
The Project Gutenberg EBook of Inorganic Plant Poisons and Stimulants, by Winifred E. Brenchley This eBook is for the use of anyone anywhere in the United States and most other parts of the world at no cost and with almost no restrictions whatsoever. You may copy it, give it away or re-use it under the terms of the Project Gutenberg License included with this eBook or online at www.gutenberg.org. If you are not located in the United States, you'll have to check the laws of the country where you are located before using this ebook. Title: Inorganic Plant Poisons and Stimulants Author: Winifred E. Brenchley Release Date: January 18, 2015 [EBook #48008] Language: English Character set encoding: UTF-8 *** START OF THIS PROJECT GUTENBERG EBOOK INORGANIC PLANT POISONS, STIMULANTS *** Produced by Chris Curnow, Rosanna Murphy and the Online Distributed Proofreading Team at http://www.pgdp.net (This file was produced from images generously made available by The Internet Archive)
Transcriber’s Notes:
Minor inconsistencies in punctuation have been standardised. Spelling has been retained as it appears in the original publication except as marked like this in the text. The original text appears when hovering the cursor over the marked text. A list of amendments is at the end of the text.

CAMBRIDGE AGRICULTURAL MONOGRAPHS
INORGANIC PLANT POISONS
AND STIMULANTS
CAMBRIDGE UNIVERSITY PRESS
C. F. CLAY, Manager
London: FETTER LANE, E.C.
Edinburgh: 100 PRINCES STREET

London: H. K. LEWIS, 136 GOWER STREET, W.C.
London: WILLIAM WESLEY AND SON, 28 ESSEX STREET, STRAND
New York: G. P. PUTNAM’S SONS
Bombay and Calcutta: MACMILLAN AND CO., Ltd.
Toronto: J. M. DENT AND SONS, Ltd.
Tokyo: THE MARUZEN-KABUSHIKI-KAISHA
All rights reserved
BY
WINIFRED E. BRENCHLEY, D.Sc., F.L.S.
Fellow of University College, London
(Rothamsted Experimental Station)
Cambridge:
at the University Press
1914
Cambridge:
PRINTED BY JOHN CLAY, M.A.
AT THE UNIVERSITY PRESS
During the last century great and widespread changes have been made in agricultural practice—changes largely associated with the increase in the use of artificial fertilisers as supplements to the bulky organic manures which had hitherto been used. The value of certain chemical compounds as artificial manures is fully recognised, yet many attempts are being made to prove the value of other substances for the same purpose, with a view to increase in efficiency and decrease in cost. The interest in the matter is naturally great, and agriculturists, botanists and chemists have all approached the question from their different standpoints. In the following pages an attempt is made to correlate the work that has been done on a few inorganic substances which gave promise of proving useful in agricultural practice. Much of the evidence put forward by different workers is conflicting, and it is clear that no definite conclusions can yet be reached. Nevertheless, examination of the evidence justifies the hope that results of practical value will yet be obtained, and it is hoped that the analysis and coordination of the available data put forward in this book will aid in clearing the ground for those investigators who are following up the problem from both the academic and the practical standpoints.
Rothamsted.
October 1914.
[vi]
[x]
[1]
Ever since the physiological side of botany began to emerge from obscurity, the question of the relation between the nutrition and the growth of the plant has occupied a foremost position. All kinds of theories, both probable and improbable, have been held as to the way in which plants obtain the various components of their foods. But quite early in the history of the subject it was acknowledged that the soil was the source of the mineral constituents of the plant food, and that the roots were the organs by which they were received into the plant.
A new chapter in the history of science was begun when Liebig in 1840 first discussed the importance of inorganic or mineral substances in plant nutrition. This discussion led to a vast amount of work dealing with the problem of nutrition from many points of view, and the general result has been the sorting out of the elements into three groups, nutritive, indifferent, and toxic. Thus calcium, phosphorus, nitrogen and potassium are classed as nutritive, arsenic, copper and boron as toxic, and many others are regarded as indifferent.
Closer examination, however, shows that this division into three classes is too rigid. Now that experiments are more refined it has become evident that no such simple grouping is possible. It has been found that typical nutrient salts are toxic when they are applied singly to the plant in certain concentrations, the toxic power decreasing and the nutritive function coming into play more fully on the addition of other nutrient salts. For instance, Burlingham found that the typical nutrient magnesium sulphate in concentrations above m/8192 (m = molecular weight) is toxic to most seedlings, the degree of toxicity varying with the type of seedling and the conditions under which growth takes place. It will be shown in the following pages that even such a typical poison as boric acid may, under suitable conditions, increase plant growth just as if it were a nutrient. A review of the[2] whole subject leads one to conclude that in general both favourable and unfavourable conditions of nutrition are present side by side, and only when a balance is struck in favour of the good conditions can satisfactory growth take place. As indicated above, experiments have shown that the very substances that are essential for plant food may be, in reality, poisonous in their action, exercising a decidedly depressing or toxic influence on the plant when they are presented singly to the roots. This toxic action of food salts is decreased when they are mixed together, so that the addition of one toxic food solution to another produces a mixture which is less toxic than either of its constituents. Consequently a balanced solution can be made in which the toxic effects of the various foods for a particular plant are reduced to a minimum, enabling optimum growth to take place. Such a mixture of plant foods occurs in the soil, the composition of course varying with the soil.
While the earliest observations set forth the poisonous action of various substances upon plants, it was not long before investigators found that under certain conditions these very substances seemed to exert a beneficial rather than an injurious action. The poisons were therefore said to act as “stimulants” when they were presented to the plant in sufficiently great dilution. This stimulation was noticed with various plants and with several poisons, and a hypothesis was brought forward that attempted to reconcile the new facts with the old conceptions. Any poison, it was suggested, might act as a stimulant, if given in sufficiently small doses. It will be seen in the following pages that this is not universally true, such substances as copper, zinc, and arsenic failing to stimulate certain plants even in the most minute quantities so far tested.
Of recent years investigators in animal physiology have brought into prominence the striking effect of minute quantities of certain substances in animal nutrition, as for example iodine in the thyroid gland (see E. Baumann, 1895). This and other work has rendered it imperative to re-examine the parallel problems in plant physiology.
The words “stimulant” and “stimulation” themselves need more precise definition. As a matter of fact the “stimulation” noticed by one observer is not necessarily held to be such by another. Stimulation may express itself in various ways—the green weight and the general appearance of the fresh plant may be improved, the dry weight may be increased, the transpiration current may be hurried up, entailing increased absorption of water and food substances by the roots, assimilation processes may be encouraged. But these benefits are[3] not of necessity correlated with one another, e.g. a plant treated with a dilute solution of poison may look much healthier and weigh far more in the green state than an untreated plant, whereas the latter may prove the heavier in the dry state. To a market gardener to whom size and appearance is so important, stimulation means an improvement in his cabbages and lettuces in the green state, even though the increased weight is chiefly due to additional water absorbed under the encouragement of the stimulative agent, whereas to a scientific observer, the dry weight may give a more accurate estimate of stimulation in that it expresses more fully an increased activity in the vital functions of the plant whereby the nutritive and assimilative processes have gone on more rapidly, with a consequent increase in the deposition of tissue.
While stimulation expresses itself in the ways detailed above poisoning action also makes itself visible to the eye. Badly poisoned plants either fail to grow at all or else make very little or weak growth. Even when less badly affected the toxic action is well shown in some cases by the flaccidity of the roots, and in others by the formation of a “strangulation” near the crown of the root, which spreads to the stem, making it into a thin thread, while the leaves usually wither and die. If such plants as peas are able to make any shoot growth at all the roots show signs of a desperate attempt to put forth laterals. The primary root gets much thickened and then bursts down four sides, the tips of the laterals all trying to force their way through in a bunch, but failing to do so on coming in contact with the poison. Most curious malformations of the root arise from this strong effort of the plant to fight against adverse circumstances.
While all the inorganic substances examined in this monograph are toxic in high concentrations, some lead to increased growth in lower concentrations, while others apparently have no effect. In this sense all substances could be classed as toxins, even the nutrients. Thus the old distinction between toxin and nutrient has now lost its sharpness, but it does not lose all its significance. The old “nutrients” had certain definite characters in common, in that they were essential to plant growth, the growth being in a great degree proportional to the supply, a relatively large amount of the nutrients being not only tolerated but necessary. The substances dealt with more particularly in this book have none of these characters. Even those that cause increased growth do not appear to be essential, at any rate not in the quantities that potassium, phosphorus, nitrogen, &c., are essential,[4] while there is no evidence that growth is proportional to supply. The substances fall into two groups:
(1) Those that apparently become indifferent in high dilutions and never produce any increase in plant growth.
(2) Those that cause a small, but quite distinct, increased growth when applied in quantities sufficiently small.
The former group may be legitimately regarded as toxins; the latter present more difficulty and even now their function is not settled. It is not clear whether they stimulate the protoplasm or in some way hasten the metabolic processes in the plant, whether they help the roots in their absorbent work, or whether they are simple nutrients needed only in infinitesimal quantities. The two groups, however, cannot be sharply separated from one another. Indeed a substance may be put into one of these classes on the basis of experiments made with one plant alone and into another when a different plant is used, while it is quite conceivable that further experiments with other plants may abolish the division between the two groups altogether. It is even impossible to speak rigidly of toxicity. The addition of the inorganic food salts to solutions of a poison reduces the toxicity of the latter, so that the plant makes good growth in the presence of far more poison than it can withstand in the absence of the nutrients. This masking effect of the inorganic food salts upon the toxicity of inorganic plant poisons is paralleled by a similar action on organic toxic agents. Schreiner and Reed (1908) found that the addition of a second solute to a solution decreases the toxicity of that solution; further the plant itself may exercise a modifying influence upon the toxic agent. Water culture experiments were made upon the toxicity of certain organic compounds, with and without the addition of other inorganic salts. Arbutin, vanillin, and cumarin were definitely toxic and the toxicity decidedly fell off after the addition of sodium nitrate and calcium carbonate, especially with the weaker solutions of the toxins. Curiously enough, while weaker solutions of vanillin alone produced stimulation, the stimulating effect of this toxic agent disappeared entirely on the addition of the inorganic substances. The results showed that the addition of certain inorganic salts to solutions of toxic organic compounds was decidedly beneficial to the plant.
Another important problem has come to the front with regard to these toxic substances—How do these substances get into the plant? Are they all absorbed if they occur in the soil, or is there any discriminatory power on the part of the root? In other words, do the[5] roots perforce take in everything that is presented to their surfaces, or have they the power of making a selection, absorbing the useful and rejecting the useless and harmful?
Daubeny (1833) described experiments in which various plants, as radish, cabbage, Vicia Faba, hemp and barley were grown actually on sulphate of strontium or on soils watered with nitrate of strontium. No strontium could be detected in the ash of any of the plants save barley, and then only the merest trace was found. Daubeny concluded that the roots were able to reject strontium even when presented in the form of a solution. “Upon the whole, then, I see nothing, so far as experiments have yet gone, to invalidate the conclusion ... that the roots of plants do, to a certain extent at least, possess a power of selection, and that the earthy constituents which form the basis of their solid parts are determined as to quality by some primary law of nature, although their amount may depend upon the more or less abundant supply of the principles presented to them from without.” Some years after, in 1862, Daubeny reverted to the idea, stating “I should be inclined to infer that the spongioles of the roots have residing in them some specific power of excluding those constituents of the soil that are abnormal and, therefore, unsuitable to the plant, but that they take up those which are normal in any proportions in which they may chance to present themselves[1].” This, however, was not held to apply to such corrosive substances as copper sulphate. De Saussure had found that Polygonum Persecaria took up copper sulphate in large quantities, a circumstance which he attributed to the poisonous and corrosive quality of this substance, owing to which the texture of the cells became disorganised and the entrance of the solution into the vegetable texture took place as freely, perhaps, as if the plants had been actually severed asunder[2]. Daubeny concluded that a plant is unable to exclude poisons of a corrosive nature, as this quality of the substance destroys the vitality of the absorbing surface of the roots and thus reduces it to the condition of a simple membrane which by[6] endosmosis absorbs whatever is presented to its external surfaces, so that whenever abnormal substances are taken up by a living plant it is in consequence of some interference with the vital functions of the roots caused in the first instance by the deleterious influence of the agent employed.
In spite of the enormous amount of work that has been done on this subject of toxic action and stimulation it is yet too early to discuss the matter in any real detail. A voluminous literature has arisen around the subject, and in the present discussion some selection has been made with a view to presenting ascertained facts as succinctly as possible. No attempt has been made to notice all the papers; many have been omitted perforce; it would have been impossible to deal with the matter within reasonable length otherwise. A full and complete account would have demanded a ponderous treatise. This widespread interest on the part of investigators is fully justified, as the problems under discussion are not only of the highest possible interest to the plant physiologist, but hold out considerable promise for the practical agriculturist.
[7]
In the course of the scattered investigations on plant poisons and stimulants, various experimental methods have been brought into use, but these all fall into the two main categories of water and soil cultures, with the exception of a few sand cultures which hold a kind of intermediate position, combining certain characteristics of each of the main groups.
The conditions of plant life appertaining to soil and water cultures are totally different, so different that it is impossible to assume that a result obtained by one of the experimental methods must of necessity hold good in respect of the other method. A certain similarity does exist, and where parallel investigations have been carried out this becomes evident, but it seems to be more or less individual, the plant, the poison and the cultural conditions each playing a part in determining the matter.
This method of cultivation represents the simplest type of experiment. Its great advantage is that the investigator has absolute control over all the experimental conditions. Nutritive salts and toxic substances can be supplied in exact quantities and do not suffer loss or change by interaction with other substances which are beyond control. Any precipitates which may form in the food solution are contained within the culture vessel and are available for use if needed. The results are thus most useful as aids in interpreting the meaning of those from the field experiments, the results of the one method frequently dovetailing in with those of the other in a remarkable way. The disadvantage of the water culture method is that it is more or less unnatural, as the roots of the plants are grown in a medium quite unlike that which they meet in[8] nature, a liquid medium replacing the solid one, so that the roots have free access to every part of the substratum without meeting any opposition to their spread until the walls of the culture vessel are reached. The conditions of aeration are also different, for while the plant roots meet with gaseous air in the interstices of the soil, in water cultures they are dependent upon the air dissolved in the solution, so that respiration takes place under unusual conditions. It is possible that the poverty of the air supply can be overcome by regular aeration of the solution, resulting in decided improvement in growth, as L. M. Underwood (1913) has shown in recent work on barley in which continued aeration was carried out.
This method has the advantage over water cultures in that the environment of the plant roots is somewhat more natural, but on the other hand the work is cumbersome and costly, while the conditions of nutrition, watering, &c., are less under control than in the water cultures. Sand cultures represent an attempt to combine the advantages of both soil and water cultures, without their respective disadvantages. Generally speaking perfectly clean sand is used varying in coarseness in different tests, and this is impregnated with nutritive solutions suitable for plant growth. The sand is practically insoluble and sets up no chemical interaction with the nutritive compounds, while it provides a medium for the growth of the plant roots which approximates somewhat to a natural soil. It is probable, however, that a certain amount of adsorption or withdrawal from solution occurs, whereby a certain proportion of the food salts are affiliated, so to speak, to the sand particles and are so held that they are removed from the nutritive solution in the interspaces and are not available for plant food, the nutritive solution being thus weakened. The same remark applies to the poisons that are added, so that the concentration of the toxic substance used in the experiment does not necessarily indicate the concentration in which it is presented to the plant roots. On the other hand, undue concentration of the solution is apt to occur on account of the excessive evaporation from the surface of the sand. The sand particles are relatively so coarse in comparison with soil particles that the water is held loosely and so is easily lost by evaporation, thus concentrating the solution at the surface, a condition that does not apply in soil work. With care this disadvantage is easily overcome as it is possible to weigh the pots regularly and to make up the evaporation loss by the addition of water.
[9]
In this case the conditions of life are still more natural, as the plant roots find themselves in their normal medium of soil. But the investigator has now far less control, and bacterial and other actions come into play, while the nutrients and poisons supplied may set up interactions with the soil which it is impossible to fathom. This method is useful in the laboratory as it is more convenient for handling and gives more exact quantitative results than plot experiments. Also the pots can be protected from many of the untoward experiences that are likely to befall the crops in the open field. The conditions are somewhat more artificial, as the root systems are confined and the drainage is not natural, but on the whole the results of pot experiments are very closely allied to those obtained in the field by similar tests.
These make a direct appeal to the practical man, but of the scientific methods employed the field experiments are the least under control. The plants are grown under the most natural conditions of cultivation it is possible to obtain, and for that reason much value has been attached to such tests. Certainly, so far as the final practical application is concerned, open field experiments are the only ones which give information of the kind required. But from the scientific point of view one very great drawback exists in the lack of control that the investigator has over the conditions of experiment. The seeds, application of poison, &c., can all be regulated to a nicety, but the constitution of the soil itself and the soil conditions of moisture, temperature and aeration introduce factors which are highly variable. No one can have any idea of the composition of the soil even in a single field, as it may vary, sometimes very considerably, at every step. Further, no one knows the complicated action that may or may not occur in the soil on the addition of extraneous substances such as manures or poisons. Altogether, one is working quite in the dark as to knowledge of what is going on round the plant roots. It is impossible to attribute the results obtained to the direct action of the poison applied. While the influence may be direct, it may also happen that certain chemical and physical interactions of soil and poison occur, and that the action on the plant is secondary and not primary, so that a deleterious or beneficial result is not necessarily due to the action of the toxic or stimulating substance directly on the plant, but it may be an indirect effect induced possibly by an increase or decrease in the available plant food, or to some[10] other physiological factor. Consequently great care is needed in interpreting the results of field experiments without the due consideration of those obtained by other methods.
Many details of the sand and soil culture methods have been published by various investigators, e.g. Hiltner gives accounts of sand cultures, while the various publications issued from Rothamsted deal largely with the soil experiments. As this is the case, and as all crucial experiments have always been and must always be done in water cultures, it is only necessary to give here full details of these.
The great essential for success in water culture work is strict attention to detail. Cleanliness of apparatus and purity of reagents are absolutely indispensable, as the failure of a set of cultures can often be traced to a slight irregularity in one of these two directions. Purity of distilled water is perhaps the greatest essential of all. Plant roots are extraordinarily sensitive to the presence of small traces of deleterious matter in the distilled water, especially when they are grown in the absence of food salts. Ordinary commercial distilled water is generally useless as the steam frequently passes through tubes and chambers which get incrusted with various impurities, metallic and otherwise, of which slight traces get into the distilled water. Loew (1891) showed that water which contained slight traces of copper, lead or zinc derived from distilling apparatus exercised a toxic influence which was not evident in glass distilled water. This poisonous effect was removed by filtering through carbon dust or flowers of sulphur. Apparently only about the first 25 litres of distilled water were toxic, in the later distillate the deleterious substance was not evident.
The best water to use is that distilled in a jena glass still, the steam being passed through a jena glass condenser. For work on a large scale, however, it is impossible to get a sufficient supply of such water, while the danger of breakage is very great. Experiments at Rothamsted were made to find a metallic still that would supply pure water. While silver salts are very injurious to plant growth it was found that water that had been in contact with pure metallic silver had no harmful action. Consequently a still was constructed in which the cooling dome and the gutters were made of pure silver without any alloy, so placed that the steam impinged upon the silver dome, condensed into the silver gutter and was carried off by a glass tube into the receptacle.[11] Such water proved perfectly satisfactory so long as any necessary repairs to the still were made with pure silver, but a toxic action set in directly ordinary solder was employed. More recently a new tinned copper still has been employed with good results, but this is somewhat dangerous for general purposes, as in the event of the tin wearing off in any place, copper poisoning sets in at once. The water is always filtered through a good layer of charcoal as a final precaution against impurity.
In the Rothamsted experiments no attempt is made to carry on the cultures under sterile conditions. Bottles of 600 c.c. capacity are used, after being thoroughly cleaned by prolonged boiling (about four hours) followed by washing and rinsing. The bottles are filled with nutritive solution and the appropriate dose of poison, carefully labelled and covered with thick brown paper coats to exclude the light from the roots and to prevent the growth of unicellular green algae. The corks to fit the bottles are either used brand new or, if old, are sterilised in the autoclave to avoid any germ contamination from previous experiments. Lack of care in this respect leads to diseased conditions due to the growth of fungi and harmful bacteria. Two holes are bored in each cork, one to admit air, the other to hold the plant, and the cork is cut into two pieces through the latter hole.
The seeds of the experimental plants are “graded,” weighed so that they only vary within certain limits, e.g. barley may be ·05–·06 gm., peas ·3–·35 gm., buckwheat ·02–·03 gm. In this way a more uniform crop is obtained. Great care is needed in selecting the seeds, the purest strain possible being obtained in each case. With barley it has always proved possible to get a pure pedigree strain, originally raised from a single ear. In this way much of the difficulty due to the great individuality of the plants is overcome, though that is a factor that must always be recognised and reckoned with. The seeds are sown in damp sawdust—clean deal sawdust, sifted and mixed up with water into a nice crumbly mass—and as soon as they have germinated and the plantlets are big enough to handle they are put into the culture solutions. Barley plants are inserted in the corks with the aid of a little cotton wool (non-absorbent) to support them, care being taken to keep the seed above the level of the water, though it is below the cork. With peas it is impossible to get a satisfactory crop if the seed is below the cork, as the plant is very prone to bacterial and fungal infection in its early stages, and damp cotyledons are fatal for this reason. Consequently the mouths of the bottles are covered with stout cartridge paper, the pea root being inserted through a hole in the paper, so that the[12] root is in the liquid while the cotyledons rest on the surface. As soon as sufficient growth has been made the papers are replaced by corks, the remnants of the seeds still being kept on top in the air. Other plants are treated according to their individual needs and mode of germination (Fig. 1).
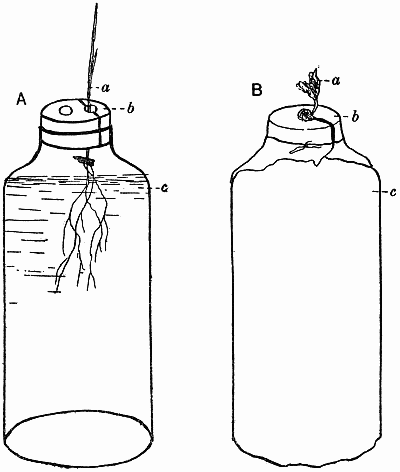
Fig. 1. Diagrammatic sketches showing methods of setting up water cultures.
| A. | a. | Seedling of cereal. |
| b. | Cork bored with two holes, and cut into two pieces through one hole. | |
| c. | Food solution. | |
| B. | a. | Pea seedling. |
| b. | Paper shield which supports the seedling. | |
| c. | Brown paper cover over bottle of food solution. |
The constitution of the nutritive solution is important, and it is becoming more and more evident that different plants have different[13] optima in this respect. For several years a solution of medium strength was used, containing the following:
| Potassium nitrate | 1·0 gram |
| Magnesium sulphate | ·5 „ |
| Sodium chloride | ·5 „ |
| Calcium sulphate | ·5 „ |
| Potassium di-hydrogen phosphate | ·5 „ |
| Ferric chloride | ·04 „ |
| Distilled water | to make up 1 litre. |
This is an excellent solution for barley plants, giving good and healthy growth. While peas grew very well in it, they showed some slight signs of over-nutrition. A weaker solution is being tested which gives very good results. Peas grow very strongly in it and it also seems to be sufficiently concentrated to allow barley to carry on its growth long enough for the purposes of experiment. The solution is as follows:
| Sodium nitrate | ·5 gram |
| Potassium nitrate | ·2 „ |
| Potassium di-hydrogen phosphate | ·1 „ |
| Calcium sulphate | ·1 „ |
| Magnesium sulphate | ·1 „ |
| Sodium chloride | ·1 „ |
| Ferric chloride | ·04 „ |
| Distilled water | to make up 1 litre. |
The latter solution was made up so that the quantity of phosphoric acid and potash approximated more or less to the amount of those substances found by analysis in an extract made from a good soil.
The experiments are usually carried on for periods varying from 4–10 weeks, six weeks being the average time. Careful notes are made during growth and eventually the plants are removed from the solutions, the roots are washed in clean water to remove adherent food salts, and then the plants are dried and weighed either separately or in sets. In order to reduce the error due to the individuality of the plants, five, ten or even twenty similar sets are grown in each experimental series, the mean dry weight being taken finally. Also the same experiment is repeated several times before any definite conclusions are drawn.
Another method of water cultures is used by some investigators, in which the experiments only last for a few hours or days, usually 24–48 hours. While such experiments may not be without value for determining the broader outlines of toxic poisoning, they fail to show the finer details. The effect of certain strengths of poison is not[14] always immediate. Too great concentrations kill the plant at once, too weak solutions fail to have any appreciable immediate action and so appear indifferent. Between the two extremes there exists a range of concentrations of which the effect varies with the plant’s growth. A solution may be of such a nature and strength that at first growth is seriously checked, though later on some recovery may be made, while it is also possible that a concentration which is apparently indifferent at first may prove more or less toxic or stimulant at a later date, according to circumstances. Consequently too much stress must not be laid upon the results of the short time experiments with regard to the ultimate effect of a poison upon a particular plant.
An examination of the various experimental methods shows that while no one of them is ideal, yet each of them has a definite contribution to make to the investigation of toxic and stimulant substances. Each method aids in the elucidation of the problem from a different standpoint, and the combination of the results obtained gives one a clearer picture of the truth than could be obtained by one method alone. Water cultures, with their exactitude of quantitative control lead on by way of sand cultures to pot cultures, and these to field experiments in which the control is largely lost, but in which the practical application is brought to the front.
[15]
Copper has been recognised as a normal constituent of certain plants for at least a century, so much so that in 1816 Meissner brought out a paper dealing solely with the copper content of various plant ashes. The ash of Cardamomum minus, of the root of Curcuma longa, and of “Paradieskörner[3],” amongst others, were tested and all yielded copper in very small quantity. Meissner was led to conclude that copper is widespread in the vegetable kingdom, but that it exists in such minute traces that its determination in plants is exceedingly difficult. In 1821 Phillips made an interesting observation as to the effect of copper on vegetation. Some oxide of copper was accidentally put near the roots of a young poplar, and soon after the plant began to fail. The lower branches died off first, but the harm gradually spread to the topmost leaves. As a proof that copper had been absorbed by the plant the record tells that the blade of a knife with which a branch was severed was covered with a film of copper where it had been through the branch, and the death of the plant was attributed to the absorbed copper.
After this preliminary breaking of the ground little more seems to have been done for some sixty years, but from about 1880 till the present day the association of copper with the vegetable kingdom has been actively investigated in its many aspects. Dieulafait (1880) showed that the quantity of copper present in the vegetation is largely determined by the nature of the soil, which thus affects the ease with which the element can be detected and estimated. Copper was shown to exist in all plants which grow on soils of “primary origin” (“roches de la formation primordiale”), the proportion being sufficient to enable[16] it to be recognised with certainty in one gram of ash, even by means of the ammonia reaction. Samples of white oak from the clay soils, and plants from the dolomitic horizons also gave evidence of copper in one gram of ash, though less was present than in the first case considered, but with plants grown on relatively pure chalk 100 grams of ash had to be examined before copper could be recognised with certainty.
E. O. von Lippman found traces of copper in beets, beet leaves, and beet products; Passerini estimated as much as ·082% copper in the stem of chickpea plants, though he regarded this figure as too high; Hattensaur determined ·266% CuO in the total ash of Molinia cærulea (·006% of total plant, air-dried).
After this Lehmann (1895, 1896) carried out more exhaustive studies on the subject of detecting and estimating the copper in various articles of food: wheat, rye, barley, oats, maize, buckwheat, and also in various makes of bread; potatoes, beans, linseed, salads, apricots and pears; cocoa and chocolate. He found that only in those plants which are grown on soil rich in copper does the copper reach any considerable value, a value which lies far above the quantity present in an ordinary soil. Plants from the former soils contained as much as 83–560 mg. Cu in 1 kilog. dry substance, whereas ordinarily the plants only contained from a trace to 20 mg. Apparently the species of the plants concerned seems to be of less importance for their copper content than is the copper content of the soil. The deposition of copper (in wheat, buckwheat and paprika) is chiefly in the stems and leaves, little being conveyed to the fruits and seeds, so that a high content of copper in the soil does not necessarily imply the presence of much copper in the grain and seed. The metal is variously distributed among the tissues, the bark of the wood being the richest of the aerial parts in that substance. The form in which the copper exists in the plant is uncertain and it is suggested that an albuminous copper compound possibly exists.
Vedrödi (1893) tackled the problem at about the same time as Lehmann but from a rather different standpoint. He ratifies the statement as to the absorption of copper by plants, and going still further he states that in some cases the percentage of copper found in the seed may be four times as great as that occurring in the soil on which the plants grow, quoting one instance in which the soil contained ·051% CuO and the seed ·26% CuO. It is assumed that copper must play some physiological rôle in the plant, but no explanation of this action is yet forthcoming. Lehmann criticised Vedrödi’s figures of the copper content of certain plant ashes, and the latter replied in a further[17] paper (1896) in which he brings most interesting facts to light. The quantity of copper in any species of plant varies with the individuals of that species, even when grown on the same soil, in the same year, and under similar conditions. The copper content of certain plants is put forward as a table, the years 1894 and 1895 being compared, and enormous differences are to be noticed in some cases. A quotation of the table will illustrate this more clearly than any amount of explanation.
| 1894 | 1895 | |||
| / | \ | / | \ | |
| “Seeds” | min. | max. | min. | max. |
| Winter wheat | 80 | 710 | 200 | 680 |
| Summer wheat | 190 | 630 | 190 | 230 |
| Maize | 60 | 90 | 10 | 30 |
| Barley | 80 | 120 | 10 | 70 |
| Oats | 40 | 190 | 40 | 200 |
| Buckwheat | 160 | 640 | 150 | 160 |
| “Fisolen” (Beans) | 160 | 320 | 110 | 150 |
| Linseed | 120 | 150 | 110 | 150 |
| Peas | 60 | 100 | 60 | 110 |
| Soy Beans | 70 | 100 | 70 | 80 |
| Lupins | 80 | 190 | 70 | 290 |
| Mustard seed | 70 | 130 | 60 | 70 |
| Paprika pods | 790 | 1350 | 230 | 400 |
The method of water cultures has been largely applied to determine the relation of copper compounds to plants. Twenty years ago (1893) Otto discovered the extreme sensitiveness of plants to this poison when grown under such conditions, as he found that growth was very soon checked in ordinary distilled water which on analysis proved to contain minute traces of copper. Controls grown in tap water gave far better plants, but this superiority was attributed partly to the minute traces of mineral salts in the tap water, and not only to the absence of the copper which occurred in the distilled water.
Tests made at Rothamsted have carried this point still further. Pisum sativum, Phaseolus vulgaris, Triticum vulgare, Zea japonica, Tropeolum Lobbianum, sweet pea (American Queen), nasturtium, and[18] cow pea—the first three of these being the species used by Otto—were grown in (1) ordinary distilled water, which was found to contain traces of copper, (2) glass distilled water, for about a month, till no more growth was possible owing to the lack of nutriment. In every single case the root growth was checked in some degree in the ordinary distilled water, the roots seeming to the eye to be less healthy and less well developed. In Pisum, Tropeolum and Zea, the shoot growth of the coppered plants appeared stronger than that of the controls, and this was borne out when the dry weights of the plants were obtained. In every other case the coppered plants were inferior, root and shoot, to those grown in the pure water. With the first three plants it appears that while the toxic water has a bad effect on the roots, yet the growth of the shoots is increased. The idea suggests itself that this apparent stimulation is in reality the result of a desperate struggle against adverse circumstances. The roots are the first to respond to the action of the poison, as they are in actual contact; their growth is checked, and hence the water absorption is decreased. No food is available in the water supply from the roots, so the plant is entirely dependent on the stores laid up in the seed and on the carbon it can derive from the air by photo-synthesis carried on by the green leaves. The result of the root checking in these particular cases seems to be so to stimulate the shoots by some physiological action or other, that this process of photo-synthesis is hastened, more carbon being converted into carbo-hydrates, so that the shoot development is increased, yielding a greater weight of dry matter. In each of the other cases observed the shoot was obviously not stimulated to increased energy by the poison, and so the whole plant fell below the normal.
Other experiments showed that barley roots are peculiarly sensitive to the presence of minute traces of copper, as very little root growth took place in the copper distilled water, and root growth was also entirely checked by the presence of one part per million copper sulphate in the pure glass distilled water. Yet again, one litre of pure distilled water was allowed to stand on a small piece of pure metallic copper foil (about 11⁄2″ × 1⁄2″) for an hour, and even such water exercised a very considerable retarding influence upon the root-growth, checking it entirely in some instances.
Some years before True and Gies published their results, Coupin (1898) had grown wheat seedlings in culture solutions with the addition of copper salts for several days in order to find the fatal concentrations of the different compounds. Taking toxic equivalent as meaning “the[19] minimum weight of salt, which, dissolved in 100 parts of water, kills the seedling,” the results were as follows:
| Toxic equivalent | Containing copper | |
| Copper bromide (CuBr2) | ·004875 | ·001387 |
| Copper chloride (CuCl2 . 2aq.) | ·005000 | ·001865 |
| Copper sulphate (CuSO4 . 5aq.) | ·005555 | ·001415 |
| Copper acetate (Cu{C2H3O2}2 . aq.) | ·005714 | ·001820 |
| Copper nitrate (Cu{NO3}2 . 6aq.) | ·006102 | ·001312 |
These numbers appear to be very close, so Coupin considered that it might be permissible to regard the differences as due to the impurities in the salts, and to the water of crystallisation which may falsify the weights, so that under these conditions one may believe that all these salts have the same toxicity. This is considerable, and is evidently due to the copper ion, the electro-negative ion not intervening with such a feeble dose. A recalculation of these toxic equivalents to determine the actual amount of copper present in each, gives results that are fairly approximate, but it is difficult to accept this hypothesis in view of other work in which different salts of the same poison are proved to differ greatly in their action on plant growth.
Kahlenberg and True (1896), working with Lupinus albus, found that the various copper salts, as sulphate, chloride and acetate, were similar in their action upon the roots. Plants placed in solutions of these salts of varying strengths for 15–24 hours showed that in each case 1/25,600 gram molecule killed the root, while with 1/51,200 gram molecule the root was just alive. These workers discuss their results from the standpoint of electrolytic dissociation, and concur in the opinion that the positive ions of the toxic salt are exceedingly poisonous.
The toxicity of the positive ion was again set forth by Copeland and Kahlenberg (1900). Their water culture experiments were carried on in glass vessels coated internally with paraffin to avoid solution of glass, and in tests with seedlings of maize, lupins, oats and soy beans it was found that such metals as copper, iron, zinc and arsenic were almost always fatal to the growth of plants. As a general rule those metals whose salts are toxic, themselves poison plants when they are present in water. The assumption made was that the injury to plants when cultivated in the presence of pure metals depends on the tendency of the metal to go into solution as a component of chemical compounds and on the specific toxicity of the metallic ion when in solution.
[20]
Experiments were carried on with barley, in which the plants were grown in the various grades of distilled water indicated above, both with and without the addition of nutrient salts. It was found that the presence of the nutrients exercises a very definite masking effect upon the action of the poisonous substance, so that the deleterious properties of the toxic substance are materially reduced. Later work, in which known quantities of such toxic salts as copper sulphate were added to pure distilled water showed that in the presence of nutrient salts a plant is able to withstand the action of a much greater concentration of poison. For instance, a concentration of 1:1,000,000 copper sulphate alone stops all growth in barley, but, if nutrient salts are present, a strength of 1:250,000 (at least four times as great) does not prevent growth, though the retarding action is very considerable (Figs. 2 and 3).

Fig. 2. Photograph showing the action of copper sulphate on barley in the presence of nutrient salts. (March 5th–April 19th, 1907.)
| 1. | Glass distilled water. | ||
| 2. | Copper distilled water. | ||
| 3. | 1/12,500 | copper | sulphate. |
| 4. | 1/25,000 | „ | „ |
| 5. | 1/50,000 | „ | „ |
| 6. | 1/100,000 | „ | „ |
| 7. | 1/250,000 | „ | „ |
| 8. | 1/500,000 | „ | „ |
| 9. | 1/1,000,000 | „ | „ |
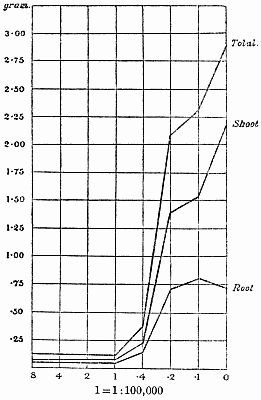
Fig. 3. Curve showing the dry weights of a series of barley plants grown in the presence of copper sulphate and nutrient salts. (March 13th–May 3rd, 1907.)
Note. In each scale of concentrations represented in the curves a convenient intermediate strength is selected as a unit, and all other concentrations in the series are expressed in terms of that unit. Thus, with 1/1,000,000 as the unit a scale of concentrations might run thus:
| 10 | 1/100,000 |
| 4 | 1/250,000 |
| 2 | 1/500,000 |
| 1 | 1/1,000,000 |
| 0·5 | 1/2,000,000 |
| 0·1 | 1/10,000,000 |
| 0·05 | 1/20,000,000 |
| 0· | Control. |
These later Rothamsted results fit in very well with those obtained ten years ago (1903) by True and Gies in their experiments on the physiological action of some of the heavy metals in mixed solutions. Plants of Lupinus albus were tested for 24–48 hours with different solutions in which the roots were immersed. Given the same strength of the same poison, the addition of different salts yielded varying results. For instance, with copper chloride as the toxic agent, the addition of magnesium chloride did not affect the toxicity, calcium chloride decreased it, while sodium chloride slightly increased the poisonous action. Calcium sulphate with copper sulphate enabled a plant to withstand four times as much copper as when the latter was used in pure solution. Calcium salts in conjunction with those of copper proved generally to accelerate but not to increase growth, but with silver salts they did not cause any improvement. Perhaps this amelioration is in inverse proportion to the activity of the heavy metals. With a complex mixture consisting of five salts—copper sulphate and salts of sodium, magnesium, calcium and potassium, all except calcium being present in concentrations strong enough to interfere with growth if used alone—it was shown that “as a result of their presence together, not only is there no addition of poisonous effects, but a neutralisation of toxicity to such degree as to permit in the mixed solutions a growth-rate equal to or greater than that seen in the check culture.” If the concentration of the copper salts was increased, the[21] other salts remaining the same, the poisonous activity of the copper became greater than could be neutralised by the other salts. If the copper remained the same and the other salts were diminished by half (i.e. below toxic concentration) the neutralising action of the added salts was markedly less, and the growth rate never exceeded that of the[22] control. This was apparently due to the action of the unneutralised copper. The indications are that the conspicuously effective part of the molecule is the cation or metal, and that the anion plays little or no part in causing the toxicity; in such great dilutions the metals act as free ions. The hypothesis is put forward that interior physiological modifications are responsible for the observed differences in growth rate, the cell processes being so affected as to bring about different results on cellular growth; in other words, the growth rate represents the physiological sum of oppositely acting stimuli or of antagonistic protoplasmic changes where mixtures of salts occur. This is really an extension of Heald’s idea that the toxic effect of a poison is due partly to changes in the turgescence of the cell, a sudden decrease causing retardation or inhibition of growth, and partly to a direct action on the protoplasm, which differs in different plants with the same salt. Heald (1896) went so far as to suggest that the poisonous action is a mere matter of adaptation and adjustment, since toxic substances are not usually present in soil, but this assertion is too sweeping to be accepted in its entirety, although it probably holds good to a certain extent with some species of plants.
Kahlenberg and True (1896) found that the addition of an organic substance produced the same effect as the addition of some nutrient salt, in that it reduced the toxicity of the copper salt, e.g. in the presence of sugar and potassium hydrate the lupins were able to withstand a concentration of 1/400 copper sulphate, part of which reduction of toxicity is attributed to the sugar.
Other investigators have shown that the presence of insoluble substances has a similar effect in reducing toxicity to an even greater degree. True and Oglevee (1904, 1905) again used Lupinus albus as a test plant in the presence of solutions of various poisons in pure distilled water, copper sulphate, silver nitrate, mercuric chloride, hydrochloric acid, sodium hydroxide, thymol and resorcinol all coming under consideration. Clean sea sand, powdered Bohemian glass, shredded filter paper, finely divided paraffin wax and pure unruptured starch grains were respectively added to the solutions, and seedlings were suspended over glass rods so that their roots were in the solutions for 24–48 hours. The solids varied in their action on the different poisons; while the toxic influence of mercuric chloride was reduced by sand and crushed glass, the action of silver nitrate was modified by nearly[23] all the solids. Lupin roots proved unable to withstand an exposure of 24 hours to a concentration of copper sulphate of 1 molecular weight in 60,000 litres of water (i.e. about 1 part by weight CuSO4 . 5H2O in 240·4 parts water), but the addition of solids caused a great decrease in toxicity. When the amount of copper was diminished an advantage was regularly obtained in favour of the cultures containing the solid bodies. On the whole the ameliorating action of solids is more clearly marked with dilute solutions of strong poisons than with relatively concentrated solutions of weaker poisons. As a general rule, filter paper and potato starch grains exert a more marked modifying action than the denser bodies, such as sand, glass or paraffin.
Breazeale (1906) tested the same point with extracts of certain soils which proved toxic to wheat seedlings grown in them as water cultures. The toxicity was wholly or partly removed by the addition of such substances as carbon black, calcium carbonate or ferric hydrate. Other experiments showed that the toxic substances of ordinary distilled water are removed by ferric hydrate and carbon black, and further that the latter substance will take out copper from copper solutions, rendering them far less poisonous.
Further corroboration of True and Oglevee’s work was obtained by Fitch (1906) who worked in a similar way with fungi, arriving at the general conclusion that insoluble substances in a solution act as agents of dilution or absorption whereby poisonous ions or molecules are in some way removed. He found that n/256 of copper sulphate in beet concoction exercised a stimulating effect on Penicillium glaucum, but the addition of fine glass to the solution increased the stimulation, while large or medium sized pieces did not have the same effect.
This action of solid bodies in reducing the deleterious effects of poisonous solutions is attributed to the process of “adsorption” whereby a layer of greater molecular density is formed on the surfaces of solids immersed in solutions. The solids presumably withdraw a certain proportion of poisonous ions or molecules from the body of the solution (retaining them in a molecularly denser layer over their own surfaces), so that the toxic properties of the solution are reduced owing to the withdrawal of part of the poison from the field of action. In some cases this reduction may be so great as to relieve the solution of its toxic properties, or even to cause an abnormal acceleration to replace a marked retardation. Also, if the solution is of such a dilution as to cause acceleration of growth in plants, the addition of insoluble substances may increase this acceleration. The progressive addition of[24] quantities of solids causes progressive dilution of the toxic medium, the underlying cause of these results being the gradual removal of molecules or ions from the solutions by the insoluble body present.
Fitch’s results are also in accordance with the well-known fact that the physical condition and properties of the added solid play a considerable part in determining its efficacy as an adsorbing agent.
As has already been shown the toxic property of copper with regard to plants was recognised almost as soon as that element was found to occur in the vegetable kingdom, but little notice was taken of the discovery for many years. In 1882 F. C. Phillips asserted, as the result of experiments with various cultivated flowering plants, including geraniums, coleas, ageratum, pansies, &c., that under favourable conditions plants will absorb small quantities of copper by their roots, and that such compounds exercise a distinctly retarding influence even if in very small amount, while if large quantities are present they tend to check root formation, either killing the plants outright or so far reducing their vitality as seriously to interfere with nutrition and growth. Two years later Knop confirmed both the absorption and the toxicity of copper by his experiments on maize.
Jensen (1907) worked with “artificial” soils, under sterile conditions, using finely ground quartz flour for his medium and wheat for a test plant, parallel experiments being carried on with solutions. Every precaution was taken to ensure sterility—the corks were boiled first in water and then in paraffin, the seeds were sterilised in 2% copper sulphate solution for 3⁄4 hour, washed in sterilised water, planted in sterilised sphagnum, the transplanting being done in a sterile chamber into sterilised solutions. The criteria used to determine the toxic and stimulation effects were the total transpiration, average length of sprout, the green weight and dry weight of plants. The results obtained with the different substrata showed that it does not follow that a salt highly toxic in solution is equally so in soil, or that one which holds a relatively high toxic position in soil should occupy the same relative position in solution cultures. For instance, while in soil cultures nickel compounds were the most toxic of all the substances tried, in solution cultures silver compounds were more poisonous than nickel. The range of concentrations, both fatal and accelerating, was found to be much greater in solution than in soil cultures.
In the sand cultures the toxicity of the copper sulphate was found[25] to decrease as the ratio of the quartz sand to the poisonous solution increased, provided that a water content suitable for growth was present. Jensen states that the fatal concentration of copper sulphate in solution cultures is approximately 1⁄10th that of the fatal concentration in his artificial soil.
When copper salts are added to soil a complication at once sets in due to the double decomposition which is always likely to occur when any soluble salt is added to soil. The reaction may be graphically expressed as follows, in a much simplified form—
Haselhoff (1892) extracted several lots of 25 kgm. soil, each with 25 litres of water in which quantities of mixed copper salts varying from 0–200 mg. had been dissolved, the mixture consisting of three parts copper sulphate and one part copper nitrate. This operation was repeated 15 times, the soils being allowed to drain thoroughly after each treatment, so that altogether each 25 kgm. soil was extracted with 375 litres water. The drainage waters were analysed, so that the amount of copper absorbed by the soils could be estimated. It was found that by extracting with water containing such soluble copper salts as sulphate and nitrate, the food salts of the soil, especially those of calcium and potassium, were dissolved and washed out, copper oxide being retained by the soil. In this way a double action was manifest, whereby the fertility of the soil was reduced by the loss of plant food, while its toxicity was increased by the accumulation of copper oxide. So long as the soil contained a good supply of undissolved calcium carbonate the harmful action of the copper-containing water was diminished, but as soon as the store was exhausted by solution and leaching, the toxic influence became far more evident.
Quite early in the investigations on the effect of copper on plants the question arose as to its mode of activity—whether the toxicity was merely due to some mechanical action on the root from outside, whereby the absorptive power of the root was impaired, or whether the poisonous substance was absorbed into the plant, so acting directly on the internal tissues. Gorup-Besanez made definite experiments towards ascertaining the truth of these theories as far back as 1863, endeavouring first of all to see whether the plants take up any appreciable quantity of poisons which exist in the soil as mixtures or combinations and which[26] are capable of solution by the cell-sap. Salts of arsenic, copper, lead, zinc and mercury were intimately mixed with soil, 30 grams of the poison being added to 30·7 cubic decimetres of soil, two plants separated by a partition being grown on this quantity. The test plants were Polygonum Fagopyrum, Pisum sativum, Secale cereale and Panicum italicum, and all the plants developed strongly and normally except the last named. The Panicum developed very badly coloured leaves in an arsenic-containing soil, and the plants were killed soon after they started in soils containing copper. After harvesting, the crops were analysed and no trace of copper was found in any one of the experimental plants by the methods adopted. Also the absorption capacity of different soils for different poisons was shown to vary, for basic salts are absorbed, while acids may pass completely through the soil into the drainage water.
These results obtained by Gorup-Besanez are possibly not altogether above criticism, for later workers showed that copper was absorbed to some extent by plants grown in water cultures, and if that is so it seems unlikely that no absorption should take place from soil. Nevertheless, the absorption is very slight, for apparently living protoplasm is very resistant to copper osmotically. Otto showed that beans, maize and peas can have their roots for a long time in a relatively concentrated solution of copper sulphate, and yet take up very little copper indeed, but analyses do reveal slight traces after a sufficient interval of time of contact has elapsed. Berlese and Sostegni indicate that the roots of plants grown in water culture in the presence of bicarbonate of copper showed traces of copper.
Verschaffelt (1905) devised an ingenious method of estimating the toxic limits of plant poisons, though it is rather difficult to see how the method can be put to practical use with water culture and soil experiments. Living tissues increase in weight when put into water on account of the absorption of water. Dead tissues do not, as they have lost their semi-permeable characteristics, so a decrease in weight takes place owing to part of the water passing out. This principle is applied by Verschaffelt to determine the “mortal limit” of external agents in their action on plant tissues. Root of beetroot, potato tuber, aloe leaves, and parts of other plants rich in sugar all came under review. The parts were cut into small pieces weighing about 3–5 grams, dried with filter paper, weighed, and plunged into solutions of copper sulphate of varying strengths from ·001–·004 gm. mol. per litre, and left for 24 hours. After drying and again weighing[27] all were heavier owing to the absorption of water. The pieces were then immersed in pure water for another period of 24 hours, when after drying and weighing, those from the weaker strengths of copper sulphate (·001–·002) had absorbed yet more water, while those from higher concentrations (·003–·004) had lost weight. So the author assumes that for such pieces of potato the limit of toxicity lies between ·002 and ·003 gm. mol. copper sulphate per litre.
These experiments may possibly give some indication as to the action of copper salts on plant roots. So long as the solution of copper salt is dilute enough, the absorption layer of the root, acting as a semi-permeable membrane and upheld by the resistant protoplasm, is able to keep the copper out of the plant and to check its toxicity. As soon as a certain limit is reached the copper exercises a corrosive influence upon the outer layer of the root whereby its functions are impaired, so that it is no longer able efficiently to resist the entry of the poison. As the concentration increases it is easy to conceive that the harmful action should extend to the protoplasm itself, so that the vital activities of the plants are seriously interfered with and growth is entirely or partially checked, death ensuing in the presence of sufficiently high concentrations.
The action of copper on the germination of seeds, spores and pollen grains has attracted a certain amount of attention, and although the results are apparently contradictory this is probably due to the different plant organs with which the observers have worked.
Miyajima (1897) showed that the germinating power of such seeds as Vicia Faba, Pisum sativum, and Zea Mays was partly destroyed by a 1% solution of copper[4], Zea Mays being the most resistant and Vicia Faba the least resistant of the three. Micheels (1904–5) stated that water distilled in a tinned copper vessel was more favourable for germination than water from a non-tinned vessel. He suggests that this is due to copper being present in the water in a colloidal form in which the particles are exceedingly small and maintain themselves in the liquid by reason of a uniform disengagement of energy in all directions, to which energy the influence on germinating seeds must be[28] attributed, the nature of the suspended substance determining whether the influence be favourable or not. It is questionable, however, whether Micheels was really dealing with a true colloidal solution of copper or with a dilute solution of some copper salt produced by oxidation of the copper vessel from which his distilled water was obtained.
Miani (1901) brought fresh ideas to bear upon the problem of the action of copper on living plant cells, in that he sought to attribute the toxic or stimulant effects to an oligodynamic action, i.e. spores and pollen grains were grown in hanging drop cultures in pure glass distilled water with the addition of certain salts or traces of certain metals. While the salts are known to be often disadvantageous to germination, Nägeli had asserted that the latter often exerted an oligodynamic action. In some cases pure copper was placed for varying times in the water from which the hanging drop cultures were eventually made, or tiny bits of copper were placed in the drop itself. Various kinds of pollen grains were tested, and as a rule, pollen was only taken from one anther in each experiment, though occasionally it was from several anthers of the same flower. It was generally found that the germination of pollen grains or Ustilago spores was not hindered by the use of coppered water or by the presence of small bits of copper in the culture solution. The only cases in which some spores or pollen grains were more or less harmed were those in which the water had stood over copper for more than two weeks, and even so the deleterious effect was chiefly noticeable when the pollen itself was old or derived from flowers in which the anther formation was nearly at an end. As a rule germination was better in the presence of copper, whether in pure water or food solution, the stimulus being indicated both by the greater number of germinated grains and by the regular and rapid growth of the pollen tubes. Miani attributes this favourable action to the mere presence of the copper, corroborating Nägeli’s idea of an oligodynamic action.
From the foregoing review it is evident that it is the toxic action of copper that is most to the front, so far as the higher plants are concerned, and that little or no evidence of its stimulative action in great dilution has so far been discussed. Kanda dealt with this question, with the deliberate intention of obtaining such evidence,[29] if it existed. He worked with Pisum sativum, var. arvense, Pisum arvense, Vicia Faba, var. equine Pers, and Fagopyrum esculentum Mönch, which were grown in glass distilled water, without any food salts, so that the plants were forced to live on the reserves in the seeds, which were carefully graded to ensure uniformity of size. It was found that in water cultures copper sulphate solutions down to ·00000249% (about 1 in 40,160,000) are harmful to peas, and still further down to ·0000000249% (about 1 in 4,016,000,000) the copper salts act as a poison rather than as a stimulant. Against this, however, is the statement that in certain soils copper sulphate acts as a stimulant when it is added in solution. Jensen again could obtain no stimulation with copper sulphate.
The Rothamsted experiments go to uphold Kanda’s statements as to the failure of copper sulphate to stimulate plants grown in water cultures. Peas are perhaps slightly more resistant to the greater strengths of copper sulphate than are barley and buckwheat, for while 1/100,000 proves mortal to the latter, peas will struggle on and fruit in 1/50,000, though this strength is very near the limit beyond which no growth can occur (Fig. 4). As a general rule, with barley the depression caused by the poison is still evident with 1/5,000,000 and 1/10,000,000, though occasionally these doses act as indifferent doses, no sign of[30] stimulation appearing in any single instance. With peas again, even 1/20,000,000 copper sulphate is poisonous, although to the eye there is little to choose between the control plants and those receiving poison up to a concentration of one part in 21⁄2 million (Fig. 5). In the case of buckwheat the matter is still undecided, as in some experiments apparent stimulation is obtained with 1 in 21⁄2 or 1 in 5 million copper sulphate, while in others a consistent depression is evident, even when the dilution is carried considerably below this limit. The reason for the variation with this particular plant is so far unexplained.

Fig. 4. Photograph showing the action of copper sulphate on pea plants in the presence of nutrient salts. (Oct. 3rd–Dec. 20th, 1912.)
| 1. | Control. | ||
| 2. | 1/50,000 | copper | sulphate. |
| 3. | 1/100,000 | „ | „ |
| 4. | 1/250,000 | „ | „ |
| 5. | 1/500,000 | „ | „ |
| 6. | 1/1,000,000 | „ | „ |
| 7. | 1/2,500,000 | „ | „ |
| 8. | 1/5,000,000 | „ | „ |
| 9. | 1/10,000,000 | „ | „ |
| 10. | 1/20,000,000 | „ | „ |
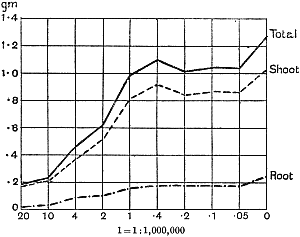
Fig. 5. Curve showing the mean values of the dry weights of four series of pea plants grown in the presence of copper sulphate and nutrient salts. (Oct. 3rd–Dec. 20th, 1912.)
Yet, in spite of all the accumulated evidence as to the consistent toxicity of copper salts in great dilution, the possibility still remains that the limit of toxicity has not yet been reached, and that a stimulating concentration does exist, so that it is still uncertain whether beyond the limits of toxicity copper salts act as indifferent or stimulative agents.
The bulk of the work on the relations of copper with the life-processes of plants has dealt with those cases in which the metal has been supplied to the roots in some form or other, and many of the results may be said to apply more strictly to the theoretical, or rather to the purely scientific aspects of the matter, than to the practical everyday life of the community. This statement is hardly correct, in that the two lines of work are so inextricably interwoven that the one could not be satisfactorily followed up without a parallel march of progress along the other. In practice, copper has proved remarkably efficient as a fungicide when applied as sprays in the form of Bordeaux mixture to infested plants and trees. Observations on the action of the fungicide have shown that the physiological processes of the treated plants are also affected to some degree, and a number of interesting theories and results have been put forward.
Frank and Krüger (1894) treated potato plants with a 2% Bordeaux mixture, and obtained a definite improvement in growth, which they attributed to the direct action of the Bordeaux mixture upon the activities of the plant. The effect of the copper was most marked in the leaves, and was chiefly indicated by increase in physiological activity rather than by morphological changes. The structure of the sprayed leaves was not fundamentally changed but they were thicker and[31] stronger in some degree, while their life was lengthened. Apparently, treatment increased the chlorophyll content, and, correlated with this, was a rise in the assimilatory capacity, more starch being produced. Rise in transpiration was also observed. While the leaves were the organs most affected, a subsidiary stimulation occurred in the tubers, since the greater quantity of starch produced required more accommodation for its storage. In different varieties the ratio of tuber formation on treated and untreated plants was 19:17 and 17:16. In discussing the meaning of this stimulation these writers, following the custom then in vogue, were inclined to hold that it was due to a catalytic rather than to a purely chemical action, an idea similar to one which later on came much into prominence in connection with the work of Bertrand’s school on manganese, boron and other substances.
The imputed increase in photo-synthesis seems to have met with approval and acceptance, but nevertheless it did not pass unchallenged. Ewert (1905) brought forward a detailed discussion and criticism of the assumption that green plants when treated with Bordeaux mixture attain a higher assimilation activity than untreated plants. His experiments were made to test the effects of differing conditions of life on plants treated in various ways, and his conclusions lead him to assert that “instead of the organic life of the plant being stimulated by treatment with Bordeaux mixture it is rather hindered.”
While Frank and Krüger indicated a rise in transpiration when copper compounds were applied to the leaves as sprays, Hattori (1901) attributed part of the toxic effect of copper salts, when applied to the roots, to a weakening action on the transpiration stream, and he maintained that the toxic effect of the copper salts is therefore connected with the humidity of the air. No further confirmation or refutation of this statement has so far come to light.
In certain plants the application of cupric solutions as sprays causes a slight increase in the quantity of sugar present in the matured fruits. Chuard and Porchet (1902, 1903) consider that such a modification in the ripe fruit during the process of maturation occurs in all plants which ripen their fruits before leaf-fall begins. Injection of solutions of copper salts into the tissues of such plants as the vine causes more vigorous growth, more intense colour and greater persistence of the leaves; in other words the copper acts as a stimulant to all the cells of the organism. A similar effect is produced by other metals such as iron or cadmium. By injecting small quantities of cupric salts into the branches of currants an acceleration of the maturation of the fruits was caused, identical[32] with that obtained by the application of Bordeaux mixture to the leaves. If the quantity of copper introduced into the vegetable organism was augmented, the toxic action of the metal began to come into play. These investigators attributed the stimulus, as shown by the earlier maturation of the fruits, to a greater activity of all the cells of the organism and not to an excitation exercised only on the chlorophyll functions.
Treboux (1903) demonstrated the harmful action of solutions of copper salts on leaves by means of experiments on shoots of Elodea canadensis. The activity of photo-synthesis was measured by the rate of emission of bubbles of oxygen. On placing the shoots first in water, then in N/1,000,000 copper sulphate (·0000159%), there was a reduction from 20 to 15 or 16 bubbles in 5 minutes. On replacing in water there was an increase to 18, but not to 20, indicating a permanent injury. With N/10,000,000 copper sulphate there was little or no reduction in the number of bubbles. This experiment had an interesting side issue in that it was noticed that not only the concentration, but also the quantity of fluid was concerned in the toxic action, indicating that both the proportion and the actual amount of poison available play their part. For instance, with a shoot 10 cm. long in 100 c.c. solution the plants were only slightly affected by ·000015% copper sulphate, but in 500 c.c. solution the shoots were killed after some days in ·0000015% copper sulphate, a concentration only one-tenth as great.
While it is evident that copper sprays have a definite action upon green leaves, whether favourable or unfavourable, the question arises as to the means whereby the copper obtains access to the plant in order to take effect. Dandeno found that solutions of copper sulphate were absorbed by the leaves of Ampelopsis, forming a brown ring. Generally speaking inorganic salts in solution are absorbed through both surfaces of the leaves, whether the leaves are detached or not, provided the surrounding atmospheric conditions are favourable, the absorption being usually more ready through the lower surface. Dilute solutions applied in drops stimulate the leaf tissue in a ring, whereas if the solutions are concentrated the entire area covered by the drop is affected. Too concentrated solutions of copper sulphate applied to leaves caused scorching, but if this was avoided while the solution was still strong enough to cause a darkening of green colour after a time, Dandeno considered that[33] the action was probably of the nature of a stimulus to growth, and produced a better development of chlorophyll and protoplasm in the region where the tissues appeared dark to the naked eye, a conclusion which tallies very closely with that of Frank and Krüger.
Amos (1907–8) experimented to see whether the application of Bordeaux mixture affected the assimilation of carbon dioxide by the leaves of plants, and whether any stimulation was produced. Brown and Escombe’s methods and apparatus were used and the summarised results indicate that the application of Bordeaux mixture to the leaves of plants diminishes the assimilation of carbon dioxide by those leaves for a time. The effect gradually passes off, whatever the age of the leaves may be. The suggestion is made that the stomata are blocked by the Bordeaux mixture, so that less air diffuses into the intercellular spaces and less carbon dioxide comes into contact with the absorptive surfaces. If this hypothesis is correct, the physiological slackening of assimilation is not due to the toxic action of the copper in the Bordeaux mixture, but to a mechanical hindrance due to blocking of the stomata.
On turning to the lower plants, especially to some species of fungi, one notices a striking contrast in their behaviour to that of the higher plants. Some species of fungi have the power of living and flourishing in the presence of relatively large quantities of copper compounds, or even of copper or bronze in the solid state. Dubois (1890) found that concentrated solutions of copper sulphate, neutralised by ammonia, which were used for the immersion of gelatine plates used in photography, showed white flocculent masses resembling the mycelium of Penicillium and Aspergillus, which grew rapidly and fructified in Raulin’s solution, but which remained as mycelium in cupric solutions. The mould proved capable of transforming copper sulphate into malachite in the presence of a piece of bronze, but it was found that the presence of the latter was not essential for the conversion into basic carbonate. The same result was obtained if the culture liquid was put in contact with a body which prevented it from becoming acid, fragments of marble acting in this way. Copper sulphate solution in the presence of the mould produced a green deposit on the marble, while without the fungus the solution simply evaporated leaving a blue stain of copper sulphate.
[34]
Trabut (1895) found that on treating smutty wheat with a 2% solution of copper sulphate he obtained a mass of flocculent white mycelium, whose surface was soon covered with aerial branches bearing pale rose-coloured spores, and he gave the provisional name of Penicillium cupricum to the species. On preparing nutritive solutions by steeping a handful of wheat in water for 24 hours, and then adding various amounts of copper sulphate to them, Penicillium was found to vegetate quite well until the amount of copper sulphate reached 91⁄2 grams in 100 c.c., after which the seedings with spores did not develope at all. De Seynes tested this Penicillium more exhaustively with different culture media under various conditions and decided that Trabut was right in only assigning the name P. cupricum provisionally, as the mould reverts to the form P. glaucum when seeded in a natural medium, indicating that P. cupricum has not an autonomous existence, but is P. glaucum which modifies the colour of its conidia under the influence of copper sulphate, in the same way that it often modifies them in other media. It is noticeable that the mycelium arising from the germination of conidia of P. cupricum in a normal medium has a very poor capacity for producing reproductive organs, but this diminished activity is attributed not to a special deleterious action of the copper sulphate but to the impulse given to the vegetative functions, at the expense of the reproductive, when the spores are seeded in a richer medium than the solutions of copper sulphate which serve as the soil for P. cupricum.
Ono found that Aspergillus and Penicillium are retarded in growth in the higher concentrations of copper sulphate, but that they are stimulated by weaker strengths. The range of stimulating concentrations is given as from ·0015%–·012%, the biggest crop being obtained with both moulds in the strongest of these solutions. Hattori gives the optimum as being considerably lower for the two fungi mentioned, Penicillium being at its best in a solution of ·008% and Aspergillus in ·004%. A. Richter (1901) opposes this absolutely so far as Aspergillus niger is concerned. In his experiments copper appears invariably as a depressant, all concentrations from 1/150 to 1/150,000,000 giving growth below the normal, no stimulative action ever being observed. Zinc however proved to be a definite stimulant and in a mixture of copper and zinc salts in appropriate concentrations the toxic effect of the copper was completely paralysed by the stimulating action of the zinc, 1/200,000 zinc salt paralysing or overcoming the copper salt at 1/1125.
Ono states that the optimal quantity of such poisons as copper salts is lower for algae than for fungi, copper failing to stimulate algae at[35] dilutions which were the most favourable to the growth of fungi. Bokorny indicates that silver and copper salts work harm in unusually dilute solutions.
Attempts have been made to utilise the poisonous action of copper on algae in clearing ponds of those plants. Lindsay (1913) describes experiments carried on in a reservoir infested with Spirogyra. A quantity of copper sulphate sufficient to make a solution of 1/50,000,000 was found necessary to kill off the Spirogyra, but it is suggested that the solution was probably weaker before it reached the algae, owing to the currents of fresh water. Anaboena needed 1/10,000,000 before it was killed off, while Oscillatoria is less sensitive still, 1/5,000,000 usually representing the mortal dose, though 1/4,000,000 was necessary in some instances. Algae seem to be peculiarly sensitive to the copper sulphate, far more so than the higher plants, as Nuphar lutea, Menyanthes trifoliata, and Polygonum amphibium grew in the water unharmed by the addition of the poisonous substance. For some unexplained reason it seems that “the concentration of copper sulphate necessary to kill off the algae in the laboratory is five to twenty times as great as that needed to destroy the same species in its natural habitat.”
Altogether, after looking at the question from many points of view, one is forced to the conclusion that under most typical circumstances copper compounds act as poisons to the higher plants, and that it is only under particular and peculiar conditions and in very great dilutions that any stimulative action on their part can be clearly demonstrated.
[36]
The presence of zinc in the ash of certain plants has been recognised for many years, especially in so far as the vegetation of soils containing much zinc is concerned. Risse, before 1865, stated that most plants when grown on such soils prove to contain greater or less quantities of zinc oxide. He states that the soil at Altenberg, near Aachen, is very rich in zinc, which rises as high as 20% in places. The flora of the soil is very diversified and zinc has been determined qualitatively in most and quantitatively in some of the plants. Viola tricolor and Thlaspi alpestre are most characteristic under such circumstances, both showing such constant habit changes that they resemble new species, while other plants such as Armeria vulgaris and Silene inflata are peculiarly luxuriant. Risse’s figures of the zinc content of these four plants may prove of interest. The figures are based on the dry weights, air dried.
| Thlaspi alpestre, var. calaminaria. | |||||||||
| Root | 6·28% ash, | 0·167% ZnO, | 1·66% ZnO in ash. | ||||||
| Stem | 11·75% | „ | 0·385% | „ | 3·28% | „ | „ | ||
| Leaves | 11·45% | „ | 1·50% | „ | 13·12% | „ | „ | ||
| Flowers | 8·49% | „ | 0·275% | „ | 3·24% | „ | „ | ||
| Viola tricolor. | |||||||||
| Root | 5·59% ash, | 0·085% ZnO, | 1·52% ZnO in ash. | ||||||
| Stem | 10·55% | „ | 0·065% | „ | 0·62% | „ | „ | ||
| Leaves | 9·42% | „ | 0·110% | „ | 1·16% | „ | „ | ||
| Flowers | 7·66% | „ | 0·075% | „ | 0·98% | „ | „ | [37] | |
| Armeria vulgaris. | |||||||||
| Root | 4·74% ash, | 0·17% ZnO, | 3·58% ZnO in ash. | ||||||
| Stem | 5·37% | „ | 0·02% | „ | 0·37% | „ | „ | ||
| Leaves | 9·36% | „ | 0·11% | „ | 1·17% | „ | „ | ||
| Flowers | 6·08% | „ | 0·07% | „ | 1·15% | „ | „ | ||
| Silene inflata. | |||||||||
| Root | 2·71% ash, | 0·02% ZnO, | 0·74% ZnO in ash. | ||||||
| Stem | ⎫ | ||||||||
| Leaves | ⎬ | 11·43% | „ | 0·22% | „ | 1·92% | „ | „ | |
| Flowers | ⎭ | ||||||||
Freytag (1868) carried out various experiments on the influence of zinc oxide and its compounds on vegetation, and found that all plants are capable of absorbing zinc oxide by their roots when grown on soils containing such oxide. Generally speaking the zinc is deposited chiefly in the leaves and stems, very little being found in the seeds, such minute traces occurring that he stated that the seeds must be harmless for men and animals. The general content of ZnO in plants is given as ·5–1·0% of ash, except in the abnormal case of plants growing on calamine.
Lechartier and Bellamy (1877) demonstrated the presence of zinc in such food substances as wheat, American maize, barley and white haricots, but they failed to find it in maize stems and beetroot, so they cautiously concluded that if it does occur in the latter cases it must be far less in quantity than in the former. Hattensaur (1891) analysed the ash of Molinia cærulea and discovered the presence of copper, manganese, zinc and lead, zinc oxide forming ·265% of the total ash, (·006% of the air dried plant).
Jensch (1894) observed that the flora on calamine soils was somewhat scanty, the chief plants that came under his notice being Taraxacum officinale, Capsella Bursa-pastoris, Plantago lanceolata, Tussilago Farfara, and Polygonum aviculare, all of which showed certain morphological peculiarities. Generally speaking the growth of these plants on the calamine soils was weak and poor, the stems and leaves being very brittle. Jensch found that the roots were deformed and showed a tendency towards a plate-like superficial spread of root. The leaves of Tussilago were uneven in shape and lacked the white hairs on the under side, the flower stalks were twisted, while the flowers themselves were a deep saturated yellow colour. The stems of Polygonum aviculare were much thickened at the nodes, the leaves weak and rolled in character, while the flowers were long-stalked, the calyces being usually of a[38] purple red colour. The following figures are given for the quantities of zinc carbonate (ZnCO3) in the ash of these two plants:—
| Tussilago Farfara. | ||
| Root | Leaf-stalk | Leaf-blade |
| 2·51%–3·26% | 1·75%–1·63% | 2·90%–2·83% ZnCO3 |
| = 1·629%–2·115% | 1·136%–1·058% | 1·882%–1·836% ZnO. |
| Polygonum aviculare. | ||
| Root | Stem | Leaves |
| 1·77%–1·93% | 2·25%–2·86% | 1·24%–1·49% ZnCO3 |
| = 1·148%–1·252% | 1·46%–1·856% | ·804%–·967% ZnO. |
Other analyses of plants from zinc soils as against controls from normal soils indicated the high water and high ash content of the zinc plants, though the dry matter was low, and it is suggested that the increase of the ash may be connected with a stimulation caused by the zinc salts, unless it is due to phosphoric-acid hunger, since the calamine soils concerned are very deficient in phosphorus.
Javillier (1908 c) corroborated the early statements of Risse as to the presence of considerable quantities of zinc in certain species of Viola, Thlaspi and Armeria, and also he cited a list of other plants in which zinc occurs in some quantity. Javillier, however, is of opinion that zinc oxide, like the oxides of iron and manganese, is very common in plant ash, being present in all plant organs. Zinc is specially abundant in Coniferae, where it is probably characteristic, as is the presence of manganese in the ash and manno-cellulose in the wood. The so-called “calamine” plants show great powers of accommodation to large amounts of zinc.
Klopsch (1908) analysed 17 species of plants grown on soil in the vicinity of zinc works, and showed that the plants evidently absorb small quantities of zinc from their surroundings. He also regarded zinc as a normal constituent of certain plants.
In comparison with copper little work has been done with regard to the action of soluble zinc salts alone on higher plants when grown in water cultures. Freytag (1868) stated that zinc salts must be very dilute if the plants are not to be harmed, and that for zinc sulphate the concentrations must not be more than 200 mg. per litre (= 1/5000).[39] Baumann (1885) carried out further experiments and concluded that zinc salts are far more toxic than Freytag suspected, 44 mg. zinc sulphate per litre[5] killing plants of 13 species belonging to 7 families (Coniferae excepted). The various plants withstand the action of the zinc salts in different degrees, the same concentration killing off the species in different times. With the 44 mg. zinc sulphate the following results were obtained:—
| Trifolium pratense | killed in 16 days | ||
| Spergula arvensis | „ | 21 | „ |
| Hordeum vulgare | „ | 30 | „ |
| Vicia sativa | „ | 31 | „ |
| Polygonum Fagopyrum | „ | 60 | „ |
| Beta vulgaris | „ | 76 | „ |
| Onobrychis sativa | „ | 194 | „ |
With still less poison, 22 mg. zinc sulphate per litre, all the species mentioned were eventually killed with the exception of Onobrychis sativa, while 4·4 mg. zinc sulphate seemed to be harmless for all the plants tested except Raphanus sativus, which is evidently exceptionally sensitive to this toxic substance.
Jensen (1907) again indicated the poisonous action of zinc salts and also found that a relatively small reduction of toxicity was obtained by the addition of finely divided quartz to the solutions.
Krauch (1882) grew various plants in the presence of nutrient solutions and quantities of zinc sulphate varying from ·1 to ·8 gm. per litre (= 1/10,000 to 8/10,000). Barley proved to be very sensitive, even to the weakest strength of the poison, as the plants soon showed reddish flecks, while all were dead within six weeks, the control plants without zinc remaining quite healthy. Certain grasses took longer to kill than barley, those with ·4 gm. zinc sulphate per litre dying in about seven weeks, while 13 weeks elapsed before the others were killed. Even after this length of time the plants with ·1 gm. zinc sulphate per litre still survived, although in a very sickly condition. With willow, again, even ·1 gm. zinc sulphate per litre made the plants very sickly after four weeks, growth being weak, the leaves yellow, and the roots brownish. In this case the solutions were renewed, but the plants treated with zinc compounds were dead within eight weeks from the start, the controls being very healthy.
[40]
The next year (1883) Storp repeated these experiments made by Krauch and corroborated his results fully. Barley and grasses (timothy and others) grown in solutions of zinc sulphate, both with and without nutrients, soon lost their green colour and became covered with rusty brown flecks, the barley dying within 14 days, and the grasses soon after. With willow, too, the toxic action was again manifested.
True and Gies (1903) showed that the addition of calcium salts in appropriate concentrations reduced the toxicity of zinc salts considerably, a result similar to that which they obtained for copper.
Recent experiments at Rothamsted have shown that zinc sulphate is very toxic to barley, though the plant is able to make some slight amount of growth even in the presence of a solution of the anhydrous salt ZnSO4 as strong as 1/5000, rapid improvement occurring as the concentration decreases to 1/2,500,000 or less (Fig. 6). On the whole the higher strengths of zinc sulphate are less poisonous to peas than they are to barley. At a concentration of 1 in 1⁄4 or 1 in 1⁄2 million in different experiments the growth was nearly as good as with the control plants, though it consistently lagged a little way behind until a dilution of 1/10,000,000 was reached (Figs. 7 and 8). Incidentally it is very striking to see the desperate efforts that badly poisoned pea plants make to reproduce themselves. Growth of the roots is nearly always checked[41] in advance of that of the shoots, probably on account of the contact of the roots with the poison. In the greater strengths of such poisons as zinc and copper sulphate root growth is checked from the outset, but usually a very little shoot growth is made, and one frequently obtains ridiculous little plants about an inch high bearing unhappy and diminutive flowers, which are occasionally replaced by equally unhappy and miniature fruits. The same thing has also been noticed when unsuccessful attempts have been made to introduce spinach as a test plant for water cultures.
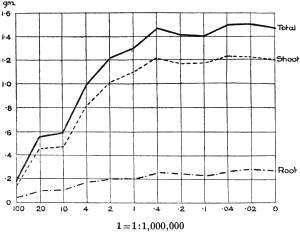
Fig. 6. Curve showing the mean value of the dry weights of ten series of barley plants grown in the presence of anhydrous zinc sulphate and nutrient salts. (March 2nd–May 8th, 1911.)

Fig. 7. Photograph showing the action of anhydrous zinc sulphate on pea plants in the presence of nutrient salts. (Sept. 30th–Dec. 20th, 1912.)
| 1. | Control. | ||
| 2. | 1/5,000 | zinc | sulphate. |
| 3. | 1/10,000 | „ | „ |
| 4. | 1/50,000 | „ | „ |
| 5. | 1/100,000 | „ | „ |
| 6. | 1/250,000 | „ | „ |
| 7. | 1/500,000 | „ | „ |
| 8. | 1/1,000,000 | „ | „ |
| 9. | 1/2,500,000 | „ | „ |
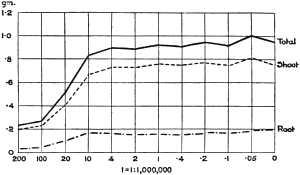
Fig. 8. Curve showing the mean values of the dry weights of nine series of pea plants grown in the presence of anhydrous zinc sulphate and nutrient salts. (May 18th–June 28th, 1910.)
As soon as the presence of zinc in members of the vegetable kingdom was established the question arose as to its effect upon both the plant and the soil.
Gorup-Besanez (1863) grew plants in soil with which 30 grams of metallic poisons such as CuSO4, ZnSO4, HgO, were intimately mixed with 30·7 litres (“cubik Decimeter”) of soil[6]. On analysing the ash of Secale cereale, Polygonum Fagopyrum, and Pisum sativum after six months growth he failed to detect the presence of zinc in any one of the three. As the results varied with different poisons on different plants he concluded that the absorption capacity of the various kinds of[42] soils for different poisons varies, that basic salts are absorbed, while the acid salts may pass completely through the soil in the drainage water.
Freytag (1868) stated that zinc is retained by the soil in the form of oxide, which is derived from dilute zinc compounds as they filter through the soil, by decomposition by the salts of the soil. For field earth the limit of absorption of zinc oxide from zinc sulphate is between ·21%–·24% of the earth.
F. C. Phillips (1882) corroborated Freytag’s statement as to the absorption of small quantities of zinc by the roots of plants, but he states as a fact that both lead and zinc may enter plant tissues without causing any disturbance in the growth, nutrition or functions of the plants, a conclusion that is obviously incorrect or at least incomplete in view of later work on the subject. His choice of plants was certainly unusual, including geraniums, coleas, ageratums and pansies, the poison used being zinc carbonate.
Holdefleiss (1883) stated that in spite of a soil content of 2% zinc the vegetation was not in any way harmed, clover fields and meadow lands on zinc soil presenting a normal appearance. This observation was quite inconclusive, as the author proceeds to say that of the plants that were able to absorb zinc salts without disadvantage the most luxuriant were the so-called zinc plants—the exceptions that prove the rule. Two years later Baumann showed that such insoluble zinc salts as the carbonate and sulphide in the soil cannot hurt plants. These salts are certainly dissolved to some extent by water containing CO2 but solution is hindered by the constitution of the soil. He also found that the various kinds of soil act differently upon zinc solutions, the absorptive power of pure humus soils (“reinem Humusboden”) for zinc solutions being the strongest. Clay and chalk soils also decompose such solutions energetically, while poor sandy soils have only a weak power of absorption. This selectivity of absorption may account for the difference in the toxicity of zinc salts to plants in the various soils.
Storp (1883) experimented to determine the changes in the various characters of the soil by the action of zinc salts on it, and he makes the remarkable statement that in some soils the presence of zinc generates free sulphuric acid, which is particularly injurious to plant life. Grasses, young oaks and figs showed a decrease in dry weight, nitrogen and fat, as the quantity of zinc compounds increased in the water added to the soil. Both the quality and the quantity of the crop were adversely affected. This decrease in the dry weight due to the presence of zinc[43] was confirmed by Jensch later on, and also by Nobbe, Baessler and Will (1884), who state that both lead and zinc compounds work disadvantageously to vegetation even when they are present in such small quantities that the plants are outwardly sound, the harmful action appearing in the decrease of dry weight. Contrary to Baumann’s opinion, zinc carbonate is said to be one of the salts that exercises this insidious poisonous action. Storp (1883) noticed that the direct poisonous action of zinc compounds is largely destroyed by their admixture with soil, but he suggests that a secondary cause of harm is introduced by the accumulation of insoluble zinc salts, so that the fertility of the soil is impaired to the detriment of the vegetation.
Ehrenberg (1908) throws out a suggestion that zinc is specially harmful to plant life when it occurs in conjunction with ammonia, but no further evidence has come to light.
The reason for the toxicity of zinc salts when present in soil forced itself upon the attention of some of the early investigators in this field. Freytag (1868) put forward the hypothesis that the zinc oxide is partly or exclusively absorbed by the roots on account of the cell walls of the root being corroded by the very thin layer of zinc salts lying in contact with it—the same theory as has been held with regard to copper. He stated also that the quantity of zinc oxide taken up by the plant through its roots is strictly limited, not being proportional to the quantity occurring in the soil, but varying between narrow limits. Krauch (1882) found himself unable to accept another hypothesis which at one time found favour, i.e. that the zinc salts kill the plants by coagulating the protoplasm. If this were so, he argued, no plants at all could grow upon soils containing zinc, and he was content to leave the cause as one yet to be explained. Even at the present time, thirty years after, we know very little more about the physiological cause of the toxicity of zinc.
In the course of his investigations on the influence of zinc on vegetation Freytag just touched upon the question of seed germination. According to his statement the presence of zinc oxide in the soil does not exercise much influence upon germination and the growth processes of plants. Little zinc is stored up in seeds and on this account seeds originating from plants containing zinc germinate quite normally and[44] do not seem to be affected by the peculiar nutritive conditions of the parent plants.
In certain cases light seems to have something to do with the harm zinc compounds work on plants. Storp found that when clover seeds were germinated in the dark on filter paper moistened with water containing ·025 gm. ZnO per litre (added in the form of zinc sulphate) no deleterious action was observed. Barley seeds were soaked for four days in (a) distilled water, (b) water with ·9 gm. ZnO per litre, which was frequently changed. These seeds were then placed in the dark on filter papers soaked respectively with water and with the solution containing ZnO. So long as no light was admitted, for a period of eleven days, germination was uniform in both sets, but directly the covers were removed the growth of the seeds with zinc ceased almost entirely, and they did not assume the green colour taken on by the unpoisoned seedlings. With maize the germination was retarded by zinc even in the dark, but the harmful action of light on the plants with zinc was again established. These results seem to indicate that the formation and activity of chlorophyll is impaired by the toxic agent, and this hypothesis is borne out by the fact that in many fungi and non-assimilating higher plants the toxic action of zinc is not evident.
Micheels (1906) approached the matter from a totally different standpoint, seeking to discover what influence the valency of a metal has upon the toxicity of its salts. In each of a series of experiments 1000 c.c. of 5⁄8 decinormal solution of sodium chloride in pure distilled water were used, with the addition of varying strengths of calcium sulphate. Grains of wheat, which previously had been soaked in distilled water, were placed in the solutions, and it was found that the stronger the calcium sulphate solution (up to 1⁄64 normal—the limit of experiment), the better the growth. The calcium sulphate was then replaced by salts of other bivalent metals, as zinc, lead and barium, with analogous results, the quantity necessary to obtain the maximum development varying with one and another; with zinc, n/128 gave the maximum. In this case the toxic action of both sodium chloride and zinc sulphate on germination were considerably reduced by their mutual presence—a result which fits in perfectly with what is known as to the masking effect of soluble substances upon toxic action. The same fact obtains in the animal kingdom, where Loeb and others have found that the toxicity of solutions of sodium chloride for marine animals is reduced by the introduction of salts of the bivalent metals.
[45]
While the toxic action of zinc on the higher plants is so obvious that it forced itself upon the attention of investigators at an early date, the question of possible stimulus is so much more subtle that it has only come into prominence during the last twelve years, during which time an extraordinary amount of experimental work has been done with regard to it. One investigator, Gustavson, was somewhat in advance of his time, for as long ago as 1881 he hinted at the possibility that zinc, aluminium and other substances might act as stimulants or rather as accelerators. He indicated that the rôle of certain mineral salts in the plant economy is to enter into combination with the existing organic compounds, the resulting product of the reaction aiding in the formation of yet other purely organic compounds which ordinarily require for their formation either a very high temperature or a long time—in other words, such a mineral salt acts as a kind of accelerator.
This work was apparently not followed up immediately, but it evidently contains the germ of the “catalytic” hypothesis of which so much has been made during recent years.
The work dealing with zinc as a stimulant to plant growth has yielded such various and apparently contradictory results that the question cannot yet be regarded as settled—it is even still more or less uncertain whether zinc compounds act as stimulants, or whether they are merely indifferent at concentrations below the toxic doses.
True and Gies (1903) suspended seedlings of Lupinus albus for 24–48 hours with their roots in solutions of zinc sulphate and calcium sulphate (m/256)[7], and found that while zinc sulphate alone at m/8192 retarded growth, yet with m/2048 ZnSO4 and m/256 calcium sulphate growth was more than twice as rapid as in controls grown in water, indicating a marked stimulation. The presence of the calcium exercised a definite ameliorating influence, reducing the toxicity of zinc to one-sixteenth at most. The hypothesis put forward is that interior physiological modifications are responsible for the observed differences in growth rate, the cell processes being so affected as to bring about different results on cellular growth—i.e. that where mixtures of salts are concerned growth rate represents the physiological sum of oppositely acting stimuli or of antagonistic protoplasmic changes.
[46]
Kanda (1904) found that peas were stimulated in dilute solutions of zinc sulphate in the absence of nutrients, the optimum concentration being between ·00000287% and ·000001435% (about 1 in 34,840,000 and 1 in 69,700,000), higher concentrations being poisonous when the solutions were changed every four days. Jensen (1907) stated that he obtained no stimulation at all with water cultures, even in a solution as dilute as n/100,000 (about 1 in 1,239,000), but he suggested that it was quite possible that in proper concentration the zinc sulphate might prove to be a stimulant.
Javillier (1910) grew wheat in nutritive solutions with quantities of zinc salts containing from 1/5,000,000–1/250,000 zinc, and found that the dry weight of the plant was increased in so far as the stems and leaves were concerned, though it remained uncertain whether a similar increase occurred in the grain.
A consideration of the Rothamsted experiments shows that up to the present time there is no conclusive evidence that zinc sulphate acts as a stimulant to barley grown in water cultures. As a general rule the growth of those plants with 1/5,000,000 ZnSO4 approximates closely to that of the controls. Beyond this the growth varies in different experiments. In some cases lower concentrations from 1/5,000,000 to 1/50,000,000 seem to cause some slight improvement in comparison with the normal, indicating a possible stimulus, but this improvement is not at all well marked. In other cases these great dilutions are apparently indifferent, neither a poisonous nor a stimulative action being exerted on the growth of the plant (Fig. 6). With peas some increase has been obtained with 1/20,000,000, and although the rise is only slight, yet it is possible that it may indicate the setting in of a stimulus which would make itself more strongly felt with still weaker concentrations (Fig. 7).
While Jensen denied stimulation in wheat grown in water cultures even when the solutions were as dilute as n/100,000 zinc sulphate, yet he found increase of growth with the same plant in artificial soil (quartz flour) to which much stronger solutions of zinc sulphate, from 5n/10,000–n/10,000, had been added.
Nakamura (1904) dealt with a few plants of agricultural importance, adding ·01 gram anhydrous zinc sulphate to 2300 grams air-dried soil.[47] The marked individuality in the response of the various plants to the poison is very striking. Allium showed signs of increased growth throughout; Pisum was apparently improved in the early stages of growth, but when the dry weights were taken at the end of the experiment no increase manifested itself in the weights of the plants treated with zinc; with Hordeum the same quantity of zinc exercised a consistently injurious action. These results with peas and barley corroborate those obtained in the Rothamsted experiments with water cultures in that zinc sulphate proved to be less toxic to peas than to barley.
Kanda found that both peas and beans when grown in soil as pot cultures were improved by larger quantities of zinc sulphate than when they were treated as water cultures—a result in full accordance with current knowledge.
Wheat is evidently peculiarly sensitive to the effects of zinc compounds under differing conditions. Javillier (1908 c) pointed out that while wheat is very susceptible to the toxic action of zinc, yet it can benefit by the presence of sufficiently small quantities of the compounds of the metal. Rice is another cereal that is said to respond to the action of zinc sulphate, as Roxas, working in pot cultures with soil both with and without the addition of nutritive salts, obtained an acceleration of growth on the addition of m/1000 zinc sulphate, a quantity so remarkably great that it might be expected to act as a toxic rather than as a stimulant.
With phanerogams the zinc question is not only concerned with the effect of the metal upon germination, but also with its effect upon the later growth of the green plants, and on the physiological functions involving the construction of substances at the expense of mineral elements and the carbon dioxide of the air. Javillier holds that the indications are that zinc would prove to be profitable if applied to crops as a “complementary” manure.
Dandeno (1900) applied zinc sulphate in drops to the leaves of Ampelopsis, and found that the solution was not all absorbed by the leaf, but that a slight dark ring of a yellow colour was produced, and he was induced to think that some local stimulation was produced if the salt was presented in sufficient dilution.
Klopsch (1908) discussed the effect on plant growth of zinc derived from industries producing zinc fumes. Zinc oxide from the fumes is deposited on the leaves, and Klopsch stated that the rain and dew containing[48] dissolved zinc compounds find entrance to the tissues by way of the stomates and work injury to the plants. Against this, however, it must be remembered that these same fumes also contain other substances which are admittedly harmful to plant life, and so the deleterious effect may be partly or even chiefly due to these substances rather than to the zinc. Yet it is probable that at least some of the depreciation is due to the zinc. Treboux (1903) tested the effect of zinc sulphate on shoots of Elodea canadensis. If the shoots were placed in n/100,000 (= ·000016%) zinc sulphate no reduction of assimilation (as observed by counting the number of oxygen bubbles emitted per minute) took place, and replacement in water apparently had no effect either way. When however the shoots were placed in (1) water, (2) ·00008% zinc sulphate, (3) fresh ·00008% zinc sulphate, (4) water again, it was found that while the first solution of zinc sulphate had apparently no effect on assimilation, yet during the second immersion a gradual reduction in assimilation set in, which reduction was continued after the return to pure water, so that the toxic action of the zinc sulphate upon the shoots was clearly demonstrated.
Among the fungi, one species stands out in special prominence on account of the great amount of work that has been done on it with regard to its reactions to zinc salts. Aspergillus niger = Sterigmatocystis nigra van Tgh was used as a test plant by Raulin (1869), who evidently considered that zinc was an essential primary constituent of the food solutions of the fungi, ·07 parts zinc sulphate being added to each 1500 parts of water. In his experiments he tested (1) ordinary nutritive solution, (2) nutritive solution with various salts added, as zinc sulphate, (3) nutritive solution and salts (as 2) and also powdered porcelain. (2) gave a crop of Aspergillus about 3·1–3·5 times better than (1), while (3) was even better still. Sulphate of iron also proved stimulating in its action, but Raulin stated that zinc cannot replace iron, as both are essential.
Ono (1900) determined the relation between the weight of the mould crop in grams and the quantity of sugar used up in the presence of varying amounts of zinc sulphate. The amount of sugar used was always greater in the crops with ·0037–·0297% zinc sulphate by weight than in the control crops, indicating a stimulation caused by zinc.
[49]
Richter (1901) carried out rather similar experiments. When grown in solutions without and with 1/700,000 gram molecule zinc sulphate the dry weights of the mould were practically the same for the first two days, then the dry weight of the zinc crop shot ahead for a day or two, a depression setting in on the fifth day. Without zinc a less increase took place, and a similar drop was noticeable about the sixth day. The conclusion drawn is that the stimulation due to the zinc occurs chiefly in the first few days and also that the rise in the sugar consumed is more rapid at first with the moulds treated with zinc. Concentrations above 1/600 are harmful, but in weaker solutions zinc is a definite stimulant.
Coupin (1903) re-investigated some of Raulin’s work under more antiseptic conditions in order to see what substances were really needed by the mould and whether certain elements declared essential were really so. He concluded that iron and zinc are of no use in the nutrition of Sterigmatocystis nigra, but that the zinc retards the development of mycelium when food is abundant, killing it if it is badly nourished. This denial of stimulation was controverted by Javillier (1907) who re-tested Raulin’s solution with extreme care, growing Sterigmatocystis in
The ratio of crops a/b varied from 2·3–3·1 in four experiments, vindicating the favourable action of zinc. With regard to the optimum value for zinc the mould seemed to be perfectly indifferent to the presence of medium quantities but very sensitive to extremes, the maximum weights being reached in dilutions between 1/10,000,000 and 1/250,000, while quantities above 1/25,000 were toxic in their action. At a dilution of 1/50,000,000 stimulation was still evident, though in a less degree than with the optimal concentrations.
Javillier maintains that zinc is fixed by the fungus, the whole of the zinc present in dilute solutions being taken up, only part being utilised in stronger solutions. The value of accordance between the quantity of zinc fixed and the quantity supplied decreases rapidly with increase of concentration. Sterigmatocystis is able to fix without harm a quantity of zinc equal to more than 1/1100 of its weight. Zinc is regarded as a catalytic element, as essential to the well-being of the plant as are the more obvious nutrients, carbon, sulphur, phosphorus, &c., in spite of the minute traces in which it occurs.
[50]
A few tests on yeasts made by Javillier showed that with vegetative yeasts zinc has a specific action, a consistent increase occurring in the amount of yeast formed and in the amount of sugar consumed as the quantity of zinc increased from 0–1/10,000,000–1/10,000. With ferment yeast, however, zinc exerted no appreciable action. These results lend force to the conclusion of Richards (1897) who carried out experiments on fungi with various nutritive media with the addition of certain salts of zinc, nickel, manganese, iron, &c. He considered that his general results showed that the fact of a chemical stimulation of certain metallic salts upon the growth of fungi is established, although it must not be considered without further investigations that all fungi react in the same degree to the same reagent.
As matters stand at the present day, it appears that it is still uncertain whether higher plants grown in water cultures are susceptible to stimulation by zinc salts. If a stimulus does exist, it must be at exceedingly great dilutions, but further evidence is needed. In soil cultures, however, the fact of increased growth seems to be more firmly established, certain species responding to zinc salts when used as manure, though no increase has been obtained with other species. It must always be remembered that the action may be an indirect one. The soil is very complex in its constitution, and it is impossible to determine the exact action of the added poison upon it, so that a stimulating effect need not necessarily be due to a direct action of a substance upon the plant, but it may be the result of more favourable conditions for life induced by the action of the substance upon the soil.
Among the fungi the stimulation of Aspergillus niger by minute traces of zinc compounds seems to be well proved, though again it does not necessarily follow that all fungi will react in the same way to zinc.
[51]
The occurrence of arsenic as an occasional constituent of plants has been recognised for many years. Chatin (1845) found that if a plant were supplied with arsenical compounds at the roots arsenic was absorbed, but that it was distributed unequally to the various tissues. The greatest accumulation of the element was in the floral receptacle and the leaves, while it was scarce in the fruits, seeds, stems, roots and petals. E. Davy (1859) commented on the presence of arsenic in plants cultivated for food. He grew peas in pots and watered them for a short time with a saturated aqueous solution of arsenious acid, the application being then discontinued. The plants, apparently uninjured by the treatment, flowered and formed seeds. On analysis arsenic was readily detected in all parts of the plant, including the seeds. Other analyses revealed the presence of the element in cabbage plants (from pots) and turnips (from field), both of which had been manured with superphosphate containing some amount of arsenic. This absorption of arsenic by the roots of plants was further established by Phillips (1882).
Various physiological workers have pointed out that this element is frequently or usually present in animal tissues. Cerný (1901) reached the general conclusion that minimal traces of arsenic can occur in animal organisms, but that these play no part in the organism and indeed are not constant in their occurrence. Bertrand (1902) established its presence in minute quantities in the thyroid glands of the ox and pig, hair and nails of the dog, and the feathers of the goose. Gautier and Clausmann (1904) realised the constant presence of arsenic in human tissues and recognised that it must inevitably be introduced into the body with the food. This led them to estimate the arsenic present in various animal and vegetable foods, some of their results being given in the following table.
[52]
Arsenic per 100 parts fresh substance in µ gr. (= thousandth part of a milligram)[8].
| Wheat | (Victoria—complete grain) | ·7 | |
| „ | (from Franche Comté) | ·85 | |
| White bread | ·71 | ||
| Whole green cabbage | ·2 | ||
| Outside leaves of cabbage | ·0 | (absent) | |
| Green haricots | ·0 | „ | |
| Turnip | ·36 | ||
| Potatoes | 1·12 | ||
Arsenic was also found in wine and beer and in considerable quantities in sea water and various kinds of salt. Since it cannot be found in some things even in the least traces, the authors conclude that it is incorrect to say that the element is always present or that it is essential to all living cells.
S. H. Collins (1902) found that barley is able to absorb relatively large quantities of arsenic. The plants were grown in pots on soil which originally contained a certain amount of the substance, and various combinations of arsenic acid, arsenious acid and superphosphate were added. Particulars and details are not given by the author, except that arsenic was detected by Reinsch’s test in the grains from all the experimental pots, and in one case (not specified) in the upper and lower halves of the straw and in the threshed ears. The analyses of the soil at the close of the experiments showed the presence of 7–22 parts arsenious acid per million.
Wehmer (1911) quotes references to the occurrence of arsenic in Vitis vinifera. The element was detected in the ash of the must and its presence was attributed to treatment of the plants with arsenical compounds. In this connection it is interesting to note the observation of Swain and Harkins (1908), who, while acknowledging the absorption of arsenic from the soil by many plants, yet indicate that in the case of those plants which are exposed to smelter smoke the arsenic is deposited on the vegetation, and is not absorbed by the latter from the soil.
The poisonous action of arsenic on plants has long been recognised. Chatin (1845) gave accounts of tissues poisoned by strong arsenical[53] solutions. Nobbe, Baessler and Will (1884) carried on water culture experiments with buckwheat, oats, maize and alder, and found that arsenic was a particularly strong poison for these plants. When small quantities of arsenious acid (As2O3) were added to the food solutions, growth was measurably hindered by a concentration of 1/1,000,000 As (reckoned as As). The element only appears in plants in very small quantity and can never be detected in notable quantities. The aerial organs show the effect of arsenical poisoning by intense withering, interrupted by periods of recovery, but eventually followed by death. It was also found that if plant roots were exposed to the action of arsenical solutions for a short period, say ten minutes, and then were transferred to normal food solutions, the action of the poison was delayed, but eventually hindering of growth or death occurred, according to the strength of the poison used in the first solution.
At the same time that Nobbe, Baessler and Will were establishing the great toxicity of the lower oxide of arsenic, Knop (1884) was carrying the matter a step further by comparing the action of arsenious and arsenic acid and their derivatives on plant growth. He established the fact that while arsenious acid is a strong poison for maize plants, arsenic acid in small quantities is not toxic to the roots and that the plants can produce flowers and fruit in its presence. Arsenic acid applied as potassium arsenate proved to be harmful to young maize seedlings if the solutions contained ·05–·1 gm. arsenic acid per litre (= 1/–2/20,000 arsenic acid). If however the plants were allowed to form 10–15 leaves in a pure food solution and then when strongly rooted were transferred to a solution of ·05 gm. arsenic acid per litre, they were found to grow strongly and develope big healthy leaves. Careful measurements indicated that the development is unchecked by the addition of the poison, though arsenic was determined in the ash of the treated plants.
Stoklasa (1896, 1898) tested the effect of arsenic compounds on plant growth with special attention to their comparative relation to phosphoric acid. He corroborated Knop’s statement as to the greater toxicity of arsenious acid and arsenites in comparison with arsenic acid and arsenates, stating that 1/100,000 mol. wt. arsenious acid per litre causes definite trouble in plants, while with arsenic acid 1/1000 mol. wt. per litre first shows a noticeable toxicity. Water culture experiments were made with and without phosphoric acid, in each case with and without the addition of arsenic and arsenious acid. It was found that the arsenic acid was unable to replace the phosphoric acid, the plants decaying in the flower in the absence of the latter. In the complete absence of[54] phosphoric acid, arsenic acid causes a strong production of organic substances up to the flowering time. The following figures were obtained with maize:—
| ·002 gm. | As2O3 | with P2O5 | 2·84 gm. dry wt. | |||
| ·005 gm. | „ | „ | „ | 2·37 | „ | |
| ·01 gm. | As2O5 | „ | „ | 67·32 | „ | |
| ·40 | „ | „ | „ | „ | 64·13 | „ |
| ·03 | „ | As2O5 | without P2O5 | 39·98 | „ | |
| ·07 | „ | „ | „ | „ | 42·13 | „ |
| normal solution | „ | „ | 12·93 | „ | ||
| „ | „ | with | „ | 65·84 | „ | |
Comparative experiments with the two arsenical oxides showed that varying times were required to kill different plants. Young seedlings were brought into solutions containing 1/10,000 mol. wt. arsenious acid (= ·019 gm. As2O3 per litre) and the plants died in a very short time.
| Hordeum distichum | 46 hours | ||
| Polygonum Fagopyrum | 84 | „ | |
| „ | Persecaria | 90 | „ |
With ten times the strength of arsenic acid (1/1000 mol. wt. = ·23 gm. per litre) the plants took much longer to kill.
| Hordeum distichum | 24·5 days | ||
| Polygonum Fagopyrum | 40 | „ | |
| „ | Persecaria | 42 | „ |
Various experiments have been carried on at Rothamsted with peas and barley. With arsenious acid on barley a depressing influence is manifest even at a concentration of 1/10,000,000, while no growth at all is possible with 1/10,000 and upwards. Apparently the toxic action on the root ceases at a higher strength than on the shoot, as with 1/1,000,000 and less the dry weight of the root remains practically constant. At this same strength the shoots look better than the controls, but this is not apparent in the dry weights (Figs. 9 and 10). With peas the depression is again evident to 1/10,000,000, but the plants are more sensitive to the higher concentrations, as no growth can take place in the presence of 1/250,000 arsenious acid (Fig. 11). A striking difference is observed with arsenic acid on barley, as apparently this does not act as a toxic even with such comparatively great concentrations as 1/100,000, though possibly the shoot is slightly depressed by this strength (Fig. 12).
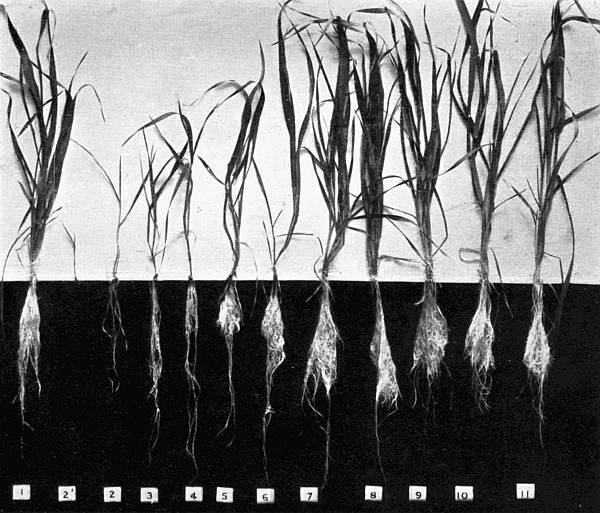
Fig. 9. Photograph showing the action of arsenious acid on barley in the presence of nutrient salts. (March 16th–May 9th, 1911.)
| 1. | Control. | ||
| 2*. | 1/50,000 | arsenious | acid. |
| 2. | 1/100,000 | „ | „ |
| 3. | 1/150,000 | „ | „ |
| 4. | 1/200,000 | „ | „ |
| 5. | 1/250,000 | „ | „ |
| 6. | 1/500,000 | „ | „ |
| 7. | 1/1,000,000 | „ | „ |
| 8. | 1/5,000,000 | „ | „ |
| 9. | 1/10,000,000 | „ | „ |
| 10. | 1/25,000,000 | „ | „ |
| 11. | 1/50,000,000 | „ | „ |
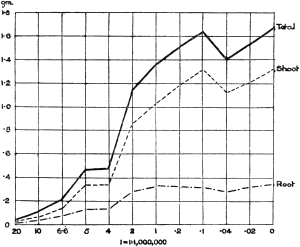
Fig. 10. Curve showing the mean value of the dry weights of ten series of barley plants grown in the presence of arsenious acid and nutrient salts. (March 16th–May 9th, 1911.)
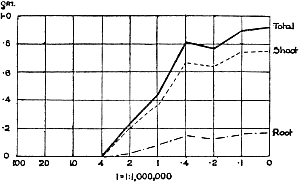
Fig. 11. Curve showing the mean value of the dry weights of ten series of pea plants grown in the presence of arsenious acid and nutrient salts. (June 8th–July 21st, 1910.)
[55]
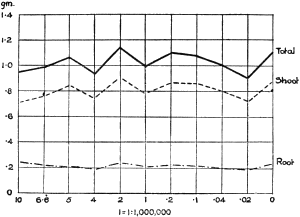
Fig. 12. Curve showing the mean value of the dry weights of ten series of barley plants grown in the presence of arsenic acid and nutrient salts. (Feb. 28th–April 24th, 1911.)
With sodium arsenite the dilutions were carried further, to 1/250,000,000, but this still depressed barley to some extent (Fig. 13). With peas the results vary somewhat in the different tests, the depression with 1/2,500,000 and less being usually slight, though occasionally it is much more strongly marked (Fig. 14). In a single series with sodium arsenate barley was apparently unaffected by a concentration[56] of 1/1,000,000, but from this point down to 1/250,000,000 a constant depression showed itself, which was paralleled by a similar depression in the sodium arsenite series from 1/25,000,000 to 1/250,000,000, the curves grading downwards instead of up towards the normal. With peas sodium[57] arsenate has little or no action, though it is just possible that the rather irregular curves indicate a very slight depression below the normal throughout.
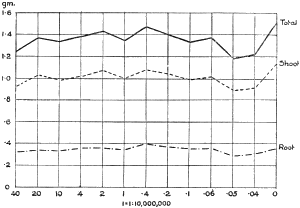
Fig. 13. Curve showing the mean value of the dry weights of ten series of barley plants grown in the presence of sodium arsenite and nutrient salts. (Feb. 10th–April 18th, 1913.)
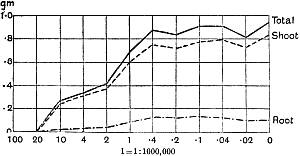
Fig. 14. Curve showing the mean value of the dry weights of ten series of pea plants grown in the presence of sodium arsenite and nutrient salts. (June 27th–Aug. 10th, 1911.)
Comparatively few tests seem to have been made as to the action of arsenical solutions in sand cultures. Stoklasa (1898) repeated his water culture work, using sand as a medium, and found analogous results by the two methods, i.e. that arsenites are far more toxic than arsenates, and also that the degree of toxicity of a salt varies with the plant to which it is applied, as was shown by the fact that different plants lived for varying times when treated with similar strengths of solution.
Daubeny (1862) watered barley plants with a solution of arsenious acid, 1 ounce in 10 gallons, five times in succession, and found that the crop arrived at maturity about a fortnight earlier than the untreated part of the crop, though the amount harvested was rather less. With turnips four waterings had no effect upon the time of maturity, but again the crop was slightly decreased. The analyses made indicated that no arsenic was taken into the tissues, but that it merely adhered to the external surfaces.
[58]
Gorup-Besanez (1863) mixed 30 grams arsenious acid with 30·7 litres[9] soil, growing two plants on this quantity of earth. Most of his experimental plants (Polygonum Fagopyrum, Pisum sativum, and Secale cereale) developed normally, but Panicum italicum died soon after the plants appeared above the surface, the leaves being very badly coloured. Analyses by Marsh’s test showed no trace of arsenic in 20 grams dry matter from Secale cereale, but in 148 grams Polygonum Fagopyrum the presence of arsenic was evident, though the mirror formed was weak. With such a large proportion of arsenious acid in the soil it seems hardly conceivable that the plants were not injured to some extent, and also it is probable that with more careful analyses arsenic would have been detected in those instances in which its presence was denied. Yet it must be remembered that Davy (1859) had treated pea plants in pots with a saturated solution of arsenious acid for a short time and had stated that the plants were uninjured. Thus both Gorup-Besanez and Davy concur in the opinion that Pisum sativum is indifferent to relatively large quantities of arsenious acid when presented in the soil, whereas the Rothamsted experiments show that in water cultures the plant is extremely sensitive even to minute traces of the substance. It is possible that the arsenic in the solution added to the soil enters into combination with other substances, forming insoluble compounds, thus being removed from the sphere of action and rendered unable to affect plant life. If this be so, the apparent immunity of certain plants to arsenious acid is explained. F. C. Phillips (1882), in his experiments on various flowering plants, such as geraniums, coleas and pansies, found that compounds of arsenic in the soil exercised a distinct poisoning influence, tending, when present in large amount, to check the formation of roots, so that the vitality of the plant was so far reduced as to interfere with nutrition and growth, or even to kill it outright. He also stated that traces of arsenic were found in all the plants grown upon the poisoned soil.
In this connection it is interesting to note that a certain proportion of arsenic is frequently present in the superphosphate used as manure. In view of the known toxicity of arsenical compounds to plant life the question arose as to whether superphosphate manuring would exercise a detrimental influence on account of its arsenic content. Experiments carried out by Stoklasa (1898), however, indicate that there is not sufficient arsenic in maximum doses of superphosphate to exercise a toxic action in the field.
[59]
The physiological action of arsenic compounds on plant life early attracted the attention of investigators. Chatin (1845) put forward some rather curious and unexpected considerations with regard to this action. He stated that the effect of arsenic on plant growth is determined more by the constitution and temperament of individual plants than by their age, and that apparently difference in the sex of plants is of no significance. The chief determining agent, however, is the species, and Chatin found that as a general rule Cryptogams are more sensitive than Phanerogams, and Monocotyledons than Dicotyledons, as is shown by the fact that under treatment the former perish first. Some extreme exceptions exist, though, as Mucor mucedo and Penicillium glaucum will grow on moist arsenious acid, whereas leguminous plants are killed by an arsenical solution in a few hours. Chatin held the view that elimination of the poison succeeded its absorption, and that this elimination is complete if the plant lives long enough. Here again the species exerts a great influence on the excretory functions of the plants. Lupins and Phaseolus are presumably able to eliminate in six weeks all the arsenious acid they can absorb without dying. Most Dicotyledons need 3–5 months, while Monocotyledons retain traces of poison for six months after its absorption. Lichens are said to eliminate it more slowly still. Again, woody species are longer in freeing themselves than herbaceous, and young plants carry out the elimination more easily than old plants. The excretory function is influenced by other physiological factors such as dryness and season. The toxic effects and elimination are supposed to act inversely and parallel, the absorbed arsenious acid combining with alkaline bases, making a very soluble salt which is excreted by the roots. Calcium chloride is given as the antidote to arsenious acid, all soluble acid being “neutralised” by it. This view of the elimination of arsenic apparently did not gain much support, as no further references to the matter have so far come to light. In view of the work of some modern investigators (Wilfarth, Römer and Wimmer) on the excretion of salts by plant roots, the idea may prove of fresh interest. Chatin also found that moving or still air influenced the working of the poison, indicating that the external physical conditions affect the toxic action considerably. Nearly forty years later Nobbe, Baessler and Will found that, if transpiration were hindered by placing plants in a dark or moist room, it was possible to keep[60] the plants turgescent in arsenic solutions for a long time without thereby increasing the toxic effect later on. The poisonous action proceeds from the roots, of which the protoplasm is disorganised and the osmotic action hindered. Finally, in the presence of sufficient of the poison, the root dies without growth.
Stoklasa (1896, 1898) again found that phanerogamic plants can withstand arsenic poisoning for some time in the dark or in CO2-free air, provided that glucose is given in the food solution. The arsenic poisoning is at its maximum during carbon assimilation by means of chlorophyll. The toxic action of arsenious and arsenic acids, especially in phanerogams, is due to injury to the chlorophyll activity. The destruction of the living molecule is far more rapid in the chlorophyll apparatus than in the protoplasm of the plant cell.
Thus it seems that the physiological cause of the toxicity of arsenic is partly a direct action on the root protoplasm, whereby its osmotic action is hindered, and partly a detrimental action upon those functions which are directly concerned with the elaboration processes of nutrition.
In view of the great toxicity of arsenic to plants in their various stages of development, one would naturally expect to find a similar action with regard to the germination of the seeds. Davy (1859) casually mentioned cases in which watering with arsenical solutions or dipping seeds in arseniated water prevented germination. Heckel (1875) found that arsenious acid checks germination and kills the embryo at relatively feeble doses, ·25 gm. to 90 gm. water.[10] Guthrie and Helms (1903–4–5) carried out a systematic series of experiments to test the effect of arsenic compounds upon different farm crops. Various amounts of arsenious acid were added to soil in pot experiments, and the seeds of the several crops were then sown. With barley, wheat and rye 0·10% arsenious acid had little or no effect on germination, while an increase in the poison exercised a retarding action. Maize could withstand 0·40% arsenious acid without retardation being perceptible. The aftergrowth with the different crops varied considerably. The wheat plants with 0·10% arsenious acid grew all right at first, but later on they developed weakly. The toxic action increased rapidly as the strength of the poison rose in the different[61] pots. Barley proved even more sensitive than wheat, for even 0·05% arsenious acid affected the growth adversely. After a time the plants with 0·05–0·06% recovered and grew strongly, though not so well as the controls, but those with 0·10% practically died off. Rye behaved in the reverse way from wheat. The plants with 0·10% were slightly checked at first but later recovered and made growth quite equal to the check plants. Growth was stunted with 0·20% arsenious acid, and the plants were killed with 0·30%, so that rye is far less sensitive than barley. With maize the growth was slightly affected with 0·05% As2O3, and increasingly so with greater quantities. It was also found that the action of 0·8% As2O3 was strongly adverse to the germination of all plants, and that above this strength germination was altogether prevented.
The results show very clearly how impossible it is to draw any general conclusions with regard to the action of arsenic compounds on plants, as they emphasise the strong individuality of the species in their reaction.
The question of stimulation due to arsenic does not seem to have engaged the attention of investigators to any extent. Water culture experiments at Rothamsted have so far yielded negative results, and no stimulation has yet been obtained with any plant, with the possible exception of white lupin with sodium arsenite. In a single series a stimulus was suggested, beginning to make itself felt at 1/500,000, rising to an optimum at 1/10,000,000. No stress can be laid on this result, as it is never safe to draw any certain conclusions without several repetitions of the same experiment. With arsenic acid on barley a possible stimulus is sometimes indicated to the eye, the plants being fine and of a particularly healthy dark colour, but this is not corroborated by the dry weights. Additional tests were made with peas and barley, treated with sodium arsenite and arsenate, the dilutions being carried down to 1/250,000,000, but no evidence of stimulus was obtained, so that it hardly seems possible that arsenic can act as a stimulative agent for these two plants when grown in water cultures. It had been thought that the failure to find a stimulation point hitherto might be due to the too great concentration of the toxic substance rather than to the actual inability of the poison to stimulate, but this hypothesis must now be dismissed so far as these plants are concerned.
[62]
Loew (1883) was sceptical concerning the specific toxicity of arsenic for plant protoplasm. He was convinced that arsenic and arsenious acid were poisonous to algae, not because of their specific character as arsenical compounds, but because of their acid nature, algae being peculiarly sensitive to any acid, and he maintained that these substances were not more poisonous than vinegar or citric acid. He placed various species of Spirogyra in solutions of ·2 gm. potassium arsenate per litre water (1/5000), and found that the algae grew well without making any abnormal growth in a fortnight, showing hardly one dead thread. Some of this alga was then transferred to a 1/1000 solution of potassium arsenate. This suited it excellently and it increased and the appearance under the microscope was very fresh and strong, which was attributed more to the potash than to the arsenic acid. Loew maintained that for the lower animals and for many of the lower plants arsenic in the form of neutral salts is not a poison. When the differentiation of the protoplasm into certain organs reaches a specific degree in the higher plants, then the poisonous action of the arsenic compounds comes into play.
Knop (1884) found that certain unicellular green algae grew luxuriantly in a neutral solution supplied with potassium arsenate. Bouilhac (1894) concerned himself chiefly with the possibility of the replacement of phosphates by arsenates. He recognised that the influence of arsenic is not the same on all species of plants, so he confined his attention to certain of the algae. Stichococcus bacillaris Naegeli was found to live and reproduce itself in a mineral solution containing arsenic acid. Even in the presence of phosphoric acid the arsenic acid favours growth, the best dose being about 1/1000. The arsenic acid is capable of partly replacing phosphoric acid. Other species of algae, Protococcus infusionum, Ulothrix tenerrima, and Phormidium Valderianum invaded the original culture of Stichococcus from the atmosphere, but with no arsenic or phosphoric acid their development was poor. The jars with arsenic compounds were invaded by still more species which grew strongly. Under these conditions it is evident that these algae are capable of assimilating arsenic, and the addition of arsenic acid to a solution free from phosphoric acid is sufficient to enable these algae to live satisfactorily, the arsenates in this case replacing the phosphates. Ono (1900) found that algae are favourably influenced by small doses of[63] poisons, the optimal quantity for algae being lower than that for fungi. Protococcus showed a possible stimulus when grown in concentrations of potassium arsenate varying from ·00002–·0005%. This possible stimulus is interesting in view of the failure to observe stimulation in higher plants by minute traces of arsenic.
The effect of arsenic on fungi is of special interest in that it has a direct bearing upon hygienic and commercial interests. Gosio (1892, 1897, 1901) found that certain of the fungi, Mucor mucedo and Aspergillus glaucum, will grow on various arsenic compounds and exercise a reducing influence on them. These moulds attack all oxygen compounds of arsenic including copper arsenite, and develope arsenical gases. Sulphur compounds of arsenic are not influenced by these fungi. The same moulds would, if cultivated in soil containing arsenic, develope hydrogen arsenide. Penicillium glaucum has such a strong and definite action on arsenic compounds that he states that there is no doubt of the possibility of poisoning by arsenical gas in a room hung with paper containing arsenic. The compounds are so extraordinarily potent that if a mouse is placed in a vessel in which the mould is strongly developed in the presence of arsenic, it dies in a few seconds. Penicillium brevicaule uses the element in its development as a food substance. If material containing arsenic is placed in contact with dead fungi no reaction occurs. The life activity of the mould is evidently necessary for the reaction by which the arsenic-containing gases are liberated. Csapodi (1894) put forward the earlier results of Gosio and noted that the so-called arsenical fungicides do not only fail to kill the mould fungi but actually favour their development. This action explains why wallpaper containing arsenic is so disadvantageous in a room. Abba (1898) severely tested Gosio’s method of detecting arsenic by means of growths of Penicillium brevicaule, whereby arsenic gases are liberated, vindicating the method completely, and establishing the test as an exceptionally delicate one. Segale (1904) applied the same method to the detection of the presence of arsenic in animal tissues.
Ono (1900) grew Penicillium cultures with solutions of potassium arsenate and found no important differences either of depression or stimulation. Orlowski (1902–3) stated that small doses of arsenic (1/1000–1/100% Sodium arsen—[11]) stimulate the growth of[64] Aspergillus niger, larger doses up to 1⁄8% retard growth, while 1⁄6% kills. Spores of the fungus taken from soil containing arsenic are said to possess an immunity against arsenic, in that they germinate in the presence of an arsenic content which rapidly kills control fungi. This immunity is not specific for arsenic, but extends also to other poisons. The chemical composition and water content are not altered.
The toxic effect of arsenic upon higher plants is much more marked with arsenious acid and its compounds than with arsenic acid and its derivatives. No definite evidence of stimulation has yet been obtained with any arsenic compound, however great the dilution at which it is applied. With certain algae a stimulus may occur, and it is possible that arsenic acid is capable of replacing phosphoric acid to some extent under certain conditions. With fungi the toxic effect of great concentrations is marked with certain species, but there are others which are capable of living happily on arsenical compounds and of liberating highly poisonous arsenic gas.
[65]
The first claim to the discovery of boron in plants was put forward in 1857 by Wittstein and Apoiger, who carried out investigations on the Abyssinian Saoria (seeds of Maasa or Maessa picta, N.O. Primulaceae[12]). In the course of analyses a crystalline mass was obtained which was found to contain chlorine, phosphoric acid, lime, and boric acid. The discovery apparently attracted little attention and for about another thirty years the matter was again allowed to sink into oblivion. Then it came to the front again, and from 1888 onwards one investigator after another demonstrated the presence of boron in various plants.
In 1888 Baumert detected boron in French, German, and Spanish wines without exception, while E. O. von Lippman (1888) demonstrated it in sugar must and also in the leaves and root of the sugar beet. In the latter case the reactions were so definite that the presence of more than a minimal amount of boric acid was conjectured.
Crampton (1889) tested various fruits, but while he found boron in every part of the watermelon, he could get no reaction with apples or with certain samples of sugar cane. He predicted, however, that the occurrence of boron would prove to be more general in the plant kingdom than had previously been supposed. The next year (1890) Hotter extended the work on fruits, testing for boron in the fruits, leaves, and twigs of certain plants, and finding it in the apple, pear, cherry, raspberry, fig, and others. His results indicated that fruits are relatively rich in boron. Later on (1895) Hotter carried his experiments further, and he stated that stone fruits are richer in boric acid than are berries and pomes. The accumulation of boron is in the fruit itself, the other parts of the plant containing little. The quantities of boric acid found in the ash of the various fruits ranged from ·58% in the “Autumn[66] Reinette” apple to ·06% in figs. Bechi had previously (1891) detected boron in the ash of figs, love-apple, and rubus fruits from Pitecio, but he attributed this to the presence of boric acid or borates in the soil at the place.
Passerini (1891) found traces of boron in the stems of chickpea plants, while in 1892 Brand determined boric acid in the ash of beer. In consequence of this various samples of hops were ashed without the addition of any alkali, and then the ash was distilled with sulphuric acid and methyl alcohol. When tested all the hops showed relatively large quantities of boric acid in comparison with beer, hence he argued that the boric acid in beer is derived from the hops. Boron was discovered in various parts of the hop plant—in the clusters, leaves, pedicels, and stems.
Jay (1895) analysed many plants and plant products grown in various soils and waters, and arrived at the conclusion that boron is of practically universal occurrence in the plant world. Of all vegetable liquids wines are the richest in this constituent, the amount varying from ·009 gram to ·33 gram per litre. He confirmed Hotter’s statement as to the richness of fruits in this substance, finding from 1·50–6·40 grams in 1 kgm. of ash. Chrysanthemums and onions, amongst other plants, are well off in this respect, containing 2·10–4·60 grams per kgm. of ash. Jay also found that the plants vary in their capacity for absorbing boric acid, those which do so the least easily being Gramineae (as wheat, barley, rice), mushrooms and watercress, the quantity in these plants never exceeding ·500 grams per kgm. of ash.
Of all the workers upon boron, Agulhon has done the most to extend and concentrate our knowledge of the subject. He used the most refined, up-to-date methods for the detection and estimation of boric acid, and so determined its presence in many plants, including angiosperms, gymnosperms, ferns, algae, and fungi. Tobacco is so rich in boron that it can be detected in the ash of one cigarette. Among the plants tested, the highest percentages of boric acid were found in Betula alba (1·175% of ash) and Laminaria saccharina (·682% of ash), the lowest in Cannabis sativa (·123% of ash). Generally speaking annual plants and parts of plants seem to have the least boron in the composition of their ashes. In one and the same plant the durable parts like bark and wood are richer than the leaves, even in evergreen trees. He indicated that plants seem to have a great affinity for boron, as even when plants are grown on soils in which the boron is practically indetectable they always seem to extract an appreciable quantity of the element.
[67]
From the foregoing results it is evident that boron is very widespread in the vegetable kingdom, entering into the composition of many plants in all the great classes. A general impression obtains that its distribution is universal, and that it will ultimately prove to enter into the composition of practically every plant, as the scope of the analyses is widened and as methods of detection are improved. On the other hand, Agulhon is inclined to think that boron may be a “particular element,” characteristic of certain groups of individuals or of life under certain conditions. The series of individuals differ among themselves as to their particular needs of nutriment (in the widest sense) and doubtless each group has special need of particular elements, a need that is possibly correlated with morphological and chemical differences. It may well be that boron is one of these elements, associated with certain vital functions in a way as yet unexplained, though it may possibly be found to play some part in the formation of vascular tissues, since it is most abundant in bark and lignified parts.
Excessive quantities of boric acid are decidedly poisonous to plants, the action being well marked in water cultures. Knop (1884) found that free boric acid was poisonous in neutral food solutions when present at the rate of ·5 gram per litre, but he was not able to detect boron in the ash of the roots of the experimental plants. Archangeli (1885) placed seedlings of maize, white lupins, Vicia sativa and Triticum vulgare in solutions of boric acid varying in concentration from 1–·05%, with controls in spring water. In the latter case the development was normal, with 1% boric acid the plants were killed, while it was found that the weaker the solution (within the indicated limits) the stronger the root and shoot growth.
Hotter (1890) stated that it was known that 1/20,000 boric acid by weight was harmful to soy beans in nutritive solutions. He experimented with peas and maize, placing the seedlings first in distilled water, later in nutritive solutions. When the peas were nineteen days old they were transferred to nutritive solutions containing 1/1000–1/100,000 boric acid by weight per litre, and within three days the plants with 1/1000 showed signs of injury. Two days later all the plants showed[68] signs of poisoning in that, even with the weakest strengths, the lower leaves were flecked with brown, especially at the edges, while with the greater strengths the lower leaves were dead and the flecking had extended to the upper leaves. In eleven days from the start the plants with 1/1000 boric acid were completely dead, while the other plants showed more or less signs of poisoning. The dry matter and ash decreased steadily with the increase in the boric acid, while the boric acid per 100,000 parts of dry matter increased steadily from 8 to 557 parts. Similar experiments were carried on with potassium borate and with borax; the results showed that, weight for weight, borax is less toxic than potassium borate, which in turn is less toxic than boric acid, while at a strength of 1/100,000 there is little to choose between the three poisons. Similar results were obtained with maize; plants treated with boric acid or potassium borate yielded about 2300 parts boric acid in 100,000 parts dry matter. The general conclusion arrived at by Hotter was that the effect is not so much that of a general poisoning as of a bleaching of parts of the leaf, mere traces of boron being harmless. The cause of injury is local inhibition of assimilation and killing of roots in stronger concentrations. Increase of the strength of boron raises the toxicity until 1/1000 practically inhibits increase in dry substance. The boron was found to be fairly evenly distributed through sound and affected organs.
Kahlenberg and True (1896) worked with seedlings of Lupinus albus L., limiting their experiments to those of 15–24 hours in duration. Various combinations of boron and other substances were tested. With boric acid alone 2⁄25 gram molecule per litre killed the plants, with 1⁄25 they were apparently just alive, while 1/100 and less had no injurious effect. Boromannitic acid was possibly more poisonous than the boric acid, while a combination of boric acid and cane sugar proved slightly less toxic. The short duration of these experiments limited their scope considerably, as with certain concentrations the toxic action would not become evident within the prescribed limits of time.
Agulhon (1910 a) worked with sterile nutrient solutions, and found that the higher strengths of boric acid hindered growth, 200 mg. boric acid per litre rendering growth impossible. He supported Hotter’s idea that the toxic action affects the roots and the formation of chlorophyll, and he stated that the plants are less green as the dose of boron increases, plants growing in doses of above 10 mg. per litre being yellowish. In other experiments he found that[69] at 100 mg. boric acid per litre life seems impossible for the plant. The roots seem to be more adversely affected by toxic doses than do the shoots. In control plants Agulhon determined the stem/root ratio as 6, with a little boron as 7, while the ratio rose to 13 as the dose of the poison increased to 50–100 mg. boron per litre.
The Rothamsted experiments show that boric acid is definitely poisonous to barley down to a strength of 1/250,000 (Fig. 15), the depressing effect frequently being evident at much smaller concentrations, while peas can withstand far more of the poison, the limit of toxicity being about 1/25–1/50 thousand (Fig. 16). With the greater strengths of poison the lower leaves of both barley and peas are badly damaged. In barley the leaves turn yellow with big brown spots, giving the leaves a curious, mottled appearance, while with peas the poisoning seems to begin at the tip and edge of the leaves, spreading inwards, without, however, showing the large spots as in barley. So far as chemical tests go at present, it is very probable that boron is deposited in the leaves in the same way as manganese, and that this is the cause of the degeneration. As with manganese, the lower leaves are attacked first, and the trouble spreads upwards, one leaf after another being involved. These observations fit in very well with those of Hotter, and the hypothesis of direct boron poisoning gains support from the fact that in dilutions which produce stimulation of the shoot the leaves show hardly any sign of dying off, even after prolonged growth in the solutions. With barley the effects of boron can be seen in the leaves[70] in concentrations as low as 1/2,500,000, and it may be significant that this is the point at which the depressant action of boric acid entirely ceases in many cases.
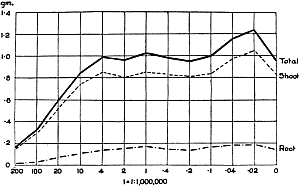
Fig. 15. Curve showing the mean value of the dry weights of ten series of barley plants grown in the presence of boric acid and nutrient salts. (May 1st–June 20th, 1911.)
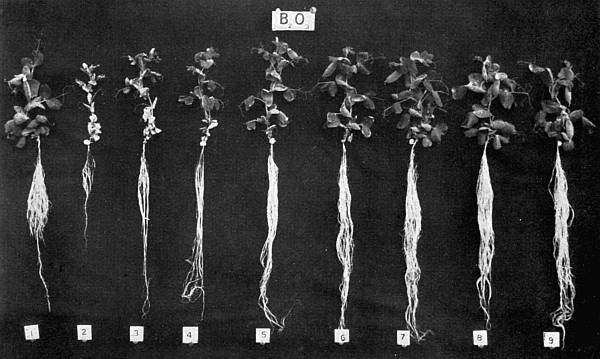
Fig. 16. Photograph showing the action of boric acid on pea plants in the presence of nutrient salts. (Sept. 30th–Dec. 20th, 1912.)
| 1. | Control. | ||
| 2. | 1/5,000 | boric | acid. |
| 3. | 1/10,000 | „ | „ |
| 4. | 1/25,000 | „ | „ |
| 5. | 1/50,000 | „ | „ |
| 6. | 1/100,000 | „ | „ |
| 7. | 1/250,000 | „ | „ |
| 8. | 1/500,000 | „ | „ |
| 9. | 1/1,000,000 | „ | „ |
Tests with white lupins gave no conclusive results, as for some reason it proved very difficult to get satisfactory plants in water cultures. When they are grown under such conditions the roots always tend to get more or less diseased and covered with slime, probably fungal in nature. In the presence of much boric acid the roots remain in a much healthier condition, which suggests that the acid has in this case a strong antiseptic action, and protects the roots. With high concentrations the lower leaves of the plant are badly affected, just as with peas and barley, turning brown and withering at an early date. Various experiments have been made with yellow lupins, but these again are very difficult to grow well in water cultures, as they are apt to drop their leaves for no apparent reason. Generally speaking, the evidence goes to prove that boric acid is toxic down to a concentration of about 500 parts in 25 million. It is difficult to get a true control with which to make comparisons as the plants without boric acid are encumbered with the slime on their roots, which naturally interferes with normal growth, while the plants in the presence of boric acid have the unfair advantage due to the probable antiseptic action of the boron. The effect of the boron poisoning is again evident in the dying off of the lower leaves, which become flaccid and drooping and finally drop off. The lupins grown with boron are very active in the putting forth of lateral roots, so much so that the cortex of the roots is split along the line of emergence of the laterals, which are very numerous and crowded.
Agulhon (1910 a) moistened 2 kgm. pure sand with 500 c.c. nutritive solution for each pot, and boron was added at the rate of 0, 0·1, 1, 10, and 50 mg. boric acid per litre of nutritive solution. Twenty wheat seeds were sown in each pot, and after twelve days the healthy plants in the first four pots were 6–8 cm. high, but those with the maximum amount of boron showed yellowish leaves only 3 cm. long. After three months’ growth the plants were harvested, when those with most boron were found to have died after making about 10 cm. growth. The toxic doses in sand proved to be weaker than those in water cultures, probably because evaporation from the surface of the sand caused concentration of the poisonous liquid.
[71]
Long before any experimental work was done with boron in water cultures, the poisonous properties of the substance were recognised with regard to plants growing in soil. Peligot (1876) grew haricots in porous earthenware pots, the plants being watered by rain and by solutions, each containing about 2 grams per litre of such substances as borax, borate of potassium, and boric acid, other pots receiving various fertilisers, as potassium nitrate, sodium nitrate, &c. This quantity of boron completely killed off the plants receiving it, whether it was applied as free or combined boric acid, while the fertilised plants completed their development well. On this account the deleterious action was attributed to the boric acid and not to the sodium or potassium base supplied. Peligot hinted at the improbability of a substance like boron, which is so poisonous to plants, being really innocuous to human beings when it is used as a preservative for foods.
Nakamura (1903) also found that borax is harmful in pot cultures if present in large quantities, 50 mg. borax per kgm. of soil exerting a very injurious influence, while even 10 mg. per kgm. did some damage. Agulhon (1910 c) found that the toxic doses of boric acid in soil cultures approached those in nutritive solutions rather than in sand cultures, a phenomenon that he attributed to the fact that the boric acid was fixed by the soil, probably as insoluble borate of calcium, so that the surface concentration obtained with sand cultures was avoided. He found that the ash of plants grown with excess of boron contained more than the normal amount of boron, while the weight of ash per 100 dry matter was also increased. He concluded that the plant thus suffers an over-mineralisation and in consequence an augmentation of its hold on water, so that the fresh weight of the plant may indicate a more favourable action of the boric acid than does the dry weight. Other investigators (Fliche and Grandeau 1874) had found the same increase in the proportion of ash in chestnut trees grown on too calcareous soil, so Agulhon concluded that one is here dealing with a general reaction of plants to an excess of a useful element.
Other experiments were carried on in the open field, maize being grown on control plots and on plots receiving 2 gm. boron per square metre. At first the latter plants were behind, the dose being too strong. Eventually, however, they pulled up level and the dry weights from the two plots proved to be nearly the same, the fresh weights being identical. Maize is evidently far less sensitive to boron poisoning than[72] are peas and oats, for with these one-half the original amount of boron (= 1 gm. per sq. metre) proved toxic.
Interesting results were obtained (Agulhon 1910 a) by repeated experiments with the same soil containing boron. It was found that sand or soil containing a proportion of boron which is lethal or toxic to a first culture will allow much better growth with a second and subsequent crops. Repeated experiments on the same soil may show the change from a lethal dose to a toxic one, thence to an indifferent and finally to an optimum concentration. Furthermore (Agulhon 1910 b) the very plants may accustom themselves to greater quantities of boron, the increased power of resistance being transmitted. He concluded from his experiments that the progeny of the second generation of maize were able to withstand quantities of boron that were toxic to control plants[13]. Agulhon once again emphasised the fact that for toxic doses of boron the first symptom is the more or less marked disappearance of chlorophyll, though the aerial parts are not affected so soon as the roots.
One of the first indications that boron compounds affect the germination of seeds was given by Heckel (1875) who found that germination was retarded for 1–3 days by weak solutions of borates (·25 gm. to 20 gm. water), and was stopped altogether by stronger solutions (·60 gm. to 20 gm. water). Archangeli (1885) tested the germination of a variety of seeds of Leguminosae, Gramineae, and of Cannabis, Iberis, Raphanus, Collinsia, and Linum in the presence of boric acid. The seeds were placed in bowls with solutions of ·25, ·5, and 1% boric acid at temperatures ranging from 16°–23° C. The bowls were covered with glass plates to prevent evaporation and consequent increase of concentration, controls in spring water being dealt with under similar conditions. 1% boric acid was found to check germination altogether, and the weaker the concentration the less was the process hindered. Morel soaked seeds of haricots and wheat in various solutions of boric acid, and found that germination was generally hindered or inhibited. The deleterious action diminishes as the strength of the solution or the time[73] of contact diminishes, but solutions of the same concentration do not act equally on all seeds. Boric acid and borax proved to be similar in their action qualitatively.
The deleterious effect of strong doses of boric acid on germination was confirmed by Agulhon (1910 a), the higher quantities (above 10 mg. boric acid per litre) retarding germination of wheat.
Of recent years a few investigators have thrown out hints as to the stimulant action exerted by boron compounds on plants. Roxas indicated that M/100,000 (M = molecular weight) of boric acid exercised a favourable action on rice. Nakamura (1903) tested the point by means of pot cultures. Peas and spinach plants were grown in soil which received 1 and 5 mg. borax per kgm. With peas the 1 mg. exerted evident stimulant action, as determined by the increase in height of the shoot over that of the control, 5 mg. seeming to be slightly depressant in action. With spinach a stimulation was observed both in weight and height with a dose of 5 mg. borax per kgm.
| Average weight | Average length of leaves | |
| 5 mg. borax | 10·35 | 38·2 |
| Control | 7·2 | 34·0 |
Agulhon (1910 c and d) took the matter up still more definitely and made many tests of various kinds, in water, sand and pot cultures.
His water cultures were made under sterile conditions, the seeds when possible being sterilised with corrosive sublimate, the germinating apparatus being also sterilised. With wheat a stimulant action was evident, maximum growth being obtained with between 2·5 and 10 mg. boric acid per litre, though the dry weight increase did not quite keep pace with that of the fresh weight, a fact to which previous reference has been made. The chief improvement is in the root, the stem/root ratio falling to 5, as against 6 in the control series. Visual observation indicated that the roots of plants receiving 5–10 mg. boric acid per litre are longer than the others, though they are less rich in adventitious roots. The increased dry weight due to boron may amount to as much as 30%.
Agulhon again observed stimulation in this case. 2 kgm. of sand were moistened with 500 c.c. nutritive solution, varying quantities[74] of boric acid being added in addition. ·1 mg. boric acid per litre of N.S. (·05 mg. per pot) gave an increase of 25% fresh weight, and 7·5% dry weight. The stimulating doses seem to be weaker than in the experiments with liquid media, probably because the evaporation from the sand increases the concentration of the boric acid at the surface. It was also noticed that the increase of weight varied in experiments made at different times. With oats the stimulating influence is greater than with wheat, showing that some plants are more sensitive than others to the influence of boron. With radish 1 mg. boric acid per litre exercised a stimulating effect, the enormous average increase of 61% in fresh weight occurring with this strength, though this only represented an average increase of 9·6% dry weight.
Here again the stimulating action was evident with higher concentrations than in sand cultures, and Agulhon obtained good results with strengths that are toxic in sand. The evaporation from earth is not so rapid as from sand, so that the concentration is not increased, and also some of the boric acid is withdrawn from the solution by interaction with the soil, so that the stimulating concentration rises in the scale.
In field experiments Agulhon found that peas were more sensitive to the toxic action of boric acid than is maize. A strength of boric acid (= 1 gm. B per sq. metre) that poisoned peas, gave an increase of 61% fresh weight and 39% dry weight with maize; half the strength proved to be indifferent for peas, the improvement with maize equalling 56% increase fresh and 50% increase dry. Curiously enough, judging by appearances in the first experiment, an unfavourable influence was at work, though in reality a great stimulation was being caused. Colza gave a good increase with similar strengths, but with turnips 1 gm. B per sq. metre only favoured the aerial parts, while ·5 gm. B per sq. metre only increased root development. Agulhon concluded that it is as yet impossible to determine with any precision the exact part that boron plays in the plant economy. He suggests that boron is a “particulier” element characteristic of a certain group of individuals or of life under particular conditions. In his summary he argues that each series of individuals adapted to different environments has doubtless need of particular elements, and that perhaps chemical causes and morphological differences are very closely connected. Boron may be of this “particulier élément” type in the higher plants of the vegetable[75] kingdom, and it may be useful commercially as a manurial agent, the “catalytic manure” of Bertrand and Agulhon.
While the higher concentrations of boric acid proved definitely toxic to both peas and barley in the Rothamsted water cultures, some evidence of stimulation was obtained with the lower strengths. With barley the question of stimulation is still an open one, as below the toxic limit growth seems fairly level in most of the experimental series. The lower limit of toxicity varies from 40–4 parts boric acid per 10,000,000 according to circumstances. Below this critical concentration the boric acid has apparently no action, either depressant or stimulant, unless the stimulation should prove to begin at a dilution of 1/50,000,000, but the evidence on this point is not sufficiently well marked or consistent to be conclusive. This failure to detect stimulation was somewhat unexpected, as when judged by the eye the plants treated with the lower concentrations of boric acid seemed better than the controls, and also exhibited a particularly healthy green colouration.
Peas on the other hand are definitely stimulated with traces of boric acid, concentrations of 1/100,000 and less causing an improvement in growth, while under some experimental conditions even higher amounts of boric acid were beneficial. All the stimulated plants showed the characteristic dark green colour which seems to be associated with the presence of minute traces of boron in the nutritive solution. An interesting morphological feature was the strong development of small side shoots from the base of the plants in the presence of medium amounts of boric acid, from 1 part in 100,000 downwards. This gave rise to a certain bushiness of growth, which was less evident as the concentration of the stimulant decreased. The general outcome of the tests seems to be that boric acid needs to be supplied in relatively great strength to be fatal to pea plants, and that the toxic action gives place to a stimulative one high up in the scale of concentration. As far as experiments have already gone it seems as though the stimulation is not a progressive one, as the effect of 1/100,000 boric acid is as good as that of 1/20,000,000, a flat curve connecting the two. This, however, needs confirmation.
Yellow lupins also give some evidence of stimulation with concentrations of about 1/50,000 boric acid, the improvement being far more strongly marked in some sets of experiments than in others.
[76]
Our knowledge of the action of boron on the lower plants is less definite and complete than with regard to the higher plants. Morel (1892) found that boric acid acts as a strong poison to the lower fungi and similar organisms, their development being completely arrested by very weak solutions of the acid. He suggested, on this account, that boric acid might be used in the same way as copper to attack such diseases as mildew, anthracnose, &c., which attack useful plants.
On the other hand Loew (1892) stated that such algae as Spirogyra and Vaucheria showed no harmful influence for many weeks when the culture water contained as much as ·2% (= 1/500) boric acid. This may be supplemented by a recent observation at Rothamsted, in which certain unicellular green algae (unidentified), were found growing at the bottom of a stoppered bottle containing a stock solution of 1/100 boric acid.
Agulhon (1910 a) dealt chiefly with yeasts and certain ferments, and found that yeasts grown in culture solutions are not influenced favourably or unfavourably by relatively large quantities of boric acid up to 1 gram per litre, while all development is checked with 10 grams per litre. The presence of boron affects the action of yeast on glucose and galactose. Galactose alone is not attacked even after 40 days in the presence of ·66% boric acid. When glucose is mixed with the galactose the latter is said to be at first left untouched, but later it disappears very slowly.
Boric acid exercises an antiseptic action on lactic ferments, 5 gm. per litre checking their action sufficiently to enable milk to remain uncoagulated. Lactic acid is still produced even with as much boric acid as 10 gm. per litre. The microbe is not actually killed by the boric acid, but its development is so arrested that reproduction cannot take place. The same phenomenon was observed with yeast. With moulds again, while no stimulation could be obtained with small quantities of boric acid, yet the toxic action does not begin to set in until 5 gm. boric acid per litre are present.
Thus it appears that such lower organisms as yeast, lactic ferment and Aspergillus niger are remarkably indifferent to the action of boric acid, as is shown by the fact that the toxic dose is remarkably high, while stimulation effects cannot be observed even in the presence of the smallest quantities yet tried.
[77]
Boric acid is less harmful to the growth of higher plants than are the compounds of copper, zinc, and arsenic. Evidence exists that below a certain limit of concentration boron exercises a favourable influence upon plant growth, encouraging the formation of stronger roots and shoots. This stimulation is more strongly marked with some species than with others, peas responding more readily than barley to the action of boric acid. Fungi are very indifferent to boron, whether it is present in large or small quantities, and there is evidence to show that certain of the green algae can also withstand large quantities of it.
[78]
The presence of manganese as a constituent of plant tissues has been known for many years, and in view of the close association between iron and manganese it was natural that the early investigators should seek for the latter element. De Saussure (1804) gives one of the earliest references to manganese in plant ash, stating that it occurs in the seeds in less great proportion than in the stems, and also that the leaves of trees contain less in autumn than in spring. At first oxides of iron and manganese were put together as “metallic oxides” and little or no attempt was made to separate them so as to get an idea of their relative abundance. John (1814) gives a number of rough analyses of plants and indicates the presence of manganese in many plants, including Solanum tuberosum, Brassica oleracea viridis L., Conium maculatum, Aesculus (in outer bark), and Arundo Sacchar. No further references presented themselves until 1847, as probably manganese was overlooked and always classed with iron in any analyses made during that time. Kane (1847) found traces of manganese in the ashes of some samples of flax, but none in others, and examinations of the soils on which the plants were grown gave similar results. Mayer and Brazier (1849) confirmed this result. Herapath (1849) analysed the ashes of various culinary vegetables, finding manganese in cauliflowers, swede turnips, beetroot, and in one variety of potato (Forty fold).
Malaguti and Durocher (1858) tried to investigate the matter quantitatively. The oxides of iron, manganese, and aluminium were all classed together, and the mean percentage of the three varied from ·85%–5·06% according to the varieties of plants concerned, Cruciferae possessing least and Leguminosae most. Different mean results with the same plant were obtained from different soils.
Wolff (1871) made other quantitative analyses including[79] Trapa natans (·15% Mn3O4), Acorus Calamus (1·52% Mn3O4), Alnus incana (trace–·73% Mn3O4), Pyrus communis (2·15% Mn3O4). Many other plants were mentioned by Wolff as containing manganese.
Campani (1876) found manganese in ash by a method in which it was detected as phosphate of manganese, and he claimed to be the first to discover manganese in wheat ash. Warden (1878) found traces of Mn3O4 in the ash of opium from Behar.
Dunnington (1878) detected manganese in the ash of wheat, ·00144 gm. (as Mn3O4?) in 300 grams of “Dark Lancaster” variety, equivalent to ·027% of the pure ash. The ash was exhausted with nitric acid, and after separating the iron the ammonium sulphide precipitate was found to contain manganese, and gave by fusion with nitre and sodium phosphate a violet coloured mass. Andreasch (1878) found slight traces of Mn3O4 in the flowers of Dianthus caryophyllus, none occurring elsewhere, while in Rosa remontana it appeared in both leaves and flowers.
Maumené (1884) tested many food plants and concluded that some quantity of manganese is frequently present in potato, rice, barley, carrot, lentil, pea, beetroot, asparagus, chicory, most fruits, tea, and also in some fodder plants, as lucerne, oats, and sainfoin. Ricciardi (1889), Hattensaur (1891) also added to the list of plants proved to contain manganese. Guerin (1897) studied the manganese content of woody tissues. Sawdust was treated with distilled water containing 1% caustic potash, expressed, and filtered after two or three days. A brown coloured liquid was obtained, which when treated with a slight excess of hydrochloric acid gave an abundant flocculent precipitate. This precipitate proved to be soluble in pure water, so it was washed with slightly acidulated distilled water, and after further purification was analysed. No trace of iron was obtained, but about ·402% Mn was found. Guerin regarded the precipitate as a “nucleinic” combination, which he supposed to occur generally in wood and to contain the manganese present in the woody tissues of all plants.
Schlagdenhauffen and Reeb (1904) detected manganese in a petrol extract of such cereals as barley, oats, and maize, and since inorganic salts of manganese are not soluble in such liquids as ether or petrol they concluded that the manganese must be present in the plant in organic combination, thereby upholding Guerin’s view. Loew and Seiroku Honda (1904) give a table of Mn3O4 in the ashes of certain trees. This is very high in some cases, rising to 11·25% in the ash of beech leaves, 6·73% in birch leaves, and 5·48% in chestnut fruits.
[80]
Gössl (1905) gives lists of the distribution of manganese in plants, both Thallophytes and Phanerogams, indicating the presence of much or little of the element. As a rule, he states, marsh and water plants gather up more manganese than do land plants.
The Gymnosperms seem to be particularly rich in their manganese content. Schröder (1878) tested for the element in firs and pines and found the following amounts of Mn3O4.
| In 100 parts ash. | In 1000 parts dry matter. | ||||
| Fir | Pine | Fir | Pine | ||
| 33·18 | 13·46 | 2·76 | ·77 | ||
He gave a table of detailed analyses showing the differing proportions of manganese in the different parts of the fir.
Baker and Smith (1910) paid special attention to manganese in their exhaustive work on the Pines of Australia. They state that “in the anatomical investigations of the timber, bark, and leaves of the various species, there was found to be present, in a more or less degree, a naturally brownish-bronze coloured substance, which invariably stained dark brown or almost black with haematoxylin.” This substance on careful investigation proved to be a compound of manganese. The quantity present varies with the species and also with the plant organs. The different species of the genus Callitris show variable percentages of manganese from a maximum of 0·230% in C. gracilis, to a minimum of 0·010% in C. robusta. The percentage of manganese in Australian Coniferae other than Callitris is given by the authors in the following table:
| Ash of timber of | Agathis robusta | 0·145% | Mn. | |||
| „ | „ | Araucaria Cunninghamii | 0·054% | „ | ||
| „ | „ | Araucaria Bidwilli | 0·077% | „ | ||
| „ | „ | Actinostrobus pyramidalis | 0·077% | „ | ||
| „ | „ | Podocarpus elata | 0·002% | „ | ||
| „ | „ | Dacrydium Franklini | 0·129% | „ | ||
| „ | „ | Athrotaxis selaginoides | 0·019% | „ | ||
| „ | „ | Phyllocladus rhomboidalis | 0·145% | „ | ||
| Air-dried | black gum of | Agathis robusta | 0·0046% | „ | ||
| „ | „ | Araucaria Cunninghamii | 0·0038% | „ | ||
Baker and Smith assume that manganese is essential to the production of the most complete growth of Coniferae. The element is found in these plants even when they grow on soils containing only traces of manganese and it is suggested that possibly the excess or deficiency of manganese in the soil helps to govern the location of certain[81] of the Australian Coniferae. The authors conclude that manganese may be essential to the growth of these plants, and that its association with plant life may be considered to date back to past geological time, as is indicated by plates illustrating fossil woods.
Little work seems to have been done on the action of manganese compounds in water cultures. Knop (1884) just indicated that manganese compounds had no effect on maize, but gave no details. Japanese investigators touched on the matter in the course of their extensive experiments with this element. Asō (1902) found that the greater concentrations of manganese sulphate exercised an injurious influence on barley. Even in solutions with as little as ·002% manganese sulphate (= 1/50,000 MnSO4) the roots gradually turned brown, the lower leaves following suit. The brown colour was concentrated at certain points of the leaves, and microscopical examination showed that the membranes of the epidermal cells, and in some cases the nuclei, were stained deeply brown. The greatest concentration endured by barley without injury seemed to be about ·01 per 1000 = 1/100,000. The presence of iron in the food solutions seems to counteract the effect of the manganese to some extent by delaying the yellowing of the leaves. Wheat proved very similar to barley in its reactions, though more iron is necessary to give good healthy growth. Asō states that wheat is able to overcome the injurious action of manganese much more readily than is barley. With peas the yellowing of the leaves was delayed, probably on account of a sufficient supply of iron in the reserve stores of the seeds.
Loew and Sawa (1902) found that ·25% = 1/400 MnSO4 (anhydrous) kills pea plants within five days and that the green colour is gradually affected with more dilute solutions. Barley and soy beans were grown in nutritive solutions with either iron sulphate or manganese sulphate or both (·01% FeSO4, ·02% MnSO4, ·01% FeSO4 + ·02% MnSO4). At first the growth was increased by the action of two salts together, but eventually the shoots turned yellowish, and assimilation was depressed, so that decreased nutrition led to relaxation in the speed of growth, indicating the toxic action due to the manganese sulphate.
[82]
The Rothamsted experiments supported Asō’s work on the action of manganese sulphate on barley, concentrations of the salt above 1/100,000 having a retarding influence on the growth, the roots being coloured brown and the leaves also showing discolouration. At an early stage in growth the lower leaves of the plants receiving the most poison began to be flecked with brown spots, which were at first attributed to an attack of rust. Suspicion was soon aroused, however, and a closer microscopic investigation showed that no disease was present, but that the cells in the affected spots were dead and brown, though they retained their shape. The dead cells at first occurred in small patches, which spread and coalesced until ultimately the whole leaf was involved. Some of the affected leaves were detached and fused with a mixture of sodium carbonate and potassium nitrate. On dissolving up the resulting mass with water a green colouration was obtained, indicating the presence of manganese in the leaves. This shows that the manganese is taken up by the roots, transferred to the leaves and then deposited in them, the lower leaves being the first affected.
The presence of manganese in the nutritive solution retarded the ripening of the grain to some extent, as when the grains from the control plants were hard and ripe, those from plants treated with 1/10,000 MnSO4 were green, those with 1/100,000 were a mixture of ripe, half-ripe, and green grains, while plants which had received 1/1,000,000 MnSO4 possessed ripe grains.
Peas give similar results to barley so far as the vegetative growth is concerned, the same retardation with the higher concentrations being observed, while the brown discoloured patches in the lower leaves are much in evidence. All traces of manganese in the leaves disappear when the concentration falls to 1/250,000. On the whole peas are more sensitive to manganese poisoning than is barley, and the higher strengths of manganese prove more deleterious to them.
Little work has been done on this aspect of the problem. Prince de Salm Horstmar (1851) grew oats in sand with various combinations of nitrogenous substances and inorganic mineral salts. He stated that until the time of fruit formation manganese does not seem to be essential to the oat unless iron is in excess in the substratum.
A large body of work has been done with manganese in soil cultures, but the toxic effect is hardly indicated, possibly because it is[83] less manifest under soil conditions, possibly because the observation of the toxic action has been almost completely overshadowed by the interest in the stimulation observed under the same circumstances. Namba stated that ·5 gm. MnSO4 added to 8 kgm. Japanese soil exerted a depressing influence on the growth of various plants. The Hills Experiments (1903) indicated some toxic effect. Various soluble and insoluble salts of manganese were added to soil in pots at the rate of 2 cwt. per acre, wheat being sown. On the whole the plants from untreated pots were as good as any with manganese except those that received manganese nitrate or phosphate. Manganese iodide distinctly retarded growth. The plants that grew did well eventually, but development of the ear was greatly or entirely retarded. If the seeds were soaked in the iodide, a concentration of 10% was found to be harmful, 5% allowing normal growth. Similar experiments with barley showed that plants treated with manganese carbonate and sulphate were both inferior to the untreated plants; with iodide less plants were obtained and their development was abnormal. Soaking the seeds in the iodide, even in 10% solution, did not do damage as it did with wheat. The oxides were apparently innocuous, but gave no increase either in corn or straw.
Kelley (1909) found that on soils in Hawaii in which excessive quantities of manganese are present (5·61% Mn3O4) pineapples do not flourish, but turn yellow and produce poor fruits, and also that if rather less manganese is present (1·36% Mn3O4) the pineapples show the toxic effect by yellowing during the winter months, but they recover completely during the hot summer months. Kelley also observed that the deleterious effect is hardly noticeable during the first twelve months of growth, and that after a time a darkening occurs in the colour of the soil, which he attributes to some change in the constitution of the manganese compounds.
Some interesting observations were made by Guthrie and Cohen (1910) on certain Australian soils. A bowling green that was initially covered with a healthy mat of couch grass developed a number of small patches after about three years growth, on which the grass died off. No reason was apparent for this phenomenon, as the cultural conditions were uniform and to all appearances the soil over the whole area was similar in character. Analyses of soil samples from the dead patches and from the neighbouring healthy parts of the green showed that the chemical composition in both cases was practically the same, except that while no manganese occurred in the soil from the unharmed part,[84] as much as ·254% Mn2O3 was found in that from the dead patches. As no other differences were found it was argued that the manganese, present in such large quantities, acted as a toxic agent and killed off the grass. Other instances of manganese poisoning in which wheat and barley were affected are quoted by these authors, the analytical results indicating that possibly barley is able to withstand without injury a greater quantity of manganese compounds in the soil than is wheat.
Nazari (1910) rolled wheat grains in a paste of manganese dioxide, iron sesquioxide (both with and without organic matter), and in what he terms “artificial oxydases.” The seeds rolled in the last-named showed the greatest energy in germination, while those with manganese gave an appreciable acceleration. The presence of organic matter decreased the action of manganese. The plants from the manganese seedlings gave an increased yield in both straw and grain, while those treated with sesquioxide of iron showed no gain over the check plants.
The Hills Experiments yielded some information as to the differing effects of various compounds of manganese on germination. With wheat plants in pot experiments manganese oxide (MnO2) distinctly retarded germination when applied at the rate of 2 cwt. per acre. With barley MnO2, manganese carbonate and sulphate all retarded germination, while with the iodide 50% of the seeds were entirely prevented from germinating.
With manganese the evidence in favour of stimulation is more weighty than with such poisons as copper, zinc and arsenic, and the literature on the subject is correspondingly plentiful.
While Asō (1902) asserted that plants can develope normally in water cultures in the absence of any trace of manganese, he further stated that manganese compounds exercise both an injurious and a stimulant action on plants. With increasing dilution of the compound the deleterious action diminishes, while the stimulant action increases, and a dilution can be reached in which only the favourable influence of the manganese becomes obvious. The addition of ·002% manganese sulphate (= 1/50,000) to culture solutions stimulated radish, barley,[85] wheat and peas. The intensity of the colour reaction of the oxidising enzyme of the manganese plants was found to exceed that of the control plants, at least with regard to those leaves on the manganese plants which had turned a yellowish colour.
Loew and Sawa (1902) obtained an initial increase of growth with barley and soy beans in nutritive solutions + ·01% ferrous sulphate + ·02% manganese sulphate, but this initial stimulation was followed by depression. These authors support Asō’s contention that manganese exerts both an injurious and a stimulative action upon plants, and that the promoting effect is still observable with manganese compounds in high dilution, while the injurious effects disappear under this condition.
The Rothamsted experiments with barley show a decided stimulation with 1/100,000 MnSO4 and less. Care was taken to utilise sublimed FeCl3 to avoid error due to the introduction of manganese into the control solution through the agency of this salt. It is interesting to notice that concentrations that are weak enough to stimulate the vegetative growth still show a depressing action in that they retard the ripening of the grain, a fact which supports Loew and Sawa’s contention that manganese exerts both a toxic and a stimulative action at one and the same time, the balance showing itself according to the concentration (Fig. 17). In the later experiments the plants were not allowed to form ears, but similar results were obtained, except that when dealing with[86] the vegetative growth only, a definite stimulus was obtained with a higher concentration than in those experiments in which the plants were allowed to form seed. This may or may not be significant, as it is possible that seasonal variation and individuality of the plants may have played some part. Barley seems to be most extraordinarily sensitive to the action of manganese, as even 1 part in 100,000,000 was found to exercise a beneficial action (Fig. 18). With peas the evidence of stimulus is less well marked. No sign of stimulation is obtained until a greater dilution is reached than is necessary with barley. Even so the resulting curves are not sufficiently conclusive to warrant the definite statement that manganese does act as a stimulant to peas when present in very small quantities (Fig. 19).
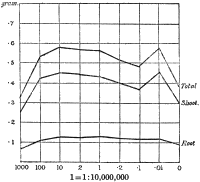
Fig. 17. Curve showing the mean value of the dry weights of ten series of barley plants grown in the presence of manganese sulphate and nutrient salts. (Feb. 5th–March 29th, 1909.)

Fig. 18. Photograph showing the action of manganese sulphate on barley plants grown in the presence of nutrient salts. (Feb. 5th–March 29th, 1909.)
| 1. | Control. | ||
| 2. | 1/10,000 | manganese sulphate. | |
| 3. | 1/100,000 | „ | „ |
| 4. | 1/1,000,000 | „ | „ |
| 5. | 1/10,000,000 | „ | „ |
| 6. | 1/100,000,000 | „ | „ |

Fig. 19. Photograph showing the action of manganese sulphate on pea plants in the presence of nutrient salts. (Oct. 2nd–Dec. 20th, 1912.)
| 1. | Control. | ||
| 2. | 1/5,000 | manganese sulphate. | |
| 3. | 1/10,000 | „ | „ |
| 4. | 1/25,000 | „ | „ |
| 5. | 1/50,000 | „ | „ |
| 6. | 1/100,000 | „ | „ |
| 7. | 1/250,000 | „ | „ |
| 8. | 1/500,000 | „ | „ |
| 9. | 1/1,000,000 | „ | „ |
Roxas carried out pot experiments with rice in soil to which was added varying proportions of manganese sulphate, with and without the addition of nutrient salts of ammonium, potassium, and calcium. The criterion of stimulation was the length of the growing leaves as measured daily, a strength of M/1000 MnSO4 (M = molecular weight) giving a favourable result.
In the Hills Experiments (1903) an increase of produce was obtained with wheat by manuring with manganese phosphate, chloride, sulphate, or oxide (MnO2), while an increase of straw was gained with nitrate, though this compound decreased the yield of corn. With barley no evidence of stimulation is set forth for any compound, except that the root growth was improved by the addition of manganese iodide, in spite of the general unfavourable action this substance exerted upon germination and growth.
Bertrand (1905) whose work will later be considered in detail, experimented on arable land, adding quantities of manganese sulphate (?) equivalent to about 1·6 gm. Mn to each square metre, growing oats from February to May. Increase of weight was found in the plants growing on the manganese plots, the differences in favour of manganese being
| For | total crops | 22·5%. |
| „ | grain only | 17·4%. |
| „ | straw only | 26·0%. |
A certain alteration in the quality of the grain was also noted from the manganese plots, the weight per hectolitre exceeding that[87] from the untreated plot, the % of water and of total nitrogen being somewhat lower than that from the untreated, while the ash and the quantity of manganese present was the same in the grain from both plots. Bertrand suggested that these results might indicate a new line to follow in the study of the causes of the soil fertility.
Strampelli (1907) tested the effect of manganese dioxide, carbonate, and sulphate, and of a manganiferous mineral from the Argentine upon wheat, and found that while all four substances exercised a favourable influence on the vegetation, the best result was obtained with the sulphate. When however other manures were used in conjunction with the manganese compounds the balance of improvement shifted. With nitrogen, applied as nitrate of soda, manganese dioxide proved the most beneficial, with farmyard manure the manganiferous mineral[14], and with blood the carbonate. It was also found that a manganese compost did not increase production when phosphatic manure was applied as basic slag.
Feilitzen (1907) indicated that the nature of the soil plays its part in determining whether manganese acts as a stimulant or not. His experiments were made in the field on poor moor soil, which carried a little Sphagnum turf and Eriophorum, and which was poor in food salts. The soil was prepared and manured and then the plots were watered with a solution of ·1 gm. MnSO4 . 4H2O per litre at the rate of 10 kgm. sulphate per hectare, six control plots being left untreated. Oats were sown and the soil rolled. During growth no difference was noted between the various plots, and after harvesting the weights of the different crops showed that the manganese had not caused increase of crop in either grain or straw on this poor moor soil.
The great bulk of the work on this problem has been carried out by various Japanese investigators, whose work extends over several years. Loew and Sawa (1902) found that small quantities of manganese sulphate in soil cultures stimulated the growth of rice, pea, and cabbage. They suggested that soils of great natural fertility contain manganese in an easily absorbed condition, and that this forms one of the characteristics of such soils.
Nagaoka (1903) dealt with plots in the rice fields which had not been manured for the three previous years and which were then treated with manure at the rate of 100 kgm. ammonium sulphate, 100 kgm.[88] potassium carbonate and 100 kgm. double superphosphate per hectare. Twelve series were worked in triplicate and received manganese sulphate in varying quantities, equivalent to 0–55 kgm. Mn2O3 per hectare, one set of three being left untreated. The cultivation was normal and the application of manganese was found to influence the yield of rice. 25 kgm. per hectare gave the best result and increased the harvest of grains by one-third; higher doses of Mn2O3 gave no better crop. The percentage of grain relative to the straw was also increased. The increase in both respects was evident all through the series from 10 to 55 kgm. Mn2O3 per hectare. The conclusion was reached that the application of this salt to soils poor in manganese would be a commercial advantage.
The next year (1904) the experiments were extended to observe the after effects of the initial doses of manganese sulphate. The harvest of grain was greatest in those plots that had received 30 kgm. Mn2O3 per hectare, while it was approached very closely by that from the plot with 25 kgm. Mn2O3, which had proved the best in the first year’s experiments. The maximum increase of yield over the unmanured plots in the first year was 37%, while in the second year it dropped to 16·9%.
Asō (1904) also obtained an increase of one-third in produce of grain when 25 kgm. Mn3O4 per hectare (as manganous chloride) was applied to rice. The development of the plants was improved and the treated plants flowered about four days before the untreated ones.
Loew and Honda (1904) grew Cryptomeria japonica in beds, treating the soil with various manures and with iron or manganese sulphate. The latter favoured increase in height, and within 11⁄2 years the cubic content of the trees had increased to double.
Fukutome (1904) grew flax in pot cultures, each pot containing 8 kgm. soil, to which was added ·4 gm. MnCl2 . 4H2O and ·4 gm. FeSO4 . 7H2O. This mixture had a marked effect on the growth of the flax, but the individual salts in doses of ·4 gm. per 8 kgm. soil had but little effect.
Namba (1908) applied manganese salts to onion plants in pots with a considerable measure of success. Pots containing 8 kgm. loamy soil were manured and received:
the manganese sulphate being applied in high dilution as top dressing.[89] The bulbs and leaves were considerably stimulated by small doses of manganese sulphate, the best results being obtained from (2), which represents a manuring of 22 kgm. MnSO4 per hectare. An increase of the dose lessens the beneficial effect, as the toxic action begins to come into play. The actual figures obtained may prove of interest.
| Wt. leaves | Wt. bulbs | Total weight | Bulbs & roots | ||
| & roots | Absolute | Relative | leaves | ||
| gm. | gm. | gm. | gm. | ||
| 1. | 29·5 | 8·5 | 38·0 | 100·0 | ·28 |
| 2. | 38·0 | 22·5 | 60·5 | 159·2 | ·59 |
| 3. | 35·5 | 16·5 | 51·0 | 134·2 | ·46 |
Uchiyama (1907) carried on a variety of experiments with manganese sulphate on several plants on different soils, both in the field and in pots, and found that the compound exercised a favourable action in most cases when applied in appropriate quantities. In summarising his results he stated that both manganese and iron stimulate the development of plants, different plants varying in their susceptibility to the action. Sometimes a joint application of the two salts is the most beneficial, sometimes an individual application is the better, in which case manganese sulphate is generally better than ferric sulphate in its action. The stimulating action of manganese varies greatly with the character of the soil, and the mode of application also affects results. As a general rule the manganese acts best when applied as a top dressing rather than when added together with the manure. Further the stimulating action differs greatly with the nature and reaction of the manurial mixture. Uchiyama concludes that 20–50 kgm. per hectare of crystallised manganese sulphate is a good general amount to apply.
Takeuchi (1909) corroborates the statements of the various writers that plants differ in their response to the manganese manuring. Pot cultures, in each of which 8 kgm. soil were similarly manured, received ·2 gm. MnSO4 . 4H2O applied as a solution of 1/100 strength, the controls receiving the same amount of water. The manganese increased the green weight of spinach by 41%, while the dry weight of barley, peas and flax rose 5·3%, 19·4%, and 13·9% respectively above that of the untreated. The control plants of flax were behind the manganese plants in growth and flowering, while barley was the least stimulated of all the test-plants. Other observations seemed to show that Leguminosae and Cruciferae are more susceptible to manganese stimulation than are the Gramineae.
[90]
The information on this point is exceedingly meagre, possibly because of the diversion of general attention to the higher plants in view of the commercial interests involved.
Richards (1897) carried out experiments with various nutritive media with the addition of certain metallic salts, including those of zinc, iron, aluminium and manganese. The fungi tested were Aspergillus niger, Penicillium glaucum and Botrytis cinerea. His general conclusion was that fungi may be stimulated, though it must not be concluded without further investigation that all fungi react in the same degree to the same reagent, but this conclusion is traversed by Loew and Sawa (1902). These writers state that fungi are not stimulated by manganese, and take this as a proof that the improvement in the growth of phanerogams, induced by manganese compounds, is not due to direct stimulation of the protoplasmic activity, but to some other more obscure cause.
The physiological cause of the stimulation exerted by manganese compounds has raised much controversy. Loew and Sawa suggested that the action of the sun’s rays upon a normal plant puts a certain check on growth, arising out of the action of certain noxious compounds which they supposed to be produced in the cells under the influence of light. The stimulation of the manganese compounds may be due to a supposed increase in the oxidising powers of the oxidising enzymes, so that destruction of the checking compounds can be accomplished as quickly as they are formed, so enabling growth to continue more rapidly.
Asō (1902) had previously stated that colorimetric tests for oxidising enzymes indicate that the yellowish leaves from plants treated with manganese compounds give reactions of higher intensity than the green leaves from control plants, the difference between the reactions being specially marked in barley, and less so in radish.
Bertrand has devoted much time to the consideration of this and allied problems. In 1897 (a, b, c) he proceeded to investigate the essential nature of manganese in the economy of the plant, his[91] experiments showing its constant presence in a ferment (laccase) obtained from plants. He also extracted from lucerne a substance very poor in manganese, which was somewhat inactive, but which regained or increased its activity on the addition of manganese. Bertrand stated that manganese was apparently not to be replaced by another metal, not even by iron, and that the small quantity of it occurring was no reason for regarding it as a secondary element in the composition of plants. The view was also put forward that in the presence of certain organic substances, such as hydroquinone, pyrogallol or similar bodies, manganese is capable of fixing free oxygen from the air, the volume of oxygen absorbed varying according to the compound of manganese used. Bertrand was led to conceive the oxydases as special combinations of manganese in which the acid radicle, probably protein in nature and variable according to the ferment considered, would have just the necessary affinity to maintain the metal in solution, i.e. the form the most suitable for the part it has to play. The manganese would then be, according to his view, the true active element of oxydase, which functions as the “activator”; the albuminous matter, on the other hand, gives to the ferment those special characters, which show themselves in their behaviour with regard to reagents and physical agents. From this point of view manganese could no longer be considered as a non-essential element, but as a substance of vital necessity to the functions of plant-life. The name “complementary” manure was suggested for compounds of such elements as manganese, which exert a physiological action and which were proposed for use as manures. Later (1905) Bertrand considered that he had still further proved the indispensable nature of manganese. The absence or insufficiency of one essential element arrests or diminishes growth. This applies not only to those substances which are present in the greatest abundance, such as C, P, N, &c., but also to those elements like manganese, boron, and iodine, which only occur in traces. These elements are usually specialised in function, and for them the name “catalytic” elements was suggested, in view of the work they are held to do. As late as 1910 the rôle of manganese in the functioning of the oxidising enzymes was again insisted on. It was concluded that manganese intervenes as a catalytic agent in the material changes of which plants are the seat, and that it participates in an indirect manner in the building up of the tissues and in the production of organic matter.
[92]
Manganese exerts a toxic influence upon the higher plants, if it is presented in high concentration, but, in the absence of great excess of the manganese compounds, the poisoning effect is overshadowed by a definite stimulation. As is the case with boron, manganese stimulates some species more than others, the action on barley being more evident than that on peas. It seems probable that manganese may prove to be an element essential to the economy of plant life, even though the quantity usually found in plants is very small.
[93]
In the foregoing chapters a very limited number of plant poisons have been considered, yet there is sufficient evidence to show that even these few differ considerably in their action upon plant-life. This action is most variable, and it is impossible to foretell the effect of any substance upon vegetative growth without experiments. The degree of toxicity of the different poisons is not the same, and also one and the same poison varies in the intensity and nature of its action on different species of plants. While certain compounds of copper, zinc and arsenic are exceedingly poisonous, compounds of manganese and boron are far less deleterious, so that a plant can withstand the presence of far more of the latter substances than of the former. Again, the tested compounds of copper, zinc and arsenic do not seem to stimulate growth, even when they are applied in the smallest quantities, whereas very dilute solutions of manganese and boron compounds decidedly increase growth. But, differentiation occurs even in this stimulative action, for while manganese is the more effective in stimulating barley, boric acid is far more potent for peas, the shoots being particularly improved.
A consideration of the experimental work that has been done on this subject of poisoning and stimulation leads one to the inevitable conclusion that it is not true to maintain the hypothesis that all inorganic plant poisons act as stimulants when they are present in very small quantities, for while some poisons do increase plant growth under such conditions, others fail to do so in any circumstances. It is probable that what has been found true with the few substances tested would prove to be similarly true over a much wider range of poisons, and at any rate the hypothesis must be dismissed in its universal application. A more accurate statement would be that some inorganic poisons act as stimulants when present in small amounts, the[94] stimulating concentrations varying both with the poisons used and the plants on which they act.
It is quite possible for a stimulation in one respect to be correlated with a retardation in another. In the Rothamsted experiments on the action of manganese sulphate on barley the weaker concentrations of the salt improved the vegetative growth, as was shown by the increase in the dry weights, but with the same strengths of the poison the ripening of the grain was retarded, so that, while certain of the physiological functions were expedited, others were hindered by the action of the poison.
Thus it is evident that it is exceedingly difficult sharply to characterise either toxic or stimulant action. In neither case is the reaction simple—many factors may come into play and many processes are concerned, while the effect of a so-called poison may vary in respect of each of the functions and processes concerned. If the poison is presented in great strength the toxic action is dominant, and probably affects many functions in the same sense, so that the action is, so to speak, cumulative. Lower down in the scale of concentration differentiation of action may set in, and while some processes may still be hindered, others may be stimulated. If the two actions balance one another an apparent indifference may be manifested, so that it seems that such strengths of the poison have no effect on growth, either harmful or beneficial. At still lower concentrations, with certain plants and certain poisons, the stimulative action overpowers the toxic effect, so that in some respect or other improvement occurs in growth.
It is quite conceivable, however, that some poisons are truly indifferent in weak concentrations, as no stimulation makes itself evident under any circumstances. In these cases one is inclined to suspect that the action is somewhat more simple, in that the toxic effects gradually diminish until no poisonous action is manifest at very weak concentrations, and as no stimulation is present to bring the growth above the normal with these very weak concentrations the plant is similar to those grown without any addition of the poison.
The modus operandi of these stimulative agents is not yet fully understood. Perhaps at the present time two main theories hold the field: (1) that they act as catalytic agents, being valueless on their own account, but valuable in that they aid in the procuring of essential food substances; (2) that the stimulants themselves are of integral value for nutrition. The French school, with Bertrand at the head, hold strongly to the catalytic theory, maintaining that[95] manganese and boron compounds are able to increase growth if they are present in small quantities, as they act as “carriers” whereby the various functions of the plant are expedited by the increased facility with which the essential nutritive elements are supplied. The manganese in laccase, for instance, is held to be an oxygen carrier, whereby the oxygen is first absorbed and then released for the benefit of the plant, the manganese being regarded as essential for the functioning of the enzyme. But, if these elements are essential, this theory seems to stop short of the truth. If certain functions are dependent for their very occurrence upon the presence of even minute traces of any element, then surely that element is as essentially a nutrient element, as vital to the well-being of the plant as is such an element as carbon or nitrogen or phosphorus, even though the latter occurs in far greater quantity. It is necessary that one should free one’s mind from the idea that the quantity of an element present in a plant is an index of its value to the plant. Naturally enough, in the early days of plant physiology, the most abundant elements first engaged the attention of investigators, and they were divided into essential and non-essential, ten elements being classed in the former category. More recent work is beginning to show that other elements are constantly present in plants, but in such small quantities that the older and cruder methods of analysis failed to reveal them, so that until lately they have been completely overlooked in work on plant nutrition. Even yet we do not know which of these other elements are essential and which are merely accidental. While we do know that the ten essential elements (C, H, O, N, S, P, K, Mg, Fe, Ca) are necessary for the well-being of all plants, it is conceivable that these other substances which only occur in very small quantities may be more individual in their action, and that while a trace of a certain element may be absolutely essential to one plant, that same element may be quite indifferent for another species. If one takes a broad outlook, the two theories seem to be in reality only parts of one, the “nutrition” theory carrying matters a little farther than the “catalytic” idea, broadening its scope and extending its application.
It seems probable that all the experimental work that has been discussed will prove to be simply preliminary to a far greater practical application of the principle of stimulation or increased growth. While the physiologists have been feeling their way towards the conclusions put forth on this subject, the agriculturists have been discovering and[96] extending the application of artificial manures, until at the present time such manuring is coming into its own and is receiving more of the widespread attention that it deserves. The possibility now exists that in some respects the two lines of work are converging and that the more purely scientific line will have a big contribution to make to the strictly practical line. Artificial manuring aims at improvement of the soil and crop by the addition of food substances that are needed in a particular soil, a result that used to be obtainable only by the use of the bulky farmyard manure, seaweed, &c. Apart from any other aspect of the matter the artificials, when intelligently used, are far more easy to handle and to regulate in supply, and they yield excellent results, especially in conjunction with a certain proportion of organic manures. The further prospect now opened up is the possibility of utilising some of these stimulating compounds as artificial manures. As only small traces are beneficial, larger amounts being poisonous, it is obvious that only small quantities would be needed, and, as the compounds are not usually very expensive, a considerable increase of crop for a relatively small outlay might be anticipated if no complicating factors intervened. Very much work will be required in the field to test the value of these substances, as their action may be influenced by the nature of the soil, climatic conditions, general conditions of manuring, and the crops grown. Some tests have already been made, especially in Japan, with boron and manganese, and these indicate a promising field for investigation.
Above all, it is most important to realise that one is approaching an entirely unexplored field, and that it is inevitable that the results of the initial experiments will be contradictory, at least in appearance, so that it is necessary to keep an open mind on the subject, being ready to modify one’s ideas as circumstances require, as improved experimental methods lead on to more accurate results.
[97]
The references are to pages on which authors are mentioned. Papers to which no page-references are appended are not dealt with in the text.
[107]
The symbols after the plant-names represent the elements referred to on the pages indicated.
[109]
CAMBRIDGE AGRICULTURAL MONOGRAPHS
Each volume of this series will contain a summary of the present position on some particular aspect or branch of agricultural science by an expert of acknowledged authority.
The treatment will be critical and impartial, and sufficiently detailed on all points of fundamental importance to be of use alike to all readers, but especially to those who are not in touch with an institution possessing a well equipped reference library. Full references will be given, and a bibliography attached for the benefit of those who wish to follow up any particular point.
The following volumes are in preparation:
THE CAMBRIDGE FARM INSTITUTE SERIES
The volumes of this series are intended to meet the needs of the many Farm Institutes already in existence or about to be formed. They are intended for the average student whose object is to farm, rather than for the exceptional man who wishes to become an agricultural expert.
Every endeavour will be made to attain a high standard educationally, by training students to take an intelligent interest in their daily work and to appreciate the beauty of the common objects among which their life will be passed. On the other hand the fact that farm students must earn their living on the land will not be lost sight of.
The following will be among the first volumes:
SELECTION FROM THE GENERAL CATALOGUE OF BOOKS PUBLISHED BY THE CAMBRIDGE UNIVERSITY PRESS
THE CAMBRIDGE BRITISH FLORA
The styles of binding and the prices will be as follows:
| Published Price per volume | Price per volume to subscribers to the whole work | |
| Paper boards, with canvas back and paper label, each volume in two parts, the first containing the text and the second the plates | £2 10s net | £2 5s net |
| Quarter morocco, in two parts divided as above | £6 net | £5 5s net |
| Paper boards, with canvas back and paper label, in one volume, the plates mounted on guards and bound interspersed with the text | £3 net | £2 15s net |
| Quarter morocco, in one volume, the plates mounted on guards and bound interspersed with the text | £6 net | £5 5s net |
“The appearance of Dr Moss’s work has been anticipated by British botanists with the greatest interest; not only to them does it appeal, for its completeness and attention to detail entitle it to rank among works of Continental importance. The Cambridge University Press has been fortunate in securing the services of Dr Moss, than whom no one more competent for the task could be found. By a combination as admirable as it is rare, Dr Moss is at once an acute field botanist, a diligent investigator of herbaria, and a student of botanical literature.... Mr Hunnybun’s drawings are all made from living plants, so that the work may be regarded as representing more fully than has been hitherto done our knowledge of British Botany at the present day.”—Journal of Botany.
Transcriber’s Notes:
Missing periods and parentheses have been supplied where obviously required. All other original errors and inconsistencies have been retained, except as follows (the first line is the original text, the second the passage as currently stands):
End of the Project Gutenberg EBook of Inorganic Plant Poisons and Stimulants, by
Winifred E. Brenchley
*** END OF THIS PROJECT GUTENBERG EBOOK INORGANIC PLANT POISONS, STIMULANTS ***
***** This file should be named 48008-h.htm or 48008-h.zip *****
This and all associated files of various formats will be found in:
http://www.gutenberg.org/4/8/0/0/48008/
Produced by Chris Curnow, Rosanna Murphy and the Online
Distributed Proofreading Team at http://www.pgdp.net (This
file was produced from images generously made available
by The Internet Archive)
Updated editions will replace the previous one--the old editions will
be renamed.
Creating the works from print editions not protected by U.S. copyright
law means that no one owns a United States copyright in these works,
so the Foundation (and you!) can copy and distribute it in the United
States without permission and without paying copyright
royalties. Special rules, set forth in the General Terms of Use part
of this license, apply to copying and distributing Project
Gutenberg-tm electronic works to protect the PROJECT GUTENBERG-tm
concept and trademark. Project Gutenberg is a registered trademark,
and may not be used if you charge for the eBooks, unless you receive
specific permission. If you do not charge anything for copies of this
eBook, complying with the rules is very easy. You may use this eBook
for nearly any purpose such as creation of derivative works, reports,
performances and research. They may be modified and printed and given
away--you may do practically ANYTHING in the United States with eBooks
not protected by U.S. copyright law. Redistribution is subject to the
trademark license, especially commercial redistribution.
START: FULL LICENSE
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full
Project Gutenberg-tm License available with this file or online at
www.gutenberg.org/license.
Section 1. General Terms of Use and Redistributing Project
Gutenberg-tm electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or
destroy all copies of Project Gutenberg-tm electronic works in your
possession. If you paid a fee for obtaining a copy of or access to a
Project Gutenberg-tm electronic work and you do not agree to be bound
by the terms of this agreement, you may obtain a refund from the
person or entity to whom you paid the fee as set forth in paragraph
1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this
agreement and help preserve free future access to Project Gutenberg-tm
electronic works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the
Foundation" or PGLAF), owns a compilation copyright in the collection
of Project Gutenberg-tm electronic works. Nearly all the individual
works in the collection are in the public domain in the United
States. If an individual work is unprotected by copyright law in the
United States and you are located in the United States, we do not
claim a right to prevent you from copying, distributing, performing,
displaying or creating derivative works based on the work as long as
all references to Project Gutenberg are removed. Of course, we hope
that you will support the Project Gutenberg-tm mission of promoting
free access to electronic works by freely sharing Project Gutenberg-tm
works in compliance with the terms of this agreement for keeping the
Project Gutenberg-tm name associated with the work. You can easily
comply with the terms of this agreement by keeping this work in the
same format with its attached full Project Gutenberg-tm License when
you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are
in a constant state of change. If you are outside the United States,
check the laws of your country in addition to the terms of this
agreement before downloading, copying, displaying, performing,
distributing or creating derivative works based on this work or any
other Project Gutenberg-tm work. The Foundation makes no
representations concerning the copyright status of any work in any
country outside the United States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other
immediate access to, the full Project Gutenberg-tm License must appear
prominently whenever any copy of a Project Gutenberg-tm work (any work
on which the phrase "Project Gutenberg" appears, or with which the
phrase "Project Gutenberg" is associated) is accessed, displayed,
performed, viewed, copied or distributed:
This eBook is for the use of anyone anywhere in the United States and
most other parts of the world at no cost and with almost no
restrictions whatsoever. You may copy it, give it away or re-use it
under the terms of the Project Gutenberg License included with this
eBook or online at www.gutenberg.org. If you are not located in the
United States, you'll have to check the laws of the country where you
are located before using this ebook.
1.E.2. If an individual Project Gutenberg-tm electronic work is
derived from texts not protected by U.S. copyright law (does not
contain a notice indicating that it is posted with permission of the
copyright holder), the work can be copied and distributed to anyone in
the United States without paying any fees or charges. If you are
redistributing or providing access to a work with the phrase "Project
Gutenberg" associated with or appearing on the work, you must comply
either with the requirements of paragraphs 1.E.1 through 1.E.7 or
obtain permission for the use of the work and the Project Gutenberg-tm
trademark as set forth in paragraphs 1.E.8 or 1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any
additional terms imposed by the copyright holder. Additional terms
will be linked to the Project Gutenberg-tm License for all works
posted with the permission of the copyright holder found at the
beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including
any word processing or hypertext form. However, if you provide access
to or distribute copies of a Project Gutenberg-tm work in a format
other than "Plain Vanilla ASCII" or other format used in the official
version posted on the official Project Gutenberg-tm web site
(www.gutenberg.org), you must, at no additional cost, fee or expense
to the user, provide a copy, a means of exporting a copy, or a means
of obtaining a copy upon request, of the work in its original "Plain
Vanilla ASCII" or other form. Any alternate format must include the
full Project Gutenberg-tm License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works
provided that
* You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is owed
to the owner of the Project Gutenberg-tm trademark, but he has
agreed to donate royalties under this paragraph to the Project
Gutenberg Literary Archive Foundation. Royalty payments must be paid
within 60 days following each date on which you prepare (or are
legally required to prepare) your periodic tax returns. Royalty
payments should be clearly marked as such and sent to the Project
Gutenberg Literary Archive Foundation at the address specified in
Section 4, "Information about donations to the Project Gutenberg
Literary Archive Foundation."
* You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or destroy all
copies of the works possessed in a physical medium and discontinue
all use of and all access to other copies of Project Gutenberg-tm
works.
* You provide, in accordance with paragraph 1.F.3, a full refund of
any money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days of
receipt of the work.
* You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project
Gutenberg-tm electronic work or group of works on different terms than
are set forth in this agreement, you must obtain permission in writing
from both the Project Gutenberg Literary Archive Foundation and The
Project Gutenberg Trademark LLC, the owner of the Project Gutenberg-tm
trademark. Contact the Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
works not protected by U.S. copyright law in creating the Project
Gutenberg-tm collection. Despite these efforts, Project Gutenberg-tm
electronic works, and the medium on which they may be stored, may
contain "Defects," such as, but not limited to, incomplete, inaccurate
or corrupt data, transcription errors, a copyright or other
intellectual property infringement, a defective or damaged disk or
other medium, a computer virus, or computer codes that damage or
cannot be read by your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH 1.F.3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium
with your written explanation. The person or entity that provided you
with the defective work may elect to provide a replacement copy in
lieu of a refund. If you received the work electronically, the person
or entity providing it to you may choose to give you a second
opportunity to receive the work electronically in lieu of a refund. If
the second copy is also defective, you may demand a refund in writing
without further opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS', WITH NO
OTHER WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT
LIMITED TO WARRANTIES OF MERCHANTABILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of
damages. If any disclaimer or limitation set forth in this agreement
violates the law of the state applicable to this agreement, the
agreement shall be interpreted to make the maximum disclaimer or
limitation permitted by the applicable state law. The invalidity or
unenforceability of any provision of this agreement shall not void the
remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in
accordance with this agreement, and any volunteers associated with the
production, promotion and distribution of Project Gutenberg-tm
electronic works, harmless from all liability, costs and expenses,
including legal fees, that arise directly or indirectly from any of
the following which you do or cause to occur: (a) distribution of this
or any Project Gutenberg-tm work, (b) alteration, modification, or
additions or deletions to any Project Gutenberg-tm work, and (c) any
Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of
computers including obsolete, old, middle-aged and new computers. It
exists because of the efforts of hundreds of volunteers and donations
from people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need are critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future
generations. To learn more about the Project Gutenberg Literary
Archive Foundation and how your efforts and donations can help, see
Sections 3 and 4 and the Foundation information page at
www.gutenberg.org
Section 3. Information about the Project Gutenberg Literary Archive Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Contributions to the Project Gutenberg Literary
Archive Foundation are tax deductible to the full extent permitted by
U.S. federal laws and your state's laws.
The Foundation's principal office is in Fairbanks, Alaska, with the
mailing address: PO Box 750175, Fairbanks, AK 99775, but its
volunteers and employees are scattered throughout numerous
locations. Its business office is located at 809 North 1500 West, Salt
Lake City, UT 84116, (801) 596-1887. Email contact links and up to
date contact information can be found at the Foundation's web site and
official page at www.gutenberg.org/contact
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
gbnewby@pglaf.org
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To SEND
DONATIONS or determine the status of compliance for any particular
state visit www.gutenberg.org/donate
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including checks, online payments and credit card donations. To
donate, please visit: www.gutenberg.org/donate
Section 5. General Information About Project Gutenberg-tm electronic works.
Professor Michael S. Hart was the originator of the Project
Gutenberg-tm concept of a library of electronic works that could be
freely shared with anyone. For forty years, he produced and
distributed Project Gutenberg-tm eBooks with only a loose network of
volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as not protected by copyright in
the U.S. unless a copyright notice is included. Thus, we do not
necessarily keep eBooks in compliance with any particular paper
edition.
Most people start at our Web site which has the main PG search
facility: www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.