CYCLOSTOMATA[23].
Petromyzon is the only type of this degenerated but primitive group of
Fishes the development of which has been as yet studied[24].
The development does not however throw any light on the relationships
of the group. The similarity of the mouth and other parts of
Petromyzon to those of the Tadpole probably indicates that there
existed a common ancestral form for the Cyclostomata and Amphibia.
Embryology does not however add anything to the anatomical evidence on
this subject. The fact of the segmentation being complete was at one
time supposed to indicate an affinity between the two groups; but the
discovery that the segmentation is also complete in the Ganoids
deprives this feature in the development of any special weight. In the
formation of the layers and in most other developmental characters
there is nothing to imply a special relationship with the Amphibia,
and in the mode of formation of the nervous system Petromyzon exhibits
a peculiar modification, otherwise only known to occur in Teleostei
and Lepidosteus.
Dohrn[25]
was the first to bring into prominence the degenerate
character of the Cyclostomata. I cannot however assent to his view
that they are[Pg 84] descended from a relatively highly-organized type of
Fish. It appears to me almost certain that they belong to a group of
fishes in which a true skeleton of branchial bars had not become
developed, the branchial skeleton they possess being simply an
extra-branchial system; while I see no reason to suppose that a true
branchial skeleton has disappeared. If the primitive Cyclostomata had
not true branchial bars, they could not have had jaws, because jaws
are essentially developed from the mandibular branchial bar. These
considerations, which are supported by numerous other features of
their anatomy, such as the character of the axial skeleton, the
straightness of the intestinal tube, the presence of a subintestinal
vein etc., all tend to prove that these fishes are remnants of a
primitive and prægnathostomatous group. The few surviving members of
the group have probably owed their preservation to their parasitic or
semiparasitic habits, while the group as a whole probably disappeared
on the appearance of gnathostomatous Vertebrata.
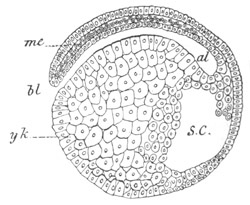
Fig. 37. Longitudinal vertical section through an
embryo of Petromyzon Planeri of 136 hours.
me. mesoblast; yk. yolk-cells; al. alimentary tract; bl.
blastopore; s.c. segmentation cavity.
The ripe ovum of Petromyzon Planeri is a slightly oval body of about 1
mm. in diameter. It is mainly formed of an opaque nearly white yolk,
invested by a membrane composed of an inner perforated layer, and an
outer structureless layer. There appears to be a pore perforating the
inner layer at the formative pole, which may be called a micropyle
(Kupffer and Benecke, No. 79). Enclosing the egg-membranes there is
present a mucous envelope, which causes the egg, when laid, to adhere
to stones or other objects.
Impregnation is effected by the male attaching itself by its suctorial
mouth to the female. The attached couple then shake together; and, as
they do so, they respectively emit from their abdominal pores ova and
spermatozoa which pass into a hole previously made[26].
[Pg 85]The segmentation is total and unequal, and closely resembles that in
the Frog’s egg (Vol. II. p. 96). The upper pole is very slightly
whiter than the lower. A segmentation cavity is formed very early, and
is placed between the small cells of the upper pole and the large
cells of the lower pole. It is proportionately larger than in the
Frog; and the roof eventually thins out so as to be formed of a single
row of small cells. At the sides of the segmentation cavity there are
always several rows of small cells, which gradually merge into the
larger cells of the lower pole of the egg. The segmentation is
completed in about fifty hours.
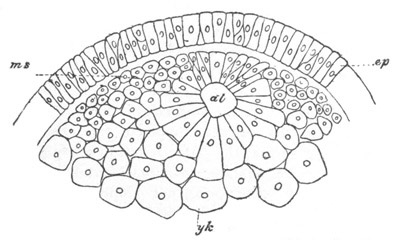
Fig. 38. Transverse section through a Petromyzon embryo 160 hours
after impregnation.
ep. epiblast; al. mesenteron; yk. yolk-cells; ms.
mesoblast.
The segmentation is followed by an asymmetrical invagination (fig. 37)
which leads to a mode of formation of the hypoblast fundamentally
similar to that in the Frog. The process has been in the main
correctly described by M. Schultze (No. 81).
On the border between the large and small cells of the embryo, at a
point slightly below the segmentation cavity, a small circular pit
appears; the roof of which is formed by an infolding of the small
cells, while the floor is formed of the large cells. This pit is the
commencing mesenteron. It soon grows deeper (fig. 37, al) and
extends as a well-defined tube (shewn in transverse section in fig.
38, al) in the direction of the segmentation cavity. In the course
of the formation of the mesenteron the segmentation cavity gradually
becomes smaller, and is[Pg 86] finally (about the 200th hour) obliterated.
The roof of the mesenteron is formed by the continued invagination of
small cells, and its floor is composed of large yolk-cells. The wide
external opening is arched over dorsally by a somewhat prominent
lip—the homologue of the embryonic rim. The opening persists till
nearly the time of hatching; but eventually becomes closed, and is not
converted into the permanent anus. On the formation of the mesenteron
the hypoblast is composed of two groups of cells, (1) the yolk-cells,
and (2) the cells forming the roof of the mesenteron.
While the above changes are taking place, the small cells, or as they
may now be called the epiblast cells, gradually spread over the large
yolk-cells, as in normal types of epibolic invagination. The growth
over the yolk-cells is not symmetrical, but is most rapid in the
meridian opposite the opening of the alimentary cavity, so that the
latter is left in a bay (cf. Elasmobranchii, p. 63). The epibolic
invagination takes place as in Molluscs and many other forms, not
simply by the division of pre-existing epiblast cells, but by the
formation of fresh epiblast cells from the yolk-cells (fig. 37); and
till after the complete enclosure of the yolk-cells there is never
present a sharp line of demarcation between the two groups of cells.
By the time that the segmentation cavity is obliterated the whole yolk
is enclosed by the epiblast. The yolk-cells adjoining the opening of
the mesenteron are the latest to be covered in, and on their enclosure
this opening constitutes the whole of the blastopore. The epiblast is
composed of a single row of columnar cells.
Mesoblast and notochord. During the above changes the mesoblast
becomes established. It arises, as in Elasmobranchs, in the form of
two plates derived from the primitive hypoblast. During the
invagination to form the mesenteron some of the hypoblast cells on
each side of the invaginated layer become smaller, and marked off as
two imperfect plates (fig. 38, ms). It is difficult to say whether
these plates are entirely derived from invaginated cells, or are in
part directly formed from the pre-existing yolk-cells, but I am
inclined to adopt the latter view; the ventral extension of the
mesoblast plates undoubtedly takes place at the expense of the
yolk-cells. The mesoblast plates soon become more definite, and form
(fig. 39, ms) well-defined[Pg 87] structures, triangular in section, on the
two sides of the middle line.
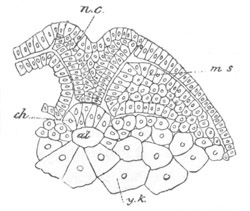
Fig. 39. Transverse section through an embryo of
Petromyzon Planeri of 208 hours.
The figure illustrates the formation of the neural cord and of the
notochord.
ms. mesoblast; nc. neural cord; ch. notochord; yk.
yolk-cells; al. alimentary canal.
At the time the mesoblast is first formed the hypoblast cells, which
roof the mesenteron, are often imperfectly two layers thick (fig. 38).
They soon however become constituted of a single layer only. When the
mesoblast is fairly established, the lateral parts of the hypoblast
grow inwards underneath the axial part, so that the latter (fig. 39,
ch) first becomes isolated as an axial cord, and is next inclosed
between the medullary cord (nc) (which has by this time been formed)
and a continuous sheet of hypoblast below (fig. 40). Here its cells
divide and it becomes the notochord. The notochord is thus bodily
formed out of the axial portion of the primitive hypoblast. Its mode
of origin may be compared with that in Amphioxus, in which an axial
fold of the archenteric wall is constricted off as the notochord. The
above features in the development of the notochord were first
established by Calberla[27]
(No. 78).
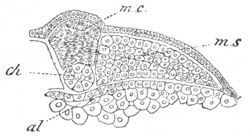
Fig. 40. Transverse section through part of an
embryo of Petromyzon Planeri of 256 hours.
m.c. medullary cord; ch. notochord; al. alimentary canal;
ms. mesoblastic plate.
General history of the development. Up to about the time when the
enclosure of the hypoblast by the epiblast is completed, no external
traces are visible of any of the organs of the embryo; but about this
time, i.e. about 180 hours after impregnation, the rudiment of[Pg 88] the
medullary plate becomes established, as a linear streak extending
forwards from the blastopore over fully one half the circumference of
the embryo. The medullary plate first contains a shallow median
groove, but it is converted into the medullary cord, not in the usual
vertebrate fashion, but, as first shewn by Calberla, in a manner much
more closely resembling the formation of the medullary cord in
Teleostei. Along the line of the median groove the epiblast becomes
thickened and forms a kind of keel projecting inwards towards the
hypoblast (fig. 39, nc). This keel is the rudiment of the medullary
cord. It soon becomes more prominent, the median groove in it
disappears, and it becomes separated from the epiblast as a solid cord
(fig. 40, mc).
By this time the whole embryo has become more elongated, and on the
dorsal surface is placed a ridge formed by the projection of the
medullary cord. At the lip of the blastopore the medullary cord is
continuous with the hypoblast, thus forming the rudiment of a
neurenteric canal.
Calberla gives a similar account of the formation of the neural canal
to that which he gives for the Teleostei (vide p. 72).
He states that the epiblast becomes divided into two layers, of which
the outer is involuted into the neural cord, a median slit in the
involution representing the neural groove. The eventual neural canal
is stated to be lined by the involuted cells. Scott (No. 87) fully
confirms Calberla on this point, and, although my own sections do not
clearly shew an involution of the outer layer of epiblast cells, the
testimony of these two observers must no doubt be accepted on this
point.
Shortly after the complete establishment of the neural cord the
elongation of the embryo proceeds with great rapidity. The processes
in this growth are shewn in fig. 41, A, B, and C. The cephalic portion
(A, c) first becomes distinct, forming an anterior protuberance free
from yolk. About the time it is formed the mesoblastic plates begin to
be divided into somites, but the embryo is so opaque that this process
can only be studied in sections. Shortly afterwards an axial lumen
appears in the centre of the neural cord, in the same manner as in
Teleostei.
The general elongation of the embryo continues rapidly, and, as shewn
in my figures, the anterior end is applied to the[Pg 89] ventral surface of
the yolk (B). With the growth of the embryo the yolk becomes entirely
confined to the posterior part. This part is accordingly greatly
dilated, and might easily be mistaken for the head. The position of
the yolk gives to the embryo a very peculiar appearance. The apparent
difference between it and the embryos of other Fishes in the position
of the yolk is due in the main to the fact that the postanal portion
of the tail is late in developing, and always small. As the embryo
grows longer it becomes spirally coiled within the egg-shell. Before
hatching the mesoblastic somites become distinctly marked (C).
The hatching takes place at between 13-21 days after impregnation; the
period varying according to the temperature.
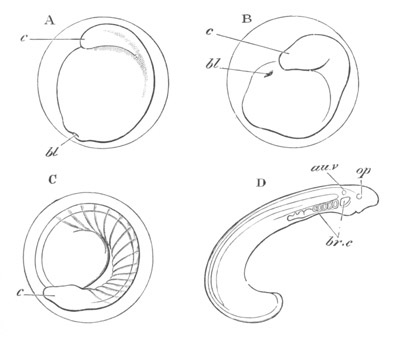
Fig. 41. Four stages in the development of Petromyzon. (After
Owsjannikoff.)
c. cephalic extremity; bl. blastopore; op. optic vesicle;
au.v. auditory vesicle; br.c. branchial clefts.
During the above changes in the external form of the embryo, the
development of the various organs makes great progress. This is
especially the case in the head. The brain becomes distinct from the
spinal cord, and the auditory sacks and the optic vesicles of the eye
become formed. The branchial region of the mesenteron becomes
established, and causes a[Pg 90] dilatation of the anterior part of the body,
and the branchial pouches grow out from the throat. The anus becomes
formed, and a neurenteric canal is also established (Scott). The
nature of these and other changes will best be understood by a
description of the structure of the just-hatched larva. The general
appearance of the larva immediately after hatching is shewn in fig.
41, D. The body is somewhat curved; the posterior extremity being much
dilated with yolk, while the anterior is very thin. All the cells
still contain yolk particles, which render the embryo very opaque. The
larva only exhibits slow movements, and is not capable of swimming
about.
The structure of the head is shewn in figs. 42 and fig. 43. Fig. 42 is a
section through a very young larva, while fig. 43 is taken from a
larva three days after hatching, and shews the parts with considerably
greater detail.
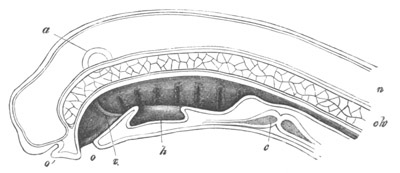
Fig. 42. Diagrammatic vertical section of a just-hatched larva of
Petromyzon. (From Gegenbaur; after Calberla.)
o. mouth; o´. olfactory pit; v. septum between stomodæum and
mesenteron; h. thyroid involution; n. spinal cord; ch.
notochord; c. heart; a. auditory vesicle.
On the ventral side of the head is placed the oral opening (fig. 43,
m) leading into a large stomodæum which is still without a
communication with the mesenteron. Ventrally the stomodæum is
prolonged for a considerable distance under the anterior part of the
mesenteron. Immediately behind the stomodæum is placed the branchial
region of the mesenteron. Laterally it is produced on each side into
seven or perhaps eight branchial pouches (fig. 43, br.c), which
extend outwards nearly to the skin but are not yet open. Between the
successive pouches are placed mesoblastic segments, of the same nature
and structure as the walls of the head cavities in the embryos of
Elasmobranchs, and like them enclosing a central cavity. A[Pg 91] similar
structure is placed behind the last, and two similar structures in
front of the first persistent pouch. This pouch is situated in the
same vertical line as the auditory sack (au.v), and would appear
therefore to be the hyobranchial cleft; and this identification is
confirmed by the fact of two head cavities being present in front of
it. At the front end of the branchial region of the mesenteron is
placed a thickened ridge of tissue, which, on the opening of the
passage between the stomodæum and the mesenteron, forms a partial
septum between the two, and is known as the velum (fig. 43, tv).
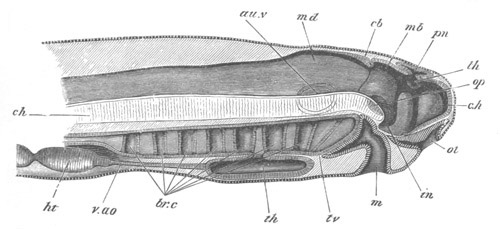
Fig. 43. Diagrammatic vertical section through the head of a larva
of Petromyzon.
The larva had been hatched three days, and was 4.8 mm. in length.
The optic and auditory vesicles are supposed to be seen through the
tissues. The letter tv pointing to the base of the velum is where
Scott believes the hyomandibular cleft to be situated.
c.h. cerebral hemisphere; th. optic thalamus; in.
infundibulum; pn. pineal gland; mb. mid-brain; cb. cerebellum;
md. medulla oblongata; au.v. auditory vesicle; op. optic
vesicle; ol. olfactory pit; m. mouth; br.c. branchial pouches;
th. thyroid involution; v.ao. ventral aorta; ht. ventricle of
heart; ch. notochord.
According to Scott (No. 87) a hyomandibular pouch forming the eighth
pouch is formed in front of the pouch already defined as the
hyobranchial. It disappears early and does not acquire gill folds[28].
The tissue forming the[Pg 92] line of insertion of the velum appears to me to
represent the mandibular arch. The grounds for this view are the
following:
(1) The structure in question has exactly the position usually
occupied by the mandibular arch.
(2) There is present in late larvæ (about 20 days after hatching) an
arterial vessel, continued from the ventral prolongation of the bulbus
arteriosus along the insertion of the velum towards the dorsal aorta,
which has the relations of a true branchial artery.
On the ventral aspect of the branchial region is placed a sack (figs.
42, h, and 43, th), which extends from the front end of the
branchial region to the fourth cleft. At first it constitutes a groove
opening into the throat above (fig. 44), but soon the opening becomes
narrowed to a pore placed between the second and third of the
permanent branchial pouches (fig. 43, th). In Ammocœtes[29]
the
simple tube becomes divided, and assumes a very complicated form,
though still retaining its opening into the branchial region of the
throat. In the adult it forms a glandular mass underneath the
branchial region of the throat equivalent to the thyroid gland of
higher Vertebrates.
On the ventral aspect of the head, and immediately in front of the
mouth, is placed the olfactory pit (fig. 43, ol). It is from the
first unpaired, and in just-hatched larvæ simply forms a shallow
groove of thickened epiblast at the base of the front of the brain. By
the stage represented in fig. 43 the ventral part of the original
groove is prolonged into a pit, extending backwards beneath the brain
nearly up to the infundibulum.
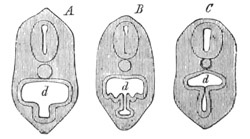
Fig. 44. Diagrammatic transverse sections through
the branchial region of a young larva of Petromyzon. (From
Gegenbaur; after Calberla.)
d. branchial region of throat.
On the side of the head, nearly on a level with the front end of the
notochord, is placed the eye (fig. 43, op). It is constituted (figs.
45 and 46) of a very shallow optic cup with a thick outer (retinal)
layer, and a thin inner choroid layer. In contact with the retinal
layer is placed the lens. The latter is formed as an invagination of
the[Pg 93] skin; to which it is still attached in the just-hatched larva
(fig. 45). The eye only differs at this stage from that of other
Vertebrata in its extraordinarily small size, and the rudimentary
character of its constituent parts.
The auditory sack is a large vesicle (fig. 43, au.v.), placed at the
side of the brain opposite the first persistent branchial pouch.
The brain is formed of the usual vertebrate parts[30], but is
characterized by the very slight cranial flexure. The fore-brain
consists (fig. 43) of a thalamencephalon (th) and an undivided
cerebral rudiment (ch). To the roof of the thalamencephalon is
attached a flattened sack (pn) which is probably the pineal gland.
The floor is prolonged into an infundibulum (in) which contains a
prolongation of the third ventricle. The lateral walls of the cerebral
rudiment are much thickened.
Behind the thalamencephalon follows the mid-brain (mb), the sides of
which form the optic lobes, and behind this again the hind-brain
(md); the front border of the roof of which is thickened to form the
cerebellum (cb). The medulla passes without any marked line of
demarcation into the spinal cord.
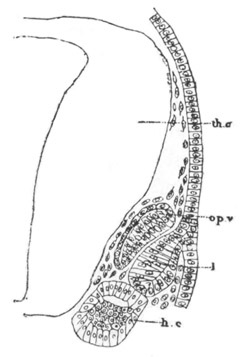
Fig. 45. Horizontal section through the head of a
just-hatched larva of Petromyzon shewing the development of the lens
of the eye.
th.c. thalamencephalon; op.v. optic vesicle; l. lens of eye;
h.c. head cavity.
The histological differentiation of the brain has already proceeded to
some extent; and it has in the main the same character as the spinal
cord. Before the larva has been hatched very long a lateral investment
of white matter is present throughout. The notochord (ch) is
continued forwards in the head to the hinder border of the
infundibulum. It is slightly flexed anteriorly.
From the hinder border of the auditory region to the end of the
branchial region the mesoblast is dorsally divided into[Pg 94] myotomes,
which nearly, though not quite, correspond in number with the
branchial pouches.
The growth of the myotomes would seem, as might be anticipated from
their independent innervation, not to be related to that of the
branchial pouches, so that there is a want of correspondence between
these parts, the extent of which varies at different periods of life.
The relation between the two in an old larva is shewn in fig. 47.
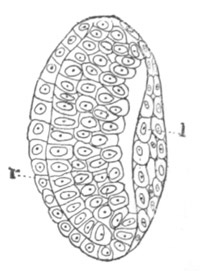
Fig. 46. Eye of a larva of Petromyzon nine days
after hatching.
l. lens; r. retina.
The section passes through one side of the lens.
The head of the larva of Petromyzon differs very strikingly in general
appearance from that of the normal Vertebrata. This is at once shewn
by a comparison of fig. 43 with fig. 29. The most important difference
between the two is due to the absence of a pronounced cranial flexure
in Petromyzon; an absence which is in its turn probably caused by the
small development of the fore-brain.
The stomodæum of Petromyzon is surprisingly large, and its size and
structure in this type militate against the view of some embryologists
that the stomodæum originated from the coalescence of a pair of
branchial pouches.
In the region of the trunk there is present an uninterrupted dorsal
fin continuous with a ventral fin round the end of the tail.
There is a well-developed body cavity, which is especially dilated in
front, in the part which afterwards becomes the pericardium. In this
region is placed the nearly straight heart, divided into an auricle
and ventricle (figs. 42 and 43), the latter continued forwards into a
bulbus arteriosus.
The myotomes are now very numerous (about 57, including those of the
head, in a three days’ larva). They are separated by septa, but do not
fill up the whole space between the septa, and have a peculiar wavy
outline. The notochord is provided with a distinct sheath, and below
it is placed a subnotochordal rod.
The alimentary canal consists of a narrow anterior section free from
yolk, and a posterior region, the walls of which are[Pg 95] largely swollen
with yolk. The anterior section corresponds to the region of the
œsophagus and stomach, but exhibits no distinction of parts.
Immediately behind this point the alimentary canal dilates
considerably, and on the ventral side is placed the opening of a
single large sack, which forms the commencement of the liver. The
walls of the hepatic sack are posteriorly united to the yolk-cells. At
the region where the hepatic sack opens into the alimentary tract the
latter dilates considerably.
The posterior part of the alimentary tract still constitutes a kind of
yolk-sack, the ventral wall being enormously thick and formed of
several layers of yolk-cells. The dorsal wall is very thin.
The excretory system is composed of two segmental ducts, each
connected in front with a well-developed pronephros (head-kidney),
with about five ciliated funnels opening into the pericardial region
of the body cavity. The segmental ducts in the larvæ open behind into
the cloacal section of the alimentary tract.
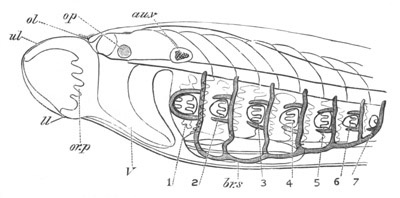
Fig. 47. Head of a larva of Petromyzon six weeks old.
(Altered from Max Schultze.)
au.v. auditory vesicle; op. optic vesicle; ol. olfactory pit;
ul. upper lip; ll. lower lip; or.p. papillæ at side of mouth;
v. velum; br.s. extra branchial skeleton; 1-7. branchial clefts.
The development of the larva takes place with considerable rapidity.
The yolk becomes absorbed and the larva becomes accordingly more
transparent. It generally lies upon its side, and resembles in general
appearance and habit a minute Amphioxus. It is soon able to swim with
vigour, but usually, unless disturbed, is during the day quite
quiescent, and chooses by[Pg 96] preference the darkest situations. It soon
straightens out, and, with the disappearance of the yolk, the tail
becomes narrower than the head. A large caudal fin becomes developed.
When the larva is about twenty days old, it bears in most anatomical
features a close resemblance to an Ammocœtes; though the histological
differences between my oldest larva (29 days) and even very young
Ammocœtes are considerable.
The mouth undergoes important changes. The upper lip becomes much more
prominent, forming of itself the anterior end of the body (fig. 47,
ul). The opening of the nasal pit is in this way relatively thrown
back, and at the same time is caused to assume a dorsal position.
This will be at once understood by a comparison of fig. 43 with fig.
47. On the inner side of the oral cavity a ring of papillæ is formed
(fig. 47, or.p). Dorsally these papillæ are continued forward as a
linear streak on the under side of the upper lip. A communication
between the oral cavity and the branchial sack is very soon
established.
The gill pouches gradually become enlarged; but it is some time before
their small external openings are established. Their walls, which are
entirely lined by hypoblast, become raised in folds, forming the
branchial lamellæ. The walls of the head cavities between them become
resolved into the contractors and dilators of the branchial sacks. The
extra-branchial basketwork becomes established very early (it is
present in the larva of 6 millimetres, about 9 days after hatching)
and is shewn in an older larva in fig. 47, br.s. It is not so
complicated in these young larvæ as in the Ammocœtes, but in Max
Schultze’s figure, which I have reproduced, the dorsal elements of the
system are omitted. On the dorsal wall of the branchial region a
ciliated ridge is formed, which may be homologous with the ridge on
the dorsal wall of the branchial sack of Ascidians. It has been
described by Schneider in Ammocœtes.
With reference to the remainder of the alimentary canal there is but
little to notice. The primitive hepatic diverticulum rapidly sprouts
out and forms a tubular gland. The opening into the duodenum changes
from a ventral to a lateral or even dorsal position. The duct leads
into a gall-bladder imbedded in the substance of the liver. Ventrally
the liver is united with the abdominal wall, but laterally passages
are left by which the pericardial and body cavities continue to
communicate.
The greater part of the yolk becomes employed in the formation of the
intestinal wall. This part of the intestine in a nine days’ larva (67
mm.) has the form of a cylindrical tube with very thick columnar cells
entirely filled with yolk particles. The dorsal wall is no longer
appreciably thinner than the ventral. In the later stages the cells of
this part of the intestine become gradually less columnar as the yolk
is absorbed.
The fate of the yolk-cells in the Lamprey is different from that in
most other Vertebrata with an equally large amount of yolk. They no
doubt[Pg 97] supply nutriment for the growth of the embryo, and although in
the anterior part of the intestine they become to some extent enclosed
in the alimentary tract and break up, yet in the posterior part they
become wholly transformed into the regular epithelium of the
intestine.
On the ninth day a slight fold filled with mesoblastic tissue is
visible on the dorsal wall of the intestine. This fold appears to
travel towards the ventral side; at any rate a similar but
better-marked fold is visible in a ventro-lateral position at a
slightly later period. This fold is the commencement of the fold which
in the adult makes a half spiral, and is no doubt equivalent to the
spiral valve of Elasmobranchs and Ganoids. It contains a prolongation
of the cœliac artery, which constitutes at first the vitelline
artery.
The nervous system does not undergo during the early larval period
changes which require a description.
The opening of the olfactory sack becomes narrowed and ciliated (fig.
47, ol). It is carried by the process already mentioned to the
dorsal surface of the head. The lumen of the sack is well developed;
and lies in contact with the base of the fore part of the brain.
The vascular system presents no very remarkable features. The heart is
two-chambered and straight. The ventricle is continued forwards as a
bulbus arteriosus, which divides into two arteries at the thyroid
body. From the bulbus and its continuations eight branches are given
off to the gills; and, as mentioned above, a vessel, probably of the
same nature, is given off in the region of the velum. The blood from
the branchial sacks is collected into the dorsal aorta. Some of it is
transmitted to the head, but the greater part flows backwards under
the notochord.
The venous system consists of the usual anterior and posterior
cardinal veins which unite on each side into a ductus Cuvieri, and of
a great subintestinal vessel of the same nature as that in embryo
Elasmobranchs, which persists however in the adult. It breaks up into
capillaries in the liver, and constitutes therefore the portal vein.
From the liver the blood is brought by the hepatic vein into the sinus
venosus. In addition to these vessels there is a remarkable unpaired
sub-branchial vein, which brings back the blood directly to the heart
from the ventral part of the branchial region.
Metamorphosis. The larva just described does not grow directly into
the adult, but first becomes a larval form, known as Ammocœtes, which
was supposed to be a distinct species till Aug. Müller (No. 80) made
the brilliant discovery of its nature.
The Ammocœtes does not differ to any marked extent from the larva
just described. The histological elements become more differentiated,
and a few organs reach a fuller development.
The branchial skeleton becomes more developed, and capsules for the
olfactory sack and auditory sacks are established.
[Pg 98]The olfactory sack is nearly divided into two by a ventral septum. The
eye (fig. 48) is much more fully developed, but lies a long way below
the surface. The optic cup forms a deep pit, in the mouth of which is
placed the lens. The retinal layers are well developed (cf.
Langerhans), and the outer layer of the optic cup or layer of retinal
pigment (rp) contains numerous pigment granules, especially on its
dorsal side. At the edge of the optic cup the two layers fall into
each other. They constitute the commencement of the pigment layer of
the iris; but at this stage they are not pigmented. The mesoblast of
the iris is hardly differentiated. The lens (l) has the normal
structure of the embryonic lens of Vertebrata. The inner wall is thick
and doubly convex, while the outer wall, which will form the anterior
epithelium, is very thin. There is a large space between the lens and
the retina containing the vitreous humour (v.h). There is no aqueous
humour, and the tissues in front of the lens bear but little
resemblance to those in higher Vertebrata. The cornea is represented
by (1) the epidermis (ep); (2) the dermis (d.c); (3) the subdermal
connective tissue (s.d.c) which passes without any sharp line of
demarcation into the dermis; (4) a thick membrane continuous with the
choroid which represents Descemet’s membrane. The subdermal connective
tissue is continued as an investment round the whole eye. There is no
specially differentiated sclerotic, and a choroid is only imperfectly
indicated[31].
The peculiar features of the eye of the young larva of
the Ammocœtes are probably due to degeneration.
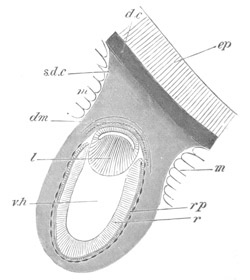
Fig. 48. Eye of an Ammocœtes lying beneath the skin.
ep. epidermis; d.c. dermal connective tissue continuous with the
subdermal connective tissue (s.d.c), which is also shaded. There
is no definite boundary to this tissue where it surrounds the eye.
m. muscles; dm. membrane of Descemet; l. lens; v.h. vitreous
humour; r. retina; rp. retinal pigment.
In the brain the two cerebral hemispheres lie one on each side of the
anterior end of the thalamencephalon. There are well-defined olfactory
lobes, and two distinct olfactory nerves are present.
The excretory system has undergone great changes. A series of
segmental tubes, which first appear in a larva of about 9 mm.,[Pg 99] becomes
established behind the pronephros, and in an Ammocœtes of 65 mm. the
pronephros has begun to atrophy. The generative organs are formed in a
larva of about 35 mm. Shortly before the metamorphosis the portion of
the cloaca into which the segmental tubes open becomes separated off
as a distinct urinogenital sinus, the walls of which become perforated
by the two abdominal pores.
The Ammocœtes of Petromyzon Planeri lives in the mud in streams.
Without undergoing any marked changes in structure it gradually grows
larger, and after three or four years undergoes a metamorphosis. The
full-grown larva may be as large or even larger than the adult. The
metamorphosis takes place from August till January. The breeding
season sets in during the second half of April; and shortly after
depositing its generative products the Lamprey dies. The changes which
take place in the metamorphosis are of a most striking kind.
The dome-shaped mouth of the larva is replaced (fig. 47) by a more
definitely suctorial mouth with horny cuticular teeth (fig. 49). The
eyes appear on the surface; and the dorsal fin becomes more prominent,
and is divided into two parts.
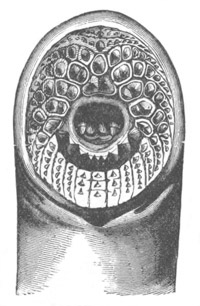
Fig. 49. Mouth of Petromyzon marinus with its
horny teeth. (From Gegenbaur; after Heckel and Kner.)
Besides these obvious external changes very great modifications are
effected in almost all the organs, which may be very briefly
enumerated.
1. Very profound changes take place in the skeleton. An elaborate
system of cartilages is developed in connection with the mouth; the
cranium itself undergoes important modifications; and neural arches
become formed.
2. Considerable changes are effected in the gill pouches, and,
according to Schneider, whose statements must however be received with
some caution, the branchial sack becomes detached posteriorly from the
œsophagus, the œsophagus then sends forwards a prolongation above
the branchial sack which is at first solid. This prolongation forms
the anterior part of the[Pg 100] œsophagus of the adult, and joins the
primitive oral cavity at the velum. The so-called bronchus of the
adult is thus the whole branchial region of the Ammocœtes, and the
anterior part of the œsophagus of the adult is an entirely new
formation.
3. The posterior part of the alimentary tract of the Ammocœtes
undergoes partial atrophy. The gall-bladder of the liver is absorbed;
and the liver itself ceases to communicate with the intestine.
4. The eye undergoes important changes in that it travels to the
surface, and acquires all the characters of the normal vertebrate eye.
5. The brain becomes relatively larger but more compact, and the optic
lobes (corpora bigemina) become more distinct.
6. The pericardial cavity becomes completely separated from the body
cavity, and a distinct pericardium is formed.
7. The mesonephros of the larva disappears, and a fresh posterior part
is formed.
Myxine. The ovum of Myxine when ready to be laid is inclosed, as shewn
by Allen Thomson[32],
in an oval horny shell in many respects similar
to that of Elasmobranchii; from its ends there project a number of
trumpet-shaped tubular processes, which no doubt serve to attach it to
marine objects. No observations have been made on the development.
Bibliography.
(77) E. Calberla. “Der Befruchtungsvorgang beim Petromyzon Planeri.”
Zeit. f. wiss. Zool., Vol. XXX. 1877.
(78) E. Calberla. “Ueb. d. Entwicklung d. Medullarrohres u. d. Chorda
dorsalis d. Teleostier u. d. Petromyzonten.” Morpholog. Jahrbuch,
Vol. III. 1877.
(79) C. Kupffer u. B. Benecke. Der Vorgang d. Befruchtung am Ei d.
Neunaugen. Königsberg, 1878.
(80) Aug. Müller. “Ueber die Entwicklung d. Neunaugen.” Müller’s
Archiv, 1856.
(81) Aug. Müller. Beobachtungen üb. d. Befruchtungserscheinungen im
Ei d. Neunaugen. Königsberg, 1864.
[Pg 101]
(82) W. Müller. “Das Urogenitalsystem d. Amphioxus u. d. Cyclostomen.”
Jenaische Zeitschrift, Vol. IX. 1875.
(83) Ph. Owsjannikoff. “Die Entwick. von d. Flussneunaugen.” Vorläuf.
Mittheilung. Mélanges Biologiques tirés du Bulletin de l'Acad. Imp.
St Pétersbourg, Vol. VII. 1870.
(84) Ph. Owsjannikoff. On the development of Petromyzon fluviatilis
(Russian).
(85) Anton Schneider. Beiträge z. vergleich. Anat. u. Entwick. d.
Wirbelthiere. Quarto. Berlin, 1879.
(86) M. S. Schultze. “Die Entwickl. v. Petromyzon Planeri.” Gekrönte
Preisschrift. Haarlem, 1856.
(87) W. B. Scott. “Vorläufige Mittheilung üb. d.
Entwicklungsgeschichte d. Petromyzonten.” Zoologischer Anzeiger,
Nos. 63 and 64. III. Jahrg. 1880.
[Pg 102]
CHAPTER VI.
GANOIDEI[33].
It is only within quite recent times that any investigations have been
made on the embryology of this heterogeneous, but primitive group of
fishes. Much still remains to be done, but we now know the main
outlines of the development of Acipenser and Lepidosteus, which are
representatives of the two important subdivisions of the Ganoids. Both
types have a complete segmentation, but Lepidosteus presents in its
development some striking approximations to the Teleostei. I have
placed at the end of the chapter a few remarks with reference to the
affinities indicated by the embryology.
Acipenser[34].
The freshly laid ovum is 2 mm. in diameter and is invested by a
two-layered shell, covered by a cellular layer derived from the
follicle[35].
The segmentation, though complete, approaches[Pg 103] the
meroblastic type more nearly than the segmentation of the frog’s egg.
The first furrow appears at the formative pole, at which the germinal
vesicle was situated. The earlier phases of the segmentation are like
those of meroblastic ova, in that the furrows only penetrate for a
certain distance into the egg. Eight vertical furrows appear before
the first equatorial furrow; which is somewhat irregular, and situated
close to the formative pole.
In the later stages the vertical furrows extend through the whole egg,
and a segmentation cavity appears between the small and the large
spheres. The segmentation is thus in the main similar to that of a
frog, from which it diverges in the fact that there is a greater
difference in size between the small and the large segments.
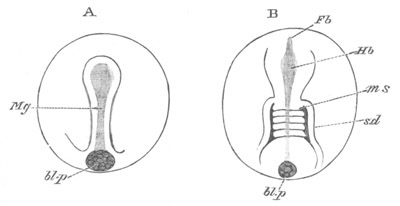
Fig. 50. Embryos of Acipenser viewed from the dorsal surface.
(After Salensky.)
A. Stage before the appearance of the mesoblastic somites.
B. Stage with five somites.
Mg. medullary groove; bl.p. blastopore; s.d. segmental duct;
Fb. fore-brain; Hb. hind-brain; m.s. mesoblastic somite.
In the final stages of the segmentation the cells become distinctly
divided into two layers. A layer of small cells is placed at the
formative pole, and constitutes the epiblast. The cells composing it
are divided, like those of Teleostei, etc., into a superficial
epidermic and a deeper nervous layer. The remaining cells constitute
the primitive hypoblast (the eventual hypoblast and mesoblast); they
form a great mass of yolk-cells at the lower pole, and also spread
along the roof of the segmentation cavity, on the inner side of the
epiblast.
A process of unsymmetrical invagination now takes place, which is in
its essential features exactly similar to that in the[Pg 104] frog or the
lamprey, and I must refer the reader for the details of the process to
the chapter on the Amphibia. The edge of the cap of epiblast forms an
equatorial line. For the greater extent of this line the epiblast
cells grow over the hypoblast, as in an epibolic gastrula, but for a
small arc they are inflected. At the inflected edge an invagination of
cells takes place, underneath the epiblast, towards the segmentation
cavity, and gives rise to the dorsal wall of the mesenteron and the
main part of the dorsal mesoblast. The slit below the invaginated
layer gradually dilates to form the alimentary cavity; the ventral
wall of which is at first formed of yolk-cells. The epiblast along the
line of the invaginated cells soon becomes thickened, and forms a
medullary plate, which is not very distinct in surface views. The
cephalic extremity of this plate, which is furthest removed from the
edge, dilates, and the medullary plate then assumes a spatula form
(fig. 50 A, Mg).
By the continued extension of the epiblast the uncovered part of the
hypoblast has in the meantime become reduced to a small circular
pore—the blastopore—and in surface views of the embryo has the form
represented in fig. 50 A, bl.p. The invagination of the mesenteron
has in the meantime extended very far forwards, and the segmentation
cavity has become obliterated. The lip of the blastopore has moreover
become inflected for its whole circumference.
The invaginated cells forming the dorsal wall of the mesenteron soon
become divided into a pigmented hypoblastic epithelium adjoining the
lumen of the mesenteron (fig. 51, En) and a mesoblastic layer
(Sgp), between the hypoblast and the epiblast. The mesoblast is
divided into two plates, between which is placed the notochord[36]
(Ch).
With the completion of the medullary plate and the germinal layers,
the first embryonic period may be considered to come to a close. The
second period ends with the hatching of the embryo. During it the
rudiments of the greater number of organs make their appearance. The
general form of the embryo during this period is shewn in figs. 50 B
and 52 A and B.
One of the first changes to take place is the conversion of the
[Pg 105]
medullary plate into the medullary canal. This, as shewn in fig. 51,
is effected in the usual vertebrate fashion, by the establishment of a
medullary groove which is then converted into a closed canal by the
folding over of the sides.
The uncovered patch of yolk in the blastoporic area soon becomes
closed over; and on the formation of the medullary canal the usual
neurenteric canal becomes established.
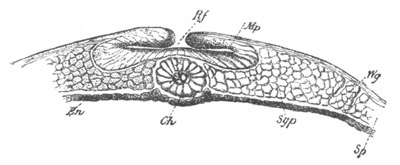
Fig. 51. Transverse section through the anterior part of an Acipenser
embryo. (After Salensky.)
Rf. medullary groove; Mp. medullary plate; Wg. segmental duct;
Ch. notochord; En. hypoblast; Sgp. mesoblastic somite; Sp.
parietal part of mesoblastic plate.
The further changes which take place are in the main similar to those
in other Ichthyopsida, but in some ways the appearance of the embryo
is, as may be gathered from fig. 52, rather strange. This is mainly
due to the fact that the embryo does not become folded off from the
yolk in the manner usual in Vertebrates; and as will be shewn in the
sequel, the relation of the yolk to the embryo is unlike that in any
other known Vertebrate. The appearance of the embryo is something like
that of an ordinary embryo slit open along the ventral side and then
flattened out. Organs which properly belong to the ventral side appear
on the lateral parts of the dorsal surface. Owing to the great forward
extension of the yolk the heart (fig. 52 B) appears to be placed
directly in front of the head.
Even before the formation of the medullary canal the cephalic portion
of the nervous system becomes marked out. This part, after the closure
of the medullary groove, becomes divided into two (fig. 50 B), and
then three lobes—the fore-, the mid-, and the hind-brain (fig. 52, A
and B). From the lateral parts of the at first undivided fore-brain
the optic vesicles (fig. 52 B, Op) soon sprout out; and in the
hind-brain a dilatation to form the fourth ventricle appears in the
usual fashion.
[Pg 106]
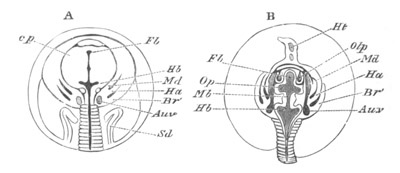
Fig. 52. Embryos of Acipenser belonging to two stages viewed from the
dorsal surface. (After Salensky.)
Fb. fore-brain; Mb. mid-brain; Hb. hind-brain; cp. cephalic
plate; Op. optic vesicle; Auv. auditory vesicle; Olp.
olfactory pit; Ht. heart; Md. mandibular arch; Ha. hyoid arch;
Br´. first branchial arch; Sd. segmental duct.
The epiblast at the sides of the brain constitutes a more or less
well-defined structure, which may be spoken of as a cephalic plate
(fig. 52 A, cp). From this plate are formed the essential parts of
the organs of special sense. Anteriorly the olfactory pits arise (fig.
52 B, Olp) as invaginations of both layers of the epiblast. The lens
of the eye is formed as an ingrowth of the nervous layer only, and
opposite the hind-brain the auditory sack (fig. 52 A and B, Auv) is
similarly formed from the nervous layer of the epiblast. At the sides
of the cephalic plate the visceral arches make their appearance; and
in fig. 52 A and B there are shewn the mandibular (Md), hyoid (Ha)
and first branchial (Br´) arches, with the hyomandibular (spiracle)
and hyobranchial clefts between them. They constitute peculiar
concentric circles round the cephalic plate; their shape being due to
the flattened form of the embryo, already alluded to.
While the above structures are being formed in the head the changes in
the trunk have also been considerable. The mesoblastic plates at the
junction of the head and trunk become very early segmented, the
segments being formed from before backwards (fig. 50 B). With their
formation the trunk rapidly increases in length. At their outer border
the segmental duct (fig. 50 B, and fig. 52 A, Sd) is very early
established. It is formed, as in Elasmobranchs, as a solid outgrowth
of the mesoblast (fig. 51, Wg); but its anterior extremity becomes
converted into a pronephros (fig. 57, pr.n).
[Pg 107]
Before hatching, the embryo has to a small extent become folded off
from the yolk both anteriorly and posteriorly; and has also become to
some extent vertically compressed. As a result of these changes, the
general form of its body becomes much more like that of an ordinary
Teleostean embryo.
The general features of the larva after hatching are illustrated by
figs. 53, 54 and 55. Fig. 53 represents a larva of about 7 mm. and
fig. 54 a lateral and fig. 55 a ventral view of the head of a larva of
about 11 mm.
Fig. 53. Larva of Acipenser of 7 mm., shortly after hatching.
ol. olfactory pit; op. optic vesicle; sp. spiracle; br.c.
branchial clefts; an. anus.
There are only a few points which call for special attention in the
general form of the body. In the youngest larva figured the ventral
part of the hyomandibular cleft is already closed: the dorsal part of
the cleft is destined to form the spiracle (sp). The arch behind is
the hyoid: on its posterior border is a membranous outgrowth, which
will develop into the operculum. In older larvæ, a very rudimentary
gill appears to be developed on the front walls of the spiracular
cleft (Parker), but I have not succeeded in satisfying myself about
its presence; and rows of gill papillæ appear on the hyoid and the
true branchial arches (figs. 54 and 55, g). The biserially-arranged
gill papillæ of the true branchial arches are of considerable length,
and are not at first covered by the operculum; but they do not form
elongated thread-like external gills similar to those of the
Elasmobranchii.
The oral cavity is placed on the ventral side of the head; it has at
first a more or less rhomboidal form. It soon however (fig. 55)
becomes narrowed to a slit with projecting lips, and eventually
becomes converted into the suctorial mouth of the adult. The most
remarkable feature connected with the mouth is the development of
provisional teeth (fig. 55) on both jaws.
[Pg 108]
These teeth were first discovered by Knock (No. 88). They do not
appear to be calcified, and might be supposed to be of the same nature
as the horny teeth of the Lamprey. They are however developed like
true teeth, as a deposit between a papilla of subepidermic tissue and
an epidermic cap. The substance of which they are formed corresponds
morphologically to the enamel of ordinary teeth. As they grow they
pierce the epidermis, and form hollow spine-like structures with a
central axis filled with subepidermic (mesoblastic) cells. They
disappear after the third month of larval life.
In front of the mouth two pairs of papillæ grow out, which appear to
be of the same nature as the papillæ on the suctorial disc in the
embryo of Lepidosteus (vide p. 115). They are very short in the
embryo represented in fig. 53; soon however they grow in length (figs.
54 and 55, st); and it is probable that they become the barbels,
since these occupy a precisely similar position[37].
Fig. 54. Side view of a larva of Acipenser of 11
millimetres.
op. eye; ol. olfactory pit; st. suctorial (?) processes; m.
mouth; sp. spiracle; g. gills.
Fig. 55. Ventral view of a larva of Acipenser of
11 millimetres.
m. mouth; st. suctorial (?) processes; op. eye; g. gills.
The openings of the nasal pits are at first single; but the opening of
each becomes gradually divided into two by the growth of a flap on the
outer side (fig. 54, ol). It is probable that this flap is
equivalent to the fold of the superior maxillary process of the
Amniota, which by its growth roofs over the open groove which
originally leads from the external to the internal nares; so that the
two openings of each nasal sack, so established in these and in other
fishes, correspond to the external and internal nares of higher
Vertebrata.
[Pg 109]
At the time of hatching there is a continuous dorso-ventral fin,
which, by atrophy in some parts, and hypertrophy in other parts, gives
rise to all the unpaired fins of the adult, except the first dorsal
and the abdominal. The caudal part of the fin is at first symmetrical,
and the heterocercal tail is produced by the special growth of the
ventral part of the fin.
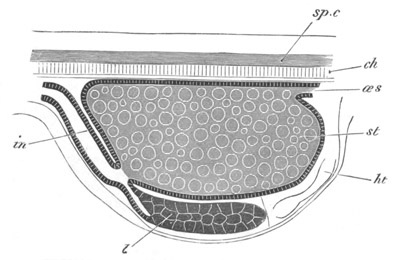
Fig. 56. Diagrammatic longitudinal section through the anterior
part of the trunk of a larva of Acipenser to shew the position occupied
by the yolk.
in. intestine; st. stomach filled with yolk; œs. œsophagus;
l. liver; ht. heart; ch. notochord; sp.c. spinal cord.
Of the internal features of development in the Sturgeon the most
important concern the relation of the yolk to the alimentary tract. In
most Vertebrata the yolk-cells form a protuberance of the part of the
alimentary canal, immediately behind the duodenum. The yolk may
either, as in the lamprey or frog, form a simple thickening of the
alimentary wall in this region, or it may constitute a well-developed
yolk-sack as in Elasmobranchii and the Amniota. In either case the
liver is placed in front of the yolk. In the Sturgeon on the contrary
the yolk is placed almost entirely in front of the liver, and the
Sturgeon appears to be also peculiar in that the yolk, instead of
constituting an appendage of the alimentary tract, is completely
enclosed in a dilated portion of the tract which becomes the stomach
(figs. 56 and 57). It dilates this portion to such extent that it
might be supposed to form a true external yolk-sack. In the stages
before hatching the glandular hypoblast, which was established on the
dorsal side of the primitive mesenteron, envelops the yolk-cells,
which fuse together into a yolk-mass, and lose all trace of their
original cellular structure.
The peculiar flattening out of the embryo over the yolk (vide p.
105) is no doubt connected with the mode in which the yolk becomes
enveloped by the hypoblast.
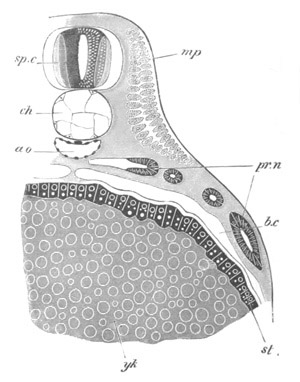
Fig. 57. Transverse section through the region of
the stomach of a larva of Acipenser 5 mm. in length.
st. epithelium of stomach; yk. yolk; ch. notochord, below
which is a subnotochordal rod; pr.n. pronephros; ao. aorta;
mp. muscle-plate formed of large cells, the outer parts of which
are differentiated into contractile fibres; sp.c. spinal cord;
b.c. body cavity.
[Pg 110]
As the posterior part of the trunk, containing the intestine, becomes
formed, the yolk is gradually confined to the anterior part of the
alimentary tract, which, as before stated, becomes the stomach. The
epithelial cells of the stomach, as well as those of the intestine,
are enormously dilated with food-yolk (fig. 57, st). Behind the
stomach is formed the liver. The subintestinal vein bringing back the
blood to the liver appears to have the same course as in Teleostei, in
that the blood, after passing through the liver, is distributed to the
walls of the stomach and is again collected into a venous trunk which
falls into the sinus venosus. As the yolk becomes absorbed, the liver
grows forwards underneath the stomach till it comes in close contact
with the heart. The relative position of the parts at this stage is
shewn diagrammatically in fig. 56. At the commencement of the
intestine there arises in the larva of about 14 mm. a great number of
diverticula, which are destined to form the compact glandular organ,
which opens at this spot in the adult. At this stage there is also a
fairly well developed pancreas opening into the duodenum at the same
level as the liver.
No trace of the air-bladder was present at the stage in question.
The spiral valve is formed, as in Elasmobranchii, as a simple fold in
the wall of the intestine.
There is a well developed subnotochordal rod (fig. 57) which,
according to Salensky, becomes the subvertebral ligament of the adult;
a statement which confirms an earlier suggestion of Bridge. The
pronephros (head-kidney) resembles in the main that of Teleostei (fig.
57); while the front end of the mesonephros, which is developed
considerably later than the pronephros, is placed some way behind it.
In my oldest larva (14 mm.) the mesonephros did not extend backwards
into the posterior part of the abdominal cavity.
[Pg 111]Bibliography.
(88) Knock. “Die Beschr. d. Reise z. Wolga Behufs d.
Sterlettbefruchtung.” Bull. Soc. Nat. Moscow, 1871.
(89) A. Kowalevsky, Ph. Owsjannikoff, and N. Wagner. “Die Entwick. d.
Störe.” Vorläuf. Mittheilung. Mélanges Biologiques tirés du Bulletin
d. l'Acad. Imp. St Pétersbourg, Vol. VII. 1870.
(90) W. Salensky. “Development of the Sterlet (Acipenser ruthenus).” 2
Parts. Proceedings of the Society of Naturalists in the imperial
University of Kasan. 1878 and 9 (Russian). Part I., abstracted in
Hoffmann and Schwalbe’s Jahresbericht for 1878.
(91) W. Salensky. “Zur Embryologie d. Ganoiden (Acipenser).”
Zoologischer Anzeiger, Vol. I., Nos. 11, 12, 13.
Lepidosteus[38].
The ova of Lepidosteus are spherical bodies of about 3 mm. in
diameter. They are invested by a tough double membrane, composed of
(1) an outer layer of somewhat pyriform bodies, radiately arranged,
which appear to be the remains of the follicular cells; and (2) of an
inner zona radiata, the outer part of which is radiately striated,
while the inner part is homogeneous.
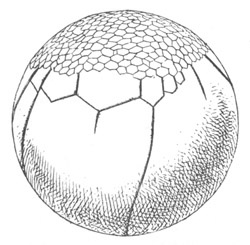
Fig. 58. Surface view of the ovum of Lepidosteus
with the membranes removed on the third day after
impregnation.
The segmentation, as in the Sturgeon, is complete, but approaches
closely the meroblastic type. It commences with a vertical furrow at
the animal pole, extending through about one-fifth of the
circumference. Before this furrow has proceeded further a second
furrow is formed at right angles[Pg 112] to it. The next stages have not been
observed, but on the third day after impregnation (fig. 58), the
animal pole is completely divided into small segments, which form a
disc similar to the blastoderm of meroblastic ova; while the
vegetative pole, which subsequently forms a large yolk-sack, is
divided by a few vertical furrows, four of which nearly meet at the
pole opposite the blastoderm. The majority of the vertical furrows
extend only a short way from the edge of the small spheres, and are
partially intercepted by imperfect equatorial furrows.
The stages immediately following the segmentation are still unknown,
and in the next stage satisfactorily observed, on the fifth day after
impregnation, the body of the embryo is distinctly differentiated. The
lower pole of the ovum is then formed of a mass in which no traces of
segments or segmentation furrows can be detected.
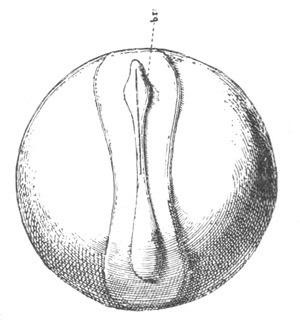
Fig. 59. Surface view of a Lepidosteus
embryo on the fifth day
after impregnation.
br. dilated extremity of medullary plate which forms the rudiment
of the brain.
The embryo (fig. 59) has a dumbbell-shaped outline, and is composed of
(1) an outer area, with some resemblance to the area pellucida of an
avian embryo, forming the lateral part of the body; and (2) a central
portion consisting of the vertebral plates and medullary plate. The
medullary plate is dilated in front to form the brain (br). Two
lateral swellings in the brain are the commencing optic vesicles. The
caudal extremity of the embryo is somewhat swollen.
Sections of this stage (fig. 60) are interesting as shewing a
remarkable resemblance between Lepidosteus and Teleostei.
The three layers are fully established. The epiblast (ep) is formed
of a thicker inner nervous stratum, and an outer flattened epidermic
stratum. Along the axial line there is a solid keel-like thickening of
the nervous layer of the epidermis, which projects towards the
hypoblast. This thickening (MC) is the[Pg 113] medullary cord; and there is
no evidence of the epidermic layer being at this or any subsequent
period concerned in its formation (vide chapter on Teleostei, p.
72). In the region of the brain the medullary cord is so thick that it
gives rise, as in Teleostei, to a projection of the whole body of the
embryo towards the yolk. Posteriorly it is flatter. The mesoblast
(Me) in the trunk has the form of two plates, which thin out
laterally. The hypoblast (hy) is a single layer of cells, and is
nowhere folded in to form a closed alimentary canal. The hypoblast is
separated from the neural cord by the notochord (Ch), which
throughout the greater part of the embryo is a distinct structure.
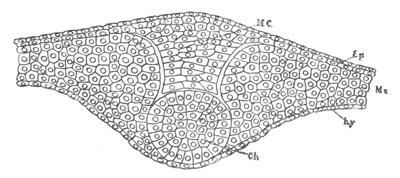
Fig. 60. Section through an embryo of Lepidosteus on the fifth day
after impregnation.
MC. medullary cord; Ep. epiblast; Me. mesoblast; hy.
hypoblast; Ch. notochord.
In the region of the tail, the axial part of the hypoblast, the
notochord, and the neural cord fuse together, the fused part so formed
is the homologue of the neurenteric canal of other types. Quite at the
hinder end of the embryo the mesoblastic plates cease to be separable
from the axial structures between them.
In a somewhat later stage the embryo is considerably more elongated,
embracing half the circumference of the ovum. The brain is divided
into three distinct vesicles.
Anteriorly the neural cord has now become separated from the
epidermis. The whole of the thickened nervous layer of the epiblast
appears to remain united with the cerebrospinal cord, so that the
latter organ is covered dorsally by the epidermic layer of the
epiblast only. The nervous layer soon however grows in again from the
two sides.
Where the neural cord is separated from the epidermis, it is[Pg 114] already
provided with a well-developed lumen. Posteriorly it remains in its
earlier condition.
In the region of the hind-brain traces of the auditory vesicles are
present in the form of slightly involuted thickenings of the nervous
layer of the epidermis.
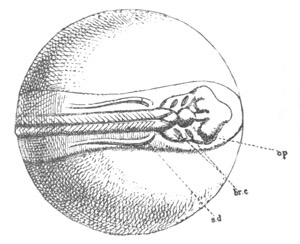
Fig. 61. Embryo of Lepidosteus on the sixth day
after impregnation.
op. optic vesicles; br.c. branchial clefts (?); s.d. segmental
duct.
N.B. The branchial clefts and segmental duct are somewhat too
prominent.
The mesoblast of the trunk is divided anteriorly into splanchnic and
somatic layers.
In the next stage, on the sixth day after impregnation (fig. 61),
there is a great advance in development. The embryo is considerably
longer, and a great number of mesoblastic somites are visible. The
body is now laterally compressed and raised from the yolk.
The region of the head is more distinct, and laterally two streaks are
visible (br.c), which, by comparison with the Sturgeon, would seem
to be the two first visceral clefts[39]:
they are not yet perforated.
In the lateral regions of the trunk the two segmental ducts are
visible in surface views (fig. 61, sd) occupying the same situation
as in the Sturgeon. Their position in section is shewn in fig. 62,
sg.
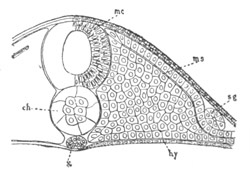
Fig. 62. Section through the trunk of a
Lepidosteus embryo on the sixth day after impregnation.
mc. medullary cord; ms. mesoblast; sg. segmental duct; ch.
notochord; x. subnotochordal rod; hy. hypoblast.
With reference to the features in development, visible in sections, a
few points may be alluded to.
[Pg 115]
The optic vesicles are very prominent outgrowths of the brain, but are
still solid, though the anterior cerebral vesicle has a well-developed
lumen. The auditory vesicles are now deep pits of the nervous layer of
the epiblast, the openings of which are covered by the epidermic
layer. They are shewn for a slightly later stage in fig. 63 (au.v).
There is now present a subnotochordal rod, which develops as in other
types from a thickening of the hypoblast (fig. 62, x).
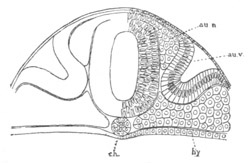
Fig. 63. Section through the head of a Lepidosteus
embryo on the sixth day after impregnation.
au.v. auditory vesicle; au.n. auditory nerve; ch. notochord;
hy. hypoblast.
In an embryo of the seventh day after impregnation, the features of
the preceding stage become generally more pronounced.
The optic vesicles are now provided with a lumen (fig. 64), and have
approached close to the epidermis. Adjoining them a thickening (l)
of the nervous layer of the epidermis has appeared, which will form
the lens. The cephalic extremity of the segmental duct, which, as
shewn in fig. 61, is bent inwards towards the middle line, has now
become slightly convoluted, and forms the rudiment of a pronephros
(head-kidney).
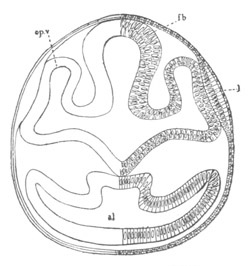
Fig. 64. Section through the front part of the
head of a Lepidosteus embryo on the seventh day after
impregnation.
al. alimentary tract; fb. thalamencephalon; l. lens of eye;
op.v. optic vesicle. The mesoblast is not represented.
During the next few days the folding off of the embryo from the yolk
commences, and proceeds till the embryo acquires the form represented
in fig. 65.
Both the head and tail are quite free from the yolk; and the embryo
presents a general resemblance to that of a Teleostean.
On the ventral surface of the front of the head there is a disc (figs.
65, 66, sd), which is[Pg 116] beset with a number of processes, formed as
thickenings of the epiblast. As shewn by Agassiz, these eventually
become short suctorial papillæ[40].
Immediately behind this disc is
placed a narrow depression which forms the rudiment of the mouth.
The olfactory pits are now developed, and are placed near the front of
the head.
A great advance has taken place in the development of the visceral
clefts and arches. The oral region is bounded behind by a well-marked
mandibular arch, which is separated by a shallow depression from a
still more prominent hyoid arch (fig. 65, hy). Between the hyoid and
mandibular arches a double lamella of hypoblast, which represents the
hyomandibular cleft, is continued from the throat to the external
skin, but does not, at this stage at any rate, contain a lumen.
The hyoid arch is prolonged backwards into a considerable opercular
fold, which to a great extent overshadows the branchial clefts behind.
The hyobranchial cleft is widely open.
Behind the hyobranchial cleft are four pouches of the throat on each
side, not yet open to the exterior. They are the rudiments of the four
branchial clefts of the adult.
The trunk has the usual compressed piscine form, and there is a
well-developed dorsal fin continuous round the end of the tail, with a
ventral fin. There is no trace of the paired fins.
Fig. 65. Embryo of Lepidosteus shortly before hatching.
ol. olfactory pit; sd. suctorial disc; hy. hyoid arch.
The anterior and posterior portions of the alimentary tract are closed
in, but the middle region is still open to the yolk. The circulation
is now fully established, and the vessels present the usual vertebrate
arrangement. There is a large subintestinal vein.
[Pg 117]
The first of Agassiz’ embryos was hatched about ten days after
impregnation. The young fish on hatching immediately used its
suctorial disc to attach itself to the sides of the vessel in which it
was placed.
Fig. 66. Ventral view of the head of a Lepidosteus
embryo shortly before hatching, to shew the large suctorial
disc.
m. mouth; op. eye; s.d. suctorial disc.
The general form of Lepidosteus shortly after hatching is shewn in
fig. 67. On the ventral part of the front of the head is placed the
large suctorial disc. At the side of the head are seen the olfactory
pit, the eye and the auditory vesicle; while the projecting vesicle of
the mid-brain is very prominent above. Behind the mouth follow the
visceral arches. The mandibular arch (md) is placed on the hinder
border of the mouth, and is separated by a deep groove from the hyoid
arch (hy). This groove is connected with the hyomandibular cleft,
but I have not determined whether it is now perforated. The posterior
border of the hyoid arch is prolonged into an opercular fold. Behind
the hyoid arch are seen the true branchial arches.
Fig. 67. Larva of Lepidosteus shortly after hatching. (After
Parker.)
ol. olfactory pit; op. optic vesicle; au.v. auditory vesicle;
mb. mid-brain; sd. suctorial disc; md. mandibular arch; hy.
hyoid arch with operculum; br. branchial arches; an. anus.
[Pg 118]
There is still a continuous dorso-ventral fin, in which there are as
yet no fin-rays, and the anterior paired fins are present.
The yolk-sack is very large, but its communication with the alimentary
canal is confined to a narrow vitelline duct, which opens into the
commencement of the intestine immediately behind the duct of the
liver, which is now a compact gland. The yolk in Lepidosteus thus
behaves very differently from that in the Sturgeon. In the first place
it forms a special external yolk-sack, instead of an internal
dilatation of part of the alimentary tract; and in the second place it
is placed behind instead of in front of the liver.
I failed to find any trace of a pancreas. There is however, opening
on the dorsal side of the throat, a well-developed appendage
continued backwards beyond the level of the commencement of the
intestine. This appendage is no doubt the air-bladder.
In the course of the further growth of the young Lepidosteus, the
yolk-sack is rapidly absorbed, and has all but disappeared after three
weeks. A rich development of pigment early takes place; and the
pigment is specially deposited on the parts of the embryonic fin which
will develop into the permanent fins.
The notochord in the tail bends slightly upwards, and by the special
development of a caudal lobe an externally heterocercal tail like that
of Acipenser is established. The ventral paired fins are first visible
after about the end of the third week, and by this time the operculum
has grown considerably, and the gills have become well developed.
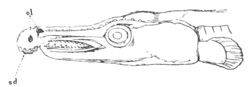
Fig. 68. Head of an advanced larva of Lepidosteus. (After Parker.)
ol. openings of the olfactory pit; sd. remains of the larval
suctorial disc.
The most remarkable changes in the later periods are those of the
mouth.
The upper and lower jaws become gradually prolonged, till they
eventually form a snout; while at the end of the upper jaw is placed
the suctorial disc, which is now considerably reduced in size (fig.
68, sd). The “fleshy globular termination of the upper jaw of the
adult Lepidosteus is the remnant of this embryonic sucking disc.”
(Agassiz, No. 92.)
[Pg 119]
The fin-rays become formed as in Teleostei, and parts of the
continuous embryonic fin gradually undergo atrophy. The dorsal limb of
the embryonic tail, as has been shewn by Wilder, is absorbed in
precisely the same manner as in Teleostei, leaving the ventral lobe to
form the whole of the permanent tail-fin.
Bibliography.
(92) Al. Agassiz. “The development of Lepidosteus.” Proc. Amer. Acad.
of Arts and Sciences, Vol. XIII. 1878.
General observations on the Embryology of the Ganoids.
The very heterogeneous character of the Ganoid group is clearly shewn
both in its embryology and its anatomy. The two known types of
formation of the central nervous system are exemplified in the two
species which have been studied, and these two species, though in
accord in having a holoblastic segmentation, yet differ in other
important features of development, such as the position of the yolk
etc. Both types exhibit Teleostean affinities in the character of the
pronephros; but as might have been anticipated Lepidosteus presents in
the origin of the nervous system, the relations of the hypoblast, and
other characters, closer approximations to the Teleostei than does
Acipenser. There are no very prominent Amphibian characters in the
development of either type, other than a general similarity in the
segmentation and formation of the layers. In the young of Polypterus
an interesting amphibian and dipnoid character is found in the
presence of a pair of true external gills covered by epiblast. These
gills are attached at the hinder end of the operculum, and receive
their blood from the hyoid arterial arch[41].
In the peculiar
suctorial disc of Lepidosteus, and in the more or less similar
structure in the Sturgeon, these fishes retain, I believe, a very
primitive vertebrate organ, which has disappeared in the adult state
of almost all the Vertebrata; but it is probable that further
investigations will shew that the Teleostei, and especially the
Siluroids, are not without traces of a similar structure.
I. Selachoidei
Acipenseridæ.
Polyodontidæ.
II. Teleostoidei
Polypteridæ.
Amiidæ.
Lepidosteidæ.
[Pg 120]
CHAPTER VII.
AMPHIBIA[42].
The eggs of most Amphibia[43]
are laid in water. They are smallish
nearly spherical bodies, and in the majority of known Anura (all the
European species), and in many Urodela (Amblystoma, Axolotl, though
not in the common Newt) part of the surface is dark or black, owing to
the presence of a superficial layer of pigment, while the remainder is
unpigmented. The pigmented part is at the upper pole of the egg, and
contains the germinal vesicle till the time of its atrophy; and the
yolk-granules in it are smaller than those in the unpigmented part.
The ovum is closely surrounded by a vitelline membrane[44],
and
receives, in its passage down the oviduct, a gelatinous investment of
varying structure.
In the Anura the eggs are fertilized as they leave the oviduct. In
some of the Urodela the mode of fertilization is still imperfectly
understood. In Salamanders and probably Newts it is internal[45];
[Pg 121]but
in Amblystoma punctatum (Clark, No. 98), the male deposits the semen
in the water. The eggs are laid by the Anura in masses or strings. By
Newts they are deposited singly in the angle of a bent blade of grass
or leaf of a water-plant, and by Amblystoma punctatum in masses
containing from four eggs to two hundred. Salamandra atra and
Salamandra maculosa are viviparous. The period of gestation for the
latter species lasts a whole year.
A good many exceptions to the above general statements have been
recorded[46].
In Notodelphis ovipara the eggs are transported (by the male?) into a
peculiar dorsal pouch of the skin of the female, which has an anterior
opening, but is continued backwards into a pair of diverticula. The
eggs are very large, and in this pouch, which they enormously distend,
they undergo their development. A more or less similar pouch is found
in Nototrema marsupiatum.
In the Surinam toad (Pipa dorsigera) the eggs are placed by the male
on the back of the female. A peculiar pocket of skin becomes developed
round each egg, the open end of which is covered by a gelatinous
operculum. The larvæ are hatched, and actually undergo their
metamorphosis, in these pockets. The female during this period lives
in water. Pipa Americana (if specifically distinct from P. dorsigera)
presents nearly the same peculiarities. The female of a tree frog of
Ceylon (Polypedates reticulatus) carries the eggs attached to the
abdomen.
Rhinoderma Darwinii[47]
behaves like some of the Siluroid fishes, in
that the male carries the eggs during their development in an
enormously developed laryngeal pouch.
Some Anura do not lay their eggs in water. Chiromantis Guineensis
attaches them to the leaves of trees; and Cystignathus mystacius lays
them in holes near ponds, which may become filled with water after
heavy rains.
The eggs of Hylodes Martinicensis are laid under dead leaves in moist
situations.
Formation of the layers.
Anura. The formation of the germinal layers has so far only been
studied in some Anura and in the Newt. The following description
applies to the Anura, and I have called[Pg 122] attention, at the end of the
section, to the points in which the Newt is peculiar.
The segmentation of the Frog’s ovum has already been described (Vol.
II. pp. 95-7), but I may remind the reader that the segmentation (fig.
69) results in the formation of a vesicle, the cavity of which is
situated excentrically; the roof of the cavity being much thinner than
the floor. The cavity is the segmentation cavity. The roof is formed
of two or three layers of smallish pigmented cells, and the floor of
large cells, which form the greater part of the ovum. These large
cells, which are part of the primitive hypoblast, will be spoken of in
the sequel as yolk-cells: they are equivalent to the food-yolk of the
majority of vertebrate ova.
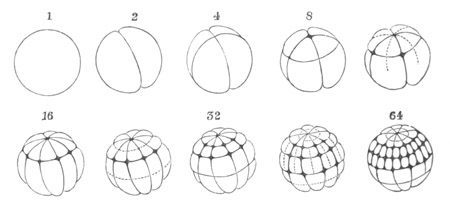
Fig. 69. Segmentation of Common Frog. Rana Temporaria.
(After Ecker.)
The numbers above the figures refer to the number of segments at the
stage figured.
The cells forming the roof of the cavity pass without any sharp
boundary into the yolk-cells, there being at the junction of the two a
number of cells of an intermediate character. The cells both of the
roof and the floor continue to increase in number, and those of the
roof become divided into two distinct strata (fig. 70, ep).
The upper of these is formed of a single row of somewhat cubical
cells, and the lower of several rows of more rounded cells. Both of
these strata eventually become the epiblast, of which they form the
epidermic and nervous layers. The roof of the segmentation cavity
appears therefore to be entirely constituted of epiblast.
The next changes which take place lead (1) to the formation[Pg 123] of the
mesenteron[48],
and (2) to the enclosure of the yolk-cells by the
epiblast.
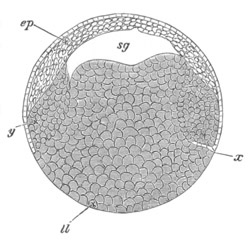
Fig. 70. Section through Frog’s ovum at the close
of segmentation. (After Götte.)
sg. segmentation cavity; ll. large yolk-containing cells; ep.
small cells at formative pole (epiblast); x. point of inflection
of epiblast; y. small cells close to junction of the epiblast and
yolk.
The mesenteron is formed as in Petromyzon and Lepidosteus by an
unsymmetrical form of invagination. The invagination first commences
by an inflection of the epiblast-cells for a small arc on the
equatorial line which marks the junction between the epiblastic cells
and the yolk-cells (fig. 70, x).
The inflected cells become continuous with the adjoining cells; and
the region where the inflection is formed constitutes a kind of lip,
below which a slit-like cavity is soon established. This lip is
equivalent to the embryonic rim of the Elasmobranch blastoderm, and
the cavity beneath it is the rudiment of the mesenteron.
The mesenteron now rapidly extends by the invagination of the cells on
its dorsal side. These cells grow inwards towards the segmentation
cavity as a layer of cells several rows deep. At its inner end, this
layer is continuous with the yolk-cells; and is divided into two
strata (fig. 71 A), viz. (1) a stratum of several rows of cells
adjoining the epiblast, which becomes the mesoblast (m), and (2) a
stratum of a single row of more columnar cells lining the cavity of
the mesenteron, which forms the hypoblast (hy). The growth inwards
of the dorsal wall of the mesenteron is no doubt in part a true
invagination, but it seems probable that it is also due in a large
measure to an actual differentiation of yolk-cells along the line of
growth. The mesenteron is at first a simple slit between the yolk and
the hypoblast (fig. 71 A), but as the involution of the hypoblast and
[Pg 124]
mesoblast extends further inwards, this slit enlarges, especially at
its inner end, into a considerable cavity; the blind end of which is
separated by a narrow layer of yolk-cells from the segmentation cavity
(fig. 71 B).
In the course of the involution, the segmentation cavity becomes
gradually pushed to one side and finally obliterated. Before
obliteration, it appears in some forms (Pelobates fuscus) to become
completely enclosed in the yolk-cells.
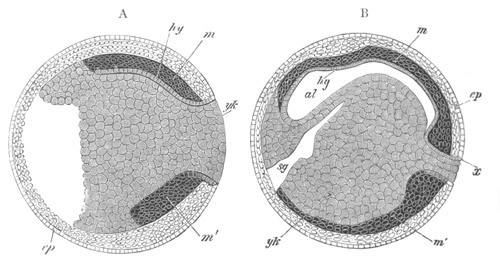
Fig. 71. Diagrammatic longitudinal sections through the embryo of
a Frog at two stages, to shew the formation of the germinal layers.
(Modified from Götte.)
ep. epiblast; m. dorsal mesoblast; m´. ventral mesoblast;
hy. hypoblast; yk. yolk; x. point of junction of the epiblast
and hypoblast at the dorsal side of the blastopore; al.
mesenteron; sg. segmentation cavity.
While the invagination to form the mesenteron takes place as above
described, the enclosure of the yolk has been rapidly proceeding. It
is effected by the epiblast growing over the yolk at all points of its
circumference. The nature of the growth is however very different at
the embryonic rim and elsewhere. At the embryonic rim it takes place
by the simple growth of the rim, so that the point x in figs. 70 and
71 is carried further and further over the surface of the yolk.
Elsewhere the epiblast at first extends over the yolk as in a typical
epibolic gastrula, without being inflected to form a definite lip.
While a considerable patch of yolk is still left uncovered, the whole
of the edge of the epiblast becomes however inflected, as at the
embryonic rim (fig. 71 A); and a circular blastopore is established,
round the[Pg 125] whole edge of which the epiblast and intermediate cells are
continuous.
From the ventral lip of the blastopore the mesoblast (fig. 71, m´),
derived from the small intermediate cells, grows inwards till it comes
to the segmentation cavity; the growth being not so much due to an
actual invagination of cells at the lip of the blastopore, as to a
differentiation of yolk-cells in situ. Shortly after the stage
represented in fig. 71 B, the plug of yolk, which fills up the opening
of the blastopore, disappears, and the mesenteron communicates freely
with the exterior by a small circular blastopore (fig. 73). The
position of the blastopore is the same as in other types, viz. at the
hinder end of the embryo.
By this stage the three layers of the embryo are definitely
established. The epiblast, consisting from the first of two strata,
arises from the small cells forming the roof of the segmentation-cavity.
It becomes continuous at the lip of the blastopore with cells
intermediate in size between the cells of which it is formed and the
yolk-cells. These latter, increasing in number by additions from the
yolk-cells, give rise to the mesoblast and to part of the hypoblast;
while to the latter layer the yolk-cells, as mentioned above, must
also be considered as appertaining. Their history will be dealt with
in treating of the general fate of the hypoblast.
Urodela. The early stages of the development of the Newt have been
adequately investigated by Scott and Osborn (No. 114). The
segmentation and formation of the layers is in the main the same as in
the Frog. The ovum is without black pigment. There is a typical
unsymmetrical invagination, but the dorsal lip of the blastopore is
somewhat thickened. The most striking feature in which the Newt
differs from the Frog is the fact that the epiblast is at first
constituted of a single layer of cells (fig. 75, ep). The roof of
the segmentation cavity is constituted, during the later stages of
segmentation, of several rows of cells (Bambeke, No. 95), but
subsequently it would appear to be formed of a single row of cells
only (Scott and Osborn, No. 114).
General history of the layers.
Epiblast: Anura. At the completion of the invagination the epiblast
forms a continuous layer enclosing the whole ovum, and constituted
throughout of two strata. The formation of the medullary canal
commences by the nervous layer along the axial dorsal line becoming
thickened, and giving rise to a somewhat[Pg 126] pyriform medullary plate, the
sides of which form the projecting medullary folds (fig. 77 A). The
medullary plate is thickened at the two sides, and is grooved in the
median line by a delicate furrow (fig. 72, r). The dilated extremity
of the medullary plate, situated at the end of the embryo opposite the
blastopore, is the cerebral part of the plate, and the remainder the
spinal. The medullary folds bend upwards, and finally meet above,
enclosing a central cerebrospinal canal (fig. 74). The point at which
they first meet is nearly at the junction of the brain and spinal
cord, and from this point their junction extends backwards and
forwards; but the whole process is so rapid that the closure of the
medullary canal for its whole length is effected nearly
simultaneously. In front the medullary canal ends blindly, but behind
it opens freely into the still persisting blastopore, with the lips of
which the medullary folds become, as in other types, continuous. Fig.
73 represents a longitudinal section through an embryo, shortly after
the closure of the medullary canal (nc); the opening of which into
the blastopore (x) is clearly seen.
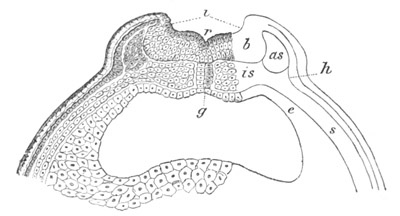
Fig. 72. Transverse section through the posterior cephalic region of
an early embryo of Bombinator. (After Götte.)
l. medullary groove; r. axial furrow in the medullary groove;
h. nervous layer of epidermis; as. outer portion of vertebral
plate; is. inner portion of vertebral plate; s. lateral plate of
mesoblast; g. notochord; e. hypoblast.
On the closure of the medullary canal, its walls become separated from
the external epiblast, which extends above it as a continuous layer.
In the formation of the central nervous system both strata of the
epiblast have a share, though the main mass is derived from the
nervous layer. After the central[Pg 127] nervous tube has become separated
from the external skin, the two layers forming it fuse together; but
there can be but little doubt that at a later period the epidermic
layer separates itself again as the central epithelium of the nervous
system.
Both the nervous and epidermic strata have a share in forming the
general epiblast; and though eventually they partially fuse together
yet the horny layer of the adult epidermis, where such can be
distinguished, is probably derived from the epidermic layer of the
embryo, and the mucous layer of the epidermis from the embryonic
nervous layer.
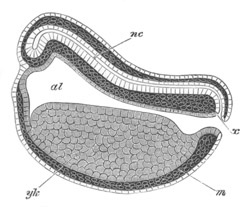
Fig. 73. Diagrammatic longitudinal section of the
embryo of a Frog. (Modified from Götte.)
nc. neural canal; x. point of junction of epiblast and hypoblast
at the dorsal lip of the blastopore; al. alimentary tract; yk.
yolk-cells; m. mesoblast. For the sake of simplicity the epiblast
is represented as if composed of a single row of cells.
In the formation of the organs of sense the nervous layer shews itself
throughout as the active layer. The lens of the eye and the auditory
sack are derived exclusively from it, the latter having no external
opening. The nervous layer also plays the more important part in the
formation of the olfactory sack.
The outer layer of epiblast-cells becomes ciliated after the close of
the segmentation, but the cilia gradually disappear on the formation
of the internal gills. The cilia cause a slow rotatory movement of the
embryo within the egg, and probably assist in the respiration after it
is hatched. They are especially developed on the external gills.
Urodela. In the Newt (Scott and Osborn, No. 114) the medullary plate
becomes established, while the epiblast is still formed of a single
row of cells; and it is not till after the closure of the neural
groove that any distinction is observable between the epithelium of
the central canal, and the remaining cells of the cerebrospinal cord
(fig. 75).
Before the closure of the medullary folds the lateral epiblast becomes
divided into the two strata present from the first in the Frog; and in
the subsequent development the inner layer behaves as the active
layer, precisely as in the Anura.
[Pg 128]The mesoblast and notochord: Anura. After the disappearance of the
segmentation cavity, the mesoblast is described by most observers,
including Götte, as forming a continuous sheet round the ovum,
underneath the epiblast. The first important differentiations in it
take place, as in the case of the epiblast, in the axial dorsal line.
Along this line a central cord of the mesoblast becomes separated from
the two lateral sheets to form the notochord. Calberla states,
however, that when the mesoblast is distinctly separated from the
hypoblast it does not form a continuous sheet, but two sheets one on
each side, between which is placed a ridge of cells continuous with
the hypoblastic sheet. This ridge subsequently becomes separated from
the hypoblast as the notochord. Against this view Götte has recently
strongly protested, and given a series of careful representations of
his sections which certainly support his original account.
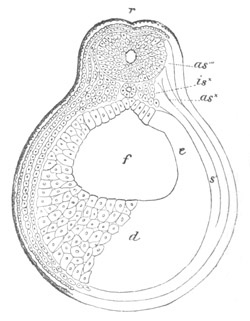
Fig. 74. Section through the anterior part of the
trunk of a young embryo of Bombinator. (After Götte.)
as´´´. medulla oblongata; isx. splanchnopleure; asx.
somatopleure in the vertebral part of the mesoblastic plate; s.
lateral plate of mesoblast; f. throat; e. passage of epithelial
cells into yolk-cells; d. yolk-cells; r. dorsal groove along the
line of junction of the medullary folds.
My own observations are in favour of Calberla’s statement, and so far
as I can determine from my sections the mesoblast never appears as a
perfectly continuous sheet, but is always deficient in the dorsal
median line. My observations are unfortunately not founded on a
sufficient series of sections to settle the point definitely.
After the formation of the notochord (fig. 72), the mesoblast may be
regarded as consisting of two lateral plates, continuous ventrally,
but separated in the median dorsal line. By the division of the dorsal
parts of these plates into segments, which commences in the region of
the neck and thence extends backwards, the mesoblast of the trunk
becomes divided into[Pg 129] a vertebral portion, cleft into separate somites,
and a lateral unsegmented portion (fig. 74).
The history of these two parts and of the mesoblast is generally the
same as in Elasmobranchs.
The mesoblast in the head becomes, according to Götte, divided into
four segments, equivalent to the trunk somites. Owing to a confusion
into which Götte has fallen from not recognizing the epiblastic origin
of the cranial nerves, his statements on this head must, I think, be
accepted with considerable reserve; but some part of his segments
appears to correspond with the head-cavities of Elasmobranchii.
Urodela. Scott and Osborn (No. 114) have shewn that in the Newt the
mesoblast (fig. 75) is formed of two lateral plates, split off from
the hypoblast, and that the ventral growth of these plates is largely
effected by the conversion of yolk-cells into mesoblast-cells. They
have further shewn that the notochord is formed of an axial portion
of the hypoblast, as in the types already considered (fig. 75). The
body cavity is continued into the region of the head; and the
mesoblast lining the cephalic section of the body cavity is divided
into the same number of head cavities as in Elasmobranchii, viz. one
in front of the mouth, and one in the mandibular and one in each of
the following arches.
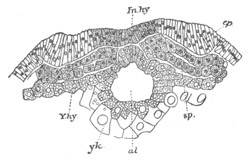
Fig. 75. Transverse section through
the cephalic region of a young Newt embryo. (After Scott and Osborn.)
In.hy. invaginated hypoblast, the dorsal part of which will form
the notochord; ep. epiblast of neural plate; sp.
splanchnopleure; al. alimentary tract; yk. and Y.hy.
yolk-cells.
The hypoblast. There are no important points of difference in the
relations of the hypoblast between the Anura and Urodela. The
mesenteron, at the stage represented in fig. 73, forms a wide cavity
lined dorsally by a layer of invaginated hypoblast, and ventrally by
the yolk-cells. The hypoblast is continuous laterally and in front
with the yolk-cells (figs. 72, 74 and 75). At an earlier stage, when
the mesenteron has a less definite form, such a continuity between the
true hypoblast and the yolk-cells does not exist at the sides of the
cavity.
The definite closing in of the mesenteron by the true hypoblast-cells
commences in front and behind, and takes place last[Pg 130] of all in the
middle (fig. 76). In front this process takes place with the greatest
rapidity. The cells of the yolk-floor become continuously
differentiated into hypoblast-cells, and very soon the whole of the
front end becomes completely lined by true hypoblastic cells, while
the yolk-cells become confined to the floor of the middle part.
The front portion of the mesenteron gives rise to the œsophagus,
stomach and duodenum. Close to its hinder boundary there appears a
ventral outgrowth, which is the commencement of the hepatic
diverticulum (fig. 76, l). The yolk is thus post-hepatic, as in
Vertebrates generally.
The stomodæum is formed comparatively late by an epiblastic
invagination (fig. 76, m).
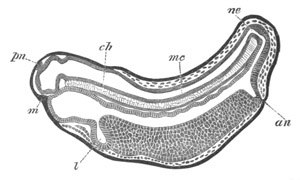
Fig. 76. Longitudinal section through an advanced
embryo of Bombinator. (After Götte.)
m. mouth; an. anus; l. liver; ne. neurenteric canal; mc.
medullary canal; ch. notochord; pn. pineal gland.
It should be noticed that the conversion of the yolk-cells into
hypoblast-cells to form the ventral wall of the anterior region of the
alimentary tract is a closely similar occurrence to the formation of
cells in the yolk-floor of the anterior part of the alimentary tract
in Elasmobranchii. This conversion is apparently denied by Götte, but
since I find cells in all stages of transition between yolk-cells and
hypoblast-cells I cannot doubt the fact of its occurrence.
At first, the mesenteron freely communicates with the exterior by the
opening of the blastopore. The lips of the blastopore gradually
approximate, and form a narrow passage on the dorsal side of which the
neural tube opens, as has already been described (fig. 73). The
external opening of this passage finally becomes obliterated, and the
passage itself is left as a narrow diverticulum leading from the hind
end of the mesenteron into the neural canal (fig. 76). It forms the
postanal gut, and gradually narrows and finally atrophies. At its
front border, on the ventral side, there may be seen a slight
ventrally directed[Pg 131] diverticulum of the alimentary tract, which first
becomes visible at a somewhat earlier stage (fig. 73). This
diverticulum becomes longer and meets an invagination of the skin
(fig. 76, an), which arises in Rana temporaria at a somewhat earlier
period than represented by Götte in Bombinator. This epiblastic
invagination is the proctodæum, and an anal perforation eventually
appears at its upper extremity.
The differentiation of the hinder end of the præanal gut proceeds in
the same fashion as that of the front end, though somewhat later. It
gives rise to the cloacal and intestinal part of the alimentary tract.
From the ventral wall of the cloacal section, there grows out the
bifid allantoic bladder, which is probably homologous with the
allantois of the higher Vertebrata. After the differentiation of the
ventral wall of the fore and hind ends of the alimentary tract has
proceeded for a certain distance, the yolk only forms a floor for a
restricted median region of the alimentary cavity, which corresponds
to the umbilical canal of the Amniota. The true hypoblastic epithelium
then grows over the outer side of the yolk, which thus constitutes a
true, though small, and internal yolk-sack. The yolk-cells enclosed in
this sack become gradually absorbed, and the walls of the sack form
part of the intestine.
General growth of the Embryo.
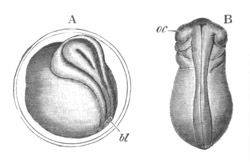
Fig. 77. Embryos of the common Frog. (After Remak.)
A. Young stage represented enclosed in the egg-membrane. The
medullary plate is distinctly formed, but no part of the medullary
canal is closed. bl. blastopore.
B. Older embryo after the closure of the medullary canal. oc.
optic vesicle. Behind the optic vesicle are seen two visceral
arches.
Anura. The pyriform medullary plate, already described, is the first
external indication of the embryo. This plate appears about the stage
represented in longitudinal section in fig. 71 B. The feature most
conspicuous in it at first is the axial groove. It soon becomes more
prominent (fig. 77 A), and ends behind at the blastopore (bl), the
lips of which are continuous with the two medullary folds. As the
sides of this plate bend upwards to form the closed medullary canal,
the embryo elongates itself and assumes a somewhat oval form. At the
same time the cranial flexure becomes apparent (fig. 73), and the
blastopore shortly afterwards becomes shut off from the exterior. The
embryo now continues to grow in length (fig. 77 B), and the mesoblast
becomes segmented. The somites are first formed in the neck, and are
added successively behind in[Pg 132] the unsegmented posterior region of the
embryo. The hind end of the embryo grows out into a rounded
prominence, which rapidly elongates, and becomes a well-marked tail
entirely formed by the elongation of the postanal section of the body.
The whole body has a very decided dorsal flexure, the ventral surface
being convex. Fig. 78 represents an embryo of Bombinator in side view,
with the tail commencing to project. The longitudinal section (fig.
76) is taken through an embryo of about the same age. In the cephalic
region important changes have taken place. The cranial flexure has
become more marked, but is not so conspicuous a feature in the
Amphibia as in most other types, owing to the small size of the
cerebral rudiment. The mid-brain is shewn at fig. 78 a forming the
termination of the[Pg 133] long axis of the body, and the optic vesicles
(a´) are seen at its sides.
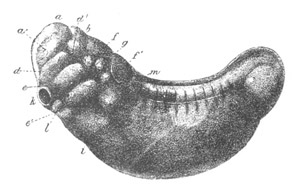
Fig. 78. Lateral view of an advanced embryo of Bombinator. (After
Götte.)
a. mid-brain, a´. eye; b. hind-brain; d. mandibular arch;
d´. Gasserian ganglion; e. hyoid arch; e´. first branchial
arch; f. seventh nerve; f´. glossopharyngeal and vagus nerve;
g. auditory vesicle; i. boundary between liver and yolk-sack;
k. suctorial disc; l. pericardial prominence; m. prominence
formed by the pronephros.
The rudiments of the mandibular (d), hyoid (e), and first
branchial (e´) arches project as folds at the side of the head, but
the visceral clefts are not yet open. Rudiments of the proctodæum and
stomodæum have appeared, but neither of them as yet communicates with
the mesenteron. Below the hyoid arch is seen a peculiar disc (k)
which is an embryonic suctorial organ, formed of a plate of thickened
epiblast. There is a pair of these discs, one on each side, but only
one of them is shewn in the figure. At a later period they meet each
other in the middle line, though they separate again before their
final atrophy. They are found in the majority of the Anura, but are
absent according to Parker in the Aglossa (Pipa and Dactylethra (fig.
83)). They are probably remnants of the same primitive organs as the
suctorial disc of Lepidosteus.
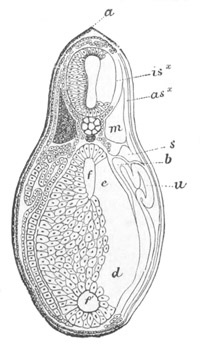
Fig. 79. Transverse section through a very young
tadpole of Bombinator at the level of the anterior end of the
yolk-sack. (After Götte.)
a. fold of epiblast continuous with the dorsal fin; isx. neural
cord; m. lateral muscle; asx. outer layer of muscle-plate; s.
lateral plate of mesoblast; b. mesentery; u. fold of the
peritoneal epithelium which forms the segmental duct; f.
alimentary tract; f´. ventral diverticulum which becomes the
liver; e. junction of yolk-cells and hypoblast-cells; d.
yolk-cells.
The embryo continues to grow in length, while the tail becomes more
and more prominent, and becomes bent round to the side owing to the
confinement of the larva within the egg-membrane. At the front of the
head the olfactory pits become distinct. The stomodæum deepens, though
still remaining blind, and three fresh branchial arches become formed;
the last two being very imperfectly differentiated, and not visible
from the exterior. There are thus six arches in all, viz. the
mandibular, the hyoid and four branchial arches. Between the
mandibular and the hyoid, and between each of the following arches,
pouches of the mesenteron push their way towards the external skin. Of
these pouches there are five, there[Pg 134] being no pouch behind the last
branchial arch. The first of these will form the hyomandibular cleft,
the second the hyobranchial, and the third, fourth and fifth the three
branchial clefts.
Although the pouches of the throat meet the external skin, an external
opening is not formed in them till after the larva is hatched. Before
this takes place there grow, in the majority of forms, from the outer
side of the first and second branchial arches small processes, each
forming the rudiment of an external gill; a similar rudiment is
formed, either before or after hatching, on the third arch; but the
fourth arch is without it (figs. 80 and 82).
These external gills, which differ fundamentally from the external
gills of Elasmobranchii in being covered by epiblast, soon elongate
and form branched ciliated processes floating freely in the medium
around the embryo (fig. 80).
Before hatching the excretory system begins to develop. The segmental
duct is formed as a fold of the somatic wall at the dorsal side of the
body cavity (fig. 79, u). Its anterior end alone remains open to the
body cavity, and gives rise to a pronephros with two or three
peritoneal openings, opposite to which a glomerulus is formed.
The mesonephros (permanent kidney of Amphibia) is formed as a series
of segmental tubes much later than the pronephros, during late larval
life. Its anterior end is situated some distance behind the
pronephros, and during its formation the pronephros atrophies.
The period of hatching varies in different larvæ, but in most cases,
at the time of its occurrence, the mouth has not yet become
perforated. The larva, familiarly known as a tadpole, is at first
enclosed in the detritus of the gelatinous egg envelopes. The tail, by
the development of a dorsal and ventral fin, very soon becomes a
powerful swimming organ. Growth, during the period before the larva
begins to feed, is no doubt carried on at the expense of the yolk,
which is at this time enclosed within the mesenteron.
The mouth and anal perforations are not long in making their
appearance, and the tadpole is then able to feed. The gill slits also
become perforated, but the hyomandibular diverticulum in most species
never actually opens to the exterior, and in all cases becomes very
soon closed.
[Pg 135]There can be but little doubt that the hyomandibular diverticulum
gives rise, as in the Amniota, to the Eustachian tube and tympanic
cavity, except when these are absent (i.e. Bombinatoridæ). Götte
holds however that these parts are derived from the hyobranchial
cleft, but his statements on this head, which would involve us in
great morphological difficulties, stand in direct contradiction to the
careful researches of Parker.
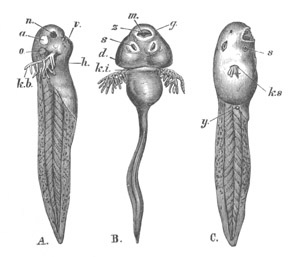
Fig. 80. Tadpoles with external branchiæ. (From Huxley; after Ecker.)
A. Lateral view of a young tadpole.
B. Ventral view of a somewhat older tadpole.
kb. external branchiæ; m. mouth; n. nasal sack; a. eye; o.
auditory vesicle; z. horny jaws; s. ventral sucker; d.
opercular fold.
C. More advanced larva, in which the opercular fold has nearly
covered the branchiæ.
s. ventral sucker; ks. external branchiæ; y. rudiment of hind
limb.
Shortly after hatching, there grows out from the hyoid arch on each
side an opercular fold of skin, which gradually covers over the
posterior branchial arches and the external gills (fig. 80 d). It
fuses with the skin at the upper part of the gill arches, and also
with that of the pericardial wall below them; but is free in the
middle, and so assists in forming a cavity, known as the branchial
cavity, in which the gills are placed. Each branchial cavity at first
opens by a separate widish pore behind (fig. 80), and in Dactylethra
both branchial apertures are preserved (Huxley). In the larva of
Bombinator, and it would seem also that of Alytes and Pelodytes, the
original widish openings of the two branchial chambers meet together
in the ventral line, and[Pg 136] form a single branchial opening or spiracle.
In most other forms, i.e. Rana, Bufo, Pelobates, etc., the two
branchial chambers become united by a transverse canal, and the
opening of the right sack then vanishes, while that of the left
remains as the single unsymmetrical spiracle. In breathing the water
is taken in at the mouth, passes through the branchial clefts into the
branchial cavities, and is thence carried out by the spiracle.
Immediately after the formation of the branchial cavities, the
original external gills atrophy, but in their place fresh gills,
usually called internal gills, appear on the outer side of the middle
region of the four branchial arches.
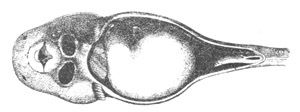
Fig. 81. Tadpole of Bombinator from the ventral
side, with the abdominal wall removed. (After Götte.)
Behind the mouth are placed the two suckers, and behind these are
seen the gills projecting through the spiracles.
There is a single row of these on the first and fourth branchial
arches, and two rows on the second and third. In addition to these
gills, which are vascular processes of the mesoblast, covered,
according to Götte, with an epiblastic (?) epithelium, branchial
processes appear on the hypoblastic walls of the three branchial
clefts. The last-named branchial processes would appear to be
homologous with the gills of Lampreys. In Dactylethra no other gills
but these are formed (Parker).
The mouth, even before the tadpole begins to feed, acquires a
transversely oval form (fig. 81), and becomes armed with provisional
structures in the form of a horny beak and teeth, which are in use
during larval life.
The beak is formed of a pair of horny plates moulded on the upper and
lower pairs of labial cartilages. The upper valve of the beak is the
larger of the two, and covers the lower. The beak is surrounded by a
projecting lip formed of a circular fold of skin, the free edge of
which is covered by papillæ. Between the papillæ and the beak rows of
horny teeth are placed on the inner surface of the lip. There are
usually two rows of these on the upper side, the inner one not
continuous across the middle line, and three or four rows on the lower
side, the inner one or two divided into two lateral parts.
[Pg 137]
As the tadpole attains its full development, the suctorial organs
behind the mouth gradually atrophy. The alimentary canal, which is
(fig. 81) at first short, rapidly elongates, and fills up with its
numerous coils the large body cavity. In the meantime, the lungs
develop as outgrowths from the œsophagus.
Various features in the anatomy of the Tadpole point to its being a
repetition of a primitive vertebrate type. The nearest living
representative of this type appears to be the Lamprey.
The resemblance between the mouths of the Tadpole and Lamprey is very
striking, and many of the peculiarities of the larval skull of the
Anura, especially the position of the Meckelian cartilages and the
subocular arch, perhaps find their parallel in the skull of the
Lamprey[49].
The internal hypoblastic gill-sacks of the Frog, with
their branchial processes, are probably equivalent to the gill-sacks
of the Lamprey[50];
and it is not impossible that the common posterior
openings of the gill-pouches in Myxine are equivalent to the
originally paired openings of the branchial sack of the Tadpole.
The resemblances between the Lamprey and the Tadpole appear to me to
be sufficiently striking not to be merely the results of more or less
similar habits; but at the same time there are no grounds for
supposing that the Lamprey itself is closely related to an ancestral
form of the Amphibia. In dealing with the Ganoids and other types
arguments have been adduced to shew that there was a primitive
vertebrate stock provided with a perioral suctorial disc; and of this
stock the Cyclostomata are the degraded, but at the same time the
nearest living representatives. The resemblances between the Tadpole
and the Lamprey are probably due to both of them being descended from
this stock. The Ganoids, as we have seen, also shew traces of a
similar descent; and the resemblance between the larva of Dactylethra
(fig. 83), the Old Red Sandstone Ganoids[51]
and Chimæra, probably
indicates that an extension of our knowledge will bring to light
further affinities between the primitive Ganoid and Holocephalous
stocks and the Amphibia.
Metamorphosis. The change undergone by the Tadpole in its passage into
the Frog is so considerable as to deserve the name of a metamorphosis.
This metamorphosis essentially consists in the reduction and atrophy
of a series of provisional embryonic organs, and the appearance of
adult organs in their[Pg 138] place. The stages of this metamorphosis are
shewn in fig. 82, 5, 6, 7, 8.
The two pairs of limbs appear nearly simultaneously as small buds; the
hinder pair at the junction of the tail and body (fig. 82, 5), and the
anterior pair concealed under the opercular membrane. The lungs
acquire a greater and greater importance, and both branchial and
pulmonary respirations go on together for some time.

Fig. 82. Tadpoles and young of the common Frog. (From Mivart.)
1. Recently-hatched Tadpoles twice the natural size. 2. Tadpole with
external gills. 2a. Same enlarged. 3 and 4. Later stages after the
enclosure of the gills by the opercular membrane. 5. Stage with
well-developed hind-limbs visible. 6. Stage after the ecdysis, with
both pairs of limbs visible. 7. Stage after partial atrophy of the
tail. 8. Young Frog.
When the adult organs are sufficiently developed an ecdysis takes
place, in which the gills are completely lost, the provisional horny
beak is thrown off, and the mouth loses its suctorial form.[Pg 139] The eyes,
hitherto concealed under the skin, become exposed on the surface, and
the front limbs appear (fig. 82, 6). With these external changes
important internal modifications of the mouth, the vascular system,
and the visceral arches take place. A gradual atrophy of the tail,
commencing at the apex, next sets in, and results in the complete
absorption of this organ.
The long alimentary canal becomes shortened, and the, in the main,
herbivorous Tadpole gradually becomes converted into the carnivorous
Frog (fig. 82, 6, 7, 8).
The above description of the metamorphosis of the Frog applies fairly
to the majority of the Anura, but it is necessary to notice a few of
the more instructive divergences from the general type.
In the first place, several forms are known, which are hatched in the
condition of the adult. The exact amount of metamorphosis which these
forms pass through in the egg is still a matter of some doubt. Hylodes
Martinicensis is one of these forms. The larva no doubt acquires
within the egg a long tail; but while Bavay[52]
states that it is
provided with external gills, which however are not covered by an
operculum, Peters[53]
was unable to see any traces of such structures.
In Pipa Americana, and apparently in Pipa dorsigera also if a distinct
species, the larva leaves the cells on the back of the mother in a
condition closely resembling the adult. The embryos of both species
develop a long tail in the egg, which is absorbed before hatching, and
according to Wyman[54]
P. Americana is also temporarily provided with
gills, which atrophy early.
The larva of Rhinoderma Darwinii is stated by Jiminez de la Espada to
be without external gills, and it appears to be hatched while still in
the laryngeal pouch of the male. In Nototrema marsupiatum the larvæ
are also stated to be without external gills.
Amongst the forms with remarkable developments Pseudis paradoxa
deserves especial mention, in that the tadpole of this form attains an
immensely greater bulk than the adult; a peculiarity which may be
simply a question of nutrition, or may perhaps be explained by
supposing that the larva resembles a real ancestral form, which was
much larger than the existing Frog.
Another form of perhaps still greater morphological interest is the
larva of Dactylethra. The chief peculiarities of this larva (fig. 83)
have been summarized by Parker (No. 107, p. 626), from whom I quote
the following passage:
a. “The mouth is not inferior in position, suctorial and small, but
is very wide like that of the ‘Siluroids and Lophius;’ has an
underhung lower[Pg 140] jaw, an immensely long tentacle from each upper lip,
and possesses no trace of the primordial horny jaws of the ordinary
kind.
b. “In conformity with these characters the head is extremely flat
or depressed, instead of being high and thick.
c. “There are no claspers beneath the chin.
d. “The branchial orifice is not confined to the left side, but
exists on the right side also.
e. “The tail, like the skull, is remarkably chimæroid; it terminates
in a long thin pointed lash, and the whole caudal region is narrow and
elongated as compared with that of our ordinary Batrachian larvæ.
f. “The fore-limbs are not hidden beneath the opercular fold.”
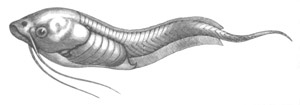
Fig. 83. Larva of Dactylethra. (After Parker.)
Although most Anurous embryos are not provided with a sufficient
amount of yolk to give rise to a yolk-sack as an external appendage of
the embryo, yet in some forms a yolk-sack, nearly as large as that of
Teleostei, is developed. One of these forms, Alytes obstetricans,
belongs to a well-known European genus allied to Pelobates. The
embryos of Pipa dorsigera (Parker) are also provided with a very large
yolk-sack, round which they are coiled like a Teleostean embryo. A
large yolk-sack is also developed in the embryo of Pseudophryne
australis.
The actual complexity of the organization of different tadpoles, and
their relative size, as compared with the adult, vary considerably.
The tadpoles of Toads are the smallest, Pseudophryne australis
excelling in this respect; those of Pseudis are the largest known.
The external gills reach in certain forms, which are hatched in late
larval stages, a very great development. It seems however that this
development is due to these gills being especially required in the
stages before hatching. Thus in Alytes, in which the larva leaves the
egg in a stage after the loss of the external gills, these structures
reach in the egg a very great development. In Notodelphis ovipara, in
which the eggs are carried in a dorsal pouch of the mother, the
embryos are provided with long vesicular gills attached to the neck by
delicate threads. The fact (if confirmed) that some of the forms which
are not hatched till post-larval stages are without external gills,
probably indicates that there may be various contrivances for
embryonic respiration[55];
and that the external gills only attain a
great development in[Pg 141] those instances in which respiration is mainly
carried on by their means. The external gills of Elasmobranchii are
probably, as stated in a previous chapter, examples of secondarily
developed structures, which have been produced by the same causes as
the enlarged gills of Alytes, Notodelphis, etc.
Urodela. Up to the present time complete observations on the
development of the Urodela are confined to the Myctodera[56].
The early stages are in the main similar to those of the Anura. The
body of the embryo is, as pointed out by Scott and Osborn, ventrally
instead of dorsally flexed. The metamorphosis is much less complete
than in the Anura. The larva of Triton may be taken as typical. At
hatching, it is provided with a powerful swimming tail bearing a
well-developed fin: there are three pairs of gills placed on the three
anterior of the true branchial arches.
Between the hyoid and first branchial arch, and between the other
branchial arches, slits are developed, there being four slits in all.
At the period just before hatching, only three of these have made
their appearance. The hyomandibular cleft is not perforated. Stalked
suckers, of the same nature as the suckers of the Anura, are formed on
the ventral surface behind the mouth. A small opercular fold,
developed from the lower part of the hyoid arch, covers over the bases
of the gills. The suctorial mouth and the provisional horny beak of
the Anura have no counterpart in these larvæ. The skin is ciliated,
and the cilia cause a rotation in the egg. Even before hatching, a
small rudiment of the anterior pair of limbs is formed, but the
hind-limbs are not developed till a later stage, and the limbs do not
attain to any size till the larva is well advanced. In the course of
the subsequent metamorphosis lungs become developed, and a pulmonary
respiration takes the place of the branchial one. The branchial slits
at the same time close and the branchiæ atrophy.
The other types of Myctodera, so far investigated, agree fairly with
the Newt.
The larva of Amblystoma punctatum (fig. 84) is provided with two very
[Pg 142]
long processes (s), like the suctorial processes in Triton, placed
on the throat in front of the external gills. They are used to support
the larva when it sinks to the bottom, and have been called by Clarke
(No. 98) balancers. On the development of the limbs, these processes
drop off. The external gills atrophy about one hundred days after
hatching.
It might have been anticipated that the Axolotl, being a larval form
of Amblystoma, would agree in development with Amblystoma punctatum.
The conspicuous suctorial processes of the latter form are however
represented by the merest rudiments in the Axolotl.
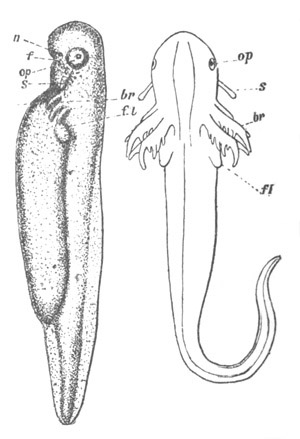
Fig. 84. Larvæ of Amblystoma punctatum.
(After Clarke.)
n. nasal pit; f. oral invagination; op. eye; s. balancers;
f.l. front limb; br. branchiæ.
The young of Salamandra maculata leave the uterus with external gills,
but those of the Alpine Salamander (Salamandra atra) are born in the
fully developed condition without gills. In the uterus they pass
through a metamorphosis, and are provided (in accordance with the
principle already laid down) with very long gill-filaments[57].
Salamandra atra has only two embryos, but there are originally a
larger number of eggs (Von Siebold), of which all but two fail to
develop, while their remains are used as pabulum by the two which
survive. Both species of Salamander have a sufficient quantity of
food-yolk to give rise to a yolk-sack.
Spelerpes only develops three post-hyoid arches, between which slits
are formed as in ordinary types. Menobranchus and Proteus agree with
Spelerpes in the number of post-hyoid arches.
One of the most remarkable recent discoveries with reference to the
metamorphosis of the Urodela was made by Dumeril[58].
He found that
some of the larvæ of the Axolotl, bred in the Jardin des Plantes, left
the water, and in the course of about a fortnight underwent a similar
metamorphosis to that of the Newt, and became converted into a form
agreeing in every[Pg 143] particular with the American genus Amblystoma.
During this metamorphosis a pulmonary respiration takes the place of a
branchial one, the gills are lost, and the gill slits close. The tail
loses its fin and becomes rounded, the colour changes, and alterations
take place in the gums, teeth, and lower jaw.
Madame von Chauvin[59]
was able, by gradually accustoming Axolotl
larvæ to breathe, artificially to cause them to undergo the above
metamorphosis.
It seems very possible, as suggested by Weismann[60],
that the
existing Axolotls are really descendants of Amblystoma forms, which
have reverted to a lower stage. In favour of this possibility a very
interesting discovery of Filippi’s[61]
may be cited. He found in a
pond in a marsh near Andermat some examples of Triton alpestris,
which, though they had become sexually mature, still retained the
external gills and the other larval characters. Similar sexually
mature larval forms of Triton tæniatus have been described by Jullien.
These discoveries would seem to indicate that it might be possible
artificially to cause the Newt to revert to a perennibranchiate
condition.
Gymnophiona. The development of the Gymnophiona is almost unknown, but
it is certain that some larval forms are provided with a single
gill-cleft, while others have external gills.
A gill-cleft has been noticed in Epicrium glutinosum (Müller), and in
Cœcilia oxyura. In Cœcilia compressicauda, Peters (No. 108) was
unable to find any trace of a gill-cleft, but he observed in the larvæ
within the uterus two elongated vesicular gills.
Bibliography.
Amphibia.
(93) Ch. van Bambeke. “Recherches sur le développement du Pélobate
brun.” Mémoires couronnés, etc. de l'Acad. roy. de Belgique, 1868.
(94) Ch. van Bambeke. “Recherches sur l'embryologie des Batraciens.”
Bulletin de l'Acad. roy. de Belgique, 1875.
(95) Ch. van Bambeke. “Nouvelles recherches sur l'embryologie des
Batraciens.” Archives de Biologie, Vol. I. 1880.
(96) K. E. von Baer. “Die Metamorphose des Eies der Batrachier.”
Müller’s Archiv, 1834.
(97) B. Benecke. “Ueber die Entwicklung des Erdsalamanders.”
Zoologischer Anzeiger, 1880.
[Pg 144]
(98) S. F. Clarke. “Development of Amblystoma punctatum,” Part I.,
External. Studies from the Biological Laboratory of the Johns Hopkins
University, No. II. 1880.
(99) H. Cramer. “Bemerkungen üb. d. Zellenleben in d. Entwick. d.
Froscheies.” Müller’s Archiv, 1848.
(100) A. Ecker. Icones Physiolog. 1851-1859.
(101) A. Götte. Die Entwicklungsgeschichte der Unke. Leipzig, 1875.
(102) C. K. Hoffmann. “Amphibia.” Klassen u. Ordnungen d.
Thierreichs, 1873-1879.
(103) T. H. Huxley. Article “Amphibia” in the Encyclopædia
Britannica.
(104) A. Moquin-Tandon. “Développement des Batraciens anures.”
Annales des Sciences Naturelles, III. 1875.
(105) G. Newport. “On the impregnation of the Ovum in Amphibia” (three
memoirs). Phil. Trans. 1851, 1853, and 1854.
(106) W. K. Parker. “On the structure and development of the Skull of
the common Frog.” Phil. Trans., CLXI. 1871.
(107) W. K. Parker. “On the structure and development of the Skull of
the Batrachia.” Phil. Trans., Vol. CXLVI., Part 2. 1876.
(108) W. C. H. Peters. “Ueber die Entwicklung der Coecilien und
besonders von Cœcilia compressicauda.” Berlin Monatsbericht, p. 40,
1874.
(109) W. C. H. Peters. “Ueber die Entwicklung der Coecilien.” Berl.
Monatsbericht, p. 483, 1875.
(110) J. L. Prevost and J. B. Dumas. “Deuxième Mém. s. l. génération.
Développement de l'œuf d. Batraciens.” Ann. Sci. Nat. II. 1824.
(111) R. Remak. Untersuchungen über die Entwicklung der
Wirbelthiere, 1850-1858.
(112) M. Rusconi. Développement de la grenouille commune depuis le
moment de sa naissance jusqu'à son état parfait, 1826.
(113) M. Rusconi. Histoire naturelle, développement et métamorphose
de la Salamandre terrestre, 1854.
(114) W. B. Scott and H. F. Osborn. “On the early development of the
common Newt.” Quart. J. of Micr. Science, Vol. XXIX. 1879.
(115) S. Stricker. “Entwicklungsgeschichte von Bufo cinereus.” Sitzb.
der kaiserl. Acad. zu Wien, 1860.
(116) S. Stricker. “Untersuchungen über die ersten Anlagen in
Batrachier-Eiern.” Zeitschrift f. wiss. Zoologie, Bd. XI. 1861.
I. Anura.
Aglossa.
Phaneroglossa.
II. Urodela.
Perennibranchiata
Trachystomata.
Proteidæ.
Caducibranchiata
Amphiumidæ.
Menopomidæ.
Myctodera
Amblystomidæ.
Salamandridæ.
III. Gymnophiona.
[Pg 145]
CHAPTER VIII.
AVES.
Introduction.
The variations in the character of the embryonic development of the
Amniota are far less important than in the case of the Ichthyopsida.
There are, it is true, some very special features in the early
developmental history of the Mammalia, but apart from these there is
such a striking uniformity in the embryos of all the groups that it
would, in many cases, be difficult to assign a young embryo to its
proper class.
Amongst the Sauropsida the Aves have for obvious reasons received a
far fuller share of attention than any other group; and an account of
their embryology forms a suitable introduction to this part of our
subject. For the convenience of the student many parts of their
developmental history will be dealt with at greater length than in the
case of the previous groups.
The development of the Aves.
Comparatively few types of Birds have been studied embryologically.
The common Fowl has received a disproportionately large share of
attention; although within quite recent times the[Pg 146] Duck, the Goose, the
Pigeon, the Starling, and a Parrot (Melopsittacus undulatus) have also
been studied. The result of these investigations has been to shew that
the variations in the early development of different Birds are
comparatively unimportant. In the sequel the common Fowl will be
employed as type, attention being called when necessary to the
development of the other forms.

Fig. 85. Yolk elements from the egg of the Fowl.
A. Yellow yolk. B. White yolk.
The ovum of the Fowl, at the time when it is clasped by the expanded
extremity of the oviduct, is a large yellow body enclosed in a
vitelline membrane. It is mainly formed of spherules of food-yolk. Of
these there are two varieties; one known as yellow yolk, and the other
as white. The white yolk spherules form a small mass at the centre of
the ovum, which is continued to the surface by a narrow stalk, and
there expands into a somewhat funnel-shaped disc, the edges of which
are continued over the surface of the ovum as a delicate layer. The
major part of the ovum is formed of yellow yolk. The yellow yolk
consists of large delicate spheres, filled with small granules (fig.
85 A); while the white yolk is formed of vesicles of a smaller size
than the yellow yolk spheres, in which are a variable number of highly
refractive bodies (fig. 85 B).
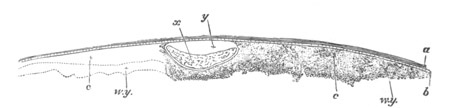
Fig. 86. Section through the germinal disc of the ripe ovarian ovum
of a Fowl while yet enclosed in its capsule.
a. Connective-tissue capsule of the ovum; b. epithelium of the
capsule, at the surface of which nearest the ovum lies the vitelline
membrane; c. granular material of the germinal disc, which becomes
converted into the blastoderm. (This is not very well represented in
the woodcut. In sections which have been hardened in chromic acid it
consists of fine granules.) w.y. white yolk, which passes
insensibly into the fine granular material of the disc; x.
germinal vesicle enclosed in a distinct membrane, but shrivelled up;
y. space originally completely filled up by the germinal vesicle,
before the latter was shrivelled up.
In addition to the yolk there is present in the ovum a small
protoplasmic region, containing the remains of the germinal vesicle,
which forms the germinal disc (fig. 86). It overlies the[Pg 147] funnel-shaped
disc of white yolk, into which it is continued without any marked line
of demarcation. It contains numerous minute spherules of the same
nature as the smallest white yolk spherules.
Impregnation takes place at the upper extremity of the oviduct.
In its passage outwards the ovum gradually receives its accessory
coverings in the form of albumen, shell-membrane, and shell (fig. 87).
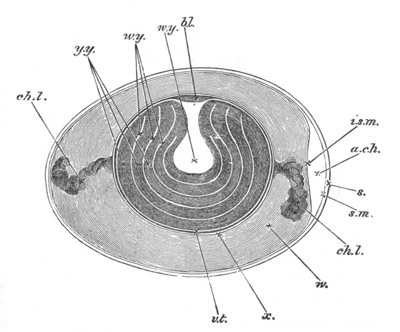
Fig. 87. Diagrammatic section of an unincubated Fowl’s egg.
bl. blastoderm; w.y. white yolk. This consists of a central
flask-shaped mass and a number of layers concentrically arranged
around it. y.y. yellow yolk; v.t. vitelline membrane; x. layer
of more fluid albumen immediately surrounding the yolk; w. albumen
consisting of alternate denser and more fluid layers; ch.l.
chalaza; a.ch. air-chamber at the broad end of the egg. This
chamber is merely a space left between the two layers of the
shell-membrane. i.s.m. internal layer of shell-membrane; s.m.
external layer of shell-membrane; s. shell.
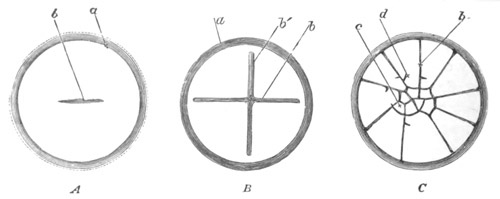
Fig 88. Surface views of the early stages of the segmentation
in a Fowl’s egg. (After Coste.)
a. edge of germinal disc; b. vertical furrow; c. small central
segment; d. larger peripheral segment.
Fig. 89. Surface view of the germinal disc
of Fowl’s egg during a late stage of the segmentation.
c. small central segmentation spheres; b. larger segments
outside these; a. large, imperfectly circumscribed, marginal
segments; e. margin of germinal disc.
The segmentation commences in the lower part of the oviduct, shortly
before the shell has begun to be formed. It is meroblastic, being
confined to the germinal disc, through the full depth of which however
the earlier furrows do not extend. It is mainly remarkable for being
constantly somewhat unsymmetrical (Kölliker)—a feature which is not
represented in fig. 88, copied from Coste. Owing to the absence of
symmetry the cells at one side of the germinal disc are larger than
those at the other, but the relations between the disc and the axis of
the[Pg 148] embryo are not known. During the later stages the segmentation is
irregular, and not confined to the surface; and towards its close the
germinal disc becomes somewhat lenticular in shape; and is formed of
segments, which are smallest in the centre and increase in size
towards the periphery (figs. 89 and 90). The superficial segments in
the centre of the germinal disc are moreover smaller than those below,
and more or less separated as a distinct layer (fig. 90). As
development proceeds the segmentation reaches its limits in the
centre, but continues at the periphery; and thus eventually the masses
at the periphery become of the same size as those at the centre. At
the time when the ovum is laid (fig. 91) the uppermost layer of
segments has given rise to a distinct membrane, the epiblast, formed
of a single row of columnar[Pg 149] cells (ep). The lower or hypoblast
segments are larger, in some cases very much larger, than those of the
epiblast, and are so granular that their nuclei can only with
difficulty be seen. They form a somewhat irregular mass, several
layers deep, and thicker at the periphery than at the centre: they
rest on a bed of white yolk, from which they are in parts separated by
a more or less developed cavity, which is probably filled with fluid
yolk matter about to be absorbed. In the bed of white yolk nuclei are
present, which are of the same character, and have the same general
fate, as those in Elasmobranchii. They are generally more numerous in
the neighbourhood of the thickened periphery of the blastoderm than
elsewhere. Peculiar large spherical bodies are to be found amongst the
lower layer cells, which superficially resemble the larger cells
around them, and have been called formative cells [vide Foster and
Balfour (No. 126)]. Their real nature is still very doubtful, and
though some are no doubt true cells, others are perhaps only nutritive
masses of yolk. In a surface view the blastoderm, as the segmented
germinal disc may[Pg 150] now be called, appears as a circular disc; the
central part of which is distinguished from the peripheral by its
greater transparency, and forms what is known in the later stages as
the area pellucida. The narrow darker ring of blastoderm, outside the
area pellucida, is the commencing area opaca.
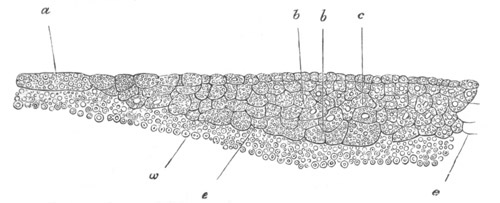
Fig. 90. Section of the germinal disc of a Fowl during the later
stages of segmentation.
The section, which represents rather more than half the breadth of
the blastoderm (the middle line being shewn at c), shews that the
upper and central parts of the disc segment faster than those below
and towards the periphery. At the periphery the segments are still
very large. One of the larger segments is shewn at a. In the
majority of segments a nucleus can be seen; and it seems probable
that the nucleus is present in them all. Most of the segments are
filled with highly refracting spherules, but these are more numerous
in some cells (especially the larger cells near the yolk) than in
others. In the central part of the blastoderm the upper cells have
commenced to form a distinct layer. No segmentation cavity is
present.
a. large peripheral cell; b. larger cells of the lower parts of
the blastoderm; c. middle line of blastoderm; e. edge of the
blastoderm adjoining the white yolk; w. white yolk.
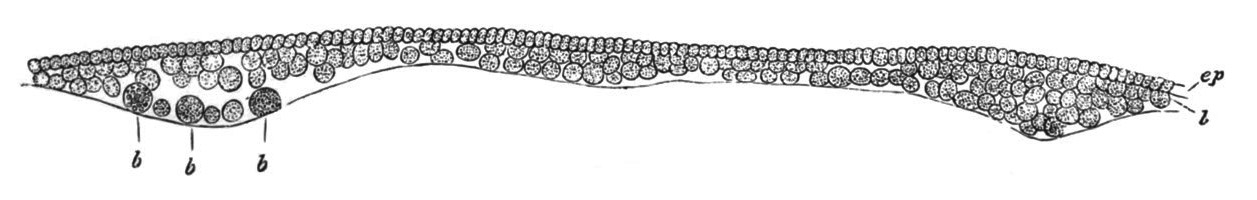
Fig. 91. Section of a blastoderm of a Fowl’s egg at
the commencement of incubation.
The thin epiblast ep composed of columnar cells rests on the
incomplete lower layer l, composed of larger and more granular
hypoblast cells. The lower layer is thicker in some places than in
others, and is especially thick at the periphery. The line below the
under layer marks the upper surface of the white yolk. The larger
so-called formative cells are seen at b, lying on the white yolk.
The figure does not take in quite the whole breadth of the
blastoderm; but the reader must understand that both to the right
hand and to the left ep is continued farther than l, so that at
the extreme edge it rests directly on the white yolk.
As a result of incubation the blastoderm undergoes a series of
changes, which end in the definite formation of three germinal layers,
and in the establishment of the chief systems of organs of the embryo.
The more important of these changes are accomplished in the case of
the common Fowl during the first day and the early part of the second
day of incubation.
There is hardly any question in development which has been the subject
of so much controversy as the mode of formation of the germinal layers
in the common Fowl. The differences in the views of authors have been
caused to a large extent by the difficulties of the investigation, but
perhaps still more by the fact that many of the observations were made
at a time when the methods of making sections were very inferior to
those of the present day. The subject itself is by no means of an
importance commensurate with the attention it has received. The
characters which belong to the formation of the layers in the
Sauropsida are secondarily derived from those in the Ichthyopsida, and
are of but little importance for the general questions which concern
the nature and origin of the germinal layers. In the account in the
sequel I have avoided as much as possible discussion of controverted
points. My statements are founded in the main on my own observations,
more especially on a recent investigation carried on in conjunction
with my pupil, Mr Deighton. It is to Kölliker (No. 135), and to Gasser
(No. 127) that the most important of the more recent advances in our
knowledge are due. Kölliker,[Pg 151] in his great work on Embryology,
definitely established the essential connection between the primitive
streak and the formation of the mesoblast; but while confirming his
statement on this head, I am obliged to differ from him with reference
to some other points.
Gasser’s work, especially that part of it which relates to the
passages leading from the neural to the alimentary canal, which he was
the first to discover, is very valuable.
The blastoderm gradually grows in size, and extends itself over the
yolk; the growth over the yolk being very largely effected by an
increase in the size of the area opaca, which during this process
becomes more distinctly marked off from the area pellucida. The area
pellucida gradually assumes an oval form, and at the same time becomes
divided into a posterior opaque region and an anterior transparent
region. The posterior opacity is named by some authors the embryonic
shield.
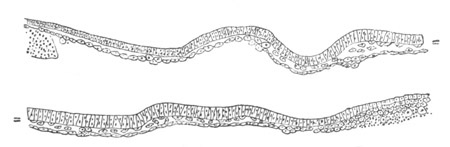
Fig. 92. Transverse section through the blastoderm of a Chick
before the appearance of the primitive streak.
The epiblast is represented somewhat diagrammatically. The hyphens
shew the points of junction of the two halves of the section.
During these changes the epiblast (fig. 92) becomes two layers deep
over the greater part of the area pellucida, though still only one
cell deep in the area opaca. The irregular hypoblast spheres of the
unincubated blastoderm flatten themselves out, and unite into a
definite hypoblastic membrane (fig. 92). Between this membrane and the
epiblast there remain a number of scattered cells (fig. 92) which
cannot however be said to form a definite layer altogether distinct
from the hypoblast. They are almost entirely confined to the posterior
part of the area pellucida, and give rise to the opacity of that part.
At the edge of the area pellucida the hypoblast becomes continuous
with a thickened rim of material, underlying the epiblast, and derived
from the original thickened edge of the blastoderm and the subjacent
yolk. It is mainly formed of yolk granules,[Pg 152] with a varying number of
cells and nuclei imbedded in it. It is known as the germinal wall, and
is spoken of more in detail on pp. 160 and 161.
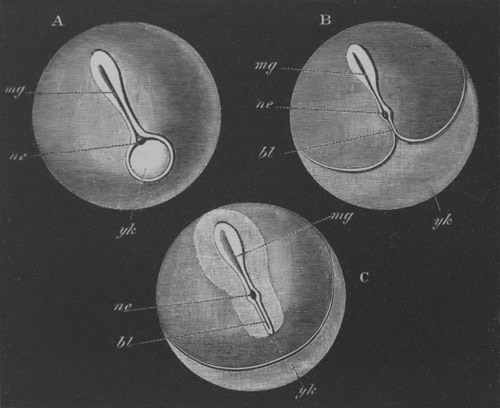
Fig. 93. Diagrams illustrating the position of the blastopore, and
the relation of the embryo to the yolk in various meroblastic vertebrate
ova.
A. Type of Frog. B. Elasmobranch type. C. Amniotic Vertebrate.
mg. medullary plate; ne. neurenteric canal; bl. portion of
blastopore adjoining the neurenteric canal. In B this part of the
blastopore is formed by the edges of the blastoderm meeting and
forming a linear streak behind the embryo; and in C it forms the
structure known as the primitive streak. yk. part of yolk not yet
enclosed by the blastoderm.
The changes which next take place result in the complete
differentiation of the embryonic layers, a process which is intimately
connected with the formation of the structure known as the primitive
streak. The meaning of the latter structure, and its relation to the
embryo, can only be understood by comparison with the development of
the forms already considered. The most striking peculiarity in the
first formation of the embryo Bird, as also in that of the embryos of
all Amniota, consists in the fact that they do not occupy a position
at the edge[Pg 153] of the blastoderm, but are placed near its centre.
Behind the embryo there is however a peculiar structure—the primitive
streak above mentioned—which is a linear body placed in the posterior
region of the blastoderm. This body, the nature of which will be more
fully explained in the chapter on the comparative development of
Vertebrates, is really a rudimentary part of the blastopore, of the
same nature as the linear streak behind the embryo in Elasmobranchii
formed by the concrescence of the edges of the blastoderm (vide p.
64); although there is no ontogenetic process in the Amniota, like the
concrescence in Elasmobranchii. The relations of the blastopore in
Elasmobranchii and Aves is shewn in figs. B and C of the diagram (fig.
93).
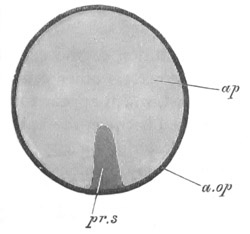
Fig. 94. Area pellucida of
a very young blastoderm of
a chick, shewing the primitive
streak at its first appearance.
pr.s. primitive streak; ap. area pellucida; a.op. area opaca.
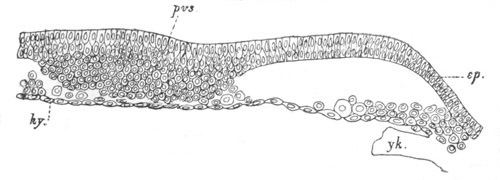
Fig. 95. Transverse section through a blastoderm of about the age
represented in fig. 94, shewing the first differentiation of the primitive
streak.
The section passes through about the middle of the primitive streak.
pvs. primitive streak; ep. epiblast; hy. hypoblast; yk. yolk
of the germinal wall.
In describing in detail the succeeding changes we may at first confine
our attention to the area pellucida. As this gradually assumes an oval
form the posterior opacity becomes replaced by a very dark median
streak, which extends forwards some distance from the posterior border
of the area (fig. 94). This is the first rudiment of the primitive
streak. In the[Pg 154] region in front of it the blastoderm is still formed of
two layers only, but in the region of the streak itself the structure
of the blastoderm is greatly altered. The most important features in
it are represented in fig. 95. This figure shews that the median
portion of the blastoderm has become very much thickened (thus
producing the opacity of the primitive streak), and that this
thickening is caused by a proliferation of rounded cells from the
epiblast. In the very young primitive streak, of which fig. 95 is a
section, the rounded cells are still continuous throughout with the
epiblast, but they form nevertheless the rudiment of the greater part
of a sheet of mesoblast, which will soon arise in this region.
In addition to the cells clearly derived from the epiblast, there are
certain other cells (vide fig. 95), closely adjoining the hypoblast,
which appear to me to be the derivatives of the cells interposed
between the epiblast and hypoblast, which gave rise to the posterior
opacity in the blastoderm during the previous stage. In my opinion
these cells also have a share in forming the future mesoblast.
The number and distribution of these cells is subject to not
inconsiderable variations. In a fair number of cases they are entirely
congregated along the line of the primitive streak, leaving the sides
of the blastoderm quite free. They then form a layer, which can only
with difficulty be distinguished from the cells derived from the
epiblast by slight peculiarities of staining, and by the presence of a
considerable proportion of large granular cells. It is, I believe, by
the study of such blastoderms that Kölliker has been led to deny to
the intermediate cells of the previous stage any share in the
formation of the mesoblast. In other instances, of which fig. 95 is a
fairly typical example, they are more widely scattered. To follow with
absolute certainty the history of these cells, and to prove that they
join the mesoblast is not, I believe, possible by means of sections,
and I must leave the reader to judge how far the evidence given in the
sequel is sufficient to justify my opinions on this subject.
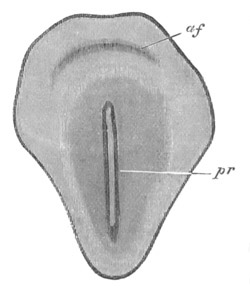
Fig. 96. Surface view of
the area pellucida of a chick’s
blastoderm shortly after the
formation of the primitive
groove.
pr. primitive streak with primitive groove; af. amniotic fold.
The darker shading round the primitive streak shews the extension of
the mesoblast.
[Pg 155]
In the course of further growth the area pellucida soon becomes
pyriform, the narrower extremity being the posterior. The primitive
streak (fig. 96) elongates considerably, so as to occupy about
two-thirds of the length of the area pellucida; but its hinder end in
many instances does not extend to the posterior border of the area
pellucida. The median line of the primitive streak becomes marked by a
shallow groove, known as the primitive groove.
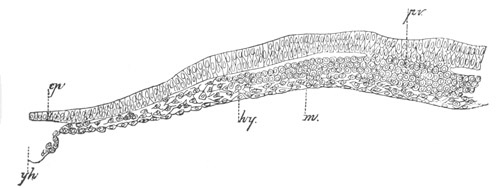
Fig. 97. Transverse section through the front end of the primitive
streak of a blastoderm of the same age as fig. 96.
pv. primitive groove; m. mesoblast; ep. epiblast; hy.
hypoblast; yh. yolk of germinal wall.
During these changes in external appearance there grow from the sides
of the primitive streak two lateral wings of mesoblast cells, which
gradually extend till they reach the sides of the area pellucida (fig.
97). The mesoblast still remains attached to the epiblast along the
line of the primitive streak. During this extension many sections
through the primitive streak give an impression of the mesoblast being
involuted at the lips of a fold, and so support the view above
propounded, that the primitive streak is the rudiment of the coalesced
lips of the blastopore. The hypoblast below the primitive streak is
always quite independent of the mesoblast above, though much more
closely attached to it in the median line than at the sides. The part
of the mesoblast, which I believe to be derived from the primitive
hypoblast, can generally be distinctly traced. In many cases,
especially at the front end of the primitive streak, it forms, as in
fig. 97, a distinct layer of stellate cells, quite unlike the[Pg 156] rounded
cells of the mesoblastic involution of the primitive streak.
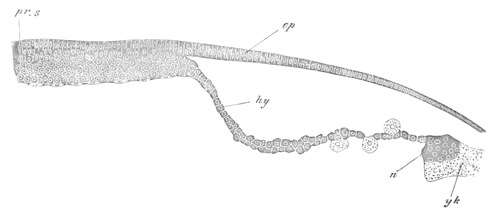
Fig. 98. Longitudinal section through the axial line of the
primitive streak, and the part of the blastoderm in front of it, of
an embryo chick somewhat younger than fig. 99.
pr.s. primitive streak; ep. epiblast; hy. hypoblast of region
in front of primitive streak; n. nuclei; yk. yolk of germinal
wall.
In the region in front of the primitive streak, where the first trace
of the embryo will shortly appear, the layers at first undergo no
important changes, except that the hypoblast becomes somewhat thicker.
Soon, however, as shewn in longitudinal section in fig. 98, the
hypoblast along the axial line becomes continuous behind with the
front end of the primitive streak. Thus at this point, which is the
future hind end of the embryo, the mesoblast, the epiblast, and the
hypoblast all unite together; just as they do in all the types of
Ichthyopsida.
Shortly afterwards, at a slightly later stage than that represented in
fig. 96, an important change takes place in the constitution of the
hypoblast in front of the primitive streak. The rounded cells, of
which it is at first composed (fig. 98), break up into (1) a layer
formed of a single row of more or less flattened elements below—the
hypoblast—and (2) into a layer formed of several rows of stellate
elements, between the hypoblast and the epiblast—the mesoblast (fig.
99). A separation between these two layers is at first hardly
apparent, and before it has become at all well marked, especially in
the median line, an axial opaque line makes its appearance in surface
views, continued forwards[Pg 157] from the front end of the primitive streak,
but stopping short at a semicircular fold—the future head-fold—near
the front end of the area pellucida. In section (fig. 100) this opaque
line is seen to be due to a special concentration of cells in the form
of a cord. This cord is the commencement of the notochord (ch). In
some instances the commencing notochord remains attached to the
hypoblast, while the mesoblast is laterally quite distinct (vide
fig. 100), and is therefore formed in the same manner as in most
Ichthyopsida; while in other instances, and always apparently in the
Goose (Gasser, No. 127), the notochord appears to become
differentiated in the already separated layer of mesoblast. In all
cases the notochord and the hypoblast below it unite with the front
end of the primitive streak; with which also the two lateral plates
of mesoblast become continuous.
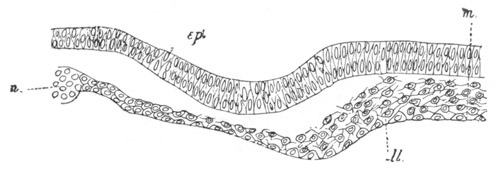
Fig. 99. Transverse section through the embryonic region of the
blastoderm of a Chick shortly prior to the formation of the medullary
groove and notochord.
m. median line of the section; ep. epiblast; ll. lower layer
cells (primitive hypoblast) not yet completely differentiated into
mesoblast and hypoblast; n. nuclei of germinal wall.
From what has just been said it is clear that in the region of the
embryo the mesoblast originates as two lateral plates split off from
the hypoblast, and that the notochord originates as a median plate,
simultaneously with the mesoblast, with which it may sometimes be at
first continuous.
Kölliker holds that the mesoblast of the region of the embryo is
derived from a forward growth from the primitive streak. There is no
theoretical objection to this view, and I think it would be impossible
to shew for certain by sections whether or not there is a growth such
as he describes; but such sections as that represented in fig. 99 (and
I have series of similar sections from several embryos) appear to me
to be conclusive in favour of the view that the mesoblast of the
region of the embryo is to a large extent derived[Pg 158] from a
differentiation of the primitive hypoblast. I am however inclined to
believe that some of the mesoblast cells of the embryonic region have
the derivation which Kölliker ascribes to all of them.
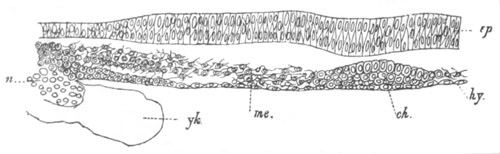
Fig. 100. Transverse section through the embryonic region of the
blastoderm of a Chick at the time of the formation of the notochord,
but before the appearance of the medullary groove.
ep. epiblast; hy. hypoblast; ch. notochord; me. mesoblast;
n. nuclei of the germinal wall yk. yolk.
As regards the mesoblast of the primitive streak, in a purely
objective description like that given above, the greater part of it
may fairly be described as being derived from the epiblast. But if it
is granted that the primitive streak corresponds with the blastopore,
it is obvious to the comparative embryologist that the mesoblast
derived from it really originates from the lips of the blastopore, as
in so many other cases; and that to describe it, without explanation,
as arising from the epiblast, would give an erroneous impression of
the real nature of the process.

Fig. 101. Transverse section of a blastoderm incubated for 18 hours.
The section passes through the medullary groove mc., at some
distance behind its front end.
A. epiblast. B. mesoblast. C. hypoblast.
m.c. medullary groove; m.f. medullary fold; ch. notochord.
The differentiation of the embryo may be said to commence with the
formation of the notochord and the lateral plates of mesoblast. Very
shortly after the formation of these structures the axial part of the
epiblast, above the notochord and in front of the primitive streak,
which is somewhat thicker than[Pg 159] the lateral parts, becomes
differentiated into a distinct medullary plate, the sides of which
form two folds—the medullary folds—enclosing between them a
medullary groove (fig. 101).
In front the two medullary folds meet, while posteriorly they thin out
and envelop between them the front end of the primitive streak. On the
formation of the medullary folds the embryo assumes a form not unlike
that of the embryos of many Ichthyopsida at a corresponding stage. The
appearance of the embryo, and its relation to the surrounding parts is
somewhat diagrammatically represented in fig. 102. The primitive
streak now ends with an anterior swelling (not represented in the
figure), and is usually somewhat unsymmetrical. In most cases its axis
is more nearly continuous with the left, or sometimes the right,
medullary fold than with the medullary groove. In sections its front
end appears as a ridge on one side or on the middle of the floor of
the widened end of the medullary groove.
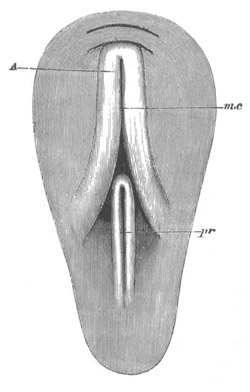
Fig. 102. Surface view of the
pellucid area of a blastoderm of 18
hours.
None of the opaque area is shewn, the pear-shaped outline indicating
the limits of the pellucid area.
At the hinder part of the area is seen the primitive groove pr.,
with its nearly parallel walls, fading away behind, but curving
round and meeting in front so as to form a distinct anterior
termination to the groove, about halfway up the pellucid area.
Above the primitive groove is seen the medullary groove m.c., with
the medullary folds A. These, diverging behind, slope away on
either side of the primitive groove, while in front they curve round
and meet each other close upon a curved line which represents the
head-fold.
The second curved line in front of and concentric with the first is
the commencing fold of the amnion.
The mesoblast and hypoblast, within the area pellucida, do not give
rise to the whole of these two layers in the surrounding area opaca;
but the whole of the hypoblast of the area opaca, and a large portion
of the mesoblast, and possibly even some of the epiblast, take their
origin from the peculiar material already spoken of, which forms the
germinal wall, and is continuous with[Pg 160] the hypoblast at the edge of the
area opaca (vide figs. 91, 94, 97, 98, 99, 100).
The exact nature of this material has been the subject of many
controversies. Into these controversies it is not my purpose to enter,
but subjoined are the results of my own examination. The germinal wall
first consists, as already mentioned, of the lower cells of the
thickened edge of the blastoderm, and of the subjacent yolk material
with nuclei. During the period before the formation of the primitive
streak the epiblast extends itself over the yolk, partly, it appears,
at the expense of the cells of the germinal wall, and possibly even of
cells formed around the nuclei in this part. This mode of growth of
the epiblast is very similar to that in the epibolic gastrulas of many
Invertebrata, of the Lamprey, etc.; but how far this process is
continued in the subsequent extension of the epiblast I am unable to
say. The cells of the germinal wall, which are at first well separated
from the yolk below, become gradually absorbed in the growth of the
hypoblast, and the remaining cells and yolk then become mingled
together, and constitute a compound structure, continuous at its inner
border with the hypoblast. This structure is the germinal wall usually
so described. It is mainly formed of yolk granules with numerous
nuclei, and a somewhat variable number of largish cells imbedded
amongst them. The nuclei typically form a special layer immediately
below the epiblast, some of which are probably enclosed by a definite
cell-body. A special mass of nuclei (vide figs. 98 and 100, n) is
usually present at the junction of the hypoblast with the germinal
wall.
The germinal wall at this stage corresponds in many respects with the
granular material, forming a ring below the edge of the blastoderm in
Teleostei.
It retains the characters above enumerated till near the close of the
first day of incubation, i.e. till several mesoblastic somites have
become established. It then becomes more distinctly separated from the
subjacent yolk, and its component parts change very considerably in
character. The whole wall becomes much less granular. It is then
mainly formed of large vesicles, which often assume a palisade-like
arrangement, and contain granular balls, spherules of white yolk, and
in an early stage a good deal of granular matter (vide fig. 115).
These bodies have some resemblance to cells, and have been regarded as
such by Kölliker (No. 135) and Virchow (No. 150): they contain however
nothing which can be considered as a nucleus. Between them however
nuclei[62]
may easily be seen in specimens hardened in picric acid,
and stained with hæmatoxylin (these nuclei are not shewn in fig. 115).
These nuclei are about the same size as those of the hypoblast cells,
and are surrounded by a thin layer of granular protoplasm,[Pg 161] which is
continuous with a mesh-work of granular protoplasm enveloping the
above described vesicles. The germinal wall is still continuous with
the hypoblast at its edge; and close to the junction of the two the
hypoblast at first forms a layer of moderately columnar cells, one or
two deep and directly continuous with the germinal wall, and at a
later period usually consists of a mass of rounder cells lying above
the somewhat abrupt inner edge of the germinal wall.
The germinal wall certainly gives rise to the hypoblast cells, which
mainly grow at its expense. They arise at the edge of the area
pellucida, and when first formed are markedly columnar, and enclose in
their protoplasm one of the smaller vesicles of the germinal wall.
In the later stages (fourth day and onwards) the whole germinal wall
is stated to break up into columnar hypoblast cells, each of them
mainly formed of one of the vesicles just spoken of. After the
commencing formation of the embryo the mesoblast becomes established
at the inner edge of the area opaca, between the germinal wall and the
epiblast; and gives rise to the tissue which eventually forms the area
vasculosa. It seems probable that the mesoblast in this situation is
mainly derived from cells formed around the nuclei of the germinal
wall, which are usually specially aggregated close below the epiblast.
Disse (No. 122) has especially brought evidence in favour of this
view, and my own observations also support it.
The mesoblastic somites begin to be formed in the lateral plates of
the mesoblast before the closure of the medullary folds. The first
somite arises close to the foremost extremity of the primitive streak,
but the next is stated to arise in front of this, so that the first
formed somite corresponds to the second permanent vertebra[63].
The
region of the embryo in front of the second formed somite—at first
the largest part of the embryo—is the cephalic region. The somites
following the second are formed in the regular manner, from before
backwards, out of the unsegmented posterior part of the embryo, which
rapidly grows in length to supply the necessary material (fig. 103).
As the somites retain during the early stages of development an
approximately constant breadth, their number is a fair test of the
length of the trunk. With the growth of the embryo the primitive
streak is continually carried back, the lengthening of the embryo
always taking place between the front end of the primitive streak and
the last somite; and during this[Pg 162] process the primitive streak
undergoes important changes both in itself and in its relation to the
embryo. Its anterior thicker part, which is enveloped in the diverging
medullary folds, soon becomes distinguished in structure from the part
behind this, and placed symmetrically in relation to the axis of the
embryo (fig. 103, a.pr), and at the same time the medullary folds,
which at first simply diverge on each side of the primitive streak,
bend in again and meet behind so as completely to enclose the front
part of the primitive streak. The region of the embryo bird, where the
medullary folds diverge, is known as the sinus rhomboidalis, though it
has no connection with the similarly named structure in the adult. By
the time that ten somites are formed the sinus rhomboidalis is
completely established, and the medullary groove has become converted
into a tube till close up to the front end of the sinus. In the
following stages the closure of the medullary canal extends to the
sinus rhomboidalis, and the folding off of the hind end of the embryo
from the yolk commences. Coincidently with the last-named changes the
sides of the front part of the primitive streak become thickened, and
give rise to conspicuous caudal swellings; in which the layers of the
embryo are indistinguishably fused. The apparently hinder part of the
primitive streak becomes, as more particularly explained in the
sequel, folded downwards and forwards on the ventral side.
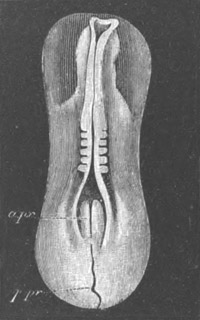
Fig. 103. Dorsal view of the hardened blastoderm
of a Chick with five mesoblastic somites. The medullary folds have met
for part of their extent, but have not united.
a.pr. anterior part of the primitive streak; p.pr. posterior
part of the primitive streak.
This is a convenient place to notice remarkable appearances which
present themselves close to the junction of the neural plate and the
primitive streak. These are temporary passages leading from the hinder
end of the neural tube into the alimentary canal. They vary somewhat
in different species of birds, and it appears that in the same species
there may be several openings of the kind, which appear one after the
other and then[Pg 163] close again. They were first discovered by Gasser (No.
127). In all cases[64]
they lead round the posterior end of the
notochord, or through the point where the notochord falls into the
primitive streak.
If the primitive streak is, as I believe, formed of the lips of the
blastopore, there can be but little doubt that these structures are
disappearing, and functionless rudiments of the opening of the
blastopore, and they thus lend support to my view as to the nature of
the primitive streak. That, in part, they correspond with the
neurenteric canal of the Ichthyopsida is clear from the detailed
statements below. Till their relations have been more fully worked out
it is not possible to give a more definite explanation of them.
According to Braun (No. 120) three independent communications are to
be distinguished in Birds. These are best developed in the Duck. The
first of these is a small funnel-shaped diverticulum leading from the
neural groove through the hypoblast. It is visible when eight
mesoblastic somites are present, and soon disappears. The second,
which is the only one I have myself investigated, is present in the
embryo duck with twenty-six mesoblastic somites, and is represented in
the series of sections (fig. 104). The passage leads obliquely
backwards and ventralwards from the hind end of the neural tube into
the notochord, where the latter joins the primitive streak (B). A
narrow diverticulum from this passage is continued forwards for a
short distance along the axis of the notochord (A, ch). After
traversing the notochord, the passage is continued into a hypoblastic
diverticulum, which opens ventrally into the future lumen of the
alimentary tract (C). Shortly behind the point where the neurenteric
passage communicates with the neural tube the latter structure opens
dorsally, and a groove on the surface of the primitive streak is
continued backwards from it for a short distance (C). The first part
of this passage to appear is the hypoblastic diverticulum above
mentioned.
This passage does not long remain open, but after its closure, when
the tail-end of the embryo has become folded off from the yolk, a
third passage is established, and leads round the end of the notochord
from the closed medullary canal into the postanal gut. It is shewn
diagrammatically in fig. 106, ne, and, as may be gathered from that
figure, has the same relations as the neurenteric canal of the
Ichthyopsida.
In the goose a passage has been described by Gasser, which appears
when about fourteen or fifteen somites are present, and lasts till
twenty-three are formed. Behind its opening the medullary canal is
continued back as a small diverticulum, which follows the course of
the primitive groove and is apparently formed by the conversion of
this groove into a canal. It is at first open to the exterior, but
soon becomes closed, and then atrophies.
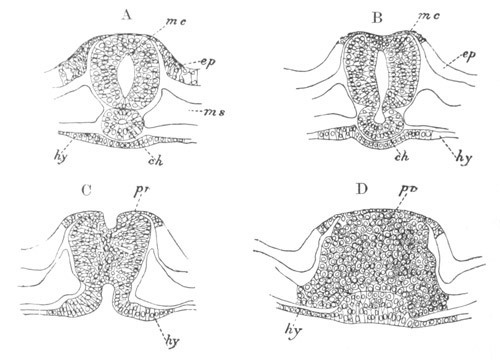
Fig. 104. Four transverse sections through the neurenteric passage
and adjoining parts in a Duck embryo with twenty-six mesoblastic
somites.
A. Section in front of the neurenteric canal shewing a lumen in the
notochord.
B. Section through the passage from the medullary canal into the
notochord.
C. Section shewing the hypoblastic opening of the neurenteric canal,
and the groove on the surface of the primitive streak, which opens
in front into the medullary canal.
D. Primitive streak immediately behind the opening of the
neurenteric passage.
mc. medullary canal; ep. epiblast; hy. hypoblast; ch.
notochord; pr. primitive streak.
In the chick there is a perforation on the floor of the neural canal,
[Pg 164]
which is not so marked as those in the goose or duck, and never
results in a complete contin164uity between the neural and alimentary
tracts; but simply leads from the floor of the neural canal into the
tissues of the tail swelling, and thence into a cavity in the
posterior part of the notochord. The hinder diverticulum of the neural
canal along the line of the primitive groove is, moreover, very
considerable in the chick, and is not so soon obliterated as in the
goose. The incomplete passage in the chick arises when about twelve
somites are present. It is regarded by Braun as equivalent to the
first formed passage in the duck, but I very much doubt whether there
is a very exact equivalence between the openings in different types,
and think it more probable that they are variable remnants of a
primitive neurenteric canal, which in the ancestors of those forms
persisted through the whole period of the early development. The third
passage is formed in the chick (Kupffer) during the third day of
incubation. In[Pg 165]
Melopsittacus undulatus the two first communications
are stated by Braun (No. 120) to be present at the same time, the one
in front of the other.
It is probable, from the above description, that the front portion of
the primitive streak in the bird corresponds with that part of the
lips of the blastopore in Elasmobranchii which becomes converted into
the tail swelling and the lining of the neurentic canal; while the
original groove of the front part of the primitive streak appears to
be converted into the posterior diverticulum of the neural canal. The
hinder part of the primitive streak of the bird corresponds, in a very
general way, with the part of the blastopore in Elasmobranchii, which
shuts off the embryo from the edge of the blastoderm (vide p. 64),
though there is of course no genetic relation between the two
structures. When the anterior part of the streak is becoming converted
into the tail swelling, the groove of the posterior part gradually
shallows and finally disappears. The hinder part itself atrophies from
behind forwards, and in the course of the folding off of the embryo
from the yolk the part of the blastoderm where it was placed becomes
folded in, so as to form part of the ventral wall of the embryo. The
apparent hinder part of the primitive streak is therefore in reality
the ventral and anterior part[65].
It has generally been maintained that the primitive streak and groove
become wholly converted into the dorsal portion of the trunk of the
embryo, i.e. into the posterior part of the medullary plate and
subjacent structures. This view appears to me untenable in itself, and
quite incompatible with the interpretation of the primitive streak
given above. To shew how improbable it is, apart from any theoretical
considerations, I have compiled two tables of the relative lengths of
the primitive streak and the body of the embryo, measured by the
number of sections made through them, in a series of examples from the
data in Gasser’s important memoir (No. 127). In these tables each
horizontal line relates to a single embryo. The first column shews the
number of somites, and the second the number of sections[Pg 166] through the
primitive streak. Where the primitive streak becomes divided into two
parts the sections through the two parts are given separately: the
left column (A) referring to the anterior part of the streak; the
right column (P) to the posterior part. The third column gives the
number of sections through the embryo. The first table is for fowl
embryos, the second for goose embryos.
| No. of Somites. |
No. of sections through the Primitive
Streak. |
No. of sections through the Embryo. |
|---|
| 0 | 29 | 7 |
| 0 | 45 | 10 |
| 0 | 39 | 23 |
| 2 | 30 | 30 |
| 4 | 30 | 30 |
| A P | |
| 5 or 6 | 10 + 17 = 27 | |
| 8 | 12 + 20 = 32 | 48 |
| 12 | 13 + 10 = 23 | |
| 14 | 9 + 12 = 21 | |
| 18 | 10 + 7 = 17 | 70 |
| 8 + 4 = 12 | |
| 8 + 3 = 11 | |
| No. of Somites. | No. of sections
through the
Primitive
Streak. |
No. of sections
through the
Embryo. |
|---|
| 0 | 10 | 4 |
| 0 | 28 | 5 |
| 0 | 44 | 12 |
| 2 | 36 | 32 |
| 4 | 24 | 42 |
| A P | |
| 9 | 10 + 10 = 20 | 61 |
| 14 | 8 + 10 = 18 | 68 |
| 17 | 8 + 5 = 13 | |
| 22 | 9 + 6 = 15 | |
| 26 | 6 + 5 = 11 | |
An inspection of these two tables shews that an actual diminution in
the length of the primitive streak takes place just about the time
when the first somites are being formed, but there is no ground for
thinking that the primitive streak becomes then converted into the
medullary plate. Subsequently the primitive streak does not for a
considerable time become markedly shorter, and certainly its
curtailment is not really sufficient to account for the increased
length of the embryo—an increase in length, which (with the exception
of the head) takes place entirely by additions at the hind end. At the
stage with fourteen somites the primitive streak is still pretty long.
In the later stages, as is clearly demonstrated by the tables, the
diminution in the length of the primitive streak mainly concerns the
posterior part and not that adjoining the embryo.
General history of the germinal layers.
The epiblast. The epiblast of the body of the embryo, though several
rows of cells deep, does not become divided into two strata till late
in embryonic life; so that the organs of sense formed from the
epiblast, which are the same as in the types already described, are
not specially formed from an inner nervous stratum. The medullary
canal is closed in the same[Pg 167] manner as in Elasmobranchii, the Frog,
etc., by the simple conversion of an open groove into a closed canal.
The closure commences first of all in the region of the mid-brain, and
extends rapidly backwards and more slowly forwards. It is completed in
the Fowl by about the time that twelve mesoblastic somites are formed.
The mesoblast. The general changes of this layer do not exhibit any
features of special interest—the division into lateral and vertebral
plates, etc., being nearly the same as in the lower forms.
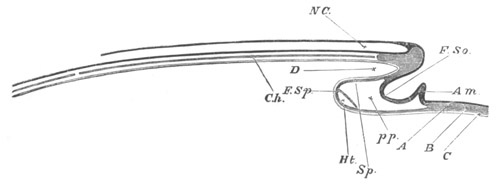
Fig. 105. Diagrammatic longitudinal section through the axis of an
Embryo Bird.
The section is supposed to be made at a time when the head-fold has
commenced but the tail-fold has not yet appeared.
F.So. head-fold of the somatopleure. F.Sp. head-fold of the
splanchnopleure.
pp. pleuroperitoneal cavity; Am. commencing (head-) fold of the
amnion; D. alimentary tract; N.C. neural canal; Ch. notochord;
A. epiblast; B. mesoblast; C. hypoblast.
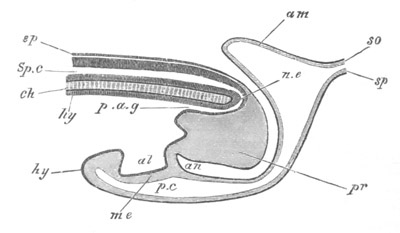
Fig. 106. Diagrammatic longitudinal section through the posterior
end of an Embryo Bird at the time of the formation of the allantois.
ep. epiblast; Sp.c. spinal canal; ch. notochord; n.e.
neurenteric canal; hy. hypoblast; p.a.g. postanal gut; pr.
remains of primitive streak folded in on the ventral side; al.
allantois; me. mesoblast; an. point where anus will be formed;
p.c. perivisceral cavity; am. amnion; so. somatopleure; sp.
splanchnopleure.
The hypoblast. The closure of the alimentary canal is entirely
effected by a process of tucking in or folding off of the embryo from
the yolk-sack. The general nature of the process is seen in the
diagrams figs. 105 and 121. The folds by which it is effected are
usually distinguished as the head-, the tail- and the lateral folds.
The head-fold (fig. 105) is the first to appear; and in combination
with the lateral folds gives rise to the anterior part of the
mesenteron (D) (including the œsophagus, stomach and duodenum),
which by its mode of formation clearly ends blindly in front. The
tail-fold, in combination with the two lateral folds, gives rise to
the hinder part of the alimentary tract, including the cloaca, which
is a true part of the mesenteron. At the junction between the two
folds there is present[Pg 168] a circular opening leading into the yolk-sack,
which becomes gradually narrowed as development proceeds. The opening
is completely closed long before the embryo is hatched. Certain
peculiarities in reference to the structure of the tail-fold are
caused by the formation of the allantois, and are described with the
embryonic appendages. The stomodæum and proctodæum are formed by
epiblastic invaginations. The communication between the stomodæum and
the mesenteron is effected comparatively early (on the 4th day in the
chick), while that between the proctodæum and mesenteron does not take
place till very late (15th day in the chick). The proctodæum gives
rise to the bursa Fabricii, as well as to the anus. Although the
opening of the anus is so late in being formed, the proctodæum itself
is very early apparent. Soon after the hinder part of the primitive
streak becomes tucked in on the ventral side of the embryo, an
invagination may be noticed where the tail of the embryo is folded
off. This gradually becomes deeper, and finally comes into contact
with the hypoblast at the front (primitively the apparent hind) border
of the posterior section of the primitive streak. An early stage in
the invagination is shewn in the diagram (fig. 106, an). It deserves
to be noted that the anus lies some way in front of the blind end of
[Pg 169]
the mesenteron, so that there is in fact a well-developed postanal
section of the gut (fig. 106, p.a.g), which corresponds with that in
the Ichthyopsida. For a short period, as mentioned above (p. 163), a
neurenteric canal is present connecting the postanal gut with the
medullary tube in the duck, fowl, and other birds. On the ventral wall
of the postanal gut there are at first two prominences. The posterior
of these is formed of part of the tail swelling, and is therefore
derived from the apparent anterior part of the primitive streak. The
anterior is formed from what was originally the apparent posterior
part of the primitive streak. The postanal gut becomes gradually less
and less prominent, and finally atrophies.
General development of the Embryo.
It will be convenient to take the Fowl as a type for the general
development of the Sauropsida.
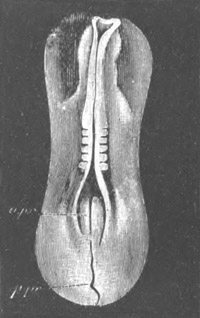
Fig. 107. Dorsal view of the hardened blastoderm of a Chick with
five mesoblastic somites. The medullary folds have met for part of
their extent, but have not united.
a.pr. anterior part of the primitive streak; p.pr. posterior
part of the primitive streak.
The embryo occupies a fairly constant position with reference to the
egg-shell. Its long axis is placed at right angles to that of the egg,
and the broad end of the egg is on the left side of the embryo. The
general history of the embryo has already been traced up to the
formation of the first formed mesoblastic somites (fig. 107). This
stage is usually reached at about the close of the first day. After
this stage the embryo rapidly grows in length, and becomes, especially
in front and to the sides, more and more definitely folded off from
the yolk-sack.
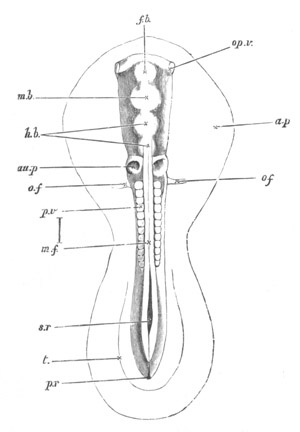
Fig. 108. Embryo of the Chick between 30 and 36
hours viewed from above as an opaque object. (Chromic acid
preparation.)
f.b. front-brain; m.b. mid-brain; h.b. hind-brain; op.v.
optic vesicle; au.p. auditory pit; o.f. vitelline vein; p.v.
mesoblastic somite; m.f. line of junction of the medullary folds
above the medullary canal; s.r. sinus rhomboidalis; t.
tail-fold; p.r. remains of primitive groove (not satisfactorily
represented); a.p. area pellucida.
The line to the side between p.v. and m.f. represents the true
length of the embryo.
The fiddle-shaped outline indicates the margin of the pellucid area.
The head, which reaches as far back as o.f., is distinctly marked
off; but neither the somatopleuric nor splanchnopleuric folds are
shewn in the figure; the latter diverge at the level of o.f., the
former considerably nearer the front, somewhere between the lines
m.b. and h.b. The optic vesicles op.v. are seen bulging out
beneath the superficial epiblast. The heart lying underneath the
opaque body cannot be seen. The tail-fold t. is just indicated; no
distinct lateral folds are as yet visible in the region midway
between head and tail. At m.f. the line of junction between the
medullary folds is still visible, being lost forwards over the
cerebral vesicles, while behind may be seen the remains of the sinus
rhomboidalis, s.r.
The general appearance of the embryo between the 30th and 40th hours
of incubation is shewn in fig. 108 from the upper surface, and in fig.
109 from the lower. The outlines of the embryo are far bolder than
during the earlier stages. Fig. 109 shews the nature of the folding,
by which the embryo is constricted off from the yolk-sack. The folds
are complicated by the fact that the mesoblast has already become
split into two layers—a splanchnic layer adjoining the hypoblast and
a somatic layer adjoining the epiblast—and that the body cavity
between these two layers has already become pretty wide in the lateral
parts of the body of the embryo and the area pellucida. The fold by
which the embryo is constricted off from the yolk-sack[Pg 170] is in
consequence a double one, formed of two limbs or laminæ, an inner limb
constituted by the splanchnopleure, and an outer limb by the
somatopleure. The relation of these two limbs is shewn in the
diagrammatic longitudinal section (fig. 105), and in the surface view
(fig. 109) the splanchnic limb being shewn at sf and the somatic at
so. Between the two limbs, and closely adjoining the splanchnopleure,
is seen the heart (ht). At the stage figured the head is well marked
off from the trunk, but the first separation between the two regions
was effected at an earlier period, on the appearance of the foremost
somite (fig. 107). Very shortly after the cephalic region is
established, and before the closure of the medullary folds, the
anterior part of the neural canal becomes enlarged to form the first
cerebral vesicle, from which two lateral diverticula—rudiments of the
optic lobes—are almost at once given off (fig. 108, op.v). By the
stage figured the cephalic part of the neural canal has become
distinctly differentiated into a fore- (f.b), a mid- (m.b) and a
hind-brain (h.b); and the hind-brain is often subdivided into
successive lobes. In the region of the hind-brain two shallow
epiblastic invaginations form the rudiments of the auditory pits
(au.p).
[Pg 172]
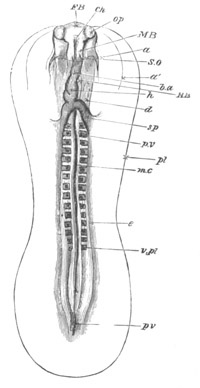
Fig. 109. An Embryo Chick of about thirty-six
hours viewed from below as a transparent object.
FB. the fore-brain or first cerebral vesicle, projecting from the
sides of which are seen the optic vesicles op. A definite head is
now constituted, the backward limit of the somatopleure fold being
indicated by the faint line S.O. Around the head are seen the two
limbs of the amniotic head-fold: one, the true amnion a, closely
enveloping the head, the other, the false amnion a´, at some
distance from it. The head is seen to project beyond the anterior
limit of the pellucid area.
The splanchnopleure fold extends as far back as sp. Along its
diverging limbs are seen the conspicuous venous roots of the
vitelline veins, uniting to form the heart h, already established
by the coalescence of two lateral halves which, continuing forward
as the bulbus arteriosus b.a, is lost in the substance of the head
just in front of the somatopleure fold.
HB. hind-brain; MB. mid-brain; p.v. and v.pl. mesoblastic
somites; ch. front end of notochord; mc. posterior part of
notochord; e. parietal mesoblast; pl. outline of area pellucida;
pv. primitive streak.
A section through the posterior part of the head of an embryo of 30
hours is represented in fig. 110. The enlarged part of the neural
tube, forming the hind-brain, is shewn at (hb). It is still
connected with the epidermis, and at its dorsal border an outgrowth on
each side forming the root of the vagus nerve is present (vg). The
notochord (ch) is seen below the brain, and below this again the
crescentic foregut (al). The commencing heart (ht), formed at this
stage of two distinct tubes, is attached to the ventral side of the
foregut.
On the dorsal side of the foregut immediately below the notochord is
[Pg 171]
seen a small body (x) formed as a thickening of the hypoblast. This
may possibly be a rudiment of the subnotochordal rod of the
Ichthyopsida.
In the trunk (fig. 108) the chief point to be noticed is the complete
closure of the neural canal, though in the posterior part, where the
open sinus rhomboidalis was situated at an earlier stage, there may
still be seen a dilatation of the canal (fig. 108, s.r), on each
side of which are the tail swellings; while the mesoblastic somites
stop short somewhat in front of it. Underneath the neural canal may be
seen the notochord (fig. 109, ch) extending into the head, as far as
the base of the mid-brain. At the sides of the trunk are seen the
mesoblastic somites (p.v), the outer edges of which mark the
boundary between the vertebral and lateral plates. A fainter line can
be seen marking off the part of the lateral plates which will become
[Pg 173]
part of the body-wall, from that which pertains to the yolk-sack.
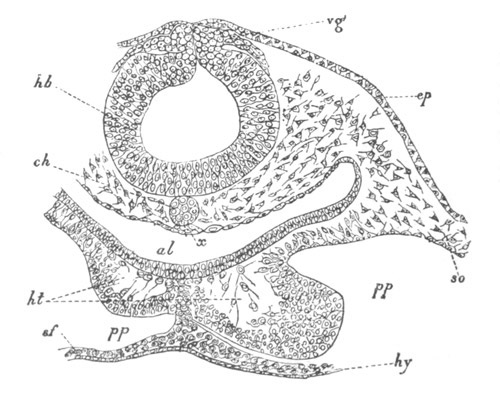
Fig. 110. Transverse section through the posterior part of the head
of an embryo chick of thirty hours.
hb. hind-brain; vg. vagus nerve; ep. epiblast; ch.
notochord; x. thickening of hypoblast (possibly a rudiment of the
subnotochordal rod); al. throat; ht. heart; pp. body cavity;
so. somatic mesoblast; sf. splanchnic mesoblast; hy.
hypoblast.
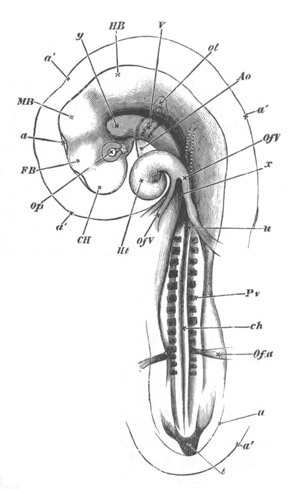
Fig. 111. Chick of the third day (54 hours) viewed from underneath
as a transparent object.
a´. the outer amniotic fold or false amnion. This is very
conspicuous around the head, but may also be seen at the tail.
a. the true amnion, very closely enveloping the head, and here
seen only between the projections of the several cerebral vesicles.
It may also be traced at the tail, t.
In the embryo of which this is a drawing the head-fold of the amnion
reached a little farther backward than the reference u, but its
limit cannot be distinctly seen through the body of the embryo.
C.H. cerebral hemisphere; F.B. vesicle of the third ventricle;
M.B. mid-brain; H.B. hind-brain; Op. eye; Ot. auditory
vesicle.
OfV. vitelline veins forming the venous roots of the heart. The
trunk on the right hand (left trunk when the embryo is viewed in its
natural position from above) receives a large branch, shewn by
dotted lines, coming from the anterior portion of the sinus
terminalis. Ht. the heart, now completely twisted on itself. Ao.
the bulbus arteriosus, the three aortic arches being dimly seen
stretching from it across the throat, and uniting into the aorta,
still more dimly seen as a curved dark line running along the body.
The other curved dark line by its side, ending near the reference
y, is the notochord ch.
About opposite the line of reference x the aorta divides into two
trunks, which running in the line of the somewhat opaque somites on
either side, are not clearly seen. Their branches however, Of.a,
the vitelline arteries, are conspicuous and are seen to curve round
the commencing side-folds.
Pv. mesoblastic somites.
x is placed at the “point of divergence” of the splanchnopleure
folds. The blind foregut begins here and extends about up to near
y, the more transparent space marked by that letter is however
mainly due to the presence there of investing mass at the base of
the brain. x marks the hind limit of the splanchnopleure folds.
The limit of the more transparent somatopleure folds cannot be seen.
It will be of course understood that all the body of the embryo
above the level of the reference x, is seen through the portion of
the yolk-sack (vascular and pellucid area), which has been removed
with the embryo from the egg, as well as through the double amniotic
fold.
The view being from below, whatever is described in the natural
position as being to the right appears here to the left, and vice
versâ.
During the latter half of the second day, and during the third day,
great progress is made in the folding off of the[Pg 174] embryo. Both the
head- and tail-ends of the embryo become quite distinct, and the
side-folds make such considerable progress that the embryo is only
connected with the yolk by a broad stalk. This stalk is double, and
consists of an inner splanchnic stalk, continuous with the walls of
the alimentary canal, and an outer somatic stalk, continuous with the
body-walls of the embryo. The somatic stalk is very much wider than
the splanchnic. (Compare fig. 121 E and F, which may be taken as
diagrammatic longitudinal and transverse sections of the embryo on the
third day.) A change also takes place in the position of the embryo.
Up to the third day it is placed symmetrically, on the yolk, with its
ventral face downwards. During this day it turns so as partially to
lie on its left side. This rotation affects first the head (fig. 111),
but in the course of the fourth day gradually extends to the rest of
the body (fig. 118). Coincidently with this change in position the
whole embryo undergoes a ventral and somewhat spiral flexure.
During the latter part of the second day and during the third day
important changes take place in the head. One of these is the cranial
flexure. This, which must not be confounded with the curvature of the
body just referred to, commences by the bending downwards of the front
part of the head round a point which may be considered as the extreme
end either of the notochord or of the alimentary canal.
The cranial flexure progresses rapidly, the front-brain being more and
more folded down till, at the end of the third day, it is no longer
the first vesicle or fore-brain, but the second cerebral vesicle or
mid-brain, which occupies the extreme front of the long axis of the
embryo. In fact a straight line through the long axis of the embryo
would now pass through the mid-brain instead of, as at the beginning
of the second day, through the fore-brain, so completely has the front
end of the neural canal been folded over the end of the notochord. The
commencement of this cranial flexure gives the body of an embryo of
the third day somewhat the appearance of a chemist’s retort, the head
of the embryo corresponding to the bulb. On the fourth day the flexure
is still greater than on the third, but on the fifth and succeeding
days it becomes less obvious.
The anterior part of the fore-brain has now become greatly[Pg 175] dilated,
and may be distinguished from the posterior part as the unpaired
rudiment of the cerebral hemispheres. It soon bulges out laterally
into two lobes, which do not however become separated by a median
partition till a much later period.
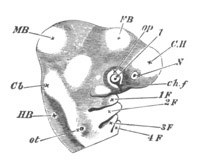
Fig. 112. Side view of the head of an Embryo Chick of the third
day as an opaque object. (Chromic acid preparation.)
CH. Cerebral hemispheres; F.B. Vesicle of third ventricle;
M.B. Mid-brain; Cb. Cerebellum; H.B. Medulla oblongata; N.
Nasal pit; ot. auditory vesicle in the stage of a pit with the
opening not yet closed up; op. Optic vesicle, with l. lens and
ch.f. choroidal fissure. The choroidal fissure, though formed
entirely underneath the superficial epiblast, is distinctly visible
from the outside.
1 F. The first visceral fold; above it is seen a slight indication
of the superior maxillary process.
2, 3, 4 F. Second, third and fourth visceral folds, with the
visceral clefts between them.
Owing to the development of the cerebral rudiment the posterior part
of the fore-brain no longer occupies the front position (fig. 111, and
112 FB), and ceases to be the conspicuous object that it was.
Inasmuch as its walls will hereafter be developed into the parts
surrounding the so-called third ventricle of the brain, it is known as
the vesicle of the third ventricle, or the thalamencephalon.
On the summit of the thalamencephalon there may now be seen a small
conical projection, the rudiment of the pineal gland, while the
centre of the floor is produced into a funnel-shaped process, the
infundibulum, which, stretching towards the extreme end of the
alimentary canal, joins the pituitary body.
Beyond an increase in size, which it shares with nearly all parts of
the embryo, and the change of position which has already been referred
to, the mid-brain undergoes no great alterations during the third day.
Its sides will ultimately become developed into the corpora bigemina
or optic lobes, its floor will form the crura cerebri, and its cavity
will be reduced to the narrow canal known as the iter a tertio ad
quartum ventriculum and two diverticula leading from this into the
optic lobes.
In the hind-brain, or third cerebral vesicle, the roof of the part
which lies nearest to the mid-brain, becomes during the third day
marked off from the rest by a slight constriction. This distinction,
which becomes much more evident later on by[Pg 176] a thickening of the walls
and roof of the front portion, separates the hind-brain into the
cerebellum and the medulla oblongata (fig. 112 Cb and HB). While
the walls of the cerebellar portion of the hind-brain become very much
thickened as well at the roof as at the sides, the roof of the
posterior portion or medulla oblongata thins out into a mere membrane,
forming a delicate covering to the cavity of the vesicle (fig. 114
IV), which here becoming broad and shallow with greatly thickened
floor and sides, is known as the fourth ventricle, subsequently
overhung by the largely-developed posterior portion of the cerebellum.
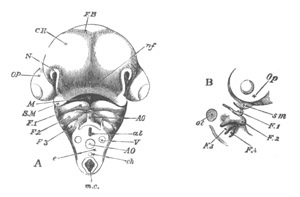
Fig. 113. Head of an Embryo Chick of the fourth day viewed
as an opaque object: from the front in A, and from the side in B. (Chromic
acid preparation.)
CH. cerebral hemispheres; FB. vesicle of the third ventricle;
Op. eyeball; nf. nasofrontal process; M. cavity of mouth;
SM. superior maxillary process of F. 1, the first visceral fold
(inferior maxillary process); F. 2, F. 3, second and third
visceral folds; N. nasal pit; ot. otic vesicle.
In order to gain the view here given the neck was cut across between
the third and fourth visceral folds. In the section e thus made,
are seen the alimentary canal al, the neural canal n.c., the
notochord ch, the dorsal aorta AO, and the vertebral veins V.
The third day, therefore, marks the distinct differentiation of the
brain into five distinct parts: the cerebral hemispheres, the central
masses round the third ventricle, the corpora bigemina, the cerebellum
and the medulla oblongata; the original cavity of the neural canal at
the same time passing from its temporary division of three single
cavities into the permanent arrangement of a series of connected
ventricles, viz. the lateral ventricles, the[Pg 177] third ventricle, the iter
(with a prolongation into the optic lobe on each side), and the fourth
ventricle.
By the third day the lens of the eye has become formed by an
invagination of the epiblast, and other changes in the eye have taken
place. The external opening of the auditory pit is closed before the
completion of the third day (fig. 114, RL); and the rudiments of the
external parts of the organ of smell have become formed as small pits
on the under surface of the fore-brain (fig. 112, N). Like the lens
and the labyrinth of the ear, they are formed as invaginations of the
external epiblast; unlike them they are never closed up.
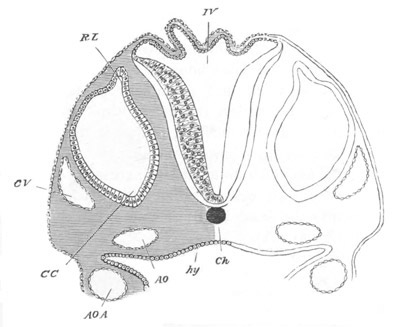
Fig. 114. Section through the hind-brain of a Chick at the end
of the third day of incubation.
IV. Fourth ventricle. The section shews the very thin roof and
thicker sides of the ventricle. Ch. Notochord; CV. Anterior
cardinal vein; CC. Involuted auditory vesicle; CC points to the
end which will form the cochlear canal; RL. Recessus labyrinthi
(remains of passage connecting the vesicle with the exterior); hy.
Hypoblast lining the alimentary canal; AO., AOA. Aorta, and
aortic arch.
During the second and third days there are formed the visceral or
branchial clefts, homologous with those of the Ichthyopsida, though
never developing branchial processes from their walls.
They are however real clefts or slits passing right through the walls
of the throat, and are placed in series on either side
[Pg 178] across the axis
of the alimentary canal, lying not quite at right angles to that axis
nor parallel to each other, but converging somewhat to the middle of
the throat in front (fig. 112 and fig. 113).
Four in number on either side, the anterior is the first to be formed,
the other three following in succession. They originate as pouches of
the hypoblast, which meet the epiblast. At the junction of the
epiblast and hypoblast an absorption of the tissue is effected,
placing the pouches in communication with the exterior.
No sooner has a cleft been formed than its anterior border (i.e. the
border nearer the head) becomes raised into a thick lip or fold, the
visceral or branchial fold. Each cleft has its own fold on its
anterior border, and in addition the posterior border of the fourth or
last visceral cleft is raised into a similar fold. There are thus
five visceral folds to four visceral clefts (figs. 112 and 113).
The last two folds however, and especially the last, are not nearly so
thick and prominent as the other three, the second being the broadest
and most conspicuous of all. The first fold meets, or nearly meets,
its fellow in the middle line in front, but the second falls short of
reaching the middle line, and the third, fourth and fifth do so in an
increasing degree. Thus in front views of the neck a triangular space
with its apex directed towards the head is observed between the ends
of the several folds (fig. 113 A).
Into this space the pleuroperitoneal cavity extends, the somatopleure
separating from the splanchnopleure along the ends of the folds; and
it is here that the aorta plunges into the mesoblast of the body.
The history of these most important visceral folds and clefts will be
dealt with in detail hereafter; meanwhile I may say that in the Chick
and higher Vertebrates the first three pairs of folds are those which
call for most notice.
The first fold on either side, increasing rapidly in size and
prominence, does not, like the others, remain single, but sends off in
the course of the third day a branch or bud-like process from its
upper edge (fig. 113). This branch, starting from near the outer end
of the fold, runs forwards and upwards in front of the stomodæum,
tending to meet the corresponding branch
[Pg 179] from the fold on the other
side, at a point in the middle line nearer the front of the head than
the junction of the main folds (fig. 113, sm). The two branches do
not quite meet, being separated by a median process, which at the same
time grows down from the extreme front of the head, and against which
they abut (fig. 120, k). Between the main folds, which are directed
somewhat downwards and their branches which slant upwards, the
somewhat lozenge-shaped stomodæum is placed, which, as the folds
become more and more prominent, grows deeper and deeper (fig. 120 A).
The main folds form the mandibular arch, and their branches the
maxillary processes, and the descending process which helps to
complete the anterior margin of the stomodæum or oral cavity is
called, from the parts which will be formed out of it, the
frontonasal process.
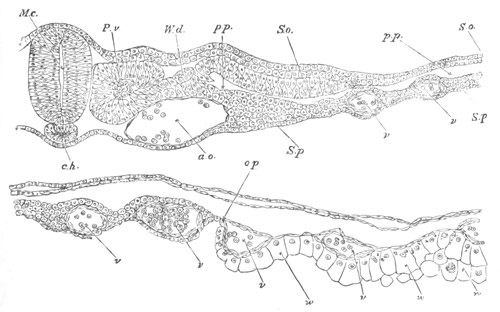
Fig. 115. Transverse section through the dorsal region of an Embryo
Chick of 45 hours.
M.c. medullary canal; P.v. mesoblastic somite; W.d. Wolffian
duct; So. Somatopleure; S.p. Splanchnopleure; p.p.
pleuroperitoneal cavity; ao. aorta; v. blood-vessels; w.
germinal wall; ch. notochord; op. junction between area opaca
and area pellucida.
In two succeeding pairs of visceral folds, which correspond with the
hyoid and first branchial arches of the Ichthyopsida, are developed
the parts of the hyoid bone, which will be best
[Pg 180] considered in
connection with the development of the skull. The last two disappear
in the Chick without giving rise to any permanent structures. The
external opening of the first visceral i.e. hyomandibular cleft
becomes closed[66],
but the inner part of the cleft, opening into the
mouth, gives rise to the Eustachian tube and the tympanic cavity, the
latter being formed as a special diverticulum.
Part of the membranous mandibular and hyoid arches form a wall round
the dorsal part of the original opening of this cleft, and so give
rise to the meatus auditorius externus. At the bottom of this is
placed the tympanic membrane, which is probably derived from the
tissue which grows over the dorsal part of the opening of the first
cleft. It is formed of an external epiblast epithelium, a middle layer
of mesoblast, and an internal hypoblastic epithelium.
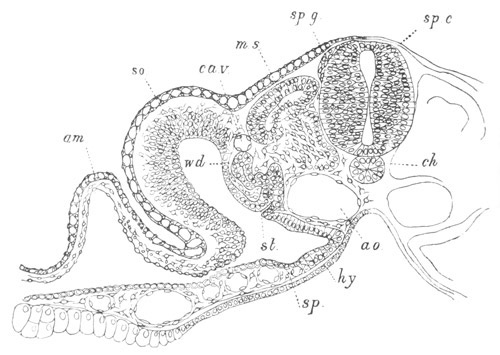
Fig. 116. Transverse section through the trunk of a Duck embryo
with about twenty-four mesoblastic somites.
am. amnion; so. somatopleure; sp. splanchnopleure; wd.
Wolffian duct; st. segmental tube; ca.v. cardinal vein; ms.
muscle-plate; sp.g. spinal ganglion; sp.c. spinal cord; ch.
notochord; ao. aorta; hy. hypoblast.
[Pg 181]
The general nature of the changes, which take place in the trunk
between the commencement of the second half of the second day and the
end of the third day, is illustrated by the sections figs. 115, 116,
117.
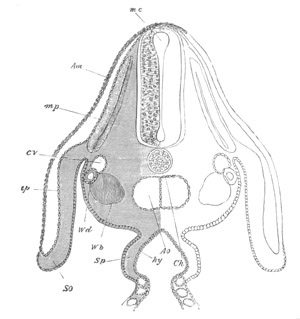
Fig. 117. Section through the dorsal region of an embryo Chick at
the end of the third day.
Am. amnion; m.p. muscle-plate. C.V. cardinal vein. Ao.
dorsal aorta. The section passes through the point where the dorsal
aorta is just commencing to divide into two branches. Ch.
notochord; W.d. Wolffian duct; W.b. commencing differentiation
of the mesoblast cells to form the Wolffian body; ep. epiblast;
So. somatopleure; Sp. splanchnopleure; hy. hypoblast. The
section passes through the point where the digestive canal
communicates with the yolk-sack, and is consequently still open
below.
In the earliest of these sections there is not a trace of a folding
off of the embryo from the yolk, and the body walls are quite
horizontal. In the second section (fig. 116), from an embryo of about
two days, the body walls are already partially inclined, and the
splanchnopleure is very distinctly folded inwards. There is a
considerable space between the notochord and the hypoblast, which
forms the rudiment of the mesentery.
[Pg 182]
In the third section (fig. 117) the body walls have become nearly
vertical, the folding of the splanchnopleure is nearly completed, and
it is only for a small region that the alimentary tract is open, by
the vitelline duct, to the yolk-sack.
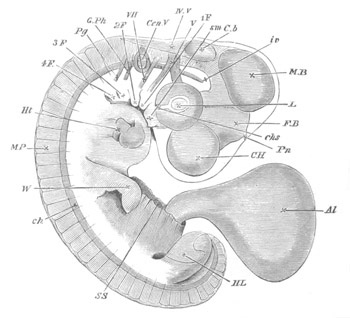
Fig. 118. Embryo Chick at the end of the fourth day seen as a
transparent object.
The amnion has been completely removed, the cut end of the somatic
stalk is shewn at S.S. with the allantois (Al) protruding from
it.
C.H. cerebral hemisphere; F.B. vesicle of the third ventricle
with the pineal gland (Pn) projecting from its summit; M.B.
mid-brain; Cb. cerebellum. IV. V. fourth ventricle; L. lens;
ch.s. choroid slit. Owing to the growth of the optic cup the two
layers of which it is composed cannot any longer be seen from the
surface, but the retinal surface of the layer alone is visible.
Cen. V. auditory vesicle; s.m. superior maxillary process; 1
F, 2 F, etc. first, second, third and fourth visceral arches;
V. fifth nerve sending one branch to the eye, the ophthalmic
branch, and another to the first visceral arch; VII. seventh nerve
passing to the second visceral arch; G.Ph. glossopharyngeal nerve
passing towards the third visceral arch; Pg. pneumogastric nerve
passing towards the fourth visceral arch; iv. investing mass. No
attempt has been made in the figure to indicate the position of the
dorsal wall of the throat, which cannot be easily made out in the
living embryo; ch. notochord. The front end of this cannot be seen
in the living embryo. It does not end however as shewn in the
figure, but takes a sudden bend downwards and then terminates in a
point. Ht. heart seen through the walls of the chest; M.P.
muscle-plates. W. wing; H.L. hind limb. Beneath the hind limb is
seen the curved tail.
These three sections further illustrate (1) the gradual
differentiation[Pg 183]
of the mesoblastic somites (fig. 115, P.v) into
(a) the muscle-plates (figs. 116, ms and 117, m.p), and (b)
the tissue to form the vertebral bodies and adjacent connective
tissue; (2) the formation of a mass of tissue between the lateral
plates and the mesoblastic somites (fig. 115), known as the
intermediate cell mass, on the dorsal side of which the Wolffian duct
is formed, while the intermediate cell mass itself breaks up into the
segmental tubes (fig. 116, st) and connective tissue of the Wolffian
body.
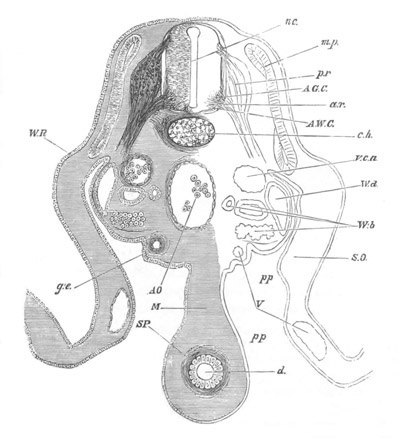
Fig. 119. Section through the lumbar region of an embryo Chick at the
end of the fourth day.
n.c. neural canal; p.r. posterior root of spinal nerve with
ganglion; a.r. anterior root of spinal nerve; A.G.C. anterior
grey column of spinal cord; A.W.C. anterior white column of spinal
cord just commencing to be formed, and not very distinctly marked in
the figure; m.p. muscle-plate; ch. notochord; W.R. Wolffian
ridge; AO. dorsal aorta; V.c.a. posterior cardinal vein; W.d.
Wolffian duct; W.b. Wolffian body, consisting of tubules and
Malpighian bodies; g.e. germinal epithelium; d. alimentary
canal; M. commencing mesentery; SO. somatopleure; SP.
splanchnopleure; V. blood-vessels; pp. pleuroperitoneal cavity.
[Pg 184]
Various other features in the development of the vascular system,
general mesoblast, etc., are also represented in these sections. It
may more especially be noted that there are at first two widely
separated dorsal aortæ, which gradually approach (figs. 115 and 116);
and meeting first of all in front finally coalesce (figs. 117 and 119)
for their whole length.
The general appearance of the embryo of the fourth day may be gathered
from fig. 118.
Fig. 120. Head of a Chick from below on the sixth and seventh days
of incubation. (From Huxley.)
I a. cerebral vesicles; a. eye, in which the remains of the
choroid slit can still be seen in A; g. nasal pits; k.
frontonasal process; l. superior maxillary process; 1. inferior
maxillary process or first visceral arch; 2. second visceral arch;
x. first visceral cleft.
In A the cavity of the mouth is seen enclosed by the frontonasal
process, the superior maxillary processes and the first pair of
visceral arches. At the back of it is seen the opening leading into
the throat. The nasal grooves leading from the nasal pits to the
mouth are already closed over and converted into canals.
In B the external opening of the mouth has become much constricted,
but it is still enclosed by the frontonasal process and superior
maxillary processes above, and by the inferior maxillary processes
(first pair of visceral arches) below.
The superior maxillary processes have united with the frontonasal
process, along nearly the whole length of the latter.
The changes which have taken place consist for the most part in the
further development of the parts already present, and do not need to
be specified in detail. The most important event of the day is perhaps
the formation of the limbs. They appear as outgrowths from a slightly
marked lateral ridge (fig. 119, WR), which runs on the level of the
lower end of the muscle-plates for
[Pg 185] nearly the whole length of the
trunk. This ridge is known as the Wolffian ridge. The first trace of
the limbs can be seen towards the end of the third day; and their
appearance at the end of the fourth day is shown in fig. 118, W and
HL.
A section through the trunk of the embryo on the fourth day is
represented in fig. 119. The section passes through the region of the
trunk behind the vitelline duct. The mesentery (M) is very much
deeper and thinner than on the previous day. The notochord has become
invested by a condensed mesoblastic tissue, which will give rise to
the vertebral column. The two dorsal aortæ have now completely
coalesced into the single dorsal aorta, and the Wolffian body has
reached a far more complete development.
In the course of the fifth day the face begins to assume a less
embryonic character, and by the sixth and succeeding days presents
distinctive avian characters.
The general changes which take place between the sixth day and the
time of hatching do not require to be specified in detail.
Fœtal Membranes.
The Reptilia, Aves and Mammalia are distinguished from the
Ichthyopsida by the possession of certain provisional fœtal
membranes, known as the amnion and allantois.
As the mode of development of these membranes may be most conveniently
studied in the Chick, I have selected this type for their detailed
description.
The Amnion. The amnion is a peculiar sack which envelopes and protects
the embryo.
At the end of the first day of incubation, when the cleavage of the
mesoblast has somewhat advanced, there appears, a little way in front
of the semilunar head-fold, a second fold (fig. 102, also fig. 121 C,
af and fig. 122, Am), running more or less parallel or rather
concentric with the first and not unlike it in general appearance,
though differing widely from it in nature. This second fold gives rise
to the amnion, and is limited entirely to the somatopleure. Rising up
as a semilunar fold with its concavity directed towards the embryo
(fig. 121 C, af), as it increases in height it
[Pg 186] is gradually drawn
backwards over the developing head of the embryo. The fold thus
covering the head is in due time accompanied by similar folds of
somatopleure, starting at some little distance behind the tail, and at
some little distance from the side (fig. 121 C, D, E, F, and 116,
am). In this way the
[Pg 190] embryo becomes surrounded by a series of folds
of thin somatopleure, which form a continuous wall all round it. All
are drawn gradually over the body of the embryo, and at last meet and
completely coalesce (fig. 121, H, I, and 117, Am), all traces of
their junction being removed. Beneath these united folds there is
therefore a cavity, within which the embryo lies (fig. 121 H, ae).
This cavity is the cavity of the amnion.
Fig. 121.
A to N forms a series of purely diagrammatic
representations introduced to facilitate the comprehension of the
manner in which the body of the embryo is formed, and of the various
relations of the yolk-sack, amnion, and allantois.
In all vt is the vitelline membrane, placed, for convenience sake,
at some distance from its contents, and represented as persisting in
the later stages; in reality it is in direct contact with the
blastoderm or yolk, and early ceases to have a separate existence.
In all e indicates the embryo proper; pp the general
pleuroperitoneal space with its extension between the membranes;
af the folds of the amnion; a the amnion proper; ae or ac
the cavity holding the liquor amnii; al the allantois; a´ the
alimentary canal; y or ys the yolk or yolk-sack.
A, which may be considered as a vertical section taken
longitudinally along the axis of the embryo, represents the
relations of the parts of the egg at the time of the first
appearance of the head-fold, seen on the right-hand side of the
embryo e. The blastoderm is spreading both behind (to the left
hand in the figure), and in front (to right hand) of the head-fold,
its limits being indicated by the shading and thickening for a
certain distance of the margin of the yolk y. As yet there is no
fold on the left side of e corresponding to the head-fold on the
right.
B is a vertical transverse section of the same period drawn for
convenience sake on a larger scale (it should have been made flatter
and less curved). It shews that the blastoderm (vertically shaded)
is extending laterally as well as fore and aft, in fact in all
directions; but there are no lateral folds, and therefore no lateral
limits to the body of the embryo as distinguished from the
blastoderm.
[Pg 187]
Incidentally it shews the formation of the medullary groove by the
rising up of the laminæ dorsales. Beneath the section of the groove
is seen the rudiment of the notochord. On either side a line
indicates the cleavage of the mesoblast just commencing.
In C, which represents a vertical longitudinal section of later
date, both head-fold (on the right) and tail-fold (on the left) have
advanced considerably. The alimentary canal is therefore closed in,
both in front and behind, but is in the middle still widely open to
the yolk y below. Though the axial parts of the embryo have become
thickened by growth, the body-walls are still thin; in them however
is seen the cleavage of the mesoblast, and the divergence of the
somatopleure and splanchnopleure. The splanchnopleure both at the
head and at the tail is folded in to a greater extent than the
somatopleure, and forms the still wide splanchnic stalk. At the end
of the stalk, which is as yet short, it bends outwards again and
spreads over the surface of the yolk. The somatopleure, folded in
less than the splanchnopleure to form the wider somatic stalk,
sooner bends round and runs outwards again. At a little distance
from both the head and the tail it is raised up into a fold, af,
af, that in front of the head being the highest. These are the
amniotic folds. Descending from either fold,
[Pg 188] it speedily joins the
splanchnopleure again, and the two, once more united into an uncleft
membrane, extend some way downwards over the yolk, the limit or
outer margin of the opaque area not being shewn. All the space
between the somatopleure and the splanchnopleure is shaded with
dots, pp. Close to the body this space may be called the
pleuroperitoneal cavity; but outside the body it runs up into either
amniotic fold, and also extends some little way over the yolk.
D represents the tail end at about the same stage on a more enlarged
scale, in order to illustrate the position of the allantois al
(which was for the sake of simplicity omitted in C), shewn as a bud
from the splanchnopleure, stretching downwards into the
pleuroperitoneal cavity pp. The dotted area representing as before
the whole space between the splanchnopleure and the somatopleure, it
is evident that a way is open for the allantois to extend from its
present position into the space between the two limbs of the
amniotic fold af.
E, also a longitudinal section, represents a stage still farther
advanced. Both splanchnic and somatic stalks are much narrowed,
especially the former, the cavity of the alimentary canal being now
connected with the cavity of the yolk by a mere canal. The folds of
the amnion are spreading over the top of the embryo and nearly meet.
Each fold consists of two walls or limbs, the space between which
(dotted) is as before merely a part of the space between the
somatopleure and splanchnopleure. Between these arched amniotic
folds and the body of the embryo is a space not as yet entirely
closed in.
F represents on a different scale a transverse section of E taken
through the middle of the splanchnic stalk. The dark ring in the
body of the embryo shews the position of the neural canal, below
which is a black spot, marking the notochord. On either side of the
notochord the divergence of somatopleure and splanchnopleure is
obvious. The splanchnopleure, more or less thickened, is somewhat
bent in towards the middle line, but the two sides do not unite, the
alimentary canal being as yet open below at this spot; after
converging somewhat they diverge again and run outwards over the
yolk. The somatopleure, folded in to some extent to form the
body-walls, soon bends outwards again, and is almost immediately
raised up into the lateral folds of the amnion af. The continuity
of the pleuroperitoneal cavity, within the body, with the interior
of the amniotic fold, outside the body, is evident; both cavities
are dotted.
G, which corresponds to D at a later stage, is introduced to shew
the manner in which the allantois, now a considerable hollow body,
whose cavity is continuous with that of the alimentary canal,
becomes directed towards the amniotic fold.
In H a longitudinal, and I a transverse section of later date, great
changes have taken place. The several folds of the amnion have met
and coalesced above the body of the embryo. The inner limbs of the
several folds have united into a single membrane (a), which
encloses a space (ae or ac) round the embryo. This membrane a
is the amnion proper, and the cavity within it, i.e. between it
and the embryo, is the cavity of the amnion containing the liquor
amnii. The allantois is omitted for the sake of simplicity.
It will be seen that the amnion a now forms in every direction the
termination of the somatopleure; the peripheral portions of the
somatopleure, the united outer or descending limbs of the folds af
in C, D, F, G having been cut adrift, and now forming an independent
continuous membrane, the serous membrane, immediately underneath the
vitelline membrane.
In I the splanchnopleure is seen converging to complete the closure
of the alimentary canal a´ even at the stalk (elsewhere the canal
has of course long been closed
[Pg 189] in), and then spreading outwards as
before over the yolk. The point at which it unites with the
somatopleure, marking the extreme limit of the cleavage of the
mesoblast, is now much nearer the lower pole of the diminished yolk.
As a result of these several changes, a great increase in the dotted
space has taken place. It is now possible to pass from the actual
peritoneal cavity within the body, on the one hand round a great
portion of the circumference of the yolk, and on the other hand
above the amnion a, in the space between it and the serous
envelope.
Into this space the allantois is seen spreading in K at al.
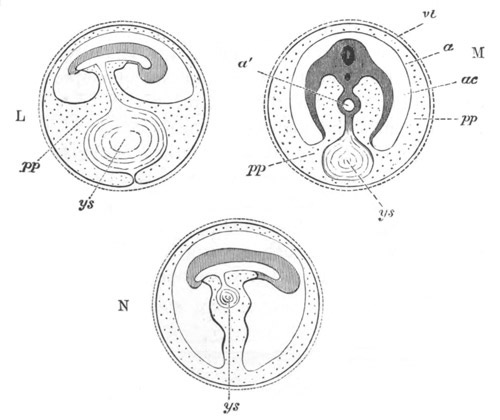
In L the splanchnopleure has completely invested the yolk-sack, but
at the lower pole of the yolk is still continuous with that
peripheral remnant of the somatopleure now called the serous
membrane. In other words, cleavage of the mesoblast has been carried
all round the yolk (ys) except at the very lower pole.
In M the cleavage has been carried through the pole itself; the
peripheral portion of the splanchnopleure forms a complete
investment of the yolk quite unconnected with the peripheral portion
of the somatopleure, which now exists as a continuous membrane
lining the interior of the shell. The yolk-sack (ys) is therefore
quite loose in the pleuroperitoneal cavity, being connected only
with the alimentary canal (a´) by a solid pedicle.
Lastly, in N the yolk-sack (ys) is shewn being withdrawn into the
cavity of the body of the embryo. The allantois is as before, for
the sake of simplicity, omitted; its pedicle would of course lie by
the side of ys in the somatic stalk marked by the usual dotted
shading.
It may be repeated that the above are diagrams, the various spaces
being shewn distended, whereas in many of them in the actual egg the
walls have collapsed, and are in near juxtaposition.
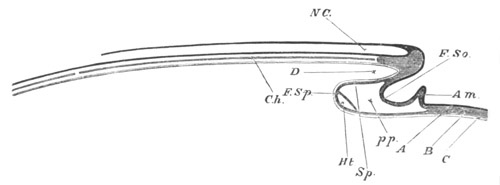
Fig. 122. Diagrammatic longitudinal section through the axis of an
embryo.
The section is supposed to be made at a time when the head-fold has
commenced but the tail-fold has not yet appeared.
F.So. fold of the somatopleure. F.Sp. fold of the
splanchnopleure; D. foregut.
pp. pleuroperitoneal cavity between somatopleure and
splanchnopleure; Am. commencing (head) fold of the amnion. For
remaining reference letters vide p. 167.
Each fold is necessarily formed of two limbs, both limbs consisting of
epiblast and a very thin layer of mesoblast; but in one limb the
epiblast looks towards the embryo, while in the other it looks away
from it. The space between the two limbs of the fold, as can easily be
seen in fig. 121, is really part of the space between the somatopleure
and splanchnopleure; it is therefore continuous with the general
space, part of which afterwards becomes the pleuroperitoneal cavity of
the body, shaded with dots in the figure and marked (pp); so that it
is possible to pass from the cavity between the two limbs of the
amniotic folds into the cavity which surrounds the alimentary canal.
When the several folds meet and coalesce together above the embryo,
they unite in such a way that all their inner limbs unite to form a
continuous inner membrane or sack, and all their outer limbs a
similarly continuous outer membrane or sack. The inner membrane thus
built up forms a completely closed sack round the body of the embryo,
and is called the amniotic
[Pg 191] sack, or amnion proper (fig. 121, H, I,
&c., a), and the fluid which it afterwards contains is called the
amniotic fluid, or liquor amnii. The space between the inner and
outer sack is, from the mode of its formation, simply a part of the
general cavity found everywhere between somatopleure and
splanchnopleure. The outer sack over the embryo lies close under the
vitelline membrane, and the cavity between it and the true amnion is
gradually extended over the whole yolk-sack.
The actual manner in which the amniotic folds meet is somewhat
peculiar (His and Kölliker). The head-fold of the amnion is the
earliest formed, and completely covers over the head before the end of
the second day. The side and tail folds are later in developing. The
side-folds finally meet in the dorsal line, and their coalescence
proceeds backwards from the head-fold in a linear direction, till
there is only a small opening left over the tail. This also becomes
closed early on the third day.
The allantois[67]
is essentially a diverticulum of the alimentary
tract into which it opens immediately in front of the anus. Its walls
are formed of splanchnic mesoblast with blood-vessels, within which is
a lining of hypoblast. It becomes a conspicuous object on the third
day of incubation, but its first development takes place at an earlier
period, and is intimately connected with the formation of the
posterior section of the gut.
At the time of the folding in of the hinder end of the mesenteron the
splitting of the mesoblast into somatopleure and splanchnopleure has
extended up to the border of the hinder division of the primitive
streak. As has been already mentioned, the ventral wall of the
postanal section of the alimentary tract is formed by the primitive
streak. Immediately in front of this is the involution which forms the
proctodæum; while the wall of the hindgut in front of the anus owes
its origin to a folding in of the splanchnopleure.
The allantois first appears as a protuberance of the splanchnopleure
just in front of the anus. This protuberance arises, however, before
the splanchnopleure has begun to be tucked in so as
[Pg 192] to form the
ventral wall of the hindgut; and it then forms a diverticulum (fig.
123 A, All) the open end of which is directed forward, while its
blind end points somewhat upwards and towards the peritoneal space
behind the embryo.
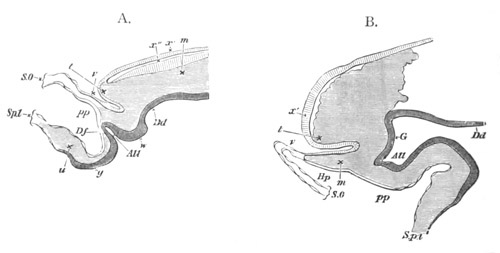
Fig. 123. Two longitudinal sections of the tail-end of an embryo
Chick to shew the origin of the allantois. A at the Beginning Of
The Third Day; B at the Middle of the Third Day. (After Dobrynin.)
t. the tail; m. the mesoblast of the body, about to form the
mesoblastic somites; x´. the roof of x´´. the neural canal;
Dd. the hind end of the hindgut; So. somatopleure; Spl.
splanchnopleure; u. the mesoblast of the splanchnopleure carrying
the vessels of the yolk-sack; pp. pleuroperitoneal cavity; Df.
the epithelium lining the pleuroperitoneal cavity; All. the
commencing allantois; w. projection formed by anterior and
posterior divisions of the primitive streak; y. hypoblast which
will form the ventral wall of the hindgut; v. anal invagination;
G. cloaca.
As the hindgut becomes folded in the allantois shifts its position,
and forms (figs. 123 B and 124) a rather wide vesicle lying
immediately below the hind end of the digestive canal, with which it
communicates freely by a still considerable opening; its blind end
projects into the pleuroperitoneal cavity below.
Still later the allantois grows forward, and becomes a large spherical
vesicle, still however remaining connected with the cloaca by a narrow
canal which forms its neck or stalk (fig. 121 G, al). From the first
the allantois lies in the pleuroperitoneal cavity. In this cavity it
grows forwards till it reaches the front limit of the hindgut, where
the splanchnopleure turns back to enclose the yolk-sack. It does not
during the third day project beyond this point; but on the fourth day
begins to pass out beyond the body of the chick, along the as yet wide
space between the
[Pg 193] splanchnic and somatic stalks of the embryo, on its
way to the space between the external and internal folds of the
amnion, which it will be remembered is directly continuous with the
pleuroperitoneal cavity (fig. 121 K). In this space it eventually
spreads out over the whole body of the chick. On the first half of the
fourth day the vesicle is still very small, and its growth is not very
rapid. Its mesoblast wall still remains very thick. In the latter half
of the day its growth becomes very rapid, and it forms a very
conspicuous object in a chick of that date (fig. 118, Al). At the
same time its blood-vessels become important. It receives its supply
of blood from two branches of the iliac arteries known as the
allantoic arteries[68],
and the blood is brought back from it by two
allantoic veins which run along in the body walls (fig. 119) and after
uniting into a single trunk fall into the vitelline vein close behind
the liver.
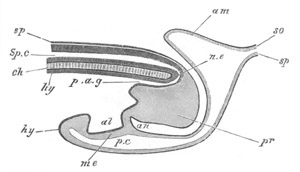
Fig. 124. Diagrammatic longitudinal section through the posterior
end of an embryo Bird at the time of the formation of the Allantois.
ep. epiblast; Sp.c. spinal canal; ch. notochord; n.e.
neurenteric canal; hy. hypoblast; p.a.g. postanal gut; pr.
remains of primitive streak folded in on the ventral side; al.
allantois; me. mesoblast; an. point where anus will be formed;
p.c. perivisceral cavity; am. amnion; so. somatopleure; sp.
splanchnopleure.
Before dealing with the later history of the fœtal membranes, it will
be convenient to complete the history of the yolk-sack.
Yolk-Sack. The origin of the area opaca has already been described. It
rapidly extends over the yolk underneath the vitelline membrane; and
is composed of epiblast and of the
[Pg 194] hypoblast of the germinal wall
continuous with that of the area pellucida, which on the fourth day
takes the form of a more or less complete layer of columnar cells[69].
Between the epiblast and hypoblast there is a layer of mesoblast,
which does not extend as far as the two other layers. The yolk is
completely surrounded by the seventh day.
Fig. 125. Diagram of the circulation of the Yolk-Sack at the end of
the third day of incubation.
H. heart; AA. the second, third and fourth aortic arches; the
first has become obliterated in its median portion, but is continued
at its proximal end as the external carotid, and at its distal end
as the internal carotid; AO. dorsal aorta; L.Of.A. left
vitelline artery; R.Of.A. right vitelline artery; S.T. sinus
terminalis; L.Of. left vitelline vein; R.Of. right vitelline
vein; S.V. sinus venosus; D.C. ductus Cuvieri; S.Ca.V.
superior cardinal vein; V.Ca. inferior cardinal vein. The veins
are marked in outline and the arteries are black. The whole
blastoderm has been removed from the egg and is supposed to be
viewed from below. Hence the left is seen on the right, and vice
versâ.
Towards the end of the first day blood-vessels begin to be
[Pg 195] developed
in the inner part of the mesoblast of the area opaca. Their
development is completed on the second day; and the region through
which they extend is known as the area vasculosa. The area vasculosa
also grows round the yolk, and completely encloses it not long after
the area opaca. The part of the blastoderm which thus encloses the
yolk forms the yolk-sack. The splitting of the mesoblast gradually
extends to the mesoblast of the yolk-sack, and eventually the
somatopleure of the sack, which is continuous, it will be remembered,
with the outer limb of the amnion, separates completely from the
splanchnopleure; and between the two the allantois inserts itself.
These features are represented in fig. 121 E, K, and L.
The circulation of the yolk-sack is most important during the third
day of incubation. The arrangement of the vessels during that day is
shewn in fig. 125.
The blood leaving the body of the embryo by the vitelline arteries
(fig. 125, R.Of.A, L.Of.A), which are branches of the dorsal
aortæ, is carried to the small vessels and capillaries of the vascular
area, a small portion only being appropriated by the pellucid area.
From the vascular area part of the blood returns directly to the sinus
venosus by the main lateral trunks of the vitelline veins (R.Of,
L.Of), and so to the heart. During the second day these venous
trunks join the body of the embryo considerably in front of, that is
nearer, the head than the corresponding arterial ones. Towards the end
of the third day, owing to the continued lengthening of the heart, the
veins and arteries run not only parallel to each other, but almost in
the same line, the points at which they respectively join and leave
the body being nearly at the same distance from the head.
The rest of the blood brought by the vitelline arteries finds its way
into the lateral portions of a venous trunk bounding the vascular
area, which is known as the sinus terminalis, S.T., and there
divides on each side into two streams. Of these, the two which, one on
either side, flow backward, meet at a point about opposite to the tail
of the embryo, and are conveyed along a distinct vein which, running
straight forward parallel to the axis of the embryo, empties itself
into the left vitelline vein. The
[Pg 196] two forward streams reaching a gap
in the front part of the sinus terminalis fall into either one, or in
some cases two veins, which run straight backwards parallel to the
axis of the embryo, and so reach the roots of the heart. When one such
vein only is present it joins the left vitelline trunk; where there
are two they join the left and right vitelline trunks respectively.
The left vein is always considerably larger than the right; and the
latter when present rapidly gets smaller and speedily disappears.
After the third day, although the vascular area goes on increasing in
size until it finally all but encompasses the yolk, the prominence of
the sinus terminalis becomes less and less.
The fœtal membranes and the yolk-sack may conveniently be treated of
together in the description of their later changes and final fate.
On the sixth and seventh days they exhibit changes of great
importance.
The amnion, at its complete closure on the fourth day, very closely
invested the body of the chick: the true cavity of the amnion was then
therefore very small. On the fifth day fluid begins to collect in the
cavity, and raises the membrane of the amnion to some distance from
the embryo. The cavity becomes still larger by the sixth day, and on
the seventh day is of very considerable dimensions, the fluid
increasing with it. On the sixth day Von Baer observed movements of
the embryo, chiefly of the limbs; he attributes them to the
stimulation of the cold air on opening the egg. By the seventh day
very obvious movements begin to appear in the amnion itself; slow
vermicular contractions creeping rhythmically over it. The amnion in
fact begins to pulsate slowly and rhythmically, and by its pulsation
the embryo is rocked to and fro in the egg. This pulsation is probably
due to the contraction of involuntary muscular fibres, which seem to
be present in the attenuated portion of the mesoblast, forming part of
the amniotic fold. Similar movements are also seen in the allantois at
a considerably later period.
The growth of the allantois has been very rapid, and it forms a
flattened bag, covering the right side of the embryo, and rapidly
spreading out in all directions between the primitive folds of the
[Pg 197]
amnion, that is, between the amnion proper and the false amnion or
serous envelope. It is filled with fluid, so that in spite of its
flattened form its opposite walls are distinctly separated from each
other.
The vascular area has become still further extended than on the fifth
day, but with a corresponding loss in the definite character of its
blood-vessels. The sinus terminalis has indeed by the end of the
seventh day lost all its previous distinctness; and the vessels which
brought back the blood from it to the heart are no longer to be seen.
Both the vitelline arteries and veins now pass to and from the body of
the chick as single trunks, assuming more and more the appearance of
being merely branches of the mesenteric vessels.
The yolk is still more fluid than on the previous day, and its bulk
has (according to von Baer) increased. This can only be due to its
absorbing the white of the egg, which indeed is diminishing rapidly.
During the eighth, ninth, and tenth days, the amnion does not undergo
any very important changes. Its cavity is still filled with fluid, and
on the eighth day its pulsations are at their height, henceforward
diminishing in intensity.
The splitting of the mesoblast has now extended to the outer limit of
the vascular area, i.e. over about three-quarters of the yolk-sack.
The somatopleure at this point is continuous (as can be easily seen by
reference to fig. 121) with the original outer fold of the amnion. It
thus comes about that the further splitting of the mesoblast merely
enlarges the cavity in which the allantois lies. The growth of this
organ keeps pace with that of the cavity in which it is placed. Spread
out over the greater part of the yolk-sack as a flattened bag filled
with fluid, it now serves as the chief organ of respiration. It is
indeed very vascular and a marked difference may be observed between
the colour of the blood in the outgoing and the returning vessels.
The yolk now begins to diminish rapidly in bulk. The yolk-sack becomes
flaccid, and on the eleventh day is thrown into a series of internal
folds, abundantly supplied by large venous trunks. By this means the
surface of absorption is largely increased, and the yolk is more and
more rapidly taken up by the
[Pg 198] blood-vessels, and in a partially
assimilated condition transferred to the body of the embryo[70].
By the eleventh day the abdominal parietes, though still much looser
and less firm than the walls of the chest, may be said to be
definitely established; and the loops of intestine, which have
hitherto been hanging down into the somatic stalk, are henceforward
confined within the cavity of the abdomen. The body of the embryo is
therefore completed; but it still remains connected with its various
appendages by a narrow somatic umbilicus, in which run the stalk of
the allantois and the solid cord suspending the yolk-sack.
The cleavage of the mesoblast is still progressing, and the yolk is
completely invested by a splanchnopleural sack.
The allantois meanwhile spreads out rapidly, and lies over the embryo
close under the shell, being separated from the shell membrane by
nothing more than the attenuated serous envelope, formed out of the
outer primitive fold of the amnion and the remains of the vitelline
membrane. With this membrane the allantois partially coalesces, and in
opening an egg at the later stages of incubation, unless care be
taken, the allantois is in danger of being torn in the removal of the
shell-membrane. As the allantois increases in size and importance, the
allantoic vessels are correspondingly developed.
On about the sixteenth day, the white having entirely disappeared, the
cleavage of the mesoblast is carried right over the pole of the yolk
opposite the embryo, and is thus completed (fig. 121). The yolk-sack
now, like the allantois which closely wraps it all round, lies loose
in a space bounded outside the body by the serous membrane, and
continuous with the pleuroperitoneal cavity of the body of the embryo.
Deposits of urates now become abundant in the allantoic fluid.
The loose and flaccid walls of the abdomen enclose a space which the
empty intestines are far from filling, and on the nineteenth day the
yolk-sack, diminished greatly in bulk but still of some considerable
size, is withdrawn through the somatic stalk into the abdominal
cavity, which it largely distends. Outside the embryo there now
remains nothing but the highly vascular
[Pg 199] allantois and the bloodless
serous membrane and amnion. The amnion, whose fluid during the later
days of incubation rapidly diminishes, is continuous at the umbilicus
with the body-walls of the embryo. The serous membrane (or outer
primitive amniotic fold) is, by the completion of the cleavage of the
mesoblast and the withdrawal of the yolk-sack, entirely separated from
the embryo. The cavity of the allantois, by means of its stalk passing
through the umbilicus, is of course continuous with the cloaca.
When the chick is about to be hatched it thrusts its beak through the
egg-membranes and begins to breathe the air contained in the air
chamber. Thereupon the pulmonary circulation becomes functionally
active, and at the same time blood ceases to flow through the
allantoic arteries. The allantois shrivels up, the umbilicus becomes
completely closed, and the chick, piercing the shell at the broad end
of the egg with repeated blows of its beak, casts off the dried
remains of allantois, amnion and serous membrane, and steps out into
the world.
Bibliography.
(117) K. E. von Baer. Ueb. Entwicklungsgeschichte d. Thiere.
Königsberg, 1828-1837.
(118) F. M. Balfour. “The development and growth of the layers of the
Blastoderm,” and “On the disappearance of the Primitive Groove in the
Embryo Chick.” Quart. J. of Micros. Science, Vol. XIII. 1873.
(119) M. Braun. “Die Entwicklung d. Wellenpapagei’s.” Part I. Arbeit.
d. zool.-zoot. Instit. Würzburg. Vol. V. 1879.
(120) M. Braun. “Aus d. Entwick. d. Papageien; I. Rückenmark; II.
Entwicklung d. Mesoderms; III. Die Verbindungen zwischen Rückenmark u.
Darm bei Vögeln.” Verh. d. phys.-med. Ges. zu Würzburg. N. F. Bd.
XIV. and XV. 1879 and 1880.
(121) J. Disse. “Die Entwicklung des mittleren Keimblattes im
Hühnerei.” Archiv für mikr. Anat., Vol. XV. 1878.
(122) J. Disse. “Die Entstehung d. Blutes u. d. ersten Gefässe im
Hühnerei.” Archiv f. mikr. Anat., Vol. XVI. 1879.
(123) Fr. Durante. “Sulla struttura della macula germinativa delle
uova di Gallina.” Ricerche nel Laboratorio di Anatomia della R.
Università di Roma.
(124) E. Dursy. Der Primitivstreif des Hühnchens. 1867.
(125) M. Duval. “Etude sur la ligne primitive de l'embryon de Poulet.”
Annales des Sciences Naturelles, Vol. VII. 1879.
(126) M. Foster and F. M. Balfour. Elements of Embryology. Part I.
London, 1874.
(127) Gasser. “Der Primitivstreifen bei Vogelembryonen.” Schriften
d.
[Pg 200] Gesell. zur Beförd. d. gesammten Naturwiss. zu Marburg, Vol. II.
Supplement I. 1879.
(128) A. Götte. “Beiträge zur Entwicklungsgeschichte d. Wirbelthiere.
II. Die Bildung d. Keimblatter u. d. Blutes im Hühnerei.” Archiv für
mikr. Anat., Vol. X. 1874.
(129) V. Hensen. “Embryol. Mitth.” Archiv f. mikr. Anat., Vol. III.
1867.
(130) W. His. Untersuch. üb. d. erste Anlage d. Wirbelthierleibes.
Leipzig, 1868.
(131) W. His. Unsere Körperform und das physiol. Problem ihrer
Entstehung. Leipzig, 1875.
(132) W. His. “Der Keimwall des Hühnereies u. d. Entstehung d.
parablastischen Zellen.” Zeit. f. Anat. u. Entwicklungsgeschichte.
Bd. I. 1876.
(133) W. His. “Neue Untersuchungen üb. die Bildung des Hühnerembryo
I.” Archiv f. Anat. u. Phys. 1877.
(134) E. Klein. “Das mittlere Keimblatt in seiner Bezieh. z. Entwick.
d. ers. Blutgefässe und Blutkörp. im Hühnerembryo.” Sitzungsber.
Wien. Akad., Vol. LXIII. 1871.
(135) A. Kölliker. Entwicklungsgeschichte d. Menschen u. d. höheren
Thiere. Leipzig, 1879.
(136) C. Kupffer. “Die Entsteh. d. Allantois u. d. Gastrula d.
Wirbelth.” Zoolog. Anzeiger, Vol. II. 1879, pp. 520, 593, 612.
(137) C. Kupffer and B. Benecke. “Photogramme z. Ontogenie d. Vögel.”
Nov. Act. d. k. Leop.-Carol.-Deutschen Akad. d. Naturforscher, Vol.
XLI. 1879.
(138) J. Oellacher. “Untersuchungen über die Furchung u.
Blatterbildung im Hühnerei.” Stricker’s Studien. 1870.
(139) C. H. Pander. Beiträge z. Entwick. d. Hühnchens im Eie.
Würzburg, 1817.
(140) A. Rauber. “Ueber die Embryonalanlage des Hühnchens.”
Centralblatt für d. medic. Wissenschaften. 1874-75.
(141) A. Rauber. Ueber die Stellung des Hühnchens im
Entwicklungsplan. 1876.
(142) A. Rauber. “Primitivrinne und Urmund. Beiträge zur
Entwicklungsgeschichte des Hühnchens.” Morphol. Jahrbuch, B. II.
1876.
(143) A. Rauber. Primitivstreifen und Neurula der Wirbelthiere in
normaler und pathologischer Beziehung. 1877.
(144) R. Remak. Untersuch. üb. d. Entwicklung d. Wirbelthiere.
Berlin, 1850-55.
(145) S. L. Schenk. “Beiträge z. Lehre v. d. Organanlage im
motorischen Keimblatt.” Sitz. Wien. Akad., Vol. LVII. 1860.
(146) S. L. Schenk. “Beiträge z. Lehre v. Amnion.” Archiv f. mikr.
Anat., Vol. VII. 1871.
(147) S. L. Schenk. Lehrbuch d. vergleich. Embryol. d. Wirbelthiere.
Wien, 1874.
(148) S. Stricker. “Mittheil. üb. d. selbstständigen Bewegungen
embryonaler Zellen.” Sitz. Wien. Akad., Vol. XLIX. 1864.
(149) S. Stricker. “Beiträge zur Kenntniss des Hühnereies.” Wiener
Sitzungsber., Vol. LIV. 1866.
[Pg 201]
(150) H. Virchow. Ueber d. Epithel d. Dottersackes im Hühnerei.
Inaug. Diss. Berlin, 1875.
(151) W. Waldeyer. “Ueber die Keimblätter und den Primitivstreifen bei
der Entwicklung des Hühnerembryo.” Zeitschrift für rationelle
Medicin. 1869.
(152) C. F. Wolff. Theoria generationis. Halæ, 1759.
(153) C. F. Wolff. Ueb. d. Bildung d. Darmcanals im bebrüteten
Hühnchen. Halle, 1812.
[Pg 202]
CHAPTER IX.
REPTILIA.
The formation of the germinal layers in the Reptilia is very
imperfectly known. The Lizard has been studied in this respect more
completely than other types, and there are a few scattered
observations on Turtles and Snakes.
The ovum has in all Reptilia a very similar structure to that in
Birds. Impregnation is effected in the upper part of the oviduct, and
the early stages of development invariably take place in the oviduct.
A few forms are viviparous, viz. some of the blindworms amongst
Lizards (Anguis, Seps), and some of the Viperidæ and Hydrophidæ
amongst the Serpents. In the majority of cases, however, the eggs are
laid in moist earth, sand, &c. Around the true ovum an egg-shell (of
the same general nature as that in birds, though usually soft), and a
variable quantity of albumen, are deposited in the oviduct. The extent
to which development has proceeded in the oviparous forms before the
eggs are laid varies greatly in different species.
The general features of the development (for a knowledge of which we
are mainly indebted to Rathke’s beautiful memoirs), the structure of
the amnion and allantois, &c. are very much the same as in Birds.
The Lizards will be taken as type of the class, and a few noteworthy
points in the development of other groups will be dealt with at the
close of the Chapter. The following description, taken in the main
from my own observations, applies to Lacerta muralis.
The segmentation is meroblastic, and similar to that in Birds. At its
close the resulting blastoderm becomes divided into two layers, a
superficial epiblast formed of a single row of cells, and
[Pg 203] a layer
below this several rows deep. Below this layer fresh segments continue
for some time to be added to the blastoderm from the subjacent yolk.
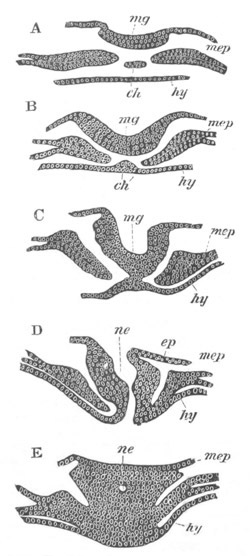
Fig. 126. Sections through an embryo of Lacerta muralis
represented in fig. 129.
m.g. medullary groove; mep. mesoblastic plate; ep. epiblast;
hy. hypoblast; ch´. notochordal thickening of hypoblast; ch.
notochord; ne. neurenteric canal (blastopore). In E. ne points a
diverticulum of the neurenteric canal into the primitive streak.
The blastoderm, which is thickened at its edge, spreads rapidly over
the yolk. Shortly before the yolk is half enclosed a small embryonic
shield (area pellucida) makes its appearance near the centre of the
blastoderm. The embryonic shield is mainly distinguished from the
remainder of the blastoderm by the more columnar character of its
constituent epiblast cells. It is somewhat pyriform in shape, the
narrower end corresponding with the future posterior end of the
embryo. At the hind end of the shield a somewhat triangular primitive
streak is formed, consisting of epiblast continuous below with a great
mass of rounded mesoblast cells, probably mainly formed, as in the
bird, by a proliferation of the epiblast. To this mass of cells the
hypoblast is also partially adherent. At the front end of the streak
an epiblastic involution appears, which soon becomes extended into a
passage open at both extremities, leading obliquely forwards through
the epiblast to the space below the hypoblast. The walls of the
passage are formed of a layer of columnar cells continuous both with
epiblast and hypoblast. In front of the primitive streak the body of
the embryo becomes first differentiated by the formation of a
medullary plate; and at the same time there grows out from the
primitive streak a layer of mesoblast, which spreads out in all
directions between the epiblast and
[Pg 204] hypoblast. In the region of the
embryo the mesoblast plate is stated by Kupffer and Benecke to be
continuous across the middle line, but this appears very improbable.
In a slightly later stage the medullary plate becomes marked by a
shallow groove, and the mesoblast of the embryo is then undoubtedly
constituted of two lateral plates, one on each side of the median
line. In the median line the notochord arises as a ridge-like
thickening of the hypoblast, which is continued posteriorly into the
front wall of the passage mentioned above.
The notochord does not long remain attached to the hypoblast, and the
separation between the two is already effected for the greater part of
the length of the embryo by the stage represented in fig. 129. Fig.
126 represents a series of sections through this embryo.
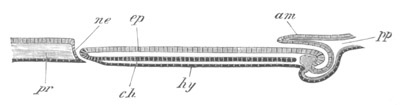
Fig. 127. Diagrammatic longitudinal section of an embryo of Lacerta.
pp. body cavity; am. amnion; ne. neurenteric canal; ch.
notochord; hy. hypoblast; ep. epiblast of the medullary plate;
pr. primitive streak. In the primitive streak all the layers are
partially fused.
In a section (A) through the trunk of the embryo a short way in front
of the primitive streak, there is a medullary plate with a shallow
groove (mg), well-developed mesoblastic plates (mep), already
divided into somatic and splanchnic layers, and a completely formed
notochord independent of the hypoblast (hy). In the next section
(B), taken just in front of the primitive streak, the notochord is
attached to the hypoblast, and the medullary groove is deeper; while
in the section following (C), which passes through the front border of
the primitive streak, the notochord and hypoblast have become fused
with the epiblast. The section behind (D) shews the neurenteric
passage leading through the floor of the medullary groove and through
the hypoblast (ne). On the right side the mesoblastic plate has
become continuous with the walls of the passage. The last section (E)
passes through the front part of the primitive streak
[Pg 205] behind the
passage. The mesoblast, epiblast, and to some extent the hypoblast,
are now fused together in the axial line, and in the middle of the
fused mass is seen a narrow diverticulum (ne) which is probably
equivalent to the posterior diverticulum of the neural canal in Birds
(vide p. 164).
The general features of the stage will best be understood by an
examination of the diagrammatic longitudinal section represented in
fig. 127. In front is shewn the amnion (am), growing over the head
of the embryo. The notochord (ch) is seen as an independent cord for
the greater part of the length of the embryo, but falls into the
hypoblast shortly in front of the neurenteric passage. The neurenteric
passage is shewn at ne, and behind it is the front part of the
primitive streak.
It is interesting to notice the remarkable relations of the notochord
to the walls of the neurenteric passage. More or less similar
relations are also well marked in the case of the goose and the fowl,
and support the conclusion, deducible from the lower forms of
Vertebrata, that the notochord is essentially hypoblastic.
The passage at the front end of the primitive streak forms the
posterior boundary of the medullary plate, though the medullary groove
is not at first continued back to it. The anterior wall of this
passage connects together the medullary plate and the notochordal
ridge of the hypoblast. In the stage represented in fig. 126 and 129
the medullary groove has become continued back to the opening of the
passage, which thus becomes enclosed in the medullary folds, and forms
a true neurenteric passage[71].
It will be convenient at this point to say a few words as to what is
known of the further fate of the neurenteric canal, and the early
development of the allantois. According to Strahl, who has worked on
Lacerta vivipara, the canal gradually closes from below upwards, and
is obliterated
[Pg 206] before the completion of the neural canal. The hind end
of the alimentary tract appears also to become a closed canal before
this stage.
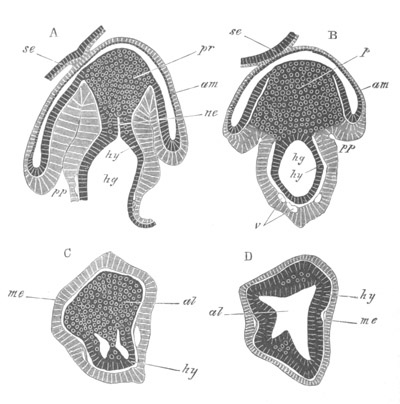
Fig. 128. Four transverse sections through the hinder end of a young
embryo of Lacerta muralis.
Sections A and B pass through the whole embryo, while C and D only
pass through the allantois, which at this stage projects backwards
into the section of the body cavity behind the primitive streak.
ne. neurenteric canal; pr. primitive streak; hg. hindgut;
hy. hypoblast; pp. body cavity; am. amnion; se. serous
envelope (outer limb of the amnion fold not yet separated from the
inner limb or true amnion); al. allantois; me. mesoblastic wall
of the allantois; v. vessels passing to the allantois.
In Lacerta muralis the history appears to be somewhat different, and
it is more especially to be noticed that in this species the hindgut
does not become closed till considerably after the completion of the
neural canal. In a stage shortly after that last described, the
neurenteric passage becomes narrower. The next stage which I have
observed is considerably later. The neural canal has become completely
closed, and the flexure of the embryo has already made its appearance.
There is still a well-developed, though somewhat slit-like,
neurenteric passage, but from the analogy of birds, it is not
impossible that it may have in the meantime closed up
[Pg 207] and opened
again. It has, in any case, the same relations as in the previous
stage.
It leads from the end of the medullary canal (at the point where its
walls are continuous with the cells of the primitive streak) round the
end of the notochord, which here becomes continuous with the medullary
cord, and so through the hypoblast. The latter layer is still a flat
sheet without any lateral infolding; but it gives rise, behind the
neurenteric passage, to a blind posteriorly directed diverticulum,
placed in the body cavity behind the embryo, and opening at the
ventral face of the apparent hind end of the primitive streak. There
is very little doubt that this diverticulum is the commencing
allantois.
At a somewhat later stage the arrangement of these parts has undergone
some changes. Their relations are shewn in the sections represented in
fig. 128.
The foremost section (A) passes through the alimentary opening of the
neurenteric passage (ne). Above this opening the section passes
through the primitive streak (pr) close to its junction with the
walls of the medullary canal. The hypoblast is folded in laterally,
but the gut is still open below. The amnion is completely established.
In the next section figured (B), the fourth of my series, the gut is
completely closed in; and the mesoblast has united laterally with the
axial tissue of the primitive streak. Vessels to supply the allantois
are shewn at v.
The three following sections are not figured, but they present the
same features as B, except that the primitive streak gets rapidly
smaller, and the lumen of the gut narrower. The section following (C)
represents, I believe, only the stalk of the allantoic diverticulum.
This diverticulum appears to be formed as usual of hypoblast (hy)
enveloped by splanchnic mesoblast (me), and projects into the
section of the body cavity present behind the embryo. Its position in
the body cavity is the cause of its somewhat peculiar appearance in
the figure. Had the whole section been represented the allantois would
have been enclosed in a space between the serous membrane (se) and a
layer of splanchnic mesoblast below which has also been omitted in
fig. B[72].
It still points directly backwards, as it primitively does
in the chick, vide fig. 123 A, and Gasser,
No. 127, Pl. V.
figs. 1
and 2. I do not understand the apparently double character of the
lumen of the allantois. In the next section (not figured) the lumen of
the allantoic stalk is larger, but still apparently double, while in
the last section (D) the lumen is considerably enlarged and single.
The neurenteric canal appears to close shortly after the stage last
described, though its further history has not been followed in detail.
[Pg 208]
General development of the Embryo.
The formation of the embryo commences with the appearance of the
medullary plate, the sides of which soon grow up to form the medullary
folds. The medullary groove is developed anteriorly before any trace
of it is visible behind. In a general way the closure of the groove
takes place as in Birds, but the anterior part of the body is very
early folded off, sinks into the yolk, and becomes covered over by the
amnion as by a hood (figs. 127 and 129). All this takes place before
the closure of the medullary canal; and the changes of this part are
quite concealed from view.
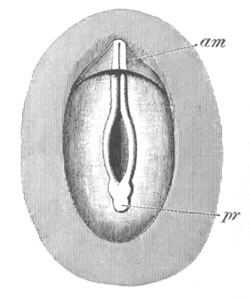
Fig. 129. Surface view of a young embryo of Lacerta muralis.
am. amnion; pr. primitive streak.
The closure of the medullary canal commences in the neck, and extends
forwards and backwards; and the whole region of the brain becomes
closed in, while the groove is still largely open behind.
The later stages in the development of the Lacertilian embryo do not
require a detailed description, as they present the closest analogy
with those already described for Aves. The embryo soon turns on to its
left side; and then, becoming continuously folded off from the yolk,
passes through the series of changes of form with which the reader is
already familiar. An advanced embryo is represented in fig. 130. The
early development and great length of the tail, which is spirally
coiled on the ventral surface, is a special feature to which the
attention of the reader may be called.
Embryonic Membranes and Yolk-Sack.
The early development of the cephalic portion of the amnion has
already been alluded to. The first traces of it become apparent while
the medullary groove is still extremely shallow. The medullary plate
in the region of the head forms an axial strip of a thickish plate of
epiblast. The edge of this plate
[Pg 209] coincides with the line of the
amniotic fold, and as this fold rises up the two sides of the plate
become bent over the embryo and give rise to the inner limb of the
amnion or amnion proper. The section (fig. 127), representing the
origin of the amniotic hood of the head, shews very well how the space
between the two limbs of the amnion is continuous with the body
cavity. The amnion very early completely encloses the embryo (fig. 128
A and B), and its external limb or serous membrane, after separating
from the true amnion, soon approaches and fuses with the vitelline
membrane.
Fig. 130. Advanced embryo of Lacerta
muralis as an opaque object[73].
The embryo was 7 mm. in length in the curled up state.
fb. fore-brain; mb. mid-brain; cb. cerebellum; au. auditory
vesicle (closed); ol. olfactory pit; md. mandible; hy. hyoid
arch; br. branchial arches; fl. fore-limb; hl. hind-limb.
The first development of the allantois as a diverticulum of the
hypoblast covered by splanchnic mesoblast, at the apparent posterior
end of the primitive streak, has been described on p. 207. The
allantois continues for some time to point directly backwards; but
gradually assumes a more ventral direction; and, as it increases in
size, extends into the space between the serous membrane and amnion,
eventually to form a large, highly vascular, flattened sack
immediately below the serous membrane.
The Yolk-Sack. The blastoderm spreads in the Lizard with very great
rapidity over the yolk to form the yolk-sack. The early appearance of
the area pellucida, or as it has been called by Kupffer and Benecke
the embryonic shield, has already been noted. Outside this a vascular
area, which has the same function as
[Pg 210] in the chick, is not long in
making its appearance. In all Reptilia the vascular channels which
arise in the vascular area, and the vessels carrying the blood to and
from the vascular area, are very similar to those in the chick. In the
Snake the sinus terminalis never attains so conspicuous a development
and in Chelonia the stage with a pair of vitelline arteries is
preceded by a stage in which the vascular area is supplied, as it
permanently is in many Mammals, by numerous transverse arterial
trunks, coming off from the dorsal aorta (Agassiz, No. 164). The
vascular area gradually envelops the whole yolk, although it does so
considerably more slowly than the general blastoderm.
Ophidia. There is, as might have been anticipated, a very close
correspondence in general development between the Lacertilia and
Ophidia. The embryos of all the Amniota are, during part of their
development, more or less spirally coiled about their long axis. This
is well marked in the chick of the third day; it is still more
pronounced in the Lizard (fig. 130); but it reaches its maximum in the
Snake. The whole Snake embryo has at the time when most coiled
(Dutrochet, Rathke) somewhat the form of a Trochus. The base of the
spiral is formed by the head, while the majority of the coils are
supplied by the tail. There are in all at this stage seven coils, and
the spiral is right-handed.
Another point, which deserves notice in the Snake, is the absence in
the embryo of all external trace of the limbs. It might have been
anticipated, on the analogy of the branchial arches, that rudiments of
the limbs would be preserved in the embryo even when limbs were absent
in the adult. Such, however, is not the case. It is however very
possible that rudiments of the branchial arches and clefts have been
preserved because these structures were functional in the larva
(Amphibia) after they ceased to have any importance in the adult; and
that the limbs have disappeared even in the embryo because in the
course of their gradual atrophy there was no advantage to the organism
in their being specially preserved at any period of life[74].
Fig. 131. Chelone midas, first stage.
Au. auditory capsule; br. 1 and 2, branchial arches; C.
carapace; E. eye; f.b. fore-brain; f.l. fore-limb; H. heart;
h.b. hind-brain; h.l. hind-limb; hy. hyoid; m.b. mid-brain;
mn. mandible; mx.p. maxillopalatine; N. nostril; u.
umbilicus.
Fig. 132. Chelone midas, second stage.
Letters as in fig. 131.
Chelonia[75].
In their early development the Chelonia resemble,
[Pg 211] so far
as is known, the Lacertilia. The amnion arises early, and soon forms a
great cephalic hood. Before development has proceeded very far the
embryo turns over on to its left side. The tail in many species
attains a very considerable
[Pg 212] development (fig. 133). The chief
peculiarity in the form of the embryo (figs. 131, 132, and 133) is
caused by the development of the carapace. The first rudiment of the
carapace appears in the form of two longitudinal folds, extending
above the line of insertion of the fore- and hind-limbs, which have
already made their appearance (fig. 131). These folds are subsequently
prolonged so as to mark out the area of the carapace on the dorsal
surface. On the surface of this area there are formed the horny plates
(tortoise shell), and in the mesoblast below the bony elements of the
carapace (figs. 132 and 133).
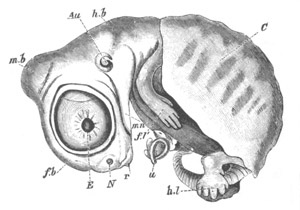
Fig. 133. Chelone midas, third stage.
Letters as in fig. 131. r. rostrum.
Immediately after hatching the yolk-sack becomes withdrawn into the
body; while the external part of the allantois shrivels up.
Bibliography.
General.
(154) C. Kupffer and Benecke. Die erste Entwicklung am Ei d.
Reptilien. Königsberg, 1878.
(155) C. Kupffer. “Die Entstehung d. Allantois u. d. Gastrula d.
Wirbelthiere.” Zoologischer Anzeiger, Vol. II. 1879, pp. 520, 593,
612.
Lacertilia.
(156) F. M. Balfour. “On the early Development of the Lacertilia,
together with some observations, etc.” Quart. J. of Micr. Science,
Vol. XIX. 1879.
[Pg 213]
(157) Emmert u. Hochstetter. “Untersuchung üb. d. Entwick. d.
Eidechsen in ihren Eiern.” Reil’s Archiv, Vol. X. 1811.
(158) M. Lereboullet. “Développement de la Truite, du Lézard et du
Limnée. II. Embryologie du Lézard.” An. Sci. Nat., Ser. IV., Vol.
XXVII. 1862.
(159) W. K. Parker. “Structure and Devel. of the Skull in Lacertilia.”
Phil. Trans., Vol. 170, p. 2. 1879.
(160) H. Strahl. “Ueb. d. Canalis myeloentericus d. Eidechse.”
Schrift. d. Gesell. z. Beför. d. gesam. Naturwiss. Marburg. July 23,
1880.
Ophidia.
(161) H. Dutrochet. “Recherches s. l. enveloppes du fœtus.” Phil.
Trans., Paris, Vol. VIII. 1816.
(162) W. K. Parker. “On the skull of the common Snake.” Phil.
Trans., Vol. 169, Part II. 1878.
(163) H. Rathke. Entwick. d. Natter. Königsberg, 1839.
Chelonia.
(164) "L. Agassiz. Contributions to the Natural History of the United
States, Vol. II. 1857. Embryology of the Turtle.
(165) W. K. Parker. “On the development of the skull and nerves in the
green Turtle.” Proc. of the Roy. Soc., Vol. XXVIII. 1879. Vide
also Nature, April 14, 1879, and Challenger Reports, Vol. I. 1880.
(166) H. Rathke. Ueb. d. Entwicklung d. Schildkröten. Braunschweig,
1848.
Crocodilia.
(167) H. Rathke. Ueber die Entwicklung d. Krokodile. Braunschweig,
1866.
[Pg 214]
CHAPTER X.
MAMMALIA.
The classical researches of Bischoff on the embryology of several
mammalian types, as well as those of other observers, have made us
acquainted with the general form of the embryos of the Placentalia,
and have shewn that, except in the earliest stages of development,
there is a close agreement between them. More recently Hensen,
Schäfer, Kölliker, Van Beneden and Lieberkühn have shed a large amount
of light on the obscurer points of the earliest developmental periods,
especially in the rabbit. For the early stages the rabbit necessarily
serves as type; but there are grounds for thinking that not
inconsiderable variations are likely to be met with in other species,
and it is not at present easy to assign to some of the developmental
features their true value. We have no knowledge of the early
development of the Ornithodelphia or Marsupialia.
The ovum on leaving the ovary is received by the fimbriated extremity
of the Fallopian tube, down which it slowly travels. It is still
invested by the zona radiata, and in the rabbit an albuminous envelope
is formed around it in its passage downwards. Impregnation takes place
in the upper part of the Fallopian tube, and is shortly followed by
the segmentation, which is remarkable amongst the Amniota for being
complete.
Although this process (the details of which have been made known by
the brilliant researches of Ed. van Beneden) has already been shortly
dealt with as it occurs in the rabbit (Vol. II. p. 98) it will be
convenient to describe it again with somewhat greater detail.
The ovum first divides into two nearly equal spheres, of which one is
slightly larger and more transparent than the
[Pg 215] other. The larger sphere
and its products will be spoken of as the epiblastic spheres, and the
smaller one and its products as the hypoblastic spheres, in accordance
with their different destinations.
Both the spheres are soon divided into two, and each of the four so
formed into two again; and thus a stage with eight spheres ensues. At
the moment of their first separation these spheres are spherical, and
arranged in two layers, one of them formed of the four epiblastic
spheres, and the other of the four hypoblastic. This position is not
long retained, but one of the hypoblastic spheres passes to the
centre; and the whole ovum again takes a spherical form.
In the next phase of segmentation each of the four epiblastic spheres
divides into two, and the ovum thus becomes constituted of twelve
spheres, eight epiblastic and four hypoblastic. The epiblastic spheres
have now become markedly smaller than the hypoblastic.
The four hypoblastic spheres next divide, giving rise, together with
the eight epiblastic spheres, to sixteen spheres in all; which are
nearly uniform in size. Of the eight hypoblastic spheres four soon
pass to the centre, while the eight superficial epiblastic spheres
form a kind of cup partially enclosing the hypoblastic spheres. The
epiblastic spheres now divide in their turn, giving rise to sixteen
spheres which largely enclose the hypoblastic spheres. The
segmentation of both epiblastic and hypoblastic spheres continues, and
in the course of it the epiblastic spheres spread further and further
over the hypoblastic, so that at the close of segmentation the
hypoblastic spheres constitute a central solid mass almost entirely
surrounded by the epiblastic spheres. In a small circular area however
the hypoblastic spheres remain for some time exposed at the surface
(fig. 134 A).
The whole process of segmentation is completed in the rabbit about
seventy hours after impregnation. At its close the epiblast cells, as
they may now be called, are clear, and have an irregularly cubical
form; while the hypoblast cells are polygonal and granular, and
somewhat larger than the epiblast cells.
The opening in the epiblastic layer where the hypoblast cells are
exposed on the surface may for convenience be called with
[Pg 216] Van Beneden
the blastopore, though it is highly improbable that it in any way
corresponds with the blastopore of other vertebrate ova[76].
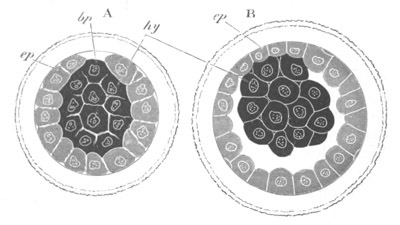
Fig. 134. Optical sections of a Rabbit’s ovum at two stages closely
following upon the segmentation. (After E. van Beneden.)
ep. epiblast; hy. primary hypoblast; bp. Van Beneden’s
blastopore. The shading of the epiblast and hypoblast is
diagrammatic.
After its segmentation the ovum passes into the uterus. The epiblast
cells soon grow over the blastopore and thus form a complete
superficial layer. A series of changes next take place which result in
the formation of what has been called the blastodermic vesicle. To Ed.
van Beneden we owe the fullest account of these changes; to Hensen and
Kölliker however we are also indebted for valuable observations,
especially on the later stages in the development of this vesicle.
The succeeding changes commence with the appearance of a narrow cavity
between the epiblast and hypoblast, which extends so as completely to
separate these two layers except in the region adjoining the original
site of the blastopore (fig. 134 B)[77].
The cavity so formed rapidly
enlarges, and with it the ovum also; which soon takes the form of a
thin-walled vesicle with a large central cavity. This vesicle is the
blastodermic
[Pg 217] vesicle. The greater part of its walls are formed of a
single row of flattened epiblast cells; while the hypoblast cells form
a small lens-shaped mass attached to the inner side of the epiblast
cells (fig. 135).
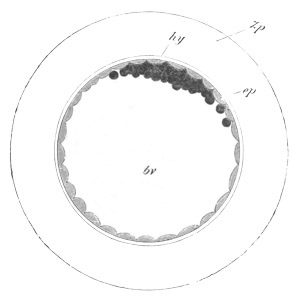
Fig. 135. Rabbit’s ovum between 70-90 hours after
impregnation. (After E. van Beneden.)
bv. cavity of blastodermic vesicle (yolk-sack); ep. epiblast;
hy. primitive hypoblast; Zp. mucous envelope (zona pellucida).
In the Vespertilionidæ Van Beneden and Julin have shewn that the ovum
undergoes at the close of segmentation changes of a more or less
similar nature to those in the rabbit; the blastopore would however
appear to be wider, and to persist even after the cavity of the
blastodermic vesicle has commenced to be developed.
Although by this stage, which occurs in the rabbit between seventy and
ninety hours after impregnation, the blastodermic vesicle has by no
means attained its greatest dimensions, it has nevertheless grown from
about 0.09 mm.—the size of the ovum at the close of segmentation—to
about 0.28. It is enclosed by a membrane formed from the zona radiata
and the mucous layer around it. The blastodermic vesicle continues to
enlarge rapidly, and during the process the hypoblastic mass undergoes
important changes. It spreads out on the inner side of the epiblast
and at the same time loses its lens-like form and becomes flattened.
The central part of it remains however thicker, and is constituted of
two rows of cells, while the peripheral part, the outer boundary of
which is irregular, is formed of an imperfect layer of amœboid cells
which continually spread further and further within the epiblast. The
central thickening of the hypoblast forms an opaque circular spot on
the blastoderm, which constitutes the commencement of the embryonic
area.
[Pg 218]
The history of the stages immediately following, from about the
commencement of the fifth day to the seventh day, when a primitive
streak makes its appearance, is imperfectly understood, and has been
interpreted very differently by Van Beneden (No. 171) on the one hand
and by Kölliker (184), Rauber (187) and Lieberkühn (186) on the other.
I have myself in conjunction with my pupil, Mr Heape, also conducted
some investigations on these stages, which have unfortunately not as
yet led me to a completely satisfactory reconciliation of the opposing
views.
Van Beneden states that about five days after impregnation the
hypoblast cells in the embryonic area become divided into two distinct
strata, an upper stratum of small cells adjoining the epiblast and a
lower stratum of flattened cells which form the true hypoblast. At the
edge of the embryonic area the hypoblast is continuous with a
peripheral ring of the amœboid cells of the earlier stage, which now
form, except at the edge of the ring, a continuous layer of flattened
cells in contact with the epiblast. During the sixth day the flattened
epiblast cells are believed by Van Beneden to become columnar. The
embryonic area gradually extends itself, and as it does so becomes
oval. A central lighter portion next becomes apparent, which gradually
spreads, till eventually the darker part of the embryonic area forms a
crescent at the posterior part of the now somewhat pyriform embryonic
area. The lighter part is formed of columnar epiblast and hypoblast
only, while in the darker area a layer of the mesoblast, derived from
the intermediate layer of the fifth day, is also found. In this darker
area the primitive streak originates early on the seventh day.
Kölliker, following the lines originally laid down by Rauber, has
arrived at very different results. He starts from the three-layered
condition described by Van Beneden for the fifth day, but does not
give any investigations of his own as to the origin of the middle
layer. He holds the outer layer to be a provisional layer of
protective cells, forming part of the wall of the original vesicle,
the middle layer he regards as the true epiblast and the inner layer
as the hypoblast.
During the sixth day he finds that the cells of the outer layer
gradually cease to form a continuous layer and finally disappear;
while the cells of the middle layer become columnar, and form the
columnar epiblast present in the embryonic area at the end of the
sixth day. The mesoblast first takes its origin in the region and on
the formation of the primitive streak.
The investigations of Heape and myself do not extend to the first
formation of the intermediate layer found on the fifth day. We find on
the sixth day in germinal vesicles of about 2.2-2.5 millimetres in
diameter with embryonic areas of about .8 mm. that the embryonic area
(fig. 136) is throughout composed of:
[Pg 219]
(1) A layer of flattened hypoblast cells;
(2) A somewhat irregular layer of more columnar elements, in some
places only a single row deep and in other places two or more
rows deep.
(3) Flat elements on the surface, which do not, however, form
a continuous layer, and are intimately attached to the columnar
cells below.
Our results as to the structure of the blastoderm at this stage
closely correspond therefore with those of Kölliker, but on one
important point we have arrived at a different conclusion. Kölliker
states that he has never found the flattened elements in the act of
becoming columnar. We believe that we have in many instances been able
to trace them in the act of undergoing this change, and have attempted
to shew this in our figure.
Our next oldest embryonic areas were somewhat pyriform measuring about
1.19 mm. in length and .85 in breadth. Of these we have several, some
from a rabbit in which we also met with younger still nearly circular
areas. All of them had a distinctly marked posterior opacity forming a
commencing primitive streak, though decidedly less advanced than in
the blastoderm represented in fig. 140. In the younger specimens the
epiblast in front of the primitive streak was formed of a single row
of columnar cells (fig. 138 A), no mesoblast was present and the
hypoblast formed a layer of flattened cells. In the region immediately
in front of the primitive streak, an irregular layer of mesoblast
cells was interposed between the epiblast and hypoblast. In the
anterior part of the primitive streak itself (fig. 138 B) there was a
layer of mesoblast with a considerable lateral extension, while in the
median line there was a distinct mesoblastic proliferation of epiblast
cells. In the posterior sections the lateral extension of the
mesoblast was less, but the mesoblast cells formed a thicker cord in
the axial line.
Owing to the unsatisfactory character of our data the following
attempt to fill in the history of the fifth and sixth days must be
regarded as tentative[78].
At the commencement of the fifth day the
central thickening, of what has been called above the primitive
hypoblast, becomes divided into two layers: the lower of these is
continuous with the peripheral hypoblast and is formed of flattened
cells, while the upper one is formed of small rounded elements. The
superficial epiblast again is formed of flattened cells.
During the fifth day remarkable changes take place in the epiblast of
the embryonic area. It is probable that its constituent
[Pg 220] cells increase
in number and become one by one columnar; and that in the process they
press against the layer of rounded elements below them, so that the
two layers cease to be distinguishable, and the whole embryonic area
acquires in section the characters represented in fig. 136[79].
Towards the end of the sixth day the embryonic area becomes oval, but
the changes which next take place are not understood. In the front
part of the area only two layers of cells are found, (1) an hypoblast,
and (2) an epiblast of columnar cells probably derived from the
flattened epiblast cells of the earlier stages. In the posterior part
of the blastoderm a middle layer is present (Van Beneden) in addition
to the two other layers; and this layer probably originates from the
middle layer which extended throughout the area at the beginning of
the fifth day, and then became fused with the epiblast. The middle
layer does not give rise to the whole of the eventual mesoblast, but
only to part of it. From its origin it may be called the hypoblastic
mesoblast, and it is probably equivalent to the hypoblastic mesoblast
already described in the chick (pp. 154 and 155). The stage just
described has only been met with by Van Beneden[80].
Fig. 136. Section through the nearly circular embryonic area of a
Rabbit’s ovum of six days, nine hours and .8 mm. in diameter.
The section shews the peculiar character of the upper layer with a
certain number of superficial flattened cells; and represents about
half the breadth of the area.
A diagrammatic view of the whole blastodermic vesicle at about the
beginning of the seventh day is given in fig. 137. The embryonic area
is represented in white. The line ge in B shews the extension of the
hypoblast round the inner side of the vesicle. The blastodermic
vesicle is therefore formed of three areas, (1)
[Pg 221] the embryonic area
with three layers: this area is placed where the blastopore was
originally situated. (2) The ring around the embryonic area where the
walls of the vesicle are formed of epiblast and hypoblast. (3) The
area beyond this again where the vesicle is formed of epiblast
only[81].
Fig. 137. Views of the blastodermic vesicle of a Rabbit on the seventh
day without the zona. A. from above, B. from the side. (From
Kölliker.)
ag. embryonic area; ge. boundary of the hypoblast.
The changes which next take place begin with the formation of a
primitive streak, homologous with, and in most respects similar to,
the primitive streak in Birds. The formation of the streak is preceded
by that of a clear spot near the middle of the blastoderm, forming the
nodal point of Hensen. This spot subsequently constitutes the front
end of the primitive streak.
The history of the primitive streak was first worked out in a
satisfactory manner by Hensen (No. 182), from whom however I differ in
admitting the existence of a certain part of the mesoblast before its
appearance.
Early on the seventh day the embryonic area becomes pyriform, and at
its posterior and narrower end a primitive streak makes its
appearance, which is due to a proliferation of rounded cells from the
epiblast. At the time when this proliferation
[Pg 222] commences the layer of
hypoblastic mesoblast is present, especially just in front of, and at
the sides of, the anterior part of the streak; but no mesoblast is
found in the anterior part of the embryonic area. These features are
shewn in fig. 138 A and B. The mesoblast derived from the
proliferation of the epiblast soon joins the mesoblast already
present; though in many sections it
[Pg 223] seems possible to trace a
separation between the two parts (fig. 139 B) of the mesoblast.
Fig. 138. Two sections through oval blastoderms of a Rabbit on
the seventh day. The length of the area was about 1.2 mm. and its
breadth about .86 mm.
A. Through the region of the blastoderm in front of the primitive
streak; B. through the front part of the primitive streak; ep.
epiblast; m. mesoblast; hy. hypoblast; pr. primitive streak.
Fig. 139. Two transverse sections through the embryonic area of an
embryo Rabbit of seven days.
The embryo has nearly the structure represented in fig. 140.
A. is taken through the anterior part of the embryonic area. It
represents about half the breadth of the area, and there is no trace
of a medullary groove or of the mesoblast.
B. Is taken through the posterior part of the primitive streak.
ep. epiblast; hy. hypoblast.
During the seventh day the primitive streak becomes a more pronounced
structure, the mesoblast in its neighbourhood increases in quantity,
while an axial groove—the primitive groove—is formed on its upper
surface. The mesoblastic layer in front of the primitive streak
becomes thicker, and, in the two-layered region in front, the epiblast
becomes several rows deep (fig. 139 A).
Fig. 140. embryonic area of an eight days'
Rabbit. (After Kölliker.)
arg. embryonic area; pr. primitive streak.
In the part of the embryonic area in front of the primitive streak
there arise during the eighth day two folds bounding a shallow median
groove, which meet in front, but diverge behind, and enclose between
them the foremost end of the primitive streak (fig. 141). These folds
are the medullary folds and they constitute the first definite traces
of the embryo. The medullary plate bounded by them rapidly grows in
length, the primitive streak always remaining at its hinder end. While
the lateral epiblast is formed of several rows of cells, that of the
medullary plate is at first formed of but a single row (fig. 142,
mg). The mesoblast, which appears to grow forward from the primitive
streak, is stated to be at first a continuous sheet between the
epiblast and hypoblast (Hensen). The evidence on this point does not
however appear to me to be quite conclusive. In any case, as soon as
ever the medullary groove is formed, the mesoblast becomes divided,
exactly as in Lacerta and Elasmobranchii, into two independent lateral
plates, which are not continuous across the middle line (fig. 142,
me). The hypoblast cells are flattened laterally, but become
columnar beneath the medullary plate (fig. 142).
In tracing the changes which take place in the relations of the
layers, in passing from the region of the embryo to that of the
primitive streak, it will be convenient to follow the account given by
Schäfer for the guinea-pig (No. 190), which on this point is far
fuller and more satisfactory than that of other observers.
[Pg 224] In doing so
I shall leave out of consideration the fact (fully dealt with later in
this chapter) that the layers in the guinea-pig are inverted. Fig. 143
represents a series of sections through this part in the guinea-pig.
The anterior section (D) passes through the medullary groove near its
hinder end. The commencement of the primitive streak is marked by a
slight prominence on the floor of the medullary groove between the two
diverging medullary folds (fig. 143 C, ae). Where this prominence
becomes first apparent the epiblast and hypoblast are united together.
The mesoblast plates at the two sides remain
[Pg 225] in the meantime quite
free. Slightly further back, but before the primitive groove is
reached, the epiblast and hypoblast are connected together by a cord
of cells (fig. 143 B. f), which in the section next following
becomes detached from the hypoblast and forms a solid keel projecting
from the epiblast. In the following section the hitherto independent
mesoblast plates become united with this keel (fig. 143 A); and in the
posterior sections, through the part of the primitive streak with the
primitive groove, the epiblast and mesoblast continue to be united in
the axial line, but the hypoblast remains distinct. These peculiar
relations may shortly be described by saying that in the axial line
the hypoblast becomes united with the epiblast at the posterior end
of the embryo; and that the cells which connect the hypoblast and
epiblast are posteriorly continuous with the fused epiblast and
mesoblast of the primitive streak, the hypoblast in the region of the
primitive streak having become distinct from the other layers.
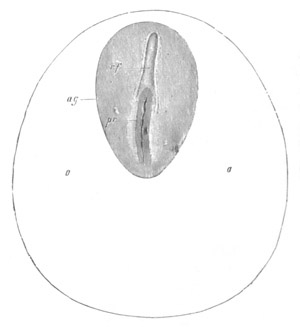
Fig. 141. Embryonic area of a seven days’ embryo Rabbit. (From
Kölliker.)
o. place of future area vasculosa; rf. medullary groove; pr.
primitive streak; ag. embryonic area.

Fig. 142. Transverse section through an embryo Rabbit of eight days.
ep. epiblast; me. mesoblast; hy. hypoblast; mg. medullary
groove.
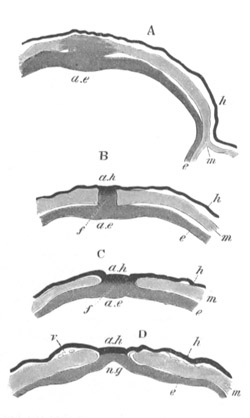
Fig. 143. A series of transverse sections through the junction of
the primitive streak and medullary groove of a young Guinea-pig.
(After Schäfer.)
A. is the posterior section.
e. epiblast; m. mesoblast; h. hypoblast; ae. axial epiblast
of the primitive streak; ah. axial hypoblast attached in B. and C.
to the epiblast at the rudimentary blastopore; mg. medullary
groove; f. rudimentary blastopore.
The peculiar relations just described, which hold also for the rabbit,
receive their full explanation by a comparison of the Mammal with the
Bird and the Lizard, but before entering into this comparison, it will
be well to describe the next stage in the rabbit, which is in many
respects very instructive. In this stage
[Pg 226] the thickened axial portion
of the hypoblast in the region of the embryo becomes separated from
the lateral part as the notochord. Very shortly after the formation of
the notochord, the hypoblast grows in from the two sides, and becomes
quite continuous across the middle line. The formation of the
notochord takes place from before backwards; and at the hinder end of
the embryo the notochord is continued into the mass of cells which
forms the axis of the primitive streak, becoming therefore at this
point continuous with the epiblast. The notochord in fact behaves
exactly as did the axial hypoblast in the earlier stage.
In comparison with Lacerta (pp. 203-205) it is obvious that the axial
hypoblast and the notochord derived from it have exactly the same
relations in Mammalia and Lacertilia. In both they are continued at
the hind end of the embryo into the epiblast; and close to where they
join it, the mesoblast and epiblast fuse together to form the
primitive streak. The difference between the two types consists in the
fact that in Reptilia there is formed a passage connecting the neural
and alimentary canals, the front wall of which is constituted by the
cells which form the above junction between the notochord and
epiblast; and that in Mammalia this passage—which is only a
rudimentary structure in Reptilia—has either been overlooked or else
is absent. In any case the axial junction of the epiblast and
hypoblast in Mammalia is shewn by the above comparison with Lacertilia
to represent the dorsal lip of the true vertebrate blastopore. The
presence of this blastopore seems to render it clear that the
blastopore discovered by Ed. van Beneden cannot have the meaning he
assigned to it in comparing it with the blastopore of the frog.
Kölliker adduces the fact that the notochord is continuous with the
axial cells of the primitive streak as an argument against its
hypoblastic origin. The above comparison with Lacertilia altogether
deprives this argument of any force.
At the stage we have now reached the three layers are definitely
established. The epiblast (on the view adopted above) clearly
originates from epiblastic segmentation cells. The hypoblast without
doubt originates from the hypoblastic segmentation spheres which give
rise to the lenticular mass within the epiblast on the appearance of
the cavity of the blastodermic vesicle; while, though the history of
the mesoblast is still obscure, part of it appears to originate from
the hypoblastic mass, and part is undoubtedly formed from the epiblast
of the primitive streak.
[Pg 227]
While these changes have been taking place the rudiments of a vascular
area become formed, and it is very possible that part of the
hypoblastic mesoblast passes in between the epiblast and hypoblast,
immediately around the embryonic area, to give rise to the area
vasculosa. From Hensen’s observation it seems at any rate clear that
the mesoblast of the vascular area arises independently of the
primitive streak: an observation which is borne out by the analogy of
Birds.
General growth of the Embryo.
We have seen that the blastodermic vesicle becomes divided at an early
stage of development into an embryonic area, and a non-embryonic
portion. The embryonic area gives rise to the whole of the body of the
embryo, while the non-embryonic part forms an appendage, known as the
umbilical vesicle, which becomes gradually folded off from the embryo,
and has precisely the relations of the yolk-sack of the Sauropsida. It
is almost certain that the Placentalia are descended from ancestors,
the embryos of which had large yolk-sacks, but that the yolk has
become reduced in quantity owing to the nutriment received from the
wall of the uterus taking the place of that originally supplied by the
yolk. A rudiment of the yolk-sack being retained in the umbilical
vesicle, this structure may be called indifferently umbilical vesicle
or yolk-sack.
The yolk which fills the yolk-sack in Birds is replaced in Mammals by
a coagulable fluid; while the gradual extension of the hypoblast round
the wall of the blastodermic vesicle, which has already been
described, is of the same nature as the growth of the hypoblast round
the yolk-sack in Birds.
The whole embryonic area would seem to be employed in the formation of
the body of the embryo. Its long axis has no very definite relation to
that of the blastodermic vesicle. The first external trace of the
embryo to appear is the medullary plate, bounded by the medullary
folds, and occupying at first the anterior half of the embryonic area
(fig. 141). The two medullary folds diverge behind and enclose the
front end of the primitive streak. As the embryo elongates, the
medullary folds
[Pg 228] nearly meet behind and so cut off the front portion of
the primitive streak, which then appears as a projection in the hind
end of the medullary groove. In an embryo rabbit, eight days after
impregnation, the medullary groove is about 1.80 mm. in length. At
this stage a division may be clearly seen in the lateral plates of
mesoblast into a vertebral zone adjoining the embryo and a more
peripheral lateral zone; and in the vertebral zone indications of two
somites, about 0.37 mm. from the hinder end of the embryo, become
apparent. The foremost of these somites marks the junction, or very
nearly so, of the cephalic region and trunk. The small size of the
latter as compared with the former is very striking, but is
characteristic of Vertebrates generally. The trunk gradually elongates
relatively to the head, by the addition behind of fresh somites. The
embryo has not yet begun to be folded off from the yolk-sack. In a
slightly older embryo of nine days there appears (Hensen, Kölliker)
round the embryonic area a delicate clear ring which is narrower in
front than behind (fig. 144 A, ap). This ring is regarded by these
authors as representing the peripheral part of the area pellucida of
Birds, which does not become converted into the body of the embryo.
Outside the area pellucida, an area vasculosa has become very well
defined. In the embryo itself (fig. 144 A) the disproportion between
head and trunk is less marked than before; the medullary plate dilates
anteriorly to form a spatula-shaped cephalic enlargement; and three or
four somites are established. In the lateral parts of the mesoblast of
the head there may be seen on each side a tube-like structure (hz).
Each of these is part of the heart, which arises as two independent
tubes. The remains of the primitive streak (pr) are still present
behind the medullary groove.
In somewhat older embryos (fig. 144 B) with about eight somites, in
which the trunk considerably exceeds the head in length, the first
distinct traces of the folding-off of the head end of the embryo
become apparent, and somewhat later a fold also appears at the hind
end. In the formation of the hind end of the embryo the primitive
streak gives rise to a tail swelling and to part of the ventral wall
of the postanal gut. In the region of the head the rudiments of the
heart (h) are far more definite. The medullary groove is still open
for its whole length, but in
[Pg 229] the head it exhibits a series of
well-marked dilatations. The foremost of these (vh) is the rudiment
of the fore-brain, from the sides of which there project the two optic
vesicles (ab); the next is the mid-brain (mh), and the last is the
hind-brain (hh), which is again divided into smaller lobes by
successive constrictions. The medullary groove behind the region of
the somites dilates into an embryonic sinus rhomboidalis like that of
the Bird. Traces of the amnion (af) are now apparent both in front
of and behind the embryo.
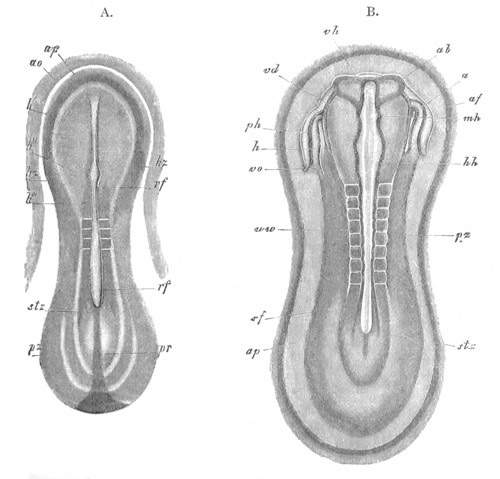
Fig. 144. Embryo Rabbits of about nine days from the dorsal side.
(From Kölliker.)
A. magnified 22 times, and B. 21 times.
ap. area pellucida; rf. medullary groove; h´. medullary plate
in the region of the future fore-brain; h´´. medullary plate in the
region of the future mid-brain; vh. fore-brain; ab. optic
vesicle; mh. mid-brain; hh. and h´´´. hind-brain; uw.
mesoblastic somite; stz. vertebral zone; pz. lateral zone; hz.
and h. heart; ph. pericardial section of body cavity; vo.
vitelline vein; af. amnion fold.
[Pg 230]
The structure of the head and the formation of the heart at this age
are illustrated in fig. 145. The widely-open medullary groove (rf)
is shewn in the centre. Below it the hypoblast is thickened to form
the notochord dd´; and at the sides are seen the two tubes, which,
on the folding-in of the foregut, give rise to the unpaired heart.
Each of these is formed of an outer muscular tube of splanchnic
mesoblast (ahh), not quite closed towards the hypoblast, and an
inner epithelioid layer (ihh); and is placed in a special section of
the body cavity (ph), which afterwards forms the pericardial cavity.
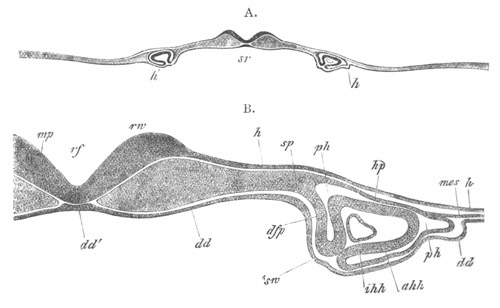
Fig. 145. Transverse section through the head of a Rabbit of the same
age as fig. 144 b. (From Kölliker.)
B. is a more highly magnified representation of part of A.
rf. medullary groove; mp. medullary plate; rw. medullary fold;
h. epiblast; dd. hypoblast; dd´. notochordal thickening of
hypoblast; sp. undivided mesoblast; hp. somatic mesoblast;
dfp. splanchnic mesoblast; ph. pericardial section of body
cavity; ahh. muscular wall of heart; ihh. epithelioid layer of
heart; mes. lateral undivided mesoblast; sw. fold of hypoblast
which will form the ventral wall of the pharynx; sr. commencing
throat.
Before the ninth day is completed great external changes are usually
effected. The medullary groove becomes closed for its whole length
with the exception of a small posterior portion. The closure
commences, as in Birds, in the region of the mid-brain. Anteriorly the
folding-off of the embryo proceeds so far
[Pg 231] that the head becomes quite
free, and a considerable portion of the throat, ending blindly in
front, becomes established. In the course of this folding the, at
first widely separated, halves of the heart are brought together,
coalesce on the ventral side of the throat, and so give rise to a
median undivided heart. The fold at the tail end of the embryo
progresses considerably, and during its advance the allantois is
formed in the same way as in Birds. The somites increase in number to
about twelve. The amniotic folds nearly meet above the embryo.
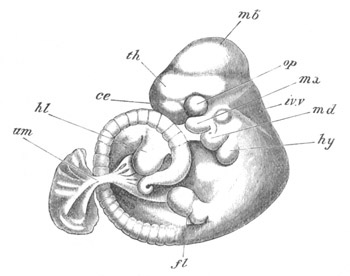
Fig. 146. Advanced embryo of a Rabbit (about twelve days)[82].
mb. mid-brain; th. thalamencephalon; ce. cerebral hemisphere;
op. eye; iv.v. fourth ventricle; mx. maxillary process; md.
mandibular arch; hy. hyoid arch; fl. fore-limb; hl. hind-limb;
um. umbilical stalk.
The later stages in the development proceed in the main in the same
manner as in the Bird. The cranial flexure soon becomes very marked,
the mid-brain forming the end of the long axis of the embryo (fig.
146). The sense organs have the usual development. Under the
fore-brain appears an epiblastic involution giving rise both to the
mouth and to the pituitary body. Behind the mouth are three
well-marked pairs of visceral arches. The first of these is the
mandibular arch (fig. 146, md), which meets its fellow in the middle
line, and forms the posterior boundary of the mouth. It sends forward
on each side a superior
[Pg 232] maxillary process (mx) which partially forms
the anterior margin of the mouth. Behind the mandibular arch are
present a well-developed hyoid (hy) and a first branchial arch (not
shewn in fig. 146). There are four clefts, as in other Amniota, but
the fourth is not bounded behind by a definite arch. Only the first of
these clefts persists as the tympanic cavity and Eustachian tube.
At the time when the cranial flexure appears, the body also develops a
sharp flexure immediately behind the head, which is thus bent forwards
upon the posterior straight part of the body (fig. 146). The amount of
this flexure varies somewhat in different forms. It is very marked in
the dog (Bischoff). At a later period, and in some species even before
the stage figured, the tail end of the body also becomes bent (fig.
146), so that the whole dorsal side assumes a convex curvature, and
the head and tail become closely approximated. In most cases the
embryo, on the development of the tail, assumes a more or less
definite spiral curvature (fig. 146); which however never becomes
nearly so marked a feature as it commonly is in Lacertilia and
Ophidia. With the more complete development of the lower wall of the
body the ventral flexure partially disappears, but remains more or
less persistent till near the close of intra-uterine life. The limbs
are formed as simple buds in the same manner as in Birds. The buds of
the hind-limbs are directed somewhat forwards, and those of the
fore-limb backwards.
Embryonic membranes and yolk-sack.
The early stages in the development of the embryonic membranes are
nearly the same as in Aves; but during the later stages in the
Placentalia the allantois enters into peculiar relations with the
uterine walls, and the two, together with the interposed portion of
the subzonal membrane or false amnion, give rise to a very
characteristic Mammalian organ—the placenta—into the structure of
which it will be necessary to enter at some length. The embryonic
membranes vary so considerably in the different forms that it will be
advantageous to commence with a description of their development in an
ideal case.
[Pg 233]
We may commence with a blastodermic vesicle, closely invested by the
delicate remnant of the zona radiata, at the stage in which the
medullary groove is already established. Around the embryonic area a
layer of mesoblast would have extended for a certain distance; so as
to give rise to an area vasculosa, in which however the blood-vessels
would not have become definitely established. Such a vesicle is
represented diagrammatically in fig. 147, 1. Somewhat later the embryo
begins to be folded off, first in front and then behind (fig. 147, 2).
These folds result in a constriction separating the embryo and the
yolk-sack (ds), or as it is known in Mammalian embryology, the
umbilical vesicle. The splitting of the mesoblast into a splanchnic
and a somatic layer has taken place, and at the front and hind end of
the embryo a fold (ks) of the somatic mesoblast and epiblast begins
to rise up and grow over the head and tail of the embryo. These two
folds form the commencement of the amnion. The head and tail folds of
the amnion are continued round the two sides of the embryo, till they
meet and unite into a continuous fold. This fold grows gradually
upwards, but before it has completely enveloped the embryo, the
blood-vessels of the area vasculosa become fully developed. They are
arranged in a manner not very different from that in the chick.
The following is a brief account of their arrangement in the Rabbit:—
The outer boundary of the area, which is continually
extending further and further round the umbilical vesicle,
is marked by a venous sinus terminalis (fig. 147, st).
The area is not, as in the chick, a nearly complete circle,
but is in front divided by a deep indentation extending
inwards to the level of the heart. In consequence of this
indentation the sinus terminalis ends in front in two
branches, which bend inwards and fall directly into the
main vitelline veins. The blood is brought from the dorsal
aortæ by a series of lateral vitelline arteries, and not by
a single pair as in the chick. These arteries break up into
a more deeply situated arterial network, from which the
blood is continued partly into the sinus terminalis, and
partly into a superficial venous network. The hinder end of
the heart is continued into two vitelline veins, each of
which divides into an anterior and a posterior branch. The
anterior branch is a limb of the sinus terminalis, and the
posterior and smaller branch is continued towards the hind
part of the sinus, near which it ends. On its way it
receives, on its outer side, numerous branches from the venous
network,
[Pg 235] which connect by their anastomoses the posterior branch
of the vitelline vein and the sinus terminalis.
[Pg 234]
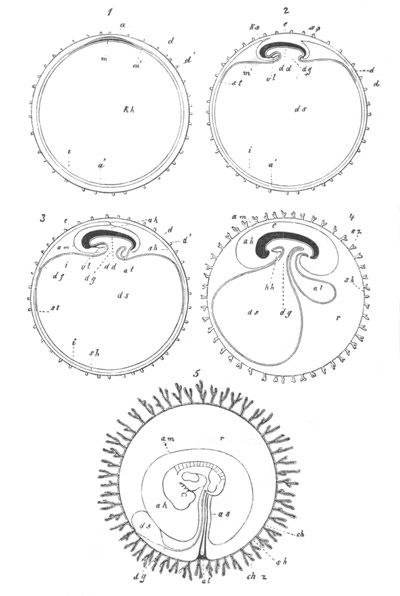
Fig. 147. Five diagrammatic figures illustrating the formation of
the fœtal membranes of a Mammal. (From Kölliker.)
In 1, 2, 3, 4 the embryo is represented in longitudinal section.
1. Ovum with zona pellucida, blastodermic vesicle, and embryonic
area.
2. Ovum with commencing formation of umbilical vesicle and amnion.
3. Ovum with amnion about to close, and commencing allantois.
4. Ovum with villous subzonal membrane, larger allantois, and mouth
and anus.
5. Ovum in which the mesoblast of the allantois has extended round
the inner surface of the subzonal membrane and united with it to
form the chorion. The cavity of the allantois is aborted. This fig.
is a diagram of an early human ovum.
d. zona radiata; d´. processes of zona; sh. subzonal membrane;
ch. chorion; ch.z. chorionic villi; am. amnion; ks.
head-fold of amnion; ss. tail-fold of amnion; a. epiblast of
embryo; a´. epiblast of non-embryonic part of the blastodermic
vesicle; m. embryonic mesoblast; m´. non-embryonic mesoblast;
df. area vasculosa; st. sinus terminalis; dd. embryonic
hypoblast; i. non-embryonic hypoblast; kh. cavity of
blastodermic vesicle, the greater part of which becomes the cavity
of the umbilical vesicle ds.; dg. stalk of umbilical vesicle;
al. allantois; e. embryo; r. space between chorion and amnion
containing albuminous fluid; vl. ventral body wall; hh.
pericardial cavity.
While the above changes have been taking place the whole blastodermic
vesicle, still enclosed in the zona, has become attached to the walls
of the uterus. In the case of the typical uterus with two tubular
horns, the position of each embryo, when there are several, is marked
by a swelling in the walls of the uterus, preparatory to the changes
which take place on the formation of the placenta. In the region of
each swelling the zona around the blastodermic vesicle is closely
embraced, in a ring-like fashion, by the epithelium of the uterine
wall. The whole vesicle assumes an oval form, and it lies in the
uterus with its two ends free. The embryonic area is placed close to
the mesometric attachment of the uterus. In many cases peculiar
processes or villi grow out from the ovum (fig. 147, 4, sz), which
fit into the folds of the uterine epithelium. The nature of these
processes requires further elucidation, but in some instances they
appear to proceed from the zona (the Rabbit) and in other instances
from the subzonal membrane (the Dog). In any case the attachment
between the blastodermic vesicle and the uterine wall becomes so close
at the time when the body of the embryo is first formed out of the
embryonic area, that it is hardly possible to separate them without
laceration; and at this period—from the 8th to the 9th day in the
Rabbit—it requires the greatest care to remove the ovum from the
uterus without injury. It will be understood of course that the
attachment above described is at first purely superficial and not
vascular.
Shortly after the establishment of the circulation of the yolk-sack
[Pg 236]
the folds of the amnion meet and coalesce above the embryo (fig. 147,
3 and 4, am). After this the inner or true amnion becomes severed
from the outer or false amnion, though the two sometimes remain
connected by a narrow stalk. Between the true and false amnion is a
continuation of the body cavity. The true amnion consists of a layer
of epiblastic epithelium and generally also of somatic mesoblast,
while the false amnion consists, as a rule, of epiblast only; though
it is possible that in some cases (the Rabbit?) the mesoblast may be
continued along its inner face.
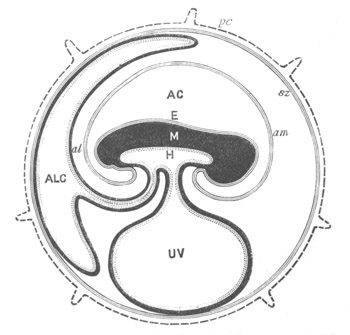
Fig. 147*. Diagram of the fœtal membranes of a Mammal. (From
Turner.)
Structures which either are or have been at an earlier period of
development continuous with each other are represented by the same
character of shading.
pc. zona with villi; sz. subzonal membrane; E. epiblast of
embryo; am. amnion; AC. amniotic cavity; M. mesoblast of
embryo; H. hypoblast of embryo; UV. umbilical vesicle; al.
allantois; ALC. allantoic cavity.
Before the two limbs of the amnion are completely severed, the
epiblast of the umbilical vesicle becomes separated from the mesoblast
and hypoblast of the vesicle (fig. 147, 3), and, together with the
false amnion (sh), with which it is continuous, forms a complete
lining for the inner face of the zona radiata.
[Pg 237] The space between this
membrane and the umbilical vesicle with the attached embryo is
obviously continuous with the body cavity (vide figs. 147, 4 and
147*). To this membrane Turner has given the appropriate name of
subzonal membrane: by Von Baer it was called the serous envelope. It
soon fuses with the zona radiata, or at any rate the zona ceases to be
distinguishable.
While the above changes are taking place in the amnion, the allantois
grows out from the hind gut as a vesicle lined by hypoblast, but
covered externally by a layer of splanchnic mesoblast (fig. 147, 3 and
4, al)[83].
The allantois soon becomes a flat sack, projecting into
the now largely developed space between the subzonal membrane and the
amnion, on the dorsal side of the embryo (fig. 147*, ALC). In some
cases it extends so as to cover the whole inner surface of the
subzonal membrane; in other cases again its extension is much more
limited. Its lumen may be retained or may become nearly or wholly
aborted. A fusion takes place between the subzonal membrane and the
adjoining mesoblastic wall of the allantois, and the two together give
rise to a secondary membrane round the ovum, known as the chorion.
Since however the allantois does not always come in contact with the
whole inner surface of the subzonal membrane, the term chorion is apt
to be somewhat vague; and in the rabbit, for instance, a considerable
part of the so-called chorion is formed by a fusion of the wall of the
yolk-sack with the subzonal membrane (fig. 148). The placental region
of the chorion may in such cases be distinguished as the true chorion,
from the remaining part which will be called the false chorion.
The mesoblast of the allantois, especially that part of it which
assists in forming the chorion, becomes highly vascular; the blood
being brought to it by two allantoic arteries continued from the
terminal bifurcation of the dorsal aorta, and returned to the body by
one, or rarely two, allantoic veins, which join the vitelline veins
from the yolk-sack. From the outer surface of the true chorion (fig.
147, 5, d, 148) villi grow out and fit into crypts or depressions
which have in the meantime made their
[Pg 238] appearance in the walls of the
uterus[84].
The villi of the chorion are covered by an epithelium
derived from the subzonal membrane, and are provided with a connective
tissue core containing an artery and vein and a capillary plexus
connecting them. In most cases they assume a more or less arborescent
form, and have a distribution on the surface of the chorion varying
characteristically in different species. The walls of the crypts into
which the villi are fitted also become highly vascular, and a
nutritive fluid passes from the maternal vessels of the placenta to
the fœtal vessels by a process of diffusion; while there is probably
also a secretion by the epithelial lining of the walls of the crypts,
which becomes absorbed by the vessels of the fœtal villi. The above
maternal and fœtal structures constitute together the organ known as
the placenta. The maternal portion consists essentially of the
vascular crypts in the uterine walls, and the fœtal portion of more
or less arborescent villi of the true chorion fitting into these
crypts.
While the placenta is being developed, the folding-off of the embryo
from the yolk-sack becomes more complete; and the yolk-sack remains
connected with the ileal region of the intestine by a narrow stalk,
the vitelline duct (fig. 147, 4 and 5 and fig. 147*), consisting of
the same tissues as the yolk-sack, viz. hypoblast and splanchnic
mesoblast. While the true splanchnic stalk of the yolk-sack is
becoming narrow, a somatic stalk connecting the amnion with the walls
of the embryo is also formed, and closely envelops the stalk both of
the allantois and the yolk-sack. The somatic stalk together with its
contents is known as the umbilical cord. The mesoblast of the
somatopleuric layer of the cord develops into a kind of gelatinous
tissue, which cements together the whole of the contents. The
allantoic arteries in the cord wind in a spiral manner round the
allantoic vein. The yolk-sack in many cases atrophies completely
before the close of intra-uterine life, but in other cases it is only
removed with the other embryonic membranes at birth. The
intra-embryonic portion of the allantoic stalk gives rise to two
structures, viz. to (1) the urinary bladder
[Pg 239] formed by a dilatation of
its proximal extremity, and to (2) a cord known as the urachus
connecting the bladder with the wall of the body at the umbilicus. The
urachus, in cases where the cavity of the allantois persists till
birth, remains as an open passage connecting the intra- and
extra-embryonic parts of the allantois. In other cases it gradually
closes, and becomes nearly solid before birth, though a delicate but
interrupted lumen would appear to persist in it. It eventually gives
rise to the ligamentum vesicæ medium.
At birth the fœtal membranes, including the fœtal portion of the
placenta, are shed; but in many forms the interlocking of the fœtal
villi with the uterine crypts is so close that the uterine mucous
membrane is carried away with the fœtal part of the placenta. It thus
comes about that in some placentæ the maternal and fœtal parts simply
separate from each other at birth, and in others the two remain
intimately locked together, and both are shed together as the
afterbirth. These two forms of placenta are distinguished as
non-deciduate and deciduate, but it has been shewn by Ercolani and
Turner that no sharp line can be drawn between the two types;
moreover, a larger part of the uterine mucous membrane than that
forming the maternal part of the placenta is often shed in the
deciduate Mammalia, and in the non-deciduate Mammalia it is probable
that the mucous membrane (not including vascular parts) of the
maternal placenta either peels or is absorbed.
Comparative history of the Mammalian fœtal membranes.
Two groups of Mammalia—the Monotremata and the Marsupialia—are
believed not to be provided with a true placenta.
The nature of the fœtal membranes in the Monotremata is not known.
Ova, presumably in an early stage of development, have been found free
in the uterus of Ornithorhyncus by Owen. The lining membrane of the
uterus was thickened and highly vascular. The females in which these
were found were killed early in October[85].
[Pg 240]
Marsupialia. Our knowledge of the fœtal membranes of the Marsupialia
is almost entirely due to Owen. In Macropus major he found that birth
took place thirty-eight days after impregnation. A fœtus at the
twentieth day of gestation measured eight lines from the mouth to the
root of the tail. The fœtus was enveloped in a large subzonal
membrane, with folds fitting into uterine furrows, but not adhering
to the uterus, and without villi. The embryo was enveloped in an
amnion reflected over the stalk of the yolk-sack, which was attached
by a filamentary pedicle to near the end of the ileum. The yolk-sack
was large and vascular, and was connected with the fœtal vascular
system by a vitelline artery and two veins. The yolk-sack was
partially adherent, especially at one part, to the subzonal membrane.
No allantois was observed. In a somewhat older fœtus of ten lines in
length there was a small allantois supplied by two allantoic arteries
and one vein. The allantois was quite free and not attached to the
subzonal membrane. The yolk-sack was more closely attached to the
subzonal membrane than in the younger embryo[86].
All Mammalia, other than the Monotremata and Marsupialia, have a true
allantoic placenta. The placenta presents a great variety of forms,
and it will perhaps be most convenient first to treat these varieties
in succession, and then to give a general exposition of their mutual
affinities[87].
Amongst the existing Mammals provided with a true placenta, the most
primitive type is probably retained by those forms in which the
placental part of the chorion is confined to a comparatively
restricted area on the dorsal side of the embryo; while the false
chorion is formed by the
[Pg 241] vascular yolk-sack fusing with the remainder
of the subzonal membrane. In all the existing forms with this
arrangement of fœtal membranes, the placenta is deciduate. This,
however, was probably not the case in more primitive forms from which
these are descended[88].
The placenta would appear from Ercolani’s
description to be simpler in the mole (Talpa) than in other species.
The Insectivora, Cheiroptera, and Rodentia are the groups with this
type of placenta; and since the rabbit, amongst the latter, has been
more fully worked out than other species, we may take it first.
The Rabbit. In the pregnant female Rabbit several ova are generally
found in each horn of the uterus. The general condition of the
egg-membranes at the time of their full development is shewn in fig.
148.
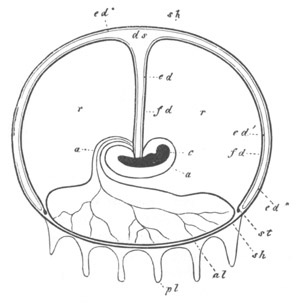
Fig. 148. diagrammatic longitudinal section of a
Rabbit’s ovum at an advanced stage of pregnancy. (From Kölliker
after Bischoff.)
e. embryo; a. amnion; a. urachus; al. allantois with
blood-vessels; sh. subzonal membrane; pl. placental villi; fd.
vascular layer of yolk-sack; ed. hypoblastic layer of yolk-sack;
ed´. inner portion of hypoblast, and ed´´. outer portion of
hypoblast lining the compressed cavity of the yolk-sack; ds.
cavity of yolk-sack; st. sinus terminalis; r. space filled with
fluid between the amnion, the allantois and the yolk-sack.
The embryo is surrounded by the amnion, which is comparatively small.
The yolk-sack (ds) is large and attached to the embryo by a long
stalk. It has the form of a flattened sack closely applied to about
two-thirds of the surface of the subzonal membrane. The outer wall of
this sack, adjoining the subzonal membrane, is formed of hypoblast
only; but the inner wall is covered by the mesoblast of the area
vasculosa, as indicated by the thick black line (fd). The vascular
area is bordered by the sinus terminalis (st). In an earlier stage
of development the yolk-sack had not the compressed form represented
in the figure. It is, however, remarkable that the vascular area never
extends over the whole yolk-sack; but the inner vascular wall of the
yolk-sack fuses with the outer, and with the subzonal membrane, and so
forms a false chorion, which receives its blood supply from the
yolk-sack. This part of the chorion does not develop vascular villi.
The allantois (al) is a simple vascular sack with a large cavity.
Part of its wall is applied to the subzonal membrane, and gives rise
to
[Pg 242] the true chorion, from which there project numerous vascular villi.
These fit into corresponding uterine crypts. It seems probable, from
Bischoff’s and Kölliker’s observations, that the subzonal membrane in
the area of the placenta becomes attached to the uterine wall, by
means of villi, even before its fusion with the allantois. In the
later periods of gestation the intermingling of the maternal and
fœtal parts of the placenta becomes very close, and the placenta is
truly deciduate. The cavity of the allantois persists till birth.
Between the yolk-sack, the allantois, and the embryo, there is left a
large cavity filled with an albuminous fluid.
The Hare does not materially differ in the arrangement of its fœtal
membranes from the Rabbit.
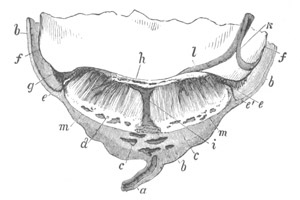
Fig. 149. Section through the placenta and adjacent parts of a Rat
one inch and a quarter long. (From Huxley.)
a. uterine vein; b. uterine wall; c. cavernous portion of
uterine wall; d. deciduous portion of uterus with cavernous
structure; i. large vein passing to the fœtal portion of the
placenta; f. false chorion supplied by vitelline vessels; k.
vitelline vessel; l. allantoic vessel; g. boundary of true
placenta; e, m, m, e. line of junction of the deciduate and
non-deciduate parts of the uterine wall.
In the Rat (Mus decumanus) (fig. 149) the sack of the allantois
completely atrophies before the close of fœtal life[89],
and there is
developed, at the junction of the maternal part of the placenta and
the unaltered mucous membrane of the uterus, a fold of the mucous
membrane which completely encapsules the whole chorion, and forms a
separate chamber for it, distinct from the general lumen of the
uterus. Folds of this nature, which are specially developed in Man and
Apes, are known as a decidua reflexa. The decidua reflexa of the Rat
is reduced to extreme tenuity, or even vanishes before the close of
gestation.
Guinea-pig. The development of the Guinea-pig is dealt with elsewhere,
but, so far as its peculiarities permit a comparison with the Rabbit,
the agreement between the two types appears to be fairly close.
[Pg 243]
The blastodermic vesicle of the Guinea-pig becomes completely
enveloped in a capsule of the uterine wall (decidua reflexa) (fig.
150). The epithelium of the blastodermic vesicle in contact with the
uterine wall is not epiblastic, but corresponds with the hypoblast of
the yolk-sack of other forms, and the mesoblast of the greater part of
the inner side of this becomes richly vascular (yk); the vascular
area being bounded by a sinus terminalis.
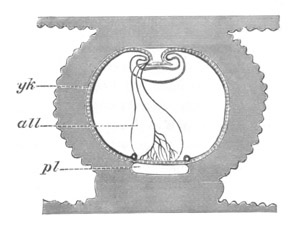
Fig. 150. Diagrammatic longitudinal section of an
ovum of a Guinea-pig and the adjacent uterine walls at an advanced
stage of pregnancy. (After Bischoff.)
yk. yolk-sack (umbilical vesicle) formed of an external
hypoblastic layer (shaded) and an internal mesoblastic vascular
layer (black). At the end of this layer is placed the sinus
terminalis; all. allantois; pl. placenta.
The external shaded parts are the uterine walls.
The blastodermic vesicle is so situated within its uterine capsule
that the embryo is attached to the part of it adjoining the free side
of the uterus. From the opposite side of the uterus, viz. that to
which the mesometrium is attached, there grow into the wall of the
blastodermic vesicle numerous vascular processes of the uterine wall,
which establish at this point an organic connection between the two
(pl). The blood-vessels of the blastodermic vesicle (yolk-sack) stop
short immediately around the area of attachment to the uterus; but at
a late period the allantois grows towards, and fuses with this area.
The blood-vessels of the allantois and of the uterus become
intertwined, and a disc-like placenta more or less similar to that in
the Rabbit becomes formed (pl). The cavity of the allantois, if
developed, vanishes completely.
In all the Rodentia the placenta appears to be situated on the
mesometric side of the uterus.
Insectivora. In the Mole (Talpa) and the Shrew (Sorex), the fœtal
membranes are in the main similar to those in the rabbit, and a
deciduate discoidal placenta is always present. It may be situated
anywhere in the circumference of the uterine tube. The allantoic
cavity persists (Owen), but the allantois only covers the placental
area of the chorion. The yolk-sack is persistent, and fuses with the
non-allantoic part of the subzonal membrane; which is rendered
vascular by its blood-vessels. There would seem to be (Owen) a small
decidua reflexa. A similar arrangement is found in the Hedgehog
(Erinaceus Europæus) (Rolleston), in which the placenta occupies the
typical dorsal position. It is not clear from Rolleston’s description
whether the yolk-sack persists till the close of fœtal life, but it
seems probable that it does so. There is a considerable reflexa which
does not,
[Pg 244] however, cover the whole chorion. In the Tenrec (Centetes)
the yolk-sack and non-placental part of the chorion are described by
Rolleston as being absent, but it seems not impossible that this may
have been owing to the bad state of preservation of the specimen. The
amnion is large. In the Cheiroptera (Vespertilio and Pteropus),
the yolk-sack is large, and coalesces with part of the chorion. The
large yolk-sack has been observed in Pteropus by Rolleston, and in
Vespertilio by Owen. The allantoic vessels supply the placenta only.
The Cheiroptera are usually uniparous.
Simiadæ and Anthropidæ. The fœtal membranes of Apes and Man, though
in their origin unlike those of the Rodentia and Insectivora, are in
their ultimate form similar to them, and may be conveniently dealt
with here. The early stages in the development of these membranes in
the human embryo have not been satisfactorily observed; but it is
known that the ovum, shortly after its entrance into the uterus,
becomes attached to the uterine wall, which in the meantime has
undergone considerable preparatory changes. A fold of the uterine wall
appears to grow round the blastodermic vesicle, and to form a complete
capsule for it, but the exact mode of formation of this capsule is a
matter of inference and not of observation. During the first fortnight
of pregnancy villi grow out, according to Allen Thomson over its whole
surface, but according to Reichert in a ring-like fashion round the
edge of the somewhat flattened ovum, and attach it to the uterus. The
further history of the early stages is extremely obscure, and to a
large extent a matter of speculation: what is known with reference to
it will be found in a special section, but I shall here take up the
history at about the fourth week.
At this stage a complete chorion has become formed, and is probably
derived from a growth of the mesoblast of the allantois (unaccompanied
by the hypoblast) round the whole inner surface of the subzonal
membrane. From the whole surface of the chorion there project branched
vascular processes, covered by an epithelium. The allantois is without
a cavity, but a hypoblastic epithelium is present in the allantoic
stalk, through which it does not, however, form a continuous tube. The
blood-vessels of the chorion are derived from the usual allantoic
arteries and vein. The general condition of the embryo and of its
membranes at this period is shewn diagrammatically in fig. 147, 5.
Around the embryo is seen the amnion, already separated by a
considerable interval from the embryo. The yolk-sack is shewn at ds.
Relatively to the other parts it is considerably smaller than it was
at an earlier stage. The allantoic stalk is shewn at al. Both it and
the stalk of the yolk-sack are enveloped by the amnion (am). The
chorion with its vascular processes surrounds the whole embryo.
It may be noted that the condition of the chorion at this stage is
very similar to that of the normal diffused type of placenta,
described in the sequel.
While the above changes are taking place in the embryonic membranes,
the blastodermic vesicle greatly increases in size, and forms a
considerable projection from the upper wall of the uterus. Three
regions of the uterine
[Pg 245] wall, in relation to the blastodermic vesicle,
are usually distinguished; and since the superficial parts of all of
these are thrown off with the afterbirth, each of them is called a
decidua. They are represented at a somewhat later stage in fig. 151.
There is (1) the part of the wall reflected over the blastodermic
vesicle, called the decidua reflexa (dr); (2) the part of the wall
forming the area round which the reflexa is inserted, called the
decidua serotina (ds); (3) the general wall of the uterus, not
related to the embryo, called the decidua vera (du).
The decidua reflexa and serotina together envelop the chorion, the
processes of which fit into crypts in them. At this period both of
them are highly and nearly uniformly vascular. The general cavity of
the uterus is to a large extent obliterated by the ovum, but still
persists as a space filled with mucus, between the decidua reflexa and
the decidua vera.
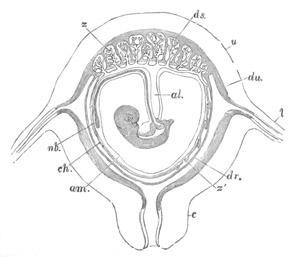
Fig. 151. Diagrammatic section of pregnant human uterus with
contained fœtus. (From Huxley after Longet.)
al. allantoic stalk; nb. umbilical vesicle; am. amnion; ch.
chorion; ds. decidua serotina; du. decidua vera; dr. decidua
reflexa; l Fallopian tube; c. cervix uteri; u. uterus; z.
fœtal villi of true placenta; z´. villi of non-placental part of
chorion.
The changes which ensue from this period onwards are fully known. The
amnion continues to dilate (its cavity being intensely filled with
amniotic fluid) till it comes very close to the chorion (fig. 151,
am); from which, however, it remains separated by a layer of
gelatinous tissue. The villi of the chorion in the region covered by
the decidua reflexa, gradually cease to be vascular, and partially
atrophy, but in the region in contact with the decidua serotina
increase and become more vascular and more arborescent (fig. 151,
z). The former region becomes known as the chorion læve, and the
latter as the chorion frondosum. The chorion frondosum, together with
the decidua serotina, gives rise to the placenta.
[Pg 246]
Although the vascular supply is cut off from the chorion læve, the
processes on its surface do not completely abort. It becomes, as the
time of birth approaches, more and more closely united with the
reflexa, till the union between the two is so close that their exact
boundaries cannot be made out. The umbilical vesicle (fig. 151, nb),
although it becomes greatly reduced in size and flattened, persists in
a recognisable form till the time of birth.
As the embryo enlarges, the space between the decidua vera and decidua
reflexa becomes reduced, and finally the two parts unite together. The
decidua vera is mainly characterised by the presence of peculiar
roundish cells in its subepithelial tissue, and by the disappearance
of a distinct lining of epithelial cells. During the whole of
pregnancy it remains highly vascular. The decidua reflexa, on the
disappearance of the vessels in the chorion læve, becomes
non-vascular. Its tissue undergoes changes in the main similar to
those of the decidua vera, and as has been already mentioned, it fuses
on the one hand with the chorion, and on the other with the decidua
vera. The membrane resulting from its fusion with the latter structure
becomes thinner and thinner as pregnancy advances, and is reduced to a
thin layer at the time of birth.
The placenta has a somewhat discoidal form, with a slightly convex
uterine surface and a concave embryonic surface. At its edge it is
continuous both with the decidua reflexa and decidua vera. Near the
centre of the embryonic surface is implanted the umbilical cord. As
has already been mentioned, the placenta is formed of the decidua
serotina and the fœtal villi of the chorion frondosum. The fœtal and
maternal tissues are far more closely united (fig. 152) than in the
forms described above. The villi of the chorion, which were originally
comparatively simple, become more and more complicated, and assume an
extremely arborescent form. Each of them contains a vein and an
artery, which subdivide to enter the complicated ramifications; and
are connected together by a rich anastomosis. The villi are formed
mainly of connective tissue, but are covered by an epithelial layer
generally believed to be derived from the subzonal membrane; but, as
was first stated by Goodsir, and has since been more fully shewn by
Ercolani and Turner, this epithelial layer is really a part of the
cellular decidua serotina of the uterine wall, which has become
adherent to the villi in the development of the placenta (fig. 161,
g). The placenta is divided into a number of lobes, usually called
cotyledons, by septa which pass towards the chorion. These septa,
which belong to the serotina, lie between the arborescent villi of the
chorion. The cotyledons themselves consist of a network of tissue
permeated by large vascular spaces, formed by the dilatation of the
maternal blood-vessels of the serotina, into which the ramifications
of the fœtal villi project. In these spaces they partly float freely,
and partly are attached to delicate trabeculæ of the maternal tissue
(fig. 161, G). They are, of course, separated from the maternal
blood by the uterine epithelial layer before mentioned. The blood is
brought to the maternal part of the placenta by spirally coiled
arteries, which do not divide into capillaries, but
[Pg 247] open into the
large blood-spaces already spoken of. From these spaces there pass off
oblique utero-placental veins, which pierce the serotina, and form a
system of large venous sinuses in the adjoining uterine wall (fig.
152, F), and eventually fall into the general uterine venous system.
At birth the whole placenta, together with the fused decidua vera, and
reflexa, with which it is continuous, is shed; and the blood-vessels
thus ruptured are closed by the contraction of the uterine wall.
Fig. 152. Section of the human uterus and placenta at the thirtieth
week of pregnancy. (From Huxley after Ecker.)
A. umbilical cord; B. chorion; C. fœtal villi separated by
processes of the decidua serotina, D; E, F, G. walls of
uterus.
The fœtal membranes and the placenta of the Simiadæ (Turner, No. 225)
are in most respects closely similar to those in Man; but the placenta
is, in most cases, divided into two lobes, though in the Chimpanzee,
Cynocephalus, and the Apes of the New World, it appears to be single.
The types of deciduate placenta so far described, are usually
classified by anatomists as discoidal placentæ, although it must be
borne in mind that they differ very widely. In the Rodentia,
Insectivora, and Cheiroptera there is a (usually) dorsal placenta,
which is co-extensive with the area of contact between the allantois
and the subzonal membrane, while the yolk-sack adheres to a large part
of the subzonal membrane. In Apes and Man the allantois spreads over
the whole inner surface of the subzonal membrane; the placenta is on
the ventral side of the embryo, and occupies only a small part of the
surface of the allantois. The placenta of Apes and Man might be
[Pg 248] called
metadiscoidal, in order to distinguish it from the primitive discoidal
placenta of the Rodentia and Insectivora.
In the Armadilloes (Dasypus) the placenta is truly discoidal and
deciduate (Owen and Kölliker). Alf. Milne Edwards states that in
Dasypus novemcinctus the placenta is zonary, and both Kölliker and he
found four embryos in the uterus, each with its own amnion, but the
placenta of all four united together; and all four enclosed in a
common chorion. A reflexa does not appear to be present. In the Sloths
the placenta approaches the discoidal type (Turner, No. 218). It
occupies in Cholæpus Hoffmanni about four-fifths of the surface of the
chorion, and is composed of about thirty-four discoid lobes. It is
truly deciduate, and the maternal capillaries are replaced by a system
of sinuses (fig. 161). The amnion is close to the inner surface of the
chorion. A dome-shaped placenta is also found amongst the Edentata in
Myrmecophaga and Tamandua (Milne Edwards, No. 208).
Zonary Placenta. Another form of deciduate placenta is known as the
zonary. This form of placenta occupies a broad zone of the chorion,
leaving the two poles free. It is found in the Carnivora, Hyrax,
Elephas, and Orycteropus.
It is easy to understand how the zonary placenta may be derived from
the primitive arrangement of the membranes (vide p. 240) by the
extension of a discoidal placental area to a zonary area, but it
is possible that some of the types of zonary placenta may have been
evolved from the concentration of a diffused placenta (vide p. 261)
to a zonary area. The absence of the placenta at the extreme poles of
the chorion is explained by the fact of their not being covered by a
reflection of the uterine mucous membrane. In the later periods of
pregnancy the placental area becomes, however, in most forms much more
restricted than the area of contact between the uterus and chorion.
In the Dog[90],
which may be taken as type, there is a large vascular
yolk-sack formed in the usual way, which does not however fuse with
the chorion. It extends at first quite to the end of the citron-shaped
ovum, and persists till birth. The allantois first grows out on the
dorsal side of the embryo, where it coalesces with the subzonal
membrane, over a small discoidal area.
Before the fusion of the allantois with the subzonal membrane, there
grow out from the whole surface of the external covering of the ovum,
except the poles, numerous non-vascular villi, which fit into uterine
crypts. When the allantois adheres to the subzonal membrane vascular
processes grow out from it into these villi. The vascular villi so
formed are of course at first confined to the disc-shaped area of
adhesion between the allantois and the subzonal membrane; and there
is thus formed a rudimentary discoidal placenta, closely resembling
that of the Rodentia. The view previously stated, that the zonary
placenta is derived from the discoidal one, receives from this fact a
strong support.
The cavity of the allantois is large, and its inner part is in contact
with
[Pg 249] the amnion. The area of adhesion between the outer part of the
allantois and subzonal membrane gradually spreads over the whole
interior of the subzonal membrane, and vascular villi are formed over
the whole area of adhesion except at the two extreme poles of the egg.
The last part to be covered is the ventral side where the yolk-sack
adjoins the subzonal membrane.
During the extension of the allantois its cavity persists, and its
inner part covers not only the amnion, but also the yolk-sack. It
adheres to the amnion and supplies it with blood-vessels (Bischoff).
With the full growth of the allantois there is formed a broad
placental zone, with numerous branched villi, fitting into
corresponding pits which become developed in the uterine walls. The
maternal and fœtal structures become closely interlocked and highly
vascular; and at birth a large part of the maternal part is carried
away with the placenta; some of it however still remains attached to
the muscular wall of the uterus. The villi of the chorion do not fit
into uterine glands. The zone of the placenta diminishes greatly in
proportion to the chorion as the latter elongates, and at the full
time the breadth of the zone is not more than about one-fifth of the
whole length of the chorion.
At the edge of the placental zone there is a very small portion of the
uterine mucous membrane reflected over the non-placental part of the
chorion, which forms a small reflexa analogous with the reflexa in
Man.
The Carnivora generally closely resemble the Dog, but in the Cat the
whole of the maternal part of the placenta is carried away with the
fœtal parts, so that the placenta is more completely deciduate than
in the Dog. In the Grey Seal (Halichœrus gryphus, Turner, No. 219)
the general arrangement of the fœtal membranes is the same as in the
other groups of the Carnivora, but there is a considerable reflexa
developed at the edge of the placenta. The fœtal part of the placenta
is divided by a series of primary fissures which give off secondary
and tertiary fissures. Into the fissures there pass vascular laminæ of
the uterine wall. The general surface of the fœtal part of the
placenta between the fissures is covered by a greyish membrane formed
of the coalesced terminations of the fœtal villi.
The structure of the placenta in Hyrax is stated by Turner (No. 221)
to be very similar to that in the Felidæ. The allantoic sack is large,
and covers the whole surface of the subzonal membrane. The amnion is
also large, but the yolk-sack would seem to disappear at an early
stage, instead of persisting, as in the Carnivora, till the close of
fœtal life.
The Elephant (Owen, Turner, Chapman) is provided with a zonary
deciduate placenta, though a villous patch is present near each pole
of the chorion.
Turner (No. 220) has shewn that in Orycteropus there is present a
zonary placenta, which differs however in several particulars from the
normal zonary placenta of the Carnivora; and it is even doubtful
whether it is truly deciduate. There is a single embryo, which fills
up the body of the uterus and also projects into only one of the
horns. The placenta forms a
[Pg 250] broad median zone, leaving the two poles
free. The breadth of the zone is considerably greater than is usual in
Carnivora, one-half or more of the whole longitudinal diameter of the
chorion being occupied by the placenta. The chorionic villi are
arborescent, and diffusely scattered, and though the maternal and
fœtal parts are closely interwoven, it has not been ascertained
whether the adhesion between them is sufficient to cause the maternal
subepithelial tissue to be carried away with the fœtal part of the
placenta at birth. The allantois is adherent to the whole chorion, the
non-placental parts of which are vascular. In the umbilical cord a
remnant of the allantoic vesicle was present in the embryos observed
by Turner, but in the absence of a large allantoic cavity the Cape
Ant-eater differs greatly from the Carnivora. The amnion and allantois
were in contact, but no yolk sack was observed.
Non-deciduate placenta. The remaining Mammalia are characterized by a
non-deciduate placenta; or at least by a placenta in which only parts
of the maternal epithelium and no vascular maternal structures are
carried away at parturition. The non-deciduate placentæ are divided
into two groups: (1) The polycotyledonary placenta, characteristic of
the true Ruminantia (Cervidæ, Antilopidæ, Bovidæ, Camelopardalidæ);
(2) the diffused placenta found in the other non-deciduate Mammalia,
viz. the Perissodactyla, the Suidæ, the Hippopotamidæ, the Tylopoda,
the Tragulidæ, the Sirenia, the Cetacea, Manis amongst the Edentata,
and the Lemuridæ. The polycotyledonary form is the most
differentiated; and is probably a modification of the diffused form.
The diffused non-deciduate placenta is very easily derived from the
primitive type (p. 240) by an extension of the allantoic portion of
the chorion; and the exclusion of the yolk-sack from any participation
in forming the chorion.
The possession in common of a diffused type of placenta is by no means
to be regarded as a necessary proof of affinity between two groups,
and there are often, even amongst animals possessing a diffused form
of placenta, considerable differences in the general arrangement of
the embryonic membranes.
Ungulata. Although the Ungulata include forms with both cotyledonary
and diffused placentæ, the general arrangement of the embryonic
membranes is so similar throughout the group, that it will be
convenient to commence with a description of them, which will fairly
apply both to the Ruminantia and to the other forms.
The blastodermic vesicle during the early stages of development lies
freely in the uterus; and no non-vascular villi, similar to those of
the Dog or the Rabbit, are formed before the appearance of the
allantois. The blastodermic vesicle has at first the usual spherical
form, but it grows out at an early period, and with prodigious
rapidity, into two immensely long horns; which in cases where there is
only one embryo are eventually prolonged for the whole length of the
two horns of the uterus. The embryonic area is formed in the usual
way, and its long axis is placed at right angles to that of the
vesicle. On the formation of an amnion there
[Pg 251] is formed the usual
subzonal membrane, which soon becomes separated by a considerable
space from the yolk-sack (fig. 153). The yolk-sack is, however,
continued into two elongated processes (yk), which pass to the two
extremities of the subzonal membrane. It is supplied with the normal
blood-vessels. As soon as the allantois appears (fig. 153 all), it
grows out into a right and a left process, which rapidly fill the
whole free space within the subzonal membrane and in many cases,
e.g. the Pig (Von Baer), break through the ends of the membrane,
from which they project as the diverticula allantoidis. The cavity of
the allantois remains large, but the lining of hypoblast becomes
separated from the mesoblast, owing to the more rapid growth of the
latter. The mesoblast of the allantois applies itself externally to
the subzonal membrane to form the chorion[91],
and internally to the
amnion, the cavity of which remains very small. The chorionic portion
of the allantoic mesoblast is very vascular, and that applied to the
amnion also becomes vascular in the later developmental periods.
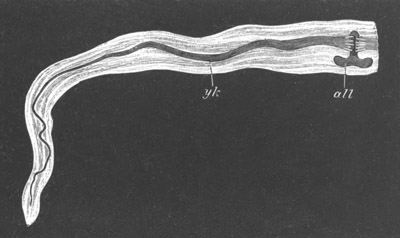
Fig. 153. Embryo and fœtal membranes of a young embryo Roe-deer.
(After Bischoff.)
yk. yolk-sack; all. allantois just sprouting as a bilobed sack.
The horns of the yolk-sack gradually atrophy, and the whole yolk-sack
disappears some time before birth.
Where two or more embryos are present in the uterus, the chorions of
the several embryos may unite where they are in contact.
From the chorion there grow out numerous vascular villi, which fit
into corresponding pits in the uterine walls. According to the
distribution of these villi, the allantois is either diffused or
polycotyledonary.
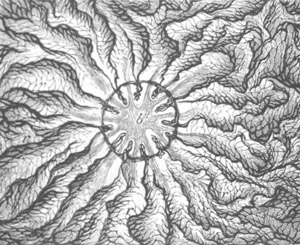
Fig. 154. Portion of the injected chorion of a Pig, slightly magnified. (From Turner.)
The figure shews a minute circular spot (b) (enclosed by a
vascular ring) from which villous ridges (r) radiate.
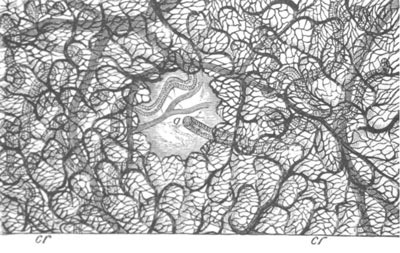
Fig. 155. Surface-view of the injected uterine mucosa of a gravid Pig. (From Turner.)
The fig. shews a circular non-vascular spot where a gland opens
(g) surrounded by numerous vascular crypts (cr).
The pig presents the simplest type of diffused placenta. The villi of
[Pg 252]
the surface of the chorion cover a broad zone, leaving only the two
poles free; their arrangement differs therefore from that in a zonary
placenta in the greater breadth of the zone covered by them. The villi
have the form of simple papillæ, arranged on a series of ridges, which
are highly vascular as compared with the intervening valleys. If an
injected chorion is examined (fig. 154), certain clear non-vascular
spots are to be seen (b), from which the ridges of villi radiate.
The surface of the uterus adapts itself exactly to the elevations of
the chorion; and the furrows which receive the
[Pg 253] chorionic ridges are
highly vascular (fig. 155). On the other hand, there are non-vascular
circular depressions corresponding to the non-vascular areas on the
chorion; and in these areas, and in these alone, the glands of the
uterus open (fig. 155 g) (Turner). The maternal and fœtal parts of
the placenta in the pig separate with very great ease.
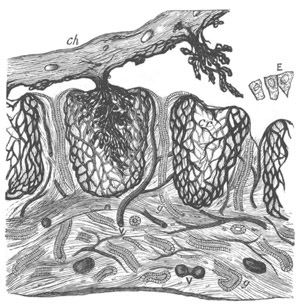
Fig. 156. Vertical section through the injected placenta of a Mare.
(From Turner.)
ch. chorion with its villi partly in situ and partly drawn out
of the crypts (cr); E. loose epithelial cells which formed the
lining of the crypt; g. uterine glands; v. blood-vessels.
In the mare (Turner), the fœtal villi are arranged in a less definite
zonary band than in the pig, though still absent for a very small area
at both poles of the chorion, and also opposite the os uteri. The
filiform villi, though to the naked eye uniformly scattered, are, when
magnified, found to be clustered together in minute cotyledons, which
fit into corresponding uterine crypts (fig. 156). Surrounding the
uterine crypts are reticulate ridges on which are placed the openings
of the uterine glands. The remaining Ungulata with diffused placentæ
do not differ in any important particulars from those already
described.
The polycotyledonary form of placenta is found in the Ruminantia
alone. Its essential character consists in the fœtal villi not being
uniformly distributed, but collected into patches or cotyledons which
form as it were so many small placentæ (fig. 157). The fœtal villi of
these patches fit into corresponding pits in thickened patches of the
wall of the uterus (figs. 158 and 159). In many cases (Turner), the
interlocking of the maternal and fœtal structures is so close that
large parts of the maternal
[Pg 254] epithelium are carried away when the
fœtal villi are separated from the uterus. The glands of the uterus
open in the intervals between the cotyledons. The character of the
cotyledons differs greatly in different types. The maternal parts are
cup-shaped in the sheep, and mushroom-shaped in the cow. There are
from 60-100 in the cow and sheep, but
[Pg 255] only about five or six in the
Roe-deer. In the Giraffe there are, in addition to larger and smaller
cotyledons, rows and clusters of short villi, so that the placenta is
more or less intermediate between the polycotyledonary and diffused
types (Turner). A similarly intermediate type of placenta is found in
Cervus mexicanus (Turner).
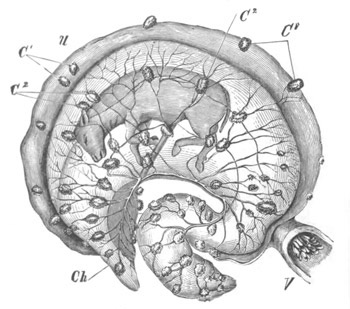
Fig. 157. Uterus of a Cow in the middle of pregnancy laid open.
(From Huxley after Colin.)
V. vagina; U. uterus; Ch. chorion; C1. uterine cotyledons;
C2. fœtal cotyledons.
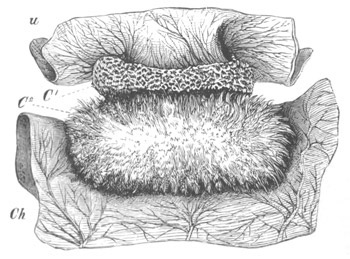
Fig. 158. Cotyledon of a Cow, the fœtal and maternal parts half
separated. (From Huxley after Colin.)
u. uterus; Ch. chorion; C1. maternal part of cotyledon;
C2. fœtal part.
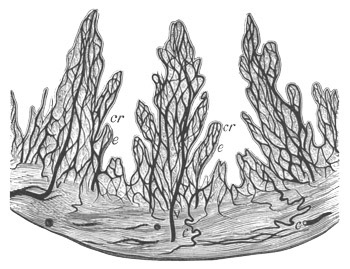
Fig. 159. Semi-diagrammatic vertical section through a portion of a
maternal cotyledon of a Sheep. (From Turner.)
cr. crypts; e. epithelial lining of crypts; v. veins and c.
curling arteries of subepithelial connective tissue.
The groups not belonging to the Ungulata which are characterized by
the possession of a diffused placenta are the Sirenia, the Cetacea,
Manis, and the Lemuridæ.
Sirenia. Of the Sirenia, the placentation of the Dugong is known from
some observations of Harting (No. 201).
It is provided with a diffuse and non-deciduate placenta; with the
villi generally scattered except at the poles. The umbilical vesicle
vanishes early.
Cetacea. In the Cetacea, if we may generalize from Turner’s
observations on Orca Gladiator and the Narwhal, and those of Anderson
(No. 191) on Platanista and Orcella, the blastodermic vesicle is very
much elongated, and prolonged unsymmetrically into two horns. The
mesoblast (fig. 160) of the allantois would appear to grow round the
whole inner surface of the subzonal membrane, but the cavity of the
allantois only persists as a widish sack on the ventral aspect of the
embryo (al). The amnion (am) is enormous, and is dorsally in
apposition with, and apparently coalesces with the chorion, and
ventrally covers the inner wall of the persistent allantoic sack. The
chorion, except for a small area at the two poles and opposite the os
uteri, is nearly uniformly covered with villi, which are more numerous
[Pg 256] than in fig. 160. In the large size of the amnion, and small
dimensions of the persistent allantoic sack, the Cetacea differ
considerably from the Ungulata.
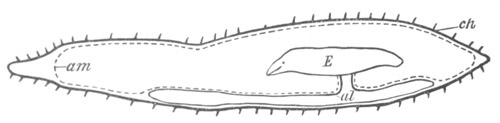
Fig. 160. Diagram of the fœtal membranes in Orca gladiator. (From
Turner.)
ch. chorion; am. amnion; al. allantois; E. embryo.
Manis. Manis amongst the Edentata presents a type of diffused
placenta[92].
The villi are arranged in ridges which radiate from a
non-villous longitudinal strip on the concave surface of the chorion.
Manis presents us with the third type of placenta found amongst the
Edentata. On this subject, I may quote the following sentence from
Turner (Journal of Anat. and Phys., vol. X., p. 706).
“The Armadilloes (Dasypus), according to Professor Owen, possess a
single, thin, oblong, disc-shaped placenta; a specimen, probably
Dasypus gymnurus, recently described by Kölliker[93],
had a
transversely oval placenta, which occupied the upper 2⁄3rds of the
uterus. In Manis, as Dr Sharpey has shewn, the placenta is diffused
over the surfaces of the chorion and uterine mucosa. In Myrmecophaga
and Tamandua, as MM. Milne Edwards have pointed out, the placenta is
set on the chorion in a dome-like manner. In the Sloths, as I have
elsewhere described, the placenta is dome-like in its general form,
and consists of a number of aggregated, discoid lobes. In Orycteropus,
as I have now shewn, the placenta is broadly zonular.”
Lemuridæ. The Lemurs in spite of their affinities with the Primates
and Insectivora have, as has been shewn by Milne Edwards and Turner,
an apparently very different form of placenta. There is only one
embryo, which occupies the body and one of the cornua of the uterus.
The yolk-sack disappears early, and the allantois (Turner) bulges out
into a right and left lobe, which meet above the back of the embryo.
The cavity of the allantois persists, and the mesoblast of the outer
wall fuses with the subzonal membrane (the hypoblastic epithelium
remaining distinct) to give rise to the chorion.
On the surface of the chorion are numerous vascular villi, which fit
into uterine crypts. They are generally distributed, though absent at
the two
[Pg 257] ends of the chorion and opposite the os uteri. Their
distribution accords with Turner’s diffused type. Patches bare of
villi correspond with smooth areas on the surface of the uterine
mucosa in which numerous utricular glands open. There is no reflexa.
Although the Lemurian type of placenta undoubtedly differs from that
of the Primates, it must be borne in mind that the placenta of the
Primates may easily be conceived to be derived from a Lemurian form of
placenta. It will be remembered that in Man, before the true placenta
becomes developed, there is a condition with simple vascular villi
scattered over the chorion. It seems very probable that this is a
repetition of the condition of the placenta of the ancestors of the
Primates which has probably been more or less retained by the Lemurs.
It was mentioned above that the resemblance between the metadiscoidal
placenta of Man and that of the Cheiroptera, Insectivora and Rodentia
is rather physiological than morphological.
Comparative histology of the Placenta.
It does not fall within the province of this work to treat from a
histological standpoint the changes which take place in the uterine
walls during pregnancy. It will, however, be convenient to place
before the reader a short statement of the relations between the
maternal and fœtal tissues in the different varieties of placenta.
This subject has been admirably dealt with by Turner (No. 222), from
whose paper fig. 161 illustrating this subject is taken.
The simplest known condition of the placenta is that found in the pig
(B). The papilla-like fœtal villi fit into the maternal crypts. The
villi (v) are formed of a connective tissue cone with capillaries,
and are covered by a layer of very flat epithelium (e) derived from
the subzonal membrane. The maternal crypts are lined by the uterine
epithelium (e´), immediately below which is a capillary flexus. The
maternal and fœtal vessels are here separated by a double epithelial
layer. The same general arrangement holds good in the diffused
placentæ of other forms, and in the polycotyledonary placenta of the
Ruminantia, but the fœtal villi (C) in the latter acquire an
arborescent form. The maternal vessels retain the form of capillaries.
In the deciduate placenta a considerably more complicated arrangement
is usually found. In the typical zonary placenta of the fox and cat (D
and E), the maternal tissue is broken up into a complete trabecular
mesh-work, and in the interior of the trabeculæ there run dilated
maternal capillaries (d´). The trabeculæ are covered by a more or
less columnar uterine epithelium (e´), and are in contact on every
side with fœtal villi. The capillaries of the fœtal villi preserve
their normal size, and the villi are covered by a flat epithelial
layer (e).
In the sloth (F) the maternal capillaries become still more dilated,
and the epithelium covering them is formed of very flat polygonal
cells.
[Pg 258]
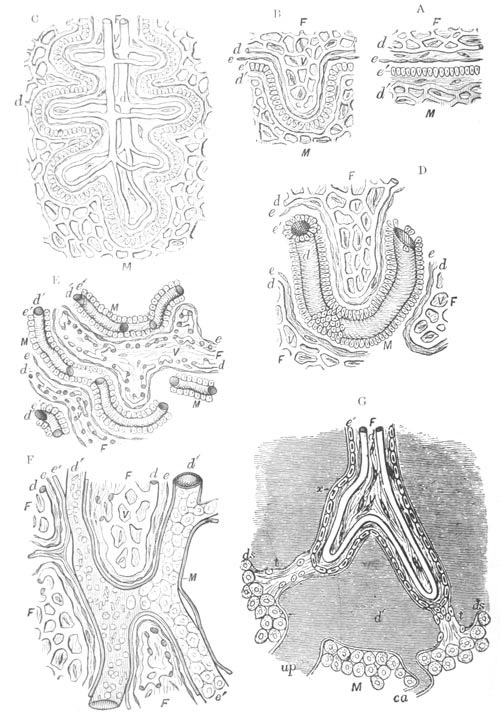
Fig. 161. Diagrammatic representations of the minute structure of
the placenta. (From Turner.)
F. the fœtal; M. the maternal placenta; e. epithelium of
chorion; e´. epithelium of maternal placenta; d. fœtal
blood-vessels; d´. maternal blood-vessels; v. villus.
A. Placenta in its most generalized form.
B. Structure of placenta of a Pig.
C. Structure of placenta of a Cow.
D. Structure of placenta of a Fox.
E. Structure of placenta of a Cat.
F. Structure of placenta of a Sloth. On the right side of the figure
the flat maternal epithelial cells are shewn in situ. On the
left side they are removed, and the dilated maternal vessel with
its blood-corpuscles is exposed.
G. Structure of Human placenta. In addition to the letters already
referred to ds, ds. represents the decidua serotina of the
placenta; t, t. trabeculæ of serotina passing to the fœtal
villi; ca. curling artery; up. utero-placental vein; x. a
prolongation of maternal tissue on the exterior of th villus
outside the cellular layer e´, which may represent either the
endothelium of the maternal blood-vessel or delicate connective
tissue belonging to the serotina, or both. The layer e´
represents maternal cells derived from the serotina. The layer of
fœtal epithelium cannot be seen on the villi of the fully-formed
human placenta.
In the human placenta (G), as in that of Apes, the greatest
modification
[Pg 259] is found in that the maternal vessels have completely
lost their capillary form, and have become expanded into large freely
communicating sinuses (d´). In these sinuses the fœtal villi hang
for the most part freely, though occasionally attached to their walls
(t). In the late stages of fœtal life there is only one epithelial
layer (e´) between the maternal and fœtal vessels, which closely
invests the fœtal villi, but, as shewn by Turner and Ercolani, is
part of the uterine tissue. In the fœtal villi the vessels retain
their capillary form.
Evolution of the Placenta.
From Owen’s observations on the Marsupials it is clear that the
yolk-sack in this group plays an important, if not the most important
part, in absorbing the maternal nutriment destined for the fœtus. The
fact that in Marsupials both the yolk-sack and the allantois are
functional in rendering the chorion vascular makes it à priori
probable that this was also the case in the primitive types of the
Placentalia, and this deduction is supported by the fact that in the
Rodentia, Insectivora and Cheiroptera this peculiarity of the fœtal
membranes is actually found. In the primitive Placentalia there was
probably present a discoidal allantoic region of the chorion, from
which simple fœtal villi, like those of the pig (fig. 161 B),
projected into uterine crypts; but it is not certain how far the
umbilical part of the chorion, which was no doubt vascular, may also
have been
[Pg 260] villous. From such a primitive type of fœtal membranes
divergences in various directions have given rise to the types of
fœtal membranes now existing.
In a general way it may be laid down that variations in any direction
which tended to increase the absorbing capacities of the chorion would
be advantageous. There are two obvious ways in which this might be
done, viz. (1) by increasing the complexity of the fœtal villi and
maternal crypts over a limited area, (2) by increasing the area of the
part of the chorion covered by placental villi. Various combinations
of the two processes would also of course be advantageous.
The most fundamental change which has taken place in all the existing
Placentalia is the exclusion of the umbilical vesicle from any
important function in the nutrition of the fœtus.
The arrangement of the fœtal parts in the Rodentia, Insectivora and
Cheiroptera may be directly derived from the primitive form by
supposing the villi of the discoidal placental area to have become
more complex, so as to form a deciduate discoidal placenta; while
the yolk-sack still plays a part, though physiologically an
unimportant part, in rendering the chorion vascular.
In the Carnivora again we have to start from the discoidal placenta,
as shewn by the fact that the allantoic region of the placenta is at
first discoidal (p. 248). A zonary deciduate placenta indicates an
increase both in area and in complexity. The relative diminution of
the breadth of the placental zone in late fœtal life in the zonary
placenta of the Carnivora is probably due to its being on the whole
advantageous to secure the nutrition of the fœtus by insuring a more
intimate relation between the fœtal and maternal parts, than by
increasing their area of contact. The reason of this is not obvious,
but as mentioned below, there are other cases where it can be shewn
that a diminution in the area of the placenta has taken place,
accompanied by an increase in the complexity of its villi.
The second type of differentiation from the primitive form of
discoidal placenta is illustrated by the Lemuridæ, the Suidæ, and
Manis. In all these cases the area of the placental villi appears to
have increased so as to cover nearly the whole subzonal membrane,
without the villi increasing to any great
[Pg 261] extent in complexity. From
the diffused placenta covering the whole surface of the chorion,
differentiations appear to have taken place in various directions. The
metadiscoidal placenta of Man and Apes, from its mode of ontogeny
(p. 248), is clearly derived from a diffused placenta—very probably
similar to that of Lemurs—by a concentration of the fœtal villi,
which are originally spread over the whole chorion, to a disc-shaped
area, and by an increase in their arborescence.
The polycotyledonary forms of placenta are due to similar
concentrations of the fœtal villi of an originally diffused placenta.
In the Edentata we have a group with very varying types of placenta.
Very probably these may all be differentiations within the group
itself from a diffused placenta, such as that found in Manis. The
zonary placenta of Orycteropus is capable of being easily derived from
that of Manis, by the disappearance of the fœtal villi at the two
poles of the ovum. The small size of the umbilical vesicle in
Orycteropus indicates that its discoidal placenta is not, like that in
Carnivora, directly derived from a type with both allantoic and
umbilical vascularization of the chorion. The discoidal and
dome-shaped placentæ of the Armadilloes, Myrmecophaga, and the Sloths
may easily have been formed from a diffused placenta, just as the
discoidal placenta of the Simiadæ and Anthropidæ appears to have been
formed from a diffused placenta like that of the Lemuridæ.
The presence of zonary placentæ in Hyrax and Elephas does not
necessarily afford any proof of affinity of these types with the
Carnivora. A zonary placenta may quite easily be derived from a
diffused placenta; and the presence of two villous patches at the
poles of the chorion in Elephas indicates that this was very probably
the case with the placenta of this form.
Although it is clear from the above considerations that the placenta
is capable of being used to some extent in classification, yet at the
same time the striking resemblances which can exist between such
essentially different forms of placenta, as for instance those of Man
and the Rodentia, are likely to prevent it being employed, except in
conjunction with other characters.
[Pg 262]Special types of development.
The Guinea-pig, Cavia cobaya. Many years ago Bischoff (No. 176) shewed
that the development of the guinea-pig was strikingly different from
that of other Mammalia. His statements, which were at first received
with some doubt, have been in the main fully confirmed by Hensen (No.
182) and Schäfer (No. 190), but we are still as far as ever from
explaining the mystery of the phenomenon.
The ovum, enclosed by the zona radiata, passes into the Fallopian tube
and undergoes a segmentation which has not been studied with great
detail. On the close of segmentation, about six days after
impregnation, it assumes (Hensen) a vesicular form not unlike that of
other Mammalia. To the inner side of one wall of this vesicle is
attached a mass of granular cells similar to the hypoblastic mass in
the blastodermic vesicle of the rabbit. The egg still lies freely in
the uterus, and is invested by its zona radiata. The changes which
next take place are in spite of Bischoff’s, Reichert’s (No. 188) and
Hensen’s observations still involved in great obscurity. It is
certain, however, that during the course of the seventh day a
ring-like thickening of the uterine mucous membrane, on the free side
of the uterus, gives rise to a kind of diverticulum of the uterine
cavity, in which the ovum becomes lodged. Opposite the diverticulum
the mucous membrane of the mesometric side of the uterus also becomes
thickened, and this thickening very soon (shortly after the seventh
day) unites with the wall of the diverticulum, and completely shuts
off the ovum in a closed capsule.
The history of the ovum during the earlier period of its inclusion in
the diverticulum of the uterine wall is not satisfactorily elucidated.
There appears in the diverticulum during the eighth and succeeding
days a cylindrical body, one end of which is attached to the uterine
walls at the mouth of the diverticulum. The opposite end of the
cylinder is free, and contains a solid body.
With reference to the nature of this cylinder two views have been put
forward. Reichert and Hensen regard it as an outgrowth of the uterine
wall, while the body within its free apex is regarded as the ovum.
Bischoff and Schäfer maintain that the cylinder itself is the ovum
attached to the uterine wall. The observations of the latter authors,
and especially those of Schäfer, appear to me to speak for the
correctness of their view[94].
The cylinder gradually elongates up to the twelfth day. Before this
period it becomes attached by its base to the mesometric thickening of
the uterus, and enters into vascular connection with it. During its
elongation it
[Pg 263] becomes hollow, and is filled with a fluid not
coagulable in alcohol, while the body within its apex remains
unaltered till the tenth day.
Fig. 162. Diagrammatic longitudinal section through the embryo of
a Guinea-pig with its membranes. (After Schäfer.)
e. epiblast; h. hypoblast; m´. amniotic mesoblast; m´´.
splanchnic mesoblast; am. amnion; ev. cavity of amnion; all.
allantois; f. rudimentary blastopore; mc. cavity of vesicle
continuous with body cavity; mm. mucous membrane of uterus;
m´m´. parts where vascular uterine tissue perforates hypoblast of
blastodermic vesicle; vt. uterine vascular tissue; l. limits of
uterine tissue.
On this day a cavity develops in the interior of this body which at
the same time enlarges itself. The greater part of its wall next
attaches itself to the free end of the cylinder, and becomes
considerably thickened. The remainder of the wall adjoining the cavity
of the cylinder becomes a comparatively thin membrane. At the free end
of the cylinder there appears on the thirteenth day an embryonic area
similar to that of other Mammalia. It is at first round but soon
becomes pyriform, and in it there appear a primitive streak and
groove; and on their appearance it becomes obvious that the outer
layer of the cylinder is the hypoblast[95],
instead of, as in all
other Mammalia, the epiblast; and that the epiblast is formed by the
wall of the inner vesicle, i.e. the original solid body placed at the
end of the cylinder. Thus the dorsal surface of the embryo is turned
inwards, and the ventral surface outwards, and the ordinary position
of the layers is completely inverted.
[Pg 264]
The previously cylindrical egg next assumes a spherical form, and the
mesoblast arises in connection with the primitive streak in the manner
already described. A splanchnic layer of mesoblast attaches itself to
the inner side of the outer hypoblastic wall of the egg, a somatic
layer to the epiblast of the inner vesicle, and a mass of mesoblast
grows out into the cavity of the larger vesicle forming the
commencement of the allantois. The general structure of the ovum at
this stage is represented on fig. 162, copied from Schäfer; and the
condition of the whole ovum will best be understood by a description
of this figure.
It is seen to consist of two vesicles, (1) an outer larger one
(h)—the original egg-cylinder—united to the mesometric wall of the
uterus by a vascular connection at m´m´, and (2) an inner smaller
one (ev)—the originally solid body at the free end of the
egg-cylinder. The outer vesicle is formed of (1) an external lining of
columnar hypoblast (h) which is either pierced or invaginated at the
area of vascular connection with the uterus, and (2) of an inner layer
of splanchnic mesoblast (m´´) which covers without a break the
vascular uterine growth. At the upper pole of the ovum is placed the
smaller epiblastic vesicle, and where the two vesicles come together
is situated the embryonic area with the primitive streak (f), and
the medullary plate seen in longitudinal section. The thinner wall of
the inner vesicle is formed of epiblast and somatic mesoblast, and
covers over the dorsal face of the embryo just like the amnion. It is
in fact usually spoken of as the amnion. The large cavity of the outer
vesicle is continuous with the body cavity, and into it projects the
solid mesoblastic allantois (all), so far without hypoblast[96].
The outer vesicle corresponds exactly with the yolk-sack, and its
mesoblastic layer receives the ordinary vascular supply.
The embryo becomes folded off from the yolk-sack in the usual way, but
comes to lie not outside it as in the ordinary form, but in its
interior, and is connected with it by an umbilical stalk. The
yolk-sack forms the substitute for part of the subzonal membrane of
other Mammalia. The so-called amnion appears to me from its
development and position rather to correspond with the non-embryonic
part of the epiblastic wall (true subzonal membrane) of the
blastodermic vesicle of the ordinary mammalian forms than with the
true amnion; and a true amnion would seem not to be developed.
The allantois meets the yolk-sack on about the seventeenth day at the
region of its vascular connection with the uterine wall, and gives
rise to the placenta. A diagrammatic representation of the structure
of the embryo at this stage is given in fig. 163.
The peculiar inversion of the layers in the Guinea-pig has naturally
excited the curiosity of embryologists, but as yet no satisfactory
explanation has been offered of it.
[Pg 265]
At the time when the ovum first becomes fixed it will be remembered
that it resembles the early blastodermic vesicle of the Rabbit, and it
is natural to suppose that the apparently hypoblastic mass attached to
the inner wall of the vesicle becomes the solid body at the end of the
egg-cylinder. This appears to be Bischoff’s view, but, as shewn above,
the solid mass is really the epiblast! Is it conceivable that the
hypoblast in one species becomes the epiblast in a closely allied
species? To my mind it is not conceivable, and I am reduced to the
hypothesis, put forward by Hensen, that in the course of the
attachment of the ovum to the wall of the uterus a rupture of walls of
the blastodermic vesicle takes place, and that they become completely
turned inside out. It must be admitted, however, that in the present
state of our knowledge of the development of the ovum on the seventh
and eighth days it is not possible to frame a satisfactory explanation
how such an inversion can take place.
Fig. 163. Diagrammatic longitudinal section of an ovum of a
Guinea-pig and the adjacent uterine walls at an advanced stage of
pregnancy. (After Bischoff.)
yk. inverted yolk-sack (umbilical vesicle) formed of an external
hypoblastic layer (shaded) and an internal vascular layer (black).
At the end of this layer is placed the sinus terminalis; all.
allantois; pl. placenta.
The external shaded parts are the uterine walls.
The Human Embryo. Our knowledge as to the early development of the
human embryo is in an unsatisfactory state. The positive facts we know
are comparatively few, and it is not possible to construct from them a
history of the development which is capable of satisfactory comparison
with that in other forms, unless all the early embryos known are to be
regarded as abnormal. The most remarkable feature in the development,
which was first clearly brought to light by Allen Thomson in 1839, is
the very early appearance of branched villi. In the last few years
several ova, even younger than those described by Allen Thomson, have
been met with, which exhibit this peculiarity.
The best-preserved of these ova is one described by Reichert (No.
237). This ovum, though probably not more than thirteen days old, was
completely enclosed by a decidua reflexa. It had (fig. 164 A and B) a
flattened oval form, measuring in its two diameters 5.5 mm. and 3.5
mm. The edge was covered with branched villi, while in the centre of
each of the flattened surfaces there was a spot free from villi. On
the surface adjoining the uterine wall was a darker area (e) formed
of two layers of cells, which is interpreted by Reichert as the
embryonic area, while the membrane forming
[Pg 266] the remainder of the ovum,
including the branched villi, was stated by Reichert to be composed of
a single row of epithelial cells.
Whether or no Reichert is correct in identifying his darker spot as
the embryonic area, it is fairly certain from the later observations
of Beigel and Löwe (No. 228), Ahlfeld (No. 227), and Kollmann (No.
234) on ova nearly as young as that of Reichert, that the wall of very
young ova has a more complicated structure than Reichert is willing to
admit. These authors do not however agree amongst themselves, but from
Kollmann’s description, which appears to me the most satisfactory, it
is probable that it is composed of an outer epithelial layer, and an
inner layer of connective tissue, and that the connective tissue
extends at a very early period into the villi; so that the latter are
not hollow, as Reichert supposed them to be.
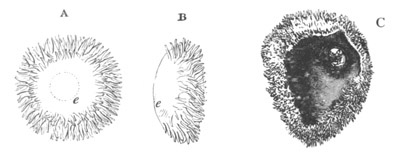
Fig. 164. The human ova during early stages of development. (From
Quain’s Anatomy.)
A. and B. Front and side view of an ovum figured by Reichert,
supposed to be about thirteen days. e. embryonic area.
C. An ovum of about four or five weeks shewing the general structure
of the ovum before the formation of the placenta. Part of the wall
of the ovum is removed to shew the embryo in situ. (After Allen
Thomson.)
The villi, which at first leave the flattened poles free, seem soon to
extend first over one of the flat sides, and finally over the whole
ovum (fig. 164 C).
Unless the two-layered region of Reichert’s ovum is the embryonic
area, nothing which can clearly be identified as an embryo has been
detected in these early ova. In an ovum described by Breus (No. 228),
and in one described long ago by Wharton-Jones a mass found in the
interior of the egg may perhaps be interpreted (His) as the remains of
the yolk. It is, however, very probable that all the early ova so far
discovered are more or less pathological.
The youngest ovum with a distinct embryo is one described by His (No.
232). This ovum, which is diagrammatically represented in fig. 168 in
longitudinal section, had the form of an oval vesicle completely
covered by villi, and about 8.5 mm. and 5.5 mm. in its two diameters,
and flatter on one side than on the other. An embryo with a yolk-sack
was attached to the inner side of the flatter wall of the vesicle by a
stalk, which must be
[Pg 267] regarded as the allantoic stalk[97],
and the
embryo and yolk-sack filled up but a very small part of the whole
cavity of the vesicle.
The embryo, which was probably not quite normal (fig. 165 A), was very
imperfectly developed; a medullary plate was hardly indicated, and,
though the mesoblast was unsegmented, the head fold, separating the
embryo from the yolk-sack (um), was already indicated. The amnion
(am) was completely formed, and vitelline vessels had made their
appearance.
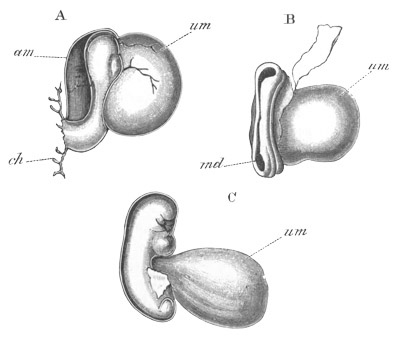
Fig. 165. Three early human embryos. (Copied from His.)
A. An early embryo described by His from the side. am. amnion;
um. umbilical vesicle; ch. chorion, to which the embryo is
attached by a stalk.
B. Embryo described by Allen Thomson about 12-14 days. um.
umbilical vesicle; md. medullary groove.
C. Young embryo described by His. um. umbilical vesicle.
Two embryos described by Allen Thomson (No. 239) are but slightly
older than the above embryos of His. Both of them probably belong to
the first fortnight of pregnancy. In both cases the embryo was more or
less folded off from the yolk-sack, and in one of them the medullary
groove was still widely open, except in the region of the neck (fig.
165 B). The allantoic stalk, if present, was not clearly made out, and
the condition of the amnion was also not fully studied. The smaller of
the two ova was just 6 mm. in
[Pg 268] its largest diameter, and was nearly
completely covered with simple villi, more developed on one side than
on the other.
In a somewhat later period, about the stage of a chick at the end of
the second day, the medullary folds are completely closed, the region
of the brain already marked, and the cranial flexure commencing. The
mesoblast is divided up into numerous somites, and the mandibular and
first two branchial arches are indicated. The embryo is still but
incompletely folded off from the yolk-sack below.
In a still older stage the cranial flexure becomes still more
pronounced, placing the mid-brain at the end of the long axis of the
body. The body also begins to be ventrally curved (fig. 165 C).
Externally human embryos at this age are characterised by the small
size of the anterior end of the head.
Fig. 166. Two views of a human embryo of between the third and
fourth week.
A. Side view. (From Kölliker; after Allen Thomson.) a. amnion;
b. umbilical vesicle; c. mandibular arch; e. hyoid arch;
f. commencing anterior limb; g. primitive auditory vesicle;
h. eye; i. heart.
B. Dorsal view to shew the attachment of the dilated allantoic stalk
to the chorion. (From a sketch by Allen Thomson.) am. amnion;
all. allantois; ys. yolk-sack.
The flexure goes on gradually increasing, and in the third week of
pregnancy in embryos of about 4 mm. the limbs make their appearance.
The embryo at this stage (fig. 166), which is about equivalent to that
of a chick on the fourth day, resembles in almost every respect the
normal embryos of the Amniota. The cranial flexure is as pronounced as
usual, and the cerebral region has now fully the normal size. The
whole body soon becomes flexed ventrally, and also somewhat spirally.
The yolk-sack (b) forms a small spherical appendage with a long wide
stalk, and the embryo (B) is attached by an allantoic stalk with a
slight swelling (all), probably indicating the presence of a small
hypoblastic diverticulum, to the inner face of the chorion.
A remarkable exception to the embryos generally observed is afforded
by an embryo which has been described by Krause (No. 235). In this
[Pg 269]
embryo, which probably belongs to the third week of pregnancy, the
limbs were just commencing to be indicated, and the embryo was
completely covered by an amnion, but instead of being attached to the
chorion by an allantoic cord, it was quite free, and was provided with
a small spherical sack-like allantois, very similar to that of a
fourth-day chick, projected from its hind end.
Fig. 167. Figures shewing the early changes in the form of the
human head. (From Quain’s Anatomy.)
A. Head of an embryo of about four weeks. (After Allen Thomson.)
B. Head of an embryo of about six weeks. (After Ecker.)
C. Head of an embryo of about nine weeks.
1. mandibular arch; 1´. persistent part of hyomandibular cleft; a.
auditory vesicle.
No details are given as to the structure of the chorion or the
presence of villi upon it. The presence of such an allantois at this
stage in a human embryo is so unlike what is usually found that
Krause’s statements have been received with considerable scepticism.
His even holds that the embryo is a chick embryo, and not a human one;
while Kölliker regards Krause’s allantois as a pathological structure.
The significance to be attached to this embryo is dealt with below.
A detailed history of the further development of the human embryo does
not fall within the province of this work; while the later changes in
the embryonic membranes have already been dealt with (pp. 244-248).
For the changes which take place on the formation of the face I may
refer the reader to fig. 167.
The most obscure point connected with the early history of the human
ovum concerns the first formation of the allantois, and the nature of
the villi covering the surface of the ovum. The villi, if really
formed of mesoblast covered by epiblast, have the true structure of
chorionic villi; and can hardly be compared to the early villi of the
dog which are derived from the subzonal membrane, and still less to
those of the rabbit formed from the zona radiata.
Unless all the early ova so far described are pathological, it seems
to [Pg 270]
follow that the mesoblast of the chorion is formed before the
embryo is definitely established, and even if the pathological
character of these ova is admitted, it is nevertheless probable
(leaving Krause’s embryo out of account), as shewn by the early
embryos of Allen Thomson and His, that it is formed before the closure
of the medullary groove. In order to meet this difficulty His supposes
that the embryo never separates from the blastodermic vesicle, but
that the allantoic stalk of the youngest embryo (fig. 168) represents
the persistent attachment between the two[98].
His’ view has a good
deal to be said for it. I would venture, however, to suggest that
Reichert’s embryonic area is probably not in the two-layered stage,
but that a mesoblast has already become established, and that it has
grown round the inner face of the blastodermic vesicle from the
(apparent) posterior end of the primitive streak. This growth I regard
as a precocious formation of the mesoblast of the allantois—an
exaggeration of the early formation of the allantoic mesoblast which
is characteristic of the Guinea-pig (vide p. 264). This mesoblast,
together with the epiblast, forms a true chorion, so that in fig. 168,
and probably also in fig. 164 A and B, a true chorion has already
become established. The stalk connecting the embryo with the chorion
in His’ earliest embryo (fig. 168) is therefore a true allantoic stalk
into which the hypoblastic allantoic diverticulum grows in for some
distance. How the yolk-sack (umbilical vesicle) is formed is not
clear. Perhaps, as suggested by His, it arises from the conversion of
a solid mass of primitive hypoblast directly into a yolk-sack. The
amnion is probably formed as a fold over the head end of the embryo in
the manner indicated in His’ diagram (fig. 168 Am).
Fig. 168. Diagrammatic longitudinal
section of the ovum to
which the embryo (fig. 165 a) belonged. (After His.)
Am. amnion; Nb. umbilical vesicle.
These speculations have so far left Krause’s embryo out of account.
How is this embryo to be treated? Krause maintains that all the other
embryos shewing an allantoic stalk at an early age are pathological.
This, though not impossible, appears to me, to say the least of it,
improbable; especially when it is borne in mind that embryos, which
have every appearance of being normal, of about the same age and
younger than Krause’s, have been frequently observed, and have always
been found attached to the chorion by an allantoic stalk.
We are thus provisionally reduced to suppose either that the structure
figured by Krause is not the allantois, or that it is a very abnormal
allantois. It is perhaps just possible that it may be an abnormally
developed hypoblastic vesicle of the allantois artificially detached
from the mesoblastic layer,—the latter having given rise to the
chorion at an earlier date.
[Pg 271]
Bibliography.
General.
(168) K. E. von Baer. Ueb. Entwicklungsgeschichte d. Thiere.
Königsberg, 1828-1837.
(169) Barry. “Researches on Embryology.” First Series. Philosophical
Transactions, 1838, Part II. Second Series, Ibid. 1839, Part II.
Third Series, Ibid. 1840.
(170) Ed. van Beneden. La maturation de l'œuf, la fécondation et les
premières phases du développement embryonaire d. Mammifères.
Bruxelles, 1875.
(171) Ed. van Beneden. “Recherches sur l'embryologie des Mammifères.”
Archives de Biologie, Vol. I. 1880.
(172) Ed. v. Beneden and Ch. Julin. “Observations sur la maturation
etc. de l'œuf chez les Cheiroptères.” Archives de Biologie, Vol. I.
1880.
(173) Th. L. W. Bischoff. Entwicklungsgeschichte d. Säugethiere u.
des Menschen. Leipzig, 1842.
(174) Th. L. W. Bischoff. Entwicklungsgeschichte des Kanincheneies.
Braunschweig, 1842.
(175) Th. L. W. Bischoff. Entwicklungsgeschichte des Hundeeies.
Braunschweig, 1845.
(176) Th. L. W. Bischoff. Entwicklungsgeschichte des
Meerschweinchens. Giessen, 1852.
(177) Th. L. W. Bischoff. Entwicklungsgeschichte des Rehes. Giessen,
1854.
(178) Th. L. W. Bischoff. “Neue Beobachtungen z. Entwicklungsgesch.
des Meerschweinchens.” Abh. d. bayr. Akad., Cl. II. Vol. X. 1866.
(179) Th. L. W. Bischoff. Historisch-kritische Bemerkungen z. d.
neuesten Mittheilungen üb. d. erste Entwick. d. Säugethiereier.
München, 1877.
(180) M. Coste. Embryogénie comparée. Paris, 1837.
(181) E. Haeckel. Anthropogenie, Entwicklungsgeschichte des
Menschen. Leipzig, 1874.
(182) V. Hensen. “Beobachtungen üb. d. Befrucht. u. Entwick. d.
Kaninchens u. Meerschweinchens.” Zeit. f. Anat. u. Entwick., Vol. I.
1876.
(183) A. Kölliker. Entwicklungsgeschichte d. Menschen u. d. höheren
Thiere. Leipzig, 1879.
(184) A. Kölliker. “Die Entwick. d. Keimblätter des Kaninchens.”
Zoologischer Anzeiger, Nos. 61, 62, Vol. III. 1880.
(185) N. Lieberkühn. Ueber d. Keimblätter d. Säugethiere.
Doctor-Jubelfeier d. Herrn. H. Nasse. Marburg, 1879.
(186) N. Lieberkühn. “Z. Lehre von d. Keimblättern d. Säugethiere.”
Sitz. d. Gesell. z. Beförd d. gesam. Naturwiss. Marburg, No. 3.
1880.
(187) Rauber. “Die erste Entwicklung d. Kaninchens.” Sitzungsber. d.
naturfor. Gesell. z. Leipzig. 1875.
(188) C. B. Reichert. “Entwicklung des Meerschweinchens.” Abh. der.
Berl. Akad. 1862.
(189) E. A. Schäfer. “Description of a Mammalian ovum in an early
condition of development.” Proc. Roy. Soc., No. 168. 1876.
[Pg 272]
(190) E. A. Schäfer. “A contribution to the history of development of
the guinea-pig.” Journal of Anat. and Phys., Vol. X. and XI. 1876
and 1877.
Fœtal Membranes and Placenta.
(191) John Anderson. Anatomical and Zoological Researches in Western
Yunnan. London, 1878.
(192) K. E. von Baer. Untersuchungen über die Gefässverbindung
zwischen Mutter und Frucht, 1828.
(193) C. G. Carus. Tabulae anatomiam comparativam illustrantes.
1831, 1840.
(194) H. C. Chapman. “The placenta and generative apparatus of the
Elephant.” Journ. Acad. Nat. Sc., Philadelphia. Vol. VIII. 1880.
(195) C. Creighton. “On the formation of the placenta in the
guinea-pig.” Journal of Anat. and Phys., Vol. XII. 1878.
(196) Ecker. Icones Physiologicae. 1852-1859.
(197) G. B. Ercolani. The utricular glands of the uterus, etc.,
translated from the Italian under the direction of H. O. Marcy.
Boston, 1880. Contains translations of memoirs published in the Mem.
dell'Accad. d. Scienze d. Bologna, and additional matter written
specially for the translation.
(198) G. B. Ercolani. Nuove ricerche sulla placenta nei pesci
cartilaginosi e nei mammiferi. Bologna, 1880.
(199) Eschricht. De organis quae respirationi et nutritioni fœtus
Mammalium inserviunt. Hafniae, 1837.
(200) A. H. Garrod and W. Turner. “The gravid uterus and placenta of
Hyomoschus aquaticus.” Proc. Zool. Soc., London, 1878.
(201) P. Harting. Het ei en de placenta van Halicore Dugong. Inaug.
diss. Utrecht. “On the ovum and placenta of the Dugong.” Abstract by
Prof. Turner. Journal of Anat. and Phys., Vol. XIII.
(202) Th. H. Huxley. The Elements of Comparative Anatomy. London,
1864.
(203) A. Kölliker. “Ueber die Placenta der Gattung Tragulus.” Verh.
der Würzb. phys.-med. Gesellschaft, Bd. X.
(204) C. D. Meigs. “On the reproduction of the Opossum (Didelphis
Virginiana).” Amer. Phil. Soc. Trans., Vol. X. 1853.
(205) H. Milne-Edwards. “Sur la Classification Naturelle.” Ann.
Sciences Nat., Sér. 3, Vol. I. 1844.
(206) Alf. Milne-Edwards. “Recherches sur la famille des Chevrotains.”
Ann. des Sciences Nat., Séries V., Vol. II. 1864.
(207) Alf. Milne-Edwards. “Observations sur quelques points de
l'Embryologie des Lemuriens, etc.” Ann. Sci. Nat., Sér. V., Vol. XV.
1872.
(208) Alf. Milne-Edwards. “Sur la conformation du placenta chez le
Tamandua.” Ann. des Sci. Nat., XV. 1872.
(209) Alf. Milne-Edwards. “Recherches s. l. enveloppes fœtales du
Tatou à neuf bandes.” Ann. Sci. Nat., Sér. VI., Vol. VIII. 1878.
(210) R. Owen. “On the generation of Marsupial animals, with a
description of the impregnated uterus of the Kangaroo.” Phil.
Trans., 1834.
(211) R. Owen. “Description of the membranes of the uterine fœtus of
the Kangaroo.” Mag. Nat. Hist., Vol. I. 1837.
[Pg 273]
(212) R. Owen. “On the existence of an Allantois in a fœtal Kangaroo
(Macropus major).” Zool. Soc. Proc., v. 1837.
(213) R. Owen. “Description of the fœtal membranes and placenta of
the Elephant.” Phil. Trans., 1857.
(214) R. Owen. On the Anatomy of Vertebrates, Vol. III. London,
1868.
(215) G. Rolleston. “Placental structure of the Tenrec, etc.”
Transactions of the Zoological Society, Vol. V. 1866.
(216) W. Turner. “Observations on the structure of the human
placenta.” Journal of Anat. and Phys., Vol. VII. 1868.
(217) W. Turner. “On the placentation of the Cetacea.” Trans. Roy.
Soc. Edinb., Vol. XXVI. 1872.
(218) W. Turner. “On the placentation of Sloths (Cholœpus
Hoffmanni).” Trans. of R. Society of Edinburgh, Vol. XXVII. 1875.
(219) W. Turner. “On the placentation of Seals (Halichœrus gryphus).”
Trans. of R. Society of Edinburgh, Vol. XXVII. 1875.
(220) W. Turner. “On the placentation of the Cape Ant-eater
(Orycteropus capensis).” Journal of Anat. and Phys., Vol. X. 1876.
(221) W. Turner. Lectures on the Anatomy of the Placenta. First
Series. Edinburgh, 1876.
(222) W. Turner. “Some general observations on the placenta, with
special reference to the theory of Evolution.” Journal of Anat. and
Phys., Vol. XI. 1877.
(223) W. Turner. “On the placentation of the Lemurs.” Phil. Trans.,
Vol. 166, p. 2. 1877.
(224) W. Turner. “On the placentation of Apes.” Phil. Trans., 1878.
(225) W. Turner. “The cotyledonary and diffused placenta of the
Mexican deer (Cervus Americanus).” Journal of Anat. and Phys., Vol.
XIII. 1879.
Human Embryo.
(226) Fried. Ahlfeld. “Beschreibung eines sehr kleinen menschlichen
Eies.” Archiv f. Gynaekologie, Bd. XIII. 1878.
(227) Herm. Beigel und Ludwig Loewe. “Beschreibung eines menschlichen
Eichens aus der zweiten bis dritten Woche der Schwangerschaft.”
Archiv f. Gynaekologie, Bd. XII. 1877.
(228) K. Breus. “Ueber ein menschliches Ei aus der zweiten Woche der
Gravidität.” Wiener medicinische Wochenschrift, 1877.
(229) M. Coste. Histoire générale et particulière du développement
des corps organisés, 1847-59.
(230) A. Ecker. Icones Physiologicae. Leipzig, 1851-1859.
(231) V. Hensen. “Beitrag z. Morphologie d. Körperform u. d. Gehirns
d. menschlichen Embryos.” Archiv f. Anat. u. Phys., 1877.
(232) W. His. Anatomie menschlicher Embryonen, Part I. Embryonen d.
ersten Monats. Leipzig, 1880.
(233) J. Kollmann. “Die menschlichen Eier von 6 MM. Grösse.” Archiv
f. Anat. und Phys., 1879.
(234) W. Krause. “Ueber d. Allantois d. Menschen.” Archiv f. Anat.
und Phys., 1875.
(235) W. Krause. “Ueber zwei frühzeitige menschliche Embryonen.”
Zeit. f. wiss. Zool., Vol. XXXV. 1880.
[Pg 274]
(236) L. Loewe. “Im Sachen der Eihäute jüngster menschlicher Eier.”
Archiv für Gynaekologie, Bd. XIV. 1879.
(237) C. B. Reichert. “Beschreibung einer frühzeitigen menschlichen
Frucht im bläschenformigen Bildungszustande (sackformiger Keim von
Baer) nebst vergleichenden Untersuchungen über die bläschenformigen
Früchte der Säugethiere und des Menschen.” Abhandlungen der königl.
Akad. d. Wiss. zu Berlin, 1873.
(238) Allen Thomson. “Contributions to the history of the structure of
the human ovum and embryo before the third week after conception; with
a description of some early ova.” Edinburgh Med. Surg. Journal, Vol.
LII. 1839.
[Pg 275]
CHAPTER XI.
COMPARISON OF THE FORMATION OF THE GERMINAL LAYERS AND OF THE EARLY
STAGES IN THE DEVELOPMENT OF VERTEBRATES.
Although the preceding chapters of this volume contain a fairly
detailed account of the early developmental stages of different groups
of the Chordata, it will nevertheless be advantageous to give at this
place a short comparative review of the whole subject.
In this review only the most important points will be dwelt upon, and
the reader is referred for the details of the processes to the
sections on the development of the individual groups.
The subject may conveniently be treated under three heads.
(1) The formation of the gastrula and behaviour of the blastopore:
together with the origin of the hypoblast.
(2) The mesoblast and notochord.
(3) The epiblast.
At the close of the chapter is a short summary of the organs derived
from the several layers, together with some remarks on the growth in
length of the vertebrate embryo, and some suggestions as to the origin
of the allantois and amnion.
Formation of the gastrula. Amphioxus is the type in which the
developmental phenomena are least interfered with by the presence of
food-yolk.
In this form the segmentation results in a uniform, or nearly uniform,
blastosphere, one wall of which soon becomes thickened and
invaginated, giving rise to the hypoblast; while the larva takes the
form of a gastrula, with an archenteric cavity opening by a
blastopore. The blastopore rapidly narrows, while the
[Pg 276] embryo assumes
an elongated cylindrical form with the blastopore at its hinder
extremity (fig. 169 A). The blastopore now passes to the dorsal
surface, and by the flattening of this surface a medullary plate is
formed extending forwards from the blastopore (fig. 169 B). On the
formation of the medullary groove and its conversion into a canal, the
blastopore opens into this canal, and gives rise to a neurenteric
passage, leading from the neural canal into the alimentary tract (fig.
169 C and E). At a later period this canal closes, and the neural and
alimentary canals become separated.
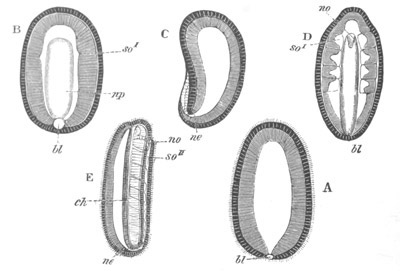
Fig. 169. Embryos of Amphioxus. (After Kowalevsky.)
The parts in black with white lines are epiblastic; the shaded parts
are hypoblastic.
A. Gastrula stage in optical section.
B. Slightly later stage after the neural plate np has become
differentiated, seen as a transparent object from the dorsal side.
C. Lateral view of a slightly older larva in optical section.
D. Dorsal view of an older larva with the neural canal completely
closed except for a small pore (no) in front.
E. Older larva seen as a transparent object from the side.
bl. blastopore (which becomes in D the neurenteric canal); ne.
neurenteric canal; np. neural or medullary plate; no. anterior
opening of neural canal; ch. notochord; so´, so´´. first and
second mesoblastic somites.
Such is the simple history of the layers in Amphioxus. In the simplest
types of Ascidians the series of phenomena is almost the same, but the
blastopore assumes a more definitely dorsal position.
[Pg 277]
Here also the blastopore lies at the hinder end of the medullary
groove, and on the closure of the groove becomes converted into a
neurenteric passage.
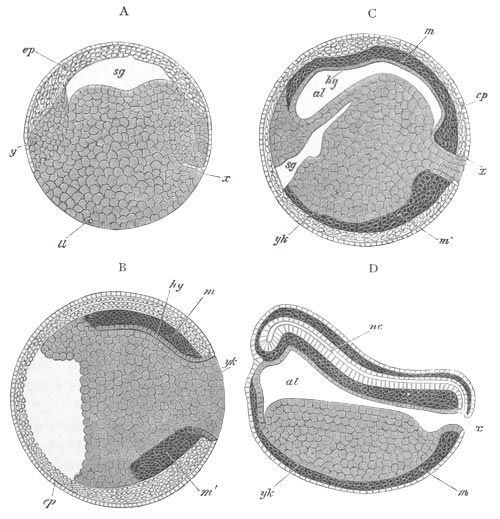
Fig. 170. Diagrammatic longitudinal sections through the embryo of
Bombinator at two stages, to shew the formation of the germinal layers. (Modified from Götte.)
ep. epiblast; m. dorsal mesoblast; m´. ventral mesoblast;
hy. hypoblast; yk. yolk; x. point of junction of the epiblast
and hypoblast at the dorsal side of the blastopore; al.
mesenteron; sg. segmentation cavity.
In the true Vertebrates the types which most approach Amphioxus are
the Amphibia, Acipenser and Petromyzon. We may take the first of these
as typical (though Petromyzon is perhaps still more so) and fig. 170 A
B C D represents four diagrammatic longitudinal vertical sections
through a form
[Pg 278] belonging to this group (Bombinator). The food-yolk is
here concentrated in what I shall call the lower pole of the egg,
which becomes the ventral aspect of the future embryo. The part of the
egg containing the stored-up food-yolk is, as has already been
explained in the chapter on segmentation (Vol. II. pp. 94 and 95), to
be regarded as equivalent to part of those eggs which do not contain
food-yolk; a fact which requires to be borne in mind in any attempt to
deal comparatively with the formation of the layers in the Vertebrata.
It may be laid down as a general law, which holds very accurately for
the Vertebrata, that in eggs in which the distribution of food-yolk is
not uniform, the size of the cells resulting from segmentation is
proportional to the quantity of food-material they contain. In
accordance with this law the cells of the Amphibian ovum are of
unequal size even at the close of segmentation. They may roughly be
divided into two categories, viz. the smaller cells of the upper pole
and the larger of the lower (fig. 170 A). The segmentation cavity
(sg) lies between the two, but is unsymmetrically placed near the
upper pole of the egg, owing to the large bulk of the ventrally placed
yolk-segments. In the inequality of the cells at the close of
segmentation the Amphibia stand in contrast with Amphioxus. The upper
cells are mainly destined to form the epiblast, and the lower the
hypoblast and mesoblast.
The next change which takes place is an invagination, the earliest
traces of which are observable in fig. 170 A. The invagination is not
however so simple as in Amphioxus. Owing in fact to the presence of
the food-yolk it is a mixture of invagination by epibole and by
embole.
At the point marked x in fig. 170 A, which corresponds with the
future hind end of the embryo, and is placed on the equatorial line
marking the junction of the large and small cells, there takes place a
normal invagination, which gives rise solely to the hypoblast of the
dorsal wall of the alimentary tract and to part of the dorsal
mesoblast. The invaginated layer grows inwards from the point x
along what becomes the dorsal side of the embryo; and between it and
the yolk-cells below is formed a slit-like space (fig. 170 B and C).
This space is the mesenteron. It is even better shewn in fig. 171
representing the
[Pg 279] process of invagination in Petromyzon. The point x
in fig. 170 where epiblast, mesoblast and hypoblast are continuous, is
homologous with the dorsal lip of the blastopore in Amphioxus. In the
course of the invagination the segmentation cavity, as in Amphioxus,
becomes obliterated.
While the above invagination has been taking place, the epiblast cells
have been simply growing in an epibolic fashion round the yolk; and by
the stage represented in fig. 170 C and D the exposed surface of yolk
has become greatly diminished; and an obvious blastopore is thus
established. Along the line of the growth a layer of mesoblast cells
(m´), continuous at the sides with the invaginated mesoblast layer,
has become differentiated from the small cells (fig. 170 A)
intermediate between the epiblast cells and the yolk.
Owing to the nature of the above process of invagination the
mesenteron is at first only provided with an epithelial wall on its
dorsal side, its ventral wall being formed of yolk-cells (fig. 170).
At a later period some of the yolk-cells become transformed into the
epithelial cells of the ventral wall, while the remainder become
enclosed in the alimentary cavity and employed as pabulum. The whole
of the yolk-cells, after the separation of the mesoblast, are however
morphologically part of the hypoblast.
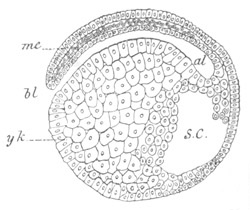
Fig. 171. Longitudinal vertical section
through an embryo of Petromyzon
of 136 hours.
me. mesoblast; yk. yolk-cells; al. alimentary tract; bl.
blastopore; s.c. segmentation cavity.
The final fate of the blastopore is nearly the same as in Amphioxus.
It gradually narrows, and the yolk-cells which at first plug it up
disappear (fig. 170 C and D). The neural groove, which becomes formed
on the dorsal surface of the embryo, is continued forwards from the
point x in fig. 170 C. On the conversion of this groove into a canal
the canal freely opens behind into the blastopore; and a condition is
reached in which the blastopore still opens to the exterior and also
into the neural canal fig. 170 D. In a later stage (fig. 172) the
external opening of the blastopore becomes closed by the medullary
folds meeting behind it, but the passage connecting the neural and
alimentary canals is left. There is one small difference between the
Frog and Amphioxus in the relation of the neural canal to the
blastopore. In both types the medullary folds embrace and meet behind
it, so that it comes to occupy a position at the hind extremity of the
medullary groove. In Amphioxus the closure
[Pg 280] of the medullary folds
commences behind, so that the external opening of the blastopore is
obliterated simultaneously with the commencing formation of the
medullary canal; but in the Frog the closure of the medullary folds
commences anteriorly and proceeds backwards, so that the obliteration
of the external opening of the blastopore is a late event in the
formation of the medullary canal.
The anus is formed (vide fig. 172) some way in front of the
blastopore, and a postanal gut, continuous with the neurenteric canal,
is thus established. Both the postanal gut and the neurenteric canal
eventually disappear.
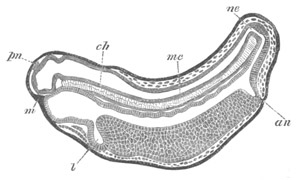
Fig. 172. Longitudinal section through an advanced embryo of
Bombinator. (After Götte.)
medullary canal; ch. notochord; pn. pineal gland.
The two other types classed above with the Amphibia, viz. Petromyzon
and Acipenser, agree sufficiently closely with them to require no
special mention; but with reference to both types it may be pointed
out that the ovum contains relatively more food-yolk than that of the
Amphibian type just described, and
[Pg 281] that this leads amongst other
things to the lower layer cells extending up the sides of the
segmentation cavity, and assisting in forming its roof.
The next type to be considered is that of Elasmobranchii. The yolk in
the ovum of these forms is enormously bulky, and the segmentation is
in consequence a partial one. At first sight the differences between
their development and that of Amphibia would appear to be very great.
In order fully to bridge over the gulf which separates them I have
given three diagrammatic longitudinal sections of an ideal form
intermediate between Amphibia and Elasmobranchii, which differs
however mainly from the latter in the smaller amount of food-yolk; and
by their aid I trust it will be made clear that the differences
between the Amphibia and Elasmobranchii are of an insignificant
character. In fig. 174 A B C are represented three diagrammatic
longitudinal sections of Elasmobranch embryos, and in fig. 173 A B C
three longitudinal sections of the ideal intermediate form. The
diagrams correspond with the Amphibian diagrams already described
(fig. 170). In the first stage figured there is present in all of
these forms a segmentation cavity (sg) situated not centrally but
near the surface of the egg. The roof of the cavity is thin, being
composed in the Amphibian embryo of epiblast alone, and in the
Elasmobranch of epiblast and lower layer cells. The floor of the
cavity is formed of so-called yolk, which forms the main mass of the
embryo. In Amphibia the yolk is segmented. In Elasmobranchii there is
at first a layer of primitive hypoblast cells separating the
segmentation cavity from the yolk proper; this however soon
disappears, and an unsegmented yolk with free nuclei fills the place
of the segmented yolk of the Amphibia. The small cells at the sides of
the segmentation cavity in Amphibia correspond exactly in function and
position with the lower layer cells of the Elasmobranch blastoderm.
The relation of the yolk to the blastoderm in the Elasmobranch embryo
at this stage of development very well suits the view of its homology
with the yolk-cells of the Amphibian embryo. The only essential
difference between the two embryos arises from the roof of the
segmentation cavity being formed in the Elasmobranch embryo of lower
layer cells, which are absent
[Pg 282] in the Amphibian embryo. This difference
no doubt depends upon the greater quantity of yolk in the Elasmobranch
ovum, and a similar distribution of the lower layer cells is found in
Acipenser and in Petromyzon.
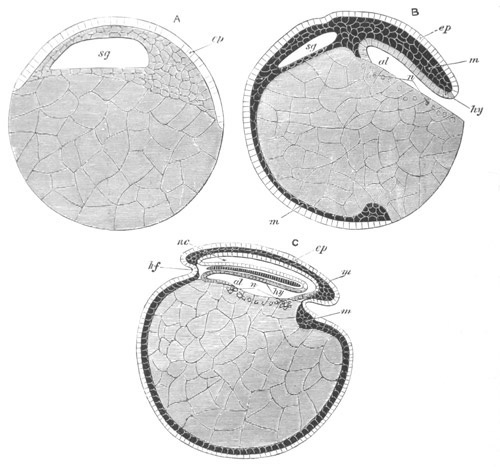
Fig. 173. Three diagrammatic longitudinal sections through an
ideal type of Vertebrate embryo intermediate in the mode of formation
of its layers between Amphibia or Petromyzon and Elasmobranchii.
sg. segmentation cavity; ep. epiblast; m. mesoblast; hy.
hypoblast; nc. neural canal; al. mesenteron; n. nuclei of the
yolk.
In the next stage for the Elasmobranch (fig. 173 and 174 B) and for
the Amphibian (fig. 170 C) or better still Petromyzon (fig. 171) the
agreement between the three types is again very close. For a small arc
(x) of the edge of the blastoderm the epiblast and hypoblast become
continuous, while at all other
[Pg 283] parts the epiblast, accompanied by
lower layer cells, grows round the yolk or round the large cells which
correspond to it. The yolk-cells of the Amphibian embryo form a
comparatively small mass, and are therefore rapidly enveloped; while
in the case of the Elasmobranch embryo, owing to the greater mass of
the yolk, the same process occupies a long period. The portion of the
blastoderm, where epiblast and hypoblast become continuous, forms the
dorsal lip of an opening—the blastopore—which leads into the
alimentary cavity. This cavity has the same relation in all the three
cases. It is lined dorsally by lower layer cells, and ventrally by
yolk-cells or what corresponds with yolk-cells; a large part of the
ventral epithelium of the alimentary canal being in both cases
eventually derived from the yolk. In Amphibia this epithelium is
formed directly from the existing cells, while in Elasmobranchii it is
derived from cells formed around the nuclei of the yolk.
As in the earlier stage, so in the present one, the anatomical
relations of the yolk to the blastoderm in the one case
(Elasmobranchii) are nearly identical with those of the yolk-cells to
the blastoderm in the other (Amphibia).
The main features in which the two embryos differ, during the stage
under consideration, arise from the same cause as the solitary point
of difference during the preceding stage.
In Amphibia the alimentary cavity is formed coincidently with a true
ingrowth of cells from the point where epiblast and hypoblast become
continuous; and from this ingrowth the dorsal wall of the alimentary
cavity is formed. The same ingrowth causes the obliteration of the
segmentation cavity.
In Elasmobranchs, owing probably to the larger bulk of the lower layer
cells, the primitive hypoblast cells arrange themselves in their final
position during segmentation, and no room is left for a true
invagination; but instead of this there is formed a simple space
between the blastoderm and the yolk. The homology of this space with
the primitive invagination cavity is nevertheless proved by the
survival of a number of features belonging to the ancestral condition
in which a true invagination was present. Amongst the more important
of these are the following:—(1) The continuity of epiblast and
hypoblast at the dorsal lip of the blastopore. (2) The continuous
conversion of primitive
[Pg 284] hypoblast cells into permanent hypoblast,
which gradually extends inwards towards the segmentation cavity, and
exactly represents the course of the invagination whereby in Amphibia
the dorsal wall of the alimentary cavity is formed. (3) The
obliteration of the segmentation cavity during the period when the
pseudo-invagination is occurring.
In the next stage there appear more important differences between the
two types than in the preceding stages, though here again the points
of resemblance predominate.
Figs. 170 D and 174 C represent longitudinal sections through embryos
after the closure of the medullary canal. The neurenteric canal is
established; and in front and behind the epithelium of the ventral
wall of the mesenteron has begun to be formed.
The mesoblast is represented as having grown in between the medullary
canal and the superjacent epiblast.
There are at this stage two points in which the embryo Elasmobranch
differs from the corresponding Amphibian embryo. (1) In the formation
of the neurenteric canal, there is no free passage leading into the
mesenteron from the exterior as in Amphibia (fig. 170 D). (2) The
whole yolk is not enclosed by the epiblast, and therefore part of the
blastopore is still open.
The difference between Amphibia and Elasmobranchii in the first of
these points is due to the fact that in Elasmobranchii, as in
Amphioxus, the neural canal becomes first closed behind; and
simultaneously with its closure the lateral parts of the lips of the
blastopore, which are continuous with the medullary folds, meet
together and shut in the hindmost part of the alimentary tract.
The second point is of some importance for understanding the relations
of the formation of the layers in the amniotic and the non-amniotic
Vertebrates. Owing to its large size the whole of the yolk in
Elasmobranchii is not enclosed by the epiblast at the time when the
neurenteric canal is established; in other words a small posterior and
dorsal portion of the blastopore is shut off in the formation of the
neurenteric canal. The remaining ventral portion becomes closed at a
later period. Its closure takes place in a linear fashion, commencing
at the hind end of the embryo, and proceeding apparently backwards;
though, as this part eventually becomes folded in to form the ventral
wall of the embryo, the closure of it really travels forwards. The
[Pg 285]
process causes however the embryo to cease to lie at the edge of the
blastoderm, and while situated at some distance from the edge, to be
connected with it by a linear streak, representing the coalesced lips
of the blastopore. The above process is diagrammatically represented
in fig. 175 B; while as it actually occurs it is shewn in fig. 30, p.
63. The whole closure of the blastopore in Elasmobranchii is
altogether unlike what takes place in Amphibia, where the blastopore
remains as a circular opening which
[Pg 286] gradually narrows till it becomes
completely enveloped in the medullary folds (fig. 175 A).
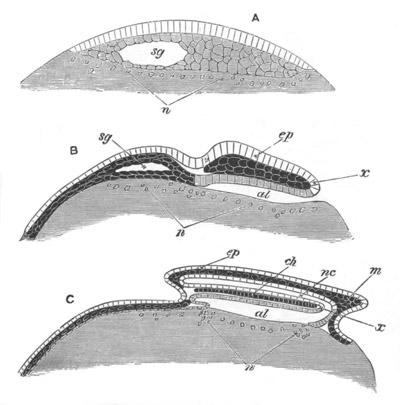
Fig. 174. Diagrammatic longitudinal sections of an Elasmobranch
embryo.
Epiblast without shading. Mesoblast black with clear
outlines to the cells. Lower layer cells and hypoblast
with simple shading.
ep. epiblast; m. mesoblast; al. alimentary cavity; sg.
segmentation cavity; nc. neural canal; ch. notochord; x. point
where epiblast and hypoblast become continuous at the posterior end
of the embryo; n. nuclei of yolk.
A. Section of young blastoderm, with the segmentation cavity
enclosed in the lower layer cells (primitive hypoblast).
B. Older blastoderm with embryo in which hypoblast and mesoblast are
distinctly formed, and in which the alimentary cavity has
appeared. The segmentation cavity is still represented, though by
this stage it has in reality disappeared.
C. Older blastoderm with embryo in which the neural canal is formed,
and is continuous posteriorly with the alimentary canal. The
notochord, though shaded like mesoblast, belongs properly to the
hypoblast.
On the formation of the neurenteric canal the body of the embryo
Elasmobranch becomes gradually folded off from the yolk, which, owing
to its great size, forms a large sack appended to the ventral side of
the body. The part of the somatopleure, which grows round it, is to be
regarded as a modified portion of the ventral wall of the body. The
splanchnopleure also envelops it, so that, morphologically speaking,
the yolk lies within the mesenteron.
The Teleostei, so far as the first formation of the layers is
concerned, resemble in all essential features the Elasmobranchii, but
the neurenteric canal is apparently not developed (?), owing to the
obliteration of the neural canal; and the roof of the segmentation
cavity is formed of epiblast only.
* * * * *
In the preceding pages I have attempted to shew that the Amphibia,
Acipenser, Petromyzon, the Elasmobranchii and the Teleostei agree very
closely in the mode of formation of the gastrula. The unsymmetrical
gastrula or pseudo-gastrula which is common to them all is, I believe,
to be explained by the form of the vertebrate body. In Amphioxus,
where the small amount of food-yolk present is distributed uniformly,
there is no reason why the invagination and resulting gastrula should
not be symmetrical. In true Vertebrates, where more food-yolk is
present, the shape and structure of the body render it necessary for
the food-yolk to be stored away on the ventral side of the alimentary
canal. It is this fact which causes the asymmetry of the gastrula,
since it is not possible for the part of the ovum, which will become
the ventral wall of the alimentary tract, and which is loaded with
food-yolk, to be invaginated in the same fashion as the dorsal wall.
Sauropsida. The comparison of the different types of the Ichthyopsida
is fairly simple, but the comparison of the Sauropsida with the
Ichthyopsida is a far more difficult matter. In all the Sauropsida
there is a large food-yolk, and the segmentation agrees closely with
that in the Elasmobranchii. It might have been anticipated that the
resemblance would continue in the subsequent development. This however
is far from being the
[Pg 287] case. The medullary plate, instead of lying at
the edge of the blastoderm, lies in the centre, and its formation is
preceded by that of a peculiar structure, the primitive streak, which,
on the formation of the medullary plate, is found to lie at the hinder
end of the latter and to connect it with the edge of the blastoderm.
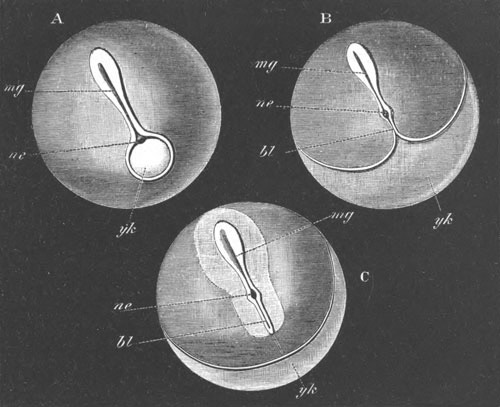
Fig. 175. Diagrams illustrating the position of the blastopore, and
the relation of the embryo to the yolk in various meroblastic Vertebrate
ova.
A. Type of Frog. B. Elasmobranch type. C. Amniotic Vertebrate.
mg. medullary plate; ne. neurenteric canal; bl. portion of
blastopore adjoining the neurenteric canal. In B this part of the
blastopore is formed by the edges of the blastoderm meeting and
forming a linear streak behind the embryo; and in C it forms the
structure known as the primitive streak. yk. part of the yolk not
yet enclosed by the blastoderm.
The possibility of a comparison between the Sauropsida and the
Elasmobranchii depends upon the explanation being possible of (1) the
position of the embryo near the centre of the blastoderm, and (2) the
nature of the primitive streak.
The answers to these two questions are, according to my view,
intimately bound together.
[Pg 288]
I consider that the embryos of the Sauropsida have come to occupy a
central position in the blastoderm owing to the abbreviation of a
process similar to that by which, in Elasmobranchii, the embryo is
removed from the edge of the blastoderm; and that the primitive streak
represents the linear streak connecting the Elasmobranch embryo with
the edge of the blastoderm after it has become removed from its
previous peripheral position, as well as the true neurenteric part of
the Elasmobranch blastopore.
This view of the nature of the primitive streak, which is
diagrammatically illustrated in fig. 175, will be rendered more clear
by a brief review of the early developmental processes in the
Sauropsida.
After segmentation the blastoderm becomes divided, as in
Elasmobranchii, into two layers. It is doubtful whether there is any
true representative of the segmentation cavity. The first structure to
appear in the blastoderm is a linear streak placed at the hind end of
the blastoderm, known as the primitive streak (figs. 175 C, bl and
176, pr). At the front end of the primitive streak the epiblast and
hypoblast become continuous, just as they do at the dorsal lip of the
blastopore in Elasmobranchii. Continued back from this point is a
streak of fused mesoblast and epiblast to the under side of which a
linear thin layer of hypoblast is more or less definitely attached.
A further structure, best developed in the Lacertilia, appears in the
form of a circular passage perforating the blastoderm at the front end
of the primitive streak (fig. 176, ne). This passage is bounded
anteriorly by the layer of cells forming the continuation of the
hypoblast into the epiblast.
In the next stage the medullary plate becomes formed in front of the
primitive streak (fig. 175 C), and the medullary folds are continued
backwards so as to enclose the upper opening of the passage through
the blastoderm. On the closure of the medullary canal (fig. 177) this
passage leads from the medullary canal into the alimentary tract, and
is therefore the neurenteric canal; and a postanal gut also becomes
formed. The latter part of the above description applies especially to
the Lizard: but in Chelonia and most Birds distinct remnants (vide
pp. 162-164) of the neurenteric canal are developed.
On the hypothesis that the Sauropsidan embryos have come
[Pg 289] to occupy
their central position, owing to an abbreviation of a process
analogous to the linear closing of the blastopore behind the embryos
of Elasmobranchii, all the appearances above described receive a
satisfactory explanation. The passage at the front end of the
primitive streak is the dorsal part of the blastopore, which in
Elasmobranchii becomes converted into the neurenteric canal. The
remainder of the primitive streak represents, in a rudimentary form,
the linear streak in Elasmobranchii, formed by the coalesced edges of
the blastoderm, which connects the hinder end of the embryo with the
still open yolk blastopore. That it is in later stages not continued
to the edge of the blastoderm, as in Elasmobranchii, is due to its
being a rudimentary organ. The more or less complete fusion of the
layers in the primitive streak is simply to be explained by this
structure representing the coalesced edges of the blastopore; and the
growth outwards from it of the mesoblast is probably a remnant of a
primitive dorsal invagination of the mesoblast and hypoblast like that
in the Frog.
Fig. 176. Diagrammatic longitudinal section of an embryo of Lacerta.
pp. body cavity; am. amnion; ne. neurenteric canal; ch.
notochord; hy. hypoblast; ep. epiblast; pr. primitive streak.
In the primitive streak all the layers are partially fused.
The final enclosure of the yolk in the Sauropsida takes place at the
pole of the yolk-sack opposite the embryo, so that the blastopore is
formed of three parts, (1) the neurenteric canal, (2) the primitive
streak behind this, (3) the blastopore at the pole of the yolk-sack
opposite the embryo.
Mammalia. The features of the development of the placental Mammalia
receive their most satisfactory explanation on the hypothesis that
their ancestors were provided with a large-yolked ovum like that of
the Sauropsida. The food-yolk must be supposed to have ceased to be
developed on the establishment of a maternal nutrition through the
uterus.
On this hypothesis all the developmental phenomena subsequently
[Pg 290] to the
formation of the blastodermic vesicle receive a satisfactory
explanation.
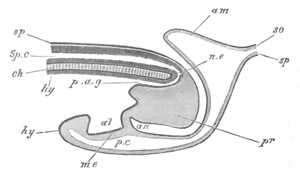
Fig. 177. Diagrammatic longitudinal section through the posterior
end of an embryo Bird at the time of the formation of the Allantois.
ep. epiblast; Sp.c. spinal canal; ch. notochord; n.e.
neurenteric canal; hy. hypoblast; p.a.g. postanal gut; pr.
remains of primitive streak folded in on the ventral side; al.
allantois; me. mesoblast; an. point where anus will be formed;
p.c. perivisceral cavity; am. amnion; so. somatopleure; sp.
splanchnopleure.
The whole of the blastodermic vesicle, except the embryonic area,
represents the yolk-sack, and the growth of the hypoblast and then of
the mesoblast round its inner wall represents the corresponding
growths in the Sauropsida. As in the Sauropsida it becomes constricted
off from the embryo, and the splanchnopleuric stalk of the sack opens
into the ileum in the usual way.
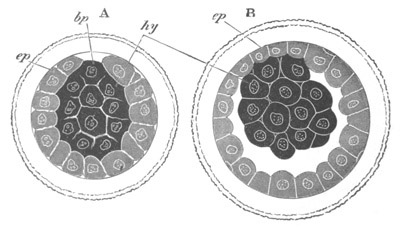
Fig. 178. Optical sections of a Rabbit’s ovum at two stages closely
following upon the segmentation. (After E. van Beneden.)
ep. epiblast; hy. primary hypoblast; bp. Van Beneden’s
so-called blastopore. The shading of the epiblast and hypoblast is
diagrammatic.
[Pg 291]
In the formation of the embryo out of the embryonic area the phenomena
which distinguish the Sauropsida from the Ichthyopsida are repeated.
The embryo lies in the centre of the area; and before it is formed
there appears a primitive streak, from which there grows out the
greater part of the mesoblast. At the front end of the primitive
streak the hypoblast and epiblast become continuous, though a
perforated neurenteric blastopore has not yet been detected.
All these Sauropsidan features are so obvious that they need not be
insisted on further. The embryonic evidence of the common origin of
Mammalia and Sauropsida, both as concerns the formation of the layers
and of the embryonic membranes, is as clear as it can be. The only
difficulty about the early development of Mammalia is presented by the
epibolic gastrula and the formation of the blastodermic vesicle (figs.
178 and 179). That the segmentation is a complete one is no doubt a
direct consequence of the reduction of the food-yolk, but the growth
of the epiblast cells round the hypoblast and the final enclosure of
the latter, which I have spoken of as giving rise to the epibolic
gastrula, are not so easily explained.
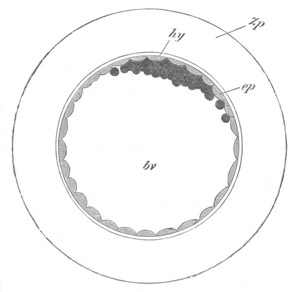
Fig. 179. Rabbit’s ovum between 70-90 hours after impregnation.
(After E. van Beneden.)
bv. cavity of blastodermic vesicle (yolk-sack); ep. epiblast;
hy. primitive hypoblast; Zp. mucous envelope.
[Pg 292]
It might have been supposed that this process was equivalent to the
growth of the blastoderm round the yolk in the Sauropsida, but then
the blastopore ought to be situated at the pole of the egg opposite to
the embryonic area, while, according to Van Beneden, the embryonic
area corresponds approximately to the blastopore.
Van Beneden regards the Mammalian blastopore as equivalent to that in
the Amphibia, but if the position previously adopted about the
primitive streak is to be maintained, Van Beneden’s view must be
abandoned. No satisfactory phylogenetic explanation of the Mammalian
gastrula by epibole has in my opinion as yet been offered.
The formation of the blastodermic vesicle may perhaps be explained on
the view that in the Proto-mammalia the yolk-sack was large, and that
its blood-vessels took the place of the placenta of higher forms. On
this view a reduction in the bulk of the ovarian ovum might easily
have taken place at the same time that the presence of a large
yolk-sack was still necessary for the purpose of affording surface of
contact with the uterus.
The formation of the Mesoblast and of the Notochord.
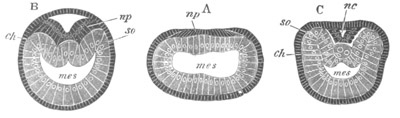
Fig. 180. Sections of an Amphioxus embryo at three stages.
(After Kowalevsky.)
A. Section at gastrula stage.
B. Section of an embryo slightly younger than that represented in
fig. 169 D.
C. Section through the anterior part of an embryo at the stage
represented in fig. 169 E.
np. neural plate; nc. neural canal; mes. archenteron in A and
B, and mesenteron in C; ch. notochord; so. mesoblastic somite.
Amphioxus. The mesoblast originates in Amphioxus, as in several
primitive invertebrate types, from a pair of lateral
[Pg 293] diverticula,
constricted off from the archenteron (fig. 180). Their formation
commences at the front end of the body and is thence carried
backwards, and each diverticulum contains a prolongation of the cavity
of the archenteron. After their separation from the archenteron the
dorsal parts of these diverticula become divided by transverse septa
into successive somites, the cavities of which eventually disappear;
while the walls become mainly converted into the muscle-plates, but
also into the tissue around the notochord which corresponds with the
vertebral tissue of the higher Chordata.
The ventral part of each diverticulum, which is prolonged so as to
meet its fellow in the middle ventral line, does not become divided
into somites, but contains a continuous cavity, which becomes the body
cavity of the adult. The inner layer of this part forms the splanchnic
mesoblast, and the outer layer the somatic mesoblast.
The notochord would almost appear to arise as a third median and
dorsal diverticulum of the archenteron (fig. 180 ch). At any rate it
arises as a central fold of the wall of this cavity, which is
gradually constricted off from before backwards.
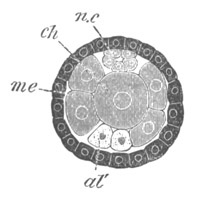
Fig. 181. Transverse optical
section of the tail of an
embryo of Phallusia mammillata. (After Kowalevsky.)
The section is from an embryo of the same age as fig. 8 IV.
ch. notochord; n.c. neural canal; me. mesoblast; al´.
hypoblast of tail.
Urochorda. In simple Ascidians the above processes undergo a slight
modification, which is mainly due (1) to a general simplification of
the organization, and (2) to the non-continuation of the notochord
into the trunk.
The whole dorsal wall of the posterior part of the archenteron is
converted into the notochord (fig. 181 ch), and the lateral walls
into the mesoblast (me); so that the original lumen of the posterior
part of the archenteron ceases to be bounded by hypoblast cells, and
disappears as such. Part of the ventral wall remains as a solid cord
of cells (al´) The anterior part of the archenteron in front of the
notochord passes wholly into the permanent alimentary tract.
The derivation of the mesoblast from the lateral walls of the
[Pg 294]
posterior part of the archenteron is clearly comparable with the
analogous process in Amphioxus.
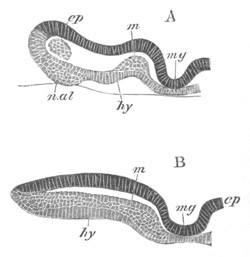
Fig. 182. Two transverse sections
of an embryo Pristiurus of the same
age as fig. 17.
A. Anterior section.
B. Posterior section.
mg. medullary groove; ep. epiblast; hy. hypoblast; n.al.
cells formed round the nuclei of the yolk which have entered the
hypoblast; m. mesoblast.
The sections shew the origin of the mesoblast.
Vertebrata. In turning from Amphioxus to the true Vertebrata we find
no form in which diverticula of the primitive alimentary tract give
rise to the mesoblast. There is reason to think that the type
presented by the Elasmobranchii in the formation of the mesoblast is
as primitive as that of any other group. In this group the mesoblast
is formed, nearly coincidently with the hypoblast of the dorsal wall
of the mesenteron, as two lateral sheets, one on each side of the
middle line (fig. 182 m). These two sheets are at first solid
masses; and their differentiation commences in front and is continued
backwards. After their formation the notochord arises from the axial
portion of the hypoblast (which had no share in giving rise to the two
mesoblast plates) as a solid thickening (fig. 183 ch´), which is
separated from it as a circular rod. Its differentiation, like that of
the mesoblastic plates, commences in front. The mesoblast plates
subsequently become divided for their whole length into two layers,
between which a cavity is developed (fig. 184). The dorsal parts of
the plates become divided by transverse partitions into somites, and
these somites with their contained cavities are next separated from
the more ventral parts of the plates (fig. 185 mp). In the somites
the cavities become eventually obliterated, and from their inner sides
plates of tissue for the vertebral bodies (fig. 186 Vr) are
separated; while the outer parts, consisting of two sheets, containing
the remains of the original cavity, form the muscle-plates (mp).
[Pg 295]
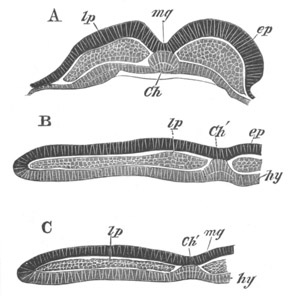
Fig. 183. Three sections of a Pristiurus embryo slightly older than
fig. 28 B.
The sections shew the development of the notochord.
Ch. notochord; Ch´. developing notochord; mg. medullary
groove; lp. lateral plate of mesoblast; ep. epiblast; hy.
hypoblast.
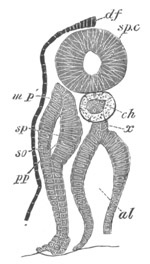
Fig. 184. Transverse section through the
Tail-region of a Pristiurus embryo of the
same age as fig. 28 E.
df. dorsal fin; sp.c. spinal cord; pp. body cavity; sp.
splanchnic layer of mesoblast; so. somatic layer of mesoblast;
mp´. commencing differentiation of muscles; ch. notochord; x.
subnotochordal rod arising as an outgrowth of the dorsal wall of the
alimentary tract; al. alimentary tract.
The undivided ventral portion gives rise to the general somatic and
splanchnic mesoblast (fig. 185), and the cavity between its two layers
constitutes the body cavity. The originally separate halves of the
body cavity eventually meet and unite in the ventral median line
throughout the greater part of the body, though in the tail they
remain distinct and are finally obliterated. Dorsally they are
separated by the mesentery. From the mesoblast at the junction of the
dorsal and
[Pg 296] ventral parts of the primitive plates is formed the
urinogenital system.
That the above mode of origin of the mesoblast and notochord is to be
regarded as a modification of that observable in Amphioxus seems
probable from the following considerations:—
In the first place, the mesoblast is split off from the hypoblast not
as a single mass but as a pair of distinct masses, comparable with the
paired diverticula in Amphioxus. Secondly, the body cavity, when it
appears in the mesoblast plates, does not arise as a single cavity,
but as a pair of cavities, one for each plate of mesoblast; and these
cavities remain permanently distinct in some parts of the body, and
nowhere unite till a comparatively late period. Thirdly, the primitive
body cavity of the embryo is not confined to the region in which a
body cavity exists in the adult, but extends to the summit of the
muscle-plates, at first separating parts which become completely
fused in the adult to form the great lateral muscles of the body.
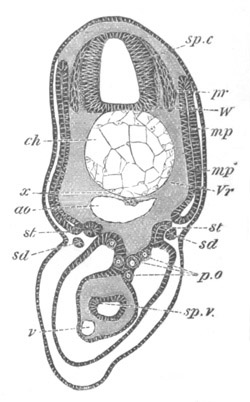
Fig. 185. Section through the trunk of a Scyllium
embryo slightly younger than 28 F.
sp.c. spinal canal; W. white matter of spinal cord; pr.
posterior nerve-roots; ch. notochord; x. subnotochordal rod;
ao. aorta; mp. muscle-plate; mp´. inner layer of muscle-plate
already converted into muscles; Vr. rudiment of vertebral body;
st. segmental tube; sd. segmental duct; sp.v. spiral valve;
v. subintestinal vein; p.o. primitive generative cells.
It is difficult to understand how the body cavity could thus extend
into the muscle-plates on the supposition that it represents a
primitive split in the mesoblast between the wall of the gut and the
body-wall; but its extension to this part is quite intelligible, on
the hypothesis that it represents the cavities of two diverticula of
the alimentary tract, from the muscular walls of which the voluntary
muscular system has been derived; and it may be pointed out that the
derivation of part of the muscular system from what is apparently
splanchnic mesoblast is easily explained on the above hypothesis, but
not, so far as I see, on any other.
[Pg 297]
Such are the main features, presented by the mesoblast in
Elasmobranchii, which favour the view of its having originally formed
the walls of the alimentary diverticula. Against this view of its
nature are the facts (1) of the mesoblast plates being at first solid,
and (2) of the body cavity as a consequence of this never
communicating with the alimentary canal. These points, in view of our
knowledge of embryological modifications, cannot be regarded as great
difficulties in my hypothesis. We have many examples of organs, which,
though in most cases arising as involutions, yet appear in other cases
as solid ingrowths. Such examples are afforded by the optic vesicle,
auditory vesicle, and probably also by the central nervous system of
Osseous Fishes. In most Vertebrates these organs are formed as hollow
involutions from the exterior; in Osseous Fishes, however, as solid
involutions, in which a cavity is secondarily established.
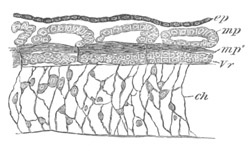
Fig. 186. Horizontal section through the trunk of an embryo of Scyllium
considerably younger than 28 F.
The section is taken at the level of the notochord, and shews the
separation of the cells to form the vertebral bodies from the
muscle-plates.
ch. notochord; ep. epiblast; Vr. rudiment of vertebral body;
mp. muscle-plate; mp´. portion of muscle-plate already
differentiated into longitudinal muscles.
There are strong grounds for thinking that in all Vertebrates the
mesoblast plates on each side of the notochord originate
independently, much as in Elasmobranchii, and that the notochord is
derived from the axial hypoblast; but there are some difficulties in
the application of this general statement to all cases. In Amphibia,
Ganoids, and Petromyzon, where the dorsal hypoblast is formed by a
process of invagination as in Amphioxus, the dorsal mesoblast also
owes its origin to this invagination, in that the indifferent
invaginated layer becomes divided into hypoblast and mesoblast.
Amongst these forms the mesoblast sheet, when separated from the
hypoblast, is certainly not continuous across the middle line in
Petromyzon (Calberla) and the Newt (Scott and Osborn), and doubtfully
so
[Pg 298] in the other forms. It arises, in fact, as in Elasmobranchii, as
two independent plates. The fact of these plates originating from an
invaginated layer can only be regarded in the light of an
approximation to the primitive type found in Amphioxus.
In Petromyzon and the Newt the whole axial plate of dorsal hypoblast
becomes separated off from the rest of the hypoblast as the notochord,
and this mode of origin for the notochord resembles more closely that
in Amphioxus than the mode of origin in Elasmobranchii.
In Teleostei, there is reason to think that the processes in the
formation of the mesoblast accord closely with what has been described
as typical for the Ichthyopsida, but there are still some points
involved in obscurity.
Leaving the Ichthyopsida, we may pass to the consideration of the
Sauropsida and Mammalia. In both of these types there is evidence to
shew that a part of the mesoblast is formed in situ at the same time
as the hypoblast, from the lower strata of segmentation spheres. This
mesoblast is absent in the front part of the area pellucida, and on
the formation of the primitive streak (blastopore), an outgrowth of
mesoblast arises from it as in Amphibia, etc. From this region the
mesoblast spreads as a continuous sheet to the sides and posterior
part of the blastoderm. In the region of the embryo, its exact
behaviour has not in some cases been quite satisfactorily made out.
There are reasons for thinking that it appears as two sheets not
united in the axial line in both Lacertilia (fig. 126) and Mammalia
(fig. 187), and this to some extent holds true for Aves (vide p.
156). In Lacertilia (fig. 188) and Mammalia, the axial hypoblast
becomes wholly converted into the notochord, which at the posterior
end of the body is continued into the epiblast at the dorsal lip of
the blastopore; while in Birds the notochord is formed by a very
similar (fig. 189 ch) process.

Fig. 187. Transverse section through an embryo Rabbit of eight days.
ep. epiblast; me. mesoblast; hy. hypoblast; mg. medullary
groove.
[Pg 299]
The above processes in the formation of the mesoblast are for the most
part easily explained by a comparison with the lower types. The
outgrowth of the mesoblast from the sides of the primitive streak is a
rudiment of the dorsal invagination of hypoblast and mesoblast found
in Amphibia; and the apparent outgrowth of the mesoblast from the
epiblast in the primitive streak is no more to be taken as a proof of
the epiblastic origin of the mesoblast, than the continuity of the
epiblast with the invaginated hypoblast and mesoblast at the lips of
the blastopore in the Frog of the derivation of these layers from the
epiblast in this type.
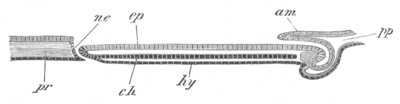
Fig. 188. Diagrammatic longitudinal section through an embryo
Lizard to shew the relations of the neurenteric canal (ne) and of
the primitive streak (pr).
am. amnion; ep. epiblast; hy. hypoblast; ch. notochord;
pp. body cavity; ne. neurenteric canal; pr. primitive streak.
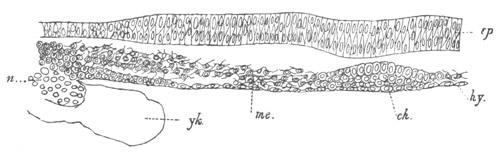
Fig. 189. Transverse section through the embryonic region of the
blastoderm of a Chick at the time of the formation of the notochord,
but before the appearance of the medullary groove.
ep. epiblast; hy. hypoblast; ch. notochord; me. mesoblast;
n. nuclei in the yolk of the germinal wall yk.
The division of the mesoblast into two plates along the dorsal line of
the embryo, and the formation of the notochord from the axial
hypoblast, are intelligible without further explanation. The
appearance of part of the mesoblast before the formation of the
primitive streak is a process of the same nature as the
[Pg 300]
differentiation of hypoblast and mesoblast in Elasmobranchii without
an invagination.
In the Sauropsida, some of the mesoblast of the vascular area would
appear to be formed in situ out of the germinal wall, by a process
of cell-formation similar to that which takes place in the yolk
adjoining the blastoderm in Elasmobranchii and Teleostei. The
mesoblast so formed is to be compared with that which arises on the
ventral side of the embryo in the Frog, by a direct differentiation of
the yolk-cells.
What was stated for the Elasmobranchii with reference to the general
fate of the mesoblast holds approximately for all the other forms.
The Epiblast.
The epiblast in a large number of Chordata arises as a single row of
more or less columnar cells. Since the epidermis, into which it
becomes converted, is formed of two more or less distinct strata in
all Chordata except Amphioxus and Ascidians, the primitive row of
epiblast cells, when single, necessarily becomes divided in the course
of development into two layers.
In some of the Vertebrata, viz. the Anurous Amphibia, Teleostei,
Acipenser, and Lepidosteus, the epiblast is from the first formed of
two distinct strata. The upper of these, formed of a single row of
cells, is known as the epidermic stratum, and the lower, formed of
several rows, as the nervous stratum. In these cases the two original
strata of the epiblast are equivalent to those which appear at a later
period in the other forms. Thus Vertebrates may be divided into groups
according to the primitive condition of their epiblast, viz. a larger
group with but a single stratum of cells at first; and a smaller group
with two strata.
While there is no great difficulty in determining the equivalent parts
of the epidermis in these two groups, it still remains an open
question in which of them the epiblast retains its primitive
condition.
Though it is not easy to bring conclusive proofs on the one side or
the other, the balance of argument appears to me to be
[Pg 301] decidedly in
favour of regarding the condition of the epiblast in the larger group
as primitive, and its condition in the smaller group as secondary, and
due to the throwing back of the differentiation of the epiblast to a
very early period of development.
In favour of this view may be urged (1) the fact that the simple
condition is retained in Amphioxus through life. (2) The correlation
in Amphibia, and the other forms belonging to this group, between a
closed auditory pit and the early division of the epiblast into two
strata; there being no doubt that the auditory pit was at first
permanently open, a condition of the epiblast which necessitates its
never having an external opening must clearly be secondary. (3) It
appears more likely that a particular genetic feature should be thrown
back in development, than that such an important feature, as a
distinction between two primary layers, should be absolutely lost
during an early period of development, and then reappear in later
stages.
The fact of the epiblast of the neural canal being divided, like the
remainder of the layer, into nervous and epidermic parts, cannot, I
think, be used as an argument in favour of the opposite view to that
here maintained. It seems probable that the central canal of the
nervous system arose phylogenetically as an involution from the
exterior, and that the epidermis lining it is merely part of the
original epidermis, which has retained its primitive structure as a
simple stratum, but is naturally distinguishable from the nervous
structures adjacent to it.
Where the epiblast is divided at an early period into two strata, the
nervous stratum is always the active one, and takes the main share in
forming all the organs derived from the layer.
Formation of the central nervous system. In all Chordata an axial
strip of the dorsal epiblast, extending from the lip of the blastopore
to the anterior extremity of the head, and known as the medullary
plate, becomes isolated from the remainder of the layer to give rise
to the central nervous axis.
According to the manner in which this takes place, three types may,
however, be distinguished. In Amphioxus the axial
[Pg 302] strip becomes first
detached from the adjoining epiblast, which then meets and forms a
continuous layer above it (fig. 190 A and B np). The sides of the
medullary plate, which is thus shut off from the surface, bend over
and meet so as to convert the plate into a canal (fig. 190 C nc). In
the second and ordinary type the sides of the medullary plate fold
over and meet so as to form a canal before the plate becomes isolated
from the external epiblast.
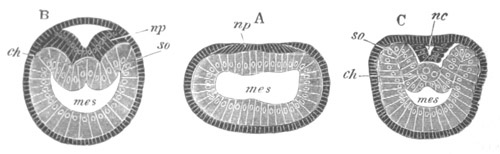
Fig. 190. Sections of an Amphioxus embryo at three stages. (After
Kowalevsky.)
A. Section at gastrula stage.
B. Section of an embryo slightly younger than that represented in
fig. 169 D.
C. Section through the anterior part of an embryo at the stage
represented in fig. 169 E.
np. neural plate; nc. neural canal; mes. archenteron in A and
B, and mesenteron in C; ch. notochord; so. mesoblastic somite.
The third type is characteristic of Lepidosteus, Teleostei, and
Petromyzon. Here the axial plate becomes narrowed in such a way that
it forms a solid keel-like projection towards the ventral surface
(fig. 191 Me). This keel subsequently becomes separated from the
remainder of the epidermis, and a central canal is afterwards
developed in it. Calberla and Scott hold that the epidermic layer of
the skin is involuted into this keel in Petromyzon, and Calberla
maintains the same view for Teleostei (fig. 32), but further
observations on this subject are required. In the Teleostei a very
shallow depression along the axis of the keel is the only indication
of the medullary groove of other forms.
In Amphioxus (fig. 190), the Tunicata, Petromyzon (?), Elasmobranchii
(fig. 182), the Urodela and Mammalia (fig. 187), the epiblast of the
medullary plate is only formed of a single row of cells at the time
when the formation of the central nervous system commences; but,
except in Amphioxus and the Tunicata,
[Pg 303] it becomes several cells deep
before the completion of the process. In other types the epiblast is
several cells deep even before the differentiation of a medullary
plate. In the Anura, the nervous layer of the epidermis alone is
thickened in the formation of the central nervous system (fig. 72);
and after the closure of the medullary canal, the epidermic layer
fuses for a period with the nervous layer, though on the subsequent
formation of the central epithelium of the nervous canal, there can be
little doubt that it becomes again distinct.
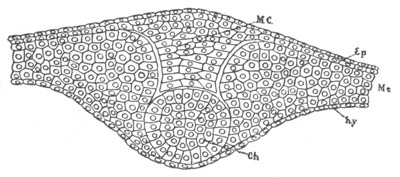
Fig. 191. Section through an embryo of Lepidosteus on the fifth day
after impregnation.
MC. medullary cord; Ep. epiblast; Me. mesoblast; hy.
hypoblast; Ch. notochord.
It seems almost certain that the formation of the central nervous
system from a solid keel-like thickening of the epidermis is a derived
and secondary mode; and that the folding of the medullary plate into a
canal is primitive. Apart from its greater frequency the latter mode
of formation of the central nervous system is shewn to be the
primitive type by the fact that it offers a simple explanation of the
presence of the central canal of the nervous system; while the
existence of such a canal cannot easily be explained on the assumption
that the central nervous system was originally developed as a
keel-like thickening of the epiblast.
It is remarkable that the primitive medullary plate rarely exhibits
any indication of being formed of two symmetrical halves. Such
indications are, however, found in the Amphibia (fig. 192 and fig.
72); and, since in the adult state the nervous cord exhibits nearly as
distinct traces of being formed of two united strands as does the
ventral nerve-cord of many Chætopods, it is
[Pg 304] quite possible that the
structure of the medullary plate in Amphibia may be more primitive
than that in other types[99].
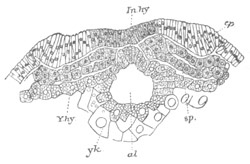
Fig. 192. Transverse section through
the cephalic region of a young Newt embryo. (After Scott and Osborn.)
In.hy. invaginated hypoblast, the dorsal part of which will form
the notochord; ep. epiblast of neural plate; sp.
splanchnopleure; al. alimentary tract; yk. and Y.hy.
yolk-cells.
Formation of the organs of special sense. The more important parts of
the organs of smell, sight, and hearing are derived from the epiblast;
and it has been asserted that the olfactory pit, optic vesicles and
auditory pit take their origin from a special sense plate, continuous
at first with this medullary plate. In my opinion this view cannot be
maintained.
In the case of the group of forms in which the epiblast is early
divided into nervous and epidermic layers, the former layer alone
becomes involuted in the formation of the auditory pit and the lens,
the external openings of which are never developed, while it is also
mainly concerned in the formation of the olfactory pit.
Summary of the more important Organs derived from the three
germinal layers.
The epiblast primarily gives origin to two very important parts of the
body, viz. the central nervous system and the epidermis.
It is from the involuted epiblast of the neural tube that the whole of
the grey and white matter of the brain and spinal cord appears to be
developed, the simple columnar cells of the epiblast being directly
transformed into the characteristic multipolar nerve cells. The whole
of the sympathetic nervous system
[Pg 305] and the peripheral nervous elements
of the body, including both the spinal and the cranial nerves and
ganglia, are epiblastic in origin.
The epithelium (ciliated in the young animal) lining the canalis
centralis of the spinal cord, together with that lining the ventricles
of the brain, is the undifferentiated remnant of the primitive
epiblast.
The epiblast also forms the epidermis; not however the dermis, which
is of mesoblastic origin. The line of junction between the epiblast
and the mesoblast coincides with that between the epidermis and the
dermis. From the epiblast are formed all such tegumentary organs or
parts of organs as are epidermic in nature.
In addition to the above, the epiblast plays an important part in the
formation of the organs of special sense.
According to their mode of formation, these organs may be arranged
into two divisions. In the first come the organs where the sensory
expansion is derived from the involuted epiblast of the medullary
canal. To this class belongs the retina, including the pigment
epithelium of the choroid, which is formed from the original optic
vesicle budded out from the fore-brain.
To the second class belong the epithelial expansions of the membranous
labyrinth of the ear, and the cavity of the nose, which are formed by
an involution of the epiblast covering the external surface of the
embryo. These accordingly have no primary connection with the brain.
‘Taste bulbs’ and other terminal nervous organs, such as those of the
lateral line in fishes, are also structures formed from the external
epiblast.
In addition to these we have the crystalline lens formed of involuted
epiblast as well as the cavity of the mouth and anus, and the glands
derived from them. The pituitary body is also epiblastic in origin.
From the hypoblast are derived the epithelium of the digestive canal,
the epithelium of the trachea, bronchial tubes and air cells, the
cylindrical epithelium of the ducts of the liver, pancreas, thyroid
body, and other glands of the alimentary canal, as well as the hepatic
cells constituting the parenchyma of the liver, developed from the
hypoblast cylinders given off around the primary hepatic diverticula.
[Pg 306]
Homologous probably with the hepatic cells, and equally of hypoblastic
origin, are the spheroidal ‘secreting cells’ of the pancreas and other
glands. The epithelium of the salivary glands, though these so closely
resemble the pancreas, is probably of epiblastic origin, inasmuch as
the cavity of the mouth is entirely lined by epiblast.
The hypoblast also lines the allantois. To these parts must be added
the notochord and subnotochordal rod. From the mesoblast are formed
all the remaining parts of the body. The muscles, the bones, the
connective tissue and the vessels, both arteries, veins, capillaries
and lymphatics with their appropriate epithelium, are entirely formed
from the mesoblast.
The generative and urinary organs are entirely derived from the
mesoblast. It is worthy of notice that the epithelium of the urinary
glands, though resembling the hypoblastic epithelium of the alimentary
canal, is undoubtedly mesoblastic.
From the mesoblast are lastly derived all the muscular, connective
tissue, and vascular elements, as well of the alimentary canal and its
appendages as of the skin and the tegumentary organs. Just as it is
only the epidermic moiety of the latter which is derived from the
epiblast, so it is only the epithelium of the former which comes from
the hypoblast.
Growth in length of the Vertebrate Embryo.
With reference to the formation and growth in length of the body of
the Vertebrate embryo two different views have been put forward, which
can be best explained by taking the Elasmobranch embryo as our type.
One of these views, generally held by embryologists and adopted in the
previous pages, is that the Elasmobranch embryo arises from a
differentiation of the edge of the blastoderm; which extends inwards
from the edge for some little distance. This differentiation is
supposed to contain within itself the rudiments of the whole of the
embryo with the exception of the yolk-sack; and the hinder extremity
of it, at the edge of the blastoderm, is regarded as corresponding
with the hind end of the body of the adult. The growth in length takes
place by a process of intussusception, and, till there are formed the
full number of mesoblastic somites, it is effected, as in Chætopods,
by the continual addition of fresh somites between the last-formed
somite and the hind end of the body.
A second and somewhat paradoxical view has been recently brought into
prominence by His and Rauber. This view has moreover since been taken
up by many embryologists, and has led to strange comparisons between
the
[Pg 307] formation of the mesoblastic plates of the Chætopods and the
medullary folds of Vertebrata. According to this view the embryo grows
in length by the coalescence of the two halves of the thickened edges
of the blastoderm in the dorsal median line. The groove between the
coalescing edges is the medullary groove, which increases in length by
the continued coalescence of fresh portions of the edge of the
blastoderm.
The following is His’ own statement of his view: “I have shewn that
the embryo of Osseous Fishes grows together in length from two
symmetrically-placed structures in the thickened edge of the
blastoderm. Only the foremost end of the head and the hindermost end
of the tail undergo no concrescence, since they are formed out of that
part of the edge of the blastoderm which, together with the two
lateral halves, completes the ring. The whole edge of the blastoderm
is used in the formation of the embryo.”
The edges of the blastoderm which meet to form the body of the embryo
are regarded as the blastopore, so that, on this view, the blastopore
primitively extends for the whole length of the dorsal side of the
embryo, and the groove between the coalesced lips becomes the
medullary groove.
It is not possible for me to enter at any great length into the
arguments used to support this position.
They may be summarised as (1) The general appearance; i.e. that the
thickened edge of the blastoderm is continuous with the medullary
fold.
(2) Certain measurements (His) which mainly appear to me to prove that
the growth takes place by the addition of fresh somites between that
last formed and the end of the body.
(3) Some of the phenomena of double monsters (Rauber).
None of these arguments appear to be very forcible, but as the view of
His and Rauber, if true, would certainly be important, I shall attempt
shortly to state the arguments against it, employing as my type the
Elasmobranchii, by the development of which, according to His, the
view which he adopts is more conclusively proved than by that of any
other group.
(1) The general appearance of the thickened edge of the blastoderm
becoming continuous with the medullary folds has been used as an
argument for the medullary folds being merely the coalesced thickened
edges of the blastoderm. Since, however, the medullary folds are
merely parts of the medullary plate, and since the medullary plate is
continuous with the adjoining epiblast of the embryonic rim, the
latter structure must be continuous with the medullary folds however
they are formed, and the mere fact of their being so continuous cannot
be used as an argument either way. Moreover, were the concrescence
theory true, the coalescing edges of the blastoderm might be expected
to form an acute angle with each other, which they are far from doing.
(2) The medullary groove becomes closed behind earlier than in front,
and the closure commences while the embryo is still quite short, and
before the hind end has begun to project over the yolk. After the
medullary canal becomes closed, and is continued behind into the
alimentary canal by the neurenteric passage, it is clearly impossible
for any further increase in length
[Pg 308] to take place by concrescence. If
therefore His’ and Rauber’s view is accepted, it will have to be
maintained that only a small part of the body is formed by concrescence,
while the larger posterior part grows by intussusception. The
difficulty involved in this supposition is much increased by the fact
that long after the growth by concrescence must have ceased the yolk
blastopore still remains open, and the embryo is still attached to the
edge of the blastoderm; so that it cannot be maintained that the
growth by concrescence has come to an end because the thickened edges
of the blastoderm have completely coalesced.
The above are arguments derived simply from a consideration of the
growth of the embryo; and they prove (1) that the points adduced by
His and Rauber are not at all conclusive; (2) that the growth in
length of the greater part of the body takes place by the addition of
fresh somites behind, as in Chætopods, and it would therefore be
extremely surprising that a small middle part of the body should grow
in quite a different way.
Many minor arguments used by His might be replied to, but it is hardly
necessary to do so, and some of them depend upon erroneous views as to
the course of development, such as an argument about the notochord,
which depends for its validity upon the assumption that the notochord
ridge appears at the same time as the medullary plate, while, as a
matter of fact, the ridge does not appear till considerably later. In
addition to the arguments of the class hitherto used, there may be
brought against the His-Rauber view a series of arguments from
comparative embryology.
(1) Were the vertebrate blastopore to be co-extensive with the dorsal
surface, as His and Rauber maintain, clear evidence of this ought to
be apparent in Amphioxus. In Amphioxus, however, the blastopore is at
first placed exactly at the hind end of the body, though later it
passes up just on to the dorsal side (vide p. 4). It nearly closes
before the appearance of the medullary groove or mesoblastic somites;
and the medullary folds have nothing to do with its lips, except in so
far as they are continuous with them behind, just as in
Elasmobranchii.
(2) The food-yolk in the Vertebrata is placed on the ventral side of
the body, and becomes enveloped by the blastoderm; so that in all
large-yolked Vertebrates the ventral walls of the body are obviously
completed by the closure of the lips of the blastopore, on the ventral
side.
If His and Rauber are right the dorsal walls are also completed by the
closure of the blastopore, so that the whole of the dorsal, as well as
of the ventral wall of the embryo, must be formed by the concrescence
of the lips of the blastopore; which is clearly a reductio ad
absurdum of the whole theory. To my own arguments on the subject I
may add those of Kupffer, who has very justly criticised His'
statements, and has shewn that growth of the blastoderm in Clupea and
Gasterosteus is absolutely inconsistent with the concrescence theory.
The more the theory of His and Rauber is examined by the light of
comparative embryology, the more does it appear quite untenable; and
it may be laid down as a safe conclusion from a comparative study of
vertebrate
[Pg 309] embryology that the blastopore of Vertebrates is
primitively situated at the hind end of the body, but that, owing to
the development of a large food-yolk, it also extends, in most cases,
over a larger or smaller part of the ventral side.
The origin of the Allantois and Amnion.
The development and structure of the allantois and amnion have already
been dealt with at sufficient length in the chapters on Aves and
Mammalia; but a few words as to the origin of these parts will not be
out of place here.
The Allantois. The relations of the allantois to the adjoining organs,
and the conversion of its stalk into the bladder, afford ample
evidence that it has taken its origin from a urinary bladder such as
is found in Amphibia. We have in tracing the origin of the allantois
to deal with a case of what Dohrn would call ‘change of function.’ The
allantois is in fact a urinary bladder which, precociously developed
and enormously extended in the embryo, has acquired respiratory
(Sauropsida) and nutritive (Mammalia) functions. No form is known to
have been preserved with the allantois in a transitional state between
an ordinary bladder and a large vascular sack.
The advantage of secondary respiratory organs during fœtal life, in
addition to the yolk-sack, is evinced by the fact that such organs are
very widely developed in the Ichthyopsida. Thus in Elasmobranchii we
have the external gills (cf. p. 62). Amongst Amphibia we have the tail
modified to be a respiratory organ in Pipa Americana; and in
Notodelphis, Alytes and Cæcilia compressicanda the external gills are
modified and enlarged for respiratory purposes within the egg (cf. pp. 140 and 143).
The Amnion. The origin of the amnion is more difficult to explain than
that of the allantois; and it does not seem possible to derive it from
any pre-existing organ.
It appears to me, however, very probable that it was evolved pari
passu with the allantois, as a simple fold of the somatopleure round
the embryo, into which the allantois extended itself as it increased
in size and became a respiratory organ. It would be obviously
advantageous for such a fold, having once started, to become larger
and larger in order to give more and more room for the allantois to
spread into.
The continued increase of this fold would lead to its edges meeting on
the dorsal side of the embryo, and it is easy to conceive that they
might then coalesce.
To afford room for the allantois close to the surface of the egg,
where respiration could most advantageously be carried on, it would be
convenient that the two laminæ of the amnion—the true and false
amnion—should then separate and leave a free space above the embryo,
and thus it may have come about that a separation finally takes place
between the true and false amnion.
This explanation of the origin of the amnion, though of course
hypothetical, has the advantage of suiting itself in most points to
the actual ontogeny
[Pg 310] of the organ. The main difficulty is the early
development of the head-fold of the amnion, since, from the position
of the allantois, it might have been anticipated that the tail-fold
would be the first formed and most important fold of the amnion.
Bibliography.
(239) F. M. Balfour. “A comparison of the early stages in the
development of Vertebrates.” Quart. J. of Micr. Science, Vol. XV.
1875.
(240) F. M. Balfour. “A monograph on the development of Elasmobranch
Fishes.” London, 1878.
(241) F. M. Balfour. “On the early development of the Lacertilia
together with some observations, etc.” Quart. J. of Micr. Science,
Vol. XIX. 1879.
(242) A. Götte. Die Entwicklungsgeschichte d. Unke. Leipzig, 1875.
(243) W. His. “Ueb. d. Bildung d. Haifischembryonen.” Zeit. f. Anat.
u. Entwick., Vol. II. 1877. Cf. also His’ papers on Teleostei, Nos.
65 and 66.
(244) A. Kowalevsky. “Entwick. d. Amphioxus lanceolatus.” Mém. Acad.
des Sciences St Pétersbourg, Ser. VII. Tom. XI. 1867.
(245) A. Kowalevsky. “Weitere Studien üb. d. Entwick. d. Amphioxus
lanceolatus.” Archiv f. mikr. Anat., Vol. XIII. 1877.
(246) C. Kupffer. “Die Entstehung d. Allantois u. d. Gastrula d.
Wirbelthiere.” Zool. Anzeiger, Vol. II. 1879, pp. 520, 593, 612.
(247) R. Remak. Untersuchungen üb. d. Entwicklung d. Wirbelthiere,
1850-1858.
(248) A. Rauber. Primitivstreifen u. Neurula d. Wirbelthiere.
Leipzig, 1877.
[Pg 311]
CHAPTER XII.
OBSERVATIONS ON THE ANCESTRAL FORM OF THE CHORDATA.
The present section of this work would not be complete without some
attempt to reconstruct, from the materials recorded in the previous
chapters, and from those supplied by comparative anatomy, the
characters of the ancestors of the Chordata; and to trace as far as
possible from what invertebrate stock this ancestor was derived.
The second of these questions has been recently dealt with in a very
suggestive manner by both Dohrn (No. 250) and Semper (Nos. 255 and
256), but it is still so obscure that I shall refrain from any
detailed discussion of it.
While differing very widely in many points both Dohrn and Semper have
arrived at the view, already tentatively put forward by earlier
anatomists, that the nearest allies of the Chordata are to be sought
for amongst the Chætopoda, and that the dorsal surface of the Chordata
with the spinal cord corresponds morphologically with the ventral
surface of the Chætopods with the ventral ganglion chain. In
discussing this subject some time ago[100]
I suggested that we must
look for the ancestors of the Chordata, not in allies of the present
Chætopoda, but in a stock of segmented forms descended from the same
unsegmented types as the Chætopoda, but in which two lateral
nerve-cords, like those of Nemertines, coalesced dorsally, instead of
ventrally to form a median nervous cord. This group of forms, if my
suggestion as to its existence is well founded, appears now to have
perished. The recent researches of Hubrecht on the anatomy of the
Nemertines[101]
have, however, added somewhat to the probability of my
views, in that they shew that in some existing Nemertines the
nerve-cords approach each other very closely in the dorsal line.
With reference to the characters of the ancestor of the Chordata the
following pages contain a few tentative suggestions rather than an
attempt to deal with the whole subject; while the
[Pg 312] origin of certain of
the organs is dealt with in a more special manner in the chapters on
organogeny which form the second part of this work.
Before entering upon the more special subject of this chapter, it will
be convenient to clear the ground by insisting on a few morphological
conclusions to be drawn from the study of Amphioxus,—a form which,
although probably in some respects degenerate, is nevertheless capable
of furnishing on certain points very valuable evidence.
(1) In the first place it is clear from Amphioxus that the ancestors
of the Chordata were segmented, and that their mesoblast was divided
into myotomes which extended even into the region in front of the
mouth. The mesoblast of the greater part of what is called the head in
the Vertebrata proper was therefore segmented like that of the trunk.
(2) The only internal skeleton present was the unsegmented
notochord—a fact which demonstrates that the skeleton is of
comparatively little importance for the solution of a large number of
fundamental questions, as for example the point which has been mooted
recently as to whether gill-clefts existed at one time in front of the
present mouth; and for this reason:—that from the evidence of
Amphioxus and the lower Vertebrata[102]
it is clear that such clefts,
if they ever existed, had atrophied
[Pg 313] completely before the
formation of cartilaginous branchial bars; so that any skeletal
structures in front of the mouth, which have been interpreted by
morphologists as branchial bars, can never have acted in supporting
the walls of branchial clefts.
(3) The region which, in the Vertebrata, forms the œsophagus and
stomach, was, in the ancestors of the Chordata, perforated by
gill-clefts. This fact, which has been clearly pointed out by
Gegenbaur, is demonstrated by the arrangement of the gill-clefts in
Amphioxus, and by the distribution of the vagus nerve in the
Vertebrata[103].
On the other hand the insertion of the liver, which
was probably a very primitive organ, appears to indicate with
approximate certainty the posterior limit of the branchial clefts.
With these few preliminary observations we may pass to the main
subject of this section. A fundamental question which presents itself
on the threshold of our enquiries is the differentiation of the head.
In the Chætopoda the head is formed of a præoral lobe and of the oral
segment; while in Arthropods a somewhat variable number of segments
are added behind to this primitive head, and form with it what may be
called a secondary compound head. It is fairly clear that the section
of the trunk, which, in Amphioxus, is perforated by the visceral
clefts, has become the head in the Vertebrates proper, so that the
latter forms are provided with a secondary head like that of
Arthropods. There remain however difficult questions (1) as to the
elements of which this head is composed, and (2) as to the extent of
its differentiation in the ancestors of the Chordata.
In Arthropods and Chætopods there is a very distinct element in the
head known as the procephalic lobe in the case of Arthropods, and the
præoral lobe in that of Chætopods; and this lobe is especially
characterized by the fact that the supraœsophageal ganglia and optic
organs are formed as differentiations
[Pg 314] of part of the epiblast covering
it. Is such an element to be recognized in the head of the Chordata?
From a superficial examination of Amphioxus the answer would
undoubtedly be no; but then it has to be borne in mind that Amphioxus,
in correlation with its habit of burying itself in sand, is especially
degenerate in the development of its sense-organs; so that it is not
difficult to believe that its præoral lobe may have become so reduced
as not to be recognizable. In the true Vertebrata there is a portion
of the head which has undoubtedly many features of the præoral lobe in
the types already alluded to, viz. the part containing the cerebral
hemispheres and the thalamencephalon. If there is any part of the
brain homologous with the supraœsophageal ganglia of the
Invertebrates, and it is difficult to believe there is not such a
part, it must be part of, or contain, the fore-brain. The fore-brain
resembles the supraœsophageal ganglia in being intimately connected
in its development with the optic organs, and in supplying with nerves
only organs of sense. Its connection with the olfactory organs is an
argument in the same direction. Even in Amphioxus there is a small
bulb at the end of the nervous tube supplying what is very probably
the homologue of the olfactory organ of the Vertebrata; and it is
quite possible that this bulb is the reduced rudiment of what forms
the fore-brain in the Vertebrata.
The evidence at our disposal appears to me to indicate that the third
nerve belongs to the cranio-spinal series of segmental nerves, while
the optic and olfactory nerves appear to me equally clearly not to
belong to this series[104].
The mid-brain, as giving origin to the
third nerve, would appear not to have been part of the ganglion of the
præoral lobe.
These considerations indicate with fair probability that the part of
the head containing the fore-brain is the equivalent of the præoral
lobe of many Invertebrate forms; and the primitive position of the
Vertebrate mouth on the ventral side of the head affords a distinct
support for this view. It must however be admitted that this part of
the head is not sharply separated in development from that behind;
and, though the fore-brain is
[Pg 315] usually differentiated very early as a
distinct lobe of the primitive nervous tube, yet that such
differentiation is hardly more marked than in the other parts of the
brain. The termination of the notochord immediately behind the
fore-brain is, however, an argument in favour of the morphological
distinctness of the latter structure.
The evidence at our disposal appears to indicate that the posterior
part of the head was not differentiated from the trunk in lower
Chordata; but that, as the Chordata rose in the scale of development,
more and more centralizing work became thrown on the anterior part of
the nervous cord, and pari passu this part became differentiated
into the mid- and hind-brain. An analogy for such a differentiation is
supplied in the compound subœsophageal ganglion of many Arthropods;
and, as will be shewn in the chapter on the nervous system, there is
strong embryological evidence that the mid- and hind-brains had
primitively the same structure as the spinal cord. The head appears
however to have suffered in the course of its differentiation a great
concentration in its posterior part, which becomes progressively more
marked, even within the limits of the surviving Vertebrata. This
concentration is especially shewn in the structure of the vagus nerve,
which, as first pointed out by Gegenbaur, bears evidence of having
been originally composed of a great series of nerves, each supplying a
visceral cleft. Rudiments of the posterior nerves still remain as the
branches to the œsophagus and stomach[105].
The atrophy of the posterior visceral clefts seems to have taken place
simultaneously with the concentration of the neural part of the head;
but the former process did not proceed so rapidly as the latter, so
that the visceral region of the head is longer in the lower Vertebrata
than the neural region, and is dorsally overlapped by the anterior
part of the spinal cord and the anterior muscle-plates (videfig.
47).
On the above view the posterior part of the head must have been
originally composed of a series of somites like those of the
[Pg 316] trunk,
but in existing Vertebrata all trace of these, except in so far as
they are indicated by the visceral clefts, has vanished in the adult.
The cranial nerves however, especially in the embryo, still indicate
the number of anterior somites; and an embryonic segmentation of the
mesoblast has also been found in many lower forms in the region of the
head, giving rise to a series of cavities known as head-cavities,
enclosed by mesoblastic walls which afterwards break up into muscles.
These cavities correspond with the nerves, and it appears that there
is a præmandibular cavity corresponding with the third nerve (fig.
193, 1pp) and a mandibular cavity (2pp) and a cavity in each of
the succeeding visceral arches. The fifth nerve, the seventh nerve,
the glossopharyngeal nerve, and the successive elements of the vagus
nerve correspond with the posterior head-cavities.
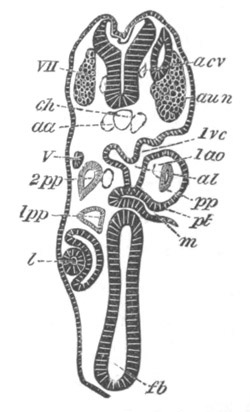
Fig. 193. Transverse section through the front part of the head of
a young Pristiurus embryo.
The section, owing to the cranial flexure, cuts both the fore- and
the hind-brain. It shews the præmandibular and mandibular
head-cavities 1pp and 2pp, etc.
fb. fore-brain; l. lens of eye; m. mouth; pt. upper end of
mouth, forming pituitary involution; 1ao. mandibular aortic arch;
1pp. and 2pp. first and second head-cavities; 1vc. first
visceral cleft; V. fifth nerve; aun. ganglion of auditory nerve;
VII. seventh nerve; aa. dorsal aorta; acv. anterior cardinal
vein; ch. notochord.
The medullary canal. The general history of the medullary plate seems
to point to the conclusion that the central canal of the nervous
system has been formed by a groove having appeared in the ancestor of
the Chordata along the median dorsal line, which caused the sides of
the nervous plate, which was placed immediately below the skin, or may
perhaps at that stage not have been distinctly differentiated from the
skin, to be bent upwards; and that this groove subsequently became
converted into a canal. This view is not only supported by the actual
development of the central canal of the nervous system (the types of
Teleostei, Lepidosteus and Petromyzon being undoubtedly secondary),
but also (1) by the presence of cilia in the epithelium lining the
canal, probably inherited from cilia coating the external skin, and
(2) by
[Pg 317] the posterior roots arising from the extreme dorsal line (fig.
194), a position which can most easily be explained on the supposition
that the two sides of the plate, from which the nerves originally
proceeded have been folded up so as to meet each other in the median
dorsal line[106].
The medullary plate, before becoming folded to form the medullary
groove, is (except in Amphibia) without any indication of being
composed of two halves. In both the embryo and adult the walls of the
tube have however a structure which points to their having arisen from
the coalescence of two lateral, and most probably at one time
independent, cords; and as already indicated this is the view I am
myself inclined to adopt; vide pp. 303 and 304.
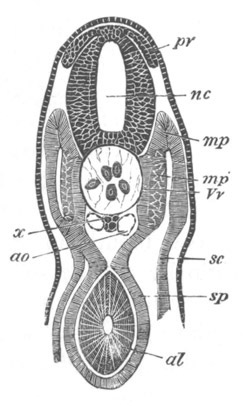
Fig. 194. Transverse section
through the trunk of
an embryo slightly older
than fig. 28 e.
nc. neural canal; pr. posterior root of spinal nerve; x.
subnotochordal rod; ao. aorta; sc. somatic mesoblast; sp.
splanchnic mesoblast; mp. muscle-plate; mp´. portion of
muscle-plate converted into muscle; Vv. portion of the vertebral
plate which will give rise to the vertebral bodies; al. alimentary
tract.
The origin and nature of the mouth. The most obvious point connected
with the development of the mouth is the fact that in all vertebrate
embryos it is placed ventrally, at some little distance from the front
end of the body. This feature is retained in the adult stage in
Elasmobranchii, the Myxinoids, and some Ganoids, but is lost in other
vertebrate forms. A mouth, situated as is the embryonic vertebrate
mouth, is very ill adapted for biting; and though it acquires in this
position a distinctly biting character in the Elasmobranchii, yet it
is almost certain that it had not such a character in the ancestral
Chordata, and that its terminal position in higher types indicates a
step in advance of the Elasmobranchii.
On the structure of the primitive mouth there appears to me
[Pg 318] to be some
interesting embryological evidence, to which attention has already
been called in the preceding chapters. In a large number of the larvæ
or embryos of the lower Vertebrates the mouth has a more or less
distinctly suctorial character, and is connected with suctorial organs
which may be placed either in front of or behind it. The more
important instances of this kind are (1) the Tadpoles of the Anura,
with their posteriorly placed suctorial disc, (2) Lepidosteus larva
(fig. 195) with its anteriorly placed suctorial disc, (3) the adhesive
papillæ of the larvæ of the Tunicata. To these may be added the
suctorial mouth of the Myxinoid fishes[107].
All these considerations point to the conclusion that in the ancestral
Chordata the mouth had a more or less definitely suctorial
character[108],
and was placed on the ventral surface immediately
behind the præoral lobe; and that this mouth has become in the higher
types gradually modified for biting purposes, and has been carried to
the front end of the head.
The mouth in Elasmobranchii and other Vertebrates is originally a wide
somewhat rhomboidal cavity (fig. 28 G); on the development of the
mandibular and its maxillary (pterygo-quadrate) process the opening of
the mouth becomes narrowed to a slit. The wide condition of the mouth
may not improbably be interpreted as a remnant of the suctorial state.
The fact that no more definite remnants of the suctorial mouth are
found in so primitive a group as the Elasmobranchii is probably to be
explained by the fact that the members of this group undergo an
abbreviated development within the egg.
[Pg 319]
While the embryological data appear to me to point to the existence of
a primitive suctorial mouth, very different conclusions have been put
forward by other embryologists, more especially by Dohrn, which are
sufficiently striking and suggestive to merit a further discussion.
As mentioned above, both Dohrn and Semper hold that the Vertebrata are
descended from Chætopod-like forms, in which the ventral surface has
become the dorsal. In consequence of this view Dohrn has arrived at
the following conclusions: (1) that primitively the alimentary canal
perforated the nervous system in the region of the original
œsophageal nerve-ring; (2) that there was therefore an original
dorsal mouth (the present ventral mouth of the Chætopoda); and (3)
that the present mouth was secondary and derived from two visceral
clefts which have ventrally coalesced.
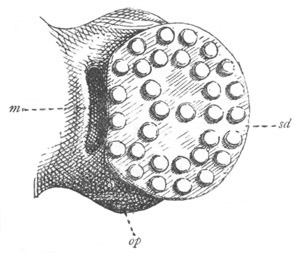
Fig. 195. Ventral view of the head of a Lepidosteus embryo shortly
before hatching, to shew the large suctorial disc.
m. mouth; op. eye; sd. suctorial disc.
A full discussion of these views[109]
is not within the scope of this
work; but, while recognizing that there is much to be said in favour
of the interchange of the dorsal and ventral surfaces, I am still
inclined to hold that the difficulties involved in this view are so
great that it must, provisionally at least, be rejected; and that
there are therefore no reasons against supposing the present
vertebrate mouth to be the primitive mouth. There is no embryological
evidence in favour of the view adopted by Dohrn that the present mouth
was formed by the coalescence of two clefts.
If it is once admitted that the present mouth is the primitive mouth,
and is more or less nearly in its original situation, very strong
evidence will be required to shew that any structures originally
situated in front of it are the remnants of visceral clefts; and if it
should be proved that such remnants of visceral clefts were present,
the views so far arrived at in this section would, I think, have to be
to a large extent reconsidered.
The nasal pits have been supposed by Dohrn to be remnants of visceral
[Pg 320]
clefts, and this view has been maintained in a very able manner by
Marshall. The arguments of Marshall do not, however, appear to me to
have any great weight unless it is previously granted that there is an
antecedent probability in favour of the presence of a pair of
gill-clefts in the position of the nasal pits; and even then the
development of the nasal pits as epiblastic involutions, instead of
hypoblastic outgrowths, is a serious difficulty which has not in my
opinion been successfully met. A further argument of Marshall from the
supposed segmental nature of the olfactory nerve has already been
spoken of.
While most of the structures supposed to be remains of gill-clefts in
front of the mouth do not appear to me to be of this nature, there is
one organ which stands in a more doubtful category. This organ is the
so-called choroid gland. The similarity of this organ to the
pseudobranch of the mandibular or hyoid arch was pointed out to me by
Dohrn, and the suggestion was made by him that it is the remnant of a
præmandibular gill which has been retained owing to its functional
connection with the eye[110].
Admitting this explanation to be true
(which however is by no means certain) are we necessarily compelled to
hold that the choroid gland is the remnant of a gill-cleft originally
situated in front of the mouth? I believe not. It is easy to conceive
that there may originally have been a præmandibular cleft behind the
suctorial mouth, but that this cleft gradually atrophied (for the same
reasons that the mandibular cleft shews a tendency to atrophy in
existing fishes, &c.), the rudiment of the gill (choroid gland) alone
remaining to mark its situation. After the disappearance of this cleft
the suctorial mouth may have become relatively shifted backwards. In
the meantime the branchial bars became developed, and as the mouth was
changed into a biting one, the
[Pg 321] bar (the mandibular arch) supporting
the then first cleft became gradually modified and converted into a
supporting apparatus for the mouth, and finally formed the skeleton of
the jaws. In the hyostylic Vertebrata the hyoid arch also became
modified in connection with the formation of the jaws.
The conclusions arrived at may be summed up as follows:
The relations which exist in all jaw-bearing Vertebrates between the
mandibular arch and the oral aperture are secondary, and arose pari
passu with the evolution of the jaws[111].
Fig. 196. The heads of Elasmobranch embryos at two stages viewed
as transparent objects.
A. Pristiurus embryo of the same stage as fig. 28 F. B. Somewhat
older Scyllium embryo.
III. third nerve; V. fifth nerve; VII. seventh nerve; au.n.
auditory nerve; gl. glossopharyngeal nerve; Vg. vagus nerve;
fb. fore-brain; pn. pineal gland; mb. mid-brain; hb.
hind-brain; iv.v. fourth ventricle; cb. cerebellum; ol.
olfactory pit; op. eye; au.V. auditory vesicle; m. mesoblast
at base of brain; ch. notochord; ht. heart; Vc. visceral
clefts; eg. external gills; pp. sections of body cavity in the
head.
The cranial flexure and the form of the head in vertebrate embryos.
All embryologists who have studied the embryos of the various
vertebrate groups have been struck with the remarkable similarity
[Pg 322]
which exists between them, more especially as concerns the form of the
head. This similarity is closest between the members of the Amniota,
but there is also a very marked resemblance between the Amniota and
the Elasmobranchii. The peculiarity in question, which is
characteristically shewn in fig. 196, consists in the cerebral
hemispheres and thalamencephalon being ventrally flexed to such an
extent that the mid-brain forms the termination of the long axis of
the body. At a later period in development the cerebral hemispheres
come to be placed at the front end of the head; but the original nick
or bend of the floor of the brain is never got rid of.
It is obvious that in dealing with the light thrown by embryology on
the ancestral form of the Chordata the significance of this peculiar
character of the head of many vertebrate embryos must be discussed. Is
the constancy of this character to be explained by supposing that at
one period vertebrate ancestors had a head with the same features as
the embryonic head of existing Vertebrata?
This is the most obvious explanation, but it does not at the same time
appear to me satisfactory. In the first place the mouth is so situated
at the time of the maximum cranial flexure that it could hardly have
been functional; so that it is almost impossible to believe that an
animal with a head such as that of these embryos can have existed.
Then again, this type of embryonic head is especially characteristic
of the Amniota, all of which are developed in the egg. It is not
generally so marked in the Ichthyopsida. In Amphibia, Teleostei,
Ganoidæ and Petromyzontidæ, the head never completely acquires the
peculiar characteristic form of the head of the Amniota, and all these
forms are hatched at a relatively much earlier phase of development,
so that they are leading a free existence at a stage when the embryos
of the Amniota are not yet hatched. The only Ichthyopsidan type with a
head like that of the Amniota is the Elasmobranchii, and the
Elasmobranchii are the only Ichthyopsida which undergo the major part
of their development within the egg.
These considerations appear to shew that the peculiar characters of
the embryonic head above alluded to are in some way connected with an
embryonic as opposed to a larval development; and for reasons which
are explained in the section on larval forms, it is probable that a
larval development is a more faithful record of ancestral history than
an embryonic development. The flexure at the base of the brain appears
however to be a typical vertebrate character, but this flexure never
led to a conformation of the head in the adult state similar to that
of the embryos of the Amniota. The form of the head in these embryos
is probably to be explained by supposing that some advantage is gained
by a relatively early development of the brain, which appears to be
its proximate cause; and since these embryos had not to lead a free
existence (for which such a form of the head would have been unsuited)
there was nothing to interfere with the action of natural selection in
bringing about this form of head during fœtal life.
Postanal gut and neurenteric canal. One of the most
[Pg 323] remarkable
structures in the trunk is the postanal gut (fig. 197). Its structure
is fully dealt with in the chapter on the alimentary tract, but
attention may here be called to the light which it appears to throw on
the characters of the ancestor of the Chordata.
In face of the facts which are known with reference to the postanal
section of the alimentary tract, it can hardly be doubted that this
portion of the alimentary tract must have been at one time functional.
This seems to me to be shewn (1) by the constancy and persistence of
this obviously now functionless rudiment, (2) by its greater
development in the lower than in the higher forms, (3) by its relation
to the formation of the notochord and subnotochordal rod.
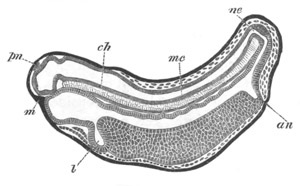
Fig. 197. Longitudinal section through an advanced embryo of Bombinator.
m. mouth; an. anus; l. liver; ne. neurenteric canal; mc.
medullary canal; ch. notochord; pn. pineal gland.
If the above position be admitted, it is not permissible to shirk the
conclusions which seem necessarily to follow, however great the
difficulties may be which are involved in their acceptance. These
conclusions have in part already been dealt with by Dohrn in his
suggestive tract (No. 250). In the first place the alimentary canal
must primitively have been continued to the end of the tail; and if
so, it is hardly credible that the existing anus can have been the
original one. Although, therefore, it is far from easy, on the
physiological principles involved in the Darwinian theory, to
understand the formation of a new anus[112];
it is nevertheless
necessary to believe that the present
[Pg 324] vertebrate anus is a formation
acquired within the group of the Chordata, and not inherited from some
older group. This involves a series of further consequences. The
opening of the urinogenital ducts into the cloaca must also be
secondary, and it is probable that the segmental tubes were
primitively continued along the whole postanal region of the
vertebrate tail, opening into the body cavity which embryology proves
to have been originally present there. They are in fact continued in
many existing forms for some distance behind the present anus. If the
present anus is secondary, there must have been a primitive anus,
which was probably situated behind the postanal vesicle; and therefore
in the region of the neurenteric canal. The neurenteric canal is,
however, the remnant of the blastopore (vide p. 277). It follows,
therefore, that the vertebrate blastopore is probably almost, if not
exactly identical in position with the primitive anus. This
consideration may assist in explaining the remarkable phenomenon of
the existence of the neurenteric canal. The attempt has already been
made to shew that the central canal of the nervous system is really a
groove converted into a tube and lined by the external epidermis. This
tube (as may be concluded from embryological considerations) was
probably at first open posteriorly, and no doubt terminated at the
primitive anus. On the closure of the primitive anal opening, the
terminal portions of the postanal gut and the neural tube, may
conceivably have been so placed that both of them opened into a common
cavity, which previously had communication with the exterior by the
anus. Such an arrangement would necessarily result in the formation of
a neurenteric canal. It seems not impossible that a dilated vesicle,
often present at the end of the postanal gut (vide fig. 28*, p. 58),
may have been the common cavity into which both neural and alimentary
tubes opened[113].
[Pg 325] Till further light is thrown by fresh discoveries
upon the primitive condition of the posterior continuation of the
vertebrate alimentary tract, it is perhaps fruitless to attempt to
work out more in detail the above speculation.
Body cavity and mesoblastic somites. The Chordata, or at least the
most primitive existing members of the group, are characterized by the
fact that the body cavity arises as a pair of outgrowths of the
archenteric cavity. This feature[114]
in the development is a nearly
certain indication that the Chordata are a very primitive stock. The
most remarkable point with reference to the development of the two
outgrowths is, however, the fact that the dorsal part of each
outgrowth becomes separated from the ventral. Its walls become
segmented and form the mesoblastic somites, which eventually, on the
obliteration of their cavity, give rise to the muscle-plates and to
the tissue surrounding the notochord. It is not easy to understand the
full significance of the processes concerned in the formation of the
mesoblastic somites (vide p. 296). The mesoblastic somites have no
doubt a striking resemblance to the mesoblastic somites of the
Chætopods, and most probably the segmentation of the mesoblast in the
two groups is a phenomenon of the same nature; but the difference in
origin between the two types of mesoblastic somites is so striking,
and the development of the muscular system from them is so dissimilar
in the two groups, as to render a direct descent of the Chordata from
the Chætopoda very improbable. The ventral parts of the original
outgrowth give rise to the permanent body cavity, which appears
originally to have been divided into two parts by a dorsal and a
ventral mesentery.
The notochord. The most characteristic organ of the Chordata is
without doubt the notochord. The ontogenetic development of this organ
probably indicates that it arose as a differentiation of the dorsal
wall of the archenteron; at the same time it is not perhaps safe to
lay too much stress upon its mode of development. Embryological and
anatomical evidence demonstrate, however, in the clearest manner that
the early Chordata were provided with this organ as their sole axial
skeleton;
[Pg 326] and no invertebrate group can fairly be regarded as
genetically related to the Chordata till it can be shewn to possess
some organ either derived from a notochord, or capable of having
become developed into a notochord. No such organ has as yet been
recognized in any invertebrate group[115].
Gill-clefts. The gill-clefts, which are essentially pouches of the
throat opening externally, constitute extremely characteristic organs
of the Chordata, and have always been taken into consideration in any
comparison between the Chordata and the Invertebrata.
Amongst the Invertebrata organs of undoubtedly the same nature are, so
far as I know, only found in Balanoglossus, where they were discovered
by Kowalevsky. The resemblance in this case is very striking; but
although it is quite possible that the gill-clefts in Balanoglossus
are genetically connected with those of the Chordata, yet the
organization of Balanoglossus is as a whole so different from that of
the Chordata that no comparison can be instituted between the two
groups in the present state of our knowledge.
Other organs of the Invertebrata have some resemblance to the
gill-clefts. The lateral pits of the Nemertines, which appear to grow
out as a pair of œsophageal diverticula, which are eventually placed
in communication with the exterior by a pair of ciliated canals
(vide Vol. II. pp. 200 and 202), are such organs.
Semper (No. 256) has made the interesting discovery that in the
budding of Nais and Chætogaster two lateral masses of cells, in each
of which a lumen may be formed, unite with the oral invagination and
primitive alimentary canal to form the permanent cephalic gut. The
lateral masses of cells are regarded by him as branchial passages
homologous in some way with those in the Chordata. The somewhat scanty
observations on this subject which he has recorded do not appear to me
to lend much support to this interpretation.
It is probable that the part of the alimentary tract in which
gill-clefts are present was originally a simple unperforated tube
provided with highly vascular walls; and that respiration was carried
on in it by the alternate introduction and expulsion of sea water. A
more or less similar mode of respiration has been recently shewn by
Eisig[116]
to take place in the fore part
[Pg 327] of the alimentary tract of
many Chætopods. This part of the alimentary tract was probably
provided with paired cæcal pouches with their blind ends in contiguity
with the skin.
Perforations placing these pouches in communication with the exterior
must be supposed to have been formed; and the existence of openings
into the alimentary tract at the end of the tentacles of many Actiniæ
and of the hepatic diverticula of some nudibranchiate Molluscs (Eolis,
&c.[117])
shews that such perforations may easily be made. On the
formation of such perforations the water taken in at the mouth would
pass out by them; and the respiration would be localized in the walls
of the pouches leading to them, and thus the typical mode of
respiration of the Chordata would be established.
Phylogeny of the Chordata. It may be convenient to shew in a definite
way the bearing of the above speculations on the phylogeny of the
Chordata. For this purpose, I have drawn up the subjoined table, which
exhibits what I believe to be the relationships of the existing groups
of the Chordata. Such a table cannot of course be constructed from
embryological data alone, and it does not fall within the scope of
this work to defend its parts in detail.
In the above table the names printed in large capitals are
hypothetical groups. The other groups are all in existence at the
present day, but those printed in Italics are probably degenerate.
[TN1]
The ancestral forms of the Chordata, which may be called the
Protochordata, must be supposed to have had (1) a
[Pg 328] notochord as their
sole axial skeleton, (2) a ventral mouth, surrounded by suctorial
structures, and (3) very numerous gill-slits. Two degenerate offshoots
of this stock still persist in Amphioxus (Cephalochorda), and the
Ascidians (Urochorda).
The direct descendants of the ancestral Chordata, were probably a
group which may be called the Protovertebrata, of which there is no
persisting representative. In this group, imperfect neural arches were
probably present; and a ventral suctorial mouth without a mandible and
maxillæ was still persistent. The branchial clefts had, however,
become reduced in number, and were provided with gill-folds; and a
secondary head (vide p. 313), with brain and organs of sense like
those of the higher Vertebrata, had become formed.
The Cyclostomata are probably a degenerate offshoot of this group.
With the development of the branchial bars, and the conversion of the
mandibular bar into the skeleton of the jaws, we come to the
Proto-gnathostomata. The nearest living representatives of this group
are the Elasmobranchii, which still retain in the adult state the
ventrally placed mouth. Owing to the development of food-yolk in the
Elasmobranch ovum the early stages of development are to some extent
abbreviated, and almost all trace of a stage with a suctorial mouth
has become lost.
We next come to an hypothetical group which we may call the
Proto-ganoidei. Bridge, in his memoir on Polyodon[118],
which contains
some very interesting speculations on the affinities of the Ganoids,
has called this group the Pneumatocœla, from the fact that we find
for the first time a full development of the air-bladder, though it is
possible that a rudiment of this organ, in the form of a pouch opening
on the dorsal side of the stomachic extremity of the œsophagus, was
present in the earlier type.
Existing Ganoids are descendants of the Proto-ganoidei. Some of them
at all events retain in larval life the suctorial mouth of the
Protovertebrata; and the mode of formation of their germinal layers,
resembling as it does that in the Lamprey
[Pg 329] and the Amphibia, probably
indicates that they are not descended from forms with a large
food-yolk like that of Elasmobranchii, and that the latter group is
therefore a lateral offshoot from the main line of descent.
Of the two groups into which the Ganoidei may be divided it is clear
that certain members of the one (Telcostoidei), viz. Lepidosteus and
Amia, shew approximations to the Teleostei, which no doubt originated
from the Ganoids; while the other (Selachoidei or Sturiones) is more
nearly related to the Dipnoi. Polypterus has also marked affinities in
this direction, e.g. the external gills of the larva (vide p.
118).
The Teleostei, which have in common a meroblastic segmentation, had
probably a Ganoid ancestor, the ova of which were provided with a
large amount of food-yolk. In most existing Teleostei, the ovum has
become again reduced in size, but the meroblastic segmentation has
been preserved. It is quite possible that Amia may also be a
descendant of the Ganoid ancestor of the Teleostei; but Lepidosteus,
as shewn by its complete segmentation, is clearly not so.
The Dipnoi as well as all the higher Vertebrata are descendants of the
Proto-ganoidei.
The character of the limbs of higher Vertebrata indicates that there
was an ancestral group, which may be called the Proto-pentadactyloidei,
in which the pentadactyle limb became established; and that to this
group the common ancestor of the Amphibia and Amniota belonged.
It is possible that the Plesiosauri and Ichthyosauri of Mesozoic times
may have been more nearly related to this group than either to the
Amniota or the Amphibia. The Proto-pentadactyloidei were probably much
more closely related to the Amphibia than to the Amniota. They
certainly must have been capable of living in water as well as on
land, and had of course persistent branchial clefts. It is also fairly
certain that they were not provided with large-yolked ova, otherwise
the mode of formation of the layers in Amphibia could not be easily
explained.
The Mammalia and Sauropsida are probably independent offshoots from a
common stem which may be called the Protoamniota.
[Pg 330]
Bibliography.
(249) F. M. Balfour. A Monograph on the development of Elasmobranch
Fishes. London, 1878.
(250) A. Dohrn. Der Ursprung d. Wirbelthiere und d. Princip. d.
Functionswechsel. Leipzig, 1875.
(251) E. Haeckel. Schöpfungsgeschichte. Leipzig. Vide also
Translation. The History of Creation. King and Co., London, 1876.
(252) E. Haeckel. Anthropogenie. Leipzig. Vide also Translation.
Anthropogeny. Kegan Paul and Co., London, 1878.
(253) A. Kowalevsky. “Entwicklungsgeschichte d. Amphioxus
lanceolatus.” Mém. Acad. d. Scien. St Pétersbourg, Ser. VII. Tom.
XI. 1867, and Archiv f. mikr. Anat., Vol. XIII. 1877.
(254) A. Kowalevsky. “Weitere Stud. üb. d. Entwick. d. einfachen
Ascidien.” Archiv f. mikr. Anat., Vol. VII. 1871.
(255) C. Semper. “Die Stammesverwandschaft d. Wirbelthiere u.
Wirbellosen.” Arbeit. a. d. zool.-zoot. Instit. Würzburg, Vol. II.
1875.
(256) C. Semper. “Die Verwandschaftbeziehungen d. gegliederten
Thiere.” Arbeit. a. d. zool.-zoot. Instit. Würzburg, Vol. III.
1876-1877.
[Pg 331]
CHAPTER XIII.
GENERAL CONCLUSIONS.
I. THE MODE OF ORIGIN AND HOMOLOGIES OF THE GERMINAL LAYERS.
It has already been shewn in the earlier chapters of the work that
during the first phases of development the history of all the Metazoa
is the same. They all originate from the coalescence of two cells, the
ovum and spermatozoon. The coalesced product of these cells—the
fertilized ovum—then undergoes a process known as the segmentation,
in the course of which it becomes divided in typical cases into a
number of uniform cells. An attempt was made from the point of view of
evolution to explain these processes. The ovum and spermatozoon were
regarded as representing phylogenetically two physiologically
differentiated forms of a Protozoon; their coalescence was equivalent
to conjugation: the subsequent segmentation of the fertilized ovum was
the multiplication by division of the organism resulting from the
conjugation; the resulting organisms, remaining, however, united to
form a fresh organism in a higher state of aggregation.
In the systematic section of this work the embryological history of
the Metazoa has been treated. The present chapter contains a review of
the cardinal features of the various histories, together with an
attempt to determine how far there are any points common to the whole
of these histories; and the phylogenetic interpretation to be given to
such points.
Some years ago it appeared probable that a definite answer
[Pg 332] would be
given to the questions which must necessarily be raised in the present
chapter; but the results of the extended investigations made during
the last few years have shewn that these expectations were premature,
and in spite of the numerous recent valuable contributions to this
branch of Embryology, amongst which special attention may be called to
those of Kowalevsky (No. 277), Lankester (Nos. 278 and 279), and
Haeckel (No. 266), there are few embryologists who would venture to
assert that any answers which can be given are more than tentative
gropings towards the truth.
In the following pages I aim more at summarising the facts, and
critically examining the different theories which can be held, than at
dogmatically supporting any definite views of my own.
In all the Metazoa, the development of which has been investigated,
the first process of differentiation, which follows upon the
segmentation, consists in the cells of the organism becoming divided
into two groups or layers, known respectively as epiblast and
hypoblast.
These two layers were first discovered in the young embryos of
vertebrated animals by Pander and Von Baer, and have been since known
as the germinal layers, though their cellular nature was not at first
recognised. They were shewn, together with a third layer, or
mesoblast, which subsequently appears between them, to bear throughout
the Vertebrata constant relations to the organs which became developed
from them. A very great step was subsequently made by Remak (No. 287),
who successfully worked out the problem of vertebrate embryology on
the cellular theory.
Rathke in his memoir on the development of Astacus (No. 286) attempted
at a very early period to extend the doctrine of the derivation of the
organs from the germinal layers to the Invertebrata. In 1859 Huxley
made an important step towards the explanation of the nature of these
layers by comparing them with the ectoderm and endoderm of the
Hydrozoa; while the brilliant researches of Kowalevsky on the
development of a great variety of invertebrate forms formed the
starting point of the current views on this subject.
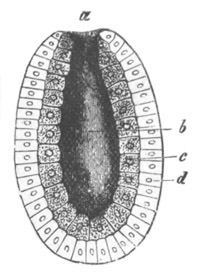
Fig. 198. Diagram of a Gastrula. (From Gegenbaur.)
a. mouth; b. archenteron; c. hypoblast; d. epiblast.
The differentiation of the epiblast and hypoblast may commence during
the later phases of the segmentation, but is generally not completed
till after its termination. Not only do the cells of the blastoderm
become differentiated
[Pg 333] into two layers, but these two layers, in the
case of a very large number of ova with but little food-yolk,
constitute a double-walled sack—the gastrula (fig. 198)—the
characters of which are too well known to require further description.
Following the lines of phylogenetic speculation above indicated, it
may be concluded that the two-layered condition of the organism
represents in a general way the passage from the protozoon to the
metazoon condition. It is probable that we may safely go further, and
assert that the gastrula reproduces, with more or less fidelity, a
stage in the evolution of the Metazoa, permanent in the simpler
Hydrozoa, during which the organism was provided with (1) a fully
developed digestive cavity (fig. 198 b) lined by the hypoblast with
digestive and assimilative functions, (2) an oral opening (a), and
(3) a superficial epiblast (d). These generalisations, which are now
widely accepted, are no doubt very valuable, but they leave unanswered
the following important questions:
(1) By what steps did the compound Protozoon become differentiated
into a Metazoon?
(2) Are there any grounds for thinking that there is more than one
line along which the Metazoa have become independently evolved
from the Protozoa?
(3) To what extent is there a complete homology between the two
primary germinal layers throughout the Metazoa?
Ontogenetically there is a great variety of processes by which the
passage from the segmented ovum to the two-layered or diploblastic
condition is arrived at.
These processes may be grouped under the following heads:
1. Invagination. Under this term a considerable number of closely
connected processes are included. When the segmentation results in the
formation of a blastosphere, one half of the blastosphere may be
pushed in towards the opposite half, and a gastrula be thus produced
(fig. 199, A and B). This process is known as embolic invagination.
Another process, known as epibolic invagination, consists in epiblast
cells growing round and enclosing
[Pg 334] the hypoblast (fig. 200). This
process replaces the former process when the hypoblast cells are so
bulky from being distended by food-yolk that their invagination is
mechanically impossible.
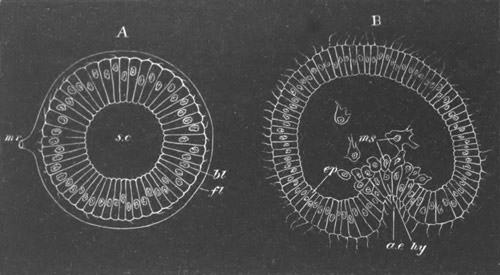
Fig. 199. Two stages in the development of Holothuria tubulosa,
viewed in optical section. (After Selenka.)
A. Stage at the close of segmentation. B. Gastrula stage.
mr. micropyle; fl. chorion; s.c. segmentation cavity; bl.
blastoderm; ep. epiblast; hy. hypoblast; ms. amœboid cells
derived from hypoblast; a.e. archenteron.
There are various peculiar modifications of invagination which cannot
be dealt with in detail.
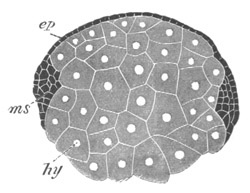
Fig. 200. Transverse section through the ovum of Euaxes during an
early stage of development, to shew the nature of epibolic
invagination. (After Kowalevsky.)
ep. epiblast; ms. mesoblastic band; hy. hypoblast.
Invagination in one form or other occurs in some or all the members of
the following groups:
The Dicyemidæ, Calcispongiæ (after the amphiblastula stage) and
Silicispongiæ, Cœlenterata, Turbellaria, Nemertea, Rotifera,
Mollusca, Polyzoa, Brachiopoda, Chætopoda, Discophora, Gephyrea,
Chætognatha, Nematelminthes, Crustacea, Echinodermata, and Chordata.
The gastrula of the Crustacea is peculiar, as is also that of many of
the Chordata (Reptilia, Aves, Mammalia), but there is every reason to
suppose
[Pg 335] that the gastrulæ of these groups are simply modifications of
the normal type.
2. Delamination. Three types of delamination may be distinguished:
a. Delamination where the cells of a solid morula become divided
into a superficial epiblast, and a central solid mass in which the
digestive cavity is subsequently hollowed out (fig. 201).
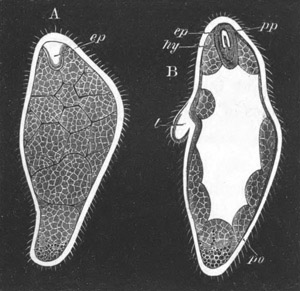
Fig. 201. Two stages in the development of Stephanomia pictum,
to illustrate the formation of the layers by delamination. (After
Metschnikoff.)
A. Stage after the delamination; ep. epiblastic invagination to
form pneumatocyst.
B. Later stage after the formation of the gastric cavity in the
solid hypoblast. po. polypite; t. tentacle; pp. pneumatocyst;
ep. epiblast of pneumatocyst; hy. hypoblast surrounding
pneumatocyst.
b. Delamination where the segmented ovum has the form of a
blastosphere, the cells of which give rise by budding to scattered
cells in the interior of the vesicle, which, though they may at
first form a solid mass, finally arrange themselves in the form of a
definite layer around a central digestive cavity (fig. 202).
c. Delamination where the segmented ovum has the form of a
blastosphere in the cells of which the protoplasm is differentiated
into an inner and an outer part. By a subsequent
[Pg 336] process the inner
parts of the cells become separated from the outer, and the walls of
the blastosphere are so divided into two distinct layers (fig. 205).
Although the third of these processes is usually regarded as the type
of delamination, it does not, so far as I know, occur in nature, but
is most nearly approached in Geryonia (fig. 203).
The first type of delamination is found in the Ceratospongiæ, some
Silicispongiæ (?), and in many Hydrozoa and Actinozoa, and in Nemertea
and Nematelminthes (Gordioidea?). The second type occurs in many
Porifera [Calcispongiæ (Ascetta), Myxospongiæ], and in some
Cœlenterata, and Brachiopoda (Thecidium).
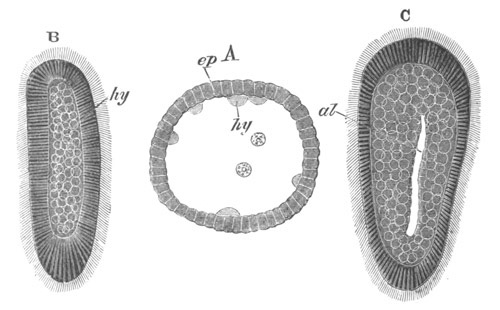
Fig. 202. Three larval stages of Eucope polystyla. (After
Kowalevsky.)
A. Blastosphere stage with hypoblast spheres becoming budded off
into central cavity. B. Planula stage with solid hypoblast. C.
Planula stage with a gastric cavity. ep. epiblast; hy.
hypoblast; al. gastric cavity.
Delamination and invagination are undoubtedly the two most frequent
modes in which the layers are differentiated, but there are in
addition several others. In the first place the whole of the Tracheata
(with the apparent exception of the Scorpion) develop, so far as is
known, on a plan peculiar to them, which approaches delamination. This
consists in the appearance of a superficial layer of cells enclosing a
central yolk mass, which corresponds to the hypoblast (figs. 204 and
214). This mode of development might be classed under delamination,
were it not for the fact that the early development
[Pg 337] of many Crustacea
is almost the same, but is subsequently followed by an invagination
(fig. 208), which apparently corresponds
[Pg 338] to the normal invagination of
other types. There are strong grounds for thinking that the tracheate
type of formation of the epiblast and hypoblast is a secondary
modification of an invaginate type (vide Vol. II. p. 457).
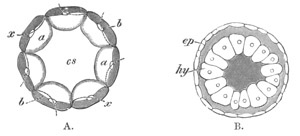
Fig. 203. Diagrammatic figures shewing the delamination of the
embryo of Geryonia. (After Fol.)
A. Stage at the commencement of the delamination; the dotted lines
x shew the course of the next planes of division. B. Stage at the
close of the delamination. cs. segmentation cavity; a.
endoplasm; b. ectoplasm; ep. epiblast; hy. hypoblast.
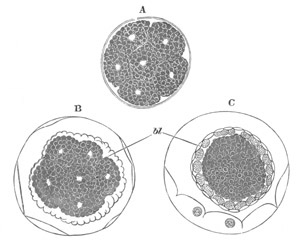
Fig. 204. Segmentation and formation of the blastoderm in Chelifer.
In A the ovum is divided into a number of separate segments. In B a
number of small cells have appeared (bl) which form a blastoderm
enveloping the large yolk-spheres. In C the blastoderm has become
divided into two layers.
The type of some Turbellaria (Stylochopsis ponticus) and that of
Nephelis amongst the Discophora is not capable of being reduced to the
invaginate type.
The development of almost all the parasitic groups, i.e. the
Trematoda, the Cestoda, the Acanthocephala, and the Linguatulida, and
also of the Tardigrada, Pycnogonida, and other minor groups, is too
imperfectly known to be classed with either the delaminate or
invaginate types.
It will, I think, be conceded on all sides that, if any of the
ontogenetic processes by which a gastrula form is reached are
repetitions of the process by which a simple two-layered gastrula was
actually evolved from a compound Protozoon, these processes are most
probably of the nature either of invagination or of delamination.
The much disputed questions which have been raised about the gastrula
and planula theories, originally put forward by Haeckel and Lankester,
resolve themselves then into the simple question, whether any, and if
so which, of the ontogenetic processes by which the gastrula is formed
are repetitions of the phylogenetic origin of the gastrula.
It is very difficult to bring forward arguments of a conclusive kind
in favour of either of these processes. The fact that delaminate and
invaginate gastrulæ are in several instances found coexisting in the
same group renders it certain that there are not two independent phyla
of the Metazoa, derived respectively from an invaginate and a
delaminate gastrula[119].
[Pg 339]
The four most important cases in which the two processes coexist are
the Porifera, the Cœlenterata, the Nemertea, and the Brachiopoda. In
the cases of the Porifera and Cœlenterata, there do not appear to me
to be any means of deciding which of these processes is derived from
the other; but in the Nemertea and the Brachiopoda the case is
different. In all the types of Nemertea in which the development is
relatively not abbreviated there is an invaginate gastrula, while in
the types with a greatly abbreviated development there is a delaminate
gastrula. It would seem to follow from this that a delaminate gastrula
has here been a secondary result of an abbreviation in the
development. In the Brachiopoda, again, the majority of types develop
by a process of invagination, while Thecidium appears to develop by
delamination; here also the delaminate type would appear to be
secondarily derived from the invaginate.
If these considerations are justified, delamination must be in some
instances secondarily derived from invagination; and this fact is so
far an argument in favour of the more primitive nature of
invagination; though it by no means follows that in the invaginate
process the steps by which the Metazoa were derived from the Protozoa
are preserved.
It does not, therefore, seem possible to decide conclusively in favour
of either of these processes by a comparison of the cases where they
occur in the same groups.
The relative frequency of the two processes supplies us with another
possible means for deciding between them; and there is no doubt that
here again the scale inclines towards invagination. It must, however,
be borne in mind that the frequency of the process of invagination
admits of another possible explanation. There is a continual tendency
for the processes of development to be abbreviated and simplified, and
it is quite possible that the frequent occurrence of invagination is
due to the fact of its being, in most cases, the simplest means by
which the two-layered condition can be reached. But this argument can
have but little weight until it can be shewn in each case that
invagination is a simpler process than delamination; and it is
rendered improbable by the cases already mentioned in which
delamination has been secondarily derived from invagination.
If it were the case that the blastopore had in all types the
[Pg 340] same
relation to the adult mouth, there would be strong grounds for
regarding the invaginate gastrula as an ancestral form; but the fact
that this is by no means so is an argument of great weight in favour
of some other explanation of the frequency of invagination.
The force of this consideration can best be displayed by a short
summary of the fate of the blastopore in different forms.
The fate of the blastopore is so variable that it is difficult even to
classify the cases which have been described.
(1) It becomes the permanent mouth in the following forms[120]:
Cœlenterata.—Pelagia, Cereanthus.
Turbellaria.—Leptoplana (?), Thysanozoon.
Nemertea.—Pilidium, larvæ of the type of Desor.
Mollusca.—In numerous examples of most Molluscan groups, except
the Cephalopoda.
Chætopoda.—Most Oligochæta, and probably many Polychæta.
Gephyrea.—Phascolosoma, Phoronis.
Nematelminthes.—Cucullanus.
(2) It closes in the position where the mouth is subsequently
formed.
Cœlenterata.—Ctenophora (?).
Mollusca.—In numerous examples of most Molluscan groups, except
the Cephalopoda.
Crustacea.—Cirripedia (?), some Cladocera (Moina) (?).
(3) It becomes the permanent anus.
Mollusca.—Paludina.
Chætopoda.—Serpula and some other types.
Echinodermata.—Almost universally, except amongst the Crinoidea.
(4) It closes in the position where the anus is subsequently formed.
Echinodermata.—Crinoidea.
(5) It closes in a position which does not correspond or is not
known to correspond[121]
either with the future mouth or
anus.—Porifera—Sycandra. Cœlenterata—Chrysaora*,
Aurelia*. Nemertea*—Some larvæ which develop without a
metamorphosis. Rotifera*. Mollusca—Cephalopoda. Polyzoa*.
Brachiopoda—Argiope, Terebratula, Terebratulina.
Chætopoda—Euaxes. Discophora—Clepsine.
Gephyrea—Bonellia*. Chætognatha. Crustacea—Decapoda.
Chordata.
The forms which have been classed together under the last heading vary
considerably in the character of the blastopore. In some cases the
fact of its not coinciding either with the mouth
[Pg 341] or anus appears to be
due simply to the presence of a large amount of food-yolk. The cases
of the Cephalopoda, of Euaxes, and perhaps of Clepsine and Bonellia,
are to be explained in this way: in the case of all these forms,
except Bonellia, the blastopore has the form of an elongated slit
along the ventral surface. This type of blastopore is characteristic
of the Mollusca generally, of the Polyzoa, of the Nematelminthes, and
very possibly of the Chætopoda and Discophora. In the Chætognatha
(fig. 209 B) the blastopore is situated, so far as can be determined,
behind the future anus. In many Decapoda the blastopore is placed
behind, but not far from, the anus. In the Chordata it is also placed
posteriorly to the anus, and, remarkably enough, remains, in a large
number of forms, for some time in connection with the neural tube by a
neurenteric canal.
The great variations in the character of the gastrula, indicated in
the above summary, go far to shew that if the gastrulæ, as we find
them in most types, have any ancestral characters, these characters
can only be of the most general kind. This may best be shewn by the
consideration of a few striking instances. The blastopore in Mollusca
has an elongated slit-like form, extending along the ventral surface
from the mouth to the anus. In Echinodermata it is a narrow pore,
remaining as the anus. In most Chætopoda it is a pore remaining as the
mouth, but in some as the anus. In Chordata it is a posteriorly-placed
pore, opening into both the archenteron and the neural canal.
It is clearly out of the question to explain all these differences as
having connection with the characters of ancestral forms. Many of them
can only be accounted for as secondary adaptations for the convenience
of development.
The epibolic gastrula of Mammalia (vide pp. 215 and 291) is a still
more striking case of a secondary embryonic process, and is not
directly derived from the gastrula of the lower Chordata. It probably
originated in connection with the loss of food-yolk which took place
on the establishment of a placental nutrition for the fœtus. The
epibolic gastrula of the Scorpion, of Isopods, and of other
Arthropoda, seems also to be a derived gastrula. These instances of
secondary gastrulæ are very probably by no
[Pg 342] means isolated, and should
serve as a warning against laying too much stress upon the frequency
of the occurrence of invagination. The great influence of the
food-yolk upon the early development might be illustrated by numerous
examples, especially amongst the Chordata (vide Chapter XI.).
If the descendants of a form with a large amount of food-yolk in its
ova were to produce ova with but little food-yolk, the type of
formation of the germinal layers which would thereby result would be
by no means the same as that of the ancestors of the forms with much
food-yolk, but would probably be something very different, as in the
case of Mammalia. Yet amongst the countless generations of ancestors
of most existing forms, such oscillations in the amount of the
food-yolk must have occurred in a large number of instances.
The whole of the above considerations point towards the view that the
formation of the hypoblast by invagination, as it occurs in most forms
at the present day, can have in many instances no special phylogenetic
significance, and that the argument from frequency, in favour of
invagination as opposed to delamination, is not of prime importance.
A third possible method of deciding between delamination and
invagination is to be found in the consideration as to which of these
processes occurs in the most primitive forms. If there were any
agreement amongst primitive forms as to the type of their development
this argument might have some weight. On the whole, delamination is,
no doubt, characteristic of many primitive types, but the not
infrequent occurrence of invagination in both the Cœlenterata and the
Porifera—the two groups which would on all hands be admitted to be
amongst the most primitive—deprives this argument of much of the
value it might otherwise have.
To sum up—considering the almost indisputable fact that both the
processes above dealt with have in many instances had a purely
secondary origin, no valid arguments can be produced to shew that
either of them reproduces the mode of passage between the Protozoa and
the ancestral two-layered Metazoa. These conclusions do not, however,
throw any doubt upon the fact that the gastrula, however evolved, was
a primitive form of the Metazoa; since this conclusion is founded upon
the actual
[Pg 343] existence of adult gastrula forms independently of their
occurrence in development.
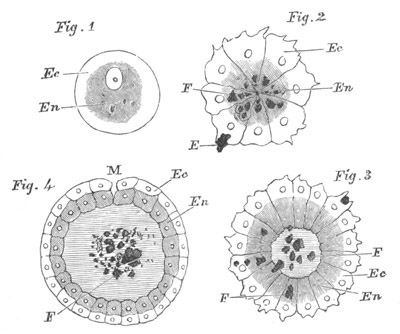
Fig. 205. Diagram shewing the formation of a Gastrula by
delamination. (From Lankester.)
Fig. 1, ovum; fig. 2, stage in segmentation; fig. 3, commencement of
delamination after the appearance of a central cavity; fig. 4,
delamination completed, mouth forming at M. In figs. 1, 2, and 3,
Ec. is ectoplasm, and En. is endoplasm. In fig. 4, Ec. is
epiblast, and En. hypoblast. E. and F. food particles.
Though embryology does not at present furnish us with a definite
answer to the question how the Metazoa became developed from the
Protozoa, it is nevertheless worth while reviewing some of the
processes by which this can be conceived to have occurred.
On purely à priori grounds there is in my opinion more to be said
for invagination than for any other view.
On this view we may suppose that the colony of Protozoa in the course
of conversion into Metazoa had the form of a blastosphere; and that at
one pole of this a depression appeared. The cells lining this
depression we may suppose to have been amœboid, and to have carried
on the work of digestion; while the remaining cells were probably
ciliated. The digestion may be supposed to have been at first carried
on in the interior of the cells, as in the Protozoa; but, as the
depression became deeper (in order to increase the area of nutritive
cells and to retain the food) a digestive secretion probably became
poured out from the cells lining it, and the mode of digestion
generally characteristic of the Metazoa was thereby inaugurated. It
may be noted that an intracellular protozoon type of digestion
persists in the Porifera, and appears also to occur in many
Cœlenterata, Turbellaria,
[Pg 344] &c., though in most of these cases both
kinds of digestion probably go on simultaneously[122].
Another hypothetical mode of passage, which fits in with delamination,
has been put forward by Lankester, and is illustrated by fig. 205. He
supposes that at the blastosphere stage the fluid in the centre of the
colony acquired special digestive properties; the inner ends of the
cells having at this stage somewhat different properties from the
outer, and the food being still incepted by the surface of the cells
(fig. 205, 3). In a later stage of the process the inner portions of
the cells became separated off as the hypoblast; while the food,
though still ingested in the form of solid particles by the
superficial cells, was carried through the protoplasm into the central
digestive cavity. Later (fig. 205, 4), the point where the food
entered became localised, and eventually a mouth became formed at this
point.
The main objection which can be raised against Lankester’s view is
that it presupposes a type of delamination which does not occur in
nature except in Geryonia.
Metschnikoff has propounded a third view with reference to
delamination. He starts as before with a ciliated blastosphere. He
next supposes the cells from the walls of this to become budded off
into the central cavity, as in Eucope (fig. 202), and to lose their
cilia. These cells give rise to an internal parenchyma, which carries
on an intracellular digestion. At a later stage a central digestive
cavity is supposed to be formed. This view of the passage from the
protozoon to the metazoon state, though to my mind improbable in
itself, fits in very well with the ontogeny of the lower Hydrozoa.
Another view has been put forward by myself in the chapter on the
Porifera[123],
to the effect that the amphiblastula larva of
Calcispongiæ may be a transitional form between the Protozoa and the
Metazoa, composed of a hemisphere of nutritive amœboid cells, and a
hemisphere of ciliated cells. The absence of such a larval form in the
Cœlenterata and higher Metazoa is opposed, however, to this larva
being regarded as a transitional form, except for the Porifera.
It is obvious that so long as there is complete uncertainty as to the
value to be attached to the early developmental processes, it is not
possible to decide from these processes whether there is only a single
metazoon phylum or whether there may not be two or more such phyla. At
the same time there appear to be strong
[Pg 345] arguments for regarding the
Porifera as a phylum of the Metazoa derived independently from the
Protozoa. This seems to me to be shewn (1) by the striking larval
peculiarities of the Porifera; (2) by the early development of the
mesoblast in the Porifera, which stands in strong contrast to the
absence of this layer in the embryos of most Cœlenterata; and above
all, (3) by the remarkable characters of the system of digestive
channels. A further argument in the same direction is supplied by the
fact that the germinal layers of the Sponges very probably do not
correspond physiologically to the germinal layers of other types. The
embryological evidence is insufficient to decide whether the
amphiblastula larva is, as suggested above, to be regarded as the
larval ancestor of the Porifera.
Homologies of the germinal layers. The question as to how far there is
a complete homology between the two primary germinal layers throughout
the Metazoa was the third of the questions proposed to be discussed
here.
Since there are some Metazoa with only two germinal layers, and other
Metazoa with three, and since, as is shewn in the following section,
the third layer or mesoblast can only be regarded as a derivative of
one or both the primary layers, it is clear that a complete homology
between the two primary germinal layers does not exist.
That there is a general homology appears on the other hand hardly open
to doubt.
The primary layers are usually continuous with each other, near one or
both (when both are present) the openings of the alimentary tract.
As a rule an oral and anal section of the alimentary tract—the
stomodæum and proctodæum—are derived from the epiblast; but the
limits of both these sections are so variable, sometimes even in
closely allied forms, that it is difficult to avoid the conclusion
that there is a border-land between the epiblast and hypoblast, which
appears by its development to belong in some forms to the epiblast and
in other forms to the hypoblast. If this is not the case it is
necessary to admit that there are instances in which a very large
portion of the alimentary canal is phylogenetically an epiblastic
structure. In some of the Isopods, for example, the stomodæum and
proctodæum give
[Pg 346] rise to almost the whole of the alimentary canal with
its appendages, except the liver.
The origin of the Mesoblast. A diploblastic condition of the organism
preceded, as we have seen, the triploblastic. The epiblast during the
diploblastic condition was, as appears from such forms as Hydra,
especially the sensory and protective layer, while the hypoblast was
the secretory and assimilating layer; both layers giving rise to
muscular elements. It must not, however, be supposed that in the early
diploblastic ancestors there was a complete differentiation of
function, but there is reason to think that both the primary layers
retained an indefinite capacity for developing into any form of
tissue[124].
The fact of the triploblastic condition being later than
the diploblastic proves in a conclusive way that the mesoblast is a
derivative of one or both the primary layers. In the Cœlenterata we
can study the actual origin from the two primary layers of various
forms of tissue which in the higher types are derived from the
mesoblast[125].
This fact, as well as general à priori
considerations, conclusively prove that the mesoblast did not at first
originate as a mass of independent cells between the two primary
layers, but that in the first instance it gradually arose as
differentiations of the two layers, and that its condition in the
embryo as an independent layer of undifferentiated cells is a
secondary condition, brought about by the general tendency
[Pg 347] towards a
simplification of development, and a retardation of histological
differentiation[126].
The Hertwigs have recently attempted (No. 271) to distinguish two
types of differentiation of the mesoblast, viz. (1) a direct
differentiation from the primitive epithelial cells; (2) a
differentiation from primitively indifferent cells budded off into the
gelatinous matter between the two primary layers.
It is quite possible that this distinction may be well founded, but no
conclusive evidence of the occurrence of the second process has yet
been adduced. The Ctenophora are the type upon which special stress is
laid, but the early passage of amœboid cells into the gelatinous
tissue, which subsequently become muscular, is very probably an
embryonic abbreviation; and it is quite possible that these cells may
phylogenetically have originated from epithelial cells provided with
contractile processes passing through the gelatinous tissue.
The conversion of non-embryonic connective-tissue cells into muscle
cells in the higher types has been described, but very much more
evidence is required before it can be accepted as a common occurrence.
In addition to the probably degraded Dicyemidæ and Orthonectidæ, the
Cœlenterata are the only group in which a true mesoblast is not
always present. In other words, the Cœlenterata are the only group in
which there is not found in the embryo an undifferentiated group of
cells from which the majority of the organs situated between the
epidermis and the alimentary epithelium are developed.
The organs invariably derived, in the triploblastic forms, from the
mesoblast, are the vascular and lymphatic systems, the muscular
system, and the greater part of the connective tissue and the
excretory and generative (?) systems. On the other hand, the nervous
systems (with a few possible exceptions) and organs of sense, the
epithelium of most glands, and a few exceptional connective-tissue
organs, as for example the notochord, are developed from the two
primary layers.
The fact of the first-named set of organs being invariably derived
from the mesoblast points to the establishment of the two following
propositions:—(1) That with the differentiation
[Pg 348] of the mesoblast as a
distinct layer by the process already explained, the two primary
layers lost for the most part the capacity they primitively possessed
of giving rise to muscular and connective-tissue differentiations[127],
to the epithelium of the excretory organs, and to generative cells.
(2) That the mesoblast throughout the triploblastic Metazoa, in so far
as these forms have sprung from a common triploblastic ancestor, is an
homologous structure.
The second proposition follows from the first. The mesoblast can only
have ceased to be homologous throughout the triploblastica by
additions from the two primary layers, and the existence of such
additions is negatived by the first proposition.
These two propositions, which hang together, are possibly only
approximately true, since it is quite possible that future
investigations may shew that differentiations of the two primary
layers are not so rare as has been hitherto imagined.
Ranvier[128]
finds that the muscles of the sweat-glands are developed
from the inner part of the layer of epiblast cells, invaginated to
form these glands.
Götte[129]
describes the epiblast cells of the larva of Comatula as
being at a certain stage contractile and compares them with the
epithelio-muscular cells of Hydra. These cells would appear
subsequently to be converted into a simple cuticular structure.
It is moreover quite possible that fresh differentiations from the two
primary layers may have arisen after the triploblastic condition had
been established, and by the process of simplification of development
and precocious segregation, as Lankester calls it, have become
indistinguishable from the normal mesoblast. In spite of these
exceptions it is probable that the major part of the muscular system
of all existing triploblastic forms has been differentiated from the
muscular system of the ancestor or ancestors (if there is more than
one phylum) of the triploblastica.
[Pg 349] In the case of other tissues there
are a few instances which might be regarded as examples of an organ
primitively developed in one of the two primary layers having become
secondarily carried into the mesoblast. The notochord has sometimes
been cited as such an organ, but, as indicated in a previous chapter,
it is probable that its hypoblastic origin can always be demonstrated.
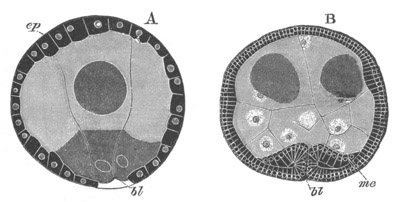
Fig. 206. Epibolic gastrula of Bonellia. (After Spengel.)
A. Stage when the four hypoblast cells are nearly enclosed.
B. Stage after the formation of the mesoblast has commenced by an
infolding of the lips of the blastopore.
ep. epiblast; me. mesoblast; bl. blastopore.
The nervous system, although imbedded in mesoblastic derivates in the
adults of all the higher triploblastica, retains with marvellous
constancy its epiblastic origin (though it is usually separated from
the epiblast prior to its histogenic differentiation); yet in the
Cephalopoda, and some other Mollusca, the evidence is in favour of its
developing in the mesoblast. Should future investigations confirm
these conclusions, a good example will be afforded of an organ
changing the layer from which it usually develops[130].
The
explanation of such a change would be precisely the same as that
already given for the mesoblast as a whole.
The actual mode of origin of various tissues, which in the true
triploblastic forms arise in mesoblast, can be traced in the
[Pg 350]
Cœlenterata[131].
In this group the epiblast and hypoblast both give
rise to muscular and connective-tissue elements; and although the main
part of the nervous system is formed in the epiblast, it seems certain
that in some types nerves may be derived from the hypoblast[132].
These facts are extremely interesting, but it is by no means certain
that any conclusions can be directly drawn from them as to the actual
origin of the mesoblast in the triploblastic forms, till we know from
what diploblastic forms the triploblastica originated. All that they
shew is that any of the constituents of the mesoblast may have
originated from either of the primitive layers.
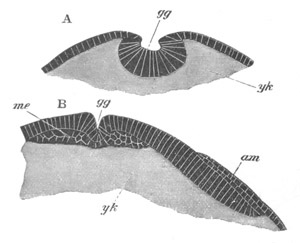
Fig. 207. Two transverse sections through embryos of Hydrophilus
piceus. (After Kowalevsky.)
A. Section through an embryo at the point where the two germinal
folds most approximate.
B. Section through an embryo, in the anterior region where the folds
of the amnion have not united.
gg. germinal groove; me. mesoblast; am. amnion; yk. yolk.
[Pg 351]
For further light as to the origin of the mesoblast, it is necessary
to turn to its actual development.
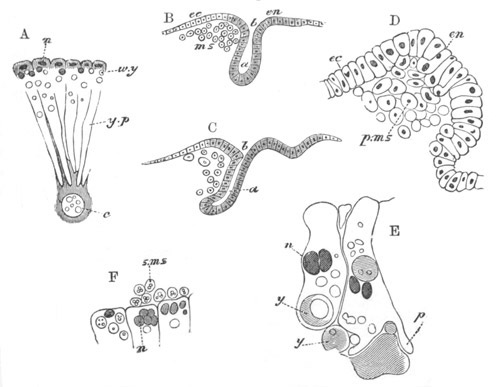
Fig. 208. Figures illustrating the development of Astacus. (From
Parker; after Reichenbach.)
A. Section through part of the ovum during segmentation. n.
nuclei; w.y. white yolk; y.p. yolk pyramids; c. central
yolk mass.
B. and C. Longitudinal sections of the gastrula stage. a.
archenteron; b. blastopore; ms. mesoblast; ec. epiblast;
en. hypoblast, distinguished from epiblast by shading.
D. Highly magnified view of anterior lip of blastopore, to shew the
origin of the primary mesoblast from the wall of the archenteron.
p.ms. primary mesoblast; ec. epiblast; en. hypoblast.
E. Two hypoblast cells to shew the amœeba-like absorption of yolk
spheres. y. yolk; n. nucleus; p. pseudopodial process.
F. Hypoblast cells giving rise endogenously to the secondary
mesoblast (s.ms.); n. nucleus.
The following summary illustrates the more important modes in which
the mesoblast originates.
1. It grows inwards from the lips of the blastopore as a pair of
bands. In these cases it may originate (a) from cells which are
clearly hypoblastic, (b) from cells which are clearly epiblastic,
(c) from cells which cannot be regarded as belonging to either
layer.
Mollusca.—Gasteropoda, Cephalopoda, and Lamellibranchiata. In
Gasteropoda and Lamellibranchiata the mesoblast sometimes originates
[Pg 352]
from a pair of cells at the lips of the blastopore, though very
probably some of the elements subsequently come from the epiblast; and
in Cephalopoda it begins as a ring of cells round the edge of the
blastoderm.
Polyzoa Entoprocta.—It originates from a pair of cells at the lips of
the blastopore.
Chætopoda.—Euaxes. It arises as a ridge of cells at the lips of the
blastopore (fig. 200).
Gephyrea.—Bonellia. It arises (fig. 206) as an infolding of the
epiblastic lips of the blastopore.
Nematelminthes.—Cucullanus. It grows backwards from the hypoblast
cells at the persistent oral opening of the blastopore.
Tracheata.—Insecta. It grows inwards from the lips of the germinal
groove (fig. 207), which probably represent the remains of a
blastopore. Part of the mesoblast is probably also derived from the
yolk-cells. A similar though more modified development of the
mesoblast occurs in the Araneina (fig. 214).
Crustacea.—Decapoda. It partly grows in from the hypoblastic lips of
the blastopore, and is partly derived from the yolk-cells (fig. 208).
2. The mesoblast is developed from the walls of hollow outgrowths of
the archenteron, the cavities of which become the body cavity.
[Pg 353]
Brachiopoda.—The walls of a pair of outgrowths form the whole of the
mesoblast.
Chætognatha.—The mesoblast arises in the same manner as in the
Brachiopoda (fig. 209).
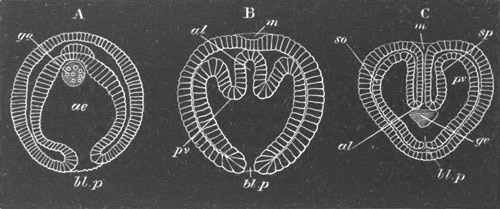
Fig. 209. Three stages in the development of Sagitta. (A. and C.
after Bütschli, and B. after Kowalevsky.)
The three embryos are represented in the same positions.
A. Represents the gastrula stage.
B. Represents a succeeding stage, in which the primitive archenteron
is commencing to be divided into three.
C. Represents a later stage, in which the mouth involution (m) has
become continuous with the alimentary tract, and the blastopore has
become closed.
m. mouth; al. alimentary canal; ae. archenteron; bl.p.
blastopore; pv. perivisceral cavity; sp. splanchnic mesoblast;
so. somatic mesoblast; ge. generative organs.
Echinodermata.—The lining of the peritoneal cavity is developed from
the walls of outgrowths of the archenteron, but the greater part of
the mesoblast is derived from the amœboid cells budded off from the
walls of the archenteron (fig. 210).
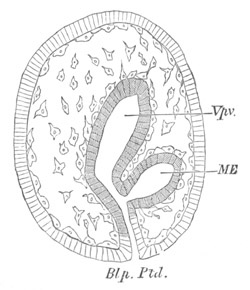
Fig. 210. Longitudinal section through an embryo of Cucumaria
doliolum at the end of the fourth day.
Vpv. vaso-peritoneal vesicle; ME. mesenteron; Blp., Ptd.
blastopore, proctodæum.
Enteropneusta (Balanoglossus).—The body cavity is derived from two
pairs of alimentary diverticula, the walls of which give rise to the
greater part of the mesoblast.
Chordata.—Paired archenteric outgrowths give rise to the whole
mesoblast in Amphioxus (fig. 211), and the mode of formation of the
mesoblast in other Chordata is probably secondarily derived from this.
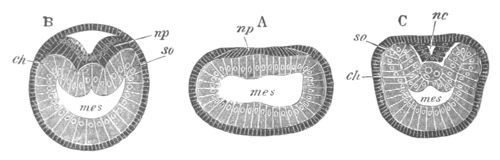
Fig. 211. Sections of an Amphioxus embryo at three stages. (After
Kowalevsky.)
A. Section at gastrula stage.
B. Section of a somewhat older embryo.
C. Section through the anterior part of still older embryo.
np. neural plate; nc. neural canal; mes. archenteron in A, and
mesenteron in B and C; ch. notochord; so. mesoblastic somite.
3. The cells which will form the mesoblast become marked out very
early, and cannot be regarded as definitely springing from either of
the primary layers.
Turbellaria.—Leptoplana (fig. 212), Planaria polychroa (?).
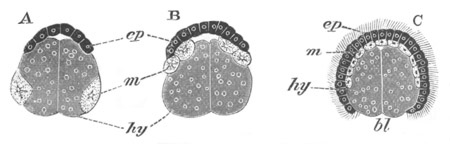
Fig. 212. Sections through the ovum of Leptoplana tremellaris in
three stages of development. (After Hallez.)
ep. epiblast; m. mesoblast; hy. yolk-cells (hypoblast); bl.
blastopore.
Chætopoda.—Lumbricus, &c.
Discophora.
It is very possible that the cases quoted under this head ought more
properly to belong to group 1.
4. The mesoblast cells are split off from the epiblast.
Nemertea.—Larva of Desor. The mesoblast is stated to be split off
from the four invaginated discs.
[Pg 354]
5. The mesoblast is split off from the hypoblast.
Nemertea.—Some of the types without a metamorphosis.
Mollusca.—Scaphopoda. It is derived from the lateral and ventral
cells of the hypoblast.
Gephyrea.—Phascolosoma.
Vertebrata.—In most of the Ichthyopsida the mesoblast is derived from
the hypoblast (fig. 213). In some types (i.e. most of the Amniota)
the mesoblast might be described as originating at the lips of the
blastopore (primitive streak).
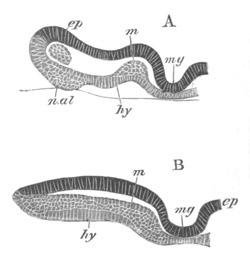
Fig. 213. Two sections of a young Elasmobranch embryo, to shew the
mesoblast split off as two lateral masses from the hypoblast.
mg. medullary groove; ep. epiblast; m. mesoblast; hy.
hypoblast; n.al. cells formed around the nuclei of the yolk which
have entered the hypoblast.
6. The mesoblast is derived from both germinal layers.
Tracheata.—Araneina (fig. 214). It is derived partly from cells split
off from the epiblast and partly from the yolk-cells; but it is
probable that the statement that the mesoblast is derived from both
the germinal layers is only formally accurate; and that the derivation
of part of the mesoblast from the yolk-cells is not to be interpreted
as a derivation from the hypoblast.
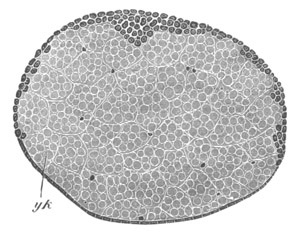
Fig. 214. Section through an embryo of Agelena labyrinthica.
The section is represented with the ventral plate upwards. In the
ventral plate is seen a keel-like thickening, which gives rise to
the main mass of the mesoblast.
yk. yolk divided into large polygonal cells, in several of which
are nuclei.
Amniota.—The derivation of the mesoblast of the Amniota from both the
primary germinal layers is without doubt a secondary process.
The conclusions to be drawn from the above summary are by no means
such as might have been anticipated. The analogy of the Cœlenterata
would lead us to expect that the mesoblast would be derived partly
from the epiblast and partly from the hypoblast. Such, however, is not
for the most part the case, though more complete investigations may
shew that there are a greater number of instances in which the
mesoblast has a mixed origin than might be supposed from the above
summary.
[Pg 355]
I have attempted to reduce the types of development of the mesoblast
to six; but owing to the nature of the case it is not always easy to
distinguish the first of these from the last four. Of the six types
the second will on most hands be admitted to be the most remarkable.
The formation of hollow outgrowths of the archenteron, the cavities of
which give rise to the body cavity, can only be explained on the
supposition that the body cavity of the types in which such outgrowths
occur is derived from diverticula cut off from the alimentary tract.
The lining epithelium of the diverticula—the peritoneal
epithelium—is clearly part of the primitive hypoblast, and this part
of the mesoblast is clearly hypoblastic in origin.
In the case of the Chætognatha (Sagitta), Brachiopoda, and Amphioxus,
the whole of the mesoblast originates from the walls of the
diverticula; while in the Echinodermata the walls of the diverticula
only give rise to the vaso-peritoneal epithelium, the remainder of the
mesoblast being derived from amœboid cells which spring from the
walls of the archenteron before the origin of the vaso-peritoneal
outgrowths (figs. 199 and 210).
Reserving for the moment the question as to what conclusions can be
deduced from the above facts as to the origin of the mesoblast, it is
important to determine how far the facts of embryology warrant us in
supposing that in the whole of the triploblastic forms the body cavity
originated from the alimentary diverticula. There can be but little
doubt that the mode of origin of the mesoblast in many Vertebrata, as
two solid plates split off from the hypoblast, in which a cavity is
secondarily developed, is an abbreviation of the process observable in
Amphioxus; but this process approaches in some forms of
[Pg 356] Vertebrata to
the ingrowth of the mesoblast from the lips of the blastopore.
It is, therefore, highly probable that the paired ingrowths of the
mesoblast from the lips of the blastopore may have been in the first
instance derived from a pair of archenteric diverticula. This process
of formation of the mesoblast is, as may be seen by reference to the
summary, the most frequent, including as it does the Chætopoda, the
Mollusca, the Arthropoda, &c.[133]
While there is no difficulty in the view that the body cavity may have
originated from a pair of enteric diverticula in the case of the forms
where a body cavity is present, there is a considerable difficulty in
holding this view, for forms in which there is no body cavity distinct
from the alimentary diverticula.
Of these types the Platyelminthes are the most striking. It is, no
doubt, possible that a body cavity may have existed in the
Platyelminthes, and become lost; and the case of the Discophora, which
in their muscular and connective tissue systems as well as in the
absence of a body cavity resemble the Platyelminthes, may be cited in
favour of this view, in that, being closely related to the Chætopoda,
they are almost certainly descended from ancestors with a true body
cavity. The usual view of the primitive character of the
[Pg 357]
Platyelminthes, which has much to support it, is, however, opposed to
the idea that the body cavity has disappeared.
If Kowalevsky[134]
is right in stating that he has found a form
intermediate between the Cœlenterata and the Platyelminthes, there
will be strong grounds for holding that the Platyelminthes are, like
the Cœlenterata, forms the ancestors of which were not provided with
a body cavity.
Perhaps the triploblastica are composed of two groups, viz. (1) a more
ancestral group (the Platyelminthes), in which there is no body cavity
as distinct from the alimentary, and (2) a group descended from these,
in which two of the alimentary diverticula have become separated from
the alimentary tract to form a body cavity (remaining triploblastica).
However this may be, the above considerations are sufficient to shew
how much there is that is still obscure with reference even to the
body cavity.
If embryology gives no certain sound as to the questions just raised
with reference to the body cavity, still less is it to be hoped that
the remaining questions with reference to the origin of the mesoblast
can be satisfactorily answered. It is clear, in the first place, from
an inspection of the summary given above, that the process of
development of the mesoblast is, in all the higher forms, very much
abbreviated and modified. Not only is its differentiation relatively
deferred, but it does not in most cases originate, as it must have
done to start with, as a more or
[Pg 358] less continuous sheet, split off from
parts of one or both the primary layers. It originates in most cases
from the hypoblast, and although the considerations already urged
preclude us from laying very great stress on this mode of origin, yet
the derivation of the mesoblast from the walls of archenteric
outgrowths suggests the view that the whole, or at any rate the
greater part, of the mesoblast primitively arose by a process of
histogenic differentiation from the walls of the archenteron or rather
from diverticula of these walls. This view, which was originally put
forward by myself (No. 260), appears at first sight very improbable,
but if the statement of the Hertwigs (No. 270), that there is a large
development of a hypoblastic muscular system in the Actinozoa, is well
founded, it cannot be rejected as impossible. Lankester (No. 279), on
the other hand, has urged that the mode of origin of the mesoblast in
the Echinodermata is more primitive; and that the amœboid cells which
here give rise to the muscular and connective tissues represent cells
which originally arose from the whole inner surface of the epiblast.
It is, however, to be noted that even in the Echinodermata the
amœboid cells actually arise from the hypoblast, and their mode of
origin may, therefore, be used to support the view that the main part
of the muscular system of higher types is derived from the primitive
hypoblast.
The great changes which have taken place in the development of the
mesoblast would be more intelligible on this view than on the view
that the major part of the mesoblast primitively originated from the
epiblast. The presence of food-yolk is much more frequent in the
hypoblast than in the epiblast; and it is well known that a large
number of the changes in early development are caused by food-yolk.
If, therefore, the mesoblast has been derived from the hypoblast, many
more changes might be expected to have been introduced into its early
development than if it had been derived from the epiblast. At the same
time the hypoblastic origin of the mesoblast would assist in
explaining how it has come about that the development of the nervous
system is almost always much less modified than that of the mesoblast,
and that the nervous system is not, as might, on the grounds of
analogy, have been anticipated, as a rule secondarily developed in the
mesoblast.
[Pg 359]
The Hertwigs have recently suggested in their very interesting memoir
(No. 271) that the Triploblastica are to be divided into two phyla,
(1) the Enterocœla, and (2) the Pseudocœla; the former group
containing the Chætopoda, Gephyrea, Brachiopoda, Nematoda, Arthropoda,
Echinodermata, Enteropneusta and Chordata; and the latter the
Mollusca, Polyzoa, the Rotifera, and Platyelminthes.
The Enterocœla are forms in which the primitive alimentary
diverticula have given origin to the body cavity, while the major part
of the muscular system has originated from the epithelial walls of
these diverticula, part however being in many cases also derived from
the amœboid cells, called by them mesenchyme, by the second process
of mesoblastic differentiation mentioned on p. 347.
In the Pseudocœla the muscular system has become differentiated from
mesenchyme cells; while the body cavity, where it exists, is merely a
split in the mesenchyme.
It is impossible for me to attempt in this place to state fully, or do
justice to, the original and suggestive views contained in this paper.
The general conclusion I cannot however accept. The views of the
Hertwigs depend to a large extent upon the supposition that it is
possible to distinguish histologically muscle cells derived from
epithelial cells, from those derived from mesenchyme cells. That in
many cases, and strikingly so in the Chordata, the muscle cells retain
clear indications of their primitive origin from epithelial cells, I
freely admit; but I do not believe either that its histological
character can ever be conclusive as to the non-epithelial origin of a
muscle cell, or that its derivation in the embryo from an indifferent
amœboid cell is any proof that it did not, to start with, originate
from an epithelial cell.
I hold, as is clear from the preceding statements, that such immense
secondary modifications have taken place in the development of the
mesoblast, that no such definite conclusions can be deduced from its
mode of development as the Hertwigs suppose.
In support of the view that the early character of embryonic cells is
no safe index as to their phylogenetic origin, I would point to the
few following facts.
(1) In the Porifera and many of the Cœlenterata (Eucope polystyla,
Geryonia, &c.) the hypoblast (endoderm) originates from cells, which
according to the Hertwigs’ views ought to be classed as mesenchyme.
(2) In numerous instances muscles which have, phylogenetically, an
undoubted epithelial origin, are ontogenetically derived from cells
which ought to be classed as mesenchyme. The muscles of the head in
all the higher Vertebrata, in which the head cavities have
disappeared, are examples of this kind; the muscles of many of the
Tracheata, notably the Araneina, must also be placed in the same
category.
(3) The Mollusca are considered by the Hertwigs to be typical
Pseudocœla. A critical examination of the early development of the
mesoblast in these forms demonstrates however that with reference to
the mesoblast they
[Pg 360] must be classed in the same group as the Chætopoda.
The mesoblast (Vol. II. p. 227) clearly originates as two bands of
cells which grow inwards from the blastopore, and in some forms
(Paludina, Vol. II. fig. 107) become divided into a splanchnic and
somatic layer, with a body cavity between them. All these processes
are such as are, in other instances, admitted to indicate
Enterocœlous affinities.
The subsequent conversion of the mesoblast elements into amœboid
cells, out of which branched muscles are formed, is in my opinion
simply due to the envelopment of the soft Molluscan body within a hard
shell.
In addition to these instances I may point out that the distinction
between the Pseudocœla and Enterocœla utterly breaks down in the
case of the Discophora, and the Hertwigs have made no serious attempt
to discuss the characters of this group in the light of their theory,
and that the derivation of the Echinoderm muscles from mesenchyme
cells is a difficulty which is very slightly treated.
II. Larval forms: their nature, origin and affinities.
Preliminary considerations. In a general way two types of development
may be distinguished, viz. a fœtal type and a larval type. In the
fœtal type animals undergo the whole or nearly the whole of their
development within the egg or within the body of the parent, and are
hatched in a condition closely resembling the adult; and in the larval
type they are born at an earlier stage of development, in a condition
differing to a greater or less extent from the adult, and reach the
adult state either by a series of small steps, or by a more or less
considerable metamorphosis.
The satisfactory application of embryological data to morphology
depends upon a knowledge of the extent to which the record of
ancestral history has been preserved in development. Unless secondary
changes intervened this record would be complete; it becomes therefore
of the first importance to the embryologist to study the nature and
extent of the secondary changes likely to occur in the fœtal or the
larval state.
The principles which govern the perpetuation of variations which occur
in either the larval or the fœtal state are the same as those for the
adult condition. Variations favourable to the survival of the species
are equally likely to be perpetuated, at whatever period of life they
occur, prior to the loss of the reproductive powers. The possible
nature and extent of the
[Pg 361] secondary changes which may have occurred in
the developmental history of forms, which have either a long larval
existence, or which are born in a nearly complete condition, is
primarily determined by the nature of the favourable variations which
can occur in each case.
Where the development is a fœtal one, the favourable variations which
can most easily occur are—(1) abbreviations, (2) an increase in the
amount of food-yolk stored up for the use of the developing embryo.
Abbreviations take place because direct development is always simpler,
and therefore more advantageous; and, owing to the fact of the fœtus
not being required to lead an independent existence till birth, and of
its being in the meantime nourished by food-yolk, or directly by the
parent, there are no physiological causes to prevent the characters of
any stage of the development, which are of functional importance
during a free but not during a fœtal existence, from disappearing
from the developmental history. All organs of locomotion and nutrition
not required by the adult will, for this reason, obviously have a
tendency to disappear or to be reduced in fœtal developments; and a
little consideration will shew that the ancestral stages in the
development of the nervous and muscular systems, organs of sense, and
digestive system will be liable to drop out or be modified, when a
simplification can thereby be effected. The circulatory and excretory
systems will not be modified to the same extent, because both of them
are usually functional during fœtal life.
The mechanical effects of food-yolk are very considerable, and
numerous instances of its influence will be found in the earlier
chapters of this work[135].
It mainly affects the early stages of
development, i.e. the form of the gastrula, &c.
The favourable variations which may occur in the free larva are much
less limited than those which can occur in the fœtus. Secondary
characters are therefore very numerous in larvæ, and there may even be
larvæ with secondary characters only, as, for instance, the larvæ of
Insects.
In spite of the liability of larvæ to acquire secondary characters,
there is a powerful counterbalancing influence tending
[Pg 362] towards the
preservation of ancestral characters, in that larvæ are necessarily
compelled at all stages of their growth to retain in a functional
state such systems of organs, at any rate, as are essential for a
free and independent existence. It thus comes about that, in spite of
the many causes tending to produce secondary changes in larvæ, there
is always a better chance of larvæ repeating, in an unabbreviated
form, their ancestral history, than is the case with embryos, which
undergo their development within the egg.
It may be further noted as a fact which favours the relative retention
by larvæ of ancestral characters, that a secondary larval stage is
less likely to be repeated in development than an ancestral stage,
because there is always a strong tendency for the former, which is a
secondarily intercalated link in the chain of development, to drop out
by the occurrence of a reversion to the original type of
development.
The relative chances of the ancestral history being preserved in the
fœtus or the larva may be summed up in the following way:—There is a
greater chance of the ancestral history being lost in forms which
develop in the egg; and of its being masked in those which are
hatched as larvæ.
The evidence from existing forms undoubtedly confirms the a priori
considerations just urged[136].
This is well shewn by a study of the
development of Echinodermata, Nemertea, Mollusca, Crustacea, and
Tunicata. The free larvæ of the four first groups are more similar
amongst themselves than the embryos which develop directly, and since
this similarity cannot be supposed to be due to the larvæ having been
modified by living under precisely similar conditions, it must be due
to their retaining common ancestral characters. In the case of the
Tunicata the free larvæ retain much more completely than the embryos
certain characters such as the notochord, the cerebrospinal canal,
etc., which are known to be ancestral.
[Pg 363]
Types of Larvæ.—Although there is no reason to suppose that all
larval forms are ancestral, yet it seems reasonable to anticipate that
a certain number of the known types of larvæ would retain the
characters of the ancestors of the more important phyla of the animal
kingdom.
Before examining in detail the claims of various larvæ to such a
character, it is necessary to consider somewhat more at length the
kind of variations which are most likely to occur in larval forms.
It is probable a priori that there are two kinds of larvæ, which may
be distinguished as primary and secondary larvæ. Primary larvæ are
more or less modified ancestral forms, which have continued
uninterruptedly to develop as free larvæ from the time when they
constituted the adult form of the species. Secondary larvæ are those
which have become introduced into the ontogeny of species, the young
of which were originally hatched with all the characters of the adult;
such secondary larvæ may have originated from a diminution of
food-yolk in the egg and a consequently earlier commencement of a free
existence, or from a simple adaptive modification in the just hatched
young. Secondary larval forms may resemble the primary larval forms in
cases where the ancestral characters were retained by the embryo in
its development within the egg; but in other instances their
characters are probably entirely adaptive.
Causes tending to produce secondary changes in larvæ.—The modes of
action of natural selection on larvæ may probably be divided more or
less artificially into two classes.
1. The changes in development directly produced by the existence of
a larval stage.
2. The adaptive changes in a larva acquired in the ordinary course
of the struggle for existence.
The changes which come under the first head consist essentially in a
displacement in the order of development of certain organs. There is
always a tendency in development to throw back the differentiation of
the embryonic cells into definite tissues to as late a date as
possible. This takes place in order to enable the changes of form,
which every organ undergoes, in repeating even in an abbreviated way
its phylogenetic history, to be effected with the least expenditure of
energy. Owing to
[Pg 364] this tendency it comes about that when an organism is
hatched as a larva many of the organs are still in an undifferentiated
state, although the ancestral form which this larva represents had all
its organs fully differentiated. In order, however, that the larva may
be enabled to exist as an independent organism, certain sets of
organs, e.g. the muscular, nervous, and digestive systems, have to
be histologically differentiated. If the period of fœtal life is
shortened, an earlier differentiation of certain organs is a necessary
consequence; and in almost all cases the existence of a larval stage
causes a displacement in order of development of organs, the complete
differentiation of many organs being retarded relatively to the
muscular, nervous, and digestive systems.
The possible changes under the second head appear to be unlimited.
There is, so far as I see, no possible reason why an indefinite number
of organs should not be developed in larvæ to protect them from their
enemies, and to enable them to compete with larvæ of other species,
and so on. The only limit to such development appears to be the
shortness of larval life, which is not likely to be prolonged, since,
ceteris paribus, the more quickly maturity is reached the better it
is for the species.
A very superficial examination of marine larvæ shews that there are
certain peculiarities common to most of them, and it is important to
determine how far such peculiarities are to be regarded as adaptive.
Almost all marine larvæ are provided with well-developed organs of
locomotion, and transparent bodies. These two features are precisely
those which it is most essential for such larvæ to have. Organs of
locomotion are important, in order that larvæ may be scattered as
widely as possible, and so disseminate the species; and transparency
is very important in rendering larvæ invisible, and so less liable to
be preyed upon by their numerous enemies[137].
These considerations, coupled with the fact that almost all
free-swimming animals, which have not other special means of
protection, are transparent, seem to shew that the transparency
[Pg 365] of
larvæ at all events is adaptive; and it is probable that organs of
locomotion are in many cases specially developed, and not ancestral.
Various spinous processes on the larvæ of Crustacea and Teleostei are
also examples of secondarily acquired protective organs.
These general considerations are sufficient to form a basis for the
discussion of the characters of the known types of larvæ.
The following table contains a list of the more important of such
larval forms:
Dicyemidæ.—The Infusoriform larva (vol. II. fig. 62).
Porifera.—(a) The Amphiblastula larva (fig. 215), with one-half
of the body ciliated, and the other half without cilia; (b) an
oval uniformly ciliated larva, which may be either solid or have
the form of a vesicle.
Cœlenerata.—The planula (fig. 216).
Turbellaria.—(a) The eight-lobed larva of Müller (fig. 222);
(b) the larva of Götte and Metschnikoff, with some Pilidium
characters.
Nemertea.—The Pilidium (fig. 221).
Trematoda.—The Cercaria.
Rotifera.—The Trochosphere-like larvæ of Brachionus (fig. 217) and
Lacinularia.
Mollusca.—Mollusca.—The Trochosphere larva (fig. 218), and the subsequent
Veliger larva (fig. 219).
Brachiopoda.—The three-lobed larva, with a postoral ring of cilia
(fig. 220).
Polyzoa.—A larval form with a single ciliated ring surrounding the
mouth, and an aboral ciliated ring or disc (fig. 228).
Chætopoda.—Various larval forms with many characters like those of
the molluscan Trochosphere, frequently with distinct transverse
bands of cilia. They are classified as Atrochæ, Mesotrochæ,
Telotrochæ (fig. 225 A and fig. 226), Polytrochæ, and Monotrochæ
(fig. 225 B).
Gephyrea Nuda.—Larval forms like those of preceding groups. A
specially characteristic larva is that of Echiurus (fig. 227).
Gephyrea Tubicola.—Actinotrocha (fig. 230), with a postoral
ciliated ring of arms.
Myriapoda.—A functionally hexapodous larval form is common to all
the Chilognatha (vol. II. fig. 174).
Insecta.—Various secondary larval forms.
Crustacea.—The Nauplius (vol. II. fig. 208) and the Zoæa
(vol. II. fig. 210).
Echinodermata.—The Auricularia (fig. 223 A), the Bipinnaria (fig.
223 B), and the Pluteus (fig. 224), and the transversely-ringed
larvæ of Crinoidea (vol. II. fig. 268). The three first of which
can be reduced to a common type (fig. 231 C).
Enteropneusta.—Tornaria (fig. 229).
Urochorda (Tunicata).—The tadpole-like larva (vol. III. fig. 8).
Ganoidei.—A larva with a disc with adhesive papillæ in front of the
mouth (vol. III. fig. 67).
Anurus Amphibia.—The tadpole (vol. III. fig. 80).
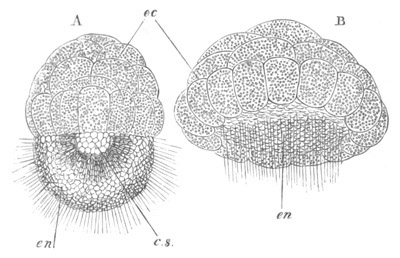
Fig. 215. Two free stages in the development of Sycandra raphanus.
(After Schultze.)
A. Amphiblastula stage.
B. Stage after the ciliated cells have commenced to be invaginated.
c.s. segmentation cavity; ec. granular epiblast cells; en.
ciliated hypoblast cells.
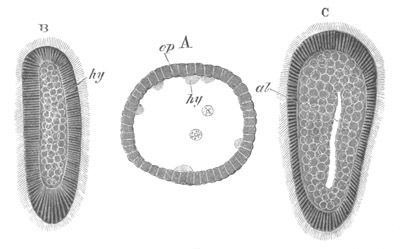
Fig. 216. Three larval stages of Eucope ploystyla. (After Kowalevsky.)
A. Blastosphere stage with hypoblast spheres becoming budded into
the central cavity.
B. Planula stage with solid hypoblast.
C. Planula stage with a gastric cavity.
ep. epiblast; hy. hypoblast; al. gastric cavity.
[Pg 366]
Of the larval forms included in the above list a certain number are
probably without affinities outside the group to which they belong.
This is the case with the larvæ of the
[Pg 367] Myriapoda, the Crustacean
larvæ, and with the larval forms of the Chordata. I shall leave these
forms out of consideration.
There are, again, some larval forms which may possibly turn out
hereafter to be of importance, but from which, in the present state of
our knowledge, we cannot draw any conclusions. The infusoriform larva
of the Dicyemidæ, and the Cercaria of the Trematodes, are such forms.
Excluding these and certain other forms, we have finally left for
consideration the larvæ of the Cœlenterata, the Turbellaria, the
Rotifera, the Nemertea, the Mollusca, the Polyzoa, the Brachiopoda,
the Chætopoda, the Gephyrea, the Echinodermata, and the Enteropneusta.
The larvæ of these forms can be divided into two groups. The one group
contains the larva of the Cœlenterata or Planula, the other group the
larvæ of all the other forms.
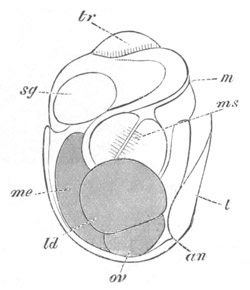
Fig. 217. Embryo of Brachionus urceolaris, shortly before it is
hatched. (After Salensky.)
m. mouth; ms. masticatory apparatus; me. mesenteron; an.
anus; ld. lateral gland; ov. ovary; t. tail (foot); tr.
trochal disc; sg. supraœsophageal ganglion.
The Planula (fig. 216) is characterised by its extreme simplicity. It
is a two-layered organism, with a form varying from cylindrical to
oval, and usually a radial symmetry. So long as it remains free it is
not usually provided with a mouth, and it is as yet uncertain whether
or no the absence of a mouth is to be regarded as an ancestral
character. The Planula is very probably the ancestral form of the
Cœlenterata.
The larvæ of almost all the other groups, although they may be
subdivided into a series of very distinct types, yet agree in the
possession of certain common characters[138].
There is a more or less
dome-shaped dorsal surface, and a flattened or concave ventral
surface, containing the opening
[Pg 368] of the mouth, and usually extending
posteriorly to the opening of the anus, when such is present.
The dorsal dome is continued in front of the mouth to form a large
præoral lobe.
There is usually present at first an uniform covering of cilia; but in
the later larval stages there are almost always formed definite bands
or rings of long cilia, by which locomotion is effected. These bands
are often produced into arm-like processes.
The alimentary canal has, typically, the form of a bent tube with a
ventral concavity, constituted (when an anus is present) of three
sections, viz. an œsophagus, a stomach, and a rectum. The œsophagus
and sometimes the rectum are epiblastic in origin, while the stomach
always and the rectum usually are derived from the hypoblast[139].
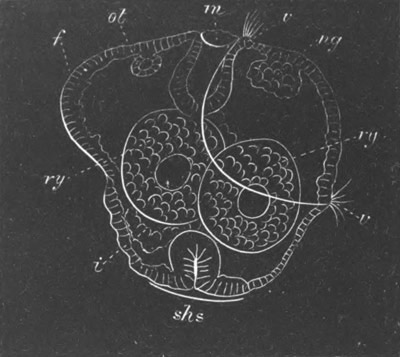
Fig. 218. Diagram of an embryo of Pleurobranchidium. (From
Lankester.)
f. foot; ot. otocyst; m. mouth; v. velum; ng. nerve
ganglion; ry. residual yolk spheres; shs. shell-gland; i.
intestine.
To the above characters may be added a glass-like transparency; and
the presence of a widish space possibly filled with gelatinous tissue,
and often traversed by contractile cells, between the alimentary tract
and the body wall.
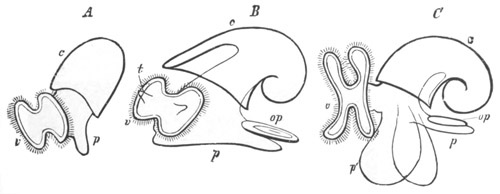
Fig. 219. Larvæ of Cephalophorous Mollusca in the veliger stage.
(From Gegenbaur.)
A. and B. Earlier and later stage of Gasteropod. C. Pteropod
(Cymbulia). v. velum; c. shell; p. foot; op. operculum; t.
tentacle.
[Pg 369]
Considering the very profound differences which exist between many of
these larvæ, it may seem that the characters just enumerated are
hardly sufficient to justify my grouping them together. It is,
however, to be borne in mind that my grounds for doing so depend quite
as much upon the fact that they constitute a series without any great
breaks in it, as upon the existence of characters common to the whole
of them. It is also worth noting that most of the characters which
have been enumerated as common to the whole of these larvæ are not
such secondary characters as (in accordance with the considerations
used above) might be expected to arise from the fact of their being
subjected to nearly similar conditions of life. Their transparency is,
no doubt, such a secondary character, and it is not impossible that
the existence of ciliated bands may be so also; but it is quite
possible that if, as I suppose, these larvæ reproduce the characters
of some ancestral form, this form may have existed at a time when all
marine animals were free-swimming, and that it may, therefore, have
been provided with at least one ciliated band.
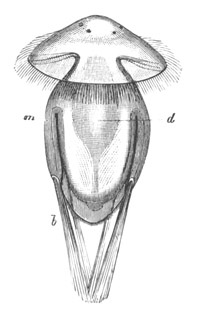
Fig. 220. Larva of Argiope. (From Gegenbaur; after
Kowalevsky.)
m. mantle; b. setæ; d. archenteron.
[Pg 370]
The detailed consideration of the characters of these larvæ, given
below, supports this view.
This great class of larvæ may, as already stated, be divided into a
series of minor subdivisions. These subdivisions are the following:
1. The Pilidium Group.—This group is characterised by the mouth being
situated nearly in the centre of the ventral surface, and by the
absence of an anus. It includes the Pilidium of the Nemertines (fig.
221), and the various larvæ of marine Dendrocœla (fig. 222). At the
apex of the præoral lobe a thickening of epiblast may be present, from
which (fig. 232) a contractile cord sometimes passes to the
œsophagus.
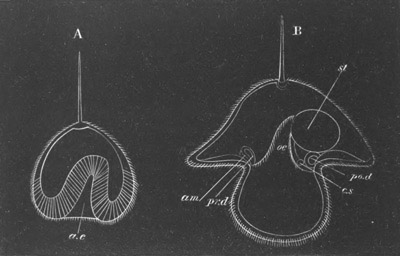
Fig. 221. Two stages in the development of Pilidium.(After
Metschnikoff.)
ae. archenteron; oe. œsophagus; st. stomach; am. amnion;
pr.d. prostomial disc; po.d. metastomial disc; c.s. cephalic
sack (lateral pit).
2. The Echinoderm Group.—This group (figs. 223, 224 and 231 C) is
characterised by the presence of a longitudinal postoral band of
cilia, by the absence of special sense organs in the præoral region,
and by the development of the body cavity as an outgrowth of the
alimentary tract. The three typical divisions of the alimentary tract
are present, and there is a more or less developed præoral lobe. This
group only includes the larvæ of the Echinodermata.
[Pg 371]
3. The Trochosphere Group.—This group (figs. 225, 226) is
characterised by the presence of a præoral ring of long cilia, the
region in front of which forms a great part of the præoral lobe. The
mouth opens immediately behind the præoral ring of cilia, and there is
very often a second ring of short cilia parallel to the main ring,
immediately behind the mouth. The function of the ring of short cilia
is nutritive, in that its cilia are employed in bringing food to the
mouth; while the function of the main ring is locomotive. A perianal
patch or ring of cilia is often present (fig. 225 A), and in many
forms intermediate rings are developed between the præoral and
perianal rings.
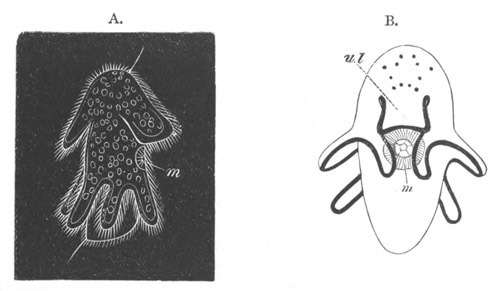
Fig. 222. A. Larva of Eurylepta auriculata immediately after
hatching. Viewed from the side. (After Hallez.) m. mouth.
B. Müller’s Turbellarian larva (probably Thysanozoon). Viewed
from the ventral surface. (After Müller.) The ciliated band is
represented by the black line. m. mouth; u.l. upper lip.
The præoral lobe is usually the seat of a special thickening of
epiblast, which gives rise to the supraœsophageal ganglion of the
adult. On this lobe optic organs are very often developed in
connection with the supraœsophageal ganglion, and a contractile band
frequently passes from this region to the œsophagus.
The alimentary tract is formed of the three typical divisions.
The body cavity is not developed directly as an outgrowth of the
alimentary tract, though the process by which it originates is very
probably secondarily modified from a pair of alimentary outgrowths.
[Pg 372]
Paired excretory organs, opening to the exterior and into the body
cavity, are often present (fig. 226 nph).
This type of larva is found in the Rotifera (fig. 217) (in which it is
preserved in the adult state), the Chætopoda (figs. 225 and 226), the
Mollusca (fig. 218), the Gephyrea nuda (fig. 227), and the Polyzoa
(fig. 228)[140].
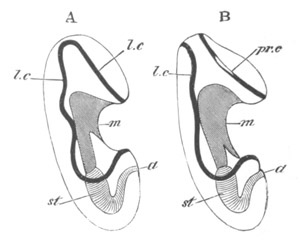
Fig. 223. A. The larva of a Holothuroid.
B. The larva of an Asteroid.
m. mouth; st. stomach; a. anus; l.c. primitive longitudinal
ciliated band; pr.c. præoral ciliated band.
4. Tornaria.—This larva (fig. 229) is intermediate in most of its
characters between the larvæ of the Echinodermata (more especially the
Bipinnaria) and the Trochosphere. It resembles Echinoderm larvæ in the
possession of a longitudinal ciliated band (divided into a præoral and
a postoral ring), and in the derivation of the body cavity and
water-vascular vesicle from alimentary diverticula; and it resembles
the Trochosphere in the presence of sense organs on the præoral lobe,
in the existence of a perianal ring of cilia, and in the possession of
a contractile band passing from the præoral lobe to the œsophagus.
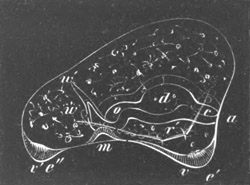
Fig. 224. A larva of Strongylocentrus. (From Agassiz.)
m. mouth; a. anus; o. œsophagus; d. stomach; c.
intestine; v´. and v. ciliated ridges; w. water-vascular
tube; r. calcareous rods.
[Pg 373]
5. Actinotrocha.—The remarkable larva of Phoronis (fig. 230), known
as Actinotrocha, is characterised by the presence of (1) a postoral
and somewhat longitudinal ciliated ring produced into tentacles, and
(2) a perianal ring. It is provided with a præoral lobe, and a
terminal or somewhat dorsal anus.
6. The larva of the Brachiopoda articulata (fig. 220).
The relationships of the six types of larval forms thus briefly
characterised have been the subject of a considerable amount of
controversy, and the following suggestions on their affinities must be
viewed as somewhat speculative. The Pilidium type of larva is in some
important respects less highly differentiated than the larvæ of the
five other groups. It is, in the first place, without an anus; and
there are no grounds for supposing that the anus has become lost by
retrogressive changes. If for the moment it is granted that the
Pilidium larva represents more nearly than the larvæ of the other
groups the ancestral type of larva, what characters are we led to
assign to the ancestral form which this larva repeats?
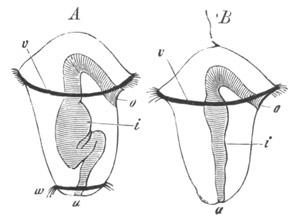
Fig. 225. Two chætopod larvæ. (From Gegenbaur.)
o. mouth; i. intestine; a. anus; v. præoral ciliated band;
w. perianal ciliated band.
In the first place, this ancestral form, of which fig. 231 A is an
ideal representation, would appear to have had a dome-shaped body,
with a flattened oral surface and a rounded aboral surface. Its
symmetry was radial, and in the centre of the flattened oral surface
was placed the mouth, and round its edge was a ring of cilia. The
passage of a Pilidium-like larva into the vermiform bilateral
Platyelminth form, and therefore it may be presumed of the ancestral
form which this larva repeats, is effected by the
[Pg 374] larva becoming more
elongated, and by the region between the mouth and one end of the body
becoming the præoral region, and by an outgrowth between the mouth and
the opposite end developing into the trunk, an anus becoming placed at
its extremity in the higher forms.
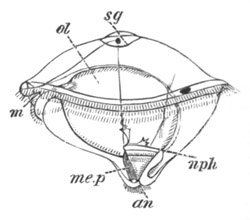
Fig. 226. Polygordius larva.(After Hatschek.)
m. mouth; sg. supraœsophageal ganglion; nph. nephridion;
me.p. mesoblastic band; an. anus; ol. stomach.
If what has been so far postulated is correct, it is clear that this
primitive larval form bears a very close resemblance to a simplified
free-swimming Cœlenterate (Medusa), and that the conversion of such a
radiate form into the bilateral took place, not by the elongation of
the aboral surface, and the formation of an anus there, but by the
unequal elongation of the oral face, an anterior part, together with
the dome above it, forming a præoral lobe, and a posterior outgrowth
the trunk (figs. 226 and 233); while the aboral surface became the
dorsal surface.
This view fits in very well with the anatomical resemblances between
the Cœlenterata and the Turbellaria[141],
and shews, if true, that
the ventral and median position of the mouth in many Turbellaria is
the primitive one.
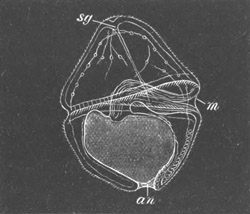
Fig. 227. Larva of Echiurus. (After Salensky.)
m. mouth; an. anus; sg. supraœsophageal ganglion (?).
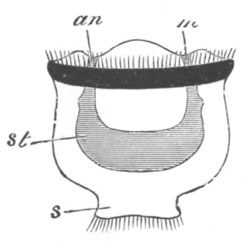
Fig. 228. Diagram of a larva of the Polyzoa.
m. mouth; an. anus; st. stomach; s. ciliated disc.
The above suggestion as to the mode of passage from the radial into
the bilateral form differs largely from that usually held.
Lankester[142],
for instance, gives the following account of this
passage:
≴It has been recognised by various writers, but notably by Gegenbaur
and Haeckel, that a condition of radiate symmetry must have preceded
the condition of bilateral symmetry in animal evolution. The
Diblastula may be conceived to have been at first absolutely spherical
with spherical symmetry. The establishment of a mouth led necessarily
to the establishment of a structural axis passing through the mouth,
around which axis the body was arranged with radial symmetry. This
condition is more or less perfectly maintained by many Cœlenterates,
and is reassumed by degradation
[Pg 375] of higher forms (Echinoderms, some
Cirrhipedes, some Tunicates). The next step is the differentiation of
an upper and a lower surface in relation to the horizontal position,
with mouth placed anteriorly, assumed by the organism in locomotion.
With the differentiation of a superior and inferior surface, a right
and a left side, complementary one to the other, are necessarily also
differentiated. Thus the organism becomes bilaterally symmetrical. The
Cœlentera are not wanting in indications of this bilateral symmetry,
but for all other higher groups of animals it is a fundamental
character. Probably the development of a region in front of, and
dorsal to the mouth, forming the Prostomium, was accomplished pari
passu with the development of bilateral symmetry. In the radially
symmetrical Cœlentera we find very commonly a series of lobes of the
body-wall or tentacles produced equally—with radial symmetry, that
is to say—all round the mouth, the mouth terminating the main axis of
the body—that is to say, the organism being ‘telostomiate.’ The later
fundamental form, common to all animals above the Cœlentera, is
attained by shifting what was the main axis of the body—so that it
may be described now as the ‘enteric’ axis; whilst the new main axis,
that parallel with the plane of progression, passes through the dorsal
region of the body running obliquely in relation to the enteric axis.
Only one lobe or outgrowth of those radially disposed in the
telostomiate organisms now persists. This lobe lies dorsally to the
mouth, and through it runs the new main axis. This lobe is the
Prostomium, and all the organisms which thus develop a new main
axis, oblique to the old main axis, may be called prostomiate.”
[Pg 376]
It will be seen from this quotation that the aboral part of the body
is supposed to elongate to form the trunk, while the præoral region is
derived from one of the tentacles.
Before proceeding to further considerations as to the origin of the
Bilateralia, suggested by the Pilidium type of larva, it is necessary
to enter into a more detailed comparison between our larval forms.
A very superficial consideration of the characters of these forms
brings to light two important features in which they differ, viz.:
(1) In the presence or absence of sense organs on the præoral lobe.
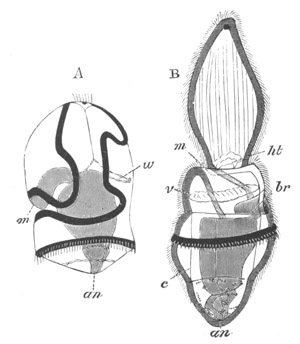
Fig. 229. Two stages in the development of Tornaria. (After
Metschnikoff.)
The black lines represent the ciliated bands.
m. mouth; an. anus; br. branchial cleft; ht. heart; c.
body cavity between splanchnic and somatic mesoblast layers; w.
so-called water-vascular vesicle; v. circular blood-vessel.
(2) In the presence or absence of outgrowths from the alimentary tract
to form the body cavity.
The larvæ of the Echinodermata and Actinotrocha (?) are without sense
organs on the præoral lobe, while the other types
[Pg 377] of larvæ are
provided with them. Alimentary diverticula are characteristic of the
larvæ of the Echinodermata and of Tornaria.
If the conclusion already arrived at to the effect that the prototype
of the six larval groups was descended from a radiate ancestor is
correct, it appears to follow that the nervous system, in so far as it
was differentiated, had primitively a radiate form; and it is also
probably true that there were alimentary diverticula in the form of
radial pouches, two of which may have given origin to the paired
diverticula which become the body cavity in such types as the
Echinodermata, Sagitta, etc. If these two points are granted, the
further conclusions seem to follow—(1) that the ganglion and sense
organs of the præoral lobe were secondary structures, which arose
(perhaps as differentiations of an original circular nerve ring) after
the assumption of a bilateral form; and (2) that the absence of these
organs in the larvæ of the Echinodermata and Actinotrocha (?) implies
that these larvæ retain, so far, more primitive characters than the
Pilidium. The same may be said of the alimentary diverticula. There
are thus indications that in two important points the Echinoderm larvæ
are more primitive than the Pilidium.
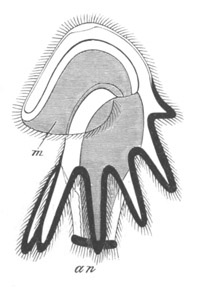
Fig. 230. Actinotrocha. (After Metschnikoff.)
m. mouth; an. anus.
The above conclusions with reference to the Pilidium and Echinoderm
larvæ involve some not inconsiderable difficulties, and suggest
certain points for further discussion.
In the first place it is to be noted that the above speculations
render it probable that the type of nervous system from which that
found in the adults of the Echinodermata, Platyelminthes, Chætopoda,
Mollusca, etc., is derived, was a circumoral ring, like that of
Medusæ, with which radially arranged sense organs may have been
connected; and that in the Echinodermata this form of nervous system
has been retained, while in the other types it has been modified. Its
anterior part may have given rise to supraœsophageal ganglia and
organs of vision; these being
[Pg 378] developed on the assumption of a
bilaterally symmetrical form, and the consequent necessity arising for
the sense organs to be situated at the anterior end of the body. If
this view is correct, the question presents itself as to how far the
posterior part of the nervous system of the Bilateralia can be
regarded as derived from the primitive radiate ring.
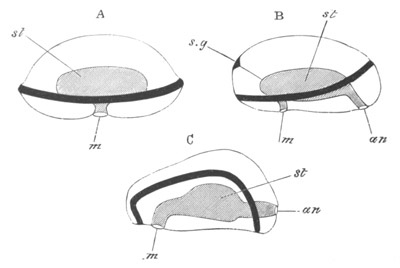
Fig. 231. Three diagrams representing the ideal evolution of various
larval forms.
A. Ideal ancestral larval form.
B. Larval form from which the Trochosphere larva may have been
derived.
C. Larval form from which the typical Echinoderm larva may have been
derived.
m. mouth; an. anus; st. stomach; s.g. supraœsophageal
ganglion. The black lines represent the ciliated bands.
A circumoral nerve-ring, if longitudinally extended, might give rise
to a pair of nerve-cords united in front and behind—exactly such a
nervous system, in fact, as is present in many Nemertines[143]
(the
Enopla and Pelagonemertes), in Peripatus[144],
and in primitive
molluscan types (Chiton, Fissurella, etc.). From the lateral parts of
this ring it would be easy to derive the ventral cord of the Chætopoda
and Arthropoda. It is especially deserving of notice in connection
with the nervous system of the
[Pg 379] above-mentioned Nemertines and
Peripatus, that the commissure connecting the two nerve-cords behind
is placed on the dorsal side of the intestine. As is at once
obvious, by referring to the diagram (fig. 231 B), this is the
position this commissure ought, undoubtedly, to occupy if derived from
part of a nerve-ring which originally followed more or less closely
the ciliated edge of the body of the supposed radiate ancestor.
The fact of this arrangement of the nervous system being found in so
primitive a type as the Nemertines tends to establish the views for
which I am arguing; the absence or imperfect development of the two
longitudinal cords in Turbellarians may very probably be due to the
posterior part of the nerve-ring having atrophied in this group.
It is by no means certain that this arrangement of the nervous system
in some Mollusca and in Peripatus is primitive, though it may be so.
In the larvæ of the Turbellaria the development of sense organs in the
præoral region is very clear (fig. 222 B); but this is by no means so
obvious in the case of the true Pilidium. There is in Pilidium (fig.
232 A) a thickening of epiblast at the summit of the dorsal dome,
which might seem, from the analogy of Mitraria, etc. (fig. 233), to
correspond to the thickening of the præoral lobe, which gives rise to
the supraœsophageal ganglion; but, as a matter of fact, this part of
the larva does not apparently enter into the formation of the young
Nemertine (fig. 232). The peculiar metamorphosis, which takes place in
the development of the Nemertine out of the Pilidium[145],
may,
perhaps, eventually supply an explanation of this fact; but at present
it remains as a still unsolved difficulty.
The position of the flagellum in Pilidium, and of the supraœsophageal
ganglion in Mitraria, suggests a different view of the origin of the
supraœsophageal ganglion from that adopted above. The position of the
ganglion in Mitraria corresponds closely with that of the auditory
organ in Ctenophora; and it is not impossible that the two structures
may have had a common origin. If this view is correct, we must suppose
that the apex of the aboral lobe has become the centre of the præoral
field of the Pilidium and Trochosphere larval forms[146]—a view which
fits in very well with their structure (figs. 226 and 233). The whole
of the questions concerning the nervous system are still very obscure,
and until further facts are brought to light no definite conclusions
can be arrived at.
[Pg 380]
The absence of sense organs on the præoral lobe of larval
Echinodermata, coupled with the structure of the nervous system of the
adult, points to the conclusion that the adult Echinodermata have
retained, and not, as is now usually held, secondarily acquired,
their radial symmetry; and if this is admitted it follows that the
obvious bilateral symmetry of Echinoderm larvæ is a secondary
character.
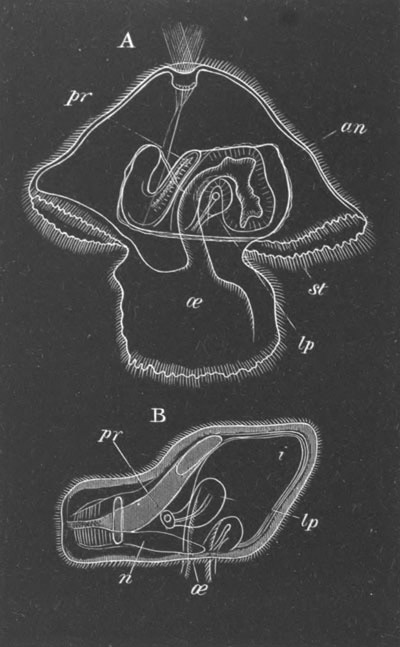
Fig. 232. A. Pilidium with an advanced Nemertine Worm. B. Ripe
embryo of Nemertes in the position it occupies in Pilidium. (Both
after Bütschli.)
œ. œsophagus; st. stomach; i. intestine; pr. proboscis;
lp. lateral pit (cephalic sack); an. amnion; n. nervous
system.
The bilateral symmetry of many Cœlenterate larvæ (the larva of
Æginopsis, of many Acraspeda, of Actinia, &c.), coupled with the fact
that a bilateral symmetry is obviously advantageous
[Pg 381] to a free-swimming
form, is sufficient to shew that this supposition is by no means
extravagant; while the presence of only two alimentary diverticula in
Echinoderm larvæ is quite in accord with the presence of a single pair
of perigastric chambers in the early larva of Actinia, though it must
be admitted that the derivation of the water-vascular system from the
left diverticulum is not easy to understand on this view.
A difficulty in the above speculation is presented by the fact of the
anus of the Echinodermata being the permanent blastopore, and arising
prior to the mouth. If this fact has any special significance, it
becomes difficult to regard the larva of Echinoderms and that of the
other types as in any way related; but if the views already urged, in
a previous section on the germinal layers, as to the unimportance of
the blastopore, are admitted, the fact of the anus coinciding with the
blastopore ceases to be a difficulty. As may be seen, by referring to
fig. 231 C, the anus is placed on the dorsal side of the ciliated
band. This position for the anus adapts itself to the view that the
Echinoderm larva had originally a radial symmetry, with the anus
placed at the aboral apex, and that, with the elongation of the larva
on the attainment of a bilateral symmetry, the aboral apex became
shifted to the present position of the anus.
It may be noticed that the obscure points connected with the absence
of a body cavity in most adult Platyelminthes, which have already been
dealt with in the section of this chapter devoted to the germinal
layers, present themselves again here; and that it is necessary to
assume either that alimentary diverticula, like those in the
Echinodermata, were primitively present in the Platyelminthes, but
have now disappeared from the ontogeny of this group, or that the
alimentary diverticula have not become separated from the alimentary
tract.
So far the conclusion has been reached that the archetype of the six
types of larvæ had a radiate form, and that amongst existing larvæ it
is most nearly approached in general shape and in the form of the
alimentary canal by the Pilidium group, and in certain other
particulars by the Echinoderm larvæ.
The edge of the oral disc of the larval archetype was probably armed
with a ciliated ring, from which the ciliated ring of the Pilidium
type and of the Echinodermata was most likely derived. The ciliated
ring of the Pilidium varies greatly in its characters,
[Pg 382] and has not
always the form of a complete ring. In Pilidium proper (fig. 232 A) it
is a simple ring surrounding the edge of the oral disc. In Müller’s
larva of Thysanozoon (fig. 222 B) it is inclined at an axis to the
oral disc, and might be called præoral, but such a term cannot be
properly used in the absence of an anus.
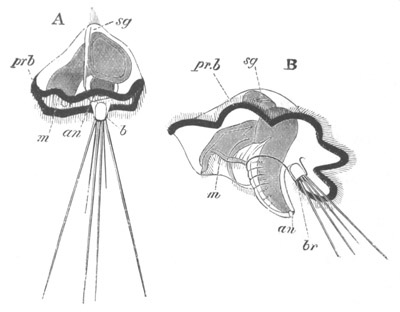
Fig. 233. Two stages in the development of Mitraria. (After
Metschnikoff.)
m. mouth; an. anus; sg. supraœsophageal ganglion; br. and
b. provisional bristles; pr.b. præoral ciliated band.
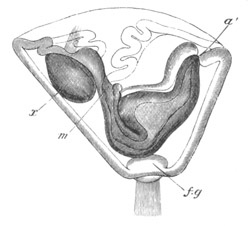
Fig. 234. Cyphonautes (larva of Membranipora). (After Hatschek.)
m. mouth; a´. anus; f.g. foot gland; x. problematical body
(probably a bud). The aboral apex is turned downwards.
[Pg 383]
The Echinoderm ring is oblique to the axis of the body, and, owing to
the fact of its passing ventrally in front of the anus, must be called
postoral.
The next point to be considered is that of the affinities of the other
larval types to these two types.
The most important of all the larval types is the Trochosphere, and
this type is undoubtedly more closely related to the Pilidium than to
the Echinoderm larva. Mitraria amongst the Chætopods (fig. 233) has,
indeed, nearly the form of a Pilidium, and mainly differs from a
Pilidium in the possession of an anus and of provisional bristles; the
same may be said of Cyphonautes (fig. 234) amongst the Polyzoa.
The existence of these two forms appears to shew that the præoral
ciliated ring of the Trochosphere may very probably be derived
directly from the circumoral ciliated ring of the Pilidium; the other
ciliated rings or patches of the Trochosphere having a secondary
origin.
The larva of the Brachiopoda (fig. 220), in spite of its peculiar
characters, is, in all probability, more closely related to the
Chætopod Trochosphere than to any other larval type. The most
conspicuous point of agreement between them is, however, the
possession in common of provisional setæ.
Echinoderm larvæ differ from the Trochosphere, not only in the points
already alluded to, but in the character of the ciliated band. The
Echinoderm band is longitudinal and postoral. As just stated, there is
reason to think that the præoral band of the Trochosphere and the
postoral band of the Echinoderm larva are both derived from a ciliated
ring surrounding the oral disc of the prototype of these larvæ (vide
fig. 231). In the case of the Echinodermata the anus must have been
formed on the dorsal side of this ring, and in the case of the
Trochosphere on the ventral side; and so the difference in position
between the two rings was brought about. Another view with reference
to these rings has been put forward by Gegenbaur and Lankester, to the
effect that the præoral ring of the Trochosphere is derived from the
breaking up of the single band of most Echinoderm larvæ into the two
bands found in Bipinnaria (vide fig. 223) and the atrophy of the
posterior band. There is no doubt a good deal to be said for this
origin of the præoral ring, and it is
[Pg 384] strengthened by the case of
Tornaria; but the view adopted above appears to me more probable.
Actinotrocha (fig. 230) undoubtedly resembles more closely Echinoderm
larvæ than the Trochosphere. Its ciliated ring has Echinoderm
characters, and the growth along the line of the ciliated ring of a
series of arms is very similar to what takes place in many
Echinoderms. It also agrees with the Echinoderm larvæ in the absence
of sense organs on the præoral lobe.
Tornaria (fig. 229) cannot be definitely united either with the
Trochosphere or with the Echinoderm larval type. It has important
characters in common with both of these groups, and the mixture of
these characters renders it a very striking and well-defined larval
form.
Phylogenetic conclusions. The phylogenetic conclusions which follow
from the above views remain to be dealt with. The fact that all the
larvæ of the groups above the Cœlenterata can be reduced to a common
type seems to indicate that all the higher groups are descended from a
single stem.
Considering that the larvæ of comparatively few groups have persisted,
no conclusions as to affinities can be drawn from the absence of a
larva in any group; and the presence in two groups of a common larval
form may be taken as proving a common descent, but does not
necessarily shew any close affinity.
There is every reason to believe that the types with a Trochosphere
larva, viz. the Rotifera, the Mollusca, the Chætopoda, the Gephyrea,
and the Polyzoa, are descended from a common ancestral form; and it is
also fairly certain there was a remote ancestor common to these forms
and to the Platyelminthes. A general affinity of the Brachiopoda with
the Chætopoda is more than probable. All these types, together with
various other types which are nearly related to them, but have not
preserved an early larval form, are descended from a bilateral
ancestor. The Echinodermata, on the other hand, are probably directly
descended from a radial ancestor, and have more or less completely
retained their radial symmetry. How far Actinotrocha[147]
is related
to the Echinoderm larvæ cannot be settled. Its characters may possibly
be secondary, like those of the
[Pg 385] mesotrochal larvæ of Chætopods, or
they may be due to its having branched off very early from the stock
common to the whole of the forms above the Cœlenterata. The position
of Tornaria is still more obscure. It is difficult, in the face of the
peculiar water-vascular vesicle with a dorsal pore, to avoid the
conclusion that it has some affinities with the Echinoderm larvæ. Such
affinities would seem, on the lines of speculation adopted in this
section, to prove that its affinities to the Trochosphere, striking as
they appear to be, are secondary and adaptive. From this conclusion,
if justified, it would follow that the Echinodermata and Enteropneusta
have a remote ancestor in common, but not that the two groups are in
any other way related.
General conclusions and summary. Starting from the demonstrated fact
that the larval forms of a number of widely separated types above the
Cœlenterata have certain characters in common, it has been
provisionally assumed that the characters have been inherited from a
common ancestor; and an attempt has been made to determine (1) the
characters of the prototype of all these larvæ, and (2) the mutual
relations of the larval forms in question. This attempt started with
certain more or less plausible suggestions, the truth of which can
only be tested by the coherence of the results which follow from them,
and their capacity to explain all the facts.
The results arrived at may be summarised as follows:
1. The larval forms above the Cœlenterata may be divided into six
groups enumerated on pages 370 to 373.
2. The prototype of all these groups was an organism something like a
Medusa, with a radial symmetry. The mouth was placed in the centre of
a flattened ventral surface. The aboral surface was dome-shaped. Round
the edge of the oral surface was a ciliated ring, and probably a
nervous ring provided with sense organs. The alimentary canal was
prolonged into two or more diverticula, and there was no anus.
3. The bilaterally symmetrical types were derived from this larval
form by the larva becoming oval, and the region in front of the mouth
forming a præoral lobe, and that behind the mouth growing out to form
the trunk. The aboral dome became the dorsal surface.
On the establishment of a bilateral symmetry the anterior
[Pg 386] part of the
nervous ring gave rise (?) to the supraœsophageal ganglia, and the
optic organs connected with them; while the posterior part of the
nerve-ring formed (?) the ventral nerve-cords. The body cavity was
developed from two of the primitive alimentary diverticula.
The usual view that radiate forms have become bilateral by the
elongation of the aboral dome into the trunk is probably erroneous.
4. Pilidium is the larval form which most nearly reproduces the
characters of the larval prototype in the course of its conversion
into a bilateral form.
5. The Trochosphere is a completely differentiated bilateral form, in
which an anus has become developed. The præoral ciliated ring of the
Trochosphere is probably directly derived from the ciliated ring of
Pilidium, which is itself the original ring of the prototype of all
these larval forms.
6. Echinoderm larvæ, in the absence of a nerve-ganglion or special
organs of sense on the præoral lobe, and in the presence of alimentary
diverticula, which give rise to the body cavity, retain some
characters of the prototype larva which have been lost in Pilidium.
The ciliated ring of Echinoderm larvæ is probably derived directly
from that of the prototype by the formation of an anus on the dorsal
side of the ring. The anus was very probably originally situated at
the aboral apex.
Adult Echinoderms have probably retained the radial symmetry of the
forms from which they are descended, their nervous ring being directly
derived from the circular nervous ring of their ancestors. They have
not, as is usually supposed, secondarily acquired their radial
symmetry. The bilateral symmetry of the larva is, on this view,
secondary, like that of so many Cœlenterate larvæ.
7. The points of similarity between Tornaria and (1) the Trochosphere
and (2) the Echinoderm larvæ are probably adaptive in the one case or
the other; and, while there is no difficulty in believing that those
to the Trochosphere are adaptive, the presence of a water-vascular
vesicle with a dorsal pore renders probable a real affinity with
Echinoderm larvæ.
8. It is not possible in the present state of our knowledge to decide
how far the resemblances between Actinotrocha and Echinoderm larvæ are
adaptive or primary.
[Pg 387]
Bibliography.
(257) Allen Thomson. British Association Address, 1877.
(258) A. Agassiz. “Embryology of the Ctenophoræ.” Mem. Amer. Acad. of
Arts and Sciences, Vol. X. 1874.
(259) K. E. von Baer. Ueb. Entwicklungsgeschichte d. Thiere.
Königsberg, 1828-1837.
(260) F. M. Balfour. “A Comparison of the Early Stages in the
Development of Vertebrates.” Quart. Journ. of Micr. Sci., Vol. XV.
1875.
(261) C. Claus. Die Typenlehre u. E. Haeckel’s sg. Gastræa-theorie.
Wien, 1874.
(262) C. Claus. Grundzüge d. Zoologie. Marburg und Leipzig, 1879.
(263) A. Dohrn. Der Ursprung d. Wirbelthiere u. d. Princip des
Functionswechsels. Leipzig, 1875.
(264) C. Gegenbaur. Grundriss d. vergleichenden Anatomie. Leipzig,
1878. Vide also Translation. Elements of Comparative Anatomy.
Macmillan & Co., 1878.
(265) A. Götte. Entwicklungsgeschichte d. Unke. Leipzig, 1874.
(266) E. Haeckel. Studien z. Gastræa-theorie, Jena, 1897; and also
Jenaische Zeitschrift, Vols. VIII. and IX. 1874-5.
(267) E. Haeckel. Schöpfungsgeschichte. Leipzig. Vide also
Translation, The History of Creation. King & Co., London, 1878.
(268) E. Haeckel. Anthropogenie. Leipzig. Vide also Translation,
Anthropogeny. Kegan Paul & Co., London, 1878.
(269) B. Hatschek. “Studien üb. Entwicklungsgeschichte d. Anneliden.”
Arbeit. a. d. zool. Instit. d. Univer. Wien. 1878.
(270) O. and R. Hertwig. “Die Actinien.” Jenaische Zeitschrift,
Vols. XIII. and XIV. 1879.
(271) O. and R. Hertwig. Die Cœlomtheorie. Jena, 1881[148].
(272) O. Hertwig. Die Chætognathen. Jena, 1880.
(273) R. Hertwig. Ueb. d. Bau d. Ctenophoren. Jena, 1880.
(274) T. H. Huxley. The Anatomy of Invertebrated Animals. Churchill,
1877.
(274*) T. H. Huxley. “On the Classification of the Animal Kingdom.”
Quart. J. of Micr. Science, Vol. XV. 1875.
(275) N. Kleinenberg. Hydra, eine
anatomisch-entwicklungsgeschichtliche Untersuchung. Leipzig, 1872.
(276) A. Kölliker. Entwicklungsgeschichte d. Menschen u. d. höh.
Thiere. Leipzig, 1879.
(277) A. Kowalevsky. “Embryologische Studien an Würmern u.
Arthropoden.” Mém. Acad. Pétersbourg, Series VII. Vol. XVI. 1871.
[Pg 388]
(278) E. R. Lankester. “On the Germinal Layers of the Embryo as the
Basis of the Genealogical Classification of Animals.” Ann. and Mag.
of Nat. Hist. 1873.
(279) E. R. Lankester. “Notes on Embryology and Classification.”
Quart. Journ. of Micr. Sci., Vol. XVII. 1877.
(280) E. Metschnikoff. “Zur Entwicklungsgeschichte d. Kalkschwämme.”
Zeit. f. wiss. Zool., Vol. XXIV. 1874.
(281) E. Metschnikoff. “Spongiologische Studien.” Zeit. f. wiss.
Zool., Vol. XXXII. 1879.
(282) A. S. P. Packard. Life Histories of Animals, including Man, or
Outlines of Comparative Embryology. Holt and Co., New York, 1876.
(283) C. Rabl. “Ueb. d. Entwick. d. Malermuschel.” Jenaische
Zeitsch., Vol. X. 1876.
(284) C. Rabl. “Ueb. d. Entwicklung. d. Tellerschnecke (Planorbis).”
Morph. Jahrbuch, Vol. V. 1879.
(285) H. Rathke. Abhandlungen z. Bildung und Entwicklungsgesch. d.
Menschen u. d. Thiere. Leipzig, 1833.
(286) H. Rathke. Ueber die Bildung u. Entwicklungs. d. Flusskrebses.
Leipzig, 1829.
(287) R. Remak. Untersuch. üb. d. Entwick. d. Wirbelthiere. Berlin,
1855.
(288) Salensky. “Bemerkungen üb. Haeckels Gastræa-theorie.” Archiv.
f. Naturgeschichte, 1874.
(289) E. Schäfer. “Some Teachings of Development.” Quart. Journ. of
Micr. Science, Vol. XX. 1880.
(290) C. Semper. “Die Verwandtschaftbeziehungen d. gegliederten
Thiere.” Arbeiten a. d. zool.-zoot. Instit. Würzburg, Vol. III.
1876-7.
[Pg 389]
PART II.
ORGANOGENY.
[Pg 390]
[Pg 391]
PART II.
ORGANOGENY.
Introduction.
Our knowledge of the development of the organs in most of the
Invertebrate groups is so meagre that it would not be profitable to
attempt to treat systematically the organogeny of the whole animal
kingdom.
For this reason the plan adopted in this section of the work has been
to treat somewhat fully the organogeny of the Chordata, which is
comparatively well known; and merely to indicate a few salient facts
with reference to the organogeny of other groups. In the case of the
nervous system, and of some other organs which especially lend
themselves to this treatment, such as the organs of special sense and
the excretory system, a wider view of the subject has been taken; and
certain general principles underlying the development of other organs
have also been noticed.
The classification of the organs is a matter of some difficulty.
Considering the character of this treatise it seemed desirable to
arrange the organs according to the layers from which they are
developed. The compound nature of many organs, e.g. the eye and ear,
renders it, however, impossible to carry out consistently such a mode
of treatment. I have accordingly adopted a rough classification of the
organs according to the layers, dropping the principle where
convenient, as, for instance, in the case of the stomodæum and
proctodæum.
The organs which may be regarded as mainly derived from
[Pg 392] the epiblast
are (1) the skin; (2) the nervous system; (3) the organs of special
sense.
Those from the mesoblast are (1) the general connective tissue and
skeleton; (2) the vascular system and body cavity; (3) the muscular
system; (4) the urinogenital system.
Those from the hypoblast are the alimentary tract and its derivates;
with which the stomodæum and proctodæum and their respective derivates
are also dealt with.
Bibliography.
General works dealing with the development of the organs of the
Chordata.
(291) K. E. von Baer. Ueber Entwicklungsgeschichte d. Thiere.
Königsberg, 1828-1837.
(292) F. M. Balfour. A Monograph on the development of Elasmobranch
Fishes. London, 1878.
(293) Th. C. W. Bischoff. Entwicklungsgesch. d. Säugethiere U. d.
Menschen. Leipzig, 1842.
(294) C. Gegenbaur. Grundriss d. vergleichenden Anatomie. Leipzig,
1878. Vide also English translation, Elements of Comp. Anatomy.
London, 1878.
(295) M. Foster and F. M. Balfour. The Elements of Embryology. Part
I. London, 1874.
(296) Alex. Götte. Entwicklungsgeschichte d. Unke. Leipzig, 1875.
(297) W. His. Untersuch. üb. d. erste Anlage d. Wirbelthierleibes.
Leipzig, 1868.
(298) A. Kölliker. Entwicklungsgeschichte d. Menschen u. der höheren
Thiere. Leipzig, 1879.
(299) H. Rathke. Abhandlungen ü. Bildung und Entwicklungsgeschichte
d. Menschen u. d. Thiere. Leipzig, 1838.
(300) H. Rathke. Entwicklungs. d. Natter. Königsberg, 1839.
(301) H. Rathke. Entwicklungs. d. Wirbelthiere. Leipzig, 1861.
(302) R. Remak. Untersuchungen üb. d. Entwicklung d. Wirbelthiere.
Berlin, 1850-1855.
(303) S. L. Schenk. Lehrbuch d. vergleich. Embryologie d.
Wirbelthiere. Wien, 1874.
[Pg 393]
CHAPTER XIV.
THE EPIDERMIS AND ITS DERIVATIVES.
In many of the Cœlenterata the outermost layer of the blastoderm is
converted as a whole into the skin or ectoderm. The cells composing it
become no doubt in part differentiated into muscular elements and in
part into nervous elements, &c.; but still it may remain through life
as a simple external membrane. This membrane contains in itself
indefinite potentialities for developing into various organs, and in
all the true Triploblastica these potentialities are more or less
realized. The embryonic epiblast ceases in fact, in the higher forms,
to become converted as a whole into the epidermis, but first gives
rise to parts of the nervous system, organs of special sense, and
other parts.
After the formation of these parts the remnant of the epiblast gives
rise to the epidermis, and often unites more or less intimately with a
subjacent layer of mesoblast, known as the dermis, to form with it the
skin.
Various differentiations may arise in the epidermis forming protective
or skeletal structures, terminal sense organs, or glands. The
structure of the epidermis itself varies greatly, and for Vertebrates
its general modifications have been already sufficiently dealt with in
chapter XII. Of its special differentiations those of a protective or
skeletal nature and those of a glandular nature may be considered in
this place.
Protective epidermal structures. These structures constitute a general
cuticle or an exoskeleton of scales, hairs, feathers, nails, hoofs,
&c. They may be entirely formed from
[Pg 394] the epidermis either as (1) a
cuticular deposit, or as (2) a chitinization, a cornification, or
calcification of its constituent cells. These two processes run into
each other, and are in many cases not easily distinguished. The
protective structures of the epidermis may be divided into two groups
according as they are formed on the outer or the inner side of the
epidermis. Dermal skeletal structures are in many cases added to them.
Amongst the Invertebrata the most widely distributed type of
exoskeleton is a cuticle formed on the outer surface of the epidermis,
which reaches its highest development in the Arthropoda. In the same
class with this cuticle must be placed the molluscan and brachiopod
shells, which are developed as cuticular plates on special regions of
the epidermis. They differ, however, from the more usual form of
cuticle in their slighter adhesion to the subjacent epidermis, and in
their more complicated structure. The test of Ascidians is an abnormal
form of exoskeleton belonging to this type. It is originally formed
(Hertwig and Semper) as a cuticle on the surface of the epidermis; but
subsequently epidermic cells migrate into it, and it then constitutes
a tissue similar to connective tissue, but differing from ordinary
epidermic cuticles in that the cells which deposit it do so over their
whole surface, instead of one surface, as is usually the case with
epithelial cells.
In the Vertebrata the two types of exoskeleton mentioned above are
both found, but that developed on the inner surface of the epidermis
is always associated with a dermal skeleton, and that on the outer
side frequently so. The type of exoskeleton developed on the inner
side of the general epidermis is confined to the Pisces, where it
appears as the scales; but a primitive form of these structures
persists as the teeth in the Amphibia and Amniota. The type developed
on the outer side of the epidermis is almost entirely[149]
confined
to the Amphibia and Amniota, where it appears as scales, feathers,
hairs, claws, nails, &c. For the histological details as to the
formation of these various organs I must refer the reader to treatises
on histology, confining my attention here to the general embryological
processes which take place in their development.
[Pg 395]
The most primitive form of the first type of dermal structures is that
of the placoid scales of Elasmobranchii[150].
These consist, when
fully formed, of a plate bearing a spinous projection. They are
constituted of an outer enamel layer on the projecting part, developed
as a cuticular deposit of the epidermis (epiblast), and an underlying
basis of dentine (the lower part of which may be osseous) with a
vascular pulp in its axis. The development (fig. 235) is as follows
(Hertwig, No. 306). A papilla of the dermis makes its appearance, the
outer layer of which gradually calcifies to form the dentine and
osseous tissue. This papilla is covered by the columnar mucous layer
of the epidermis (e), from which it is separated by a basement
membrane, itself a product of the epidermis. This membrane gradually
thickens and calcifies, and so gives rise to the enamel cap (o). The
spinous point gradually forces its way through the epidermis, so as to
project freely at the surface.
The scales of other forms of fishes are to be derived from those of
Elasmobranchii. The great dermal plates of many fishes have been
formed by the concrescence of groups of such scales. The dentine in
many cases partially or completely atrophies, leaving the major part
of the scale formed of osseous tissue; such plates often become parts
of the internal skeleton.
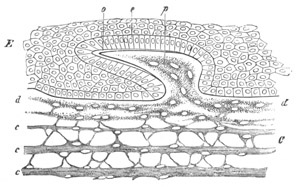
Fig. 235. Vertical section through the skin of an embryonic Shark,
to shew a developing placoid scale. (From Gegenbaur; after O.
Hertwig.)
E. epidermis; C. layers of dermis; d. uppermost layer of
dermis; p. papilla of dermis; e. mucous layer of epidermis; o.
enamel layer.
[Pg 396]
The teeth, as will be more particularly described in the section on
the alimentary tract, are formed by a modification of the same process
as the placoid scales, in which a ridge of the epithelium grows
inwards to meet a connective tissue papilla, so that the development
of the teeth takes place entirely below the superficial layer of
epidermis.
In most Teleostei the enamel and dentine layers have disappeared, and
the scales are entirely formed of a peculiar calcified tissue
developed in the dermis.
The cuticle covering the scales of Reptiles is the simplest type of
protective structure formed on the outer surface of the epidermis. The
scales consist of papillæ of the dermis and epidermis; and are covered
by a thickened portion of a two-layered cuticle, formed over the whole
surface of the body from a cornification of the superficial part of
the epidermis. Dermal osseous plates may be formed in connection with
these scales, but are never of course united with the superficial
cuticle.
Feathers are probably special modifications of such scales. They arise
from an induration of the epidermis of papillæ containing a vascular
core. The provisional down, usually present at the time of hatching,
is formed by the cornification of longitudinal ridges of the mucous
layer of the epidermis of the papillæ; each cornified ridge giving
rise to a barb of the feather. The horny layer of the epidermis forms
a provisional sheath for the developing feather below. When the barbs
are fully formed this sheath is thrown off, the vascular core dries
up, and the barbs become free except at their base.
Without entering into the somewhat complicated details of the
formation of the permanent feathers, it may be mentioned that the
calamus or quill is formed by a cornification in the form of a tube of
both layers of the epidermis at the base of the papilla. The quill is
open at both ends, and to it is attached the vexillum or plume of the
feather. In a typical feather this is formed at the apex of the
papilla from ridge-like thickenings of the mucous layer of the
epidermis, arranged in the form of a longitudinal axis, continuous
with the cornified mucous layer of the quill, and from lateral ridges.
These subsequently become converted into the axis and barbs of the
plume. The external epidermic layer becomes converted into a
provisional horny sheath for the true feather beneath.
On the completion of the plume of the feather the external sheath is
thrown off, leaving it quite free, and the vascular core belonging to
it shrivels up. The papilla in which the feather is formed becomes at
a very early period secondarily enveloped in a pit or follicle which
gradually deepens as the development of the feather is continued.
Hairs (Kölliker, No. 298) are formed in solid processes of the mucous
layer of the epidermis, which project into
[Pg 397] the subjacent dermis. The
hair itself arises from a cornification of the cells of the axis of
one of the above processes; and is invested by a sheath similarly
formed from the more superficial epidermic cells. A small papilla of
the dermis grows into the inner end of the epidermic process when the
hair is first formed. The first trace of the hair appears close to
this papilla, but soon increases in length, and when the end of the
hair projects from the surface, the original solid process of the
epidermis becomes converted into an open pit, the lumen of which is
filled by the root of the hair. Hairs differ in their mode of
formation from scales in a manner analogous to that in which the teeth
differ from ordinary placoid scales; i.e. they are formed in
inwardly directed projections of the epidermis instead of upon free
papillæ at the surface.
Nails (Kölliker, No. 298) are developed on special regions of the
epidermis, known as the primitive nail beds. They are formed by the
cornification of a layer of cells which makes its appearance between
the horny and mucous layers of the epidermis. The distal border of the
nail soon becomes free, and the further growth is effected by
additions to the under side and attached extremity of the nail.
Although the nail at first arises in the interior of the epidermis,
yet its position on the outer side of the mucous layer clearly
indicates with which group of epidermic structures it should be
classified.
Dermal skeletal structures. We have seen that in the Chordata skeletal
structures, which were primitively formed of both an epidermic and
dermic element, may lose the former element and be entirely developed
in the dermis. Amongst the Invertebrata there are certain dermal
skeletal structures which are evolved wholly independently of the
epidermis. The most important of these structures are the skeletal
plates of the Echinodermata.
Glands. The secretory part of the various glandular structures
belonging to the skin is invariably formed from the epidermis. In
Mammalia it appears that these glands are always formed as solid
ingrowths of the mucous layer (Kölliker, No. 298). The ends of these
ingrowths dilate to form the true glandular part of the organs, while
the stalks connecting the glandular portions with the surface form the
ducts. In the case of the sweat-glands the lumen of the duct becomes
first established. Its formation is inaugurated by the appearance of
[Pg 398]
the cuticle, and appears first at the inner end of the duct and thence
extends outwards (Ranvier, No. 311). In the sebaceous glands the first
secretion is formed by a fatty modification of the whole of the
central cells of the gland.
The muscular layer of the secreting part of the sweat-glands is
formed, according to Ranvier (No. 311), from a modification of the
deeper layer of the epidermic cells.
The Mammary Glands arise in essentially the same manner as the other
glands of the skin[151].
The glands of each side are formed as a solid
bud of the mucous layer of the epidermis. From this bud processes
sprout out, each of which gives rise to one of the numerous glands of
which the whole organ is formed. Two very distinct types in the
relation of the ducts of the glands to the nipple are found
(Gegenbaur, No. 313).
Bibliography of Epidermis.
General.
(304) T. H. Huxley. “Tegumentary organs.” Todd’s Cyclopædia of Anat.
and Physiol.
(305) P. Z. Unna. “Histol. u. Entwick. d. Oberhaut.” Archiv f. mikr.
Anat. Vol. XV. 1876. Vide also Kölliker (No. 298).
Scales of the Pisces.
(306) O. Hertwig. “Ueber Bau u. Entwicklung d. Placoidschuppen u. d.
Zähne d. Selachier.” Jenaische Zeitschrift, Vol. VIII. 1874.
(307) O. Hertwig. “Ueber d. Hautskelet d. Fische.” Morphol.
Jahrbuch, Vol. II. 1876. (Siluroiden u. Acipenseridæ.)
(308) O. Hertwig. “Ueber d. Hautskelet d. Fische (Lepidosteus u.
Polypterus).” Morph. Jahrbuch, Vol. V. 1879.
Feathers.
(309) Th. Studer. Die Entwick. d. Federn. Inaug. Diss. Bern, 1873.
(310) Th. Studer. “Beiträge z. Entwick. d. Feder.” Zeit. f. wiss.
Zool., Vol. XXX. 1878.
Sweat-glands.
(311) M. S. Ranvier. “Sur la structure des glandes sudoripares.”
Comptes Rendus, Dec. 29, 1879.
[Pg 399]Mammary glands.
(312) C. Creighton. “On the development of the Mamma and the Mammary
function.” Jour. of Anat. and Phys., Vol. XI. 1877.
(313) C. Gegenbaur. “Bemerkungen üb. d. Milchdrüsen-Papillen d.
Säugethiere.” Jenaische Zeit., Vol. VII. 1873.
(314) M. Huss. “Beitr. z. Entwick. d. Milchdrüsen b. Menschen u. b.
Wiederkäuern.” Jenaische Zeit., Vol. VII. 1873.
(315) C. Langer. “Ueber d. Bau u. d. Entwicklung d. Milchdrüsen.”
Denk. d. k. Akad. Wiss. Wien, Vol. III. 1851.
[Pg 400]
CHAPTER XV.
NERVOUS SYSTEM.
Origin of the Nervous System.
One of the most important recent embryological discoveries is the fact
that the central nervous system, in all the Metazoa in which it is
fully established, is (with a few doubtful exceptions) derived from
the primitive epiblast[152].
As we have already seen that the epiblast
represents to a large extent the primitive epidermis, the fact of the
nervous system being derived from the epiblast implies that the
functions of the central nervous system, which were originally taken
by the whole skin, became gradually concentrated in a special part of
the skin which was step by step removed from the surface, and has
finally become in the higher types a well-defined organ imbedded in
the subdermal tissues.
Before considering in detail the comparative development of the
nervous system, it will be convenient shortly to review the present
state of our knowledge on the general process of its evolution.
This process may be studied either embryologically, or by a comparison
of the various stages in its evolution preserved in living forms. Both
the methods have led to important results.
[Pg 401]
The embryological evidence shews that the ganglion-cells of the
central part of the nervous system are originally derived from the
simple undifferentiated epithelial cells of the surface of the body,
while the central nervous system itself has arisen from the
concentration of such cells in special tracts. In the Chordata at any
rate the nerves arise as outgrowths of the central organ.
Another important fact shewn by embryology is that the central nervous
system, and percipient portions of the organs of special sense,
especially of optic organs, are often formed from the same part of the
primitive epidermis. Thus the retina of the Vertebrate eye is formed
from the two lateral lobes of the primitive fore-brain.
The same is true for the compound eyes of some Crustacea. The
supraœsophageal ganglia of these animals are formed in the embryo
from two thickened patches of the epiblast of the procephalic lobes.
These thickened patches become gradually detached from the surface,
remaining covered by a layer of epidermis. They then constitute the
supraœsophageal ganglia; but they form not only the ganglia, but also
the retinulæ of the eye—the parts in fact which correspond to the
rods and cones in our own retina. The accessory parts of these organs
of special sense, viz. the crystalline lens of the Vertebrate eye, and
the corneal lenses and crystalline cones of the Crustacean eye, are
independently formed from the epiblast after the separation of the
part which becomes the central nervous system.
In the Acraspedote Medusæ the rudimentary central nervous system has
the form of isolated rings, composed of sense-cells prolonged into
nervous fibres, surrounding the stalks of tentacle-like organs, at the
ends of which are placed the sense-organs.
This close connection between certain organs of special sense and
ganglia is probably to be explained by supposing that the two sets of
structures actually originated pari passu.
We may picture the process as being somewhat as follows:—
It is probable that in simple ancestral organisms the whole body was
sensitive to light, but that with the appearance of pigment-cells in
certain parts of the body, the sensitiveness to light became
localised to the areas where the pigment-cells were present. Since,
however, it was necessary that stimuli received by such organs
should be communicated to other parts
[Pg 402] of the body, some of the
epidermic cells in the neighbourhood of the pigment-spots, which
were at first only sensitive in the same manner as other cells of
the epidermis, became gradually differentiated into special
nerve-cells. As to the details of this differentiation embryology
does not as yet throw any great light; but from the study of
comparative anatomy there are grounds for thinking that it was
somewhat as follows:—Cells placed on the surface sent protoplasmic
processes of a nervous nature inwards, which came into connection
with nervous processes from similar cells placed in other parts of
the body. The cells with such processes then became removed from the
surface, forming a deeper layer of the epidermis below the sensitive
cells of the organ of vision. With the latter cells they remained
connected by protoplasmic filaments, and thus they came to form a
thickening of the epidermis underneath the organ of vision, the
cells of which received their stimuli from those of the organ of
vision, and transmitted the stimuli so received to other parts of
the body. Such a thickening would obviously be the rudiment of a
central nervous system, and is in fact very similar to the
rudimentary ganglia of the Acraspeda mentioned above. It is easy to
see by what steps it might become larger and more important, and
might gradually travel inwards, remaining connected with the
sense-organ at the surface by protoplasmic filaments, which would
then constitute nerves. The rudimentary eye would at first merely
consist of cells sensitive to light, and of ganglion-cells connected
with them; while at a later period optical structures, constituting
a lens capable of throwing an image of external objects upon it,
would be developed, and so convert the whole structure into a true
organ of vision. It has thus come about that, in the development of
the individual, the retina is often first formed in connection with
the central nervous system, while the lenses of the eye are
independently evolved from the epidermis at a later period.
A series of forms of the Cœlenterata and Platyelminthes affords us
examples of various stages in the differentiation of a central nervous
system[153].
In sea-anemones (Hertwigs, No. 321) there are, for instance, no organs
of special sense, and no definite central nervous system. There are,
however, scattered throughout the skin, and also throughout the lining
of the digestive tract, a number of specially modified epithelial
cells, which are no doubt delicate organs of sense. They are provided
at their free extremity with a long hair, and are prolonged on their
inner side into fine processes which penetrate into the deeper part of
the epithelial layer of the skin or digestive wall. They eventually
join a fine network of protoplasmic fibres which forms a special layer
immediately within the epithelium. The fibres of this network are no
doubt essentially nervous. In addition to fibres there are,
[Pg 403] moreover,
present in the network cells of the same character as the multipolar
ganglion-cells in the nervous system of Vertebrates, and some of these
cells are characterised by sending a process into the superjacent
epithelium. Such cells are obviously intermediate between
neuro-epithelial cells and ganglion-cells; and it is probable that the
nerve-cells are, in fact, sense-cells which have travelled inwards and
lost their epithelial character.
Fig. 236. Neuro-epithelial sense-cells of Aurelia aurita. (From
Lankester; after Schäfer.)
In the Craspedote Medusæ (Hertwigs, No. 320) the differentiation of
the nervous system is carried somewhat further. There is here a
definite double ring, placed at the insertion of the velum, and
usually connected with sense-organs. The two parts of the ring belong
respectively to the epithelial layers on the upper and lower surfaces
of the velum, and are not separated from these layers; they are formed
of fine nerve-fibres and ganglion-cells. The epithelium above the
nerve rings contains sense-cells (fig. 237) with a stiff hair at their
free extremity, and a nervous prolongation at the opposite end, which
joins the nerve-fibres of the ring. Between such cells and true
ganglion-cells an intermediate type of cell has been found (fig. 237
B) which sends a process upwards amongst the epithelial cells, but
does not reach the surface. Such cells, as the Hertwigs have pointed
out, are clearly sense-cells partially transformed into
ganglion-cells.
A still higher type of nervous system has been met with amongst some
primitive Nemertines (Hubrecht, No. 323), consisting of a pair of
large cephalic ganglia, and two well-developed lateral ganglionic
cords placed close beneath the epidermis. These cords, instead of
giving off definite nerves, as in animals with a fully differentiated
nervous system, are connected with a continuous subdermal nervous
plexus.
The features of the embryology and the anatomy of the nervous system,
to which attention has just been called, point to the following
general conclusions as to the evolution of the nervous system.
(1) The nervous system of the higher Metazoa appears to have been
evolved in the course of a long series of generations from a
differentiation of some of the superficial epithelial cells of the
body, though it is possible that some parts of the system may have
been formed by a differentiation of the alimentary epithelium.
(2) An early feature in the differentiation consisted in the growth of
a series of delicate processes of the inner ends of
[Pg 404] certain epithelial
cells, which became at the same time especially differentiated as
sense-cells (figs. 236 and 237).
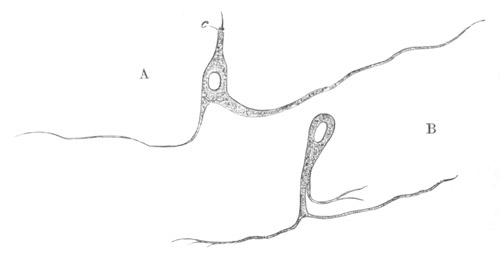
Fig. 237. Isolated cells belonging to the upper nerve-ring of Carmarina
hastata. (After O. and R. Hertwig.)
A. Neuro-epithelial sense-cell. c. sense-hair.
B. Transitional cell between a neuro-epithelial cell and a
ganglion-cell.
(3) These processes gave rise to a subepithelial nervous
plexus, in which ganglion-cells, formed from sense-cells which
travelled inwards and lost their epithelial character (fig. 237 B),
soon formed an important part.
(4) Local differentiations of the nervous network, which was
no doubt distributed over the whole body, took place partly in
the formation of organs of special sense, and partly in other
ways, and such differentiations gave rise to a central nervous
system. The central nervous system was at first continuous
with the epidermis, but became separated from it and travelled
inwards.
(5) Nerves, such as we find them in the higher types,
originated from special differentiations of the nervous network,
radiating from the parts of the central nervous system.
The following points amongst others are still very obscure:—
(1) The steps by which the protoplasmic processes from the primitive
epidermic cells became united together so as to form a network
of nerve-fibres, placing the various parts of the body in
nervous communication.
(2) The process by which nerves became connected with muscles, so
that a stimulus received by a nerve-cell could be communicated
to and cause a contraction in a muscle.
It is probable, as stated in the above summary, that the nervous
network
[Pg 405] took its origin from processes of the sense-cells. The
processes of the different cells probably first met and then fused
together, and, becoming more arborescent, finally gave rise to a
complicated network.
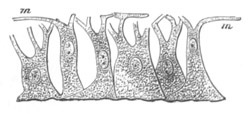
Fig. 238. Myo-epithelial cells of Hydra. (From Gegenbaur; after
Kleinenberg.)
m. contractile fibres; processes of cells.
The primitive relations between the nervous network and the muscular
system are matters of pure speculation. The primitive muscular cells
consist of epithelial cells with muscular processes (fig. 238), but
the branches of the nervous network have not been traced into
connection with the muscles in any Cœlenterata except Ctenophora. In
the higher types a continuity between nerves and muscles in the form
of motorial end plates has been widely observed. Even in the case of
the Cœlenterata it is quite clear from Romanes’ experiments that
stimuli received by the nerves are capable of being transmitted to the
muscles, and that there must therefore be some connection between
nerves and muscles. How did this connection originate?
Epithelial cells with muscular processes (fig. 238) were discovered by
Kleinenberg (No. 324) in Hydra before epithelial cells with nervous
processes were known, and Kleinenberg pointed out that Hydra shewed
the possibility of nervous and muscular tissues existing without a
central nervous system, and suggested that the epithelial part of the
myoepithelial cells was a sense-organ, and that the connecting part
between this and the contractile processes was a rudimentary nerve. He
further supposed that in the subsequent evolution of these elements
the epithelial part of the cell became a ganglion-cell, while the part
connecting this with the muscular tail became prolonged so as to form
a true nerve. The discovery of neuro-epithelial cells existing side by
side with myoepithelial cells demonstrates that this theory must in
part be abandoned, and that some other explanation must be given of
the continuity between nerves and muscles. The hypothetical
explanation which most obviously suggests itself is that of fusion.
It seems quite possible that many of the epithelial cells of the
epidermis and walls of the alimentary tract were originally provided
with processes, the protoplasm of which, like that of the Protozoa,
carried on the functions of nerves and muscles at the same time, and
that these processes united amongst themselves into a network. Such
cells would be very similar to Kleinenberg’s neuro-muscular cells. By
a subsequent differentiation some of the cells forming this network
may have become specially contractile, the epithelial parts of the
cells ceasing to have a nervous function, and other cells may have
lost their contractility and become solely nervous. In this way we
should get neuro-epithelial cells and myoepithelial cells both
differentiated from the primitive network, and the connection between
the two would also be explained. This hypothesis fits in moreover very
well with the condition of the neuro-muscular system as we find it in
the Cœlenterata.
[Pg 406]
Bibliography.
Origin of the Nervous System.
(316) F. M. Balfour. “Address to the Department of Anat. and Physiol.
of the British Association.” 1880.
(317) C. Claus. “Studien üb. Polypen u. Quallen d. Adria. 1.
Acalephen, Discomedusen,” Denk. d. math.-naturwiss. Classe d. k.
Akad. Wiss. Wien, Vol. XXXVIII. 1877.
(318) Th. Eimer. Zoologische Studien a. Capri. 1. Ueber Beroë ovatus.
Ein Beitrag z. Anat. d. Rippenquallen. Leipzig, 1873.
(319) V. Hensen. “Zur Entwicklung d. Nervensystems.” Virchow’s
Archiv, Vol. XXX. 1864.
(320) O. and R. Hertwig. Das Nervensystem u. d. Sinnesorgane d.
Medusen. Leipzig, 1878.
(321) O. and R. Hertwig. “Die Actinien anat. u. histol. mit besond.
Berücksichtigung d. Nervenmuskelsystem untersucht.” Jenaische Zeit.,
Vol. XIII. 1879.
(322) R. Hertwig. “Ueb. d. Bau d. Ctenophoren.” Jenaische
Zeitschrift, Vol. XIV. 1880.
(323) A. W. Hubrecht. “The Peripheral Nervous System in Palæo- and
Schizonemertini, one of the layers of the body-wall.” Quart. J. of
Micr. Science, Vol. XX. 1880.
(324) N. Kleinenberg. Hydra, eine
anatomisch-entwicklungsgeschichtliche Untersuchung. Leipzig, 1872.
(325) A. Kowalevsky. “Embryologische Studien an Würmern u.
Arthropoden.” Mém. Acad. Pétersbourg, Series VII., Vol. XVI. 1871.
(326) E. A. Schäfer. “Observations on the nervous system of Aurelia
aurita.” Phil. Trans. 1878.
Nervous system of the Invertebrata. Our knowledge of the development
of the central nervous system is still very imperfect in the case of
many Invertebrate groups. In the Echinodermata and some of the
Chætopoda it is never detached from the epidermis, and in such cases
its origin is clear without embryological evidence.
In the majority of groups the central nervous system may be reduced to
the type of a pair of cephalic ganglia, continued posteriorly into two
cords provided with nerve-cells, which may coalesce ventrally or be
more or less widely separated, and be unsegmented or segmented.
Various additional visceral ganglia may be added, and in different
instances parts of the system may be much reduced, or peculiarly
modified. The nervous system of the Platyelminthes (when present), of
the
[Pg 407] Rotifera, Brachiopoda, Polyzoa (?), the Mollusca, the Chætopoda,
the Discophora, the Gephyrea, the Tracheata, and the Crustacea, the
various small Arthropodan phyla (Pœcilopoda, Pycnognida, Tardigrada,
&c.), the Chætognatha (?), and the Myzostomea, probably belongs to
this type.
The nervous system of the Echinodermata cannot be reduced to this
form; nor in the present state of our knowledge can that of the
Nematelminthes or Enteropneusta.
It is only in the case of members of the former set of groups that any
adequate observations have yet been made on the development of the
nervous system, and even in the case of these groups observations
which have any claim to completeness are confined to certain members
of the Chætopoda, the Arthropoda and the Mollusca. An account of
imperfect observations on other forms, where such have been made, will
be found in the systematic part of this work.
Chætopoda. We are indebted to Kleinenberg (No. 329) for the most
detailed account which we have of the development of the central
nervous system in the Chætopoda.
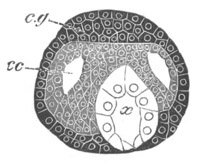
Fig. 239. Section through the head of a young embryo of Lumbricus
trapezoides. (After Kleinenberg.)
c.g. cephalic ganglion; cc. cephalic portion of the body cavity;
x. œsophagus.
The supraœsophageal ganglion with the œsophageal commissure
developes independently of the ventral cord. It arises as an unpaired
thickening of the epiblast, close to the dorsal side of the œsophagus
at the front end of the head (fig. 239), which becomes separated from
the epiblast, and extends obliquely backwards and downwards in a
somewhat arched form; its lower extremities being somewhat swollen.
The inner portion of this curved rudiment becomes converted into
commissural nerve-fibres, while the cells of the outer and upper
portion assume the characters of ganglion-cells. The commissural
fibres are continued downwards to meet the ventral chord, but their
junction with the latter structure is not effected till late in
embryonic life.
The ventral cord is formed by the coalescence of a pair of linear
cords, the development of which takes place from before backwards, so
that when their anterior part is well developed their posterior part
is hardly differentiated. These cords arise, one on
[Pg 408] each side of a
ventral ciliated furrow, first as a single row of epiblast cells, and
subsequently as several rows (fig. 240, Vg). While still united to
the external epiblast, they extend themselves below the cells lining
the ventral furrow, and unite into a single nervous band, which
however exhibits its double origin by its bilobed section. Before the
two cords unite, the groove between them becomes somewhat deep, but
subsequently shallows out and disappears. The nervous band, before
separating from the epiblast, exhibits, in correspondence with the
mesoblastic segments, alternate swellings and constrictions. The
former become the ganglia, and the latter the connecting trunks.
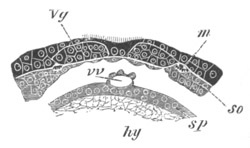
Fig. 240. Section through part of the ventral wall of the trunk of
an embryo of Lumbricus trapezoides. (After Kleinenberg.)
m. longitudinal muscles; so. somatic mesoblast; sp. splanchnic
mesoblast; hy. hypoblast; Vg. ventral nerve-cord; vv. ventral
vessel.
As soon as the cord becomes free from the epiblast, it becomes
surrounded by a sheath, formed of somatic mesoblast. In each of the
ganglionic enlargements there next appears on the dorsal surface a
pair of areas of punctiform material, the substance of which soon
differentiates itself into nerve-fibres. These areas, by uniting from
side to side, give rise to the transverse commissures, and also by a
linear coalescence to the longitudinal commissures of the cord. The
cellular parts of the band surrounding them become converted into a
ganglionic covering of the cord.
In each ganglion the cells of this ganglionic investment penetrate as
a median septum into the cord. A fissure is next formed, dividing this
septum into two; it is subsequently continued for the whole length of
the cord.
Arthropoda. In the Tracheata and the Crustacea the development of the
ventral cord is in the main similar to that in the Chætopods, while
that of the supraœsophageal ganglia is as a rule somewhat more
complicated. No such clear evidence of an independent development of
these two parts, as in the case of the Chætopods, has as yet been
produced.
The most primitive type of nervous system amongst the
[Pg 409] Tracheata is
that of Peripatus, where it consists of large supraœsophageal
ganglia, continuous with a pair of widely separated but large ventral
cords united posteriorly above the anus. These cords have an
investment of ganglion-cells for their whole length, and are
imperfectly divided into ganglia corresponding in number with the
feet.
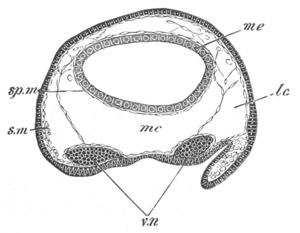
Fig. 241. Section through the trunk of an embryo of
Peripatus. The embryo from which the section is taken was
somewhat younger than that of fig. 242.
sp.m. splanchnic mesoblast; s.m. somatic mesoblast; mc. median
section of body cavity; lc. lateral section of body cavity; v.n.
ventral nerve cord; me. mesenteron.
The ventral cords are formed as two separate epiblastic ridges (fig.
241, v.n), continued in front into a pair of thickenings of the
procephalic lobes, which are at first independent of each other, and
from which a large part of the supraœsophageal ganglia takes its
origin. After the latter have become separated from the epiblast an
invagination of the epiblast covering them grows into each lobe (fig.
242), and becoming constricted from the superficial epiblast, which
remains as the epidermis, forms a not unimportant part of the
permanent supraœsophageal ganglia.
In the Arachnida the mode of development of the nervous system is
essentially the same, and the reader will find a detailed account of
it for Spiders in Vol. II. pp. 447-451. The ventral cords are here
formed as independent and at first widely separated strands (fig. 243,
vn), which for a long time remain far apart; they are subsequently
divided into ganglia and become united by transverse commissures.
The supraœsophageal ganglia are formed as two independent
[Pg 410] thickenings
of the procephalic lobes (fig. 244), which eventually separate from
the superficial skin. There is formed however in each of them a
semicircular groove (fig. 244, gr) lined by the superficial
epiblast, which becomes detached from the skin, and is involuted to
form part of the ganglia.
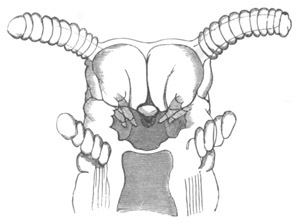
Fig. 242. Head of an embryo Peripatus. (From Moseley.)
The figure shews the jaws (mandibles), and close to them epiblastic
involutions, which grow into the supraœsophageal ganglia. The
antennæ, oral cavity, and oral papillæ are also shewn.
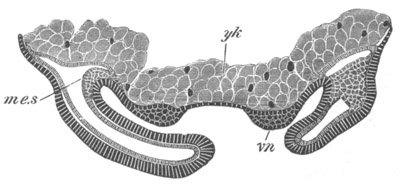
Fig. 243. Transverse section through the Ventral plate of Agelena
labyrinthica.
The ventral cords have begun to be formed as thickenings of the
epiblast, and the limbs are established.
me.s. mesoblastic somite; vn. ventral nerve-cord; yk. yolk.
A similar mode of formation of both the ventral cords and the
supraœsophageal ganglia obtains in Insects (fig. 245). The ventral
cords are however much less widely separated than in Spiders, and
early unite in the median line. In the supraœsophageal ganglia the
invaginated epiblast has in Lepidoptera (Hatschek) the form of a pit
on the dorsal border of the antennæ.
[Pg 411]
Hatschek states that there takes place an invagination of a median
part of the skin between the two ventral cords, for the details of
which I must refer the reader to Vol. II. p. 410. He has made more or
less similar statements for the earthworm, but his observations in
both instances are open to serious doubt.
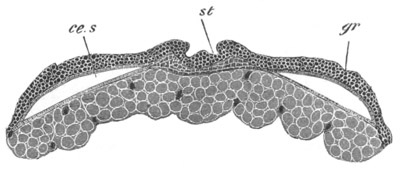
Fig. 244. Section through the procephalic lobes of an embryo of
Agelena labyrinthica.
st. stomadæum; gr. section through semicircular groove in
procephalic lobe; ce.s. cephalic section of body cavity.
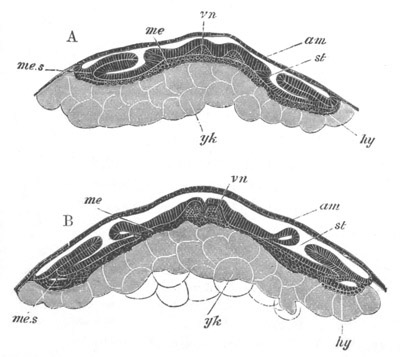
Fig. 245. Two transverse sections through the embryo of Hydrophilus. (After Kowalevsky.)
A. Transverse section through an embryo in the region of one of the
stigmata.
B. Transverse section through an older embryo.
vn. ventral nerve-cord; am. amnion and serous membrane; me.
mesoblast; me.s. somatic mesoblast; hy. hypoblast (?); yk.
yolk-cells (true hypoblast); st. stigma of trachea.
Full details as to the development of the nervous system in the
Crustacea are still wanting; a fairly complete account of
[Pg 412] what is
known on the subject is given in Vol. II. pp. 521-2. It appears that
the ventral cord may either arise as an unpaired thickening of the
epiblast (Isopoda), marked however by a shallow median furrow, or from
two cords which eventually coalesce[154].
It is not certain how far
the supraœsophageal ganglia are usually in the first instance
continuous with the ventral cord. In Astacus, the early stages of
which have been elaborately investigated by Reichenbach (No. 331),
they are stated to be so; the supraœsophageal ganglia are moreover
described by this author as having a somewhat complicated origin. Five
elements enter into their composition. There is first formed a pair of
pits on the procephalic lobes, which become very deep during the
Nauplius stage, and are continuous with a pair of epiblastic ridges
which pass round the mouth, and join the ventral cords just described.
The walls of the pits are believed to form a part of the embryonic
ganglia which gives rise to the retina as well as to the optic
ganglia. The ridges form the remainder of the ganglia and the
œsophageal commissures; while the fifth element is supplied by a
median invagination in front of the mouth, which appears at a much
later date than the other parts.
In the Isopoda supraœsophageal ganglia are stated to arise as
thickenings of the procephalic lobes, which become eventually detached
from the epidermis.
The ventral cord is at first unsegmented, but soon becomes partially
divided by a series of constrictions into a number of ganglia,
corresponding with the segments. The development of the commissural
and ganglionic portions takes place much as in the Chætopoda.
The Gephyrea approach closely the types so far dealt with, but the
ventral cord in the Inermia is formed as an unpaired thickening of the
epiblast. In Echiurus, as has been shewn by Hatschek in an interesting
paper on the larva of this species, published since the appearance of
the first volume, there is a pair of ventral cords[155].
In
correspondence with a general segmentation of the body, which is
subsequently lost, these cords become
[Pg 413] segmented. The two cords unite
in the median line, and Hatschek, in accordance with his general view
on this subject, states that their junction is effected by means of a
median cord of invaginated epiblast. The segmentation of the cords
subsequently becomes lost. The supraœsophageal ganglia arise as an
unpaired median thickening of the procephalic lobe. No traces of
segmentation in the ventral cord have been observed by Spengel in
Bonellia, and the supraœsophageal ganglion is formed in this genus as
an unpaired band.
In all the groups above considered the nervous system clearly presents
the same type of development with various modifications.
It is formed of two parts, viz. (1) the supraœsophageal ganglia, and
(2) the ventral cord.
In the simpler forms, Chætopoda and Gephyrea, the supraœsophageal
ganglia are usually stated to be formed as an unpaired thickening at
the apex of the præoral lobe, which in most cases becomes subsequently
bilobed.
In the Arthropoda the unpaired præoral lobe of the Chætopoda is
replaced by the so-called procephalic lobes, which are themselves
bilobed; and the supraœsophageal ganglia are formed of two
independent halves; further complications in development are also
generally found.
There is not as yet sufficient evidence to decide whether the
supraœsophageal ganglia were primitively developed continuously with,
or independently of, the ventral cords.
The ventral cord appears in the embryo as two independent unsegmented
strands, although in a few cases (some Crustacea and Gephyrea) these
cords, by an abbreviation in development, arise as an unpaired median
thickening of the epiblast.
The form of nervous system of the Chætopoda, Arthropoda, and Gephyrea
is clearly therefore to be derived, as was first pointed out by
Gegenbaur, from a more or less similar type to that now found in the
Nemertines; and as suggested in the chapter on larval forms (vide p.
378) may perhaps be derived from the elongation of a circular ring, of
which the anterior end has become developed into the supraœsophageal
ganglia, the lateral parts into the two lateral strands, while the
posterior part persists in some forms in the junction of the ventral
cords above the anus (Enopla and Peripatus).
[Pg 414]
Mollusca. While study of the anatomy of the nervous system of the
Mollusca, especially of certain primitive genera (Chiton, Haliotis,
Fissurella, &c.) leaves little doubt that it is formed on the same
type as that of the groups just spoken of, the development, so far as
our imperfect knowledge enables us to make definite statements on the
subject, is somewhat abnormal[156].
In the Gasteropoda and Pteropoda the supraœsophageal ganglia appear
most probably to be developed either as paired thickenings of the
epiblast of the velar area, or as invaginated pits of the velar area,
which become detached from the surface, and then become solid
(Hyaleacea and Limax). In either case the supraœsophageal ganglia
appear to be developed quite independently of the pedal ganglia. The
latter, as might be anticipated, are earlier in their development and
more constant than the various visceral ganglia; and, if the views
above expressed are correct, are homologous with the ventral cord of
the Chætopods and Arthropods. Their actual development is very
imperfectly known.
The most precise statements on the subject, viz. those of Bobretzky
and Fol, would lead us to suppose that they arise in the mesoblast,
but it seems more probable that they are formed as thickenings of the
sides of the foot.
In the Cephalopods all the ganglia are stated to be differentiated in
the mesoblast (Lankester, Bobretzky).
Hatschek[157]
has recently given a detailed description of the
development of the supraœsophageal and pedal ganglia of Teredo. He
finds that the former ganglia arise as an unpaired thickening of the
epiblast in the centre of the velar area, and the latter as an
unpaired thickening of the epiblast of the ventral side of the body
between the mouth and the anus. The two ganglia would thus seem to be
disconnected with each other in their development.
(327) F. M. Balfour. “Notes on the development of the Araneina.”
Quart. J. of Micr. Science, Vol. XX. 1880.
(328) B. Hatschek. “Beitr. z. Entwicklung d. Lepidopteren.” Jenaische
Zeitschrift, Vol. XI. 1877.
(329) N. Kleinenberg. “The development of the Earthworm, Lumbricus
Trapezoides.” Quart. J. of Micr. Science, Vol. XIX. 1879.
(330) A. Kowalevsky. “Embryologische Studien an Würmern u.
Arthropoden.” Mém. Acad. Pétersbourg, Series VIII., Vol. XVI. 1871.
(331) H. Reichenbach. “Die Embryonalanlage u. erste Entwick. d.
Flusskrebses.” Zeit. f. wiss. Zool., Vol. XXIX. 1877.
[Pg 415]
The Central Nervous System of the Vertebrata[158].
The formation of the cerebrospinal axis of the Chordata from the
medullary plate has already been treated at length (pp. 301-304).
Before entering into the consideration of the morphological value of
the various parts of this cord, it will be convenient to describe the
more important features of its ontogeny. For this purpose the two
parts into which the nervous axis becomes at an early period divided,
viz. the spinal cord and the brain, may be dealt with separately.
The Spinal Cord, shortly after the closure of the medullary canal,
has, in all the true Vertebrata, the form of an oval tube; the walls
of which are of a fairly uniform thickness, and are composed of
several rows of elongated cells. This cord, as development proceeds,
usually becomes vertically prolonged in transverse section, and the
central canal which it contains also becomes vertically elongated. The
variations in shape of the spinal canal are very great at different
periods and in different parts of the body, and an attempt to
chronicle them would appear, in the present state of our knowledge, to
be quite valueless[159].
fig. 117, in which the spinal cord of the
chick of the third day is shewn in transverse section, illustrates the
character of the cord at the stage just described. Up to this time the
walls of the spinal canal have exhibited an uniform structure. A
series of changes now however takes place, which results in the
differentiation (1) of the epithelium of the central canal, (2) of the
grey matter of the cord, and (3) of the external coating of white
matter.
The relative time at which each of these parts becomes developed is
not constant in the different forms.
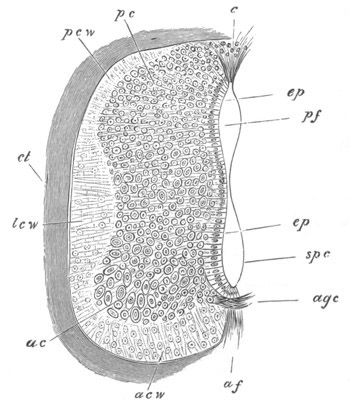
Fig. 246. Section through the spinal cord of a seven days’ Chick.
pcw. dorsal white column; lcw. lateral white column; acw.
ventral white column; c. dorsal tissue filling up the part where
the dorsal fissure will be formed; pc. dorsal grey cornu; ac.
anterior grey cornu; ep. epithelial cells; agc. anterior
commissure; pf. dorsal part of spinal canal; spc. ventral part
of spinal canal; af. anterior fissure.
The white matter is apparently the result of a differentiation of the
outermost parts of the superficial cells of the cord into
[Pg 416] longitudinal
nerve-fibres, which remain for a long period without a medullary
sheath. These fibres appear in transverse sections as small dots. The
white matter forms a transparent investment of the grey matter and
would seem to contain neither nuclei nor cells[160].
The white matter
may from the first form only two masses, one on each side, forming a
layer on the ventral and lateral parts of the spinal cord but not
extending to the dorsal surface (Elasmobranchii, fig. 185, W); or it
may form four patches, viz. an anterior and a posterior white column
on each side, which lie on a level with the origin of the anterior and
posterior nerve-roots (the Fowl, Human embryo, etc.). In whichever of
these forms the white matter appears, it is always, at first, a layer
of extreme tenuity, which rapidly increases
[Pg 417] in thickness in the
subsequent stages, and extends so as gradually to cover the whole cord
(fig. 246).
The anterior white commissure is formed very shortly after the first
appearance of the white matter. The grey matter and the central
epithelium are formed by a differentiation of the main mass of the
spinal cord. The outer cells lose their epithelial-like arrangement,
and, becoming prolonged into fibres, give rise to the grey matter,
while the innermost cells retain their primitive arrangement, and
constitute the epithelium of the canal. The process of formation of
the grey matter would appear to proceed from without inwards, so that
some of the cells, which have, on the formation of the grey matter, an
epithelial-like arrangement, subsequently become converted into true
nerve-cells.
As has already been mentioned, the central epithelium of the nervous
system probably corresponds with the so-called epidermic layer of the
epiblast.
The grey matter soon becomes prolonged dorsally and ventrally into the
posterior and anterior horns. Its fibres may especially be traced in
two directions:—(1) round the anterior end of the spinal canal,
immediately outside its epithelium and so to the grey matter on the
opposite side, forming in this way an anterior grey commissure,
through which a decussation of the fibres from the opposite sides is
effected: (2) dorsalwards along the outside of the lateral walls of
the canal.
There is at this period no trace of the ventral or dorsal fissure, and
the shape of the central canal is not very different to what it was at
an earlier period. This condition of the spinal cord is especially
instructive, as it is very nearly that which is permanent in
Amphioxus.
The next event of importance is the formation of the ventral or
anterior fissure. This owes its origin to a downgrowth of the anterior
horns of the cord on each side of the middle line. The two downgrowths
enclose between them a somewhat linear space—the anterior
fissure—which increases in depth in the succeeding stages (fig. 246,
af).
The dorsal or posterior fissure is formed at a later period than the
anterior, and accompanies the atrophy of the dorsal section of the
embryonically large canal of the spinal cord.
[Pg 418]
The exact mode of its formation appears to me to be still involved in
some obscurity.
In the Elements of Embryology the development of the posterior
fissure was described in the following way:
“On the seventh day the most important event is the formation of the
posterior fissure.
“This is brought about by the absorption of the roof of the
posterior of the two parts into which the neural canal has become
divided.
“Between the posterior horns of the cord, the epithelium forming the
roof of the, so to speak, posterior canal is along the middle line
covered neither by grey nor by white matter, and on the seventh day
is partially absorbed, thus transforming the canal into a
wedge-shaped fissure, whose mouth however is seen in section to be
partially closed by a triangular clump of elongated cells (fig. 246,
c). Below this mass of cells the fissure is open. It is separated
from the ‘true spinal canal’ by a very narrow space along which the
side walls have coalesced. In the lumbar and sacral regions the two
still communicate.
“We thus find, as was first pointed out by Lockhart Clarke, that the
anterior and posterior fissures of the spinal cord are,
morphologically speaking, entirely different. The anterior fissure
is merely the space left between two lateral downward growths of the
cord, while the posterior fissure is part of the original neural
canal separated from the rest of the cavity (which goes to form the
true spinal canal) by a median coalescence of the side walls.”
I confess that I have some doubts as to the complete accuracy of the
above statement.
Kölliker gives a full account of the gradual atrophy of the central
canal; but I do not fully understand his statements with reference to
the formation of the posterior fissure, which in fact appears to be
only incidentally mentioned. It would seem from his account that a
shallow and somewhat wide dorsal fissure is formed to start with, in
the human embryo, by two projections of the posterior white horns. On
the atrophy of the central canal this furrow becomes narrowed, but
Kölliker does not definitely state how it becomes deepened so as to
give rise to the permanent dorsal fissure.
It seems to me probable, though further investigations on the point
are still required, that the dorsal fissure is a direct result of the
atrophy of the dorsal part of the central canal of the spinal cord.
The walls of the canal coalesce dorsally, and the coalescence
gradually extends ventralwards, so as finally to reduce the central
canal to a minute tube, formed of the ventral part of the original
canal. The epithelial wall formed by the coalesced walls on the dorsal
side of the canal is gradually absorbed.
The epithelium of the central canal, at the period when its
[Pg 419] atrophy
commences, is not covered dorsally either by grey or white matter, so
that, with the gradual reduction of the dorsal part of the canal, and
the absorption of the epithelial wall formed by the fusion of its two
sides, a fissure between the two halves of the spinal cord becomes
formed. This fissure is the posterior or dorsal fissure. In the
process of its formation the white matter of the dorsal horns becomes
prolonged so as to line its walls; and shortly after its formation the
dorsal grey commissure makes its appearance, which is not improbably
derived from part of the epithelium of the original central canal.
Development of the Brain.
The brain is formed from the anterior portion of the medullary plate.
When the medullary plate first becomes differentiated it is not
possible to distinguish between the region of the brain and that of
the spinal cord. The brain region is however usually very early
indicated by a widening of the medullary plate, but does not become
sharply marked off from the region of the spinal cord. In many
Ichthyopsida (Elasmobranchii (fig. 28, C) and Amphibia (fig. 77, A))
the anterior dilatation gives to the medullary plate, before its sides
meet to form a canal, a spatula-like form; which is either not present
or less marked in Reptilia, Aves and Mammalia.
The length of the brain as compared to the spinal cord is always very
great in the embryo, and in the earliest developmental periods the
disproportion in the size of the brain is specially marked, owing to
the full number of the somites of the trunk not having been formed. In
Elasmobranchii the brain is about one-third of the whole length of the
embryo at the stage immediately following the closure of the medullary
canal.
The first differentiation of the brain into distinct parts is a very
early occurrence, and may take place before (Mammalia) or during the
closure of the medullary folds. The brain first becomes divided into
two successive lobes or vesicles by a single transverse constriction,
and subsequently the posterior of these again becomes divided into
two, so that three lobes
[Pg 420] are formed—known as the fore- the mid- and
the hind-brain; of these the hind-brain is usually the longest. In
some instances a bilobed stage can hardly be recognised. This
primitive division of the brain is shewn in many of the figures
already given. The reader may perhaps best refer to fig. 108. On the
closure of the medullary groove the lumen of the medullary canal is
continued uninterruptedly through the brain, but dilates considerably
in each of the cerebral vesicles.
The anterior lobe of the brain becomes converted into the cerebral
hemispheres, the thalamencephalon, the primary optic vesicles, and the
parts connected with them. The middle lobe becomes the optic lobes
(corpora bigemina or corpora quadrigemina in Mammalia) and the crura
cerebri; while the posterior lobe becomes converted into the
cerebellum and medulla oblongata.
Before describing in detail the changes by which the primary vesicles
of the brain become converted into the above parts, it will be
convenient to say a few words about the general development of the
brain.
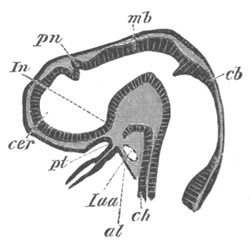
Fig. 247. Longitudinal section through the brain of a young
Pristiurus embryo.
cer. commencement of the cerebral hemisphere; pn. pineal gland;
In. infundibulum; pt. ingrowth from mouth to form the pituitary
body; mb. mid-brain; cb. cerebellum; ch. notochord; al.
alimentary tract; Iaa. artery of mandibular arch.
The most striking peculiarity with reference to the general
development of the brain is a curvature which appears in its axis,
known as the cranial flexure. The flexure takes place through the
mid-brain, and causes the fore-brain to be gradually bent downwards so
that the axis of its floor forms, first, a right angle with that of
the hinder part of the brain, and subsequently, as a rule, an acute
angle.
During these changes the brain, in most Amniota at any rate, becomes
in the first instance retort-shaped, the cerebral vesicle forming the
swollen part of the retort, but subsequently the retort-shape is lost
owing to the great development of the vesicle of the mid-brain, which
forms the termination of the long axis of the embryo. Figs. 29, 76,
[Pg 421]
and 118, are representative figures of embryos of various vertebrate
forms at a period when the mid-brain forms the termination of the long
axis of the body.
It is generally stated that the cranial flexure is at its maximum at
the stage represented in these figures, and there can be no doubt that
viewed from the exterior the cranial flexure ceases to be so marked a
feature, and finally disappears as the embryo gradually grows older;
but though the mid-brain ceases to form the termination of the long
axis of the embryo, the flexure of the brain becomes in many forms
absolutely more marked; while in other forms, though stated to
diminish, it does not entirely vanish.
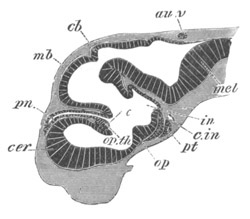
Fig. 248. Longitudinal section through the brain of Scyllium
canicula at an advanced stage of development.
cer. cerebral hemisphere; pn. pineal gland; op.th. optic
thalamus, connected with its fellow by a commissure (the middle
commissure). In front of it is seen a fold of the roof of the
fore-brain, which is connected with the choroid plexus of the third
ventricle; op. optic chiasma; pt. pituitary body; in.
infundibulum; cb. cerebellum; au.v. passage leading from the
auditory vesicle to the exterior; mel. medulla oblongata; c.in.
internal carotid artery.
The general nature of the changes which take place will perhaps best
be understood by a comparison of figs. 247 and 248 representing
longitudinal sections at two stages through the brain of an embryo
Elasmobranch. The actual cranial flexure, i.e. flexure of the floor
of the brain, is obviously greater in the older of the two brains,
though viewed from the exterior the axis of this brain appears to be
quite straight. In the younger stage, fig. 247, the mid-brain (mb)
forms the end of the long axis of the body, while in the older one the
cerebral hemispheres (cer) have grown very greatly, especially
forwards and dorsalwards. They have thus come to lie in front of the
mid-brain, and to form the end of the long axis of the body, and have
at the same time compressed the originally large thalamencephalon
against the mid-brain. The same general features may be seen in fig.
250 representing a longitudinal section of the brain of an embryo
fowl, and fig. 255 representing a longitudinal section of the brain of
a Mammal.
[Pg 422]
The infundibulum or perhaps rather the point of origin of the optic
nerves is to be regarded as the anterior termination of the axis of
the base of the brain.
The cranial flexure is least marked in Cyclostomata (fig. 253),
Teleostei, Ganoidei, and Amphibia, while it is very pronounced in
Elasmobranchii, Reptilia, Aves, and Mammalia. In Teleostei, and still
more in Cyclostomata, it permanently remains slight, owing to the
small development of the cerebral hemispheres.
In addition to the cranial flexures, two other flexures make their
appearance in the base of the brain. A posterior at the junction of
the brain and spinal cord, and an anterior at the boundary between the
cerebellum and medulla oblongata, just at the point where the pons
Varolii is formed in Mammalia. The anterior of these is the most
marked and constant; it is shewn in fig. 250. It arises considerably
later than the main cranial flexure, and since it is turned the
opposite way it assists to a considerable extent in causing the
apparent straightening of the cranial axis.
Histogenetic changes[161].
The walls of the brain are at first very
thin and, like those of the spinal cord, are formed of a number of
ranges of spindle-shaped cells. The processes of each of these cells
are stated to be continued through the whole thickness of the wall. In
the floor of the hind- and mid-brain a superficial layer of delicate
nerve-fibres is formed at an early period. This layer appears in the
first instance on the floor and sides of the hind-brain, and very
slightly, if at all, later on the floor and the sides of the
mid-brain. The cells internal to the nerve-fibres become
differentiated into an innermost epithelial layer lining the cavities
of the ventricles, and an outer layer of grey matter.
The similarity of the primitive arrangement and histological character
of the parts of the brain behind the cerebral hemispheres to that of
the spinal cord is very conclusively shewn by the examination of any
good series of sections. In both brain and spinal cord the white
matter forms a cap on the ventral and lateral parts considerably
before it extends to the dorsal surface. In the medulla the white
matter does not eventually extend to the roof owing to the peculiar
degeneration which that part undergoes.
[Pg 423]
In the case of the fore-brain the earliest histological changes,
except possibly in Mammals, take place on the same general plan as
those of the remainder of the central nervous system[162];
but though
the general plan is the same, yet the early histological distinction
between the fore-brain, and the mid- and hind-brain is more marked
than the distinction between the latter and the spinal cord.
On the floor and sides of the thalamencephalon, and apparently the
whole of the hemispheres of the lower types, there is formed, somewhat
later than in the remainder of the brain, a very delicate layer of
white matter. The inner part of the wall, which still remains
comparatively thin, is not at first clearly divided into an epithelial
and nervous layer. This distinction soon however becomes more or less
apparent, though it is not so marked as in most other parts of the
brain; and it appears that in the subsequent growth the greater part
of the original epithelial layer becomes converted into nervous
tissue.
In Mammals the same plan of differentiation would seem to be followed,
though somewhat less obviously than in the lower types. The walls of
the hemispheres become first divided (Kölliker) into a superficial
thinner layer of rounded elements, and a deeper and thicker epithelial
layer, and between these the fibres of the crura cerebri soon
interpose themselves. At a slightly later period a thin superficial
layer of white matter, homologous with that of the remainder of the
brain, becomes established.
The inner layer, together with the fibres from the crura cerebri,
gives rise to the major part of the white matter of the hemispheres
and to the epithelium lining the lateral ventricles.
The outer layer of rounded cells becomes divided into (1) a
superficial part with comparatively few cells, which, together with
its coating of white matter, forms the cortical part of the grey
matter, and (2) a deeper layer with numerous cells which forms the
main mass of the grey matter of the hemispheres.
The development of the several parts of the brain will now be
described.
[Pg 424]
The hind-brain. The hind-brain is at first an elongated, funnel-shaped
tube, the walls of which are of a nearly uniform thickness, though the
roof and floor are somewhat thinner than the sides. It forms a direct
continuation of the spinal cord, into which it passes without any
sharp line of demarcation. The ventricle it contains is known as the
fourth ventricle.
The sides become in the chick marked by a series of transverse
constrictions, dividing it into lobes, which are somewhat indefinite
in number. The first of these remains permanent, and its roof gives
rise to the cerebellum. It is uncertain whether the other
constrictions have any morphological significance. More or less
similar constrictions are present in Teleostei. In Elasmobranchii the
medulla presents on its inner face at a late period a series of lobes
corresponding with the roots of the vagus and glossopharyngeal nerves,
and it is possible that the earlier constrictions may potentially
correspond to so many nerve-roots.
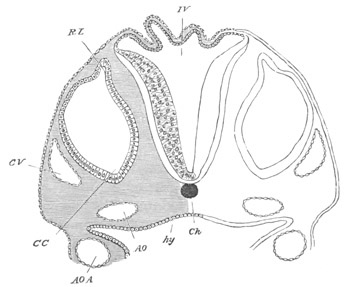
Fig. 249. Section through the hind-brain of a Chick at the end
of the third day of incubation.
IV. Fourth ventricle. The section shews the very thin roof and
thicker sides of the ventricle. Ch. Notochord; CV. Anterior
cardinal vein; CC. Involuted auditory vesicle; CC points to the
end which will form the cochlear canal; RL. Recessus labyrinthi
(remains of passage connecting the vesicle with the exterior); hy.
Hypoblast lining the alimentary canal; AO., AOA. Aorta, and
aortic arch.
Throughout the Vertebrata an anterior lobe of the hind-brain becomes
very early marked off, so that the primitive hind-brain becomes
divided into two regions which may be
[Pg 425] conveniently spoken of as the
cerebellum (figs. 247 and 248, cb) and medulla oblongata. The floor
of these regions is quite continuous and is also prolonged without any
break into the floor of the mid-brain.
The posterior section of the hind-brain, which forms the medulla,
undergoes changes of a somewhat complicated character. In the first
place its roof becomes in front very much extended and thinned out. At
the raphe, where the two lateral halves of the brain originally
united, a separation, as it were, takes place, and the two sides of
the brain become pushed apart, remaining united by only a very thin
layer of nervous matter, consisting of a single row of flattened cells
(fig. 249). As a result of this peculiar growth in the brain, the
roots of the nerves of the two sides, which were originally in contact
at the dorsal summit of the brain, become carried away from one
another, and appear to arise at the sides of the brain.
The thin roof of the fourth ventricle is triangular, or, in Mammalia,
somewhat rhomboidal in shape. The apex of the triangle is directed
backwards.
At a later period the blood-vessels of the pia mater form a rich
plexus over the anterior part of the thin roof of the medulla, which
becomes at the same time somewhat folded. The whole structure is known
as the tela vasculosa, or choroid plexus of the fourth ventricle (fig.
250, chd 4). The floor of the whole hind-brain becomes thickened,
and there very soon appears on its outer surface a layer of
non-medullated nerve-fibres, similar to those which first appear on
the spinal cord. They are continuous with a similar layer of fibres on
the floor of the mid-brain, where they constitute the crura cerebri.
On the ventral floor of the medulla is a shallow continuation of the
anterior fissure of the spinal cord.
In Elasmobranchii and many Teleostei the restiform tracts are well
developed, and are anteriorly continued into the cerebellum, of which
they form the peduncles. Near their junction with the cerebellum they
form prominent bodies, which are regarded by Miklucho-Maclay as
representing the true cerebellum of Elasmobranchii.
In Elasmobranchii a dorsal pair of ridges projects into the cavity of
the fourth ventricle, corresponding apparently with the fasciculi
teretes of the Mammalia.
In Mammalia there develop, subsequently to the longitudinal fibres
[Pg 426]
already spoken of, first the olivary bodies of the ventral side of the
medulla, and at a still later period the pyramids. The fasciculi
teretes in the cavity of the fourth ventricle are developed shortly
before the pyramids.
When the hind-brain becomes divided into two regions the roof of the
anterior part does not become thinned out like that of the posterior,
but on the contrary, becomes somewhat thickened and forms a band-like
structure roofing over the anterior part of the fourth ventricle (fig.
247 and fig. 253, cb).
This is a rudiment of the cerebellum, and in all Craniate Vertebrates
it at first presents this simple structure and insignificant size. In
Cyclostomata, Amphibia and many Reptilia this condition is permanent.
In Elasmobranchii, on the other hand, the cerebellum assumes in the
course of development a greater and greater prominence (fig. 248,
cb), and eventually overlaps both the optic lobes in front and the
medulla behind. In the later embryonic stages it exhibits in
surface-views the appearance of a median constriction, and the portion
of the ventricle contained in it is prolonged into two lateral
outgrowths.
Miklucho-Maclay, from his observations on the brains of adult
Elasmobranchii, was led to regard what is here called the cerebellum
as identical with the mid-brain, and the true mid-brain as part of the
thalamencephalon. Miklucho-Maclay was no doubt misled by the large
size of the cerebellum, but, as we have seen, this body does not begin
to be conspicuous till late in embryonic life.
The mid-brain and thalamencephalon (according to the ordinary
interpretations) have in the embryo of Elasmobranchs exactly the same
relations as in the embryos of other Vertebrates; so that the
embryological evidence appears to me to be conclusive against
Miklucho-Maclay’s view.
In Birds the cerebellum attains a very considerable development (fig.
250, cbl), consisting of a folded central lobe with an arbor vitæ,
into which the fourth ventricle is prolonged. There are two small
lateral lobes, apparently equivalent to the flocculi. Anteriorly the
cerebellum is connected with the roof of the mid-brain by a delicate
membrane, the velum medullæ anterius, or valve of Vieussens (fig. 250,
vma). The pons Varolii of Mammalia is represented by a small number
of transverse fibres on the floor of the hind-brain immediately below
the cerebellum.
In Mammalia the cerebellum attains a still greater development.
[Pg 427] The
median lobe or vermiform process is first developed. In the higher
Mammalia the lateral parts forming the hemispheres of the cerebellum
become formed as swellings at the sides at a considerably later
period, and are hardly developed in the Monotremata and Marsupialia.
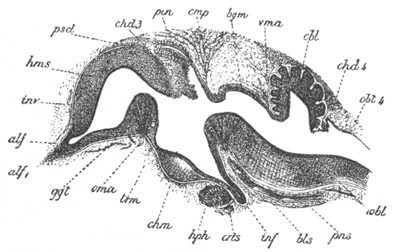
Fig. 250. Longitudinal section through the brain of a Chick of ten
days. (After Mihalkovics.)
hms. cerebral hemispheres; alf. olfactory lobe; alf1.
olfactory nerve; ggt. corpus striatum; oma. anterior commissure;
chd3. choroid plexus of the third ventricle; pin. pineal
gland; cmp. posterior commissure; trm. lamina terminalis; chm.
optic chiasma; inf. infundibulum; hph. pituitary body; bgm.
commissure of Sylvius (roof of iter a tertio ad quartum
ventriculum); vma. velum medullæ anterius (valve of Vieussens);
cbl. cerebellum; chd4. choroid plexus of the fourth
ventricle; obl4. roof of fourth ventricle; obl. medulla
oblongata; pns. commissural part of medulla; inv. sheath of
brain; bls. basilar artery; crts. internal carotid.
The cerebellum is connected with the roof of the mid-brain in front
and with the choroid plexus of the fourth ventricle behind by delicate
membranous structures, known as the velum medullæ anterius (valve of
Vieussens) and the velum medullæ posterius.
The pons Varolii is formed on the ventral side of the floor of the
cerebellar region as a bundle of transverse fibres at about the same
time as the olivary bodies.
The mid-brain. The changes undergone by the mid-brain are simpler than
those of any other part of the brain. We have already seen that the
mid-brain, on the appearance of the cranial flexure, forms an
unpaired vesicle with a vaulted roof and curved floor, at the front
end of the long axis of the body (fig. 118, MB). It is at this
period in most Vertebrates relatively much larger than in the adult;
and it is only in the Teleostei that it more or less retains in the
adult its embryonic proportions.
[Pg 428]
The cavity of the mid-brain, greatly reduced in size in the higher
forms, is known as the iter a tertio ad quartum ventriculum, or
aqueductus Sylvii.
The roof of the mid-brain is sharply constricted off from the
divisions of the brain in front of and behind it, but these
constrictions do not extend to the floor.
In some Vertebrates the region of the mid-brain is stated to undergo
hardly any further development. In the Axolotl it remains according to
Stieda[163]
as a simple tube with nearly uniformly thick walls. In the
majority of forms it undergoes, however, a more complicated
development.
In Elasmobranchs the sides become thickened to form the optic lobes,
which are soon separated by a median longitudinal groove. The floor
becomes thickened to form the crura cerebri. The primitive simple
median cavity becomes imperfectly divided into a median portion below,
and two lateral diverticula in the optic lobes.
In Teleostei the changes, resulting in the formation of (1) a pair of
longitudinal ridges projecting from the roof into the cavity of the
iter, constituting the fornix of Gottsche, and (2) of the two
swellings on the floor, forming the tori semicirculares, are more
complicated, but have not been satisfactorily worked out. In
Bombinator and the Anura generally the changes are of the same nature
as those in Elasmobranchii, except that the prolongations of the
ventricle into the optic lobes are still further constricted off from
the median portion, which forms the true iter.
In Reptilia and Aves the development of the mid-brain takes place on
the same type as in Elasmobranchii and the Anura. In Birds the optic
lobes are pushed very much aside, and the roof of the iter is greatly
thinned out. In Mammalia the sides of the mid-brain give rise to two
pairs of prominences—the corpora quadrigemina—instead of the two
optic lobes of other Vertebrata. The prominences, which do not contain
prolongations of the iter, become first visible on the appearance of
an oblique transverse furrow, while the anterior pair alone are
separated by a longitudinal furrow. In the later stages of development
the longitudinal furrow is continued so as to bisect the posterior
pair.
The floor, which is bounded posteriorly by the pons Varolii, becomes
the crura cerebri. The corpora geniculata interna also belong to this
division of the brain.
Fore-brain. In its earliest condition the fore-brain forms a single
vesicle without a trace of separate divisions, but very early it buds
off the optic vesicles, whose history is described with that of the
eye.
[Pg 429]
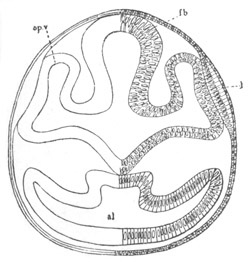
Fig. 251. Section through the front part of the head of a Lepidosteus embryo on the
seventh day after impregnation.
al. alimentary tract; fb. thalamencephalon; l. lens of eye;
op.v. optic vesicle. The mesoblast is not represented.
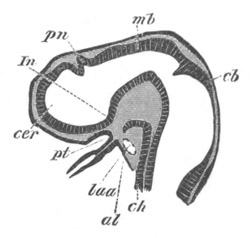
Fig. 252. Longitudinal section through the brain of a young
Pristiurus embryo.
cer. commencement of cerebral hemisphere; pn. pineal gland;
In. infundibulum; pt. ingrowth of mouth to form the pituitary
body; mb. mid-brain; cb. cerebellum; ch. notochord; al.
alimentary tract; Iaa. artery of mandibular arch.
The optic vesicles become gradually constricted off from the
fore-brain in a direction obliquely backwards and downwards. They
remain, however, attached to it at the anterior extremity of the base
of the fore-brain (fig. 251, op.v.). While the above changes are
taking place in the optic vesicles the anterior part of the fore-brain
becomes prolonged, and at the same time somewhat dilated. At first
there is no sharp boundary between the primitive fore-brain and its
anterior prolongation, but there shortly appears a constriction which
passes from above obliquely forwards and downwards. This constriction
is shallow at first, but soon becomes much deeper, leaving however the
cavities of the two divisions of the fore-brain united ventrally by a
somewhat wide canal (fig. 252).
Of these two divisions the posterior becomes the thalamencephalon,
while the anterior and larger division (cer) forms the rudiment of
the cerebral hemispheres and olfactory lobes. For a considerable
period this rudiment remains perfectly simple, and exhibits no signs,
either externally or internally, of a longitudinal constriction
dividing it into two lobes.
From the above description it may be concluded that the
[Pg 430] rudiment of
the cerebral hemispheres is contained in the original fore-brain. In
spite however of their great importance in all the Craniata, it is
probable that the hemispheres were either not present as distinct
structures, or only imperfectly separated from the thalamencephalon,
in the primitive vertebrate stock.
The thalamencephalon. The thalamencephalon varies so slightly in
structure throughout the Vertebrate series that a general description
will suffice for all the types.
It forms at first a simple vesicle, the walls of which are of a nearly
uniform thickness and formed of the usual spindle-shaped cells.
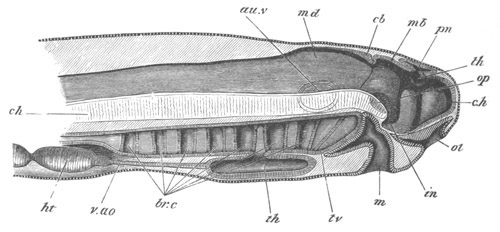
Fig. 253. Diagrammatic vertical section through the head of a
larva of Petromyzon.
The larva had been hatched three days, and was 4.8 mm. in length.
The optic and auditory vesicles are supposed to be seen through the
tissues.
c.h. cerebral hemisphere; th. optic thalamus; in.
infundibulum; pn. pineal gland; mb. mid-brain; cb. cerebellum;
md. medulla oblongata; au.v. auditory vesicle; op. optic
vesicle; ol. olfactory pit; m. mouth; br.c. branchial pouches;
th. thyroid involution; v.ao. ventral aorta; ht. ventricle of
heart; ch. notochord.
The cavity it contains is known as the third ventricle. Anteriorly it
opens widely into the cerebral rudiment, and posteriorly into the
ventricle of the mid-brain. The opening into the cerebral rudiment
becomes the foramen of Munro.
For convenience of description I shall divide it into three regions,
viz. (1) the floor, (2) the sides, and (3) the roof.
The floor becomes divided into two parts, an anterior part, giving
origin to the optic nerves, in which is formed the optic chiasma; and
a posterior part, which becomes produced into
[Pg 431] an at first
inconspicuous prominence—the rudiment of the infundibulum (fig. 252,
In). This comes in contact with an involution from the mouth, which
gives rise to the pituitary body (fig. 252, pt), the development of
which will be dealt with separately.
In the later stages of development the infundibulum becomes gradually
prolonged, and forms an elongated diverticulum of the third ventricle,
the apex of which is in contact with the pituitary body (figs. 252,
254, in, and figs. 250 and 255, inf).
Along the sides of the infundibulum run the commissural fibres
connecting the floor of the mid-brain with the cerebrum.
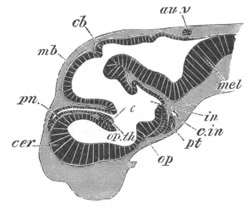
Fig. 254. Longitudinal section through the brain of Scyllium canicula at an advanced
stage of development.
cer. cerebral hemisphere; pn. pineal gland; op. th. optic
thalamus, connected with its fellow by a commissure (the middle
commissure). In front of it is seen a fold of the roof of the
fore-brain, which is the choroid plexus of the third ventricle;
op. optic chiasma; pt. pituitary body; in. infundibulum; cb.
cerebellum; au.v. passage leading from the auditory vesicle to the
exterior; mel. medulla oblongata; c.in. internal carotid
artery.
In its later stages the infundibular region presents considerable
variations in the different vertebrate types. In Fishes it generally
remains very large, and permanently forms a marked diverticulum of the
floor of the thalamencephalon. In Elasmobranchii the distal end
becomes divided into three lobes—a median and two lateral. The
lateral lobes appear to become the sacci vasculosi of the adult.
In Teleostei peculiar bodies known as the lobi inferiores (hypoaria)
make their appearance at the sides of the infundibulum. They appear to
correspond in position with the tuber cinereum of Mammalia[164].
In
Birds, Reptiles, and Amphibia the lower part of the embryonic
infundibulum becomes atrophied and reduced to a mere finger-like
process—the processus infundibuli.
In Mammalia the posterior part of the primitive infundibulum becomes
the corpus albicans, which is double in Man and the higher Apes; the
ventral part of the posterior wall forms the tuber cinereum.
Laterally, at the junction of the optic thalami and infundibulum,
there are placed the fibres of the crura cerebri, which are probably
derived from the walls of the infundibulum. A special process grows
out from the base of the infundibulum, which undergoes peculiar
changes, and becomes intimately united with the pituitary body; in
which connection it will be more fully described.
[Pg 432]
The sides of the thalamencephalon become very early thickened to form
the optic thalami, which constitute the most important section of the
thalamencephalon. They are separated, in Mammalia at all events, on
their inner aspect from the infundibular region by a somewhat S-shaped
groove, known as the sulcus of Munro, which ends in the foramen of
Munro. They also become in Mammalia secondarily united by a transverse
commissure, the grey or middle commissure, which passes across the
cavity of the third ventricle. This commissure is probably homologous
with, and derived from, a commissural band in the roof of the
thalamencephalon, placed immediately in front of the pineal gland
which is well developed in Elasmobranchii (fig. 254).
The roof undergoes more complicated changes. It becomes divided, on
the appearance of the pineal gland as a small papilliform outgrowth
(the development of which is dealt with separately), into two
regions—a longer anterior in front of the pineal gland and a shorter
posterior. The anterior region becomes at an early period excessively
thin, and at a later period, when the roof of the thalamencephalon is
shortened by the approach of the cerebral hemispheres to the
mid-brain, it becomes (vide figs. 250 and 255, chd 3, and 254)
considerably folded, while at the same time a vascular plexus is
formed in the pia mater above it. On the accomplishment of these
changes it is known as the tela choroidea of the third ventricle.
In the roof of the third ventricle behind the pineal gland there
appear in Elasmobranchii, the Sauropsida and Mammalia transverse
commissural fibres, forming a structure known as the posterior
commissure, which connects together the two optic thalami.
The most remarkable organ in the roof of the thalamencephalon is the
pineal gland, which is developed in most Vertebrates as a simple
papilliform outgrowth of the roof, and is at first composed of cells
similar to those of the other parts of the central nervous system
(figs. 250, 252, 254 and 255, pn or pin). In the lower Vertebrata
it is directed forwards, but in Mammalia, and to some extent in Aves,
it is directed backwards.
In Amphibia it is described by Götte (No. 296) as being a product of
the point where the roof of the brain remains latest attached to the
external skin.
[Pg 433]
The figure which Götte gives to prove this does not appear to me fully
to bear out his conclusion; which if true is very important. Although
I directed my attention specially to this point, I could find no
indication in Elasmobranchii of a process similar to that described by
Götte, and his observations have not as yet been confirmed for other
Vertebrates. Götte compares the pineal gland to the long-persisting
pore which leads into the cavity of the brain in the embryo of
Amphioxus, and we might add the Ascidians, and, should his facts be
confirmed, the conclusion he draws from them would appear to be well
founded.
The later stages in the development of the pineal gland in different
Vertebrates have not in all cases been fully worked out[165].
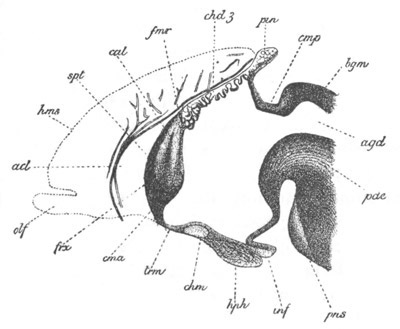
Fig. 255. Longitudinal vertical section through the anterior part
of the brain of an embryo rabbit of four centimetres. (After
Mihalkovics.)
The section passes through the median line so that the cerebral
hemispheres are not cut; their position is however indicated in
outline.
spt. septum lucidum formed by the coalescence of the inner walls
of part of the cerebral hemispheres; cna. anterior commissure;
frx. vertical pillars of the fornix; cal. genu of corpus
callosum; trm. lamina terminalis; hms. cerebral hemispheres;
olf. olfactory lobes; acl. artery of corpus callosum; fmr.
position of foramen of Munro; chd3. choroid plexus of third
ventricle; pin. pineal gland; cmp. posterior commissure; bgm.
lamina uniting the lobes of the mid-brain; chm. optic chiasma;
hph. pituitary body; inf. infundibulum; pns. pons Varolii;
pde. cerebral peduncles; agd. iter.
In Elasmobranchii the pineal gland becomes in time very long, and
extends far forwards over the roof of the cerebral
[Pg 434] hemispheres (fig.
254 pn). Its distal extremity dilates somewhat, and in the adult the
whole organ forms (Ehlers, No. 337) an elongated tube, enlarged at its
free extremity, and opening at its base into the brain. The enlarged
extremity may either be lodged in a cavity in the cartilage of the
cranium (Acanthias), or be placed outside the cranium (Raja).
In Petromyzon its form is very different. It arises (fig. 253 pn) as
a sack-like diverticulum of the thalamencephalon extending at first
both backwards and forwards. In the Ammocœte the walls of this sack
are deeply infolded.
The embryonic form of the pineal gland in Amphibia is very much like
that which remains permanent in Elasmobranchii; the stalk connecting
the enlarged terminal portion with the brain soon however becomes
solid and very thin except at its proximal extremity. The enlarged
portion also becomes solid, and is placed in the adult externally to
the skull, where it forms a mass originally described by Stieda as the
cerebral gland.
In Birds the primitive outgrowth to form the pineal gland becomes,
according to Mihalkovics, deeply indented by vascular connective
tissue ingrowths, so that it assumes a dendritic structure (fig. 250
pin).
The proximal extremity attached to the roof of the thalamencephalon
forms a special section, known as the infra-pineal process. The
central lumen of the free part of the gland finally atrophies, but the
branches still remain hollow. The infra-pineal process becomes reduced
to a narrow stalk, connecting the branched portion of the body with
the brain. The branched terminal portion and the stalk obviously
correspond with the vesicle and distal part of the stalk of the types
already described. In Mammalia the development of the pineal gland is,
according to Mihalkovics, generally similar to that of Birds. The
original outgrowth becomes branched, but the follicles or lobes to
which the branching gives rise eventually become solid (fig. 255
pin). An infra-pineal process is developed comparatively late, and
is not sharply separated from the roof of the brain.
No satisfactory suggestions have yet been offered as to the nature of
the pineal gland, unless the view of Götte be regarded as such. It
appears to possess in all forms an epithelial structure, but, except
at the base of the stalk (infra-pineal process) in
[Pg 435] Mammalia, in the
wall of which there are nerve-fibres, no nervous structures are
present in it in the adult state.
The pituitary body. Although the pituitary body is not properly a
nervous structure, yet from its intimate connection with the brain it
will be convenient to describe its development here. The pituitary
body is in fact an organ derived from the epiblast of the stomodæum.
This fact has been demonstrated for Mammalia, Aves, Amphibia and
Elasmobranchii, and may be accepted as holding good for all the
Craniata[166].
The epiblast in the angle formed by the cranial flexure
becomes involuted to form the cavity of the mouth. This cavity is
bordered on its posterior surface by the front wall of the alimentary
tract, and on its anterior by the base of the fore-brain. Its
uppermost end does not at first become markedly constricted off from
the remainder, but is nevertheless the rudiment of the pituitary body.
Fig. 256 represents a transverse section through the head of an
Elasmobranch embryo, in which, owing to the cranial flexure, the fore
part of the head is cut longitudinally and horizontally, and the
section passes through both the fore-brain (fb) and the hind-brain.
Close to the base of the fore-brain are seen the mouth (m), and the
pituitary involution from this (pt). In contact with the pituitary
involution is the blind anterior termination of the throat (al)
which a little way back opens to the exterior by the first visceral
cleft (1 v.c.). This figure alone suffices to demonstrate the
correctness of the above account of the pituitary body; but its truth
is still further confirmed by fig. 252; in which the mouth involution
(pt) is in contact with, but still separated from, the front end of
the alimentary tract. Very shortly after the septum between the mouth
and throat becomes pierced, and the two are placed in communication,
the pituitary involution becomes very partially constricted off from
the mouth involution, though still in direct communication with it. In
later stages the pituitary involution becomes longer
[Pg 436] and is dilated
terminally; while the passage connecting it with the mouth becomes
narrower and narrower, and is finally reduced to a solid cord, which
in its turn disappears.
Before the connection between the pituitary vesicle and the mouth is
obliterated the cartilaginous cranium becomes developed, and it may
then be seen that the infundibulum projects through the pituitary
space to come into close juxtaposition with the pituitary body.
After the pituitary vesicle has lost its connection with the mouth it
lies just in front of the infundibulum (figs. 250 and 255 hph and
fig. 254 pt); and soon becomes surrounded by vascular mesoblast,
which grows in and divides it into a number of branching tubes. In
many forms the cavity of the vesicle completely disappears, and the
branches become for the most part solid [Cyclostomata and some
Mammalia (the rabbit), Elasmobranchii, Teleostei and Amphibia]. In
Reptilia, Aves and most Mammalia the lumen of the organ is more or
less retained (W. Müller, No. 344).
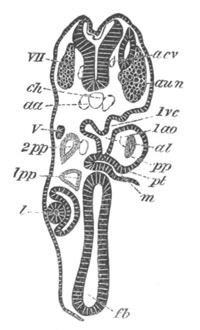
Fig. 256. Transverse section through the front part of the head of a young
Pristiurus embryo.
The section, owing to the cranial flexure, cuts both the fore- and
the hind-brain. It shews the premandibular and mandibular head
cavities 1pp and 2pp, etc. The section is moreover somewhat
oblique from side to side.
fb. fore-brain; l. lens of eye; m. mouth; pt. upper end of
mouth, forming pituitary involution; 1ao. mandibular aortic arch;
1pp. and 2pp. first and second head cavities; 1vc. first
visceral cleft; V. fifth nerve; aun. auditory nerve; VII.
seventh nerve; aa. roots of dorsal aorta; acv. anterior cardinal
vein; ch. notochord.
Although in the majority of the Vertebrata there is a close connection
between the pituitary body and the infundibulum, there is no actual
fusion between the two. In Mammalia the case is different. The part of
the infundibulum which lies at the hinder end of the pituitary body is
at first a simple finger-like process of the brain (fig. 255 inf),
but its end becomes swollen, and the lumen in this part becomes
obliterated. Its cells, originally similar to those of the other parts
of the nervous system and even (Kölliker) containing differentiated
nerve-fibres, partly atrophy, and partly assume an indifferent form,
while at the same time
[Pg 437] there grow in amongst them numerous vascular
and connective-tissue elements. The process of the infundibulum thus
metamorphosed becomes inseparably connected with the true pituitary
body, of which it is usually described as the posterior lobe. The part
of the infundibulum which undergoes this change is very probably
homologous with the saccus vasculosus of Fishes.
The true nature of the pituitary body has not yet been made out. It is
clearly a rudimentary organ in existing craniate Vertebrates, and its
development indicates that when functional it was probably a sense
organ opening into the mouth, its blind end reaching to the base of
the brain. No similar organ has as yet been found in Amphioxus, but it
seems possible perhaps to identify it with the peculiar ciliated sack
placed at the opening of the pharynx in the Tunicata, the development
of which was described at p. 18. If the suggestion is correct, the
division of the body into lobes in existing Vertebrata must be
regarded as a step towards a retrogressive metamorphosis.
Another possible view is to regard the pituitary body as a glandular
structure which originally opened into the mouth in the lower
Chordata, but which has in all existing forms ceased to be functional.
The intimate relation of the organ to the brain appears to me opposed
to this view of its nature, while on the other hand its permanent
structure is more easily explained on this view than on that
previously stated. In the Ascidians a glandular organ has been
described by Lacaze Duthiers[167]
in juxtaposition to the ciliated
sack, and it is possible that this organ as well as the ciliated sack
may be related to the pituitary body. In view of this possibility
further investigations ought to be carried out in order to determine
whether the whole pituitary body is derived from the oral involution,
or whether there may not be a nervous part and a glandular part of the
organ.
The Cerebral Hemispheres. It will be convenient to treat separately
the development of the cerebral hemispheres proper, and that of the
olfactory lobes.
Although the cerebral hemispheres vary more than any other part of the
brain, they are nevertheless developed from the unpaired cerebral
rudiment in a nearly similar manner throughout the series of
Vertebrata.
In the cerebral rudiment two parts may be distinguished, viz. the
floor and the roof. The former gives rise to the ganglia at the base
of the hemispheres—corpora striata, etc.—the latter to the
hemispheres proper.
[Pg 438]
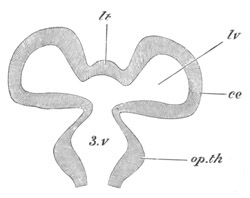
Fig. 257. Diagrammatic longitudinal horizontal section through the
fore-brain.
3.v. third ventricle; lv. lateral ventricle; lt. lamina
terminalis; ce. cerebral hemisphere; op.th. optic thalamus.
The first change which takes place consists in the roof growing out
into two lobes, between which a shallow median constriction makes its
appearance (fig. 257). The two lobes thus formed are the rudiments of
the two hemispheres. The cavity of each of them opens by a widish
aperture into the vestibule at the base of the cerebral rudiment,
which again opens directly into the cavity of the third ventricle (3
v). The Y-shaped aperture thus formed, which leads from the cerebral
hemispheres into the third ventricle, is the foramen of Munro. The
cavity (lv) in each of the rudimentary hemispheres is a lateral
ventricle. The part of the cerebrum which lies between the two
hemispheres, and passes forwards from the roof of the third ventricle
round the end of the brain to the optic chiasma, is the rudiment of
the lamina terminalis (figs. 257 lt and 255 trm). Up to this point
the development of the cerebrum is similar in all Vertebrata, but in
some forms it practically does not proceed much further.
In Elasmobranchii, although the cerebrum reaches a considerable size
(fig. 254 cer), and grows some way backwards over the
thalamencephalon, yet it is not in many forms divided into two
distinct lobes, but its paired nature is only marked by a shallow
constriction on the surface. The lamina terminalis in the later stages
of development grows backwards as a thick median septum which
completely separates the two lateral ventricles[168]
(fig. 263).
There are, it may be mentioned, considerable variations in
[Pg 439] the
structure of the cerebrum in Elasmobranchii into which it is not
however within the scope of this work to enter.
In the Teleostei the vesicles of the cerebral hemispheres appear at
first to have a wide lumen, but it subsequently becomes almost or
quite obliterated, and the cerebral rudiment forms a small bilobed
nearly solid body. In Petromyzon (fig. 253 ch) the cerebral rudiment
is at first an unpaired anterior vesicle, which subsequently becomes
bilobed in the normal manner. The walls of the hemispheres become much
thickened, but the lateral ventricles persist.
In all the higher Vertebrates the division of the cerebral rudiment
into two distinct hemispheres is quite complete, and with the
deepening of the furrow between the two hemispheres the lamina
terminalis is carried backwards till it forms a thin layer bounding
the third ventricle anteriorly, while the lateral ventricles open
directly into the third ventricle.
In Amphibians the two hemispheres become united together immediately
in front of the lamina terminalis by commissural fibres, forming the
anterior commissure. They also send out anteriorly two solid
prolongations, usually spoken of as the olfactory lobes, which
subsequently fuse together.
In all Reptilia and Aves there is formed an anterior commissure, and
in the higher members of the group, especially Aves (fig. 250), the
hemispheres may obtain a considerable development. Their outer walls
are much thickened, while their inner walls become very thin; and a
well-developed ganglionic mass, equivalent to the corpus striatum, is
formed at their base.
Fig. 258. Brain of a three months’ human embryo: natural
size. (From Kölliker.)
1. From above with the dorsal part of hemispheres and mid-brain
removed; 2. From below. f. anterior part of cut wall of the
hemisphere; f´. cornu ammonis; tho. optic thalamus; cst.
corpus striatum; to. optic tract; cm. corpora mammillaria; p.
pons Varolii.
The cerebral hemispheres undergo in Mammalia the most complicated
development. The primitive unpaired cerebral rudiment becomes, as in
lower Vertebrates, bilobed, and at the same time divided by the
ingrowth of a septum of connective tissue into two distinct
hemispheres (figs. 260 and 261 f and 258 1). From this septum is
formed the falx cerebri and other parts.
The hemispheres contain at first very large cavities, communicating by
a wide foramen of Munro with the third ventricle (fig. 260). They grow
rapidly in size, and extend, especially backwards, and gradually
cover the thalamencephalon and the
[Pg 440] mid-brain (fig. 258 1, f). The
foramen of Munro becomes very much narrowed and reduced to a mere
slit.
The walls are originally nearly uniformly thick, but the floor becomes
thickened on each side, and gives rise to the corpus striatum (figs.
260 and 261 st). The corpus striatum projects upwards into each
lateral ventricle, giving to it a somewhat semilunar form, the two
horns of which constitute the permanent anterior and descending cornua
of the lateral ventricles (fig. 262 st).
Fig. 259. Transverse section through the brain of a rabbit of five
centimetres. (After Mihalkovics.)
The section passes through nearly the posterior border of the septum
lucidum, immediately in front of the foramen of Munro.
hms. cerebral hemispheres; cal. corpus callosum; amm. cornu
ammonis (hippocampus major); cms. superior commissure of the
cornua ammonis; spt. septum lucidum; frx 2. vertical fibres of
the fornix; cma. anterior commissure; trm. lamina terminalis;
str. corpus striatum; ltf. nucleus lenticularis of corpus
striatum; vtr 1. lateral ventricle; vtr 3. third ventricle;
ipl. slit between cerebral hemispheres.
With the further growth of the hemisphere the corpus
[Pg 441] striatum loses
its primitive relations to the descending cornu. The reduction in size
of the foramen of Munro above mentioned is, to a large extent, caused
by the growth of the corpora striata.
Fig. 260. Transverse section through the brain of a sheep’s embryo
of 2.7 cm. in length. (From Kölliker.)
The section passes through the level of the foramen of Munro.
st. corpus striatum; m. foramen of Munro; t. third ventricle;
pl. choroid plexus of lateral ventricle; f. falx cerebri; th.
anterior part of optic thalamus; ch. optic chiasma; o. optic
nerve; c. fibres of the cerebral peduncles; h. cornu ammonis;
p. pharynx; sa. presphenoid bone; a. orbitosphenoid bone; s.
points to part of the roof of the brain at the junction between the
roof of the third ventricle and the lamina terminalis; l. lateral
ventricle.
The corpora striata are united at their posterior border with the
optic thalami. In the later stages of development the area of contact
between these two pairs of ganglia increases to an immense extent
(fig. 261), and the boundary between them becomes somewhat obscure, so
that the sharp distinction which exists in the embryo between the
thalamencephalon and cerebral hemispheres becomes lost. This change is
usually (Mihalkovics, Kölliker) attributed to a fusion between the
corpora striata and optic thalami, but it has recently been attributed
by Schwalbe (No. 349), with more probability, to a growth of the
original surface of contact, and an accompanying change in the
relations of the parts.
[Pg 442]
The outer wall of the hemispheres gradually thickens, while the inner
wall becomes thinner. In the latter, two curved folds, projecting
towards the interior of the lateral ventricle, become formed. These
folds extend from the foramen of Munro along nearly the whole of what
afterwards becomes the descending cornu of the lateral ventricle.
The upper fold becomes the hippocampus major (cornu ammonis) (figs.
259 amm, 260 and 261 h, and 262 am). When the rudiment of the
descending cornu has become transformed into a simple process of the
lateral ventricle the hippocampus major forms a prominence upon its
floor.
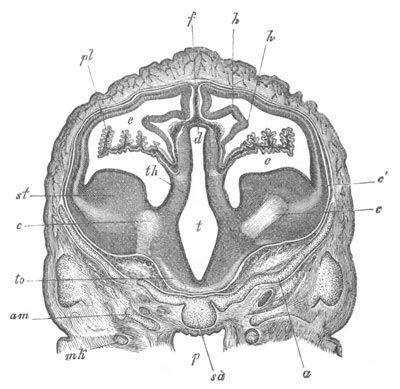
.
Fig. 261. Transverse section through the brain of a sheep’s embryo
of 2.7 cm. in length. (From Kölliker.)
The section is taken a short distance behind the section represented
in fig. 260, and passes through the posterior part of the
hemispheres and the third ventricle.
st. corpus striatum; th. optic thalamus; to. optic tract; t.
third ventricle; d. roof of third ventricle; c. fibres of
cerebral peduncles; c´. divergence of these fibres into the walls
of the hemispheres; e. lateral ventricle with choroid plexus pl; h
cornu ammonis; f. primitive falx; am. alisphenoid; a.
orbitosphenoid; sa. presphenoid; p. pharynx; mk. Meckel’s
cartilage.
The wall of the lower fold becomes very thin, and a vascular plexus,
derived from the connective-tissue septum between the hemispheres, and
similar to that of the roof of the third ventricle,
[Pg 443] is formed outside
it. It constitutes a fold projecting far into the cavity of the
lateral ventricle, and together with the vascular connective tissue in
it gives rise to the choroid plexus of the lateral ventricle (figs.
260 and 261 pl).
It is clear from the above description that a marginal fissure leading
into the cavity of the lateral ventricle does not exist in the sense
often implied in works on human anatomy, in that the epithelium
covering the choroid plexus, which forms the true wall of the brain,
is a continuous membrane. The epithelium of the choroid plexus of
the lateral ventricle is quite independent of that of the choroid
plexus of the third ventricle, though at the foramen of Munro the roof
of the third ventricle is of course continuous with the inner wall of
the lateral ventricle (fig. 260 s). The vascular elements of the
two plexuses form however a continuous structure.
The most characteristic parts of the Mammalian cerebrum are the
commissures connecting the two hemispheres. These commissures are (1)
the anterior commissure, (2) the fornix, and (3) the corpus callosum,
the two latter being peculiar to Mammalia.
By the fusion of the inner walls of the hemispheres in front of the
lamina terminalis a solid septum is formed, known as the septum
lucidum, continuous behind with the lamina terminalis, and below with
the corpora striata (figs. 255 and 259 spt). It is by a series of
differentiations within this septum that the above commissures
originate. In Man there is a closed cavity left in the septum known as
the fifth ventricle, which has however no communication with the true
ventricles of the brain.
In the septum lucidum there become first formed, below, the transverse
fibres of the anterior commissure (fig. 255 and fig. 259 cma), and
in the upper part the vertical fibres of the fornix (fig. 255 and fig.
259 frx 2). The vertical fibres meet above the foramen of Munro, and
thence diverge backwards, as the posterior pillars, to lose themselves
in the cornu ammonis (fig. 259 amm). Ventrally they are continued,
as the descending or anterior pillars of the fornix, into the corpus
albicans, and thence into the optic thalami.
The corpus callosum is not formed till after the anterior commissure
and fornix. It arises in the upper part of the region
[Pg 444] (septum lucidum)
formed by the fusion of the lateral walls of the hemispheres (figs.
255 and 259 cal), and at first only its curved anterior portion—the
genu or rostrum—is developed. This portion is alone found in
Monotremes and Marsupials. The posterior portion, which is present in
all the Monodelphia, is gradually formed as the hemispheres are
prolonged further backwards.
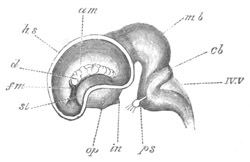
Fig. 262. Lateral view of the brain
of a calf embryo of 5 cm. (After Mihalkovics.)
The outer wall of the hemisphere is removed, so as to give a view of
the interior of the left lateral ventricle.
hs. cut wall of hemisphere; st. corpus striatum; am.
hippocampus major (cornu ammonis); d. choroid plexus of lateral
ventricle; fm. foramen of Munro; op. optic tract; in.
infundibulum; mb. mid-brain; ch. cerebellum; IV.V. roof of
fourth ventricle; ps. pons Varolii, close to which is the fifth
nerve with Gasserian ganglion.
Primitively the Mammalian cerebrum, like that of the lower Vertebrata,
is quite smooth. In many of the Mammalia, Monotremata, Insectivora,
etc., this condition is nearly retained through life, while in the
majority of Mammalia a more or less complicated system of fissures is
developed on the surface. The most important, and first formed, of
these is the Sylvian fissure. It arises at the time when the
hemispheres, owing to their growth in front of and behind the corpora
striata, have assumed a somewhat bean-shaped form. At the root of the
hemispheres—the hilus of the bean—there is formed a shallow
depression, which constitutes the first trace of the Sylvian fissure.
The part of the brain lying in this fissure is known as the island of
Reil.
The olfactory lobes. The olfactory lobes, or rhinencephala, are
secondary outgrowths of the cerebral hemispheres, and contain
prolongations of the lateral ventricles, but may however be solid in
the adult state. According to Marshall they develop in Birds and
Elasmobranchs and presumably other forms later than the olfactory
nerves, so that the olfactory region of the hemispheres is indicated
before the appearance of the olfactory lobes.
In most Vertebrates the olfactory lobes arise at a fairly early
[Pg 445] stage
of development from the under and anterior part of the hemispheres
(fig. 250 olf). In Elasmobranchs they arise, not from the base, but
from the lateral parts of the brain (fig. 263), and become
subsequently divided into a bulbous portion and a stalk. They vary
considerably in their structure in the adult.
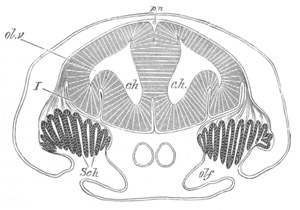
Fig. 263. Section through the brain and olfactory organ of an
embryo of Scyllium. (Modified from figures by Marshall and myself.)
ch. cerebral hemispheres; ol.v. olfactory vesicle; olf.
olfactory pit; Sch. Schneiderian folds; I. olfactory nerve. The
reference line has been accidentally taken through the nerve to the
brain; pn. anterior prolongation of pineal gland.
In Amphibia the solid anterior prolongations of the cerebral
hemispheres already spoken of are usually regarded as the olfactory
lobes, but according to Götte, whose view appears to me well founded,
small papillæ, situated at the base of these prolongations, from which
olfactory nerves spring, and which contain a process of the lateral
ventricle, should properly be regarded as the olfactory lobes. These
papillæ arise prior to the solid anterior prolongations of the
hemispheres.
In Birds the olfactory lobes are small. In the chick they arise
(Marshall) on the seventh day of incubation.
General conclusions as to the Central Nervous System.
It has been shewn above that both the brain and spinal cord are
primitively composed of a uniform wall of epithelial cells, and that
the first differentiation results in the formation of an external
layer of white matter, a middle layer of grey matter (ganglion cells),
and an inner epithelial layer. This primitive
[Pg 446] histological
arrangement, which in many parts of the brain at any rate, is only to
be observed in the early developmental stages, has a simple
phylogenetic explanation.
As has been already explained in an earlier part of this chapter the
central nervous system was originally a differentiated part of the
superficial epidermis.
This differentiation (as may be concluded from the character of the
nervous system in the Cœlenterata and Echinodermata) consisted in the
conversion of the inner ends of the epithelial cells into
nerve-fibres; that is to say, that the first differentiation resulted
in the formation of a layer of white matter on the inner side of the
epidermis. The next stage was the separation of a deeper layer of the
epidermis as a layer of ganglion cells from the superficial epithelial
layer, i.e. the formation of a middle layer of ganglion cells and an
outer epithelial layer. Thus, phylogenetically, the same three layers
as those which first make their appearance in the ontogeny of the
vertebrate nervous system became successively differentiated, and in
both cases they are clearly placed in the same positions, because the
central canal of the vertebrate nervous system, as formed by an
involution, is at the true outer surface, and the external part of the
cord is at the true inner surface.
It is probable that a very sharp distinction between the white and
grey matter is a feature acquired in the higher Vertebrata, since in
Amphioxus there is no such sharp separation; though the nerve-fibres
are mainly situated externally and the nerve-cells internally.
As already stated in Chapter XII. the primitive division of the
nervous axis was probably not into brain and spinal cord, but into (1)
a fore-brain, representing the ganglion of the præoral lobe, and (2)
the posterior part of the nervous axis, consisting of the mid- and
hind-brains and the spinal cord. This view of the division of the
central nervous system fits in fairly satisfactorily with the facts of
development. The fore-brain is, histologically, more distinct from the
posterior part of the nervous system than the posterior parts are from
each other; the front end of the notochord forms the boundary between
these two parts of the central nervous system (vide fig. 253),
ending as it does at the front termination of the floor of the
mid-brain, and finally,
[Pg 447] the nerves of the fore-brain have a different
character to those of the mid- and hind-brain.
This primitive division of the central nervous system is lost in all
the true Vertebrata, and in its place there is a secondary
division—corresponding with the secondary vertebrate head—into a
brain and spinal cord. The brain, as it is established in these forms,
is again divided into a fore-brain, a mid-brain and a hind-brain. The
fore-brain is, as we have already seen, the original ganglion of the
præoral lobe. The mid-brain appears to be the lobe, or ganglion, of
the third pair of nerves (first pair of segmental nerves), while the
hind-brain is a more complex structure, each section of which (perhaps
indicated by the constrictions which often appear at an early stage of
development) giving rise to a pair of segmental nerves is, roughly
speaking, homologous with the whole mid-brain.
The type of differentiation of each of the primitively simple vesicles
forming the fore-, the mid- and the hind-brains is very uniform
throughout the Vertebrate series, but it is highly instructive to
notice the great variations in the relative importance of the parts of
the brain in the different types. This is especially striking in the
case of the fore-brain, where the cerebral hemispheres, which on
embryological grounds we may conclude to have been hardly
differentiated as distinct parts of the fore-brain in the most
primitive types now extinct, gradually become more and more prominent,
till in the highest Mammalia they constitute a more important section
of the brain than the whole of the remaining parts put together.
The little that is known with reference to the significance of the
more or less corresponding outgrowths of the floor and roof of the
thalamencephalon, constituting the infundibulum and pineal gland, has
already been mentioned in connection with the development of these
parts.
Bibliography.
(332) C. J. Carus. Versuch einer Darstellung d. Nervensystems, etc.
Leipzig, 1814.
(333) J. L. Clark. “Researches on the development of the spinal cord
in Man, Mammalia and Birds.” Phil. Trans., 1862.
[Pg 448]
(334) E. Dursy. “Beiträge zur Entwicklungsgeschichte des
Hirnanhanges.” Centralblatt f. d. med. Wissenschaften, 1868. Nr. 8.
(335) E. Dursy. Zur Entwicklungsgeschichte des Kopfes des Menschen
und der höheren Wirbelthiere. Tübingen, 1869.
(336) A. Ecker. “Zur Entwicklungsgeschichte der Furchen und Windungen
der Grosshirn-Hemisphären im Foetus des Menschen.” Archiv f.
Anthropologie, v. Ecker und Lindenschmidt. Vol. III. 1868.
(337) E. Ehlers. “Die Epiphyse am Gehirn d. Plagiostomen.” Zeit. f.
wiss. Zool. Vol. XXX., suppl. 1878.
(338) P. Flechsig. Die Leitungsbahnen im Gehirn und Rückenmark des
Menschen. Auf Grund entwicklungsgeschichtlicher Untersuchungen.
Leipzig, 1876.
(339) V. Hensen. “Zur Entwicklung des Nervensystems.” Virchow’s
Archiv, Bd. XXX. 1864.
(340) L. Löwe. “Beiträge z. Anat. u. z. Entwick. d. Nervensystems d.
Säugethiere u. d. Menschen.” Berlin, 1880.
(341) L. Löwe. “Beiträge z. vergleich. Morphogenesis d. centralen
Nervensystems d. Wirbelthiere.” Mittheil. a. d. embryol. Instit.
Wien, Vol. II. 1880.
(342) A. M. Marshall. “The Morphology of the Vertebrate Olfactory
organ.” Quart. J. of Micr. Science, Vol. XIX. 1879.
(343) V. v. Mihalkovics. Entwicklungsgeschichte d. Gehirns. Leipzig,
1877.
(344) W. Müller. “Ueber Entwicklung und Bau der Hypophysis und des
Processus infundibuli cerebri.” Jenaische Zeitschrift. Bd. VI. 1871.
(345) H. Rahl-Rückhard. “Die gegenseitigen Verhältnisse d. Chorda,
Hypophysis etc. bei Haifischembryonen, nebst Bemerkungen üb. d.
Deutung d. einzelnen Theile d. Fischgehirns.” Morphol. Jahrbuch,
Vol. VI. 1880.
(346) H. Rathke. “Ueber die Entstehung der glandula pituitaria.”
Müller’s Archiv f. Anat. und Physiol., Bd. V. 1838.
(347) C. B. Reichert. Der Bau des menschlichen Gehirns. Leipzig,
1859 u. 1861.
(348) F. Schmidt. “Beiträge zur Entwicklungsgeschichte des Gehirns.”
Zeitschrift f. wiss. Zoologie, 1862. Bd. XI.
(349) G. Schwalbe. “Beitrag z. Entwick. d. Zwischenhirns.” Sitz. d.
Jenaischen Gesell. f. Med. u. Naturwiss. Jan. 23, 1880.
(350) Fried. Tiedemann. Anatomie und Bildungsgeschichte des Gehirns
im Foetus des Menschen. Nürnberg, 1816.
The development of the Cranial and Spinal Nerves[169].
All the nerves are outgrowths of the central nervous system, but the
differences in development between the cranial and spinal nerves are
sufficiently great to make it convenient to treat them separately.
[Pg 449]
Spinal nerves. The posterior roots of the spinal nerves, as well as
certain of the cranial nerves, arise in the same manner, and from the
same structure, and are formed considerably before the anterior roots.
Elasmobranch fishes may be taken as the type to illustrate the mode of
formation of the spinal nerves.
The whole of the nerves in question arise as outgrowths of a median
ridge of cells, which makes its appearance on the dorsal side of the
spinal cord (fig. 264 A, pr). This ridge has been called by Marshall
the neural crest. At each point, where a pair of nerves will be
formed, two pear-shaped outgrowths project from it, one on each side;
and apply themselves closely to the walls of the spinal cord (fig. 264 B,
pr). These outgrowths are the rudiments of the posterior nerves.
While still remaining attached to the dorsal summit of the neural cord
they grow to a considerable size (fig. 264 B, pr).
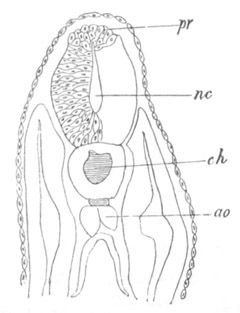
Fig. 264 A. Transverse section through a pristiurus embryo shewing
the proliferation of cells to form the neural crest.
pr. neural crest; nc. neural canal; ch. notochord; ao.
aorta.
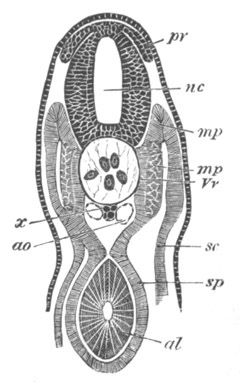
Fig. 264 B. Transverse section through the trunk of an embryo
slightly older than fig. 28 E.
nc. neural canal; pr. posterior root of spinal nerve; x.
subnotochordal rod; ao. aorta; sc. somatic mesoblast; sp.
splanchnic mesoblast; mp. muscle-plate; mp´. portion of
muscle-plate converted into muscle; Vv. portion of the vertebral
plate which will give rise to the vertebral bodies; al. alimentary
tract.
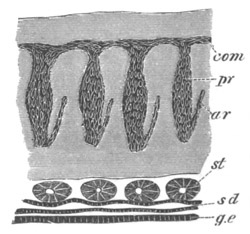
Fig. 265. Vertical longitudinal section through part of the trunk
of a young Scyllium embryo.
com. commissure uniting the dorsal ends of the posterior
nerve-roots; pr. ganglia of posterior roots; ar. anterior roots;
st. segmental tubes; sd. segmental duct; g.e. epithelium
lining the body cavity in the region of the future germinal ridge.
The attachment to the dorsal summit is not permanent, but
[Pg 450] before
describing the further fate of the nerve-rudiments it is necessary to
say a few words as to the neural crest. At the period when the nerves
have begun to shift their attachment to the spinal cord, there makes
its appearance, in Elasmobranchii, a longitudinal commissure
connecting the dorsal ends of all the spinal nerves (figs. 265, 266
com), as well as those of the vagus and glossopharyngeal nerves.
This commissure has as yet only been found in a complete form in
Elasmobranchii;
[Pg 451] but it is nevertheless to be regarded as a very
important morphological structure.
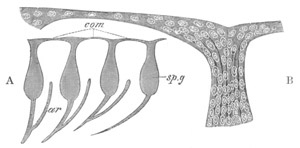
Fig. 266. Spinal Nerves of Scyllium in longitudinal section to shew
the commissure connecting them.
A. Section through a series of nerves.
B. Highly magnified view of the dorsal part of a single nerve, and
of the commissure connected with it.
com. commissure; sp.g. ganglion of posterior root; ar.
anterior root.
It is probable, though the point has not yet been definitely made out,
that this commissure is derived from the neural crest, which appears
therefore to separate into two cords, one connected with each set of
dorsal roots.
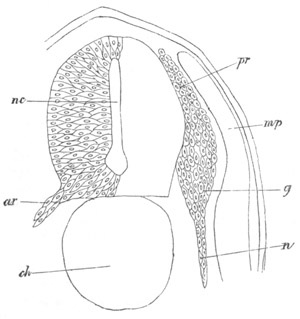
Fig. 267. Section through the dorsal part of the trunk of a
Torpedo embryo.
pr. posterior root of spinal nerve; g. spinal ganglion; n.
nerve; ar. anterior root of spinal nerve; ch. notochord; nc.
neural canal; mp. muscle-plate.
[Pg 452]
Returning to the original attachment of the nerve-rudiments to the
medullary wall, it has been already stated that this attachment is not
permanent. It becomes, in fact, at about the time of the appearance of
the above commissure, either extremely delicate or absolutely
interrupted.
The nerve-rudiment now becomes divided into three parts (figs. 267 and
268), (1) a proximal rounded portion, to which is attached the
longitudinal commissure (pr); (2) an enlarged portion, forming the
rudiment of a ganglion (g and sp g); (3) a distal portion, forming
the commencement of the nerve (n). The proximal portion may very
soon be observed to be united with the side of the spinal cord at a
very considerable distance from its original point of attachment.
Moreover the proximal portion of the nerve is attached, not by its
extremity, but by its side, to the spinal cord (fig. 268 x). The
dorsal extremities of the posterior roots are therefore free.
This attachment of the posterior nerve-root to the spinal cord is, on
account of its small size, very difficult to observe. In favourable
specimens there may however be seen a distinct cellular prominence
from the spinal cord, which becomes continuous with a small prominence
on the lateral border of the nerve-root near its proximal extremity.
The proximal extremity of the nerve is composed of cells, which, by
their small size and circular form, are easily distinguished from
those which form the succeeding or ganglionic portion of the nerve.
This part has a swollen configuration, and is composed of large
elongated cells with oval nuclei. The remainder of the rudiment forms
the commencement of the true nerve. This also is, at first, composed
of elongated cells[170].
[Pg 453]
It is extremely difficult to decide whether the permanent attachment
of the posterior nerve-roots to the spinal cord is entirely a new
formation, or merely due to the shifting of the original point of
attachment. I am inclined to adopt the former view, which is also held
by Marshall and His, but may refer to fig. 269, shewing the roots
after they have become attached to the side, as distinct evidence in
favour of the view that the attachment simply becomes shifted, a
process which might perhaps be explained by a growth of the dorsal
part of the spinal cord. The change of position in the case of some of
the cranial nerves is, however, so great that I do not think that it
is possible to account for it without admitting the formation of a new
attachment.
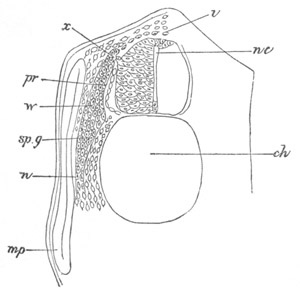
Fig. 268. Section through the dorsal region of a Pristiurus embryo.
pr. posterior root; sp.g. spinal ganglion; n. nerve; x.
attachment of ganglion to spinal cord; nc. neural canal; mp.
muscle-plate; ch. notochord; i. investment of spinal cord.
The anterior roots of the spinal nerves appear somewhat later than the
posterior roots, but while the latter are still quite small. Each of
them (fig. 269 ar) arises as a small but distinct conical outgrowth
from a ventral corner of the spinal cord, before the latter has
acquired its covering of white matter. From the very first the
rudiments of the anterior roots have a somewhat fibrous appearance and
an indistinct form of peripheral termination, while the protoplasm of
which they are composed becomes attenuated towards its end. They
differ from the posterior roots in never shifting their point of
attachment to the spinal cord, in not being united with each other by
a commissure, and in never developing a ganglion.
[Pg 454]
The anterior roots grow rapidly, and soon form elongated cords of
spindle-shaped cells with wide attachments to the spinal cord (fig.
267). At first they pass obliquely and nearly horizontally outwards,
but, before reaching the muscle-plates, they take a bend downwards.
One feature of some interest with reference to the anterior roots is
the fact that they arise not vertically below, but alternately with
the posterior roots: a condition which persists in the adult. They are
at first quite separate from the posterior roots; but about the stage
represented in fig. 267 a junction is effected between each posterior
root and the corresponding anterior root. The anterior root joins the
posterior at some little distance below its ganglion (figs. 265 and
266).
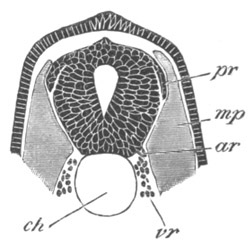
Fig. 269. Transverse section through the dorsal region of a young
Torpedo embryo to shew the origin of the anterior and posterior roots
of the spinal nerves.
pr. posterior root of spinal nerve; ar. anterior root of spinal
nerve; mp. muscle-plate; ch. notochord; vr. mesoblast cells
which will form the vertebral bodies.
Although I have made some efforts to determine the eventual fate of
the commissure uniting the dorsal roots, I have not hitherto met with
success. It grows thinner and thinner, becoming at the same time
composed of fibrous protoplasm with imbedded nuclei, and finally
ceases to be recognisable. I can only conclude that it gradually
atrophies, and ultimately vanishes.
After the junction of the posterior and anterior roots the compound
nerve extends downwards, and may easily be traced for a considerable
distance. A special dorsal branch is given off from the ganglion on
the posterior root (fig. 275 dn). According to Löwe the fibres of
the anterior and posterior roots can easily be distinguished in the
higher types by their structure and behaviour towards colouring
reagents, and can be separately traced in the compound nerve.
So far as has been made out, the development of the spinal nerves of
other Vertebrates agrees in the main with that in Elasmobranchii, but
no dorsal commissure has yet been discovered, except in the case of
the first two or three spinal nerves of the Chick.
In the Chick (Marshall, No. 353) the posterior roots, during their
early stages, closely resemble those in Elasmobranchii, though their
relatively smaller size makes them difficult to observe. They at first
extend more or
[Pg 455] less horizontally outwards above the muscle-plates (as
a few of the nerves also do to some extent in Elasmobranchii), but
subsequently lie close to the sides of the neural canal. They are
shewn in this position in fig. 116 sp.g. There does not appear to be
a continuous crest connecting the roots of the posterior nerves. The
later stages of the development are precisely like those in
Elasmobranchii.
The anterior roots have not been so satisfactorily investigated as the
posterior, but they grow out, possibly by several roots for each
nerve, from the ventral corners of the spinal cord, and subsequently
become attached to the posterior nerves.
I have observed the development of the posterior roots in Lepidosteus,
in which they appear as projections from the dorsal angles of the
spinal cord, extending laterally outwards and, at first, having their
extremities placed dorsally to the muscle-plates.
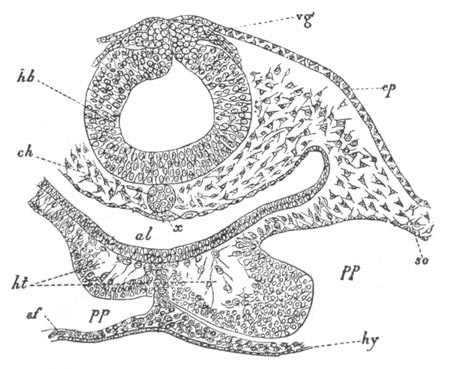
Fig. 270. Transverse section through the posterior part of the
head of an embryo chick of thirty hours.
hb. hind-brain; vg. vagus nerve; ep. epiblast; ch.
notochord; x. thickening of hypoblast (possibly a rudiment of the
subnotochordal rod); al. throat; ht. heart; pp. body cavity;
so. somatic mesoblast; sf. splanchnic mesoblast; hy.
hypoblast.
The cranial nerves[171].
The earliest stages in the development of the
cranial nerves have been most satisfactorily studied, especially by
Marshall (No. 354), in the Chick, while the later stages have been
more fully worked out in Elasmobranchii, where, moreover, they present
a very primitive arrangement.
[Pg 456] In the Chick certain of the cranial
nerves arise before the complete closure of the neural groove. These
nerves are formed as paired outgrowths of a continuous band composed
of two laminæ, connecting the dorsal end of the incompletely closed
medullary canal with the external epiblast. This mode of development
will best be understood by an examination of fig. 270, where the two
roots of the vagus nerve (vg) are shewn growing out from the neural
band. Shortly after this stage the neural band, becoming separated
from the epiblast, constitutes a crest attached to the roof of the
brain, while its two laminæ become fused. The relation of the cranial
nerves to the brain then becomes exactly the same as that of the
posterior roots of the spinal nerves to the spinal cord.
It does not appear possible to decide whether the mode of development
of the cranial nerves in the Chick, or that of the posterior roots of
the spinal nerves, is the more primitive. The difference in
development between the two sets of nerves probably depends upon the
relative time of the closure of the neural canal. The neural crest
clearly belongs to the brain, from the fact of its remaining connected
with the latter when the medullary tube separates from the external
epiblast.
It is not known whether the cranial nerves originate before the
closure of the neural canal in other forms besides the Chick.
The neural crest of the brain is continuous with that of the spinal
cord, and on its separation from the central nervous axis forms on
each side a commissure, uniting the posterior cranial nerves with the
spinal nerves, and continuous with the commissure connecting together
the latter nerves.
Anteriorly, the neural crest extends as far as the roof of the
mid-brain[172].
The pairs of nerves which undoubtedly grow out from it
are the third pair (Marshall), the fifth, the seventh and auditory (as
a single root), the glossopharyngeal, and the various elements of the
vagus (as separate roots in Elasmobranchii, but as a single root in
Aves). Marshall holds that the olfactory
[Pg 457] nerve probably also
originates from this crest. It will however be convenient to deal
separately with this nerve, after treating of the other nerves which
undoubtedly arise from the neural crest.
The cranial nerves just enumerated present in their further
development many points of similarity; and the glossopharyngeal nerve,
as it develops in Elasmobranchii, may perhaps be taken as typical.
This nerve is connected by a commissure with those behind, but this
fact may for the moment be left out of consideration. Springing at
first from the dorsal line of the hind-brain immediately behind the
level of the auditory capsule, it apparently loses this primitive
attachment and acquires a secondary attachment about halfway down the
side of the hind-brain. The primitive undifferentiated rudiment soon
becomes divided, exactly like a true posterior root of a spinal nerve,
into a root, a ganglion and a nerve. The main branch of the nerve
passes ventralwards, and supplies the first branchial arch (fig. 271
gl). Shortly afterwards it sends forwards a smaller branch, which
passes to the hyoid arch in front; so that the nerve forks over the
hyobranchial cleft. A typical cranial nerve appears therefore, except
as concerns its relations to the clefts, to develop precisely like the
posterior root of the spinal nerve.
Most of the cranial nerves of the above group, in correlation with the
highly differentiated character of the head, acquire secondary
differentiations, and render necessary a brief description of what is
known with reference to their individual development.
The Glossopharyngeal and Vagus Nerves. Behind the ear there are
formed, in Scyllium, a series of five nerves which pass down to
respectively the first, second, third, fourth and fifth branchial
arches.
For each arch there is thus one nerve, whose course lies close to the
posterior margin of the preceding cleft; a second anterior branch,
forking over the cleft and passing to the arch in front, being
developed later. These nerves are connected with the brain by roots at
first attached to the dorsal summit, but eventually situated about
halfway down the sides. The foremost of them is the glossopharyngeal.
The next four are, as has been shewn by Gegenbaur[173],
equivalent to
four independent nerves, but form together a compound nerve, which we
may briefly call the vagus.
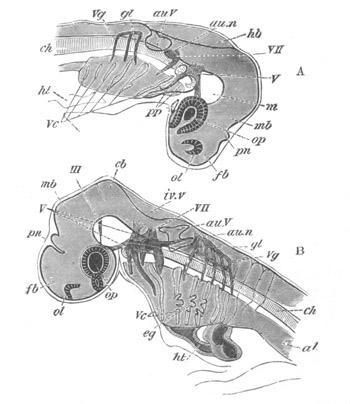
Fig. 271. Views of the head of Elasmobranch embryos at two stages
as transparent objects.
A. Pristiurus embryo of the same stage as fig. 28 F.
B. Somewhat older Scyllium embryo.
III. third nerve; V. fifth nerve; VII. seventh nerve; au.n.
auditory nerve; gl. glossopharyngeal nerve; Vg. vagus nerve;
fb. fore-brain; pn. pineal gland; mb. mid-brain; hb.
hind-brain; iv.v. fourth ventricle; cb. cerebellum; ol.
olfactory pit; op. eye; au.V. auditory vesicle; m. mesoblast
at base of brain; ch. notochord; ht. heart; Vc. visceral
clefts; eg. external gills; pp. sections of body cavity in the
head.
[Pg 458]
This compound nerve together with the glossopharyngeal soon attains a
very complicated structure, and presents several remarkable features.
There are present five branches (fig. 271 B), viz. the glossopharyngeal
(gl) and four branches of the vagus, the latter probably arising by
a considerably greater number of strands from the brain[174].
All the
strands from the brain are united together by a thin commissure (fig.
271 B, vg), continuous with the commissure of the posterior roots of
the spinal nerves, and from this commissure the five branches are
continued obliquely ventralwards and backwards, and each of them
dilates into a ganglionic swelling. They all become again united
together by a second thick commissure, which is continued backwards as
the intestinal branch of the vagus nerve. The nerves, however, are
continued ventralwards each to its respective arch.
[Pg 459] From the lower
commissure springs the lateral nerve, at a point whose relations to
the branches of the vagus I have not certainly determined.
With reference to the dorsal commissure, which is almost certainly
derived from the original neural crest, it is to be noted that there
is a longish stretch of it between the last branch of the vagus and
the first spinal nerve, which is probably the remains of a part of the
commissure which connected the posterior branches of the vagus, at a
stage in the evolution of the Vertebrata, when the posterior visceral
clefts were still present. These branches of the vagus are probably
partially preserved in the ramifications of the intestinal stem of the
vagus (Gegenbaur). The origin of the ventral commissure, continued as
the intestinal branch of the vagus, has not been embryologically
worked out.
The lateral nerve may very probably be a dorsal sensory branch of the
vagus, whose extension into the posterior part of the trunk has been
due to the gradual backward elongation of the lateral line[175],
causing the nerve supplying it to elongate at the same time (vide
Section on lateral line).
In the Chick the common rudiment for the vagus and glossopharyngeal
nerves (Marshall), which has already been spoken of, subsequently
divides into two parts, an anterior forming the glossopharyngeal
nerve, and a posterior forming the vagus nerve.
The seventh and auditory nerves. As shewn by Marshall’s and my own
observations there is a common rudiment for the seventh and auditory
nerves. This rudiment divides almost at once into two branches. The
anterior of these pursues a straight course to the hyoid arch (fig.
271 A, VII.) and forms the rudiment of the facial nerve; the second
of the two (fig. 271 A, au.n), which is the rudiment of the auditory
nerve, develops a ganglionic enlargement and, turning backwards,
closely hugs the ventral wall of the auditory involution (fig. 272).
The seventh or facial nerve soon becomes more complicated. It early
develops, like the glossopharyngeal and vagus nerves, a branch, which
forks over the cleft in front (spiracle), and supplies the mandibular
arch (fig. 271 B). This branch forms the præspiracular nerve of the
adult, and is homologous with the chorda tympani of Mammalia. Besides
however giving rise to this typical branch it gives origin, at a very
early period, to two other rather remarkable branches; one of these,
arising from its dorsal anterior border, passes forwards to the front
part of the head, immediately dorsal to the ophthalmic branch of the
fifth to be described directly. This nerve is the portio major or
superficialis of the nerve usually known as the ramus ophthalmicus
superficialis in the adult[176].
[Pg 460]
The other branch of the seventh is the palatine branch—superficial
petrosal of Mammalia—the course of which has been more fully
investigated by Marshall than by myself. He has shewn that it arises
"just below the root of the ophthalmic branch,” and “runs downwards
and forwards, lying parallel and immediately superficial to the
maxillary branch of the fifth nerve.” This branch of the seventh nerve
appears to bear the same sort of relation to the superior maxillary
branch of the fifth nerve, that the ophthalmic branch of the seventh
does to the ophthalmic branch of the fifth.
Both the root of the seventh and its main branches are gangliated.
The auditory nerve is probably to be regarded as a specially
differentiated part of a dorsal branch of the seventh, while the
ophthalmic branch may not improbably be a dorsal branch comparable to
a dorsal branch of one of the spinal nerves.
The fifth nerve. Shortly after its development the root of the fifth
nerve shifts so as to be attached about halfway down the side of the
brain. A large ganglion becomes developed close to the root, which
forms the rudiment of the Gasserian ganglion. The main branch of the
nerve grows into the mandibular arch (fig. 271 A, V), maintaining
towards it similar relations to those of the posterior nerves to their
respective arches.
Two other branches very soon become developed, which were not properly
distinguished in my original account. The dorsal one takes a course
parallel to the ophthalmic branch of the seventh nerve, and forms,
according to the nomenclature already adopted, the portio profunda of
the ophthalmicus superficialis of the adult.
The second nerve (fig. 271 A) passes forwards, above the mandibular
head cavity, and is directed straight towards the eye, near which it
meets and unites with the third nerve, where the ciliary ganglion is
developed (Marshall). This branch is usually called the ophthalmic
branch of the fifth nerve, but Marshall rightly prefers to call it the
communicating branch between the fifth and third nerves[177].
Later than these two branches there is developed a third branch,
passing to the front of the mouth, and forming the superior maxillary
branch of the adult (fig. 271 B).
Of the branches of the fifth nerve the main mandibular branch is
obviously comparable to the main branch of the posterior nerves. The
superficial ophthalmic branch is clearly equivalent to the ophthalmic
branch of the seventh. The superior maxillary is usually held to be
equivalent to that branch of the posterior nerves which forms the
anterior limb of the fork over a cleft. The similarity between the
course of this nerve and that of the palatine branch of the seventh,
resembling as it does the similar course of the ophthalmic branches of
the two nerves, suggests that it may perhaps really be the homologue
of the palatine branch of the seventh, there
[Pg 461] being no homologue of the
typical anterior branch of the other cranial nerves.
The third nerve. Our knowledge of the development of the third nerve
is entirely due to Marshall. He has shewn that in the Chick there is
developed from the neural crest, on the roof of the mid-brain, an
outgrowth on each side, very similar to the rudiment of the posterior
nerves. This outgrowth, the presence of which I can confirm, he
believes to be the third nerve, but although he is probably right in
this view, it must be borne in mind that there is no direct evidence
on the point, the fate of the outgrowth in question not having been
satisfactorily followed.
At a very considerably later period a nerve may be found springing
from the floor of the mid-brain, which is undoubtedly the third
nerve, and which Marshall supposes to be the above rudiment, which has
shifted its position. It is shewn in Scyllium in fig. 271 B, III. A
few intermediate stages between this and the earliest condition of the
nerve have been imperfectly traced by Marshall.
The nerve at the stage represented in fig. 271 B arises from a
ganglionic root, and “runs as a long slender stem almost horizontally
backwards, then turns slightly outwards to reach the interval between
the dorsal ends of the first and second head cavities, where it
expands into a small ganglion.” This ganglion, as first suggested by
Schwalbe (No. 357), and subsequently proved embryologically by
Marshall, is the ciliary ganglion. From the ciliary ganglion two
branches arise; one branch continuing the main stem of the nerve, and
obviously homologous with the main branch of the other nerves, and the
other passing directly forwards “along the top of the first head
cavity, then along the inner side of the eye, and finally terminating
at the anterior extremity of the head, just dorsal of the olfactory
pit.”
The partial separation, in many forms, of the ciliary ganglion from
the stem of the third nerve has led to the erroneous view (disproved
by the researches of Marshall and Schwalbe) that the ciliary ganglion
belongs to the fifth nerve. The connecting branch of the fifth nerve
often becomes directly continuous with the anterior branch of the
third nerve, and the two together probably constitute the nerve known
as the ramus ophthalmicus profundus (Marshall). Further embryological
investigations will be required to shew whether this nerve is
homologous with the nasal branch of the fifth nerve in Mammalia.
Relations of the nerves to the head-cavities. The cranial nerves,
whose development has just been given, bear certain very definite
relations to the mesoblastic structures in the head, of the nature of
somites, which are known as the head-cavities. Each cranial nerve is
typically placed immediately behind the head-cavity of its somite.
Thus the main branch of the fifth nerve lies in contact with the
posterior wall of the mandibular cavity, as shewn in section in fig.
272 V. 2pp and in surface view in fig. 271; the main branch of the
seventh nerve occupies a similar position in relation to the hyoid
cavity; and, as Marshall has recently shewn, the main branch of the
third nerve adjoins the posterior border of the front
[Pg 462] cavity,
described by me as the premandibular cavity. Owing to the early
conversion of the walls of the posterior head-cavities into muscles,
their relations to the nerves are not quite so clear as in the case of
the anterior cavities, though, as far as is known, they are precisely
the same.
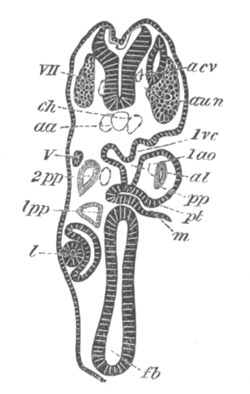
Fig. 272. Transverse section through the front part of the head of
a young Pristiurus embryo.
The section, owing to the cranial flexure, cuts both the fore- and
the hind-brain. It shews the præmandibular and mandibular
head-cavities 1pp and 2pp, etc.
fb. fore-brain; l. lens of eye; m. mouth; pt. upper end of
mouth, forming pituitary involution; 1ao. mandibular aortic arch;
1pp. and 2pp. first and second head-cavities; 1vc. first
visceral cleft; V. fifth nerve; aun. ganglion of auditory nerve;
VII. seventh nerve; aa. dorsal aorta; acv. anterior cardinal
vein; ch. notochord.
Anterior nerve-roots in the brain.
During my investigations on the development of the cranial nerves I
was unable to find any roots comparable with the anterior roots of the
spinal nerves, and propounded an hypothesis (suggested by the absence
of anterior spinal roots in Amphioxus[178])
that the head and trunk
had become differentiated from each other at a stage when mixed motor
and sensory posterior roots were the only roots present, and I
supposed the cranial and spinal nerves to have been independently
evolved from a common ground form, the resulting types of nerves being
so different that no roots strictly comparable with the anterior roots
of spinal nerves were to be found in the cranial nerves.
The views put forward by me on this subject, though accepted by
Schwalbe (No. 357), have in other quarters not met with much favour.
Wiedersheim holds that it is impossible to believe that the cranial
nerves are simpler than the spinal nerves. Such simplicity, which is
clearly not found, I have never asserted to exist; I have only stated
that the cranial nerves, in acquiring the complicated character they
have in the adult, do not develop anterior roots comparable with those
of the spinal nerves. Marshall also strongly objects to my views, and
has made some observations for the purpose of testing them, leading to
some very interesting results, which I proceed to state, and I will
then explain my opinion concerning them.
The most important observation of Marshall on this subject concerns
the sixth nerve. In both the Chick and Scyllium he has detected a
nerve (the first development of which has unfortunately not been made
out) arising by a series of roots from the base of the hind-brain. By
tracing this nerve to the external rectus muscle of the eye he has
satisfactorily identified
[Pg 463] it as the sixth nerve. “Neither in the
nerve nor in its roots are there any ganglion cells." This nerve he
finds to be placed vertically below the roots of the seventh nerve;
and it is not visible till much later than the cranial nerves above
described.
In addition to this nerve Marshall has found, both in the third nerve
and in the fifth nerve, a series of non-gangliated roots, which arise
in a manner not yet satisfactorily elucidated, considerably later
than, and in front of, the main roots. These roots join the gangliated
roots on the proximal side of the ganglion or in the ganglion[179];
and Marshall believes them to be homologous with the anterior roots of
spinal nerves, while he holds the sixth nerve to be an anterior root
of the seventh nerve.
In addition to these nerves Marshall holds certain ventral roots,
which occur in Elasmobranchs close to the boundary of the spinal cord
and medulla, and which probably form the hypoglossal nerve of higher
types, to be anterior roots of the vagus. It is very difficult to
prove anything definitely about these nerves, but, for reasons stated
in my work on Elasmobranch Fishes, I am inclined to regard them as
anterior roots of one or more spinal nerves.
Before attempting to decide how far Marshall’s views about the
so-called anterior roots of the seventh, the fifth and the third
nerves are well founded it will conduce to clearness to state the
characters and relations of the two roots of spinal nerves.
The posterior root is (1) always purely sensory; (2) it always
develops a ganglion. The anterior root is (1) always purely motor; (2)
it always joins the posterior root below the ganglion, except in
Petromyzon (though not in Myxine) where the two roots are stated to be
independent.
How far do Marshall’s anterior and posterior roots of the cranial
nerves exhibit these respective peculiarities?
With reference to the sixth and seventh nerves he states “we must
regard the sixth nerve as having the same relation to the seventh that
the anterior root of a spinal nerve has to the posterior root.” On
this I would remark (1) that the posterior root of this nerve is a
mixed sensory and motor nerve and therefore differs in a very
fundamental point from that of a spinal nerve; (2) the sixth nerve
though resembling the anterior root of a spinal nerve in being motor
and without a ganglion, differs from the nearly universal arrangement
of spinal nerves in not uniting with the seventh.
With reference to the fifth nerve it is to be observed that it is by
no means certain that the whole of the motor fibres are supplied by
the so-called anterior roots, and that these roots differ again in the
most marked manner from the anterior roots of spinal nerves in joining
the main root of the nerve above (nearer the brain), and not as in a
spinal nerve below the
[Pg 464] ganglion. The gangliated root of the third
nerve is purely motor[180],
and its so-called anterior roots again
differ from the anterior roots of spinal nerves, in the same manner as
those of the fifth nerve.
With reference to the glossopharyngeal and vagus nerves I would merely
remark that no anterior root has even been suggested for the
glossopharyngeal nerve and that the posterior roots of both these
nerves contain a mixture of sensory and motor fibres.
In view of these facts, my original hypothesis appears to me to be
confirmed by Marshall’s observations.
The fact of all the posterior roots of the above cranial nerves
(except the third which may be purely motor) being mixed motor and
sensory roots appears to me to demonstrate that the starting-point of
their differentiation was a mixed nerve with a single dorsal root; and
that they did not therefore become differentiated from nerves built on
the same type as the spinal nerves with dorsal sensory and ventral
motor roots. The presence of such non-gangliated roots as those of the
third and fifth nerves is not a difficulty to this view. Considering
that the cranial nerves are more highly differentiated than the spinal
nerves, and have more complicated functions to perform, it would be
surprising if there had not been developed nonganglionated roots
analogous to, but not of course homologous with, the anterior roots
of the spinal nerves[181].
As to the sixth nerve further embryological investigations are
requisite before its true position in the series can be determined;
but it appears to me very probable that it is a product of the
differentiation of the seventh nerve.
The fourth nerve. No embryological investigations have been made with
reference to the fourth nerve. It is possible that it is a segmental
nerve comparable with the third nerve, and that the only remnant still
left of the segment to which it belongs is the superior oblique muscle
of the eye. If this is the case there must have been two præmandibular
segments, viz. that belonging to the third nerve, and that belonging
to the fourth nerve. Against this view of the fourth nerve is the
fact, urged with great force by Marshall, that the superior oblique
muscle is in front of the other eye muscles, and that the fourth nerve
therefore crosses the third nerve to reach its destination.
The Olfactory nerve. It was shewn in my monograph on Elasmobranch
Fishes that the olfactory nerve grew out from the brain in the
[Pg 465] same
manner as other nerves; and Marshall (No. 355), to whom we are
indebted for the greater part of our knowledge on the development of
this nerve, has proved that it arises prior to the differentiation of
the olfactory lobes.
The earliest stages in the development of the nerve have not been made
out. Marshall, as already stated, finds that in the Chick the neural
crest is continued in front of the optic vesicles, and holds that this
fact is strong a priori evidence in favour of the nerve growing out
from it. As mentioned above, note on p. 456, I cannot without further
evidence accept Marshall’s statements on this point. In any case
Marshall has not yet been able again to find an olfactory nerve till
long after the disappearance of the neural crest. The olfactory nerve
at the next stage observed forms an outgrowth of fusiform cells
springing on either side from near the summit of the fore-brain; and
at fifty hours it ends close to a slight thickening of the epiblast
forming the first rudiment of the olfactory pit, with the walls of
which it soon becomes united.
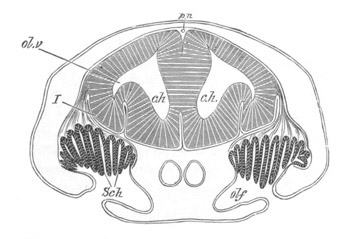
Fig. 273. Section through the brain and olfactory organ of an
embryo of Scyllium. (Modified from figures by Marshall and myself.)
c.h. cerebral hemispheres; ol.v. olfactory vesicle; olf.
olfactory pit; Sch. Schneiderian folds; I. olfactory nerve. The
reference line has been accidentally taken through the nerve to the
brain; pn. pineal gland.
The growth of the cerebral hemispheres causes its point of insertion
in the brain to be relatively shifted; and on the development of the
olfactory lobes (vide pp. 444, 445) it arises from them (fig. 273).
In Elasmobranchs there is a large development of ganglion cells near
its root. From Marshall’s figures these appear also to be present in
the Chick, but they do not seem to have been found in other forms. In
both Teleostei and Amphibia the olfactory nerves are at first
extremely short.
Marshall holds that the olfactory nerve is a segmental nerve
equivalent to the third, fifth, seventh etc. nerves. It has been
already stated that in my opinion the origin of the olfactory nerves
from the fore-brain, which I hold to be the ganglion of the præoral
lobe, negatives this view. The mere fact
[Pg 466] of these nerves originating
as an outgrowth from the central nervous system is no argument in
favour of Marshall’s view of their nature; and even if Marshall’s
opinion that they arise from the neural crest should turn out to be
well founded, this fact would not prove their segmental nature,
because their origin from this crest would, as indicated in the next
paragraph, merely seem to imply that they primitively arose from the
lateral borders of the nerve-plate from which the cerebrospinal tube
has been formed.
Situation of the dorsal roots of the cranial and spinal nerves. The
probable explanation of the origin of nerves from the neural crest has
already been briefly given (p. 316). It is that the neural crest
represents the original lateral borders of the nervous plate, and
that, in the mechanical folding of the nervous plate to form the
cerebrospinal canal, its two lateral borders have become approximated
in the median dorsal line to form the neural crest. The subsequent
shifting of the nerves I am unable to explain, and the meaning of the
transient longitudinal commissure connecting the nerves is also
unknown. The folding of the neural plate must have extended to the
region of the origin of the olfactory nerves, so that, as just stated,
there would be no special probability of the olfactory nerves
belonging to the same category as the other dorsal nerves from the
fact of their springing from the neural crest.
Bibliography of the Peripheral Nervous System.
(351) F. M. Balfour. “On the development of the spinal nerves in
Elasmobranch Fishes.” Philosophical Transactions, Vol. CLXVI. 1876;
vide also, A monograph on the development of Elasmobranch Fishes.
London, 1878, pp. 191-216.
(352) W. His. “Ueb. d. Anfänge d. peripherischen Nervensystems.”
Archiv f. Anat. u. Physiol., 1879.
(353) A. M. Marshall. “On the early stages of development of the
nerves in Birds.” Journal of Anat. and Phys., Vol. XI. 1877.
(354) A. M. Marshall. “The development of the cranial nerves in the
Chick.” Quart. J. of Micr. Science, Vol. XVIII. 1878.
(355) A. M. Marshall. “The morphology of the vertebrate olfactory
organ.” Quart. J. of Micr. Science, Vol. XIX. 1879.
(356) A. M. Marshall. “On the head-cavities and associated nerves in
Elasmobranchs.” Quart. J. of Micr. Science, Vol. XXI. 1881.
(357) C. Schwalbe. “Das Ganglion oculomotorii.” Jenaische
Zeitschrift, Vol. XIII. 1879.
Sympathetic nervous system.
The discovery that the spinal and cranial nerves together with their
ganglia were formed from the epiblast was shortly afterwards extended
to the sympathetic nervous system, which has now been shewn to arise
in connection with the spinal and
[Pg 467] cranial nerves. The earliest
observations on this subject were those contained in my Monograph on
Elasmobranch Fishes (p. 173), while Schenk and Birdsell (No. 361)
have since arrived at the same result for Aves and Mammalia.
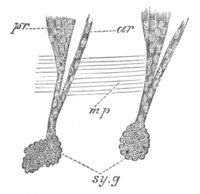
Fig. 274. Longitudinal vertical section through part of the body
wall of an Elasmobranch embryo shewing part of two spinal nerves and
the sympathetic ganglia belonging to them.
ar. anterior root; pr. posterior root; sy.g. sympathetic
ganglion; mp. part of muscle-plate.
In my account of the development of these ganglia, it is stated that
they were first met with as small masses situated at the ends of short
branches of the spinal nerves (fig. 275 sy.g). More recent
investigations have shewn me that the sympathetic ganglia are at first
simply swellings on the main branches of the spinal nerves some way
below the ganglia. Their situation may be understood from fig. 274,
sy.g, which belongs however to a somewhat later stage. Subsequently
the sympathetic ganglia become removed from the main stem of their
respective nerves, remaining however connected with those stems by a
short branch (fig. 275, sy.g). I have been unable to find a
longitudinal commissure connecting them in their early stages; and I
presume that they are at first independent, and become subsequently
united into a continuous cord on each side.
The observations of Schenk and Birdsell on the Mammalia seem to
indicate that the main parts of the sympathetic system arise in
continuity with the posterior spinal ganglia: they also shew that in
the neck and other parts the sympathetic cords arise as a continuous
ganglionic chain. The observations on the topographical features of
the development of the sympathetic system in higher types are however
as yet very imperfect.
The later history of the sympathetic ganglia is intimately bound up
with that of the so-called suprarenal bodies, which are dealt with in
another chapter.
[Pg 468]
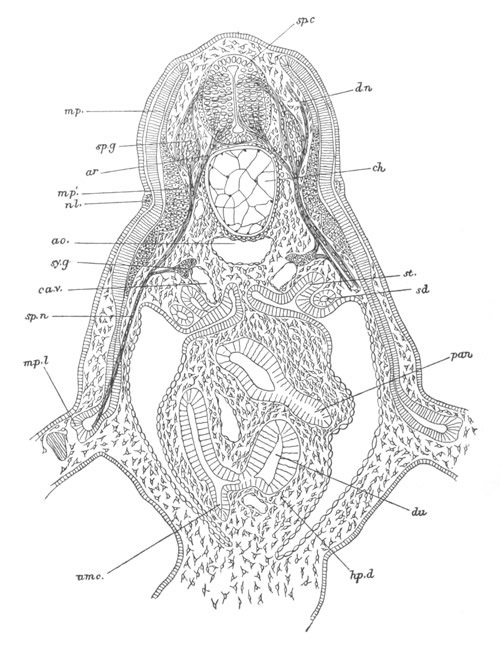
Fig. 275. Transverse section through the anterior part of the trunk
of an embryo of Scyllium slightly older than fig. 29 B.
The section is diagrammatic in the fact that the anterior
nerve-roots have been inserted for their whole length; whereas they
join the spinal cord halfway between two posterior roots.
sp.c. spinal cord; sp.g. ganglion of posterior root; ar.
anterior root; d.n. dorsally directed nerve springing from
posterior root; mp. muscle plate; mp´. part of muscle plate
already converted into muscles; mp.l. part of muscle plate which
gives rise to the muscles of the limbs; nl. nervus lateralis;
ao. aorta; ch. notochord; sy.g. sympathetic ganglion; ca.v.
cardinal vein; sp.n. spinal nerve; sd. segmental (archinephric)
duct; st. segmental tube; du. duodenum; pan. pancreas; hp.d.
point of junction of hepatic duct with duodenum; umc. umbilical
canal.
[Pg 469]
Bibliography of the Sympathetic Nervous System.
(360) F. M. Balfour. Monograph on the development of Elasmobranch
Fishes. London, 1878, p. 173.
(361) S. L. Schenk and W. R. Birdsell. “Ueb. d. Lehre von d.
Entwicklung d. Ganglien d. Sympatheticus.” Mittheil. a. d.
embryologischen Instit. Wien. Heft III. 1879.
[Pg 470]
CHAPTER XVI.
ORGANS OF VISION.
In the lowest forms of animal life the whole surface is sensitive to
light, and organs of vision have no doubt arisen in the first instance
from limited areas becoming especially sensitive to light in
conjunction with a deposit of pigment. Lens-like structures, formed
either as a thickening of the cuticle, or as a mass of cells, were
subsequently formed; but their function was not, in the first
instance, to throw an image of external objects on the perceptive part
of the eye, but to concentrate the light on it. From such a simple
form of visual organ it is easy to pass by a series of steps to an eye
capable of true vision.
There are but few groups of the Metazoa which are not provided with
optic organs of greater or less complexity.
In a large number of instances these organs are placed on the anterior
part of the head, and are innervated from the anterior ganglia. It is
possible that many of the eyes so situated may be modifications of a
common prototype. In other instances organs of vision are situated in
different regions of the body, and it is clear that such eyes have
been independently evolved in each instance.
The percipient elements of the eye would invariably appear to be
cells, one end of each of which is continuous with a nerve, while the
other terminates in a cuticular structure, or indurated part of the
cell forming what is known as the rod or cone.
The presence of such percipient elements in various eyes is therefore
no proof of genetic relationship between these eyes, but merely of
similarity of function.
Embryological data as to the development of the eye do not
[Pg 471] exist
except in the case of the Arthropoda, Mollusca and Chordata. From such
data as there are, combined with study of the adult structure of the
eye, it can be shewn that two types of development are found. In one
of these the percipient elements are formed from the central nervous
system, in the other from the epidermis. The former may be called
cerebral eyes. It is probable however that this distinction is not, in
all cases at any rate, so fundamental as might be supposed; but that
in both instances the eye may have taken its origin from the
epidermis. In the eyes in which the retina is continuous with the
central nervous system, these two organs were probably evolved
simultaneously as differentiations of the epidermis, and continue to
develop together in the ontogenetic growth of the eye.
Some of the eyes in which the retina is formed from the epidermis have
also probably arisen simultaneously with part of the central nervous
system, while in other instances they have arisen as later formations
subsequently to the complete establishment of a central nervous
system.
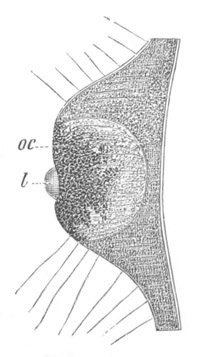
Fig. 276. Eye Of Lizzia Koellikeri. (From Lankester; after
Hertwig.)
l. lens; oc. perceptive part of eye.
Cœlenterata. The actual evolution of the eye is best shewn in the
Hydrozoa. The simplest types are those found in Oceania and
Lizzia[182].
In Lizzia the eye is placed at the base of a tentacle and
consists of (fig. 276) a lens (l) and a percipient bulb (oc). The
lens is a simple thickening of the cuticle, while the percipient part
of the eye is formed of three kinds of elements:—(1) pigment cells;
(2) sense cells, forming the true retinal elements, and consisting of
a central swelling with the nucleus, a peripheral process representing
a hardly differentiated rod, and a central process continuous with (3)
ganglion cells at the base of the eye. In this eye there is present a
commencing differentiation of a ganglion as well as of a retina.
The eye of Oceania is simpler than that of Lizzia in the absence of a
lens. Claus has shewn that in
[Pg 472] Charybdea amongst the Acraspeda a more
highly differentiated eye is present, with a lens formed of cells like
the vertebrate eye.
Mollusca. In a large number of the odontophorous Mollusca eyes,
innervated by the supraœsophageal ganglia, are present on the dorsal
side of the head. These eyes exhibit very various degrees of
complexity, but are shewn both by their structure and development to
be modifications of a common prototype.
The simplest type of eye is that found in the Nautilus, and although
the possibility of this eye being degenerated must be borne in mind,
it is at the same time very interesting to note (Hensen) that it
retains permanently the early embryonic structure of the eyes of the
other groups.
It has (fig. 277 A) the form of a vesicle, with a small opening in the
outer wall, placing the cavity of the vesicle in free communication
with the exterior. The cells lining the posterior face of the vesicle
form a retina (R); and are continuous with the fibres of the optic
nerve (N.op). We have no knowledge of the development of this eye.
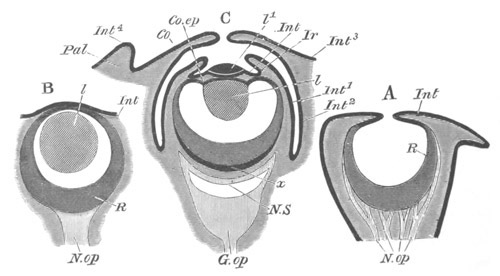
Fig. 277. Three diagrammatic sections of the eyes of Mollusca.
(After Grenacher.)
A. Nautilus. B. Gasteropod (Limax or Helix). C. Dibranchiate
Cephalopod.
Pal. eyelid; Co. cornea; Co.ep. epithelium of ciliary body;
Ir. iris; Int, Int1 ... Int4. different parts of the
integument; l. lens; l1. outer segment of lens; R. retina;
N.op. optic nerve; G.op. optic ganglion; x. inner layer of
retina; N.S. nervous stratum of retina.
In the Gasteropods the eye (fig. 277 B) has the form of a closed
vesicle: the cells lining the inner side form the retina, while the
outer wall of the vesicle constitutes the cornea. A
[Pg 473] cuticular lens is
placed in the cavity, on the side adjoining the cornea. This eye
originates from the ectoderm, within the velar area, and close to the
supraœsophageal ganglia, usually at the base of the tentacles.
According to Rabl (Vol. II. No. 268) it is formed as an invagination,
the opening of which soon closes; while according to Bobretzky (Vol.
II. No. 242) and Fol it arises as a thickening of the epiblast, which
becoming detached takes the form of a vesicle. It is quite possible
that both types of development may occur, the second being no doubt
abbreviated. The vesicle, however formed, soon acquires a covering of
pigment, except for a small area of its outer wall, where the lens
becomes formed as a small body projecting into the lumen of the
vesicle. The lens seems to commence as a cuticular deposit, and to
grow by the addition of concentric layers. The inner wall of the
vesicle gives rise to the retina.
The most highly differentiated molluscan eye is that of the
Dibranchiate Cephalopoda, which is in fact more highly organized than
any other invertebrate eye.
A brief description of its adult structure[183]
will perhaps render
more clear my account of the development. The most important features
of the eye are shewn in fig. 277 C. The outermost layer of the optic
bulb forms a kind of capsule, which may be called the sclerotic.
Posteriorly the sclerotic abuts on the cartilaginous orbit, which
encloses the optic ganglion (G. op); and in front it becomes
transparent and forms the cornea Co, which may be either completely
closed, or (as represented in the diagram) perforated by a larger or
smaller opening. Behind the cornea is a chamber known as the anterior
optic chamber. This chamber is continued back on each side round a
great part of the circumference of the eye, and separates the
sclerotic from a layer internal to it.
In the anterior optic chamber there are placed (1) the anterior part
of the lens (l1) and (2) the folds of the iris (Ir). The whole
chamber, except the part formed by the lens, is lined by the epidermis
(Int1 and Int2). Bounding the inner side of the anterior optic
chamber is a layer which is called the choroid (Int1) which is
continued anteriorly into the fold of the iris (Ir). The most
superficial layer of the choroid is the epithelium already mentioned,
next comes a layer of obliquely placed plates known as the argentea
externa, then a layer of muscles, and finally the argentea interna.
The argentea interna abuts on a cartilaginous capsule, which
completely invests the inner part of the eye.
The lens is a nearly spherical body composed of concentric lamellæ of
a structureless material. It is formed of a small outer (l1) and
large inner
[Pg 474] (l) segment, the two being separated by a thin membrane.
It is supported by a peculiar projection of the wall of the optic cup,
known as the ciliary body (Co.ep), inserted at the base of the iris,
and mainly formed of a continuation of the retina. This body is
however muscular, and presents a series of folds on its outer and
inner surfaces, which are especially developed on the latter.
The membrane dividing the lens into two parts is continuous with the
ciliary body. Within the lens is the inner optic chamber, bounded in
front by the lens and the ciliary body, and behind by the retina.
The retina is formed of two main divisions, an anterior division
adjoining the inner optic chamber, and a posterior division (N.S)
adjoining the cartilage of the choroid. The two layers are separated
by a membrane. Passing from within outwards the following layers in
the retina may be distinguished:
Anterior division of retina.
(1) Homogeneous membrane.
(2) Layer of rods.
(3) Layer of granules imbedded in pigment.
Posterior layer of retina.
(4) Cellular layer.
(5) Connective tissue layer
(6) Layer of nerve-fibres.
At the side of the optic ganglion is a peculiar body, known as the
white body (not shewn in the figure), which has the histological
characters of glandular tissue.
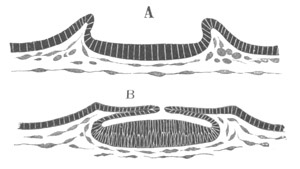
Fig. 278. Two sections through the developing eye of a Cephalopod
to shew the formation of the optic cup. (After Lankester.)
The first satisfactory account of the development of the eye is due to
Lankester (No. 365). The more important features in it were also
independently worked out by Grenacher (No. 363), and are beautifully
illustrated in Bobretzky’s paper (No. 362). The eye first appears as
an oval pit of the epiblast, the edge of which is formed by a
projecting rim (fig. 278 A). The epiblast layer lining the floor of
the pit soon becomes considerably thickened. By the growth inwards of
the rim the mouth of the pit
[Pg 475] is gradually narrowed (fig. 278 B),
resembling at this stage the eye of Nautilus, and finally closed.
There is thus formed a flattened sack, lined by epiblast, which may be
called the primary optic vesicle. Its cavity eventually forms the
inner optic chamber. The anterior wall of the sack is lined by a much
less columnar layer than the posterior, the former giving rise to the
epithelium on the inner side of the ciliary processes, the latter to
the retina.
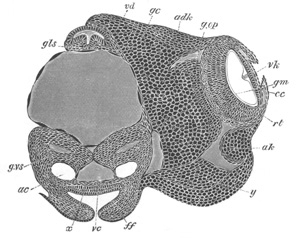
Fig. 279. Transverse section through the head of an advanced
embryo of Loligo. (After Bobretzky.)
gls. salivary gland; g.vs. visceral ganglion; gc. cerebral
ganglion; g.op. optic ganglion; adk. optic cartilage; ak. and
y. lateral cartilage or (?) white body; rt. retina; gm.
limiting membrane of retina; vk. ciliary region of eye; cc.
iris; ac. auditory sack (the epithelium lining the auditory sacks
is not represented); vc. vena cava; ff. folds of funnel; x.
epithelium of funnel.
The cavity of the sack rapidly enlarges, and assumes a spherical form.
At the same time a layer of mesoblast grows in between the walls of
the sack and the external epiblast. Two new structures soon arise
nearly simultaneously (fig. 279),—which become in the adult eye the
iris (cc) and the posterior segment of the lens. The iris is formed
as a circular fold of the skin in front of the optic vesicle. It
consists both of epiblast and mesoblast, and gives rise to a pit lined
by epiblast. The posterior segment of the lens arises as a
structureless rod-like body, which is shewn in fig. 279 depending from
the inner side
[Pg 476] of the anterior wall of the optic vesicle. Its exact
mode of origin is somewhat obscure. The following is Lankester’s
account of it[184]:
“It is formed entirely within the primitive optic
chamber, and at first depends as a short cylindrical rod from the
middle point of the anterior wall of that chamber, that is to say,
from the point at which the chamber finally closed up. It grows
subsequently by the deposition of concentric layers of a horny
material round this cone. No cells appear to be immediately concerned
in effecting the deposition, and it must be looked upon as an organic
concretion, formed from the liquid contained in the primitive optic
chamber.”
The lens would thus appear to be a cuticular structure. It gradually
assumes a nearly spherical form; and is then composed of
concentrically arranged layers (fig. 280, hl).
While the lens is being formed, the ciliary epithelium of the optic
vesicle becomes divided into two layers, an outer layer of large cells
and an inner of small cells. Both layers are at first continuous
across the anterior wall of the optic chamber in front of the lens,
but soon become confined to the sides (fig. 280 A, cc and gz). The
inner layer is stated by Lankester to give rise to the muscles present
in the adult. The mesoblast cells also disappear from the region in
front of the lens, and the outer epithelium is converted into a kind
of cuticular membrane. By these changes the original layers of cells
in front of the lens become reduced to mere membranes,—a change which
appears to be preparatory to the appearance of the anterior segment of
the lens. The formation of the latter has not been fully followed out
by any investigator except Bobretzky. His figures would seem to
indicate that it is formed as a cuticular deposit in front of the
membrane already spoken of (fig. 280 B, vl). The two segments of the
lens appear at any rate to be separated by a membrane continuous with
the ciliary region of the optic vesicle.
Grenacher believes that the front part of the lens is formed in a
pocket-like depression of the epiblastic layer covering the outer side
of the optic cup; and Lankester thinks that the lens “pushes its way
through the median anterior area of the primitive optic chamber, and
projects into the second or anterior optic chamber where the iridian
folds lie closely upon it.”
[Pg 477]
While the lens is attaining its complete development there appears a
fresh fold round the circumference of the eye, which gradually grows
inwards so as to form a chamber outside the parts already present.
This chamber is the anterior optic chamber of the adult. In most
Cephalopods (fig. 277 C) the edges of the fold do not quite meet, but
leave a larger or smaller aperture leading into the chamber containing
the iris, outer segment of the lens, etc. In some forms however they
meet and coalesce, and so shut off this chamber from communication
with the exterior. The edge of the fold constitutes the cornea while
the remainder of it gives rise to the sclerotic.
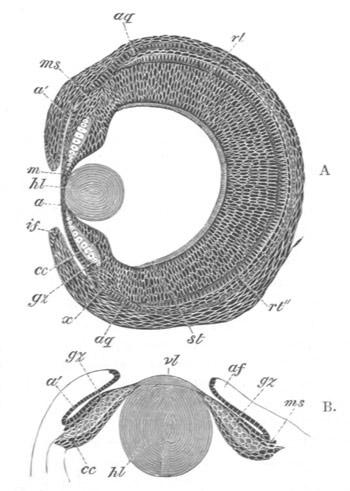
Fig. 280. Sections through the developing eye of Loligo
at two stages. (After Bobretzky.)
hl. inner segment of lens; vl. outer segment of lens; a and
a´. epithelium lining the anterior optic chamber; gz. large
epiblast cells of ciliary body; cc. small epiblast cells of
ciliary body; ms. layer of mesoblast between the two epiblastic
layers of the ciliary body; af. and if. fold of iris; rt.
retina; rt´´. inner layer of retina; st. rods; aq. cartilage of
the choroid.
The retina is at first a thick layer of numerous rows of oval
[Pg 478] cells
(fig. 279). When the inner segment of the lens is far advanced towards
its complete formation pigment becomes deposited in the anterior part
of the retina, and a layer of rods grows out from the surface turned
towards the cavity of the optic vesicle (fig. 280 A, st). At a
slightly later stage the retina becomes divided into two layers
(Bobretzky), a thicker anterior layer, and a thinner posterior layer
(fig. 280, rt and rt´´). The former is composed of two strata, (1)
the rods and (2) a stratum with numerous rows of nuclei which becomes
in the adult the granular layer with its pigment. The posterior layer
gives rise to the cellular part of the posterior division of the
retina, while layers of connective tissue around it give rise to the
connective tissue of this portion of the retina (layer 6 in the scheme
on p. 474). The nervous layer is derived from the optic ganglion which
attaches itself to the inner side of the connective tissue layer.
The greater part of the choroid is formed from the mesoblast adjoining
the retina, but the epithelium covering its outer wall is of
epiblastic origin.
It is difficult to decide from development whether the Molluscan eyes,
so far dealt with, originated in the first instance pari passu with
the supraœsophageal ganglia or independently at a later period. On
purely à priori ground I should be inclined to adopt the former
alternative.
In addition to the above eyes there occur amongst Mollusca highly
complicated eyes, of a very different kind, in two widely separated
groups, viz. certain species of a genus of slug (Onchidium), and
certain Lamellibranchiata. These eyes, though they have no doubt been
evolved independently of each other, present certain remarkable points
of agreement. In both of them the rods of the retina are turned away
from the surface, and the nerve-fibres are placed, as in the
Vertebrate eye, on the side of the retina which faces outwards.
The peculiar eyes of Onchidium, investigated by Semper[185],
are
scattered on the dorsal surface, there being normal eyes in the usual
situation on the head. The eyes on the dorsal surface are formed of a
cornea, a lens composed of 1-7 cells, and a retina surrounded by
pigment; which is perforated in the centre by an optic nerve, the
retinal elements being in the inverted position above mentioned.
The development of these eyes has been somewhat imperfectly studied in
the adult, in which they continue to be formed anew. They arise by a
[Pg 479]
differentiation of the epidermis at the end of a papilla. At first a
few glandular cells appear in the epidermis in the situation where an
eye is about to be formed. Then, by a further process of growth, an
irregular mass of epidermic cells becomes developed, which pushes the
glandular cells to one side, and constitutes the rudiment of the eye.
This mass, becoming surrounded by pigment, unites with the optic
nerve, and its cells then differentiate themselves, in situ, into
the various elements of the eye. No explanation is offered by Semper
of the inverted position of the rods, nor is any suggested by his
account of the development. As pointed out by Semper these eyes are no
doubt modifications of the sensory epithelium of the papillæ.
The eyes of Pecten and Spondylus[186]
are placed on short stalks at
the edge of the mantle, and are probably modifications of the
tentacular processes of the mantle edge. They are provided with a
cornea, a cellular lens, a vitreous chamber, and a retina. The retinal
elements are inverted, and the optic nerve passes in at the side, but
occupies, in reference to its ramifications, the same relative
situation as the optic nerve in the Vertebrate eye. The development
has unfortunately not yet been studied.
Our knowledge of the structure or still more of the development of the
organ of vision of the Platyelminthes, Rotifera, and Echinodermata is
too scanty to be of any general interest.
Chætopoda. Amongst the Chætopoda the cephalic eyes of Alciope (fig.
281) have been adequately investigated as to their anatomy by Greeff.
These are provided with a large cuticular lens (l), separated from
the retina by a wide cavity containing the vitreous humour. The retina
is formed of a single row of cells, with rods at their free
extremities, continuous at their opposite ends with nerve-fibres. The
development of this eye has not been worked out. Eyes not situated
on the head are found in Polyophthalmus, and have probably been
evolved from the more indifferent type of sense-organ found by Eisig
in the allied Capitellidæ.
Chætognatha[187].
The paired cephalic eyes of Sagitta are spherical
bodies imbedded in the epidermis. They are formed of a central mass of
pigment with three lenses partially imbedded in it. The outer covering
of the eye is the retina, which is mainly composed of rod-bearing
cells; the rods being placed in contact with the outer surface of each
of the lenses. In the presence of three lenses the eye of Sagitta
approaches in some respects the eye of the Arthropoda.
Arthropodan eye. A satisfactory elucidation of the phylogeny of
Arthropodan eyes has not yet been given.
Fig. 281. Eye of an Alciopid (Neophanta Celox). (From Gegenbaur;
after Greef.)
i. cuticle; c. continuation of cuticle in front of eye; l.
lens; h. vitreous humour; o. optic nerve; o´. expansion of the
optic nerve; b. layer of rods; p. pigment layer.
All the types of eyes found in the group (with exception of
[Pg 480] that of
Peripatus)[188]
present marked features of similarity, but I am
inclined to view this similarity as due rather to the character of the
exoskeleton modifying in a more or less similar way all the forms of
visual organs, than to the descent of all these eyes from a common
prototype. In none of these eyes is there present a chamber filled
with fluid between the lens and the retina, but the space in question
is filled with cells. This character sharply distinguishes them from
such eyes as those of Alciope (fig. 281). The types of eyes which are
found in the Arthropoda are briefly the following:
(1) Simple eyes. In all simple eyes the corneal lens is formed by a
thickening of the cuticle. Such eyes are confined to the Tracheata.
There are three types of simple eyes. (a) A type in which the
retinal cells are placed immediately behind the lens, found
[Pg 481] (Lowne) in
the larvæ of some Diptera (Eristalis), and also in some Chilognatha.
(b) A type of simple eye found in some Chilopoda, and in some Insect
larvæ (Dytiscus, etc.) (fig. 282), the parts of which are entirely
derived from the epidermis. There is present a lens (l) formed as a
thickening of the cuticle, a so-called vitreous humour (gl) formed
of modified hypodermis cells, and a retina (r) derived from the same
source. The outer ends of the retinal cells terminate in rods, and
their inner ends are continuous with nerve-fibres.
Fig. 282. Section through the simple eye of a young Dytiscus
larva. (From Gegenbaur; after Grenacher.)
l. corneal lens; g. vitreous humour; r. retina; o. optic
nerve; h. hypodermis.
(c) A type of simple eye found in the Arachnida, and apparently some
Chilopoda, and forming the simple eyes of most Insects, which differs
from type (a) in the cells of the retina forming a distinct layer
beneath the hypodermis; the latter only obviously giving rise to the
vitreous humour.
The development of the simple eyes has not yet been studied.
The simple eyes so far described are always placed on the head, and
are usually rather numerous.
(2) Compound eyes. Compound eyes are almost always present in the
Crustacea, and are usually found in adult Insects. In both groups they
are paired, though in the Crustacea a median much simplified compound
eye may either take the place of the paired eyes in the Nauplius larva
and lower forms, or be present together with them during a period in
the development of higher forms.
The typical compound eye is formed (fig. 283) of a series of corneal
lenses (c) developed from the cuticle; below which are placed bodies
known as the crystalline cones, one to each corneal lens; and below
the crystalline cones are placed bodies known as the retinulæ (r)
constituting the percipient elements of the eye, each of them being
formed of an axial rod, the rhabdom, and a number of cells surrounding
it.
[Pg 482]
The crystalline cones are formed from the coalescence of cuticular
deposits in several cells, the nuclei of which usually remain as
Semper’s nuclei. These cells are probably simple hypodermis cells, but
in some forms, e.g. Phronima, there may be a continuous layer of
hypodermis cells between them and the cuticle. In various Insect eyes
the cells which usually give rise to a crystalline cone may remain
distinct, and such eyes have been called by Grenacher aconous eyes,
while eyes with incompletely formed crystalline cones are called by
him pseudoconous eyes.
The rhabdom of the retinulæ is, like the crystalline cone, developed
by the coalescence of a series of parts, which are primitively
separate rods placed each in its own cell: this condition of the
retinulæ is permanently retained in the eyes of the Tipulidæ.
The development of the compound eye has so far only been
satisfactorily studied in some Crustacea by Bobretzky (No. 367); by
whom it has been worked out in Palæmon and Astacus, but more fully in
the latter, to which the following account refers:
Fig. 283. Diagrammatic representations of parts of a compound
Arthropod eye. (From Gegenbaur.)
A. Section through the eye.
B. Corneal facets.
C. Two segments of
the eye.
c. corneal (cuticular) lenses; r. retinulæ with rhabdoms; n.
optic nerve; g. ganglionic swelling of optic nerve.
The eye of Astacus takes its origin from two distinct parts, (1) the
external epidermis of the procephalic lobes which will be spoken of as
the epidermic layer of the eye, (2) a portion of the supraœsophageal
ganglia, which will be spoken of as the neural layer of the eye. The
mesoblast is moreover the source of some of the pigment between the
two above layers. The epidermic layer gives rise to the corneal
lenses, the crystalline cones, and the pigment around the latter. The
neural layer on the other hand seems to give rise to the retinulæ with
their rhabdoms, and to the optic ganglion.
After the separation of the supraœsophageal ganglia from the
superficial epiblast, the cells of the epidermis in the region of the
future eye become columnar, and so form the above-mentioned epidermic
layer of the eye. This layer soon becomes two or three cells deep. At
the same time the most superficial part of the adjoining
supraœsophageal ganglion becomes partially constricted off from the
remainder as the neural layer of the eye, but is separated by a small
space from the thickened patch of epidermis.
[Pg 483] Into this space some
mesoblast cells penetrate at a slightly later period. Both the
epidermic and neural layers next become divided into two strata. The
outer stratum of the epidermic layer gives rise to the crystalline
cones and Semper’s nuclei; each crystalline cone being formed from
four coalesced rods, developed as cuticular differentiations of four
cells, the nuclei of which may be seen in the embryo on its outer
side. The lower ends of the cones pass through the inner stratum of
the epidermic disc, the cells of which become pigmented, and
constitute the pigment cells surrounding the lower part of the
crystalline cones in the adult. The outer end of each of the
crystalline cones is surrounded by four cells, believed by Bobretzky
to be identical with Semper’s nuclei[189].
These cells give rise in a
later stage (not worked out in Astacus) to the cuticular corneal
lenses.
Of the two strata of the neural layer the outer is several cells deep,
while the inner is formed of elongated rod-like cells. Unfortunately
however the fate of the two neural layers has not been worked out,
though there can be but little doubt that the retinulæ originate from
the outer layer.
The mesoblast which grows in between the neural and epidermic layers
becomes a pigment layer, and probably also forms the perforated
membrane between the crystalline cones and the retinulæ.
The above observations of Bobretzky would appear to indicate that the
paired compound eyes of Crustacea belong to the type of cerebral eyes.
How far this is also the case with the compound eyes of Insects is
uncertain, in that it is quite possible that the latter eyes may have
had an independent origin.
The relation between the paired and median eye of the Crustacea is
also uncertain.
In the genus Euphausia amongst the Schizopods there is present a
series of eyes placed on the sides of some of the thoracic legs and on
the sides of the abdomen. The structure of these eyes, though not as
yet satisfactorily made out, would appear to be very different from
that of other Arthropodan visual organs.
The Eye of the Vertebrata. In view of the various structures which
unite to form it, the eye is undoubtedly the most complicated organ of
the Vertebrata; and though its mode of development is fairly constant
throughout the group, it will be convenient shortly to describe what
may be regarded as its typical development, and then to proceed to a
comparative view of the origin of its various parts, and to enter into
greater detail with reference to some of them. At the end of the
section
[Pg 484] there is an account of the accessory structures connected with
the eye.
The formation of the eye commences with the appearance of a pair of
hollow outgrowths from the anterior cerebral vesicle or
thalamencephalon, which arise in many instances, even before the
closure of the medullary canal. These outgrowths, known as the optic
vesicles, at first open freely into the cavity of the anterior
cerebral vesicle. From this they soon however become partially
constricted, and form vesicles (fig. 284, a), united to the base of
the brain by comparatively narrow hollow stalks, the rudiments of the
optic nerves. The constriction to which the stalk or optic nerve is
due takes place obliquely downwards and backwards, so that the optic
nerves open into the base of the front part of the thalamencephalon
(fig. 284, b).
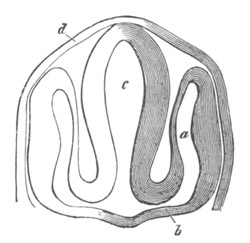
Fig. 284. Section through the head of an embryo Teleostean, to
shew the formation of the optic vesicles, etc. (From Gegenbaur;
after Schenk.)
c. fore-brain; a. optic vesicle; b. stalk of optic vesicle;
d. epidermis.
After the establishment of the optic nerves, there take place (1) the
formation of the lens, and (2) the formation of the optic cup from the
walls of the primary optic vesicle.
The external or superficial epiblast which covers, and is in most
forms in immediate contact with, the most projecting portion of the
optic vesicle, becomes thickened. This thickened portion is then
driven inwards in the form of a shallow open pit with thick walls
(fig. 285 A, o), carrying before it the front wall (r) of the
optic vesicle. To such an extent does this involution of the
superficial epiblast take place, that the front wall of the optic
vesicle is pushed close up to the hind wall, and the cavity of the
vesicle becomes almost obliterated (fig. 285 B).
The bulb of the optic vesicle is thus converted into a cup with double
walls, containing in its cavity the portion of involuted epiblast.
This cup, in order to distinguish its cavity from that of the original
optic vesicle, is generally called the secondary optic vesicle. We
may, for the sake of brevity, speak of it as the optic cup; in
reality it never is a vesicle, since it
[Pg 485] always remains widely open in
front. Of its double walls the inner or anterior (fig. 285 B, r) is
formed from the front portion, the outer or posterior (fig. 285 B,
u) from the hind portion of the wall of the primary optic vesicle.
The inner or anterior (r), which very speedily becomes thicker than
the other, is converted into the retina: in the outer or posterior
(u), which remains thin, pigment is eventually deposited, and it
ultimately becomes the tesselated pigment-layer of the choroid.
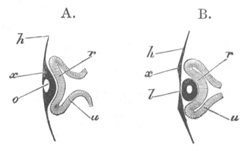
Fig. 285. Diagrammatic sections illustrating the formation of the
eye. (After Remak.)
In A the thin superficial epiblast h is seen to be thickened at
x, in front of the optic vesicle, and involuted so as to form a
pit o, the mouth of which has already begun to close in.
Accompanying this involution, which forms the rudiment of the lens,
the optic vesicle is doubled in, its front portion r being pushed
against the back portion u, and the original cavity of the vesicle
thus reduced in size. The stalk of the vesicle is shewn as still
broad.
In B the optic vesicle is still further doubled in so as to form a
cup with a posterior wall u and an anterior wall r. In the
hollow of this cup lies the lens l, now completely detached from
the superficial epiblast xh.
By the closure of its mouth the pit of the involuted epiblast becomes
a completely closed sac with thick walls and a small central cavity
(fig. 285 B, l). At the same time it breaks away from the external
epiblast, which forms a continuous layer in front of it, all traces of
the original opening being lost. There is thus left lying in the cup
of the secondary optic vesicle, an isolated elliptical mass of
epiblast. This is the rudiment of the lens. The small cavity within it
speedily becomes still less by the thickening of the walls, especially
of the hinder one.
At its first appearance the lens is in immediate contact with the
anterior wall of the secondary optic vesicle (fig. 285 B). In a short
time however, the lens is seen to lie in the mouth of the cup (fig.
288 D), a space (vh) (which is occupied by the vitreous humour)
making its appearance between the lens and anterior wall of the
vesicle.
In order to understand how this space is developed, the position of
the optic vesicle and the relations of its stalk must be borne in
mind.
The vesicle lies at the side of the head, and its stalk is directed
downwards, inwards and backwards. The stalk in fact
[Pg 486] slants away from
the vesicle. Hence, when the involution of the lens takes place, the
direction in which the front wall of the vesicle is pushed in is not
in a line with the axis of the stalk, as for simplicity’s sake has
been represented in the diagram (fig. 285), but forms an obtuse angle
with that axis, after the manner of fig. 286, where s´ represents
the cavity of the stalk leading away from the almost obliterated
cavity of the primary vesicle.
Fig. 286 represents the early stage at which the lens fills the whole
cup of the secondary vesicle. The subsequent condition is brought
about through the rapid growth of the walls of the cup. This growth
however does not take place equally in all parts of the cup. The walls
of the cup rise up all round except that point of the circumference of
the cup which adjoins the stalk. While elsewhere the walls increase
rapidly in height, carrying so to speak the lens with them, at this
spot, which in the natural position of the eye is on its under
surface, there is no growth: the wall is here imperfect, and a gap is
left. Through this gap, which afterwards receives the name of the
choroidal fissure, a way is open from the mesoblastic tissue
surrounding the optic vesicle and stalk into the interior of the
cavity of the cup.
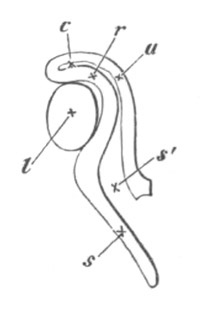
Fig. 286. Diagrammatic section of the eye and the optic nerve at
an early stage. (From Lieberkühn.)
To shew the lens l occupying the whole hollow of the optic cup,
the inclination of the stalk s to the optic cup, and the
continuity of the cavity of the stalk s´ with that of the primary
vesicle c; r. anterior, u. posterior wall of the optic cup.
From the manner of its formation the gap or fissure is evidently in a
line with the axis of the optic stalk, and in order to be seen must be
looked for on the under surface of the optic vesicle. In this position
it is readily recognised in the embryo seen as a transparent object
(fig. 118, chs).
Bearing in mind these relations of the gap to the optic stalk, the
reader will understand how sections of the optic vesicle at this stage
present very different appearances according to the plane in which the
sections are taken.
When the head is viewed from underneath as a transparent
[Pg 487] object the
eye presents very much the appearance represented in the diagram (fig.
287).
A section of such an eye taken along the line y, perpendicular to
the plane of the paper, would give a figure corresponding to that of
fig. 288 D. The lens, the cavity and double walls of the secondary
vesicle, the remains of the primary cavity, would all be represented
(the superficial epiblast of the head would also be shewn); but there
would be nothing seen of either the stalk or the fissure. If on the
other hand the section were taken in a plane parallel to the plane of
the paper, at some distance above the level of the stalk, some such
figure would be obtained as that shewn in fig. 288 E. Here the fissure
f is obvious, and the communication of the cavity vh of the
secondary vesicle with the outside of the eye evident; the section of
course would not go through the superficial epiblast. Lastly, a
section, taken perpendicular to the plane of the paper along the line
z, i.e. through the fissure itself, would present the appearances
of fig. 288 F, where the wall of the vesicle is entirely wanting in
the region of the fissure marked by the position of the letter f.
The external epiblast has been omitted in this figure.
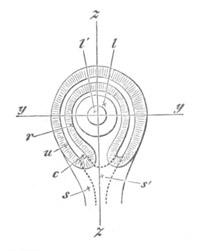
Fig. 287. Diagrammatic representation of the eye of the Chick of
about the third day as seen when the head is viewed from underneath as
a transparent object.
l. the lens; l´. the cavity of the lens, lying in the hollow of
the optic cup; r. the anterior, u. the posterior wall of the
optic cup; c. the cavity of the primary optic vesicle, now nearly
obliterated. By inadvertence u has been drawn in some places
thicker than r, it should have been thinner throughout. s. the
stalk of the optic cup with s´ its cavity, at a lower level than
the cup itself and therefore out of focus; the dotted line indicates
the continuity of the cavity of the stalk with that of the primary
vesicle.
The line z z, through which the section shewn in fig. 288 F is
supposed to be taken, passes through the choroidal fissure.
With reference to the above description, taken with very slight
alterations from the Elements of Embryology, Pt. 1., two points
require to be noticed. Firstly it is extremely doubtful whether the
invagination of the secondary optic vesicle is to be viewed as an
actual mechanical result of the ingrowth of the lens. Secondly it
seems probable that the choroid fissure is not simply due to an
inequality in the growth of the walls of the secondary optic cup, but
is partly due to a doubling up of the primary vesicle from the side
[Pg 488]
along the line of the fissure, at the same time that the lens is being
thrust in in front. In Mammalia, the doubling up involves the optic
stalk, which becomes flattened (whereby its original cavity is
obliterated) and then folded in on itself, so as to embrace a new
central cavity continuous with the cavity of the vitreous humour. And
in other forms a partial phenomenon of the same kind is usually
observable, as is more particularly described in the sequel.
Before describing the development of the cornea, aqueous humour, etc.
we may consider the further growth of the parts, whose first
development has just been described, commencing with the optic cup.
During the above changes the mesoblast surrounding the optic cup
assumes the character of a distinct investment, whereby the outline of
the eyeball is definitely formed. The internal portions of this
investment, nearest to the retina, become the choroid (i.e. the
chorio-capillaris, and the lamina fusca; the pigment epithelium,
as we have seen, being derived from the epiblastic optic cup), and
pigment is subsequently deposited in it. The remaining external
portion of the investment forms the sclerotic.
The complete differentiation of these two coats of the eye does not
however take place till a late period.
The cavity of the original optic vesicle was left as a nearly
obliterated space between the two walls of the optic cup. By the end
of the third day the obliteration is complete, and the two walls are
in immediate contact.
The inner or anterior wall is, from the first, thicker than the outer
or posterior; and over the greater part of the cup this contrast
increases with the growth of the eye, the anterior wall becoming
markedly thicker and undergoing changes of which we shall have to
speak directly (fig. 289).
In the front portion however, along, so to speak, the lip of the cup,
anterior to a line which afterwards becomes the ora serrata, both
layers cease to take part in the increased thickening, accompanied by
peculiar histological changes, which the rest of the cup is
undergoing. Thus a hind portion or true retina is marked off from a
front portion.
The front portion, accompanied by the mesoblast which immediately
overlies it, is behind the lens thrown into folds, the
[Pg 489] ciliary ridges;
while further forward it bends in between the lens and the cornea to
form the iris. The original wide opening of the optic cup is thus
narrowed to a smaller orifice, the pupil; and the lens, which before
lay in the open mouth of the cup, is now inclosed in its cavity. While
in the hind portion of the cup or retina proper no deposit of black
pigment takes place in the layer formed out of the inner or anterior
wall of the vesicle; in the front portion forming the region of the
iris, pigment is largely deposited throughout both layers, though
first of all in the outer one, so that eventually this portion seems
to become nothing more than a forward prolongation of the pigment
epithelium of the choroid.
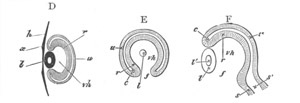
Fig. 288.
D. Diagrammatic section taken perpendicular to the plane of the
paper, along the line yy, fig. 287. The stalk is not seen, the
section falling quite out of its region. vh. hollow of optic cup
filled with vitreous humour; other letters as in fig. 285 B. (After
Remak.)
E. Section taken parallel to the plane of the paper through fig.
287, so far behind the front surface of the eye as to shave off a
small portion of the posterior surface of the lens l, but not so
far behind as to be carried at all through the stalk. Letters as
before; f. the choroidal fissure.
F. Section along the line zz, perpendicular to the plane of the
paper, to shew the choroidal fissure f, and the continuity of the
cavity of the optic stalk with that of the primary optic vesicle.
Had this section been taken a little to one side of the line zz,
the wall of the optic cup would have extended up to the lens below
as well as above. Letters as before. The external epiblast is
omitted in this section.
Thus, while the hind moiety of the optic cup becomes the retina
proper, including the choroid-pigment in which the rods and cones are
imbedded, the front moiety is converted into the ciliary portion of
the retina, covering the ciliary processes, and into the uvea of the
iris; the bodies of the ciliary processes and the substance of the
iris, their vessels, muscles, connective tissue and ramified pigment,
being derived from the mesoblastic choroid. The margin of the pupil
marks the extreme lip of the optic
[Pg 490] vesicle, where the outer or
posterior wall turns round to join the inner or anterior.
The ciliary muscle and the ligamentum pectinatum are both derived from
the mesoblast between the cornea and the iris.
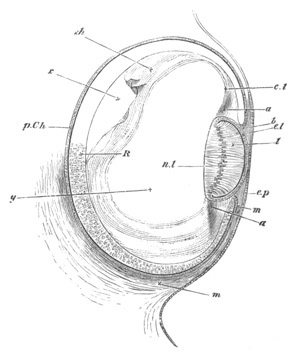
Fig. 289. Section of the eye of Chick at the fourth day.
e.p. superficial epiblast of the side of the head; R. true
retina: anterior wall of the optic cup; p.Ch. pigment-epithelium
of the choroid: posterior wall of the optic cup. b is placed at
the extreme lip of the optic cup at what will become the margin of
the iris. l. the lens. The hind wall, the nuclei of whose
elongated cells are shewn at nl, now forms nearly the whole mass
of the lens, the front wall being reduced to a layer of flattened
cells el. m. the mesoblast surrounding the optic cup and about
to form the choroid and sclerotic. It is seen to pass forward
between the lip of the optic cup and the superficial epiblast.
Filling up a large part of the hollow of the optic cup is seen a
hyaline mass, the rudiment of the hyaloid membrane, and of the
coagulum of the vitreous humour, y. In the neighbourhood of the
lens it seems to be continuous as at cl with the tissue a, which
appears to be the rudiment of the capsule of the lens and suspensory
ligament.
The Retina. At first the two walls of the optic cup do not greatly
differ in thickness. On the third day the outer or posterior becomes
much thinner than the inner or anterior, and by the middle of the
fourth day is reduced to a single layer of flattened
[Pg 491] cells (fig. 289,
p.Ch). At about the 80th hour its cells commence to receive a
deposit of pigment, and eventually form the so-called pigmentary
epithelium of the choroid; from them no part of the true retina (or no
other part of the retina, if the pigment-layer in question be supposed
to belong more truly to the retina than to the choroid) is derived.
On the fourth day, the inner (anterior) wall of the optic cup (fig.
289, R) has a perfectly uniform structure, being composed of
elongated somewhat spindle-shaped cells, with distinct nuclei. On its
external (posterior) surface a distinct cuticular membrane, the
membrana limitans externa, early appears.
As the wall increases in thickness, its cells multiply rapidly, so
that it soon becomes several cells thick: each cell being however
probably continued through the whole thickness of the layer. The wall
at this stage corresponds closely in its structure with the brain, of
which it may properly be looked upon as part. According to the usual
view, which is not however fully supported by the development, the
retina becomes divided in the subsequent growth into (1) an outer
part, corresponding morphologically to the epithelial lining of the
cerebrospinal canal, composed of what may be called the visual cells
of the eye, i.e. the cells forming the outer granular (nuclear)
layer and the rods and cones attached to them; and (2) an inner
portion consisting of the inner granular (nuclear) layer, the inner
molecular layer, the ganglionic layer and the layer of nerve-fibres
corresponding morphologically to the walls of the brain. According to
Löwe, however, only the outer limbs of the rods and cones, which he
holds to be metamorphosed cells, correspond to the epithelial layer of
the brain.
The actual development of the retina is not thoroughly understood.
According to the usual statements (Kölliker, No. 298, p. 693) the
layer of ganglion cells and the inner molecular layer are first
differentiated, while the remaining cells give rise to the rest of the
retina proper, and are bounded externally by the membrana limitans
externa. On the inner side of the ganglionic layer the stratum of
nerve-fibres is also very early established. The rods and cones are
formed as prolongations (Kölliker, Babuchin), or cuticularizations
(Schultze, W. Müller) of the cells which eventually form the outer
granular layer. The layer of cells external to the molecular layer is
not divided till comparatively late into the inner and outer granular
(nuclear) layers, and the interposed outer molecular layer.
[Pg 492]
Löwe’s account of the development of the retina in the Rabbit is in
many points different from the above. He finds that three stages in
the differentiation of the layers of the retina may be distinguished.
In the first stage, in an embryo of four or five millimetres, the
following layers are present, commencing at the outer side, adjoining
the external wall of the secondary optic cup.
(1) A membrane, which does not however, as usually believed, become
the membrana limitans externa.
(2) A layer of clear elements, derived from metamorphosed cells,
constituting the outer limbs of the rods and cones.
(3) A layer of dark rounded elements.
(4) An indistinctly striated layer, the future layer of nerve-fibres.
The third of these layers gives rise to all the eventual strata of the
retina proper, except the outer limbs of the rods and cones.
In the next stage, when the embryo has reached a length of 2 cm., this
layer becomes divided into three strata: viz. an outer and inner layer
of dark elements and a middle one of clearer elements. The two inner
of these layers become respectively the inner molecular layer and the
layer of ganglion cells, while the outer layer gives rise to the parts
of the retina external to the inner molecular layer.
In the newly born animal the outer darker layer of the previous stage
has become considerably subdivided. Its outermost part forms a stratum
of darkly coloured elements, which develop into the inner limbs of the
rods and cones. It is bounded internally by a membrane—the true
membrana elastica externa. The part of the layer within this is soon
divided into the outer and inner granular layers, separated from each
other by the delicate outer molecular layer. Thus, shortly after
birth, all the layers of the retina are established in the Rabbit. It
is important to notice that, according to Löwe’s views, the outer and
inner limbs of the rods and cones are metamorphosed cells. The outer
limbs at first form a continuous layer, in which separate elements
cannot be recognised.
At a very early period there appears a membrane on the side of the
retina adjoining the vitreous humour. This membrane is the hyaloid
membrane. The investigations of Kessler and myself lead to the
conclusion that it may be formed at a time when there is no trace of
mesoblastic structures in the cavity of the vitreous humour, and that
it is therefore necessarily developed as a cuticular deposit of the
cells of the optic cup. Lieberkühn, Arnold, Löwe, and other authors
regard it however as a mesoblastic product; and Kölliker believes that
a primitive membrane is developed from the cells of the optic cup, and
that a true hyaloid membrane is developed much later as a product of
the mesoblast.
For fuller information on this subject the reader is referred to the
authors quoted above.
The optic nerve. The optic nerves are derived, as we have said, from
the at first hollow stalks of the optic vesicles. Their
[Pg 493] cavities
gradually become obliterated by a thickening of the walls, the
obliteration proceeding from the retinal end inwards towards the
brain. While the proximal ends of the optic stalks are still hollow
the rudiments of the optic chiasma are formed from fibres at the roots
of the stalks, the fibres of the one stalk growing over into the
attachment of the other. The decussation of the fibres would appear to
be complete. The fibres arise in the remainder of the nerves somewhat
later. At first the optic nerve is equally continuous with both walls
of the optic cup; as must of necessity be the case, since the interval
which primarily exists between the two walls is continuous with the
cavity of the stalk. When the cavity within the optic nerve vanishes,
and the fibres of the optic nerve appear, all connection is ruptured
between the outer wall of the optic cup and the optic nerve, and the
optic nerve simply perforates the outer wall, and becomes continuous
with the inner one.
There does not appear to me any ground for doubting (as has been done
by His and Kölliker) that the fibres of the optic nerve are derived
from a differentiation of the epithelial cells of which the nerve is
at first formed.
Choroid Fissure. With reference to the choroid fissure we may state
that its behaviour varies somewhat in the different types. It becomes
for the greater part of its extent closed, though its proximal end is
always perforated by the optic nerve, and in many forms by a
mesoblastic process also.
The lens when first formed is an oval vesicle with a small central
cavity, the front and hind walls being of nearly equal thickness, and
each consisting of a single layer of elongated columnar cells. In the
subsequent stages the mode of growth of the hind wall is of precisely
an opposite character to that of the front wall. The hind wall becomes
much thicker, and tends to obliterate the central cavity by becoming
convex on its front surface. At the same time its cells, still
remaining as a single layer, become elongated and fibre-like. The
front wall on the contrary becomes thinner and thinner and its cells
flattened.
These modes of growth continue until, as shewn in fig. 289, the hind
wall l is in absolute contact with the front wall el, and the
cavity thus becomes entirely obliterated. The cells of the hind wall
have by this time become veritable fibres, which, when
[Pg 494] seen in
section, appear to be arranged nearly parallel to the optic axis,
their nuclei nl being seen in a row along their middle. The front
wall, somewhat thickened at either side where it becomes continuous
with the hind wall, is now a single layer of flattened cells
separating the hind wall of the lens, or as we may now say the lens
itself, from the front limb of the lens-capsule; of the latter it
becomes the epithelium.
The subsequent changes undergone consist chiefly in the continued
elongation and multiplication of the lens-fibres, with the partial
disappearance of their nuclei.
During their multiplication they become arranged in the manner
characteristic of the adult lens of the various forms. The
lens-capsule, as was originally stated by Kölliker, appears to be
formed as a cuticular membrane deposited by the epithelial cells of
the lens.
The views of Lieberkühn, Arnold, Löwe and others, according to which
the lens-capsule is a mesoblastic structure, do not appear to be well
founded. The contrary view, held by Kölliker, Kessler, etc., is
supported mainly by the fact that at the time when the lens-capsule
first appears there are no mesoblast cells to give rise to it. It
should however be stated that W. Müller has actually found cellular
elements in what he believes to be the lens-capsule of the Ammocœte
lens. Considering the degraded character of the Ammocœte eye,
evidence derived from its structure must be accepted with caution.
The vitreous humour. The vitreous humour is derived (except in
Cyclostomata) from a vascular ingrowth, which differs considerably in
different types, through the choroid slit. Its real nature is very
much disputed. According to Kessler’s view, it is of the nature of a
fluid transudation, but the occasional presence in it of ordinary
embryonic mesoblast cells, in addition to more numerous
blood-corpuscles, gives it a claim to be regarded as intercellular
substance. The number of cells in it is however at best extremely
small and in many cases there is no trace of them. In Mammals there
appear to be some mesoblast cells invaginated with the lens, which are
not improbably employed in the formation of the vessels of the
so-called membrana capsulo-pupillaris. In the Ammocœte the vitreous
humour originates from a distinct mesoblastic ingrowth, though the
cells which give rise to it subsequently disappear.
[Pg 495]
The development of the zonula of Zinn in Mammalia, which ought to
throw some light on the nature of the vitreous humour, has not been
fully investigated. According to Lieberkühn (No. 373, p. 43), this
structure appears in half-grown embryos of the sheep and calf.
He says “At the point where the ciliary processes and the ciliary part
of the retina are entirely removed, one sees in the meridian bundles
of fine fibres, which correspond to the valleys between the ciliary
processes and fill them; also between these bundles there extend, as a
thin layer, similar finely striated masses, and these would have been
on the top of the ciliary processes.” He further states that these
fibres may be traced to the anterior and posterior limb of the
lens-capsule, and that amongst them are numerous cells. Kölliker
confirms Lieberkühn’s statements. There can be little doubt that the
fibres of the zonula are of the nature of connective tissue: they are
stated to be elastic. By Löwe they are believed to be developed out of
the substance of the vitreous humour, but this does not appear to me
to follow from the observations hitherto made. It seems quite possible
that they arise from mesoblast cells which have grown into the cavity
of the vitreous humour, solely in connection with their production.
The integral parts of the eye in front of the lens are the cornea, the
aqueous humour, and the iris. The development of the latter has
already been described, and there remain to be dealt with the cornea,
and the cavity containing the aqueous humour.
The cornea. The cornea is formed by the coalescence of two structures,
viz. the epithelium of the cornea and the cornea proper. The former is
directly derived from the external epiblast, which covers the eye
after the invagination of the lens. The latter is formed in a somewhat
remarkable manner, first clearly made out by Kessler.
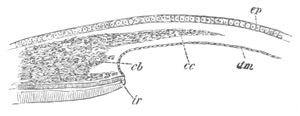
Fig. 290. Section through the eye of a Fowl on the eighth day
of development, to shew the iris and cornea in the process of
formation. (After Kessler.)
ep. epiblastic epithelium of cornea; cc. corneal corpuscles
growing into the structureless matrix of the cornea; dm.
Descemet’s membrane; ir. iris; cb. mesoblast of the iris (this
reference letter points a little too high).
The space between the layers dm. and ep. is filled with the
structureless matrix of the cornea.
When the lens is completely separated from the epidermis its outer
wall is directly in contact with the external epiblast (future corneal
epithelium). At its edge there is a small ring-shaped space bounded by
the outer skin, the lens and the edge of the optic cup. In the chick,
which we may take as typical, there appears at about the time when the
cavity of the lens is completely obliterated a structureless layer
external to the above ring-like space and immediately adjoining the
inner face of the epiblast. This layer, which forms the commencement
of the cornea proper, at first only forms a ring at the border of the
lens, thickest at its outer edge, and gradually thinning off to
[Pg 496]
nothing towards the centre. It soon however becomes broader, and
finally forms a continuous stratum of considerable thickness,
interposed between the external skin and the lens. As soon as this
stratum has reached a certain thickness, a layer of flattened cells
grows in along its inner side from the mesoblast surrounding the optic
cup (fig. 290, dm). This layer is the epithelioid layer of the
membrane of Descemet. After it[190]
has become completely established,
the mesoblast around the edge of the cornea becomes divided into two
strata; an inner one (fig. 290, cb) destined to form the mesoblastic
tissue of the iris already described, and an outer one (fig. 290,
cc) adjoining the epidermis. The outer stratum gives rise to the
corneal corpuscles, which are the only constituents of the cornea not
yet developed. The corneal corpuscles make their way through the
structureless corneal layer, and divide it into two strata, one
adjoining the epiblast, and the other adjoining the inner epithelium.
The two strata become gradually thinner as the corpuscles invade a
larger and larger portion of their substance, and finally the
outermost portion of them alone remains as the membrana elastica
anterior and posterior (Descemet’s membrane) of the cornea. The
corneal
[Pg 497] corpuscles, which have grown in from the sides, thus form a
layer which becomes continually thicker, and gives rise to the main
substance of the cornea. Whether the increase in the thickness of the
layer is due to the immigration of fresh corpuscles, or to the
division of those already there, is not clear. After the cellular
elements have made their way into the cornea, the latter becomes
continuous at its edge with the mesoblast which forms the sclerotic.
The derivation of the original structureless layer of the cornea is
still uncertain. Kessler derives it from the epiblast, but it appears
to me more probable that Kölliker is right in regarding it as derived
from the mesoblast. The grounds for this view are, (1) the fact of its
growth inwards from the border of the mesoblast round the edge of the
eye, (2) the peculiar relations between it and the corneal corpuscles
at a later period. This view would receive still further support if a
layer of mesoblast between the lens and the epiblast were really
present as believed by Lieberkühn. It must however be admitted that
the objections to Kessler’s view of its epiblastic nature are rather
a priori than founded on definite observation.
The observations of Kessler, which have been mainly followed in the
above account, are strongly opposed by Lieberkühn (No. 374) and Arnold
(No. 370), and are not entirely accepted by Kölliker. It is especially
on the development of these parts in Mammalia (to be spoken of in the
sequel) that the above authors found their objections. I have had
through Kessler’s kindness an opportunity of looking through some of
his beautiful preparations, and have no hesitation in generally
accepting his conclusions, though as mentioned above I cannot agree
with all his interpretations.
The aqueous humour. The cavity for the aqueous humour has its origin
in the ring-shaped space round the front of the lens, which, as
already mentioned, is bounded by the external skin, the edge of the
optic cup, and the lens. By the formation of the cornea this space is
shut off from the external skin, and on the appearance of the
epithelioid layer of Descemet’s membrane a continuous cavity is
developed between the cornea and the lens. This cavity enlarges and
receives its final form on the full development of the iris.
Comparative view of the development of the Vertebrate Eye.
The organ of vision, when not secondarily aborted, contains in all
Vertebrata the essential parts above described. The most interesting
cases of partial degeneration are those of Myxine and the Ammocœte.
The development of such aborted eyes has as yet been studied only in
the
[Pg 498] Ammocœte[191],
in which it resembles in most important features
that of other Vertebrata.
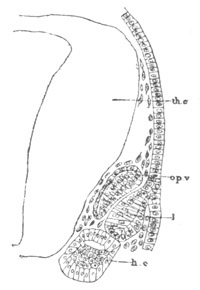
Fig. 291. Horizontal section through the head of a just hatched
larva of Petromyzon shewing the development of the lens of the
eye.
th.c. thalamencephalon; op.v. optic vesicle; l. lens of eye;
h.c. head cavity.
Eye of Ammocœtes. The optic vesicle arises as an outgrowth of the
fore-brain, but the secondary optic cup is remarkable in the young
larva for its small size (fig. 291, opv). The thicker outer wall
gives rise to the retina, and the thinner inner wall to the choroid
pigment. The lens is formed as an invagination of the single-layered
epidermis (fig. 291, l). As development proceeds the parts of the
eye gradually enlarge, and the mesoblast around the hinder and dorsal
part of the optic cup becomes pigmented. There is at first no cavity
for the vitreous humour, but eventually the growth of the optic cup
gives rise to a space, into which a cellular process of mesoblast
grows at a slight notch in the ventral edge of the optic cup (W.
Müller, No. 377). This notch is the only rudiment of the choroid
fissure of other types. The mesoblastic process is probably the
homologue of the processus falciformis and pecten, and appears to give
rise to the vitreous humour; for a long time it retains its connection
with the surrounding mesoblast. Its cells eventually disappear, and it
never contains any vascular structures.
The lens for a long time remains as an oval vesicle with a central
cavity. In a later stage, when the Ammocœte is fully developed,
the secondary optic cup forms a deep pit (fig. 292, r); in the
mouth of which is placed the lens (l). The two walls of the
retina have now the normal vertebrate structure, though the pigment is
as yet imperfectly present in the choroid layer. The lens has the
embryonic forms of higher types (cf. fig. 289), consisting of an inner
thicker segment, the true lens, and an outer layer forming the
epithelium of the lens capsule. The edge of the optic cup, which forms
the rudiment of the epiblast of the iris, is imperfectly separated
from the remainder of the optic cup; and a mesoblastic element of the
iris, distinct from Descemet’s membrane (dm), can hardly be
spoken of.
There is no cavity for the aqueous humour in front of the lens; and
there is no cornea as distinct from the epidermis and subepidermic
tissues. The elements in front of the lens are (1) the epidermis
(ep); (2) the dermis (dc); (3) the subdermal connective tissue
(sdc) which passes without any sharp line of demarcation into the
dermis; (4) a thick membrane, continuous with the mesoblastic part of
the choroid, which appears to represent Descemet’s membrane. The
subdermal connective tissue is continued as an
[Pg 499] investment round the
whole eye; and there is no differentiated sclerotic and only an
imperfect choroid.
In a still later stage a distinct mesoblastic element for the iris is
formed. When the Ammocœte is becoming a Lamprey, the eye approaches
the surface; an anterior chamber is established; and the eye differs
from that of the higher types mainly in the fact that the cornea is
hardly distinguished from the remainder of the skin, and that a
sclerotic is very imperfectly represented.
Optic vesicles. The development of the primitive optic vesicles, so
far as is known, is very constant throughout the Vertebrata. In
Teleostei and Lepidosteus alone is there an important deviation from
the ordinary type, dependent however upon the mode of formation of the
medullary keel, the optic vesicles arising while the medullary keel is
still solid, and being at first also solid. They subsequently acquire
a lumen and undergo the ordinary changes.
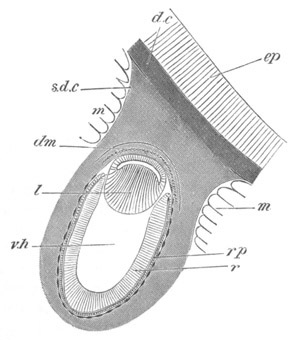
Fig. 292. Eye of an Ammocœtes lying beneath the skin.
ep. epidermis; d.c. dermal connective tissue continuous with the
subdermal connective tissue (s.d.c), which is also shaded. There
is no definite boundary to this tissue where it surrounds the eye.
m. muscles; dm. membrane of Descemet; l. lens; v.h. vitreous
humour; r. retina; rp. retinal pigment.
The lens. In the majority of groups, viz. Elasmobranchii, Reptilia,
Aves, and Mammalia, the lens is formed by an open invagination of the
epiblast, but in Amphibia, Teleostei and Lepidosteus, where the
nervous layer of the skin is early established, this layer alone takes
part in the formation of the lens (fig. 293, l). The lens is however
formed even in these types as a hollow body by an invagination; but
its opening remains permanently shut off from communication with the
exterior by the epidermic
[Pg 500] layer of the epiblast. Götte describes the
lens as formed by a solid thickening of the nervous layer in
Bombinator. This is probably a mistake.
The cornea. The mode of formation of the cornea already described
appears to be characteristic of most Vertebrata except the Ammocœte.
It has been found by Kessler in Aves, Reptilia and Amphibia, and
probably also occurs in Pisces. In Mammals it is not however so easy
to establish. There are at first no mesoblast cells between the lens
and the epiblast (fig. 295) but in many Mammals (vide Kessler, No.
372, pp. 91-94) a layer of rounded mesoblast cells, which forms
Descemet’s membrane, grows in between the two, at a time when it is
not easy to recognise a corneal lamina, as distinct from a simple
coagulum.
After the formation of this layer the mesoblast cells grow into the
corneal lamina from the sides, and becoming flattened arrange
themselves in rows between the laminæ of the cornea. The cornea
continues to increase in thickness by the addition of laminæ on the
side adjoining the epiblast.
We have already seen that in the Lamprey the cornea is nothing else
but the slightly modified and more transparent epidermis and dermis.
The optic nerve and the choroid fissure. It will be convenient to
consider together the above structures, and with them the vascular and
other processes which pass into the cavity of the optic cup through
the choroid fissure. These parts present on the whole a greater amount
of variation than any other parts of the eye.
I commence with the Fowl which is both a very convenient general type
for comparison, and also that in which these structures have been most
fully worked out.
During the third day of incubation there passes in through the choroid
slit a vascular loop, which no doubt supplies the transuded material
for the growth of the vitreous humour. Up to the fifth day this
vascular loop is the only structure passing through the choroid slit.
On this day however a new structure appears, which remains permanently
through life, and is known as the pecten. It consists of a lamellar
process of the mesoblast cells round the eye, passing through the
choroid slit near the optic nerve, and enveloping part of the afferent
branch of the vascular loop above mentioned. The proximal part of the
free edge of the pecten is somewhat swollen, and sections through this
part have a club-shaped form. On the sixth day the choroid slit
becomes rapidly closed, so that at the end of the sixth day it is
reduced to a mere seam. There are however two parts of this seam where
the edges of the optic cup have not coalesced. The proximal of these
adjoins the optic nerve, and permits the passage of the pecten and at
a later period of the optic nerve; and the second or distal one is
placed near the ciliary edge of the slit, and is traversed by the
efferent branch of the above-mentioned vascular loop. This vessel soon
atrophies, and with it the distal opening in the choroid slit
completely vanishes. In some varieties of domestic Fowl (Lieberkühn)
the opening however persists. The seam which marks the original site
of the choroid slit is at first
[Pg 501] conspicuous by the absence of pigment,
and at a later period by the deep colour of its pigment. Finally, a
little after the ninth day, no trace of it is to be seen.
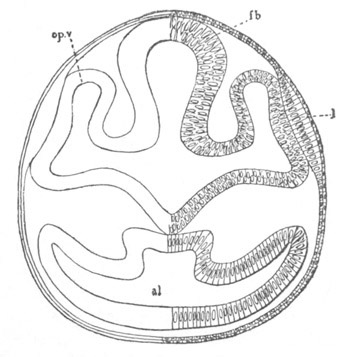
Fig. 293. Section through the front part of the head of a Lepidosteus
embryo on the seventh day after impregnation.
al. alimentary tract; fb. thalamencephalon; l. lens of eye;
op.v. optic vesicle. The mesoblast is not represented.
Up to the eighth day the pecten remains as a simple lamina; by the
tenth or twelfth day it begins to be folded or rather puckered, and by
the seventeenth or eighteenth day it is richly pigmented and the
puckerings have become nearly as numerous as in the adult, there being
in all seventeen or eighteen. The pecten is almost entirely composed
of vascular coils, which are supported by a sparse pigmented
connective tissue; and in the adult the pecten is still extremely
vascular. The original artery which became enveloped at the formation
of the pecten continues, when the latter becomes vascular, to supply
it with blood. The vein is practically a fresh development after the
atrophy of the distal portion of the primitive vascular loop of the
vitreous humour.
There are no true retinal blood-vessels.
In the formation of the optic cup the extreme peripheral part of the
optic nerve, which is in immediate proximity with the artery of the
pecten, becomes folded. The permanent opening in the choroid fissure
for the pecten is intimately related to the entrance of the optic
nerve into the eyeball; the fibres of the optic nerve passing in at
the inner border of the pecten, coursing along its sides to its outer
border, and radiating from it as from a centre to all parts of the
retina.
In the Lizard the choroid slit closes considerably earlier than in the
Fowl. The vascular loop in the vitreous humour is however more
developed. The pecten long remains without vessels, and does not in
fact become at all
[Pg 502] vascular till after the very late disappearance of
the distal part of the vascular loop of the vitreous humour.
The arrangement of the ingrowth through the choroid slit in
Elasmobranchii (Scyllium) has been partially worked out, and so far as
is at present known the agreement between the Avian and Elasmobranch
type is fairly close.
At the time when the cavity between the lens and the secondary optic
cup is just commencing to be formed, a process of mesoblast
accompanied by a vascular loop passes into the vitreous humour,
through the choroid slit, close to the optic nerve. The vessel in this
process is no doubt equivalent to the vascular loop in the Avian eye,
but I have not made out that it projects beyond the mesoblastic
process accompanying it. As the cavity of the vitreous humour enlarges
and the choroid slit elongates, the process through it takes the form
of a lamina with a somewhat swollen border, and projects for some
distance into the cavity of the vitreous humour.
At a later stage, after the outer layer of the optic cup has become
pigmented, the distal part of the choroid slit adjoining the border of
the lens closes up; but along the line where it was present the walls
of the optic cup remain very thin and are thrown into three folds, two
lateral and one median, projecting into the cavity of the vitreous
humour. The median fold is in contact with the lens, and the vascular
mesoblast surrounding the eye projects into the space between the two
laminæ of which it is formed. In passing from the region of the lens
to that of the optic nerve the lateral folds of the optic cup
disappear, and the median fold forms a considerable projection into
the cavity of the vitreous humour. It consists of a core of mesoblast
covered by a delicate layer derived from both strata of the optic cup.
Still nearer the optic nerve the choroid slit is no longer closed, and
the mesoblast, which in the neighbourhood of the lens only extended
into the folds of the wall of the optic cup, now projects freely into
the cavity of the vitreous humour, and forms the lamina already
described. It is not very vascular, but close to the optic nerve there
passes into it a considerable artery.
In the young animal the choroid slit is no longer perforated by a
mesoblastic lamina. At its inner end it remains open to allow of the
passage of the optic nerve. The line of the slit can easily be traced
along the lower side of the retina; and close to the lens the retinal
wall continues, as in the embryo, to be raised into a projecting fold.
Traces of these structures are visible even in the fully grown
examples of Scyllium.
As has been pointed out by Bergmeister the mesoblastic lamina
projecting into the vitreous humour resembles the pecten at an early
stage of development, and is without doubt homologous with it. The
artery which supplies it is certainly equivalent to the artery of the
pecten.
There can be no doubt that the mesoblastic lamina projecting into the
vitreous humour is equivalent to the processus falciformis of
Teleostei, and it seems probable that the whole of it, including the
free part as well as that covered by epiblast, ought to be spoken of
under this title. The optic nerve
[Pg 503] in Elasmobranchii is not included in
the folding to which the secondary optic vesicle owes its origin, and
would seem to perforate the walls of the optic cup only at the distal
end of the processus falciformis.
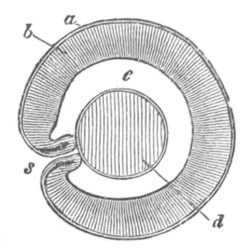
Fig. 294. Horizontal section through the eye of a Teleostean
embryo. (From Gegenbaur; after Schenk.)
s. choroid fissure, with two folds forming part of the processus
falciformis; a. choroid layer of optic cup; b. retinal layer of
optic cup; c. cavity of vitreous humour; d. lens.
In Teleostei there is at first a vascular loop like that in Birds,
passing through the choroid fissure. This has been noticed by Kessler
in the Pike, and by Schenk in the Trout. At a later period a
mesoblastic ingrowth with a blood-vessel makes its way in many forms
into the cavity of the vitreous humour, accompanied by two folds in
the walls of the free edges of the choroid fissure (fig. 294). These
structures, which constitute the processus falciformis, clearly
resemble very closely the mesoblastic process and folds of the optic
cup in Elasmobranchii. The processus falciformis comes in contact
with, and perhaps becomes attached to the wall of the lens; and
persists through life.
In Triton there is no vascular ingrowth through the choroid fissure,
but a few mesoblastic cells pass in which represent the vascular
ingrowth of other types. The optic nerve perforates the proximal
extremity of the original choroid slit.
The absence of an embryonic blood-vessel does not however hold good
for all Amphibia, as there is present in the embryo Alytes
(Lieberkühn) an artery, which breaks up into a capillary system on the
retinal border of the vitreous humour.
In the Ammocœte the choroid slit is merely represented by a slight
notch on the ventral edge of the optic cup, and the mesoblastic
process which passes through the choroid slit in most types is
represented by a large cellular process, from which the vitreous
humour would appear to be derived.
Mammalia differ from all the types already described in the immense
fœtal development of the blood-vessels of the vitreous humour. There
are however some points in connection with the development of these
vessels which are still uncertain. The most important of these points
concerns the presence of a prolongation of the mesoblast around the
eye into the cavity of the vitreous humour. It is maintained by
Lieberkühn, Arnold, Kölliker, etc., that in the invagination of the
lens a thin layer of mesoblast is carried before it; and is thus
transported into the cavity of the vitreous humour. This is denied by
Kessler, but the layer is so clearly figured by the above
embryologists, that the existence of it in some Mammalia (the Rabbit,
etc.) must I think be accepted.
In the folding in of the optic vesicle, which accompanies the
formation of the lens, the optic nerve becomes included, and on the
development of the cavity of the vitreous humour an artery, running in
the fold of the optic
[Pg 504] nerve, passes through the choroid slit into the
cavity of the vitreous humour (fig. 295, acr). The sides of the
optic nerve subsequently bend over, and completely envelope this
artery, which at a later period gives off branches to the retina, and
becomes known as the arteria centralis retinæ. It is homologous with
the arterial limb of the vascular loop projecting into the vitreous
humour in Birds, Lizards, Teleostei, etc.
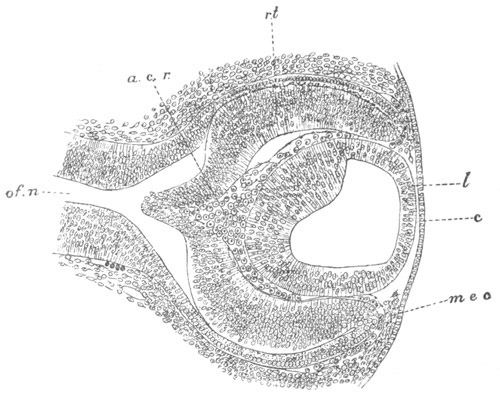
Fig. 295. Section through the eye of a Rabbit embryo of
about twelve days.
c. epithelium of cornea; l. lens; mec. mesoblast growing in
from the side to form the cornea; rt. retina; a.c.r. arteria
centralis retinæ; of.n. optic nerve.
The figure shews (1) the absence at this stage of mesoblast between
the lens and the epiblast: the interval between the two has however
been made too great; (2) the arteria centralis retinæ forming the
vascular capsule of the lens and continuous with vascular structures
round the edges of the optic cup.
Before becoming enveloped in the optic nerve this artery is continued
through the vitreous humour (fig. 295), and when it comes in close
proximity to the lens it divides into a number of radiating branches,
which pass round the edge of the lens, and form a vascular sheath
which is prolonged so as to cover the anterior wall of the lens. In
front of the lens they anastomose with vessels, coming from the iris,
many of which are venous (fig. 295)—and the whole of the blood from
the arteria centralis is carried away by these veins. The vascular
sheath surrounding the lens receives the name of the membrana
capsulo-pupillaris. The posterior part of it appears (Kessler, No.
372) to be formed of vessels without the addition of any other
structures and is either formed simply by branches of the arteria
centralis, or out of
[Pg 505] the mesoblast cells involuted with the lens. The
anterior part of the vascular sheath is however inclosed in a very
delicate membrane, the membrana pupillaris, continuous at the sides
with the epithelium of Descemet’s membrane. On the formation of the
iris this membrane lies superficially to it, and forms a kind of
continuation of the mesoblast of the iris over the front of the lens.
The origin of this membrane is much disputed. By Kessler, whose
statements have been in the main followed, it is believed to appear
comparatively late as an ingrowth of the stroma of the iris; while
Kölliker believes it to be derived from a mesoblastic ingrowth between
the front wall of the lens and the epiblast. According to Kölliker
this ingrowth subsequently becomes split into two laminæ, one of which
forms the cornea, and the other the anterior part of the vascular
sheath of the lens with its membrana pupillaris. Between the two
appears the aqueous humour.
The membrana capsulo-pupillaris is simply a provisional embryonic
structure, subserving the nutrition of the lens. The time of its
disappearance varies somewhat for the different Mammalia in which this
point has been investigated. In the human embryo it lasts from the
second to the seventh month and sometimes longer. As a rule it is
completely absorbed at the time of birth. The absorption of the
anterior part commences in the centre and proceeds outwards.
In addition to the vessels of the vascular capsule round the lens,
there arise from the arteria centralis retinæ, just after its exit
from the optic nerve, in many forms (Dog, Cat, Calf, Sheep, Rabbit,
Man) provisional vascular branches which extend themselves in the
posterior part of the vitreous humour. Near the ciliary end of the
vitreous humour they anastomose with the vessels of the membrana
capsulo-pupillaris.
In Mammals the choroid slit closes very early, and is not perforated
by any structure homologous with the pecten. The only part of the slit
which remains open is that perforated by the optic nerve; and in the
centre of the latter is situated the arteria centralis retinæ as
explained above. From this artery there grow out the vessels to supply
the retina, which have however nothing to do with the provisional
vessels of the vitreous humour just described (Kessler). On the
atrophy of the provisional vessels the whole of the blood of the
arteria centralis passes into the retina.
It is interesting to notice (Kessler, No. 372, p. 78) that there seems
to be a blood-vessel supplying the vitreous humour in the embryos of
nearly all vertebrate types, which is homologous throughout the
Vertebrata. This vessel often exhibits a persisting and a provisional
part. The latter in Mammalia is the membrana capsulo-pupillaris and
other vessels of the vitreous humour; in Birds and Lizards it is the
part of the original vascular loop, not included in the pecten, and in
Osseous Fishes that part (?) not involved in the processus
falciformis. The permanent part is formed by the retinal vessels of
Mammalia, by the vessels of the pecten in Birds and Lizards, and by
those of the processus falciformis in Fishes.
[Pg 506]
The Iris and Ciliary processes. The walls of the edge of the optic cup
become very much thinner than those of the true retinal part. In many
Vertebrates (Mammalia, Aves, Reptilia, Elasmobranchii, etc.) the
thinner part, together with the mesoblast covering it, becomes divided
into two regions, viz. that of the iris, and that of the ciliary
processes. In the Newt and Lamprey this differentiation does not take
place, but the part in question simply becomes the iris.
Accessory Organs connected with the Eye.
Eyelids. The most important accessory structures connected with the
eye are the eyelids. They are developed as simple folds of the
integument with a mesoblastic prolongation between their two laminæ.
They may be three in number, viz. an upper and lower, and a lateral
one—the nictitating membrane—springing from the inner or anterior
border of the eye. Their inner face is lined by a prolongation of
conjunctiva, which is the modified epiblast covering the cornea and
part of the sclerotic.
In Teleostei and Ganoidei eyelids are either not present or at most
very rudimentary. In Elasmobranchii they are better developed, and the
nictitating membrane is frequently present. The latter is also usually
found in Amphibia. In the Sauropsida all three eyelids are usually
present, but in Mammalia the nictitating membrane is rudimentary.
In many Mammalia the two eyelids meet together during a period of
embryonic life, and unite in front of the eye. A similar arrangement
is permanent through life in Ophidia and some Lacertilia; and there is
a chamber formed between the coalesced eyelids and the surface of the
cornea, into which the lacrymal ducts open.
Lacrymal glands. Lacrymal glands are found in the Sauropsida and
Mammalia. They arise (Remak, Kölliker) as solid ingrowths of the
conjunctival epithelium. They appear in the chick on the eighth day.
Lacrymal duct. The lacrymal duct first appears in Amphibia, and is
present in all the higher Vertebrates. Its mode of development in the
Amphibia, Lacertilia and Aves has recently been very thoroughly worked
out by Born (Nos. 380 and 381).
In Amphibia he finds that the lacrymal duct arises as a solid ridge of
the mucous layer of the epidermis, continued from the external opening
of the nasal cavity backwards towards the eye. It usually appears at
about the time when the nasal capsule is beginning to be chondrified.
As this ridge is gradually prolonged backwards towards the eye its
anterior end becomes separated from the epidermis, and grows inwards
in the mesoblast to become continuous with the posterior part of the
nasal sack. The posterior end which joins the eye becomes divided into
the two collecting branches of the adult. Finally the whole structure
becomes separated from the skin except at the external opening, and
develops a lumen.
[Pg 507]
In Lacertilia the lacrymal duct arises very much in the same manner as
in Amphibia, though its subsequent growth is somewhat different. It
appears as an internal ridge of the epithelium, at the junction of the
superior maxillary process and the fold which gives rise to the lower
eyelid. A solid process of this ridge makes its way through the
mesoblast on the upper border of the maxillary process till it meets
the wall of the nasal cavity, with the epithelium of which it becomes
continuous. At a subsequent stage a second solid growth from the upper
part of the epithelial ridge makes its way through the lower eyelid,
and unites with the inner epithelium of the eyelid; and at a still
later date a third growth from the lower part of the structure forms a
second junction with the epithelium of the eyelid. The two latter
outgrowths form the two upper branches of the duct. The ridge now
loses its connection with the external skin, and, becoming hollow,
forms the lacrymal duct. It opens at two points on the inner surface
of the eyelid, and terminates at its opposite extremity by opening
into the nasal cavity. It is remarkable, as pointed out by Born, that
the original epithelial ridge gives rise directly to a comparatively
small part of the whole duct.
In the Fowl the lacrymal duct is formed as a solid ridge of the
epidermis, extending along the line of the so-called lacrymal groove
from the eye to the nasal pit (fig. 120). At the end of the sixth day
it begins to be separated from the epidermis, remaining however united
with it on the inner side of the lower eyelid. After its separation
from the epidermis it forms a solid cord, the lower end of which
unites with the wall of the nasal cavity. The cord so formed gives
rise to the whole of the duct proper and to the lower branch of the
collecting tube. The upper branch of the collecting tube is formed as
an outgrowth from this cord. A lumen begins to be formed on the
twelfth day of incubation, and first appears at the nasal end. It
arises by the formation of a space between the cells of the cord, and
not by an absorption of the central cells.
In Mammalia Kölliker states that he has been unable to observe
anything similar to that described by Born in the Sauropsida and
Amphibia, and holds to the old view, originally put forward by Coste,
that the duct is formed by the closure of a groove leading from the
eye to the nose between the outer nasal process and the superior
maxillary process. The upper extremity of the duct dilates to form a
sack, from which two branches pass off to open on the lacrymal
papillæ. In view of Born’s discoveries Kölliker’s statements must be
received with some caution.
The Eye of the Tunicata.
The unpaired eye of the larva of simple Ascidians is situated somewhat
to the right side of the posterior part of the dorsal wall of the
anterior cephalic vesicle (fig. 296, O). It consists of a refractive
portion, turned towards the cavity of the vesicle of
[Pg 508] the brain, and a
retinal portion forming part of the wall of the brain. The refractive
parts consist of a convex-concave meniscus in front, and a spherical
lens behind, adjoining the concave side of the meniscus. The posterior
part of this lens is imbedded in a layer of pigment. The retina is
formed of columnar cells, with their inner ends imbedded in the
pigment which encloses the posterior part of the lens. The retinal
part of the eye arises in the first instance as a prominence of the
wall of the cerebral vesicle: its cells become very columnar and
pigmented at their inner extremities (fig. 8, V, a). The lens is
developed at a later period, after the larva has become hatched, but
the mode of its formation has not been made out.
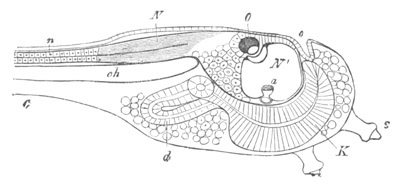
Fig. 296. Larva of Ascidia mentula. (From Gegenbaur; after Kupffer.)
Only the anterior part of the tail is represented.
N´. anterior swelling of neural tube; N. anterior swelling of
spinal portion of neural tube; n. hinder part of neural tube;
ch. notochord; K. branchial region of alimentary tract; d.
œsophageal and gastric region of alimentary tract; O. eye; a.
otolith; o. mouth; s. papilla for attachment.
General considerations on the Eye of the Chordata.
There can be but little doubt that the eye of the Tunicata belongs to
the same phylum as that of the true Vertebrata, different as the two
eyes are. The same may also be said with reference to the degenerate
and very rudimentary eye of Amphioxus.
The peculiarity of the eye of all the Chordata consists in the retina
being developed from part of the wall of the brain. How is this
remarkable feature of the eye of the Chordata to be explained?
Lankester, interpreting the eye in the light of the Tunicata, has made
the interesting suggestion[192]
“that the original Vertebrate must
have been a transparent animal, and had an eye or pair of eyes inside
the brain, like that of the Ascidian Tadpole.”
[Pg 509]
This explanation may possibly be correct, but another explanation
appears to me possible, and I am inclined to think that the vertebrate
eyes have not been derived from eyes like those of Ascidians, but that
the latter is a degenerate form of vertebrate eye.
The fact of the retina being derived from the fore-brain may perhaps
be explained in the same way as has already been attempted in the case
of the retina of the Crustacea; i.e. by supposing that the eye was
evolved simultaneously with the fore part of the brain.
The peculiar processes which occur in the formation of the optic
vesicle are more difficult to elucidate; and I can only suggest that
the development of a primary optic vesicle, and its conversion into an
optic cup, is due to the retinal part of the eye having been involved
in the infolding which gave rise to the canal of the central nervous
system. The position of the rods and cones on the posterior side of
the retina is satisfactorily explained by this hypothesis, because, as
may be easily seen from figure 285, the posterior face of the retina
is the original external surface of the epidermis, which is infolded
in the formation of the brain; so that the rods and cones are, as
might be anticipated, situated on what is morphologically the external
surface of the epiblast of the retina.
The difficulty of this view arises in attempting to make out how the
eye can have continued to be employed during the gradual change of
position which the retina must have undergone in being infolded with
the brain in the manner suggested. If however the successive steps in
this infolding were sufficiently small, it seems to me not impossible
that the eye might have continued to be used throughout the whole
period of change, and a transparency of the tissues, such as Lankester
suggests, may have assisted in rendering this possible.
The difficulty of the eye continuing to be in use when undergoing
striking changes in form is also involved in Lankester’s view, in that
if, as I suppose, he starts from the eye of the Ascidian Tadpole with
its lenses turned towards the cavity of the brain; it is necessary
for him to admit that a fresh lens and other optical parts of the eye
became developed on the opposite side of the eye to the original
lens; and it is difficult to understand such a change, unless we can
believe that the refractive media on the two sides were in operation
simultaneously. It may be noted that the same difficulty is involved
in supposing, as I have done, that the eye of the Ascidian Tadpole was
developed from that of a Vertebrate. I should however be inclined to
suggest that the eye had in this case ceased for a period to be
employed; and that it has been re-developed again in some of the
larval forms. Its characters in the Tunicata are by no means constant.
Accessory eyes in the Vertebrata.
In addition to the paired eyes of the Vertebrata certain organs are
found in the skin of a few Teleostei living in very deep water, which,
though clearly not organs of true vision, yet present characters which
indicate that
[Pg 510] they may be used in the perception of light. The most
important of such organs are those found in Chauliodus, Stomias, etc.,
the significance of which was first pointed out by Leuckart, while the
details of their structure have been recently worked out by
Leydig[193]
and Ussow. They are distributed not only in the skin, but
are also present in the mouth and respiratory cavity, a fact which
appears to indicate that their main function must be something else
than the perception of light. It has been suggested that they have the
function of producing phosphorescence.
Another organ, probably of the same nature, is found on the head of
Scopelus.
The organs in Chauliodus are spherical or nearly spherical bodies
invested in a special tunic. The larger of them, which alone can have
any relation to vision, are covered with pigment except on their outer
surface. The interior is filled with two masses, named by Leuckart the
lens and vitreous humour. According to Leydig each of them is cellular
and receives a nerve, the ultimate destination of which has not
however been made out. According to Ussow the anterior mass is
structureless, but serves to support a lens, placed in the centre of
the eye, and formed of a series of crystalline cones prolonged into
fibres, which in the posterior part of the eye diverge and terminate
by uniting with the processes of multipolar cells, placed near the
pigmented sheath. These cells, together with the fibres of the
crystalline cones which pass to them, are held by Ussow to constitute
a retina.
Eye of the Mollusca.
(362) N. Bobretzky. “Observations on the development of the
Cephalopoda” (Russian). Nachrichten d. kaiserlichen Gesell. d.
Freunde der Naturwiss. Anthropolog. Ethnogr. bei d. Universität
Moskau.
(363) H. Grenacher. “Zur Entwicklungsgeschichte d. Cephalopoden.”
Zeit. f. wiss. Zool., Bd. XXIV. 1874.
(364) V. Hensen. “Ueber d. Auge einiger Cephalopoden.” Zeit. f. wiss.
Zool., Vol. XV. 1865.
(365) E. R. Lankester. “Observations on the development of the
Cephalopoda.” Quart. J. of Micr. Science, Vol. XV. 1875.
(366) C. Semper. Ueber Sehorgane von Typus d. Wirbelthieraugen.
Wiesbaden, 1877.
Eye of the Arthropoda.
(367) N. Bobretzky. Development of Astacus and Palaemon. Kiew, 1873.
(368) A. Dohrn. “Untersuchungen üb. Bau u. Entwicklung d. Arthropoden.
Palinurus und Scyllarus.” Zeit. f. wiss. Zool., Bd. XX. 1870, p. 264
et seq.
[Pg 511]
(369) E. Claparède. “Morphologie d. zusammengesetzten Auges bei den
Arthropoden.” Zeit. f. wiss. Zool., Bd. X. 1860.
(370) H. Grenacher. Untersuchungen üb. d. Sehorgane d. Arthropoden.
Göttingen, 1879.
Vertebrate Eye.
(371) J. Arnold. Beiträge zur Entwicklungsgeschichte des Auges.
Heidelberg, 1874.
(372) Babuchin. “Beiträge zur Entwicklungsgeschichte des Auges.”
Würzburger naturwissenschaftliche Zeitschrift, Bd. 8.
(373) L. Kessler. Zur Entwicklung d. Auges d. Wirbelthiere. Leipzig,
1877.
(374) N. Lieberkühn. Ueber das Auge des Wirbelthierembryo. Cassel,
1872.
(375) N. Lieberkühn. “Beiträge z. Anat. d. embryonalen Auges.” Archiv
f. Anat. und Phys., 1879.
(376) L. Löwe. “Beiträge zur Anatomie des Auges” and “Die Histogenese
der Retina.” Archiv f. mikr. Anat., Vol. XV. 1878.
(377) V. Mihalkowics. “Untersuchungen über den Kamm des Vogelauges.”
Archiv f. mikr. Anat., Vol. IX. 1873.
(378) W. Müller. “Ueber die Stammesentwickelung des Schorgans der
Wirbelthiere.” Festgabe Carl Ludwig. Leipzig, 1874.
(379) S. L. Schenk. “Zur Entwickelungsgeschichte des Auges der
Fische.” Wiener Sitzungsberichte, Bd. LV. 1867.
Accessory organs of the Vertebrate Eye.
(380) G. Born. “Die Nasenhöhlen u. d. Thränennasengang d. Amphibien.”
Morphologisches Jahrbuch, Bd. II. 1876.
(381) G. Born. “Die Nasenhöhlen u. d. Thränennasengang d. amnioten
Wirbelthiere. I. Lacertilia. II. Aves.” Morphologisches Jahrbuch,
Bd. V. 1879.
Eye of the Tunicata.
(382) A. Kowalevsky. “Weitere Studien üb. d. Entwicklung d. einfachen
Ascidien.” Archiv f. mikr. Anat., Vol. VII. 1871.
(383) C. Kupffer. “Zur Entwicklung d. einfachen Ascidien.” Archiv f.
mikr. Anat., Vol. VII. 1872.
[Pg 512]
CHAPTER XVII.
AUDITORY ORGAN, OLFACTORY ORGAN AND SENSE
ORGANS OF THE LATERAL LINE.
Auditory Organs.
A great variety of organs, very widely distributed amongst aquatic
forms, and also found, though less universally, in land forms, are
usually classed together as auditory organs.
In the case of all aquatic forms, or of forms which have directly
inherited their auditory organs from aquatic forms, these organs are
built upon a common type; although in the majority of instances the
auditory organs of the several groups have no genetic relations. All
the organs have their origin in specialized portions of the epidermis.
Some of the cells of a special region become provided at their free
extremities with peculiar hairs, known as auditory hairs; while in
other cells concretions, known as otoliths, are formed, which appear
often to be sufficiently free to be acted upon by vibrations of the
surrounding medium, and to be so placed as to be able in their turn to
transmit their vibrations to the cells with auditory hairs[194].
The
auditory regions of the epidermis are usually shut off from the
surface in special sacks.
The actual function of these organs is no doubt correctly described,
in the majority of instances, as being auditory; but it appears to me
very possible that in some cases their function may be to enable the
animals provided with them to detect the presence of other animals in
their neighbourhood, through the
[Pg 513] undulatory movements in the water,
caused by the swimming of the latter.
Auditory organs with the above characters, sometimes freely open to
the external medium, but more often closed, are found in various
Cœlenterata, Vermes and Crustacea, and universally or all but
universally in the Mollusca and Vertebrata.
In many terrestrial Insects a different type of auditory organ has
been met with, consisting of a portion of the integument modified to
form a tympanum or drum, and supported at its edge by a chitinous
ring. The vibrations set up in the membranous tympanum stimulate
terminal nerve organs at the ends of chitinous processes, placed in a
cavity bounded externally by the tympanic membrane.
The tympanum of Amphibia and Amniota is an accessory organ added, in
terrestrial Vertebrata, to an organ of hearing primitively adapted to
an aquatic mode of life; and it is interesting to notice the presence
of a more or less similar membrane in the two great groups of
terrestrial forms, i.e. terrestrial Vertebrata and Insecta.
Nothing is known with reference to the mode of development or
evolution of the tympanic type of auditory organ found in Insects,
and, except in the case of Vertebrates, but little is known with
reference to the development of what may be called the vesicular type
of auditory organ found in aquatic forms. Some very interesting facts
with reference to the evolution of such organs have however been
brought to light by the brothers Hertwig in their investigations on
the Cœlenterata; and I propose to commence my account of the
development of the auditory organs in the animal kingdom by a short
statement of the results of their researches.
Cœlenterata. Three distinct types of auditory organ have been
recognised in the Medusæ; two of them resulting from the
differentiation of a tentacle-like organ, and one from ectoderm cells
on the under surface of the velum. We may commence with the latter as
the simplest. It is found in the Medusæ known as the Vesiculata. The
least differentiated form of this organ, so far discovered, is present
in Mitrotrocha, Tiaropsis and other genera. It has the form of an open
pit; and a series of such organs are situated along the attached edge
[Pg 514]
of the velum with their apertures directed downwards. The majority of
the cells lining the outer, i.e. peripheral side of the pit, contain
an otolith, while a row of the cells on the inner, i.e. central
side, are modified as auditory cells. The auditory cells are somewhat
strap-shaped, their inner ends being continuous with the fibres of the
lower nerve-ring, and their free ends being provided with bent
auditory hairs, which lie in contact with the convex surfaces of the
cells containing the otoliths.
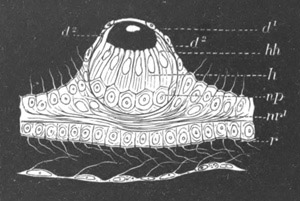
Fig. 297. Auditory vesicle of Phialidium after treatment with
dilute osmic acid. (From Lankester; after O. and R. Hertwig.)
d1. epithelium of the upper surface of the velum; d2.
epithelium of the under surface of the velum; r. circular canal at
the edge of the velum; nr1. upper nerve-ring; h. auditory
cells; hh. auditory hairs; np. nervous cushion formed of a
prolongation of the lower nerve-ring. Close to the nerve-ring is
seen a cell, shewn as black, containing an otolith.
By the conversion of such open pits into closed sacks a more
complicated type of auditory organ, which is present in many of the
Vesiculata, viz. Æquorea, Octorchis, Phialidium, &c., is produced. A
closed vesicle of this type is shewn in fig. 297. Such organs form
projections on the upper surface of the velum. They are covered by a
layer of the epithelium (d1) of the upper surface of the velum, but
the lining of the vesicle (d2) is derived from what was originally
part of the epithelium of the lower surface of the velum, homologous
with that lining the open pits in the type already described. The
general arrangement of the cells lining such vesicles is the same as
that of the cells lining the open pits.
A second type of auditory organ, found in the Trachymedusæ, appears in
its simplest condition as a modified tentacle.
[Pg 515] It is formed of a basal
portion, covered by auditory cells with long stiff auditory hairs,
supporting at its apex a club-shaped body, attached to it by a
delicate stalk. An endodermal axis is continued through the whole
structure, and in one or more of the endoderm cells of the club-shaped
body otoliths are always present. The tails of the auditory cells are
directly continued into the upper nerve-ring.
In more complicated forms of this organ the tentacle becomes enclosed
in a kind of cup, by a wall-like upgrowth of the surrounding parts
(fig. 298); and in some forms, e.g. Geryonia, by the closure of the
cup, the whole structure takes the form of a completely closed
vesicle, in the cavity of which the original tentacle forms an
otolith-bearing projection.
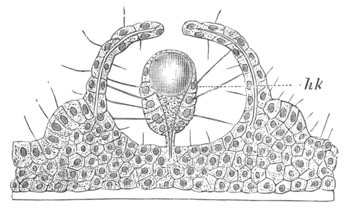
Fig. 298. Auditory organ of Rhopalonema. (From Lankester; after O.
and R. Hertwig.)
The organ consists of a modified tentacle (hk) with auditory cells
and concretions, partially enclosed in a cup.
The auditory organs found in the Acraspedote Medusæ approach in many
respects to the type of organ found in the Trachymedusæ. They consist
of tentacular organs placed in grooves on the under surface of the
disc. They have a swollen extremity, and are provided with an
endodermal axis for half the length of which there is a diverticulum
of the gastrovascular canal system. The terminal portion of the
endoderm is solid, and contains calcareous concretions. The ectodermal
cells at the base of these organs have the form of auditory cells.
Mollusca. Auditory vesicles are found in almost all Mollusca on the
ventral side of the body in close juxtaposition to the pedal ganglia.
Except possibly in some Cephalopods, these
[Pg 516] vesicles are closed. They
are provided with free otoliths, supported by the cilia of the walls
of the sack, but in addition some of the cells of the sack are
provided with stiff auditory hairs.
In many forms these sacks have been observed to originate by an
invagination of the epiblast of the foot (Paludina, Nassa,
Heteropoda, Limax, Clio, Cephalopoda and Lamellibranchiata). In
other instances (some Pteropods, Lymnæus, &c.) they appear, by a
secondary modification in the development, to originate by a
differentiation of a solid mass of epiblast.
According to Fol the otocysts in Gasteropods are formed by cells of
the wall of the auditory sacks; and the same appears to hold good for
Cephalopoda (Grenacher)[195]
shewing that free otoliths have in these
instances originated from otoliths originally placed in cells.
Crustacea. In the decapodous Crustacea organs, which have been
experimentally proved to be true organs of hearing, are usually
present on the basal joint of the anterior antennæ. They may have
(Hensen, No. 384) the form either of closed or of open sacks, lined by
an invagination of the epidermis. They are provided with chitinous
auditory hairs and free otoliths. In the case of the open sacks the
otoliths appear to be simply stones transported into the interior of
the sacks, but in the closed sacks the otoliths, though free, are no
doubt developed within the sacks.
The Schizopods, which, as mentioned in the last chapter, are
remarkable as containing a genus (Euphausia) with abnormally situated
eyes, distinguish themselves again with reference to their auditory
organs, in that another genus (Mysis) is characterized by the presence
of a pair of auditory sacks in the inner plates of the tail. These
sacks have curved auditory hairs supporting an otolith at their
extremity.
The development of the auditory organs in the Crustacea has not been
investigated.
The Vertebrata. The Cephalochorda are without organs of hearing, and
the auditory organ of the Urochorda is constructed on a special type
of its own. The primitive auditory organs of the true Vertebrata have
the same fundamental characters as those of the majority of aquatic
invertebrate forms. They consist of a vesicle, formed by the
invagination of a patch of epiblast, and usually shut off from the
exterior, but occasionally (Elasmobranchii)
[Pg 517] remaining open. The walls
of this vesicle are always much complicated and otoliths of various
forms are present in its cavity. To this vesicle accessory structures,
derived from the walls of the hyomandibular cleft, are added in the
majority of terrestrial Vertebrata.
The development of the true auditory vesicle will be considered
separately from that of the accessory structures derived from the
hyomandibular cleft.
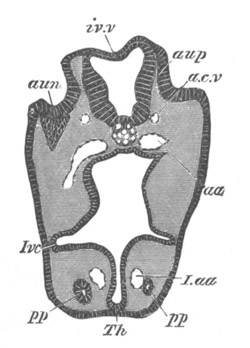
Fig. 299. Section through the head of an Elasmobranch embryo, at
the level of the auditory involution.
aup. auditory pit; aun. ganglion of auditory nerve; iv.v. roof
of fourth ventricle; a.c.v. anterior cardinal vein; aa. aorta;
I.aa. aortic trunk of mandibular arch; pp. head cavity of
mandibular arch; Ivc. alimentary pouch which will form the first
visceral cleft; Th. rudiment of thyroid body.
In all Vertebrata the development of the auditory vesicle commences
with the formation of a thickened patch of epiblast, at the side of
the hind-brain, on the level of the second visceral cleft. This patch
soon becomes invaginated in the form of a pit (fig. 299, aup), to
the inner side of which the ganglion of the auditory nerve (aun),
which as shewn in a previous chapter is primitively a branch of the
seventh nerve, closely applies itself.
In those Vertebrata (viz. Teleostei, Lepidosteus and Amphibia) in
which the epiblast is early divided into a nervous and epidermic
stratum, the auditory pit arises as an invagination of the nervous
stratum only, and the mouth of the auditory pit is always closed (fig.
300) by the epidermic stratum of the skin. Since the opening of the
pit is retained through life in Elasmobranchii the closed form of pit
in the above forms is clearly secondary.
In Teleostei the auditory pit arises as a solid invagination of the
epiblast.
The mouth of the auditory vesicle gradually narrows, and in most forms
soon becomes closed, though in Elasmobranchii it remains permanently
open. In any case the vesicle is gradually removed from the surface,
remaining connected with it by an elongated duct, either opening on
the dorsal aspect of the head (Elasmobranchii), or ending blindly
close beneath the skin.
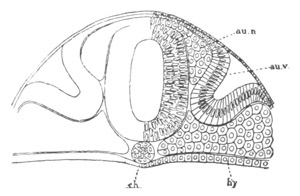
Fig. 300. Section through the head of a Lepidosteus embryo on
the sixth day after impregnation.
au.v. auditory vesicle; au.n. auditory nerve; ch. notochord;
hy. hypoblast.
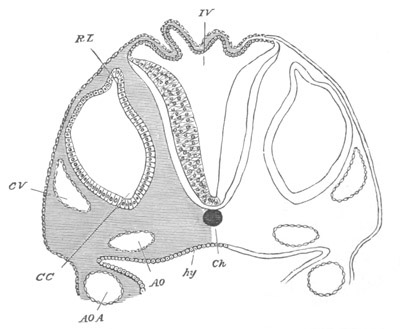
Fig. 301. Section through the hind-brain of a Chick at the end
of the third day of incubation.
IV. fourth ventricle. The section shews the very thin roof and
thicker sides of the ventricle. Ch. notochord; CV. anterior
cardinal vein; CC. involuted auditory vesicle (CC points to the
end which will form the cochlear canal); RL. recessus labyrinthi
(remains of passage connecting the vesicle with the exterior); hy.
hypoblast lining the alimentary canal; AO., AO.A. aorta, and
aortic arch.
In all Vertebrata the auditory vesicle undergoes further
[Pg 518] changes of a
complicated kind. In the Cyclostomata these changes are less
complicated than in other forms, though whether this is due to
degeneration, or to the retention of a primitive state of the auditory
organ, is not known. In the Lamprey the auditory vesicle is formed in
the usual way by an invagination
[Pg 519] of the epiblast, which soon becomes
vesicular, and for a considerable period retains a simple character.
As pointed out by Max Schultze, a number of otoliths appears in the
vesicle during larval life, and, although such otoliths are stated by
J. Müller to be absent both in the full-grown Ammocœte and in the
adult, they have since been found by Ketel (No. 387). The formation of
the two semicircular canals has not been investigated.
In all the higher Vertebrates the changes of the auditory sacks are
more complicated. The ventral end of the sack is produced into a short
process (fig. 301, CC); while at the dorsal end there is the
canal-like prolongation of the lumen of the sack (RL), derived from
the duct which primitively opened to the exterior, and which in most
cases persists as a blind diverticulum of the auditory sack, known as
the recessus labyrinthi or aqueductus vestibuli. The parts thus
indicated give rise to the whole of the membranous labyrinth of the
ear. The main body of the vesicle becomes the utriculus and
semicircular canals, while the ventral process forms the sacculus
hemisphericus and cochlear canal.
The growth of these parts has been most fully studied in Mammalia,
where they reach their greatest complexity, and it will be convenient
to describe their development in this group, pointing out how they
present, during some of the stages in their growth, a form permanently
retained in lower types.
The auditory vesicle in Mammalia is at first nearly spherical, and is
imbedded in the mesoblast at the side of the hind-brain. It soon
becomes triangular in section, with the apex of the triangle pointing
inwards and downwards. This apex gradually elongates to form the
rudiment of the cochlear canal and sacculus hemisphericus (fig. 302,
CC). At the same time the recessus labyrinthi (R.L) becomes
distinctly marked, and the outer wall of the main body of the vesicle
grows out into two protuberances, which form the rudiments of the
vertical semicircular canals (V.B). In the lower forms (fig. 305)
the cochlear process of the vestibule hardly reaches a higher stage of
development than that found at this stage in Mammalia.
The parts of the auditory labyrinth thus established soon increase in
distinctness (fig. 303); the cochlear canal (CC) becomes longer and
curved; its inner and concave surface being
[Pg 520] lined by a thick layer of
columnar epiblast. The recessus labyrinthi also increases in length,
and just below the point where the bulgings to form the vertical
semicircular canals are situated, there is formed a fresh protuberance
for the horizontal semicircular canal. At the same time the central
parts of the walls of the flat bulgings of the vertical canals grow
together, obliterating this part of the lumen, but leaving a canal
round the periphery; and, on the absorption of their central parts,
each of the original simple bulgings of the wall of the vesicle
becomes converted into a true semicircular canal, opening at its two
extremities into the auditory vesicle. The vertical canals are first
established and then the horizontal canal.
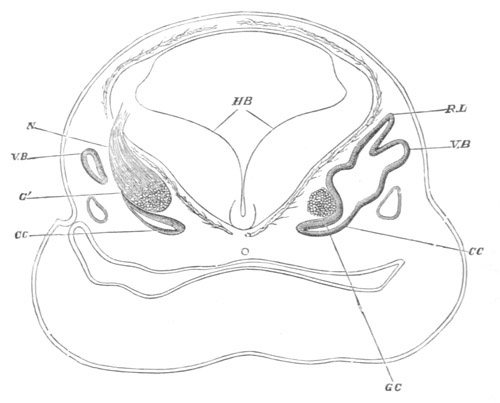
Fig. 302. Transverse section of the head of a fœtal Sheep (16 mm. in
length) in the region of the hind-brain. (After Böttcher.)
HB. the hind-brain.
The section is somewhat oblique, hence while on the right side the
connections of the recessus vestibuli R.L., and of the commencing
vertical semicircular canal V.B., and of the ductus cochlearis
CC., with the cavity of the primary otic vesicle are seen; on the
left side, only the extreme end of the ductus cochlearis CC, and
of the semicircular canal V.B. are shewn.
Lying close to the inner side of the otic vesicle is seen the
cochlear ganglion GC; on the left side the auditory nerve G and
its connection N with the hind-brain are also shewn.
Below the otic vesicle on either side lies the jugular vein.
[Pg 521]
Shortly after the formation of the rudiment of the horizontal
semicircular canal a slight protuberance becomes apparent on the inner
commencement of the cochlear canal. A constriction arises on each side
of the protuberance, converting it into a prominent hemispherical
projection, the sacculus hemisphericus (fig. 304, S.R).
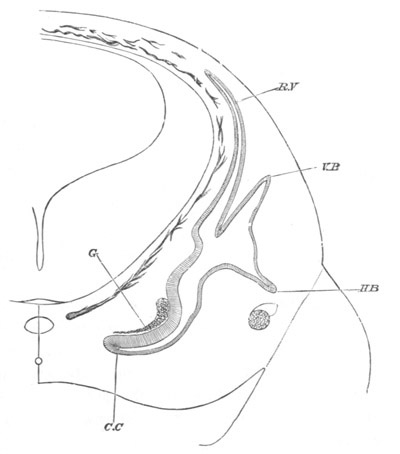
Fig. 303. Section of the head of a fœtal Sheep 20 mm. in length.
(After Böttcher.)
R.V. recessus labyrinthi; V.B. vertical semicircular canal;
H.B. horizontal semicircular canal; C.C. cochlear canal; G.
cochlear ganglion.
The constrictions are so deep that the sacculus is only connected with
the cochlear canal on the one hand, and with the general cavity of the
auditory vesicle on the other, by, in each case, a narrow though short
canal.
The former of these canals (fig. 304, b) is known as the canalis
reuniens. At this stage we may call the remaining cavity of the
original otic vesicle, into which all the above parts open, the
utriculus.
Soon after the formation of the sacculus hemisphericus, the
[Pg 522] cochlear
canal and the semicircular canals become invested with cartilage. The
recessus labyrinthi remains however still enclosed in undifferentiated
mesoblast.
Between the cartilage and the parts which it surrounds there remains a
certain amount of indifferent connective tissue, which is more
abundant around the cochlear canal than around the semicircular
canals.
As soon as they have acquired a distinct connective-tissue coat, the
semicircular canals begin to be dilated at one of their terminations
to form the ampullæ. At about the same time a constriction appears
opposite the mouth of the recessus labyrinthi, which causes its
opening to be divided into two branches—one towards the utriculus and
the other towards the sacculus hemisphericus; and the relations of the
parts become so altered that communication between the sacculus and
utriculus can only take place through the mouth of the recessus
labyrinthi (fig. 305).
When the cochlear canal has come to consist of two and a half coils,
the thickened epithelium which lines the lower surface of the canal
forms a double ridge from which the organ of Corti is subsequently
developed. Above the ridge there appears a delicate cuticular
membrane, the membrane of Corti or membrana tectoria.
The epithelial walls of the utricle, the recessus labyrinthi, the
semicircular canals, and the cochlear canal constitute together the
highly complicated product of the original auditory vesicle. The whole
structure forms a closed cavity, the various parts of which are in
free communication. In the adult the fluid present in this cavity is
known as the endolymph.
In the mesoblast lying between these parts and the cartilage, which at
this period envelopes them, lymphatic spaces become established, which
are partially developed in the Sauropsida, but become in Mammals very
important structures.
They consist in Mammals partly of a space surrounding the utricle and
semicircular canals, and partly of two very definite channels, which
largely embrace between them the cochlear canal. The latter channels
form the scala vestibuli on the upper side of the cochlear canal and
the scala tympani on the lower. The scala vestibuli is in free
communication with the lymphatic cavity surrounding the vestibule, and
opens at the apex of the cochlea
[Pg 523] into the scala tympani. The latter
ends blindly at the fenestra rotunda.
The fluid contained in the two scalæ, and in the remaining lymphatic
cavities of the auditory labyrinth, is known as perilymph.
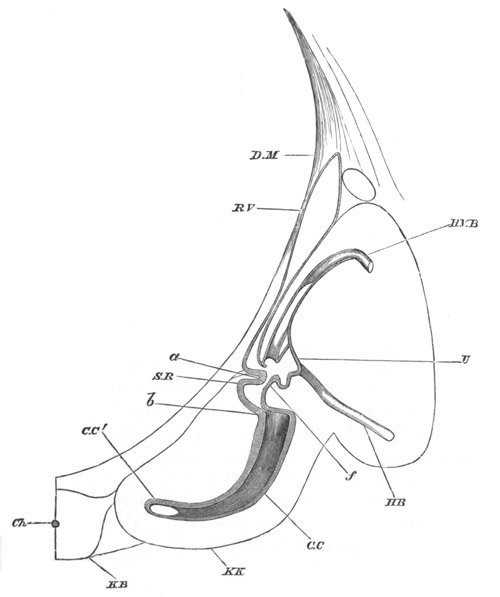
Fig. 304. Section through the internal ear of an embryonic Sheep
28 mm. in length. (After Böttcher.)
D.M. dura mater; R.V. recessus labyrinthi; H.V.B. posterior
vertical semicircular canal; U. utriculus; H.B. horizontal
semicircular canal; b. canalis reuniens; a. constriction by
means of which the sacculus hemisphericus S.R. is formed; f.
narrowed opening between sacculus hemisphericus and utriculus;
C.C. cochlea; C.C´. lumen of cochlea; K.K. cartilaginous
capsule of cochlea; K.B. basilar plate; Ch. notochord.
The cavities just spoken of are formed by an absorption of
[Pg 524] parts of
the embryonic mucous tissue between the perichondrium and the walls of
the membranous labyrinth.
The scala vestibuli is formed before the scala tympani, and both scalæ
begin to be developed at the basal end of the cochlea: the cavity of
each is continually being carried forwards towards the apex of the
cochlear canal by a progressive absorption of the mesoblast. At first
both scalæ are somewhat narrow, but they soon increase in size and
distinctness.
The cochlear canal, which is often known as the scala media of the
cochlea, becomes compressed on the formation of the scalæ so as to be
triangular in section, with the base of the triangle outwards. This
base is only separated from the surrounding cartilage by a narrow
strip of firm mesoblast, which becomes the stria vascularis, etc. At
the angle opposite the base the canal is joined to the cartilage by a
narrow isthmus of firm material, which contains nerves and vessels.
This isthmus subsequently forms the lamina spiralis, separating the
scala vestibuli from the scala tympani.
The scala vestibuli lies on the upper border of the cochlear canal,
and is separated from it by a very thin layer of mesoblast, bordered
on the cochlear aspect by flat epiblast cells. This membrane is called
the membrane of Reissner. The scala tympani is separated from the
cochlear canal by a thicker sheet of mesoblast, called the basilar
membrane, which supports the organ of Corti and the epithelium
adjoining it. The upper extremity of the cochlear canal ends in a
blind extremity called the cupola, to which the two scalæ do not for
some time extend. This condition is permanent in Birds, where the
cupola is represented by a structure known as the lagena (fig. 305,
II. L). Subsequently the two scalæ join at the extremity of the
cochlear canal; the point of the cupola still however remains in
contact with the bone, which has now replaced the cartilage, but at a
still later period the scala vestibuli, growing further round,
separates the cupola from the adjoining osseous tissue.
The ossification around the internal ear is at first confined to the
cartilage, but afterwards extends into the thick periosteum between
the cartilage and the internal ear, and thus eventually makes its way
into the lamina spiralis, etc.
The organ of Corti. In Mammalia there is formed from the
[Pg 525] epithelium of
the cochlear canal a very remarkable organ known as the organ of
Corti, the development of which is of sufficient importance to merit a
brief description. A short account of this organ in the adult state
may facilitate the understanding of its development.
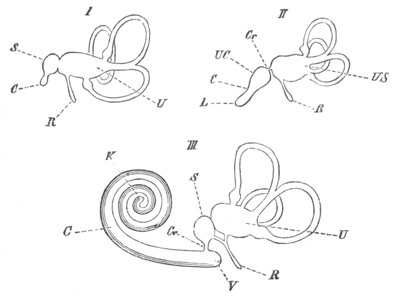
Fig. 305. Diagrams of the Membranous labyrinth. (From Gegenbaur.)
I. Fish. II. Bird. III. Mammal.
U. utriculus; S. sacculus; US. utriculus and sacculus; Cr.
canalis reuniens; R. recessus labyrinthi; UC. commencement of
cochlea; C. cochlear canal; L. lagena; K. cupola at apex of
cochlear canal; V. cæecal sack of the vestibulum of the cochlear
canal.
The cochlear canal is bounded by three walls, the outer one being the
osseous wall of the cochlea. The membrane of Reissner bounds it
towards the scala vestibuli, and the basilar membrane towards the
scala tympani. This membrane stretches from the margin of the lamina
spiralis to the ligamentum spirale; the latter being merely an
expanded portion of the connective tissue lining the osseous cochlea.
The lamina spiralis is produced into two lips, called respectively the
labium tympanicum and labium vestibulare; it is to the former and
longer of these that the basilar membrane is attached. At the margin
of the junction of the labium tympanicum with the basilar membrane the
former is perforated for the passage of the nervous fibres, and this
region is called the habenula perforata.
The labium vestibulare, so called from its position, is shorter than
the labium tympanicum and is raised above into numerous blunt teeth.
Partly springing out from the labium vestibulare, and passing from
near the inner attachment of the membrane of Reissner towards the
outer wall of the cochlea, is an elastic membrane, the membrana
tectoria. Resting on the basilar membrane is the organ of Corti.
Considering for the moment that a transverse section of the cochlear
[Pg 526]
canal only one cell deep is being dealt with, the organ of Corti will
be found to consist of a central part composed of two peculiarly
shaped rods widely separated below, but in contact above. These are
the rods or fibres of Corti. On their outer side, i.e. on the side
towards the osseous wall of the canal, is a reticulate membrane which
passes from the inner rod of Corti towards the osseous wall of the
canal. With their upper extremities fixed in that membrane, and their
lower resting on the basilar membrane are three (four in man) cells
with auditory hairs known as the outer ‘hair cells,’ which alternate
with three other cells known as Deiters’ cells. Between these and the
outer attachment of the basilar membrane is a series of cells
gradually diminishing in height in passing outwards. On the inner side
of the rods of Corti is one hair cell, and then a number of peculiarly
modified cells which fill up the space between the two lips of the
lamina spiralis.
It will not be necessary to say much in reference to the development
of the labium tympanicum and the labium vestibulare.
The labium vestibulare is formed by a growth of the connective tissue
which fuses with and passes up between the epithelial cells. The
epithelial cells which line its upper (vestibular) border become
modified, and remain as its teeth.
The labium tympanicum is formed by the coalescence of the connective
tissue layer separating the scala tympani from the cochlear canal with
part of the connective tissue of the lamina spiralis. At first these
two layers are separate, and the nerve fibres to the organ of Corti
pass between them. Subsequently however they coalesce, and the region
where they are penetrated by the nervous fibres becomes the habenula
perforata.
The organ of Corti itself is derived from the epiblast cells lining
the cochlear canal, and consists in the first instance of two
epithelial ridges or projections. The larger of them forms the cells
on the inner side of the organ of Corti, and the smaller the rods of
Corti together with the inner and outer hair cells and Deiters’ cells.
At first both these ridges are composed of simple elongated epithelial
cells one row deep. The smaller ridge is the first to shew any change.
The cells adjoining the larger ridge acquire auditory hairs at their
free extremities, and form the row of inner hair cells; the next row
of cells acquires a broad attachment to the basilar membrane, and
gives origin to the inner and outer rods of Corti.
Outside the latter come several rows of cells adhering together so as
to form a compact mass which is quadrilateral in section. This mass is
composed of three upper cells with nuclei at the same level, which
form the outer hair cells, each of them ending above in auditory
hairs, and three lower cells which form the cells of Deiters. Beyond
this the cells gradually pass into ordinary cubical epithelial cells.
As just mentioned, the cells of the second row, resting with their
broad bases on the basilar membrane, give rise to the rods of Corti.
The breadth of the bases of these cells rapidly increases, and
important changes take place in the structure of the cells themselves.
[Pg 527]
The nucleus of each cell divides; so that there come to be two nuclei
or sometimes three which lie close together near the base of the cell.
Outside the nuclei on each side a fibrous cuticular band appears. The
two bands pass from the base of the cell to its apex, and there meet
though widely separated below. The remaining contents of the cell,
between the two fibrous bands, become granular, and are soon to a
great extent absorbed; leaving at first a round, and then a triangular
space between the two fibres. The two nuclei, surrounded by a small
amount of granular matter, come to lie, each at one of the angles
between the fibrous bands and the basilar membrane.
The two fibrous bands become, by changes which need not be described
in detail, converted into the rods of Corti—each of their upper ends
growing outwards into the processes which the adult rods possess.
Each pair of rods of Corti is thus (Böttcher) to be considered as the
product of one cell; and the nuclei embedded in the granular mass
between them are merely the remains of the two nuclei formed by the
division of the original nucleus of that cell[196].
The larger ridge
is for the most part not permanent, and from being the most
conspicuous part of the organ of Corti comes to be far less important
than the smaller ridge. Its cells undergo a partial degeneration; so
that the epithelium in the hollow between the two lips of the lamina
spiralis, which is derived from the larger ridge, comes to be composed
of a single row of short and broad cells. In the immediate
neighbourhood however of the inner hair cell, one or two of the cells
derived from the larger ridge are very much elongated.
The membrana reticularis is a cuticular structure derived from the
parts to which it is attached.
Accessory structures connected with the organ of hearing in
Terrestrial Vertebrata.
In all the Amphibia, Sauropsida and Mammalia, except the Urodela and a
few Anura and Reptilia, the first visceral or hyomandibular cleft
enters into intimate relations with the organs of hearing, and from it
and the adjoining parts are formed the tympanic cavity, the Eustachian
tube, the tympanic membrane and the meatus auditorius externus. The
tympanic membrane serves to receive from the air the sound vibrations,
which are communicated to fluids contained in the true auditory
labyrinth by one ossicle or by a chain of auditory ossicles.
The addition to the organ of hearing of a tympanic membrane to receive
aerial sound vibrations is an interesting case of the
[Pg 528] adaptation of a
structure, originally required for hearing in water, to serve for
hearing in air; and as already pointed out, the similarity of this
membrane to the tympanic membrane of some Insects is also striking.
There is much that is obscure with reference to the actual development
of the above parts of the ear, which has moreover only been carefully
studied in Birds and Mammals.
The Eustachian tube and tympanic cavity seem to be derived from the
inner part of the first visceral or hyomandibular cleft, the external
opening of which becomes soon obliterated. Kölliker holds that the
tympanic cavity is simply a dorsally and posteriorly directed
outgrowth of the median part of the inner section of this cleft; while
Moldenhauer (No. 392) holds, if I understand him rightly, that it is
formed as an outgrowth of a cavity called by him the sulcus
tubo-tympanicus, derived from the inner aperture of the first visceral
cleft together with the groove of the pharynx into which it opens; and
Moldenhauer is of opinion that the greater part of the original cleft
atrophies.
The meatus auditorius externus is formed at the region of a shallow
depression where the closure of the first visceral cleft takes place.
It is in part formed by the tissue surrounding this depression growing
up in the form of a wall, and Moldenhauer believes that this is the
whole process. Kölliker states however that the blind end of the
meatus becomes actually pushed in towards the tympanic cavity.
The tympanic membrane is derived from the tissue which separates the
meatus auditorius externus from the tympanic cavity. This tissue is
obviously constituted of an hypoblastic epithelium on its inner
aspect, an epiblastic epithelium on its outer aspect, and a layer of
mesoblast between them, and these three layers give rise to the three
layers of which this membrane is formed in the adult. During the
greater part of fœtal life it is relatively very thick, and presents
a structure bearing but little resemblance to that in the adult.
A proliferation of the connective tissue-cells in the vicinity of the
tympanic cavity causes in Mammalia the complete or nearly complete
obliteration of the cavity during fœtal life.
The tympanic cavity is bounded on its inner aspect by the osseous
investment of the internal ear, but at one point, known
[Pg 529] as the
fenestra ovalis, the bone is deficient in the Amphibia, Sauropsida and
Mammalia, and its place is taken by a membrane; while in Mammalia and
Sauropsida a second opening, the fenestra rotunda, is also present.
These two fenestræ appear early, but whether they are formed by an
absorption of the cartilage, or by the nonchondrification of a small
area, is not certainly known. The upper of the two, or fenestra
ovalis, contains the base of a bone, known in the Sauropsida and
Amphibia as the columella. The main part of the columella is formed of
a stalk which is held by Parker to be derived from part of the
skeleton of the visceral arches, but its nature is discussed in
connection with the skeleton, while the base, forming the stapes,
appears to be derived from the wall of the periotic cartilage.
In all Amphibia and Sauropsida with a tympanic cavity, the stalk of
the columella extends to the tympanic membrane; its outer end becoming
imbedded in this membrane, and serving to transmit the vibrations of
the membrane to the fluid in the internal ear. In Mammalia there is a
stapes not directly attached to the tympanic membrane by a stalk, and
two additional auditory ossicles, derived from parts of the skeleton
of the visceral arches, are placed between the stapes and the tympanic
membrane. These ossicles are known as the malleus and incus, and the
chain of the three ossicles replaces physiologically the single
ossicle of the lower forms.
These ossicles are at first imbedded in the connective tissue in the
neighbourhood of the tympanic cavity, but on the full development of
this cavity, become apparently placed within it; though really
enveloped in the mucous membrane lining it.
The fenestra ovalis is in immediate contiguity with the walls of the
utricle, while the fenestra rotunda adjoins the scala tympani.
Hunt (No. 391) holds, from his investigations on the embryology of the
pig, that “the Eustachian tube is an involution of the pharyngeal
mucous membrane;” and that “the meatus is an involution of the
integument” while “the drum is formed by the Eustachian tube
overlapping the extremity of the meatus.” Urbantschitsch also holds
that the first visceral cleft has nothing to do with the formation of
the tympanic cavity and Eustachian tube, and that these parts are
derived from lateral outgrowths of the oral cavity.
[Pg 530]
The evolution of the accessory parts of the ear would be very
difficult to explain on Darwinian principles if the views of Hunt and
Urbantschitsch were correct; and the accepted doctrine, originally
proposed by Huschke (No. 389), according to which these structures
have originated by a ‘change of function’ of the parts of the first
visceral cleft, may fairly be held till more conclusive evidence has
been brought against it than has yet been done.
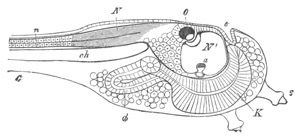
Fig. 306. Larva of Ascidia mentula. (From Gegenbaur; after
Kupffer.) Only the anterior part of the tail is represented.
N´. anterior swelling of neural tube; N. anterior swelling of
spinal portion of neural tube; n. hinder part of neural tube;
ch. notochord; K. branchial region of alimentary tract; d.
œsophageal and gastric region of alimentary tract; O. eye; a.
otolith; o. mouth; s. papilla for attachment.
Tunicata. The auditory organ of the Tunicata (fig. 306) is placed on
the under surface of the anterior vesicle of the brain. It consists of
two parts (1) a prominence of the cells of the floor of the brain
forming a crista acustica, and (2) an otolith projecting into the
cavity of the brain, and attached to the crista by delicate hairs.
The crista acustica is formed of very delicate cylindrical cells, and
in its most projecting part is placed a vesicle with clear contents.
The otolith is an oval body with its dorsal half pigmented, and its
ventral half clear and highly refractive. It is balanced on the
highest point of the crista.
The crista acustica would seem to be developed from the cells of the
lower part of the front vesicle of the brain. The otolith however is
developed from a single cell on the dorsal and right side of the
brain. This cell commences to project into the cavity of the brain and
its free end becomes pigmented. It gradually grows inwards till it
forms a spherical prominence in the cavity of the brain, to the wall
of which it is attached by a
[Pg 531] stalk. At the same time it travels round
the right side of the vesicle of the brain (in a way not fully
explained) till it reaches the summit of the crista, which has become
in the meantime established.
The auditory organ of the simple Ascidians can hardly be brought into
relation with that of the other Chordata, and has most probably been
evolved within the Tunicate phylum.
Bibliography.
Invertebrata.
(384) V. Hensen. “Studien üb. d. Gehörorgan d. Decapoden.” Zeit. f.
wiss. Zool., Vol. XIII. 1863.
(385) O. and R. Hertwig. Das Nervensystem u. d. Sinnesorgane d.
Medusen. Leipzig, 1878.
Vertebrata.
(386) A. Boettcher. “Bau u. Entwicklung d. Schnecke.” Denkschriften
d. kaiserl. Leop. Carol. Akad. d. Wissenschaft., Vol. XXXV.
(387) C. Hasse. Die vergleich. Morphologie u. Histologie d. häutigen
Gehörorgane d. Wirbelthiere. Leipzig, 1873.
(388) V. Hensen. “Zur Morphologie d. Schnecke.” Zeit. f. wiss.
Zool., Vol. XIII. 1863.
(389) E. Huschke. “Ueb. d. erste Bildungsgeschichte d. Auges u. Ohres
beim bebrüteten Küchlein.” Isis von Oken, 1831, and Meckel’s
Archiv, Vol. VI.
(390) Reissner. De Auris internæ formatione. Inaug. Diss. Dorpat,
1851.
Accessory parts of Vertebrate Ear.
(391) David Hunt. “A comparative sketch of the development of the ear
and eye in the Pig.” Transactions of the International Otological
Congress, 1876.
(392) W. Moldenhauer. “Zur Entwick. d. mittleren u. äusseren Ohres.”
Morphol. Jahrbuch, Vol. III. 1877.
(393) V. Urbantschitsch. “Ueb. d. erste Anlage d. Mittelohres u. d.
Trommelfelles.” Mittheil. a. d. embryol. Instit. Wien, Heft I. 1877.
Olfactory organ.
Amongst the Invertebrata numerous sense organs have been described
under the title of olfactory organs. In aquatic animals they often
have the form of ciliated pits or grooves, while in the Insects and
Crustacea delicate hairs and other structures present on the antennæ
are usually believed to be organs of smell. Our knowledge of all these
organs is however so vague that it
[Pg 532] would not be profitable to deal
with them more fully in this place. Amongst the Chordata there are
usually well developed olfactory organs.
Amongst the Urochorda (Tunicata) it is still uncertain what organs (if
any) deserve this appellation. The organ on the dorsal side of the
opening of the respiratory pharynx may very possibly have an olfactory
function, but it is certainly not homologous with the olfactory pits
of the true Vertebrata, and as mentioned above (pp. 436 and 437), may
perhaps be homologous with the pituitary body.
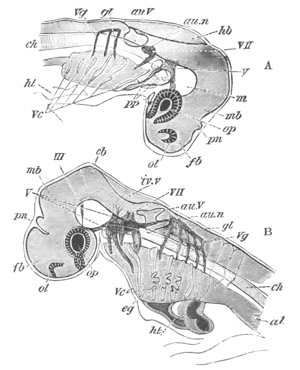
Fig. 307. Views of the head of Elasmobranch embryos at two stages
as transparent objects.
A. Pristiurus embryo of the same stage as fig. 28 F.
B. Somewhat older Scyllium embryo.
III. third nerve; V. fifth nerve; VII. seventh nerve; au.n.
auditory nerve; gl. glossopharyngeal nerve; Vg. vagus nerve; fb.
fore-brain; pn. pineal gland; mb. mid-brain; hb. hind-brain;
iv.v. fourth ventricle; cb. cerebellum; ol. olfactory pit; op.
eye; au.V. auditory vesicle; m. mesoblast at base of brain; ch.
notochord; ht. heart; Vc. visceral clefts; eg. external gills;
pp. sections of body cavity in the head.
In the Cephalochorda (Amphioxus) there is a shallow ciliated pit,
discovered by Kölliker, which is situated on the left side of the
head, and is closely connected with a special process of the
[Pg 533] front end
of the brain. It is most probably the homologue of the olfactory pits
of the true Vertebrata.
In the true Vertebrata the olfactory organ has usually the form of a
pair of pits, though in the Cyclostomata the organ is unpaired.
In all the Vertebrata with two olfactory pits these organs are formed
from a pair of thickened patches of the epiblast, on the under side of
the fore-brain, immediately in front of the mouth (fig. 307, ol).
Each thickened patch of epiblast soon becomes involuted as a pit (fig.
308, N), the lining cells of which become the olfactory or
Schneiderian epithelium. The surface of this epithelium is usually
much increased by various foldings, which in the Elasmobranchii arise
very early, and are bilaterally symmetrical, diverging on each side
like the barbs of a feather from the median line. They subsequently
become very pronounced (fig. 309), serving greatly to increase the
surface of the olfactory epithelium. At a very early stage the
olfactory nerve attaches itself to the olfactory epithelium.
In Petromyzon the olfactory organ arises as an unpaired thickening
of the epiblast, which in the just hatched larva forms a shallow pit,
on the ventral side of the head, immediately in front of the mouth.
This pit rapidly deepens, and soon extends itself backwards nearly as
far as the infundibulum (fig. 310, ol). By the development of the
upper lip the opening of the olfactory pit is gradually carried to the
dorsal surface of the head, and becomes at the same time narrowed and
ciliated (fig. 47, ol). The whole organ forms an elongated sack, and
in later stages becomes nearly divided by a median fold into two
halves.
It is probable that the unpaired condition of the olfactory organ in
the Lamprey has arisen from the fusion of two pits into one; there is
however no evidence of this in the early development; but the division
of the sack into two halves by a median fold may be regarded as an
indication of such a paired character in the later stages.
In Myxine the olfactory organ communicates with the mouth through the
palate, but the meaning of this communication, which does not appear
to be of the same nature as the communication between the olfactory
pits and the mouth by the posterior nares in the higher types, is not
known.
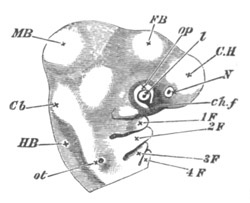
Fig. 308. Side view of the head of an embryo Chick of the third
day as an opaque object. (Chromic acid preparation.)
C.H. cerebral hemispheres; F.B. vesicle of third ventricle;
M.B. mid-brain; Cb. cerebellum; H.B. medulla oblongata; N.
nasal pit; ot. auditory vesicle in the stage of a pit with the
opening not yet closed up; op. optic vesicle, with l. lens and
ch.f. choroidal fissure.
1 F. The first visceral fold; above it is seen the superior
maxillary process.
2, 3, 4 F. Second, third and fourth visceral folds, with the
visceral clefts between them.
The opening of the olfactory pit does not retain its embryonic
characters. In Elasmobranchii and Chimæra it becomes enclosed by a
wall of integument, often deficient on the side of the mouth, so that
there is formed a groove leading from the nasal pit towards the angle
of the mouth. This groove is
[Pg 534] usually constricted in the middle, and
the original single opening of the nasal sack thus becomes nearly
divided into two. In Teleostei and Ganoids the division of the nasal
opening into two parts becomes complete, but the ventral opening is
generally carried off some distance from the mouth, and placed, by the
growth of the snout, on the upper surface of the head (figs. 54 and
68). In all these instances it is probable that the dorsal opening of
the external nares, and the ventral opening with the posterior nares
of higher types. Thus the posterior nares would in fact seem to be
represented in all Fishes by a ventral part of the opening of the
original nasal pit which either adjoins the border of the mouth (many
Elasmobranchii) or is quite separate from the mouth (Teleostei and
Ganoidei). In the Dipnoi, Amphibia and all the higher types the oral
region becomes extended so as to enclose the posterior nares, and then
each nasal pit acquires two openings; viz. one outside the mouth, the
external nares, and one within the mouth, the internal or posterior
nares. In the Dipnoi the two nasal openings are very similar to those
in Ganoidei and Teleostei, but both are placed on the under surface of
the head, the inner one being within the mouth, and the external one
is so close to the outer border of the upper lip that it also has been
considered by some anatomists to lie within the mouth.
In all the higher types the nasal pits have originally only a single
opening, and the ontogenetic process by which the posterior nasal
opening is formed has been studied in the Amniota and Amphibia.
Amongst the Amniota we may take the Chick as representing the process
in a very simple form. The general history of the process was first
made out by Kölliker.
[Pg 535]
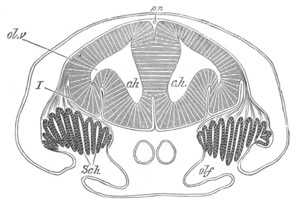
Fig. 309. Section through the brain and olfactory organ of an
embryo of Scyllium. (Modified from figures by Marshall and myself.)
c.h. cerebral hemispheres; ol.v. olfactory vesicle; olf.
olfactory pit; Sch. Schneiderian folds; I. olfactory nerve. The
reference line has been accidentally taken through the nerve to the
brain.
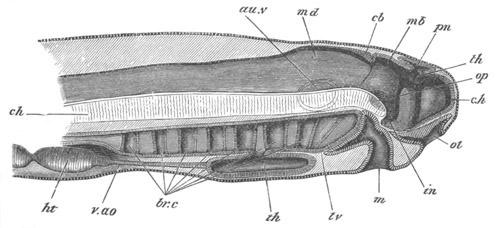
Fig. 310. Diagrammatic vertical section through the head of a
larva of Petromyzon.
The larva had been hatched three days, and was 4.8 mm. in length.
The optic and auditory vesicles are supposed to be seen through the
tissues.
c.h. cerebral hemisphere; th. optic thalamus; in.
infundibulum; pn. pineal gland; mb. mid-brain; cb. cerebellum;
md. medulla oblongata; au.v. auditory vesicle; op. optic
vesicle; ol. olfactory pit; m. mouth; br.c. branchial pouches;
th. thyroid involution; v.ao. ventral aorta; ht. ventricle of
heart; ch. notochord.
The opening of the nasal pit becomes surrounded by a ridge except on
its oral side. The deficiency of this ridge on the side of the mouth
gives rise to a kind of shallow groove leading from the nasal pit to
the mouth. The ridge enveloping the opening of the nasal pit next
becomes prolonged along the sides of this groove, especially on its
inner one; and at the same time the superior maxillary process grows
forwards so as to bound the lower
[Pg 536] part of its outer side. The inner
and outer ridges, together with the superior maxillary process,
enclose a deep groove, connecting the original opening of the nasal
pit with the mouth. The process just described is illustrated by fig.
311 A, and it may be seen that the ridge on the inner side of the
groove forms the edge of the frontonasal process (k).
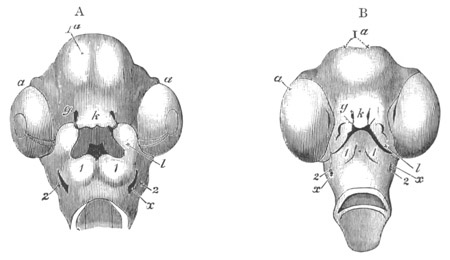
Fig. 311. Head of a Chick from below on the sixth and seventh days
of incubation. (From Huxley.)
Ia. cerebral vesicles; a. eye, in which the remains of the
choroid slit can still be seen in A; g. nasal pits; k.
frontonasal process; l. superior maxillary process; 1. inferior
maxillary process or first visceral arch; 2. second visceral arch;
x. first visceral cleft.
In A the cavity of the mouth is seen enclosed by the frontonasal
process, the superior maxillary processes and the first pair of
visceral arches. At the back of it is seen the opening leading into
the throat. The nasal grooves leading from the nasal pits to the
mouth are already closed over.
In B the external opening of the mouth has become much constricted,
but it is still enclosed by the frontonasal process and superior
maxillary processes above, and by the inferior maxillary processes
(first pair of visceral arches) below.
The superior maxillary processes have united with the frontonasal
process, along nearly the whole length of the latter.
On the sixth day (Born, 394) the sides of this groove unite together
in the middle, and convert it into a canal open at both ends—the
ventral openings of the canals of the two sides being placed just
within the border of the mouth, and forming the posterior nares; while
the external openings form the anterior nares. The upper part of the
canal, together with the original nasal pit, is alone lined by
olfactory epithelium; the remaining epithelium of the nasal cavity
being indifferent epiblastic epithelium.
[Pg 537] Further changes subsequently
take place in connection with the posterior nares, but these are
described in the section dealing with the mouth.
In Mammalia the general formation of the anterior and posterior nares
is the same as in Birds; but, as shewn by Dursy and Kölliker, an
outgrowth from the inner side of the canal between the two openings
arises at an early period; and becoming separate from the posterior
nares and provided with a special opening into the mouth, forms the
organ of Jacobson. The general relations of this organ when fully
formed are shewn in fig. 312.
In Lacertilia the formation of the posterior nares differs in some
particulars from that in Birds (Born). A groove is formed leading from
the primitive nasal pit to the mouth, bordered on its inner side by
the swollen edge of the frontonasal process, and on its outer by an
outer-nasal process; while the superior maxillary process does not
assist in bounding it. On the inner side of the narrowest part of this
groove there is formed a large lateral diverticulum, which is lined by
a continuation of the Schneiderian epithelium, and forms the rudiment
of Jacobson’s organ. The nasal groove continues to grow in length, but
soon becomes converted into a canal by the junction of the outer-nasal
process with the frontonasal process. This canal is open at both ends:
at its dorsal end is placed the original opening of the nasal pit, and
its ventral opening is situated within the cavity of the mouth. The
latter forms the primitive posterior nares. The superior maxillary
process soon grows inwards on the under side of the posterior part of
the nasal passage, and assists in forming its under wall. This
ingrowth of the superior maxillary process is the rudiment of the hard
palate.
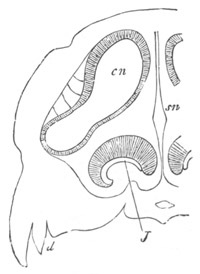
Fig. 312. Section through the nasal cavity and Jacobson’s
organ. (From Gegenbaur.)
sn. septum nasi; cn. nasal cavity; J. Jacobson’s organ; d.
edge of upper jaw.
On the conversion of the nasal groove into a closed passage, the
opening of Jacobson’s organ into the groove becomes concealed; and at
a later period Jacobson’s organ becomes completely shut off from the
nasal cavity, and opens into the mouth at the front end of an
elongated groove leading back to the posterior nares.
In Amphibia the posterior nares are formed in a manner very different
from that of the Amniota. At an early stage a shallow groove is formed
leading from the nasal pit to the mouth; but this groove instead
[Pg 538] of
forming the posterior nares soon vanishes, and by the growth of the
front of the head the nasal pits are carried farther away from the
mouth.
The actual posterior nares are formed by a perforation in the palate,
opening into the blind end of the original nasal pit.
Considering that the various stages in the formation of the posterior
nares of the Amniota are so many repetitions of the adult states of
lower forms, it may probably be assumed that the mode of formation of
the posterior nares in Amphibia is secondary, as compared with that in
the Amniota.
A diverticulum of the front part of the nasal cavity of the Anura is
probably to be regarded as a rudimentary form of Jacobson’s organ.
Bibliography.
(394) G. Born. “Die Nasenhöhlen u. d. Thränennasengang d. amnioten
Wirbelthiere.” Parts I. and II. Morphiologisches Jahrbuch, Bd. V.,
1879.
(395) A. Kölliker. “Ueber die Jacobson’schen Organe des Menschen.”
Festschrift f. Rienecker, 1877.
(396) A. M. Marshall. “Morphology of the Vertebrate Olfactory Organ.”
Quart. Journ. of Micr. Science, Vol. XIX., 1879.
Sense organs of the lateral line.
Although I do not propose dealing with the general development of
various sense organs of the skin, there is one set of organs, viz.
that of the lateral line, which, both from its wide extension amongst
the Ichthyopsida and from the similarity of some of its parts to
certain organs found amongst the Chætopoda[197],
has a great
morphological importance.
The organs of the lateral line consist as a rule of canals, partly
situated in the head, and partly in the trunk. These canals open at
intervals on the surface, and their walls contain a series of
nerve-endings. The branches of the canal in the head are innervated
for the most part by the fifth pair, and those of the trunk by the
nervus lateralis of the vagus nerve. There is typically but a single
canal in the trunk, the openings and nerve-endings of which are
segmentally arranged.
Two types of development of these organs have been found. One of these
is characteristic of Teleostei; the other of Elasmobranchii.
In just hatched Teleostei, Schulze (No. 402) found that instead of the
normal canals there was present a series of sense bulbs, projecting
freely on the surface and partly composed of cells with stiff hairs.
In most
[Pg 539] cases each bulb is enclosed in a delicate tube open at its
free extremity; while the bulbs correspond in number with the
myotomes. In some Teleostei (Gobius, Esox, etc.) such sense organs
persist through life; in most forms however each organ becomes covered
by a pair of lobes of the adjacent tissue, one formed above and the
other below it. The two lobes of each pair then unite and form a tube
open at both ends. The linear series of tubes so formed is the
commencement of the adult canal; while the primitive sense bulbs form
the sensory organs of the tubes. The adjacent tubes partially unite
into a continuous canal, but at their points of apposition pores are
left, which place the canal in communication with the exterior.
Besides these parts, I have found that there is present in the just
hatched Salmon a linear streak of modified epidermis on the level of
the lateral nerve, and from the analogy of the process described below
for Elasmobranchii it appears to me probable that these streaks play
some part in the formation of the canal of the lateral line.
In Elasmobranchii (Scyllium) the lateral line is formed as a linear
thickening of the mucous layer of the epidermis. This thickening is at
first very short, but gradually grows backwards, its hinder end
forming a kind of enlarged growing point. The lateral nerve is formed
shortly after the lateral line, and by the time that the lateral line
has reached the level of the anus the lateral nerve has grown back for
about two-thirds of that distance. The lateral nerve would seem to be
formed as a branch of the vagus, but is at first half enclosed in the
modified cells of the lateral line (fig. 275, nl)[198],
though it
soon assumes a deeper position.
A permanent stage, more or less corresponding to the stage just
described in Elasmobranchii, is retained in Chimæra, and Echinorhinus
spinosus, where the lateral line has the form of an open groove
(Solger, No. 404).
The epidermic thickening, which forms the lateral line, is converted
into a canal, not as in Teleostei by the folding over of the sides,
but by the formation of a cavity between the mucous and epidermic
layers of the epiblast, and the subsequent enclosure of this cavity by
the modified cells of the mucous layer of the epiblast which
constitute the lateral line. The cavity first appears at the hind end
of the organ, and thence extends forwards.
After its conversion into a canal the lateral line gradually recedes
from the surface; remaining however connected with the epidermis at a
series of points corresponding with the segments, and at these points
perforations are eventually formed to constitute the segmental
apertures of the system.
The manner in which the lumen of the canal is formed in Elasmobranchs
bears the same relation to the ordinary process of conversion of a
groove into a canal that the formation of the auditory involution
[Pg 540] in
Amphibia does to the same process in Birds. In both Elasmobranchii and
Amphibia the mucous layer of the epiblast behaves exactly as does the
whole epiblast in the other types, but is shut off from the surface by
the passive epidermic layer of the epiblast.
The mucous canals of the head and the ampullæ are formed from the
mucous layer of the epidermis in a manner very similar to the lateral
line; but the nerves to them arise as simple branches of the fifth and
seventh nerves, which unite with them at a series of points, but do
not follow their course like the lateral nerve.
It is clear that the canal of the lateral line is secondary, as
compared with the open groove of Chimæra or the segmentally arranged
sense bulbs of young Teleostei; and it is also clear that the
phylogenetic mode of formation of the canal consisted in the closure
of a primitively open groove. The abbreviation of this process in
Elasmobranchii was probably acquired after the appearance of food-yolk
in the egg, and the consequent disappearance of a free larval stage.
While the above points are fairly obvious it does not seem easy to
decide à priori whether a continuous sense groove or isolated sense
bulbs were the primitive structures from which the canals of the
lateral line took their origin. It is equally easy to picture the
evolution of the canal of the lateral line either from (1) a
continuous unsegmented sense line, certain points of which became
segmentally differentiated into special sense bulbs, while the whole
subsequently formed a groove and then a canal; or from (2) a series of
isolated sense bulbs, for each of which a protective groove was
developed; and from the linear fusion of which a continuous canal
became formed.
From the presence however of a linear streak of modified epidermis in
larval Teleostei, as well as in Elasmobranchii, it appears to me more
probable that a linear sense streak was the primitive structure from
which all the modifications of the lateral line took their origin, and
that the segmentally arranged sense bulbs of Teleostei are secondary
differentiations of this primitive structure.
The, at first sight remarkable, distribution of the vagus nerve to the
lateral line is probably to be explained in connection with the
evolution of this organ. As is indicated both by its innervation from
the vagus, as also from the region where it first becomes developed,
the lateral line was probably originally restricted to the anterior
part of the body. As it became prolonged backwards it naturally
carried with it the vagus nerve, and thus a sensory branch of this
nerve has come to innervate a region which is far beyond the limits of
its original distribution.
Bibliography.
(397) F. M. Balfour. A Monograph on the development of Elasmobranch
Fishes, pp. 141-146. London, 1878.
(398) H. Eisig. “Die Segmentalorgane d. Capitelliden.” Mittheil. a.
d. zool. Station zu Neapel, Vol. I. 1879.
[Pg 541]
(399) A. Götte. Entwicklungsgeschichte d. Unke. Leipzig, 1875.
(400) Fr. Leydig. Lehrbuch d. Histologie des Menschen u. d. Thiere.
Hamm. 1857.
(401) Fr. Leydig. Neue Beiträge z. anat. Kenntniss d. Hautdecke u.
Hautsinnesorgane d. Fische. Halle, 1879.
(402) F. E. Schulze. “Ueb. d. Sinnesorgane d. Seitenlinie bei Fischen
und Amphibien.” Archiv f. mikr. Anat., Vol. VI. 1870.
(403) C. Semper. “Das Urogenitalsystem d. Selachier.” Arbeit. a. d.
zool.-zoot. Instit. Würzburg, Vol. II.
(404) B. Solger. “Neue Untersuchungen zur Anat. d. Seitenorgane d.
Fische.” Archiv f. mikr. Anat., Vol. XVII. and XVIII. 1879 and 1880.
[Pg 542]
CHAPTER XVIII.
THE NOTOCHORD, THE VERTEBRAL COLUMN, THE
RIBS AND THE STERNUM.
Introduction.
Amongst the products of that part of the mesoblast which constitutes
the connective tissue of the body special prominence must be given to
the skeleton of the Vertebrata, from its importance in relation to
numerous phylogenetic and morphological problems.
The development of the skeleton is however so large a subject that it
cannot be satisfactorily dealt with except in a special treatise
devoted to it; and the following description must be regarded as a
mere sketch, from which detail has been as far as possible excluded.
In the lowest Chordata the sole structure present, which deserves to
be called a skeleton, is the notochord. Although the notochord often
persists as an important organ in the true Vertebrata, yet there are
always added to it various skeletal structures developed in the
mesoblast. Before entering into a systematic description of these, it
will be convenient to say a few words as to the general characters of
the skeleton.
Two elements, distinct both in their genesis and structure, are to be
recognized in the skeleton. The one, forming the true primitive
internal skeleton or endoskeleton, is imbedded within the muscles and
is originally formed in cartilage. In many instances it retains a
cartilaginous consistency through life, but in the majority of cases
it becomes gradually ossified, and
[Pg 543] converted into true bone. Bones so
formed are known as cartilage bones.
The other element is originally formed by the fusion of the ossified
bases of the dermal placoid scales already described in Chapter XIV.,
or by the fusion of the ossified bases of teeth situated in the mucous
membrane of the mouth. In both instances the plates of bone so formed
may lose the teeth or spines with which they were in the first
instance covered, either by absorption in the individual, or
phylogenetically by their gradually ceasing to be developed. The
plates of bone, which originated by the above process, become in
higher types directly developed in the connective tissue beneath the
skin; and gradually acquire a deeper situation, and are finally so
intimately interlocked with parts of the true internal skeleton, that
the two sets of elements can only be distinguished by the fact of the
one set ossifying in cartilage and the other in membrane.
It seems probable that in the Reptilia, and possibly the extinct
Amphibia, dermal bones have originated in the skin without the
intervention of superjacent spinous structures.
In cases where a membrane bone, as the dermal ossifications are
usually called, overlies a part of the cartilage, it may set up
ossification in the latter, and the cartilage bone and membrane bone
may become so intimately fused as to be quite inseparable. It seems
probable that in cases of this kind the compound bone may in the
course of further evolution entirely lose either its cartilaginous
element or its membranous element; so that cases occasionally occur
where the development of a bone ceases to be an absolutely safe guide
to its evolution.
As to the processes which take place in the ossification of cartilage
there is still much to be made out. Two processes are often
distinguished, viz. (1) a process known as ectostosis, in which the
ossification takes place in the perichondrium, and either simply
surrounds or gradually replaces the cartilage, and (2) a process known
as endostosis, where the ossification actually takes place between the
cartilage cells. It seems probable however (Gegenbaur, Vrolik) that
there is no sharp line to be drawn between these two processes; but
that the ossification almost always starts from the perichondrium. In
the higher types, as a rule, the vessels of the perichondrium extend
into
[Pg 544] the cartilage, and the ossification takes place around these
vessels within the cartilage; but in the lower types (Pisces,
Amphibia) ossification is often entirely confined to the
perichondrium; and the cartilage is simply absorbed.
The regions where ossification first sets in are known as centres of
ossification; and from these centres the ossification spreads
outwards. There may be one or more centres for a bone.
The actual causes which in the first instance gave rise to particular
centres of ossification, or to the ossification of particular parts of
the cartilage, are but little understood; nor have we as yet any
satisfactory criterion for determining the value to be attached to the
number and position of centres of ossification. In some instances such
centres appear to have an important morphological significance, and in
other instances they would seem to be determined by the size of the
cartilage about to be ossified.
There is no doubt that the membrane bones and cartilage bones can as a
rule be easily distinguished by their mode of development; but it is
by no means certain that this is always the case. It is necessarily
very difficult to establish the homology between bones, which develop
in one type from membrane and in another type from cartilage; but
there are without doubt certain instances in which the homology
between two bones would be unhesitatingly admitted were it not for the
difference in their development. The most difficult cases of this kind
are connected with the shoulder-girdle.
The possible sources of confusion in the development of bones are
obviously two. (1) A cartilage bone by origin may directly ossify in
membrane, without the previous development of cartilage, and (2) a
membrane bone may in the first instance be formed in cartilage.
The occurrence of the first of these is much more easy to admit than
that of the second; and there can be little doubt that it sometimes
takes place. In a large number of cases it would moreover cause no
serious difficulty to the morphologist.
Bibliography of the origin of the Skeleton.
(405) C. Gegenbaur. “Ueb. primäre u. secundäre Knochenbildung mit
besonderer Beziehung auf d. Lehre von dem Primordialcranium.”
Jenaische Zeitschrift, Vol. III. 1867.
(406) O. Hertwig. “Ueber Bau u. Entwicklung d. Placoidschuppen u. d.
Zähne d. Selachier.” Jenaische Zeitschrift, Vol. VIII. 1874.
[Pg 545]
(407) O. Hertwig. “Ueb. d. Zahnsystem d. Amphibien u. seine Bedeutung
f. d. Genese d. Skelets d. Mundhöhle.” Archiv f. mikr. Anat., Vol.
XI. Supplementheft, 1874.
(408) O. Hertwig. “Ueber d. Hautskelet d. Fische.” Morphol.
Jahrbuch, Vol. II. 1876. (Siluroiden u. Acipenseriden.)
(409) O. Hertwig. “Ueber d. Hautskelet d. Fische (Lepidosteus u.
Polypterus).” Morph. Jahrbuch, Vol. V. 1879.
(410) A. Kölliker. “Allgemeine Betrachtungen üb. die Entstehung d.
knöchernen Schädels d. Wirbelthiere.” Berichte v. d. königl. zoot.
Anstalt z. Würzburg, 1849.
(411) Fr. Leydig. “Histologische Bemerkungen üb. d. Polypterus
bichir.” Zeit. f. wiss. Zool., Vol. V. 1858.
(412) H. Müller. “Ueber d. Entwick. d. Knochensubstanz nebst
Bemerkungen, etc.” Zeit. f. wiss. Zool., Vol. IX. 1859.
(413) Williamson. “On the structure and development of the Scales and
Bones of Fishes.” Phil. Trans., 1851.
(414) Vrolik. “Studien üb. d. Verknöcherung u. die Knochen d. Schädels
d. Teleostier.” Niederländisches Archiv f. Zoologie, Vol. I.
Notochord and Vertebral column.
The primitive axial skeleton of the Chordata consists of the notochord
and its sheath. It persists as such in the adult in Amphioxus, and
constitutes, in embryos of all Vertebrata, for a considerable period
of their early embryonic life, the sole representative of the axial
skeleton.
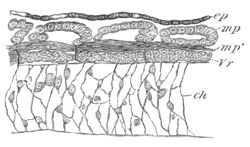
Fig. 313. Horizontal section through the trunk of an embryo of
Scyllium considerably younger than F in fig. 28.
The section is taken at the level of the notochord, and shews the
separation of the cells to form the vertebral bodies from the
muscle-plates.
ch. notochord; ep. epiblast; Vr. rudiment of vertebral body;
mp. muscle-plate; mp´. portion of muscle-plate already
differentiated into longitudinal muscles.
The Notochord. The early formation of the notochord has already been
described in detail (pp. 292-300). It is developed, in most if not all
cases, as an axial differentiation of the hypoblast, and forms at
first a solid cord of cells, without a sheath, placed between the
nervous system and the dorsal wall of the alimentary tract, and
extending from the base of the front of the mid-brain to the end of
the tail. The section in the region of the brain will be dealt with by
itself. That
[Pg 546] in the trunk forms the basis round which the vertebral
column is moulded.
The early histological changes in the cells of the notochord are
approximately the same in all the Craniata. There is formed by the
superficial cells of the notochord a delicate sheath, which soon
thickens, and becomes a well-defined structure. Vacuoles (one or more
to each cell) are formed in the cells of the notochord, which enlarge
till the whole notochord becomes almost entirely formed of large
vacuoles separated by membranous septa which form a complete
sponge-like reticulum (fig. 313). In the Ichthyopsida most of the
protoplasm with the nuclei is carried to the periphery, where it forms
a special nucleated layer sometimes divided into definite
epithelial-like cells (fig. 314), while in the meshes of the reticulum
a few nuclei surrounded by a little protoplasm still remain. In the
Amniotic Vertebrata, probably owing to the early atrophy of the
notochord, the distribution of the nuclei in the spaces of the
mesh-work remains fairly uniform.
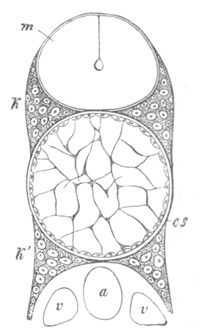
Fig. 314. Section through the spinal column of a young
Salmon. (From Gegenbaur.)
cs. sheath of notochord; k. neural arch; k´. hæmal arch; m.
spinal cord; a. dorsal aorta; v. cardinal veins.
In the early stages of development the spaces in the notochordal
sponge-work, each containing a nucleus and protoplasm, probably
represent cells. In the types in which the notochord persists in the
adult the mesh-work becomes highly complicated, and then forms a
peculiar reticulum filled with gelatinous material, the spaces in
which do not indicate the outlines of definite cells (figs. 315 and
318).
Around the sheath of the notochord there is formed in the
Cyclostomata, Ganoidei, Elasmobranchii and Teleostei an elastic
membrane usually known as the membrana elastica externa.
In most Vertebrates the notochord and its sheath either atrophy
completely or become a relatively unimportant part of the axial
skeleton; but in the Cyclostomata (fig. 315) and in the Selachioidean
Ganoids (Acipenser, etc.) they persist as the sole representative of
the true vertebral axis. The sheath becomes very much thickened; and
on the membrana elastica covering
[Pg 547] it the vertebral arches directly
rest. In Elasmobranchii the sheath of the notochord undergoes a more
complicated series of changes, which result first of all in the
formation of a definite unsegmented cartilaginous tube[199]
round
the notochord, and subsequently (in most forms) in the formation of
true vertebral bodies.
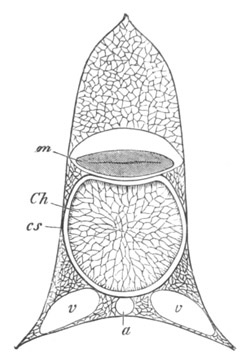
Fig. 315. Section through the vertebral column of
Ammocœtes. (From Gegenbaur.)
Ch. notochord; cs. notochordal sheath; m. spinal cord; a.
aorta; v. cardinal veins.
Between the membrana elastica externa and the sheath of the notochord
a layer of cells becomes interposed (fig. 316, n), which lie in a
matrix not sharply separated from the sheath of the notochord. The
cells which form this layer appear to be derived from a special
investment of the notochord, and to have penetrated through the
membrana elastica externa to reach their final situation. The layer
with these cells soon increases in thickness, and forms a continuous
unsegmented tube of fibrous tissue with flattened concentrically
arranged nuclei (fig. 317, Vb). Externally is placed the membrana
elastica externa (mel), while within is the cuticular sheath of the
notochord. This tube is the cartilaginous tube spoken of above and is
known as the cartilaginous sheath of the notochord.
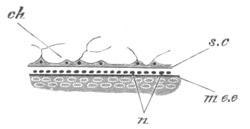
Fig. 316. Longitudinal section through a small part of the
notochord and adjoining parts of a Scyllium embryo, at the time of the
first formation of the cartilaginous sheath.
ch. notochord; sc. sheath of notochord; n. nuclei of
cartilaginous sheath; me.e. membrana elastica externa.
The exact origin of the cartilaginous tube just described is a
question of fundamental importance with reference to the origin of the
vertebral column and the homologies of its constituent parts; but is
by no means easy to settle. In the account of the subject in my memoir
on Elasmobranch Fishes I held with Gegenbaur that it arose from
[Pg 548] a
layer of cells outside the sheath of the notochord, on the exterior
of which the membrana elastica externa was subsequently formed. To
this view Götte (No. 419) also gave his adhesion. Schneider has since
(No. 429) stated that this is not the case, but that, as described
above, the membrana elastica externa is formed before the layer of
cartilage. I have since worked over this subject again, and am on the
whole inclined to adopt Schneider’s correction.
It follows from the above description that the cartilaginous tube in
question is an essential part of the sheath of the notochord, and that
it is to some extent homologous with the notochordal sheath of the
Sturgeon and the Lamprey, and not an entirely new formation.
Fig. 317. Transverse section through the ventral part of the
notochord and adjoining structures of an advanced Scyllium embryo
at the root of the tail.
Vb. cartilaginous sheath of the notochord; ha. hæmal arch; vp.
process to which the rib is articulated; mel. membrana elastica
externa; ch. notochord; ao. aorta; V.cau. caudal vein.
This sheath forms the basis of the centra of the future vertebræ. In a
few adult forms, i.e. Chimæra and the Dipnoi, it retains its
primitive condition, except that in Chimæra there are present delicate
ossified rings more numerous than the arches; while in the Notidani,
Læmargi and Echinorhini the
[Pg 549] indications of vertebræ are imperfectly
marked out. The further history of this sheath in the forms in which
true vertebræ are formed can only be dealt with in connection with the
formation of the vertebral arches.
In Teleostei there is present, as in Elasmobranchii, an elastica
externa, and an inner notochordal sheath. The elastica externa
contains, according to Götte, cells. These cells, if present, are
however very difficult to make out, but in any case the so-called
elastica externa appears to correspond with the cartilaginous sheath
of Elasmobranchii together with its enveloping elastica, since
ossification, when it sets in, occurs in this layer. The sheath within
becomes unusually thick.
In the Amphibia and in the Amniota no membrane is present which can be
identified with the membrana elastica externa of the Elasmobranchii,
Teleostei, etc. In Amphibia (Götte) there is formed round the
notochord a cellular sheath, which has very much the relations of the
cartilaginous tube around the notochord of Elasmobranchii, and is
developed in the same way from the perichordal connective tissue
cells. It is only necessary to suppose that the membrana elastica
externa has ceased to be developed (which in view of its extreme
delicacy and unimportant function in Elasmobranchii is not difficult
to do) and this cellular sheath would then obviously be homologous
with the cartilaginous tube in question. In the Amniota an external
sheath of the notochord cannot be traced as a distinct structure, but
the connective tissue surrounding the notochord and spinal cord is
simply differentiated into the vertebral bodies and vertebral arches.
Vertebral arches and Vertebral bodies.
Cyclostomata. The Cyclostomata are the most primitive forms in which
true vertebral arches are present. Their ontogeny in this group has
not been satisfactorily worked out. It is however noticeable in
connection with them that they form for the most part isolated pieces
of cartilage, the segmental arrangement of which is only imperfect.
Elasmobranchii. In the Elasmobranchii the cells forming the vertebral
arches are derived from the splanchnic layer of the mesoblastic
somites. They have at first the same segmentation
[Pg 550] as the somites (fig.
313, Vr), but this segmentation is soon lost, and there is formed
round the notochord a continuous sheath of embryonic connective tissue
cells, which gives rise to the arches of the vertebræ, the tissue
forming the dura mater, the perichondrium, and the general investing
connective tissue.
The changes which next follow result in what has been known since
Remak as the secondary segmentation of the vertebral column. This
segmentation, which occurs in all Vertebrata with true vertebræ, is
essentially the segmentation of the continuous investment of the
notochord and spinal cord into vertebral bodies and vertebral arches.
It does not however follow the lines of the segmentation of the
muscle-plates, but is so effected that the centres of the vertebral
bodies are opposite the septa between the muscle-plates.
The explanation of this character in the segmentation is not difficult
to find. The primary segmentation of the body is that of the
muscle-plates, which were present in the primitive forms in which
vertebræ had not appeared. As soon however as the notochordal sheath
was required to be strong as well as flexible, it necessarily became
divided into a series of segments.
The condition under which the lateral muscles can best cause the
flexure of the vertebral column is clearly that each myotome shall be
capable of acting on two vertebræ; and this condition can only be
fulfilled when the myotomes are opposite the intervals between the
vertebræ. For this reason, when the vertebræ became formed, their
centres were opposite not the middle of the myotomes but the
intermuscular septa.
These considerations fully explain the characters of the secondary
segmentation of the vertebral column. On the other hand the primary
segmentation (fig. 313) of the vertebral rudiments is clearly a
remnant of a condition when no vertebral bodies were present; and has
no greater morphological significance than the fact that the cells of
the vertebræ were derived from the segmented muscle-plates, and then
became fused into a continuous sheath around the notochord and nervous
axis; till finally they became in still higher forms differentiated
into vertebræ and their arches.
During the stage represented in fig. 28 g, and somewhat before the
cartilaginous sheath of the notochord is formed, there appear four
special concentrations of the mesoblastic tissue adjoining the
notochord, two of them dorsal (neural) and two of them ventral
(hæmal). They are not segmented, and form four ridges, seated on the
sides of the notochord. They are united
[Pg 551] with each other by a delicate
layer of tissue, and constitute the substance in which the neural and
hæmal arches subsequently become differentiated.
Fig. 318. Section through the vertebral column of an advanced
embryo of Scyllium in the region of the tail.
na. neural arch; ha. hæmal arch; ch. notochord; sh. inner
sheath of notochord; ne. membrana elastic externa.
At about the time when the first traces of the cartilaginous sheath of
the notochord arise, differentiations take place in the neural and
hæmal ridges. In the neural ridge two sets of arches are formed for
each myotome, and resting on the cartilaginous sheath of the notochord
in the region which will afterwards form the centrum of a vertebra,
and constituting a true neural arch; and a second separate from the
cartilaginous sheath, forming an intercalated piece[200].
Both of them
soon become hyaline cartilage.
There is a considerable portion of the original tissue of the neural
ridge, especially in the immediate neighbourhood of the notochord,
which is not employed in the formation of the neural arches. This
tissue has a fibrous character and becomes converted into the
perichondrium and other parts.
The hæmal arches are formed from the hæmal ridge in precisely the same
way as the neural arches, but interhæmal intercalated pieces are often
present. In the region of the tail the hæmal arches are continued into
ventral processes which meet below, enclosing the aorta and caudal
veins.
[Pg 552]
Since primitively the postanal gut was placed between the aorta and
the caudal vein, the hæmal arches potentially invest a caudal section
of the body cavity. In the trunk region they do not meet ventrally,
but give support to the ribs. The structures just described are shewn
in section in fig. 318, in which the neural (na) and hæmal (ha)
arches are shewn resting upon the cartilaginous sheath of the
notochord.
While these changes are being effected in the arches the cartilaginous
sheath of the notochord undergoes important differentiations. In the
vertebral regions opposite the origin of the neural and hæmal arches
(fig. 318) its outer part becomes hyaline cartilage, while the inner
parts adjoining the notochord undergo a somewhat different
development, the notochord in this part becomes at the same time
somewhat constricted. In the intervertebral regions the
cartilaginous sheath of the notochord becomes more definitely fibrous,
while the notochord is in no way constricted. A diagrammatic
longitudinal section through the vertebral column, while these changes
are being effected, is shewn in fig. 320 B.
These processes are soon carried further. The notochord within the
vertebral body becomes gradually constricted, especially in the median
plane, till it is here reduced to a fibrous band, which gradually
enlarges in either direction till it reaches its maximum thickness in
the median plane of the intervertebral region. The hyaline cartilage
of the vertebral region forms a vertebral body in which calcification
may to some extent take place. The cartilage of the base of the arches
gradually spreads over it, and on the absorption of the membrana
elastica externa, which usually takes place long before the adult
state is reached, the arch tissue becomes indistinguishably fused with
that of the vertebral bodies, so that the latter are compound
structures, partly formed of the primitive cartilaginous sheath, and
partly of the tissue of the bases of the neural and hæmal arches.
Owing to the beaded structure of the notochord the vertebral bodies
take of necessity a biconcave hourglass-shaped form.
The intervertebral regions of the primitive sheath of the notochord
form fibrous intervertebral ligaments enclosing the unconstricted
intervertebral sections of the notochord.
[Pg 553]
A peculiar fact may here be noticed with reference to the formation of
the vertebral bodies in the tail of Scyllium, Raja, and possibly other
forms, viz. that there are double as many vertebral bodies as there
are myotomes and spinal nerves. This is not due to a secondary
segmentation of the vertebræ but, as I have satisfied myself by a
study of the development, takes place when the vertebral bodies first
become differentiated. The possibility of such a relation of parts is
probably to be explained by the fact that the segmentation of the
vertebral column arose subsequently to that of the nerves and
myotomes.
Ganoidei. In Acipenser and other cartilaginous Ganoids the hæmal and
neural arches are formed as in Elasmobranchii, and rest upon the outer
sheath of the notochord. Since however the sheath of the notochord is
never differentiated into distinct vertebræ, this primitive condition
is retained through life.
Teleostei. In Teleostei the formation of the vertebral arches and
bodies takes place in a manner, which can be reduced, except in
certain minor points, to the same type as that of Elasmobranchii.
There are early formed (fig. 314 k and k´) neural and hæmal arches
resting upon the outer sheath of the notochord. The latter structure,
which, as mentioned on p. 549, corresponds to the cartilaginous sheath
of the notochord of Elasmobranchii, soon becomes divided into
vertebral and intervertebral regions. In the former ossification
directly sets in without the sheath acquiring the character of hyaline
cartilage (Götte, 419). The latter forms the fibrous intervertebral
ligaments. The notochord exhibits vertebral constrictions.
The ossified outer sheath of the notochord forms but a small part of
the permanent vertebræ. The remainder is derived partly from an
ossification of the connective tissue surrounding the sheath, and
partly from the bases of the arches, which do not spread round the
primitive vertebral bodies as in Elasmobranchii. The ossifications in
the tissue surrounding the sheath usually (fig. 319) take the form of
a cross, while the bases of the arches (k and k´) remain as four
cartilaginous radii between the limbs of the osseous cross. In some
instances the bases of the arches also become ossified, and are then
with difficulty distinguishable from the other parts of the secondary
vertebral body. The parts of the arches outside the vertebral bodies
are for the most part ossified (fig. 319). In correlation with the
vertebral constrictions of the notochord the vertebral bodies are
biconcave.
Amphibia. Of the forms of Amphibia so far studied embryologically the
Salamandridæ present the most primitive type of formation of the
vertebral column.
It has already been stated that in Amphibia there is present
[Pg 554] around
the notochord a cellular sheath, equivalent to the cartilaginous
sheath of Elasmobranchii. In the tissue on the dorsal side of this
sheath a series of cartilaginous processes becomes formed. These
processes are the commencing neural arches; and they rest on the
cellular sheath of the notochord opposite the middle of the vertebral
regions.
Fig. 319. Vertical section through the middle of a vertebra of
Esox lucius (Pike). (From Gegenbaur.)
ch. notochord; cs. notochordal sheath; k. and k´.
cartilaginous tissue of the neural and hæmal arches; h. osseous
hæmal process; n. spinal canal.
A superficial osseous layer becomes very early formed in each
vertebral region of the cellular sheath; while in each of the
intervertebral regions, which are considerably shorter than the
vertebral, there is developed a ring-like cartilaginous thickening of
the sheath, which projects inwards so as to constrict the notochord.
At a period before this thickening has attained considerable
dimensions the notochord becomes sufficiently constricted in the
centre of each vertebral region to give a biconcave form to the
vertebræ for a very short period of fœtal life.
The stage with biconcave vertebræ is retained through life in the
Perennibranchiata and Gymnophiona.
The chief peculiarity which distinguishes the later history of their
vertebral column from that of fishes consists in the immense
development of the intervertebral thickenings just mentioned, which
increase to such an extent as to reduce the notochord, where it passes
through them, to a mere band; while the cartilage of which they are
composed becomes differentiated into two regions, one belonging to the
vertebra in front, the other to that behind, the hinder one being
convex, and the anterior concave. The two parts are not however
absolutely separated from each other.
By these changes each vertebra comes to be composed of (1) a thin
osseous somewhat hourglass-shaped cylinder with a dilated portion of
the notochord in its centre, and (2 and 3) of two
[Pg 555] halves of two
intervertebral cartilages, viz. an anterior convex half and a
posterior concave half. The vertebræ thus come to be opisthocœlous. A
longitudinal section through the vertebral column at this stage is
diagrammatically shewn in fig. 320 C.
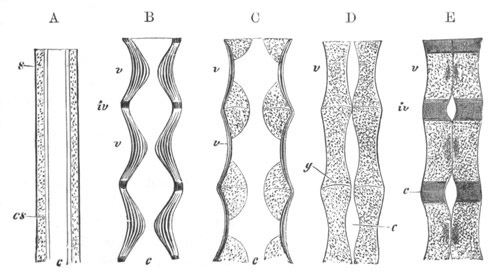
Fig. 320. Diagram representing the mode of development of the
vertebræ in the different types. (From Gegenbaur.)
A. Ideal type in which distinct vertebræ are not established.
B. Type of Pisces with vertebral constrictions of the notochord.
C. Amphibian type, with intervertebral constrictions of the
notochord by the intervertebral parts of the cellular sheath.
D. Intervertebral constriction of the notochord as effected in
Reptilia and Aves.
E. Vertebral constriction of the notochord as effected in Mammalia,
the intervertebral parts of the cartilaginous sheath being
converted into intervertebral ligaments.
c. notochord; cs. cuticular sheath of notochord; s.
cartilaginous sheath; v. vertebral regions; iv. intervertebral
regions; g. intervertebral joints.
To the centre of each of these vertebræ the neural arches, the origin
of which was described above, become in the meantime firmly attached;
and grow obliquely upwards and backwards, so as to meet and unite
above the spinal cord. The transverse processes of the vertebræ would
seem (Fick) to be developed independently of the arches, though they
very soon fuse with them. According to Götte the transverse processes
are double in the trunk, there being two pairs, one vertically above
the other for each vertebra. The pair on each side eventually fuse
together.
In the tail hæmal arches are formed, which are similar in their mode
of development to the neural arches.
The unconstricted portion of the notochord, which persists in each
vertebra, becomes in part converted into cartilage.
[Pg 556]
Anura. In the Anura the process of formation of the vertebral column
is essentially the same as that in the Salamandridæ. Two types may
however be observed. One of these occurs in the majority of the Anura,
and mainly differs from that in Salamandra in (1) the earlier fusion
of the arches with the cellular sheath of the notochord; (2) the more
rapid growth of the intervertebral thickenings of the cellular sheath,
which results in the early and complete obliteration of the
intervertebral parts of the notochord; (3) the complete division of
these intervertebral thickenings into anterior and posterior portions,
which unite with and form the articular surfaces of two contiguous
vertebræ. The vertebræ are moreover procœlous instead of being
opisthocœlous.
The unconstricted vertebral sections of the notochord always persist
till the ossification of the vertebræ has taken place. In some forms
they remain through life (Rana), while in other cases they eventually
either wholly or partially disappear.
The second type of vertebral development is found in Bombinator,
Pseudis, Pipa, and Pelobates. In these genera the formation of the
vertebra takes place almost entirely on the dorsal side of the
notochord; so that the latter forms a band on the ventral side of the
vertebral column. In other respects the history of the vertebral
column is the same in the two cases; the vertebral unconstricted parts
of the notochord appear however to become in part converted into
cartilage. The type of formation of the vertebral column in these
genera has been distinguished as epichordal in contradistinction to
the more normal or perichordal type.
Amniota. In the Amniota all trace of a distinction between a cellular
notochord sheath and an arch tissue is lost, and the two are developed
together as a continuous whole forming an unsegmented tube round the
notochord, with a neural ridge which does not at first nearly invest
the neural cord. This tube becomes differentiated, in the manner
already described for other types, into (1) vertebral regions with
true arches, and (2) intervertebral regions.
Reptilia. In Reptilia (Gegenbaur, No. 416) a cartilaginous tube is
formed round the notochord, which is continuous with the cartilaginous
neural arches. The latter are placed in the vertebral regions, and in
these regions ossification very early sets in, while the notochord
remains relatively unconstricted. In the intervertebral regions the
cartilage becomes thickened, as in Amphibia, and gradually constricts
the notochord. The cartilage in each of the intervertebral regions
soon becomes divided into two parts which form the articular faces of
two contiguous vertebræ.
[Pg 557]
The general character of the vertebral column on the completion of
these changes is shewn in fig. 320 D. The later changes are relatively
unimportant. The constricted intervertebral sections of the notochord
rapidly disappear, while the vertebral sections become partially
converted into cartilage, and only cease to be distinguishable at a
considerably later period.
The ossification extends from the bodies of the vertebræ into the
arches and into the articular surfaces, so that the whole vertebræ
eventually become ossified.
The Ascalabotæ (Geckos) present an exceptional type of vertebral
column which has many of the characters of a developmental stage in
other Lizards. The body of the vertebra is formed of a slightly
hourglass-shaped osseous tube, united with adjoining vertebræ by a
short intervertebral cartilage. There is a persistent and continuous
notochord which, owing to the small development of the intervertebral
cartilages, is narrower in the vertebral than in the intervertebral
regions.
Aves. In Birds the cellular tube formed round the notochord is far
thicker than in the Reptilia. It is continuous in the regions of the
future vertebræ with neural arches, which do not at first nearly
enclose the spinal cord.
On about the fifth day, in the case of the chick, it becomes
differentiated into vertebral regions opposite the attachments of the
neural arches, and intervertebral regions between them; the two sets
of regions being only distinguished by their histological characters.
Very shortly afterwards each intervertebral region becomes segmented
into two parts, which respectively attach themselves to the contiguous
vertebral regions. A part of each intervertebral region, immediately
adjoining the notochord, does not however undergo this division, and
afterwards gives rise to the ligamentum suspensorium.
The notochord during these changes at first remains indifferent, but
subsequently, on about the seventh day in the chick, a slight
constriction of each vertebral region takes place; so that the
vertebræ have temporarily, as they have also in Amphibia, a biconcave
form which repeats the permanent condition of most fishes. By the
ninth and tenth days, however, this condition has completely
disappeared, and in all the intervertebral portions the notochord has
become distinctly constricted, and at the same time in each vertebral
portion there
[Pg 558] have also appeared two constrictions of the notochord
giving rise to a central and to two terminal enlargements.
On the twelfth day the ossification of the cartilaginous centra
commences.
The first vertebra to ossify is the second or third cervical, and the
ossification gradually extends to those behind. It does not commence
in the arches till somewhat later than in the bodies. For each arch
there are two centres of ossification, one on each side.
The notochord persists for the greater part of fœtal life and even
into post-fœtal life. The larger vertebral portions are often the
first completely to vanish. They would seem in many cases at any rate
(Gegenbaur) to be converted into cartilage, and so form an integral
part of the permanent vertebræ. Rudiments of the intervertebral
portions of the notochord may long be detected in the ligamenta
suspensoria.
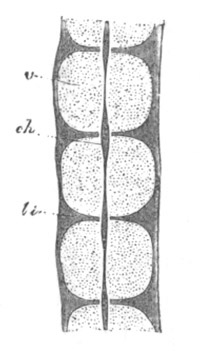
Fig. 321. Longitudinal section through the vertebral column of an
eight weeks’ human embryo in the thoracic region. (From
Kölliker.)
v. cartilaginous vertebral body; li. intervertebral ligament;
ch. notochord.
Schwarck (No. 420) states that in both the intervertebral and the
vertebral regions, though less conspicuously in the former, the
cartilage is divided into two layers, an inner and an outer. He
holds that the inner layer corresponds to the cartilaginous
notochordal sheath of the lower types, and the outer to the arch
tissue. Ossification (Gegenbaur) of the centra appears in a special
inner layer of cartilage, which is probably the same as the inner
layer of the earlier stage, though this point has not been definitely
established.
Mammalia. The early development of the perichordal cartilaginous tube
and rudimentary neural arches is almost the same in Mammals as in
Birds. The differentiation into vertebral and intervertebral regions
is the same in both groups; but instead of becoming divided as in
Reptilia and Birds into two segments attached to two adjoining
vertebræ, the intervertebral regions become in Mammals wholly
converted into the intervertebral ligaments (fig. 322 li). There
are three centres of ossifications for each vertebra, two in the arch
and one in the centrum.
[Pg 559]
Fig. 322. Longitudinal section through the intervertebral ligament
and adjacent parts of two vertebræ from the thoracic region of an
advanced embryo of a Sheep. (From Kölliker.)
la. ligamentum longitudinale anterius; lp. ligamentum long.
posterius; li. ligamentum intervertebrale; k, k´. epiphysis of
vertebra; w. and w´. anterior and posterior vertebræ; c.
intervertebral dilatation of notochord; c´. and c´´. vertebral
dilatation of notochord.
The fate of the notochord is in important respects different from that
in Birds. It is first constricted in the centre of the vertebræ
(figs. 320 E and 321) and disappears there shortly after the
ossification; while in the intervertebral regions it remains
relatively unconstricted (figs. 320 E, 321 and 322 c) and after
undergoing certain histological changes remains through life as part
of the nucleus pulposus in the axis of the invertebral ligaments[201].
There is also a slight swelling of the notochord near the two
extremities of each vertebra (fig. 322 c´ and c´´). In the
persistent vertebral constriction of the notochord Mammals retain a
more primitive and piscine mode of formation of the vertebral column
than the majority either of the Reptilia or Amphibia.
[Pg 560]
Bibliography of Notochord and Vertebral column.
(415) Cartier. “Beiträge zur Entwicklungsgeschichte der Wirbelsäule.”
Zeitschrift für wiss. Zool., Bd. XXV. Suppl. 1875.
(416) C. Gegenbaur. Untersuchungen zur vergleichenden Anatomie der
Wirbelsäule der Amphibien und Reptilien. Leipzig, 1862.
(417) C. Gegenbaur. “Ueber die Entwickelung der Wirbelsäule des
Lepidosteus mit vergleichend anatomischen Bemerkungen.” Jenaische
Zeitschrift, Bd. III. 1863.
(418) C. Gegenbaur. “Ueb. d. Skeletgewebe d. Cyclostomen.” Jenaische
Zeitschrift, Vol. V. 1870.
(419) Al. Götte. “Beiträge zur vergleich. Morphol. des Skeletsystems
d. Wirbelthiere.” II. “Die Wirbelsäule u. ihre Anhänge.” Archiv f.
mikr. Anat., Vol. XV. 1878 (Cyclostomen, Ganoiden, Plagiostomen,
Chimaera), and Vol. XVI. 1879 (Teleostier).
(420) Hasse und Schwarck. “Studien zur vergleichenden Anatomie der
Wirbelsäule u. s. w.” Hasse, Anatomische Studien, 1872.
(421) C. Hasse. Das natürliche System d. Elasmobranchier auf
Grundlage d. Bau. u. d. Entwick. ihrer Wirbelsäule. Jena, 1879.
(422) A. Kölliker. “Ueber die Beziehungen der Chorda dorsalis zur
Bildung der Wirbel der Selachier und einiger anderen Fische.”
Verhandlungen der physical. medicin. Gesellschaft in Würzburg, Bd.
X.
(423) A. Kölliker. “Weitere Beobachtungen über die Wirbel der
Selachier insbesondere über die Wirbel der Lamnoidei.” Abhandlungen
der senkenbergischen naturforschenden Gesellschaft in Frankfurt, Bd.
V.
(424) H. Leboucq. “Recherches s. l. mode de disparition de la corde
dorsale chez les vertébrés supérieurs.” Archives de Biologie, Vol.
I. 1880.
(425) Fr. Leydig. Anatomisch-histologische Untersuchungen über Fische
und Reptilien. Berlin, 1853.
(426) Aug. Müller. “Beobachtungen zur vergleichenden Anatomie der
Wirbelsäule.” Müller’s Archiv. 1853.
(427) J. Müller. “Vergleichende Anatomie der Myxinoiden u. der
Cyklostomen mit durchbohrtem Gaumen, I. Osteologie und Myologie.”
Abhandlungen der königlichen Akademie der Wissenschaften zu Berlin.
1834.
(428) W. Müller. “Beobachtungen des pathologischen Instituts zu Jena,
I. Ueber den Bau der Chorda dorsalis.” Jenaische Zeitschrift, Bd.
VI. 1871.
(429) A. Schneider. Beiträge z. vergleich. Anat. u. Entwick. d.
Wirbelthiere. Berlin, 1879.
Ribs and Sternum.
Ribs. Embryological evidence on the development of the ribs, though
somewhat inadequate, indicates that they arise as cartilaginous bars
in the connective tissue of the intermuscular septa, and that they are
placed, in Elasmobranchii and
[Pg 561] Amphibia, on the level of division
between the dorso-lateral and ventro-lateral divisions of the
muscle-plates. This does not appear to hold true for either Ganoidei
or Teleostei. In Teleostei they are entirely below the muscles along
the lines of the intermuscular septa, and this is partially true for
Ganoidei, though not wholly so in Lepidosteus. They may be attached
either to the hæmal (Pisces) or neural (Amphibia and Amniota) arches.
The connective tissue from which they are formed is continuous with
the processes of the vertebræ to which they are attached; but the
conversion of the tissue into cartilage takes place more or less
independently of that of the arches, although in many cases the
cartilage of the two becomes continuous, the separation of the ribs
being then effected by a subsequent process of segmentation (Fick, No.
431). It is possible that the ribs of Pisces may not be homologous
with those of Amphibia and the Amniota, but till the reverse can be
proved it is more convenient to assume that the ribs are homologous
structures throughout the vertebrate series.
In Elasmobranchii the ribs are relatively of less importance in the
adult than in the embryo. By a careful examination of their early
development, I have satisfied myself that the differentiation of the
ribs is independent of that of the hæmal processes to which they are
attached, although the differentiation proceeds in such a manner that,
when both are converted into cartilage, they are quite continuous.
Subsequently the ribs become segmented off from the hæmal processes.
At the junction of the tail and trunk, where the hæmal processes
commence to be ventrally prolonged, eventually to unite in the region
of the tail below the caudal vein, the ribs are attached to short
processes which spring from the sides of the hæmal arches (fig. 317).
The ventral hæmal arches of these fishes are therefore clearly in no
part formed by the ribs.
In Ganoidei and Teleostei there is very great difficulty in
determining the homologies of the ribs.
In the cartilaginous Ganoidei there are well developed rib-like
structures, which might be regarded as homologous with Elasmobranch
ribs, and indeed probably are so; but at the same time their relations
are in some respects very different from those of Elasmobranch ribs in
the caudal region. In Ganoids the ribs, in approaching the tail,
become shorter and then fuse with the ends of the hæmal processes, and
finally in the caudal region form together with the hæmal arches a
closed hæmal canal which superficially resembles that in
Elasmobranchii.
In Lepidosteus and Amia, especially the former, the same phenomenon is
still more marked; and in Lepidosteus it is easy, in passing
backwards,
[Pg 562] to trace the ribs bending ventralwards, and uniting
ventrally in the caudal region to form, with the hæmal processes, a
complete hæmal canal.
It might have been anticipated that the Teleostean Ganoids would
resemble the Teleostei, but, from an examination of adult Teleostei,
it would seem to be clear that the relations of the parts are the same
as in Elasmobranchii, i.e. that the ribs have no share in forming
the hæmal canal in the tail. Aug. Müller and Götte have however
brought embryological evidence (though not of a conclusive character),
to shew that in the embryo the ribs really fuse with the hæmal
processes in the tail, and so assist, as in the Ganoids, in forming
the hæmal canal. Götte moreover holds that the ribs in Elasmobranchii
are not homologous with those of Teleostei and Ganoids; but that the
hæmal arches in the tail are homologous in the three groups.
Without necessarily following Götte in these views it is worth
pointing out that the undoubtedly close affinity between the bony
Ganoids and the Teleostei is in favour of the view on the hæmal arches
of Teleostei at which he has arrived on embryological grounds.
In Amphibia the formation of the ribs from the connective tissue of
the intermuscular septa, their secondary attachment to the transverse
processes of the neural arches, and their subsequent separation was
first clearly established by Fick (No. 431), whose statements have
since been confirmed by Hasse, Born, &c., and in part by Götte, who
holds however that, though converted into cartilage independently of
the transverse processes, they are formed in membrane as outgrowths of
these processes.
In the Amniota the ribs are also independently established (Hasse and
Born), though they subsequently become united to the transverse
processes and to the bodies of the vertebræ, or to the transverse
processes only. This junction is however stated by the majority of
authorities, never to be effected by the fusion of the cartilage of
the two parts, but always by fibrous tissue; though Hoffmann (No. 435)
takes a different view on this subject, holding that the ribs are at
first continuous with the intervertebral regions of the primitive
cartilaginous tube surrounding the notochord.
Sternum. In dealing with the development of the sternum it will be
convenient to leave out of consideration the interclavicle or
episternum which is, properly speaking, only part of the
shoulder-girdle and to confine my statements to the sternum proper.
This structure is found in all the Amniota except the Ophidia,
Chelonia, and some of the Amphisbænæ.
From the older researches of Rathke, and from the newer ones of Götte,
etc., it appears that the sternum is always formed from the fusion of
the ventral extremities of a certain number of ribs. The extremities
of the ribs unite with each other from
[Pg 563] before backwards, and thus give
rise to two cartilaginous bands. These bands become segmented off from
the ribs with which they are at first continuous, and subsequently
fuse in the median ventral line to form an unpaired sternum. The
Mammalian presternum (manubrium sterni) and xiphosternum have the same
origin as the main body of the sternum (Ruge, No. 438).
In the Amphibia there is no structure which admits from its mode of
development of a complete comparison with the sternum of the Amniota;
and it must for this reason be considered doubtful whether the median
structure placed behind the coracoids in the Anura, which is usually
known as the sternum, is really homologous with the sternum of the
Amniota[202].
The remaining Ichthyopsida are undoubtedly not provided with a sternum.
Bibliography of Ribs and Sternum.
(430) C. Claus. “Beiträge z. vergleich. Osteol. d. Vertebraten. I.
Rippen u. unteres Bogensystem.” Sitz. d. kaiserl. Akad. Wiss. Wien,
Vol. LXXIV. 1876.
(431) A. E. Fick. “Zur Entwicklungsgeschichte d. Rippen und
Querfortsätze.” Archiv f. Anat. und Physiol. 1879.
(432) C. Gegenbaur. “Zur Entwick. d. Wirbelsäule des Lepidosteus mit
vergleich. anat. Bemerk.” Jenaische Zeit., Vol. III. 1867.
(433) A. Götte. “Beiträge z. vergleich. Morphol. d. Skeletsystems d.
Wirbelthiere Brustbein u. Schultergürtel.” Archiv f. mikr. Anat.,
Vol. XIV. 1877.
(434) C. Hasse u. G. Born. “Bemerkungen üb. d. Morphologie d. Rippen.”
Zoologischer Anzeiger, 1879.
(435) C. K. Hoffmann. “Beiträge z. vergl. Anat. d. Wirbelthiere.”
Niederländ. Archiv Zool., Vol. IV. 1878.
(436) W. K. Parker. “A monograph on the structure and development of
the shoulder-girdle and sternum.” Ray Soc. 1867.
(437) H. Rathke. Ueb. d. Bau u. d. Entwicklung d. Brustbeins d.
Saurier. 1853.
(438) G. Ruge. “Untersuch. üb. Entwick. am Brustbeine d. Menschen.”
Morphol. Jahrbuch., Vol. VI. 1880.
[Pg 564]
CHAPTER XIX.
THE SKULL.
Three distinct sets of elements may enter into the composition of the
skull. These are (1) the cranium proper, composed of true endoskeletal
elements originally formed in cartilage, to which are usually added
exoskeletal osseous elements, formed in the manner already described
p. 542, and known in the higher types as membrane bones. (2) The
visceral arches formed primitively as cartilaginous bars, but in the
higher types largely supplemented or even replaced by exoskeletal
elements. (3) The labial cartilages.
These parts present themselves in the most various forms, and their
study constitutes one of the most important departments of vertebrate
morphology, and one which has always been a favourite subject of study
with anatomists. At the end of the last century and during the first
half of the present century the morphology of the skull was handled
from the point of view of the adult anatomy by Goethe, Oken, Cuvier,
Owen, and many other anatomists, while Dugés and, nearer to our own
time, Rathke, laid the foundation of an embryological study of its
morphology. A new era in the study of the skull was inaugurated by
Huxley in his Croonian lecture in 1858, and in his lectures on
Comparative Anatomy subsequently delivered before the Royal College of
Surgeons. In these lectures Huxley disproved the then widely accepted
view that the skull was composed of four vertebræ; and laid the
foundation of a more satisfactory method of dealing with the
homologies of its constituent parts. Since then the knowledge of the
development of the skull has made great progress. In this country a
number
[Pg 565] of very interesting memoirs have been published on the subject
by Parker, which together constitute a most striking contribution to
our knowledge of the ontogeny of the skull in a series of types; and
in Germany Gegenbaur’s monograph on the cephalic skeleton of
Elasmobranchii has greatly promoted a scientific appreciation of the
nature of the skull.
In the present chapter only the most important features in the
development of the skull will be touched on.
It will be convenient to describe, in the first instance, the
development of the cartilaginous elements of the skull.
Fig. 323. head of embryo Dogfish, second stage; basal view of
cranium from above, the contents having been removed. (From Parker.)
ol. olfactory sacs; au. auditory capsule; nc. notochord; py.
pituitary body; pa.ch. parachordal cartilage; tr. trabecula;
inf. infundibulum; C.tr. cornua trabeculæ; pn. prenasal
element; sp. spiracular cleft; br. external branchiæ; Cl. 2,
4. visceral clefts.
The Cranium. The brain is at first enveloped in a continuous layer of
mesoblast known as the membranous cranium, into the base of which the
anterior part of the notochord is prolonged for some distance. The
primitive cartilaginous cranium is formed by a differentiation within
the membranous cranium, and is always composed of the following parts
(fig. 323):
(1) A pair of cartilaginous plates on each side of the cephalic
section of the notochord, known as the parachordals (pa.ch). These
plates together with the notochord (nc) enclosed between them form a
floor for the hind- and mid-brain. The continuous plate, formed by
them and the notochord, is known as the basilar plate.
(2) A pair of bars forming the floor for the fore-brain, known as the
trabeculæ (tr). These bars are continued forward from the
parachordals. They meet behind and embrace the front end of the
notochord; and after separating for some distance bend in again in
such a way
[Pg 566] as to enclose a space—the pituitary space. In front of
this space they remain in contact and generally unite. They extend
forwards into the nasal region (pn).
(3) The cartilaginous capsules of the sense organs. Of these the
auditory (au) and olfactory capsules (ol) unite more or less
intimately with the cranial walls; while the optic capsules, forming
the usually cartilaginous sclerotics, remain distinct.
The parachordals and notochord. The first of these sets of elements,
viz. the parachordals and notochord, forming together the basilar
plate, is always an unsegmented continuation of the axial tissue of
the vertebral column. It forms the floor for that section of the brain
which belongs to the primitive postoral part of the head (vide p.
314), and its extension is roughly that of the basioccipital of the
adult skull. Its mode of development is almost identical with that of
the vertebral column, except that the notochord, even in many forms
where it persists in the vertebral column, disappears in the basilar
plate; though in a certain number of cases remnants of it are found in
the adult state.
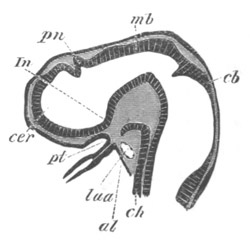
Fig. 324. Longitudinal section through the brain of a young
Pristiurus embryo.
cer. commencement of the cerebral hemisphere; pn. pineal gland;
In. infundibulum; pt. ingrowth from mouth to form the pituitary
body; mb. mid-brain; cb. cerebellum; ch. notochord; al.
alimentary tract; Iaa. artery of mandibular arch.
It will be convenient to say a few words here with reference to the
notochord in the head. It always extends along the floor of the mid-
and hind-brains, but ends immediately behind the infundibulum. The
limits of its anterior extension are clearly shewn in fig. 43. The
front end of the notochord often becomes more or less ventrally flexed
in correspondence with the cranial flexure; its anterior end being in
some instances (Elasmobranchii) almost bent backwards (fig. 324).
Kölliker has shewn that in the Rabbit[203],
and I believe that a more
or less similar phenomenon may also be observed in Birds, the anterior
end of the notochord is united to the hypoblast of the throat in
immediate contiguity with the opening of the pituitary body; but it is
not clear whether this is to be looked upon as the remnant of a
primitive attachment of the notochord to the hypoblast, or as a
secondary attachment.
[Pg 567]
Before the parachordals are formed the anterior end of the notochord
has usually undergone a partial atrophy; and its front end often
becomes somewhat dorsally flexed. Within the basilar plate it often
exhibits two or more dilatations, which have been regarded by Parker
and Kölliker as indicative of a segmentation of this plate; but they
hardly appear to me to be capable of this interpretation.
In Elasmobranchs where, as shewn above, a very primitive type of
development of the vertebral column is retained, we find that the
basilar plate is at first formed of (1) the notochord invested by its
cartilaginous sheath, and (2) of lateral masses of cartilage, the
parachordals, homologous with the arch tissue of the vertebral column.
This development probably indicates that the basilar plate contains in
itself the same elements as those from which the neural arches and the
centra of the vertebral column are formed; but that it never passes
beyond the unsegmented stage at first characteristic of the vertebral
column. The hinder end of each parachordal forms a condyle
articulating with the first vertebra; so that in the cartilaginous
skull there are always two occipital condyles. The basilar plate
always grows up behind (fig. 326, so), and gives rise to a complete
cartilaginous ring enveloping the medulla oblongata, in the same
manner that the neural arches envelope the spinal cord. This ring
forms an occipital cartilaginous ring; in front of it the basilar
plate becomes laterally continuous with the periotic cartilaginous
capsules, and the occipital ring above usually spreads forward to form
a roof for the part of the brain between these capsules. In the higher
Vertebrates the periotic cartilages may be developed continuously with
the basilar plate (fig. 325).
The trabeculæ. The trabeculæ, so far as their mere anatomical
relations are concerned, play the same part in forming the floor for
the front cerebral vesicle as the parachordals for the mid- and
hind-brains. They differ however from the parachordals in one
important feature, viz. that, except at their hinder end (fig. 323),
they do not embrace between them the notochord.
The notochord constitutes, as we have seen, the primitive axial
skeleton of the body, and its absence in the greater part of the
region of the trabeculæ would probably seem to indicate, as
[Pg 568] pointed
out by Gegenbaur, that these parts, in spite of their similarity to
the parachordals, have not the same morphological significance.
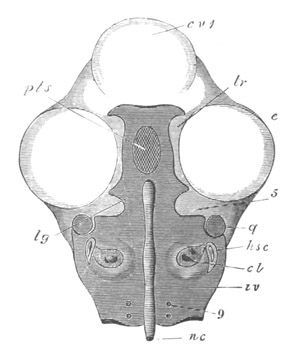
Fig. 325. View From above of the investing mass and of the trabeculæ
of a chick on the fourth day of incubation. (After Parker.)
In order to shew this, the whole of the upper portion of the head
has been sliced away. The cartilaginous portions of the skull are
marked with the dark horizontal shading.
cv 1. cerebral vesicle (sliced off); e. eye; nc. notochord;
iv. investing mass; 9. foramen for the exit of the ninth nerve;
cl. cochlea; hsc. horizontal semicircular canal; q. quadrate;
5. notch for the passage of the fifth nerve; lg. expanded anterior
end of the investing mass; pts. pituitary space; tr. trabeculæ.
The reference line tr. has been accidentally made to end a little
short of the cartilage.
The nature of the trabeculæ has been much disputed by morphologists.
The view that they cannot be regarded as the anterior section of the
vertebral axis is supported by the consideration that the forward
limit of the primitive skeletal axis, as marked by the notochord,
coincides exactly with the distinction we have found it necessary to
recognise, on entirely independent grounds, between the fore-brain,
and the remainder of the nervous axis. But while this distinction
between the parachordals and the trabeculæ must I think be admitted, I
see no reason against supposing that the trabeculæ may be plates
developed to support the floor of the fore-brain, for the same
physiological reasons that the parachordals have become formed at the
sides of the notochord to support the floor of the hind-brain. By some
anatomists the trabeculæ have been held to be a pair of branchial
bars; but this view has now been generally given up. They have also
been regarded as equivalent to a complete pair of neural arches
enveloping the front end of the brain. The primitive extension of the
base of the fore-brain through the pituitary
[Pg 569] space is an argument, not
without force, which has been appealed to in support of this view.
In the majority of the lower forms the trabeculæ arise quite
independently of the parachordals, though the two sets of elements
soon unite; while in Birds (fig. 325) and Mammals the parachordals and
trabeculæ are formed as a continuous whole. The junction between the
trabeculæ and parachordals becomes marked by a cartilaginous ridge
known as the posterior clinoid.
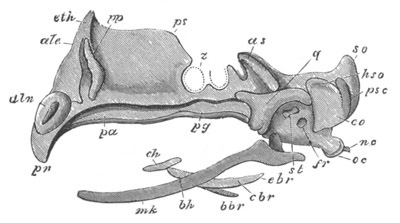
Fig. 326. Side view of the cartilaginous cranium of a Fowl on the
seventh day of incubation. (After Parker.)
pn. prenasal cartilage; aln. alinasal cartilage; ale.
aliethmoid; immediately below this is the aliseptal cartilage.
eth. ethmoid; pp. pars plana; ps. presphenoid or interorbital;
pa. palatine; pg. pterygoid; z. optic nerve; as.
alisphenoid; q. quadrate; st. stapes; fr. fenestra rotunda;
hso. horizontal semicircular canal; psc. posterior vertical
semicircular canal: both the anterior and the posterior semicircular
canals are seen shining through the cartilage. so. supraoccipital;
eo. exoccipital; oc. occipital condyle; nc. notochord; mk.
Meckel’s cartilage; ch. ceratohyal; bh. basihyal; cbr. and
ebr. ceratobranchial; bbr. basibranchial.
The trabeculæ are usually somewhat lyre-shaped, meeting in front and
behind, and leaving a large pituitary space between their middle parts
(figs. 323 and 325). Into this space there primitively projects the
whole base of the fore-brain, but the space itself gradually becomes
narrowed, till it usually contains only the pituitary body. The
carotid arteries always pass through it in the embryo; but in the
higher forms it ceases to be perforated in the adult. The trabeculæ
soon unite together both in front and behind and form a complete plate
underneath the fore-brain, and extending into the nasal region[204].
A special
[Pg 570] vertical growth of this plate in the region of the orbit forms
the interorbital plate of Teleostei, Lacertilia and Aves (fig. 326,
ps), on the upper surface of which the front part of the brain
rests. The trabecular floor of the brain does not long remain simple.
Its sides grow vertically upwards, forming a lateral wall for the
brain, in which in the higher types two regions may be distinguished,
viz. an alisphenoidal region (fig. 326, as) behind, growing out from
what is known as the basisphenoidal region of the primitive trabeculæ,
and an orbitosphenoidal region in front growing out from the
presphenoidal region of the trabeculæ. These plates form at first a
continuous lateral wall of the cranium. At the front end of the brain
they are continued inwards, and more or less completely separate the
true cranial cavity from the nasal region in front. The region of the
cartilage forming the anterior boundary of the cranial cavity is known
as the lateral ethmoid region, and it is always perforated for the
passage of the olfactory nerves.
The cartilaginous walls which grow up from the trabecular floor of the
cranium generally extend upwards so as to form a roof, though almost
always an imperfect roof, for the cranial cavity. In the higher types,
in Mammals more especially, this roof can hardly be said to be formed
at all. The region of the trabeculæ in front of the brain is the
ethmoid region. The basal part of this region forms an internasal
plate, from which an internasal septum may grow up (fig. 326). To its
sides the olfactory capsules are attached, and there are usually
lateral outgrowths in front forming the trabecular cornua, while from
the posterior part of the ethmoidal plate, forming the anterior
boundary of the cranial cavity, there often grows out a prefrontal or
lateral ethmoidal process.
These and other processes growing out from the trabeculæ have
occasionally been regarded as rudimentary præoral branchial arches. I
have already stated it as my view that the existence of branchial
arches in this region is highly improbable, and I may add that the
development of these structures as outgrowths of the skull is in
itself to my mind a nearly conclusive argument against their being
branchial arches, in that true branchial arches hardly ever or perhaps
never arise in this way.
The sense capsules. The most important of these is the auditory
capsule, which, as we have seen, fuses intimately with
[Pg 571] the lateral
walls of the skull. In front there is usually a cleft separating it
from the alisphenoid region of the skull, through which the third
division of the fifth nerve passes out. This cleft becomes narrowed to
a small foramen (fig. 327, V). The sclerotic cartilage is always
free, but profoundly modifies the region of the cranium near which it
is placed. The nasal investment forms in Elasmobranchs (fig. 327,
Na) a capsule open below, and continuous with the ethmoid region of
the trabeculæ. In most types however it becomes more closely united
with the ethmoid region and the accessory parts belonging to it.
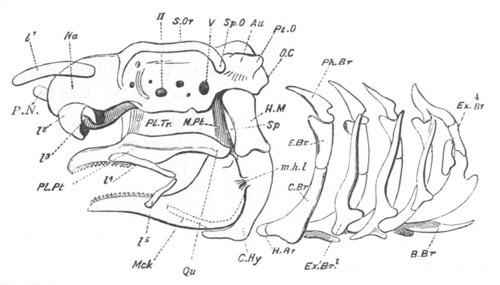
Fig. 327. Skull of adult Dogfish, side view. (From Parker.)
O.C. occipital condyle; Au. periotic capsule; Pt.O. pterotic
ridge; Sp.O. sphenotic process; S.Or. supraorbital ridge; Na.
nasal capsule; P.N. prenasal cartilage; II. optic foramen; V.
trigeminal foramen; Pl.Pt., Qu. pterygo-quadrate arcade; M.Pt.
metapterygoid ligament (including a small cartilage); Pl.Tr.
ethmo-palatine or palato-trabecular ligament; Mck. lower jaw;
Sp. spiracle; H.M. hyomandibular; C.Hy. ceratohyal; m.h.l.
mandibulohyoid ligament; Ph.Br. pharyngobranchial; E.Br.
epibranchial; C.br. ceratobranchial; H.Br. hypobranchial;
B.Br. basibranchial; Ex.Br. extra-branchial; l1, 2, 3,
4, 5. labial cartilages; the dotted lines within Mck.
indicate the basihyal.
The cartilaginous cranium, the development of which has been thus
briefly traced, persists in the adult without even the addition of
membrane bones in the Cyclostomata, Elasmobranchii (fig. 327) and
Holocephali. In the Selachioid Ganoids it is also found in the adult,
but is covered over by membrane bones. In all other types it is
invariably present in the embryo, but becomes in the adult more or
less replaced by osseous tissue.
[Pg 572]Branchial skeleton.
The most primitive type of branchial skeleton in any existing form
would appear to be that of the Petromyzonidæ, which is developed in a
superficial subdermal tissue, and consists of a series of bars united
by transverse pieces, so as to form a basketwork. It is known as an
extra-branchial system, and an early stage of its development in the
Lamprey is shewn in fig. 47. In the higher forms this system is
replaced by a series of bars, known as the branchial bars, so situated
as to afford support to the successive branchial pouches. Outside
these bars there may be present in some primitive forms
(Elasmobranchii) cartilaginous elements, which are supposed to be
remnants of the extra-branchial system (fig. 327, Ex.Br); while a
series of membrane bones is also usually added to them, which will be
dealt with in a separate section. The branchial bars are developed as
simple cartilaginous rods in the deeper parts of the mesoblast which
constitutes the primitive branchial arches.
The position of the branchial bars in relation to the somatopleure and
splanchnopleure can be determined from their relation to the so-called
head cavities. These cavities atrophy before the formation of the
cartilaginous branchial bars, but it will be observed (fig. 328), that
the artery of each arch (aa) is placed on the inner side of the head
cavity (pp). The cartilaginous bar arises at a later period on the
inner side of the artery, and therefore on the inner side of the
section of the body cavity primitively present in the arches.
An anterior arch, known as the mandibular arch, placed in front of the
hyomandibular cleft, and a second arch, known as the hyoid arch,
placed in front of the hyobranchial cleft, are developed in all types.
The succeeding arches are known as the true branchial arches, and are
only fully developed in the Ichthyopsida.
In some Sharks (Notidani) seven branchial arches may be present (not
including the hyoid and mandibular). In other Ichthyopsida five are
usually present, in the embryo at any rate, while in the Amniota there
are usually two or three post-hyoid membranous arches, in the interior
of which a cartilaginous bar is usually formed. The general form of
these bars at an early
[Pg 573] stage of development is shewn in the dogfish
(Scyllium) in fig. 329.
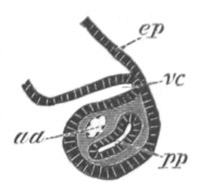
Fig. 328. Horizontal section through the penultimate visceral arch
of an embryo of Pristiurus.
ep. epiblast; vc. pouch of hypoblast which will form the walls
of a visceral cleft; pp. segment of body-cavity in visceral arch;
aa. aortic arch.
The simple condition of these bars in the embryo renders it highly
probable that forms existed at one time with a simple branchial
skeleton of this kind: at the present day however such forms no longer
exist. The first arch has in all cases changed its function and has
become converted into a supporting skeleton for the mouth; the hyoid
arch, though retaining in some forms its branchial function, has in
most acquired additional functions and has undergone in consequence
various peculiar modifications. The true branchial arches retain their
branchial functions in Pisces and some Amphibia, but are secondarily
modified and largely aborted in the abranchiate forms. Since the
changes undergone by the true branchial bars are far less complicated
than those of the hyoid and mandibular bars it will be convenient to
treat of them in the first instance.
Fig. 329. Head of embryo Dogfish, 11 lines long. (From Parker.)
Tr. trabecula; Pl.Pt. pterygo-quadrate; M.Pt. metapterygoid
region; Mn. mandibular cartilage; Hy. hyoid arch; Br. 1. first
branchial arch; Sp. mandibulohyoid cleft; Cl1. hyobranchial
cleft; Lch. groove below the eye; Na. olfactory rudiment; E.
eyeball; Au. auditory mass; C 1, 2, 3. cerebral vesicles; Hm.
hemispheres; f.n.p. nasofrontal process.
These bars are, as already mentioned, most numerous in certain very
primitive forms (seven in Notidanus), while as we ascend the series
there is a gradual tendency for the posterior of them to disappear.
This tendency is the result of a gradual atrophy of the posterior
branchial pouches, which commenced at
[Pg 574] a stage in the evolution of the
Chordata long prior to the appearance of cartilaginous or osseous
branchial bars, and reaches its climax in the Amniota.
In a fully developed branchial bar the primitively simple rod of
cartilage becomes divided into a series of segments, usually four,
articulated so as to be more or less mobile: and either remaining
cartilaginous or becoming partially or wholly ossified. Each bar (fig.
327) forms a somewhat curved structure, embracing the pharynx. The
dorsal and somewhat horizontally placed segment is known as the
pharyngobranchial (Ph.Br), the next two as the epibranchial (E.Br)
and ceratobranchial (C.Br), and the ventral segment as the
hypobranchial (H.Br). There is also typically present a basal
unpaired segment, uniting the bars of the two sides, known as the
basibranchial (B.Br). The arches often bear cartilaginous rays which
support the gill lamellæ.
In Teleostei dental plates are usually developed as an exoskeletal
covering on parts of the branchial arches.
In the Amphibia four or three branchial arches are present in the
embryo. These parts are more or less completely retained in the
Perennibranchiata and Caducibranchiata, but in the Myctodera and Anura
they become largely reduced, and entirely connected with the hyoid.
In the Anura they never reach any considerable development, and are
soon reduced to a plate (fig. 330)—the coalesced basihyal and
basibranchial plate—the posterior processes of which represent the
remnants of the branchial arches.
Fig. 330. Young Frog, with tail just absorbed; side view of
skull. (From Parker.)
Au. auditory capsule; in front of it is the cranial side wall;
A.N. external nostril; St. stapes; Mck. Meckelian cartilage;
B.Hy. basihyobranchial plate; St.Hy. stylohyal or ceratohyal;
Br. 1. first branchial arch.
Bones: E.O. exoccipital; Pr.O. prootic; Pa. parietal; Fr.
frontal; Na. nasal; Pmx. premaxillary; Mx. maxillary; Pt.
pterygoid; Sq. squamosal; Qu.Ju. quadratojugal; Art.
articular; D. dentary.
According to Parker the posterior process of this plate in the adult
is a remnant of the fourth branchial bar; the next one is the third
branchial bar, while the anterior lamina behind the hyoid is stated by
him (though this is somewhat doubtful) to be a remnant of the first
two bars.
In the Amniota, the branchial arches become still more
[Pg 575] degenerated, in
correlation with the total disappearance of a branchial respiration at
all periods of life. Their remnants become more or less important
parts of the hyoid bone, and are solely employed in support of the
tongue. Their basal portions are best preserved, forming parts of the
body of the hyoid. The posterior (thyroid) cornua of the hyoid are
remnants of the true arches. Of these there are two in the Chelonia
and Lacertilia, and one in the Aves and Mammalia. In Aves the cornu
formed from the first branchial arch (fig. 331, cbr) is always
larger than that of the true hyoid arch (ch).
Mandibular and Hyoid arches. The adaptations of both the mandibular
and hyoid bars, to functions entirely distinct from those which they
primitively served, are most remarkable; and the adaptations of the
two bars are in many cases so intimately bound together, that it is
not possible to treat them separately.
Fig. 331. View from below of the branchial skeleton of the skull
of a Fowl on the fourth day of incubation. (After Parker.)
cv 1. cerebral vesicles; e. eye; fn. frontonasal process; n.
nasal pit; tr. trabeculæ; pts. pituitary space; mr. superior
maxillary process; pg. pterygoid; pa. palatine; q. quadrate;
mk. Meckel’s cartilage; ch. ceratohyal; bh. basihyal; cbr.
ceratobranchial; ebr. proximal portion of the cartilage in the
third visceral (first branchial) arch; bbr. basibranchial; 1. first
visceral cleft; 2. second visceral cleft; 3. third visceral arch.
The most important change of function is undoubtedly that of the
mandibular arch, which becomes entirely converted into a skeleton for
the jaws. It may be noted as a peculiarity of the
[Pg 576] mandibular arch that
it is never provided with an unpaired basal element.
The simplest forms of metamorphosis are those undergone by
Elasmobranchii, of which the Dogfish (Scyllium) and Skate (Raja) have
been studied (Parker, No. 456). In some of these forms, e.g. the
Skate, part of the mandibular bar is still related to the
hyomandibular cleft (the spiracle).
Elasmobranchii. In Scyllium the hyoid and mandibular arches are at
first very similar to those which follow. Soon however each of them
sends an anteriorly directed dorsal process (fig. 329). The regions
which may be distinguished owing to the growth of these processes have
received names from ossifications in them which are found in other
types. The anterior process of the mandibular arch is known as the
pterygo-quadrate bar (Pl.Pt); the dorsal end of the primitive bar
from which it starts (M.Pt) is known as the metapterygoid process;
while the ventral end of the bar forms the Meckelian cartilage. The
upper end of the hyoid arch is known as the hyomandibular.
In a somewhat later stage changes take place which cause these parts
practically to assume the adult form (fig. 327). The mandibular arch
becomes segmented at its bend into (1) a pterygo-quadrate bar
(Pl.Pt) which grows forwards in front of the mouth and forms an
upper jaw, and (2) a Meckelian cartilage (Mck) which is placed
behind the mouth, and forms a lower jaw. The two jaws are articulated
together, and the cartilages of the two sides composing them meet each
other distally.
At the articulation of the Meckelian cartilage with the quadrate part
of the pterygo-quadrate is situated a ligament (M.Pt), which takes
the place of the metapterygoid process of the previous stage, and
passes up on the anterior side of the spiracle, to be attached to the
cranium in the front part of the auditory region. This ligament, which
is supplemented by a second ligament, the ethmo-palatine ligament,
passing from the pterygo-quadrate bar to the antorbital region of the
skull, is not the most important support of the jaw. The main support
is, on the contrary, given by the hyoid arch; the hyomandibular
segment of which (H.M) as well as the adjoining segment (ceratohyoid
C.Hy) are firmly attached by ligament to the mandibular
[Pg 577] arch. The
hyomandibular is articulated with the cranium beneath the pterotic
ridge (Pt.O).
In the type just described, the hyoid and mandibular arches undergo
less modification than in almost any other case. The hyoid arch has
altered its form, but retains its respiratory function. It has however
acquired the secondary function of supporting the mandibular arch. The
mandibular arch is divided into two elements, which form respectively
the upper and lower jaws. It is not directly articulated with the
skull, and its mode of support by the hyoid arch has been called by
Huxley (No. 445) hyostylic.
Fig. 333. Head of embryo Skate, 11⁄3 in. long. (From
Parker.)
Tr. trabecula; Pl.Pt. pterygo-quadrate bar; Mn. mandibular
bar; M.Pt. metapterygoid cartilage; H.M. hyomandibular; Hy.
remainder of hyoid arch; Br. 1. first branchial arch; Sp.
mandibulohyoid cleft or spiracle; Pn. pineal gland; Au. auditory
vesicle; C. 1, C. 2, and C. 3. vesicles of the brain.
The development of the hyoid and mandibular arches in the Skate is
characterised by a few important features (fig. 333). The anterior
element of the hyoid arch, which forms the hyomandibular (H.M),
becomes entirely separate from the posterior part of the arch, and
only serves to support the jaws. The posterior part of the arch (Hy)
carries on the respiratory functions of the hyoid, and is closely
connected with the first branchial arch. The upper or metapterygoid
element of the mandibular arch (M.Pt) has a considerable
development, and, becoming separated from the remainder of the arch,
forms a mass of cartilage with one or two branchial rays, in the front
wall of the spiracle, and constitutes a section of the mandibular arch
still retaining traces of its primitive function in supporting the
wall of a branchial pouch.
Although the development of other Elasmobranch types is not known, it
is necessary to call attention to the mode of support of the
mandibular arch in certain forms, notably Notidanus, Hexanchus and
Cestracion, where the pterygo-quadrate region of the mandibular arch
is directly articulated to the
[Pg 578] cranium between the optic and
trigeminal foramina. In the two former genera the metapterygoid region
of the arch is moreover continuous with the pterygo-quadrate, and
articulates with the postorbital process of the auditory region of the
skull. In spite of these attachments the mandibular arch continues to
be partially supported by the hyomandibular. The skulls in which the
mandibular arch has this double form of support have been called by
Huxley amphistylic.
Fig. 334. Cranial skeleton of a Salmon fry, second week after
hatching; membrane bones, eyeballs, and nasal sacs removed. (From
Parker.)
T.Cr. tegmen cranii; S.Or. supraorbital band; Fo. superior
fontanelle; Au. auditory capsule; Pa.ch. parachordal cartilage;
Ch. notochord; Tr. trabecula; above the trabecula, the
interorbital septum is seen, passing into the cranial wall above and
reaching the supraorbital band; H. optic foramen; V. trigeminal
foramen; l1, l2. labial cartilages; Pl.Pt. palatopterygoid
bar; M. Pt. metapterygoid tract; Qu. quadrate region; Mck.
Meckelian cartilage; H.M. hyomandibular cartilage; Sy.
symplectic tract; I.Hy. interhyal; C.Hy. ceratohyal; H.Hy.
hypohyal; G.Hy. glossohyal; Br.1. first branchial arch.
Considering the in many respects primitive characters of the forms
with amphistylic skulls it seems not improbable that they preserve the
original mode of support of the mandibular arch; from which
differentiations in two directions have taken place, viz.
differentiations in the direction of a complete support of the
mandibular arch by the hyoid, which is characteristic of most
Elasmobranchii and, as will be shewn below, of Ganoidei and Teleostei;
and differentiations towards a direct articulation or attachment of
the mandibular arch to the cranium, without the
[Pg 579] intervention of the
hyoid. The latter mode of attachment is called by Huxley autostylic.
It is found in Holocephala, Dipnoi, Amphibia and the Amniota.
Teleostei. In addition to that of Elasmobranchii, the skull of the
Salmon is the only hyostylic skull in which, by the admirable
investigation of Parker (No. 451), the ontogeny of the hyoid and
mandibular bars has been satisfactorily worked out. Apart from the
presence of a series of membrane bones, the development of these bars
agrees on the whole with the types already described.
Fig. 335. Young Salmon of the first summer, about 2 inches long;
side view of skull, excluding branchial arches. (From Parker.)
The palato-mandibular and hyoid tracts are detached from their
proper situations, a line indicating the position where the
hyomandibular is articulated beneath the pterotic ridge.
ol. olfactory fossa; c.tr. trabecular cornu; ula. ulb.
upper labial cartilages; p.s. presphenoid tract; t.cr. tegmen
cranii; s.o.b. supraorbital band; fo. superior fontanelle;
n.c. notochord; b.o. basilar cartilage; tr. trabecula; p.c.
condyle for palatine cartilage; 5. trigeminal foramen; 7a. facial
foramen; 8. foramen for glossopharyngeal and vagus nerves; mk.
Meckelian cartilage; op.c. opercular condyle.
Bones: e.o. exoccipital; s.o. supraoccipital; e.p. epiotic;
pt.o. pterotic; sp.o. sphenotic; op. opisthotic; pro.
prootic; b.s. basisphenoid; al.s. alisphenoid; o.s.
orbitosphenoid; l.e. ectethmoid or lateral ethmoid; pa.
palatine; pg. pterygoid; m.pg. mesopterygoid; mt.pg.
metapterygoid; qu. quadrate; ar. articular; h.m.
hyomandibular; sy. symplectic; i.h. interhyal; ep.h.
epiceratohyal; c.h. ceratohyal; h.h. hypohyal; g.h. glosso- or
basihyal.
The hyoid arch, though largely ossified, undergoes a process of
development very similar to that in Raja. It is formed as a simple
cartilaginous bar, which soon becomes segmented longitudinally
[Pg 580] into an
anterior and a posterior part (fig. 334). The former constitutes the
hyomandibular (H.M), while the latter, becoming more and more
separated from the hyomandibular, constitutes the hyoid arch proper;
owing to the disappearance of the hyobranchial cleft, it loses its
primitive function, and serves on the one hand to support the
operculum covering the gills, and on the other to support the tongue.
It becomes segmented into a series of parts which are ossified (fig.
335) as the epiceratohyal (ep.h) above, then a large ceratohyal
(ch), followed by a hypohyal (hh), while the median ventral
element forms the basi- or glossohyal (g.h).
The hyomandibular itself is articulated with the skull below the
pterotic process (fig. 334, H.M). Its upper element ossifies as the
hyomandibular (fig. 335, h.m.), while its lower part (fig. 334,
Sy), which is firmly connected with the mandibular arch, ossifies as
the symplectic (fig. 335, sy). A connecting element between the two
parts of the hyoid bar forms an interhyal (ih).
There are more important differences in the development of the
mandibular arch in Elasmobranchii and the Salmon than in that of the
hyoid arch, in that, instead of the whole arcade of the upper jaw
being formed from the mandibular arch, a fresh element, in the form of
an independently developed bar of cartilage, completes the upper
arcade in front; but even with this bar the two halves of the upper
branch of the arch do not meet anteriorly, but are separated by the
ends of the trabeculæ.
The anterior bar of the upper arcade is known as the palatine; but it
appears to me as yet uncertain how far it is to be regarded as an
element, primitively belonging to the upper arcade of the mandibular
arch, which has become secondarily independent in its development; or
as an entirely distinct structure which has no counterpart in the
Elasmobranch upper jaw. The latter view is adopted by Parker and
Bridge, and a cartilage attached to the hinder wall of the nasal
capsule of many Elasmobranchii is identified by them with the palatine
rod of the Teleostei.
The arch itself is at first very similar to the succeeding arches; its
dorsal extremity soon however becomes broadened, and provided with an
anteriorly directed process. This part (fig. 334, M.Pt and Qu) is
then segmented from the lower region,
[Pg 581] and forms what may be called the
pterygo-quadrate cartilage, though not completely homologous with the
similarly named cartilage in Elasmobranchs; while the lower region
forms the Meckelian cartilage (Mck), which has already grown
inwards, so as to meet its fellow ventrally below the mouth. The whole
arch becomes at the same time widely separated from the axial parts of
the skull.
Nearly simultaneously with the first differentiation of the mandibular
arch, a bar of cartilage—the palatine bar already spoken of—is
formed on each side, below the eye, in front of the mouth. The dilated
anterior extremity of this bar soon comes in contact with an anterior
process of the trabeculæ, known as the ethmo-palatine process.
In a later stage the pterygoid end of the pterygo-quadrate cartilage
unites with the distal end of the palatine bar (fig. 334, Pl.Pt),
and there is then formed a continuous cartilaginous arcade for the
upper jaw, which is strikingly similar to the cartilaginous upper jaw
of Elasmobranchii.
A large dorsal process of the primitive pterygo-quadrate now forms a
large metapterygoid tract (M.Pt); while the whole arch becomes
firmly bound to the hyomandibular (H.M).
In the later stages the parts formed in cartilage become ossified
(fig. 335). The palatine is first ossified, the pterygoid region of
the pterygo-quadrate is next ossified as a dorsal mesopterygoid
(m.pg) and a ventral pterygoid proper (pg). The quadrate region,
articulating with the Meckelian cartilage, becomes ossified as a
distinct quadrate (qu), while the dorsal region becomes also
ossified as a metapterygoid (mt.pg).
In the Meckelian cartilage a superficial ossification of the ventral
edge and inner surface forms an articulare (ar); but the greater
part of the cartilage persists through life.
Some of the above ossifications, at any rate those of the palatine and
pterygoid, seem to be started by dental osseous plates adjoining the
cartilage. They will be spoken of further in the section dealing with
the membrane bones.
Amphibia. The development of the autostylic piscine skulls
has unfortunately not yet been studied; and the most primitive
autostylic types whose development we are acquainted with are
[Pg 582]
those of the Amphibia; on which a large amount of light has
been shed by the researches of Huxley and Parker.
The modifications of the hyoid arch are comparatively simple
and uniform. It forms a rod of cartilage, which soon articulates
in front with the quadrate element of the mandibular arch, and
is subsequently attached by ligaments both to the quadrate and
to the cranium. In those Amphibia in which external gills and
gill clefts are lost, it fuses with the basal element of the hyoid
(fig. 330), which, together with the basal portions of the following
arches, forms a continuous cartilaginous plate. On the completion
of these changes the paired parts of the hyoid arch have
the form of two elongated rods, known as the anterior cornua of
the hyoid, which attach the basihyal plate to the cranium behind
the auditory capsule.
It is still uncertain whether there is any distinct element corresponding
to the hyomandibular of fishes.
Parker holds that the columella auris of the Anura is the homologue of
the hyomandibular. The columella develops comparatively late and
independently of the remainder of the hyoid arch, but the similarity
between its relations to the nerves and those of the hyomandibular is
put forward by Parker as an argument in favour of his view. The early
ligamentous connection between the quadrate and the upper end of the
primitive hyoid is however an argument in favour of regarding the
upper end of the primitive hyoid as the hyomandibular element, not
separated from the remainder of the arch.
The history of the mandibular arch is more complicated than that of
the hyoid. The part of it which corresponds with the upper jaw of
Elasmobranchii exhibits most striking variations in development; so
striking indeed as to suggest that the secondary modifications it has
undergone are sufficiently considerable to render great caution
necessary in drawing morphological conclusions from the processes
which are in some instances observable. A more satisfactory judgment
on this point will be possible after the publication of a memoir with
which Parker is now engaged on the skulls of the different Anura.
The membrane bones applying themselves to the sides of the mandibular
arch are relatively far more important than in the lower types. This
is especially the case with the upper jaw where the maxillary and
premaxillary bones functionally replace the primitive cartilaginous
jaw; while membranous pterygoids
[Pg 583] and palatines apply themselves to,
and largely take the place of, the cartilaginous palatine and
pterygoid bars.
Two types worked out by Parker, viz. the Axolotl and the common Frog,
may be selected to illustrate the development of the mandibular arch.
Fig. 336. Young Axolotl, 2¼ inches long; under view of skull,
dissected, the lower jaw and gill arches having been removed.
(From Parker.)
nc. notochord; oc.c. occipital condyle; f.o. fenestra ovalis;
st. stapes; tr. trabecular cartilage; i.n. internal nares;
c.tr. cornu trabeculæ; pd. pedicle of quadrate; q. quadrate;
pg. outline of pterygoid cartilage; 5´. orbito-nasal nerve; 7.
facial nerve.
Bones: pa.s. parasphenoid; e.o. exoccipital; v. vomer; px.
premaxillary; mx. maxillary; pa. palatine; pg. pterygoid.
In the Axolotl, which may be taken as the type for the Urodela, the
mandibular arch is constituted at a very early stage of (1) an
enlarged dorsal element, corresponding with the pterygo-quadrate of
the lower types, but usually known as the quadrate; and (2) a ventral
or Meckelian element. The Meckelian bar very early acquires its
investing bones, while the dorsal part of the quadrate becomes divided
into two characteristic processes, viz. an anterior dorsal process
which grows towards and soon permanently fuses with the trabecular
crest, and a posterior process known as the otic process, which
applies itself to the outer side of the auditory region. The anterior
of these processes, as pointed out by Huxley, is probably homologous
with the anterior process of the pterygo-quadrate bar in Notidanus,
which articulates with the trabecular region of the cranium, while the
otic process is homologous with the metapterygoid
[Pg 584] process. Hardly any
trace is present of an anterior process to form a pterygoid bar, but
dentigerous plates forming a dermal palatopterygoid bar have already
appeared.
At a somewhat later stage a fresh process, called by Huxley the
pedicle, grows out from the quadrate, and articulates with the ventral
side of the auditory region (fig. 336, pd). Shortly afterwards a rod
of cartilage grows forward from the quadrate under the membranous
pterygoid (pg), which corresponds with the cartilaginous pterygoid
bar of other types (fig. 336), and an independent palatine bar,
arising even before the pterygoid process, is formed immediately
dorsal to the dentigerous palatine plate (pa), and is attached to
the trabecula. These two bars eventually meet, but never become firmly
united to the more important membrane bones placed superficially to
them.
The mandibular arch in the Frog stands, so far as development is
concerned, in striking contrast to the mandibular arch of the Axolotl,
in spite of the obvious similarity in the arrangement of the adult
parts in the two types.
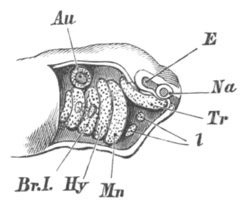
Fig. 337. Embryo Frog, just before
hatching; side view of head,
with skin removed. (From Parker.)
Na. olfactory sack; E. involution for eyeball; Au. auditory
sack; Tr. trabecula; Mn. mandibular; Hy. hyoid; Br.I. first
branchial arch; the gill-buds are seen on the first two branchial
arches; l. labial cartilages.
In the earliest stage it forms a simple bar in the membranous
mandibular arch, parallel to and very similar to the hyoid bar behind
(fig. 337, Mn). In the next stage observed, that is to say in
Tadpoles of four, five, to six lines long, an astonishing
transformation has taken place. The mandibular arch (fig. 338) is
turned directly forwards parallel to the trabecula, to which it is
attached in front (p.pg) and behind (pd). The proximal part of the
arch thus forms a subocular bar, and the space between it and the
trabecula a subocular fenestra. In front of the anterior attachment it
is continued forwards for a short distance, and to the free end of
this projecting part is articulated a small Meckelian cartilage
directed upwards (mk). The Meckelian cartilage is at this stage
placed in front of the nasal sacks, in the lower lip of the suctorial
[Pg 585]
mouth. The greater part of the arch, parallel with the trabeculæ, is
equivalent to what has been called in the Axolotl the quadrate, while
its anterior attachment to the trabeculæ is the rudiment of the
palatopterygoid cartilage. The posterior attachment is known as the
pedicle.
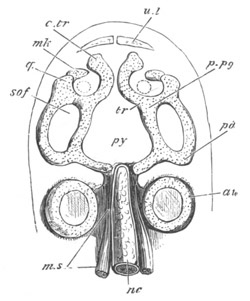
Fig. 338. Tadpole of Common Toad, one-third of an inch long;
cranial and mandibular cartilages seen from above; the parachordal
cartilages are not yet definite. (From Parker.)
nc. notochord; ms. muscular segments; au. auditory capsule;
py. region of pituitary body; tr. trabecula; c.tr. cornu
trabeculæ; p.pg. palatopterygoid bar; pd. pedicle; q. quadrate
condyle; mk. Meckelian piece of mandibular arch; so.f. subocular
fenestra; u.l. upper labial cartilage. The dotted circle within
the quadrate region indicates the position of the internal nostril.
The condition of the mandibular arch during this and the next stage
(fig. 339) is very perplexing. Its structure appears adapted in some
way to support the suctorial mouth of the Tadpole.
Reasons have been offered in a previous part of this volume for
supposing that the suctorial mouth of the Tadpole is probably not
simply a structure secondarily acquired by this larva, but is an organ
inherited from an ancestor provided through life with a suctorial
mouth.
The question thus arises, is the peculiar modification of the
mandibular arch of the Tadpole an inherited or an acquired
feature?
If the first alternative is accepted we should have to admit that the
mandibular arch became first of all modified in connection with the
suctorial mouth, before it was converted into the jaws of the
Gnathostomata; and that the peculiar history of this arch in the
Tadpole is a more or less true record of its phylogenetic development.
In favour of this
[Pg 586] view is the striking similarity which Huxley has
pointed out between the oral skeleton of the Lamprey and that of the
Tadpole; and certain peculiarities of the mandibular arch of Chimæra
and the Dipnoi can perhaps best be explained on the supposition that
the oral skeleton of these forms has arisen in a manner somewhat
similar to that in the Frog; though with reference to this point
further developmental data are much required.
On the other hand the above suppositions would necessitate our
admitting that a great abbreviation has occurred in the development of
the mandibular arch of the otherwise more primitive Urodela; and that
the simple mode of growth of the jaws in Elasmobranchii, from the
primitive mandibular arch, is phylogenetically a much abbreviated and
modified process, instead of being, as usually supposed, a true record
of ancestral history.
If the view is accepted that the characters of the mandibular arch of
the Tadpole are secondary, it will be necessary to admit that the
adaptation of the mandibular arch to the suctorial mouth took place
after the suctorial mouth had come to be merely a larval organ.
In view of our imperfect knowledge of the development of most Piscine
skulls I would refrain from expressing a decided opinion in favour of
either of these alternatives.
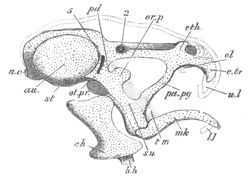
Fig. 339. Tadpole with tail beginning to shrink; side view of skull
without the branchial arches. (From Parker.)
n.c. notochord; au. auditory capsule; between it and eth. the
low cranial side wall is seen; eth. ethmoidal region; st.
stapes; 5. trigeminal foramen; 2. optic foramen; ol. olfactory
capsules, both seen owing to slight tilting of the skull; c.tr.
cornu trabeculæ; u.l. upper labial, in outline; su. suspensorium
(quadrate); pd. its pedicle; ot.pr. its otic process; or.p.
its orbitar process; t.m. temporal muscle, indicated by dotted
lines passing beneath the orbitar process; pa.pg. palatopterygoid
bar; mk. Meckelian cartilage; l.l. lower labial, in outline;
c.h. ceratohyal; b.h. basihyal. The upper outline of the head is
shewn by dotted lines.
As the tail of the Tadpole gradually disappears, and the metamorphosis
into the Frog becomes accomplished, the mandibular arch undergoes
important changes (fig. 339): the
[Pg 587] palatopterygoid attachment (pa.pg)
of the quadrate subocular bar becomes gradually elongated; and, as it
is so, the front end of the subocular bar (su) rotates outwards and
backwards, and soon forms a very considerable angle with the
trabeculæ. The Meckelian cartilage (mk) at its free end becomes at
the same time considerably elongated. These processes of growth
continue till (fig. 330) the palatopterygoid bar (Pt) forms a
subocular bar, and is considerably longer than the original subocular
region of the quadrate; while the Meckelian cartilage (Mck) has
assumed its permanent position on the hinder border of the no longer
suctorial mouth, and has grown forwards so as nearly to meet its
fellow in the median line.
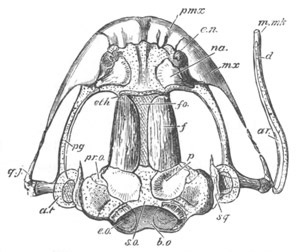
Fig. 340. Young Frog, near end of first summer; upper view of
skull, with left mandible removed, and the right extended outwards.
(From Parker.)
b.o. basioccipital tract; s.o. supraoccipital tract; fo.
frontal fontanelle; e.n. external nostril; internal to it,
internasal plate; a.t. tympanic annulus.
Bones: e.o. exoccipital; pr.o. prootic, partly overlapped by
p. parietal; f. frontal; eth. rudiment of sphenethmoid; na.
nasal; pmx. premaxillary; mx. maxillary; pg. pterygoid, partly
ensheathing the reduced cartilage; q.j. quadratojugal; sq.
squamosal; ar. articular; d. dentary; m.mk. mento-Meckelian.
The metapterygoid region of the quadrate gives rise to a posterior and
dorsal process (fig. 339, ot.pr), the end of which is constricted
off as the tympanic annulus (fig. 340, a.t); while the proximal part
of the process remains as the otic (metapterygoid) process,
articulating with the auditory cartilage.
The pedicle (pd) retains its original attachment to the skull.
[Pg 588]
The palatopterygoid soon becomes segmented into a transversely placed
palatine, and a longitudinally placed pterygoid (fig. 340). With the
exception of a few ossifications, which present no features of special
interest, the parts of the mandibular arch have now reached their
final condition, which is not very different from that in the Axolotl.
Sauropsida. In the Sauropsida the modifications of the hyoid and
mandibular arches are fairly uniform.
The lower part of the hyoid arch, including the basihyoid, unites with
the remnants of the arches behind to form the hyoid bone, to which it
contributes the anterior cornu and anterior part of the body.
The columella is believed by Huxley and Parker to represent, as in the
Anura, the independently developed dorsal (hyomandibular) element of
the hyoid, together with the stapes with which it has become
united[205].
The membranous mandibular arch gives off in the embryos of all the
Sauropsida an obvious bud to form the superior maxillary process, and
the formation of this bud appears to represent the growth forwards of
the pterygoid process in Elasmobranchii, which is indeed accompanied
by the formation of a similar bud; but the skeletal rod, which appears
in the axis of this bud, is as a rule independent of that in the true
arch (fig. 331, pa, pg). The former is the pterygo-palatine bar;
the latter the Meckelian and quadrate cartilages.
The pterygo-palatine bar is usually if not always ossified directly,
without the intervention of cartilage.
Born has recently shewn that Parker was mistaken in supposing that the
palatopterygoid bone is cartilaginous in Birds. In the Turtle a short
cartilaginous pterygoid process of the quadrate would seem to be
present (Parker, No. 458).
The quadrate and Meckelian cartilages are either from the first
separate, or very early become so.
[Pg 589]
The quadrate cartilage ossifies as the quadrate bone, and supplies the
permanent articulation for the lower jaw. Its upper end exhibits a
tendency to divide into two processes, corresponding with the pedicle
and otic processes of the Amphibia. The Meckelian cartilage becomes
soon covered by investing bones, and its proximal end ossifies as the
articulare. The remainder of the cartilage usually disappears.
Mammalia. The most extraordinary metamorphosis of the hyoid and
mandibular arches occurs in the Mammalia, and has been in part known
since the publication of the memoir of Reichert (No. 461).
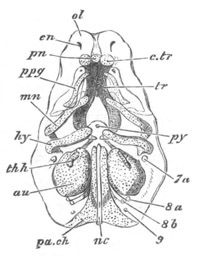
Fig. 341. Embryo Pig, two-thirds of an inch long; elements of the
skull seen somewhat diagrammatically from below. (From Parker.)
pa. ch. parachordal cartilage; nc. notochord; au. auditory
capsule; py. pituitary body; tr. trabeculæ; c.tr. trabecular
cornu; pn. prenasal cartilage; e.n. external nasal opening;
ol. nasal capsule; p.pg. palatopterygoid tract enclosed in the
maxillopalatine process; mn. mandibular arch; hy. hyoid arch;
th.h. first branchial arch; 7a. facial nerve; 8a.
glossopharyngeal; 8b. vagus; 9. hypoglossal.
Both the hyoid and mandibular arches develop at first more completely
than in any of the other types above Fishes; and are articulated to
each other above, while the pterygo-palatine bar is quite distinct.
The main features of the subsequent development are undisputed, with
the exception of that of the upper end of the hyoid, which is still
controverted. The following is Parker’s (No. 452) account for the Pig,
which confirms in the main the view originally put forward by Huxley
(No. 445).
The mandibular and hyoid arches are at first very similar
[Pg 590] (fig. 341
mn and hy), their dorsal ends being somewhat incurved, and
articulating together.
In a somewhat later stage (fig. 342) the upper end of the mandibular
bar (mb), without becoming segmented from the ventral part, becomes
distinctly swollen, and clearly corresponds to the quadrate region of
other types. The ventral part of the bar constitutes the Meckelian
cartilage (mk).
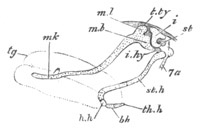
Fig. 342. Embryo Pig, an inch and a third long; side view of
mandibular and hyoid arches. The main hyoid arch is seen as displaced
backwards after segmentation from the incus. (From Parker.)
tg. tongue; mk. Meckelian cartilage; ml. body of malleus;
mb. manubrium or handle of the malleus; t.ty. tegmen tympani;
i. incus; st. stapes; i.hy. interhyal ligament; st.h.
stylohyal cartilage; h.h. hypohyal; b.h. basibranchial; th.h.
rudiment of first branchial arch; 7a. facial nerve.
The hyoid arch has in the meantime become segmented into two parts, an
upper part (i), which eventually becomes one of the small bones of
the ear—the incus—and a lower part which remains permanently as the
anterior cornu of the hyoid (st.h). The two parts continue to be
connected by a ligament.
The incus is articulated with the quadrate end of the mandibular arch,
and its rounded head comes in contact with the stapes (fig. 342, st)
which is segmented from the fenestra ovalis. The main arch of the
hyoid becomes divided into a hypohyal (h.h) below and a stylohyal
(st.h) above, and also becomes articulated with the basal element of
the arch behind (bh).
In the course of further development the Meckelian part of the
mandibular arch becomes enveloped in a superficial ossification
forming the dentary. Its upper end, adjoining the quadrate region,
becomes calcified and then absorbed, and its lower, with the exception
of the extreme point, is ossified and subsequently incorporated in the
dentary.
The quadrate region remains relatively stationary in growth
[Pg 591] as
compared with the adjacent parts of the skull, and finally ossifies to
form the malleus bone of the ear. The processus gracilis of the
malleus is the primitive continuation into Meckel’s cartilage.
The malleus and incus are at first embedded in the connective tissue
adjoining the tympanic cavity (hyomandibular cleft, vide p. 528);
and externally to them a bone known as the tympanic bone becomes
developed so that they become placed between the tympanic bone and the
periotic capsule. In late fœtal life they become transported
completely within the tympanic cavity, though covered by a reflection
of the tympanic mucous membrane.
The dorsal end of the part of the hyoid separated from the incus
becomes ossified as the tympano-hyal, and is anchylosed with the
adjacent parts of the periotic capsule. The middle part of the bar
just outside the skull forms the stylohyal (styloid process in Man)
which is attached by ligament to the anterior cornu of the hyoid
(ceratohyal).
While the account of the formation of the malleus, incus, and stapes
just given is that usually accepted in this country, a somewhat
different view of the development of these parts has as a rule been
adopted in Germany. Reichert (No. 461) held that both the malleus and
the incus were derived from the mandibular bar; and this view has been
confirmed by Günther, Kölliker and other observers, and has recently
been adopted by Salensky (No. 462) after a careful research especially
directed towards this point. Reichert also held that the stapes was
derived from the hyoid bar; but, though his observations on this point
have been very widely accepted, they have not met with such universal
recognition as his views on the origin of the malleus and incus.
Salensky has recently arrived at a view, which is in accord with that
of Parker, in so far as the independence of the stapes of both the
hyoid and mandibular arches is concerned. Salensky however holds that
it is formed from a mass of mesoblast surrounding the artery of the
mandibular arch, and that the form of the stapes is due to its
perforation by the mandibular artery. A product of this artery
permanently perforates the stapes in a few Mammalia, though in the
majority it atrophies.
In view of the different accounts of the origin of the incus the exact
nature of this bone must still be considered as an open question, but
should Reichert’s view be confirmed the identification of the incus
with the columella of the Amphibia and Sauropsida must be abandoned.
[Pg 592]Membrane bones and ossifications of the cranium.
The membrane bones of the skull may be divided into two classes, viz.
(1) those derived from dermal osseous plates, which as explained above
(p. 542) are primitively formed by the coalescence of the osseous
plates of scales; and (2) those formed by the coalescence of the
osseous plates of teeth lining the oral cavity. Some of the bones
sheathing the edge of the mouth have been formed partly by the one
process and partly by the other.
In the Fishes there are found all grades of transition between simple
dermal scutes, and true subdermal osseous plates forming an integral
part of the internal skeleton. Dermal scutes are best represented in
Acipenser and some Siluroid Fishes.
Where the membrane bones still retain the character of dermal plates,
those on the dorsal surface of the cranium are usually arranged in a
series of longitudinal rows, continuing in the region of the head the
rows of dermal scutes of the trunk; while the remaining cranial scutes
are connected with the visceral arches. The dermal bones on the dorsal
surface of the head are very different in number, size, and
arrangement in different types of Fishes; but owing to their linear
disposition it is usually possible to find a certain number both of
the paired and unpaired bones which have a similar situation in the
different forms. These usually receive the same names, but both from
general considerations as to their origin, as well as from a
comparison of different species, it appears to me probable that there
is no real homology between these bones in different species, but only
a kind of general correspondence[206].
It is not in fact till we get to the types above the Fishes that we
can find a series of homologous dorsal membrane bones covering the
roof of the skull. In these types three paired sets of such bones are
usually present, viz. from behind forwards the parietals, frontals and
nasals, the latter bounding the posterior surface of the external
nasal opening. Even in the higher
[Pg 593] types these bones are liable to vary
very greatly from the usual arrangement.
Besides these bones there is usually present in the higher forms a
lacrymal bone on the anterior margin of the orbit derived from one of
a series of periorbital membrane bones frequently found in Fishes.
Various supraorbital and postorbital bones, etc. are also frequently
found in Lacertilia, etc. which are not impossibly phylogenetically
independent of the membrane bones inherited from Fishes; and may have
been evolved as bony scutes in the subdermal tissue of the papillæ of
the sauropsidan scales.
The visceral arches of Fishes, especially of the Teleostei, are
usually provided with a series of membrane bones. In the true
branchial arches these take the form of dentigerous plates; but no
such plates are found in the Amphibia or Amniota.
The opercular flap attached to the hyoid arch is usually supported by
a series of membrane bones, which attain their highest development in
the Teleostei. One of these bones, the præopercular, is very constant
and is primitively attached along the outer edge of the hyomandibular.
It seems to be retained in Amphibia as a membrane bone, overlapping
the attachment of the quadrate and known as the squamosal; though it
is not impossible that this bone may be derived from a superficial
membrane bone, widely distributed in Teleostei and Ganoids, which is
known as the supra-temporal. In Dipnoi the bone which appears to be
clearly homologous with the squamosal would seem from its position to
belong to the series of dorsal plates, and therefore to be the
supra-temporal; but it is regarded by Huxley (No. 446) as the
præopercular[207].
In the Amniota the squamosal forms an integral part of the osseous
roof of the skull; but in the Sauropsida it continues, as in Amphibia,
to be closely related to the quadrate.
A larger series of persistent membrane bones are related to the
mandibular, and its palato-quadrate process.
Overlying the palato-quadrate process are two rows of bones,
[Pg 594] one row
lying at the edge of the mouth, on the outer side of the
pterygo-palatine process, and the other set on the roof of the mouth
superficial to the pterygo-palatine process.
The outer row is formed of the præmaxilla, maxilla, jugal, and very
often quadratojugal. Of these bones the maxilla and præmaxilla, as is
more especially demonstrated by their ontogeny in the Urodela, are
partly derived from dentigerous plates and partly from membrane plates
outside the mouth; while the jugal, and quadratojugal when present,
are entirely extra-oral. In the Amphibia and Amniota the præmaxillæ
and maxillæ are the most important bones in the facial region, and are
quite independent of any cartilaginous substratum.
The second row of bones is clearly constituted in the Dipnoi and
Amphibia by the vomer in front, then the palatine, and finally the
pterygoid behind. Of these bones the vomer is never related to a
cartilaginous tract below, while the palatines and pterygoids usually
are so. The position and growth of the three bones in many Urodela
(Axolotl) are especially striking (Hertwig. No. 442). In the Axolotl
they form a continuous series, the vomer and palatine being covered by
teeth, but the pterygoid being without teeth. The vomer and palatine
originate from the united osseous plates of the bases of the teeth,
while the pterygoid is in the first instance continuous with the
palatine.
In Teleostei, Amia, etc., there are dentigerous plates forming a
palatine and pterygoid, which in position, at any rate, closely
correspond with the similarly named bones in Amphibia; and there is
also a dentigerous vomer which may fairly be considered as equivalent
to that in Amphibia.
In the Amniota the three bones found in Amphibia are always present,
but with a few exceptions amongst the Lacertilia and Ophidia, are no
longer dentigerous. The cartilaginous bars, which in the lower types
are placed below the palatine and pterygoid membrane bones, are
usually imperfectly or not at all developed.
On Meckel’s cartilage important membrane bones are almost always
grafted. On the outside and distal part of the cartilage a dentary is
usually developed, which may envelope and replace the cartilage to a
larger or smaller extent. Its oral edge
[Pg 595] is usually dentigerous. The
splenial membrane bone is the most important bone on the inner side of
Meckel’s cartilage, but other elements known as the coronoid and
angular may also be added. In Mammalia the dentary is the only element
present (vide p. 590).
On the roof of the mouth a median bone, the parasphenoid, is very
widely present in the Amphibia and Fishes, except the Elasmobranchii
and Cyclostomata, and has no doubt the same phylogenetic origin as the
vomer and membranous palatines and pterygoids.
It is less important in the Sauropsida, and becomes indistinguishably
fused with the sphenoid in the adult, while in Mammalia it is no
longer found.
Ossification of the Cartilaginous Cranium. In certain Fishes the
cartilaginous cranium remains quite unossified, while completely
enveloped in dermal bones. Such for instance is its condition in the
Selachioid Ganoids. In most instances, however, the investment of the
cartilaginous cranium by membrane bones is accompanied by a more or
less complete ossification of the cartilage itself.
In the Dipnoi this occurs to the smallest extent, the only
ossifications occurring in the lateral parts of the occipital region,
and forming the exoccipitals.
In Teleostei and bony Ganoids, a considerably greater number of
ossifications occur in the cartilage.
In the region of the occipital cartilaginous ring there appears a
basioccipital and supraoccipital and two exoccipitals. The
basioccipital is the only bone on the floor of the skull ossifying
that part into which the notochord is primitively continued[208].
In the region of the periotic cartilage a large number of bones may
appear. In front there is the prootic, which often meets the
exoccipital behind; behind there is above and in close connection with
the supraoccipital the epiotic, and below in close connection with the
exoccipital the opisthotic. On the dorsal side of the cartilage there
is a projecting ridge composed mainly of a bone known as the pterotic,
sometimes erroneously
[Pg 596] called the squamosal, and continued in front by
the sphenotic. The pterotic, or the cartilaginous region corresponding
to it, always supplies the articular surface for the hyomandibular.
In the floor of the skull, in the region of the pituitary body, there
is formed a basisphenoid; while in the lateral parts of the wall of
this part of the cranium, there is a bone known as the alisphenoid.
In front, parts of the lateral walls of the cranium ossify as the
orbitosphenoids.
In view of the very imperfect ossification of the cartilaginous
cranium of the Dipnoi, and of the fact that there is certainly no
direct genetic connection between the Teleostei on the one hand, and
the Amphibia and Amniota on the other, it is very difficult to believe
that most of the ossifications of the cranium in the Amphibia and
Amniota have more than a general correspondence with those in the
Teleostei.
In the Amphibia the ossifications in the cartilage are comparatively
few. In the occipital region there is a lateral ossification on each
side of the exoccipital, the basioccipital region being unossified,
and the supraoccipital at the utmost indurated by a calcareous
deposit.
The periotic capsule is ossified by a prootic centre, which meets the
exoccipital behind.
The front part of the cartilaginous cranium is ossified by a complete
ring of bone—the sphenethmoid bone—which embraces part of the
ethmoid region, and of the orbitosphenoid and presphenoid regions.
In the Amphibia the cartilaginous cranium, with its centres of
ossification, is easily separable from the membranous investing bones.
In the Amniota the cartilaginous cranium, whose development in the
embryo has already been described, becomes in the adult much more
largely ossified, and the bones which replace the primitive cartilage
unite with the membrane bones to form a continuous bony cranium.
The centres of ossification become again much more numerous. In the
occipital segment analogous centres to those of Teleostei are again
found; and it is probable that the exoccipitals are homologous
throughout the series, the supraoccipital and basioccipital
[Pg 597] bones of
the higher types being merely identical in position with the similarly
named bones in Fishes.
In the periotic there are usually three centres of ossification, first
recognised by Huxley. These are the prootic, the epiotic and
opisthotic, the situations of which have already been defined. Of
these the prootic is the most constant.
In Reptiles, the prootic and opisthotic frequently remain distinct
even in the adult.
In Birds, the epiotic and opisthotic are early united with the supra-
and exoccipital; and at a later period the prootic is also
indistinguishably fused with the adjacent parts.
In Mammals the three ossifications fuse into a continuous whole—the
periotic bone—which may be partially united with the adjacent parts.
In the pituitary region of the base of the cranium a pair of osseous
centres or in the higher types a single centre (Parker[209]) gives
rise to the basisphenoid bone, and in front of this another basal or
pair of basal ossifications forms the presphenoid, while laterally to
these two centres there are formed centres of ossification in the
alisphenoid and orbitosphenoid regions, which may be extremely reduced
in various Sauropsida, leaving the side walls of the skull almost
entirely formed of membrane or cartilage.
In the ethmoid region there may arise a median ossification forming
the mesethmoid and lateral ossifications forming the lateral ethmoids
or prefrontals; which may assist in forming the front wall of the
brain-case, or be situated quite externally to the brain-case and be
only related to the olfactory capsules.
The labial cartilages. In most Fishes a series of skeletal structures,
known as the labial cartilages, are developed at the front and sides
of the mouth, and in connection with the olfactory capsules; and these
cartilages still persist in connection with the olfactory capsules,
though in a reduced form, in the higher types. They are more developed
in the Cyclostomata than in any other Vertebrate type.
The meaning of these cartilages is very obscure; but, from their being
in part employed to support the lips and horny teeth of the
Cyclostomata and the Tadpole, I should be inclined to regard them as
remnants of a primitive skeleton supporting the suctorial mouth, with
which, on the grounds already stated (p. 317), I believe the ancestors
of the present Vertebrata to have been provided.
[Pg 598]Bibliography.
(439) A. Dugès. “Recherches sur l'Ostéologie et la myologie des
Batraciens à leur différents âges.” Paris, Mém. savans étrang. 1835,
and An. Sci. Nat. Vol. I. 1834.
(440) C. Gegenbaur. Untersuchungen z. vergleich. Anat. d.
Wirbelthiere, III. Heft. Das Kopfskelet d. Selachier. Leipzig,
1872.
(441) Günther. Beob. üb. die Entwick. d. Gehörorgans. Leipzig, 1842.
(442) O. Hertwig. “Ueb. d. Zahnsystem d. Amphibien u. seine Bedeutung
f. d. Genese d. Skelets d. Mundhöhle.” Archiv f. mikr. Anat., Vol.
XI. 1874, suppl.
(443) T. H. Huxley. “On the theory of the vertebrate skull.” Proc.
Royal Soc., Vol. ix. 1858.
(444) T. H. Huxley. The Elements of Comparative Anatomy. London,
1869.
(445) T. H. Huxley. “On the Malleus and Incus.” Proc. Zool. Soc.,
1869.
(446) T. H. Huxley. “On Ceratodus Forsteri.” Proc. Zool. Soc., 1876.
(447) T. H. Huxley. “The nature of the craniofacial apparatus of
Petromyzon.” Journ. of Anat. and Phys., Vol. X. 1876.
(448) T. H. Huxley. The Anatomy of Vertebrated Animals. London,
1871.
(449) W. K. Parker. “On the structure and development of the skull of
the Common Fowl (Gallus Domesticus).” Phil. Trans., 1869.
(450) W. K. Parker. “On the structure and development of the skull of
the Common Frog (Rana temporaria).” Phil. Trans., 1871.
(451) W. K. Parker. “On the structure and development of the skull in
the Salmon (Salmo salar).” Bakerian Lecture, Phil. Trans., 1873.
(452) W. K. Parker. “On the structure and development of the skull in
the Pig (Sus scrofa).” Phil. Trans., 1874.
(453) W. K. Parker. “On the structure and development of the skull in
the Batrachia.” Part II. Phil. Trans., 1876.
(454) W. K. Parker. “On the structure and development of the skull in
the Urodelous Amphibia.” Part III. Phil. Trans., 1877.
(455) W. K. Parker. “On the structure and development of the skull in
the Common Snake (Tropidonotus natrix).” Phil. Trans., 1878.
(456) W. K. Parker. “On the structure and development of the skull in
Sharks and Skates.” Trans. Zoolog. Soc., 1878. Vol. X. pt. IV.
(457) W. K. Parker. “On the structure and development of the skull in
the Lacertilia.” Pt. I. Lacerta agilis, L. viridis and Zootoca
vivipara. Phil. Trans., 1879.
(458) W. K. Parker. “The development of the Green Turtle.” The
Zoology of the Voyage of H. M. S. Challenger. Vol. I. pt. V.
(459) W. K. Parker. “The structure and development of the skull in the
Batrachia.” Pt. III. Phil. Trans., 1880.
(460) W. K. Parker and G. T. Bettany. The Morphology of the Skull.
London, 1877.
(460*) H. Rathke. Entwick. d. Natter. Königsberg, 1839.
(461) C. B. Reichert. “Ueber die Visceralbogen d. Wirbelthiere.”
Müller’s Archiv, 1837.
(462) W. Salensky. “Beiträge z. Entwick. d. knorpeligen
Gehörknöchelchen.” Morphol. Jahrbuch, Vol. VI. 1880.
Vide also Kölliker (No. 298), especially for the human and mammalian
skull; Götte (No. 296).
[Pg 599]
CHAPTER XX.
THE PECTORAL AND PELVIC GIRDLES AND THE SKELETON OF THE LIMBS.
The Pectoral girdle.
Pisces. Amongst Fishes the pectoral girdle presents itself in its
simplest form in Elasmobranchii, where it consists of a bent band of
cartilage on each side of the body, of somewhat variable form, meeting
and generally uniting with its fellow ventrally. Its anterior border
is in close proximity with the last visceral arch, and a transverse
ridge on its outer and posterior border, forming the articular surface
for the skeleton of the limb, divides it into a dorsal part, which may
be called the scapula, and a ventral part which may be called the
coracoid.
In all the remaining groups of Fishes there is added to the
cartilaginous band, which may wholly or partially ossify, an osseous
support composed of a series of membrane bones.
In the types with such membrane bones the cartilaginous parts do not
continue to meet ventrally, except in the Dipnoi where there is a
ventral piece of cartilage, distinct from that bearing the
articulation of the limb. The cartilage is moreover produced into two
ventral processes, an anterior and a posterior, below the articulation
of the limb; which may be called, in accordance with Gegenbaur’s
nomenclature, the præcoracoid and coracoid. Of these the præcoracoid
is far the most
[Pg 600] prominent, and in the majority of cases the coracoid
can hardly be recognised. The coracoid process is however well
developed in the Selachioid Ganoids, and the Siluroid Teleostei. In
Teleostei the scapular region often ossifies in two parts, the smaller
of which is named by Parker præcoracoid, though it is quite distinct
from Gegenbaur’s præcoracoid. The membrane bones, as they present
themselves in their most primitive state in Acipenser and the
Siluroids, are dermal scutes embracing the anterior edge of the
cartilaginous girdle. In Acipenser there are three scutes on each
side. A dorsal scute known as the supra-clavicle, connected above with
the skull by the post-temporal; a middle piece or clavicle, and a
ventral or infra-clavicle (interclavicle), which meets its fellow
below.
In most Fishes the primitive dermal scutes have become subdermal
membrane bones, and the infra-clavicle is usually not distinct, but
the two clavicles form the most important part of the membranous
elements of the girdle. Additional membrane bones (post-clavicles) are
often present behind the main row.
The development of these parts in Fishes has been but little studied.
In Scyllium, amongst the Elasmobranchii, I find that each half of the
pectoral girdle develops as a vertical bar of cartilage at the front
border of the rudimentary fin, and externally to the muscle plates.
Before the tissue forming the pectoral girdle has acquired the
character of true cartilage, the bars of the two sides meet ventrally
by a differentiation in situ of the mesoblastic cells, so that, when
the girdle is converted into cartilage, it forms an undivided arc,
girthing the ventral side of the body. There is developed in
continuity with the posterior border of this arc on the level of the
fin a horizontal bar of cartilage, which is continued backwards along
the insertion of the fin, and, as will be shewn in the sequel, becomes
the metapterygium of the adult (figs. 344, bp and 348, mp). With
this bar the remaining skeletal elements of the fin are also
continuous.
The foramina of the pectoral girdle are not in the first instance
formed by absorption, but by the non-development of the cartilage in
the region of pre-existing nerves and vessels.
[Pg 601]
The development of these parts in Teleostei has been recently
investigated by ’Swirski (No. 472) who finds in the Pike (Esox) that
the cartilaginous pectoral girdle is at first continuous with the
skeleton of the fin. It forms a rod with a dorsal scapular and ventral
coracoid process. An independent mass of cartilage gives rise to a
præcoracoid, which unites with the main mass, forming a triradiate bar
like that of Acipenser or the Siluroids. The coracoid process becomes
in the course of development gradually reduced.
’Swirski concludes that the so-called præcoracoid bar is to some
extent a secondary element, and that the coracoid bar corresponds to
the whole of the ventral part of the girdle of Elasmobranchii, but his
investigations do not appear to me to be as complete as is desirable.
Amphibia and Amniota. The pectoral girdle contains a more or less
constant series of elements throughout the Amphibia and Amniota; and
the differences in structure between the shoulder girdle of these
groups and that of Fishes are so great that it is only possible to
make certain general statements respecting the homologies of the parts
in the two sets of types.
The generally accepted view, founded on the researches of Parker,
Huxley, and Gegenbaur, is to the effect that there is a primitively
cartilaginous coraco-scapular plate, homologous with that in Fishes,
and that the membrane bones in Fishes are represented by the clavicle
and interclavicle in the Sauropsida and Mammalia, which are however
usually admitted to be absent in Amphibia. These views have recently
been challenged by Götte (No. 466) and Hoffmann (No. 467), on the
ground of a series of careful embryological observations; and until
the whole subject has been worked over by other observers it does not
seem possible to decide satisfactorily between the conflicting views.
It is on all hands admitted that the scapulo-coracoid elements of the
shoulder girdle are formed as a pair of cartilaginous plates, one on
each side of the body. The dorsal half of each plate becomes the
scapula, which may subsequently become divided into a supra-scapula
and scapula proper; while the ventral half forms the coracoid, which
is not always separated from the scapula, and is usually divided into
a coracoid proper, a præcoracoid, and an epicoracoid. By the
conversion of parts of the primitive cartilaginous plates into
membranous tissue various fenestræ may be formed in the cartilage, and
the bars
[Pg 602] bounding these fenestræ both in the scapula and coracoid
regions have received special names; the anterior bar of the coracoid
region, forming the præcoracoid, being especially important. At the
boundary between the scapula and the coracoid, on the hinder border of
the plate, is placed the glenoid articular cavity to carry the head of
the humerus.
The grounds of difference between Götte and Hoffmann and other
anatomists concern especially the clavicle and interclavicle. The
clavicle is usually regarded as a membrane bone which may become to
some extent cartilaginous. By the above anatomists, and by Rathke
also, it is held to be at first united with the coraco-scapular plate,
of which it forms the anterior limb, free ventrally, but united
dorsally with the main part of the plate; and Götte and Hoffmann hold
that it is essentially a cartilage bone, which however in the
majority of the Reptilia ossifies directly without passing through the
condition of cartilage.
The interclavicle (episternum) is held by Götte to be developed from a
paired formation at the free ventral ends of the clavicles, but he
holds views which are in many respects original as to its homologies
in Mammalia and Amphibia. Even if Götte’s facts are admitted, it does
not appear to me necessarily to follow that his deductions are
correct. The most important of these is to the effect that the dermal
clavicle of Pisces has no homologue in the higher types. Granting that
the clavicle in these groups is in its first stage continuous with the
coraco-scapular plate, and that it may become in some forms
cartilaginous before ossifying, yet it seems to me all the same quite
possible that it is genetically derived from the clavicle of Pisces,
but that it has to a great extent lost even in development its
primitive characters, though these characters are still partially
indicated in the fact that it usually ossifies very early and
partially at least as a membrane bone[210].
In treating the development of the pectoral girdle systematically it
will be convenient to begin with the Amniota, which may be considered
to fix the nomenclature of the elements of the shoulder girdle.
[Pg 603]
Lacertilia. The shoulder girdle is formed as two membranous plates,
from the dorsal part of the anterior border of each of which a bar
projects (Rathke, Götte), which is free at its ventral end. This bar,
which is usually (Gegenbaur, Parker) held to be independent of the
remaining part of the shoulder girdle, gives rise to the clavicle and
interclavicle. The scapulo-coracoid plate soon becomes cartilaginous,
while at the same time the clavicular bar ossifies directly from the
membranous state. The ventral ends of the two clavicular bars enlarge
to form two longitudinally placed plates, which unite together and
ossify as the interclavicle.
Parker gives a very different account of the interclavicle in Anguis.
He states that it is formed of two pairs of bones ‘strapped on to the
antero-inferior part of the præsternum,’ which subsequently unite into
one.
Chelonia. The shoulder girdle of the Chelonia is formed (Rathke) of a
triradiate cartilage on each side, with one dorsal and two ventral
limbs. It is admitted on all hands that the dorsal limb is the
scapular element, and the posterior ventral limb the coracoid; but,
while the anterior ventral limb is usually held to be the præcoracoid,
Götte and Hoffmann maintain that, in spite of its being formed of
cartilage, it is homologous with the anterior bar of the primitive
shoulder-plates of Lacertilia, and therefore the homologue of the
clavicle.
Parker and Huxley (doubtfully) hold that the three anterior elements
of the ventral plastron (entoplastron and epiplastra) are homologous
with the interclavicle and clavicles, but considering that these
plates appear to belong to a secondary system of dermal ossifications
peculiar to the Chelonia, this homology does not appear to me probable.
Aves. There are very great differences of view as to the development
of the pectoral arch of Aves.
About the presence in typical forms of the coraco-scapular plate and
two independent clavicular bars all authors are agreed. With reference
to the clavicle and interclavicle Parker (No. 468) finds that the
scapular end of the clavicle attaches itself to and ossifies a mass of
cartilage, which he regards as the mesoscapula, while the
interclavicle is formed of a mass of tissue between the ends of the
clavicles where they meet ventrally, which becomes the dilated plate
at their junction.
Gegenbaur holds that the two primitive clavicular bars are simply
clavicles, without any element of the scapula; and states that the
clavicles are not entirely ossified from membrane, but that a delicate
band of cartilage precedes the osseous bars. He finds no
interclavicle.
Götte and Rathke both state that the clavicle is at first continuous
with the coraco-scapular plate, but becomes early separated, and
ossifies entirely as a membrane bone. Götte further states that the
interclavicles are formed as outgrowths of the median ends of the
clavicles, which extend themselves at an early period of development
along the inner edges of the two halves of the sternum. They soon
separate from the clavicles, which subsequently meet to form the
furculum; while the interclavicular rudiments give rise, on the
junction of the two halves of the sternum, to its keel, and to the
ligament
[Pg 604] connecting the furculum with the sternum. The observations of
Götte, which tend to shew the keel of the sternum is really an
interclavicle, appear to me of great importance.
A præcoracoid, partially separated from the coracoid by a space, is
present in Struthio. It is formed by a fenestration of a primitively
continuous cartilaginous coracoid plate (Hoffmann). In Dromæus and
Casuarius clavicles are present (fused with the scapula in the adult
Dromæus), though absent in other Ratitæ (Parker, etc.).
Mammalia. The coracoid element of the coraco-scapular plate is much
reduced in Mammalia, forming at most a simple process (except in the
Ornithodelphia) which ossifies however separately[211].
With reference to the clavicles the same divergencies of opinion met
with in other types are found here also.
The clavicle is stated by Rathke to be at first continuous with the
coraco-scapular plate. It is however soon separated, and ossifies very
early, in the human embryo before any other bone. Gegenbaur however
shewed that the human clavicle is provided with a central axis of
cartilage, and this observation has been confirmed by Kölliker, and
extended to other Mammalia by Götte. The mode of ossification is
nevertheless in many respects intermediate between that of a true
cartilage bone and a membrane bone. The ends of the clavicles remain
for some time, or even permanently, cartilaginous, and have been
interpreted by Parker, it appears to me on hardly sufficient grounds,
as parts of the mesoscapula and præcoracoid. Parker’s so-called
mesoscapula may ossify separately. The homologies of the episternum
are much disputed. Götte, who has worked out the development of the
parts more fully than any other anatomist, finds that paired
interclavicular elements grow out backwards from the ventral ends of
the clavicles, and uniting together form a somewhat T-shaped
interclavicle overlying the front end of the sternum. This condition
is permanent in the Ornithodelphia, except that the anterior part of
the sternum undergoes atrophy. But in the higher forms the
interclavicle becomes almost at once divided into three parts, of
which the two lateral remain distinct, while the median element fuses
with the subjacent part of the sternum and constitutes with it the
presternum (manubrium sterni). If Götte’s facts are to be trusted, and
they have been to a large extent confirmed by Hoffmann, his homologies
appear to be satisfactorily established. As mentioned on p. 563 Ruge
(No. 438) holds that Götte is mistaken as to the origin of the
presternum.
Gegenbaur admits the lateral elements as parts of the interclavicle,
while Parker holds that they are not parts of an interclavicle but are
homologous with the omosternum of the Frog, which is however held by
Götte to be a true interclavicle.
[Pg 605]
Amphibia. In Amphibia the two halves of the shoulder girdle are each
formed as a continuous plate, the ventral or coracoid part of which is
forked, and is composed of a larger posterior and a smaller anterior
bar-like process, united dorsally. In the Urodela the two remain
permanently free at their ventral ends, but in the Anura they become
united, and the space between them then forms a fenestra. The anterior
process is usually (Gegenbaur, Parker) regarded as the præcoracoid,
but Götte has pointed out that in its mode of development it strongly
resembles the clavicle of the higher forms, and behaves quite
differently to the so-called præcoracoid of Lizards. It is however to
be noticed that it differs from the clavicle in the fact that it is
never segmented off from the coraco-scapular plate, a condition which
has its only parallel in the equally doubtful case of the Chelonia.
Parker holds that there is no clavicle present in the Amphibia, while
Gegenbaur maintains that an ossification which appears in many of the
Anura (though not in the Urodela) in the perichondrium on the anterior
border of the cartilaginous bar above mentioned is the representative
of the clavicle. Götte’s observations on the ossification of this bone
throw doubt upon this view of Gegenbaur; while the fact that the
cartilaginous bar may be completely enclosed by the bone in question
renders Gegenbaur’s view, that there is present both a clavicle and
præcoracoid, highly improbable.
No interclavicle is present in Urodela, but in this group and in a
number of the Anura, a process grows out from the end of each of the
bars (præcoracoids) which Götte holds to be the clavicles. The two
processes unite in the median line, and give rise in front to the
anterior unpaired element of the shoulder girdle (omosternum of
Parker). They sometimes overlap the epicoracoids behind, and fusing
with them bind them together in the median line. Parker who has
described the paired origin of the so-called omosternum, holds that it
is not homologous with the interclavicle, but compares it with his
omosternum in Mammals.
Bibliography.
(463) Bruch. “Ueber die Entwicklung der Clavicula und die Farbe des
Blutes.” Zeit. f. wiss. Zool., iv. 1853.
(464) A. Dugès. “Recherches sur l'ostéologie et la myologie des
Batraciens à leurs différens âges.” Mémoires des savants étrang.
Académie royale des sciences de l'institut de France, Vol. VI. 1835.
(465) C. Gegenbaur. Untersuchungen zur vergleichenden Anatomie der
Wirbelthiere, 2 Heft. Schultergürtel der Wirbelthiere. Brustflosse
der Fische. Leipzig, 1865.
(466) A. Götte. “Beiträge z. vergleich. Morphol. d. Skeletsystems d.
Wirbelthiere, Brustbein u. Schultergürtel.” Archiv f. mikr. Anat.
Vol. XIV. 1877.
(467) C. K. Hoffmann. “Beiträge z. vergleichenden Anatomie d.
Wirbelthiere.” Niederländisches Archiv f. Zool., Vol. V. 1879.
(468) W. K. Parker. “A Monograph on the Structure and Development of
the Shoulder-girdle and Sternum in the Vertebrata.” Ray Society,
1868.
[Pg 606]
(469) H. Rathke. Ueber die Entwicklung der Schildkröten.
Braunschweig, 1848.
(470) H. Rathke. Ueber den Bau und die Entwicklung des Brustbeins der
Saurier, 1853.
(471) A. Sabatier. Comparaison des ceintures et des membres
antérieurs et postérieurs d. la Série d. Vertébrés. Montpellier,
1880.
(472) Georg ’Swirski. Untersuch. üb. d. Entwick. d. Schultergürtels
u. d. Skelets d. Brustflosse d. Hechts. Inaug. Diss. Dorpat, 1880.
Pelvic girdle.
Pisces. The pelvic girdle of Fishes is formed of a cartilaginous band,
to the outer and posterior side of which the basal element of the
pelvic fin is usually articulated. This articulation divides it into a
dorsal iliac, and ventral pubic section. The iliac section never
articulates with the vertebral column.
In Elasmobranchii the two girdles unite ventrally, but the iliac
section is only slightly developed. In Chimæra there is a well
developed iliac process, but the pubic parts of the girdle are only
united by connective tissue.
In the cartilaginous Ganoids the pelvic girdle is hardly to be
separated from the skeleton of the fin. It is not united with its
fellow, and is represented by a plate with slightly developed pubic
and iliac processes.
In the Dipnoi there is a simple median cartilage, articulated with the
limb, but not provided with an iliac process. In bony Ganoids and
Teleostei there is on each side a bone meeting its fellow in the
ventral line, which is usually held to be the rudiment of the pelvic
girdle; while Davidoff attempts to shew that it is the basal element
of the fin, and that, except in Polypterus, a true pelvic girdle is
absent in these types.
From my own observations I find that the mode of development of the
pelvic girdle in Scyllium is very similar to that of the pectoral
girdle. There is a bar on each side, continuous on its posterior
border with the basal element of the fin (figs. 345 and 347). This bar
meets and unites with its fellow ventrally before becoming converted
into true cartilage, and though the iliac process (il) is never very
considerable, yet it is better developed in the embryo than in the
adult, and is at first directed nearly horizontally forwards.
Amphibia and Amniota. The primitive cartilaginous pelvic
[Pg 607] girdle of the
higher types exhibits the same division as that of Pisces into a
dorsal and a ventral section, which meet to form the articular cavity
for the femur, known as the acetabulum. The dorsal section is always
single, and is attached by means of rudimentary ribs to the sacral
region of the vertebral column, and sometimes to vertebræ of the
adjoining lumbar or caudal regions. It always ossifies as the ilium.
The ventral section is usually formed of two more or less separated
parts, an anterior which ossifies as the pubis, and a posterior which
ossifies as the ischium. The space between them is known as the
obturator foramen. In the Amphibia the two parts are not separated,
and resemble in this respect the pelvic girdle of Fishes. They
generally meet the corresponding elements of the opposite side
ventrally, and form a symphysis with them. The symphysis pubis, and
symphysis ischii may be continuous (Mammalia, Amphibia).
The observations on the development of the pelvic girdle in the
Amphibia and Amniota are nearly as scanty as on those of Fishes.
Amphibia. In the Amphibia (Bunge, No. 473) the two halves of the
pelvic girdle are formed as independent masses of cartilage, which
subsequently unite in the ventral line.
In the Urodelous Amphibia (Triton) each mass is a simple plate of
cartilage divided into a dorsal and ventral section by the acetabulum.
The ventral parts, which are not divided into two regions, unite in a
symphysis comparatively late.
The dorsal section ossifies as the ilium. The ventral usually contains
a single ossification in its posterior part which forms the ischium;
while the anterior part, which may be considered as representing the
pubis, usually remains cartilaginous; though Huxley (No. 475) states
that it has a separate centre of ossification in Salamander, which
however does not appear to be always present (Bunge). There is a small
obturator foramen between the ischium and pubis, which gives passage
to the obturator nerve. It is formed by the part of the tissue where
the nerve is placed not becoming converted into cartilage.
There is a peculiar cartilage in the ventral median line in front of
the pubis, which is developed independently of and much later than the
true parts of the pelvic girdle. It may be called the præpubic
cartilage.
Reptilia. In Lacertilia the pelvic girdle is formed as a somewhat
triradiate mass of cartilage on each side, with a dorsal (iliac)
process, and two ventral (pubic and ischiad) processes. The acetabulum
is placed on the outer side at the junction of the three processes,
each of which may
[Pg 608] be considered to have a share in forming it. The
distal ends of the pubis and ischium are close together when first
formed, but subsequently separate. Each of them unites at a late stage
with the corresponding process of the opposite side in a ventral
symphysis. A centre of ossification appears in each of the three
processes of the primitive cartilage.
Aves. In Birds the parts of the pelvic girdle no longer develop as a
continuous cartilage (Bunge). Either the pubis may be distinct, or, as
in the Duck, all the elements. The ilium early exhibits a short
anterior process, but the pubis and ischium are at first placed with
their long axes at right angles to that of the ilium, but gradually
become rotated so as to lie parallel with it, their distal ends
pointing backwards, and not uniting ventrally excepting in one or two
Struthious forms.
Mammalia. In Mammalia the pelvic girdle is formed in cartilage as in
the lower forms, but in Man at any rate the pubic part of the
cartilage is formed independently of the remainder (Rosenberg). There
are the usual three centres of ossification, which unite eventually
into a single bone—the innominate bone. The pubis and ischium of each
side unite with each other ventrally, so as completely to enclose the
obturator foramen.
Huxley holds that the so-called marsupial bones of Monotremes and
Marsupials, which as shewn by Gegenbaur (No. 474) are performed in
cartilage, are homologous with the præpubis of the Urodela; but
considering the great gap between the Urodela and Mammalia this
homology can only be regarded as tentative. He further holds that the
anterior prolongations of the cartilaginous ventral ends of the pubis
of Crocodilia are also structures of the same nature.
Bibliography.
(473) A. Bunge. Untersuch. z. Entwick. d. Beckengürtels d. Amphibien,
Reptilien u. Vögel. Inaug. Diss. Dorpat, 1880.
(474) C. Gegenbaur. “Ueber d. Ausschluss des Schambeins von d. Pfanne
d. Hüftgelenkes.” Morph. Jahrbuch, Vol. II. 1876.
(475) Th. H. Huxley. “The characters of the Pelvis in Mammalia, etc.”
Proc. of Roy. Soc., Vol. XXVIII. 1879.
(476) A. Sabatier. Comparaison des ceintures et des membres
antérieurs et postérieurs dans la Série d. Vertébrés. Montpellier,
1880.
Comparison of Pectoral and Pelvic girdles.
Throughout the Vertebrata a more or less complete serial homology may
be observed between the pectoral and pelvic girdles.
In the cartilaginous Fishes each girdle consists of a continuous band,
a dorsal and ventral part being indicated by the articulation of the
fin; the former being relatively undeveloped in the pelvic
[Pg 609] girdle,
while in the pectoral it may articulate with the vertebral column. In
the case of the pectoral girdle secondary membrane bones become added
to the primitive cartilage in most Fishes, which are not developed in
the case of the pelvic girdle.
In the Amphibia and Amniota the ventral section of each girdle becomes
divided into an anterior and a posterior part, the former constituting
the præcoracoid and pubis, and the latter the coracoid and ischium;
these parts are however very imperfectly differentiated in the pelvic
girdle of the Urodela. The ventral portions of the pelvic girdle
usually unite below in a symphysis. They also meet each other
ventrally in the case of the pectoral girdle in Amphibia, but in most
other types are separated by the sternum, which has no homologue in
the pelvic region, unless the præpubic cartilage is to be regarded as
such. The dorsal or scapular section of the pectoral girdle remains
free; but that of the pelvic girdle acquires a firm articulation with
the vertebral column.
If the clavicle of the higher types is derived from the membrane bones
of the pectoral girdle of Fishes, it has no homologue in the pelvic
girdle; but if, as Götte and Hoffmann suppose, it is a part of the
primitive cartilaginous girdle, the ordinary view as to the serial
homologies of the ventral sections of the two girdles in the higher
types will need to be reconsidered.
Limbs.
It will be convenient to describe in this place not only the
development of the skeleton of the limbs but also that of the limbs
themselves. The limbs of Fishes are moreover so different from those
of the Amphibia and Amniota that the development of the two types of
limb may advantageously be treated separately.
In Fishes the first rudiments of the limbs appear as slight
longitudinal ridge-like thickenings of the epiblast, which closely
resemble the first rudiments of the unpaired fins.
These ridges are two in number on each side, an anterior immediately
behind the last visceral fold, and a posterior on the level of the
cloaca. In most Fishes they are in no way connected, but in some
Elasmobranch embryos, more especially in Torpedo, they are connected
together at their first development
[Pg 610] by a line of columnar epiblast
cells[212].
This connecting line of columnar epiblast is a very
transitory structure, and after its disappearance the rudimentary fins
become more prominent, consisting (fig. 343, b) of a projecting
ridge both of epiblast and mesoblast, at the outer edge of which is a
fold of epiblast only, which soon reaches considerable dimensions. At
a later stage the mesoblast penetrates into this fold and the fin
becomes a simple ridge of mesoblast, covered by epiblast. The pectoral
fins are usually considerably ahead of the pelvic fins in development.
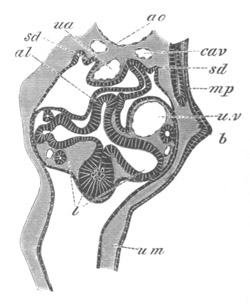
Fig. 343. Section through the ventral part of the trunk of a young
embryo of scyllium at the level of the umbilical cord.
b. pectoral fin; ao. dorsal aorta; cav. cardinal vein; ua.
vitelline artery; u.v. vitelline vein; al. duodenum; l. liver;
sd. opening of segmented duct into the body cavity; mp. muscle
plate; um. umbilical canal.
For the remaining history it is necessary to confine ourselves to
Scyllium as the only type which has been adequately studied.
The direction of the original ridge which connects the two fins of
each side is nearly though not quite longitudinal, sloping somewhat
obliquely downwards. It thus comes about that the attachment of each
pair of limbs is somewhat on a slant, and that the pelvic pair nearly
meet each other in the median ventral line a little way behind the
anus.
The elongated ridge, forming the rudiment of each fin, gradually
projects more and more, and so becomes broader in proportion to its
length, but at the same time its actual attachment to the side of the
body becomes shortened from behind forwards, so that what was
originally the attached border becomes in part converted into the
posterior border. This process is much more completely carried out
in the case of the pectoral fins than in that of the pelvic, and the
changes of form undergone by the pectoral fin in its development may
be gathered from figs. 344 and 348.
[Pg 611]
Before proceeding to the development of the skeleton of the fin it may
be pointed out that the connection of the two rudimentary fins by a
continuous epithelial line suggests the hypothesis that they are the
remnants of two continuous lateral fins[213].
Shortly after the view that the paired fins were remnants of
continuous lateral fins had been put forward in my memoir on
Elasmobranch Fishes, two very interesting papers were published by
Thacker (No. 489) and Mivart (No. 484) advocating this view on the
entirely independent grounds of the adult structure of the skeleton of
the paired fins in comparison with that of the unpaired fins[214].
The development of the skeleton has unfortunately not been as yet very
fully studied. I have however made some investigations on this subject
on Scyllium, and ’Swirski has also made some on the Pike.
In Scyllium the development of both the pectoral and pelvic fins is
very similar.
In both fins the skeleton in its earliest stage consists of a bar
springing from the posterior side of the pectoral or pelvic girdle,
and running backwards parallel to the long axis of the body. The outer
side of this bar is continued into a plate which
[Pg 612] extends into the fin,
and which becomes very early segmented into a series of parallel rays
at right angles to the longitudinal bar.
In other words, the primitive skeleton of both the fins consists of a
longitudinal bar running along the base of the fin, and giving off at
right angles series of rays which pass into the fin. The longitudinal
bar, which may be called the basipterygium, is moreover continuous in
front with the pectoral or pelvic girdle as the case may be.
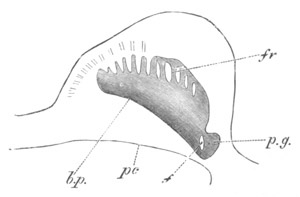
Fig. 344. Pectoral fin of a young embryo of Scyllium in longitudinal
and horizontal section.
The skeleton of the fin was still in the condition of embryonic
cartilage.
b.p. basipterygium (eventual metapterygium); fr. fin rays;
p.g. pectoral girdle in transverse section; f. foramen in
pectoral girdle; pc. wall of peritoneal cavity.
The primitive skeleton of the pectoral fin is shewn in longitudinal
section in fig. 344, and that of the pelvic fin at a slightly later
stage in fig. 345.
A transverse section shewing the basipterygium (mpt) of the pectoral
fin, and the plate passing from it into the fin, is shewn in fig. 346.
Before proceeding to describe the later history of the two fins it may
be well to point out that their embryonic structure completely
supports the view which has been arrived at from the consideration of
the soft parts of the fin.
My observations shew that the embryonic skeleton of the paired fin
consists of a series of parallel rays similar to those of the unpaired
fins. These rays support the soft part of the fin which has the form
of a longitudinal ridge, and are continuous at their base with a
longitudinal bar, which may very probably
[Pg 613] be due to secondary
development. As pointed out by Mivart, a longitudinal bar is also
occasionally formed to support the cartilaginous rays of unpaired
fins. The longitudinal bar of the paired fins is believed by both
Thacker and Mivart to be due to the coalescence of the bases of
primitively independent rays, of which they believe the fin to have
been originally composed. This view is probable enough in itself, but
there is no trace in the embryo of the bar in question being formed by
the coalescence of rays, though the fact of its being perfectly
continuous with the bases of the rays is somewhat in favour of this
view[215].
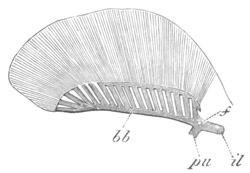
Fig. 345. Pelvic fin of a very young female embryo of Scyllium
stellare.
bb. basipterygium; pu. pubic process of pelvic girdle; il.
iliac process of pelvic girdle.
A point may be noticed here which may perhaps appear to be a
difficulty, viz. that to a considerable extent in the pectoral, and to
some extent in the pelvic fin the embryonic cartilage from which the
fin-rays are developed is at first a continuous lamina, which
subsequently segments into rays. I am however inclined to regard this
merely as a result of the mode of conversion of the indifferent
mesoblast into cartilage; and in any case no conclusion adverse to the
above view can be drawn from it, since I find that the rays of the
unpaired fin are similarly segmented from a continuous lamina. In all
cases the segmentation of the rays is to a large extent completed
before the tissue in question is sufficiently differentiated to be
called cartilage by an histologist.
Thacker and Mivart both hold that the pectoral and pelvic girdles have
been evolved by ventral and dorsal growths of the anterior end of the
longitudinal bar supporting the fin-rays.
There is, so far as I see, no theoretical objection to be taken to
this view, and the fact of the pectoral and pelvic girdles originating
continuously, and long remaining united with the
[Pg 614] longitudinal bars of
their respective fins is in favour of rather than against this view.
The same may be said of the fact that the first part of each girdle to
be formed is that in the neighbourhood of the longitudinal bar
(basipterygium) of the fin, the dorsal and ventral prolongations being
subsequent growths.
The later development of the skeleton of the two fins is more
conveniently treated separately.
Fig. 346. Transverse section through the pectoral fin of a young
embryo of Scyllium stellare.
mpt. basipterygial bar (metapterygium); fr. fin ray; m.
muscles; hf. horny fibres.
The pelvic fin. The changes in the pelvic fin are comparatively
slight. The fin remains through life as a nearly horizontal lateral
projection of the body, and the longitudinal bar—the basipterygium—at
its base always remains as such. It is for a considerable period
attached to the pelvic girdle, but eventually becomes segmented from
it. Of the fin rays the anterior remains directly articulated with the
pelvic girdle on the separation of the basipterygium (fig. 347), and
the remaining rays finally become segmented from the basipterygium,
though they remain articulated with it. They also become to some
extent transversely segmented. The posterior end of the basipterygial
bar also becomes segmented off as the terminal ray.
The pelvic fin thus retains in all essential points its primitive
arrangement.
[Pg 615]
The pectoral fin. The earliest stage of the pectoral fin differs from
that of the pelvic fin only in minor points. There is the same
longitudinal or basipterygial bar to which the fin-rays are attached,
whose position at the base of the fin is clearly seen in the
transverse section (fig. 346, mpt). In front the bar is continuous
with the pectoral girdle (figs. 344 and 348).
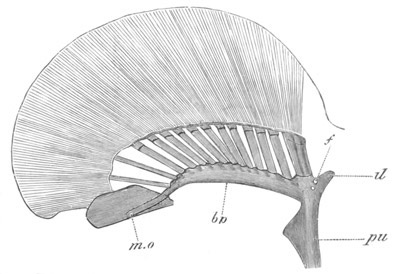
Fig. 347. Pelvic fin of a young male embryo of Scyllium stellare.
bp. basipterygium; m.o. process of basipterygium continued into
clasper; il. iliac process of pectoral girdle; pu. pubis.
Fig. 348. Pectoral fin of an embryo of
Scyllium stellare.
mp. metapterygium (basipterygium of earlier stage); me.p.
rudiment of future pro- and mesopterygium; sc. cut surface of
scapular process; cr. coracoid process; fr. foramen; f. horny
fibres.
The changes which take place in the course of the further development
are however very much more considerable in the case of the pectoral
than in that of the pelvic fin.
By the process spoken of above, by which the attachment of the
pectoral
[Pg 616] fin to the body wall becomes shortened from behind forwards,
the basipterygial bar is gradually rotated outwards, its anterior end
remaining attached to the pectoral girdle. In this way this bar comes
to form the posterior border of the skeleton of the fin (figs. 348 and
349, mp), constituting what Gegenbaur called the metapterygium, and
eventually becomes segmented off from the pectoral girdle, simply
articulating with its hinder edge.
The plate of cartilage, which is continued outwards from the
basipterygium, or as we may now call it, the metapterygium, into the
fin, is not nearly so completely divided up into fin-rays as in the
case of the pelvic fin, and this is especially the case with the basal
part of the plate. This basal part becomes in fact at first only
divided into two parts (fig. 348) a small anterior part at the front
end (me.p), and a larger posterior along the base of the remainder
of the fin. The anterior part directly joins the pectoral girdle at
its base, resembling in this respect the anterior fin-ray of the
pelvic girdle. It constitutes the rudiment of the mesopterygium and
propterygium of Gegenbaur. It bears four fin-rays at its extremity,
the anterior not being well marked. The remaining fin-rays are borne
by the edge of the plate continuous with the metapterygium.
The further changes in the cartilages of the limb are not important,
and are easily understood by reference to fig. 349 representing the
limb of a nearly full-grown embryo. The front end of the anterior
basal cartilage becomes segmented off as a propterygium, bearing a
single fin-ray, leaving the remainder of the cartilage as a
mesopterygium. The remainder of the now considerably segmented
fin-rays are borne by the metapterygium.
The mode of development of the pectoral fin demonstrates that, as
supposed by Mivart, the metapterygium is the homologue of the basal
cartilage of the pelvic fin.
From the mode of development of the fins of Scyllium conclusions may
be drawn adverse to the views recently put forward on the structure of
the fin by Gegenbaur and Huxley, both of whom consider the primitive
type of fin to be most nearly retained in Ceratodus, and to consist of
a central multisegmented axis with numerous rays. Gegenbaur derives
the Elasmobranch pectoral fin from a form which he calls the
archipterygium, nearly like that of Ceratodus, with a median axis and
two
[Pg 617] rows of rays; but holds that in addition to the rays attached to
the median axis, which are alone found in Ceratodus, there were other
rays directly articulated to the shoulder-girdle. He considers that in
the Elasmobranch fin the majority of the lateral rays on the posterior
(median or inner according to his view of the position of the limb)
side have become aborted, and that the central axis is represented by
the metapterygium; while the pro- and mesopterygium and their rays
are, he believes, derived from those rays of the archipterygium which
originally articulated directly with the shoulder-girdle.
Gegenbaur’s view appears to me to be absolutely negatived by the facts
of development of the pectoral fin in Scyllium; not so much because
the pectoral fin in this form is necessarily to be regarded as
primitive, but because what Gegenbaur holds to be the primitive axis
of the biserial fin is demonstrated to be really the base, and it is
only in the adult that it is conceivable that a second set of lateral
rays could have existed on the posterior side of the metapterygium. If
Gegenbaur’s view were correct we should expect to find in the embryo,
if anywhere, traces of the second set of lateral rays; but the fact is
that, as may easily be seen by an inspection of figs. 344 and 346,
such a second set of lateral rays could not possibly have existed in a
type of fin like that found in the embryo[216].
With this view of
Gegenbaur’s it appears to me that the theory held by this anatomist to
the effect that the limbs are modified gill arches also falls; in that
his method of deriving the limbs from gill arches ceases to be
admissible, while it is not easy to see how a limb, formed on the type
of the embryonic limb of Elasmobranchs, could be derived from a
visceral arch with its branchial rays[217].
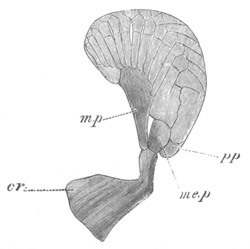
Fig. 349. Skeleton of the pectoral fin and part of pectoral girdle
of a nearly ripe embryo of Scyllium stellare.
m.p. metapterygium; me.p. mesopterygium; pp. propterygium;
cr. coracoid process.
Gegenbaur’s older view
[Pg 618] that the Elasmobranch fin retains a primitive
uniserial type appears to me to be nearer the truth than his more
recent view on this subject; though I hold that the fundamental point
established by the development of these parts in Scyllium is that the
posterior border of the adult Elasmobranch fin is the primitive base
line, i.e. the line of attachment of the fin to the side of the
body.
Huxley holds that the mesopterygium is the proximal piece of the axial
skeleton of the limb of Ceratodus, and derives the Elasmobranch fin
from that of Ceratodus by the shortening of its axis and the
coalescence of some of its elements. The secondary character of the
mesopterygium, and its total absence in the embryo Scyllium, appears
to me as conclusive against Huxley’s view, as the character of the
embryonic fin is against that of Gegenbaur; and I should be much more
inclined to hold that the fin of Ceratodus has been derived from a fin
like that of the Elasmobranchii by a series of steps similar to those
which Huxley supposes to have led to the establishment of the
Elasmobranch fin, but in exactly the reverse order.
With reference to the development of the pectoral fin in the Teleostei
there are some observations of ’Swirski (No. 488) which unfortunately
do not throw very much light upon the nature of the limb.
’Swirski finds that in the Pike the skeleton of the limb is formed of
a plate of cartilage, continuous with the pectoral girdle; which soon
becomes divided into a proximal and a distal portion. The former is
subsequently segmented into five basal rays, and the latter into
twelve parts, the number of which subsequently becomes reduced.
These investigations might be regarded as tending to shew that the
basipterygium of Elasmobranchii is not represented in Teleostei, owing
to the fin rays not having united into a continuous basal bar, but the
observations are not sufficiently complete to admit of this conclusion
being founded upon them with any certainty.
The cheiropterygium.
Observations on the early development of the pentadactyloid limbs of
the higher Vertebrata are comparatively scanty.
The limbs arise as simple outgrowths of the sides of the body, formed
both of epiblast and mesoblast. In the Amniota, at all events, they
are processes of a special longitudinal ridge known as the Wolffian
ridge. In the Amniota they also bear at their extremity a thickened
cap of epiblast, which may be compared with the epiblastic fold at the
apex of the Elasmobranch fin.
Both limbs have at first a precisely similar position, both being
directed backwards and being parallel to the surface of the body.
[Pg 619]
In the Urodela (Götte) the ulnar and fibular sides are primitively
dorsal, and the radial and tibial ventral: in Mammalia however
Kölliker states that the radial and tibial edges are from the first
anterior.
The exact changes of position undergone by the limbs in the course of
development are not fully understood. To suit a terrestrial mode of
life the flexures of the two limbs become gradually more and more
opposite, till in Mammalia the corresponding joints of the two limbs
are turned in completely opposite directions.
Within the mesoblast of the limbs a continuous blastema becomes
formed, which constitutes the first trace of the skeleton of the limb.
The corresponding elements of the two limbs, viz. the humerus and
femur, radius and tibia, ulna and fibula, carpal and tarsal bones,
metacarpals and metatarsals, and digits, become differentiated within
this, by the conversion of definite regions into cartilage, which may
either be completely distinct or be at first united. These
cartilaginous elements subsequently ossify.
The later development of the parts, more especially of the carpus and
tarsus, has been made the subject of considerable study; and important
results have been thereby obtained as to the homology of the various
carpal and tarsal bones throughout the Vertebrata; but this subject is
too special to be treated of here. The early development, including
the succession of the growth of the different parts, and the extent of
continuity primitively obtaining between them, has on the other hand
been but little investigated; recently however the development of the
limbs in the Urodela has been worked out in this way by two
anatomists, Götte (No. 482) and Strasser (No. 487), and their results,
though not on all points in complete harmony, are of considerable
interest, more especially in their bearing on the derivation of the
pentadactyloid limb from the piscine fin. Till however further
investigations of the same nature have been made upon other types, the
conclusions to be drawn from Götte and Strasser’s observations must be
regarded as somewhat provisional, the actual interpretation of various
ontological processes being very uncertain.
The forms investigated are Triton and Salamandra. We may remind the
reader that the hand of the Urodela has four digits, and the foot
five, the fifth digit being absent in the hand[218].
In Triton the
proximal row of carpal bones consists (using Gegenbaur’s nomenclature)
of (1) a radiale, and (2 and 3) an intermedium and ulnare, partially
united. The distal row is formed of four carpals, of which the first
often does not support the first
[Pg 620] metacarpal; while the second
articulates with both the first and second metacarpals. In the foot
the proximal row of tarsals consists of a tibiale, an intermedium and
a fibulare. The distal row is formed of four tarsals, the first, like
that in the hand, often not articulating with the first metatarsal,
the second supporting the first and second metatarsals; and the fourth
the fourth and fifth metatarsals.
The mode of development of the hand and foot is almost the same. The
most remarkable feature of development is the order of succession of
the digits. The two anterior (radial or tibial) are formed in the
first instance, and then the third, fourth and fifth in succession.
As to the actual development of the skeleton Strasser, whose
observations were made by means of sections, has arrived at the
following results.
The humerus with the radius and ulna, and the corresponding parts in
the hind limb, are the first parts to be differentiated in the
continuous plate of tissue from which the skeleton of the limb is
formed. Somewhat later a cartilaginous centre appears at the base of
the first and second fingers (which have already appeared as
prominences at the end of the limb) in the situation of the permanent
second carpal of the distal row of carpals; and the process of
chondrification spreads from this centre into the fingers and into the
remainder of the carpus. In this way a continuous carpal plate of
cartilage is established, which is on the one hand continuous with the
cartilage of the two metacarpals, and on the other with the radius and
ulna.
In the cartilage of the carpus two special columns may be noticed, the
one on the radial side, most advanced in development, being continuous
with the radius; the other less developed column on the side of the
ulna being continuous both with the ulna and with the radius. The ulna
and radius are not united with the humerus.
In the further growth the third and fourth digits, and in the foot the
fifth digit also, gradually sprout out in succession from the ulnar
side of the continuous carpal plate. The carpal plate itself becomes
segmented from the radius and ulna, and divided up into the carpal
bones.
The original radial column is divided into three elements, a proximal
the radiale, a middle element the first carpal, and a distal the
second carpal already spoken of. The first carpal is thus situated
between the basal cartilage of the second digit and the radiale, and
would therefore appear to be the representative of a primitive middle
row of carpal bones, of which the centrale is also another
representative.
The centrale and intermedium are the middle and proximal products of
the segmentation of the ulnar column of the primitive carpus, the
distal second carpal being common both to this column and to the
radial column.
The ulnar or fibular side of the carpus or tarsus becomes divided into
a proximal element—the ulnare or fibulare—the ulnare remaining
partially united with the intermedium. There are also formed from this
plate two carpals to articulate with digits 3 and 4; while in the foot
the corresponding elements articulate respectively with the third
digit, and with the fourth and fifth digits.
[Pg 621]
Götte, whose observations were made in a somewhat different method to
those of Strasser, is at variance with him on several points. He finds
that the primitive skeleton of the limb consists of a basal portion,
the humerus, continued into a radial and an ulnar ray, which are
respectively prolonged into the two first digits. The two rays next
coalesce at the base of the fingers to form the carpus, and thus the
division of the limb into the brachium, antebrachium and manus is
effected.
The ulna, which is primitively prolonged into the second digit, is
subsequently separated from it and is prolonged into the third; from
the side of the part of the carpus connecting the ulna with the third
digit the fourth digit is eventually budded out, and in the foot the
fourth and fifth digits arise from the corresponding region. Each of
the three columns connected respectively with the first, second, and
third digits becomes divided into three successive carpal bones, so
that Götte holds the skeleton of the hand or foot to be formed of a
proximal, a middle, and a distal row of carpal bones each containing
potentially three elements. The proximal row is formed of the radiale,
intermedium and ulnare; the middle row of carpal 1, the centrale and
carpal 4, and the distal of carpal 2 (consisting according to Götte of
two coalesced elements) and carpal 3.
The derivation of the cheiropterygium from the ichthyopterygium. All
anatomists are agreed that the limbs of the higher Vertebrata are
derived from those of Fishes, but the gulf between the two types of
limbs is so great that there is room for a very great diversity of
opinion as to the mode of evolution of the cheiropterygium. The most
important speculations on the subject are those of Gegenbaur and
Huxley.
Gegenbaur holds that the cheiropterygium is derived from a uniserial
piscine limb, and that it consists of a primitive stem, to which a
series of lateral rays are attached on one (the radial) side; while
Huxley holds that the cheiropterygium is derived from a biserial
piscine limb by the “lengthening of the axial skeleton, accompanied by
the removal of its distal elements further away from the
shoulder-girdle and by a diminution in the number of the rays.”
Neither of these theories is founded upon ontology, and the only
ontological evidence we have which bears on this question is that
above recorded with reference to the development of the Urodele limb.
Without holding that this evidence can be considered as in any way
conclusive, its tendency would appear to me to be in favour of
regarding the cheiropterygium as derived from a uniserial type of fin.
The humerus or femur would appear to be the basipterygial bars
(metapterygium), which have become directed outwards instead of
retaining their original position parallel to the length of the body
at the base of the fin. The anterior (proximal) fin-rays and the pro-
and mesopterygium must be supposed to have become aborted, while the
radius or ulna, and tibia or fibula are two posterior fin-rays
(probably each representing several coalesced rays like the pro- and
mesopterygium) which support at their distal extremities more numerous
fin-rays consisting of the rows of carpal and tarsal bones.
[Pg 622]
This view of the cheiropterygium corresponds in some respects with
that put forward by Götte as a result of his investigations on the
development of the Urodele limbs, though in other respects it is very
different. A difficulty of this view is the fact that it involves our
supposing that the radial edge of the limb corresponds with the
metapterygial edge of the piscine fin. The difficulties of this
position have been clearly pointed out by Huxley, but the fact that in
the primitive position of the Urodele limbs the radius is ventral and
the ulna dorsal shews that this difficulty is not insuperable, in that
it is easy to conceive the radial border of the fin to have become
rotated from its primitive Elasmobranch position into the vertical
position it occupies in the embryos of the Urodela, and then to have
been further rotated from this position into that which it occupies in
the adult Urodela and in all higher forms.
Bibliography of the Limbs.
(477) M. v. Davidoff. “Beiträge z. vergleich. Anat. d. hinteren
Gliedmaassen d. Fische I.” Morphol. Jahrbuch, Vol. V. 1879.
(478) C. Gegenbaur. Untersuchungen z. vergleich. Anat. d.
Wirbelthiere. Leipzig, 1864-5. Erstes Heft. Carpus u. Tarsus. Zweites
Heft. Brustflosse d. Fische.
(479) C. Gegenbaur. “Ueb. d. Skelet d. Gliedmaassen d. Wirbelthiere im
Allgemeinen u. d. Hintergliedmaassen d. Selachier insbesondere.”
Jenaische Zeitschrift, Vol. V. 1870.
(480) C. Gegenbaur. “Ueb. d. Archipterygium.” Jenaische Zeitschrift,
Vol. VII. 1873.
(481) C. Gegenbaur. “Zur Morphologie d. Gliedmaassen d. Wirbelthiere.”
Morphologisches Jahrbuch, Vol. II. 1876.
(482) A. Götte. Ueb. Entwick. u. Regeneration d. Gliedmaassenskelets
d. Molche. Leipzig, 1879.
(483) T. H. Huxley. “On Ceratodus Forsteri, with some observations on
the classification of Fishes.” Proc. Zool. Soc. 1876.
(484) St George Mivart. “On the Fins of Elasmobranchii.” Zoological
Trans., Vol. X.
(485) A. Rosenberg. “Ueb. d. Entwick. d. Extremitäten-Skelets bei
einigen d. Reduction ihrer Gliedmaassen charakterisirten
Wirbelthieren.” Zeit. f. wiss. Zool., Vol. XXIII. 1873.
(486) E. Rosenberg. “Ueb. d. Entwick. d. Wirbelsäule u. d. centrale
carpi d. Menschen.” Morphologisches Jahrbuch, Vol. I. 1875.
(487) H. Strasser. “Z. Entwick. d. Extremitätenknorpel bei Salamandern
u. Tritonen.” Morphologisches Jahrbuch, Vol. V. 1879.
(488) G. ’Swirski. Untersuch. üb. d. Entwick. d. Schultergürtels u.
d. Skelets d. Brustflosse d. Hechts. Inaug. Diss. Dorpat, 1880.
(489) J. K. Thacker. “Median and paired fins. A contribution to the
history of the Vertebrate limbs.” Trans. of the Connecticut Acad.,
Vol. III. 1877.
(490) J. K. Thacker. “Ventral fins of Ganoids.” Trans. of the
Connecticut Acad., Vol. IV. 1877.
[Pg 623]
CHAPTER XXI.
THE BODY CAVITY, THE VASCULAR SYSTEM, AND THE VASCULAR GLANDS.
The Body cavity.
In the Cœlenterata no body cavity as distinct from the alimentary
cavity is present; but in the remaining Invertebrata the body cavity
may (1) take the form of a wide space separating the wall of the gut
from the body wall, or (2) may be present in a more or less reduced
form as a number of serous spaces, or (3) only be represented by
irregular channels between the muscular and connective-tissue cells
filling up the interior of the body. The body cavity, in whatever form
it presents itself, is probably filled with fluid, and the fluid in it
may contain special cellular elements. A well developed body cavity
may coexist with an independent system of serous spaces, as in the
Vertebrata and the Echinodermata; the perihæmal section of the body
cavity of the latter probably representing the system of serous
spaces.
In several of the types with a well developed body cavity it has been
established that this cavity originates in the embryo from a pair of
alimentary diverticula, and the cavities resulting from the formation
of these diverticula may remain distinct, the adjacent walls of the
two cavities fusing to form a dorsal and a ventral mesentery.
It is fairly certain that some groups, e.g. the Tracheata, with
imperfectly developed body cavities are descended from ancestors which
were provided with well developed body cavities, but how far this is
universally the case cannot as yet be definitely decided, and for
additional information on this subject the
[Pg 624] reader is referred to pp. 355-360 and to the literature there referred to.
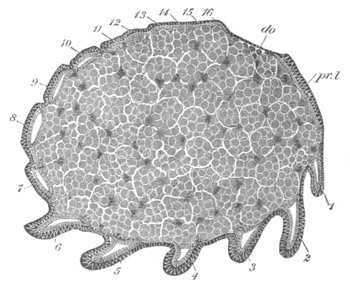
Fig. 350. Longitudinal section through an embryo of Agelina
labyrinthica.
The section is taken slightly to one side of the middle line so as
to shew the relation of the mesoblastic somites to the limbs. In the
interior are seen the yolk segments and their nuclei.
1-16. the segments; pr.l. procephalic lobe; do. dorsal
integument.
In the Chætopoda and the Tracheata the body cavity arises as a series
of paired compartments in the somites of mesoblast (fig. 350) which
have at first a very restricted extension on the ventral side of the
body, but eventually extend dorsalwards and ventralwards till each
cavity is a half circle investing the alimentary tract; on the dorsal
side the walls separating the two half cavities usually remain as the
dorsal mesentery, while ventrally they are in most instances absorbed.
The transverse walls, separating the successive compartments of the
body cavity, generally become more or less perforated.
Chordata. In the Chordata the primitive body cavity is either directly
formed from a pair of alimentary diverticula (Cephalochorda) (fig. 3)
or as a pair of spaces in the mesoblastic plates of the two sides of
the body (fig. 20).
As already explained (pp. 294-300) the walls of the dorsal sections of
the primitive body cavity soon become separated from those of the
ventral, and becoming segmented constitute the muscle plates, while
the cavity within them becomes
[Pg 625] obliterated: they are dealt with in a
separate chapter. The ventral part of the primitive cavity alone
constitutes the permanent body cavity.
The primitive body cavity in the lower Vertebrata is at first
continued forwards into the region of the head, but on the formation
of the visceral clefts the cephalic section of the body cavity becomes
divided into a series of separate compartments. Subsequently these
sections of the body cavity become obliterated; and, since their walls
give rise to muscles, they may probably be looked upon as equivalent
to the dorsal sections of the body cavity in the trunk, and will be
treated of in connection with the muscular system.
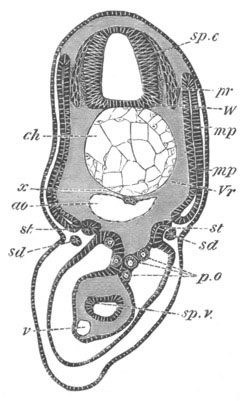
Fig. 351. Section through the trunk of a Scyllium embryo slightly
younger than 28 f.
sp.c. spinal canal; W. white matter of spinal cord; pr.
posterior nerve-roots; ch. notochord; x. subnotochordal rod;
ao. aorta; mp. muscle-plate; mp´. inner layer of muscle-plate
already converted into muscles; Vr. rudiment of vertebral body;
st. segmental tube; sd. segmental duct; sp.v. spiral valve;
v. subintestinal vein; p.o. primitive generative cells.
As a result of its mode of origin the body cavity in the trunk is at
first divided into two lateral halves; and part of the mesoblast
lining it soon becomes distinguished as a special layer of epithelium,
known as the peritoneal epithelium, of which the part bounding the
outer wall forms the somatic layer, and that bounding the inner wall
the splanchnic layer. Between the two splanchnic layers is placed the
gut. On the ventral side, in the region of the permanent gut, the two
halves of the body cavity soon coalesce, the septum between them
becoming absorbed, and the splanchnic layers of epithelium of the two
sides uniting at the ventral side of the gut, and the somatic layers
at the median ventral line of the body wall (fig. 351).
In the lower Vertebrata the body cavity is originally present even in
the postanal region of the trunk, but usually atrophies early,
frequently before the two halves coalesce.
On the dorsal side of the gut the
[Pg 626] two halves of the body cavity never
coalesce, but eventually the splanchnic layers of epithelium of the
two sides, together with a thin layer of interposed mesoblast, form a
delicate membrane, known as the mesentery, which suspends the gut from
the dorsal wall of the body (figs. 119 and 351). On the dorsal side
the epithelium lining of the body cavity is usually more columnar than
elsewhere (fig. 351), and its cells partly form a covering for the
generative organs, and partly give rise to the primitive germinal
cells. This part of the epithelium is often known as the germinal
epithelium.
Over the greater part of the body cavity the lining epithelium becomes
in the adult intimately united with a layer of the subjacent
connective tissue, and constitutes with it a special lining membrane
for the body cavity, known as the peritoneal membrane.
Abdominal pores. In the Cyclostomata, the majority of the
Elasmobranchii, the Ganoidei, a few Teleostei, the Dipnoi, and some
Sauropsida (Chelonia and Crocodilia) the body cavity is in
communication with the exterior by a pair of pores, known as abdominal
pores, the external openings of which are usually situated in the
cloaca[219].
The ontogeny of these pores has as yet been but very slightly
investigated. In the Lamprey they are formed as apertures leading from
the body cavity into the excretory section of the primitive cloaca.
This section would appear from Scott’s (No. 87) observations to be
derived from part of the hypoblastic cloacal section of the alimentary
tract.
In all other cases they are formed in a region which appears to belong
to the epiblastic region of the cloaca; and from my observations on
Elasmobranchs it may be certainly concluded that they are formed there
in this group. They may appear as perforations (1) at the apices of
papilliform prolongations of the body cavity, or (2) at the ends of
cloacal pits directed from the exterior towards the body cavity, or
(3) as simple slit-like openings.
Considering the difference in development between the abdominal pores
of most types, and those of the Cyclostomata, it is open to doubt
whether these two types of pores are strictly homologous.
In the Cyclostomata they serve for the passage outwards of the
generative products, and they also have this function in some of the
few Teleostei in which they are found; and Gegenbaur and Bridge hold
that the primitive mode of exit of the generative products, prior to
the development of the Müllerian ducts, was probably by means of these
pores. I have elsewhere
[Pg 627] suggested that the abdominal pores are perhaps
remnants of the openings of segmental tubes; there does not however
appear to be any definite evidence in favour of this view, and it is
more probable that they may have arisen as simple perforations of the
body wall.
Pericardial cavity, pleural cavities, and diaphragm. In all Vertebrata
the heart is at first placed in the body cavity (fig. 353 A), but the
part of the body cavity containing it afterwards becomes separated as
a distinct cavity known as the pericardial cavity. In Elasmobranchii,
Acipenser, etc. a passage is however left between the pericardial
cavity and the body cavity; and in the Lamprey a separation between
the two cavities does not occur during the Ammocœte stage.
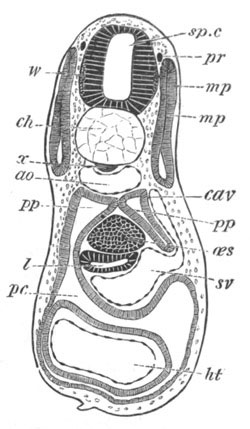
Fig. 352. Section through the trunk of a Scyllium embryo slightly
younger than 28 F.
The figure shews the separation of the body cavity from the
pericardial cavity by a horizontal septum in which runs the ductus
Cuvieri; on the left side is seen the narrow passage which remains
connecting the two cavities.
sp.c. spinal canal; w. white matter of spinal cord; pr.
commissure connecting the posterior nerve-roots; ch. notochord;
x. subnotochordal rod; ao. aorta; sv. sinus venosus; cav.
cardinal vein; ht. heart; pp. body cavity; pc. pericardial
cavity; œs. solid œsophagus; l. liver; mp. muscle-plate.
In Elasmobranchii the pericardial cavity becomes established as a
distinct space in front of the body cavity in the following way. When
the two ductus Cuvieri, leading transversely from the sinus venosus to
the cardinal veins, become developed, a horizontal septum, shewn on
the right side in fig. 352, is formed to support them, stretching
across from the splanchnic to the somatic side of the body cavity, and
dividing the body cavity (fig. 352) in this part into (1) a dorsal
section formed of a right and left division constituting the true body
cavity (pp), and (2) a ventral part the pericardial cavity (pc).
The septum is at first of a very small longitudinal extent, so that
both in front and behind it (fig. 352 on the left side) the dorsal and
ventral sections of the body cavity are in free communication. The
septum soon however becomes prolonged, and ceasing to be quite
horizontal, is directed obliquely upwards and forwards till it meets
the dorsal wall of the body.
[Pg 628] Anteriorly all communication is thus early
shut off between the body cavity and the pericardial cavity, but the
two cavities still open freely into each other behind.
The front part of the body cavity, lying dorsal to the pericardial
cavity, becomes gradually narrowed, and is wholly obliterated long
before the close of embryonic life, so that in adult Elasmobranch
Fishes there is no section of the body cavity dorsal to the
pericardial cavity. The septum dividing the body cavity from the
pericardial cavity is prolonged backwards, till it meets the ventral
wall of the body at the point where the liver is attached by its
ventral mesentery (falciform ligament). In this way the pericardial
cavity becomes completely shut off from the body cavity, except, it
would seem, for the narrow communications found in the adult. The
origin of these communications has not however been satisfactorily
worked out.
The septum between the pericardial cavity and the body cavity is
attached on its dorsal aspect to the liver. It is at first nearly
horizontal, but gradually assumes a more vertical position, and then,
owing to the obliteration of the primitive anterior part of the body
cavity, appears to mark the front boundary of the body cavity. The
above description of the mode of formation of the pericardial cavity,
and the explanation of its relations to the body cavity, probably
holds true for Fishes generally.
In the higher types the earlier changes are precisely the same as
those in Elasmobranch Fishes. The heart is at first placed within the
body cavity attached to the ventral wall of the gut by a mesocardium
(fig. 353 A). A horizontal septum is then formed, in which the ductus
Cuvieri are placed, dividing the body cavity for a short distance into
a dorsal (p.p) and ventral (p.c) section (fig. 353 B). In Birds
and Mammals, and probably also in Reptilia, the ventral and dorsal
parts of the body cavity are at first in free communication both in
front of and behind this septum. This is shewn for the Chick in fig.
353 A and B, which are sections of the same chick, A being a little in
front of B. The septum is soon continued forwards so as completely to
separate the ventral pericardial and the dorsal body cavity in front,
the pericardial cavity extending at this period considerably further
forwards than the body cavity.
Since the horizontal septum, by its mode of origin, is
[Pg 629] necessarily
attached to the ventral side of the gut, the dorsal part of the
primitive body space is divided into two halves by a median vertical
septum formed of the gut and its mesentery (fig. 353 B). Posteriorly
the horizontal septum grows in a slightly ventral direction along the
under surface of the liver (fig. 354), till it meets the abdominal
wall of the body at the insertion of the falciform ligament, and thus
completely shuts off the pericardial cavity from the body cavity. The
horizontal septum forms, as is obvious from the above description, the
dorsal wall of the pericardial cavity[220].
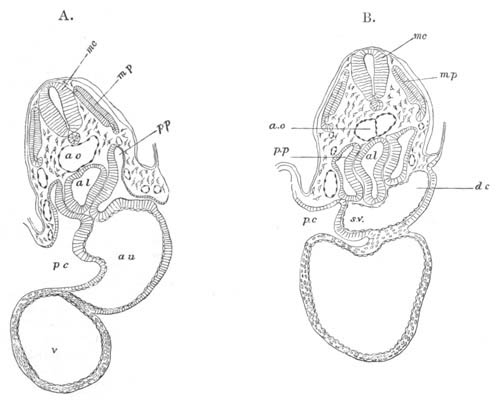
Fig. 353. Transverse sections through a Chick embryo with twenty-one
mesoblastic somites to shew the formation of the pericardial
cavity, A. being the anterior section.
p.p. body cavity; p.c. pericardial cavity; al. alimentary
cavity; au. auricle; v. ventricle; s.v. sinus venosus; d.c.
ductus Cuvieri; ao. aorta; mp. muscle-plate; mc. medullary
cord.
With the complete separation of the pericardial cavity from the body
cavity, the first period in the development of these parts is
completed, and the relations of the body cavity to the
[Pg 630] pericardial
cavity become precisely those found in the embryos of Elasmobranchii.
The later changes are however very different. Whereas in Fishes the
right and left sections of the body cavity dorsal to the pericardial
cavity soon atrophy, in the higher types, in correlation with the
relatively backward situation of the heart, they rapidly become
larger, and receive the lungs which soon sprout out from the throat.
The diverticula which form the lungs grow out into the splanchnic
mesoblast, in front of the body cavity; but as they grow, they extend
into the two anterior compartments of the body cavity, each attached
by its mesentery to the mesentery of the gut (fig. 354, lg). They
soon moreover extend beyond the region of the pericardium into the
undivided body cavity behind. This holds not only for the embryos of
the Amphibia and Sauropsida, but also for those of Mammalia.
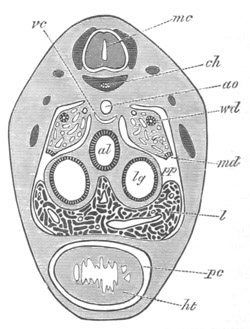
Fig. 354. Section through the cardiac region of an embryo of
Lacerta Muralis of 9 mm. to shew the mode of formation of the
pericardial cavity.
ht. heart; pc. pericardial cavity; al. alimentary tract; lg.
lung; l. liver; pp. body cavity; md. open end of Müllerian
duct; wd. Wolffian duct; vc. vena cava inferior; ao. aorta;
ch. notochord; mc. medullary cord.
To understand the further changes in the pericardial cavity it is
necessary to bear in mind its relations to the adjoining parts. It
lies at this period completely ventral to the two anterior
prolongations of the body cavity containing the lungs (fig. 354). Its
dorsal wall is attached to the gut, and is continuous with the
mesentery of the gut passing to the dorsal abdominal wall, forming the
posterior mediastinum of human anatomy.
The changes which next ensue consist essentially in the enlargement of
the sections of the body cavity dorsal to the pericardial cavity. This
enlargement takes place partly by the elongation of the posterior
mediastinum, but still more by the two divisions of the body cavity
which contain the lungs extending themselves ventrally round the
outside of the pericardial
[Pg 631] cavity. This process is illustrated by fig.
355, taken from an embryo Rabbit. The two dorsal sections of the body
cavity (pl.p) finally extend so as completely to envelope the
pericardial cavity (pc), remaining however separated from each other
below by a lamina extending from the ventral wall of the pericardial
cavity to the body wall, which forms the anterior mediastinum of human
anatomy.
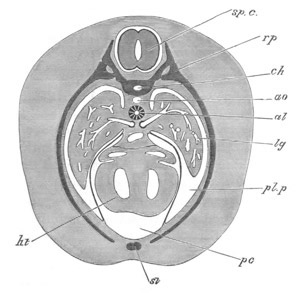
Fig. 355. Section through an advanced embryo of a Rabbit to shew
how the pericardial cavity becomes surrounded by the pleural
cavities.
ht. heart; pc. pericardial cavity; pl.p pleural cavity; lg.
lung; al. alimentary tract; ao. dorsal aorta; ch. notochord;
rp. rib; st. sternum; sp.c. spinal cord.
By these changes the pericardial cavity is converted into a closed
bag, completely surrounded at its sides by the two lateral halves of
the body cavity, which were primitively placed dorsally to it. These
two sections of the body cavity, which in Amphibia and Sauropsida
remain in free communication with the undivided peritoneal cavity
behind, may, from the fact of their containing the lungs, be called
the pleural cavities.
In Mammalia a further change takes place, in that, by the formation of
a vertical partition across the body cavity, known as the diaphragm,
the pleural cavities, containing the lungs,
[Pg 632] become isolated from the
remainder of the body or peritoneal cavity. As shewn by their
development the so-called pleuræ or pleural sacks are simply the
peritoneal linings of the anterior divisions of the body cavity, shut
off from the remainder of the body cavity by the diaphragm.
The exact mode of formation of the diaphragm is not fully made out;
the account of it recently given by Cadiat (No. 491) not being in my
opinion completely satisfactory.
Bibliography.
(491) M. Cadiat. “Du développement de la partie céphalothoracique de
l'embryon, de la formation du diaphragme, des pleures, du péricarde,
du pharynx et de l'œsophage.” Journal de l'Anatomie et de la
Physiologie, Vol. XIV. 1878.
Vascular System.
The actual observations bearing on the origin of the vascular system,
using the term to include the lymphatic system, are very scanty. It
seems probable, mainly it must be admitted on à priori grounds, that
vascular and lymphatic systems have originated from the conversion of
indefinite spaces, primitively situated in the general connective
tissue, into definite channels. It is quite certain that vascular
systems have arisen independently in many types; a very striking case
of the kind being the development in certain parasitic Copepoda of a
closed system of vessels with a red non-corpusculated blood (E. van
Beneden, Heider), not found in any other Crustacea. Parts of vascular
systems appear to have arisen in some cases by a canalization of
cells.
The blood systems may either be closed or communicate with the body
cavity. In cases where the primitive body cavity is atrophied or
partially broken up into separate compartments (Insecta, Mollusca,
Discophora, etc.) a free communication between the vascular system and
the body cavity is usually present; but in these cases the
communication is no doubt secondary. On the whole it would seem
probable that the vascular system has in most instances arisen
independently of the body cavity, at least in types where the body
cavity is
[Pg 633] present in a well-developed condition. As pointed out by the
Hertwigs, a vascular system is always absent where there is not a
considerable development of connective tissue.
As to the ontogeny of the vascular channels there is still much to be
made out both in Vertebrates and Invertebrates.
The smaller channels often rise by a canalization of cells. This
process has been satisfactorily studied by Lankester in the
Leech[221],
and may easily be observed in the blastoderm of the Chick
or in the epiploon of a newly born Rabbit (Schäfer, Ranvier). In
either case the vessels arise from a network of cells, the superficial
protoplasm and part of the nuclei giving rise to the walls, and the
blood-corpuscles being derived either from nucleated masses set free
within the vessels (the Chick) or from blood-corpuscles directly
differentiated in the axes of the cells (Mammals).
Larger vessels would seem to be formed from solid cords of cells, the
central cells becoming converted into the corpuscles, and the
peripheral cells constituting the walls. This mode of formation has
been observed by myself in the case of the Spider’s heart, and by
other observers in other Invertebrata. In the Vertebrata a more or
less similar mode of formation appears to hold good for the larger
vessels, but further investigations are still required on this
subject. Götte finds that in the Frog the larger vessels are formed as
longitudinal spaces, and that the walls are derived from the
indifferent cells bounding these spaces, which become flattened and
united into a continuous layer.
The early formation of vessels in the Vertebrata takes place in the
splanchnic mesoblast; but this appears due to the fact that the
circulation is at first mainly confined to the vitelline region, which
is covered by splanchnic mesoblast.
The Heart.
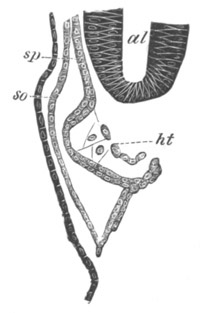
Fig. 356. Section through the developing heart of an embryo of an
Elasmobranch (Pristiurus).
al. alimentary tract; sp. splanchnic mesoblast; so. somatic
mesoblast; ht. heart.
The heart is essentially formed as a tubular cavity in the splanchnic
mesoblast, on the ventral side of the throat, immediately behind the
region of the visceral clefts. The walls of this cavity are formed of
two layers, an outer thicker layer, which has at first only the form
of a half tube, being incomplete on its dorsal side; and an inner
lamina formed of delicate flattened cells. The latter is the
epithelioid lining of the heart, and the cavity it contains the true
cavity of the heart. The outer layer gives rise to the muscular wall
and peritoneal covering of the heart. Though at first it has only the
form of a half tube (fig.
[Pg 634] 356), it soon becomes folded in on the
dorsal side so as to form for the heart a complete muscular wall. Its
two sides, after thus meeting to complete the tube of the heart,
remain at first continuous with the splanchnic mesoblast surrounding
the throat, and form a provisional mesentery—the mesocardium—which
attaches the heart to the ventral wall of the throat. The superficial
stratum of the wall of the heart differentiates itself as the
peritoneal covering. The inner epithelioid tube takes its origin at
the time when the general cavity of the heart is being formed by the
separation of the splanchnic mesoblast from the hypoblast. During this
process (fig. 357) a layer of mesoblast remains close to the
hypoblast, but connected with the main mass
[Pg 635] of the mesoblast by
protoplasmic processes. A second layer next becomes split from the
splanchnic mesoblast, connected with the first layer by the
above-mentioned protoplasmic processes. These two layers form together
the epithelioid lining of the heart; between them is the cavity of the
heart, which soon loses the protoplasmic trabeculæ which at first
traverse it. The cavity of the heart may thus be described as being
formed by a hollowing out of the splanchnic mesoblast, and resembles
in its mode of origin that of other large vascular trunks.
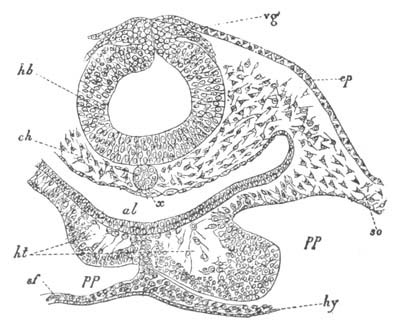
Fig. 357. Transverse section through the posterior part of the
head of an embryo Chick of thirty hours.
hb. hind-brain; vg. vagus nerve; ep. epiblast; ch.
notochord; x. thickening of hypoblast (possibly a rudiment of the
subnotochordal rod); al. throat; ht. heart; pp. body cavity;
so. somatic mesoblast; sf. splanchnic mesoblast; hy.
hypoblast.
Fig. 358. Transverse section through the head of a Rabbit of the
same age as fig. 144 B. (From Kölliker.)
B is a more highly magnified representation of part of A.
rf. medullary groove; mp. medullary plate; rw. medullary fold;
h. epiblast; dd. hypoblast; dd´. notochordal thickening of
hypoblast; sp. undivided mesoblast; hp. somatic mesoblast;
dfp. splanchnic mesoblast; ph. pericardial section of body
cavity; ahh. muscular wall of heart; ihh. epithelioid layer of
heart; mes. lateral undivided mesoblast; sw. part of the
hypoblast which will form the ventral wall of the pharynx.
The above description applies only to the development of the heart in
those types in which it is formed at a period after the throat has
become a closed tube (Elasmobranchii, Amphibia, Cyclostomata, Ganoids
(?)). In a number of other cases, in which the heart is formed before
the conversion of the throat into a closed tube, of which the most
notable is that of Mammals (Hensen, Götte, Kölliker), the heart arises
as two independent
[Pg 636] tubes (fig. 358), which eventually coalesce into an
unpaired structure.
In Mammals the two tubes out of which the heart is formed appear at
the sides of the cephalic plates, opposite the region of the mid- and
hind-brain (fig. 358). They arise at a time when the lateral folds
which form the ventral wall of the throat are only just becoming
visible. Each half of the heart originates in the same way as the
whole heart in Elasmobranchii, etc.; and the layer of the splanchnic
mesoblast, which forms the muscular wall for each part (ahh), has at
first the form of a half tube open below to the hypoblast.
Fig. 359. Two diagrammatic sections through the region of the
hind-brain of an embryo Chick of about 36 hours illustrating the
formation of the heart.
hb. hind-brain; nc. notochord; E. epiblast; so.
somatopleure; sp. splanchnopleure; d. alimentary tract; hy.
hypoblast; hz. heart; of. vitelline veins.
On the formation of the lateral folds of the splanchnic walls, the two
halves of the heart become carried inwards and downwards, and
eventually
[Pg 637] meet on the ventral side of the throat. For a short time
they here remain distinct, but soon coalesce into a single tube.
In Birds, as in Mammals, the heart makes its appearance as two tubes,
but arises at a period when the formation of the throat is very much
more advanced than in the case of Mammals. The heart arises
immediately behind the point up to which the ventral wall of the
throat is established and thus has at first a Lambda-shaped form. At
the apex of the Lambda, which forms the anterior end of the heart, the
two halves are in contact (fig. 357), though they have not coalesced;
while behind they diverge to be continued as the vitelline veins. As
the folding in of the throat is continued backwards the two limbs of
the heart are brought together and soon coalesce from before backwards
into a single structure. Fig. 359 A and B shews the heart during this
process. The two halves have coalesced anteriorly (A) but are still
widely separated behind (B). In Teleostei the heart is formed as in
Birds and Mammals by the coalescence of two tubes, and it arises
before the formation of the throat.
The fact that the heart arises in so many instances as a double tube
might lead to the supposition that the ancestral Vertebrate had two
tubes in the place of the present unpaired heart.
The following considerations appear to me to prove that this
conclusion cannot be accepted. If the folding in of the
splanchnopleure to form the throat were deferred relatively to the
formation of the heart, it is clear that a modification in the
development of the heart would occur, in that the two halves of the
heart would necessarily be formed widely apart, and only eventually
united on the folding in of the wall of the throat. It is therefore
possible to explain the double formation of the heart without having
recourse to the above hypothesis of an ancestral Vertebrate with two
hearts. If the explanation just suggested is the true one the heart
should only be formed as two tubes when it arises prior to the
formation of the throat, and as a single tube when formed after the
formation of the throat. Since this is invariably found to be so, it
may be safely concluded that the formation of the heart as two
cavities is a secondary mode of development, which has been brought
about by variations in the period of the closing in of the wall of the
throat.
The heart arises continuously with the sinus venosus, which in the
Amniotic Vertebrata is directly continued into the vitelline veins.
Though at first it ends blindly in front, it is very soon connected
with the foremost aortic arches.
[Pg 638]
The simple tubular heart, connected as above described, grows more
rapidly than the chamber in which it is contained, and is soon doubled
upon itself, acquiring in this way an S-shaped curvature, the
posterior portion being placed dorsally, and the anterior ventrally. A
constriction soon appears between the dorsal and ventral portions.
The dorsal section becomes partially divided off behind from the sinus
venosus, and constitutes the relatively thin-walled auricular section
of the heart; while the ventral portion, after becoming distinct
anteriorly from a portion continued forwards from it to the origin of
the branchial arteries, which may be called the truncus arteriosus,
acquires very thick spongy muscular walls, and becomes the ventricular
division of the heart.
The further changes in the heart are but slight in the case of the
Pisces. A pair of simple membranous valves becomes established at the
auriculo-ventricular orifice, and further changes take place in the
truncus arteriosus. This part becomes divided in Elasmobranchii,
Ganoidei, and Dipnoi into a posterior section, called the conus
arteriosus, provided with a series of transverse rows of valves, and
an anterior section, called the bulbus arteriosus, not provided with
valves, and leading into the branchial arteries. In most Teleostei
(except Butirinus and a few other forms) the conus arteriosus is all
but obliterated, and the anterior row of its valves alone preserved;
and the bulbus is very much enlarged[222].
In the Dipnoi important changes in the heart are effected, as compared
with other Fishes, by the development of true lungs. Both the
auricular and ventricular chamber may be imperfectly divided into two,
and in the conus a partial longitudinal septum is developed in
connection with a longitudinal row of valves[223].
In Amphibia the heart is in many respects similar to that of the
Dipnoi. Its curvature is rather that of a screw than of a simple S.
The truncus arteriosus lies to the left, and is continued into the
ventricle which lies ventrally and more to the right, and this again
into the dorsally placed auricular section.
After the heart has reached the piscine stage, the auricular section
(Bombinator) becomes prolonged into a right and left auricular
appendage. A septum next grows from the roof of the auricular portion
of the heart
[Pg 639] obliquely backwards and towards the left, and divides it
in two chambers; the right one of which remains continuous with the
sinus venosus, while the left one is completely shut off from the
sinus, though it soon enters into communication with the newly
established pulmonary veins. The truncus arteriosus[224]
is divided
into a posterior conus arteriosus (pylangium) and an anterior
bulbus (synangium). The former is provided with a proximal row of
valves at its ventricular end, and a distal row at its anterior end
near the bulbus. It is also provided with a longitudinal septum, which
is no doubt homologous with the septum in the conus arteriosus of the
Dipnoi. The bulbus is well developed in many Urodela, but hardly
exists in the Anura.
In the Amniota further changes take place in the heart, resulting in
the abortion of the distal rows of valves of the conus
arteriosus[225],
and in the splitting up of the whole truncus
arteriosus into three vessels in Reptilia, and two in Birds and
Mammals, each opening into the ventricular section of the heart, and
provided with a special set of valves at its commencement. In Birds
and Mammals the ventricle becomes moreover completely divided into two
chambers, each communicating with one of the divisions of the
primitive truncus, known in the higher types as the systemic and
pulmonary aortæ. The character of the development of the heart in the
Amniota will be best understood from a description of what takes place
in the Chick.
In Birds the originally straight heart (fig. 109) soon becomes doubled
up upon itself. The ventricular portion becomes placed on the ventral
and right side, while the auricular section is dorsal and to the left.
The two parts are separated from each other by a slight constriction
known as the canalis auricularis. Anteriorly the ventricular cavity is
continued into the truncus, and the venous or auricular portion of the
heart is similarly connected behind with the sinus venosus. The
auricular appendages grow out from the auricle at a very early period.
The general appearance of the heart, as seen from the ventral side on
the fourth day, is shewn in fig. 360. Although the external divisions
of the heart are well marked even before this stage, it is not till
the end of the third day that the internal partitions become apparent;
and, contrary to what might have been anticipated from the evolution
of these parts in the lower types, the ventricular septum is the first
to be established.
[Pg 640]
It commences on the third day as a crescentic ridge or fold springing
from the convex or ventral side of the rounded ventricular portion of
the heart, and on the fourth day grows rapidly across the ventricular
cavity towards the concave or dorsal side. It thus forms an incomplete
longitudinal partition, extending from the canalis auricularis to the
commencement of the truncus arteriosus, and dividing the twisted
ventricular tube into two somewhat curved canals, one more to the left
and above, the other to the right and below. These communicate with
each other, above the free edge of the partition, along its whole
length.
Fig. 360. Heart of a Chick on the fourth day of incubation viewed
from the ventral surface.
l.a. left auricular appendage; C.A. canalis auricularis; v.
ventricle; b. truncus arteriosus.
Externally the ventricular portion as yet shews no division into two
parts.
By the fifth day the venous end of the heart, though still lying
somewhat to the left and above, is placed as far forwards as the
arterial end, the whole organ appearing to be drawn together. The
ventricular septum is complete.
The apex of the ventricles becomes more and more pointed. In the
auricular portion a small longitudinal fold appears as the rudiment of
the auricular septum, while in the canalis auricularis, which is now
at its greatest length, there is also to be seen a commencement of the
valvular structures tending to separate the cavity of the auricles
from those of the ventricles.
About the 106th hour, a septum begins to make its appearance in the
truncus arteriosus in the form of a longitudinal fold, which according
to Tonge (No. 495) starts at the end of the truncus furthest removed
from the heart. It takes origin from the wall of the truncus between
the fourth and fifth pairs of arches, and grows downwards in such a
manner as to divide the truncus into two channels, one of which leads
from the heart to the third and fourth pairs of arches, and the other
to the fifth pair. Its course downwards is not straight but spiral,
and thus the two channels into which it divides the truncus arteriosus
wind spirally the one round the other.
At the time when the septum is first formed, the opening of the
truncus arteriosus into the ventricles is narrow or slit-like,
apparently in order to prevent the flow of the blood back into the
heart. Soon after the appearance of the septum, however, semilunar
valves (Tonge, No. 495) are developed from the wall of that portion of
the truncus which lies between the free edge of the septum and the
cavity of the ventricles[226].
[Pg 641]
The ventral and the dorsal pairs of valves are the first to appear:
the former as two small solid prominences separated from each other by
a narrow groove; the latter as a single ridge, in the centre of which
is a prominence indicating the point where the ridge will subsequently
become divided into two. The outer valves appear opposite each other,
at a considerably later period.
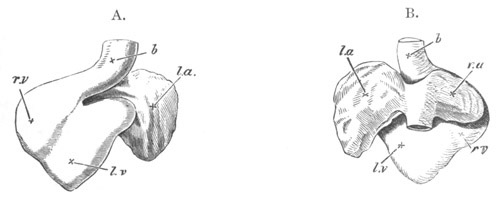
Fig. 361. Two views of the heart of a Chick upon the fifth day
of incubation.
A. from the ventral, B. from the dorsal side.
l.a. left auricular appendage; r.a. right auricular appendage;
r.v. right ventricle; l.v. left ventricle; b. truncus
arteriosus.
As the septum grows downwards towards the heart, it finally reaches
the position of these valves. One of its edges then passes between the
two ventral valves, and the other unites with the prominence on the
dorsal valve-ridge. At the same time the growth of all the parts
causes the valves to appear to approach the heart, and thus to be
placed quite at the top of the ventricular cavities. The free edge of
the septum of the truncus now fuses with the ventricular septum, and
thus the division of the truncus into two separate channels, each
provided with three valves, and each communicating with a separate
side of the heart, is complete; the position of the valves not being
very different from that in the adult heart.
That division of the truncus which opens into the fifth pair of arches
is the one which communicates with the right ventricle, while that
which opens into the third and fourth pairs communicates with the left
ventricle. The former becomes the pulmonary artery, the latter the
commencement of the systemic aorta.
The external constriction actually dividing the truncus into two
vessels does not begin to appear till the septum has extended some way
back towards the heart.
The semilunar valves become pocketed at a period considerably later
than their first formation (from the 147th to the 165th hour) in the
order of their appearance.
At the end of the sixth day, and even on the fifth day (figs. 361 and
362), the appearance of the heart itself, without reference to the
vessels which come from it, is not very dissimilar from that of the
adult. The original
[Pg 642] protuberance to the right now forms the apex of
the ventricles, and the two auricular appendages are placed at the
anterior extremity of the heart. The most noticeable difference (in
the ventral view) is the still externally undivided condition of the
truncus arteriosus.
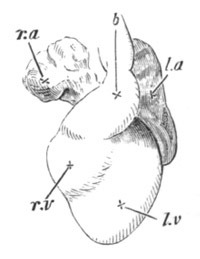
Fig. 362. Heart of a Chick upon the sixth day of incubation, from
the ventral surface.
l.a. left auricular appendage; r.a. right auricular appendage;
r.v. right ventricle; l.v. left ventricle; b. truncus
arteriosus.
The subsequent changes which the heart undergoes are concerned more
with its internal structure than with its external shape. Indeed,
during the next three days, viz. the eighth, ninth, and tenth, the
external form of the heart remains nearly unaltered.
In the auricular portion, however, the septum which commenced on the
fifth day becomes now more conspicuous. It is placed vertically, and
arises from the ventral wall; commencing at the canalis auricularis
and proceeding towards the opening into the sinus venosus.
This latter structure gradually becomes reduced so as to become a
special appendage of the right auricle. The inferior vena cava enters
the sinus obliquely from the right, so that its blood has a tendency
to flow towards the left auricle of the heart, which is at this time
the larger of the two.
The valves between the ventricles and auricles are now well developed,
and it is about this time that the division of the truncus arteriosus
into the aorta and pulmonary artery becomes visible from the exterior.
By the eleventh to the thirteenth day the right auricle has become as
large as the left, and the auricular septum much more complete, though
there is still a small opening, the foramen ovale, by which the two
cavities communicate with each other.
The most important feature in which the development of the Reptilian
heart differs from that of Birds is the division of the truncus into
three vessels, instead of two. The three vessels remain bound up in a
common sheath, and appear externally as a single trunk. The vessel not
represented in Birds is that which is continued into the left aortic
arch.
In Mammals the early stages in the development of the heart present no
important points of difference from those of Aves. The septa in the
truncus, in the ventricular, and in the auricular cavities are formed,
so far as is known, in the same way and at the same relative periods
in both groups. In the embryo Man, the Rabbit, and other Mammals the
division of the ventricles is made apparent externally by a deep
cleft, which, though evanescent in these forms, is permanent in the
Dugong.
The attachment of the auriculo-ventricular valves to the wall of the
ventricle, and the similar attachment of the left auriculo-ventricular
valves in Birds, have been especially studied by Gegenbaur and Bernays
(No. 492),
[Pg 643] and deserve to be noticed. In the primitive state the
ventricular walls have throughout a spongy character; and the
auriculo-ventricular valves are simple membranous projections like the
auriculo-ventricular valves of Fishes. Soon however the spongy
muscular tissue of both the ventricular and auricular walls, which at
first pass uninterruptedly the one into the other, grows into the
bases of the valves, which thus become in the main muscular
projections of the walls of the heart. As the wall of the ventricle
thickens, the muscular trabeculæ, connected at one end with the
valves, remain at the other end united with the ventricular wall, and
form special bands passing between the two. The valves on the other
hand lose their muscular attachment to the auricular walls. This is
the condition permanent in Ornithorhynchus. In higher Mammalia the
ends of the muscular bands inserted into the valves become fibrous,
from the development of intermuscular connective tissue, and the
atrophy of the muscular elements. The fibrous parts now form the
chordæ tendineæ, and the muscular the musculi papillares.
The sinus venosus in Mammals becomes completely merged into the right
auricle, and the systemic division of the truncus arteriosus is
apparently not homologous with that in Birds.
In the embryos of all the Craniata the heart is situated very far
forwards in the region of the head. This position is retained in
Pisces. In Amphibia the heart is moved further back, while in all the
Amniota it gradually shifts its position first of all into the region
of the neck and finally passes completely within the thoracic cavity.
The steps in the change of position may be gathered from figs. 109,
111, and 118.
Bibliography of the Heart.
(492) A. C. Bernays. “Entwicklungsgeschichte d.
Atrioventricularklappen.” Morphol. Jahrbuch, Vol. II. 1876.
(493) E. Gasser. “Ueber d. Entstehung d. Herzens beim Hühn.” Archiv
f. mikr. Anat., Vol. XIV.
(494) A. Thomson. “On the development of the vascular system of the
fœtus of Vertebrated Animals.” Edinb. New Phil. Journal, Vol. IX.
1830 and 1831.
(495) M. Tonge. “Observations on the development of the semilunar
valves of the aorta and pulmonary artery of the heart of the Chick.”
Phil. Trans. CLIX. 1869.
Vide also Von Baer (291), Rathke (300), Hensen (182), Kölliker
(298), Götte (296), and Balfour (292).
Arterial System.
In the embryos of Vertebrata the arterial system consists of a forward
continuation of the truncus arteriosus, on the ventral
[Pg 644] side of the
throat (figs. 363, abr, and 364, a), which, with a few exceptions
to be noticed below, divides into as many branches on each side as
there are visceral arches. These branches, after traversing the
visceral arches, unite on the dorsal side of the throat into a common
trunk on each side. This trunk (figs. 363 and 364) after giving off
one (or more) vessels to the head (c´ and c) turns backwards, and
bends in towards the middle line, close to its fellow, immediately
below the notochord (figs. 21 and 116) and runs backwards in this
situation towards the end of the tail. The two parallel trunks below
the notochord fuse very early into a single trunk, the dorsal aorta
(figs. 363, ad, and 364, a´´). There is given off from each
collecting trunk from the visceral arches, or from the commencement of
the dorsal aorta, a subclavian artery to each of the anterior limbs;
from near the anterior end of the dorsal aorta a vitelline artery (or
before the dorsal aortæ have united a pair of arteries fig. 125, R
of A and L of A) to the yolk-sack, which subsequently becomes the
main visceral artery[227];
and from the dorsal aorta opposite the hind
limbs one (or two) arteries on each side—the iliac arteries—to the
hind limbs; from these arteries the allantoic arteries are given off
in the higher types, which remain as the hypogastric arteries after
the disappearance of the allantois.
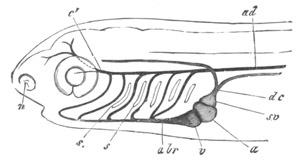
Fig. 363. Diagrammatic view of the head of an embryo Teleostean,
with the primitive vascular trunks. (From Gegenbaur.)
a. auricle; v. ventricle; abr. branchial artery; c´.
carotid; ad. dorsal aorta; s. branchial clefts; sv. sinus
venosus; dc. ductus Cuvieri; n. nasal pit.
The primitive arrangement of the arterial trunks is with a few
modifications retained in Fishes. With the development of the gills
the vessels to the arches become divided into two parts connected by a
capillary system in the gill folds, viz. into the
[Pg 645] branchial arteries
bringing the blood to the gills from the truncus arteriosus, and the
branchial veins transporting it to the dorsal aorta. The branchial
vessels to those arches which do not bear gills, either wholly or
partially atrophy; thus in Elasmobranchii the mandibular trunk, which
is fully developed in the embryo (fig. 193, 1av), atrophies, except
for a small remnant bringing blood to the rudimentary gill of the
spiracle from the branchial vein of the hyoid arch. In Ganoids the
mandibular artery atrophies, but the hyoid is usually preserved. In
Teleostei both mandibular[228]
and hyoid arteries are absent in the
adult, except that there is usually left a rudiment of the hyoid,
supplying the pseudobranch, which is similar to the rudiment of the
mandibular artery in Elasmobranchii. In Dipnoi the mandibular artery
atrophies, but the hyoid is sometimes preserved (Protopterus), and
sometimes lost.
In Fishes provided with a well developed air-bladder this organ
receives arteries, which arise sometimes from the dorsal aorta,
sometimes from the cæliac arteries, and sometimes from the dorsal
section of the last (fourth) branchial trunk. The latter origin is
found in Polypterus and Amia, and seems to have been inherited by the
Dipnoi where the air-bladder forms a true lung.
The pulmonary artery of all the air-breathing Vertebrata is derived
from the pulmonary artery of the Dipnoi.
In all the types above Fishes considerable changes are effected in the
primitive arrangement of the arteries in the visceral arches.
In Amphibia the piscine condition is most nearly retained[229].
The
mandibular artery is never developed, and the hyoid artery is
imperfect, being only connected with the cephalic vessels and never
directly joining the dorsal aorta. It is moreover developed later than
the arteries of the true branchial arches behind. The subclavian
arteries spring from the common trunks which unite to form the dorsal
aorta.
In the Urodela there are developed, in addition to the hyoid,
[Pg 646] four
branchial arteries. The three foremost of these at first supply gills,
and in the Perennibranchiate forms continue to do so through life. The
fourth does not supply a gill, and very early gives off, as in the
Dipnoi, a pulmonary branch.
The hyoid artery soon sends forward a lingual artery from its ventral
end, and is at first continued to the carotid which grows forward from
the dorsal part of the first branchial vessel.
In the Caducibranchiata, where the gills atrophy, the following
changes take place. The remnant of the hyoid is continued entirely
into the lingual artery. The first branchial is mainly continued into
the carotid and other cephalic branches, but a narrow remnant of the
trunk, which originally connected it with the dorsal aorta, remains,
forming what is known as a ductus Botalli. A rete mirabile on its
course is the remnant of the original gill.
The second and third branchial arches are continued as simple trunks
into the dorsal aorta, and the blood from the fourth arch mainly
passes to the lungs, but a narrow ductus Botalli still connects this
arch with the dorsal aorta.
In the Anura the same number of arches is present in the embryo as in
the Urodela, all four branchial arteries supplying branchiæ, but the
arrangement of the two posterior trunks is different from that in the
Urodela. The third arch becomes at a very early period continued into
a pulmonary vessel, a relatively narrow branch connecting it with the
second arch. The fourth arch joins the pulmonary branch of the third.
At the metamorphosis the hyoid artery loses its connection with the
carotid, and the only part of it which persists is the root of the
lingual artery. The first branchial artery ceases to join the dorsal
aorta, and forms the root of the carotid: the so-called carotid gland
placed on its course is the remnant of the gill supplied by it before
the metamorphosis.
The second artery forms a root of the dorsal aorta. The third, as in
all the Amniota, now supplies the lungs, and also sends off a
cutaneous branch. The fourth disappears. The connection of the
pulmonary artery with both the third and fourth branchial arches in
the embryo appears to me clearly to indicate that this artery was
primitively derived from the fourth arch as in the Urodela, and that
its permanent connection
[Pg 647] with the third arch in the Anura and in all
the Amniota is secondary.
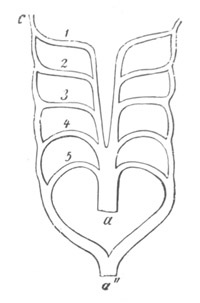
Fig. 364. Diagram of the arrangement of the arterial arches in an
embryo of one of the Amniota. (From Gegenbaur; after
Rathke.)
a. ventral aorta; a´´. dorsal aorta; 1, 2, 3, 4, 5. arterial
arches; c. carotid artery.
In the Amniota the metamorphosis of the arteries is in all cases very
similar. Five arches, viz. the mandibular, hyoid, and three branchial
arches are always developed (fig. 364), but, owing to the absence of
branchiæ, never function as branchial arteries. Of these the main
parts of the first two, connecting the truncus arteriosus with the
collecting trunk into which the arterial arches fall, always
disappear, usually before the complete development of the arteries in
the posterior arches.
The anterior part of the collecting trunk into which these vessels
fall is not obliterated when they disappear, but is on the contrary
continued forwards as a vessel supplying the brain, homologous with
that found in Fishes. It constitutes the internal carotid. Similarly
the anterior part of the trunk from which the mandibular and hyoid
arteries sprang is continued forwards as a small vessel[230],
which at
first passes to the oral region and constitutes in Reptiles the
lingual artery, homologous with the lingual artery of the Amphibia;
but in Birds and Mammals becomes more important, and is then known as
the external carotid (fig. 125). By these changes the roots of the
external and internal carotids spring respectively from the ventral
and dorsal ends of the primitive third artery, i.e. the artery of
the first branchial arch (fig. 365, c and c´); and thus this
arterial arch persists in all types as the common carotid,
[Pg 648] and the
basal part of the internal carotid. The trunk connecting the third
arterial arch with the system of the dorsal aorta persists in some
Reptiles (Lacertilia, fig. 366 A) as a ductus Botalli, but is lost in
the remaining Reptiles and in Birds and Mammals (fig. 366 B, C, D). It
disappears earliest in Mammals (fig. 365 C), later in Birds (fig. 365
B), and still later in the majority of Reptiles.
The fourth arch always continues to give rise, as in the Anura, to the
system of the dorsal aorta.
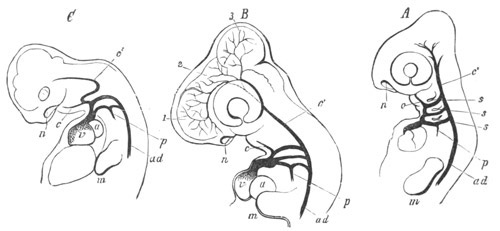
Fig. 365. Development of the great arterial trunks in the embryos
of A. a Lizard; B. the common Fowl; C. the Pig. (From Gegenbaur; after
Rathke.)
The first two arches have disappeared in all three. In A and B the
last three are still complete, but in C the last two are alone
complete.
p. pulmonary artery springing from the fifth arch, but still
connected with the system of the dorsal aorta by a ductus Botalli;
c. external carotid; c´. internal carotid; ad. dorsal aorta;
a. auricle; v. ventricle; n. nasal pit; m. rudiment of
fore-limb.
In all Reptiles it persists on both sides (fig. 366 A and B), but with
the division of the truncus arteriosus into three vessels one of
these, i.e. that opening furthest to the left side of the ventricle
(e and d), is continuous with the right fourth arch, and also
with the common carotid arteries (c); while a second springing from
the right side of the ventricle is continuous with the left fourth
arch (h and f). The right and left divisions of the fourth arch
meet however on the dorsal side of the œsophagus to give origin to
the dorsal aorta (g).
In Birds (fig. 366 C) the left fourth arch (h) loses its
connection with the dorsal aorta, though the ventral part remains as
[Pg 649]
the root of the left subclavian. The truncus arteriosus is moreover
only divided into two parts, one of which is continuous with all the
systemic arteries. Thus it comes about that in Birds the right fourth
arch (e) alone gives rise to the dorsal aorta.
In Mammals (fig. 366 D) the truncus arteriosus is only divided into
two, but the left fourth arch (e), instead of the right, is that
continuous with the dorsal aorta, and the right fourth arch (i) is
only continued into the right vertebral and right subclavian arteries.
The fifth arch always gives origin to the pulmonary artery (fig. 365,
p) and is continuous with one of the divisions of the truncus
arteriosus. In Lizards (fig. 366 A, i), Chelonians and Birds (fig.
366 C, i) and probably in Crocodilia, the right and left pulmonary
arteries spring respectively from the right and left fifth arches, and
during the greater part of embryonic life the parts of the fifth
arches between the origins of the pulmonary arteries and the system of
the dorsal aorta are preserved as ductus Botalli. These ductus Botalli
persist for life in the Chelonia. In Ophidia (fig. 366 B, h) and
Mammalia (fig. 366 D, m) only one of the fifth arches gives origin
to the two pulmonary arteries, viz. that on the right side in Ophidia,
and the left in Mammalia.
The ductus Botalli of the fifth arch (known in Man as the ductus
arteriosus) of the side on which the pulmonary arteries are formed,
may remain (e.g. in Man) as a solid cord connecting the common stern
of the pulmonary aorta with the systemic aorta.
The main history of the arterial arches in the Amniota has been
sufficiently dealt with, and the diagram, fig. 366, copied from
Rathke, shews at a glance the character of the metamorphosis these
arches undergo in the different types. It merely remains for me to say
a few words about the subclavian and vertebral arteries.
The subclavian arteries in Fishes usually spring from the trunks
connecting the branchial veins with the dorsal aorta. This origin,
which is also found in Amphibia, is typically found in the embryos of
the Amniota. In the Lizards this origin persists through life, but
both subclavians spring from the right
[Pg 650] side. In most other types the
origin of the subclavians is carried upwards, so that they usually
spring from a trunk common to them and the carotids (arteria anonyma)
(Birds and some Mammals); or the left one, as in Man and some other
Mammals, arises from the systemic aorta just beyond the carotids.
Various further modifications in the origin of the subclavians of the
same general nature are found in Mammalia,
[Pg 651] but they need not be
specified in detail. The vertebral arteries usually arise in close
connection with the subclavians, but in Birds they arise from the
common carotids.
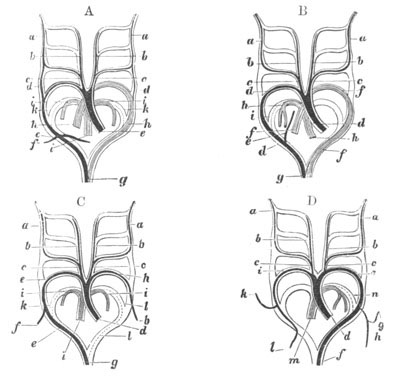
Fig. 366. Diagrams illustrating the metamorphosis of the arterial
arches in a Lizard A, a Snake B, a Bird C and a Mammal D. (From
Mivart; after Rathke.)
A. a. internal carotid; b. external carotid; c. common
carotid; d. ductus Botalli between the third and fourth arches;
e. right aortic trunk; f. subclavian; g. dorsal aorta; h.
left aortic trunk; i. pulmonary artery; k. rudiment of ductus
Botalli between the pulmonary artery and the system of the dorsal
aorta.
B. a. internal carotid; b. external carotid; c. common
carotid; d. right aortic trunk; e. vertebral artery; f. left
aortic trunk of dorsal aorta; h. pulmonary artery; i. ductus
Botalli of pulmonary artery.
C. a. internal carotid; b. external carotid; c. common
carotid; d. systemic aorta; e. fourth arch of right side (root
of dorsal aorta); f. right subclavian; g. dorsal aorta; h.
left subclavian (fourth arch of left side); i. pulmonary artery;
k. and l. right and left ductus Botalli of pulmonary arteries.
D. a. internal carotid; b. external carotid; c. common carotid;
d. systemic aorta; e. fourth arch of left side (root of dorsal
aorta); f. dorsal aorta; g. left vertebral artery; h. left
subclavian artery; i. right subclavian (fourth arch of right side);
k. right vertebral; l. continuation of right subclavian; m.
pulmonary artery; n. ductus Botalli of pulmonary artery.
Bibliography of the Arterial System.
(496) H. Rathke. “Ueb. d. Entwick. d. Arterien w. bei d. Säugethiere
von d. Bogen d. Aorta ausgehen.” Müller’s Archiv, 1843.
(497) H. Rathke. “Untersuchungen üb. d. Aortenwurzeln d. Saurier.”
Denkschriften d. k. Akad. Wien, Vol. XIII. 1857.
Vide also His (No. 232) and general works on Vertebrate Embryology.
The Venous System.
The venous system, as it is found in the embryos of Fishes, consists
in its earliest condition of a single large trunk, which traverses the
splanchnic mesoblast investing the part of the alimentary tract behind
the heart. This trunk is directly continuous in front with the heart,
and underlies the alimentary canal through both its præanal and
postanal sections. It is shown in section in fig. 367, v, and may be
called the subintestinal vein. This vein has been found in the embryos
of Teleostei, Ganoidei, Elasmobranchii and Cyclostomata, and runs
parallel to the dorsal aorta above, into which it is sometimes
continued behind (Teleostei, Ganoidei, etc.).
In Elasmobranch embryos the subintestinal vein terminates, as may be
gathered from sections (fig. 368, v.cau), shortly before the end of
the tail. The same series of sections also shews that at the cloaca,
where the gut enlarges and comes in contact with the skin, this vein
bifurcates, the two branches uniting into a single vein both in front
of and behind the cloaca.
In most Fishes the anterior part of this vein atrophies, the caudal
section alone remaining, but the anterior section of it persists in
the fold of the intestine in Petromyzon, and also remains in the
spiral valve of some Elasmobranchii. In Amphioxus, moreover, it forms,
as in the embryos of higher types, the main venous trunk, though even
here it is usually broken up into two or three parallel vessels.
It no doubt represents one of the primitive longitudinal trunks of the
vermiform ancestors of the Chordata. The heart and the branchial
artery constitute a specially modified anterior continuation of this
vein. The
[Pg 652] dilated portal sinus of Myxine is probably also part of it;
and if this is really rhythmically contractile[231]
the fact would be
interesting as shewing that this quality, which is now localised in
the heart, was once probably common to the subintestinal vessel for
its whole length.
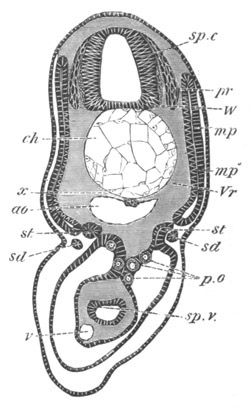
Fig. 367. Section through the trunk of a Scyllium embryo slightly
younger than 28 F.
sp.c. spinal canal; W. white matter of spinal cord; pr.
posterior nerve-roots; ch. notochord; x. subnotochordal rod;
ao. aorta; mp. muscle plate; mp´. inner layer of muscle-plate
already converted into muscles; Vr. rudiment of vertebral body;
st. segmental tube; sd. segmental duct; sp.v. spiral valve;
v. subintestinal vein; p.o. primitive generative cells.
On the development of the cardinal veins (to be described below)
considerable changes are effected in the subintestinal vein. Its
postanal section, which is known in the adult as the caudal vein,
unites with the cardinal veins. On this junction being effected
retrogressive changes take place in the præanal section of the
original subintestinal vessel. It breaks up in front into a number of
smaller vessels, the most important of which is a special vein, which
lies in the fold of the spiral valve, and which is more conspicuous in
some Elasmobranchii than in Scyllium, in which the development of the
vessel has been mainly studied. The lesser of the two branches
connecting it round the cloaca with the caudal vein first vanishes,
and then the larger; and the two posterior cardinals are left as the
sole forward continuations of the caudal vein. The latter then becomes
prolonged forwards, so that the two cardinals open into it some little
distance in front of the hind end of the kidneys. By these changes,
and by the disappearance of the postanal section of the gut, the
caudal vein is made to appear as a supraintestinal and not, as it
really is, a subintestinal vessel.
From the subintestinal vein there is given off a branch which supplies
the yolk-sack. This leaves the subintestinal vein close
[Pg 653] to the liver.
The liver, on its development, embraces the subintestinal vein, which
then breaks up into a capillary system in the liver, the main part of
its blood coming at this period from the yolk-sack.
The portal system is thus established from the subintestinal vein; but
is eventually joined by the various visceral, and sometimes by the
genital, veins as they become successively developed.
The blood from the liver is brought back to the sinus venosus by veins
known as the hepatic veins, which, like the hepatic capillary system,
are derivatives of the subintestinal vessel.
There join the portal system in Myxinoids and many Teleostei a number
of veins from the anterior abdominal walls, representing a
commencement of the anterior abdominal or epigastric vein of higher
types[232].
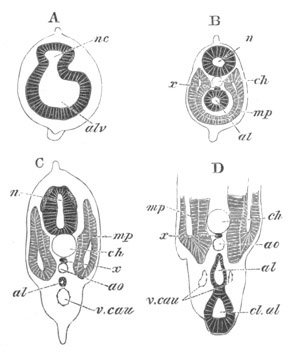
Fig. 368. Four sections through the postanal part of the tail
of an embryo of the same age as fig. 28 F.
A. is the posterior section.
nc. neural canal; al. postanal gut; alv. caudal vesicle of
postanal gut; x. subnotochordal rod; mp. muscle-plate; ch.
notochord; cl.al. cloaca; ao. aorta; v.cau. caudal vein.
In the higher Vertebrates the original subintestinal vessel never
attains a full development, even in the embryo. It is represented by
(1) the ductus
[Pg 654] venosus, which, like the true subintestinal vein, gives
origin (in the Amniota) to the vitelline veins to the yolk-sack, and
(2) by the caudal vein. Whether the partial atrophy of the
subintestinal vessel was primitively caused by the development of the
cardinal veins, or for some other reason, it is at any rate a fact
that in all existing Fishes the cardinal veins form the main venous
channels of the trunk.
Their later development than the subintestinal vessel as well as their
absence in Amphioxus, probably indicate that they became evolved, at
any rate in their present form, within the Vertebrate phylum.
The embryonic condition of the venous system, with a single large
subintestinal vein is, as has been stated, always modified by the
development of a paired system of vessels, known as the cardinal
veins, which bring to the heart the greater part of the blood from the
trunk.
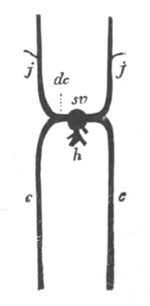
Fig. 369. Diagram of the paired venous system of a Fish.
(From Gegenbaur.)
j. jugular vein (anterior cardinal vein); c. posterior cardinal
vein; h. hepatic veins; sv. sinus venosus; dc. ductus
Cuvieri.
The cardinal veins appear in Fishes as four paired longitudinal trunks
(figs. 363 and 369), two anterior (j) and two posterior (c). They
unite into two transverse trunks on either side, known as the ductus
Cuvieri (dc), which fall into the sinus venosus, passing from the
body wall to the sinus by a lateral mesentery of the heart already
spoken of (p. 627, fig. 352). The anterior pair, known as the anterior
cardinal or jugular veins, bring to the heart the blood from the head
and neck. They are placed one on each side above the level of the
branchial arches (fig. 299, a.cv). The posterior cardinal veins lie
immediately dorsal to the mesonephros (Wolffian body), and are mainly
supplied by the blood from this organ and from the walls of the body
(fig. 275, c.a.v). In many forms (Cyclostomata, Elasmobranchii and
many Teleostei) they unite posteriorly with the caudal veins in the
manner already described, and in a large number of instances the
connecting branch between the two systems, in its passage through the
mesonephros, breaks up into a capillary network, and so gives rise to
a renal portal system.
The vein from the anterior pair of fins (subclavian) usually unites
with the anterior jugular vein.
[Pg 655]
The venous system of the Amphibia and Amniota always differs from that
of Fishes in the presence of a new vessel, the vena cava inferior,
which replaces the posterior cardinal veins; the latter only being
present, in their piscine form, during embryonic life. It further
differs from that of all Fishes, except the Dipnoi, in the presence of
pulmonary veins bringing back the blood directly from the lungs.
In the embryos of all the higher forms the general characters of the
venous system are at first the same as in Fishes, but with the
development of the vena cava inferior the front sections of the
posterior cardinal veins atrophy, and the ductus Cuvieri, remaining
solely connected with the anterior cardinals and their derivatives,
constitute the superior venæ cavæ. The inferior cava receives the
hepatic veins.
Apart from the non-development of the subintestinal vein the visceral
section of the venous system is very similar to that in Fishes.
The further changes in the venous system must be dealt with separately
for each group.
Amphibia. In Amphibia (Götte, No. 296) the anterior and posterior
cardinal veins arise as in Pisces. From the former the internal
jugular vein arises as a branch; the external jugular constituting the
main stem. The subclavian with its large cutaneous branch also springs
from the system of the anterior cardinal. The common trunk formed by
the junction of these three veins falls into the ductus Cuvieri.
The posterior cardinal veins occupy the same position as in Pisces,
and unite behind with the caudal veins, which Götte has shewn to be
originally situated below the postanal gut. The iliac veins unite with
the posterior cardinal veins, where the latter fall into the caudal
vein. The original piscine condition of the veins is not long
retained. It is first of all disturbed by the development of the
anterior part of the important unpaired venous trunk which forms in
the adult the vena cava inferior. This is developed independently, but
unites behind with the right posterior cardinal. From this point
backwards the two cardinal veins coalesce for some distance, to give
rise to the posterior section of the vena cava inferior, situated
between the kidneys[233].
The anterior sections of the cardinal veins
subsequently atrophy. The posterior part of the cardinal veins, from
their junction with the vena cava inferior to the caudal veins, forms
a rhomboidal figure. The iliac vein joins the outer angle of this
figure, and is thus in direct communication with the inferior vena
cava, but it is also connected with a longitudinal
[Pg 656] vessel on the outer
border of the kidneys, which receives transverse vertebral veins and
transmits their blood to the kidneys, thus forming a renal portal
system. The anterior limbs of the rhomboid formed by the cardinal
veins soon atrophy, so that the blood from the hind limbs can only
pass to the inferior vena cava through the renal portal system. The
posterior parts of the two cardinal veins (uniting in the Urodela
directly with the unpaired caudal vein) still persist. The iliac veins
also become directly connected with a new vein, the anterior abdominal
vein, which has meanwhile become developed. Thus the iliac veins
become united with the system of the vena cava inferior through the
vena renalis advehens on the outer border of the kidney, and with the
anterior abdominal veins by the epigastric veins.
The visceral venous system begins with the development of two
vitelline veins, which at first join the sinus venosus directly. They
soon become enveloped in the liver, where they break up into a
capillary system, which is also joined by the other veins from the
viscera. The hepatic system has in fact the same relations as in
Fishes. Into this system the anterior abdominal vein also pours itself
in the adult. This vein is originally formed of two vessels, which at
first fall directly into the sinus venosus, uniting close to their
opening into the sinus with a vein from the truncus arteriosus. They
become prolonged backwards, and after receiving the epigastric veins
above mentioned from the iliac veins, and also veins from the
allantoic bladder, unite behind into a single vessel. Anteriorly the
right vein atrophies and the left continues forward the unpaired
posterior section.
A secondary connection becomes established between the anterior
abdominal vein and the portal system; so that the blood originally
transported by the former vein to the heart becomes diverted so as to
fall into the liver. A remnant of the primitive connection is still
retained in the adult in the form of a small vein, the so-called vena
bulbi posterior, which brings the blood from the walls of the truncus
arteriosus directly into the anterior abdominal vein.
The pulmonary veins grow directly from the heart to the lungs.
For our knowledge of the development of the venous system of the
Amniota we are mainly indebted to Rathke.
Reptilia. As an example of the Reptilia the Snake may be selected, its
venous system having been fully worked out by Rathke in his important
memoir on its development (No. 300).
The anterior (external jugular) and posterior cardinal veins are
formed in the embryo as in all other types (fig. 370, vj and vc);
and the anterior cardinal, after giving rise to the anterior vertebral
and to the cephalic veins, persists with but slight modifications in
the adult; while the two ductus Cuvieri constitute the superior venæ
cavæ.
The two posterior cardinals unite behind with the caudal veins. They
are placed in the usual situation on the dorsal and outer border of
the kidneys.
[Pg 657]
With the development of the vena cava inferior, to be described below,
the blood from the kidneys becomes mainly transported by this vessel
to the heart; and the section of the posterior cardinals opening into
the ductus Cuvieri gradually atrophies, their posterior parts
remaining however on the outer border of the kidneys as the venæ
renales advehentes[234].
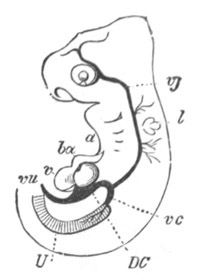
Fig. 370. Anterior portion of the venous system of an embryonic
Snake. (From Gegenbaur; after Rathke.)
vc. posterior cardinal vein; vj. jugular vein; DC. ductus
Cuvieri; vu. allantoic vein; v. ventricle; ba. truncus
arteriosus; a. visceral clefts; l. auditory vesicle.
While the front part of the posterior cardinal veins is undergoing
atrophy, the intercostal veins, which originally poured their blood
into the posterior cardinal veins, become also connected with two
longitudinal veins—the posterior vertebral veins—which are
homologous with the azygos and hemiazygos veins of Man; and bear the
same relation to the anterior vertebral veins that the anterior and
posterior cardinals do to each other.
These veins are at first connected by transverse anastomoses with the
posterior cardinals, but, on the disappearance of the front part of
the latter, the whole of the blood from the intercostal veins falls
into the posterior vertebral veins. They are united in front with the
anterior vertebral veins, and the common trunk of the two veins on
each side falls into the jugular vein.
The posterior vertebral veins are at first symmetrical, but after
becoming connected by transverse anastomoses, the right becomes the
more important of the two.
The vena cava inferior, though considerably later in its development
than the cardinals, arises fairly early. It constitutes in front an
unpaired trunk, at first very small, opening into the right allantoic
vein, close to the heart. Posteriorly it is continuous with two veins
placed on the inner border of the kidneys[235].
The vena cava inferior passes through the dorsal part of the liver,
and in doing so receives the hepatic veins.
The portal system is at first constituted by the vitelline vein, which
is directly continuous with the venous end of the heart, and at first
receives the two ductus Cuvieri, but at a later period unites with the
left ductus.
[Pg 658] It soon receives a mesenteric vein bringing the blood
from the viscera, which is small at first but rapidly increases in
importance.
The common trunk of the vitelline and mesenteric veins, which may be
called the portal vein, becomes early enveloped by the liver, and
gives off branches to this organ, the blood from which passes by the
hepatic veins to the vena cava inferior. As the branches in the liver
become more important, less and less blood is directly transported to
the heart, and finally the part of the original vitelline vein in
front of the liver is absorbed, and the whole of the blood from the
portal system passes from the liver into the vena cava inferior.
The last section of the venous system to be dealt with is that of the
anterior abdominal vein. There are originally, as in the Anura, two
veins belonging to this system, which owing to the precocious
development of the bladder to form the allantois, constitute the
allantoic veins (fig. 370, vu).
These veins, running along the anterior abdominal wall, are formed
somewhat later than the vitelline vein, and fall into the two ductus
Cuvieri. They unite with two epigastric veins (homologous with those
in the Anura), which connect them with the system of the posterior
cardinal veins. The left of the two eventually atrophies, so that
there is formed an unpaired allantoic vein. This vein at first
receives the vena cava inferior close to the heart, but eventually the
junction of the two takes place in the region of the liver, and
finally the anterior abdominal vein (as it comes to be after the
atrophy of the allantois) joins the portal system and breaks up into
capillaries in the liver[236].
In Lizards the iliac veins join the posterior cardinals, and so pour
part of their blood into the kidneys; they also become connected by
the epigastric veins with the system of the anterior abdominal or
allantoic vein. The subclavian veins join the system of the superior
venæ cavæ.
The venous system of Birds and Mammals differs in two important points
from that of Reptilia and Amphibia. Firstly the anterior abdominal
vein is only a fœtal vessel, forming during fœtal life the allantoic
vein; and secondly a direct connection is established between the vena
cava inferior and the veins of the hind limbs and posterior parts of
the cardinal veins, so that there is no renal portal system.
Aves. The Chick may be taken to illustrate the development of the
venous system in Birds.
On the third day, nearly the whole of the venous blood from the body
of the embryo is carried back to the heart by two main venous trunks,
the anterior (fig. 125, S.Ca.V) and posterior (V.Ca) cardinal
veins, joining on each side to form the short transverse ductus
Cuvieri (DC), both of which unite with the sinus venosus close to
the heart. As the head and neck continue to enlarge, and the wings
become developed, the single anterior
[Pg 659] cardinal or jugular vein (fig.
371, J), of each side, is joined by two new veins: the vertebral
vein, bringing back blood from the head and neck, and the subclavian
vein from the wing (W).
On the third day the posterior cardinal veins are the only veins which
return the blood from the hinder part of the body of the embryo.
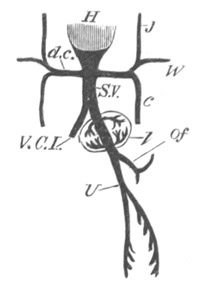
Fig. 371. Diagram of the venous circulation in the Chick at the
commencement of the fifth day.
H. heart; d.c. ductus Cuvieri. Into the ductus Cuvieri of each
side fall J. the jugular vein, W. the vein from the wing, and
c. the inferior cardinal vein; S.V. sinus venosus; Of.
vitelline vein; U. allantoic vein, which at this stage gives off
branches to the body-walls; V.C.I. inferior vena cava; l. liver.
About the fourth or fifth day, however, the vena cava inferior (fig.
371, V.C.I.) makes its appearance. This, starting from the sinus
venosus not far from the heart, is on the fifth day a short trunk
running backward in the middle line below the aorta, and speedily
losing itself in the tissues of the Wolffian bodies. When the true
kidneys are formed it also receives blood from them, and thenceforward
enlarging rapidly becomes the channel by which the greater part of the
blood from the hinder part of the body finds its way to the heart. In
proportion as the vena cava inferior increases in size, the posterior
cardinal veins diminish.
The blood originally coming to them from the posterior part of the
spinal cord and trunk is transported into two posterior vertebral
veins, similar to those in Reptilia, which are however placed dorsally
to the heads of the ribs, and join the anterior vertebral veins. With
their appearance the anterior parts of the posterior cardinals
disappear. The blood from the hind limbs becomes transported directly
through the kidney into the vena cava inferior, without forming a
renal portal system[237].
On the third day the course of the vessels from the yolk-sack is very
simple. The two vitelline veins, of which the right is already the
smaller, form the ductus venosus, from which, as it passes through the
liver on its way to the heart, are given off the two sets of venæ
advehentes and venæ revehentes (fig. 371).
With the appearance of the allantois on the fourth day, a new feature
is introduced. From the ductus venosus there is given off a vein which
quickly divides into two branches. These, running along the ventral
walls of the body from which they receive some amount of blood, pass
to the allantois. They are the allantoic veins (fig. 371, U)
homologous with the anterior abdominal vein of the lower types. They
unite in front to form a single vein, which becomes, by reason of the
rapid growth of the allantois, very long. The right branch soon
diminishes in size and finally disappears. Meanwhile the left on
reaching the allantois bifurcates; and, its two
[Pg 660] branches becoming
large and conspicuous, there still appear to be two main allantoic
veins. At its first appearance the allantoic vein seems to be but a
small branch of the vitelline, but as the allantois grows rapidly, and
the yolk-sack dwindles, this state of things is reversed, and the less
conspicuous vitelline appears as a branch of the larger allantoic
vein.
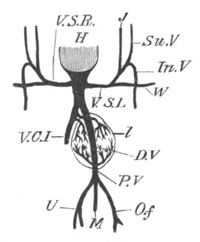
Fig. 372. Diagram of the venous circulation in the Chick during
the later days of incubation.
H. heart; V.S.R. right vena cava superior; V.S.L. left vena
cava superior. The two venæ cavæ superiores are the original ‘ductus
Cuvieri,’ they open into the sinus venosus. J. jugular vein;
Su.V. anterior vertebral vein; In.V. inferior vertebral vein;
W. subclavian; V.C.I. vena cava inferior; D.V. ductus venosus;
P.V. portal vein; M. mesenteric vein bringing blood from the
intestines into the portal vein; O.f. vitelline vein; U.
allantoic vein. The three last mentioned veins unite together to
form the portal vein; l. liver.
On the third day the blood returning from the walls of the intestine
is insignificant in amount. As however the intestine becomes more and
more developed, it acquires a distinct venous system, and its blood is
returned by veins which form a trunk, the mesenteric vein (fig. 372,
M) falling into the vitelline vein at its junction with the
allantoic vein.
These three great veins, in fact, form a large common trunk, which
enters at once into the liver, and which we may now call the portal
vein (fig. 372, P.V). This, at its entrance into the liver, partly
breaks up into the venæ advehentes, and partly continues as the
ductus venosus (D.V) straight through the liver, emerging from which
it joins the vena cava inferior. Before the establishment of the vena
cava inferior, the venæ revehentes, carrying back the blood which
circulates through the hepatic capillaries, join the ductus venosus
close to its exit from the liver. By the time however that the vena
cava has become a large and important vessel it is found that the venæ
revehentes, or as we may now call them the hepatic veins, have
shifted their embouchment, and now fall directly into that vein, the
ductus venosus making a separate junction rather higher up (fig. 372).
This state of things continues with but slight changes till near the
end of incubation, when the chick begins to breathe the air in the
air-chamber of the shell, and respiration is no longer carried on by
the allantois. Blood then ceases to flow along the allantoic vessels;
they become obliterated. The vitelline vein, which as the yolk becomes
gradually absorbed proportionately diminishes in size and importance,
comes to appear as a mere branch of the portal vein. The ductus
venosus becomes obliterated; and hence the whole of the blood coming
through the portal vein flows into the substance of the liver, and so
by the hepatic veins into the vena cava.
Although the allantoic (anterior abdominal) vein is obliterated in the
adult, there is nevertheless established an anastomosis between the
portal system and the veins bringing the blood from the limbs to the
vena cava
[Pg 661] inferior, in that the caudal vein and posterior pelvic veins
open into a vessel, known as the coccygeo-mesenteric vein, which joins
the portal vein; while at the same time the posterior pelvic veins are
connected with the common iliac veins by a vessel which unites with
them close to their junction with the coccygeo-mesenteric vein.
Mammalia. In Mammals the same venous trunks are developed in the
embryo as in other types (fig. 373 A). The anterior cardinals or
external jugulars form the primitive veins of the anterior part of the
body, and the internal jugulars and anterior vertebrals are
subsequently formed. The subclavians (fig. 373 A, s), developed on
the formation of the anterior limbs, also pour their blood into these
primitive trunks. In the lower Mammalia (Monotremata, Marsupialia,
Insectivora, some Rodentia, etc.), the two ductus Cuvieri remain as
the two superior venæ cavæ, but more usually an anastomosis arises
between the right and left innominate veins, and eventually the whole
of the blood of the left superior cava is carried to the right side,
and there is left only a single superior cava (fig. 373 B and C). A
small rudiment of the left superior cava remains however as the sinus
coronarius and receives the coronary vein from the heart (figs. 373 C,
cor and 374, cs).
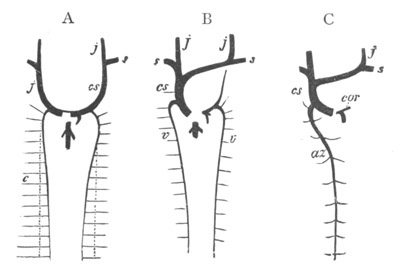
Fig. 373. Diagram of the development of the paired venous system of
Mammals (Man). (From Gegenbaur.)
j. jugular vein; cs. vena cava superior; s. subclavian veins;
c. posterior cardinal vein; v. vertebral vein; az. azygos
vein; cor. coronary vein.
A. Stage in which the cardinal veins have already disappeared. Their
position is indicated by dotted lines.
B. Later stage when the blood from the left jugular vein is carried
into the right to form the single vena cava superior; a remnant
of the left superior cava being however still left.
C. Stage after the left vertebral vein has disappeared; the right
vertebral remaining as the azygos vein. The coronary vein remains
as the last remnant of the left superior vena cava.
The posterior cardinal veins form at first the only veins receiving
the
[Pg 662] blood from the posterior part of the trunk and kidneys; and on the
development of the hind limbs receive the blood from them also.
As in the types already described an unpaired vena cava inferior
becomes eventually developed, and gradually carries off a larger and
larger portion of the blood originally returned by the posterior
cardinals. It unites with the common stem of the allantoic and
vitelline veins in front of the liver.
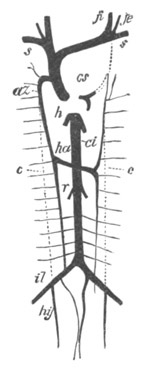
Fig. 374. Diagram of the chief venous trunks of Man. (From
Gegenbaur.)
cs. vena cava superior; s. subclavian vein; ji. internal
jugular; je. external jugular; az. azygos vein; ha. hemiazygos
vein; c. dotted line shewing previous position of cardinal veins;
ci. vena cava inferior; r. renal veins; il. iliac; hy.
hypogastric veins; h. hepatic veins.
The dotted lines shew the position of embryonic vessels aborted in
the adult.
At a later period a pair of trunks is established bringing the blood
from the posterior part of the cardinal veins and the crural veins
directly into the vena cava inferior (fig. 374, il). These vessels,
whose development has not been adequately investigated, form the
common iliac veins, while the posterior ends of the cardinal veins
which join them become the hypogastric veins (fig. 374, hy). Owing
to the development of the common iliac veins there is no renal portal
system like that of the Reptilia and Amphibia.
Posterior vertebral veins, similar to those of Reptilia and Birds, are
established in connection with the intercostal and lumbar veins, and
unite anteriorly with the front part of the posterior cardinal veins
(fig. 373 A)[238].
On the formation of the posterior vertebral veins, and as the inferior
vena cava becomes more important, the middle part of the posterior
cardinals becomes completely aborted (fig. 374, c), the anterior and
posterior parts still persisting, the former as the continuations of
the posterior vertebrals into the anterior vena cava (az), the
latter as the hypogastric veins (hy).
Though in a few Mammalia both the posterior vertebrals persist, a
transverse connection is usually established between them, and the one
(the right) becoming the more important constitutes the azygos vein
(fig. 374, az), the persisting part of the left forming the
hemiazygos vein (ha).
The remainder of the venous system is formed in the embryo of the
vitelline and allantoic veins, the former being eventually joined by
the mesenteric vein so as to constitute the portal vein.
[Pg 663]
The vitelline vein is the first part of this system established, and
divides near the heart into two veins bringing back the blood from the
yolk-sack (umbilical vesicle). The right vein soon however aborts.
The allantoic (anterior abdominal) veins are originally paired. They
are developed very early, and at first course along the still widely
open somatic walls of the body, and fall into the single vitelline
trunk in front. The right allantoic vein disappears before long, and
the common trunk formed by the junction of the vitelline and allantoic
veins becomes considerably elongated. This trunk is soon enveloped by
the liver.
The succeeding changes have been somewhat differently described by
Kölliker and Rathke. According to the former the common trunk of the
allantoic and vitelline veins in its passage through the liver gives
off branches to the liver, and also receives branches from this organ
near its anterior exit. The main trunk is however never completely
aborted, as in the embryos of other types, but remains as the ductus
venosus Arantii.
With the development of the placenta the allantoic vein becomes the
main source of the ductus venosus, and the vitelline or portal vein,
as it may perhaps be now conveniently called, ceases to join it
directly, but falls into one of its branches in the liver.
The vena cava inferior joins the continuation of the ductus venosus in
front of the liver, and, as it becomes more important, it receives
directly the hepatic veins which originally brought back blood into
the ductus venosus. The ductus venosus becomes moreover merely a small
branch of the vena cava.
At the close of fœtal life the allantoic vein becomes obliterated up
to its place of entrance into the liver; the ductus venosus becomes a
solid cord—the so-called round ligament—and the whole of the venous
blood is brought to the liver by the portal vein[239].
Owing to the allantoic (anterior abdominal) vein having merely a
fœtal existence an anastomosis between the iliac veins and the portal
system by means of the anterior abdominal vein is not established.
Bibliography of the Venous System.
(498) J. Marshall. “On the development of the great anterior veins.”
Phil. Trans., 1859.
(499) H. Rathke. “Ueb. d. Bildung d. Pfortader u. d. Lebervenen b.
Säugethieren.” Meckel’s Archiv, 1830.
(500) H. Rathke. “Ueb. d. Bau u. d. Entwick. d. Venensystems d.
Wirbelthiere.” Bericht. üb. d. naturh. Seminar. d. Univ. Königsberg,
1838.
Vide also Von Baer (No. 291), Götte (No. 296), Kölliker (No. 298),
and Rathke (Nos. 299, 300, and 301).
[Pg 664]Lymphatic System.
The lymphatic system arises from spaces in the general parenchyma of
the body, independent in their origin of the true body cavity, though
communicating both with this cavity and with the vascular system.
In all the true Vertebrata certain parts of the system form definite
trunks communicating with the venous system; and in the higher types
the walls of the main lymphatic trunks become quite distinct.
But little is known with reference to the ontogeny of the lymphatic
vessels, but they originate late in larval life, and have at first the
form of simple intercellular spaces.
The lymphatic glands appear to originate from lymphatic plexuses, the
cells of which produce lymph corpuscles. It is only in Birds and
Mammals, and especially in the latter, that the lymphatic glands form
definite structures.
The Spleen. The spleen, from its structure, must be classed with the
lymphatic glands, though it has definite relations to the vascular
system. It is developed in the mesoblast of the mesogastrium, usually
about the same time and in close connection with the pancreas.
According to Müller and Peremeschko the mass of mesoblast which forms
the spleen becomes early separated by a groove on the one side from
the pancreas and on the other from the mesentery. Some of its cells
become elongated, and send out processes which uniting with like
processes from other cells form the trabecular system. From the
remainder of the tissue are derived the cells of the spleen pulp,
which frequently contain more than one nucleus. Especial accumulations
of these cells take place at a later period to form the so-called
Malpighian corpuscles of the spleen.
Bibliography of Spleen.
(501) W. Müller. “The Spleen.” Stricker’s Histology.
(502) Peremeschko. “Ueb. d. Entwick. d. Milz.” Sitz. d. Wien. Akad.
Wiss., Vol. LVI. 1867.
Suprarenal bodies.
In Elasmobranch Fishes two distinct sets of structures are found, both
of which have been called suprarenal bodies. As shewn in the sequel
both of these structures probably unite in the higher types to form
the suprarenal bodies.
One of them consists of a series of paired bodies, situated on the
branches of the dorsal aorta, segmentally arranged, and forming a
chain extending from close behind the heart to the hinder end of the
body cavity. Each body is formed of a series of lobes, and exhibits a
well-marked distinction into a cortical layer of columnar cells, and a
medullary substance formed of irregular polygonal cells. As first
shewn by Leydig, they are
[Pg 665] closely connected with the sympathetic
ganglia, and usually contain numerous ganglion cells distributed
amongst the proper cells of the body.
The second body consists of an unpaired column of cells placed between
the dorsal aorta and unpaired caudal vein, and bounded on each side by
the posterior parts of the kidney. I propose to call it the interrenal
body. In front it overlaps the paired suprarenal bodies, but does not
unite with them. It is formed of a series of well-marked lobules, etc.
In the fresh state Leydig (No. 506) finds that “fat molecules form the
chief mass of the body, and one finds freely imbedded in them clear
vesicular nuclei.” As may easily be made out from hardened specimens
it is invested by a tunica propria, which gives off septa dividing it
into well-marked areas filled with polygonal cells. These cells
constitute the true parenchyma of the body. By the ordinary methods of
hardening, the oil globules, with which they are filled in the fresh
state, completely disappear.
The paired suprarenal bodies (Balfour, No. 292, pp. 242-244) are
developed from the sympathetic ganglia. These ganglia, shewn in an
early stage in fig. 380, sy.g, become gradually divided into a
ganglionic part and a glandular part. The former constitutes the
sympathetic ganglia of the adult; the latter the true paired
suprarenal bodies. The interrenal body is however developed (Balfour,
No. 292, pp. 245-247) from indifferent mesoblast cells between the two
kidneys, in the same situation as in the adult.
The development of the suprarenal bodies in the Amniota has been most
fully studied by Braun (No. 503) in the Reptilia.
In Lacertilia they consist of a pair of elongated yellowish bodies,
placed between the vena renalis revehens and the generative glands.
They are formed of two constituents, viz. (1) masses of brown cells
placed on the dorsal side of the organ, which stain deeply with
chromic acid, like certain of the cells of the suprarenals of
Mammalia, and (2) irregular cords, in part provided with a lumen,
filled with fat-like globules[240],
amongst which are nuclei. On
treatment with chromic acid the fat globules disappear, and the cords
break up into bodies resembling columnar cells.
The dorsal masses of brown cells are developed from the sympathetic
ganglia in the same way as the paired suprarenal bodies of the
Elasmobranchii, while the cords filled with fat-like globules are
formed of indifferent mesoblast cells as a thickening in the lateral
walls of the inferior vena cava, and the cardinal veins continuous
with it. The observations of Brunn (No. 504) on the Chick, and
Kölliker (No. 298, pp. 953-955) on the Mammal, add but little to those
of Braun. They shew that the greater part of the gland (the cortical
substance) in these two types is derived from the mesoblast, and that
the glands are closely connected with sympathetic ganglia; while
Kölliker also states that the posterior part of the organ is unpaired
in the embryo rabbit of 16 or 17 days.
The structure and development of what I have called the interrenal
body
[Pg 666] in Elasmobranchii so closely correspond with that of the
mesoblastic part of the suprarenal bodies of the Reptilia, that I have
very little hesitation in regarding them as homologous[241];
while the
paired bodies in Elasmobranchii, derived from the sympathetic ganglia,
clearly correspond with the part of the suprarenals of Reptilia having
a similar origin; although the anterior parts of the paired suprarenal
bodies of Fishes have clearly become aborted in the higher types.
In Elasmobranch Fishes we thus have (1) a series of paired bodies,
derived from the sympathetic ganglia, and (2) an unpaired body of
mesoblastic origin. In the Amniota these bodies unite to form the
compound suprarenal bodies, the two constituents of which remain,
however, distinct in their development. The mesoblastic constituent
appears to form the cortical part of the adult suprarenal body, and
the nervous constituent the medullary part.
Bibliography of the Suprarenal bodies.
(503) M. Braun. “Bau u. Entwick. d. Nebennieren bei Reptilien.”
Arbeit. a. d. zool.-zoot. Institut Würzburg, Vol. V. 1879.
(504) A. v. Brunn. “Ein Beitrag z. Kenntniss d. feinern Baues u. d.
Entwick. d. Nebennieren.” Archiv f. mikr. Anat., Vol. VIII. 1872.
(505) Fr. Leydig. Untersuch. üb. Fische u. Reptilien. Berlin, 1853.
(506) Fr. Leydig. Rochen u. Haie. Leipzig, 1852.
Vide also F. M. Balfour (No. 292), Kölliker (No. 298), Remak (No.
302), etc.
[Pg 667]
CHAPTER XXII.
THE MUSCULAR SYSTEM.
In all the Cœlenterata, except the Ctenophora, the contractile
elements of the body wall consist of filiform processes of ectodermal
or entodermal epithelial cells (figs. 375 and 376 B). The elements
provided with these processes, which were first discovered by
Kleinenberg, are known as myoepithelial cells. Their contractile parts
may either be striated (fig. 376) or non-striated (fig. 375). In some
instances the epithelial part of the cell may nearly abort, its
nucleus alone remaining (fig. 376 A); and in this way a layer of
muscles lying completely below the surface may be established.
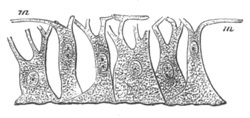
Fig. 375. Myo-epithelial cells of Hydra. (From Gegenbaur;
after Kleinenberg.)
m. contractile fibres.
There is embryological evidence of the derivation of the voluntary
muscular system of a large number of types from myoepithelial cells of
this kind. The more important of these groups are the Chætopoda, the
Gephyrea, the Chætognatha, the Nematoda, and the Vertebrata[242].
While there is clear evidence that the muscular system of a large
number of types is composed of cells which had their origin in
myoepithelial cells, the mode of evolution of the
[Pg 668] muscular system of
other types is still very obscure. The muscles may arise in the embryo
from amœboid or indifferent cells, and the Hertwigs[243]
hold that in
many of these instances the muscles have also phylogenetically taken
their origin from indifferent connective-tissue cells. The subject is
however beset with very serious difficulties, and to discuss it here
would carry me too far into the region of pure histology.
The voluntary muscular system of the Chordata.
The muscular fibres. The muscular elements of the Chordata undoubtedly
belong to the myoepithelial type. The embryonic muscle-cells are at
first simple epithelial cells, but soon become spindle-shaped: part of
their protoplasm becomes differentiated into longitudinally placed
striated muscular fibrils, while part, enclosing the nucleus, remains
indifferent, and constitutes the epithelial element of the cells. The
muscular fibrils are either placed at one side of the epithelial part
of the cell, or in other instances (the Lamprey, the Newt, the
Sturgeon, the Rabbit) surround it. The latter arrangement is shewn for
the Sturgeon in fig. 57.
Fig. 376. Muscle-cells of Lizzia Köllikeri. (From Lankester; after
O. and R. Hertwig.)
A. Muscle-cell from the circular fibres of the subumbrella.
B. Myoepithelial cells from the base of a tentacle.
The number of the fibrils of each cell gradually increases, and the
protoplasm diminishes, so that eventually only the nucleus, or nuclei
resulting from its division, are left. The products of each cell
probably give rise, in conjunction with a further division of the
nucleus, to a primitive bundle, which,
[Pg 669] except in Amphioxus,
Petromyzon, etc., is surrounded by a special investment of sarcolemma.
The voluntary muscular system. For the purposes of description the
muscular system of the Vertebrata may conveniently be divided into two
sections, viz. that of the head and that of the trunk. The main part,
if not the whole, of the muscular system of the trunk is derived from
certain structures, known as the muscle-plates, which take their
origin from part of the primitive mesoblastic somites.
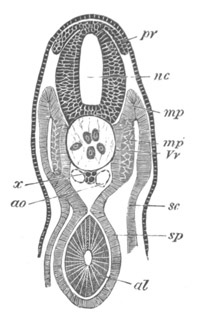
Fig. 377. Transverse section through the trunk of an embryo
slightly older than fig. 28 E.
nc. neural canal; pr. posterior root of spinal nerve; x.
subnotochordal rod; ao. aorta; sc. somatic mesoblast; sp.
splanchnic mesoblast; mp. muscle-plate; mp´. portion of
muscle-plate converted into muscle; Vr. portion of the vertebral
plate which will give rise to the vertebral bodies; al. alimentary
tract.
It has already been stated (pp. 292-296) that the mesoblastic somites
are derived from the dorsal segmented part of the primitive
mesoblastic plates. Since the history of these bodies is presented in
its simplest form in Elasmobranchii it will be convenient to commence
with this group. Each somite is composed of two layers—a somatic and
a splanchnic—both formed of a single row of columnar cells. Between
these two layers is a cavity, which is at first directly continuous
with the general body cavity, of which indeed it merely forms a
specialised part (fig. 377). Before long the cavity becomes however
completely constricted off from the permanent body cavity.
Very early (fig. 377) the inner or splanchnic wall of the somites
loses its simple constitution, owing to the middle part of it
undergoing peculiar changes. The meaning of the changes is at once
shewn by longitudinal horizontal sections, which prove (fig. 378) that
the cells in this situation (mp´) have become extended in a
longitudinal direction, and, in fact, form typical spindle-shaped
embryonic muscle-cells, each with a large nucleus. Every muscle-cell
extends for the whole length of a somite. The inner layer of each
somite, immediately within the muscle-band just described, begins to
proliferate, and produce
[Pg 670] a mass of cells, placed between the muscles
and the notochord (Vr). These cells form the commencing vertebral
bodies, and have at first (fig. 378) the same segmentation as the
somites from which they sprang.
After the separation of the vertebral bodies from the somites the
remaining parts of the somites may be called muscle-plates; since they
become directly converted into the whole voluntary muscular system of
the trunk (fig. 379, mp).
According to the statements of Bambeke and Götte, the Amphibians
present some noticeable peculiarities in the development of their
muscular system, in that such distinct muscle-plates as those of other
vertebrate types are not developed. Each side-plate of mesoblast is
divided into a somatic and a splanchnic layer, continuous throughout
the vertebral and parietal portions of the plate. The vertebral
portions (somites) of the plates soon become separated from the
parietal, and form independent masses of cells constituted of two
layers, which were originally continuous with the somatic and
splanchnic layers of the parietal plates (fig. 79). The outer or
somatic layer of the vertebral plates is formed of a single row of
cells, but the inner or splanchnic layer is made up of a kernel of
cells on the side of the somatic layer and an inner layer. The kernel
of the splanchnic layer and the outer or somatic layer together
correspond to a muscle-plate of other Vertebrata, and exhibit a
similar segmentation.
Osseous Fishes are stated to agree with Amphibians in the development
of their somites and muscular systems[244],
but further observations
on this point are required.
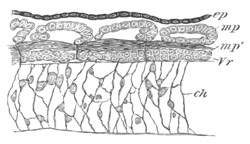
Fig. 378. Horizontal section through the trunk of an embryo of
Scyllium considerably younger than 28 F.
ch. notochord; ep. epiblast; Vr. rudiment of vertebral body;
mp. muscle-plate; mp´. portion of muscle-plate already
differentiated into longitudinal muscles.
In Birds the horizontal splitting of the mesoblast extends at first to
the dorsal summit of the mesoblastic plates, but after the isolation
of the somites the split between the somatic and splanchnic layers
becomes to a large extent obliterated, though in the anterior somites
it appears in part to persist. The somites on the second day, as seen
in a transverse section (fig. 115, P.v.), are somewhat quadrilateral
in form but broader than they are deep.
Each at that time consists of a somewhat thick cortex of radiating
[Pg 671]
rather granular columnar cells, enclosing a small kernel of spherical
cells. They are not, as may be seen in the above figure, completely
separated from the ventral (or lateral as they are at this period)
parts of the mesoblastic plate, and the dorsal and outer layer of the
cortex of the somites is continuous with the somatic layer of
mesoblast, the remainder of the cortex, with the central kernel, being
continuous with the splanchnic layer. Towards the end of the second
and beginning of the third day the upper and outer layer of the
cortex, together probably with some of the central cells of the
kernel, becomes separated off as a muscle-plate (fig. 116). The
muscle-plate when formed (fig. 117) is found to consist of two layers,
an inner and an outer, which enclose between them an almost
obliterated central cavity; and no sooner is the muscle-plate formed
than the middle portion of the inner layer becomes converted into
longitudinal muscles. The avian muscle-plates have, in fact, precisely
the same constitution as those of Elasmobranchii. The central space is
clearly a remnant of the vertebral portion of the body cavity,
which, though it wholly or partially disappears in a previous stage,
reappears again on the formation of the muscle-plate.
The remainder of the somite, after the formation of the muscle-plate,
is of very considerable bulk; the cells of the cortex belonging to it
lose their distinctive characters, and the major part of it becomes
the vertebral rudiment.
In Mammalia the history appears to be generally the same as in
Elasmobranchii. The split which gives rise to the body cavity is
continued to the dorsal summit of the mesoblastic plates, and the
dorsal portions of the plates with their contained cavities become
divided into somites, and are then separated off from the ventral. The
later development of the somites has not been worked out with the
requisite care, but it would seem that they form somewhat cubical
bodies in which all trace of the primitive slit is lost. The further
development resembles that in Birds.
The first changes of the mesoblastic somites and the formation of the
muscle-plates do not, according to existing statements, take place on
quite the same type throughout the Vertebrata, yet the comparison
which has been instituted between Elasmobranchs and other Vertebrates
appears to prove that there are important common features in their
development, which may be regarded as primitive, and as having been
inherited from the ancestors of Vertebrates. These features are (1)
the extension of the body cavity into the vertebral plates, and
subsequent enclosure of this cavity between the two layers of the
muscle-plates; (2) the primitive division of the vertebral plate into
an outer (somatic) and an inner (splanchnic) layer, and the formation
of a large part of the voluntary muscular system out of the inner
[Pg 672]
layer, which in all cases is converted into muscles earlier than the
outer layer.
The conversion of the muscle-plates into muscles. It will be
convenient to commence this subject with a description of the changes
which take place in such a simple type as that of the Elasmobranchii.
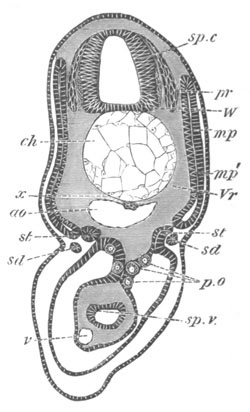
Fig. 379. Section through the trunk of a Scyllium embryo slightly
younger than 28 F.
sp.c. spinal canal; W. white matter of spinal cord; pr.
posterior nerve-roots; ch. notochord; x. subnotochordal rod;
ao. aorta; mp. muscle-plate; mp´. inner layer of muscle-plate
already converted into muscles; Vr. rudiment of vertebral body;
st. segmental tube; sd. segmental duct; sp.v. spiral valve;
v. subintestinal vein; p.o. primitive generative cells.
At the time when the muscle-plates have become independent structures
they form flat two-layered oblong bodies enclosing a slit-like central
cavity (fig. 379, mp). The outer or somatic wall is formed of simple
epithelial-like cells. The inner or splanchnic wall has however a
somewhat complicated structure. It is composed dorsally and ventrally
of a columnar epithelium, but in its middle portion of the
muscle-cells previously spoken of. Between these and the central
cavity of the plates the epithelium forming the remainder of the layer
commences to insert itself; so that between the first-formed muscle
and the cavity of the muscle-plate there appears a thin layer of
cells, not however continuous throughout.
When first formed the muscle-plates, as viewed from the exterior, have
nearly straight edges; soon however they become bent in the middle, so
that the edges have an obtusely angular form, the apex of the angle
being directed forwards. They are so arranged that the anterior edge
of the one plate fits into the posterior edge of the one in front. In
the lines of junction between the plates layers of connective-tissue
cells appear, which form the commencements of the intermuscular septa.
The growth of the plates is very rapid, and their upper ends
[Pg 673] soon
extend to the summit of the neural canal, and their lower ones nearly
meet in the median ventral line. The original band of muscles, whose
growth at first is very slow, now increases with great rapidity, and
forms the nucleus of the whole voluntary muscular system (fig. 380,
mp´). It extends upwards and downwards by the continuous conversion
of fresh cells of the splanchnic layer into muscle-cells. At the same
time it grows rapidly in thickness by the addition of fresh
spindle-shaped muscle-cells from the somatic layer as well as by the
division of the already existing cells.
Thus both layers of the muscle plate are concerned in forming the
great longitudinal lateral muscles, though the splanchnic layer is
converted into muscles very much sooner than the somatic[245].
Each muscle-plate is at first a continuous structure, extending from
the dorsal to the ventral surface, but after a time it becomes divided
by a layer of connective tissue, which becomes developed nearly on a
level with the lateral line, into a dorso-lateral and a ventro-lateral
section. The ends of the muscle-plates continue for a long time to be
formed of undifferentiated columnar cells. The complicated outlines of
the intermuscular septa become gradually established during the later
stages of development, causing the well-known appearances of the
muscles in transverse sections, which require no special notice here.
[Pg 674]
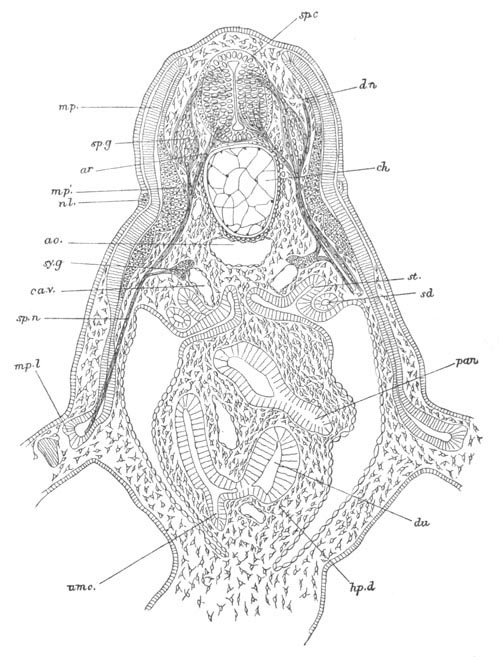
Fig. 380. Transverse section through the anterior part of the trunk
of an embryo of Scyllium slightly older than fig. 29 B.
The section is diagrammatic in so far that the anterior nerve-roots
have been inserted for the whole length; whereas they join the
spinal cord halfway between two posterior roots.
sp.c. spinal cord; sp.g. ganglion of posterior root; ar.
anterior root; dn. dorsally directed nerve springing from
posterior root; mp. muscle-plate; mp´. part of muscle-plate
already converted into muscles; m.pl. part of muscle-plate which
gives rise to the muscles of the limbs; nl. nervus lateralis;
ao. aorta; ch. notochord; sy.g. sympathetic ganglion; ca.v.
cardinal vein; sp.n. spinal nerve; sd. segmental (archinephric)
duct; st. segmental tube; du. duodenum; pan. pancreas; hp.d.
point of junction of hepatic duct with duodenum; umc. umbilical
canal.
The muscles of the limbs. The limb muscles are formed in
Elasmobranchii, coincidently with the cartilaginous skeleton, as two
bands of longitudinal fibres on the dorsal and ventral surfaces of the
limbs (fig. 346). The cells, from which these muscles originate, are
derived from the muscle-plates. When the ends of the muscle-plates
reach the level of the limbs they bend outwards and enter the tissue
of the limbs (fig. 380). Small portions of several muscle-plates
(m.pl) come in this way to be situated within the limbs, and are
very soon segmented off from the remainder of the muscle-plates. The
portions of the muscle-plates thus introduced soon lose their original
distinctness.
[Pg 675] There can however be but little doubt that they supply
the tissue for the muscles of the limbs. The muscle-plates themselves,
after giving off buds to the limbs, grow downwards, and soon cease to
shew any trace of having given off these buds.
In addition to the longitudinal muscles of the trunk just described,
which are generally characteristic of Fishes, there is found in
Amphioxus a peculiar transverse abdominal muscle, extending from the
mouth to the abdominal pore, the origin of which has not been made
out.
It has already been shewn that in all the higher Vertebrata
muscle-plates appear, which closely resemble those in Elasmobranchii;
so that all the higher Vertebrata pass through, with reference to
their muscular system, a fish-like stage. The middle portion of the
inner layers of their muscle-plates becomes, as in Elasmobranchii,
converted into muscles at a very early period, and the outer layer for
a long time remains formed of indifferent cells. That these
muscle-plates give rise to the main muscular system of the trunk, at
any rate to the episkeletal muscles of Huxley, is practically certain,
but the details of the process have not been made out.
In the Perennibranchiata the fish-like arrangement of muscles is
retained through life in the tail and in the dorso-lateral parts of
the trunk. In the tail of the Amniotic Vertebrata the primitive
arrangement is also more or less retained, and the same holds good for
the dorso-lateral trunk muscles of the Lacertilia. In the other
Amniota and the Anura the dorso-lateral muscles have become divided up
into a series of separate muscles, which are arranged in two main
layers. It is probable that the intercostal muscles belong to the same
group as the dorso-lateral muscles.
The abdominal muscles of the trunk, even in the lowest Amphibia,
exhibit a division into several layers. The recti abdominis are the
least altered part of this system, and usually retain indications of
the primitive intermuscular septa, which in many Amphibia and
Lacertilia are also to some extent preserved in the other abdominal
muscles.
In the Amniotic Vertebrates there is formed underneath the vertebral
column and the transverse processes a system of muscles, forming part
of the hyposkeletal system of Huxley, and called by Gegenbaur the
subvertebral muscles. The development of this system has not been
worked out, but on the whole I am inclined to believe that it is
derived from the muscle-plates. Kölliker, Huxley and other
embryologists believe however that these muscles are independent of
the muscle-plates in their origin.
[Pg 676]
Whether the muscle of the diaphragm is to be placed in the same
category as the hyposkeletal muscles has not been made out.
It is probable that the cutaneous muscles of the trunk are derived
from the cells given off from the muscle-plates. Kölliker however
believes that they have an independent origin.
The limb-muscles, both extrinsic and intrinsic, as may be concluded
from their development in Elasmobranchii, are derived from the
muscle-plates. Kleinenberg found in Lacertilia a growth of the
muscle-plates into the limbs, and in Amphibia Götte finds that the
outer layer of the muscle-plates gives rise to the muscles of the
limbs.
In the higher Vertebrata on the other hand the entrance of the
muscle-plates into the limbs has not been made out (Kölliker). It
seems therefore probable that by an embryological modification, of
which instances are so frequent, the cells which give rise to the
muscles of the limbs in the higher Vertebrata can no longer be traced
into a direct connection with the muscle-plates.
The Somites and muscular system of the head.
The extension of the somites to the anterior end of the body in
Amphioxus clearly proves that somites, similar to those of the trunk,
were originally present in a region, which in the higher Vertebrata
has become differentiated into the head. In the adult condition no
true Vertebrate exhibits indications of such somites, but in the
embryos of several of the lower Vertebrata structures have been found,
which are probably equivalent to the somites of the trunk: they have
been frequently alluded to in the previous chapters of this volume.
These structures have been most fully worked out in Elasmobranchii.
The mesoblast in Elasmobranch embryos becomes first split into somatic
and splanchnic layers in the region of the head; and between these
layers there are formed two cavities, one on each side, which end in
front opposite the blind anterior extremity of the alimentary canal;
and are continuous behind with the general body-cavity (fig. 20 A,
vp). I propose calling them the head-cavities. The cavities of the
two sides have no communication with each other.
Coincidently with the formation of an outgrowth from the throat to
form the first visceral cleft, the head-cavity on each side becomes
divided into a section in front of the cleft and a section behind the
cleft; and at a later period it becomes, owing to the formation of a
second cleft, divided into three sections:
[Pg 677] (1) a section in front of
the first or hyomandibular cleft; (2) a section in the hyoid arch
between the hyomandibular cleft and the hyobranchial or first
branchial cleft; (3) a section behind the first branchial cleft.
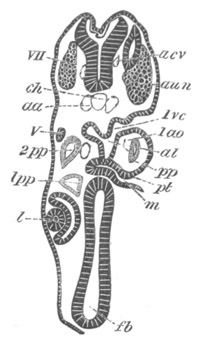
Fig. 381. Transverse section through the front part of the head of
a young Pristiurus embryo.
The section, owing to the cranial flexure, cuts both the fore- and
the hind-brain. It shews the premandibular and mandibular
head-cavities 1pp and 2pp, etc. The section is moreover somewhat
oblique from side to side.
fb. fore-brain; l. lens of eye; m. mouth; pt. upper end of
mouth, forming pituitary involution; 1ao. mandibular aortic arch;
1pp. and 2pp. first and second head-cavities; 1vc. first
visceral cleft; V. fifth nerve; aun. auditory nerve; VII.
seventh nerve; aa. dorsal aorta; acv. anterior cardinal vein;
ch. notochord.
The front section of the head-cavity grows forward, and soon becomes
divided, without the intervention of a visceral cleft, into an
anterior and posterior division. The anterior lies close to the eye,
and in front of the commencing mouth involution. The posterior part
lies completely within the mandibular arch.
As the rudiments of the successive visceral clefts are formed, the
posterior part of the head-cavity becomes divided into successive
sections, there being one section for each arch. Thus the whole
head-cavity becomes on each side divided into (1) a premandibular
section; (2) a mandibular section (vide fig. 29 A, pp); (3) a
hyoid section; (4) sections in each of the branchial arches.
The first of these divisions forms a space of a considerable size,
with epithelial walls of somewhat short columnar cells (fig. 381,
1pp). It is situated close to the eye, and presents a rounded or
sometimes a triangular figure in section. The two halves of the cavity
are prolonged ventralwards, and meet below the base of the fore-brain.
The connection between them appears to last for a considerable time.
These two cavities are the only parts of the body-cavity within the
head which unite ventrally. The section of the head-cavity just
described is so similar to the remaining sections that it must be
considered as serially homologous with them.
The next division of the head-cavity, which from its position
[Pg 678] may be
called the mandibular cavity, presents a spatulate shape, being
dilated dorsally, and produced ventrally into a long thin process
parallel to the hyomandibular gill-cleft (fig. 20, pp). Like the
previous space it is lined by a short columnar epithelium.
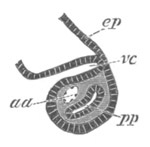
Fig. 382. Horizontal section through the penultimate visceral arch
of an embryo of Pristiurus.
ep. epiblast; vc. pouch of hypoblast which will form the walls
of a visceral cleft; pp. segment of body-cavity in visceral arch;
aa. aortic arch.
The mandibular aortic arch is situated close to its inner side (fig.
381, 2pp). After becoming separated from the lower part (Marshall),
the upper part of the cavity atrophies about the time of the
appearance of the external gills. Its lower part also becomes much
narrowed, but its walls of columnar cells persist. The outer or
somatic wall becomes very thin indeed, the splanchnic wall, on the
other hand, thickens and forms a layer of several rows of elongated
cells. In each of the remaining arches there is a segment of the
original body-cavity fundamentally similar to that in the mandibular
arch (fig. 382). A dorsal dilated portion appears, however, to be
present in the third or hyoid section alone (fig. 20), and even there
disappears very soon, after being segmented off from the lower part
(Marshall). The cavities in the posterior parts of the head become
much reduced like those in its anterior part, though at rather a later
period.
It has been shewn that the divisions of the body-cavity in the head,
with the exception of the anterior, early become atrophied, not so
however their walls. The cells forming the walls both of the dorsal
and ventral sections of these cavities become elongated, and finally
become converted into muscles. Their exact history has not been
followed in its details, but they almost unquestionably become the
musculus constrictor superficialis and musculus interbranchialis[246];
and probably also musculus levator mandibuli and other muscles of the
front part of the head.
The anterior cavity close to the eye remains unaltered much longer
than the remaining cavities.
[Pg 679]
Its further history is very interesting. In my original account of
this cavity (No. 292, p. 208) I stated my belief that its walls gave
rise to the eye-muscles, and the history of this process has been to
some extent worked out by Marshall in his important memoir (No. 509).
Marshall finds that the ventral portion of this cavity, where its two
halves meet, becomes separated from the remainder. The eventual fate
of this part has not however been followed. Each dorsal section
acquires a cup-like form, investing the posterior and inner surface of
the eye. The cells of its outer wall subsequently give rise to three
sets of muscles. The middle of these, partly also derived from the
inner walls of the cup, becomes the rectus internus of the eye, the
dorsal set forms the rectus superior, and the ventral the rectus
inferior. The obliquus inferior appears also to be in part developed
from the walls of this cavity.
Marshall brings evidence to shew that the rectus externus (as might be
anticipated from its nerve supply) has no connection with the walls of
the premandibular head-cavity, and finds that it arises close to the
position originally occupied by the second and third cavities.
Marshall has not satisfactorily made out the mode of development of
the obliquus superior.
The walls of the cavities, whose history has just been recorded, have
definite relations with the cranial nerves, an account of which has
already been given at p. 461.
Head-cavities, in the main similar to those of Elasmobranchii, have
been found in the embryo of Petromyzon (fig. 45, hc), the Newt
(Osborn and Scott), and various Reptilia (Parker).
Bibliography.
(507) G. M. Humphry. “Muscles in Vertebrate Animals.” Journ. of Anat.
and Phys., Vol. VI. 1872.
(508) J. Müller. “Vergleichende Anatomie d. Myxinoiden. Part I.
Osteologie u. Myologie.” Akad. Wiss., Berlin, 1834.
(509) A. M. Marshall. “On the head cavities and associated nerves of
Elasmobranchs.” Quart. J. of Micr. Science, Vol. XXI. 1881.
(510) A. Schneider. “Anat. u. Entwick. d. Muskelsystems d.
Wirbelthiere.” Sitz. d. Oberhessischen Gesellschaft, 1873.
(511) A. Schneider. Beiträge z. vergleich. Anat. u. Entwick. d.
Wirbelthiere. Berlin, 1879.
Vide also Götte (No. 296), Kölliker (No. 298), Balfour (No. 292),
Huxley, etc.
[Pg 680]
CHAPTER XXIII.
EXCRETORY ORGANS.
Excretory organs consist of coiled or branched and often ciliated
tubes, with an excretory pore opening on the outer surface of the
body, and as a rule an internal ciliated orifice placed in the
body-cavity. In forms provided with a true vascular system, there is a
special development of capillaries around the glandular part of the
excretory organs. In many instances the glandular cells of the organs
are filled with concretions of uric acid or some similar product of
nitrogenous waste.
There is a very great morphological and physiological similarity
between almost all the forms of excretory organ found in the animal
kingdom, but although there is not a little to be said for holding all
these organs to be derived from some common prototype, the attempt to
establish definite homologies between them is beset with very great
difficulties.
Platyelminthes. Throughout the whole of the Platyelminthes these
organs are constructed on a well-defined type, and in the Rotifera
excretory organs of a similar form to those of the Platyelminthes are
also present.
These organs (Fraipont, No. 513) are more or less distinctly paired,
and consist of a system of wide canals, often united into a network,
which open on the one hand into a pair of large tubes leading to the
exterior, and on the other into fine canals which terminate by
ciliated openings, either in spaces between the connective-tissue
cells (Platyelminthes), or in the body-cavity (Rotifera). The fine
canals open directly into the larger ones, without first uniting into
canals of an intermediate size.
[Pg 681]
The two large tubes open to the exterior, either by means of a median
posteriorly placed contractile vesicle, or by a pair of vesicles,
which have a ventral and anterior position. The former type is
characteristic of the majority of the Trematoda, Cestoda, and
Rotifera, and the latter of the Nemertea and some Trematoda. In the
Turbellaria the position of the external openings of the system is
variable, and in a few Cestoda (Wagner) there are lateral openings on
each of the successive proglottides, in addition to the terminal
openings. The mode of development of these organs is unfortunately not
known.
Mollusca. In the Mollusca there are usually present two independent
pairs of excretory organs—one found in a certain number of forms
during early larval life only[247],
and the other always present in
the adult.
The larval excretory organ has been found in the pulmonate Gasteropoda
(Gegenbaur, Fol[248],
Rabl), in Teredo (Hatschek), and possibly also
in Paludina. It is placed in the anterior region of the body, and
opens ventrally on each side, a short way behind the velum. It is
purely a larval organ, disappearing before the close of the veliger
stage. In the aquatic Pulmonata, where it is best developed, it
consists on each side of a V-shaped tube, with a dorsally-placed apex,
containing an enlargement of the lumen. There is a ciliated cephalic
limb, lined by cells with concretions, and terminating by an internal
opening near the eye, and a non-ciliated pedal limb opening to the
exterior[249].
Two irreconcilable views are held as to the development of this
system. Rabl (Vol. ii. No. 268) and Hatschek hold that it is developed
in the mesoblast; and Rabl states that in Planorbis it is formed from
the anterior mesoblast cells of the mesoblastic bands. A special
mesoblast cell on each side elongates into two processes, the
commencing limbs of the future organ. A lumen is developed in this
cell, which is continued into each limb, while
[Pg 682] the continuations of
the two limbs are formed by perforated mesoblast cells.
According to Fol these organs originate in aquatic Pulmonata as a pair
of invaginations of the epiblast, slightly behind the mouth. Each
invagination grows in a dorsal direction, and after a time suddenly
bends on itself, and grows ventralwards and forwards. It thus acquires
its V-shaped form.
In the terrestrial Pulmonata the provisional excretory organs are,
according to Fol, formed as epiblastic invaginations, in the same way
as those in the aquatic Pulmonata, but have the form of simple
non-ciliated sacks, without internal openings.
The permanent renal organ of the Mollusca consists typically of a pair
of tubes, although in the majority of the Gasteropoda one of the two
tubes is not developed. It is placed considerably behind the
provisional renal organ.
Each tube, in its most typical form, opens by a ciliated funnel into
the pericardial cavity, and has its external opening at the side of
the foot. The pericardial funnel leads into a glandular section of the
organ, the lining cells of which are filled with concretions. This
section is followed by a ciliated section, from which a narrow duct
leads to the exterior.
As to the development of this organ the same divergence of opinion
exists as in the case of the provisional renal organ.
Rabl’s careful observations on Planorbis (Vol. II. No. 268) tend to
shew that it is developed from a mass of mesoblast cells, near the end
of the intestine. The mass becomes hollow, and, attaching itself to
the epiblast on the left side of the anus, acquires an opening to the
exterior. Its internal opening is not established till after the
formation of the heart. Fol gives an equally precise account, but
states that the first rudiment of the organ arises as a solid mass of
epiblast cells. Lankester finds that this organ is developed as a
paired invagination of the epiblast in Pisidium, and Bobretzky also
derives it from the epiblast in marine Prosobranchiata. In Cephalopoda
on the other hand Bobretzky’s observations (I conclude this from his
figures) indicate that the excretory sacks of the renal organs are
derived from the mesoblast.
Polyzoa. Simple excretory organs, consisting of a pair of ciliated
canals, opening between the mouth and the anus, have
[Pg 683] been found by
Hatschek and Joliet in the Entoproctous Polyzoa, and are developed,
according to Hatschek, by whom they were first found in the larva,
from the mesoblast.
Brachiopoda. One or rarely two (Rhynchonella) pairs of canals, with
both peritoneal and external openings, are found in the Brachiopoda.
They undoubtedly serve as genital ducts, but from their structure are
clearly of the same nature as the excretory organs of the Chætopoda
described below. Their development has not been worked out.
Chætopoda. Two forms of excretory organ have been met with in the
Chætopoda. The one form is universally or nearly universally present
in the adult, and typically consists of a pair of coiled tubes
repeated in every segment. Each tube has an internal opening, placed
as a rule in the segment in front of that in which the greater part of
the organ and the external opening are situated.
There are great variations in the structure of these organs, which
cannot be dealt with here. It may be noted however that the internal
opening may be absent, and that there may be several internal
openings for each organ (Polynoe). In the Capitellidæ moreover
several pairs of excretory tubes have been shewn by Eisig (No. 512) to
be present in each of the posterior segments.
The second form of excretory organ has as yet only been found in the
larva of Polygordius, and will be more conveniently dealt with in
connection with the development of the excretory system of this form.
There is still considerable doubt as to the mode of formation of the
excretory tubes of the Chætopoda. Kowalevsky (No. 277), from his
observations on the Oligochæta, holds that they develop as outgrowths
of the epithelial layer covering the posterior side of the
dissepiments, and secondarily become connected with the epidermis.
Hatschek finds that in Criodrilus they arise from a continuous linear
thickening of the somatic mesoblast, immediately beneath the
epidermis, and dorsal to the ventral band of longitudinal muscles.
They break up into S-shaped cords, the anterior end of each of which
is situated in front of a dissepiment, and is formed at first of a
single large cell, while the posterior part is
[Pg 684] continued into the
segment behind. The cords are covered by a peritoneal lining, which
still envelopes them, when in the succeeding stage they are carried
into the body-cavity. They subsequently become hollow, and their
hinder ends acquire openings to the exterior. The formation of their
internal openings has not been followed.
Kleinenberg is inclined to believe that the excretory tubes take their
origin from the epiblast, but states that he has not satisfactorily
worked out their development.
The observations of Eisig (No. 512) on the Capitellidæ support
Kowalevsky’s view that the excretory tubes originate from the lining
of the peritoneal cavity.
Hatschek (No. 514) has given a very interesting account of the
development of the excretory system in Polygordius.
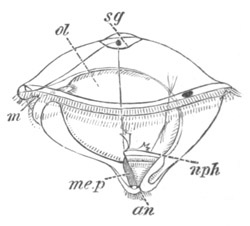
Fig. 383. Polygordius larva. (After Hatschek.)
m. mouth; sg. supraœsophageal ganglion; nph. nephridion;
me.p. mesoblastic band; an. anus; ol. stomach.
The excretory system begins to be formed, while the larva is still in
the trochospere stage (fig. 383, nph), and consists of a provisional
excretory organ, which is placed in front of the future segmented part
of the body, and occupies a position very similar to that of the
provisional excretory organ found in some Molluscan larvæ (vide p.
681).
Hatschek, with some shew of reason, holds that the provisional
excretory organs of Polygordius are homologous with those of the
Mollusca.
In its earliest stage the provisional excretory organ of Polygordius
consists of a pair of simple ciliated tubes, each with an anterior
funnel-like opening situated in the midst of the mesoblast cells, and
a posterior external opening. The latter is placed immediately in
front of what afterwards becomes the segmented region of the embryo.
While the larva is still unsegmented, a second internal opening is
formed for each tube (fig. 383, nph) and the two openings so formed
may eventually become divided into five (fig. 384 A), all
communicating by a single pore with the exterior.
When the posterior region of the embryo becomes segmented,
[Pg 685] paired
excretory organs are formed in each of the posterior segments, but the
account of their development, as given by Hatschek, is so remarkable
that I do not think it can be definitely accepted without further
confirmation.
From the point of junction of the two main branches of the larval
kidney there grows backwards (fig. 384 B), to the hind end of the
first segment, a very delicate tube, only indicated by its ciliated
lumen, its walls not being differentiated. Near the front end of this
tube a funnel, leading into the larval body cavity of the head, is
formed, and subsequently the posterior end of the tube acquires an
external opening, and the tube distinct walls. The communication with
the provisional excretory organ is then lost, and thus the excretory
tube of the first segment is established.
The excretory tubes in the second and succeeding segments are formed
in the same way as in the first, i.e. by the continuation of the
lumen of the hind end of the excretory tube from the preceding
segment, and the subsequent separation of this part as a separate
tube.
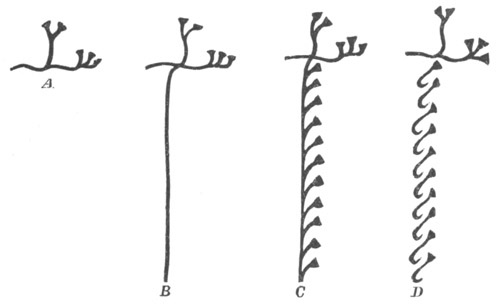
Fig. 384. Diagram illustrating the development of the excretory
system of Polygordius. (After Hatschek.)
The tube may be continued with a sinuous course through several
segments without a distinct wall. The external and internal openings
of the permanent excretory tubes are thus secondarily acquired. The
internal openings communicate with the permanent body-cavity. The
development of the permanent
[Pg 686] excretory tubes is diagrammatically
represented in fig. 384 C and D.
The provisional excretory organ atrophies during larval life.
If Hatschek’s account of the development of the excretory system of
Polygordius is correct, it is clear that important secondary
modifications must have taken place in it, because his description
implies that there sprouts from the anterior excretory organ, while it
has its own external opening, a posterior duct, which does not
communicate either with the exterior or with the body-cavity! Such a
duct could have no function. It is intelligible either (1) that the
anterior excretory organ should lead into a longitudinal duct, opening
posteriorly; that then a series of secondary openings into the
body-cavity should attach themselves to this, that for each internal
opening an external should subsequently arise, and the whole break up
into separate tubes; or (2) that behind an anterior provisional
excretory organ a series of secondary independent segmental tubes
should be formed. But from Hatschek’s account neither of these modes
of evolution can be deduced.
Gephyrea. The Gephyrea may have three forms of excretory organs, two
of which are found in the adult, and one, similar in position and
sometimes also in structure, to the provisional excretory organ of
Polygordius, has so far only been found in the larvæ of Echiurus and
Bonellia.
In all the Gephyrea the so-called ‘brown tubes’ are apparently
homologous with the segmented excretory tubes of Chætopods. Their main
function appears to be the transportation of the generative products
to the exterior. There is but a single highly modified tube in
Bonellia, forming the oviduct and uterus; a pair of tubes in the
Gephyrea inermia, and two or three pairs in most Gephyrea armata,
except Bonellia. Their development has not been studied.
In the Gephyrea armata there is always present a pair of posteriorly
placed excretory organs, opening in the adult into the anal extremity
of the alimentary tract, and provided with numerous ciliated
peritoneal funnels. These organs were stated by Spengel to arise in
Bonellia as outgrowths of the gut; but in Echiurus Hatschek (No.
515) finds that they are developed from the somatic mesoblast of the
terminal part of the trunk. They soon become hollow, and after
attaching themselves to the epiblast on each side of the anus, acquire
external openings. They are not at first provided with peritoneal
funnels, but these parts of the organs become developed from a ring of
cells at
[Pg 687] their inner extremities; and there is at first but a single
funnel for each vesicle. The mode of increase of the funnels has not
been observed, nor has it been made out how the organs themselves
become attached to the hindgut.
The provisional excretory organ of Echiurus is developed at an early
larval stage, and is functional during the whole of larval life. It at
first forms a ciliated tube on each side, placed in front of that part
of the larva which becomes the trunk of the adult. It opens to the
exterior by a fine pore on the ventral side, immediately in front of
one of the mesoblastic bands, and appears to be formed of perforated
cells. It terminates internally in a slight swelling, which represents
the normal internal ciliated funnel. The primitively simple excretory
organ becomes eventually highly complex by the formation of numerous
branches, each ending in a slightly swollen extremity. These branches,
in the later larval stages, actually form a network, and the inner end
of each main branch divides into a bunch of fine tubes. The whole
organ resembles in many respects the excretory organ of the
Platyelminthes.
In the larva of Bonellia Spengel has described a pair of provisional
excretory tubes, opening near the anterior end of the body, which are
probably homologous with the provisional excretory organs of Echiurus
(vide Vol. II., fig. 162 C, se).
Discophora. As in many of the types already spoken of, permanent and
provisional excretory organs may be present in the Discophora. The
former are usually segmentally arranged, and resemble in many respects
the excretory tubes of the Chætopoda. They may either be provided with
a peritoneal funnel (Nephelis, Clepsine) or have no internal opening
(Hirudo).
Bourne[250]
has shewn that the cells surrounding the main duct in the
medicinal Leech are perforated by a very remarkable network of
ductules, and the structure of these organs in the Leech is so
peculiar that it is permissible to state with due reserve their
homology with the excretory organs of the Chætopoda.
The excretory tubes of Clepsine are held by Whitman to be developed in
the mesoblast.
[Pg 688]
There are found in the embryos of Nephelis and Hirudo certain
remarkable provisional excretory organs the origin and history of
which are not yet fully made out. In Nephelis they appear as one
(according to Robin), or (according to Bütschli) as two successive
pairs of convoluted tubes on the dorsal side of the embryo, which are
stated by the latter author to develop from the scattered mesoblast
cells underneath the skin. At their fullest development they extend,
according to Robin, from close to the head to near the ventral sucker.
Each of them is U-shaped, with the open end of the U forwards, each
limb of the U being formed by two tubes united in front. No external
opening has been clearly made out. Fürbringer is inclined from his own
researches to believe that they open laterally. They contain a clear
fluid.
In Hirudo, Leuckart has described three similar pairs of organs, the
structure of which he has fully elucidated. They are situated in the
posterior part of the body, and each of them commences with an
enlargement, from which a convoluted tube is continued for some
distance backwards; the tube then turns forwards again, and after
bending again upon itself opens to the exterior. The anterior part is
broken up into a kind of labyrinthic network.
The provisional excretory organs of the Leeches cannot be identified
with the anterior provisional organs of Polygordius and Echiurus.
Arthropoda. Amongst the Arthropoda Peripatus is the only form with
excretory organs of the type of the segmental excretory organs of the
Chætopoda[251].
These organs are placed at the bases of the feet, in the lateral
divisions of the body-cavity, shut off from the main median division
of the body-cavity by longitudinal septa of transverse muscles.
Each fully developed organ consists of three parts:
(1) A dilated vesicle opening externally at the base of a foot. (2) A
coiled glandular tube connected with this, and subdivided again into
several minor divisions. (3) A short terminal portion opening at one
extremity into the coiled tube
[Pg 689] and at the other, as I believe, into
the body cavity. This section becomes very conspicuous, in stained
preparations, by the intensity with which the nuclei of its walls
absorb the colouring matter.
In the majority of the Tracheata the excretory organs have the form of
the so-called Malpighian tubes, which always (vide Vol. II.)
originate as a pair of outgrowths of the epiblastic proctodæum. From
their mode of development they admit of comparison with the anal
vesicles of the Gephyrea, though in the present state of our knowledge
this comparison must be regarded as somewhat hypothetical.
The antennary and shell-glands of the Crustacea, and possibly also the
so-called dorsal organ of various Crustacean larvæ appear to be
excretory, and the two former have been regarded by Claus and Grobben
as belonging to the same system as the segmental excretory tubes of
the Chætopoda.
Nematoda. Paired excretory tubes, running for the whole length of the
body in the so-called lateral line, and opening in front by a common
ventral pore, are present in the Nematoda. They do not appear to
communicate with the body cavity, and their development has not been
studied.
Very little is known with reference either to the structure or
development of excretory organs in the Echinodermata and the other
Invertebrate types of which no mention has been so far made in this
Chapter.
Excretory organs and generative ducts of the Craniata.
Although it would be convenient to separate, if possible, the history
of the excretory organs from that of the generative ducts, yet these
parts are so closely related in the Vertebrata, in some cases the same
duct having at once a generative and a urinary function, that it is
not possible to do so.
The excretory organs of the Vertebrata consist of three distinct
glandular bodies and of their ducts. These are (1) a small glandular
body, usually with one or more ciliated funnels opening into the body
cavity, near the opening of which there projects into the body cavity
a vascular glomerulus. It is situated very far forwards, and is
usually known as the head-kidney,
[Pg 690] though it may perhaps be more
suitably called, adopting Lankester’s nomenclature, the pronephros.
Its duct, which forms the basis for the generative and urinary ducts,
will be called the segmental duct.
(2) The Wolffian body, which may be also called the mesonephros. It
consists of a series of, at first, segmentally (with a few exceptions)
arranged glandular canals (segmental tubes) primitively opening at
one extremity by funnel-shaped apertures into the body cavity, and at
the other into the segmental duct. This duct becomes in many forms
divided longitudinally into two parts, one of which then remains
attached to the segmental tubes and forms the Wolffian or mesonephric
duct, while the other is known as the Müllerian duct.
(3) The kidney proper or metanephros. This organ is only found in a
completely differentiated form in the amniotic Vertebrata. Its duct is
an outgrowth from the Wolffian duct.
The above parts do not coexist in full activity in any living adult
member of the Vertebrata, though all of them are found together in
certain embryos. They are so intimately connected that they cannot be
satisfactorily dealt with separately.
Elasmobranchii. The excretory system of the Elasmobranchii is by no
means the most primitive known, but at the same time it forms a
convenient starting point for studying the modifications of the system
in other groups. The most remarkable peculiarity it presents is the
absence of a pronephros. The development of the Elasmobranch excretory
system has been mainly studied by Semper and myself.
The first trace of the system makes its appearance as a knob of
mesoblast, springing from the intermediate cell-mass near the level of
the hind end of the heart (fig. 385 A, pd). This knob is the
rudiment of the abdominal opening of the segmental duct, and from it
there grows backwards to the level of the anus a solid column of
cells, which constitutes the rudiment of the segmental duct itself
(fig. 385 B, pd). The knob projects towards the epiblast, and the
column connected with it lies between the mesoblast and epiblast. The
knob and column do not long remain solid, but the former acquires an
opening into the body cavity (fig. 421, sd) continuous with a lumen,
which
[Pg 691] makes its appearance in the column (fig. 386, sd). The knob
forms the only structure which can be regarded as a rudiment of the
pronephros.
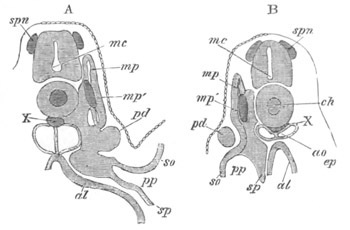
Fig. 385. Two sections of a Pristiurus embryo with three visceral
clefts.
The sections illustrate the development of the segmental duct (pd)
or primitive duct of the pronephros. In A (the anterior of the two
sections) this appears as a solid knob (pd) projecting towards the
epiblast. In B is seen a section of the column which has grown
backwards from the knob in A.
spn. rudiment of a spinal nerve; mc. medullary canal; ch.
notochord; X. subnotochordal rod; mp. muscle-plate; mp´.
specially developed portion of muscle-plate; ao. dorsal aorta;
pd. segmental duct; so. somatopleure; sp. splanchnopleure;
pp. body cavity; ep. epiblast; al. alimentary canal.
While the lumen is gradually being formed, the segmental tubes of the
mesonephros become established. They appear to arise as
differentiations of the parts of the primitive lateral plates of
mesoblast, placed between the dorsal end of the body cavity and the
muscle-plate (fig. 386, st)[252],
which are usually known as the
intermediate cell-masses.
The lumen of the segmental tubes, though at first very small, soon
becomes of a considerable size. It appears to be established in the
position of the section of the body cavity in the intermediate
cell-mass, which at first unites the part of the body cavity in the
muscle-plates with the permanent body cavity. The lumen of each tube
opens at its lower end into the dorsal part of the body cavity (fig.
386, st), and each tube curls obliquely
[Pg 692] backwards round the inner
and dorsal side of the segmental duct, near which it at first ends
blindly.
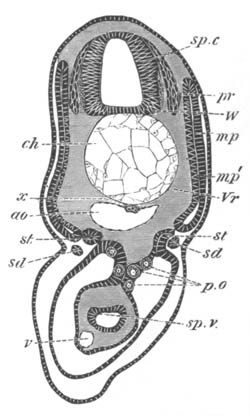
Fig. 386. Section through the trunk of a Scyllium embryo slightly
younger than 28 F.
sp.c. spinal canal; W. white matter of spinal cord; pr.
posterior nerve-roots; ch. notochord; x. subnotochordal rod;
ao. aorta; mp. muscle-plate; mp´. inner layer of muscle-plate
already converted into muscles; Vr. rudiment of vertebral body;
st. segmental tube; sd. segmental duct; sp.v. spiral valve;
v. subintestinal vein; p.o. primitive generative cells.
One segmental tube makes its appearance for each somite (fig. 265),
commencing with that immediately behind the abdominal opening of the
segmental duct, the last tube being situated a few segments behind the
anus. Soon after their formation the blind ends of the segmental tubes
come in contact with, and open into the segmental duct, and each of
them becomes divided into four parts. These are (1) a section carrying
the peritoneal opening, known as the peritoneal funnel, (2) a dilated
vesicle into which this opens, (3) a coiled tubulus proceeding from
(2), and terminating in (4) a wider portion opening into the segmental
duct. At the same time, or shortly before this, each segmental duct
unites with and opens into one of the horns of the cloaca, and also
retires from its primitive position between the epiblast and
mesoblast, and assumes a position close to the epithelium lining the
body cavity (fig. 380, sd). The general features of the excretory
organs at this period are diagrammatically represented in the woodcut
(fig. 387). In this fig. pd is the segmental duct and o its
abdominal opening; s.t points to the segmental tubes, the finer
details of whose structure are not represented in the diagram. The
mesonephros thus forms at this period an elongated gland composed of a
series of isolated coiled tubes, one extremity of each of which opens
into the body cavity, and the other into the segmental duct, which
forms the only duct of the system, and communicates at its front end
with the body cavity, and behind with the cloaca.
[Pg 693]
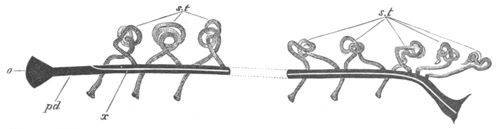
Fig. 387. Diagram of the primitive condition of the kidney in an
Elasmobranch embryo.
pd. segmental duct. It opens at o into the body cavity and at
its other extremity into the cloaca; x. line along which the
division appears which separates the segmental duct into the
Wolffian duct above and the Müllerian duct below; s.t. segmental
tubes. They open at one end into the body cavity, and at the other
into the segmental duct.
The next important change concerns the segmental duct, which becomes
longitudinally split into two complete ducts in the female, and one
complete duct and parts of a second duct in the male. The manner in
which this takes place is diagrammatically represented in fig. 387 by
the clear line x, and in transverse section in figs. 388 and 389.
The resulting ducts are (1) the Wolffian duct or mesonephric duct
(wd), dorsally, which remains continuous with the excretory tubules
of the mesonephros, and ventrally (2) the oviduct or Müllerian duct in
the female, and the rudiments of this duct in the male. In the female
the formation of these ducts takes place (fig. 389) by a nearly solid
rod of cells being gradually split off from the ventral side of all
but the foremost part of the original segmental duct. This nearly
solid cord is the Müllerian duct (od). A very small portion of the
lumen of the original segmental duct is perhaps continued into it, but
in any case it very soon acquires a wide lumen (fig. 389 A). The
anterior part of the segmental duct is not divided, but remains
continuous with the Müllerian duct, of which its anterior pore forms
the permanent peritoneal opening[253]
(fig. 387). The remainder of the
segmental duct (after the loss of its anterior section, and the part
split off from its ventral side) forms the Wolffian duct. The process
of formation of these ducts in the male differs from that in the
female chiefly
[Pg 694] in the fact of the anterior undivided part of the
segmental duct, which forms the front end of the Müllerian duct, being
shorter, and in the column of cells with which it is continuous being
from the first incomplete.
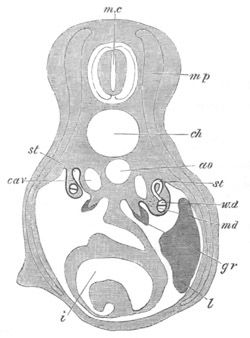
Fig. 388. Diagrammatic representation of a transverse section of a
Scyllium embryo illustrating the formation of the Wolffian and
Müllerian ducts by the longitudinal splitting of the segmental
duct.
mc. medullary canal; mp. muscle-plate; ch. notochord; ao.
aorta; cav. cardinal vein; st. segmental tube. On the left side
the section passes through the opening of a segmental tube into the
body cavity. On the right this opening is represented by dotted
lines, and the opening of the segmental tube into the Wolffian duct
has been cut through; w.d. Wolffian duct; m.d. Müllerian duct.
The section is taken through the point where the segmental duct and
Wolffian duct have just become separate; gr. the germinal ridge
with the thickened germinal epithelium; l. liver; i. intestine
with spiral valve.
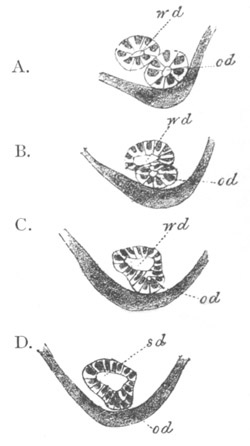
Fig. 389. Four sections through the anterior part of the segmental
duct of a female embryo of Scyllium canicula.
The figure shews how the segmental duct becomes split into the
Wolffian or mesonephric duct above, and Müllerian duct or oviduct
below.
wd. Wolffian or mesonephric duct; od. Müllerian duct or oviduct;
sd. segmental duct.
The segmental tubes of the mesonephros undergo further important
changes. The vesicle at the termination of each peritoneal funnel
sends a bud forwards towards the preceding tubulus, which joins the
fourth section of it close to the opening
[Pg 695] into the Wolffian duct (fig.
390, px). The remainder of the vesicle becomes converted into a
Malpighian body (mg). By the first of these changes a tube is
established connecting each pair of segments of the mesonephros, and
though this tube is in part aborted (or only represented by a fibrous
band) in the anterior part of the excretory organs in the adult, and
most probably in the hinder part, yet it seems almost certain that the
secondary and tertiary Malpighian bodies of the majority of segments
are developed from its persisting blind end. Each of these secondary
and tertiary Malpighian bodies is connected with a convoluted tubulus
(fig. 391, a.mg), which is also developed from the tube connecting
each pair of segmental tubes, and therefore falls into the primary
tubulus close to its junction with the
[Pg 696] segmental duct. Owing to the
formation of the accessory tubuli the segments of the mesonephros
acquire a compound character.
Fig. 390. Longitudinal vertical section through part of the
mesonephros of an embryo of Scyllium.
The figure contains two examples of the budding of the vesicle of a
segmental tube (which forms a Malpighian body in its own segment) to
unite with the tubulus in the preceding segment close to its opening
into the Wolffian (mesonephric) duct.
ge. epithelium of body-cavity; st. peritoneal funnel of
segmental tube with its peritoneal opening; mg. Malpighian body;
px. bud from Malpighian body uniting with preceding segment.
Fig. 391. Three segments of the anterior part of the mesonephros of a
nearly ripe embryo of Scyllium canicula as a transparent object.
The figure shews a fibrous band passing from the primary to the
secondary Malpighian bodies in two segments, which is the remains of
the outgrowth from the primary Malpighian body.
st.o. peritoneal funnel; p.mg. primary Malpighian body; a.mg.
accessory Malpighian body; w.d. mesonephric (Wolffian) duct.
The third section of each tubulus becomes by continuous growth,
especially in the hinder segments, very bulky and convoluted.
The general character of a slightly developed segment of the
mesonephros at its full growth may be gathered from fig. 391. It
commences with (1) a peritoneal opening, somewhat oval in form
(st.o) and leading directly into (2) a narrow tube, the segmental
tube, which takes a more or less oblique course backwards, and,
passing superficially to the Wolffian duct (w.d), opens into (3) a
Malpighian body (p.mg) at the anterior extremity of an isolated coil
of glandular tubuli. This coil forms the third section of each
segment, and starts from the Malpighian body. It consists of a
considerable number of rather definite convolutions, and after uniting
with tubuli from one, two, or more (according to the size of the
segment) accessory Malpighian bodies (a.mg) smaller than the one
into which the segmental tube falls, eventually opens by (4) a
narrowish collecting tube into the Wolffian duct at the posterior end
of the segment. Each segment is probably completely isolated from the
adjoining segments, and never has more than one peritoneal funnel and
one communication with the Wolffian duct.
Up to this time there has been no distinction between the anterior and
posterior tubuli of the mesonephros, which alike open into the
Wolffian duct. The collecting tubes of a considerable number of the
hindermost tubuli (ten or eleven in Scyllium canicula), either in some
species elongate, overlap, while at the same time their openings
travel backward so that they eventually open by apertures (not usually
so numerous as the separate tubes), on nearly the same level, into the
hindermost section of the Wolffian duct in the female, or into the
urinogenital cloaca, formed by the coalesced terminal parts of the
Wolffian ducts, in the male; or in other species become modified, by a
peculiar process of splitting from the Wolffian duct, so as to pour
their secretion into a single duct on each side, which opens in a
position corresponding with the numerous ducts of the other species
(fig. 392). In both cases the modified posterior kidney-segments are
probably equivalent to the permanent
[Pg 697] kidney or metanephros of the
amniotic Vertebrates, and for this reason the numerous collecting
tubes or single collecting tube, as the case may be, will be spoken of
as ureters. The anterior tubuli of the primitive excretory organ
retain their early relation to the Wolffian duct, and form the
permanent Wolffian body or mesonephros.
The originally separate terminal extremities of the Wolffian ducts
always coalesce, and form a urinal cloaca, opening by a single
aperture, situated at the extremity of the median papilla behind the
anus. Some of the peritoneal openings of the segmental tubes in
Scyllium, or in other cases all the openings, become obliterated.
In the male the anterior segmental tubes undergo remarkable
modifications, and become connected with the testes. Branches appear
to grow from the first three or four or more of them (though probably
not from their peritoneal openings), which pass to the base of the
testis, and there uniting into a longitudinal canal, form a network,
and receive the secretion of the testicular ampullæ (fig. 393, nt).
These ducts, the vasa efferentia, carry the semen to the Wolffian
body, but before opening into the tubuli of this body they unite into
a canal known as the longitudinal canal of the Wolffian body
(l.c), from which pass off ducts equal in number to the vasa
efferentia, each of which normally ends in a Malpighian corpuscle.
From the Malpighian corpuscles so connected there spring the
convoluted tubuli, forming the generative segments of the Wolffian
body, along which the semen is conveyed to the Wolffian duct (v.d).
The Wolffian duct itself becomes much contorted and acts as vas
deferens.
Figs. 392 and 393 are diagrammatic representations of the chief
constituents of the adult urinogenital organs in the two sexes. In the
adult female (fig. 392), there are present the following parts:
(1) The oviduct or Müllerian duct (m.d) split off from the segmental
duct of the kidneys. Each oviduct opens at its anterior extremity into
the body cavity, and behind the two oviducts have independent
communications with the general cloaca.
(2) The mesonephric ducts (w.d), the other product
[Pg 698] of the segmental
ducts of the kidneys. They end in front by becoming continuous with
the tubulus of the anterior persisting segment of the mesonephros on
each side, and unite behind to open by a common papilla into the
cloaca. The mesonephric duct receives the secretion of the anterior
tubuli of the primitive mesonephros.
(3) The ureter which carries off the secretion of the kidney proper or
metanephros. It is represented in my diagram in its most rare and
differentiated condition as a single duct connected with the posterior
segmental tubes.
(4) The segmental tubes (s.t) some of which retain their
[Pg 699] original
openings into the body cavity, and others are without them. They are
divided into two groups, an anterior forming the mesonephros or
Wolffian body, which pours its secretion into the Wolffian duct; and a
posterior group forming a gland which is probably equivalent to the
kidney proper of amniotic Craniata, and is connected with the ureter.
Fig. 392. Diagram of the arrangement of the urinogenital organs
in an adult female Elasmobranch.
m.d. Müllerian duct; w.d. Wolffian duct; s.t. segmental tubes;
five of them are represented with openings into the body cavity, the
posterior segmental tubes form the mesonephros; ov. ovary.
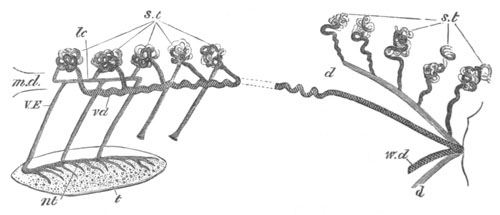
Fig. 393. Diagram of the arrangement of the urinogenital organs
in an adult male Elasmobranch.
m.d. rudiment of Müllerian duct; w.d. Wolffian duct, marked vd
in front and serving as vas deferens; s.t. segmental tubes; two of
them are represented with openings into the body cavity; d.
ureter; t. testis; nt. canal at the base of the testis; VE.
vasa efferentia; lc. longitudinal canal of the Wolffian body.
In the male the following parts are present (fig. 393):
(1) The Müllerian duct (m.d), consisting of a small rudiment
attached to the liver, representing the foremost end of the oviduct of
the female.
(2) The mesonephric duct (w.d) which precisely corresponds to the
mesonephric duct of the female, but, in addition to serving as the
duct of the Wolffian body, also acts as a vas deferens (vd). In the
adult male its foremost part has a very tortuous course.
(3) The ureter (d), which has the same fundamental constitution as
in the female.
(4) The segmental tubes (s.t). The posterior tubes have the same
arrangement in both sexes, but in the male modifications take place in
connection with the anterior tubes to fit them to act as transporters
of the semen.
Connected with the anterior tubes there are present (1) the vasa
efferentia (VE), united on the one hand with (2) the central canal
in the base of the testis (nt), and on the other with the
longitudinal canal of the Wolffian body (lc). From the latter are
seen passing off the successive tubuli of the anterior segments of the
Wolffian body, in connection with which Malpighian bodies are
typically present, though not represented in my diagram.
Apart from the absence of the pronephros the points which deserve
notice in the Elasmobranch excretory system are (1) The splitting of
the segmental duct into Wolffian (mesonephric) and Müllerian ducts.
(2) The connection of the former with the mesonephros, and of the
latter with the abdominal opening of the segmental duct which
represents the pronephros of other types. (3) The fact that the
Müllerian duct serves as oviduct, and the Wolffian duct as vas
deferens. (4) The differentiation of a posterior section of the
mesonephros into a special gland foreshadowing the metanephros of the
Amniota.
[Pg 700]
Cyclostomata. The development of the excretory system amongst the
Cyclostomata has only been studied in Petromyzon (Müller, Fürbringer,
and Scott).
The first part of the system developed is the segmental duct. It
appears in the embryo of about 14 days (Scott) as a solid cord of
cells, differentiated from the somatic mesoblast near the dorsal end
of the body cavity. This cord is at first placed immediately below the
epiblast, and grows backwards by a continuous process of
differentiation of fresh mesoblast cells. It soon acquires a lumen,
and joins the cloacal section of the alimentary tract before the close
of fœtal life. Before this communication is established, the front
end of the duct sends a process towards the body cavity, the blind end
of which acquires a ciliated opening into the latter. A series of
about four or five successively formed outgrowths from the duct, one
behind the other, give rise to as many ciliated funnels opening into
the body cavity, and each communicating by a more or less elongated
tube with the segmental duct. These funnels, which have a metameric
arrangement, constitute the pronephros, the whole of which is situated
in the pericardial region of the body cavity.
On the inner side of the peritoneal openings of each pronephros there
is formed a vascular glomerulus, projecting into the body cavity, and
covered by peritoneal epithelium. For a considerable period the
pronephros constitutes the sole functional part of the excretory
system.
A mesonephros is formed (Fürbringer) relatively late in larval life,
as a segmentally arranged series of solid cords, derived from the
peritoneal epithelium. These cords constitute the rudiments of the
segmental tubes. They are present for a considerable portion of the
body cavity, extending backwards from a point shortly behind the
pronephros. They soon separate from the peritoneal epithelium, become
hollowed out into canals, and join the segmental duct. At their blind
extremity (that originally connected with the peritoneal epithelium) a
Malpighian body is formed.
The pronephros is only a provisional excretory organ, the atrophy of
which commences during larval life, and is nearly completed when the
Ammocœte has reached 180 mm. in length.
[Pg 701] Further changes take place in
connection with the excretory system on the conversion of the
Ammocœte into the adult.
The segmental ducts in the adult fall into a common urinogenital
cloaca, which opens on a papilla behind the anus. This cloaca also
communicates by two apertures (abdominal pores) with the body cavity.
The generative products are carried into the cloaca by these pores; so
that their transportation outwards is not performed by any part of the
primitive urinary system. The urinogenital cloaca is formed by the
separation of the portion of the primitive cloaca containing the
openings of the segmental ducts from that connected with the
alimentary tract.
The mesonephros of the Ammocœte undergoes at the metamorphosis
complete atrophy, and is physiologically replaced by a posterior
series of segmental tubes, opening into the hindermost portion of the
segmental duct (Schneider).
In Myxine the excretory system consists (1) of a highly developed
pronephros with a bunch of ciliated peritoneal funnels opening into
the pericardial section of the body cavity. The coiled and branched
tubes of which the pronephros is composed open on the ventral side of
the anterior portion of the segmental duct, which in old individuals
is cut off from the posterior section of the duct. On the dorsal side
of the portion of the segmental duct belonging to the pronephros there
are present a small number of diverticula, terminating in glomeruli:
they are probably to be regarded as anterior segmental tubes. (2) Of a
mesonephros, which commences a considerable distance behind the
pronephros, and is formed of straight extremely simple segmental tubes
opening into the segmental duct (fig. 385).
The excretory system of Myxine clearly retains the characters of the
system as it exists in the larva of Petromyzon.
Teleostei. In most Teleostei the pronephros and mesonephros coexist
through life, and their products are carried off by a duct, the nature
of which is somewhat doubtful, but which is probably homologous with
the mesonephric duct of other types.
The system commences in the embryo (Rosenberg, Oellacher, Götte,
Fürbringer) with the formation of a groove-like fold of the somatic
layer of peritoneal epithelium, which becomes gradually constricted
into a canal; the process of constriction commencing in the middle and
extending in both directions. The canal does not however close
anteriorly, but remains open to the body cavity, thus giving rise to a
funnel equivalent to the pronephric funnels of Petromyzon and Myxine.
On the inner side of this
[Pg 702] funnel there is formed a glomerulus,
projecting into the body cavity; and at the same time that this is
being formed the anterior end of the canal becomes elongated and
convoluted. The above structures constitute a pronephros, while the
posterior part of the primitive canal forms the segmental duct.
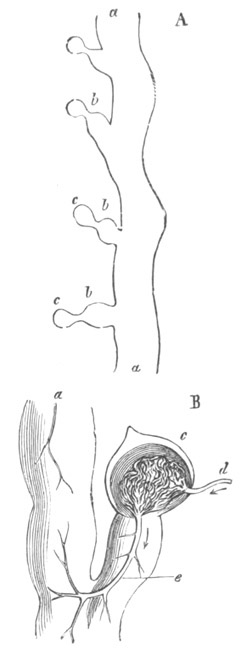
Fig. 394. Portions of the mesonephros of Myxine. (From
Gegenbaur; after J. Müller.)
a. segmental duct; b. segmental tube; c. glomerulus; d.
afferent, e. efferent artery.
B represents a portion of A highly magnified.
The portion of the body cavity with the glomerulus and peritoneal
funnel of the pronephros (fig. 395, po) soon becomes completely
isolated from the remainder, so as to form a closed cavity (gl). The
development of the mesonephros does not take place till long after
that of the pronephros. The segmental tubes which form it are stated
by Fürbringer to arise from solid ingrowths of peritoneal epithelium,
developed successively from before backwards, but Sedgwick informs me
that they arise as differentiations of the mesoblastic cells near
the peritoneal epithelium. They soon become hollow, and unite with the
segmental duct. Malpighian bodies are developed on their median
portions. They grow very greatly in length, and become much
convoluted, but the details of this process have not been followed
out.
The foremost segmental tubes are situated close behind the pronephros,
while the hindermost are in many cases developed in the postanal
continuations of the body cavity. The pronephros appears to form the
swollen cephalic portion of the kidney of the adult, and the
mesonephros the remainder; the so-called caudal portion, where
present, being derived (?) from the postanal segmental tubes.
In some cases the cephalic portion of the kidneys is absent
[Pg 703] in the
adult, which probably implies the atrophy of the pronephros; in other
instances the cephalic portion of the kidneys is the only part
developed. Its relation to the embryonic pronephros requires however
further elucidation.
In the adult the ducts in the lower part of the kidneys lie as a rule
on their outer borders, and almost invariably open into a urinary
bladder, which usually opens in its turn on the urinogenital papilla
immediately behind the genital pore, but in a few instances there is a
common urinogenital pore.
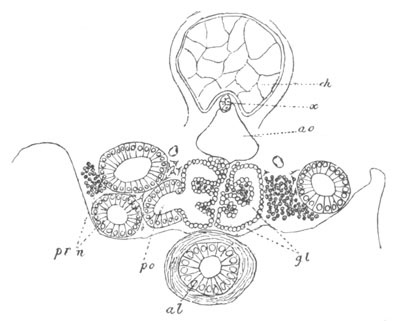
Fig. 395. Section through the pronephros of a Trout and adjacent
parts ten days before hatching.
pr.n. pronephros; po. opening of pronephros into the isolated
portion of the body cavity containing the glomerulus; gl.
glomerulus; ao. aorta; ch. notochord; x. subnotochordal rod;
al. alimentary tract.
In most Osseous Fish there are true generative ducts continuous with
the investment of the generative organs. It appears to me most
probable, from the analogy of Lepidosteus, to be described in the next
section, that these ducts are split off from the primitive segmental
duct, and correspond with the Müllerian ducts of Elasmobranchii, etc.;
though on this point we have at present no positive embryological
evidence (vide general considerations at the end of the Chapter). In
the female Salmon and the male and female Eel the generative products
are carried to the exterior by abdominal pores. It is possible that
this may represent a primitive condition, though it
[Pg 704] is more probably a
case of degeneration, as is indicated by the presence of ducts in the
male Salmon and in forms nearly allied to the Salmonidæ.
The coexistence of abdominal pores and generative ducts in Mormyrus
appears to me to demonstrate that the generative ducts in Teleostei
cannot be derived from the coalescence of the investment of the
generative organs with the abdominal pores.
Ganoidei. The true excretory gland of the adult Ganoidei resembles on
the whole that of Teleostei, consisting of an elongated band on each
side—the mesonephros—an anterior dilatation of which probably
represents the pronephros.
There is in both sexes a Müllerian duct, provided, except in
Lepidosteus, with an abdominal funnel, which is however situated
relatively very far back in the abdominal cavity. The Müllerian ducts
appear to serve as generative canals in both sexes. In Lepidosteus
they are continuous with the investment of the generative glands, and
thus a relation between the generative ducts and glands, very similar
to that in Teleostei, is brought about.
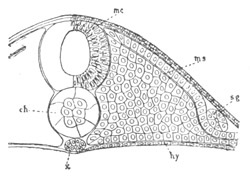
Fig. 396. Section through the trunk of a Lepidosteus embryo on the
sixth day after impregnation.
mc. medullary cord; ms. mesoblast; sg. segmental duct; ch.
notochord; x. subnotochordal rod; hy. hypoblast.
Posteriorly the Müllerian ducts and the ducts of the mesonephros
remain united. The common duct so formed on each side is clearly the
primitive segmental duct. It receives the secretion of a certain
number of the posterior mesonephric tubules, and usually unites with
its fellow to form a kind of bladder, opening by a single pore into
the cloaca, behind the anus. The duct which receives the secretion of
the anterior mesonephric tubules is the true mesonephric or Wolffian
duct.
The development of the excretory system, which has been partially
worked out in Acipenser and Lepidosteus[254],
is on the whole very
similar to that in the Teleostei. The first portion of the system to
[Pg 705]
be formed is the segmental duct. In Lepidosteus this duct is formed as
a groove-like invagination of the somatic peritoneal epithelium,
precisely as in Teleostei, and shortly afterwards forms a duct lying
between the mesoblast and the epiblast (fig. 396, sg). In Acipenser
(Salensky) however it is formed as a solid ridge of the somatic
mesoblast, as in Petromyzon and Elasmobranchii (fig. 397, Wg).
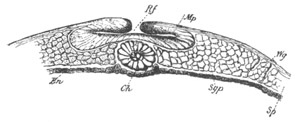
Fig. 397. Transverse section through the anterior part of an Acipenser
embryo. (After Salensky.)
Rf. medullary groove; Mp. medullary plate; Wg. segmental duct;
Ch. notochord; En. hypoblast; Sgp. mesoblastic somite; Sp.
parietal part of mesoblastic plate.
In both forms the ducts unite behind with the cloaca, and a pronephros
of the Teleostean type appears to be developed. This gland is provided
with but one[255]
peritoneal opening, which together with the
glomerulus belonging to it becomes encapsuled in a special section of
the body cavity. The opening of the pronephros of Acipenser into this
cavity is shewn in fig. 398, pr.n. At this early stage of Acipenser
(larva of 5 mm.) I could find no glomerulus.
The mesonephros is formed some distance behind, and some time after
the pronephros, both in Acipenser and Lepidosteus, so that in the
larvæ of both these genera the pronephros is for a considerable period
the only excretory organ. In Lepidosteus especially the development of
the mesonephros occurs very late.
The development of the mesonephros has not been worked out in
Lepidosteus, but in Acipenser the anterior segmental tubes become
first established as (I believe) solid cords of cells, attached at one
extremity to the peritoneal epithelium on each
[Pg 706] side of the insertion
of the mesentery, and extending upwards and outwards round the
segmental duct[256].
The posterior segmental tubes arise later than
the anterior, and (as far as can be determined from the sections in my
possession) they are formed independently of the peritoneal
epithelium, on the dorsal side of the segmental duct.
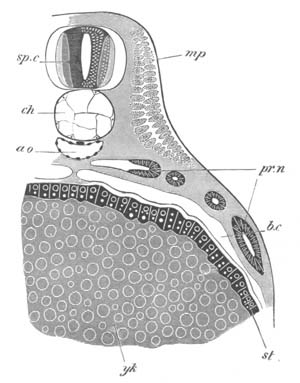
Fig. 398. Transverse section through the region of the stomach of a
larva of Acipenser 5 mm. in length.
st. epithelium of stomach; yk. yolk; ch. notochord, below
which is a subnotochordal rod; pr.n. pronephros; ao. aorta;
mp. muscle-plate formed of large cells, the outer parts of which
are differentiated into contractile fibres; sp.c. spinal cord;
b.c. body cavity.
In later stages (larvæ of 7-10 mm.) the anterior segmental tubes
gradually lose their attachment to the peritoneal epithelium. The
extremity near the peritoneal epithelium forms a Malpighian body, and
the other end unites with the segmental duct. At a still later stage
wide peritoneal funnels are established, for at any rate a
considerable number of the tubes, leading from the body cavity to the
Malpighian bodies. These
[Pg 707] funnels have been noticed by Fürbringer,
Salensky and myself, but their mode of development has not, so far as
I know, been made out. The funnels appear to be no longer present in
the adult. The development of the Müllerian ducts has not been worked
out.
Dipnoi. The excretory system of the Dipnoi is only known in the adult,
but though in some respects intermediate in character between that of
the Ganoidei and Amphibia, it resembles that of the Ganoidei in the
important feature of the Müllerian ducts serving as genital ducts in
both sexes.
Amphibia. In Amphibia (Götte, Fürbringer) the development of the
excretory system commences, as in Teleostei, by the formation of the
segmental duct from a groove formed by a fold of the somatic layer of
the peritoneal epithelium, near the dorsal border of the body cavity
(fig. 399, u). The anterior end of the groove is placed immediately
behind the branchial region. Its posterior part soon becomes converted
into a canal by a constriction which commences a short way from the
front end of the groove, and thence extends backwards. This canal at
first ends blindly close to the cloaca, into which however it soon
opens.
The anterior open part of the groove in front of the constriction
(fig. 399, u) becomes differentiated into a longitudinal duct, which
remains in open communication with the body cavity by two (many
Urodela) three (many Anura) or four (Cœciliidæ) canals. This
constitutes the dorsal part of the pronephros. The ventral part of the
gland is formed from the section of the duct immediately behind the
longitudinal canal. This part grows in length, and, assuming an
S-shaped curvature, becomes placed on the ventral side of the first
formed part of the pronephros. By continuous growth in a limited space
the convolutions of the canal of the pronephros become more numerous,
and the complexity of the gland is further increased by the outgrowth
of blindly ending diverticula.
At the root of the mesentery, opposite the peritoneal openings of the
pronephros, a longitudinal fold, lined by peritoneal epithelium, and
attached by a narrow band of tissue, makes its appearance. It soon
becomes highly vascular, and constitutes a glomerulus homologous with
that in Petromyzon and Teleostei.
[Pg 708]
The section of the body cavity which contains the openings of the
pronephros and the glomerulus, becomes dilated, and then temporarily
shut off from the remainder. At a later period it forms a special
though not completely isolated compartment. For a long time the
pronephros and its duct form the only excretory organs of larval
Amphibia. Eventually however the formation of the mesonephros
commences, and is followed by the atrophy of the pronephros. The
mesonephros is composed, as in other types, of a series of segmental
tubes, but these, except in Cœciliidæ, no longer correspond in number
with the myotomes, but are in all instances more numerous. Moreover,
in the posterior part of the mesonephros in the Urodeles, and through
the whole length of the gland in other types, secondary and tertiary
segmental tubes are formed in addition to the primary tubes.
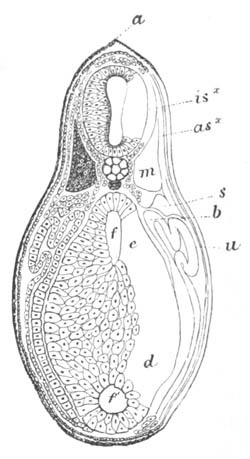
Fig. 399. Transverse section through a very young tadpole of
Bombinator at the level of the anterior end of the yolk-sack.
(After Götte.)
a. fold of epiblast continuous with the dorsal fin; isx. neural
cord; m. lateral muscle; asx. outer layer of muscle-plate; s.
lateral plate of mesoblast; b. mesentery; u. open end of the
segmental duct, which forms the pronephros; f. alimentary tract;
f´. ventral diverticulum which becomes the liver; e. junction of
yolk cells and hypoblast cells; d. yolk cells.
The development of the mesonephros commences in Salamandra
(Fürbringer) with the formation of a series of solid cords, which in
the anterior myotomes spring from the peritoneal epithelium on the
inner side of the segmental duct, but posteriorly arise independently
of this epithelium in the adjoining mesoblast. Sedgwick informs me
that in the Frog the segmental tubes are throughout developed in the
mesoblast, independently of the peritoneal epithelium. These cords
next become detached from the peritoneal epithelium (in so far as they
are primitively united to it), and after first assuming a vesicular
form, grow out into coiled tubes, with a median limb the blind end of
which assists in forming a Malpighian body, and a lateral limb which
comes in contact with and opens into the segmental duct, and an
intermediate portion connecting the two. At the junction of the median
with the intermediate portion, and therefore at the neck of the
Malpighian body, a canal grows out in a ventral direction, which meets
the
[Pg 709] peritoneal epithelium, and then develops a funnel-shaped opening
into the body cavity, which subsequently becomes ciliated. In this way
the peritoneal funnels which are present in the adult are established.
The median and lateral sections of the segmental tubes become highly
convoluted, and the separate tubes soon come into such close proximity
that their primitive distinctness is lost.
The first fully developed segmental tube is formed in Salamandra
maculata in about the sixth myotome behind the pronephros. But in the
region between the two structures rudimentary segmental tubes are
developed.
The number of primary segmental tubes in the separate myotomes of
Salamandra is as follows:
In the 6th myotome (i.e. the first with a true
segmental tube): 1-2 segmental tubes
In the 7th-10th myotome: 2-3 segmental tubes
In the 11th myotome: 3-4 segmental tubes
In the 12th myotome: 3-4 4-5 segmental tubes
In the 13th myotome: 4-5 segmental tubes
In the 13th-16th myotome: 5-6 segmental tubes
It thus appears that the segmental tubes are not only more numerous
than the myotomes, but that the number in each myotome increases from
before backwards. In the case of Salamandra there are formed in the
region of the posterior (10-16) myotomes secondary, tertiary, etc.
segmental tubes out of independent solid cords, which arise in the
mesoblast dorsally to the tubes already established.
The secondary segmental tubes appear to develop out of these cords
exactly in the same way as the primary ones, except that they do not
join the segmental duct directly, but unite with the primary segmental
tubes shortly before the junction of the latter with the segmental
duct. In this way compound segmental tubes are established with a
common collecting tube, but with numerous Malpighian bodies and
ciliated peritoneal openings. The difference in the mode of origin of
these compound tubes and of those in Elasmobranchii is very striking.
The later stages in the development of the segmental tubes have not
been studied in the other Amphibian types.
In Cœciliidæ the earliest stages are not known, but the tubes present
in the adult (Spengel) a truly segmental arrangement, and in the young
each of them is single, and provided with only a single peritoneal
funnel. In the adult however many of the segmental organs become
compound, and may have as many as twenty funnels, etc. Both simple and
compound segmental tubes occur in all parts of the mesonephros, and
are arranged in no definite order.
In the Anura (Spengel) all the segmental tubes are compound, and an
enormous number of peritoneal funnels are present on the ventral
surface, but it has not yet been definitely determined into what part
of the segmental tubes they open.
[Pg 710]
Before dealing with the further changes of the Wolffian body it is
necessary to return to the segmental duct, which, at the time when the
pronephros is undergoing atrophy, becomes split into a dorsal Wolffian
and ventral Müllerian duct. The process in Salamandra (Fürbringer) has
much the same character as in Elasmobranchii, the Müllerian duct being
formed by the gradual separation, from before backwards, of a solid
row of cells from the ventral side of the segmental duct, the
remainder of the duct constituting the Wolffian duct. During the
formation of the Müllerian duct its anterior part becomes hollow, and
attaching itself in front to the peritoneal epithelium acquires an
opening into the body cavity. The process of hollowing is continued
backwards pari passu with the splitting of the segmental duct. In
the female the process is continued till the Müllerian duct opens,
close to the Wolffian duct, into the cloaca. In the male the duct
usually ends blindly. It is important to notice that the abdominal
opening of the Müllerian duct in the Amphibia (Salamandra) is a
formation independent of the pronephros, and placed slightly behind
it; and that the undivided anterior part of the segmental duct (with
the pronephros) is not, as in Elasmobranchii, united with the
Müllerian duct, but remains connected with the Wolffian duct.
The development of the Müllerian duct has not been satisfactorily
studied in other forms besides Salamandra. In Cœciliidæ its abdominal
opening is on a level with the anterior end of the Wolffian body. In
other forms it is usually placed very far forwards, close to the root
of the lungs (except in Proteus and Batrachoseps, where it is placed
somewhat further back), and some distance in front of the Wolffian
body.
The Müllerian duct is always well developed in the female, and serves
as oviduct. In the male it does not (except possibly in Alytes) assist
in the transportation of the genital products, and is always more or
less rudimentary, and in Anura may be completely absent.
After the formation of the Müllerian duct, the Wolffian duct remains
as the excretory channel for the Wolffian body, and, till the atrophy
of the pronephros, for this gland also. Its anterior section, in front
of the Wolffian body, undergoes a more or less complete atrophy.
The further changes of the excretory system concern (1) the junction
in the male of the anterior part of the Wolffian body with the testis;
(2) certain changes in the collecting tubes of the
[Pg 711] posterior part of
the mesonephros. The first of these processes results in the division
of the Wolffian body into a sexual and a non-sexual part, and in
Salamandra and other Urodeles the division corresponds with the
distribution of the simple and compound segmental tubes.
Since the development of the canals connecting the testes with the
sexual part of the Wolffian body has not been in all points
satisfactorily elucidated, it will be convenient to commence with a
description of the adult arrangement of the parts (fig. 400 B). In
most instances a non-segmental system of canals—the vasa efferentia
(ve)—coming from the testis, fall into a canal known as the
longitudinal canal of the Wolffian body, from which there pass off
transverse canals, which fall into, and are equal in number to, the
primary Malpighian bodies of the sexual part of the gland. The
spermatozoa, brought to the Malpighian bodies, are thence transported
along the segmental tubes to the Wolffian duct, and so to the
exterior. The system of canals connecting the testis with the
Malpighian bodies is known as the testicular network. The number of
segmental tubes connected with the testis varies very greatly. In
Siredon there are as many as from 30-32 (Spengel).
The longitudinal canal of the Wolffian body is in rare instances
(Spelerpes, etc.) absent, where the sexual part of the Wolffian body
is slightly developed. In the Urodela the testes are united with the
anterior part of the Wolffian body. In the Cœciliidæ the junction
takes place in an homologous part of the Wolffian body, but, owing to
the development of the anterior segmental tubes, which are rudimentary
in the Urodela, it is situated some way behind the front end. Amongst
the Anura the connection of the testis with the tubules of the
Wolffian body is subject to considerable variations. In Bufo cinereus
the normal Urodele type is preserved, and in Bombinator the same
arrangement is found in a rudimentary condition, in that there are
transverse trunks from the longitudinal canal of the Wolffian body,
which end blindly, while the semen is carried into the Wolffian duct
by canals in front of the Wolffian body. In Alytes and Discoglossus
the semen is carried away by a similar direct continuation of the
longitudinal canal in front of the Wolffian body, but there are no
rudimentary transverse canals passing into the Wolffian body, as in
Bombinator. In Rana the transverse ducts which pass off from the
longitudinal canal of the Wolffian body, after dilating to form (?)
rudimentary Malpighian bodies, enter directly into the collecting
tubes near their opening into the Wolffian duct.
[Pg 712]
In most Urodeles the peritoneal openings connected with the primary
generative Malpighian bodies atrophy, but in Spelerpes they persist.
In the Cœciliidæ they also remain in the adult state.
With reference to the development of these parts little is known
except that the testicular network grows out from the primary
Malpighian bodies, and becomes united with the testis. Embryological
evidence, as well as the fact of the persistence of the peritoneal
funnels of the generative region in the adults of some forms, proves
that the testicular network is not developed from the peritoneal
funnels.
Rudiments of the testicular network are found in the female Cœciliidæ
and in the females of many Urodela (Salamandra, Triton). These
rudiments may in their fullest development consist of a longitudinal
canal and of transverse canals passing from this to the Malpighian
bodies, together with some branches passing into the mesovarium.
Amongst the Urodela the collecting tubes of the hinder non-sexual part
of the Wolffian body, which probably represents a rudimentary
metanephros, undergo in the male sex a change similar to that which
they usually undergo in Elasmobranchii. Their points of junction with
the Wolffian duct are carried back to the hindermost end of the duct
(fig. 400 B), and the collecting tubes themselves unite together into
one or more short ducts (ureters) before joining the Wolffian duct.
In Batrachoseps only the first collecting tube becomes split off in
this way; and it forms a single elongated ureter which receives all
the collecting tubes of the posterior segmental tubes. In the female
and in the male of Proteus, Menobranchus, and Siren the collecting
tubes retain their primitive transverse course and open laterally into
the Wolffian duct. In rare cases (Ellipsoglossus, Spengel) the
ureters open directly into the cloaca.
The urinary bladder of the Amphibia is an outgrowth of the ventral
wall of the cloacal section of the alimentary tract, and is homologous
with the allantois of the amniotic Vertebrata.
The subjoined diagram (fig. 400) of the urogenital system of Triton
illustrates the more important points of the preceding description.
In the female (A) the following parts are present:
(1) The Müllerian duct or oviduct (od) derived from the splitting
of the segmental duct.
(2) The Wolffian duct (sug) constituting the portion of the
segmental duct left after the formation of the Müllerian duct.
(3) The mesonephros (r), divided into an anterior sexual part
[Pg 713]
connected with a rudimentary testicular network, and a posterior
part. The collecting tubes from both parts fall transversely
into the Wolffian duct.
(4) The ovary (ov).
(5) The rudimentary testicular network.
In the male (B) the following parts are present:
(1) The functionless though fairly developed Müllerian duct (m).
(2) The Wolffian duct (sug).
(3) The mesonephros (r) divided into a true sexual part, through
the segmental tubes of which the semen passes, and a non-sexual
part. The collecting tubes of the latter do not enter the
Wolffian duct directly, but bend obliquely backwards and only
fall into it close to its cloacal aperture, after uniting to
form one or two primary tubes (ureters).
(4) The testicular network (ve) consisting of (1) transverse ducts
from the testes, falling into (2) the longitudinal canal of the
Wolffian body, from which (3) transverse canals are again given
off to the Malpighian bodies.
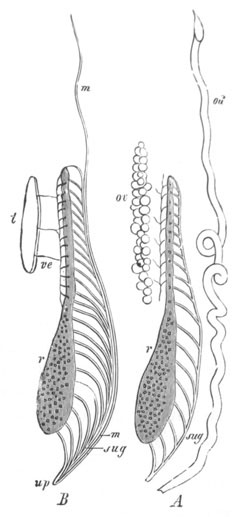
Fig. 400. Diagram of the urinogenital system of Triton. (From
Gegenbaur; after Spengel.)
A. Female. B. Male.
r. mesonephros, on the surface of which numerous peritoneal
funnels are visible; sug. mesonephric or Wolffian duct; od.
oviduct (Müllerian duct); m. Müllerian duct of male; ve. vasa
efferentia of testis; t. testis; ov. ovary; up. urinogenital
pore.
Amniota. The amniotic Vertebrata agree, so far as is known, very
closely amongst themselves in the formation of the urinogenital
system.
The most characteristic feature of the system is the full development
of a metanephros, which constitutes the functional kidney on the
atrophy of the mesonephros or Wolffian body, which is a purely
embryonic organ. The first part of the system to develop is a duct,
which is usually spoken of as the Wolffian duct, but which is really
the homologue of the segmental
[Pg 714] duct. It apparently develops in all the
Amniota nearly on the Elasmobranch type, as a solid rod, primarily
derived from the somatic mesoblast of the intermediate cell mass (fig.
401 W.d)[257].
The first trace of it is visible in an embryo Chick with eight
somites, as a ridge projecting from the intermediate cell mass towards
the epiblast in the region of the seventh somite. In the course of
further development it continues to constitute such a ridge as far as
the eleventh somite (Sedgwick), but from this point it grows backwards
in the space between the epiblast and mesoblast. In an embryo with
fourteen somites a small lumen has appeared in its middle part and in
front it is connected with rudimentary Wolffian tubules, which develop
in continuity with it (Sedgwick). In the succeeding stages the lumen
of the duct gradually extends backwards and forwards, and the duct
itself also passes inwards relatively to the epiblast (fig. 402). Its
hind-end elongates till it comes into connection with, and opens into,
the cloacal section of the hindgut[258].
It might have been anticipated that, as in the lower types, the
anterior end of the segmental duct would either open into the body
cavity, or come into connection with a pronephros. Neither of these
occurrences takes place, though in some types (the Fowl) a structure,
which is probably the rudiment of a pronephros, is developed; it does
not however appear till a later stage, and is then unconnected with
the segmental duct. The next part of the system to appear is the
mesonephros or Wolffian body.
This is formed in all Amniota as a series of segmental tubes, which in
Lacertilia (Braun) correspond with the myotomes, but in Birds and
Mammalia are more numerous.
In Reptilia (Braun, No. 542), the mesonephric tubes develop as
segmentally-arranged masses on the inner side of the Wolffian duct,
and appear to be at first united with the peritoneal epithelium. Each
mass soon becomes an oval vesicle, probably opening for a very short
period into the
[Pg 715] peritoneal cavity by a peritoneal funnel. The vesicles
become very early detached from the peritoneal epithelium, and lateral
outgrowths from them give rise to the main parts of the segmental
tubes, which soon unite with the segmental duct.
In Birds the development of the segmental tubes is more
complicated[259].
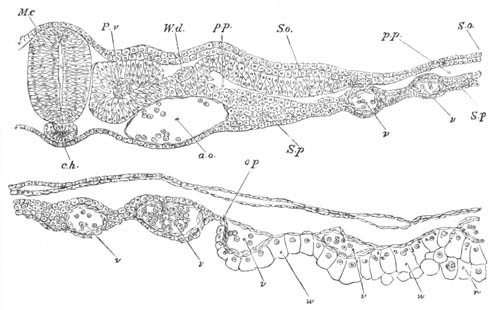
Fig. 401. Transverse section through the dorsal region of an
embryo Chick of 45 hours.
M.c. medullary canal; P.v. mesoblastic somite; W.d. Wolffian
duct which is in contact with the intermediate cell mass; So.
somatopleure; S.p. splanchnopleure; p.p. pleuroperitoneal
cavity; ch. notochord; op. boundary of area opaca; v.
blood-vessel.
The tubules of the Wolffian body are derived from the intermediate
cell mass, shewn in fig. 401, between the upper end of the body cavity
and the muscle-plate. In the Chick the mode of development of this
mass into the segmental tubules is different in the regions in front
of and behind about the sixteenth segment. In front of about the
sixteenth segment the intermediate cell mass becomes detached from the
peritoneal epithelium at certain points, remaining attached to it at
other points, there being several such to each segment. The parts of
the intermediate cell mass attached to the peritoneal epithelium
become converted into S-shaped cords (fig. 402, st) which soon unite
with the segmental duct (wd). Into the commencement of each of these
cords the lumen of the body cavity is for a short distance prolonged,
so that this part constitutes a rudimentary peritoneal funnel.
[Pg 716] In the
Duck the attachment of the intermediate cell mass to the peritoneal
epithelium is prolonged further back than in the Chick.
In the foremost segmental tubes, which never reach a very complete
development, the peritoneal funnels widen considerably, while at the
same time they acquire a distinct lumen. The section of the tube
adjoining the wide peritoneal funnel becomes partially invaginated by
the formation of a glomerulus, and this glomerulus soon grows to such
an extent as to project through the peritoneal funnel, the neck of
which it completely fills, into the body cavity (fig. 403, gl).
There is thus formed a series of free peritoneal glomeruli belonging
to the anterior Wolffian tubuli[260].
These tubuli become however
early aborted.
In the case of the remaining tubules developed from the S-shaped cords
the attachment to the peritoneal epithelium is very soon lost. The
cords acquire a lumen, and open into the segmental duct. Their blind
extremities constitute the rudiments of Malpighian bodies.
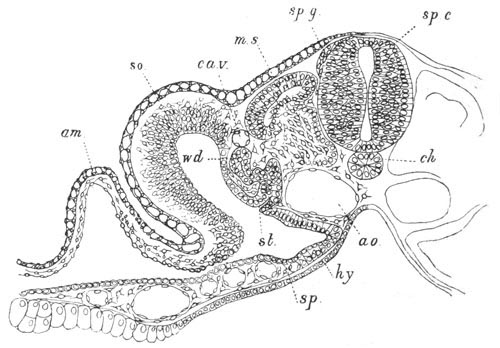
Fig. 402. Transverse section through the trunk of a Duck embryo with
about twenty-four mesoblastic somites.
am. amnion; so. somatopleure; sp. splanchnopleure; wd.
Wolffian duct; st. segmental tube; ca.v. cardinal vein; m.s.
muscle-plate; sp.g. spinal ganglion; sp.c. spinal cord; ch.
notochord; ao. aorta; hy. hypoblast.
[Pg 717] In the posterior part of the Wolffian body of the Chick the
intermediate cell mass becomes very early detached from the peritoneal
epithelium, and at a considerably later period breaks up into oval
vesicles similar to those of the Reptilia, which form the rudiments of
the segmental tubes.
Fig. 403. Section through the external
glomerulus of one of the anterior segmental tubes of an embryo Chick
of about 100 h.
gl. glomerulus; ge. peritoneal epithelium; Wd. Wolffian duct;
ao. aorta; me. mesentery. The segmental tube, and the connection
between the external and internal parts of the glomerulus are not
shewn in this figure.
Secondary and tertiary segmental tubules are formed in the Chick, on
the dorsal side of the primary tubules, as direct differentiations of
the mesoblast. They open independently into the Wolffian duct.
In Mammalia the segmental tubules (Egli) are formed as solid masses in
the same situation as in Birds and Reptiles. It is not known whether
they are united with the peritoneal epithelium. They soon become oval
vesicles, which develop into complete tubules in the manner already
indicated.
Fig. 404. Sections shewing two of the peritoneal invaginations which
give rise to the anterior part of the Müllerian duct (pronephros).
(After Balfour and Sedgwick.)
A is the 11th section of the series.
B is the 15th section of the series.
C is the 18th section of the series.
gr2. second groove; gr3. third groove; r2. second ridge; wd.
Wolffian duct.
After the establishment of the Wolffian body there is formed in both
sexes in all the Amniota a duct, which in the female becomes the
oviduct, but which is functionless and disappears more or less
completely in the male. This duct, in spite of certain peculiarities
in its development, is without doubt homologous with the Müllerian
duct of
[Pg 718] the Ichthyopsida. In connection with its anterior extremity
certain structures have been found in the Fowl, which are probably, on
grounds to be hereafter stated, homologous with the pronephros
(Balfour and Sedgwick).
The pronephros, as I shall call it, consists of a slightly convoluted
longitudinal canal with three or more peritoneal openings. In the
earliest condition, it consists of three successive open involutions
of the peritoneal epithelium, connected together by more or less
well-defined ridge-like thickenings of the epithelium. It takes its
origin from the layer of thickened peritoneal epithelium situated near
the dorsal angle of the body cavity, and is situated some considerable
distance behind the front end of the Wolffian duct.
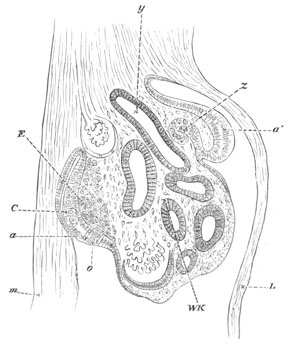
Fig. 405. Section of the Wolffian body developing pronephros and
genital gland of the fourth day. (After Waldeyer.) Magnified 160 times.
m. mesentery; L. somatopleure; a´. portion of the germinal
epithelium from which the involution (z) to form the pronephros
(anterior part of Müllerian duct) takes place; a. thickened
portion of the germinal epithelium in which the primitive germinal
cells C and o are lying; E. modified mesoblast which will form
the stroma of the ovary; WK. Wolffian body; y. Wolffian duct.
In a slightly later stage the ridges connecting the grooves become
partially constricted off from the peritoneal epithelium,
[Pg 719] and develop
a lumen. The condition of the structure at this stage is illustrated
by fig. 404, representing three transverse sections through two
grooves, and through the ridge connecting them.
The pronephros may in fact now be described as a slightly convoluted
duct, opening into the body cavity by three groove-like apertures, and
continuous behind with the rudiment of the true Müllerian duct.
The stage just described is that of the fullest development of the
pronephros. In it, as in all the previous stages, there appear to be
only three main openings into the body cavity; but in some sections
there are indications of the possible presence of one or two
additional rudimentary grooves.
In an embryo not very much older than the one last described the
pronephros atrophies as such, its two posterior openings vanishing,
and its anterior opening remaining as the permanent opening of the
Müllerian duct.
The pronephros is an extremely transitory structure, and its
development and atrophy are completed between the 90th and 120th hours
of incubation.
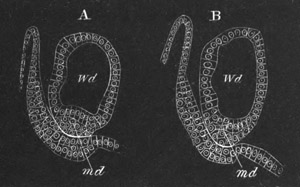
Fig. 406. Two sections shewing the junction of the terminal solid
portion of the Müllerian duct with the Wolffian duct. (After Balfour
and Sedgwick.)
In A the terminal portion of the duct is quite distinct; in B it has
united with the walls of the Wolffian duct.
md. Müllerian duct; Wd. Wolffian duct.
The position of the pronephros in relation to the Wolffian body is
shewn in fig. 405, which probably passes through a region between two
of the peritoneal openings. As long as the pronephros persists, the
Müllerian duct consists merely of a very
[Pg 720] small rudiment, continuous
with the hindermost of the three peritoneal openings, and its solid
extremity appears to unite with the walls of the Wolffian duct.
After the atrophy of the pronephros, the Müllerian duct commences to
grow rapidly, and for the first part of its course it appears to be
split off as a solid rod from the outer or ventral wall of the
Wolffian duct (fig. 406). Into this rod the lumen, present in its
front part, subsequently extends. Its mode of development in front is
thus precisely similar to that of the Müllerian duct in Elasmobranchii
and Amphibia.
This mode of development only occurs however in the anterior part of
the duct. In the posterior part of its course its growing point lies
in a bay formed by the outer walls of the Wolffian duct, but does not
become definitely attached to that duct. It seems however possible
that, although not actually split off from the walls of the Wolffian
duct, it may grow backwards from cells derived from that duct.
The Müllerian duct finally reaches the cloaca though it does not in
the female for a long time open into it, and in the male never does
so.
The mode of growth of the Müllerian duct in the posterior part of its
course will best be understood from the following description quoted
from the paper by Sedgwick and myself.
“A few sections before its termination the Müllerian duct appears as
a well-defined oval duct lying in contact with the wall of the
Wolffian duct on the one hand and the germinal epithelium on the
other. Gradually, however, as we pass backwards, the Müllerian duct
dilates; the external wall of the Wolffian duct adjoining it becomes
greatly thickened and pushed in in its middle part, so as almost to
touch the opposite wall of the duct, and so form a bay in which the
Müllerian duct lies. As soon as the Müllerian duct has come to lie
in this bay its walls lose their previous distinctness of outline,
and the cells composing them assume a curious vacuolated appearance.
No well-defined line of separation can any longer be traced between
the walls of the Wolffian duct and those of the Müllerian, but
between the two is a narrow clear space traversed by an irregular
network of fibres, in some of the meshes of which nuclei are
present.
“The Müllerian duct may be traced in this condition for a
considerable number of sections, the peculiar features above
described becoming more and more marked as its termination is
approached. It continues to dilate and attains a maximum size in the
section or so before it disappears. A lumen may be observed in it up
to its very end, but is usually irregular in outline and frequently
traversed by strands of protoplasm. The Müllerian
[Pg 721] duct finally
terminates quite suddenly, and in the section immediately behind its
termination the Wolffian duct assumes its normal appearance, and the
part of its outer wall on the level of the Müllerian duct comes into
contact with the germinal epithelium.”
Before describing the development of the Müllerian duct in other
Amniotic types it will be well to say a few words as to the
identifications above adopted. The identification of the duct, usually
called the Wolffian duct, with the segmental duct (exclusive of the
pronephros) appears to be morphologically justified for the following
reasons: (1) that it gives rise to part of the Müllerian duct as well
as to the duct of the Wolffian body; behaving in this respect
precisely as does the segmental duct of Elasmobranchii and Amphibia.
(2) That it serves as the duct for the Wolffian body, before the
Müllerian duct originates from it. (3) That it develops in a manner
strikingly similar to that of the segmental duct of various lower
forms.
With reference to the pronephros it is obvious that the organ
identified as such is in many respects similar to the pronephros of
the Amphibia. Both consist of a somewhat convoluted longitudinal
canal, with a certain number of peritoneal openings.
The main difficulties in the homology are:
(1) the fact that the pronephros in the Bird is not united with the
segmental duct;
(2) the fact that it is situated behind the front end of the
Wolffian body.
It is to be remembered in connection with the first of these
difficulties that in the formation of the Müllerian duct in
Elasmobranchii the anterior undivided extremity of the primitive
segmental duct, with the peritoneal opening, which probably represents
the pronephros, is attached to the Müllerian duct, and not to the
Wolffian duct; though in Amphibia the reverse is the case. To explain
the discontinuity of the pronephros with the segmental duct it is only
necessary to suppose that the segmental duct and pronephros, which in
the Ichthyopsida develop as a single formation, develop in the Bird as
two independent structures—a far from extravagant supposition,
considering that the pronephros in the Bird is undoubtedly quite
functionless.
With reference to the posterior position of the pronephros it is only
necessary to remark that a change in position might easily take place
after the acquirement of an independent development, and that the
shifting is probably correlated with a shifting of the abdominal
opening of the Müllerian duct.
The pronephros has only been observed in Birds, and is very possibly
not developed in other Amniota. The Müllerian duct is also usually
stated to develop as a groove of the peritoneal epithelium, shewn in
the Lizard in fig. 354, md., which is continued backward as a
primitively solid rod in the space between
[Pg 722] the Wolffian duct and
peritoneal epithelium, without becoming attached to the Wolffian duct.
On the formation of the Müllerian duct, the duct of the mesonephros
becomes the true mesonephric or Wolffian duct.
After these changes have taken place a new organ of great importance
makes its appearance. This organ is the permanent kidney, or
metanephros.
Metanephros. The mode of development of the metanephros has as yet
only been satisfactorily elucidated in the Chick (Sedgwick, No. 549).
The ureter and the collecting tubes of the kidney are developed from a
dorsal outgrowth of the hinder part of the Wolffian duct. The
outgrowth from the Wolffian duct grows forwards, and extends along the
outer side of a mass of mesoblastic tissue which lies mainly behind,
but somewhat overlaps the dorsal aspect of the Wolffian body.
This mass of mesoblastic cells may be called the metanephric blastema.
Sedgwick, of the accuracy of whose account I have satisfied myself,
has shewn that in the Chick it is derived from the intermediate cell
mass of the region of about the thirty-first to the thirty-fourth
somite. It is at first continuous with, and indistinguishable in
structure from, the portion of the intermediate cell mass of the
region immediately in front of it, which breaks up into Wolffian
tubules. The metanephric blastema remains however quite passive during
the formation of the Wolffian tubules in the adjoining blastema; and
on the formation of the ureter breaks off from the Wolffian body in
front, and, growing forwards and dorsalwards, places itself on the
inner side of the ureter in the position just described.
In the subsequent development of the kidney collecting tubes grow out
from the ureter, and become continuous with masses of cells of the
metanephric blastema, which then differentiate themselves into the
kidney tubules.
The process just described appears to me to prove that the kidney of
the Amniota is a specially differentiated posterior section of the
primitive mesonephros.
According to the view of Remak and Kölliker the outgrowths from the
ureter give rise to the whole of the tubuli uriniferi and the capsules
of the Malpighian bodies, the mesoblast around them forming
blood-vessels, etc. On the other hand some observers (Kupffer,
Bornhaupt, Braun) maintain, in
[Pg 723] accordance with the account given
above, that the outgrowths of the ureter form only the collecting
tubes, and that the secreting tubuli, etc. are formed in situ in the
adjacent mesoblast.
Braun (No. 542) has arrived at the conclusion that in the Lacertilia
the tissue, out of which the tubuli of the metanephros are formed, is
derived from irregular solid ingrowths of the peritoneal epithelium,
in a region behind the Wolffian body, but in a position corresponding
to that in which the segmental tubes take their origin. These
ingrowths, after separating from the peritoneal epithelium, unite
together to form a cord into which the ureter sends the lateral
outgrowths already described. These outgrowths unite with secreting
tubuli and Malpighian bodies, formed in situ. In Lacertilia the
blastema of the kidney extends into a postanal region. Braun’s account
of the origin of the metanephric blastema does not appear to me to be
satisfactorily demonstrated.
The ureter does not long remain attached to the Wolffian duct, but its
opening is gradually carried back, till (in the Chick between the 6th
and 8th day) it opens independently into the cloaca.
Of the further changes in the excretory system the most important is
the atrophy of the greater part of the Wolffian body, and the
conversion of the Wolffian duct in the male sex into the vas deferens,
as in Amphibia and the Elasmobranchii.
The mode of connection of the testis with the Wolffian duct is very
remarkable, but may be derived from the primitive arrangement
characteristic of Elasmobranchii and Amphibia.
In the structures connecting the testis with the Wolffian body two
parts have to be distinguished, (1) that equivalent to the testicular
network of the lower types, (2) that derived from the segmental tubes.
The former is probably to be found in peculiar outgrowths from the
Malpighian bodies at the base of the testes.
These were first discovered by Braun in Reptilia, and consist in this
group of a series of outgrowths from the primary (?) Malpighian bodies
along the base of the testis: they unite to form an interrupted cord
in the substance of the testis, from which the testicular tubuli (with
the exception of the seminiferous cells) are subsequently
differentiated. These outgrowths, with the exception of the first two
or three, become detached from the Malpighian bodies. Outgrowths
similar to those in the male are found in the female, but subsequently
atrophy.
Outgrowths homologous with those found by Braun have
[Pg 724] been detected by
myself (No. 555) in Mammals. It is not certain to what parts of the
testicular tubuli they give rise, but they probably form at any rate
the vasa recta and rete vasculosum.
In Mammals they also occur in the female, and give rise to cords of
tissue in the ovary, which may persist through life.
The comparison of the tubuli, formed out of these structures, with the
Elasmobranch and Amphibian testicular network is justified in that
both originate as outgrowths from the primary Malpighian bodies, and
thence extend into the testis, and come into connection with the true
seminiferous stroma.
As in the lower types the semen is transported from the testicular
network to the Wolffian duct by parts of the glandular tubes of the
Wolffian body. In the case of Reptilia the anterior two or three
segmental tubes in the region of the testis probably have this
function. In the case of Mammalia the vasa efferentia, i.e. the coni
vasculosi, appear, according to the usually accepted view, to be of
this nature, though Banks and other investigators believe that they
are independently developed structures. Further investigations on this
point are required. In Birds a connection between the Wolffian body
and the testis appears to be established as in the other types. The
Wolffian duct itself becomes, in the males of all Amniota, the vas
deferens and the convoluted canal of the epididymis—the latter
structure (except the head) being entirely derived from the Wolffian
duct.
In the female the Wolffian duct atrophies more or less completely.
In Snakes (Braun) the posterior part remains as a functionless canal,
commencing at the ovary, and opening into the cloaca. In the Gecko
(Braun) it remains as a small canal joining the ureter; in Blindworms
a considerable part of the canal is left, and in Lacerta (Braun) only
interrupted portions.
In Mammalia the middle part of the duct, known as Gaertner’s canal,
persists in the females of some monkeys, of the pig and of many
ruminants.
The Wolffian body atrophies nearly completely in both sexes; though,
as described above, part of it opposite the testis persists as the
head of the epididymis. The posterior part of the gland from the level
of the testis may be called the sexual part of the gland, the anterior
part forming the non-sexual part.
[Pg 725] The latter, i.e. the anterior
part, is first absorbed; and in some Reptilia the posterior part,
extending from the region of the genital glands to the permanent
kidney, persists till into the second year.
Various remnants of the Wolffian body are found in the adults of both
sexes in different types. The most constant of them is perhaps the
part in the female equivalent to the head of the epididymis and to
parts also of the coiled tube of the epididymis, which may be called,
with Waldeyer, the epoophoron[261].
This is found in Reptiles, Birds
and Mammals; though in a very rudimentary form in the first-named
group. Remnants of the anterior non-sexual part of the Wolffian bodies
have been called by Waldeyer parepididymis in the male, and
paroophoron in the female. Such remnants are not (Braun) found in
Reptilia, but are stated to be found in both male and female Birds, as
a small organ consisting of blindly ending tubes with yellow pigment.
In some male Mammals (including Man) a parepididymis is found on the
upper side of the testis. It is usually known as the organ of
Giraldes.
The Müllerian duct forms, as has been stated, the oviduct in the
female. The two ducts originally open independently into the cloaca,
but in the Mammalia a subsequent modification of this arrangement
occurs, which is dealt with in a separate section. In Birds the right
oviduct atrophies, a vestige being sometimes left. In the male the
Müllerian ducts atrophy more or less completely.
In most Reptiles and in Birds the atrophy of the Müllerian ducts is
complete in the male, but in Lacerta and Anguis a rudiment of the
anterior part has been detected by Leydig as a convoluted canal. In
the Rabbit (Kölliker)[262]
and probably other Mammals the whole of the
ducts probably disappears, but in some Mammals, e.g. Man, the lower
fused ends of the Müllerian ducts give rise to a pocket opening into
the urethra, known as the uterus masculinus; and in other cases,
e.g. the Beaver and the Ass, the rudiments are more considerable,
and may be continued into horns homologous with the horns of the
uterus (Weber).
The hydatid of Morgani in the male is supposed (Waldeyer) to represent
the abdominal opening of the Fallopian tube in the female, and
therefore to be a remnant of the Müllerian duct.
Changes in the lower parts of the urinogenital ducts in the Amniota.
The genital cord. In the Monodelphia the lower part of the Wolffian
ducts becomes enveloped in both sexes in a special
[Pg 726] cord of tissue,
known as the genital cord (fig. 407, gc), within the lower part of
which the Müllerian ducts are also enclosed. In the male the Müllerian
ducts in this cord atrophy, except at their distal end where they
unite to form the uterus masculinus. The Wolffian ducts, after
becoming the vasa deferentia, remain for some time enclosed in the
common cord, but afterwards separate from each other. The seminal
vesicles are outgrowths of the vasa deferentia.
In the female the Wolffian ducts within the genital cord atrophy,
though rudiments of them are for a long time visible or even
permanently persistent. The lower parts of the Müllerian ducts unite
to form the vagina and body of the uterus. The junction commences in
the middle and extends forwards and backwards; the stage with a median
junction being retained permanently in Marsupials.
The urinogenital sinus and external generative organs. In all the
Amniota, there open at first into the common cloaca the alimentary
canal dorsally, the allantois ventrally, and the Wolffian and
Müllerian ducts and ureters laterally. In Reptilia and Aves the
embryonic condition is retained. In both groups the allantois serves
as an embryonic urinary bladder, but while it atrophies in Aves, its
stalk dilates to form a permanent urinary bladder in Reptilia. In
Mammalia the dorsal part of the cloaca with the alimentary tract
becomes first of all partially constricted off from the ventral, which
then forms a urinogenital sinus (fig. 407, ug). In the course of
development the urinogenital sinus becomes, in all Mammalia but the
Ornithodelphia, completely separated from the intestinal cloaca, and
the two parts obtain separate external openings. The ureters (fig.
407, 3) open higher up than the other ducts into the stalk of the
allantois which dilates to form the bladder (4). The stalk connecting
the bladder with the ventral wall of the body constitutes the urachus,
and loses its lumen before the close of embryonic life. The part of
the stalk of the allantois below the openings of the ureters narrows
to form the urethra, which opens together with the Wolffian and
Müllerian ducts into the urinogenital cloaca.
In front of the urinogenital cloaca there is formed a genital
prominence (fig. 407, cp), with a groove continued from the
[Pg 727]
urinogenital opening; and on each side a genital fold (ls). In the
male the sides of the groove on the prominence coalesce together,
embracing between them the opening of the urinogenital cloaca; and the
prominence itself gives rise to the penis, along which the common
urinogenital passage is continued. The two genital folds unite from
behind forwards to form the scrotum.
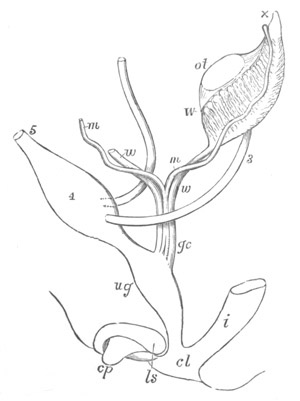
Fig. 407. Diagram of the urinogenital organs of a Mammal at an
early stage. (After Allen Thomson; from Quain’s Anatomy.)
The parts are seen chiefly in profile, but the Müllerian and
Wolffian ducts are seen from the front.
3. ureter; 4. urinary bladder; 5. urachus; ot. genital ridge
(ovary or testis); W. left Wolffian body; x. part at apex from
which coni vasculosi are afterwards developed; w. Wolffian duct;
m. Müllerian duct; gc. genital cord consisting of Wolffian and
Müllerian ducts bound up in a common sheath; i. rectum; ug.
urinogenital sinus; cp. elevation which becomes the clitoris or
penis; ls. ridge from which the labia majora or scrotum are
developed.
In the female the groove on the genital prominence gradually
disappears, and the prominence remains as the clitoris, which is
therefore the homologue of the penis: the two genital folds form the
labia majora. The urethra and vagina open independently into the
common urinogenital sinus.
[Pg 728]
General conclusions and Summary.
Pronephros. Sedgwick has pointed out that the pronephros is always
present in types with a larval development, and either absent or
imperfectly developed in those types which undergo the greater part of
their development within the egg. Thus it is practically absent in the
embryos of Elasmobranchii and the Amniota, but present in the larvæ of
all other forms.
This coincidence, on the principles already laid down in a previous
chapter on larval forms, affords a strong presumption that the
pronephros is an ancestral organ; and, coupled with the fact that it
is the first part of the excretory system to be developed, and often
the sole excretory organ for a considerable period, points to the
conclusion that the pronephros and its duct—the segmental duct—are
the most primitive parts of the Vertebrate excretory system. This
conclusion coincides with that arrived at by Gegenbaur and Fürbringer.
The duct of the pronephros is always developed prior to the gland, and
there are two types according to which its development may take place.
It may either be formed by the closing in of a continuous groove of
the somatic peritoneal epithelium (Amphibia, Teleostei, Lepidosteus),
or as a solid knob or rod of cells derived from the somatic mesoblast,
which grows backwards between the epiblast and the mesoblast
(Petromyzon, Elasmobranchii, and the Amniota).
It is quite certain that the second of these processes is not a true
record of the evolution of the duct, and though it is more possible
that the process observable in Amphibia and the Teleostei may afford
some indications of the manner in which the duct was established, this
cannot be regarded as by any means certain.
The mode of development of the pronephros itself is apparently partly
dependent on that of its duct. In Petromyzon, where the duct does not
at first communicate with the body cavity, the pronephros is formed as
a series of outgrowths from the duct, which meet the peritoneal
epithelium and open into the body cavity; but in other instances it is
derived from the anterior open end of the groove which gives rise to
the segmental duct. The open end of this groove may either remain
single
[Pg 729] (Teleostei, Ganoidei) or be divided into two, three or more
apertures (Amphibia). The main part of the gland in either case is
formed by convolutions of the tube connected with the peritoneal
funnel or funnels. The peritoneal funnels of the pronephros appear to
be segmentally arranged.
The pronephros is distinguished from the mesonephros by developmental
as well as structural features. The most important of the former is
the fact that the glandular tubules of which it is formed are always
outgrowths of the segmental duct; while in the mesonephros they are
always or almost always[263]
formed independently of the duct.
The chief structural peculiarity of the pronephros is the absence from
it of Malpighian bodies with the same relations as those in the meso-
and metanephros; unless the structures found in Myxine are to be
regarded as such. Functionally the place of such Malpighian bodies is
taken by the vascular peritoneal ridge spoken of in the previous pages
as the glomerulus.
That this body is really related functionally to the pronephros
appears to be indicated (1) by its constant occurrence with the
pronephros and its position opposite the peritoneal openings of this
body; (2) by its atrophy at the same time as the pronephros; (3) by
its enclosure together with the pronephridian stoma in a special
compartment of the body-cavity in Teleostei and Ganoids, and its
partial enclosure in such a compartment in Amphibia.
The pronephros atrophies more or less completely in most types, though
it probably persists for life in the Teleostei and Ganoids, and in
some members of the former group it perhaps forms the sole adult organ
of excretion.
The cause of its atrophy may perhaps be related to the fact that it is
situated in the pericardial region of the body-cavity, the dorsal part
of which is aborted on the formation of a closed pericardium; and its
preservation in Teleostei and Ganoids may on this view be due to the
fact that in these types its peritoneal funnel and its glomerulus are
early isolated in a special cavity.
Mesonephros. The mesonephros is in all instances composed of a series
of tubules (segmental tubes) which are developed independently of the
segmental duct. Each tubule is
[Pg 730] typically formed of (1) a peritoneal
funnel opening into (2) a Malpighian body, from which there proceeds
(3) a coiled glandular tube, finally opening by (4) a collecting tube
into the segmental duct, which constitutes the primitive duct for
the mesonephros as well as for the pronephros.
The development of the mesonephridian tubules is subject to
considerable variations.
(1) They may be formed as differentiations of the intermediate cell
mass, and be from the first provided with a lumen, opening into the
body-cavity, and directly derived from the section of the body-cavity
present in the intermediate cell mass; the peritoneal funnels often
persisting for life (Elasmobranchii).
(2) They may be formed as solid cords either attached to or
independent of the peritoneal epithelium, which after first becoming
independent of the peritoneal epithelium subsequently send downwards a
process, which unites with it and forms a peritoneal funnel, which may
or may not persist (Acipenser, Amphibia).
(3) They may be formed as in the last case, but acquire no secondary
connection with the peritoneal epithelium (Teleostei, Amniota). In
connection with the original attachment to the peritoneal epithelium,
a true peritoneal funnel may however be developed (Aves, Lacertilia).
Physiological considerations appear to shew that of these three
methods of development the first is the most primitive. The
development of the tubes as solid cords can hardly be primary.
A question which has to be answered in reference to the segmental
tubes is that of the homology of the secondarily developed peritoneal
openings of Amphibia, with the primary openings of the Elasmobranchii.
It is on the one hand difficult to understand why, if the openings are
homologous in the two types, the original peritoneal attachment should
be obliterated in Amphibia, only to be shortly afterwards reacquired.
On the other hand it is still more difficult to understand what
physiological gain there could be, on the assumption of the
non-homology of the openings, in the replacement of the primary
opening by a secondary opening exactly similar to it. Considering the
great variations in development which occur in undoubtedly homologous
parts I incline to the view that the openings in the two types are
homologous.
[Pg 731]
In the majority of the lower Vertebrata the mesonephric tubes have at
first a segmental arrangement, and this is no doubt the primitive
condition. The coexistence of two, three, or more of them in a single
segment in Amphibia, Aves and Mammalia has recently been shewn, by an
interesting discovery of Eisig, to have a parallel amongst Chætopods,
in the coexistence of several segmental organs in a single segment in
some of the Capitellidæ.
In connection with the segmental features of the mesonephros it is
perhaps worth recalling the fact that in Elasmobranchii as well as
other types there are traces of segmental tubes in some of the
postanal segments. In the case of all the segmental tubes a Malpighian
body becomes established close to the extremity of the tube adjoining
the peritoneal opening, or in an homologous position in tubes without
such an opening. The opposite extremity of the tube always becomes
attached to the segmental duct.
In many of the segments of the mesonephros, especially in the hinder
ones, secondary and tertiary tubes become developed in certain types,
which join the collecting canals of the primary tubes, and are
provided, like the primary tubes, with Malpighian bodies at their
blind extremities.
There can it appears to me be little or no doubt that the secondary
tubes in the different types are homodynamous if not homologous. Under
these circumstances it is surprising to find in what different ways
they take their origin. In Elasmobranchii a bud sprouts out from the
Malpighian body of one segment, and joins the collecting tube of the
preceding segment, and subsequently, becoming detached from the
Malpighian body from which it sprouted, forms a fresh secondary
Malpighian body at its blind extremity. Thus the secondary tubes of
one segment are formed as buds from the segment behind. In Amphibia
(Salamandra) and Aves the secondary tubes develop independently in the
mesoblast. These great differences in development are important in
reference to the homology of the metanephros or permanent kidney,
which is discussed below.
Before leaving the mesonephros it may be worth while putting forward
some hypothetical suggestions as to its origin and relation to the
pronephros,
[Pg 732] leaving however the difficult questions as to the homology
of the segmental tubes with the segmental organs of Chætopods for
subsequent discussion.
It is a peculiarity in the development of the segmental tubes that
they at first end blindly, though they subsequently grow till they
meet the segmental duct with which they unite directly, without the
latter sending out any offshoot to meet them[264].
It is difficult to
believe that peritoneal infundibula ending blindly and unprovided with
some external orifice can have had an excretory function, and we are
therefore rather driven to suppose that the peritoneal infundibula
which become the segmental tubes were either from the first provided
each with an orifice opening to the exterior, or were united with the
segmental duct. If they were from the first provided with external
openings we may suppose that they became secondarily attached to the
duct of the pronephros (segmental duct), and then lost their external
openings, no trace of these structures being left, even in the
ontogeny of the system. It would appear to me more probable that the
pronephros, with its duct opening into the cloaca, was the only
excretory organ of the unsegmented ancestors of the Chordata, and
that, on the elongation of the trunk and its subsequent segmentation,
a series of metameric segmental tubes became evolved opening into the
segmental duct, each tube being in a sort of way serially homologous
with the primitive pronephros. With the segmentation of the trunk the
latter structure itself may have acquired the more or less definite
metameric arrangement of its parts.
Another possible view is that the segmental tubes may be modified
derivatives of posterior lateral branches of the pronephros, which may
at first have extended for the whole length of the body-cavity. If
there is any truth in this hypothesis it is necessary to suppose that,
when the unsegmented ancestor of the Chordata became segmented, the
posterior branches of the primitive excretory organ became segmentally
arranged, and that, in accordance with the change thus gradually
introduced in them, the time of their development became deferred, so
as to accord to a certain extent with the time of formation of the
segments to which they belonged. The change in their mode of
development which would be thereby introduced is certainly not greater
than that which has taken place in the case of segmental tubes, which,
having originally developed on the Elasmobranch type, have come to
develop as they do in the posterior part of the mesonephros of
Salamandra, Birds, etc.
Genital ducts. So far the origin and development of the excretory
organs have been considered without reference to the modifications
introduced by the excretory passages coming to serve as generative
ducts. Such an unmodified state of the
[Pg 733] excretory organs is perhaps
found permanently in Cyclostomata[265]
and transitorily in the embryos
of most forms.
At first the generative products seem to have been discharged freely
into the body-cavity, and transported to the exterior by the abdominal
pores (vide p. 626).
The secondary relations of the excretory ducts to the generative
organs seem to have been introduced by an opening connected with the
pronephridian extremity of the segmental duct having acquired the
function of admitting the generative products into it, and of carrying
them outwards; so that primitively the segmental duct must have
served as efferent duct both for the generative products and the
pronephric secretion (just as the Wolffian duct still does for the
testicular products and secretion of the Wolffian body in
Elasmobranchii and Amphibia).
The opening by which the generative products entered the segmental
duct can hardly have been specially developed for this purpose, but
must almost certainly have been one of the peritoneal openings of the
pronephros. As a consequence (by a process of natural selection) of
the segmental duct having both a generative and a urinary function, a
further differentiation took place, by which that duct became split
into two—a ventral Müllerian duct and a dorsal Wolffian duct.
The Müllerian duct was probably continuous with one or more of the
abdominal openings of the pronephros which served as generative pores.
At first the segmental duct was probably split longitudinally into two
equal portions, and this mode of splitting is exceptionally retained
in some Elasmobranchii; but the generative function of the Müllerian
duct gradually impressed itself more and more upon the embryonic
development, so that, in the course of time, the Müllerian duct
developed less and less at the expense of the Wolffian duct. This
process appears partly to have taken place in Elasmobranchii, and
still more in Amphibia, the Amphibia offering in this respect a less
primitive condition than the Elasmobranchii; while in Aves it has been
carried even further, and it seems possible that in some Amniota the
Müllerian and segmental
[Pg 734] ducts may actually develop independently, as
they do exceptionally in individual specimens of Salamandra
(Fürbringer). The abdominal opening no doubt also became specialised.
At first it is quite possible that more than one pronephric abdominal
funnel may have served for the entrance of the generative products;
this function being, no doubt, eventually restricted to one of them.
Three different types of development of the abdominal opening of the
Müllerian duct have been observed.
In Amphibia (Salamandra) the permanent opening of the Müllerian duct
is formed independently, some way behind the pronephros.
In Elasmobranchii the original opening of the segmental duct forms the
permanent opening of the Müllerian duct, and no true pronephros
appears to be formed.
In Birds the anterior of the three openings of the rudimentary
pronephros remains as the permanent opening of the Müllerian duct.
These three modes of development very probably represent
specialisations of the primitive state along three different lines. In
Amphibia the specialisation of the opening appears to have gone so far
that it no longer has any relation to the pronephros. It was probably
originally one of the posterior openings of this gland.
In Elasmobranchii, on the other hand, the functional opening is formed
at a period when we should expect the pronephros to develop. This
state is very possibly the result of a differentiation by which the
pronephros gradually ceased to become developed, but one of its
peritoneal openings remained as the abdominal aperture of the
Müllerian duct. Aves, finally, appear to have become differentiated
along a third line; since in their ancestors the anterior (?) pore of
the head-kidney appears to have become specialised as the permanent
opening of the Müllerian duct.
The Müllerian duct is usually formed in a more or less complete manner
in both sexes. In Ganoids, where the separation between it and the
Wolffian duct is not completed to the cloaca, and in the Dipnoi, it
probably serves to carry off the generative products of both sexes. In
other cases however only the female
[Pg 735] products pass out by it, and the
partial or complete formation of the Müllerian duct in the male in
these cases needs to be explained. This may be done either by
supposing the Ganoid arrangement to have been the primitive one in the
ancestors of the other forms, or, by supposing characters acquired
primitively by the female to have become inherited by both sexes.
It is a question whether the nature of the generative ducts of
Teleostei can be explained by comparison with those of Ganoids. The
fact that the Müllerian ducts of the Teleostean Ganoid Lepidosteus
attach themselves to the generative organs, and thus acquire a
resemblance to the generative ducts of Teleostei, affords a powerful
argument in favour of the view that the generative ducts of both sexes
in the Teleostei are modified Müllerian ducts. Embryology can however
alone definitely settle this question.
In the Elasmobranchii, Amphibia, and Amniota the male products are
carried off by the Wolffian duct, and they are transported to this
duct, not by open peritoneal funnels of the mesonephros, but by a
network of ducts which sprout either from a certain number of the
Malpighian bodies opposite the testis (Amphibia, Amniota), or from the
stalks connecting the Malpighian bodies with the open funnels
(Elasmobranchii). After traversing this network the semen passes
(except in certain Anura) through a variable number of the segmental
tubes directly to the Wolffian duct. The extent of the connection of
the testis with the Wolffian body is subject to great variations, but
it is usually more or less in the anterior region. Rudiments of the
testicular network have in many cases become inherited by the female.
The origin of the connection between the testis and Wolffian body is
still very obscure. It would be easy to understand how the testicular
products, after falling into the body-cavity, might be taken up by the
open extremities of some of the peritoneal funnels, and how such open
funnels might have groove-like prolongations along the mesorchium,
which might eventually be converted into ducts. Ontogeny does not
however altogether favour this view of the origin of the testicular
network. It seems to me nevertheless the most probable view which has
yet been put forward.
The mode of transportation of the semen by means of the mesonephric
tubules is so peculiar as to render it highly improbable that it was
twice acquired, it becomes therefore necessary to suppose that the
Amphibia and
[Pg 736] Amniota inherited this mode of transportation of the
semen from the same ancestors as the Elasmobranchii. It is remarkable
therefore that in the Ganoidei and Dipnoi this arrangement is not
found.
Either (1) the arrangement (found in the Ganoidei and Dipnoi) of the
Müllerian duct serving for both sexes is the primitive arrangement,
and the Elasmobranch is secondary, or (2) the Ganoid arrangement is a
secondary condition, which has originated at a stage in the evolution
of the Vertebrata when some of the segmental tubes had begun to serve
as the efferent ducts of the testis, and has resulted in consequence
of a degeneration of the latter structures. Although the second
alternative is the more easy to reconcile with the affinities of the
Ganoid and Elasmobranch types, as indicated by the other features of
their organization, I am still inclined to accept the former; and
consider that the incomplete splitting of the segmental duct in
Ganoidei is a strong argument in favour of this view.
Metanephros. With the employment of the Wolffian duct to transport the
semen there seems to be correlated (1) a tendency of the posterior
segmental tubes to have a duct of their own, in which the seminal and
urinary fluids cannot become mixed, and (2) a tendency on the part of
the anterior segmental tubes to lose their excretory function. The
posterior segmental tubes, when connected in this way with a more or
less specialised duct, have been regarded in the preceding pages as
constituting a metanephros.
This differentiation is hardly marked in the Anura, but is well
developed in the Urodela and in the Elasmobranchii; and in the latter
group has become inherited by both sexes. In the Amniota it
culminates, according to the view independently arrived at by Semper
and myself, (1) in the formation of a completely distinct metanephros
in both sexes, formed however, as shewn by Sedgwick, from the same
blastema as the Wolffian body, and (2) in the atrophy in the adult of
the whole Wolffian body, except the part uniting the testis and the
Wolffian duct.
The homology between the posterior metanephridian section of the
Wolffian body, in Elasmobranchii and Urodela, and the kidney of the
Amniota, is only in my opinion a general one, i.e. in both cases a
common cause, viz. the Wolffian duct acting as vas deferens, has
resulted in a more or less similar differentiation of parts.
Fürbringer has urged against Semper’s and my view that no satisfactory
proof of it has yet been offered. This proof has however, since
Fürbringer wrote his paper, been supplied by Sedgwick’s observations.
The development of the kidney in the Amniota is no doubt a direct as
opposed to a phylogenetic development; and the substitution of a
direct for
[Pg 737] a phylogenetic development has most probably been rendered
possible by the fact that the anterior part of the mesonephros
continued all the while to be unaffected and to remain as the main
excretory organ during fœtal life.
The most serious difficulty urged by Fürbringer against the homology
is the fact that the ureter of the metanephros develops on a type of
its own, which is quite distinct from the mode of development of the
ureters of the metanephros of the Ichthyopsidan forms. It is however
quite possible, though far from certain, that the ureter of Amniota
may be a special formation confined to that group, and this fact would
in no wise militate against the homology I have been attempting to
establish.
Comparison of the Excretory organs of the Chordata and Invertebrata.
The structural characters and development of the various forms of
excretory organs described in the preceding pages do not appear to me
to be sufficiently distinctive to render it possible to establish
homologies between these organs on a satisfactory basis, except in
closely related groups.
The excretory organs of the Platyelminthes are in many respects
similar to the provisional excretory organ of the trochosphere of
Polygordius and the Gephyrea on the one hand, and to the Vertebrate
pronephros on the other; and the Platyelminth excretory organ with an
anterior opening might be regarded as having given origin to the
trochosphere organ, while that with a posterior opening may have
done so for the Vertebrate pronephros[266].
Hatschek has compared the provisional trochosphere excretory organ of
Polygordius to the Vertebrate pronephros, and the posterior Chætopod
segmental tubes to the mesonephric tubes; the latter homology having
been already suggested independently by both Semper and myself. With
reference to the comparison of the pronephros with the provisional
excretory organ of Polygordius there are two serious difficulties:
(1) The pronephric (segmental) duct opens directly into the cloaca,
while the duct of the provisional trochosphere excretory organ opens
anteriorly, and directly to the exterior.
(2) The pronephros is situated within the segmented region of the
trunk, and has a more or less distinct metameric arrangement of its
parts; while the provisional trochosphere organ is placed in front
of the segmented region of the trunk, and is in no way segmented.
The comparison of the mesonephric tubules with the segmental excretory
organs of the Chætopoda, though not impossible, cannot be
satisfactorily admitted till some light has been thrown upon the loss
of the supposed external openings of the tubes, and the origin of
their secondary connection with the segmental duct.
[Pg 738]
Confining our attention to the Invertebrata it appears to me fairly
clear that Hatschek is justified in holding the provisional
trochosphere excretory organs of Polygordius, Echiurus and the
Mollusca to be homologous. The atrophy of all these larval organs may
perhaps be due to the presence of a well-developed trunk region in the
adult (absent in the larva), in which excretory organs, probably
serially homologous with those present in the anterior part of the
larva, became developed. The excretory organs in the trunk were
probably more conveniently situated than those in the head, and the
atrophy of the latter in the adult state was therefore brought about,
while the trunk organs became sufficiently enlarged to serve as the
sole excretory organs.
Bibliography of the Excretory Organs.
Invertebrata.
(512) H. Eisig. “Die Segmentalorgane d. Capitelliden.” Mitth. a. d.
zool. Stat. z. Neapel, Vol. I. 1879.
(513) J. Fraipont. “Recherches s. l'appareil excréteur des Trematodes
et d. Cestoïdes.” Archives de Biologie, Vol. I. 1880.
(514) B. Hatschek. “Studien üb. Entwick. d. Anneliden.” Arbeit. a. d.
zool. Instit. Wien, Vol. I. 1878.
(515) B. Hatschek. “Ueber Entwick. von Echiurus,” etc. Arbeit. a. d.
zool. Instit. Wien, Vol. III. 1880.
Excretory Organs of Vertebrata.
General.
(516) F. M. Balfour. “On the origin and history of the urinogenital
organs of Vertebrates.” Journal of Anat. and Phys., Vol. X. 1876.
(517) Max. Fürbringer[267].
“Zur vergleichenden Anat. u. Entwick. d.
Excretionsorgane d. Vertebraten.” Morphol. Jahrbuch, Vol. IV. 1878.
(518) H. Meckel. Zur Morphol. d. Harn-u. Geschlechtswerkz. d.
Wirbelthiere, etc. Halle, 1848.
(519) Joh. Müller. Bildungsgeschichte d. Genitalien, etc.
Düsseldorf, 1830.
(520) H. Rathke. “Beobachtungen u. Betrachtungen ü. d. Entwicklung d.
Geschlechtswerkzeuge bei den Wirbelthieren.” N. Schriften d. naturf.
Gesell. in Dantzig, Bd. I. 1825.
(521) C. Semper[267].
“Das Urogenitalsystem d. Plagiostomen u. seine
Bedeutung f. d. übrigen Wirbelthiere.” Arb. a. d. zool.-zoot.
Instit. Würzburg, Vol. II. 1875.
(522) W. Waldeyer[267].
Eierstock u. Ei. Leipzig, 1870.
[Pg 739] Elasmobranchii.
(523) A. Schultz. “Zur Entwick. d. Selachiereies.” Archiv f. mikr.
Anat., Vol. XI. 1875.
Vide also Semper (No. 521) and Balfour (No. 292).
Cyclostomata.
(524) J. Müller. “Untersuchungen ü. d. Eingeweide d. Fische.” Abh. d.
k. Ak. Wiss. Berlin, 1845.
(525) W. Müller. “Ueber d. Persistenz d. Urniere b. Myxine glutinosa.”
Jenaische Zeitschrift, Vol. VII. 1873.
(526) W. Müller. “Ueber d. Urogenitalsystem d. Amphioxus u. d.
Cyclostomen.” Jenaische Zeitschrift, Vol. IX. 1875.
(527) A. Schneider. Beiträge z. vergleich. Anat. u. Entwick. d.
Wirbelthiere. Berlin, 1879.
(528) W. B. Scott. “Beiträge z. Entwick. d. Petromyzonten.” Morphol.
Jahrbuch, Vol. VII. 1881.
Teleostei.
(529) J. Hyrtl. “Das uropoetische System d. Knochenfische.” Denkschr.
d. k. k. Akad. Wiss. Wien, Vol. II. 1850.
(530) A. Rosenberg. Untersuchungen üb. die Entwicklung d.
Teleostierniere. Dorpat, 1867.
Vide also Oellacher (No. 72).
Amphibia.
(531) F. H. Bidder. Vergleichend-anatomische u. histologische
Untersuchungen ü. die männlichen Geschlechts- und Harnwerkzeuge d.
nackten Amphibien. Dorpat, 1846.
(532) C. L. Duvernoy. “Fragments s. les Organes genito-urinaires des
Reptiles,” etc. Mém. Acad. Sciences. Paris. Vol. XI. 1851, pp.
17-95.
(533) M. Fürbringer. Zur Entwicklung d. Amphibienniere. Heidelberg,
1877.
(534) F. Leydig. Anatomie d. Amphibien u. Reptilien. Berlin, 1853.
(535) F. Leydig. Lehrbuch d. Histologie. Hamm, 1857.
(536) F. Meyer. “Anat. d. Urogenitalsystems d. Selachier u.
Amphibien.” Sitz. d. naturfor. Gesellsch. Leipzig, 1875.
(537) J. W. Spengel. “Das Urogenitalsystem d. Amphibien.” Arb. a. d.
zool.-zoot. Instit. Würzburg. Vol. III. 1876.
(538) Von Wittich. “Harn- u. Geschlechtswerkzeuge d. Amphibien.”
Zeit. f. wiss. Zool., Vol. IV.
Vide also Götte (No. 296).
Amniota.
(539) F. M. Balfour and A. Sedgwick. “On the existence of a
head-kidney in the embryo Chick,” etc. Quart. J. of Micr. Science,
Vol. XIX. 1878.
(540) Banks. On the Wolffian bodies of the fœtus and their remains
in the adult. Edinburgh, 1864.
[Pg 740]
(541) Th. Bornhaupt. Untersuchungen üb. die Entwicklung d.
Urogenitalsystems beim Hühnchen. Inaug. Diss. Riga, 1867.
(542) Max Braun. “Das Urogenitalsystem d. einheimischen Reptilien.”
Arbeiten a. d. zool.-zoot. Instit. Würzburg. Vol. IV. 1877.
(543) J. Dansky u. J. Kostenitsch. “Ueb. d. Entwick. d. Keimblätter u.
d. Wolff’schen Ganges im Hühnerei.” Mém. Acad. Imp. Pétersbourg,
VII. Series, Vol. XXVII. 1880.
(544) Th. Egli. Beiträge zur Anat. und Entwick. d.
Geschlechtsorgane. Inaug. Diss. Zürich, 1876.
(545) E. Gasser. Beiträge zur Entwicklungsgeschichte d. Allantois,
der Müller’schen Gänge u. des Afters. Frankfurt, 1874.
(546) E. Gasser. “Beob. üb. d. Entstehung d. Wolff’schen Ganges bei
Embryonen von Hühnern u. Gänsen.” Arch. für mikr. Anat., Vol. XIV.
1877.
(547) E. Gasser. “Beiträge z. Entwicklung d. Urogenitalsystems d.
Hühnerembryonen.” Sitz. d. Gesell. zur Beförderung d. gesam.
Naturwiss. Marburg, 1879.
(548) C. Kupffer. “Untersuchung über die Entwicklung des Harn- und
Geschlechtssystems.” Archiv für mikr. Anat., Vol. II. 1866.
(549) A. Sedgwick. “Development of the kidney in its relation to the
Wolffian body in the Chick.” Quart. J. of Micros. Science, Vol. XX.
1880.
(550) A. Sedgwick. “On the development of the structure known as the
glomerulus of the head-kidney in the Chick.” Quart. J. of Micros.
Science, Vol. XX. 1880.
(551) A. Sedgwick. “Early development of the Wolffian duct and
anterior Wolffian tubules in the Chick; with some remarks on the
vertebrate excretory system.” Quart. J. of Micros. Science, Vol.
XXI. 1881.
(552) M. Watson. “The homology of the sexual organs, illustrated by
comparative anatomy and pathology.” Journal of Anat. and Phys., Vol.
XIV. 1879.
(553) E. H. Weber. Zusätze z. Lehre von Baue u. d. Verrichtungen d.
Geschlechtsorgane. Leipzig, 1846.
Vide also Remak (No. 302), Foster and Balfour (No. 295), His (No.
297), Kölliker (No. 298).
[Pg 741]
CHAPTER XXIV.
GENERATIVE ORGANS AND GENITAL DUCTS.
Generative organs.
The structure and growth of the ovum and spermatozoon were given in
the first chapter of this work, but their derivation from the germinal
layers was not touched on, and it is this subject with which we are
here concerned. If there are any structures whose identity throughout
the Metazoa is not open to doubt these structures are the ovum and
spermatozoon; and the constancy of their relations to the germinal
layers would seem to be a crucial test as to whether the latter have
the morphological importance usually attributed to them.
The very fragmentary state of our knowledge of the origin of the
generative cells has however prevented this test being so far very
generally applied.
Porifera. In the Porifera the researches of Schulze have clearly
demonstrated that both the ova and the spermatozoa take their origin
from indifferent cells of the general parenchyma, which may be called
mesoblastic. The primitive germinal cells of the two sexes are not
distinguishable; but a germinal cell by enlarging and becoming
spherical gives rise to an ovum; and by subdivision forms a
sperm-morula, from the constituent cells of which the spermatozoa are
directly developed.
Cœlenterata. The greatest confusion prevails as to the germinal layer
from which the male and female products are derived in the
Cœlenterata[268].
[Pg 742]
The following apparent modes of origin of these products have been
observed.
(1) The generative products of both sexes originate in the ectoderm
(epiblast): Hydra, Cordylophora, Tubularia, all (?) free Gonophores of
Hydromedusæ, the Siphonophora, and probably the Ctenophora.
(2) The generative products of both sexes originate in the entoderm
(hypoblast): Plumularia and Sertularella, amongst the Hydroids, and
the whole of the Acraspeda and Actinozoa.
(3) The male cells are formed in the ectoderm, and the female in the
entoderm: Gonothyræa, Campanularia, Hydractinia, Clava.
In view of the somewhat surprising results to which the researches on
the origin of the genital products amongst the Cœlenterata have led,
it would seem to be necessary either to hold that there is no definite
homology between the germinal layers in the different forms of
Cœlenterata, or to offer some satisfactory explanation of the
behaviour of the genital products, which would not involve the
acceptance of the first alternative.
Though it can hardly be said that such an explanation has yet been
offered, some observations of Kleinenberg (No. 557) undoubtedly point
to such an explanation being possible.
Kleinenberg has shewn that in Eudendrium the ova migrate freely from
the ectoderm into the endoderm, and vice versa; but he has given
strong grounds for thinking that they originate in the ectoderm. He
has further shewn that the migration in this type is by no means an
isolated phenomenon.
Since it is usually only possible to recognise generative elements
after they have advanced considerably in development, the mere
position of a generative cell, when first observed, can afford, after
what Kleinenberg has shewn, no absolute proof of its origin. Thus it
is quite possible that there is really only one type of origin for the
generative cells in the Cœlenterata.
Kleinenberg has given reasons for thinking that the migration of the
ova into the entoderm may have a nutritive object. If this be so, and
there are numerous facts which shew that the position of generative
cells is often largely influenced by their nutritive requirements, it
seems not impossible
[Pg 743] that the endodermal position of the generative
organs in the Actinozoa and acraspedote Medusæ may have arisen by a
continuously earlier migration of the generative cells from the
ectoderm into the endoderm; and that the migration may now take place
at so early a period of the development, that we should be justified
in formally holding the generative products to be endodermal in
origin.
We might perhaps, on this view, formulate the origin of the generative
products in the Cœlenterata in the following way:—
Both ova and spermatozoa primitively originated in the ectoderm, but
in order to secure a more complete nutrition the cells which give rise
to them exhibit in certain groups a tendency to migrate into the
endoderm. This migration, which may concern the generative cells of
one or of both the sexes, takes place in some cases after the
generative cells have become recognisable as such, and very probably
in other cases at so early a period that it is impossible to
distinguish the generative cells from indifferent embryonic cells.
Very little is known with reference to the origin of the generative
cells in the triploblastic Invertebrata.
Chætopoda and Gephyrea. In the Chætopoda and Gephyrea, the germinal
cells are always developed in the adult from the epithelial lining of
the body cavity; so that their origin from the mesoblast seems fairly
established.
If we are justified in holding the body cavity of these forms to be a
derivative of the primitive archenteron (vide pp. 356 and 357) the
generative cells may fairly be held to originate from a layer which
corresponds to the endoderm of the Cœlenterata[269].
Chætognatha. In Sagitta the history of the generative cells, which was
first worked out by Kowalevsky and Bütschli, has been recently treated
with great detail by O. Hertwig[270].
The generative cells appear during the gastrula stage, as two large
cells with conspicuous nuclei, which are placed in the hypoblast
lining the archenteron, at the pole opposite the blastopore. These
cells soon divide, and at the same time pass out of the hypoblast, and
enter the archenteric cavity (fig. 408 A, ge). The division into
four cells, which is not satisfactorily represented in my diagram,
takes place in such a way that two
[Pg 744] cells are placed nearer the median
line, and two externally. The two inner cells form the eventual
testes, and the outer the ovaries, one half of each primitive cell
thus forming an ovary, and the other a testis.
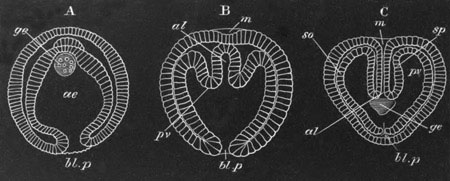
Fig. 408. Three stages in the development of Sagitta. (A and C
after Bütschli, and B after Kowalevsky.)
The three embryos are represented in the same positions.
A. Represents the gastrula stage.
B. Represents a succeeding stage, in which the primitive archenteron
is commencing to be divided into three.
C. Represents a later stage, in which the mouth involution (m) has
become continuous with the alimentary tract, and the blastopore is
closed.
m. mouth; al. alimentary canal; ae. archenteron; bl.p.
blastopore; pv. perivisceral cavity; sp. splanchnic mesoblast;
so. somatic mesoblast; ge. generative organs.
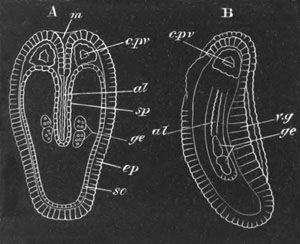
Fig. 409. Two views of a late embryo of Sagitta. A, from the dorsal
surface. B, from the side. (After Bütschli.)
m. mouth; al. alimentary canal; v.g. ventral ganglion
(thickening of epiblast); ep. epiblast; c.pv. cephalic section
of body cavity; so. somatopleure; sp. splanchnopleure; ge.
generative organs.
[Pg 745]
When the archenteric cavity is divided into a median alimentary tract,
and two lateral sections forming the body cavity, the generative
organs are placed in the common vestibule into which both the body
cavity and alimentary cavity at first open (fig. 408).
The generative organs long retain their character as simple cells.
Eventually (fig. 409) the two ovaries travel forwards, and apply
themselves to the body walls, while the two testes also become
separated by a backward prolongation of the median alimentary tract.
On the formation of the transverse septum dividing the tail from the
body, the ovarian cells lie immediately in front of this septum, and
the testicular cells in the region behind it.
Polyzoa. In Pedicellina amongst the entoproctous Polyzoa Hatschek
finds that the generative organs originate from a pair of specially
large mesoblast cells, situated in the space between the stomach and
the floor of the vestibule. The two cells undergo changes, which have
an obvious resemblance to those of the generative cells of the
Chætognatha. They become surrounded by an investment of mesoblast
cells, and divide so as to form two masses. Each of these masses at a
later period separates into an anterior and a posterior part. The
former becomes the ovary, the latter the testis.
Nematoda. In the Nematoda the generative organs are derived from the
division of a single cell which would appear to be mesoblastic[271].
Insecta. The generative cells have been observed at a very early
embryonic stage in several insect forms (Vol. II. p. 404), but the
observations so far recorded with reference to them do not enable us
to determine with certainty from which of the germinal layers they are
derived.
Crustacea. In Moina, one of the Cladocera, Grobben[272]
has shewn that
the generative organs are derived from a single cell, which becomes
differentiated during the segmentation. This cell, which is in close
contiguity with the cells from which both the mesoblast and hypoblast
originate, subsequently divides;
[Pg 746] but at the gastrula stage, and after
the mesoblast has become formed, the cells it gives rise to are
enclosed in the epiblast, and do not migrate inwards till a later
stage. The products of the division of the generative cell
subsequently divide into two masses. It is not possible to assign the
generative cell of Moina to a definite germinal layer. Grobben,
however, thinks that it originates from the division of a cell, the
remainder of which gives rise to the hypoblast.
Chordata. In the Vertebrata, the primitive generative cells (often
known as primitive ova) are early distinguishable, being imbedded
amongst the cells of two linear streaks of peritoneal epithelium,
placed on the dorsal side of the body cavity, one on each side of the
mesentery (figs. 405 C and 410, po). They appear to be derived from
the epithelial cells amongst which they lie; and are characterized by
containing a large granular nucleus, surrounded by a considerable body
of protoplasm. The peritoneal epithelium in which they are placed is
known as the germinal epithelium.
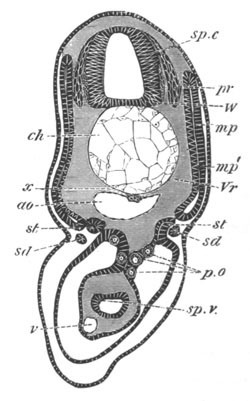
Fig. 410. Section through the trunk of a Scyllium embryo slightly
younger than 28 F.
sp.c. spinal cord; W. white matter of spinal cord; pr.
posterior nerve-roots; ch. notochord; x. subnotochordal rod;
ao. aorta; mp. muscle-plate; mp´. inner layer of muscle-plate
already converted into muscles; Vr. rudiment of vertebral body;
st. segmental tube; sd. segmental duct; sp.v. spiral valve;
v. subintestinal vein; p.o. primitive generative cells.
It is at first impossible to distinguish the germinal cells which will
become ova from those which will become spermatozoa.
The former however remain within the peritoneal epithelium (fig. 411),
and become converted into ova in a manner more particularly described
in Vol. II. pp. 54-59.
The history of the primitive germinal cells in the male has not been
so adequately worked out as in the female.
The fullest history of them is that given by Semper (No. 559) for the
Elasmobranchii, the general accuracy of which I can fully support;
[Pg 747]
though with reference to certain stages in the history further
researches are still required[273].
In Elasmobranchii the male germinal cells, instead of remaining in the
germinal epithelium, migrate into the adjacent stroma, accompanied I
believe by some of the indifferent epithelial cells. Here they
increase in number, and give rise to masses of variable form, composed
partly of true germinal cells, and partly of smaller cells with deeply
staining nuclei, which are, I believe, derived from the germinal
epithelium.
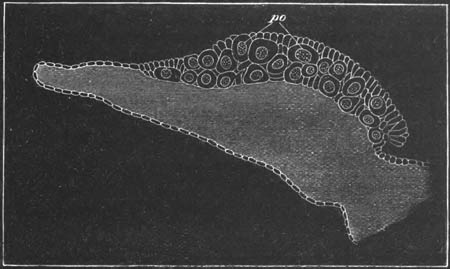
Transverse section through the ovary of a young embryo of
scyllium canicula, to shew the primitive germinal cells (po) lying
in the germinal epithelium on the outer side of the ovarian ridge.
These masses next break up into ampullæ, mainly formed of germinal
cells, and each provided with a central lumen; and these ampullæ
attach themselves to tubes derived from the smaller cells, which are
in their turn continuous with the testicular network. The spermatozoa
are developed from the cells forming the walls of the primitive
ampullæ; but the process of their formation does not concern us in
this chapter.
In the Reptilia Braun has traced the passage of the primitive germinal
cells into the testicular tubes, and I am able to confirm his
observations on this point: he has not however traced their further
history.
[Pg 748]
In Mammalia the evidence of the origin of the spermatospores from the
germinal epithelium is not quite complete, but there can be but little
doubt of its occurrence[274].
In Amphioxus Langerhans has shewn that the ova and spermatozoa are
derived from similar germinal cells, which may be compared to the
germinal epithelium of the Vertebrata. These cells are however
segmentally arranged as separate masses (vide Vol. II. p. 54).
Bibliography.
(554) G. Balbiani. Leçons s. la génération des Vertébrés. Paris,
1879.
(555) F. M. Balfour. “On the structure and development of the
Vertebrate ovary.” Quart. J. of Micr. Science, Vol. XVIII.
(556) E. van Beneden. “De la distinction originelle du tecticule et de
l'ovaire, etc.” Bull. Ac. roy. belgique, Vol. XXXVII. 1874.
(557) N. Kleinenberg. “Ueb. d. Entstehung d. Eier b. Eudendrium.”
Zeit. f. wiss. Zool., Vol. XXXV. 1881.
(558) H. Ludwig. “Ueb. d. Eibildung im Theirreiche.” Arbeit. a. d.
zool.-zoot. Instit. Würzburg, Vol. I. 1874.
(559) C. Semper. “Das Urogenitalsystem d. Plagiostomen, etc.” Arbeit.
a. d. zool.-zoot. Instit. Würzburg, Vol. II. 1875.
(560) A. Weismann. “Zur Frage nach dem Ursprung d. Geschlechtszellen
bei den Hydroiden.” Zool. Anzeiger, No. 55, 1880.
Vide also O. and R. Hertwig (No. 271), Kölliker (No. 298), etc.
Genital ducts.
The development and evolution of the generative ducts is as yet very
incompletely worked out, but even in the light of our present
knowledge a comparative review of this subject brings to light
features of considerable interest, and displays a fruitful field for
future research.
In the Cœlenterata there are no generative ducts.
In the Hydromedusæ and Siphonophora the generative products are
liberated by being dehisced directly into the surrounding medium;
while in the Acraspeda, the Actinozoa and the Ctenophora, they are
dehisced into parts of the gastrovascular system, and carried to the
exterior through the mouth.
The arrangement in the latter forms indicates the origin of
[Pg 749] the
methods of transportation of the genital products to the exterior in
many of the higher types.
It has been already pointed out that the body cavity in a very large
number of forms is probably derived from parts of a gastrovascular
system like that of the Actinozoa.
When the part of the gastrovascular system into which the generative
products were dehisced became, on giving rise to the body cavity, shut
off from the exterior, it would be essential that some mode of
transportation outwards of the generative products should be
constituted.
In some instances simple pores (probably already existing at the time
of the establishment of a closed body cavity) become the generative
ducts. Such seems probably to have been the case in the Chætognatha
(Sagitta) and in the primitive Chordata.
In the latter forms the generative products are sometimes dehisced
into the peritoneal cavity, and thence transported by the abdominal
pores to the exterior (Cyclostomata and some Teleostei, vide p.
626). In Amphioxus they pass by dehiscence into the atrial cavity, and
thence through the gill slits and by the mouth, or by the abdominal
pore (?) to the exterior. The arrangement in Amphioxus and the
Teleostei is probably secondary, as possibly also is that in the
Cyclostomata; so that the primitive mode of exit of the generative
products in the Chordata is still uncertain. It is highly improbable
that the generative ducts of the Tunicata are primitive structures.
A better established and more frequent mode of exit of the generative
products when dehisced into the body cavity is by means of the
excretory organs. The generative products pass from the body cavity
into the open peritoneal funnels of such organs, and thence through
their ducts to the exterior. This mode of exit of the generative
products is characteristic of the Chætopoda, the Gephyrea, the
Brachiopoda and the Vertebrata, and probably also of the Mollusca. It
is moreover quite possible that it occurs in the Polyzoa, some of the
Arthropoda, the Platyelminthes and some other types.
The simple segmental excretory organs of the Polychæta, the Gephyrea
and the Brachiopoda serve as generative canals, and in many instances
they exhibit no modification, or but a very slight one, in connection
with their secondary generative
[Pg 750] function; while in other instances,
e.g. Bonellia, such modification is very considerable.
The generative ducts of the Oligochæta are probably derived from
excretory organs. In the Terricola ordinary excretory organs are
present in the generative segments in addition to the generative
ducts, while in the Limicola generative ducts alone are present in the
adult, but before their development excretory organs of the usual type
are found, which undergo atrophy on the appearance of the generative
ducts (Vedjovsky).
From the analogy of the splitting of the segmental duct of the
Vertebrata into the Müllerian and Wolffian ducts, as a result of a
combined generative and excretory function (vide p. 728), it seems
probable that in the generative segments of the Oligochæta the
excretory organs had at first both an excretory and a generative
function, and that, as a secondary result of this double function,
each of them has become split into two parts, a generative and an
excretory. The generative part has undergone in all forms great
modifications. The excretory parts remain unmodified in the Earthworms
(Terricola), but completely abort on the development of the generative
ducts in the Limicola. An explanation may probably be given of the
peculiar arrangements of the generative ducts in Saccocirrus amongst
the Polychæta (vide Marion and Bobretzky), analogous to that just
offered for the Oligochæta.
The very interesting modifications produced in the excretory organs of
the Vertebrata by their serving as generative ducts were fully
described in the last chapter; and with reference to this part of our
subject it is only necessary to call attention to the case of
Lepidosteus and the Teleostei.
In Lepidosteus the Müllerian duct appears to have become attached to
the generative organs, so that the generative products, instead of
falling directly into the body cavity and thence entering the open end
of a peritoneal funnel of the excretory organs, pass directly into the
Müllerian duct without entering the body cavity. In most Teleostei the
modification is more complete, in that the generative ducts in the
adult have no obvious connection with the excretory organs.
The transportation of the male products to the exterior in all the
higher Vertebrata, without passing into the body cavity, is in
principle similar to the arrangement in Lepidosteus.
The above instances of the peritoneal funnels of an excretory organ
becoming continuous with the generative glands, render it highly
probable that there may be similar instances amongst the Invertebrata.
[Pg 751]
As has been already pointed out by Gegenbaur there are many features
in the structure of the genital ducts in the more primitive Mollusca,
which point to their having been derived from the excretory organs. In
several Lamellibranchiata[275]
(Spondylus, Lima, Pecten) the
generative ducts open into the excretory organs (organ of Bojanus), so
that the generative products have to pass through the excretory organ
on their way to the exterior. In other Lamellibranchiata the genital
and excretory organs open on a common papilla, and in the remaining
types they are placed close together.
In the Cephalopoda again the peculiar relations of the generative
organs to their ducts point to the latter having primitively had a
different, probably an excretory, function. The glands are not
continuous with the ducts, but are placed in special capsules from
which the ducts proceed. The genital products are dehisced into these
capsules and thence pass into the ducts.
In the Gasteropoda the genital gland is directly continuous with its
duct, and the latter, especially in the Pulmonata and Opisthobranchiata,
assumes such a complicated form that its origin from the excretory
organ would hardly have been suspected. The fact however that its
opening is placed near that of the excretory organ points to its being
homologous with the generative ducts of the more primitive types.
In the Discophora, where the generative ducts are continuous with the
glands, the structure both of the generative glands and ducts points
to the latter having originated from excretory organs.
It seems, as already mentioned, very possible that there are other
types in which the generative ducts are derived from the excretory
organs. In the Arthropoda for instance the generative ducts, where
provided with anteriorly placed openings, as in the Crustacea,
Arachnida and the Chilognathous Myriapoda, the Pœcilopoda, etc., may
possibly be of this nature, but the data for deciding this point are
so scanty that it is not at present possible to do more than frame
conjectures.
The ontogeny of the generative ducts of the Nematoda and
[Pg 752] the Insecta
appears to point to their having originated independently of the
excretory organs.
In the Nematoda the generative organs of both sexes originate from a
single cell (Schneider, Vol. I. No. 390).
This cell elongates and its nuclei multiply. After assuming a somewhat
columnar form, it divides into (1) a superficial investing layer, and
(2) an axial portion.
In the female the superficial layer is only developed distinctly in
the median part of the column. In the course of the further
development the two ends of the column become the blind ends of the
ovary, and the axial tissue they contain forms the germinal tissue of
nucleated protoplasm. The superficial layer gives rise to the
epithelium of the uterus and oviduct. The germinal tissue, which is
originally continuous, is interrupted in the middle part (where the
superficial layer gives rise to the uterus and oviduct), and is
confined to the two blind extremities of the tube.
In the male the superficial layer, which gives rise to the epithelium
of the vas deferens, is only formed at the hinder end of the original
column. In other respects the development takes place as in the
female.
In the Insecta again the evidence, though somewhat conflicting,
indicates that the generative ducts arise very much as in Nematodes,
from the same primitive mass as the generative organs. In both of
these types it would seem probable that the generative organs were
primitively placed in the body cavity, and attached to the epidermis,
through a pore in which their products passed out; and that, acquiring
a tubular form, the peripheral part of the gland gave rise to a duct,
the remainder constituting the true generative gland. It is quite
possible that the generative ducts of such forms as the Platyelminthes
may have had a similar origin to those in Insecta and Nematoda, but
from the analogy of the Mollusca there is nearly as much to be said
for regarding them as modified excretory organs.
In the Echinodermata nothing is unfortunately known as to the ontogeny
of the generative organs and ducts. The structure of these organs in
the adult would however seem to indicate that the most primitive type
of echinoderm generative organ consists of a blind sack, projecting
into the body cavity, and opening by
[Pg 753] a pore to the exterior. The sack
is lined by an epithelium, continuous with the epidermis, the cells of
which give rise to the ova or spermatozoa. The duct of these organs is
obviously hardly differentiated from the gland; and the whole
structure might easily be derived from the type of generative organ
characteristic of the Hydromedusæ, where the generative cells are
developed from special areas of the ectoderm, and, when ripe, pass
directly into the surrounding medium.
If this suggestion is correct we may suppose that the generative ducts
of the Echinodermata have a different origin to those of the majority
of[276]
the remaining triploblastica.
Their ducts have been evolved in forms in which the generative
products continued to be liberated directly to the exterior, as in the
Hydromedusæ; while those of other types have been evolved in forms in
which the generative products were first transported, as in the
Actinozoa, into the gastrovascular canals[277].
[Pg 754]
CHAPTER XXV.
THE ALIMENTARY CANAL AND ITS APPENDAGES, IN THE CHORDATA.
The alimentary canal in the Chordata is always formed of three
sections, analogous to those so universally present in the
Invertebrata. These sections are (1) the mesenteron lined by
hypoblast; (2) the stomodæum or mouth lined by epiblast, and (3) the
proctodæum or anal section lined like the stomodæum by epiblast.
Mesenteron.
The early development of the epithelial wall of the mesenteron has
already been described (Chapter XI.). It forms at first a simple
hypoblastic tube extending from near the front end of the body, where
it terminates blindly, to the hinder extremity where it is united with
the neural tube by the neurenteric canal (fig. 420, ne). It often
remains for a long time widely open in the middle towards the
yolk-sack.
It has already been shewn that from the dorsal wall of the mesenteron
the notochord is separated off nearly at the same time as the lateral
plates of mesoblast (pp. 292-300).
The subnotochordal rod. At a period slightly subsequent to the
formation of the notochord, and before any important differentiations
in the mesenteron have become apparent, a remarkable rod-like body,
which was first discovered by Götte, becomes split off from the dorsal
wall of the alimentary tract in all the Ichthyopsida. This body, which
has a purely provisional existence, is known as the subnotochordal
rod.
[Pg 755]
It develops in Elasmobranch embryos in two sections, one situated in
the head, and the other in the trunk.
The section in the trunk is the first to appear. The wall of the
alimentary canal becomes thickened along the median dorsal line (fig.
412, x), or else produced into a ridge into which there penetrates a
narrow prolongation of the lumen of the alimentary canal. In either
case the cells at the extreme summit become gradually constricted off
as a rod, which lies immediately dorsal to the alimentary tract, and
ventral to the notochord (fig. 413, x).
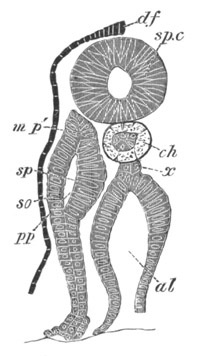
Fig. 412. Transverse section through the tail region of a
Pristiurus embryo of the same age as fig. 28 E.
df. dorsal fin; sp.c. spinal cord; pp. body cavity; sp.
splanchnic layer of mesoblast; so. somatic layer of mesoblast;
mp´. portion of splanchnic mesoblast commencing to be
differentiated into muscles; ch. notochord; x. subnotochordal
rod arising as an outgrowth of the dorsal wall of the alimentary
tract; al. alimentary tract.
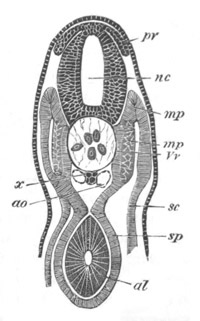
Fig. 413. Transverse section through the trunk of an embryo
slightly older than fig. 28 E.
nc. neural canal; pr. posterior root of spinal nerve; x.
subnotochordal rod; ao. aorta; sc. somatic mesoblast; sp.
splanchnic mesoblast; mp. muscle-plate; mp´. portion of
muscle-plate converted into muscle; Vv. portion of the vertebral
plate which will give rise to the vertebral bodies; al. alimentary
tract.
In the hindermost part of the body its mode of formation differs
somewhat from that above described. In this part the alimentary wall
is very thick, and undergoes no special growth prior to the formation
of the subnotochordal rod; on the contrary, a small linear portion of
the wall becomes scooped out along the median dorsal line, and
eventually separates from the remainder as the rod in question. In the
trunk the splitting off of the rod takes place from before backwards,
so that the anterior part of it is formed before the posterior.
The section of the subnotochordal rod in the head would appear to
develop in the same way as that in the trunk, and the splitting off
from the throat proceeds from before backwards.
[Pg 756]
On the formation of the dorsal aorta, the subnotochordal rod becomes
separated from the wall of the gut and the aorta interposed between
the two (fig. 367, x).
When the subnotochordal rod attains its fullest development it
terminates anteriorly some way in front of the auditory vesicle,
though a little behind the end of the notochord; posteriorly it
extends very nearly to the extremity of the tail and is almost
co-extensive with the postanal section of the alimentary tract,
though it does not reach quite so far back as the caudal vesicle (fig.
424, b x). Very shortly after it has attained its maximum size it
begins to atrophy in front. We may therefore conclude that its
atrophy, like its development, takes place from before backwards.
During the later embryonic stages not a trace of it is to be seen. It
has also been met with in Acipenser, Lepidosteus, the Teleostei,
Petromyzon, and the Amphibia, in all of which it appears to develop in
fundamentally the same way as in Elasmobranchii. In Acipenser it
appears to persist in the adult as the subvertebral ligament (Bridge,
Salensky). It has not yet been found in a fully developed form in any
amniotic Vertebrate, though a thickening of the hypoblast, which may
perhaps be a rudiment of it, has been found by Marshall and myself in
the Chick (fig. 110, x).
Eisig has instituted an interesting comparison between it and an organ
which he has found in a family of Chætopods, the Capitellidæ. In these
forms there is a tube underlying the alimentary tract for nearly its
whole length, and opening into it in front, and probably behind. A
remnant of such a tube might easily form a rudiment like the
subnotochordal rod of the Ichthyopsida, and as Eisig points out the
prolongation into the latter during its formation of the lumen of the
alimentary tract distinctly favours such a view of its original
nature. We can however hardly suppose that there is any direct genetic
connection between Eisig’s organ in the Capitellidæ and the
subnotochordal rod of the Chordata.
Splanchnic mesoblast and mesentery. The mesenteron consists at first
of a simple hypoblastic tube, which however becomes enveloped by a
layer of splanchnic mesoblast. This layer, which is not at first
continued over the dorsal side of the mesenteron, gradually grows in,
and interposes itself between the hypoblast of the mesenteron, and the
organs above. At the same time it becomes differentiated into two
layers, viz. an outer epithelioid layer which gives rise to part of
the peritoneal epithelium, and an inner layer of undifferentiated
cells which in time becomes converted into the connective tissue and
muscular walls of the mesenteron. The connective tissue layers become
first formed, while of the muscular layers the circular is the first
to make its appearance.
[Pg 757]
Coincidently with their differentiation the connective tissue stratum
of the peritoneum becomes established.
The Mesentery. Prior to the splanchnic mesoblast growing round the
alimentary tube above, the attachment of the latter structure to the
dorsal wall of the body is very wide. On the completion of this
investment the layer of mesoblast suspending the alimentary tract
becomes thinner, and at the same time the alimentary canal appears to
be drawn downwards and away from the vertebral column.
In what may be regarded as the thoracic division of the general
pleuroperitoneal space, along that part of the alimentary canal which
will form the œsophagus, this withdrawal is very slight, but it is
very marked in the abdominal region. In the latter the at first
straight digestive canal comes to be suspended from the body above by
a narrow flattened band of mesoblastic tissue. This flattened band is
the mesentery, shewn commencing in fig. 117, and much more advanced
in fig. 119, M. It is covered on either side by a layer of flat
cells, which form part of the general peritoneal epithelioid lining,
while its interior is composed of indifferent tissue.
The primitive simplicity in the arrangement of the mesentery is
usually afterwards replaced by a more complicated disposition, owing
to the subsequent elongation and consequent convolution of the
intestine and stomach.
The layer of peritoneal epithelium on the ventral side of the stomach
is continued over the liver, and after embracing the liver, becomes
attached to the ventral abdominal wall (fig. 380). Thus in the region
of the liver the body cavity is divided into two halves by a membrane,
the two sides of which are covered by the peritoneal epithelium, and
which encloses the stomach dorsally and the liver ventrally. The part
of the membrane between the stomach and liver is narrow, and
constitutes a kind of mesentery suspending the liver from the stomach:
it is known to human anatomists as the lesser omentum.
The part of the membrane connecting the liver with the anterior
abdominal wall constitutes the falciform or suspensory ligament of the
liver. It arises by a secondary fusion, and is not a remnant of a
primitive ventral mesentery (vide pp. 624 and 625).
[Pg 758]
The mesentery of the stomach, or mesogastrium, enlarges in Mammalia to
form a peculiar sack known as the greater omentum.
The mesenteron exhibits very early a trifold division. An anterior
portion, extending as far as the stomach, becomes separated off as the
respiratory division. On the formation of the anal invagination the
portion of the mesenteron behind the anus becomes marked off as the
postanal division, and between the postanal section and the
respiratory division is a middle portion forming an intestinal and
cloacal division.
The respiratory division of the mesenteron.
This section of the alimentary canal is distinguished by the fact that
its walls send out a series of paired diverticula, which meet the
skin, and after a perforation has been effected at the regions of
contact, form the branchial or visceral clefts.
In Amphioxus the respiratory region extends close up to the opening of
the hepatic diverticulum, and therefore to a position corresponding
with the commencement of the intestine in higher types. In the
craniate Vertebrata the number of visceral clefts has become reduced,
but from the extension of the visceral clefts in Amphioxus, combined
with the fact that in the higher Vertebrata the vagus nerve, which is
essentially the nerve of the branchial pouches, supplies in addition
the walls of the œsophagus and stomach, it may reasonably be
concluded, as has been pointed out by Gegenbaur, that the true
respiratory region primitively included the region which in the higher
types forms the œsophagus and stomach.
In Ascidians the respiratory sack is homologous with the respiratory
tract of Amphioxus.
The details of the development of the branchial clefts in the
different groups of Vertebrata have already been described in the
systematic part of this work.
In all the Ichthyopsida the walls of a certain number of clefts become
folded; and in the mesoblast within these folds a rich capillary
network, receiving its blood from the branchial arteries, becomes
established. These folds constitute the true internal gills.
[Pg 759]
In addition to internal gills external branchial processes covered
by epiblast are placed on certain of the visceral arches in the larva
of Polypterus, Protopterus and many Amphibia. The external gills have
probably no genetic connection with the internal gills.
The so-called external gills of the embryos of Elasmobranchii are
merely internal gills prolonged outwards through the gill clefts.
The posterior part of the primitive respiratory division of the
mesenteron becomes, in all the higher Vertebrata, the œsophagus and
stomach. With reference to the development of these parts the only
point worth especially noting is the fact that in Elasmobranchii and
Teleostei their lumen, though present in very young embryos, becomes
at a later stage completely filled up, and thus the alimentary tract
in the regions of the œsophagus and stomach becomes a solid cord of
cells (fig. 23 A, œs): as already suggested (p. 61) it seems not
impossible that this feature may be connected with the fact that the
œsophageal region of the throat was at one time perforated by gill
clefts.
In addition to the gills two important organs, viz. the thyroid body
and the lungs, take their origin from the respiratory region of the
alimentary tract.
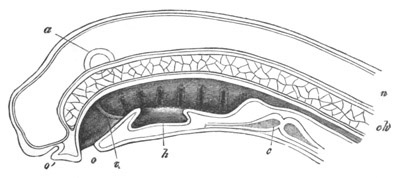
Fig. 414. Diagrammatic vertical section of a just-hatched larva
of Petromyzon. (From Gegenbaur; after Calberla.)
o. mouth; o´. olfactory pit; v. septum between stomodæum and
mesenteron; h. thyroid involution; n. spinal cord; ch.
notochord; c. heart; a. auditory vesicle.
Thyroid body. In the Ascidians the origin of a groove-like
diverticulum of the ventral wall of the branchial sack, bounded by two
lateral folds, and known as the endostyle or hypopharyngeal groove,
has already been described (p. 18). This groove remains permanently
open to the pharyngeal sack,
[Pg 760] and would seem to serve as a glandular
organ secreting mucus. As was first pointed out by W. Müller there is
present in Amphioxus a very similar and probably homologous organ,
known as the hypopharyngeal groove.
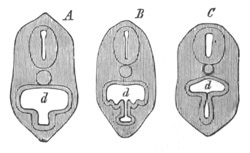
Fig. 415. Diagrammatic transverse sections through the branchial
region of young larvæ of Petromyzon. (From Gegenbaur; after
Calberla.)
d. branchial region of throat.
In the higher Vertebrata this organ never retains its primitive
condition in the adult state. In the larva of Petromyzon there is,
however, present a ventral groove-like diverticulum of the throat,
extending from about the second to the fourth visceral cleft. This
organ is shewn in longitudinal section in fig. 414, h, and in
transverse section in fig. 415, and has been identified by W. Müller
(Nos. 565 and 566) with the hypopharyngeal groove of Amphioxus and
Ascidians. It does not, however, long retain its primitive condition,
but its opening becomes gradually reduced to a pore, placed between
the third and fourth of the permanent clefts (fig. 416, th). This
opening is retained throughout the Ammocœte condition, but the organ
becomes highly complicated, with paired anterior and posterior horns
and a median spiral portion. In the adult the connection with the
pharynx is obliterated, and the organ is partly absorbed and partly
divided up into a series of glandular follicles, and eventually forms
the thyroid body.
From the consideration of the above facts W. Müller was led to the
conclusion that the thyroid body of the Craniata was derived from the
endostyle or hypopharyngeal groove. In all the higher Vertebrata the
thyroid body arises as a diverticulum of the ventral wall of the
throat in the region either of the mandibular or hyoid arches (fig.
417, Th), which after being segmented off becomes divided up into
follicles.
In Elasmobranch embryos it appears fairly early as a diverticulum from
the ventral surface of the throat in the region of the mandibular
arch, extending from the border of the mouth to the point where the
ventral aorta divides into the two aortic branches of the mandibular
arch (fig. 417, Th).
[Pg 761] Somewhat later it becomes in Scyllium and
Torpedo solid, though still retaining its attachment to the wall of
the œsophagus. It continues to grow in length, and becomes divided up
into a number of solid branched lobules separated by connective tissue
septa. Eventually its connection with the throat becomes lost, and the
lobules develop a lumen. In Acanthias the lumen of the gland is
retained (W. Müller) till after its detachment from the throat. It
preserves its embryonic position through life. In Amphibia it
originates, as in Elasmobranchii, from the region of the mandibular
arch; but when first visible it forms a double epithelial wall
connecting the throat with the nervous layer of the epidermis. It
subsequently becomes detached from the epidermis, and then has the
usual form of a diverticulum from the throat. In most Amphibians it
becomes divided into two lobes, and so forms a paired body. The
peculiar connection between the thyroid diverticulum and the epidermis
in Amphibia has been noted by Götte in Bombinator, and by Scott and
Osborn in Triton. It is not very easy to see what meaning this
connection can have.
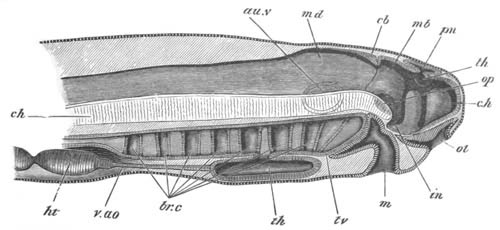
Fig. 416. Diagrammatic vertical section through the head of a
larva of Petromyzon.
The larva had been hatched three days, and was 4.8 mm. in length.
The optic and auditory vesicles are supposed to be seen through the
tissues. The letter tv pointing to the base of the velum is where
Scott believes the hyomandibular cleft to be situated.
c.h. cerebral hemisphere; th. optic thalamus; in.
infundibulum; pn. pineal gland; mb. mid-brain; cb. cerebellum;
md. medulla oblongata; au.v. auditory vesicle; op. optic
vesicle; ol. olfactory pit; m. mouth; br.c. branchial pouches;
th. thyroid involution; v.ao. ventral aorta; ht. ventricle of
heart; ch. notochord.
In the Fowl (W. Müller) the thyroid body arises at the end of the
second or beginning of the third day as an outgrowth from the
hypoblast of the throat, opposite the point of origin of the anterior
arterial arch. This outgrowth becomes by the fourth day a solid mass
of cells, and by the fifth ceases to be connected with the epithelium
of the throat, becoming at the same time bilobed. By the seventh day
it has travelled somewhat backwards, and the two lobes have completely
separated from each other. By
[Pg 762] the ninth day the whole is invested by a
capsule of connective tissue, which sends in septa dividing it into a
number of lobes or solid masses of cells, and by the sixteenth day it
is a paired body composed of a number of hollow branched follicles,
each with a ‘membrana propria,’ and separated from each other by septa
of connective tissue. It finally travels back to the point of origin
of the carotids.
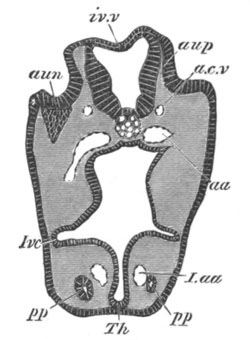
Fig. 417. Section through the head of an Elasmobranch embryo, at
the level of the auditory involution.
Th. rudiment of thyroid body; aup. auditory pit; aun. ganglion
of auditory nerve; iv.v. roof of fourth ventricle; a.c.v.
anterior cardinal vein; aa. aorta; I.aa. aortic trunk of
mandibular arch; pp. head cavity of mandibular arch; Ivc.
alimentary pouch which will form the first visceral cleft.
Amongst Mammalia the thyroid arises in the Rabbit (Kölliker) and Man
(His) as a hollow diverticulum of the throat at the bifurcation of the
foremost pair of aortic arches. It soon however becomes solid, and is
eventually detached from the throat and comes to lie on the ventral
side of the larynx or windpipe. The changes it undergoes are in the
main similar to those in the lower Vertebrata. It becomes partially
constricted into two lobes, which remain however united by an
isthmus[278].
The fact that the thyroid sometimes arises in the region
of the first and sometimes in that of the second cleft is probably to
be explained by its rudimentary character.
The Thymus gland. The thymus gland may conveniently be dealt with
here, although its origin is nearly as obscure as its function. It has
usually been held to be connected with the lymphatic system. Kölliker
was the first to shew that this view was probably erroneous, and he
attempted to prove that it was derived in the Rabbit from the walls of
one of the visceral clefts, mainly on the ground of its presenting in
the embryo an epithelial character.
[Pg 763]
Stieda (No. 569) has recently verified Kölliker’s statements. He finds
that in the Pig and the Sheep the thymus arises as a paired outgrowth
from the epithelial remnants of a pair of visceral clefts. Its two
lobes may at first be either hollow (Sheep) or solid (Pig), but
eventually become solid, and unite in the median line. Stieda and His
hold that in the adult gland, the so-called corpuscles of Hassall are
the remnants of the embryonic epithelial part of the gland, and that
the lymphatic part of it is of mesoblastic origin; but Kölliker
believes the lymphatic cells to be direct products of the embryonic
epithelial cells.
The posterior visceral clefts in the course of their atrophy give rise
to various more or less conspicuous bodies of a pseudo-glandular
nature, which have been chiefly studied by Remak[279].
Swimming bladder and lungs. A swimming bladder is present in all
Ganoids and in the vast majority of Teleostei. Its development however
is only imperfectly known.
In the Salmon and Carp it arises, as was first shewn by Von Baer, as
an outgrowth of the alimentary tract, shortly in front of the liver.
In these forms it is at first placed on the dorsal side and slightly
to the right, and grows backwards on the dorsal side of the gut,
between the two folds of the mesentery.
The absence of a pneumatic duct in the Physoclisti would appear to be
due to a post-larval atrophy.
In Lepidosteus the air-bladder appears to arise, as in the Teleostei,
as an invagination of the dorsal wall of the œsophagus.
In advanced embryos of Galeus, Mustelus and Acanthias, Miklucho-Maclay
detected a small diverticulum opening on the dorsal side of the
œsophagus, which he regards as a rudiment of a swimming bladder. This
interpretation must however be regarded as somewhat doubtful.
The lungs. The lungs originate in a nearly identical way in all the
Vertebrate forms in which their development has been observed. They
are essentially buds or processes of the ventral wall of the primitive
œsophagus.
At a point immediately behind the region of the visceral clefts the
cavity of the alimentary canal becomes compressed laterally, and at
the same time constricted in the middle, so that its transverse
section (fig. 418 1) is somewhat hourglass-shaped, and shews an upper
or dorsal chamber d, joining on to a lower or ventral chamber l by
a short narrow neck.
[Pg 764]
The hinder end of the lower tube enlarges (fig. 418 2), and then
becomes partially divided into two lobes (fig. 418 3). All these parts
at first freely communicate, but the two lobes, partly by their own
growth, and partly by a process of constriction, soon become isolated
posteriorly; while in front they open into the lower chamber of the
œsophagus (fig. 422).
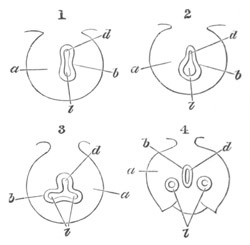
Fig. 418. Four diagrams illustrating the formation of the
Lungs. (After Götte.)
a. mesoblast; b. hypoblast; d. cavity of digestive canal; l.
cavity of the pulmonary diverticulum.
In (1) the digestive canal has commenced to be constricted into an
upper and lower canal; the former the true alimentary canal, the
latter the pulmonary tube; the two tubes communicate with each
other in the centre.
In (2) the lower (pulmonary) tube has become expanded.
In (3) the expanded portion of the tube has become constricted into
two tubes, still communicating with each other and with the
digestive canal.
In (4) these are completely separated from each other and from the
digestive canal, and the mesoblast has also begun to exhibit
externally changes corresponding to the internal changes which
have been going on.
By a continuation forwards of the process of constriction the lower
chamber of the œsophagus, carrying with it the two lobes above
mentioned, becomes gradually transformed into an independent tube,
opening in front by a narrow slit-like aperture into the œsophagus.
The single tube in front is the rudiment of the trachea and larynx,
while the two diverticula behind become (fig. 419, lg) the bronchial
tubes and lungs.
While the above changes are taking place in the hypoblastic walls of
the alimentary tract, the splanchnic mesoblast surrounding these
structures becomes very much thickened; but otherwise bears no marks
of the internal changes which are going on, so that the above
formation of the lungs and trachea cannot be seen from the surface. As
the paired diverticula of the lungs grow backwards, the mesoblast
around them takes however the form of two lobes, into which they
gradually bore their way.
There do not seem to be any essential differences in the mode of
formation of the above structures in the types so far observed, viz.
Amphibia, Aves and Mammalia. Writers differ as to whether the lungs
first arise as
[Pg 765] paired diverticula, or as a single diverticulum; and as
to whether the rudiments of the lungs are established before those of
the trachea. If the above account is correct it would appear that any
of these positions might be maintained. Phylogenetically interpreted
the ontogeny of the lungs appears however to imply that this organ was
first an unpaired structure and has become secondarily paired, and
that the trachea was relatively late in appearing.
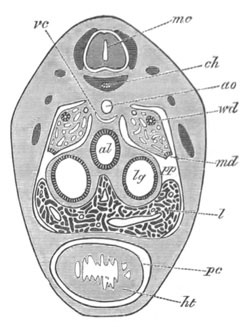
Fig. 419. Section through the cardiac region of an embryo of
Lacerta Muralis of 9 mm. to shew the mode of formation of the
pericardial cavity.
ht. heart; pc. pericardial cavity; al. alimentary tract; lg.
lung; l. liver; pp. body cavity; md. open end of Müllerian
duct; wd. Wolffian duct; vc. vena cava inferior; ao. aorta;
ch. notochord; mc. medullary cord.
The further development of the lungs is at first, in the higher types
at any rate, essentially similar to that of a racemose gland. From
each primitive diverticulum numerous branches are given off. In Aves
and Mammalia (fig. 355) they are mainly confined to the dorsal and
lateral parts. These branches penetrate into the surrounding mesoblast
and continue to give rise to secondary and tertiary branches. In the
mesoblast around them numerous capillaries make their appearance, and
the further growth of the bronchial tubes is supposed by Boll to be
due to the mutual interaction of the hitherto passive mesoblast and of
the hypoblast.
The further changes in the lungs vary somewhat in the different forms.
The air sacks are the most characteristic structures of the avian
lung. They are essentially the dilated ends of the primitive
diverticula or of their main branches.
In Mammalia (Kölliker, No. 298) the ends of the bronchial tubes become
dilated into vesicles, which may be called the primary air-cells. At
first, owing to their development at the ends of the bronchial
branches, these are confined to the surface of the lungs. At a later
period the primary air-cells divide each into two or three parts, and
give rise to secondary air-cells, while at the same time the smallest
bronchial tubes, which continue all the while to divide, give rise at
all points to fresh air-cells. Finally the bronchial tubes cease to
become more branched, and the air-cells belonging to each minute lobe
come in their further growth to open into a common chamber.
[Pg 766] Before the
lungs assume their function the embryonic air-cells undergo a
considerable dilatation.
The trachea and larynx. The development of the trachea and larynx
does not require any detailed description. The larynx is formed as a
simple dilatation of the trachea. The cartilaginous structures of the
larynx are of the same nature as those of the trachea.
It follows from the above account that the whole pulmonary structure
is the result of the growth by budding of a system of branched
hypoblastic tubes in the midst of a mass of mesoblastic tissue, the
hypoblastic elements giving rise to the epithelium of the tubes, and
the mesoblast providing the elastic, muscular, cartilaginous,
vascular, and other connective tissues of the tracheal and bronchial
walls.
There can be no doubt that the lungs and air-bladder are homologous
structures, and the very interesting memoir of Eisig on the
air-bladder of the Chætopoda[280]
shews it to be highly probable that
they are the divergent modifications of a primitive organ, which
served as a reservoir for gas secreted in the alimentary tract, the
gas in question being probably employed for respiration when, for any
reason, ordinary respiration by the gills was insufficient.
Such an organ might easily become either purely respiratory, receiving
its air from the exterior, and so form a true lung; or mainly
hydrostatic, forming an air-bladder, as in Ganoidei and Teleostei.
It is probable that in the Elasmobranchii the air-bladder has become
aborted, and the organ discovered by Micklucho-Maclay may perhaps be a
last remnant of it.
The middle division of the mesenteron. The middle division of the
mesenteron, forming the intestinal and cloacal region, is primitively
a straight tube, the intestinal region of which in most Vertebrate
embryos is open below to the yolk-sack.
Cloaca. In the Elasmobranchii, the embryos of which probably retain a
very primitive condition of the mesenteron, this region is not at
first sharply separated from the postanal section behind. Opposite the
point where the anus will eventually
[Pg 767] appear a dilatation of the
mesenteron arises, which comes in contact with the external skin (fig.
28 E, an). This dilatation becomes the hypoblastic section of the
cloaca. It communicates behind with the postanal gut (fig. 424 D), and
in front with the intestine; and may be defined as the dilated
portion of the alimentary tract which receives the genital and urinary
ducts and opens externally by the proctodæum.
In Acipenser and Amphibia the cloacal region is indicated as a ventral
diverticulum of the mesenteron even before the closure of the
blastopore. It is shewn in the Amphibia at an early stage in fig. 73,
and at a later period, when in contact with the skin at the point
where the anal invagination is about to appear, in fig. 420.
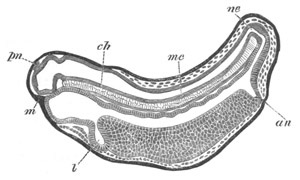
Fig. 420. Longitudinal section through an advanced embryo of
Bombinator. (After Götte.)
m. mouth; an. anus; l. liver; ne. neurenteric canal; mc.
medullary canal; ch. notochord; pn. pineal gland.
In the Sauropsida and Mammalia the cloaca appears as a dilatation of
the mesenteron, which receives the opening of the allantois almost as
soon as the posterior part of the mesenteron is established.
The eventual changes which it undergoes have been already dealt with
in connection with the urinogenital organs.
Intestine. The region in front of the cloaca forms the intestine. In
certain Vertebrata it nearly retains its primitive character as a
straight tube; and in these types its anterior part is characterised
by the presence of a peculiar fold, which in a highly specialised
condition is known as the spiral valve. This structure appears in its
simplest form in Ammocœtes. It
[Pg 768] there consists of a fold in the wall
of the intestine, giving to the lumen of this canal a semilunar form
in section, and taking a half spiral.
In Elasmobranchii a similar fold to that in Ammocœtes first makes its
appearance in the embryo. This fold is from the first not quite
straight, but winds in a long spiral round the intestine. In the
course of development it becomes converted into a strong ridge
projecting into the lumen of the intestine (fig. 388, l). The spiral
it makes becomes much closer, and it thus acquires the form of the
adult spiral valve. A spiral valve is also found in Chimæra and
Ganoids. No rudiment of such an organ is found in the Teleostei, the
Amphibia, or the higher Vertebrata.
The presence of this peculiar organ appears to be a very primitive
Vertebrate character. The intestine of Ascidians exhibits exactly the
same peculiarity as that of Ammocœtes, and we may probably conclude
from embryology that the ancestral Chordata were provided with a
straight intestine having a fold projecting into its lumen, to
increase the area of the intestinal epithelium.
In all forms in which there is not a spiral valve, with the exception
of a few Teleostei, the intestine becomes considerably longer than the
cavity which contains it, and therefore necessarily more or less
convoluted.
The posterior part usually becomes considerably enlarged to form the
rectum or in Mammalia the large intestine.
In Elasmobranchii there is a peculiar gland opening into the dorsal
side of the rectum, and in many other forms there is a cæcum at the
commencement of the rectum or of the large intestine.
In Teleostei, the Sturgeon and Lepidosteus there opens into the front
end of the intestine a number of cæcal pouches known as the pancreatic
cæca. In the adult Sturgeon these pouches unite to form a compact
gland, but in the embryo they arise as a series of isolated outgrowths
of the duodenum.
Connected with the anterior portion of the middle region of the
alimentary canal, which may be called the duodenum, are two very
important and constant glandular organs, the liver and the pancreas.
[Pg 769]
The liver. The liver is the earliest formed and largest glandular
organ in the embryo.
It appears in its simplest form in Amphioxus as a single unbranched
diverticulum of the alimentary tract, immediately behind the
respiratory region, which is directed forwards and placed on the left
side of the body.
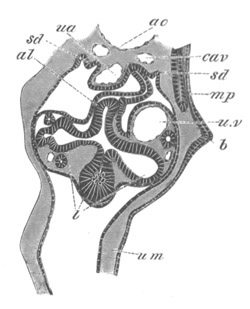
Fig. 421. Section through the ventral part of the trunk of a young
embryo of Scyllium at the level of the umbilical cord.
b. pectoral fin; ao. dorsal aorta; cav. cardinal vein; ua.
vitelline artery; uv. vitelline vein united with subintestinal
vein; al. duodenum; l. liver; sd. opening of segmental duct
into the body-cavity; mp. muscle-plate; um. umbilical canal.
In all true Vertebrata the gland has a much more complicated
structure. It arises as a ventral outgrowth of the duodenum (fig. 420,
l). This outgrowth may be at first single, and then grow out into
two lobes, as in Elasmobranchii (fig. 421) and Amphibia, or have from
the first the form of two somewhat unequal diverticula, as in Birds
(fig. 422), or again as in the Rabbit (Kölliker) one diverticulum may
be first formed, and a second one appear somewhat later. The hepatic
diverticula, whatever may be their primitive form, grow into a special
thickening of the splanchnic mesoblast.
From the primitive diverticula there are soon given off a number of
hollow buds (fig. 421) which rapidly increase in length and number,
and form the so-called hepatic cylinders. They soon anastomose and
unite together, and so constitute an irregular network. Coincidently
with the formation of the hepatic network the united vitelline and
visceral vein or veins (u.v), in their passage through the liver,
give off numerous branches, and gradually break up into a plexus of
channels which form a secondary network amongst the hepatic cylinders.
In Amphibia these channels are stated by Götte to be lacunar, but in
Elasmobranchii, and probably Vertebrata generally, they are from the
first provided with distinct though delicate walls.
[Pg 770]
It is still doubtful whether the hepatic cylinders are as a rule
hollow or solid. In Elasmobranchii they are at first provided with a
large lumen, which though it becomes gradually smaller never entirely
vanishes. The same seems to hold good for Amphibia and some Mammalia.
In Aves the lumen of the cylinders is even from the first much more
difficult to see, and the cylinders are stated by Remak to be solid,
and he has been followed in this matter by Kölliker. In the Rabbit
also Kölliker finds the cylinders to be solid.
The embryonic hepatic network gives rise to the parenchyma of the
adult liver, with which in its general arrangement it closely agrees.
The blood-channels are at first very large, and have a very irregular
arrangement; and it is not till comparatively late that the hepatic
lobules with their characteristic vascular structures become
established.
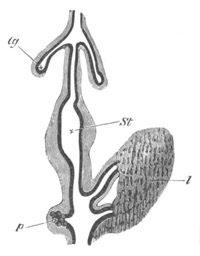
Fig. 422. Diagram of the digestive tract of a Chick upon the
fourth day. (After Götte.)
The black line indicates the hypoblast. The shaded part around it is
the splanchnic mesoblast.
lg. lung; st. stomach; p. pancreas; l. liver.
The biliary ducts are formed either from some of the primitive hepatic
cylinders, or, as would seem to be the case in Elasmobranchii and
Birds (fig. 422), from the larger diverticula of the two primitive
outgrowths.
The gall-bladder is so inconstant, and the arrangement of the ducts
opening into the intestine so variable, that no general statements can
be made about them. In Elasmobranchii the primitive median
diverticulum (fig. 421) gives rise to the ductus choledochus. Its
anterior end dilates to form a gall-bladder.
In the Rabbit a ductus choledochus is formed by a diverticulum from
the intestine at the point of insertion of the two primitive lobes.
The gall-bladder arises as a diverticulum of the right primitive lobe.
The liver is relatively very large during embryonic life and has, no
doubt, important functions in connection with the circulation.
[Pg 771]
The pancreas. So far as is known the development of the pancreas takes
place on a very constant type throughout the series of craniate
Vertebrata, though absent in some of the Teleostean fishes and
Cyclostomata, and very much reduced in most Teleostei and in
Petromyzon.
It arises nearly at the same time as the liver in the form of a hollow
outgrowth from the dorsal side of the intestine nearly opposite but
slightly behind the hepatic outgrowth (fig. 422, p). It soon
assumes, in Elasmobranchii and Mammalia, somewhat the form of an
inverted funnel, and from the expanded dorsal part of the funnel there
grow out numerous hollow diverticula into the passive splanchnic
mesoblast.
As the ductules grow longer and become branched, vascular processes
grow in between them, and the whole forms a compact glandular body in
the mesentery on the dorsal side of the alimentary tract. The
funnel-shaped receptacle loses its original form, and elongating,
assumes the character of a duct.
From the above mode of development it is clear that the glandular
cells of the pancreas are derived from the hypoblast.
Into the origin of the varying arrangements of the pancreatic ducts it
is not possible to enter in detail. In some cases, e.g. the Rabbit
(Kölliker), the two lobes and ducts arise from a division of the
primitive gland and duct. In other cases, e.g. the Bird, a second
diverticulum springs from the alimentary tract. In a large number of
instances the primitive condition with a single duct is retained.
Postanal section of the mesenteron. In the embryos of all the Chordata
there is a section of the mesenteron placed behind the anus. This
section invariably atrophies at a comparatively early period of
embryonic life; but it is much better developed in the lower forms
than in the higher. At its posterior extremity it is primitively
continuous with the neural tube (fig. 420), as was first shewn by
Kowalevsky.
The canal connecting the neural and alimentary canals has already been
described as the neurenteric canal, and represents the remains of the
blastopore.
In the Tunicata the section of the mesenteron, which in all
probability corresponds to the postanal gut of the Vertebrata, is that
immediately
[Pg 772] following the dilated portion which gives rise to the
branchial cavity and permanent intestine. It has already been shewn
that from the dorsal and lateral portions of this section of the
primitive alimentary tract the notochord and muscles of the Ascidian
tadpole are derived. The remaining part of its walls forms a solid
cord of cells (fig. 423, al´), which either atrophies, or, according
to Kowalevsky, gives rise to blood-vessels.
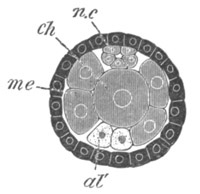
Fig. 423. Transverse optical section of the tail of an embryo of
Phallusia mammillata. (After Kowalevsky.)
The section is from an embryo of the same age as fig. 8 IV.
ch. notochord; n.c. neural canal; me. mesoblast; al´.
hypoblast of tail.
In Amphioxus the postanal gut, though distinctly developed, is not
very long, and atrophies at a comparatively early period.
In Elasmobranchii this section of the alimentary tract is very well
developed, and persists for a considerable period of embryonic life.
The following is a history of its development in the genus Scyllium.
Shortly after the stage when the anus has become marked out by the
alimentary tract sending down a papilliform process towards the skin,
the postanal gut begins to develop a terminal dilatation or vesicle,
connected with the remainder of the canal by a narrower stalk.
The walls both of the vesicle and stalk are formed of a fairly
columnar epithelium. The vesicle communicates in front by a narrow
passage with the neural canal, and behind is continued into two horns
corresponding with the two caudal swellings previously spoken of (p.
55). Where the canal is continued into these two horns, its walls lose
their distinctness of outline, and become continuous with the adjacent
mesoblast.
In the succeeding stages, as the tail grows longer and longer, the
postanal section of the alimentary tract grows with it, without
however undergoing alteration in any of its essential characters. At
the period of the maximum development, it has a length of about 1⁄3 of
that of the whole alimentary tract.
Its features at a stage shortly before the external gills have become
prominent are illustrated by a series of transverse sections through
the tail (fig. 424). The four sections have been selected for
illustration out of a fairly-complete series of about one hundred and
twenty.
Posteriorly (A) there is present a terminal vesicle (alv) .25 mm. in
diameter, which communicates dorsally by a narrow opening with the
neural canal (nc); to this is attached a stalk in the form of a
tube, also lined by columnar epithelium, and extending through about
thirty sections (B al). Its average diameter is about .084 mm., and
its walls are very thick. Overlying its front end is the
subnotochordal rod (x), but this does not extend as far back as the
terminal vesicle.
The thick-walled stalk of the vesicle is connected with the cloacal
section
[Pg 773] of the alimentary tract by a very narrow thin-walled tube (C
al). This for the most part has a fairly uniform calibre, and a
diameter of not more than .035 mm. Its walls are formed of flattened
epithelial cells. At a point not far from the cloaca it becomes
smaller, and its diameter falls to .03 mm. In front of this point it
rapidly dilates again, and, after becoming fairly wide, opens on the
dorsal side of the cloacal section of the alimentary canal just behind
the anus (D al).
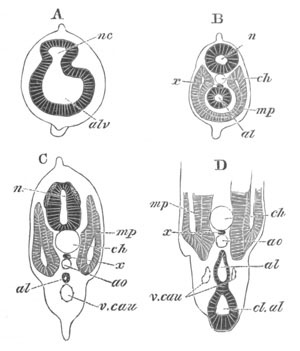
Fig. 424. Four sections through the postanal part of the tail
of an embryo of the same age as fig. 28 F.
A. is the posterior section.
nc. neural canal; al. postanal gut; alv. caudal vesicle of
postanal gut; x. subnotochordal rod; mp. muscle-plate; ch.
notochord; cl.al. cloaca; ao. aorta; v.cau. caudal vein.
Very shortly after the stage to which the above figures belong, at a
point a little behind the anus, where the postanal section of the
canal was thinnest in the previous stage, it becomes solid, and a
rupture here occurs in it at a slightly later period.
The atrophy of this part of the alimentary tract having once commenced
proceeds rapidly. The posterior part first becomes reduced to a small
rudiment near the end of the tail. There is no longer a terminal
vesicle, nor a neurenteric canal. The portion of the postanal section
of the alimentary tract, just behind the cloaca, is for a short time
represented by a small rudiment of the dilated part which at an
earlier period opened into the cloaca.
In Teleostei the vesicle at the end of the tail, discovered by
Kupffer,
[Pg 774] (fig. 34, hyv) is probably the equivalent of the vesicle at
the end of the postanal gut in Elasmobranchii.
In Petromyzon and in Amphibia there is a well-developed postanal gut
connected with a neurenteric canal which gradually atrophies. It is
shewn in the embryo of Bombinator in fig. 420.
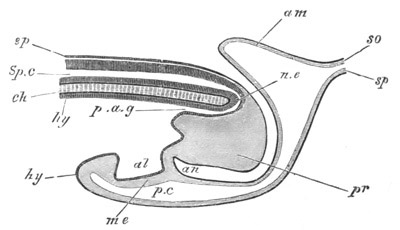
Fig. 425. Diagrammatic longitudinal section through the posterior
end of an embryo Bird at the time of the formation of the Allantois.
ep. epiblast; Sp.c. spinal canal; ch. notochord; n.e.
neurenteric canal; hy. hypoblast; p.a.g. postanal gut; pr.
remains of primitive streak folded in on the ventral side; al.
allantois; me. splanchnic mesoblast; an. point where anus will
be formed; p.c. perivisceral cavity; am. amnion; so.
somatopleure; sp. splanchnopleure.
Amongst the amniotic Vertebrata the postanal gut is less developed
than in the Ichthyopsida. A neurenteric canal is present for a short
period in various Birds (Gasser, etc.) and in the Lizard, but
disappears very early. There is however, as has been pointed out by
Kölliker, a well-marked postanal gut continued as a narrow tube from
behind the cloaca into the tail both in the Bird (fig. 425, p.a.g.)
and Mammals (the Rabbit), but especially in the latter. It atrophies
early as in lower forms.
The morphological significance of the postanal gut and of the
neurenteric canal has already been spoken of in Chapter XII., p. 323.
The Stomodæum.
The anterior section of the permanent alimentary tract is formed by an
invagination of epiblast, constituting a more or less considerable
pit, with its inner wall in contact with the blind anterior extremity
of the alimentary tract.
In Ascidians this pit is placed on the dorsal surface (fig. 9, o),
and becomes the permanent oral cavity of these forms. In the larva of
Amphioxus it is stated to be formed unsymmetrically
[Pg 775] (vide p. 5), but
further observations on its development are required.
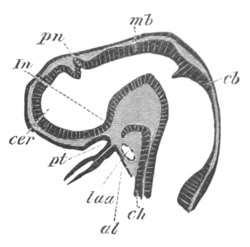
Fig. 426. Longitudinal section through the brain of a young
Pristiurus embryo.
cer. unpaired rudiment of the cerebral hemispheres; pn. pineal
gland; In. infundibulum; pt. ingrowth from mouth to form the
pituitary body; mb. mid-brain; cb. cerebellum; ch. notochord;
al. alimentary tract; Iaa. artery of mandibular arch.
In the true Vertebrata it is always formed on the ventral surface of
the head, immediately behind the level of the fore-brain (fig. 426),
and is deeper in Petromyzon (fig. 416, m) than in any other known
form.
From the primary buccal cavity or stomodæum there grows out the
pituitary pit (fig. 426, pt), the development of which has already
been described (p. 435).
The wall separating the stomodæum from the mesenteron always becomes
perforated, usually at an early stage of development, and though in
Petromyzon the boundary between the two cavities remains indicated by
the velum, yet in the higher Vertebrata all trace of this boundary is
lost, and the original limits of the primitive buccal cavity become
obliterated; while a secondary buccal cavity, partly lined by
hypoblast and partly by epiblast, becomes established.
This cavity, apart from the organs which belong to it, presents
important variations in structure. In most Pisces it retains a fairly
simple character, but in the Dipnoi its outer boundary becomes
extended so as to enclose the ventral opening of the nasal sack, which
thenceforward constitutes the posterior nares.
In Amphibia and Amniota the posterior nares also open well within the
boundary of the buccal cavity.
In the Amniota further important changes take place.
In the first place a plate grows inwards from each of the superior
maxillary processes (fig. 427, p), and the two plates, meeting in
the middle line, form a horizontal septum dividing the front part of
the primitive buccal cavity into a dorsal respiratory section (n),
containing the opening of the posterior nares, and a ventral cavity,
forming the permanent mouth. The
[Pg 776] two divisions thus formed open into a
common cavity behind. The horizontal septum, on the development within
it of an osseous plate, constitutes the hard palate.
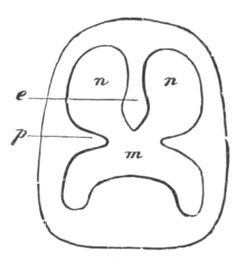
Fig. 427. Diagram shewing the division of the primitive buccal
cavity into the respiratory section above and the true mouth
below. (From Gegenbaur.)
p. palatine plate of superior maxillary process; m. permanent
mouth; n. posterior part of nasal passage; e. internasal
septum.
An internasal septum (fig. 427, e) may more or less completely
divide the dorsal cavity into two canals, continuous respectively with
the two nasal cavities.
In Mammalia a posterior prolongation of the palate, in which an
osseous plate is not formed, constitutes the soft palate.
The second change in the Amniota, which also takes place in some
Amphibia, is caused by the section of the mesenteron into which the
branchial pouches open, becoming, on the atrophy of these structures,
converted into the posterior part of the buccal cavity.
The organs derived from the buccal cavity are the tongue, the various
salivary glands, and the teeth; but the latter alone will engage our
attention here.
The teeth. The teeth are to be regarded as a special product of the
oral mucous membrane. It has been shewn by Gegenbaur and Hertwig that
in their mode of development they essentially resemble the placoid
scales of Elasmobranchii, and that the latter structures extend in
Elasmobranchii for a certain distance into the cavity of the mouth.
As pointed out by Gegenbaur, the teeth are therefore to be regarded as
more or less specialised placoid scales, whose presence in the mouth
is to be explained by the fact that the latter structure is lined by
an invagination of the epidermis; The most important developmental
point of difference between teeth and placoid scales consists in the
fact, that in the case of the former there is a special ingrowth of
epiblast to meet a connective tissue papilla which is not found in the
latter.
Although the teeth are to be regarded as primitively epiblastic
structures, they are nevertheless found in Teleostei and Ganoidei on
the hyoid
[Pg 777] and branchial arches; and very possibly the teeth on some
other parts of the mouth are developed in a true hypoblastic region.
The teeth are formed from two distinct organs, viz. an epithelial cap
and a connective tissue papilla.
The general mode of development, as has been more especially shewn by
the extended researches of Tomes, is practically the same for all
Vertebrata, and it will be convenient to describe it as it takes place
in Mammalia.
Along the line where the teeth are about to develop, there is formed
an epithelial ridge projecting into the subjacent connective tissue,
and derived from the innermost columnar layer of the oral epithelium.
At the points where a tooth is about to be formed this ridge undergoes
special changes. It becomes in the first place somewhat thickened by
the development of a number of rounded cells in its interior; so that
it becomes constituted of (1) an external layer of columnar cells, and
(2) a central core of rounded cells; both of an epithelial nature. In
the second place the organ gradually assumes a dome-shaped form (fig.
428, e), and covers over a papilla of the subepithelial connective
tissue (p) which has in the meantime been developed.
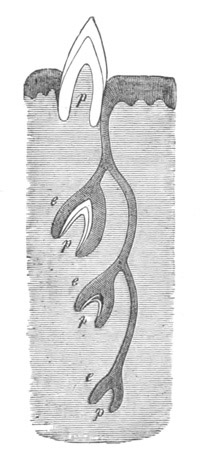
Fig. 428. Diagram shewing the development of the teeth. (From
Gegenbaur.)
p. dental papilla; e. enamel organ.
From the above epithelial structure, which may be called the enamel
organ, and from the papilla it covers, which may be spoken of as the
dental papilla, the whole tooth is developed. After these parts have
become established there is formed round the rudiment of each tooth a
special connective tissue capsule; known as the dental capsule.
Before the dental capsule has become definitely formed the enamel
organ and the dental papilla undergo important changes. The rounded
epithelial cells forming the core of the enamel organ undergo a
peculiar transformation into a tissue closely resembling ordinary
embryonic connective tissue, while at the same time the epithelium
adjoining the dental papilla and covering the inner surface of the
enamel organ, acquires a somewhat different structure to the
epithelium on the outer side of the organ. Its cells become very
markedly columnar, and form a very regular cylindrical epithelium.
This layer alone is concerned in forming the enamel. The cells of the
outer epithelial layer of the enamel organ become somewhat flattened,
and the surface of the layer is raised into a series of short papillæ
which project into the highly vascular tissue of the dental sheath.
Between
[Pg 778] the epithelium of the enamel organ and the adjoining
connective tissue there is everywhere present a delicate membrane
known as the membrana præformativa.
The dental papilla is formed of a highly vascular core and a
non-vascular superficial layer adjoining the inner epithelium of the
enamel organ. The cells of the superficial layer are arranged so as
almost to resemble an epithelium.
The first formation of the hard structures of the tooth commences at
the apex of the dental papilla. A calcification of the outermost layer
of the papilla sets in, and results in the formation of a thin layer
of dentine. Nearly simultaneously a thin layer of enamel is deposited
over this, from the inner epithelial layer of the enamel organ (fig.
428). Both enamel and dentine continue to be deposited till the crown
of the tooth has reached its final form, and in the course of this
process the enamel organ is reduced to a thin layer, and the whole of
the outer layer of the dental papilla is transformed into
dentine—while the inner portion remains as the pulp.
The root of the tooth is formed later than the crown, but the enamel
organ is not prolonged over this part, so that it is only formed of
dentine.
By the formation of the root the crown of the tooth becomes pushed
outwards, and breaking through its sack projects freely on the
surface.
The part of the sack which surrounds the root of the tooth gives rise
to the cement, and becomes itself converted into the periosteum of the
dental alveolus.
The general development of the enamel organs and dental papillæ is
shewn in the diagram (fig. 428). From the epithelial ridge three
enamel organs are represented as being developed. Such an arrangement
may occur when teeth are successively replaced. The lowest and
youngest enamel organ (e) has assumed a cap-like form enveloping a
dental papilla, but no calcification has yet taken place.
In the next stage a cap of dentine has become formed, while in the
still older tooth this has become covered by a layer of enamel. As may
be gathered from this diagram, the primitive epithelial ridge from
which the enamel organ is formed is not necessarily absorbed on the
formation of a tooth, but is capable of giving rise to fresh enamel
organs. When the enamel organ has reached a certain stage of
development, its connection with the epithelial ridge is ruptured
(fig. 428).
The arrangement represented in fig. 428, in which successive enamel
organs are formed from the same epithelial ridge, is found in most
Vertebrata except the Teleostei. In the Teleostei, however (Tomes), a
fresh enamel organ grows inwards from the epithelium for each
successively formed tooth.
The Proctodæum.
In all Vertebrata the cloacal section of the alimentary tract which
receives the urinogenital ducts is placed in communication
[Pg 779] with the
exterior by means of an epiblastic invagination, constituting a
proctodæum.
This invagination is not usually very deep, and in most instances the
boundary wall between it and the hypoblastic cloaca is not perforated
till considerably after the perforation of the stomodæum; in
Petromyzon, however, its perforation is effected before the mouth and
pharynx are placed in communication.
The mode of formation of the proctodæum, which is in general extremely
simple, is illustrated by fig. 420 an.
In most forms the original boundary between the epiblast of the
proctodæum and the hypoblast of the primitive cloaca becomes
obliterated after the two have become placed in free communication.
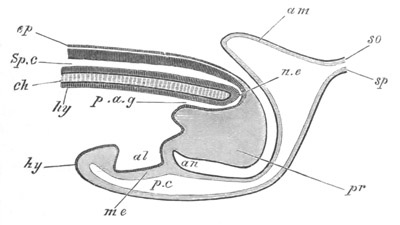
Fig. 429. Diagrammatic longitudinal section through the posterior
end of an embryo Bird at the time of the formation of the Allantois.
ep. epiblast; Sp.c. spinal canal; ch. notochord; n.e.
neurenteric canal; hy. hypoblast; p.a.g. postanal gut; pr.
remains of primitive streak folded in on the ventral side; al.
allantois; me. mesoblast; an. point where anus will be formed;
p.c. perivisceral cavity; am. amnion; so. somatopleure; sp.
splanchnopleure.
In Birds the formation of the proctodæum is somewhat more complicated
than in other types, owing to the outgrowth from it of the bursa
Fabricii.
The proctodæum first appears when the folding off of the tail end of
the embryo commences (fig. 429, an) and is placed near the front
(originally the apparent hind) end of the primitive streak. Its
position marks out the front border of the postanal section of the
gut.
The bursa Fabricii first appears on the seventh day (in the chick), as
a dorsal outgrowth of the proctodæum. The actual perforation of the
septum between the proctodæum and the cloacal section of the
alimentary tract is not effected till about the fifteenth day of
fœtal life, and the approximation
[Pg 780] of the epithelial layers of the two
organs, preparatory to their absorption, is partly effected by the
tunneling of the mesoblastic tissue between them by numerous spaces.
The hypoblastic section of the cloaca of birds, which receives the
openings of the urinogenital ducts, is permanently marked off by a
fold from the epiblastic section or true proctodæum, with which the
bursa Fabricii communicates.
Bibliography.
Alimentary Canal and its appendages.
(561) B. Afanassiew. “Ueber Bau u. Entwicklung d. Thymus d. Säugeth.”
Archiv f. mikr. Anat. Bd. XIV. 1877.
(562) Fr. Boll. Das Princip d. Wachsthums. Berlin, 1876.
(563) E. Gasser. “Die Entstehung d. Cloakenöffnung bei
Hühnerembryonen.” Archiv f. Anat. u. Physiol., Anat. Abth. 1880.
(564) A. Götte. Beiträge zur Entwicklungsgeschichte d. Darmkanals im
Hühnchen. 1867.
(565) W. Müller. “Ueber die Entwickelung der Schilddrüse.” Jenaische
Zeitschrift, Vol. VI. 1871.
(566) W. Müller. “Die Hypobranchialrinne d. Tunicaten.” Jenaische
Zeitschrift, Vol. VII. 1872.
(567) S. L. Schenk. “Die Bauchspeicheldrüse d. Embryo.”
Anatomischphysiologische Untersuchungen. 1872.
(568) E. Selenka. “Beitrag zur Entwicklungsgeschichte d. Luftsäcke d.
Huhns.” Zeit. f. wiss. Zool. 1866.
(569) L. Stieda. Untersuch. üb. d. Entwick. d. Glandula Thymus,
Glandula thyroidea, u. Glandula carotica. Leipzig, 1881.
(570) C. Fr. Wolff. “De formatione intestinorum.” Nov. Comment. Akad.
Petrop. 1766.
(571) H. Wölfler. Ueb. d. Entwick. u. d. Bau d. Schilddrüse. Berlin,
1880.
Vide also Kölliker (298), Götte (296), His (232 and 297), Foster and
Balfour (295), Balfour (292), Remak (302), Schenk (303), etc.
Teeth.
(572) T. H. Huxley. “On the enamel and dentine of teeth.” Quart. J.
of Micros. Science, Vol. III. 1855.
(573) R. Owen. Odontography. London, 1840-1845.
(574) Ch. S. Tomes. Manual of dental anatomy, human and comparative.
London, 1876.
(575) Ch. S. Tomes. “On the development of teeth.” Quart. J. of
Micros. Science, Vol. XVI. 1876.
(576) W. Waldeyer. “Structure and development of teeth.” Stricker’s
Histology. 1870.
Vide also Kölliker (298), Gegenbaur (294), Hertwig (306), etc.
[Pg 781]
INDEX TO VOLUME III.
- Abdominal muscles, 675
- Abdominal pore, 626, 749
- Acipenser, development of, 102;
- affinities of, 118;
- comparison of gastrula of, 279;
- pericardial cavity of, 627;
- Actinotrocha, 373
- Air-bladder of Teleostei, 77;
- Lepidosteus, 117;
- blood supply of, 645;
- general account of, 763;
- homologies of, 766;
- Alciope, eye of, 480
- Alisphenoid region of skull, 569
- Alimentary canal and appendages, development of, 754
- Alimentary tract of Ascidia, 18;
- Molgula, 22;
- Pyrosoma, 24;
- Salpa, 31;
- Elasmobranchii, 52;
- Teleostei, 75;
- Petromyzon, 93, 97;
- Acipenser, 110;
- Amphibia, 129, 136;
- Chick, 167;
- respiratory region of, 754;
- temporary closure of oesophageal region of, 759;
- Allantois, development of in Chick, 191, 198;
- blood-vessels of in Chick, 193;
- Lacerta, 205, 209;
- early development of in Rabbit, 229;
- of Guinea-pig, 264;
- origin of, 309.
- _See also_ ‘Placenta’ and ‘Bladder’
- Alternation of generations in Ascidians, origin of, 35;
- in Botryllus, 35;
- Pyrosoma, 36;
- Salpa, 36;
- Doliolum, 36;
- Alytes, branchial chamber of, 136;
- yolk-sack of, 139;
- branchiæ, 141;
- Müllerian duct of, 710;
- Amblystoma, ovum of, 120;
- Amia, ribs of, 561
- Ammocoetes, 95;
- metamorphosis of, 97;
- eye of, 498;
- Amnion, early development of in Chick, 185;
- Amphibia, development of, 120;
- viviparous, 121;
- gastrula of, 277;
- suctorial mouth of, 317;
- cerebellum of, 426;
- infundibulum of, 431;
- pineal gland of, 433;
- cerebrum of, 439;
- olfactory lobes of, 444;
- nares of, 553;
- notochord and its sheath, 548;
- vertebral column of, 554;
- ribs of, 561;
- branchial arches of, 574;
- mandibular and hyoid arches of, 582;
- columella of, 582;
- pectoral girdle of, 605;
- pelvic girdle of, 607;
- limbs of, 619;
- heart of, 638;
- arterial system of, 645;
- venous system of, 655;
- excretory system of, 707;
- vasa efferentia of, 711;
- liver of, 769;
- postanal gut of, 774;
- stomodæum of, 778;
- Amphiblastula larva of Porifera, 344
- Amphioxus, development of, 1;
- gastrula of, 275;
- formation of mesoblast of, 292;
- development of notochord of, 293;
- head of, 314;
- spinal nerves of, 461;
- olfactory organ of, 462;
- venous system of, 651;
- transverse abdominal muscle of, 673;
- generative cells of, 748;
- liver of, 769;
- postanal gut of, 772;
- stomodæum of, 777;
- Amphistylic skulls, 578
- Angular bone, 594
- Anterior abdominal vein, 653
- Anura, development of, 121;
- epiblast of, 125;
- mesoblast of, 128;
- notochord of, 128;
- hypoblast of, 129;
- general growth of embryo of, 131;
- larva of, 134;
- vertebral column of, 556;
- mandibular arch of, 584;
- Anus of Amphioxus, 7;
- Ascidia, 18;
- Pyrosoma, 28;
- Salpa, 31;
- Elasmobranchii, 57;
- Amphibia, 130, 132;
- Chick, 167;
- primitive, 324;
- Appendicularia, development of, 34
- Aqueductus vestibuli, 519
- Aqueous humour, 497
- Arachnida, nervous system of, 409;
- Area, embryonic, of Rabbit, 218;
- epiblast of, 219;
- origin of embryo from, 228;
- area opaca of Chick, 150;
- epiblast, hypoblast, and mesoblast of, 159;
- area pellucida of Chick, 150;
- of Lacerta, 202 area vasculosa of Chick, 194;
- mesoblast of, 160;
- of Lizard, 209;
- Rabbit, 228, 229;
- Arteria centralis retinæ, 503
- Arterial system of Petromyzon, 97;
- constitution of in embryo, 643;
- of Fishes, 644;
- of Amphibia, 645;
- of Amniota, 647;
- Arthropoda, head of, 313;
- nervous system of, 409;
- eye of, 480;
- excretory organs of, 688;
- Articular bone of Teleostei, 581;
- Ascidia, development of, 9
- Ascidians. _See_ ‘Tunicata’
- Ascidiozooids, 25
- Atrial cavity of Amphioxus, 7;
- Ascidia, 18;
- Pyrosoma, 24;
- Atrial pore of Amphioxus,7;[Pg 782]
- Ascidia, 20;
- Pyrosoma, 28;
- Salpa, 32;
- Auditory capsules, ossifications in, 595, 596
- Auditory involution of Elasmobranchii, 57;
- Teleostei, 73;
- Petromyzon, 89, 92;
- Acipenser, 106;
- Lepidosteus, 114;
- Amphibia, 127;
- Chick, 170;
- Auditory nerve, development of, 459
- Auditory organs, of Ascidia, 15;
- of Salpa, 31;
- of Ammocoetes, 98;
- Ganoidei, 108, 114;
- of Amphibia, 127;
- of Aves, 170;
- general development of, 512;
- of aquatic forms, 512;
- of land forms, 513;
- of Coelenterata, 513;
- of Mollusca, 515;
- of Crustacea, 516;
- of Vertebrata, 517;
- of Cyclostomata, 89, 92, 518;
- of Teleostei, Lepidosteus and Amphibia, 518;
- of Mammalia, 519;
- accessory structures of, 527;
- of Tunicata, 528;
- Auriculo-ventricular valves, 642
- Autostylic skulls, 579
- Aves, development of, 145;
- cerebellum of, 426;
- mid-brain of, 427;
- infundibulum of, 431;
- pineal gland of, 434;
- pituitary body of, 436;
- cerebrum of, 439;
- olfactory lobes of, 444;
- spinal nerves of, 449;
- cranial nerves of, 455;
- vagus of, 458;
- glossopharyngeal of, 458;
- vertebral column of, 557;
- ossification of vertebral column of, 558;
- branchial arches of, 572, 573;
- pectoral girdle of, 603;
- pelvic girdle of, 608;
- heart of, 637;
- arterial system of, 647;
- venous system of, 658;
- muscle-plates of, 670;
- excretory organs of, 714;
- mesonephros of, 715;
- pronephros of, 718;
- Müllerian duct of, 718, 720;
- nature of pronephros of, 721;
- connection of Müllerian duct with Wolffian in, 720;
- kidney of, 722;
- lungs of, 764;
- liver of, 769;
- postanal gut of, 774;
- Axolotl, 142, 143;
- ovum of, 120;
- mid-brain of, 427;
- mandibular arch of, 583;
- Basilar membrane, 524
- Basilar plate, 565
- Basipterygium, 612
- Basisphenoid region of skull, 569
- Bilateral symmetry, origin of, 373-376
- Bile duct, 770
- Bladder, Amphibia, 131;
- Blastodermic vesicle, of Rabbit, first development of, 217;
- of 7th day, 222;
- Guinea-pig, 263;
- meaning of, 291;
- Blastoderm of Pyrosoma, 24;
- Elasmobranchii, 41;
- Chick, 150;
- Lacerta 202;
- Blastopore, of Amphioxus, 2;
- of Ascidia, 11;
- Elasmobranchii, 42, 54, 62;
- Petromyzon, 87;
- Acipenser, 104;
- Amphibia, 125, 130;
- Chick, 153;
- Rabbit, 216;
- true Mammalian, 226;
- comparative history of closure of, 284, 288;
- summary of fate of, 340;
- relation of to primitive anus, 324;
- Blood-vessels, development of, 633
- Body cavity, of Ascidia, 21;
- Molgula, 21;
- Salpa, 31;
- Elasmobranchii, 47;
- of Teleostei, 75;
- Petromyzon, 94;
- Chick, 169;
- development of in Chordata, 325;
- views on origin of, 356-360, 377;
- of Invertebrata, 623;
- of Chordata, 624;
- of head, 676;
- Bombinator, branchial chamber of, 136;
- vertebral column of, 556;
- Bonellia, excretory organs of, 687
- Bones, origin of cartilage bones, 542;
- origin of membrane bones, 543;
- development of, 543;
- homologies of membrane bones, 542;
- homologies of cartilage bones, 545;
- Brachiopoda, excretory organs of, 683;
- generative ducts of, 749;
- Brain, of Ascidia, 11, 15;
- Elasmobranchii, 56, 59, 60;
- Teleostei, 77;
- Petromyzon, 89, 92;
- Acipenser, 105;
- Lepidosteus, 113;
- early development of in Chick, 170;
- flexure of in Chick, 175;
- later development of in Chick, 176;
- Rabbit, 229;
- general account of development of, 419;
- flexure of, 420;
- histogeny of, 422;
- Branchial arches, præoral, 570;
- disappearance of posterior, 573;
- dental plates of in Teleostei, 574;
- relation of to head cavities, 571;
- _See_ ‘Visceral arches’
- Branchial chamber of Amphibia, 136
- Branchial clefts, of Amphioxus, 7;
- of Ascidia, 18, 20;
- Molgula, 23;
- Salpa, 32;
- of Elasmobranchii, 57, 59-61;
- Teleostei, 77;
- Petromyzon, 91, 96;
- Acipenser, 105;
- Lepidosteus, 114, 116;
- Amphibia, 132, 133;
- Chick, 178;
- Rabbit, 231;
- præoral, 312, 318;
- of Invertebrata, 326;
- origin of, 326;
- Branchial rays, 574
- Branchial skeleton, development of, 572, 592;
- of Petromyzon, 96, 312, 571;
- of Ichthyopsida, 572;
- dental plates of in Teleostei, 574;
- relation of to head cavities, 572;
- Branchiæ, external of Elasmobranchii, 61, 62;
- Brood-pouch, of Salpa, 29;
- Teleostei, 68;
- Amphibia, 121;
- Brown tubes of Gephyrea, 686
- Bulbus arteriosus, of Fishes, 638;
- Bursa Fabricii, 167, 779
- Canalis auricularis, 639
- Canalis reuniens, 521
- Capitellidæ, excretory organs of, 683
- Carcharias, placenta of, 66
- Cardinal vein, 652
- Carnivora, placenta of, 250
- Carpus, development of, 620
- Cartilage bones of skull, 595;[Pg 783]
- Cat, placenta of, 250
- Caudal swellings of Elasmobranchii, 46, 55;
- Cephalic plate of Elasmobranchii, 55
- Cephalochorda, development of, 1
- Cephalopoda, eyes of, 473-477
- Cerebellum, Petromyzon, 93;
- Chick, 176;
- general account of development of, 424, 425;
- Cerebrum of Petromyzon, 93, 97;
- Chick, 175;
- general development of, 429, 438;
- transverse fissure of, 443;
- Cestoda, excretory organs of, 681
- Cetacea, placenta, 255
- Chætognatha, nervous system of, 349;
- eye of, 479;
- generative organs of, 743;
- generative ducts of, 749;
- Chætopoda, head of, 313;
- eyes of, 479;
- excretory organs of, 683;
- generative organs of, 743;
- generative ducts of, 749;
- Charybdæa, eye of, 472
- Cheiroptera, placenta of, 244
- Cheiropterygium, 618;
- relation of to ichthyopterygium, 621;
- Chelonia, development of, 210;
- pectoral girdle of, 603;
- arterial system of, 649;
- Chick, development of, 145;
- general growth of embryo of, 170;
- rotation of embryo of, 173;
- foetal membranes of, 185;
- epiblast of, 150, 166;
- optic nerve and choroid fissure of, 500;
- Chilognatha, eye of, 481
- Chilopoda, eye of, 481
- Chimæra, lateral line of, 539;
- vertebral column of, 548;
- nares of, 533;
- Chiromantis, oviposition of, 121
- Chorda tympani, development of, 460
- Chordata, ancestor of, 311;
- branchial system of, 312;
- evidence from Ammocoetes, 312;
- head of, 312;
- mouth of, 318;
- table of phylogeny of, 327;
- Chorion, 237;
- Choroid coat, Ammocoetes, 99;
- Choroid fissure, of Vertebrate eye, 486, 493;
- of Ammocoetes, 498;
- comparative development of, 500;
- of Chick, 501;
- of Lizards, 501;
- of Elasmobranchii, 502;
- of Teleostei, 503;
- Amphibia, 503;
- Mammals, 503, 504;
- Choroid gland, 320
- Choroid pigment, 489
- Choroid plexus, of fourth ventricle, 425;
- of third ventricle, 432;
- of lateral ventricle, 442;
- Ciliated sack of Ascidia, 18;
- Ciliary ganglion, 461
- Ciliary muscle, 490
- Ciliary processes, 488;
- comparative development of, 506;
- Clavicle, 600
- Clitoris, development of, 727
- Clinoid ridge, 569
- Cloaca, 766
- Coccygeo-mesenteric vein, 661
- Cochlear canal, 519
- Coecilia, development of, 143;
- pronephros of, 707;
- mesonephros of, 709;
- Müllerian duct of, 710;
- Coelenterata, larvæ of, 367;
- eyes of, 471;
- auditory organs of, 513;
- generative organs of, 741;
- Columella auris, 529;
- of Amphibia, 582;
- of Sauropsida, 588;
- Commissures, of spinal cord, 417;
- Coni vasculosi, 724
- Conus arteriosus, of Fishes, 638;
- Coracoid bone, 599
- Cornea, of Ammocoetes, 99;
- general development of, 495;
- corpuscles of, 496;
- comparative development of, 499;
- of Mammals, 499;
- Coronoid bone, 595
- Corpora geniculata interna, 428
- Corpora quadrigemina, 428
- Corpora striata, development of, 437
- Corpus callosum, development of, 443
- Corti, organ of, 522;
- structure of, 525;
- fibres of, 525;
- development of, 526;
- Cranial flexure, of Elasmobranchii, 58, 60;
- of Teleostei, 77;
- Petromyzon, 93, 94;
- of Amphibia, 131, 132;
- Chick, 174;
- Rabbit, 231;
- characters of, 321;
- significance of, 322;
- Cranial nerves, development of, 455;
- relation of to head cavities, 461;
- anterior roots of, 462-464;
- view on position of roots of, 466;
- Crocodilia, arterial system of, 649
- Crura cerebri, 429
- Crustacea, nervous system of, 411;
- eye of, 481;
- auditory organs of, 515;
- generative cells of, 745;
- generative ducts of, 751;
- Cupola, 524
- Cutaneous muscles, 676
- Cyathozooid, 25
- Cyclostomata, auditory organs of, 517;
- olfactory organ of, 532;
- notochord and vertebral column of, 546, 549;
- abdominal pores of, 626;
- segmental duct of, 700;
- pronephros of, 700;
- mesonephros of, 700;
- generative ducts of, 733, 749;
- venous system of, 651;
- excretory organs of, 700;
- Cystignathus, oviposition of, 122
- Dactylethra, branchial chamber of, 136;
- branchiæ of, 136;
- tadpole of, 140;
- Decidua reflexa, of Rat, 242;
- of Insectivora, 243;
- of Man, 245;
- Deiter’s cells, 526
- Dental papilla, 777
- Dental capsule, 777
- Dentary bone, 595
- Dentine, 780
- Descemet’s membrane, 496[Pg 784]
- Diaphragm, 631;
- Dipnoi, nares of, 534;
- vertebral column of, 548;
- membrane bones of skull of, 592;
- heart of, 638;
- arterial system of, 645;
- excretory system of, 707;
- stomodæum of, 777;
- Diptera, eye of, 481
- Discophora, excretory organs of, 687
- Dog, placenta of, 248
- Dohrn, on relations of Cyclostomata, 84;
- on ancestor of Chordata, 311, 319;
- Doliolum, development of, 28
- Ductus arteriosus, 649
- Ductus Botalli, 648
- Ductus Cuvieri, 654
- Ductus venosus Arantii, 663
- Dugong, heart of, 642
- Dysticus, eye of, 481
- Ear, _see_ ‘Auditory organ’
- Echinodermata, secondary symmetry of larva of, 380;
- excretory organs of, 689;
- generative ducts of, 752;
- Echinorhinus, lateral line of, 539;
- vertebral column of, 548;
- Echiurus, excretory organs of, 686
- Ectostosis, 543
- Edentata, placenta of, 248, 250, 256
- Eel, generative ducts of, 703
- Egg-shell of Elasmobranchii, 40;
- Elasmobranchii, development of, 40;
- viviparous, 40;
- general features of development of, 55;
- gastrula of, 281;
- development of mesoblast of, 294;
- notochord of, 294;
- meaning of formation of mesoblast of, 295;
- restiform tracts of, 425;
- optic lobes of, 427;
- cerebellum of, 425;
- pineal gland of, 432;
- pituitary body of, 435;
- cerebrum of, 438;
- olfactory lobes of, 444;
- spinal nerves, 449;
- cranial nerves of, 457;
- sympathetic nervous system of, 466;
- nares of, 533;
- lateral line of, 539;
- vertebral column of, 549;
- ribs of, 560;
- parachordals of, 567;
- mandibular and hyoid arches of, 576;
- pectoral girdle of, 600;
- pelvic girdle of, 607;
- limbs of, 609;
- pericardial cavity of, 627;
- arterial system of, 644;
- venous system of, 651;
- muscle-plates of, 668;
- excretory organs of, 690;
- constitution of excretory organs in adult of, 697;
- spermatozoa of, 747;
- swimming-bladder of, 763;
- intestines of, 767;
- liver of, 769;
- postanal gut of, 772;
- Elæoblast of Pyrosoma, 28;
- Elephant, placenta of, 249
- Embolic formation of gastrula, 333
- Enamel organ, 777
- Endolymph of ear, 522
- Endostosis, 543
- Endostyle of Ascidia, 18, 759;
- Epiblast, of Elasmobranchii, 47;
- Teleostei, 71, 75;
- Petromyzon, 86;
- Lepidosteus, 112;
- Amphibia, 122, 125;
- Chick, 149, 166;
- Lacerta, 203;
- Rabbit, 216, 219;
- origin of in Rabbit, 221;
- comparative account of development of, 300;
- Epibolic formation of gastrula, 334
- Epichordal formation of vertebral column, 556
- Epicrium glutinosum, 143
- Epidermis, in Coelenterata, 393;
- protective structures of, 394;
- Epididymis, 724
- Epigastric vein, 653
- Episkeletal muscles, 676
- Episternum, 602
- Epoophoron, 725
- Ethmoid bone, 597
- Ethmoid region of skull, 570
- ethmo-palatine ligament of Elasmobranchs, 576
- Euphausia, eye of, 483
- Eustachian tube, of Amphibia, 135;
- Chick, 180;
- Rabbit, 232;
- general development of, 528;
- Excretory organs, general constitution of, 680;
- of Platyelminthes, 680;
- of Mollusca, 681;
- of Polyzoa, 682;
- of Brachiopoda, 683;
- of Chætopoda, 683;
- of Gephyrea, 686;
- of Discophora, 687;
- of Arthropoda, 688;
- of Nematoda, 689;
- of Echinodermata, 689;
- constitution of in Craniata, 689;
- of Elasmobranchii, 690;
- constitution of in adult Elasmobranch, 697;
- of Petromyzon, 700;
- of Myxine, 701;
- of Teleostei,701;
- of Ganoidei, 704;
- of Dipnoi, 707;
- of Amphibia, 707;
- of Amniota, 713;
- comparison of Vertebrate and Invertebrate, 737;
- Excretory system, of Elasmobranchii, 49;
- Teleostei, 78;
- Petromyzon, 95, 98;
- Acipenser, 99;
- Amphibia, 133;
- Exoccipital bone, 595
- Exoskeleton, dermal, 393-395;
- External generative organs, 726
- Extra-branchial skeleton, 572
- Eye, of Ascidia, 16;
- Salpa, 31;
- Elasmobranchii, 56, 57, 58;
- Teleostei, 73;
- Petromyzon, 92, 98;
- Aves, 170;
- Rabbit, 229;
- general development of, 470;
- evolution of, 470, 471;
- simple, 480;
- compound, 481;
- aconous, 482;
- pseudoconous, 482;
- of Invertebrata, 471;
- of Vertebrata, 483;
- comparative development of Vertebrate, 497;
- of Ammocoetes, 497;
- of Tunicata, 507;
- of Chordata, general views on, 508;
- accessory eyes of Fishes, 509;
- muscles of, 677;
- Eyelids, development of, 506
- Falciform ligament, 757
- Falx cerebri, 439
- Fasciculi teretes, of Elasmobranchii, 426
- Feathers, development of, 396[Pg 785]
- Fenestra rotunda and ovalis, 529
- Fertilization, of Amphioxus, 2;
- of Urochorda, 9;
- Salpa, 29;
- Elasmobranchii, 46;
- of Teleostei, 68;
- Petromyzon, 84;
- Amphibia, 120;
- Chick, 145;
- Reptilia, 202;
- meaning of, 331;
- Fifth nerve, development of, 460
- Fifth ventricle, 443
- Fins, of Elasmobranchii, 62;
- Teleostei, 78;
- Petromyzon, 94, 95;
- Acipenser, 109;
- Lepidosteus, 118;
- relation of paired to unpaired, 611, 612;
- development of pelvic, 614;
- development of pectoral, 615;
- views on nature of paired fins, 616;
- Fissures of spinal cord, 417
- Foetal development, 360;
- secondary variations in, 361;
- Foot, 618
- Foramen of Munro, 430, 438
- Foramen ovale, 642
- Fore-brain, of Elasmobranchii, 55, 59, 60;
- Petromyzon, 93;
- general development of, 428;
- Formative cells, of Chick, 154
- Fornix, development of, 443
- Fornix of Gottsche, 428
- Fourth nerve, 464
- Frontals, 592
- Frontonasal process of Chick, 179
- Gaertner’s canals, 724
- Gall-bladder, 770
- Ganoidei, development of, 102;
- relations of, 118;
- nares of, 534;
- notochord of, 546;
- vertebral column of, 546, 553;
- ribs of, 561;
- pelvic girdle of, 606;
- arterial$ system of, 645;
- excretory organs of, 704;
- generative ducts of, 734;
- Gastropoda, eye of, 472
- Gastrula, of Amphioxus, 2;
- of Ascidia, 10;
- Elasmobranchii, 43, 44;
- Petromyzon, 86;
- Acipenser, 103;
- Amphibia, 123;
- comparative development of, in Invertebrata, 275;
- comparison of Mammalian, 291;
- phylogenetic meaning of, 333;
- ontogeny of (general), 333;
- phylogeny of, 338-343;
- secondary types of, 341;
- Geckos, vertebral column of, 557
- Generative cells, development of, 741;
- origin of in Coelenterata, 741;
- of Invertebrata, 743;
- of Vertebrata, 746;
- Generative ducts, of Teleostei, 704, 735;
- of Ganoids, 704;
- of Cyclostomata, 733;
- origin of, 733;
- of Lepidosteus, 735, 750;
- development and evolution of, 748;
- of Coelenterata, 748;
- of Sagitta, 749;
- of Tunicata, 749;
- Chætopoda, Gephyrea, etc., 749;
- of Mollusca, 751;
- of Discophora, 751;
- of Echinodermata, 752;
- Generative system of Elasmobranchii, 51
- Gephyrea, nervous system of, 412;
- excretory organs of, 686;
- generative cells of, 743;
- generative ducts of, 749;
- Germinal disc, of Elasmobranchii, 40;
- Teleostei, 68;
- Chick, 147;
- Germinal epithelium, 746
- Germinal layers, summary of organs derived from, in Vertebrata, 304;
- historical account of views of, 332;
- homologies of in the Metazoa, 345;
- Germinal wall of Chick, 152, 159;
- structure and changes of, 160;
- Geryonia, auditory organ of, 515
- Gill of Salpa, 31
- Giraldes, organ of, 725
- Glands, epidermic, development of, 397
- Glomerulus, external, of Chick, 716
- Glossopharyngeal nerve, development of, 456, 457
- Grey matter of spinal cord, 417;
- Growth in length of Vertebrate embryo, 306
- Guinea-pig, primitive streak of, 223;
- notochord of, 226;
- placenta of, 242;
- development of, 262;
- Gymnophiona, _see_ ‘Coecilia’
- Habenula perforata, 525
- Hairs, development of, 396
- Halichærus, placenta of, 250
- Hand, 619
- Head, comparative account of, 313;
- Head cavities, of Elasmobranchii, 50;
- Petromyzon, 90, 96;
- Amphibia, 127;
- general development of, 676;
- Head-fold of Chick, 157, 167
- Head kidney, _see_ ‘Pronephros’
- Heart, of Pyrosoma, 25;
- Elasmobranchii, 50, 58;
- Petromyzon, 94, 97;
- Acipenser, 106;
- Chick, 170;
- first appearance of in Rabbit, 230;
- general development of, 633;
- of Fishes, 635, 637;
- of Mammalia, 638;
- of Birds, 637, 639;
- meaning of development of, 637;
- of Amphibia, 638;
- of Amniota, 639;
- change of position of, 643;
- Hind-brain, Elasmobranchii, 55, 59, 60;
- Petromyzon, 93;
- general account of, 424;
- Hippocampus major, development of, 442
- Hirudo, development of blood-vessels of, 633;
- excretory organs of, 688;
- Horse, placenta of, 253
- Hyaloid membrane, 492
- Hylodes, oviposition of, 121;
- Hyobranchial cleft, 572
- Hyoid arch, of Chick, 179;
- general account of, 572, 575;
- modifications of, 573, 577;
- of Elasmobranchii, 576;
- of Teleostei, 577;
- of Amphibia, 582;
- of Sauropsida, 588;
- of Mammalia, 589;
- Hyomandibular bar of Elasmobranchii, 576, 577;786.png
- of Teleostei, 579;
- of Amphibia, 582;
- Hyomandibular cleft, of Petromyzon, 91;
- Chick, 179;
- general account of, 572;
- Hyostylic skulls, 582
- Hypoblast of Elasmobranchii, 51;
- Teleostei, 71, 75;
- Petromyzon, 86;
- Acipenser, 104;
- Lepidosteus, 113;
- Amphibia, 122, 129;
- Chick, 151, 167;
- Lacerta, 203;
- Rabbit, 215, 216, 219;
- origin of in Rabbit, 220;
- Hyposkeletal muscles, 675
- Hyrax, placenta of, 249
- Incus, 529, 590
- Infraclavicle, 600
- Infundibulum of Petromyzon, 92;
- Chick, 175;
- general development of, 430;
- Insectivora, placenta of, 243
- Insects, nervous system of, 410;
- eye of, 481;
- generative organs of, 745;
- generative ducts of, 751;
- Intercalated pieces of vertebral column, 551
- Interclavicle, homologies of, 602
- Intermediate cell-mass of Chick, 183
- Intermuscular septa, 672
- Interorbital septum, 570
- Interrenal bodies, 665
- Iris, 489;
- comparative development of, 506;
- Iris of Ammocoetes, 98
- Island of Reil, 444
- Jacobson’s organ, 537
- Jugal bone, 594
- Kidney, _see_ ‘Metanephros’
- Labia majora, development of, 727
- Labial cartilages, 597
- Labium tympanicum, 525;
- Lacertilia, general development of, 202;
- nares of, 537;
- pectoral girdle of, 603;
- pelvic girdle of, 607;
- arterial system of, 649;
- Lacrymal bone, 593
- Lacrymal duct, 506
- Lacrymal glands, 506
- Læmargus, vertebral column of, 548
- Lagena, 524
- Lamina spiralis, 524
- Lamina terminalis, 438
- Larva of Amphioxus, 2;
- of Ascidia, 15-21;
- Teleostei, 81;
- Petromyzon, 89, 95;
- Lepidosteus, 117, 318;
- Amphibia, 134, 142;
- types of, in the Invertebrata, 363;
- Larvæ, nature, origin, and affinities of, 360-386;
- secondary variations of less likely to be retained, 362;
- ancestral history more fully recorded in, 362;
- secondary variations in development of, 363;
- ontogenetic record of secondary variations in, 361;
- of freshwater and land animals, 362;
- types of, 362;
- phosphorescence of, 364;
- of Coelenterata, 367;
- table of, 365;
- of Invertebrata, 367 et seq.
- Larynx, 766
- Lateral line sense organs, 538;
- comparison of, with invertebrate, 538;
- development of, in Teleostei, 538;
- development of, in Elasmobranchii, 539;
- Lateral ventricle, 438;
- anterior cornu of, 440;
- descending cornu of, 440;
- choroid plexus of, 443;
- Layers, formation of, in Elasmobranchii, 41, 56;
- Teleostei, 71;
- Petromyzon, 85;
- Acipenser, 103;
- Lepidosteus, 111;
- Amphibia, 121;
- Chick, 150, 152;
- Lacerta, 202;
- Rabbit, 215-227;
- comparison of Mammalia with lower forms, 226, 289;
- comparison of formation of in Vertebrata, 275;
- origin and homologies of, in the Metazoa, 331;
- Leech, _see_ ‘Hirudo’
- Lemuridæ, placenta, 256
- Lens, of Elasmobranchii, 57, 58;
- Petromyzon, 94, 99;
- Acipenser, 106;
- Lepidosteus, 115;
- Amphibia, 127;
- Chick, 177;
- of Vertebrate eyes, 485;
- general account of, 493;
- capsule of, 493;
- comparative development of, 499;
- of Amphibia, Teleostei, Lepidosteus, 499;
- Lepidosteus, development of, 111;
- larva of, 117;
- relations of, 119;
- spinal nerves of, 455;
- ribs of, 561;
- generative ducts of, 704, 735;
- swimming-bladder of, 763;
- Ligamentum pectinatum, 490
- Ligamentum suspensorium, 557, 558
- Ligamentum vesicæ medium, 239
- Limbs, of Elasmobranchii, 59;
- Teleostei, 80;
- first appearance of in Chick, 184;
- Rabbit, 232;
- muscles of, 673;
- of Fishes, 609;
- relation of, to unpaired fins of Fishes, 611, 612;
- of Amphibia, 618;
- Liver of Teleostei, 78;
- Petromyzon, 95, 96;
- Acipenser, 110;
- Amphibia, 130;
- general account of, 769;
- Lizard, development of, 202;
- general growth of embryo of, 208;
- Müllerian duct of, 721;
- Lizzia, eye of, 471
- Lobi inferiores, 431
- Lungs of Amphibia, 137;
- development of, 763;
- homology of, 766;
- Lymphatic system, 664
- Malleus, 529, 591;
- Malpighian bodies, development of accessory in Elasmobranchs, 695
- Mammalia, development of, 214;
- comparison of gastrula of, 291;
- cerebellum of, 427;
- infundibulum of, 431;
- pineal gland of, 434;
- pituitary body of, 436;
- cerebrum of, 439;
- spinal nerves of, 449;
- sympathetic of, 466;
- vertebral column of, 558;
- branchial arches of, 573, 574;
- mandibular and hyoid arches of, 589;
- pectoral girdle of, 604;
- pelvic girdle of, 608;[Pg 787]
- heart of, 636;
- arterial system of, 647;
- venous system of, 661;
- muscle-plates of, 671;
- mesonephros of, 714;
- testicular network of, 724;
- urinogenital sinus of, 727;
- spermatozoa of, 747;
- lungs of, 765;
- intestines of, 768;
- liver of, 769;
- postanal gut of, 774;
- stomodæum of, 775;
- Mammary gland, development of, 398
- Man, placenta of, 244;
- general account of development of, 265;
- characters of embryo of, 270;
- Mandibular arch of Elasmobranchii, 62, 576;
- Petromyzon, 91;
- Acipenser, 106, 116;
- Chick, 179;
- general account of, 572, 575;
- modification of to form jaws, 573, 575;
- of Teleostei, 580;
- of Amphibia, 582;
- Sauropsida, 588;
- Mammalia, 589;
- Mandibular bar, evolution of, 311, 321
- Manis, placenta of, 256
- Marsupial bones, 608
- Marsupialia, foetal membranes of, 240;
- cerebellum of, 426;
- corpus callosum of, 443;
- uterus of, 726;
- Maxilla, 594
- Meatus auditorius externus, of Chick, 181;
- Meckelian cartilage, of Elasmobranchii, 576;
- of Teleostei, 581;
- of Amphibia, 584, 585;
- of Sauropsida, 588;
- of Mammalia, 590;
- Mediastinum anterior and posterior, 630
- Medulla oblongata, of Chick, 176;
- general development of, 425;
- Medullary plate of Amphioxus, 4, 5;
- of Ascidia, 11;
- Elasmobranchii, 44, 47, 55;
- Teleostei, 72;
- Petromyzon, 88;
- Acipenser, 104;
- Lepidosteus, 111;
- Amphibia, 126, 127, 131;
- Chick, 159;
- Lacerta, 204;
- Rabbit, 223, 227, 228;
- primitive bilobed character of, 303, 317;
- Medusæ, auditory organs of, 513
- Membrana capsulo-pupillaris, 494, 504, 507
- Membrana elastica externa, 546
- Membrana limitans of retina, 491
- Membrana tectoria, 522, 525
- Membrane bones, of Amphibia, 582;
- of Sauropsida, 588;
- of Mammalia, 590;
- of mandibular arch, 593;
- of pectoral girdle, 599, 602;
- origin of, 592;
- homologies of, 593;
- Membranous labyrinth, development of in Man, 519
- Menobranchus, branchial arches of, 142
- Mesenteron of Elasmobranchii, 43;
- Mesentery, 626, 756
- Mesoblast, of Amphioxus, 6;
- Ascidia, 17, 20;
- Pyrosoma, 24;
- Salpa, 30;
- Elasmobranchii, 44, 47;
- Teleostei, 75;
- Petromyzon, 86;
- Acipenser, 105;
- Lepidosteus, 113;
- Amphibia, 125, 128, 129;
- of Chick, 154, 167;
- double origin of in Chick, 154, 158, 159;
- origin of from lips of blastopore in Chick, 158;
- of area vasculosa of Chick, 160;
- Lacerta, 203;
- origin of in Rabbit, 218, 223;
- of area vasculosa in Rabbit, 227;
- comparative account of formation of, 292;
- discussion of development of in Vertebrata, 297;
- meaning of development of in Amniota, 298;
- phylogenetic origin of, 346;
- summary of ontogeny of, 349-352;
- views on ontogeny of, 352-360;
- Mesoblastic somites, of Amphioxus, 6;
- Elasmobranchii, 48, 55;
- Petromyzon, 88;
- Acipenser, 105;
- Lepidosteus, 114;
- Amphibia, 129, 131;
- Chick, 161, 180;
- Rabbit, 228;
- development of in Chordata, 325;
- meaning of development of, 331;
- of head, 676
- Mesogastrium, 758
- Mesonephros, of Teleostei, 78, 702;
- Petromyzon, 95, 98, 700;
- Acipenser, 110, 705;
- Amphibia, 134, 708;
- Chick, 184, 714;
- general account of, 690;
- development of in Elasmobranchs 691;
- of Cyclostomata, 700;
- Ganoidei, 705;
- sexual and non-sexual part of in Amphibia, 710;
- of Amniota, 713, 724;
- summary and general conclusions as to, 729;
- relation of to pronephros, 731;
- Mesopterygium, 616
- Metagenesis of Ascidians, 34
- Metamorphosis of Amphibia, 137, 140
- Metanephros, 690;
- development of in Elasmobranchii, 697;
- of Amphibia, 712;
- of Amniota, 713;
- of Chick, 722;
- of Lacertilia, 723;
- phylogeny of, 736;
- Metapterygium, 616
- Metapterygoid, of Elasmobranchii, 576;
- Metazoa, evolution of, 339, 342;
- Mid-brain, of Elasmobranchii, 55, 58, 59;
- Petromyzon, 92;
- general account of development of, 427;
- Moina, generative organs of, 745
- Molgula, development of, 22
- Mollusca, nervous system of, 414;
- eyes of, 472;
- auditory organs of, 515;
- excretory organs of, 681;
- Monotremata, foetal membranes of, 240;
- cerebellum of, 426;
- corpus callosum of, 443;
- cerebrum of, 443;
- urinogenital sinus of, 726;
- Mormyrus, generative ducts of, 704
- Mouth, of Amphioxus, 7;
- of Ascidia, 18;
- Pyrosoma, 27;
- Salpa, 31;
- Elasmobranchii, 57, 60, 61, 62;
- Petromyzon, 92, 94, 95, 99;
- Acipenser, 107;
- Lepidosteus, 118;
- Amphibia, 129, 132, 134;
- Rabbit, 231;
- origin of, 317;
- Mouth, suctorial, of Petromyzon, 99;
- Müllerian duct, 690;[Pg 788]
- of Elasmobranchs, 693;
- of Ganoids, 704;
- of Amphibia, 710;
- of Aves, 717, 720;
- opening of into cloaca, 727;
- origin of, 733;
- summary of development of, 733;
- relation of to pronephros, 733;
- Muscle-plates, of Amphioxus, 6;
- Elasmobranchii, 49, 668;
- Teleostei, 670;
- Petromyzon, 94;
- Chick, 183, 670;
- general development of, 669;
- of Amphibia, 670;
- Aves, 670;
- of Mammalia, 671;
- origin of muscles from, 672;
- Muscles, of Ascidia, 11, 17;
- development of from muscle-plates, 672;
- of limbs, 673;
- of head, 676;
- of branchial arches, 678;
- of eye, 678;
- Muscular fibres, epithelial origin of, 667
- Muscular system, development of, 667;
- Mustelus, placenta of, 66
- Myoepithelial cells, 667
- Mysis, auditory organ of, 517
- Myxine, ovum of, 100;
- olfactory organ of, 533;
- portal sinus of, 652;
- excretory system of, 701;
- Nails, development of, 397
- Nares, of Acipenser, 108;
- of Ichthyopsida, 534;
- development of in Chick, 535;
- development of in Lacertilia, 537;
- development of in Amphibia, 537;
- Nasal bones, 592
- Nasal pits, Acipenser, 108;
- Chick, 176;
- general development of, 531;
- Nematoda, excretory organs of, 689;
- generative organs of, 745;
- generative ducts of, 752;
- Nemertines, nervous system of, 311;
- excretory organs of, 681;
- Nerve cord, origin of ventral, 378
- Nerves, spinal, 449;
- Nervous system, central,
- general account of development of in Vertebrata, 415;
- conclusions as to, 445;
- sympathetic, 466;
- Nervous system, of Amphioxus, 4;
- Ascidia, 15, 16;
- Molgula, 22;
- Pyrosoma, 24, 25;
- Salpa, 30, 31;
- Elasmobranchii, 44;
- Teleostei, 77;
- Petromyzon, 89, 93;
- Acipenser, 105;
- Amphibia, 126;
- comparative account of formation of central, 301;
- of Sagitta, 349;
- origin of in Coelenterata, 349;
- of præoral lobe, 377, 380;
- evolution of, 400-405;
- development of in Invertebrates, 406;
- of Arthropoda, 408;
- of Gephyrea, 412;
- Mollusca, 414;
- Neural canal, of Ascidia, 10;
- Teleostei, 72;
- Petromyzon, 88;
- Acipenser, 105;
- Lepidosteus, 114;
- Amphibia, 126, 131;
- Chick, 166, 171;
- Lacerta, 208;
- closure of in Frog and Amphioxus, 279;
- closure of in Elasmobranchii, 284;
- phylogenetic origin of, 316;
- Neural crest, 449, 456, 457
- Neurenteric canal, of Amphioxus, 4, 5;
- Ascidia, 10;
- Elasmobranchii, 54;
- Petromyzon, 88;
- Acipenser, 105;
- Lepidosteus, 113;
- Aves, 162;
- Lacerta, 203, 206;
- general account of, 323;
- meaning of, 323;
- Newt, ovum of, 120;
- development of, 125;
- general growth of, 141;
- Notidanus, vertebral column of, 548;
- branchial arches of, 572;
- Notochord of Amphioxus, 6;
- Ascidia, 11, 17;
- Elasmobranchii, 51;
- Teleostei, 74;
- Petromyzon 86, 94;
- Acipenser, 104;
- Lepidosteus, 113;
- Amphibia, 128, 129;
- Chick, 157;
- canal of, in Chick, 163;
- Lacerta, 204, 205;
- Guinea-pig, 226;
- comparative account of formation of, 292, 325;
- sheath of, 545;
- later histological changes in, 546;
- cartilaginous sheath of, 547;
- in head, 566;
- absence of in region of trabeculæ, 567;
- Notodelphys, brood-pouch of, 121;
- Nototrema, brood-pouch of, 121
- Nucleus pulposus, 559
- Oceania, eye of, 471
- Occipital bone, 595
- OEsophagus, solid, of Elasmobranchii, 61, 759;
- Olfactory capsules, 571
- Olfactory lobes, development of, 444
- Olfactory nerves, Ammocoetes, 99;
- general development of, 464;
- Olfactory organ, of aquatic forms, 531;
- Insects and Crustacea, 531;
- of Tunicata, 532;
- of Amphioxus, 532;
- of Vertebrata, 533;
- Petromyzon, 533;
- of Myxine, 533;
- Olfactory sacks, of Elasmobranchii, 60;
- Oligochæta, excretory organs of, 683
- Olivary bodies, 426
- Omentum, lesser and greater, 757
- Onchidium, eye of, 478
- Opercular bones, 593
- Operculum, of Teleostei, 77;
- Ophidia, development of, 210;
- arterial system of, 649;
- venous system of, 656;
- Optic chiasma, 430, 493
- Optic cup, retinal part of, 488;
- Optic lobes, 428
- Optic nerve, development of, 492;
- comparative development of, 500;
- Optic thalami, development of, 431
- Optic vesicle, of Elasmobranchii, 57-59;
- Teleostei, 74, 499;
- Petromyzon, 89, 92;
- Acipenser, 106;
- Lepidosteus, 115;
- Chick, 170;
- Rabbit, 229;
- general development of, 429;
- formation of secondary, 487;
- obliteration of cavity of, 488;[Pg 789]
- comparative development of, 499;
- of Lepidosteus and Teleostei, 499.
- _See also_ ‘Eye’
- Ora serrata, 488
- Orbitosphenoid region of skull, 570
- Organs, classification of, 391;
- derivation of from germinal layers, 392;
- Orycteropus, placenta of, 249
- Otic process of Axolotl, 583;
- Otoliths, 512
- Oviposition, of Amphioxus, 1;
- Elasmobranchii, 40;
- Teleostei, 68;
- Petromyzon, 84;
- Amphibia, 121;
- Reptilia, 202;
- Ovum, of Amphioxus, 1;
- Pyrosoma, 23;
- Elasmobranchii, 40;
- Teleostei, 68;
- Petromyzon, 83;
- Myxine, 100;
- Acipenser, 102;
- Lepidosteus, 111;
- Amphibia, 120;
- Chick, 146;
- Reptilia, 202;
- Mammalia, 214;
- of Porifera, 741;
- migration of in Coelenterata, 742;
- Vertebrata, 746;
- Palatine bone, of Teleostei, 580;
- Pancreas, Acipenser, 110;
- general development of, 770;
- Pancreatic cæca, of Teleostei, etc. 768
- Papillæ, oral, of Acipenser, 108;
- Parachordals, 565, 566
- Parasphenoid bone, 594
- Parepididymis, 725
- Parietal bones, 592
- Paroophoron, 725
- Parovarium, 725
- Pectoral girdle, 599;
- of Elasmobranchs, 600;
- of Teleostei, 600;
- of Amphibia and Amniota, 601;
- comparison of with pelvic, 608;
- Pecten, eye of, 479
- Pecten, of Ammocoetes, 498;
- of Chick, 501;
- Lizard, 501;
- Elasmobranchs, 501;
- Pedicle, of Axolotl, 484;
- Pelobates, branchial apertures of, 136;
- vertebral column of, 556;
- Pelodytes, branchial chamber of, 135
- Pelvic girdle, 606;
- of Fishes, 606;
- Amphibia and Amniota, 607;
- of Lacertilia, 607;
- of Mammalia, 608;
- comparison with pectoral, 608;
- Penis, development of, 727
- Peribranchial cavity, of Amphioxus, 7;
- of Ascidia, 18;
- Pyrosoma, 24;
- Pericardial cavity, of Pyrosoma, 26;
- Elasmobranchii, 49;
- Petromyzon, 94;
- general account of, 626;
- of Fishes, 627;
- of Amphibia, Sauropsida and Mammalia, 628;
- Perichordal formation of vertebral column, 556
- Perilymph of ear, 523
- Periotic capsules, ossifications in, 595, 596
- Peripatus, nervous system of, 409;
- eye of 480;
- excretory organs of, 688;
- Peritoneal membrane, 626
- Petromyzon, development of, 83;
- affinities of, 83, 84;
- general development of, 87;
- hatching of, 89;
- comparison of gastrula of, 280;
- branchial skeleton of, 312, 572;
- cerebellum of, 425;
- pineal gland of, 434;
- pituitary body of, 436;
- cerebrum of, 439;
- auditory organ of, 517;
- olfactory organ of, 533;
- comparison of oral skeleton of with Tadpole, 586;
- pericardial cavity of, 627;
- abdominal pores of, 626;
- venous system of, 651;
- excretory organs of, 700;
- segmental duct of, 700;
- pronephros of, 700;
- mesonephros of, 700;
- thyroid body of, 760;
- postanal gut of, 774;
- stomodæum of, 775;
- Phosphorescence of larvæ, 364
- Phylogeny, of the Chordata, 327;
- Pig, placenta of, 251;
- mandibular and hyoid arches of, 589;
- Pineal gland, of Petromyzon, 93;
- Chick, 175;
- general development of, 432;
- nature of, 432, 434;
- Pipa, brood-pouch of, 121;
- metamorphosis of, 139;
- yolk-sack of, 140;
- vertebral column of, 556;
- Pituitary body, of Rabbit, 231;
- general development of, 435;
- meaning of, 436;
- Placenta, of Salpa, 29;
- Elasmobranchii, 66;
- of Mammalia, 232;
- villi of, 235;
- deciduate and non-deciduate, 239;
- comparative account of, 239-259;
- characters of primitive type of, 240;
- zonary, 248;
- non-deciduate, 250;
- histology of, 257;
- evolution of, 259;
- Placoid scales, 395
- Planorbis, excretory organs of, 681
- Planula, structure of, 367
- Pleural cavities, 631
- Pleuronectidæ, development of, 80
- Pneumatocoela, characters of, 327
- Polygordius, excretory organs of, 684
- Polyophthalmus, eye of, 479
- Polypedates, brood-pouch of, 121
- Polyzoa, excretory organs of, 682;
- generative cells of, 745;
- generative ducts of, 751;
- Pons Varolii, 426, 427
- Pori abdominales, Ammocoetes, 99
- Porifera, ancestral form of, 345;
- development of generative cells of, 741;
- Portal vein, 653
- Postanal gut of Elasmobranchii, 58, 59, 60;
- Teleostei, 75;
- Chick, 169;
- general account of, 323, 772;
- Præmaxilla, 594
- Præopercular bone, 593
- Præoral lobe, ganglion of, 377, 380
- Prefrontals, 597
- Presphenoid region of skull, 570
- Primitive groove of Chick, 155
- Primitive streak, of Chick, 152, 161;[Pg 790]
- meaning of, 153;
- origin of mesoblast form in Chick, 154;
- continuity of hypoblast with epiblast at anterior end of, in Chick, 156;
- comparison of with blastopore, 165;
- fate of, in Chick, 165;
- of Lacerta, 203;
- of Rabbit, 221;
- of Guinea-pig, 223;
- fusion of layers at, in Rabbit, 224;
- comparison of with blastopore of lower forms, 226, 287;
- of Mammalia, 290;
- Processus falciformis of Ammocoetes, 498;
- of Elasmobranch, 502;
- of Teleostei, 503;
- Proctodæum, 778
- Pronephros, of Teleostei, 78, 701;
- Petromyzon, 95, 99, 700;
- Acipenser, 106, 110;
- Amphibia, 134, 707;
- general account of, 689;
- of Cyclostomata, 700;
- of Myxine, 701;
- Ganoidei, 705;
- of Amniota, 714;
- of Chick, 718;
- summary of and general conclusions as to, 728;
- relation of, to mesonephros, 731;
- cause of atrophy of, 729;
- Prootic, 596, 597
- Propterygium, 616
- Proteus, branchial arches of, 142
- Protochordata, characters of, 327
- Proto-ganoidei, characters of, 328
- Proto-gnathostomata, characters of, 328
- Proto-pentadactyloidei, characters of, 329
- Protovertebrata, characters of, 328
- Pseudis, Tadpole of, 139;
- vertebral column of, 556;
- Pseudophryne, yolk-sack of, 140;
- Pterygoid bone, of Teleostei, 581;
- Pterygoquadrate bar, of Elasmobranchii, 576;
- of Teleostei, 581;
- Axolotl, 584;
- Frog, 584;
- of Sauropsida, 588;
- of Mammalia, 589;
- Pulmonary artery, origin of, 645;
- of Amphibia, 645;
- of Amniota, 649;
- Pulmonary vein, 655
- Pupil, 489
- Pyrosoma, development of, 23
- Quadrate bone of Teleostei, 581;
- Quadratojugal bone, 594
- Rabbit, development of, 214;
- general growth of embryo of, 227;
- placenta of, 248;
- Radiate symmetry, passage from to bilateral symmetry, 373-376
- Raja, caudal vertebræ of, 553
- Rat, placenta of, 242
- Recessus labyrinthi, 519
- Reissner’s membrane, 524
- Reptilia, development of, 202;
- viviparous, 202;
- cerebellum of, 426;
- infundibulum of, 431;
- pituitary body of, 436;
- cerebrum of, 439;
- vertebral column of, 556;
- arterial system of, 648;
- venous system of, 656;
- mesonephros of, 713;
- testicular network of, 723;
- spermatozoa of, 747;
- Restiform tracts of Elasmobranchii and Teleostei, 425
- Retina, histogenesis of, 490
- Retinulæ, 482
- Rhabdom, 482
- Rhinoderma, brood-pouch of, 121;
- Ribs, development of, 560
- Rosenmüller’s organ, 725
- Rotifera, excretory organs of, 680
- Round ligament of liver, 663
- Ruminantia, placenta of, 253
- Sacci vasculosi, 437
- Sacculus hemisphericus, 519;
- Sagitta. _See_ ‘Chætognatha’
- Salpa, sexual development of, 29;
- asexual development of, 33;
- Salamandra, larva of, 142;
- vertebral column of, 553;
- limbs of, 619;
- mesonephros of, 708;
- Müllerian duct of, 710;
- Salmonidæ, hypoblast of, 71;
- generative ducts of, 704;
- Sauropsida, gastrula of, 286;
- meaning of primitive streak of, 288;
- blastopore of, 289;
- mandibular and hyoid arches of, 588;
- pectoral girdle of, 601;
- Scala, vestibuli, 522;
- Scales, general development of, 396;
- development of placoid scales, 395;
- Scapula, 599
- Sclerotic, 488
- Scrotum, development of, 727
- Scyllium, caudal vertebræ of, 553;
- mandibular and hyoid arches of, 578;
- pectoral girdle of, 600;
- limbs of, 610;
- pelvic fin of, 614;
- pectoral fin of, 615;
- Segmental duct, 690;
- development of in Elasmobranchs, 690;
- of Cyclostomata, 700;
- of Teleostei, 701;
- of Ganoidei, 704, 705;
- of Amphibia, 707;
- of Amniota, 713;
- Segmental organs, 682
- Segmental tubes, 690;
- development of in Elasmobranchs, 691;
- rudimentary anterior in Elasmobranchs, 693;
- development of secondary, 731;
- Segmentation cavity, of Elasmobranchii, 42-44;
- Segmentation, meaning of, 331
- Segmentation of ovum, in Amphioxus, 2;
- Ascidia, 9;
- Molgula, 22;
- Pyrosoma, 23;
- Salpa, 30;
- Elasmobranchii, 40;
- Telostei, 69;
- Petromyzon, 84;
- Acipenser, 102;
- Lepidosteus, 111;
- Amphibia, 122, 124;
- Newt, 125;
- Chick, 146;
- Lizard, 202;
- Rabbit, 214;
- Semicircular canals, 519[Pg 791]
- Sense organs, comparative account of development of, 304
- Septum lucidum, 443
- Serous membrane, Lacerta, 209;
- Seventh nerve, development of, 459
- Shell-gland of Crustacea, 689
- Shield, embryonic, of Chick, 151;
- Simiadæ, placenta of, 247
- Sinus rhomboidalis, of Chick, 162
- Sinus venosus, 637
- Sirenia, placenta of, 255
- Sixth nerve, 463
- Skate, mandibular and hyoid arches of, 577
- Skeleton, elements of found in Vertebrata, 542
- Skull, general development of, 564;
- historical account of, 564;
- development of cartilaginous, 566;
- cartilaginous walls of, 570;
- composition of primitive cartilaginous cranium, 565;
- Somatopleure, of Chick, 170
- Spelerpes, branchial arches of, 142
- Spermatozoa, of Porifera, 741;
- Sphenoid bone, 595
- Sphenodon, hyoid arch of, 588
- Spinal cord, general account of, 415;
- white matter of, 415;
- central canal of, 417, 418;
- commissures of, 417;
- grey matter of, 417;
- fissures of, 418;
- Spinal nerves, posterior roots of, 449;
- Spiracle, of Elasmobranchii, 62;
- Acipenser, 105;
- Amphibia, 136;
- Spiral valve. _See_ ‘Valve’
- Spleen, 664
- Splenial bone, 595
- Squamosal bone, 593
- Stapes, 529;
- Sternum, development of, 562
- Stolon of Doliolum, 29;
- Stomodæum, 774
- Stria vascularis, 524
- Styloid process, 591
- Subintestinal vein, 651;
- Syngnathus, brood-pouch of, 68
- Subnotochordal rod, of Elasmobranchii, 54;
- Petromyzon, 94;
- Acipenser, 110;
- Lepidosteus, 115;
- general account of, 754;
- comparison of with siphon of Chætopods, 756;
- Subzonal membrane, 237;
- Sulcus of Munro, 432
- Supraclavicle, 600
- Suprarenal bodies, 664
- Supra-temporal bone, 593
- Swimming bladder, _see_ Air bladder
- Sylvian aqueduct, 428
- Sylvian fissure, 444
- Sympathetic ganglia, development of, 467
- Tadpole, 134, 139, 140;
- phylogenetic meaning of, 137;
- metamorphosis of, 137;
- meaning of suctorial mouth of, 585;
- Tail of Teleostei, 80;
- Acipenser, 109;
- Lepidosteus, 109;
- Amphibia, 132;
- Tarsus, development of, 620
- Teeth, horny provisional, of Amphibia, 136;
- general development of, 776;
- origin of, 777;
- Teleostei, development of, 68;
- viviparous, 68;
- comparison of formation of layers in, 286;
- restiform tracts of, 425;
- mid-brain of, 425;
- infundibulum of, 431;
- cerebrum of, 439;
- nares of, 534;
- lateral line of, 538;
- notochord and membrana elastica of, 549;
- vertebral column of, 553;
- ribs of, 561;
- hyoid and mandibular arches of, 579;
- pectoral girdle of, 601;
- pelvic girdle of, 606;
- limbs of, 618;
- heart of, 637;
- arterial system of, 645;
- muscle-plates of, 670;
- excretory organs of, 701;
- generative ducts of, 704, 735, 749;
- swimming bladder of, 763;
- postanal gut of, 774;
- Teredo, nervous system of, 414
- Test of Ascidia, 14;
- Testicular network, of Elasmobranchs, 697;
- of Amphibia, 712;
- Reptilia, 723;
- of Mammals, 724;
- Testis of Vertebrata, 746
- Testis, connection of with Wolffian body, in Elasmobranchii, 697;
- in Amphibia, 710;
- in Amniota, 723;
- origin of, 735;
- Thalamencephalon of Chick, 175;
- general development of, 430;
- Third nerve, development of, 461
- Thymus gland, 762
- Thyroid gland, Petromyzon, 92;
- general account of, 759;
- nature of, 760;
- development of in Vertebrata, 761;
- Tooth. _See_ ‘Teeth’
- Tori semicirculares, 428
- Tornaria, 372
- Trabeculæ, 565, 567;
- Trachea, 766
- Trematoda, excretory organs of, 681
- Triton alpestris, sexual larva of, 143
- Triton, development of limbs of, 619;
- urinogenital organs of, 712;
- Truncus arteriosus, 638;
- of Amphibia, 638;
- of Birds, 639;
- Tunicata, development of mesoblast of, 293;
- test of, 394;
- eye of, 507;
- auditory organ of, 530;
- olfactory organ of, 532;
- generative duct of, 749;
- intestine of 767;
- postanal gut of, 771;
- stomodæum of, 775;
- Turbellaria, excretory organs of, 681
- Tympanic annulus of Frog, 587
- Tympanic cavity, of Amphibia, 135;
- Chick, 180;
- Rabbit, 232;
- general development of, 528;
- of Mammals, 591;
- Tympanic membrane, of Chick, 180;
- general development of, 528;
- Tympanohyal, 591[Pg 792]
- Umbilical canal of Elasmobranchii, 54, 57, 58, 59
- Umbilical cord, 238;
- Ungulata, placenta of, 250
- Urachus, 239, 726
- Ureters, of Elasmobranchii, 696;
- Urethra, 727
- Urinary bladder of Amphibia, 712;
- Urinogenital organs, _see_ Excretory organs
- Urinogenital sinus of Petromyzon, 700;
- of Sauropsida, 726;
- of Mammalia, 727;
- Urochorda, development of, 9
- Uterus, development of, 726;
- Uterus masculinus, 726
- Utriculus, 519
- Uvea of iris, 489
- Vagus nerve, development of, 456, 457;
- intestinal branch of, 458;
- branch of to lateral line, 459;
- Valve, spiral, of Petromyzon, 97;
- Acipenser, 110;
- general account of, 767;
- Valves, semilunar, 641;
- auriculo-ventricular, 642;
- Vasa efferentia, of Elasmobranchs, 697;
- of Amphibia, 711;
- general origin of, 724;
- Vascular system, of Amphioxus, 8;
- Petromyzon, 97;
- Lepidosteus, 116;
- general development of, 632;
- Vas deferens, of Elasmobranchii, 697;
- Vein, subintestinal of Petromyzon, 97;
- Acipenser, 110;
- Lepidosteus, 116;
- Velum of Petromyzon, 91
- Vena cava inferior, development of, 655
- Venous system of Petromyzon, 97;
- general development of, 651;
- of Fishes, 651;
- of Amphibia and Amniota, 655;
- of Reptilia, 656;
- of Ophidia, 656;
- of Aves, 658;
- of Mammalia, 661;
- Ventricle, fourth, of Chick, 176;
- Ventricle, lateral, 438, 440;
- Ventricle, third, of Chick, 175
- Vertebral bodies, of Chick, 183
- Vertebral column, development of, 545, 549;
- epichordal and perichordal development of in Amphibia, 556;
- Vespertilionidæ, early development of, 217
- Vieussens, valve of, 426
- Villi, placental, of zona radiata, 235;
- subzonal membrane, 235;
- chorion, 237;
- Man, 246;
- comparative account of, 257;
- of young human ovum, 265, 269;
- Visceral arches, Amphioxus, 7;
- Elasmobranchii, 57-60;
- Teleostei, 77;
- Acipenser, 106;
- Lepidosteus, 116;
- Amphibia, 133;
- Chick, 177;
- Rabbit, 231;
- præoral, 570;
- relation of to head cavities, 572;
- disappearance of posterior, 573;
- dental plates of in Teleostei, 574;
- Visual organs, evolution of, 470
- Vitelline arteries of Chick, 195
- Vitelline veins of Chick, 195
- Vitreous humour, of Ammocoetes, 98;
- general development of, 494;
- blood-vessels of in Mammals 503;
- mesoblastic ingrowth in Mammals, 503;
- Vomer, 594
- White matter, of spinal cord, 415;
- Wolffian body, _see_ ‘Mesonephros’
- Wolffian duct, first appearance of in Chick, 183;
- general account of, 690;
- of Elasmobranchs, 693;
- of Ganoids, 704;
- of Amphibia, 710;
- of Amniota, 713;
- atrophy of in Amniota, 724;
- Wolffian ridge, 185
- Yolk blastopore, of Elasmobranchii, 64
- Yolk, folding off of embryo from, in Elasmobranchii, 55;
- Yolk nuclei, of Elasmobranchii, 41, 53;
- Yolk, of Elasmobranchii, 40;
- Teleostei, 68;
- Petromyzon, 96;
- Acipenser, 109;
- Amphibia, 122, 129;
- Chick, 146;
- influence of on formation of layers, 278;
- influence of on early development, 341, 342;
- Yolk-sack, Amphibia, 131, 140, 141;
- Yolk-sack, development of in Rabbit, 227;
- of Mammalia reduced, 227;
- circulation of in Rabbit, 233;
- enclosure of in Sauropsida, 289;
- Yolk-sack, enclosure of, Petromyzon, 86
- Yolk-sack, Lepidosteus, 118
- Yolk-sack of Chick, enclosure of, 160;
- stalk of, 174;
- general account of, 193;
- circulation of, 195;
- later history of, 198;
- Yolk-sack of Elasmobranchii, enclosure of, 62, 283;
- Yolk-sack of Lacerta, 209;
- Yolk-sack, Teleostei, 75, 81;
- enclosure of, 75;
- circulation of, 81;
- Zona radiata, villi of, 237
- Zonula of Zinn, 495
[Pg i]
BIBLIOGRAPHY.
Cephalopoda.
(1) A. Kowalevsky. “Entwicklungsgeschichte des Amphioxus lanceolatus.”
Mém. Acad. Impér. des Sciences de St Pétersbourg, Series VII. Tom.
XI. 1867.
(2) A. Kowalevsky. “Weitere Studien über die Entwicklungsgeschichte
des Amphioxus lanceolatus.” Archiv f. mikr. Anat., Vol. XIII. 1877.
(3) Leuckart u. Pagenstecher. “Untersuchungen über niedere Seethiere.”
Müller’s Archiv, 1858.
(4) Max Schultze. “Beobachtung junger Exemplare von Amphioxus.” Zeit.
f. wiss. Zool., Bd. III. 1851.
(5) A. M. Marshall. “On the mode of Oviposition of Amphioxus.” Jour.
of Anat. and Phys., Vol. X. 1876.
Urochorda.
(6) P. J. van Beneden. “Recherches s. l'Embryogénie, l'Anat. et la
Physiol. des Ascidies simples.” Mém. Acad. Roy. de Belgique,
Tom. XX.
(7) W. K. Brooks. “On the development of Salpa.” Bull. of the Museum
of Comp. Anat. at Harvard College, Cambridge, Mass.
(8) H. Fol. Etudes sur les Appendiculaires du détroit de Messine.
Genève et Bâle, 1872.
(9) Ganin. “Neue Thatsachen a. d. Entwicklungsgeschichte d. Ascidien.”
Zeit. f. wiss. Zool., Vol. XX. 1870.
(10) C. Gegenbaur. “Ueber den Entwicklungscyclus von Doliolum nebst
Bemerkungen über die Larven dieser Thiere.” Zeit. f. wiss. Zool.,
Bd. VII. 1856.
(11) A. Giard. “Etudes critiques des travaux d'embryogénie relatifs à
la parenté des Vertebrés et des Tuniciers.” Archiv Zool.
expériment., Vol. I. 1872.
(12) A. Giard. “Recherches sur les Synascidies.” Archiv Zool.
expér., Vol. I. 1872.
(13) O. Hertwig. “Untersuchungen üb. d. Bau u. d. Entwicklung des
Cellulose-Mantels d. Tunicaten.” Jenaische Zeitschrift, Bd. VII.
1873.
(14) Th. H. Huxley. “Remarks upon Appendicularia and Doliolum.” Phil.
Trans., 1851.
(15) Th. H. Huxley. “Observations on the anatomy and physiology of
Salpa and Pyrosoma.” Phil. Trans., 1851.
(16) Th. H. Huxley. “Anatomy and development of Pyrosoma.” Linnean
Trans., 1860, Vol. XXIII.
(17) Keferstein u. Ehlers. Zoologische Beiträge, 1861. Doliolum.
(18) A. Kowalevsky. “Entwicklungsgeschichte d. einfachen Ascidien.”
Mém. Acad. Pétersbourg, VII. série, T. X. 1866.
(19) A. Kowalevsky. “Beitrag z. Entwick. d. Tunicaten.” Nachrichten
d. königl. Gesell. zu Göttingen. 1868.
(20) A. Kowalevsky. “Weitere Studien üb. d. Entwicklung d. einfachen
Ascidien.” Archiv f. mikr. Anat., Vol. VII. 1871.
(21) A. Kowalevsky. “Ueber Knospung d. Ascidien.” Archiv f. mikr.
Anat., Vol. X. 1874.
(22) A. Kowalevsky. “Ueber die Entwicklungsgeschichte d. Pyrosoma.”
Archiv f. mikr. Anat., Vol. XI. 1875.
(23) A. Krohn. “Ueber die Gattung Doliolum u. ihre Arten.” Archiv f.
Naturgeschichte, Bd. XVIII. 1852.
[Pg ii]
(24) A. Krohn. “Ueber die Entwicklung d. Ascidien.” Müller’s Archiv,
1852.
(25) A. Krohn. “Ueber die Fortpflanzungsverhältnisse d. Botrylliden.”
Archiv f. Naturgeschichte, Vol. XXXV. 1869.
(26) A. Krohn. “Ueber die früheste Bildung d. Botryllenstöcke.”
Archiv f. Naturgeschichte, Vol. XXXV. 1869.
(27) C. Kupffer. “Die Stammverwandschaft zwischen Ascidien u.
Wirbelthieren.” Archiv f. mikr. Anat., Vol. VI. 1870.
(28) C. Kupffer. “Zur Entwicklung d. einfachen Ascidien.” Archiv f.
mikr. Anat., Vol. VIII. 1872.
(29) H. Lacaze Duthiers. “Recherches sur l'organisation et
l'Embryogénie des Ascidies (Molgula tubulosa).” Comptes rendus, May
30, 1870, p. 1154.
(30) H. Lacaze Duthiers. “Les Ascidies simples des Côtes de France”
(Development of Molgula). Archiv Zool. expér., Vol. III. 1874.
(31) R. Leuckart. “Salpa u. Verwandte.” Zoologische Untersuchungen,
Heft II.
(32) E. Metschnikoff. “Observations sur le développement de quelques
animaux (Botryllus and Simple Ascidians).” Bull. d. l'Acad.
Pétersbourg, Vol. XIII. 1869.
(33) H. Milne-Edwards. “Observations s. l. Ascidies composées des
côtes de la Manche.” Mémoires d. l'Institut, T. XVIII. 1842.
(34) W. Salensky. “Ueber d. embryonale Entwicklungsgeschichte der
Salpen.” Zeit. f. wiss. Zool., B. XXVII. 1877.
(35) W. Salensky. “Ueber die Knospung d. Salpen.” Morphol. Jahrbuch,
Bd. III. 1877.
(36) W. Salensky. “Ueber die Entwicklung d. Hoden u. über den
Generationswechsel d. Salpen.” Zeit. f. wiss. Zool., Bd. XXX. Suppl.
1878.
(37) C. Semper. “Ueber die Entstehung d. geschichteten
Cellulose-Epidermis d. Ascidien.” Arbeit. a. d. zool.-zoot. Instit.
Würzburg, Vol. II. 1875.
(38) Fr. Todaro. Sopra lo sviluppo e l'anatomia delle Salpe. Roma,
1875.
(39) Fr. Todaro. “Sui primi fenomeni dello sviluppo delle Salpe.”
Reale Accademia dei Lincei, Vol. IV. 1880.
Elasmobranchii.
(40) F. M. Balfour. “A preliminary account of the development of the
Elasmobranch Fishes.” Quart. J. of Micr. Science, Vol. XIV. 1876.
(41) F. M. Balfour. “A Monograph on the development of Elasmobranch
Fishes.” London, 1878. Reprinted from the Journal of Anat. and
Physiol. for 1876, 1877, and 1878.
(42) Z. Gerbe. Recherches sur la segmentation de la cicatrule et la
formation des produits adventifs de l'œuf des Plagiostomes et
particulièrement des Raies. Vide also Journal de l'Anatomie et de
la Physiologie, 1872.
(43) W. His. “Ueb. d. Bildung v. Haifischenembryonen.” Zeit. für
Anat. u. Entwick., Vol. II. 1877.
(44) A. Kowalevsky. “Development of Acanthias vulgaris and Mustelus
lævis.” (Russian.) Transactions of the Kiew Society of
Naturalists, Vol. I. 1870.
(45) R. Leuckart. “Ueber die allmählige Bildung d. Körpergestalt bei
d. Rochen.” Zeit. f. wiss. Zool., Bd. II., p. 258.
(46) Fr. Leydig. Rochen u. Haie. Leipzig, 1852.
(47) A. W. Malm. “Bidrag till kännedom om utvecklingen af Rajæ.”
Kongl. vetenskaps akademiens förhandlingar. Stockholm, 1876.
(48) Joh. Müller. Glatter Haie des Aristoteles und über die
Verschiedenheiten unter den Haifischen und Rochen in der Entwicklung
des Eies. Berlin, 1840.
(49) S. L. Schenk. “Die Eier von Raja quadrimaculata innerhalb der
Eileiter.” Sitz. der k. Akad. Wien, Vol. LXXIII. 1873.
(50) Alex. Schultz. “Zur Entwicklungsgeschichte des Selachiereies.”
Archiv für micro. Anat., Vol. XI.? 1875.
(51) Alex. Schultz. “Beitrag zur Entwicklungsgeschichte d.
Knorpelfische.” Archiv für micro. Anat., Vol. XIII. 1877.
[Pg iii]
(52) C. Semper. “Die Stammesverwandschaft d. Wirbelthiere u.
Wirbellosen.” Arbeit. a. d. zool.-zoot. Instit. Würzburg, Vol. II.
1875.
(53) C. Semper. “Das Urogenitalsystem d. Plagiostomen, etc.” Arbeit.
a. d. zool.-zoot. Instit. Würzburg, Vol. II. 1875.
(54) Wyman. “Observations on the Development of Raja batis.” Memoirs
of the American Academy of Arts and Sciences, Vol. IX. 1864.
Teleostei.
(55) Al. Agassiz. “On the young Stages of some Osseous Fishes. I.
Development of the Tail.” Proceedings of the American Academy of Arts
and Sciences, Vol. XIII. Presented Oct. 11, 1877.
(56) Al. Agassiz. “II. Development of the Flounders.” Proceedings of
the American Acad. of Arts and Sciences, Vol. XIV. Presented June,
1878.
(57) K. E. v. Baer. Untersuchungen über die Entwicklungsgeschichte
der Fische. Leipzig, 1835.
(58) Ch. van Bambeke. “Premiers effets de la fécondation sur les œufs
de Poissons: sur l'origine et la signification du feuillet muqueux ou
glandulaire chez les Poissons Osseux.” Comptes Rendus des Séances de
l'Académie des Sciences, Tome LXXIV. 1872.
(59) Ch. van Bambeke. “Recherches sur l'Embryologie des Poissons
Osseux.” Mém. couronnés et Mém. de savants étrangers de l'Académie
roy. Belgique, Vol. XL. 1875.
(60) E. v. Beneden. “A contribution to the history of the Embryonic
development of the Teleosteans.” Quart. J. of Micr. Sci., Vol.
XVIII. 1878.
(61) E. Calberla. “Zur Entwicklung des Medullarrohres u. d. Chorda
dorsalis d. Teleostier u. d. Petromyzonten.” Morphologisches
Jahrbuch, Vol. III. 1877.
(62) A. Götte. “Beiträge zur Entwicklungsgeschichte der Wirbelthiere.”
Archiv f. mikr. Anat., Vol. IX. 1873.
(63) A. Götte. “Ueber d. Entwicklung d. Central-Nervensystems der
Teleostier.” Archiv f. mikr. Anat., Vol. XV. 1878.
(64) A. Götte. “Entwick. d. Teleostierkeime.” Zoologischer Anzeiger,
No. 3. 1878.
(65) W. His.. “Untersuchungen über die Entwicklung von Knochenfischen,
etc.” Zeit. f. Anat. u. Entwicklungsgeschichte, Vol. I. 1876.
(66) W. His. “Untersuchungen über die Bildung des Knochenfischembryo
(Salmen).” Archiv f. Anat. u. Physiol., 1878.
(67) E. Klein. “Observations on the early Development of the Common
Trout.” Quart. J. of Micr. Science, Vol. XVI. 1876.
(68) C. Kupffer. “Beobachtungen über die Entwicklung der
Knochenfische.” Archiv f. mikr. Anat., Bd. IV. 1868.
(69) C. Kupffer. Ueber Laichen u. Entwicklung des Ostsee-Herings.
Berlin, 1878.
(70) M. Lereboullet. “Recherches sur le développement du brochet de la
perche et de l'écrevisse.” Annales des Sciences Nat., Vol. I.,
Series IV. 1854.
(71) M. Lereboullet. “Recherches d'Embryologie comparée sur le
développement de la Truite.” An. Sci. Nat., quatrième série, Vol.
XVI. 1861.
(72) T. Oellacher. “Beiträge zur Entwicklungsgeschichte der
Knochenfische nach Beobachtungen am Bachforellenei.” Zeit. f. wiss.
Zool., Vol. XXII., 1872, and Vol. XXIII., 1873.
(72*) H. Rathke. Abh. z. Bildung u. Entwick. d. Menschen u. Thiere.
Leipzig, 1832-3. Part II. Blennius.
(73) Reineck. “Ueber die Schichtung des Forellenkeims.” Archiv f.
mikr. Anat., Bd. V. 1869.
(74) S. Stricker. “Untersuchungen über die Entwicklung der
Bachforelle.” Sitzungsberichte der Wiener k. Akad. d. Wiss., 1865.
Vol. LI. Abth. 2.
(75) Carl Vogt. “Embryologie des Salmones.” Histoire Naturelle des
Poissons de l'Europe Centrale. L. Agassiz. 1842.
(76) C. Weil. “Beiträge zur Kenntniss der Knochenfische.”
Sitzungsber. der Wiener kais. Akad. der Wiss., Bd. LXVI. 1872.
Cyclostomata.[Pg iv]
(77) E. Calberla. “Der Befruchtungsvorgang beim Petromyzon Planeri.”
Zeit. f. wiss. Zool., Vol. XXX. 1877.
(78) E. Calberla. “Ueb. d. Entwicklung d. Medullarrohres u. d. Chorda
dorsalis d. Teleostier u. d. Petromyzonten.” Morpholog. Jahrbuch,
Vol. III. 1877.
(79) C. Kupffer u. B. Benecke. Der Vorgang d. Befruchtung am Ei d.
Neunaugen. Königsberg, 1878.
(80) Aug. Müller. “Ueber die Entwicklung d. Neunaugen.” Müller’s
Archiv, 1856.
(81) Aug. Müller. Beobachtungen üb. d. Befruchtungserscheinungen im
Ei d. Neunaugen. Königsberg, 1864.
(82) W. Müller. “Das Urogenitalsystem d. Amphioxus u. d. Cyclostomen.”
Jenaische Zeitschrift, Vol. IX. 1875.
(83) Ph. Owsjannikoff. “Die Entwick. von d. Flussneunaugen.” Vorläuf.
Mittheilung. Mélanges Biologiques tirés du Bulletin de l'Acad. Imp.
St Pétersbourg, Vol. VII. 1870.
(84) Ph. Owsjannikoff. On the development of Petromyzon fluviatilis
(Russian).
(85) Anton Schneider. Beiträge z. vergleich. Anat. u. Entwick. d.
Wirbelthiere. Quarto. Berlin, 1879.
(86) M. S. Schultze. “Die Entwickl. v. Petromyzon Planeri.” Gekrönte
Preisschrift. Haarlem, 1856.
(87) W. B. Scott. “Vorläufige Mittheilung üb. d.
Entwicklungsgeschichte d. Petromyzonten.” Zoologischer Anzeiger,
Nos. 63 and 64. III. Jahrg. 1880.
Ganoidei.
Acipenserdæ
(88) Knock. “Die Beschr. d. Reise z. Wolga Behufs d.
Sterlettbefruchtung.” Bull. Soc. Nat. Moscow, 1871.
(89) A. Kowalevsky, Ph. Owsjannikoff, and N. Wagner. “Die Entwick. d.
Störe.” Vorläuf. Mittheilung. Mélanges Biologiques tirés du Bulletin
d. l'Acad. Imp. St Pétersbourg, Vol. VII. 1870.
(90) W. Salensky. “Development of the Sterlet (Acipenser ruthenus).” 2
Parts. Proceedings of the Society of Naturalists in the imperial
University of Kasan. 1878 and 9 (Russian). Part I., abstracted in
Hoffmann and Schwalbe’s Jahresbericht for 1878.
(91) W. Salensky. “Zur Embryologie d. Ganoiden (Acipenser).”
Zoologischer Anzeiger, Vol. I., Nos. 11, 12, 13.
Lepidosteidæ
(92) Al. Agassiz. “The development of Lepidosteus.” Proc. Amer. Acad.
of Arts and Sciences, Vol. XIII. 1878.
Amphibia.
(93) Ch. van Bambeke. “Recherches sur le développement du Pélobate
brun.” Mémoires couronnés, etc. de l'Acad. roy. de Belgique, 1868.
(94) Ch. van Bambeke. “Recherches sur l'embryologie des Batraciens.”
Bulletin de l'Acad. roy. de Belgique, 1875.
(95) Ch. van Bambeke. “Nouvelles recherches sur l'embryologie des
Batraciens.” Archives de Biologie, Vol. I. 1880.
(96) K. E. von Baer. “Die Metamorphose des Eies der Batrachier.”
Müller’s Archiv, 1834.
(97) B. Benecke. “Ueber die Entwicklung des Erdsalamanders.”
Zoologischer Anzeiger, 1880.
[Pg v]
(98) S. F. Clarke. “Development of Amblystoma punctatum,” Part I.,
External. Studies from the Biological Laboratory of the Johns Hopkins
University, No. II. 1880.
(99) H. Cramer. “Bemerkungen üb. d. Zellenleben in d. Entwick. d.
Froscheies.” Müller’s Archiv, 1848.
(100) A. Ecker. Icones Physiolog. 1851-1859.
(101) A. Götte. Die Entwicklungsgeschichte der Unke. Leipzig, 1875.
(102) C. K. Hoffmann. “Amphibia.” Klassen u. Ordnungen d.
Thierreichs, 1873-1879.
(103) T. H. Huxley. Article “Amphibia” in the Encyclopædia
Britannica.
(104) A. Moquin-Tandon. “Développement des Batraciens anures.”
Annales des Sciences Naturelles, III. 1875.
(105) G. Newport. “On the impregnation of the Ovum in Amphibia” (three
memoirs). Phil. Trans. 1851, 1853, and 1854.
(106) W. K. Parker. “On the structure and development of the Skull of
the common Frog.” Phil. Trans., CLXI. 1871.
(107) W. K. Parker. “On the structure and development of the Skull of
the Batrachia.” Phil. Trans., Vol. CXLVI., Part 2. 1876.
(108) W. C. H. Peters. “Ueber die Entwicklung der Coecilien und
besonders von Cœcilia compressicauda.” Berlin Monatsbericht, p. 40,
1874.
(109) W. C. H. Peters. “Ueber die Entwicklung der Coecilien.” Berl.
Monatsbericht, p. 483, 1875.
(110) J. L. Prevost and J. B. Dumas. “Deuxième Mém. s. l. génération.
Développement de l'œuf d. Batraciens.” Ann. Sci. Nat. II. 1824.
(111) R. Remak. Untersuchungen über die Entwicklung der
Wirbelthiere, 1850-1858.
(112) M. Rusconi. Développement de la grenouille commune depuis le
moment de sa naissance jusqu'à son état parfait, 1826.
(113) M. Rusconi. Histoire naturelle, développement et métamorphose
de la Salamandre terrestre, 1854.
(114) W. B. Scott and H. F. Osborn. “On the early development of the
common Newt.” Quart. J. of Micr. Science, Vol. XXIX. 1879.
(115) S. Stricker. “Entwicklungsgeschichte von Bufo cinereus.” Sitzb.
der kaiserl. Acad. zu Wien, 1860.
(116) S. Stricker. “Untersuchungen über die ersten Anlagen in
Batrachier-Eiern.” Zeitschrift f. wiss. Zoologie, Bd. XI. 1861.
Aves.
(117) K. E. von Baer. Ueb. Entwicklungsgeschichte d. Thiere.
Königsberg, 1828-1837.
(118) F. M. Balfour. “The development and growth of the layers of the
Blastoderm,” and “On the disappearance of the Primitive Groove in the
Embryo Chick.” Quart. J. of Micros. Science, Vol. XIII. 1873.
(119) M. Braun. “Die Entwicklung d. Wellenpapagei’s.” Part I. Arbeit.
d. zool.-zoot. Instit. Würzburg. Vol. V. 1879.
(120) M. Braun. “Aus d. Entwick. d. Papageien; I. Rückenmark; II.
Entwicklung d. Mesoderms; III. Die Verbindungen zwischen Rückenmark u.
Darm bei Vögeln.” Verh. d. phys.-med. Ges. zu Würzburg. N. F. Bd.
XIV. and XV. 1879 and 1880.
(121) J. Disse. “Die Entwicklung des mittleren Keimblattes im
Hühnerei.” Archiv für mikr. Anat., Vol. XV. 1878.
(122) J. Disse. “Die Entstehung d. Blutes u. d. ersten Gefässe im
Hühnerei.” Archiv f. mikr. Anat., Vol. XVI. 1879.
(123) Fr. Durante. “Sulla struttura della macula germinativa delle
uova di Gallina.” Ricerche nel Laboratorio di Anatomia della R.
Università di Roma.
(124) E. Dursy. Der Primitivstreif des Hühnchens. 1867.
(125) M. Duval. “Etude sur la ligne primitive de l'embryon de Poulet.”
Annales des Sciences Naturelles, Vol. VII. 1879.
(126) M. Foster and F. M. Balfour. Elements of Embryology. Part I.
London, 1874.
[Pg vi]
(127) Gasser. “Der Primitivstreifen bei Vogelembryonen.” Schriften
d. Gesell. zur Beförd. d. gesammten Naturwiss. zu Marburg, Vol. II.
Supplement I. 1879.
(128) A. Götte. “Beiträge zur Entwicklungsgeschichte d. Wirbelthiere.
II. Die Bildung d. Keimblatter u. d. Blutes im Hühnerei.” Archiv für
mikr. Anat., Vol. X. 1874.
(129) V. Hensen. “Embryol. Mitth.” Archiv f. mikr. Anat., Vol. III.
1867.
(130) W. His. Untersuch. üb. d. erste Anlage d. Wirbelthierleibes.
Leipzig, 1868.
(131) W. His. Unsere Körperform und das physiol. Problem ihrer
Entstehung. Leipzig, 1875.
(132) W. His. “Der Keimwall des Hühnereies u. d. Entstehung d.
parablastischen Zellen.” Zeit. f. Anat. u. Entwicklungsgeschichte.
Bd. I. 1876.
(133) W. His. “Neue Untersuchungen üb. die Bildung des Hühnerembryo
I.” Archiv f. Anat. u. Phys. 1877.
(134) E. Klein. “Das mittlere Keimblatt in seiner Bezieh. z. Entwick.
d. ers. Blutgefässe und Blutkörp. im Hühnerembryo.” Sitzungsber.
Wien. Akad., Vol. LXIII. 1871.
(135) A. Kölliker. Entwicklungsgeschichte d. Menschen u. d. höheren
Thiere. Leipzig, 1879.
(136) C. Kupffer. “Die Entsteh. d. Allantois u. d. Gastrula d.
Wirbelth.” Zoolog. Anzeiger, Vol. II. 1879, pp. 520, 593, 612.
(137) C. Kupffer and B. Benecke. “Photogramme z. Ontogenie d. Vögel.”
Nov. Act. d. k. Leop.-Carol.-Deutschen Akad. d. Naturforscher, Vol.
XLI. 1879.
(138) J. Oellacher. “Untersuchungen über die Furchung u.
Blatterbildung im Hühnerei.” Stricker’s Studien. 1870.
(139) C. H. Pander. Beiträge z. Entwick. d. Hühnchens im Eie.
Würzburg, 1817.
(140) A. Rauber. “Ueber die Embryonalanlage des Hühnchens.”
Centralblatt für d. medic. Wissenschaften. 1874-75.
(141) A. Rauber. Ueber die Stellung des Hühnchens im
Entwicklungsplan. 1876.
(142) A. Rauber. “Primitivrinne und Urmund. Beiträge zur
Entwicklungsgeschichte des Hühnchens.” Morphol. Jahrbuch, B. II.
1876.
(143) A. Rauber. Primitivstreifen und Neurula der Wirbelthiere in
normaler und pathologischer Beziehung. 1877.
(144) R. Remak. Untersuch. üb. d. Entwicklung d. Wirbelthiere.
Berlin, 1850-55.
(145) S. L. Schenk. “Beiträge z. Lehre v. d. Organanlage im
motorischen Keimblatt.” Sitz. Wien. Akad., Vol. LVII. 1860.
(146) S. L. Schenk. “Beiträge z. Lehre v. Amnion.” Archiv f. mikr.
Anat., Vol. VII. 1871.
(147) S. L. Schenk. Lehrbuch d. vergleich. Embryol. d. Wirbelthiere.
Wien, 1874.
(148) S. Stricker. “Mittheil. üb. d. selbstständigen Bewegungen
embryonaler Zellen.” Sitz. Wien. Akad., Vol. XLIX. 1864.
(149) S. Stricker. “Beiträge zur Kenntniss des Hühnereies.” Wiener
Sitzungsber., Vol. LIV. 1866.
(150) H. Virchow. Ueber d. Epithel d. Dottersackes im Hühnerei.
Inaug. Diss. Berlin, 1875.
(151) (151) W. Waldeyer. “Ueber die Keimblätter und den Primitivstreifen bei
der Entwicklung des Hühnerembryo.” Zeitschrift für rationelle
Medicin. 1869.
(152) C. F. Wolff. Theoria generationis. Halæ, 1759.
(153) C. F. Wolff. Ueb. d. Bildung d. Darmcanals im bebrüteten
Hühnchen. Halle, 1812.
Reptilia.
(154) C. Kupffer and Benecke. Die erste Entwicklung am Ei d.
Reptilien. Königsberg, 1878.
[Pg vii]
(155) C. Kupffer. “Die Entstehung d. Allantois u. d. Gastrula d.
Wirbelthiere.” Zoologischer Anzeiger, Vol. II. 1879, pp. 520, 593,
612.
Lacertilia.
(156) F. M. Balfour. “On the early Development of the Lacertilia,
together with some observations, etc.” Quart. J. of Micr. Science,
Vol. XIX. 1879.
(157) Emmert u. Hochstetter. “Untersuchung üb. d. Entwick. d.
Eidechsen in ihren Eiern.” Reil’s Archiv, Vol. X. 1811.
(158) M. Lereboullet. “Développement de la Truite, du Lézard et du
Limnée. II. Embryologie du Lézard.” An. Sci. Nat., Ser. IV., Vol.
XXVII. 1862.
(159) W. K. Parker. “Structure and Devel. of the Skull in Lacertilia.”
Phil. Trans., Vol. 170, p. 2. 1879.
(160) H. Strahl. “Ueb. d. Canalis myeloentericus d. Eidechse.”
Schrift. d. Gesell. z. Beför. d. gesam. Naturwiss. Marburg. July 23,
1880.
Ophidia.
(161) H. Dutrochet. “Recherches s. l. enveloppes du fœtus.” Phil.
Trans., Paris, Vol. VIII. 1816.
(162) W. K. Parker. “On the skull of the common Snake.” Phil.
Trans., Vol. 169, Part II. 1878.
(163) H. Rathke. Entwick. d. Natter. Königsberg, 1839.
Chelonia.
(164) "L. Agassiz. Contributions to the Natural History of the United
States, Vol. II. 1857. Embryology of the Turtle.
(165) W. K. Parker. “On the development of the skull and nerves in the
green Turtle.” Proc. of the Roy. Soc., Vol. XXVIII. 1879. Vide
also Nature, April 14, 1879, and Challenger Reports, Vol. I. 1880.
(166) H. Rathke. Ueb. d. Entwicklung d. Schildkröten. Braunschweig,
1848.
Crocodilia.
(167) H. Rathke. Ueber die Entwicklung d. Krokodile. Braunschweig,
1866.
Mammalia
(168) K. E. von Baer. Ueb. Entwicklungsgeschichte d. Thiere.
Königsberg, 1828-1837.
(169) Barry. “Researches on Embryology.” First Series. Philosophical
Transactions, 1838, Part II. Second Series, Ibid. 1839, Part II.
Third Series, Ibid. 1840.
(170) Ed. van Beneden. La maturation de l'œuf, la fécondation et les
premières phases du développement embryonaire d. Mammifères.
Bruxelles, 1875.
(171) Ed. van Beneden. “Recherches sur l'embryologie des Mammifères.”
Archives de Biologie, Vol. I. 1880.
(172) Ed. v. Beneden and Ch. Julin. “Observations sur la maturation
etc. de l'œuf chez les Cheiroptères.” Archives de Biologie, Vol. I.
1880.
(173) Th. L. W. Bischoff. Entwicklungsgeschichte d. Säugethiere u.
des Menschen. Leipzig, 1842.
(174) Th. L. W. Bischoff. Entwicklungsgeschichte des Kanincheneies.
Braunschweig, 1842.
(175) Th. L. W. Bischoff. Entwicklungsgeschichte des Hundeeies.
Braunschweig, 1845.
(176) Th. L. W. Bischoff. Entwicklungsgeschichte des
Meerschweinchens. Giessen, 1852.
[Pg viii]
(177) Th. L. W. Bischoff. Entwicklungsgeschichte des Rehes. Giessen,
1854.
(178) Th. L. W. Bischoff. “Neue Beobachtungen z. Entwicklungsgesch.
des Meerschweinchens.” Abh. d. bayr. Akad., Cl. II. Vol. X. 1866.
(179) Th. L. W. Bischoff. Historisch-kritische Bemerkungen z. d.
neuesten Mittheilungen üb. d. erste Entwick. d. Säugethiereier.
München, 1877.
(180) M. Coste. Embryogénie comparée. Paris, 1837.
(181) E. Haeckel. Anthropogenie, Entwicklungsgeschichte des
Menschen. Leipzig, 1874.
(182) V. Hensen. “Beobachtungen üb. d. Befrucht. u. Entwick. d.
Kaninchens u. Meerschweinchens.” Zeit. f. Anat. u. Entwick., Vol. I.
1876.
(183) A. Kölliker. Entwicklungsgeschichte d. Menschen u. d. höheren
Thiere. Leipzig, 1879.
(184) A. Kölliker. “Die Entwick. d. Keimblätter des Kaninchens.”
Zoologischer Anzeiger, Nos. 61, 62, Vol. III. 1880.
(185) N. Lieberkühn. Ueber d. Keimblätter d. Säugethiere.
Doctor-Jubelfeier d. Herrn. H. Nasse. Marburg, 1879.
(186) N. Lieberkühn. “Z. Lehre von d. Keimblättern d. Säugethiere.”
Sitz. d. Gesell. z. Beförd d. gesam. Naturwiss. Marburg, No. 3.
1880.
(187) Rauber. “Die erste Entwicklung d. Kaninchens.” Sitzungsber. d.
naturfor. Gesell. z. Leipzig. 1875.
(188) C. B. Reichert. “Entwicklung des Meerschweinchens.” Abh. der.
Berl. Akad. 1862.
(189) E. A. Schäfer. “Description of a Mammalian ovum in an early
condition of development.” Proc. Roy. Soc., No. 168. 1876.
(190) E. A. Schäfer. “A contribution to the history of development of
the guinea-pig.” Journal of Anat. and Phys., Vol. X. and XI. 1876
and 1877.
Fœtal Membranes and Placenta of Mammalia.
(191) John Anderson. Anatomical and Zoological Researches in Western
Yunnan. London, 1878.
(192) K. E. von Baer. Untersuchungen über die Gefässverbindung
zwischen Mutter und Frucht, 1828.
(193) C. G. Carus. Tabulae anatomiam comparativam illustrantes.
1831, 1840.
(194) H. C. Chapman. “The placenta and generative apparatus of the
Elephant.” Journ. Acad. Nat. Sc., Philadelphia. Vol. VIII. 1880.
(195) C. Creighton. “On the formation of the placenta in the
guinea-pig.” Journal of Anat. and Phys., Vol. XII. 1878.
(196) Ecker. Icones Physiologicae. 1852-1859.
(197) G. B. Ercolani. The utricular glands of the uterus, etc.,
translated from the Italian under the direction of H. O. Marcy.
Boston, 1880. Contains translations of memoirs published in the Mem.
dell'Accad. d. Scienze d. Bologna, and additional matter written
specially for the translation.
(198) G. B. Ercolani. Nuove ricerche sulla placenta nei pesci
cartilaginosi e nei mammiferi. Bologna, 1880.
(199) Eschricht. De organis quae respirationi et nutritioni fœtus
Mammalium inserviunt. Hafniae, 1837.
(200) A. H. Garrod and W. Turner. “The gravid uterus and placenta of
Hyomoschus aquaticus.” Proc. Zool. Soc., London, 1878.
(201) P. Harting. Het ei en de placenta van Halicore Dugong. Inaug.
diss. Utrecht. “On the ovum and placenta of the Dugong.” Abstract by
Prof. Turner. Journal of Anat. and Phys., Vol. XIII.
(202) (202) Th. H. Huxley. The Elements of Comparative Anatomy. London,
1864.
(203) A. Kölliker. “Ueber die Placenta der Gattung Tragulus.” Verh.
der Würzb. phys.-med. Gesellschaft, Bd. X.
(204) C. D. Meigs. “On the reproduction of the Opossum (Didelphis
Virginiana).” Amer. Phil. Soc. Trans., Vol. X. 1853.
(205) H. Milne-Edwards. “Sur la Classification Naturelle.” Ann.
Sciences Nat., Sér. 3, Vol. I. 1844.
[Pg ix]
(206) Alf. Milne-Edwards. “Recherches sur la famille des Chevrotains.”
Ann. des Sciences Nat., Séries V., Vol. II. 1864.
(207) Alf. Milne-Edwards. “Observations sur quelques points de
l'Embryologie des Lemuriens, etc.” Ann. Sci. Nat., Sér. V., Vol. XV.
1872.
(208) Alf. Milne-Edwards. “Sur la conformation du placenta chez le
Tamandua.” Ann. des Sci. Nat., XV. 1872.
(209) Alf. Milne-Edwards. “Recherches s. l. enveloppes fœtales du
Tatou à neuf bandes.” Ann. Sci. Nat., Sér. VI., Vol. VIII. 1878.
(210) R. Owen. “On the generation of Marsupial animals, with a
description of the impregnated uterus of the Kangaroo.” Phil.
Trans., 1834.
(211) R. Owen. “Description of the membranes of the uterine fœtus of
the Kangaroo.” Mag. Nat. Hist., Vol. I. 1837.
(212) R. Owen. “On the existence of an Allantois in a fœtal Kangaroo
(Macropus major).” Zool. Soc. Proc., v. 1837.
(213) R. Owen. “Description of the fœtal membranes and placenta of
the Elephant.” Phil. Trans., 1857.
(214) R. Owen. On the Anatomy of Vertebrates, Vol. III. London,
1868.
(215) G. Rolleston. “Placental structure of the Tenrec, etc.”
Transactions of the Zoological Society, Vol. V. 1866.
(216) W. Turner. “Observations on the structure of the human
placenta.” Journal of Anat. and Phys., Vol. VII. 1868.
(217) W. Turner. “On the placentation of the Cetacea.” Trans. Roy.
Soc. Edinb., Vol. XXVI. 1872.
(218) W. Turner. “On the placentation of Sloths (Cholœpus
Hoffmanni).” Trans. of R. Society of Edinburgh, Vol. XXVII. 1875.
(219) W. Turner. “On the placentation of Seals (Halichœrus gryphus).”
Trans. of R. Society of Edinburgh, Vol. XXVII. 1875.
(220) W. Turner. “On the placentation of the Cape Ant-eater
(Orycteropus capensis).” Journal of Anat. and Phys., Vol. X. 1876.
(221) W. Turner. Lectures on the Anatomy of the Placenta. First
Series. Edinburgh, 1876.
(222) W. Turner. “Some general observations on the placenta, with
special reference to the theory of Evolution.” Journal of Anat. and
Phys., Vol. XI. 1877.
(223) W. Turner. “On the placentation of the Lemurs.” Phil. Trans.,
Vol. 166, p. 2. 1877.
(224) W. Turner. “On the placentation of Apes.” Phil. Trans., 1878.
(225) W. Turner. “The cotyledonary and diffused placenta of the
Mexican deer (Cervus Americanus).” Journal of Anat. and Phys., Vol.
XIII. 1879.
Human Embryo.
(226) Fried. Ahlfeld. “Beschreibung eines sehr kleinen menschlichen
Eies.” Archiv f. Gynaekologie, Bd. XIII. 1878.
(227) Herm. Beigel und Ludwig Loewe. “Beschreibung eines menschlichen
Eichens aus der zweiten bis dritten Woche der Schwangerschaft.”
Archiv f. Gynaekologie, Bd. XII. 1877.
(228) K. Breus. “Ueber ein menschliches Ei aus der zweiten Woche der
Gravidität.” Wiener medicinische Wochenschrift, 1877.
(229) M. Coste. Histoire générale et particulière du développement
des corps organisés, 1847-59.
(230) A. Ecker. Icones Physiologicae. Leipzig, 1851-1859.
(231) V. Hensen. “Beitrag z. Morphologie d. Körperform u. d. Gehirns
d. menschlichen Embryos.” Archiv f. Anat. u. Phys., 1877.
(232) W. His. Anatomie menschlicher Embryonen, Part I. Embryonen d.
ersten Monats. Leipzig, 1880.
(233) J. Kollmann. “Die menschlichen Eier von 6 MM. Grösse.” Archiv
f. Anat. und Phys., 1879.
(234) W. Krause. “Ueber d. Allantois d. Menschen.” Archiv f. Anat.
und Phys., 1875.
(235) W. Krause. “Ueber zwei frühzeitige menschliche Embryonen.”
Zeit. f. wiss. Zool., Vol. XXXV. 1880.
[Pg x]
(236) L. Loewe. “Im Sachen der Eihäute jüngster menschlicher Eier.”
Archiv für Gynaekologie, Bd. XIV. 1879.
(237) C. B. Reichert. “Beschreibung einer frühzeitigen menschlichen
Frucht im bläschenformigen Bildungszustande (sackformiger Keim von
Baer) nebst vergleichenden Untersuchungen über die bläschenformigen
Früchte der Säugethiere und des Menschen.” Abhandlungen der königl.
Akad. d. Wiss. zu Berlin, 1873.
(238) Allen Thomson. “Contributions to the history of the structure of
the human ovum and embryo before the third week after conception; with
a description of some early ova.” Edinburgh Med. Surg. Journal, Vol.
LII. 1839.
Comparison of the formation of the germinal layers
in the Vertebrata.
(239) F. M. Balfour. “A comparison of the early stages in the
development of Vertebrates.” Quart. J. of Micr. Science, Vol. XV.
1875.
(240) F. M. Balfour. “A monograph on the development of Elasmobranch
Fishes.” London, 1878.
(241) F. M. Balfour. “On the early development of the Lacertilia
together with some observations, etc.” Quart. J. of Micr. Science,
Vol. XIX. 1879.
(242) A. Götte. Die Entwicklungsgeschichte d. Unke. Leipzig, 1875.
(243) W. His. “Ueb. d. Bildung d. Haifischembryonen.” Zeit. f. Anat.
u. Entwick., Vol. II.
1877. Cf. also His’ papers on Teleostei, Nos.
65 and 66.
(244) A. Kowalevsky. “Entwick. d. Amphioxus lanceolatus.” Mém. Acad.
des Sciences St Pétersbourg,
Ser. VII.
Tom. XI. 1867.
(245) A. Kowalevsky. “Weitere Studien üb. d. Entwick. d. Amphioxus
lanceolatus.” Archiv f. mikr. Anat., Vol. XIII. 1877.
(246) C. Kupffer. “Die Entstehung d. Allantois u. d. Gastrula d.
Wirbelthiere.” Zool. Anzeiger, Vol. II. 1879, pp. 520, 593, 612.
(247) R. Remak. Untersuchungen üb. d. Entwicklung d. Wirbelthiere,
1850-1858.
(248) A. Rauber. Primitivstreifen u. Neurula d. Wirbelthiere.
Leipzig, 1877.
Phylogeny of the Chordata.
(249) F. M. Balfour. A Monograph on the development of Elasmobranch
Fishes. London, 1878.
(250) A. Dohrn. Der Ursprung d. Wirbelthiere und d. Princip. d.
Functionswechsel. Leipzig, 1875.
(251) E. Haeckel. Schöpfungsgeschichte. Leipzig. Vide also
Translation. The History of Creation. King and Co., London, 1876.
(252) E. Haeckel. Anthropogenie. Leipzig. Vide also Translation.
Anthropogeny. Kegan Paul and Co., London, 1878.
(253) A. Kowalevsky. “Entwicklungsgeschichte d. Amphioxus
lanceolatus.” Mém. Acad. d. Scien. St Pétersbourg, Ser. VII. Tom.
XI. 1867, and Archiv f. mikr. Anat., Vol. XIII. 1877.
(254) A. Kowalevsky. “Weitere Stud. üb. d. Entwick. d. einfachen
Ascidien.” Archiv f. mikr. Anat., Vol. VII. 1871.
(255) C. Semper. “Die Stammesverwandschaft d. Wirbelthiere u.
Wirbellosen.” Arbeit. a. d. zool.-zoot. Instit. Würzburg, Vol. II.
1875.
(256) C. Semper. “Die Verwandschaftbeziehungen d. gegliederten
Thiere.” Arbeit. a. d. zool.-zoot. Instit. Würzburg, Vol. III.
1876-1877.
General works on Embryology.
(257) Allen Thomson. British Association Address, 1877.
(258) A. Agassiz. “Embryology of the Ctenophoræ.” Mem. Amer. Acad. of
Arts and Sciences, Vol. X. 1874.
(259) K. E. von Baer. Ueb. Entwicklungsgeschichte d. Thiere.
Königsberg, 1828-1837.
[Pg xi]
(260) F. M. Balfour. “A Comparison of the Early Stages in the
Development of Vertebrates.” Quart. Journ. of Micr. Sci., Vol. XV.
1875.
(261) C. Claus. Die Typenlehre u. E. Haeckel’s sg. Gastræa-theorie.
Wien, 1874.
(262) C. Claus. Grundzüge d. Zoologie. Marburg und Leipzig, 1879.
(263) A. Dohrn. Der Ursprung d. Wirbelthiere u. d. Princip des
Functionswechsels. Leipzig, 1875.
(264) C. Gegenbaur. Grundriss d. vergleichenden Anatomie. Leipzig,
1878. Vide also Translation. Elements of Comparative Anatomy.
Macmillan & Co., 1878.
(265) A. Götte. Entwicklungsgeschichte d. Unke. Leipzig, 1874.
(266) E. Haeckel. Studien z. Gastræa-theorie, Jena, 1897; and also
Jenaische Zeitschrift, Vols. VIII. and IX. 1874-5.
(267) E. Haeckel. Schöpfungsgeschichte. Leipzig. Vide also
Translation, The History of Creation. King & Co., London, 1878.
(268) E. Haeckel. Anthropogenie. Leipzig. Vide also Translation,
Anthropogeny. Kegan Paul & Co., London, 1878.
(269) B. Hatschek. “Studien üb. Entwicklungsgeschichte d. Anneliden.”
Arbeit. a. d. zool. Instit. d. Univer. Wien. 1878.
(270) O. and R. Hertwig. “Die Actinien.” Jenaische Zeitschrift,
Vols. XIII. and XIV. 1879.
(271) O. and R. Hertwig. Die Cœlomtheorie.
Jena, 1881.
(272) O. Hertwig. Die Chætognathen. Jena, 1880.
(273) R. Hertwig. Ueb. d. Bau d. Ctenophoren. Jena, 1880.
(274) T. H. Huxley. The Anatomy of Invertebrated Animals. Churchill,
1877.
(274*) T. H. Huxley. “On the Classification of the Animal Kingdom.”
Quart. J. of Micr. Science, Vol. XV. 1875.
(275) N. Kleinenberg. Hydra, eine
anatomisch-entwicklungsgeschichtliche Untersuchung. Leipzig, 1872.
(276) A. Kölliker. Entwicklungsgeschichte d. Menschen u. d. höh.
Thiere. Leipzig, 1879.
(277) A. Kowalevsky. “Embryologische Studien an Würmern u.
Arthropoden.” Mém. Acad. Pétersbourg, Series VII. Vol. XVI. 1871.
(278) E. R. Lankester. “On the Germinal Layers of the Embryo as the
Basis of the Genealogical Classification of Animals.” Ann. and Mag.
of Nat. Hist. 1873.
(279) E. R. Lankester. “Notes on Embryology and Classification.”
Quart. Journ. of Micr. Sci., Vol. XVII. 1877.
(280) E. Metschnikoff. “Zur Entwicklungsgeschichte d. Kalkschwämme.”
Zeit. f. wiss. Zool., Vol. XXIV. 1874.
(281) E. Metschnikoff. “Spongiologische Studien.” Zeit. f. wiss.
Zool., Vol. XXXII. 1879.
(282) A. S. P. Packard. Life Histories of Animals, including Man, or
Outlines of Comparative Embryology. Holt and Co., New York, 1876.
(283) C. Rabl. “Ueb. d. Entwick. d. Malermuschel.” Jenaische
Zeitsch., Vol. X. 1876.
(284) C. Rabl. “Ueb. d. Entwicklung. d. Tellerschnecke (Planorbis).”
Morph. Jahrbuch, Vol. V. 1879.
(285) H. Rathke. Abhandlungen z. Bildung und Entwicklungsgesch. d.
Menschen u. d. Thiere. Leipzig, 1833.
(286) H. Rathke. Ueber die Bildung u. Entwicklungs. d. Flusskrebses.
Leipzig, 1829.
(287) R. Remak. Untersuch. üb. d. Entwick. d. Wirbelthiere. Berlin,
1855.
(288) Salensky. “Bemerkungen üb. Haeckels Gastræa-theorie.” Archiv.
f. Naturgeschichte, 1874.
(289) E. Schäfer. “Some Teachings of Development.” Quart. Journ. of
Micr. Science, Vol. XX. 1880.
(290) C. Semper. “Die Verwandtschaftbeziehungen d. gegliederten
Thiere.” Arbeiten a. d. zool.-zoot. Instit. Würzburg, Vol. III.
1876-7.
General works dealing with the development of the organs of the
Chordata.[Pg xii]
(291) K. E. von Baer. Ueber Entwicklungsgeschichte d. Thiere.
Königsberg, 1828-1837.
(292) F. M. Balfour. A Monograph on the development of Elasmobranch
Fishes. London, 1878.
(293) Th. C. W. Bischoff. Entwicklungsgesch. d. Säugethiere U. d.
Menschen. Leipzig, 1842.
(294) C. Gegenbaur. Grundriss d. vergleichenden Anatomie. Leipzig,
1878. Vide also English translation, Elements of Comp. Anatomy.
London, 1878.
(295) M. Foster and F. M. Balfour. The Elements of Embryology. Part
I. London, 1874.
(296) Alex. Götte. Entwicklungsgeschichte d. Unke. Leipzig, 1875.
(297) W. His. Untersuch. üb. d. erste Anlage d. Wirbelthierleibes.
Leipzig, 1868.
(298) A. Kölliker. Entwicklungsgeschichte d. Menschen u. der höheren
Thiere. Leipzig, 1879.
(299) H. Rathke. Abhandlungen ü. Bildung und Entwicklungsgeschichte
d. Menschen u. d. Thiere. Leipzig, 1838.
(300) H. Rathke. Entwicklungs. d. Natter. Königsberg, 1839.
(301) H. Rathke. Entwicklungs. d. Wirbelthiere. Leipzig, 1861.
(302) R. Remak. Untersuchungen üb. d. Entwicklung d. Wirbelthiere.
Berlin, 1850-1855.
(303) S. L. Schenk. Lehrbuch d. vergleich. Embryologie d.
Wirbelthiere. Wien, 1874.
Epidermis and its derivatives.
General.
(304) T. H. Huxley. “Tegumentary organs.” Todd’s Cyclopædia of Anat.
and Physiol.
(305) P. Z. Unna. “Histol. u. Entwick. d. Oberhaut.” Archiv f. mikr.
Anat. Vol. XV. 1876. Vide also Kölliker (No. 298).
Scales of the Pisces.
(306) O. Hertwig. “Ueber Bau u. Entwicklung d. Placoidschuppen u. d.
Zähne d. Selachier.” Jenaische Zeitschrift, Vol. VIII. 1874.
(307) O. Hertwig. “Ueber d. Hautskelet d. Fische.” Morphol.
Jahrbuch, Vol. II. 1876. (Siluroiden u. Acipenseridæ.)
(308) O. Hertwig. “Ueber d. Hautskelet d. Fische (Lepidosteus u.
Polypterus).” Morph. Jahrbuch, Vol. V. 1879.
Feathers.
(309) Th. Studer. Die Entwick. d. Federn. Inaug. Diss. Bern, 1873.
(310) Th. Studer. “Beiträge z. Entwick. d. Feder.” Zeit. f. wiss.
Zool., Vol. XXX. 1878.
Sweat-glands.
(311) M. S. Ranvier. “Sur la structure des glandes sudoripares.”
Comptes Rendus, Dec. 29, 1879.
Mammary glands.[Pg xiii]
(312) C. Creighton. “On the development of the Mamma and the Mammary
function.” Jour. of Anat. and Phys., Vol. XI. 1877.
(313) C. Gegenbaur. “Bemerkungen üb. d. Milchdrüsen-Papillen d.
Säugethiere.” Jenaische Zeit., Vol. VII. 1873.
(314) M. Huss. “Beitr. z. Entwick. d. Milchdrüsen b. Menschen u. b.
Wiederkäuern.” Jenaische Zeit., Vol. VII. 1873.
(315) C. Langer. “Ueber d. Bau u. d. Entwicklung d. Milchdrüsen.”
Denk. d. k. Akad. Wiss. Wien, Vol. III. 1851.
The Nervous System.
Evolution of the Nervous System.
(316) F. M. Balfour. “Address to the Department of Anat. and Physiol.
of the British Association.” 1880.
(317) C. Claus. “Studien üb. Polypen u. Quallen d. Adria. 1.
Acalephen, Discomedusen,” Denk. d. math.-naturwiss. Classe d. k.
Akad. Wiss. Wien, Vol. XXXVIII. 1877.
(318) Th. Eimer. Zoologische Studien a. Capri. 1. Ueber Beroë ovatus.
Ein Beitrag z. Anat. d. Rippenquallen. Leipzig, 1873.
(319) V. Hensen. “Zur Entwicklung d. Nervensystems.” Virchow’s
Archiv, Vol. XXX. 1864.
(320) O. and R. Hertwig. Das Nervensystem u. d. Sinnesorgane d.
Medusen. Leipzig, 1878.
(321) O. and R. Hertwig. “Die Actinien anat. u. histol. mit besond.
Berücksichtigung d. Nervenmuskelsystem untersucht.” Jenaische Zeit.,
Vol. XIII. 1879.
(322) R. Hertwig. “Ueb. d. Bau d. Ctenophoren.” Jenaische
Zeitschrift, Vol. XIV. 1880.
(323) A. W. Hubrecht. “The Peripheral Nervous System in Palæo- and
Schizonemertini, one of the layers of the body-wall.” Quart. J. of
Micr. Science, Vol. XX. 1880.
(324) N. Kleinenberg. Hydra, eine
anatomisch-entwicklungsgeschichtliche Untersuchung. Leipzig, 1872.
(325) A. Kowalevsky. “Embryologische Studien an Würmern u.
Arthropoden.” Mém. Acad. Pétersbourg, Series VII., Vol. XVI. 1871.
(326) E. A. Schäfer. “Observations on the nervous system of Aurelia
aurita.” Phil. Trans. 1878.
Nervous System of the Invertebrata.
(327) F. M. Balfour. “Notes on the development of the Araneina.”
Quart. J. of Micr. Science, Vol. XX. 1880.
(328) B. Hatschek. “Beitr. z. Entwicklung d. Lepidopteren.” Jenaische
Zeitschrift, Vol. XI. 1877.
(329) N. Kleinenberg. “The development of the Earthworm, Lumbricus
Trapezoides.” Quart. J. of Micr. Science, Vol. XIX. 1879.
(330) A. Kowalevsky. “Embryologische Studien an Würmern u.
Arthropoden.” Mém. Acad. Pétersbourg, Series VIII., Vol. XVI. 1871.
(331) H. Reichenbach. “Die Embryonalanlage u. erste Entwick. d.
Flusskrebses.” Zeit. f. wiss. Zool., Vol. XXIX. 1877.
Central Nervous System of the Vertebrata.
(332) C. J. Carus. Versuch einer Darstellung d. Nervensystems, etc.
Leipzig, 1814.
(333) J. L. Clark. “Researches on the development of the spinal cord
in Man, Mammalia and Birds.” Phil. Trans., 1862.
[Pg xiv]
(334) E. Dursy. “Beiträge zur Entwicklungsgeschichte des
Hirnanhanges.” Centralblatt f. d. med. Wissenschaften, 1868. Nr. 8.
(335) E. Dursy. Zur Entwicklungsgeschichte des Kopfes des Menschen
und der höheren Wirbelthiere. Tübingen, 1869.
(336) A. Ecker. “Zur Entwicklungsgeschichte der Furchen und Windungen
der Grosshirn-Hemisphären im Foetus des Menschen.” Archiv f.
Anthropologie, v. Ecker und Lindenschmidt. Vol. III. 1868.
(337) E. Ehlers. “Die Epiphyse am Gehirn d. Plagiostomen.” Zeit. f.
wiss. Zool. Vol. XXX., suppl. 1878.
(338) P. Flechsig. Die Leitungsbahnen im Gehirn und Rückenmark des
Menschen. Auf Grund entwicklungsgeschichtlicher Untersuchungen.
Leipzig, 1876.
(339) V. Hensen. “Zur Entwicklung des Nervensystems.” Virchow’s
Archiv, Bd. XXX. 1864.
(340) L. Löwe. “Beiträge z. Anat. u. z. Entwick. d. Nervensystems d.
Säugethiere u. d. Menschen.” Berlin, 1880.
(341) L. Löwe. “Beiträge z. vergleich. Morphogenesis d. centralen
Nervensystems d. Wirbelthiere.” Mittheil. a. d. embryol. Instit.
Wien, Vol. II. 1880.
(342) A. M. Marshall. “The Morphology of the Vertebrate Olfactory
organ.” Quart. J. of Micr. Science, Vol. XIX. 1879.
(343) V. v. Mihalkovics. Entwicklungsgeschichte d. Gehirns. Leipzig,
1877.
(344) W. Müller. “Ueber Entwicklung und Bau der Hypophysis und des
Processus infundibuli cerebri.” Jenaische Zeitschrift. Bd. VI. 1871.
(345) H. Rahl-Rückhard. “Die gegenseitigen Verhältnisse d. Chorda,
Hypophysis etc. bei Haifischembryonen, nebst Bemerkungen üb. d.
Deutung d. einzelnen Theile d. Fischgehirns.” Morphol. Jahrbuch,
Vol. VI. 1880.
(346) H. Rathke. “Ueber die Entstehung der glandula pituitaria.”
Müller’s Archiv f. Anat. und Physiol., Bd. V. 1838.
(347) C. B. Reichert. Der Bau des menschlichen Gehirns. Leipzig,
1859 u. 1861.
(348) F. Schmidt. “Beiträge zur Entwicklungsgeschichte des Gehirns.”
Zeitschrift f. wiss. Zoologie, 1862. Bd. XI.
(349) G. Schwalbe. “Beitrag z. Entwick. d. Zwischenhirns.” Sitz. d.
Jenaischen Gesell. f. Med. u. Naturwiss. Jan. 23, 1880.
(350) Fried. Tiedemann. Anatomie und Bildungsgeschichte des Gehirns
im Foetus des Menschen. Nürnberg, 1816.
Peripheral Nervous System of the Vertebrata.
(351) F. M. Balfour. “On the development of the spinal nerves in
Elasmobranch Fishes.” Philosophical Transactions, Vol. CLXVI. 1876;
vide also, A monograph on the development of Elasmobranch Fishes.
London, 1878, pp. 191-216.
(352) W. His. “Ueb. d. Anfänge d. peripherischen Nervensystems.”
Archiv f. Anat. u. Physiol., 1879.
(353) A. M. Marshall. “On the early stages of development of the
nerves in Birds.” Journal of Anat. and Phys., Vol. XI. 1877.
(354) A. M. Marshall. “The development of the cranial nerves in the
Chick.” Quart. J. of Micr. Science, Vol. XVIII. 1878.
(355) A. M. Marshall. “The morphology of the vertebrate olfactory
organ.” Quart. J. of Micr. Science, Vol. XIX. 1879.
(356) A. M. Marshall. “On the head-cavities and associated nerves in
Elasmobranchs.” Quart. J. of Micr. Science, Vol. XXI. 1881.
(357) C. Schwalbe. “Das Ganglion oculomotorii.” Jenaische
Zeitschrift, Vol. XIII. 1879.
Sympathetic Nervous System.
(360) F. M. Balfour. Monograph on the development of Elasmobranch
Fishes. London, 1878, p. 173.
(361) S. L. Schenk and W. R. Birdsell. “Ueb. d. Lehre von d.
Entwicklung d. Ganglien d. Sympatheticus.” Mittheil. a. d.
embryologischen Instit. Wien. Heft III. 1879.
The Eye.[Pg xv]
Eye of the Mollusca.
(362) N. Bobretzky. “Observations on the development of the
Cephalopoda” (Russian). Nachrichten d. kaiserlichen Gesell. d.
Freunde der Naturwiss. Anthropolog. Ethnogr. bei d. Universität
Moskau.
(363) H. Grenacher. “Zur Entwicklungsgeschichte d. Cephalopoden.”
Zeit. f. wiss. Zool., Bd. XXIV. 1874.
(364) V. Hensen. “Ueber d. Auge einiger Cephalopoden.” Zeit. f. wiss.
Zool., Vol. XV. 1865.
(365) E. R. Lankester. “Observations on the development of the
Cephalopoda.” Quart. J. of Micr. Science, Vol. XV. 1875.
(366) C. Semper. Ueber Sehorgane von Typus d. Wirbelthieraugen.
Wiesbaden, 1877.
Eye of the Arthropoda.
(367) N. Bobretzky. Development of Astacus and Palaemon. Kiew, 1873.
(368) A. Dohrn. “Untersuchungen üb. Bau u. Entwicklung d. Arthropoden.
Palinurus und Scyllarus.” Zeit. f. wiss. Zool., Bd. XX. 1870, p. 264
et seq.
(369) E. Claparède. “Morphologie d. zusammengesetzten Auges bei den
Arthropoden.” Zeit. f. wiss. Zool., Bd. X. 1860.
(370) H. Grenacher. Untersuchungen üb. d. Sehorgane d. Arthropoden.
Göttingen, 1879.
Vertebrate Eye.
(371) J. Arnold. Beiträge zur Entwicklungsgeschichte des Auges.
Heidelberg, 1874.
(372) Babuchin. “Beiträge zur Entwicklungsgeschichte des Auges.”
Würzburger naturwissenschaftliche Zeitschrift, Bd. 8.
(373) L. Kessler. Zur Entwicklung d. Auges d. Wirbelthiere. Leipzig,
1877.
(374) N. Lieberkühn. Ueber das Auge des Wirbelthierembryo. Cassel,
1872.
(375) N. Lieberkühn. “Beiträge z. Anat. d. embryonalen Auges.” Archiv
f. Anat. und Phys., 1879.
(376) L. Löwe. “Beiträge zur Anatomie des Auges” and “Die Histogenese
der Retina.” Archiv f. mikr. Anat., Vol. XV. 1878.
(377) V. Mihalkowics. “Untersuchungen über den Kamm des Vogelauges.”
Archiv f. mikr. Anat., Vol. IX. 1873.
(378) W. Müller. “Ueber die Stammesentwickelung des Schorgans der
Wirbelthiere.” Festgabe Carl Ludwig. Leipzig, 1874.
(379) S. L. Schenk. “Zur Entwickelungsgeschichte des Auges der
Fische.” Wiener Sitzungsberichte, Bd. LV. 1867.
Accessory organs of the Vertebrate Eye.
(380) G. Born. “Die Nasenhöhlen u. d. Thränennasengang d. Amphibien.”
Morphologisches Jahrbuch, Bd. II. 1876.
(381) G. Born. “Die Nasenhöhlen u. d. Thränennasengang d. amnioten
Wirbelthiere. I. Lacertilia. II. Aves.” Morphologisches Jahrbuch,
Bd. V. 1879.
Eye of the Tunicata.
(382) A. Kowalevsky. “Weitere Studien üb. d. Entwicklung d. einfachen
Ascidien.” Archiv f. mikr. Anat., Vol. VII. 1871.
(383) C. Kupffer. “Zur Entwicklung d. einfachen Ascidien.” Archiv f.
mikr. Anat., Vol. VII. 1872.
Auditory Organs.[Pg xvi]
Auditory organs of the Invertebrata.
(384) V. Hensen. “Studien üb. d. Gehörorgan d. Decapoden.” Zeit. f.
wiss. Zool., Vol. XIII. 1863.
(385) O. and R. Hertwig. Das Nervensystem u. d. Sinnesorgane d.
Medusen. Leipzig, 1878.
Auditory organs of the Vertebrata.
(386) A. Boettcher. “Bau u. Entwicklung d. Schnecke.” Denkschriften
d. kaiserl. Leop. Carol. Akad. d. Wissenschaft., Vol. XXXV.
(387) C. Hasse. Die vergleich. Morphologie u. Histologie d. häutigen
Gehörorgane d. Wirbelthiere. Leipzig, 1873.
(388) V. Hensen. “Zur Morphologie d. Schnecke.” Zeit. f. wiss.
Zool., Vol. XIII. 1863.
(389) E. Huschke. “Ueb. d. erste Bildungsgeschichte d. Auges u. Ohres
beim bebrüteten Küchlein.” Isis von Oken, 1831, and Meckel’s
Archiv, Vol. VI.
(390) Reissner. De Auris internæ formatione. Inaug. Diss. Dorpat,
1851.
Accessory parts of Vertebrate Ear.
(391) David Hunt. “A comparative sketch of the development of the ear
and eye in the Pig.” Transactions of the International Otological
Congress, 1876.
(392) W. Moldenhauer. “Zur Entwick. d. mittleren u. äusseren Ohres.”
Morphol. Jahrbuch, Vol. III. 1877.
(393) V. Urbantschitsch. “Ueb. d. erste Anlage d. Mittelohres u. d.
Trommelfelles.” Mittheil. a. d. embryol. Instit. Wien, Heft I. 1877.
Olfactory Organ.
(394) G. Born. “Die Nasenhöhlen u. d. Thränennasengang d. amnioten
Wirbelthiere.” Parts I. and II. Morphiologisches Jahrbuch, Bd. V.,
1879.
(395) A. Kölliker. “Ueber die Jacobson’schen Organe des Menschen.”
Festschrift f. Rienecker, 1877.
(396) A. M. Marshall. “Morphology of the Vertebrate Olfactory Organ.”
Quart. Journ. of Micr. Science, Vol. XIX., 1879.
Sense-organs of the lateral line.
(397) F. M. Balfour. A Monograph on the development of Elasmobranch
Fishes, pp. 141-146. London, 1878.
(398) H. Eisig. “Die Segmentalorgane d. Capitelliden.” Mittheil. a.
d. zool. Station zu Neapel, Vol. I. 1879.
(399) A. Götte. Entwicklungsgeschichte d. Unke. Leipzig, 1875.
(400) Fr. Leydig. Lehrbuch d. Histologie des Menschen u. d. Thiere.
Hamm. 1857.
(401) Fr. Leydig. Neue Beiträge z. anat. Kenntniss d. Hautdecke u.
Hautsinnesorgane d. Fische. Halle, 1879.
(402) F. E. Schulze. “Ueb. d. Sinnesorgane d. Seitenlinie bei Fischen
und Amphibien.” Archiv f. mikr. Anat., Vol. VI. 1870.
(403) C. Semper. “Das Urogenitalsystem d. Selachier.” Arbeit. a. d.
zool.-zoot. Instit. Würzburg, Vol. II.
(404) B. Solger. “Neue Untersuchungen zur Anat. d. Seitenorgane d.
Fische.” Archiv f. mikr. Anat., Vol. XVII. and XVIII. 1879 and 1880.
Origin of the Skeleton.
(405) C. Gegenbaur. “Ueb. primäre u. secundäre Knochenbildung mit
besonderer Beziehung auf d. Lehre von dem Primordialcranium.”
Jenaische Zeitschrift, Vol. III. 1867.
[Pg xvii]
(406) O. Hertwig. “Ueber Bau u. Entwicklung d. Placoidschuppen u. d.
Zähne d. Selachier.” Jenaische Zeitschrift, Vol. VIII. 1874.
(407) O. Hertwig. “Ueb. d. Zahnsystem d. Amphibien u. seine Bedeutung
f. d. Genese d. Skelets d. Mundhöhle.” Archiv f. mikr. Anat., Vol.
XI. Supplementheft, 1874.
(408) O. Hertwig. “Ueber d. Hautskelet d. Fische.” Morphol.
Jahrbuch, Vol. II. 1876. (Siluroiden u. Acipenseriden.)
(409) O. Hertwig. “Ueber d. Hautskelet d. Fische (Lepidosteus u.
Polypterus).” Morph. Jahrbuch, Vol. V. 1879.
(410) A. Kölliker. “Allgemeine Betrachtungen üb. die Entstehung d.
knöchernen Schädels d. Wirbelthiere.” Berichte v. d. königl. zoot.
Anstalt z. Würzburg, 1849.
(411) Fr. Leydig. “Histologische Bemerkungen üb. d. Polypterus
bichir.” Zeit. f. wiss. Zool., Vol. V. 1858.
(412) H. Müller. “Ueber d. Entwick. d. Knochensubstanz nebst
Bemerkungen, etc.” Zeit. f. wiss. Zool., Vol. IX. 1859.
(413) Williamson. “On the structure and development of the Scales and
Bones of Fishes.” Phil. Trans., 1851.
(414) Vrolik. “Studien üb. d. Verknöcherung u. die Knochen d. Schädels
d. Teleostier.” Niederländisches Archiv f. Zoologie, Vol. I.
Notochord and Vertebral Column.
(415) Cartier. “Beiträge zur Entwicklungsgeschichte der Wirbelsäule.”
Zeitschrift für wiss. Zool., Bd. XXV. Suppl. 1875.
(416) C. Gegenbaur. Untersuchungen zur vergleichenden Anatomie der
Wirbelsäule der Amphibien und Reptilien. Leipzig, 1862.
(417) C. Gegenbaur. “Ueber die Entwickelung der Wirbelsäule des
Lepidosteus mit vergleichend anatomischen Bemerkungen.” Jenaische
Zeitschrift, Bd. III. 1863.
(418) C. Gegenbaur. “Ueb. d. Skeletgewebe d. Cyclostomen.” Jenaische
Zeitschrift, Vol. V. 1870.
(419) Al. Götte. “Beiträge zur vergleich. Morphol. des Skeletsystems
d. Wirbelthiere.” II. “Die Wirbelsäule u. ihre Anhänge.” Archiv f.
mikr. Anat., Vol. XV. 1878 (Cyclostomen, Ganoiden, Plagiostomen,
Chimaera), and Vol. XVI. 1879 (Teleostier).
(420) Hasse und Schwarck. “Studien zur vergleichenden Anatomie der
Wirbelsäule u. s. w.” Hasse, Anatomische Studien, 1872.
(421) C. Hasse. Das natürliche System d. Elasmobranchier auf
Grundlage d. Bau. u. d. Entwick. ihrer Wirbelsäule. Jena, 1879.
(422) A. Kölliker. “Ueber die Beziehungen der Chorda dorsalis zur
Bildung der Wirbel der Selachier und einiger anderen Fische.”
Verhandlungen der physical. medicin. Gesellschaft in Würzburg, Bd.
X.
(423) A. Kölliker. “Weitere Beobachtungen über die Wirbel der
Selachier insbesondere über die Wirbel der Lamnoidei.” Abhandlungen
der senkenbergischen naturforschenden Gesellschaft in Frankfurt, Bd.
V.
(424) H. Leboucq. “Recherches s. l. mode de disparition de la corde
dorsale chez les vertébrés supérieurs.” Archives de Biologie, Vol.
I. 1880.
(425) Fr. Leydig. Anatomisch-histologische Untersuchungen über Fische
und Reptilien. Berlin, 1853.
(426) Aug. Müller. “Beobachtungen zur vergleichenden Anatomie der
Wirbelsäule.” Müller’s Archiv. 1853.
(427) J. Müller. “Vergleichende Anatomie der Myxinoiden u. der
Cyklostomen mit durchbohrtem Gaumen, I. Osteologie und Myologie.”
Abhandlungen der königlichen Akademie der Wissenschaften zu Berlin.
1834.
(428) W. Müller. “Beobachtungen des pathologischen Instituts zu Jena,
I. Ueber den Bau der Chorda dorsalis.” Jenaische Zeitschrift, Bd.
VI. 1871.
(429) A. Schneider. Beiträge z. vergleich. Anat. u. Entwick. d.
Wirbelthiere. Berlin, 1879.
Ribs and Sternum.[Pg xviii]
(430) C. Claus. “Beiträge z. vergleich. Osteol. d. Vertebraten. I.
Rippen u. unteres Bogensystem.” Sitz. d. kaiserl. Akad. Wiss. Wien,
Vol. LXXIV. 1876.
(431) A. E. Fick. “Zur Entwicklungsgeschichte d. Rippen und
Querfortsätze.” Archiv f. Anat. und Physiol. 1879.
(432) C. Gegenbaur. “Zur Entwick. d. Wirbelsäule des Lepidosteus mit
vergleich. anat. Bemerk.” Jenaische Zeit., Vol. III. 1867.
(433) A. Götte. “Beiträge z. vergleich. Morphol. d. Skeletsystems d.
Wirbelthiere Brustbein u. Schultergürtel.” Archiv f. mikr. Anat.,
Vol. XIV. 1877.
(434) C. Hasse u. G. Born. “Bemerkungen üb. d. Morphologie d. Rippen.”
Zoologischer Anzeiger, 1879.
(435) C. K. Hoffmann. “Beiträge z. vergl. Anat. d. Wirbelthiere.”
Niederländ. Archiv Zool., Vol. IV. 1878.
(436) W. K. Parker. “A monograph on the structure and development of
the shoulder-girdle and sternum.” Ray Soc. 1867.
(437) H. Rathke. Ueb. d. Bau u. d. Entwicklung d. Brustbeins d.
Saurier. 1853.
(438) G. Ruge. “Untersuch. üb. Entwick. am Brustbeine d. Menschen.”
Morphol. Jahrbuch., Vol. VI. 1880.
The Skull.
(439) A. Dugès. “Recherches sur l'Ostéologie et la myologie des
Batraciens à leur différents âges.” Paris, Mém. savans étrang. 1835,
and An. Sci. Nat. Vol. I. 1834.
(440) C. Gegenbaur. Untersuchungen z. vergleich. Anat. d.
Wirbelthiere, III. Heft. Das Kopfskelet d. Selachier. Leipzig,
1872.
(441) Günther. Beob. üb. die Entwick. d. Gehörorgans. Leipzig, 1842.
(442) O. Hertwig. “Ueb. d. Zahnsystem d. Amphibien u. seine Bedeutung
f. d. Genese d. Skelets d. Mundhöhle.” Archiv f. mikr. Anat., Vol.
XI. 1874, suppl.
(443) T. H. Huxley. “On the theory of the vertebrate skull.” Proc.
Royal Soc., Vol. ix. 1858.
(444) T. H. Huxley. The Elements of Comparative Anatomy. London,
1869.
(445) T. H. Huxley. “On the Malleus and Incus.” Proc. Zool. Soc.,
1869.
(446) T. H. Huxley. “On Ceratodus Forsteri.” Proc. Zool. Soc., 1876.
(447) T. H. Huxley. “The nature of the craniofacial apparatus of
Petromyzon.” Journ. of Anat. and Phys., Vol. X. 1876.
(448) T. H. Huxley. The Anatomy of Vertebrated Animals. London,
1871.
(449) W. K. Parker. “On the structure and development of the skull of
the Common Fowl (Gallus Domesticus).” Phil. Trans., 1869.
(450) W. K. Parker. “On the structure and development of the skull of
the Common Frog (Rana temporaria).” Phil. Trans., 1871.
(451) W. K. Parker. “On the structure and development of the skull in
the Salmon (Salmo salar).” Bakerian Lecture, Phil. Trans., 1873.
(452) W. K. Parker. “On the structure and development of the skull in
the Pig (Sus scrofa).” Phil. Trans., 1874.
(453) W. K. Parker. “On the structure and development of the skull in
the Batrachia.” Part II. Phil. Trans., 1876.
(454) W. K. Parker. “On the structure and development of the skull in
the Urodelous Amphibia.” Part III. Phil. Trans., 1877.
(455) W. K. Parker. “On the structure and development of the skull in
the Common Snake (Tropidonotus natrix).” Phil. Trans., 1878.
(456) W. K. Parker. “On the structure and development of the skull in
Sharks and Skates.” Trans. Zoolog. Soc., 1878. Vol. X. pt. IV.
(457) W. K. Parker. “On the structure and development of the skull in
the Lacertilia.” Pt. I. Lacerta agilis, L. viridis and Zootoca
vivipara. Phil. Trans., 1879.
[Pg xix]
(458) W. K. Parker. “The development of the Green Turtle.” The
Zoology of the Voyage of H. M. S. Challenger. Vol. I. pt. V.
(459) W. K. Parker. “The structure and development of the skull in the
Batrachia.” Pt. III. Phil. Trans., 1880.
(460) W. K. Parker and G. T. Bettany. The Morphology of the Skull.
London, 1877.
(460*) H. Rathke. Entwick. d. Natter. Königsberg, 1839.
(461) C. B. Reichert. “Ueber die Visceralbogen d. Wirbelthiere.”
Müller’s Archiv, 1837.
(462) W. Salensky. “Beiträge z. Entwick. d. knorpeligen
Gehörknöchelchen.” Morphol. Jahrbuch, Vol. VI. 1880.
Vide also Kölliker (No. 298), especially for the human and mammalian
skull; Götte (No. 296).
The Pectoral Girdle.
(463) Bruch. “Ueber die Entwicklung der Clavicula und die Farbe des
Blutes.” Zeit. f. wiss. Zool., iv. 1853.
(464) A. Dugès. “Recherches sur l'ostéologie et la myologie des
Batraciens à leurs différens âges.” Mémoires des savants étrang.
Académie royale des sciences de l'institut de France, Vol. VI. 1835.
(465) C. Gegenbaur. Untersuchungen zur vergleichenden Anatomie der
Wirbelthiere, 2 Heft. Schultergürtel der Wirbelthiere. Brustflosse
der Fische. Leipzig, 1865.
(466) A. Götte. “Beiträge z. vergleich. Morphol. d. Skeletsystems d.
Wirbelthiere, Brustbein u. Schultergürtel.” Archiv f. mikr. Anat.
Vol. XIV. 1877.
(467) C. K. Hoffmann. “Beiträge z. vergleichenden Anatomie d.
Wirbelthiere.” Niederländisches Archiv f. Zool., Vol. V. 1879.
(468) W. K. Parker. “A Monograph on the Structure and Development of
the Shoulder-girdle and Sternum in the Vertebrata.” Ray Society,
1868.
(469) H. Rathke. Ueber die Entwicklung der Schildkröten.
Braunschweig, 1848.
(470) H. Rathke. Ueber den Bau und die Entwicklung des Brustbeins der
Saurier, 1853.
(471) A. Sabatier. Comparaison des ceintures et des membres
antérieurs et postérieurs d. la Série d. Vertébrés. Montpellier,
1880.
(472) Georg ’Swirski. Untersuch. üb. d. Entwick. d. Schultergürtels
u. d. Skelets d. Brustflosse d. Hechts. Inaug. Diss. Dorpat, 1880.
The Pelvic Girdle.
(473) A. Bunge. Untersuch. z. Entwick. d. Beckengürtels d. Amphibien,
Reptilien u. Vögel. Inaug. Diss. Dorpat, 1880.
(474) C. Gegenbaur. “Ueber d. Ausschluss des Schambeins von d. Pfanne
d. Hüftgelenkes.” Morph. Jahrbuch, Vol. II. 1876.
(475) Th. H. Huxley. “The characters of the Pelvis in Mammalia, etc.”
Proc. of Roy. Soc., Vol. XXVIII. 1879.
(476) A. Sabatier. Comparaison des ceintures et des membres
antérieurs et postérieurs dans la Série d. Vertébrés. Montpellier,
1880.
Skeleton of the Limbs.
(477) M. v. Davidoff. “Beiträge z. vergleich. Anat. d. hinteren
Gliedmaassen d. Fische I.” Morphol. Jahrbuch, Vol. V. 1879.
(478) C. Gegenbaur. Untersuchungen z. vergleich. Anat. d.
Wirbelthiere. Leipzig, 1864-5. Erstes Heft. Carpus u. Tarsus. Zweites
Heft. Brustflosse d. Fische.
(479) C. Gegenbaur. “Ueb. d. Skelet d. Gliedmaassen d. Wirbelthiere im
Allgemeinen u. d. Hintergliedmaassen d. Selachier insbesondere.”
Jenaische Zeitschrift, Vol. V. 1870.
[Pg xx]
(480) C. Gegenbaur. “Ueb. d. Archipterygium.” Jenaische Zeitschrift,
Vol. VII. 1873.
(481) C. Gegenbaur. “Zur Morphologie d. Gliedmaassen d. Wirbelthiere.”
Morphologisches Jahrbuch, Vol. II. 1876.
(482) A. Götte. Ueb. Entwick. u. Regeneration d. Gliedmaassenskelets
d. Molche. Leipzig, 1879.
(483) T. H. Huxley. “On Ceratodus Forsteri, with some observations on
the classification of Fishes.” Proc. Zool. Soc. 1876.
(484) St George Mivart. “On the Fins of Elasmobranchii.” Zoological
Trans., Vol. X.
(485) A. Rosenberg. “Ueb. d. Entwick. d. Extremitäten-Skelets bei
einigen d. Reduction ihrer Gliedmaassen charakterisirten
Wirbelthieren.” Zeit. f. wiss. Zool., Vol. XXIII. 1873.
(486) E. Rosenberg. “Ueb. d. Entwick. d. Wirbelsäule u. d. centrale
carpi d. Menschen.” Morphologisches Jahrbuch, Vol. I. 1875.
(487) H. Strasser. “Z. Entwick. d. Extremitätenknorpel bei Salamandern
u. Tritonen.” Morphologisches Jahrbuch, Vol. V. 1879.
(488) G. ’Swirski. Untersuch. üb. d. Entwick. d. Schultergürtels u.
d. Skelets d. Brustflosse d. Hechts. Inaug. Diss. Dorpat, 1880.
(489) J. K. Thacker. “Median and paired fins. A contribution to the
history of the Vertebrate limbs.” Trans. of the Connecticut Acad.,
Vol. III. 1877.
(490) J. K. Thacker. “Ventral fins of Ganoids.” Trans. of the
Connecticut Acad., Vol. IV. 1877.
Pleural and pericardial cavities.
(491) M. Cadiat. “Du développement de la partie céphalothoracique de
l'embryon, de la formation du diaphragme, des pleures, du péricarde,
du pharynx et de l'œsophage.” Journal de l'Anatomie et de la
Physiologie, Vol. XIV. 1878.
Vascular System.
The Heart.
(492) A. C. Bernays. “Entwicklungsgeschichte d.
Atrioventricularklappen.” Morphol. Jahrbuch, Vol. II. 1876.
(493) E. Gasser. “Ueber d. Entstehung d. Herzens beim Hühn.” Archiv
f. mikr. Anat., Vol. XIV.
(494) A. Thomson. “On the development of the vascular system of the
fœtus of Vertebrated Animals.” Edinb. New Phil. Journal, Vol. IX.
1830 and 1831.
(495) M. Tonge. “Observations on the development of the semilunar
valves of the aorta and pulmonary artery of the heart of the Chick.”
Phil. Trans. CLIX. 1869.
Vide also Von Baer (291), Rathke (300), Hensen (182), Kölliker
(298), Götte (296), and Balfour (292).
The Arterial System.
(496) H. Rathke. “Ueb. d. Entwick. d. Arterien w. bei d. Säugethiere
von d. Bogen d. Aorta ausgehen.” Müller’s Archiv, 1843.
(497) H. Rathke. “Untersuchungen üb. d. Aortenwurzeln d. Saurier.”
Denkschriften d. k. Akad. Wien, Vol. XIII. 1857.
Vide also His (No. 232) and general works on Vertebrate Embryology.
The Venous System.
(498) J. Marshall. “On the development of the great anterior veins.”
Phil. Trans., 1859.
[Pg xxi]
(499) H. Rathke. “Ueb. d. Bildung d. Pfortader u. d. Lebervenen b.
Säugethieren.” Meckel’s Archiv, 1830.
(500) H. Rathke. “Ueb. d. Bau u. d. Entwick. d. Venensystems d.
Wirbelthiere.” Bericht. üb. d. naturh. Seminar. d. Univ. Königsberg,
1838.
Vide also Von Baer (No. 291), Götte (No. 296), Kölliker (No. 298),
and Rathke (Nos. 299, 300, and 301).
The Spleen.
(501) W. Müller. “The Spleen.” Stricker’s Histology.
(502) Peremeschko. “Ueb. d. Entwick. d. Milz.” Sitz. d. Wien. Akad.
Wiss., Vol. LVI. 1867.
The Suprarenal bodies.
(503) M. Braun. “Bau u. Entwick. d. Nebennieren bei Reptilien.”
Arbeit. a. d. zool.-zoot. Institut Würzburg, Vol. V. 1879.
(504) A. v. Brunn. “Ein Beitrag z. Kenntniss d. feinern Baues u. d.
Entwick. d. Nebennieren.” Archiv f. mikr. Anat., Vol. VIII. 1872.
(505) Fr. Leydig. Untersuch. üb. Fische u. Reptilien. Berlin, 1853.
(506) Fr. Leydig. Rochen u. Haie. Leipzig, 1852.
Vide also F. M. Balfour (No. 292), Kölliker (No. 298), Remak (No.
302), etc.
The Muscular System of the Vertebrata.
(507) G. M. Humphry. “Muscles in Vertebrate Animals.” Journ. of Anat.
and Phys., Vol. VI. 1872.
(508) J. Müller. “Vergleichende Anatomie d. Myxinoiden. Part I.
Osteologie u. Myologie.” Akad. Wiss., Berlin, 1834.
(509) A. M. Marshall. “On the head cavities and associated nerves of
Elasmobranchs.” Quart. J. of Micr. Science, Vol. XXI. 1881.
(510) A. Schneider. “Anat. u. Entwick. d. Muskelsystems d.
Wirbelthiere.” Sitz. d. Oberhessischen Gesellschaft, 1873.
(511) A. Schneider. Beiträge z. vergleich. Anat. u. Entwick. d.
Wirbelthiere. Berlin, 1879.
Vide also Götte (No. 296), Kölliker (No. 298), Balfour (No. 292),
Huxley, etc.
Excretory Organs.
invertebrata.
(512) H. Eisig. “Die Segmentalorgane d. Capitelliden.” Mitth. a. d.
zool. Stat. z. Neapel, Vol. I. 1879.
(513) J. Fraipont. “Recherches s. l'appareil excréteur des Trematodes
et d. Cestoïdes.” Archives de Biologie, Vol. I. 1880.
(514) B. Hatschek. “Studien üb. Entwick. d. Anneliden.” Arbeit. a. d.
zool. Instit. Wien, Vol. I. 1878.
(515) B. Hatschek. “Ueber Entwick. von Echiurus,” etc. Arbeit. a. d.
zool. Instit. Wien, Vol. III. 1880.
vertebrata.
General.
(516) F. M. Balfour. “On the origin and history of the urinogenital
organs of Vertebrates.” Journal of Anat. and Phys., Vol. X. 1876.
[Pg xxii]
(517) Max. Fürbringer[281].
“Zur vergleichenden Anat. u. Entwick. d.
Excretionsorgane d. Vertebraten.” Morphol. Jahrbuch, Vol. IV. 1878.
(518) H. Meckel. Zur Morphol. d. Harn-u. Geschlechtswerkz. d.
Wirbelthiere, etc. Halle, 1848.
(519) Joh. Müller. Bildungsgeschichte d. Genitalien, etc.
Düsseldorf, 1830.
(520) H. Rathke. “Beobachtungen u. Betrachtungen ü. d. Entwicklung d.
Geschlechtswerkzeuge bei den Wirbelthieren.” N. Schriften d. naturf.
Gesell. in Dantzig, Bd. I. 1825.
(521) C. Semper[281].
“Das Urogenitalsystem d. Plagiostomen u. seine
Bedeutung f. d. übrigen Wirbelthiere.” Arb. a. d. zool.-zoot.
Instit. Würzburg, Vol. II. 1875.
(522) W. Waldeyer[281].
Eierstock u. Ei. Leipzig, 1870.
Elasmobranchii.
(523) A. Schultz. “Zur Entwick. d. Selachiereies.” Archiv f. mikr.
Anat., Vol. XI. 1875.
Vide also Semper (No. 521) and Balfour (No. 292).
Cyclostomata.
(524) J. Müller. “Untersuchungen ü. d. Eingeweide d. Fische.” Abh. d.
k. Ak. Wiss. Berlin, 1845.
(525) W. Müller. “Ueber d. Persistenz d. Urniere b. Myxine glutinosa.”
Jenaische Zeitschrift, Vol. VII. 1873.
(526) W. Müller. “Ueber d. Urogenitalsystem d. Amphioxus u. d.
Cyclostomen.” Jenaische Zeitschrift, Vol. IX. 1875.
(527) A. Schneider. Beiträge z. vergleich. Anat. u. Entwick. d.
Wirbelthiere. Berlin, 1879.
(528) W. B. Scott. “Beiträge z. Entwick. d. Petromyzonten.” Morphol.
Jahrbuch, Vol. VII. 1881.
Teleostei.
(529) J. Hyrtl. “Das uropoetische System d. Knochenfische.” Denkschr.
d. k. k. Akad. Wiss. Wien, Vol. II. 1850.
(530) A. Rosenberg. Untersuchungen üb. die Entwicklung d.
Teleostierniere. Dorpat, 1867.
Vide also Oellacher (No. 72).
Amphibia.
(531) F. H. Bidder. Vergleichend-anatomische u. histologische
Untersuchungen ü. die männlichen Geschlechts- und Harnwerkzeuge d.
nackten Amphibien. Dorpat, 1846.
(532) C. L. Duvernoy. “Fragments s. les Organes genito-urinaires des
Reptiles,” etc. Mém. Acad. Sciences. Paris. Vol. XI. 1851, pp.
17-95.
(533) M. Fürbringer. Zur Entwicklung d. Amphibienniere. Heidelberg,
1877.
(534) F. Leydig. Anatomie d. Amphibien u. Reptilien. Berlin, 1853.
(535) F. Leydig. Lehrbuch d. Histologie. Hamm, 1857.
(536) F. Meyer. “Anat. d. Urogenitalsystems d. Selachier u.
Amphibien.” Sitz. d. naturfor. Gesellsch. Leipzig, 1875.
(537) J. W. Spengel. “Das Urogenitalsystem d. Amphibien.” Arb. a. d.
zool.-zoot. Instit. Würzburg. Vol. III. 1876.
(538) Von Wittich. “Harn- u. Geschlechtswerkzeuge d. Amphibien.”
Zeit. f. wiss. Zool., Vol. IV.
Vide also Götte (No. 296).
Amniota.[Pg xxiii]
(539) F. M. Balfour and A. Sedgwick. “On the existence of a
head-kidney in the embryo Chick,” etc. Quart. J. of Micr. Science,
Vol. XIX. 1878.
(540) Banks. On the Wolffian bodies of the fœtus and their remains
in the adult. Edinburgh, 1864.
(541) Th. Bornhaupt. Untersuchungen üb. die Entwicklung d.
Urogenitalsystems beim Hühnchen. Inaug. Diss. Riga, 1867.
(542) Max Braun. “Das Urogenitalsystem d. einheimischen Reptilien.”
Arbeiten a. d. zool.-zoot. Instit. Würzburg. Vol. IV. 1877.
(543) J. Dansky u. J. Kostenitsch. “Ueb. d. Entwick. d. Keimblätter u.
d. Wolff’schen Ganges im Hühnerei.” Mém. Acad. Imp. Pétersbourg,
VII. Series, Vol. XXVII. 1880.
(544) Th. Egli. Beiträge zur Anat. und Entwick. d.
Geschlechtsorgane. Inaug. Diss. Zürich, 1876.
(545) E. Gasser. Beiträge zur Entwicklungsgeschichte d. Allantois,
der Müller’schen Gänge u. des Afters. Frankfurt, 1874.
(546) E. Gasser. “Beob. üb. d. Entstehung d. Wolff’schen Ganges bei
Embryonen von Hühnern u. Gänsen.” Arch. für mikr. Anat., Vol. XIV.
1877.
(547) E. Gasser. “Beiträge z. Entwicklung d. Urogenitalsystems d.
Hühnerembryonen.” Sitz. d. Gesell. zur Beförderung d. gesam.
Naturwiss. Marburg, 1879.
(548) C. Kupffer. “Untersuchung über die Entwicklung des Harn- und
Geschlechtssystems.” Archiv für mikr. Anat., Vol. II. 1866.
(549) A. Sedgwick. “Development of the kidney in its relation to the
Wolffian body in the Chick.” Quart. J. of Micros. Science, Vol. XX.
1880.
(550) A. Sedgwick. “On the development of the structure known as the
glomerulus of the head-kidney in the Chick.” Quart. J. of Micros.
Science, Vol. XX. 1880.
(551) A. Sedgwick. “Early development of the Wolffian duct and
anterior Wolffian tubules in the Chick; with some remarks on the
vertebrate excretory system.” Quart. J. of Micros. Science, Vol.
XXI. 1881.
(552) M. Watson. “The homology of the sexual organs, illustrated by
comparative anatomy and pathology.” Journal of Anat. and Phys., Vol.
XIV. 1879.
(553) E. H. Weber. Zusätze z. Lehre von Baue u. d. Verrichtungen d.
Geschlechtsorgane. Leipzig, 1846.
Vide also Remak (No. 302), Foster and Balfour (No. 295), His (No.
297), Kölliker (No. 298).
Generative Organs.
(554) G. Balbiani. Leçons s. la génération des Vertébrés. Paris,
1879.
(555) F. M. Balfour. “On the structure and development of the
Vertebrate ovary.” Quart. J. of Micr. Science, Vol. XVIII.
(556) E. van Beneden. “De la distinction originelle du tecticule et de
l'ovaire, etc.” Bull. Ac. roy. belgique, Vol. XXXVII. 1874.
(557) N. Kleinenberg. “Ueb. d. Entstehung d. Eier b. Eudendrium.”
Zeit. f. wiss. Zool., Vol. XXXV. 1881.
(558) H. Ludwig. “Ueb. d. Eibildung im Theirreiche.” Arbeit. a. d.
zool.-zoot. Instit. Würzburg, Vol. I. 1874.
(559) C. Semper. “Das Urogenitalsystem d. Plagiostomen, etc.” Arbeit.
a. d. zool.-zoot. Instit. Würzburg, Vol. II. 1875.
(560) A. Weismann. “Zur Frage nach dem Ursprung d. Geschlechtszellen
bei den Hydroiden.” Zool. Anzeiger, No. 55, 1880.
Vide also O. and R. Hertwig (No. 271), Kölliker (No. 298), etc.
Alimentary Canal and its Appendages.
(561) B. Afanassiew. “Ueber Bau u. Entwicklung d. Thymus d. Säugeth.”
Archiv f. mikr. Anat. Bd. XIV. 1877.
[Pg xxiv]
(562) Fr. Boll. Das Princip d. Wachsthums. Berlin, 1876.
(563) E. Gasser. “Die Entstehung d. Cloakenöffnung bei
Hühnerembryonen.” Archiv f. Anat. u. Physiol., Anat. Abth. 1880.
(564) A. Götte. Beiträge zur Entwicklungsgeschichte d. Darmkanals im
Hühnchen. 1867.
(565) W. Müller. “Ueber die Entwickelung der Schilddrüse.” Jenaische
Zeitschrift, Vol. VI. 1871.
(566) W. Müller. “Die Hypobranchialrinne d. Tunicaten.” Jenaische
Zeitschrift, Vol. VII. 1872.
(567) S. L. Schenk. “Die Bauchspeicheldrüse d. Embryo.”
Anatomischphysiologische Untersuchungen. 1872.
(568) E. Selenka. “Beitrag zur Entwicklungsgeschichte d. Luftsäcke d.
Huhns.” Zeit. f. wiss. Zool. 1866.
(569) L. Stieda. Untersuch. üb. d. Entwick. d. Glandula Thymus,
Glandula thyroidea, u. Glandula carotica. Leipzig, 1881.
(570) C. Fr. Wolff. “De formatione intestinorum.” Nov. Comment. Akad.
Petrop. 1766.
(571) H. Wölfler. Ueb. d. Entwick. u. d. Bau d. Schilddrüse. Berlin,
1880.
Vide also Kölliker (298), Götte (296), His (232 and 297), Foster and
Balfour (295),
Balfour (292), Remak (302), Schenk (303), etc.
Teeth.
(572) T. H. Huxley. “On the enamel and dentine of teeth.” Quart. J.
of Micros. Science, Vol. III. 1855.
(573) R. Owen. Odontography. London, 1840-1845.
(574) Ch. S. Tomes. Manual of dental anatomy, human and comparative.
London, 1876.
(575) Ch. S. Tomes. “On the development of teeth.” Quart. J. of
Micros. Science, Vol. XVI. 1876.
(576) W. Waldeyer. “Structure and development of teeth.” Stricker’s
Histology. 1870.
Vide also Kölliker (298), Gegenbaur (294), Hertwig (306), etc.
CAMBRIDGE: PRINTED BY C. J. CLAY, M.A. & SON, AT THE UNIVERSITY PRESS.
Transcriber’s Note:
Footnotes were renumbered sequentially and moved to the end of the
chapter in which the related anchor occurs. In the bibliography for
Chapter 23, entries (517), (521), and (522) have the same footnote
anchor, [267]; in the final bibliography, these appear as duplicate
footnote anchors [281].
Section headers in Chapter 24 were changed from small caps to italics,
for consistency with formatting in the remaining chapters.
Punctuation, hyphenation, and use of small caps were standardized;
missing letters were added. Authors names in the bibliography sections
were changed from gesperrt to bold.
The layout of several tables was adjusted to accommodate display on
ebook readers and handheld devices. An alpha jump table was added at
the beginning of the index for the convenience of users.
Changes to text:
Chapter 6 - ‘mesoblastis’ to ‘mesoblast is’
... mesoblast is divided ...
Chapter 10 - ‘vescicle’ to ‘vesicle’
... blastodermic vesicle ...
Chapter 12 - added italic markup to ‘e.g.’
Chapter 15 - changed reference from (359) to (357)
...This ganglion, as first suggested by Schwalbe (No. 357)
Chapter 16 - removed duplicate ‘in’ from
... being thrust in in front ...
Chapter 22 - ‘contrictor’ to ‘constrictor’
... musculus constrictor superficialis ...
Chapter 23 - removed duplicate ‘in’ from
... and pushed in in its middle ...
Footnote 163 - added final ‘s’ to ‘Nervensystems’
Fig. 100 - added ‘yolk’ as definition to abbreviation ‘yk.’ in caption
Fig. 191 - ‘Mc’ to ‘Me’ in reference to ventral surface
Fig. 390 - ‘Malphigian’ to ‘Malpighian’ in 2nd paragraph of caption
Fig. 416 - removed italics from ‘thyroid involution’ for consistency
Bibliography item (139) - ‘Hünchens’ to ‘Hühnchens’
Bibliography item (168) - ‘Jhiere’ to ‘Thiere’
Bibliography item (466) - ‘Brustbien’ to ‘Brustbein’
Anomalies noted, but left unchanged:
Periods after abbreviations of Mr., Dr., St., etc. were omitted.
Many illustrations are slightly out of focus in the original.
Abbreviations in the captions usually conclude with a period, which
is missing in most illustrations, and is usually missing in the text
discussing the illustration; occasionally, periods are used in the
abbreviation in the illustration, but not in the caption.
Fig. 73 - reference to ‘medullary canal’ in text is shown as ‘neural
canal’ in the figure.
Fig. 113 - the abbreviation for neural canal is identified in the
illustration as ‘m.c.’
Fig. 324 - ‘Iaa’ in caption doesn't match ‘laa’ in illustration.
Fig. 148 - abbr ‘a.’ is used for both amnion and urachus in the caption
and illustration.
There is no Figure 332 in the original.
Bibliography header is missing between Footnote 157 and reference (327).
Bibliography item (208) does not have a series or volume number
identified in the original.
There are no bibliography entries numbered (358) or (359).
End of the Project Gutenberg EBook of The Works of Francis Maitland Balfour,
Volume III (of 4), by Francis Maitland Balfour
*** END OF THIS PROJECT GUTENBERG EBOOK WORKS OF FRANCIS MAITLAND BALFOUR, VOL III ***
***** This file should be named 45019-h.htm or 45019-h.zip *****
This and all associated files of various formats will be found in:
http://www.gutenberg.org/4/5/0/1/45019/
Produced by Bryan Ness, Carol Brown, and the Online
Distributed Proofreading Team at http://www.pgdp.net (This
file was produced from images generously made available
by The Internet Archive/Canadian Libraries)
Updated editions will replace the previous one--the old editions
will be renamed.
Creating the works from public domain print editions means that no
one owns a United States copyright in these works, so the Foundation
(and you!) can copy and distribute it in the United States without
permission and without paying copyright royalties. Special rules,
set forth in the General Terms of Use part of this license, apply to
copying and distributing Project Gutenberg-tm electronic works to
protect the PROJECT GUTENBERG-tm concept and trademark. Project
Gutenberg is a registered trademark, and may not be used if you
charge for the eBooks, unless you receive specific permission. If you
do not charge anything for copies of this eBook, complying with the
rules is very easy. You may use this eBook for nearly any purpose
such as creation of derivative works, reports, performances and
research. They may be modified and printed and given away--you may do
practically ANYTHING with public domain eBooks. Redistribution is
subject to the trademark license, especially commercial
redistribution.
*** START: FULL LICENSE ***
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full Project
Gutenberg-tm License available with this file or online at
www.gutenberg.org/license.
Section 1. General Terms of Use and Redistributing Project Gutenberg-tm
electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or destroy
all copies of Project Gutenberg-tm electronic works in your possession.
If you paid a fee for obtaining a copy of or access to a Project
Gutenberg-tm electronic work and you do not agree to be bound by the
terms of this agreement, you may obtain a refund from the person or
entity to whom you paid the fee as set forth in paragraph 1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this agreement
and help preserve free future access to Project Gutenberg-tm electronic
works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the Foundation"
or PGLAF), owns a compilation copyright in the collection of Project
Gutenberg-tm electronic works. Nearly all the individual works in the
collection are in the public domain in the United States. If an
individual work is in the public domain in the United States and you are
located in the United States, we do not claim a right to prevent you from
copying, distributing, performing, displaying or creating derivative
works based on the work as long as all references to Project Gutenberg
are removed. Of course, we hope that you will support the Project
Gutenberg-tm mission of promoting free access to electronic works by
freely sharing Project Gutenberg-tm works in compliance with the terms of
this agreement for keeping the Project Gutenberg-tm name associated with
the work. You can easily comply with the terms of this agreement by
keeping this work in the same format with its attached full Project
Gutenberg-tm License when you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are in
a constant state of change. If you are outside the United States, check
the laws of your country in addition to the terms of this agreement
before downloading, copying, displaying, performing, distributing or
creating derivative works based on this work or any other Project
Gutenberg-tm work. The Foundation makes no representations concerning
the copyright status of any work in any country outside the United
States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other immediate
access to, the full Project Gutenberg-tm License must appear prominently
whenever any copy of a Project Gutenberg-tm work (any work on which the
phrase "Project Gutenberg" appears, or with which the phrase "Project
Gutenberg" is associated) is accessed, displayed, performed, viewed,
copied or distributed:
This eBook is for the use of anyone anywhere at no cost and with
almost no restrictions whatsoever. You may copy it, give it away or
re-use it under the terms of the Project Gutenberg License included
with this eBook or online at www.gutenberg.org
1.E.2. If an individual Project Gutenberg-tm electronic work is derived
from the public domain (does not contain a notice indicating that it is
posted with permission of the copyright holder), the work can be copied
and distributed to anyone in the United States without paying any fees
or charges. If you are redistributing or providing access to a work
with the phrase "Project Gutenberg" associated with or appearing on the
work, you must comply either with the requirements of paragraphs 1.E.1
through 1.E.7 or obtain permission for the use of the work and the
Project Gutenberg-tm trademark as set forth in paragraphs 1.E.8 or
1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any additional
terms imposed by the copyright holder. Additional terms will be linked
to the Project Gutenberg-tm License for all works posted with the
permission of the copyright holder found at the beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including any
word processing or hypertext form. However, if you provide access to or
distribute copies of a Project Gutenberg-tm work in a format other than
"Plain Vanilla ASCII" or other format used in the official version
posted on the official Project Gutenberg-tm web site (www.gutenberg.org),
you must, at no additional cost, fee or expense to the user, provide a
copy, a means of exporting a copy, or a means of obtaining a copy upon
request, of the work in its original "Plain Vanilla ASCII" or other
form. Any alternate format must include the full Project Gutenberg-tm
License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works provided
that
- You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is
owed to the owner of the Project Gutenberg-tm trademark, but he
has agreed to donate royalties under this paragraph to the
Project Gutenberg Literary Archive Foundation. Royalty payments
must be paid within 60 days following each date on which you
prepare (or are legally required to prepare) your periodic tax
returns. Royalty payments should be clearly marked as such and
sent to the Project Gutenberg Literary Archive Foundation at the
address specified in Section 4, "Information about donations to
the Project Gutenberg Literary Archive Foundation."
- You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or
destroy all copies of the works possessed in a physical medium
and discontinue all use of and all access to other copies of
Project Gutenberg-tm works.
- You provide, in accordance with paragraph 1.F.3, a full refund of any
money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days
of receipt of the work.
- You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project Gutenberg-tm
electronic work or group of works on different terms than are set
forth in this agreement, you must obtain permission in writing from
both the Project Gutenberg Literary Archive Foundation and Michael
Hart, the owner of the Project Gutenberg-tm trademark. Contact the
Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
public domain works in creating the Project Gutenberg-tm
collection. Despite these efforts, Project Gutenberg-tm electronic
works, and the medium on which they may be stored, may contain
"Defects," such as, but not limited to, incomplete, inaccurate or
corrupt data, transcription errors, a copyright or other intellectual
property infringement, a defective or damaged disk or other medium, a
computer virus, or computer codes that damage or cannot be read by
your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH 1.F.3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium with
your written explanation. The person or entity that provided you with
the defective work may elect to provide a replacement copy in lieu of a
refund. If you received the work electronically, the person or entity
providing it to you may choose to give you a second opportunity to
receive the work electronically in lieu of a refund. If the second copy
is also defective, you may demand a refund in writing without further
opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS', WITH NO OTHER
WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO
WARRANTIES OF MERCHANTABILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of damages.
If any disclaimer or limitation set forth in this agreement violates the
law of the state applicable to this agreement, the agreement shall be
interpreted to make the maximum disclaimer or limitation permitted by
the applicable state law. The invalidity or unenforceability of any
provision of this agreement shall not void the remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in accordance
with this agreement, and any volunteers associated with the production,
promotion and distribution of Project Gutenberg-tm electronic works,
harmless from all liability, costs and expenses, including legal fees,
that arise directly or indirectly from any of the following which you do
or cause to occur: (a) distribution of this or any Project Gutenberg-tm
work, (b) alteration, modification, or additions or deletions to any
Project Gutenberg-tm work, and (c) any Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of computers
including obsolete, old, middle-aged and new computers. It exists
because of the efforts of hundreds of volunteers and donations from
people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need are critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future generations.
To learn more about the Project Gutenberg Literary Archive Foundation
and how your efforts and donations can help, see Sections 3 and 4
and the Foundation information page at www.gutenberg.org
Section 3. Information about the Project Gutenberg Literary Archive
Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Contributions to the Project Gutenberg
Literary Archive Foundation are tax deductible to the full extent
permitted by U.S. federal laws and your state's laws.
The Foundation's principal office is located at 4557 Melan Dr. S.
Fairbanks, AK, 99712., but its volunteers and employees are scattered
throughout numerous locations. Its business office is located at 809
North 1500 West, Salt Lake City, UT 84116, (801) 596-1887. Email
contact links and up to date contact information can be found at the
Foundation's web site and official page at www.gutenberg.org/contact
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
gbnewby@pglaf.org
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To
SEND DONATIONS or determine the status of compliance for any
particular state visit www.gutenberg.org/donate
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including checks, online payments and credit card donations.
To donate, please visit: www.gutenberg.org/donate
Section 5. General Information About Project Gutenberg-tm electronic
works.
Professor Michael S. Hart was the originator of the Project Gutenberg-tm
concept of a library of electronic works that could be freely shared
with anyone. For forty years, he produced and distributed Project
Gutenberg-tm eBooks with only a loose network of volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as Public Domain in the U.S.
unless a copyright notice is included. Thus, we do not necessarily
keep eBooks in compliance with any particular paper edition.
Most people start at our Web site which has the main PG search facility:
www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.










































































































































































































































































 .
.







































































































































































