Fig. 1
(Continuation of The Ohio Naturalist)
Official Organ of the
Ohio State University Scientific Society
and of the
Ohio Academy of Science
COLUMBUS, OHIO
Annual Subscription Price, $2.00 Single Number, 30 Cents
Entered at the Post-Office at Columbus, Ohio, as Second-Class Matter.
The Ohio Journal of Science
PUBLISHED BY THE
Ohio State University Scientific Society
Issued Monthly during the Academic Year, from November to June (eight numbers).
Official Organ of the Ohio Academy of Science
Subscription Price: $2.00 per Year, payable in advance; to Foreign Countries, $2.50.
Single Copies, 30 Cents.
| Editor, | John H. Schaffner |
| Associate Editor, | James S. Hine |
| Associate Editor, | Frederick W. Ives |
EDITORIAL BOARD |
|
| J. F. Lyman | Agricultural Chemistry |
| F. W. Ives | Agricultural Engineering |
| A. G. McCall | Agronomy |
| F. L. Landacre | Anatomy |
| J. H. Schaffner | Botany |
| Carl B. Harrop | Ceramic Engineering |
| Jas. R. Withrow | Chemistry |
| F. H. Eno | Civil Engineering |
| N. W. Scherer | Forestry |
| C. S. Prosser | Geology |
| V. H. Davis | Horticulture |
| W. A. Knight | Industrial Arts |
| C. J. West | Mathematics |
| Horace Judd | Mechanical Engineering |
| Jonathan Forman | Pathology |
| F. C. Blake | Physics |
| R. J. Seymour | Physiology (General) |
| Clayton McPeek | Physiology (Medical) |
| E. R. Hayhurst | Public Health & Sanitation |
| J. S. Hine | Zoology and Entomology |
The Ohio Journal of Science is owned and controlled by the Ohio State University Scientific Society. By a special arrangement with the Ohio Academy of Science, the Ohio Journal of Science is sent without additional expense to all members of the Academy who are not in arrears for annual dues.
The first fifteen volumes of the old Ohio Naturalist may be obtained at $1.00 per volume.
Remittances of all kinds should be made payable to the Business Manager, J. S. Hine.
Address The Ohio Journal of Science
Ohio State University, COLUMBUS
| Ohio Academy of Science Publications. | |
First and Second Annual Reports |
Price 30 cts. each |
| Third and Fourth Annual Reports | Price 25 cts. each |
| Fifth to Sixteenth Annual Reports | Price 20 cts. each |
| Seventeenth Annual Report | Price 40 cts. each |
| SPECIAL PAPERS. | ||
| 1. | Sandusky Flora. pp. 107. E. L. Moseley | 60 cts. |
| 2. | The Odonata of Ohio. pp. 116. David S. Kellicott | 60 cts. |
| 3. | The Preglacial Drainage of Ohio. pp. 75. W. G. Tight, | |
| J. A. Bownocker, J. H. Todd and Gerard Fowke | 50 cts. | |
| 4. | The Fishes of Ohio. pp. 105. Raymond C. Osburn | 60 cts. |
| 5. | Tabanidæ of Ohio. pp. 63. James S. Hine | 50 cts. |
| 6. | The Birds of Ohio. pp. 241. Lynds Jones | 75 cts. |
| 7. | Ecological Study of Big Spring Prairie. pp. 96. Thomas A. Bonser | 50 cts. |
| 8. | The Coccidæ of Ohio, i. pp. 66. James G. Sanders | 50 cts. |
| 9. | Batrachians and Reptiles of Ohio. pp. 54. Max Morse | 50 cts. |
| 10. | Ecological Study of Brush Lake. pp. 20. J. H. Schaffner, | |
| Otto E. Jennings, Fred J. Tyler | 35 cts. | |
| 11. | The Willows of Ohio. pp. 60. Robert F. Griggs | 50 cts. |
| 12. | Land and Fresh-water Mollusca of Ohio. pp. 35. V. Sterkj | 50 cts. |
| 13. | The Protozoa of Sandusky Bay and Vicinity. F. L. Landacre | 60 cts. |
| 14. | Discomycetes in the Vicinity of Oxford, Ohio. pp. 54. | |
| Freda M. Bachman | 50 cts. | |
| 15. | Trees of Ohio and Surrounding Territory. pp. 122. John H. Schaffner | 75 cts. |
| 16. | The Pteridophytes of Ohio. pp. 41. John H. Schaffner | 50 cts. |
| 17. | Fauna of the Maxville Limestone. pp. 65. W. C. Morse | 60 cts. |
| 18. | The Agaricaceæ of Ohio. pp. 116. W. G. Stover | 75 cts. |
| 19. | An Ecological Study of Buckeye Lake. pp. 138. Frederica Detmers | 75 cts. |
Address: C. W. REEBE, Librarian, Ohio Academy of Science. |
||
| Library, Ohio State University, Columbus, Ohio. | ||
THE
Ohio Journal of Science
PUBLISHED BY THE
Ohio State University Scientific Society
Volume XVI NOVEMBER, 1915 No. 1
| TABLE OF CONTENTS | |
| Introductory | 1 |
| Lord—The Making of a Photographic Objective | 3 |
| Transeau—Notes on the Zygnemales | 17 |
| Organization of the Ohio State University Scientific Society | 32 |
Fifteen years ago the Biological Club of the Ohio State University began publishing The Ohio Naturalist. This Journal has had a continuous existence and has been an important medium in advancing the knowledge of the natural history of the state. A number of years ago the Naturalist became the official organ of the Ohio Academy of Science and was thus sent to every member of the Academy. At that time the Ohio Academy was largely composed of Biologists and Geologists, but has now widened its scope to include Physicists, Mathematicians, and others. It was, therefore, thought desirable by many that the scope of the Naturalist should be enlarged so as to make it representative of all of the activities of the Academy. In accordance with this desire, committees were appointed by the various departments interested and a plan for future publication was proposed which was finally adopted.
The Ohio State University Scientific Society was thus organized at the Ohio State University and will take over the control of the new publication. This Society is to have somewhat the same relationship to The Ohio Journal of Science as the Biological Club had to the Ohio Naturalist. The management of the Journal is under an Editorial Board made up of representatives of various scientific departments of the University. This Board elects annually the Editor and two Associate Editors. [2]
Editorial Board.
Agricultural Chemistry, J. F. Lyman; Agricultural Engineering, F. W. Ives; Agronomy, A. G. McCall; Anatomy, F. L. Landacre; Botany, J. H. Schaffner; Ceramic Engineering, Carl B. Harrop; Chemistry, Jas. R. Withrow; Civil Engineering, F. H. Eno; Forestry, N. W. Scherer; Geology, C. S. Prosser; Horticulture, V. H. Davis; Industrial Arts, W. A. Knight; Mathematics, C. J. West; Mechanical Engineering, Horace Judd; Pathology, Jonathan Forman; Physics, F. C. Blake; Physiology (General) R. J. Seymour; Physiology (Medical), Clayton McPeek; Public Health and Sanitation, E. R. Hayhurst; Zoology and Entomology, J. S. Hine.
The Ohio Journal of Science is to be considered as a continuation of The Ohio Naturalist. It is hoped that with the wider field covered, it may interest a much larger number of the scientific people of the state, and be financially supported so that it may soon develop into a journal of high standard. It is the intention of the present Editors, with the large field before them, to publish results of research as well as articles of general interest in the advancement of Science. On the natural history side the aim at present will be to pay more especial attention to the biology, geology and geography of Ohio, but articles dealing with any other region will be acceptable.
Being a Description of a Course in Applied Optics Offered at the
Emerson McMillin Observatory of the Ohio State University.
H. C. Lord.
Photography, in its more serious phase, has taken an important place in almost every field of human activity while in its lighter mood, through the development of the “Kodak” and the roll film, is giving us one of our most delightful pastimes. As a condition for the best work, a high grade lens is a necessity and especially so for those extremely short exposures required in the photography of rapidly moving objects. It often happens that some of the most perfect and at the same time most difficult specimens of optical design are found on cameras so small that they can be easily carried in one’s coat pocket. These so called anastigmats furnish to the optician a difficult and yet at the same time most fascinating problem for mathematical investigation. Thousands of photographic objectives are placed on the market every year, yet though almost every branch of engineering is covered by our technical schools, I know of no place outside of Germany where a student can be instructed in the design and construction of a simple photographic objective. Professor Silvanus P. Thompson in his inaugural address as President of the British Optical Convention held in London in 1912, states: “In the Universities and Colleges the only people who are learning Optics are merely taking it as a part of Physics for the sake of passing an examination for a degree, and care nothing for the application of Optics in the industries. They are being taught Optics by men who are not opticians, who never ground a lens or calculated even an achromatic doublet, who never worked an opthalmoscope or measured a cylindrical lens.” Further on he speaks as follows: “What is wanted is an establishment where the whole atmosphere is one of optical interest; where theory and practice go hand in hand; where the mathematician will himself grind lenses and measure their performance on the test bench; where braincraft will be married to handcraft; where precision, whether in computation or workmanship, will be the dominating ambition.” [4]
Some four years before the above quotations were written, the author started to work up a course in Optics which should aim, not only to give to the student a knowledge of the fundamental theory of lenses, but should also apply those principles to the methods of optical design and thus enable him to compute the curves of the component lenses of a photographic objective. This has now been fairly well worked out and is given in the Arts college under the official titles “Astronomy, 107, 108, 109 and 110.” The basis of this course is “A System of Applied Optics,” by H. Dennis Taylor, the inventor of the Cooke lens. This splendid volume develops, from the standpoint of geometric optics, a complete discussion of the formation of an image by a combination of any number of lenses, but does not apply the methods and formulae there developed to the actual design of a photographic objective. The writer of this paper was, therefore, compelled to work out this part of the theory for himself and, as he had always felt that all mathematics should ultimately end in arithmetic and that all arithmetic should ultimately end in doing something, he resolved at the outset that the course should end in laboratory work in the actual computation, grinding and polishing of lenses. As to how well this has succeeded, I will let the illustrations which accompany this article speak for themselves. Suffice it to say that the half tone cuts were made from five by seven enlargements from negatives, one and three quarters by two and one-eighth inches, taken with a lens designed and built at this observatory and working at an aperture of F six. A peculiar feature of this lens is that it is composed of four lenses all cut from the same piece of crown glass. This lens beautifully illustrates the importance of adding to the theoretical side of the course, the practical work in the laboratory in construction and testing as this lens, though in the main satisfactory, has one serious defect and a defect which is very instructive in that it shows that at a certain point in the design, the theory was weak and needed to be extended and enlarged. It should be stated that this theoretical investigation is now completed and ready to be put to the test of practice. [5]
This Observatory possesses a well equipped instrument shop, which was used for the practical side of this work and it has seemed to me that a description of how we used the ordinary tools of a machine shop, of what special appliances we were compelled to make, and how we finally ground and polished our lenses would be of general interest. These methods do not pretend to be the best, nor those actually employed by the manufacturer, but they do illustrate how a lens can be made and how a little ingenuity will enable one if he has the standard tools of a machine shop to carry out almost any kind of experimental work.
As a preliminary to this, a brief outline of the problem before the lens designer may be of interest. A simple lens consists of a piece of glass bounded by either plane or spherical surfaces as these, except in large reflecting surfaces, are the only kind that can be made with sufficient accuracy. Such a lens would have a great many defects or errors and would be unable to give a sharp image on the photographic plate unless stopped down to a very small aperture. By changing the radii of the surfaces, and the thickness of the lens, the designer can vary these errors, but after all is said and done he can do but little to improve the single lens. He then combines lenses of different forms and of different kinds of glass into a single objective, in this way making the positive errors of some of the lenses balance the negative errors of the others, until he arrives at a combination which is more or less perfect according to his skill as a designer. How this is accomplished is far beyond the limits of this paper, so I will now proceed to the mechanical side of the problem.
The first consideration is the glass; of course it must be what is known as optical glass and its selection is really part of the work of the designer. Optical glass is nothing more than a very perfect kind of glass which has been exquisitely annealed. You are all familiar with the intense green of window glass when seen edgewise; a piece of white paper will hardly be changed in color when seen through twelve inches of a good optical crown. The best optical glass is not made in this country, but must be purchased from either Schott & Gen. of Jena or Mantois of France. The Jena glass has become very celebrated and most of the lens makers state that their lenses are made out of it and as a consequence most people think that Jena glass means a certain kind, while, as a matter of fact, their catalogue for 1909 shows about seventy different varieties. These differ in optical qualities and chemical composition, and cost from about a dollar to five dollars a pound, with a few special varieties costing as much as fifteen dollars. This glass comes in slabs, but will be cut by the makers with either a diamond saw or a sand saw, the purchaser paying for the “saw dust.” [6]
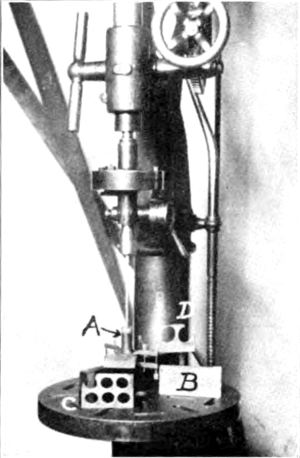
The slabs that were used here were 2" × 6" × ½" and the first operation was to cut from these round disks a little larger than the finished lens. This was accomplished in the following manner and is illustrated in Fig. 1. In the chuck of a drill speeder on a Barnes drill press was placed a ¼" steel rod which carried at its lower end a copper tube, A, which was steadied at the bottom by a steel washer, bored to a loose fit to the tube, and clamped to the glass as shown. Number 40 Carborundum was used and lubricated with plenty of water. The tube must be lifted frequently to allow the abrasive to flow to the cutting edge. This is done so often that it seems almost a continuous motion of lifting and pressing down again, the tool resting on the glass hardly more than two or three seconds at a time. The cutting may be done at such a speed as to allow of a slight heating. As soon as the tube has cut itself about a sixteenth of an inch into the glass, the guiding [7] washer may be removed and the glass will then act as its own guide. A disk about one inch in diameter and a half of an inch thick could be cut out in a little over a half of an hour. At B Fig. 1 is shown one of the uncut slabs and at C and D two that are about used up. Though working rather slowly this proved quite satisfactory though wasteful of glass as it cut a rather wide scarf, copper must be used; brass was tried but the wear was so great as to render it almost useless while the copper shows almost none.
As these disks are cut out they are not only cone shaped but the edges are very rough so that the next operation was to grind these to smooth and true circular disks. This was done on a Wells tool grinder shown in Fig. 2, which was slowed way down by placing a large pulley on the counter shaft. The glass to be ground was held by cementing it with pitch onto a piece of brass rod which in turn was held in the drawing collet of the head A. A special wheel B, made by the Norton people for grinding the rims of spectacle lenses, was used and the machine slowed until the wheel would keep wet when running against a sponge, C, resting in water. The glass disk was in this way kept dripping and heating entirely prevented. The grinding was then carried out just as with any other material and the edge was made beautifully smooth and true in a few minutes. The beauty of pitch as a cement for holding the glass is that a slight heating will soften it so that the disk can be shifted to any position and then a dash of cold water clamps it in place and at the same time the pitch will slowly yield to the slightest pressure so that in a few minutes the glass is entirely free from strain. In manufacturing this sort of work is done with a diamond and is of course done much more quickly.
The disks were thick enough to make two lenses each so we sawed them into two as illustrated in Fig. 3. A is an old polishing head upon which was mounted a pulley at one end and a copper disk, B, at the other, the disk being held between large washers. C is a cast iron box fastened to an arm, D, hinged at E and kept pressed against the copper disk by a cord passing over two pulleys on the ceiling. This made a most excellent automatic feed. The glass to be split was fastened to a block of pine with pitch and the wood held in the iron box, C, with wedges. Number 40 Carborundum was used with [8] plenty of water and the glass was cut through faster than a power hack saw would cut through steel. The glass should be cut half way through and then reversed so that the final break will come in the middle and thus prevent the edges from spawling off. The chief defect of this machine was the way it scattered emery.
The disks are now ready for the grinding which is done on the machine on the right of Fig. 3, which consists simply of a vertical spindle run by a quarter twist belt from the counter shaft against the wall. The end of this spindle is tapered at the upper end to receive the grinding tool or laps, shown on the table in Fig. 5 which also shows the spindle raised so that the grinding lap is seen above the tin box, C, which surrounds the spindle to catch the abrasive that is thrown off in grinding. The glass is first smoothed down on a flat lap until it is of equal thickness at all points as measured by a micrometer when it is ready to be ground to the proper curves. For this purpose the spherical laps, shown in Fig. 5, are turned in the special machine illustrated in Fig. 4. The compound rest of an [9] old Seller’s lathe was removed and in its place, on the cross slide of the carriage, was mounted the sphere turning rest. This consists of a base, A, in which the slide, B, is so mounted that it can be rotated about the center, C, by turning the milled head, D, which carries a worm at the opposite end. E is the tool post with the cutting tool T and L the lap to be turned. A hole was drilled at C into which was fitted a round piece of steel the upper end being pointed and then half cut away like a center reamer. This was used in finding the zero; the rod, pointed end up, was placed in the hole at C and the cutting tool adjusted against the flattened side. The zero position is then determined by measuring, with an inside micrometer, the distance from the tool post to a stop placed at the end of the slide B. By adding to or subtracting from the zero reading of the micrometer the length of the radius of the grinding lap, the tool post may be set to the proper position for either a convex or a concave surface. This, however, is only approximate, for these laps must be made with the highest possible accuracy. After sufficient cuts have been taken to give a spherical surface, the radius is carefully measured with a special spherometer and the error in the radius corrected by changing the position of the cutting tool by an amount calculated from the readings of the spherometer. This spherometer we were compelled to build as we could find none of sufficient accuracy on the market and it is described in a note at the end of this article.
In Fig. 4, R is simply a steady rest made with the large overhang to allow the slide B to swing under it in turning a convex surface. Two master laps, male and female, must be made and carefully ground together. Every effort should be taken to make these as accurate as possible since upon these depends the goodness of our lens. This special tool is easy to make and leaves nothing to be desired in its operation. Detail drawings and directions for making it are given in a note at the end.
We now come to the grinding or lapping of the lenses themselves. This is done in a lap turned as above and carefully fitted to the master laps and which must be trued from time to time as the work progresses. This lapping of glass is entirely different from the lapping of metals in that, while in metals the lap is to be kept almost free from the abrasive, in glass the lap must be freely supplied with emery and water or deep scratches will result. The best way to apply the emery is with a paint brush; the brush, saturated with emery, being held in front of [11] the lens as it is ground. The lens may be held in the hand or cemented to a disk of brass having a center hole drilled in the back in which is placed a pointed piece of steel held in the hand, the lens being free to rotate about the pointed steel holder. Of course where the lens has to be ground to a definite thickness it must be held by hand. Flour of emery was used to rough grind though coarser grades would have worked faster. The final smooth grinding was done with a special fine emery made for this purpose by Bausch and Lomb. Great care must be taken in the grinding to keep the lens as nearly centered as possible. A lens is said to be centered when the line which joins the centers of curvature of the surfaces passes through the center of figure. Obviously if a double convex lens could be ground to a knife edge it would be centered but if this were done the edge would be almost certain to crumble in the final polishing and deep scratches result. The centering of a convex lens can be watched by keeping the edge as nearly uniform of thickness as possible with a concave lens, if the original blank is made larger than necessary and care is taken to make the sides parallel, the centering can be watched by keeping a flat edge of equal width around the concave portion, the lens being placed back on the flat tool, from time to time, as the work progresses. If care is used the lens need be made but little larger than the finished size to allow for the final accurate centering to be described later.
After being smooth ground the lens is beautifully smooth and velvety to the touch but is just as much ground glass as ever, that is, it is absolutely opaque. We now come to the polishing. This is done with specially prepared rouge and only an excessively small amount of glass is taken off. Lord Rayleigh in a paper on “Polishing of Glass Surfaces” read before the British Optical Convention held in 1905, states: “I started with a finely ground surface, rather more finely ground I think than is used in practice, and I found that in order to obtain a pretty good polish it was necessary to remove a weight of glass, corresponding to a depth of about 6 wavelengths. I do not pretend that such a polish would satisfy the requirements of commerce; probably the 6 would have to be raised to 10 or 12 in order to get to the bottom of the deepest pits.” When it is remembered that a wave length is about the fifty [12] thousandth part of an inch we realize how very delicate such lapping must be. For this work the lap is covered with pitch which has been brought to the proper degree of hardness either by boiling, to harden it or by adding asfalt varnish to soften it. The proper degree of hardness is very important and must be adjusted to the temperature of the room. Obviously if the pitch is too soft it will not hold its shape and it will be impossible to hold the polishing tool to the proper radius. I have put three different curves on a lens about an inch in diameter in a few minutes and it had to go back on the grinding machine before it could be finished.
The polishing tool is prepared as follows: A disk of pitch, about ¼" thick, is cast by pouring it in a mold made by a strip of brass bent to a circle, the ends clamped with a tool maker’s clamp, and rested on a piece of cold cast iron which has been planed smooth. This should be of such size that when bent to the proper shape it can be molded over a tool similar to the grinding tool but with a radius changed by about the thickness of the pitch. This tool is then heated and painted with a stick of pitch, the disk is warmed, and the two pressed together, when cooled the pitch will stick tight to the iron but will be far from a smooth surface. This and the master tool of the opposite curvature are placed in warm water and pressed together and at the same time one slowly rotated, one about the other. When a good fit is secured they are cooled and a number of small holes, about 1-8" in diameter, are drilled all over the pitch to distribute the abrasive, which of course spoils the surface and the tool must be again pressed. This pressing to shape must be done repeatedly and requires great care and some practice in order to have the pitch come to the exact opposite of the pressing tool. The most important thing is to do the pressing slowly and in fact in the whole process of this work one must never get in a hurry. Ritchey, in his memoir on the construction of the great 60" at Mt. Wilson, recommends covering the pitch with beeswax, and for quicker and poorer work a cloth polisher may be used, the cloth being a special felt and cemented to the cast iron tool with a thin layer of pitch.
The abrasive is rouge or red oxide of iron and its preparation is fully described in the above mentioned work by Ritchey. We purchased the anhydrous red oxide of iron from Merck & Co. This was mixed with plenty of water in the jars shown at E, Fig. 5. [13] The rouge will rapidly precipitate, the coarse particles falling to the bottom, and leaving clear water above the precipitated rouge. The upper two-thirds of the rouge will be almost perfect and will give a beautiful polish when carefully siphoned off. This should be kept in tightly corked bottles, one of the best things is a horse radish jar as this has a place for the handle of the brush in the glass stopper, and all dust and grit can be easily washed off before the jar is opened. For polishing, the lens is cemented to a handle at whose end is a piece of brass turned to fit the lens in the sphere turning machine already described. Even in a small lens the polishing tool must be run slowly, the speeds of our machines run from 170 to 300 revolutions per minute and the fastest can seldom be used. The reason of this is that the lens fits the polisher so perfectly that almost a perfect vacuum is formed and the lens hugs the polished so closely that it is impossible to hold it in small sizes by hand alone and in the case of a convex surface, if the cavity is carried clear out to the edge of the glass disk, this may be broken simply by the friction due to this grip of the glass and pitch. Fig. 5 shows a horizontal polishing head at B and a vertical one at C. There is little choice except that for convex surfaces B seems the best, as it can be run faster, while for concave C seems better.
The lenses are now ready to be centered, that is, the circumference so turned that the line which joins the centers of curvature of the two spherical surfaces shall pass through the center of figure. In order to accomplish this, the lens is first cleaned from the pitch used to cement it to the handle used in holding the lens for polishing. For a long time I could find no way of doing this satisfactorily when pitch was the cement; finally, I laid my troubles before Dr. A. M. Bleile, Head of the Department of Physiology, and he suggested to first soak the lens in lard and then wash it in benzol (C6H6). This worked like magic though the first time I tried it I used some lard that had been heated with some pitch in it which made the lard very soft in fact almost as soft as it could be and yet not be an oil, and this same lard was used over and over again. The action is rather peculiar; the lard does not apparently effect the pitch at all but after a few minutes in the benzene it all flakes off and leaves the lens perfectly clean. The actual centering is then carried out on the grinding machine shown in Fig. 2; A holder, D, whose front face has [14] been turned in the spherical turning machine to fit one of the surfaces of the lens, is held in the head A. If the lens be cemented to this with a thin coat of pitch, it is obvious that the surface of the lens next to the holder will have its center of curvature coincide with the axis of rotation of the spindle of the head, A, but the center of curvature of the other lens surface will probably fall outside of this axis. A lamp, L, has a tin chimney with a pin hole in it turned towards the lens, this pin hole forming a brilliant point of light, an image of which is formed by each surface and reflected by the total reflecting prism, P, into the telescope, T, where it is seen through the eyepiece. If the centers of curvature of both surfaces do not accurately coincide with the axis of rotation of the head, A, the images of the pin hole will describe circles as this axis is rotated. The back surface will of course be centered if the layer of the pitch used as cement is of uniform thickness which will generally be the case if the work has been carefully done; but in any case the image formed by it should be examined. If the front surface is out of center, as it generally will be, the holder should be warmed and the lens shifted, care being used to keep it tight against the surface of the holder as it is being shifted. As soon as both images remain stationary as the head, A, is rotated, the lens is fed against the wheel, B, and ground true and to size. This worked beautifully and the tests were wonderfully sensitive. As soon as the component lenses of the objective have all been thus centered, they are ready to be assembled in the cell or shutter in which they are to be used; but as this is simply a matter of careful machine work, I need not describe it further.
I know of no literature on the grinding of small lenses though the following memoirs on the making of large reflecting telescopes should be in the hands of any one interested in this work:
Note 1—A Spherometer for Short Radii.
In Fig. 6, A is a regular Brown & Sharpe Micrometer Head with the measuring point ground to an angle of 60° and slightly rounded; B is a round steel base all machined at one setting in which the micrometer head is clamped by a set screw not shown.
Let r be the radius of the spherical surface, MNO, and we will have at once r = (a2 + d2) ⁄ 2d. The advantage of this form of spherometer is that it is very easy to make the point of the micrometer exactly central with the base and the value of 2a can be accurately determined by means of an ordinary micrometer calliper. For a convex surface, 2a should obviously be the inside diameter of the base, B.
In using the instrument, two tables, one for concave and one for convex surfaces, should be prepared; these tables to give the power in dioptres for each one thousandth of an inch in the value of d. Using the American Optical Co.’s Standard Index, namely, μ equal to 1.5000 and one dioptre as being the power of a lens of 40 inches focus, we have, for a plano lens, p = 40⁄f = 40d ⁄ (a2 + d2) since f = r ⁄ (μ-1).
The advantage of forming the table in dioptres in place of radii directly is that the tabular differences are small at all parts of the table so that interpolation can be readily done and this is not the case in tables which give the radii directly.
If upon measuring the radius of the tool or lap being turned in the sphere turning machine, Fig. 4, with this spherometer, the tool is found to be in error by an amount Δp this may be corrected by changing the position of the cutting tool by an amount 20 (Δp ⁄ p2).
Note 2—Cross Section of the Sphere Turning Rest.
In Fig. 7 is shown a cross section of the sphere turning rest further illustrated in Fig. 4. In machining this the following suggestions should be followed. The piece M should be cast with a lug projecting from the face PQ to chuck it by and all the turning done at one chucking. It should be made a close fit to R and bolted tight against DG and ED´ with the bolts S3 and S4, clearance being given along the line HF. To compensate for ware the face DG and ED´ can be releaved from time to time with a file. The base N, should be planed along AB, where it fastens to the cross slide of the carriage, then bolted to a face plate of the lathe and finished, care being used to leave the setting of the compound rest unchanged between machining the faces CD and C´D´ of the pieces M and N. The dove tail on R should be first planed and then this bolted to a face plate and the boss GHFE and the faces KG and EL turned at one setting. If these directions are followed almost no hand work will be needed. W is a brass worm wheel held by screws not shown and J is the sliding tool post clamped at X with the tool at K´.
The following notes principally concerning North American Zygnemales are based on a study of the specimens accumulated in the course of eight years collecting in central Illinois; a collection made by Mr. Charles Bullard, of Cambridge, Mass., in Massachusetts and New Hampshire; the specimens distributed in the Phycotheca Boreali-Americana by Collins, Holden and Setchell; the specimens distributed in American Algae, by Miss Josephine E. Tilden; the specimens in the U. S. National Herbarium; and small collections sent me by Professor Farlow, Miss Tilden, Professor A. B. Klugh, Professor D. S. Johnson and Miss Grace Stone. They have been compared with the species distributed by Wittrock and Nordstedt in their “Algae Aquae dulcis exsiccatae,” and other valuable European and South American specimens sent me by Professors O. Borge and O. Nordstedt.
In determining almost any species of the Zygnemales it is absolutely essential that the specimens show both the vegetative cells and the mature spores. With the exception of a few species of Mougeotia the spores are colored either yellow, brown, or blue when they are mature. The characteristic markings of the median spore wall do not develop usually until this color appears. Consequently it is useless to attach names to vegetative specimens based on dimensions and number of chromatophores. Keys based on such characters are not only useless, but misleading.
Judging from my experience in Illinois it is highly probable that the list of North American forms will be considerably augmented, when intensive studies have been made at localities in the Southern United States. The most satisfactory method of collecting these forms is to take samples from the various ponds and streams at regular intervals of ten days, or two weeks, throughout the growing season. Many of the species show local variations and considerable experience is needed before many of the forms can be satisfactorily classified. The writer has in course of preparation an illustrated key to the group, in which figures for all of the species will be published. [18]
This genus is in many respects the most generalized of all the Zygnemales. It is distinguished by three important characteristics: (1) the entire contents of the gametangia enter into the making of the zygospore; (2) the zygospore is formed in the conjugating tube and is not cut off from the other parts of the gametangia by partition walls; (3) as the gametes move toward the tube during conjugation, their place is taken by a secretion of cellulose, which renders the gametangia solid and highly refractive. This secretion also occurs when a vegetative cell forms an aplanospore.
This species has been recorded for America. It is not uncommon in Massachusetts and has also been found in Minnesota and Florida. In P. B.-A. No. 808 from Boswell, California is a somewhat smaller variety with blue spores associated with Zygnema peliosporum Wittr. The spores are common in the material and the vegetative cells and filaments occasional. Following is a diagnosis for this variety:
Var. formosa nov. var. Cellulis vegetativis 7.5-9µ latis; zygosporis 24-30µ × 30-42µ, caeruleis; ceterum ut in typo.
Vegetative cells 7.5-9µ in diameter, zygospores 24-30µ × 30-42µ steel blue, otherwise like the type.
Cellulis vegetativis 9-12µ × 27-120µ, ad dissepimenta constrictis; chromatophoris cum pyrenoidibus 2; cellulis fructiferis, 10-14µ × 75-180µ; zygosporis ovoideis vel quadrato-ovoideis, 20-40µ × 30-40µ, angulis rotundatis, productis, vel retusis; parthenosporis 15-20µ × 20-30µ, oblique ellipticis, cum polis retusis; mesosporio subtiliter et irregulariter verrucoso, maturitate luteo-brunneo. [19]
Vegetative cells 9-12µ × 27-120µ constricted at the end walls, chromatophore with two pyrenoids; fertile cells 10-14µ × 75-180µ; zygospores ovoid or quadrately ovoid, 20-40µ × 30-40µ, with angles rounded, produced, or retuse; parthenospores 15-20µ-× 20-30µ unilaterally ellipsoid with retuse ends; median spore wall minutely and irregularly verrucose, yellow-brown at maturity.
This species was collected by Professor A. B. Klugh, Kingston, Ontario. It is the material upon which the Ontario record for Mougeotia calcarea (Cleve) Wittr. is based. Of special interest is the chromatophore with two pyrenoids, which although an axile plate is distinctly two-lobed and forms an easy transition to the next species, in which the chromatophore resembles Zygnema. Type in herb. E. N. T. Collection No. 2950.
Cellulis vegetativis 16-20µ × 25-50µ cylindraceis; chromatophoris asteroidiis duobus, singulis cum pyrenoidibus (ut in Zygnemate); zygosporis vel ovoideis, vel irregularibus, 24-30µ × 30-48µ cum angulis vel rotundatis, vel retusis, vel productis; aplanosporis uno latere ovoideis, 17-25µ × 20-40µ; parthenosporis 15-20µ × 20-30µ; membrana media sporarum scrobiculata, luteo-brunnea; akinetis ad dissepimenta constrictis, membrana subcrassa et glabra, 18-20µ × 20-36µ.
Vegetative cells 16-20µ × 25-50µ cylindrical; chromatophores two, stellate, each with a pyrenoid (as in Zygnema); zygospores ovoid, quadrate-ovoid, or irregular, 24-30µ × 30-48µ, with rounded, retuse, or produced angles; aplanospores unilaterally ovoid, 17-25µ × 20-40µ; parthenospores 15-20µ × 20-30µ; median spore walls scrobiculate, yellow-brown; akinetes with smooth heavy walls, 18-20µ × 20-30µ.
Type in herb, E. N. T. Collections No. 1177, 1939, 1949, 2686 and 2918. I have specimens from several localities in central Illinois; Williamsport, Pa.; Minnesota; Mackinaw, Mich. and Kingston, Ontario.
This form is of great interest because of its resemblance, in the vegetative condition, to Zygnema decussatum (Vauch.) Transeau. Also because it shows not only the zygospores, but aplanospores and parthenospores. In all cases the secretion of cellulose accompanies the process of spore formation. The unilaterally placed aplanospores are strikingly different from those formed by the Zygnemas. In some of the Illinois ponds it regularly produces only zygospores, in other ponds from which I have collections covering a period of several years it fruited only asexually, producing aplanospores and akinetes. But several of the collections show all the forms of reproduction in different cells of the same filament. [20]
The characteristics of this species suggest that the peculiar Zygnema reticulatum, which was described by Hallas in 1895[B], is in reality a Debarya. The fact that the reproductive cells become filled with cellulose, that the aplanospores are very irregular in form and that the vegetative cells contain as high as seven chromatophores, are all in harmony with this idea. On this basis it is also easy to understand the most notable peculiarity of the species—that spores derived from cells with several chromatophores produce two or three sporelings.
With the addition of the two new American species and this Danish species Debarya reticulata (Hallas) nov. comb. the description of the genus needs to be modified as follows:
Vegetative cells cylindrical or constricted at the ends, varying from 1-16 diameters in length; chromatophore varying from an axile plate with two or more pyrenoids to stellate chromatophores, each with a central pyrenoid. Reproduction by zygospores formed of the complete contents of the gametangia; not cut off from the gametangia by partition walls; but in the process of conjugation, as the gametes pass into the conjugating tube, their place is taken by a secretion of cellulose. Aplanospores occupying only part of the sporogenous cell, the remainder being filled with cellulose. All spores variable in form. Parthenospores and akinetes occur not infrequently in some of the species. The walls of the aplanospores and parthenospores resemble the zygospores of the same species in their markings.
There are now eleven described species belonging to this genus. D. immersa W. West and D. africana G. S. West bear a close resemblance to Mongeotia sphaerocarpa Wolle. D. Hardyi G. S. West has much the same appearance as Mongeotia viridis (Kutz) Wittrock. D. desmidiodes W. & G. S. West, D. calospora (Palla) W. & G. S. West, D. reticulata, D. americana, and D. decussata have characters in common with the Zygnemas. D. glyptosperma has the vegetative characters common to several of the species, but its spores are quite unique among the Zygnemales. [21]
This is probably common in the eastern half of the United States at least. In Illinois along with the type occurs the variety conspicuum (Hass.) Kirchner, and a variety with large spores. This latter variety in fact is more common than the type.
var. crassum nov. var. Cellulis vegetativis 30-40µ × 20-80µ; zygosporis 40-55µ × 50-60µ, ceterum ut in typo.
Vegetative cells 30-40µ × 20-80µ; zygospores 40-55µ × 50-60µ, otherwise like the type. Type in herb. E. N. T. Collections No. 2350, 2392, 2660, 2685.
Professor G. S. West has studied the reproduction of this species and finds that it is a true Zygnema and that the description and figure by De Bary, which shows the cutting off of two special gametangia before the union of the gametes is at fault, consequently there is no longer any need of maintaining the genus Zygogonium Kützing.
Specimens of this species have been distributed under the name of Z. chalybeospernum Hansgirg, in P. B.-A. No. 808 from Boswell, Calif. (N. L. Gardner); Amer. Alg. No. 156 from Ft. Collins, Colo. (J. H. Cowan); and Amer. Alg. No. 392 from Vancouver, B. C. (J. E. Tilden). Z. chalybeospermum has the median wall smooth, but the spores of all of the above specimens have distinctly scrobiculate median walls. In size the specimens show a somewhat greater variation in dimensions than has been recorded for European localities.
Specimens of this species have been found at Fath Pond, north of Coffeen, Ill., in which both zygospores and aplanospores occurred in abundance. The aplanospores fill or slightly enlarge the vegetative cells as in Z. Collinsianum Transeau,[C] but the ends of the spores are usually more nearly truncate, 34-50µ × 30-80µ. At Casey, Ill., a variety with the same dimensions but steel blue spores occurs in the old Ice Plant Pond. [22]
var. caeruleum nov. var. Cellulis vegetativis et sporis ut in typo, sed membrana media sporarum caerulea.
Vegetative cells and spores as in the type, except that the median spore wall is steel blue. Type in Collection E. N. T. No. 495.
The specimen distributed under the name Z. insigne (Hass.) Kütz. in the P. B.-A. No. 457, from Chestnut Hill, Mass. (G. F. Moore), belongs to this species as shown by the scattered mature spores. This species is common everywhere in central Illinois. In the U. S. Natl. Herb, is a specimen from Baltimore Co., Md., (J. D. Smith). In Amer. Alg. No. 157, a specimen from St. Paul, Minn., (J. E. Tilden) shows both zygospores and aplanospores. The aplanospores are cylindric ovoid in form, occupying the entire cell 30-33µ × 40-88µ, median wall scrobiculate.
Cellulis vegetativis 28-33µ × 28-66µ; zygosporis incognitis; aplanosporis cylindricis vel tumido-cylindricis, 30-33µ × 24-58µ, sporangia complentibus; membrana media brunnea scrobiculata.
Vegetative cells 28-33µ × 28-66µ; zygospores unknown; aplanospores cylindric or tumid-cylindric, 30-33µ × 24-58µ, filling the sporangia, median wall brown, scrobiculate. Type in Herb. E. N. T. No. 1164, 1177.
This species is not uncommon in ponds, and pools throughout central Illinois. It was at first classified as aplanosporic material of Z. stellinum (Müller) Agardh. On going over the specimens in all my collections, however, it was found that in no case were the filaments containing the aplanospores connected with the filaments containing zygospores. This must be the final test of the identity of the species, as it occurs in some collections alone, sometimes associated only with fruiting Zygnema pectinatum, and sometimes with Z. stellinum.
In a collection of algae made at the Minnesota Seaside Station, Vancouver Island, B. C., in 1901, by Professor Tilden, is a form which perhaps belongs here. The vegetative cells are from 22-28µ in diameter, and 32-52µ in length, while the European specimens are described as [23] 16-20µ in diameter and 2-6 diameters long. The Vancouver specimens are producing both aplanospores (globose, 24-26µ in diam.), and zygospores (ovoid 24-28µ × 36-44µ) by the union of gametes through the partition wall separating the two gametangia. The specimens show some evidences of being in an abnormal condition.
The specimen in P. B.-A. No. 510 from Knightsville, R. I., distributed under the name of S. longata (Vauch) Kütz. with cell diameter 27-30µ, and ellipsoid spores 30-33µ in diameter, fertile cells enlarged, evidently belongs to this species. The spores of S. longata are distinctly ovoid with rounded ends. In the Illinois specimens the spores of S. Juergensii frequently occur with diameters up to 33µ.
The varieties scrobiculata Stockman and minor Teodoresco have not been reported from America. They both occur rarely in Illinois. The latter I have also seen in material collected by Mr. Charles Bullard, at Lynnfield, Mass. The former is characterized by its scrobiculate spores, the latter by its smaller dimensions throughout. In my herbarium S. varians scrobiculata is represented in Collections No. 1799, and 1881; and S. varians minor in Collection No. 2951.
Cellulis vegetativis 30-35µ × 50-200µ, dissepimentis planis, chromatophoris singulis anfractibus arctis 1.5-5; cellulis fractiferis altero latere inflatis, altero latere (in quo conjugatio sequitur) rectis; zygosporis ellipticis, 33-40µ × 54-70µ, membrana media flava, glabra.
Vegetative cells 30-35µ × 50-200µ, end walls plane, 1 chromatophore making 1.5-5 turns; fertile cells inflated on the outer side, straight on the conjugating side; zygospores ellipsoid 33-40µ × 54-70µ, median wall yellow, smooth. Type in herb. E. N. T. Coll. No. 1883, 1890. Charleston, Illinois. [24]
This species bears some resemblance to a form of S. varians figured by Professor Borge.[D] It differs from his figure in that the conjugating side of the fertile cells is not at all swollen, and the dimensions are somewhat larger. If this form had been found but once it would have been passed over as a variation intermediate between S. Juergensii and S. varians. But it has been found several successive years in a small stream south, and at a small pond west of Charleston, Illinois.
So far as I am aware no specimens of this species have been found in America. The Illinois record in my list[E] is an error. The P. B.-A. specimen labelled S. lutetiana is S. fallax (Hansg.) Wille, as shown by its often replicate cell walls, verrucose spores and the number of chromotophores.
Specimens of this variety have been distributed in the P. B.-A. No. 96, under the name of S. dubia Kütz. var. longiarticulata Kütz. from Oak Bay, Victoria, British Columbia (N. L. Gardner). The spores are for the most part not mature but they show the characteristic scrobiculate markings of the median wall.
This species is not uncommon in central Illinois. The specimen labelled S. communis in P. B.-A. No. 1416, from Winchester, Mass., has a cell diameter over 30µ, and the spores are ellipsoid instead of ovoid. The median spore wall in the mature spores is punctate. Here also belongs the P. B.-A. specimen No. 365, Falmouth, Mass. Both the vegetative cells and the spores are considerably below the lower dimensions for S. porticalis. The P. B.-A. specimen No. 1668, S. porticalis var. tenuispira Collins establishes this name as a synonym of S. Lagerheimii. Professor Farlow has recently sent me a specimen of this species from Chocorua, N. H.
This species has recently been found in a pond south of Coffeen, Ill. The spores show the characteristic markings and the dimensions are near those of the original collection. The spores are slightly more rhomboidal than in the type material, which I have seen. In herb. E. N. T. Collection No. 2912, 2850. [25]
This species previously known only from the tropics has been found in the collection of Mr. Charles Bullard, from Wellfleet, Mass. In herb. E. N. T. Collection No. 2954.
This species was described by Collins as a variety of S. decimina (Müller) Kütz, which it somewhat resembles in the form of the vegetative cells. The spores, however, are distinctly ellipsoid, while those of S. decimina are ovoid. The dimensions are much smaller than those of S. decimina. It seems better therefore to recognize this as a distinct species. It has been collected in Massachusetts, Connecticut and Bermuda.
Described originally from Illinois, I have seen specimens during the past year from Maine, Massachusetts and Minnesota. Professor G. S. West[F] described about the same time a species from Columbia, South America, which appears to be a form of this same species. The vegetative cells are considerably larger, the chromatophores are six (or five) in number, and the spores are at the upper limit of size of the North American form. As our specimens all show, a wider range of dimensions and number of chromatophores, the South American form is best classified as a variety under the name S. ellipsospora var. splendida (G. S. West) nov. comb.
Cellulis vegetativis 60-68µ × 80-150µ, dissepimentis planis; chromatophoris 3, anfractibus arctis .5-1; cellulis fructiferis cylindricis; zygosporis ellipticis 42-60µ × 80-120µ; membrana media sporarum scrobiculis irregularis ornata, luteo-brunnea.
Vegetative cells 60-68µ × 80-150µ, end walls plane; 3 chromatophores making .5-1 turn in the cell; fertile cells cylindrical; zygospores ellipsoid, 42-60µ × 80-120µ, median wall irregularly pitted, yellow-brown. Type in herb. E. N. T. Coll. No. 2666. Coffeen, Illinois. [26]
This species is very distinct in the form of its spores and their position in the fertile cells. Lateral conjugation only has been observed. It is possible that the number of chromatophores is more variable, but in all the vegetative cells in which they could be counted there were three.
Owing to the indefinite and imperfect description of S. lineata Suring., the variety Braziliensis Nordstedt, of which we have a perfect description and specimens (W. & N. Alg. aq. dulc. exsicc. No. 360), should be given specific rank. Its connection with S. lineata is very problematical.
In all the published descriptions of this species the spores are described as smooth, and the number of chromatophores is given as four. I have seen many specimens from Illinois, and collections from the upper peninsula of Michigan (T. L. Hankinson), Minnesota (J. E. Tilden), Hawaii (J. E. Tilden), Massachusetts (P. B.-A. No. 1217), Pennsylvania (E. N. T.) and Guatemala (W. A. Kellerman). In all cases the mature spores are brown and scrobiculate, and the number of chromatophores is three or four.
Cellulis vegetativis 50-60µ × 200-350µ, dissepimentis planis; chromatophoris 3-5, anfractibus arctis 2.5-4.5; cellulis fructiferis non inflatis; zygosporis ovoideis 50-65µ × 80-120µ: membrana media sporarum reticulata et dense punctata, flava.
Vegetative cells 50-60µ × 200-350µ, end walls plane; 3-5 chromatophores making 2.5-4.5 turns; fertile cells not inflated; zygospores ovoid 50-65µ × 80-120µ: median wall reticulate and densely punctate, yellow in color.
This species was first found in the collections of Mr. Bullard from Beaver Dam, Brook Pond, Natick; the pond west of Winter Pond, Winchester; and the Middlesex Fells, Mass. Recently the same form was found in a large prairie pond south of Coffeen, Illinois. Its position in the genus is near S. malmeana Hirn. In herb. E. N. T. Collections No. 2952, 2953 and 2900. [27]
I first came across this species in Mr. Bullard’s collection from the pond west of Winter Pond, Winchester, Mass. On going over Wood’s description, its identity with S. diluta is unmistakable. The position, color and form of the spore, and the shape of the fertile cells is perfectly represented in Wood’s figure. The dimensions also correspond. Wolle is responsible for confusing this species with S. nitida (Dillw.) Link, but a glance at Wood’s figure is sufficient to show that it is very different from that species. The P. B. A. specimen No. 513 (labelled S. nitida) from Bridgeport, Conn., belongs here. Miss Grace Stone also sent me a collection of this species from near New York City. In the U. S. National Herbarium is another specimen from Bois Sabbi, Louisiana, April 7th, 1891, (A. B. Langlois). Recently the species has been collected at Donnelson, Illinois, by Mr. Frank Harris.
The vegetative cells are usually shorter than in S. nitida, the spores are ovoid, not ellipsoid, and the spore wall is verrucose, or reticulate-verrucose, chestnut brown in color. In herb. E. N. T. Coll. No. 2900.
Var. formosa nov. var. Varietas gracilis, cellulis vegetativis 80-95µ × 80-270µ; zygosporis 88-100µ × 120-150µ × 70-90µ; ceterum ut in typo.
A small variety, vegetative cells 80-95µ × 80-270µ: zygospores 88-100µ × 120-150µ × 70-90µ; otherwise similar to the type. Type in herb. E. N. T. Coll. No. 1939. This variety occurs in a pond east of Ashmore, Ill.
This species which was described from Illinois has been found with nearly the same dimensions in the collections from Middlesex Fells, and South Peabody Station, Mass., sent me by Mr. Chas. Bullard.
Cellulis vegetativis 30-36µ × 120-300µ, dissepimentis planis, chromatophoris singulis anfractibus arctis 3-7; cellulis fructiferis modo binis vel quaternis inter cellulas vegetativas distributis, modo continuis, altero latere (in quo conjugatio sequitur) inflatis, altero rectis; tubo conjugationis plerumque ex cellula mascula emisso; zygosporis ellipticis 37-42µ × 57-100µ membrana media micropunctata et lutea. [28]
Vegetative cells 30-36µ × 120-300µ, end walls plane; 1 chromatophore making 3-7 turns; fertile cells scattered in twos or fours among vegetative cells, or continuous, inflated on the conjugating side, outer side straight; conjugating tubes formed almost wholly by the male cell, zygospores ellipsoid 37-42µ × 57-70µ, median wall minutely punctate, yellow. Type in herb. E. N. T. Coll. No. 2470, 2953.
This species was first found in the West Big Four Pond, east of Charleston, Illinois. It has since been found in a collection from Chocorua, N. H., sent me by Mr. Chas. Bullard. It evidently belongs in the punctata group of the Spirogyras, but in form and markings of the spore, and the shape of the fertile cells it is amply distinct from its nearest allies; S. punctiformis Transeau and the next species to be described.
Cellulis vegetativis 30-40µ × 120-300µ, dissepimentis planis; chromatophoris singulis anfractibus arctis 3-8 cellulis fructiferis binis vel quaternis inter cellulas vegetativas distributis, inflatis et valde reflexis; tubo conjugationis ex cellula mascula emisso; zygosporis ellipticis, 44-54µ × 90-150µ, membrana media glabra et luteo-brunnea.
Vegetative cells 30-40µ × 120-300µ, with plane end wall; 1 chromatophore making 3-8 turns; fertile cells in groups of 2 or 4, inflated or enlarged and strongly reflexed; conjugating tube formed by the male cells; zygospores ellipsoid, 44-54µ × 90-150µ, median wall smooth, yellow-brown. Type in herb. E. N. T. Collection No. 2661, 2664, 2912.
This species has been under observation for four years and has been collected from ponds near Casey, Lerna, Coffeen and Donnellson, Illinois. The large, smooth spores, the reflexed conjugating cells, and the tube produced wholly by the male cells are the distinguishing characteristics.
Cellulis vegetativis 75-100µ × 210-360µ, dissepimentis planis, chromatophoris 7-10, modo subrectis longitudinalibus, modo spiralibus anfractibus arctis .1-.5; cellulis fructiferis inflatis vel subinflatis; tubo conjugationis ex cellula mascula emisso; zygosporis lenticularibus vel globoso-lenticularibus, 80-120µ × 110-195µ, membrana media scrobiculis obsita, brunnea. [29]
Vegetative cells 75-100µ × 210-360µ, end walls plane, 7-10 chromatophores, either straight, or spiral making .1-.5 turns; fertile cells inflated or subinflated; conjugating tube formed by the male cell; zygospores lenticular or globose-lenticular 80-120µ × 110-195µ, median wall brown, pitted. Type in herb. E. N. T. Coll. No. 2661, 2665. Coffeen, Illinois.
This is one of the most remarkable forms described in this genus. It combines large size, the lenticular spore form, and the habit of forming the conjugating tube entirely by the male cell. The conjugating tube has walls heavier than those of any known species. Conjugation is both lateral and scalariform, and occurs between scattered cells, very rarely continuous for 6-8 cells. In the fruiting condition the filaments form a mesh-work which suggests the specific name. It has thus far been found only in the Fath Pond, north of Coffeen, Illinois.
A study of American specimens of this species from Massachusetts, Connecticut, New Jersey, Michigan and Illinois, shows that like S. Grevilleana there are always some cells with two chromatophores. I have twice found this species producing aplanospores.
P. B.-A. specimen No. 456, Easton’s Pt., Newport, R. I., belongs to this variety rather than the type, as shown by the scrobiculate spore wall. In Mr. Bullard’s collection there are also specimens of the variety from Pennannock, N. J., and from Spy Pond, Lake St., Arlington, Mass.
Cellulis vegetativis 24-30µ × 70-180µ, dissepimentis replicatis; chromatophoris singulis, rarius duobus, anfractibus arctis 2.5-6; cellulis fructiferis inflatis (ad 39-60µ); zygosporis ellipticis, polis plus minus acuminatis, 32-45µ × 48-93µ, membrana media glabra, lutea. [30]
Vegetative cells 24-30µ × 70-180µ, end walls replicate; 1 (rarely 2) chromatophore making 2.5-6 turns; fertile cells inflated to 39-60µ; zygospores ellipsoid, ends more or less pointed, 32-45µ × 48-93µ, median wall smooth, yellow. Type in herb. E. N. T. Coll. No. 2955, 2956, 2957.
In Mr. Bullard’s collection there are specimens of this species from Lexington, Arlington, and Middlesex Fells, Mass. The P. B.-A. specimen No. 362, labeled S. Grevilleana, from Medford, Mass., belongs here, rather than to S. Grevilleana, in which the spores are distinctly ovoid with broad rounded ends.
This interesting form is characterized by quadrately inflated fertile cells, highly refractive cell walls, and unusually long cells and spores. In Mr. Bullard’s collection there are specimens from Stony Brook, South Framingham, Middlesex Fells, Wayside Inn, North Eastham, and Malden Fells, Massachusetts. The P. B.-A. specimen No. 363 labelled S. inflata, Orange, Conn., belongs to this species.
This species is one of several forms near S. insignis (Hass.) Kützing. If Wille’s description is correct and identical with Hansgirg’s material, then S. inconstans Collins becomes a synonym of S. fallax. Hansgirg’s figure suggests that the filaments in his material are homosexual. While Wille’s description and figure suggests that the filaments are reflexed and that conjugation does not regularly occur between parallel filaments, with the spores all in one filament. It is difficult to decide just where these rough-spored forms belong as the earlier authors did not pay much attention to spore markings. In this connection the note by Professor Nordstedt in connection with specimen No. 958 in Wittrock and Nordstedt’s Algae Exsiccatae is of interest. Until these forms have been clearly separated by a study of the original collections it seems best to use S. fallax for S. inconstans, of which the type is P. B.-A. No. 1568. Here also belongs P. B.-A. No. 1570, Middlesex Fells, Mass., and P. B.-A. No. 1571, Wakefield, Mass.
Cellulis vegetativis 56-66µ × 120-335µ, dissepimentis planis; chromatophoris 4-5, subrectis vel anfractibus arctis .5; cellulis conjugatis abbreviatis, inflatis (ad 135µ) et geniculatis; canalis conjugationis brevis et latis; zygosporis ellipticis, 75-105µ × 95-135µ membrana media glabra, lutea. [31]
Vegetative cells 56-66µ × 120-335µ, end walls plane; 4-5 chromatophores, nearly straight or making a half turn; conjugating cells geniculate, shortened; fertile cells inflated up to 135µ; conjugating tube very short and broad; zygospores ellipsoid, 75-105µ × 95-135µ median wall smooth, yellow. Type in U. S. National Herbarium, collected by J. D. Smith, in S. W. Florida, March, 1878.
In its dimensions S. floridana is intermediate between S. stictica (Eng. Bot.) Wille and S. ceylanica Wittrock. In several publications the statement is made that S. ceylanica is intermediate between S. stictica and the common forms of Spirogyra. A study of authentic material of this species has shown that it has not intermediate characters, but with its spores having a minutely pitted median wall, it seems to be intermediate between S. floridana and S. illinoiensis Transeau, the most specialized form in the Sirogonium group of the genus.
Throughout the study of these collections the writer has been greatly assisted by Mr. Hanford Tiffany, now a teacher in the Charleston, Illinois, High School. It is a pleasure to acknowledge my indebtedness to the many collectors who have sent me specimens for study.
As the result of the sentiment expressed at the 1914 meeting of the Ohio Academy of Science that the official organ of the Academy, “The Ohio Naturalist,” should be broadened and made more comprehensive in scope, and feeling that the Ohio State University had no publication representing the scientific work being done at the institution, the members of the Biological Club of the University, in whom the publication of the “Ohio Naturalist” had been vested, called a meeting of representatives of the various departments interested in science at the university to discuss the advisability of publishing as successor to the “Naturalist” a journal to be known as the Ohio Journal of Science.
The first meeting was held in May, 1915, and committees appointed to outline preliminary plans. At subsequent meetings the reports of the committees were discussed, interest in the plan continued to develop, until at a meeting held October 13 the following self-explanatory Constitution was adopted. The society as now constituted represents twenty-four departments of pure or applied science at the university.
The name of this society shall be the Ohio State University Scientific Society.
It shall be the purpose of the Society to promote scientific work in the University by holding meetings for the presentation and discussion of the results of scientific work; by co-operating with other agencies in arranging for scientific lectures and in the entertainment of visiting scientists and scientific societies; by publishing the Ohio Journal of Science and by furnishing opportunity for the discussion and promotion of any project of scientific interest which may properly come within the scope of such an organization and, in general, by furthering in every way possible the interests of scientific work in the University and the State.
Any member of the instructional staff in the Ohio State University interested in scientific work shall upon application be eligible to election to membership in the Society. Students of the Ohio State University interested in scientific work shall be eligible to membership when endorsed by two faculty members of the society.
Section 1. The officers of the society shall consist of President, Vice-President, Secretary and Treasurer. These officers shall perform the duties common to such positions.
Section 2. The Executive Committee shall consist of the officers and the Editor of the Ohio Journal of Science. It shall have power to arrange programs for meetings, to represent the society when co-operating with other organizations and to conduct all affairs of the society not otherwise provided for. [34]
The Editorial Board shall be responsible for the management of the Ohio Journal of Science. It shall consist of representatives, one from each department of science in the university represented in the society membership. This board shall elect annually an Editor and two Associate Editors.
Election to membership shall be by vote of the Executive Committee.
The officers shall be elected by ballot at the annual meeting in May. Nominations shall be presented by a nominating committee which shall consist of the Editorial Board.
One member of the Editorial Board shall be elected by each department from among the members of such department represented in the society and in case any department fails to elect a member for this board the Executive Committee shall elect for the department.
The Editor and Associate Editors of the Ohio Journal of Science shall have immediate direction of the publication. The department editors shall be responsible for the approval of papers from their several departments, and all papers offered for publication shall be submitted to such department editors.
The selection for publication from available material shall be determined by the Editorial Board.
A quorum for the transaction of regular business shall consist of at least fifteen members with a representation of at least one-third of the departments included in the society.
Amendments to the constitution may be made by the concurrence of three-fourths of the members present at a duly called meeting, notice of such amendment having been given to all members at least one week in advance.
The membership fees of the society shall be twenty-five cents per year or one dollar for a period of five years and such fee shall entitle the members to participation in all activities of the society but shall not include the subscription to the Ohio Journal of Science.
The subscription price to the Ohio Journal of Science shall be two dollars to non-members, and one dollar and seventy-five cents to members.
The fiscal year of the society shall coincide with that of the University—July 1st to June 30th. The publication to be issued during eight months, beginning with November.
Regular meetings shall be held on the second Tuesday evening of the months of October, November, March, April and May. The meeting in May shall be the annual meeting for the election of officers and an editorial board. Other meetings may be called by the Executive Committee, or by the President on petition of five members.
The University Instructional Staff shall be understood to include any member of the teaching force.
Amendments to the By-laws may be adopted at any regular meeting by vote of a majority of the members present, notice of proposed amendment having been given at time meeting is called.
The College Book Store
Reference books in all departments of Higher Education.
Biological Supplies and Advanced Text Books
new and secondhand.
OPPOSITE THE UNIVERSITY ENTRANCE.
COLUMBUS, OHIO.
The Bucher Engraving Co.,
COLUMBUS, OHIO
Scientific Illustrations given extremely
careful attention by highly skilled
artisans using the most modern equipment
DIE STAMPING. PLATE AND LETTER PRESS PRINTING.
SPAHR & GLENN,
PRINTERS and PUBLISHERS.
50 EAST BROAD STREET. COLUMBUS, OHIO.
The Ohio State University
COLUMBUS
WILLIAM OXLEY THOMPSON, President.
Ten Colleges and a Graduate School |
|
| College of Agriculture | |
| College of Arts, Philosophy and Science | |
| College of Education | |
| College of Engineering | |
| College of Homeopathic Medicine | |
| College of Law | |
| College of Medicine | |
| College of Dentistry | |
| College of Pharmacy | |
| College of Veterinary Medicine | |
| Graduate School | |
| Summer Session (Eight weeks) | |
For general information, catalogue, or special bulletin describing each college, with fees and announcement of courses.
Address:
L. E. WOLFE, Secretary Entrance Board,
THE OHIO STATE UNIVERSITY
FOOTNOTES:
[A] Contribution from the Botanical Laboratory of the Ohio State University, No. 91.
[B] Hallas, E., Om en ny Zygnema-Art med Azygosporer. Bot. Tidsskrift 20:1-16. 1895.
[C] See Fig. 3, Plate XXV, Amer. Jour. Bot. 1:301. 1914.
[D] Borge, O., Beitrage zur Algenflora von Schweden.
[E] Transeau, E. N., Annotated list of the Algae of Eastern Illinois. Trans. Ill. Acad. Sci. 6:69-89, 1913.
[F] West, G. S., A contribution to our knowledge of the Freshwater Algae of Columbia. Memoires de la Societe neuchateloise des Sciences Naturelles 5:1013-1051. Neuchatel, 1914.
Transcriber's Notes:
The cover image was created by the transcriber, and is in the public domain.
Uncertain or antiquated spellings or ancient words were not corrected.
The illustrations have been moved so that they do not break up paragraphs and so that they are next to the text they illustrate.
Errors in punctuation and inconsistent hyphenation were not corrected unless otherwise noted.
Typographical errors have been silently corrected but other variations in spelling and punctuation remain unaltered.