
The Project Gutenberg eBook of Political and commercial geology and the world's mineral resources, by Various
Title: Political and commercial geology and the world's mineral resources
Author: Various
Editor: J. E. Spurr
Release Date: July 1, 2023 [eBook #71082]
Language: English
Credits: Charlene Taylor, Harry Lamé and the Online Distributed Proofreading Team at https://www.pgdp.net (This file was produced from images generously made available by The Internet Archive/American Libraries.)
Please see the Transcriber’s Notes at the end of this text.
New original cover art included with this eBook is granted to the public domain.


McGraw-Hill Book Co. Inc.
PUBLISHERS OF BOOKS FOR
Coal Age ▽ Electric Railway Journal
Electrical World ▽ Engineering News-Record
American Machinist ▽ Ingeniería Internacional
Engineering & Mining Journal ▽ Power
Chemical & Metallurgical Engineering
Electrical Merchandising
POLITICAL AND COMMERCIAL
GEOLOGY
AND THE
WORLD’S MINERAL RESOURCES
A SERIES OF STUDIES BY SPECIALISTS
J. E. SPURR, Editor
First Edition
Second Impression
Royalties received from the sale of this book will be
assigned to an institution of learning to finance
further studies along the lines followed in the volume.
McGRAW-HILL BOOK COMPANY, Inc.
NEW YORK: 370 SEVENTH AVENUE
LONDON: 6 & 8 BOUVERIE ST., E. C. 4
1920
Copyright, 1920, by the
McGraw-Hill Book Company, Inc.
THE MAPLE PRESS YORK PA.
[v]
The purpose of the accompanying series of studies is to shed light upon the vast importance of commercial control of raw materials by different powers, or by the citizens of those powers, through invested capital. The question of domestic and foreign governmental policies of the United States is closely involved. It appeared to many of us who were engaged (as all the authors of these papers were) in studying the mineral problems during the war, that our Government had never grasped the vast political significance of commercial domination, and especially of the control of mineral wealth; and that other more seasoned nations had done so, and thereby affected the interests of America and her policy very deeply, without her being aware of the circumstance.
With the rapid increase of the world’s population and the exploring and exploiting of the hitherto undeveloped natural resources, competition for this wealth has become and will still become keener. In past ages war, pestilence, and starvation held down the earth’s population; and in the last few years all these grim spectres have returned in force, suggesting the possibility of a permanent return of the old primitive days. Nevertheless, modern science and organization, if not quenched by vast social disorders, will so safeguard life, as in recent times, that the world is in a fair way to become crowded. All of us, like Germany, yearn for our “place in the sun,” and our share of comfort and power. Of all the fundamental necessities for this, nothing is so much in the nature of a fixed and unmultipliable quality as the metals; they constitute the basis and foundation of our modern civilization and power over man and natural forces. Other raw materials are of vegetable or animal origin; they propagate and duplicate themselves in successive incarnations according to the law of life; they are born in some magical fashion of air and water, with a minimum of the earth, and they return their loans faithfully to air and water and earth with the passing of each generation and the dawning of a new. There is the hint of such a law of growth in the mineral kingdom, but it is so vastly slow that the evanescent animal man has no personal interest in it; for all his purposes and by all his standards of measurement it is inert, and these riches, once dug and used, will never again be available. The treasures of commercially valuable ore-deposits have been hid by nature whimsically throughout the earth, here and there, by no rule of geography or latitude, and with a great disregard of equality. A nation’s needs or desires for mineral wealth have no stated relation to its actual mineral possessions; what it needs[vi] is often in the territory of another nation which does not need it. Commerce is thus born, and the nation which must have the metal or ore in question backs up its commerce and helps it to fasten its claims for permanent control of the deposits in question, by legislation, by diplomacy, and, if need be, by war. In the case of war, the metallic prize falls to the strongest—usually the nation which before, through its necessities, exercised only commercial control, but which, as the result of the trial of strength, now frankly asserts its sovereignty.
Have we as Americans realized these forces? Absolutely not, I should say. How many realize that the Alsace-Lorraine question is and was not a sentimental one, but a struggle for the greatest iron deposits of Europe and the second largest in the world, which gave Germany her immense growth and power, and may now transfer that wealth and power to France? That the dispute between Poland and Germany as to Upper Silesia is not a question of nationality, sentiment, or even territory, but concerns the greatest coal field of Europe as well as great deposits of lead and zinc? If Poland gets this, she may rival Germany in wealth and importance; if Germany loses it, she may drop into the position of a second-rate power, now that she has also had to give up Alsace-Lorraine. To submit such a question to the vote of the native population is of the same order of fitness as tossing a coin for it; but how many of us have understood this? Population shifts and changes, swells or shrinks, may be at one time predominantly Polish and at another time mainly German; but the coal deposits are fixed. To clarify these things we should in place of Silesia say Coal, in the place of Alsace-Lorraine, Iron, and so on.
The reason we have not realized these facts is on account of our own vast mineral wealth, so abundant that not till recently has American capital and enterprise found it necessary to adventure into the outside world, as the European nations had long ago done. Their natural wealth was limited so that they have become familiar with those fundamental principles and laws of which we have been unconscious. From this has arisen European foreign policies, the protection of their national commerce and national capital in foreign enterprises and consequently at home; governmental participation in business combinations, as in Germany, England, France, and Italy; while the United States has been engaged in “trust-busting” and has neglected the protection of its investors in foreign countries. This illustrates the difference between European diplomacy and American guilelessness. How well this played into the hands of foreign powers it is unnecessary to explain. The spectacle of the United States maintaining a Monroe Doctrine of protection over Latin-American republics which she took no vigorous steps to unite with her by the powerful bonds of commerce, must well have excited the amusement of those European commercial nations like[vii] Germany who have been strengthening themselves in those countries by the closest commercial, and hence political, ties.
This volume simply takes up the study of the actual situation, as to the distribution and ownership of mineral supplies in the world, and the author of each chapter is a well-known specialist.
First is considered the question of petroleum, source of power and light, the key to the mastery of the air, and, on account of its fluid and easily transportable condition, of extraordinary future importance. Next are taken up the great fuel mineral, coal, and its ally, the great metallic mineral, iron, which must go together for the manufacture of iron and steel, the backbone of all our mechanical achievements. Next come those metals indispensable in steel making and in the manufacture of specially hard or tough steels. These are of great importance, and include manganese, chromium, nickel, tungsten, vanadium, antimony, molybdenum, uranium, and zirconium. Radium is closely associated with uranium and is considered with it. Closely allied with zirconium are thorium and mesothorium, and their treatment therefore closely follows that of zirconium.
The next great group is that of the major metals, other than iron and the ferro-alloy metals: copper, lead, zinc, tin and mercury, and aluminum. Aluminum ores are used not only as sources of the metal, but for the manufacture of refractories and abrasives. Therefore they are classed partly with the metallic and partly with the non-metallic minerals; and the other non-metallic minerals, used likewise for abrasives, refractories, and other uses—such minerals as emery and corundum, magnesite, graphite, mica, and asbestos—follow.
The next great group is that of the fertilizer minerals—phosphate rock, potash, nitrates and nitrogen, and pyrite and sulphur, all essential for agriculture.
The last group is that of the precious metals, gold, silver, and platinum, essential for coinage and in the arts.
These various studies are essentially both inclusive and elementary: together they form almost the first contribution to the branch of investigation—that of the relation of geology to industry, commerce, and political economy—which they cover; and it is natural that beginnings should be rather crude. Moreover, many of the chapters were written a year or more previous to the publication of the volume, and although brought to date to the extent possible in the brief time available, are considered inadequate by the authors themselves. Apologies for shortcomings and possible inaccuracies are therefore very much in order. Nevertheless, it is felt that the volume merits publication, and that the beginning here made is far better than no start at all.
Josiah Edward Spurr.
| Page | ||
| Preface | v | |
| Chapter | ||
| I. | Petroleum, by John D. Northrop | 1 |
| II. | Coal, by George S. Rice and Frank F. Grout | 22 |
| III. | Iron, by E. C. Harder and F. T. Eddingfield | 55 |
| IV. | Manganese, by D. F. Hewett | 90 |
| V. | Chromium, by E. C. Harder | 109 |
| VI. | Nickel, by C. S. Corbett | 129 |
| VII. | Tungsten, by Frank L. Hess | 142 |
| VIII. | Vanadium, by R. B. Moore | 163 |
| IX. | Antimony, by H. G. Ferguson and D. A. Hall | 172 |
| X. | Molybdenum, by R. B. Moore | 191 |
| XI. | Radium and Uranium, by R. A. F. Penrose, Jr. | 201 |
| XII. | Zirconium, by H. C. Morris | 209 |
| XIII. | Monazite, Thorium, and Mesothorium, by R. B. Moore | 216 |
| XIV. | Copper, by F. W. Paine | 223 |
| XV. | Lead, by Frederick B. Hyder | 261 |
| XVI. | Zinc, by Frederick B. Hyder | 294 |
| XVII. | Tin, by James M. Hill | 317 |
| XVIII. | Mercury, by F. L. Ransome | 337 |
| XIX. | Bauxite and Aluminum, by James M. Hill | 349 |
| XX. | Emery and Corundum, by Frank J. Katz | 356 |
| XXI. | Magnesite, by R. W. Stone | 363 |
| XXII. | Graphite, by H. G. Ferguson, Frank F. Grout, and George D. Dub | 372 |
| XXIII. | Mica, by Durand A. Hall | 380 |
| XXIV. | Asbestos, by Oliver Bowles | 388 |
| XXV. | Phosphate Rock, by R. W. Stone | 402 |
| XXVI. | Potash, by Hoyt S. Gale and A. W. Stockett | 411 |
| XXVII. | Nitrogen, by Chester G. Gilbert | 421 |
| XXVIII. | Pyrite and Sulphur, by A. G. White | 447 |
| XXIX. | Gold, by John E. Orchard | 462 |
| XXX. | Silver, by F. W. Paine | 495 |
| XXXI. | Platinum, by James M. Hill | 506 |
| XXXII. | Who Owns the Earth? by J. E. Spurr | 522 |
[1] In this article, prepared in June, 1918, by Mr. Northrop, have been incorporated certain notes and additions; as, for example, information furnished by E. Russell Lloyd, of the United States Geological Survey; A. G. White and W. E. Perdue, of the Bureau of Mines, and others. (J. E. S.)
Coal and iron are the backbone of industrial civilization, and should be considered first in any attempt to analyze the ownership and control, as between nations, of the world’s mineral resources. Kin to coal in growing importance, however, is the lighter, fluid and volatile mineral substance, petroleum, whose significance is vast and as yet not wholly defined. More easily transportable than coal, and yielding refined products whose explosive action in internal-combustion engines furnishes greater power in proportion to weight than was once deemed possible, petroleum and its products, apart from their immense direct economic importance, may, in the automobile, the submarine, and the air plane, and through numerous other applications, control strategically, from a nationalistic standpoint, the more inert foundations of civilization. Moreover, the use of crude petroleum as fuel, especially for ships, is of the most vital importance in these days of greater competitive plans for expanding world-wide commerce, and establishing the strength and ready efficiency of navies. Great maritime nations must have, for their oil-burning ships, oil-bunkering stations under their own control in all parts of the world where they wish their commerce to dominate, and their navies to protect their interests efficiently.
The recognition by certain strong and aggressive nationalities of this critical factor has brought about a situation that is perhaps unparalleled[2] in the mineral history of the world. Coal and iron have always been decidedly static as to control—they have remained largely under the supervision and direction of the countries in which they occur. Transportation costs, the conjunction of iron and coal deposits, and other factors have prevented these minerals, in spite of their vast importance, from being fully used as a world commodity. By contrast, petroleum is coming to be universal, like gold, in its acceptance and applicability; but, unlike gold, it is essential in the highest degree to the advance of modern civilization. The fluidity of this mineral, its consequent amazingly cheap transportation and handling by pipe lines, the completeness with which it can be utilized, all combine toward making it in the future the crucial factor of commercial and of political control. Moreover, this fluidity of form and ease of application facilitate the control of petroleum by vast commercial organizations, like the Standard Oil of America, and others in various parts of the world; and even make its world control feasible and probable. Recognizing this tendency, many nations, like England, France, Holland, Argentina, and Mexico, have taken steps looking toward a partial nationalization of their petroleum resources, in order to protect themselves against foreign commercial aggression in this particular. England has gone farthest in this direction, and has reached and is reaching out aggressively into other countries to secure, through commercial control, backed where necessary by political pressure, a world empire of petroleum to serve her world-wide colonial empire. The United States, on the other hand, has dominated the world’s petroleum industry through her own vast resources, worked by interests which have grown without conscious governmental help or even in spite of governmental and popular opposition, and have reached out and secured footholds in other countries.
In the past the mineral development of the world has led to great changes in political sovereignty. Important as these have been, the events that may result from the nationalistic competitive exploitation and control of the world’s petroleum supply bid fair to exceed in importance all similar changes of the past. The perception of the problem and of the necessity, and the advantage of the initiative, naturally belongs to those nations with restricted area and resources, that have grown great by trading and by exploiting the resources of other countries. Such a nation, for example, is England, a country that is fortunately the natural ally of the United States. By contrast, in the United States, a nation concerned hitherto only with the development of its own vast resources, commercial enterprise in foreign countries has been backed by no fixed national policy, and indeed has often been treated as unworthy. In the new international era that was initiated by the World War, however, this policy of Chinese self-sufficiency and exclusion can not be safely continued, and the United States must not only perceive clearly the[3] tendencies and movements of other nationalities, but consider how best to direct its own commercial and political plans so as to uphold its independence and power. Such a policy would naturally lead to international agreements as to the distribution and division of petroleum lands, resources, and production, and probably no one thing would contribute more to the promotion of frank understanding between nations and the removal of obstacles to permanent peace.
Mr. Northrop’s paper follows:
In its crude or semi-refined state, petroleum is extensively utilized as fuel under locomotive and marine boilers and to a small extent in internal-combustion engines of the Diesel type. Certain grades of petroleum are utilized in the crude state as lubricants.
The principal use of petroleum is for the manufacture of refined products, of which the number and uses are legion. The lightest gravity, etherial products are employed as anaesthetics in surgery. The gasolines are the universal fuels of internal-combustion engines, and the naphthas are widely used as solvents and for blending with raw casinghead gasoline in the manufacture of commercial gasoline. The kerosene group includes a variety of products utilized primarily as illuminants, but in annually increasing quantities as fuel in farm tractors. The lubricating oils and the greases derived from petroleum are indispensable to the operation of all types of machinery. The waxes derived from petroleum of paraffin base are utilized in many forms as preservatives and as sources of illumination, and in the last three years have become indispensable constituents of surgical dressings in the treatment of burns. Petroleum coke, because of its purity, is in demand for use in certain metallurgical processes and for the manufacture of battery carbons and arc-light pencils. Fuel oils obtained as by-products of petroleum refining satisfy the fuel needs of many industrial plants, railroads and ocean steamers. Road oils, as the name implies, are employed for minimizing dust on streets and highways; and artificial asphalt, a product of certain types of petroleum, has in many localities superseded the use of other forms of asphalt for paving purposes.
—For petroleum as a fuel under boilers in the generation of steam there are numerous substitutes, including wood, charcoal, coal, peat, natural gas, artificial gas, and electricity; as a fuel in internal-combustion engines some demonstrated substitutes are natural gas, artificial gas, benzol, and alcohol, and in the Diesel type of engine certain vegetable and fish oils can be utilized.
For illuminating purposes, animal fats, oils distilled from coal, natural[4] gas, artificial gas, acetylene gas and electricity may be substituted for kerosene.
For certain types of lubrication carefully refined vegetable and mineral oils are acceptable, but for lubricating high-speed bearings and for all lubrication in the presence of high temperature and of steam no satisfactory substitutes for mineral lubricants derived from petroleum are known.
Substitutes for petroleum asphalt are available in the form of native asphalts, bituminous rocks, and coal-tar residues. For petrolatum, animal fats and vegetable oils can be substituted, and for paraffin wax, ozokerite might be made to satisfy such essential requirements as could not be met by refrigeration or by vegetable and animal oils.
Probable changes in practice that may be expected to affect the petroleum industry within the next ten years include an increased dependence by oil producers on geologic investigations in advance of drilling, the development of methods for deeper drilling than is now practicable, and the more efficient handling of individual wells and of entire properties, with a view to the ultimate recovery, at minimum cost, of a higher percentage of the oil originally present.
The tendency toward amalgamation of individual producing, transporting, refining and marketing interests into strong units capable of competition in domestic and foreign markets on relatively equal terms with each other and with pre-existing combinations of equivalent strength will doubtless increase, and with the growing strength of the several units will come an efficient and thorough quest for petroleum in all parts of the world.
In the refining of petroleum it is probable that methods will be devised and perfected for recovering more of the light-gravity products from low-grade petroleum and for the conversion of the less-salable products of petroleum into products of greatest current demand. Moreover, it is believed that internal-combustion engines will be so modified as to run successfully on petroleum products of lower volatility than gasoline. The use of petroleum as railroad, marine, and industrial fuel is destined to increase enormously in the next decade.
Although an important contributor to the oil-supply of Great Britain, the shale-oil industry has received little attention in recent years outside of Scotland. Investigations by the United States Geological Survey have demonstrated that the United States contains vast deposits of oil shale in Utah, Colorado, Wyoming and Nevada, much of which will average higher in oil content than the Scottish shale. Efforts already begun to develop methods for the recovery of shale oil on a commercial[5] scale in the United States will undoubtedly result in the establishment of a shale-oil industry in this country within the next two or three years. The future growth of this industry will depend largely on the rapidity of the decline in the domestic production of petroleum.
Commercial accumulations of petroleum are everywhere restricted to strata of sedimentary origin. In the United States petroleum is produced commercially from strata of all periods from Cambrian to Quaternary, the most prolific sources being in strata of the Carboniferous and Tertiary systems. The geological age of the chief sources of petroleum production in each of the other oil-producing countries of the world is indicated in the table following:
Table 1.—Geologic Age of Petroleum-bearing Formations
| Country | System |
|---|---|
| North America | |
| Canada | Silurian and Devonian |
| Mexico | Cretaceous and basal Tertiary |
| Alaska | Tertiary (?) |
| West Indies | |
| Trinidad | Tertiary |
| Cuba | Cretaceous and pre-Cretaceous |
| South America | |
| Colombia | Cretaceous and Tertiary |
| Venezuela | Cretaceous and Tertiary |
| Peru | Tertiary |
| Argentina | Jurassic, Cretaceous and Tertiary |
| Europe | |
| Russia | Tertiary |
| Roumania | Tertiary |
| Galicia | Tertiary |
| Italy | Tertiary |
| Germany (Alsace) | Tertiary and pre-Tertiary |
| Asia | |
| India | Tertiary |
| Turkestan | Tertiary |
| Persia | Tertiary |
| Africa | |
| Algeria | Tertiary |
| Egypt | Tertiary |
| Oceania | |
| Japan | Tertiary |
| Dutch East Indies | Tertiary |
| New Zealand | Cretaceous and Tertiary |
From the foregoing table one might conclude that a direct relation exists between the distribution of Tertiary rocks and the supply of petroleum, but in the United States, which produces two-thirds of the world’s[6] current supply, the quest for petroleum has, under scientific direction, included the entire range of the stratigraphic column, and has found petroleum in considerable quantities in the rocks of each geologic system younger than the Cambrian.
The fact that seeps and other surface indications of petroleum are generally more pronounced in the relatively younger Mesozoic strata than in the older Paleozoic formations, and the further fact that geologic exploration for oil and gas in countries other than the United States has been restricted in the main to areas containing the most pronounced indications of petroleum, tend to account for the predominance of the Tertiary system in the foregoing table and to indicate the fallacy of attempts to estimate the world’s reserves of petroleum on stratigraphic evidence alone.
Despite the broad geologic range of petroleum, its occurrence in specific members, formations, groups, series or systems is by no means universal. On the contrary, its occurrence is restricted to specific localities in which are fulfilled certain variable relations, as yet but little understood, that involve (1) the constitution, sequence and content of organic matter of the sediments; (2) the nature and degree of metamorphism they have undergone; (3) their structure; and (4) their degree of saturation with salt water. Because the most detailed geologic work is insufficient to provide a basis for the appropriate evaluation of the numerous factors involved, and because only a relatively small percentage of the areas of sedimentary rocks in the world have been examined geologically in appreciable detail, any estimate of the future supply of petroleum in the world is peculiarly hazardous.
The geographical distribution of petroleum is as wide relatively as its geologic range. The oil fields of present commercial significance are situated, in the order of their importance as contributors to the world’s production of petroleum in 1917, in the United States, Russia, Galicia, Mexico, Dutch East Indies, India, Persia, Japan and Formosa, Roumania, Peru, Trinidad, Argentina, Egypt, Germany, Canada, Venezuela and Italy. Small quantities of petroleum have also been reported from Guatemala, Honduras, Costa Rica, Panama, Haiti, Porto Rico, Bolivia, Chile, Spain, Arabia, China, Australia, Papua, Philippine Islands, Nigeria, Belgian Congo, Gold Coast, Madagascar, and elsewhere. The geographical distribution of petroleum in the world is shown on the accompanying map. (Plate I.)
[7]
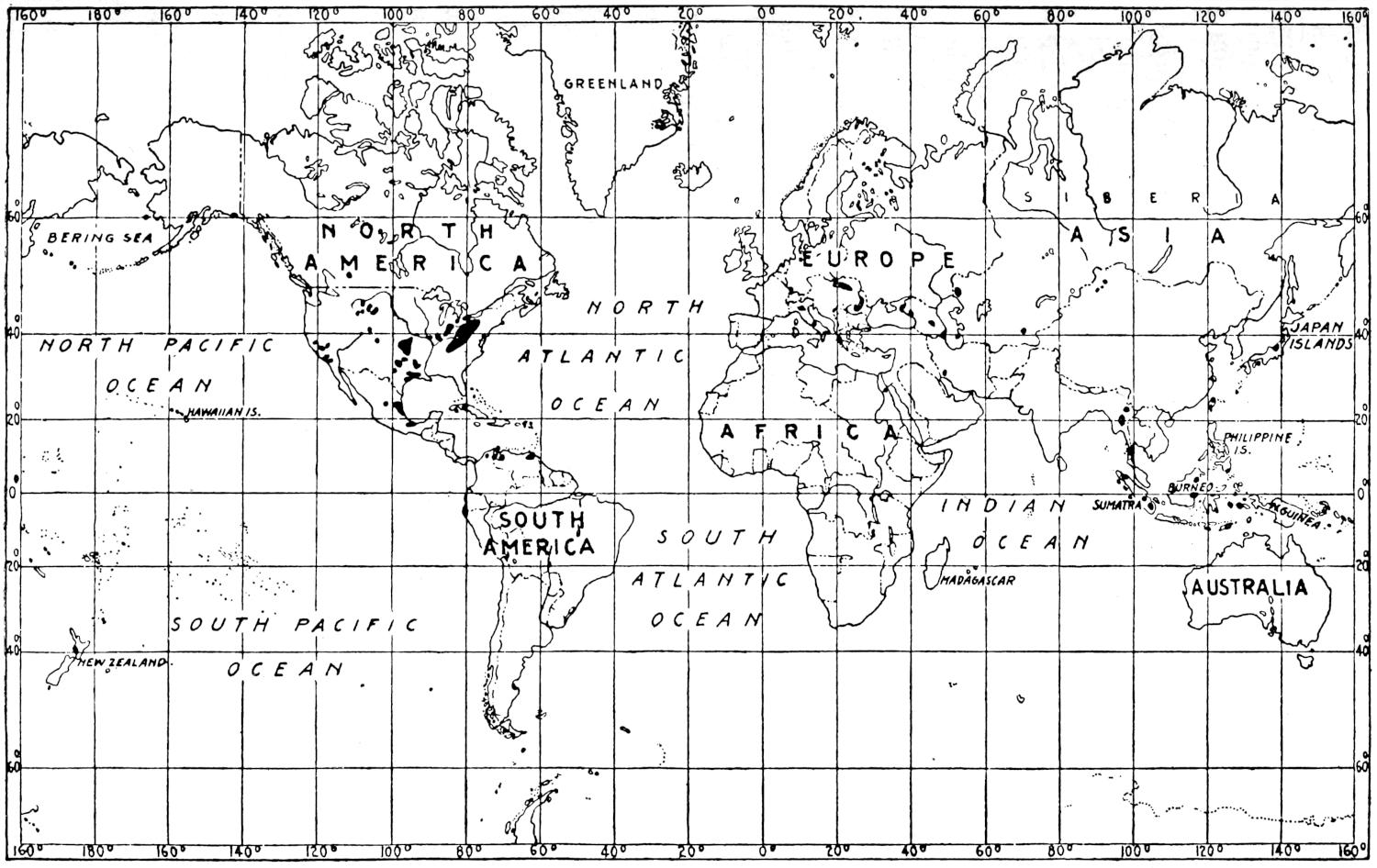
Plate I.—Geographical distribution of the producing petroleum fields of the world. By John D. Northrop.
[8]
In the opinion of the author the most conspicuous developments of the world’s supply of petroleum in the next decade will take place in the countries that border the Caribbean Sea and the Gulf of Mexico. The trend in this direction is unmistakable. From 1913 to 1917, the annual production of petroleum in Mexico increased from 21,000,000 barrels to 56,000,000 barrels, and the potentialities of future production in that country have been demonstrated to be almost beyond comprehension. The output, originally considered valuable only as a source of fuel oil, is now yielding, by modern refining methods, increasingly important percentages of illuminating oils and gasoline. The only obstacles to enormously increased production are unsettled political conditions and inadequate facilities for marine transportation. These obstacles will doubtless be overcome within the next few years, and barring unforeseen contingencies Mexico will soon rank second among the oil-producing countries of the world.[2] Judged by the results of exploratory work already done in Venezuela and Colombia, both of those countries are destined to contribute appreciably to the world’s supply of petroleum within the next decade. Recently Colombia has given enough evidence of ability to furnish high-grade petroleum from wells of large individual capacity to warrant the large interests holding concessions there to exert every effort to overcome the adverse natural conditions that have so long barred the way to exploitation. Enough drilling has already been done in Venezuela to demonstrate that the resources of heavy-gravity asphalt-base petroleum in that country are large, and the recent installation of a modern petroleum refinery for the treatment of these oils on the island of Curacao, off the Venezuelan coast, has provided the market necessary to active field development.
[2] Mexico ranked second in 1918 and 1919.
In Trinidad the production of petroleum exceeds 1,500,000 barrels a year and has doubled in the last few years. With the increased facilities for ocean transport of petroleum that are becoming available, a large output is assured.
Cuba is not expected to become an important producer of petroleum, and present knowledge concerning the petroleum resources of the Central American countries is not such as to warrant the belief that oil fields of material consequence will be developed in any of them.
Petroleum production in the United States is expected to reach its maximum within the next two or three years and to decline steadily thereafter, although this country is expected to remain the leading oil-producing country of the world for the greater part, if not all, of the coming decade.
As regards those oil-producing countries of North and South America that have not been already mentioned, no significant changes in their present status are anticipated.
The petroleum resources of Russia (including Asiatic Russia) are believed sufficient to assure that country retaining its position as the leading producer of petroleum in the Eastern Hemisphere far beyond the[9] next decade. During the last few years the output has been obtained under increasing difficulties, and as a consequence there has been no measure either of present productive capacity or of potentialities. Concerning the future of Russia as a source of petroleum Arnold[3] says: “Such large areas, both in European and Asiatic Russia, yield unmistakable evidence of the presence of oil in large quantities that it is to this country, among those of Europe and Asia, to which the future must look for a supply.”
[3] Arnold, Ralph: “The World’s Oil Supply”: Report Am. Min. Cong., 19th annual session, 1917, pp. 485-486.
Russia being endowed with petroleum reserves, both proved and prospective, of great magnitude, the ultimate position of that country as the leading oil-producer of the world seems reasonably assured. Its immediate future is too intimately dependent on the progress from political turmoil to warrant a forecast.
The oil fields of both Roumania and Galicia are believed to have passed their maximum yield, and the possibilities of opening new fields of consequence in those countries are not considered large enough to justify a forecast of anything but a moderate decline of production in future years. No material change in the status of the negligible oil fields of Italy or of Alsace is anticipated at any time in the future.
With regard to the situation in Asia, the writer believes that the next decade will witness a steady increase in the output of petroleum in India, and the probable development of one or more important oil fields in Persia and possibly of fields in Asia Minor, Turkestan and China. In Oceania the same period will doubtless witness a material increase in the production of petroleum in Japan and Formosa and in the Dutch East Indies, together with the possible opening of new fields in Papua. Africa will doubtless receive considerable attention from oil operators in the next ten years, but on the basis of available evidence the results obtained in that period will probably not be large enough to affect the petroleum situation of the world.
The status of the political control of the world’s output of petroleum in 1917, as determined by the best data now available, is indicated in the table following.
The accompanying diagram (Figure 1) shows the proportion of the world’s production of petroleum contributed annually by each of the principal producing countries in each of the last ten years.
[10]
Table 2.—Political Control of the World’s Production of Petroleum in 1917
| Source of production |
Quantity of production (barrels) |
Percentage of total |
Country exercising political control |
|---|---|---|---|
| United States | 335,315,601 | 66.17 | United States |
| Russia | 69,000,000 | 13.62 | Russia |
| Mexico | 55,292,770 | 10.91 | Mexico |
| Dutch East Indies | 12,928,955 | 2.55 | Holland |
| India | 8,078,843 | 1.59 | Great Britain |
| Persia | 6,856,063 | 1.36 | Persia |
| Galicia | 5,965,447 | 1.18 | Poland (?) |
| Japan and Formosa | 2,898,654 | 0.57 | Japan |
| Roumania | 2,681,870 | 0.55 | Roumania |
| Peru | 2,533,417 | 0.50 | Peru |
| Trinidad | 1,599,455 | 0.32 | Great Britain |
| Argentina | 1,144,737 | 0.23 | Argentina |
| Egypt | 1,008,750 | 0.20 | Great Britain |
| Germany | 995,764 | 0.20 | Germany |
| Canada | 205,332 | 0.04 | Great Britain |
| Venezuela | 127,743 | 0.03 | Venezuela |
| Italy | 50,334 | 0.01 | Italy |
| Cuba | 19,167 | ... | Cuba |
| 506,702,902 | 100.00 |
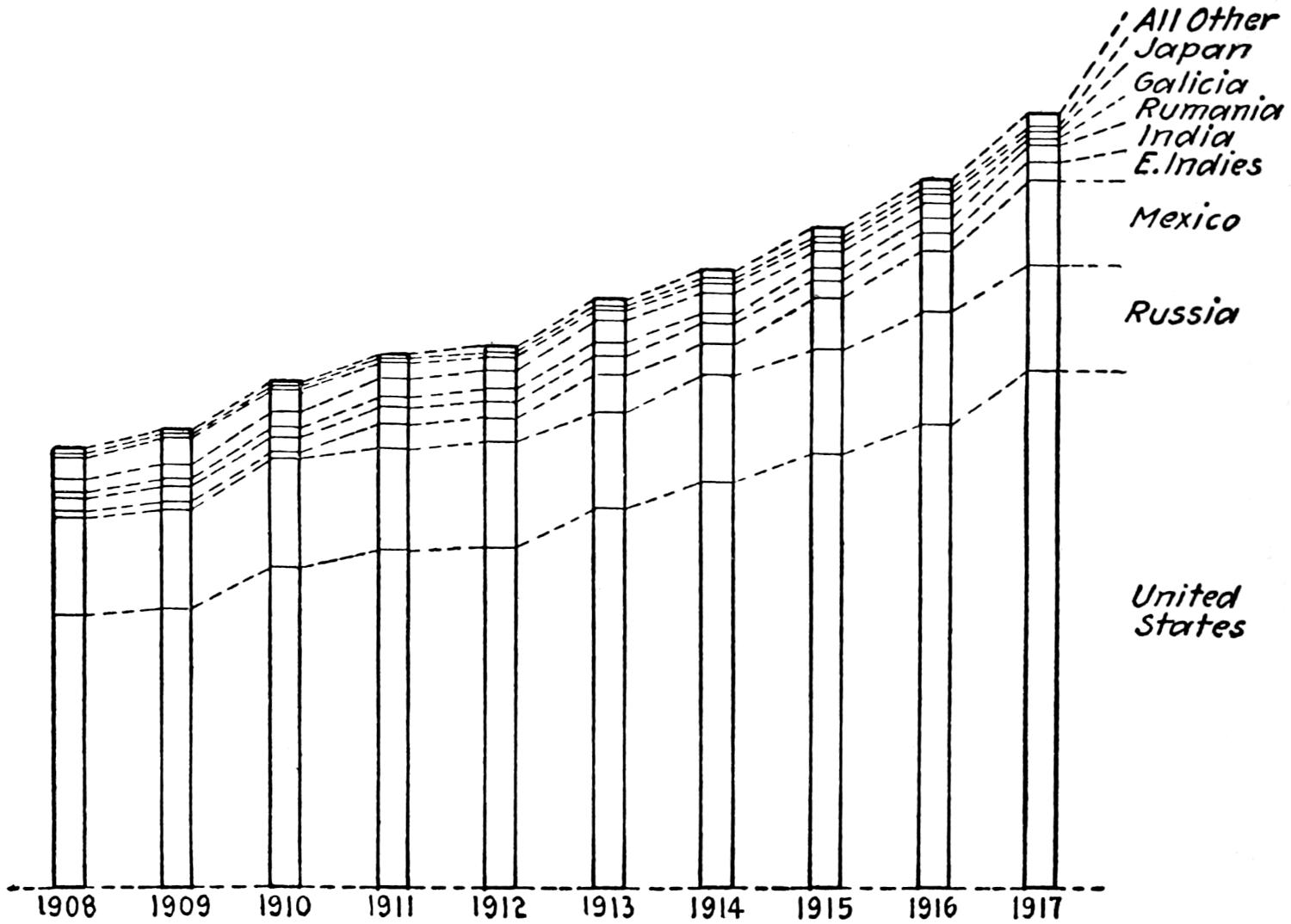
Fig. 1.—Proportion of the world’s output of petroleum contributed annually by each of the chief producing countries, 1908-1917.
[11]
Aside from the control exercised by Great Britain through its protectorate relation over the petroleum resources of Egypt, control of the petroleum resources of the various countries is mainly by virtue of state sovereignty. This political control is in proportion to the strength of the government in the country exercising it. Recent developments whereby the British government becomes the majority stockholder of a corporation controlling the oil resources of Persia, practically transfer the political control, as well as the commercial control, of Persian petroleum from Persia to England. Mexico’s recently attempted firm political control of her vast petroleum resources depends for its success upon her diplomatic ability in dealing with the stronger governments of England and the United States, whose nationals have acquired a commercial control that is threatened by Mexico’s new and decided nationalistic policy.
The commercial control of the world’s production of petroleum, as far as nations are involved, is determined in the main through direct ownership, of lands, leases and concessions, or by the control, through holding corporations, of subsidiary companies holding fee, leases, mineral rights or concessions of petroleum land. Except in Argentina, where the domestic petroleum industry is owned and operated by the state; in Germany, where the government participates directly in the financing of petroleum enterprises through the Deutsche Bank; and in Persia, where the British government owns a substantial interest in a company owning and operating extensive concessions, the commercial control of the petroleum industry is determined almost wholly by aggregations of private capital acting in their own interests.
Table 3.—Nationality and Extent of Control of Dominant Interest
| Country | Production in 1917 (barrels) |
Nationality of dominant interests |
Approximate extent of control by dominant interests (per cent.) |
|
|---|---|---|---|---|
| United States | 335,315,601 | United States | 96 | |
| Russia | 69,000,000 | British-Dutch | 40 | + |
| Mexico | 55,292,770 | United States | 65 | |
| Dutch East Indies | 12,928,955 | British-Dutch | 100 | |
| India | 6,078,843 | British | 100 | |
| Persia | 6,856,063 | British | 100 | |
| Galicia | 5,965,447 | German | 100 | |
| Japan and Formosa | 2,898,654 | Japanese | 100 | |
| Roumania | 2,681,870 | British-Dutch | 36 | |
| Peru | 2,533,417 | United States | 70 | |
| Trinidad | 1,599,455 | British | 80 | |
| Argentina | 1,144,737 | Argentinian | 100 | |
| Egypt | 1,008,750 | British-Dutch | 100 | |
| Germany and Alsace | 995,746 | German | 100 | |
| Canada | 205,332 | United States[4] | 80 | - |
| Venezuela | 127,743 | British-Dutch | 80 | (?) |
| Italy | 50,000 | French | 96 | |
[4] By control of refining facilities.
[12]
So far as the author is aware, Canada is the only country in which the petroleum industry may be said to be controlled by foreign (United States) interests, this control being by virtue of an essential monopoly of pipe-line and refining facilities.
The preceding table shows, according to the best information available, the nationality and approximate extent of control exercised by the dominant interest in each of the principal oil-producing countries of the world in 1917.
The accompanying diagram (Figure 2) shows graphically the approximate commercial control of the world’s production of petroleum in 1917.
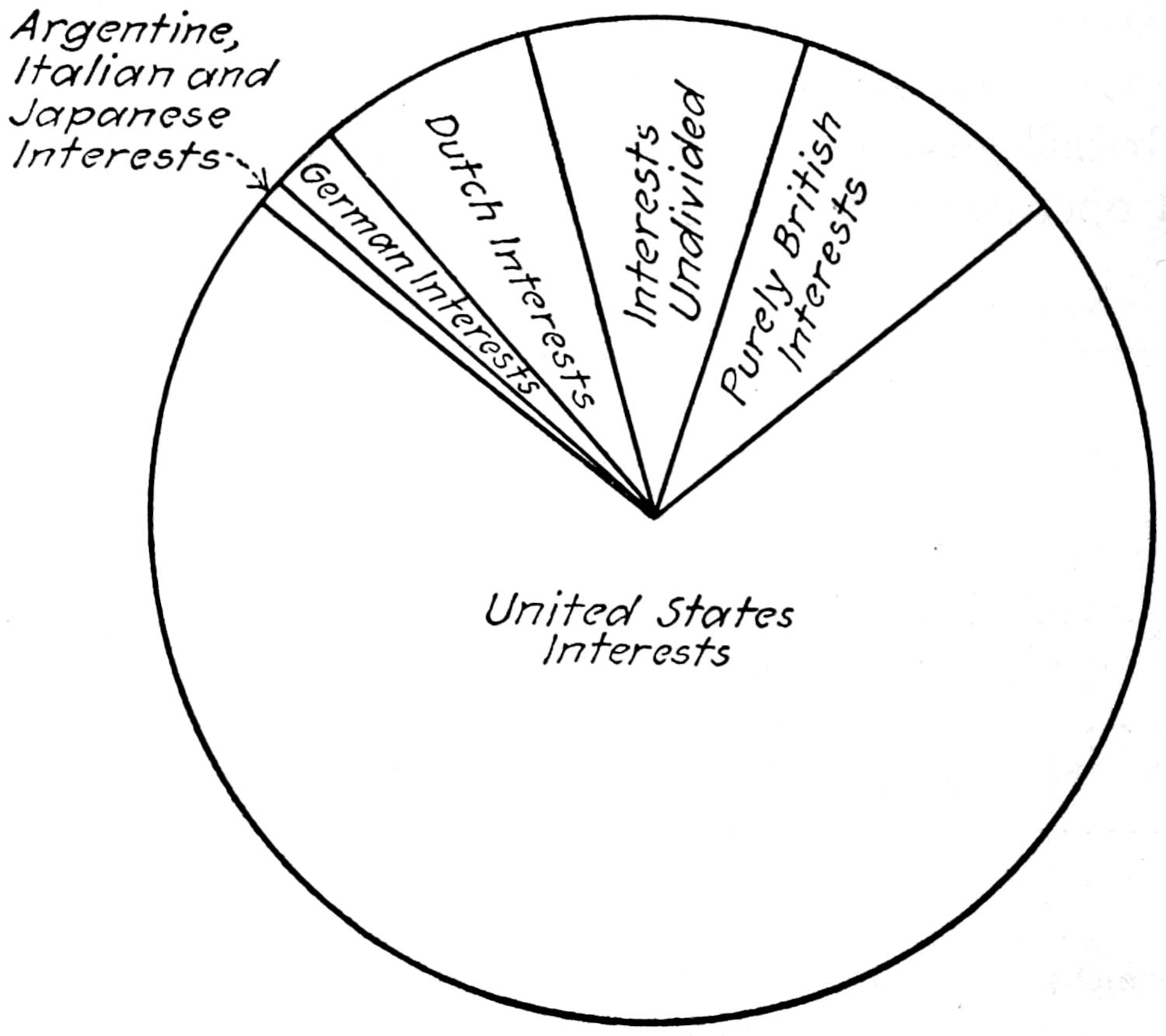
Fig. 2.—Approximate commercial control of the world’s production of petroleum in 1917.
Commercial control of the petroleum industry in the United States is in the hands of the so-called “Standard Oil Group” of companies, through their control of most of the great pipe-line systems of the country, of probably 75 per cent. of the refining facilities and of a substantial part of the actual production. Other domestic interests having important shares in the control of the petroleum industry in the United States include the Southern Pacific Railroad Co., Cities Service Co. (Doherty interests), General Petroleum Corporation, Gulf Oil Corporation, Ohio Cities Gas Co., Cosden & Co., Sinclair Oil & Refining[13] Corporation, The Sun Co., the Texas Co., the Tide Water Oil Co., and the Union Oil Co. Foreign interests in the United States include purely British companies, which control a production of about 2,000,000 barrels a year; British-Dutch companies represented by the Royal Dutch-Shell Syndicate, which control a production of about 9,000,000 barrels a year, together with refining and marketing facilities; and Franco-Belgian companies controlling a yearly production of about 1,000,000 barrels. Aside from the very probable holdings by individual Germans of shares in companies engaged in one or more phases of the petroleum industry of the United States, the author is aware of no organized German interest in any phase of the domestic industry.
Commercial control of the petroleum industry of Russia is, under the political conditions now existing in central Europe, largely a matter of speculation. As nearly as can be ascertained, the dominant control is in the hands of purely British, Franco-British, and British-Dutch (Royal Dutch-Shell Syndicate) interests. Certain of the second-named interests are allied closely with an additional group of capitalists represented by the firm Nobel Bros., of much importance, the present control of which is by no means clear, from the literature available on the subject. Though originally Swedish, the financial interests now involved in Nobel Bros. are believed to include representatives of financial groups in England, France, and Germany as well, with control probably lying with the Anglo-Swedish interests. Before the war, direct German interest in Russian petroleum included control by the Deutsche Bank through a Belgian company (the Petrole de Grosny) of the important producing and refining company, A. I. Akverdoff & Co., control of which is now in British or British-Dutch hands. As in the United States, a considerable part of the actual production of petroleum in Russia is distributed among a large number of individually weak companies, dominated, through the control of pipe-line or refining facilities, by one or another of the principal groups.
Of considerable importance in Russian petroleum affairs at one time was the European Petroleum Union, organized for combat in the world markets with the Standard Oil trust. This union included among others such important petroleum operators as Nobel Bros., the Rothschild interests (now Dutch-Shell), Mantaschoff (now Russian General Oil Corporation) (British), and the Deutsche Bank, the latter controlling Akverdoff and Spies in Russia, together with important companies in Roumania and Galicia. How far this union controlled the affairs of its constituent companies is not evident from available data, and its present influence on companies now operating in Russia is uncertain.
Conditions in Russia make impossible any definite statement on the petroleum situation. A decree of the Bolsheviki government, dated June 20, 1918, on the nationalization of the petroleum industry, declared as the property of the state all movable and immovable property employed[14] in and belonging to that industry. Trading in oil was declared a state monopoly and was delegated to the chief petroleum committee of the fuel department of the supreme Council of National Economy. As the chief producing areas are now under British military control, this decree is ineffective.
Commercial control of petroleum in Mexico is divided among United States, British, and British-Dutch interests, which controlled about 65, 30 and 2 per cent., respectively, of the production in 1917. The interests of the United States include the Doheny group, operating principally as the Huasteca and Mexican Petroleum companies; the Standard Oil Co. of New Jersey, operating as the Penn-Mex Fuel Co.; the Sinclair interests, operating as the Freeport and Mexican Fuel Oil Co.; the Texas Co.; Gulf Co.; Southern Pacific Railroad; and others. The British interests are represented by the Pearsons, operating as the Mexican Eagle Oil Co.; and the British-Dutch interests by La Corona Petroleum Co., and Chijoles Oil, Ltd., controlled by the Royal Dutch-Shell Syndicate. No exclusively German interests are known to hold a substantial portion of any important company operating in Mexico.
Formerly concessions were freely granted to foreign individuals and companies for the exploitation of mineral deposits, and oil lands were sold by the native owners to foreigners. Article 27 of the constitution of 1917 expressly forbids any but Mexican companies acquiring directly or operating directly petroleum lands in Mexico.
All recent concessions for the exploitation of oil properties contain a provision stating that the concession will be declared null if any of the rights are transferred to any foreign government. The provisions and the intent of a series of presidential decrees issued on February 19, 1918, July 8, 1918, July 31, 1918, and August 1, 1918, are to nationalize all petroleum lands and to permit them to be worked only by Mexican citizens or by companies that agree to consider themselves Mexican and further agree not to invoke the protection of their governments. A bill was presented in December, 1918, to carry out Article 27 of the new constitution, but thus far no action has been taken in the matter. The decrees and legislation growing out of Article 27 have been protested by the chief petroleum companies operating in Mexico and by their respective governments.
Commercial control of the petroleum resources of the Dutch East Indies is in the hands of the Royal Dutch-Shell Syndicate and is essentially absolute by reason of the restrictions contained in the Netherlands East India Mining Act and subsequent supplements on foreign acquisition of mining rights in the East Indian Archipelago. Actual control is in the hands of the Bataafsche Petroleum Maatschappij, which has a capital of $56,000,000 divided into five shares, three of which are owned by the Royal Dutch Petroleum Co., and two by the Shell Transport[15] Trading Co. (British). Purely British interests control an inconsequential production of petroleum in British North Borneo and in Sarawak.
Prospecting licenses and concessions are granted only to Dutch subjects and to Dutch companies. It is officially stated that the object of these restrictions is not to exclude foreign capital; this is precisely their effect, and on account of the economic monopoly which the Royal Dutch-Shell now has of the petroleum industry of the Dutch East Indies, it would be very difficult for any new enterprise to gain a foothold.
Commercial control of the petroleum resources of India is exercised by the Burma Oil Co. through its dominance of production, refineries, and pipe-line facilities, and by reason of agreements as to marketing with its principal competitor, the British Burma Petroleum Co., both controlled by British capital. The Burma Oil Co. is allied with, if not directly controlled by, a group of British financiers, one or more of whom is interested in companies in Trinidad and in Persia.
During the war the petroleum industry of Roumania was temporarily wholly in control of German and Austrian interests. The advanced stage of development of the oil fields prior to the war and the intentional damage, much of which is irreparable, wrought in the fields by British detachments in 1916, when capture of the fields by Austro-German forces became inevitable, are believed, however, to have deprived Germany of a large part of the fruits of her conquest, as it is considered doubtful if the Roumanian fields can ever again be made to yield petroleum at the pre-war rate of 12,000,000 barrels per annum.
The American Petroleum Institute states that “Roumania is considering the erection of a state monopoly of both production and distribution on the ruins of the monopoly which Germany sought to establish there but was compelled by the armistice to renounce.”
Prior to the war Dutch or rather British-Dutch (Dutch-Shell) interests controlled about 30 per cent. of the annual production of petroleum in Roumania, German interests about 26 per cent., United States interests (Standard Oil Co. of New Jersey) about 18 per cent., French interests about 16 per cent., purely British interests about 6 per cent., and Belgian and Roumanian interests the remainder.
Through the Austrian “Society Gaz” and the German “Deutsche Erdoel Aktien-gesellschaft,” German interests have dominated the petroleum industry of Galicia for years through the direct control of the larger producing and refining interests and by reason of the fact that the smaller scattered interests were dependent almost entirely on the two leading companies, the Galizische Karpathen Petroleum A. G. (controlled by Society Gaz), and the Premier Oil & Pipe Line Co. (controlled by the Deutsche Erdoel A. G., which is in turn controlled by the Diskonto und Bleichroeder, a branch of the Deutche Bank) for their transportation and refining facilities. British and Dutch capital were involved in the[16] Galician fields prior to the war, but not, it is believed, to a controlling extent in either of the dominant companies.
The petroleum industry of Japan is controlled wholly by Japanese interests and to a preponderant extent by a single company, the Nippon Oil Co. So far as the author is aware, no foreign interests share in any way in the development or control of the Japanese petroleum industry.
Commercial control of the petroleum industry of Peru is exercised by the Standard Oil Co. of New Jersey through its subsidiary, the Imperial Oil Co. of Canada. This control involves about 70 per cent. of the annual production, the remaining 30 per cent. being divided in the ratio of 27 to 3 between British and Italian interests respectively. So far as is known no other interests are involved.
The interests engaged in the petroleum industry of Trinidad include financial groups purely British, controlling about 57 per cent. of the production; British-Dutch interests (Dutch-Shell) controlling about 23 per cent., and United States interests (General Asphalt Co.), controlling the remainder. The leading operator in Trinidad is the Trinidad Leaseholds, Ltd., a British company that in 1917 produced about 42 per cent. of the petroleum output credited to Trinidad that year.
Commercial control of the petroleum resources of lower Alsace has been in the hands of the Vereinigte Pechelbronner Oelbergwerke Gesellschaft and the Deutsche Tiefbohr A. G. Both of these companies are believed to be controlled by the Deutsche Bank through the Deutsche Erdoel A. G., and the Diskonto und Bleichroeder. The negligible production of petroleum in Hanover is doubtless under the same financial control, although data that would warrant a positive statement to that effect are not at hand.
The petroleum reserves of Argentina, which comprise the only areas from which petroleum is being commercially produced in that country, are operated by the state through the Comodora Rivadavia Petroleum Commission. German interests are thought to have been involved in two or three unsuccessful efforts in the last decade to obtain petroleum on tracts adjacent to the government reserves in the Comodora Rivadavia district.
The petroleum industry in Egypt is controlled wholly by British-Dutch capital operating as the Anglo-Egyptian Oilfields, Ltd., a subsidiary of the Royal Dutch-Shell Syndicate, through the Anglo-Saxon Petroleum Co., the last-named company being predominantly British.
Commercial control of the petroleum industry in Canada is exercised in effect by the Standard Oil Co. of New Jersey, through its subsidiary the Imperial Oil Co. of Canada. This control is exercised through a virtual monopoly of pipe-line and refining facilities, and by the producing interests, though British and Canadian, being individually small and unorganized.
[17]
The production of petroleum in Italy, which is small, represents the output of two companies, the Petroli d’Italia, in which French capital is predominant, and the Petrolifera Italiana, which is believed to be essentially Italian.
Financial groups interested in petroleum in Venezuela include the Royal Dutch-Shell Syndicate (British-Dutch), the General Asphalt Co., (United States), and a group of British financiers who control properties in Trinidad as well as the most important group of companies, other than Nobels and the Dutch-Shell, in Russia.
United States interests, including the Standard Oil Co., the Doherty interests, the Texas Co., the Gulf Corporation, and the Island Oil Transport Corporation, are predominant in the quest for petroleum in Colombia. The Venezuelan Oil Concessions, Ltd., an English company operating in Venezuela, is reported to have obtained a concession to explore for oil in the northwest district of British Guiana.
The Sinclair interests (United States) are particularly active in the search for petroleum in Costa Rica and Panama; and the Sun Co. (United States) is understood to be investigating petroleum possibilities in other Central American republics.
The Pearson interests (British) have expended considerable effort in the quest of petroleum in Algeria and Morocco, and in the former country American interests (E. E. Smith) are reported to have recently sought petroleum concessions from the French government.
British interests, including the British government, control extensive petroleum concessions in Persia, from which oil in unreported quantities is now being produced.
The most promising oil territory of Persia has recently been closed to American activity through the granting of a concession aggregating approximately 500,000 square miles to a British concern, the Anglo-Persian Oil Co., a majority of whose voting stock is owned by the British government. This concession runs until 1961. The importance of the oil territory is indicated by its reported potential capacity of 30,000,000 barrels yearly, with tremendous reserves undeveloped.
United States interests (Standard Oil Co. of New York) are understood to still retain control over the petroleum rights in certain provinces in China, where active prospecting in two or three localities a few years ago was reported to have yielded unfavorable results.
Petroleum in small quantities is produced in New Zealand by purely British interests.
—As regards probable developments in the petroleum industry within the next decade, the United States, thanks to the enterprise and foresightedness of financial interests of domestic origin, seems[18] to have a strong position. United States interests are practically supreme in the commercial control of the petroleum resources of the Western Hemisphere, dominating the petroleum industry in the United States, Canada, Mexico, and Peru, holding substantial interests in Trinidad and Venezuela and in the prospective petroliferous areas in Central America and Colombia. Its only competitors are British and British-Dutch interests, which control the petroleum situation in Trinidad and are not only strongly intrenched in the United States, Mexico, and Venezuela, but are aggressively seeking to enlarge their holdings in those countries and to gain footholds elsewhere. Unless the United States adopts measures, such as Federal operation of the trunk pipe-lines, to limit the aggressions of foreign capital in this country, and erects a firm forward-looking governmental policy toward the protection of investments of its citizens in petroleum properties in other countries, particularly Latin-American countries, it may witness its commercial supremacy in petroleum affairs wane and disappear, while it is yet the largest political contributor to the world’s supply of petroleum.
As contrasted with the strongly nationalistic and deliberately aggressive governmental policy adopted by Great Britain, France, Holland and some other nations, the United States has never adopted any policy founded on recognition of the importance of political and commercial control of petroleum. American companies may not own and operate oil lands in the British Empire, in the French possessions, or the Dutch colonies, but the only American restrictions on foreign activity in the petroleum industry are those which cover all minerals contained in public lands. Only American citizens, or those who have declared their intention of becoming American citizens, can apply for patents to such land. However, after the application is made, there is no restriction on transfer of the mineral rights thus secured.
—British and British-Dutch interests easily dominate the petroleum situation in the Eastern Hemisphere by supremacy in the petroleum industries of Russia, Persia, India, and the Netherlands East Indies. Domination of the petroleum situation in Russia alone is believed tantamount to dominion of the petroleum situation in the entire Eastern Hemisphere for the greater part of the next century. The strength of Great Britain’s present position in the world’s petroleum affairs lies in a strong governmental policy and in the wide scope of British petroleum investments, embracing practically every country where petroleum is an important product and nearly every country where it is a product of potential importance. The general policy of the British Empire seems to be to control all oil development and restrict operations by foreign capital. In Australia licenses are required for the exploitation of oil lands, and only companies incorporated in the United Kingdom or a British possession may receive such licenses. The Governor General[19] has the right of pre-emption of all oil produced and in case of war may take control of all oil properties. In Canada, in those western provinces where minerals are the property of the Crown, petroleum and natural gas lands may be leased only to British companies. A similar restriction exists in Burma. In Burma a monopoly of the petroleum industry for 99 years was granted to the Burma Oil Co. in 1865. This grant seems to have been inspired by fear of the Standard Oil Co. of the United States, for the agreement between the company and the government stipulates that the former shall not amalgamate with other oil companies. Regulations of like effect exist in other British colonies where oil exists; in Barbados the British government has the right of pre-emption of all oil residues; in British Guiana, non-British companies can only hold lands by special license of the Governor; in British Honduras all mineral oil is reserved to the Crown; in southern Nigeria, the Gold Coast, Trinidad, and Tobago the British government has the right of pre-emption over all petroleum.
The recent granting of a concession amounting to a monopoly in the most promising oil district of Persia (a region that many oil experts believe likely to become one of the most important in the world) to a British company controlled directly (by stock ownership) by the British government, signifies an aggressive policy of England, outside of her own dominions, to secure and hold, under government control, oil lands in all parts of the globe.
It is understood that the best-known oil territories in Venezuela are already covered by concessions that are practically all controlled either directly or indirectly by British interests, chiefly the Dutch-Shell Syndicate.
So far as observed, German interests actually dominate the petroleum industry in Galicia and at home. Whether forced back on its own petroleum resources or on these reinforced by those of Galicia, Germany will obviously have an inadequate supply, and in consequence German interests are likely to be particularly aggressive in seeking petroleum in Mesopotamia, Africa and South America.
—Since control of the petroleum interests of the Rothschilds passed into the hands of the Royal Dutch-Shell Syndicate (British-Dutch), the influence of French finances in petroleum affairs has been negligible, outside Galicia and Italy, where its potency was not great. French capital will undoubtedly participate in efforts to determine the petroleum reserve of the Barbary States, French dependencies, but it will hardly be much involved in organized efforts to control the world situation with respect to petroleum.
The French mining law holds that oil and gas belong to the state, and may be exploited under concessions, the area and time limit of which are matters of negotiations between the applicant and the authorities.[20] It is understood that the French government is unwilling to grant oil concessions except to companies the majority of whose stock is held by French citizens. A company incorporated recently to work the Algerian oil fields contains in its articles of incorporation the provision that 60 per cent. of its stock must be held by French citizens.
—Japanese investments in the world’s petroleum industry have not yet attained significant proportions outside Japan itself, though the Japanese government is officially alive to the importance of Japanese investments in petroleum properties in Mexico, particularly Lower California and Sonora; China; and undoubtedly Russia. Hence large investments of Japanese capital in the petroleum industry in one or all of those countries may be expected in the near future.
Petroleum in its crude or semi-refined state is used as fuel under locomotive and marine boilers and as a lubricant. The principal use of petroleum, however, is in the manufacture of numerous refined products. Some of the more important products and their uses are as follows: ether, as an anæsthetic in surgery; gasoline, as fuel in internal-combustion engines; naphthas, as solvents and in the manufacture of commercial gasoline; kerosene, as an illuminant and as a fuel for farm tractors; lubricating oils; waxes, as preservatives, illuminants, and surgical dressings in treatment of burns; petroleum coke, in metallurgical processes and in the manufacture of battery carbons and arc-light pencils; heavy fuel oils; road oils; artificial asphalts, for pavements. The use of petroleum and its products as fuel, as a lubricant, and for illumination may be considered essential. Substitutes for most of these uses are known, but they are either inefficient or not readily available.
The most prolific sources of petroleum are in sedimentary strata of the Carboniferous and Tertiary periods. Because the most detailed geologic work is insufficient to provide for the appropriate evaluation of the numerous factors involved in the occurrence of petroleum, and because only a relatively small percentage of the areas of sedimentary rocks throughout the world have been examined geologically in any appreciable detail, it is difficult to estimate the future supply of petroleum or to predict that large accumulations will be discovered in any particular region.
The principal countries contributing to the world’s production of petroleum rank as follows in general order of importance: United States, Russia, Mexico, Dutch East Indies, Roumania, India, Persia and Galicia. Other countries produce less than 2 per cent. of the annual total. The greatest change that is likely to come in the geographical distribution of production is a larger output from the countries bordering the Caribbean[21] Sea and the Gulf of Mexico, and from the Persian and Mesopotamian fields. Mexico now ranks second to the United States, and South American countries promise to become more important contributors to the world’s production than they now are. Russia is expected to become ultimately one of the chief producers of petroleum.
Within the next decade, through improved methods of production and through the further amalgamation of producing, transporting, refining, and marketing companies into strong units, the output will undoubtedly be larger and will be more economically produced. In the refining of petroleum it is probable that improved methods will make possible the recovery of a larger percentage of lighter products from low-grade petroleum. Internal-combustion engines are being modified so as to run on petroleum products of lower volatility than gasoline. The use of petroleum as fuel under railroad and marine boilers is expected to increase enormously in the next decade. As the output of the producing fields declines, the vast deposits of oil shale in the western United States will be developed as a source of oil.
So far as is known, political control of the petroleum resources of the world is determined by state sovereignty (see Plate I, page 7). In normal times, the United States controls politically over 66 per cent. of the present output of petroleum. Russia and Mexico ranked second and third in 1917, controlling 13.6 per cent. and 10.9 per cent., respectively. The remaining 9 per cent. was controlled by Great Britain, Holland, Persia (British government owned), Roumania, Austria-Hungary, Japan, Peru, Germany, Argentina and Italy in the order named.
The table showing the nationality and the approximate extent of the commercial control exercised by the dominant interests in each of the principal oil-producing countries, and Fig. 2 (page 12) are the best possible summaries of commercial control. United States capital is supreme in the commercial control of the petroleum industry of the Western Hemisphere. British and British-Dutch interests easily dominate the petroleum situation in the Eastern Hemisphere. France no longer exercises control over any important fields. Japanese interests, controlling at present all the oil fields of Japan, may be expected to make large investments in the petroleum fields of Mexico, China and Russia.
[22]
Coal is among the most important of all minerals. It furnishes power and heat, and its distillation yields a great number of useful materials, such as gas for lighting and fuel, explosives, ammonia, aniline dyes, etc. Coke, which is bituminous coal with the more volatile constituents removed by distillation, is used for smelting metallic ores; and thus the contiguity of fields of high-grade coking coal and of iron ore determined the location of the centers of steel industry, which are the very main-springs of our modern machine-made civilization. Near such coal districts, other manufactures of all kinds naturally developed, the coal being cheaply available for power and constituting practically the only source of power in regions where cheap hydro-electric power is not available. About 66 per cent. of the coal mined goes to the production of power, including transportation; about 12 per cent. to coking and the by-products; and about 22 per cent. to the heating of buildings.
Commercial coal is of three varieties: (1) anthracite (Pennsylvania anthracite is popularly termed hard coal), and semi-anthracite containing a high percentage of fixed carbon and a relatively low percentage of the volatile constituents (3 to 12 per cent.); (2) bituminous (ambiguously termed “soft coal” in the United States), containing less fixed carbon and more volatile matter (12 to 40 per cent.); and (3) lignite, containing a still smaller proportion of fixed carbon and a large proportion of water. Of the bituminous coals, some coke satisfactorily, but many do not, so that good coking coals are highly prized. Anthracite, because it makes no smoke, is in great demand for house heating; whereas bituminous coal is chiefly used for power production, including locomotive and steamship firing. Lignites as a rule are used only where the better grades of coal are not available.
Coal was first used for heating before steam power came into use, and iron was smelted with charcoal instead of with coke as at present.
Ship bunkering calls for the best grades of bituminous coal, low in ash and preferably high in fixed carbon, because the use of low-grade coals would require carrying larger amounts, leaving less space for cargo. However, no country that has enough coal to bunker ships, need be dependent[23] on foreign supplies; the low grade of coal would simply reduce efficiency and thus increase expense.
—The proportion of coal used for power, as distinct from that used for heat and coal products, is increasing, and is now two-thirds of the total. As a source of power there is really no complete substitute for coal. All the great industrial nations, like England, Germany and the United States, have developed their industries on the basis of large coal supplies. Some countries make large use of hydro-electric power, but for most it is an insufficient substitute. Wood and other fuels are rarely sufficient to maintain an industry built up on a supply of coal. Oil is being successfully substituted in some industries, notably in shipping, but the importance of coaling stations will no doubt persist.
The technique of coal mining in many districts, and the development of heat, power and coal products are not far advanced. Wasteful methods are used, mostly as a result of competition and lack of co-operation and organization among producers. Economies are being advocated, however. Labor-saving machinery has been installed in many mines. A number of power plants have been erected near the mine mouth and the power distributed electrically, thus eliminating freight charges on coal. Central heating and power plants that can burn coal efficiently will no doubt be more popular and numerous in a few years. Government control and legislation may be expected to hasten the changes. In Europe the technique of coal mining, except in undercutting machinery, is further advanced than in the United States, as regards mining all the coal and in supporting the surface.
Improvements in coking ovens may soon make possible the manufacture of some sort of coke from almost any bituminous coal. While all coke may not be satisfactory for modern blast-furnace practice, any future lack of coke will probably be offset by the development of electric smelting, so the seriousness of the metallurgical need is doubtful. The proportion of by-product coke ovens, which make for cheaper coke by providing for other marketable products, is increasing.
Coals are found in the sedimentary deposits of several geological eras: Paleozoic, Mesozoic, and Tertiary. The Paleozoic era, embracing the Carboniferous period, is by far the most important as regards quality and availability of its coal resources; but the lower-grade and chiefly lignitic coals of the Mesozoic and Tertiary are of great importance locally, and there are enormous reserves that exceed in quantity the generally higher-grade coals of the earlier periods.
The geologic distribution of coal is described in “The Coal Resources[24] of the World,” the most important and comprehensive compilation on coal reserves ever made, which was undertaken by the Executive Committee of the Twelfth International Geologic Congress, held in Canada in 1913. As the compilation was made with the assistance of geological surveys and mining geologists of the several countries of the world, it is cited in this paper as authoritative on geologic distribution and resources.
The geographic distribution of the chief coal fields of the world is shown in Plate II.
In North America the most important coals in the Central and Eastern part are of Paleozoic age, but in the Rocky Mountain region vast quantities of coal occur in the Cretaceous (Mesozoic) strata. In the Gulf province and in the Northern Great Plains province of the United States, which extends into Canada, are coals of Triassic (Mesozoic) age that are relatively unimportant at present.
In beds of the Eocene period of the Tertiary era are large deposits of brown lignite locally converted by mountain-building forces into bituminous and semi-bituminous coal, and also a little anthracite under difficult mining conditions. Such locally altered beds are found in the State of Washington, in British Columbia, and in Alaska.
The limited coal resources of South America, in those deposits east of the Andes and in southern and eastern Brazil, are of Paleozoic age. Small areas of Tertiary coals are found in southern Argentina and in Chile.
Key to Plate II.
World’s Coal Reserves as of 1916—Coal Fields in Solid Black.
1. Countries possessing coal reserves of the first magnitude (4,000,000 million to 1,000,000 million tons): The United States (3,527,000 million), Canada (1,234,000 million), and China (1,500,000 million).
2. Countries possessing coal reserves of the second degree of magnitude (500,000 million to 100,000 million): The British Isles (189,533 million), Germany (before the war) (423,356 million), Siberia (173,879 million), and Australia (165,572 million).
3. Countries possessing coal reserves of the third degree of magnitude (80,000 million to 16,000 million tons): France (before the war) (17,583 million), Alaska (16,293 million), Colombia (27,000 million), Austria-Hungary (before the war) (55,553 million), Russia in Europe (before the war) (60,106 million), India (79,001 million), Indo-China (20,000 million) and South Africa (56,200 million).
4. Countries possessing coal reserves of the fourth degree of magnitude (16,000 million to 6,000 million tons): Spain (8,768 million), Japan (7,970 million), Belgium (11,000 million), Spitzbergen (8,750 million).
5. Countries possessing coal reserves, but of inferior magnitude (less than 4,000 million tons): Brazil, Argentina, Chile, Peru, Ecuador, Venezuela, Greenland, Holland, Denmark, Sweden, Italy, Bulgaria, Turkey, Greece, Roumania, Asia Minor, Persia, Arabia, various islands of Malaysia and various countries in Africa. Coal fields shown in black—country not shaded.
[25]
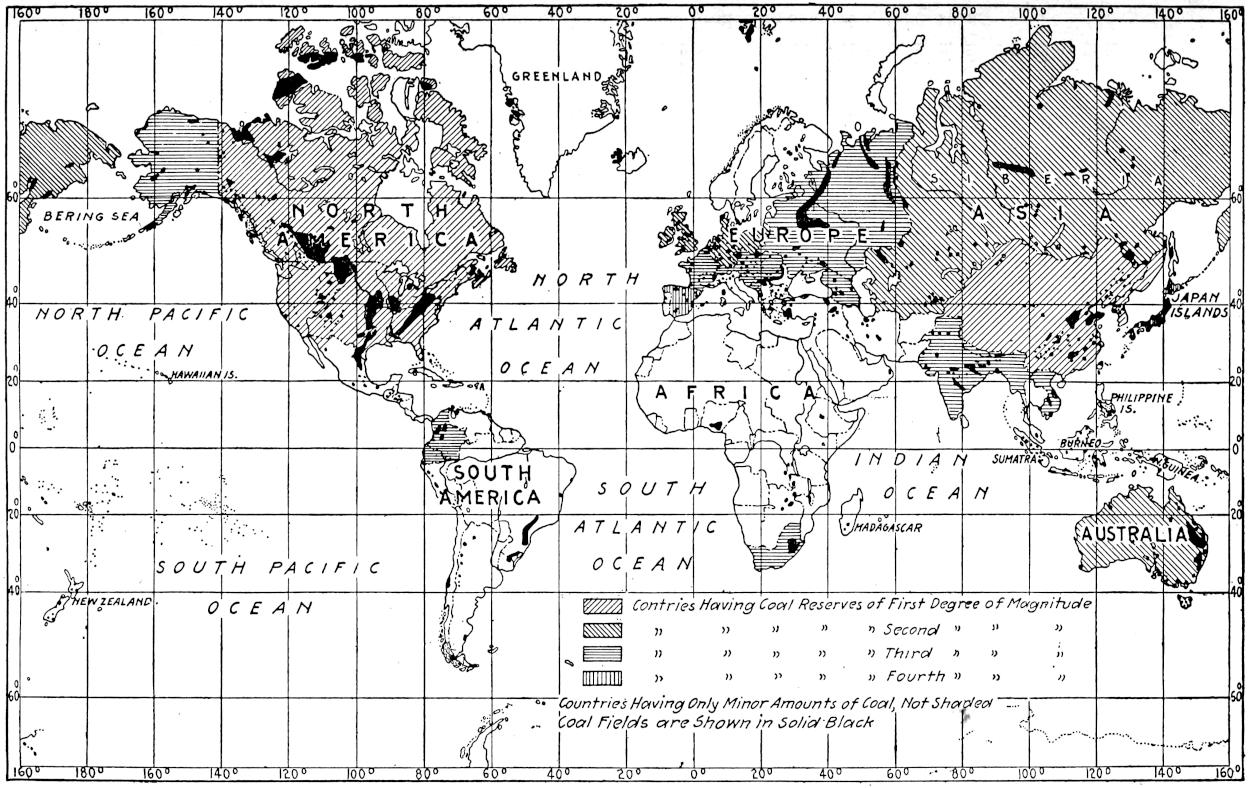
Plate II.—Geographical distribution of the coal deposits of the world, and relative reserves. By F. F. Grout.
[26]
In Europe the principal coal deposits occur in the Carboniferous system, either in the upper or the lower part. The Lower Carboniferous is the principal series in which coals occur in Scotland, whereas the most important coals in England and in Wales lie in Upper Carboniferous rocks. In northern France, in Belgium, and in Westphalia, Germany, the middle Carboniferous measures contain the most important reserves. Mesozoic coals are found in northern Australia and in central France. The lignites or brown coals of middle Europe are locally very important in Germany; those of Austria are found in numerous small but thick deposits of the Tertiary age.
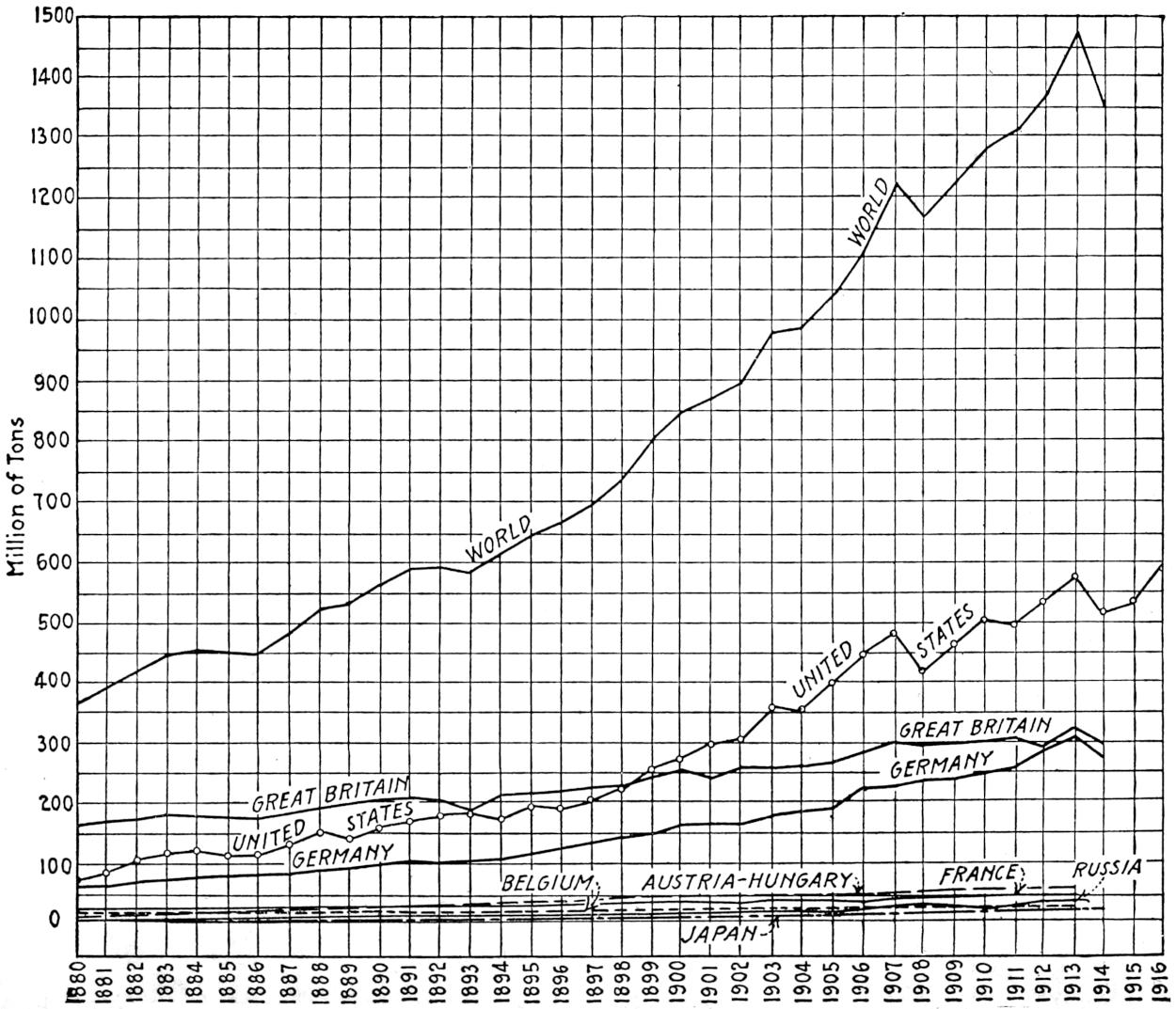
Fig. 3.—Coal output of the chief coal-producing countries, 1880-1916.
The principal coal resources of Africa are in the southern part of the continent and are chiefly in deposits whose ages range from Carboniferous to Triassic.
[27]
In Asia the coal fields are not well defined. There are coal basins of note in India and China. In China important coals are found in the Upper Carboniferous. Coals of the Lower Carboniferous are found east of the Urals and also in Turkestan. In Japan the Mesozoic coals are important. Tertiary coals are widely distributed in Asia but are not high-grade nor of importance.
It may be safely stated that geological reconnoissance has covered the world so well that further development is not likely to disclose coal resources of great magnitude not now known with more or less exactitude. Estimates of resources of some regions will undoubtedly be revised many times, especially those of reserves in the middle portion of Africa, in South America, and China.
As the great World War began on July 31, 1914, the last normal production figures were for 1913. The following table of the world’s production of coal for the years 1911-1914 is from “Mineral Resources” of the U. S. Geological Survey, the compilation being credited by Mr. Lesher, of the Survey, to Mr. Wm. G. Gray, statistician of the American Iron and Steel Institute, and Prof. G. A. Roush, editor of “Mineral Industry.”
The output (1880-1916) of the chief coal-producing countries of the world is shown graphically in Figure 3.
Table 4.—The World’s Production of Coal (in Short Tons)
| Country | 1911 | 1912 | 1913 | 1914 | ||||
|---|---|---|---|---|---|---|---|---|
| United States | 496,371,126 | 534,466,580 | 569,960,219 | 513,525,477 | ||||
| Great Britain | 304,518,927 | 291,666,299 | 321,922,130 | 297,698,617 | ||||
| Germany | 259,223,763 | 281,979,467 | 305,714,664 | 270,594,952 | ||||
| Austria-Hungary | 54,960,298 | 56,954,579 | 59,647,957 | |||||
| France | 43,242,778 | 45,534,448 | 45,108,544 | |||||
| Russia | 29,361,764 | 33,775,754 | 35,500,674 | |||||
| Belgium | 25,411,917 | 25,322,851 | 25,196,869 | |||||
| Japan | 19,436,536 | 21,648,902 | 23,988,292 | 21,700,572 | ||||
| India | 13,494,573 | 16,471,100 | 18,163,856 | |||||
| China | 16,534,500 | 16,534,500 | 15,432,200 | [5] | ||||
| Canada | 11,323,388 | 14,512,829 | 15,115,089 | 13,597,982 | ||||
| New South Wales | 9,374,596 | 10,897,134 | 11,663,865 | 11,644,476 | ||||
| Transvaal | 4,343,680 | 8,119,288 | [6] | 5,225,036 | ||||
| Spain | 4,316,245 | 4,559,453 | 4,731,647 | |||||
| Natal | 2,679,551 | See note 6 | 2,898,726 | |||||
| New Zealand | 2,315,390 | 2,438,929 | 2,115,834 | |||||
| Holland | 1,628,097 | 1,901,902 | 2,064,608 | |||||
| Chile | 1,277,191 | 1,470,917 | 1,362,334 | |||||
| Queensland | 998,556 | 1,010,426 | 1,162,497 | 1,180,825 | ||||
| Mexico[28] | 1,400,000 | [5] | 982,396 | |||||
| Bosnia and Herzegovina | 848,510 | 940,174 | 927,244 | |||||
| Turkey | 799,168 | 909,293 | ||||||
| Italy | 614,132 | 731,720 | 772,802 | |||||
| Victoria | 732,328 | 664,334 | 668,524 | |||||
| Orange Free State (Orange River Colony) | 482,690 | 609,973 | ||||||
| Dutch East Indies | 600,000 | [5] | 622,669 | 453,136 | ||||
| Indo-China | 460,000 | [5] | 471,259 | |||||
| Serbia | 335,495 | 335,000 | ||||||
| Sweden | 343,707 | 397,149 | 401,199 | |||||
| Western Australia | 300,000 | [5] | 330,488 | 351,687 | ||||
| Peru | 300,000 | [5] | 307,461 | 301,970 | ||||
| Formosa | 280,999 | 306,941 | ||||||
| Bulgaria | 270,410 | 324,511 | ||||||
| Rhodesia | 212,529 | 216,140 | 237,728 | |||||
| Roumania | 266,784 | |||||||
| Cape Colony (Cape of Good Hope) | 89,023 | See note 6 | 67,481 | |||||
| Korea | 138,508 | |||||||
| Tasmania | 70,000 | [5] | 59,987 | 61,648 | 68,130 | |||
| British Borneo | 100,000 | [5] | 49,762 | |||||
| Spitzbergen | 44,092 | |||||||
| Brazil | 16,535 | |||||||
| Portugal | 10,000 | [5] | 16,938 | 27,053 | ||||
| Venezuela | 10,000 | [5] | 12,000 | [5] | 13,355 | |||
| Switzerland | 8,267 | |||||||
| Philippine Islands | 2,000 | [5] | 2,998 | |||||
| Unspecified | 1,016,947 | [5] | ||||||
| Total | 1,309,565,000 | [7] | 1,377,000,000 | [7] | 1,478,000,000 | [7] | 1,346,000,000 | |
Table 5.—Reserves
Total coal reserves in millions of metric tons have been estimated, by continents, as follows:
| Continent | Millions of tons |
|---|---|
| North America | 5,073,000 |
| Asia | 1,280,000 |
| Europe | 784,000 |
| Australia and Oceania | 170,000 |
| Africa | 58,000 |
| South America | 32,000 |
[29]
The countries on pre-war basis having the greatest reserves are as follows:
| Country | Millions of tons |
|---|---|
| United States (half lignite) | 3,527,000 |
| Canada (three-fourths lignite) | 1,234,000 |
| China | 996,000 to 1,500,000 |
| British Isles | 190,000 |
| Siberia (largely lignite) | 173,000 |
| Germany (including Upper Silesia and the Saar) | 423,000 |
| New South Wales | 118,000 |
| India | 79,000 |
| Russia including Dombrova field (Poland) | 60,000 |
| Austria (chiefly in Bohemia, Silesia and Galicia) | 54,000 |
| France | 17,600 |

Fig. 4.—Coal reserves of chief producing countries, according to “Coal Resources of the World,” in millions of metric tons. Squares are to scale: lines showing relative production are not on same scale as squares.
The reserves of the principal productive coal fields are graphically shown in Figure 4.
The distribution of the coal deposits of the world and the estimated reserves in these deposits are shown in Plate II.
[30]
The districts with coal for export have been chiefly the British Isles, United States and Germany; there might be included also New South Wales, British South Africa, Japan, French Indo-China, Canada, New Zealand and Spitzbergen. China, with her large reserves, may become an exporter in the future; or, if her industries develop, may find use for her coal at home.
Anthracite of good grade is found in large amounts in Pennsylvania and South Wales only. Poorer supplies are known in Germany, France, Italy, Indo-China, and also in the states of Colorado and New Mexico.
Coking coals in large amounts are found in the eastern United States, Germany and the United Kingdom and are coked extensively. Smaller amounts of coke are made in France, Belgium and old Austria. Relatively very small amounts are made in Canada, Chile, New South Wales, Japan and Spain.
The coal reserve of a country bears no direct relation to its present production, for the latter, which has to be developed in competition with other countries, depends upon relative facility of transportation and proximity of iron-ore deposits, which render steel making and other industries economically feasible.
Great Britain is particularly favored through the possession of high-grade coal immediately adjacent to coast ports, as in the North of England, Scotland and Wales. Hence Great Britain became a great exporter of coal. First, coal for heating purposes traveled by sea from Newcastle to London; next coal was carried to European ports, and finally to all parts of the world. The possession of easily worked iron deposits in the north of England and the discovery that iron could be smelted with coke rapidly accelerated the development of the coal industry in Great Britain, so that by the middle of the nineteenth century Great Britain had a commanding lead.
No other country possesses high-grade coal in such quantity immediately adjacent to the coast, and this fact has enabled Great Britain to remain the great exporter. The average length of haul of export coal, from mine to ship, is less than 20 miles. In Germany coal for ocean export must be hauled 118 miles to 168 miles; in the United States from 150 to 375 miles, except for Washington coals, which are within 40 miles of tidewater, but are small in quantity and of indifferent quality.
After the war with France in 1871, when Germany annexed Alsace and Lorraine, the coal industry of the German Empire developed with tremendous rapidity, largely through the discovery—from the investigations of Thomas, of Great Britain—of a method of utilizing the high-phosphorus iron ores of German and French Lorraine, and the nearness of these iron-ore deposits to the high-grade bituminous coals of Westphalia.[31] The coal industry was also developed by fostering the export trade with adjacent countries, which have small coal resources or none, this trade nearly all going by rail.
However, the long rail haul and the correspondingly high cost of mining have retarded the ocean export business of Germany, in spite of the fostering care of the government. With the transfer of practically all its iron deposits and its important Lorraine potash deposits, as well as the ownership of its Saar coal mines, to France, and the possible loss of its Upper Silesian coal and zinc deposits to Poland, the balance of commercial prosperity, as well, may be handed over.
No other countries except the United States, Canada, Australia and China have reserves for extensive export trade. In Canada the coals are mostly inland, and those near the coast, as on Vancouver Island and in Nova Scotia, are limited in quantity and difficult to mine, so that export business is perforce restricted.
One change that seems likely is a rapid increase in output of coal in China. The resources are enormous, the reserves of the higher classes of coal being surpassed by those of no country but the United States. The ambitious and aggressive Japanese, with their strategic neighboring location, have given every indication that they will take advantage of an opportunity to develop such a resource. It may be a question how soon Chinese coal will be developed, but great changes are inevitable when development begins. Some of the coal fields are so near the coast and have coals of such good quality as to permit an extensive development of export business in the Orient.
Australia, particularly in Queensland and New South Wales, has coal resources that are considerable, in comparison with the needs of the small population. In New South Wales the coals are excellent and are adjacent or close to harbors, so that the coal is extensively supplied for bunkerage and export trade to the South Pacific and has become a large factor in the ocean trade of that part of the world.
A large factor in the coal trade of the Pacific Ocean is the carrying of coal as return cargo. This is also important in South American trade. South Africa during the war sent considerable coal to the western Mediterranean, but its coal cannot be a large factor in ocean trade in normal times.
Of all the continents South America is the poorest in coal resources. There is coal in Brazil and Chile and other South American countries, but it is difficult to reach and the fields so far known do not give promise of being able to take care of the needs of the countries in which they occur. The total annual production of South America is less than two million tons, mostly from Chile. It is probable that for the next generation South America will continue to import coal as it has in the past, to the extent of 15 million tons or more per annum, although developments in[32] Brazil are now promising for that country’s future supply. For estimates of reserves, see Table 5.
—By way of summary, it may be said that the United States leads the world in coal resources; moreover, its resources are most immediately available, because of their shallow depth and general undisturbed (geologic) condition and their accessibility through railway systems. This is particularly true in the Appalachian region, which contains by far the best coal, is the nearest to the coast, and hence is the most available for ocean trade. On the other hand, the average haul to export points, as already noted, is far greater than that of Great Britain and greater than that of Westphalia; but to offset this the coal has been and can be mined more cheaply than in either Westphalia or Great Britain. The resources of Upper Silesia are large, and the coal is easily mined, but the output will all be needed for central Europe. As regards both quality and quantity, Pennsylvania anthracite is unique; nevertheless, the home needs will continue to be a brake on extensive exports. United States steam and coking coals available for shipment average a little poorer than the corresponding coals of Great Britain, but are superior to the German coals.
The total resources of Great Britain, possible, actual, and probable, are only 190,000 million tons, as contrasted with 423,000 million tons in the old German Empire and 3,838,000 million tons in the United States.
—The possible depletion of coal resources is of course important in considering the future, but it can be safely stated that in none of the principal fields now being mined are the resources so depleted that the output therefrom will be reduced for another generation at least.
Of the great coal-producing countries, Great Britain, with its increasing rate of production, most nearly approaches the point of ultimate depletion, but that point has been variously placed at one hundred to several hundred years in the future, much depending upon whether with better methods of use the output will continue to increase at the same rate as in the past. Meantime, certain areas in Great Britain with shallower coal beds will be depleted much sooner and the remaining coal will become more and more difficult to get, because of the increasing depth of mining, which in turn will cause a continually increasing cost.
Although the exhaustion of coal is a distant prospect, there are clear signs of the approaching exhaustion of certain grades of coal. It has been estimated that the output of American anthracite will seriously decline in 60 to 100 years. Probably this will result in a change in practice, the use of coke, and other methods of house heating. If the rate of production continues to increase, high-grade American steam coal, New River and similar grades, will probably be exhausted in a little over 150 years. American coking coal of the best Connellsville quality is of[33] equally short duration. British coal of similar high grade may last longer than American, as the production rate is less rapid, even though the total American coal should last much longer than the British.
In several countries state ownership of coal mines is established; as, for example, in parts of Prussia, Australia, Chile and Bulgaria. In others the undeveloped coal lands are still largely owned by the government, as in Alaska and the western United States. In some countries the government retains control of all mineral rights, simply leasing the property and granting a mining concession. Many governments have a department or administration of mines. In every country, of course, in an emergency, the sovereign state would exercise control of coal resources as fully as was necessary. Where the state owns mines, and favors organization, as in Germany, the more drastic regulation of war time is easily effected. It is quite possible that coal may in time be generally considered a public utility.
In England a movement toward nationalization of mines, with miners as well as the government having a hand in the control, seems to be making progress.
England’s colonies excel all others in extent of coal resources. There is coal in Australia, Canada, India, New Zealand, South Africa, Rhodesia, Newfoundland, South Nigeria and British North Borneo—the total being many times greater than that in the British Isles. The colonies and possessions of France, Denmark, Portugal, United States and Japan have relatively small amounts of coal.
Coal resources, along with other raw materials, have been influenced by some trade treaties. However, no permanent advantage in commerce has been secured to any nation by a trade treaty. Nearly all such treaties are made for short periods and renewed. If not satisfactory to one party they are soon corrected. Various informal or tacit agreements might be mentioned. When shipping was scarce during the war, England and the United States divided the coal business of South America according to the requirements of ship economy. Germany made agreements to send coal to Austria-Hungary and Switzerland during the period of the war. England had agreements with France, Spain, Portugal and Italy, these agreements changing with conditions, about supplies of English coal.
Although the ownership of mines in most countries is nominally open to citizens and aliens alike, exceptions and restrictions tend to keep the control in the hands of citizens. For example, it is impossible for foreigners[34] to control any mining company in Japan. Concessions in Holland and the Dutch colonies are limited to Dutch subjects and in Bolivia to Bolivian citizens. The legislation suggested since the outbreak of the World War may develop a similar condition in the possessions of Great Britain and those of her allies.
In peace the relations of two countries may be largely determined by the ownership of property; owners of property in a foreign country may strongly influence the policy of the two governments toward each other. In emergencies, the political and the commercial control are put to the test, and there results either a deadlock or the victory of one over the other.
An important commercial relation exercising political influence is the incorporation of companies under the laws of different countries. Mining companies in China, like the Kailan Mining Administration, organize at Hong Kong to obtain British protection and are thus subject to British control, in spite of the fact that Belgian money finances the company. Some companies organized in Japan may also own Chinese coal mines.
As relatively few regions of the world have coal in excess of their own needs, the larger number are dependent on imports. If a country with coal controls also steamship lines, it may completely control the coal situation in the importing country. England, with about half the total world’s shipping and a good supply of seaport coal, has been in a position to dominate coal exports, even to handling the excess American coal. During the last three or four years the scarcity of shipping has given increased importance to American and Japanese shipping, but the English still exert a strong influence. Their docks and storage facilities are the best, and their ships are still numerous.
Railroad shipping rights over the National Lines in Mexico give a certain amount of control over the coal industry. The National Lines are state owned and have a special agreement giving trackage rights to two companies, the American Smelting and Refining Co., and the Peñoles Company, of German ownership. Since the Mexican railway service has been disturbed, the German company has been operating with cars and engines of its own. It has many coke ovens and large coal reserves, and has been the chief competitor of the American company in Mexican metallurgy.
No patent is likely to limit coal industries, except as regards the by-products of coke. Before the war, coal-tar products were largely developed by Germans, who patented their processes in many countries, but offered no such protection to foreign inventions by patents in Germany. They limited production chiefly to their German plants, and exported about $50,000,000 worth a year.
During the war, these patents in the United States were taken over[35] by the Alien Property Custodian, and the American industries that sprung up in consequence may be permanently protected. Other allied countries took the same steps to free themselves from German control, which has retarded the development of the by-product coke industry in non-German countries.
In Germany the large mining companies generally own the coal rights. The only government that has mined coal on a commercial scale and for commercial purposes is Germany, and even there the government production covered only a small part of the total output of the German Empire. Mines of the Saar coal field and a group of mines in the Upper Silesian field owned by the Prussian government have been the only extensive state-operated mines of the world, and now, under the terms of the Treaty of Peace, the ownership of the Saar mines will pass to France. (The details are given on p. 39).
On the other hand, the government exercised a quiet but real control over the whole German coal industry. Through its ownership of the Koenigen Louise mines it acted as a member of the Upper Silesian coal syndicate. Formerly, through its ownership of the Saar mines, it was also a member of the Westphalian coal syndicate, but whether a member or not it practically approved the syndicate operations and the fixing of prices in advance; also, it co-operated with the syndicate in hauling the latter’s coal over state-owned railway systems. In certain undeveloped coal fields in Germany, the northern extension of the Westphalian field, the Prussian government retains most of the coal rights.
The Rhenish Westphalian coal syndicate is a classic example of a great interlocking trade combination. Capitalized at $571,000, it covered an enormous capitalization of individual members. The mines have votes in proportion to production, which in turn is limited for certain periods. Prices are fixed and coal is marketed for the syndicate as a unit, but other affairs are left to the companies. The syndicate as a whole is a member of a transporting and exporting combine, and disposes of its product through a combine of coal dealers. The several minor combines interlock and are practically merged in a larger organization, the Kohlen Kontor. Thus the combination had exceptional power to study the various problems of the industry, and became very powerful. It maintains a research department and an explosion testing gallery near Dortmund. In several countries German companies through the control of advertising contracts have been able to influence the editorial policy of the leading newspapers, even during the war. Over half of the German coal mined before and during the war was syndicate coal. All of the 600 other German cartels have not been as moderate in their action and regulations as the coal syndicate. When the coal syndicate, however, at one time, found it could not supply its German market with coal, it is reported to have bought inferior British coal for[36] its customers, so that they would not themselves get good British coal and refuse to return to the German supply.
Efforts were made by the cartel to absorb and control foreign coal trade, where political and financial reasons served to render this advisable, and therefore these efforts were out of all proportion to the intrinsic value of the trade. When it was planned to capture such a trade, the German-invented “dumping” system was used, coal being sold cheaper abroad than it could be sold or produced at home, the difference being met by export bonuses. This German dumping became so serious in some countries, as Canada, New Zealand and South Africa, that special import duties were imposed to counteract it. When desirable, the syndicate purchased collieries abroad, including even a South Yorkshire plant in England, the Heraclea collieries, in Turkey, and many mines in Australia.
In Great Britain is a powerful coal combine, the Cambria, closely allied with great shipping concerns. An important trade asset in this organization, like that in the German cartel, is a banking connection by which the combine can offer long-term credit.
Coal syndicates are mentioned in Belgium. Some sort of central organization interested in coal is known in Italy, Russia, Austria-Hungary, Sweden, Greece, Argentina, Chile, and Ecuador.
France has a modified form of ownership of coal resources: this was vested formerly in the Crown and now in the Republic. The government gives concessions for mining the mineral and charges a royalty. The mineral is not considered to be owned by the surface owner or by the original surface owner, as is the case in all other important countries. The concessions granted are liberal and for large areas. The royalty or rental is small and is now paid on the basis of so much per superficial unit of area (hectare) in the concession, but the chief returns received by the public are through a percentage of the net earnings, that is, earnings available for dividends. Under the conditions prevailing in France the system seems eminently fair, and the undertakings have been profitable both to the operators and to the country. The books of the company are open to inspection by government officials, and the annual reports are published in detail. The system virtually makes the government a partner in the business.
In Great Britain most of the coal ownership is vested in entailed estates, and the royalties are a shilling a ton and upwards.
Both private and government ownership exist in the United States. Throughout the greater part of the country the large operating companies own the coal rights, although in the anthracite district of Pennsylvania[37] the fortunate owners of the surface, or their assigns, receive large royalties, 25 to 50 cents a ton. In the Middle West most of the operators have bought the coal rights from the surface owners. When leased, the royalty is relatively small, 2 to 6¹⁄₄ cents a ton. Operators in the Rocky Mountains generally own the coal they mine. The government has sold the coal rights, but the state school lands of Colorado and Wyoming have generally been leased at royalties of about 10 cents a ton. In Oklahoma the Five Civilized Tribes have until recently, under government control, leased their lands and coal rights at about 10 cents per ton, but these rights are now being sold.
In the State of Washington a considerable amount of the bituminous coal district now opened is owned by the Northern Pacific Railway, which secured these lands as grants when the railroad was constructed. The royalty is about 15 to 25 cents a ton.
In Alaska in the Matanuska and Bering River bituminous fields, and in the Nenana lignite field, the government has offered the coal for leasing purposes at 2 cents a ton for the first period, under restrictions providing for conservation of coal and reasonable prices to consumers. Some units have been taken up in the Matanuska and Bering River fields, but, as the measures are badly contorted and the coal beds difficult to trace, progress has been slow and production has scarcely begun. Temporarily, the Alaskan Railway Commission is working some mines at Chickaloon and Eska Creek, to obtain a supply of coal pending the development of other mines by lessees. Congress, in opening the coal lands in the Matanuska and Bering River fields for leasing, has reserved tracts of not exceeding 7,680 acres and 5,120 acres respectively for the use of the Navy.
The United States still owns large areas of coal and lignite lands in the western states. Most of these lands are remote from railroads and difficult of access, but they contain enormous reserves. At present, outside of Alaska, only one mine, the Gebo mine, Gebo, Wyoming, is leased by the government, but extension of a leasing system similar to that of Alaska has been recently effected.
In the United States the anthracite industry is well organized, and its railroad connections make it notably efficient and powerful. Bituminous coal, on the other hand, is so widely distributed on both public and private lands that no private organization has attempted to control the industry. Such control has always been opposed by Congress and the general public.
Except during the war, neither Great Britain nor the United States has attempted any control over commercial mining and the sale of coal. Each country created a fuel administration, and the coal was shipped under government instructions and paid for at prices fixed by the fuel administration. In the United States this government control has practically disappeared with the war, but in England the Coal Control[38] has so far been continued, and the tendency is for the government to retain for the present a strong guiding hand on the various “key” industries. Certainly, in the final analysis, coal mining is a public utility and should be supervised and adjusted by the government accordingly, allowing free latitude for private initiative.
Of the important coal-producing countries, only the German Empire, more or less openly, has fostered in peace times the coal industry and to some extent controlled it. In France there was only an indirect control, through the control by the government of the concessions and taxation of revenues and through tacit knowledge of the operations of the French coal syndicate, which ostensibly at least obtains and disseminates information and conducts mine safety investigations. In the United States, Great Britain, and other countries free competition has been permitted. Free competition does not seem serious in countries like France, where the supply of coal is limited, but it has had more or less serious financial effects where the supply of coal has been very large. In Germany before the formation of the syndicates the coal mining industry had periods of overproduction and serious financial depression; and at other, rarer, periods there was great prosperity. In Great Britain there have been similar times of depression and prosperity, but generally the business has been profitable.
In the United States, except in the anthracite district, where for more than twenty years the operations have been in the hands of comparatively few companies, depression and prosperity have alternated rapidly. The statistics obtained by the census show that the average profits of the bituminous industry prior to 1917 were smaller than those of any other great industry, and this has had an unfortunate effect on the best development of the coal resources. The companies generally have had little or no surplus to develop properly in the lean years; hence they have mined only the best or thickest coal, and in short periods of great prosperity many mines not directly owned by the railroads and steel companies have been worked so as to lead to “squeezes” and great loss of coal. Moreover, these conditions have also been unfortunate for labor; in times of prosperity too many new mines were opened, because of the tremendous and easily accessible resources, and in times of depression the number of days the miners worked has been so reduced that their monthly or yearly earnings have been low enough to make their living a hard one. The average number of days worked per year from 1901 to 1915 was 213. Some system of limited control of trade combinations by the government would appear to be highly advantageous for both the operators and the miners, and should insure a steady supply of coal to the consumers and steady prices with reasonable profits.
As regards trade relations between the United States and other countries concerning fuel supplies, except for a possible agreement on[39] non-subsidy of the coal-carrying shipping, any attempt at a general agreement on so vital a necessity as coal seems unwise, except for non-duplication of elaborate coal storage and rehandling plants in ports requiring small tonnages, and preventing ruinous competition by systematic “dumping” of surplus coal to drive a competitor out of business.
Of all the continents, South America has the smallest coal resources. Although there is coal in Brazil and Chile and other South American countries, it is difficult to reach, and the fields so far known do not give promise of being able to take care of the needs of the countries in which they occur.
—Immediately after the opening of the war in Europe, July 31, 1914, the German military forces attacked and advanced in the east through Russian Poland, promptly securing the important Dombrova field, which is an extension of the Upper Silesian coal fields. The German forces also advanced in the west through the Belgian coal fields and thence through the extension of these fields in northern France, at the same time seizing the important Briey iron-ore deposits north of Verdun.
The economic effect of these advances was of enormous importance in securing all the productive coal mines of Belgium, the most productive coal mines of Russia, and most of the coal fields of northern France. The French coal and iron mines seized produced one-half of the coal output of France (20 million tons out of 40 million tons), and 95 per cent. (20 million tons) of the output of iron ore. Necessarily under these conditions France had to rely upon England and the United States to meet the military and economic need for iron, leaning chiefly upon Great Britain for the necessary supply of coal. Great Britain during the war continued to supply coal to Italy; also to Spain and other neutral nations.
The armistice ended Germany’s occupation of the coal fields of Belgium and northern France. On the other hand, the French took charge of the important Saar coal field, and the Allies occupied German territory reaching to the Rhine and beyond the Rhine at certain bridgeheads, this occupation including the supervision of the mines in the coal and brown lignite basins near Aix-la-Chapelle and Cologne and the western margin of the Westphalian basin on the left bank of the Rhine.
The treaty of peace gives to the French the important iron resources of former German Lorraine, which together with imports from French Lorraine were the chief sources of iron ore for German iron works, and the ownership of the Saar coal mines.
The terms under which the Saar mines are transferred, and the future government of the district, are indicated in the following extracts from the treaty:
“As compensation for the destruction of the coal mines in the north[40] of France, and as part payment towards the total reparation due from Germany for the damages resulting from the war, Germany cedes to France in full and absolute possession, with exclusive rights of exploitation, unencumbered and free from all debts and charges of any kind, the coal mines situated in the Saar basin.”
This is exclusive of that part of the Saar basin in Lorraine which belonged to France prior to 1870, and which now reverts to France with some minor rectifications of boundary. The treaty further specifies, “all the deposits of coal situated within the Saar basin will become the complete and absolute property of the French state. * * * The right of ownership of the French state will apply not only to the deposits which are free and for which concessions have not yet been granted, but also to the deposits for which concessions have already been granted, whoever may be the present proprietors, irrespective of whether they belong to the Prussian state, to the Bavarian state, to other states or bodies, to companies or to individuals. * * * The value of the property thus ceded to the French state will be determined by the Reparation Commission. * * * This value shall be credited to Germany in part payment of the amount due for reparation. It will be for Germany to indemnify the proprietors or parties concerned, whoever they may be.”
As concerns the government of the territory of the Saar, at the termination of a period of fifteen years, the population will be called upon to indicate their desires, and then, “The League of Nations shall decide on the sovereignty under which the territory is to be placed, taking into account the wishes of the inhabitants as expressed by the voting.” In the meantime, the territory will be governed by a commission of five members chosen by the Council of the League of Nations.
In addition to turning over the ownership of the mines and minerals in the Saar basin, Germany accords the following options for the delivery of coal to the undermentioned signatories of the present treaty:
“Germany undertakes to deliver to France seven million tons of coal per year for ten years (it is understood that this is to provide fuel for the Alsace Lorraine territory ceded back to France). In addition, Germany undertakes to deliver to France annually for a period not exceeding ten years, an amount of coal equal to the difference between the annual production before the war of the coal mines of the Nord and Pas de Calais, destroyed as a result of the war, and the production of the mines of the same area during the years in question; such delivery not to exceed twenty million tons in any one year of the first five years, and eight million tons in any one year of the succeeding five years. It is understood that due diligence will be exercised in the restoration of the destroyed mines in the Nord and the Pas de Calais.”
Besides furnishing France with coal, “Germany undertakes to deliver to Belgium eight million tons of coal annually for ten years;” and to[41] Italy from four and one-half to eight and one-half million tons annually; and also to Luxemburg, “a quantity of coal equal to the pre-war annual consumption of German coal in Luxemburg.”
The prices to be paid for coal under these options shall be as follows:
“(a) For overland delivery, including delivery by barge, the German pithead price to German nationals, plus the freight to French, Belgian, Italian or Luxemburg frontiers, provided that the pithead price does not exceed the pithead price of British coal for export. In the case of Belgian bunker coal, the price shall not exceed the Dutch bunker price. Railroad and barge tariffs shall not be higher than the lowest similar rates paid in Germany.
“(b) For sea delivery, the German export price f.o.b. German ports, or the British export price f.o.b. British ports, whichever may be lower.
“The allied and associated governments interested may demand the delivery, in place of coal, of metallurgical coke in the proportion of 3 tons of coke to 4 tons of coal.”
Germany undertakes to deliver to France during each of the three years following the coming into force of this treaty,
| Benzol | 35,000 | tons |
| Coal tar | 50,000 | tons |
| Sulphate of ammonia | 30,000 | tons |
The price paid for coke and for the articles referred to shall be the same as the price paid by German nationals under the same conditions of shipment.
The ownership of the Saar mines is a most welcome addition to the coal resources of France; and the Saar basin, as it is capable of further development, may in the future make France more nearly self-sustaining as regards coal production.
The requirement of furnishing coal to France during the rehabilitation of the French mines wrecked by the Germans is a most equitable arrangement. Germany at first, owing to the drop in the output of the Westphalian fields, claimed not to be able to furnish coal, but this situation will no doubt right itself in time, especially as France holds the whip hand through control of the iron ores necessary for the great iron and steel plants of the Rhine district. In the meantime it is hoped Great Britain, with the assistance of the United States, will be able to supply the deficiency in the coal requirements.
The problems connected with the Russian coal fields are complicated, but at least the Dombrova coal field would seem to be in the hands of the new Poland, and this carries coal resources estimated at 2,525 million tons, with an output before the war probably exceeding 7 million metric tons per annum.
According to the terms of the peace treaty, a plebiscite will determine the political control of the Upper Silesian coal fields.
[42]
—In the ocean coal trade of the world the greatest change likely is that the United States will more largely supply South America, its coal being substituted for that of Great Britain. The ocean distance is markedly in favor of the United States, particularly on the west coast of South America by vessels passing through the Canal. With the increased shipping facilities of the United States, there is every reason to believe exports of coal to South America will be equally shared between the United States and Great Britain.
At the present time, it is evident that the British coal-mining industry is in a bad way, and publicists are expressing serious alarm at the possible loss of the greater part of the export trade and the curtailment of home industries through a great decrease in production accompanied by a rapid increase in cost.
In 1913 Great Britain produced 287,000,000 long tons, and exported 77,000,000 long tons of coal. During the war, owing to the large number of miners entering the military service, the output greatly declined, but was expected to recover rapidly with the signing of the armistice and the return of the miners. But labor unrest, resulting in strikes and absenteeism, kept the output down, and on July 16, 1919, the so-called Sankey award went into effect. This award shortened the miners’ working day from eight to seven hours, exclusive of the time taken in hoisting and lowering, but inclusive of the time taken in reaching the working place. Rates were raised so that the miner received more in a day with the seven-hour day than formerly with the eight-hour day, and the Controller raised the price of coal six shillings a ton to offset the increased cost.
Sir Richard Redland, chief inspector of mines, predicted that the output for 1919 would be 230,000,000 tons, and for 1920, 217,000,000 tons, or a reduction of 70,000,000 tons from the output of 1913. Presumably, in the course of time, by using additional shifts, Great Britain may recover its former output, though manifestly at greatly increased cost; so that unless the cost in the United States goes up correspondingly, there is every probability that this country will be able to compete successfully in export business, not only in South America, but also in Mediterranean ports.
At the present time, demands for coal are reaching the United States not only from those parts of the world, but also from Scandinavia, Switzerland, Denmark, and The Netherlands. On account of nearness, however, Great Britain should be able to take care of the fuel requirements of northern Europe.
In the Pacific, it is not probable that either the State of Washington or the Territory of Alaska will produce coal in such quantity and at such a price that the output can be a general factor in the Pacific Coast trade. The demands of Alaska, Washington, and adjoining states will absorb[43] the local production; and California will continue to import in ballast more or less coal from Vancouver Island, British Columbia, China, Japan, New Zealand, and Australia.
The immediate changes in Asia are more likely to be in the development of mines in the interior of China and in India to supply domestic needs rather than extensive exports, although, as before stated, it is possible that China will gradually get into the Pacific Coast markets.
—The United States has the best coal reserve of any country—about 3,527,000 million out of a total world reserve of 7,900,000 million tons—and good reserves of each of the several classes of coal. For many years there will be no danger of a shortage except for anthracite, good coking coal and the highest grades of steam coal, which are now actively mined. About 600,000,000 tons a year, or nearly 40 per cent. of the annual output of the world, is mined in the United States.
In contrast with the reserves and production, the exports in 1913 were only about 12 per cent. of the exports of coal from all countries; and a large part of the American exports goes to Canada by rail. Of sea-borne coal, the United States sent out only 4 per cent. This small proportion of international trade is due to the distance of our coal from seaports, the lack of organization and related shipping organizations; and, further, to the relative independence of the United States, which, from most countries, requires only a small amount of import as a return cargo for coal-carrying ships. We use our coal at home, but the advantage of exporting a considerable quantity of coal for its effects on increasing trade relationships with other countries is now becoming evident.
Correlated with the large supply and small export of coal is the remarkable development of home industries using our own coal. From the curves of production (shown in Figure 3) it seems that within a century the United States will surpass all Europe in coal production. As our industries have kept pace with coal production, our consumption of coal is indicated roughly by the production curve. Hence it seems that the United States is likely to be a center of manufacturing and wealth; and with this will come an equally certain continual increase in population and power.
The second great world supply of coal is likely to be that of China. To be sure, European production is large, but it will be divided among several powers. The main part of the world’s power and industry for the next century is so definitely located by the coal deposits (and by associated iron in most cases) that the part the United States should take in the world’s program is clear. Every precaution should be observed[44] to have the Chinese resources controlled by powers that will not abuse them to make the world “unsafe for democracy.”
The war opened several foreign markets, especially in South America, to United States coal. Some of these markets may be permanent, but Welsh coal is still likely to dominate sea-borne trade. The United States has coaling stations as far away as Manila and the Samoan Islands, but little coal reaches them from this country. American coal supplies our government coaling stations in Alaska; Hawaii; our home ports, both Atlantic and Pacific; Cuba; Porto Rico; Nicaraguan ports; Panama Canal ports; Mazatlan, Mexico; and some South American ports. No attempt seems to have been made to establish strategic ports around the world, such as may be needed if the present increase in American shipping is to be maintained under the American flag.
No foreign control has been influential in mining or handling coal in the United States. The ownership of coal mines by aliens has been possible, but apparently has not become important.
American coal resources are so great that no single organization, foreign or domestic, has been able to dominate the situation. The lack of a strong trade combination made it possible (in 1916) for a combination of British shippers to fix the price of bunker coal in Atlantic ports, so that the mines got even less for it than for industrial coal. This was the result of competitive bids, and the lack of organization here, but it is expected that organization will develop now.
Since the war began the development of industries based on coal tar has been remarkable. There are signs, however, of an unhealthy competition in this country, and the government should be careful that internal squabbles do not open the door for German control again.
—The British Isles have only one-fortieth of the total world’s supply of coal, but this is of better than average quality. High-grade steam coal is abundant and there is a fair supply of coking coal. The annual production, 300 million tons, is about one-fifth the world’s production, and is second only to that of the United States. England before the war exported about one-fourth of the production, overseas exports from England being six times as much as from any other country in the world. Coal has constituted about three-fourths of all English exports. The coal mines are near seaports, and ocean freight rates are low, because the demand for imports gives return cargoes to England from all parts of the world. There are large supplies of coal also in the colonies, especially India, Australia and South Africa.
The coal business and shipping of Great Britain grew up together. About one-fourth of the coal shipped goes for bunkering. In 1916 England owned 40 per cent. of the world’s shipping and exported nearly 70 per cent. of the world’s sea-borne coal. The maintenance of the shipping requires bunkering ports all around the world. Coal from Wales[45] and British colonies was sufficient to supply them all, and they constitute by far the most strategic system that any country possesses.
England, Gibraltar, Greece, Malta, Suez, Port Said, Aden, Maskat, Colombo, Singapore, Bombay, Hong Kong, Shanghai, Sydney, Fiji Islands, Vancouver, Valparaiso, St. Lucia, Jamaica, Halifax, Newfoundland, St. Helena, Bermuda, Cape Town and Durban, and others encircle the globe. France, Japan, Holland and the United States have each a few stations, but no such comprehensive system. The German proposals of terms of peace (during the war) recognized the importance of these stations by specifying that England should give up Aden, Malta and similar ports.
Trade arrangements between Great Britain and other countries have been such as to grant “most favored nation” treatment to both parties, even with Germany, where no formal treaty was in force. The free-trade policy of England is well established, and on that basis England’s commercial growth has been very great. Studies since the war began show that Germany took advantage of the freedom in British countries and the protection at home. For example, German capital controlled some collieries in South Yorkshire, through Mr. Stinnes, one of the largest components of the German Kohlen Kontor. This organization had branches in Newcastle, Cardiff, Glasgow, Hull, and many foreign ports. The French also had purchased an English colliery before the war, the Stonehall colliery, at Lydden, near Dover.
The Australian colonies and probably others found that German financiers owned and controlled most of the mines when war broke out. It took some time to destroy this influence. Early in the war, British sentiment seemed to call for action against all such German commercial aggression, and at an Allied Economic Conference at Paris in June, 1916, plans were suggested for protection by tariff and exclusion of alien ownership in allied countries. More recently it seems that the British plan is to keep British certain key industries at all hazards and at any expense, but not to abandon free trade or in any way decrease the amount of trading done.
Commercial control of the Welsh steam-coal export trade is largely in the hands of the Cambria Coal Combine, but in the trade there are several other large combinations. The anthracite industry of England is not as well organized as that of America.
Commercial control of coal exported from Wales is largely in the hands of the large combinations of British shippers, in agreement with the Cambria. Even when the war interfered with shipping, it is estimated that two-thirds of the South American coal trade was in British control.
Many instances of British financial control of coal in countries other than British colonies have not been noted. British capital is invested in a few mines in Siberia and there are extensive holdings in China.
[46]
—The reserves of coal in Germany before the war were greater than those in England, counting possible reserves, and of fair quality. Germany formerly controlled 70 per cent. of the coal on the Continent. Austrian coal was controlled, and the coal of Spitzbergen has been claimed, though now in British and Norwegian control.
Although the most important deposits of coking coal of the Continent are in Westphalia, those of Belgium and northern France are very important to a general control of the coal situation. It cannot be assumed that these important coal fields were seized by the Germans for other reasons, or that the Germans included coal and iron by accident. In 1911 the Rhenish Westphalian Zeitung advised that French Lorraine and Luxemburg should be dominated as thoroughly as Westphalia was. In 1915 the six greatest associations of business men in Germany petitioned the Chancellor to consider the control of the coal (and iron) of northern France as a military as well as economic necessity.
The annual production of coal in Germany before the war was about one-fifth of the world’s total output, closely approaching the production of Great Britain. In the first years of the war the coal output declined somewhat. In coal exports, Germany has been second only to the British Isles, but no country exports by sea one-sixth as much as Great Britain. Before the war Germany had established some fourteen “Kohlen depots” abroad and had a large amount of shipping. These bunkering ports were taken by the Allies.
An example of German industrial penetration is furnished by the case of Kiau Chau, China. In 1899 the Shantung Eisenbahn Gesellschaft was formed in Berlin with headquarters at Tsingtao. It acquired exclusive rights for 5 years to search for minerals in a zone 10 miles each side of the railway and to acquire claims. Chinese mines were not to be allowed to adopt modern methods and compete, unless they bought German material and employed German men. The mines that were developed produced good steamship coal and good enough coke, so that a blast furnace was planned. The Germans lost their control when war broke out, and these rights have passed, under the terms of the peace treaty, to Japan. German capital that had been invested some years ago in the Chung Hsing Coal Co. was bought out in 1908.
—Before the war France was fifth in the list of world’s coal producers, but for many years needed more coal than she produced. Possibly enough coal could have been mined in France by greater developments, but to import it was cheaper. French capital was invested in some foreign coal deposits. A company of French control owned the Stonehall colliery, near Dover. Before the war a French company was one of the largest operators in Turkey and was steadily acquiring new mines. A French company owned an important colliery in the Dombrova field, now part of Poland.
[47]
France has maintained government coaling stations for shipping in Indo-China, Tahiti, Society Islands, Martinique, and Madagascar; but no attempt is made to supply them wholly with French coal, or to be independent of other coaling stations.
—Italy has a poor supply of low-grade coal, and the normal production is insignificant. Imports have been large, those from Great Britain amounting in 1914 to 10,000,000 tons.
—Russia has several important coal fields. The production was 31,000,000 tons in 1915 and 23,000,000 in 1916, so that Russia, including the province of Ukraine, has ranked fifth or sixth among the world’s producers. If the demand for coal develops under the stimulus of industrial stability, the output will all be consumed in Russia. About two-thirds of the production has been raised in the Donetz basin. Half of this is coking coal.
—Japan has on the main island, Hondo, enough coal to permit considerable exports. Supplies in Korea and Formosa are less abundant. In all Japan’s coal there is very little of good coking quality.
When the war reduced the amount of British shipping in the North Pacific, Japanese ships supplied Japanese coal to a number of new coaling stations, and the British may find it difficult to regain their former prominence in bunkering there. Japanese coal now gets as far west also as Colombo, Ceylon.
Japanese law specifies Japanese control of the policy of mining companies, though some foreign financial interests are allowed. Japan controls a part of the coal produced in China, and by presenting insistent demands is increasing her control in that country. If given a free hand, Japan is in a position to exercise industrial and military control in Asia almost as thoroughly as the United States can in America. English and Belgian interests have some Chinese coal, and thus are about the only real competition at present with Japanese control.
—Outside the United States, China has the largest coal reserves of any country in the world. The coal varies in quality and grade; some of it is excellent, but the production has reached only about 18,000,000 tons a year. Some districts export and others import coal. The country could easily be independent if there were internal means of distribution. There might even be large exports if production began in advance of other industrial development. Coking coal is available only in certain areas, mostly in the south.
The largest and best-equipped producer is the Kailan Mining Administration, operating British and Chinese properties. The ownership of the company is mostly in Belgian hands, but the incorporation is under Hong Kong law, so that the company is under British control.
The chief producers in China are:
[48]
Table 6.—Chief Producers of Coal in China
| Company | Tons a year | Control |
|---|---|---|
| Kailan Mining Administration | 3,000,000 | British and Chinese |
| Funshun Collieries | 2,000,000 | Owned by the South Manchuria Railway Co. (Japanese) |
| Pingshieng Collieries | 1,000,000 | Chinese |
| Pekin Syndicate | 500,000 | British |
| Pingshihu | 300,000 | Japanese |
| Lincheng | 800,000 | Belgian |
The Mining Company of Shantung, producing 400,000 tons a year, formerly owned by the Germans, is now run by the Japanese military organization. Thus only one of the large producers is under Chinese control, though many smaller mines are worked by Chinese. The Chinese law designed to prevent foreign control of this sort is not effective. It requires that the share of a mining industry held by foreigners shall not be over one-half; but if a foreign company owns half the shares and finances the Chinese half by a loan, the foreign control may be complete. There is a further control of mining, through the ownership of railways. All the larger producers must ship coal by rail, and foreign nations are allowed to finance railways. The difficulty of a government exercising adequate political and commercial control when it grants concessions in this way is evident.
General mining affairs in China are supervised by a Bureau of Mining Affairs. Any specific enterprise is controlled by a commissioner of finance in each province. It is questionable, however, whether governmental control will be strong enough to overbalance commercial and financial control, and diplomatic pressure from outside. Those companies incorporated under Hong Kong law can count on British protection. Japanese demands on China have been very insistent, and it is said that about a third of the production of the country is now controlled by Japan.
As a whole, China seems to take a small part in the control of her own coal. The opportunity for other powers to get financial, and, through that, industrial favors may be involved in the problem of financing the central government.
[49]
Table 7.—Production, Exports and Imports
for 1913[8]
Millions of metric tons
| Country | Kind of coal | Production | Exports | Imports | Remarks (1919) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| United States | Anthracite | 85 | 4.1 | ... | Resources greater than those of any other country; can easily increase ocean exports with more shipping available. Present exports chiefly to Canada. Value of coal-tar products imported in 1913, $10,962,000. | ||||||||
| Bituminous | 432 | 18.0 | 1.4 | ||||||||||
| Coke | 42 | 1.0 | 0.1 | ||||||||||
| Bunker coal | ... | (7.7) | ... | ||||||||||
| Great Britain | Anthracite | 5 | ... | ... | Chief coal-exporting country; before war had virtual monopoly of ocean exports. Export control imperiled by shortage from labor conditions. | ||||||||
| Bituminous | 282 | 73.4 | ... | ||||||||||
| Coke | 20.5 | 1.2 | ... | ||||||||||
| Briquettes | ... | 2.1 | ... | ||||||||||
| Bunker coal | (21.0) | ... | |||||||||||
| German Empire | Bituminous | 191 | 34.6 | 10.5 | Coal needed for Central Europe. Exports by rail and canal. Distance from seaports prevents oversea exports. Westphalia has largest coking coal resources in Europe. Ownership of Saar mines transferred to France by Treaty of Peace. | ||||||||
| Lignite | 87 | ... | 7 | ||||||||||
| Coke | 32 | 6.4 | 0.6 | ||||||||||
| Coal Briquettes | 5.8 | 2.3 | 0.3 | ||||||||||
| Lignite Briquettes | 21.4 | 0.9 | 0.1 | ||||||||||
| Saar District (Included under German Empire, above) | Bituminous | 17.0 | |||||||||||
| Coke | 2.0 | ||||||||||||
| Upper Silesia (Included under German Empire, above) | Bituminous | 49.1 | Nationality of Upper Silesia to be determined by plebiscite; coal production vital to eastern Germany, Poland and Austria. | ||||||||||
| Lignite | 2.3 | ... | ... | ||||||||||
| Coke | 3.1 | ... | ... | ||||||||||
| Austria-Hungary | Bituminous | 17.6 | 0.7 | 13.7 | Austria, already deficient in bituminous coal, under the Peace Treaty loses practically all coal fields to Poland and Czecho-Slovakia. | ||||||||
| Lignite | 36.4 | 7.0 | ... | ||||||||||
| Austria (Included under Austria-Hungary, above)[50] | Bituminous | 16.3 | ... | ... | Hungary always lacked enough bituminous coal, and under any political control must continue to import coal from Upper Silesia. | ||||||||
| Lignite | 27.4 | ... | ... | ||||||||||
| Coke | 2.6 | ... | ... | ||||||||||
| Hungary (Included under Austria-Hungary, above) | Bituminous | 1.3 | |||||||||||
| Lignite | 9.0 | ||||||||||||
| Coke | 0.2 | ||||||||||||
| France | Bituminous | 40.0 | 1.3 | 18.7 | France consumed in 1913 (millions of tons)
|
||||||||
| Lignite | 0.8 | ... | ... | ||||||||||
| Coke | 4.0 | 0.2 | 3.0 | ||||||||||
| Briquettes | 3.7 | 0.1 | 1.1 | ||||||||||
| Russia (Included in above is the Dombrova field of Poland) |
Bituminous chiefly | 32.3 | ... | 8.1 | Russia, with poorly developed fields and great future needs, has imported from Great Britain and Germany; through loss of the Dombrova field (extension of Upper Silesian basin) needs more coal than can produce and is unlikely ever to be an exporting country. | ||||||||
| Bituminous (Some brown coal) | 7.0 | ||||||||||||
| Belgium | Bituminous | 22.8 | 4.9 | 8.9 | Belgium has high-grade steam coals and some coking coal; beds are deep and difficult to mine. Its exports to Holland and France probably will in future continue to be exceeded by imports from Westphalia and Great Britain. | ||||||||
| Coke | 3.5 | 1.1 | 0.4 | ||||||||||
| Briquettes | 2.6 | 0.6 | 1.1 |
[8] Compiled by George S. Rice.
[51]
Table 8.—Countries in Europe Largely Dependent on Imports of Fuel.
Production and Imports, 1913[9]
Millions of metric tons
| Country | Kind of coal | Production | Imports | Remarks (1919) |
|---|---|---|---|---|
| Holland | Bituminous | 2.0 | 12.0 | Holland, in the small Limbourg basin, has an extension of the Aix-la-Chapelle basin of Germany. The output is increasing, but as the basin is small Holland will import from Westphalia, Belgium and Great Britain or America. |
| Italy | Anthracite and Lignite | 0.7 | 10. | Italy has insignificant and poor resources in thin anthracite beds and in lignite deposits; has depended on Great Britain for imports, but now the United States is furnishing some coal. Water-power developments are large; opportunity for further development. |
| Spain | Bituminous | 4.3 | 3.6 | Spain has a number of small coal basins. It must continue to import. |
| Sweden | Bituminous | 0.4 | 4.8 | Sweden has a few thin impure beds, but has relied on imports from Great Britain. |
| Norway | 2.3 | Norway has no coal resources and has imported coal from Great Britain. | ||
| Switzerland | 1.6 | Switzerland has no coal worthy of mention; it has relied on imports from Westphalia. Since the armistice, Switzerland has imported coal from the United States, but this movement is abnormal. |
[9] Compiled by George S. Rice.
—As regards political control, three great national or race factors loom in the future of the coal industry and in the development of wealth and power: the European, dominated by England; the American, by the United States; and the Asiatic, by Japan. The efforts of England during the war temporarily prostrated her, and diminished the grip of her export coal and bunkering trade, but conditions late in 1919 indicated that recovery might be rapid. The immediate growth in the mining of coal for use at home, with consequent progress in steel and other industries, however, will be greatest in the United States, because of our gigantic resources. In the East, however, are indications of a development of China’s coal and the growth of attendant industries on a scale which may in time outstrip those of any country except America, and transfer the bulk of wealth and power to the two great civilizations, on either side of the Pacific—the newest, that of America, and the oldest, that of China and Japan. The war and the settlements after the war proved an unmixed benefit and opportunity to Japan,[52] and enabled her so to strengthen herself in China and Korea that she is not only the preponderating power in the East, but may claim a sphere of influence and a wide protectorate for Asia, far more effective than the American Monroe Doctrine.
Having regard to national internal economy and external and internal effectiveness, the United States will clearly neglect the main function of government if it fails to exercise an effective supervision and regulation of the coal industry. This industry is national; every citizen has an interest in it, and a right to expect its administration for the highest benefit of all.
Table 9.—Production and Imports of Fuel of South American Countries
in 1913[10]
Millions of metric tons
| Country | Production | Imports | Remarks (1919) |
|---|---|---|---|
| Argentina | 4.0 | Argentina has no coal resources. Imports chiefly from Great Britain; Cardiff coal. | |
| Brazil | 2.2 | Brazil has coal, but it is inaccessible for transportation. | |
| Chile | 1.23 | 0.6 | Chile has some coal, but does not mine enough high-grade coal for its needs; has imported from Great Britain. |
| Colombia | Colombia has some undeveloped coal resources; has imported a little coal from time to time from the United States. | ||
| Peru | 0.28 | 0.02 | Peru has small coal resources and mines a little coal, practically enough for its needs. |
| Uruguay | 0.8 | Uruguay has no coal resources and imported some in the past, chiefly from Great Britain. | |
| Total South America | 1.51 | 7.6 |
[10] Compiled by George S. Rice.
[53]
Table 10.—1913 Production, Exports, and Imports of Coal of Principal
Countries of Asia and Australia[11]
Millions of metric tons
| Country | Kind of coal | Production | Exports | Imports | Remarks (1919) |
|---|---|---|---|---|---|
| Japan | Bituminous | 21.8 | 3.5 | ... | Japan does not have large coal resources and coal is not of high grade, but deposits are advantageously located for exporting. |
| India | ... | 16.5 | ... | ... | India has considerable good coal, but this will be needed for domestic purposes and bunkering. |
| China | Bituminous | 14.0 | ... | about 2.0 |
Coal is imported from Japan, but China has great coal resources, and these are being developed by Japanese, British and American capital. Germany had large interests, which have reverted, it is understood, to Japan. |
| Indo-China | ... | 0.4 | ... | ... | Indo-China has some coal, both anthracite and lignite. |
| Siberia | ... | ... | ... | ... | Little is known of the coal resources of Siberia. There are many indications of lignite deposits. |
| New South Wales | ... | 10.5 | 6.0 | ... | New South Wales has large resources of good coal. One-half of the 6 million tons exported goes to other Australian states; the other half to Pacific ports. |
| New Zealand[54] | ... | 1.9 | ... | ... | New Zealand has small coal basins, but they are close to the sea, permitting ready export. |
| Queensland | ... | 1.1 | ... | ... | Queensland, Victoria, and Western Australia have considerable coal resources, but not of grade or quality to be a factor in export trade. |
| Victoria | ... | 0.6 | ... | ... | |
| Western Australia | ... | 0.3 | ... | ... |
[11] Compiled by George S. Rice.
[55]
The uses of iron ore are so well known that their enumeration is hardly necessary. From iron ore are manufactured cast iron, wrought iron, and steel. By the addition of one or more other elements, chiefly silicon, carbon, chromium, nickel, manganese, vanadium, sulphur, and phosphorus, in quantities less than 5 per cent. and usually less than 1 per cent., various qualities, such as hardness, toughness, elasticity, durability, brittleness, density, porosity, endurance, resistance to oxidation or corrosion, malleability, and fusibility, can be controlled and given to the cast iron or steel in the desired degree.
The uses of the products of iron ore are so common that the finding of objects which do not contain some of them is difficult. Besides being used as a metal, iron enters into the manufacture of paints (especially red, yellow, and blue), chemicals of various kinds, medicines, coloring matter in glass and pottery, and in the form of specular hematite it is made into jewelry. Considerable amounts of iron ore are also consumed annually for flux in the smelting of silver, copper, lead, and other metalliferous ores.
Iron and its products are more widely used than any other metal; and the yearly production of pig iron makes up 94 to 96 per cent. of the total amount of all the metals produced in the world, and in normal times averages about 80,000,000 tons annually.
Iron ores are associated with many different classes of rocks—sedimentary, igneous, and metamorphic. Where associated with sedimentary rocks the ores may be the result of direct sedimentation or may be later replacements of sedimentary beds by magmatic or meteoric iron-bearing waters. Many iron-ore deposits associated with sedimentary rocks are formed by the enrichment of original iron-bearing beds, either by solution and transportation of iron compounds or by the removal of other associated mineral constituents.
Among those important iron-ore deposits of sedimentary origin that have undergone little or no further enrichment since deposition, except perhaps directly at the surface, are the iron ores of the Clinton[56] type of the eastern United States, the Wabana iron ores of Newfoundland, the “minette” ores of the Lorraine district in northern France, Luxemburg, and southern Germany, the oolitic siderite beds of the Cleveland district in northern England, and the hematite ores of Minas Geraes in Brazil. The most important of the sedimentary iron ores that are the result of further enrichment since deposition are those of the Lake Superior district in the United States.
Iron ores associated with igneous rock are mostly of deep-seated origin, usually having been formed by solutions that accompanied or followed the intrusion of the rocks with which the ores are associated. These ores are of two main classes: (1) Those associated with siliceous igneous rocks; and (2) those associated with basic igneous rocks. The ores associated with siliceous igneous rocks consist either of hematite or, more commonly, magnetite. They occur in granite, syenite, and monzonite, and in gneiss derived from these by metamorphism. Many important ore deposits in different countries belong to this class, among them being the magnetite and hematite deposits of Swedish Lapland and of central Sweden, the magnetite bodies of the Adirondacks and northern New Jersey in the eastern United States, various magnetite and hematite bodies in California and elsewhere in the western United States, the mixed hematite and magnetite deposits of the south coast of Cuba, most of the iron-ore bodies of Chile, and the newly developed iron ores of Manchuria. As a class, the iron ores associated with siliceous igneous rocks rank next in importance to iron ores of sedimentary origin.
Iron-ore deposits associated with basic igneous rocks are nearly all of a distinct type known as titaniferous magnetites. These ores consist of a mixture of magnetite and ilmenite in varying proportions, and therefore carry a variable amount of titanium. Many large ore deposits of this class are found in different parts of the world, among the larger ones being certain ore bodies in Wyoming, in the Adirondack region, and elsewhere in the United States, and several deposits in Norway and in northern and southern Sweden.
An important group of iron-ore deposits has resulted from mineral replacement along the contact of sedimentary rocks with igneous intrusives. These ores usually occur in limestones not far from intrusive masses of granite, monzonite, syenite or diorite, but they may be found within the igneous rocks themselves, near the contact. They are rarely associated with the more basic igneous rocks. These ores are known as igneous contact ores, and their origin is ascribed to iron-bearing solutions that accompanied or followed the intrusion of the igneous rocks with which the ores are associated. Such ores are extremely widespread, occurring in practically every continent. Locally, extensive deposits exist, as in the Cornwall district of Pennsylvania, in the western United States and British Columbia, in Chile, and in China and Japan. Igneous[57] contact ores have furnished only a relatively small percentage of the world’s total production of iron ore, however.
There are also widespread replacement deposits in sedimentary rocks that are not associated with igneous rocks. These are believed to be formed by ordinary meteoric waters which dissolve disseminated iron minerals from certain beds or masses of rock, and redeposit the mineral elsewhere in a more concentrated form. Such ore deposits may be roughly tabular and resemble bedded deposits, or they may be very irregular. Most deposits of this type consist of siderite which has replaced limestone, but hematite and limonite deposits formed by replacement also exist. Among the important deposits of this group are the siderite ores of Bilbao, Spain, largely altered to limonite near the surface; the siderite ore of Eisenerz, Styria; and the hematite deposits near Hartville, Wyoming. Small deposits of siderite, hematite, and limonite of this type are found in many parts of the world.
Besides the classes of iron ores already mentioned, widely distributed iron ores occur as residual products derived from the weathering of either igneous or sedimentary rocks. These ores have been formed by the concentration of iron-bearing materials originally disseminated through the rocks whose weathered products they now constitute. They are mainly in the form of limonite and occur either as large bodies of relatively pure ore or as aggregates of irregular masses of various sizes imbedded in clays. To this class belong the brown iron ores associated with clay in the Appalachian region of the United States, the limonite ores of parts of Russia, and similar ores in Korea. In this class should also be included the extensive limonite deposits derived from the weathering of serpentine which have recently been developed along the north coast of Cuba, as well as the lateritic iron-ore deposits found in many tropical countries. Limonite ores associated with clays have been smelted since early ages, owing to their accessibility and the ease with which they could be smelted by crude methods. They have, however, furnished a decidedly minor percentage of the world’s production of iron ore.
The iron ore consumed by the world has been obtained principally from the four great iron-producing countries: United States, Germany, France and Great Britain.
Other countries that yield important quantities of iron ore are, in the order of their importance: Spain, Russia, Sweden, Luxemburg, Austria-Hungary, Cuba, Newfoundland, and Algeria. The normal annual output in each one of these countries is more than one million tons. Minor amounts of iron ore are produced in many other countries.
[58]
Table 11.—Iron-Ore Output of Principal Producing Countries, 1910-1917, in Gross Tons[12]
| Country | 1910 | 1911 | 1912 | 1913 | 1914 | 1915 | 1916 | 1917 |
|---|---|---|---|---|---|---|---|---|
| North America: | ||||||||
| Canada[13] | 231,623 | 187,807 | 192,753 | 274,673 | 218,620 | 355,457 | 245,693 | 192,210 |
| Cuba[13] | 1,462,498 | 1,163,714 | 1,397,797 | 1,582,431 | 821,110 | 827,448 | 712,716 | 553,485 |
| Newfoundland[13] | 1,108,762 | 1,171,992 | 1,251,968 | 1,433,858 | 566,000 | 775,403 | 903,625 | 788,820 |
| United States | 57,014,906 | 43,876,552 | 55,150,147 | 61,980,437 | 41,439,761 | 55,526,490 | 75,167,672 | 75,288,851 |
| South America: | ||||||||
| Chile | ... | 28,150 | 6,546 | 13,878 | 62,506 | 144,783 | 55,281 | 4,921 |
| Venezuela[14] | ... | ... | 12,100 | 57,225 | 2,400 | |||
| Europe: | ||||||||
| Austria-Hungary | 4,592,572 | 4,779,851 | 4,997,311 | 5,233,055 | 3,939,248 | [15]1,218,367 | ([16]) | ([16]) |
| Belgium | 121,024 | 148,130 | 164,734 | 147,048 | 81,063 | 4,646 | 29,951 | 16,732 |
| France | 14,375,984 | 16,376,967 | 18,858,668 | 21,572,835 | ([16]) | ([16]) | ([16]) | ([16]) |
| Germany | [17]28,257,579 | [17]29,408,812 | [17]33,180,258 | 26,771,598 | ([16]) | ([16]) | ([16]) | ([16]) |
| Greece | 527,040 | 493,106 | 424,835 | 308,640 | 294,573 | 154,951 | 83,647 | 62,366 |
| Italy | 542,578 | 367,900 | 572,900 | 593,618 | 695,124 | 669,262 | 927,406 | 978,174 |
| Luxemburg | ([18]) | ([18]) | ([18]) | 7,215,514 | 4,926,980 | 6,040,765 | 6,643,689 | 4,436,682 |
| Norway | 100,834 | 217,051 | 401,665 | 535,869 | 641,790 | 706,379 | 865,700 | ([16]) |
| Portugal | 3,307 | 19,233 | 28,947 | 48,392 | 6,532 | ([16]) | ([16]) | ([16]) |
| Russia | ([19]) | ([19]) | ([19]) | 7,947,191 | ([16]) | ([16]) | ([16]) | ([16]) |
| Spain | 8,530,310 | 8,635,523 | [20]8,990,743 | 9,703,177 | 6,710,357 | 5,527,552 | 5,762,733 | 5,461,857 |
| Sweden | 5,465,234 | 6,056,868 | 6,595,044 | 7,357,845 | 6,482,904 | 6,774,909 | 6,876,278 | 6,119,263 |
| United Kingdom | 15,226,015 | 15,519,424 | 13,790,391 | 15,997,328 | 14,867,582 | 14,235,012 | 13,494,658 | 14,845,734 |
| Asia: | ||||||||
| China | [21]130,472 | [21]109,542 | [21]201,561 | [22]416,342 | [22]488,258 | 537,047 | 274,078 | 299,465 |
| Chosen (Korea) | 104,627 | 96,902 | 121,224 | 139,370 | 179,062 | 206,510 | 241,412 | ([16]) |
| India | 54,626 | 366,212 | 580,029 | 370,845 | 441,674 | 390,270 | 411,758 | 413,273 |
| Japan | 132,921 | 144,001 | 168,479 | 168,897 | 134,193 | 133,933 | 156,263 | ([16]) |
| Philippine Islands | 148 | 216 | 347 | 546 | 392 | ([16]) | ([16]) | ([16]) |
| Africa: | ||||||||
| Algeria | 1,048,228 | 1,057,087 | 1,171,252 | 1,327,320 | 1,097,101 | 805,547 | 923,598 | 1,048,722 |
| Morocco | 186,149 | ([16]) | ([16]) | ... | ... | ... | ... | ... |
| Madagascar | ([23]) | ([23]) | 22 | |||||
| Natal | 50 | ... | ... | ... | ... | ... | ... | ... |
| Togoland | ([16]) | 394 | ([16]) | |||||
| Tunis | 327,756 | 397,638 | 470,866 | 584,649 | 244,528 | 281,304 | 361,593 | 596,261 |
| Australia: | 157,821 | 122,361 | 113,989 | [24]90,712 | 177,938 | 370,887 | 390,108 | [25]328,310 |
[12] Burchard, E. F., “Iron Ore, Pig Iron and Steel”: U. S. Geol. Survey “Mineral Resources,” 1915 and 1917, with additions and revisions. (The above figures are, so far as possible, based on official reports and, where these are lacking, on the technical press.)
[13] Shipments.
[14] Exports to United States.
[15] For Hungary only.
[16] Statistics not available.
[17] Includes Luxemburg.
[18] Included in Germany.
[19] Russia produced 2,936,024 long tons of pig iron in 1910; 3,536,417 long tons in 1911, and 4,131,890 long tons in 1912.
[20] Includes 1,563 long tons of argentiferous iron ore.
[21] Exports.
[22] From Tayeh deposits only.
[23] Madagascar produced about 8 long tons of iron in 1910 and 1.5 long tons in 1911.
[24] For South Australia and Queensland.
[25] For South Australia only.
[59]
Table 12.—Pig Iron Manufactured in Principal Countries in 1850, 1890, 1900, and 1910 to 1916, in Gross Tons[26]
| Country | 1850 | 1890 | 1900 | 1910 | 1911 | 1912 | 1913 | 1914[27] | 1915[27] | 1916[28] |
|---|---|---|---|---|---|---|---|---|---|---|
| United States | 563,755 | 9,202,703 | 13,789,242 | 27,303,567 | 23,649,547 | 29,726,937 | 30,966,152 | 23,332,244 | 29,916,213 | 39,434,797 |
| Germany | 350,000 | 4,584,882 | 8,381,373 | 14,559,509 | 15,404,648 | 17,586,521 | 19,004,022 | 14,162,147 | 11,603,874 | 13,000,000 |
| Great Britain | 2,300,000 | 7,904,214 | 8,959,691 | 10,217,022 | 9,718,638 | 8,889,124 | 10,481,917 | 9,005,898 | 8,793,659 | 9,047,983 |
| France | 405,653 | 1,931,188 | 2,669,966 | 3,974,478 | 4,309,498 | 4,870,913 | 5,227,378 | 3,500,000 | 4,000,000 | 5,000,000 |
| Russia | 227,555 | 912,561 | 2,889,789 | 2,992,058 | 3,531,807 | 4,133,000 | 4,474,757 | 4,190,000 | 3,638,000 | 4,300,000 |
| Austria-Hungary | 250,000 | 910,685 | 1,472,695 | 2,153,788 | 2,056,839 | 2,276,141 | 2,335,170 | 1,500,000 | 1,929,000 | 2,000,000 |
| Belgium | 144,452 | 775,385 | 1,001,872 | 1,822,821 | 2,072,836 | 2,307,853 | 2,318,767 | 1,500,000 | 500,000 | 1,000,000 |
| Canada | ... | 19,439 | 86,090 | 740,210 | 824,368 | 912,878 | 1,015,118 | 705,972 | 825,420 | 1,069,541 |
| Sweden | 150,000 | 483,155 | 518,263 | 594,385 | 624,367 | 688,757 | 728,103 | 629,608 | 755,000 | 750,000 |
| Spain | ... | 176,598 | 289,315 | 367,423 | 402,209 | 396,872 | 418,061 | 400,000 | 421,000 | 440,000 |
| Italy | ... | 14,094 | 23,569 | 347,657 | 298,144 | 373,960 | 420,011 | 379,028 | 389,000 | 454,923 |
| Japan | ... | ... | ... | 186,794 | 200,000 | 200,709 | 236,491 | 295,428 | 312,957 | 379,574 |
| Other countries[28] | 10,000 | 80,000 | 100,000 | 200,000 | 250,000 | 350,000 | 250,000 | 200,000 | 200,000 | 500,000 |
| Total | 4,401,415 | 26,994,904 | 40,181,865 | 65,459,712 | 63,342,901 | 72,713,565 | 77,876,347 | 59,800,325 | 63,284,123 | 77,000,000 |
[26] “Metal Statistics,” 1914 to 1918, with some additions and changes.
[27] Partly estimated.
[28] Largely estimated.
[60]
More than two-fifths of the total annual output of iron ore in the world has come from the United States, and of the American production more than 80 per cent. is generally produced in the Lake Superior district. This district is, therefore, by far the most important iron-ore district in the world, producing annually more than 30 per cent. of the world’s total of iron ore.
Germany and France have been next in importance to the United States as iron ore-producing countries, about 80 per cent. of the ore mined in these two countries being obtained from the Lorraine iron fields situated on the border. The annual output of these fields, which includes also the ores of Luxemburg, has been about 25 per cent. of the world’s production. The Lorraine district and the Lake Superior district together, therefore, produce somewhat more than one-half of the total iron ore annually mined in the world.
The iron ore produced in Great Britain is obtained mainly from the Cleveland district of northern England, this district furnishing about 40 per cent. of the British total, equivalent to about 2.6 per cent. of the world’s annual production. In comparison, the Birmingham district of Alabama and the Krivoi-Rog district of southern Russia, which are next in importance to the Lorraine and Lake Superior districts, furnish about 3.5 per cent. and 3.2 per cent., respectively, of the world’s annual production.
Table 13.—Production and Movement of Iron Ore and Production of Pig
Iron, 1913[29]
Gross Tons
| Production of iron ore |
Iron ore imports |
Iron ore exports |
Apparent consumption (in part stocks) |
Production of pig iron |
|
|---|---|---|---|---|---|
| United Kingdom | 15,997,328 | 8,028,532 | 21,223 | 24,004,637 | 10,260,315 |
| Canada | 307,634 | 2,110,828 | 126,124 | 2,292,338 | 1,015,118 |
| Belgium | 149,450 | 4,400,000 | ... | 4,549,450 | 2,484,690 |
| France | 19,160,407 | 1,410,424 | 10,066,627 | 10,504,264 | 5,311,316 |
| Italy | 603,116 | 7,666 | 20,000 | 590,772 | 426,755 |
| Russia | 8,077,000 | ... | 565,000 | 7,512,000 | 4,557,000 |
| Austria | 3,039,324 | 942,312 | 106,071 | 3,875,565 | 1,757,864 |
| Hungary | 2,059,000 | ... | 700,000 | 1,359,000 | 623,000 |
| Germany (including Luxemburg) | 35,941,285 | 14,019,045 | 2,613,158 | 47,347,172 | 19,291,920 |
| Spain | 9,861,668 | ... | 8,907,202 | 954,466 | 424,774 |
| Sweden | 7,475,571 | ... | 6,440,000 | 1,035,571 | 730,257 |
| United States | 61,980,437 | 2,594,770 | ... | 64,575,207 | 30,966,152 |
| Algeria | 1,349,000 | ... | 1,350,000 | ||
| Chile | 70,000 | ... | 65,000 | ||
| Cuba | 1,500,000 | ... | 1,582,431 | ||
| Newfoundland | 1,605,900 | ... | 1,605,920 |
[29] Advisory Council, Dept. of Sci. and Indust. Research: “Report on the sources and production of iron and other metalliferous ores used in the iron and steel industry,” London, 1918, p. 12.
[61]
Table 11 shows the world’s production of iron ore from 1910 to 1917.
Table 12 shows the production of pig iron in 1850, 1890, 1900, and 1910 to 1916.
Table 13 shows the production and movement of iron ore and the production of pig iron for the year 1913.
—The principal iron ores of the United States are the extensive pre-Cambrian hematite deposits of the Lake Superior region; the bedded fossiliferous ores of the Clinton type of Alabama and other southern states; the magnetite deposits of New York, northern New Jersey, and southeastern Pennsylvania; the limonite ores of the eastern and southern states; and the mixed hematite and magnetite ores of the West. The United States is the largest producer of iron ore in the world, and annually yields more than two-fifths of the world’s supply. More than 80 per cent. of the output comes from the Lake Superior district and most of the remainder from Alabama, New York, and Pennsylvania. In 1917 there were mined in the United States 75,000,000 tons of iron ore, of which 63,000,000 came from the Lake Superior region, 7,000,000 from Alabama, 1,000,000 from New York, and about 500,000 each from Pennsylvania, New Jersey, Tennessee, Virginia, and Wyoming; in 1918 the total production was 69,000,000 tons, of which 60,000,000 came from the Lake Superior district, 6,000,000 from Alabama, 900,000 from New York, 500,000 from Pennsylvania, and 400,000 each from Tennessee, Virginia, New Jersey and Wyoming.
The following table shows the approximate reserves of iron ore in the principal districts of the United States:
Table 14.—Iron-Ore Reserves of the United States in Gross Tons[30]
| Millions of tons. |
|
|---|---|
| Lake Superior District (hematite) | 3,500 |
| Birmingham District (fossil hematite) | 355 |
| Tennessee and Virginia (fossil hematite) | 100 |
| Adirondack District (non-titaniferous magnetite) | 40 |
| Adirondack District (titaniferous magnetite) | 90 |
| Northern New Jersey and Southeastern New York (magnetite) | 15 |
| Southeastern Pennsylvania (magnetite) | 40 |
| Appalachian region (magnetite) | 50 |
| Northeastern Texas (limonite) | 260 |
| Western United States (magnetite and hematite) | 100 |
| Other Districts | 150 |
| Total | 4,700 |
[30] Kemp, J. F.: “The Iron-Ore Resources of the World,” Stockholm, 1910 (with minor revisions).
[62]
The greatest single iron and steel industry in the United States is that of the United States Steel Corporation, which controls the following iron- and steel-producing companies: Carnegie Steel Co., Illinois Steel Co., Indiana Steel Co., American Steel & Wire Co., American Sheet & Tinplate Co., National Tube Co., The National Tube Co. of Ohio, Minnesota Steel Co., The Lorain Steel Co., Tennessee Coal, Iron & Railroad Co., and the Shelby Steel Tube Co., with a total of 124 blast furnaces, having an annual capacity of about 18,000,000 tons of pig iron. Most of the blast furnaces are in Pennsylvania, Ohio, Illinois, and Alabama.
With the United States Steel Corporation is connected the Oliver Iron Mining Co., which produces about 43 per cent. of the iron ore mined annually in the Lake Superior district, this being equivalent to nearly 37 per cent. of all the iron ore mined annually in the United States. The Tennessee Coal, Iron & Railroad Co. is the chief producer of iron ore in Alabama.
Next in importance to the United States Steel Corporation as a producer of iron and steel is the Bethlehem Steel Corporation, with its subsidiaries, the Bethlehem Steel Co., Pennsylvania Steel Co., Maryland Steel Co., Jurugua Iron Co., Spanish-American Iron Co., and Bethlehem Iron Mines Co.
The works of the Bethlehem Steel Corporation have a total pig iron-producing capacity of 3,060,000 tons annually from 23 blast furnaces. Seven of the furnaces are in South Bethlehem, Pa., seven in Steelton, Pa., four in Lebanon, Pa., three in Cornwall, Pa., and four in Sparrow’s Point, Md. The Bethlehem Steel Corporation owns large iron-ore deposits in Cuba and Chile. Most of the ore consumed in its furnaces at present comes from Cuba, from the Lake Superior district, and from Cornwall, Pa.
Third in importance of the iron- and steel-producing companies of the United States is the recently organized Midvale Steel & Ordnance Co., controlling Worth Brothers, Midvale Steel Co., Remington Arms Co., Cambria Steel Co., and others. The combined pig iron-producing capacity of the 14 blast furnaces controlled by the Midvale Steel & Ordnance Co. is 2,420,000 tons of pig iron. Three of the blast furnaces are at Coatesville, Pa., and eleven are at Johnstown, Pa. This company owns important iron-ore deposits in Cuba and in the Lake Superior district.
Four other large companies produce more than a million tons of pig iron annually, these being the Republic Iron & Steel Co., with an estimated total capacity from its 11 blast furnaces in Ohio and Alabama of 1,430,000 tons of pig iron, and of 2,500,000 tons of ore from its mines in the Lake Superior district and in Alabama; the Lackawanna Steel Co., with an annual capacity in 1918 from its nine blast furnaces at Lackawanna, N. Y., of 1,440,000 tons of pig iron; the Jones & Laughlin Co., of Pittsburgh,[63] with a capacity of 1,920,000 tons from 11 blast furnaces; and the McKinney Steel Co., with eight furnaces in Ohio, New York, and Pennsylvania, and an annual capacity of 1,205,000 tons of pig iron. The last three companies named have extensive iron mines in the Lake Superior district.
Important iron and steel companies producing somewhat less than a million tons of pig iron annually are: the Youngstown Sheet & Tube Co., with an annual capacity of 990,000 tons of pig iron from six blast furnaces, all of which are at Youngstown, Ohio; the recently organized Steel & Tube Co. of America, having six blast furnaces in and near Chicago, with an annual pig-iron capacity of 900,000 tons; the Colorado Fuel & Iron Co., an important western iron and steel producer, which has six blast furnaces near Pueblo, Colo., with an annual capacity of 625,000 tons of pig iron, and has iron-ore mines in New Mexico, Wyoming, and Colorado; and the Schloss-Sheffield Steel & Iron Co. of Alabama, with seven blast furnaces and a pig-iron capacity of 530,000 tons. Many other plants with smaller capacity are scattered through eastern and central United States. So far as is known, practically the entire iron and steel business of the United States is in the hands of American capital.
—The “minette” ore of the German Lorraine district before the war constituted by far the largest iron-ore reserve of Germany and is the chief source of present supply. Next in importance of the German ore reserves are the brown hematites occurring north of the Harz, and third and fourth in importance, respectively, are the deposits of the Lahn and Dill districts in the Rhineland, and those of Siegerland. All of these districts are in western and southwestern Germany and all of them, except Lorraine, are in the region lying east of the Rhine in Hanover, Westphalia, Hesse-Nassau, and Rhenish Prussia.
The Lorraine district is on the French border and forms a part of the large ore field of Luxemburg and northern France. The deposits, of sedimentary origin and Jurassic age, comprise extensive beds of oolitic limonite varying in thickness up to 20 feet, interlayered with marl and limestone. Seven principal beds of ore are found within a thickness of sediments ranging from 75 to 150 feet, the most important being known as the Grey seam. The tonnage[31] of “minette” ore available in the German Lorraine district is estimated at 1,830 million[32] as compared with 300 million in Luxemburg and 2,975 million[33] in northern France. The “minette” ores average 30 to 40 per cent. in metallic iron content and 0.3 to 0.7 per cent. or more in phosphorus.
[31] The ore reserves in this chapter are given in metric tons unless otherwise stated.
[32] Einecke, G., and Kohler, W.: “Iron-Ore Resources of the World,” Stockholm, 1910.
[33] Nicou, L., idem.
The deposits of the Salzgitter and Ilsede districts north of the Harz Mountains are beds of brown ironstone conglomerate with an average[64] thickness of 20 to 30 feet, consisting of limonite pebbles in a clayey or calcareous cement. These deposits cover many square miles, the reserves being estimated at 248 million tons. The ores contain 30 to 40 per cent. of iron and average 0.7 to 0.8 per cent. in phosphorus.
The ores of the Lahn and Dill region are mainly red hematites, that lie in an extensive sedimentary bed. They contain about 48 per cent. of iron, 0.2 to 0.3 per cent. phosphorus, and are high in silica. The reserves are estimated at about 135 million tons.
In Siegerland the iron ores are mainly carbonate, carrying 38 to 40 per cent. metallic iron and 6 to 9 per cent. manganese. They form irregular deposits abundantly scattered through the region, the available reserves being estimated at about 100 million tons.
The total iron-ore reserves of Germany (not including those of Luxemburg) actually available have been estimated at 2,540 million tons, and the probable further reserves at 1,067 million tons. Of these amounts, however, Lorraine has by far the largest part, so that the transfer of this province to France reduces Germany’s available reserves to 28 per cent. of the pre-war figure, and her further probable reserves to one-half, altogether reducing her iron-ore resources to one-third of the former amount.
As far as is known, the German iron and steel business is in the hands of German capitalists, who, besides, have important iron-ore holdings in France, Spain, Sweden, and elsewhere. Among the important iron-ore and pig-iron producing firms in Germany are Gutehoffnungshütte, de Wendel & Co., Krupp, Gebrüder Stumm, Aschener Hütten Aktien Verein, Rombacher Hüttenwerke, Thyssen & Cie., and others. All of these firms had large ore reserves in the Lorraine district when the war began.
—The iron ores of France are divided into three distinct groups: the “minette” ores of the Briey, Longwy, Crusnes, and Nancy districts; the Silurian ores in Normandy; and the vein deposits of the eastern Pyrenees.
The “minette” ores of northern France form part of the great basin of “minette” ores of France, Luxemburg, and Germany already mentioned. In 1913 they furnished about 91 per cent. of the total production of France. The iron ores of Normandy and Brittany are of sedimentary origin and are composed of hematite or carbonate or a mixture of both. The carbonate becomes more abundant with depth. The iron-ore deposits of the eastern Pyrenees consist of both hematite and siderite and are of high grade, constituting the only considerable source of Bessemer ore in France. The iron content of the ores of Normandy and Brittany ranges from 30 per cent. to 50 per cent.; that of the Pyrenees ores from 51 per cent. to 57 per cent. The latter range includes calcined siderite.
The production of iron ore from French Lorraine was about 19,500,000[65] tons in 1913; that of Normandy and Brittany about 1,500,000 tons, and that of the eastern Pyrenees about 500,000, making a total production of more than 21,000,000 tons for France.
The following table shows the relative output of iron ore from the different iron fields of France and Germany during the last three normal years before the war:
Table 15.—Production of Iron Ore in France and Germany, 1911 to 1913
Metric tons
| District | 1911 | 1912 | 1913 |
|---|---|---|---|
| German Lorraine | 17,734,576 | 20,050,245 | 21,135,554 |
| Luxemburg | 6,059,797 | 6,553,930 | 7,331,050 |
| French Lorraine, including Briey, Longwy and Nancy | 14,878,000 | 17,235,125 | 19,499,166 |
| Germany, outside of Lorraine | 6,968,000 | 7,167,000 | 7,472,000 |
| France, outside of Lorraine | 1,584,000 | 1,925,000 | 1,686,000 |
The available reserves in the different districts of the French “minette” ore field are estimated as follows:[34]
Table 16.—Ore Reserves in French “Minette” Field
| Million tons |
|
|---|---|
| Briey | 2,000 |
| Crusnes | 500 |
| Longwy | 275 |
| Nancy | 200 |
| Total | 2,975 |
[34] Nicou, L.: “Iron Resources of the World,” Stockholm, 1910.
The list of operating companies in France shows a considerable number of German companies among the predominating French. German or probably German companies produced in 1913 six and a half million tons of iron ore, or one-third of the whole production. In the Normandy and Brittany region two German companies made 11 per cent. of the whole production; and in the eastern Pyrenees one German company produced 20 per cent. Altogether, German capital controlled over one-third of the iron and steel industry of France in 1913. The rest seems to have been in the hands of French capital.
The most important iron-producing firms in the Lorraine field in recent years have been those of de Wendel & Co., Gutehoffnungshütte, Société des Hauts Fourneaux et Fonderies de Pont-a-Moussons, Société des Forges et Acieries de la Marine et d’Homecourt, Société Anonyme des Acieries de Longwy, Société des Acieries de Micheville, and Société des Mines d’Ammermont Dommery. The first two firms are chiefly German and have controlled lands not only in German Lorraine, but also in French Lorraine. Thus, the iron-ore lands of the Lorraine district[66] will, even after the cessation of the territory to France, be owned largely by German-controlled firms. Politically, however, France will have control of the output.
—The iron ores mined in Great Britain come chiefly from the Cleveland Hills in Yorkshire and from Lincolnshire, Northamptonshire, Cumberland, Staffordshire, Leicestershire, Scotland, and Lancashire, in order of importance. More than one-third of the total production is derived from the Cleveland Hills. The ore from the Cleveland Hills, Lincolnshire, Northamptonshire, Leicestershire, and Scotland is bedded oolitic siderite of Middle and Lower Jurassic age; that from Cumberland and Lancashire is hematite, exceptionally low in phosphorus, found in pockets in Carboniferous and Silurian limestones; and that in Staffordshire is siderite of the “black band” and “clay band” varieties found in the Coal Measures.
The following table shows the production of iron ore in the United Kingdom in 1915:
Table 17.—Production of Iron Ore in the United Kingdom in 1915
| Long tons |
|
|---|---|
| Cleveland Hills | 4,746,293 |
| Lincolnshire | 3,149,079 |
| Northamptonshire | 2,517,150 |
| Cumberland | 1,323,408 |
| Staffordshire | 703,231 |
| Leicestershire | 685,137 |
| Scotland | 375,241 |
| Lancashire | 333,086 |
| Other Great Britain and Ireland | 402,387 |
| Total | 14,235,012 |
A large part of the iron ore in Great Britain can not now be worked profitably, and much of the ore that was merchantable a few years ago could not now be worked, on account of increased cost of transportation, labor, and particularly of fuel. The actual reserves of ore of present merchantable grade are estimated at 1,300 million tons; the total reserves have been estimated by H. Louis at 39,500 million tons.[35]
[35] Louis, H.: “Iron-Ore Resources of the World,” Stockholm, 1910.
In 1915 the United Kingdom produced 14,000,000 tons of iron ore. In the same year nearly 7,000,000 tons were imported, of which 4,000,000 came from Spain and between one-half and one million from Algeria and Norway each, making a total of over 20,000,000 tons smelted in the United Kingdom. The total production of pig iron was nearly 9,000,000 tons, of which nearly 7,000,000 tons were produced in England, 1,000,000 tons in Scotland, and nearly 1,000,000 tons in Wales. Ireland produces no pig iron. The iron and steel industry of Great Britain, so far as information is available, is in the hands of British subjects.
[67]
Eckel[36] reviews the British iron-ore situation as follows:
The position of Great Britain as regards iron-ore resources is peculiar—perhaps more curious than satisfactory. The matter may be summarized by saying that England has still several hundred million tons of high-grade ore which would be salable anywhere; that she has in addition perhaps double that quantity of low-grade ore, workable because of its nearness to coal and markets; and that England, Scotland, and Wales have thousands of millions of tons of ore now unworkable, but which may be serviceable in the future provided that at that future date there is still any other good reason for making steel in Great Britain. This last limitation may not be palatable, but it is really the crux of the whole question, and it seems to have been overlooked by the British geologists who have discussed the subject. People do not make iron out of low-grade ores simply to use up the ores; and with an increasing coke cost and a narrowing export market it is a very serious question whether the bulk of these British carbonates will ever be used. The duration of the British steel industry will be fixed by its coal supply, and not by its supply of local ores; for so long as coke and markets justify it, ore can be imported to good advantage. If other conditions do not justify the importation of ore, they will certainly not justify the use of these hypothetical reserve tonnages.
[36] Eckel, E. C.: “Iron Ores, Their Occurrence, Valuation and Control,” p. 320, 1914.
—Spain is rich in iron-ore reserves, but the iron and steel manufacturing industry has had little development. The annual production of iron ore in Spain during the last years before the war amounted to about 9,000,000 tons, of which more than 8,000,000 tons were exported. The consumption of iron ore by Spanish blast furnaces has been in the neighborhood of 800,000 tons annually.
The principal iron ores of Spain lie in the northwestern part, in the provinces of Viscaya, Oviedo, Lugo, and Santander; in the northeastern part, in the provinces of Teruel and Guadalajara; and in the southeastern part, in the provinces of Granada, Almeria, Murcia, Sevilla, and Huelva.
The iron ores of the Bilbao district of Viscaya are all of Bessemer grade, and for many years large amounts have been exported to England for use in Bessemer plants to supplement ores from the Cleveland and other districts of England. Because of their excellence, they have been in continuous demand, and the English iron and steel industry has depended to a considerable extent upon these and other high-grade ores of Spain. In more recent years, Germany has also become interested in the Bilbao iron fields, and in the last years before the war Germany took more than one-third of the total Spanish production, including large amounts of ore from southern Spain as well. Spanish interests own important deposits in southeastern Spain and in the Bilbao district.
Most of the ore of southeastern Spain is of high grade, being rich in iron and low in phosphorus. Nearly all of it is of Bessemer quality, and some is very low in phosphorus; the latter is exported extensively for use in the manufacture of low-phosphorus pig iron. The United States has[68] been largely dependent in past years upon Spain for this grade of ore, more than 100,000 tons being imported annually.
Spain has a number of blast furnaces and steel plants, the principal ones being at Bilbao, in the Province of Viscaya. More than 300,000 tons of pig iron are produced annually in Viscaya, this being approximately three-fourths of the total output of pig iron in Spain.
—In European Russia[37] the principal deposits of iron ore are distributed over four chief districts: Ural Mountains, central Russia, southern Russia and the Caucasus.
[37] Bogdanowitsch, K.: “The Iron Resources of the World,” Stockholm, 1910.
In the Ural Mountains for the greater part the ores are associated with igneous rocks. The most important deposits are in the neighborhood of Gora Blagodat, in the northern Ural regions, and near Gora Mongnitnaja, in the southern Urals. The ores are mainly magnetite and limonite and come from an extremely large number of small mines. In central Russia, over widely scattered areas, are deposits of calcareous ores, clay ironstones, and bog ores. Many of the deposits are thin and can not be profitably worked. The only reserves in southern Russia of any importance are divided among three centers: Krivoi-Rog, the Donetz basin, and the Kertsch peninsula. By far the most important deposits are the magnetite-hematite ores in the region of Krivoi-Rog. The ironstones of the Coal Measures in the Donetz basin and the limonite of the Kertsch peninsula are of secondary importance. The mines of Krivoi-Rog are extensively worked, and their reserves are estimated at some 86 million tons of commercial ore. The mines are controlled mainly by the following three companies: the Briansk company; Krivoi-Rog Iron Ore Co.; and the Providence company.
The following table shows the production of iron ore in different parts of Russia in 1912:
Table 18.—Output of Iron Ore in Russia
| Long tons | |
|---|---|
| Southern Russia | 5,679,000 |
| Ural | 1,817,000 |
| Central Russia | 286,000 |
| Other Russia and Siberia | 6,000 |
| Total | 7,788,000 |
The ore reserves of Russia may be summarized by districts as follows:
Iron-Ore Reserves in Russia
| Millions of tons |
|
|---|---|
| Ural | 282 |
| Central Russia | 789 |
| Southern Russia | 536 |
| Caucasus | 14 |
| Total | 1,621 |
[69]
Eckel[38] reviews the iron-ore situation in Russia as follows:
On their face the ore reserves noted seem satisfactory enough, and until the data are examined more critically it is difficult to explain why the relatively large furnishing capacity of the Moscow and other central Russian districts is so far out of line with the comparatively small ore production of that area. As a matter of fact, however, the large total ore reserves credited to central Russia are in reality less important than they seem, owing both to grade of ore and thinness of the ore bodies. From an international viewpoint the ore deposits of southern Russia are the ones which require most attention; for these are so located as to be of importance to foreign competitors, while the total reserve tonnage is high, and the grade of much of the ore is excellent.
[38] Eckel, E. C.: “Iron Ores, Their Occurrence, Valuation and Control,” 1914, p. 326.
Actual available ore reserves of merchantable grade in Russia are estimated at 865 million tons.
Before the Revolution the greater part of the Russian iron and steel industries was controlled by syndicates.[39] The oldest of these consisted of manufacturers of medium sheets (1902); then followed manufacturers of joists and U-iron (1903), axles and tires (1904), iron tubes (1906), rails (1907), and bar iron and hoops. These six syndicates were afterwards combined into one, officially styled the Association for the Sale of Products of the Metallurgical Works of Russia, but generally known as “Prodameta,” from its telegraphic address. There were separate syndicates for wire, wire nails, and roofing sheets. The “Prodameta” consisted, at last advices, of nineteen works, of which sixteen are in southern Russia, and one each in Petrograd, Moscow, and the Ural region. The “Prodameta” expired at the end of 1915, but was provisionally prolonged for one year, and again at the end of 1916 it was extended for a similar period. The aggregate capital of the eighteen works was 198,400,000 roubles, and their net profit for 1915-16 was 76,200,000 roubles.
[39] Ironmonger Metal Market Year-Book, London, 1918.
—The iron-ore fields of Sweden are among the most important in Europe and have for the last ten or fifteen years furnished a large output, which has gone mainly to England and Germany. A relatively small amount of iron ore is used in Swedish iron-smelting works. The iron mines of central Sweden have been actively worked since about the beginning of the twelfth century, whereas those of Swedish Lapland have been developed recently. At present about one-half the output of iron ore in Sweden comes from Swedish Lapland, and the other half from central Sweden.
Swedish Lapland is estimated to have iron-ore reserves amounting to 1,128 million tons. The ores are mostly magnetite associated with igneous rocks and show wide difference in phosphorus content. Certain deposits or parts of deposits are composed of ores that are moderately[70] low in phosphorus, whereas others are high enough to obtain a special bonus from German steel plants that produce high-phosphorus slag for fertilizer purposes. Practically all the ores of Swedish Lapland are exported. In recent years the total production has amounted to about 3,500,000 tons annually. The principal mines are worked by the Trafikaktiebolaget Grängesberg Oxelosund, in which English and German capital is interested with the Swedish government. The Swedish government controls the output of the mines and receives a large sum in royalties on the ore produced. The ore deposits from which ore is being produced at present are Kiruna, Gellivare, and Tuolluvarra, the first two being operated by the firm mentioned above and the last being an independent operation.
The ore reserves of central and southern Sweden are estimated at 140 million tons, included in a great number of relatively small deposits. Most of the mines of central Sweden are controlled by small Swedish operators. Some of the mines, however, such as Blotberg, are to a large extent under German control, and the largest one, Grängesberg, is operated by the same firm that controls the deposits of Swedish Lapland. Some of the ores of central Sweden, such as those of Dannemora, Norberg, Strossa, and Stripa, are very low in phosphorus, and are used in the manufacture of special low-phosphorus iron; others, like those of Grängesberg and Blotberg, contain more than 1 per cent. of phosphorus.
—The iron ores of the former Austro-Hungarian Empire are mainly low-grade hydrous iron silicates that require roasting, large deposits of iron carbonate, and some limonite. The total probable ore reserves have been estimated at 940 million tons, of which about 560 million are very low grade.
The principal sources from which the domestic iron ore used in the past in the Austro-Hungarian Empire has been obtained are the chamosite-hematite deposits at Nucitz and elsewhere in Bohemia; the siderite beds at Erzberg, in Styria, estimated to contain more than 200 million tons of ore; the siderite-limonite deposits on the slopes of the Carpathians; and deposits of various ores in northern and central Bosnia.
By far the largest of the deposits is that at Erzberg, owned and operated by the Oesterreichische Alpinen Montan Gesellschaft, presumably Austrian. The Bohemian deposits, also important, are largely under the control of the Prager Eisen Industrie Gesellschaft. The Carpathian deposits are largely controlled by local individuals and firms, among them Duke Philipp of Sachsen-Coburg-Gotha-Kohar. Thus the principal deposits have been largely under Austro-Hungarian control.
As a result of the war and the disruption of the Austro-Hungarian Empire, the Bohemian deposits, estimated to contain 35,100,000 tons of high-grade ore and 221,800,000 tons of low-grade ore, will come under the control of Czechoslovakia, whereas the Bosnian ores, with an estimated[71] reserve of 21,500,000 tons, will go to Jugoslavia. Austria will retain control of the large Erzberg deposit in Styria, and the ores of the Carpathian region will continue under Hungarian control.
—The iron ores of Morocco, Algeria, and Tunisia, in northern Africa, are mainly high-grade hematite. The reserve tonnage of Algeria and Tunisia is estimated by Nicou at 100 million to 150 million tons, and about 30 million or 40 million tons is reported in the Spanish territory of Riff, Morocco.
The deposits of Morocco and Algeria are nearly all near the north coast, and the ores are shipped from various small ports, such as Melilla, Benisaf, Arzeu, Algiers, Bougie, and Bona. The deposits of Tunisia are 180 to 200 kilometers southwest of Tunis, the shipping port, with which they are connected by rail.
The principal mines of El Riff, Morocco, are owned by the Sociedad Española de Minas de Riff. German interests, the “Netta Company,” held a large concession, but since the war these interests are controlled by the Company of Bilbao.
The North African deposits are important as a source of high-grade low-phosphorus ore for European blast furnaces. All of the ore produced is exported, the annual shipments amounting to about 1,500,000 tons.
—There are two principal groups of iron-ore deposits in Cuba—the magnetite and hematite ore on the south coast, and the brown ore, or limonite, on the north coast. All are near the eastern end of the island. The ores of Firmeza and Daiquiri, on the south coast, are mixed magnetite and hematite, averaging about 58 per cent. iron and 0.03 per cent. phosphorus. They are associated with igneous rocks. A determination of tonnage is difficult because of the irregularity of the ore bodies, and estimates of reserves range from 5 million to 9 million tons. The brown ore of the north shore is hydrated brown hematite, a laterization product of serpentine. The dried ore averages about 46 per cent. iron, 0.01 per cent. phosphorus, and 1.7 per cent. chromium. The reserve tonnage, estimated as high as 3,000 million tons, is mainly contained in the three large deposits of Camaguey, Mayari, and Moa.
The principal deposits of Cuba are owned and operated by the Bethlehem Steel Co. Important undeveloped deposits are owned by the Buena Vista Iron Co. (Midvale Steel & Ordnance Co.), United States Steel Corporation, Guantanamo Exploration Co., and Eastern Steel Co.
—The principal iron ores of Newfoundland are bedded oolitic hematites, which average 50 per cent. to 52 per cent. in metallic iron. The ore reserves of Newfoundland have been estimated as between 3,250 million and 3,500 million tons, making them among the largest and by far the most compact iron-ore reserves in the world. The output of ore has been 1,000,000 to 1,500,000 tons annually, except during the war,[72] when the production decreased. These deposits are important on account of both their size and their situation. Ore can be placed readily in American or European ports at a cost far lower per unit of iron than any competitive ore, so that the market is practically unlimited.
The ores have been mainly exported to Sydney, Nova Scotia, and to Philadelphia, while about 10 per cent. has gone to Holland (Germany). The phosphorus content is too high for normal economic basic open-hearth practice if the ores are used alone, but not too high for foundry use or for the basic Bessemer process developed in Europe.
The Wabana iron-ore deposits are owned and mined by the Dominion Iron & Steel Co., and the Nova Scotia Steel & Coal Co., two Canadian firms. Both companies operate steel plants near Sydney, Cape Breton.
—The principal iron ores of Norway are low-grade magnetite and specular hematite, much of which can profitably be concentrated. They occur in the northern part, north of the Arctic Circle. Small deposits of high-grade ores, consisting mainly of magnetite lenses, occur in southern Norway.
The Sydvaranger deposits, in the extreme north near the border of Finland, are estimated to contain 100 million tons of low-grade magnetite. The ore is treated in a large concentrating plant erected by a Norwegian company but controlled by Swedish and German capital. The concentrates analyze 70 per cent. iron and 0.02 per cent. phosphorus. The Dunderland deposits on Rannenfjord, near the Arctic Circle, are estimated to contain 80 million tons of mixed low-grade specular hematite and magnetite.
In 1914 Norway produced 652,273 tons of iron ore, of which seven-eighths came from the Sydvaranger deposit.
—The most important iron mines in Italy are the hematite mines of the island of Elba, which have furnished between 500,000 and 1,000,000 tons of ore annually in recent years. Ten or twelve large ore bodies are found in the eastern part of the island, all under control of the Elba Company, which has obtained a concession giving it exclusive iron-mining rights on the island. Important but as yet little developed magnetite deposits are found in the Aosta Valley, Piedmont, and limonite deposits are found on the island of Sardinia. These have furnished a very small output. Minor deposits of iron ore occur in Lombardy, in the Apennines of central Italy, and elsewhere. The total reserves of iron ore in Italy are estimated at about 25 million tons.
Italy has several important iron-smelting works, among them being the Elba Company furnaces on the island of Elba, the Piombino furnaces at Piombino, on the mainland opposite Elba, and the Ilva furnaces at Bagnoli, near Naples. In addition there are some small plants in northern Italy.
[73]
Italy’s iron-ore deposits and iron manufactures are controlled by commercial organizations, mainly Italian, but in part English. The Italian government exercises control over the ore deposits by granting concessions for comparatively short terms.
Agreements made among the Italian manufacturers for the rational division of work have led to the formation of a syndicate of the following firms: Ilva, Elba, Siderurgica di Savona, Metallurgica di Lestre, Ferriere Italiane, and the Piombino Steel Works. These firms undertook to maintain the syndicate for eleven years, dating from July, 1911. The affairs of the organization were directed by the Ilva Company. “In 1916 the constituent companies renewed their agreement up to the year 1930, but the Ilva Company ceased to direct the affairs of the Syndicate, and the relations between the Syndicate and the German Stahlwerks Verband were abrogated and replaced by Anglo-Italian relationships.”[40]
[40] Ironmonger Metal Market Year Book, 1918.
A further organization was made called the “Societa Ferro ed Acciaio,” a combination of steel works.
Italy is important as a producer of iron ore and as a manufacturer of iron products. She has been able to supply her own needs in iron ore for many years and at the present rate can continue to do so for probably twenty years longer. There appears to be no tendency toward expansion into other fields to control foreign ore deposits.
Italy produced 593,000 tons of iron ore in 1913; 669,000 tons in 1915; and 927,000 tons in 1916. In 1915, 408,000 tons of pig iron were produced, and 87,000 tons were imported.
—Chromiferous iron ores are found in eastern Greece and adjacent islands. They contain 46 to 52 per cent. iron, 2 to 3 per cent. chromium, and about 0.10 to 1.00 per cent. nickel and cobalt. The normal annual production of iron ore in Greece has been in the neighborhood of 400,000 tons.
—Small iron-ore deposits occur in New Brunswick and Nova Scotia. In Ontario there are two principal iron-ore districts—the Atikokan and the Michipicotan ranges. The ore in the former is magnetite, and in the latter hematite and siderite with some limonite. The ores of western British Columbia are largely magnetite. The principal deposits are on Texada and Vancouver islands, where the ore is of excellent grade, averaging 63 per cent. iron, 0.02 per cent. phosphorus and 4 to 10 per cent. silica. Low-grade magnetite ore is found at the Moose Mountain mine, Ontario, and is being concentrated.
There are a number of blast furnaces in Canada, among them being those of the Dominion Iron & Steel Co., Sydney; the Nova Scotia Steel & Coal Co., Sydney Mines, and the Londonderry Iron & Mining Co., Londonderry, all of which use ores from Newfoundland and Nova Scotia; the Algoma Steel Corporation, Sault Ste. Marie, Ontario; the Steel Company[74] of Canada, Hamilton, Ontario; the Canadian Furnace Co., Port Colborne, Ontario; the Canada Iron Foundries, Midland, Ontario; the Standard Iron Co., Deseronto and Parry Sound, Ontario; and the Atikokan Iron Co., Port Arthur, Ontario, which use largely Lake ores of the United States and Canada. The Moose Mountain Co., of Sellwood, Ontario, has a magnetic concentrating and briquetting plant using ore from the Moose Mountain mine. In May, 1920, the British Empire Steel Corporation, the second largest in the world, was formed by the merger of nine steel, coal, ship-building and transportation companies.
In 1912 Canada produced 156,000 tons of iron ore, and made 906,000 tons of pig iron.[41] In 1918 she made 1,066,071 tons of pig iron. The iron mines of Canada are largely of Canadian and partly of American ownership.
[41] Board of Trade, “Reports on Iron and Steel,” London, 1905-1918.
—Little information is available on the extent of the iron-ore deposits of the Chinese Empire. The principal producing area is that of Tayeh, south of Yangtse River in the Province of Hupeh, where a series of ore bodies, consisting of mixed hematite and magnetite, occurs along the contact of limestone and intrusive syenite. The deposits are estimated to contain about 40 million tons of ore.[42] The Han-Yeh-Ping Iron & Steel Co., largely controlled in Japan, owns these deposits and the Han Yang steel plant near Hankow. Iron ore similar to that of Tayeh is reported to occur farther down the Yangtse at Tungling, in the Province of Ngan-whei, and also along the coast near Amoy, Province of Fukien. The deposits near Amoy are said to contain about 25 million tons.
[42] Bain, H. F.: “Notes on Iron-Ore Resources of China,” Trans. Am. Inst. Min. Eng., 1918.
Of considerable importance are the mixed hematite and magnetite ores of Chin-ling-chen, near Kiaochow, Shantung Province. A series of ore bodies, some of them 100 feet in width, are said to occur along a contact zone two kilometers in length.[43] The deposits were exploited by Germans and are being developed by Japanese.
[43] Koert, W.: “Iron-Ore Resources of the World,” Stockholm, 1910.
Sedimentary beds of oolitic ore of some extent are reported in the provinces of Chih-li and Kiang-si, but they are of low grade.
Bedded siderite ores similar to the Coal Measures ores of England have been mined for many years in Shan-si Province and smelted in native furnaces. In Hunan Province, also, this type of ore is mined.
The principal iron ores of Manchuria are magnetites that occur as a series of deposits in a northwest-southeast belt south and southeast of Mukden, in southern Manchuria. They are interbedded with schist, gneiss, and porphyry. In this belt are the An-shan-chang deposits that are now being developed by Japanese interests affiliated with the South[75] Manchurian Railway, and the Miaor-kow deposits operated by a Sino-Japanese company, the Pen-hsi-hu Coal & Iron Co., Ltd. The southern Manchuria magnetite belt is reported to contain reserves amounting to about 500 million tons.[44] Much of the ore is of low grade.
[44] Wang, C. F.: “Coal and Iron Deposits of the Pen-hsi-hu District, Manchuria,” Trans. Am. Inst. Min. Eng., 1918.
—Iron ores of four types are found in India[45]: (1) lenses of specular hematite with some magnetite, occurring in quartz-hematite and quartz-magnetite schist of the Dharwar series and other older rocks; (2) granules of magnetite and hematite scattered through granite and schist; (3) clay ironstones in the Coal Measures of Bengal; and (4) lateritic ores.
[45] de la Touche, F. E.: “Iron-Ore Resources of the World,” Stockholm, 1910.
The hematite-magnetite lenses interlayered with iron-bearing schists are the most important of the Indian ores, although it is only recently that they have been exploited. The Tata Iron & Steel Co. owns the principal deposits of this type, including Mayurbhanj, in Bengal, where mining is being conducted at present, and the large undeveloped deposits of the Raipur district, Central Provinces. Important deposits of this type are also found in southern India.
Magnetite and hematite derived from the disintegration of schist and granite are now being used by the Bengal Iron & Steel Co., of Barakar. They consist of surface accumulations of iron sands and are found in various parts of India. Clay ironstones scattered through shales were formerly used by the Bengal Iron & Steel Co., but its supply of these is exhausted. Lateritic iron ores lying at the surface are widespread but undeveloped.
The principal iron and steel company of India is that of Tata & Sons, an Indian firm. Its plant, which has been mining since 1912, is at Sakchi, in Bengal, and comprises three blast furnaces with a total monthly capacity of 24,000 tons of pig iron. There are also extensive coke ovens, open-hearth furnaces, and steel mills. Many additions are being planned. The Bengal Iron & Steel Co., which has been in operation for thirty years, has three small blast furnaces at Barakar, with a monthly capacity of 12,000 tons. A steel mill and rolling mill built by this company have been abandoned.
Several new developments are being planned in the Indian steel industry, among them being the Indian Iron & Steel Co., an English firm, which is building a plant near Asanol on the East Indian Railway.
The production of iron ores for 1915 was 390,270 tons, the production of iron and steel for 1914 was 504,564 tons and for 1915 to 1916 it was 584,775 tons. Total imports of iron and steel, 1914 to 1915, amounted to 698,635 tons, and 1915 to 1916, to 424,597 tons.
[76]
—The iron ore used in Japanese furnaces is obtained in part from domestic mines and in part from Korean, Manchurian, and Chinese mines. The iron ore produced in Japan comes mainly from the Kamaishi group of deposits in the northern part of the island of Honshu. These mines yield more than one-half of the total annual production of iron ore in Japan, the remainder coming mainly from the Sennin and Kuriki mines, also in the northern part of Honshu; the Abuta and other bog ore deposits in Hokkaido, and the black sand deposits of Chugoku, in southern Honshu. The Kamaishi, Sennin, and Kuriki deposits consist of magnetite and hematite associated with sedimentary rocks near igneous intrusions. The iron-ore reserves of Japan are estimated by Inouye[46] at about 60 million tons.
[46] Inouye, K.: “Iron-Ore Resources of the World,” Stockholm, 1910.
The Korean iron ores used in Japan have come mainly from the surficial limonite deposits of Hoang-hai-do, about 100 miles northwest of Seoul, which have been actively mined for 10 years or more. Recently Japanese-controlled blast furnaces have been established at Ken-ji-pho, Korea, which use ore from the Ken-ji-pho iron mine, situated in the same region as the Hoang-hai-do mines. The pig iron produced by this plant is sent to Japan for use in Japanese steel plants.
Chinese iron ore used in Japan has been obtained from the Tayeh mines of Hupeh Province. A part of the ore from these mines goes directly to Japan, and a part goes to the Han-Yang furnaces, near Hankow, to be manufactured into pig-iron and steel products which also go to Japan. The Han-Yeh-Ping Iron & Steel Co., which owns both the Tayeh mines and the Han-Yang furnaces, is a Chinese concern, the capital of which is at present controlled largely by Japanese banking firms.
Of considerable interest at the present time is the development by Japanese of the Chin-ling-chen iron-ore deposits on the peninsula of Kiaochow, Shantung Province. These deposits were being exploited by the Germans just before the war and have recently been taken over by the Japanese, who are continuing development.
Two important Japanese-controlled iron and steel manufacturing projects are at present being developed in Manchuria. The older of these is the plant of the Pen-hsi-hu Colliery & Mining Co. at Pen-hsi-hu, southeast of Mukden, where pig iron is being manufactured from ore obtained from the neighboring Miaor-kou magnetite mines. The pig iron is being sent to Japan. The other Manchurian enterprise is the An-shan-chang iron and steel works at Sha-ho-kou, south of Mukden. The ore is derived from the An-shan-chang iron mines and the pig iron and steel products which it is later planned to manufacture are to be sent to Japan. It is also planned to send Manchurian ore to Japan.
On page 77 are shown the production and importation of iron ore into Japan in recent years:
[77]
Table 19.—Production and Importation of Iron Ore into Japan, 1914 to 1916
(Metric tons)
| Year | Produc- tion |
Importation from | Total including other countries |
|
|---|---|---|---|---|
| Korea | China | |||
| 1914 | 136,385 | 163,747 | 300,305 | 465,754 |
| 1915 | 136,121 | 204,101 | 311,310 | 516,132 |
| 1916 | 158,815 | 192,225 | 282,149 | 474,955 |
A considerable quantity of pig iron is imported into Japan from British India, Great Britain, and other countries.
The principal iron-smelting works in Japan are as follows: Imperial Steel Works, Yawata; Kamaishi Iron Works, Kamaishi; Wanishi Iron Works, Tanburi; Sennin Iron Works, Waka; Kuriki Iron Works, Kisen. All are controlled by Japanese, the first being the Japanese government works.
—In Poland the chief deposits of iron ore are the limonite deposits in the Vistula district, carrying 22 to 50 per cent. iron. The production in 1912 was 289,000 long tons. The resources are estimated at 300 million to 800 million tons.
—Belgium’s production of iron ore in recent years has amounted to about 150,000 tons annually, of which more than half was derived from the “minette” ore beds in the southeastern part along the French border, and the remainder came in part from beds of oolitic hematite of Devonian age in the Namur and Liege basins and in part from bog-ore deposits in the northern part. In the past the largest production has come from the Namur basin, and there are still large reserves of these oolitic hematite ores. The total iron-ore reserves of Belgium are estimated to be about 62,500,000 tons.
The iron ore produced in Belgium supplies only a very small part of the requirements for the Belgian iron and steel industry, most of the ore being imported from France.
—The largest deposits of iron ore in Portugal are those of Moncorvo, in the northeastern part. The ore is a bedded sedimentary deposit of low grade and the estimated reserve is 45 million tons. Small deposits of magnetite and brown hematite are found in the southern part in the Province of Alemtejo. Portugal produced 48,342 tons of iron ore in 1913.
—Minor deposits of magnetite and hematite occur in Bulgaria and former European Turkey, and in western Asia Minor several important ore bodies are known.[47] The largest of these[78] deposits occurs in the Berut Hills, 90 miles northwest of the Gulf of Alexandretta. It is reported to be capable of producing 300,000 tons annually.
[47] Edwards, G. M.: “Notes on Mines in the Ottoman Empire,” Trans. Inst. Min. Met., vol. 23, 1913-14.
Other important deposits are found near Ayazmat, on the mainland opposite the island of Mitylene, and near Tireboli and Trebizond, on the Black Sea. The only producing mine is near Ayazmat.
—Scattered iron-ore deposits occur in Chile in the coastal mountain region; the principal deposits extend a distance of about 150 miles parallel to the coast, some of them being north and some south of Coquimbo. The ore bodies are within 10 to 40 miles of the coast. Most of them are enclosed as lenses in granitic rocks; a few are in sedimentary rocks near the contact of igneous rocks.
Furnaces and a steel plant were erected by a French syndicate 10 to 15 years ago in southern Chile. The plant ran only a few months, there being apparently no market for the product. The iron ore was obtained from the Tofo deposit, and green wood was used for fuel.
Of the Chilean deposits, the largest, Algarrobo, is owned by a joint Dutch-German syndicate controlled by Wm. H. Müller & Co., and Gutehoffnungshütte. Tofo, next in size to Algarrobo, is under lease to the Bethlehem Steel Co. Most of the other deposits are owned by Chileans. The tonnage of Chilean ore controlled by different nationalities is approximately as follows:
| Millions of tons (long tons) |
|
|---|---|
| German | 50 |
| American | 40 |
| Chilean (in part English) | 50 |
| Total | 140 |
Iron ore was mined at Tofo during 1914, 1915, and 1916, and exported to the United States. In 1915 about 153,000 tons were shipped. During the war the mining practically ceased.
—The iron-ore deposits of Minas Geraes, Brazil, are among the most important in the world. The ore bodies, which as yet are practically undeveloped, lie in an area roughly 100 miles square, the center of which is 225 miles in a direct line north of Rio de Janeiro. The principal ores are hematite and are associated as beds and lenses with a laminated ferruginous quartzite known as “itabirite” that covers many square miles. The interlayered beds and lenses of ore are high grade, carrying up to 69 or 70 per cent. of metallic iron and averaging between 0.003 and 0.025 per cent. phosphorus. Nearly all ores of this type are of Bessemer or low-phosphorus grade. There are also large areas of recently formed surface ores consisting of mixed hematite and limonite moderately high in phosphorus; these average 55 to 65 per cent. in metallic iron.
On account of the distance from the coast and high cost of transportation,[79] only the high-grade bedded ores are considered at present as available. The Central Railroad of Brazil runs through the iron-ore district to the port of Rio, 310 miles from the southern edge of the district, but unfortunately, on account of heavy grades, it can not be used for extensive transportation of iron ores. As workable bodies of coal suitable for iron manufacture are not known to exist in Brazil, the Minas Geraes deposits have up to the present produced little ore.
The principal iron-ore deposits of Minas Geraes are owned by the Itabria Iron Ore Co., the St. John del Rey Gold Mining Co., Ltd., the Brazilian Iron & Steel Co., and the Compania Metallurgica, the first two being English, the third American, and the fourth Brazilian. The Deutsch-Luxemburgisches Bergwerks und Hütten Aktiengesellschaft (German), the Société Anonyme Franco-Bresiliene (French), Jules Bernard, Mathiew Goudchaux et Cie (French), the Minas Geraes Iron Syndicate (American), and others, own local deposits. The following table shows approximately the tonnage of ore controlled by each nationality:
Table 20.—Brazilian Iron Ore Controlled by Different Nationalities
| Millions of tons (long tons) |
||
|---|---|---|
| Bessemer | Non- Bessemer |
|
| English | 145 | 300 |
| American | 160 | 420 |
| French | 21 | 15 |
| German | 40 | 10 |
| Brazilian | 44 | 400 |
| Total | 410 | 1,145 |
Iron ores similar to those of Minas Geraes, and magnetite deposits of minor importance are reported in other parts of Brazil.
—Mexico has important deposits of iron ore in the States of Lower California, Coahuila, Durango, Guerrero, Michoacan, and Oaxaca. The largest iron and steel making plant in Mexico is that of the Compañia de Aciero y Fierro de Monterey, State of Nuevo Leon, operated by Spanish capital. This plant produces between 50,000 and 100,000 tons of pig iron yearly. Ore is obtained from Coahuila, and coke from nearby coal fields. At Durango is a charcoal furnace, which has been idle for many years. It is owned by the Durango Iron & Steel Co. (American). Small iron-making operations exist in Hidalgo, Puebla, Vera Cruz and Oaxaca. The best known of the Mexican iron-ore deposits is that of Iron Mountain (Cerro de Mercado), near Durango City, a large body of magnetite. In Lower California there are important deposits of iron ore at several localities. They are owned by the International Development[80] Co., an American firm with headquarters at Los Angeles, California. The deposits in Guerrero, Michoacan and Oaxaca are reported to be extensive.
—Various deposits of low-grade iron ore are found in South Africa. In Transvaal there is siliceous sedimentary hematite and magnetite in ferruginous schists of different ages, titaniferous magnetite associated with basic igneous rocks, and local clay-band ore; and in both Cape Colony and Transvaal there are lateritic surface ores. A 15-ton blast furnace has been built within the last year or two near Pretoria by the Pretoria Iron Mines Co., Ltd., for the purpose of manufacturing pig iron from local ores. This is the first attempt to establish an iron industry in South Africa.
—In Australia and New Zealand are some important iron-ore deposits, but only a few are developed. Most of the iron ore mined in Australia has been used for flux in copper, lead, zinc, and other smelting plants; a small amount has been used in the two local iron and steel works—that of the Broken Hill Proprietary Co., at Newcastle, New South Wales, and that of the Eskbank Iron Works at Lithgow, about 75 miles west of Sydney, New South Wales. The former is the more important, having in operation at the present time two blast furnaces as well as steel furnaces, rail mill, and plate mill.
Among the important Australian iron-ore deposits are the hematite ores of Coombing Park, near Carcoar, and of Cadia, near Millthorpe, both in New South Wales, estimated to contain reserves of 42 million tons of ore; the hematite deposits of the Murchison district, about 400 miles northeast of Perth, western Australia, where one single deposit—that of Wilgi Mia—has been estimated to contain more than 25 million tons; the Iron Monarch manganiferous iron-ore deposit, estimated to contain 20 million tons of ore, and the neighboring Iron Knob hematite deposit of one million tons, both about 40 miles from Port Augusta, at the head of Spencer Bay, South Australia; and the hematite deposits of Mt. Leviathan, estimated at 10 million tons, located about 250 miles from Normanton, on the Gulf of Carpentaria, Queensland. Numerous smaller and less important ore bodies are found in all the provinces.
The Coombing Park ores have been used at the Eskbank Iron Works, 90,200 tons being produced in 1916. The ore averages about 55 per cent. iron. The Iron Monarch deposit is being developed by the Broken Hills Proprietary Co., and the ore is to be used in the furnaces at Newcastle.
An important iron-ore deposit, estimated to contain 23 million tons of minable ore, is reported to occur on Blythe River, in the northwestern part of Tasmania, about 6¹⁄₂ miles from the coast. There have been rumors recently of a possible exploitation of this deposit.
In New Zealand large deposits of limonite occur in the Nelson district, in the northern part of South Island. The principal group of[81] deposits, known as Parapara, is estimated to contain about 64 million tons. Titaniferous magnetite sands, measurable in millions of tons, are reported to occur in the southwestern part of North Island near New Plymouth.
—The world’s chief iron- and steel-producing countries are, in the order of their importance: United States, Germany, Great Britain, France, Russia, Austria-Hungary, and Belgium. The annual pig-iron production of these countries ranged in 1913 from 2,300,000 tons in Belgium to 30,900,000 tons in the United States. The normal consumption of iron ore by these countries in the last years preceding the war and their recent maximum annual production are given below:
Table 21.—Maximum Annual Output and Normal Consumption of Iron Ore by Chief Iron- and Steel-Making Countries
| Countries | Consumption (long tons) |
Production (long tons) |
|---|---|---|
| United States | 62,000,000 | 75,288,851 |
| Germany | 40,600,000 | [48]33,987,112 |
| Great Britain | 19,000,000 | 15,997,328 |
| France | 12,300,000 | 21,572,835 |
| Russia | 8,900,000 | 9,362,746 |
| Belgium | 6,800,000 | 164,734 |
| Austria-Hungary | 5,200,000 | 5,233,055 |
[48] Includes production of Luxemburg.
The consumption figures represent metallic iron consumed in terms of iron ore and are obtained on the basis of production and imports of iron ore, and imports of pig iron and crude iron and steel products. Exports of iron ore, pig iron, and crude iron and steel products are not considered as forming part of the countries’ consumption.
A comparison of the consumption and production indicates that the United States, France, Russia and Austria-Hungary were self-supporting as far as raw materials for their iron and steel industry were concerned. Great Britain and Germany are dependent for a small percentage of their requirements upon foreign countries. Belgium produces a very small percentage of her consumption of iron ore, being almost entirely dependent upon foreign sources, mainly France and Germany, for her iron-ore requirements.
In several countries that produce much iron ore the iron and steel[82] industry is still in its infancy. The iron ore from these countries is nearly all exported to the large iron and steel making countries. The following table shows the recent maximum annual production and normal annual consumption in some of these:
Table 22.—Maximum Annual Output and Normal Consumption of Iron Ore in Several Countries
| Countries | Consumption (long tons) |
Production (long tons) |
|---|---|---|
| Spain | 1,000,000 | 9,705,963 |
| Sweden | 700,000 | 6,878,318 |
| Cuba | ... | 1,585,431 |
| Newfoundland | ... | 1,433,858 |
| North Africa | ... | 1,349,000 |
Thus, considerable quantities of iron ore are available from these countries for consumption in countries that have to import iron ore and iron products.
There is shown below the pig-iron and steel production in 1913 of the world’s principal iron and steel manufacturing countries.
Table 23.—Pig-Iron and Steel Output of the Chief Producing Countries, 1913
| Countries | Pig iron (long tons) |
Steel (long tons) |
|---|---|---|
| United States | 30,966,152 | 31,300,874 |
| Germany | 19,004,022 | 18,659,000 |
| Great Britain | 10,481,917 | 7,664,000 |
| France | 5,227,378 | 4,349,000 |
| Russia | 4,474,757 | 4,750,000 |
| Austria-Hungary | 2,335,170 | 2,641,000 |
| Belgium | 2,318,767 | 2,475,000 |
| Canada | 1,015,118 | 1,044,000 |
| Sweden | 728,103 | 574,000 |
| Spain | 418,061 | 359,000 |
| Italy | 420,011 | 897,000 |
| Japan | 236,491 | 251,000 |
—The United States has for many years had in the Lake Superior district the chief iron-ore producing fields in the world. In recent years the Lake Superior district has furnished more than two-fifths of the world’s output of iron ore. In 1917, 75,288,851 gross tons of iron ore, 38,647,397 gross tons of pig iron, and 45,060,607 gross tons of steel were produced in the United States, as compared with 61,980,437[83] tons of iron ore, 30,966,152 tons of pig iron, and 31,300,874 tons of steel in 1913. The imports of iron ore in 1917 amounted to 971,663 tons and of crude forms of iron and steel to 306,189 tons, as compared with 2,594,770 tons of iron ore and 250,592 tons of crude iron and steel products imported in 1913. The exports of iron ore from the United States in 1917 amounted to 1,132,313 tons and of crude forms of iron and steel to 4,744,527 tons, as compared with 1,042,151 tons of iron ore and 1,278,131 tons of crude forms of iron and steel exported in 1913.
These figures indicate that in normal times the United States consumes about 85 per cent. of the domestic output of iron ore, in the manufacture of finished iron and steel products. Fifteen per cent. is exported either as iron ore, or as crude iron and steel products which are manufactured into finished products in other countries. Of the finished iron and steel products made in this country the United States itself consumes the larger part. However, large quantities of iron and steel articles and machinery are exported to other countries as well.
The iron ore exported from this country is mainly Lake ore, which goes to Canadian furnaces. The iron ore imported is largely Cuban ore, which is used at the Sparrow’s Point plant of the Bethlehem Steel Corporation. This plant has facilities for using only ore arriving by boat and has been running almost entirely on foreign ores. The Cuban iron mines are largely under the control of this company, and an increased production is expected from them in the future.
The Bethlehem Steel Corporation has also developed an extensive iron-ore deposit in Chile, from which some shipments were made during the first years of the World War. It has been allowed to remain idle recently on account of lack of shipping facilities. Large shipments are expected from Chile in the future.
A considerable amount of Swedish ore has been imported in recent years by the Bethlehem Steel Corporation, to supplement its shipments of Cuban ore. During the war, however, the Trafikaktiebolaget Grängesberg Oxelosund decreased its ore shipments and finally refused altogether to export ore to the United States. These shipments recommenced soon after the cessation of hostilities in Europe.
Certain high-grade low-phosphorus iron ores which are not present in the United States in sufficient quantity to supply domestic needs have been imported in past years from Spain, North Africa, and to a small extent from Sweden. During the war, when the shortage of shipping facilities necessitated combing this country for supplies of high-grade low-phosphorus ores, it was shown that the United States is more or less dependent upon foreign sources for such ores.
There are a number of mines in the United States, such as the Lyon Mountain mine in New York, and the Cranberry mine in North Carolina, which produce limited amounts of high-grade low-phosphorus ore. Several[84] mines on the Menominee Range, Michigan, produce a very siliceous low-phosphorus ore that can be used to supplement in part the high-grade ores. A considerable quantity of low-phosphorus pyrite residue from sulphuric acid and fertilizer plants is also used for making low-phosphorus iron. Much of the pyrite yielding this residue is imported from Spain, some of it is of domestic origin, and some of it comes from Canada. Altogether, the United States supplies about 60 per cent. of the material required for the manufacture of its normal output of low-phosphorus pig iron.
Certain developments in progress make it probable that a greater percentage of ore used for this purpose can be supplied from domestic mines. The principal enterprise is one that plans to concentrate the siliceous magnetite ore of the eastern Mesabi Range. Experiments have yielded a high-grade product and work on a commercial scale is planned.
The reserves of the ordinary grades of iron ore in the United States are large, and no shortage of such ore is anticipated for many years. They are easily capable of taking care of a considerably increased consumption. The largest reserves are in the Lake Superior district and in the southeastern states, but large untouched reserves occur in the western states as well. The iron ores in the Pacific Coast region have remained undeveloped from the lack of sufficient demand for pig iron and crude forms of iron and steel on the Pacific Coast. Undoubtedly this demand will increase in the future, and iron and steel industries will be established there.
The reserves of ore in the Lake Superior district are large. The grade of ore mined, however, has been gradually getting lower, and it is possible that before many years Lake iron ores averaging considerably below 50 per cent. will have to be utilized. At present the average grade of the ores mined in the Lake Superior district is about 51 per cent.
It is clear that there is not likely to be a shortage of the ordinary grades of iron ore in the United States. Reserves of high-grade ores, however, are being gradually depleted, and high-grade ores from foreign countries will find an increasingly ready market. American capital controls a large reserve of high-grade iron ore in Brazil. Much of the Brazilian ore averages about 68 per cent. in metallic iron and is very low in phosphorus, making it an exceedingly desirable raw material for the manufacture of special iron and for mixing with lower-grade domestic ores. Doubtless much of the Brazilian ore will go to Europe, as British and other foreign holdings of this ore are extensive. However, it is highly desirable that a certain proportion of the ore should be diverted to American furnaces.
—The annual consumption of iron ore in Germany just before the war was about 40 million tons, and the maximum annual output at this time, including more than 7 million tons from Luxemburg, was only about 34 million long tons. In order to supply German furnaces[85] it was necessary, therefore, to import more than 6 million tons of iron ore from foreign countries. More than 58 per cent. of the iron ore mined in Germany has come from the Lorraine district. The production from German iron mines outside of the Lorraine district amounted to 6,906,809 tons in 1913. The production of pig iron during that year was 19,004,022 tons.
The pre-war imports of iron ore into Germany were large, amounting to nearly 14 million tons in 1913; against these the exports were somewhat more than 2 million tons.
The iron ore imported from Sweden is mainly high-phosphorus ore from the mines of Swedish Lapland and central Sweden. This is especially adapted to the manufacture of pig iron for the Thomas process, much used in Germany. Most of the ores from the Lorraine district are slightly too low in phosphorus to be suitable for the Thomas process; and Swedish high-phosphorus ores, phosphate rock, and phosphatic slags are in places mixed with Lorraine ore to raise the phosphorus content.
A considerable amount of low-phosphorus iron ore used in the manufacture of low-phosphorus pig iron is also imported from Sweden, and a larger amount of this ore is imported from Spain. This material is used in Germany for the manufacture of pig iron to be used in making acid open-hearth steel.
Since Germany has lost the Lorraine iron fields, the remaining domestic iron mines will be able to supply less than 20 per cent. of the requirements of iron ore for the German furnaces. However, it is likely that Germany will continue to receive most of her supplies from the Lorraine fields, in which German holdings at present predominate over French holdings and will probably continue to predominate.
—The United Kingdom has produced from 10 to 14 per cent. of the iron and steel of the world annually for the past 10 years or more, and apparently has consumed in normal times about 50 per cent. of the product and exported 50 per cent., mainly to British possessions. During the war about 75 per cent. of the British production was consumed at home and 25 per cent. was exported, largely to France. Fifty per cent. of the iron and steel products manufactured has been obtained from ores mined in Great Britain, and 50 per cent. from imported ores. Thus, normally, the domestic yield of iron ore just about equals the domestic demand for iron, whereas during the war the domestic demand for iron was greater than the domestic supply of ore. Great Britain depends upon outside sources for one-third of her iron-ore supply and this constitutes the source of about one-half of the iron products.
The iron industry in Great Britain before the war was loosely controlled by merchants who acted as intermediaries between producer and consumer, an arrangement that did not work to the advantage of the[86] consumer.[49] British manufacturers had little interest abroad and were themselves insufficiently organized to operate successfully. If the sources of foreign iron ore were cut off, the situation might become critical and exceedingly embarrassing until the domestic mining industry could be expanded. To meet this condition the British Board of Trade Committee advised a consolidation of iron interests by the formation of a syndicate for the purchase and distribution of iron ores and particularly for the acquiring of interests abroad. This syndicate would establish sales agencies and arrange for transportation and trade, similar to the organization of W. H. Müller & Co., of The Hague. The committee recommended that these operations be backed by the government and that all the resources of the British Empire be under the control of the government, especially in regard to the granting of concessions to aliens and the imposing of restrictions to favor home producers. It has recently been reported that the iron interests have organized along the lines indicated.
[49] British Board of Trade, “Reports on Iron and Steel,” London.
—The annual consumption of iron ore in France for the manufacture of pig iron and crude iron and steel products amounts to about 12 million tons under normal conditions. The productive capacity of the iron mines of France is more than 21 million, leaving a surplus of 9 million tons of ore annually available for export. More than 90 per cent. of the iron ore produced in France is obtained from the Lorraine iron mines.
Most of the ore exported from France in the past has gone to German blast furnaces. Much has gone to Belgium. Imports of iron ore are small, being mostly high-grade ore from Sweden, in which class of ore France is deficient. French possessions in North Africa have large reserves of high-grade ore, but the bulk of the ore mined there has gone to England and Germany.
As a result of the war, that part of the Lorraine iron fields within the boundaries of the disputed provinces of Alsace and Lorraine has been given to France, who thus has control of the entire output of the great Lorraine iron fields with the exception of the part included in Luxemburg. The production of the Lorraine iron fields, including the part that formerly belonged to Germany, has been nearly 48 million tons annually, of which about 7 million tons is mined in Luxemburg. Outside of the Lorraine district France produces about 1,500,000 tons of ore. Thus, unless iron and steel making expand greatly in France, much iron ore will be mined for export.
—In 1913 Russia ranked sixth in the output of iron ore and fifth in the output of pig iron, producing about 4¹⁄₂ per cent. of the world’s production of iron ore and 6 per cent. of the pig iron.
Russia’s iron-ore reserves are estimated at about 1,600 million tons, a part of which, especially in central Russia, is not economically minable.[87] The district of southern Russia is important on account of its large reserves, large output, and its location. This is particularly true at this time on account of Germany’s need of iron ore for future use.
The Russian output of iron ore grew from about 2 million tons annually in 1891-93 to 7 or 8 million tons annually in 1913-1917. Southern Russia (almost exclusively the Krivoi-Rog district) produced nearly 7 million tons in 1913, but by 1916 the production from this region had been cut down to half, its difference being made up from other regions.[50] Between 1913 and 1917, Russia produced about 4 million tons of pig iron annually, of which 3 million tons came from South Russia, and most of the remainder from the Ural region.
[50] Advisory Council, Dept. of Sci. and Indust. Research, “Report on the Sources and Production of Iron and Other Metalliferous Ores Used in the Iron and Steel Industry,” 1918. Also British Board of Trade, “Reports on Iron and Steel,” London; and Ironmonger Metal Market Year Book, 1918.
In 1916 the Central War Industry Committee estimated the monthly requirements of the whole country at 300,000 tons of pig iron for war purposes, and at 80,000 tons for the requirements of the civil population, making a total annual consumption of about 4,500,000 tons, or only about one-half of the normal consumption. In 1917, the total production in the country was estimated to amount to only 30 per cent. of these minimum requirements.
The situation in Russia is so unsettled that a statement of present conditions in the steel industry is valueless. It is reasonable to assume, however, that the iron and steel situation will not materially change as to operations and control. Moreover, it will be safe to predict that Poland will develop more rapidly as an iron-ore producer in the future, as she was handicapped in the past by restrictions on exportation of ore.
—Belgium has been negligible as a producer of iron ore but has been a comparatively large importer of iron ore and manufacturer of pig iron. The country ranked sixth as a producer of pig iron in 1913, in which year it produced 147,048 tons of ore and imported 4,400,000 tons.[51]
[51] Board of Trade, “Reports on Iron and Steel,” London.
Belgian iron works were greatly damaged by the Germans during the war, and probably some time will elapse before the industry again reaches the position it occupied before the war. The country offers a good market, however, for the iron ores of France and should in future years be a larger producer of iron and steel wares.
Belgium is practically dependent upon outside sources for ore supply, but is conveniently situated as a market for ores from many countries. The total iron-ore reserves of the country have been estimated at 62,500,000 tons, not enough to last 10 years at the present rate of consumption.
—The former Austro-Hungarian Empire yielded in recent years 2 to 3 per cent. of the annual iron-ore production of the world,[88] and about 2 per cent. of the pig-iron production; therefore it has been of minor importance in the iron industry. The ore reserves have been estimated at 284 million tons of available ore, and 807 million tons additional of probable ore.
The present unsettled conditions will probably result in considerable change in the operation and control of the iron mines and works. Eventually the upheaval may stimulate the iron industry, but the result should not materially alter the international position.
—The iron and steel industry of Japan is of small magnitude as compared with that of the United States, Germany, Great Britain, and other leading iron and steel manufacturing countries. The total reserves of iron ore are probably not much more than 60 million tons, or less than has been mined annually in the Lake Superior district in recent years. The steel-making industry is expanding rapidly, however, and at present blast furnaces, steel-making furnaces, and steel mills are being erected in Japan, Korea, Manchuria, and China by Japanese interests.
The output of iron ore in Japan is utterly inadequate to supply this expanding industry. The production of iron ore in Japan has averaged about 150,000 tons annually in recent years, whereas the consumption of crude, semi-crude and manufactured articles of iron and steel is approximately 1,500,000 tons. In order to supply her needs, therefore, from her own manufacturing plants, Japan would require in the neighborhood of 3 million tons of iron ore annually. As compared with this, Japan’s entire consumption of iron ore, both imported and domestic, is less than 700,000 tons. The remainder of the iron and steel required in Japan is being imported in the form of pig iron and crude and manufactured products.
Japan is making a strong effort to develop iron-ore deposits in neighboring countries, especially in China, Manchuria, and Korea; and the production from these sources which goes to Japanese-controlled furnaces is rapidly increasing. Among the more recent Japanese iron and steel enterprises in these countries are the blast furnaces and steel plant now being built at An-schan-chang, south of Mukden, in Manchuria; the blast furnaces at Pen-hsi-hu, southeast of Mukden, in Manchuria; and the blast furnaces at Ken-ji-pho, in Korea. The last two of these plants are now producing pig iron, which is being sent to Japan. In the future all three plants will probably build steel works. Iron-ore deposits are being mined in connection with all of them. Besides being used in the local blast furnaces, iron ore is being sent to Japan from these mines. In China, the most important iron and steel enterprise is that at Han-yang, in the Province of Hu-peh. This operation was started by the Han-Yeh-Ping Iron & Steel Co., as a Chinese enterprise in connection with the Tayeh mines in the same province. This company, however, became involved in financial difficulties, and Japanese capital was called[89] upon in order that work might continue. Considerable expansion of the plant is at present taking place under Japanese supervision. Iron ore from the Tayeh mines and pig iron from the Han-yang plant are sent to Japan for use in Japanese iron and steel works.
It is doubtful whether, with the rapid expansion of the Japanese iron and steel industry, mines in China, Manchuria and Korea can be developed fast enough to supply the raw materials necessary. There are rumors that several deposits of iron ore in eastern China are now being developed, including that of Chin-ling-chen, and these may afford some additional supply. The iron mines of India also may be called upon to furnish more iron ore to Japan than they have done in the past. The only other important iron-ore deposits known elsewhere in the Orient are in the Philippine Islands. These deposits are reported to be fairly important and they are favorably situated for supplying Japanese plants. They are controlled by Americans.
The present expansion of the Japanese iron and steel industry is such that it is a question whether the consumption of iron products in Japan will be sufficient to take care of the entire output. It seems very probable that Japan is looking for a large export trade in iron and steel products. The Japanese may be ambitious not only to displace European and American goods in the Orient, but may even attempt to secure a market on the Pacific Coast of the United States and Canada. It is quite probable that Japanese manufactured articles will be able to compete in the western United States with articles manufactured in the eastern states and subject to heavy transportation rates. On the other hand, there is an active movement to start an iron industry on the Pacific Coast, and it is hoped that plants established there will be able to manufacture iron and steel products at a low enough cost to enable them to compete with Japanese products in the Orient.
[90]
Alloys of manganese are essential in the manufacture of steel by the open-hearth and the Bessemer processes, which produce 99 per cent. of the total output of the United States. In this country, about 14 pounds of metallic manganese as alloys, equivalent to about 40 pounds of high-grade ore, is used in making a ton of average steel. Two alloys are in common use: ferromanganese and spiegeleisen. Ferromanganese, with 70 to 80 per cent. manganese, is largely used in making open-hearth steel carrying less than 0.30 per cent. carbon, whereas spiegeleisen, with 20 to 32 per cent. manganese, is used in making Bessemer steel carrying more than 0.30 per cent. carbon. The first group of low-carbon steels is used in making structural shapes, sheets, bars, wire, etc., and the second group of high-carbon steels is used in making rails, forgings, etc.
In making 70 to 80 per cent. ferromanganese, so-called “high-grade” ore with more than 35 per cent. manganese and less than 5 per cent. iron and 15 per cent. silica is needed. In making 20 to 32 per cent. spiegeleisen, so-called “low-grade” or ferruginous manganese ore with 10 to 35 per cent. manganese, 20 to 35 per cent. iron, and less than 20 per cent. silica is needed, although here and there spiegeleisen is made by mixing high-grade manganese ore with iron ore.
Several other alloys such as silico-manganese, ferro-silicon, and ferro-carbon-titanium may be used as partial substitutes for ferromanganese, but although they may be capable of wider use under stress, they are electric-furnace products and under normal conditions their cost is prohibitive.
Very pure manganese oxide is used in making the common dry battery, the production of which has greatly increased with the wide use of the internal-combustion engine. About 25,000 tons is used annually in the United States for this purpose. The manganese oxide thus used is not consumed, but becomes exhausted through the loss of oxygen. Under stress of high prices, the oxide may be regenerated by treatment or by mixture with new refined material.
Small quantities of manganese ore are used in making many chemical products and pigments.
[91]
Any consideration of the need for manganese ore and ferromanganese and of dependence upon foreign sources of supply should take account of the degree to which low-grade ore and spiegeleisen may be used as substitutes for high-grade ore and ferromanganese. Thus, although both Germany and the United States have only insignificant resources of high-grade ore, both possess unusually large reserves of low-grade ore. Under recent conditions in the United States the percentage of total manganese used as spiegeleisen increased in three years from 10 to about 18 per cent. Competent authorities have estimated that this substitution may be further increased to nearly 70 per cent. with slight modifications in practice and modest addition of equipment. Some competent engineers further contend that by other modifications of practice a large part of the manganese now needed as alloys may be eliminated by the addition of low-grade manganese ore during early stages of the smelting and refining process.
Although concentrated masses such as are useful in the arts are rather uncommon, manganese is widespread throughout the earth; it forms a part of about 100 minerals and most of the common rocks, igneous as well as sedimentary, contain 0.1 to 2 per cent.
Present requirements as to grade are such that manganese ores are largely oxides. The carbonate, rhodochrosite, contains enough manganese to permit its use in making 80 per cent. ferromanganese, but only in a few places are the masses large enough to be the basis of extensive mining. The common silicate contains 43 per cent. manganese, but the silica content is so high (23 per cent.) that it can not be used alone in making the ordinary alloys.
The common oxides of manganese are deposited under many conditions which are found near the surface of the earth. Large masses of oxides were deposited in shallow marine waters, in shallow fresh-water basins and under many other conditions in the relatively thin mantle of weathered rock that is found over the entire world. Although most of these large masses were formed in the surface zone where the underlying unweathered rocks are unusually rich in manganese, some large masses of oxides accumulate under peculiarly favorable conditions by the concentration of small quantities of manganese disseminated through the common rocks.
For purposes of geologic study, deposits of manganese oxides may be considered in two groups, as follows: (1) those derived from more or less localized masses of carbonate or silicate materials, generally with more than 5 per cent. manganese, that seem to have no relation to the surface of[92] the earth; and (2) those originally deposited near the surface as localized bodies of oxides.
Under the first group are zones of carbonate or silicate rocks in contact with intrusive igneous rocks, such as are found in Brazil and India. These zones contain manganiferous carbonates (mixed with iron, lime, and magnesia); and silicates (spessartite or manganese garnet and piedmontite or manganese epidote and possibly rhodonite, or manganese pyroxene). By the weathering of such rocks, large bodies of high-grade oxides have been formed.
Manganese also occurs in fissure veins or the adjacent wall rocks in regions that have been intruded by igneous rocks. The veins commonly contain rhodochrosite, manganiferous siderite, or rhodonite, associated with quartz and metallic sulphide minerals, including alabandite, the sulphide of manganese. Such veins are known in Philipsburg and Butte, Mont.; Silverton, Colo., and elsewhere. In such regions if the wall rocks adjacent to fissures are limestone or dolomite, they may be extensively replaced by manganiferous siderite or other carbonates which on weathering yield large bodies of manganese oxides, locally mixed with iron oxides. Such bodies are known at Leadville, Colo.
The manganese existing in sediments, notably clayey, but in part carbonate, may migrate locally after the sediments are slightly buried and form zones of manganiferous carbonate and silicate concretions parallel to the bedding. Where, as in the Batesville district, Arkansas, these zones are exposed by erosion, the manganese is further concentrated as masses of oxides in residual clay.
Metamorphic rocks, such as slates and schists, here and there, as in Spain, Newfoundland, California, and Washington, contain extensive lenses of rhodonite or other silicate with or without rhodochrosite, roughly parallel to the bedding. The origin of these lenses, which are generally rather remote from igneous intrusions, is obscure. Some are considered to be materials laid down during sedimentation, others are thought to represent concentrations effected during metamorphism when the sediments were deeply buried.
In the second group mentioned above (manganese deposits first concentrated near the surface as oxides) are included extensive deposits of oxides interbedded with marine sediments, as in the Caucasus region, Russia. Others interbedded with volcanic material, tuffs and flows, are known in the Mediterranean region and in Chile. In India important beds of oxides are interbedded with quartzite and slate.
Many deposits of oxides have recently formed and are probably now forming in bogs in many regions, notably New Brunswick, Canada. They are not important as sources of production.
Although no simple relation seems to govern the distribution of manganese in unweathered rock, there is reason for believing that the[93] accumulation of manganese oxides in the weathered mantle is favored by climates that cause unusually complete or deep rock decay. Although a few manganese oxide deposits are found within the belts of recent glaciation, and some have no relation to weathering, most of the important deposits occur in areas now or recently favored with warm, humid climates, and there is reason for suspecting that such areas will yield other important deposits.
—In the United States, the occurrence of manganese ore can be most clearly described by grouping the districts according to grade or ore: (1) high grade, containing 35 per cent. or more of manganese, which is used ordinarily for making the high-grade alloy, ferromanganese, and (2) low-grade, ferruginous manganese ore, used ordinarily for the low-grade ferro-alloy, spiegeleisen. The country is deficient in natural supplies of the former, but has abundant resources of the latter, which under the stress of necessity could be largely substituted for the high-grade ore, which is now mainly imported.
The following districts in the United States yield high-grade ore:
At Philipsburg, Mont., are bodies of manganese carbonate that replace Cambrian limestone near veins and igneous contacts. These are weathered to oxides to a depth of about 200 feet below the surface. At Butte, Mont., veins in granite contain manganese carbonate and silicate, locally weathered to oxides.
In the Shenandoah Valley in Virginia, and in similar valleys in Tennessee and Georgia, the residual clays from certain Cambrian limestones and Silurian shale and sandstone yield bodies of manganese oxides to depths that range from 200 to 250 feet below the surface. Many small deposits also occur in Arkansas, Arizona, California, Nevada, and Utah.
The total production of the United States from 1838 to 1918 was 893,734 tons, and the maximum was 305,869 tons in 1918.
Among the chief districts yielding the lower grade of ore (10 to 35 per cent. manganese) the most conspicuous is the Cuyuna district in Minnesota, where beds of iron-manganese carbonate of pre-Cambrian age are weathered to oxides to depths of 250 to 500 feet below the surface and contain ore bodies carrying 7 to 20 per cent. manganese, and 25 to 50 per cent. iron. The single deposits range from 50,000 to 7,500,000 tons each. Since the first shipments in 1913, the production through 1918 has been 1,666,677 tons of ore carrying more than 5 per cent. manganese.
Large deposits of low-grade manganese ore also occur in the Leadville district in Colorado, where irregular bodies of iron-manganese carbonate have replaced magnesian limestone of Carboniferous age and are[94] weathered to oxides to depths as great as 850 feet. From 1885 to 1918, the total production was 3,202,678 tons of material, most of which contained from 15 to 30 per cent. manganese.
Other deposits of ferruginous manganese ore have been exploited in Eagle County, Colorado; the Pioche district, Nevada; Silver City district, New Mexico; and in Arkansas, Georgia, and Virginia.
In Canada, large deposits of siliceous manganese ores occur in Newfoundland, and several small deposits in New Brunswick, Alberta, and British Columbia.
In Costa Rica, manganese occurs in four districts near Playa Real, Nicoya Peninsula, in the form of oxides that seem to be interlayered with sedimentary rocks. The most productive deposits are owned by citizens of United States and of Cuba; others are owned entirely by Cubans. They were first exploited in 1916, and to the end of 1918 had exported 18,000 tons to United States.
In Cuba, manganese is mined near Santiago and Bueycito, in the province of Oriente. Near Santiago, manganese oxides occur as lenticular or irregular bodies in tuff, clay, and limestone. Other deposits are reported in Santa Clara and Pinal del Rio provinces. The mines that have been the source of more than 90 per cent. of the exports are owned jointly by citizens of Cuba and of the United States, and the remaining mines by Cubans.
From 1888 to 1910, 266,621 tons were exported. In 1915, after four years of idleness, the mines were reopened, and imports into the United States from 1915 to 1918, inclusive, were 163,189 tons.[52]
[52] Production data, long tons, unless otherwise specified.
In Mexico, manganese ores are found four miles north of Chihuahua City and south of Palomas, in the State of Chihuahua. It is assumed that the deposits are owned by native Mexicans. Manganese also occurs near Conception Bay, Lower California, where the mines are owned by native Mexicans but are under lease to Americans. From both of these districts, 1,500 tons were produced and exported to the United States in 1917.
In the Republic of Panama, near Nombre de Dios and Madinga, there are irregular lenses of manganese oxides in decomposed sedimentary beds. Seven groups of deposits were exploited near Nombre de Dios. Five of them were exploited by Americans, one by native owners, and one by French. The Nombre de Dios deposits yielded 50,000 tons of ore from 1871 to 1902, largely during the last six years. The Madinga deposits were opened in 1916 and during 1916 and 1917 exported 11,000 tons to the United States.
—The largest deposits of manganese ore in South America are in Brazil, and especially in the important mining state of Minas Geraes.
[95]
In the Lafayette district in this state, manganese oxides occur in wide lenticular bodies, that seem to have no definite arrangement or association, except that most of them are bounded by schist or gneiss. The deposits lie in a complex of granite, gneiss, and crystalline schists; and the manganese oxides are probably derived from manganese-bearing carbonate and silicate minerals. The most productive area, known as the Morro da Mina, 2,500 feet long by 1,000 feet wide, contains four distinct bodies that range from 320 to 1,300 feet long and from 48 to 320 feet wide. Here manganese oxides persist 410 feet below the surface.
The area has yielded about 1,000,000 tons of ore and the reserves are probably between 7,000,000 and 10,000,000 tons.
In the same State of Minas Geraes, in the Miguel Burnier and Ouro Prieto districts, manganese oxides are interlayered with ferruginous sedimentary rocks of pre-Cambrian age.
In the State of Bahia, the Nazareth district contains bodies of manganese oxide in a thick surface zone of highly weathered schistose rocks. The oxides are probably derived from lenses of manganese garnet in schist. The largest deposit yielded 70,000 tons of ore. Manganese deposits are also reported near Bom Fim, in the same state.
Other deposits of manganese ore in Brazil are reported in the states of Maranhao and Matto Grosso.
The known manganese deposits of Minas Geraes lie within an area about 30 miles square, the center of which is about 300 miles north of Rio Janeiro. The ore is readily mined from open cuts, but existing transportation and loading facilities practically limit the annual exports to 550,000 tons.
Most of the important deposits in the Lafayette-Miguel Burnier and Ouro Prieto districts are owned by resident Brazilians. In 1915 a German company had worked a part of the Morro da Mina deposit for six or seven years and produced a total of 200,000 tons of ore. During 1915 a Belgian company was operating the Cocuruto mine near Ouro Prieto and was shipping 2,000 tons monthly. The largest deposit of the Nazareth district is owned by an American and the undeveloped deposits near Turyassu are owned by Norwegians.
From the beginning of the industry in 1894 to 1918, 4,660,000 tons of manganese ore were exported from Brazil. From 1900 to 1913, the annual exports ranged from 99,000 to 250,000 tons, but with the elimination of Russian sources in 1914, exports rose to 503,130 tons in 1916, 532,855 tons in 1917, and 393,388 tons in 1918. In October, 1917, the export tax on manganese ore was advanced from $0.85 to $3.00 per metric ton. Even before the war a large part of the Brazilian exports went to the United States. The destination of the 1913 exports was as follows: United States, 60 per cent.; Germany, 18 per cent.; Great Britain, 16 per cent.; France, 6 per cent.
[96]
In Chile, manganese ores occur at Corral Quemada, and nearby districts in the State of Coquimbo. In these districts, beds of manganese oxides are interlayered with sandstone, shale, and volcanic flows. Manganese is also found in the Carrizal district in the State of Atacama, where beds of manganese oxides are interbanded with shale and limestone. From 1885, when explorations were begun, to 1905, the exports of manganese ores from Chilean ports amounted to 549,716 tons, the maximum exports for one year being 50,871 tons, in 1892.
In Uruguay, deposits of ferruginous manganese ore, reported to contain 80,500,000 tons, occur at Zapucay, in the Department of Rivera.
—In the former empire of Austria-Hungary, the principal manganese district is near Dorna Vatra, in Bukowina. Here there are lenses of manganese carbonate and silicate in schists that have weathered to oxides near the surface. The deposits are owned by the Bukowina Greek Church. The average annual production from 1906 to 1912 was 13,600 tons. Other deposits are reported in Bohemia, Istria, Styria, Hungary and Bosnia. Since 1901, the production of Austria-Hungary has ranged from 18,000 to 25,000 tons annually.
In Belgium, near Chevron, in the Province of Liège, ferruginous manganese oxides have formed by the weathering of manganese and iron carbonates. Since 1901, the annual production in peace times has ranged from 2,000 to 15,000 tons.
In France, manganese occurs chiefly near Romaneche, in the Department of Saone and Loire, where several bodies of manganese oxides lie in a fault between sedimentary rocks and granite. The deposit has been known since 1823, and the production in 1901 was 9,500 tons. Other French deposits have been explored in the Departments of Hautes-Pyrenees, L’Ariege, L’Allier, L’Ande, and La Nievre. The annual production rather steadily declined from 22,000 tons in 1901 to 6,000 tons in 1913. The ownership of the French manganese deposits seems to be largely French, possibly aided by some English capital.
In Germany, manganese ore of the better type, containing over 30 per cent. of the metal, occurs in Sachsen-Gotha, Central Germany, in small veins of manganese and iron carbonates weathered near the surface to oxides. A similar grade of manganese ore occurs in small quantities at Hessen and Waldeck, in Rhenish Prussia.
Manganiferous iron ore, containing from 12 to 30 per cent. of manganese, occurs in Hessen-Nassau, Rhenish Prussia, where manganese and iron oxides form irregular flat lenses imbedded in clays derived from the weathering of underlying Devonian limestone. During the period 1907 to 1911, nine deposits yielded 262,000 to 283,000 tons annually.
Manganiferous iron ore (containing less than 12 per cent. manganese) is found at Siegerland and at Nassau, Rhenish Prussia. The veins are large and contain manganiferous siderite; they cut Devonian sediments.[97] These deposits are largely owned by the principal iron works of Rhenish Prussia. During the period 1907 to 1911, the annual production ranged from 2,200,000 to 2,600,000 tons.
In Great Britain, the principal deposits are in North Wales. Veins of manganese carbonate and silicate, as well as interlayered lenses, are found. The material contains 20 to 36 per cent. manganese. Other deposits are recorded in Devonshire, Cornwall, and Shropshire. The maximum production of Great Britain of about 23,000 tons was attained in 1906, when two mines in North Wales yielded 19,300 tons.
Manganese occurs in Greece, in the western end of the island of Melos, where nodules and masses of manganese oxides are disseminated through beds of tuffs of Pliocene age. The maximum production of 15,000 tons was recorded in 1902. The output has since steadily declined to 550 tons in 1913. Manganese also occurs on the peninsula of Kassandra, which was formerly in European Turkey. A vein explored mainly for argentiferous galena yields manganese oxides from the surface zone. The maximum production of 52,000 tons was attained in 1902, steadily declining thereafter to 12,000 tons in 1910.
In Italy there is manganese in Tuscany, where irregular bodies of manganese and iron oxides occur in Triassic limestones. Other deposits in Liguria and Sardinia have recently yielded a little ore. Of the maximum Italian production of 18,147 metric tons (18 to 45 per cent. manganese) in 1916, 14,072 metric tons was derived from the Tuscany deposits in Tuscany. Normally the annual production has ranged from 1,600 to 4,700 tons.
In Portugal there is manganese in Alemtejo. The deposits, reported to be lenses and veins in Silurian quartzites, are owned by a Portuguese company.
Russia contains the most important manganese deposits in Europe, if not in the world. The principal mining district is near Chiaturi, in the Kutais Government, on the south side of the Caucasus Mountains, in southern Russia. Layers of oolitic grains of manganese oxides are interbedded with horizontal sandstone and shale of Lower Eocene age. Within a zone that ranges in thickness from 4.5 to 7.5 feet and averages about 6.5 feet, seven distinct layers of very pure manganese oxide aggregate about 40 inches in thickness, and the remainder is low-grade material and sand. It is estimated that an area of 120 to 143 square kilometers was originally underlain by the bed of oxides, but that about half has been removed by erosion. Estimates of reserves range from 23,000,000 tons to several hundred million tons.
The mines are operated in a crude, inefficient manner and scarcely two-thirds of the ore is recovered. The number of actual producers ranged from 183 in 1902, to 376 in 1906, but declined to 96 during the political troubles in 1908. The ore is sorted by hand and the low-grade[98] material is washed in crude plants. From 20 to 25 per cent. of the exported material has been concentrated by washing.
In 1902 there were 5,000 concessions, of which 3,750 were owned by 14 persons, each with 25 to 500 concessions; the remainder belonged to 300 peasants and small merchants. By 1912 a producers’ association had been formed to permit the owners to deal collectively with the exporters. Large investments had also been made by German capitalists in mines as well as in undeveloped territory. The Gelsenkirchen Gesellschaft, a German firm, had been formed partly for the purpose of mining but largely to purchase and export ore to Germany. In 1912, this firm, although it produced only a little ore, exported nearly one-third of the total. German groups also established necessary financial agencies to facilitate export of ore as well as to make loans to mine operators. In 1913, of 16 exporting firms, only 3 were Russian.
The output of the mines of the Chiaturi district is hauled to Chiaturi (1.3 to 3.3 miles), loaded on narrow-gauge cars for transport to Sharopan (25 miles), and then reloaded on cars for shipment to Poti or Batum (107 miles), the ports of export. Large stocks ranging from 1,030,000 tons in 1912, to 1,525,000 tons in 1908, are kept at Chiaturi, Poti and Batum. From 1910 to 1912, the distribution of exports ranged as follows: Holland (for Germany), 30 to 43 per cent.; England, 22 to 23 per cent.; Belgium (largely for Germany), 15 to 21 per cent.; Germany (direct), 5 per cent.; France, 4 to 6 per cent.; United States, 4 to 10 per cent.; Austria, 4 to 10 per cent. About 1913, an export tax equal to 40 cents per long ton was levied by the Russian government.
It is estimated that from 1848 to 1914, inclusive, this deposit yielded about 11,000,000 tons of washed ore of marketable grade. The maximum production of 1,300,000 tons was attained in 1913.
Another important manganese mining region is the Nicopol district, in the Province of Ekaterinoslav, north of the Black Sea. In this district a bed of manganese oxides lies between clays and sandstone of Oligocene age. This bed is 1 to 5 feet thick, averaging nearly 3 feet. It is estimated to extend over an area of 20 square kilometers (7.5 square miles) and to contain 7,400,000 tons of manganese ore. The ore is mined and washed in a crude way to free it from the attached clay.
The deposits in this district are probably owned largely by Russians, although French capital is interested in one company and German capital in another. During the period 1901 to 1910, between 80 and 90 per cent. of the production was consumed in southern Russia and the remainder was exported. From 1886, when the deposits were first exploited, the output rose rather steadily to the maximum of 271,000 tons in 1907, then declined to 173,000 tons in 1910. The total production of the district is about 1,800,000 tons.
Manganese deposits are also known in the Province of Podolien[99] and Terek, and in the governments of Tiflis, Erwin, Elisabetpol, and Perm.
The principal manganese deposits of Spain are on the south slope of Sierra Morena, in the Province of Huelva. Vertical lenticular bodies of manganese carbonate and silicate with a little pyrite, garnet, and mica occur interlayered with slate of Paleozoic age. About one hundred bodies are known, many being 500 feet long and 100 feet wide, whereas the largest is 3,300 feet long and 330 feet wide. The manganese minerals are weathered to oxides to an average depth of 65 feet and a maximum depth of 250 feet. From 1881 to 1905, when the oxide ores were nearly exhausted, nearly 700,000 tons had been shipped. From 1906 to 1910 about 125,000 tons of mixed carbonate and silicate was produced.
Manganese is also found in the Covadonga district, Province of Oviedo, where large boulders of manganese oxide are found in clay resulting from the weathering of underlying limestone. These deposits yielded 3,800 tons in 1915. Other productive deposits occur in the provinces of Seville and Teruel. Deposits are also known in the provinces of Ciudad Real, Murcia, and Almeria.
In Sweden, there are manganese ores north of Philipstad, in Wermland, where tabular bodies of manganese oxides are interlayered with dolomite and magnetite. These deposits contributed 7,607 tons out of a total of 7,733 tons in 1915, which was the maximum recorded production of Sweden.
—In India, on the east coast in the Vizagapatam and Ganjam districts, Madras, is a unique group of rocks known as the Kodurite series, containing manganese garnet, manganese pyroxene, potash feldspar, apatite, and quartz. These rocks, supposed to be of igneous origin, have been deeply weathered and the manganese concentrated as oxides in the surface zone. The manganese ore bodies have been explored only to 100 feet in depth, but it is expected that they will extend to 500 feet. The largest ore body explored at the Garbham mine is 1,600 feet long and 100 feet wide, and, from 1896 to 1913, yielded 736,192 tons of ore. The Kodur deposit yielded 370,382 tons of ore from 1892 to 1913. Production began in 1892, reached a maximum of 111,501 tons in 1906, and slowly declined to 44,127 tons in 1913.
Manganese also occurs in the Balaghat, Bhandara, Chindwara, and Nagpur districts in the central provinces; Narukot and Panch Mahals districts, Bombay; Jhabua district, central India; and the Gangpur district in Bihar and Orissa. These districts form a belt that extends from Baroda, on the west coast, across northern India nearly to Calcutta on the east, a distance of 700 miles. In these districts, beds of manganese oxides with manganese garnet and rhodonite form a rock type known as gondite, which is interlayered with quartzite and mica schist. These rocks are considered to be sediments of the Dharwar group (Archean).[100] The manganese oxides may have been laid down as sediments, or may represent the weathering of the silicates. The ore bodies are lenses and layers. The largest single deposit, Balaghat, has the form of a shallow trough, is 1³⁄₄ miles long and 45 to 50 feet thick, and yielded from 1901 to 1913, 725,248 tons of ore. In 1913, thirteen distinct deposits had yielded more than 100,000 tons each, the range being from 101,721 to 725,248 tons.
From 1901, when the deposits of this type were first exploited, the rate of production rose steadily to the maximum of 697,035 tons in 1913. During 1907, fifty-two separate deposits contributed 598,437 tons.
Where rocks of Dharwar age (Archean), such as mica schists, that do not seem to contain the manganese-bearing Gondite series, are deeply weathered, manganese and manganiferous iron oxides form irregular but locally extensive deposits on the crests of hills. These deposits are underlain by barren clays that represent the residue of the underlying rocks. The largest deposit yielded 160,000 tons of ore in three years, 1906 to 1908, but is probably almost exhausted. The principal deposits of this class are in the Sandur Hills district, Madras; the Shimoga district, Mysore; and the Belgaum district, in Bombay; which lie within an area less than 100 miles in diameter in southwest India. From 1905, when deposits of this group were first explored, the production increased to a maximum of 11,353 tons in 1909,then declined to 62,770 tons in 1913. The total yield of this group to the end of 1913 was only 765,401 tons.
As regards the commercial control of the manganese deposits, a law recently passed forbids aliens to own more than a minor interest in mineral deposits in India. Previous to this, during 1907, the latest year for which complete data are available and in which 899,055 tons was produced, the entire output was from mines owned by resident English or natives, except for 21,500 tons produced by the Carnegie Steel Co., of Pittsburgh, U. S. A.
The annual production of manganese ore in India rose steadily from 1892, when the first shipments were made, to 1907, when 899,055 tons was shipped; and since then has ranged from 450,000 to 815,000 tons. The total production, up to and including 1916, was 8,748,000 tons.
Indian ores are transported to the shipping ports by rail for distances ranging from 56 miles for the Vizagapatam district to 783 miles for the Chindwara district, with the result that freight charges are heavy. The ports of export in order of tonnage handled are, Bombay, Mormugas, Calcutta, and Vizagapatam. The destination of exports in 1913 was as follows: United Kingdom, 36 per cent.; Belgium (largely to Germany), 26 per cent.; United States, 15 per cent.; France, 14 per cent.; Germany, 2 per cent.; others, 7 per cent.
In the Japanese Empire, in the islands of Mutsu, Echigo, Ugo, and[101] Nato, are irregular lenticular bodies of rather pure manganese oxides that occur more or less parallel to the bedding of metamorphosed Paleozoic sediments. Below water level, the oxides grade into rhodonite and are probably derived from this mineral. The ore bodies are not large, but many are known and they are the source of a small but regular production. In the islands of Mutsu, Nogo, Hokkaido and Ugo many irregular but locally large deposits of manganese oxides are associated with highly altered volcanic tuffs and flows of Tertiary age. Most of the deposits in Japan seem to be owned by natives in small holdings. The maximum production of 18,076 tons is reported for 1913, but since 1900 the range has usually been from 5,000 to 15,000 tons.
In the Philippine Islands, manganese occurs on the islands of Ilocos Norte, Masbate, Bulacan, Pangasinan and Tarloc, largely as veinlets and boulders of oxides in weathered igneous rock. On Ilocos Norte a maximum production of 3,000 tons was attained in 1916.
—On the Gold Coast of West Africa (a British colony), near Dagwin, are several deposits of manganese, the largest being 400 feet long and 70 feet wide. The concession is owned by a British exploration company. The deposit was discovered in 1914; and from the beginning of exploration in 1916 up to November 7, 1917, 28,465 tons had been shipped to England.
In the Belgian Congo, there is manganese ore in the valley of the Upper Fungwe River, and in southern Katanga. The deposits are too remote from the ocean to justify exploration, but are reported to be large. In the Union of South Africa (British) several manganese deposits are found along the sea coast, within 30 miles east and west of Capetown. Of the seven known deposits the largest is estimated to contain 15,000 tons. In Egypt, in the Sinai peninsula, are large manganiferous iron deposits as well as small manganese deposits, but none has been exploited. In Tunis, there are deposits reported to contain 4,000,000 to 5,000,000 tons of manganiferous iron ore, and also several manganese deposits which yielded 5,800 metric tons of manganese ore in 1917.
Although the known deposits of manganese in Africa are few and relatively unimportant, the continent offers an unusual prospect for the discovery of deposits that will contribute largely to the world’s supply. Inasmuch as the moist tropical climate of large areas favors extraordinary rock decay and surface concentration of manganese oxides, exploration will probably show the presence of many deposits, and where bedrock geological conditions are favorable, large bodies may be found.
—In Australia, there are deposits of manganese ore in New South Wales, in Queensland, in South Australia, and in Victoria.
In New Zealand, deposits of manganese ore occur in the Thames district, Auckland.
Deposits of manganese ore are known in Borneo, at Maruda Bay.
[102]
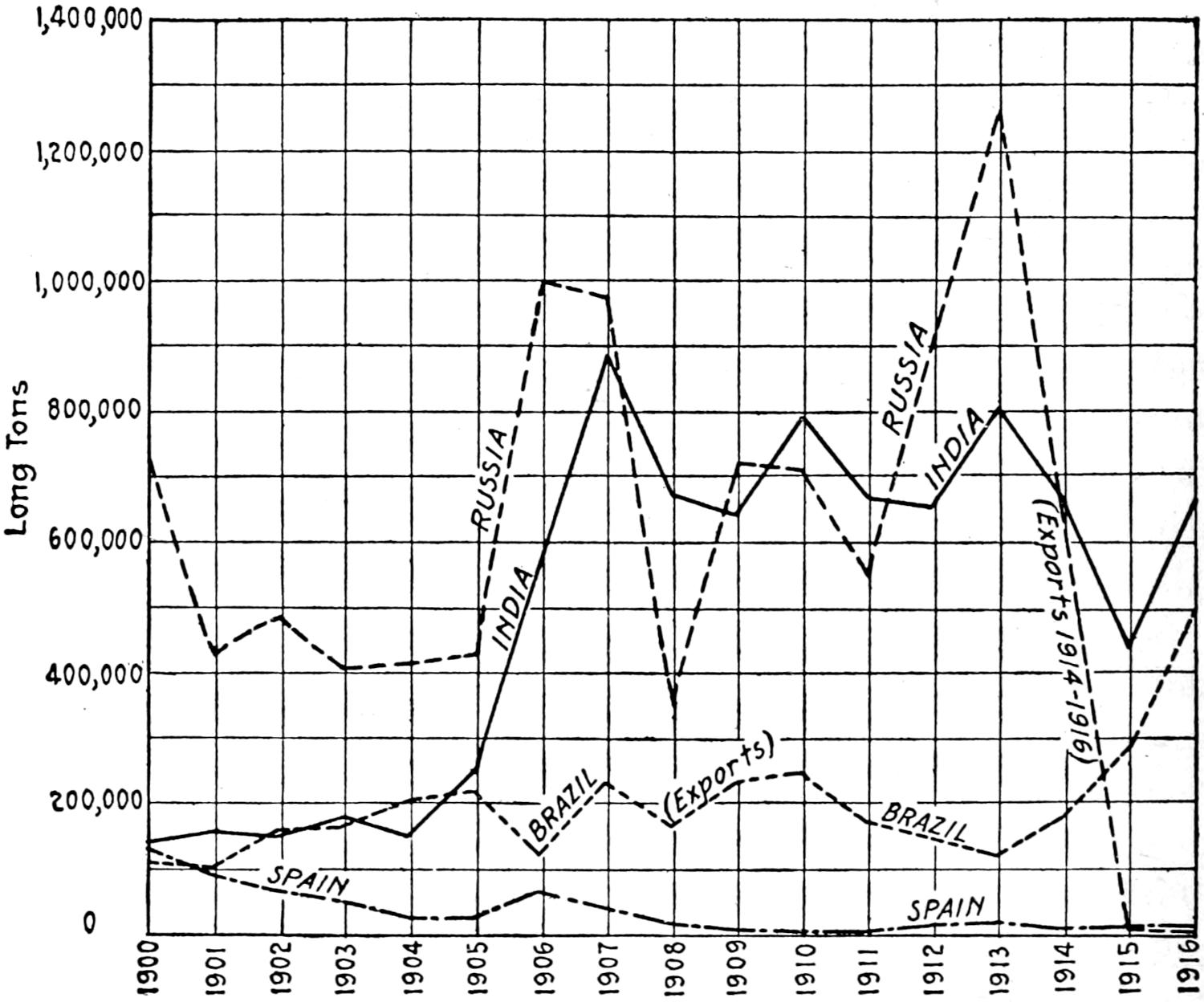
Fig. 5.—Annual output of manganese ore in chief producing countries.
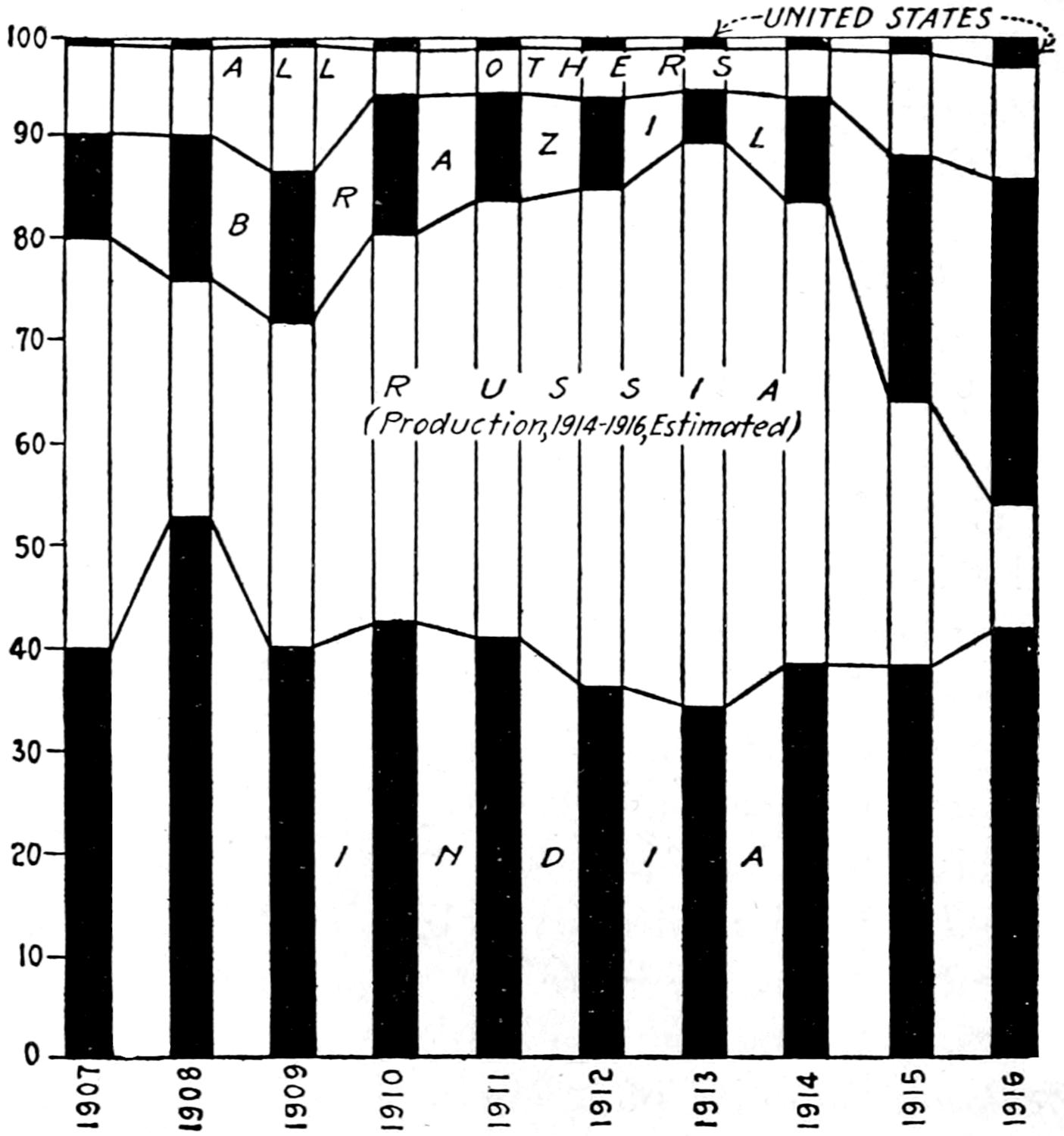
Fig. 6.—Percentage of manganese ore produced by chief producing countries.
[103]
It is reported that large deposits of high-grade ore have recently been discovered in Java.
Some statistics of the production of manganese are shown graphically in figures 5 and 6.
The known manganese resources of Russia, India, and Brazil are so large and readily available for exploitation and marketing that there is little prospect of their being displaced as the chief sources of the world’s supply for many years. Since the war began, in 1914, several important new districts have been discovered and brought to the producing stage, notably the Gold Coast of West Africa, western Costa Rica, and Java. Although the Javan deposits are reported to be large and may become an important factor in the world’s trade, all that is known concerning the other deposits does not hold out much hope that they can compete with the established sources.
There appears to be a fair chance that the equatorial belt as well as several other parts of the earth may yield additional important manganese deposits.
The distribution of the manganese deposits of the world is shown in Plate III.
The table of production for 1913 on page 105 shows the part of the total that each country contributed and the known extent of commercial control.
In contrast with deposits of several other important minerals, most of the manganese deposits throughout the world are owned by natives or residents of the respective countries in which they are found.
German companies have acquired tracts in the Chiaturi and Nicopol districts, Russia, and Queluz (Lafayette) district, Brazil. It appears that although one of these companies produces a little ore, the main purpose was to stabilize an unorganized industry by financial assistance.
In India it is difficult to distinguish between those companies composed of resident English and native Indians which were formed to exploit mines for profit and those composed of absentee English who desire to secure a supply of ore for English or other consumption. There seems to be no English capital in Brazil or Russia.
One French company owned two shipping mines in India in 1907, but there is no record of operations in 1913. French capital is interested in several companies busy in the Nicopol district, Russia. A Belgian company operates one mine in the Queluz district, Brazil.
[104]
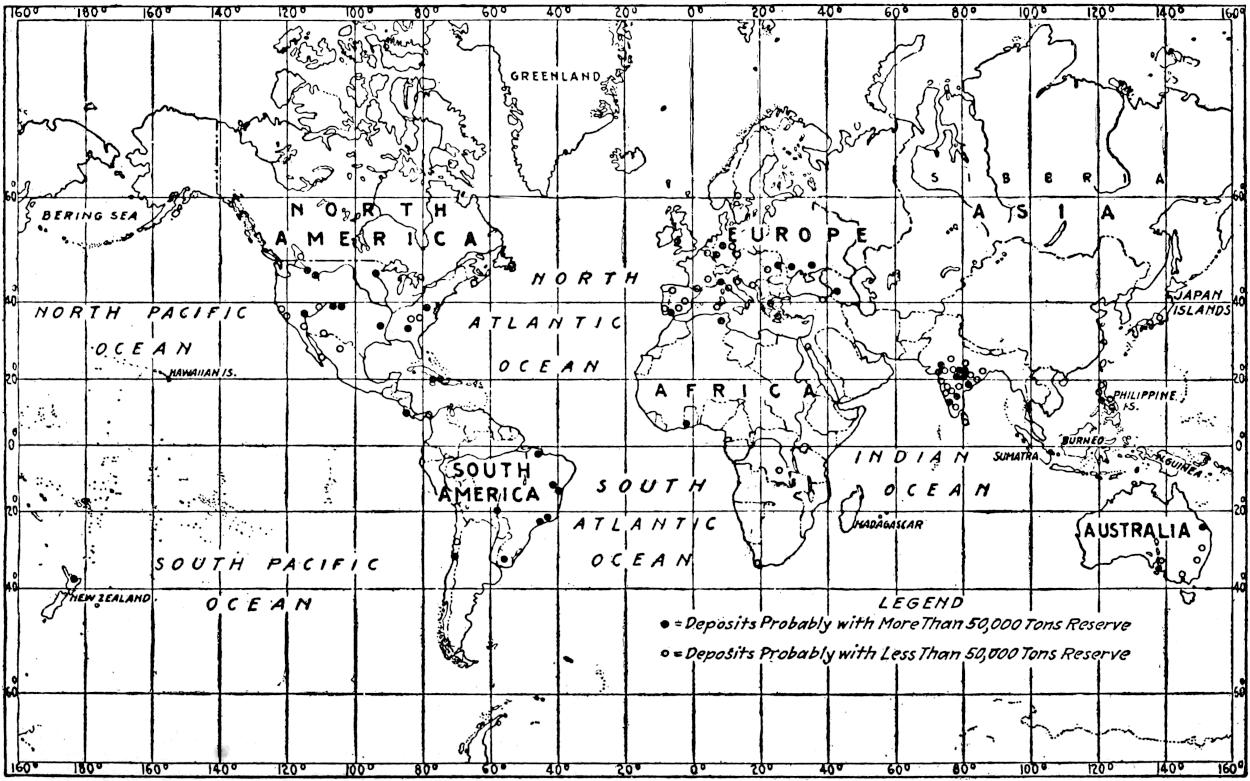
Plate III.—Geographical distribution of the principal manganese deposits of the world. By D. F. Hewett.
[105]
Table 24.—Production and Commercial Control of Manganese
| Country | Long tons | Manganese content (per cent.) |
Per cent. of total production |
Commercial control (estimated per cent.) |
||
|---|---|---|---|---|---|---|
| North America | ||||||
| United States | 4,048 | 40+ | 0.16 | United States, 100 | ||
| South America | ||||||
| Brazil | 120,368 | 38-48 | 5.10 | - | Brazil, 80 | |
| Belgium, 5 | ||||||
| Germany, 15 | ||||||
| Europe | ||||||
| Austria-Hungary | 34,986 | ? | 1.5 | Austria-Hungary, 100 | ||
| Bosnia-Herzegovina | 5,709 | ? | 0.2 | ? | ||
| France | 7,610 | 30+ | 0.3 | France, 100 | ||
| Germany | 748 | 30+ | 0.03 | Germany, 100 | ||
| Italy | 1,596 | 18-45 | 0.07 | Italy, 100 | ||
| Russia | 1,289,370 | 41-48 | 55.4 | - | Russia, 65 | |
| Germany, 30 | ||||||
| France, 5 | ||||||
| Spain | 21,254 | 29+ | 0.9 | ? | ||
| Sweden | 3,938 | ? | 0.15 | ? | ||
| United Kingdom | 5,393 | 30+ | 0.21 | England, 100 | ||
| Asia | ||||||
| India | 815,047 | 42-54 | 35.0 | - | English and native, 90 | |
| United States, 10 | ||||||
| Japan | 18,516 | ? | 0.8 | Japan, 100 | ||
| Oceania | ||||||
| Australia | 27 | ? | ||||
| Total | 2,328,110 | |||||
The Carnegie Steel Co., of Pittsburgh, Pa., U. S. A., owns and works several deposits in India. Although Americans own deposits in the Nazareth district, Brazil, in Panama, and Costa Rica, and some of those of Cuba, there is no record of American ownership of any of the most important deposits of Brazil, nor of any in Russia.
From 1902 to 1914, about half the ferromanganese used in the United States was made in this country from foreign ore and half was imported from England, where it was made from imported ore. This tendency arises out of the limitations of blast-furnace smelting of the alloy and the difference between the cost of labor in the United States and in England, but does not represent definite control, for ferromanganese may be made in any modern blast furnace used to make pig iron. In order to smelt with maximum efficiency in making ferromanganese, however, a blast furnace should run continuously for long periods, and therefore make 20,000 to 35,000 tons of alloy annually. Although it is possible to pass without interruption from making ferromanganese to spiegeleisen and then to pig iron, the change causes losses. Small steel works in the[106] United States therefore find it more advantageous to purchase imported ferromanganese than to make what they need.
—Although from 1885 to 1890, deposits in the United States supplied half or more of the needed high-grade manganese ore, from 1890 to 1916 the domestic production rather steadily declined to a negligible minimum, while imports of foreign ore and ferromanganese steadily rose in accord with the rate of total steel production. On the other hand, during the period ending about 1908, when the rate of manufacture of steel by the Bessemer process (in which spiegeleisen is largely used) exceeded that by the open-hearth process, the annual domestic contribution of spiegeleisen largely made from domestic ores greatly exceeded the imports. In advance, therefore, of the exploitation of the large deposits of low-grade ores of Minnesota, which have been the source of most of the production since 1916, the United States demonstrated independence of foreign supplies of low-grade ore and alloys.
The experience and information gained during the war, largely during 1918, show conclusively, first, with respect to metallurgy, that 20 to 30 per cent. spiegeleisen, as well as 60 to 70 per cent. ferromanganese, instead of 80 per cent., may be used to make satisfactory grades of open-hearth steel without appreciably sacrificing rate of production or quality of product; and second, with respect to ore production, that known domestic deposits can supply for at least five and probably ten or more years, much more low-grade ore than is needed to make spiegeleisen, and for at least five years and possibly ten years, about one-third the high-grade ore needed for the manufacture of alloy with 60 to 80 per cent. manganese. The reader should note, however, that capacity of mines to meet demand is in large measure determined by the prices offered for the product, which during 1918 were about five times those prevailing before the war. Beyond doubt, at pre-war prices, the United States can not supply more than several per cent. of the high-grade ore needed to make ferromanganese.
Citizens of the United States have not shown great interest in purchasing foreign deposits of manganese ore. With Cubans, they have controlled the mines yielding a large part of the Cuban output, and about 1907 one company, the Carnegie Steel Co., purchased several deposits in India. That company, however, seems to purchase ore, in addition to the output of its mines. Not until 1917 did Americans enter the Brazilian fields; then the largest deposits of the relatively unimportant Bahia district were purchased by a Philadelphia group.
—Before the war, England received about 50 per cent. of her manganese ore from India, 40 per cent. from Russia, 3 per cent. from[107] Brazil, and small quantities from Spain and Portuguese India. Some low-grade ore also came from Spain, Algeria, and Greece. Domestic production was scarcely 1 per cent. of imports. Exports of ferromanganese, largely to the United States, however, have been equivalent to 35 to 45 per cent. of the total imports of ore. Two effects of the war were to eliminate Russia as a source of ore, the deficit being made up from India, and greatly to curtail exports of ferromanganese. In contrast with the United States and Germany, Great Britain does not seem to contain deposits of low-grade ores capable of supplementing the needs of high-grade ore.
England controls fully 90 per cent. of the Indian output, probably through ownership by resident English and native Indians. On the other hand, England seems to have no control, direct or indirect, of the output of Brazil, Russia, or of other important contributions to supplies.
—Of the needed manganese ore, France imports from 35 to 45 per cent. from India, 40 to 55 per cent. from Russia, about 10 per cent. from Spain, and several per cent. from Brazil, and produces about 2 per cent. The domestic material, however, contains 30 per cent. or less manganese. In addition, France imports, as well as exports, a little ferromanganese from time to time.
So far as available data indicate, the French have made practically no foreign investments in manganese deposits, except in the Nicopol district, Russia. A company with a French name mined about 1,300 tons in India in 1907, out of a total of 899,055 long tons.
—Germany’s position with respect to manganese is very similar to that of the United States. For four years prior to 1914, Germany imported 48 to 68 per cent. of the total receipts from Russia, 25 to 35 from India, 3 to 7 from Brazil, and small quantities from Spain, Greece, and Sweden. Domestic production of ore with more than 30 per cent. manganese is negligible. Germany probably exports small quantities of ferromanganese to Sweden and other European countries, and from time to time has exported alloy to the United States.
Like the United States, however, Germany possesses extensive deposits of ferruginous manganese ore with 12 to 30 per cent. manganese; and from 1908 to 1913, produced 260,000 to 330,000 metric tons of such material, as well as 2,300,000 to 3,000,000 tons with 5 to 7 per cent. manganese. There can be little doubt that although Germany, through accumulated stocks of manganese ore and seizures in Belgium, possessed in 1914 at least two years’ supply, she was able to maintain a fairly constant rate of steel production for four years by adapting processes to economize high-grade ore and use low-grade.
Germans appear to have purchased manganese deposits in Russia and Brazil only, and these have yielded only a small part of the annual imports. In the Chiaturi district of Russia, however, where most of the[108] deposits are owned by natives, a German company, Gelsenkirchen Gesellschaft, reported to be a subsidiary of the Krupp company, was established about 1910, to purchase property as well as trade with and offer financial assistance to the producers. It is reported that this company alone exported about one-third of the output of the district. Germans are reported to own a part of one of several companies operating in the Nicopol district, Russia.
A review of the manganese ore industry, including features of the deposits, their geographic distribution and ownership, indicates several definite conclusions:
1. The surface outcrops of most manganese deposits give reliable information concerning the size and grade of the deposits, and the most desirable ore occurs in a surface zone scarcely 100 feet deep.
2. Except in Russia and Spain, the most productive mines are open-cuts, and mining is quickly and easily accomplished at minimum expense. Extensive operations in advance of production are rarely necessary.
3. The countries that possess the largest and richest deposits have an abundant and cheap labor supply.
4. The productive capacity of the known deposits so much exceeds the world’s demand for ore for steel making, that if any single source is temporarily eliminated, the demand can be wholly met by the remaining sources at prices that are only slightly higher than those previously prevailing.
5. The value of the material at the sources of production is relatively low among raw minerals, and ranges from one-third to one-eighth of the selling price at the points of consumption. It is evident that the cost of transportation represents a large part of the final price.
6. The working of most deposits yields so little profit, and therefore is so hazardous, that only a few foreigners own deposits in the chief producing countries.
7. The only case of commercial control, that of the Chiaturi district, Russia, by Germans, who offer the natural market, seems to have been established to counteract local political disorders rather than to eliminate competitive consumers.
8. No nation that contributes largely to the world’s steel production, except Russia, possesses domestic deposits of manganese ore sufficient to meet its needs, and all must import ore from rather remote sources. The United States and Germany, however, possess domestic deposits of ferruginous manganese ore that under great stress would probably permit independence of foreign sources.
[109]
Chromite is the principal ore from which metallic chromium and chromium products are obtained. The theoretical composition of chromite is represented by the formula FeO Cr2O3, which represents 32 per cent. ferrous oxide and 68 per cent. chromic oxide. In many ores, however, the ferrous oxide is partly replaced by magnesia, up to 30 per cent., and the chromic oxide by alumina and ferric oxide, up to 20 per cent. Thus the composition of chromite varies considerably. The percentage of chromic oxide may be as low as 10 per cent.; that of ferrous oxide may range from 10 to 50 per cent. Other common minerals of chromium are picotite (chrome spinel), uvarovite (chrome garnet), chrome diopside and crocoite (lead chromate).
Chrome ore is consumed mainly in the manufacture of special steels and in tanning leather. The special steels comprise chrome steel, chrome-nickel steel, chrome-tungsten steel, and chrome-vanadium steel. Metallic chromium is added to such steel in the form of ferrochrome, an alloy of chromium and iron containing 60 to 70 per cent. metallic chromium. Chrome steel is tough and hard to break. It hardens rapidly and has a fine grain and a fibrous fracture; it does not break readily upon concussion and because of its hardness is difficult or impossible to cut with ordinary machine tools. Metallic chromium is present in percentages varying from 1 to 5 per cent. Special steels containing chromium are used for guns, armor plate, armor-piercing projectiles, automobile parts, machine tools, bars for prisons, burglar-proof safes, shoes, cutlery, crusher jaws, stampmills, springs and for other articles in which hardness and toughness are necessary. During the war, when a considerable shortage of chromite threatened, less chromium was used in chrome steels, and certain other hardening materials were used in its place. The results are said to have been unsatisfactory, however.
Ferrochrome used in the manufacture of chrome steels is produced in the electric furnace by smelting a mixture, in proper proportions, of chromite, coal or coke, lime, and fluorspar or silica.
Much chrome ore is used in the steel industry for refractory materials in lining open-hearth furnaces. Some of the ore thus used is first manufactured[110] into chrome brick and some is utilized in the crude form. Chrome brick is used in open-hearth furnaces as a lining along the slag line between the magnesite bottom of the furnace and the silica-brick sides and roof. Chrome brick is used also to cover the ports of gas-fired furnaces. It is desirable for these purposes on account of its neutral reaction, which reduces the wear due to corrosion. Lump or crushed chromite is used for patching the bottoms of open-hearth furnaces, particularly the toe or apex of the bottom, the ore being either hammered in place as lump or crushed and mixed with a little water or tar and clay and then tamped into place. The use of lump chrome in repairing such furnaces is desirable on account of the rapidity with which the furnaces can be repaired and on account of the greater wear that chromite will stand. The amount of chromite used for this purpose ranges from 2 pounds to 10 pounds per ton of steel manufactured. Magnesite has been used to replace chromite for repairing furnaces, but has been found to be more expensive and to stand less wear.
Chrome brick is used in a minor way in electric furnaces for manufacturing steel, in a belt along the slag line and in the area around the pouring lips. It is also being used in furnaces manufacturing steel by the duplex process.
Chrome brick, besides being used in steel-making furnaces, is used in lining furnaces for making copper, nickel, and other metals. In these furnaces it is used in the bottoms and around the tap holes. Magnesite brick, as well as bauxite brick, have been used to replace chromite brick for this purpose.
Chromium chemicals used for tanning are mainly sodium or potassium bichromates. About half of the bichromates produced in the United States is commonly used for tanning, the remainder being used for paints, pigments, dry colors, and dyes in the paint, printing and engraving, and textile industries. Chrome yellow, chrome orange, and chrome black are used in calico printing and dyeing. Chromic oxide, or chrome green, is an indelible pigment employed in printing banknotes. Various other chrome colors are used for paints and pigments as well as in the ceramic arts. The minor uses of chrome chemicals are many.
Chromite throughout the world is associated with basic igneous rocks, such as peridotite or pyroxenite, or with the alteration products of these rocks, such as serpentine, talc schist, and related rocks. Chromite deposits are generally in the form of lenses, pods, or irregular masses that may occur singly or may be associated in groups. Besides being found as large bodies, chromite occurs as a minor constituent of these rocks, being widely disseminated through them as small specks and[111] particles. Chromite that forms workable deposits is believed to have been separated out of the molten mass of basic igneous rock by segregation and to have formed separate bodies within the rock mass during the cooling. Most chromite deposits are found along the borders of intrusive masses not far from the contact of older rocks into which they are intruded. This is probably due to the formation of peripheral fractures during the cooling of the igneous mass, chromite being forced up into these openings. The action of convection-currents in the molten magma may also have resulted in localizing chromite bodies near the borders of the mass. However, bodies of chromite are quite abundant in other parts of the igneous masses as well, often being found at long distances from bordering rocks.
By weathering of chromite-bearing igneous rock, chromite bodies are freed and occur as loose masses in resultant residual clays. Such bodies in clay are of commercial importance in many places. The breaking down of chromite-bearing rocks results in setting free disseminated specks and particles of chromite, and these may be transported and later deposited along streams flowing out of chromite-bearing areas. In this manner accumulations of chromite sands are formed. Besides chromite, these sands usually contain considerable quantities of other heavy minerals such as magnetite, ilmenite, garnet, and rutile, and the chromite in them is generally not available commercially.
The world’s chromite supply has been obtained mainly from the following sources, named roughly in order of their importance:
New Caledonia; southern Rhodesia; western and southern Asia Minor; Ural Mountains, Russia; eastern Greece, adjacent islands, Macedonia, and Serbia; Baluchistan and Mysore, India; Quebec, Canada; Atlantic and Pacific coast states, United States; State of Bahia, Brazil; Oriente, Cuba; Japan; Bosnia and Herzegovina; Austria-Hungary; and Guatemala.
The geographic distribution of the more important deposits of chromite is shown in Plate IV.
Other countries in which deposits of chromite are known but in which little or no ore has been produced are: Shetland Islands, Scotland; Norway; Sweden; Silesia; Portugal; New South Wales, Australia; New Zealand; Transvaal; Togoland; and Newfoundland.
Table 25 shows the output of chromite in the chief producing countries from 1905 to 1917.
—Important quantities of chromite occur on the island of New Caledonia, in the South Pacific, and in smaller amounts in Australia, New Zealand, and Tasmania.
[112]
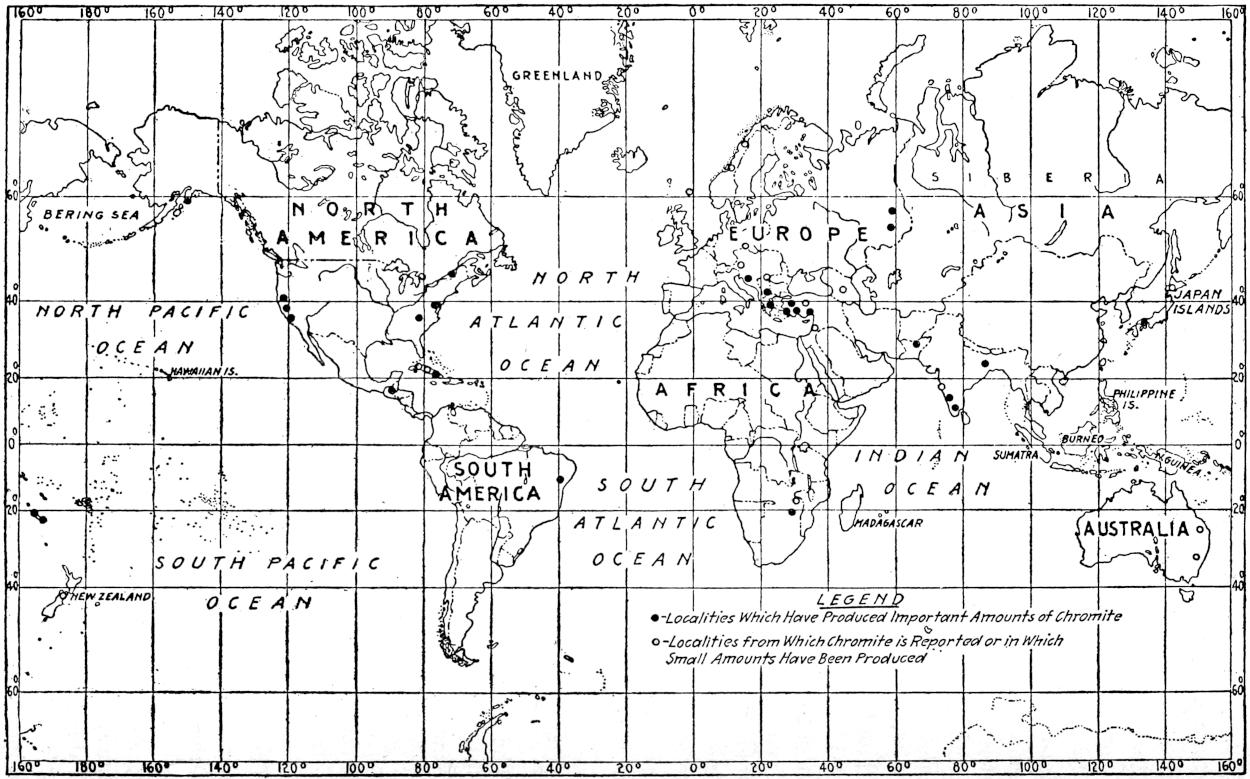
Plate IV.—Geographical distribution of the chromite deposits of the world. By E. C. Harder.
[113]
Table 25.—World’s Chromite Production 1905-1917 in Long Tons[53]
| 1905[54] | 1906 | 1907 | 1908 | 1909 | 1910 | 1911 | 1912 | 1913 | 1914 | 1915 | 1916 | 1917 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| United States | 22 | 107 | 290 | 359 | 598 | 205 | 120 | 201 | 255 | 591 | 3,281 | 47,035 | 43,725 | |||||||||||||
| Canada[55] | 7,657 | 8,068 | 6,425 | 6,451 | 2,205 | 267 | 140 | ... | ... | 121 | 11,008 | 24,545 | 32,457 | [56] | ||||||||||||
| Cuba[57] | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | 34 | 17 | [58] | ||||||||||||
| Guatemala[57] | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | 179 | [58] | ||||||||||||
| Brazil[57] | ||||||||||||||||||||||||||
| Great Britain (Shetland Islands) | ... | ... | ... | ... | ... | ... | ... | ... | ... | 100 | ||||||||||||||||
| Norway | ... | ... | 105 | [56] | ... | ... | ... | ... | 113 | [56] | ... | 80 | [56] | 344 | [56] | |||||||||||
| Sweden | ... | ... | ... | ... | ... | 31 | ||||||||||||||||||||
| Austria-Hungary (Bosnia and Herzegovina) | 183 | 315 | 305 | 492 | 327 | 315 | 246 | 197 | 300 | [56] | 474 | [56] | [59] | [59] | ||||||||||||
| Greece[60] | 8,759 | 11,348 | 11,545 | 4,281 | 9,448 | 9,311 | 4,542 | 6,209 | 6,242 | [56] | 6,947 | [56] | 10,255 | [56] | 9,724 | [56] | [59] | |||||||||
| Serbia[61] | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | [59] | [59] | [59] | |||||||||||||
| Russia | 26,620 | 16,708 | 25,940 | 10,777 | 21,857 | 14,157 | 1,218 | [56] | 20,934 | 21,401 | [59] | [59] | [59] | [59] | ||||||||||||
| Turkey[62] (mainly Asia Minor) | ... | 23,404 | 21,111 | 28,394 | 11,364 | [59] | 11,993 | 16,823 | [63] | [59] | [59] | [59] | [59] | [59] | ||||||||||||
| Rhodesia | ... | 3,256 | 8,017 | 11,927 | 22,875 | 39,287 | 46,753 | 61,840 | 62,365 | 48,235 | 60,617 | [56] | 79,350 | [56] | [59] | |||||||||||
| India | 2,708 | 4,375 | 7,274 | 4,745 | 9,250 | 1,737 | 3,804 | 2,890 | 5,580 | [56] | 5,887 | [56] | 3,768 | [56] | 20,160 | [56] | [59] | |||||||||
| Japan | ... | ... | ... | ... | ... | 2,091 | 1,500 | 1,591 | [59] | 2,075 | [56] | 2,932 | [56] | 8,149 | [56] | [59] | ||||||||||
| New South Wales | 52 | 15 | 30 | ... | ... | ... | 148 | 23 | ||||||||||||||||||
| New Caledonia | 75,717 | 82,910 | 56,461 | [56] | 24,970 | [56] | 39,368 | 39,368 | 34,447 | 41,325 | 62,352 | 41,336 | [56] | 65,941 | [56] | 74,115 | [64] | 41,230 | [56] | |||||||
[53] Figures not otherwise credited were obtained from “Mineral Resources of United States, 1914” and subsequent years, U. S. Geological Survey (figures originally obtained mainly from the British statistical publication, Mines and Quarries: General report with statistics, pt. 4, London).
[54] Figures for 1905 taken direct from Mines and Quarries, 1905 and 1906.
[55] Mineral production of Canada, 1916, Canada Department of Mines, Mines Branch.
[56] Mineral Industry, 1917.
[57] Exports from Cuba, Guatemala, and Brazil in 1918 were respectively 8,820 tons, 1,193 tons, and 17,854 tons.
[58] Exports.
[59] Not available.
[60] Production from the Saloniki district included under Turkey previous to 1914.
[61] Present Serbian deposits in Turkish domain before the war.
[62] Exports from Turkey for fiscal years beginning with March.
[63] Mines and Quarries reports production of Turkey in 1912 as 145 tons.
[64] Stateman’s Year Book, 1918, London.
[114]
Serpentine, in masses intruding metamorphic rocks and sediments ranging in age from Archean to Mesozoic, is abundant throughout the island of New Caledonia, and chromite invariably is associated with it as a minor constituent. Locally, large masses of fairly pure chromite are found, generally as lenticular bodies in the serpentine, but locally as irregular masses in residual clay derived from the decomposition of the serpentine.
Chromite deposits of three types are known in New Caledonia, as follows: Rock chrome, consisting of solid ore bodies in serpentine; residual chromite, as irregular bodies or scattered masses, frequently disintegrated, in red residual clay derived from the weathering of the serpentine; and chrome sand and gravel in surface wash and stream deposits. Ores of the first two classes are both important commercially in New Caledonia and are both present in most mines. Ores of the last named class are not worked.
Of these New Caledonian deposits, that of Mt. d’Or, in the southern part of the island, was the first to be discovered and worked, being found by Garnier in 1866. Later the deposits of Ngo Bay and others further south were exploited. Production and exportation of New Caledonian chromite began in the early 80’s and continued in a minor way until 1902, the production coming mainly from Mt. d’Or and the Ngo Bay deposits.
In 1902, L. Bernheim formed the Société le Chrome, in which French capital was largely interested. This company operated mines in the northern and southern parts of the island. It developed the Tiebaghi deposits near the port of Pagoumene, now among the most important in New Caledonia. The exportation of chromite soon increased, and gradually New Caledonian ore replaced Turkish ore in the market. In 1911 the Chrome Co., Ltd., of London, was formed and acquired from the Société le Chrome the Tiebaghi and other mines. At the same time it acquired the right from the Rhodesia Chrome Mines Co., Limited, to market the Rhodesian ore, thus securing practically a monopoly of the world’s chromite trade. The Chrome Co., Ltd., is controlled by French and English interests. Chalas & Sons, Finsbury Pavement House, London, E. C, are the largest stockholders. There were formerly German stockholders. L. Bernheim does not seem to be associated with the Chrome Co., Ltd., but it is said that he still owns chromite deposits in New Caledonia. Besides the operations of the Chrome Co., Ltd., there are a number of independent operations in different parts of the island. These are mainly under the control of inhabitants of the island.
—The important chromite deposits of southern Rhodesia are near the town of Selukwe, which is connected by railroad with the shipping port of Beira. They occur scattered through an area of schist and serpentine. One hundred and twenty chromite bodies have been mapped[115] out, but the only ones that have been developed are ten closely grouped large bodies at Chrome Mine northwest of Selukwe. One of the largest bodies is 180 feet wide and 240 feet long. The ores are high grade, averaging between 48 and 51 per cent. chromic oxide.
Recently considerable publicity has been given to the discovery of what are reported to be among the largest deposits of high-grade chromite in the world in the Umvukwe Hills in the Lomagundi district 30 miles from Banket Junction, southern Rhodesia. The ore is said to occur over a large area in bodies in serpentine. More than two million tons is reported to have been uncovered. The deposits were discovered by Albert Peake, of Umvukwe Ranch, and are owned by Peake Brothers, who are said to have offered them to the Imperial government on special terms.
Chromite was mined in Rhodesia for the first time in 1905, the ore coming from claims near Selukwe held by the Bechuanaland Exploration Company, Ltd. Production steadily increased, slowly at first but more rapidly after 1910, when the Selukwe mines were taken over by the Rhodesia Chrome Mines, Ltd. In 1910 the staking of chromite claims in the Hartley district was reported and in 1911 chromite was discovered at Victoria. Shipments from Selukwe stopped in August, 1914, after the declaration of war, but began in December and increased during 1915 and 1916. In 1917 the discovery of the large and valuable deposits of chromite in the Lomagundi district (already mentioned) was reported. Previous to 1916 the entire production of Rhodesian chromite came from the Selukwe mines of the Rhodesia Chrome Mines, Ltd. In 1917, however, another company, the Rhodesia Metals Syndicate, Ltd., entered the field and is producing important amounts of ore.
—Before Turkey lost most of her European possessions after the Balkan wars, the chromite deposits of the Kossowo, Uskub, and Monastir district of Serbia and the Saloniki district of Greece were within her borders. Now, however, only the deposits of Asia Minor remain to her.
Chromite deposits are widely scattered through many parts of Asia Minor and are said to be numbered by the hundreds. The most important deposits are grouped into three districts: In the regions of Brussa and Kutahia south of the Sea of Marmora, where the important Daghardi deposits are found; near Macri, Denislu and around the Gulf of Adalia in the southwestern part of the peninsula, as well as on the neighboring Island of Rhodes; and near Mersina, Adana, Aleppo, and elsewhere in the region around the Gulf of Alexandretta northeast of the Island of Cyprus. Smaller deposits are reported in the vilayets of Angora and Kastamuni in the north central part of Asia Minor and near Beirut and Damascus in Syria. All the ore bodies are found in more or less schistose and decomposed serpentine in groups of lenslike or irregular bodies.
The chromite mines of Asia Minor have produced important quantities[116] of ore. From about 1870, when Turkey began to supplant the United States as the world’s principal producer of chromite, to near 1900, Asia Minor furnished the bulk of the chromite for the world’s consumption. Most of the ore mined has come from the mines in the Brussa region on the south and southwest slopes of the Mysian Olympus and from the mines of the Macri region. The Brussa and Kutahia deposits are said to have produced an average of about 20,000 tons annually for many years, of which the Daghardi mine is said to have furnished nearly three-fourths. This deposit consists of high-grade ore averaging 51 to 55 per cent. chromic oxide and has been estimated to contain about 10,000,000 tons of ore. Probably this is somewhat an exaggeration, although doubtless the deposit is large and important.
The chromite mines in the Macri region have furnished a considerable part of the output of Asia Minor, but much of the ore mined in recent years has been of low grade, running as low as 40 per cent. chromic oxide. The chromite near Denislu and that near the Gulf of Adalia, on the other hand, is said to be very rich, some deposits containing ore averaging as high as 56 per cent. chromic oxide.
Most of the chromite mines of Asia Minor are probably now under the control of the Turkish government, having reverted back ten or fifteen years ago when increasing competition of New Caledonia chromite in foreign markets resulted in the shutting down of many of the mines. The taxes on both worked and undeveloped mineral properties are so heavy in Turkey that unless mines are bringing in continuous and substantial revenues, they cannot be held by private individuals.
It has been the policy of Turkey not to allow her mineral properties to fall into the hands of foreigners. Even while the exploitation of the chromite deposits was most vigorous, therefore, the mines, although in many places worked by foreign firms, were largely owned by the Turkish government or by Turkish subjects who leased them. Thus in 1904 the principal deposits near Brussa were owned by an officer of the Porte and were operated by J. W. Whittal & Co., an English firm in Constantinople, while other deposits in the same district were worked by Patterson & Canghellari, an English company located in Smyrna. The famous Daghardi deposit, in the Kutahia region, at that time was owned by the Turkish minister of marine and was operated by a Turk named Raghit Bey.
In the Macri district, a number of low-grade deposits were in 1904 under the control of Patterson & Co., of Smyrna, and the mines near the Gulf of Adalia were controlled by a French syndicate. Some large deposits near Denislu, in the interior, north of Macri, are said to be lying undeveloped owing to the refusal of the Turkish government to permit mining.
The chromite deposits in the region surrounding the Gulf of Alexandretta[117] have been worked in a small way both by Turks and foreigners. Among operators in the region are mentioned Durian Effendi, a Turk, representing the Ottoman Bank; Husni Herikizadeh Effendi, a Turk of Adana; Nader Brothers, of Mersina; Alfred Keun & Co. of Smyrna; Protopazzi Brothers, of Smyrna, and Mavrommati & Sons, of Mersina, both probably Greek firms; Loizides, of Mersina; and Hadji Kemal Bey, of Constantinople. Durian Effendi is mentioned also as having operated chromite mines near Beirut.
The most important chromite deposits of India are in the northern part of Baluchistan, but the mines of the Madras and Mysore districts in southern India have also furnished important amounts of ore. A small production has come from Bengal. The deposits of Baluchistan are large and the ore is rich, much of it averaging nearly 55 per cent. chromic oxide. One deposit is reported to be 440 feet long by 5 feet wide. The ore bodies are segregations in serpentine. The problem of transportation is difficult, as the ores are far from the coast and land transportation facilities are poor. Nevertheless, those mines have made a steady output since they were opened in 1903. The largest production was in 1907, when the yield exceeded 7,000 tons. Since then there has been a decrease owing to competition from New Caledonia and Rhodesia.
Chromite ore bodies were exploited in Madras as early as 1861 and small amounts of ore were mined intermittently. The ores are associated with magnesite veins in serpentine. Since 1907 a steady production is recorded from Madras, which has increased recently. Important deposits of chromite are found in Mysore. These deposits have produced more than 2,000 tons of ore annually in recent years, which is reported to have been sent to the United States. The chromite deposits of Bengal are said to be small and unimportant. In Bombay a large body of low-grade chromite is said to measure 1,000 by 300 feet and to average 34 per cent. chromic oxide.
Before 1910 chromite mining in Japan was sporadic and unimportant, but since 1910 the output has been steady and increasing. The principal occurrences of chrome ore are in the southwestern part, the mines of Wakamatsu, in Hoki, being the most important. The ore is said to average about 40 per cent. chromic oxide. Chromite is also reported to occur in the northern part of Japan. The Japanese chromite deposits are small and soon exhausted.
The principal chromite deposits of Russia are in the southern part of the Ural Mountains and are associated with serpentine and soapstone. The deposits are classed under three heads: Large granular masses in serpentine, finely disseminated chromite in serpentine, and chromite sand in platinum- and gold-bearing placers. The characteristic occurrence of chromite bodies in the Urals is as segregations within areas of dunite largely altered to serpentine. Platinum in scattered grains is[118] associated with chromite in the dunite in several places. Recently chromite deposits have been reported in the northern part of the Caucasus Mountains.
—The chromite deposits of the Balkan Peninsula may be grouped into four main districts: central Serbia; southern Serbia; Saloniki, in eastern Macedonia; and Magnesia, southern Thessaly, and the neighboring islands. The chromite deposits are found in serpentine derived from the alteration of peridotite.
The chromite mines of Serbia and Macedonia for many years furnished a small production, credited to European Turkey, which before the last Balkan War embraced all the chromite-bearing areas. Many of the deposits of central Serbia are poorly situated with reference to transportation, the ore being hauled on carts to the railroad stations and thence by rail to the coast. In eastern Macedonia some of the mines are relatively near the coast and the ore is carried on carts to Saloniki. The mines of Serbia and Macedonia were worked in part by individuals and in part by the same firms which mined the chrome ores of Asia Minor, such as Patterson & Co., Whittal & Co., and others.
The chromite mines of Magnesia, Thessaly, and adjacent islands in eastern Greece have furnished a more or less continuous output for the last 30 or 40 years. The ore mined, however, has been mainly of low grade, most of it averaging between 30 and 40 per cent. chromic oxide. It is said to be used largely for refractory purposes. Before 1908 most of the Grecian production of chromite came from the mines of Magnesia, but more recently the mines of southern Thessaly have furnished most of the ore. Mines in the Grecian Archipelago have also furnished some ore. The annual output of Greece has varied from a few hundred tons to more than 15,000 tons. During the past 20 years it has rarely fallen below 5,000 tons.
Chromiferous iron ore in considerable quantity is mined in Greece, most of it being exported. In 1913 ten or more mines were worked. The production has averaged more than 100,000 tons annually. The mines are operated in part by Greek and in part by French and British firms.
The chromic iron ores produced in the former Austrian Empire have come mainly from the central part of Bosnia, but the chromite mines in Upper Styria and those on the Roumanian border have also furnished an appreciable output, the ore being low-grade. The deposits of Bosnia are in serpentine. The ore is of good quality and has been mined for use as a furnace lining. On and near the left bank of the Danube River in the Banat, Hungary, there is an extensive area of serpentine containing chromite in bunches. The deposits have been worked to a slight extent.
—In Canada chromite-bearing areas of considerable extent are found in the southern part of the Province of Quebec, where nearly all the productive chromite mines of Canada are situated. The[119] ore occurs in serpentine in irregular masses and pockets without definite form, that range in size up to 75 feet along the longer axis, rarely reaching 100 feet. The amount of high-grade ore in the Quebec chromite deposits is not large, but low-grade ore bodies which under normal market conditions can not be mined at a profit are numerous and of large size. The low-grade ores range in content of chromic oxide from less than 20 per cent. to 35 or 40 per cent. Nearly all the Canadian ore mined has been exported to the United States. From 1910 to 1914 the output of chrome ore from the Quebec mines was insignificant, owing to the cost of mining low-grade ores and the lack of a market for them. When the price of chromite rose late in 1914, American firms began active developments in the field, and subsequently two concentrators were built by the Mutual Chemical Co. The output of Canadian chromite, both of crude ore and concentrates, during the war period was noteworthy, the production rising from 121 long tons in 1914, to 47,035 long tons in 1916 and 43,725 long tons in 1917.
The principal American firms interested in the development of the Canadian chromite deposits during the past few years have been the Mutual Chemical Co., the Harbison-Walker Refractories Co., the Electrometallurgical Co., and the Quebec Asbestos & Chrome Co. The last-named company purchased one of the concentrators built by the Mutual Chemical Co., and has furnished a considerable output both of crude ore and concentrates. Canadian firms have also produced considerable ore.
With the close of the war and the drop in the price of chromite, the Canadian mines have been largely abandoned. It is possible, however, that some American firms that mine ore for use in their own plants may continue work on a small scale.
When the United States was the world’s principal chromite-producing country, the output came from the eastern United States and principally from Maryland. The Wood chrome mine and neighboring deposits in Baltimore and Harford counties furnished most of the production. Smaller amounts were mined in North Carolina and Pennsylvania.
The principal chromite deposits of the United States, and those that have furnished nearly all the ore produced in recent years, are in California and Oregon. Recently deposits of some extent have been found in Montana, but these have not reached the producing stage. The chromite deposits of California are for the most part grouped into four principal districts, the Klamath Mountains region of northwestern California and southwestern Oregon; the Coast Range of west central California; the Sierra Nevada range throughout a considerable part of its length; and the San Luis Obispo district of southwestern California.
The chrome ores of California and Oregon form lenses or irregular bodies in serpentine and related rocks. Many of the ore bodies are found[120] in comparatively fresh peridotite and dunite, and the intimate relation between the chromite and the associated pyroxene or olivine is well shown. In places, also, chromite masses are found in the mantle of residual material derived from the alteration of serpentine and other rocks. Most of the chrome ores of the Pacific Coast are of low-grade, few running more than 45 per cent. chromic oxide. Concentrating plants have been built to beneficiate the ore from bodies large enough to warrant such expenditure. At some plants the grade of ore was thus raised to more than 52 per cent. Locally small bodies of high-grade ore have been found.
The chromite mines of the eastern United States were first worked about 1827 and continued to be operated for about forty years. The California deposits began to be developed about 1870, but never furnished a large output until the war raised the price of chromite to unprecedented figures and ore could be produced at a profit in spite of high costs and high freight rates to consuming centers. The chromite mines of the United States have always been worked and controlled by American capital. In California the ore has been mined mainly by private individuals working small scattered deposits. A few large firms, such as the California Chrome Co., the Adams & Maltby Co., L. H. Butcher Co., and the Union Chrome Co., worked on a larger scale during the war period.
Deposits of chromite have been known in Alaska for a number of years, but not until the war brought high prices was it possible to mine them at a profit. The deposits of present importance are near the southwestern end of the Kenai Peninsula. About 1,000 tons of ore containing 46 to 49 per cent. chromic oxide was mined in 1917.
—As far as is known, only one chromite-bearing district of importance occurs in South America, this being in the State of Bahia, in Brazil. One deposit has been worked at this locality by E. J. Lavino & Co., of Philadelphia, and the discovery of several neighboring deposits is reported. The first shipments of ore were made in February, 1918, and by July 1, 1918, 12,620 tons had been sent to the United States. The deposits are said to be owned by Newman & Co., a firm of American exporters in Bahia. They are leased to E. J. Lavino & Co., of Philadelphia.
South America, outside of Brazil, has no known chromite deposits of importance. In Colombia, chrome ore is reported to exist near Antioquia and chromiferous pig iron is said to have been produced by the blast furnace near Medellin. In Venezuela, chrome ore is said to occur on Coro Peninsula.
Important chromite deposits lie along the north coast of Cuba in the provinces of Camaguey and Oriente. A small deposit is found in the northwestern part of the Province of Matanzas. The most important deposits in Cuba are those at the Caledonia mine, south of the Bay of Nipe and northeast of the Bethlehem Steel Co.’s Mayari iron mines.[121] These deposits are estimated to contain about 40,000 tons of ore in sight. They are owned and worked by the Bethlehem Steel Co., which began exploitation in the spring of 1918. Shipments during 1918 amounted to 8,820 tons. Next in importance to the deposits at the Caledonia mine are those along the coast, northeast of Baracoa, known as the Cayoguan and Potosi deposits, where about 35,000 tons of ore is estimated to be in sight. The deposits are on the north edge of a rugged mountain range forming the eastern end of Cuba. The Cayoguan claims are owned by Brady interests, American, and the Potosi claims by the Harbison-Walker Refractories Co., of Philadelphia.
In the Province of Camaguey the deposits are found northeast of the town of Camaguey. They consist chiefly of masses of ore in residual clay and float on the surface. The underlying rock is serpentine. The Camaguey deposits are owned in part by Lehigh University and in part by Cubans. The estimated reserves are 20,000 tons.
Exploitation of the chromite deposits of Cuba began in the fall of 1917 and continued during the spring and summer of 1918. Only the Caledonia mine has produced ore. The chromite deposits are all associated with areas of serpentine.
Chromiferous iron ores that are destined to play an important part in the American iron and steel industry are found at Mayari, Camaguey, and Moa, along the northeastern coast of Cuba. The reserves are measured in hundreds of millions of tons. Only the Mayari deposits are mined at present.
Chromite deposits were developed in the interior of Guatemala in 1917 and shipments started in the autumn. The deposits were owned and operated by the International Railways of Central America, an American company, and are situated in the hills 100 miles inland from Puerto Barrios. The ore is in serpentine. It is very pure and is especially desirable for chemical purposes. The average chromic oxide content of the shipments during 1918 was 58 per cent., thus making this the highest grade ore that came to the American market. The ore was used by the Grasselli Chemical Co. Because of the distance from the railroad, these ores are very expensive to mine, and it was only on account of the high prices paid for chromite during the war that they were developed.
The country that leads in the manufacture of chromium products, such as ferrochrome and chrome chemicals, is the United States. In normal times the United States consumes more than one-third of the annual consumption of chromite by the world. In 1913 the chromite used by manufacturers of ferrochrome and chrome chemicals in the United States amounted to 65,000 tons. Owing to the war, the consumption increased[122] markedly until 1917, when it was nearly 130,000 tons. The normal consumption of chromite in England is about 25,000 tons and in France approximately 35,000 tons. These amounts have not greatly increased in recent years. Germany is an important producer of chromium products, normally consuming 30,000 tons of chromite annually. Russia and the former Austrian Empire used perhaps 5,000 tons each annually. Norway and Sweden use small amounts. Russia’s consumption has been mainly in the manufacture of chromium chemicals, and that of the former Austrian Empire was principally for refractory purposes. In Norway and Sweden small amounts of ferrochrome are produced.
—The United States, although the world’s largest consumer of chromite, is not an important producer of this mineral in normal times. During the 30 years preceding the war, the annual production never exceeded 4,000 long tons of crude ore, and during the last 15 years preceding the war the largest annual production was 598 long tons, in 1909. The production in 1913 was less than 1 per cent. of the domestic requirements.
The chromite supply of the United States has, therefore, come largely from foreign sources, and these sources have been mainly Asia Minor, Rhodesia, and New Caledonia. Before 1905 Turkey in Asia was the principal source of supply. Since then, however, Rhodesia and New Caledonia have largely replaced Turkey in the American chrome market.
Although numerous deposits of chromite occur in the western United States and locally in the eastern states, these deposits are usually small and scattered or of low grade. On account of their physical character, small size, scattered occurrence, or distance from consuming centers, domestic chromite could not be furnished to consumers in the required grade or for the price that chromite from rich foreign deposits could be furnished. For this reason the American chromite deposits remained undeveloped and no ore was mined except small quantities which were consumed for refractory purposes in neighboring metallurgical works.
When in 1914, at the beginning of the war, the price of chromite increased, production was immediately stimulated, this being shown by the rapid increase in output from 591 long tons of crude ore in 1914 to about 82,350 long tons of crude ore in 1918, equivalent to about 66,554 tons of ore on the basis of 50 per cent. chromic oxide. Even this largely increased domestic output, however, filled only little more than one-half of the American requirements, the total amount of chrome ore consumed in 1918 being about 104,000 long tons on the basis of 50 per cent. chromic oxide. Had the market for chromite kept up, however, the domestic mines would have supplied a much larger proportion of the requirements in 1919. The consumption of chromite in the United States in 1913 amounted to 65,000 tons of ore containing 50 per cent. chromic[123] oxide. From this it rose to about 127,000 tons in 1917, which represents the maximum annual consumption thus far.
The following table shows the production and imports of chromite on the basis of 50 per cent. chromic oxide, from 1913 to 1918, as well as the total quantity available for consumption for these years. No chromite is exported from the United States.
Table 26.—Production and Imports of Chromite, United States, 1913-1918
| Production (long tons) |
Imports (long tons) |
Total available for consumption (long tons) |
|
|---|---|---|---|
| 1913 | 230 | 65,180 | 65,410 |
| 1914 | 530 | 74,578 | 75,108 |
| 1915 | 2,756 | 73,762 | 76,318 |
| 1916 | 39,509 | 110,849 | 150,358 |
| 1917 | 36,729 | 64,978 | 101,707 |
| 1918 | 66,554 | 92,678 | 159,232 |
The prices paid for domestic chromite in the United States in recent years ranged from an average of $11.19 per ton in 1913 to an average of about $24.00 per ton in 1917.
When the need of increased shipping was felt in the latter part of 1917, steps were taken to reduce the imports of chromite from distant countries, such as Turkey, New Caledonia, and Rhodesia, to increase the imports from nearby sources such as Brazil, Cuba, and Canada, and to urge the maximum production from domestic mines. As a result, the imports of chromite from Brazil were 17,854 long tons of crude ore and from Cuba 8,821 long tons of crude ore in 1918. The only previous production in Cuba was 34 long tons in 1916 and 17 long tons in 1917. Brazil had no production before 1918. The imports from Canada amounted to 20,949 long tons of crude ore in 1918, as compared to 19,021 long tons of crude ore in 1917, and 12,220 long tons of crude ore in 1916. In order to reduce the importation of chromite from countries far overseas, various restrictions were put into effect by the War Trade Board in the early part of 1918.
The development of domestic chromite supplies means the depletion of limited resources, high cost of production, use of lower-grade ores, and lowered efficiency in consumption. With the free access of high-grade foreign ores, the market for domestic ores, therefore, disappears; and the domestic chromite-mining industry can not survive to any large extent. If world conservation of raw materials or the best use of the world’s resources is of chief importance, the domestic chromite-mining industry should be allowed to decline, and cheaper and higher-grade foreign ores should be allowed to replace domestic chromite. Experience during the[124] past few years has shown that the chromite deposits of the United States, supplemented by imports from Canada, Brazil, and Cuba, can largely supply the domestic requirements for a limited period.
The United States controls only a small part of the chromite reserves of the world. American firms own the principal Cuban deposits and control the Brazilian deposits through leases. The United States is, therefore, dependent to a large extent upon the good will of France and England for a continuous supply of chrome ore, these two countries jointly being largely in control of the chromite reserves of Rhodesia and New Caledonia. Turkey controls most, if not all, of the chromite deposits of Asia Minor, but because of their enclosed situation on the Mediterranean, these deposits could not be relied upon as a source of supply in time of need. The same is true of the deposits of the Ural region in Russia.
The chief chromite-consuming firms in the United States are the Electrometallurgical Co., probably the largest producer of ferrochrome in the world; the Mutual Chemical Co., and the National Electrolytic Co., large producers of chromium chemicals, and the Harbison-Walker Refractories Co., American Refractories Co., and various steel-making plants, users of chromite for refractory purposes.
—Before the war Great Britain consumed annually about 25,000 tons of chromite, most of it being used by Blackwell & Sons, Ltd., for the manufacture of ferrochrome. The ferrochrome made in England, however, is not sufficient to supply the needs of the British steel industry, and much is imported from France.
Except for unimportant occurrences in the Shetland Islands there are no chromite deposits in the British Isles, and Great Britain is therefore dependent entirely upon overseas sources of supply. British colonies, on the other hand, are rich in chromite deposits, and as long as British ships have freedom of movement on the ocean, they will have access to the more important chromite deposits of the world.
Owing to their richness and large size, the most important of all the British-controlled deposits are those of Rhodesia. Only one of many deposits in this area is being operated, and the reserves in untouched ore bodies are undoubtedly large, comparable perhaps with those of New Caledonia.
The production of the Rhodesian chrome mines has in recent years averaged in the neighborhood of 60,000 tons annually or about 35 per cent. of the world’s production, and doubtless the output could be very greatly increased if other known deposits were developed. However, even the present production is more than twice the actual chromite requirements of Great Britain. These requirements, however, do not represent the needs for metallic chromium or chromium compounds, and if France should cease to supply Great Britain with ferrochrome, a much[125] larger amount of the raw material, chromite, would be necessary for the English steel industry.
Besides the chromite deposits of South Africa, chromite deposits of importance are found in other British colonies, notably in British India and Canada. Amounts of chromite varying from 2,000 tons to 10,000 tons have been produced annually in British India for many years, coming mainly from Baluchistan, in the northwestern part, but a small production has come also from Mysore, in the southern part. In early years, India furnished a more important part of the world’s supply of chromite than at present. Transportation is a serious difficulty in the mining of these deposits and when New Caledonian and Rhodesian ores became developed, the Indian ores dropped in importance.
The Canadian deposits, while of considerable extent, and having ready accessibility to eastern American markets, have not been extensively or continuously mined, on account of their low-grade character. By concentration, a medium high-grade product can be obtained, but concentration methods are expensive and bring the cost of the material up to such an extent that it can not compete with other ores now on the market. Thus, the cost of producing both Indian and Canadian ores is such that under normal conditions it is difficult to find a market for them, but in case of necessity, a considerable tonnage can be supplied from these sources.
Other British colonies in which deposits of chromite exist are New South Wales, Tasmania, and New Zealand. As far as known, the deposits in these countries are small and only those of the first have furnished a small production.
Besides controlling chromite deposits in many parts of the world through her colonial possessions, Great Britain controls deposits through British firms with foreign possessions. Thus, the Chrome Co., Ltd., of mixed British and French interests, controls not only the Rhodesian chromite output but also owns and controls most of the important New Caledonian mines.
Great Britain has in the past received most of her supplies of chromite from Rhodesia, New Caledonia, and Turkey. Although more than enough chromite is produced in Rhodesia to supply the British needs, England has allowed most of the Rhodesian ore to be exported to other countries and has imported foreign ore for part of her own needs. Probably the largest part of the Rhodesian output before the war went to the United States. Much of the ore consumed in England has come from Turkey, where English firms have been interested in chromite mining for many years. Most of the Indian output probably has been used in Great Britain, but a part has gone to France.
—A few deposits of chromite are known in France, but they are of no importance commercially. France, therefore, like England, is[126] entirely dependent upon overseas sources for her chromite supply. Unlike England, however, France has only one colony, New Caledonia, containing important chromite deposits; but luckily this colony contains enough to make it one of the world’s principal sources of chromite.
Although the Chrome Co., Ltd., which controls the principal New Caledonian chromite deposits and is the largest shipper, represents both English and French capital, France through political means can control the output of chromite from the island. While in the past probably the major part of the chromite used in France has come from this source, France has also used Rhodesian and Turkish ores and probably Russian ores to a considerable extent, and much New Caledonian ore has gone to Germany, the United States and Great Britain.
The principal French firms manufacturing ferrochrome are the Société Electrometallurgique Française, at La Praz; Société La New Metallurgie, at Giffre; Société Anonyme Electrometallurgique, at Albertville; Keller, Leleux et Cie, at Livet; Société Electrometallurgique de Saint Beron, at Saint Beron; Ch. Betrolus, at Bellegarde; and Rochette Frères, at Epierre.
—Except for unimportant low-grade deposits in Silesia, Germany has no chromite supplies within her borders. As a user of chromite Germany ranks in importance with France, most of the ore consumed being used in the manufacture of ferrochrome, the principal manufacturers of which have been the Krupp works. Chrome chemicals are also made in abundance, however. None of Germany’s former colonies is known to have chromite deposits except Togoland, and the Togoland deposits are undeveloped and are believed to be unimportant.
In the past, Germany has received chromite from New Caledonia, Rhodesia, Turkey, Greece, and probably Russia. Because of the long rail haul from Russia and the poor state of development of the industry in Turkey, the ores from these two countries were, in the years immediately preceding the war, being largely replaced by ores from overseas. The four large chromite-consuming countries have, therefore, all been looking mainly to New Caledonia and Rhodesia for their sources of supply.
During the war, when overseas chromite was not available, Germany was enabled by her relations with Turkey to obtain chromite from Asia Minor, and probably from chromite mines in Serbia, Hungary, Bosnia, and Herzegovina. Thus in time of need, when the usual overseas sources of supply were cut off, Germany, through her relations with neighboring countries, was able to obtain by land sufficient chromite to supply her ordnance requirements. Had the war continued Germany would doubtless have developed the chromite resources of the Urals, and those, together with the deposits of Asia Minor, even in their present state of development, could have kept Germany supplied indefinitely.
It is probable that German control became important in the mines of Asia Minor during the war. By relatively small improvements in transportation[127] facilities, such as building branch railroad lines to the principal deposits, the chromite mines of Asia Minor might be rejuvenated to such an extent as to enable the ores to compete with Rhodesian and New Caledonian ore and to place them again among the world’s large producers. The deposits are large and the reserves rank in importance with those of Rhodesia and New Caledonia.
—Russia is independent as far as her requirements of chromite are concerned. Out of her production of about 20,000 tons annually, the domestic industry consumes less than one-fourth and the rest is available for export to nations less favored with chromite resources.
Most of the chromite used in Russia goes into the manufacture of chrome chemicals. The Russian bichromate works at Elabouga, east of Kazan, established in 1892, have consumed about 2,000 tons of ore annually.
The political and commercial control of the principal chromite deposits of the world is summarized in the following table:
Table 27.—Political and Commercial Control of Chromite Deposits
| Country | Political control |
Predominant commercial control |
|---|---|---|
| New Caledonia | French | French-British |
| Rhodesia | British | British |
| Asia Minor | Turkish | Turkish |
| Ural Mountains, Russia | Russian | Russian-British |
| Greece | Grecian | Uncertain |
| Serbia | Serbian | Uncertain |
| India | British | Probably British |
| Canada | British | United States-Canadian |
| United States | United States | United States |
| Brazil | Brazilian | United States |
| Cuba | Cuban | United States |
| Japan | Japanese | Japanese |
| Austria-Hungary | Austrian | Uncertain |
| Guatemala | Guatemalan | United States |
Great Britain and France produce in their colonial possessions chromite enough for their own needs and for export, but the United States, the world’s largest consumer, must depend, except in time of extreme emergency, upon imports, mainly from New Caledonia and Rhodesia.
Various countries have from time to time played important rôles in supplying the world’s demand for chromite. One country after another has been displaced, as cheaper or better grades of chromite came into the market. At times these changes were caused by the finding of larger bodies of higher-grade ores than had previously been mined, and at other times they were caused by cheaply transported ores replacing ores inconveniently situated with reference to centers of consumption.
[128]
Thus, at the beginning of the nineteenth century and up to about 1830, the Ural Mountain region of Russia supplied the major part of the world’s requirements for chromite, which were small. About 1830, important discoveries of chromite were made in the eastern United States, particularly in Maryland, and the United States soon displaced Russia as the leading producer of chromite. After this, for many years, the United States continued to lead in chromite production until about 1870, when the domestic deposits gradually approached exhaustion and important deposits discovered in European Turkey and Asia Minor began to be extensively developed.
Asia Minor was the principal chromite-producing country from about 1870 until about the beginning of the twentieth century. The deposits continued to be worked fairly steadily on account of their richness and large size, although they were inconveniently situated with reference to transportation. Only certain deposits, such as those in the Macri and Alexandretta regions, were so near to the coast that the ore could be carried on muleback or by camel to the shipping ports. From the larger deposits, such as Daghardi, the ore had to be transported by animals to railroad stations, often a distance of many miles, and then by rail to the coast. Because of these difficulties, Turkish ore was largely replaced by ore from the important New Caledonian and Rhodesian deposits as soon as those were developed.
In New Caledonia labor is cheap and the deposits are all near the coast. By means of sailing vessels and tramp steamers which charge low rates and often require such heavy materials as chromite for ballast, this ore can be delivered to points of consumption at a relatively small cost. The Rhodesian chromite deposits are not as accessible from the coast, but they are large and rich and the rail freight rates to the shipping port are exceptionally low. For this reason, Rhodesian ore has been able to successfully compete in the market with New Caledonian ore, and these two sources have, therefore, in recent years, jointly supplied most of the requirements of the principal nations for chromite.
During the war, because of abnormal conditions arising from the shortage of shipping facilities, there was considerable uncertainty as to whether it would be possible to supply American consumers of chromite with imported ores. Under the stimulus of much higher prices, the production of chromite in the United States increased from 255 long tons of crude chromite in 1913, to about 82,350 tons in 1918, mainly from California, Washington and Oregon. This production was larger than the annual production of crude ore from any single source (except 82,910 tons from New Caledonia in 1906) since the chromite-mining industry began. This production, of course, could not be continued without a rapid depletion of American chromite reserves, and does not represent a normal development.
[129]
Nickel is used chiefly in the manufacture of special steels. It is estimated[65] that about 60 per cent. of the entire nickel production goes into steel making in normal times and more under war conditions. Nickel steels are the most important of all the alloy steels and are the most used.[66] They are used where unusual tensile strength is required. Nickel-chromium steels are used in the manufacture of armor plate.
[65] Report of the Royal Ontario Nickel Commission, 1917, p. 300.
[66] Stoughton, Bradley: “The Metallurgy of Iron and Steel,” 1911.
Ordinary nickel steels commonly carry about 3¹⁄₂ per cent. nickel. “Highly nickeliferous steels carrying up to 40 per cent. nickel are used for special purposes where non-magnetic qualities, resistance to corrosion and, above all, no expansion or contraction, or any desired expansion or contraction, with change of temperature, is important.”[67]
[67] Report of Royal Ontario Nickel Commission, 1917, p. 301.
Non-ferrous nickel alloys have found extensive uses, and probably about 20 per cent. of the world’s production of nickel goes into them. By far the most important are those formed from nickel and copper. One, generally known as “cupro-nickel,” contains approximately 80 per cent. copper and 20 per cent. nickel and is used largely for bullet jackets and other munition purposes. Another, which has become well known under the trade name “Monel metal,” contains 29 per cent. copper, 67 per cent. nickel, about 2.5 per cent. iron, and a small amount of manganese. It is manufactured direct from the Sudbury matte. Monel metal is used chiefly where a strong non-corroding metal is needed, as, notably, in ship propellers.
It is estimated that about 2 per cent. of the nickel production is used for electro-plating. To a large extent nickel plating prevents corrosion of the metal plated.
Nickel is also used for storage batteries; for coinage; as a catalyser in the hardening of oils or fats (solid fats being used for soap making); for cooking utensils; and as a pigment for coloring ceramic ware. It is doubtful whether these last-named uses would take more than 3 per cent. of the world’s nickel production.
Copper gives to steel properties somewhat similar to those given[130] by nickel, notably increasing the tensile strength. Silicon also imparts similar properties to steel, increasing especially the toughness and tensile strength. A considerable proportion of chromium makes steel highly non-corroding. There is, however, nothing known that will take the place of nickel in special steels having a zero or a predetermined coefficient of expansion, such as “invar” or “platinite.”
Nickel possesses extraordinary power in giving its white color to alloys of copper; hence its use in coinage. The other white metals that might be used in its place are less plentiful and more expensive. There is no other abundant metal or alloy having the good color, great strength and resistance to corrosion possessed by Monel metal and like alloys of nickel and copper.
Nickel salts are used as substitutes for metals of the platinum group as catalysts in hardening oils and fats.
Production of nickel on a fairly large scale did not begin until the mines of New Caledonia were opened in 1875. To handle these ores, smelters and refineries were built in England and continental Europe. As the ores are of the silicate type and free from copper, they were easily treated by common processes of smelting with fluxes to get rid of the gangue and then heating with charcoal for reduction to a metallic condition. A pure metal was obtained which found a good market.
Production of nickel-copper ores in the Sudbury district began in 1887. On account of the copper with the nickel in these ores, new processes of refining had to be worked out and the trade prejudice against the product had to be overcome. This took persistent effort for several years. Only one of the first companies to operate in the Sudbury district has survived to the present time. This is the Canadian Copper Co., the producing subsidiary of the International Nickel Co.
Three new refining processes have been developed to handle nickel-copper ores. The Orford Copper Co. (subsidiary of the International company) uses the salt cake process. This process is one of fusion with sodium sulphide, the copper sulphide concentrating on top and the nickel sulphides at the bottom of the melt. Repeated fusion of the “tops” and “bottoms” effects a good separation.
The Mond company had a process before it had any mines. This process depends upon volatilization of the nickel by passing carbon monoxide over the matte, which has been previously roasted and leached of its copper content. Nickel carbonyl, Ni(CO)4, is formed and metallic nickel is thrown down by decomposition of this product, so that the nickel obtained is pure.
The Hybinette process, a process depending on electrolysis, was first[131] worked out at the plant of the Orford Copper Co. on Sudbury matte. It was used for low-grade ores at Fredericktown, Mo., and then later for the Norwegian ores at Kristiansand. It is to be used in the new refinery of the British-American Co. in Ontario.
Monel metal is made directly from Sudbury matte with the removal of a little of the copper. It is known as a natural alloy, in contrast to one made by combining the pure metals.
Increased use of nickeliferous iron ore for steel making will decrease the amount of nickel that will have to be added in making such steel. This will probably be overbalanced by the increased use of such steels.
Analyses of igneous rocks indicate that nickel is present in the average igneous rock to the extent of 0.020[68] per cent. Of its occurrence, Clarke says:[69] “Very frequently detected in igneous rocks, probably as a constituent of olivine. * * * The presence of nickel is especially characteristic of magnesian igneous rocks, and it is generally associated in them with chromium.” Regarding copper he says: “Minute traces of this metal are often detected in igneous rocks, although they are rarely determined quantitatively.” It is estimated[70] that copper is present in the average igneous rock to the proportion of about 0.010 per cent., or only about half that of nickel, and that zinc and lead are present in even smaller proportion. Notwithstanding this greater abundance of nickel, the workable deposits of copper, lead, and zinc are much more widespread and production is correspondingly greater. “It can thus be said that nickel is less amenable to concentration by the agencies that tend to produce workable deposits than are the other metals mentioned (copper, lead and zinc).”[71]
[68] F. W. Clarke, “The Data of Geochemistry,” Bull. 616, U. S. Geological Survey, 1916, p. 27.
[69] Ibid., p. 18.
[70] Report of Royal Ontario Nickel Commission, 1917, p. 95.
[71] Ibid.
One would infer from the above that nickel deposits of consequence would most likely occur in connection with igneous rocks, especially those that are basic. This is indeed true; in fact, the only known nickel deposits of commercial or prospective commercial value occur in association with basic igneous rocks. The nickeliferous metallographic provinces of the world may be said to lie within petrographic provinces. Harker says:[72] “An examination of the rocks belonging to one great period of igneous activity, and of their actual distribution, enables us to distinguish areas of greater or less extent, within which the rocks present a less or greater degree of consanguinity, the law being that more marked specialization goes with narrower localization.” On the[132] basis of this law and the fact noted by Clarke and mentioned above, nickeliferous metallographic provinces may be limited more closely than simply to areas of basic igneous rocks. They may be said to roughly coincide with areas of basic igneous rock characterized by minerals of the olivine group, these minerals having formed as a result of the high magnesium content of the rock magma.
[72] Harker, Alfred, “The Natural History of Igneous Rocks,” 1909, p. 89.
Nickel ores occur where there was an unusual segregation of the element in the igneous rock at the time of solidification. In some places further concentration by weathering has been necessary to make an ore of the rock. In still other places, the concentration by weathering has not been sufficient to make the nickel valuable in itself, but has raised the iron content of the rock, and with it the nickel content, to such an extent as to make the ore valuable in the first instance as an iron ore and of additional value because of its nickel content.
Nickel is unique among the less rare metals in that a single district contains a quantity of accessible, workable ore, far ahead of that in all other known deposits of the world combined. This is the Sudbury district of Ontario. The nickel ores there were segregated from an enormous mass of igneous rock intruded under conditions favorable for broad segregation and subsequently so eroded that large ore bodies are accessible.
In those nickel ores and nickeliferous iron ores where weathering has been a necessary agent in concentrating the nickel and iron enough to make them commercially valuable, the original deposits were nickeliferous igneous rocks—that is, they were similar to the rocks associated with the nickel deposits of the sulphide type, but contained much less nickel. The nickel-ore deposits of the New Caledonia region, which rank next in productiveness to those of the Sudbury district, are of this type. The Cuban nickeliferous iron ores best exemplify iron ore deposits of this type.
The nickeliferous ore deposits of the world may be divided into two main types—the sulphide type, in which weathering has not been of prime importance, and the garnierite or lateritic type, in which weathering has altered and concentrated the nickel as well as the chromium and iron of the original rock. In addition, nickel occurs in some places with ores of precious and semi-precious metals in veins. Nickel may be recovered from such ores as a by-product, but the ores are never mined primarily for their nickel content. The brief descriptions of the known deposits, grouped according to type, which follow, have been taken from the descriptions of the world’s nickel deposits in the report of the Royal Ontario Nickel Commission. A few direct quotations from the text of the report are given.
[133]
—The Sudbury district is situated in southeastern Ontario, Canada. In its broader outlines the geology of this district is relatively simple. An immense mass of nickeliferous rock was intruded as a “laccolithic sheet” or sill along an unconformable plane of contact between flat-lying sediments and an underlying complex of ancient rocks. During the intrusion and cooling, or perhaps soon thereafter, the underlying rocks of the central part of the laccolith, covering the reservoirs from which the magma came, subsided. Long periods of erosion then planed down the region until all that is left of the sill occupies a synclinal basin nearly 40 miles long and 10 to 15 miles wide. All of the rocks involved are of pre-Cambrian age.
The laccolithic sheet is approximately 10,000 feet thick. It differentiated on cooling into two kinds of rock—micropegmatite, a rock of the granite group, now forming the upper part of the sill, and norite, a gabbro rock, the lower part. The gradation between these two rocks is rather abrupt. At the bottom of the sill, in some places lying between the norite and the underlying rock and at other places entirely within the underlying rock, are bodies of ore consisting of pyrrhotite, pentlandite and chalcopyrite. These are segregated products of the norite, which in some places solidified at the base of the sill and in others were intruded as dikes in the underlying rocks or in previously solidified portions of the norite. They constitute commercial ore bodies where the sulphides form a preponderant part of the rock.
The ore bodies are classified as “marginal” and “offset.” The marginal deposits occur along the contact of the norite with the underlying rock. Frequently they lie entirely within the rocks adjacent to the norite. The offset deposits occur where faults cut across the limbs of the fold, forming zones of weakness into which ore or mineralized norite was intruded.
The nickel-bearing mineral is pentlandite; the copper-bearing, chalcopyrite. The other sulphide, pyrrhotite, is a sulphide of iron. The ores mined to date average roughly 3.5 per cent. nickel and 2 per cent. copper.
From the opening of the district in 1887 to the end of 1916 nearly ten and one-half million tons of Sudbury ore had been mined and smelted; and, from this, about 285,000 tons of nickel had been produced. It is estimated that there are probably fully 100,000,000 tons of ore reserves. Over a million and a half tons of ore were mined and smelted in 1916.
The Alexo Mine is situated 150 miles due north of Sudbury. The ore occurs at the contact of a large mass of peridotite (now altered to serpentine), with a pillow-lava which the peridotite intruded. The ore consists of sulphide minerals segregated from the intrusive mass. It is of two types, one, a massive, pure sulphide occupying cracks in[134] dike-like relationship, the other, disseminated sulphide in peridotite adjacent to the sulphide ore masses. The ore deposit has a proven length of 700 feet, has been opened to a depth of 120 feet, and drilling has shown ore to extend to a depth of 240 feet. The average width may be taken as approximately 10 feet. By the end of 1916, ore had been raised to the extent of 34,650 tons and more than that amount had been developed. About 12,000 tons of ore were shipped in 1915, averaging about 4.9 per cent. nickel and 0.6 per cent. copper. Several hundred thousand tons are probably available in this deposit.
The nickel ore deposits of Norway are similar mineralogically to those of Sudbury. The deposits are small, their metal content is low, and compared to the Sudbury and New Caledonia deposits they are of little consequence. Up to 1909 there had been mined and smelted in Norway about 400,000 tons of nickel ore. The hand-sorted ore carried 1.4 to 1.7 per cent. of nickel.
Deposits like those of Norway have been found in Sweden, but they have not been worked in recent years. There is evidently a nickeliferous metallographic province in the Scandinavian countries and important ore deposits may yet be found there.
In the United States, near Gap, Lancaster County, Pennsylvania, is a deposit of nickel ore of the sulphide type, which occurs as a segregation from a 300-ft. dike of amphibolite. It was worked spasmodically for copper throughout the 18th century. Nickel was discovered in the ore in 1852 and 4,000,000 pounds of nickel are estimated to have been produced up to 1882. The advent of New Caledonia and Sudbury ores caused the closing of the mine. The ore as mined carried 1 to 3 per cent. nickel and about one-third as much copper.
Near Julian, San Diego County, California, is a sulphide nickel deposit that has never been commercially productive. Assays show the ore to contain nearly 3 per cent. nickel or more.
A small deposit in Tasmania has produced a few thousand tons of rich ore. Diamond drilling has shown that little ore remains.
Nickel-copper sulphides have been found in connection with a large intrusive of basic igneous rock in the Insizwa Range, South Africa. No payable ore has been found.
Nickel sulphide deposits of unknown importance occur in India and in Southwestern China. Small deposits which were worked when nickel was scarce occur in Italy, Scotland, Germany and Austria.
—About one-third of the surface of the island of New Caledonia is occupied by serpentine, this being the weathered product of basic igneous rock. “The ore, noumeaite or garnierite, occurs as a hydrated silicate of nickel and magnesia and may best be described as an alteration product of the serpentine in which the magnesia and iron have been replaced by nickel. * * * The[135] workable deposits always occur on the saddle of spurs from the main mountain ridge, at elevations of 400 to 2,500 feet, the latter elevation being the more common. * * * The replacement of the serpentine by nickel follows the joints and fractures in the serpentine and the undecomposed blocks and boulders of serpentine are as a rule covered by a shell of ore which has to be picked off.” The ores are hand picked to bring them up to a grade profitable to treat. In the past it has not been considered economical to smelt ore of lower grade than 4.5 per cent. nickel nor to ship ore much below 6.5 per cent.
The ore bodies usually contain under 250,000 tons of ore. The largest mine yet worked produced less than 600,000 metric tons (2,204 lb.) of ore. Probably there still remains as much undeveloped ore as has been mined in the forty years of production,—equivalent to 160,000 tons of nickel. This would be equal to about four years’ output of the Sudbury district at the 1916 rate of production. “There are large bodies of lower-grade ores which it has not yet been found feasible to treat.”
Three large blanket deposits of nickeliferous iron ore occur on elevated plateaus in Cuba. These are typical lateritic (residual) deposits. The average depth of the ore is 15 feet and the combined tonnage of the three deposits is placed at one and one-half to three billion tons. The nickel content ranges from about 0.6 per cent. to 2.1 per cent. and shows progressive enrichment from the top downward. Chromium is also present and shows similar enrichment. The presence of nickel and chromium in the iron ores greatly enhances their value for making special steels.
Iron ore on the island of Seboekoe, lying off the southeast coast of Borneo, contains appreciable amounts of nickel and chromium. At least 300,000,000 tons of ore are contained in the deposit, which is a porous limonite, about 15 feet thick, overlying serpentine.
In the United States, nickel deposits of the garnierite type occur in North Carolina and Oregon. Attempts to mine these ores have never been successful.
Small nickel deposits of the garnierite type occur in Egypt, Germany (Prussian Silesia), Greece, Madagascar, Russia and Spain. Nickeliferous iron ores occur in Greece, and it is thought that chromiferous iron ores on Mindao Island, in the Philippines, may, on further exploration, prove to be nickeliferous.
—Nickel occurs with other metals, precious or semi-precious, in some vein deposits and is recovered as a by-product in the treatment of ores from them. It is notably present in the deposits at Cobalt, Ontario, and has been found in vein deposits in the United States, France, Germany, Austria, Mexico and South America. Several hundred tons are recovered annually in the refining of copper produced in the United States.
Related to the vein deposit type of nickel-bearing ore are the galena[136] deposits disseminated through dolomite in southeastern Missouri. Iron, copper, nickel, and cobalt sulphides occur with the galena. Years ago nickel was recovered electrolytically from matte from these ores.
In 1900 New Caledonia supplied 65 per cent. of the world’s production and Ontario 35 per cent. Since then the world’s production has increased six-fold, and Ontario, by the end of 1916, was producing 80 per cent. of the whole. This shows the trend of the industry. Recent discoveries of ore in the Sudbury district and construction of new smelters and refineries in Ontario to treat the ores indicate an increasing dominance of the industry by that district. New Caledonia has not the large ore bodies and is too far away to compete favorably with Ontario, though work will continue there.
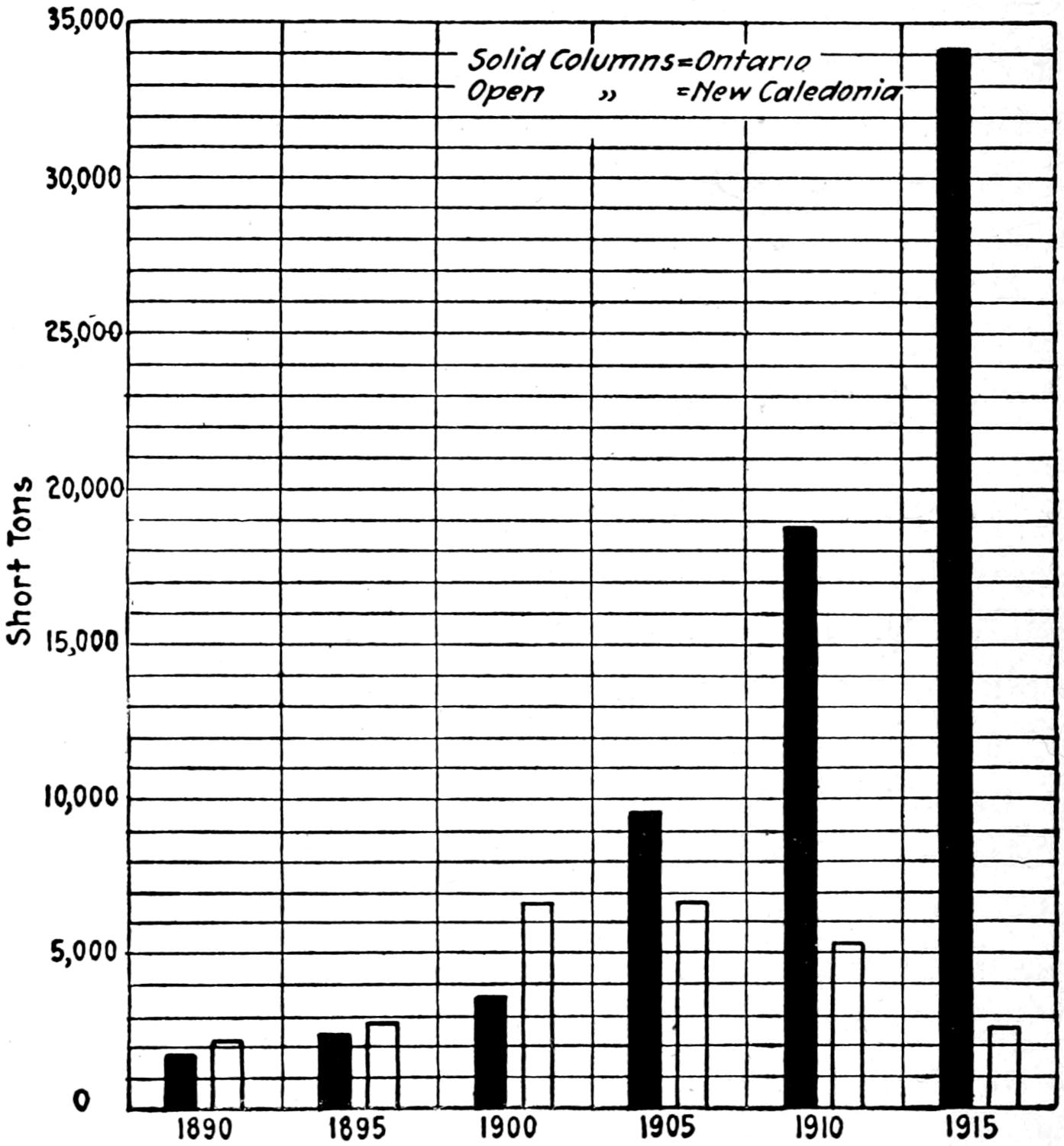
Fig. 7.—Refined nickel produced from the ores of New Caledonia and Ontario, for five-year periods; the amounts are for the calendar year indicated.
Statistics of production and ore reserves in Ontario and New Caledonia are shown graphically in figures 7 and 8.
Isolated nickel ore bodies of the sulphide type—that is, segregations from basic igneous rocks, as at Sudbury—have been found in a number of widely separated places. There is a distinct probability that others exist and may be discovered. Such undiscovered deposits might even[137] contain more accessible ore, in the aggregate, than the total of the mined and unmined ores of the Sudbury district, but in the light of present knowledge this seems a remote possibility.
Other deposits of the New Caledonian and Cuban types will probably be discovered, but it is doubtful if as large deposits of those types remain to be found. In these the nickel has remained in the material left as residuum from the partial or complete weathering by solution of the original nickeliferous rock. This material lies at the surface and covers relatively broad areas. Bodies of it are therefore not usually difficult of discovery. They seem to be found most abundantly in warm latitudes, where solution weathering has most easily gone on under conditions favorable for preservation of the residuum.
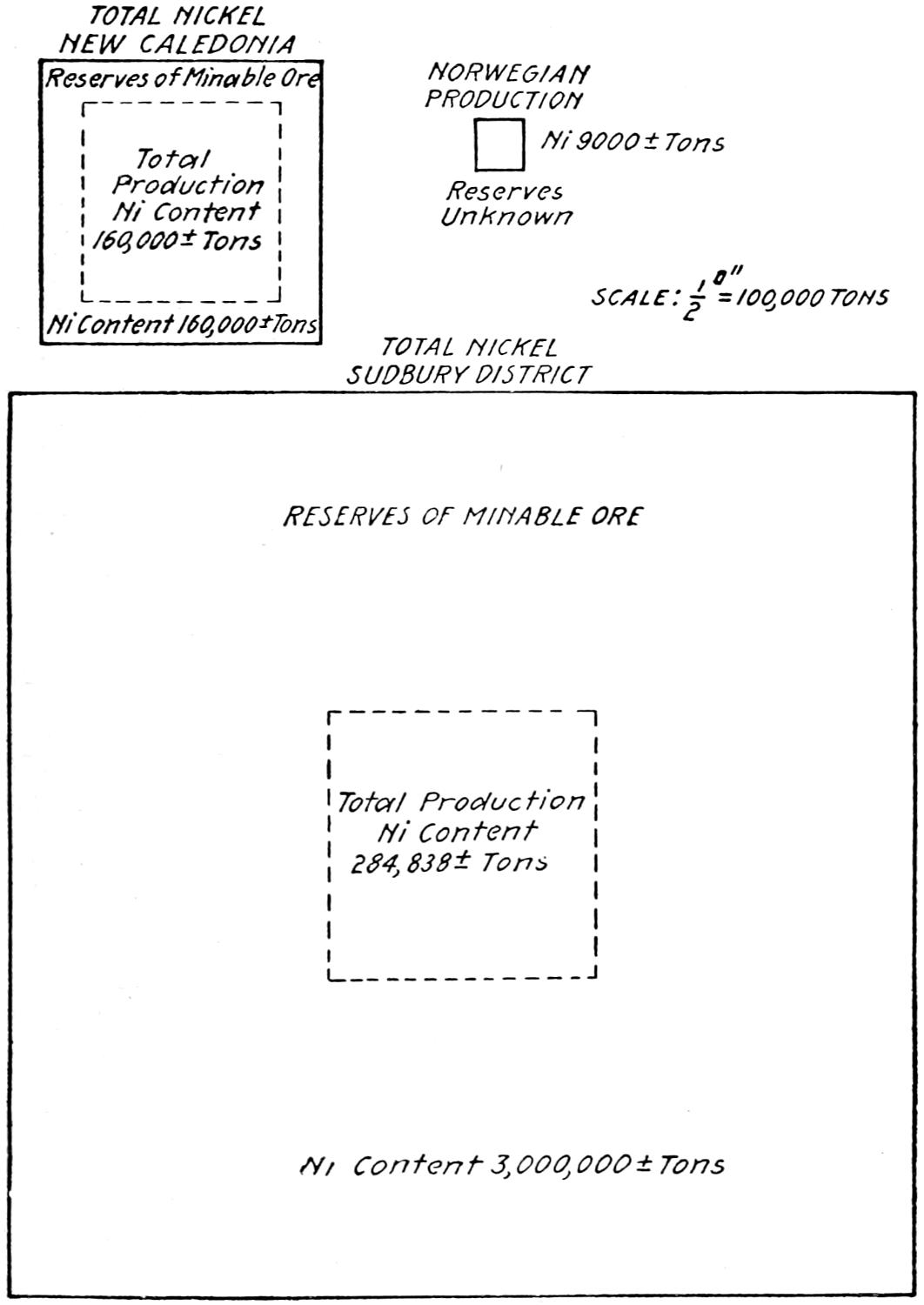
Fig. 8.—Nickel production, and nickel content of reserves, in Ontario, New Caledonia, and Norway.
The present known nickel and nickeliferous iron-ore deposits indicate roughly the locations of perhaps all or nearly all of the nickeliferous metallographic provinces of the world. It is in these provinces that new deposits are most likely to be found. The wide-spread distribution of[138] these provinces is indicated by the following statement in the Report of the Ontario Nickel Commission:
While competition is not to be feared (that is, for Ontario), it would be futile to try to shut off the supply of nickel from almost any of the great nations. * * * Nearly every important country has supplies of nickel ore which can be worked if the demand is great, thus ensuring a high price.
It is thought probable that the nickeliferous metallographic province of eastern Canada, which includes the Sudbury district, contains the greatest unknown nickel deposits, just as it contains the greatest known one, and this for three reasons. The first and most obvious reason is that the great nickel-ore bodies of the Sudbury district, the isolated Alexo ore body, 150 miles north of the district, and the appreciable content of nickel in the veins of the Cobalt and other districts near by, indicate an unusually large nickel content in the original magma from which the basic igneous rocks were derived. The second reason is that the mantle of glacial drift effectively conceals large areas of underlying rock and prevents easy discovery. The third is that the wild and unsettled nature of the country inhibits the human activities whereby accidental or other discoveries would be made.
The Sudbury nickel deposits and the Alexo ore body in eastern Ontario are under the political control of the British Empire; the New Caledonian nickel deposits are controlled politically by France, New Caledonia being a French colony; and the nickel deposits of Norway and Cuba are under the political control of those governments. The other deposits of the world, as has been noted, are commercially unimportant.
Ownership of mines and undeveloped ore bodies in the Sudbury district is divided between British and American interests. There are two companies producing ore at the present time and a third is expected to begin producing soon.
The first large company in the field was the Canadian Copper Co. This is a subsidiary of the International Nickel Co., over 90 per cent. of the stock of which is held in the United States. The Canadian Copper Co. owns the following mines: Copper Cliff, Evans, Stobie, Crean Hill, Vermilion, Creighton, No. 1, No. 2, No. 3, No. 4, No. 5, and No. 6. Four of these were being worked in 1917—the Crean Hill, Vermilion, Creighton and No. 2. In December, 1916, the president of the Canadian Copper Co. gave the following estimates of reserves of payable ore in three properties of that company: 10,000,000 tons in the Creighton; 2,000,000 tons in Crean Hill; and 45,000,000 tons in No. 3.
[139]
The Mond Nickel Co. is controlled by British interests. It owns the following mines: Garson, Worthington, Levack, Victoria and Kirkwood. The Levack has proven reserves of good ore amounting to 4,500,000 tons. The reserves of the other mines are not given.
The British-America Nickel Corporation, Ltd., has $20,000,000 of common stock, of which $14,500,000 is held by the British government in the name of Alan Anderson, trustee. By the end of 1916, this company had 11,000,000 tons of workable ore blocked out. Its mines are the Murray, Gertrude, Elsie, Blue Lake and Frood Extension. The reserves of the Murray alone are put at 9,000,000 tons.
In the southeastern part of the district an ore body 7,500 feet long, 10 to 120 feet thick, and extending to a depth of 1,020 feet in one place at least, has been found recently in diamond drilling operations by the E. J. Longyear Co., of Minneapolis. This company and its associates, all American, control the ore body. The ore tonnage of this deposit is estimated at 6,000,000 tons above the 500-ft. level. A few drill holes have gone to greater depths and found ore. It is not possible to estimate the reserves of this deposit below the 500-ft. depth.
The Alexo Mining Co. Ltd., which is mining the Alexo ore deposit north of the Sudbury district, is a Canadian concern. An estimate of the quantity of ore in this deposit is not available, but the deposit is small compared to that of the Sudbury district. The total may be several hundred thousand tons.
The largest and most important owners of nickel-holding lands in New Caledonia, in relative order of the importance of their holdings, are: (1) La Société le Nickel, a company which has been mining in the island for many years. (2) The International Nickel Co., represented in New Caledonia by its two subsidiary companies, The Nickel Corporation and La Société Minière Caledonienne. The International does not mine in the island, but some of its lands are worked on lease by persons associated with La Société le Nickel. (3) Les Hauts-Fourneaux de Noumea.[73]
[73] Report of Ontario Nickel Commission, 1917, p. 253.
La Société le Nickel was under control of the Rothschilds of France at the time of the discovery of the Sudbury deposits. Mr. F. E. Merry, an English metallurgist, in testifying to the Ontario Nickel Commission, reported that Germans were in control of the company at the outbreak of the war. The German firm of Krupp had also acquired some nickel property in New Caledonia.
The International Nickel Co., the second largest holder of New Caledonian nickel lands, is the same American firm which owns the Canadian Copper Co.
Les Hauts-Fourneaux de Noumea is owned, at least since the outbreak of the war, by French interests.
According to Mr. Merry, the nickel mines and smelters of Norway[140] were also mainly in control of the same German group that had gotten control of Le Nickel. It worked under the name “Metallgesellschaft.” One mine and smelter reopened recently were under English control.
The nickeliferous iron ores of Cuba are owned entirely by American companies, principally by steel manufacturing companies, notably the Bethlehem Steel Co.
All three companies producing or about to produce nickel in the Sudbury district own their own smelting and refining plants. The International has been smelting its ore in Canada and refining it in the United States. It has a new refinery at Port Colborne, Ontario. The Mond Nickel Co. which smelts in Canada and refines in England, has started a refinery in Canada. The British-American Nickel Corporation has built both a smelter and a refinery in Canada.
The United States Nickel Co. operates a refinery at New Brunswick, New Jersey. Les Hauts-Fourneaux de Noumea and this company belong to the same interests. They have a smelter at Noumea, New Caledonia; also a refinery at Havre, France.
La Société le Nickel has a smelter at Thio, New Caledonia, and refineries at Havre, France, and Erdington and Kirkintilloch, British Isles.
No one refining process dominates the nickel industry of the world. The two companies producing nickel from Sudbury ores are using entirely different refining processes, which they individually control; and the British-America concern, soon to begin producing, will use a third process, the Hybinette, an electrolytic process on which it has exclusive rights for North America. The Orford Copper Co., subsidiary of the International, uses what is known as the “salt-cake” process, but it also produces some nickel electrolytically.
The process of the United States Nickel Co. at New Brunswick, New Jersey, being one of fluxing and reducing from matte produced at the Noumea smelter, is not adaptable to copper-bearing sulphide ores.
The ores produced in Norway are smelted and refined in Norway by the Hybinette process.
Obviously the nickel resources of the world are controlled by a few companies. The chief one is the International Nickel Co., which has the largest holdings at Sudbury, and the second largest holdings in New Caledonia. The Mond and the British-America are the next most important, and La Société le Nickel is fourth in importance. E. J. Longyear Co. and associates do not aspire to become producers of nickel and are in the market to sell their properties. For a new concern to succeed it would have to develop a refining process of its own. The alternative would be for it to sell out to or merge itself with a firm that controls a refining process. There are no custom smelters or refineries in America. The Mond company buys the Alexo ore because its high magnesium content makes it a good fluxing material in smelting Sudbury ores.
[141]
—Though the United States has insignificant deposits of nickel ore and therefore exerts little or no political control over nickel mining, American capital plays an important if not the leading rôle in the industry. Of the four companies holding the deposits of the Sudbury district two are American, and these two possess what are doubtless the largest reserves there. One of these, the International Nickel Co., has the next to the largest holdings in New Caledonia.
—Of all nations Great Britain is in the strongest position politically with respect to the nickel industry, because of the Sudbury district being in Canada. It has used this control in an endeavor to localize the business of refining Ontario nickel ores in Canada, with the result that the International Nickel Co. is to transfer its refining operations from New Jersey to its new refinery in Ontario.
Commercially, British capital controls the two other companies having holdings at Sudbury. In one of these,—the British-America Nickel Corporation,—the government itself has a controlling interest.
The policy of the British government with relation to the Sudbury nickel ores, which give the British an overwhelmingly dominant political control over the world’s nickel, is highly significant as showing that the government is aware of the necessity for commercial as well as political control, in order to reap all the commercial and strategic advantages of its good fortune. During the war, and before the United States entered, great feeling was roused in Canada and England by the German submarine, Deutschland, loading at New York a cargo that consisted partly of metallic nickel, it being assumed that this was originally Canadian nickel. The direct participation of the British government in the Sudbury industry in such a way as to make the government practically the dominant factor, and the transfer of the refinery operations of the American-owned International Nickel Co. from New Jersey to Ontario, mark a vigorous and aggressive nationalistic policy which has attained its object without much delay.
—France owns the island of New Caledonia and has political control of the nickel deposits there. Two of the three principal companies holding New Caledonian ore deposits are presumably held by French interests. The larger one, La Société le Nickel, was for a long time controlled by the Rothschilds of France. It was reported later to have gotten into German hands.
—Germany exercises political control over no important nickel deposits. Before the war the German firm of Krupp had obtained some New Caledonian nickel properties. A German group, the Metallgesellschaft, is reported to have had control of La Société le Nickel at the outbreak of the war, and also the mines and smelters of Norway.
[142]
[74] Unless otherwise noted, the short ton of 2,000 pounds is used throughout this chapter and “tungsten ore” means materials carrying 60 per cent. WO3.
The essential uses of tungsten are as an alloy in high-speed tool steel, for the making of filaments for incandescent lamps, for targets and cathodes of Roentgen (“X”) ray tubes, and for electric contacts for explosion engines or wherever an intermittent electric contact is needed. Other uses are in saw and some other steels, as a constituent of stellite, in a tungsten-iron alloy for valves in automobile and airplane engines, for kenotrons and similar instruments, in a manganese-chromium-tungsten-iron alloy for wire-drawing dies, in wire cloth, luminescent screens for Roentgen (“X”) rays, mordants and minor chemicals.
—The use of tungsten in high-speed steels is as standard as the use of yeast in bread, and, though assiduously sought, no substitute is known that satisfactorily takes its place. According to report, in England and France molybdenum has been used to replace about half of the tungsten in some high-speed tool steels, but this is seemingly not a preferred method, being used only when the obtaining of tungsten is difficult. In the United States the practice has had few sponsors. The following quotation from the Mining Journal (London) for May 25, 1918, p. 318, shows that this sentiment is not unknown abroad:
The manufacture of ferromolybdenum is stated to have been commenced in Sweden, where the lack of ferrotungsten has forced the employment of this substitute.
During 1917, 104 (metric?) tons of molybdenite was shipped from Norway to Germany, where it probably was used as in Sweden. Henry E. Wood reported finding molybdenum in the steel of a German helmet. When tungsten was at the excessively high price of early 1916, many experiments were made to find a substitute, but apparently without full success, although lately several substitute steels containing cobalt and chromium and especially intended for cast milling-cutters and other multiple-edged tools have been placed on the market and a cobalt-chromium-molybdenum steel and a uranium steel have been offered for lathe tools. Stellite, the cobalt-chromium-tungsten alloy, in[143] which there is only one-third to one-half as much tungsten as is used in high-speed steel, has grown in favor, and cooperite, a nickel-zirconium alloy, is also a competitor, but the trade in the combined list has made no appreciable impression on the demand for tungsten steels.
A change in the manner of using tungsten steel, by which a thin plate of high-speed steel is cemented to a more ordinary steel bar so as to form the cutting edge of a lathe tool, has made the demand for tungsten less than it would have been had the old practice been followed of making the whole tool of high-speed steel.
No startling changes of practice in the metallurgy of tungsten are known to have taken place, but there has been a steady betterment of the art, improvement in the quality of ferrotungsten, a shifting in localities of reduction, and a considerable change in the manner of use. The wasteful, lazy demand for ores of high concentration and of great purity common before the war has given way before more enlightened and intelligent practice, until firms both in this country and in England make a specialty of using low-grade or impure ores, though seemingly much more advance has been made here than abroad. One firm, the Chemical Products Co. (Washington, D. C.) was organized specifically to buy ores carrying less than the 60 per cent. tungsten trioxide (WO3) demanded by other American firms, and ores containing sulphur, copper, arsenic, bismuth, tin, antimony, phosphorus, or other impurities to which most users objected. Two firms, the Black Metal Reduction Co., and the Tungsten Products Co., both of Boulder, Colorado, were organized to handle materials such as tailings carrying as little as 1 per cent. tungsten trioxide, and they said that they were able to pay for gold and silver in the ore.
Several firms make tungsten trioxide or tungstic acid (H2WO4) for ferrotungsten makers. Firms have also made artificial scheelite (CaWO4) for the same trade, using off-color ores in the process. Powdered ferrotungsten made by chemically precipitating an iron tungsten salt from solution and reducing it to a metallic powder carrying 4 to 11 per cent. iron has been produced by several firms and seems to have started a demand for powdered ferrotungsten, so that firms are now finely grinding the massive ferrotungsten. Claims for better furnace practice which greatly cuts down the consumption of current and makes a saving of 90 to 96 per cent. instead of the usual 80 per cent. are also made. Several firms are making ferrotungsten powder and tungsten powder in iron tubes or other iron containers instead of in the graphite crucibles used before the war.
In the milling of tungsten ores, the tendency in this country is to get[144] away from the uneconomic method of making concentrates very high in tungsten trioxide (carrying more than 60 per cent. WO3). Few operators really know what the heads run, and the determination of losses is mere guesswork. Some of the most careful operators in the Boulder field before closing down at the beginning of 1919 were making a high-grade product carrying nearer 50 per cent. than 60 per cent. tungsten trioxide, and a lower grade product carrying about 20 per cent. tungsten trioxide, not attempting to raise its tenor. This practice cuts down the losses largely. When the great shortage of tungsten ore came in 1916, users were compelled to take lower grade concentrates, and some improved their metallurgy accordingly. This change has made the sale of 50 per cent. concentrates easier. Even in England, 60 to 65 per cent. ores were accepted where formerly a content of 67.5 per cent. or 70 per cent. WO3 was demanded.
Formerly scheelite sold 50 cents per unit below other tungsten minerals, even though freer from bothersome impurities, but with the graduation from rule-of-thumb methods to more thoughtful, careful and scientific practice, it came to command a premium. Huebnerite seems to be still sold with and as “wolfram” in England, but in this country it must be sold according to its composition, for to most metallurgists the manganese is undesirable, though at least one firm now makes ferrotungsten from huebnerite without prejudice or difficulty. This growth in knowledge and technique has caused the price of the tungsten minerals to rank about as follows in the order named: Scheelite, ferberite, wolframite and huebnerite. This applies to tungsten used in the manufacture of tungsten and ferrotungsten for steel making. Scheelite is not wanted in this country for making filaments.
Before the war the United States imported considerable quantities of German ferrotungsten, but metallurgists claim that the ferrotungsten now made in this country is superior to any from Germany. For the present, of course, the German export trade is dead. England, formerly a small producer, is now making large quantities of ferrotungsten and tungsten powder at a number of plants. One of the producers, the High Speed Steel Alloys, Ltd., Widnes, near Liverpool, “is under government assistance and is owned by 31 of the leading consumers of Sheffield.”[75] The article quoted stated that the output was at the rate of 500 tons of tungsten per annum. The Thermo-Electric Ore Reduction Corporation,[76] Luton, was at the same time producing 140 tons of tungsten[145] per month. The ferro produced carried 80 per cent. and the powder 98 per cent. of metallic tungsten. Only one company in the United States was producing as much as 60 tons of tungsten per month during the same war period. The Thermo Electric Ore Reduction Corporation owned mines from which it expected to produce each year 4,000 long tons of concentrates carrying 65 per cent. tungstic oxide. There are at least seven other manufacturers of tungsten or ferrotungsten in England.
[75] Mining Journal, London. “High Speed Steel Alloys, Ltd., Visit of Inspection to the Works,” vol. 115, Nov. 25, 1916, p. 779.
Julius L. F. Vogel has since written an article (Min. Jour., London, vol. 20, p. 16, Jan., 1919) in which he says that government aid, though proffered, was not accepted. This statement confirms the government policy.
[76] Mining Journal. “Thermo-Electric Ore Reduction Corporation, Ltd., Visit to the Luton Works,” vol. 115, p. 797, Dec. 2, 1916.
France had a number of ferrotungsten plants before the war, and these are thought to be still in operation.
At the beginning of the war about half of the tungsten used in the United States was introduced into steel in the form of ferrotungsten and about half in the form of tungsten powder. This practice has changed, so that now more than three-fourths of the tungsten used is introduced as ferrotungsten, largely, it seems, because of the ferro being now manufactured in purer form and partly because tungsten powder could not be obtained for a while. At one time tungsten ores were put in the charge and the tungsten alloyed directly with the steel; in fact, tungsten steels were first made in this way, although the process was patented in this country as new. The practice seems to have been dropped because of the introduction into the steel of impurities that may be eliminated when tungsten or ferrotungsten is made first.
In the making of tungsten steels a considerable change has taken place through the increased use of the electric furnace. One considerable producer, the Latrobe Electric Steel Co., makes all of its high-speed tool steel in this way. The Vanadium Alloys Steel Co., one of the larger producers, makes a large part of its steel in the electric furnace, and the Crucible Steel Co. and some other steel companies are understood to make a part or all of their steels thus.
The removal of tin, copper and other impurities from ferrotungsten by grinding and chemical treatment has made possible the use of impure ores in the production of high-grade ferrotungsten in the electric furnace.
Tungsten, even more than tin, is found almost exclusively with granitic rocks. In a few places tungsten ores are found in volcanic, sedimentary or metamorphic rocks, but as is postulated with certain tin deposits, many of these deposits may be explained on the supposition that they are not far vertically above underlying granite.
Among the deposits themselves there is a considerable variety of types, and they may be classed as follows: Segregation deposits, pegmatite dikes, veins, replacement deposits, contact metamorphic deposits and placers.
Segregation deposits are few and of little importance and constitute those deposits in which wolframite is segregated in granite, like biotite[146] or hornblende. A closely related type is the occurrence of tungsten minerals in aplitic granite, and this grades almost insensibly into the second type, the pegmatites.
The pegmatites are also of comparatively small importance, but do yield certain quantities of tungsten minerals. The pegmatites also grade into the next type, the veins, which have heretofore furnished the greater part of the tungsten minerals of the world. Closely connected with the veins are the fourth type, replacement deposits, in which the country rocks alongside the veins, though the veins may be very small, are replaced by various minerals, including those of tungsten. The only known large examples are the wolframite deposits near Lead, South Dakota, which were considerable producers under the high prices of the Great War.
Among the replacement deposits are to be noted also such deposits as those in the Deep Creek Mountains, Utah, in which solutions following cracks in monzonite have replaced the rock with a mass of feldspar, quartz, tourmaline, apatite, scheelite, wolframite (very little), bismuth, copper, and molybdenum minerals, which under other conditions would be unhesitatingly called pegmatite.
Closely related to the replacement deposits are the contact metamorphic deposits, the fifth type. These only recently have begun to be of commercial importance, but promise to be among the greatest, if not the greatest, producers of this country and possibly of other countries. They are of the familiar kind—limestones or limey rocks which have been invaded by granites bringing large quantities of watery or gaseous solutions of silicon, iron, aluminum and magnesium, with less chlorine, fluorine, potash and sulphur, and, in this case, tungsten. In the Great Basin broad areas of limestone, extending from northwestern Utah to the Sierra Nevadas and around their southern extremity, have been thus intruded and metamorphosed. Some large deposits of scheelite have already been exploited in the region and others remain to be worked. Similar deposits occur in Korea, Japan and Tasmania, and probably exist, though as yet undiscovered, in China, the Malay Peninsula and other countries. The tungsten mineral in such deposits is invariably scheelite.
Placers, the sixth type, are formed from all grades of deposits, but their value depends largely on local conditions. They are both residual and fluviatile deposits and have been large producers of tungsten minerals, especially of wolframite. A very large proportion of the Burmese output has been from semi-residual and stream placers, and the Chinese output in 1918, the largest ever made by any country in one year, was almost wholly from placers.
The distribution of tungsten ores is far from being as wide as the distribution of the granitic rocks, and some regions with large areas of[147] granite have almost no tungsten minerals. Among such regions are the Scandinavian peninsula, large stretches of Canada, the eastern United States, and Brazil.
The world’s known large tungsten fields are grouped along the shores of the Pacific Ocean—not always close to it, but somewhere in the great mountain masses paralleling its margin, and the western shore is much richer than the eastern shore. In 1918, fully 92 per cent. of the world’s tungsten came from the shores of the Pacific, 61 per cent. coming from Asia, Australia, and Oceania, and 31 per cent. from North America and South America. Eastern Asia alone furnished 56 per cent. There is only one considerable tungsten-bearing area not situated close to the Pacific, that of the Iberian Peninsula, mostly in Portugal but partly in Spain. Of the less than 8 per cent. not produced around the Pacific, that area yielded nearly 5 per cent. There are, of course, small deposits in England, Germany and other places near the Atlantic, but together they produce less than 3 per cent. of the world’s tungsten ores. The huge continent of Africa has only negligible known deposits; none of consequence are found on the borders of the Arctic, Antarctic or Indian oceans except along the narrow Malay Peninsula dividing the Indian and Pacific oceans, and only minor deposits are known in Siberia.
Considered as a single metallogenic province, the region making by far the greatest production is in southeastern Asia; it includes the Malay Peninsula, Burma, the Shan States, Siam, Tonkin, and southeastern China. The second largest producing metallogenic province is the Cordilleran, including Bolivia and the adjacent closely related areas of Peru, Argentina and Chile, to which the United States and Mexico would be a close third.[77] Portugal, Spain and Italy form the fourth province, to which are closely related the Cornish-French producing areas. Australia, including Tasmania, is next in importance and is a distinct province, practically all the ores being found in the ranges of the eastern side of the continent. Japan and Korea also form a rather distinct province which may continue into Manchuria. Mexico, as has been indicated, should be included in the same province as the western United States. There are numerous small more or less isolated areas, like Connecticut, Nova Scotia, Manitoba, etc., that are at present of little importance and give no promise of future greatness.
[77] It may be quite justly objected that the idea of a metallogenic province is considerably stretched to include the Boulder and Black Hills deposits with those of the southwestern states and Mexico, but for convenience they are here so grouped—with a frank confession of the license taken.
The world’s production of tungsten ores by metallogenic provinces and political areas is shown in the following table:
[148]
Table 28.—The World’s Production of Tungsten Ore, 1913 to 1918, by Metallogenic
Provinces and Political Areas[78]
In short tons (2,000 pounds) of concentrates containing 60 per cent. WO3.
| Province and area | 1913 | 1914 | 1915 | 1916 | 1917 | 1918 |
|---|---|---|---|---|---|---|
| Africa | ||||||
| South African province: | ||||||
| Rhodesia | 4 | ... | ... | 2 | 12 | 37 |
| Union of South Africa | ... | ... | ... | 1 | 9 | 19 |
| 4 | ... | ... | 3 | 21 | 56 | |
| Asia | ||||||
| Chino-Malayan province: | ||||||
| Burma | 1,891 | 2,605 | 2,963 | 4,166 | 5,018 | 4,919 |
| China | ... | ... | ... | [79]120 | [79][80]1,500 | [80]11,662 |
| Dutch East Indies (Billiton and Singkep) | 7 | 1 | 7 | 52 | [81]61 | [81]61 |
| Federated Malay States | [80]252 | [80]292 | [80]326 | 578 | 794 | 398 |
| French Indo-China (Tonkin) | [80]101 | 119 | 220 | 183 | 422 | [82]450 |
| Siam | ... | 301 | 475 | 584 | 800 | [82]800 |
| Unfederated Malay States: | ||||||
| Johore[80] | ... | ... | 2 | 25 | 2 | 1 |
| Kedah | 32 | 27 | 14 | 19 | 139 | 582 |
| Trengganu[80] | 94 | 160 | 161 | 303 | 225 | 691 |
| Indian province: | ||||||
| India (excluding Burma) | ... | ... | ... | 47 | 69 | 45 |
| Japo-Korean province: | ||||||
| Chosen (Korea) | ... | ... | 74 | 613 | 992 | [82]1,102 |
| Japan | 272 | 215 | 411 | 770 | 808 | [79]651 |
| 2,649 | 3,720 | 4,653 | 7,460 | 10,830 | 21,362 | |
| Australia | ||||||
| Australian province: | ||||||
| New South Wales | [80]191 | [80]220 | [80]93 | [80]296 | [80]274 | [79]325 |
| Northern Territory[79] | 217 | 159 | 366 | 401 | 427 | 614 |
| Queensland | 402 | 270 | 468 | 415 | 406 | 298 |
| South Australia | ... | ... | ... | 1 | ... | ... |
| Tasmania | 76 | 53 | 106 | 119 | 270 | 416 |
| Victoria | 1 | ... | 16 | 1 | 25 | 4 |
| Western Australia[80] | 1 | 1 | ... | 4 | 1 | 5 |
| 888 | 703 | 1,049 | 1,237 | 1,403 | 1,662 | |
| Europe | ||||||
| Erzgebirgan province: | ||||||
| Austria | 57 | 62 | [82]75 | [82]150 | [82]150 | [82]150 |
| Germany | [80]318 | [82]100 | [82]150 | [82]351 | [82]200 | [82]200 |
| Franco-Cornish province: | ||||||
| England | 204 | 229 | 370 | 441 | 270 | 338 |
| France | 301 | [82]200 | [82]200 | [81]182 | [81]182 | [81]182 |
| Iberian province: | ||||||
| Italy | ... | ... | ... | 9 | 1 | ... |
| Portugal | 1,241 | 735 | 1,029 | 1,563 | 1,742 | [82]1,300 |
| Spain | 186 | 149 | 208 | 468 | 491 | 4,741 |
| Russian province: | ||||||
| Russia | ... | ... | ... | 36 | [79]108 | [82]150 |
| Scandinavian province: | ||||||
| Norway | 3 | 4 | ... | 1 | [82]10 | [82]10 |
| 2,310 | 1,479 | 2,032 | 3,201 | 3,154 | 7,071 | |
| North America | ||||||
| American province: | ||||||
| Alaska | ... | ... | ([83]) | 47 | 32 | 12 |
| United States | 1,537 | 990 | 2,332 | 5,876 | 6,112 | 5,056 |
| Canadian province: | ||||||
| Southeastern Canada | ... | ... | ... | ... | ... | 13 |
| Southwestern province: | ||||||
| Mexico | ... | ... | ... | 13 | 207 | 165 |
| 1,537 | 990 | 2,332 | 5,936 | 6,351 | 5,246 | |
| Oceania | ||||||
| Oceanic province: | ||||||
| New Zealand[80] | 248 | 228 | 217 | 298 | 181 | 190 |
| South America | ||||||
| Brazilian province: | ||||||
| Brazil | ... | ... | ... | [80]7 | ... | ... |
| Cordilleran province: | ||||||
| Argentina[80] | 679 | 499 | 201 | 963 | 1,251 | 724 |
| Bolivia[80] | 339 | 330 | 947 | 3,624 | 4,646 | 4,082 |
| Chile | ... | ... | 10 | 2 | ... | ... |
| Peru | 357 | 235 | 455 | 587 | 471 | 295 |
| 1,375 | 1,064 | 1,613 | 5,183 | 6,368 | 5,101 | |
| Total | 9,011 | 8,184 | 11,896 | 23,318 | 28,308 | 40,688 |
[78] Figures unaccompanied by footnote references are taken from official sources or other authentic publications. In all cases the original quantities have been reduced to their equivalent in concentrates containing 60 per cent. WO3.
[79] Figures partly official and partly estimated by the United States Geological Survey.
[80] Exports.
[81] Figures estimated by the Allies when it was proposed to allocate the tungsten ores of the world among themselves.
[82] Figures estimated by the United States Geological Survey.
[83] Less than half a ton.
[149]
—The increase of output from eastern Asia has been marvelous. In 1913 it amounted to 2,497 tons and in 1918, as already stated, to 20,228 tons—more than 56 per cent. of the world’s production. As elsewhere, production must decrease until the accumulated stocks in the reducing centers are used, then production will again proceed. The alluvial deposits of China are by no means exhausted, the veins are scarcely touched, the tungsten-bearing area is large and only partly prospected, and such prospecting as has been done has been almost wholly for placers; labor is cheap, and a large future output is sure. More liberal ideas of trade and government are slowly taking root in China and ultimately educated Chinese or trained foreigners will work the deposits; the output for a long time will be large, though it may never again be as large as it was in 1918.
So far as can be learned, the easily worked placers and the upper parts of the veins in Burma are becoming exhausted rather rapidly,[84] and recourse must therefore be had more and more to the mining of those parts of the veins below water level and in harder rock, and this will probably mean a diminution rather than an increase in output. Siam seemingly should give an increased production, as the mines are comparatively new, and there still should be opportunity for discoveries. The Federated Malay States and the unfederated states (Johore, Kedah and Trengganu) should produce at least as much in the immediate future as in the past—given the demand and an equal price.
[84] Burma Chamber of Commerce and Tavoy Chamber of Mines, “Memorial to Sir George Barnes,” Mining Jour., London, vol. 121, 1918, May 4, p. 261.
—Like other British possessions, Australia labored under the handicap of a comparatively low fixed price for tungsten ores during the war. This price, at first 55 shillings per long ton unit c.i.f. London, was later raised to 60 shillings. During the earlier part of the war the price paid in Australia averaged less than one-half the price paid in the United States, and only a little more than half that paid in regions other than the British provinces. In consequence the Australian tungsten production did not increase during the war as it might have done had prices been higher. The cream of the known deposits is gone except in Tasmania, where contact metamorphic deposits on King Island have quadrupled the Tasmanian output. The tungsten minerals mined in Australia are largely wolframite with smaller quantities of huebnerite and scheelite. The huebnerite seems to be rarely recognized as such in the British market, but is all sold as wolframite. Except for the contact metamorphic deposits on King Island, Tasmania, the deposits worked are mostly veins, with some pegmatites.
[150]
In New Zealand, the production is wholly scheelite; it increased considerably during the war until the last year—1918—when, apparently from a lack of efficient labor, it fell to the lowest point since 1909. The probabilities are that there will be a stoppage of output for the present.
—In Bolivia the increase of production has been great. The deposits seem to be wholly veins and derived placers. The veins are closely connected with the tin deposits, and in many veins tin and tungsten are associated, but many tungsten deposits contain little or no tin. The tungsten minerals mined are ferberite, wolframite, scheelite and some huebnerite. In some veins the minerals are mixed and in others wholly separate. The mines are in and on both sides of the eastern Cordillera of the Andes through a distance of nearly 400 miles from a point near Puerta Acosta, on the northwest, to Chorolque, on the southeast. Mining costs during the war rose greatly in sympathy with the rise in other parts of the world. Wages did not rise to great heights, but the cost of materials advanced decidedly. Transportation conditions are always bad in most of Bolivia, and heavily increase expenses. Because of circumstances the output is extremely sensitive to a decrease in demand or prices, and hence it fell quickly after the armistice, but should high prices come again, it will probably again increase quickly. Some modern plants were placed at mines before or just as the armistice was signed, and when world stocks of tungsten are used and when there is again a demand, some ore will be produced even at less than $10 a unit, though the average cost seems to be about $12 a unit, at the mine.[85]
[85] Hazeltine, Ross, United States consul, La Paz. Report dated May 14, 1919.
The output of Peru, as now produced, seems to depend upon high prices and with such prices could probably remain at the level of 1916 for several years. The Huaura deposits are reported to be large, though of low grade, and may under proper management yield much ore even at lower prices. They were under the control of German firms during the war and probably still are.
—Until 1911 the United States was the leading tungsten-producing country, but in that year it was passed by Burma, which kept the lead until 1916, when the United States again became the principal miner of tungsten ore. In 1918 China entered the excessively high-priced market with an output that exceeded by nearly 1,000 tons the world’s production of any year before 1915. North America increased its output from 1,549 tons in 1913 to 6,512 tons in 1917, but dropped back nearly 1,000 tons (to 5,406 tons) in 1918. In the United States the decrease of production was due almost wholly to the fall in price, and only partly to exhaustion of deposits. In the Boulder, Colo., tungsten field some of its best ore bodies are worked out and the cost of production has risen greatly owing to the impoverishment of others, and the same thing is true in some other places, but it seems possible that in the country as a[151] whole the production can be made about equal to what it has been before, provided prices are equally high, through the discovery of the contact-metamorphic deposits of the Great Basin.
In Mexico the tungsten deposits are seemingly a continuation of those of southern Arizona. So far as known, all the Mexican deposits carry scheelite, in places partly replaced by cuprotungstite. The known worked deposits in the Sahuaripa district of Sonora are described as veins containing scheelite with copper minerals and a pegmatite dike in which are large masses of scheelite and molybdenite.
—European production increased about 50 per cent. between 1913 and 1918, mostly in Portugal, and the output of tungsten ores in Portugal apparently did not reach its maximum. Both placers and veins have been exploited and there seems to be placer material still to be worked as well as veins that are said to be far from exhausted. The official statistics of production given by Portugal during the greater part of the war are declared by engineers conversant with the situation to have been too low, because of ore being smuggled into Spain and on board ships bound for England. On the other hand, in 1918 England and France objected to the shipment of Portuguese ore to the United States, but would not pay equivalent prices. The Portuguese government therefore issued an order preventing the export of tungsten ore except at fixed prices approaching the current American prices. American owners could not work their mines successfully under the British-French embargo, with the result that the output was probably much smaller than in 1917. In spite of the uncertainties the official estimates have been used as far as they are available, for no better figures are at hand.
As to the Spanish output, prophecy is difficult because the data concerning the mines are meager. It seems probable that under similar prices about the same output as in the past may be expected from the English and French deposits. The English output decreased in 1917 and increased only a little in 1918. No accurate data are available from France. The German and Austrian deposits were probably worked so hard during the World War that less is to be expected from them than they have heretofore produced.
—The principal changes in the distribution of production during the next few years would seem to be: Further development in Korea; possible development in Manchuria; development of deposits in southern China and Siam; further development in Bolivia; a tendency in the United States to largely increased production from deposits in the Great Basin; and development of both veins and contact-metamorphic deposits in Mexico. Production will possibly decrease in the Atolia and Boulder fields of the United States; and in Australia, Japan, Germany and Austria.
[152]
The actual control of the world’s tungsten deposits differs considerably from that indicated by the production within political areas. Actual control is justly obtained through ordinary competitive buying, ownership by nationals (sometimes by governments) of deposits, and through commercial alliance. Control through ownership of banks and transportation lines may be just or it may be by coercion and commercial brigandage, seizing ports for coaling and repair stations—methods that are merely refinements evolved since the days when “They sought their fortunes as they pleased abroad, the crown annoying them with no inquiry to embarrass their search for Spanish treasure ships, or their trade in pirated linens and silks.”[86]
[86] Wilson, Woodrow. “A History of the American People,” vol. 1, p. 25.
Table 29.—Actual Control of the World’s Tungsten Output in 1917 and 1918.
In Short Tons of 2,000 Pounds
| 1917 | 1918 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Quantity | Percent- age of world’s output |
Quantity | Percent- age of world’s output |
||||||
| British: | |||||||||
| Possessions | |||||||||
| Burma and Shan States | 4,600 | 4,870 | |||||||
| Federated Malay States | 853 | 920 | |||||||
| Trengganu | 350 | 350 | |||||||
| Johore and Kedah | 200 | 582 | |||||||
| India | 75 | 46 | |||||||
| Australia | 1,404 | 1,662 | |||||||
| New Zealand | 241 | 146 | |||||||
| England | 265 | 330 | |||||||
| South Africa | 24 | 37 | |||||||
| 8,012 | 28.4 | 8,943 | 24.9 | ||||||
| Obtained through trade and political pressure | |||||||||
| Japan and Korea (including ores for France) | 790 | None | |||||||
| China and Hongkong (including ores for France) | 1,105 | 900 | |||||||
| Siam | 600 | 600 | |||||||
| Billiton and Singkep | 60 | 60 | |||||||
| Argentina | - | (including ores for France) | 2,035 | 950 | |||||
| Bolivia | |||||||||
| Peru | |||||||||
| Portugal | 960 | 800 | |||||||
| Spain (including ores for France) | 446 | 425 | |||||||
| 5,996 | 21.3 | 3,735 | 10.4 | ||||||
| Total ores under British control | 14,008 | 49.7 | 12,678 | 35.3 | |||||
| French: | |||||||||
| France | 182 | 180 | |||||||
| Tonkin | 422 | 450 | |||||||
| Siam | 170 | 190 | |||||||
| Portugal | 650 | 440 | |||||||
| Bolivia (See Great Britain) | ? | ? | |||||||
| Argentina (See Great Britain) | ? | ? | |||||||
| 1,424 | 5.3 | 1,260 | 3.5 | ||||||
| German: | |||||||||
| Germany | 200 | 200 | |||||||
| Austria | 150 | 150 | |||||||
| Norway | ? | ||||||||
| Portugal | ? | ? | |||||||
| Spain | ? | ? | |||||||
| 350 | 1.2 | 350 | 1 | ||||||
| American:[153] | |||||||||
| Mexico | 340 | 326 | |||||||
| Peru | - | 4,320 | 4,680 | ||||||
| Bolivia | |||||||||
| Argentina | |||||||||
| Japan and Korea (including some Chinese ore) | 1,010 | 1,650 | |||||||
| China and Hongkong | 395 | 9,300 | |||||||
| Portugal | 130 | 60 | |||||||
| Siam | 30 | 12 | |||||||
| Domestic production | 6,144 | 5,068 | |||||||
| 12,369 | 43.9 | 21,150 | 59 | ||||||
| Japanese: (Quantity smelted only) | |||||||||
| China | ? | 300 | - | 1.3 | |||||
| Norwegian: | |||||||||
| Norway | 10 | 10 | |||||||
| Russian: | |||||||||
| Russia | 110 | 150 | |||||||
| Total | 28,178 | 35,832 | |||||||
Owing to the close relationships between some foreign governments and private firms—as illustrated by the German government’s interest in dye, potash, and shipping firms, and the British government’s participation in nickel mining and ferrotungsten-making companies—it is not practicable to draw a line between governmentally and privately controlled deposits. In countries with weak governments, the deposits owned by British subjects are to all intents and purposes British; but foreign deposits owned by Americans are not necessarily under American control; in fact, instead of helping and encouraging our pioneers in foreign trade we are apt to harass them and destroy their business with drastic tariff laws.
In effect, the preceding table merely shows where the ores of different countries go for treatment; it is, of course, only a generalization, for trade conditions constantly change. For instance, Japanese electric[154] furnaces are beginning to smelt tungsten ores, though at present to the extent of only 10 to 15 tons of contained tungsten per month, but it is conceivable that the output may be increased greatly. Although Japan could control the disposition of its ore, it is given credit for control only of its smelted ores. The exact distribution of ores from Argentina, Bolivia, Peru, Portugal and Spain can not be given.
—During the war the British government demanded and obtained all of the tungsten ores produced in its colonies and possessions. This restriction was later lifted as regards to Canada, and a new rule allowed Canada to ship tungsten ores to other Entente nations, but as Canada was not a producer the license granted amounted to nothing except as it eased the feelings of the Canadians. Scheelite deposits had been discovered in Manitoba, however, that for a time seemed to be potential producers. Nominally Siam has remained free from British control because more or less under the zone of influence of the French, but diplomatic pressure seems to have been exercised at Bangkok. The Siamese ores mostly contain some tin and have gone to Singapore for separation; and when once within the British possessions, of course they could not be exported. The English control of Siamese shipments, however, seems as complete as if the ores came from an English province. Mr. Nassuer, of the Siamese American Trading Co., testified before the Tariff Commission at San Francisco, June 28, 1918, that his company wished to ship ore to the United States but the British minister to Siam would give no permit. The company took the matter up with our State Department and finally got permission to ship 10 tons.
In February, 1918, the Chemical Products Co., of Washington, D. C., protested to the Department of State at Washington with reference to British interference with exportation of tungsten ore from Siam to the United States, stating that the company was working under conditions peculiar to itself in that it employed an expensive process developed to handle low-grade ores obtainable at a much lower price than the regular grades on the market; that it entered into an agreement with an American working tungsten mines in Siam for the purchase of his tungsten ore, only to find that through control of port privileges at Singapore and Hongkong the British effectually prohibited it or any other American firm from obtaining the material. Of course, this, like other incidents mentioned, took place under the shadow of a desperate war when strictness was to be expected, but the shipments asked were to an ally from a country not openly under control of Great Britain. Doubtless no such objections would be offered now, but the incidents show the efficiency of these methods of controlling commerce.
In southern China, Hongkong being the port for Kwangtung and Kwangsi, and parts of southern Kiangsi and Hunan, the British for a while exercised control over the export of ores produced in those[155] districts,[87] refusing to allow the reshipment of ores unless they were sent to England.
[87] Anderson, George E.: American Consul General, Hongkong, China. “Tungsten from South China.” Commerce Reports, Nov. 9, 1917, p. 546.
Foreigners, including Americans of course, are not allowed to own mining property in Burma, the Federated and Unfederated Malay States, or Australia, territory producing nearly all the tungsten ores of the British Empire.
In Argentina small tungsten mines are owned by English companies.[88]
[88] Sharp, Ralston C.: “Wolfram Deposits in the Argentine.” Mining Magazine, London, vol. 18, May, 1918, pp. 230-233.
In Bolivia the English and French governments during the war leased mines directly, and came into direct competition with American business men engaged in buying or producing tungsten ores.
British traders are constantly striving to increase their control of Bolivian tungsten ores. At present the English seemingly have complete control of the financial system of Bolivia, so far as foreign exchange is concerned. An American interested in a tungsten mine in Bolivia has informed the writer that it is almost impossible to do business with English banks, because they insist that if they extend commercial courtesies, even for pay, the recipient must buy only English mining machinery. The buddle, which for dressing tungsten ore is obsolete in other countries, is said to be still used in Bolivian mills under English control. If miners do not wish to deal through English banks, they are compelled to cable money to and from New York at considerable expense. The American banking interests represented in Bolivia seem conservative in advancing money on ore shipments, whereas German and English representatives are said to advance up to 80 per cent. of the market value of ores shipped. Mining corporations controlled by English firms ship to England, and Americans can not compete for the production. Such a firm is Aramayo Francke Mines, Ltd., which produced 2,050 long tons of tin concentrates, 226 tons of wolframite concentrates, and a considerable output of bismuth in the year ending May 31, 1916. Control of the Bolivian mines by the English is not yet dangerous to American interests, except through the banking system, but entire control may be passed to them, to the Germans or the French, through American tariff legislation.
In Portugal, English companies control a number of the mines, and it has been alleged by at least two Americans[89] that the English government, through its representations at Lisbon, for a period of more than two years, prevented title passing to American companies. The Thermo Electric Ore Reduction Corporation, Ltd., seems to be the chief English owner of Portuguese tungsten mines.
[89] Personal communications.
In the Dutch East Indies, the British are understood to control the present wolframite production of about 5 tons a month.
[156]
—French control of tungsten deposits is not large. It includes the production of France and of Tonkin, a part of that from Portugal, and a comparatively small interest in Bolivia. During the war, control in Portugal was attempted by England and France. The prices offered by the English and French were much below the market prices at New York, and the Portuguese government stepped in and raised prices to a point somewhat lower than those of the United States, but 20 per cent. higher than the prices offered by England and France.
—Japan has within her own borders a considerable number of tungsten deposits in the southern part of the islands, but all are small. In Korea important deposits have been discovered and actively worked, especially within the last two years. Deposits in Manchuria are said to be controlled by the Japanese; little is known of them, and if they exist they are probably small. Japanese ores have largely come to the United States for several years. As has been said, Japanese firms have erected electric furnaces in which a part of the tungsten ores are reduced, probably the equivalent of 25 to 35 tons per month of concentrates carrying 60 per cent. WO3.
—The United States controls entirely the tungsten deposits within its own borders and Alaska. Americans operating in Mexico have produced 200 to 300 short tons of scheelite concentrates per year, from deposits in the Sahuaripa district, Sonora. Wolframite is said to have been shipped from Sinaloa to the United States, but its real origin is unknown. Contact metamorphic deposits about 60 miles southwest of Nacozari carry 0.7 per cent. WO3 and 1 to 2 per cent. copper. They are owned by Americans but are not now productive.
In Bolivia, Americans own some of the more important tungsten mines. The American firms known to own tungsten properties there are W. R. Grace & Co., local address, La Paz; Stewart, Wilson & Hepburn, Oruro; Easley Inslee, La Paz; and C. Dillon, Oruro. Their total output is estimated to amount to about 1,600 tons, out of a total output of more than 4,000 tons for the country.
In southern China, American firms have largely developed the tungsten trade, so that through this source the United States (or rather American capital) controls, unless hindered, a yearly output of perhaps 9,000 short tons of tungsten concentrates.
In Siam one or two United States companies have attempted to produce tungsten, but English influence during the war made difficult the shipment of even small lots of ores to this country.
Because it offered higher prices than other countries, and because the more direct and shorter trade route made trade with this country advantageous to the Japanese, the United States largely controlled the Japanese output of tungsten ore in 1918. This trade probably has been somewhat curtailed and will be further diminished through the erection[157] of electric furnaces in Japan. The table following shows the tungsten ores imported for consumption into the United States in 1918, but gives a poor idea of the ores shipped from the countries of origin; Chinese ores lag three months and South American ores about two months. The table gives only the ores actually received during the year. Table 29 shows more nearly the ores shipped to the United States during the year.
Chinese ores are treated as averaging 67.5 per cent. WO3 and other ores 65 per cent. WO3.
Table 30.—Tungsten-bearing Ores Imported into the United States in 1918, by Countries as Listed at Ports of Entry, and by Probable Countries of Origin
| As listed at ports | Probable origin and equivalent in 60 per cent. WO3 |
||||||
|---|---|---|---|---|---|---|---|
| Country | Quantity, short tons |
Value | Country | Quantity, short tons |
Value | ||
| Argentina | 536 | $ | 730,722 | South America, including: Argentina Bolivia Chile Colombia Costa Rica Ecuador Panama Peru Salvador |
4,181 | $ | 3,746,299 |
| Bolivia | 88 | 122,357 | |||||
| Canada | 56 | 115,863 | |||||
| Chile | 1,251 | 1,209,864 | |||||
| China | 2,384 | 2,068,636 | |||||
| Colombia | 56 | 65,124 | |||||
| Costa Rica | 18 | 19,081 | |||||
| Ecuador | 6 | 9,979 | China, including: Hongkong, “Other British East Indies;” also Canada |
6,811 | 5,708,616 | ||
| France | 29 | 3,400 | |||||
| Hongkong | 3,595 | 3,511,046 | |||||
| Japan | 1,361 | 1,700,332 | Japan | 1,474 | 1,700,332 | ||
| Mexico | 264 | 224,247 | Mexico | 286 | 224,247 | ||
| “Other British East Indies” | 19 | 13,071 | Portugal, including: France |
61 | 21,160 | ||
| Panama | 37 | 40,614 | |||||
| Peru | 1,827 | 1,488,516 | Siam | 12 | 8,583 | ||
| Portugal | 27 | 17,760 | |||||
| Salvador | 40 | 60,042 | Unaccounted for | 157 | 142,981 | ||
| Siam | 11 | 8,583 | |||||
| Unaccounted for | 145 | 142,981 | |||||
| 11,750 | $ | 11,552,218 | 12,882 | $ | 11,552,218 | ||
[158]
Unhindered by other governments, this country would have imported even larger quantities of ore, because of its paying higher prices and being more liberal regarding impurities.
The table shows that the United States imported 36 per cent. of the tungsten output of the world; this amount added to the domestic output makes a total of 17,921 tons, or 50 per cent. of the world’s production. Owing to the lag in shipments from South America and China the South American ores received in January and February were from the output of 1917, as were the Chinese ores arriving up to the end of March. Subtracting the ores arriving from the two regions during the first two and three months, respectively, of 1918, and adding the ores arriving during a like period in 1919, make the quantity of ore controlled by the United States in 1918 (as shown on page 153) equivalent to 21,131 tons of concentrates carrying 60 percent. WO3, or 59 per cent. of the world’s production. These ores were all controlled through the private initiative of American firms who offered better prices and better terms than could be obtained abroad. Probably a larger proportion could be handled in the future, should interference not come from within our own borders. It is now proposed to put a tariff of $10 a unit on tungsten ores without regard to purity or quality, with a correspondingly high tariff of $1 a pound, plus the 15 per cent. ad valorem duties now in force, on metal in any form—element, alloy or salt; and such a bill has passed the House of Representatives. Its advocates believe that the price, now about $7 a unit in New York, will be raised to $17 a unit.
Hereafter the quantities of tungsten ore handled will be much smaller than during 1916, 1917 and 1918; and will be confined to peace-time needs unless some unforeseen war arises. England, according to government estimates about January, 1920, had two years’ supply, and France is probably as well supplied. The United States probably had on hand an equivalent of quite 8,000 tons of ore carrying 60 per cent. WO3. Makers of tool steel figured on a consumption of 7,500 tons during 1919, but because of the lack of market for ore this was much too high; and probably 4,000 tons is large enough, so that there will likely be little market for new supplies for nearly two years, except as ore may be bought speculatively. During this time, mines everywhere must remain idle until a demand again arises, except for those mines required to furnish tungsten for Germany, Austria and Russia and the small quantity required by Sweden, Norway and Italy. If industries in Germany, Austria and Russia recover so that they can buy and use tungsten, Germany will have regained in the ores that will be eagerly offered by producing nations needing a market, a part of the trade she has lost. Traders of England, France and the United States will be glad to sell tungsten and ferrotungsten, but Germany will undoubtedly reach out for raw material in order that she may make as much use as possible of her abundant[159] unemployed labor. Should a tariff law like that now proposed be passed, the United States will have cut off its foreign supplies and will have ended its control of any considerable part of them. However, should a high price be maintained, artificially or otherwise, the development of other alloy steels for use in multiple-edged tools may have reached a point where not so much tungsten will be needed.
—Germany had no considerable tungsten deposits at home, and none in the foreign territory she held, but in 1913 her control through business alliances covered about two-thirds of the world’s output of tungsten ore. In that year, according to the German official figures, 5,295 short tons of tungsten ores were imported. Most of this probably carried 65 per cent. or more WO3, equivalent to, say, 5,736 tons of concentrates carrying 60 per cent. WO3. Adding the 106 tons of Saxon concentrates produced in that year shows that Germany treated a total of approximately 5,840 tons out of a world’s output of 8,864 tons, or about 66 per cent. of the total. The United States in the same year produced 1,537 tons and imported 449 tons of unknown content, but the whole was probably equivalent to more than 2,000 tons of ore carrying 60 per cent. WO3, leaving only about 1,000 tons for other countries, most of which seems to have been treated in France. This trade Germany lost when with Austria she started the World War. With the cutting off of all shipments by ocean to Germany, most of the foreign ores were denied her, but undoubtedly small quantities leaked in through Sweden and Norway for some time after the war began. The small output of Austria was always available, and it is said that a considerable quantity of ore was smuggled across the border of Portugal into Spain, thence by water to the western frontier of Italy, into Switzerland, and from there shipped direct to Germany. A considerable part of the Spanish production is said to have reached Germany in this way also, and the “crippled” submarines that ran into Spanish ports are reported to have carried out cargoes of tungsten for Germany. From available data it is impossible to confirm or to disprove these reports, and, in giving them, their doubtfulness is fully recognized, but such possibilities must be acknowledged.
In the Allied countries and the United States, the German interests were taken over by the governments, but in South America the German firms still hold some control of tungsten-bearing properties. In Bolivia four German firms are said to have an output of about 600 metric tons of ore a year. In Peru what is said to be the larger part of the tungsten deposits has been controlled by firms thought to be German, E. y W. Hardt and Carlos W. Weiss y Cia. In Argentina the Hansa Mining Co., a German concern, is the principal producer. Its output is said to be about 500 tons of concentrates a year, but even this output is said to have come to the United States during the war. If the United[160] States is placed under a prohibitive tariff, Germany may easily recover a large part of her control of the world’s tungsten trade.
The spread of knowledge, particularly that regarding electric furnaces, makes the control of the tungsten trade through secret processes or superior skill extremely difficult, and so far as the United States, Great Britain and France are concerned, gives little advantage to any one. Japan is perhaps somewhat less advantageously placed. Smelting plants are so easily, quickly and cheaply erected that they do not offer any great chance for monopoly. Cheap power, high technical skill and knowledge, originality and boldness in experiment, excellence of organization, generous dealing with producers, an honest product honestly sold, good transportation facilities, and broad sane laws are the elements that will give control. The United States may have this control through reasonable effort, but selfish laws may still more easily wreck control of the larger part of the world’s trade, reduce our tungsten business to a provincial scope, and make the product high priced for all time.
In years of good business before the World War, the United States used an equivalent of 3,000 to 4,000 short tons of concentrates, carrying 60 per cent. WO3 per annum. When the war began there was a lull while the attacked countries caught their breath and prepared for a long struggle. After plans had been made, and the manufacture of munitions had begun on a grand scale, the demand for tungsten rose enormously. All kinds of ores were taken at fabulous prices. Ores carrying tin, phosphorus, sulphur and bismuth, that before would not have been considered by steel makers, were taken with avidity, and there was a great scramble for deposits. In October, 1918, the United States was using tungsten ores at the rate of 20,000 tons per annum. Meanwhile prospecting had uncovered so many new deposits and they were so actively exploited that great stocks of ores were accumulated in the Entente countries. On the other hand, in this country many of the known deposits showed signs of impoverishment, a number after being worked profitably for a short time became wholly inoperative, and it is likely that some of the deposits that have seemed to be the richest will never again produce largely. Among the new discoveries were the contact metamorphic deposits of the Great Basin, in California, Nevada and northeastern Utah. They were partly developed, and several promise well, but the irregularity of contact metamorphic ore deposits is notorious.
[161]
In 1916 with prices ranging from $15 to $93.50 per unit, the United States produced 5,969 tons of concentrates; in 1917 while still under the impetus of the 1916 boom, with prices ranging around $25 per unit, 6,144 tons; and in 1918, with prices still averaging about $25 per unit, 5,041 tons, although little was produced in December. Under a price of $17 per unit, which tariff advocates think can be reached by means of a tariff of $10 per unit, it seems improbable that the United States can depend on a production of more than 3,000 tons per annum for the next three years. There are, of course, possibilities of a larger production and there are equal possibilities of a smaller. Should another great war take place, an event that is not beyond the range of imagination, the United States would probably begin by using tungsten at the rate of 20,000 tons of concentrates per annum. Unless the price were even more extravagant than the highest price in 1916, $93.50 per unit, the United States could not produce half of its needed concentrates, and the time required to reach even that output would be far too long for safety. Of course, such a production would be much better than none, but the United States should, for safety, have within reach at least a year’s supply.
The Pacific, around the borders of which are the largest tungsten deposits, is by many looked upon as the next large theatre of war, and however vitally they were needed, the obtaining of supplies of tungsten ores might become impossible though the blocking of trade routes. It would, therefore, seem vastly better that, instead of putting a premium on the quick depletion of our own supplies, which are already too meager, we should use the rich low-priced ores now being mined in the Orient. These cheap ores we may have in trade for the asking, and it would be one of the best forms of national life insurance for the government to store 10,000 tons of these ores while they may be had.
The argument is often made that by putting a high tariff on tungsten ores we would have our own deposits so developed that quick production could be made when needed; also that with the need we would find more ores. Both arguments are specious. What is meant is not development but removal. No one will open a tungsten mine to let the ores stand against the country’s day of need. The finding of new ores is a probability, but the quantity is wholly a question. Few tungsten mines of the United States can be profitably worked at the present price of about $7 per unit, and the mines are now closed. The number of persons dependent on the mining of American tungsten ores is small, probably less than 900 in peace times. At present most tungsten miners have already obtained other employment, and practically all could obtain employment fully as profitably in other mines, many of which are short handed, so that no great hardship would be worked. As a matter of national economy, the United States can not afford to throw away its chance to buy cheap tungsten ores while they are available. Aside from the question[162] of insurance and even of existence during another war, not to buy South American ores is to throw away South American trade. In a degree this is also true of Chinese and Japanese ores.
The metallurgy of tungsten, like that of other metals, is being improved constantly, and should our ores remain in the ground for a time they will be of greater absolute value when mined, for there will be less waste in conversion. If our ores are mined now under an artificially high price, we will always pay a high price for tungsten ores, for when ours are used the ores in other countries will have diminished in quantity and increased in cost; prices would be higher and we would have to buy at the advanced rate. On the other hand, by holding our markets open to cheap ores from any quarter, we will stand on an equal footing with other countries and will always have a reserve of high-priced ores available in an emergency.
There is but one crop of ore. Deliberately to turn over all the cheap tungsten ores of the world to our competitors, allowing them this advantage in making high-speed steels with which to compete in foreign trade with our steels and with all products on and in which they are used; to put a premium on the early depletion of our own deposits of this indispensable metal; and to compel our use always of high-priced ores—these would be economic crimes.
[163]
The main use of vanadium is in steel. It is used where great toughness and torsional strength are required, as in automobile parts, gears, piston rods, tubes, boiler plates, transmission shafts, bolts, gun barrels, gun shields and forgings of any kind which have to withstand heavy wear and tear. The vanadium content of such steels varies from 0.1 to 0.4 per cent. Vanadium is occasionally used in certain tungsten alloys for making high-speed tool steel, the introduction of a small proportion of vanadium decidedly reducing the proportion of tungsten required to give such alloys the desired hardness and toughness.
Arnold has given some illustrations of the effect of vanadium on steels of different types:
One plain carbon steel containing about 1 per cent. of carbon had a yield point of 35 tons per square inch, a maximum stress of 60 tons per square inch, an elongation of 10 per cent. on 2 inches, and a reduction of area of 10 per cent. The addition to this steel of about 0.6 per cent. of vanadium raised the yield point from 35 to 65 tons, the maximum stress from 60 to 86 tons per square inch, still leaving an elongation of 7 per cent. and a reduction of area of 8 per cent.
A steel containing 0.25 per cent. of carbon and 3.3 per cent. of nickel gave a yield point of 33 tons, a maximum stress of 42 tons per square inch, an elongation of 26 per cent. on 2 inches, and a reduction of area of 53 per cent. A practically identical steel, containing in addition about 0.25 per cent. of vanadium, gave a yield point of 50 tons instead of 33, and a maximum stress of 68 instead of 42 tons per square inch. The elongation was 17 per cent. on 2 inches and the reduction of area 36 per cent.
A steel containing 0.25 per cent. of carbon and about 1 per cent. of chromium registered a yield point of 27 tons and a maximum stress of 41 tons per square inch, with an elongation of 36 per cent. on 2 inches and a reduction of area of 55 per cent. The addition of 0.25 per cent. of vanadium raised the yield point from 27 to 40 and the maximum stress from 41 to 55 tons per square inch. The elongation was lowered from 36 to 26 per cent., and the reduction of area from 55 to 53 per cent.
Vanadium, therefore, differs from tungsten in having an extremely beneficial effect, not only on tool but also on structural steel. Arnold has shown that vanadium seemingly does not form a double carbide with[164] iron, but gradually takes the carbon from the carbide of iron until, if about 5 per cent. of vanadium is present, Fe3C can not exist, and only a vanadium carbide, V4C3, containing 15 per cent. of carbon, is present; and this constituent is constant, at least in tool steels containing 5 to 14 per cent. of vanadium. The micrographic analysis of such alloys has resulted in the discovery of three new constituents, namely, vanadium pearlite, vanadium hardenite, and vanadium cementite.
Chromium-vanadium steels are the latest development in structural alloy steels that have gained an extensive market. Almost all these steels are made in the open-hearth furnace; the chromium and vanadium alloys being added shortly before casting. In their physical properties these steels are much like chrome-nickel steels, but they have a greater contraction of area for a given elastic limit than the latter. The greater part of the chrome-vanadium steels made goes into automobiles. Some manufacturers prefer such steels because of their greater freedom from surface imperfections, notably seams, which steels containing nickel are prone to have if the ingots are at all unsound. These steels are almost always used in the heat-treated condition, but even in automobiles some frames, forgings and shafts are made of the steel in its natural state.
Some chrome-vanadium steel is said to be used in armor plate of medium thickness, which is not face-hardened but has high resistance imparted by heat treatment.
Vanadium is also used to some extent in making bronzes, in medicine and in dyeing.
—Several substitutes, chiefly titanium and molybdenum, have been claimed to give the properties of vanadium in steel. Both of those metals give to steel some of the properties that are usually associated with vanadium, but neither one takes the place of vanadium entirely.
The Primos Chemical Co. (see later) has its own patented method for treating roscoelite, the ore found at Newmire, Colorado. This method consists in roasting the ore with salt containing a little pyrite, and is a method that is applicable to some extent to most vanadium ores that do not carry lead. The American Vanadium Co. has a secret process for the treatment of its Peruvian ores. This method has not been published. The treatment of vanadinite, cuprodescloizite and carnotite ores has been studied by the U. S. Bureau of Mines, at Golden, Colorado. Whatever change in practice takes place is likely to be mainly in the concentration of vanadinite and in the treatment of this mineral and cuprodescloizite.
[165]
—The largest deposits of vanadium in the world, and the most important, were until recently controlled by the American Vanadium Co. of Pittsburgh, Pennsylvania, which in 1919 was absorbed by the Vanadium Products Corporation, allied to the Bethlehem Steel Corporation. These deposits are at Minasragra, Peru, 20 miles from Cerro de Pasco. The area lies along the western limit of a broad anticline in Juratrias and Cretaceous rocks. A section shows the series in this locality to be composed of green shales, thin beds of limestone, and red shales. Vanadium is found only in the red shales. The deposit proper appears to be a lens-shaped mass, 28 feet wide and 350 feet long. The ore contains several minerals. The mineral that constitutes the large portion of the deposit is called “quisqueite”; it is a black carbonaceous substance containing sulphur. There is also a lesser quantity of a coke-like material. Neither of these contains vanadium. The vanadium is mostly at the southern end of the ore body, and to a depth of 20 feet is largely in the form of red calcium vanadate, which is brighter colored then the calcium vanadate in Colorado and Utah, and carries as much as 50 per cent. vanadium oxide. It occurs in small pockets and fills the cracks and fissures in a fine shale. Below this shale is the “mother lode,” which is 9 to 30 feet thick, extends along the greater length of the deposit, and carries as high as 10 per cent. vanadium oxide and nearly as much sulphur. On the east and south sides, below the “mother lode,” is a hard blue-black vanadium shale, carrying as much as 13 per cent. vanadium oxide and 4 to 5 per cent. sulphur. Patronite, the main vanadium mineral, is greenish black and contains 19 to 24.8 per cent. vanadium oxide and in places 50 to 55 per cent. of combined sulphur. The patronite originally almost reached the surface and is most abundant in the north half of the lens. The whole ore body is almost completely inclosed by porphyry dikes and contains two or three intrusions. These deposits are controlled by the American Vanadium Co., and its successor, the Vanadium Products Corporation, through a concession from the Peruvian government. They are large, but are by no means inexhaustible, and as they are entirely local they are not likely to be duplicated. No similar deposits are known, either in Peru or in any other part of the world.
In 1917 the American Vanadium Co. treated 5,236 gross tons of ore, from which it extracted 2,122,005 pounds of vanadic acid. From this vanadic acid the company manufactured 4,925,014 pounds of ferrovanadium. The company did not buy any ore in this country, but relied entirely upon its Peruvian production.
—The deposits in Peru are the only deposits of any commercial importance outside of the United States. Vanadium[166] is found in South Australia, associated with carnotite and other uranium minerals. Small quantities of vanadic oxide are obtained as a by-product in the treatment of these ores.
Vanadium is also associated with uranium minerals in the Andijan district, Central Asiatic Russia. The vanadium is usually found as turanite, or copper vanadate; ferganite, an ortho-vanadate of uranium; and as several other new minerals. The amount of ore seems to be reasonably large, and this district may ultimately become a source of both uranium and vanadium.
The lead ores of Mexico contain some vanadium, the best known deposits being in the northeastern part of the State of Chihuahua. Other deposits are reported in Zacatecas, Guanajuato, San Luis Potosi, and Hidalgo.
—The principal vanadium deposits of the United States occur in a metallographic province covering southern and southwestern Colorado, southeastern Utah, and parts of Arizona and New Mexico. Uranium and radium characterize the same province.
Probably the largest deposits of vanadium yet discovered in the United States are in southwestern Colorado in San Miguel County. These deposits were visited by Ransome and Spencer in 1899 and their description, together with notes on the chemical analyses and composition of roscoelite by Hillebrand, was published in 1900. Fleck and French have also described the deposits. Fleck and Haldane later published additional descriptions, with notes on mining operations. Hess, in 1912, published an excellent description of these deposits with notes on the possible origin, etc.
According to Cross and Purington, the country rock is composed of Jurassic and Triassic sediments, divisible into three formations, the Dolores below, La Plata above and McElmo above La Plata. The latter is composed of two heavy beds of light-colored sandstone, separated by a thin bed of limestone. The vanadium-bearing rock is the lower sandstone. It is a light to dull green, and fine-grained. Occasionally splotches of carnotite are found in the cracks and fissures, but the uranium content is too small to be worth saving.
According to Hillebrand, the green vanadium mineral to which the sandstone owes its color is not a chlorite, but is closely related to the mica, roscoelite. The ore mined has an average content of 1¹⁄₂ per cent. V2O5. These low-grade roscoelite deposits can be mined at a profit, because they are large and easily worked.
Undoubtedly these deposits have been feeling the effects of the rather large production of the last few years, and the average grade of the ore is now probably at least half of a per cent. lower in V2O5 than it was a few years ago. There is still considerable ore untouched that will average 1 per cent., or a little less. The British government for several[167] years, according to reports, has been interested in obtaining control of vanadium deposits.
The Primos Chemical Co., with works at Newmire, Colorado, and Primos, Pennsylvania, is mining these deposits, and in 1917 made this production:
Treated 60,907,000 pounds of ore; from this was produced 496,731 pounds of vanadium in the form of iron vanadate running about 34 per cent. metallic vanadium. From this was produced 417,770 pounds of contained vanadium in the form of regular 40 per cent. ferrovanadium. In 1918, up to and including July, this company mined 17,449,000 pounds of ore, from which was produced 149,343 pounds of contained vanadium in the form of vanadate of iron and 133,666 pounds of contained vanadium made in the form of 40 per cent. ferrovanadium.
In 1919 the Primos Chemical Co., was absorbed by the newly organized Vanadium Products Corporation.
Vanadium ore has been discovered in Huerfano County, Colorado, near the Sangre de Cristo Range. The vein is said to be well defined and 1 to 4 feet in width. A number of assays show 2 to 7 per cent. V2O5 content, and others 2 to 4 per cent. copper. The ore is heavy, black and banded; it contains small quantities of uranium oxide, but should be classed as a vanadium, rather than a uranium mineral. There has been no commercial production up to date.
In Eagle County, 7 miles southeast of the town of Eagle, a silver ore has been found that carries vanadium. This was located mostly in the Lady Bell mine. The ore, a dark-greenish sandstone similar in appearance to the darker types of roscoelite ore found in San Miguel County, assayed from 25 to 1,000 ounces of silver to the ton. The mine has been largely worked out for silver, the vanadium being lost during the smelting process. There is still, however, an appreciable amount of vanadium ore left, as the low-grade silver ore was not mined or treated.
A considerable amount of vanadium is obtained as a by-product from the treatment of carnotite (uranium and radium) ore. It is difficult to say just what the yield from this ore is, but it is probable that it averages about 200,000 pounds of vanadic oxide per annum. This is produced by five or six operating radium companies. These deposits are found in southwestern Colorado, around the Paradox Valley, and in southeastern Utah, extending as far as the San Rafael Swell, southwest of Green River, Utah.
There is considerable ore running one-half to 1 per cent. uranium oxide which carries from 4 to 10 per cent. vanadic oxide. In the past this ore has not been mined, because the extraction of radium from it would not pay. With a strong demand and a high price for vanadium, at least the higher grades of this ore could be mined at a profit. There is considerable of such ore at certain localities north of the Paradox[168] Valley; unfortunately, these deposits are somewhat scattered and some would involve not only long wagon hauls, but also transportation by burro to wagon roads. Only the higher-grade ore could be handled in this way at a profit, and the difficulty is to get enough to justify building a treatment plant.
The writer has been told that there are deposits of this same type of ore at Temple Mountain, 40 miles south of Green River, Utah.
A small vanadinite deposit, containing traces of wulfenite, has been found near Klinefelter Station, on the main line of the Santa Fe Railroad, near the eastern border of San Bernardino County, California. The ore is largely calcite and is low grade, averaging probably from 1 to 2 per cent. vanadic oxide.
A deposit of vanadinite in Sierra County, New Mexico, on the Atchison, Topeka & Santa Fe Railroad, was mined for a short period in 1912 and 1913. Besides vanadium, the veins contain galenite, copper carbonates, barite, fluorite and other minerals. The ore was treated at a mill close to the mine, but the whole undertaking was unsuccessful, probably because the ore was so low grade, and because of metallurgical difficulties. There are a number of other deposits of vanadinite in New Mexico, but none of them have been commercially developed in any way.
Vanadinite is found in a considerable number of places in Arizona, frequently associated with wulfenite, or lead molybdate. Indeed, one of the difficulties of producing both vanadium and molybdenum from vanadinite and wulfenite is the fact that the two minerals are frequently so closely associated that, because of the slight difference in specific gravity, it is not easy to separate them by mechanical methods. At the Mammoth mine, in Arizona, the upper levels are richer in vanadinite than in wulfenite, but at the lower levels, the reverse is true. Undoubtedly a considerable amount of vanadinite could be produced from this mine and others in the vicinity, but it is doubtful whether it could be done at a profit, even at a high price for vanadium.
The United States Vanadium Co. has a mine 4 miles from Ray Junction, Arizona, and at the mine a small mill to concentrate the ore, which is low grade, and produces vanadium from the concentrates. The amount of ore that can be obtained from this mine is somewhat doubtful. This is the trouble with vanadinite as a whole; it exists over a wide territory, but the deposits are all low grade and apparently are not extensive in any one locality.
One of the most promising deposits, as regards increased production of vanadium, is at the Shattuck mine, Bisbee, Arizona, where a large vug, or cavity, is lined with a vanadium mineral, probably cuprodescloizite. The ore carries about 8 to 10 per cent. vanadic oxide, in addition to copper and lead. This seems to be one of the best opportunities for an increased production of vanadium in this country. The Golden, Colo., station of the United States Bureau of Mines made a metallurgical study of the treatment of this ore.
[169]
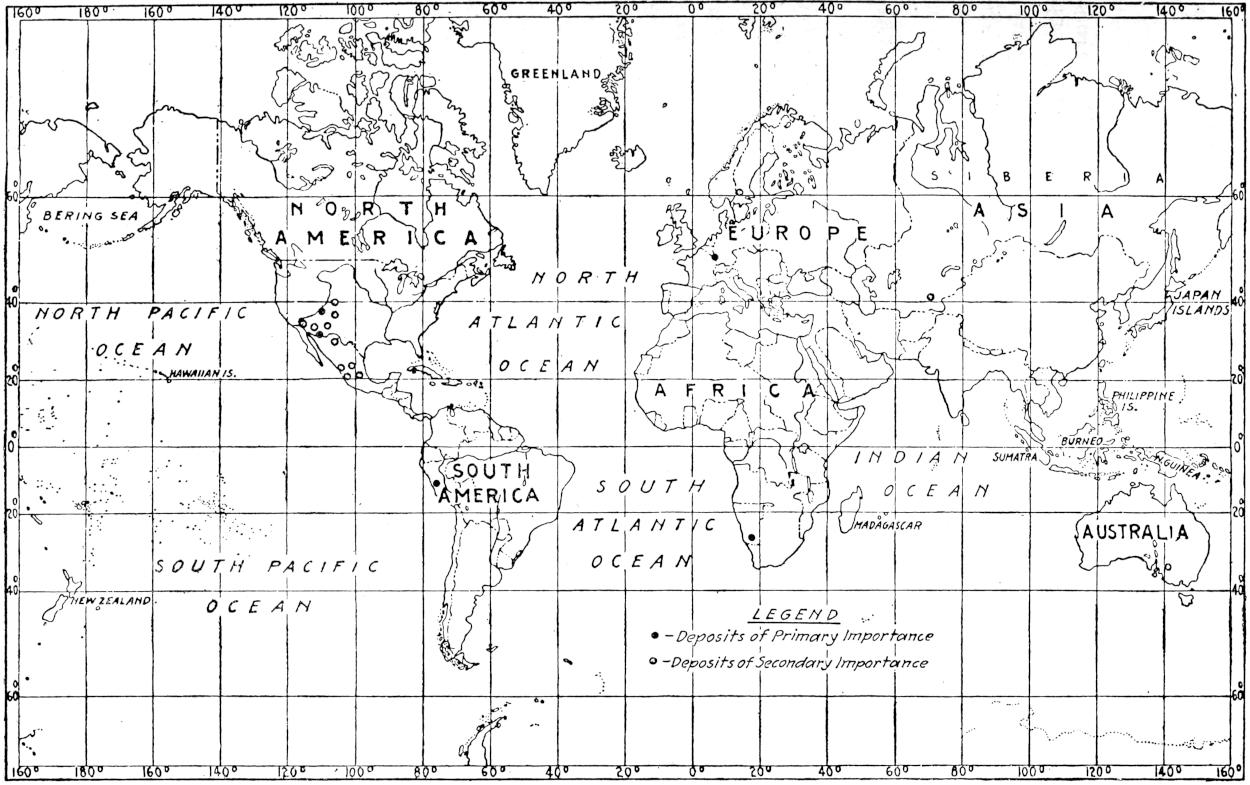
Plate V.—Geographical distribution of the vanadium deposits of the world.
[170]
The distribution of the vanadium deposits of the world is shown in Plate V.
The United States is peculiarly fortunate as regards vanadium products, for it is practically the only producer of vanadium in the world, the Peruvian deposits being under the control of an American company. Therefore, England, France, Germany, Japan and other nations are forced to buy of American companies.
The larger part of the vanadium that has been used in this country has come from the mines of the American Vanadium Co. in Peru, but the Primos Chemical Co. produced an amount of vanadium from its claims at Newmire, San Miguel County, Colorado, that at least tended to give some real competition to the American Vanadium Co. As already stated, these claims are not nearly as rich or productive as they were, but they are probably good for several years more. The ore from Peru can be counted on probably for several years at something like the present output. The same statement applies to carnotite ore, and it is likely that the production of vanadium from carnotite may increase to some extent, as the demand for radium is strong, and there may be a consequent increase in the treatment of lower-grade carnotite. As this low-grade material usually carries more vanadium, the production of vanadium from this source may increase.
Vanadinite may prove to be a source of vanadium, although it is doubtful whether any large quantity can be produced from this mineral. As already stated, cuprodescloizite is probably the best source for an immediate increase in production.
As regards foreign countries other than Peru, Russia is the only one likely to produce any appreciable amount of vanadium ores, and undoubtedly no such production will be obtained until industrial conditions are more settled.
The American Vanadium Co. holds its mines in Peru through a concession from the Peruvian government. Thus at least two-thirds of the vanadium production of the world is practically in the hands of the Peruvian government, although the company operating is American.
[171]
Formerly the American Vanadium Co. was the only producer of vanadium products and ferrovanadium in the world. The price of vanadium was then somewhere around $5.00 per pound for the metallic vanadium content of the ferrovanadium. Later the Primos Chemical Co. came into the field, and the American Vanadium Co. cut the price. On account of the large deposits of ore that the Primos Chemical Co. had in Colorado, the result was simply a lowering of the price of ferrovanadium. Undoubtedly, if it were not for this competition the price of vanadium during that period would have been higher than it was, and if it were not for the Primos Chemical Co., the American Vanadium Co. would have had practically a monopoly of the whole vanadium production, as the output from carnotite was not large enough to affect the market seriously. As it was, these two companies controlled more than 90 per cent. of the ore supply, and thus the recent change of ownership to the Vanadium Products Corporation will enable the latter to fix the price, as well as to regulate the consumption and thus prolong the availability of a useful metal which otherwise would be likely to soon become exhausted. The principal vanadium deposits of Chihuahua, Mexico, are controlled by the Madero estate (Mexican).
This dominance of control of sources of supply has made control through ownership of reduction plants, patents and secret processes of less importance.
[172]
The following summary of the uses of antimony is taken from Mineral Resources of United States, 1918.[90]
[90] Bastin, E. S.: “Antimony in 1918,” Mineral Resources of the United States, 1918. U. S. Geol. Survey, 1919.
Peace uses.—Metallic antimony unalloyed has few industrial uses. In the form of fine powder, known as “iron black,” it is used for producing the appearance of polished steel on articles made of papier-maché or pottery. For these purposes it is precipitated by the action of metallic zinc in an acid solution of antimony salts. Antimony alloys readily with most heavy metals and the alloy is harder than the two pure metals. Most of these alloys possess the property of slight expansion on solidifying. Type metal is an alloy of antimony, lead, and tin; babbitt, anti-friction, or bearing metal is usually an alloy of antimony, tin, and copper. Britannia metal, also known as “white metal,” is an alloy of antimony, tin, and copper, with some zinc, and, rarely, small quantities of other metals. It is used in making cheap domestic tableware, teapots, and spoons. Antimony alloys find minor utilization in battery plates, toys, cable coverings, and siphon tops. Lead-antimony alloy or hard lead is used in making acid-resisting valves.
White antimony oxide, mainly the tetraoxide (Sb2O4), is used for making opaque white enamel and other sanitary ware. In this use antimony oxides compete with tin oxide. Antimony oxide, mainly trioxide, is used as a coloring agent in the manufacture of glass, as it is more readily fusible than tetraoxide and does not impart opacity to the glass. Antimony oxides are further used as paint pigments.
The red sulphides of antimony are used in vulcanizing and coloring red rubber and also as paint pigments. The natural antimony trisulphide, stibnite, enters into the composition of safety matches or of the compound that is put on the match box.
Antimonate of lead containing an excess of lead oxide, known as “Naples yellow,” is used in oil paints and in the glass and ceramic industries. The antimony salt, tartar emetic (double tartrate of antimony and potassium), and antimony fluoride are employed as mordants in dyeing. Tartar emetic and antimony trioxide are employed medicinally.
War uses.—Antimonial lead carrying 12 to 13 per cent. of antimony is employed in the manufacture of shrapnel bullets. Smaller quantities of liquated antimony sulphide are used in the primers of shells. For this last purpose it is claimed the material must carry less than 2 per cent. of impurities insoluble in[173] hydrochloric acid. Antimony sulphide as a powder is used in the charge of some shells to produce on explosion a white smoke which is of service in range finding.
During the war Germany used antimony to some extent as a substitute for more important metals in the manufacture of currency, but shortage of antimony itself did not allow this use to become important.
The geological distribution of commercial antimony depends, with a few exceptions, upon the distribution of the principal ore mineral, stibnite (antimony sulphide). Cervantite, sernarmontite and valentinite are antimony oxides resulting directly from the decomposition of stibnite near the surface, and with other oxidized products form the chief ores of certain districts. Metallic antimony, also a result of oxidation of the sulphides, is rarely found, and still more rarely is it an ore mineral. Jamesonite, the sulphide of antimony and lead, is of frequent occurrence and is the principal ore of one important deposit in Mexico. Antimony is also recovered from the refining of antimonial lead.
Stibnite occurs in quartz veins and related deposits. Many of the important antimony deposits of the world occur in more or less close genetic relationship with eruptives of Tertiary age. The ore often gives way to pyrite with depth. A few important deposits occur in connection with intrusive rocks formed at considerable depth and are probably of contact metamorphic origin.
Although there is a wide diversity in the forms of the deposits and the nature of enclosing rocks, stibnite shows a distinct tendency to form replacements in limestone. The chief gangue minerals are quartz and calcite. Of other sulphides pyrite is the commonest, but cinnabar, realgar, chalcopyrite, galena, and sphalerite are often present as accessories. A characteristic feature of stibnite deposits is the relative scarcity of other sulphides; and it is equally true that important sulphide deposits of other metals rarely contain stibnite. An exception to the general rule is cinnabar, the sulphide of mercury, which is a characteristic mineral of certain stibnite veins. Likewise stibnite is one of the minerals most frequently associated with cinnabar deposits.
Several of the most important antimony districts owe their production of that metal to the presence of recoverable amounts of gold. This is true of certain French, Hungarian, Australian, and South African deposits.
Although antimony has been produced at times from a great many localities in the world, in only a few countries have deposits been developed to an important extent commercially. Under normal conditions of consumption the potential supply of antimony ore is far in excess of the demand. Consequently only those deposits that can be cheaply[174] worked and are favorably situated with regard to markets, or contain appreciable amounts of other minerals, principally gold, have been extensively exploited.
The antimony-producing countries of the world may be divided into three groups as follows:
1. Chief producing countries in order of importance: China, France and Algeria, and Mexico.
2. Countries in which production is irregular in normal times but in which potential reserves are considerable, and production becomes important at high-price levels: The former Austrian Empire, Bolivia, Australia (Victoria), Burma, South Africa, Italy, Spain, and Asia Minor.
3. Countries in which normal production is small and in which known reserves are probably less important: United States and Alaska, Canada, Peru, Germany, Turkey (Asia Minor), Serbia, Portugal, Borneo, Indo-China, and Japan.
Plate VI shows the geographical distribution of the chief antimony deposits of the world.
The production statistics of the various countries are so little in accord that it is impossible to give more than a rough comparison between the important producers. As nearly as can be estimated the output of antimony ore in 1913, the last year for which even approximately complete statistics are available, amounted to about 20,000 metric tons of recoverable antimony. The consumption is even more difficult to estimate, as customs figures for different countries vary widely. The following table shows the relative importance of the principal producing and consuming countries in terms of percentage of the world’s output in 1913:
Table 31.—Percentage of Antimony Produced and Consumed
| Country | Percentage of production |
Percentage of consumption |
||
|---|---|---|---|---|
| Austria-Hungary | 4 | 4 | ||
| France | 24 | 20 | ||
| Germany | (small) | 20 | ||
| Italy | 2 | 2 | ||
| United Kingdom | 0 | 12 | ||
| Serbia | 1 | 0 | ||
| Asia Minor | 1 | 0 | ||
| Japan | 0 | - | 10 | |
| China | 51 | |||
| Algiers | 1 | 0 | ||
| United States | 0 | 32 | ||
| Mexico | 11 | 0 | ||
| Australia (Victoria) | 4 | (small) | ||
| All other | 1 | (small) | ||
| 100 | 100 | |||
[175]
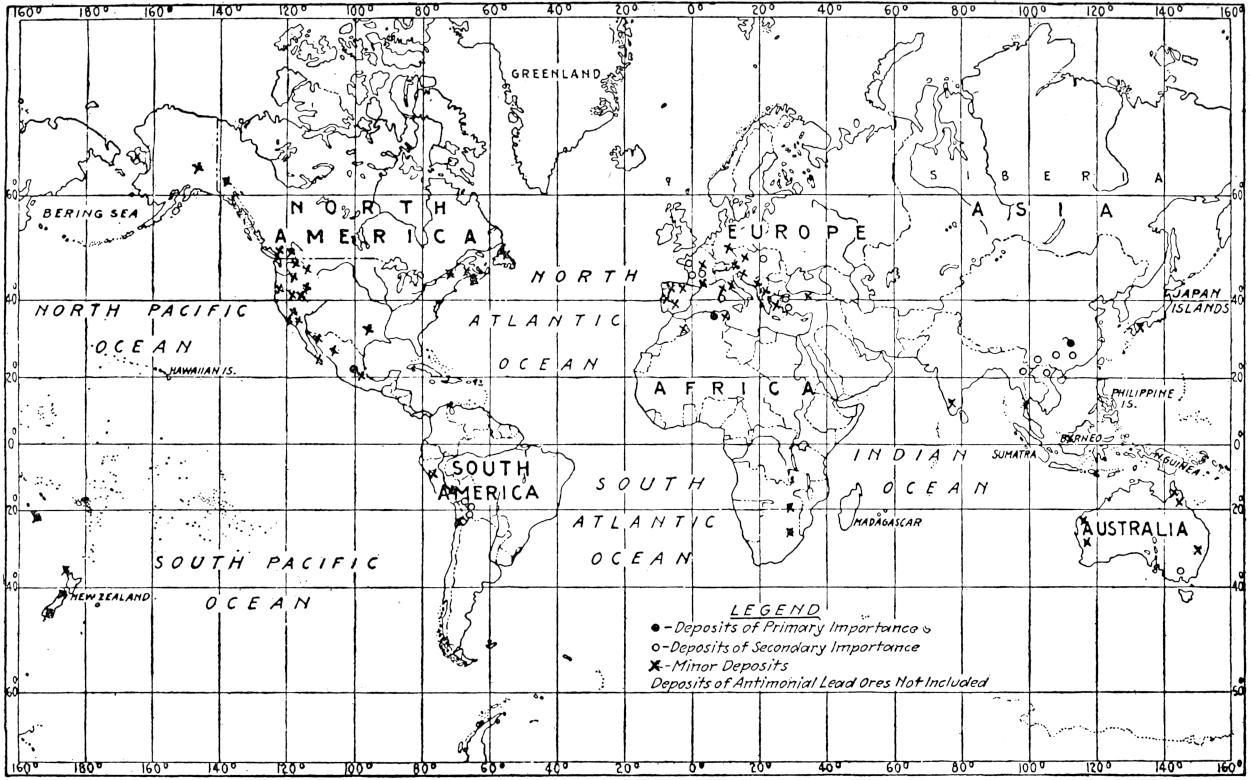
Plate VI.—Geographical distribution of the antimony deposits of the world. By H. G. Ferguson and D. A. Hall.
[176]
In the following discussion of the world’s antimony resources, political control is largely indicated by the country headings, under which are summarized the essential features of the commercial control of production, by ownership of mines and reduction plants, and by trading interests.
—Antimony deposits occur in many places in the United States, but during peace times the comparatively high costs of mining in this country do not permit competition with the Chinese and Mexican mines. A small production of antimonial lead from domestic ores was made prior to the war and a small amount of antimony recovered as a by-product of lead refining, but except for this the country was entirely dependent upon imported antimony.
High prices following the outbreak of the war brought a quick response in the production of antimony ores. The mine production of antimony ore in 1915 was about 5,000 short tons containing 2,100 short tons of metal, and in 1916 was 4,500 short tons containing about 1,770 short tons of metal. The lower prices of 1917 were reflected in the decreased output for that year, amounting to 1,060 short tons of ore containing 390 tons of metal. The 1918 production was 190 tons, containing about 50 tons of metal.
The chief producing states in order of importance were Nevada, California, Alaska, Washington, Oregon, Idaho, and Arkansas. Utah and Arizona yielded insignificant amounts. In Nevada the Sutherland mine, in Humboldt County, was the principal producer. In California, the greater part of the output was from two mines in Inyo and Kern counties operated by the Western Metals Co., of Los Angeles, the ore being shipped to San Pedro, near Los Angeles, for smelting. In Washington the antimony was produced at the property of the Gold Creek Antimony Mining & Smelting Co., in Okanogan County. In Oregon the Jim Dandy mine, near Baker City, was the principal producer. In Arkansas one property in Sevier County yielded a noteworthy output of ore in 1916 and also a small quantity of metallic antimony at a local reduction plant. In Alaska the production was mainly from the Fairbanks district, the ore being shipped to Los Angeles and Seattle for smelting.
The ownership of mines within the United States does not play an important part in the present control of the world’s resources, inasmuch as production under normal conditions is insignificant. It is possible that with the development of new uses for antimony, and a greater demand for that metal, the reserves in this country may become of commercial importance.
About 40 per cent. of the Mexican antimony output is controlled in the United States. An American-owned smelter was built during the war[177] at San Luis Potosi. Ores from mines in this region have also been shipped to the Western Metals Co. at Los Angeles. An American concern, the Antimony Corporation, owns a large deposit of jamesonite, antimony-lead sulphide, in Mexico, which constitutes an important reserve that has not yet been developed. Antimony deposits in northern Mexico were worked by American capital during the war. Prior to the year 1914 only one company in the United States had attempted the smelting of antimony. During the war considerable activity prevailed, however, and several companies undertook the smelting of foreign and domestic ores. China, Bolivia, and Mexico were the principal sources of supply. The success of all these enterprises has been only temporary, as under normal conditions the high cost of production in this country prevents successful competition with Chinese and Japanese metal. American smelting interests exert little control on the antimony of the world at present, and can not be expected to do so in the immediate future. The smelter capacity of the country is estimated at 6,000 to 7,000 tons of metal per annum, all of which is now idle. One company, the Antimony & Compounds Co. of America, is closely connected with a French company, La Lucette.
—Antimony production in Canada has been extremely irregular. During periods of high prices a considerable output was obtained, the years of maximum production being 1898, with shipments of 1,344 short tons of ore, and 1907, with 2,016 short tons. Mining ceased in 1910 and was not resumed until 1915. In 1915, 1,341 tons of 40 per cent. (metal content) ore were produced; in 1916, 885 tons of 42 per cent. ore; and 361 tons in 1917.
The principal producing district is at West Gore, Nova Scotia, where the ore in addition to its antimony content has a tenor of 2 to 4 ounces of gold per ton. Other regions that have produced antimony ore are New Brunswick (York County), British Columbia, Quebec, and Yukon Territory. In British Columbia (Slocan District) and Yukon Territory (Chieftain Hills) antimonial lead ores are also worked. The Nova Scotia ores, which furnished the bulk of the production, have been exported to England since 1915; the earlier production went to Germany. The small production of refined antimony came chiefly as a by-product of lead refining at the smelter of the Consolidated Mining & Smelting Co., at Trail, B. C.; a small amount of antimony was also smelted from antimony ores by the New Brunswick Metals, Ltd. (formerly the Canadian Antimony Co.) at Lake George, N. B. Canada appears to be in about the same position as the United States with reference to antimony mining. High prices, continued over a long period, will bring out a considerable production, but no output is to be expected at peace-time prices.
—Antimony deposits exist in many parts of Mexico and there has been a considerable production for many years. As in other countries,[178] the output increased largely during 1915 and 1916. The production of metallic antimony for 1917 is reported as 2,141 metric tons. In 1914 there were exported to England 1,543 long tons of crude antimony and regulus; there were no exports to England in 1918, but 1,449 short tons of ore and 2,660 of metal were shipped to the United States.
The principal mines are in the Sierra Catorce, in the states of San Luis Potosi and Queretaro. The ores are mixed sulphides and oxides and carry 5 to 50 per cent. antimony.
A smelter with an annual capacity of 6,000 tons of metal was built at Wadley in 1900, and most of the production formerly went to England, but since 1915 has been marketed in the United States. The smelter and most important mines are owned by Cookson’s, of England, through a subsidiary, the Republican Mining & Metal Co. American interests own other properties in the same region, and during the war a smelter was constructed at San Luis Potosi by the International Mining & Metal Co. In western Sonora, near the Gulf of California, there are deposits of oxidized ores that furnished a considerable part of the ore imported into the United States. These are owned by American capital.
A large deposit of lead-antimony ore (jamesonite) at Zimapan, Hidalgo, owned by The Antimony Corporation, an American firm, has not yet reached the producing stage. Other deposits of possible importance are known in the states of Guerrero, Durango, Sonora, Mexico, Baja California, and elsewhere.
Political disturbances during the last few years have prevented an output of antimony commensurate with the probable capacity of the deposits. Production will probably be maintained in the future, even during periods of low prices.
—The output of Bolivia was negligible before the war, but under the stimulus of high prices large amounts of high-grade ore were produced in 1915 and 1916. This ore was shipped principally to England, until an embargo was placed on Bolivian ore in 1918. The ore is high grade, that shipped averaging over 50 per cent. antimony, but the veins are small and become unproductive at shallow depth. It is possible that the known deposits have been largely exhausted; and although demand as strong as that of 1916 might result in new discoveries of importance, it is not likely that Bolivia can be an important producer when prices are under normal conditions.
Exports of ore amounted to 17,923 metric tons in 1915 (as against 186 in 1914); to 22,748 tons in 1916; and to 18,340 tons in 1917; but in 1918, for reasons given above, shipments dropped to 3,070 tons. From 75 to 90 per cent. of the ore went to England, and most of the remainder to the United States, except for about a thousand tons a year to France.
[179]
—During the antimony boom of 1906-1907 a small amount of antimony was produced in Peru. No further production was made until 1915, when 522 tons of high-grade ore was mined. In 1916 the production rose to 1,876 tons of 60 per cent. ore. The 1917 production was 902 tons. Although deposits are known in many parts of the republic, over 90 per cent. of the production has come from the department of Puno, in southern Peru. Up to the present, profitable mining has been possible only during periods of high prices, but the deposits are said to be extensive, and it is possible that improved transportation facilities would result in some production under normal conditions.
—The most important antimony deposits of the old Austrian Empire are those of Hungary and Bohemia. Others of minor importance are in Carniola, in Austria. The Hungarian deposits in 1913 furnished 11,017 tons of ore containing 1,038 tons of metal; for the rest of Austria the output was 1,270 tons of ore, but only 89 tons of metal. The low antimony content of the Hungarian deposits is compensated by the gold content, and these deposits have produced much more regularly than those of the other parts of the empire. So far as known the reserves are fairly large, but production can hardly be expected to increase greatly.
The productive capacity of both the Hungarian and Bohemian deposits is probably enough to supply local needs in normal times, and allow a surplus for export when economic conditions are favorable. Prior to the war, exports of regulus went to Germany and small amounts of ore were exported to France and England. During the war the Central Empires probably depended largely on the Austro-Hungarian deposits for their antimony supplies. All mines were worked by the government. It is known that certain mines that had been abandoned resumed operations.
—France is the most important antimony-producing country in Europe and also controls important productive deposits in Algiers and Indo-China.
The French deposits are numerous but for the most part small. The most important of these is La Lucette, in Mayenne, where stibnite associated with auriferous pyrite has been mined for many years. This deposit was considered to be approaching exhaustion, but recent work is reported to have developed new ore bodies. The La Lucette company has recently extended its holdings in other parts of France, has bought properties in Algiers and the Transvaal, and in 1911 leased a smelter at Barcelona for the treatment of ores purchased abroad. The La Lucette company is also to some degree associated with the American firm of Antimony & Compounds Co. of America. Undeveloped deposits are known in Tunis, Morocco, French Guinea, and Madagascar.
[180]
French control of foreign supplies is not of great importance. In addition to its holdings in the Transvaal, the La Lucette company purchases some foreign ores. In 1913, 4,440 tons of antimony ore was imported from China, and 205 tons from Turkey. Foreign control of French deposits is limited to a few companies. An Italian company, Minière Fonderie d’Antimonio, owns concessions in France and Corsica, from which the production before the war was about 3,500 tons of ore per year. The great Belgian smelting company, Société de la Vieille Montagne, owns the most productive Algerian deposit—the Hamman N’ Bails mine; and an unimportant Algerian mine was, prior to the war, owned by Beer, Sondheimer & Co., a German firm.
In 1913, France produced 20,872 metric tons of ore carrying about 32 per cent. metal content. The smelter output in 1913 was 6,390 tons of regulus and oxide. France is normally an exporter of metallic antimony, the average annual exports during the period of 1910-1914 amounting to about 2,000 metric tons. The principal purchasers were United States, Germany, Italy, Netherlands, and Russia. According to recent information, the surplus production of antimony in France is now so large that the industry can hardly continue to exist on a paying basis unless the producers come to an understanding among themselves. It is clear, however, that to be effective, any agreement among French producers must be either backed by a high protective tariff or must be extended to include their principal foreign competitors.
—In Germany the antimony output is too small to affect appreciably the world’s market, but a few localities have possibilities of production when the price of antimony is sufficiently high. One plant in the Eifel district in 1915 was producing 25 to 30 tons of regulus and 60 to 70 tons of oxide a month. There was, however, a production of antimonial lead from the smelters that may amount to 1,000 tons or more of antimony a year. This is derived in part from German ores, especially the lead-zinc ores, and in part from ores of foreign origin.
Germany’s interest in the antimony market is chiefly that of the smelter and middle man. Average annual imports, 1910-1913, were as follows: Ore, 3,668 tons; metal, 3,398; salts, 668. The exports averaged, ore, 566 tons; metal, 331; salts, 1,226. The principal purchasers of metal were the United States and Russia; and of salts Russia and England.
German interest in foreign deposits was not extensive. The Metallgesellschaft seems to have had some connection with an Italian company, the Minière Fonderie d’Antimonio, owning mines in Italy and France, and Beer, Sondheimer & Co. was recorded as the owner of one Algerian mine.
—Although deposits were formerly worked in Cornwall, Devon, and elsewhere, no antimony has been mined in England since 1892, but before the war England was the chief smelting center of the[181] world, and several brands of British antimony, such as Cookson’s and Hallett’s, had a world-wide reputation. Deposits of considerable importance exist in many of the British possessions.
Cookson & Co., of Newcastle, control mines in the Catorce district, San Luis Potosi, Mexico, and operate a smelter, the output from which was shipped to England for further refining until 1915, when the supply was in large part diverted to the United States.
England, through her smelting interests, has played an important part in the antimony trade of the world. Seven smelters in England refine ore and crude metals that come chiefly from China, but also from Mexico, Australia, and Hungary, and, during the war, in large quantities from Bolivia and Spain. The better British brands have been considered more pure than other grades, and before the war virtually monopolized American markets.
British trading interests have exerted important control both in securing raw material for British smelters and in obtaining markets for British metal. Until 1914 the Chinese Eastern Antimony Co., a subsidiary of Cookson & Co., held contracts for the production of the Wah Chang Mining & Smelting Co., the most important antimony producers in China. In 1914, the Wah Chang Co. established an independent selling agency in the United States. During the great demand for antimony in 1915 and 1916, British interests secured the greater proportion of the output of Bolivian mines and completely controlled the industry of that country.
—Italy is the third important antimony producer of Europe. The principal deposits are those in the southern part of the island of Sardinia. During the war, however, the Tuscan deposits were reopened and there has been also a small production from Sicily. The grade of the ore is low, probably on the average less than 25 per cent., and the production, which was 7,609 tons of ore in 1900, had fallen in 1913 to 1,822 tons. War conditions stimulated the industry, and in 1915, 1916, and 1917 the production averaged over 5,000 tons annually, although the imports of metallic antimony also increased, being as follows: 191 tons in 1914; 825 in 1915; 155 in 1916; and 1,247 in 1917.
The low metallic content of the ore, together with the fact that in the Sardinia deposits the calcite gangue makes recovery more difficult, renders it probable that under peace-time conditions and prices, Italy will not become an important factor in the world’s antimony production.
The chief producing company, Minière Fonderie d’Antimonio, was, prior to the war, closely connected with the German Metallgesellschaft, and the richest Sardinian ore went to Germany for smelting. Besides mines in Italy, this company owned several productive deposits in France.
[182]
—A small amount of antimony ore, 100 tons in 1912 and 19 tons in 1913, is produced in Portugal. Exports in 1916 exceeded 4,000 tons.
—Antimony and argentiferous lead-antimony deposits are known in the Urals and were under development in 1912. Antimony deposits also occur in the Amur province and in many localities in Siberia. In 1915, 67 tons of regulus was imported into England from Russia. Possibly, however, this represents an overland shipment of Chinese material.
—Serbia contains several antimony deposits of considerable promise. The production, however, has been small. No data are available since 1912. The output of regulus and oxide, which amounted to 4,725 metric tons in 1904, had decreased to 297 metric tons in 1912. The greater part of the product was formerly shipped to the United States. Plants at two of the mines are capable of a considerable output should conditions warrant it. It seems unlikely, however, that the Serbian deposits will play an important part in determining the control of the world’s production.
—Antimony deposits are known in many localities in Spain. Most of these are irregular and have been repeatedly worked and abandoned. A few, however, offer some promise of a continued output. The annual production has scarcely exceeded 500 metric tons of ore, even under the stimulus of war conditions (516 tons in 1916, and 502 tons in 1917). The smelter production in 1916 was 425 tons. Shortly after the outbreak of the war there were three smelters, the most important being operated by the French company, La Lucette.
Before the war Spain imported annually 800 tons of antimony ore from France, and over 100 tons of salts of antimony was exported annually to Germany.
—British Borneo was formerly a producer of considerable importance, and much ore was exported between 1859 and 1894, mainly to England. The deposits then remained idle until 1914, when 870 tons of ore was exported; in 1915 the exports amounted to about 360 tons. It is probable that no important output at peace-time prices is to be expected, although the country is largely unexplored. The Borneo company (British) seems to have been the principal if not the only producer.
—With all her vast mineral resources China has been able to obtain an important position in the world’s markets with regard to but few metals. Of these antimony is the most striking example, for since 1908 over 50 per cent. of the world’s total antimony production has come from China. In 1913 the output was estimated to be the equivalent of 10,800 tons of metallic antimony, that of the whole world being about 20,000 tons. The Chinese industry being well-established, it was able[183] to respond rapidly to the great demand of the war. Exports increased from 14,361 short tons of regulus and crude antimony, and 4,795 tons of ore, in 1913, to 38,142 tons and 8,667 tons, respectively, in 1917.
Antimony is found over widely scattered areas in the central and southern provinces, but chiefly in the provinces of Hunan, Yunnan, Kweichow and Kwangsi. In Hunan the deposits have been most extensively exploited, probably 90 per cent. of the total production of China coming from the region about Changsha, the center of the smelting industry. Here, in the Hai-Keng-Shan district, in 1915 about 70 companies mined antimony along the outcrop of the deposits. The ore, remarkable for its purity, occurs as pockets and bunches, mainly of stibnite, in a flat bed of dolomitic limestone. Several local smelters produce liquated sulphide, and the output of the district is about 1,000 tons monthly of crude antimony averaging about 70 per cent. metallic antimony. All regulus manufacture is controlled by the Wah Chang Co. In the Panshi district the ore occurs as fissure veins in slates, shales, and quartzites. The output consists of about 400 tons monthly of 30 per cent. ore, all of which is shipped to Wah Chang Co. at Changsha for treatment.
The only district in Yunnan where antimony is dealt with commercially is near Chihtsun on the Tongking-Yunnan Railroad. The Pao Hua Co., connected with the Wah Chang Co., owns a French-constructed plant and produces high-grade regulus.
The Wah Chang Mining & Smelting Co. virtually controls the production of antimony ore, regulus, and crude in the Province of Hunan. This company operates smelters in Changsha and owns low-grade mines. It possesses a complete monopoly, granted by the Peking government, for the manufacture of regulus in Hunan and owns the patent rights in China for the Herrenschmidt furnace, the most successful means of reducing low-grade antimony ores. The mines themselves are mostly native-owned, and worked in a small way.
Prior to the war, exports of Chinese antimony were chiefly in the hands of English, French, and a few German firms. The New Chinese Antimony Co. (also known as the Chinese Eastern Antimony Co.) a subsidiary of Cookson & Co., of England, held a contract for the entire output of the Wah Chang Co. This contract was broken shortly after the war began, although the Wah Chang Co. paid a percentage on all sales to the New Chinese Antimony Co. for a year thereafter. The Wah Chang Trading Co. was organized as a direct selling agency in New York, and has established a large business in this country.
With present high scale of wages for labor, and prices for material, it is difficult to see how this country can compete with China in the production of antimony. Adverse exchange conditions due to the high price of silver have probably nearly doubled the cost of production in China and[184] wages in that country have advanced. In spite of this, however, China can manufacture antimony far more cheaply than is possible in Europe or America; and probably, also, more cheaply than in Japan.
Chinese antimony suffered from lack of advertising before the war, being largely excluded from this country by the British metal, but has now become firmly established in our markets, and its quality has proved equal to the best English grades.
—Since the war, small amounts of antimony have been produced in Burma and Mysore. The total Indian ore production was 1,040 tons in 1916 and 130 tons in 1917. The most productive region was the Amherst district of Burma. Here the ore reserves are said to be considerable, but the inaccessibility of the district has made production impossible except at high prices. The production from Mysore was only 26 tons in 1916.
—There are productive deposits of possible future importance in French Indo-China. In 1916 these produced 1,437 tons of antimony ore with a metal content of 642 tons. Smelters were operated by the firm of Schön & Rhay, and both native and Chinese ore was treated. In 1914 and 1915, 883 and 630 tons of antimony ore were exported to France.
—Very little antimony ore has been produced in Japan since the development of the Chinese deposits, although, as in most other countries, there was a renewed development during the war. The smelting of Chinese ores in Japan has become extremely important; and the smelter production, which was only 32 tons in 1914, rose to 8,189 tons in 1915, and to 10,633 tons in 1916. It was 6,562 tons in 1917. The production of metal and crude from domestic ores was only 186 tons in 1915 and 286 tons in 1916. It is probable that as long as cheap ore is available in China little production from Japanese deposits is to be expected.
Japanese ownership in Chinese mines is probably small, as practically all Chinese antimony bought by Japan has been purchased in the open market in the form of crude and ore. Since 1914 Japan has played an important part in the smelting of Chinese ore and matte, and in this regard has ranked second only to China. Prior to that time, however, production was insignificant. China is now in a position to supply direct the major part of the world’s requirement of metal, having largely extended her facilities for treating antimony ores, and it is doubtful whether Japanese smelters will long be able to compete successfully.
—The antimony production of the Turkish Empire comes from Eastern Asia Minor in the vilayets of Brussa and Smyrna. The productive district of Allchar, formerly in European Turkey, passed to Serbia after the last Balkan War. The deposits seem to be rich and capable of greater development. Bad government, lack of transportation[185] facilities, and excessive export duties seem to have retarded production. Some mines were the property of the Sultan, and development was hindered by excessive royalties. Most of the mines seem to be owned or leased by Greeks. Deposits of antimony ores associated with argentiferous lead ores are reported in the vicinity of Karahissar, in Armenia. In 1914 the concession for these was held by the Asia Minor Mining Co., presumably a British corporation. Undeveloped deposits of possible importance occur in the islands of Mytelene and Chios, now in Greek ownership.
Little information is available as regards production. In 1911 the Djinli Kaya mine produced 1,500 tons of 50 per cent. ore. The 1912 production of Asia Minor is reported as 677 tons of ore. Exports of antimony ore from Turkey to Great Britain were as follows: 1910, 303 (metric) tons; 1911, 773 tons; 1912, 1,108 tons; and in 1913, 408 tons. Some ore was also shipped to Austria, and, in 1913, 205 tons went to France. It seems probable that, given good government and improved transportation facilities, an increased production could be obtained from the region even at peace-time prices, for according to the best available information the deposits are large and much of the ore is high grade.
—The Algerian deposits are probably capable of considerable development, as is shown by the response to the increased demand in 1915 and 1916. The ores are nearly all oxidized and contain various rare antimony minerals. Prior to the war the chief production consisted of antimonate of iron, mined together with lead and zinc ores at Hamman N’ Bails. During the war large deposits of oxides were developed and were supplying antimony at the rate of 300 tons per month during the early part of 1918. In 1912, there was produced 4,661 tons of ore; in 1913, 582 tons; in 1914, 1,100 tons; in 1915, 9,022 tons, and in 1916, 28,473 tons. Apparently the ore produced carries around 40 per cent. antimony (metallic content).
The mine of Hamman N’ Bails is owned by the great Belgian smelting company, the Société de la Vieille Montagne; and the La Lucette company (French) owns the productive Ain Kerma oxide deposits. Prior to the war, the German firm of Beer, Sondheimer & Co. was listed as the owner of one of the less important mines.
—Several antimony deposits are known in British South Africa, seemingly the most promising being those of the Murchison Range in the northern Transvaal. Here auriferous stibnite occurs as veins and replacements in limestone over a considerable area. The ore as mined carries 3 to 6 dwt. gold and 7 to 10 per cent. antimony. Sales and shipments of concentrated and crude antimony were as follows: 1913, 48 tons; 1914, nothing; 1915, 91 tons; 1916, 722 tons; and 1917, 617 tons.
[186]
The principal mine of the range, the United Jack, was purchased in 1917 by the La Lucette company (French). In 1916 there were four producing mines in the district. The antimony deposits of the Steynsdorp district, near the Swaziland border, were under development in 1916, and antimony deposits are known in the Forbes Reef district in Swaziland. Antimony ores are found over a considerable part of southern Rhodesia, and this district would probably be capable of a considerable output with better transportation facilities and continued high prices. Ore production in 1916 and 1917 was 38 and 15 tons. Some of the mines, such as the Hope Fountain, near Bulawayo, are chiefly gold producers, antimony being a by-product.
—The only antimony-producing district of any importance in Australia is the Costerfield district of Victoria. Here stibnite and antimony oxides occur in quartz veins cutting Ordovician slates. The antimony concentrates, which average about 48 per cent. antimony, and also contain about 2¹⁄₂ ounces of gold per ton, are shipped to England. The annual production is rather regularly 2,500 to 3,000 tons of concentrates.
Table 32.—World’s Production of Antimony (1912-1917)
Approximate recoverable metal content of ore produced, metric tons; antimonial lead ores not included
| 1912 | 1913 | 1914 | 1915 | 1916 | 1917 | Principal financial control | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| United States | 0 | 0 | 0 | 1,760 | 1,420 | 310 | United States | ||||||
| Canada | 0 | 0 | 0 | 420 | 300 | 120 | Great Britain | ||||||
| Mexico | 3,500 | 2,340 | 1,570 | 200 | [91] | 450 | [91] | 2,730 | Great Britain and United States | ||||
| Bolivia | 40 | 30 | 70 | 7,170 | 9,100 | 7,340 | Great Britain | ||||||
| Peru | 0 | 0 | 0 | 260 | 930 | 450 | Peru | ||||||
| Austria-Hungary | 1,350 | 840 | [92] | [92] | [92] | [92] | Hungary | ||||||
| Germany | 0 | 0 | [92] | 700 | [91] | [92] | [92] | Germany | |||||
| France | 2,290 | 5,170 | [92] | [92] | [92] | [92] | France | ||||||
| Italy | 310 | 360 | 110 | 720 | 1,080 | 960 | Italy | ||||||
| Spain | 170 | 0 | 0 | 100 | 170 | 160 | France | ||||||
| Portugal | 40 | 10 | [92] | [92] | 1,000 | [91] | [92] | ? | |||||
| Serbia | 300 | 250 | [91] | [92] | [92] | [92] | [92] | ? | |||||
| Algiers | 940 | 180 | 320 | 2,740 | 8,940 | [92] | France | ||||||
| British S. Africa | 0 | 30 | 0 | 50 | 380 | 300 | Great Britain and France | ||||||
| China | 10,800 | 11,000 | 15,900 | 10,500 | 42,800 | 31,000 | China (Japan), Great Britain? | ||||||
| Japan | 70 | 20 | 30 | 180 | 280 | [92] | Japan | ||||||
| India | 0 | 0 | 0 | 0 | 400 | 50 | Great Britain | ||||||
| Indo-China | 110 | 0 | 0 | 160 | 510 | [92] | Great Britain | ||||||
| Borneo | 0 | 0 | 300 | 120 | [92] | [92] | Great Britain | ||||||
| Asia Minor | 270 | 240 | [92] | [92] | [92] | [92] | Turkey (Greece) | ||||||
| Victoria | 580 | 960 | 890 | 1,300 | 1,320 | [92] | Great Britain | ||||||
| New South Wales | 30 | 10 | 20 | 320 | 310 | 150 | Great Britain | ||||||
| Queensland | 0 | 0 | 0 | 80 | 80 | [92] | Great Britain | ||||||
| West Australia | 0 | 0 | 0 | 0 | 20 | 10 | Great Britain | ||||||
| Total | 20,800 | 21,440 | 24,400 | [93] | 35,400 | [93] | 78,700 | [93] | 54,300 | [93] | |||
[91] Incomplete data; actual production probably larger.
[92] No data.
[93] Totals for years 1914-1917 include estimates of production of countries from which data are lacking.
[187]
The Hillgrove district, in New South Wales, was formerly of considerable importance, the highest annual output being 2,450 tons of ore in 1906. Recent production has been slight, and although a very large increase took place with the stimulus of war prices, the 1917 production was valued at only about 5 per cent. of that of Victoria. Insignificant amounts of antimony ore have recently been produced in Queensland and Western Australia. New Zealand yielded a small amount during the boom of 1906 and 1907, but no production is recorded since 1910.
Imports of antimony ore into Great Britain from Australia in 1915 amounted to 3,854 tons.
Statistics of production (1912-1917) are given in the table preceding.
—The United States is the largest consumer of antimony in the world, requiring under normal conditions between 7,000 and 8,000 tons of new metal, most of which, before the war, came from England. The consumption during the war was about double this amount, and was derived chiefly from the Orient, South America, and Mexico. The United States must remain dependent upon foreign sources for its supply, unless a much higher tariff is placed upon imports. Even under such conditions it is doubtful whether domestic mines would prove adequate to supply more than a small part of the country’s needs.
Chinese and Japanese antimony has largely replaced the British product since 1914 and has become so well established that it will probably continue to hold American markets. Chinese antimony in particular has shown itself equal in every way to the best British grades. With a somewhat higher level of prices the importation of ore from Mexico and South America may be undertaken by reducing plants in this country, as the experience gained by several companies during the war has made possible the production of high-grade metal.
—No figures as to the actual consumption of antimony in England are available. Judged from a balance of imports and exports, the normal consumption is about 4,500 tons annually. During the war consumption was enormously increased for the manufacture of munitions. English smelters are entirely dependent on foreign ores, most of which in the past have come from China, with smaller amounts from Mexico and Australia. The position of the industry, at least in so far as export trade is concerned, is threatened by the strong position of the Chinese industry acquired during the war, as represented particularly by the activities of the Wah Chang Mining & Smelting Co. Two-thirds of the[188] English antimony exports went to the United States before 1914. It does not seem probable that England will be able to fully recover this market, now dominated completely by Chinese and Japanese antimony.
—France is the only world power that possesses important resources of antimony within her boundaries. Including her Algerian mines, she is entirely independent of outside supply. Inasmuch as certain of the French deposits contain important amounts of gold, and the principal Algerian mine contains lead and zinc, the production of antimony in France will probably continue to be of some importance, and it is probable that she will continue to export antimony as before the war, though probably to a less extent.
—Germany, prior to 1914, consumed about 20 per cent. of the world’s annual output of antimony. Her own resources of antimony are insignificant, and German interests in foreign deposits have not been widely extended but were rather those of smelter and middleman, raw material being drawn chiefly from China, and metal and salts being exported to the United States, Russia and Great Britain. During the war Germany drew largely upon Hungary for antimony supply, but it is known that this source could not adequately meet the demand.
—Japan’s actual consumption of antimony has never been large and before the war was confined largely to the production of “white metal” boxes, trays, and other articles. During the war her importance in the antimony trade rested upon her ability to supply a large part of the needs of the Allies, principally Russia, and later the United States and Canada. How long after the war she will be able to retain her position is uncertain. Favorable freight rates to Japanese shippers, and the fact that the present high price of silver and the consequent exchange conditions affect adversely Chinese production may enable Japan to continue a factor in the antimony trade.
Owing to the very high prices prevailing for antimony during 1915 and 1916, caused by a greatly increased demand for antimony for the manufacture of munitions, several countries became large producers. The most important among these were Bolivia, Mexico, and Algeria, but Victoria and the United States, Peru, Burma, and Spain all contributed substantial amounts. With the possible exception of Algeria—whose principal mines yield considerable lead and zinc and are situated near to French reduction plants—and of Mexico, none of these countries will be important factors in the production of antimony at the usual low prices prevailing for that metal.
The sudden ending of the war found the belligerents with large stocks of antimony on hand, the English holding, according to figures published[189] by the British Ministry of Munitions on March 1, 1919, 4,325 long tons of regulus. There is reason to believe that the other Allies had stocks of the same order of magnitude, and if so there must have been about a year’s supply available on April 1, 1919, as the 1913 consumption of antimony amounted to only about 20,000 tons. In addition there were large supplies of alloys and antimonial lead available and more will undoubtedly be obtained by salvage operations. It may be expected, therefore, that until these are absorbed the production of antimony will be even less than that of pre-war years. At present there is little inducement for mining. Costs of mining have increased everywhere. China, the largest producer, faces a particularly difficult situation, for the higher price of silver has resulted in doubling costs of labor and local supplies. If silver prices remain high after the demand for primary antimony has recovered, the other antimony-producing countries, not on a silver basis, will have a corresponding advantage over China in the matter of production.
The peace-time consumption of antimony is limited rather by the relatively restricted uses to which antimony is put than by any lack of potential supply. As a consequence, steady production has been maintained only from those districts in which working expenses are low and markets readily available, or in which the deposits contain other metals of value. Modern warfare, however, creates a special use for antimony—in the manufacture of shrapnel—which requires many times the amount of antimony necessary for ordinary peace uses. In the case of each of the three important wars of the last twenty years, the Boer War, the Russo-Japanese War and the Great War, the curves of antimony prices and production have risen sharply in accordance with the demand, and have fallen as rapidly after the need for munitions was past.
China has for long been the most important source of antimony and will doubtless retain that position for many years. Steady though less important production has been maintained in France, Austria-Hungary and Mexico, while several other countries produced important amounts as a result of the largely increased demands of the war. Chief among these were Bolivia, Algeria, and Australia.
England dominated the antimony market prior to 1914 through her large smelting interest, trade agreements in the Orient, and selling agencies in America—the principal consuming country. Since that time, however, Chinese interests have become independent, and Japan has become of importance in the antimony smelting and trading field.
The United States possesses limited antimony resources which can be exploited only at very high prices, and is dependent almost entirely upon[190] outside sources of supply. In the past this supply has been drawn largely from England, but more recently from the Orient and Mexico.
Germany has insignificant antimony resources of her own, and depended for her supply during the war upon the Hungarian deposits, which were apparently inadequate to meet the demands. Her interests prior to the war were chiefly those of the smelter and middleman, and did not extend very largely to foreign deposits.
The United States, France, Germany and Great Britain normally consume 85 per cent. of the antimony of the world, and of these France alone is independent of foreign sources of supply.
The antimony trade of the world is largely controlled by a few companies, of which the most important are: Cookson’s (British), Wah Chang Co. (Chinese), and Société de La Lucette (French). The Mitsui Co. (Japanese), largely through shipping interests, has a considerable share in the Chinese and Japanese antimony trade.
[191]
Molybdenum is used in the manufacture of ferro-alloys for making steel. As wire, it is used for supporting the filament in incandescent electric lamps. The wire is also employed for winding electric resistance furnaces and for this use has proved cheaper and better than platinum because of the quicker heating and higher temperatures attainable. The metal has been successfully substituted for platinum and for platinum-iridium in electric contact-making devices. Molybdenum compounds are used in chemistry, particularly ammonium molybdate for the determination of phosphorus. Fast colors in a variety of shades may be produced on leather by employing molybdenum tannate in conjunction with logwood extracts. It has been employed for color glazes in porcelain and in coloring silks and rubber.
The addition of molybdenum to steel increases the elastic limit without diminishing the ductility. Molybdenum can be substituted for a certain percentage of tungsten in high-speed steel, as a rule one part of molybdenum taking the place of two to three parts of tungsten.
Up to about 1916, practically all of the molybdenite concentrates produced came from Queensland, New South Wales, and Norway. Shortly after the opening of the war, interest was shown in the production of molybdenite in Canada, principally in the provinces of Ontario and British Columbia. During 1917 and 1918 there was a great deal of interest in the United States in molybdenum ores, and at the present time this country can probably produce molybdenite concentrates in quantity equaling if not exceeding the rest of the world put together. Some molybdenum is produced by Spain and Peru.
—The first official record of a production of molybdenite in Queensland was in 1900, when the output amounted to 12.3 short tons of high-grade material. The production gradually rose to 119 tons in 1906, and has not varied materially since that date, although the selling prices for concentrates increased considerably. The bulk of the material was mined at Wolfram Camp, in the Chillagoe field, 120 miles southwest of Cairns, in Northern Queensland. The mines at[192] Bamford, in the same field, are credited with a small output. With the molybdenite ores are bismuth-tungsten ores, so that all three metals are produced.
The production of molybdenite was first reported in New South Wales in 1902; in that year the output was 17 short tons. The total output to the end of 1914 was 498 short tons, valued at $264,000. The chief producing molybdenite mines are at Whipstick, in the Pambula division, at Kingsgate, in the Glenn Innes division, and near Deepwater, in the Deepwater division. Molybdenite is also being produced at Rocky River, in the Tantafield division, and in the Bathhurst division. The production at all of these localities has not been large—in no one year exceeding 100 tons of concentrates.
In Norway, the production of high-grade molybdenite concentrates has averaged about 30 tons per annum since 1902. In 1906, an output of 1,129 short tons was reported. This probably refers to ore mined and not to concentrates produced.
The chief molybdenite districts in Norway are the provinces of Lister, Mandal, and Nedenes, on the extreme southern end of the peninsula. The district of Fjotland, in the former province, is probably richer in molybdenite than any yet discovered in Norway. A mine at Knaben, in this district, has been the largest and probably the only successful producer in Norway. This mine, owned by George G. Blackwell & Sons, of Liverpool, England,[94] has made an average output of about 25 short tons per annum.
[94] Reported taken over by a Norwegian company. U. S. Commerce Reports, September 24, 1918.
Table 33.—Production of Molybdenite in Queensland, New South Wales and Norway
| Year | Queensland | New South Wales | Norway | |||
|---|---|---|---|---|---|---|
| Weight (short tons) |
Value (dollars) |
Weight (short tons) |
Value (dollars) |
Weight (short tons) |
Value (dollars) |
|
| 1902 | 45.9 | 26,770 | 16.8 | 8,960 | 22 | 16,100 |
| 1903 | 26.9 | 10,220 | 32.5 | 21,960 | 34 | 21,400 |
| 1904 | 23.6 | 13,010 | 28.3 | 13,270 | 33 | 17,400 |
| 1905 | 70.8 | 41,340 | 21.7 | 12,200 | 51 | 16,300 |
| 1906 | 118.9 | 74,330 | 36.6 | 23,350 | 1,129 | 14,200 |
| 1907 | 74.0 | 41,080 | 24.2 | 17,340 | 33 | 12,900 |
| 1908 | 98.7 | 44,960 | 9.5 | 4,520 | 39 | 13,400 |
| 1909 | 103.9 | 45,120 | 31.5 | 15,810 | 33 | 12,100 |
| 1910 | 118.6 | 58,640 | 53.2 | 27,580 | ||
| 1911 | 111.4 | 64,610 | 23.1 | 12,610 | 2 | 800 |
| 1912 | 114.6 | 84,420 | 63.3 | 18,030 | 23 | 5,400 |
| 1913 | 74.3 | 92,460 | 88.3 | 33,100 | 13 | 3,200 |
| 1914 | 87.1 | 185,830 | 68.8 | 55,720 | ||
[193]
The production of molybdenite in Queensland, New South Wales and Norway, by years, is shown in the preceding table.
Figures for more recent years indicate the total production in Australia of about 330 short tons per annum and 110 short tons in Norway in 1916. In 1917 the output in Norway was three times the 1916 figures.
—As already stated, the chief molybdenite deposits in Canada are in the provinces of Ontario and British Columbia. They are low grade and of course need concentration. The Canadian and British governments have been much interested in the concentration of these ores and the Canadian government has a mill engaged in experimental work and in commercial concentration. The Department of Mines has spent a good deal of time in experimentation, believing that molybdenite has an important future in metallurgy.
The production in 1917 was about 80 short tons of high-grade concentrates and was undoubtedly larger in 1918.
Canada when properly prospected may produce a good deal more molybdenite than now.
In the United States are a very large number of small molybdenum deposits, scattered over the western states from Washington to Arizona and from Colorado to California. There are two common minerals—molybdenite, or molybdenum sulphide, and wulfenite, or lead molybdate. Generally speaking, molybdenite is found in the northern states, and wulfenite in the southern states, but this rule is not without exception. In Arizona and New Mexico, the principal mineral is wulfenite, but there are some fairly large deposits of molybdenite, probably the best being at the Leviathan mines, in Copper Canyon, Mohave County, Arizona. This is the only molybdenite deposit that is being worked in New Mexico or Arizona. It is in the Cedar Valley mining district about three miles southeast of Copperville and about 25 miles east of Yucca. This ore carries a good deal of copper, as well as small traces of gold and silver. Some analyses have shown 2 or 3 per cent. MoS2 and 1¹⁄₂ to 2 per cent. copper with 0.02 ounce of gold and 1 to 4 ounces of silver per ton. The percentage of molybdenite is undoubtedly above the average, which does not exceed 1 per cent. The country rock is medium-grained gray granite, consisting of quartz, feldspar, biotite, muscovite and small amounts of other accessory minerals, such as zircon and apatite. The company has erected a mill and has succeeded in making a satisfactory separation of the copper from the molybdenite.
The largest possibilities in Arizona and New Mexico are in mining wulfenite. This mineral is widely scattered over these two states, especially Arizona, and is, to a great extent, associated with vanadinite. One of the greatest difficulties in concentrating wulfenite has been the[194] separation from vanadinite. The Bureau of Mines has worked on this problem for some time with partial success.
The most important deposit of wulfenite is at the Mammoth and Collins mines, in Pinal County. They were originally gold mines, and when the value of molybdenum became evident, Colonel Randolph, owner of the Mammoth mine, decided that it would be worth while to run the tailings dump for wulfenite. He converted his mill to this purpose, and not only ran the Mammoth dump but also the dump at the old Yuma mine, in Pima county. The total amount of concentrates produced by Colonel Randolph and others in the vicinity during the three years 1916 to 1918 are probably represented by 1,000 to 1,200 tons of wulfenite concentrates. While these operations were going on, they represented practically the only production of molybdenum concentrates in this country except on a very small scale. One other operating company is the Rowley Copper Mines Co., Gila Bend, Arizona, the ore being wulfenite and the principal impurity barite. The company has succeeded in making satisfactory concentrates, which carry, however, a considerable amount of barite. The Golden, Colo., station of the Bureau of Mines has run some tests on this concentrate and has made a partial separation of the barite and wulfenite.
Molybdenite is found in a considerable number of places in Colorado, Montana, Washington, Nevada, Utah, Texas and other western and northwestern states, but the largest occurrence is in Colorado. Generally speaking, the individual deposits in the West are not large enough to warrant the building of a mill for any one of them, and as the deposits are widely scattered, it is difficult to find a place where a custom mill could obtain a sufficient amount of ore. This is one of the chief difficulties in producing a large tonnage of molybdenite concentrates, outside of Colorado.
Probably the largest deposits of molybdenite in the world are at Climax, Colorado. These deposits are on the southwestern slope of Bartlett Mountain, Summit County, about 15 miles from Leadville. Outcrops are practically continuous across the whole length of the mountain and at places are one hundred to two hundred feet thick. The ore is rather granular and not flaky molybdenite, the average grade running about 8 per cent. MoS2.
There are two operating companies, the Climax Molybdenum Co., a subsidiary of the American Metal Co., of New York and Denver, and the Molybdenum Products Co., of Denver. Both of these companies have erected mills having daily capacities of 200 tons. The mill of the American Metal Co. started continuous operation about March, 1918, and that of the other company was completed shortly afterwards. Both companies claim that they can enlarge the capacity at short notice. The writer was in the mine of the American Metal Co. Evidently[195] a considerable part of the mountain is molybdenite, and without doubt, a very large tonnage can be produced. The Jackling interests recently acquired the adjoining properties owned by the Pingree Mines Co., but in 1918 had not built a mill or carried out any serious development work.
Another very large group of deposits of molybdenite lies near Empire, Clear Creek County, Colorado, on the eastern slope of Red Mountain, at an altitude of about 11,000 to 12,000 feet. The deposits are 14 miles from the Empire station of the Colorado & Southern Railroad and are owned by the Primos Chemical Co., of Primos, Pennsylvania. The ore-bearing bodies consist of three veins of low-grade ore. The ore zone, that is, the ground included between the footwall of Vein No. 1 and the hanging wall of Vein No. 3 where cut by the tunnel, is about 200 feet wide. The veins vary greatly in width and are not particularly well defined, thin veinlets and stringers of ore running into the walls. The Primos Chemical Co. has worked these mines off and on for several years. About three years ago it built a mill near the mine, but production has been rather intermittent. This company has probably used all of its concentrates for making ferromolybdenum at its own works in Primos, Pennsylvania.
A small mill has been erected at Pitkin, Colorado, in connection with the mine at that place owned by the Pennsylvania Molybdenum Mines Co., of Johnstown, Pennsylvania. A number of other deposits of considerable interest are found in Colorado, especially around Breckenridge, Summit County. Here the pegmatite veins consist largely of muscovite and quartz, with some feldspar, and carry biotite, chalcopyrite and accessory minerals. The difficulty here is to separate the molybdenum from the copper minerals and also from the mica, most of which floats with the molybdenite.
Most of the developed molybdenum deposits of Mexico are in the State of Sonora. In the Sahuaripa district of eastern Sonora, the mineral occurs with scheelite in rich pockets containing very large pieces of pure mineral. Some molybdenum ore has been shipped from the Montezuma copper district to the Empire Smelting & Refining Co., of Deming, New Mexico. Molybdenum is reported in several other Sonora localities. Near Coyame and Marquez, northeastern Chihuahua, the mines of the Compañia Minera Aurora y Anexas produce molybdenum ore. Wulfenite is found abundantly with the lead ore of the Cuchillo Parado mine in the same district. The Jibosa copper mine of the American Smelters Securities Co., near Jimenez, Chihuahua, seems to carry considerable oxide of molybdenum, molybdite. It is not commercial at present.
Molybdenite deposits are also reported in the states of Sinaloa, Oaxaca, Hidalgo, and Jalisco.
[196]
As the production of molybdenum ores in the past in all countries has been relatively small, probably none of the deposits have been worked out and an increased output can be obtained. This applies particularly to the United States. All of the work that has been done so far has been on an experimental basis, and possibly the only mine whose tonnage will be decreased by past production is the Mammoth mine, near Mammoth, Arizona, and there is no certainty that this mine can not continue to produce as much for some time in the future as it did during its period of operation. Just what production can be obtained in the United States is somewhat uncertain, but it is probable that the deposits at Climax, Colorado, will yield at least one thousand tons of ore a day for several years and possibly for a good many years. The deposits of the Primos Chemical Co. near Empire, Colorado, are also extensive and should give a large output for some time to come. The rest of the deposits mentioned are small compared with these two, but the total production of all of them might be large, were a steady market assured and custom mills erected.
In concentrating wulfenite ores some form of table concentration, with the addition of slimers, is generally used. The metallurgical treatment of the concentrates varies and is still very much in the experimental stage. There is opportunity for the development of a process that will give the patentee a considerable advantage over competitors. The Bureau of Mines has been working on this problem and has recently devised a method that seems to be as efficient as any other and possibly has some additional advantages.
In the treatment of molybdenite the same methods are almost universally used. First the ore is crushed to the required fineness and the molybdenite is separated by flotation, largely oil flotation. The metallurgy of the concentrate, now more or less standardized, involves roasting the concentrate to the oxide and treating this oxide in an electric furnace for the production of ferromolybdenum. With small improvements, this practice is likely to be maintained for some time to come.
The political control of the molybdenum deposits of the world is determined by the geographical location. At present most of the known deposits are controlled by the United States and Great Britain, the latter controlling those in Canada and Australia. The British and Canadian governments actually have a government mill in Canada.
[197]
The Knaben mines, in Fjotland, the most famous and probably the only successful molybdenum mines in Norway, were acquired in 1905 by an English company, the Blackwell Developing Corporation. Later a newly organized Norwegian company, with head office in Christiania, took over the mines at a price of 2,500,000 crowns.
In Mexico the large molybdenum deposit in the Sahuaripa district of eastern Sonora is owned by George Fast, of Douglas, Arizona. The Lucky Tiger-Combination Gold Mining Co., of Kansas City, Mo., (American) owns the deposits in the Montezuma district. Another American company has acquired deposits near Poza, Sonora, about 20 miles north of Hermosillo. A deposit located 35 miles from the port of Topolabampo, northwestern Sinaloa, is owned by an American. The Compañia Minera Aurora y Anexas, operating molybdenum mines near Coyame and Marquez, northeastern Chihuahua, is owned by the Madero estate. The ore has been shipped to Leonard Worcester, El Paso, Texas, agent for the estate and also purchaser for L. Vogelstein & Co., New York, formerly a branch of the German metals combine.
In the United States, during the war the claim was made a number of times, both privately and in the press, that German interests were trying to obtain control of the molybdenum deposits of the country. This was due to the fact that the American Metal Co., which operates at Climax, Colorado, as the Climax Molybdenum Co., and which has some other molybdenum deposits, was formerly controlled by German interests. It is true that the majority of the stock of the American Metal Co. was owned in Germany, but Mr. Palmer, the Alien Property Custodian, took charge of this stock, and the affairs of the American Metal Co. were readjusted to changed conditions. The principal stockholders of the Primos Chemical Co. were four brothers by the name of Boericke. Before the war they had strong German connections, but outside of their deposits at Empire they made no special effort to get large molybdenum holdings in this country and did not seek to get a combination of any of the operating companies. The Primos Chemical Co. has since 1919 been taken over by the Vanadium Products Corporation, affiliated with the Bethlehem Steel Corporation. The Molybdenum Products Co., Denver, Colorado, which owns a part of the molybdenum deposit on the slope of Bartlett Mountain, near Climax, and operates a 200-ton mill, is a subsidiary of E. J. Longyear Co., exploring engineers, of Minneapolis, Minnesota. Both companies are owned by American stockholders.
In Canada and Australia it is certain that no one large interest has control, as the ore comes from a number of more or less independent small mines.
A large number of patents have been issued in connection with the concentration and metallurgy of molybdenum. None of these is vital[198] to production; most are valueless, and even those that have a distinct value do not necessarily give control to the owner of the patents or secret processes.
In order to insure a steady demand for molybdenum, the prime requisite is a definite knowledge of the properties and uses of molybdenum steel. In the past this has been lacking, and at present it is not possessed by the majority of operators and steel makers. Europe should assist materially in supplying this deficiency. Molybdenum steel at the present time is in the same position as vanadium steel was a number of years ago—it is on trial. This uncertainty caused a very decided slump in the demand for molybdenum concentrates in the spring of 1918. A control of molybdenum is more likely to come through the manufacture of molybdenum steel than through processes connected with the production of concentrates or ferromolybdenum.
At present the United States has the largest potential supply of molybdenum ores of any country in the world. In addition it has three of the largest and most modern mills that are handling ore from any molybdenum deposit. It is in a favorable position to equal or surpass, for some time, any other country, in output of molybdenum concentrates.
Before the war Great Britain, through political control of the Australian and Canadian deposits, was the world’s leading producer. Some molybdenum came from Norway, but the amount was small as compared to the output of Australia, Norway producing in 1913 only 13 short tons and Australia 162.6. In both Canada and Australia the known deposits have not been worked to capacity and new deposits will probably be discovered with proper prospecting, so that the future molybdenum production under British control promises to increase. The Canadian and British governments are much interested in the development of the molybdenum resources of the dominion.
France is entirely dependent upon England and the United States, although it might be able to get a small amount of concentrates direct from Norway.
Germany was much interested in molybdenum before the war. During the war it probably did not import any molybdenum concentrates at all, and as the world production before that time was small it is not likely that there was any reserve on hand in the empire in 1919.
Japan has no molybdenum deposits as far as is known, and probably is not specially interested at present in the use of this metal.
The distribution of the chief molybdenum deposits of the world is shown in Plate VII.
[199]
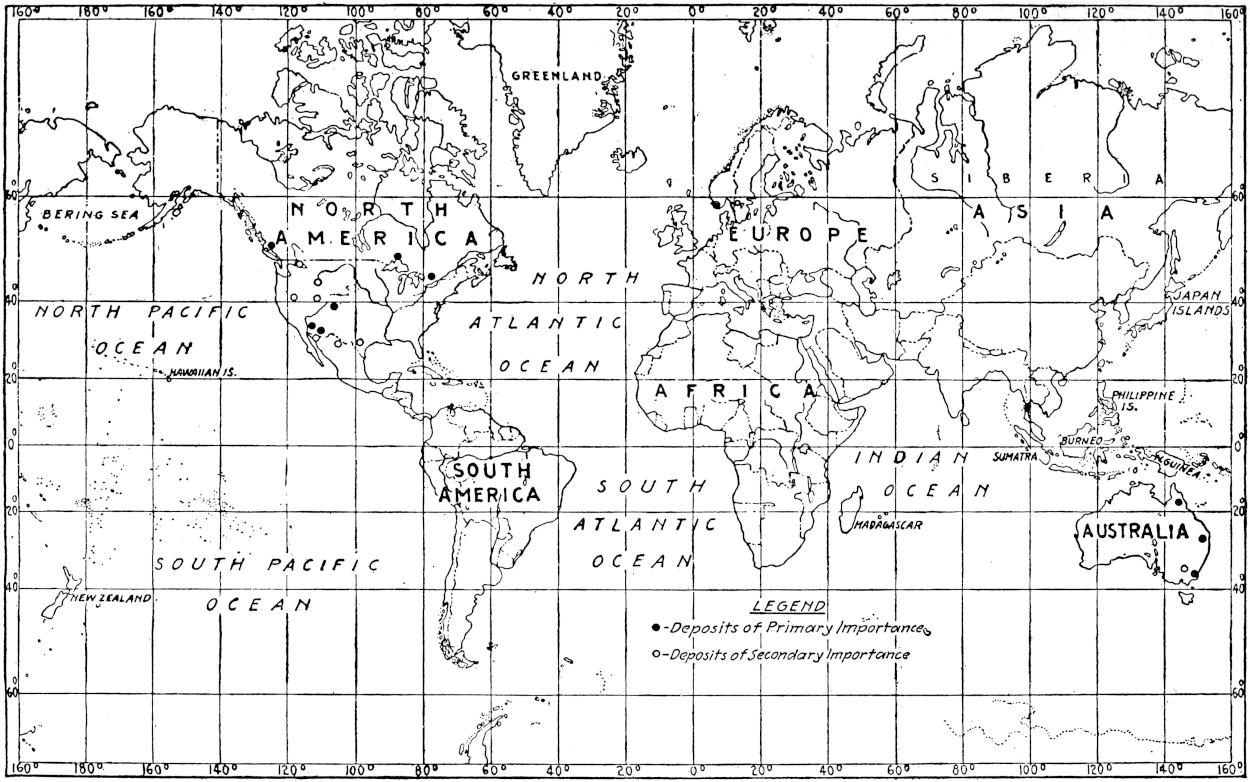
Plate VII.—Geographical distribution of the molybdenum deposits of the world. By R. B. Moore.
[200]
Molybdenum commands attention because of its growing importance as a steel alloy metal. Although the metallurgy and properties of molybdenum steels are not thoroughly understood and their use is not widespread, especially in this country, accumulating evidence indicates that molybdenum will eventually become one of the common alloy metals. It is used in the form of wire as supports for incandescent light filaments and in electrical apparatus, and may become essential in the manufacture of special steels. It cannot be easily replaced in chemistry, and for its other applications it is better and cheaper than other materials.
Up to 1915 practically all of the molybdenum produced came from Queensland, New South Wales, and Norway. At present more important deposits are being developed in Ontario, Quebec, and British Columbia, Canada, and in Colorado, Arizona, New Mexico, and other western states. The mineral is widely distributed and the discovery of additional deposits is likely if the demand is sufficient to encourage prospecting. The United States has deposits large enough to meet all domestic needs and also to produce a surplus for export. Some molybdenum is obtained from Mexico, Peru, and Spain, but the United States, Great Britain (Canada and Australia), and Norway control the important deposits.
The largest molybdenum deposit in the United States, located at Climax, Colorado, is owned by the Climax Molybdenum Co., a subsidiary of the American Metal Co. (formerly German), and by the Molybdenum Products Co., of Denver, an American company. Other deposits in Colorado are owned by the Primos Chemical Co., a company that had strong German connections before the war, but has been taken over by the Vanadium Products Corporation, an American company. Other producing deposits of the United States are owned by American citizens.
The Knaben mines, the most important in Norway, have been owned since 1905 by an English company, but, according to a report, they have been acquired by a Norwegian company. A number of the deposits of northern Mexico are owned by Americans. Others are owned by the Madero estate (Mexican). The Canadian and Australian deposits are controlled by small, independent operators. Both the United States and Great Britain have ample supplies of molybdenum; France produces none and is dependent upon other countries; Germany, which was much interested in molybdenum before the war, probably has no large stocks on hand.
[201]
Radium is a metal and is a product of the disintegration in nature of the metal uranium. Both radium and uranium are elements. Radium has been isolated in its metallic state, but is not used in that form and is known better in the form of its salts, among the most important of which, so far as their uses are concerned, are the bromide, chloride and sulphate.
Wherever uranium occurs in nature, radium is associated with it in certain definable quantities. Uranium can contain, however, only a certain maximum amount of radium at a time, and when it has reached this stage, the radium and uranium ratio is said to be in equilibrium. In this condition the amount of radium per gram of uranium has been calculated by Rutherford to be 3.4 × 10⁻⁷ gram. This corresponds to 1 gram of radium element to about 3,000 kilograms of uranium element, or 1 part of radium element to about 3,000,000 parts of uranium element. Uranium minerals as mined are usually impure and carry only a small percentage of uranium elements, so that the ratio between radium and the crude uranium ore may be 1 to several or many times 3,000,000.
The production of radium from uranium is usually stated in milligrams or grams, and even in the richest ores there is usually only a small fraction of a gram to a ton, while in the ordinary lower-grade ore there are only a few milligrams to a ton, corresponding to a small fraction of a grain to a ton. Less than twenty years ago it was estimated that probably not one gram of radium element in the form of its refined salts had been extracted in the world. Today a great many times, perhaps a hundred times or more, this amount has been extracted and is in use. The annual production of radium today in the world is probably several grams. The annual production of uranium in the world is probably several hundred pounds.
The unique position of uranium as the source of radium in nature makes it necessary to discuss both materials together.
—Radium is a heavy white metal which is very unstable, and alters rapidly in the air. It is not used in its metallic stage but only in the form of its salts. A few years ago these salts were supposed to have a general beneficial effect in the treatment of cancer[202] and other malignant growths, but more recent investigations seem to confine their influence to only certain forms of these afflictions. Their influence in other diseased conditions is often very marked, but the full extent of the field of usefulness of radium for medical purposes has not yet been very clearly defined.
In recent years radium has been applied to other important purposes, especially in luminous paint for watches, clocks, compasses and other instruments; and this use has so greatly increased in recent years, especially for military purposes, that it now consumes more radium than is used in medicine. Radium salts are more or less luminous when seen in a darkened room, and this quality is often increased by the admixture of certain other materials, notably zinc sulphide. Hence their value in luminous paints. Radium salts also cause certain minerals to fluoresce, notably the zinc minerals willemite and sphalerite. In Germany, where radium during the war became scarce on account of the shortage of the ores from which it is extracted, radium salts are said to have been preserved for medical purposes, and mesothorium and other radioactive substances used in making luminous paints.
—Uranium is a heavy white metal, which slowly tarnishes on exposure to the air. The chief use of uranium today is as a source of radium. For many years before the discovery of radium, however, uranium compounds were used in a small way in coloring glass and porcelain, in photography, in reagents for chemical analysis, in mordants for dyeing and for other minor purposes. The use of uranium metal in small quantities in steel manufacture has been tried with some degree of success.
—The principal uranium minerals at present known in nature, which are therefore the principal sources of both uranium and radium, are carnotite and uraninite, with the impure amorphous form of uraninite known as pitchblende. Torbernite, autunite and some of the rarer uranium minerals have produced a little radium and uranium.
Carnotite and uraninite or pitchblende as mined for ores are generally more or less mixed with other materials and are rarely found pure. The uranium in the ores is usually stated commercially, for convenience, in the form of the uranium oxides represented by the formula UO2 + 2UO3, briefly expressed as U3O8. Most carnotite ore varies from 1 per cent. to 3 per cent. of U3O8; a 5 to 10 per cent. ore is considered high grade; a 20 to 40 per cent. ore is remarkably rich. Uraninite and pitchblende ordinarily contain more uranium than carnotite contains, and even in the impure forms in which they are mined as ores, they often show this greater uranium content. The ordinary uraninite and pitchblende[203] ores carry from 2 to 3 per cent. to 8 or 10 per cent. U3O8, and a 20 per cent. ore is very high grade, though some ore runs 60 or 70 per cent.
—Carnotite is an amorphous, soft, powdery material, sometimes more or less coherent and of a talcose or waxy character, generally of a brilliant canary yellow color, though sometimes discolored by iron, organic matter and other substances. It is essentially a hydrous potassium uranium vanadate. Some authorities believe that carnotite is not a distinct mineral, but a mixture of different minerals.
—The terms uraninite and pitchblende are often used synonymously to designate the same mineral, but more properly the term uraninite is a general name for all forms of the mineral and especially for the purer and distinctly crystalline variety, and the term pitchblende is applicable to the impure amorphous form. It is black or grayish black in color, opaque, and often has a submetallic glossy or pitchlike luster. Uraninite is often remarkably lacking in distinctive characteristics, so that its presence might frequently be overlooked. For this reason it seems possible that this mineral, now known in only comparatively small quantities, may some time in the future be found more abundantly.
Uraninite, like carnotite, has a somewhat indefinite formula, but is essentially a combination of the two uranium oxides UO2 and UO3, in which UO2 seems to act as a base and UO3 as an acid. A number of both the rarer and commoner elements are often associated with them. The relative amounts of the two oxides vary considerably in different specimens, especially in the impure form of pitchblende, and no definite formula can at present be given. In pitchblende a notable amount of water, perhaps sometimes in chemical combination, is often present. Several other minerals much rarer than uraninite or pitchblende are related to them in composition, among them being cleveite, bröggerite and nivenite.
—Though carnotite, uraninite and pitchblende are the most abundant of all the radium and uranium materials in nature, and produce almost all the radium and uranium of commerce, yet many other minerals contain both metals, and though as yet known only in such limited quantities as to be of small commercial value, may in the future be found in quantities of importance. Among them may be mentioned tyuyamunite, a hydrous calcium uranium vanadate often associated with the hydrous potassium uranium vanadate described above as carnotite; autunite, a hydrous calcium uranium phosphate; torbernite or chalcolite, a hydrous copper uranium phosphate.
The only regions of the world that have as yet produced any large amounts of radium and uranium minerals on a commercial scale are[204] Colorado, Utah and Austria. Cornwall, Australia and Germany have produced a small quantity of these minerals. They are known in small quantities in France and Portugal, and have been reported in India and German East Africa, but in these regions they have not yet become commercially important. They occur sparingly, so far as yet known, and practically as only mineralogical curiosities, in Connecticut, North Carolina, Canada, Norway and many other regions, but may in the future be found in larger quantities.
Minute quantities of radium or its products of disintegration occur in almost all rocks and in the atmosphere, and in the waters of the sea and land, but in such small amounts as to be unavailable as a source of these substances. The source of all radium of commerce at the present time is in the certain few uranium minerals already mentioned. They are found in formations of various geologic ages, from recent superficial deposits to the older crystalline rocks, but show a tendency toward certain modes of occurrence, such as in southwestern Colorado and southeastern Utah as an impregnation in sandstone; in eastern Colorado, Cornwall, Austria and South Australia as one of the gangue minerals in veins of other ores; in North Carolina, Canada, Norway and West Australia in pegmatite or other feldspathic dikes.
—The commercially important deposits of ores of radium and uranium in the United States are, so far as yet known, confined to the carnotite regions of southwestern Colorado and southeastern Utah, and the pitchblende deposits of Gilpin County, in eastern Colorado. In Connecticut, North Carolina and elsewhere, uraninite, pitchblende and other uranium minerals have been found; and near Mauch Chunk, in Pennsylvania, small quantities of carnotite have been discovered, but these occurrences are, so far as known, in quantities too small to be of commercial value.
—The carnotite deposits of southwestern Colorado and southeastern Utah are the most important sources of radium and uranium in the world. In Colorado the largest quantities of ore have come from many mines in Montrose County, especially in Paradox Valley, while Mesa, San Miguel, Dolores, Rio Blanco, Routt and other counties have been producers. In southeastern Utah the ores are carnotite, as in southwestern Colorado, and occur especially in Grand, Emery and San Juan counties, but have not been worked to the same extent as in Colorado.
The carnotite of Colorado and Utah occurs as an impregnation in sandstones and shaly sandstones, mostly in the McElmo and the La Plata formations, lying at the top of the Jurassic beds and below the Cretaceous[205] sandstones and conglomerates of the region. The deposits seem to have been formed by the precipitation of carnotite from solution along certain strata of these formations, and the material occurs along bedding planes, in fissures and small cavities, in layers or irregular masses from a fraction of an inch to several inches in width, and sometimes as a general impregnation of the sandstone for several feet in thickness. It seems to be especially abundant in strata impregnated strongly with vegetable or animal matter, and is often in unusual quantities in lignitized or petrified trunks of trees. This phenomenon suggests the influence of organic matter in precipitating and segregating the carnotite.
The rocks carrying the carnotite lie horizontally or dip at low angles in most parts of the Colorado region; in Utah they lie often in the same way, but occasionally dip at steep angles. Where they appear on the surface, the carnotite sometimes impregnates certain strata for several hundred feet or more along the outcrops, but more generally it occurs in spots along them, with little or no carnotite in the intervening spaces. As these outcrops are followed into the hillsides, the ore appears to be even more irregular in its distribution than on the surface, and in many or most cases it becomes much scarcer the further it is explored underground, until within 10 to 40 or 50 feet from the surface it often mostly or entirely disappears. There are exceptions to this feature, but the gradual and often rapid decrease in quantity and grade of the carnotite ore as it is followed into a hill is generally recognized. This fact suggests that the carnotite may have been redissolved in the sandstone and carried to the surface by capillary action in this arid climate, forming rich, superficial efflorescences.
In many of the carnotite deposits, vanadium minerals occur independently of the vanadium in the carnotite, but this association is not always observed. They occur in sandstone and often give it a dark-gray or blackish color.
In eastern Colorado several mines near Central City, Gilpin County, have produced limited quantities of pitchblende. Among these are the Kirk, the Wood, the Belcher, the Alps, the German and the Calhoun mines. The pitchblende occurs as a subordinate constituent in the gold-bearing veins of that country. The veins intersect old metamorphic rocks intruded by igneous rocks. The mines of Gilpin County are today producing little if any pitchblende, and the total production has been small, amounting in all probably to only a few tons. Much more pitchblende, however, was let go to waste in former days when the mines were worked for other ores and the value of uranium was not recognized.
—The United States is today by far the largest producer of radium and uranium ores in the world, and is also the largest producer of manufactured radium and uranium compounds. Before the war, England, France and Germany, especially Germany, imported large[206] quantities of American ores and extracted the radium in a refined state as its different salts, much of which was returned to the United States for sale. Now, however, American ores are almost entirely treated in the United States, with the exception of a little shipped to England and possibly to France. The Standard Chemical Co., of Pittsburgh, was a pioneer in this work, and others quickly followed, among them the National Radium Institute, of Denver; the Schlesinger Radium Co., of Denver; the Chemical Products Co., of Denver; the Cummings Chemical Co., of Lansdowne, Pa.; the Radium Luminous Materials Corporation, of New York, and others.
Before the discovery of radium in 1898, but little attention was given to uranium ores in America, though some little pitchblende was shipped from the Central City, Colorado, region for use in making uranium compounds. Shortly after the discovery of radium, however, mining was begun on the carnotite of southwestern Colorado, and from 1900 to 1910 several companies were formed to work these ores both in Colorado and Utah. The pitchblende of Central City also began to attract renewed attention. For a few years active work was done in prospecting for it, but the quantities have so far proved to be small. A few tons probably represent the total amount derived from these mines since the search began. In the meantime, however, the production of carnotite increased rapidly until 1915, when it greatly decreased on account of the curtailment of shipments to Europe. In the latter part of 1916, however, the production increased again, on account of the increased consumption of ore in this country, and in 1918 the production was very active, largely on account of the increased use of radium not only in medicine but especially in luminous paints.
The amount of radium and uranium ores produced in the United States, or in fact anywhere, during a given period, is difficult to determine, on account of the different bases on which reports are made, but it may be said that the tonnage is small compared with that of ores of commoner metals, a few thousand tons being a large amount of carnotite, and simply a few tons or pounds being a large amount of pitchblende. Though the mining of radium and uranium ores in the United States began about 1900 or shortly before, no very large quantities were produced until 1912, when about 1,100 tons were mined, consisting chiefly of Colorado carnotite. The production gradually increased to several thousand tons yearly, practically all of which is carnotite from Colorado and Utah.
—The most important radium and uranium ore in Europe at present is the uraninite or pitchblende found in the mines of Joachimsthal, in Bohemia. It occurs as a subordinate gangue mineral[207] in certain silver veins of that region which intersect metamorphic and igneous rocks, and has been actively worked ever since the discovery of radium by M. and Mme. Curie in 1898. Before that time the mineral had a certain value as a source of uranium compounds.
These Austrian mines are second to those of the United States as a source of radium and uranium, but their production equals only a very small part of that of this country. Until the Great War this production was controlled largely, if not wholly, by the Austrian government, and as the production is said still to continue, it is probably still controlled in the same way.
—Next in importance in Europe to the uraninite or pitchblende ore of Joachimsthal as a source of radium and uranium, is the similar ore in some of the mines of Cornwall, England. It occurs as a subordinate mineral in the gangue of some of the old tin and copper mines, in veins intersecting metamorphic and igneous rocks, especially at St. Just, St. Ives, Grampound Road, St. Austell and elsewhere. The production and treatment of the ore has been under private or corporate auspices and the amount produced has not been large.
—In Germany the production of radium and uranium ores has always been insignificant. A small quantity of such ores has been produced at Schneeberg, Johanngeorgenstadt, Annaberg and elsewhere. Before the war, Germany was a large producer of manufactured radium and uranium compounds, but they were derived mostly from imported American ores.
—With the exception of Joachimsthal and Cornwall, Europe has produced but small quantities of radium and uranium minerals. A little uraninite or pitchblende has been found in other localities in Austria, such as Przibram and elsewhere, and sparingly in Norway. Autunite and other uranium minerals have been found in small quantities near Autun, France, and near Sabugal and Guarda, in Portugal, but no important quantities have been produced.
—In South Australia carnotite, autunite, torbernite and other rare uranium minerals occur in regions of metamorphic and igneous rocks at Radium Hill, near Olary, and at Mount Painter, in the Flinders range. A few hundred tons of ore containing these minerals have been mined by private or corporation interests. Most of this has been sent to Woolwich, near Sydney, New South Wales, or to England, for the extraction of the radium. Since the war started no very active mining operations in such ores have been carried on in the South Australian region.
At Cooglegong, in Western Australia, the uranium mineral fergusonite[208] and to a less extent the uranium mineral euxenite occur in the surface detrital material of the region. At Wodgina the minerals mackintoshite, thorogummite and pilbarite, all hydrous silicates of uranium, thorium and lead, occur in an albite pegmatite dike. No important quantities of these Western Australia ores have yet been produced.
—Radium and uranium minerals have been reported in India and German East Africa, but no important quantities have yet been produced.
The prospect for increased discoveries of radium and uranium minerals at the present time seems best in the carnotite regions of Colorado and Utah. The workable deposits seem to be more or less superficial, and perhaps no large quantity of ore may be found in any one spot, yet the great extent of the region in which the formations carrying carnotite occur, will supply an immense aggregate amount of ore.
Increased discoveries of uraninite, pitchblende and other uranium minerals in Europe seem possible, even though that continent has already been well explored for them. Moreover, new discoveries of different radium and uranium minerals may very likely be made in still other parts of the United States than those mentioned, and in less explored parts of the world, especially certain regions of South America, Australia, Asia and Africa. Many of these minerals, especially pitchblende, have no very distinctive features when first observed, and might readily be overlooked many times before their true nature was discovered. Hence the possibilities of future discoveries.
[209]
As early as 1830 an attempt was made to use zirconia buttons, heated to incandescence, for lighting the streets of Paris. In 1885 an incandescent gas mantle of zirconium oxide was patented, but was replaced in a few years by thorium. About 1900 zirconia was used in the Nernst glower, and it has also been used in place of lime and magnesia as the incandescing material in the Drummond light. It is also said to be used in the Bleriot light, and its use in flares has been suggested.
During the past few years Dr. C. M. Johnson has succeeded in manufacturing laboratory ware made from zirconium minerals mixed with other refractories. Filtering crucibles, muffles, combustion tubes and boats, pyrometer protection tubes, and Kipp generators are now on the market, competing in price with German porcelain and fused silica. Zirconia crucibles are made from the fused material ground in a suitable mill. The powder is pressed or molded into shape with an organic binder, such as starch, or perhaps, better still, with a plastic cement made by grinding the fused material to 20 mesh, when it becomes colloidal in the presence of water. After drying, the articles are burned at a very high temperature (2300 to 2400°C.) until contraction ceases.
Fused zirconia has a high thermal endurance; is not affected when heated to redness and plunged into cold water, its coefficient of expansion being as low as 0.00000084; and its resistance to crushing is many times that of quartz glass. Its hardness is between that of corundum and quartz; its specific gravity 5.89, and porosity below 1 per cent. Its melting point is 2950°C, but 0.5 per cent. impurity reduces that by 100°C. Platinum, with a melting point of about 1750°C., can be melted to a mobile liquid in zirconia crucibles, and it is claimed that the boiling point of pure iron has been determined in similar crucibles.
Chemically, zirconia is very inert, being highly resistant to acids, fused alkalies, fused quartz, or molten glass. Possibly no other material known to chemists possesses such a combination of desirable refractory properties. Its one undesirable characteristic is its tendency under certain conditions at high temperatures, in the presence of nitrogen or carbon, to become converted into nitride or carbide.
An instructive paper entitled “Zirconia as a Refractory,” by E. H.[210] Rodd, was published in the Journal of the Society of Chemical Industry, June 15, 1918.
Zirconium oxide as an opacifier has been thoroughly investigated by Hartman[95] and by Grunwald[96] with promising results; and a number of foreign patents have been granted on the use of the oxide in white ceramic enamels. Zirconia seems to be especially suitable for the manufacture of refractory bricks, or it can be applied as a lining or surfacing to other less desirable refractories. Continental practice along this line is said to have been much more highly developed than practice in this country, one example quoted being actual practice tests of a Martin Siemens furnace with a zirconia-lined hearth. After four months of continuous operation at high temperatures the hearth was still in good condition and gave promise of lasting at least an equal length of time without renewal. Statistics compiled from these tests showed a saving of about 50 per cent. in actual maintenance costs in favor of zirconia over the other refractories ordinarily used for such purposes. Bradford[97] quoted Podszus[98] as claiming to have made a furnace with the pure oxide which had first been fused in an arc furnace and then ground; and in which temperatures of 2400° to 2500°C. were obtained by firing with gas and oxygen.
[95] Hartman, Augustus, Zirconemail: Dissertations Arbeit: Techn. Hochschule, Munich, 1910.
[96] Ueber Zirkonoxyd in der Emailindustrie: Sprechsaal, No. 5, 1911.
[97] Bradford, Leopold, Birmingham Metallurgical Society (British).
[98] Podszus, Zeitsch. angew. Chem., Jahrg. 30, 1917, 1, pp. 17-19.
The most important question regarding zirconium at the present time has to do with the remarkable properties that some of its advocates claim it imparts to steel. That the Germans have had this use in mind for some time is evidenced by the numerous patents they have obtained covering the use of zirconium and its alloys.
Zirconium has been obtained in the amorphous and the graphitic state; and Wedekind has produced a metal of 99.8 per cent. purity resembling white cast iron in appearance, with a hardness of 7.8 (Mohs); specific gravity 6.40; specific heat, 0.0804; heat of combination, 1958 calories; and melting point, 1530°C.
Numerous alloys of zirconium have been made and a number of foreign and domestic patents have been issued covering various alloys, both ferrous and non-ferrous. It is stated that ferrozirconium is finding a limited application in the steel industry as a scavenger for removing nitrogen and oxides. An English patent, No. 29,376, covers the use of zirconium as a scavenger, the alloy containing 20 per cent. of the element and being used in an amount equal to about 1 per cent. of the weight of steel treated.
Another alloy containing 40 to 90 per cent. zirconium, the rest being mainly iron, is said to be free from metalloids and oxides, and malleable and ductile. Alloys covered by United States Patent No. 1,151,160 are[211] claimed to be highly resistant to oxidation and chemical reagents. They have a metallic lustre and take a high polish, are readily malleable and ductile, and it is suggested that they may find an important application in filaments for electric lamps, as they are said to have the property of selective radiation. A typical analysis of some of the alloys claimed to have been produced under this patent shows Zr. 0.65 per cent., Fe 26 per cent., Ti 0.12 per cent. and Al 7.7 per cent.
A widely circulated statement to the effect that zirconium has been used in Germany in the production of steel for armor plates and armor-piercing projectiles has not been substantiated, so far as the writer knows, by any records of analyses.
The use of zirconium for alloying in steel is so new that, pending definite determination by disinterested competent authorities of the exact properties, if any, which zirconium imparts to steel, judgment as to its value for this purpose should be withheld.
Zirconium carbide has been patented for filaments for incandescent electric lamps and it has also been used as an abrasive. Tried as a pigment, zirconia has been found to have good covering power and should be considered where protection from acids, alkalies, or gases is particularly desired. The basic acetate has been used for weighting silk, and the pure oxide is used as a substitute for bismuth subnitrate in X-ray work.
Zirconium occurs in nature in commercial quantities as a mixed oxide and silicate known as baddeleyite, sometimes called brazilite, and as the silicate, zircon. Baddeleyite, as supplied to the trade, usually carries 80 to 85 per cent. ZrO2. The silicate, zircon, carries about 65 per cent. ZrO2. The oxide deposits, containing as they do a higher percentage of zirconium and the ore being more pure and easier to reduce, will in all probability become the principal source of zirconium.
The principal known deposits of zirconium ores are in Brazil, India and the United States, the countries being named in the relative order of their commercial importance.
—The natural oxide, baddeleyite, or brazilite, occurs only in Brazil in commercial quantities and is described in Mineral Foote-Notes by Meyer,[99] who writes as follows:
[99] Meyer, H. C., Mineral Foote-Notes, November, 1916, p. 29.
There are but few commercial deposits of the unusual ores which present more interesting geologic as well as economic features than do the deposits of natural zirconium oxide in Brazil. The Caldas region (visited in 1915 by the writer) in which these zirconia deposits occur, is situated partly in the State of Minas Geraes and partly in the State of Sao Paulo, approximately 130 miles north of the city of Sao Paulo. It is a mountainous plateau, the main elevation of which is about 3,600 feet. The surface is undulating, presenting differences in level of from 300 to 600 feet.
[212]
The whole area is bounded on all sides by ridges rising abruptly from 600 to 1,200 feet above the general level and forming a roughly elliptical enclosure with a major axis of approximately 20 miles in length and a minor axis of 15 miles. This peculiar arrangement of the higher ridges is very significant when coupled with the fact that the predominant rock of the plateau is a phonolite and the presence of highly mineralized thermal water of considerable medicinal value.[100] No thorough geological survey has been made of this area with a view to determining the origin of the zirconia. The character of the ore, however, and the formation, seems to point to pneumatolitic agencies. A careful study of the relationship of the large masses of coarsely crystalline nephelite-syenite in this area, with pronounced segregations of eudialyte, might throw some light upon this subject.
[100] Derby, O. A., “Nepheline-rock in Brazil:” Quart. Jour. Geol. Soc., August, 1887.
Zirconia ore can be roughly divided into two classes:
First, alluvial pebbles ranging in size from one-half inch to three inches in diameter, generally carrying about 90 per cent. to 93 per cent. zirconium oxide. These pebbles, known as favas and having a specific gravity ranging from 4.8 to 5.2, are found along small stream beds and on the talus slopes of low ridges.
Second, zirconia ore proper, or zirkite (a trade name), which ranges in shade from a light gray to a blue black, the lighter colored material carrying a higher percentage of zirconium silicate, as evidenced by analyses, which in some cases show a minimum of 73 per cent. zirconium oxide. The blue-black ore generally carries from 80 per cent. to 85 per cent. zirconium oxide. By careful sorting, however, a uniform grade carrying about 80 per cent. is produced.
Prior to the investigations of Derby and Lee, this ore was considered identical with baddeleyite. It has now been shown, however, that it is a mechanical mixture of three minerals; namely, brazilite, zircon, and a new and unnamed zirconium silicate carrying about 75 per cent. zirconium oxide. This new mineral has the same crystal form as zircon (67 per cent. ZrO2) but is readily soluble in hydrofluoric acid, while zircon is not affected, this being a characteristic differential test. The finely powdered mineral, on being treated with a weak solution of hydrofluoric acid, leaves a residue of minute, perfect, pyramidal crystals of zircon, the brazilite and new zirconium silicate going into solution. Several large outcrops of the ore occur on the extreme westerly edge of the plateau, one or two isolated boulders weighing as much as thirty tons. This very cursory examination of the zirconia deposits makes it unsafe to venture any conjecture as to the quantity of ore available. Suffice it to say, however, that the deposits have been traced for a distance of fifteen miles between Gascata and Caldas, and if surface indications are of any significance, are of vast extent.
The oxides have also been found in the State of Montana, and in Ceylon, Sweden, and Italy, but none of these occurrences are of commercial importance.
—The simple silicate, zircon, is found in seashore and river concentrations of monazite sands, associated with ilmenite, garnet, rutile, and various other heavy minerals usually found in such places.
[213]
An important concentration of zircon occurs along the coast of Brazil, in the states of Bahia, Espirito Santo, and Rio de Janeiro, where cusps of the beaches are protected on the north by granite headlands and bordered by Tertiary bluffs, which are cut by various streams and lagoons that constantly furnish fresh material for the concentrating action of the tides and waves.
Probably the next most important occurrence of zircon is in India, in the beach sands of the province or state of Travancore at the extreme southwestern end of the Hindustan peninsula.
Next in importance is an occurrence in the United States at Pablo Beach, Florida, where not only the beach sands, but the dunes bordering them, contain appreciable quantities of the following minerals in their relative order of abundance: ilmenite, garnet, epidote, zircon, rutile, and other heavy minerals including monazite.[101]
[101] Hess, Frank L., Letter of May 1, 1918.
Other occurrences in this country, most of which are of academic interest only, are in Colorado, at St. Peter’s Dome, near Pike’s Peak; in Idaho, in the Clearwater region and elsewhere in black sands and in certain granitic rocks; in Sussex County, New Jersey, at the Williams mine, where zircon occurs abundantly in magnetite; in New York at Lyon Mountain, Clinton County; at a few places near Crown Point; abundantly in pegmatite at Old Red Mines, Mineville, Essex County, and in numerous places in Orange County on the south, and St Lawrence County on the north. In North Carolina, in Burke, McDowell, and Rutherford counties, zircon occurs in monazite sands, and also in Henderson County near Zirconia and in Fredell County near Sterling.
Large crystals of zircon occur in a small pegmatite area in Comanche County, Oklahoma, in the southwestern portion of the Wichita National Forest. Oregon has many localities in which the presence of zircon has been noted, chiefly in black sands, old and present beaches, placer gravels, etc., and the same is true of Washington. In Virginia, zircon is found in pegmatite in Aurelia County, and in sandstone near Ashland, not far from Richmond.
Zircon is also found in Norway, the Ural Mountains, Ceylon, Australia, and British South Africa.
The important zirconium resources of the world are controlled politically, in the order of their commercial importance, by Brazil, Great Britain, and the United States.
—A number of years ago John Gordon, an American mining engineer, became interested in deposits of monazite sand on the coast of Brazil in the vicinity of Prado, Bahia. A thorough investigation of all the known monazite deposits of the world showed that those[214] in Brazil were by far the most desirable, and that those in Travancore, India, were second in commercial importance.[102]
[102] See Chapter XIII.
Other firms exporting zirconium minerals from Brazil, in addition to Gordon & Rogers, of 141 Broadway, New York, are Suffern & Co., of 135 Broadway, New York; S. R. Scott & Co., 39 Broadway, New York; Foote & Co., Philadelphia, Pa.; P. S. Nicholson & Co., Caixa 91, Rio de Janeiro; E. J. Lavino & Co., Bullitt Building, Philadelphia, Pa.; Luiz de Rezende & Co., Rua Ouidor, Rio de Janeiro, Brazil; and Chas. Spitz, also of Rio de Janeiro, who is agent for the Société Minière, a French company in which Rezende & Co. are said to have a considerable interest. Sometime ago the firm of A. C. de Freitas & Co. of Hamburg, Germany, had a contract from the Brazilian government on monazite sands and agreed to export at least 1,200 tons annually. How much zircon that company exported is not known. The de Freitas properties are now being worked by the Société Minière (French).[103]
Until very recently, zirconium has been of minor interest in this country, the monazite sands having been exploited for their thorium contents only, not for zirconium. However, Germany and Austria seem to have placed considerable value upon zirconium-bearing sands, and, as shown by the attached table, more was produced in 1913 than in all the time prior, and practically all the material produced during that year went to Germany. This is in accord with the efforts of Germany, just before the war, to obtain tungsten and molybdenum, and is an evidence of preparations long before hostilities began.
—Before the war, the German users of monazite were in control of the Travancore, India, deposits. That control of course ceased and the contracts were cancelled. The India Office decided that, in the future, all directors of the company must be British born, and that the company must be ready at all times to sell monazite sand to British firms, direct, at a fair price.
—It is hardly probable that the deposits in the United States will be able to compete, on a commercial basis, with the Brazilian ores; but, if the necessity should arise, this country can produce, within its own borders, enough zirconium to manufacture many thousand tons of zirconium steel. The cordial commercial relations between the United States and Brazil will probably protect this country from any undue restraint on exports from South America.
—All of the large countries interested have facilities for making the ferro-alloy. Numerous alloys of zirconium have been made, and a number of patents, both foreign and domestic, have been issued covering various alloys, both ferrous and non-ferrous. No secret processes, as such, are known to the writer.
[215]
Table 34.—Production of Zirconium Minerals in the United States and Brazil, 1902-1916[104]
| Year | United States | Brazil | |||||
|---|---|---|---|---|---|---|---|
| Quantity (short tons) |
Value | Quantity (short tons) |
Value | ||||
| 1902 | ... | ... | 12 | $ | 3,947 | ||
| 1903 | 1 | ¹⁄₂ | $ | 570 | 7 | 1,947 | |
| 1904 | ¹⁄₂ | 200 | 9 | 3,935 | |||
| 1905 | 4 | 1600 | 18 | 5,506 | |||
| 1906 | ¹⁄₂ | 248 | 26 | 5,041 | |||
| 1907 | 46 | 38 | 8,756 | ||||
| 1908 | 0 | 0 | 275 | 15,151 | |||
| 1909 | 1 | 250 | 117 | 11,838 | |||
| 1910 | 0 | 0 | 128 | 23,271 | |||
| 1911 | 1 | ¹⁄₂ | 802 | 45 | 16,169 | ||
| 1912 | 0 | 0 | 43 | 14,772 | |||
| 1913 | 0 | 0 | 1,119 | 54,767 | |||
| 1914 | 0 | 0 | 237 | 14,903 | |||
| 1915 | 0 | 0 | 8 | 2,915 | |||
| 1916 | 0 | 0 | 104 | 16,647 | |||
[104] Adapted from Mineral Resources, U. S., 1916, Part II, U. S. Geol. Survey, 1917, pp. 377-386.
The principal use for zirconium ores at present is determined by the refractory properties of the oxide, zirconia. Refractory bricks and shapes for furnace linings, chemical ware, and other heat, acid, and alkali resisting articles are made of zirconia, and are finding a limited market.
The recent interest in zirconium is due to the remarkable properties which it is said to impart to steel. Its real worth, or function, in that direction is yet to be definitely determined, and tests conducted by the Bureau of Mines, Bureau of Standards and others are being made to ascertain the facts.
The best and most available source of supply at present is Brazil, where the natural oxide, baddeleyite, occurs in considerable quantities in the states of Minas Geraes and Sao Paulo. The deposits have not been explored sufficiently to make any reliable estimate of tonnage possible, but, judged from their surface showing, they are of vast extent. The silicate, zircon, is found in Brazil, India and in the United States in commercial quantities.
The important deposits of zirconium minerals are controlled by Brazil, Great Britain and the United States, but the actual ownership of many of the deposits is unknown. This is particularly true of the oxide ores.
[216]
Practically the only use of monazite has been as a source of thorium salts. In extracting these, by-products are recovered, such as cerium, lanthanum, and other rare earths. Thorium salts are used almost exclusively for the manufacture of Welsbach mantles, which consist of 99 per cent. thorium oxide and 1 per cent. cerium oxide. For this use there is no known substitute. The one other use of thorium salts is in ordinary chemical laboratories, but this use is very limited. The fluoride and other salts of cerium are used in connection with the flaming arc, their presence giving increased luminosity.
Mesothorium is a radio-active element always found in thorium minerals. Until quite recently it has been thrown away in the manufacture of thorium nitrate; but it is now being produced as a by-product, and is useful as a substitute for radium in luminous paints, and for therapeutic purposes.
The principal sources of monazite are Brazil and India, although it has been mined successfully in the United States in the Carolinas and in Idaho. It has been found in Swaziland, Africa, and in Australia, and also, to a limited extent, in native rock and in placers in Ekaterinburg, Russia.
Monazite is usually found in the gravels of small streams or bottom lands, although it is sometimes found in the soil of hillsides. In Brazil and India it occurs mainly in the beach sands of the sea coast. In places it is found in small crystals in gneiss, granite and pegmatite rocks. As these rocks become disintegrated, the crystals are washed into streams and with other heavy sands are deposited in the stream beds. On the coast of Brazil, the monazite grains from the crystalline rocks of the coastal mountains are concentrated by the waves of the sea.
—The deposits of the Carolinas cover an area of several hundred square miles east of the Blue Ridge Mountains. In North[217] Carolina, the counties of Cleveland, Burke, Alexander, Rutherford and Lincoln furnish the richest deposits. In South Carolina the only deposits of value are in the counties of Cherokee and Greenville. Practically all of the monazite mined in the Carolinas is derived from gravels in streams and bottom lands, the miners usually following the stream courses. The gravels vary greatly in thickness, and it is therefore difficult to make a true estimate of the average, but in general the monazite-bearing gravels are between 1¹⁄₂ and 2¹⁄₂ feet thick. The top soil in the bottom lands averages 3 to 6 feet thick and may be 7 feet or more.
The deposits in the State of Idaho are near Centerville and Idaho City. In the future, under more favorable conditions of price, transportation, etc., these deposits may possibly become a commercial source of monazite. Almost all of them contain small quantities of gold. The gravel beds are considerably thicker than those in the Carolinas, and with the gold content might be more profitable were it not for labor being higher priced.
Monazite has been found in Colorado, in the Newland Gulch district, 20 miles south of Denver, where it occurs in gravels that carry considerable gold.
—There are three kinds of monazite sand deposits in Brazil; the deposits within the government lands along the coast; deposits lying behind the government coastal lands, which are private state possessions or belong to private parties; and inland deposits. The bulk of the monazite is derived from coast sands in the states of Espirito Santo and Bahia, the sand being washed by means of oscillating tables or sluice boxes. The coast lands are the property of the federal government for 33 meters inland, measuring from the point where the sea waves wash the beach at mean high tide. This uncertain method of marking property has of course given rise to disputes when boundaries are established.
At a few places along the coast strips of monazite-bearing sands lie directly behind the government land, and some of these might be worked profitably were it not for the fact that it has been difficult to prove to the federal government that these sands were not taken from the nearby government land. One French concern has exploited such lands in the State of Rio de Janeiro. Along the banks of the large rivers, such as Parahyba, there are great quantities of black sands with traces of monazite. Near Sapucaia such deposits have been worked by a French concern. Many of the inland deposits can not be exploited on account of the expense of transportation of the products, as the deposits are many miles from a railroad.
—India is the newest source of monazite sands, the deposits lying on the sea beach like those in Brazil. The deposits are in the Travancore district near the southwestern end of the Hindustan peninsula. Very little information is available concerning these deposits.
[218]
The general method of treatment employed until quite recently has involved leaching the concentrate with concentrated sulphuric acid, thus getting the thorium, cerium, and other rare earths in solution and separating them from the silica, zircon, ilmenite and other insoluble products. From this stage different companies used slight variations in practice, such variations being kept strictly secret, although they all involved the precipitation of the thorium as oxalate by means of oxalic acid.
At the outbreak of the war the price of sulphuric acid went to four or five times the original cost, and the increased price of oxalic acid was practically prohibitive. Consequently the manufacturing companies had to change their entire procedure and to use chemicals that they could obtain at a reasonable price. In this difficult undertaking the companies were successful, and at least one company devised a process that was considerably more efficient than the old one.
At present mesothorium is being produced as a by-product by the Lindsay Light Co. and the Welsbach Co., the process used by the latter company having been originated by the Colorado Station of the United States Bureau of Mines. This process is cheap and extremely efficient, and will no doubt enable the Welsbach Co. to compete commercially with any other manufacturer of mesothorium. Of course as the process was developed by the Bureau of Mines it will ultimately be made available for anyone, but as mesothorium can only be made at a profit as a by-product of thorium nitrate, anyone who manufacturers mesothorium must first establish a thorium industry.
The important monazite resources of the world are controlled politically by three nations: the United States, Great Britain (India) and Brazil. The deposits of the United States, in normal times, probably can not be worked successfully in competition with the foreign deposits without the protection of a high tariff.
—The widely scattered monazite deposits of the United States are of importance only during a period of abnormally high prices or during the restriction of imports from Brazil and India. The known reserves are small and the deposits will probably never be an important factor in the world monazite market. As far as the writer knows, all of the American deposits are controlled by American capital.
The manufacture of thorium nitrate in this country is closely controlled by two large companies, the Welsbach Co. and the Lindsay Light[219] Co.; they own the only two commercial plants of any size in the United States.
All of the manufacturers of mantles in the United States, with one exception, must obtain their thorium nitrate from one of these two companies. As these companies supply practically all the thorium nitrate requirements of England and France, they control the thorium industry. The only other manufacturer of thorium nitrate in this country is the Block Co., of Chicago, which uses about 15 tons of monazite a year, making sufficient thorium nitrate for its own production of mantles. The Welsbach Co. uses about 1,200 tons of monazite annually and the Lindsay Light Co. about half that amount. The Welsbach Co. has contracts for the delivery of monazite, up to its requirements, from Brazil; the Lindsay Light Co. has obtained all of its monazite from India.
Most of the processes used, including those used by the Welsbach Co. and the Lindsay Light Co., are secret. As thorium can be extracted from monazite in more ways than one, a secret process does not necessarily mean commercial control unless such a process is in every way efficient and the company owning it is willing to get into a commercial war.
—As the Brazilian deposits have for so many years been the chief source of the supply of monazite, the history of their commercial control is practically a history of the commercial control of the monazite industry. About 27 years ago, John Gordon, an American now residing in New York, found monazite on the coast of Brazil. He brought large quantities to Hamburg, Germany, and was able to obtain a monoply of the monazite sand.
At that time the manufacture of thorium nitrate, the principal product of the monazite sand, was confined in Europe to a few large chemical firms in Germany and the Welsbach Co. in Vienna. They not only supplied the European market with thorium nitrate, but also sent large quantities to the United States. The American Welsbach Co. early manufactured thorium nitrates from sands mined in the Carolinas, a protective duty of 6 per cent. making this possible.
In 1902 Mr. Gordon agreed to supply the four large German manufacturers and the Austrian manufacturers with monazite at a price of $150 a ton and a profit on the manufactured nitrates. A close combination thus formed, known as the German Thorium Convention, prevented other thorium manufacturers from acquiring any of the mineral mined by Mr. Gordon, and raised the price of thorium nitrate 100 per cent.
For a considerable period Mr. Gordon exported the sand from the coast lands of Bahia, near Prado, Brazil, without interference. Finally the Brazilian government became acquainted with the value of the resources and decided that no private individual or state government had the right to mine, sell, lease, or remove any monazite on so-called state[220] lands without the consent of the federal authorities. In 1908 the government advertised that coast lands in the State of Espirito Santo would be leased to the highest bidder for the exploitation of the sands. A contract was thus obtained for the firm of A. C. de Freitas & Co., of Hamburg, Germany. By the contract the firm agreed to pay to the Brazilian government a rental equal to 50 per cent. of the selling price of the monazite sands and to export at least 1,200 tons annually.
To avoid trouble the German Thorium Convention arranged later that half of its supply should be furnished by Mr. Gordon and half by the de Freitas company. A new convention was formed by the four German chemical manufacturers with Mr. Gordon and the de Freitas company, preventing firms in other countries that had started to manufacture thorium nitrate from getting raw material. Consequently great efforts were made to find and develop new deposits of monazite. The high price for thorium nitrate made possible the mining of monazite in the Carolinas and its export to Germany, especially to one German manufacturer who was not in the German Thorium Convention.
Ultimately there was an overproduction of thorium and in 1906 the price dropped 50 per cent. Monazite mining declined in all localities where the cost of mining was high, and production in the Carolinas and the interior of Brazil practically stopped.
During the four or five years antedating the war the German Incandescent Gas Light Co., of Berlin, succeeded in controlling the largest manufacturers of thorium nitrate in Europe, except those in France. It controlled both the English and Australian companies and became the active competitor of the so-called Thorium Convention, which at that time had lost much of its power. Mr. Gordon still has extensive interests in Brazil, but he does not have a monoply. The exportation rights from Brazil are in the hands of Luis de Rezende & Co. (Rio de Janiero), Mr. Gordon, and others. Luis de Rezende & Co. is mainly a French concern, but has Brazilian and Portuguese stockholders. The company controls the French company, Société Minière, associated with the Welsbach Co.
As noted above, one French company has exploited monazite deposits in the territory immediately behind the government lands in the State of Rio de Janeiro. Another French company has worked the black sands along the Parahyba River, near Sapucaica, which contain traces of monazite.
—Before the war, the German manufacturers of thorium nitrate exercised as close control over the monazite deposits of Travancore, India, as over those of Brazil. Only a limited quantity of the sand was sold to gas-mantle manufacturers and other consumers in the United Kingdom, and then at a price nine times the price paid by the German consumers. Such a monoply of the supplies of raw material made the German monoply of the thorium nitrate industry almost complete.
[221]
According to S. J. Johnstone, in an address at the annual meeting of the Society of Chemical Industry in July, 1916, the Germans obtained practical control of the Travancore monazite deposits in the following manner: A lease for working these deposits was granted some years ago by the Travancore Durbar, with the approval of the Government of India, to the London Cosmopolitan Tin Mining Co., which contracted to sell the whole of its output to a German firm. Soon after the outbreak of war it was found that the whole of the preference shares and 11,000 of the ordinary shares of the Travancore Minerals Co. were held in trust for the Auer company, of Berlin.
The India Office decided that in the future all directors of the company working the concession must be British-born and that the company, must be ready at all times to sell monazite sand direct, and at a fair price, to British firms. German contracts were canceled. A second company, Thorium, Ltd., obtained a 20-year lease to work 150 acres in Travancore for monazite sand, and is exporting the sand and manufacturing thorium nitrate from it at works in England. A great deal of Travancore monazite has been imported by American companies.
As outlined above, it can be readily seen that the United States is dependent upon Brazil and India for its raw materials, as domestic deposits are not large enough to furnish the required supply and cannot be worked in competition with the more cheaply mined foreign deposits.
The average concentrate obtained in the Carolinas runs about 3¹⁄₂ to 4 per cent. thorium oxide; that obtained in Brazil averages somewhat over 8 per cent. Under such conditions it is difficult for the Carolina monazite to compete with that from Brazil or from India. In addition, a very considerable amount of the Carolina monazite available has been removed. The old workings are more or less covered up and the whole industry has become completely disorganized.
Whilst these deposits were being mined and operated farmers were in the habit of making their own concentrate in crude sluice boxes. The product thus obtained averaged about 35 per cent. monazite. The concentrates were then sold to a refinery, where it was best treated by electromagnetic separators, such as the Wetherill machine. The final product obtained from these machines was ready for chemical treatment for the extraction of the thorium.
Practically the same treatment is given to the monazite from Brazil and India. As the concentrate obtained is of much higher grade, the additional charges for freight and duty, which are not borne by the Carolinas product, are more than offset. Undoubtedly, unless a very high tariff is placed on the monazite from Brazil and India, our future[222] supplies will come from these two sources, at least for some time. It is very doubtful whether with a high tariff the Carolina deposits could furnish the monazite required in this country, even for a few years, and under the most favorable conditions it would take some time, possibly six months to a year, to revive the industry.
The Allies and practically the whole world are dependent upon the United States for the manufactured products, thorium nitrate and gas mantles. Whether this monoply will continue is doubtful, as there is a movement in England to encourage both the thorium nitrate and the gas-mantle industry.
[223]
One country stands pre-eminent as the world’s great producer of copper, and that is the United States, whose production was 60 per cent. of the total world output in 1917. Iron, coal, oil and copper are fundamental raw materials of which the United States produces more than any other country, but only in copper and in oil is the output greater than that of all other countries together. In copper this has been true since the early nineties. American copper, English gold, Russian platinum and Chilean nitrate are common phrases in world markets; as common as the commodities themselves.
No other country produces or has for many years ever produced one-sixth as much copper as the United States. While the world output of copper has been increasing, at the average rate of 5 per cent. annually for 10 years up to 1914 and three times as rapidly since then, the relative importance of the United States has not declined. On the contrary it has increased at a greater rate than the total world output. Certain individual countries, it is true, have since 1914 increased their output faster than the United States, but there is no indication that the United States will lose its present dominating leadership.
Because of the magnitude of the copper industry of the United States, great refining plants have been built up here. American capital also has gone largely into Canadian, Mexican and South American copper properties. As a result the United States now imports nearly one-third as much copper as is produced (18 per cent. of the total world output in 1917). Thus American capital controls, through refining in addition to ownership of mines, 78 per cent. of the world’s copper production. This control should also be equally strong as regards selling. Obviously, a large part of our domestic, and, as regards statistics, all the imported copper, is exported in finished form—copper ingots and bars, brass, electrical machinery, etc. But as regards selling and even mine ownership in Mexico and South America, there is considerable German control; although the important mines of Canada, Mexico and South America are owned by either American, British or French interests, except those owned by local foreign capital.
[224]
Table 35.—Geographical and Financial Control of the World’s Copper Mines
(Production in Metric Tons)
| Percentage of World total |
Average 1916-1917 output of copper |
Country of origin | Estimated capacity output of copper |
Per- centage of world total |
Owned by U. S. capital |
Owned by British capital |
Owned by German capital |
Owned by French capital |
Owned by Japanese capital |
Owned by local capital in producing countries |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Western Hemisphere | |||||||||||||
| 59.2 | 868,903 | United States | 928,000 | 57.5 | 899,000 | 29,000 | |||||||
| 3.3 | 49,168 | Canada | 58,000 | 3.6 | 28,000 | 30,000 | |||||||
| 3.4 | 49,478 | Mexico | 65,000 | 4.0 | 49,000 | Idle | 2,500 | plus | 13,500 | ||||
| 1.0 | 14,000 | Cuba | 10,000 | 0.6 | ... | ... | 2,000 | plus | ... | ... | 8,000 | ||
| 0.2 | 2,000 | Venezuela | 2,000 | 0.1 | ... | 2,000 | |||||||
| 4.8 | 70,000 | Chile | 110,000 | 6.8 | 86,000 | 2,500 | 4,500 | plus | 9,500 | [105] | ... | 7,500 | |
| 3. | 43,620 | Peru | 45,000 | 2.8 | 45,000 | ||||||||
| 0.4 | 6,000 | Bolivia | 12,000 | 0.8 | 6,000 | ... | ... | 6,000 | |||||
| 75.4 | 1,103,169 | Total Western Hemisphere | 1,230,000 | 76.2 | 1,113,000 | 63,500 | 9,000 | 29,000 | ... | 15,500 | |||
| Eastern Hemisphere | |||||||||||||
| 3.2 | 47,500 | Africa | 58,000 | 3.6 | ... | 58,000 | [105] | ... | ... | ||||
| 2.5 | 36,550 | Australia | 43,000 | 2.7 | ... | 43,000 | |||||||
| 7.9 | 112,900 | Japan | 125,000 | 7.7 | ... | ... | ... | ... | 125,000 | ||||
| 2.9 | 42,000 | Spain and Portugal | 42,000 | 2.6 | ... | 40,000 | ... | ... | ... | 2,000 | |||
| 1.3 | 18,500 | Russia (estimated) | 18,000 | 1.1 | ... | ... | 18,000 | (?) | |||||
| 4.8 | 71,000 | Central Powers (estimated) | 71,000 | 4.4 | ... | ... | 71,000 | ||||||
| 1.4 | 19,000 | Norway | 19,000 | 1.2 | ... | 10,000 | ... | ... | ... | 9,000 | |||
| 0.1 | 1,000 | Sweden | 1,000 | 0.1 | ... | ... | ... | ... | ... | 1,000 | |||
| 0.4 | 6,250 | Other countries | 6,250 | 0.4 | ... | 250 | ... | 2,000 | ... | (3,000) (Italy) | |||
| (1,000) (China) | |||||||||||||
| 24.6 | 354,700 | Total Eastern Hemisphere | 383,250 | 23.8 | 31,500 | ||||||||
| World Total | 1,456,869 | 1,613,250 | 100 | 1,113,000 | 212,750 | 98,000 | 31,000 | 125,000 | |||||
| 100% | 69[106] | 13.3[106] | 6.1[106] | 1.9[106] | 7.7[106] | 2.0%, divided as follows: Cuba, 0.5; Spain, 0.1; Norway, 0.6; Sweden, 0.05; Chile, 0.5; China, 0.05; Italy, 0.2 | |||||||
[225]
Table 36.—Business Control of the World’s Copper Mines
All figures metric tons
| Country of origin |
Estimated capacity output of refined copper |
Refined in the U. S. |
Refined in British Dominions |
Refined in Germany |
Refined in France |
Refined in Japan |
Refined in other countries |
Formerly sold by German houses |
|
|---|---|---|---|---|---|---|---|---|---|
| Western Hemisphere | |||||||||
| United States | 928,000 | 928,000 | ... | ... | ... | ... | ... | ( 73,000) | |
| Canada | 58,000 | 28,000 | 30,000 | ... | ... | ... | ... | ( 21,000) | |
| Mexico | 65,000 | 52,000 | ... | ... | 13,000 | ... | ... | ( 2,500) | |
| Cuba | 10,000 | 10,000 | ... | ... | ... | ... | ... | ( 10,000) | |
| Venezuela | 2,000 | 2,000 | |||||||
| Chile | 110,000 | 43,000 | 2,500 | ... | 9,500 | ... | 55,000 | ( 20,000) | |
| Peru | 45,000 | 45,000 | |||||||
| Bolivia | 12,000 | 6,000 | ... | ... | 6,000 | ||||
| Total Western Hemisphere | 1,230,000 | 1,114,000 | 32,500 | 0 | 28,500 | 0 | 55,000 | (134,500) | |
| Percent of total | 76.2%[107] | 69 | 2 | ... | 1.8 | ... | 3.4 (Chili copper) (U. S. owned) |
(8¹⁄₄%) | |
| Eastern Hemisphere | |||||||||
| (or in Belgium) | |||||||||
| Africa | 58,000 | 5,000 | 53,000 | ... | ... | ... | ... | ( 5,000) | |
| Australia | 43,000 | ... | 43,000 | ... | ... | ... | ... | ( 43,000) | |
| Japan | 125,000 | 3,000 | ... | ... | ... | 122,000 | |||
| Spain and Portugal (estimated) | 42,000 | 2,000 | 35,000 | ... | 5,000 | ... | ... | ||
| Russia (estimated) | 18,000 | ... | ... | ... | ... | ... | ... | ? | |
| Central Powers | 71,000 | ? | ... | ... | 71,000 | ... | ... | 18,000 | ? |
| Norway | 19,000 | ... | 19,000 | ... | ... | ... | ... | ? | |
| Sweden | 1,000 | ... | 1,000 | ... | ... | ... | ... | ? | |
| Other Countries | 6,250 | ... | 250 | ... | 2,000 | ... | 3,000 (Italy) 1,000 (China) |
||
| Total Eastern Hemisphere | 383,250 | 10,000 | 151,250 | 71,000 | 7,000 | 122,000 | 22,000 | ( 48,000) | |
| Percentage of total | 23.8 | .7 | 9.3 | 4.4 | .4 | 7.6 | 1.4 | (3%) | |
| Total percentage | 100% | 69.7 | 11.3 | 4.4 | 2.2 | 7.6 | 4.8 | (11¹⁄₄%) | |
[107] The United States controls the sale of substantially all this copper.
[226]
Table 37.—Future Importance of Present Copper-producing Countries as Indicated by Known Reserves of Copper Ore, and Capital Controlling These Reserves
| Producing country |
Estimated capacity output of copper (metric tons) |
Percentage of world total |
Developed reserves in terms of years’ life at capacity output |
Exten- sion[108] |
Percentage of total reserves of world |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Owned by U. S. capital |
Owned by British capital |
Owned by German capital |
Owned by French capital |
Owned by Japanese capital |
Owned by local capital in producing countries |
|||||||||
| W. Hemisphere: | ||||||||||||||
| United States | 928,000 | 57.5 | 12.4 | yrs. | 12 | .4 yrs. | ... | ... | ... | ... | 713 | 34 | . | |
| Canada | 58,000 | 3.6 | 15 | 20 | ... | ... | ... | ... | 63.3 | 3 | . | |||
| Mexico | 65,000 | 4.0 | 5 | large | large | 6 yrs. | ... | ... | 20.8 | 1 | .15 | |||
| Cuba and Venezuela | 12,000 | 0.7 | ... | ... | 3 | yrs. | ... | ... | 3 yrs. | 2.1 | 0 | .10 | ||
| Chile | 110,000 | 6.8 | 150 | 3 | ? | 5 | ... | consider- able |
795.8 | 37 | .9[109] | |||
| Peru | 45,000 | 2.8 | 4 | ... | ... | ... | ... | 11.2 | 0 | .55 | ||||
| Bolivia | 12,000 | 0.8 | ... | ... | ... | 4 | ... | ... | 2.8 | 0 | .10 | |||
| Total | 1,230,000 | 76.2 | ... | ... | ... | ... | ... | ... | 1,609.0 | |||||
| Percentage total reserves | ... | 76.8 | 73.6 | 2 | .4 | 0 | .1 | 0.4 | ... | 0.3 | ... | 76 | .8 | |
| Eastern Hemisphere: | ||||||||||||||
| Africa | 58,000 | 3.6 | ... | 66 | [110] | ... | ... | ... | ... | 237.6 | 11 | .3 | ||
| Australia | 43,000 | 2.7 | ... | 7 | .2 | ... | ... | ... | ... | 20 | 0 | .95 | ||
| Japan | 125,000 | 7.7 | ... | ... | ... | ... | 6 | ... | 46.2 | 2 | .2 | |||
| Spain and Portugal | 42,000 | 2.6 | ... | 50 | ... | ... | ... | 50 | 130 | 6 | .2 | |||
| Russia | 18,000 | 1.1 | ... | 18 | (?) | 18 | (?) | ... | ... | 18 | 19.8 | 0 | .95 | |
| Central Powers | 71,000 | 4.4 | ... | ... | ? | ... | ... | ? | 20 | 0 | .95 | |||
| Norway | 19,000 | 1.2 | ... | 10 | ... | ... | ... | 10 | 12 | 0 | .55 | |||
| Sweden | 1,000 | 0.1 | ... | ... | ... | ... | ... | 10 | 1 | 0 | .04 | |||
| Other countries | 6,250 | 0.4 | ... | ... | ... | ... | ... | ? | 1.4 | 0 | .06 | |||
| Total | 383,250 | 23.8 | ... | ... | ... | ... | ... | ... | 488.0 | |||||
| Percentage total reserves | ... | 23.2 | ... | 18 | .4 | 0 | .95 | ... | 2.2 | 1.65 | ... | 23 | .2 | |
| World total | 1,613,250 | 100 | ... | ... | ... | ... | ... | ... | 2,097.0 | |||||
| Percentage of world total reserves | ... | ... | 73.6 | 20 | .8 | 1 | .05 | 0.4 | 2.2 | 1.95 | ... | 100 | ||
[108] Extension is the product of “Percentage of World Total” and “Developed Reserves in Terms of Years Life,” and gives total ultimate relative importance of different countries.
[109] In so far as it affects production this high figure must be discounted because of the large reserves being compact, the distance from market and the unfavorable mining conditions of a thinly settled country.
[110] Katanga equals ⁶⁵⁄₆₆ of this.
Note.—Not much weight is to be attached to figures showing more than 10 years of life. Total full future value over long number of years is greatly reduced when expressed in terms of present value on normal interest discount. All countries with reserves good for ten or more years may be considered as being on an equal footing so far as copper resources are concerned. But, large reserves may mean expanding production (as in Chile and Africa) and be important on that account and only on that account.
To weigh ore reserves they have been taken in toto, but Spain would be no worse off as compared to the United States if her reserves were put at 10 years instead of at 50 (as calculated above). Her reserves happen to be of a kind easily blocked out, but her output is stationary.
[227]
Plate VIII.—Geographical distribution of the principal copper-producing districts of the world. By F. W. Paine.
[228]
Table 35 shows the different copper-producing countries of the world and the chief features of financial control of the producing mines. Table 36 shows the chief features of business control (refining and selling control) as distinct from control by mine ownership. In both tables a forecast for future conditions is made by using estimates of 1918 and 1919 production. These figures are followed out to the different forms of control. The actual outputs of 1916-17 are given as a reference and check. Table 37 shows reserves, largely those of producing mines. Plate VIII shows the location of the principal copper deposits of the world.
—The maximum output of copper from mines within the borders of the United States was in 1916, and amounted to 1,927,850,548 pounds. In 1917 the output was less, because of labor troubles, but six leading states showed an increase, and if Montana had equaled the output of 1916, the 1917 output would have been 1,962,034,512 pounds. Without good markets and favorable labor conditions, the United States production cannot reach anything like 2,000,000,000 pounds a year.
—All the productive bodies of copper in the United States are owned by Americans, except a small number controlled by English capital. Before the war, there was evidence of German affiliation and potential control in the copper industry, but this centered in refining and selling the metal. Accordingly, the German grip on the industry was highly centralized, and direct and effective measures were used in breaking it.
Five leading groups are in control of copper production in the United States. Certain of these have additional important ownerships in South America and Mexico, which will be discussed under those countries.
Table 38.—Leading Financial Groups in Control of Copper Production in United States
| Pounds | |
|---|---|
| Group 1. Hayden-Jackling “porphyries” (closely affiliated with group 2) Utah Copper, Ray Consolidated (Ariz.), Nevada Consolidated, (Nev.) Chino (N. Mex.) and Butte & Superior (Mont.); 1917 output (custom ore not included) | 448,887,253 |
| Group 2. Morgan-Guggenheim (American Smelting & Refining Co). Kennecott (Alaska) and a large number of smaller mines owned by Americans which have their ore treated at plants controlled by this group. The 1917 output of Kennecott and eleven of the chief customers of American Smelting & Refining plants (smaller custom shipments to smelters must be added) was | 156,954,722 |
| Group 3. Rockefeller-Ryan: Anaconda, Inspiration, North Butte, Utah Consolidated, Mountain Copper (Cal.), Balaklala (Cal.), Walker mine (Cal.), and Arizona Copper Co., 1917 output (custom ore not included) | 357,308,558 |
| Group 4. Phelps-Dodge and affiliations: Arizona and New Mexico[229] only. Copper Queen, Detroit, Burro Mountain, Commercial Mining Co. (Phelps-Dodge); 1917 output | 123,000,000 |
| Calumet & Arizona—New Cornelia (Briggs-Congdon); 1917 output | 79,360,000 |
| United Verde Extension (affiliated with each of above); total 1917 output | 63,242,784 |
| To which must be added custom business which is important, e.g., Arizona Commercial mine, etc.; 1917 business (other custom ore not included) about | 10,000,000 |
| Total for group 4 | 275,603,113 |
| Group 5. Calumet & Hecla: 1917 output, Calumet & Hecla mine and subsidiaries | 168,765,033 |
| Total 1917 output of these five leading groups[111] | 1,407,518,679 |
[111] Exclusive of the small mines (shippers of custom ore) partly controlled through smelting and refining contracts by same interests.
Important smaller groups may be listed as follows:
| Group 6. W. A. Clark: United Verde, Elm Orlu (Mont.) and Ophir Hill (Utah); 1917 output | 88,390,038 |
| Group 7. Adolph Lewisohn: Miami and, by sale of output, Shattuck-Arizona; 1917 output | 57,058,666 |
| Group 8. U. S. Smelting, Refining & Mining Co. Custom ore and Mammoth mine, Cal., Utah Apex, and Tintic (Utah) mines; 1917 output | 22,600,000 |
| Group 9. Lake mines (other than Calumet & Hecla): Copper Range Co., Mohawk, Quincy, etc.[112]; 1917 output | 99,700,000 |
[112] Ten different interests.
There is also a group, discussed later, of uncertain or unclassifiable affiliations, owned by Americans, but in many instances worked under smelting and refining contracts that merit special mention:
| 1917 output, pounds |
|
|---|---|
| Old Dominion (Ariz.), (perhaps group 4) | 25,758,381 |
| East Butte (and custom ore), (perhaps group 9) | 18,000,000 |
| Shannon Copper Co., (Ariz.), (independent) | 6,138,219 |
| Penn. Mining Co. (Cal.) (independent) | 3,400,000 |
| Cons. Arizona Smelting Co. (independent) | 5,000,000 |
| Ducktown Sulphur, Copper & Iron Co., Ltd. (Tenn.), (English) | 5,523,573 |
| Total unclassifiable and uncertain | 63,820,173 |
| Total of groups 1 to 5 | 1,407,518,679 |
| Total of groups 6 to 9 | 267,748,704 |
| Total of all interests | 1,739,087,556 |
| Total 1917 United States copper production (from domestic mines only) | 1,873,546,171 |
| Balance (custom ore from small shippers)[113] | 134,458,614 |
[113] This balance includes copper produced as by-product in mining of other metals in Colorado and the East, estimated at 50,000,000 pounds, and custom shipments from 1,000 small operations, chiefly to smelters controlled by groups 1, 2, 3, 4 and 6, estimated at 84,458,614 pounds.
[230]
Certain mines in the above groups are owned in England, but are classed with that interest which refines and sells the production. In group 2, for example, is listed the Tennessee Copper Co., whose copper is refined and sold at the American Smelting & Refining plant in Baltimore. In ownership, however, this property should be placed in group 7, (output 10,547,704 pounds).
In group 3 are two English-owned mines: Arizona Copper Co. (Scotch) and Mountain Copper Co., (English). Ducktown Sulphur, Copper & Iron in the last group (uncertain and unclassifiable) is also English-owned. The combined output of these three English-owned properties is 48,000,000 pounds. This comprises all properties not owned by American capital. Ducktown Sulphur, Copper & Iron Co., Ltd. is English-owned, but all its production (copper matte) is sold to the American Metal Co. The others in the group designated above as uncertain, or unclassifiable, are entirely American owned, although their production has been marketed by the American Metal Co. or L. Vogelstein, as discussed later.
None of these groups actually own the mines outright, the mines being owned by hosts of stockholders scattered all over the country. Of many companies the president and directors own a very small percentage of the stock. As regards group 6 and to a less extent group 4, however, the actual ownership is in very few hands; but this is exceptional. The copper mines of the United States, like the railroads and the largest industrial enterprises, such as the United States Steel Corporation, etc., are, in the last analysis, controlled by their stockholders.
Summarized on the basis of the 1917 output, one finds that the ownership of American copper mines is as follows: American 97¹⁄₂ per cent., English and Scotch, 2¹⁄₂ per cent.
—Ownership of smelters that treat domestic ore is substantially identical to the mine ownership given above. Interests owning active smelters are less numerous than interests owning mines, because efficient smelting requires large-scale operations.
The electrolytic refineries are all American owned. Large-scale units, representing heavy capital investments, are essential in electrolytic refining. A small refinery cannot compete successfully with large ones. The average important copper mine produces enough ore to make about 25,000,000 pounds a year, whereas the average smelter produces three or four times as much blister or casting copper, and the average electrolytic refinery can produce over 250,000,000 pounds annually. Consequently, there are only six groups (Hayden-Jackling, Morgan-Guggenheim, Rockefeller-Ryan, Phelps-Dodge, Calumet & Hecla, and U. S. Smelting, Refining & Mining Co.) interested in refinery ownership. No small producer has the capital or the size to be able to enter[231] this field. Table 39 shows electrolytic copper refineries of the United States and their ownerships:
Table 39.—Ownership and Capacity of American Copper Refineries
| Works | Ownership, group— |
1917 capacity (pounds) |
|---|---|---|
| Baltimore Copper Smelting & Rolling Co. | 1 and 2 | 720,000,000 |
| Nichols Copper Co. Independent and in part | 4 | 500,000,000 |
| Raritan Copper Works | 3 | 460,000,000 |
| American Smelting & Refining Co. | 1 and 2 | 288,000,000 |
| United States Metals Refining Co. | 8 | 250,000,000 |
| Tacoma Smelting Co. | 1 and 2 | 204,000,000 |
| Anaconda Copper Mining Co. (new plant) | 3 | 180,000,000 |
| Calumet & Hecla Mining Co. | 5 | 65,000,000 |
| Anaconda Copper Mining Co. (old plant) | 3 | 65,000,000 |
| Balbach Smelting & Refining Co.[114] (former German affiliations) | ... | 48,000,000 |
| Total capacity | 2,780,000,000 | |
| 1917 production (pounds) |
||
| Electrolytic copper | ... | 1,452,744,593 |
| Secondary electrolytic copper | ... | 66,337,771 |
| Imported copper made into electrolytic | ... | 555,000,000 |
| Total 1917 refinery production | ... | 2,074,082,364 |
[114] This company treats copper scrap and imported copper ores and matte.
The refineries control the situation to a very considerable extent. A copper producer must obtain electrolytic refining in order to market his product. Lake copper and casting copper do not require electrolytic refining, although producers of casting are often at a disadvantage when there is a big premium on electrolytic copper and casting can only be sold at a large discount.
The smelters do not control the situation in the same way. In the United States are a large number of custom smelters—32—that actively compete for ores; some have many branches. Moreover, a mine of any size will have its own smelter, as the capital investment is far less than that required for an electrolytic refinery. As the table shows, there are only seven groups (the six above enumerated and the Balbach Smelting & Refining Co.) interested in electrolytic refining, and one of these (the smallest) is to a considerable degree interested in the treatment of secondary or scrap copper.
The electrolytic refinery control of the copper production of the United States is shown by the 1917 figures. In that year the production of electrolytic copper was 1,452,744,593 pounds; of Lake copper, 238,[232] 508,091 pounds; and of casting copper, 152,293,487 pounds. Electrolytic copper thus constituted 77¹⁄₂ per cent. of the total.
—Groups owning mines, smelters, and refineries invariably also control or own the selling agencies that distribute the product to the consumer. In these cases, control through selling is the same as control through mine ownership, but is increased by the copper in ores received at custom smelters.
Control through selling, then, is identical to the control shown in Table 38, so far as groups 1, 2, 3, 4, and 8 are concerned, if certain additions at the expense of the other groups are made. But every producer of Lake copper controls the sale of its product, because Lake copper needs no electrolytic refining. Hence in groups 5 and 9 mine ownership and control through selling are identical. This is a fact of considerable interest and confirms the fact of control through refinery ownership. Groups 6 and 7 (Table 38) are large producers, and although they do not own refineries they are able to control the sales of their product. The refineries are willing to refine their copper on toll and return the marketable copper to the mine owners, who make sale to the trade. Groups 2, 3, and 7 now control copper even further, as they own brass mills, wire and rod mills, etc. They manufacture a part of their production and sell it as copper wire, finished brass, etc., instead of making sales to the brass and wire mills of ingots, bars, cakes, etc., which is and has been always the general practice.
There remains to consider the control, through selling, of the six uncertain groups (total production 63,820,173 pounds) and some of the 134,458,614 pounds of copper produced from custom ore. A large part of this, as noted, is lodged in groups 1, 2, 3, 4 and 8.
For many years three concerns affiliated with the German metal combines (Merton Co. and Metallgesellschaft, of Frankfort-on-the-Main, and Aaron Hirsch, of Halberstadt) have been active in the copper business of the United States. Their activity has been confined to selling of copper produced in this country, and to ownership and selling of copper produced in foreign countries but brought to the United States for smelting and refining. One small refinery is or was owned by this group in the United States, and two smelters, treating mainly foreign ores. It also owns smelters in Mexico and South America, and had close connections with two large refineries in New Jersey, so that it sold all the electrolytic copper produced by one plant and an important part of the production of the other plant.
—The exact German ownership of these concerns was disclosed during the war by the work of the Alien Property Custodian, and was as follows: (1) American Metal Co., with an issued capital of $7,000,000, of which the German holdings amounted to $3,336,000, or close to 50 per cent.; these holdings were taken over by[233] the United States authorities; (2) L. Vogelstein & Co., of which 80 per cent. of the stock was held by Germans (A. Hirsch & Sohn) and 15 per cent. by L. Vogelstein, a naturalized American citizen; (3) Beer, Sondheimer & Co., entirely German owned. The government seized the two last-named concerns.
The American Metal Co. markets the copper of the Old Dominion, East Butte, Shannon, Penn. Mining Co. and Ducktown Sulphur, Coal & Iron companies. Blister copper 99 per cent. pure is purchased on contract from the first-named four companies by the American Metal Co. This copper is refined for the American Metal Co. by the Nichols Copper Co., and the finished product sold to the trade by the American Metal Co. The Ducktown Sulphur, Copper & Iron Co. sells all of its production, as copper matte, to the American Metal Co., which has it treated and sells the finished product. In this manner 58,820,173 pounds was controlled through sale by the American Metal Co. in 1917. In addition, considerable domestic custom ore and a large amount of copper imported from Canada (Granby), South America and Mexico is handled by the American Metal Co. in the same way. Such imported copper totals up to three times as much as the domestic copper so controlled.
So far as domestic copper is concerned this situation will be corrected. The Nichols Copper Co. is free to refine this copper and sell it or turn it back to the producers for sale; it does this latter for Phelps-Dodge and Miami. It can be assumed that control through selling will cease as regards the 58,820,173 pounds cited above and also as regards Granby’s production—about 50,000,000 pounds.
The American Metal Co. may be sold to American interests,[115] thus clearing up the situation in imported copper to some extent. Certain Mexican and Chilean properties owned by the American Metal Co. will perhaps not be sold, and such properties can be considered as likely always to be German owned.
[115] The German holdings in the American Metal Co. are reported sold to an American syndicate in which L. Vogelstein participated.
The American Metal Co. owns the Balbach electrolytic refinery, and from treatment of scrap and imported copper (Chilean and Japanese blister and copper) at that plant obtains and sells about 50,000,000 pounds of refined copper per annum.
The concern of L. Vogelstein & Co. lost its chief hold on American copper production in 1915. Up to that time L. Vogelstein & Co. worked very closely with group 8 (United States Smelting, Refining & Mining Co.). The output of the United States Smelting Refining & Mining Co. mines and smelters was sold by Vogelstein as well as the output of its electrolytic refinery. But since 1915 this American-owned enterprise itself sells all the copper produced by its own mines and smelters.
[234]
But Vogelstein still controls the sale of the copper treated at the plant of the United States Metals Refining Co.,[116] over and above what is produced by the United States Smelting, Refining & Mining Co. from its own mines and smelters. This remaining copper consists in part of imported copper, and in part of the output of the Consolidated Arizona Smelting Co., which is an American-owned enterprise. The output was large in 1917 (about 20,000,000 pounds) but three-fourths of it was ore from the United Verde Extension mine. The United Verde Extension has since completed its own smelter and no longer turns over a part of its copper output to Vogelstein through the Consolidated Arizona Smelting Co. The output of United Verde (group 6) also was formerly sold by Vogelstein, but now is sold by the owner of the mine.
[116] The United States Metals Refining Co. was a subsidiary of the United States Smelting, Refining & Mining Co., sold, 1920, to the American Metal Co.
Therefore, the only copper controlled in 1918 by Vogelstein through selling was about 50,000,000 pounds a year, the output of mines owned by Consolidated Arizona Smelting Co. (about 5,000,000 pounds) and the imported copper, of which about 45,000,000 pounds was treated in 1917. The latter is controlled by Vogelstein not only by selling of the product but in part by smelting contracts and in part probably, as regards South America, by ownership of mines.[117]
[117] L. Vogelstein is reported to have sold his interests to the American Metal Co. and subsequently, early in 1920, to have acquired a fifth interest in that company.
has never been a large factor in the United States copper industry, although much interested in zinc. But the firm does control the sale of some copper and owns a smelter at Norfolk. This smelter treats imported ores for the most part, but also obtains some copper from pyrites (sulphur ores) coming from the United States and Canada. Perhaps as much as 10,000,000 pounds of domestic copper was sold by Beer, Sondheimer & Co. in 1917. The company owns an important Cuban mine.
As American capital owns American copper mines, smelters and refineries, German interests were able to obtain a foothold only through selling organizations (trading in metals), which later they extended to close working arrangements with electrolytic refineries, which were naturally interested in finding a good cash market for their output. The fact that Germany prior to 1914 was the biggest foreign buyer of United States copper, made easy the successful development of the carefully laid German plans.
In the future such plans can be guarded against by encouraging copper producers to sell their own output. All the large producers already do this, a change in this respect having developed since 1914. Sales in foreign markets can now be properly managed under the provisions of recent legislation permitting copper producers to enter into a combination[235] in the sale of export copper. This counteracts the old German system of a buyers’ combine against the sellers of copper, which was an important factor in forcing American producers to have German concerns sell their product. But it will be necessary for the electrolytic refineries to co-operate in this policy of American selling control of American copper. Producers whose output is only a few million pounds per annum probably cannot afford to establish their own selling agencies; such producers will include all who have no smelter but ship to custom smelting plants. The production can be sold by the large custom smelting plants, which are American owned, or these small producers could establish a common sales agency. Other larger producers, as those whose copper is now sold by the American Metal Co., should be enabled to do their own selling. In this they have been blocked by the lack of refining facilities, except on a basis that took away from them selling control of their product. This situation can be corrected by regulations that put electrolytic refineries on a recognized toll basis for all American customers. All refineries have about the same costs and their combined capacity is ample to treat all the blister copper that will be produced. They should receive good profits on the business, but it should be unlawful for refineries to refuse to treat blister copper on toll and insist that copper must be sold to them outright. The toll system is already in use at several refineries and has proved satisfactory.
As shown above, there are in the United States a few very large refineries whose ownership is in few hands. If these refineries control the sale of all the production of copper several objectionable features develop. The few sales agencies handle so much copper that there is a tendency to co-operate with representatives of foreign consumers who can buy in large quantities, and it is not difficult to manipulate the market temporarily, in disregard of actual conditions of demand and supply. Thus the entire output of copper, one of our great natural resources, is placed in the hands of groups who, while interested in mining, are more interested in refining. The best interests of the industry are more nearly those of the miner than of the refiner. Therefore, the most positive dislodgment of former German control of copper through selling will come from the breaking up of the former system and transferring each unit of that system to other hands; rather than transferring the old units in block to non-German hands. Sales of American copper should be handled by a large number of separate agencies actively competing for the domestic market; this is essential in the interests of the consumer and of the country. But export copper business should be handled through one agency or association representing all the sales agencies, as is now legal, and this should be done in the interests of the producer and of the country.
[236]
—The developed reserves of United States copper deposits are fully equal, in proportion to output, to such reserves in foreign copper deposits. This insures the fact that for the next ten years, at least, the copper production of the United States will maintain its present relative dominance over all foreign countries.
A large proportion of all copper deposits are of such a deep-seated character that at no time can large reserves be positively developed, even when they exist. On the other hand, all over the world the mines with large known reserves are horizontal deposits, lying near the surface, because only in such occurrences is it possible to block out easily and cheaply big tonnages of ore. There are in the United States six very important deposits of this type, the so-called “porphyries.” These are:
Table 40.—“Porphyry” Copper Mines in the United States
| Mine | Reserves (tons) |
Years of life at present production |
|---|---|---|
| Utah Copper Co. | 200,000,000 | 31 |
| Ray Consolidated Copper Co. | 90,000,000 | 30 |
| Chino Copper Co. | 80,000,000 | 27 |
| Inspiration Consolidated | 120,000,000 | 20 |
| Nevada Consolidated | 80,000,000 | 20 |
| Miami Copper Co. | 50,000,000 | 20 |
| Total | 620,000,000 | 26 |
These mines in 1917 produced 31.5 per cent. of the total United States output.
The New Cornelia mine is of similar type and has already developed 75,000,000 tons, but as it is new its 1917 output was small. The Arizona Copper Co., Ltd., is also of this type, as are certain new developments in the Phelps-Dodge properties.
These “porphyry” deposits occur in or near intrusive igneous rocks of various ages. Fully one-third of the United States production is now and will continue to be obtained from such deposits. On the average, about 1 per cent. copper is recovered from the ore.
Distinct from the shallow and horizontal-lying disseminated ores or “porphyry coppers,” are the deep mines. The two oldest and most important deep-mine districts in the United States are Butte, Montana, and the Keweenaw Peninsula, Michigan. The mines of Butte work steeply dipping veins. It cannot be considered that over five years of ore reserves are known, and probably not over 2¹⁄₂ years of reserves are actually blocked out on three sides. However, there are no indications of early exhaustion, as the veins are profitable at more than 3,000 feet,[237] the greatest depth to which mining has yet progressed. The deposits of northern Michigan are in pre-Cambrian rocks. They have been important producers of copper for over 50 years, and several mines have reached a vertical depth of more than 5,000 feet. Certainly not over five years of ore reserves are fully developed, but there are no signs of early exhaustion. These two districts, Butte and Michigan, now produce about 30 per cent. of the total United States output, a smaller proportion than before the development of the “porphyries.” The average copper content of the Michigan deposits ranges from 0.5 per cent. to 2 per cent. and of the Butte deposits 2.5 to 5 per cent.
Certain ore deposits (usually massive but irregular) which are situated mainly in Arizona constitute the third important general class. These deposits produce about one-quarter of the total United States output. Bisbee, Jerome and Globe (Arizona), and Kennecott (Alaska), are the main localities. Owing to the irregular nature of the deposits and the distance from the surface at which the ore is found, large developed reserves cannot be blocked out in advance. Such reserves are assumed to be five years.
Apart from the three classes of deposits described above are many smaller deposits, of which the most distinct class are the pyritic bodies, notably those of Tennessee and California. Such deposits are often profitable even when of low grade, because the sulphur as well as the copper is recovered. Reserves in such deposits are large: equal, say, to ten years’ life. Deposits of this class are important in Spain, Norway and in part in Japan. Mines producing copper as a by-product should also be grouped here.
From the above outline the table below has been compiled:
Table 41.—Developed Reserves of United States Copper Mines
| General group | Percentage of total output |
Years of life |
Extension[118] |
|---|---|---|---|
| The “porphyries” | 35 | 26 | 9.10 |
| Deep mines | 30 | 5 | 1.50 |
| Rich ore bodies (Arizona and Alaska) | 25 | 5 | 1.25 |
| Pyritic ore bodies | 5 | 10 | 0.50 |
| Others | 5 | 2 | 0.10 |
| Average | 100 | 12.4 | 12.45 |
[118] “Extension” is the Percentage of Total Output multiplied by the Years of Life, giving the relative importance of each group.
The known ore reserves serve as a basis for the assumption that the production of copper in the United States will continue at the present figures for at least ten years.
[238]
Canadian copper-producing properties are entirely controlled by American and British capital in about equal proportion, changes involving construction of new refineries and a shift in selling control being assumed to be already effective. Up to the present time the natural development has been for Canada to depend largely on the United States for refining facilities. It is likely that in the future local or English control in this field will be closer than heretofore, although the Canadian copper industry will always be closely identified with that of the United States.
There are three chief copper properties in Canada which are controlled by United States capital, all in British Columbia, as well as several small mines in this province and in others. By far the largest is the Granby Consolidated Mining, Smelting & Power Co. This company has mines in two districts. One of these properties is nearly exhausted; the other is a new and vigorous producer. Smelters are operated at each place. The Granby company is controlled by the same interests that own the Nichols Copper Co. (electrolytic refinery). Considerable custom ore is treated by Granby, a large amount coming from Alaska. The other two mines are those of the Canada Copper Co. and the Howe Sound Co. Their production is refined and sold in the United States by American concerns. The developed reserves at these three mines are all large, being fully adequate for fifteen years at the present rate of production.
The only property in Canada which has established facilities for producing copper ready for the consumer is the Consolidated Mining & Smelting Co., of Trial, B. C. This Canadian company owns and operates its own mines, smelter and electrolytic refinery. The capacity of the refinery is now 14,000,000 pounds refined copper annually. The ores are massive pyritic bodies without great developed reserves, but the probable reserves are large.
The English and American properties at Sudbury yield nearly as much copper as the Granby company, although their main business is nickel production. A small part of the output is refined in Wales, producing copper sulphate; but the largest part has been refined in New Jersey. A refinery is being completed in Canada that will treat these copper-nickel mattes and produce refined copper. These copper-nickel deposits occur in pre-Cambrian rocks, and the known or potential reserves are very large. They are described in more detail in Chapter VI.
In Ontario and Quebec there are a number of pyrite mines where some of the pyrite contains considerable copper. Most of the pyrite is shipped to the United States, where the copper is recovered, refined and sold.
The production of Canada is growing, and the country will become of increasing importance as a source of copper. Known reserves are larger[239] in proportion to output than in the United States and there will probably be important developments of new districts. The northern British Columbia region is of exceptional promise.
The copper output of Cuba has increased in an extraordinary degree during the past few years, but there are no indications that this increase will continue. Two mines are responsible for nearly all the production. One is an old mine near Santiago, the El Cobre, which yielded about one-quarter of the total output. It is owned by the German metal combine, which ships the ore and concentrates to Norfolk, where they are treated by the smelter owned by Beer, Sondheimer & Co., who have always marketed the production. The Matahambre mine, in Pinar del Rio Province, yields nearly the entire remaining Cuban output; it is owned by Cubans. Ore is shipped to the United States Smelting, Refining & Mining Co., of New York, and is believed to be sold by L. Vogelstein & Co. The reserves of copper ore in Cuba can not be considered large. Statistics of Cuban output are conflicting: producers’ reports, statistical authorities and United States commerce reports not being in agreement. All production is shipped in crude form to the United States for refining.
There are copper deposits in Central America,[119] and at one or two points in the West Indies outside of Cuba. To date production has been insignificant, although future possibilities are considerable.
[119] The Rosita mine, in Nicaragua, has 1,500,000 tons of ore blocked out, running over 5 per cent. copper. It has not been equipped.
The future importance of Mexico as a producer of copper or of other metals will be determined not only by the character of her natural deposits but by political conditions. The latter have materially decreased the output during the past few years, so that in 1917 production was about 100,000,000 pounds, probably not much over half what it would have been had normal conditions been continuous since 1912.
—Three companies now produce about three-fourths of the total copper. Two of these are owned by American capital and their product is refined and sold in the United States. Situated near the Arizona border, they have not suffered from the revolution as much as the properties farther south. These two companies are the Greene Cananea Copper Co. (Ryan-Rockefeller, group 3 of Table 38) and Montezuma Copper Co. (Phelps-Dodge, etc.; group 4 of Table 38). The developed reserves at the Montezuma Copper Co. are equivalent to five years at present production and those at Cananea can be considered about[240] the same. Both districts have important possibilities. Near Greene Cananea is another American property, Democrata Cananea, which is a producer of moderate size. The Cananea district is now producing at the rate of over 50,000,000 pounds per annum. Another American property of note is the Teziutlan Copper Co., Puebla, now idle due to revolutions. It has a smelter which normally ships 12,000,000 pounds of blister copper annually to the Anaconda company’s electrolytic plant. Considerable copper is produced at the plants of the American Smelting & Refining Co., which has three copper smelters, one at Matehuala, San Luis Potosi; one at Asarco and another at Aguascalientes, Durango.
controls the third important property (among the three most important in Mexico), which is on the peninsula of Lower California. This is the Boleo, owned by the Rothschilds; as it is far removed from the heart of Mexico, operations have not been greatly disturbed. The mine is an old producer with large potential reserves, and the actual developed reserves will maintain present output for six years. The Boleo ore deposits, which are of an uncommon type, are Tertiary sediments that contain 3.5 per cent. copper ore, usually as oxides. Smelters near the mine produce blister copper and matte, which normally is shipped to France. During the war a large part of the blister and matte came to the United States and passed into the hands of the American Smelting & Refining Co. French interests also own the Compagnie d’Inguaran, near Ario, Michoacan. There are large developed reserves, but the property is idle because of political conditions and absence of equipment or rail connections. French capital also controls the Magistral Ameca Co., in Jalisco, where large ore reserves have been developed. This property is also idle due to political conditions.
controls the Mazapil Copper Co., which has large plants, including smelters, in Zacatecas and Coahuila. This is one of the largest copper companies in Mexico as well as one of the oldest important mines. It was idle for some time because of the Mexican revolutions, but has been reopened recently.
To sum up, the production in Mexico at present is three-quarters American controlled (of which one-half may be assigned to group 3 and one-quarter to groups 2 and 4), while the remaining one-quarter is French controlled. But present output and known reserves give no true picture of future possibilities. Mexico can become of first importance as a copper producer, ranking possibly second only to the United States or equaling Chile and Japan among the world’s producers. The natural resources are there and in the future will surely be developed much more extensively than ever before.
must be taken into consideration, and especially the activities of the American Metal Co. in Mexico, notably more vigorous since 1914 than before. It is believed that the company has made[241] substantial profits from Mexican mining investments in this period and has obtained a very strong foothold. The Compañia Metallurgica de Torreon is one of the American Metal Co. subsidiaries, owning promising mines, chiefly in the development state, and smelters already equipped to produce 20,000,000 pounds of copper yearly. The Mapimi smelter is also owned by the American Metal Co., as are many other companies. What is the future Mexican political situation to be and what part will the American Metal Co. or the German metal combine play in the Mexican copper industry? There is no more pertinent question in the entire field of the political and commercial control of the copper resources of the world, particularly as American capital is largely interested in Mexican copper mines and has made enormous investments there. Also the natural tendency is for most of the Mexican copper to be shipped to the United States for refining and marketing. American refineries, with cheap fuel and efficient methods, are the natural destination for Mexican raw copper.
South America is the second largest copper-producing continent of the world, but at present stands far behind North America. The copper-producing countries of South America, in the order of their importance, are Chile, Peru, Bolivia, Venezuela and Argentina.
Chile is a copper producer of rapidly increasing importance, as indicated by production records of recent years.[120]
[120] According to Chilean statistics, and estimate for 1917.
| 1914 | 1915 | 1916 | 1917 | |
|---|---|---|---|---|
| Metric tons | 44,665 | 52,341 | 71,288 | 95,000 |
The largest mines of Chile are controlled by American capital. Group 2 of Table 38 (the Morgan-Guggenheim interests) controls the Chile Copper Co., Braden Copper Co. and the Caldera and Carrizal custom smelters. The developed ore reserves of the Chile Copper Co. are the largest in any known copper deposits in the world and the reserves of Braden are among the largest known. Group 3 of Table 38 (Anaconda Copper Co.) has a property with large developed ore reserves—the Andes Copper Co. This mine is not yet (1918) producing.
Table 43 (p. 243) gives data concerning these American-owned mines.
These three mines are allied to the “porphyries” of the United States in character, but they are less profitable because the external conditions make operation more difficult.
[242]
Table 42.—Chief Copper Producers of Chile. Their Ownership and Relative Importance
| Controlled by capital of |
Company | Plant capacity, 1918 (pounds a year) |
Nature of product |
Source of product |
Remarks | ||
|---|---|---|---|---|---|---|---|
| United States | Chile Copper Co. | 120,000,000 | Refined copper | Mines of the Company | Capacity will be increased | ||
| Do | Braden Copper Co. | 72,000,000 | Blister copper | Do | Do | ||
| Do | A. S. & R. Co. | 24,000,000 | Blister copper and matte | Local owned mines | Caldera plant | ||
| Do | A. S. & R. Co., custom smelters | 24,000,000 | Do | Carrizal plant | |||
| Total United States | 240,000,000 | ||||||
| United States properties’ reserves | 150 years | ||||||
| England | Central Chile Co. | 6,000,000 | Matte | Panulcillo mine ¹⁄₃ | Balance custom ore | ||
| Do | Poderosa mine | 2,000,000 | Ore (rich) | Shipped to U. S. | |||
| Do | Lota custom smelter | 8,000,000 | Blister | Largely custom ore | Treat custom matte | ||
| Total British | 16,000,000 | ||||||
| British properties’ reserves | 3 years | ||||||
| France | Chanaral | 15,000,000 | Blister and matte | Mines of the company | - | Product treated at Balbach plant (U. S.) in 1917 | |
| Do | Naltagua | 10,000,000 | Blister | Mines of the company | |||
| Total French | 25,000,000 | ||||||
| French properties’ reserves | 5 years | ||||||
| Belgium | Catemo | 11,000,000 | Blister | Mines of the company | Refined by Balbach, 1917. | ||
| Reserves | 5 years | ||||||
| Germany | Gatico smelter | 8,000,000 | Blister | Custom ore and mines controlled. | Tributary to rich mining district. | ||
| Guayacan smelter | 3,000,000 | Matte | Formerly shipped matte to England. | ||||
| Chile | 6 mines with smelters | 5,000,000 | Matte | Locally owned mines | Shipped to big smelters or exported. | ||
| Great numbers of mines | large | Ore | Locally owned mines | Shipped to big smelters or exported. | |||
| Grand total | 308,000,000 | ||||||
| Expected output 1918-1919 | 244,000,000 (110,000 metric tons) |
||||||
[243]
Table 43.—Output, Reserves, and Life of Three American-owned Copper Mines in Chile
| Company | Output, First six months of 1918, (pounds) |
Ore reserves (tons) |
Life at present output, (years) |
|---|---|---|---|
| Chile | 50,000,000 | 350,000,000 | 200 |
| Braden Copper Co. | 36,000,000 | 150,000,000 | 125 |
| Andes Copper Co. | non-producing (capacity 24,000,000) |
50,000,000 | Work suspended for present |
The Caldera and Carrizal custom smelters of the American Smelting & Refining Co. treat ores shipped from various smaller mines. The Carrizal plant has been closed. Two chief properties are tributary to this plant,—the Carrizal Alto and the Astilla mines. A large number of properties are tributary to the Caldera plant, among them: Dulcinea, Flamenco, Morado, San Juan, El Gallo, etc. Throughout northern Chile there are a great many small copper mines. From the Braden property east of Valparaiso to the Chili Copper Co., southeast of Iquique, the entire country seems to be unusually rich in copper.
There are five important Chilean mines controlled by French or British capital. See Table 42. All are vein mines with no considerable tonnage of developed ore reserves, and the combined output is nearly 50,000,000 pounds of copper a year (a considerable part being from custom ore shipped by small mines) in the form of blister or matte. This production is normally shipped to France or England.
The American Metal Co. and L. Vogelstein, representing German capital, are believed to control, through selling and refining, much of the output of the French, British and Belgian mines. These concerns also own custom smelters in Chile and probably have interests in many mining properties there. The big business in Chilean ores shipped into the United States is done by these concerns, although the American Smelting, Refining & Mining Co. is also an important factor. These ores come from many small mines, although there are a few important ore shippers, one of which is British owned. Most of the mines are ostensibly Chilean owned, but much of the financing, marketing of products, etc., is done by American houses with German affiliations.
It seems that Chile may soon become the second largest copper producer of the world. American capital has led in the development of the Chilean deposits, as American interests have discovered and furnished funds for the equipment of the two leading producers, and the third most important ore body is American owned. This is the more remarkable in light of the fact that American capital has so far been conspicuous by its absence in the development of the other important industries of[244] Chile. Were it not for the present difficulties of building up plant facilities, due to shipping shortage, high cost of equipment, etc., the American-owned copper mines would today be still larger producers than they are. However, American capital now completely controls about seven-eighths of the total output. Substantially all the copper produced in Chile is shipped to the United States. The Chili Copper Co. produces in Chile a refined electrolytic copper, and this is the only finished refined copper produced in South America. Braden can produce a grade of copper that does not compete with electrolytic but is refined enough to go directly to the consumer.
Peru is one of the important copper-producing countries of the world. The chief mines are at extreme altitudes, however, and their character and location is such as to indicate that production will probably remain stationary or at least not show any important increases in the next few years. There are two important districts: Cerro de Pasco (elevation, 14,300 feet) and Morococha (elevation 13,700 feet).
The chief mines of these districts are now controlled by American capital and may be classed with group 2 of Table 38. The Morococha district was formerly English controlled, but the majority interests have lately been acquired by the Cerro de Pasco Copper Co. (American). The ores in both districts are exceedingly rich and the properties are well fortified with reserves. Developed reserves are adequate to insure four years’ production, and everything indicates that the mines are working extensive and persistent ore deposits. Blister copper is shipped to the American Smelting & Refining Co. in New York for treatment and marketing. Both mines have fully equipped plants, including smelters.
At the plant of Backus & Johnson Co., Morococha, some custom ore and matte from locally owned mines is treated. Among these mines are those of J. Galliver, producing 600 tons matte a year. Backus & Johnson also have a smelter and mines in the Casapalca district. The Sayapullo Syndicate (English) is working the Sayapullo mine under option from the Peruvian owners. Some of this matte is shipped to the Casapalca smelter, and the rest is exported to the United States. The output so far is small; at present 800 tons per year.
Certain small companies ship ores to the United States: from these the copper content amounted to about 4,000,000 pounds in 1917. This goes (1919) chiefly to the American Metal Co. and L. Vogelstein for refining and selling. This ore comes from Salaverry and Trujillo (Casapalca district), Mollendo (southern district, including Ferrobamba, see below), and Callao. All the blister copper is shipped from Callao except small amounts shipped by Backus & Johnson from the Casapalca district. An American company has developed a large body of low-grade ore at[245] Ferrobamba (Cotobamba Province), southern Peru. The property is inaccessible and no work is now being done.
The following résumé gives some of the salient facts concerning the Peruvian copper producers:
Table 44.—Production of Copper in Peru in 1918
| Output | Developed Reserves | Product | Control | ||
|---|---|---|---|---|---|
| Cerro de Pasco | 72,000,000 | (-) | 4 years | Blister Copper | United States |
| Backus & Johnson | 28,000,000 | (-) | 4 years | Do | Do |
| Huaron (new) | 5,000,000 | (?) | 4 years | Do | French |
| Ore shippers | 5,000,000 | ? | Ore | Local | |
| 110,000,000 | |||||
There is one important copper producing locality in Bolivia—Coro Coro, central Bolivia (elevation 12,000 feet). Beds of sandstone carry native copper and veins of sulphides. The mines are owned by French capital. There is no smelter, but concentrates running up to 85 per cent. copper are exported to France for treatment. Production is about 12,000,000 pounds annually. Reserves are large, but the almost inaccessible location of the mines has retarded development. Recently, there has been more activity, a flotation concentrator having been installed.
Some of the Bolivian tin and silver mines produce small amounts of copper, but outside of Coro Coro total copper production is insignificant.
The Aroa Mines, an English syndicate, owns a copper mine and smelter producing low-grade matte. The property is located along the railroad which terminates at the port of Tucacas. In 1917 the output was 3,500,000 pounds. The product was shipped to the United States and refined and sold there by L. Vogelstein & Co.
There are some copper prospects in the extreme western portion of the country, which are really extensions from the Chilean copper-producing areas. One company, Famatina, Ltd., is an English concern which has had an unfortunate career. The company operates the only copper smelter in Argentina, a small affair that mines irregularly. At last accounts a few hundred tons of blister copper was the annual output;[246] this has always been shipped to England, but recently attempts have been made to ship to New York. This blister is exceedingly rich in silver (6 per cent. silver).
Africa is a copper producer of growing importance, almost entirely because of the development of one group of deposits (Katanga), which overshadows all others to such an extent that the African situation is almost completely described by a discussion of the properties of the Tanganyika Concession, Ltd. The mines of this company yield now three-quarters of the copper production of Africa, and their importance in the future will be still greater.
The copper production of Africa comes from south of the Equator and from six general districts, as follows:
Table 45.—Copper Production of Africa
(Production, pounds of fine copper)
| District | Province and chief mines |
1917 output | 1918-1919, estimated output |
Control | Reserves, years of life at this rate of output |
||
|---|---|---|---|---|---|---|---|
| 1. | Congo: Katanga[121] | 60,000,000 | 80,000,000 | English-Belgian | 100 | ||
| Bwan M’Kubwa | 4,000,000 | ? | 3,000,000 | English | large | ||
| 2. | Transvaal: Messina | 14,000,000 | 12,000,000 | English | 1¹⁄₂ | ||
| 3. | Rhodesia: Falcon | 7,000,000 | 7,000,000 | English | 4 | ||
| 4. | Cape Colony: Cape Copper-Namaqua | 7,000,000 | 6,000,000 | English | 2 | ||
| 5. | Former German Southwest-Africa: Tsumeb (Otavi) | 10,000,000 | ?[122] | 15,000,000 | ? | (English since conquest of German Southwest Africa) | 4 |
| Khan, etc. | 2,000,000 | 4,000,000 | ? | 4 | |||
| 6. | Miscellaneous northern Africa not specified | 1,000,000 | ? | 1,000,000 | ? | French | ? |
| Total | 105,000,000 | 128,000,000 | Almost entirely English | ||||
[121] Kanshanshi included in Katanga.
[122] Basis 1914. Present political conditions govern extent of work. Shipped 1,000,000 pounds copper in ore to United States in 1917.
In District 1, the Katanga district, which includes the Belgian Congo and adjacent territory, the Union Minière du Haut Katanga has[247] acquired from the Belgian Special Committee the ownership of all of the Katanga copper belt but not the Kanshanshi deposit in Rhodesia. Equal, but not the entire, share interests of this company are owned by the Tanganyika Concessions, Ltd., and the Katanga (Belgian) Special Committee. The Lubumbashi smelter of U. M. du Haut Katanga, at Elisabethville, close to the Rhodesian boundary, has seven blast furnaces with a daily capacity of 2,000 tons. The ore treated runs 15 per cent. copper, and yields 96 per cent. blister copper. The past production is as follows: 1911, 996 tons blister copper; 1912, 2,492 tons; 1913, 7,407 tons; 1914, 10,722 tons; 1915, 14,190 tons; 1916, 22,165 tons; 1917, 30,000 tons. This copper is shipped to England, and is consumed there and in France after further refining. There is very little gold and silver in the copper, so it goes to market largely as refined, best selected and tough copper.
Tanganyika Concessions, Ltd., owns concessions in northern Rhodesia containing the Kanshanshi mine (a deposit similar to those in Katanga) owned by a subsidiary railroad company, 70 per cent. of which is owned by Tanganyika Concessions, Ltd. A small blast furnace there is producing. The actual railroad connection with Katanga is north from Rhodesia and thence to the eastern coast of Africa. An uncompleted line from the West Coast is planned to connect these mineral deposits with Lobito Bay (Benguella By. Co.). The Portuguese government has a small interest in certain railway lines in its territory. It seems that the English group, (R. Williams, who was an associate of Cecil Rhodes, T. White and others) with the Belgian Special Committee, control the entire group; but a considerable interest has been sold to the public, mainly British and Belgian investors.
The Bwan M’Kubwa mine farther south on the Rhodesia railroad is a deposit of the same general character. This is owned by British capital, two of the Rhodesian development companies holding a large block of the stock.
Ore bodies are found in Katanga over a district extending 250 miles east-west and 50 miles north-south, and also at scattered localities in Rhodesia. Malachite chiefly and other oxidized ores impregnate certain sediments and constitute the ore bodies, which are very rich (10 to 15 per cent. copper). At Luushia sulphides (3 per cent. copper as chalcopyrite) occur beneath the oxidized ore. Cobalt is common; there is some nickel but not much gold and silver. The developed reserves of the mines of Katanga are estimated at 40,000,000 tons of 8 per cent. copper ore above water level, equal to 100 years’ production at the present rate of output; of the Bwan M’Kubwa mine in Rhodesia at 3,000,000 tons of 4 per cent. ore, besides ore of lesser grade. The reserves of the Kanshanshi mine, in Rhodesia, are not included in the estimates.
In District 2, near the Rhodesian boundary, is the Messina mine, the[248] only copper producer of the Transvaal. Along veins in very old gneissic rocks occur shoots and lenses of chalcocite and enargite with some oxide. A little matte is made at the mine but the chief product is concentrates (45 to 50 per cent. copper), all of which were shipped to England. Lately they have been in part sent to the United States. The production was about 14,000,000 pounds in 1915 and the same in 1916. Reserves of 208,000 tons of 5 per cent. ore are reported developed, or not much over one year’s supply. The company is strictly English.
In District 3, the Falcon mines in Central Rhodesia belong to an English company which is treating copper-gold ore occurring in old schists. The production was about 7,000,000 pounds in 1916. The ore carries 2 per cent. copper, $5 in gold, and the reserves are stated at 862,000 tons of this grade, or about four years’ supply.
In the French Congo, just north of the mouth of Congo River, is a belt of copper deposits 60 miles long, which have been worked for centuries by the natives. Diabase rocks occur in this vicinity. The output is very small.
District 4, Cape Colony, is the oldest important copper producer of modern Africa, but its chief deposits, worked since 1852, are nearly exhausted. The Cape Copper Co. and Namaqua Copper Co. are south of the Orange River in a district 90 miles by rail from a port on the West Coast. The ore is bornite and chalcopyrite, in irregular lenses; the reserves are equivalent to only two years of production. Each mine has a mill and smelter. Further refining is done at the Briton Ferry smelter of the Cape Copper Co. in Wales, which has a capacity of 12,000 tons per year of refined copper and electrolytic copper. All these companies, including the railroad, are entirely British. Cape Colony copper production is dependent on this one district, from which the 1917 output was 6,000,000 to 7,000,000 pounds.
In District 5, the former colony of German Southwest Africa, copper deposits are numerous, and next to diamond mining the production of copper is the most important industry of the province. The main deposit is the Tsumeb in the northeast part of the country, where solid sulphides of lead and copper occur in dolomite. Fifty thousand tons of ore carrying 13 per cent. copper and 40 per cent. lead was shipped in 1917. There is a smelter here and the mine has rail connection. The company working these deposits was the Otavi Minen und Eisenbahn, but through the Southwest Africa Co. the English had an interest in the property. The English now have control. The Khan mine, second in importance to Tsumeb, is working a pegmatite vein in schist. In 1914 a mill treating 50 tons a day was shipping 60 to 70 per cent. concentrates to Europe. The ores are chalcopyrite and chalcocite.
In District 6, northern Africa, there are some deposits of copper in Algeria, Egypt, and Morocco. They are not of importance at present.[249] De Launay describes them as chiefly veins in rocks of much younger age than the important deposits of Africa which lie south of the Equator.
Matte and blister produced in Africa are normally treated at small English plants or at the Briton Ferry smelter, Wales, which is a plant of some size.
The general economic situation as regards African copper ores in 1917 is shown in the table.
Table 46.—Production and Shipments of Copper from Africa in 1917
| Mine | Production, (pounds) |
Chief product shipped | Shipped to— |
|---|---|---|---|
| Katanga | 60,000,000 | matte and blister | England |
| Cape Copper-Namaqua | 7,000,000 | matte | England |
| Messina | 14,000,000 | concentrates, matte, hand-picked ore | England |
| Falcon | 7,000,000 | concentrates or ore | England |
| Bwan M’Kubwa | 4,000,000 | Katanga plants | |
| German Southwest Africa, Otavi, Khan | 12,000,000 | ore | England or Germany |
| North Africa | 1,000,000 | ore | France |
| 105,000,000 |
Considerable of this copper goes to the United States. In 1917, as shown below, the total amount of copper received in this country was 7.5 per cent. of the total African production.
Table 47.—Shipments to the United States in 1917
| Grade Shipped | Shipments (tons) |
Copper content (pounds) |
|
|---|---|---|---|
| Otavi to New York | Ore (12 per cent.) | 3,900 | 1,048,000 |
| Messina to New York | Ore (45 per cent.) | 703 | 715,000 |
| Do | Matte (45 per cent.) | 306 | 310,000 |
| Cape Copper to New York | Concentrates (55 per cent.) | 375 | 460,000 |
| 5,284 | 2,533,000 | ||
| Katanga | Blister | ... | 5,437,000 |
| Total | 8,000,000 |
Copper production in Australasia is not now increasing. In recent years the production (metric tons) has been as follows:
[250]
| 1912 | 1913 | 1914 | 1915 | 1916 | 1917 |
|---|---|---|---|---|---|
| 16,600 | 22,900 | 37,592 | 32,512 | 35,000 | 38,100 |
Formerly German metal buying and refining companies controlled the Australian copper output (as that of lead and zinc) by virtue of refining and selling contracts. Hence the year 1914 brought disorganization to Australian mining. War-profits taxation on Australian mines has been severe; and government aid, formerly granted, has been largely withdrawn. Lack of labor, inability of new properties to obtain railroad connections, and absence of government encouragement to prospecting, have retarded the copper industry since August, 1914. Aside from former German control based on selling and refining contracts, all Australian copper mining is and has been under British control. The properties are owned by British and Australian capital and present refining and selling arrangements will prevent the German metal companies from again getting any foothold in the field. All former contracts with German agencies were abrogated. The government took this phase of the matter in hand, and one step was to purchase the entire production of Australia for the first half of 1918 at £106 to £108 per ton.
The Australian copper situation in 1917 is exhibited in the table following:
Table 48.—Production of Copper and Refining Plants in Australia in 1917
| Chief mines | Production, 1917, (long tons) |
Refineries | |
|---|---|---|---|
| Queensland: | |||
| Mount Morgan | 8,000 | [123] | Port Kembla plant, Electrolyte Refining & Smelting Co., Ltd. |
| Hampden Cloncurry | 8,000 | [123] | Capacity, 29,000 tons electrolyte copper per annum; 12,000 tons fire-refined copper per annum. |
| Other Cloncurry district mines | 4,000 | [123] | Mount Elliott completed refinery at Bowen, Queensland, in 1917; capacity 10,000 tons refined copper. |
| South Australia: | |||
| Wallaroo & Moonta | 7,000 | Wallaroo & Moonta smelter, Bowen, Queensland; capacity increased from 7,000 to 10,000 tons per annum. | |
| Tasmania: | |||
| Mount Lyell | 5,000 | Ships to Port Kembla. | |
| New South Wales: | |||
| Great Cobar | 2,500 | [123] | |
| C. S. A. and Mt. Hope, etc. | 2,250 | ||
| West Australia and Papua: | |||
| New pyrite ore bodies of promise opened | 1,250 | ||
| 38,000 | |||
[123] Ores mined are primary, consisting of chalcopyrite and chalcocite. Secondarily enriched ores near the surface have been entirely mined out.
[251]
As stated, the ownership of these mines is strictly British, and at present the refining and marketing is now British instead of German. Two refineries, both recently enlarged and improved, now have a capacity of over 60,000 tons a year, which is well in excess of the present output of the country. Towards the end of 1917, the Copper Producers’ Association Proprietary, Ltd., was formed in Australia for the purpose of selling and shipping copper on a co-operative basis. All copper producers were invited to join. Wallaroo & Moonta, Mount Morgan, Mount Lyell and the Cloncurry mines are members of this association, as is the Electrolytic Refining & Smelting Co., which treats the ores of these mines. The company markets its products in England and Australia. Its chief brands are E. S. A. (electrolytic) and E. S.A.F.R. (fire refined). These brands are also sold by Elder Smith & Co., Ltd., of London and Australia, which has had a financial interest in the Wallaroo & Moonta Co. since the early days, and has financed and marketed the copper production of this company since 1915.
Metals Products, Ltd., has recently been formed, with works at Port Kembla producing copper wire, brass goods, etc. Importation of copper and brass products into Australia should entirely disappear if the plans of the Australian government and copper producers do not miscarry.
The oldest mine and one of the two leading mines in Australasia is the Wallaroo & Moonta, in South Australia. This deposit occurs in a pre-Cambrian complex, intersected by pegmatitic dikes. Ore reserves are believed to be ample: say five years’ supply of developed ore. In recent years, production has been very steady at about 7,000 tons per year of refined copper. This is the only important copper deposit of pre-Cambrian age in Australia.
The other leading mine in Australia is Mount Morgan, in Queensland, which until 1910 was one of the largest gold mines in the world. The gold was in the oxidized top of a large low-grade copper deposit, which is now being mined from open cuts. Primary chalcocite, chalcopyrite and pyrite with quartz constitutes the ore deposit. The ore runs 2¹⁄₂ per cent. copper and is concentrated up to 7 per cent. The reserves exceed 4,000,000 tons of 2¹⁄₂ per cent. copper, or 10 years’ supply.
The Cloncurry district is a large area, 200 by 40 miles in extent, containing several copper deposits, which occur in schist and are mainly small bonanzas. The future of this district seems very promising. The chief mines are Hampden Cloncurry, Mount Elliott, Mount Cuthbert; and the developed ore reserves insure five years’ production. The Hampden Cloncurry mine has 300,000 tons of 7 per cent. ore in reserve, a smelter producing blister copper, and railroad connections. The district is hampered by bad climate, etc., and especially by labor conditions. Recent government rulings (1918), as to very short hours of labor and[252] higher wages, are reducing outputs. The Mount Elliott mine, which has a 300,000-ton deposit of 10 per cent. ore, is now (1919) idle because of labor conditions. The Mount Cuthbert mine, which completed a new smelter in 1917, has reserves of 150,000 tons of 6¹⁄₂ per cent. copper, and railroad connections.
The Mount Lyell mine, in Tasmania, is an old and steady producer. One class of ore runs 0.5 per cent. of copper and 1.25 ounces silver; the other contains 6 per cent. copper ore, with the same amount of silver. This is a disseminated deposit in schist, near a conglomerate contact. No igneous rocks are known in the vicinity. The reserves are 2,000,000 tons of low-grade ore and 1,000,000 tons of high grade; insuring the present output for 15 years. Considerable of the ore is mined open cut, and this is one of the lowest grade profitable mines in the world. Ores are smelted direct after being mixed, so that the product going to the furnace runs 2¹⁄₂ per cent. copper.
The Great Cobar mine, in New South Wales, works cupriferous pyrite carrying 2 to 2¹⁄₂ per cent. copper. Cobar used to produce 4,000 to 5,000 tons of copper a year. After erecting a 1,200-ton smelter in 1912, the company was placed in the hands of a receiver, April, 1914. The reserves are believed to be large. The other chief mines in New South Wales (the C. S. A., Mount Hope, Nymagee, etc.) produce together about as much as Great Cobar. These mines have smelters, and for the most part were formerly of much greater importance than they are today. Several have no railway connections, and this is an important handicap to New South Wales mines, which once produced twice the present output.
In West Australia, there have been developed recently a number of promising deposits of pyritic copper ores. There are no important producers, there being no railways.
The present conditions in Australia, in which labor conditions are most important, and the absence of new railroad construction, have seriously affected the development of the copper industry, but the future seems promising. Several important deposits have proved large and the ore bodies persistent. Much of the country has not been explored and many good showings could be worked and some probably developed into profitable mines if conditions were favorable to new ventures. Just the reverse is the case, however. The Mount Lyell company has been exploring northern Tasmania, and recently found in the mine of the Tasmania Copper Co. about 1,000,000 tons of ore carrying copper, zinc, gold and silver and worth $20 to $30 per ton, gross.
It is believed that Australia will continue to be an important copper producer; probably its importance will increase. Weighted reserves, in terms of ore supply to maintain present output, insure copper production continuing undiminished for 7.2 years. These reserves are well distributed among the various producers.
[253]
Copper deposits are found over a large part of central Japan. The ores, which occur in Tertiary volcanics, consist of chalcopyrite and pyrite running 2¹⁄₂ to 3¹⁄₂ per cent. copper, and are commonly concentrated before smelting. The gangue is usually quartzose. Lenticular deposits of cupriferous pyrite in Paleozoic schists and sediments occur on the west and the south side of Japan. These mines yield smelting ore carrying about 3¹⁄₂ to 4 per cent., but contain very little silica. Pyritic smelting is extensively practiced. Over one-half the copper production comes from four chief mines: Ashio and Kosaka of the Tertiary type; and Hitachi and Beshi of the Paleozoic schist type.
The state reserves to itself the right of original ownership in all ores, including copper. The right to work them is granted to individuals or companies of Japanese nationality. Copper mining, smelting and refining companies seem to be entirely Japanese in ownership and policy. The number of mines is considerable, but their ownership is concentrated into a few hands and the smelting and refining industry is still more concentrated. Japanese producers sell their own copper, all foreign selling agencies being strictly Japanese. The mines in Japan are not generally worked as joint-stock enterprises, but are mostly family properties inherited by the present owners. A table showing the 1917 copper production of Japan indicates these facts. (See Table 49.)
Because of labor conditions, abundant fuel near the mines and water transportation, Japanese copper production has increased rapidly in recent years. High prices and the adoption of modern methods of mining and smelting have been important contributing factors. There seems no reason to expect that Japan’s production will decrease, but not enough is known of geological conditions to enable one to discuss the future outlook. The only mine whose reserves are known is the Beshi, which has reserves adequate for 100 years of production at the present rate, which is 10 per cent. of the Japanese output. The reserves at other mines are not developed far ahead, but must insure several years of continued production at the present rate.
The production and exports of Japanese copper in recent years are as follows (in terms of metric tons):
| Year | 1912 | 1913 | 1914 | 1915 | 1916 | 1917 |
|---|---|---|---|---|---|---|
| Production | 63,893 | 67,697 | 71,046 | 76,039 | 101,467 | 124,306 |
| Exports | 30,000 | 42,000 | 45,500 | 59,500 | 62,000 | 72,000 |
[254]
Table 49.—Japanese Copper Production
Producing companies and brands of copper marketed. The companies named have selling offices in London and in other
foreign consuming centers.
| Company and chief mines owned | Smelters | Electrolytic refineries | ||
|---|---|---|---|---|
| Location | 1917 production (pounds) of refined and casting[124] and brands sold | Location | 1917 estimated capacity (pounds) and brands sold | |
| Mitsu Bishi & Co. | Osaka | 15,000,000 | Osaka | 15,000,000 |
| Osaruzawa | ||||
| Arakawa | ||||
| Ikuni and 6 smaller mines | ||||
| Brands sold | Arakawa, Mitsui Bishi, casting, etc. (rough copper) | M. B. (Mitsu Bishi) | ||
| Furukawa & Co. | Osaka (Amagasaki) | 17,000,000 | Osaka (Nikko Plant) | 67,000,000 |
| Ashio | ||||
| also Ani, Furukura, Kune and 6 smaller mines | ||||
| Brands sold | Furukawa, Ani Tiles, refined Maragata Best Selected | F. M. (Furukawa Mines) | ||
| Fujita & Co. | Inushimo | 3,000,000 | Mosaka | 20,000,000 |
| Kosaka (North end Honshu) | ||||
| Brands sold | Obiye casting, etc. (rough copper) | Kosaka | ||
| Kuhara Mining Co. | None | Hitach | 100,000,000 | |
| Hitachi (near Tokio) | Reported capacity at least 20% in excess of 1917 output. | |||
| 3 smaller mines 30 per cent. custom ore | ||||
| Brands sold | H. M. (Hiatachi Mine) | |||
| Baron Sumitomo | Shikoku Island | 22,000,000 | ||
| Beshi | Beshi Best Selected | |||
| Brands sold | ||||
| Nippon Metals Co. of Kobe | Moji | 19,000,000 | ||
| Brands sold | S. Z. K. (Susuki) and some casting | |||
| Denkibundo Co. | Bundo | 13,000,000 | ||
| Total casting rough copper and best selected sold[255] | 57,000,000 | |||
| Total electrolytic refining capacity[125] | 235,000,000 | |||
| 1917 output of electrolytic estimated | 217,000,000 | |||
| Total | 214,000,000 or 124,306 metric tons Eng. & Mining Journal figure 1917 output. | |||
[124] Balance smelted to cathodes and treated electrolytically.
[125] Plant capacity. Evidently not reached in 1917.
Since the high copper prices of 1916 there was a heavy importation of Chinese copper coins into Japan. In 1917 one concern alone had contracted for 200,000 tons of such coins, which contain about 85 per cent. copper, and the rate of importation at that time would mean 60,000 tons refined copper a year from this secondary source. This development has enabled Japan to make heavy exports of copper.
The collapse of Russia removed one of Japan’s big copper markets. Japan will probably not be able profitably to produce a large exportable surplus of copper unless the price obtained is fairly high compared to quotations ruling in 1912 to 1914. Under normal conditions Japan will supply her own needs for copper with little or nothing to spare.
The Seoul Mining Co. (Collbran and Bostwick) is producing from contact deposits, one 50 miles and the other 100 miles from a harbor. This American concern is a dominant trading and banking house in Korea and is working mines formerly operated by Koreans. The copper ores are sulphides between limestone and granite. Five hundred tons of 60 per cent. concentrates were shipped from Chosen to the United States in 1917.
The present production of China is 2,000 tons a year, and the chief deposits are in Yunnan Province. A great many localities are reported to show copper ores, mainly cupriferous pyrite in very old schists, or in Permian basalt. The latter deposits are too small for modern methods. In the last hundred years, lack of wood to make charcoal has restricted output to a nominal amount. Small seams of native copper are highly esteemed by the natives. The ores are carefully hand-picked, and the small-scale methods are wasteful.
The Tungschuanfu mine, Yunnan, the chief mine in China, has a yearly output of about 1,000 tons of copper. The district has been worked for hundreds of and probably is very rich. The ores, replacements in limestone[256] and veins in shale, are 8 per cent. copper and the reserves are large. The Yaoki Kansu government smelter is a modern plant with a capacity of four tons of copper a day.
There are several other mines, all controlled by the government. Outside engineers have reported favorably on some of them, but the government closely regulates copper mining in China because it affects currency and government profits on coinage of copper. There are also some copper mines worked with the contact iron deposits of China (formed by contact action of diorite); and deposits of malachite in Triassic sandstone are worked at several points.
India is not a copper producer. The Rakha Hills mine of the Cape Copper Co. (see Cape Colony) has 400,000 tons of 4 per cent. copper ore developed and a smelter is being built. India is an enormous copper consumer, and it is surprising she has never been a producer.
Political and commercial control of much of the copper production of Europe is obviously uncertain in the extreme, as are any figures of production for the Central Powers and Russia. (See Table 49.)
Spain is the oldest and steadiest producer of copper in the world. The chief deposits are controlled by English capital and their developed reserves insure present production for 30 to 60 years. The copper is largely refined to finished form in England and enters the market there under various brands. The Rio Tinto is an enormous deposit worked by open pits.
[257]
Table 50.—Production and Control of Copper in Europe
| Country and chief localities in which copper is produced | Output 1916-1917 (metric tons) |
Estimated output 1918-1919 (metric tons) |
Estimated output 1918-1919 (pounds) |
Control by: | Reserves of ore |
Remarks | |||
|---|---|---|---|---|---|---|---|---|---|
| Financial[126] | Political | Handling of sales |
|||||||
| Germany | |||||||||
| Mansfeld | 30,000 | 30,000 | ... | Now entirely German | Output in future should not be nearly as large. | ||||
| Mitterberg, etc. | 10,000 | 10,000 | ... | Do | |||||
| Austria-Hungary | 10,000 | 10,000 | ... | Do | |||||
| Serbia, Bor, etc. | 10,000 | 10,000 | ... | Do | |||||
| Turkey, Aghano, etc. | 10,000 | 10,000 | ... | Do | |||||
| Bulgaria and Roumania | 1,000 | 1,000 | ... | Do | |||||
| Total Central Powers (est.) | 71,000 | 71,000 | ... | ||||||
| Spain and Portugal[127] | 42,000 | 42,000 | ... | No statistics since 1914. | |||||
| Mason & Barry | ... | ... | 5,000,000 | English | Portuguese | English | 32 years’ supply | ||
| Rio Tinto | ... | ... | 62,000,000 | Do | Spanish | Do | Very large | 60 years’ reserves known. | |
| Tharsis | ... | ... | 12,000,000 | Do | Do | Do | Do | ||
| Misc. (United Alkali, Huelva, Cordoba, etc.) | ... | ... | 11,000,000 | Do | Do | Do | Do | ||
| Miscellaneous (Calva, Los Guardos, etc.) | ... | ... | 3,000,000 | Spanish | Do | Do | Do | ||
| Russia (See Table 52) | 18,500 | 18,000 | ? | ... | ? | ? | ? | 1914 output 36,340 tons. | |
| Norway[128] (Sulitolma 40 per cent.) | 19,000 | 19,000 | ... | ¹⁄₂ English and ¹⁄₂ Norwegian | Norwegian | Important | Like Spanish deposits. | ||
| Sweden | 1,000 | 1,000 | ... | Swedish | Swedish | Swedish | |||
| Other countries | |||||||||
| Italy | 3,000 | 3,000 | ... | Italian | Italian | Italian | Some English capital. | ||
| France | 1,000 | 1,000 | ... | French | French | French | French pyrite mines. | ||
| England | 250 | 250 | ... | English | English | English | Tin mines. | ||
[126] All companies have foreign stockholders, but dominant nationality is as indicated.
[127] Export copper to France and England in form of blister, alloys, cement, pyrite ore and copper ore.
[128] Export copper to England and Sweden chiefly in form of cupriferous pyrite.
Note. Production for 1919 in Germany probably smaller than estimate.
[258]
The copper deposits, chiefly massive cupriferous pyrite, in Norway and Sweden, are similar to those of Spain geologically as well as economically. Their commercial value depends not only on their copper content, but on the sulphur and iron recovered. In many cases the sulphur used in sulphuric acid manufacture is of greater money value than the recovered copper. Hence the exported copper from Spain, Portugal and Norway is in several forms: pyrite, matte, ingot copper from Norway and considerable cement copper or precipitates from Spain and Portugal. Pyrite is exported to England, the United States, and perhaps a little to France; the matte, ingot, etc., to various European countries. Sweden imports as well as produces pyrite. France, Italy and Russia produce considerable pyrite and before the war France exported pyrite. Under normal conditions, the copper in all this pyrite is shipped back and forth over Europe and can hardly be traced. The table shows the location of raw materials but not the place where marketable copper is produced. The chief imports and exports of pyrite are normally as follows:
Table 51.—Normal Exports and Imports of Pyrite for Certain European Countries
| Exports from— | Amount (tons) |
Imports to— | Amount (tons) |
|---|---|---|---|
| Spain | 1,500,000 | England | 1,050,000 |
| Norway | 500,000 | Sweden | 100,000 |
| Portugal | 250,000 | United States | 1,000,000 |
| ... | France | 100,000 | |
| Estimated total | 2,250,000 | 2,250,000 |
Under existing conditions the present output of Germany can not be closely estimated. The figures in Table 49 are guesses based on the information available. The Mansfeld deposits are clearly the most important, and as they are in shales that extend over a large area, the reserves must be considered large. The Mitterberg mine, owned by the Krupps, had a pre-war output of only 1,000 tons yearly. Several copper deposits in the Austrian Tyrol had a pre-war output of 1,000 tons annually.
The chief source of copper in Germany during the war, however, must have been from conversion of articles containing copper which were in use before the war. It is doubtful if 10 per cent. of the yearly copper production of peace times was destroyed in use. Consequently, in all countries there is normally a big store of copper in the form of wire, brass, machine parts, etc. This is what Germany used during the war, and its replacement is essential to her industrial success in peace.
The copper production of Russia was rapidly increasing before the war and reached a maximum in 1913. The ownership of the mines and refineries was largely English but in part French. Enough development had been done to indicate that Russia will probably be a large producer of copper when consistent industrial progress is possible. In 1918 the mines had been seized by workmen and operations were nearly or entirely suspended. The copper districts are all in the Urals, the Caucasus or Siberia. The Russian copper production (in long tons) has been as follows:
| 1905 | 1910 | 1913 | 1914 | 1915 | 1916 | 1917 |
| 8,700 | 22,310 | 33,794 | 31,435 | 25,472 | 20,557 | 15,700 |
| (34,911) | (27,295) | |||||
[259]
Table 52.—Copper Production and Ore Reserves of Russia
(All figures long tons)
| District | Company | Original investments made by: |
1913 production |
1915 production, (estimated) |
Ore reserves (developed) |
Copper in ore reserves (per cent.) |
Remarks | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urals | Kyshtim | British | 7,000 | 7,600 | 3,150,000 | 2.75 | Obtains gold as by-product. | ||||||||||||||||||
| Do | Bogoslovsk | Do | 4,300 | 4,100 | ... | ... | Ore hard to follow; excellent possibilities. | ||||||||||||||||||
| Do | Sissert | Do | 1,100 | 1,500 | 5,000,000 | (2³⁄₄)-4 | |||||||||||||||||||
| Do | Tanalyk | Do | 600 | 600 | 126,000 | ... | Developing into a gold rather than a copper deposit. | ||||||||||||||||||
| Do | Verch-Isselz | Russian | 3,000 | 2,500 | large reserves | 2-3 | Massive pyrite ore. | ||||||||||||||||||
| Do | Nishni Tagilsh (Demidov) | Do | 2,000 | 1,500 | |||||||||||||||||||||
| Urals total | 18,000 | 17,800 | |||||||||||||||||||||||
| Caucasus | Caucasus Copper Co. | British | 5,500 | 3,000 | 3,600,000 | 3 | Mines at Khot Eli and Katar. | ||||||||||||||||||
| Do | Allah Verde | French | 2,000 | 700 | ... | ... | Industrial & Metallurgical Co. of Caucasus. | ||||||||||||||||||
| Do | Siemens Co. | Russian | 1,500 | 800 | ... | ... | Mines at Tcherek and Kedabeck. | ||||||||||||||||||
| Caucasus total | 9,000 | 4,500 | |||||||||||||||||||||||
| Siberia | Spassky | British | 4,756 | 3,450 | 543,000 | 10.7 | |||||||||||||||||||
| Do | Miscellaneous | Do | 734 | ... | ... | Development companies, see below. | |||||||||||||||||||
| Do | Irkysh (Ridder) | Do | (New) | ... | 2,300,000 | 5 | 1,000,000 low-grade ore also in reserves. | ||||||||||||||||||
| Do | Russian Mining Co. | Do | 410 | ... | 500,000 | 2.6 | Ore carries other metals. | ||||||||||||||||||
| Do | Alexeieff Co. | Do | 377 | ... | |||||||||||||||||||||
| Do | Russo-Asiatic Corporation | Do | |||||||||||||||||||||||
| Siberia total | 5,553 | 4,184 | |||||||||||||||||||||||
| Russian chemical works | 1,358 | 811 | Ouchkoff, Kreiners, etc. | ||||||||||||||||||||||
| Grand total Russia | 34,911 | 27,295 | |||||||||||||||||||||||
|
|||||||||||||||||||||||||
[260]
Russia has always been in large part dependent on foreign copper and there was a tariff premium on domestic production. It is likely that ultimately Russia may more nearly be self-supporting as regards copper requirements, even if consumption increases greatly. Table 52 shows the important developed properties, their production in 1913 (maximum) and in 1915 (estimated), their developed ore reserves, and the nationality of original capital that made the developments possible.
Copper, the red metal, is surpassed only by gold and silver in malleability and by silver alone in electrical conductivity. Next to iron it is industrially the most important of all metals, as its value per pound is much greater than that of lead or zinc, and the world requires and consumes much greater quantities of copper, lead, and zinc than of any other non-ferrous metals.
The uses of copper are many, but the electrical industry is the largest consumer. Brass, bronze, and other copper alloys are second in importance. A considerable quantity of copper sheets, tubes and other wares are used outside of the electrical industry. Copper is used in coinage, and in China it is the money standard of the working population.
The United States, producing a major part of the world’s copper, has also been responsible for financing and developing more successful copper mines abroad than any other country. Success has been facilitated by the presence in the Western Hemisphere of the world’s chief copper deposits and also by the advances in mining, milling and metallurgy that have been in great measure the work of United States engineers. England and Japan control considerable copper production, but it is small compared to that controlled by the United States.
In the consumption of copper Germany is a big factor. Because of this fact, German interests and international metal houses have in the past secured a considerable control over copper supplies through refining and selling contracts with mining companies. Such control is based entirely on refining and selling companies and does not extend to ownership of producing mines, and only to a small degree to ownership of smelters.
Chile, Mexico, Canada, and the Belgian Congo should become of increasing importance as copper producers not only because of known reserves but, in the case of Canada and Mexico particularly, because of the likelihood of new and important discoveries. The position of the United States, including Alaska, should be maintained as at present, neither gaining nor losing as compared to the rest of the world.
[261]
Lead is used in the form of the metal, of alloys with other metals, and of various chemical compounds. As metal its chief uses are as pipe for water and corrosive solutions; for protective covering of electrical cables; as sheet lead for lining chambers for the manufacture of sulphuric acid and vats for chemical manufacturing processes. In smelting, lead is used as a collector of other metals, particularly of gold and silver, from which it is later separated, now most generally by the use of zinc by the Parkes process of desilverization.
Lead alloys readily with nearly all other metals in all proportions. Its alloys of industrial importance comprise type metal, bearing or babbitt metals, shot, solders, casting metals, some brasses, and the fusible alloys used for the protection of electrical apparatus and in automatic sprinklers for the protection of buildings against fire. Type metal, originally composed of 83 per cent. lead and 17 per cent. antimony, now often contains bismuth and sometimes a little copper and iron. An alloy of 9 parts lead, 2 antimony and 2 bismuth is used for stereotype plates. Less than 2 per cent. of arsenic is added to lead used to make shot to increase the hardness and sphericity of the product. Antimony also imparts the hardness essential to shrapnel, etc. Bearing metals comprise alloys of lead and antimony or these with copper, tin, and zinc. Antimony imparts to lead the property of expansion on solidification, essential to type metal and casting materials generally. Lead makes a brass that is soft and machines easily. Solder is commonly an alloy of lead and tin. The melting point varies with the proportions of these constituents and others, sometimes added for special purposes. The cheapest solder in general use is 30 per cent. tin and 70 per cent. lead. Solders seldom contain more than 50 per cent. tin. The addition of bismuth, cadmium, or mercury lowers the melting below the boiling point of water. Fuses can thus be obtained which interrupt electric circuits at any desired temperature.
The largest uses of lead compounds are as pigments. White lead or basic carbonate, 2(PbCO3)Pb(OH)2, is the most extensively consumed, being used alone or mixed with zinc oxide and barytes. Red lead (Pb3O4) is used for painting structural steel, as a pipe-joint cement, and in the[262] manufacture of glass. Litharge, another oxide, is used in assaying as a flux, in rubber manufacture, and in making glass. The acetate, carbonate and other chemical compounds are used in medicine.
The relative amounts of lead consumed in the various uses in 1913 were: In pigment, comprising white lead, red lead, litharge, and orange mineral, 38.0 per cent.; in alloys such as type metal, bearing metals, and solders, 29.7 per cent.; in pipe, 15.2 per cent.; in shot, 10.4 per cent.; and in sheets, 6.7 per cent.
The most revolutionary advance in ore dressing of recent years has been the development of oil-flotation, electromagnetic, and electrostatic processes for the concentration of lead-zinc ores. These processes permit the elimination of the objectionable zinc content of many ores and render it an additional credit of great importance in the exploitation of low-grade complex ores.
The Murex process applied to the treatment of complex ores consists of coating the metallic sulphide minerals with oil and particles of magnetite and pyrite roasted to magnetic sulphide, then separating them from the gangue by an electromagnetic machine. The Lyster preferential flotation of galena depends on the presence of various salts in the water used. In the Broken Hill mines these salts are present in the mine waters. After removal of galena the blende may be preferentially floated by the Bradford copper salt or Bradford hyposulphite or sulphurous acid processes.
In the reduction of lead ores, there are improvements constantly being made. These are chiefly in the mechanical appliances, such as mechanical ore hearths and continuous roasting machinery, such as the Dwight-Lloyd, and in details of furnace construction and the handling of materials, rather than in processes or recognized principles. Various new processes have been proposed, most of which are intended to make available the ores now of too low grade, or the complex ores of lead and zinc whose separation is difficult or commercially impracticable. These processes are now the subject of experiment, with some indications that successful applications may be found. One process involves the volatilization by chloridizing roast of sulphide ores, the precipitation of the lead chloride fume by Cottrell electric precipitation, and smelting the fume with lime. At least 50 per cent. of the chlorine is recovered as calcium chloride, which can be substituted for salt in the further operations. With oxidized ores it is proposed to dissolve the lead by means of brine acidified with sulphuric acid and to precipitate metal sponge by electrolysis.
Gillies’s process consists in roasting the complex sulphides to a low sulphur content, mixing with carbonaceous matter, distilling in excess of air, and volatilizing the lead, zinc, bismuth, cadmium, arsenic, etc.,[263] as oxides and sulphates, the lead in form of sulphate, the zinc chiefly as oxide. The fume is caught and digested with a solution of ZnSO4 and free H2SO4 from the electrolytic vats; PbSO4 remains, which can be used as pigment or smelted. The ZnSO4 solution is electrolyzed, in the presence of gum arabic in the electrolyte, rendering the zinc deposit more dense.
The Ganlin process, reported successful on Burma zinc-lead middlings, consists in feeding the dry pulverized ore into a molten bath of Zn and NaCl in equal parts. Zinc replaces lead and silver, which are dissolved as chlorides, ZnS being precipitated. When this reaction is complete the lead and silver are precipitated as metals by granulated spelter added to the amount of 35 per cent. of their weight, the dissolved spelter forming ZnCl2. The silver-bearing lead is tapped off, the residue granulated, the salts leached with water, and the zinc-bearing gangue freed from lead shot by tabling, leaving a zinc ore free from lead.
Electrolytic refining has been one of the greatest advances in the industry; it makes possible the preparation of pure lead from any source and the recovery of numerous by-product metals.
Zinc and lead are commonly associated in mineral deposits, sometimes intimately mixed, sometimes segregated enough so that one metal predominates, but seldom free entirely one from the other. The geological and geographical distribution of the two metals is, therefore, nearly identical. Galena is the most common and important of the lead minerals. Cerussite, anglesite and pyromorphite usually result from the oxidation of galena, the sulphate being usually an intermediate state in the oxidation to the carbonate. Pyromorphite and wulfenite are of minor importance. Jamesonite is more an ore of antimony than of lead. Sphalerite (zinc blende) weathers more readily than galena, and therefore zinc in many places is carried below water level more rapidly and completely than lead. For this reason some mines change from predominantly lead mines to zinc mines with greater depth. Apart from the effect of such secondary enrichment, this change is often encountered in primary ores with increase in depth.
Lead ores occur in deposits of several distinct genetic types. Many deposits lie at shallow depth in sedimentary rocks, without apparent connection with igneous rocks, occurring as tabular replacements of receptive beds. In regions of slightly disturbed strata the ore shoots tend to follow pitching troughs. Ores of this type usually contain lead (galena), zinc (sphalerite), and iron (pyrite) minerals; many contain manganese and cadmium; some contain cobalt and nickel; but few carry gold, silver, copper, or antimony. Deposits of this type are of world-wide distribution, and many are extensive and commercially important. The greater purity of the ore and the simplicity of treatment (particularly[264] for the ores in the oxidized zones), caused them to be exploited first and most extensively, and to dominate formerly the world production of lead. To this type belong, with many others, the deposits of the Mississippi Valley and Silesia, that together yielded 15 per cent. of the world’s production in 1913.
Other important deposits are closely associated with igneous rocks, and are characterized by complex ores. They comprise vein deposits, disseminated replacements of igneous rocks, and silver-lead replacements in limestone.
The chief lead-ore deposits of the world are situated in the countries that are listed below in the order of their importance in 1913.
Recoverable Lead Content of the Lead Ores of the World Produced in 1913[129]
| Rank | Country | Short tons |
Metric tons |
Percentage of world’s total production |
|---|---|---|---|---|
| 1. | United States | 484,880 | 440,000 | 36.0 |
| 2. | Australia | 267,169 | 242,440 | 19.8 |
| 3. | Spain | 209,193 | 189,830 | 16.4 |
| 4. | Germany | 79,344 | 72,000 | 5.9 |
| 5. | Mexico | 68,343 | 62,000 | 5.1 |
| 6. | Tunis | 31,076 | 28,200 | 2.3 |
| 7. | Italy | 24,905 | 22,600 | 1.8 |
| 8. | Canada | 24,244 | 22,000 | 1.8 |
| 9. | Austria | 22,591 | 20,500 | 1.7 |
| 10. | Great Britain | 20,277 | 18,400 | 1.5 |
| 11. | Greece | 19,836 | 18,000 | 1.5 |
| 12. | Turkey-in-Asia | 15,428 | 14,000 | 1.1 |
| 13. | China | 13,995 | 12,700 | 1.0 |
| 14. | German S. W. Africa | 13,224 | 12,000 | 1.0 |
| 15. | Algeria | 12,893 | 11,700 | 1.0 |
| 16. | France | 9,587 | 8,700 | 0.7 |
| 17. | India (Burma) | 6,502 | 5,900 | 0.5 |
| 18. | Peru | 4,331 | 3,930 | 0.3 |
| 19. | Japan | 4,143 | 3,760 | 0.3 |
| 20. | Egypt | 3,196 | 2,900 | 0.2 |
| 21. | Russia | 3,083 | 2,800 | 0.2 |
| 22. | Bulgaria | 2,204 | 2,000 | 0.2 |
| 23. | Sweden | 2,094 | 1,900 | 0.2 |
| 24. | Hungary | 1,256 | 1,140 | 0.1 |
| 25. | Bolivia | 1,102 | 1,000 | 0.1 |
| 26. | Portugal | 661 | 600 | |
| 27. | Rhodesia | 361 | 330 | |
| Total | 1,345,918 | 1,221,390 | 100.0 |
[129] Adapted from compilations by Adolph Knopf, of the U. S. Geological Survey.
[265]
The four districts now of pre-eminent importance are, in order, Broken Hill in New South Wales, Australia; southeastern Spain; southeastern Missouri and Coeur d’Alene, in Idaho: which are credited respectively with about 19, 16, 12 and 10 per cent. of the world’s production in 1913.
—The chief producing regions and their percentage of the domestic lead production in 1915 are as follows:
| Region | Percentage of total domestic production |
|---|---|
| Southeastern Missouri | 33 |
| Coeur d’Alene, Idaho | 27 |
| Utah | 18 |
| Joplin (in Mo., Kans., Ark., and Okla.) | 6 |
| Colorado | 5 |
As regards the types of ores and the character of the lead produced, there are two metallographic provinces: the Mississippi Valley, including southeastern Missouri and Joplin, and the minor district of Wisconsin, producing as soft lead 39 to 45 per cent. of the total domestic production; and the Western province, in which the ores are complex, carrying precious metals and often antimony and copper. All of the output from the Western province, but only a part of the soft lead, is desilverized.
Ninety per cent. of the ore mined in southeastern Missouri comes from St. Francois and Madison counties. The ore deposits contain predominantly galena, and are disseminated in Cambrian limestone over large areas at depths of 100 to 550 feet. Copper, nickel, and cobalt occur in the Madison County ores, and copper concentrates are separated and shipped by nearly all the companies in the region. The principal operating companies, with the names of companies absorbed by them or now subsidiaries, shown in parenthesis, are: St. Joseph Lead Co. (Doe Run Lead Co.), Federal Lead Co., National Lead Co. (St. Louis Smelting & Refining Co.), Desloge Consolidated Lead Co., Baker Lead Co. (St. Francois Lead Co.), Boston Elvins Lead Co., Missouri Metals Co. (Mine La Motte Co.), and Missouri Cobalt Co. (North American Lead Co.). The St. Joseph Lead Co. is normally the second largest lead-producing company in the United States. Its holdings have a conservatively estimated life of 20 years, at a rate of production of 2,000,000 tons of ore, or 80,000 short tons of lead, per annum. In 1917 this company mined 2,485,431 tons of ore, nearly half the total output of the region. The Federal Lead Co., a subsidiary of the American Smelting & Refining Co., is the next largest producing company in the region. In 1915 it mined and milled 1,355,000 tons of ore. The National Lead Co., through its subsidiary, the St. Louis Smelting & Refining Co., works three mines near Flat River and has a concentration plant with a daily capacity of 2,400 tons. Its smelter at Collinsville treats its own concentrates, as well as those of the Baker Lead Co. and the Boston Elvins Lead Co. The Desloge Consolidated[266] Lead Co. operates three mines and a mill of 1,700 tons’ daily capacity; its ores are smelted by the Federal Lead Co. The Missouri Metals Co. operates the Mine La Motte and a mill treating 700,000 tons annually at the 1917 rate. In 1915 it was estimated that this mine could produce 3,000,000 tons of ore annually for sixty years.
In the Joplin region, which is chiefly in Missouri but also includes adjacent areas in Kansas, Arkansas, and Oklahoma, the ores lie at three horizons in horizontal limestone and chert beds of Lower Carboniferous age. At the upper horizon, usually 100 to 150 feet below the surface, the ore occurs in clayey chert breccias. The ore bodies are characteristically “runs” up to 300 feet wide, and continuous in one horizon for several hundred feet and, rarely, for more than a mile. The middle horizon, or “sheet ground,” at a depth of 150 to 300 feet usually ranges from 6 to 15 feet in thickness. The ore, mixed galena and blende, cements brecciated chert. The third and lowest horizon, in sandy limestones, contains disseminated ores mainly, and as yet is little exploited. In 1915, about 4,000,000 tons of ore was mined from the upper horizon, and 6,500,000 tons from the middle horizon or “sheet ground.” The average lead content of the ore as mined was about 0.25 per cent. Most of the lead concentrates are sold in open market. The Webb City district is the most important in the Joplin region, and the American Zinc, Lead & Smelting Co. is the largest galena producer. Most of the output is by lessees and small operators. The Joplin district ranks second in importance. The A. W. C. Mining Co. is the largest miner of “sheet ground.” The Ravenswood and Ritz mines of the United States Smelting, Refining & Mining Co., in Jasper County, produce 218,000 tons of ore annually. The concentrates of this region are chiefly smelted by the plants of the Eagle-Picher Lead Co. at Galena, Kansas; Joplin, Missouri; Webb City, Missouri; and by the Granby Mining & Smelting Co. at Granby, Missouri.
The Coeur d’Alene region is in Shoshone County, Idaho. The deposits are metasomatic veins formed by replacement of siliceous sedimentary rocks along zones of fissuring, and carry mainly galena and siderite with some pyrite and sphalerite. In 1915 the crude ore shipments amounted to 95,169 tons with a lead content of 35,271 short tons. The remainder of the ore, or nearly 96 per cent. of the total, is concentrated to carry about 50 per cent. lead. The 1915 yield of concentrates of all kinds amounted to 329,530 tons, having a lead content of 128,928 short tons, making the total lead content of crude and concentrate shipments 164,199 short tons. The mining companies form three groups, determined chiefly by their relations or affiliations with the smelters, as follows: The Bunker Hill group, comprising the Bunker Hill & Sullivan Mining & Concentrating Co., and the Hecla Mining Co.; the Day group, comprising the Tamarack & Custer Mining Co., the Amazon-Manhattan[267] mine, and the Hercules Mining Co.; and the American Smelting & Refining group, comprising the Federal Mining & Smelting Co. and various small producers.
The mines of the Bunker Hill & Sullivan Mining & Concentrating Co. had reserves on December 31, 1917, of 3,457,634 tons. The ore bodies are replacements of quartzite. This company’s production in 1917 was 493,030 tons of ore, the metallic lead recovered by smelting being 46,996 tons. The Bunker Hill & Sullivan Co., while still shipping its own ores to the Helena plant of the American Smelting & Refining Co., built its own smelter at Kellogg, Idaho, where it smelts the Hecla and other ores. The Day family controls the Hercules, and Tamarack & Custer companies, the Northport smelter at Northport, Washington (now closed down), and the Pennsylvania Smelting & Refining Co. at Pittsburgh, Pa. The 1916 shipments of the Hercules had a lead content of about 22,000 tons. Both companies are close corporations and make public little information as to their operations. The Tamarack & Custer probably has large reserves of ore averaging about 9 per cent. lead; it has produced as much as 3,000 tons of shipping ore and concentrates per month. The Federal Mining & Smelting Co. operates several mines. One-sixth of the stock is owned by the American Smelters Securities Co. and all its silver-lead ores and concentrates are contracted to the American Smelting & Refining Co. The Success Mining Co., working two mines, has been an important producer. The Interstate-Callahan has been chiefly a zinc producer but ships some lead concentrates to the Salida plant of the Ohio & Colorado Smelting Co.
The lead production of Utah is chiefly from the Park City, Bingham Canyon, and Tintic districts. The ores, composed of galena, tetrahedrite and pyrite, and in places sphalerite, with their oxidized derivatives, occur in lodes cutting limestones, sandstones and shales, chiefly of Carboniferous age, and also as bedded deposits in limestone. Both types are frequently associated with porphyry and form irregular ore bodies in contact-metamorphosed limestone. In many mines copper is an important constituent of the ores and the silver content is always important.
The production of Colorado in 1917 comprised 33,995 short tons of lead, of which 9,293 short tons came from the Leadville district in Lake County, 10,412 short tons from the San Juan district in San Juan, San Miguel and Ouray counties, and 6,816 tons from the Aspen district in Pitkin County. The Leadville deposits are in the Mosquito range. The chief producing companies are the Iron Silver Mining Co., the Yak Mining, Milling & Tunnel Co. and its subsidiary, the Leadville Exploration & Mining Co.; the Western Mining Co.; the Downtown Mines Co.; the Ibex Mining Co.; and the United States Smelting, Refining & Mining Co. The Yak Mining, Milling & Tunnel Co., and the Western Mining Co. are subsidiaries of the American Smelting & Refining Co. In the[268] San Juan district, the principal producers are the Liberty Bell; Smuggler-Union; Tomboy; Black Bear; Iowa-Gold Tiger; Dives; Shenandoah; and Silver Lake mines. The veins penetrate all the clastic and igneous rocks of this region, and the ores are exceedingly complex. In Pitkin County, the Smuggler Leasing Co. operates most of the producing mines at Aspen. The ores are peculiarly free from other metals than lead, antimony, and silver.
—The lead resources of the Commonwealth of Australia are chiefly in New South Wales, Western Australia, Tasmania, and Queensland. New South Wales has been the chief producer in the past, but the Tasmanian deposits are now being rapidly developed and equipped for production.
The most important source of ore in New South Wales is the great Broken Hill lode, situated in the arid Barrier Ranges at an elevation of about 1,000 feet above sea level. The lode ranges in width from a few inches to 400 feet and has been worked over a distance of three miles. Mining began in 1884 and now is conducted by several mining companies which, in the order of the importance of their production and ore reserves, are: Broken Hill South Silver Mining Co.; Broken Hill North Mining Co.; Zinc Corporation; Sulphide Corporation; British Broken Hill Proprietary Co.; Broken Hill Proprietary Co.; Broken Hill Proprietary Co., Block 10; and Broken Hill Proprietary Co., Block 14.
Although the deepest workings are 1,815 feet deep, the ore still continues downward. For many years the estimated ore reserves of all the mines have approximated 12,000,000 tons. The upper part of the lode consisted of a gossan 20 to 100 feet wide of siliceous and manganiferous limonite, hematite, and kaolin. Below the gossan were great masses of cerussite, anglesite, cuprite, and malachite, with abundant cerargyrite, embolite, and iodyrite. Between the oxidized and primary sulphide ores was a thin zone of secondary sulphides. The early operations in the district were conducted for the purpose of obtaining lead ores, and immense dumps were accumulated of zinc-bearing ores sorted out or zinc-bearing tailings left after concentration of the lead ores. In 1903 these dumps were estimated at 5,687,400 tons, carrying 18.6 per cent. zinc. With the development of a demand for zinc sulphide ores and of oil-flotation methods of separation and concentration, these dumps have been important sources of zinc. There are two classes of sulphide ores, distinguished as silicate gangue ore, and calcite gangue ore. The sulphide ores are a close mixture of galena and zinc blende, carrying silver. The silicate gangue ore bodies carry rhodonite, garnet, and quartz; and are richer in zinc and silver than those with calcite gangue.
The Broken Hill South Silver Mining Co. has ore reserves estimated at 3,350,000 tons and is the largest ore producer in the field. Broken Hill North, Broken Hill South, Amalgamated Zinc (De Bavay), Zinc[269] Corporation, and Barrier South, Ltd., are controlled by the Hoover-Govett-Bailliau group of British and Australian capitalists.
The Amalgamated Zinc Co. in 1913 treated 498,289 tons of tailings containing 17.1 per cent. zinc, 3.7 per cent. lead, and 4.4 ounces silver, obtaining 140,098 tons zinc concentrates carrying zinc, 48.9 per cent., lead 5.9 per cent., and silver 8.5 ounces per ton. The Zinc Corporation, a company formed by Bewick, Moreing & Co., has ore reserves estimated at 1,504,211 tons, averaging 14.8 per cent. lead, 9.2 per cent. zinc, and 2.5 ounces of silver per ton.
The largest lead-producing district of Tasmania is on the West Coast, where the largest producers, the Hercules of the Dundas group and the Primrose and Tasmanian copper mines of the Rosebery company group, are now controlled by the Mount Read-Rosebery Co., affiliated with the Mount Lyell Mining & Railway Co., Ltd. The deposits contain complex sulphide ores, the reserves being estimated by the state geological staff at 1,272,500 tons, averaging 29.79 per cent. zinc, 8.89 per cent. lead, 12.16 ounces of silver, and 0.17 ounces gold per ton. This estimate has since been revised and made more conservative.
In 1913 Western Australia produced 26,589 long tons of lead ore and 125 tons of silver-lead ore, almost wholly from the Northampton district on the West Coast. The only company working on a large scale is the Fremantle Trading & Smelting Co., operating the Baddera and Narra Tarra mines and, formerly, a smelter at Fremantle. The Chillagoe district is the largest producer in Queensland, its output amounting to 2,550 long tons of pig lead in 1913, chiefly from the Girofla mine of the Mungana company, but in part from lead-copper concentrates. The Chillagoe operated a small smelter. The total pig lead production of Queensland was 3,603 long tons in 1913.
—Spain yielded in 1913, 314,369 short tons of lead concentrates, from which were smelted 189,559 tons of pig lead. In 1915 only 1,010 short tons of ore or concentrates was exported, and 161,912 short tons of desilverized lead was exported, mostly to England. Over 90 per cent. of the production of ore came from the provinces of Jaen, Murcia, Cordoba, and Ciudad Real. In 1913 the Province of Almeria occupied fourth place, but its mines are now nearly exhausted. These provinces are in the southeastern part of Spain and cover the Sierra Morena and Sierra Nevada mountain ranges.
In the Province of Jaen are two principal districts—the Linares-Santa-Elena and the La Carolina. Many years ago Linares was the greatest lead-producing district in the world. The veins cut granite and thin overlying sandstone and are very narrow. The Arrayanes, a state-owned mine, has been exploited over a length of two and a half miles and to 1,500 feet in depth. The gangue is granite, quartz and calcite. Iron and copper pyrites and sphalerite are present, but a 79 per cent.[270] lead concentrate is easily made. The deepest mine is 1,800 ft. deep and is still in rich ore. In the Santa Elena vicinity, the San Fernando, Ojo Vecino, and Santa Ana mines are owned locally. The Caridad is owned by French capital and the Santa Susanna by a Belgian concern, the Compagnie Real Asturienne des Mines. In La Carolina district the nearly vertical lodes cut Silurian quartzites and Cambrian and Silurian slates. The ore attains a greater width than in the veins of the Linares district. The Nuevo Centenillo mine (English owned) produces 27,000 short tons of concentrates annually. The great Guindo lode runs through six mines, two of which are owned by Spanish companies, and three by the Guindo Co., a German-Spanish corporation having an output of 27,000 short tons of concentrates yearly. The Castillo La Vieja, owned by a French company, has a yearly output of 20,000 tons of marketable ore.
In the Province of Murcia the Mazarron and Cartagena districts are important. Most of the veins are nearly vertical but many have spurs or branches forming lenticular and bedded deposits in the sedimentaries. This district extends southwestward along the coast from Cabo de Palos a few miles north of Cartagena. The production of this province has been steadily decreasing.
In the Province of Cordoba (district of Posadas) many silver-lead-zinc mines were worked by the Romans and are still profitable. Near Alcaracejos are the mines of Anglo-Vask and Penarroya, the latter owned by a French company of the same name.
The development of the lead ores in the Province of Ciudad Real has been retarded by lack of transportation facilities. The best known district is that of El Hoyo-San Lorenzo, which in 1915 had risen to fourth place among the lead-producing districts of Spain.
—In imperial Germany the lead-producing districts in the order of their importance were as follows: Upper Silesia, Rhenish Prussia, Westphalia, Saxony, Hanover, and Nassau. Rhenish Prussia and Westphalia are usually grouped together as one metallographic province. At Gladbach, east of Cologne in Rhenish Prussia, are ore deposits lying in troughs and basins in limestone. The ore is smithsonite and galena mixed with shale. The chief deposits of Westphalia are at Iserlohn and Brilon. At Iserlohn ores containing calamine, galena, and blende are found in irregular pockets. The deposits of Brilon are similar, but most of the ore is found in crevices in the limestone. Rhenish Prussia and Westphalia are the source of about one-third the German production of lead.
The greater part of Upper Silesia lay within the boundaries of Germany in 1914, although formerly part of the kingdom of Poland, the population being still predominantly Polish; but portions were included in the old empires of Russia and Austria. The pre-war production of[271] lead ores from Russian Poland was entirely from this metallographic province. The deposits, which contain lead and zinc together, lie in Triassic beds that overlie Carboniferous rocks carrying important seams of coal. This juxtaposition of ore and fuel furnish an ideal basis for the great smelting industry that developed locally, for the conditions permit smelting of low-grade ores.
The historic mines at Freiberg, in Saxony (Erzgebirge) yield a small quantity of blende in connection with the concentration of galena ores from a remarkable series of intersecting veins, which number more than 900, although few are more than 2 feet thick. They have been worked to a depth of 2,100 feet. More than 10 per cent. of the lead production of Germany is derived from Saxony. These mines are owned and operated by the Saxon government, which also owns the smelting plants.
Lead predominates over zinc in the ores of the Upper Harz, in Hanover. These ores occur in veins and zones in slates of Devonian and Lower Carboniferous age. These mines are worked by the Prussian Department of Mines, which also operates two smelting plants, the output being about 10 per cent. of the total German output. In Nassau, in the valley of the Lahn, lead ores are produced as a by-product, with zinc blende concentrates.
—In Mexico lead ores are mined in several states, the more important being Chihuahua, Durango, Coahuila, Nuevo Leon, Sonora, San Luis Potosi, and Zacatecas. In many districts during a considerable part of the past eight years work has been intermittent and occasionally suspended for long periods.
The Santa Eulalia district in Chihuahua is largely owned by American companies, including the American Smelting & Refining Co., operating the Mina Vieja, Sin Nombre, Velardeña, San Antonio and Santo Domingo mines; and El Potosi Mining Co., operating the mines of the same name. The San Toy is under lease to the American Metal Co., now purged of German interests. The Santa Eulalia Mining Co. belongs to the Hearst estate. The Buena Tierra Mining Co. is a British concern. The mines of the Santa Barbara and Parral districts are also largely under American control, among many others being the Montezuma Lead Mining Co. of the R. S. Towne interests, Granadeña Mining Co., American Smelters Securities Co. and American Zinc Extraction Co. The American Smelters Securities Co. operates the Tecolotes, Montezuma, San Diego, Guadalupe and Alfarena mines. The San Francisco mines are owned by British capital. In the San Isidro district the Calera, Prieta and Buena Vista mines are operated by the American Smelting & Refining Co. The Lago mine is operated by C. M. de Las Plomosas (French). In the Parado district are mines of the Compañia Minera Aurora y Anexas, controlled by the Madero family (Mexican). Other Chihuahua mines[272] of the American Smelting & Refining Co. are Orizaba and La Union at Magistral, the Jibosa at Dolores, La Luz and Parcionera at Cordera, the Veta Grande and Veta Colorado.
The largest operators in the state of Durango are the American Smelters Securities Co. at Velardeña and the Cia. Minera de Peñoles at Mapimi, both now American since the selling of the German-held stock of the American Metals Co. by the Alien Property Custodian.
In Sonora, the Carnegie Lead & Zinc Co. worked a mine near Cananea, during the war, but the best part of the deposit is now depleted.
The Tiro General mines, in San Luis Potosi, belong to the American Smelting & Refining Co.
The Cabrilla and Paloma mines, in the Cabrillas district in Coahuila, are owned by the Compañia Minera de Peñoles, controlled by the American Metal Co. The Sierra Mojada district is dominated by American companies, the principal mines being owned by the Consolidated Kansas City Smelting & Refining Co., a subsidiary of the American Smelting & Refining Co. The Boquillas de Carmen mine has been acquired by an American company.
In the state of Nueva Leon, deposits lying within a radius of 50 miles of Monterrey, at Villadama, Vallecillo, Ladera Occidental de Minas Viejas, etc., have been exploited by German and American companies, including the Compañia Metalurgica Mexicana (American), Joplin-Mexican Mining Co. (American), and the Metallgesellschaft (German).
—The output of lead ore in Tunis in 1913, almost wholly by French companies, was 56,072 metric tons. It is all exported.
Practically all the output of lead ore in Italy is derived from the Inglesias district of Sardinia, which in 1915 produced 40,829 metric tons of ore averaging 55 per cent. lead, out of a total national production of 41,590 metric tons. The principal operators are the Monteponi and Pertusola companies, the former Italian, the latter English. The remainder of the ore comes from the provinces of Bergamo, Brescia Cuneo, and Grosseto, and the operating companies are the English Crown Spelter Co. (English), and the Societa Austro-Belga and Société de la Vieille Montagne (Belgian).
In Czecho-Slovakia, the most important district is that of Przibram, in Bohemia. Rich lead ores were once mined at Mies, but the district is now exhausted. The district of Joachimsthal was for centuries an important producer.
In German-Austria are the silver-lead mining districts of Schneeberg, in Tyrol, and Raibl, in Upper Carinthia. In both districts the mines were before the war owned and operated by the Austrian state. Miess, in Carinthia, is one of the chief sources of ore in recent years.
The lead mines of Great Britain in 1916 produced 17,083 tons of dressed lead ore. The largest operator is the Weardale Lead Co., operating[273] the Boltsburn and Stanhopeburn mines and smelting its own and some custom ores.
In Greece the only important lead deposit is that of Laurium, which was worked on a large scale in ancient times. It is now controlled and operated by a French company, the Compagnie Française des Mines de Laurium.
The lead production of Canada is chiefly from British Columbia, the most important producers being in the Slocan district. The largest operator is the Consolidated Mining & Smelting Co. of Canada, Ltd., proprietor also of the Trail smelter. This company operates the Sullivan and other mines and produced during the year ended September 30, 1917, 29,542 tons of lead ore from the Sullivan mine, and 1,100 to 1,500 tons from several others. The Sullivan mine has been reported to have reserves of 3,500,000 tons of galena-sphalerite ore. Numerous smaller properties in the same district ship ore to the Trail smelter, which produced some 22,000 tons of lead during the year ended September 30, 1917.
The most important lead-silver mines of Asiatic Turkey are those at Hodsha Gernish (Balia), belonging to the Société des Mines de Balia-Kara-Aidin (French), which yield about 12,000 tons of lead annually. There is a state-owned mine at Bulgardagh producing lead, gold, and silver. The English company, Asia Minor Mining Co., produces about 3,000 tons of ore annually.
In China the ten lead mines in the Province of Hunan are controlled by Chinese. The Wah Chang Mining & Smelting Co., Ltd., operates the Tien For Tai mines. The Shui-Ko-Shan mine, controlled by the Hunan Mining Board, from a deposit in limestone, produced in 1913, 51,561 net tons of ore, which yielded 3,762 tons of lead concentrates and 12,275 tons of zinc concentrates. Since 1913 the production has been increased, but the possibilities of the deposit are limited. The Japanese have endeavored to secure control of this mine, but without success. The pig-lead output of China is chiefly consumed in the country. The only modern lead smelter is at Changsha and is owned by Japanese.
The lead production of Southwest Africa (formerly German) has been derived chiefly from the Tsumeb deposit in the Grootfontein district in the Otavi Mountains. The ores exported in the fiscal year 1913-14 amounted to 48,000 long tons, averaging 13 per cent. copper, 25 per cent. lead, and 7.7 ounces silver. The ore is a coarsely crystalline aggregate of argentiferous galena and chalcocite with minor amounts of other minerals. The Otavi Mines & Railway Co. owns and operates this mine, the ore having been exported in 1913-14 to the United States for smelting.
In 1913 all the lead-ore production of Algeria was from the Department of Constantine, and amounted to 21,442 tons. Practically the whole production was by French companies.
[274]
Lead ore is produced in several scattered districts in France, chiefly in the south. Among the mines are the Chaliac et Chassezac (Ardeche) mines of the Société Metallurgique et Minière des Cevennes, producing 2,200 metric tons in 1913; the mines of the Société Civile des Mines des Malines; La Londe mine of the Société des Mines des Bormettes; that of the Société des Mines de Bleymard, producing 2,470 tons of galena ore in 1913; and the Pierrefitte (Haute Pyrenees), Peybrune, and Bulard de Sentein-Saint Lary (Ariege) mines. All of these appear to be French companies, except the Pierrefitte, which is English controlled.
In Burma, the chief deposits are those of the Bawdwin mines, in the Northern Shan States (Burma), now connected with the Burma Railway from Rangoon. The ore bodies of present interest are nearly vertical shoots in a feldspathic grit (rhyolitic tuff or silicified rhyolite) and rhyolite series. The Chinaman and the smaller Shan ore body are believed to have been one, though now separated by faulting. Estimated reserves on December 31, 1917, were 4,033,000 tons of lead-zinc ore assaying 24.7 ounces of silver, 27.4 per cent. lead, 19.1 per cent. zinc, and 0.4 per cent. copper; and 105,000 tons of copper-silver ore assaying 21.0 ounces of silver, 19.9 per cent. lead, 8.8 per cent. zinc, and 8.9 per cent. copper. Since then development has considerably increased these reserves. In addition there is estimated to be 1,600,000 tons of low-grade ore averaging 5.1 ounces of silver, 7.5 per cent. lead, 4.8 per cent. zinc, and 0.2 per cent. copper per ton, with excellent prospects of larger developments. A large tonnage of gossan outcrop ore containing 4 or 5 ounces silver, 4 to 5 per cent. lead, and a little zinc is cheaply mined and available as siliceous flux. The essential constituents of the ores are galena and sphalerite with a little pyrite and chalcopyrite. All of the ore is argentiferous.
The lead and zinc concentrates are available for the customary methods of smelting. A zinc-distilling and sulphuric acid plant is being constructed at Sakchi, with the aid of the Indian government, to treat the table zinc concentrates. Its initial capacity of 25,000 tons of concentrates is expected to be increased to 75,000 tons. The company operates a lead smelter at Nam-Tu, 11 miles from the mines, using a mixture of ore and ancient slags. Treatment of the middlings by the Ganlin process is proposed and a 100-ton unit is under construction at Avonmouth, England. The Bawdwin deposits may be expected to be an important factor in the world’s production of lead in the immediate future. They are owned by the Burma Mines, Ltd., an English corporation representing the R. Tilden Smith-Govett-Hoover interests and some American capital.
The lead production of Peru is largely in the form of ancient high-lead slags from the Cerro de Pasco district, Department of Junin, shipped to[275] plants of the American Smelting & Refining Co. in the United States. Lead occurs as a minor constituent of copper deposits.
The domestic lead-ore deposits of Japan are all owned and operated by Japanese. During the war Australian concentrates and Chinese ore were imported and smelted, 10,666 short tons of the former, carrying 56 per cent. lead, during the fiscal year 1915-16, and 9,829 short tons of the latter during the year 1916. The Fujita company, mining in Japan, Korea, and Formosa, produces 382 short tons of lead yearly. Its principal mine is the Kosaka, at the northern end of Hondo, the main island of Japan. The ore is a complex sulphide mixture of lead, zinc, iron, and copper minerals. The annual output of this mine is about 335 short tons of lead. The Mitsui Mining Co., Ltd., is the largest producer, its output in 1915 having been 3,561, and in 1916, 8,098 short tons of pig lead. This is derived wholly from the Kamioka zinc-lead mine, in the Province of Hida, on a contact metamorphic deposit in limestone lenses enclosed in Archean gneiss near a quartz porphyry contact.
The only deposit exploited in Egypt is that known to the ancients, Gebel Rosas, now operated by the French company, Compagnie Française des Mines de Laurium. As regards the former empire of Russia, the lead production of Russian Poland and the Caucasus Mountains is small, the output in 1913 coming chiefly from the Caucasus Mountains and being made by the Elboruss Co., and the Compagnie d’Alagir (Belgian).
In the Altai Mountains, in Siberia, a zinc-lead-silver deposit, the Zmeinogorsk, formerly belonging to the Russian Mining Corporation (British), is now owned by a Russian company, Altai Mines, Ltd., though part of the capital is probably British. The Nerchinski district, in eastern Siberia, comprises many known deposits. The Akatonevski, Kadaenski, Algachinski and Klichinski deposits are veins, whereas many of the Zerentniski, Gasimoura Valley, Koultoumski and Maltzevski are lenticular masses of disseminated ore. In the Kadaenski deposits two massive disseminated ore bodies occur in dolomite between two veins. These deposits were controlled by the Imperial Cabinet, and their exploitation was being seriously considered by British and American capital shortly before the revolution. The deposits of largest present importance in Siberia are those of the Ridder Mining Co., controlled by the Irtysh Corporation of London, which has developed two mines, the Ridder and the Sokolni, on the same mineralized zone. The deposits are replacements by complex sulphide ore of members of a conformable series of slates, tuffs, and igneous sills. In 1916 the reserves were estimated at 945,000 tons of a grade of 31.2 per cent. zinc, 18.1 per cent. lead, 1.5 per cent. copper, 9.7 ounces of silver, and 0.47 ounces of gold; and also 2,229,000 tons averaging 6.7 per cent. zinc, 3.5 per cent. lead, 0.5 per cent. copper, 1.7 ounces of silver, and 0.7 ounces of gold per ton. Other known mineralized zones have not been developed, but the possibilities of the[276] property are immense, being limited chiefly by general political and economic conditions.
A small quantity of lead is produced in Bulgaria from a few small deposits containing intimate mixtures of lead, copper and zinc minerals; usually either zinc or copper predominate.
The most important lead deposit in Sweden is the Sala, in Vestmanland, where irregular masses and veins of galena and blende with minor amounts of pyrite, etc. occur in limestone. Similar deposits occur at Lofas and Guldmedshyttan.
Hungary has no lead mines of importance, although galena occurs in some of the veins exploited, notably at Schemnitz, where the larger mines are the property of the Hungarian state.
Most of the ore from Bolivia exported to the United States comes from the Majo mine, which produced 884 tons, lead content, in 1912. This mine is in southern Bolivia in the region of La Quiaca.
The most important lead-producing area in Portugal is Merlota, near the Guadiana River, where silver-bearing galena and oxidized ores are found. Other districts are those of Villa Real, Vizen, Aveiro, Portalagre, and Beja. The deposits are similar to those of Spain.
The only deposit of importance in Rhodesia (South Africa) is the Rhodesia Broken Hill. The large ore bodies are, so far as developed, almost wholly oxidized. One ore body is estimated to contain 250,000 long tons of ore averaging 26 per cent. lead and 22.5 per cent. zinc; another ore body is estimated to contain 300,000 long tons, averaging 32 per cent. zinc, with little lead, but much iron oxide and carbonate. These ore bodies are controlled by British capital. Considerable difficulty has been experienced in developing a commercial treatment. Reports indicate this deposit is of greater magnitude than is generally recognized.
The most important lead deposit of Belgium, or rather of the neutral district of Moresnet adjoining Belgium, belonged to the Belgian company, Société de la Vieille Montagne at Moresnet. It was exhausted in 1882.
—No marked change in the pre-war rate of production of ores by the countries of Europe or northern Africa is anticipated when normal conditions are resumed. Most of the districts in those countries have been exploited a long time and have passed their zenith of production; many are approaching exhaustion. A possible exception to this statement is the Ciudad Real Province of Spain, which is being energetically developed by the Penarroya company. The division of Austria-Hungary will not materially affect the control of the lead industry.
The United States will continue to be the largest source of lead in the world, and the only change anticipated is a slight increase in the relative importance of the hard-lead output of the Western States.
[277]
The chief new factor in production will be the Bawdwin mines of Burma, which are being developed on a large scale for the production of 300,000 tons of ore annually.
Recent developments in the Altai Mountains of southwestern Siberia have proved immense bodies of complex zinc-lead ores. Their geographic isolation will prevent them from becoming an important factor in the world market for a long period, notwithstanding their large size and excellent grade. The extent of their exploitation will be determined largely by how far the Russian market is affected by internal social and political conditions. The loss of the Polish industrial region, with its market protected by stringent tariffs, materially restricts the extent of visible outlets.
In the future, but not soon, it is anticipated that there will be developments in the Andes region of South America of complex lead-ore deposits similar to those of the Western United States.
Political control of the lead resources of the world up to the outbreak of the war in 1914 seems to have been a minor factor in the industry, and to have made itself felt chiefly through imposition of protective tariffs or bonuses designed to stimulate domestic production, smelting, and refining. Such measures resulted in the establishment of lead smelting in British Columbia and were an inducement to the development of the Altai district of Siberia. As will be described later in detail, the growth of international commercial relations had permitted the establishment by German interests of organizations which, although not all-inclusive, gave effective control of the industry.
During the World War, however, political jurisdiction was largely invoked to restore control of national resources to citizens. This movement was particularly marked in the British Empire, where there now exists a joint political and commercial control. Alien interests have been eliminated by governmental action and the government retains a share in the control through its interest in marketing organizations or its financial participation in reduction works. In France, the consortium Société Minerais et Metaux, organized by government action, is the arbiter of the industry and comprises all the important French companies both at home and abroad. In the United States the Alien Property Custodian was active in eliminating all enemy-alien interests.
Copper, lead, and zinc form a class by themselves, with respect to industrial utility and tonnage produced, ranking after iron, which far outclasses them, as the most important base metals. The total yearly production of each throughout the world is considerably more than a million tons.
[278]
Table 53.—Ore Production, Smelting, and Consumption of Lead in 1913
(Tons of 2,000 Pounds)
| Country | Recoverable lead content of ores and concentrates |
Primary pig lead | Recoverable lead in ores and pig lead |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mine production |
Exports | Imports | Smelter production |
Imports | Exports | Consumption | Net imports |
Net exports |
||||||||||
| Net tons |
Per cent. [130] |
Net tons |
Per cent. [130] |
Net tons |
Per cent. [130] |
Net tons |
Per cent. [131] |
Net tons |
Per cent. [131] |
Net tons |
Per cent. [131] |
Net tons |
Per cent. |
Net tons |
Per cent. [132] |
Net tons |
Per cent. [132] |
|
| Algeria | 12,900 | 1.0 | 12,900 | 1.0 | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | 12,900 | 1.3 |
| Australia | [133]244,500 | 19.7 | 132,000 | 10.6 | ... | ... | 112,500 | 9.0 | ... | ... | 101,900 | 8.1 | 10,600 | 0.8 | ... | ... | 233,900 | 18.7 |
| Austria-Hungary | 23,800 | 1.9 | ... | ... | 2,800 | 0.2 | 26,600 | 2.1 | 12,500 | 1.0 | ... | ... | 39,100 | 3.0 | 15,300 | 1.2 | ... | ... |
| Belgium | ... | ... | ... | ... | 56,000 | 4.5 | 56,000 | 4.5 | ... | ... | 8,700 | 0.7 | 47,300 | 3.7 | 47,300 | 3.8 | ... | ... |
| Bolivia | 1,100 | 0.1 | 1,100 | 0.1 | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | 1,100 | 0.1 |
| Bulgaria | 2,200 | 0.2 | 2,200 | 0.2 | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | 2,200 | 0.2 |
| Canada | 18,800 | 1.5 | ... | ... | ... | ... | 18,800 | 1.5 | 6,400 | 0.5 | ... | ... | 25,200 | 2.0 | 6,400 | 0.5 | ... | ... |
| China | 14,000 | 1.1 | 14,000 | 1.1 | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | 14,000 | 1.1 |
| Egypt | 3,200 | 0.3 | 3,200 | 0.3 | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | 3,200 | 0.2 |
| France | 9,600 | 0.8 | ... | ... | 21,300 | 1.7 | 30,900 | 2.5 | 87,700 | 7.0 | ... | ... | 118,600 | 9.1 | 109,000 | 8.7 | ... | ... |
| German S. W. Africa | 13,200 | 1.0 | 13,200 | 1.0 | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | 13,200 | 1.0 |
| Germany | 79,300 | 6.4 | ... | ... | 120,300 | 9.7 | 199,600 | 15.9 | 46,800 | 3.7 | ... | ... | 246,400 | 19.1 | 167,100 | 13.4 | ... | ... |
| Great Britain | 20,300 | 1.6 | ... | ... | 13,300 | 1.1 | 33,600 | 2.7 | 177,400 | 14.1 | ... | ... | 211,000 | 16.4 | 190,700 | 15.2 | ... | ... |
| Greece | 20,300 | 1.6 | ... | ... | ... | ... | 20,300 | 1.6 | ... | ... | 20,300 | 1.6 | ... | ... | ... | ... | 20,300 | 1.6 |
| Holland | ... | ... | ... | ... | ... | ... | ... | ... | 10,500 | 0.8 | ... | ... | 10,500 | 0.8 | 10,500 | 0.1 | ... | ... |
| India (Burma) | 6,500 | 0.5 | [134]2,200 | 0.2 | ... | ... | 4,300 | 0.3 | ... | ... | 4,300 | 0.3 | ... | ... | ... | ... | 6,500 | 0.5 |
| Italy | 24,900 | 2.0 | [134]1,000 | 0.1 | ... | ... | 23,900 | 1.9 | 12,000 | 1.0 | ... | ... | 35,900 | 2.8 | 11,000 | 0.1 | ... | ... |
| Japan | 4,200 | 0.3 | ... | ... | ... | ... | 4,200 | 0.3 | 17,000 | 1.3 | ... | ... | 20,400 | 1.6 | 17,000 | 1.3 | ... | ... |
| Mexico | 68,300 | 5.5 | 7,100 | 0.6 | ... | ... | 61,200 | 4.9 | ... | ... | 61,200 | 4.9 | ... | ... | ... | ... | 68,300 | 5.5 |
| Norway | ... | ... | ... | ... | ... | ... | ... | ... | 500 | ... | ... | ... | 500 | ... | 500 | ... | ... | ... |
| Peru | 4,300 | 0.3 | 4,300 | 0.3 | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | 4,300 | 0.3 |
| Portugal | 600 | ... | 600 | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | 600 | ... |
| Rhodesia | 400 | ... | 400 | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | 400 | ... |
| Russia | 3,100 | 0.2 | [134]2,000 | 0.2 | ... | ... | 1,000 | 0.1 | 63,700 | 5.1 | ... | ... | 64,800 | 5.0 | 61,700 | 5.0 | ... | ... |
| Spain | 209,200 | 16.8 | ... | ... | 9,900 | 0.8 | 219,100 | 17.4 | ... | ... | 219,100 | 17.5 | ... | ... | ... | ... | 209,200 | 16.7 |
| Sweden | 2,100 | 0.2 | 500 | ... | ... | ... | 1,600 | 0.1 | 1,200 | 0.1 | ... | ... | 2,800 | 0.2 | 700 | ... | ... | ... |
| Switzerland | ... | ... | ... | ... | ... | ... | ... | ... | 6,400 | 0.5 | ... | ... | 6,400 | 0.5 | 6,400 | 0.5 | ... | ... |
| Tunis | 31,100 | 2.5 | 31,000 | 2.5 | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | 31,100 | 2.5 |
| Turkey-in-Asia | 15,300 | 1.2 | ... | ... | ... | ... | 15,300 | 1.2 | ... | ... | 15,300 | 1.2 | ... | ... | ... | ... | 15,300 | 1.2 |
| United States | 411,900 | 33.3 | ... | ... | 13,200 | 1.0 | 425,100 | 33.8 | ... | ... | 10,800 | 0.9 | 414,300 | 32.1 | 2,400 | 0.2 | ... | ... |
| Other countries | ... | ... | ... | ... | 2,600 | 0.2 | 2,600 | 0.2 | 34,100 | 2.7 | ... | ... | 36,700 | 2.8 | 36,700 | 2.9 | ... | ... |
| World[135] | 1,245,100 | 100.0 | 227,800 | 18.2 | 239,400 | 19.2 | 1,256,700 | 100.0 | 476,200 | 37.8 | 441,600 | 35.2 | 1,290,500 | 100.0 | 682,700 | 52.9 | 636,500 | 50.9 |
[130] Percentages of World production of recoverable lead content of ores.
[131] Percentages of World smelter production of pig lead.
[132] Percentage of mean of totals used in a and b.
[133] Total recoverable lead content of ore production reported, less lead content of Australian production in forms other than pig lead.
[134] Probably represents ore surplus rather than exports.
[135] Lack of proper balance in these totals shows error or incompleteness, or lack of comparability, due in part to lag in the statistics, which, however, show correctly the general situation.
[279]
The ownership or operative control of mines is a minor factor in the commercial control of the lead industry and in general is of importance only as determining the distribution of ore to smelters competing otherwise on nearly equal terms. Most of the large smelter interests engage in, or have subsidiaries engaged in, mining or have sufficient holdings in large mining companies to influence their smelting contracts. The actual basis of control of the industry is, therefore, often through combined ownership or control of mines and of reduction works.
The ownership or control of reduction plants has been the dominating factor in the commercial control of the industry. The production of each country in 1913, the principal lead reduction works of the world, and their affiliations and control are discussed below.
—The control of the lead industry of the United States through ownership or control is vested in ten groups as follows:
1. The Morgan-Guggenheim group, comprising the American Smelting & Refining Co.; American Smelters Securities Co.; and their subsidiaries: Selby Smelting & Lead Co.; Tacoma Smelting Co.; Garfield Smelting Co.; Consolidated Kansas City Smelting & Refining Co.; Federal Lead Co.; Federal Mining & Smelting Co.; Western Mining Co.; Silver Lake Mining Co.; and the Yak Mining, Milling & Tunnel Co.
2. The St. Joseph Lead Co.
3. National Lead Co.; St. Louis Smelting & Refining Co.; United Lead Co.
4. The Rockefeller-Ryan group, comprising the International Smelting Co.—Tooele smelter, Utah; and the International Lead Refining Co.,—refinery at East Chicago, Ind.
5. The United States Smelting, Refining & Mining Co. group, comprising the United States Smelting Co.—Mid vale smelter, Utah; U. S. Metals Refining Co.,—electrolytic lead refinery at Grasselli, Ind.; Needles Mining & Smelting Co.,—zinc-lead custom concentrator at Needles, Calif.; Bingham Mines Co. and other mines in Utah (Bullion, Beck, Champion, Utah-Apex, Centennial-Eureka and Tintic); Sunnyside and Gold Prince mines at Silverton, Colorado, and formerly a half interest in International Metals Selling Co.; and the Leadville Unit.
6. The Day group, comprising the Northport Smelting & Refining Co.; Pennsylvania Smelting & Refining Co.; Hercules Mining Co.; Tamarack & Custer Mining Co.; and the Amazon-Manhattan mines et al.
7. The American Metal Co. group, controlling the Ohio & Colorado Smelting Co. (owning 65 per cent. of its stock), and the Balbach Smelting & Refining Co. (owning one-third of its capital stock and selling the entire output).
8. Hayden-Stone-Clark-Coolidge group, comprising the American Zinc, Lead & Smelting Co.; American Zinc Co. of Tennessee; American Zinc. Co of Wisconsin; American Zinc Co. of Illinois; Granby Mining & Smelting Co. of Missouri; and the Butte & Superior Mining Co.
[280]
9. The Eagle-Picher group, comprising the Eagle-Picher Lead Co., Joplin, Mo. with smelter at Joplin, Mo., and controlling the Galena Smelting & Manufacturing Co., Galena, Kan.; and the Webb City Smelting & Manufacturing Co., Webb City, Mo.
10. The Bunker Hill & Sullivan Mining & Concentrating Co., controlled by Bradley-Crocker interests, and probably representing a certain proportion of English capital.
11. The Desloge Consolidated Lead Co., which formerly operated its own smelter but now has its ores smelted on toll by the Federal Lead Co. and markets its own lead.
All of the above companies are owned and controlled by American citizens so far as known, except certain interests in the American Metal Co. and perhaps the Bunker Hill & Sullivan Mining & Concentrating Co.
The American Metal Co. has 70,000 shares of capital stock, of which 34,644 shares were owned by the Metallgesellschaft, one of the German “Trio,” 18,620 shares belong to Germans who have become naturalized American citizens and have had the management, and the remaining 16,736 belonged to Henry L. Merton & Co., of London, a firm that is in process of liquidation. The Alien Property Custodian took over the 34,644 shares referred to above and sold them at public auction. The control of the company is vested in three voting trustees selected by agreement between the Alien Property Custodian and the American shareholders.
Vogelstein & Co. at one time linked groups 5, 7, 8, and 10, chiefly through selling contracts. Vogelstein & Co. owned the International Metals Selling Co., formerly marketing the production of the United States Smelting, Mining & Refining Co., but this connection is now broken. A contract for sale of the entire output of the American Zinc, Lead & Smelting Co. was held by L. Vogelstein & Co., partly owned by Aron Hirsch & Sohn, another member of the great German metal combine. L. Vogelstein & Co. (Inc). was seized by the Alien Property Custodian and was controlled by five directors, two of whom were appointees of the Custodian.[136] The operative control of the Bunker Hill & Sullivan Mining & Concentrating Co. is vested in Americans, but Vogelstein & Co. sells the output. Groups 5 and 8 are probably linked to some extent by large stockholders in common, Clark & Coolidge of Boston.
[136] L. Vogelstein is reported to have sold his interests to the American Metal Co. and subsequently, early in 1920, to have acquired a fifth interest in that company.
As a result of the activities of the Alien Property Custodian, the ownership and control of substantially all the mines and reduction works in the United States are vested in American citizens. Further information regarding the activities of German interests in the American metal trade is given in the chapter on copper, pages 232-235.
Table 54 lists the important lead smelting and refining plants of the United States at the present time.
[281]
Table 54.—Silver-lead Smelters in the United States
| Financial Group No. |
Operating company |
Location | Number of furnaces |
Annual capacity (tons of charge) |
|---|---|---|---|---|
| 1 | American Smelters Securities Co. | Selby, Calif. | 3 | 210,000 |
| 1 | American Smelting & Refining Co. | Denver, Colo. | 7 | 510,000 |
| 1 | American Smelting & Refining Co. | Pueblo, Colo. | 7 | 380,000 |
| 1 | American Smelting & Refining Co. | Durango, Colo. | 4 | 210,000 |
| 1 | American Smelting & Refining Co. | Leadville, Colo. | 10 | 510,000 |
| 1 | American Smelting & Refining Co. | Murray, Idaho | 8 | 657,000 |
| 1 | American Smelting & Refining Co. | East Helena, Mont. | 4 | 306,000 |
| 1 | American Smelting & Refining Co. | Omaha, Neb.[137] | 2 | 82,000 |
| 1 | American Smelting & Refining Co. | Perth Amboy, N. J.[137] | 4 | 170,000 |
| 1 | Con. Kansas City Smelting & Refining Co. | El Paso, Texas | 6 | 380,000 |
| 10 | Bunker Hill & Sullivan Mining & Concentrating Co. | Kellogg, Idaho | 3 | 600,000 |
| 7 | Ohio & Colorado Smelting Co. | Salida, Colo. | 3 | 200,000 |
| 5 | U. S. Smelting, Refining & Mining Co. | Midvale, Utah | 7 | 530,000 |
| 6 | Northport Smelting & Refining Co. | Northport, Wash. | 2 | 216,000 |
| 6 | Pennsylvania Smelting Co. | Carnegie, Pa. | 2 | 60,000 |
| 4 | International Smelting Co. | Tooele, Utah | 5 | 500,000 |
| Total | 77 | 5,521,000 |
[137] Refinery also.
The following Table 55 shows the relative importance of the various financial groups controlling the production of lead in the United States.
Table 55.—Financial Groups Controlling the Production of Lead in the United States
| Financial group No. |
Company | Percentage of production |
|
|---|---|---|---|
| Oct.-Nov., 1918 |
1917 | ||
| 1 | American Smelting & Refining Co | 34.1 | 37.7 |
| 3 | National Lead Co. (St. Louis Smelting & Refining Co.) | 5.9 | 9.0 |
| 2 | St. Joseph Lead Co. | 20.1 | 16.4 |
| 4 | International Smelting & Refining Co. | 3.7 | 6.5 |
| 5 | U. S. Smelting Refining & Mining Co. | 5.5 | 8.3 |
| 6 | Pennsylvania Smelting Co. | 6.2 | 7.2 |
| 7 | American Metal Co. | 1.8 | 2.1 |
| 8 | American Zinc, Lead & Smelting Co. | 1.7 | 1.3 |
| 9 | Eagle-Picher Lead Co. | 9.3 | 6.2 |
| 10 | Banker Hill & Sullivan Mining & Concentrating Co. | 6.3 | 1.7 |
| 11 | Desloge Consolidated Lead Co. | 2.6 | 3.3 |
| Ontario Smelting Co. | 1.9 | 0.0 | |
[282]
The American Smelting & Refining Co., through the preponderance of its production and the wide distribution of its interests, dominates the domestic industry, although its proportion of the total output has decreased greatly during the past few years.
—More than 60 per cent. of the pig lead made in Spain comes from the smelters of the Penarroya company (French), which within the past few years has absorbed the Spanish smelters of Escombrera-Bleyberg at Esconbrera, and the two smelters of Figueroa, at Linares and Cartagena. This gives the Penarroya company a dominant position in the Spanish lead industry. English interests, E. J. Enthoven & Co., have a smelter producing about 25,000 short tons of lead annually. All the smelting companies have made a combination to stop competition in ore buying. The Spanish government imposes a duty of about $1.80 per ton on lead concentrates exported.
—Smelting plants in Australia that produce lead have the capacities shown below.
| Company | Location | 1913 production, short tons pig lead |
|---|---|---|
| Broken Hill Associated Smelters | Port Pirie, South Australia | 92,824 |
| Sulphide Corporation | Cockle Creek, New South Wales | 19,660 |
| Total | 112,484 |
The annual capacity of the Port Pirie plant is now 165,000 short tons of pig lead.
Table 56.—Silver Lead Smelters in Mexico
| Company | Location | Number of furnaces |
Annual capacity tons of charge |
|
|---|---|---|---|---|
| American Smelting & Refining Co. | Monterrey | 7 | 410,000 | |
| American Smelting & Refining Co. | Aguascalientes | 1 | (lead) | 40,000 |
| American Smelting & Refining Co. | Chihuahua | 7 | 400,000 | |
| American Smelters Securities Co. | Velardeña | 3 | 150,000 | |
| Compañia de Minerales y Metales | Cerralvo | 2 | 35,000 | |
| Compañia de Minerales y Metales | Guadalupe, N. L. | 1 | 70,000 | |
| Compañia Fundadora y Refinadora de Monterrey (leased to American Metal Co.) | Monterrey | 4 | (or 8?) | 210,000 |
| Compañia Metalurgica Mexicana (Towne) | San Luis Potosi | 10 | 250,000 | |
| Compañia de Minerales y Metales (reported about to be dismantled) | Torreon | 9 | 350,000 | |
| Compañia Minera de Peñoles | Mapimi | 6 | 350,000 | |
| Mazapil Copper Co., Ltd. (English) | Saltillo, Coahuila | 1 | 36,000 | |
| Total | 51 | 2,301,000 | ||
[283]
—Practically all the Mexican smelting works are owned and operated by American companies. Prior to the war several of these were controlled by German interests through the American Metal Co. (incorporated in the United States), but the action of the Alien Property Custodian eliminated all foreign control in those companies, which comprise Compañia Minera de Peñoles, Compañia de Minerales y Metales, and Compañia Metalurgica de Torreon, leaving only the Compañia Fundadora y Refinadora de Monterrey still under German control. The American Metal Co. normally controlled 60,000 to 75,000 tons of bonded Mexican lead and 12,000 to 15,000 tons of lead smelted in Mexico, together with over a thousand tons of antimonial lead.
The Aguascalientes and Velardeña plants are chiefly copper smelters. There are small lead smelters at Terrazas and Santa Rosalia, Chihuahua, but they are probably abandoned.
—During the war the British lead-smelting capacity is reported to have been increased. Before the war a large amount of foreign lead bullion from Spain, Belgium and Germany was desilverized in the British Isles.
—The lead-smelting plants of France escaped destruction during the war, being outside the war zone. The capacity of the Pontgibaud plant was greatly increased. They are all French owned. The most important lead smelting plant in Italy is that of the Societa di Pertusola (English), with a pig-lead output in 1915 of 16,625 metric tons; the other plant is that of the Societa di Monteponi (Italian), with an output of 5,187 tons. The only lead smelting of Greece is that of the Compagnie des Mines de Laurium (French).
The only important lead smelter of Canada is that of Trail, British Columbia, which is controlled by the Canadian government. This plant includes a refinery using the Betts electrolytic process.
The only lead smelter in Turkey is one controlled by French interests, that of the Société des Mines de Balia-Kara-Aidin, at Kara-Aidin. The lead smelting plant of the Burma Mines Corporation, Ltd., at Bawdwin, Burma, has a capacity of 22,000 short tons lead.
In Siberia the Ridder Mining Co., controlled by the Irtysh Corporation, of London, has constructed 165 miles of railroad, which, together with river transport, brings the ore from the Ridder concession and the coal of the Ekibastus coal fields to the smelting plant at Ermak, which has a capacity of 15,000 tons of lead annually.
—National and even international control of the lead market has at times been attained through marketing organizations or trade combinations of reduction works, ore-buying and metal-selling agencies with interlocking directorates, joint ownership, and long-term contracts for ores, concentrates, and metals.
In 1909 the so-called Lead Convention, or officially the International[284] Sales Association, was formed in Paris. It was composed of German, Spanish, and Belgian producers and metal-selling agencies controlling directly about one-half of the European production. Some companies not nominally in the association acted in concert with it. This convention was renewed in 1910 and again in 1913 for three years. The association was dominated by the same interests as the German Zinc Syndicate,—the Merton group, comprising the Metallgesellschaft, the Metallbank and Metallurgische Gesellschaft of Frankfort and the Merton companies of London; Beer, Sondheimer & Co., controlling a dozen German metal and chemical concerns, and Aron Hirsch & Sohn at Halberstadt. The Merton group of London and Frankfort were the selling agents of the association, and the dominant “Trio,” through the Australian Metal Co., controlled the lead exported as metal or concentrates from Australia.
In the United States and Mexico all lead exported was controlled by the American Metal Co., affiliated with the association, or the American Smelting & Refining Co., acting in concert with it. The German “Trio” maintained and was rapidly extending and strengthening its ascendancy throughout the world through banks, holding companies, affiliations with syndicates and cartels, interlocking directorates, joint share holdings and purchase in whole or in part of mines and smelters. Some of the English lead refineries were closely associated with the Convention, and all lead exported from the Port Pirie smelter was sold by Merton & Co. The association was able to fix the price of lead in Europe and thus affect the output and, it has been claimed, so manipulated the market that lead outside the Convention was controlled just as effectively as that inside. The membership was international, brought together by mercenary motives. That the strongest and most aggressive interests were German citizens was purely incidental.
As a result of the war, however, the production of Australian lead concentrates and bullion is permanently diverted from German control. The activities of the Alien Property Custodian tended to eliminate all German interests in the United States. Most of the Australian lead bullion production is now marketed by the Broken Hill Associated Smelters, Prop., Ltd., of whose capital stock the Broken Hill Proprietary owns one-third and the Broken Hill South, Broken Hill North, and Zinc Corporation each own two-ninths. The plant of this company is now the largest smelter in the world.
The nationalist movement in France resulted in the formation of trade associations known as “consortiums,” comprising the principal factor in each industry. These “consortiums,” were recognized by the government, and each was made virtually arbiter of its particular field. The consortium of the mineral industry has been perfected into the Société Minerals et Metaux, 154 Boulevard Hausmann, Paris. The[285] official announcement states that this society is organized under the auspices of the French government in order to group the French metal producers operating both at home and abroad into a co-operative association for the purchase and sale of metallurgical products.
The participants comprise the principal companies in France producing or refining metals and also the mining and smelting companies controlled by French capital in Spain, Algeria, Tunis, and other foreign countries. A highly centralized organization of that part of the mineral and metal industry under French control is thus achieved for mutual protection and advantage in competition with other nationalities. Members of this organization will produce most of the ore from Algiers and Tunis and probably 75 per cent. of the Spanish lead bullion. The present annual output of the participating companies is about 200,000 metric tons of lead, 50,000 metric tons of zinc, and 40,000 tons of copper, besides iron, antimony, platinum, etc. The association comprises the following companies: Société Minière et Metallurgique de Penarroya; Société d’Affinage de Metaux; Compagnie des Mines d’Ain-Barbar; Association Minière; Société des Mines de Zinc d’Ain-Arko; Compagnie du Boleo; Compagnie Française des Mines de Bor; Corocoro United Copper Mines, Ltd.; Société des Mines de Fedj-el-Adoum; Société des Mines de Zinc de Guergour; Compagnie des Mines de Huaron; Société Minière du Kanguet; Compagnie des Minerais de Fer Magnetique de Mokta-et-Hadid; Comptoir Lyon Allemand; Société Anonyme des Mines de Malfidano; Compagnie des Mines d’Ouasta et de Desloula; Société des Mines de Parzan; Compagnie Industrielle du Platine; Société Anonyme des Mines et Fonderies de Pontgibaud; Société des Mines d’Antimoine de Rochetrejoux; Société des Anciens Etablissements Sopwith; Société Française d’Etudes et d’Enterprises; Société des Mines du Djebel-Ressas; Société Anonyme Française du Djebel-Hallouf; Société Anonyme des Mines et Usines de Peyrebrune; and Compagnie La Cruz.
One of the results of the war was the stimulation of co-operative enterprises between British companies, the movement being encouraged by the government, which in some cases participated financially. The British Australian Lead Manufacturers Proprietary, Ltd., was formed as a consolidation of the Australian interests of British firms, with the object of establishing a white-lead industry in that country. Those participating comprise: Alexander Ferguson & Co.; Cookson & Co.; Foster, Blockett & Wilson; Locke, Blockett & Co.; Locke-Lancaster; W. W. & R. Johnson & Sons, Ltd.; Mersey White Lead Co., Ltd.; and Walkers Parker & Co.
The Chloride Syndicate comprising the Zinc Corporation, Ltd., Burma Mines Corporation, and the Swansea Vale Smelter, Ltd., is conducting experimental work on the Ganlin process with the object of perfecting it.
[286]
Richard Tilden Smith, having acquired controlling interests in J. H. Enthoven & Sons, Locke-Lancaster, Walkers Parker & Co. and a one-third interest in the Burma Mines Corporation, became a strong link between these important companies and thus secured an arrangement with the Imperial Government for financial assistance with the new plant of the National Smelting Co., Ltd., at Avonmouth to the extent of £500,000 out of the total estimated cost of £750,000, the government being particularly interested in the production of by-product acid. The National Smelting Co. agreed to take 25,000 tons yearly of Broken Hill concentrate and smelt no foreign ore without government permission. Enthoven & Sons control through ownership the output of one of the Spanish smelters and through desilverizing and marketing contracts the output of the French Laurium company of Greece. An attempt to bring about a closer combination of all the above-mentioned English and Australian companies failed.
It is reported that in 1917 Vivian, Younger & Bond, and Morgan, Grenfeld & Co. were instrumental in forming a “Metal Bank” in London. Broken Hill Associated Smelters Prop. Ltd., and the Penarroya company were said to have agreed to market their production through this bank, but it is probable that the arrangement was never made effective. A Chemical and Metallurgical Bank has been formed in London, among the shareholders being Richard Tilden Smith. Its proposed field is not clear, but it will probably endeavor to influence the control of lead in the United Kingdom.
A British Metals Corporation was formed in 1918 by Vivian, Younger & Bond, Chas. Tennant Sons & Co., Cookson & Co., the British Aluminum Co., Morgan, Grenfeld & Co., and large stockholders of the Rio Tinto Co., with a capital of £5,000,000. The Imperial Treasury will be represented on the board of directors. The purposes are to finance British non-ferrous metal companies, bring all the production within the empire of those metals under one marketing organization and preserve British control. If plans are successfully carried out, the smelting both in England and on the Continent of Europe, should any British concentrates be allotted there, will practically be on toll, the metal remaining in the control of the Metals Corporation or the affiliated bank.
The accompanying table presents available statistics of production since 1913 in order to show the extent to which the industry in each country is controlled by domestic or foreign capital, and attempts to show the extent of commercial control by each of the great powers. Such statistics are at best only approximate; the control varies from year to year and the data are difficult to obtain and verify.
[287]
Table 57.—Commercial Control of the World’s Output of Pig Lead
| Country | Recent pig-lead production (short tons) |
Year | Per cent. of world |
Financial control by | ||||
|---|---|---|---|---|---|---|---|---|
| Home capital |
United States |
Great Britain |
France | Germany | ||||
| United States | 612,200 | 1917 | 44.6 | ... | 612,200 | |||
| Australia | 127,800 | 1913 | 9.4 | ... | ... | 127,800 | ||
| British Isles | 12,600 | 1916 | 0.9 | ... | ... | 12,600 | ||
| Canada | 20,400 | 1916 | 1.5 | ... | ... | 20,400 | ||
| Burma | 20,000 | 1917 | 1.5 | ... | ... | 20,000 | ||
| France | 30,800 | 1913 | 2.2 | ... | ... | ... | 30,800 | |
| Italy | 24,000 | ... | 1.8 | 5,700 | ... | 18,300 | ||
| Belgium | 39,400 | 1913 | 2.9 | 15,000 | ... | ... | 24,400 | |
| Venezuela | 200 | 1917 | ... | ... | ... | ... | ... | 200 |
| Bolivia | 2,400 | 1917 | 0.2 | 400 | ... | ... | 2,000 | |
| Greece | 12,800 | 1916 | 0.9 | ... | ... | ... | 12,800 | |
| Japan | 12,300 | 1916 | 0.9 | 12,300 | ||||
| Peru | 3,000 | 1917 | 0.2 | 500 | 500 | 2,000 | ||
| Russia | 1,100 | 1913 | 0.1 | 1,100 | ||||
| Chile | 6,000 | 1917 | 0.4 | 3,000 | ... | 1,000 | ... | 2,000 |
| Mexico | 61,200 | 1913 | 4.4 | ... | 53,200 | 8,000 | ||
| Spain | 157,000 | 1917 | 11.4 | 20,000 | ... | 25,000 | 105,000 | 7,000 |
| Sweden | 2,100 | 1915 | 0.1 | 1,100 | ... | ... | ... | 1,000 |
| Germany | 200,000 | 1913 | 14.5 | ... | ... | ... | ... | 200,000 |
| Austria-Hungary | 26,600 | 1913 | 1.9 | 26,600 | ||||
| Total | 1,371,900 | ... | 100.0 | 665,900 | 235,100 | 175,000 | 210,200 | |
| Per cent. of world production. | 100.0 | 48.6 | 17.2 | 12.7 | 16.3 | |||
—During the war the United States demonstrated that its lead-ore deposits were capable of a production greatly in excess of the normal. That the increase was not greater was chiefly because of insufficient incentive in the market price of lead, which did not attain the early inflation of many of the other metals; and later, when the price was right, was restricted by lack of refining capacity. The existing capacity was at times not fully utilized because of the congestion of railway traffic. The lead-ore deposits in the United States have large possibilities, but with the exception of the disseminated lead deposits of southeastern Missouri the developed ore reserves will supply production for only a few years. Control of the mines in the United States is vested almost exclusively in Americans or American companies, some of the latter, however, having foreign stockholders, chiefly British or Canadian.
The United States imports lead ores, to a small percentage of its total production, from Mexico, Canada, South America and Africa. The Mexican ore is largely imported to meet the especial needs of the El Paso lead smelter, and not because of lack of smelting capacity in Mexico. The smelting capacity of the United States has always been in excess of the actual production of ore and is now very greatly in excess of the normal production. The ownership of most of the important smelters has always been vested in American capital, and as a result of the activity of the Alien Property Custodian all of them are now owned[288] and controlled by American citizens. A considerable amount of lead bullion is imported, chiefly from Mexico, for refining. Most of this is re-exported. United States capital through ownership of smelters controls the greater part of the pig lead produced in Mexico and Peru. The importation of ore and bullion for refining from Canada is restricted by the government bounty on lead smelted and refined in Canada. United States capital also controls a considerable percentage of the mines of Mexico and has some interest in the Burma Mines Corporation, and the Irtysh Corporation.
Notwithstanding the enlarged smelting capacity and the possibilities of production from American mines, it is not expected that there will be any considerable export of domestic lead after the period of readjustment following the World War.
—The countries of the British Empire in the order of the importance of their lead output in 1913 are Australia, British Isles, Canada, Burma, and Egypt.
British capital controls the mines and smelters of the British Isles, Canada, Australia, Burma, and the Altai Mountains in Siberia, and, by ownership of mines and smelters, some 25,000 tons of lead annually from Spain and over 70 per cent. of the production of Italy. By contracts for desilverization and marketing it has controlled the greater part of the Spanish output and part of the production of Greece. It is reported also to control small tonnages of ore from Peru and Chile. In view of the recent increase of refining capacity in Spain by the Penarroya company (French), and the organization of all French interests into the Société Minerais et Metaux, future control by English capital of Spanish output above the 25,000 tons produced by the English smelter is doubtful, although it has been reported that the Penarroya company had agreed to market its lead through the new Metal Bank of London. Prior to the war much base bullion from Spain, Belgium, and Germany was imported and desilverized, and in large part re-exported.
The ore production of the British Empire is about 25 per cent. greater than its consumption, but insufficient smelting capacity has caused dependence on foreign-smelted lead. The smelting capacity has been increased, and the additional capacity contemplated will make the total equal the consumption. The empire can be made independent of the rest of the world should the policy of “Imperial preference” be adopted.
All the large interests in the lead industry of the British Empire have shown during the war period a strong tendency to co-operate and organize for mutual protection and benefit. A culmination of this movement is apparently the formation of the British Metals Corporation, intended to vest the marketing control of the production of the whole British Empire in one organization under governmental auspices.
—The Australian production of lead ore and concentrates, derived chiefly from New South Wales, makes Australia rank second in[289] world production of lead ores. In 1913 little more than half the output was exported to Europe for smelting, the remainder being smelted at Cockle Creek and Port Pirie. The pig lead produced, less a small local consumption, was also exported.
Australian mines have always been owned by British and Australian capital. Prior to the war the marketing of concentrates and of all lead bullion exported was controlled by the Australian Metal Co., affiliated with the German metal “Trio” headed by the Metallgesellschaft. During the war, however, the governments of Great Britain and Australia annulled the long-time ore- and metal-purchasing contracts, and the ore producers and smelters have organized for mutual protection and to keep the industry permanently under exclusive British control. The capacity of the Port Pirie reduction works has been increased by capital contributed jointly by the principal mining companies, and the plant is now the largest lead smelter in the world.
—The small output of lead ore in Great Britain is obtained from a few scattered deposits of little importance. Some ore is imported and smelted with the domestic production. The consumption of the British Isles is so large that very large imports of pig lead are necessary, amounting to 14 per cent. of the world’s production. All of the mines and smelters of the British Isles are owned and controlled, so far as known, by British capital.
—With the assistance, through bounties, of the Canadian government, the Trail smelter has become firmly established and is the only important lead producer of Canada. It handles most of the British Columbia output of ores, which otherwise would have to go to the United States for reduction. The Canadian consumption is, however, about one-third greater than the production.
—Although the production of ore and pig lead in the Northern Shan States by the Burma Mines Corporation was in 1913 an insignificant part of the world’s output, the company has developed its deposits so as to be capable of a very much greater yield, which may amount in the near future to 75,000 tons annually. This company is controlled by British with probably some American capital.
—The small ore production of Egypt is exported for smelting and is controlled by French capital.
—Germany, a large producer of lead ores, imported in 1913 for smelting nearly 10 per cent. of the world’s output and in addition imported a considerable amount of pig lead, being the second largest consumer of lead in the world. Should Germany lose Upper Silesia, which produced nearly half the domestic ores, it will be still more dependent on imports to supply its smelters. Prior to the war the German lead industry was closely organized, much of the lead mining and smelting being conducted by departments of the state governments, although some of[290] the largest concerns were private corporations. The German metal “Trio” headed by the Metallgesellschaft through the International Sales Association controlled directly about one-half the European production, comprising in this so-called Lead Convention, besides the German concerns, most of the Spanish and Belgian producers, most of the lead exported from the United States and Mexico, and the pig lead and concentrates exported from Australia. This system of control outside of Germany has now been permanently destroyed, and the magnitude and the organization of the German lead industry in the near future cannot be anticipated.
—In France the output of lead ore is small and is controlled, with probably one exception, by French capital. Some ores are imported for smelting, Tunis and Algeria being capable of supplying even more. To provide for the large consumption, France ranking fourth among lead-consuming countries, there is imported in the form of pig lead some 7 per cent. of the world’s output. Recently smelting capacity has been increased. French capital controls more than half the production of Belgium through ownership of smelters, and through ownership of mines and smelters controls more than half the production of Spain and most, if not all, of the output of Greece. It also controls the ore production of Egypt and most of the ores produced in Algeria and Tunis. Under government auspices a strong organization of all the metal-producing companies controlled by French capital in France and foreign countries has recently been effected under the name of the Société Minerais et Metaux, which controls the sale of all the production of its members, as well as acting as a purchasing agent for them.
—Belgium produces no ores but smelts about 4¹⁄₂ per cent. of the world’s output, nearly the whole of which is consumed within her borders. A little less than half of this production is controlled by Belgian capital, the remainder by French interests. Belgian capital is also interested to a minor extent in Spain, Algeria, the Caucasus Mountains and Tunis. It is believed that a part of the Australian concentrates may be allotted to Belgium for smelting.
—The Italian ore production, amounting to about 2 per cent. of the world’s total, is smelted in that country. The product is consumed, together with about 50 per cent. more, imported as pig lead. More than three-fourths of the domestic output is controlled by English capital; nearly all the remainder is controlled by Italian capital, but other English, French and Belgian companies produce insignificant amounts of ore.
—The Austro-Hungarian Empire produced and smelted ores to the amount of about 2 per cent. of the world’s output in 1913, and consumed this with about 50 per cent. more metal imported in the form of pig lead. The several lead-producing districts and smelters, some of which belong to the states of Austria and Hungary,[291] with the partition of the empire fall within three or more distinct political jurisdictions, the lead production of none of which will be of importance. One of the important lead smelters is at Fiume.
—Spain ranks third in content of ores produced and second in smelter production. The domestic consumption being negligible, all of the lead is exported. Prior to the war most of the production went to England to be desilverized. During the war, however, the refining capacity of Spain was greatly increased, and it now seems likely that any silver lead which the domestic plants can not take care of will be shipped to France for desilverization. More than half the Spanish production is controlled by French capital, and to a minor degree by Belgian and German interests.
—Japanese lead-ore resources are meagre. The output of lead ore and pig lead in 1913 was about 25 per cent. of the domestic consumption. Since then ores and concentrates have been imported from China, Formosa, Korea, Siberia, and Australia, but importation of pig lead has still been necessary. The Japanese are endeavoring to secure control of ore deposits in China and Siberia, and supply the raw material for the increase of their domestic smelting and manufacturing industries. It is probable, however, that Japan will be dependent for many years on imports from other sources for most of the lead consumed.
The chief uses of lead are as metal and in the form of white lead or basic carbonate, as a pigment. Metallic lead is used for water pipe, covering for electrical cables, lining acid chambers and vats, and for shot, bullets and shrapnel. Alloys with various other metals are used in type, bearings, and fuses. The red and orange oxides are used for pigments. The largest single form of consumption is white lead.
Lead and zinc ores are commonly associated and are widely distributed over the world. Galena is the chief lead mineral, the other ore minerals, cerussite and anglesite, being derived from it by oxidation. Galena is a persistent mineral, being found in nearly all types of ore deposits. The countries producing the most ore are, in order of importance, United States, Australia, Spain, Germany, and Mexico. Districts of major importance are Broken Hill, New South Wales; southeastern Spain; southeastern Missouri; and Coeur d’Alene, Idaho.
The developments in selective oil flotation, by which the detrimental zinc content is eliminated from complex ores and made an asset, constitute the greatest recent advance in the dressing of lead ores. Electrolytic refining is the greatest recent advance in the metallurgy of lead.
The readiness with which lead is reduced from its ores and its utility as a collector of the precious metals in smelting have resulted in a wide[292] distribution of reduction plants. Nevertheless a large percentage of the world’s output of ores is transported from the countries of origin to others, for reduction near the market and where skilled labor and fuel are more abundant and cheaper.
The countries of largest smelter production are, in order, United States, Spain, Germany and Australia; those of much less importance are Mexico, Belgium, Great Britain, France, Austria-Hungary, Italy, Greece, Canada and, recently, Burma. Other countries are of little importance.
The countries of major consumption are, in order, United States, Germany, Great Britain, and France. The United States produces its own requirements, but the other three countries import ores for smelting and also pig lead in large quantity. Australia and northern Africa have supplied the bulk of the ores, and Spain, Australia, and Mexico most of the pig lead. This is the situation at last analysis, the actual trade movement being, of course, much more complex.
The political control of the world’s lead production, in terms of the lead content of ores, in 1913 was as follows: United States, 36.0 per cent.; British Empire, 23.9 per cent.; Spain, 6.4 per cent.; Germany, 5.9 per cent.; Mexico, 5.1 per cent.; and France, 4.0 per cent. Three powers thus control 76 per cent. of the production.
The ownership of mines is important chiefly as determining the distribution of ores to smelters. Ore-purchasing contracts may modify or annul the effect of political jurisdiction and mine ownership. The ownership or control of reduction plants has been the most effective basis for commercial control of the industry. In the United States the American Smelting & Refining Co. dominates the market, but controls directly only one-third of the output. In Spain the French company of Penarroya controls more than half the production. In Germany the “Trio” headed by the Metallgesellschaft controlled the domestic industry prior to the war, and through the “Lead Convention” extended its domination to half the European output and most of the exports of Australia, Mexico, and the United States. This control was, however, never absolute. In Australia at present the Broken Hill Associated Smelters controls the marketing of most of the pig lead exported.
Joint political and commercial control has been established in the British Empire and France. During the war, political jurisdiction was quite generally invoked to modify or eliminate commercial control, particularly with regard to alien-enemy interests.
The various combinations of British companies to insure British control of the resources of the empire, particularly of Australia and Burma, culminated in the formation of the British Metals Corporation for the sale of the output. A representative of the Imperial Treasury will be on the board of directors and either the Metal Bank of London or[293] the Chemical & Metallurgical Bank may finance its operation, unless it absorbs their functions. This organization is doubtless intended to take the place of the Metallgesellschaft as the dominant factor in the lead industry of the world. It will have much influence, as British capital controls all the production of the British Isles, Australia, Burma, Canada, and normally Siberia, most of that of Italy, part of that of Spain and Mexico, and perhaps of Greece. Probably the British Isles will not in the future desilverize as much foreign lead as formerly, so that the importance of this basis of commercial control will be greatly decreased.
The Société Minerais et Metaux, comprising all producers controlled by French capital, under government auspices is selling and purchasing agent for its members, and controls all the French and most of the Grecian production, more than half of that of Spain and Belgium, besides the ores of northern Africa and of Egypt.
Since the elimination of alien-enemy holdings by the Alien Property Custodian, United States capital controls substantially all the domestic production and nearly all that of Mexico, besides some ores imported from Mexico, Canada, and South America. Notwithstanding large ore reserves and reduction capacity, the United States is expected, after the period of readjustment, to supply its domestic needs, but to export little lead, as was the case prior to the war.
The position of Germany will depend largely upon arrangements for foreign ore supply. Some Mexican ore production may still be under German control. Belgium is wholly dependent on foreign ore, which presumably will be obtained largely from Australia; and with more than half her production controlled by French capital, she will be under British and French domination; but she consumes most of her smelter output. Italy consumes 50 per cent. more than the domestic production, which is chiefly controlled by English capital. None of the states formed by the disintegration of the Austro-Hungarian Empire will be of importance in the lead industry.
The commercial control of the smelter production of lead, calculated from the latest statistics available, is approximately as follows: United States, 48.6 per cent.; British Empire, 17.2 per cent.; Germany, 15.3 per cent.; and France, 12.7 per cent.; or a total for the four powers of 93.8 per cent. of the pig-lead output of the world.
[294]
Metallic zinc, or spelter, as the commercial metal is often known in the trade, is chiefly used in the form of rolled sheets; in galvanizing; in alloys forming brass and bronze; and in the desilverization of lead bullion. Rolled sheets are used for roofs, tanks, conduits, and protective linings. Iron and steel objects are dipped into baths of molten spelter and coated with the metal or galvanized, being thereby protected from oxidizing agencies. Other methods of applying this protective coating are also in use.
Zinc and copper unite in all proportions, forming alloys known as brass which are of widespread industrial application. There is only one definite alloy; it corresponds approximately to CuZn2, contains 33 per cent. copper and 67 per cent. zinc, is hard and brittle and of little practical value. All other brasses may be considered as solid solutions of this definite alloy in an excess of one of its constituents. Brasses in use vary in zinc content from 20 to 85 per cent. and differ greatly in their properties according to the composition. Alloys of zinc and aluminum have valuable properties, especially those containing 25 to 35 per cent. zinc. Other alloys used contain, besides copper and zinc, either lead, tin or nickel.
The Parkes process of desilverizing lead bullion has superseded the older Pattinson and cupellation process, except where bismuth is present, owing to the avidity with which zinc robs the bullion of gold, silver, copper and tellurium. This purification may be made as perfect as desired or only to a commercially profitable point, generally being brought down to a content of one-half ounce of silver per ton of lead.
Among the miscellaneous uses of zinc are these: ornamental castings; in galvanic batteries; in photo-engraving; in plates hung in boilers to prevent formation of scale; precipitation of gold in the cyanide process; in the form of powder, as a reducing agent in organic chemistry, especially in the reduction of indigo-blue and in a paint for structural steel. Zinc is also used in the form of numerous salts, such as the chloride as a wood preservative, and the sulphate, employed in medicine, dyeing and glue manufacture.
[295]
Zinc oxide, produced both from the metal and from ores, is used as a pigment both in combination with white lead and barytes, and as a competitor of them. Considerable amounts of oxide are also used in the rubber manufacturing industry. Lithopone (an intimate mixture, obtained by chemical precipitation of zinc sulphide and barium sulphate) is of growing importance as a pigment.
All the chief uses of zinc, comprising galvanizing, rolled sheets, brass-making and the desilverization of lead bullion, may be considered essential. Brass belongs with steel in the category of indispensable materials of modern industry. No satisfactory substitute as regards both physical qualities and cost is available for many important parts of machinery and for manufacturing purposes. Its wide use depends on a number of qualities. The excellent sharp castings made from certain brasses are readily machined or otherwise finished and electro-plated if desired. The electrical conductivity of brass is good. Certain brasses are easily rolled into sheets and cut and stamped in desired shapes. Lubricated surfaces of steel on brass make satisfactory and durable bearings.
The other large uses of zinc depend on its resistance to oxidation and on the possibility of rolling it into fairly thin sheets. In both these qualities, however, it is surpassed by other metals, notably nickel and tin. Alloyed with lead it may be rolled into a substitute for tin-foil. It is in some cases a fairly satisfactory, cheap substitute for metals of higher quality. In times of scarcity or high prices, substitution of metals of inferior quality is feasible, and in many cases zinc may be temporarily dispensed with altogether. Its field is therefore largely fixed by commercial conditions of supply and price, which determine broadly the total consumption and especially the percentages devoted to the various uses. It may be assumed, however, that the percentages for the domestic consumption in the United States in 1910 represent approximately those of normal peace times. In that year, of the total consumption, 60 per cent. was used in galvanizing; 20 per cent. in brass-making; 11 per cent. in rolled sheets; and 1 per cent. in lead desilverization; leaving 8 per cent. for miscellaneous uses. During the war the percentage used in galvanizing was greatly reduced and that used for brass-making much increased. The use of rolled sheets will increase.
A large part of the European production in normal times is rolled into sheets used chiefly for roofing.
In the extraction of zinc from its ores the most important changes in practice during recent years have been adaptations of retort smelting for the purpose of utilizing zinc concentrates from complex ores, the increased production of zinc oxide and lithopone through the application[296] of volatilization methods to the re-treatment of retort residues and base ores, and the electrolytic and electro-thermic processes of extraction of the metal.
The most revolutionary advance has been the development of the oil flotation, the electro-magnetic, and electro-static processes for the concentration of ores. These processes are being widely introduced and in connection with electrolytic reduction are particularly adapted to the production of spelter of the highest quality from complex ores. As the electrolytic and electro-thermic processes find their field only where power is relatively cheap, the tendency is to put installations where hydro-electric power is available, effecting a redistribution of zinc-smelting centers.
Zinc and lead are commonly associated in mineral deposits, sometimes intimately mixed, sometimes so segregated that one metal predominates, but ores of one are seldom free entirely from the other. The geological and geographical distribution of the two metals is therefore nearly identical.
The sulphide ores, chiefly sphalerite or blende, are the most important, but in the oxidized zone they are often altered to carbonates—smithsonite, calamine and hydrozincite. The oxides—franklinite, willemite and zincite—are important in only one district in New Jersey. The carbonates, although they carry a low percentage of zinc, often occur in concentrated ore bodies, and yield readily to metallurgical treatment. Therefore, calamine-smithsonite ores form a large proportion of the production of many important districts, but blende will hereafter be of increasing importance in the world production.
Zinc ores occur in deposits of several distinct genetic types. In the order of their importance they are:
(a) Deposits formed in sedimentary rocks, without apparent connection with igneous rocks, as bedded replacements usually of limestones and dolomites. The ores of this type usually contain lead (galena) and iron (pyrite or marcasite) minerals, often those of manganese and cadmium, sometimes those of arsenic, cobalt and nickel, but seldom gold, silver, copper, or antimony. Barite and fluorite are sometimes present.
The deposits of this type are of world-wide distribution. Their greater purity and the simplicity of the treatment necessary, particularly of the ores in their oxidized zones, have caused them to be exploited first and most extensively and to be until recently the dominant factor in the world production of zinc. To this type belong the deposits of the Mississippi Valley and Silesia, which together produced 34 per cent. of the world’s output in 1913.
(b) Veins associated with igneous rocks and disseminated sulphide[297] replacements of igneous rock. In this group come the deposits of Butte, Leadville, and the Coeur d’Alene, and the disseminated deposits of the Bawdwin (Burma Mines Co.), Ridder (Siberia) and Salmon River (B.C.). These ores are usually complex, comprising minerals of zinc, lead, copper, iron, gold and silver, and often arsenic, antimony and other metals. In one group, the silver-lead deposits, zinc seems a minor factor, but with depth replaces lead as the predominant metal. Deposits of these complex ores have in recent years become important sources of zinc through recognition of the zinc ores in their oxidized zones, through zinc becoming the dominant metal with increasing depth at many mines, and especially through improvements in methods of concentrating complex ores and extracting metal from the concentrates. Ores of this type will be of increasing importance in the future because of their world-wide distribution.
(c) Igneous metamorphic deposits containing franklinite, willemite, zincite, a little blende, and a gangue of calcite, rhodonite, garnet, pyroxene, hornblende, magnetite and tremolite. This type is characteristic of the Franklin and Adirondack deposits of the northeastern metallographic province of the United States and is also found at Magdalena and Hanover, New Mexico and the Horn Silver mine, in Utah.
(d) Metamorphosed deposits. Originally these may have been of any of the preceding types but are now disguised by regional metamorphism. The best example is the important deposit of Ammeberg, Sweden. Blende there occurs disseminated in bands in gneissoid granulite, which also contains bands of disseminated pyrrhotite and arsenopyrite.
The chief zinc ore deposits of the world are in the countries listed in the table below; the order of the countries is that of their importance in the industry in 1913, as nearly as can be estimated from incomplete data.
The three major metallographic provinces of the world as indicated by present exploitation are those of Broken Hill, N.S.W., Silesia, and the Mississippi Valley.
The condition of the zinc industry in the principal countries, in 1913, is shown in Table 59.
—The zinc deposits of the United States may be assigned to three metallographic provinces, which in the order of their present importance are: the Mississippi Valley province; the Western province; and the Northeastern province.
Judged by present knowledge, the Mississippi Valley province is one of the three major zinc-bearing metallographic provinces of the world. It occupies an area underlain with slightly disturbed Paleozoic[298] limestone, ranging from Ordovician to Lower Carboniferous in age, that comprises most of the great mid-continental valley of the Mississippi in the states of Missouri, Arkansas, Oklahoma, Kansas, Illinois, Kentucky, Wisconsin, Iowa, also Tennessee and the western part of Virginia. Igneous rocks are generally absent. There are three principal subprovinces: the Ozark province, comprising Missouri, Oklahoma, Kansas, Arkansas; the upper Mississippi valley, comprising Wisconsin, Northern Illinois, and Iowa; and the regions of Tennessee and Virginia.
Table 58.—Chief Zinc-ore Deposits of the World
| Approximate order |
Country | Percentage of world’s production in 1913 |
|---|---|---|
| 1 | United States | 35.0 |
| 2 | Germany | 25.0 |
| 3 | Australia | 15.0 |
| 4 | Italy | 5.0 |
| 5 | Algeria | 3.0 |
| 6 | Japan | 2.0 |
| 7 | Spain | 2.0 |
| 8 | Russia (including Russian Poland) | 2.0 |
| 9 | France | 1.5 |
| 10 | Greece | 1.0 |
| 11 | Sweden | 1.0 |
| 12 | Mexico | 1.0 |
| 13 | Austria | 1.0 |
| 14 | Tunis | 1.0 |
| 15 | Indo-China (Tonkin) | |
| 16 | Great Britain | |
| 17 | China | |
| 18 | Bolivia | |
| 19 | Canada | |
| 20 | India (Burma) | |
| 21 | Egypt | |
| 22 | South Africa | |
| 23 | Peru |
In the Ozark subprovinces the Joplin district is most important. The ores lie at three horizons in flat-lying lower Carboniferous limestones. In the upper horizon, below the surface, the ore lies in clayey chert breccias, and galena predominates. The middle horizon, or “sheet ground,” carries mixed galena and blende, which cements brecciated chert. The ore is low grade, the average recovery of zinc being 1.9 per cent. This horizon has been the most important source of ore. The third and lowest horizon, as yet little exploited, contains disseminated ores. Thirty mills were busy in the Joplin district in 1918. The Athletic Mining & Smelting Co. was the largest producer, operating the Athletic mine, at Duenweg, the Bertha A., at Webb City, and Mutual mine, at Oronogo. Miami Zinc Syndicate, affiliated with the Butte & Superior and American Zinc, Lead & Smelting Co.; the Commerce Mining & Royalty Co.; the Century Zinc Co.; and the Tri-State Mining Co., are large operators. Much of the production is by lessees and small operators.
[299]
Table 59.—Zinc Industry in 1913
| Country | Available zinc in ore (short tons) |
Spelter (short tons) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Produced | For export |
To be imported |
Annual con- sumption |
||||||
| Produced | Smelted | Exported | Imported | Primary | Secondary | ||||
| United States | 305,500 | 310,500 | 8,500 | 13,500 | 310,500 | 50,000 | 21,000 | 339,500 | |
| Australasia | 206,000 | 4,000 | 202,000 | ... | 4,000 | ... | ... | ... | 4,000 |
| British Isles | 50,000 | 65,000 | ... | 15,000 | 65,000 | 6,000 | ... | 143,500 | 214,500 |
| Canada | 24,000 | ... | 24,000 | ... | ... | ... | ... | 20,000 | 20,000 |
| Burma, Indo China, etc. | 18,000 | ... | 18,000 | ... | |||||
| France and French Africa | 69,000 | 63,000 | 6,000 | ... | 63,000 | 20,500 | ... | 5,500 | 89,000 |
| Italy | 40,000 | ... | 40,000 | ... | ... | ... | ... | 12,000 | 12,000 |
| Belgium | 11,000 | 218,000 | ... | 207,000 | 218,000 | 3,500 | 7,500 | ... | 84,000 |
| China | 5,000 | 2,500 | 2,500 | ... | 2,500 | ... | ... | 7,500 | 10,000 |
| Greece | 12,000 | ... | 12,000 | ||||||
| Japan | 10,000 | 8,000 | 2,000 | ... | 8,000 | ... | ... | 4,000 | 12,000 |
| Russia | 35,000 | 13,000 | 22,000 | ... | 13,000 | ... | ... | 23,500 | 36,500 |
| Holland | ... | 27,000 | ... | 27,000 | 27,000 | 3,000 | 25,500 | ... | 4,500 |
| Mexico | 30,000 | ... | 30,000 | ||||||
| Norway | 15,000 | 10,000 | 5,000 | ... | 10,000 | ... | 10,000 | ||
| Spain | 50,000 | 15,000 | 35,000 | ... | 15,000 | ... | 8,500 | ... | 6,500 |
| Sweden | 10,000 | 6,000 | 4,000 | ... | 6,000 | ... | 6,000 | ... | |
| Austria-Hungary | 24,000 | 24,000 | ... | ... | 24,000 | 3,000 | ... | 17,500 | 44,500 |
| Germany | 163,500 | 312,000 | ... | 148,500 | 312,000 | 18,500 | 75,000 | ... | 255,500 |
| Other | ... | ... | ... | ... | ... | 1,500 | ... | 45,000 | 46,500 |
| Total | 1,078,000 | 1,078,000 | 411,000 | 411,000 | 1,078,000 | 106,000 | 283,500 | 278,500 | 1,179,000 |
[300]
In Arkansas the ores are of similar character and mode of occurrence and are found in the same formation and also in the Ordovician limestone. The Lavender Mining & Milling Co. is the largest operator and the production has been largely carbonate ores.
The Upper Mississippi region comprises deposits in nearly horizontal limestones of Ordovician age. Three-fourths of the output of this district is made by five companies: the Mineral Point Zinc Co., a subsidiary of the New Jersey Zinc Co., with seven mines; the Vinegar Hill Zinc Co., with six mines; the Wisconsin Zinc Co., a subsidiary of the American Zinc, Lead & Smelting Co., with four mines; the Frontier Mining Co., with five producing mines; and the Cleveland Mining Co., with two mines. Other important companies are Burr Mining Co., Block House Mining Co., M. & A. Mining Co., B. M. & B. Mining Co., and Oliver Mining Co., a subsidiary of the U. S. Steel Corporation. All of the mines are equipped with milling plants. The production of the district shows a steady growth.
The zinc deposits of southwest Virginia and northeastern Tennessee occur as disseminated replacement breccia along crushed and faulted zones in folded Cambro-Ordovician limestones, and also as oxidized ores in clays residual from the weathering of the same limestones. The gangue is calcite and dolomite. The American Zinc Co. is the largest operator in Tennessee; it has a milling capacity of 3,000 tons daily and zinc-blende ore reserves greater than 6,000,000 tons averaging between 4 and 5 per cent. zinc, from which 60 per cent. zinc-blende concentrates are made. This company is a subsidiary of the American Zinc, Lead & Smelting Co.
The Western province comprises most of the western states; it extends north into British Columbia and south into Mexico. The chief subprovinces are those of Leadville, Butte, and Coeur d’Alene.
The Leadville deposits are found in strata varying in age from Archean to Cretaceous, which have been intruded by igneous rocks. The ores are mainly replacements of limestone and occur in large masses. This district first became of importance in zinc production upon the recognition of smithsonite and calamine in the large masses of oxidized ores. Of recent years sulphides form an increasing part of the production, now coming largely from deeper levels. The ores carry gold, silver, manganese, copper and sometimes bismuth. The United States Smelting Refining & Mining Co., the Empire Zinc Co., a subsidiary of the New Jersey Zinc Co.; the Western Mining Co., the Downtown Mines Co., the[301] Wellington Mines Co., at Breckenridge; and the Mary Murphy mine, at Chalk Creek, are the largest operators in this region.
The Butte ores occur as veins in igneous rocks. The area in which zinc ores predominate surrounds that of important copper veins on three sides. On the border of the two areas, zinc-silver ores predominate in the upper levels and copper in the deeper workings. Many of the present zinc mines were formerly worked for silver. These complex zinc ores have been made available by the successful application of oil flotation and electrolytic deposition. The Black Rock mine of the Butte & Superior Mining Co., the Elm Orlu of W. A. Clark & Son, the Alice, and several other mines of the Anaconda Copper Mining Co., and the North Butte Mining Co. are among the most important producers. The twelve mines yielding zinc in 1915 together have immense reserves. The ores all carry lead and silver and some pyrite, and many contain copper and gold.
The Coeur d’Alene subprovince comprises a number of mining districts in Idaho, at least five being zinc producers. The Interstate-Callahan, in the Beaver district, is the largest zinc mine in the state. The ore is remarkable for the small percentage of minerals other than sphalerite, averaging 28 per cent. zinc.
The Northeastern province comprises important deposits at Franklin, New Jersey, and in the Adirondack Mountains in New York, and others of minor importance in the New England states. It is characterized by deposits of igneous metamorphic origin in Pre-Cambrian limestone. The Franklin deposits, in New Jersey, consist chiefly of franklinite, willemite and zincite in a gangue of calcite, rhodonite, garnet, pyroxene, magnetite and hornblende. Willemite is separated magnetically from these ores and used to produce a very high-grade spelter free from lead and cadmium and therefore in great demand for certain purposes. The other classes of ore are smelted for the production of zinc white and spiegeleisen. These mines, owned and operated by the New Jersey Zinc Co., have produced more than 1,500,000 tons of zinc in the form of spelter and zinc oxide. The Edwards-Balmat district, in St. Lawrence County, New York, comprises an area two to three miles wide and fifteen miles long, of Pre-Cambrian limestone. The ore occurs in lenses and is a mixture of sphalerite, pyrite and a little galena with a gangue of dolomite. Separation is effected by magnetic tables. The typical ore contains: sphalerite, 25.5 per cent.; galena, 1.43 per cent.; pyrite, 12.4 per cent.; barite, 3.9 per cent. The ore reserves of the Northern Ore Co., the largest operator, are known to exceed one million tons.
—Imperial Germany comprised most of one metallographic province of major importance, Silesia, and other districts ranking as follows: Upper Silesia; Rhenish Prussia; Westphalia; Saxony; Hanover; and Nassau.
[302]
The major part of the mineral province of Upper Silesia lay within the boundaries of Germany in 1914. Once it was part of the Kingdom of Poland, except for portions included in the old empires of Russia and Austria. The pre-war production of zinc ores from Russian Poland was entirely from this metallographic province. The deposits, which contain lead and zinc together, occur in Triassic formation overlying Carboniferous rocks that carry important seams of coal. This juxtaposition of ore and fuel has furnished an ideal basis for the great smelting industry that has been developed, and facilitates the smelting of low-grade ores. The ores are said to average 17 per cent. zinc and 5 per cent. lead. They come from two ore horizons. The lower is characterized by blende, with a little galena and marcasite; the upper or lead horizon comprises a very persistent sheet of galena 0.05 to 0.30 meters thick, which generally is underlain by red calamine. The blende deposits are extensive and will be productive for a long time.
In Rhenish Prussia, zinc ore (smithsonite) deposits are found in strata of Devonian age. These deposits are approaching exhaustion. The chief zinc deposits of Westphalia are in Devonian strata. The historic mines at Freiberg (Erzgebirge), in Saxony, produce a small quantity of blende from the concentration of galena ores. The blende carries considerable iron and silver and some of it contains traces of tin. These mines are controlled by the Saxon government.
A considerable quantity of blende ore is concentrated as a by-product in the dressing of the lead ore of the Upper Harz, Hanover. The ore of Rammelsberg, in the Lower Harz, occurs in a bed interstratified with lower Devonian slates and sandstones; it is an intimate mixture of blende, galena, pyrite, chalcopyrite, and barite and some calcite and quartz. Zinc is produced as a by-product of lead ores in the valley of Lahn (Nassau), where a series of strong veins are found in Lower Devonian strata.
—The zinc resources of the Commonwealth of Australia are chiefly in New South Wales and Tasmania. The former has been the chief source of zinc in the past, but the Tasmania deposits are now being rapidly developed and equipped for production.
The most important source of zinc ore in New South Wales is the great Broken Hill lode, where operations began in 1884. The country rock comprises crystalline schists and gneisses. Between the oxidized and primary sulphide ores was a thin zone of secondary sulphides. The early operations in the district were for lead, and immense dumps accumulated of zinc-bearing ores sorted out or of zinc-bearing tailings from the concentration of the lead ores. In 1903 these dumps were estimated at 5,687,400 tons carrying 18.6 per cent. zinc. With the development of demand for zinc sulphide ores and of oil flotation methods of separation and concentration these dumps became important sources of zinc concentrates, but many of them are approaching exhaustion. The sulphide[303] ores are a close mixture of galena and zinc blende, carrying silver. There are two classes of these ores, distinguished as silicate-gangue ore, and calcite-gangue ore. The silicate gangue ore bodies carry rhodonite, garnet and quartz and are richer in zinc and silver than those of calcite gangue.
Eight mining companies are now at work. In the order of the importance of their output and ore reserves, these companies are: Broken Hill South Silver Mining Co.; Broken Hill North Mining Co.; Zinc Corporation; Sulphide Corporation; British Broken Hill Proprietary Co.; Broken Hill Proprietary Co., Block 10; and Broken Hill Proprietary Co., Block 14. The estimated ore reserves of all the mines approximate 12,000,000 tons.
The Broken Hill South Silver Mining Co. has ore reserves estimated at 3,350,000 tons, and is the largest producer. Broken Hill North, Amalgamated Zinc (de Bavay), Zinc Corporation, and Barrier South Ltd. are controlled by Govett and associates, a group of Australian capitalists. The Amalgamated Zinc Co. in 1913 treated 498,289 tons of tailings, obtaining 140,098 tons zinc concentrates, carrying zinc, 48.9 per cent.; also lead concentrates amounting to 1,584 tons, carrying 57.1 per cent. lead. The Zinc Corporation, a company formed by Bewick, Moreing Co., has ore reserves estimated at 1,504,211 tons, averaging 14.8 per cent. lead, 9.2 per cent. zinc, and 2.5 ounces of silver per ton. The mine of the Broken Hill Proprietary Co. is, according to last reports, nearly exhausted.
The principal deposits of Tasmania are those of the Primrose, Hercules, and Tasmania Copper Mines, all owned by the Mount Reed Rosebery Co., affiliated with the Mount Lyell Mining & Railway Co. The state geological staff estimates reserves at 1,272,500 tons averaging 29.79 per cent. zinc, 8.89 per cent. lead, 12.16 ounces of silver and 0.17 ounces of gold per ton.
—The zinc production of Italy is derived from the Iglesias district of Sardinia, and the Province of Bergamo. The Iglesias district is in the southeastern part of the island of Sardinia. The ores are oxidized and mostly worked by open pits. They are mined and milled by two Italian companies, Societa di Monteponi, and the Societa di Pertusola. The ores have usually been smelted in Germany, England or Belgium. The Bergamo mines, in the Province of Lombardy, are worked by the English Crown Spelter Co., which ships the ore to Swansea, Wales, for smelting.
—Algeria produced 82,256 tons of zinc ore in 1913. Of the 78,973 tons whose origin is known, 31 per cent. of the total was extracted by Belgian companies and the remainder was produced by French operators.
—The only important zinc-producing district in Japan is the Province of Hida. The principal companies are the Osaka Zinc Mining[304] & Smelting Co., Takata & Co., the Rhuara Mining Co., and the Mitsui Mining Co. The largest producer is the Kamioka Mine of the last named, which produces annually about 10,000 tons. The Osaka Mining Co. also produces from Korea (Chosen) about 15,000 tons of ore annually.
The annual smelting capacity in Japan, with all projected construction completed, is estimated at 300,000 tons of zinc ore, whereas the domestic output of ore is about 50,000 tons. The difference has been imported chiefly from Siberia, China, Tonkin and Australia. In the future it is expected that the foreign ores will come chiefly from China and Siberia. The domestic spelter production has reached about 60,000 tons annually and the domestic consumption 29,000 tons.
—Zinc ores are produced in the provinces of Santander, Murcia, Tereul, Biscay and Guipuzcoa. The only district of importance is that of Santander, where there is a zinc smelter owned by the Compagnie Royale Asturienne des Mines (French). Some of the ore is smelted in France. Most of the Spanish ores are calamine and occur almost without exception in limestone. Eighty per cent. of the Spanish production comes from Santander and Murcia. In the latter the mines are worked primarily for lead.
—The zinc output of the Russian Empire was derived from Russian Poland, eastern Siberia, the Altai Mountains in southwestern Siberia, and the northern Caucasus Mountains. The Polish deposits are part of the Silesian field. The ores are largely carbonates and silicates. Some of the mines and plants were owned by the Russian government, others apparently by French companies. In eastern Siberia, the Tyuticha mine has a calamine orebody containing at least 200,000 tons averaging 48 per cent. zinc. Some ore has come from the Ussurisk district. It is believed that the Mitsui Mining Co. has made arrangements to ship ores from this district to Japan for treatment.
In the Altai Mountains of southwestern Siberia the Ridder Mining Co., controlled by the Irtysh Corporation, Ltd., of London, has developed two large deposits on the same mineralized zone, with ore reserves estimated in 1917 at over 3,500,000 tons. The possibilities of this property are immense. The company has acquired the Ekibastus coal fields, constructed about 165 miles of railroad and provided river transport, thus bringing the ore and coal together at a smelting plant having a capacity of 15,000 tons lead and 5,000 tons spelter annually.
In the northern Caucasus Mountains, the Sadon mine, belonging to the Société d’Alagir (French), has been worked for a long time. The ores from this mine are smelted locally.
—The zinc-ore production of France comes from several scattered districts. Of the 45,929 long tons reported in 1912, nearly all came from six mines which are all controlled by French capital with perhaps some Belgian participation.
The only important source of zinc in Greece is the famous Laurium[305] deposit, which was worked in ancient times. The ancients, however, rejected, as far as possible, the zinc ores. These deposits are now controlled and operated by a French company, the Compagnie Française des Mines de Laurium, which also has reopened the ancient workings of Gebel Rosas, near the Red Sea, in Egypt.
The chief zinc mines of Sweden are the Ammeberg, the Rylls Wytland and the Sala. At the Sala immense piles of tailings made in centuries of operation, containing quantities of zinc, can now be treated, as well as zinciferous areas left in mining silver-lead ores. The important Ammeberg deposit consists of bands and lenses of disseminated blende in gneissoid granulite and is exploited by the Société de la Vieille Montagne (Belgian).
The zinc production of Mexico has come chiefly from the states of Nuevo Leon, Chihuahua and Sonora. Various zinc deposits have been worked in Nuevo Leon by German and American capital. Most of them are within 50 miles of Monterrey. Zinc-producing districts are scattered over the State of Chihuahua. In the Santa Eulalia district, El Potosi Mining Co., controlled by Americans, works the mine of the same name, which has carbonate ores to a depth of 1,700 feet. The Buena Tierra, in the same district, is controlled by British capital. The principal mines in the Parral district are owned by Americans and British companies, including the American Smelters Securities Co., American Zinc Extraction Co., and San Francisco Mines of Mexico, Ltd. (British). The Carnegie Lead & Zinc Co. owns the largest zinc mine in Sonora, located near Cananea. The Calumet & Arizona Co. and the Mexican Metal Co. own zinc deposits in the Arizpe district. All of these companies are American.
Zinc ores are produced in Austria in the provinces of Carinthia, Styria, Carniole, Tyrol and Galicia. The Raibl and Schneeberg mines of Carinthia are government owned, and the ores are smelted at the government works at Cilli. Tyrolean ores were shipped to Frankfort-on-Main and Aix-la-Chapelle. Styrian ores were shipped to Silesia and Rhenish Prussia for smelting.
The considerable production of calamine with some blende from Tunis is under French political and commercial control. The chief zinc ore producers appear to be French companies, in general paying a royalty of 5 per cent. of the net proceeds to the government of Tunis. The total production for 1916 was 12,544 tons.
Four districts of Indo-China produce zinc ores with an aggregate total annual output of about 46,000 tons.
The largest zinc mine in Great Britain is the Nenthead, in Cumberland, worked by the Société de la Vieille Montagne (Belgian). The second largest producer is the Carshield mine, in Northumberland. With the exception of the Nenthead mine all the important producers are, so far as known, owned and operated by British capital.
[306]
The chief mine in China is the Shui K’ou Shan, under control of the Hunan Board of Mines. Prior to the war this mine was dominated by German capital, which had provided machinery and shipped the product to Europe. Japanese have sought diligently and with partial success to secure control of this mine.
The chief zinc-mining district in Bolivia is Huanchaca, the production of which has recently decreased because of great quantities of water entering the workings. The largest operator is the Compañia Huanchaca de Bolivia, the capital and control of which is French. Its zinc production is incidental, the principal metal produced being silver.
The zinc production of Canada is chiefly from British Columbia, but a small amount is from Ontario and Quebec. The only production of any moment is from the southern part of British Columbia, where the Slocan district is of greatest importance. British, Canadian and American capital are largely interested. In 1915, the mines of the Slocan district were estimated by the management of the Trail smelter to be capable of producing 10,000 to 15,000 tons of ore carrying 40 to 45 per cent. zinc. Apparently this was in addition to the possible production from the Sullivan mine, owned by the Consolidated Mining & Smelting Co., Ltd. (Trail smelter), which had proved reserves of 3,500,000 tons of galena-sphalerite ore. The principal mines of Ontario and Quebec produced 580 tons of zinc, or one-thirtieth of the production of Canada. These are operated at least in part by American capital, and the ores are smelted by the Zinc Co., Ltd., owned by Americans.
The chief deposits of India are those of the Bawdwin mines, located in the Northern Shan States (Burma). There was estimated December 31, 1917, 4,033,000 tons lead-zinc ore assaying 24.7 oz. silver, 27.4 per cent. lead, and 19.1 per cent. zinc. The essential constituents of the ores are galena and sphalerite with a little pyrite and chalcopyrite. The lead and zinc concentrates are available for the customary methods of smelting. A zinc-distilling and sulphuric acid plant is being constructed at Sakohi, with the aid of the Indian government, to treat the table zinc concentrate. The company operates a lead smelter at Nam-Tu, 11 miles from the mines. The Bawdwin deposits may be expected to be an important factor in the world’s production of zinc in the immediate future. They are owned by the Burma Mines, Ltd., an English corporation of the R. Tilden Smith-Govett-Hoover interests, in which some American capital is interested.
The only zinc deposit of note in Egypt is the Gebel Rosas, operated by the Compagnie Française de Laurium. This deposit is located near the Red Sea and was worked in ancient times.
The only deposit of importance in South Africa is the Rhodesia Broken Hill. The large ore bodies are, so far as developed, almost wholly oxidized. One ore body is estimated at 250,000 long tons, averaging 26[307] per cent. lead, 22¹⁄₂ per cent. zinc, and another at 300,000 long tons, averaging 32 per cent. zinc, with little lead, but much iron oxide and carbonate. They are controlled by British capital. Considerable difficulty has been experienced in developing a commercial treatment.
In Peru a French company, the Association Minière, has interests in the Compagnie des Mines de Huaron.
So far as information is available no marked change in the rate of production in the countries of Europe and northern Africa seems probable. Most of the districts in those countries have been exploited over a long period and have passed their zenith of production; many are approaching exhaustion. The decrease in most of them will, however, be gradual. The most important change in Europe probably will be the transfer to Polish jurisdiction of the whole of the Silesian field, making the new state of Poland, if established as proposed, the largest single source of zinc ore in Europe.
In the United States, which will continue to be the largest producer of zinc in the world, the greatest increase in relative importance may be expected from the western metallographic province, particularly in the northwest, in the Butte and Coeur d’Alene districts. The Leadville district has already passed the zenith of production and has dropped to second place after Butte, which is capable of still further increase.
In Australia the Broken Hill district may be expected to yield about 450,000 tons of concentrates annually for some three years, after which the output should drop to about 300,000 tons. The increase of production from Tasmania will largely depend upon the construction of electrolytic zinc plants, but the island will be a factor in production in the immediate future.
The greatest new factor in the world’s output of zinc will be the Bawdwin mines, in Burma, which within a short time will be equipped to produce about 300,000 tons of ore annually or about 75,000 tons of high-grade zinc concentrates and 100,000 tons of low-grade concentrates or middlings for treatment by the Ganlin process. The extent to which the zinc concentrates from this ore will be treated in India will be determined by the development of the local market for sulphuric acid. The remainder of the concentrates will be treated in England.
Recent developments in the Altai Mountains of southwestern Siberia have proved immense bodies of complex zinc-lead ores. The extent of their exploitation will, however, be determined largely by the extent of the Russian market as affected by social and political conditions. Removal of the stringent tariff on importations from abroad to the manufacturing[308] centers of Warsaw, Petrograd and Moscow might greatly restrict the market for the output of these mines. Their extreme geographic isolation will prevent the deposits from becoming an important factor in the world market for a long period, notwithstanding their large size and excellent grade.
The utilization of the Rhodesia Broken Hill deposit in South Africa will be delayed by the difficulty of separating the oxidized lead and zinc minerals, the lack of fuel, and geographic and commercial isolation.
Political control of the zinc resources of the world up to the outbreak of the war in 1914 seems to have had only a minor effect upon the industry. Economic factors made ineffective any control not international in scope. A very large percentage of the zinc ores of the world were transported from the country of production to another for treatment, in some cases even being re-exported, sometimes after calcination, for the purpose of utilizing the sulphur content in the production of acid. Tariffs were imposed by some countries, as, for example, by the United States, on certain classes of zinc ores. Such measures had some effect on production in Mexico and Canada. Russia had imposed heavy import duties which subsidized domestic production and stimulated exploration and development. The chief European countries importing and smelting zinc ores admitted them free of duty.
During the World War, however, political jurisdiction was largely invoked to restore control of national resources to citizens of the given country or its allies. This movement was particularly marked in the British Empire, wherein there now exists a joint political and commercial control. Alien interests have been eliminated by government action and the government retains a share in the control through interests in marketing organizations or through financial participation in treatment works. Canada has established a small bounty on zinc produced in Canada from domestic ores and given financial aid to attempts to establish domestic smelting and refining plants.
In the United States the Alien Property Custodian has been active in eliminating all alien enemy control. His appointees will control many important companies for several years. Such action as has been taken does not appear to have disturbed such centralization of control as had been effected.[138]
[138] See Chapter on Copper, pages 232-235, for discussion of German interests in the American metal trade.
Copper, lead and zinc form a class by themselves as regards industrial utility and tonnage produced, ranking after iron, which far outclasses[309] them, as the most important base metals. The annual production of each is considerably more than a million tons. In view of the industrial importance of zinc and the fact that brass, an alloy of copper and zinc, is an indispensable material in modern industry, it is not strange that the great industrial nations have contrived many expedients in the endeavor to control the zinc industry.
The ownership and operative control of the mines has been a minor factor in the commercial control of this industry. Economic factors force the location of retort smelting plants in industrial districts adjacent to coal fields, the most important factors being availability and cost of fuel and labor, and proximity to a market for spelter. A lack of fuel and scarcity or high cost of labor has prevented smelting in some regions producing considerable ore. On the other hand, the smelting done in some countries has been entirely disproportionate to their ore output. The revival of nationalist sentiment as a result of the World War may make ownership and operative control a more important factor in the future.
Electrolytic and electro-thermic reduction of ores makes possible the economic production of spelter where previously impossible and may bring about some decentralization of the industry.
Ownership or control of the reduction plants has been an important basis for control of the industry. The most effective control has been exercised through marketing organizations or trade combinations of reduction works, ore-buying and metal-selling agencies, with interlocking directorates, joint ownership and long-term contracts for ores, concentrates and metals. In recent years these became world-wide in their scope and completely dominated the industry.
—Below are listed the most important zinc smelting companies in the United States, together with the percentage of the total reduction capacity that each controls:
| Percentage of production controlled |
|
|---|---|
| New Jersey Zinc Co. | 13.4 |
| American Metal Co. | 15.2 |
| Grasselli Chemical Co. | 10.1 |
| Anaconda Copper Mining Co. | 10.0 |
| United States Steel Corporation | 7.7 |
| American Smelting & Refining Co. | 7.2 |
| American Zinc, Lead & Smelting Co. | 5.7 |
| Beer, Sondheimer & Co. | 4.7 |
| L. Vogelstein & Co. | 1.7 |
| Various independents or of unknown affiliation | 24.3 |
The close affiliations of L. Vogelstein & Co., Beer, Sondheimer & Co., the American Metal Co., and their subsidiary companies, comprising 27.3 per cent. of the smelting capacity, and various ore producers, with[310] their metal-selling contracts with other smelters, enabled them to dominate the American metal market in the interest of the German “Trio.” The Alien Property Custodian took over all three companies during the war and eliminated all alien-enemy ownership and control by disposing of German-owned stock at auction.
The American Metal Co. completely owns the American Zinc & Chemical Co., South American Metal Transport Co., Bartlesville Zinc Co., and South American Metal Co.; it has large holdings of stock in the Ohio and Colorado Smelting & Refining Co., Compañia Minera de Peñoles, Compañia de Minerales y Metales, Compañia Metalurgica de Torreon, Compañia Minera Palmo y Cabrillos, Fundicion de Guayacan, Balbach Smelting & Refining Co., and Nichols Copper Co., some of which operate in Mexico and South America. The capital stock of the American Metal Co. comprised 70,000 shares, of which 34,644 were owned by aliens affiliated with the German “Trio”; 18,620 shares belong to American citizens who have had control of the management, and the remainder belonged to the Merton interests, of London, which has been reorganized by the British government, eliminating alien ownership. The alien-owned shares have been sold by the Alien Property Custodian and the control of the business for a period of five years was vested in a board of five trustees named by him.
The National Zinc Co. was owned by Beer, Sondheimer & Co., one of the German “Trio,” which also owned other metal-producing companies in the United States and Cuba.
The operative control of the American Zinc, Lead & Smelting Co. has been acquired by the Butte & Superior Mining Co., dominated by Hayden, Stone & Co., of New York and Boston. A large interest in the American Zinc, Lead & Smelting Co. was held by L. Vogelstein & Co., formerly the representative of the German Metallgesellschaft in the United States. The American Zinc, Lead & Smelting Co. controls the Wisconsin Zinc Co.; the American Zinc Co., of Illinois; the American Zinc Co., of Tennessee; and the American Zinc Ore Separating Co.
The New Jersey Zinc Co., an American concern, controls the New Jersey field, and, through its subsidiary, the Empire Zinc Co., owns and operates a number of mines in the western states and Mexico, and the Mineral Point Zinc Co., of Wisconsin, one of the three largest producers in that region, and four zinc smelters.
The Anaconda company operates a number of zinc-producing mines in the Butte district and has erected electrolytic plants for the treatment of its own concentrates, one of 25-tons’ capacity as an experimental plant at Anaconda and a plant of 200-tons’ daily capacity at Great Falls, Montana. It has treated some of the production of other companies, notably of the Butte & Superior Mining Co. It is an American company under American control but has many European stockholders.
[311]
—The ambition of German commercial interests to control the metal markets and resources of the world was more nearly realized in the case of zinc than of any other metal.
The German Zinc Syndicate (Zinkhüttenverband) was organized in 1909, by three powerful interests: (1) The Merton group, comprising the Metallgesellschaft, the Metallbank and Metallurgische Gesellschaft, all of Frankfurt, and the Merton companies of London; (2) Beer, Sondheimer & Co., which through the Tellus stock company controlled over a dozen metal and chemical concerns; and (3) Aron Hirsch & Sohn, at Halberstadt; and was immediately joined by all the important Silesian and Rhenish-Westphalian zinc concerns. The organizers were made exclusive selling agencies for the syndicate and purchases from any foreign sources were made in unison. The syndicate immediately made agreements with Austrian and Belgian producers including the Vieille Montagne, which has mines, works and agencies in many parts of the world. It was also joined by a Dutch concern, Zincs de la Campine. The syndicate embraced altogether 18 firms and regulated both their output and prices. By the end of 1912 this German syndicate controlled directly one-half of the world’s output of zinc and three-fourths of the European production.
Two Belgian and some French works formed another syndicate. A third was formed by six English works, and the competition of United States firms drove all three into the International Zinc Syndicate, which endured up to April, 1914. The International regulated the output of its members but did not fix exact prices. Through the American Metal Co., and affiliated companies, the German syndicate was rapidly becoming a factor of importance in the control of American, Mexican, and Australian zinc production at its source. The German syndicate maintained its dominancy and was rapidly extending and strengthening it throughout the world through banks, holding companies, affiliations with syndicates and cartels, interlocking directorates, and joint shareholdings.
As a result of the war, the Australian zinc concentrates are permanently diverted from German reduction works, and all German control is eliminated. The activities of the Alien Property Custodian have tended to eliminate German interests in the United States. The re-establishment of the nation of Poland may take from Germany all the Silesian deposits and reduction plants, leaving only those of the Rhenish-Westphalian, Harz and Erzgebirge regions, with only one-third the former domestic production of ore. Poland should be twice as important as Germany in the zinc industry, unless Germany should be able to import ores and concentrates on a large scale.
—At the outbreak of the World War the Australian zinc industry was in the grip of the great German zinc syndicate, described in more detail above under “Germany.” This controlled the world’s[312] spelter market, determined output and prices and manipulated the market in the interests of Germany. This was possible through long-term contracts for zinc concentrates, in which form nearly the whole zinc product was exported for reduction in Belgium and Germany. Only 10 per cent. of the concentrates was smelted at Port Pirie or in England.
During the war these contracts were cancelled by the Australian and British governments and the work of reconstructing the industry on a purely Australian and British basis was undertaken, the idea being to provide for the treatment, so far as possible, of all ores in Australia, so that they could be marketed in their finished state. The Australian government first formed a metals exchange, through which all metals produced in Australia must pass for sale. The Zinc Producers’ Association Proprietary, Ltd., was registered May 20, 1916, in Victoria, Australia, to market products of the member companies producing zinc ores, concentrates, and metals in the Commonwealth of Australia and Tasmania, all of which have sold their entire output to the association for fifty years. The shares, 100,000 of £1 each, are held by the following companies: Amalgamated Zinc (De Bavay’s); Broken Hill Proprietary, Block 14; Zinc Corporation; Broken Hill Proprietary; Electrolytic Zinc Smelters; Junction North; North Broken Hill; Sulphide Corporation; British Broken Hill; Broken Hill Associated Smelters; Broken Hill Junction; Broken Hill South Silver; Broken Hill Proprietary, Block 10; Mount Lyell Mining & Railway Co.
The Australian federal government, acting through the Zinc Producers’ Association, contracted to sell to the British government the whole output of zinc concentrates in Australia for the period of the war and ten years thereafter. Previously the Prime Minister had contracted for the sale to the imperial government of 100,000 tons of zinc concentrates and 45,000 tons of electrolytic zinc and spelter for ten years. The arrangement provided for the British government taking the stocks of zinc concentrates on hand December 31, 1917, less a definite percentage reserve, and thereafter 250,000 tons per annum for the period of the war, and 300,000 tons annually for the following nine years. Provision was also made for supplying the requirements of the Australian zinc-refining works and the fulfilling of Japanese contracts during the period covered by the British contract. Under normal conditions the Australian output of zinc concentrates, averaging from 46 to 48 per cent. zinc, is about 450,000 tons per annum, valued at $25,000,000.
The effect of the above arrangements is to transfer permanently the control of the Australian zinc industry from German to British citizens under a system jointly commercial and political. The mines and works always were owned by British and Australian capital.
The Zinc Producers’ Association is co-operative in character, guaranteeing to all members equality of treatment irrespective of tonnage.[313] The commonwealth is represented on the board. The federal government is also encouraging in every possible way the establishment of reduction works, particularly electrolytic zinc works. It has been reported that the Burma Corporation was a stockholder of the Zinc Producers’ Association, but this report has not been satisfactorily confirmed. In case it should be true the association may ultimately control the sale of 85 to 95 per cent. of the zinc ores of the British Empire. Having smelting capacity for a considerable part of their production and acting as a unit in selling the surplus, the Australian zinc producers should hereafter be in a strong position in dealing with German, Dutch and Belgian smelters. The smelting capacity will not be largely increased in the near future.
—The nationalist movement in France during the war resulted in the formation of trade associations known as “consortiums,” comprising the principal factor in each industry.[139]
[139] For a description of the consortium covering the mineral industry, the Société Minerais et Metaux, see the Chapter on Lead, pages 284 and 285.
Since the elimination of the alien-enemy interests in the American Metal Co., L. Vogelstein & Co., and Beer, Sondheimer & Co., the industry in the United States is controlled and the mines and works are owned by American capital, which also controls some zinc production in Mexico, Canada and Peru. Future production and consumption will probably balance as before the war. There is now excess smelting capacity, but it has been conclusively demonstrated that the country is capable of supplying the ore for even greater capacity. The United States will retain first place as a producer. During the period of readjustment some concentrates may be shipped to Belgium and Holland.
With the permanent diversion of the Australian concentrates, and the probable loss of the Silesian deposits and reduction works, Germany will lose its second place in the industry. With only the Rhenish, Westphalian, Harz, and Erzgebirge districts as sources of domestic ores, the supply will be reduced to one-third of that produced before 1914, which was only two-thirds of all the ore smelted in the country. Unless ores or concentrates can be imported, Germany will be only a small factor in zinc production. Whereas in 1912 Germany had 50,000 tons of spelter available for export, importation may now be necessary. As a result of the treaty of peace the Polish industry may be dominated by French capital.
Not over 5 per cent. of the ore smelted in Belgium is of domestic production. The mines are owned by French and German companies. The works are owned by French, Belgian and Germans in about equal[314] proportions. Part of the Australian concentrates will be allotted to Belgium for smelting. The largest single factor in the Belgian industry, the Société de la Vieille Montagne, owned and worked mines in Belgium, Moresnet, Germany, Sweden, England, Algiers and Tunis and reduction works in Belgium, Germany and France. Although formerly a member of the German zinc convention this company seems to be controlled by Belgian capital affiliated with the strongest Belgian financial interests. Several other Belgian companies have important interests in France, Spain, Algiers, and Tunis. Close affiliation seems to exist, and may be expected to continue, between French and Belgian capital in the zinc industry.
Let us now consider the British Empire. The domestic production of zinc ores in the British Isles is insignificant, but the smelting works have made England an important factor in the industry, although before 1914 they produced only 32 per cent. of the zinc consumption of the empire. The capacity of these works, which are British owned, has been largely increased and new plants are being constructed to treat the concentrates from Australia and Burma, from which sources a supply more than sufficient is assured by the contracts of the imperial government with the Zinc Producers’ Association of Australia. The imperial government is interested in some of the plants. Reduction plants will also be in operation in Australia, Tasmania, India, and Canada. The excess Australian concentrates are to be allotted to French and Belgian works. With the organization now in effect, British domination of the European zinc industry seems certain.
The mines and works of Australia are entirely controlled by English and Australian capital. An organization under Australian government control has been made the sole marketing agency for the producers. The mines of India and Burma are English controlled and the smelter being constructed at Sakchi is partly financed by the Indian government. The mines of Canada are mostly British owned, although there are some American interests. The potential capacity of the mines is large. In spite of government subsidies, the capacity and future of the reduction works is uncertain.
French capital controls all the domestic works and mines of France; also those of Greece, Indo-China, Tunis, most of the mines of Algiers, where some Belgian capital is also interested, part of the Belgian and Polish mines and reduction works, some of the Spanish mines and smelters, and probably the Caucasus mines in Russia. France should be an important factor in the industry during the near future.
The largest mines of Italy are owned by Italian companies and some domestic reduction works are under construction by them. The chief producing company in northern Italy is English.
In Japan, Japanese capital owns all the domestic mines and works; also those of Chosen (Korea), and is rapidly securing control of the[315] deposits of China and eastern Siberia. The present smelting capacity is greater than domestic consumption and much larger than the domestic ore supply. Ore is imported from China, Siberia, Indo-China and Australia.
As to Russia, the ownership of deposits in the Polish regions was divided between Russians and French before 1914, and this condition presumably will be restored, modified by Polish political control. The Russian interests were doubtless under German influence. The Altai Mountains region is controlled by British capital and its development depends wholly upon how internal social and political conditions affect the domestic market to which the product of this region is limited by geographic isolation. Eastern Siberia seems to be rapidly coming under the commercial control of the Japanese.
Holland has no mines but has considerable smelting capacity. It is dependent on its neighbors for coal also. Its future is difficult to forecast. The mines and works of Spain are largely under French and Belgian control, which may be modified by contracts with English interests. The control in Norway and Sweden is not definitely known. It is nominally by local capital but some English and German interests are probable. The Sulphide and the Zinc corporations, both British, are reported interested in the Hydraulic Power & Smelting Co.
The mines and works in Austria, which were owned by the Austrian government and Austrian companies, perhaps under German domination, will now be distributed among two or three political jurisdictions. Little ore is imported or exported, and the region as a whole is not an important factor in the industry.
The mines in Mexico are largely owned by Mexicans and Americans. German control is reported to be strong and growing. The possibilities of ore production are large.
The essential uses of zinc are: in brass, the alloy with copper, an indispensable material in modern industry; in galvanizing as rolled sheets; and in desilverizing lead bullion. The consumption is greatest in galvanizing. The amount used in the form of rolled sheets will increase.
Zinc and lead are commonly associated in their ores and are widely distributed over the world, but the countries of largest ore production are, in order, United States, Germany, and Australia. Burma will soon be of importance. Siberia can produce much ore in the near future, but exploitation will be retarded by political conditions and geographic isolation. Canada, Mexico, Chile, Peru and Bolivia may be expected to increase their output.
The successful commercial application of electro-magnetic, electro-static, and more especially oil flotation processes to the separation and concentration of complex ores has made available vast resources, adding[316] to the list of regions of ore production and materially affecting their relative importance.
Economic factors, particularly availability and cost of fuel and skilled labor in retort smelting and market for spelter, determined the location of reduction works in populous industrial districts adjacent to coal fields. The mineral resources, however, in many instances occur in countries either undeveloped industrially or without abundant and cheap fuel, and a large percentage of the zinc ores are transported from the producing country to another for treatment. In the order of their importance the countries making the most spelter are: the United States, Germany, Belgium, Great Britain and France.
Commercial control of the industry has been based chiefly on the control of reduction plants and ore-buying and metal-selling organizations rather than on ownership of deposits, and to be effective it necessarily became international in scope. Before 1914 an organization, apparently international in character, but really dominated by Germans, had through control on this basis achieved a commanding position in the industry, influencing output and prices quite effectively. During the World War, however, political jurisdiction was largely invoked to restore control of the resources of a country and industries to citizens of that country or its allies. This movement has been particularly marked in the British Empire, wherein there now exists a joint political and commercial control through the Zinc Producers’ Association and contracts in which the governments are participants, and in France, where the Société Minerais et Metaux, under government auspices, comprises all the principal mining and metallurgical companies controlled by French capital and is the arbiter of the metal industry.
A decentralization of the industry and redistribution of reduction works is to some extent resulting from the successful application of electrolytic reduction and to the dissemination of knowledge of the practice of retort smelting.
The British Empire as a unit should be able to dominate the industry in Europe during the near future. France, through political and commercial control of Algeria and Tunis, and large financial control in Belgium, Spain and Poland, will be in a strong position. Belgium, with minor but widespread financial interests in mines and works in France, Germany, England, Sweden, Spain, Algiers and Tunis, is an important factor in the European situation. Its interests appear closely affiliated with those of France. The United States, with large reduction capacity and ore reserves, while maintaining its position as largest producer, is expected to supply domestic consumption but to export little, as was the case prior to the war. The position of Germany will depend mainly upon the supply of foreign ore, which may have to be imported largely from Mexico and the United States.
[317]
[140] The writer hereby expresses his thanks to Adolph Knopf, of the United States Geological Survey, and R. R. Horner, of the United States Bureau of Mines, for their assistance in the preparation of this article.
Tin, ordinarily considered one of the minor metals, is nevertheless a metal of prime importance in the world’s present state of development. In 1913 the value of the world’s output of tin was $131,000,000, which was greater than the value of the world’s output of either lead or zinc. Without tin it is very doubtful if the present methods of food packing and distribution could have been accomplished. The principal use of tin is in the manufacture of tin plate, from which are fabricated the so-called “tins” or “tin cans” that everyone knows. The second largest consumption of tin is for the alloys, solder and babbitt made with lead, and brass and bronzes made with copper. Minor amounts of tin are used for making fine metal tubing, tin foil, and collapsible tubes for packing such materials as dental and toilet creams, artists colors, etc. Tin is consumed by the makers of silk, principally to give weight and “rustle” to their product.
In 1917 the consumption of tin in the United States was approximately 93,000 tons,[141] of which 19,000 tons was recovered from scrap materials. Of the total consumption 31,000 short tons was used for making tin plate, 20,400 short tons for solder, 13,800 short tons for bearing metals, (babbitt, bronzes, etc.,), and 27,700 short tons for the many minor uses, items of which are 1,000 tons for the silk industry, 5,000 tons for foil, 4,000 tons for collapsible tubes, 3,000 tons for white metal.
[141] In this report the figures for tons refer to metric tons (2,204 pounds avoirdupois) unless otherwise stated, and are given in round numbers because errors in statistics and in conversion do not warrant closer figuring.
It is difficult to distinguish between the essential and the non-essential uses of tin in the industries. Surely tin plate is essential, yet some saving of tin containers was made during the war shortage by curtailing the use of tin and substituting paper and other substances for packages carrying non-perishable products. Solders, bearing metals, and bronzes are unquestionably essential, but variation in alloy specifications made possible, during the war period, a considerable saving of tin without detriment to[318] the results. In fact some of the standards set under the emergency were superior to those used before. Aluminum foil is to some extent replacing tin foil, but no suitable substitutes have been found for tin in the manufacture of collapsible tubes, which are necessities.
Over 70 per cent. of the tin produced in the world is won from placer deposits, although in the last few years the exploitation of tin-bearing lodes has become of considerable importance. Tin ores are intimately connected with siliceous igneous rocks. Practically all of the known lode deposits are either in or lie near siliceous igneous rocks such as granite, granite-porphyry, quartz-porphyry or monzonitic types. In Mexico and the United States unimportant tin deposits have been found in rhyolite. In only one or two places in the world are tin lodes known where siliceous igneous rocks do not show on the surface, and in these places geologic evidence points to the presence of granitic rocks at no great depth. In the world’s chief centers of tin production—the Malay Peninsula, Bolivia, Australia, Nigeria, Cornwall, and South Africa—the granitic rocks are everywhere in evidence, and the tin lodes are so closely related to these granites that there is no question of their origin.
Fluorine-bearing minerals such as fluorite and topaz, tourmaline, and the tungsten mineral, wolframite, are found in practically all tin deposits. Molybdenite and bismuth minerals are present in many tin deposits, though their distribution is not so general as that of the former minerals. Copper, lead, zinc, and iron sulphides, the latter often arsenical, are common in tin lodes, and quartz and feldspar are the chief gangue minerals.
It is generally accepted that the tin lodes were formed near the close of intrusive activity by the final differentiates of the acid magmas. These final solutions are notable for their pneumatolitic action and their ability to cause the profound change of granite to greisen and the formation of stanniferous pegmatite and quartz veins. Greisen, an alteration product of granite, consists of quartz, mica and varying amounts of topaz and tourmaline. It is commonly developed along fractures, and in favorable places large masses of rock may be greisenized.
Tin deposits are most often found as lodes, both fissure and pegmatitic, or stockworks, but some segregations are known. A peculiar pipe-like form of deposit is found at places in the Transvaal and Tasmania.
Generally tin deposits he close to the contact of intrusive and intruded rocks and are mainly found near the top of the intruding mass. It therefore follows that in deeply eroded granite masses the chance of finding lode tin deposits is smaller than where search is made in the tops of granitic intrusions. It has also been noted that deposits in intruded[319] rocks generally lie where the dip of the intrusive contact is low and are rarely present along a steeply dipping intrusive contact.
Practically the sole ore mineral of tin is cassiterite (tin oxide), which carries 78.6 per cent. of the metal. Cassiterite is known commercially by various names, such as tinstone, black-oxide of tin, black tin, or, where it occurs in placers, stream tin. The tin concentrates from placer mining normally carry 60 to 75 per cent. metallic tin, 70 per cent. being a fair average. The concentrates from the mills treating Bolivian lode tin make a product called barilla that averages about 62 per cent. tin; the concentrates produced from lodes in Cornwall average about 65 per cent.; and from the lodes and placers of the Malay Peninsula carry about 72 per cent. tin.
In many parts of the world the lodes do not carry sufficient tin to be worked profitably. In Cornwall and in Tasmania, lodes carrying about 1 per cent. of tin are being mined; but in general a content of 1 to 2 per cent. tin is the lower limit for commercial lode mining. In Bolivia the tin lodes average 5 to 8 per cent. tin and some bodies of ore carrying as much as 40 per cent. tin have been opened. In the places where low-grade tin ores have been mined the by-products, principally arsenic and wolfram, have helped to pay expenses, and most of these mines are advantageously situated with respect to transportation and supplies. In the placers of the Malay Peninsula, including Banca and Billiton, and those of Australia, which are worked by dredges, the tin content ranges from one-half pound to as high as 3 pounds, but averages less than a pound of cassiterite to the cubic yard. Advantageous location and cheap labor make profitable exploitation possible.
As will be seen from the map, Plate IX, tin deposits are found in every part of the world, though an inspection of Table 60 and figure 9 will show that the deposits within the British Empire are the most important sources of the world supply. Bolivia and the Dutch East Indies have been the chief producers of tin outside of the British Empire, though China and Siam are steadily gaining importance as tin producers.
—The British Empire has tin deposits in England, Asia, Australia, and Africa. The largest production is from the deposits in the Malay Peninsula. The African deposits, those in Nigeria and the South African Union, yield the second largest output of the empire, the Australian deposits rank third, and the Cornwall deposits, formerly the largest producer of tin in the world, now rank fourth.
—The Federated Malay States and the British Protected Malay States occupy the southern end of the Malay Peninsula. This region, which is entirely British controlled, produced for many years one-half of the world’s output of tin, but in the last few years the output has declined steadily. The decline seems to be due to the exhaustion of the easily worked placer deposits, though in 1917 and 1918 an additional cause was the scarcity of labor.
[320]
Plate IX.—Tin-producing localities of the world. By James M. Hill.
[321]
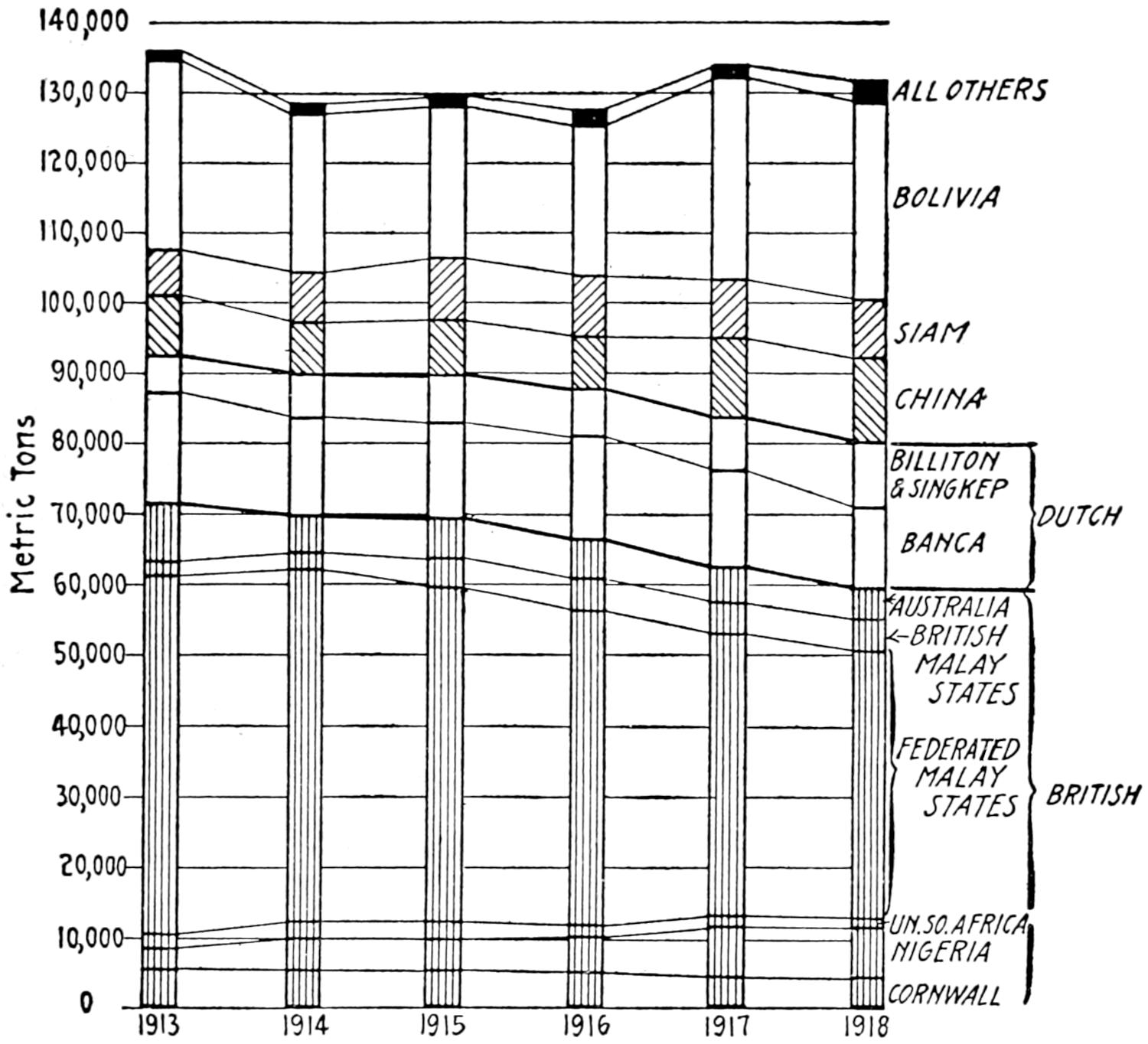
Fig. 9.—World production of tin, 1913-1918, in metric tons.
Table 60.—World’s Output of Metallic Tin, 1913-1918, in Metric
Tons[142]
(Metal obtainable by smelting from concentrates)
| Country | 1913 | 1914 | 1915 | 1916 | 1917 | 1918 |
|---|---|---|---|---|---|---|
| Cornwall | 5,370 | 5,140 | 5,060 | 4,770 | 4,000 | 4,000 |
| Nigeria | 2,950 | 4,590 | 4,630 | 5,150 | 7,070 | 7,000 |
| Union of South Africa | 2,050 | 2,000 | 2,050 | 1,900 | 1,540 | 1,500 |
| Federated Malay States | 50,930 | 49,820 | 47,520 | 44,570 | 40,470 | 37,970 |
| British Protected Malay States | 1,800 | 2,700 | 4,170 | 4,450 | 4,500 | 4,500 |
| Australia | 8,160 | 5,520 | 5,680 | 5,550 | 4,970 | 4,900 |
| Total British Empire | 71,260 | 69,770 | 69,110 | 66,390 | 62,550 | 59,870 |
| Percentage world total | 52.6 | 54.1 | 53.7 | 52. | 47. | 45.8 |
| Banca | 15,940 | 14,630 | 13,660 | 14,460 | 13,540 | 11,000 |
| Billiton and Singkep | 5,300 | 6,090 | 6,760 | 6,780 | 7,300 | 9,200 |
| Total Dutch | 21,240 | 20,720 | 20,420 | 21,240 | 20,840 | 20,200 |
| Percentage world total | 15.4 | 16.1 | 15.4 | 16.7 | 16. | 15.5 |
| China | 8,390 | 7,120 | 8,000 | 7,630 | 11,800 | 12,000 |
| Siam | 6,660 | 6,740 | 8,520 | 8,960 | 8,600 | 8,600 |
| Bolivia | 26,760 | 22,360 | 21,900 | 21,330 | 28,320 | 28,000 |
| Other countries | 1,400 | 1,500 | 1,500 | 1,700 | 1,800 | 2,000 |
| Total other control | 43,210 | 37,720 | 39,920 | 39,620 | 50,520 | 50,600 |
| Percentage world total | 32. | 29.8 | 30.9 | 31.3 | 38. | 38.7 |
| World total | 135,710 | 128,210 | 129,450 | 127,250 | 133,910 | 130,670 |
[142] Knopf, A., “Tin in 1918,” U. S. Geological Survey, “Mineral Resources of the United States in 1918.”
[322]
The largest tin-smelting center of the world is Singapore, where the Straits Trading Co., and the Eastern Smelting Co., both British owned, and a Chinese-owned smelter, have a combined capacity of 58,000 metric tons of metal a year.
A large number of the Malaysian mines are worked by Chinese, though much English and Australian capital is invested in tin mining companies in the Peninsula, and the financial control of the industry is in the hands of British subjects. Political control is exercised by a prohibitive export duty ($285 per ton) on all tin ore exported for treatment except to the Straits Settlements, United Kingdom, or Australia.
As will be seen from Table 60 the Federated Malay States produce much more tin than the Protected States. Practically all of the tin in the Peninsula is taken from placer deposits, some of which are still worked by hand methods, though part of the black tin is now being mined by dredges.
The backbone of the Malay Peninsula is composed of granite which is intrusive into limestone, shale, and quartzite. Tin has been found in place in practically all of the rock formations. Owing to the intense weathering and erosion of the tin-bearing formations great accumulations of detritus, more or less mixed with clay, all of which carry cassiterite, are found in almost all parts of the Peninsula. The original deposits are so softened by weathering that they can be worked by hydraulic methods.
The provinces of Perak, Selangor, Pahang, and Negri Sembilan, in the Federated Malay States, produce tin. The following table shows their relative importance.
Table 61.—Production of Tin in the Federated Malay States in 1917
| Metric tons | |
|---|---|
| Perak | 25,075 |
| Selangor | 10,595 |
| Pahang | 3,750 |
| Negri Sembilan | 1,055 |
In Negri Sembilan, quartz veins in decomposed pegmatite are worked by hydraulicking and the mixed tin-tungsten concentrate obtained is further separated by magnetic machines. The principal mines are near Titi and Seremban.
Pahang, on the eastern side of the mountains, has many widely scattered tin deposits, both lodes and placers. The chief workings at present are in the mountains near the Selangor boundary, at Bentong, Tras, and Machi. Some mining is also done at various places along the Kuantan River and its tributaries. Transportation is a serious item in working tin mines in Pahang.
Kuala Lumpur is the center of the more important tin-mining operations[323] in Selangor. Both decomposed lode-stuff and gravels are being worked. Near Serendah soft greisenized granite is worked by monitors. Near Tanjong, Malim, and on the Kalumpang and Selangor rivers in the northern part of the state both gravels and decomposed vein materials are worked.
The Kinta district, in the State of Perak, is the most important tin-producing area in the Federated States. A structural valley eroded in soft limestones between granite ridges is the location of most of the workings. The valley is filled with clays and boulder clays carrying tin, and the present stream channels are also stanniferous. Mining is in progress around 15 or more settlements in this district; much of the mining is by open cuts and dredges, but some lode mining is done on pipes in limestones. Next in importance to Kinta is the Larut district, northwest of the former. Placer deposits are the chief source of tin in the district but lodes are worked at Selama and Blanda Mabok. In the south of Perak, at Bruseh, stockworks in schist are worked by hydraulicking, yielding about three-fourths of a pound to the cubic yard of material worked.
Development of the tin deposits in the Protected Malay States has been hampered by transportation difficulties. Until recently the alluvial tin was won by crude native methods. The principal producing comes from the states of Johore, Kedah, Kelantan, Perlis, and Trengganu.
Near Setul, in the State of Perlis, peculiar gravel-filled caves in limestone have been mined for tin. Some of these caves have been followed for four or five miles. In the State of Trengganu, lode mining under European management is under way. The lodes seem to be decomposed stockworks in granite.
An insignificant amount of tin is mined from the beach deposits on the Island of Malacca, Straits Settlements. The tin was derived from schists intruded by granite in which there are many stanniferous veinlets.
—As will be seen from Table 60, the principal production of tin in Africa is from British Nigeria. The district was worked by natives in the early days, but no important production was made until 1904, after the subjugation of the Emir of Bauchi. The production of Nigeria has grown steadily till it reached 8,500 tons of concentrates in 1917. Seemingly all of the mines are controlled by British capital and the exports have been largely to the smelters in England.
The alluvial deposits of Nigeria are in the valleys of the Bauchi Plateau. Soda granite and pegmatites, intrusive into older crystalline rocks, seem to be the source of the cassiterite that has been concentrated by the present streams. Sluicing is the principal mining method, though some deposits are suitable only for dredging. Tin is also known in northern Nigeria in the Ningi and Burra hills, and other localities. In southern Nigeria tin has been found near Akwa-Ibami, in the Uwet district.
[324]
The tin output of the Union of South Africa is chiefly from the Waterberg-Zaaiplaats district, in the western Transvaal, a little tin being mined in Swaziland and the Cape province. The production has ranged from 2,950 tons to 3,450 tons of concentrate a year, most of which before the war went to England for smelting, but since 1915 to the Straits Settlements. A small smelter, rated at 250 tons a year, was built at Zaaiplaats in 1917; it is expected to supply the tin needed in South Africa.
The Waterberg district contains several tin fields. Tin ores are found in the Red Granite and Waterberg felsites, sandstones, and conglomerates. In the former the ores occur in pipes, in irregular bodies of altered granite, disseminated in the granite, in impregnations along fissures, and in pegmatites and quartz veins. In the Waterberg series the tin ores are in lodes, and in irregular lenses and pockets whose position is determined by fissures or bedding planes.
In the Potgietersrust district the principal mines are largely pipe deposits in the Red Granite. These pipes, which are very erratic in direction, range from a few inches to 20 feet in diameter; some have been followed for 3,000 feet. The filling material varies greatly, ranging from slightly altered granite to a greenish homogeneous rock; the outer zones are tourmaline-quartz rock. In the smaller pipes the cassiterite is fairly evenly distributed but in the larger pipes it occurs near the outer edges.
In the Nylstroom district the principal mines are working ore deposits in felsites and shales of the Lower Waterberg series. The deposits are brecciated country rock cemented by quartz, tourmaline, cassiterite, and fluorite.
The Warmbaths field includes several mines located along the junction of the Red Granite and the felsites. Tin is found in lodes in both types of rocks and some alluvial tin has been mined. In the Rooiberg field, the tin deposits are practically all in fissures in quartzite intruded by Red Granite. Tin occurs in the Red Granite 40 miles north of Pretoria.
In the Cape Province, near Kuils River, cassiterite is found disseminated in granite and in veins at the contact of granite and slates. Most of the small amount of tin won has been obtained from gravels derived from these deposits.
In northwestern Swaziland alluvial deposits have been worked for a number of years, producing around 500 tons of concentrates a year. At Forbes Reef, schists and slates have been intruded by granite and tin lodes are found near the contact of the two formations.
Tin has been reported in placer deposits in the Winnebah district and in pegmatite dikes in the Mankofa and Mount Mankwadi districts of the Gold Coast. Tin has been found in placer concentrates from streams in Nyasaland. Tin deposits seemingly of little value have been found in the Enterprise district, east of Salisbury, and in the Ndanga district, east[325] of Victoria, in Rhodesia. These deposits are stanniferous pegmatites which are found in schists near granite.
—Tin is produced in the following provinces of Australia: Tasmania, Queensland, New South Wales, West Australia, Northern Territory, Victoria, named in the order of their importance. In 1907, the output of Tasmania was about 14,000 tons of concentrates, but production since then steadily declined until it became nearly stationary at 3,000 tons annually for the last few years, and it is believed that this output can be maintained for some time.
There are tin-smelting works at Launceston, in Tasmania; Woolwich, near Sydney, New South Wales; and Irvinebank, near Herberton, Queensland, capable of producing over 4,200 tons of metallic tin a year. Of recent years tin concentrate is being sent to the Straits Settlements (Singapore) for smelting. The exports of metallic tin from Australian ports in 1917 came to about 3,100 tons.
Practically all of the mining companies are controlled by Australian and English capital, and as the tin is smelted either locally or at Singapore the total Australian output can be considered as under the direct political and commercial control of England.
The total production of tin ore from Tasmania[143] from 1880 to 1918, inclusive, is stated to be approximately 128,200 tons.
[143] Tasmania, Report Secretary for Mines for the year ending December 31, 1918, p. 46.
The most important tin mine is Mount Bischoff, 45 miles southwest of Emu Bay. The deposits, discovered in 1871, are credited with a total production of about 75,000 tons of tin ore. There are several deposits, soft altered quartz porphyries intrusive into schists. Topaz and cassiterite are disseminated in the porphyries, and veins carrying tin and wolframite, together with pyrite and arsenopyrite, are found both in the porphyry and schists.
The Shepherd and Murphy mine, near Middlesex, is in a zone of metamorphism at the contact of granite, intrusive into sandstone and quartzite. Tin, tungsten, and bismuth are produced from this ore. Placer tin deposits on the Ringarooma River (Derby district) supply about 1,000 tons of tin concentrates a year. The principal placer mines are the Pioneer and Briseis. Near Gladstone, placers and lode deposits carrying tin and tungsten are worked. At the Anchor mine, in the Blue Tier district, a tin-bearing granite averaging one-half per cent. tin is worked, but mining has not been profitable. The Renison Bell, Dreadnought-Boulder and Montana mines, in the North Dundas field, are in slates cut by dikes of quartz porphyry. Zinc, lead, and iron sulphides are important in the lodes. In the Heemskirt district, southwestern Tasmania, the tin deposits are in granite and in overlying slate and sandstone. At the Federation mine the ore is in a pipe measuring 25 by 15 feet at the surface, but contracting to only 1 by 5 feet at 115 feet down.
[326]
Tin was first produced in Queensland in 1872, and the total output, including 1917, is estimated to be about 144,008 tons. The chief producing districts are Herberton, Cooktown, Chillagoe, near the port of Cairns; Stannhills, Kangaroo Hills, and Stanthorpe, the latter being near the New South Wales border. In the Herberton-Cooktown districts the tin occurs in greisenized granite intruded into slates, schists, and quartzite; bismuth and tungsten minerals are associated with it. Placer deposits are worked by hydraulicking, and in places the tin-bearing greisen is broken down by hydraulic giants. In the Stannhills field, near Croydon, cassiterite is found in veins in granite with galena, sphalerite, and chalcopyrite. The Kangaroo Hills, 100 miles south of Herberton, produces both lode and placer tin. In the Stanthorpe district most of the output is from placer deposits, some of which are buried under basalts.
Tin was first mined in New South Wales in 1872, and the total production, including the output of 1917, is estimated at 84,230 tons of tin and 34,510 tons of tin concentrates.
The chief producing districts are the Vegetable Creek and Emmaville-Tingha-Inverell region, in the northeast near the Queensland border, and the Ardlethan district, 40 miles west of Temora, in the south. In the Emmaville-Inverell region the erosion of stanniferous greisenized granite, intrusive into slates, has resulted in a widespread distribution of tin placers, both in the present streams and in what are believed to be Tertiary stream beds that are now capped by lavas. The Vegetable Creek mines, near Emmaville, are typical of the older placer deposits. Since 1900, dredging has become important, and it is estimated that the dredge production up to 1917 was 18,854 tons of concentrates. Lode mining, although not as important, has been done in this district in pipes and stock works in granite; the typical fluorine-bearing gangue minerals are common, and tungsten, bismuth, copper, and lead minerals are found.
Tin was discovered in the Ardlethan district in 1912 in lodes in granite and schist. Molybdenum, bismuth, and tungsten are commonly associated with tin in the greisenized granite lodes. The Barrier district, in the western part of the province, has not been a large contributor, because of lack of water. Cassiterite is found in dikes of coarsely crystalline granite intrusive into greisen and mica schist.
In West Australia the most important tin-producing districts are Greenbushes, near the southwest, and Pilbara, on the northwest, though there has been a very small recovery of tin in the Murchison goldfield, and Coolgardie. In the Greenbushes district cassiterite is found in pegmatite and quartz-tourmaline veins in granite, but the tin won is from stream deposits and from laterite. In the Pilbara field the alluvial tin has been derived from pegmatite dikes that cut granite and metamorphic rocks.
The production of tin in Northern Territory has amounted to about[327] 200 tons a year, most of it being obtained from pegmatitic deposits in granite in the vicinity of Burrundie.
A few tons of tin concentrates are saved each year in the operation of gold placers in the Northeastern and Gippsland divisions of Victoria.
—The principal output of tin in India is from the Mergui and Tavoy districts, southern Lower Burma; Tharton and Amherst districts, northern Lower Burma; and the Southern Shan States. The production amounts to about 150 tons of metallic tin a year, and is sent to the Straits Settlements for smelting.
In the Mergui district cassiterite is found in alluvial deposits near granite hills, the granite being intrusive into sedimentary rocks of uncertain age. Tin ore is also found in pegmatite and quartz veins. In the Tavoy district tin is obtained as a by-product of wolfram mining. The deposits occur in pegmatite and quartz veins cutting granite and sedimentary rocks. In the Tharton district the tin-bearing alluvium is said to be rich and its development is awaited with interest. Production of tin began in 1912 from the deposits of Bawlake State, Karenni, Southern Shan States, and in 1917 these deposits were the chief producers in India.
—In the extreme southwest of England is the famous Cornwall tin region, which includes the Camborne, St. Austell, and Liskeard districts, in Cornwall, and the Tavistock district, in Devon. The mines have produced about 8,000 tons of concentrates a year, but at present the output seems to be diminishing; in 1915 the production of metallic tin was approximately 5,000 tons, but in 1918 was only about 4,000 tons.
Tin mining in Cornwall dates back to prehistoric times. In the sixteenth century the mines produced about 700 tons of tin a year; the maximum output was reached in the period 1860 to 1890, when about 10,000 tons was produced annually. It is estimated that the total output of tin from this district is approximately 1,750,000 tons. The mining companies are without exception controlled by British capital.
The second largest tin-smelting capacity in the world is in the Cornwall district. The following companies, Williams Harvey & Co., Penpoll, Cornish Tin Smelting Co., Copper Pass, Redruth Tin Smelting Co., and the London Smelting Co. operate smelters having a combined output of approximately 31,100 tons of tin a year.
The tin deposits of Cornwall and Devon lie about five masses of granite, which are intruded into slates (killas) and greenstones. Quartz porphyry dikes are closely connected with the granite, and the tin lodes are found in both slates and granite, being particularly abundant near intrusive contacts with low dips. The principal lodes are wide zones of fissured rock that are tourmalinized, the less important fissures containing tin and gangue minerals. Copper and tungsten minerals are produced[328] from these lodes, and arsenic is an important by-product of smelting. The lodes in slates are as a rule richer in copper than in the granite, and in depth the lodes contain a larger proportion of tin than nearer the surface. The mines about the Camborne granite mass yield about 85 per cent. of the tin mined, those about the Lands End granite mass 12 per cent., and the mines about St. Austell, Bodmin Moor, and Dartmoor about 1 per cent. each.
Practically all of the tin produced in recent years has been from lodes, but placer tin was mined near St. Austell. The lodes have been worked to a depth of 3,000 feet, which seems to be about the greatest depth to which commercially profitable ore extends. As considerable ground above this level remains to be developed, the district should be productive for some time.
—Outside of the British Empire the principal tin deposits of the world are in Bolivia, the Dutch East Indies, China, and Siam, named in the order of their importance as producers in 1918. There are small outputs of tin from deposits in Japan, Spain, Portugal, and the United States, and tin deposits are known in Germany, Italy, Russia, Belgian-Congo, and Southwest Africa.
—Practically all of the tin ore shipped from Bolivia is mined from lodes. Mining began late in the last century. Exports are in the form of barilla, a tin concentrate carrying 60 to 65 per cent. and averaging about 62 per cent. tin. The output has been steadily increasing, and since 1913 Bolivia has been the second largest producer of tin in the world. (See Table 60, and Figure 9). The majority of the companies working in Bolivia are controlled by Chilean or local capital, though a little English, French, Swiss, and German capital was invested in Bolivian tin mines before the war. Recently English and American capital has become interested in the deposits.
Prior to the war practically all of the barilla was sent to Germany and England to be smelted, but lately exports have been to the United States and England. A small Chilean-owned smelter, estimated capacity 900 tons a year, has recently been built at Arica to handle the concentrates from one of the larger mines.
There are four important tin-producing districts in east central Bolivia, in the provinces of Potosi, Oruro, and La Paz. The region lies on the high plateau (elevation, 12,000 feet) and the principal mines are near or in the mountains on the east of the pampa rather than in the western range of the Cordillera. Schists, slates, and quartzites have been intruded by acid igneous rocks, and the tin deposits are found in the granites, the quartz porphyries, and the sedimentary rocks near the contacts. The quartz veins are strong and carry between 3 and 8 per cent. tin in most of the productive mines, though some bodies of ore have carried as much as 40 per cent. tin. Some of the mines were worked[329] for silver by the Spanish, but the silver ores seem to be limited to the upper zones, the lodes becoming relatively richer in tin at depth. Wolframite and bismuth are won as by-products at some of the mines. Pyrite, sphalerite, chalcopyrite, and galena are usually abundant in the tin ores, and tourmaline and fluorite are not uncommon.
The Bolivian deposits are of considerable future importance. Many mines and prospects, either through lack of knowledge or finances, have not been developed; the local management of most of the mines has been notoriously poor; and it is thought that with proper technical direction the output of tin can be greatly increased.
—On the islands of Banca, Billiton, and Singkep, south of the Malay Peninsula, are important tin mines. As will be seen from Table 60 the output of tin from these islands has been approximately 21,000 tons a year. Mining began on Banka about 1718, but the Billiton deposits were not worked until about 1860. The mines of Banka are worked by the government, but on Billiton and Singkep the deposits are leased by private concerns, mostly Dutch. At Banka the Dutch government operates smelters having a yearly capacity of 16,000 tons. The concentrates produced on Billiton and Singkep are in part sent to the Straits Settlements for treatment, but some are smelted locally.
Practically all of the tin mined in the Dutch East Indies is from placer deposits, some of which are alluvial. There is, however, a little lode mining on Billiton. The cassiterite was formed in greisenized granite and sediments, and the original deposits are similar to those of the Malay Peninsula. A little tungsten and gold are obtained as by-products of the tin mining.
—Tin deposits in the Mengtze district, near Kochiu, Province of Yunnan, southeastern China, have been worked for many years. During recent years about 8,000 tons of tin have been exported, and it is known that considerable tin ore produced from these deposits is smelted locally, the metal being consumed in China. The exports go out through the French port of Haifong. The mining industry is entirely under Chinese control. Most of the tin ore is obtained by placer and open-cut methods from decomposed granitic and pegmatitic lodes which are found near the contact of granite that is intrusive into limestone. There are less important tin deposits in the Fuchuan and Tungchwan districts, the former producing a very pure metal.
The tin concentrates exported go mostly to Hong Kong and the Straits Settlements for treatment, so the Chinese tin output is more or less at the disposal of England.
—In that part of Siam lying in the Malay Peninsula, tin deposits, similar in origin and occurrence to those in the British provinces, are being worked, and as shown by Table 60 are yearly becoming larger factors in the world’s output. The largest operations are near Renong[330] and Tongkah, where dredging by British companies is active. The chief producing companies are Tongkah Harbor Tin Dredging Co., Tin Benbong, Bangnon Valley, Ronpibon Extended, Beebook Dredging Co., and Katoo Syndicate.
—The tin-producing localities in Japan are near Kagoshima, Satsuma, on Kyushu Island; about 50 miles north of Kobe in Tajima province; and near Nayegi, Mino Province, near the center of the main island. Placer deposits near Nayegi have yielded some tin, seemingly derived from pegmatite dikes in granite. The Akinobe mine, in Tajima Province, was developed as a copper mine, but about 1912 tin and tungsten minerals were found in the ore. The veins are in slates and quartzites intruded by diorites. It is said that in 1917 about 40 tons of mixed tin-tungsten ores was produced daily. A small smelter at Ikuno handles the tin concentrates and produces about 250 tons of tin a year. The Susuijama mine, in Satsuma, produces tin from veins, in shales and sandstones, that also carry lead and zinc. Apparently the output is smelted and used locally.
—In the provinces of Salamanca, Zamora, Orense, Pontevedra, and Coruña, northwest Spain, there are tin deposits. Lode deposits are found near the contact of granite intrusive into schists and gneisses, and placer deposits have been worked since ancient times. In 1913 about 6,700 tons of ore is said to have been produced, but since then the output has been around 100 tons a year.
—In Portugal, just south of the Spanish border, tin lodes in granite and slates have been found and placer deposits worked in the gravels adjacent to the lodes. The yearly output of these deposits is around 300 tons. It is reported that American capital is interested in some of the Portuguese tin and tungsten deposits.
—In the United States the domestic output is only nominal, being equivalent to 60 to 100 tons of tin a year. The productive deposits, placers worked by dredges, are in the York district of Seward Peninsula, Alaska. They occur near the contact of granite intrusive into limestones, in peculiar rocks of contact metamorphic origin.
Cassiterite has been mined from gravels derived from pegmatite dikes intrusive in pre-Cambrian rocks of the Black Hills near Tinton and Hill City, South Dakota, and various attempts have been made to mine the lode deposits. These deposits are of more scientific interest than commercial importance. A little stream tin has also been mined on the North Carolina-South Carolina boundary near King’s Mountain, the cassiterite being an original constituent of pegmatite dikes intrusive into pre-Cambrian schists. At Irish Creek, Rockbridge County, Virginia, there are known stanniferous veins in coarse granites. In the Franklin Mountains 14 miles north of El Paso, Texas, quartz veins in granite[331] carrying cassiterite were worked at one time but have not been productive of late. In the Temescal Mountains, Riverside County, California, small quartz veins carrying cassiterite are found in acid granitic rocks that are intrusive into metamorphosed sediments. Considerable work was done in this locality in the years 1880 to 1890, but the irregularity of the deposits and their low tin content do not hold much promise for future production.
Prior to the war the United States, although the largest consumer of tin in the world, produced practically no tin ore, and imported only metallic tin, having no smelters for treating tin ore. Since 1916 smelters have been erected by the American Smelting & Refining Co., and the Williams Harvey Corporation, their estimated capacity being 18,000 tons of tin a year. Presumably these smelters must rely largely on Bolivian concentrates.
—Germany has produced practically no tin ores in recent years, though the country had a smelting industry, estimated at about 16,000 tons of tin a year, dependent on foreign ores. The normal imports of tin ore before the war were 17,000 to 18,000 metric tons a year, most of which came from Bolivia.
In the Erzgebirge, on the German-Austrian frontier, in the Altenberg-Zinnwald district, there was formerly some tin mining. The deposits, which are typical greisen lying near the tops and sides of bodies of granite intrusive into schists and gneisses, have made almost no production for several years and they are considered to be exhausted.
—At Campiglia Marittima, Tuscany, iron and tin have been produced from veins in limestone and shale. The output is variable and cannot be relied upon.
—In the former Empire of Russia tin has been found in the Trans-Baikal Province, Siberia, and in the Urals and Finland in European Russia. The Siberian deposits are placers in the basin of the Onon River. A German company was formed before the war to work lode tin deposits near Olovianoy, southwest of Nerchinsk, in the Urals. The Finnish deposits are at Pitkaranta, north of Lake Ladoga. The ores are a mixture of magnetite, cassiterite, and chalcopyrite, occurring in altered limestone and schist.
—Alluvial tin derived from veins in granite and intruded sedimentary rocks has been found along Lualaba River and on Busanga Ridge. There are no records of production, but the field holds considerable promise.
—Cassiterite occurs in pegmatite, intrusive into granite, in the Erongo Mountains east of Brandberg, and some placer tin deposits have been worked. On the whole the region does not seem to be particularly promising.
[332]
It seems reasonably certain that England is in a position to keep producing a large part of the world’s tin for some time, the lessening output from the Malaysian provinces being offset by the increased production of the African colonies. Both Cornwall and Australia, it is believed, will be able to maintain for a number of years a rather steady output of about the present size.
Bolivia will doubtless be able to increase her output of tin, and probably both Siam and China can be expected to produce larger quantities in the future. Production of the Dutch East Indies can probably be maintained at about its present rate for a number of years.
As will be seen from Table 62 and Figure 10, Great Britain controls politically over 50 per cent. of the tin output of the world, in that her political influence is absolute in England, Africa, Australia, and all of her colonial possessions on the east side of the Indian Ocean; and there can be little doubt that the strong British policy with regard to the eastern colonies is also potent with respect to Siam and China.
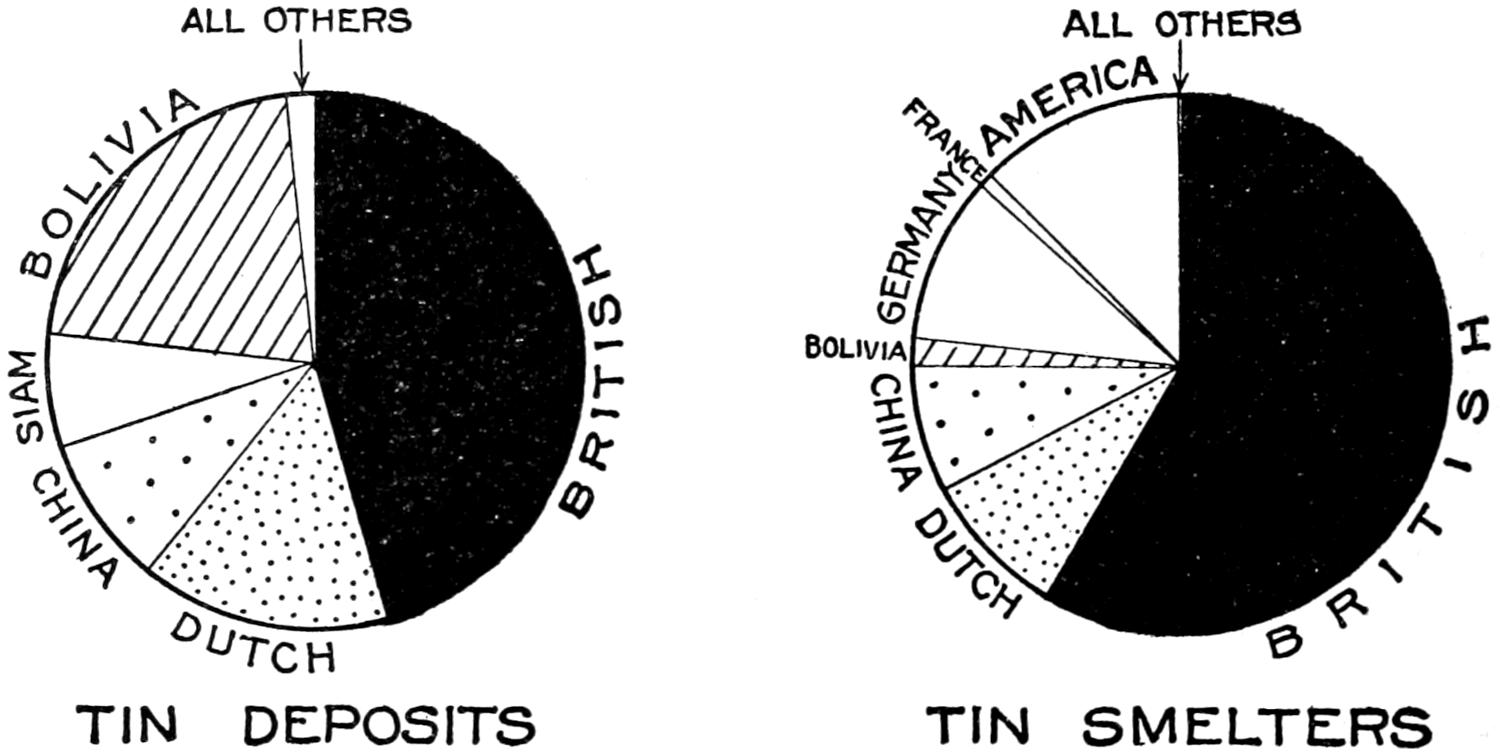
Fig. 10.—Political control of tin deposits and tin smelters, based on estimates for 1918.
Holland controls the tin output of the East Indian island colonies, in which there are smelting works that seem capable of taking care of most of the ore mined. Holland consumes little tin herself and has approximately 16 per cent. of the world’s supply at her disposal. Prior to the war Holland was a large distributor of tin, but during the war tin from her colonies was sent direct to America and England, the largest consuming countries.
China has a rather feeble political control of the output of the Yunnan[333] tin mines, but as that part of her production which reaches the rest of the world is exported through French territory, largely through English middlemen, her actual control is not particularly great.
Siam controls some important tin fields. The very strong British influence on the Malay Peninsula, coupled with the fact that the Siamese ore is smelted in the Straits Settlements, seems to indicate that British policy will largely dominate the tin-mining industry of Siam.
Bolivia, using little tin and producing nearly a quarter of the world’s output, is really the only considerable producer that can act more or less independently. Her mines are mostly controlled by Chilian-Bolivian capital and she has the world for a market. It would seem that Bolivian barilla might be smelted locally, but as Bolivia has no fuel, the tin smelting capacity of Bolivia amounts thus far to almost nothing. Her nearest market at present is the United States, but the future will show whether Bolivian ore will continue to be smelted in the United States, as during the past few years, or will be sent to England and Germany, as before the war.
The relation of political control of tin deposits and tin smelting is shown in the following table, and diagrammatically in Figure 10.
Table 62.—Political Control of Tin Deposits and Smelters Based on Estimates for 1918
| Country | Control of tin deposits, annual output (metric tons) |
Percentage of world output |
Control of tin smelters, annual capacity (metric tons) |
Percentage of world capacity |
|---|---|---|---|---|
| Great Britain | 59,900 | 45.5 | 88,300 | 57.3 |
| Holland | 20,200 | 15.2 | 16,000 | 10.2 |
| China | 12,000 | 9.3 | 12,000 | 7.4 |
| Siam | 8,600 | 7.0 | ||
| Bolivia | 28,000 | 21.3 | 2,700 | 1.6 |
| Germany | ... | ... | 16,000 | 10.2 |
| France | ... | ... | 1,500 | 0.7 |
| America | ... | ... | 18,000 | 12.4 |
| All others | 2,000 | 1.7 | 500 | 0.2 |
| Total | 130,700 | 154,000 |
British capital is the dominant controlling factor of approximately 57 per cent. of the world’s tin output, and through affiliations with capital of other countries it has a partial control of about 15 per cent. more. British capital is dominant in all of the British possessions and Siam, and through buying agencies practically controls the export tin from China. Bolivian tin mines are the only ones in the world in which British[334] control is not strongly felt. The largest part of the Bolivian output is under the financial control of Chilean financiers, with local capital the next strongest factor. French and German money has been invested to a limited extent in Bolivian mines.
Recently the firm of Guggenheim Brothers, of New York, connected with the American tin-smelting industry, has acquired certain tin mines in Bolivia.
British capital controls tin smelters with a yearly capacity of approximately 88,300 tons of tin a year. These are situated in England, Straits Settlements, and Australia. The tin deposits of undoubted British control can produce ore to furnish only 62,550 tons, so that England has a smelting capacity of 15,750 tons a year in excess of her supply.
The Dutch control the smelters, having a capacity of 16,000 tons, of their East Indian colonies, but the annual output of ore from these colonies is equivalent to 20,200 tons of tin, so that an excess of 4,200 tons must be smelted elsewhere, and most of this goes to the smelters in the Straits Settlements, which are English owned.
Chinese capital controls smelters that are seemingly capable of handling the entire output of China, about 12,000 tons of tin a year.
American capital, since the war, has developed tin smelters in the United States and Bolivia, which have an annual capacity of 18,000 metric tons. This capacity is being enlarged and should be able shortly to take care of the entire Bolivian tin output, provided it receives the ore. But Chilean capital has built a smelter at Arica which could handle about 10 per cent. of the Bolivian output, and if this smelter is favored by Chilean mine owners the American smelters may find themselves short of ore.
German capital is interested in tin smelters in Germany that have a producing capacity of 15,000 tons a year. All of the ore treated must be imported, but it hardly seems possible that much ore from outside sources can be expected for some time, as the smelting capacity of the world exceeds the output of the mines.
The tin-smelting capacity of the world is approximately 154,000 tons, whereas the world’s production of tin ore is equivalent to approximately 130,700 tons. It is evident that, unless greater production is forthcoming, some smelters will be idle, and it is a reasonable surmise that neither the British nor Dutch smelters will lack ore. The United States, owing to its favorable situation with respect to Bolivian supply, may hope to have a large part of its smelter capacity at work, though there is some question whether enough ore will be available to assure the maximum operation of the tin smelters in the United States.
[335]
Great Britain produces more tin than she consumes and is therefore in a position to dispose of tin to the rest of the world. From a study of import and export tables it seems that England consumes about 20,000 tons of tin a year and that she imports about 55,000 tons and therefore has 35,000 tons for export. She is in position through her large political and commercial control of tin deposits and smelters to practically dictate the world’s tin policy.
The Dutch colonies produce about 16 per cent. of the world’s tin, and as Holland is normally a very small consumer of tin, she has supplied a considerable part of the tin used in Germany and the United States.
Prior to the war a considerable tin-plate industry, dependent on foreign tin, was built up in southern Russia. The consumption was about 8,000 tons of tin a year, which was largely supplied by Great Britain, Holland, and Germany. If this industry is maintained Russia will still be under the necessity of importing considerable tin.
Tin users in Germany, who, before the war, apparently consumed about 22,000 tons of tin, must purchase all supplies from others. Before the war the principal supply of tin ore was Bolivia, and of metallic tin the Dutch East Indies. It seems reasonable that Germany’s supply of Bolivian ore may be curtailed in the future, as the United States is now in position to treat the ore, and freight rates should favor shipments of Bolivian barilla to the United States rather than to Germany. Whether the German tin-smelting industry will survive or not remains to be seen.
France has a small tin-smelting industry, treating about 1,500 tons a year. The apparent consumption is about 7,000 tons of tin a year, most of which was formerly imported from British India, England, and the Dutch East Indies.
The United States annually consumes over 80,000 tons of tin, including secondary metal, and produces from domestic ores about 100 tons. Prior to the war, metallic tin was obtained through England and Holland, as there were no tin smelters in this country. During the war there was established a tin-smelting industry, which is dependent entirely on foreign ore, most of which so far has come from Bolivia. The estimated capacity of tin smelters in America is about 18,000 tons a year or about 20 per cent. of the estimated yearly requirement. A combination of English, Bolivian and American capital is interested in one of these smelters, and also in Bolivian tin mines, and probably this smelter can be supplied. There is, however, considerable question whether the other smelters can obtain supplies of Bolivian ore. Certainly they will have competition from both English and German smelting concerns, which will be somewhat offset by cheaper freight to the United States than across the Atlantic. This difference is probably not large, and it would[336] seem that if American smelters are to get Bolivian tin ore their charges must be low. A surer method of meeting their ore requirements would be to obtain financial control of enough ore deposits in Bolivia to supply the demand.
Evidently the United States must in the future, as in the past, import considerable quantities of tin from both Great Britain and Holland. It is to be hoped that the tin trade routes established during the war may be maintained and that American consumers will not have to pay the additional charges necessitated by Eastern tin going to Europe and back to the United States.
[337]
Under normal conditions the chief uses of quicksilver (mercury) or its salts, stated in order of decreasing importance, are as follows: In the manufacture of drugs and chemicals, including calomel and corrosive sublimate; in the manufacture of certain chemicals, such as glacial acetic acid, phthalic acid and phthalic anhydride, into which mercury itself does not enter; as mercury fulminate ((C:N.O2)Hg, ¹⁄₂H2O), made by treating mercury with alcohol and nitric acid, which is used as a detonator for high explosives, and, though less than formerly, in small-arms ammunition.
The discovery of mercury fulminate by Howard in 1799 led to the invention of the percussion cap in place of the old flint-lock, and fulminate still remains the best-known and most-used detonator for gunpowder and high explosives. It is often combined with other substances, particularly an abrasive such as powdered glass, to increase its sensitiveness, and with compounds or mixtures that themselves have the property of detonating, such as sulphide of antimony and chlorate of potassium. Recently a large part of the mercury fulminate in detonators for modern high explosives has been replaced by picric acid, trinitrotoluene, or tetranitromethylamine, whereby a much stronger initial effect is obtained, and one part of mercury fulminate is made to detonate a charge that would have required six times as much fulminate used alone. Other substances have been found, which seem likely to replace mercury fulminate entirely for certain uses. One of these is lead azide, a salt of hydronitric acid. Large dry crystals of this salt are so sensitive as to explode when brushed with a feather, but smaller crystals are less sensitive.
As mercuric sulphide, mercury forms the brilliant red pigment vermilion. The metal is employed extensively in electrical apparatus, including rectifiers for changing alternating into direct current, mercury vapor lamps, and storage batteries. In the manufacture of felt hats from rabbits’ fur, mercuric nitrate is used to roughen the hairs so that they will adhere together, a process technically known as “carroting.” Metallic quicksilver is employed in the amalgamation of gold and silver ores, although of late years the wide application of the cyanide process[338] has decreased this use. The metal is also utilized in the manufacture of instruments, thermostats, gas governors, and other appliances. Mercury enters into the composition of some anti-fouling marine paints for ship bottoms, a modern and at present rapidly increasing use. The mercury for this purpose is generally employed as red mercuric oxide, its efficiency depending upon the gradual conversion of the oxide to the poisonous bichloride by the sodium chloride of salt water. Mercury is also used in certain compounds for preventing boiler scale, in cosmetics, and in dental amalgam. Silver nitrate has to a large extent replaced mercury in silvering mirrors. A small quantity of quicksilver, not more than two or three flasks annually, is used in floating certain types of revolving lights in lighthouses. Quicksilver is also used as the cathode in certain electrolytic processes for manufacturing chlorine and caustic soda from common salt. Mercuric oxide parts with oxygen readily and is a useful oxidizing agent in certain chemical processes. An important modern utilization of this property is in the manufacture of glacial acetic acid by the oxidation of acetylene.
Experiments to determine the possible advantages of using mercury vapor with steam in turbine power generators are reported to have been encouraging and a 4,000-kilowatt unit has been built by the General Electric Co. to test further this application. Except for incidental losses, the mercury so used is recoverable, but if in practice the increase of power is as much as the experimental work has indicated a large consumption of the metal is likely to result.
The production of quicksilver in this country in 1917 was 35,954 flasks (of 75 pounds) and in 1918 it was 32,883 flasks.
The ores of quicksilver, like those of most metals, show on the whole a close association with igneous rocks and with zones of fissuring. More commonly than with other metals, with the possible exception of antimony, they are associated with volcanism as opposed to plutonic igneous activity and were deposited comparatively near the surface. It follows that quicksilver deposits as a rule are found in regions of Tertiary and Quaternary volcanic activity which have not been subjected to long and deep erosion, that they are more likely to be in the younger geologic formations than in the older rocks, and that as a class, compared for example with the hypogene ores of gold or copper, do not extend to great depth. It must be noted, however, that there are some conspicuous exceptions to these generalizations. Although the California deposits are in a region of late volcanic activity and many of them are closely associated with active hot springs, the ore bodies that are now most productive, those at New Idria (Idria post office) and the great deposits, at New Almaden, that formerly yielded so richly, have no obvious connection with volcanism.[339] The greatest quicksilver mine in the world, that at Almaden, Spain, has no known connection with volcanism or massive igneous rocks, has been worked to a depth of 1,150 feet, and the ore bodies have been found to grow larger and richer downward. The deepest quicksilver mine in the world is the New Almaden in California, worked to a depth of 2,200 feet. The part of the mine below the 600-foot level was abandoned at a time when the price of quicksilver was low, but it is doubtful whether, under any conditions that can now be foreseen, it will be profitable to reopen and work the deep levels of this mine.
Although most of the known quicksilver deposits are in regions of geologically late volcanic eruptions it is probable that ores of quicksilver were deposited during or closely following epochs of similar igneous activity in the older geologic periods, but that many of them have been removed by erosion. Some of the deposits in the older rocks, which do not appear to be related to Tertiary or later volcanic eruptions, may have had such earlier origin.
The quicksilver deposits of the Adriatic region in Europe, including those at Idria, in Austria; Avala, in Serbia; and Monte Amiata, in Italy, have been shown by De Launay to belong to a single metallogenetic province characterized by Tertiary eruptions. Similarly, the somewhat scattered occurrences of quicksilver in Alaska, Washington, Idaho, Montana, Oregon, Nevada, Utah, California, Arizona, Mexico, Peru, and Chile coincide in part with the belt of Tertiary and Quaternary volcanic activity along the western sides of the continents of North and South America. The deposits at Almaden, Spain, in the Donetz basin, Russia, in Asiatic Turkey, and in China appear to be isolated occurrences that can not at present be assigned to recognizable provinces of eruptive activity and metallization.
Quicksilver deposits are not confined to rocks of any particular kind or of any particular geologic age.
At Oviedo the ore averages 0.33 per cent. and yields arsenic compounds as by-products. At Idria the ore yields 0.65 per cent. The ore of the Abbadia-San Salvatore, the principal mine in the Monte Amiata district, in Italy, yielded about 0.9 per cent. in 1915. In California few mines have over 2 per cent. ore, and the average yield of the ore worked is about 0.5 per cent. The lowest yield that was profitably obtained in that state in 1917 was 0.185 per cent. The ores worked in Texas are generally of higher grade than those mined in California. In the principal mine of the Terlingua district, Texas, the won tenor of the ore in 1916 was 2.5 per cent. and in 1917, 3.9 per cent.
—The largest and richest deposit of quicksilver ore known is at Almaden, in central Spain. There are three nearly parallel ore bodies[340] standing vertically side by side, each consisting of a portion of a bed of quartzite of Silurian age, impregnated with cinnabar. The ore bodies have been mined to a depth of 350 meters. The production in 1917 was probably about 25,000 flasks. The mine is said to have ore opened up that insures a future production of at least 40,000 metric tons of quicksilver. Other productive deposits in Spain are those near Oviedo, where the ore, which contains cinnabar, pyrite, orpiment, and realgar, is said to average about one-third of 1 per cent. of quicksilver, with arsenic compounds as by-products. According to a report from Vice Consul General H. A. McBride, written in Barcelona in 1911, the principal companies operating in the Oviedo districts were the Oviedo Mercury Mines Co., Ltd., of London, the Sociedad Fabrica de Mieres, of Oviedo, and the Sociedad Union Asturiana, of Mieres. The production from the district in 1915 was 608 flasks (20.7 metric tons). A third group of deposits lies on the south slope of the Sierra Nevada in the provinces of Granada and Almeria, southern Spain. The production from Granada in 1915 was 41 flasks (1.4 metric tons).
A small quantity of quicksilver was produced in Portugal in the nineteenth century from a mine not far from Lisbon. Cinnabar occurs at a number of localities in France and also in Corsica, but the deposits are not of economic character.
In South Germany, north of Zweibrucken, are quicksilver deposits that had considerable importance near the end of the thirteenth century, but at the beginning of the World War the mines had been closed for many years. Zinc ores mined near Bensberg, east of Cologne, yield annually about 90 flasks of quicksilver, won as a by-product in zinc smelting. In the former Austrian Empire the principal deposit is at Idria, about 28 miles from Trieste. The ore body occurs chiefly as an impregnation of Triassic dolomite and shale. The output in Austria in 1916, probably all from Idria, is believed to have been about 25,000 flasks. Reserves capable of yielding 20,000 metric tons, or 587,733 flasks, are known. At latest reports these mines were in the possession of Italy. At Zips, in northern Hungary, quicksilver is obtained as a by-product from iron ore (siderite) that carries mercurial tetrahedrite and some cinnabar. The production in 1913 was 2,615 flasks.
To the west of Idria, quicksilver deposits belonging to the same general belt of metallization extend into northern Italy. The principal deposit of this belt in Venetia is the Vallalta. The mine produced 9,550 flasks (325 metric tons) between 1856 and 1870, but has long been idle. The most productive deposits of quicksilver in Italy are those of the Monte Amiata district, in Tuscany, about half way between Rome and Florence. Monte Amiata is apparently a post-Pliocene volcano, and traces of recent volcanic activity survive. The most productive mine is the Abbadia-San Salvatore, which yields about 65 per cent. of[341] the output from the district, which in 1917 amounted to about 29,300 flasks.
At Mount Avala, near Belgrade in Serbia, deposits of quicksilver ore have been known since 1883, which resemble many of those in California. The Avala deposits were worked between 1889 and 1895, but seemingly have not been productive in late years.
The only quicksilver deposits of note in European Russia are those in the Donetz coal basin, southern Russia. The essential mineral is cinnabar, accompanied by stibnite and pyrite. The deposits were discovered in 1879, the maximum output, 18,102 flasks (616 metric tons), was reached in 1897, and work was abandoned in 1911, but has been resumed since in a small way.
—The Konia mine, in south-central Asia Minor, is in silicified limestone. The quicksilver occurs as cinnabar and most of the ore carries from 1 to 2.5 per cent. of the metal. The known reserves were estimated in 1908 at 13,000 metric tons of 1 per cent. ore. The production in 1911 was only 90 flasks of 75 pounds. The Kara-Burnu mine, said to be the only important quicksilver mine in Turkey, is situated southeast of Smyrna. In 1906 and 1907 the mine was producing about 3,000 flasks annually, but of late years the output has declined and in 1912 amounted to only 811 flasks (31 metric tons).
The Ildekansk quicksilver mine, in southeastern Siberia, east of Lake Baikal, has gained notoriety from the fact that political exiles were condemned to mine the ore. The deposit appears to be of slight economic importance.
That quicksilver deposits occur in the Province of Kweichow, south-central China, has long been known, but the locality is remote from ordinary routes of travel and comparatively little is on record concerning their character. The ore bodies of the Wan San Chang mines are the most extensively worked. For several years prior to 1905 the output averaged about 4,000 pounds of quicksilver a month. This would be equivalent to about 640 flasks annually. More recent figures of production are not available.
—The quicksilver deposits of North America are confined to the Cordilleran region from Alaska to Central America. The most productive deposits are in California and western Texas. In Alaska minerals containing quicksilver have been found in a number of the placer-mining districts, but deposits in place have been discovered in the central Kuskokwim region only. The ore occurs as cinnabar accompanied by stibnite, quartz, and a ferruginous dolomite. Development has been hindered by transportation difficulties, and only a few hundred pounds of quicksilver have been produced for local consumption. In Washington quicksilver ores have been prospected in various places, but the production is as yet inconsiderable. In Oregon cinnabar is[342] widely distributed, but only one deposit (at Blackbutte, in Lane County) is at present productive. In the Black Butte mine the ore averages about 0.25 per cent. of quicksilver, and the quantity available above the 500-foot level is estimated by the company at about 150,000 tons. The production of Oregon in 1917 was 388 flasks, all but 3 flasks being from the Black Butte mine.
In California the principal deposits occur in the Coast Ranges within a belt that is about 400 miles long and has a maximum width of about 75 miles. The known deposits within this area are numerous. About twenty-five of these are at present productive, while probably three times that number which were once productive are now idle. With a few exceptions, the deposits of this main quicksilver belt are in rocks of probable Jurassic age, or in serpentine which is the alteration product of peridotites. The most notable exceptions are the deposits of the Oceanic mine, San Luis Obispo County, and of the Sulphur Bank mine, in Lake County. Many of the most productive mines of the past have yielded no quicksilver from underground work for years.
The most productive mine in California at present is the New Idria, in San Benito County, which in 1917 yielded 11,000 flasks out of a total for the state of 23,733 flasks and for the United States of 35,954 flasks. The New Idria ore comes from two mines, the New Idria proper and the San Carlos. The New Idria has been extensively opened to a depth of about 1,000 feet. In the San Carlos practically all of the known ore lies within 200 feet of the surface. The two mines contributed nearly equally to the total production in 1917, and the average winnable tenor of the ore in that year was 0.32 per cent. It has been estimated that in the two mines there is available 2,400,000 tons of ore averaging 0.253 per cent. of quicksilver.
The New Almaden mine is in Santa Clara County. At present, all the levels below the 800-foot are under water and of late years very little ore has been taken from the old mine. Most of the recent production of the New Almaden Co., Inc., which for 1917 amounted to 2,683 flasks, has come from the El Senador mine, northeast of the old mine, and from quicksilver recovered from ground under old furnaces and condensers. In the New Almaden, the El Senador, and in the neighboring New Guadalupe, which produced 3,100 flasks in 1917, the ore occurs as irregular bodies in serpentine. Close to the mine now being worked by the New Guadalupe Mining Co., and owned by the same company, is the original Guadalupe mine, once highly productive but now long idle.
The Oceanic mine, in San Luis Obispo County, ranked fourth in productiveness in California in 1917, with an output of 1,246 flasks. The ore occurs as an impregnation of sandstone. The average winnable tenor of the ore in 1917 was 0.185 per cent. Other mines in California[343] which yielded from 500 to 1,000 flasks in 1917 are the Great Eastern, the Cloverdale, and the Culver-Baer, all in Sonoma County. Those whose output was between 400 and 500 flasks are the St. Johns, and the Helen, in Lake County.
Nevada contains many widely scattered deposits of quicksilver ore, no one of which has yet been worked on an extensive scale, although a few have been fairly productive for short periods. The ores occur in rhyolite of Tertiary age and in limestone or associated sedimentary beds of various ages from Paleozoic to Mesozoic. The total yield from Nevada in 1917 was 997 flasks, nearly half of which came from the Farnham and Drew mine, east of Mina, which closed for lack of ore near the end of the year. The next mine in point of yield, the Goldbanks, in Humboldt county, is also at present non-productive.
In Texas the principal quicksilver deposits are in the Terlingua district, in Brewster County. The ore occurs along fissure zones in Cretaceous limestones and shales, generally in proximity to intrusive rock. The principal mines are the Chisos, Mariposa, Big Bend, and Dallas.
In Mexico quicksilver deposits in the states of San Luis Potosi, Guerrero, and Durango are said to be yielding considerable quicksilver, even in the present disturbed condition of the country. A quicksilver dealer, testifying at the Tariff Commission hearing in San Francisco, on June 26, 1918, said that 400 flasks a month was being exported into the United States, but a considerable part of this was probably reclaimed quicksilver that has been used in the amalgamation of silver ores.
—Quicksilver deposits are known in Colombia, Ecuador, Bolivia, Chile, Brazil, Argentina, and Peru, but only those in Peru seem to be of present economic importance, and the production of that country in 1916 was only 62 flasks (2.1 metric tons). The most famous deposits in Peru are those at Huancavelica, particularly those of the Santa Barbara mine, on the east flank of the western chain of the Andes. These have been worked since 1566 and are said to have yielded 46,500 metric tons (1,366,480 flasks of 75 pounds) before 1790. The production in the 19th century has been estimated at 3,500 metric tons (102,865 flasks). The ore bodies are numerous, irregular, and occur in stratified rocks that are cut by igneous rocks. In 1916 the greater part of the quicksilver-bearing ground in the Huancavelica district was purchased by E. E. Fernandini, of Lima, and there appears to be some prospect of a resumption of active mining.
As with most metalliferous ores that have been formed later than the deposition or solidification of their inclosing rocks, the ores of quicksilver are most likely to be found in regions of eruptive activity and complex geologic structure, especially in regions of comparatively late volcanic[344] disturbance. It follows that new deposits are most likely to be discovered within the areas of Tertiary or post-Tertiary volcanic activity, as in the Cordilleran belts of North and South America, the eastern coast of Asia, certain parts of Oceania, and the shores of the Mediterranean. Alaska, Mexico, and the western part of South America seem to offer the greatest possibilities of future productivity, but there is little probability of any important changes in the sources of quicksilver taking place in the near future. The value of a quicksilver deposit can be ascertained as a rule only by mining exploration, and in very few quicksilver mines can any safe estimate be made of “undeveloped” ore. The known facts afford no secure basis for predicting that in the near future some now unimportant district will, within the next ten years, wrest the supremacy in production from Spain, or compete with Austria, Italy, California, or Texas. As regards the principal known sources, it appears that the high-water mark of productivity in California has long been passed, although the mines are still capable of increasing their present production under sufficient stimulus. The Italian output has been increasing of late years, but whether this represents the discovery of new ore bodies or indicates a longer life for the Monte Amiata district is uncertain. A permanent improvement in the political conditions in Mexico, with a continuance of the present, or higher, prices, would probably lead to a notable increase in yield from that country. There is some probability also that Peru may again become an important source of quicksilver.
The quicksilver industry is less likely to be modified by changes in mining methods than by improvements in metallurgy. Although very simple in principle, the treatment of quicksilver ores, owing to the mobility and elusiveness of the metal both in the liquid and vaporized condition, is beset with many practical difficulties.
Coarsely broken ore is generally treated in various types of simple shaft furnaces, the fuel being either mixed with the charge or burned in a firebox. Finely broken or pulverulent ore, however, such as forms the larger part of the material from most quicksilver mines, requires different treatment. In Europe the common type of furnace for fine ore is the Spirek and in the United States the Scott-Hütner, or, as more commonly called, the Scott furnace. In both, the ore descends by gravity over tiles of fire-clay so shaped and placed as to permit the flame to pass back and forth through passages under tiles, the passageways or flues being formed partly by the tiles and partly by the ore itself. From the furnace the mercury-laden vapors are conducted through a series of condensing chambers of brick, iron, wood, or other material, in which the metal collects.
[345]
When intelligently operated, the Scott furnace is remarkably economical and efficient; but its construction is expensive and requires specially skilled masons. Moreover the furnace is difficult to repair, and once erected can not be moved. These are serious disadvantages to the man of small capital who is developing a new mine, and he usually has to fall back on retorts which are expensive to operate and are unsatisfactory except for relatively small quantities of rich ore.
Of late years attempts have been made in California and Texas to use slightly modified rotary cement-kilns for treating quicksilver ores. This innovation is promising and seems likely to prove successful. Such a furnace, although it may not displace the Scott under some conditions, does not require elaborate masonry structure, and its use may lead to a considerably increased production from the smaller mines.
The condensing systems used with quicksilver furnaces differ greatly and at no two mines in the United States are they identical. The brick condensing chambers formerly so extensively used with the Scott furnace are expensive to build; also the bricks are poor conductors of heat and absorb large quantities of quicksilver. The recent tendency in California has been to replace the brick chambers with large boxes or cylindrical tanks of wood. European practice, followed by one mine in Oregon and one in Texas, favors condensers constructed of vitrified earthenware pipe. The whole question of quicksilver condensation calls for study and skillful experiment. The establishment of a standard of practice would increase production by elimination of much of the loss and discouragement that come from inefficient individual efforts to collect the mercury from the furnace vapors and gases in the most complete and economical way.
The quicksilver industry offers two conspicuous examples of the direct political control of mineral resources. The Almaden mine, whose output is such as in normal times to determine the market for quicksilver, has been owned and worked by the Spanish government since 1645, and the Idria mine up until the close of the war was owned by the Austro-Hungarian government.
The Spanish government, on the basis of competitive proposals, contracts with the successful bidder for the sale of the quicksilver for periods of ten years. For a number of successive periods the contract has been awarded to the Rothschilds of London, the present one dating from June 1, 1912. The contractors bind themselves to sell, in London, the greatest possible quantity of quicksilver, which they take f.o.b. at the reduction plant at Almaden, at prices above 7 pounds per flask, They receive a commission of 1¹⁄₄ per cent. of the amount of the sale; 6 shillings for each flask shipped from Spain to London; and 10 per cent.[346] of the amount by which the sales price exceeds 8 pounds 2 shillings per flask. The Spanish government reserves from the operation of this contract 500 flasks[144] annually for the national requirements of Spain. By this arrangement, although the mine is owned by Spain, the market has been controlled in London. During the war the sale of Almaden mercury was taken over by the Admiralty through Messrs. Rothschild. The quantity received in London from Almaden in 1917 was about 25,000 flasks.
[144] Increased to 10,000 flasks in 1919.
The Konia mine, in Asia Minor, reverted to the Turkish government in 1912, but its output, as previously noted, is inconsiderable.
The quicksilver mines of the Monte Amiata district, Italy, although less obviously illustrative of political control than those just mentioned, should perhaps be referred to in the present connection. German capital has been dominant in their development in the late years before the war, and the most productive mines are credibly reported to have been owned wholly or in part by the German Emperor. With the entry of Italy into the war they were seized by the Italian government. The Italian mines produced about 28,000 flasks, of which from 12,000 to 15,000 flasks were purchased by the British Admiralty.
Under present practice the reduction of quicksilver ores is almost invariably at the mine, both mine and reduction works being under the same ownership. They must therefore be considered together for the purpose of the present inquiry.
The most conspicuous example of commercial control is that exercised by the Rothschilds of London, who do not own the resources that give them this pre-eminence. The yield of the remarkably rich Almaden mine, whose annual output surpasses that of any other mine and in 1916 exceeded that of any country except Spain (whose ore, even when carelessly worked, yields quicksilver at low cost, and whose known reserves are large), enables the Rothschilds, in time of peace and subject to the minimum fixed price in their contract, to determine the price at which quicksilver shall be sold in the world’s markets. Another but much smaller factor in making London the leading quicksilver mart of the world is the control by British capital of the principal mines in the Oviedo district, in northern Spain.
In the United States, the country which ranks next to Spain as a producer of quicksilver, the mines are all owned by corporations or individuals, and so far as known, there is at present no formal combination or understanding between these owners to control output or sales. Some years ago most of the leading producers in California formed the[347] Eureka company, which acted as selling agent and to some extent was able to control prices. Often referred to by those outside of it as the “quicksilver trust,” this organization was abandoned after a brief existence. The firm of Haas Brothers, of San Francisco (distinct from Haas Brothers, of New York), took a leading part in the Eureka company, and since its dissolution has fulfilled many of the functions that the company was to perform. The firm owns stock in the leading mine on the Pacific Coast and acts as selling agents for the New Idria Quicksilver Mining Co., whom it charges 1 per cent.[145], whereas it charges others 2¹⁄₂ per cent. It also buys the metal for itself, usually from the smaller producers, at prices generally much below the current market quotation, and is to supply operators with empty flasks, but only on condition that Haas Brothers shall buy the product or sell it on commission. Other brokers who handle important quantities of quicksilver on the Pacific Coast are Atkins, Kroll & Co., and the Braun-Knecht-Hiemann Co., both of San Francisco. So far as known these two firms have no ownership in quicksilver mines and sell only on commission.
[145] This connection is reported to have been broken in 1919.
The quicksilver mines in Texas are owned by American citizens or American corporations. The ore from each is independently worked and the quicksilver is sold by the individual producers.
The mercury deposits of Mexico are owned, as far as is known, by native Mexicans. British capital is probably interested in some of the larger mines. The formerly productive quicksilver mines at Huancavelica, Peru, have been purchased by E. E. Fernandini, of Lima, and may again contribute to the world’s supply.
So far as known to the writer of this article, patents, secret processes or trade agreements play no part in the control of quicksilver resources.
During the war Germany and her allies controlled the quicksilver deposits of Australia, Serbia, Turkey and probably European Russia. Only the Idria deposit, and perhaps the Zips deposit, in Austria, are important, and the available annual supply for the Teutonic allies was probably 25,000 to 30,000 flasks. The Entente allies controlled the deposits of the United States, yielding about 36,000 flasks annually; of Italy, yielding about 28,000 flasks; and controlled, although they did not own, the deposits of neutral Spain, yielding from 30,000 to 41,000 flasks annually. The Chinese mercury deposits were possibly drawn upon to some extent by Japan, who, like Britain and France, has no deposits of her own that are worth mentioning.
The chief uses of mercury and mercury compounds, in general order of decreasing importance, are as follows: In the manufacture of drugs and chemicals, including calomel, corrosive sublimate, and glacial acetic[348] acid; as a detonator for high explosives; as vermilion pigment; in electrical apparatus, thermostats, gas governors, and other appliances; in the amalgamation of gold and silver ores; in anti-fouling marine paint; in compounds to prevent boiler scale; in cosmetics; and in dental amalgam. There are comparatively few applications of mercury where a substitute could not be employed, although the substitute might not be as economical or as satisfactory.
In general the ores of quicksilver do not extend to great depths and show on the whole a close association with Tertiary and Quaternary igneous rocks that have not been subjected to long and deep erosion. There are some notable exceptions, however, to these generalizations. New deposits of mercury are most likely to be discovered on the eastern coast of Asia, in certain parts of Oceania, on the shores of the Mediterranean, or in the Cordilleran belts of North and South America. The known facts afford no secure basis for predicting that there will be within the next ten years any marked shift from the present main sources of supply to some newly discovered deposit.
The richest mercury deposits are at Almaden, central Spain; at Idria, Austria-Hungary; and in the Monte Amiata district of Italy. Other productive deposits are situated near Oviedo and Granada, Spain; in the Donetz coal basin, Russia; near Aidin, Turkey; in the province of Kweichow, China; in Oregon, California, Nevada, and Texas; in San Luis Potosi, Guerrero, and Durango, Mexico; and in Peru. Many deposits at present unproductive are known in other parts of the world.
The political control of the quicksilver deposits corresponds for the most part with geographic location. The rich deposits of Almaden, in Spain, and Idria, in old Austria-Hungary, are government-owned. The Spanish government, on the basis of competitive proposals, contracts with the successful bidder for the sale of the quicksilver for a period of ten years. The contract has been awarded to the Rothschilds of London for a number of successive periods. This control of the output of the Spanish mines gives the Rothschilds a control of the world’s quicksilver market. The Spanish government reserves a sufficient number of flasks annually for the national requirements of Spain. The Konia mine, in Asia Minor, has been the property of the Turkish government since 1912. It is believed that the most productive mines of the Monte Amiata district, Italy, were owned wholly or in part by the German Emperor; with the entry of Italy into the war they were seized by the Italian government. The mines of the United States are all controlled by corporations or individuals. It is believed that the mines of Mexico are owned by Mexican citizens, although British capital may be invested in some of them. The mines at Huancavelica, Peru, have been purchased by Señor E. E. Fernandini, of Lima.
[349]
Bauxite, aluminum oxide, besides being the chief ore of aluminum, has an important use in the manufacture of artificial abrasives which are of wide application in all metal-fabricating industries. Bauxite is also the basis of an extensive chemical industry, being the crude material from which alum, aluminum sulphate, and several other chemicals used for water purification, dyeing, and tanning are made. A rapidly growing use for bauxite is in the manufacture of bauxite brick for furnace linings. The more essential uses of bauxite are for the manufacture of aluminum and abrasives, though it seems doubtful whether the utilization of bauxite for chemicals could be much restricted. The use of bauxite for refractories is relatively small. In 1917 nearly 65 per cent. of the domestic output of bauxite went into aluminum, nearly 13 per cent. was taken by manufactures of aluminum salts, 19 per cent. was consumed in the manufacture of bauxite abrasives, and 3 per cent. was used by makers of bauxite refractories.
The uses of aluminum are myriad, chief among them are in the manufacture of parts of internal-combustion engines, and the fabrication of industrial and household utensils.
Heretofore bauxites low in silica (2 to 5 per cent. SiO2) have been used for the preparation of alumina for the manufacture of aluminum. Many experimenters have endeavored to utilize low-grade (high-silica) bauxites, or aluminum silicates for the recovery of alumina. These experiments show that it is chemically possible to produce low-silica alumina from many aluminous materials, but not on a commercially profitable basis. It seems reasonably certain that one or more of the methods of handling low-grade bauxite or even aluminous silicates will be developed to the commercial stage, even under ordinary conditions, in the near future. That event should tend to revolutionize the aluminum industry, as clays and shales carrying from 25 to 35 per cent. Al2O3 are of widespread occurrence. Whether it would materially lower the price of aluminum is more doubtful, for the costs of manufacture would be raised by the increased cost of treating the low-grade crude material.
[350]
The European bauxite deposits are in folded sedimentary rocks, mainly of Cretaceous age. In the United States bauxite deposits are surficial and have resulted from the alteration of either sedimentary kaolin (aluminum silicate) or kaolin derived from the weathering of syenite or of dolomitic limestones. In the tropical fields, which have as yet been little exploited and are in fact little known, the bauxites seemingly are surficial deposits derived from the alteration of feldspathic rocks.
The chief bauxite deposits of Europe are in the provinces of Var and Herault, in southern France, though other deposits are known in Bouches du Rhone and several other southern provinces. In central Italy bauxite has been mined for some years. In Germany low-grade bauxite has been mined in the Vogelsberg Mountains, Hesse, near Königswinter, in the lower Rhine country, and is known in Hanover. In the former Empire of Austria-Hungary there are extensive bauxite deposits in the Bihar Mountains and in the provinces of Istria, Croatia, and Dalmatia. Bauxite is also known in northwestern Russia, about 200 miles southeast of Petrograd. Bauxite has been mined for a number of years from beds in northwestern Ireland.
In the United States, bauxite has been mined for years in central Arkansas, northwestern Georgia, northeastern Alabama, and southeastern Tennessee, and more recently from the central Georgia field, which is being extended into west-central Georgia.
In South America, extensive deposits of good bauxite have been found in British and Dutch Guiana, and it is reported that there are evidences of bauxite in eastern Venezuela, western French Guiana, and northeastern Brazil.
In Africa, bauxite of good quality is reported to have been developed near the coast of French Guinea and to have been found in a number of inland localities in that colony. Vague rumors are current of large areas of bauxitic laterite[146] at many places in equatorial Africa.
[146] Laterite is the general name for rock of any kind that is in a thoroughly softened and decomposed state, due to alteration or weathering at the surface.
In the literature of the geology of India there are many references to bauxitic laterites, and it is reported that recently some of the Deccan bauxite deposits are being exploited.
In southwestern and eastern Australia some of the laterites are reported to be bauxitic, though so far as known no bauxite has been developed.
There is a persistent rumor, without confirmation, that bauxite has[351] been discovered recently in China. As to this deposit no information is available.
The table below shows the world’s production of bauxite for a number of years. Although the figures give an idea of the relative importance of the deposits with respect to consuming centers, it is not believed that they represent relative importance with regard to the future. It seems unavoidable to conclude that the tropical countries hold immense reserves of bauxite and that some day the aluminum industry will be nearer the tropics than it is at present.
Table 63.—World’s Output of Bauxite, 1910-1916
(Output in tons)
| Country | 1910 | 1911 | 1912 | 1913 | 1914 | 1915 | 1916 |
|---|---|---|---|---|---|---|---|
| United States | 148,932 | 155,618 | 159,865 | 210,241 | 219,318 | 297,041 | 425,100 |
| France | 192,913 | 250,818 | 254,851 | 304,407 | ([147]) | ([147]) | ([147]) |
| United Kingdom (Ireland) | 3,792 | 6,007 | 5,790 | 6,055 | 8,286 | 11,723 | 10,329 |
| Italy | 4,524 | 5,600 | 6,596 | 6,843 | 3,844 | 6,504 | 8,746 |
| India | 66 | 12 | 950 | 1,184 | 514 | 400 | 750 |
| Total | 350,277 | 418,055 | 428,052 | 528,730 |
[147] No statistics available.
The production of bauxite in the United States in 1917 was 568,690 long tons.
France and the United States hold within their boundaries the largest deposits of bauxite that have been worked in the past. England controls, through her colonial possessions, a large share of the equatorial areas that probably contain much of the undeveloped bauxite. France and Holland each have possessions in the tropics in which bauxite is known, and it seems probable that bauxite may be found in the colonies of Portugal and of Belgium and in those formerly controlled by Germany.
It is reported that England has placed certain restrictions on the acquisition of bauxite deposits in India and Guiana by foreign individuals or corporations. It is known that she has restricted the destination of bauxite exported from British Guiana. The Dutch government is understood to have examined recently the bauxite deposits of Dutch Guiana, probably with a view to restricting acquisition of property not already acquired. Evidently there is some understanding between the British, French, and Italian governments which permits the sending of French bauxite to the aluminum works of both Italy and England.
The aluminum works of the world are largely under the political domination of the United States, England, France, Germany, Switzerland,[352] Italy and Norway. The producing capacity of the various countries has been estimated as follows:[148]
Producing Capacity of Aluminum Works
| Short tons |
|
|---|---|
| United States and Canada | 87,500 |
| France | 20,000 |
| Switzerland, Germany and Austria | 20,000 |
| Norway | 16,000 |
| England | 12,000 |
| Italy | 7,000 |
| Japan | 250 |
| Total | 162,750 |
[148] Hill, J. M., “Bauxite and Aluminum in 1916;” United States Geological Survey; “Mineral Resources of the United States,” Part I, 1917, p. 167.
The actual output of the plants included in the above table does not represent full capacity, and it seems more reasonable to assume that the production at present is probably nearer 150,000 tons a year than the total given. There is little question that the United States and Canadian plants are producing over half of the world’s supply of aluminum. It is reported that during 1917 and 1918 extensions of the British and Italian works brought their output nearly to the rated plant capacity.
It is safe to say that the aluminum industries of the various countries control to a large extent the bauxite deposits of the world. The principal aluminum companies are as follows: Aluminum Company of America, British Aluminum Co., L’Aluminium Française, Aluminum Industries A. G.
It is commonly known that before the war agreements between these four companies stabilized the prices of aluminum throughout the world. At present (1919) the outlook is that the Aluminum Company of America should be in position to dominate the aluminum industry of the world for some years through the expansion of its electrical plants and its initiative in acquiring newly discovered deposits of exceptionally pure bauxite in South America. It seems quite conceivable that the British and French and possibly the German interests may seek to adjust their relations so that they can offset the American dominance.
—The great bulk of the bauxite deposits of the United States seems controlled by the Aluminum Company of America, through its subsidiaries, the American Bauxite Co. and the Republic Mining & Manufacturing Co. There are small holdings of bauxite lands controlled by the National Bauxite Co., a subsidiary of the E. I. du Pont de Nemours Co., and by the Norton Co., of Worcester, Mass.,[353] makers of artificial abrasives. Aside from these more important holders, there are a few independent operators of bauxite mines, but their combined output is so small that it can be disregarded.
All of the aluminum works of the United States and Canada are controlled by the Aluminum Company of America, which is dominated by the Mellen banking interests, of Pittsburgh, Pa.
—It is reported that the Aluminum Company of America controls about 2,030,000 acres of bauxite land in the British and Dutch colonies. In British Guiana the ownership is seemingly in the Canadian Bauxite Co. Associated with the Aluminum Company of America in the British Guiana holdings is the Merrimac Chemical Co., of Boston. It is also reported that the Norton Co., of Worcester, Mass., has acquired in Dutch Guiana small holdings of bauxite lands. There are no works utilizing bauxite in Dutch Guiana.
—Prior to the war, some of the large deposits of high-grade bauxite in the Province of Var were controlled by the “Bauxites de France,” a German enterprise, but this control was naturally suspended at the beginning of hostilities, and it will probably not be resumed. The French bauxite industry is largely in the hands of the French producers of aluminum mentioned below, though some deposits are said to be controlled by the British Aluminum Co. through its control of the Union des Bauxites company.
The French aluminum industry is centralized under one selling agency, L’Aluminium Française, in which the following five companies participate:
Compagnie des Produits Chemiques d’Alais et de la Camargue, Société Electro-Metallurgique Française, Société d’Electro-Chemie, Société des Forces Motrices de l’Arve, Société Electro-Metallurgique de Pyrenees.
It is said that the stock of the selling company is owned by participating companies in proportion to their output of aluminum, which would indicate that the control of l’Aluminium Française rests with the first two companies above.
—The bauxite deposits in County Antrim, Ireland, are seemingly controlled exclusively by the British Aluminium Co. The British Aluminium Co. is the sole producer of the metal in England, operating plants at Foyers and Loch Leven, in the British Isles, and plants in Norway.
—All the bauxite used by the aluminum works in Norway is of French or British origin. There are no deposits of bauxite in the country. The British Aluminium Co. controls the aluminum plants at Higeland and Strangfiord through the Anglo-Norwegian Co. The Compagnie des Produits Chemiques d’Alais et de la Camargue (French) largely controls the Société Norvegienne des Nitrures, which operates aluminum[354] works at Arendal and Tyssedal. A Norwegian company, the Norske Aluminum Co., has been recently organized to make aluminum.
—There is little information concerning the ownership of the Italian bauxite deposits, but presumably they are controlled by the producers of aluminum. The principal aluminum manufacturer is the Societa Italiana per la Fabricazione dell’Alluminio, which is under Italian-French control.
The new aluminum company, L’Allumino Italiano, recently organized in Italy, if reports are true, may be in part controlled by German and Swiss interests. As the company was organized during the war, it does not seem reasonable to suppose that German participation would be permitted.
—Apparently most of the aluminum industry of these countries is controlled by a German-Swiss company, Société Swisse pour l’Industrie de l’Aluminium, or Aluminum Industrie, A. G., which operates plants at Neuhausen, Chippes, Navisance, and Borgne, in Switzerland; at Rheinfelden, Germany, and at Lend and Rauris, in Austria. A small quantity of aluminum is also made by the German firm, Gebrüder Guilini, at its plant at Martigny. It is reported that the Aluminum Fabrik-Martigny, A. G., has recently been formed with G. Guilini at its head, which is possibly a reorganization of the former concern.
By far the largest and most important use of bauxite is for the extraction of aluminum, a metal used mainly in the manufacture of parts for internal-combustion engines and of industrial and household utensils. Bauxite is also used in the manufacture of artificial abrasives, as a source of certain aluminum salts, and in the manufacture of refractory bricks. The first two uses, the manufacture of aluminum and abrasives, are the most essential, though it would be difficult to restrict to any great extent the use in the chemical industry.
The principal bauxite deposits of the world are in the provinces of Var and Herault, southern France; in the former empire of Austria-Hungary; in Arkansas, Georgia, and Alabama; in British and Dutch Guiana; and in northwestern Ireland. Minor deposits are located in Germany, Russia, Venezuela, French Guiana, Brazil, Africa, Australia, and probably China. It is believed that the tropical countries hold immense reserves of bauxite.
Experiments have shown that it is chemically possible to manufacture aluminum from the low-grade (high-silicate) bauxite ores. No commercial process has been perfected, but it seems certain that one or more methods will be developed to the commercial stage in the near future. A reduction in the price of aluminum is not to be expected as a result of[355] this change in practice, however, for the use of low-grade materials will undoubtedly increase the manufacturing costs.
The largest producing bauxite deposits are controlled politically by the United States and France. Great Britain controls a large share of the equatorial regions that probably contain most of the undeveloped deposits. Bauxite may also be found in the colonial possessions of Portugal and Belgium, and in those formerly owned by Germany. The aluminum works of the world are controlled by the United States, Great Britain, France, Germany, Switzerland, Italy and Norway.
Most of the bauxite deposits of the United States are owned by the Aluminum Company of America, which is dominated by the Mellen banking interests, of Pittsburgh, and controls all of the aluminum works of the United States and Canada. Small holdings in the United States are controlled by a subsidiary of the E. I. du Pont de Nemours Co., and by the Norton Co., of Worcester, Mass. The Aluminum Company of America also controls, through subsidiaries, large areas of bauxite land in British and Dutch Guiana. Before the war some of the large French deposits were controlled by German interests. The French industry is largely in the hands of French producers of aluminum, although some of the deposits are said to be controlled by British capital. The main French companies have organized a selling company, L’Aluminium Française.
The British Aluminium Co., controls the deposits in Ireland and is the sole producer of aluminum in England. British capital also controls aluminum works in Norway. The principal bauxite deposits of Italy are probably controlled by the Societa Italiana per la Fabricazione dell’ Alluminio, an Italian-French company. Apparently most of the aluminum industry of the Central Powers is controlled by a German-Swiss company. American interests are reported to have explored bauxite deposits in French Guinea, Africa, but so far as known have produced no bauxite.
[356]
Corundum is the natural (mineral) crystalline oxide of aluminum. Emery is a very fine-grained and intimate intergrowth of corundum and other minerals, chiefly magnetite, some varieties containing also important amounts of hematite, spinel, and chlorite. Both emery and corundum are very hard, and break into rough, sharp grains; hence they are used as abrasives for grinding, dressing, and polishing metals—chiefly iron and steel—and glass, and, to a less extent, stone, wood, and other materials. Emery and corundum are used loose in the form of grains, powders, and flours, and also as grains made up into solid wheels, cylinders, blocks, and files of many shapes by means of a great variety of binders. The essential uses are in work on iron and steel and glass. The softer metals and other materials can be worked in many cases to better advantage with other abrasives, such as quartz, tripoli, garnet and pumice.
The essential operations for which emery and corundum are used can be performed with the artificial carbide and alumina abrasives. For some work, however, such substitutions appear not to be advisable, as the abrasive quality and efficiency of both the natural and artificial abrasives depend not only on the hardness of these materials, but also on a number of other factors: among these being the physical qualities of the materials worked; the sharpness of edges and angles of broken particles of the abrasive; the manner in which the abrasive breaks down under use; the manner of, and materials used in, binding the abrasive particles; and the speed and pressure with which they are applied to the work.
Of the various kinds of material abraded, each calls for different grades and kinds of abrasives, and for variation in the above factors in the use of these abrasives in order to insure most efficient use. Consequently, it is almost impossible to determine arbitrarily the uses for which each of the various abrasive materials is essential. This much, at least, seems certain—that for finishing and polishing glass, particularly optical glass and plate glass, there is as yet no general agreement that satisfactory substitutes are available for the better grades of Turkish and Greek emery, although experiments in manufacture and use of suitable artificial abrasives have been successful.
[357]
The known emery deposits are products of magmatic differentiation or of regional metamorphism, or of combined contact and regional metamorphism of limestone, presumably argillaceous, and of argillaceous sediments. A study of certain individual deposits, therefore, makes possible some forecast as to future supplies in some regions, particularly those in which the emery deposits are intimately related to certain beds in metamorphic sedimentary formations in close proximity to igneous rocks. The emery bodies are, however, as a rule, spotted or irregularly distributed, and reliable estimates of reserves are difficult.
Corundum, in a number of associations, is an original constituent of a great variety of igneous rocks, such as peridotites, anorthosites, syenites, nepheline syenites, and syenite pegmatites. It is also abundantly found in regionally metamorphosed rocks and in contact metamorphic zones, occurring in serpentines, mica schists, quartz schists, and crystalline limestones. A third important source of corundum is alluvial deposits. Corundum is not a characteristic or essential constituent in any of these types of rocks and is present in alluvial deposits in restricted localities only. Furthermore, its distribution and its concentration, when present, are irregular and unsystematic, and there are, therefore, no geologic guides by which future supplies can be forecast without intensive study of each individual occurrence.
The chief deposits of emery and corundum in the United States are in the eastern seaboard or Appalachian states.
The emery deposits of Chester, Massachusetts, are in a narrow band less than 500 feet wide that has been traced for nearly five miles. The Chester deposits have been worked at various times since the eighties and up to 1913.
Emery deposits near Peekskill, New York, are associated with igneous rocks in an area of 20 to 25 square miles. These deposits have been worked since 1889. Some of the material mined is a true emery, that is, an intimate mixture of corundum and magnetite, but most of it is largely a mixture of spinel and magnetite, which, while not a true emery, makes an excellent abrasive. In 1916 and 1917, the annual output of ore was approximately 15,000 tons.
In the vicinity of Whittle, Pittsylvania County, Virginia, spinel emery, somewhat like the New York emery, but containing more corundum, is abundant. The deposits in this region have already produced considerable emery and may be counted on for a large supply.
Corundum is associated with a serpentine belt extending through[358] Lancaster, Chester, Delaware, Montgomery, and Bucks counties, Pennsylvania, and through adjoining counties in Delaware and Maryland. The deposits in this region do not seem to be of commercial importance.
Corundum is found in a large number of localities in North Carolina. The most important occurrences of corundum are in or near peridotite masses, also in schists, and in alluvial deposits. Active mining work was first begun in 1871 and continued until about 1906, when North Carolina corundum was driven from the market by the competition of Canadian corundum and artificial abrasives. Corundum mining was revived in 1915, and the three properties worked in 1917 made an output of 820 tons. There is unquestionably a very large reserve of corundum in this region, but transportation is difficult and efficient labor is scarce; so that there is little immediate prospect of a large development.
The only commercially important deposits of abrasive corundum west of the Appalachian Mountains are in the central part of Gallatin County near Salesville, Montana, where corundum occurs in syenite and syenite pegmatite. The deposits have been worked by three companies, and up to 1903, when operations ceased, had produced several hundred tons of corundum.
The sources in Canada of abrasive corundum of commercial importance are limited to the corundum syenites and anorthosites in central Ontario. These deposits have been developed and mined only at the Burgess mines and at Craigmont. Corundum mining as an industry in Canada began in 1900. The production reached a maximum in 1906 and was smaller and fairly uniform from 1907 to 1913, in which year operations were practically suspended.
The reported occurrences of corundum in Mexico and Central and South America are chiefly of the gem variety. Emery is reported from Musco, Colombia, and common corundum is reported as especially abundant at localities in the State of Sao Paulo, Brazil.
The only occurrence of emery and corundum of commercial importance in Europe is in the islands of the Grecian Archipelago, particularly the Island of Naxos. The deposits occur there as lenses and masses in limestones and in the vicinity of granites. Exploration and development work has been superficial, but reserves or future supplies there are probably enormous. Annual exports during the years 1897 to 1914 averaged 6,800 metric tons.
In Asia there are commercial corundum deposits in Asiatic Turkey and in India.
The emery deposits of Asiatic Turkey are near Smyrna. The Turkish emery is similar in origin and general character to the Greek emery, except that none is found in Asia Minor of quite the superior quality of that of Naxos. The main source of supply seems so far to have been from the detrital deposits. It seems almost certain that supplies are[359] large. The emery mines of Asia Minor are very old and have annually contributed large quantities to the world’s supply, their output having been larger than from any other region in the world. Statistics for recent years are lacking. The recorded output in official reports of the Turkish government was about 62,000 metric tons in 1908 and about 25,000 in 1909.
The corundum deposits of India are numerous and include not only the common abrasive varieties but also the most highly prized gem varieties. Commercially important deposits of the abrasive variety occur in the presidency of Madras. The following provinces and native states also contain the mineral in more or less abundance: Afghanistan, Bengal, Burma, Central Provinces, Punjab, and Travancore.
Available data on the production of corundum in India indicate an output between 100 and 500 long tons a year, up to and including 1915. In 1916 the production was approximately 2,000 long tons and since then it has probably equaled or exceeded that figure. Nothing definite is known as to the corundum resources of India, except that they are undoubtedly large.
The only recorded deposits of abrasive corundum in commercial quantities on the continent of Africa are in the vicinity of Pretoria, in the Transvaal, where corundum, probably occurring originally in schists, is concentrated from residual material on the land surface. Little information concerning these corundum deposits is available, but it is probable that large reserves may be developed. The production, which had been slight or negligible prior to 1912, expanded greatly in 1917 to about 3,000 long tons.
Corundum appears to be abundant on the Island of Madagascar, where large amounts of gem stuff and abrasive materials have been found in alluvial deposits. The production of abrasive corundum, which was very small in 1910, expanded to about 1,100 metric tons in 1913, and approximately 1,000 metric tons in 1916.
No material changes in geographic distribution of resources appear probable in the near future. None of the deposits now productive is approaching exhaustion, and only concerning emery in Virginia, where somewhat larger emery production may be expected, is information at hand upon which to forecast changes in output.
Substitution of artificial abrasives for emery and corundum may be extended. Experiments conducted early in 1918 looking toward the development of an artificial abrasive suitable for use in optical and plate-glass work have been successful, so that there may remain no industrial operation wholly dependent on emery and corundum. The complete[360] supplanting of the natural abrasives, however, will depend in part on the supply of bauxite available for manufacture into artificial abrasives. At present the United States supplies of bauxite are sufficient for such use.
The demand in Britain and France for Indian and South African corundum and Greek emery would undoubtedly diminish if the French artificial abrasive plants were in full operation. Such a change would also probably cut down exports of artificial abrasives from the United States, and correspondingly affect the demand for emery and corundum.
Emery and corundum resources within the United States are owned, so far as known, by American citizens, and are in no way state controlled.
The Greek emery industry was formerly a monopoly controlled by the Greek government, but the inhabitants of the emery region had always maintained their sole right to mine the emery. This right was respected, and the Greek government merely regulated and managed sales and exports, exacted high royalties, which were changed from time to time, fixed prices, and maintained high quality and uniform standards of emery for export. The French government during the war assumed control of the Naxos emery supply and presumably continued the regulations of the Greek government. Supplies of Greek emery were available only to France and her allies, through allocation by the French government.
In the United States the various emery and corundum deposits are in small holdings that are mostly owned by local residents. The mines and quarries have been worked by lessees on royalty, generally. A considerable number of operators are and have been engaged in several localities, and there are no trade coalitions. Crushing and grading are in the hands of eight independent competitive companies, except in so far as they were welded during the war into a trade association by the War Trade Board for the purpose of allocating, under Government supervision, the small imports of Greek emery to essential industries.
In Canada the better portions of the corundum deposits seem to be controlled largely by one company—Manufacturers Corundum Co.—whose owners seem to be dominantly or entirely of Canadian nationality.
The Greek emery deposits, particularly those of Naxos, are claimed to be the inalienable property of the families resident upon the island.
There is no control of emery and corundum resources through ownership of crushing, milling, and grading plants, nor through patents or[361] secret processes of preparation. Trade combinations as affecting emery and corundum supply are unknown. There are a number of milling companies in the United States, Britain, France, and Germany, who compete for the world’s supply of raw material, and those of each country compete with one another for markets for the graded, prepared material.
The United States has supplies of inferior emery and resources of corundum which are not developed adequately to meet the demands for natural abrasive materials. During the war the United States was short of the amount of emery and corundum desired by consumers. However, this shortage was offset by an excess supply of artificial abrasives.
England, in India and South Africa, has corundum supplies probably more than sufficient for her needs. England is probably well enough supplied with these abrasives, particularly as long as she continues to import from Canada and the United States the needed artificial abrasives.
France has no home supply of emery and corundum, but has large resources in her colony, Madagascar, and during the war controlled the Greek emery supply. Furthermore, France has in reserve rich bauxite deposits and hydro-electric power for manufacture of artificial abrasives.
Germany depended upon Turkish emery during the war. She is short of bauxite, but makes large quantities of carborundum.
Japan probably can supply her needs by drawing on Indian corundum resources and on the United States for artificial abrasives. During the stringency of supply in 1917 some material was exported to the United States from Japan.
Corundum and emery, the latter a close association of corundum with certain other minerals, are used as abrasives for grinding and polishing metals, glass, stone, and wood. Many of the operations formerly performed with emery and corundum are now being performed with artificial carbide and alumina abrasives.
The deposits of emery and corundum are few in number, but their product is ample for all present needs. Commercially important deposits are situated in the Appalachian region of the United States; on the islands of the Grecian Archipelago, especially the Island of Naxos; in the Province of Aidin, in Asia Minor; in the presidency of Madras and the provinces of Punjab, Bengal, and Travancore, India; in Madagascar; and in the Transvaal near Pretoria.
The geological formation of emery and corundum deposits makes impossible any accurate estimate of reserves or any forecast of future discoveries.
[362]
The political control of the emery and corundum resources of the world corresponds to the geographical location except as regards the deposits on the Island of Naxos. This island is Grecian territory, but the French government during the war assumed control of the emery industry and allocated supplies of the abrasive only to the industries of France and her allies. The deposits of Asia Minor are at present (1920) nominally in the control of Turkey, but actually partly in the coastal strips seized and held by the Italians and the Greeks after the armistice. Madagascar is a French possession, and the Transvaal and India are parts of the British Empire.
The deposits of the United States are owned by a number of small independent operators, all American as far as is known. The Canadian deposits are controlled largely by one company, the Manufacturers Corundum Co., whose owners are predominantly or entirely Canadian. The Greek emery deposits, particularly those of Naxos, are claimed to be the inalienable property of the families resident on the island.
Table 64.—Production of Emery and Corundum, 1910-1917
| Emery | Corundum | |||||||
|---|---|---|---|---|---|---|---|---|
| United States [149] |
Greece [149] |
Turkey | United States [149] |
Canada [149] |
India [149] |
Mada- gascar [149] |
So. Africa [149] |
|
| Ore produced (long tons) |
Ore shipped from Syria (metric tons) |
Grains produced (long tons) |
Grains shipped (long tons) |
Grains produced (long tons) |
Grains or ore produced (metric tons) |
Grains or ore produced (long tons) |
||
| 1910 | 940 | 12,939 | [150] | [151] | 1,660 | 218 | 11 | [150] |
| 1911 | 620 | 9,845 | [150] | [151] | 1,270 | 275 | 150 | [150] |
| 1912 | 870 | 7,687 | [150] | [151] | 1,600 | 345 | 496 | 99 |
| 1913 | 900 | 1,440 | [150] | [151] | 1,090 | 355 | 1,099 | [150] |
| 1914 | 460 | 10,226 | [150] | [151] | 500 | 105 | 556 | [150] |
| 1915 | 2,840 | [150] | [150] | [152] | 220 | 62 | 327 | [150] |
| 1916 | 14,400 | [150] | [150] | [152] | 60 | 1,868 | 914 | [150] |
| 1917 | 15,400 | [150] | [150] | 770 | 160 | [150] | [151] | [153]4,051 |
[149] Statistics for United States from U. S. Geol. Survey; for Canada, Canada Dept. Mines; for Greece, British Consular Reports quoted in “Mineral Industry;” for India, Records India Geol. Survey; for Madagascar, Service des Mines, Madagascar; for South Africa, American Consular Reports.
[150] Figures not available.
[151] No production.
[152] Small unrecorded amount.
[153] Nine months, Jan.-June and Oct.-Dec.
[363]
Magnesite and its derived products are used in a variety of industries, the most essential of which, beyond doubt, is metallurgy. Owing to the high fusion point and chemical inertness of the oxide of magnesium, magnesite is one of the principal minerals used in the metallurgical and other industries where highly refractory material is required. For this purpose dead-burned magnesite is used in the form of brick or of grains. Brick and shapes are employed for lining open-hearth steel furnaces, welding, heating, and melting furnaces, reverberatories, settlers, and furnaces for refining lead, copper converters, and electrical furnaces. Crushed or granular magnesite is used for lining the bottoms of open-hearth steel furnaces, and in making crucibles and cupels.
In the manufacture of the cement known as oxychloride or Sorel cement the quantity of magnesite used is exceeded only by that used for refractory purposes. This cement is employed largely for sanitary flooring, and to a less extent for wall plaster, both interior and exterior. It is used also instead of Portland cement for some forms of exterior construction where quick and strong set is required. Magnesite is used in the manufacture of wood-pulp paper on the Pacific Coast, in fire-resisting paint, as a non-conductor of heat in pipe and furnace coverings, and in the manufacture of magnesium chloride, light carbonate, and other products, including metallic magnesium.
Deposits of magnesite are widely distributed throughout the world and occur in two distinct forms, amorphous and crystalline. Amorphous magnesite, the most common form, is fine-grained, and compact; it is usually found in veins or masses in serpentine resulting from the alteration of magnesia-rich rocks of the peridotite family. To this group belong the Grecian deposits, nearly all the California deposits, and those in Mexico, Venezuela, and other parts of the world. Crystalline magnesite is medium to coarse grained, and occurs as masses in limestone, dolomite or associated sediments which have been metamorphosed.[364] The principal deposits of this class are those in Austria, Hungary, Quebec, and Washington.
Deposits of magnesite are regarded as having originated in three ways. The massive non-crystalline variety, such as that in California and Greece, is believed to have been formed by the decomposition of serpentine. Magnesite deposits near Bissel, California, and on Muddy River, near St. Thomas, Nevada, are said to be of sedimentary origin. The Austro-Hungarian, Washington, and Quebec deposits are regarded as resulting from the replacement of calcareous sedimentary rocks by magnesian-bearing solutions.
Magnesite deposits that occur as veins in connection with serpentinized magnesian rocks probably are formed both from the breaking down of the serpentine-making minerals and from the serpentine itself. It seems probable that usually both serpentine and magnesite are formed in the process of decay of the original minerals in peridotite and the allied basic rocks, and that during the decay of the serpentine the formation of magnesite continues. In any case the magnesia or magnesian mineral is changed to carbonate, dissolved by percolating water charged with carbon dioxide, and precipitated in cracks and crevices as veins. When formed in this way the magnesite occurs in large and small veins, lenses, and stockwork, and its distribution and extent are erratic. It seems fair to assume that these deposits may extend to the limit of depth of easily circulating surface waters, which in favorable conditions may be several hundred feet. Faulting, on the other hand, is as likely to cut the veins off in depth as in length. Any estimate of available tonnage of magnesite in deposits of this type therefore is unwarranted in advance of development work.
Sedimentary deposits such as those of Bissel, California, and near St. Thomas, Nevada, are by their nature more regular in occurrence, and their tonnage can be estimated from the outcrop in natural exposures and prospects.
Replacement deposits like those in Washington and Quebec are not so regular as the sedimentary deposits, but are more regular than the veins, and tonnage estimates may be based on the surface exposure and an assumed depth of 50 to 100 feet.
The known distribution of magnesite deposits is as follows:
The following description by countries is in the order given above:
—The principal magnesite deposits in Canada are in the Grenville district, Argenteuil County, Quebec, where the mineral is associated with serpentine, dolomite, and other minerals. The magnesite in the Grenville district is a glistening cream-white to milk-white or gray material that occurs in extensive masses associated with bands or lenses of dark green to light-yellow serpentine. Throughout the great mass of the deposits the magnesite and dolomite are so similar in appearance that the detecting of dolomite is difficult. There is considerable positive evidence in support of the hypothesis that the deposits have been formed by the solution and replacement of crystalline limestone through the agency of magnesia-rich solutions. Outcrops of the deposits are up to 1,000 feet long and 300 feet wide. It is estimated that there are in sight 686,900 tons of magnesite containing less than 12 per cent. CaO and 483,700 tons of magnesite-dolomite containing more than 12 per cent. CaO.
In the Atlin mining district, in British Columbia, both magnesite and hydromagnesite have been noted, but the extensive masses of hydromagnesite near the town of Atlin are the most important. These deposits are superficial beds of fine powdery white hydromagnesite 6 to 8 feet thick, that cover areas up to 18 acres in extent. Two groups of these deposits are estimated to contain 180,000 tons of hydromagnesite.
—Magnesite in commercial quantity occurs in California, Nevada, and Washington. Reports of workable deposits in other states have not been verified.
In California there are magnesite deposits in many places throughout the Coast Range and on the west slope of the Sierras, from Mendocino and Placer counties on the north to Riverside County on the south. Before the war, mining was limited to a few localities and the annual output was about 10,000 tons, but the demand caused by large reduction in imports started active prospecting and development, with the result that in 1917 thirteen counties yielded a total of 211,663 tons, valued at[366] $2,116,630. In nine counties the deposits are large and in four counties only small deposits have been found as yet. The most important deposits are in Napa, Santa Clara, San Benito, and Tulare counties. In 1917, 63 per cent. of the crude magnesite produced was mined in Tulare County. Practically all of the California magnesite deposits are irregular veins in serpentine, resulting from the alteration of magnesian igneous rocks.
In the state of Washington deposits of crystalline magnesite have been found at several places. The Washington magnesite differs greatly from the California deposits and occurs in larger masses. It is coarsely crystalline, like marble or coarse textured dolomite, and is red, pink, black, white, and gray. The Stevens County magnesite has been formed by the replacement of lenses of dolomite in sedimentary rocks. The recrystallization of the purer magnesian carbonate may have been secondary and influenced by the intrusion of basic magnesian rock which occurs above and below the magnesite in some places. The larger deposits are 200 or more feet thick and 1,000 or more feet long. Estimates of one million tons within 100 feet of the surface are reasonable for at least three of the deposits. Mining in Washington began in December, 1916, with a production of 715 tons. The output in 1917 was 105,175 tons, valued at $783,188.
The only known deposit of magnesite in Nevada is an extensive sedimentary bed in the valley of Muddy River, Clark County. The magnesite carries more than 5 per cent. lime and more than 11 per cent. silica. It has not been developed.
The total production of magnesite in the United States in 1915 was 30,499 short tons; in 1916, 154,974 short tons; in 1917, 316,838 short tons; and in 1918, 231,605 short tons.
—On the Island of Santa Margarita, in Magdalena Bay, Lower California, are extensive deposits of magnesite from which exports have been made to the United States. Walls of canyons in the mountains show masses of magnesite several feet thick, and magnesite boulders strew the stream beds. Large quantities can be obtained without mining and need only to be broken up for shipment. An analysis of calcined magnesite from Santa Margarita Island shows practically no silica, lime, or iron.
—The deposits on Margarita Island, Venezuela, are of the amorphous or California type and occur in veins and stockwork. No information is available regarding their extent, but 500 tons were exported to the United States in 1915.
—The magnesite deposits of Austria and Hungary, which until recently furnished much of the world’s supply, extend along a northeast line for several hundred miles across the two countries. The mineral occurs in lenses. A large deposit near Veitsch, Austria, measures[367] 700 to 800 feet from the top to the base. The ore is quarried in a series of benches. Another very large deposit in Austria is at Radenthein. The magnesite is quarried by great cuts, and lowered by gravity to rotary kilns. Calcining is done near the mine and both grain and magnesite bricks are shipped. The property was owned by Americans before the war and much of the output went to American ports.
The magnesite in these deposits is crystalline and occurs in dolomite, probably of Carboniferous age, from which it was derived by the infiltration of magnesium carbonate solutions and the leaching out of soluble calcium carbonate. It is finely to coarsely crystalline, yellow or bluish-white, carries 3 to 4.5 per cent. iron oxide, less than 2 per cent. silica, and less than 3 per cent. lime. It calcines readily to the dead-burned state and makes satisfactory grain magnesite and brick for refractory purposes.
Deposits of magnesite were worked for many years near Frankenstein, Silesia, Germany.
In the Province of Santander, in northern Spain, coarse crystalline magnesite lying in Lower Cretaceous limestone and dolomite has been mined for a number of years. The production in 1915 was 1,400 tons.
In Greece, magnesite is of the non-crystalline type and occurs associated with serpentine in veins and masses. The most important deposits are on the Island of Euboea. The Euboean deposits are all close to the seashore, and under normal conditions cheap water transportation to the principal magnesite markets of the world is available. The production of Greek magnesite in 1914 was mainly in the hands of three companies: the Anglo-Greek Magnesite Co., 24 Finsbury Sq., London; the Société Hellenique des Mines, Athens; and the Hellenic Magnesite Co., Athens. The distribution of the magnesite is controlled by the London company. The Anglo-Greek Magnesite Co. works mines at Galataki and Afration, in Euboea. At the Galataki mines the vein of ore exposed is known to be 1,300 feet long and 50 to 60 feet wide. The Société Hellenique des Mines (now called The Financial Corporation of Greece, Ltd.,) controls the production of several mines at Mantoudi, Limni, Larimna, etc. The Hellenic Magnesite Co. obtains most of its ore from surface excavations. In 1912 the production of magnesite by several companies in Greece, (not including the Hellenic Magnesite Co.) was as follows: Raw magnesite, 87,338 tons; calcined magnesite, 30,645 tons; dead-burned magnesite, 3,201 tons. This is equivalent to about 150,000 tons of crude ore, and does not include the product of one of the three largest producers.
The magnesite from Greece and that from California are practically identical in physical and chemical character, but prior to 1915 the California material could not compete with the Grecian in the New York market, because of the transcontinental freight rate being so high in comparison with the ocean freight on material brought as ballast.
[368]
Magnesite is found in large quantities in Macedonia, occurring as veins in serpentine.
Magnesite deposits, formerly worked, occur in Italy in the Turin district, and on the Island of Elba. None of the deposits seems to be large. An analysis of magnesite from the Island of Elba shows over 8 per cent. silica, a trace of iron, and from 1 to 3.5 per cent. lime.
Magnesite occurs in Norway as small veins in serpentine, but, unlike other magnesite in serpentine, it is crystalline. It is remarkable in that it shows no lime, but it carries over 4 per cent. iron and 9 per cent. silica. It is calcined and made into brick.
Deposits similar to those in Norway are found in Sweden, but on account of their situation, which entails heavy operating and transportation expenses, it is doubtful if they will ever be able to compete with cheaper European magnesite.
Magnesite has been mined in the Orenburg government, Russia. One mine yielded 26,320 metric tons in 1906. Magnesite occurs also on the north slope of the Caucasus Mountains.
—Extensive deposits of magnesite occur in the Transvaal, as veins, that range up to 4 feet in thickness. The rock is used for making carbon dioxide and oxychloride cement. Great deposits of magnesite are reported in Portuguese West Africa. The deposits are near, or associated with, boiling springs.
—The most important occurrence of magnesite in India seems to be in the Madras presidency, in the southern part of the peninsula of Hindustan, where the mineral occurs in interlacing veins. The main deposits have produced more than 2,000 tons in a single year.
Crystalline magnesite occurs in limestone in the Manchuria mountains, and is mined at Daisetsukyo for refractory purposes.
Magnesite is reported in Asiatic Turkey about 75 kilometers from Smyrna.
—The deposits of magnesite in Queensland are so small that they probably have no commercial value. Rounded blocks of pure white magnesite outcrop in one locality in New South Wales, where many thousands of tons are available at small cost. An analysis shows 99.01 per cent. magnesium carbonate and no lime. Large deposits of magnesite are reported in South Australia. Extensive deposits also occur on the north end of the west coast of New Caledonia. A small quantity has been exported.
The magnesite deposits that play a notable part in the world’s economy are situated in Canada, United States, Austria-Hungary, and Greece.
—The principal magnesite deposits in the State of Washington are owned by the Northwest Magnesite Co., Spokane,[369] Wash.; Valley Magnesite Co., Spokane, Wash.; and American Mineral Production Co., Chicago, Ill. The Northwest Magnesite Co. is the largest producer in the Washington field and probably has made the largest investment. The American Mineral Production Co. has a plant at Valley, Wash.; the Valley Magnesite Co. has a deposit near Valley, but is not operating it.
The principal producers of magnesite in California in the summer of 1918 were the Tulare Mining Co., with a mine near Porterville; Porterville Magnesite Co. of California, with mine at Porterville; Western Magnesite Development Co., with a mine at Red Mountain; and Frank R. Sweasy, working the White Rock mine, Pope Valley, Napa County.
—The Veitscher Magnesite Co., of Vienna, and the Magnesite Co., Ltd., of Budapest, formed a combination or cartel, with the understanding or agreement that all the sales of magnesite outside of Austria and Hungary had to be made through Carl Spater & Co., a German firm in Coblenz, Germany. The firm of Carl Spater & Co. formerly owned the works of the Veitscher Magnesite Co., but sold out and obtained the perpetual selling agency when the stock company was formed. The Harbison-Walker Refractories Co., Pittsburgh, Penn., is said to have been the American representative of Spater & Co.
The Austro-American Magnesite Co., whose deposit and works at Radenthein, Austria, represent an investment of $1,800,000, is owned by Americans. The entire stock is absolutely controlled by the principal stockholders of the American Refractories Co., or was so controlled before the war. The Austro-American Magnesite Co., it is claimed, was doing virtually 95 per cent. of the magnesite business in England before the war, and about 65 to 70 per cent. of the business in the United States. This company has a capacity of 150,000 tons of calcined magnesite a year.
When it was found that Carl Spater & Co., who handle both magnesite and magnesite brick made by the Veitscher Magnesite Co. and the Magnesite Co., Ltd., would not sell magnesite to the English refractory brick makers, the Austro-American Magnesite Co. formed a selling company in England, called the Anglo-Austrian Magnesite Co., of Sheffield, England. The English company sold magnesite from the Austro-American Magnesite Co. works at Radenthein, Austria, to all the refractory brick manufacturers of England, and virtually took all the English trade from the Germans.
The General Magnesite Co., of Budapest, has a magnesite deposit and plant at Hizonvich (Hisnyoviz?), Hungary. The stock of this company is believed to be owned principally by stockholders of the General Refractories Co., 111 Broadway, New York. The balance of the stock, believed to be about 40 per cent., is owned by some Hungarians[370] of Budapest, represented by Mr. Gunst, of Budapest, president of the company.
According to the United States Department of Commerce,[154] there are seven companies exploiting magnesite in Greece: The Anglo-Greek Magnesite Co., Ltd., with head offices in London, England; the Société Financière de Grèce, Solon and Lycabettus Streets, Athens; The Internationale Magnesite Werken, with head offices in Rotterdam, Netherlands; L. Carambelas, Limni, Euboea; N. Papantonatos, Limni, Euboea; G. A. Georgidades, Athens, (exploiting a concession on behalf of the General Magnesite & Magnesia Co., of Philadelphia); and Alexiou, Daphnopotamos, Euboea. Most of the producers in 1917 had an abundance of orders for magnesite to be used in the steel industry in France and England.
[154] Commerce Reports, May 1, 1917.
The principal and most essential use of magnesite is in metallurgy, as a refractory material for lining furnaces. Magnesite is also used in the manufacture of Sorel cement and of paper from wood-pulp, in fire-resisting paints, as a non-conductor of heat in pipe and furnace coverings, and in the manufacture of magnesium chloride, light carbonate and other products, including metallic magnesium.
Magnesite occurs in two forms, amorphous and crystalline, and the deposits originate in three ways: by the decomposition of serpentine, as sedimentary deposits, and by the replacement of calcareous sedimentary rocks by magnesium-bearing solutions. In advance of development work it is impossible to make reliable estimates of available tonnage of the first type, but fairly accurate estimates can be made of deposits of the second and third types.
Developed magnesite deposits that have been productive at one time are situated in California and Washington; in Quebec and British Columbia, Canada; on Santa Margarita Island, Lower California; on the Island of Margarita, Venezuela; in Austria-Hungary, Germany, Spain, Greece, Macedonia, Russia, Norway, Transvaal, and India. Other deposits, some of which have produced small amounts, are situated in Nevada, Ontario, New Brunswick, on Cedros Island, Lower California; in Asia Minor, Sweden, Rhodesia, Portuguese West Africa, Australia, Tasmania, and New Caledonia. There is no reason to believe that there will be in the near future any marked shift in the important sources of supply. In 1916 and 1917 the production from the deposits of the Pacific Coast of the United States increased very rapidly, but since January, 1918, there has been a severe slump in California production.
The magnesite deposits of California and Washington are owned by a number of companies, all of them American. American refractory manufacturers[371] are believed to be interested in some of the Canadian deposits. The deposits on Santa Margarita and Cedros islands, Lower California, seem to be owned or operated for the most part by residents of California. The deposits off the coast of Venezuela are held by a Philadelphia company. Two of the large magnesite companies of Austria-Hungary have agreed to make all of their sales outside of Austria-Hungary through a German firm in Coblenz. Two other companies, the Austro-American Magnesite Co. and the General Magnesite Co., are owned mainly by Americans. The magnesite deposits of Greece are controlled by seven companies, one of them being English, one American, one Dutch, and the remainder seemingly Greek. The other magnesite deposits of the world are of little importance at present.
[372]
Graphite is produced in several grades which are adapted to different purposes. Amorphous graphite is a trade term applied to non-crystalline or very fine-grained graphite of varying degrees of purity. If crystalline graphite is produced in flakes or scales, it is flake graphite; but if mined from veins it may have other forms, and be known as vein graphite. Lump, chip, and dust refer to products of larger crystals of Ceylon vein graphite more or less broken in mining and treatment. All those three are spoken of as crystalline. Artificial graphite, made from coal or other carbonaceous matter, resembles the amorphous variety.
Graphite for crucible use must be high grade, either lump, chip, or flake graphite, contain at least 85 per cent. graphitic carbon and be free from fluxing impurities. Vein graphite is considered especially desirable for this use. Possibly the increased development of the electric furnace in the steel industry and in non-ferrous metallurgy will reduce the demand for crucibles. Both crystalline and amorphous graphite are used as lubricants. For this purpose the graphite should be free from quartz or other gritty impurities. For foundry facings, amorphous graphite and Ceylon dust are chiefly used; high-grade material is not required. For the better grades of pencils, mixtures of crystalline and high-grade amorphous are needed; for the poorer grades, amorphous is used alone. The graphite used as polish for high explosives is amorphous. This use does not consume large amounts. For the manufacture of electrodes, artificial graphite is considered the most suitable. The graphite used as dry battery filler may be either amorphous, artificial or crystalline. Pure material is required, but the size of grain is not a factor.
Amorphous graphite is used in boiler compounds for preventing hard scale; pure material is not essential. For paints, either amorphous or crystalline graphite may be used and need not be high grade.
For stove polish and shoe polish amorphous graphite is chiefly used; imperial graphite is used as an adulterant in fertilizers, to give the desired dark color.
For amorphous graphite and dust, artificial graphite may be substituted. For crystalline graphite used in the manufacture of crucibles[373] no good substitute is available. However, the use of electric furnaces or open-flame furnaces in non-ferrous metallurgy may reduce the need of crucibles. For lubricating, mica is used in somewhat the same way as graphite but is much inferior. Many other boiler compounds serve the same purpose as graphite. In paints, lampblack is a substitute. Talc is used in connection with and as a partial substitute for graphite in foundry work. Blast furnace graphite, or “kish,” offers possibilities as a substitute for flake graphite for lubricating purposes. Developments along this line, however, have not proceeded far enough to be conclusive.
Amorphous graphite may occur wherever coal or other carbonaceous beds have undergone regional or igneous metamorphism. Crystalline graphite has two principal geologic occurrences, as flakes in schists and as larger crystals in veins. Flake graphite in schists is usually associated with granitic intrusions which appear to have aided recrystallization of original carbonaceous material in the sediments. Vein graphite in commercial quantities is rather rare. It is found associated with granitic intrusives and generally with graphitic sediments containing the flake variety. Such rocks in most parts of the world have not been prospected enough to make sure that all important bodies of graphite are discovered.
In the order of their importance the following table lists the various countries which produce graphite or in which graphite deposits have been reported:
Although no definite data are available, it is believed that Ceylon can not produce much longer at the present rate, and it is possible that the virtual exhaustion of the deposits is not far distant. Madagascar is capable of greatly increased production. It is doubtful if American deposits with less than 5 per cent. graphite will be able to meet free competition from Madagascar, where over 20 per cent. of the rock as mined is graphite. Too little is known of the Greenland and Brazilian deposits to hazard a guess as to their future importance, but it is reported that immense reserves exist in Greenland. The German flake deposits will hardly survive free competition from Madagascar, unless modern methods are introduced. Amorphous graphite will probably be chiefly produced, as at present, by Austria, Bohemia, Mexico and Chosen. Mexico and Chosen are likely to supply most of the pencil graphite of the world. Increased production of artificial graphite may reduce the demand for amorphous graphite. In 1918, notices in the German technical press told of the discovery of immense deposits of flake graphite in Roumania. These were to be worked jointly by the German and Austrian governments under a 75 year lease from the Roumanian government. The outcome of the war of course annulled this arrangement.
Vein graphite (that is, Ceylon graphite) is preferred for crucible manufacture, but increasing amounts of flake are being used successfully. European manufacturers seem to use flake almost exclusively; and American manufacturers will no doubt find it possible, if necessary. Increasing amounts of amorphous graphite are being employed for various industrial uses, chiefly foundry facings. The development of the electric steel furnace, by reducing the use of crucibles, may tend in some degree to reduce the difference in value between crystalline and amorphous graphite.
[375]
Amorphous graphite is so widely distributed that no serious difficulty is likely to be encountered by any of the great commercial nations in filling their vital needs. Mexican graphite is the chief supply of good material for pencils. Interest centers in the material capable of being made into crucibles.
Crucible graphite has been produced in the past mostly in Ceylon. Its granular form (characteristic of vein graphite) has been assumed by crucible makers to be the best. The deposits are worked mostly by small local owners in Ceylon, and the output is controlled by the British through state sovereignty and shipping. Control by ownership and operation with modern plant was attempted by an English company, but met native opposition until a few years ago.
Recently Madagascar flake graphite has largely replaced Ceylon material in European practice. This deposit is under French sovereignty and the French exercise a large degree of commercial control. The flake graphite of the United States has not yet reached a high development. There are large reserves of schist with about 5 per cent. graphite. Bavarian material is crystalline though not of such good quality. It is under German control. Prior to the war, mining and milling methods were primitive, and the product correspondingly poor. Canada, Norway, Sweden, Greenland, Brazil and possibly others have reserves of flake within their boundaries. Japan has a small production of flake graphite and controls a supply in Chosen.
American control of Mexican mines brings the entire Mexican output to this country for refining and re-export. One of the large Canadian mines is also owned in the United States.
When the British supply in Ceylon declined and the Madagascar production increased, the British, who had always controlled the world’s main supply, did not readily relinquish control. They bought a large part of the Madagascar product and have a concentrating plant on the island. The Morgan Crucible Co., of London, operates on the island as the French company “Graphites Maskar,” but this is a subsidiary of the London company and the control is entirely British. This is not the only plant, however, working on the island. One large company before the war had its main office in Hamburg, Germany, one at Antwerp, and there were several French companies. Some Austrian mines were owned by Belgian companies prior to the war, and English interests own a part of the Italian deposits.
During the war the French and British, having adopted the Madagascar graphite successfully, apparently arranged an agreement by which that supply should be used in Europe, while Ceylon graphite was sent mostly to the United States. The control was only possible by a combination[376] of British and French, apparently a commercial rather than a political matter, though subject to government control.
There appears to be a combination among Madagascar graphite producers, as evidenced by a statement from the consul in Tananarive in November, 1918, that the “Union des Producteurs de Graphite” could furnish to this country annually 15,000-20,000 tons of 85 per cent. graphite at a definite price, f.o.b. Tamatave. The object of the combine appears to be to protect producers against unfair practices of the manufacturer.
The Colombo Graphite Union is mentioned in some sources as a local combination, probably interrelated with the Ceylon Chamber of Commerce. It has not been active in improving mining and milling methods, and has also objected to modernization by outside capital.
The Graphite Producers’ Association of Alabama was organized in 1917 and had for its objects the furthering of the interests of the graphite miners of the state. An effort was made to sample and analyze shipments honestly and thus help remove the most serious objections that manufacturers had against using domestic flake—unreliability of product.
Low-grade amorphous graphite is abundant in the United States. There are supplies in many states and in Alaska, which have not been developed to any extent. An excellent grade of material from Mexico is available in large amounts, making extensive domestic development unprofitable, except when the deposits are very favorably situated. The most productive Mexican deposit is owned by the United States Graphite Co., of Saginaw, Mich. Artificial graphite is made at Niagara Falls in large amounts, making the country independent in the matter of electrodes.
This country is not yet independent in the matter of crucible graphite. Crucible makers have insisted on having Ceylon graphite, about 15,000 tons or more a year. The only supply of similar graphite in the country is a very small deposit in Montana. However, there is a fair supply of flake graphite in Alabama, Pennsylvania, New York, Alaska, Texas and possibly other states. Some 3,500 tons a year were produced prior to 1919. This resembles Madagascar rather than Ceylon graphite, though the flakes are smaller. American crucible makers are slow to make use of it, though there is evidence that it might serve very well. If the demand for crucible graphite continues, the demand for imports will probably continue.
Our deposits are not so high grade or so favorably situated as to compete successfully with those of Ceylon and Madagascar.
Canadian companies producing flake similar to that in the United States are in part owned by United States capital.
[377]
The flake graphite supply for crucible makers in normal times may come from Madagascar, but we can be fairly independent in case of necessity if we are prepared to stimulate mining in this country.
England controls the graphite from Ceylon. However, there seems to be a general opinion that Ceylon production is likely to decrease unless the control passes into the hands of some one who will introduce modern efficient mining methods. England normally allows about two-thirds of the Ceylon product to come to the United States. England’s own supply of crucible graphite is now mainly obtained from Madagascar, where an English owned and controlled company operates on the island. Amorphous graphite is obtained from Italy and Chosen. A London company operates in Quebec, producing some flake and dust. English capital is invested in Italian graphite deposits and apparently also in the Spanish.
Through her sovereignty over Madagascar, France probably controls the world’s best future supplies of flake graphite. The deposits are large, conveniently situated, and remarkably rich—20 per cent. or more graphite. They are capable of greatly increased production. Already the output exceeds that of any other country, though the deposits have been developed but recently. France has a small local production of amorphous graphite, and obtains some from Italy.
Germany is now chiefly dependent on Bavarian flake graphite for crucibles. The efforts made during the war to get Ceylon graphite in through Holland and Switzerland indicate that the Bavarian supply is not wholly satisfactory. Amorphous graphite is supplied from Austria. Overproduction has made it possible at times to sell the products in America below cost.
Japan produces some flake graphite. Chosen has an abundant supply of the amorphous variety. In Chosen there have been recent discoveries of crystalline graphite which may be of importance.
Figure 11 shows the changes in the annual output of graphite in the chief producing countries, and Figure 12 shows the percentage of crucible graphites produced by the main sources of supply.
Graphite occurs in nature in two forms, crystalline and amorphous, each form having its own peculiar uses. Crystalline graphite is used in the manufacture of crucibles, as a lubricant, and in paints. Amorphous graphite is used as a lubricant, for foundry facings, in pencils, in paints, as a polish for high explosives, in boiler compounds, in electrodes, in dry batteries, as a stove and shoe polish, and as a filler for fertilizers. Most of the above uses are essential and cannot easily be eliminated. Artificial graphite made from coal or other carbonaceous matter can be substituted for the natural amorphous graphite.
[378]
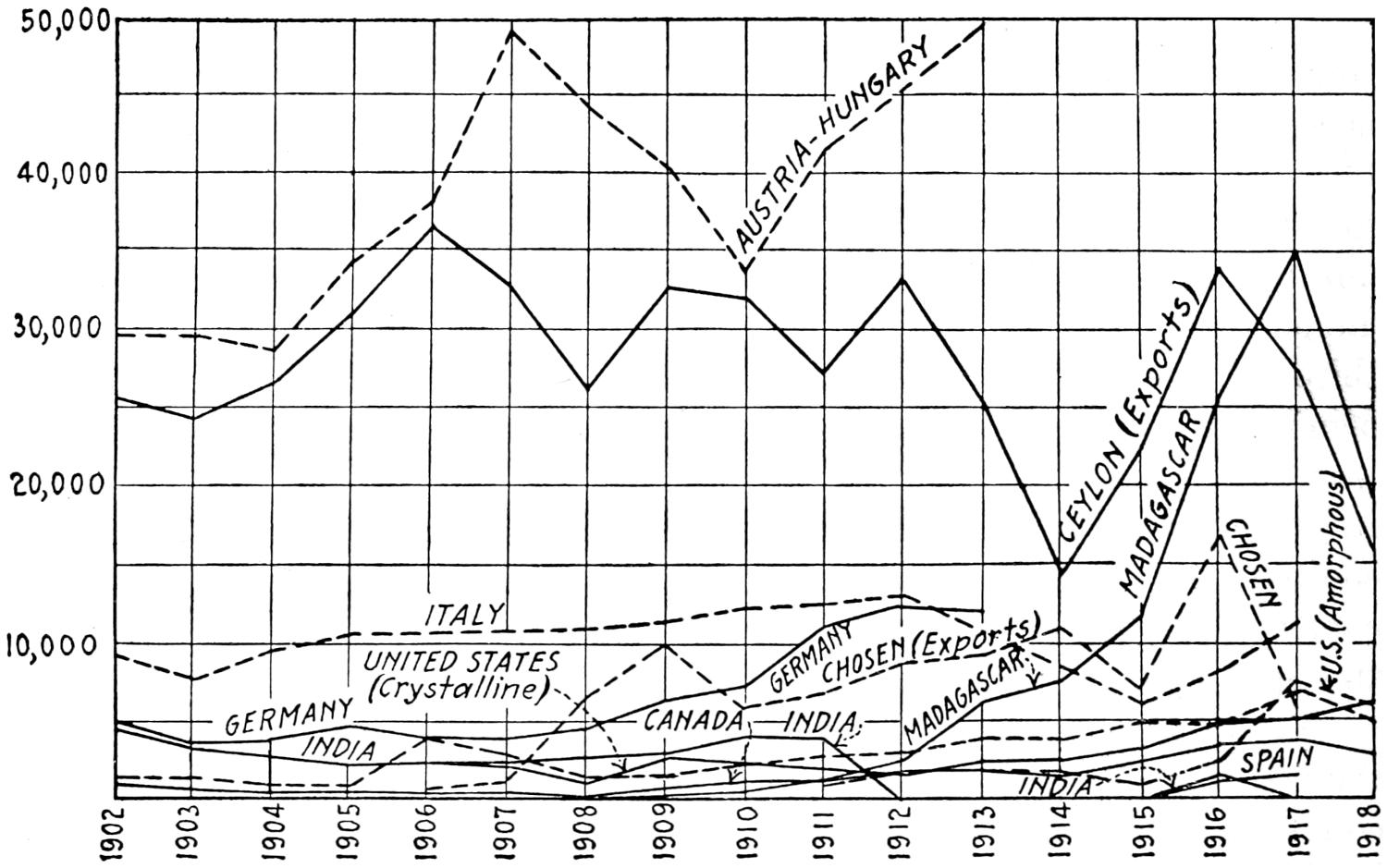
Fig. 11.—Annual output of graphite in chief producing countries, 1902-18. Full lines indicate crystalline graphite; dotted lines principally amorphous graphite.
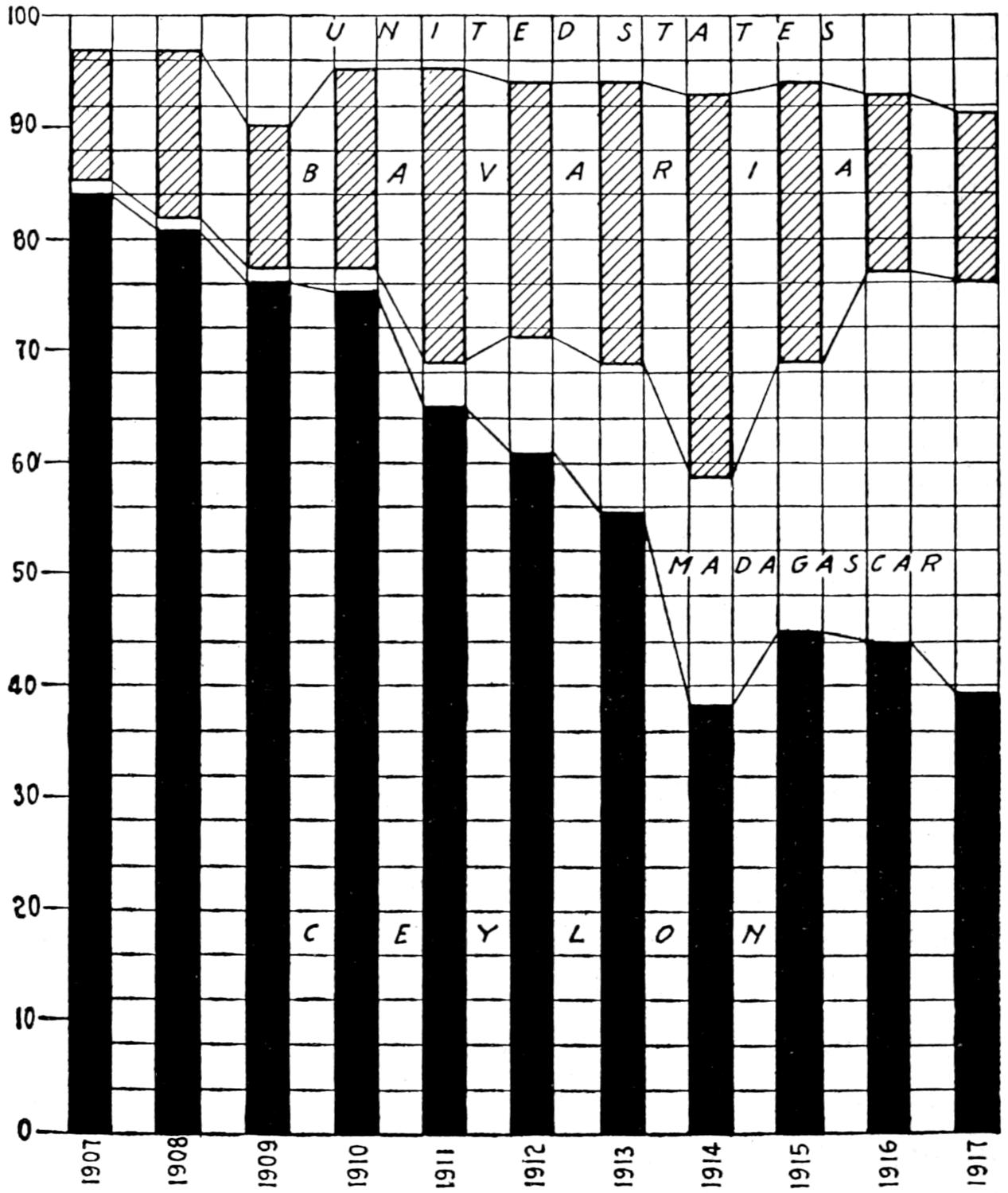
Fig. 12.—Percentage of crucible graphite produced by main sources of supply, 1907-1917. Bavarian data since 1913 doubtful, but since completion of the graph it has been found that the 1917 production was much larger than shown.
[379]
Amorphous graphite may occur wherever coal or other carbonaceous beds have undergone regional or igneous metamorphism. Crystalline graphite, both flake and vein, is usually found in association with granitic intrusives. Since such rocks have not been thoroughly prospected in all parts of the world, it is probable that important new deposits of graphite will be discovered, especially in Canada, Siberia and parts of South America and Africa.
Ceylon is the chief source of supply of the best grades of crystalline graphite, viz., vein graphite. The crystalline graphite obtained in Madagascar, Bavaria, and in small quantities in the United States, Canada, Norway, Sweden, Japan and Chosen is chiefly of the flake variety and for that reason it is considered by manufacturers inferior in grade to that obtained in Ceylon. Large undeveloped deposits are reported in Greenland and Brazil. The discovery of the large deposits of flake graphite in Roumania was reported some time since. From time to time discoveries are reported from other localities but the importance is questionable, chiefly because the deposits are usually situated in places difficult to reach. It is believed that the Ceylon deposits have passed the maximum of their production and if deposits of vein graphite of equal grade and richness could be found, Ceylon producers might be hard pressed. During the last few years Madagascar has become the leading producer of crystalline graphite, and the influence of this potential supply should exert a stabilizing effect on prices of Ceylon graphite.
Austria, Chosen, Mexico, Italy and the United States are the principal producers of amorphous graphite; Chosen and Mexico supply most of the pencil graphite of the world.
The development of the electric furnace will no doubt decrease the demand for crucibles in steel making.
Great Britain, through sovereignty over Ceylon and Canada, and France, through sovereignty over Madagascar, control politically the world’s most important deposits of crystalline graphite. Japan controls the deposits of Chosen.
American capital controls the deposits of the United States, the deposits of Mexico, and in part the deposits of Canada. The Ceylon deposits have been worked mainly by small local owners, who opposed until a few years ago the attempts of an English company to gain control through the erection and operation of a modern plant. The Graphites Maskar, owning a part of the Madagascar deposits, is a subsidiary of a British company, the Morgan Crucible Co. Another large Madagascar company before the war had its main office in Hamburg, Germany. Other companies are controlled by Belgian and French capital. British interests own a part of the Italian and probably a part of the Spanish deposits.
[380]
Two varieties of mica are of particular economic importance: muscovite or white mica, and phlogopite or amber mica. Three other varieties, lepidolite, zinnwaldite and biotite, find occasional commercial use.
Mica is marketed as sheet or block mica, mica splittings, thin sheets split chiefly from smaller sizes of block mica, and scrap or ground mica. The uses to which sheet or block mica may be put depend upon the size, thickness and shape of the piece which can be cut from it and the quality of the material itself. Factors entering into the quality of mica are: presence or absence of stains, spots, inclusions, cracks or pin holes; flexibility and elasticity; hardness; degree of distortion of the sheets; transparency; and dielectric strength.
An essential use of sheet or block muscovite is in electrical work; from the mica are made condensers for radio equipment, magnetos and certain telephone equipment; also to a less extent for resonators in sounding boxes. This mica is also used in making spark plugs, particularly plugs in high-compression engines, for winding cores and as washers in place of porcelain. These uses were widely extended by the war to meet requirements for motor transport, airplanes and radio equipment.
A great deal of this variety of mica is used for other insulating purposes. There are a vast variety of uses, such as for sheets, washers and disks in dynamos, electric-light sockets, guards in rheostats, fuse boxes, telephones, etc.
Sheet or block phlogopite is used for general electrical insulation—particularly where mica softer than muscovite is required.
Mica splittings of both muscovite and phlogopite are employed for the manufacture of “built-up mica,” which is used widely for electric insulation in many different forms, such as sheets, tubes, cups, etc. Mica board built up from phlogopite splittings is used extensively for insulation between the copper segments of commutators.
Among the less essential uses of sheet or block mica (mainly muscovite) are in windows for stove fronts and ovens; chimneys and shades for lamps and lanterns; and for many other purposes where a transparent non-inflammable, non-shattering material is required. It is also used for heat insulation, in various electric heating devices.
[381]
Ground mica is also used for heat insulation, as in pipe and boiler covering, etc.; and as a patent roofing, both as a coating to prevent sticking when rolled, and as a filler in the roofing itself. It is also used in annealing steel, and as a lubricant for wooden bearings.
Among the non-essential uses of mica, those for which a satisfactory substitute is known, are the uses of sheet or block mica for phonograph diaphragms, and for decorative purposes, chiefly in India. On a similar basis are the uses of ground mica (mixed with oil) as a lubricant for metal bearings; as a filler for rubber goods, etc.; and for decorative purposes—in wall paper, decorative paints, ornamental stone, etc.
—No other substance possesses the combination of elasticity, toughness, flexibility, transparency, ability to withstand excessive heat and sudden changes in temperature, high dielectric strength, flatness and amenability to splitting into thin films, which belongs to mica.
For the vast variety of electrical equipment in which mica is used, no satisfactory substitute has been found. In the manufacture of certain low-tension condensers sheets of oiled paper have been used instead of mica films, but attempts to substitute this material more widely have met with little success. According to one report a compressed paper product called “Pertinax” was developed in Germany during the war which is claimed to be “most satisfactory” for all electrical purposes, even for the manufacture of high-tension condensers. The fact, however, that Germany was paying $75 a pound for mica from Norway, and continued to use mica in the manufacture of condensers for airplane magnetos, indicates that complete substitution was not possible.
For a great many glazing purposes it is possible to substitute heat-withstanding or non-shattering varieties of glass.
From the nature of mica deposits there has been little encouragement for the application of any but rather crude and simple methods of mining. These methods proved sufficient as long as there remained new and easily accessible deposits. There is at present a tendency in India, Canada, and the United States to apply more scientific methods to exploration and extraction, where the exhaustion of the easily worked deposits is threatened.
Sorting, cleaning, grading, trimming and cutting of mica for the market are all essentially hand processes, and from the nature of the product will continue to be. For this reason producing localities possessing abundant cheap labor have a distinct advantage over those where labor is high and scarce. In one important direction attempts have been made to apply a mechanical device to a process which has always required[382] hand labor. This is in the manufacture of mica splittings, widely used in the manufacture of built-up mica. These inventions have not yet been demonstrated as commercially successful on a broad scale.
Mica is being used to an increasing extent for electrical purposes. The war created a large demand, particularly for the better grades of material for the manufacture of magnetos and radio condensers and spark plugs, and many of these uses will continue to require much mica. The use of mica for glazing purposes, however, particularly in the fronts of stoves, is diminishing. This is due to the decreasing manufacture of the type of stove in which mica is used.
It has been estimated that mica constitutes about 4 per cent. of the igneous rocks of the world. Segregation into deposits of workable size containing mica of commercial size and quality is comparatively rare. Mica mines are worked for the small percentage of sheet or block mica they contain, the large amount of waste mica being utilized only where a ready market warrants the grinding.
Individual deposits of both muscovite and phlogopite are characterized by their extreme irregularity, so that any prediction as to reserve is uncertain. This fact is responsible for the crude methods of mining which have for so long been almost universally employed. The output of a district as a whole is from many small mines rather than from any single large one.
An important consideration in the geological distribution of mica from an economic standpoint is the degree of dynamic metamorphism to which the region has been subjected during or subsequent to the formation of the mica-bearing deposits. This is due to the fact that the value of sheet mica depends, among many other factors, upon the freedom from distortion of the sheets.
Muscovite mica in commercial quantities invariably occurs in dikes and lenses of pegmatite which are considered to represent segregations of certain portions of granitic intrusions. The principal associated minerals are quartz and feldspar, both of which are usually considerably in excess of the mica. The dikes or lenses of pegmatite may be within the granite itself or in other inclosing rocks that may seem unrelated to a parent intrusive. Schists or gneisses form the inclosing rock of the pegmatite in most commercial deposits.
Phlogopite is much rarer than muscovite and occurs in deposits structurally similar to granitic pegmatites. The important associated minerals are pyroxene, apatite and calcite, although in certain deposits the mass is almost entirely of mica. The Canadian deposits, which are best known, consist of veins and pockets in pyroxenite dikes and the[383] inclosing rock is usually limestone, a significant fact as regards the origin of the deposits.
India, Canada, and the United States produce about 98 per cent. of the sheet mica of the world. The output of India and of the United States is entirely muscovite, whereas the Canadian production is almost entirely phlogopite. Brazil and Argentina have become important producers in the last two years, and German East Africa was becoming of considerable importance immediately before the war. South Africa, Guatemala, Ceylon, Madagascar and Australia have produced small amounts of mica and may be considered as potential sources of supply.
India produces about 65 per cent. of the sheet mica of the world, and is the most important source of high quality muscovite. The two principal producing regions are the Hazaribagh district, in the Province of Behar, and Orissa, in Bengal, and the Nellore district, Madras Presidency.
The output of Canada is practically all phlogopite or amber mica, of which that country is the world’s principal source. The important producing districts are north of the city of Ottawa, in the Province of Quebec, and in the central part of the Province of Ontario. The Lacey mine, in Ontario, has been the largest producer of amber mica in Canada for many years.
The United States produces about 15 per cent. of the sheet mica muscovite of the world. In terms of value, about 70 per cent. of the American product comes from North Carolina, and 23 from New Hampshire. Other districts are insignificant: 3 per cent. comes from Virginia, 1 from South Dakota, and 3 from Georgia, Alabama, Idaho, and Colorado taken together.
The principal sources of mica in Brazil are the states of Bahia, Sao Paulo, Goyaz and Minas Geraes. The two last mentioned are particularly important. The deposits are widely scattered over a large area.
Production of mica in Argentina has been incidental until very recently and the development has been slight. Deposits are numerous but are of special importance in the provinces of Cordoba, San Luis and San Juan. As far as is known muscovite is the only mica produced.
Considerable activity has been shown in mica mining in Guatemala and shipments have been made quite regularly to this country. The mica, however, is of inferior grade and has not found favor in American markets.
The production of the former German East Africa is confined entirely to muscovite mica found in the districts of the Ulguru Mountains and neighboring ranges. Considerable quantities of high-grade material were exported to Germany before the war. The mica mines in German East Africa were worked during the war under the direction of the[384] British Ministry of Munitions. The district is of importance as a future source of high-grade muscovite.
The principal locality in South Africa is near Leydersdorp, in the Northern Transvaal. Both muscovite and amber mica are reported from this area.
Small amounts of high-quality amber mica have been shipped from Ceylon to England.
Deposits of excellent quality mica are known to exist in the McDonald range, in South Australia, from which small shipments have been made sporadically. This is the only district from which lepidolite or lithia mica has been noted in sheets of commercial size.
In China, large deposits of muscovite mica are reported from the vicinity of Kiao-Chau bay and also from Shantung, but no commercial development has taken place, and it is believed that the deposits are worthless as a source of sheet mica.
Prior to 1914 efforts to produce mica in Norway on a commercial scale met with no success and little is known of the deposits. The great need of mica in Germany during the war stimulated production in Norway, tremendous prices being paid for the material.
The most important change in known geographic distribution of mica for the near future concerns the increased development of the South American fields. During the war Brazilian mica found considerable favor both in this country and Europe, and the better grades are considered equal to the best India mica. Importations of mica from Brazil were of much importance in meeting the large demands of the United States for mica of high quality to fill government needs. Owing to the nature of mica deposits in general, it is not safe to make estimates concerning reserves. This is particularly true of Brazil, where the industry is young, and careful prospecting and development have not been carried far. From the large number of known deposits and the rapidity with which the industry responded to war demands, despite difficulties in transportation from the mines to the seaboard, it would appear that Brazilian mica will play an increasingly important part in the mica markets of the world, particularly of the United States. No matter how great the merit of the mines of a district may be, however, unless the mica is carefully prepared, graded as to size and quality, and shipments are standardized, it cannot expect to attain permanent favor among consumers in this country or in Europe.
Less is known concerning the future possibilities of Argentina as a mica-producing country of importance. Many deposits are known and considerable shipments have been made to the United States and to[385] Europe. The material received here has not been equal to Indian or Brazilian mica.
There is every reason to expect that India will retain its position as the most important mica-producing country. The great number of deposits offer almost every grade of muscovite required in the trade. The industry is well-established, and labor conditions are extremely favorable for the production of a commodity requiring such a large amount of hand labor before being ready for market. It is true, however, that the richest and most easily accessible deposits have been mined out, and scientific methods must be applied to mining if production is to retain its former place.
It is doubtful whether the immediate future will see any important change in the position of the United States as a mica producer. Although the reserves of the country as a whole are probably large, the deposits are small and the percentage of high-quality mica is not great. Production of important amounts of medium-grade mica will continue. Labor conditions are not favorable to producing cheaply an article that has a large part of its value determined by preparation and careful grading.
Scientific methods have been applied to the mining and preparation of a considerable part of the Canadian output. Reserves are large and Canada will undoubtedly retain for many years its position as the principal source of amber mica.
The British Empire, through state sovereignty, controls 75 per cent. of the present sheet-mica production of the world. In Brazil and Argentina, which have important potential resources of mica, and in the United States, political control is determined by state sovereignty.
For the most part, the ownership of mines and concessions in India has been in the hands of natives. In the Hazaribagh district, F. F. Christen & Co., an English firm, owns rights over large areas of land outside the government forest and has mined on a considerable scale. At the outbreak of the war, Germany, through commercial interests, had obtained a large measure of control over many Indian mines. S. O. Fillion & Co., of New York, is the one American firm known to be working mines in India.
The most important producing phlogopite mine in Canada, at Sydenham, Ontario, is owned and operated by the General Electric Co., of Schenectady, New York. The same company owns several other properties in the vicinity and has a large mica-manufacturing plant at Ottawa, Ontario. Other smaller interests are held chiefly in Canada.
Davol & Co., of New York, is the only foreign firm known to be actively working mica mines in Brazil at present.
Mica mines in the United States are, as far as is known, all owned by Americans.
[386]
The United States was before the war the world’s largest consumer of mica of all kinds. The development of her electrical industry is dependent upon her supply of mica, a large part of which is imported from India and Canada. Production in this country has been considerable, but has not proved nearly adequate to supply the demands, particularly the demand for the higher grades of mica for use in magnetos, radio condensers and spark plugs, and for mica splittings used in making built-up mica.
The large demands and high prices created by the war did not increase domestic production enough to warrant the belief that this country can become independent as regards sheet mica under any but the most artificial conditions. The producer is protected by a 25 per cent. import duty on unmanufactured (rough or knife-trimmed) mica and a 30 per cent. duty on cut mica, splittings and other manufactured forms. Production in this country is largely in the hands of individuals and small companies, who are financially incapable of increasing output spasmodically even under very favorable conditions. Moreover, many consumers have a decided prejudice in favor of imported mica.
If the United States is to take a dominant position in the electrical industry of the world an adequate supply of mica must be assured. The most promising field for the development of such a supply is undoubtedly South America. This is particularly true because the important development of the electrical industry in England during the war places that country in the dominating position formerly occupied by Germany and Austria. This development will require a large part of the Indian mica formerly available for export trade.
The British Empire possesses in India the most important source of muscovite mica in the world and in Canada the only important supply of amber mica. In spite of England’s tremendous advantage with regard to raw material, Germany, through her important position in the electrical industry and the large measure of control acquired in the Indian mines, threatened to dominate the mica market of the world at the outbreak of the war. Since the outbreak of the war England has secured her position not only as the controlling center for raw mica but as the chief producing nation of electrical equipment. London is the distributing center of the world for Indian mica and London prices regulate the market. During the war Indian mica was controlled by the British Ministry of Munitions, and allotments were made to the associated nations at fixed prices.
In the development of the South American fields lies the best possibility of the world lessening its dependence upon England for this most essential raw material.
France is entirely dependent upon outside sources for her supply of[387] mica. Before the war her demands were not large and were filled by Indian mica.
Although Germany before the war was entirely dependent on outside sources for mica, her leading position in the electrical industry enabled her to gain control of much of the Indian production. Every advantage was taken of this opportunity, and in 1914, according to the British Secretary of Munitions, the mica market of the world was at the point of being transferred from London to Hamburg. The deposits of German East Africa were being actively exploited and German commercial interests were being extended to South America.
Although probably possessing very large stocks in 1914, Germany felt very acutely the shortage of mica during the war. High prices were paid to Norway for the output of that country, but this source is probably entirely incapable of meeting the normal demands of Germany.
Sheet mica is essential to the manufacture of a vast variety of electrical equipment, and must, therefore, be classed as one of the important raw materials of the world. The magneto, such a vital factor in modern transport on land, air and sea, depends upon mica for its construction, and mica condensers are indispensable in modern radio equipment.
India, Canada, and the United States are at present the most important mica-producing countries. Deposits of future importance from which production has thus far not been great are known in German East Africa, Brazil, Argentina and the Transvaal; from several other parts of the world more sporadic production is reported.
Unlike the development of many other raw materials the production of mica has not been universally undertaken on a large scale, nor have scientific methods been applied to its extraction. This has been largely due to the irregular nature of the deposits and their scattered position within a district, making considerable investment of capital in mining a particularly hazardous venture. Trading interests have, therefore, played an important part in controlling production and markets.
The British Empire, having within its boundaries a large proportion of the important mica-producing districts, at present dominates the situation politically. British commercial control, threatened by Germany’s leading position in the electrical industry and wide interests in Indian mines at the outbreak of war, is now firmly re-established, and Great Britain has taken the place in the electrical industry formerly held by Germany and Austria.
The United States, the largest consumer of mica among the nations of the world, has relied upon imports to supply a considerable part of the consumption and will probably continue to do so in the future, although steady development of domestic sources of supply may be expected. Brazil offers the most promising foreign field for the development of an independent source of supply for American markets.
[388]
Asbestos is useful because of its incombustibility, insulating qualities, and fibrous structure. High-grade asbestos is spun or woven into ropes and fabrics, the fabrics being used for many purposes, such as safety curtains, mats, mattresses, upholstering, firemen’s suits and gloves. Of late years much high-grade asbestos has been used for friction facings in brakes and for packings. Low-grade asbestos is used for a great many purposes, which may be classed in three groups—building, insulating, and miscellaneous.
For building purposes asbestos is employed in many ways. A mixture of asbestos and cement is used to make fireproof shingles or slates. Asbestos is also used with Portland cement to make a protective surface on metal sheathing; asbestos paper is used for weather and sound proofing and also for fire-protection purposes. It is used widely for plaster, which not only is fireproof but also improves the acoustic properties of auditoriums, churches, etc. Asbestos is also used for floor tiling and in the manufacture of paints. Asbestos lumber and millboard are employed for many structural purposes.
Asbestos cement, a mixture of asbestos fiber and clay, is much used as a covering for boilers and steam pipes to prevent heat radiation. Other coverings are made from a mixture of asbestos and magnesia. Varieties of asbestos having a low iron content are useful for electrical insulating purposes.
Some of the many miscellaneous uses of asbestos are for fire brick, acid filters, lead-fume collectors, stove mats, cooking-utensil linings, etc.
The most essential of the above uses are those in which the long-fiber asbestos is employed. For fireproof ropes and fabrics nothing can take its place. Its use in friction facings for brakes is essential in all motor vehicles.
The uses for which low-grade asbestos is employed may be regarded as less essential and might be eliminated in case of necessity, though such elimination would no doubt involve many serious difficulties. Fire risk would thereby be increased and many boilers and heating plants would be rendered less efficient and more wasteful, though these difficulties could be overcome to some degree by the use of substitutes.
[389]
—Slag wool or mineral wool is a fireproof material and a good non-conductor of heat and sound. It is also highly porous, and hence it is useful as an absorbent. It can, therefore, be used to some extent as a substitute for asbestos for heat insulation and fireproofing. However, as it is brittle and cannot be woven as readily as asbestos, it is not to be regarded as a satisfactory substitute for the higher grades. Talc may be employed in the manufacture of fireproof and corrosive-proof paints, and also as a lining for furnaces and fireplaces. Infusorial earth is used to some extent as a substitute for asbestos for insulating and fireproofing. But it is important to note that none of the substitutes mentioned above can replace the high-grade spinning fiber.
As has been indicated, there is a probability of the demand for asbestos increasing. The wide use of motor-transport equipment demands a large amount of high-grade fiber for brake linings, while the increasing use of steam equipment, and electrical equipment and appliances, demands more and more material, both for electrical and heat-insulating purposes. Although substitutes may be employed for the lower grades, no substitutes are known for the spinning grades of asbestos, which are now in strongest demand. Consequently there are no changes in practice that will reduce the demand for asbestos.
Asbestos originates for the most part from rocks consisting largely of olivine, such as peridotites or dunites, or from rocks consisting largely of pyroxene. Hence it is only in altered rocks of this nature that asbestos of the common types is to be expected. Two distinct types of alteration are common: Alteration of the olivine or pyroxene to serpentine, with development of chrysotile in places; and alteration of olivine or pyroxene to amphibole with development of anthophyllite (a variety of amphibole) and related forms. Both types of alteration are well represented in the great North American belt of asbestos-bearing peridotites that extends from central Alabama along the Piedmont Plateau to the Gaspé Peninsula of Quebec, a distance of more than 1,600 miles. In the northern part of the belt, in Vermont and Quebec, the alteration has been to serpentine with chrysotile asbestos in places, while in the southern part the alteration has been largely to amphibolite with development of anthophyllite asbestos and talc.
Chrysotile asbestos (Quebec type), as represented in most deposits, is formed by serpentinization, with subsequent prismatic crystallization in cracks, the veins thus formed representing a recrystallization along the[390] walls and thus being replacement veins rather than fissure veins. Contact metamorphism evidently plays an important part in the process, for in most regions intrusive dikes are associated with the deposits and evidently had a definite influence on the development of the commercial product. As serpentinization is a deep-seated process, chrysotile deposits may occur at considerable depth.
Anthophyllite asbestos (Georgia type) results from alteration of peridotites or pyroxenites to amphibole, giving the fibrous anthophyllite and related forms. This type of asbestos does not occur in veins but constitutes the major part of the rock mass.
While the modes of alteration noted above account for most of the asbestos deposits known, there are two important exceptions—the crocidolite, or blue asbestos, of South Africa, and the chrysotile deposits of Arizona, both of which occur in sedimentary rocks. Crocidolite is interbedded in jasper and ironstone, and the Arizona chrysotile has resulted from the alteration of cherty limestone influenced to some extent by the action of diabase intrusions.
As pointed out in the discussion of uses, for various select purposes anthophyllite cannot be employed as a substitute for chrysotile of spinning grade. As the uses of anthophyllite are thus restricted, and as the supply seems to be ample for many years to come, the problem of supply centers about the deposits of high-grade chrysotile.
Deposits of serpentinized basic rocks are by no means rare, and even the development of a fibrous structure is common, but in all chrysotile-bearing deposits only a small part of the serpentine is fibrous, and of the fibrous part only a small percentage can be utilized as high-grade asbestos. The value of deposits of most ores depends largely on the percentage of metal and of impurities present, and little attention need be given to the physical character of an ore. The value of asbestos, however, depends not only on purity of composition, but on very definite physical properties, such as length of fiber, flexibility, and tensile strength. Such properties result from a combination of favorable conditions of crystallization, conditions that are at present little understood.
A large body of serpentinized basic rock bears, therefore, no certain promise of an asbestos supply, though it may offer encouragement for prospecting. High-grade fiber is evidently formed under a peculiar combination of geologic conditions which involve the presence of certain primary rock types, their alteration to secondary minerals and recrystallization of these in veins, such recrystallization being induced by a combination of metamorphic agencies. Although it is quite probable, therefore, that new fields will be discovered, there is no probability of an abundant supply.
An important point to be considered in connection with future supplies is that for most uses of asbestos there is little or no scrap recovery;[391] that which is once used is for the most part gone beyond recall. It is wise, therefore, to maintain a conservative view of the asbestos reserve, for although there is evidence of a supply in the Canadian deposits and elsewhere to last for many years, the probability is that deposits of high-grade material are neither numerous nor extensive.
The total production and estimated reserves of spinning asbestos in the important producing countries are shown in Figure 13.
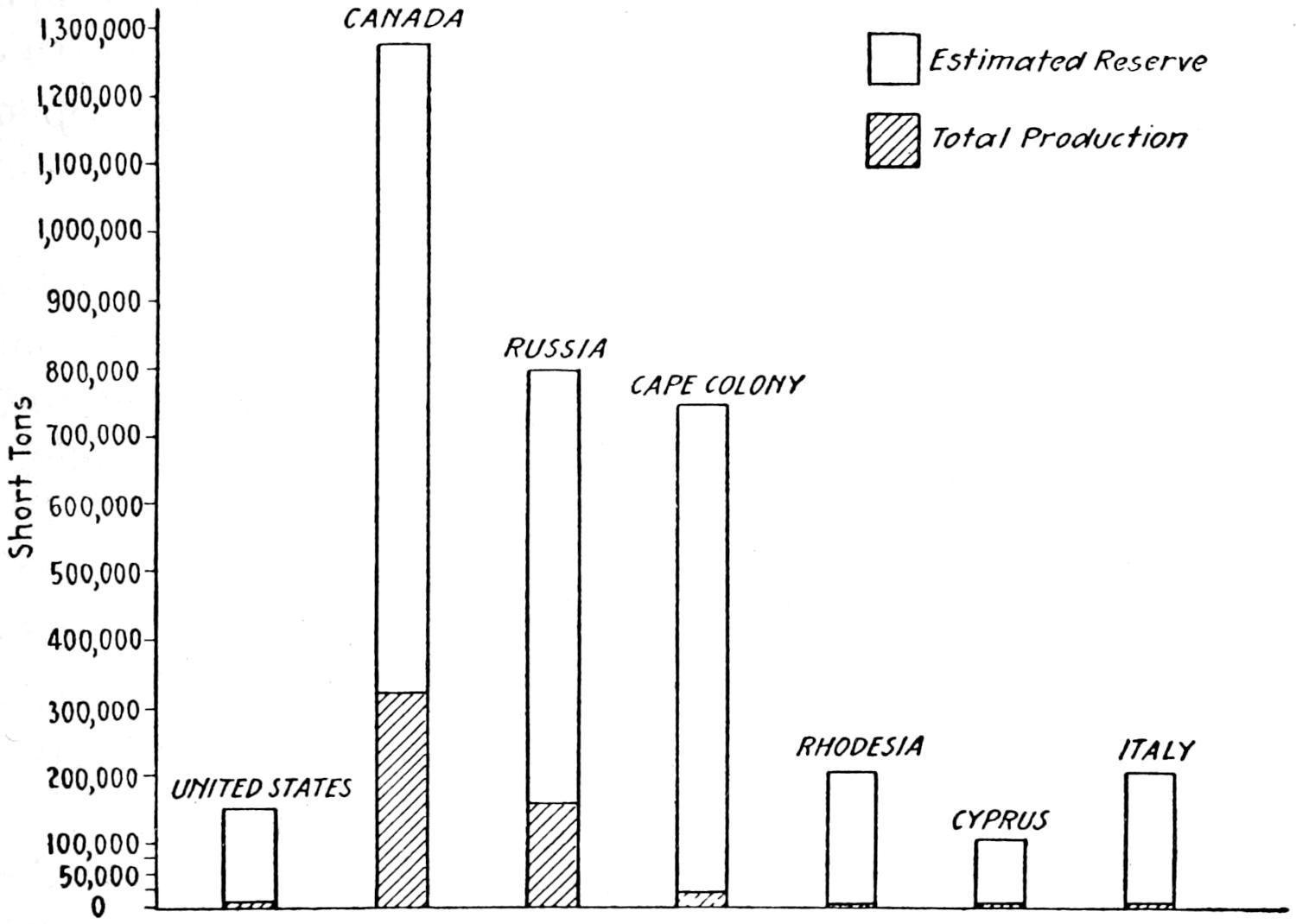
Fig. 13.—Total production and estimated reserves of spinning asbestos.
—Asbestos in the United States is of two types, chrysotile and anthophyllite. The chrysotile variety occurs in Arizona, Vermont, Wyoming, and California, and the anthophyllite in Georgia, Virginia, Maryland, Massachusetts, Connecticut, Rhode Island, and Idaho.
—The asbestos deposits of Arizona are unusual in being cross-fiber chrysotile veins in cherty limestone, and thus quite distinct in origin from the Quebec deposits. The asbestos-bearing beds are thin and some of them are almost inaccessible.
Asbestos was first discovered in Arizona at two points in the Grand Canyon of the Colorado, west of the mouth of the Little Colorado River. The mineral occurs in a single bed, 12 to 14 inches thick, the veins being parallel with the bedding. In some places the veins are 4 inches thick, but usually they do not exceed 2¹⁄₂ inches. The visible supply, therefore, is not great, and as the district is difficult of access production has been slight. Asbestos also occurs in Ash Creek Canyon near Globe. Production began in 1914, and has increased considerably since. The[392] asbestos carries only 0.5 per cent. iron oxide, while Canadian asbestos runs 2.2 per cent. to 2.6 per cent. iron oxide. The Arizona material is, therefore, superior to Canadian asbestos for electrical insulation. It is estimated that under favorable conditions the region can supply 1,000 tons annually for many years to come, but that the output will never be large as compared with that of Canada. In 1914 an asbestos deposit similar to those of Ash Creek was reported near Young postoffice. It is 80 miles from the nearest railroad station and will probably be inaccessible for many years. Near the summit of Mount Baker, north of the Roosevelt Reservoir, a mine producing good chrysotile was opened in 1917. The asbestos occurs in cross-fiber veins in limestone.
The asbestos deposits of Vermont are situated at Lowell, in the same formation as the Quebec deposits. The asbestos occurs in numerous irregular veins, the supply is probably large, and some of it compares favorably with the Canadian product. There was a considerable production in former years, but after 1912 the quarries were idle. The reserves of low-grade rock are probably large.
The main deposits of Wyoming occur south of Casper in igneous Archean rocks. The mineral is chrysotile asbestos, mostly of the cross-fiber type, though some slip-fiber is present. It is claimed that the Wyoming asbestos has better heat-resisting qualities than the Canadian fiber. However, a very small proportion of the fiber is of spinning grade.
Deposits of chrysotile asbestos have been noted in many parts of California, scattered over 13 counties. The deposits seem to be small, and thus far the production has not exceeded a few tons a year. A small production is recorded for 1917 from Nevada and Inyo counties. There was an increased output in 1918, the total being 229 tons. Some No. 1 spinning fiber was obtained in Nevada County.
—The anthophyllite asbestos deposits in Georgia yield the largest production of any state in the Union. The material is not of spinning grade and is practically all used for fireproofing and insulating. There is a considerable demand for such material, however, and the industry is established on a firm basis. A notable feature of the Georgia deposits is that approximately 95 per cent. of the rock quarried is fibrous anthophyllite of commercial quality, whereas in Quebec only about 6¹⁄₂ per cent. of the material quarried can be utilized.
Anthophyllite asbestos deposits occur in Virginia near Bedford. The material is low-grade and the amount is probably small. A little is consumed in the manufacture of “tenax,” a preparation used by dentists. Amphibole slip-fiber asbestos has lately been mined on a small scale in Maryland, near Pylesville. It is used for filters. Several years ago a small production was recorded from Dalton, Massachusetts, and New Hartford, Connecticut.
Anthophyllite asbestos occurs in Idaho, near Kamiah. It is not of[393] spinning grade, is of low tensile strength, and is inferior to all but the lowest grades of chrysotile. The deposit is evidently large, but the production is almost negligible.
—The most important asbestos-producing deposits of the world are those of the province of Quebec, Canada, chiefly in the region of Thetford and Broughton. Asbestos occurs in serpentine of Cambrian age, the area in which the important mines are found extending from southern Vermont to Gaspé, in the Province of Quebec. The serpentine lies in three prominent belts. The Danville-Eastman-Vermont serpentine belt is about 62 miles long. The scattered outcrops probably are connected beneath the heavy drift deposits and forest growth. Prospects have been worked in six places, but the production attained is small. The belt is an uncertain quantity, that gives fair promise of a large future supply.
The Thetford-Black Lake area is the important area of Quebec and now the most productive asbestos district of the world. In 1917 there were 17 active mines in the district. In 1918 the quantity of rock mined was 2,445,745 tons, and the total asbestos production was 159,225 tons, valued at $9,053,945. The Broughton and the central and eastern Thetford areas are mainly slip-fiber asbestos. The West Thetford, South Ireland, and North Coleraine Township deposits constitute the vein-fiber belt which yields the high-grade spinning asbestos that has a world-wide reputation.
Serpentine rocks bearing chrysotile asbestos are reported near Port au Port, in Newfoundland, probably representing a continuation of the Quebec belt. The possibility of commercial development is uncertain.
—Asbestos deposits have been reported in Brazil, but aside from this no commercial deposits have been noted in South America.
There is no record of asbestos deposits in Mexico or in Central America.
—The asbestos deposits of Russia probably rank next to those of Canada. The principal mines are 57 miles north of Ekaterinburg, in the Ural Mountains. The quarries can be worked only from May to November in each year. Transportation is over the Perm Railroad, and the output is exported via Riga. The fiber is of the same type as the Canadian, a chrysotile asbestos, chiefly of the cross-fiber type. The richest ore yields 42 to 55 pounds of asbestos per cubic yard. Production has also been reported by the South Urals Asbestos Co., operating in the Orsk district, in Orenburg. Russian asbestos is said to be harsher than Canadian and less suitable for spinning, but a great deal of high-grade fiber has been produced and Russia is likely to be an important future source of asbestos.
The asbestos of Italy is of the anthophyllite variety. There are three main districts: the Susa Valley deposits, near the French border,[394] which lie 6,000 to 10,000 feet above sea level and are, therefore, not readily available; the Aosta Valley deposits, of wide extent, with long-fibered, strong, and soapy product; and the deposits in Lombardy, also of great extent. Italian asbestos may be used to some extent as a substitute for Canadian fiber, or to mix with it, but the supply of high-grade fiber is not great, and it is more difficult and expensive to work than the Canadian material. The United Asbestos Co., of London, England, is the largest producer.
Large deposits of asbestos are known on the island of Cyprus. The material is derived from serpentine and is of the amphibole anthophyllite type. Much of it is short-fibered but some of it can be used to mix with Canadian fiber.
Good spinning asbestos has been noted in central Finland but production up to the present time is almost negligible.
Asbestos deposits have been noted in England, Scotland, Ireland, France, Portugal, Spain, Switzerland, Germany, Norway, Greece, and Turkey, but all the above deposits are said to consist of coarse and brittle material of little commercial value. A large deposit of good asbestos is reported in the island of Corsica.
—Deposits are known in various districts of India. Asbestos is also reported in Afghanistan. Indian asbestos is of inferior tensile strength, and the lack of development renders it an uncertain resource.
The United States Geological Survey reported in 1912 that three asbestos mines were in operation 45 miles northeast of Antung, China. The product, which was shipped into Manchuria, is of the amphibole (anthophyllite) type and quite brittle. Chrysotile asbestos of good quality is reported south of Lake Baikal, in Mongolia. It has never been mined, and on account of its remoteness is not likely to be developed. Deposits yielding a small output are reported from several other provinces.
Asbestos deposits occur in several localities in Japan. The output is of inferior quality and is mixed with imported material for asbestos packing. The Japan Asbestos Co., of Osaka, is the chief manufacturer of asbestos products.
Deposits have been opened at Minusinck, on the Yenesei River, in Siberia, but production is reported for the year 1905 only. Transportation is difficult.
—The asbestos of Cape Colony is crocidolite, or blue asbestos; it is of the amphibole type and will not bear high temperature, probably on account of its iron content, but is longer, stronger, finer, and more elastic than chrysotile. On account of its low fusibility it is useful in electric welding. The mineral occurs in three important districts and outcrops at numerous points from the Orange River north to Bechuanaland. Government engineers report it to be the largest asbestos-bearing[395] area in the world. The principal deposits are at Koejas, where the Cape Asbestos Co., Ltd., produced in 1916 about two-thirds of the total amount of asbestos mined in South Africa. Blue asbestos is gaining favor in foreign markets, and this fact, in connection with the great extent of the deposits, indicates that these deposits constitute an important factor in world supply.
Large and probably extensive deposits of chrysotile asbestos of the finest quality occur in the Transvaal. Three companies have recently operated in this district. Production, which began about 1914, in which year 30 tons were reported, had increased to a total of 407 tons in 1916.
A new and important development is the mining of a long-fibered amphibole asbestos known as “amosite.”
A small output of asbestos from Natal has been reported for several years; however, the fiber is not high grade and an increase of production is not likely.
Important deposits occur in southern Rhodesia. The Southern Rhodesia Geological Survey reports that there is in sight several years’ supply for an output of 200 tons per month without going deeper than 60 feet. The “probable ore” supply is very great. It has been stated that the Rhodesian fiber is the only class of chrysotile asbestos that can compete successfully with the best grade of Canadian fiber.
—A chrysotile-bearing serpentine belt covers a considerable area in Queensland. A deposit occurs near Rockwell, South Australia. The Australia Asbestos Manufacturing Co. has produced a small amount of material similar to Italian asbestos. A small amount of chrysotile has also been found. A deposit of chrysotile prospected in New South Wales is claimed to have the longest asbestos fiber in the world. No production has been reported. In the Pilbarra district, West Australia, there is chrysotile asbestos of spinning grade which is said to be superior to either the Russian or Italian product. Some years ago a mine was worked to considerable depth by the Pilbarra Asbestos Co., of London, England, but in recent years the production has been almost negligible. In New Zealand chrysotile asbestos of spinning grade occurs in Nelson Province. The Australasian Asbestos Co., of Sydney, has recently prospected the serpentine belt of Tasmania, which contains both chrysotile and anthophyllite asbestos.
The United States leads all nations in the manufacture of asbestos products, and the large supply of asbestos in Quebec is readily available. There is, therefore, little prospect of any radical change in the geographical distribution of American asbestos mines. As there is always a possibility of such changes, however, it is well to consider the controlling factors.
[396]
As to the future of the Quebec deposits, the present source of supply, little definite information is available. The fact that serpentinization is a deep-seated process has led Canadian geologists to conclude that commercial fiber may be formed to the full depth of the original peridotite rock. For all practical purposes, however, the depth of the deposit is limited to the depth at which asbestos can be extracted profitably. One mine is now working at a depth of 300 feet and drill holes sunk 400 feet farther indicate a thickness of at least 700 feet of good fiber-bearing rock. Cirkel has stated that in one of the Black Lake quarries there is 44,377,500 tons of asbestos rock in sight above the railway tracks, ready for immediate exploitation. A deduction of 50 per cent. for waste rock would leave 22,000,000 tons of mill rock available, or enough to supply for 22 years a plant capable of producing 4,000 tons a day. As this includes only the visible ore it may be inferred that the reserve is very great. Cirkel estimates the total acreage of productive vein fiber as 12,420, of which 1,100 acres is under development at the present time. A geologist who spent two summers studying the geology of the region states that second-grade fiber is very abundant and that the high-grade deposits are not more than 25 per cent. exhausted. There is, therefore, no prospect for many years of any change in the geographical distribution of working mines through the exhaustion of present deposits.
During 1916 and 1917 the production of high-grade fiber in the United States grew steadily, but is still far from meeting domestic demands, as the total United States output in 1917 was only one-eightieth of the amount imported. The most important development has been in the high-grade chrysotile districts of Arizona, but these new deposits do not give promise of an abundant supply, and it is unlikely that they will constitute a dominating factor in American production.
Legislation may have a profound effect on the development of deposits. A high export duty placed on raw asbestos by a country now producing it in large quantities would have the effect of encouraging prospecting in other countries and the development of deposits that might supply substitute material. In this connection it is interesting to note that the Board of Trade of the eastern townships of Quebec proposed measures to protect the export of raw asbestos, in order to force the manufacture of asbestos products in Canada. The Canadian Mining Institute Bulletin (August, 1916) pointed out the dangers of such action, for other countries would immediately search for asbestos deposits elsewhere, and as good asbestos occurs in Russia, South Africa, Cyprus, and other localities, substitutes for Canadian material could probably be found. It is evident, therefore, that Canada does not control the supply, but that so long as Canadian fiber is available at reasonable prices there is no strong incentive for the development of new deposits.
In the Old World the situation is less stable than in America. European[397] countries import considerable material from Canada, and the balance of their requirements is filled from various sources, chiefly from Russia, Cyprus, Italy, South Africa, and Australia. High-grade asbestos deposits exist in various countries and are for the most part developed to a small extent. The factors determining changes in production are somewhat different from those outlined for America. The lack of a strong central government greatly hampers production in any country. Thus under recent conditions in Russia the output fell from nearly 20,000 tons in 1913 to about 9,000 in 1916. Stabilized conditions and more efficient governments would tend to increase the output of several eastern countries. The most important factor contributing to the slow development of the Old World deposits is poor transportation. Russian asbestos for the English market has to pay transportation costs of $25 to $30 per ton. The important crocidolite deposits of South Africa are likewise hampered by poor transportation. Not only are the roads poor, but the most important deposits of Koegas are 18 miles from a traveled road, and other deposits are about 100 miles from roads. This drawback is offset to some extent by cheap labor.
The present political upheaval in Europe, involving the formation of new states and new forms of government, may have a pronounced effect on the development of asbestos deposits, but until progressive governments are established and vast improvements made in transportation, no great increase in production is possible.
A large share of the asbestos deposits of the world, being situated in British colonial possessions, are under the political control of Great Britain. The deposits of Cyprus are at present under British political control, but this may not be permanent. Before the war, Cyprus was nominally Turkish, though administered by Great Britain; in 1914, Great Britain formally annexed the island. Cyprus was offered to Greece in return for her assistance in the war, but the offer was not accepted.
From information available it is evident that the governments of Russia and Italy have in the past imposed no serious restrictions on the development of their asbestos properties through either domestic or foreign capital.
Below is set forth the commercial control of the asbestos deposits of the world, by countries, in the following order: United States, Canada, Russia, South Africa, Italy, Cyprus, and Australia.
In the Globe district of Arizona, in the United States, spinning asbestos is produced by the Johns-Manville Co., working the Snell & Fisk property, and by the American Ores & Asbestos Co. The Sierra Asbestos Co., near Nevada City, California, produced spinning fiber in 1918. As far as[398] is known all the companies operating in the United States are American owned.
The asbestos mining industry in Canada is confined to the eastern townships of the Province of Quebec. The largest company is the Asbestos Corporation of Canada, Ltd., with head office in Montreal. This company operates the Kings and Beaver mines, at Thetford Mines, the British Canadian mine, at Black Lake, and the Frazer mine, at East Broughton. The company is controlled by English, Canadian, and United States capital. The Bell Asbestos Mines Co., the Asbestos & Asbestic Corporation, and the Manville Asbestos Co. are wholly or largely owned in the United States. The remaining companies are mostly controlled by English or Canadian capital, though United States interests are represented in some of them. Evidently, therefore, the ownership of the companies is divided between English, Canadian and United States capital, with British interests probably predominating.
The most important of the Ekaterinburg mines in Russia are the Voznesensky and Zoe-Anonsky, near Bazhenof. About one-third of the total output of the Urals came from these mines in 1916. It is reported that prior to the war a German syndicate controlled several Russian mines which produced in all more than 80 per cent. of the entire Russian output. Germany and Austria were the chief buyers of Russian asbestos before the war.
In South Africa the crocidolite of Cape Colony is mined largely by the Cape Asbestos Co., a British firm with mines at Koegas and Westerberg, and having factories in England, Turin, and Hamburg. A sister company in France, Compagnie Française de l’Amiante du Cap, handled in 1916 about two-thirds of the total South African production and was the chief manufacturer of blue asbestos products. In the Transvaal, asbestos is mined by three companies, The Transvaal Asbestos Syndicate, now absorbed by the Consolidated Gold Fields; the South African Minerals Option Syndicate, a subsidiary of the Bechuanaland Exploration Co., and the Anglo-Swiss Asbestos Co. British capital predominates. The Rhodesia Asbestos Co., Ltd., was the chief Rhodesian producer until recently, but in 1917 the Rhodesia and General Asbestos Corporation was organized with a capital of £400,000 to take over the operating mines. The commercial control is, therefore, British.
The chief producer in Italy is the United Asbestos Co., of London, England.
Prior to the war the Cyprus deposits were worked by the Cyprian Mining Co., an Austrian corporation. As mining concessions are obtained from the British government by lease on a royalty basis, it is probable that the lease has now been cancelled.
The deposits in Australasia are practically all controlled by English or Australian capital.
[399]
Mines that have no milling equipment can produce crude fiber which may be treated at manufacturing plants. The various grades of mill fiber may be produced only where mills are located at or near the mines. As the utilization of all grades can be accomplished only with the assistance of mills, such mills are necessary for efficient mining. Consequently, with other factors equal, mines with near-by mills have a distinct commercial advantage over mines that produce crude fiber only. Although mills are not essential factors in the asbestos-mining industry, they exert a secondary influence in commercial control through the increase in mining efficiency that they render possible. For deposits remote from centers of manufacture, mills are of little advantage, as fiber below spinning grade will not bear heavy transportation charges. Most of the United States and Canadian mines have mills for treatment of rock bearing short fiber. Several of the Russian mines are similarly equipped, but in other parts of the world little or no milling is done.
A number of important manufacturers of asbestos products in the United States are owners of or have intimate trade agreements with large Canadian asbestos mines, and also with some of the domestic mines. Hence as regards commercial control the United States is practically assured of a supply of raw material.
Although the United States is the largest manufacturer of asbestos products in the world, in 1917 less than 1 per cent. of the raw material was mined in this country. The country is, therefore, largely dependent on foreign sources of supply. The abundant deposits of Quebec, Canada, are conveniently near, and so long as the present amicable relations with Canada continue, an ample supply seems to be assured. In 1916 the United States used 86 per cent. of the Canadian output. During 1916 and 1917 there was marked activity in developing the high-grade chrysotile deposits of Arizona. While there is as yet no evidence of an abundant supply, the material is an important supplementary source of supply because of its quality.
While no commercial asbestos is mined in the British Isles, British colonial possessions hold control of about 88 per cent. of the annual asbestos output of the world and approximately 70 per cent. of the world’s reserves. Thus, although the supply within the British Empire is ample, the home requirements of the nation can be met only under favorable shipping conditions, as all necessary material must be transported several thousand miles.
Russia is the second largest producer of asbestos in the world, and seemingly has large reserves. As little manufacturing is done in the country, practically the entire output is exported. Being independent[400] as regards her own needs for raw asbestos, Russia requires only the maintenance of an active foreign market to assure a permanent industry.
No commercial asbestos deposits are known to exist in Germany. Prior to the war asbestos was imported chiefly from Russia and Canada. The chief Russian mines are said to have been controlled by German capital.
Italy has large deposits of amphibole (anthophyllite) asbestos, some of which is of spinning grade, but as production has always been small and has, except for minor fluctuations, been stationary for the past 18 years, it is unlikely that the deposits can supply domestic requirements of high-grade fiber. A small amount has been exported for filter use, for which Italian asbestos is well adapted. As the chief mine is operated by a British company, considerable Italian asbestos is shipped to England.
No asbestos deposits are worked in France. Supplies are obtained from Russia, Canada, and South Africa. France is the leading nation in the manufacture of blue asbestos products.
Several deposits of asbestos occur in Japan, but all are of inferior quality. The material mined is mixed with imported fiber for the manufacture of asbestos packing.
Asbestos is a unique mineral for the reason that it combines incombustibility and insulating qualities with a fibrous structure that makes possible its manufacture into fabrics, felts, and similar wares. The spinning grades of asbestos are most in demand and the problem of supply hinges largely on the deposits of high-grade chrysotile. Such material is used for the manufacture of ropes, safety curtains, mats, packings and friction facings in brakes. The lower grades are used for making fireproof shingles and other building materials, for insulating, and for fire brick, acid filters, etc. Although some substitutes may be found for the lower grades, no substitutes are known for spinning fiber.
Asbestos occurs in three main types, chrysotile, crocidolite, and anthophyllite; the first and second provide most of the spinning fiber, and the third is almost all of non-spinning quality. The most important deposit of chrysotile asbestos is in Quebec, Canada, but large deposits are worked in Russia and Rhodesia. Crocidolite is mined only in Cape Colony, South Africa. Large deposits of anthophyllite occur in the United States, Italy, and Cyprus.
The United States is by far the largest manufacturer of asbestos products in the world, but produces only a small fraction of the necessary raw material; it is practically assured of an ample supply of this because the largest deposits in the world are in the adjacent Province of Quebec, Canada. The Arizona deposits provide an excellent grade of fiber and constitute a promising supplementary source of supply, though the[401] estimated reserves are not great. The British Empire holds a dominating position, controlling about 88 per cent. of the annual asbestos production of the world and approximately 70 per cent. of the estimated reserves. Canada is far in the lead of all countries, supplying about 85 per cent. of the world’s output. Russia was, before the revolution, second to Canada as a producer; because of the cost of transportation the chief output is spinning fiber. South Africa has large reserves of good fiber, but the output is handicapped by poor transportation.
Exhaustion of the chief sources of supply is not likely for many years, nor is there immediate prospect of any material shift in the centers of production, though with improved transportation a shift to South Africa is possible. The demand for high-grade asbestos will probably increase at a steady rate.
All the asbestos quarries in the United States seem to be American owned. The Canadian deposits are controlled by Canadian, English, and American capital, British interests probably being predominant. British companies evidently hold exclusive control of the present output in South Africa, Australasia, and Italy. Before the war the Russian output was largely controlled by a German syndicate, and the Cyprus output by an Austrian company.
[402]
Phosphate rock is chiefly used, after treatment with sulphuric acid, as an ingredient of artificial fertilizers. A small quantity is finely ground and used directly as fertilizer. Lesser quantities are used for making phosphoric acid and phosphorus. Phosphorus plays an important part in military operations, being used for incendiary bullets and smoke screens. Phosphorus also is a common ingredient of matches and the striking surface on boxes of safety matches, and it enters in small proportion into phosphor-bronze, phosphor-copper and phosphor-tin.
—Substitutes for phosphate rock may be classed as natural and artificial. Natural substitutes are phosphatic limestone; other phosphate-bearing minerals, such as apatite, nelsonite, and wavellite; guano; marl; animal excrement and bones. Artificial substitutes include basic slag and manufactured compounds, like ammonium phosphate.
Phosphate rock is a sedimentary deposit containing phosphate of lime. It occurs as a hard rock interstratified with beds of sandstone, shale, or other sediments; as amorphous nodular concretions or pebbles in stream deposits; and as a residuum from the decomposition of phosphatic dolomite or limestone, or other rocks containing phosphate of lime. Another type of deposit commonly classed as phosphate rock is the porous coralline or other limestone of tropical islands which has been permeated with phosphate leached from guano.
Phosphate deposits of the western United States, Algeria, Tunis, and Egypt are hard rock beds of the first type. Amorphous nodular deposits occur in South Carolina, part of Florida, Wales, England, Belgium, north-central and eastern France, and Russia. The deposits in Tennessee, Kentucky, and some of those in Florida are residual. Leached guano deposits are found on the islands of Aruba, Curacao, and Sombrero, in the West Indies, and on Christmas, Ocean, Makatea, Angaur and other islands in the Indian and South Pacific oceans.
The reserves in the United States are fairly well known and are[403] estimated at 6,000,000,000 tons. Reserves of high-grade rock in Algeria and Tunis have been estimated at 300,000,000 tons. No information is at hand regarding the quantity of phosphate rock in Egypt or in Europe, except that Russia is believed to have 80,000,000 tons in one of its fields. The deposits in the South Pacific islands are estimated at 70,000,000 tons. Before the war the world’s output of phosphate rock was about 6,000,000 tons annually, of which about one-half was mined in the United States. The next largest production is made in northern Africa.
Phosphoric acid is derived also from apatite, a calcium phosphate that occurs in veins. Apatite has been mined in the Province of Quebec, Canada, and in Spain.
In the Western Hemisphere phosphate rock is produced in the United States, in Canada, the Dutch West Indies, and French Guiana, and occurs in Peru and Chile.
—The principal deposits of phosphate rock in the United States are in Florida, South Carolina, Tennessee, Kentucky, Arkansas, Montana, Idaho, Wyoming, and Utah. Although by far the largest deposits are in the western states, those deposits yield less than 1 per cent. of the whole because of the lack of a large near-by market and because of high freight rates on the crude rock. It is not a matter of common knowledge, but it is, nevertheless, a fact, that the western rock phosphate deposits are so extensive as to be practically inexhaustible, even if the entire world depended on them for its supply of phosphate.
The Florida phosphate deposits, which are the most extensively developed in the United States, comprise three classes of phosphate—hard rock, land pebble, and river pebble. The first is highest grade, the second is produced in largest quantity, and the third is not mined at present. The hard-rock deposits lie in a narrow strip along the western part of the Florida peninsula from Suwanee County to Pasco County, a distance of approximately 100 miles. The land-pebble phosphate area, just east of Tampa, is about 30 miles long and 10 miles wide. Sales of Florida phosphate declined tremendously after 1913 through the restriction on exports by the war. In 1913 the sales were 2,500,000 tons, valued at $9,500,000, and in 1915 the production was 1,350,000 tons, valued at $3,700,000.
South Carolina produces land rock phosphate in the vicinity of Charleston. River-pebble phosphate occurs in the same area but is not mined. Some of the South Carolina output has been exported annually. Sales decreased from 169,000 tons in 1911 to 83,000 in 1915, and the value in the same years from $673,000 to $311,000.
[404]
Tennessee deposits of rock phosphate are in the west-central part and extreme northeast corner of the state; the latter have not been mined. Three types are recognized and known by their colors as brown, blue, and white rock; the last has not been mined recently. The brown rock is sold under guarantee of 70 to 80 per cent. tricalcium phosphate; the blue rock varies considerably in its phosphatic content. Sales of Tennessee phosphate in 1914 were 483,000 tons, valued at $1,823,000; by 1915 they had fallen to 390,000 tons, valued at $1,328,000.
Kentucky has been an insignificant producer of phosphate rock in recent years. Arkansas phosphate deposits are in the north-central part of the state. The output is small.
Four western states possess enormous deposits of high-grade rock phosphate, but their output is as yet insignificant, being only 3,000 to 5,000 tons a year. The producing states are Idaho, Utah, and Wyoming. Montana is not a producer, although it contains extensive deposits easy of access and close to rail transportation.
Idaho has an unlimited supply of high-grade phosphate in the southeast part of the state. A small quantity is mined in Bear Lake County. The Utah deposits are east of Great Salt Lake, in the Wasatch and Uintah ranges, and east of Bear Lake. These deposits are extensive, but the rock is leaner than the general run of the Idaho phosphate, averaging nearer 60 per cent. than 80 per cent. tricalcium phosphate. Western Wyoming also is rich in rock phosphate, the deposits being mostly in the Owl Creek, Wind River, Gros Ventre, and Salt River ranges. Some of the beds are thick, carrying 80 per cent. tricalcium phosphate, and extend for many miles. They constitute a reserve supply that may be called inexhaustible. Small local demand for fertilizer and lack of cheap transportation may retard for some years the development of the great and rich western deposits.
An estimate of the quantity of rock phosphate available in the United States was made several years ago and need not be revised to account for that mined in the meantime. It is repeated here:
Reserves of Phosphate Rock in the United States
| Long tons | |
|---|---|
| Florida | 227,000,000 |
| Tennessee | 88,000,000 |
| South Carolina | 9,000,000 |
| Kentucky | 1,000,000 |
| Arkansas | 20,000,000 |
| 345,000,000 | |
| Western States: Montana, Idaho, Utah, and Wyoming | 5,367,082,000 |
| Total | 5,712,082,000 |
[405]
—The principal phosphatic rock in Canada is apatite, which occurs in workable quantity in two main districts—one in the Province of Ontario, the other in the Province of Quebec. These deposits, which were worked mainly by quarrying, are now practically abandoned. Rock phosphate occurs in a thin bed near Banff, Alberta, but is not used.
—In Aruba and Curacao, islands of the Dutch West Indies, off the coast of Venezuela, are deposits of phosphate rock, from which a small quantity is mined and shipped to Europe. It is reported that the output in 1914 was about 100,000 tons, averaging 85 to 90 per cent. of calcium phosphate.
In Peru, in the Department of Ica, is a deposit of nodular lime phosphate, which is not used because of a local preference for guano.
A large, rich deposit of phosphate is reported in Chile, about 300 miles north of Valparaiso, but has not been developed as yet.
Phosphate deposits occur on the Island of Salut and on the Connetables, close to the coast of French Guiana. The rock is exported.
—The high-grade phosphate deposits of Belgium are exhausted, only low-grade deposits remaining. The rock is found in layers and pockets, and carries between 25 and 65 per cent. of bone phosphate. The production from 1911 to 1913 averaged more than 200,000 tons annually.
The principal deposits in France are in the Somme and Oise basins. The best French deposits are higher grade than the Belgian, as they carry 50 to 80 per cent. of bone phosphate, but they are nearly exhausted, only low-grade material remaining. The production from 1910 to 1914 was about 300,000 tons annually.
Important deposits of phosphate rock in Russia can be divided into the northern, central, and southern groups. The deposits of the southern group were the only ones exploited before the war. Their output was about 25,000 tons a year—very small in comparison with the size of the deposits, which are estimated to contain more than 1,500,000,000 tons. Some of the rock is high grade, carrying as much as 75 per cent. tricalcium phosphate, but the normal grade is about 50 per cent.
The only deposits worked extensively in Spain are apatite veins in the Province of Caceras. After lying idle many years these deposits were reopened and produced 28,000 tons in 1917.
Low-grade phosphate in the form of beds of nodules occurs in England, and in Wales. The production has been slight because the deposits are too small for commercial exploitation.
—The principal deposits of phosphate rock in Tunis are the Gafsa fields, in the southern half of the country. There phosphate occurs in beds several feet thick, but only those carrying more than 58 per cent. phosphate of lime are exploited. The deposits can be traced for several hundred miles, and constitute a reserve of hundreds of millions of tons.[406] Tunis now produces more phosphate than any other foreign country, its annual output being between 1,500,000 and 2,000,000 tons, most of which goes to southern Europe.
The deposits of phosphate rock in Algeria are continuations of those in Tunis, the important mining districts being in eastern Algeria. The production is over 500,000 tons a year, and the exported rock carries 58 to 68 per cent. of lime phosphate.
Extensive deposits of phosphate occur in Egypt, near the Red Sea, in thin and irregular beds of the same geologic age (Eocene) as the deposits in Tunis and Algeria. The best deposits average 70 per cent. lime phosphate and the output in 1916 was 125,000 tons. There are mines 20 miles from Port Safalga, and concessions 12 miles inland from Kosseir and also at Sebaia, on the eastern bank of the Nile between Keneh and Assouan. Beds of phosphate are found in other districts on both sides of the Nile valley. Practically all the raw rock phosphate produced contains 65 per cent. or more of tricalcium phosphate and is exported mainly to Japan.
Deposits of phosphate occur 80 to 120 kilometers from the city of Tripoli in beds more than 1 meter thick. These beds probably are a continuation of the phosphate deposits in southern Tunis.
Deposits of phosphate are reported in Morocco 125 kilometers south-southwest of Casa Blanca on the west coast and 70 kilometers from the end of a railroad. These deposits are said to be comparable to the Gafsa field, in Tunis.
It is reported that at Dielor, in Senegal, about the latitude of Cape Verde, the westernmost point on the African coast, there is a phosphate bed which is 2 meters thick to a depth of 64 meters. The rock carries only 50 per cent. tricalcium phosphate, so it is not workable under present conditions, especially in view of the abundant high-grade rock in Algeria and Tunis.
Phosphates have been found in Natal, near Weenen, Ladysmith, and Byrnetown, in the form of phosphatic shales and of nodules. The percentage of tricalcium phosphate in the phosphatic shales is too low for use in making superphosphates; the phosphatic nodules are of higher grade but not abundant enough to be of value.
—In the government of Uralsk, in southwestern Siberia, bordering on the north end of the Caspian Sea, there is reported to be 600,000,000 tons of phosphate rock. It is said that the greater part of this material carries 17 to 20 per cent. phosphoric acid, which is equivalent to 36 to 43 per cent. tricalcium phosphate. The government of Turgai, which borders Uralsk on the east, is reported to contain 67,000,000 tons of phosphate rock, most of which carries 18 to 19 per cent. phosphoric acid, or about 40 per cent. tricalcium phosphate. The highest-grade material reported is 24 per cent. phosphoric acid, equivalent[407] to about 52 per cent. tricalcium phosphate. All the phosphate therefore is low grade. No production is reported from either of the localities.
Low-grade phosphate rock, in sedimentary beds of considerable extent, and high-grade vein deposits are reported in Palestine, on the east side of the Jordan. The sedimentary deposits occur also on the west side of the Jordan. The known reserves are about 3,500,000 tons. The sedimentary deposits average about 48 per cent. and the vein deposits 77 per cent. tricalcium phosphate. As the vein material is suitable for export, these deposits have been explored by a French company, but available information indicates there has been no output.
—After the discovery of phosphate rock on Rasa Island, 500 miles east of Formosa, a number of years ago, a Japanese company was formed to exploit the deposits. The rock is rich, carrying 75 per cent. phosphate of lime, and the reserves are estimated at 2,800,000 tons. In 1915 Rasa Island yielded 50,000 tons. A former German supply of phosphate is on Angaur Island, in the Pelew group, east of the southern end of the Philippines. Reserves on this island are estimated at 2,000,000 to 4,000,000 tons of phosphate rock, mostly of high grade. Germany increased the output from 45,000 tons in 1910 to 90,000 tons in 1913. Japan has held this island since October, 1914, and is mining 30,000 tons or more phosphate annually.
Deposits of phosphate, consisting of replacements of dolomitic coralline limestone, and phosphatic guano are reported on several other islands in Oceania, as Baker and Fanning Islands, in Polynesia, and Fais Island, in the West Caroline Islands. It is probable that on other islands there are commercial deposits as yet undiscovered.
—North of Adelaide, in Australia, are pockety deposits of phosphate; they are without regular stratification and are of varying quality. The annual output has been 4,000 to 6,000 tons for several years. In the Otago district, near Clarendon, New Zealand, beds of phosphate 3 to 12 feet thick rest in pockets in limestone. There has been very little if any production.
On Christmas Island (Straits Settlements), which lies in the Indian Ocean 190 miles south of Java, rock carrying 80 per cent. of bone phosphate is quarried and shipped to Australia and Japan. The deposits seem to be irregular, but are estimated to contain several million tons of rock of very high grade. The island belongs to the government of Singapore. Exploitation of the deposits by the British began in 1900. Exports in 1913 were 150,000 tons.
Phosphate rock of high grade is mined on Ocean Island, in the Gilbert Archipelago, between the Marshall and Solomon Islands, east of New Guinea and north of New Zealand. On this and other so-called coral islands in the equatorial belt which for ages have been sea-bird rookeries, leachings from the guano have impregnated the limestone, forming phosphate[408] rock many feet deep. The deposits on this island are said to be many millions of tons and are among the richest in the world. They have been mined since 1901, and have produced as high as 300,000 tons a year. In 1916 the output was 70,000 tons of rock carrying about 85 per cent. tricalcium phosphate. The island is a British possession.
Another British possession in the Gilbert Archipelago containing phosphate rock is Pleasant Island, which is also known as Nauru, or Ngaru, Island. The deposits are similar to those on Ocean and Christmas Islands, being very high in calcium phosphate and low in iron and alumina. Germany formerly owned this island, but it was taken over by the British in 1917.
Makatea, near Tahiti, in the Society Islands, is estimated to contain 10,000,000 tons of very high-grade phosphate rock, irregularly distributed between reefs and pinnacles of dolomite. The deposits were developed as recently as 1910 and yielded more than 300,000 tons before 1917. Some of the rock carries 85 per cent. lime phosphate. The island is a French possession.
When the World War began, exports of phosphate rock from the United States, ordinarily about 1,000,000 tons a year, were cut off and the annual production of the United States fell from 3,000,000 tons to 1,800,000 tons. There has been a strong recovery in the domestic industry and if labor and transportation conditions improve there should shortly be an annual production of nearly 3,000,000 tons for domestic consumption, or as much phosphate rock for our own use as formerly was produced for ourselves and a large export trade.
It is surmised that northern Africa will yield larger quantities in the future than during the pre-war period. Production in Algeria, Tunis, and Egypt was probably stimulated during the war on account of the large reduction in the quantity of American rock sent to Europe.
Japan doubtless will make a large output from the German deposits in Polynesia which came into her possession at the beginning of the war.
Ownership of the phosphate deposits in the United States is largely domestic; some of the Florida hard-rock deposits are owned by French and (before the war) German companies. The German-owned deposits were taken over by the Custodian of Alien-Enemy Property, and have doubtless passed into other hands. The phosphate deposits on Curacao, Dutch West Indies, are worked by the Curacao Phosphate Mining Co., which ships the output to England and Germany. Phosphate deposits in[409] Algeria and Tunis are exploited by French companies. Some of the companies work under lease. La Compagnie des Phosphates de Paris and La Compagnie Algerienne des Phosphates have been mentioned as engaged in these fields. Deposits on the lower Nile and Red Sea are worked by the Egyptian Phosphate Co., a British concern, and by the Societa Egiziana per l’Estrazione de il Commercio dei Phosphati, a company managed by Italians. It is reported that much of the output goes to Japan. The Pacific Phosphate Co., Ltd., of London, operates under concession the phosphate deposits on Ocean and Pleasant islands. Japanese companies are mining phosphate on Rasa and Angaur islands.
The United States has the largest known deposits of phosphate rock in the world, and, as before the war, can supply the needs of other countries as well as her own. Since mining began about 55,000,000 tons have been mined, or less than 1 per cent. of reserves. Great Britain possesses phosphate in Egypt and on Christmas, Ocean and Pleasant islands, and has imported from the United States and probably from northern Africa. France and the other Mediterranean countries have an ample supply in Algeria and Tunis. Germany formerly possessed rich deposits of phosphate on Angaur Island, in the Pelew group, Polynesia, and on Pleasant and Ocean islands, but so far as known now lacks a source of supply. Japan has a large supply of high-grade phosphate at her disposal on Rasa and Angaur islands.
The principal use of phosphate rock is as an ingredient of fertilizers. Lesser quantities are consumed in the manufacture of phosphoric acid, in phosphorus used in military operations, in the manufacture of matches, and in metallurgy. Both natural and artificial substitutes are available for many of the uses of phosphate rock.
Phosphate rock is a sedimentary deposit containing phosphate of lime. It occurs as a hard rock between beds of sandstone or shale, as amorphous nodular phosphates in stream deposits, and as a residuum from the decomposition of phosphatic dolomite, limestone, and other phosphate-bearing rocks. The porous limestone of tropical islands, where it is permeated with phosphate leached from guano, is commonly classed as phosphate rock.
The phosphate rock deposits of present commercial importance are situated in the United States, Algeria, Tunis, Egypt, and the islands of the Indian and South Pacific oceans, the United States possessing by far the largest reserves. Smaller deposits, either undeveloped or nearly[410] exhausted, are in Canada, Venezuela, Chile, Belgium, France, Russia, England, Spain, South Australia, and New Zealand.
During the war the exports of phosphate rock from the United States decreased greatly. With the return to normal conditions, however, the United States should experience little difficulty in becoming once more the principal source of phosphate rock.
The principal phosphate-rock deposits are controlled politically by the United States, France (Algeria and Tunis), and Great Britain (Egypt). A number of phosphate-bearing islands in the Pacific Ocean were owned by Germany before the war, but have been seized by Great Britain and Japan.
The commercial control of the deposits of the United States is mainly in the hands of Americans, although German (before the war) and French interests own some of the Florida hard-rock deposits. The deposits of Algeria and Tunis are controlled by French companies. The Egyptian deposits are controlled by two companies, one British and the other Italian.
Germany will be without a source of supply under her own control now that she has lost her colonies.
[411]
The term potash is commonly used to designate any of the salts of the element potassium, particularly those soluble in water, which are largely used in agriculture and manufacturing. The element potassium is widely distributed as a component of rocks, soils, and vegetable and animal substances, but large quantities of potassium salts in forms suitable for the uses of man have been found in only a few places. Ashes of wood, which were formerly the principal source of potash in commerce, now supply an inconsiderable part of the world’s requirements. Since 1860, the principal, in fact almost the only, commercial source of potash salts has been the immense deposits in northern Germany. The crude potassium salts obtained from these deposits by mining operations are used either as fertilizer in the form in which they are taken from the ground, or are purified by crystallization or manufactured into various compounds of potassium needed for both agriculture and other industrial uses.
Use in agriculture as an ingredient of the so-called artificial or chemical fertilizers accounts for 90 per cent. or more of the world’s consumption. Potassium is not only one of the ten or more chemical elements essential to plant life, but of these ten it is one of three that frequently become so lacking in soils that the yield of crops is not profitable. Even if potassium exists in soils, it may be and often is present in some form not readily available for plant use, so that the addition of fresh, readily water-soluble salts of potassium shows prompt reaction in stimulating the growth of the crop. In a general way potash is supposed to supply a necessary plant food, that strengthens the stalk and fills the kernels of the growing plant. Also it is a general belief that some destructive plant diseases, such as blight and rust of cotton and potatoes, are largely favored by improper nutrition as well as by poor physical condition of the soil, for which potash seems to be a specific. Thus the so-called potash industry, by which is meant the mining and marketing of the principal or commonest compounds of potash, is based chiefly on use in agriculture.
The other uses of the salts of potassium are many and diverse. Most[412] of the potassium salts have properties similar to the corresponding salts of sodium, and for most industrial purposes the salts of these two elements may be interchanged, a generalization which does not, however, apply to any extent whatever to the agricultural application of potassium. Some industrial preferences for the potassium salt depend on more favorable physical properties of the potassium over the sodium salt, such as a less tendency of the potassium salt to absorb moisture from the air. Other preferences depend on slight chemical differences, as for instance a somewhat greater solubility, which renders the purification of the potassium salt more easy. During the scarcity and high prices of the wartime period many substitutions have been made, which either directly or by some modification of practice are now proving so satisfactory that they will probably be continued.
One of the most urgent demands for potash has come from the match manufacturers. Potassium chlorate is an important ingredient of most matches, and this use consumes a surprisingly large amount of potash. Certain varieties of glass, especially cut glass tableware (flint glass) and some optical glass, are made from potash, generally in the form known as glass-makers’ carbonate. Most soap is made from soda, but potash (as caustic or hydroxide) is used for some of the finer grades, such as shaving, toilet, and shampoo soaps, especially the liquid forms. Caustic potash has had a considerable use in laundries, and for the scouring or washing of wool. The old form of black powder was made from potash as potassium nitrate or saltpeter; hence the commonly assumed military importance of potash. Black powder, though now largely superseded by modern high-explosive powders, still has a relatively small though nevertheless important application in modern warfare. There are other uses, which, though requiring a small total amount, include some important requirements, among these being the medicinal or pharmaceutical, tannery, dye, photographic, electroplate, and metallurgical needs.
The great deposits of potash in northern Germany underlie an extensive area in the Prussian provinces of Saxony, Hanover, and in the duchies of Anhalt and Brunswick. The most important mining regions lie in an area practically encircling the Harz Mountains, 75 to 150 miles southwest of Berlin, and 100 to 150 miles south to southeast of Hamburg. This area is outlined more exactly on the accompanying index map (Fig. 14).
The field originally opened at Stassfurt has since been explored by deep boring and developed by the sinking of mining shafts, with the result that there has now been outlined an estimated reserve of 20,000,000,000 metric tons of crude potash salts, which at the present rate of the world’s[413] consumption should be sufficient to last almost 2,000 years. Thus for all practical purposes the field may be considered as inexhaustible.
Fig. 14.—Distribution of potash deposits in northern Germany.
In 1904 another important and extensive deposit of water-soluble potash salts was discovered by boring in the valley of the Rhine River in southern Alsace. Alsace, then in possession of Germany, has now been restored to France. The deposit is in two essentially continuous beds that underlie within accessible depth an area of 70 to 80 square miles of the flat bottom lands of the Rhine Valley. The beds are estimated to contain about 1,500,000,000 metric tons of crude potash salts, which average considerably richer in potash than the output of the north German deposits, and, being of simpler chemical composition, are more readily refined. The mines opening these deposits are readily accessible to water transportation by way of the Rhine River and the canals of the Rhine Valley. The distance to ocean ports is considerably longer than it is from the north German deposits, being about 375 miles, as compared[414] with 150 miles by canal and river boats from the latter, but the amount of handling necessary to transport similar cargoes from the two districts seems to be about the same. The deposit in Alsace lies in an elliptical area centering about 5 miles northwest of the city of Mulhouse.
Some potash has been produced from a deposit in Galicia, near Kalusz, south of Lemberg, from deposits reported to be of a type similar to those of north Germany, but the field has never yielded even enough potash to satisfy the local demand, and is thought not to be large.
During the war relatively small outputs of potash salts were obtained from many independent sources, in the United States, Abyssinia, Tunis, and other countries. Under stress of war necessities and the complete shutting off of other supplies, these outputs in the aggregate formed a considerable amount. Much of the development probably will not be permanent, when strict competition with the potash from more available sources is renewed, but each of these fields and doubtless many others contain the possibilities of development that may give the world situation a new aspect at any time. Chief among the most immediate prospects for important development is a rather extensive field in eastern Spain, near Barcelona. This field has not as yet produced on a commercial scale. One estimate of the reserves in the Spanish field claims a proved area of 13.5 square miles containing 200,000,000 tons of potassium oxide.
The nitrate deposits of Chile contain a small percentage of potash, and this is being recovered separately from the sodium nitrate at several of the refineries. It has been estimated that a total production of 240,000 tons of potassium oxide annually might be derived from this source.
Many different sources in the United States are yielding potash salts. The largest known deposit of soluble potash in fairly concentrated form is at Searles Lake, California. This is a dried saline lake, now represented by a bed of crystalline salts with a large amount of saturated brine rich in potash. The body of salts carrying the brine underlies an area of about 25 square miles and extends to an average depth of 70 feet. It is estimated that the brine alone in this deposit carries nearly 20,000,000 tons of potassium oxide, which would be enough potash to supply the needs of the country for about 60 years at the present normal rate. Numerous small lakes in western Nebraska carry brines exceptionally rich in potash, and these are now yielding a considerable production of potash fertilizer salts. No satisfactory estimate of the reserves in this field is available.
Under present operating conditions about one-third of the annual requirements of the United States is recoverable from the cement mills. About 380,000 short tons of potash, most of which is volatilized, is annually charged into the blast furnaces of the country. The best available estimates indicate that about 30,000 tons of potash have formerly gone to waste in molasses distillery slop, and-about 8,000 tons in Steffens[415] waste water at the beet-sugar refineries of the country. Kelp and alunite are available in quantities sufficient to continue to yield a substantial production. Enormous quantities of leucite lava, carrying 10 per cent. of potash in an insoluble silicate form; greensand carrying 6 to 7 per cent. of insoluble potash; sericite with from 7 to 12 per cent., and feldspar with similar content, are available as raw materials of production if satisfactory commercial processes can be developed. Thus potential supplies of potash in the United States are practically inexhaustible. The future of the American potash industry, therefore, depends on the development of processes or methods of separation economical enough to permit the domestic product to compete with imported potash.
The potash deposits of northern Germany lie in the midst of a series of formations known as the Zechstein, the geologic age of which is Permian. Both the Alsatian and Spanish deposits are found in Oligocene Tertiary rocks, although the deposits were not necessarily strictly synchronous in origin. The Galician deposits are described as of lower Miocene (Tertiary) age. These are the principal known bedded deposits of potash in the world, for which as a class the distinction as to geologic age seems to have a special significance. The association of potash with the large salt and gypsum deposits of the world seems for some reason to have been exceptional in geologic history. But the determination that these somewhat analogous occurrences have been formed at various times and places is a good basis for expecting that similar deposits may yet be discovered elsewhere.
Before the war the world’s consumption of potash was practically the measure of the German production, the statistics of which are available in considerable detail. In round numbers, Germany produced about 10,000,000 metric tons of potash salts, averaging about 10 per cent. of K2O, of which she used more than 60 per cent. at home, and exported about 25 per cent. to the United States. For two years a very small production from Alsace had been lumped with the rest. Shortly after the outbreak of the war the German government placed an embargo on the export of potash, presumably in the hope of thereby injuring crop production of the Allies, and possibly because of the small military significance that it has. The production of potash in Germany was continued, however, at practically the same rate as before the war, the portion formerly exported being distributed for fertilizer use at home. This naturally gave a tremendous stimulus to the efforts of other countries to develop sources of potash. Although no huge natural resources comparable[416] to the already known deposits were discovered, the efforts nevertheless had a reasonable amount of success, so that the world has not suffered in any vital way because of deprivation of potash. The cost of potash in the United States and its Allied countries increased to as much as ten times the pre-war price, but the supply was sufficient to meet all of the most pressing needs. Many possibilities for production from various sources have been opened, for which it is still too soon to predict the final outcome. It appears that a number of these new enterprises have entered the field permanently, although there will necessarily be some uncertainty during the period of reconstruction.
The ceding of Alsace to France, which is discussed in a subsequent paragraph, undoubtedly entails the largest and most significant change in the commercial situation as regards the world market for potash. A possibility of production from the deposits in eastern Spain holds similar although more remote significance.
The output of potash during 1917, summarized by countries according to the best available data, is as follows:
Table 65.—World Production of Potash in 1917
| Country | Source of data | Production (short tons) |
Average content K2O (per cent.) |
|
|---|---|---|---|---|
| Germany | Kalisyndikat statistics. | 9,853,171 | 11.2 | |
| Alsace | (Included under Germany.) | |||
| United States | U. S. Geological Survey. | 126,961 | 25.6 | |
| India | Rec. Geol. Surv. India, vol. 49, part 2, p. 71. | 23,838 | 40.0 | [155] |
| Tunis | Jour. Soc. Chem. Ind., vol. 37, p. 294 T. | 20,000 | 40.0 | |
| Galicia | “Kali,” vol. 7, p. 9. | 10,000 | 10.0 | |
| Russia | War Industrial Comm. of Russia report. | 5,000 | ||
| Abyssinia | Com. Fertilizer, Jan. 18, 1918, p. 44. | 5,000 | ? | |
| Japan | Commerce Rept., Dec. 1, 1916, p. 830. | 4,000 | ? | |
| Chile | U. S. imports potassium nitrate. | 1,750 | 25.0 | [155] |
| China | Commerce Rept., Nov. 27, 1918, p. 790. | 1,000 | ||
| France | Saline-de-Giraud. | 300 | ||
| Mexico | U. S. imports saltpeter. | 115 | 40.0 | [155] |
| England | Kelp and blast furnace recoveries. | ? | ? | |
| Spain | ... | No production | ||
| Total | 10,051,135 | |||
[155] Estimated from data indicated.
[417]
—The potash industry in Germany is reported to have represented an investment of M.500,000,000 ($119,000,000). In 1918 there were 209 mines capable of producing, as indicated by the Kalisyndikat list assigning quotas for anticipated production from the individual mines for the year.
Most of these mines are privately owned under a variety of laws in the several German states and provinces. Originally the developments are said to have taken place under various local regulations; for example, in Hanover the mining rights belonged to the property holders, in Saxony the prospecting rights were free and the mining concessions belonged to the first discoverer of the deposits without regard to the owner of the surface soil, and in Anhalt mining was at first declared to be a state monopoly, which was later contracted to a few private companies. Some properties are, however, owned and operated by the state. The Prussian government owns mines at Stassfurt, Bleicherode, and Vienenburg; the Duchy of Anhalt has large works at Leopoldshall; and the Duchy of Brunswick holds interest in some potash properties.
Two instances of participation by American concerns in the German industry are known. The International Agricultural Corporation, an American company organized in 1909, was for a time the owner of all the capital stock of the Sollstedt Works in Germany. Later, one-half interest in this American corporation was transferred to the Kaliwerke Aschersleben, another German concern, under conditions which seemed to complicate the matter of ownership. The Virginia-Carolina Chemical Co., of Richmond, Virginia, is reported to have purchased a controlling interest in the Einigkeit Works, presumably by a direct cash transaction.
Under a law of the German Reichstag, known as the potash act of the 25th of May, 1884, an obligatory control of the potash industry in Germany was ordained. A common agency, known as the Kalisyndikat, was established, to represent all of the mines, with power to control the opening of new mines, the output of each mine, and the selling prices of potash salts. The industry has thus become a trust re-enforced by the German government, although the private ownership of individual properties remains. This syndicate differs from an American syndicate in that it is formed for a limited period of time, in the present case 5 years, and its working capital is small, only enough to provide for the actual working needs of the organization. The object of the syndicate is to prevent disastrous competition, to insure that the supply will conform to the demand, and that reasonable profits may be obtained by producers.
The mines or works composing the potash syndicate, as with most mining syndicates in Germany, may be either of the limited liability sort, the shares of which are not assessable, or those whose capital stock[418] is divided into shares called “kuxe,” which are assessable at anytime and are of unlimited liability. The shares of both kinds are dealt in on the exchanges in the large cities of Germany.
Each company that is a member of a German syndicate has its representative on the board of management of the trust, and this board fixes the quota of production allowed each mine, and generally administers the affairs of the entire combination under its constitution and by-laws (statuten).[156] The constitution and by-laws must be signed by each concern entering the syndicate and the provisions therein contained strictly observed under penalties enforceable in courts of law.
[156] Abstract from Daily Consular and Trade Reports, No. 265, vol. 4, Nov. 11, 1911, p. 760.
The weak point in this form of organization is the dissension that often arises over the quotas allowed the different members. Each company wants as large an allotment as it can get. Upon the expiration of the life of a syndicate there is always uncertainty as to whether it will be renewed, owing to competition among the various constituent firms.
Before the war, under the terms of the contracts of the five large fertilizer companies with the German potash syndicate, one-half of the maximum discount allowed was deducted from the amount of the invoice covering each shipment, and the remainder was paid to the buyer at the end of each half year, provided he made a statement in writing to the syndicate to the effect that he had purchased his entire requirements of potash from the syndicate. Thus by allowing maximum discounts to the large buyers, and preventing them from dealing in potash from any source except the syndicate, this provision aimed to stifle the development of any competitive sources of supply.
—The potash deposits of Alsace were developed under the German mining law for Alsace-Lorraine of December 16, 1843, amended July 14, 1908, with specific reference to potash. Since the armistice the German mines have been operated under the sequestration régime, under charge of the French military authorities, directed by the ministry of industrial reconstruction in France. Now that the treaty of peace has been ratified by Germany, Alsace may be regarded as having formally and finally passed into the possession of France, and with this naturally the political and commercial control of the Alsatian potash deposits. It remains therefore for the French Parliament to determine what the final disposition of the former German ownership of these properties shall be, and questions such as nationalization or French-Alsatian control were being discussed in the summer of 1920.
Although most of the concessions in the Alsatian potash field had been granted to German interests, the development was started by Alsatians, and two of the concessions are in French-Alsatian ownership.[419] These have not been and probably will not be altered, unless they are to enter some voluntary combination.
An agency for the sale and distribution of the Alsatian potash has been arranged in the United States, and for the present at least the product is coming in direct competition with the salts from the older German mines.
—The potash deposits recently discovered in the provinces of Barcelona and Lerida, of Cataluña, in eastern Spain, are subject to special regulations of the Spanish government. A large area of concessions already granted to private interests covers a considerable part of the field outlined by exploratory borings. Operations on these concessions are permitted, but the state reserves the right to subordinate the exploitation to the interests of the country, and impose special conditions in favor of the consumption of the potash produced in Spain. Recently, unexplored state lands have been opened, by royal decree, to bids for exploration and lease. The scheme follows in general the plan of governmental control of the German potash industry. According to it, all concessions for the working and sale of salts are subject in many details to governmental control. Among other conditions, working must be continuous save in certain exempted circumstances; the state shall fix annually the home and export prices, as well as the maximum and minimum quota for each mine.
—Before the war, the Austrian potash syndicate, which consisted of the Austrian government and a group of capitalists, controlled the deposits near Kalusz, in Galicia. No specific governmental regulation is reported for the minor operations connected with the production of potash in other countries.
More than 90 per cent. of the potash handled in the world’s commerce is used as a fertilizer. The rest is used as a chemical in various industries, chief among which are the manufacture of matches, certain kinds of glass and soap, and the better grades of black powder. The uses specifically mentioned in this paragraph are essential, as no satisfactory substitutes are known.
Up to 1914 practically all of the potash used in the world came from the deposits in northern Germany, which are substantially inexhaustible. Next in importance are the deposits of Alsace, which contain enough potash to meet the world’s present demands for almost 300 years. Other resources are known in Spain, Galicia, Abyssinia, the nitrate beds of Chile, and in deposits in the United States, but it is too early to predict with assurance what part they will play in the expansion of the potash industry.
Germany will no longer be able to maintain a world monopoly of[420] the potash market. The passing of control of the important resources in Alsace from Germany to France foreshadows competition from recognized adequate sources of supply. There are many other possibilities, the mere potentialities of which are sufficient to restrain any tendency to unreasonable extortion by those who control the German fields. Moreover, attention has been so directed to the desirability of developing independent sources, and so much able technical talent is now being devoted toward bringing successful issue from the many undertakings in progress, that it is very unlikely that this country, or any other, will in the future be dependent on one or two arbitrarily handled monopolies.
[421]
Plant life requires nitrogen and gets it in the normal cycle of events. But when the occasion calls for stimulating the growth of plant life by feeding, by soil fertilization in other words, nitrogen in available form is indispensable. Further down along the channels of food supply it exercises another and equally important function in providing the chemical (ammonia) around which the modern practice of refrigeration is built. Likewise, the chemistry of explosives is basically the chemistry of nitrogen compounds. Nor is this all, for chemical operations in general—hence research and industrial chemistry in general—involve the employment of nitrogen compounds. Such, in brief, is its status. On each of three major counts, the interests of food production, of food distribution, and of national defense, it is indispensable; and of no less consequence is the retinue of less conspicuous agencies serving the interests of chemistry at every turn.
The development of fixed nitrogen sources is conditioned by three simple chemical facts. With these three simple facts in mind the rest follows largely as a matter of inference. The facts are:
That under all ordinary conditions of temperature and pressure, free nitrogen is a gas.
That it is extremely inert and indisposed to participate with other elements in the formation of chemical compounds.
That such combinations when they do occur are characteristically soluble.
In consequence of these three governing principles, along with its relationship to organic matter, as alluded to under the preceding caption, nitrogen has four habits of occurrence, worth considering as at least potential sources of supply.
—Being indisposed to participate in chemical combinations, nitrogen in the course of world evolution was left largely to itself; and since in the free state it is normally gaseous, it established its home in the atmosphere. Thus it comes about that the atmosphere today is approximately four-fifths nitrogen gas, and after all is said and[422] done the atmosphere is bound to constitute the great source to which we must turn for our supplies. With a source so boundlessly ever-present, the question of supply at first glance looks simple enough. But atmospheric nitrogen, it must be remembered, is nitrogen uncombined, and the demand is not for nitrogen itself but for nitrogen-bearing compounds. Once in a state of combination, it may remain so indefinitely, and the form of combination may be changed more or less readily to suit the demand. Before it can be put to use, however, it must be induced to surrender its gaseous freedom and affix itself in some such state of combination. The free nitrogen must become fixed nitrogen—hence the terms fixed nitrogen, nitrogen fixation, and the like in common use. Toward this end it must be induced to do what it has not seen fit to do of its own accord, and the very trait of aloofness responsible for the inexhaustible resources of atmospheric nitrogen stands as an obstacle opposing their utilization. The obstacle has not proven insuperable, as will appear later; but it is sufficiently a source of trouble even to this day, so that the fixed-nitrogen situation may with peculiar appropriateness be characterized as distinctly in the air.
—The disposition on the part of nitrogen to take up its abode in the atmosphere has an obvious result in minimizing the development of mineral nitrates. Atmospheric nitrogen is not entirely stagnant, however. Natural processes are constantly at work effecting substantial fixation. The processes are not obtrusively energetic, as in the case of atmospheric oxygen, whose fixation processes constitute the ever-present phenomena of oxidation. Still, in various ways, the most prominent among which is undoubtedly a form of bacterial action, nitrification and the building up of nitrate minerals everywhere in the soil goes quietly forward, and their concentration in ore deposits of more or less plentiful occurrence is thus to be looked for in the natural course of geologic events. In attempting to trace their further course, however, we are confronted at the outset by the principle of solubility. The nitrate minerals are in the nature of soluble salts. They leach from the immediate environment in which they form, just as do the soluble minerals in general. Mostly these latter are carried in solution to the ocean, adding themselves to its salinity; but under exceptional conditions of topography, where the drainage feeds into land-locked basins, the water finds itself entrapped with no avenue of escape except through evaporation. Here the salts accumulate, become concentrated, and finally give rise to deposits.
This, in outline, is the course set for the soluble mineral salts as a class, and it is along this course that we must expect to trace the development of nitrate ore deposits. But the ocean, with its 3¹⁄₂ per cent. of salinity, has only traces of nitrate minerals; and the same is true for the waters of land-locked basins, in all the various stages of concentration.[423] Their solubility is such that they can not have escaped in substantial form along the way. There is only one inference to be drawn. Evidently the inherent trait of aloofness is not lost to nitrogen when it does combine. The compounds do not survive for any length of time, but undergo dissociation, releasing their nitrogen and returning it to the atmosphere even as other processes are slowly withdrawing it from the atmosphere.
With this the eternal cycle is closed for nitrogen, and closed without apparent provision for any considerable side-tracking, such as would be required in the building up of ore deposits. So much for the rule; now as to the exceptions: Mostly they are of minor consequence. Pockety enrichments in the soil are common. Accumulations tend to build up in caves, and may even grow to be of consequence in a small way, as during the Civil War, when they helped materially toward relieving the nitrogen troubles of the blockaded Confederacy. In arid country, too, they not infrequently assume sufficient prominence to be of interest, especially at the hands of the promoter. Finally, there are the Chilean nitrate fields, which far from being of minor consequence, go to the other extreme in catering to the needs of the entire world.
These occurrences, especially the last named, have served to keep alive the hope that others of economic importance await discovery. The Chilean deposits alone among them all deserve more than passing notice. The origin of these deposits is veiled in uncertainty. Just why or how the natural forces, which elsewhere as a matter of universal observation have been seen to oppose both the formation of nitrogen salts and the accumulation of such as do manage to form, should have failed in this particular instance remains wholly conjectural. A conclusive explanation would be of the utmost value in determining the likelihood of similar occurrences elsewhere. But none has been forthcoming, and nothing is to be gained to the present purpose from stopping to inquire into the plausibility of the various attempts that have been made. Confronting us on the one hand are the evidences of a nitrogen cycle established, seemingly, without affording any visible loophole of opportunity for the accumulation of extensive deposits; on the other hand stands the bare fact of enormous deposition. This fact of existence unquestionably carries with it the possibility of duplication elsewhere. However, the fact of occurrence merely suggests the possibility, but does not determine the chances of recurrence. These are recorded in the prevalence of the conditions requisite to extensive deposition. In the case of nitrogen they are unique beyond comprehension, and the prospect of recurrence is to precisely the same degree unlikely. Accordingly, to all practical purposes, a review of the world’s nitrate ore deposits, both real and potential, resolves itself down to a review of the Chilean occurrence.
[424]
The Chilean nitrate fields lie in the arid valley basin to the east of the lofty coast range and just south of the present Peruvian boundary line. They do not occur as a single expansive area of deposition, but as deposits scattered here and there along the desert land at the bases of the mountain slopes. The formation consists of a conglomerate or breccia of rock material from the adjacent slopes, cemented with a mixture of soluble salts in which sodium chloride, common salt, is the dominant member, with sodium nitrate ranking second. The formation is called caliche. It lies for the most part just below the surface of the ground and varies from a few feet to many feet in thickness. Only in scattered patches is the caliche high enough in content of sodium nitrate to warrant treatment. These patches are sought out and excavated, and the picked ore is loaded in carts, which haul it to the extraction plant for treatment. Here the soluble salts as a whole are extracted in solution, and the nitrate in turn is segregated from the other salts by crystallization. Aside from haulage, hand labor is used throughout.
The caliche regarded as worth treating contains not less than 10 per cent. nitrate and ranges up to 25 per cent. and over, with an average of around 18 per cent. The product marketed is of two general grades—the ordinary, listed as 95 per cent. nitrate, and the refined, a guaranteed 96 per cent. nitrate, low in sodium chloride. The deposits have been worked more or less consistently, and with steadily increasing output, since about 1830. Their importance in the scheme of nitrogen supply may be gathered from Figures 16 to 18.
—Another source of fixed nitrogen grows out of its relationship to life processes, and is consequent on the very requirements of organized society which earlier it is called upon to assist in meeting. In other words, fixed nitrogen participates in the material cycle of life. It enters into the material demands of life for food, and it is yielded up among the material discards available to absorption. All manner of residuum, animal and vegetable alike, affords at least a potential source of fixed-nitrogen supply. Some of these are in service; others for one reason or another are not. Prominent among those in the former class are animal excreta, the so-called tankage from animal rendering plants, slaughter-house refuse, fish scrap, and vegetable-product refinery refuse. Most prominent among those still largely potential are sewage and garbage disposal.
The nitrogen from these organic sources does not appear on the market as such. Instead, the products enter in bulk into the make-up of fertilizer. They are of miscellaneous character, and only part of what is contributed collects to pass through industrial channels where its flow may be measured. The industrial flow goes on record and the records are available, but even here the nitrogen content has never been systematically computed, so the record is inadequate. For the rest,[425] the portion that does not reach the channels of industry, there is nothing whatever in the way of data to go by. Taken all in all, then, the significance of the organic nitrogen resources is largely conjectural. This is unfortunate. Approximate figures covering the use of organic nitrogen would be of value in various connections, as in the interests of intelligent allocation in times of nitrogen shortage, as helping to determine the extent to which the growing demands of agriculture incident to the growth of population may be discounted from the consequent expansion of scavenging opportunity, or as affording a basis for estimating the very considerable influence of motorization toward increasing the demand for chemically prepared fertilizers.
As things stand, all such questions of relationship lead only to profitless speculation. Even the relative importance of the organic sources as a whole in the economics of nitrogen supply is uncertain. What they have to offer of undeveloped reserves, now taking the form of wasteful sanitation procedure, will be taken up later. Under existing conditions, it is probably fair to assume that 40 to 50 per cent. of the nitrogen normally put to use in the United States is organically associated.
—Nitrogen in its organic relationships is bound up with carbon, of which organic matter is largely composed, and the bond between the two is entirely disestablished only as the carbon itself loses its substantial form through oxidation. In consequence of this enduring alliance, nitrogen is characteristically present in carboniferous deposits, a form of occurrence giving rise to still another, a fourth type of nitrogen resource. Coal and oil-shale loom up as the outstanding representatives of this class. In each, the nitrogen content is variable, but amounts to 1 per cent. or over. With so low a percentage of nitrogen, it goes without saying that neither of these is to be regarded as a possible source of direct supply. The cost would be prohibitive, even under the stress of the most extreme emergency. The nitrogen in a coal bed or an oil-shale formation is as worthless as the iron in any ordinary rock. But coal has other uses, and so has oil shale, or at least will shortly. The nitrogen does not have to be extracted; it gets released incidentally, and when its release is effected under conditions that prevent its escape, the result is a productive nitrogen resource. The nitrogen from this type of resource is in the form of ammonia, the relative importance of which is shown in Figures 16 to 18 and Table 66.
Such is the nature of the nitrogen resources. The resource situation as a whole is represented graphically in Figure 15. None other can compare with it for inclusiveness. Its sources are animal, vegetable, mineral, and atmospheric, which is to say, universal; and out of this unparalleled diversity has grown an industrial development as complex as it is diversified, and, incidentally, in view of its bearing on food and munitions supply, as important as it is complex. The situation at best can be but imperfectly grasped, for it has been but inadequately studied. In transgressing all set rules of resource occurrence, it transgresses the limits set for organized investigation. Geologists have studied one phase of the situation, electrochemists another, sanitation experts another, and so on; and the various commercial interests involved have seen to the giving of publicity where publicity would do the most good.
[426]
Table 66.—Statistics of Nitrogen Production
| Year | Organic nitrogen | Chemical nitrogen | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dried blood; tankage; guano; fish scrap; cotton seed cake and meal; etc. | Sodium nitrate | Fixation compounds | By-product ammonium sulphate | |||||||||
| World’s production, tons |
Domestic production, tons |
Imports, tons |
World’s production | Domestic production, tons |
Imports, tons |
World’s production, tons |
Domestic production, tons |
Imports, tons |
||||
| Ammon. sulph. (Haber), tons |
Calcium nitrate (Arc), tons |
Cyanamid, tons |
||||||||||
| 1900 | Data for organic nitrogen indefinite, but probably about equal to the total for chemical nitrogen. | ... | ... | ... | ... | ... | ... | ... | ... | 540,000 | 27,600 | 8,411 |
| 1901 | 1,328,664 | ... | 203,960 | ... | ... | ... | ... | ... | 580,000 | 29,279 | 14,486 | |
| 1902 | 1,349,300 | ... | 205,245 | ... | ... | ... | ... | ... | 600,000 | 36,124 | 18,146 | |
| 1903 | 1,485,279 | ... | 272,947 | ... | ... | ... | ... | ... | 640,000 | 41,873 | 16,777 | |
| 1904 | 1,559,091 | ... | 228,012 | ... | ... | ... | ... | ... | 650,000 | 54,664 | 16,667 | |
| 1905 | 1,754,605 | ... | 321,231 | ... | ... | ... | ... | ... | 694,575 | 65,296 | 15,288 | |
| 1906 | 1,800,500 | ... | 372,222 | ... | ... | 386 | ... | ... | 778,365 | 75,000 | 9,182 | |
| 1907 | 1,846,036 | ... | 364,610 | ... | ... | 1,874 | ... | ... | 906,255 | 99,300 | 30,114 | |
| 1908 | 1,970,974 | ... | 310,713 | ... | ... | 2,767 | ... | ... | 970,200 | 83,400 | 38,238 | |
| 1909 | 2,110,961 | ... | 428,429 | ... | 45,450 | 12,734 | ... | ... | 987,840 | 106,500 | 42,914 | |
| 1910 | 2,465,415 | ... | 529,172 | ... | ... | 22,596 | ... | 764 | 1,104,705 | 116,000 | 92,342 | |
| 1911 | 2,521,023 | ... | 544,878 | ... | ... | 59,479 | ... | 5,617 | 1,206,135 | 127,000 | 94,633 | |
| 1912 | 2,585,850 | ... | 486,352 | ... | ... | 115,688 | ... | 7,134 | 1,356,075 | 165,000 | 59,542 | |
| 1913 | 2,772,254 | ... | 625,862 | 20,000 | 181,800 | 173,026 | ... | 14,656 | 1,532,475 | 195,000 | 65,775 | |
| 1914 | 2,463,356 | ... | 541,715 | 60,000 | ... | 208,070 | ... | 29,536 | 1,320,000 | 183,000 | 75,010 | |
| 1915 | 1,755,291 | ... | 772,190 | 150,000 | ... | 845,388 | ... | 20,564 | 1,690,000 | 249,000 | 36,370 | |
| 1916 | 2,912,893 | ... | 1,218,423 | 300,000 | ... | 1,053,439 | ... | 38,023 | 2,000,000 | 285,000 | 12,962 | |
| 1917 | 2,950,000 | ... | 1,555,839 | 500,000 | 300,000 | 954,765 | ... | 44,146 | ... | 325,000 | ||
| 1918 | 2,900,000 | ... | 1,845,192 | ... | ... | ... | ... | 43,070 | ||||
[427]
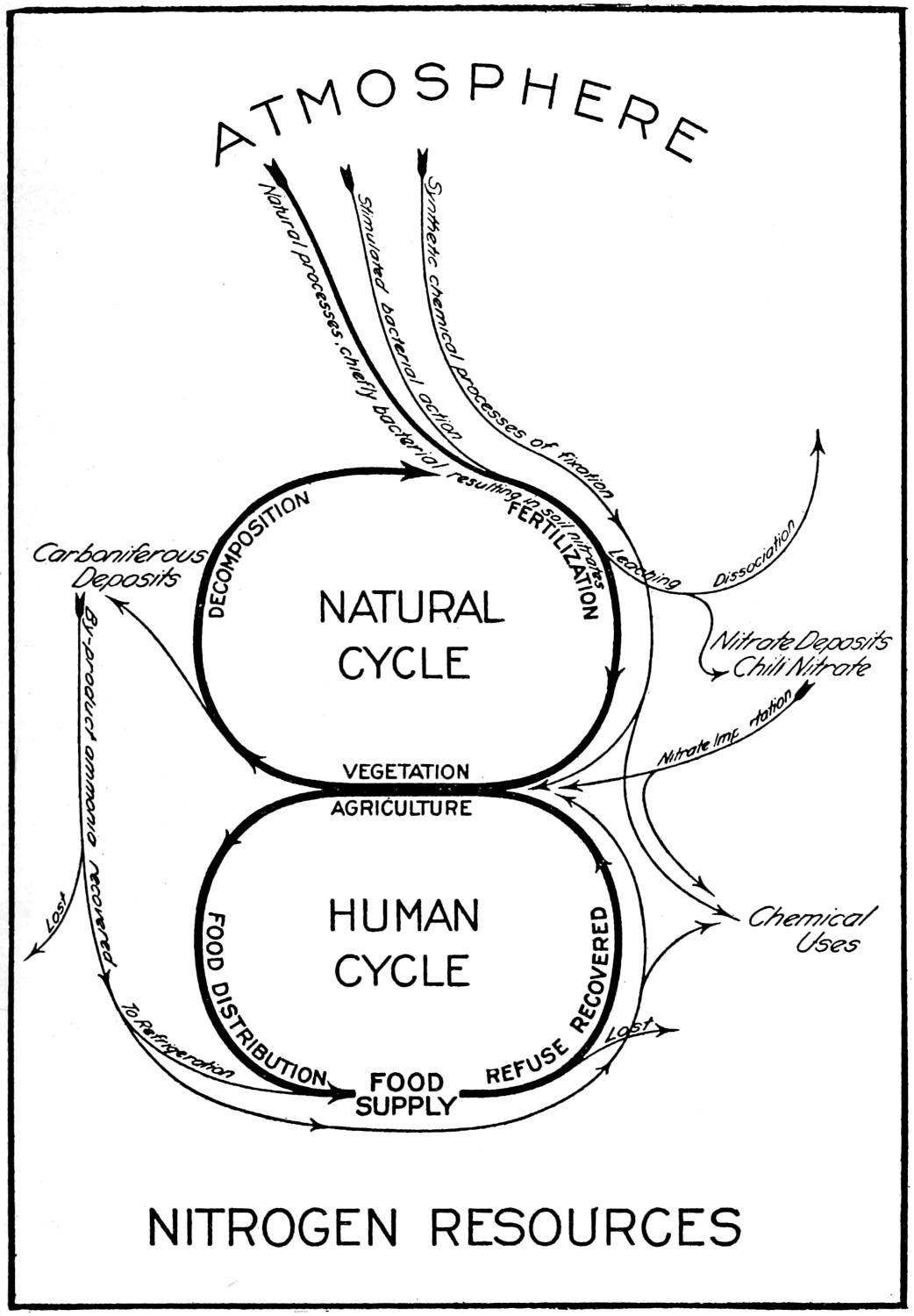
Fig. 15.—Nitrogen sources and their cycles of utilization.
[428]
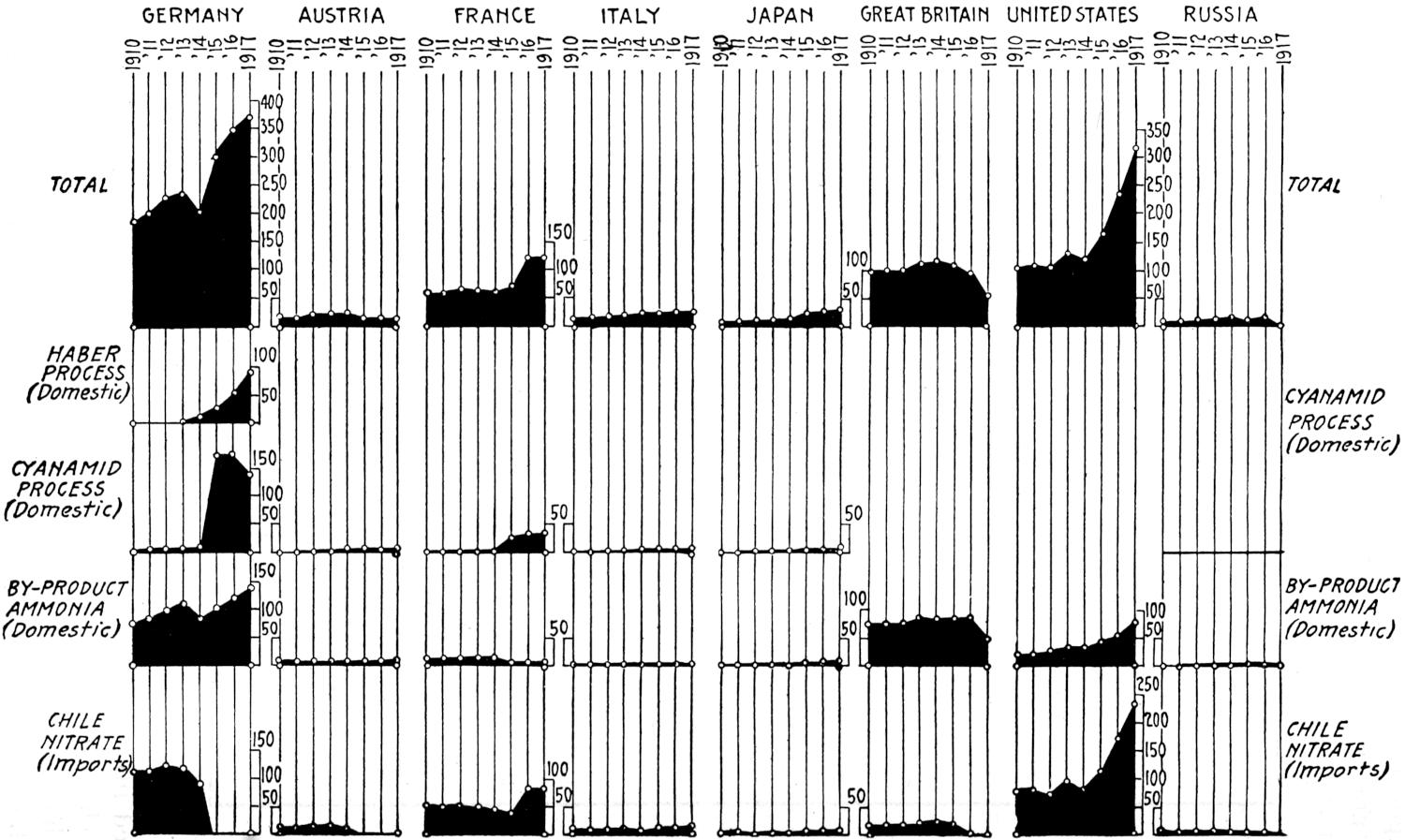
Fig. 16.—Wartime developments in the production of nitrogen. Figures are thousands of tons.
But an investigation working on the basis of geology alone can not cope with the situation; neither can one on the basis of technology alone; nor one on the basis of organic chemistry, or bacteriology alone; nor yet one prepared to employ any or all of these means, but only with a view to some special end. Nor yet again does the discordant grinding of many axes make a noise from which it is possible to gather an adequate comprehension.[429] The nitrogen situation has been inadequately treated because it has been inadequately studied. It has been studied piecemeal, always through the medium of limited means or with some special end in view. It is not a series of technical problems in geology, in bacteriology, in fixation, in munitions supply, and the like. It has to do with a composite economic structure, building for the dependence of society in peace and war alike. Until treated as such, the needs of the situation are bound to be inadequately met and its control a matter of perilous uncertainty. The present discussion makes no pretense of supplying this deficiency or of doing much of anything more than to show the extent to which it exists.
Figure 15 is designed to show not so much the scope of the resources as their composite functioning in the system of nitrogen supply. The influence of geography in the control of resources so universally available is bound to be subordinate. True, it enables Chile to exercise monopolistic control over the mineral nitrate supply, but it leaves the way open for the development of others; and while acknowledging the fullness of our dependence, as shown in Figures 16 to 18 and Table 66, we must not lose sight of the fact that it is so not of necessity, but because we have been content to leave it so rather than undertake to develop supplies of our own. So, too, with political control; what is gained in one direction is, potentially at least, offset by the possibilities opening up in others. Control of the sea gives a control over the mineral nitrate supply as absolute as that in Chile’s territorial monopoly. Yet in the recent great war, Germany, with her shipping obliterated at the outset, was not made to suffer materially from a nitrogen shortage. Britain’s supremacy of the sea went for naught. In the years before the war the force due in season to exercise control over the nitrate supply served only to stimulate the development of domestic potentialities, with the result that when the test came Germany’s proved actually to be the more advantageous equipment.
So it goes. Control over the nitrogen resources themselves is impossible. They are too universally available. Their only susceptibility to control is in the shaping of their development. This is too important a matter to be disregarded with impunity and left to develop without guidance. The modern nation that does so courts the irrepressible disaster of a nation at war but bereft of the means not only of waging war but of maintaining a food supply as well. From Figure 16 may be gathered the quality of attention given the matter of domestic supply by the different nations immediately before and during the war. Germany, it will be observed, heeded the call to give the matter special attention well before the war and had an independent system of supply developed in readiness, drawing upon the atmosphere and coal-product nitrogen with the results already chronicled. Great Britain did not ignore the importance of nitrogen, but placed reliance on her supremacy[430] of the sea and paid little or no attention to shaping the course of developments. Nor did its importance go unheeded elsewhere abroad, and the foothold gained for fixation in France, Italy, Austria, Russia, and Japan was, it is safe to say, not wholly automatic. The United States alone among the great nations up to the outbreak of hostilities in Europe in 1914 neglected to take any special precautions whatever.
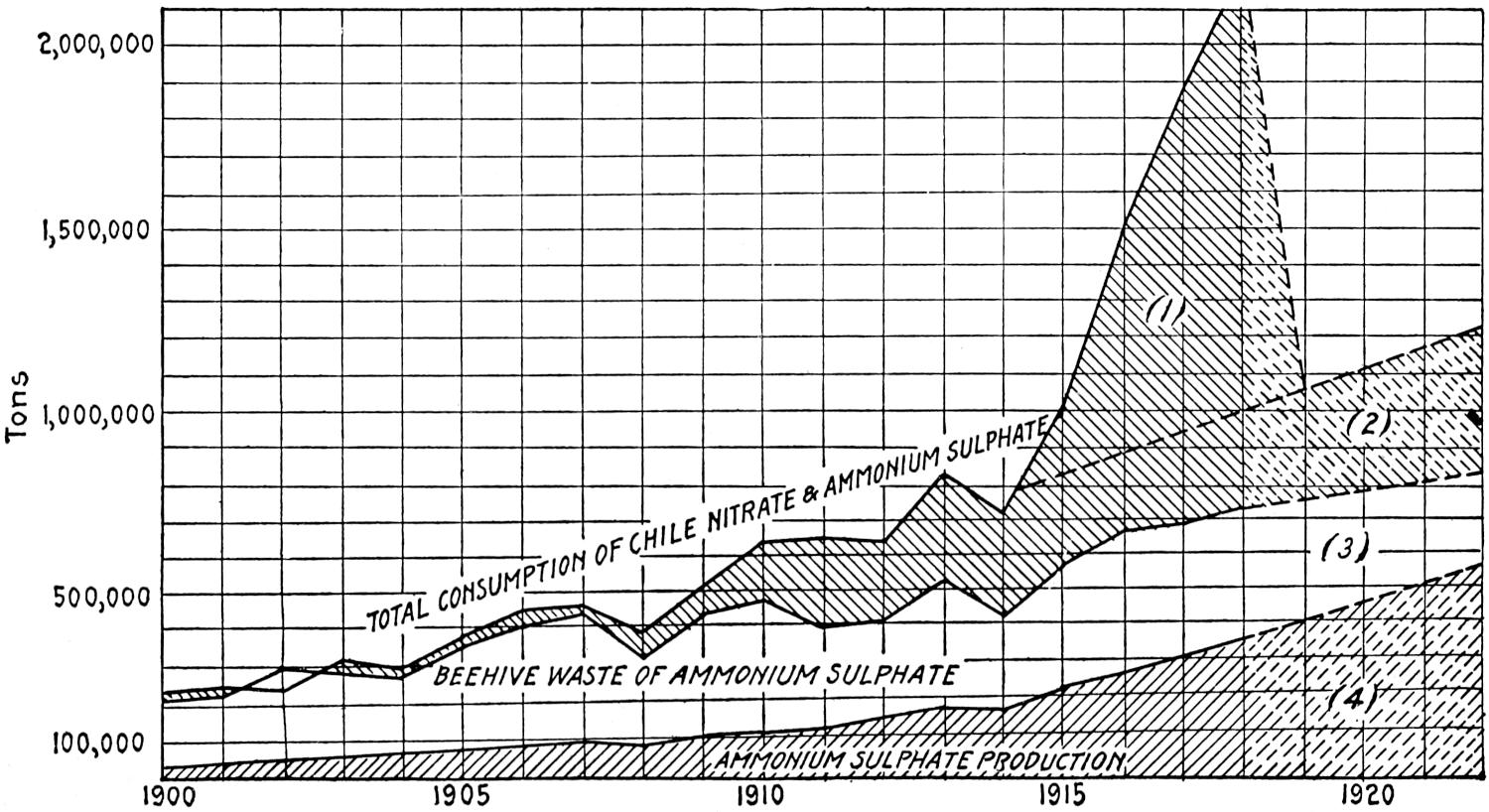
Fig. 17.—Nitrogen developments in the United States. 1. Wartime expansion for munitions manufacture. 2. Field of competitive opportunity for Chilean nitrate, air nitrate fixation, improved coal-fuel practice, shale-oil ammonia, bacterial fixation, improved sanitation, etc. 3. Field of opportunity reserved to coke-oven recovery. 4. Developments indicated for coke-oven recovery.
[431]
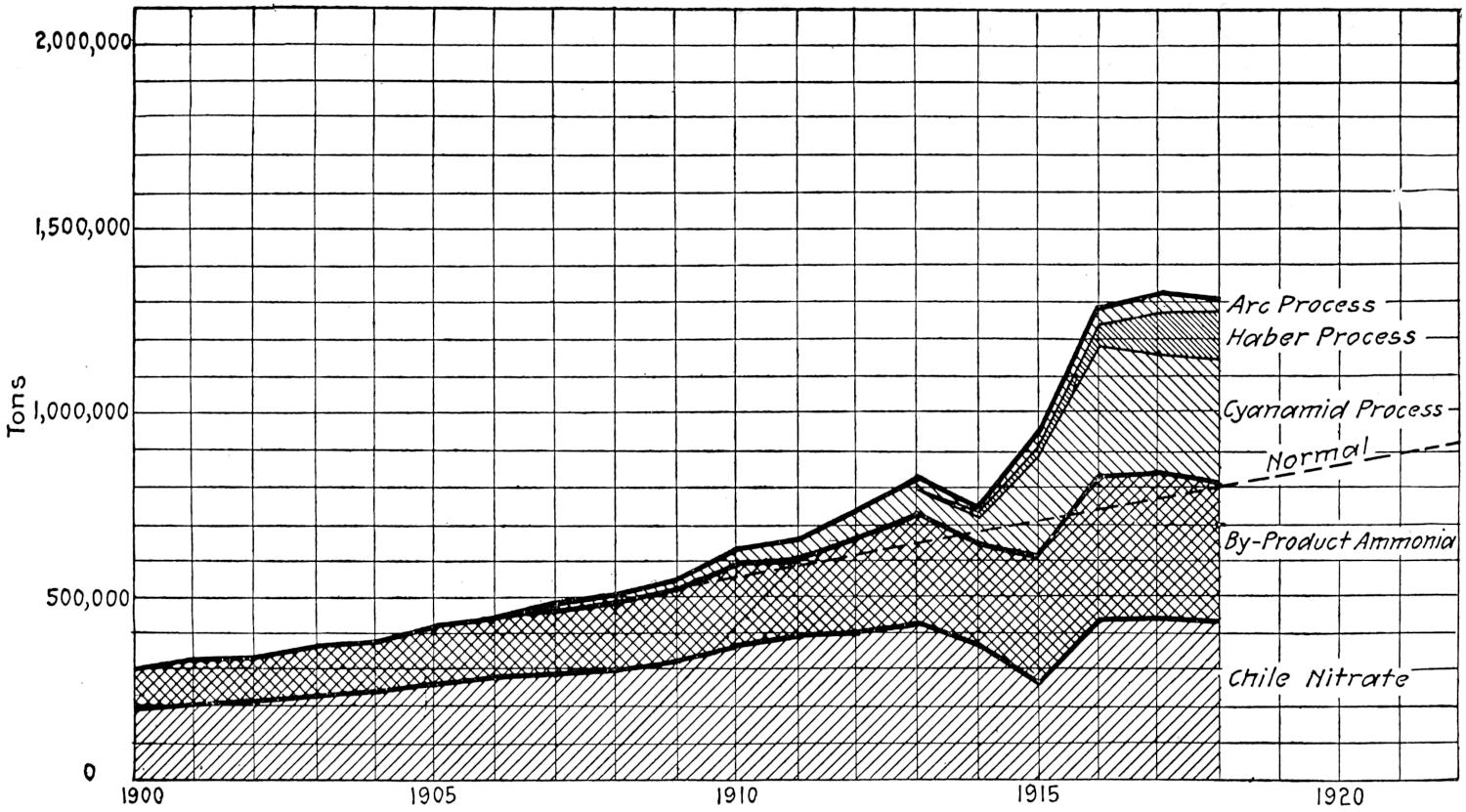
Fig. 18.—Nitrogen developments for the world, 1900-1918.
The war, when it came, far exceeded all expectations as to magnitude, and so in consequence did the demand for specially developed nitrogen supplies. To meet the emergency, some could be deflected from agricultural channels, but nothing like what was required, for food was just as important as munitions. The organic sources offered no help. Rather they were a hindrance; for organic nitrogen, broadly speaking, comes as a by-product of sanitation, and as such develops as the outgrowth of civilization’s refinements. There was a measurable response from the carboniferous sources, but these could not be made to meet the emergency,[432] for, being of by-product order, the supply is determined not in response to the demand for nitrogen but for the major products. Dependence on the native mineral source in Chile was out of the question, or at least precarious for any country except Great Britain. Accordingly, of the four great sources it remained for atmospheric nitrogen to meet the emergency. Thus, the war in bringing the nitrogen situation emphatically to the fore, communicated practically the whole weight of its tremendous impetus to development in the one direction of fixation. The result is shown in Figure 18.
Roused by the nightmare of war in 1914, even the United States awoke to the perils if not to the real needs of the domestic situation. It is a striking and highly significant fact that despite the fundamental importance of nitrogen, the awakening found us absolutely without any formulated program of action, even military or agricultural, let alone anything of comprehensive economic scope. A hysterical effort at improvising a program ensued. We were not yet in the war, and public interest was just roused to the gullible stage. The opportunity for private pickings from public favors was too promising to go by the board. The only prospect opening up lay in the direction of fixation developments, and fixation in the hands of the promoter is one of the most appealing propositions imaginable. Its major requirements are nitrogen and power. With the former inexhaustibly present in the air and the latter inexhaustibly available in the wasting waterpowers of the country, nothing it would seem, could offer greater promise. Add to this the reflection that cheap nitrogen means cheap fertilizer, and cheap fertilizer means lowered cost of foodstuffs, and the proposition broadcasted over the country is complete. Out of the confusion of interests, public, political, and private, a program was finally evolved, following our entry into the war, calling for the erection of a series of fixation plants with an aggregate producing capacity of around 85,000 tons of fixed nitrogen annually. For the present is must suffice to say that the war ended before any of these had reached the producing stage, and the United States, like Great Britain, depended on imports.
The charts comprising Figure 16 show the influence of the war in the development of nitrogen distributively among the countries concerned. The Scandinavian developments, while actuated from wholly commercial motives, were so largely influenced by the politically stimulated market that they may well enough be included in that general class of politically controlled developments. The same is true for the neutral countries in general. Figure 18, based on the best information obtainable, is designed to bring out the collective influence of the war in contributing to the world’s supply. Organic nitrogen is disregarded both because it involves too many uncertainties and because the wartime emphasis was all in the direction of chemical nitrogen. This figure takes into consideration[433] only the actual production and leaves out what was in process of construction when the war ended. Accordingly, while in one respect it overrates the effect of the war by including strictly commercial operations that very possibly might have transpired anyway, in another it underrates the situation by disregarding developments like those in this country. The best that can be done is to consider these as balancing each other, which, all things considered, is probably fair enough for all practical purposes. Taken on this basis, the net effect of the war, it will be observed, was to swell the production of fixed nitrogen some 40 or 50 per cent. above the figures indicated for the normal rate of expansion.
Thus the wartime shortage was made up; but all this is history. Now that the war is over, the question arises as to whether the world is due to face the situation in reverse. In making ready for war, and finally in meeting its demands, has the world been building up a 50 per cent. over-production beyond the needs of peace? Offhand, the answer would seem to be in the affirmative, but the question is not one that can be answered offhand. Agriculture is capable of absorbing an indefinite amount of nitrogen, and the war has wrought a lasting change in the agricultural situation. The changed agricultural conditions make room for much, perhaps for all, of the increment to nitrogen production. The development cannot be sustained, however, on its present arbitrary preferential basis of political expediency. Least of all can it be sustained on that basis in this country. Normally, we do not and can not be made to think in terms of war. The reason is evident enough, and its recurrent force is already apparent. Distasteful as the fact may be in some of its extremes of application, the only rational procedure is to accept it and fashion our measures of economic preparedness so that the normal activities of peace will keep our economic forces exercised and in trim for the test of war. It was recognized all along before the war that without an assured source of nitrogen supply, our system of defense was hollow; but we succeeded in building up no means of supply in direct response to political needs. We managed to get comfortably started during the war, but it remains to be seen to what extent this artificially nourished development is fitted to withstand the bitter strife of competition ahead.
Free nitrogen, it will be recalled, has no economic significance. To be available in the industrial arts it must be in a state of chemical combination. The form of compound is of secondary importance, since this may be modified more or less readily to suit the need, but its value is conditioned in terms of its availability in the form of nitrogen compounds. In consequence, the several sources are classifiable industrially under three heads:
[434]
Natural compounds—nitrogen occurring naturally in the form of marketable compounds.
By-product compounds—nitrogen rendered available incidentally in the course of operations otherwise directed.
Fixation compounds—nitrogen whose availability is dependent on special fixation treatment.
—Chile nitrate is the outstanding representative of the natural compounds. The guano industry, or what there is left of it, and a few other odds and ends of production from organic sources, belong here as well, but their combined output is so relatively small that the Chilean industry comprises what amounts to a monopoly of the natural resources. It is not operated as such, however, but by private capital, which owns and operates the oficinas, paying the Chilean government a royalty or export tax amounting to about $11.20 per ton. British and Chilean interests share about equally in making up the far greater part of the capital invested. The balance is largely German and American. The total capitalization in 1909 amounted to approximately $134,000,000, representing an actual valuation of about $30,000,000. Various efforts on the part of the commercial interests involved to effect combinations for the purpose of stabilizing production have been attempted, but have not been entirely successful, and the general tendency has all along been toward overproduction.
The operations, as already outlined on page 424, are crude, and the cost of production is correspondingly high, amounting to around $25 to $30 per ton at seaboard, inclusive of the $11 export tax. The nitrate is marketed largely through commission houses. The American situation is mostly in the hands of three companies, W. R. Grace & Co., E. I. du Pont de Nemours Powder Co., and Wessel, Duval & Co. The magnitude of the Chilean industry as a whole and its relative importance are shown in Figures 16 to 18 and Table 66.
—To this class of compounds belong, with the few minor exceptions already noted, the nitrogenous products of organic derivation as a whole, and those from carboniferous sources such as coal and oil shale. From the former source comes a miscellany of organic refuse resulting from activities dealing with animal, vegetable, and fish products, and carrying nitrogen in the form of organic ammoniates commonly left as such for use in agriculture. From the latter the nitrogen recovered is all chemical nitrogen in the form of ammonia or ammonium salts, mostly ammonium sulphate, and is available in all capacities.
The organic production is impossible of definite analysis from any angle. The lack of systematically compiled records, and back of that the miscellaneous largely decentralized character of the output, along with the fact that the producing costs are for the most part indistinguishable,[435] leaves altogether too much to the imagination. Much of the supply is derived from connections of sanitation, especially of local sanitation, such as the rural practice, for which there is no measure whatever. Another prominent source of supply is represented in what is known as tankage, the refuse from animal-rendering plants; but here too the issue is lost in the scattering of the production, the indefiniteness of composition, and the fact that not all of the product is used as a source of nitrogen, some of it going into the preparation of animal food. The same is true of cottonseed meal and various other less prominent forms of organic waste resulting from industrial activities. Fish scrap and slaughter-house refuse from meat packing also contribute prominently and at the same time rather more definitely to the supply of agricultural nitrogen; but even here adequate figures are not available. The Federal Trade Commission undertook to analyze the 1913 consumption, with results given in the following table:
Estimated Consumption of Nitrogen in Commercial Fertilizers for the Year 1913
| Materials | Fertilizing substance |
Consumption (tons) |
Content (per cent.) |
Units consumed [157] |
|---|---|---|---|---|
| Nitrate of soda | Ammonia | 260,000 | 18.0 | 4,680,000 |
| Sulphate of ammonia | Ammonia | 130,000 | 25.0 | 3,250,000 |
| Cyanamid | Ammonia | 15,488 | 18.0 | 278,784 |
| High-grade tankage | Ammonia | 210,000 | 10.5 | 2,205,000 |
| Concentrated | Ammonia | 18,351 | 14.5 | 266,090 |
| Dried blood | Ammonia | 40,000 | 17.0 | 680,000 |
| Dried fish scrap | Ammonia | 50,000 | 11.0 | 550,000 |
| Cottonseed meal | Ammonia | 660,000 | 7.5 | 950,000 |
| Total | ... | ... | ... | 16,859,874 |
[157] A unit is 1 per cent. of a ton, or 20 pounds.
This estimate, however, takes into account only the more strictly industrial sources, leaving rural sanitation and the like out of the reckoning.
Aside from the conversion of organic ammoniates, which is practiced on a large scale only in a few instances, notably that of the Paris system of sewage disposal, four general types of industrial operation figure more or less in the production of by-product ammonia. They include coal distillation, bone carbonization, oil-shale distillation, and blast-furnace operations. The American production, however, is all derived from the first two types. Both the others are active producers abroad, especially in Scotland, but neither of them has as yet obtained a foothold in this country. The American recovery in connection with bone carbonization is of minor consequence. Practically the whole supply comes from gas works and by-product coking operations. Figure 17, in the shaded area bearing the designation “ammonium sulphate production,” shows the magnitude and trend of the production from year to year since 1900.
[436]
The organic nitrogen recovered in all of the various by-product connections taken together probably constitutes 40 to 50 per cent. of the total supply. Coal product ammonia in this country adds another 12 to 15 per cent. So over half of our supply is of by-product derivation. The domestic output is supplemented in the case of the organic form by considerable importations from South America, and, until interfered with by the war, small amounts of ammonium sulphate were imported annually from Europe. Essentially, however, the by-product supply is of domestic origin. Despite its magnitude, it occupies an anomalous sort of position industrially. It is recovered incidentally for what it is worth, and sold for what it will bring. The cost of production is largely charged off against the major operations with which its recovery is associated, and the returns are credited in conformance, as a saving in the cost of the major operations. This is equally true whether the source be that of the domestic animal on the farm, a coke oven, or a packing house.
The industrial output is built up as a sequence to industrial concentration. This is evidenced all down the line, notably in the output of coke-oven ammonia from the steel industry and in that of organic ammoniates from the meat-packing industry. It is this influence of co-ordinated industrial concentration, along with the call for the major operations, that controls the supply of by-product nitrogen; so the development and handling of the industrial output comes naturally to be largely in the hands of trade combinations. Thus, the coal-product ammonia situation is largely at the disposal of the Barrett Co., the tankage and other animal-product ammoniates gather for disposal at the hands of the packing interests, and the nitrogenous fertilizers from cottonseed are for the most part prepared and marketed by interests subsidiary to the Cotton Oil Co.
The manufacturing interests involved are concerned primarily in the manufacture of other than nitrogen products. The by-product nitrogen recovered has to compete for its market against what comes from the other two industrial classes of supply, and its price goes just low enough to enable it to do so. The limits set in the incidental character of the output leave no special incentive to carry the price competition further. Whatever additional latitude of advantage as to cost of production it possesses goes not to promoting a further reduction in the price of nitrogen but to lowering costs with reference to the major theme of production. Gas-house ammonia, for instance, does not affect the nitrogen market so much as it does the cost of gas, and the organic ammoniates recovered in connection with meat packing have not lowered fertilizer costs so much as they have kept down the cost of meat to the consumer. Thus the by-product class of supply, though the leading one in the point of magnitude, and by far the cheapest to produce, has little to do with determining the price of nitrogen. The selling price of by-product nitrogen is[437] determined by the price the product from competing sources brings. In this country it is controlled by the price of Chile nitrate, and not, as commonly imputed, by the trade combinations that develop and handle the output.
—Nitrogen has five general habits of combination: with oxygen, giving rise to nitric acid and its retinue of nitrate salts; with hydrogen, giving ammonia and the ammonium salts; with carbon, to form cyanogen and the cyanides; with basic elements, yielding nitrides; and organically, in the form of organic ammoniates. Various projects have been advanced for turning these to account in the fixation of atmospheric nitrogen. For the most part they have met with little or no practical success, but there are exceptions to the rule of failure in all five directions.
—Nitrogen does not oxidize at all readily under any ordinary conditions, but its natural indisposition to combine with oxygen may be overcome by passing a mixture of the two gases through an electric arc. The atmosphere furnishes the nitrogen and oxygen ready mixed, so all that is needed in the way of raw materials is an abundant power supply. Arc fixation was developed in Norway, where the possibilities in the way of hydro-electric power give the best setting to be found anywhere in the world. Efforts to introduce it elsewhere have resulted unsatisfactorily, and arc fixation has made relatively little headway, as may be deduced from Table 66 and Figure 18. The reason is two-fold. So far, its use of power has proved uneconomical, and its product unsatisfactory. The former of these two objections depends for its force on the demand for power, but the latter is more decisive. The immediate end product is nitric acid, which is both difficult to transport and limited as to use. To be put in shape for agricultural use it must be neutralized in the form of a nitrate salt. Limestone is the only cheap neutralizing agent. This gives a salt, calcium nitrate, which absorbs moisture, cakes, and is thus unsuited to the American agricultural practice of machine drilling. An experimental plant near Seattle, Wash., aims to overcome this difficulty by turning out its arc product in the form of sodium nitrate, but the project is of no commercial significance as it stands.
—Nitrogen is no more disposed to combine of its own accord with hydrogen to give ammonia than with oxygen to give nitric acid. In the case of the Haber process, the only synthetic ammonia process that has stood the test of industrial application, the native indisposition to combine is overcome by subjecting a properly proportioned mixture of the two gases to heat and pressure in the presence of a catalyzer. This process was instituted in Germany shortly before the outbreak of the war, and as shown in Figure 16 and Table 66 has developed steadily since then. Little seems to be known as to the efficiency of the[438] German Haber practice. Apparently, careful manipulation is necessary to obtain results. This means a skilled attention, which is incompatible with mechanical volume production and is thus unsuited to American practice. What aims to be an adaptation to American conditions was worked out by the General Chemical Co., and a plant with a rated capacity of 60,000 pounds of anhydrous ammonia per day was projected at Sheffield, Ala., at the instance of the Government. The plant was completed, but before it could be tuned up for actual production the war ended.
—Nitrogen, in passing through a red-hot mixture of finely divided soda ash, coke, and iron, reacts with the sodium and carbon to give sodium cyanide. This principle of fixation is being extensively experimented with, but has not been developed commercially, except in a small plant with a rated daily capacity of 10 tons of sodium cyanide at Saltville, Va.
—Hot calcium carbide will absorb nitrogen, forming a compound of calcium, carbon, and nitrogen, according to the formula Ca CN2, known as cyanamid. The cyanamid process, based on this reaction, has been extensively developed, far more so than any other of the various processes, as will be seen by referring to Figure 16 and Table 66. Offhand, it looks to be the most adaptable and consequently the most promising of the lot commercially. In this connection, however, it is interesting to examine the several charts of its growth in the warring countries given in Figure 16. In none of these is the showing indicative of a strong, healthy development. Worst of all is the case of Germany, with the contrast offered in the Haber and cyanamid charts. Until the war, cyanamid manufacture was unable to obtain a competitive foothold in the United States, although a small plant has been in operation at Niagara Falls in Canada for some years. The problem it has faced is similar to that already chronicled for arc fixation, in that it draws heavily on power in the preparation of the necessary carbide, and the cost of power in this country has been prohibitive. Under the stress of the wartime demand for nitrogen, however, the Government contracted for the erection of three plants—one at Muscle Shoals, Ala., one near Toledo, Ohio, and one near Cincinnati, Ohio, with a total rated capacity amounting to 220,000 tons of ammonium nitrate per year. The work on all three was well under way, but none of the plants had reached the producing stage when the signing of the armistice brought the nitrate activities of the War Department to an end.
—The only process of any prominence aiming to fix nitrogen in the nitride form is one developed by the Aluminum Company of America. This has for its working principle the fact that a mixture of alumina and carbon, highly heated, will absorb nitrogen by reacting to give aluminum nitride. The nitride when heated with caustic soda[439] gives its end product in the form of pure ammonia. The outstanding difficulty encountered in applying this process commercially seems to be that of providing a furnace capable of standing the temperature requirements. At all events the process has not succeeded in making good industrially.
—The artificial attempts at fixation have been directed almost wholly toward employing chemical principles. In view of the difficulties experienced and the uncertain value of the results as a whole, it is interesting and perhaps highly significant to reflect that after all, as indicated in Figure 15, inorganic chemical principles seemingly have little to do with developing the natural supply, probably because of the activities of nitrifying bacteria. Little attention has been given to the possibilities in this direction. This is only natural so far as commercially actuated research is concerned, since it does not lead in the definite direction of patent rights; but the failure to institute an adequate investigation governmentally can be attributed only to lack of comprehension with reference to the scope of the nitrogen issue as brought out under “General Aspects of Control” on pages 425 to 433. The subject has received just enough attention to show that bacterial fixation represents a tremendous field of grossly neglected possibilities.
The whole matter of fixation must be regarded as in process of development. True, it was instituted some fifteen or twenty years ago and has grown to represent the largest producing source of chemical nitrogen, with operations in practically all the important industrial countries in the world and with responsible financial backing. But no one can examine the charts in Figure 16 without recognizing the premature, mushroom quality of the upgrowth, induced primarily in response to the political conditions leading to and through the war. This is especially true for the American situation. When the war broke out, fixation here was confessedly still in the dependent stage of its development, unable in every effort it had made to stand alone industrially. In the main, the developments that have transpired subsequently have followed along pre-existing lines. In so far as they have done so, little actual economic significance is to be attached to them. For the rest, the new developments, all that can be said at this juncture is that they are disappointingly meager.
Just one wartime achievement, the oxidation of ammonia, stands out as affording a worth that is unmistakably clear. The nitrogen situation, it will be recalled, has two aspects, the military and the agricultural. The military focus is on nitric acid, and the readiest means of insuring a supply; the agricultural focus is on ammonium compounds or their equivalent in neutral nitrogen salts and the most economical means of supply.[440] Here, then, is a parting of the ways to expediency, and it is at this juncture that with military influences to the fore the nitrogen developments of the past few years were led off on an uneconomical tangent of military control. The Bureau of Mines, however, taking up the work of others, has perfected a simple, effective means for oxidizing ammonia to nitric acid. This, beyond question, is the most important contribution of the day. Its significance may perhaps best be brought out graphically in the accompanying sketch.
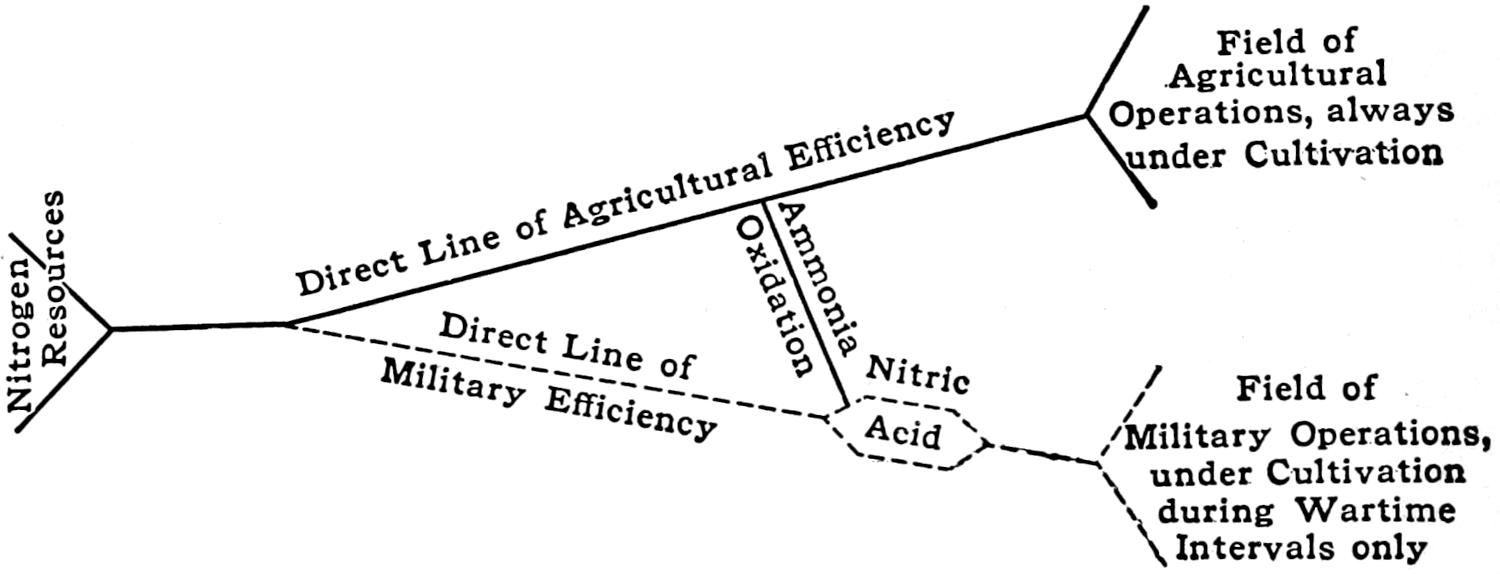
Ammonia oxidation, it will be observed from the foregoing sketch, gives a means of supplying the military requirement from the direct line of agricultural efficiency. From the strictly military viewpoint, it has the objection of being a roundabout procedure. The dotted line of direct military procedure, however, has no peace-time function, and consequently cannot be maintained in time of peace in trim for war, but must instead be built up expressly to meet wartime exigencies. We have had an illustration of what this means in the way of time and money, and this one ought to suffice. The agricultural channel, once built up on a basis of economic efficiency, is open at all times. At the most, all that is required is to keep an eye to the emergency needs in the way of oxidation equipment, a relatively simple matter. Thus, instead of the precarious procedure of trusting to luck which characterized our pre-war attitude toward nitrogen on the one hand, or of attempting the impossible in the way of maintaining a military program of industrial procedure in time of peace on the other, all that is needed is a constructive program devoted expressly to the interests of economic efficiency.
There is no import duty on nitrogen, nor is there likely to be any, for nitrogen is an important cog in the mechanism of food supply, and the peacetime emphasis, reversing the wartime order, is primarily on cheapness and only secondarily on the point of origin. Accordingly, looking ahead, the American market conditions, once world trade is fully restored, are due to reflect the world conditions. As indicated in Figure 18, the[441] sudden ending of the war, with its calling off of the military requirement which had been building up steadily since even before the outbreak of hostilities in 1914, left the world with a producing capacity 30 to 40 per cent. above normal. To what extent this apparent overproduction, amounting to some half million tons of nitrogen, will prove real is impossible to foretell; not all of it certainly, for under the stimulus of a food shortage the curve of normal consumption will doubtless bend upward. On the other hand, however, there is the producing capacity of the plants not yet in operation to be taken into consideration. Whatever may be the capacity of agriculture to absorb from the surplus, it cannot be expected to take up the full amount immediately or without special inducement. The inference follows that price and production will come down, to stimulate and co-ordinate with the increase in demand. Where the meeting point will be between the upcurve of demand and the downcurve of production it is impossible to predict. It is of interest, however, to figure in review on how the three types of industrial source, the natural, the by-product, and the fixation types, are equipped for the very evident strife of competition implied in the situation.
With the development of fixation, there have been a lot of unfounded statements to the effect that the day of Chile nitrate is passing. Whenever a synthetic development comes to the fore, a peculiar fallacy of reasoning is indulged in which ignores the fact of inherent natural worth, disregards the inescapable cost of its duplication, and regards the synthetic achievement as giving open sesame to the natural treasure. By way of substantiation in the case of nitrogen, the cost of producing Chile nitrate is high, amounting to around $30 per ton. This, however, is largely contributed to by the unsystematized crudity of the operations, by the high export tax, and by overcapitalization. But these, it will be observed, are variable factors, susceptible of indefinite modification in keeping with the need. Chile nitrate has never made any pretense of competing against by-product nitrogen, with its advantages in the way of low incidental producing costs and proximity to the market. The discrepancy between the by-product supply and the total demand for nitrogen has all along comprised the field of opportunity opening to Chile nitrate. In this its only noteworthy competitor is the fixation industry.
The fixation sources are impossible of analysis on a definite basis of cost. Too many variable factors and uncertainties are involved. Repeated attempts have been made, but all they have served to bring out is that under certain conditions, as for instance of power supply, and for certain express purposes, one form of project has an apparent margin of advantage over another, and vice versa for other conditions; but that at best the cost of production, if not actually prohibitive, is dangerously close to the normal pre-war price of fixed nitrogen. Back of it all is the[442] fact that fixation has to deal with the problem of overcoming the native chemical inertia of nitrogen, and the problem has not yet been solved at all convincingly. Always the solution advanced has called for some special measure of relief from industrial competition, whether natural, as in the case of the Scandinavian power supply, or political, as in the case of the American and German projects.
Fixation has been widely heralded of recent years as due not only to emancipate the world from its dependence upon the Chilean source, but to reduce materially the cost of nitrogenous fertilizer, hence the cost of food production, to the marked betterment of living conditions as well. In its promise of political and economic betterment in one, it has claimed the attention of all. However, the claim of special economic advantage coming from an industry barely, if at all, able to meet conditions even as they are, has been overdrawn.
This does not aim to imply that there is nothing but failure ahead for fixation in the test of competition. It has its possibilities of development into something commercially and economically as well as politically worth while, but the existing hothouse order of upgrowth is unquestionably due for a lot of training down, and much that is worthless is as certainly due to go. The American developments have a particularly inauspicious economic setting in the prevailing scale of costs. The only saving alternative for them would seem to be in one form or another of federal provision for their continued support on some such basis as that on which they were projected, and this is unlikely, for there is no apparent reason. True, lowered nitrogen costs tend to make for a lowering in the cost of foodstuffs, but so, for that matter, would a lowering in the cost of agricultural implements, and any arguments that apply in the case of nitrogen apply as equally for potash, for phosphate, for agricultural implements, for coal—in fact for industry in general.
With reference to by-product nitrogen the situation is very different. In general, the by-product sources are of an order such that they were not materially affected one way or the other by the war, and consequently are not due to be materially affected in the process of readjustment, except in the case of coke-oven ammonia, where the temporary slump in the steel industry will result in a temporary slump of probably 15 to 20 per cent. from the 1918 output. Of special significance in connection with what lies on beyond for the by-production of nitrogen is its relationship to the progress of industrial co-ordination. The whole current trend of industrialism, as represented in integration, volume production, and the like, is actuated in the interests of co-ordination and the overcoming of lost motion; and nitrogen comes in for an important share in these developments.
With reference to the organic group of compounds, the outlook for the future is as uncertain as are the actual conditions of today. The[443] centralized development of meat packing, of animal-rendering establishments, and of cotton ginning gave rise in their time to highly important recoveries of nitrogenous waste; but with the forward progress of developments this usage in turn is giving way to a more advanced order. Cottonseed as a fertilizer is giving place to a cottonseed-products industry; tankage as a fertilizer is giving place to the artificial compounding of animal food; horses, an important contributor of agricultural nitrogen in times past, are yielding much of their place in the sun to the automotive engine. Meanwhile, with the factor of dilution to be overcome, our sewage disposal is employed to pollute streams and destroy the fish supply instead of being put to useful ends. So it goes. Developments are on foot that lead in both directions, and there is no telling how the balance is due to shift. Probably the best guess is that relatively at least it will be downward rather than upward.
The outlook for by-product ammonia is more definite. Ammonia is the end point of material refinement; so here the nitrogen developments hold all they get, rather than go on to lose out again in a further refinement of usage, as in the case of the organic group. The output has increased consistently and rapidly, owing to the transition from beehive to by-product coking operations, to the progress of centralization and co-ordination; in other words, with reference to coke manufacture. Even now, less than half of the coal coked is treated in the retort oven; but the beehive oven is out of the line of progress and is due to be entirely displaced. Also, the industry is still expanding as the process of transition, with its separate potentiality for doubling the present output of coal-product ammonia, goes forward.
So far, the recovery of by-products in connection with the use of coal has been confined in the one direction of coke making, along with the analogous procedure of gas manufacture. But the development which has thus started in the coke industry will not stop there. The loss of motion resulting from lack of co-ordination in the use of coal is just as great in other directions as in that of coke making, and the advantages of integral usage may confidently be expected to assert themselves. Already projects of the kind are coming under serious contemplation in proposals such as those for furnishing gas to cities, for employing by-product operations located at the mine in support of the waning natural-gas supply, and for integrated heat, light, and power projects operating on coal with by-product recovery. Meanwhile, the motor-fuel situation is suggestive of interesting developments ahead. There is every reason to believe that the petroleum resources can not continue to meet the growing demand, in fact, that the occasion for support is already at hand. Whatever the nature of these supporting developments, whether they take the form of a shale-oil industry or what, it seems certain they will usher in an important source of by-product nitrogen.
[444]
Figure 17 summarizes the American situation with reference to chemical nitrogen, as does Figure 18 in less detail that for the world. Organic nitrogen is omitted, partly because of the lack of information, partly because the issues more directly involved in the situation as it stands are those of chemical nitrogen.
Nitrogen, itself, is an inert gas of no particular use, but nitrogenous compounds are necessary to agriculture, to refrigeration, to munitions manufacture, and to the applications of chemistry in general. In the native gaseous state, it makes up about four-fifths of the atmosphere, and combined it occurs as nitrate minerals, as organic compounds, and in carboniferous deposits. Atmospheric nitrogen is of use only after it has been artificially compounded or fixed, a proposition which the natural inertness of nitrogen renders difficult and expensive. The only mineral deposits of consequence are those comprising the nitrate fields of northern Chile. The organic resources include all manner of animal and vegetable refuse. Coal-tar ammonia from retort-coke and gas manufacture, along with some shale-oil ammonia, makes up practically the whole supply derived from the carboniferous sources. This range of associations, including animal, vegetable, mineral, and atmospheric sources, transgresses all established rules of resource occurrence, and consequently all regularly constituted research. As a result the nitrogen resources and their needs for attention have never been comprehensively investigated. This became strikingly apparent when the war, threatening swift disaster in the guise of a nitrogen shortage, showed us up to be quite devoid of any systematic nitrogen program and precipitated an hysterical effort to devise a makeshift one instead. The atmosphere was found to provide the only independent source of supply available on an emergency rating; so, following the lead of the European countries, several plants for the fixation of atmospheric nitrogen were projected governmentally.
Industrially, the nitrogen sources may be classified as natural, by-product, and fixation. The natural supply is almost wholly in the form of sodium nitrate from the Chile nitrate deposits. These are controlled and operated by British, Chilean, German, and American capital. The American imports are largely handled by three companies, whose system of control is effected through the medium of shipping and warehouse facilities.
The by-product sources include nearly all of the organic nitrogen used, and the nitrogen from coal and oil shale as well. The supply is governed as to magnitude by the progress of industrial co-ordination through the medium of centralization in the preparing of animal, vegetable, and coal[445] products. Thus the development and marketing of the by-product supply tends naturally to gather to industrial combinations. These, however, are natural developments, not developments artificially created in the interest of price control. The price of by-product nitrogen is controlled not by trade combinations, but by the price of the product from other sources, which is to say, by the price of Chile nitrate. Beyond that, the advantages accruing in the way of low-producing costs do not go wholly to commercial profit but to the saving of costs with reference to the major production, as for instance in the case of gas-works ammonia, which makes its chief contribution toward lowering the price of gas to the consumer. The rapid development of fixation is attributable largely to political influences, activated by conditions leading to and through the war. There are a number of projects for fixing nitrogen, but only three have any genuine measure of industrial achievement to their credit, are fixation in Norway, Haber synthetic ammonia fixation in Germany, and cyanamid fixation in a number of places. Three of the four large American plants are of the last-named order; the other is a synthetic ammonia proposition. All four were contracted for by the Government, and so far as fixation can be said to have gained an industrial foothold in the United States it is wholly in response to the dictates of political control.
Probably rather less than half of the nitrogen consumed is organically associated, and rather more than half of it chemically combined. Practically all of the organic nitrogen and around one-fourth of the chemical nitrogen is of domestic by-product derivation. So far, the balance has been supplied from Chile nitrate, supplemented by small imports of guano, animal refuse, by-product ammonia, and cyanamid from abroad.
There is no apparent likelihood of this adjustment being materially affected as an immediate outcome of developments with reference to fixation. These have shown themselves to be of the utmost political significance as affording an unlimited, independent source of nitrogen supply. Their genuine economic significance at the present stage of enforced expansion, however, is questionable. In this country, especially, the scale of costs gives an unpromising setting. The by-product sources growing out of centralized industrial co-ordination are in line with the trend of modern industrialism and may be looked to as assuring a steady increase in yield, especially if the process of industrial evolution in the direction of co-ordinated economic efficiency is adequately cultivated instead of being interfered with. In this same connection the most significant accomplishment recorded for nitrogen, lies in the working out of a means for the oxidation of by-product ammonia, thus rendering the growing by-product supply available for the full range of nitrogen uses.
With reference to the economic and political aspects of the outlook[446] ahead, all else is obscured and lost to view in the pressing need for a constructive program worked out on a comprehensive basis, in keeping with the comprehensiveness of the resources themselves, with which to supplant the uneconomical makeshift program brought into being by the war. The program called for is one calculated to bring out, and bring out co-ordinately, the best there is in bacterial as well as chemical fixation, in the industrial by-product sources of organic and chemical nitrogen, and in the province of sanitation.
[447]
Pyrite and sulphur are closely related in their most essential uses, and one material can in many cases be substituted for the other. The largest and most important use of these minerals is in the manufacture of sulphuric acid, which is an essential material required for a very wide variety of purposes, including the production of acid phosphate for fertilizer, the manufacture of modern high-power explosives, the refining of petroleum, pickling of iron and steel, and for a vast number of chemical industries. The competition between pyrite and sulphur for this purpose has gone through several stages. With the large-scale development of Italian deposits sulphur was largely used for acid manufacture. As the prices of sulphur were increased it became cheaper to use pyrite, which in many localities then displaced sulphur, for this purpose. With the rapid expansion of the American production, and particularly with the tremendous increase in the capacity of sulphuric acid plants for war purposes, sulphur has again been very largely used for the manufacture of acid. The prospects since the close of the war are that, due to the tremendous capacity of the sulphur mines of the United States, sulphur may continue to compete with pyrite in this use.
Probably the second most important use of these materials is in the manufacture of sulphite wood pulp. In Europe pyrite is largely used for this purpose, while in the United States and Canada sulphur is the principal material used. For every ton of sulphite pulp manufactured, under the best practice, about 250 pounds of sulphur is required. In the United States and Canada about 175,000 tons of sulphur is used annually for this purpose, representing about 50 per cent. of the total sulphur consumption of these countries. There are also a number of other important purposes where sulphur is used rather than pyrite, the most important of which are in the manufacture of agricultural sprays and insecticides, and in the hardening of rubber. Sulphur is a primary ingredient of black powder, and considerable quantities are still used for that purpose. While most of the explosives used in modern warfare require the use of sulphuric acid in their manufacture, they do not use sulphur in its elemental form. There are many other minor chemical uses.
[448]
—Because of the large deposits of sulphur now available, and of the extent and wide distribution of pyrite deposits, and of the cheapness of both these materials, there are no adequate commercial substitutes for them. The increase of by-product acid, from the copper and zinc smelters and possibly the nickel smelters of Sudbury, might be considered as the most important factors in replacing pyrite and sulphur. As a general thing, the factors of cost and transportation are the governing ones rather than any present or probable scarcity of materials.
While pyrite is a very widely distributed mineral, there are relatively few deposits which are of sufficient importance to enter into the world’s commerce. This is generally due to the relatively small value of its sulphur content per ton, usually from 40 to 45 per cent. of recoverable sulphur; which means that it takes almost 2¹⁄₂ tons of pyrite to be equivalent to the ton of sulphur which competes with it for many uses. Consequently pyrite seldom moves far, unless it is so situated as to take advantage of cheap ballast rates where little other freight is available for ships, or unless it carries important copper or gold values, which can be recovered after the sulphur content has been utilized. Many known deposits, such as those in Mexico and the Western United States, remain undeveloped because of their distance from market. In countries such as Russia, France, Italy, Germany, Sweden, Japan, and the Eastern United States production is absorbed by the local market. Spain and Portugal, the most important source of world supply, are favorably located to ship to near-by European countries or to secure cheap ballast rates to the United States, and in addition much of the ore carries several per cent. of copper.
Norway is second in export, with a high-grade pyrite carrying copper values, which is shipped to Sweden or across the Baltic to near-by countries. Canada ships considerable pyrite to the near-by markets of the United States, the Quebec product having copper values, while the product of Ontario takes advantage of boat shipments on the Great Lakes.
—The deposits of Spain and Portugal are the largest and most important in the world, furnishing approximately two-thirds of the world supply. The district is essentially a unit, and the principal deposits occur in a zone extending from Rio Tinto, Spain, to San Domingo, Portugal. The combined annual production of iron and copper pyrites for the two countries is normally almost 4 million metric tons, 90 per cent. of which is furnished by Spain and 10 per cent. by Portugal. About two-thirds of the total output carries copper values,[449] which may be recovered before the pyrite is roasted for its sulphur value or after. When copper is to be recovered at the mine the ore is leached by spreading it out in beds exposed to the weather and frequently stirring it and wetting it down. The copper goes into solution and is precipitated on scrap iron, forming cement copper. The process takes about 3 years and the pyrite residue is shipped as washed ore. The ore is compact and finely crystalline and carries from 48 to 51 per cent. sulphur. Conservative estimates of ore reserves for the district give it from 300 to 400 million tons, or enough to last for one hundred years at the present rate of production. Consequently this district is destined to long remain the chief pyrite-producing center of the world.
The Rio Tinto Co. is the principal producer, contributing about one-third of the total output of the whole district (Spain-Portugal). It is owned by British and French capital. Mining is largely by open-pit methods, and the company employs 25,000 men. The ore carries about 2 per cent. copper, making this company the largest European producer of that metal. The reserves are estimated as 250 to 300 million tons, representing the major part of the whole district. The Rio Tinto Co. furnishes about 60 per cent. of the 1,000,000 tons of Spanish pyrite normally imported by the United States.
The second principal producer is the Tharsis Sulphur and Copper Mines (British), with about one-eighth of the total production. British capital is predominant in the district as a whole, with the balance French and Spanish. Huelva, Spain, is the principal point of export, located from 30 to 40 miles from the mines. Under normal conditions the pyrite moves at cheap ballast rates, and has been sold at from $6 to $7 per long ton (12 to 16 cents per unit of sulphur), delivered in United States ports. Normally this Spain-Portugal district exported one-quarter of its output to the United States, one-eighth to England, one-eighth to Holland, one-eighth to Germany, and most of the balance to France and Belgium.
—Norway produces from 400 to 500 thousand tons of pyrite per year (about 8 per cent. of the world’s total), and her output is steadily increasing. The ore usually carries from 1 to 3 per cent. copper and from 42 to 49 per cent. sulphur; and is free from arsenic. Seven-eighths of the output is exported to Sweden, Germany, England and Russia. When Sweden’s import of sulphur (about 40,000 tons) was cut off during the war, she changed the equipment of her cellulose plants to burn pyrite instead of sulphur and took about one-half of the Norwegian output, since her own production of pyrite (about 30,000 tons) was of minor importance.
The Norwegian deposits are widely distributed from south of Bergen to the extreme northern end of the peninsula. The ore is generally massive cupriferous pyrite, occurring in flat lenses in chlorite schists in[450] areas of regional metamorphism. About 250,000 tons comes from the Trondhjem district, where the Lokken mines of the Orkla Mine Co. are the largest producers.
The northern district is second in importance, with about 150,000 tons annual production, chiefly from the Sulitjelma mine at the Swedish frontier, near the Polar Circle. In the eastern district the Fodal Copper & Sulphur Co. has a production of from 75,000 to 100,000 tons. Norway has sufficient known reserves to last for thirty years at the present rate of production and probably for much longer. The largest reserves are in the Trondhjem district. Sweden is also reported to have large reserves, although there has been little development so far.
The commercial control of the mines is principally English and Norwegian. It was reported that mines with large reserves near Narvik were owned by German interests, but were purchased by Swedish interests during the war.
—For many years France has produced about 300,000 tons of pyrite per annum, or about 5 per cent. of the world output. The principal deposits are at Sain-Bel, near Lyons, in the Department of Rhône. The product is high in sulphur. The known reserves are probably from ten to twelve million tons. The output is used for home consumption, and in the past was supplemented by the import of Spanish pyrite, and Sicilian and United States sulphur.
—In addition to her large sulphur production Italy has produced a considerable quantity of pyrite, which has been used locally in the manufacture of sulphuric acid. Pyrite production was about 300,000 tons before the war and increased to 400,000 tons in 1916, so that Italy produces about 6 per cent. of the world output. The pyrite contains about 45 per cent. of sulphur and a small part of it carries copper values. The principal production comes from a district near Florence, although a number of smaller mines are widely scattered.
—Russia has large pyrite deposits located in a belt parallel to the eastern slope of the Ural Mountains. The Kyshtim and Sissert districts furnish the principal output. Reports indicate a good grade of pyrite with high sulphur content. The production has been in the neighborhood of 150,000 tons, or about 2 per cent. of the world total. Production had been steadily increasing up to the time of Russia’s economic collapse, but has been limited, due to the remote location of the deposits from the chief centres of consumption at Petrograd, Moscow, and Odessa. It is to be expected that Russia, after she regains her balance, will continue to import pyrite to a considerable extent, as she has done in the past.
—The pre-war German production was from 200,000 to 250,000 tons of pyrite per annum, or about 4 per cent. of the world output. About two-thirds of the output comes from[451] deposits near Meggen. The pyrite is estimated to run about 43 per cent. sulphur. It is reported that the pyrite output was largely increased during the war, as Germany had been importing from 800,000 to 1,000,000 tons of pyrite. She continued to import some Norwegian pyrite, which is especially desirable because of its recoverable copper content. Germany secures a considerable amount of sulphuric acid as a by-product from zinc smelters, which helped to make up the deficiency in her sulphur resources.
Hungary normally produces about 100,000 tons of pyrite per annum, chiefly from the deposits of Schemnitz.
—An important deposit of cupriferous pyrite is under development in an old copper-mining region on the northwest coast of Cyprus. Several million tons of ore are reported, containing a high sulphur content and high copper values. It is being developed on a large scale by the Cyprus Mines Corporation, representing United States capital, and may be expected to become an important factor in pyrite export.
—The pre-war production of the United States was about 350,000 long tons, or 6 per cent. of the world’s production, compared to an import of about 1,000,000 tons. About 40 per cent. of the total was produced in Virginia and largely sold for use in acid-phosphate plants from Maryland to Georgia; about 25 per cent. was produced in California and used for local acid manufacture in the vicinity of San Francisco; about 15 per cent. was produced in New York State; and the balance was scattered, coming as a by-product from coal mines in Ohio, Illinois, and Indiana, and from the zinc-mining region of southern Wisconsin. During the war, production was increased by about 50 per cent., but with no discoveries which promise to greatly increase the permanent production of the country. On the whole the deposits are not of very high quality, averaging about 40 per cent. sulphur content. Very large reserves of pyrrhotite are located in western Virginia and eastern Tennessee, but have not been very extensively utilized. Large reserves of pyrite exist in Colorado, Arizona, Utah and other western states, but are too far from the acid plants located in the East and South to compete. On the whole, the scanty development of pyrite in the United States is due to the competition of high-grade Spanish pyrite coming in to the Atlantic ports at cheap ballast rates; to the import of Canadian pyrite either to near-by points in New England or to the Great Lakes ports; to the large production of cheap sulphur from Louisiana and Texas, which has monopolized the sulphite pulp trade; and to the recovery of by-product acid from copper and zinc smelters. The great increase in the production of sulphur during and since the war is very likely still further to curtail the market for pyrite. The production of pyrite has been in the hands of American companies, several of the larger operations being controlled by concerns either in the acid or fertilizer business.
[452]
—The production of pyrite in Canada has increased rapidly, particularly during the war, to about 300,000 tons. This is due to an increased export to the United States, principally to sulphuric-acid plants. The principal producing areas in Canada are: (1) The district in Quebec, not far north of the Vermont border, where there are two operating mines and a number of promising prospects. There are large ore reserves and the ore carries considerable copper. The principal mines are controlled by American capital. (2) The Goudreau district, located some 40 miles north of Sault Ste. Marie, has large ore reserves, but of rather low grade. Thus far an American company is the principal producer. (3) The North Pine district near Graham, Ontario, and a considerable distance west of Port Arthur, has been a large producer of good-grade pyrite. The principal producer was a subsidiary company of the General Chemical Co.
There is a large reserve of pyrrhotite, estimated at about 50,000,000 tons, much of which will average over 25 per cent. sulphur, in connection with the Sudbury nickel deposits. At present it is not commercially important. There are considerable deposits of pyrites in various parts of British Columbia, but these are unimportant commercially because of their distance from any available market. The larger part of the Canadian product is controlled by American interests, chiefly the American Chemical Co., whose headquarters are in New York City. A large part of the Canadian output is imported to the United States through Chicago, Cleveland, and Buffalo; and by rail through Vermont, Boston and to New York City.
—An important pyrite property is being developed about twenty miles from Cienfuegos, Province of Santa Clara, Cuba. It is reported as containing several million tons of good-grade ore, which will average at least 40 per cent. sulphur and may contain a recoverable copper content. The property is being developed by United States capital, interests connected with the Davison Chemical Corporation, of Baltimore, Md., who are one of the largest producers of sulphuric acid on the Atlantic Coast. This property promises to be an important near-by source of pyrite for the United States.
—Important pyrite deposits are known to exist in Mexico, but they are of no present commercial importance because of inaccessibility and high freight rates, and unsettled political conditions. A large deposit is reported about 30 miles inland in the State of Guerrero, containing several million tons of high-grade pyrite of approximately 48 per cent. sulphur content and free from arsenic.
There is no prospect that Mexico will be of any immediate importance in the world pyrite situation.
—Japan has a small pyrite production of from 75,000 to 100,000 tons per year, or about 1¹⁄₂ per cent. of the world output. Much of it[453] carries copper values. The production comes from several scattered localities. The state has reserved the ownership of the original mineral rights, and the operators to whom they have been leased appear to be entirely Japanese. Japan consumes her pyrite for local purposes, and exports most of her sulphur.
—Italy had practically a monopoly of the world’s sulphur supply until 1904, when large-scale production began in the United States. The importance of sulphur as a world mineral began with the use of gunpowder in the fourteenth century. Considerable export trade was early developed and has been of increasing importance since 1830. Ninety per cent. of the Italian production has come from the Island of Sicily.
The sulphur-bearing district of Sicily is a central belt running across the island, extending about 100 miles east and west and 50 miles north and south. The richer deposits are scattered as irregular lenses or basin-like bodies, in this extensive area. The deposits of commercial value are of sedimentary type, occurring as stratified beds or sheets in limestone, associated with gypsum and bituminous marl. There are generally three or four sulphur-bearing layers, separated by a few feet of barren rock. The average thickness of the sulphur beds is from 10 to 15 feet, although in a few places they run as high as several hundred feet. The sulphur occurs as incrustations, pockets, or thin bands intimately associated with the limestone. The average sulphur content of the ore mined is from 20 to 25 per cent., with a range from 8 to 50 per cent.; and in a few places it reaches up to 90 per cent. Estimates as to the reserves of ore vary greatly, but seem to indicate that there is from 40 to 60 million tons of ore still unmined, which will average about 23 per cent. of sulphur content.
Mining has been mostly by hand and the ore brought out on the backs of men. A few mines had modern hoisting machinery and trams. The shortage of labor during the war has increased the introduction of modern appliances in some of the newer mines. With increasing depth the cost of mining has increased to the point where American sulphur can compete in European markets.
The methods of extracting the sulphur from the ore have also been extremely crude and wasteful, but in the last few years better types of ovens have been installed, giving a much higher recovery through improved distillation and the use of superheated steam for melting the sulphur.
The sulphur industry of Sicily furnishes a notable example of an attempted commercial control which developed into a governmental[454] control of the industry. The recent history of the industry falls into three periods. The first extends from 1875 to 1895 and is characterized by a rapid increase in production, from 200,000 to 400,000 tons a year, with a corresponding decrease in selling price from $25 a ton to as low as $12 a ton. It was a period of overproduction, due to the ease with which shallow mining could be carried on and to the abundant supply of cheap labor available. These conditions resulted in the development of a great number of small mines, whose competition reduced prices. The second period, from 1896 to 1906, begins with the formation of the Anglo-Sicilian Sulphur Co., financed by English capital, which entered into a five-year agreement with the principal producers, which was later extended for an additional five years. It was primarily a marketing organization, formed by the union of Italian and English interests to control production, stabilize the industry, and maintain prices. It eventually controlled from 75 to 85 per cent. of the industry. All sulphur was purchased at about $16 per ton f.o.b. ship and the selling price remained practically stable during the ten-year period, at an average of $18 to $19 per ton. In spite of efforts to restrict production, the annual output reached 550,000 tons during most of this period. At the same time the higher prices maintained for sulphur had stimulated the use of pyrite as a substitute. In order to maintain prices under these conditions the excess production had to be purchased and stored, so that in 1906 a stock of over 500,000 tons of sulphur had been accumulated in Sicily. Toward the end of this period large-scale production began in the United States (1904). In 1903 the United States produced less than 10,000 tons of sulphur and was Italy’s best customer, buying over 170,000 tons in that year. Within three years the United States was producing more than enough to supply its own needs and was accumulating a large reserve stock. The sudden loss of the American market and the threat of competition in other markets brought on a crisis in the Sicilian industry, which was intensified by the large number (30,000) of people employed in the industry. At the termination of the agreement with the Anglo-Sicilian company (July 31, 1906) steps were taken by the Italian government to control the situation. The third period, from 1906 to the present, is one of government intervention and control of the industry. All the producers were compelled to join a company called the “Consorzia Obbligatoria per l’Industria Solfiefera Siciliana,” organized under a law passed in the Italian Parliament. The organization was managed by a commission appointed by the government, and had complete control over exports and prices. All sulphur had to be sold at fixed price to this organization. A minimum interest was guaranteed on the capital invested; local freight rates on sulphur for export were reduced; sulphur stocks accumulated by the Anglo-Sicilian company were taken over; and a campaign of price-cutting was started in the American market,[455] which resulted in a decrease of several dollars a ton in the selling price of sulphur. A market agreement was soon reached and prices recovered. A number of the smaller mines closed down and a law was passed controlling and restricting the granting of new concessions. Production declined to 350,000 tons in 1913. At the opening of the war the principal United States producer was preparing to enter into more active competition with Italian sulphur, particularly in the French markets. As a result of the war, Italian production dropped to only 180,000 tons in 1917, largely due to labor shortage; about half of the surplus stocks were used up, leaving only 160,000 tons on hand at the end of 1917; and prices increased so that refined sulphur sold at about $80 per ton and inferior grades at $55 per ton, f.o.b. Sicilian ports. The increasing cost of producing sulphur, due to deeper mining and increased labor costs, will make it difficult to compete in the European markets with the greatly expanded production of the cheaper American article.
Sulphur has been produced in several districts in the Italian peninsula, particularly Romagna, Marches, Campania and Calabria. The yield from these districts has been decreasing in recent years and has generally been only from 25,000 to 30,000 tons. The sulphur content of the ores ranges from 20 to 30 per cent. The deposits are of limited extent and are being mined at greater depths. The production has been largely used for local agricultural purposes, in preparations for use against vine diseases.
—Until 1904, the production of sulphur in the United States was considerably less than 10,000 tons per year and the bulk of our requirements had to be met by import from Sicily. From 1904 to 1914 the United States produced enough for its own use and at the end of this period was supplying Canada, had begun to actively enter the French and German market, and in addition had accumulated a reserve stock, in the hands of the producers, of approximately one million long tons. Figures recently made public in connection with litigation over patent rights show that half of this stock was accumulated in a single year, 1912, when production reached 790,000 long tons, of which only 300,000 tons was marketed and the balance of 490,000 tons went into storage. The United States production has exceeded that of Italy since 1912, although the sales have been less, because sulphur was being withdrawn from stocks in Italy while stocks in the United States were being increased. The net effect of the war was a four-fold increase in the amount of sulphur sold in the United States, without any reduction in stocks; while in Italy production fell off 50 per cent. and stocks on hand were reduced by the same percentage.
From 98 to 99 per cent. of the United States production has come from the Gulf Coast region of the states of Louisiana and Texas. A number of other localities in West Texas, Colorado, Wyoming, Idaho,[456] and Nevada have surface deposits, usually of limited extent, which have been worked on a small scale, but have declined in importance with the development of the better-grade and more accessible deposits of the Gulf Coastal region.
The occurrence of sulphur in the Gulf Coast region is in connection with a peculiar formation known as “Saline Domes” or “Mounds.” Over twenty of these domes have been located, scattered in an area 200 miles long, extending through western Louisiana and eastern Texas, and generally within 50 miles of the Gulf of Mexico. Commercial deposits of petroleum, sulphur, and salt have been developed in connection with these domes, but so far not more than one of the minerals has been developed to commercial degree in a single dome. Sulphur was discovered when drilling was being carried on for oil. So far, three domes have been developed for sulphur, namely that owned by the Union Sulphur Co. at Sulphur, La. (1903), that owned by the Freeport Sulphur Co. at Freeport, Texas (1912), and that of the Texas-Gulf Sulphur Co. near Matagordo, Texas (1919). Two other domes are under exploration and a number of others may possibly contain sulphur.
The sulphur occurs at a depth of 300 to 1,200 feet and is associated with limestone and underlain by gypsum. The surface area of the producing domes varies from 200 to 1,500 acres. Exploration is done by drilling at a cost of $200,000 to $300,000, and the cost of a complete plant is several million dollars. The sulphur cannot be mined by shafts, due to the quicksands and the poisonous gases encountered. The deposit at Sulphur, Louisiana, remained unworked for almost 40 years after its discovery before a satisfactory process was developed to mine it. This is known as the “Frasch Process” and consists of the sinking of wells to the sulphur deposit, each well being lined with a 10 to 12 in. pipe. Smaller pipes are placed inside, so that superheated water can be brought in contact with the sulphur ore, which is melted and forced to the surface by compressed air. The sulphur on cooling is ready for market and is over 99 per cent. pure. Each of the three plants in operation is equipped with a boiler capacity of over 20,000 h.p. for superheating the water, and requires about a million and a quarter barrels of fuel oil per year. The origin of these domes is believed to be due to deep-seated igneous intrusions, resulting in the alteration of gypsum and the crystallization of salt and sulphur, which has caused an upbowing of the strata. Because of the nature of the formation and the irregularity of the deposits it is impossible to accurately estimate the reserves of sulphur.
With the addition of two new plants since 1912, the United States now has a sulphur-producing capacity of about 1¹⁄₄ million tons per year, or four times the normal sales before the war. If an outlet is to be found for this excess sulphur, it must compete with pyrite in the domestic market or with Sicilian sulphur in the European markets. In the latter[457] part of 1919 prices of $14 to $15 per ton f.o.b. mines were quoted, which indicated that an effort was being made to secure part of the acid trade which formerly used pyrite.
There is no element of political control in the United States sulphur industry, beyond the temporary measures taken during the war in licensing export and allocation of consumption. The commercial control is entirely in the hands of American companies. The Union Sulphur Co. has been endeavoring to prevent the use of the improved “Frasch Process” by the other companies which are competing with it. If the claim of infringement of patent rights should be sustained, it would give the Union company control of the situation similar to that which it had before the development of the two newer companies, and might result in the restriction of output and maintenance of prices.
—Japan takes third rank in the production of sulphur, although it is of minor importance, compared to either the United States or Italy. The production of sulphur in Japan has slowly increased from 15,000 long tons in 1900 to 60,000 tons in 1913, or about 7 per cent. of the world output. The domestic consumption is very small and about 90 per cent. of the output was exported, chiefly to Australia, the west coast of the United States and Canada, and to China and India. During the war the output increased to a maximum of about 100,000 tons, but in 1918 production was considerably curtailed by the great advance in freight rates to Australia, which had been purchasing about one-half of the Japanese output.
The sulphur occurs in surface deposits of limited extent and seldom reaches 100 feet in thickness. The deposits are generally of the solfataric type and occur in the numerous areas of volcanic activity. The majority of the productive areas are nearly circular in outline, and indicate that they were formed by deposition in crater lakes. In some cases they are stratified and overlain by fine brown clayey or tufaceous material derived partly from the surrounding rocks and partly from the sulphur itself. Other deposits of minor importance may have been produced by impregnation. The ore mined runs from 50 to 60 per cent. sulphur. Deposits below 40 per cent. sulphur are seldom worked.
Approximately two-thirds of the production has come from the southwestern section of the Island of Hokkaido. Four mines average about 10,000 tons production each per year, and the remainder of the production comes from 10 to 12 smaller operations, ranging from the vicinity of Mount Daiton, in Taiwan (Formosa), to the Kurile Islands.
There is no accurate estimate of ore reserves available. One of the most important mines was estimated as containing several million tons of 50 per cent. ore. The reserves are probably sufficient to maintain present production for many years. The lack of shipping facilities has handicapped production, and there seems little likelihood that the relative[458] importance of Japan in the sulphur industry will increase to any great extent.
The sulphur mines are all operated by Japanese. The state reserves the right of original ownership of all minerals, except a few placer deposits. Right of working is granted to Japanese companies or individuals according to priority of application. The mining law, however, acknowledges the rights of any corporation organized by aliens under Japanese law.
—Great Britain has an estimated annual by-product recovery of from 30,000 to 40,000 tons of elemental sulphur. The process of recovery is known as the Chance-Claus process, and is applied in connection with the Le Blanc soda process. It is based upon the decomposition of calcium sulphate in vat waste by means of carbon dioxide, and the recovery of sulphur from the sulphuretted hydrogen gas thus generated.
—The production of sulphur outside of the United States, Italy and Japan is of minor importance.
Northern Chile has a small production of sulphur from the volcanoes of Tacora and Chupiquina. The reserves are estimated as quite large, but the high elevation (14,000 to 20,000 ft.) and poor transportation have restricted production to local uses in the vineyard districts of Chile. The production as reported had gradually increased to about 6,000 tons in 1913 and is reported to have doubled since then.
Spain produces about 10,000 tons of sulphur annually, from low-grade deposits located in the neighborhood of Almeria. The larger figures often reported are in terms of low-grade ore mined.
Austria is credited with a production of from 10 to 15 thousand tons of crude sulphur ore, probably representing only 2 or 3 thousand tons of actual sulphur.
The largest sulphur mine in Mexico is located at Cerritos, 25 miles south of Guadalcazar, San Luis Potosi. Fifteen years ago it was purchased by an American company, the Virginia-Carolina Chemical Co. It was later leased to German interests. The small output of a few thousand tons was shipped to Germany before the war. There are a number of deposits in San Luis Potosi in addition to the one at Cerritos.
It has been reported several times that a British company was about to operate the sulphur deposits of the Mexican volcano, Popocatepetl, near Mexico City. Statements regarding the deposits in the volcano are conflicting, but investigations indicate that their magnitude has been much exaggerated. Many other deposits occur in connection with local volcanic areas, but so far are of little economic value because of inaccessibility.
There are a number of deposits of sulphur in the Aleutian Islands[459] (Alaska), probably containing considerable sulphur, but partially covered by glaciers and difficult of access. It is doubtful whether they could be developed in the face of the competition of the cheap sulphur from the coastal plain district of the United States.
No very far-reaching changes in practice are likely to occur in the near future. The exhaustion of the surface sulphur deposits in Italy and the necessity for deep mining is making necessary improvement and installation of more modern methods there. Improved methods in the refining of sulphur are also being installed whereby the losses under the old calcarone method will be largely eliminated. The consumption of sulphur for sulphite wood pulp can be considerably reduced by the general utilization of the improved practice which is already used by the best plants. In localities where pyrite is available this material may be used in the pulp industry to replace sulphur. In case competition develops between the three large sulphur companies in the United States, and the price of sulphur is considerably reduced, it may result in the use of this material to a larger extent in the sulphuric-acid industry. The sulphur burners can be installed much more quickly and cheaply than the furnaces required to roast pyrite, and after once being installed the amount of labor and care required in their operation is less. The increased recovery of sulphuric acid as a by-product from copper and zinc smelters will probably represent an increasing factor in competition with acid made either from pyrite or sulphur. The further increase of this source in the United States is handicapped by the location of many of the copper smelters in the west, at long distances from the market for sulphuric acid, which is largely in the eastern and southern states. New processes are being experimented with, for the production of elementary sulphur from these sulphur fumes. If these are successful on a large scale, material from this source may supply any future markets located in the west, and might compete with the Japanese sulphur which has formerly been imported in our Pacific Coast States.
The political control of the important sulphur deposits of the world primarily corresponds to the countries in which they are located. In the case of the United States, the deposits are controlled by private companies. As a strictly war measure, control was exercised over the allocation and distribution of the output. In Italy the government had assumed control of the output and marketing of sulphur. This was largely brought about by the competition of American sulphur and[460] the consequent depression of the Italian industry. In 1906 what was known as the Consorzia Obbligatoria was organized under a law passed by the Italian Parliament, which provided that this company should be administered by a royal commissioner appointed by the Italian government. Under this law producers were obliged to sell their output to this company, which had control of prices and exports. In 1910 restrictions on the granting of new concessions were made. The arguments recently presented for the continuance of government control were the increasing foreign competition, the large war increase in United States production, the minor increase of Japanese production and the possibilities of developments in northern Africa. The intent of this governmental control of the industry is to combine and regulate the efforts of individual producers in order to effectively meet future competition.
Before 1906 the Italian deposits were largely controlled by the Anglo-Sicilian Sulphur Co., representing English capital, but since that time, when the Italian government undertook to control the industry, the commercial control has been primarily Italian.
In the United States the commercial control of sulphur output is in the hands of three companies, one of which started producing just about the time of the outbreak of the European war and another whose production was just beginning in 1919. So far as is known, there is no combination among these three interests. The Union Sulphur Co., which was the first and principal producer, controls certain patents covering the “Frasch Process.” During the war period an agreement was entered into by which alleged infringement of patents was not pushed. Since the close of the war it remains to be seen to what extent the patent rights involved may affect the production of the other two companies, the Freeport and the Texas Gulf, which in general use a similar process.
The production of sulphur in Japan is commercially controlled by Japanese interests.
An American company, the Virginia-Carolina Chemical Co., owns a sulphur deposit in Mexico, which was leased, before the war, to German interests. Several other deposits in Mexico and South America were reported as controlled by German interests, but thus far the production from all these sources has been relatively of minor importance and there is no immediate prospect of any great change.
The significant factor of commercial control in the pyrite situation is the large investment of British capital in the Spanish deposits and to a less extent the investment of French capital. United States capital controls the principal pyrite developments in Canada, Cuba, Mexico and Cyprus. English, French and Swedish capital is invested in Norway.
[461]
The United States is the most favorably situated of any nation in its supply of sulphur. In the years preceding the European war it produced about one-half the world’s supply. Since that time its production has increased several fold and it is now the dominating factor in the world situation.
The relative position of Italy, which was formerly of equal importance with the United States, is declining. Her cost of production is increasing, and American sulphur will enter into keen competition with the European markets. The resources of Japan are comparatively small and the larger part of her production has been exported. The post-war conditions will probably curtail the markets for Japanese sulphur, and there is no likelihood of any increase in Japan’s position. England, France and Germany must primarily rely upon other countries for their supply of both pyrite and sulphur. England secures a part of her sulphur supply from a by-product source known as the Chance-Claus process, which produces from 30 to 40 thousand tons annually. In addition she secures large amounts of pyrite from Spain. France produces some local pyrite, but imports large quantities of this material from Spain; and before the outbreak of the war was securing increasing amounts of sulphur from the United States. Germany and Austria have some resources in pyrite and probably had considerable stocks of sulphur at the opening of the war. It has been stated that in order to secure her needs of these materials Germany was forced to use expensive processes, to reduce the sulphur content of gypsum, to expand her pyrite production, and to increase the output of smelter acid. Under normal conditions she will probably have to return to imports of pyrite from Norway and Sweden, or sulphur from the United States and Italy.
[462]
The physical properties of gold and the difficulties connected with obtaining it have made gold through all the ages one of the most precious of the metals. Because of its luster, its color, and its indestructibility, it was shaped by primitive man into rings, bracelets, and other ornaments. These same properties, together with its divisibility, early led to the use of gold as a trading counter, and in all but the most primitive societies it has been regarded as a measure of value and a medium of exchange. The earliest known reference to gold is a reference to its use in trade, and is contained in an edict of Menes of Egypt (perhaps 3800 B. C.) fixing the ratio of the value of gold to silver at 2¹⁄₂ to 1.
As a medium of exchange gold is used in a variety of forms. The primitive trader accepted and gave ornaments of gold in exchange for commodities. In mining camps, gold dust and nuggets have been the circulating medium. In a settled community under a government possessing the confidence of the governed, gold coined into counters of convenient denominations by the government is accepted without question in all transactions. For the settlement of balances in international trade, gold bullion is used, its value being determined by weight. Today practically all nations are on a gold standard, and even those on a silver standard in domestic trade have adopted the gold standard for foreign trade.
But gold, though it has many excellent qualities, is one of the heaviest of metals, and carrying it on the person is inconvenient, especially in amounts sufficient for large transactions. Also, through constant handling, gold wears away and coins deteriorate in value. Accordingly a large part of the coins or the gold bullion is kept in the national treasury or in the vaults of a bank, and some form of paper currency is used as the circulating medium. Upon this reserve of gold, seen only by a few employees of the government or bank, who have no ownership in it, is built an intricate system of credits and trade.
It is true that little gold is in actual circulation and that for domestic trade mediums of exchange secured only in part by gold are being used in increasing amounts. Even international exchange is no longer on the[463] gold standard, but this situation is undoubtedly temporary and with the improvement in financial conditions in Europe gold will be restored to its position as foundation of trade and finance, national and international. The circulating medium, whatever its nature, must be freely convertible, and credit, to remain sound, must be founded upon an adequate gold reserve.
It has been officially estimated that between the years 1492 and 1894 the world’s production of gold equaled about $8,000,000,000. Of this amount a little less than $4,000,000,000 was held as gold reserve in 1894, the remainder having been lost or absorbed in the arts or in the manufacture of jewelry. Since 1894 the consumption of gold in the arts has been between $50,000,000 and $100,000,000 annually, or for the 27 years about $2,100,000,000 out of a total production of $9,000,000,000—almost 25 per cent.[158]
[158] Jennings, Hennen: “The Gold Industry and Gold Standard,” 1919.
Gold is widely distributed, both geographically and geologically. It is found in about 60 countries in all parts of the world and occurs in rocks of all ages, from Archean to Quaternary, often in association with other metals, as silver, copper, tellurium, lead, and iron.
Gold deposits may be broadly classified as lodes and placers. Lode deposits are also known as vein or quartz deposits; and in them the gold is found with silica or quartz in irregular masses, strings, scales, plates, and crystals, or more often in microscopic particles in the sulphide minerals or the gangue. Much the same methods are used for extracting the ore as are used in mining silver, lead, or zinc.
Placer or alluvial deposits are sedimentary beds of gravel or sand in which the particles of gold, washed from the mother lode, have been concentrated by the action of water. Most of the placer deposits lie in the open, along the bed of a river, but some are covered by a sheet of lava or other non-productive rock. The gold occurs as scales, grains, slugs or nuggets. A number of large nuggets have been found, the largest being the Australian “Welcome Stranger,” weighing 2,520 ounces and valued at about $42,000. Usually, however, the particles of gold are minute.
Placer deposits are worked by one of the following methods, depending on the nature and richness of the alluvium: sluice mining, hydraulic mining, dredging, or drift mining.
During the early years of what has been called the modern era of gold mining, beginning with the discovery of gold in California in 1848, most of the world’s annual production came from placer deposits. De Launay estimates that from 1848 to 1875 placers contributed 87 per cent. In more recent years, with the exhaustion of the easily discovered and[464] the easily worked placer mines, the proportion has decreased and at present it is probably not more than 10 per cent. Today the great gold mines of the world are lode mines. The world’s deepest gold mine has attained a vertical depth of 5,900 feet.
After making all allowances for further discoveries, students of the subject are of the opinion that the world’s gold production has already reached its zenith and that a decline may be expected.[159]
[159] The above discussion of the geological occurrence of gold is based mainly on “The Gold Industry and Gold Standard” and other articles by Hennen Jennings, consulting engineer, U. S. Bureau of Mines.
Commercially important mines are found in every continent, and there are few regions on the earth’s surface that do not contain deposits profitably worked now or formerly. The distribution of gold has played an important part in the settlement of new lands, notably California, western Canada, Alaska, Mexico, Australia, and South Africa, and will undoubtedly greatly influence the future movements of peoples. Yet although gold is distributed thus widely, $323,950,000, or over 75 per cent. of the total amount of gold produced in 1917, came from four countries, Transvaal, United States, Australia and Russia.
The outputs of the chief producing countries are given in Table 67 and Figure 19.
—Although at present surpassed by the Transvaal, the United States formerly led the world as a producer of gold. It is estimated that this country since 1792 has contributed about $3,913,000,000 to the gold supply of the world, a little less than 25 per cent. of the total produced, and an output greater than that of any other nation.[160] The United States first became an important producer in 1850, following the discovery of gold in California. Previous to that time some gold was mined in the Appalachian states, the total probably reaching $50,000,000.
[160] Report of the committee appointed by the Secretary of the Interior to study the gold situation.
In 1915 the output of the United States was $101,035,000, the highest mark so far reached. Since that year there has been an annual drop of about $8,000,000 in output. The yield in 1918 was $68,646,700 and in 1919, $58,488,800.
During the past four years, eight states, California, Colorado, Alaska, South Dakota, Nevada, Arizona, Montana, and Utah, have produced more than 90 per cent. of the gold mined in the United States.
[465]
Table 67.—Gold Production of the Principal Producing Countries, 1880-1917[161]
| Year | United States |
Australasia | Transvaal [162] |
Russia- Siberia |
Rhodesia [162] |
Mexico | Canada | British India |
World [163] |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1880 | $36,000,000 | $28,765,000 | $1,993,800 | $28,551,028 | ... | $989,160 | $815,089 | ... | $106,436,800 | ||
| 1881 | 34,700,000 | 30,690,000 | 1,993,800 | 24,371,343 | ... | 858,909 | 1,094,926 | ... | 103,023,100 | ||
| 1882 | 32,500,000 | 31,955,017 | 1,993,800 | 23,867,935 | ... | 936,223 | 1,094,926 | ... | 101,996,600 | ||
| 1883 | 30,000,000 | 27,150,000 | 717,000 | 20,119,000 | ... | 951,000 | 954,000 | ... | 95,392,000 | ||
| 1884 | 30,800,000 | 28,284,000 | 830,000 | 21,874,000 | ... | 1,183,000 | 954,000 | ... | 101,729,000 | ||
| 1885 | 31,801,000 | 27,439,000 | 1,384,000 | 25.338,000 | ... | 867,000 | 1,116,000 | $135,000 | 108 435,000 | ||
| 1886 | 34,869,000 | 26,425,000 | 1,438,000 | 20,518,000 | ... | 614,000 | 1,330,442 | 421,600 | 106,163,000 | ||
| 1887 | 33,136,000 | 27,327,600 | 1,919,600 | 20,092,000 | ... | 824,000 | 1,178,637 | 320,000 | 105,774,900 | ||
| 1888 | 33,167,000 | 28,560,660 | 4,500,000 | 21,302,000 | ... | 974,000 | 1,111,959 | 685,720 | 110,196,000 | ||
| 1889 | 32,967,000 | 33,086,700 | 7,788,372 | 23,905,600 | ... | 700,000 | 1,295,000 | 1,502,600 | 123,489,200 | ||
| 1890 | 32,845,000 | 29,808,000 | 10,438,356 | 25,484,000 | ... | 767,000 | 1,666,000 | 2,000,000 | 118,848,700 | ||
| 1891 | 33,175,000 | 31,399,000 | 14,885,639 | 24,162,500 | ... | 1,000,000 | 930,666 | 2,495,000 | 130,650,000 | ||
| 1892 | 33,015,000 | 34,159,000 | 23,220,108 | 24,806,200 | ... | 1,129,200 | 907,600 | 3,318,300 | 146,651,500 | ||
| 1893 | 35,955,000 | 35,688,600 | 28,293,831 | 27,808,200 | ... | 1,305,300 | 927,200 | 3,813,600 | 157,494,800 | ||
| 1894 | 39,500,000 | 41,760,800 | 39,696,330 | 24,133,400 | ... | 4,500,000 | [164] | 1,042,100 | 3,882,900 | 181,175,600 | |
| 1895 | 46,610,000 | 44,798,300 | 43,893,300 | 28,894,400 | ... | 6,000,000 | [164] | 1,910,900 | 4,656,200 | 198,763,600 | |
| 1896 | 53,088,000 | 45,181,900 | 43,779,669 | 21,535,800 | ... | 8,331,700 | [164] | 2,810,200 | 6,130,500 | 202,251,600 | |
| 1897 | 57,363,000 | 52,665,700 | 57,633,861 | 23,245,700 | ... | 7,500,000 | [164] | 6,089,500 | 7,247,200 | 236,083,700 | |
| 1898 | 64,463,000 | 64,860,800 | 79,213,953 | 25,463,400 | 444,617 | 8,500,000 | [164] | 13,838,700 | 7,781,500 | 286,879,700 | |
| 1899 | 71,053,400 | 79,321,600 | 71,384,561 | 22,167,100 | 1,129,773 | 8,500,000 | [164] | 21,324,300 | 8,658,800 | 306,724,100 | |
| 1900 | 79,171,000 | 73,498,900 | 6,124,226 | 20,145,500 | 1,158,815 | 9,000,000 | [164] | 27,880,500 | 9,435,500 | 254,576,300 | |
| 1901 | 78,666,700 | 76,880,200 | 5,333,994 | 22,850,900 | 2,974,943 | 10,284,800 | 24,128,500 | 9,395,900 | 260,992,900 | ||
| 1902 | 80,000,000 | 81,578,800 | 34,901,140 | 22,533,400 | 3,366,561 | 10,153,100 | 21,336,700 | 9,588,100 | 296,737,600 | ||
| 1903 | 73,591,700 | 89,210,100 | 61,454,439 | 24,632,200 | 4,065,489 | 10,677,500 | 18,834,500 | 11,428,900 | 327,702,700 | ||
| 1904 | 80,464,700 | 87,767,300 | 78,004,559 | 24,803,200 | 4,794,208 | 12,605,300 | 16,462,500 | 11,722,900 | 347,377,200 | ||
| 1905 | 88,180,700 | 85,926,500 | 101,489,199 | 22,291,600 | 7,337,211 | 16,107,100 | 14,610,400 | 11,950,200 | 380,288,700 | ||
| 1906 | 94,373,800 | 82,391,400 | 119,618,507 | 19,496,500 | 10,082,747 | 18,534,700 | 12,023,900 | 12,087,700 | 402,503,000 | ||
| 1907 | 90,435,700 | 75,677,700 | 133,361,943 | 26,684,300 | 11,199,181 | 18,681,100 | 8,382,800 | 10,383,600 | 412,966,600 | ||
| 1908 | 94,560,000 | 73,327,300 | 145,862,971 | 28,052,200 | 13,022,460 | 22,371,200 | 9,842,100 | 10,598,500 | 442,476,900 | ||
| 1909 | 99,673,400 | 71,007,900 | 150,799,880 | 32,381,300 | 13,223,955 | 23,842,900 | 9,382,200 | 10,358,600 | 454,059,100 | ||
| 1910 | 96,269,100 | 65,470,600 | 155,597,202 | 35,579,600 | 13,590,658 | 24,910,600 | 10,205,800 | 10,718,400 | 455,239,100 | ||
| 1911 | 96,890,000 | 60,184,200 | 170,566,159 | 32,151,600 | 13,823,733 | 24,880,100 | 9,762,100 | 11,054,100 | 461,939,700 | ||
| 1912 | 93,451,500 | 54,509,400 | 188,293,100 | 22,199,000 | 14,226,900 | 24,500,000 | 12,648,800 | 11,055,700 | 466,136,100 | ||
| 1913 | 88,884,400 | 53,113,200 | 181,885,300 | 26,508,700 | 14,274,700 | 19,308,800 | 16,598,900 | 12,178,000 | 459,941,100 | ||
| 1914 | 94,531,800 | 47,569,023 | 173,559,940 | 28,586,392 | 17 663,686 | 4,788,175 | 15,983,004 | 11,378,400 | 439,078,260 | ||
| 1915 | 101,035,700 | 49,397,797 | 188,033,156 | 26,322,745 | 18,915,324 | 6,559,275 | 18,977,901 | 11,522,457 | 468,724,918 | ||
| 1916 | 92,315,363 | 38,213,328 | 192,182,900 | 22,300,000 | 19,232,200 | 7,690,700 | 19,235,000 | 11,206,500 | 454,176,500 | ||
| 1917 | 84,456,600 | 35,275,000 | 186,254,256 | 18,000,000 | [164] | 14,988,600 | 9,000,000 | [164] | 15,449,426 | 10,756,800 | 423,590,200 |
[161] Compiled from reports of the Director of the Mint, 1880-1917.
[162] Previous to 1898 production of Rhodesia and Mozambique included in Transvaal returns.
[163] Total production of world, 1492-1917, $16,916,735,394.
[164] Estimated.
[466]
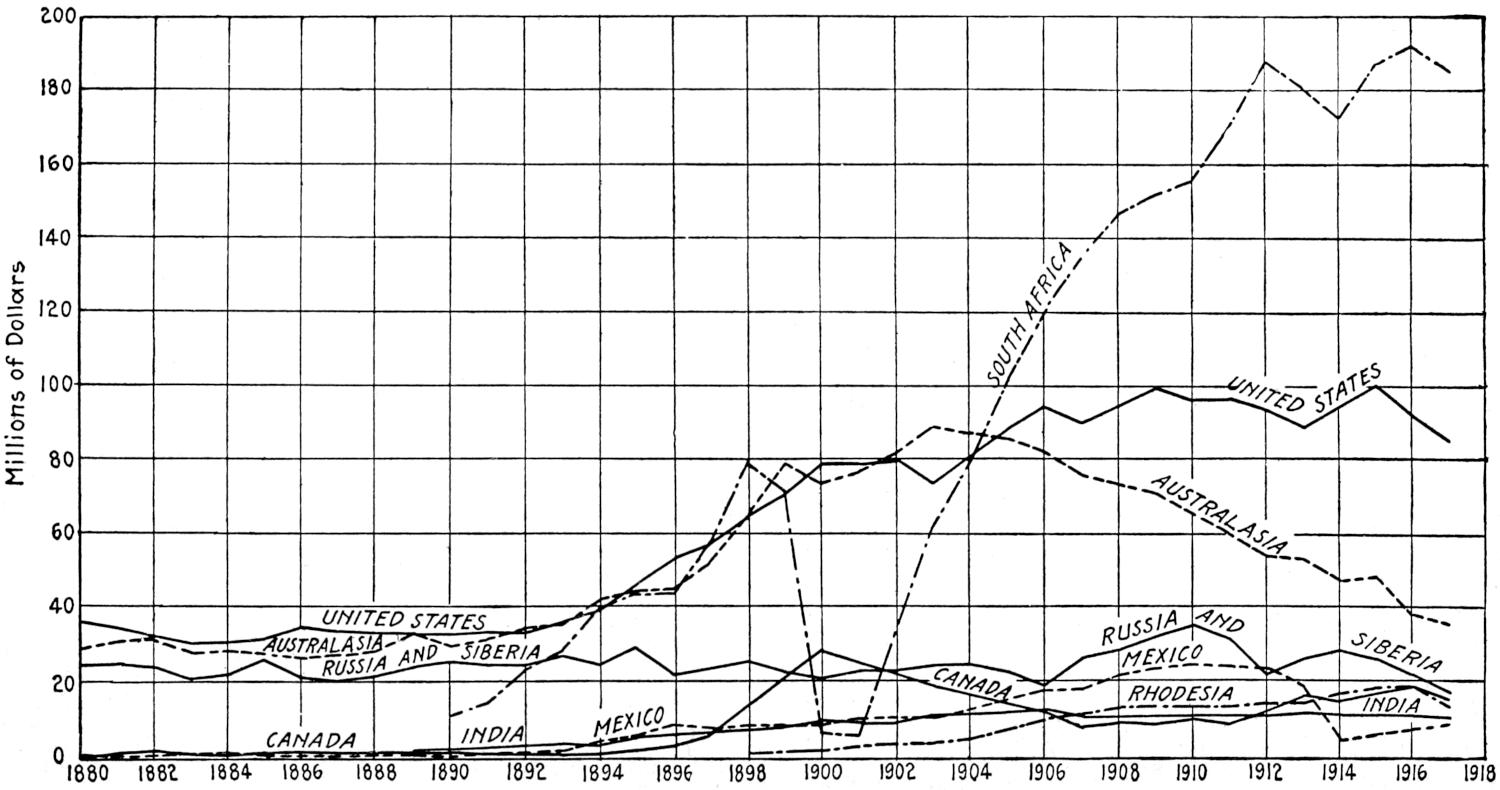
Fig 19.—Production of gold in chief producing countries, 1880-1917, based on reports of the Director of the Mint.
With the discovery of gold in a mill race at Coloma, Eldorado County, California, by J. A. Marshall in 1848, the history of modern gold production began. The great rush to California followed and the state became the leading gold-producing region of the world. The output[467] for 1860 amounted to $45,320,000, as compared with a total output of only $1,000,000 for the remaining states. For many years the gold production of California has been declining, the total yield in 1917 amounting to only $20,000,000, a decrease of almost $2,000,000 from the output of 1916. About three-fifths of the output comes from lode mines and the remainder from placers.
For a number of years Colorado was the leading gold-producing state, principally because of the output from Cripple Creek, Teller County, which was from 1893 to 1908 the leading gold camp in the United States. Recently the output has fallen below that of California. Over half of the yield of the state in 1916 came from Teller County.
The earliest discovery of gold in Alaska was probably about 1849 along the shores of Cook Inlet. In 1879 the gold-quartz veins near Sitka were discovered, and a year later the placers of Juneau. The discovery of the gold placers in the valley of the Yukon was made in 1886 and of those in the Klondike in 1896. Most of the Alaskan output is obtained from placer deposits, the richest being in the Seward Peninsula near Nome and in the Yukon basin. The largest producing lode mines have been those near Juneau; of these the Treadwell was for long the greatest gold mine in the world, but it is now inactive. During the past few years the working of large low-grade disseminated deposits of the Treadwell type near Juneau has been undertaken on a scale hitherto undreamed of, but thus far these ventures (Alaska Gold and Alaska Juneau mines) have not been financially successful.
The gold-producing area of South Dakota is confined to an area of less than 100 square miles, lying in the Black Hills. The gravels of Whitewood and Deadwood gulches were first washed in 1875, and in 1876 the famous Homestake lodes were discovered. At present about 94 per cent. of the total output of the state is controlled by one company, the Homestake Mining Co., the largest producing company in the United States.
Following the exhaustion of the famous Comstock lode, demarcated in 1851, Nevada was of little importance as a gold-producing state until the discovery of the rich deposits of Tonopah in 1900. At present practically all the gold obtained in the state comes from the vein deposits of Tonopah and Goldfield. Divide is a newly developed camp between these two.
There are three main auriferous areas in Arizona: the vicinity of Bisbee and Tombstone, Cochise County; the Oatman district, Mohave County; and the Verde district, Yavapai County. Arizona was one of the few states to show a larger output in 1917 than in 1916. The United Eastern mine, a new property opened in 1913 in the Oatman district of Mohave County, produced heavily and was responsible for the increase.[468] The gold output of Cochise and Yavapai counties is obtained largely from copper ore.
During the sixties and seventies, Montana was second only to California in its yield of placer gold. The most famous placers were those of Bannack and Alder Gulch and later Helena. Much of the present output is obtained as a by-product from copper ores.
Few mines in Utah are worked exclusively for their gold content, the greater part of the yield of the state being derived from copper and lead ores.
Gold is also mined in Idaho, New Mexico, Oregon, and Washington. Small amounts were produced in 1917 in a number of the Appalachian states. Before the discovery of gold in California, practically all of the gold coined in the United States mint came from the mines of Virginia, Maryland, Alabama, Georgia, and the Carolinas, but since the Civil War their output has not been important. The gold production of the Philippine Islands in 1917 amounted to $1,404,000.
—The total gold yield of Canada in 1900 amounted to $28,000,000, but with the exhaustion of the placer deposits it declined to $8,382,000 in 1907. With the development of vein deposits, production increased steadily from this low point, and reached $19,235,000 in 1916, and $15,272,992 in 1917, placing Canada sixth in the list of gold producers. Three provinces, Ontario, British Columbia, and the Yukon Territory, yield most of the gold, the remaining provinces producing less than 1 per cent. of the total.
More than one-half of the Canadian production comes from the Porcupine district in Temiskaming, Ontario, developed in 1912. Other producing districts, though of minor importance, are Kirkland Lake and Munro Township, also in Temiskaming, and Long Lake, near Naughton, Sudbury district.[165]
[165] “Production of Copper, Gold, Lead, Nickel, Silver, Zinc, and Other Metals in Canada, 1916.” Canadian Department of Mines, Mines Branch, 1917.
At one time most of the gold output of British Columbia was derived from placers, chiefly from those in the Atlin and Cariboo districts, but less than 5 per cent. came from that source in 1917. The main lode mining districts are West Kootenay and Yale, in the southern part of the province.[166] Gold production in 1917 amounted to about half of the total for 1916.
[166] “Production of Copper, Gold, Lead, Nickel, Silver, Zinc, and Other Metals in Canada, 1917.” Canadian Department of Mines, Mines Branch, 1919.
Gold has been known in the Yukon Territory since 1869 and the deposits have been actively worked since 1881. The greater part of the placers of Forty-mile River and all of Sixty-mile River are within Canadian jurisdiction. In 1897 came the discovery of the Klondike. Gold production reached its height in Yukon Territory in 1900, when the output[469] was 1,077,649 fine ounces, valued at $22,000,000. Practically all of the 1917 production was derived from placer deposits.
Gold was mined in Quebec as early as 1823, but Canada was of little importance as a gold-producing region prior to the discovery of the British Columbia placers in 1857. Gold deposits of little economic value are still worked in Quebec, New Brunswick, Nova Scotia, and Newfoundland. Prospecting and development work in Manitoba, Saskatchewan, and Alberta indicate that these provinces may become important producers of gold.
—For many years Mexico ranked fourth among the gold-producing countries of the world, being surpassed only by the Transvaal, the United States, and Australasia. Revolutions and bandit warfare have seriously interfered with mining operations since 1911, and the output of gold in 1917 was little more than one-third of the normal annual yield. With the establishment of a stable government, able to protect foreign investments, Mexico will no doubt regain its former position.
A large part of the gold output of Mexico is obtained as a by-product from lead, silver, zinc, and copper ores. The only true gold-mining district is the El Oro district of the states of Mexico and Michoacan. The chief producing mines are the Esperanza, El Oro, Mexico Mines of El Oro, and Dos Estrellas. In 1906 the Esperanza was considered, in respect to both actual output and profits earned, the most productive gold mine in the world. Since then it has been surpassed by mines of the United States and the Transvaal. Another famous gold mine of Mexico, the Dolores, situated in western Chihuahua, yields ore whose silver content is almost equal in value to the gold. Lower California, Sonora, Durango, Hidalgo, San Luis Potosi, Guanajuato, and Chiapas are all gold-producing states.
—At the time of the discovery of America, the Spanish were attracted to the region now included in the states of Central America by reports of fabulously rich mines and of the wealth of the Indians. A number of expeditions were sent out in search of the gold and some rich mines were discovered and worked. Gold is still produced in Central America, but the total amount is small, being less than 1 per cent. of the world total in 1913. Honduras is the richest of the states in production and Nicaragua ranks second, the principal centers of the gold-mining industry being in the Departments of Matagalpa and Chontales, and in the district of Cabo Gracias and Prinzapolka.[167]
[167] U. S. Commerce Reports, Supplement 34a, 1915, p. 4.
South America was also an important source of gold during the years following the discovery of America. The early conquerors obtained gold[470] by plundering the temples, churches, and even the graves of the natives. Following the conquest, the Spaniards by means of their slaves systematically searched much of the continent for gold deposits. About 15 per cent. ($2,266,000,000) of all the gold produced between 1492 and 1917 came from South America.
Today South America is of little importance as a gold producer, the combined yield of all the countries for 1913 being about 2.5 per cent. of the world total. The great bulk of the South American production has so far been alluvial gold. With the establishment of more stable governments and the improvement of transportation facilities it is likely that the production of South America will increase.
According to Maclaren[168] the gold fields of South America are disposed in three somewhat sharply separated areas. The chief area is that extending the length of the Andes from the Isthmus of Panama to central Chile and including the deposits of Colombia. The second area is contained in a well-marked petrographic and metallographic province extending across the rearland of the Guianas and including also the mines of Venezuela and northern Brazil. The third area is contained in the province of Minas Geraes, Brazil. Some gold is obtained from Chile, Argentina, Bolivia, Peru, Ecuador, and Uruguay. The chief gold-bearing areas of Colombia are Choco, the Department of Antioquia, and the district lying between the Cauca and Magdalena rivers. Fairly rich deposits have also been found in Colombia near the Ecuadorian border.[169]
[168] Maclaren, J. Malcolm: “Gold: Its Geological Occurrence and Geographical Distribution, 1908,” p. 619.
[169] U. S. Commerce Reports, Supplement 42b, 1915, p. 12.
The principal mines of Brazil are the St. John del Rey mine, and the mines owned by the Ouro Preto Gold Mines of Brazil, Ltd., both in the State of Minas Geraes. The former mine is said to be the deepest in the world.
Although Europe in the past has played an important part in the production of gold, very little is mined at the present time. Previous to the discovery of America, much of the gold in use came from the Alps and from Hungary. Deposits were also worked in England, Ireland, Wales, Spain, and Germany.
The only gold mines in Europe of any present economic importance are in the former empire of Austria-Hungary, France, and the Urals of Russia. In the south of France gold has been produced along the streams flowing from the Pyrenees, and also in the eastern provinces. At present only three mines are in operation, which in 1911 produced 2,554 kilograms of gold. The Hungarian deposits are very old, as they were worked by the Romans 2,000 years ago. Most of the producing mines of today are[471] in Transylvania. Russia is credited with producing 5.8 per cent. of the world total in 1913. A small part of this production came from the Urals of European Russia, chiefly as a by-product from other ores, but the greater part was obtained in Siberia. The Siberian deposits are discussed below, under Asia.
Since the decline in the Mexican production, Russia ranks fourth among the gold-producing countries. Most of the output comes from Siberia, which has long been a source of gold, the mines of the Altai Mountains being considered among the oldest in the world. The production of gold in Siberia from 1830 to date has been estimated by Russian authorities at approximately $1,000,000,000.
Maclaren[170] has divided the auriferous areas of the country into two distinct regions, the eastern and western. About four-fifths of the country’s production is derived from the former region, which extends in one fairly narrow auriferous belt from Lake Baikal to the southwestern shores of the Sea of Okhotsk.
[170] Maclaren, J. Malcolm: “Gold: Its Geological Occurrence and Geographical Distribution,” p. 210.
A number of dredges have been operated in the Ural district. The most promising property is the Riderlinsky, east of Omsk. In the southern Urals, most of the mines produce copper, pyrite, or zinc, the gold by-product making the profits. The Ridder mine, in the Altai Mountains, is the best-known lode mine in Siberia.
Placers are located along the Manchurian frontier on the Onon and Amur rivers. They are among the most important in Siberia and have produced over $100,000,000 in gold. The deposits of Irkutsk along the Lena River are the most important deposits so far exploited in Siberia and are probably the richest placers ever discovered. The district produces about one-third of the Siberian output. The placers are at present about worked out as drift mines, but will continue to produce with the installation of dredges.
Many engineers believe that Siberia is the richest remaining potential source of gold in the world. It has been estimated that the country will produce in the future, say in the next 30 or 40 years, about $6,000,000,000. The unsettled political conditions have greatly interfered with mining. Many of the mines were taken over by the Bolshevists, and although it is unlikely that any attempt at systematic mining will be made, they will probably be robbed of the richer exposed ore before they can be recovered by the owners.
India has long been regarded as a land of riches. Philologists have proved, to their own satisfaction, that Ophir, the source of the stores of gold of Solomon, was located there. In the deserts of northern India[472] lived the gold-digging ants, described by the Greek historians and later writers and as yet unexplained. Present facts do not bear out legend and tradition. In 1913 India contributed only 2.6 per cent. of the world’s total production of gold, an amount hardly proportionate to the extent of the country. The land offers to the prospector an extremely uninviting field. It has been carefully prospected and its deposits have been worked assiduously for at least twenty-five centuries by a people possessing great patience and considerable mining skill.[171] The principal modern producing gold mines of India are in the Kolar field, where the main reef carries five large mines along its strike.
[171] Maclaren, J. Malcolm: “Gold: Its Geological Occurrence and Geographical Distribution,” p. 238-240.
Japan was one of the chief contributors to the stream of gold that poured into Europe during the sixteenth century. Portuguese and Dutch traders came to the islands to exchange European products for the gold mined by thousands of natives. The Japanese finally revolted against this domination of their trade and expelled the last of the Portuguese in 1624. A few Dutch were permitted to remain and to trade through certain ports, but under most humiliating conditions. Between the years 1601 and 1764, it is estimated that about 3,763,572 ounces of gold was exported from Japan. At the present the annual gold production of Japan, including Formosa, is about $6,000,000.
Most of the gold-quartz veins of Japan have been worked for many generations and only the poorer sulphide zones remain.
The Island of Formosa, which was acquired by Japan in 1895, had been represented by early European travelers as a storehouse of untold riches. Not until 1890 were the sites of the old workings rediscovered, the discovery of flakes of gold during the construction of a railway precipitating a rush of Chinese miners to the island.[172]
[172] “Mining in Japan, Past and Present,” the Bureau of Mines, Department of Agriculture and Commerce, Japan, 1909, pp. 17 and 64.
The total output of gold in Chosen (Korea) in 1914 approximated $5,000,000, but at present the output is declining because of the abnormal rise in the price of chemicals and materials necessary for mining. The yield for 1918 will probably not exceed $3,500,000, 60 per cent. of which will be obtained from three mines operated by foreign capital. The chief gold-quartz mines of Chosen are included in the American concession at Unsan in North Pyong-an Province, northwestern Korea. From these mines is derived about half of the annual gold production of Chosen. Korea also contains a large number of placer deposits, mostly small, that have been extensively mined by Koreans. The placer deposits of the Unsan district have not yet been worked, but those of the Chiksan district are being exploited by the Japanese.[173]
[173] U. S. Commerce Reports, February 26, 1914.
[473]
The small, scattered gold deposits of China, both vein and placer, are worked almost exclusively by the Chinese, as none has been discovered of sufficient richness to attract foreign capital. On the Island of Hainan, southeast of the mainland, the Chinese government is operating mines.
Exploration work carried on in China up to the present does not indicate that the country will ever become an important producer of gold. Even in the provinces contributing most of the present output—Manchuria, Yunnan, and Szechuen—the gold industry gives little promise of growth. During the years 1911, 1912, and 1913, 700 streams were examined in the last two provinces and gold was found in 430, but in no case in sufficient quantity to pay for working.
There have been many rumors concerning the gold deposits of the vast and unexplored territory of Tibet since the very earliest expeditions to that country but the field is still closed to modern enterprise and even to careful scientific examination. Despite previous reports, it has been stated by the geologist accompanying a British expedition about 10 years ago that the mineral value of Tibet was not easily apparent. Near the frontier of the State of Bhutan there are many colonies of gold washers. The Tibetan gold is found in nuggets as well as in spangles and dust, but the Tibetans are said to be careful to leave the nuggets intact, or to replace them if disturbed, under the belief that they are living and are the parents of the spangles and dust, or the roots from which new gold grows, which latter would disappear were the lumps removed.[174]
[174] U. S. Commerce Reports, February 26, 1914.
Siam, Burma, Indo-China, and the Federated Malay States at present contribute only a small part of the gold production of Asia. Exploration and prospecting are proceeding actively and the results to date are favorable. Future developments may substantiate the statements of the ancient writers and cartographers that there was much gold in this region. Gold and other minerals are known to exist in Afghanistan, but with the exception of a gold mine near Kandahar, in charge of a European, the mineral resources are almost entirely undeveloped.
Since 1905 South Africa, including Transvaal, Cape Colony, and Natal, has been the leading gold producer of the world, a position that would have been attained several years earlier had not the Boer War interfered. In 1917, gold valued at $186,255,000, or nearly half of the world’s output, was produced, the greater part being derived from the Witwatersrand, or Rand, near Johannesburg, Transvaal. Production in 1918 fell to $174,068,000.
Gold was first discovered in the Transvaal in 1870, but production was not important previous to the discovery of gold on the farm Langlaagte, Witwatersrand, in 1885. The mines are spread along a belt extending some 62 miles from Randfontein on the west to Holfontein on[474] the east. This belt contains the largest deposit of gold that has ever been found in one place, and its gold content probably equals that of all the other known gold fields of the world combined. The ore is not exceptionally high grade, but can be economically treated in large quantities. The deposit, it has been estimated, may represent $3,000,000,000 to $4,000,000,000 from about 40 square miles, of which about half had been extracted by 1916.
Gold occurs both in vein and placer deposits in Natal and Cape Colony, but the output is small.
Rhodesia, in seventh place in 1913, has developed rapidly in recent years as a producer of gold and now outranks Mexico. According to Portuguese records, gold was mined in Rhodesia as early as 1788. The discovery in 1866 of ancient ruins and of ancient gold mines at Zimbabwe gave rise to the hypothesis that Rhodesia was the Ophir of the Scriptures. Although numerous attempts were made to open the gold fields, no measure of success was obtained until 1891, and only during the present century has the true character of the Rhodesian gold-quartz veins been recognized. The settlement of the country and the development of the mines is the result of the efforts of Cecil Rhodes and the Charter Company.
The gold is widely distributed and most of the mines are small. The future of the country as a gold producer would seem to depend upon the operation of the lower-grade ore bodies by large companies. The Shamva mine is a conspicuous example.
West Africa, especially the Gold Coast, is the only region of Africa, other than the Transvaal and Rhodesia, producing gold in important quantities. The output of the British West Africa colonies in 1917 amounted to about $7,500,000. The unhealthful climate of the region will probably prevent for some years any extensive mining operations by white men.
Gold mines are also worked in Abyssinia, Belgian Congo, Egypt, French East Africa, the former German East Africa, Madagascar, and the Sudan, but the total annual production from all these countries is little more than $2,000,000. The rock carvings and the hieroglyphics of ancient Egypt indicate that northern Africa and the region along the Nile River were important sources of the gold of the ancient world.
For many years Australasia was a close rival of the United States as a gold producer, frequently outranking this country. Since 1903 there has been a rapid decline, and Australasian production in 1917 was less than one-half the production of the United States. However, Australasia still ranks third and produced 11.3 per cent. of the world’s output in 1913 and about 8.3 per cent. in 1917.
[475]
—Gold was discovered in Australia in 1839 and perhaps even as early as 1823. Fearing the unsettling effect of gold-seeking on the progress of the colony, the government authorities kept these early discoveries a secret for a number of years. In 1851, however, a miner recently returned from California, discovered gold near Bathurst, in New South Wales, and a rush similar to the Californian rush began. Discoveries in other parts of the country followed. The vein deposits of Australia occur in two distinct areas, well separated both geographically and geologically. The first includes the gold fields of the west and northwest and the other lies along the great Eastern Cordillera of Australia and stretches northward from Tasmania through Victoria, New South Wales, and Queensland.
For many years Victoria, the smallest of the states, was the largest producer of gold, and up to 1908 had produced about half of the total output of the commonwealth. Among the earlier returns were some of the largest nuggets known. With the discovery in 1892 of the sensational field of Coolgardie, in Western Australia, a state previously believed to be without mineral wealth, Victoria dropped to second place. Kalgoorlie is the chief town and center of the gold-mining area in Western Australia.
Queensland ranks next to Victoria. A disastrous rush occurred in 1858, and 15,000 to 20,000 men were left starving on the banks of the Fitzroy River. The men were rescued by steamers sent by the governments of New South Wales and Victoria. A few years later alluvial gold was found near Peak Down, Clermont, to the present day the principal placer region of Queensland. Charters Towers, the present leading field of the state, was discovered in 1872. The Mount Morgan mine is an isolated mine lying not far from the scene of the ill-fated rush of 1858. It is by far the most productive mine of Queensland, both in gold and copper. New South Wales, although the first Australian state to yield gold in any important quantity, now ranks fourth. Tasmania and South Australia produce only a small amount of gold.
—In 1852, the year following the rich discoveries in Australia, gold dust and gold enclosed in quartz were found in New Zealand, about 40 miles from Auckland, but this discovery proved to be of little importance. Ten years later the rich placers of Gabriel’s Gully were discovered, a discovery which attracted a rush from the Australian fields. The gold fields of New Zealand may be divided into three well-defined and well-separated areas: the Huaraki gold field, which contains valuable vein deposits but no placers; the West Coast area, in which the vein and alluvial occurrences are of equal importance; and the Otago area, in which the auriferous alluvial gravels are important and the few known quartz veins have little economic value.
Some gold is produced in other parts of Australasia, notably in the[476] British and Dutch East Indies, and in British New Guinea. Deposits of little importance are reported in New Caledonia, the Fiji Islands, and in the former German New Guinea.
Specific predictions regarding changes in the geographical distribution of the sources of the world’s gold seem valueless. During the last few years the gold production of the principal fields has decreased. Aside from any decline due to the war and the attending scarcity of labor and high mining costs, it seems probable that the gold output of the world has reached its zenith and that further decline is to be expected unless new ore bodies are added to known reserves or unless some revolutionary method of extracting gold from the low-grade ores is discovered.
Although the leading fields, South Africa, the United States, and Australia, seem to have reached or passed their period of greatest output, there are a number of fields in unsettled and unexplored regions of the world which may be expected to show increased production. The possibilities of Siberia have already been mentioned, and it is the opinion of many engineers that this region will eventually rival South Africa. South America has produced an enormous quantity of gold, chiefly alluvial, and is expected to yield an increased output in the future. The future of South Africa is problematical.
During the last half century the greatly increased gold production has been due largely to the exploitation of the low-grade properties, this being made possible by improvements in mining and metallurgy. It seems, however, that this development has reached a point where excessive labor costs prevent the use of ores of a still lower grade.
In general, therefore, the gold output of the world may be expected to remain static or to decline, at least until the level of prices is so changed that it is considered profitable to expend capital in the prospecting and developing of regions hitherto unexplored.
The political control may be summarized as follows:
| Country | Percentage of 1913 production |
|---|---|
| British Empire | 62.9 |
| United States | 19.3 |
| Russia and Siberia | 5.8 |
| Japan | 1.8 |
| France | 1.4 |
| Belgium | 0.2 |
| Central Power | 0.55 |
| Mexico | 4.2 |
| Other Countries | 3.7 |
[477]
Great Britain controlled politically 62.9 per cent. of the 1913 production, through state sovereignty over the Transvaal, Australia, Rhodesia, Canada, and India. Other British possessions contributing small amounts to the world output are British Honduras, British Guiana, British West Africa, British New Guinea, New Zealand, British East Indies, Egypt, and the British Isles. All gold produced in the British Empire must be sent to England.
The United States controlled politically 19.3 per cent. of the 1913 output, practically all of which came from continental United States. The Philippine Islands and Porto Rico produced small amounts.
Russia is one of the principal contributors to the world’s gold supply, producing 5.8 per cent. of the 1913 output. Most of this 5.8 per cent. came from the mines of Siberia, and the political control of that region is still unsettled. Mexico, with an output equal to 4.2 per cent. of the world total, was the largest producer among the neutral powers during the war. France controlled 1.4 per cent. of the world total, a third coming from the mines in France and the remainder from the French colonies in South America and Africa. Japan controlled 1.8 per cent., 1 per cent. coming from the Japanese islands and Formosa, and the remainder from Korea. The rest of the 1913 production, amounting to about 4 per cent., was widely scattered, and was controlled by a number of nations of South America, Europe and Asia.
It should be pointed out that although Great Britain and the United States control politically the important producing fields of the present, all of which seem to have reached their maximum output, the control of the fields expected to be important producers in the future is in different hands.
During the war, particularly during its later months, most of the belligerent nations and some of the neutrals placed restrictions on the exportation of gold, either as bullion or in manufactured form. In normal times gold is allowed to flow freely between nations, as needed to settle international trade balances.
The commercial control of the gold resources of the world is shown in Table 68 and in Figure 20. In examining the table and the diagram the reader should remember that the figures and percentages are at best only estimates. Most gold mines are owned by companies whose shares are bearer’s shares; these are bought and sold in the stock markets of many financial centers. Today the control may be in the hands of one group, tomorrow in the hands of another. Because of this ever-changing ownership, it is difficult to determine the nationality of the commercial control, particularly when there is any attempt to conceal the nationality of the capital invested. Table and diagram, however, are believed to be[478] approximately correct. The information upon which they are based was obtained from mining manuals, the reports of mining companies, the reports of consular agents in the various countries, and by personal interviews with mining engineers.
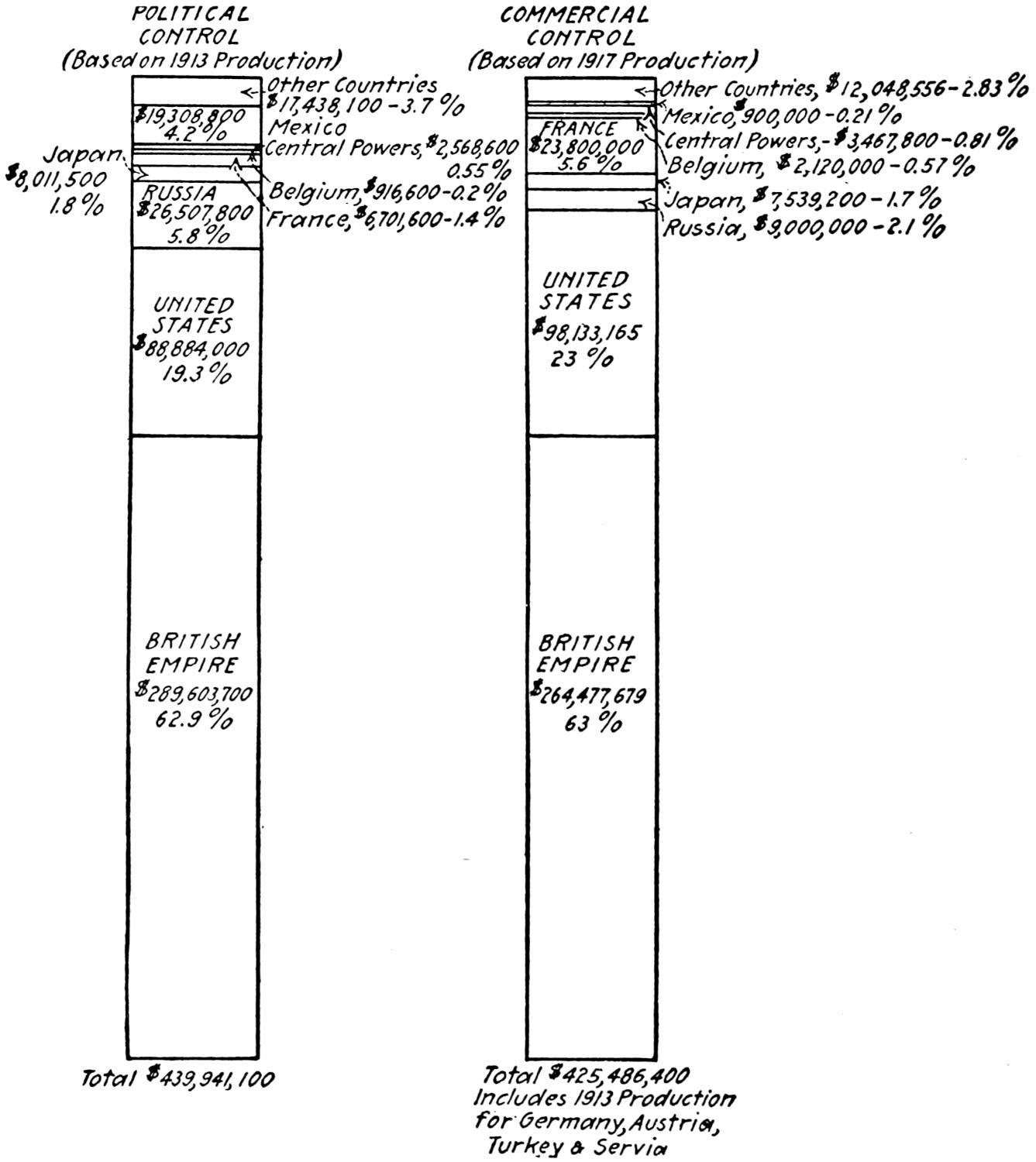
Fig. 20.—Political and commercial control of the gold production of the world.
Table 68 may be summarized as follows:
Commercial Control of Gold Resources
| Country | Percentage of 1917 production |
|---|---|
| British Empire | 63.0 |
| United States | 23.0 |
| France | 5.6 |
| Russia | 2.1 |
| Japan | 1.7 |
| China | 0.84 |
| Belgium | 0.5 |
| Brazil | 0.07 |
| Germany | 0.81 |
| Mexico | 0.21 |
| Unclassified | 1.9 |
[479]
It is interesting to note that political control and commercial control are closely identical in the case of the British Empire and the United States. French control is much more important commercially than politically. In other countries, little domestic capital is available, and political control is much greater than commercial control.
Commercial control, if different from political control, joins in a test of strength with it in periods of emergency or unrest, with the result that the stronger subdues the weaker. As regards governments that are strong and stable enough, the recent war has demonstrated that the elimination of foreign capital invested in a country is easy. The United States and Great Britain, in particular, have taken over a great number of companies formerly owned by German interests. Where the government is not so strong, however, the commercial control, backed in many instances by the more vigorous home governments from which the invested capital comes, wins the day, and political events are thus determined, even to the extent of overturning the weaker government. Thus the overturning of the Boer government and the birth of British South Africa was the result of the clash between British commercial control and Boer political control.
The question of the control of gold stocks is discussed in the report of the gold committee of the Department of the Interior.[175] The control of stocks in 1916 is shown graphically in Figure 21 and is discussed more in detail in the section following, “Position of Leading Commercial Nations.”
[175] Report of the committee appointed by the Secretary of the Interior to study the gold situation.
The gold mines of the United States are controlled mainly by American capital. A few of the mines are predominantly British, and shares in other mining companies are undoubtedly held in London, but the total production controlled by Great Britain is small. It has been estimated that in recent years 95 per cent. of the annual output was controlled by Americans and the remainder by British.
Over half of the gold mined in Canada comes from the Province of Ontario, chiefly from the Porcupine district. In 1917 and 1918, the Hollinger mine, owned by the Hollinger Gold Mines, Ltd., was the main producer. It is one of the greatest gold mines in the world, having paid over $9,000,000 in dividends up to the end of 1918. The control of the company is held in the United States. Another mine of importance in the Porcupine district is the McIntyre, owned by the McIntyre Porcupine Mines, Ltd., also American. In the Province of British Columbia, the greatest producing camp is Rossland, operated by the Consolidated Mining & Smelting Co., controlled by United States and Canadian capital. It has been estimated that about two-thirds of the gold mines of Canada are controlled by United States capital and the remaining one-third by British capital, including Canadian.
Table 68.—Financial Control of the Gold Production of the World, 1917
(Percentages are only approximate)
| Country | Production 1917 (in dollars) |
1917 Production controlled financially by— | Remarks | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| United States |
United Kingdom |
France | Belgium | Japan | China | Russia | Brazil | Total Allies |
Germany | Mexico | Unclassified | |||
| North America: | ||||||||||||||
| United States | $83,750,700 | $79,563,165 | $4,187,535 | ... | ... | ... | ... | ... | ... | $83,750,700 | ... | ... | ... | 95 per cent. by United States capital; 5 per cent. by British. |
| Canada | 15,200,000 | 10,000,000 | 5,200,000 | ... | ... | ... | ... | ... | ... | 15,200,000 | ... | ... | ... | 66 per cent. by United States capital; 33 per cent. by British. |
| Mexico | 9,000,000 | 2,700,000 | 3,600,000 | $900,000 | ... | ... | ... | ... | ... | 7,200,000 | $900,000 | $900,000 | ... | Capital control: British 40 per cent.; United States 30 per cent.; French 10 per cent.; German 10 per cent. and Mexican 10 per cent. |
| Central America | 3,122,000 | 750,000 | 750,000 | ... | ... | ... | ... | ... | ... | 1,500,000 | ... | ... | $1,622,000 | 50 per cent. by natives; 25 per cent. each by United States and Great Britain. |
| South America: | ||||||||||||||
| Argentina | 4,600 | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | 4,600 | |
| Bolivia | 115,000 | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | 115,000 | |
| Chile | 200,000 | 200,000 | ... | ... | ... | ... | ... | ... | ... | 200,000 | ... | ... | ... | |
| Brazil | 2,958,000 | ... | 2,662,200 | ... | ... | ... | ... | ... | $295,800 | 2,958,000 | ... | ... | ... | 90 per cent. by British capital. |
| Colombia | 6,200,000 | 620,000 | 3,000,000 | 900,000 | $620,000 | ... | ... | ... | ... | 5,140,000 | ... | ... | 1,060,000 | Capital control: 50 per cent. British; 15 per cent. French; 10 per cent. Belgian; 10 per cent. United States; and 15 per cent. local. |
| Guiana: | ||||||||||||||
| British | 600,000 | ... | 600,000 | ... | ... | ... | ... | ... | ... | 600,000 | ... | ... | ... | |
| Dutch | 400,000 | ... | 125,000 | 125,000 | ... | ... | ... | ... | ... | 250,000 | ... | ... | 150,000 | |
| French | 1,500,000 | ... | ... | 1,500,000 | ... | ... | ... | ... | ... | 1,500,000 | ... | ... | ... | |
| Peru | 1,300,000 | 1,300,000 | ... | ... | ... | ... | ... | ... | ... | 1,300,000 | ... | ... | ... | |
| Other S. America | 1,357,000 | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | 1,357,000 | |
| Europe: | ||||||||||||||
| Austria-Hungary | [176]2,179,000 | ... | ... | ... | ... | ... | ... | ... | ... | ... | [176]2,179,000 | ... | ... | |
| France | 700,000 | ... | ... | 700,000 | ... | ... | ... | ... | ... | 700,000 | ... | ... | ... | |
| Germany | [176]135,600 | ... | ... | ... | ... | ... | ... | ... | ... | ... | [176]135,600 | ... | ... | |
| Great Britain | 5,000 | ... | 5,000 | ... | ... | ... | ... | ... | ... | 5,000 | ... | ... | ... | |
| Italy | 2,000 | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | 2,000 | |
| Russia and Siberia | 18,000,000 | ... | 9,000,000 | ... | ... | ... | ... | $9,000,000 | ... | 18,000,000 | ... | ... | ... | Large part of Russian production probably controlled by German capital. Situation uncertain. |
| Serbia | [176]328,000 | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | [176]328,000 | |
| Sweden | 10,000 | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | 10,000 | |
| Turkey | [176]500 | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | [176]500 | |
| Asia: | ||||||||||||||
| China | $3,600,000 | ... | ... | ... | ... | ... | 3,600,000 | ... | ... | 3,600,000 | ... | ... | ... | Probably controlled largely by Japanese and other foreign capital. |
| Chosen (Korea) | 4,444,000 | $2,500,000 | ... | ... | ... | $1,944,000 | ... | ... | ... | 4,444,000 | ... | ... | ... | Part of production credited to Japan controlled by British. |
| Dutch East Indies | 2,818,000 | ... | $2,100,000 | ... | ... | ... | ... | ... | ... | 2,100,000 | ... | ... | 718,000 | |
| Federated Malay States | 342,300 | ... | 342,300 | ... | ... | ... | ... | ... | ... | 342,300 | ... | ... | ... | |
| French Indo-China | 50,000 | ... | ... | $50,000 | ... | ... | ... | ... | ... | 50,000 | ... | ... | ... | |
| Japan and Formosa | 5,595,200 | ... | ... | ... | ... | 5,595,200 | ... | ... | ... | 5,595,200 | ... | ... | ... | |
| India and British Indies | 10,756,800 | ... | 10,756,800 | ... | ... | ... | ... | ... | ... | 10,756,800 | ... | ... | ... | |
| Australasia | 35,945,500 | ... | 35,945,500 | ... | ... | ... | ... | ... | ... | 35,945,500 | ... | ... | ... | Before war considerable capital controlled by Germans. Small blocks now owned by Dutch and Belgians. |
| Africa: | ||||||||||||||
| Belgian Congo | 2,000,000 | 500,000 | ... | ... | 1,500,000 | ... | ... | ... | ... | 2,000,000 | ... | ... | ... | 25 per cent. by American capital. |
| Egypt and Sudan | 150,000 | ... | 150,000 | ... | ... | ... | ... | ... | ... | 150,000 | ... | ... | ... | |
| German East Africa | [176]253,200 | ... | ... | ... | ... | ... | ... | ... | ... | [176]253,200 | ... | ... | ||
| Madagascar | 1,000,000 | ... | ... | 1,000,000 | ... | ... | ... | ... | ... | 1,000,000 | ... | ... | ... | |
| Rhodesia | 14,988,600 | ... | 14,988,600 | ... | ... | ... | ... | ... | ... | 14,988,600 | ... | ... | ... | |
| Transvaal | 186,254,256 | ... | 167,629,256 | 18,625,000 | ... | ... | ... | ... | ... | 186,254,256 | ... | ... | ... | Before war controlled in large part by German interests. At present: British, 90 per cent.; French, 10 per cent. |
| West Africa (British) | 7,435,488 | ... | 7,435,488 | ... | ... | ... | ... | ... | ... | 7,435,488 | ... | ... | ... | Probably all controlled by British capital. |
| Undistributed | 2,785,656 | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | 2,785,656 | |
| Total (1917) | $422,590,100 | $98,133,165 | $268,477,679 | $23,800,000 | $2,120,000 | $7,539,200 | $3,600,000 | $9,000,000 | $295,800 | $412,965,844 | $3,467,800 | $900,000 | $8,152,756 | |
| Total (including 1913 figures) | $425,486,400 | |||||||||||||
| Per cent. of world production | 100 | 23 | 63 | 5.6 | 0.5 | 1.7 | 0.84 | 2.1 | 0.07 | 96.81 | 0.81 | 0.21 | 1.9 | |
[176] Production in 1913. Later figures not available.
[484]
The gold mines of Mexico are owned by British, American and French capital in the order named. The principal mining companies of the El Oro district, the leading gold district of the republic, are El Oro Mining & Railway Co., Ltd., Esperanza, Ltd., and the Dos Estrellas Mining Co. British capital controls El Oro Mining & Railway Co., Ltd., and 51 per cent. of the shares of Esperanza, Ltd., the remaining 49 per cent. being controlled by Americans. The French have been acquiring control of mining properties in El Oro district in recent years. They now own Dos Estrellas Mining Co., and the Mexico Mines of El Oro, Ltd. Both companies were previously controlled in London. The Dolores mine, in the western part of the State of Chihuahua, one of the famous gold mines of Mexico, is owned by the Mines Company of America, an American corporation.
Fig. 21.—Stocks of gold in banks, public treasuries and in circulation in principal countries of the world in 1916.
In the State of Durango, the gold mines, most of which also produce silver, are owned by the American Smelting & Refining Co. (American), the Bacis Gold & Silver Mines Co. (British), and the Inde Gold Mining Co. (American). Most of the gold-silver mines of the State of Guanajuato have been developed by American capital. The State of Sinaloa is becoming a gold-producing region; the Palmarito mines of the Mocorito district and the Minas de Tajo at Rosario are owned by American interests.[485] Gold is found in the copper ores of Sonora. Most of the mines of the district are controlled by Americans.
The mines of Chihuahua, Hidalgo, and Zacatecas are primarily silver mines but their ores carry some gold. In Chihuahua, American capital is probably predominant, with British ranking second. During recent years, German interests, through the American Metal Co. and its subsidiaries, have been acquiring control of properties in Chihuahua and in other parts of Mexico. German capital is also invested in Zacatecas, the main company being controlled by A. Goerz & Co., Ltd. The mines of the State of Hidalgo, the leading silver-producing district of Mexico, are owned by American, British and Mexican capital.
The gold mines of Central America[177] are of some importance at present, and production may increase somewhat in the future. The mines are owned for the most part by American and British capital, in the order named. Subsidiaries of the United Fruit Co., and the Tonopah Mining Co., both American, operate properties in Costa Rica and Nicaragua.
[177] “Investments in Latin America and the British West Indies,” Special Agent Series No. 169, Bureau of Foreign and Domestic Commerce, U. S. Department of Commerce, 1918.
In Colombia, the leading gold-producing country of South America, most of the large companies are British, the most important being the Pato Mines, the Nechi Mines, and the Frontino and Bolivia Mines. A Belgian company, the Platinum & Gold Concessions of Colombia, Ltd., and a French company, the San Antonio Gold Mines Co., Ltd., have recently acquired gold-mining concessions. American capital is also invested in the industry.
The two most important mining companies of Brazil, the St. John del Rey Mining Co., Ltd., and the Ouro Preto Gold Mines of Brazil, Ltd., are under British management. Some French capital is interested in the latter property. Most of the mines of French Guiana are controlled by French companies. In the remaining South American countries gold mining is of comparatively little importance. Production is controlled largely by British and American capital.
The foreign capital invested in the mines of Siberia and Russia is mainly British, though Germany is known to have been much interested in the industry. Some Russian capital, especially that furnished by the large Russian banks, was invested in Siberian mining properties. The mining laws of the old Russian Empire provided that mineral properties could be held both by foreign and Russian companies under concessions or leases granted by the crown or under leases from Russian estates. In many cases foreign capitalists formed holding companies to acquire a controlling interest in a Russian company in whose name the property was held and operated. Most of these holding companies had their main offices in London and most of the directors were British citizens.[486] Control was apparently British. The shares of the companies were bearers’ shares, however, and were traded in extensively on the Petrograd exchange.
The most important gold-mining district of Siberia is in the Province of Irkutsk, on the Lena River. A Russian company, the Lenskoie Gold Industrial Co., has been operating the principal properties of this district since 1863. The directors of the company are Russian Jews. A British company, the Lena Goldfields, Ltd., held 70 per cent. of the shares of the Lenskoie Co., but in 1917 completed the sale of 51,000 shares of stock to a Petrograd group including the Imperial Foreign Corporation, Ltd., and the Russian and English Bank. The Lena Goldfields, Ltd., now owns less than 10 per cent. of the Lenskoie stock.
A pyrite-copper mine yielding enough gold as a by-product to make the profit is located in the government of Perm, southern district of the Ural Mountains, and is owned by the Kyshtim Mining Works, a Russian company controlled by the Kyshtim Corporation, Ltd., of London. The company holds a large concession, including forests, farms, and mineral land. The control of the Kyshtim Corporation is apparently British.
The Tanalyk Corporation is controlled by practically the same interests as those controlling the Kyshtim Corporation and was organized to acquire entire control of the South Urals Mining & Smelting Co. The property of the company, situated in the County of Orsk, Orenburg government, southern Urals, is only a prospect and has not been fully developed. It includes a total area of 28,350 acres of timber and mineral land. The ore contains copper, gold, and silver, the copper content being the most valuable. During 1917, the shares held in the Kyshtim Mining Works and the South Urals Mining & Smelting Co. were vested in the Canadian Development Corporation, Ltd., a company registered in Canada, probably for the purpose of escaping the high British income taxes. The transfer involved no change of control, as the two British holding companies, the Kyshtim Corporation and the Tanalyk Corporation, Ltd., control the Canadian company.
The Kolchan placer property, in the Okhotsk mining province of Eastern Siberia, has been leased, for a royalty based on the gross output of gold, by the Orsk Goldfields, Ltd., a British company, from the Russo-Asiatic Bank. The Orsk Goldfields, Ltd., originally controlled a gold-mining property in the Province of Orenburg, southern Urals, but abandoned the lease and option.
A great deal of gold is produced in Siberia as a by-product of copper, lead, and zinc mines. The control of these mines is much the same as the control of the above-mentioned gold properties—British with Russian shareholders, chiefly representative of Russian banks.
Japanese control is being expanded, especially in eastern Siberia.[487] It was reported in the Japanese press, in November, 1918, that the Japanese special financial mission was planning the establishment of a Japan-Russian partnership enterprise similar to the Japan-China Industrial Association for the purpose of obtaining from the Russian authorities rights in the Siberian mining and forest areas and of exploiting the natural resources of Siberia. It was proposed that among the investors in the partnership should be included Russian capitalists, the South Manchurian Railway, the East Asia Development Co., the Japan-China Mercantile Association, and capitalists of the Entente countries.
Practically all of the output of India comes from the mines of the Kolar reef of Mysore. There are six large producing companies, as follows: Champion Reefs Mining Co., of India, Ltd.; Gold Fields of Mysore and General Exploration, Ltd.; Mysore Gold Mining Co., Ltd.; Nundydroog Co., Ltd.; Balaghat Gold Mining Co., Ltd.; and Ooregum Gold Mining Co. of India, Ltd. The companies are all British and are owned by closely connected interests. Other operating companies in India are the Hutti (Nizam’s) Gold Mines, Ltd., of Hyderabad, southern India; and the Northern Anantapur Gold Mines, Ltd., and Jibutil (Anantapur) Gold Mines, Ltd., Madras Presidency, all being British companies.
In Japan the state reserves to itself the right of original ownership in all ores, and grants to individuals or companies the right to work the deposits. Under the law of 1890, a foreigner was disqualified from working a mine and was not permitted to become a member of a mining establishment, so that the right of working mines was exclusively reserved for Japanese subjects. By an amendment of the mining regulations in 1900, business establishments organized by Japanese or foreigners or by both are now permitted to work mines, provided such establishments are placed under Japanese laws. A bill was recently introduced into the Japanese Parliament proposing to grant the privilege of mining and property rights to foreigners. Japanese capital has almost complete control of the gold mines of the empire.
The principal gold mines are owned by the following companies: Tanak Chobei (Formosa), Mitsubishi & Co. (Sado), Shimadzu Tadashige (Satsuma), Fujita & Co. (Rikuchu and Formosa), Kimura Kintaro (Formosa), and Ushio Gold Mine Co. (Satsuma).
The most valuable of the mineral concessions of Chosen (Korea), including gold, are in the hands of foreign companies, the majority of which are American. The Oriental Consolidated Mining Co., an American company, controls the Unsan mines in North Pyong-an Province, northwestern Korea, the most important gold-producing property of the country. The concession includes 600 square miles on the Anju River and has about 21 years to run, with the option of[488] renewal for 15 years. The operating company pays $15,000 annually to the Korean government. Ore reserves were estimated on July 1, 1916, at 852,000 tons, valued at $4,823,000.
The Chiksan mines, in South Choonchong Province, are also managed by American capital. The Suan mines, in Whanghai Province, were originally controlled by a British company but have been leased to an American company. An Italian company owns mines in North Pyong-an Province, and a British company is operating in North Choonchong Province. A German company owned mines, not in full working order, in North Pyong-an Province, but the properties have doubtless been seized by the Japanese government. Japanese and Korean miners own and operate the smaller mines of the country, the output of which has decreased greatly during recent years.
Although the gold output of China in 1917 amounted to $3,600,000, the deposits are small and widely scattered and are owned and operated almost exclusively by the native miners. Some of the larger mines have been financed by the governments of the provinces. In some provinces the owners of mining property are not permitted to seek investments of foreign capital unless they are unable to finance the property within the province. Prospecting has been carried on in many parts of the country in recent years, but no deposits have been discovered sufficiently promising to attract foreign investors. In view of their activity in developing the other natural resources of China, it is probable that the Japanese will attempt to control the industry should any important deposits be discovered.
In the Transvaal all minerals belong to the government and not to the owners of the surface of the land. When gold, or any other mineral, is discovered, a part of the territory (less than half) is reserved for the owner of the land, another part for the discoverer of the mineral, and the remainder is open to location. At present the ownership of mineral lands is being retained by the government. The right to exploit the mineral wealth is granted on a lease to the highest bidder, the bids being based upon a percentage of profits, graded according to working costs and grade of ore. A tax of 10 per cent. on profits, allowing for amortization of capital, is levied on all gold-mining properties.
Before the war, practically all the gold mines of the Witwatersrand were controlled by British and German capital. The first American company entered the field in 1917 and some French capital has also been invested. The German position was strong, as the German companies controlled half, and perhaps more, of the capital invested in gold mines. The report of the South African Custodian of Enemy Property issued early in 1918 showed that no less than 26,000 enemy shareholders in gold, coal, and other mining concerns in the Union owned stock to the aggregate nominal value of $37,500,000. As gold is by far the most[489] important mineral of the Transvaal, it is probable that the greater part of this German capital was invested in gold mines. The enemy firms were wound up or went into voluntary liquidation, and enemy shares in mining corporations to the face value of $24,000,000 were taken charge of by the Custodian of Enemy Property.[178]
[178] “Engineering and Mining Journal,” May 11, 1918, p. 888.
The control of the gold mines of the Transvaal is centered in the following six companies: Central Mining & Investment Corporation, the Consolidated Mines Selection Co., Johannesburg Consolidated Investment Co., the Consolidated Goldfields of South Africa, the Union Corporation (formerly Ed Goerz & Co.), and the General Mining & Finance Corporation. The last two companies were absolutely German before the war. The Central Mining & Investment Corporation controls probably 50 per cent. of the output of the district. American capital is interested in the Consolidated Mines Selection Co. Another American company, the Anglo-American Corporation of South Africa, Ltd., was registered at Pretoria on September 25, 1917, with an issued capital of £1,000,000. The immediate object of the corporation is to participate in tendering for leases of certain Far East Rand gold-mining areas.[179]
[179] U. S. Commerce Reports, December 15, 1917, p. 1033.
It has been reported that French capital controls about 10 per cent. of the annual output. For many years the majority of the mining engineers in the Transvaal were Americans.
The Germans had not only acquired a considerable control through the investment of capital in mining properties, but they also exercised control through their trade in mine supplies. Orenstein-Arthur Koppel Co. had established an important trade in rails, mine trucks, and similar supplies, and continued to do business even after the beginning of the war. Soon after the sinking of the Lusitania, a mob destroyed the offices of the company in Johannesburg and burned a stock of much-needed supplies. At the outbreak of the war it was feared that there might be serious obstacles in the way of the gold-mining industry continuing a normal course, because of the possible difficulty in obtaining enough of the cyanide, mercury, and zinc used in gold extraction, much of which had been previously supplied by Germany. The necessary materials were obtained from other sources, however.
Rhodesia is controlled by the British South African Co., organized by Cecil John Rhodes, for whom the colony was named. The company is entirely British; it is the government, levies the taxes, administers the laws, and controls the mining industry. Companies and individuals are permitted to locate mining claims much the same as they are in California. The Goldfields Rhodesian Development Co., which has large Rhodesian mining interests, is an offshoot of the Consolidated Goldfields of South Africa. It is safe to say that the gold mines of Rhodesia are[490] owned and worked entirely by British capital. A percentage tax is levied on the output of the gold mines.
In Australia, before the war, much the same conditions existed as in South Africa. The large gold-mining companies were seemingly British; their headquarters were either in Australia or in London and their directors and managers were British. It is certain, however, that a considerable amount of German capital was invested in the companies. Among the important producing companies are the Associated Gold Mines of Western Australia, Great Boulder Proprietary Mines, Ltd., Golden Horseshoe Estates Co., Ltd., Ivanhoe Gold Corporation, Ltd., all of Western Australia; the Mount Morgan Gold Mining Co., of Queensland; the Mount Boppy Gold Mining Co., and the Mount Lyell Mining & Railway Co., Ltd., of Tasmania.
Through the vigorous action of the Australian prime minister, William M. Hughes, German capital was expelled early in the war from all the industries of the commonwealth, including gold, lead, and zinc mines, smelters and refineries, and buying organizations. In January, 1916, to safeguard the financial position of the commonwealth, the prime minister issued decrees providing that no new flotations of companies or increases in the capital of existing companies would be allowed without the consent of the treasurer of the commonwealth; that companies incorporated in Australia would have three months to discontinue the holding of their shares by persons of enemy nationality or origin, whether naturalized or not; and that in the future no transfers of shares to persons of enemy nationality or origin would be permitted.[180]
[180] U. S. Commerce Reports, February 21, 1916, p. 733.
The remaining countries of the world are of little importance as gold producers; hence a discussion of the commercial control of their mines would be of little value. In most of them the mining properties are owned and operated by domestic capital, the former Empire of Austria-Hungary being one exception to this general statement. Prior to the war, British capital controlled gold mines in Transylvania.
The ownership of smelters and reduction plants has little influence upon the commercial control of the gold industry. Most of the large gold-mining companies have their own refineries at the mines, and smelter control is therefore identical with mine control. Some gold is produced as a by-product in the smelting of lead, silver, and copper, particularly in the United States, where the smelters are controlled almost entirely by American capital. Further discussion of the commercial control of smelters and refineries will be found in Chapters XIV, XV, and XXX.
The control of secret processes and patents is of less importance in the commercial control of the gold-mining industry than that of any other mineral. The industry is peculiar in that the price of gold is always fixed and there is no competition for a market. Control of an improved[491] method of extracting or refining gold might enable one company to reduce mining and refining costs and thus insure a larger profit, but giving the improved process to a second company would not necessarily reduce the profits of the company that had discovered it.
The United States controlled politically through state sovereignty 19.3 per cent. of the gold production in 1913, ranking next to Great Britain. Commercial control was somewhat greater, amounting to approximately 23 per cent. of the 1917 production. The mines of the United States are owned almost exclusively by Americans, and American capital is invested in many foreign fields. Over half, perhaps as much as two-thirds, of the mines of Canada are owned by United States corporations. The largest of the Korean companies are owned or managed by American capital. Two American companies were established recently in the Transvaal. Probably 30 to 40 per cent. of the gold produced in Mexico in normal times comes from the United States owned mines. The production of Central and South America, although at present relatively unimportant, is largely controlled by Americans.
Although outranked in production by Great Britain, the United States controls by far the largest stocks of gold coin and bullion of any nation. In 1916 there was in the banks, the national treasuries, and in circulation in the United States $2,299,454,000, or almost twice the amount possessed by any other nation. In 1918 this amount had increased to $3,050,000,000. The monetary reserve probably exceeds the total gold output of the United States since the recording of statistics of production began.
In view of the fact that the national debt of the United States is low as compared with the debts of the other principal nations, the financial system of this country is on a relatively sound basis. When the time comes to redeem the outstanding bonds and other forms of indebtedness that have accrued during the war, the United States will undoubtedly be in a more favorable position than any of the other belligerents.
The stocks of gold in the chief gold-producing countries in 1916 are shown diagrammatically in Figure 21.
Great Britain is the leading gold-producing nation of the world. The British Empire controlled politically 62.9 per cent. of the 1913 production, and British commercial control approximated 63 per cent. An insignificant amount of gold is produced in the British Isles, but the empire includes such important gold producers as South Africa, Australia, Rhodesia, India, and Canada. British capital also controls gold mines in Siberia, in Mexico, in South America, and in the United States.
Despite the large political and commercial control exercised by the[492] British, the gold stocks of the British Empire have been much smaller than those of the United States and in 1916 were surpassed by those of France.
The lands under French control produce little gold, the output in 1913 being only 1.4 per cent. of the world’s total production. Less than 0.5 per cent. came from France itself and the remainder was obtained from French Guiana and Madagascar. French capital has not stopped at national boundaries, however, and has been invested in many of the principal fields, as is shown by the fact that commercial control amounted to 5.6 per cent. of the 1917 production. In Mexico at least 10 per cent. of the capital controlling gold mines is French, and the amount is steadily increasing. In South America—particularly in Colombia, and Dutch and French Guiana—some of the large companies are French, but most of the gold controlled commercially by the French comes from the Transvaal. It has been estimated that French interests control about 10 per cent. of the production of South Africa since German control has been eliminated. The gold reserve of France was large before the war and ranked second to that of the United States in 1916. It is now (1920) reduced to very small proportions.
Although Russia, with Siberia, is one of the most promising potential sources of the future gold supply and produced 5.8 per cent. of the total in 1913, Russian capital seemingly controls only a small part of the gold production of the world. The British are most active in Siberia, and French interests are known to have acquired property there. So far as is known, Russian capitalists control no gold deposits in foreign countries. In 1916 the gold reserve in Russian banks, treasuries, and in circulation amounted to $1,058,000,000, but it is not known what has become of this stock since the Bolshevists have been in control. At present the total is probably much smaller.
Japan controls only a small part of the gold output of the world, either politically or commercially. Some gold is obtained from Japan and Formosa, all of which is probably under Japanese financial control. The output of Korea, claimed by the Japanese as a part of their empire since 1910 and at present attempting to secure its independence, is controlled by Americans and other nationalities. The smaller mines are owned by Japanese, but the larger concessions were granted to foreigners by the old Korean government prior to 1910. Gold stocks of Japan were estimated at $143,128,000 in 1916. By December 26, 1918, they had increased to $225,820,000. In addition Japanese had on deposit in London, New York, and Paris a total of about $550,000,000.
The gold production controlled, either politically or commercially, by Germany and the countries of the former empire of Austria-Hungary is relatively insignificant, amounting in both cases to less than 1 per cent. Germany has produced annually in its European territory a little over[493] $100,000, and its only colony yielding an appreciable amount was German East Africa, lost during the war. The mines of Austria-Hungary have been important in the past, but they are now very old and in 1913 produced only 0.5 per cent. of the world’s total production. It is understood that one of the largest operating companies in Transylvania before the war was British.
The principal and most essential uses of gold are as a measure of value and a medium of exchange. Gold is also used in dentistry, and in the arts for jewelry, gilding, and other forms of ornamentation. In an emergency other materials can be substituted for the latter uses, but any considerable substitution for the principal uses would involve radical changes in our monetary and financial system.
Gold is found in rocks of all geological ages from Archean to Quaternary, and also in minute quantities in ocean water. Only the more concentrated deposits can be worked profitably. It is believed that the production from the present main deposits has reached its zenith, but further important discoveries will probably be made in the Arctic regions, in the tropics, or in other regions hitherto unexplored by man. Siberia, South America, and perhaps China are the most promising regions, as the more settled lands have been already thoroughly prospected.
Gold is widely distributed geographically. It is found in all parts of the world and commercially important mines are worked in every continent. Over 75 per cent. of the total annual output of the world comes from four countries, the Transvaal, the United States, Australia, and Russia. Other important producers are Rhodesia, Mexico, Canada, India, West Africa, and New Zealand. Little gold is produced in Europe or in South America. The deposits of the former continent, at one time important, are becoming exhausted. With improved transportation facilities and with further exploration work, the production of South America is expected to increase materially.
Great Britain controls politically over 60 per cent. of the annual gold production of the world, through state sovereignty over South Africa, Australasia, Rhodesia, Canada, and India. The United States ranks second, controlling about 20 per cent. Russia controls about 6 per cent. and Mexico 4 per cent. The remaining 10 per cent. is controlled by many countries, among them being Japan, France, Belgium, and the Central Powers.
The extent of British political and commercial control is almost identical, British interests controlling over 60 per cent. of the production and owning in addition to the mines in the empire, property in Siberia, South and Central America, Mexico, and the United States. United States interests control financially about 23 per cent. of the world’s[494] production. The Korean deposits are predominantly American owned. United States capital is also invested in Canada, Mexico, Central and South America, and recently in the Transvaal. Although the gold controlled politically by France is of little importance, French interests control commercially about 5.6 per cent. of the total annual production, owning mines in the Transvaal, in Mexico, and in Central and South America.
Before the war, German interests had extensive holdings in South Africa and Australia.
[495]
The chief and essential use of silver is as money. This form of consumption takes place mainly in India and China, where silver serves as a basis for the settlement of foreign exchange balances. In China, silver is the money standard of the country. In India a gold standard is used, but from time immemorial the natives have hoarded silver and invested their savings in silver coins and ornaments rather than making use of banks, bonds or other securities. Silver is used for subsidiary coinage in all countries, but such coins in Europe and the United States can normally be replaced to a considerable extent by paper, as they circulate at more than their intrinsic value. In Mexico, Peru, and other silver-producing countries silver money is extensively used.
A large amount of silver is used in the arts. To a small degree such consumption is for photographic or chemical work but mainly for the production of jewelry and luxurious household wares. The use of silver jewelry in India is intimately related to silver hoards, the bank balance of the natives. Such hoards are now mainly coin, however, because coin has become of more stable value than ornaments since India adopted the gold standard. Before 1914 it was estimated by the Director of the United States Mint that two-thirds of the new silver annually produced went into the arts. In 1912, however, coinage absorbed over half of the 224,310,000 ounces produced by the world. During the war the payment by the Allies for goods purchased in the Orient diverted enormous stores of silver to India and China. In Europe greater amounts of silver coin were needed under war conditions. Moreover, the large issues of paper money make corresponding increases in the number of silver coins desirable. In 1915 only 20 per cent. of the world’s silver was used in the arts; in 1916, 15 per cent. was so used, the balance being coined.
The importance of silver for essential uses during the war is best indicated by the fact that old dead stocks of silver coin, United States dollars in the Treasury, Manila dollars, etc., had to be called into service. A stage not reached was the melting down of silver plate and ornaments.[496] This stage was reached in Germany in the case of gold and probably of silver, just as the United States had begun to adopt such a program to obtain platinum. During 1916 our exports of silver exceeded $210,000,000, surpassing by $58,000,000 the total copper exports for the same period.
As long as the centuries-old customs of India and China fail to change, silver must be considered as ranking with gold as an essential money metal of intranational and international trade.
The large use of silver in the arts, in the period 1900 to 1914, a use naturally considered as entirely a luxury, leads to emphasis upon the non-essential character of silver consumption as a whole. When the European war broke out, prices for silver, in common with most metals and other commodities, declined. But silver did not increase in price a few months later, as did base metals. It was not until the end of 1915 that silver sold as high as before the war, and during the early part of the war it sold so low that producers felt discouraged and regarded silver as, in the main, an article of luxury.
On the other side is the fact that coinage of silver in Europe increased tremendously in 1914 and 1915, although it did not seem to offset fully the curtailment in manufacture of silverware and jewelry. This need for coinage continued during the war at an accelerated rate, just as did the demands for munitions and men. The “silver bullet” was important in Europe, but still more essential in bringing supplies from the Far East to the battle fronts. This fact is confirmed by the advance in the price of silver to substantially twice the pre-war quotation, and had a price not then been fixed the advance would probably have been greater. The normal annual silver production of the world is around 159,000,000 ounces, whereas the actual demand for silver during 1918 exceeded 500,000,000 ounces.
All silver used in the arts by no means represents consumption in luxuries. Silver enters the essential chemical and photographic industries to a considerable degree. At least one-third of the silver consumed in the arts before the war represented jewelry used in India, and this use is much more a form of investment by the natives than it is in Europe and the United States.
The United States Treasury Department has been much concerned over the declining output of gold. Priorities during the war were granted to the gold industry to place it in the position of a preferred war industry. Silver cannot be considered as being in a different position, although silver producers received priorities more as producers of base metals and gold than of silver alone.
The credit and finance of the world is greatly helped by maximum gold production, but of little less importance is a large supply of silver, for although gold has become the general standard of money, silver is[497] still, as throughout man’s history, one of the two precious or money metals. It is at least a crutch to aid gold.
In many parts of the world silver ranks equal with or as more important than gold for a money standard. Elsewhere, in countries where paper currency is freely accepted by sellers, it is not an essential money metal, e.g., in the United States. However, silver with gold helps to support the credit and standing of paper money in Europe today, and in countries with less elaborate financial systems it directly replaces and so conserves gold.
Silver then is considered to be an essential metal of the world’s finance, trade, and industry. It belongs with the group of vital mineral raw materials and can not be classed with diamonds or other non-essential mineral products.
The unlimited mining and production of silver cannot be considered as an end in itself, as Spain found out in the days of the conquerors. But the present silver production is not in excess of the real needs. In fact there may be expected to be a great shortage of capital because of the great destructive effects of the world war. Silver production adds to the stock of money, increases confidence in financial conditions and furnishes, with and subsidiary to gold, a basis for credit. In the immediate future a maximum silver production may be of as great or greater importance to the world than ever before.
About one-half of the silver output of the world is obtained as a by-product in the winning of other metals, notably lead and copper. Such production comes from deposits of all geologic ages. The silver obtained from high-grade silver ores associated with minor amounts of gold or base metals is now derived chiefly from Tertiary deposits. Pre-Cambrian deposits are of some importance, e.g. Cobalt, Ontario; but the Tertiary is the great source of silver ores.
The Mexican mines, which are by far the most important as producers of silver ores, are associated with Tertiary volcanic rocks. A similar association occurs to the south, in Central America, Peru, and Bolivia. The deposits of the United States, Nevada, Colorado, Utah, and Montana, occur under similar conditions. This is also true of the chief worked-out deposits of recent silver-mining history—the Comstock Lode.
A great deal of silver has been mined in Europe in the past, chiefly from rich silver deposits which are now largely exhausted. Geologic conditions here are obscure, because the mining was completed so long ago.
In the future, Mexico and the Rocky Mountain-Andes Cordillera[498] will be the chief region for the mining of rich silver ores. The pre-Cambrian areas of Canada may continue to be of some importance.
A generalized table of normal outputs is as follows:
Table 69.—Present Normal Output of Silver
| Country | By-product silver |
Straight silver deposits |
Notes |
|---|---|---|---|
| Western Hemisphere: | |||
| Mexico and Central America | 12,000,000 | 60,000,000 | Tertiary |
| Peru and Bolivia | 10,000,000 | 5,000,000 | Tertiary |
| Chile | 2,000,000 | ... | Miscellaneous |
| United States | ... | 23,000,000 | Tertiary |
| United States lead ores | 24,000,000 | ... | Miscellaneous |
| United States copper ores | 23,000,000 | ... | Miscellaneous |
| Canada | ... | 21,000,000 | Pre-Cambrian |
| Canada | 4,000,000 | ... | Miscellaneous |
| Total Western Hemisphere | 75,000,000 | 109,000,000 | |
| Eastern Hemisphere: | |||
| Spain | ... | 4,500,000 | |
| Austria and Turkey | ... | 3,000,000 | |
| India | 5,000,000 | ... | Burma |
| Australia | 16,000,000 | ... | Broken Hill |
| Japan | 7,000,000 | ... | |
| Miscellaneous | 2,000,000 | 1,500,000 | |
| Total Eastern Hemisphere | 30,000,000 | 9,000,000 |
Summary
| Total output, ounces |
Percentage of total output |
|
|---|---|---|
| Rich silver mines | 118,000,000 | 53 |
| By-product silver | 105,000,000 | 47 |
| Total | 223,000,000 | 100 |
Mexico, the great center for rich silver mines, is now producing less than one-half of its normal output, due to the unsettled political and social conditions. In 1907 the Mexican production declined to 31,000,000 ounces, a loss of over 50 per cent. Consequently in 1917 the world’s silver output was only 170,000,000 ounces; and of this amount more than one-half was derived as a by-product in refining other metals.
A striking feature of the distribution of silver deposits is the large number of great producing areas in Mexico and the western United States. An argentiferous metallographic province is thus indicated; and is well shown in the following table:
[499]
Table 70.—Geographical Distribution of Silver Deposits, by Regions
| Region | Proportion from Rocky Mountain- Andes region |
Normal output (ounces) |
Percentage of world total |
|
|---|---|---|---|---|
| 1. | Rocky Mountain-Andes Region: | |||
| United States | 99 per cent. of total | 70,000,000 | ||
| Mexico | All | 70,000,000 | ||
| Central America | All | 2,000,000 | ||
| Peru and Bolivia | All | 15,000,000 | ||
| Chile | All | 2,000,000 | ||
| Canada | 16 per cent. of total | 4,000,000 | ||
| Total | ... | 163,000,000 | 73 | |
| 2. | Cobalt, Ontario | ... | 21,000,000 | 9.45 |
| 3. | India and Australia (2 mines) | ... | 21,000,000 | 9.45 |
| 4. | Japan | ... | 7,000,000 | 3.1 |
| 5. | Miscellaneous | ... | 11,000,000 | 5.0 |
| Total for world | ... | 223,000,000 | 100.00 | |
The relative geologic age of silver deposits is exhibited in the tabulation below:
| Probable future output (ounces) |
Future percentage of total |
Present proportion |
|
|---|---|---|---|
| Tertiary | 170,000,000 | 76 | 73.0 |
| Japanese copper (largely Tertiary) | 5,000,000 | 2 | 3.1 |
| India and Australia (age of deposits not stated) | 25,000,000 | 11 | 9.45 |
| Pre-Cambrian | 15,000,000 | 7 | 9.45 |
| Unknown | 10,000,000 | 4 | 5.0 |
| Total | 225,000,000 | 100 | 100.0 |
Examination of the above data shows that by far the greater part of the world’s silver occurs in the great petrographic and metallographic province which forms a bordering zone around the Pacific Ocean, and is most productive in the western Cordilleras of North and South America.
Decreases may occur in Cobalt (Ontario) and in Japan; in Cobalt because of exhaustion of ore, in Japan because of lower output of copper and lead if prices of these metals fall. The Rocky Mountain-Andes output should be maintained relatively at the proportion shown above—Mexico being back to normal in this assumption. This Rocky Mountain-Andes production is all, or substantially all, associated with Tertiary igneous rocks.
[500]
The above figures allow in part for the increase in Indian output from the new Burma mines.
It is doubtful if silver production will be materially increased in the next few years by improvement in metallurgy, milling, or mining methods. Silver production is perhaps unique in that a great part of the output, produced as a by-product, comes on the market at a rate determined more by the volume of lead and copper production than by current quotations for silver. Also rich silver mines when discovered can be operated at a large profit per ounce. However, high prices for silver will stimulate that part of the production which comes from silver mines proper.
The world’s production increased over one-third during the period of 1904 to 1913. At the same time prices had declined even below the 1893-1894 figures. The increased output was due to production from the United States and from Canada.
Independent of price, the Cobalt discovery poured silver on the market. Independent of price, the increased lead and copper of the United States poured by-product silver on the market.
It seems clear that discoveries of new silver deposits or enlarged and improved base-metal mining operations are the factors that will influence silver output. Changes in methods of silver recovery and even changed silver prices have no tremendous effect. Even the big drop in silver prices in the early nineties was accompanied by no decrease in silver production. Instead there was an increase.
The silver output of the world is divided among the various political groups as shown in Table 71:
Table 71.—Production of Silver in 1917 and 1913
| Country | 1917 production (ounces) |
1913 production (ounces) |
1913 percentage of total |
|
|---|---|---|---|---|
| United States | 71,740,000 | 66,801,000 | 30.0 | |
| British Empire (chiefly Canada and Australia) | 34,001,000 | 50,429,000 | 22.6 | |
| France | 235,000 | 521,000 | 0.2 | |
| Italy | 450,000 | 424,000 | 0.2 | |
| 106,426,000 | 118,175,000 | 53.0 | ||
| Japan | 6,922,000 | 4,716,000 | 2.1 | |
| Peru | 11,000,000 | 8,351,000 | 3.7 | |
| Central America | 2,369,000 | 2,135,000 | 1.0 | |
| Bolivia | 2,434,000 | 2,410,000 | 1.1 | |
| Russia, Greece, etc. | 1,000,000 | 1,000,000 | 0.4 | |
| Mexico | 31,214,000 | 70,704,000 | 31.7 | |
| Chile | 1,673,000 | 2,000,000 | 0.9 | |
| Spain | 4,500,000 | 4,232,000 | 1.9 | |
| Germany, Austria | 1,500,000 | plus | 7,195,000 | 3.2 |
| Turkey | 1,509,000 | 0.7 | ||
| Miscellaneous | 1,700,000 | 700,000 | 0.3 | |
| Total | 170,038,000 | 223,126,000 | 100.00 | |
—The production of silver in the United States is all controlled by United States capital. One-third is controlled by lead-mining interests, one-third by copper-mining interests, and the remaining third by silver miners. Moreover, United States capital owns Mexican mining property normally capable of producing over half of that country’s output. Central American production and the by-product silver of Peru are similarly controlled. About one-quarter of the Canadian production comes from properties owned in the United States. In all, the capital of the United States controls over half of the yearly output of silver throughout the world.
Most of the Canadian and all of the Australian, Indian, and African silver is controlled by British capital, as is one-quarter of the Mexican production and some from Bolivia, Peru, and Chile. In all, Great Britain controls a third of the world’s output.
[501]
Germany probably controls close to 10 per cent. of the world’s silver production. A part of this is produced locally, but the main German control is in Mexico, the mines owned by Mexicans being taken as, in the main, German properties.
Mines owned by Japanese, Spanish, French, or Chilean capital are responsible for substantially all the remaining 5 per cent. of the world’s silver output.
Financial Control Through Ownership of Mines
| Capital | 1913 output, percentage controlled |
|---|---|
| United States | 52 |
| British | 33 |
| German | 10 |
| Japanese | 2 |
| Spanish | 2 |
| French, etc. | 1 |
| 100 |
—As would be expected from the geographic location of the silver deposits, the United States and[502] Mexico are the centers of silver smelting and refining. Important silver-smelting interests are as follows
| Company | Situation of smelters |
|---|---|
| The American Smelting & Refining Co. | United States and Mexico |
| The United States S., R. & M. Co. | United States and Mexico |
| The International Smelting Co. | United States |
| Anaconda Copper Mining Co. | United States |
| Consolidated Mining & Smelting Co. | Canada |
| Compañia Metalurgica Mexicana. | Mexico |
| Compañia Metalurgica de Torreon. | Mexico |
| Compañia Minera de Peñoles. | Mexico |
Through ownership of reduction plants, the United States exercises control over a somewhat larger share of the world’s silver than it does through mine ownership. Much of the Canadian and Mexican as well as most of the South and Central American silver production enters the United States either as refined bullion or as ore and base bullion.
It is estimated that control through reduction plants is about as follows:
United States, ⁴⁄₇; Mexico, ¹⁄₇; Canada, ¹⁄₁₄; Europe and Asia, ³⁄₁₄.
As regards silver, this type of control is not at all powerful. Silver-bearing materials can bear a high transportation charge as soon as the first process of freeing from gangue has been completed. Consequently, ownership of mines, rather than of reduction plants, is the vital factor of control over silver resources, so far as production is concerned.
—The world’s output of silver is controlled by the London market. To a small extent this may be due to a trade combination; to a large extent it is due to the relations of the London market with consumers.
Four firms form the London silver market. Silver prices are fixed daily in London, and this “fixed” quotation controls the price of the metal in every important financial center throughout the world.
There are three refineries in London that handle practically the whole of the silver bullion that comes on the London market. No silver can be bought or sold in London unless assayed by one of the four official assayers to the Mint, Bank of England, etc. Silver treated by the London refineries and certain bars from European government refineries are exceptions to this rule.
The London refineries produce silver of the fineness ⁹⁹⁶⁄₁₀₀₀ to suit the Indian market. Other silver current in the London market has a fineness of ⁹⁹⁹⁄₁₀₀₀.
In the words of Benjamin White: “The care taken to safeguard the reputation of the London silver market, the high standing of the firms that comprise it, and the confidence built up by the methods and practices[503] adopted to protect the interests of buyer and seller alike, provide a strong guarantee that in the future, as well as in the past, silver will find its business center in London.”
—As already indicated, India and China are the great consumers of silver. For the five years preceding 1914, fully 40 per cent. of the world’s silver output was shipped from London to those two countries, which, with a combined population of over 700,000,000, represent the buying side of the world’s silver market, just as North America represents the selling side.
Since 1914 the capacity of these countries to absorb silver has steadily increased and in 1918 it was mainly a question of where the silver could be obtained. Current production was inadequate to meet the demands, and old stocks of the precious metal were of necessity put on the market.
The world’s silver business consists in getting the metal from the Americas to the East. Why send it via London, exposing the precious metal to greater marine hazards and losing interest while in transit? The main reason is that the chief trade of China and India with western nations is with England, and the great banking houses that finance this trade are in London. These banks purchase silver in London to adjust exchange balances. Funds to purchase such silver usually originate in London, whether from bills on London, loans from London banks or in other ways. In addition, London has been the center of the world’s finance and foreign trade, and also the center from which the mail steamers, the swiftest and cheapest routes to the principal consumers of silver, have radiated.
When the submarine became a menace the United States shipped silver direct from San Francisco to India and China. A large part of the United States production was thus diverted from London, and in 1918 exports of silver from London were far below normal. However, the officials of the American Smelting & Refining Co., refiners of over one-third of the world’s silver output, say that they do not expect these conditions to continue. The established business of the London silver trade, and, of more importance, the relationship between commercial London and the silver-consuming countries, will no doubt quickly re-establish the normal status of London—importer of American silver, exporter of the silver needed in the Far East.
The American refiner or silver producer is glad to have a steady and broad market for his silver, such as is furnished by the four London firms. Smelting interests and mines are able to dispose of their product through London; otherwise they would have to deal with brokers or banks in the Far East. The London firms keep in close touch with the big bullion dealers of Bombay, Calcutta, and other cities, which are the centers from which silver is distributed throughout India. Silver for the Indian imperial coinage, however, is purchased in London by the government.
[504]
—The above silver trade flow-sheet has recently (1920) been materially changed by the program of the Government of the United States to purchase domestic silver at $1 an ounce. If the world price for silver remains below this figure, all American silver will be absorbed by the Government for about four years, or until the 207,000,000 ounces specified in the Pittman Act are bought. If silver, however, should rise above $1 the flow to market as sketched above would be resumed.
—Chinese foreign exchange rates depend to a large extent on the price of silver. All importers or exporters dealing with China must deal in the silver market. Although copper “cash” is the basis of Chinese currency, silver is the standard legal tender for transactions involving large amounts, and weights of silver are used as units. Fineness and weight of Chinese coins are manipulated so that coins issued by the silver-producing states, e.g., Mexican dollars (two varieties), British dollars, Spanish dollars, etc., are prized by the Chinese because they are uniform in value.
Japan produces considerable silver, a great deal coming from her electrolytic copper refineries. Gold is the money standard and silver is not used extensively in the arts. Consequently Japan produces more than enough silver to satisfy local consumption. The government is alive to the importance of silver in connection with all dealings with China. In 1918, Japanese banks bought up Chinese silver supplies, paying prices in excess of all other traders. It is believed that American silver has been flowing into Japan via China and that Japan seeks to control the Chinese silver market. A large silver reserve is being built up in Japan. On account of the international importance of the whole Chinese problem, Japanese activity in silver should be noted.
Silver is used both for money and in the arts, the former use being the more important and more essential. In some countries, especially those producing silver in large amounts, it is the money standard, either alone or with gold. In other countries on a gold standard, silver is used for subsidiary coinage. In India and China it is used for the settlement of foreign trade exchange balances. Normally about two-thirds of the silver produced annually is used in the arts, mainly for the manufacture of jewelry and luxurious household wares, though some is consumed in the photographic, chemical, and other essential industries. During the war more silver went into coinage and less into the arts than formerly, as the large issues of paper money made corresponding increases in the number of silver coins desirable. Large amounts have been exported to the Far East. The war has shown that silver should still be considered an[505] essential metal of the world’s finance and trade, despite the increasing amounts consumed in non-essential uses.
About one-half of the silver output of the world is obtained as a secondary product in the mining of other metals, notably lead and copper, from deposits of all geologic ages. The high-grade silver ores are derived chiefly from Tertiary deposits, and it is probable that in the future Mexico and the Rocky Mountain-Andes region will be the chief sources of ores of this type.
Of the total world silver production over 80 per cent. comes from the mines of the Western Hemisphere. For many years Mexico was the leading silver-producing country of the world, but the unsettled political conditions have so interfered with mining operations that the production for 1917 was less than a half of that for 1911 or 1912. The United States, a close second to Mexico in pre-revolutionary years, now occupies the leading position. Canada and Central and South American countries also produce important quantities of silver. In the Eastern Hemisphere, Australia is the leading silver producer. Smaller amounts are contributed by Spain, Austria, Turkey, India, and Japan.
Changes in practice of silver recovery and even silver prices have little influence on silver production. It will be stimulated rather by discoveries of new silver deposits or by enlarged and improved base-metal milling operations.
The principal silver deposits of the world are controlled politically by the United States, Mexico, and Great Britain, the three nations controlling about 85 per cent. of the total production in 1913. United States capital controls the entire silver output of the United States, and mines producing half of the Mexican output, one-fourth of the Canadian output, and much of that of the Central and South American countries, in all something over one-half of the world’s normal silver production. Great Britain controls probably one-third of the world production, chiefly in Canada, Australia, India, Africa, and Mexico. German capital owns probably one-tenth, located partly in Germany but mainly in Mexico. The remaining silver deposits of the world are owned by Japanese, Spanish, and French capital. Ownership of reduction plants is not a powerful form of control in the case of silver. The United States owns about four-sevenths of the total smelting and refining capacity of the world, the remaining three-sevenths being controlled largely by German, British, and Mexican capital.
Although the greater part of the silver produced each year comes from North and South America, the world’s silver market is located in London because of the close relations between English business interests and India and China, the chief consumers of the metal.
The United States Government, however, will (1920) purchase all American silver offered at $1 per ounce, up to 207,000,000 ounces.
[506]
In past discussion of the uses of platinum some confusion has resulted from the lack of appreciation that all commercial platinum is not the pure metal. The pure metal is required for chemical work of all sorts, but for other uses the iridium alloys are used. Electrical platinum contains 15 to 50 per cent. iridium, but averages 25 per cent., and jeweler’s platinum carries about 10 per cent. iridium. Palladium, another of the platinum group metals, is also of importance, chiefly in the form of palladium-gold alloys, which can be used to replace platinum in the dental and jewelry industries. Rhodium, one of the rarer elements of crude platinum, has a limited use in electrical pyrometers. Osmium and ruthenium, the remaining members of the platinum group, appear to have little use, though osmium, when properly used, can be employed as a substitute of iridium to harden platinum alloys.
The essential uses of platinum metals are in the chemical and electrical industries, and probably the dental industry should be classed as essential. Pure platinum is required in the chemical industry for catalysers in the manufacture of sulphuric acid (about 75,000 ounces now in use in the United States) and in the manufacture of nitric acid from ammonia. For the sulphuric-acid industry, platinum chloride is the primary material containing platinum. Asbestos or anhydrous magnesium sulphate soaked in platinum chloride, and then baked to drive off the chlorine, forms what is known as “contact mass,” which is charged into the chambers of contact acid plants. Very fine-mesh platinum gauze is used for the catalyser in nitric-acid plants. Some gauze used for this purpose has a reinforcing edge of platinum-iridium wire. Pure platinum utensils of various kinds, including crucibles, dishes, tongs, and triangles, are required in every chemical laboratory. It is possible to substitute palladium-gold alloys, or even gold, nickel, nichrome, and silica, for some utensils, but no substitutes have yet been found which will entirely replace platinum chemical ware.
Platinum-iridium alloys have been used extensively by the electrical industry, but substitutes are constantly being developed. Tungsten, molybdenum, and nickel-chrome alloys are the principal substitutes used[507] so far, but their use has not done away with the necessity of platinum in the industry. The principal use of platinum-iridium alloys in electrical work is in contact points, and the proportion of iridium necessary in the alloys is directly dependent on the intensity of the current passing through the contacts and the speed at which the contacts move. Probably the largest consumption of platinum alloy is in the manufacture of telephone and telegraph equipment, including sending and receiving instruments, switch boards and relays. There is also a large consumption of platinum for contacts in magnetos used for various kinds of internal-combustion engines. Automobile makers are, however, developing starting systems that do not require platinum, so we can hope for a lessening future demand from that quarter.
Platinum has an important use in dentistry, though in emergencies palladium-gold alloys have been used as substitutes. Seemingly, however, the substitutes are not entirely satisfactory, and it may be necessary to go back to platinum for certain dental uses. The chief uses are for pins for crown work, pins for fastening artificial teeth to plates, and foil for making molds of cavities in which to bake porcelain fillings. For the time being, the palladium-gold substitutes can be used and perhaps they will be developed so that the use of pure platinum in the future may not be necessary.
The non-essential use of platinum metals is in jewelry, and it seems certain that this misuse of platinum metals must be stopped in order that industrial development may continue. It is estimated that for a number of years 50 per cent. of the platinum consumed in the world went into jewelry. A large part of platinum-mounted jewelry is in private ownership, and as the value of the metal in a jewel is approximately 35 per cent. of the total cost, it is evident that it would be difficult, if not impossible, in case of necessity to recover more than a small proportion of the large quantity of platinum that is in the form of jewelry.
The relative importance of the platinum-producing countries of the world can best be judged by the past output, which is shown in the following table,[181] and graphically in the chart.
[181] Hill, J. M.: “Platinum and Allied Metals,” U. S. Geol. Survey, “Mineral Resources, 1916,” Pt. 1, 1917, p. 3.
The platinum field of Russia is in the Ural Mountains, north of Ekaterinburg. In Colombia, South America, the chief production of platinum has come from the headwater streams of the San Juan River, which enters the Pacific near Buena Ventura; some platinum is found in the upper reaches of the Atrato River, which enters the Caribbean Sea near the east end of Panama. A small amount of platinum and osmiridium has come from New South Wales and some osmiridium from Tasmania. Some of the placers in southwestern Oregon and northern California carry platinum as well as gold. Platinum has recently been determined in concentrates from several localities in Alaska and Canada, and is known in some placers in Borneo and India.
[508]
Table 72.—Estimated World’s Production of Crude Platinum, 1909-1917
(In troy ounces)
| Country | 1909 | 1910 | 1911 | 1912 | 1913 | 1914 | 1915 | 1916 | 1917 |
|---|---|---|---|---|---|---|---|---|---|
| Borneo and Sumatra | 500 | 200 | ... | ... | 200 | [182] | [182] | [182] | [182] |
| Canada | 30 | 30 | 30 | 30 | 50 | 30 | 100 | 60 | 80 |
| New South Wales and Tasmania | 440 | 332 | 470 | 778 | 1,500 | 1,248 | 303 | 222 | [182] |
| Russia | 264,000 | 275,000 | 300,000 | 300,000 | 250,000 | 241,200 | 124,000 | 63,000 | 50,000 |
| United States | 672 | 390 | 628 | 721 | 483 | 570 | 742 | 750 | 605 |
| Totals | 271,642 | 285,952 | 313,128 | 313,529 | 267,233 | 260,548 | 143,145 | 89,932 | 82,685 |
[182] No basis for estimate.
Practically all of the platinum metals produced in the world to date have been derived from placer deposits, though a little platinum and palladium have been obtained as a by-product from the electrolytic refining of copper and nickel matte.
The platinum placers of the world are so definitely related to intrusive basic igneous rocks, including pyroxenites, peridotites and dunites, that there is practically no question of the origin of the metal. In fact, in British Columbia, Spain, and Russia platinum has been found in place in these types of rocks, and it is reported that owing to the great value of platinum at present, a project is on foot to crush and wash certain of the bodies of dunite in Russia.
Platinum is not found in all placers derived from basic rocks, but so far as known it is rarely found in placers derived from other types of rocks. Prospecting for platinum placers therefore resolves itself first into a search for deposits of gravel derived in a large part from pyroxenite, peridotite, and dunite. Chromite is a characteristic heavy constituent of platinum sands and in some deposits can be recovered as a by-product in mining. Olivine is also present in considerable amounts. Magnetite and ilmenite are ordinarily present in the concentrates from platinum placers, but are more characteristic of placers in which gold is the most valuable constituent.
A large amount of prospecting has been done in Russia and Spain, based on the theory of origin outlined above. Much of this prospecting has been successful in locating platiniferous placers, but many of these recent discoveries do not seem to be of commercial worth. It is not possible to foretell whether placers derived from basic igneous rocks will[509] be productive. The most that can be told is that certain placers derived from these rocks hold the greatest promise for the searcher for platinum. Platinum is such a rare metal and is found in such small quantities in its mother rock that it is necessary to have certain physiographic conditions present to predicate commercial deposits. Most important of these conditions is extremely prolonged or very rapid weathering of the primary deposits. In the Russian field, rock-weathering has been in progress for great geologic time; on the other hand, in Colombia, South America, the period of weathering seems to have been relatively short, but so rapid that the same result has been obtained.
Ordinarily, platinum is not found in commercial quantities in gravels that have not been reconcentrated, and the richer deposits of the world seem to be the results of repeated reconcentrations of platinum-bearing material. Crude or placer platinum is not pure metal, but contains, besides other metals of the platinum group, more or less iron, nickel, and copper. Russian crude platinum is ordinarily sold on the assumption that it contains 83 per cent. platinum metals; Colombian crude, 85 per cent. platinum metals. Some placer platinum, so-called, carries a large proportion of osmiridium. Thus the Oregon and California crude platinum carries from 25 to 45 per cent. iridium, and Tasmanian platinum is really nearly pure osmiridium.
The following analyses of Russian,[183] Colombian,[184] and American[184] platinum serve to illustrate the wide divergence of metal content of crude platinum.
Table 73.—Analyses of Crude Platinum from Various Parts of the World
| North America[183] | South America[183] | ||||||
|---|---|---|---|---|---|---|---|
| California | California | Oregon | British Columbia |
Colombia | Colombia | Colombia | |
| Pt | 85.50 | 63.30 | 51.45 | 72.07 | 86.20 | 86.16 | 80.00 |
| Ir | 1.05 | 0.70 | 0.40 | 1.14 | 0.85 | 1.09 | 1.55 |
| Rh | 1.00 | 1.80 | 0.65 | 2.57 | 1.40 | 2.16 | 2.50 |
| Pd | 0.60 | 0.10 | 0.15 | 0.19 | 0.50 | 0.35 | 1.00 |
| Os | ... | ... | ... | ... | ... | 0.97 | |
| Io3[186] | 1.10 | 22.55 | 27.30 | 10.51 | 0.95 | 1.19 | 1.40 |
| Au | 0.80 | 0.30 | 0.85 | ... | 1.00 | ... | 1.50 |
| Fe | 6.75 | 6.40 | 4.30 | 8.59 | 7.80 | 8.03 | 7.20 |
| Cu | 1.40 | 4.25 | 2.15 | 3.39 | 0.60 | 0.40 | 0.65 |
| Sand | 2.95 | ... | 3.00 | 1.69 | 0.40 | ... | 4.35 |
| 101.15 | 99.40 | 100.25 | 100.15 | 100.25 | 100.35 | 100.15 | |
| Oceania[185] | Russia[187][510] | |||||||
|---|---|---|---|---|---|---|---|---|
| Borneo | N. S. W. | Australia Tasmania? |
Taguil | Iso | Kamenonchy | Koswinsky | Kanjakowaky | |
| Pt | 82.60 | 75.90 | 61.40 | 76.16 | 80.10 | 82.46 | 83.50 | 60.39 |
| Ir | 0.66 | 1.30 | 1.10 | 2.68 | 1.38 | 1.79 | 2.74 | 6.90 |
| Rh | ... | 1.30 | 1.85 | 0.54 | 0.30 | 0.69 | 0.62 | 0.80 |
| Pd | ... | Trace | 1.80 | 0.27 | 0.30 | 0.18 | 0.28 | 0.19 |
| Os | ||||||||
| Io3[186] | 3.80 | 9.30 | 26.00 | 1.50 | 4.47 | 4.99 | 0.79 | 20.21 |
| Au | 0.20 | ... | 1.20 | ... | 0.09 | 0.27 | 0.07 | |
| Fe | 10.67 | 10.15 | 4.55 | 14.72 | 7.68 | 9.49 | 11.05 | 11.16 |
| Cu | 0.13 | 0.41 | 1.10 | 3.39 | 0.63 | 0.54 | 1.14 | 0.49 |
| Sand | ... | 1.22 | 1.20 | |||||
| 98.06 | 99.58 | 100.20 | 99.26 | 94.95 | 100.41 | 100.19 | 100.04 | |
[183] Duparc, Louis: “Le Platine et les gîtes platiniferes de l’Oural”: Soc. des Eng. Civ. de France. Bull. Jan.-Mar., 1916.
[184] Kemp, J. F.: “The Geological Relation and Distribution of Platinum and Associated Metals”: U. S. Geol. Survey Bull. 193.
[185] Analyses cited by Kemp.
[186] Io3 is abbreviation used for osmiridium.
[187] Analyses cited by Duparc.
The relative importance of the platinum-producing countries of the world can best be judged by the past output, which is shown in the following table[188] and graphically in Figure 22.
[188] Hill, J. M.: “Platinum and Allied Metals”: U. S. Geol. Survey “Mineral Resources, 1916,” Pt. I, 1917, p. 3.
Table 74.—Estimated World’s Production of Crude Platinum, 1909-1917
(Troy ounces)
| 1909 | 1910 | 1911 | 1912 | 1913 | 1914 | 1915 | 1916 | 1917 | |
|---|---|---|---|---|---|---|---|---|---|
| Borneo and Sumatra | 500 | 200 | ... | ... | 300 | ||||
| Canada | 30 | 30 | 30 | 30 | 50 | 30 | 100 | 60 | 80 |
| Colombia | 6,000 | 10,000 | 12,000 | 12,000 | 15,000 | 17,500 | 18,000 | 25,000 | 32,000 |
| New South Wales and Tasmania | 440 | 332 | 470 | 778 | 1,500 | 1,248 | 303 | 222 | |
| Russia | 264,000 | 275,000 | 300,000 | 300,000 | 250,000 | 241,000 | 125,000 | 63,900 | 50,000 |
| United States | 672 | 390 | 628 | 721 | 483 | 570 | 742 | 750 | 605 |
| 271,642 | 285,952 | 313,128 | 313,529 | 267,233 | 260,548 | 143,145 | 89,932 | 82,685 |
Fig. 22.—Sources of crude platinum produced from 1909 to 1917.
—Russian placer deposits have supplied approximately 95 per cent. of the platinum in the world. The principal placer deposits rich in platinum are in the central Urals, in the Perm government, near Nishni-Tagilsk, Nishni-Turinsk, and Verkhoturshi. The richer deposits are on the eastern slope of the mountains, principally on the Iss and Veeya tributaries of the Tura River of the Obi drainage. Several important[511] placers are found on the west slope of the mountains on the headwaters of the Chusovaia and Kama rivers of the Volga drainage. Near Nishni-Tagilsk platiniferous placers occur both on the Taguil, a tributary of the Obi, and on the Martian, a headwater stream of the Volga drainage. In these placers platinum is the predominant metal, but gold is also found. The greater part of the output in the past has been by hand-washing, but of recent years much of the ground has been reworked by dredges, though it is still safe to say that over 75 per cent. of the output is from hand labor. It is hardly practicable in a report of this sort to discuss in detail the individual deposits, even were the information at hand. The best information available can be summarized as follows: most of the platinum has undoubtedly originated from the disintegration of dunitic, pyroxenitic, or peridotitic rocks. The period of weathering has been[512] very long and there have been many changes in the drainage systems, which have now reached maturity. The stream grades are low; the inter-stream relief is relatively slight. The platinum has probably been reconcentrated many times and is at present won principally from present valley gravels, though the pay channels do not always follow the present river channels. Some bench-ground representing old river channels is worked, particularly in the Nishni-Tagilsk region. The pay gravels ordinarily rest on bed-rock, though concentration on clay beds forming false bed-rock is fairly common. The pay dirt ranges from a few inches to as much as 6 feet in thickness. It has few large boulders, but has considerable clay in many places. The overburden ranges from 2 to 16 feet in thickness, averaging 8 to 10 feet. It consists of a thick basal portion of practically barren gravel and sand, or clay and sand lenses interlayered with gravel, which is overlain by 2 to 3 feet of clay sand and vegetable matter similar to the “muck” of the Alaskan mines.
In shallow ground, up to 3 feet deep, mining is carried on by open-cut methods; in the deeper ground shafts and drifting have been employed. For the deposits in river channels crude hand dredges were and still are used for raising the gravels. About 35 more or less modern dredges were engaged in platinum mining prior to the war. Clay is so generally found in the gravels that specially designed machines have been used to save the platinum and the newer dredges have special devices to cope with this problem.
—The platinum district of Colombia covers the upper waters of the Atrato and San Juan rivers in the Choco district of northwestern Colombia. Platinum is known as far north as Bete, on the Atrato, and in many of the tributaries of the San Juan which enter from the east. Most of the platinum exported from Colombia has come from the Condoto River, a headwater stream of the San Juan. It is stated that in this stream the platinum constitutes about 75 per cent. of the precious metal in the gravel. On the Atrato the platinum content is lower, ranging from 5 to 15 per cent. Platinum and osmiridium also occur in the streams south of the San Juan drainage, which enter directly into the Pacific, though little authentic data concerning them are available.
According to Dr. Tulio Ospina,[189] Director of the School of Mines, Medellin, Colombia, platinum is found widespread in conglomerates which cover an extensive area in the Atrato and San Juan basins. The metal has been reconcentrated in the present stream channels from which the major output of platinum comes. According to the statements of various observers, there are places on the interstream areas in which platinum has been concentrated. These areas are, from all accounts, old stream channels. The primary platinum deposits evidently are to be[513] looked for on the west slope of the western ridge of the Andes, though no literature which gives detailed information on the geology of this range has been found. Peridotite, dunite, and other basic igneous rocks are represented in the platiniferous gravels.
[189] Proceedings Second Pan-American Scientific Congress, vol. 8, 1917.
There is almost no information upon which to base an estimate as to the possible reserves of platinum in Colombia. Little has been published on the subject that gives good data on the geology of the country, but from all accounts it seems safe to assume that Colombia holds much promise and should be more carefully prospected. Over 90 per cent. of the platinum mined in Colombia is won by natives, mostly women, who raise the gravel in calabaches and wash the gravels in bateas. The dry season is utilized, for then the low places in the river are more exposed. There is one dredge in operation in Colombia at present, though other dredging operations have been tried, which, for various causes, were not successful.
—A small quantity of platinum is produced each year by the placer operations on the Tulameen River, in British Columbia. The metal was derived from a mass of peridotite and dunite, which outcrops west of the main drainage. The gravels of this area are apparently very deep, but the platinum is found in the upper 8 to 20 feet, in part concentrated on a false bed-rock. Recent information indicates the possibility of dredging a considerable acreage of gravels on the Tulameen in the vicinity of Princeton. Reports were current during 1917 of discoveries of platiniferous placers further north in the Canadian Rockies, but no definite information concerning the size or value of the deposits is available.
By far the greater part of the yearly output of platinum and palladium of Canadian origin is a by-product of refining the nickel ores of the Sudbury district, Ontario. That a far greater production of both metals from this source is possible has been shown by the Royal Ontario Nickel Commission.
—Crude platinum is won in California, Oregon, and Washington. The dredges at the base of the Sierra Nevada Mountains in California produce a large part of the placer platinum output of the United States, principally because of the great yardage handled rather than because of any particular concentration of platinum in the gravels derived from the Mother Lode belt. In northern California the Klamath and Trinity rivers, particularly the Hay Fork of the Trinity, carry platiniferous gravels. In southwestern Oregon platiniferous gravels have been found at several places on the Illinois and Rogue rivers. Along the beach from Bandon, Oregon, to Eureka, California, platinum occurs with the black sands and has been won at a number of mines located both on the present strand line and on ancient elevated beaches. In the Blue Mountains of eastern Oregon and in the Strawberry Range[514] south of John Day River a few placers have been worked which carried platinum. In Washington, particularly on the south fork of Lewis River, near Yacolt, platinum has been found, and it is reported on the beaches from the Straits of Juan de Fuca south.
All of the platinum-bearing placers in the United States are closely associated with chromiferous serpentine derived from peridotites or pyroxenites. The concentration of the platinum has not been great and the original quantity was not large, so none of the areas seems capable of much production. Most of the crude platinum from the Pacific Coast placers carries considerable osmiridium. As stated before, the great bulk of the platinum won in the United States is from the dredging fields at the base of the Sierra Nevada Mountains from Butte to Stanislaus County. The gravels in these fields are the result of several reconcentrations, but their platinum content can not be considered high. In practically all of the other stream placer areas the gravels have not been subject to such extensive reconcentration, and in some places recent gravels carry platinum. The beach deposits are the result of repeated concentration. The platinum and gold are excessively small, flaky, and difficult to separate from the heavy sands; they are concentrated in the dark bands of sand caused by tidal and wave concentration. The lenses are rarely over one foot thick and taper out in short distances. Correct estimates of reserves are consequently almost impossible.
Within the past few years a little platinum has been won from widely scattered localities in Alaska, chiefly Dime, Bear, and Sweepstake Creek placers in eastern Seward Peninsula; the Boob Creek placers, Tolstoi district, Lower Yukon; and the beach deposits of Kodiak Island. Platinum also occurs in the Upper Kahiltna drainage, north of Anchorage.
Copper ores rich in palladium are produced at the Rambler mine, Wyoming, from the Salt Chuck Mine, Alaska, and from the Boss mine, in Nevada. At the two former mines the ore is associated with basic igneous rocks; in the latter mine the ore bodies are in dolomite. The oft-repeated reports of platinum in certain sandstones exposed near the Bright Angel trail in the Grand Canyon of the Colorado, Arizona, and in certain shale beds in Chester County, Pennsylvania, have not been verified, though in June, 1918, the United States Platinum Co. was organized to work the deposits in Arizona.
—Platinum and osmiridium have been won in small quantities from Queensland, New South Wales, and Tasmania. Platinum is also reported from New Zealand. The greater production has been from the osmiridium-bearing gravels of the Savage River drainage in the Bald Hills mining district in the northwestern part of Tasmania. Little has been published concerning the extent of these gravels, which were derived from the weathering of a series of sediments intruded by basic igneous rocks.
[515]
In New South Wales, beach deposits much like those of California and Oregon are found from Beachy Head north past Clarence and Richmond rivers into Queensland. Commercial exploitation of these deposits seems to have had the ups and many downs of similar undertakings on our West Coast. Some platinum was obtained from an old buried channel in the Platina or Fifield district in central New South Wales, where pay gravel 6 to 10 feet thick lies under an overburden of 20 to 80 feet. The water supply was not in sufficient quantities to warrant large-scale operations. The channel seems small and has been mined about as far as is commercially possible.
—What may prove to be commercial placer deposits have been found in the Sierra Ronda, in southern Spain, about 50 miles northwest of the port of Malaga. The deposits have been discussed in some detail by Duparc.[190] Apparently the gravels have not been rearranged by nature many times, the concentration of platinum is not great, and it is problematical whether extensive development will be warranted.
[190] Duparc, Louis, and Grossett, Augustine: “Etude comparée des gîtes platiniferes de la Sierra de Ronda et de l’Oural”: Soc. Phys. et Hist. Nat. Geneve, Mem., t. 38, fasc. 5, 1916.
—A small amount of platinum is produced as a by-product from gold dredges on the Irawadi River, in India, and from the tin dredges in the Dutch East Indies. Unconfirmed reports have been received of discoveries of platinum in southern Siberia, at various places in Mexico, and from several localities in Ecuador and Peru. Platinum is known to occur in some of the streams as well as in certain of the gold deposits of the Minas Geraes district of Brazil.
In southwestern Borneo platinum occurs in the Tanath-Laut district. Several Russian writers have given information on this region.
At present the Russian platinum fields are practically idle. The dredges are for the most part not running and the industry is disorganized. It will require considerable expense and several months’ time to rehabilitate the Russian platinum industry. The known deposits of the Russian field are becoming exhausted, and the reserves of known platiniferous gravels are stated by Duparc to have a life of 12 years, based on the pre-war rate of production; or, stated differently, the known deposits are capable of producing between 3,000,000 and 3,600,000 ounces of platinum before they are exhausted.
Colombia seems to have large reserves of unworked platinum-bearing ground, though so little detailed information is available that it is unsafe to predict their future. It is safe, however, to point out that all reports[516] indicate that careful prospecting in the Choco district will probably be repaid by the discovery of considerable areas of platinum-bearing ground.
The Canadian deposits hold some promise of future production. Several recent discoveries of platinum along the Rocky Mountains in British Columbia, from the Tulameen to the Stikeen, indicate that further search may be rewarded. If reports are true, there is a considerable area on the Tulameen, Willow and Peace rivers which can be dredged for the recovery of gold and platinum. The most important Canadian platinum reserves are in the Sudbury nickel deposits, but present metallurgical practice will have to be changed to obtain the maximum output of platinum and palladium from these ores.
In the United States there does not seem to be hope for a considerable increase in the output of platinum; in fact, it may be that the production will be materially less when the new refineries for the treatment of Sudbury ores are completed in Canada. The placer deposits carrying platinum are for the most part relatively small; many of those in northern California and Oregon can not be worked economically and few are available for dredging. As the gold-dredging field along the base of the Sierras becomes exhausted, the output of platinum in this country will decline in proportion, barring the discovery of new ground and deposits of gravel richer in platinum than those now known.
The various Australian platinum deposits do not seem particularly promising, as regards production, with the possible exception of the Bald Hill dredging field, in Tasmania. The Fifield deposits seem to be nearly exhausted and the beach deposits in New South Wales and Queensland are too low grade and the valuable minerals are too erratic in distribution to appear of much commercial interest.
The Spanish deposits have not yet been sufficiently explored to determine their extent, but published reports do not seem to indicate that they will prove very large or particularly rich.
As Table 74 shows, Russia has political control of approximately 90 per cent. of the world’s supply of platinum. It seems probable that Canada, in the nickel ore of the Sudbury district and in the known placers in the Tulameen and Barkersville districts, has control of the third largest reserve of platinum. The deposits under the political control of the United States in Alaska and on the Pacific Coast are relatively insignificant. Great Britain naturally controls the platinum in her colonial possessions, Australia, Tasmania, and India. The output of the Dutch East Indies, from Borneo and Sumatra, is relatively small, although there is a possibility that the production from these countries may be increased. Spain may, perhaps, control a small output of platinum, though it does[517] not seem probable that the production from this country will ever be large. A large area in the Sierra Ronda Mountains has been set aside by the government for further prospecting under the auspices of the government, and it seems reasonable to believe, judging from the general mineral policy of Spain, that in the event of the proving of commercial deposits they will be worked under government auspices rather than by private persons.
Although the deposits in Colombia are politically controlled by that country, they are, nevertheless, owned largely by American interests.
—Prior to the war, French commercial interests practically dominated the platinum industry of Russia, through the operations of the Compagnie Internationale du Platine. This company not only had extensive mining holdings but also had contracts with the two largest independent Russian platinum producers, namely, the Shouvaloff and Demidoff companies, for their entire output. The contracts were suspended by the French company shortly after the declaration of war and may not be remade. There are, however, two Russian companies which are more or less independent of French control and there are a large number of small miners and peasants who know no allegiance to any particular buying concern. It appears that at least 75 per cent. of the platinum production of Russia is (or was, previous to the Bolshevist domination) controlled by the following companies: Compagnie Internationale du Platine (French), Shouvaloff’s company (Russian), Demidoff company (Russian), Nicolo-Pavdinski company (Russian), and the Platina company (Russian). During 1914 these companies were operating approximately 35 dredges in the platinum field, though from the best reports now available it does not seem that more than two or three of the dredges were at work during 1917. The reader should realize, however, that the production from dredges has always been relatively small, as compared with the output made by other methods. It is estimated that about 80 per cent. of the platinum won from the Russian placers is recovered by hand labor by lessees (starateli) who contract to dispose of their production to the companies owning the ground and pay a royalty for the privilege of working. Since the war the peasants and miners are virtually in control of all the mines and the original operators have little to do with operation or management.
The most important platinum-bearing placers in Colombia are controlled by American financial interests. The General Development Co., of New York, through two subsidiary companies, controls a large area in the headwater region of the San Juan River, particularly on the Condoto River. Recently these interests have organized the South American[518] Gold & Platinum Co. The Quito Mining Co. controls a considerable acreage on the Quito River between Quibdo and Istimina. There are other small American holdings in the vicinity of Negua, on the Atrato drainage, and on the Tamana and Sipi, on the San Juan drainage. Late in 1917 a British company was organized for the development of extensive holdings on the Opogodo River, in the upper San Juan drainage. If the present conditions are not changed by special legislation in Colombia it would seem that American financial interests will continue to dominate the Colombia platinum field.
The platinum deposits of the United States are apparently largely in the hands of small holders, who are citizens of this country. A few of the large dredges in California, which are producing platinum as a by-product, are, in part, owned by British capital.
Apparently the beach deposits in Australia (in New South Wales and Queensland) are worked in a small way by local capital, as are the deposits near Platina and Fifield. The Tasmania deposits are controlled by local capital.
As to Canada, it is understood that a large part of the gravel area of the Tulameen River, near Princeton, British Columbia, is controlled by American capital. A few claims on the upper Tulameen are controlled by Canadian capital. An American company has recently been organized for the purpose of exploiting certain prospective areas in the Barkersville region, in north-central British Columbia, and it is understood that Canadian capital has rather extensive holdings on the Peace River, in northern British Columbia, which are reported to contain considerable quantities of platinum. The nickel deposits of Ontario, which have a considerable prospective value as producers of both platinum and palladium, are operated by the Mond Nickel Co., under British control, and the International Nickel Co., under American control. However, the Canadian government is regulating the operations of both of these companies.
—It is a peculiar fact that while the larger part of the Russian crude platinum is sold through a French company, nevertheless England has refined the greater part of the output of Russia. The Johnson Matthey Co., of London, is the largest platinum refiner in England. Prior to the war this company is said to have refined about 70 per cent. of the Russian platinum production.
In Germany the chief platinum refiner is W. C. Heraeus, of Hanau. This company is said to be owned chiefly by Dr. Heraeus and the estate of Heinrich Heraeus. The firm of F. Eisennad & Co., at Offenbach, a small platinum refiner, was acquired by Heraeus’ interests just prior to the war. G. Seibert, of Hanau, also refines platinum, the operations being financed by the Seibert Bros., and the Deutsche Gold und Silberscheidenanstalt,[519] of Frankfort. According to Russian figures, about 25 per cent. of the Russian output was refined by Germany and presumably a large part of the work was done by Heraeus. Heraeus interests without doubt predominate the platinum-refining industry of Germany.
The chief platinum refiner of France is Quenessen, de Belmont, Legendre et Cie., which is controlled by the estate of MM. Desmoutis and Lamaire. Other small refineries are the Lyon Allemand, the Credit Lyonnais, Herique Marrett & Bonnin, and Hesse Fils, all of Paris. Apparently the first company is the controlling factor in the French industry.
In the United States the platinum industry is controlled by Baker & Co., American Platinum Works, Irvington Smelting & Refining Works, Hanovia Chemical Co., and Charles Englehard. There are several independent platinum refiners in the United States, though their combined output is less than a quarter of the domestic industry. These are J. Bishop & Co., Malvern, Pennsylvania; Wilson Co., Newark, New Jersey; Belais & Cohn, New York City; Kastenhuber & Lehrfeld, New York City, and Goldsmith Bros., Smelting & Refining Co., New York City and Chicago, which are operated financially by American capital. The Rossler & Hasslacher Chemical Co., of New York City, also refines some platinum.
Prior to the war there was more or less interlocking of the interests of Johnson and Matthey of London, Quenessen of Paris, Heraeus of Hanau, and Baker & Co. of New York. It is generally conceded that prior to the war Heraeus actually controlled the American interests now dominated by Englehard. However, when war was declared these various companies, by interchange of their stock, were separated, so that it now appears that German money is no longer interested in the English, French, or American platinum industry. It is probable, however, that both the English and French companies still hold stock in the American company, though the control of the American interests is now held by Charles Englehard through ownership of the majority of the stock of the companies mentioned above.
It is reported that there were two government-owned platinum refineries in Russia prior to the war, though apparently they handle only a very small quantity of the platinum produced in Russia and no platinum from any foreign countries.
As explained above, owing to the location of the chief platinum-producing regions, Russia has been the source of practically all of the world’s platinum, though commercially the French controlled the marketing of the bulk of the Russian output. Since 1914 practically no platinum has[520] been exported and what little did get out came mostly to the United States. The situation of the various countries can be summarized as follows:
Russia normally used little of her own platinum, exporting it to England, Germany, and France. The country had almost no platinum-refining capacity, the industry being controlled by French and Russian capital with more or less German influence. Since the war the platinum mines have not been extensively worked and in fact their production has decreased greatly. Any accumulated stocks of platinum that may have been in Russia probably found their way into Germany and into Allied lands.
Before the war Germany refined about 25 per cent. of the Russian production of platinum; she has no deposits within her own territory. She had built up great chemical and electrical industries, which required large stocks of platinum, and probably was in a fair position with regard to the metal when war was declared. It seems probable that there is a shortage of platinum in Germany at present, for any great expansion of either chemical or electrical industries.
France through her control of the bulk of the Russian output was in position to have accumulated considerable stocks of platinum, and that she did so is indicated by the fact that the government did not undertake any regulation of the platinum industry until early in 1918, and it does not appear that any great expansion of the chief industries requiring platinum was necessitated.
About 70 per cent. of the Russian, probably half of the Colombian, and all of the Australian and Indian platinum was sold in England prior to the war. It is believed that not all of this was refined in England, for considerable amounts of crude platinum were exported from England to the United States; however, large stocks of the platinum metals were on hand in England when war was declared. England had to build a great chemical industry during the war, and quickly used what reserves she had, so that the government early in the war saw the necessity of controlling the use of platinum metals.
The United States has been and will continue to be dependent on foreign platinum. At present all of the Colombian platinum is coming to this country. When we entered the war the stocks of platinum in the United States were about 50 per cent. of the normal, and as we had to build large chemical and electrical industries, those stocks were rapidly exhausted.
Colombia has no platinum refineries; apparently she has use for none of the output of her mines. Before the war her crude platinum was shipped to England and the United States for refining, but at present it is all coming to the United States.
[521]
About 90 per cent. of the crude platinum produced annually has come from the Ural Mountains, Russia. The deposits of next importance are situated in Colombia, South America. Small amounts are produced in Canada (chiefly as a by-product in the refining of nickel ore), in New South Wales, Tasmania, the United States, Dutch East Indies, India and Spain.
The political control of the platinum deposits of the world corresponds to geographical distribution. Russia and Colombia control the principal deposits, and the United States, Great Britain, Holland, and Spain the minor ones.
Prior to the war, a French company had extensive holdings in Russia, and in addition had contracts with two Russian platinum producers for their entire output. These contracts were canceled shortly after the declaration of war. The remaining deposits of the Russian fields are controlled by two independent Russian companies and by a large number of small miners. At present the peasants and miners are virtually in control of the mines and the owners have little to do with their operation or management.
The principal platinum-bearing placers of Colombia are controlled by American interests. A British company was organized recently to develop holdings in the upper San Juan drainage. With the exception of a few of the large dredges of California, owned in part by British capital, the platinum deposits of the United States are owned by American citizens. The platinum deposits of Australia are probably held for the most part by British capital.
American capital controls a large part of the platinum gravel area of British Columbia. A few claims are controlled by Canadians. The companies operating the nickel deposits of Ontario, from which platinum is produced as a by-product, are American and British.
About 75 per cent. of the Russian platinum has been refined in England and most of the remainder in Germany. Before the war there was an interlocking of the interests of the platinum refiners of the United States, Germany and Great Britain, which has been broken as far as Germany is concerned.
[522]
Let us glance over the preceding studies one by one and see what salient features each one contains.
is of the utmost present importance and its future importance will be even greater. Recently 98 per cent. of the world’s production has been contributed by the following countries in this general order of importance: The United States, Russia, Mexico, Dutch East Indies, Roumania, India, Persia, and Galicia. It is believed that the region around the Caribbean Sea and the Gulf of Mexico will be of increasing importance, as will also the Persian and Mesopotamian fields.
United States capital is supreme in the commercial control of the petroleum industry in the Western Hemisphere; while British and British-Dutch interests easily dominate the petroleum situation in the Eastern Hemisphere.
Commercial control of petroleum is determined mainly through ownership by operating companies of lands, leases, or concessions. State ownership is rare, although in Argentina the petroleum industry is owned and operated by the state. The British government controls by direct ownership of a majority of the voting stock, the Anglo-Persian Oil Co., which gives it a monopoly of the Persian field, through the concession of an area of 500 square miles from Persia to the company, and closes the field to the enterprise of the United States or other nations; moreover through ramifications of this company, the British government is extending its hold to other parts of the world.
In the United States the commercial control of the petroleum industry is in the hands of the “Standard Oil Group.” British and British-Dutch companies in the United States control a production of about 11,000,000 barrels a year, out of a total of 335,000,000. In the important region of Mexico, which now takes second place in production, the commercial control is entirely in the hands of foreigners: American interests control 65 per cent. and British and Dutch interests 32 per cent.
[523]
In the Eastern Hemisphere, the productive field of the Dutch East Indies is under absolute control of British-Dutch interests, the Royal Dutch-Shell Syndicate. Prospecting licenses and concessions are granted only to Dutch subjects and to Dutch companies, and this, with the economic monopoly of the controlling British-Dutch interests, prevents foreign enterprise.
The absolute and exclusive control of the great oil fields of Persia and Mesopotamia by the British government will be confirmed and extended by the extension of the British Empire over those portions of the Turkish Empire which she won by force of arms.
In Russia the commercial control of the great petroleum industry seems to be British, the predominant interests being British, Franco-British, and British-Dutch (Royal Dutch-Shell Syndicate). The principal producing areas in Russia are or were till recently under British military control.
The production of India (Burma) is entirely in British hands.
“The general policy of the British Empire seems to be to control all oil development and restrict operations by foreign capital.” Such restrictions by government regulations exist in Australia, Canada, India, Barbados, British Guiana, British Honduras, Trinidad and other colonies.
In the oil industry, then, we have a remarkable and striking division of the world’s wealth between the two great Anglo-Saxon nations, America and Great Britain. No mineral lends itself so readily as oil to transportation and hence to commercial control. According to the present production, American interests are largely in excess. However, the British control of the great fields of the Eastern Hemisphere, many of them only partly developed, together with her growing hold in the Western Hemisphere, indicates the likelihood that the British grip of the world’s oil resources and production may in the future become predominant.
Striking phases of the situation are that in the case of Great Britain the government and the oil monopolies are united, so that to all intents and purposes the control being obtained is by the British government direct; while in the United States the control is in the hands of purely commercial interests, operating without the control, assistance, or sympathy of the government. American companies may not own and operate oil lands in the British Empire, in the French possessions, or the Dutch colonies, but there are no American restrictions on foreign ownership or operation.
The policy of Great Britain, furnishing her petroleum and oil bunkering stations all over the world, and assuring her control of the seas, will further immensely increase her already extensive world domination.
The United States has no such program of imperial expansion, but[524] she has her Monroe Doctrine, which is to a mild degree an assumed protectorate over the Western Hemisphere.
—Next let us take up coal, among the most important of all minerals—source of power, light, and heat, and smelter of iron and other metals. Here again, as in oil, we find the United States wonderfully endowed by nature. She is credited with reserves of 3,527,000 million tons out of a total 7,909,000 million in the world, or practically half of the whole world’s supply. As the world’s coal reserves are large, the high-grade varieties, so situated as to have cheap transportation, are of most immediate importance. Great Britain has such coal close to seaboard, and so, until the war, controlled the export trade all over the world. The industries of America leave her little coal for export, and her coal is farther from seaboard. The efforts of Germany before the war to build up a coal-export trade were hindered by the long rail haul; and these deposits are now being handed over to France. Besides France, Great Britain, and the United States, only Canada, Australia, and China have sufficient reserves for extensive export trade. Of these countries, China is the one most likely to increase exports, on account of nearness to the coast, and good quality of coal.
Although the coals of the United States are not so close to the coast as in England, they not only constitute by far the largest reserves, as above stated, but are also most immediately available, owing to their shallow depth and the good railway transportation facilities.
No natural substance is so universally used, and so necessary to every individual, as coal, and hence every individual feels a natural right to it, and believes that it should be available at a minimum cost. This has resulted in several countries in the nationalization of the coal industry, as in parts of Chile, Bulgaria, Prussia, and Australia. In other countries, as in parts of the United States, the government retains the ownership of coal lands, leasing them to private operators. In England the present conditions point toward the nationalization of the coal industry. In France the coal lands belong to the government, which gives concessions for their operation, and receives a royalty or rental and a percentage of the net earnings of the operator.
Although the United States is pre-eminent among the world’s nationalities as regards coal, England has the advantage of having adequate supplies scattered all over the world, in her colonies of Australia, Canada, India, New Zealand, South Africa, Rhodesia, and Borneo.
Unlike petroleum, coal is a mineral which does not lend itself readily to control by commercial combination. The mining and marketing of coal is a simple matter, requiring relatively little skill or equipment, so that it is a business open to everybody; and the vast extent of coal lands assures a multitude of owners. Therefore the effect of the control of coal on the world’s commerce and history is almost entirely a matter of political[525] control. Organization among producers exists in various countries, as in the case of the anthracite industry of the United States, but this does not as a rule extend to a central ownership, nor does it usually extend to foreign countries.
In coal, then, as well as in petroleum, we find the two dominating nations are the great Anglo-Saxon powers, England and the United States. The United States mines about 40 per cent., or two-fifths of the world’s annual production, while the British Isles have produced one-fifth of the production, making them second only to the United States. In neither case have the respective governments in the past attempted to control the mining and the sale of coal, but in England, at least, it is likely that some form of joint control, participated in by the government and the miners, will come.
The methods of mining necessary for maximum production of British coal mines during the war resulted in putting the mines in such poor condition that it will be a year or two before they can supply all of the former British export trade. The demands of British workmen for shorter hours (resulting in decreased production) will hinder still further a resumption of large exports. One of the important phases of this, to England and America alike, is the South American trade. England has always supplied this market, but the United States will probably do so for the present, and should take care to do so if she desires South American trade, and on the commercial theory of the Monroe Doctrine. Up till recently, the United States has exported by sea only about 4 per cent. of her production, whereas England sent out 25 per cent. Our own expanding industries have provided an ample market.
Aside from America and England, there is no dominating factor in the future control of the coal industry in the lands surrounding the Atlantic Ocean. Germany was a strong factor before the war, but the loss to France of the Saar coal district, and the possible loss, to Poland, of the Dombrova field, in Silesia, will deprive her of her importance; and the division among several nations of these resources will prevent any one of them from becoming a world’s factor in the coal trade.
In the lands about the Pacific Ocean, however, the most important future factor is the coal fields of China. No country except the United States has larger reserves of high-grade coal ready for development and not far from ocean transport. It is likely that the Japanese may attempt this development and the fostering of an export trade in the Orient. The high-grade coals of the United States are remote from the Pacific Ocean, and could only be available for Pacific trade by the long route of the Panama Canal. It is not unlikely, therefore, that Chinese coal may in the future be supplied to our own Pacific ports by the Japanese at a less price than American coal can be put there, and that through this development Japan may be able to dominate the Pacific trade, as England has dominated that of the Atlantic and the Pacific in the past.
[526]
—The iron supplies for the world’s consumption have been obtained principally from four countries: the United States, Germany, France, and Great Britain. More than one-third of the total production has come from the United States, and of the American output about 85 per cent. has come from the Lake Superior district, which alone produces annually over 30 per cent. of the world’s total. Next in importance to the United States have been Germany and France, and about 80 per cent. of the production of these two countries has come from the Lorraine fields on the border. The annual output of these fields has been 25 per cent. of the world’s production, or nearly as much as the Lake Superior district.
Linked with the coal industry as it is, no world-wide or even national monopolies of iron ore have been attempted. The greatest single commercial organization in the world is the United States Steel Corporation, with a total annual capacity (in 1913) of over 17 million tons of pig iron, or about half of the total American production. But this organization is not a monopoly, and there are a large number of powerful independent companies. In France and Germany no dominating organizations have been noted. In England up to the time of the war the iron industry was controlled by middlemen, and the manufacturers were insufficiently organized. To meet this condition (page 86) the British Board of Trade Committee advised a consolidation of iron interests.
Extension of commercial control by the dominant iron-producing nations to the ore reserves and to the iron industry of foreign countries, so establishing that commercial penumbra of empire which is so apt to deepen into actual sovereignty, is, however, much more marked in the case of iron than of coal, though less significant than in the case of oil. The control by ownership of great iron fields in South America by England and the United States, and the extension of Japanese control of iron-ore reserves in China, are the most significant features of this situation. In France, even before the war, Germany controlled over one-third of the iron and steel business. With the passing of German Lorraine to France, it is likely that much German capital will remain.
Japan has an iron and steel industry which, although small as compared with that of the United States, Germany, and Great Britain, and the other leading iron and steel manufacturing countries, is rapidly expanding. Blast furnaces, steel-making furnaces, and steel mills are being erected in Japan and in Korea, Manchuria, and China by Japanese interests. Japan is still very far from supplying her own consumption of iron and steel, which is a million and a half tons annually.
In brief, as regards the world’s iron and steel, the United States has a greatly preponderant position, which it will tend to increase, with the desirable[527] tendency of drawing North and South America more closely together. In Europe, France and Germany are oddly yoked in the control of the second greatest steel industry in the world. In the future great arena of the Pacific, Japan is patiently building up her steel industry, with far-reaching Oriental vision. Coal mines and an iron blast furnace are included in the German “rights” recently acquired by Japan. Will Japan return Shantung? Did Germany return Alsace Lorraine? In her forward-looking plans, Japan has two national rivals—England and America. She has a vast fertile field to work in, except for these (especially England)—all of eastern Asia. She has a great disorganized nation which is no longer a rival—but a field whereon to feed and grow stronger—Siberia and Russia.
In the train of steel, and next after the problems of coal and iron, come a number of less-known and less-abundant metals—the ferro-alloys, metals that alloy with iron to make steels of special hardness or toughness, or with some other special quality. Relatively inconspicuous as they are, they are indispensable in the industries.
—Manganese is far more than a ferro-alloy. It is essential in the manufacture of all open-hearth process and Bessemer process steel, which make up 99 per cent. of the total United States production, for it acts as a remover of the carbon which makes the difference in quality between steel and cast iron. For this purpose it is mixed with the iron in the form of alloys. One of these is high in manganese—ferromanganese—and one low—spiegeleisen.
The principal manganese fields are those of Russia, India, and Brazil, which are so large and readily available for exploitation and transportation to markets that there is little prospect that they will be displaced as the principal sources of the world’s supply for many years. In contrast with the situation regarding other important minerals, most manganese deposits throughout the world are owned by residents of the countries in which they occur. This is due to the superficial and irregular character of the oxide deposits (the only ones as a rule of high enough grade to find a market) and the simple nature of the mining and washing of ores, which does not require much capital.
The United States is poorly provided with high-grade manganese ores, and hence has always been and will always be a heavy importer. Previous to the war, the supplies were mostly drawn from Russia and India; and during the war from Brazil, in addition to an increased domestic production under the stimulus of high war prices. England, France, and Germany—in fact the whole industrial world—have the same sources of supply. There is little necessity of sharp competition, leading to commercial combinations, or of strict governmental control, since the productive capacity of the principal deposits is very large, and far exceeds the world’s demand for steel making.
[528]
—Next in importance in the ferro-alloy group of metals is chromium, found in nature on a commercial scale only as the oxide chromite. Chrome is used extensively in the steel industry and the leather industry—in the former for making a specially tough steel (and also a refractory lining for iron furnaces); in the latter, for tanning.
Chromite is found in many countries, but in most (as in the United States and Canada) in small and scattered deposits, easily exhausted. The largest and most important sources of supply are in the French colony of New Caledonia, in the South Pacific; in Rhodesia, in Africa; in Asia Minor; and in the Ural Mountains, Russia. Up to 1830 the Ural region supplied the world’s chromium; from 1830 to 1870, the Eastern United States (Maryland and Pennsylvania) became the chief source; from 1870 to about 1900 the scene of chief activity shifted to Asia Minor; and since then New Caledonian and Rhodesian ores have occupied the world’s markets. New Caledonian ore is produced with cheap labor, and the deposits are near the coast; and the Rhodesian deposits are large and rich. High prices during the war, due to lack of shipping, brought about a great increase of production in the Pacific States of the United States; but with a return to normal conditions this region cannot survive competition, unless especially protected by legislation.
In normal times, the United States consumes more than one-third of the world’s annual consumption of chromite, but depends upon foreign sources—Rhodesia and New Caledonia. During the war, deposits of limited extent in Brazil and Cuba were drawn on, as well as Canadian and domestic ores. So far as developed, however, the Western Hemisphere is relatively poor in chromite deposits. The chrome industry in the United States is highly centralized, the Electrometallurgical Company having an almost absolute monopoly of the ferrochrome industry, and being probably the largest producers of ferrochrome in the world, and the Mutual Chemical Company having a great preponderance in the chemical chrome industry.
The chromite supply of the world is therefore at present essentially a monopoly controlled by British-French capital, and the great supplies occur in the colonies of England and France.
—The position of nickel is rather unusual, in that workable deposits are rarely met with, and deposits of great importance are confined to a few places. The only really commercially important deposits are those of Sudbury, in Canada, and of New Caledonia; although small deposits of workable ore have been mined in Norway, and nickeliferous and chromiferous iron ore occurs in Cuba.
The deposits of Sudbury are relatively far more important than those of any other field. Therefore Great Britain (through Canada) possesses by all means the largest and most important nickel deposits, amounting practically to an exclusive control. Previously American capital exerted[529] a dominant commercial control over the nickel industry, through its ownership of the largest ore reserves and its control of smelting and refining plants in the United States. One of these American companies has also the second largest holdings in New Caledonia. The British government plainly has taken means to overcome this commercial domination. A large company has gone into business at Sudbury, in which the British government has the controlling interest. The government has also brought about the transfer of the refining operations of the International Nickel Company from New Jersey to Ontario, so that the entire industry will be confined to Canada.
—The greatest tungsten-producing region is that of eastern Asia; the region of the United States and Mexico second; that of Bolivia and neighboring countries in South America third; and fourth comes the province of Portugal, Spain, and Italy. There seem to be no very large and concentrated tungsten deposits; and nearly all of those worked give signs of being easily exhausted. There may be therefore a world tungsten shortage in the future. Possibly Bolivia will prove to be the most durable field.
As to the commercial control, it is entirely in British hands, and this through the active policy of the British government. Actual control is obtained through the ferrotungsten makers, to whom the ores go for treatment. On this basis the commercial control in 1917 was: British 14,606 tons; American 9,479 tons; Japanese 1,165 tons; French 1,057 tons; and Germany 360 tons.
American capital controls the tungsten deposits within its own borders and in Mexico, and is largely interested in Bolivia and China. Before the war Germany controlled probably half the tungsten output, the other half being divided among the United States, England and France. At the present time, the control through ownership of mines and smelters is as follows: Great Britain 54 per cent.; United States 35 per cent.; Japan 4 per cent.; France 4 per cent.; and Germany 1 per cent.
—Vanadium is an important ferro-alloy metal. Vanadium steel has great toughness and torsional strength, and is used in automobile parts, gun barrels, and the like. Chromium-vanadium steels have an extensive market.
The largest and most important deposits of vanadium in the world are in Peru, and until recently were controlled by the American Vanadium Company (an American firm), which has a concession from the Peruvian government. Otherwise the most important deposits are found in southwest Colorado, and were till very lately controlled by the Primos Chemical Company, of Pennsylvania. The American Vanadium Company had an absolute world monopoly of vanadium products and ferrovanadium, until the entrance into the field of the Primos company.[530] Quite recently the holdings of both these companies have been taken over by the Vanadium Corporation, allied to the Bethlehem Steel Corporation.
—Antimony has a relatively restricted use in peace-time, but war creates (for the manufacture of shrapnel) a vastly increased demand. Under normal circumstances the supply is far in excess of the demand. China has long been the most important source of supply, and is likely so to continue. France and Algeria are also producers, as is Mexico; and other countries produce under the stimulus of high prices. The United States, as well as Canada, has relatively small reserves and normally small production. In the early part of the European war, however, in 1915 and 1916, countries like Bolivia, Mexico, Australia, the United States, Peru, Burma, and Spain contributed important amounts; but none of these will be important factors at the usual low prices.
Prior to the war, England was the chief antimony-smelting center of the world. Ores from all over the world were there treated, and the British brands were considered purer than others, and virtually monopolized the world’s markets, including those of the United States. During the great demand in 1915 and 1916, British interests completely controlled the Bolivian industry. Until 1914 one of the principal English companies held contracts for the production of the Wah Chang Company, the most important antimony producers in China; but in 1914 this company established an independent selling agency in the United States. This tends to transfer the control of the antimony market from England to China. With all her vast mineral resources, China has been able to obtain an important position in the world’s markets with regard to but few metals. Of these antimony is the most striking example. Since 1908 over 50 per cent. of the world’s total antimony production has come from China.
—The use of molybdenum in steel making is as yet almost in the experimental stage, but it is likely to become important. It is valuable in electric work.
Up to about 1916 practically all the molybdenum ore (molybdenite, a sulphide of molybdenum) came from Australia and Norway. Shortly after the opening of the war, the molybdenite in Canada became prominent; and later the United States came to the fore as a producer. At present the United States can probably produce as much if not more molybdenum than all the rest of the world put together, principally from the great newly discovered deposits at Climax, Colorado. Before this development, Great Britain was the largest producer, in Australia and Canada. Both the British and the Canadian governments have been much interested in the development of the Canadian molybdenum, and the Canadian government has built a mill for the concentration of the ores. Prior to the war, the German-controlled American Metal Company, a branch of the German “metal octopus,” obtained, through a[531] subsidiary, a large share in the control of the Climax deposits; and the Primos Chemical Company, which had strong German connections before the war, produced ferromolybdenum from ore from its own mine at Empire, Colorado. This, together with the great interest taken by Germany and German capital in molybdenum elsewhere, led to the rumor of attempted German control of American molybdenum.
—Uranium is valuable for the manufacture of special steel, although only used in small quantities. It is of extraordinary interest on account of its association with radium, both being obtained principally from the minerals carnotite and uraninite (including pitchblende). Radium is used in medicine, and for luminous paint. The only regions which have yet produced large amounts of radium and uranium on a commercial scale are in the United States and Austria. At the present time the United States is producing several times as much as all other countries combined.
—Zirconium is used in electric lighting, and experiments have been made with zirconium steel. During the war it was at one time thought to be of unusual value as a ferro-alloy. Zirconium occurs in nature as the mixed oxide and silicate, baddeleyite, and as the silicate zircon. The baddeleyite deposits, having a higher percentage of zirconium, will probably become the chief source of the metal. It occurs in commercial quantities only in Brazil. Zircon deposits are found in Brazil, and also in India; and a deposit of minor note occurs in the United States (Florida).
—With zirconium must be considered monazite, a mineral which is the source of thorium and mesothorium. Thorium nitrate is used in the manufacture of Welsbach mantles for gas burners; mesothorium is a by-product of its manufacture from monazite, and is a radioactive substance used as a substitute for radium in making luminous paints and for therapeutic purposes. The zircon minerals and monazite typically occur together in river or beach sands. Like the zirconium minerals, monazite comes mainly from Brazil and India; although it has in the past been mined successfully in the United States, the industry is now extinct.
The thorium nitrate industry of the United States is closely controlled by two companies, the American Welsbach Company, and the Lindsay Light Company. During the war they furnished thorium nitrate also to England and France, thus exercising a world-wide control.
—The United States stands out predominantly as the world’s great copper producer, producing in 1917 60 per cent. of the world’s output. No other country produces one-sixth as much as the United States. American capital controls (in part through control of refining) 78 per cent. of the world’s production.
[532]
Germany has been one of the largest consumers of copper, though not a large producer. Because of this, German interests have in the past secured a considerable control over copper supplies, as well as those of lead, zinc, and other metals, through refining and selling contracts with mining companies. Such control does not as a rule extend to ownership of mines or smelters. Thus for many years companies affiliated with the great German Metal Combine (Metallgeselschaft) were influential in the copper business of the United States. There were three of these companies in the United States, the American Metal Company, L. Vogelstein & Co., and Beer-Sondheimer & Co. Recently the first two have consolidated; and all were Americanized during the war.
The commercial control of the copper in the world, as based on ownership of mines, is, in even figures: United States capital, 69 per cent. (entirely in the Western Hemisphere); British capital, 13 per cent. (in both hemispheres, but mainly in the Eastern); Japanese, 8 per cent. (entirely domestic); German, 6 per cent.; and French, 2 per cent. It will be noted that of the present production three-quarters comes from the Western Hemisphere (North and South America) and only one-quarter from the rest of the world. It is probable that this is a fair index of the relative wealth. The future production of South America will probably increase more rapidly than that of North America, which was earlier developed. It is necessary for the permanent control of the copper situation by the United States that American capital should continue to be foremost in the development of South America.
—The United States is the largest producer of lead in the world and has large resources. Next to the United States, in the order named, come Australia, Spain, Germany, and Mexico. Three powers—the United States, British Empire, and Spain—produce 76 per cent. of the total; and of these the United States and Great Britain produce 60 per cent.
The most striking feature about the lead industry is the fact that as the German system of far-reaching commercial control under government auspices—through smelting, refining, and selling—was destroyed, this system was at once adopted by the British and French. In other words, they found that the German plan had been so effective that they not only blocked it permanently so far as their own countries were concerned, but organized similar commercial-political combinations which should not only take care of all their own lead business, but, like the German organization, should reach out into other countries. The German combination still remains active outside the territory of the former Allies.
Of all the great lead-producing powers the United States is the only one which does not possess a government-controlled lead monopoly. Threatened commercial world monopolies of lead, as of other minerals, have therefore, through the revival of nationalistic spirit due to the war,[533] given place to national-commercial monopolies by three powers (Germany, France, and Great Britain), each intended to become as world-wide as possible, and thus competitive with each other and with the purely commercial organizations of the American lead industry. In England this movement has taken the form of a British Metals Corporation (covering not only lead but other metals). The British Treasury is represented on the Board of Directors.
In France, the nationalist movement has resulted in the formation of consortiums or trade monopolies for each industry, organized under government auspices. That of the mineral industry is the Société Minerais et Metaux. The official announcement states that this society is organized under the auspices of the French government, in order to group the French metal producers, operating both at home and abroad, into a co-operative association for the purchase and sale of metallurgical products.
In America, the principal commercial factor is the American Smelting & Refining Co., dominating the market through its control of reduction plants, although it controls directly only one-third the production.
—Zinc and lead are commonly associated in mineral deposits, so that their geological and geographical distribution is nearly identical. Of the world’s production of zinc, the United States produces 35 per cent., Germany 25 per cent., Australia 15 per cent., and Italy 5 per cent.
Up to the outbreak of the war in 1914, the position of zinc was extreme among the metals, in that political control or state sovereignty exercised only a minor effect upon the industry. “Economic factors,” says our author, “made ineffective any control not international in scope. A very large percentage of the zinc ores of the world were transported from the country of production to another for treatment, in some cases even being re-exported.” During the war, however, political control was largely invoked to strengthen and restore commercial control to the chief belligerent nations. This movement was particularly marked in the British Empire, where there now exists, as above noted in the paragraphs discussing lead, a joint political and commercial control. Alien interests were eliminated by government action, and the government retained a share in the control through interests in marketing organizations or financial participation in treatment works.
In the zinc industry, as in that of its closely associated metals, copper and lead, the ownership or control of reduction plants, and more particularly marketing organizations, have been more important in determining commercial control than state sovereignty or commercial ownership of mines. In recent years the marketing organizations became world-wide and completely dominated the industry. The ambition of German commercial interests to control the metal markets and resources of the world was more nearly realized in the case of zinc than of any other metal.
[534]
In France, as noted above in the case of lead, a government-controlled metal marketing organization has been formed for the same purpose—protection and advantage in competition. However, British domination of the European zinc industry seems certain. Only the American industry remains untouched by close organization under government auspices. Should Germany lose Silesia, she will probably become a small factor in the zinc industry. With so many doors closed in her face by the British and French political and commercial combinations, there should be governmental precautions taken by the American Government that she should not re-establish herself in the United States, nor so far as possible (following out to its logical conclusion the Monroe Doctrine) in the rest of North and South America.
Note that in the zinc industry, as well as in every other industry, Japan is rapidly expanding, and having reached the limit of her own resources, her field of growth is in Korea, China, and Siberia. Japan’s present zinc-smelting capacity is greater than her domestic consumption, and much greater than the domestic ore supply; and ore is imported from China, Siberia, Indo-China, and Australia.
—Tin belongs to a group of minerals that are classifiable together by the fact that they are not of widespread distribution, but are found in really commercial quantity only on a few spots of the globe, and yet are absolutely necessary for our industrial civilization. Such also are chromium, platinum, potash salts, nitrates, and nickel. Of this political-commercial group, tin is an important member. It is noteworthy of this group that the United States is not the lucky holder of the first prize in any of these cases. In the case of chromium, it is mainly the French and British colonies, of platinum it is Russia and Colombia, of potash salts it is Germany, of nitrates it is Chili, of nickel again the British and French colonies, and in the case of tin it is southeastern Asia and Bolivia.
The United States produces less than one-fifth of 1 per cent. of its requirements, and its control of foreign tin resources through mine ownership is negligible. On the other hand, the United States consumes over half the tin of the world, and is the largest manufacturer and distributor of tin products. The tin-mining and smelting industry of the world is dominated by Great Britain.
Tin is used in the manufacture of tin plate, in solder, brass, and many other essential uses, and no satisfactory substitute is available. In war as well as peace, tin cans are as necessary as rifles. Aluminum is the most likely possible substitute for tin in containers, and the United States controls the aluminum industry. About 68 per cent. of the tin is produced at present from southeastern Asia and neighboring islands, 21 per cent. from Bolivia, 4 per cent. from Nigeria and South Africa, and 3 per cent. from England. The Bolivian production will probably tend to increase.
[535]
—Mercury, or quicksilver, is a mineral of some importance, although by far not in the class of the last four discussed above. It is useful for drugs and chemicals; as a detonator for high explosives; as a pigment; for treating gold and silver ores; and for many other uses. The greatest quicksilver deposits in the world are in Spain. The United States comes second. The important Idria mine, near Trieste, formerly Austrian, but at last accounts in possession of Italy, takes third place. The production from the rest of the world is small. Spain, Italy, and the United States, therefore, divide the production and the control through state sovereignty. The great mine of Spain, the Almaden (the greatest in the world), is also owned and worked by the Spanish government. The Spanish government contracts, on the basis of competitive proposals, with the successful bidder for the sale of quicksilver for a term of ten years. For a number of successive periods, the contract has been awarded to the Rothschilds of London. By this arrangement the market is controlled in London; and during the war the sale was taken over by the British government. The control of the marketing of the product of this mine enables those in control to fix the price of quicksilver in the world’s markets.
—An important metal at present, and one bound to be eventually still more important, is aluminum. While one of the principal constituents of all rocks, in the form of silicates, its release from that combination is so difficult that it has not been solved on a commercial scale. Since there is much more aluminum than iron in the earth’s compounds, however, there will never be a shortage, if cost is disregarded. Commercial aluminum is manufactured from the oxide, bauxite. Bauxite is also used directly as an abrasive and also as a refractory. The largest bauxite deposits are controlled politically by the United States and France, with the British Empire in a favorable prospective position. The aluminum works of the world are controlled by Great Britain, France, and Germany, and also Switzerland, Italy, and Norway. The aluminum industry of the United States and Canada is practically in the hands of one company, the Aluminum Company of America, which also holds interests in South America and other countries. The French producers of aluminum have effected a central organization through the incorporation of a selling company, L’Aluminium Française. The British Aluminium Company is the sole producer in England, and controls the Irish deposits.
—Abrasives are essential and important in the industries. Chief, perhaps, in the group of natural abrasives are emery and corundum, which are superior in hardness to other abrasives such as quartz, tripoli, garnet, and pumice. They are used in grinding and polishing metals—chiefly iron and steel—and glass. Commercially important[536] deposits of emery and corundum are located in the Appalachian region of the United States, on the islands of the Grecian Archipelago (especially Naxos), in Asia Minor, India, Madagascar, and South Africa. There is little control other than that inherent in state sovereignty.
—Magnesite is a mineral of some importance, used mainly in metallurgical operations and as a refractory material for lining furnaces; also for the manufacture of a cement for flooring. Magnesite is not a rare mineral, and deposits are widespread. Productive and commercial deposits are located in the United States (California and Washington), Canada, Mexico (Lower California), Venezuela, Austria, Germany, Spain, Greece, and other countries. Not rare enough to be the subject of great combinations, the interesting international feature of the trade is that which centers in the United States. Until recently large magnesite deposits were not known in the United States, but in the last few years vast deposits have been developed in California and Oregon. Previous to the war, the deposits of Austria were mainly drawn on by consumers in the Eastern United States, and during the war Canadian magnesite was mainly used. American firms own considerable interests in the deposits in Austria, and probably in some of those in Canada, Mexico and Venezuela.
—Graphite is used for crucibles for steel and brass making, for foundry facings, pencils, shoe polish, as a lubricant, etc. Crystalline graphite only is used for crucibles. The supply of this for American consumption was one of the problems of the war. Of the crystalline graphite deposits, it is believed that those of the French colony of Madagascar will, on account of their richness—if competition is free—supplant American and German deposits; the deposits of Ceylon are regarded as on the wane. Amorphous graphite will probably come from Austria, Mexico and Korea. The deposits of the United States are extensive but of low grade.
—Mica is an essential mineral, especially in electrical work. One of the commonest minerals of nearly all rocks, it becomes valuable only when it occurs in crystals or sheets of large size, which are of comparatively rare occurrence, being found only in certain pegmatite dikes. India, Canada, and the United States produce about 98 per cent. of the sheet mica of the world. Brazil, Argentina, and the former German East Africa are becoming important. India produces 65 per cent. of the total world production; the United States only 15 per cent. Brazilian mica is expected to be of much greater importance in the future than in the past, although India will doubtless retain its position as the most important producer.
The British Empire controls 75 per cent. of the sheet-mica production. Before the war, Germany had obtained a large measure of commercial control in Indian mines, and by virtue of her dominant position in the electrical industry, threatened to control the mica market of the world.[537] The United States is now the largest consumer; but as the important development of the electrical industry in England during the war places it in the position formerly occupied by Germany and Austria, it requires a larger supply of the mica from India, and this may lead to a permanent British control. London is the distributing center for Indian mica, and London prices regulate the market. During the war Indian mica was controlled by the British government and allotted to the Allied nations at fixed prices. A permanent British monopoly of the mica market can probably best be obviated by the development of the Brazilian field by American electrical manufacturers.
—Asbestos is an essential mineral, on account of its incombustibility and insulating qualities, together with its fibrous structure, which enables it to be spun or woven into ropes and fabrics; and on this account it has a wide and varied use. There are mineralogically three kinds of asbestos—chrysotile, crocidolite, and anthophyllite—the last being, as a rule, of non-spinning quality. Chrysotile is the most valuable commercially: crocidolite, or blue asbestos, will not bear high temperature like the other varieties, and on account of its low fusibility is useful for electric welding. Therefore, the main asbestos problem centers about the deposits of high-grade chrysotile, especially as the supplies of anthophyllite asbestos are abundant, and with its restricted uses, ample for an indefinite period. The most important deposits of chrysotile asbestos are in Quebec, Canada, but large deposits are also worked in Russia and Rhodesia.
The United States is by far the largest manufacturer of asbestos products in the world, but produces only a small fraction of the necessary materials. The presence of the deposits in Canada, however, provides the American industry with an ample supply. British companies hold exclusive control of the production of South Africa, Australasia, and Italy: of these, South Africa includes the Rhodesian deposits of chrysotile, which are among the most important in the world. Altogether, the British Empire is in a dominating position, controlling about 88 per cent. of the annual asbestos production of the world and approximately 70 per cent. of the estimated reserves. Canada is in the lead of all countries, supplying about 85 per cent. of the world’s production. Should the British policy as to other mineral industries be carried out in the case of asbestos we may expect action on the part of the British or Canadian government to transfer the center of asbestos manufacture from the United States to Canada or England.
We have above touched on four great groups of minerals—the fuel minerals, the steel and ferro-alloy minerals, the major non-ferrous minerals, and the non-metallic minerals. Next comes a group by itself—the fertilizer minerals or elementary substances, chief among which are[538] phosphate rock, potash, nitrates, and sulphur and sulphuric acid. All of these appear essential to the re-invigorating of the soil as successive crops are removed, and so to securing a permanency of its original productivity.
—Phosphate rock is a natural substance which is used mainly as an ingredient of fertilizers, being finely ground and used directly. Large quantities are also used for making phosphoric acid and phosphorus, the latter being used in matches, etc. Phosphate rock is a bedded or sedimentary deposit containing phosphate of lime; phosphate of lime also occurs as nodules in stream beds. Another type of deposit commonly classed as phosphate rock is the porous coral or limestone of tropical islands, permeated with phosphate leached from guano. The phosphate rock deposits of present commercial importance are located in the United States, Algeria, Tunis, and Egypt, the United States possessing by far the largest reserves. The United States has also the largest industry of production. Politically the principal deposits are controlled by the United States, France (Tunis and Algeria) and Great Britain (Egypt). The commercial control of the deposits of the United States is mainly in the hands of Americans, although German and French interests own some of the hard-rock deposits. The deposits of Algeria and Tunis are controlled by two companies, one British and the other Italian. Germany will be without a source of supply under her own control now that her colonies have been forfeited.
From the above it will be seen that there is no probability of a world control or monopoly of phosphates. The United States is in a position to command the market unless nationalistic legislation in the various countries is enacted to protect and advance their own industries.
—Potash is a most important fertilizer, over 90 per cent. of all potash used being so employed, the remainder going into the manufacture of explosives and glass, and into the chemical industry.
Up to 1914 practically all the world’s supply of potash came from the great natural rock-salt deposits of Stassfurt, Germany. Next in importance come the deposits of Alsace, containing sufficient to supply the world’s present demands for 300 years. Large undeveloped deposits exist in northeastern Spain. Germany made active practical use of her natural potash wealth in erecting a government monopoly, which supplied the world. This advantage was made much of in her plans for further political and commercial conquest, and in the writings of the vainglorious Teutons. In potash, they openly boasted, they had an all-powerful commercial weapon which would oblige other nations to supply in exchange raw materials such as Germany needed, as cotton and copper from the United States. With her political collapse, however, her commercial potash monopoly has also gone. The vast deposits of Alsatian potash have gone to France, and while German potash may still perhaps[539] be produced and sold more cheaply, the Alsatian deposits will act as a check. A commercial combination between the two, and including the Spanish deposits, is, however, not at all out of the question. Potash is one of the commonest elements in the earth, and in the United States there is an abundant supply but it is largely in the form of silicates, and so more difficult and expensive to extract than from the soluble natural salts of Germany.
—Nitrates are essential in an extraordinary degree, in various ways: as fertilizer, and so essential to food and life; as the source of ammonia, and therefore necessary to the modern system of food refrigeration; as an essential ingredient in explosives, and thus indispensable for the national defense. Just as potash is one of the commonest elements of the earth, so nitrogen is one of the commonest elements of the air, of which it constitutes four-fifths. It should not, therefore, be hard to get; but to isolate it and catch it in usable form—in technical terms, to “fix” it—is difficult, slow and expensive. Nature has not done much toward “fixing” nitrogen in her mineral supplies; and although it is constantly being “fixed” in animal and vegetable organisms, it is largely soon returned to the atmosphere as ammonia or in other forms, or, being in the form of soluble salts, is leached from the soil and carried away, either to be transformed again to the atmosphere, or, rarely, to be accumulated under arid conditions by evaporation into mineral deposits. For some hitherto unexplained reason, only in Chili have mineral deposits of importance thus been formed; and the Chilean deposits have till lately supplied the world, giving Chile even a far more exclusive position as regards nitrates than was held by Germany as regards potash; but there has never been any monopolistic control of the Chilean nitrate supply. Besides this mineral source, and the obtaining of nitrogen from the air by fixation, important sources of fertilizer nitrogen are contained in organic matter—refuse vegetable or animal remains, or animal excreta—and also from coal, as a by-product of coke manufacture.
Previous to the war, Germany anticipated being deprived of Chilean nitrate by developing fixation and by-product processes, through which she supplied herself during the war. Other countries have not been so thorough. During the war, the danger of the United States being cut off from supplies of Chilean nitrates by the German submarine campaign, led to the Government projecting and starting four large and expensive plants for nitrogen fixation. They were unfinished when the war closed.
About five-eighths or less of the nitrogen consumed has been from various organic sources, including the by-production from coke making. By-product nitrogen in the United States is estimated at around one-eighth of the entire supply. The remaining three-eighths has been furnished largely by Chilean nitrates.
[540]
—In the group of precious metals, gold is of the most importance, mainly as the time-honored and unreplaceable measure of value and medium of exchange. This position it has sometimes shared with silver, but no country has ever refused to thus recognize gold.
Gold is found all over the world, but the British Empire produces 60 per cent. of it, while the United States produces 20 per cent. Political and commercial control are nearly identical in the case of gold, which is easily reduced to the metal state and thenceforth passes current, requiring no selling agencies. Due to the commerce brought on by the war, the United States now has a larger gold reserve than any other nation.
—“Silver,” says our author, “is used both for money and in the arts, the former use being the more important and more essential. In some countries, especially those producing silver in large amounts, it is the money standard, either alone or with gold. In other countries on a gold standard silver is used for subsidiary coinage. In India and China it is used for the settlement of foreign trade exchange balances.”
In countries with elaborate financial systems, where paper currency is freely accepted, as in the United States and Europe, it is not such an essential money metal; but in other countries it directly replaces and conserves gold. On this account silver is not an article of luxury, but an essential. The coinage of silver increased in Europe as the war progressed, and was essential in bringing supplies from the Far East to the battle fronts. While the normal annual silver production is around 159,000,000 ounces, the demand during 1918 exceeded 500,000,000 ounces. Silver production adds to the stock of money, increases confidence in financial conditions, and furnishes, with and subsidiary to gold, a basis for credit. About half of the world’s silver output is as a by-product of the production of other metals, notably lead and copper; and accordingly the production of silver is largely independent of the price.
Of the world’s total silver production, over 80 per cent. comes from the mines of the Western Hemisphere. For many years Mexico was the leading producer, until all its industries became disorganized by revolution. The United States, always a close second, is now in a leading position. Canada, Central America, and South America are important. In the Eastern Hemisphere, Australia is the leading producer.
The principal silver deposits of the world are controlled politically by the United States, Mexico, and Great Britain, these three controlling 85 per cent. of the total production in 1913.
—Platinum is controlled chiefly by Russia, being produced otherwise in important quantity only in Colombia. American interests predominate in Colombia. Before the war there was an interlocking of the interests of the platinum refiners of the United States, Germany, and Great Britain, with the German influence very marked.
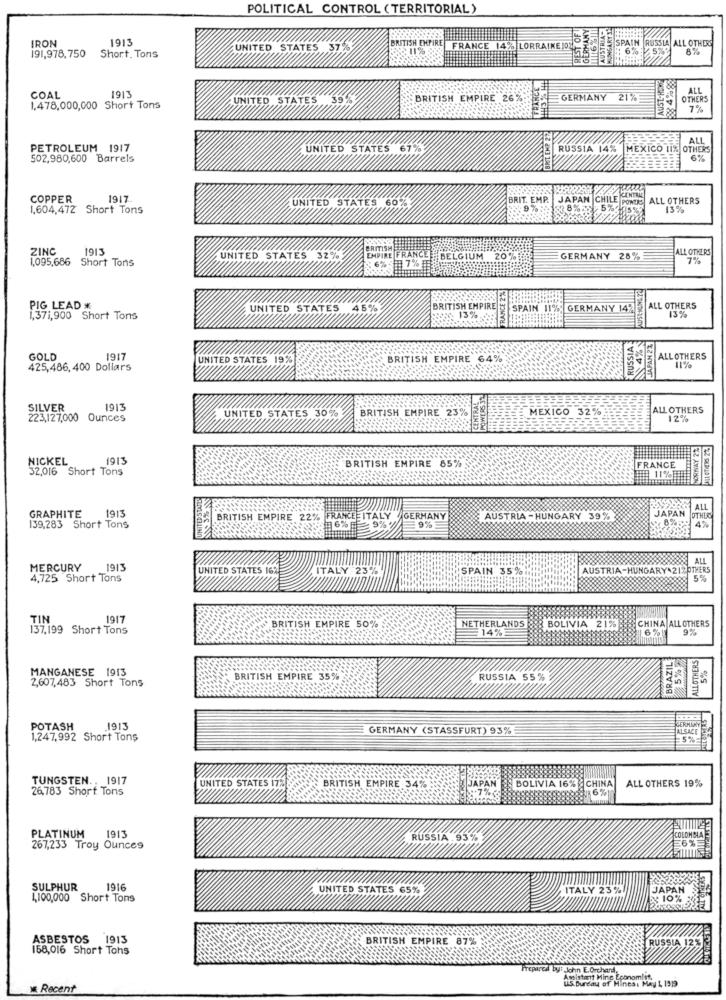
Fig. 23.—The control according to political sovereignty (territorial
ownership) of the world’s mineral production.
(Facing page 540)
POLITICAL CONTROL (TERRITORIAL)
Prepared by: John E. Orchard,
Assistant Mine Economist,
U.S. Bureau of Mines, May 1, 1919
[191] Recent
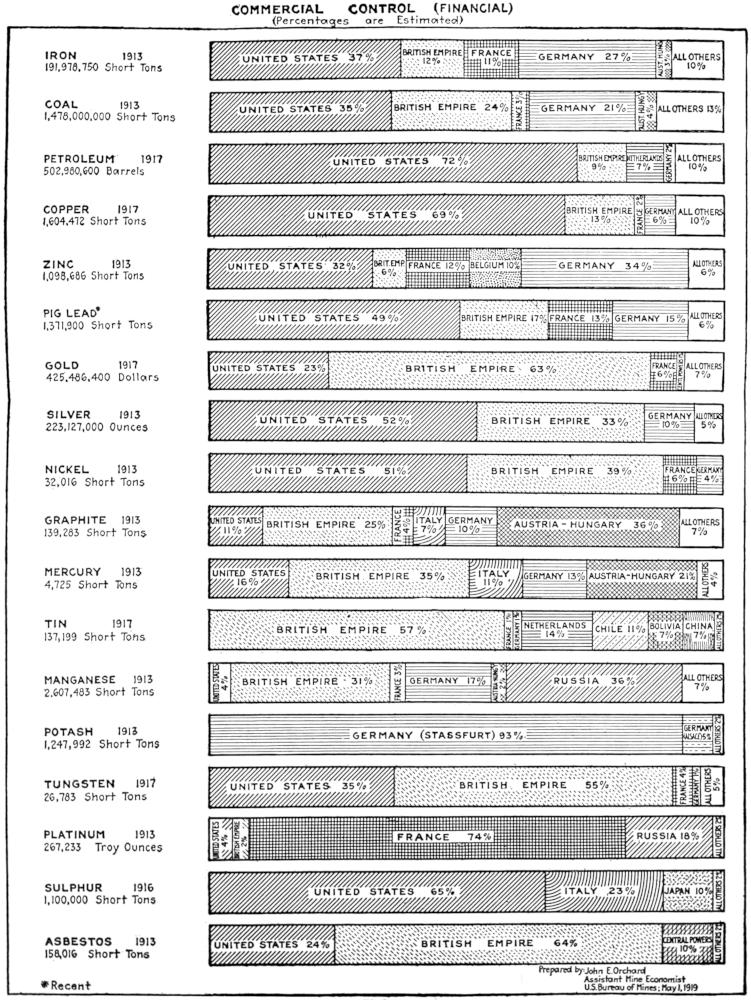
Fig. 24. The control, according to commercial or financial ownership of the world’s mineral production. (Insert following Fig. 23)
COMMERCIAL CONTROL (FINANCIAL)
(Percentages are Estimated)
Prepared by John E. Orchard
Assistant Mine Economist
U.S. Bureau of Mines, May 1, 1919
[192] Recent
[541]
—The answer to our inquiry at the head of this chapter, “Who Owns the Earth?” is therefore answered by these summaries, and is further set forth in the accompanying charts, one showing political (or, rather, in this case, strictly territorial) control, and the other commercial control, Figs. 23 and 24. As based upon the territorial and commercial control of the fundamental minerals, it appears that the earth is owned by the two great Anglo-Saxon nations, the United States and the British Empire: the former by destiny and good fortune and without political plan or policy, in that such a vast store of mineral wealth was found in the great sparsely populated wilderness of America which it occupied; the latter through the imperial policy of Britain, developed through hundreds of years by the need of extending commerce and the flag into far-off lands to supplant the slender resources of its own limited islands. Of the two, the United States is rather in the lead, and possesses and controls more of the world’s mineral wealth than any other nation; but Great Britain is a close second.
Rapid changes occur, however, in these days, and the future must be inspected. The imperial policy of expansion and increasing political control has become a tradition and an instinct with Great Britain; she learned since the loss of her American colonies to give full autonomy to her more intelligent colonies, so that she strengthens her dominion thereby, and persistently goes on her way putting more and more of the earth under the British flag. The wealth and resources of the United States being so far greater than its necessities, foreign problems have resolved into occasional questions of self-protection; and from this condition a directly resulting theory has sprung, of non-interference in the rest of the world. Like China, we declare ourselves apart from the world, and simply ask to be left alone, in consideration of which we agree to leave the world alone. Our Monroe Doctrine as originated is part of that theory—we wanted the world to leave all the Americas alone, but took no responsibility for the Americas otherwise—a selfish and one-sided position.
The manner in which we cling to this doctrine is stupid and ineffective: while we have conceived of it only as applying to military or political encroachment, we have overlooked the modern phase of commercial conquest. Thereby we gain the suspicion of our Latin-American neighbors, who accordingly welcome more gladly European or Japanese rather than American capital; and thus we encourage the very encroachments we have thought to prevent. We should either abandon the Monroe Doctrine entirely, or define it also in terms of political control.
Therefore, as regards our great industries, and, more specifically, our mineral industries, we have never had any definite policy; our troubles and problems were purely internal, and the Government’s efforts were largely directed to preventing such solidarity of any one industry that its power should be too great, although American organizing genius first successfully developed these colossal business combinations, rising without[542] government support. England, too, and France, with their democratic traditions, tended to resist the overwhelming power of great business organizations, as leading to the destruction of equal opportunity and threatening the power of the state. It remained for Germany, pressing impudently toward the conquest of the world, to see the advantage of combining the powers of the state with those of business monopolies, as a means of regulating industry at home and overpowering other nations. Thus the old question of the union or separation of Church and State becomes one of Business and State.
The success of this plan of German penetration was most clearly and disagreeably brought home to the British mind, as well as to the French and the American perception, by the war, and during the war England took under government control her mineral industries more definitely and systematically than did we. Moreover, perceiving the success of the German system as a means of penetration and as a method against competitors, she has adopted it, there being a striking tendency to put her key industries under syndicates, unions, cartels, or trusts controlled directly by the state.
A system of state socialism thus takes the place of the freedom of individual competition. As regards the mineral industries, much the same action has been taken by France. But in America, dropping all the problems and half-learned lessons of the war, we return to the status quo ante. If this difference continues, it is certain that British control of the earth—whereby we mean its minerals—will eventually preponderate. As far as we are concerned, we should perhaps rather see it in the hands of Great Britain than of any other power, but must we not decide upon our own course as a rich and populous nation of an increasingly close-packed but seething and yet unorganized globe?
Our statesmen, newspapers, and financiers proclaim to the world that we intend to take the lion’s share of the world’s shipping and commerce. England says nothing, but puts her government directly behind her own industries, while the American Government still holds aloof from them. Nationalism has been revived in Europe, and especially in England and France, as the result of the struggle to prevail against the intense German nationalistic spirit, which all but subjugated a world drifting comfortably into internationalism. It is conceivably a step backward, a reversion, but what attitude shall America take? The British and French nationalism need not disquiet us so much as that of the Japanese, still more intense and purposeful, and working with the same German tools (not invented in Germany, but in America, but, like most German arts, successfully copied and utilized), now adopted by England and France in self-defense. America also has had a rebirth of nationalism, quite necessary in the existing state of affairs.
As the case now stands, the United States largely predominates in the petroleum industry, with 71 per cent. of the world’s production in[543] 1917; England, far behind, is in a way to overtake us with giant strides under her new system. In the basic necessities of coal and iron, the United States also leads. The second place in the steel industry, held by Germany, was presumably lost as a result of the war, and probably passes to England, already second in coal and with her iron industries in charge of a government-controlled syndicate for purposes of protection and expansion.
In copper, the United States is far away in the lead, with England a long second, and in lead, also, with half the world’s production, with England second. Before the war England and Germany were about tied for second place, and the latter was rapidly drawing ahead through her state-controlled commercial methods; but the war will set her back greatly. In zinc, the United States had the greatest production (32 per cent.) before the war, slightly more than Germany (28 per cent.), but German methods gave it the preponderance of actual commercial control. The result of the war will restore the commercial supremacy to the United States, and the importance of England will increase. In silver, the United States now leads in production and in both territorial and commercial control, and, by her commercial control over Mexican production, controls one-half of the world’s output, with Great Britain a strong commercial second, having nearly 40 per cent. of it.
In the production of the important mineral, sulphur, the United States is far in advance of the world, with 65 per cent. of the world’s production in 1916. Italy is second, Japan third, and England is practically unrepresented. Phosphate rock is dominated, both territorially and commercially, by the United States, but there are other supplies for England, and France has abundance in her own territory. Vanadium is commercially controlled by the United States, although territorially by Peru. Molybdenum has very lately come to be controlled by the United States, with Great Britain, formerly first, now second. In uranium and radium the United States also has first place, with Austria a long second. The aluminum industry is strongest in the United States, although very important also in France.
In the following, however, Great Britain has control: the important “key industry” of tin, where her territorial control is one-half and her commercial control absolute, whereas the United States is not represented; in the important nickel industry, by territorial control of 80 per cent., and by a commercial control that is now probably predominant over the strong American interests, as a result of an active government policy; in tungsten, where she controls territorially the greatest production (34 per cent.), and where she has commercial supremacy, controlling 54 per cent. (1917), the United States being second, with a commercial control of 35 per cent. (although its territorial control is only 17 per cent., about equal to that of Bolivia).
In manganese, Russia nominally leads, with 36 per cent. commercial[544] control (55 per cent. of the world’s production in 1913); but under present conditions the effect is to give England the lead, with the United States in a position of minor importance. In chromium, Great Britain and France control through a syndicate, in which the British interest is in the majority, and the United States occupies a subordinate position with regard to both these countries. In gold production the British Empire controls 63 per cent.; the United States 23 per cent. In graphite in 1913 the British Empire was second to Austria-Hungary. It will now take the first place, and the United States will be a competitor. In asbestos, the British Empire produces 87 per cent. and controls commercially 63 per cent. of the world’s total, the United States being negligible in production, but second in commercial control (of Canadian asbestos). Mercury, territorially, is mainly in the hands of Spain, but the industry is actually dominated by England, under selling arrangements. Antimony has long been controlled by England, but control may revert to China, which means the possibility of its becoming Japanese. Mica, essential for electrical work, is controlled by Great Britain.
Only a few minerals remain in which most of the industry is not in the hands of either the United States or England: potash, formerly a German monopoly, and now divided between Germany and France, with Germany likely predominant; and mineral nitrates, in Chilean territory, with no marked national commercial control other than that of Chile.
Both America and England are strong in their grip on the world’s mineral industries—England much stronger than before the war and with a freshly set purpose to expand. A combination of these two countries would amount to a practical world control of minerals, and, with France, a little stronger control. This much for the present, but uncertain quantities loom shadowy, in the destiny of Russia, the future of Asia, and the progress of Japan. Japan is intently embarking on a course toward the domination of Asia, politically and commercially. Her present position is not so significant as the consideration of her rapid progress, the knowledge of the rich field in which she is to work, and a study of her militaristic methods, which remind one of those of Germany. Japan holds to no ally that will not (temporarily) aid her in her forward march, and in the weakness of Russia, China, and Korea she sees her opportunity. The war to her was an unmixed blessing. She took no chances, and seized enormous advantages.
There are three great figures of nations which seem to have been destined to be, in these times, of critical importance to the United States: in the past Germany; in the present, Great Britain; in the future, Japan. The German question was settled in the only possible way; for England the only sane solution is a closer-knit alliance; and for Japan, watchfulness and friendly intentions.
[545]
Depending on the hard- and software used to read this text and their settings, not all elements may display as intended. Some of the tables may be illegible on small screens or in narrow windows. Illustrations may be enlarged by opening them in a new tab or window.
Spelling, capitalisation and hyphenation as used in the source document (including the errors and inconsistencies in company, geographic and proper names, titles of publications, etc.) have not been corrected or standardised, except as mentioned under Changes made below. Results of calculations in tables are given in this text as they appear in the source document, even though they appear to contain occasional calculation and/or rounding off errors or are based on incomplete or contradictory data.
Page 50, Table 7, table row Austria: the remark in the last column may be better suited for the row Hungary.
Page 278, Footnote [132] Percentage of mean of totals used in a and b: a and b refer to the columns with footnotes [130] and [131] respectively.
Pages 508 and 510, Tables 72 and 74: these tables are (almost) identical in the source document as well.
Changes made
Tables and illustrations have been moved out of text paragraphs; footnotes have been renumbered sequentially and moved to directly under the paragraph where they are referenced.
In several of the tables data have been aligned more consistently than in the source document. Multi-page tables in the source document have been recombined into single tables in this text.
Some obvious minor typographical and punctuation errors have been corrected silently.
Text in a dashed box is text that does not occur as such in the source document, but that has been transcribed from the accompanying illustration.
Page ix: Platinum, by James H. Hill changed to Platinum, by James M. Hill.
Page 35: Dorfmund changed to Dortmund.
Page 45: Alden changed to Aden.
Page 50, Table 7: References to page 49 (Austria, Hungary) replaced with “above” cf. other entries.
Page 148: footnote anchor [78] inserted in table heading.
Page 224, Table 35: column header Western Hemisphere moved to separate row; row Total Eastern Hemisphere: values 1,113,000, 212,750, 98,000, 31,000 and 125,000 moved to row World Total.
Page 278, Table 53: first column Per cent. under Primary pig lead, Smelter production (values 0.2, 4.5, etc.) considered to be intended as second column under Imports, Recoverable lead content of ores and concentrate.
Page 432: ... and the latter in, exhaustibly available ... changed to ... and the latter inexhaustibly available ....
Page 509-510: Footnote anchor [186] inserted after both occurrences of IO₃.
This eBook is for the use of anyone anywhere in the United States and most other parts of the world at no cost and with almost no restrictions whatsoever. You may copy it, give it away or re-use it under the terms of the Project Gutenberg License included with this eBook or online at www.gutenberg.org. If you are not located in the United States, you will have to check the laws of the country where you are located before using this eBook.