

by
Bernard Keisch
United States Atomic Energy Commission
Office of Information Services
Library of Congress Catalog Card Number: 70-606040
1970; 1974 (rev.)

Dr. Bernard Keisch received his B.S. degree from Rensselaer Polytechnic Institute and his Ph.D. from Washington University. He is a Senior Fellow with the Division of Sponsored Research of Carnegie-Mellon University in Pittsburgh. He is presently engaged in a project that deals with the applications of nuclear technology to art identification. This is jointly sponsored by the U. S. Atomic Energy Commission and the National Gallery of Art. Previously he was a nuclear research chemist with the Phillips Petroleum Company and senior scientist at the Nuclear Science and Engineering Corporation. He has contributed articles on art authentication to a number of journals. For the AEC, in addition to this booklet, he has written The Atomic Fingerprint: Neutron Activation Analysis, Secrets of the Past: Nuclear Energy Applications in Art and Archaeology, and Lost Worlds: Nuclear Science and Archaeology.
Nuclear energy is playing a vital role in the life of every man, woman, and child in the United States today. In the years ahead it will affect increasingly all the peoples of the earth. It is essential that all Americans gain an understanding of this vital force if they are to discharge thoughtfully their responsibilities as citizens and if they are to realize fully the myriad benefits that nuclear energy offers them.
The United States Atomic Energy Commission provides this booklet to help you achieve such understanding.
This painting, originally believed to be the work of the Dutch artist Frans Hals (1580-1666), is a fake. Measurements of the naturally radioactive isotopes, polonium-210 and radium-226, in lead white from the paint proved that it was no more than 50 years old.

A Van Meegeren forgery of a Vermeer.
The New Jersey sun was high overhead and the day was hot. The three boys walking along a deserted stretch of beach didn’t mind because they were barefoot and in their swimsuits. Occasionally they would dash in and out of the surf to cool off.
Suddenly Martin let out a yell as his toe hit something hard hidden in the sand at the water’s edge. A moment later Bill and Harley were helping Martin dig out a large wooden case. It was heavy, well built, tightly sealed, and had foreign words written on it.
“Maybe it’s a pirate treasure chest,” said Martin, who was almost eight and had just read Treasure Island for the first time the week before.
“You’re crazy,” said Harley, who, nearly ten, was much older and wiser.
Bill, going on twelve, thought aloud, “It must be something worthwhile; maybe we can sell it and buy those model rockets we wanted.”
The three boys soon found that they couldn’t open the box and that it was too heavy to drag along the sand easily.
“Martin,” said Bill, “get Dad while Harley and I stand guard.”
Two hours later the box was at their house and everyone in the family was trying to read what was written on it. About all that was readable was a large “U” followed by what appeared to be two numbers. Some 3 of the other marks looked like old German script and there was a date, 1945.
“You know,” said Bill, “I bet that came from a World War II German submarine that our Coast Guard or Navy sank.”
“Let’s open it up!” said Harley as Martin ran to get the screwdrivers.
Inside they found a thoroughly waxed carton that they had to cut open. Everyone held their breath as their father lifted the top.
“Nothing but a bunch of pictures,” said Martin who was still hoping for pirate treasure.
“Paintings can be worth a lot of money,” said Dad, “thousands or even millions of dollars.”
“Well then we’re rich!” yelled Harley and Bill together.
“Not so fast,” said Dad. “First of all, we don’t know if the paintings are really valuable. Also, it looks like these might be part of the art treasures that the Nazis stole from the countries they conquered in World War II. Maybe someone was trying to get them by submarine to a neutral country, like Argentina, just before the end of the war, and the sub was sunk. If they are real and stolen, they’ll have to go back to their rightful owners. But cheer up, maybe there’s a reward.”
“How do we collect it?” asked Bill. “If the Nazis grabbed them, aren’t they real for sure?”
“Not necessarily,” Dad continued. “The Nazis were fooled sometimes by people who sold them fakes. There was one painting that 4 Hitler’s sidekick, Göring, bought that was supposed to be a 17th century painting by Vermeer, a Dutch painter. Because Vermeer’s work is so valuable, it’s usually impossible to buy one for any amount of money.
“Vermeer is regarded as a national hero by the Dutch. The matter was investigated and the painting traced to Han Van Meegeren, a modern Dutch painter who had only a fair talent. When Van Meegeren realized he might be charged with treason by the Dutch for selling a Vermeer to the Nazis, he confessed that he had painted it himself. He also confessed that he had painted other forgeries that fooled some of the experts and were sold for a lot of money.
“Many people, however, thought Van Meegeren was only lying to save himself from the charge of treason, and the whole thing had to be decided by a committee of scientific art experts appointed by a court of law. Using the methods that were then available, the experts showed that Van Meegeren had done a remarkable job of forgery and they were convinced that he had been telling the truth about painting those pictures.
“At the time, the important ways the experts used to examine a painting included studying the work with X rays, which could show another painting underneath, analyzing the pigments (or coloring materials) used in the paint, and examining the painting for certain signs of old age.

Han Van Meegeren listens to the evidence at his trial in Amsterdam. In the background is “The Blessing of Jacob”, which was sold in 1942 as the work of Vermeer.

An authentic Pieter de Hooch work, “The Card Players”, painted in the 17th century.

A forgery of a Pieter de Hooch picture painted in the 20th century by Han Van Meegeren.

“Head of Christ” by Van Meegeren.
“Van Meegeren was well acquainted with these methods. He scraped the paint from old paintings that weren’t worth much just to get the canvas and tried to use pigments that Vermeer would have used. He knew that old paint was very, very hard and impossible to dissolve; so he cleverly mixed a chemical (phenolformaldehyde) into his paint, and this hardened into Bakelite when he heated the finished painting in an oven.
“For some of the paintings, Van Meegeren became careless and the experts did find traces of a modern pigment (cobalt blue) in the paint. They also found the Bakelite. For one or more paintings, Van Meegeren did so well that, in spite of all this evidence, a few people still weren’t convinced that these paintings were painted by Van Meegeren and not by Vermeer.”
Bill, who by this time was bursting with questions, interrupted, “You mean they still aren’t sure about some of those paintings after 25 years? Aren’t there better ways of telling whether a painting is genuine or not? You’re a scientist. Can’t scientists like you do something about it now?”
“Yes, recently a method was developed to settle just such a question. It’s based on measurements of natural radioactivity in one pigment that all artists used hundreds of years ago. And the method was applied to some of the Van Meegeren paintings including the best one of them all.”
“How did it come out?” asked Martin.
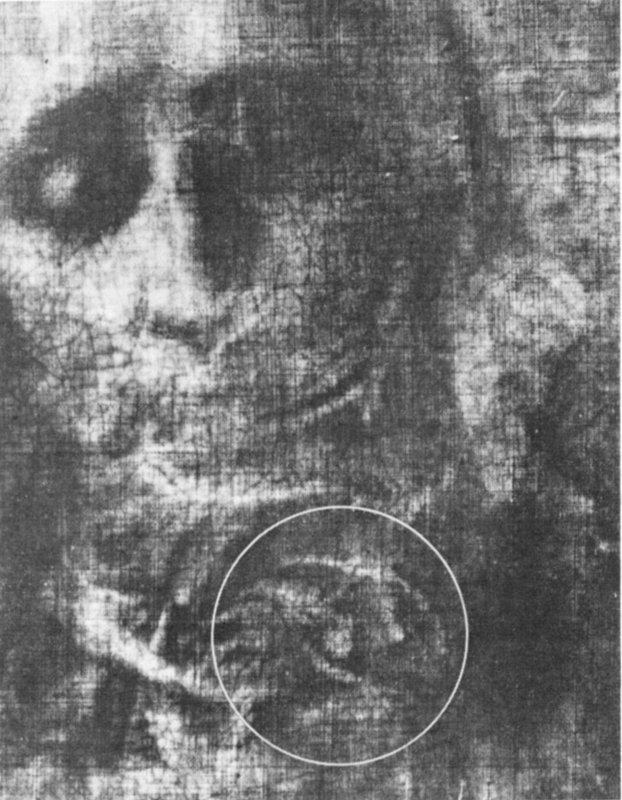
An X ray of part of the Van Meegeren forgery, “Christ and His Disciples at Emmaus”. In the white circle are traces of paint from the original painting that Van Meegeren scraped off to obtain the old canvas. When the painting was believed to be a genuine Vermeer, it was sold for about $300,000.

The complete painting.

A Van Meegeren forgery of a Vermeer.
“How does it work?” asked Harley.
“You mean paintings are radioactive?” exclaimed Bill.
“Can we do it to the paintings we found?” asked all three together.
“One question at a time. I’ll tell you how the method works and what it does if you’re really interested.”
“We’re interested! We’re interested!” chorused the boys.
“In the first place, this method works only in certain cases of suspected forgery. Over the last 50 or 100 years, a number of paintings have turned up that seemed, even to the best art experts, to be several hundred years old. Some of these were genuine, and some were painted by forgers who could not resist the high prices paid for works of art. The National Gallery of Art, in Washington, D. C., thinking that there might be a way of detecting these forgeries, gave its support to a group of scientists who developed a method for this purpose.
“To understand how the method works, you need to know a little about how radioactive atoms disintegrate to form atoms of other elements. In this case we are interested in the natural radioactivity that occurs in certain rocks. As a matter of fact, in almost all rocks in the earth’s crust there is a certain small quantity of uranium.”
“I thought uranium was rare,” interrupted Bill.
“It is, but we’re talking about such small quantities that its difficult for scientists using the most sensitive equipment to detect it. The uranium in the rock decays to another radioactive element and that one decays to another, and another, and another, and so 12 forth, in a series of elements that results in lead, which is not radioactive. In this series are two radioactive elements, radium and a radioactive isotope of lead, that help us to date paintings. To understand this, we must first understand how radioactive elements decay.
“All radioactive elements have what is known as a ‘half-life’; that is, in a certain period of time, half of the element disintegrates to another form. In another equal period of time, half of what is left disintegrates, and then half again, and so on. In the case of the uranium, which starts the series I am describing, the half-life is over 4,000,000,000 years. Because of its long half-life there is plenty of uranium around and will be for a long, long time. On the other hand, radium, which I mentioned a moment ago, has a half-life of only 1600 years. In 1600 years, half of it would be gone, and in another 1600 years half of that would be gone, and so on.
“The radioactive lead that we’re interested in has a half-life of only 22 years. This means that if you start with a small quantity of this radioactive isotope of lead, which is called lead-210,[1] then in only a few hundred years it would have disappeared. However, in rock, where there is uranium, the uranium keeps feeding the elements following it in the series, so that as fast as they decay they are reproduced by the element before them.”
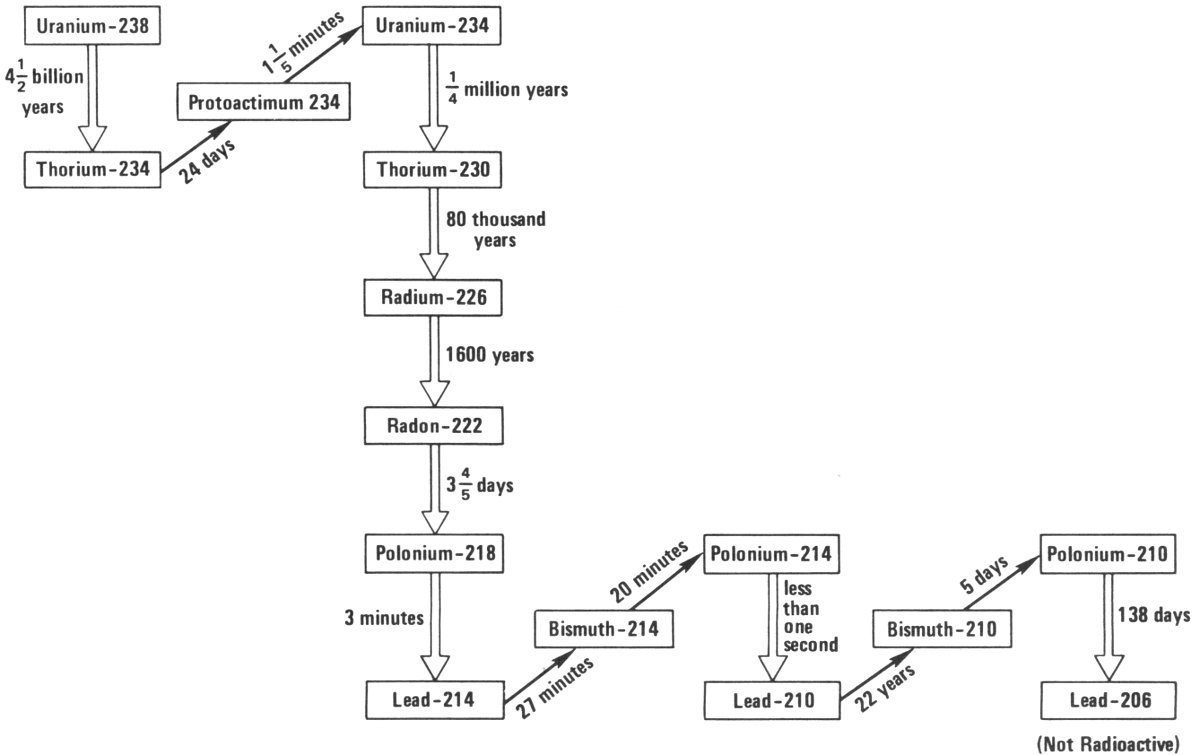
The Uranium Series. In this simplified diagram, the double vertical arrows represent alpha radioactivity and the single slanted arrows represent beta radioactivity. The times shown on the arrows are the half-lives for each step.
“I don’t quite understand how that works,” said Harley. “What do you mean ‘it keeps feeding it’?”
“Well, think of a series of lakes connected by waterfalls. At the top, the highest lake has an enormous supply of water. Following the waterfall coming out of the lake you find a smaller lake and then maybe a medium-sized lake, and after another waterfall, a smaller lake, then a tiny lake, and so on.
“As long as that big lake on top is full or nearly full, all the other lakes, whether they are small or medium-sized, will still be getting water as fast as it pours out. But if you cut off the supply of water from the upper lake to the next lake, then the smaller lakes will in time run dry. The same thing works with the radioactivity. In this series headed by uranium, as long as uranium is present all the other elements below it are kept supplied so that they don’t run out.”
“I understand that,” said Bill, “but how do we use that to date a painting?”
“One of the pigments used by artists for over 2000 years is known as lead white and it is made from lead metal. The lead metal in turn is extracted from a rock called lead ore, in a process called smelting. The radioactive lead, this lead-210 that I mentioned, behaves like ordinary lead metal and goes along with it.
“The radium, which has a fairly long half-life, doesn’t follow the lead metal, but is removed with other waste products in a material called slag. Since the longer-lived ancestor of the lead-210 is removed, the 15 supply of lead-210 is cut off. (Or we can say that one of the waterfalls is shut off.) The lead-210 will then decay with its 22-year half-life.”
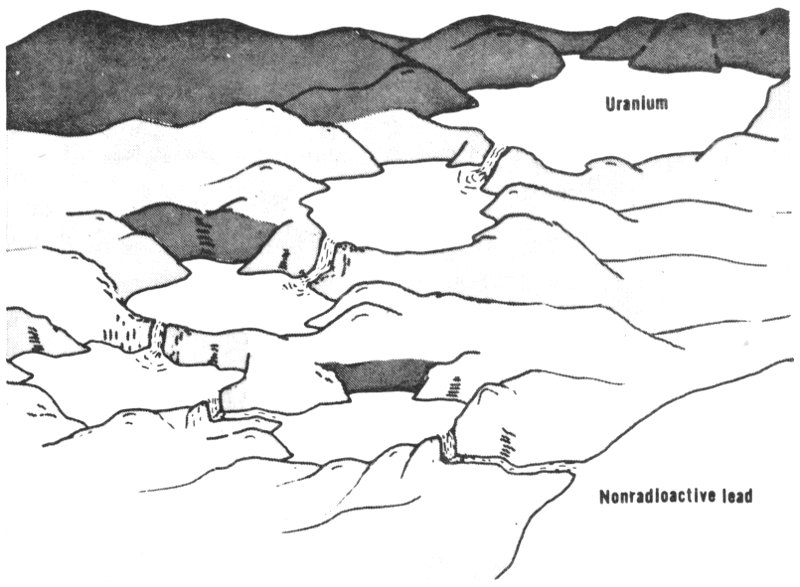
The radioactive series that starts with uranium is like a series of lakes connected by waterfalls. As long as uranium, the big one on top, has water in it, the others will be full and the falls will keep flowing. But when the first waterfall is shut off, the small lakes below it will run dry.
“I get it,” said Bill. “That means that when you take a sample of old lead white paint, there shouldn’t be any radioactive lead-210 left.”
“That’s right. But that would only be true if you removed all the radium. Actually, in the smelting process it’s more usual to remove only 90 or 95% of the radium. In that case, the lead-210 would decay only until the amount left would be equal to the small amount of radium that wasn’t removed. In 16 effect, this would be like shutting off only part of the waterfall.”
“So what do you find,” asked Harley, “if you measure the radioactivity in a sample of lead white paint?”
“We find that if the paint is old, compared to the 22-year half-life of the lead, let’s say 100 years old or more, then the amount of radioactivity from the lead-210 in the sample of paint will be equal to the amount of radioactivity from the radium in the sample. But if the paint is modern, let’s say only 20 years old or so, then the amount of radioactivity from the lead-210 will be greater than the amount of radioactivity from the radium.”
Martin, who had been quiet through all this explanation, finally spoke up. “Well, was it finally tried out? How did it work?”
“Hundreds of samples were analyzed. These samples were taken from paintings of all ages, from some over 300 years old right up to others only a couple of years old. The old samples always showed equal amounts of radioactivity from lead-210 and radium while the modern ones always showed larger amounts of radioactivity from lead-210 than from radium. That meant that scientists had a way of definitely telling if a lead white paint was modern or not.
“Eventually, the method was tried on a number of paintings believed to be by Van Meegeren. Sure enough, every one of them showed that the paint couldn’t possibly have been more than 30 or 40 years old and that Van Meegeren probably was telling the truth 17 when he said that he had painted them. The paintings certainly were not genuine Vermeers from the 17th century.”
“Okay, Dad,” said Martin, “can we use the method on any of the paintings we found? Are any of these paintings supposed to be old enough so that we can use this test?”
“Not so fast. To find that out we have to do a lot of checking first.”
“How do we go about it?” asked Bill.
“Let’s see now. There are nine paintings in the box you found. The first thing we should do is take them down to a museum or gallery and let the art experts look at them. Since we have a few weeks of vacation time left, what do you say we take a trip down to Washington, D. C., and show them to some experts at the National Gallery of Art?”
Over the next few weeks quite a few things happened to the boys and their paintings. Three of them were discarded right away because they were immediately recognized as being copies of no value. Two were relatively modern paintings with the signature Alfred Sisley; if genuine, they were less than 100 years old. The remaining four appeared to be very old paintings. Two of them seemed to correspond to paintings that disappeared during the Second World War. Photographs and X rays were taken and sent to the museum in Holland, which had owned the missing pictures, so that they could make a preliminary examination.

Lead-210 decaying with a half-life of 22 years. When no radium is present there is almost none left after 6 half-lives or 132 years.
Over the same period of time, a small amount of radium decays very little because its half-life is about 1600 years.
But when lead-210 decays in the presence of radium-226, the radioactivity of the lead-210 only decreases until it is equal to the radioactivity of the radium.
That left two that could have been old but whose origins were unknown. A series of simple chemical tests were begun on these and the boys watched experts take very small samples of paint for examination under the microscope. After several months a list of the pigments present in the paintings was prepared. All the pigments found were typical of old paintings and the ordinary examinations and tests couldn’t prove whether the works were old or not. Finally, it was decided that 19 the only way to tell if these paintings were truly old was to apply the test that Dad had described to the boys.
The boys watched a painting restorer remove samples of nearly white paint right at the edge of the paintings. He worked carefully, using a very sharp scalpel and a stereo-binocular microscope, through which objects appeared to be sixty times larger than they really were. The sample of paint weighed approximately twenty-thousandths of a gram. The boys and their father took the samples to a radiochemical laboratory where they watched a radiochemist do the required analysis for lead-210 and radium in the samples.
First the chemist dissolved the paint in acetic acid. This removed the lead white from the oil and from the small amounts of other pigments in the paint. The solutions were then heated and stirred with a silver disc hanging in the liquid. After several hours the disc still looked clean, but the chemist said that a radioactive element, polonium-210, was now plated onto the silver. Polonium-210 is a member of the uranium series following the lead-210, and a measurement of its radioactivity would be an accurate measurement of the radioactivity of lead-210.
The silver discs prepared from the two samples were each placed in an instrument called an alpha-particle spectrometer. This instrument is extremely sensitive and can measure the very small amounts of polonium-210 prepared from the tiny sample of paint that they started with.
While the instruments were making the measurements, which took a couple of days, the chemist turned to the remaining solutions and began the analyses for radium.

A painting being sampled under a stereo-binocular microscope.
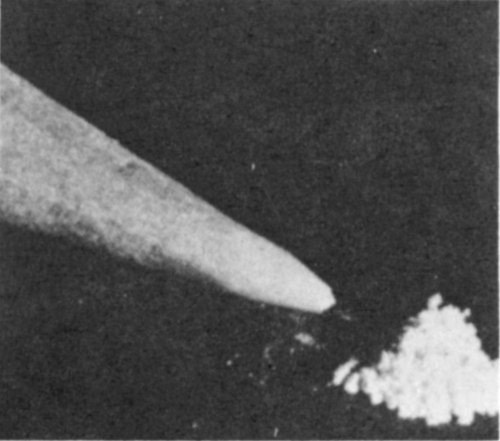
Lead white weighing twenty-thousandths of a gram (20 milligrams). This is the amount needed to measure lead-210 and radium-226 to determine if the lead white is old.
In a series of chemical steps, he purified the solutions, removing the lead and other materials so that finally he had a small amount of solution that contained little else but the original radium and a very small amount of barium (an element that he deliberately added and one which is very similar to radium in its chemical properties). By adding dilute sulfuric acid, he prepared an insoluble 21 material, barium sulfate, which was barely visible suspended in the solution.

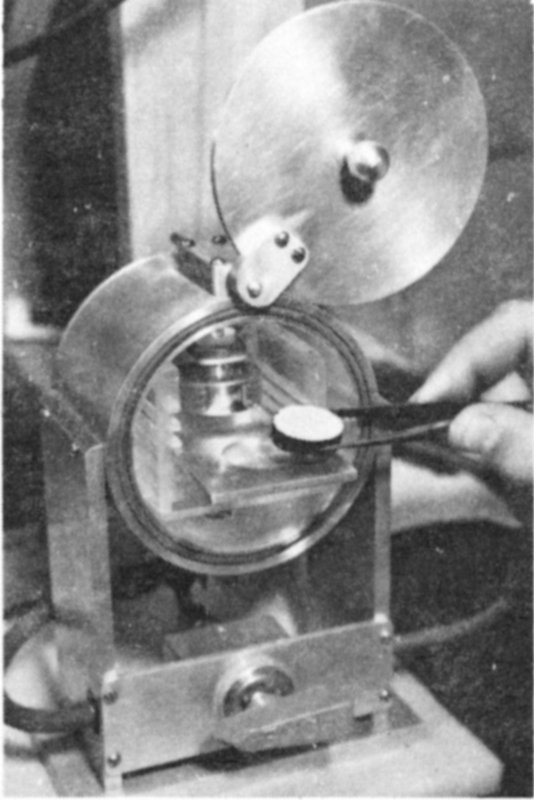
Polonium plating apparatus. A heated solution of lead white in acetic acid is stirred with silver discs for 4 to 8 hours.
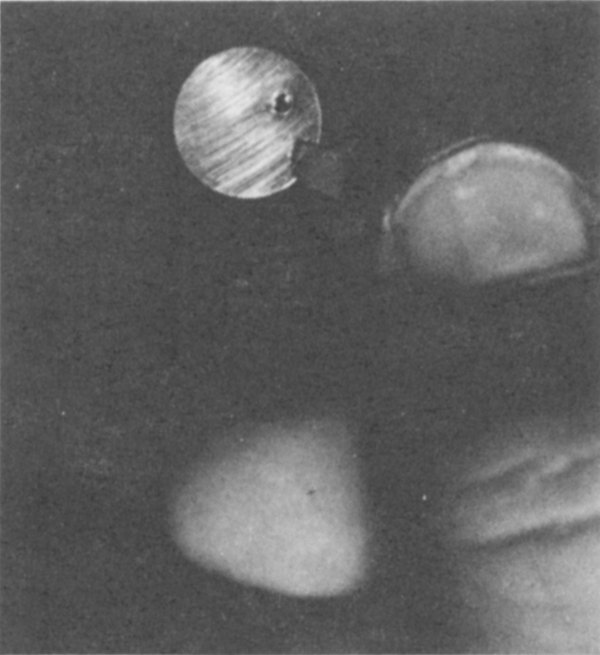
The disc above appears clean after removal, but on its surface it retains a minute amount of polonium which can be measured.
By forcing the solution through a special thin plastic filter having tiny holes, the particles of barium sulfate together with the radium that had been in the solution were caught on the surface of the filter. This was mounted on a solid disc so that it too could be placed in the alpha-particle spectrometer for the measurement of radioactivity from the radium.
Two weeks later the results were ready. Dad, the boys, and one of the experts from the museum met with the chemist to discuss them. For one of the two paintings, the 22 polonium-210 radioactivity was about ten times that of the radium activity. The boys were disappointed because this meant that the painting could not have been 300 or 400 years old as it first appeared to be.

An alpha-particle spectrometer is used to measure the radioactivity of the radium and polonium prepared from the lead white.
A plastic disc on which is cemented a filter containing a nearly invisible deposit of barium sulfate (BaSO₄) that “carried” the radium.
But in the second painting the radioactivity from the polonium-210 and from the radium-226 were just about equal. That meant that this painting was at least 100 years old and, from its appearance, probably more. The boys were excited.
“We have a really valuable painting!” said Martin.
“Not so fast, boys,” cautioned Dad. “We don’t know who painted it and we don’t know exactly how old it is.”
The Gallery’s expert was happy too. He believed that the second picture was a genuine Dutch painting from the 17th century. It was 23 a landscape and the artist might have been Aelbert Cuyp.

“The Maas at Dordrecht”, a genuine painting by Aelbert Cuyp.
“What do we do now?” asked Harley. “How can we prove that the painting was painted in Holland in the 17th century by Cuyp?”
“There is a method now being developed,” said Dad, “that could give us that kind of information.”
“How does it work?” Martin asked.
“Do you know how criminals are caught by using fingerprints?” asked Dad.
“Sure we do,” said Martin. “Each person has a set of fingerprints that is different from anyone else’s.”
Harley spoke up. “Did the artist leave his fingerprints on the paintings?”
“Probably not,” said Dad. “Besides, they would have been wiped off long ago. Also, who knows what each artist’s fingerprints were like?”
“Then what do you mean?” asked Bill.
“What I mean is, there is another kind of ‘fingerprint’ that scientists are just now learning to use in all kinds of identification problems. It’s not really a fingerprint, but it’s just as distinctive as a real fingerprint.
“You see, in every material, no matter how pure you try to make it, there are always other substances contained in it in very, very small quantities, which are there only by chance. Usually the person making or using that material doesn’t even know they are there, and the quantities are so small they don’t do any harm. During the last several years, scientists have developed extremely sensitive methods of analysis, which have been applied to all kinds of problems.
“One such method is called neutron activation analysis. In this method these small amounts of impurities can be detected in tiny samples of material. This is quite important because only very small samples can be taken from a precious painting without damaging it. 25 Normally, a scientist or an art restorer takes samples that are no bigger than the head of a pin.”
“How can you do anything with a sample that small?” asked Bill.

“With neutron activation analysis you can do a great deal. To give you an example of how sensitive this method is, think of a bathtub containing 500 quarts of milk. Add 1 drop of an acid containing a speck of gold dissolved in it. After you mix the acid and milk thoroughly, you won’t be able to tell by looking at it that anything was added. But if you take a thimble full of liquid out of the bathtub, you can easily tell with neutron activation analysis that gold was added to the milk.
“Scientists call low concentrations of accidental impurities ‘trace elements’, and the amounts that are present are measured in parts per million rather than percent. One part per million is one ten-thousandth of a percent.”
Bill spoke up again. “So how does that make a fingerprint, Dad?”
“It works this way. Suppose an artist used lead white in several paintings. Now if the lead white were absolutely pure it would contain only lead, carbon, oxygen, and hydrogen. But the lead white the artist used would also contain very small quantities of other elements, these trace elements that I spoke of. In that particular batch of lead white, certain trace elements will be present in a certain quantity. The kind and amount of the trace elements will be present in that exact pattern only in that batch of lead white.
“Now suppose you analyze the lead white from several paintings that you know were painted by that particular artist, and you find that there is silver, mercury, antimony, tin, and barium in every one of the samples. Also, each of these elements is always present in a certain concentration. Suppose also, that you have a painting which looks like it was painted by that particular artist but you’re not quite sure.
“Well, if you take a sample of lead white from that unknown painting and you find that the pattern of impurities is the same as in the paintings you knew were genuine, then the ‘fingerprints’ match. The chances of duplicating impurities of this kind by pure accident are extremely small, just about as small as the chances of finding two people with the same fingerprints. That’s why we call this a ‘fingerprint method’.”
“That sounds like a good idea,” said Harley. “Who thought it up?”
| x = one part per million (ppm) | |||||
|---|---|---|---|---|---|
| A known Rembrandt. | |||||
| x | |||||
| x | |||||
| x | x | x | |||
| x | x | x | x | ||
| x | x | x | x | ||
| x | x | x | x | x | |
| x | x | x | x | x | |
| x | x | x | x | x | x |
| silver | chromium | zinc | manganese | iron | cobalt |
| Unknown painting A | |||||
| x | |||||
| x | |||||
| x | x | ||||
| x | x | ||||
| x | x | x | |||
| x | x | x | x | ||
| x | x | x | x | ||
| x | x | x | x | x | |
| x | x | x | x | x | x |
| silver | chromium | zinc | manganese | iron | cobalt |
| Unknown painting B | |||||
| x | |||||
| x | |||||
| x | x | x | |||
| x | x | x | x | ||
| x | x | x | x | ||
| x | x | x | x | x | |
| x | x | x | x | x | |
| x | x | x | x | x | x |
| silver | chromium | zinc | manganese | iron | cobalt |
| Known forgery | |||||
| x | |||||
| x | |||||
| x | x | ||||
| x | x | ||||
| x | x | x | |||
| x | x | x | x | ||
| x | x | x | x | ||
| x | x | x | x | x | |
| x | x | x | x | x | x |
| silver | chromium | zinc | manganese | iron | cobalt |
Match the patterns of these lead white “fingerprints”. Unknown painting A is not a Rembrandt; it is by the same forger who painted the known forgery at the bottom. Unknown painting B is either by Rembrandt, one of his fellow citizens, or one of his students using the same paint.
“It was thought of many times by many people. But, it’s never been used for identifying paintings. In 1964 in the Netherlands, two scientists, named Houtman and Turkstra, analyzed about 40 different samples of lead white, 20 of which came from Dutch and Flemish paintings. The rest were samples of lead white not taken from paintings but obtained directly from the manufacturers. They analyzed these samples for different elements. These included silver, mercury, chromium, manganese, tin, antimony, and a couple of others.
“They found that the concentrations of these elements in the lead white from all the old Dutch and Flemish paintings were very similar. And the trace element concentrations were quite different in the modern lead white samples analyzed in the same way. At the time, they presumed that it was because the lead white in the paintings was manufactured so long ago. They may have been right to a certain extent.
“For example, they found that in all the old paintings there were from 10 to 30 parts per million of silver in the lead white, while in the modern samples of this pigment there were generally less than 10 parts per million of silver. All of them had been painted before the 19th century, and all the samples of pure lead white were manufactured during the latter part of the 19th century or during the 20th century. They believed that the reason the silver concentration was lower in the more modern material was because during the 19th century, lead refiners were doing a better job of removing all the valuable silver from lead.
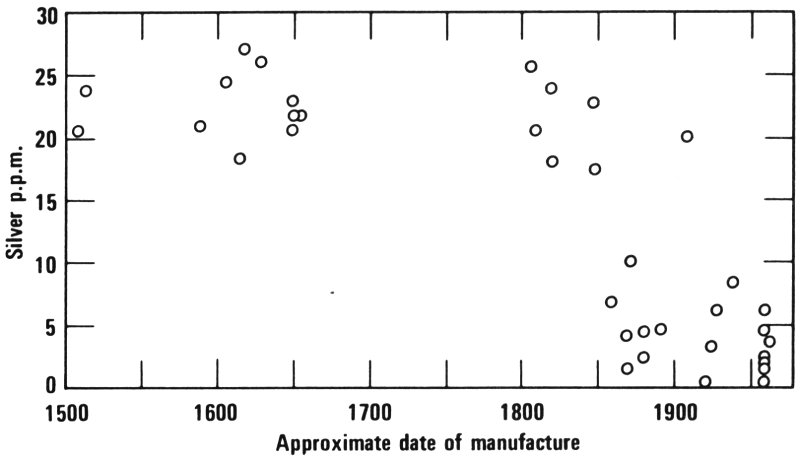
Silver concentrations in lead white. The concentrations generally decreased after the middle 1800s. Notice also how the concentrations were very similar for all the older paintings (before 1700) which were Dutch or Flemish.
“However, in 1967 in Germany, two men, named Lux and Braunstein, discovered that in some old paintings produced in Italy, lead white also contained low quantities of silver just like modern material. They believed that the higher concentrations of silver in lead white were typical of Dutch and Flemish painters while the lower concentrations were typical of Italian paintings of about the same age.
“The whole case is still unsettled because not enough measurements have been made to show how reliable this method can be. That is, no one knows if samples of paint from several paintings by one artist would all have the same pattern of impurities in the same pigment. It may be that of the many pigments present in an artist’s paintings only a few will 30 be suitable for use in this ‘fingerprinting’ method.”
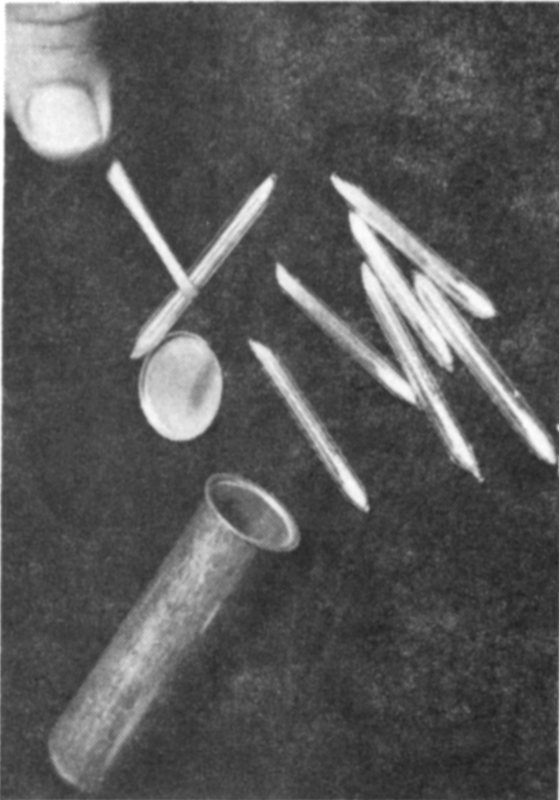
Quartz vials (right) containing samples are sealed in the aluminum can on the left. They are then bombarded with neutrons in a reactor like the one in the picture below.
“It sounds complicated,” said Bill.
“It is, and it’s going to take years of work before the method is proven, if it is at all. It may turn out that you can’t tell one artist from another, but only groups of artists like 17th century Dutch painters or 19th century English painters.”
“Tell us something about neutron activation analysis,” said Martin. “How do you measure such small amounts of impurities?”
“The best way to tell you how this works is to show you. How would you boys like to visit a laboratory where neutron activation analysis is being done?”
“Do you have to ask?” said Harley. “Of course we would!”
A few weeks later it was all arranged. At a laboratory close by a nuclear reactor, the boys watched a radiochemist place a few specks of material inside small quartz tubes that were then sealed. The tubes were put in an aluminum can and placed in the nuclear reactor. The can was fastened on the end of a long pole that was then submerged in a deep pool of water. At the bottom of the pool the boys could see a bright blue glow.
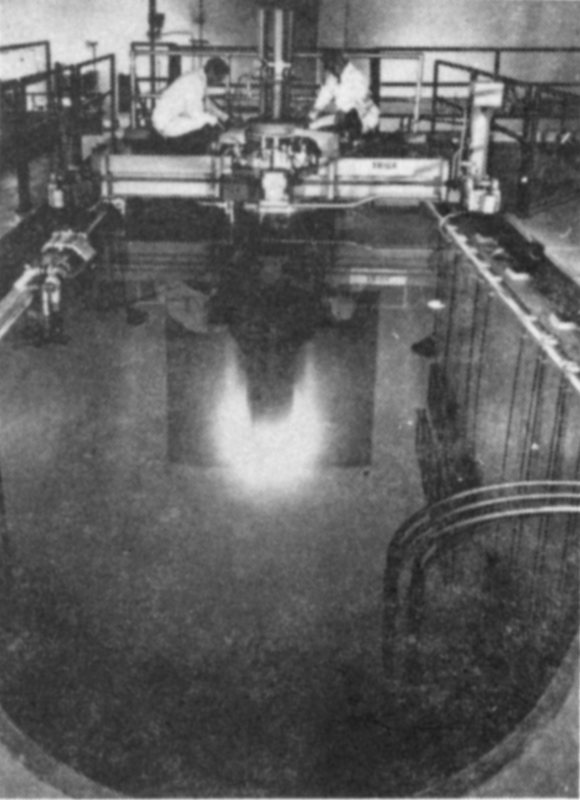
This type of nuclear reactor is used for neutron activation analysis.
“So that’s what a nuclear reactor looks like!” said Bill.
“Yes,” said Dad. “Where you see the blue glow you can also see rows of fuel elements. Each one contains slugs of uranium encased in aluminum. This is one of a number of different types of reactors. But every nuclear reactor is arranged so that the uranium atoms 31 divide (or fission) many, many times each second.
“When this happens, heat is produced that is carried away by the water, and also many, many free neutrons are produced. Those samples, placed down next to the reactor in the bottom of the pool are being bombarded by the neutrons, and some of the elements in the samples absorb the neutrons and become radioactive.”
After a while the samples were removed and carried back to the laboratory in a lead box. A short while later, the radiochemist opened the aluminum can, broke open the quartz capsules, and removed the samples for analysis. The boys watched the chemist mount each sample on a card and take it to a room where there was equipment for measuring radioactivity.

Gamma-ray spectrometer. The sample to be measured is placed on a stand over a gamma-ray detector. The pulse-height analyzer is a device that sorts electrical impulses from the detector according to the energy of the gamma rays causing the impulses. The screen displays the gamma-ray spectrum and the electric typewriter automatically types out the data collected when the measurement is complete.
One by one the samples were placed inside a shield consisting of a big pile of lead bricks. When the heavy door was opened, the boys could see a metal can inside the shield, which housed a detector (called a lithium-drifted germanium detector) that measured the gamma rays emitted by the sample. As each sample was placed near the detector the chemist turned on a gamma-ray spectrometer to which the detector was connected.
A tiny sample of lead white ![]() is sealed in a quartz vial
is sealed in a quartz vial ![]() which
is bombarded with neutrons in a reactor.
which
is bombarded with neutrons in a reactor.

Many of the atoms become radioactive, emitting gamma rays.

The sample is placed in a gamma-ray spectrometer and the gamma rays are separated according to their energy.

The location (energy) of each peak indicates what is present and the height indicates how much!
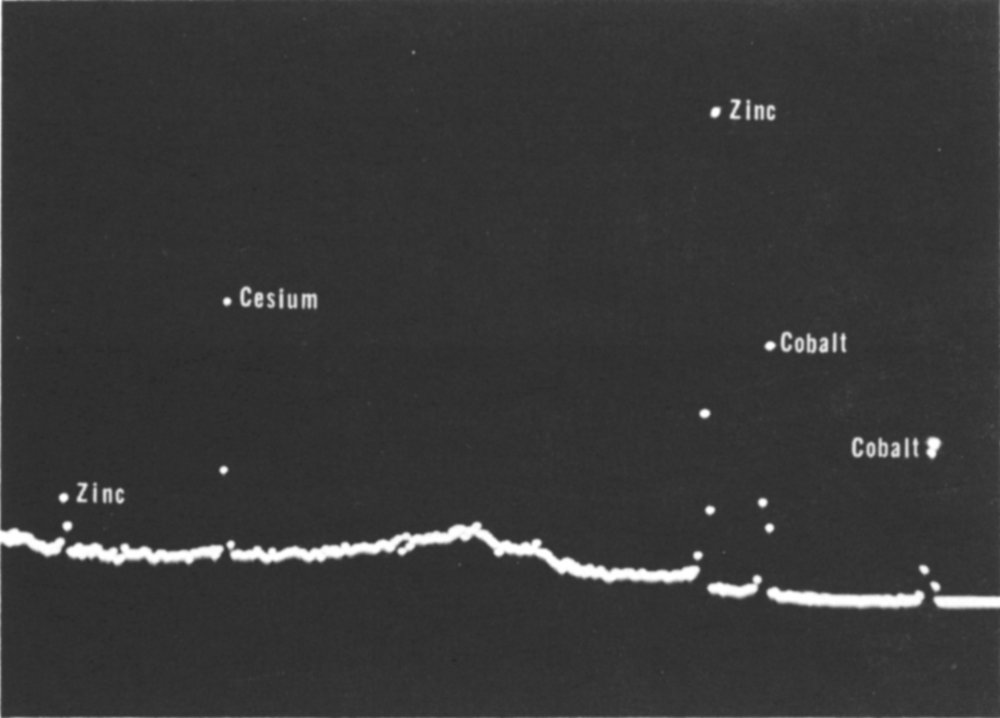
A gamma-ray spectrum as it appears on the screen of a pulse-height analyzer. The gamma-ray peaks are marked with the name of the element whose radioactive isotope emits the gamma ray; two for cobalt and zinc and one for cesium.
There, on what looked like a small television screen, flashes of light appeared that gradually formed a curve with many peaks and valleys. After a few minutes the spectrometer was stopped and an electric typewriter automatically typed out rows and columns of numbers.
The chemist explained, “This curve, which you see on the screen, is a gamma-ray spectrum and tells us what elements are in the sample. The typed-out data give us an accurate measure of the shape of the curve on the screen. By measuring the gamma-rays’ energies we know what elements in the sample were made radioactive. The height of 34 each gamma-ray peak tells us how much of that element is present in the sample.
“That gives us the information we need to calculate the concentrations of the small quantities of materials in our samples. We can do this because at the same time I irradiated a set of standards. Standards are materials that are just like the samples except that they contain known amounts of the impurities I am trying to measure.”
As the boys were leaving the laboratory, the chemist apologized for not having enough time to explain the activation analysis procedure more thoroughly, but he did give the boys a list of books to read on the subject of radioactivity and radioisotopes.[2] They thanked him for his help.
During the ride home, they discussed the paintings that were still unproven.
“It’s too bad that the method of activation analysis fingerprinting hasn’t been fully developed yet,” said Dad.
“Yes,” said Bill. “Then we could prove whether or not that last old painting was really by Aelbert Cuyp as the expert from the gallery believed. But what about those paintings that we found in the box that were not so old?”
“Well,” said Dad, “if the activation analysis method were workable, we might be able to prove if they were painted by Alfred Sisley. Meanwhile, until the method is really developed we don’t know if we can do it that way or not.”
“So what do we do now?” asked Martin.
“We’ll have to wait until scientists can thoroughly investigate this method and several others that they’re working on.”
“Other methods!” exclaimed Bill. “What other methods?”
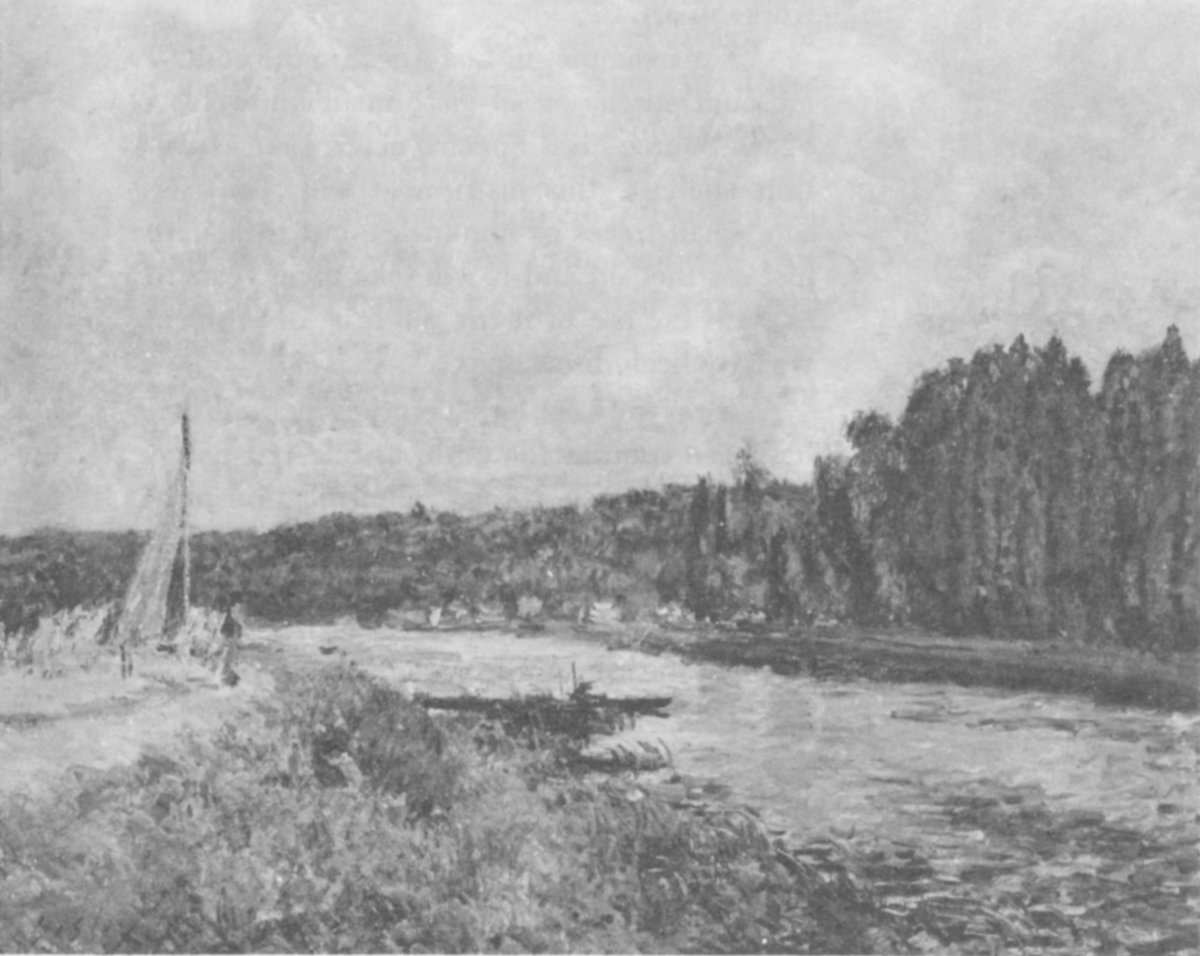
“The Banks of the Oise”, a genuine painting by Alfred Sisley.
“There are several new tools that scientists are working on now,” said Dad. “These involve methods that have been developed by scientists for other purposes, but are now being explored for use in authenticating works of art.
“For example, in Los Angeles, the county museum purchased an instrument known as a Spark Source Mass Spectrometer. Like activation analysis, this instrument will also measure small traces of impurities, but they have just set that up and it will take them years to explore the use of it for the type of problem we have been discussing.
“X-ray diffraction is another method that has been around for quite awhile but hasn’t been used much for art identification until recently. With X-ray diffraction, samples of pigments can be identified by the pattern formed when X rays are bent by passing through the sample of pigment.”
“How’s that?” asked Harley.
“There are 3 or 4 different compounds with about the same chemical composition as lead white. Chemically, they are almost impossible to distinguish. But with X-ray diffraction, a chemist can easily tell them apart. The hope is that the type of lead white will indicate how it was manufactured. Until the middle of the 19th century, lead white was produced mainly by packing strips of lead in clay pots with a little vinegar in the bottom. The clay pots were stacked in a large building with layers of decaying organic matter on the 37 floor. The building was sealed for several weeks during which time the lead corroded in the fumes and became covered with a white substance. The white substance, lead white, was scraped off, ground, and washed to make the pigment.
“But, in the 19th century, when people began to learn more about chemistry, they looked for faster ways of making lead white and some of these methods produced a lead white of somewhat different composition. By using X-ray diffraction, chemists now hope that they can tell how the lead white was manufactured. This may provide another means of dating the lead white in a painting.”
“Are there any other methods?” asked Harley.
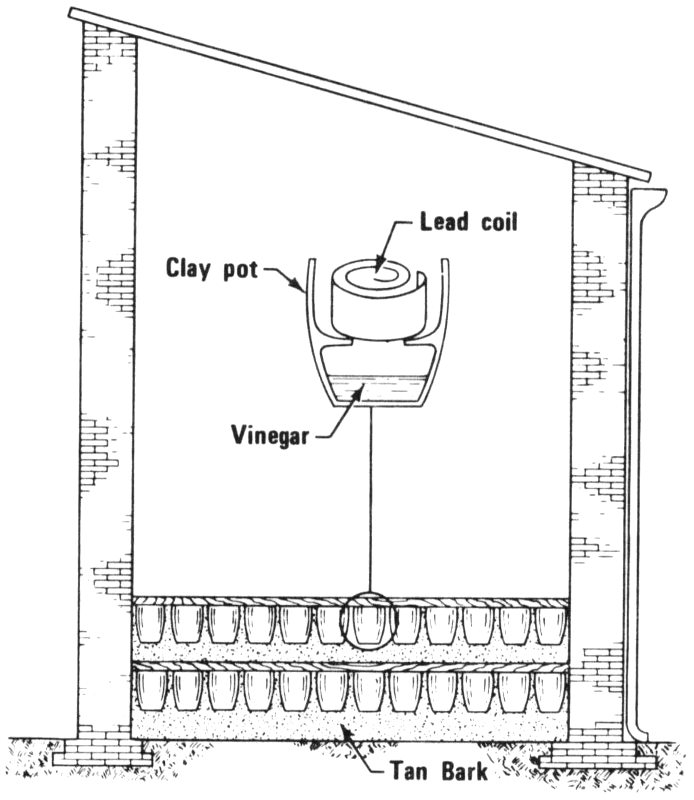
The stack process for making lead white. Rows of clay pots containing lead and vinegar are packed to the ceiling of the building, and fermenting tanbark on the floor produces carbon dioxide and heat. The fumes of vinegar and the carbon dioxide corrode the lead in 2 to 4 months, and the corrosion is lead white.
“Yes, isotope mass spectrometry is one. All lead consists of 4 different isotopes or atoms of different weights. Three of these 4 38 are the end products of a radioactive decay chain. Depending upon the history of the rock formation in which the lead ore occurred, the relative amounts of the lead isotopes vary in a special way. In other words, if we know the different amounts of lead isotopes in the world’s lead ore deposits, and we have a sample of lead white from a painting, we can tell from which deposit the lead, which formed the lead white, came. If, for example, we find that the isotope pattern in a sample from a painting is the same as in lead ore from Australia, then the painting can’t be very old because lead white wasn’t produced from lead mined in Australia until about 100 years ago.”
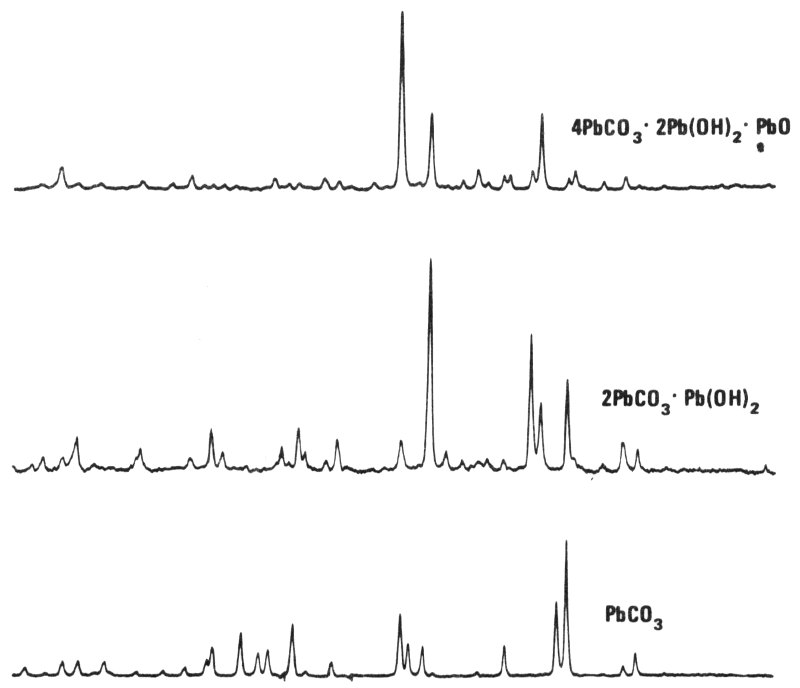
X-ray diffraction patterns from three different lead compounds that might occur in lead white. The middle one is the ideal lead white produced for over 2000 years. While some of the bottom compound may be found mixed with it, the compound shown at the top is only a 20th-century invention.
“How do you measure lead isotopes?” asked Harley.
“With an instrument called a mass spectrometer. This instrument is capable of separating the lead isotopes. First, the atoms of lead in the sample are electrically charged and ‘fired’ in a beam down the length of a tube between the poles of a strong magnet. There, the charged atoms (or ions) in the beam are deflected by different amounts according to how heavy they are. Thus the different isotopes are separated. This method is also still being studied and, although it shows great promise, it will be some time before it can solve problems of art identification. Also the study of the natural variation in isotopes of other elements, such as sulfur, is useful for identification of other pigments as well.
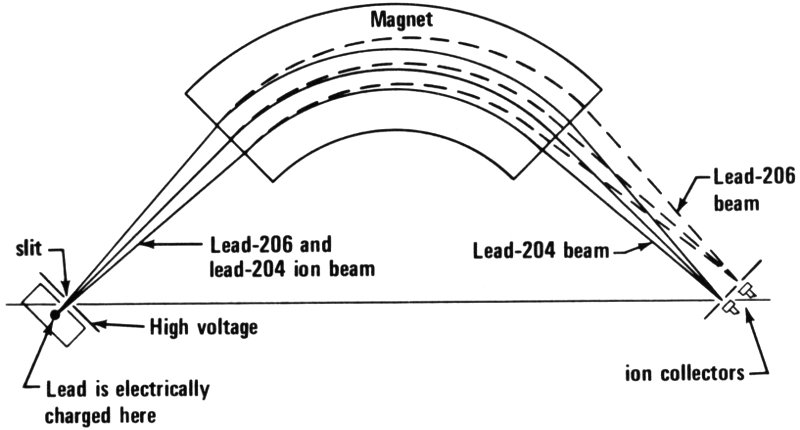
Diagram of a simple mass spectrometer. The ionized atoms of lead travel in a beam at the same speed. The heavier atoms bend less than the lighter ones when the beam passes the magnet. Thus two beams emerge instead of one. Actually there are four isotopes of lead so there will be four beams.

“Agostina”, a genuine painting by Jean Baptiste Camille Corot.
“Another new method that shows great promise has been developed, but this one is not applicable to the paintings that you boys found in the box.”
“Why not?” asked Bill.
“Since the Second World War, the art forgery business has been growing rapidly. For example, it has been said that of the 2000 pictures that Corot, a 19th century Frenchman, is known to have painted, more than 5000 of them are in the United States. This may be only a humorous exaggeration, but a large number of forgeries have been produced in the last several years. These are usually supposed to be paintings that are less than 100 years old. Present-day forgers like to forge paintings that aren’t very old because it’s easier to get away with. Now this new method, which will detect such recent forgeries, is based upon the presence of carbon-14, a radioactive isotope of carbon, in our atmosphere and in all things that grow on our planet.
“Ordinarily, carbon-14 is produced only by cosmic rays, and its concentrations in the atmosphere and in growing things would remain at a constant level. But since the middle of the 1950s the testing of nuclear weapons has increased the amount of radioactive carbon in our atmosphere by quite a bit. Many artist’s materials, such as linseed oil, canvas, paper, and so on, come from plants or animals, and so will contain the same concentrations of carbon-14 as the atmosphere up to the time that the plant or animal dies.
“Therefore, linseed oil (from the flax plant), for example, produced during the last few years will have a much greater concentration of carbon-14 in it than linseed oil produced more than 20 years ago. Scientists at Carnegie-Mellon University have shown that this method will work. It is only a matter of making the measurements on the small samples available from presumably valuable paintings.”
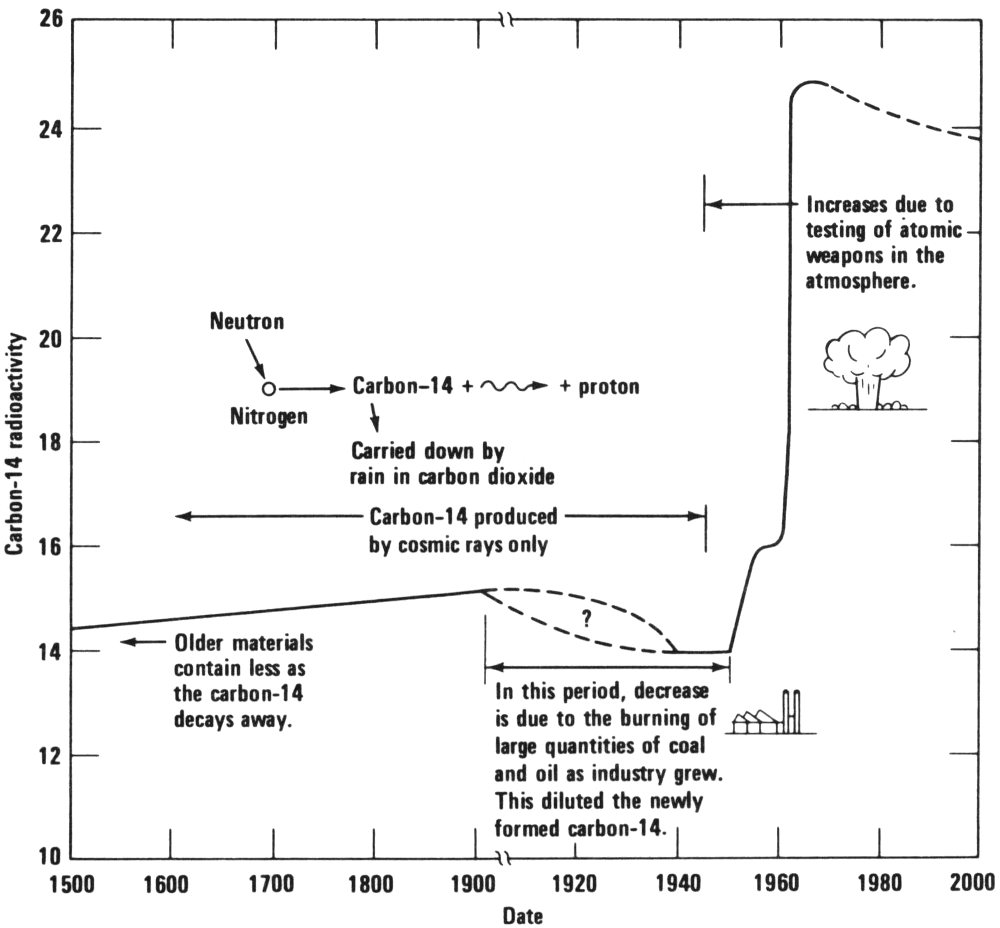
The changing concentrations of carbon-14 in our atmosphere. High levels of carbon-14 in linseed oil and other painting materials will indicate that a work of art is only a few years old.
“There are also a number of other methods being studied including the use of Messbauer Effect Spectroscopy to study pigments 42 that contain iron, thermoluminescent dating of pottery and terra-cotta statuary, X-ray fluorescence analysis as a general tool, and neutron autoradiography as a means of studying the technique of artists. You can read all about them if you wish.”[3]
“It sounds like forgers are going to have a tough time in the future,” said Harley.
“That’s right. It may even turn out that producing forgeries to pass all these new tests will be so difficult and expensive that forgers will stop trying.”
A year later an important letter arrived at the boys’ house. Dad opened it, read it quickly, and said, “Good news, boys! This letter is from the Dutch government. Remember those two paintings that we thought might have been stolen from a Dutch museum?”
“Yes,” said Bill.
“Well, it seems that after a year of studying them, the Dutch have decided that they really are the paintings that were stolen.”
“That is good news,” said Harley. “At least we know that two of the paintings we found are genuine.”
“What are they going to do with them?” asked Martin.
“Of course, they have to go back to their original owners. But this letter says that the 43 Dutch government wants us to come to Holland as their guests as a reward for finding those paintings.”

These two paintings “The Lacemaker” and “The Smiling Girl” were thought to have been by Vermeer. A series of tests, including some of those described in this booklet, showed that the paintings are fairly old. However, some of the materials used are not typical of Vermeer, and the pictures are now thought to have been painted by a follower of the artist.
“That’s great!” said Bill. “Looks like we’re getting something out of finding that box after all.”
“Yes,” said Dad. “And don’t forget the other unidentified paintings may also be genuine. We’ve proved that one is a fake, the experts believe that three of the others are copies, and then there are the two that might be Sisleys and are only waiting for a method to prove it. And we have one more that science managed to prove was really old. I’m sure that in a few years methods will be developed to tell us exactly who painted it.
“And now let’s make arrangements for our trip to Holland.”
About Atomic Power for People, Edward and Ruth S. Radlauer, Childrens Press, Chicago, Illinois 60607, 1960, 47 pp., $2.50. Grades 5-9.
All About the Atom, Ira M. Freeman, Random House, Inc., New York 10022, 1955, 146 pp., $2.50. Grades 4-6.
Atoms at Your Service, Henry A. Dunlap and Hans N. Tuch, Harper and Row, Publishers, New York 10016, 1957, 167 pp., $4.00. Grades 7-9.
Carbon-14 and Other Science Methods that Date the Past, Lynn and Gray Poole, McGraw-Hill Book Company, New York 10036, 1961, 160 pp., $3.95. Grades 9-12.
Experiments with Atomics (revised edition), Nelson F. Beeler and Franklyn M. Branley, Crowell Collier and Macmillan, Inc., New York 10022, 1965, 160 pp., $3.50. Grades 5-8.
The Fabulous Isotopes: What They Are and What They Do, Robin McKown, Holiday House, Inc., New York 10022, 1962, 189 pp., $4.50. Grades 7-10.
Inside the Atom (revised edition), Isaac Asimov, Abelard-Schuman, Ltd., New York 10019, 1966, 197 pp., $4.00. Grades 7-10.
Introducing the Atom, Roslyn Leeds, Harper and Row, Publishers, New York 10016, 1967, 224 pp., $3.95. Grades 7-9.
Our Friend the Atom, Heinz Haber, Golden Press, Inc., New York 10022, 1957, 165 pp., $4.95 (out of print but available through libraries); $0.35 (paperback) from 45 Dell Publishing Company, Inc., New York 10017. Grades 7-9.
Radioisotopes, John H. Woodburn, J. B. Lippincott Company, Philadelphia, Pennsylvania 19105, 1962, 128 pp., $3.50. Grades 7-10.
The Story of Atomic Energy, Laura Fermi, Random House, Inc., New York 10022, 1961, 184 pp., $1.95. Grades 7-11.
The Useful Atom, William R. Anderson and Vernon Pizer, The World Publishing Company, New York 10022, 1966, 185 pp., $5.75. Grades 7-12.
Working with Atoms, Otto R. Frisch, Basic Books, Inc., Publishers, New York 10016, 1965, 96 pp., $3.50. Grades 9-12.
Cover courtesy Groninger Museum voor stad en Lande
| Page | |
|---|---|
| 5 | Yale Joel, Life magazine, copyright © Time, Inc. |
| 6 | Her Majesty the Queen, copyright © reserved |
| 7 & 8 | Ullstein Bilderdienst |
| 10 | Rijksmuseum, Amsterdam |
| 23 | National Gallery of Art, Washington, D. C., Andrew Mellon Collection |
| 35 & 40 | National Gallery of Art, Washington, D. C., Chester Dale Collection |
| 43 | National Gallery of Art, Washington, D. C., Andrew Mellon Collection |
★ U.S. GOVERNMENT PRINTING OFFICE: 1974—747-556/15
The U. S. Atomic Energy Commission publishes this series of information booklets for the general public. The booklets are listed below by subject category.
If you would like to have copies of these booklets, please write to the following address for a booklet price list:
USAEC—Technical Information Center
P. O. Box 62
Oak Ridge, Tennessee 37830
School and public libraries may obtain a complete set of the booklets without charge. These requests must be made on school or library stationery.
| Chemistry | |
|---|---|
| IB-303 | The Atomic Fingerprint: Neutron Activation Analysis |
| IB-301 | The Chemistry of the Noble Gases |
| IB-302 | Cryogenics: The Uncommon Cold |
| IB-304 | Nuclear Clocks |
| IB-306 | Radioisotopes in Industry |
| IB-307 | Rare Earths: The Fraternal Fifteen |
| IB-308 | Synthetic Transuranium Elements |
| Biology | |
| IB-101 | Animals in Atomic Research |
| IB-102 | Atoms in Agriculture |
| IB-105 | The Genetic Effects of Radiation |
| IB-110 | Preserving Food with Atomic Energy |
| IB-106 | Radioisotopes and Life Processes |
| IB-107 | Radioisotopes in Medicine |
| IB-109 | Your Body and Radiation |
| The Environment | |
| IB-201 | The Atom and the Ocean |
| IB-202 | Atoms, Nature, and Man |
| IB-414 | Nature’s Invisible Rays |
| General Interest | |
| IB-009 | Atomic Energy and Your World |
| IB-010 | Atomic Pioneers—Book 1: From Ancient Greece to the 19th Century |
| IB-011 | Atomic Pioneers—Book 2: From the Mid-19th to the Early 20th Century |
| IB-012 | Atomic Pioneers—Book 3: From the Late 19th to the Mid-20th Century |
| IB-002 | A Bibliography of Basic Books on Atomic Energy |
| IB-004 | Computers |
| IB-008 | Electricity and Man |
| IB-005 | Index to AEC Information Booklets |
| IB-310 | Lost Worlds: Nuclear Science and Archeology |
| IB-309 | The Mysterious Box: Science and Art |
| IB-006 | Nuclear Terms: A Glossary |
| IB-013 | Secrets of the Past: Nuclear Energy Applications in Art and Archaeology |
| IB-017 | Teleoperators: Man’s Machine Partners |
| IB-014, 015, & 016 | Worlds Within Worlds: The Story of Nuclear Energy Volumes 1, 2, and 3 |
| Physics | |
| IB-401 | Accelerators |
| IB-402 | Atomic Particle Detection |
| IB-403 | Controlled Nuclear Fusion |
| IB-404 | Direct Conversion of Energy |
| IB-410 | The Electron |
| IB-405 | The Elusive Neutrino |
| IB-416 | Inner Space: The Structure of the Atom |
| IB-406 | Lasers |
| IB-407 | Microstructure of Matter |
| IB-415 | The Mystery of Matter |
| IB-411 | Power from Radioisotopes |
| IB-413 | Spectroscopy |
| IB-412 | Space Radiation |
| Nuclear Reactors | |
| IB-501 | Atomic Fuel |
| IB-502 | Atomic Power Safety |
| IB-513 | Breeder Reactors |
| IB-503 | The First Reactor |
| IB-505 | Nuclear Power Plants |
| IB-507 | Nuclear Reactors |
| IB-510 | Nuclear Reactors for Space Power |
| IB-508 | Radioactive Wastes |
| IB-511 | Sources of Nuclear Fuel |
| IB-512 | Thorium and the Third Fuel |

U. S. ATOMIC ENERGY COMMISSION
Office of Information Services