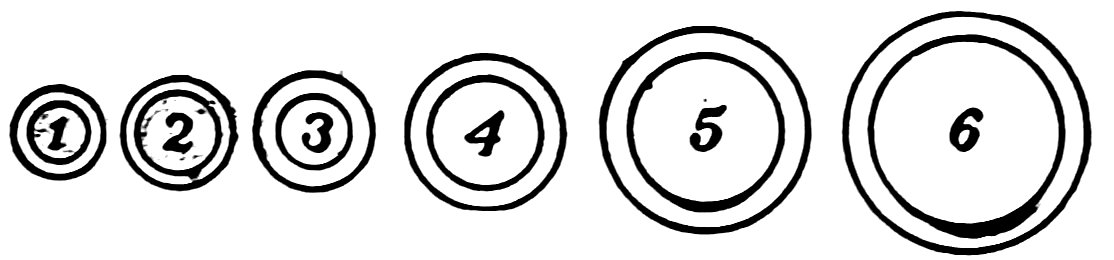
FIG. 2
SIZES OF GLASS TUBING
Transcriber’s Note:
New original cover art included with this eBook is granted to the public domain.
Boys, glass tubes are made in the sizes shown in Fig. 2, and in larger sizes. You will use sizes 2, 4, and 6 in the following experiments.

FIG. 2
SIZES OF GLASS TUBING
Hold a piece of No. 2, with both hands, in the flame of the alcohol lamp, and turn it constantly (Fig. 3). Do you find that when the glass becomes nearly red hot, it becomes soft and bends easily?

FIG. 3
HEATING GLASS TO SOFTEN IT
Take the tube out of the flame, bend it into any shape you wish (Fig. 4), and allow it to cool. Do you find that the glass hardens when it cools and retains the bent shape?
Heat the tube near the first bend, turn it constantly, take it out of the flame, and make another bend.
Repeat this and make all kinds of fantastic shapes.
Place all hot glass on the cooling blocks, not on the table.
Glass is used in many, many ways by the human race; for example, to make bottles, tumblers, window glass, and so on, and 2all of these uses depend upon the facts which you have just illustrated, namely, that glass becomes soft when heated and hard when cooled again.
FIG. 4
BENDING GLASS
The wick should be cut straight across and should project above the wick holder about ⅛ inch (Fig. 5), or a little more if you require more heat. Burn wood alcohol or grain alcohol, because they give flames without soot or smoke. Fill the lamp to within a ½ inch of the top only; it will burn one hour. The hottest part of the flame is not down close to the wick, as most beginners suppose, but up just beneath the tip.
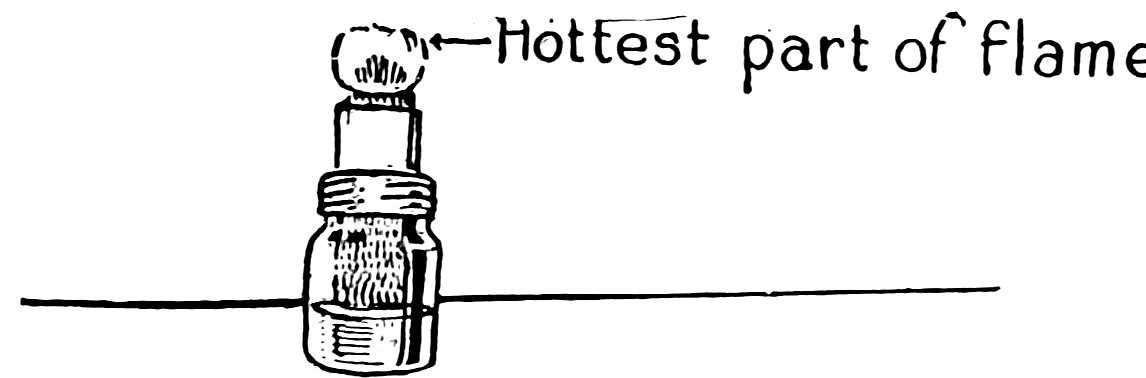
FIG. 5
THE LAMP
Buy your alcohol at a drug store in quantities of one pint or more. When you are through experimenting for the day pour the alcohol from the lamp back into the pint bottle and cork the bottle tightly. Alcohol left in the lamp gradually evaporates and is lost.
Do not let the lamp stand with alcohol in it for any considerable time—overnight for example—because fuel alcohol contains water and when it evaporates from the wick, the alcohol evaporates 3first and leaves the water in the wick. Then when you try to light the wick again, you will find that you cannot do so, because, of course, water does not burn. If this happens to you, take the wick out, dry it, and start the lamp again.

FIG. 6
MAKING A SCRATCH
It is perfectly safe to use kerosene in the lamp, but it gives a very smoky flame which deposits soot on the glass and fills the air with soot particles. Your mother will object very strenuously to this because the soot particles settle and blacken everything. Burn alcohol only, at least in the house.
FIG. 7
BREAKING THE TUBE
Cut off a six-inch length of No. 2 as follows: Lay the tube flat on the table, mark the six-inch length and draw the file across the tube at this point, pressing hard enough to make a good scratch (Fig. 6). Grasp the tube with both hands near the scratch, as in Fig. 7, pull apart and bend slightly. Do you find that the tube breaks across easily?
Repeat this with No. 4 and No. 6 tubes.
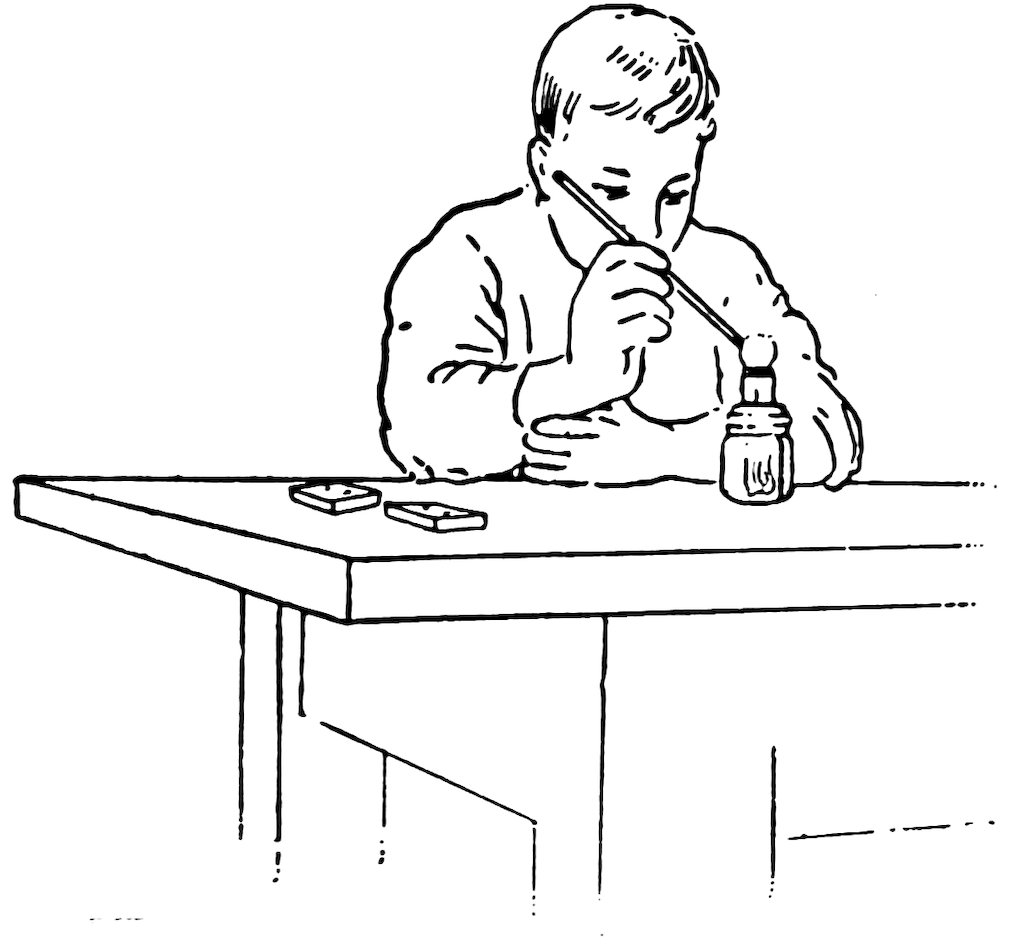
FIG. 8
MAKING THE EDGES SMOOTH
Hold one end of the six-inch piece of No. 2 in the tip of the flame (Fig. 8), and turn constantly until it is just red hot. Take it out and let it cool on the blocks. Do you find that the edges are smooth?
Repeat with the other end.
Repeat with both ends of the six-inch piece of No. 4.
If thick glass is heated quickly it may crack, because the hot exterior expands more quickly than the cooler interior and produces internal strains.
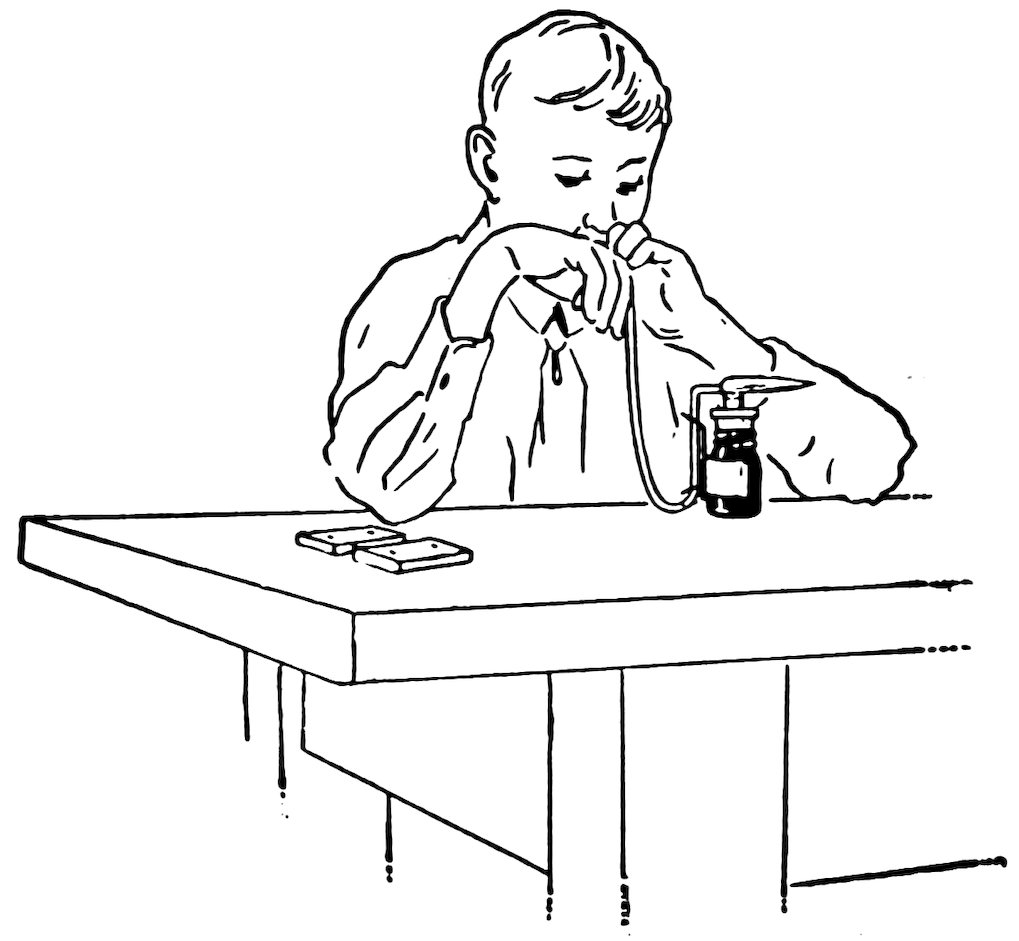
FIG. 9
THE BLOWPIPE FLAME
The No. 6 tube is comparatively thick and should be heated gradually as follows: Hold the end in the flame for about 1 second, then withdraw it for about 1 second; hold it in the flame again for 1 second, and withdraw it for 1 second. Repeat this eight or ten times, then hold and turn it in the flame until red hot.
5Smooth both ends of the No. 6 piece in this way.
Hold the small end of the blowpipe just inside the flame at one edge, about ⅛ inch above the wick (Fig. 9), and blow air through the flame parallel to the top of the wick.
Keep your mouth closed on the blowpipe, breathe through your nose, and practice keeping a steady stream of air going for a long time. You will be able to do this with a little practice.
Do you observe that the blowpipe flame is pointed, also that it is made up of a pointed cone inside and a lighter-colored cone outside? The hottest part of the flame is inside the outer cone just beyond the point of the inner cone.
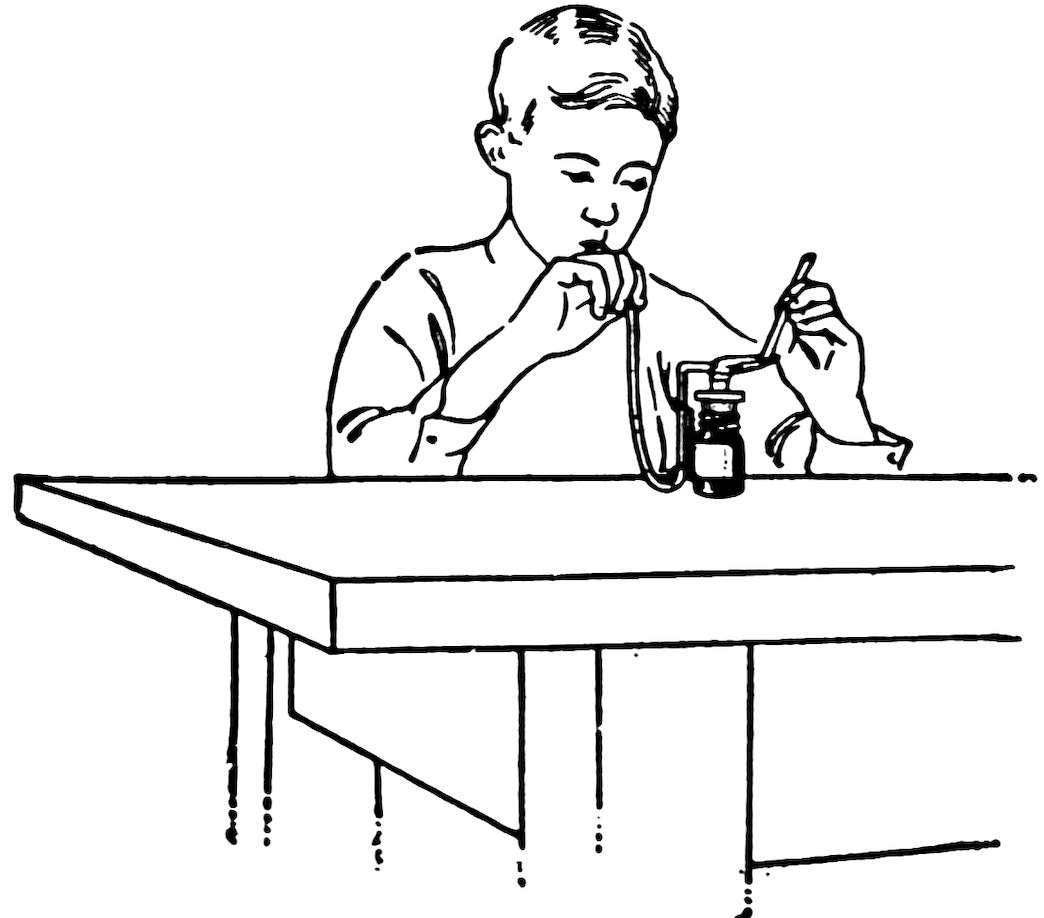
FIG. 10
CLOSING ONE END OF A TUBE
The blowpipe flame is hotter than the lamp flame because the heat of the burning alcohol is concentrated at one point by means of the air blast, and because the alcohol is more completely burned by the extra air.
Hold one end of a piece of No. 2 tube in the blowpipe flame (Fig. 10), turn it slowly, and heat until the end closes. Does it close nicely?
Close one end of a piece of No. 4 in the same way.
You can close No. 6 tubing in this way, but it leaves a large lump of glass which may crack on cooling or on reheating. You will practice closing No. 6 tubing later.
The glass becomes soft when heated because it becomes almost a liquid, and if it is heated sufficiently it becomes entirely a liquid. In this respect it acts very much as pitch, rosin, and wax act when heated by the sun or by a fire.
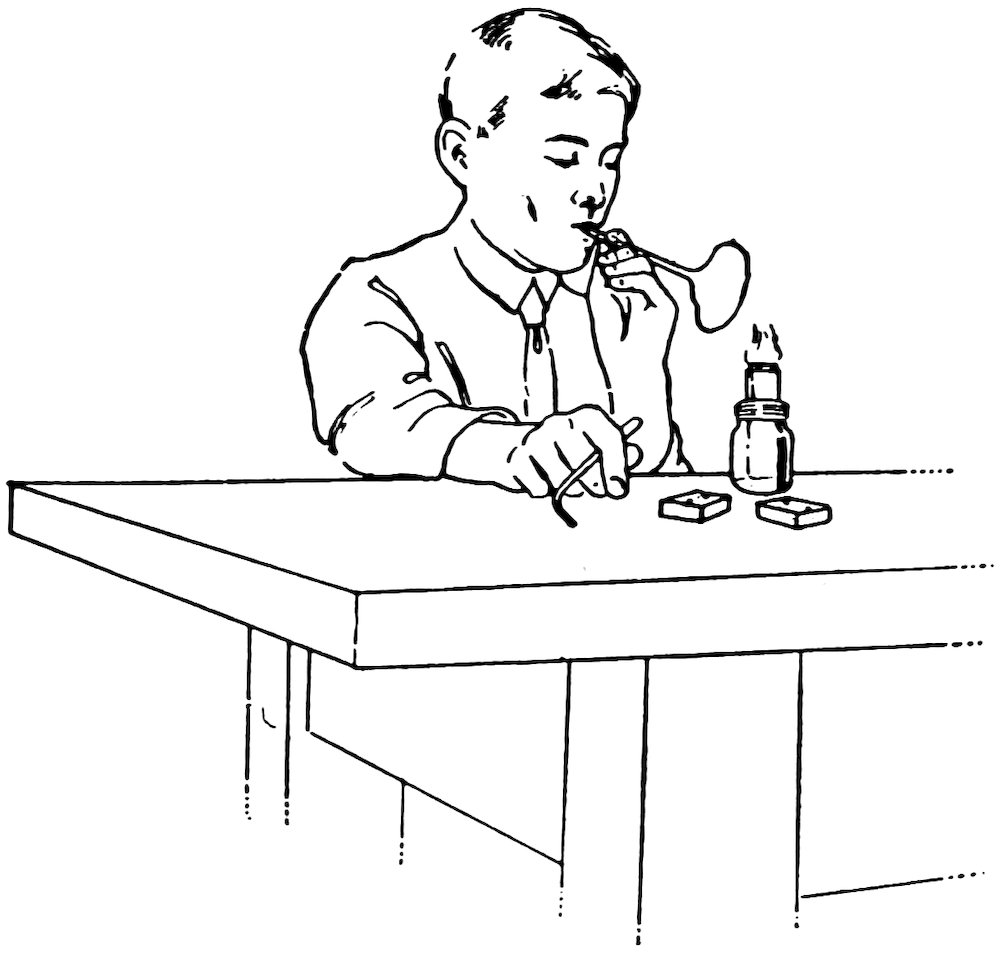
FIG. 11
MAKING A GLASS BUBBLE
The end of a glass tube becomes smooth, or closes entirely, when heated, for the following reason: The surface of any liquid tries to take the smallest possible area (this is explained in detail under “Surface Tension” in the Gilbert book on “Experimental Mechanics”), for example, a small particle of water takes the shape of a drop, a sphere, and the surface of a sphere has the least area for a given amount of water. Now when the end of the glass tube is heated it becomes a liquid, and the surface of this liquid contracts the glass into a smooth rounded surface of least area. If the tube is heated still more, the surface contracts still more and closes the end.
Smooth one end of a piece of No. 2 tube and allow it to cool. Close the other end in the blowpipe flame, turn it slowly, and heat until it is very hot. Take the tube out of the flame, put the smooth end into your mouth quickly, and blow as hard as you can (Fig. 11). Do you get a fine big glass bubble which bursts with a pop?
7If you get only a small bulb at the first trial, heat the end, and try again. Do you find that the bulb shrinks when heated but blows out again readily?
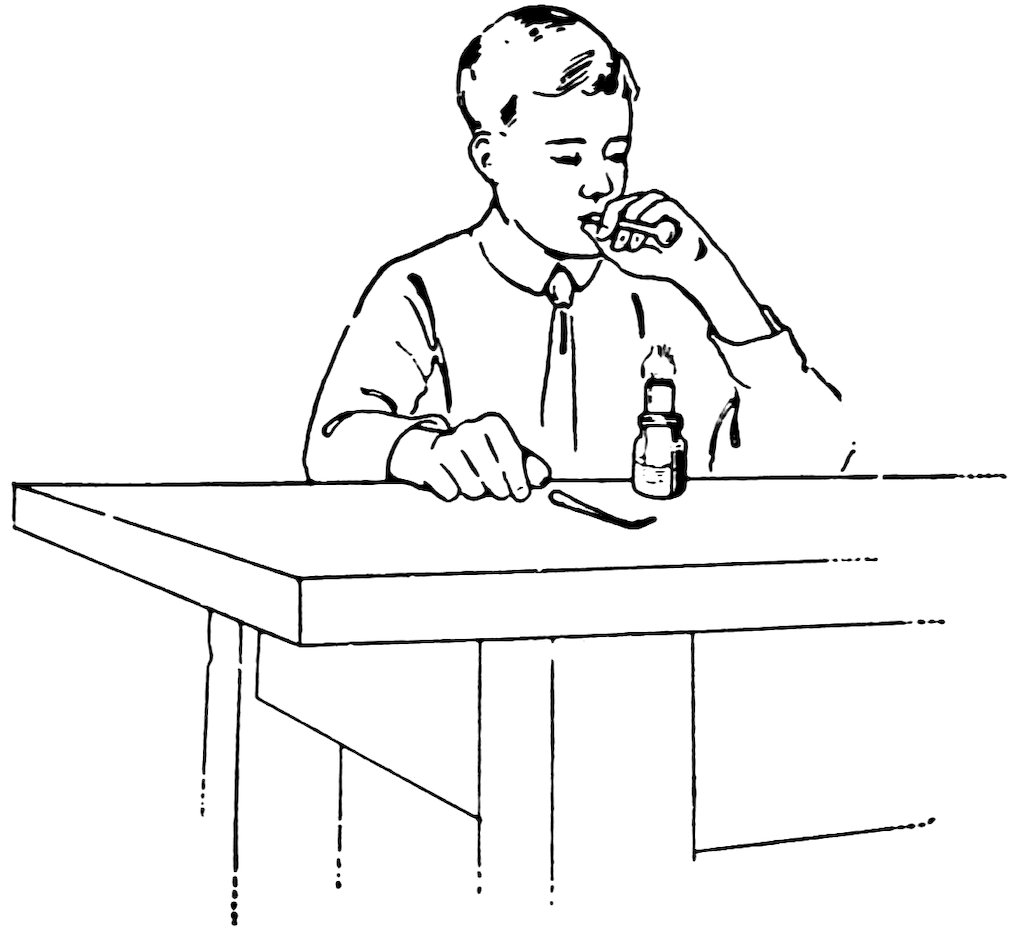
FIG. 12
BLOWING A BULB
When you get a big bubble, place the bubble end of the tube on a cooling block and break all the thin glass away from the tube by striking it with the file or blowpipe. Then close the end and blow another bubble.
Repeat until you can blow bubbles easily.
Repeat with a piece of No. 4 tube.
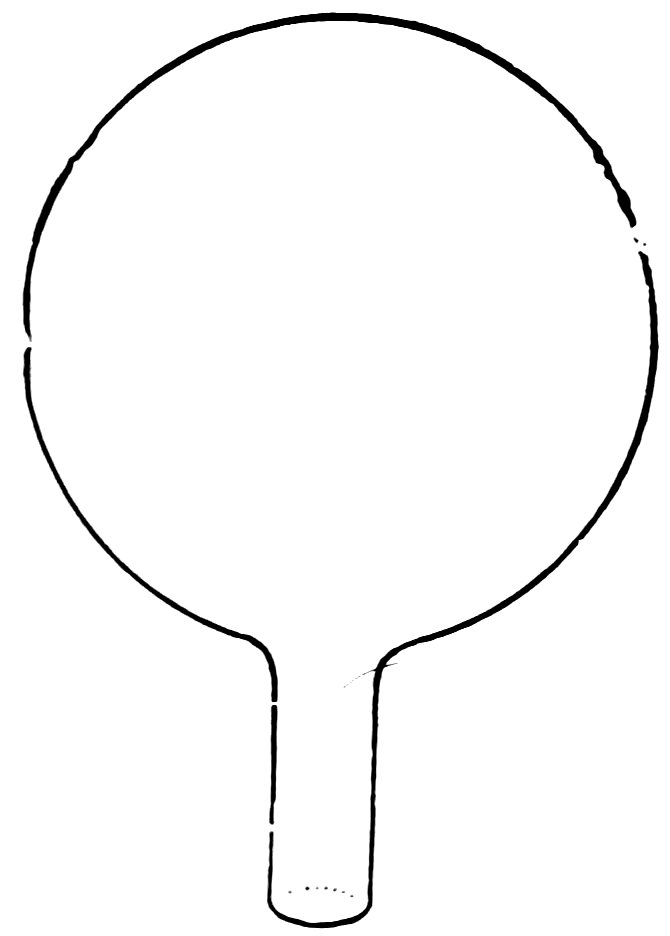
FIG. 13
A WATER BALLOON
Do you find that the thin glass of the bubbles shows colors, especially in sunlight, just as soap bubbles do? You boys who have had the Gilbert set on “Light Experiments” will know that these colors are due to “interference.” The colors produced by a thin film of oil on water are also produced by “interference.”
Close one end of the No. 2 tube in the blowpipe flame again and while it is still hot blow carefully into the open end until you have a bulb about ½ inch in diameter (Fig. 12). Now let it cool. 8Make a scratch with the file about ¼ inch from the bulb, break the tube at this point (Fig. 13), and smooth the rough edge.
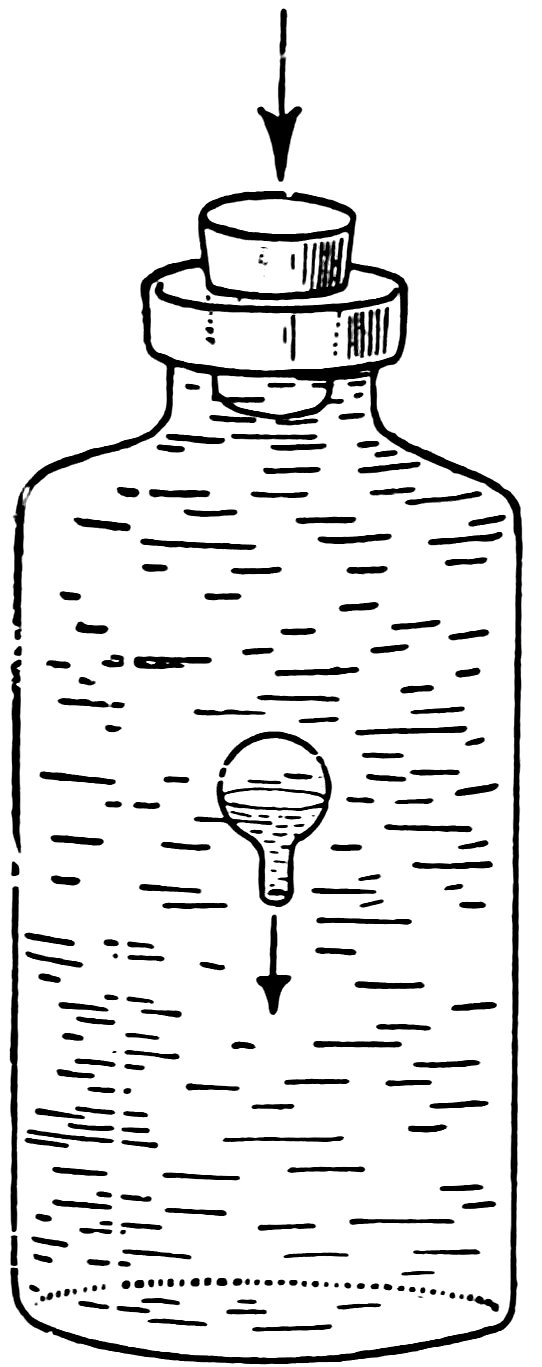
FIG. 14
THE BALLOON SINKS AND RISES
Put the bulb in a tumbler of water. Does it float? If not, make another balloon with a larger bulb.
Find a large bottle made of clear glass, the neck of which will fit your solid rubber stopper.
Fill the bottle with water to overflowing, insert the balloon, and then the stopper.
Now press down hard on the stopper. Does the balloon sink in a most magical manner (Fig. 14)?
Release the stopper. Does the balloon rise in an equally magical manner?
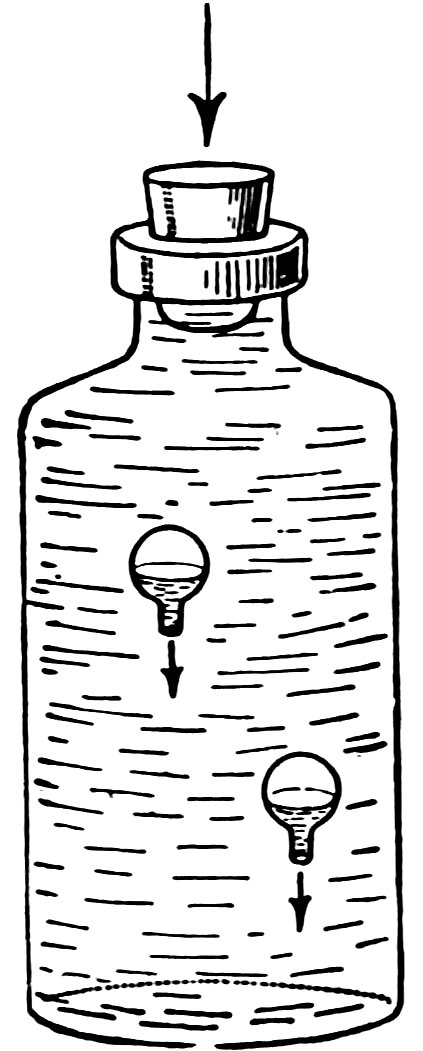
FIG. 15
A BALLOON RACE
Make another water balloon. Put the two balloons together in the bottle filled to overflowing with water.
Insert the stopper and press down hard. Do the balloons sink (Fig. 15), and does one sink more quickly than the other?
Release the stopper. Do the balloons rise, and does one rise more quickly than the other?
The most buoyant balloon sinks last and rises first.
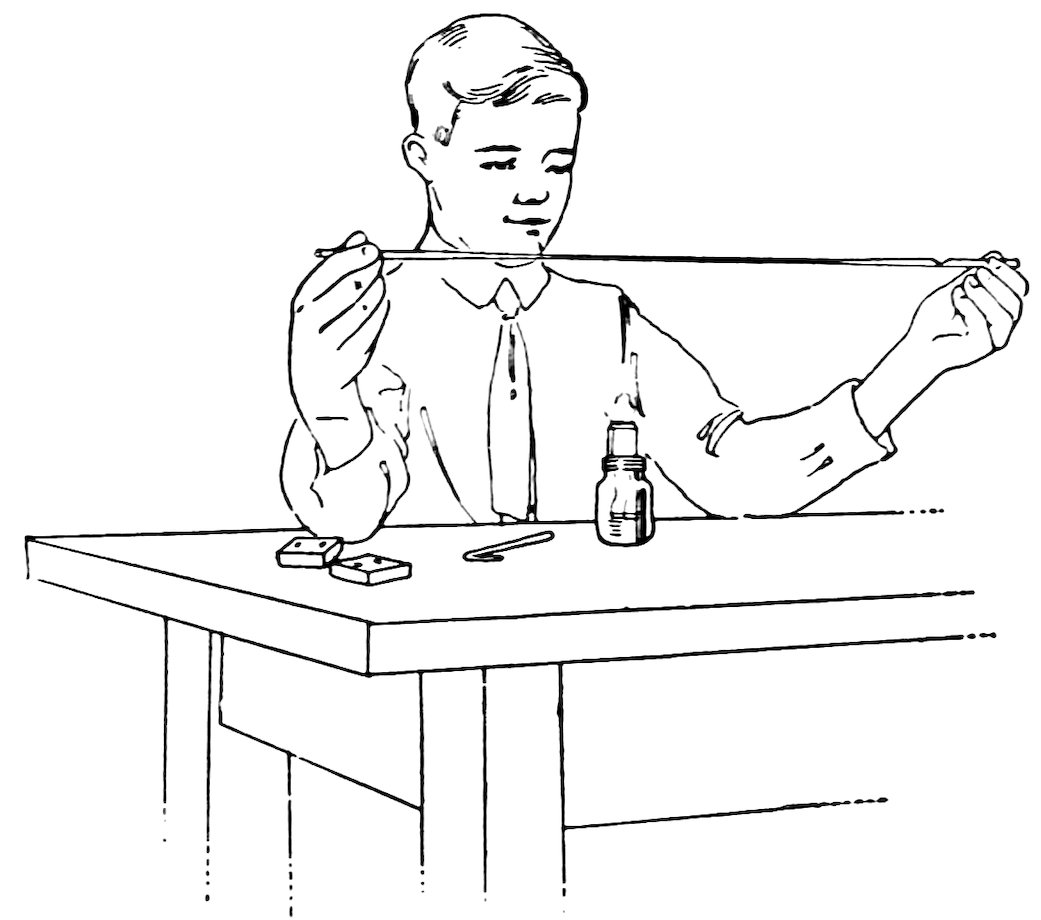
FIG. 16
DRAWING A THIN TUBE
You boys who have the Gilbert set on “Hydraulic and Pneumatic Engineering” will know the “why” of the last three experiments. Any body floats in water if it is lighter than an equal volume of water, and it sinks if it is heavier than an equal volume of water. Water is practically incompressible but air is very compressible: thus when you press down on the stopper, you force water into the balloon and compress the air in it; when you release the stopper, the compressed air in the balloon expands and drives the water out. When the weight of the balloon and the weight of the water in it are together greater than the weight of water displaced by the balloon, the balloon sinks; when they are less, it rises.
Hold a piece of No. 2 tubing in the lamp flame and turn it constantly. When it is red hot and soft, take it out of the flame and pull your hands apart until the tube is stretched ten or twelve inches (Fig. 16). Is the tube in the shape shown in Fig. 17?

FIG. 17
A GLASS TUBE STRETCHED
Allow the tube to cool, break the large ends away from the thin tube, place one end of the thin tube in a glass of water, and 10blow into the other end to make air bubbles in the water (Fig. 18). If you can do so, it is a real tube.
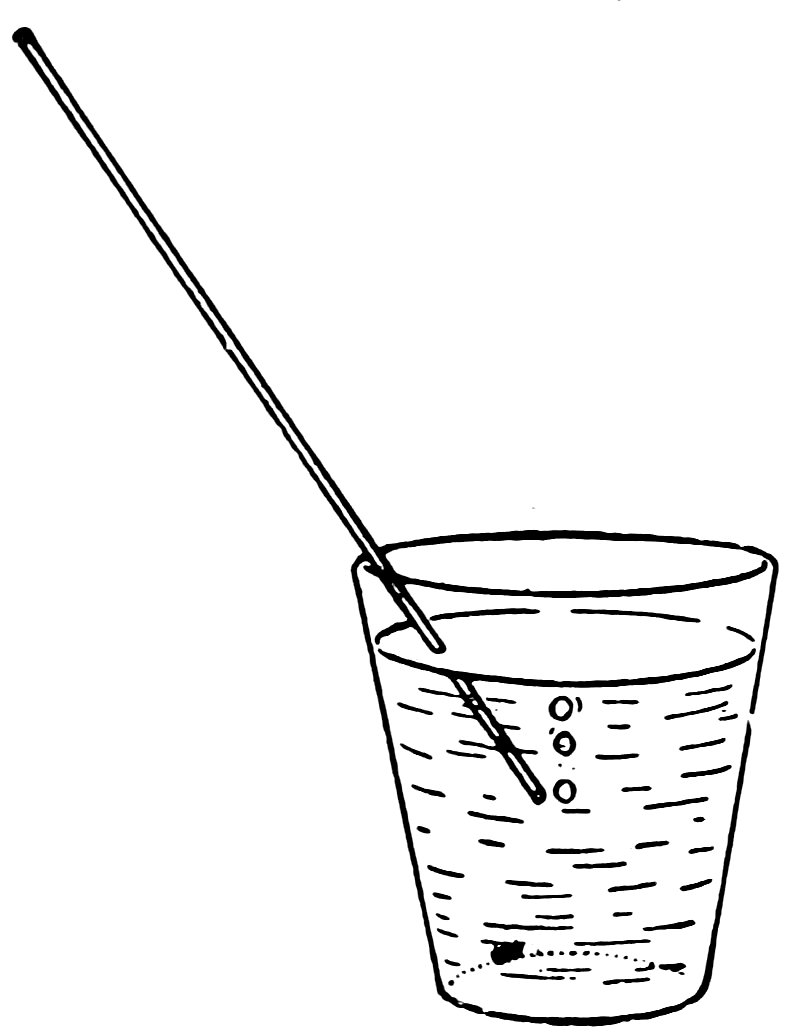
FIG. 18
AIR THROUGH TUBE
Does the thin tube bend easily and does it spring back when released?
Repeat the experiment with another piece of No. 2 tubing, but make the thin tube as long as you can.
Can you blow air through the thin tube, and does it bend very easily indeed?
Repeat with a piece of No. 4 tubing.
These thin hairlike tubes are called “capillary” tubes, from the Latin word capillus, meaning a hair.
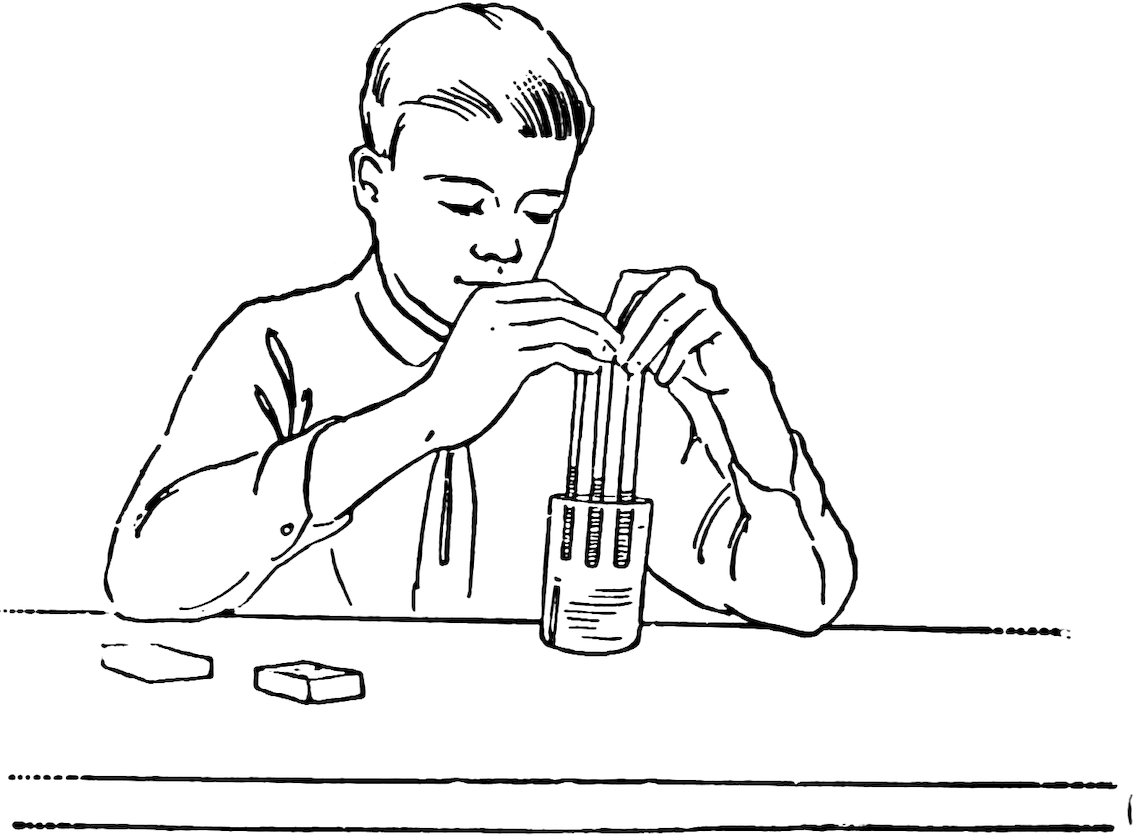
FIG. 19
WATER RUNS UPHILL
You have always heard that water runs downhill, but you will now see it run uphill and remain there in a most magical manner.
Cut off 5-inch lengths of No. 6, No. 4, and No. 2 tubing, stand them side by side in a glass full of water (Fig. 19), and move them up and down in the water to wet the inside of the tubes.
11Now look at the water level in each of the tubes. Is it above the level of the water in the glass, and is it higher the smaller the inside diameter of the tube, that is, is it higher in the No. 2 than in No. 4, and in No. 4 than in No. 6?
Now take the thin capillary tube which has the largest inside diameter, place one end in the glass of water, suck it full of water and blow it out. Now with one end in the glass of water notice quickly how the water rises inside the tube. Does it run uphill in a most magical manner (Fig. 20), and does it remain there?
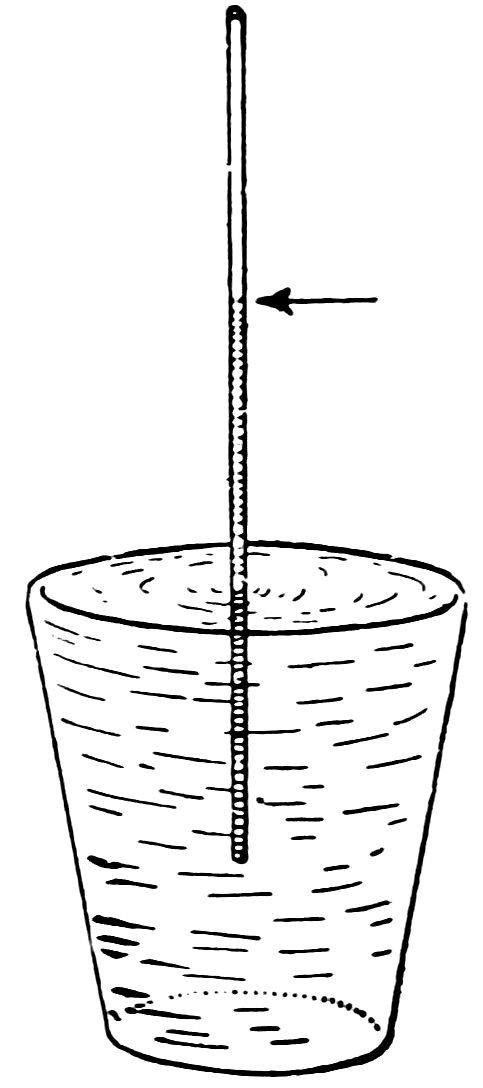
FIG. 20
WATER RUNS UP TUBE
Repeat this with your other capillary tubes. Does the water run uphill in each, and does it rise higher the smaller the inside diameter of the tube?
The “why” of this is explained in Gilbert’s “Experimental Mechanics” under “Capillarity.”
Common glass is made from three substances with which you are all more or less familiar; namely, sand, sodium carbonate (washing soda), and lime.
If sand and soda or potash are mixed and heated to a high temperature, they melt together and produce a glass which dissolves in water. This is known as “water glass” and it is used in many ways: to preserve eggs, to cement fire bricks, to make fireproof cement, and so on. If, however, lime is added and the mixture is heated to a high temperature, a glass is produced which is not soluble in water. This is the glass you know.
The three most common kinds of glass are: Venetian glass, made from sand, soda, and lime; Bohemian glass, from sand, potash, and lime; and crystal or flint glass, from sand, potash, and lead oxide.
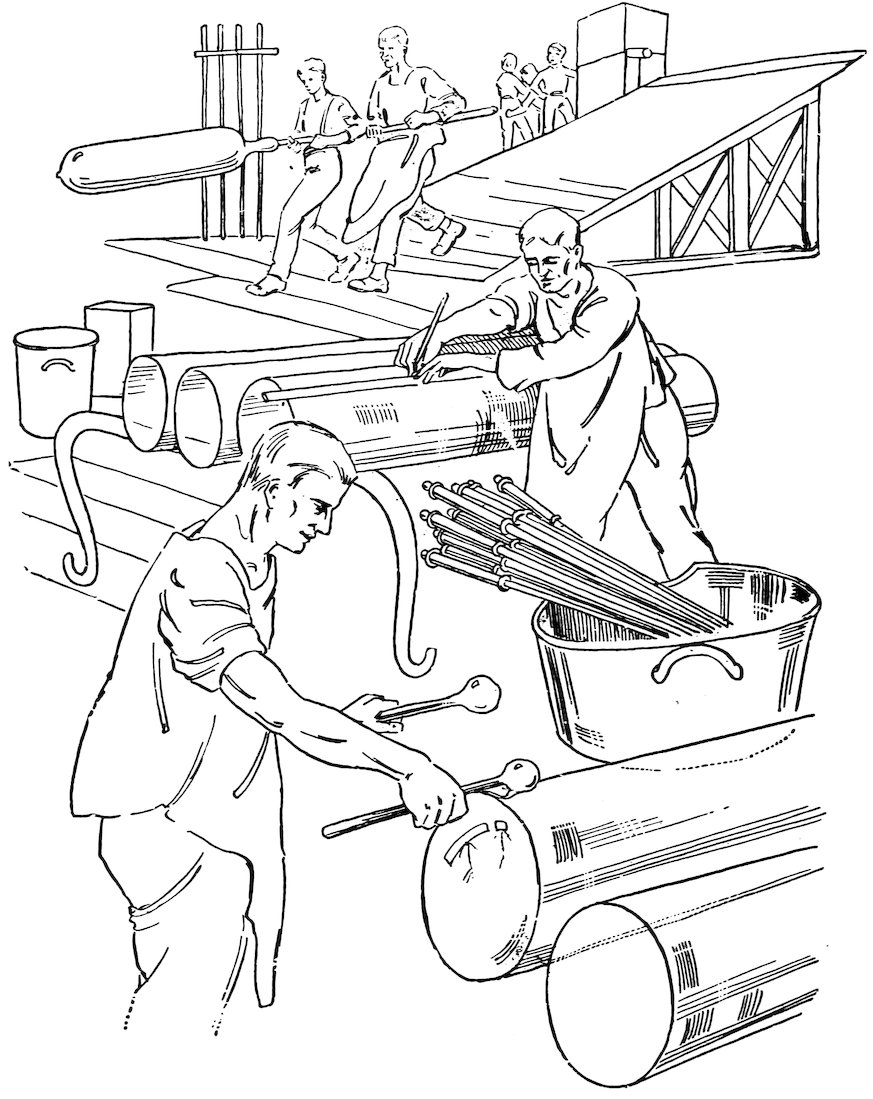
FIG. 21
SECOND STEP IN MAKING WINDOW PANES
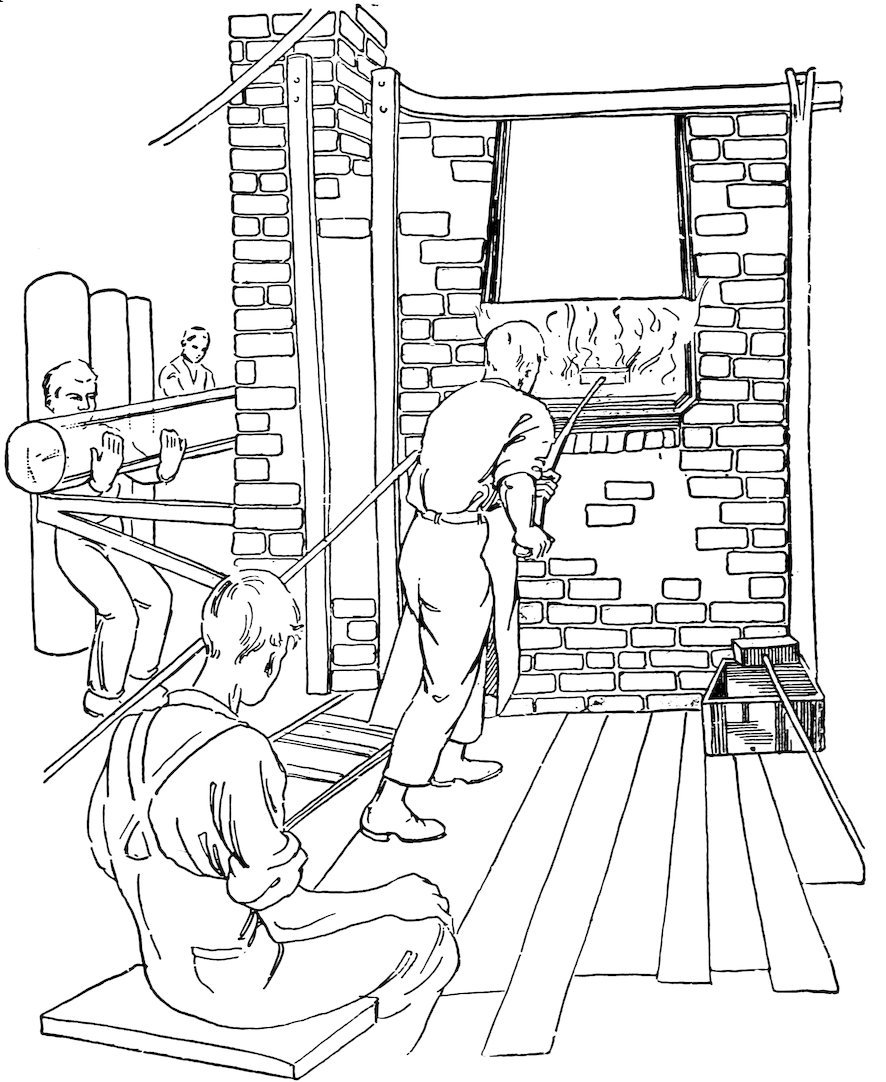
FIG. 22
IRONING THE CYLINDERS FLAT
The glass mixture is heated to a high temperature in fire clay pots or tanks in large ovens. The surface is skimmed from time to time and the heating is continued until all air bubbles have escaped from the mixture, usually about three days.
The glass is now quite fluid and it is allowed to cool somewhat until it is viscous; then the objects are made by blowing, pressing, or rolling, as described below.
The finished articles are finally “annealed,” that is, they are placed while still hot in a second hot oven, which is then sealed and allowed to cool slowly, for four or five days or for as many weeks, according to the kind of glass.
If a glass object cools quickly, it cools more rapidly on the surface than in the interior. This produces a condition of strain in the glass and the object may drop to pieces when jarred or scratched. This condition of strain is avoided by allowing the objects to cool very slowly, that is, by annealing.
Window glass is blown in exactly the same way as you have blown glass balloons; the process is illustrated in Fig. 1.
The glass mixture is heated for about three days in fire clay pots and is allowed to cool until it is viscous. The glass blower then attaches a lump of the viscous glass to the end of a straight iron blowpipe about five feet long and blows a bulb. He then reheats the glass and blows a larger pear-shaped bulb and in doing so rests the glass on a pear-shaped mold of charred wood (see center of Fig. 1). He again reheats the glass, holds the pear-shaped bulb over a pit, and blows a long cylinder (see left of Fig. 1).
The ends of the cylinder are now cut off and the edges are smeared with molten glass to prevent splitting (see right, Fig. 21). The cylinder is next cut lengthwise with a diamond 15(center, Fig. 21), and is placed in a second hot oven, where it is ironed out flat (Fig. 22).
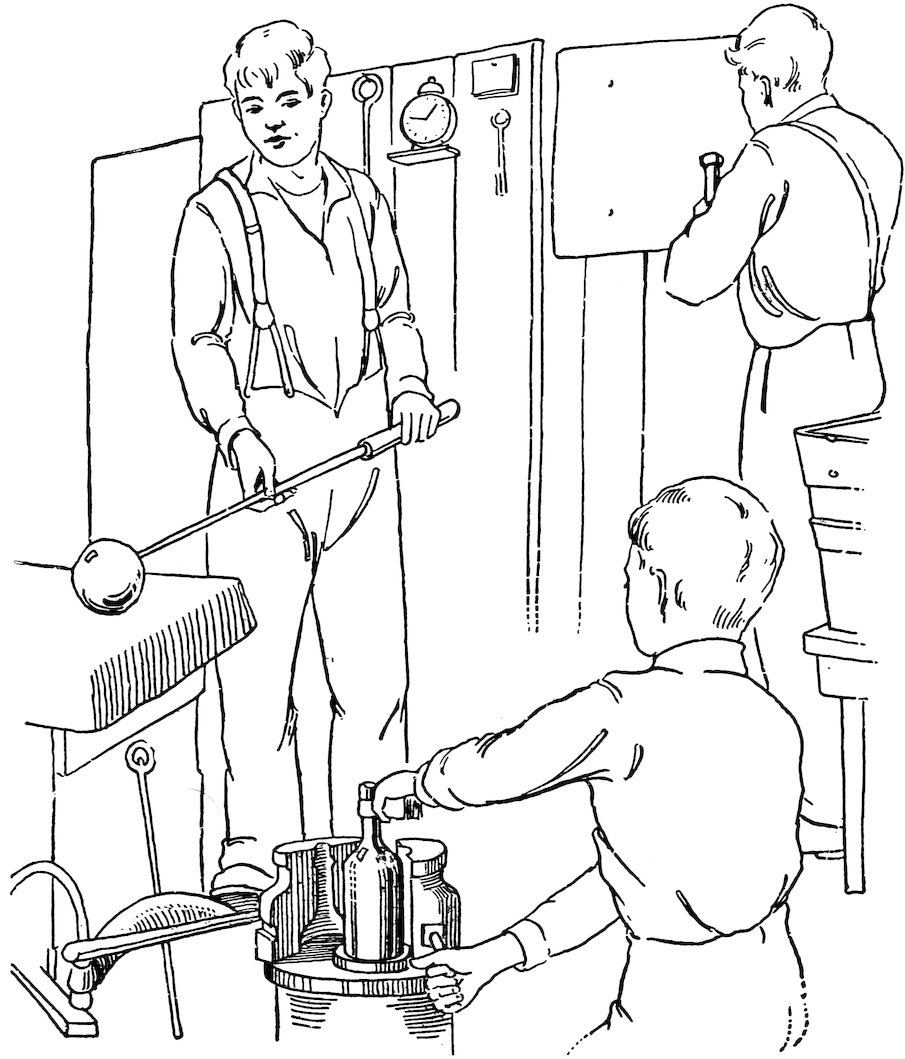
FIG. 23
BOTTLES BLOWN IN A MOLD
The flat sheets are finally annealed in a third oven for a number of days and are then cut into panes, sorted, and packed.
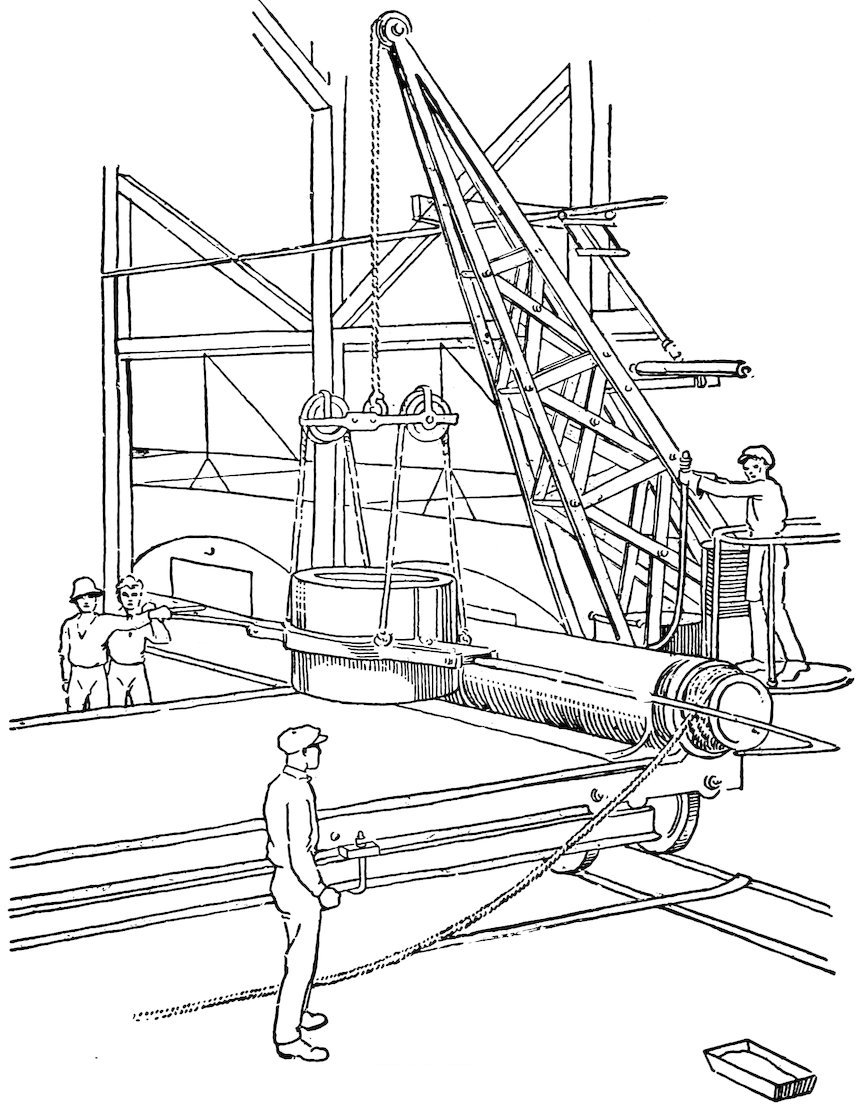
FIG. 24
ROLLING PLATE GLASS
17The glass tubes with which you do the experiments in this book are made in the same way as window glass up to the stage of blowing the cylinder; then the blower’s helper attaches an iron rod to the opposite end of the cylinder (see right of Fig. 1), and the blower and helper walk backward away from each other to pull the cylinder into a tube. Of course, they use a small amount of glass to make small tubes, and larger amounts for large tubes.
Many articles of glass are made by blowing the glass in molds. Bottles are made in this way (Fig. 23), and large machines are now in use which mold many bottles at one time in this way.
Many articles are made by pressing glass into molds, that is, the molten glass is poured into molds and is pressed against the sides of the mold by means of a plunger. Imitation cut glass is pressed in this way.
The large sheets of plate glass used in store windows are not blown, but rolled. The molten glass is poured from the fire clay pots upon a cast-iron table and is rolled flat by means of a large iron roller (Fig. 24). The glass is then in the shape of plate glass, but is rough on both sides. It is annealed for a number of days and then is ground smooth on both sides, first with coarse emery, then with finer and finer emery, and is finally polished with rouge. The result is the beautifully polished plate glass we see in large windows.
The United States and Great Britain made great strides in the manufacture of optical glass during the war and there are now many kinds on the market. They are used in making the lenses, prisms, and mirrors for optical instruments.
Optical glass is made in much the same way as ordinary glass, 18but great care is taken: first, to see that the materials are pure; second, to stir the glass constantly, as it cools from the molten to the viscous state, to make it as uniform as possible; and third, to cool it very slowly in the annealing process, to avoid strains.
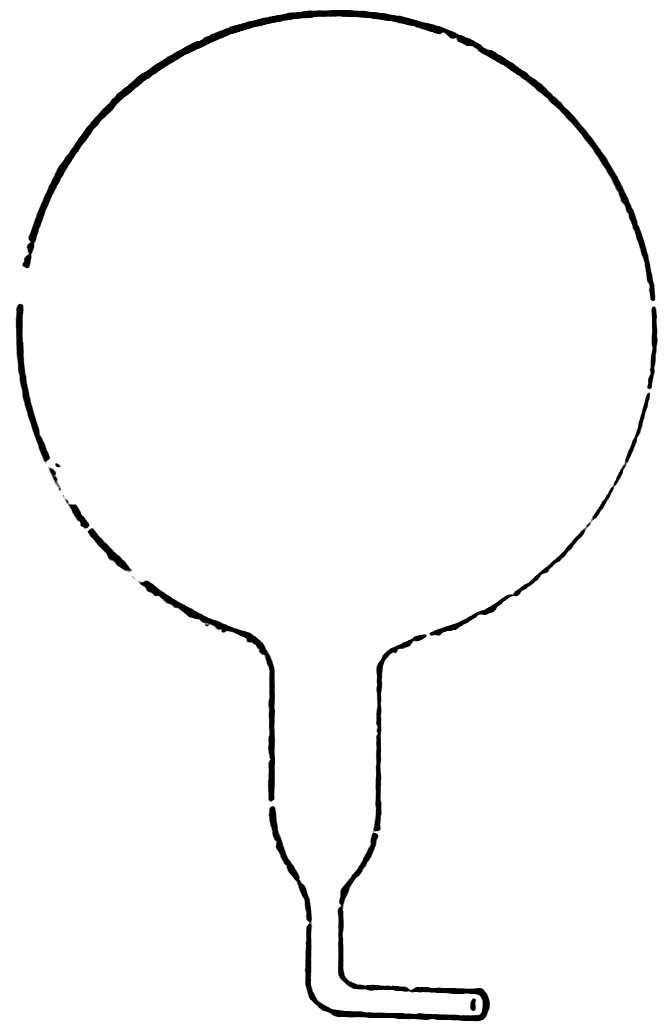
FIG. 25
A POLLYWOG
An entirely new glass has been placed on the market in quantity in recent years. It is made by melting very pure quartz sand at a temperature of 3000° F. and cooling it fairly rapidly. It has the very valuable property of expanding and contracting very, very slightly when heated and cooled. Thus there is practically no internal strain set up when it is heated or cooled quickly and it does not break. It can be heated red hot, for example, and then plunged into cold water without breaking. It is probable that this glass will be in universal use in a very few years.
Smooth one end of a piece of No. 2 tube to put in your mouth, close the other end in the blowpipe flame, take it out and blow a bulb about ½ inch in diameter.
Allow the bulb to cool, then heat the tube about ¼ inch from the bulb and draw it out into a thin tube. Now bend the thin tube at right angles near the bulb and break it off (Fig. 25).
Place the bulb in water. Does it float? If not, blow another with a larger bulb.
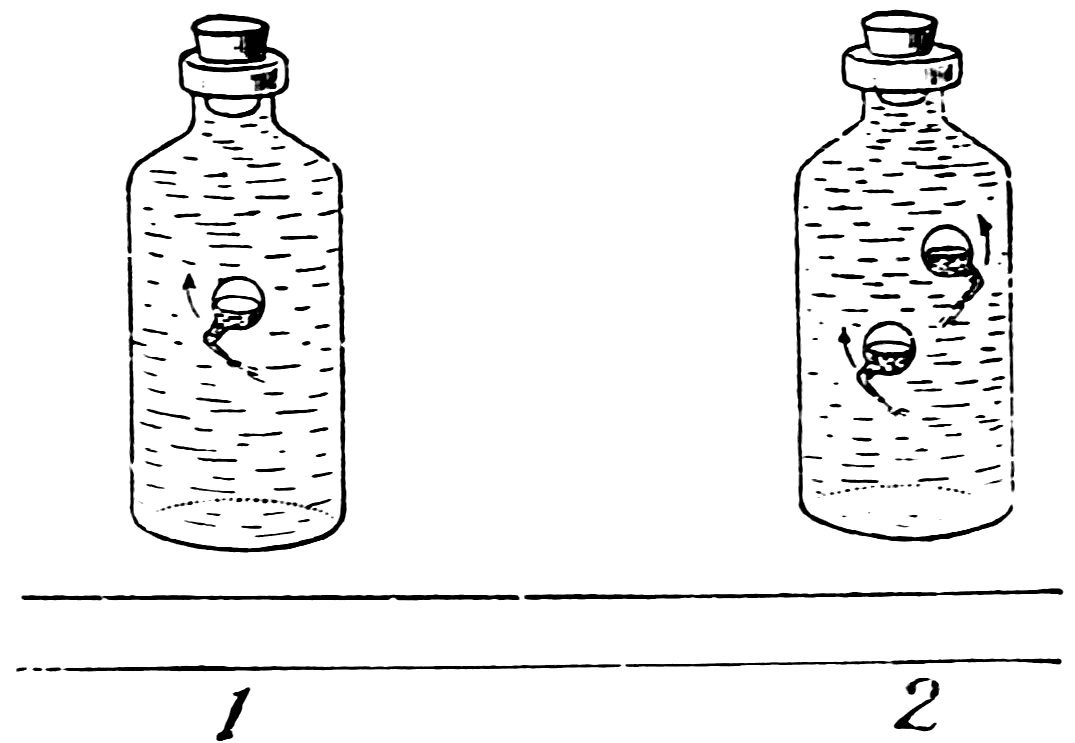
FIG. 26
ACROBATS
Place the pollywog in a bottle filled to overflowing with water, insert the solid rubber stopper, and press it down hard. Does the pollywog sink?
Now release the stopper quickly. Does the pollywog turn somersaults in a most magical manner (1, Fig. 26), and also rise?
Make one or two more pollywogs, place them all in the bottle together (2, Fig. 26), and entertain your friends with a pollywog circus.
The pollywog sinks when you press down on the stopper because you compress the air in it and force water in until it weighs more than the water it displaces.
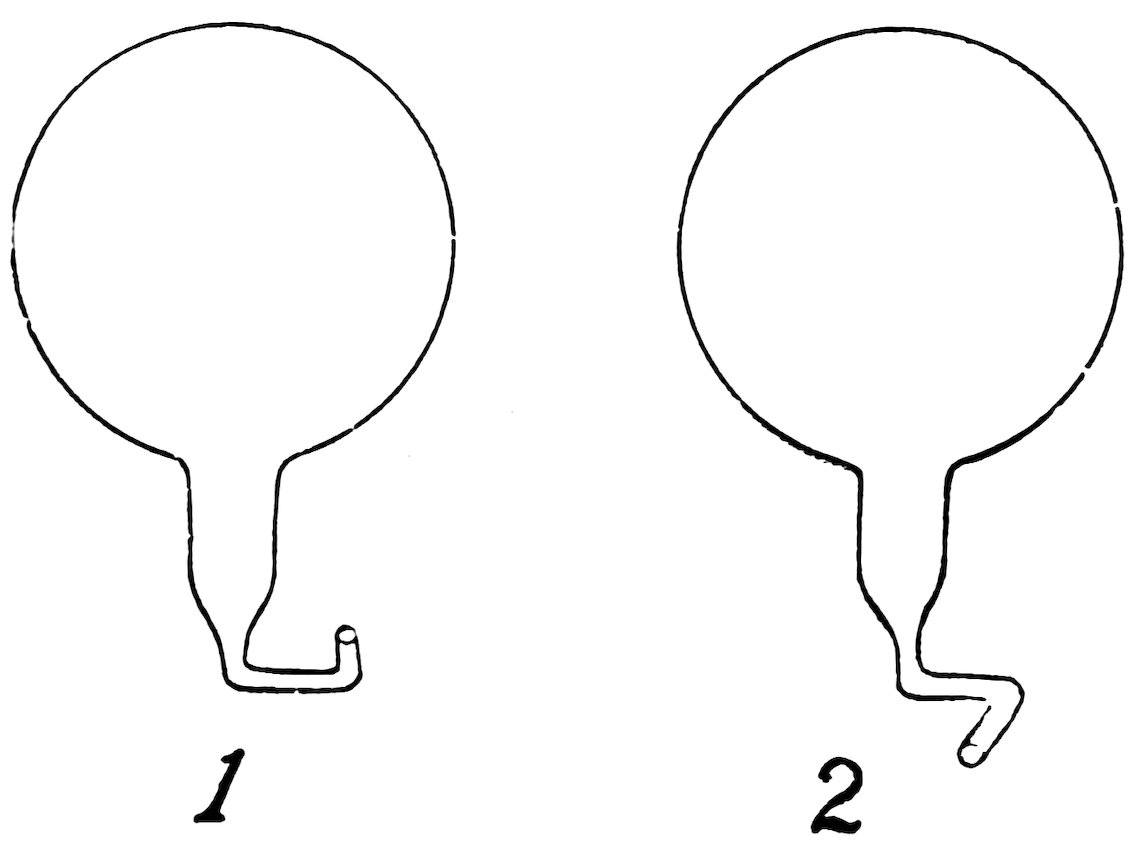
FIG. 27
DANCING POLLYWOGS
The pollywog rises when you release the stopper because the compressed air drives the water out until the pollywog weighs less than the water it displaces.
The pollywog turns a somersault because the water rushes out sidewise in one direction and forces the nozzle in the other direction.
20Air may escape from the pollywog when it is turning a somersault; if so, water will take its place, and may make the pollywog too heavy to float. You can restore its buoyancy by sucking out the water.
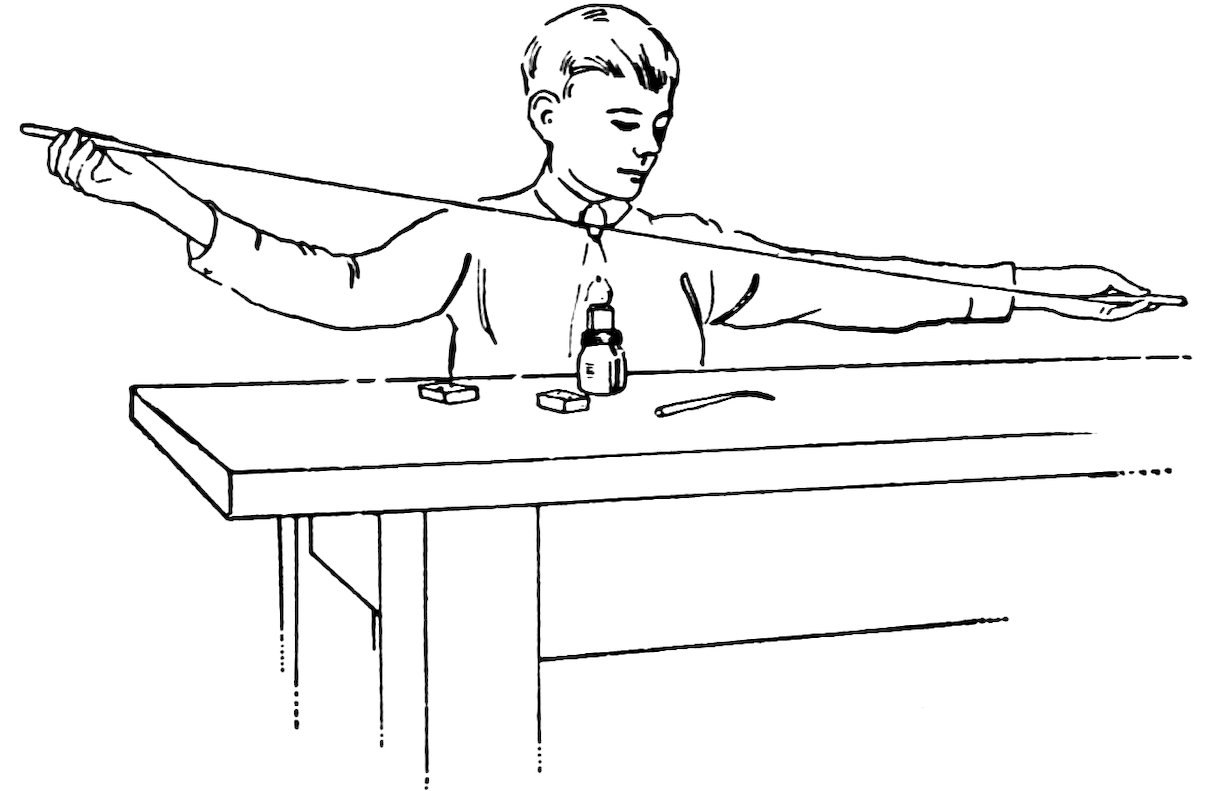
FIG. 28
DRAWING GLASS SPIDER-WEBS
Make a pollywog as in Experiment 12, but bend its tail twice as shown in 1, Fig. 27; the nozzle is at one side and points sidewise.
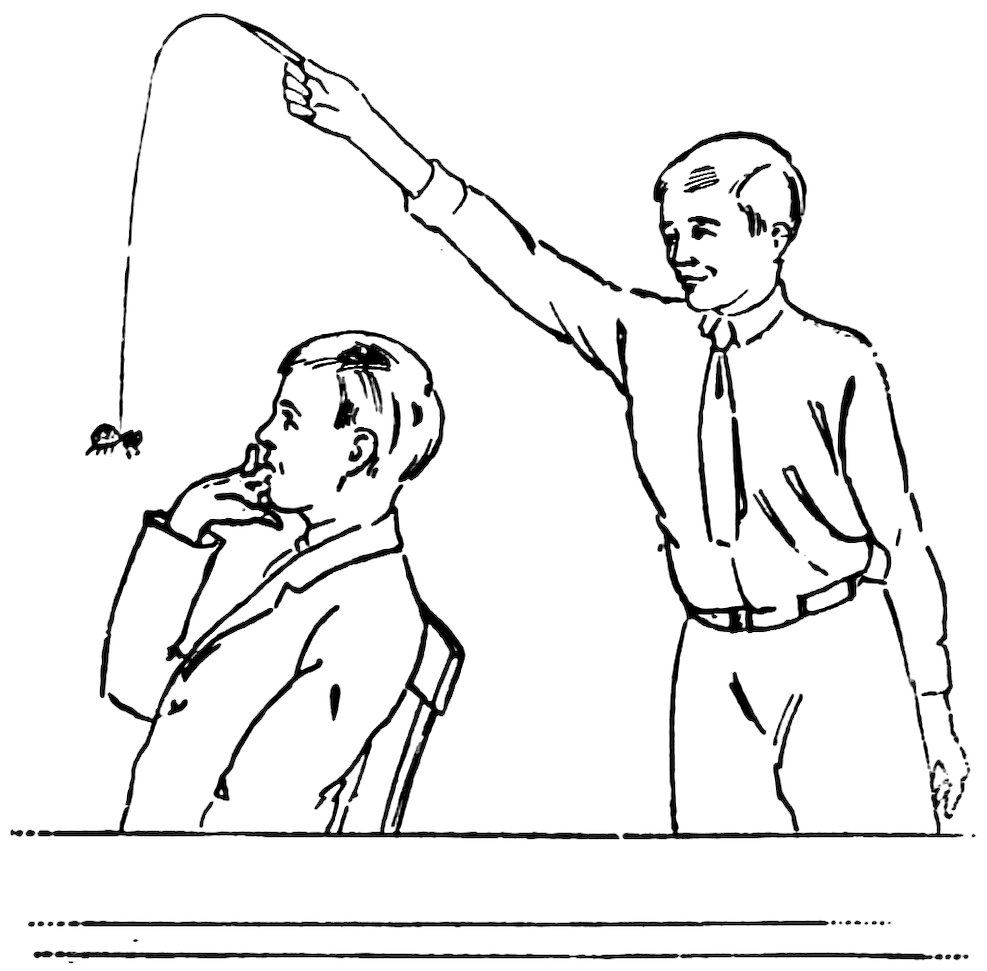
FIG. 29
THE SPIDER TRICK
Put it in the bottle full of water, then press down and release the stopper. Does it sink and rise, and does it also whirl around most beautifully as it rises?
Make another pollywog (2, Fig. 27), but bend its nozzle in the opposite direction. Does it whirl in a direction opposite to that of the first pollywog?
Put them in the bottle together and treat your friends to a pollywog dance.
The pollywog whirls because the water rushes out of the nozzle in one direction and forces the nozzle in the opposite direction.
Heat the end of a piece of No. 2 tube in the blowpipe flame until it is melted and very hot. Now touch the end of another piece of glass to the melted glass, remove from the flame, and quickly pull the two pieces apart as far as you can (Fig. 28). Do you find that you have pulled part of the melted glass out into a very fine glass spider-web?
Repeat, but ask a friend to touch the second piece of glass to the first and run away as fast as he can.
Do you get a much finer spider-web?
Is the glass spider-web fairly strong and very flexible?
FIG. 30
ATTACHING A HANDLE
Attach an imitation spider—or the dead body of a real spider—to the end of the glass spider-web and surprise your friends, as shown in Fig. 29. The glass spider-web is much less visible than a thread for this purpose.
You can save glass in many cases by attaching a short piece of glass to the piece you intend to work with, as follows: Heat an end of each piece in the lamp flame until red hot, press them together, remove from the flame, and hold until solid. The short piece then serves as a working handle (Fig. 30) for the large piece.
You closed small tubes in Experiment 5 by simply heating the end in the blowpipe flame. This method does not serve for 22large tubes, however, because it leaves a very large lump of glass which may crack on cooling or reheating.
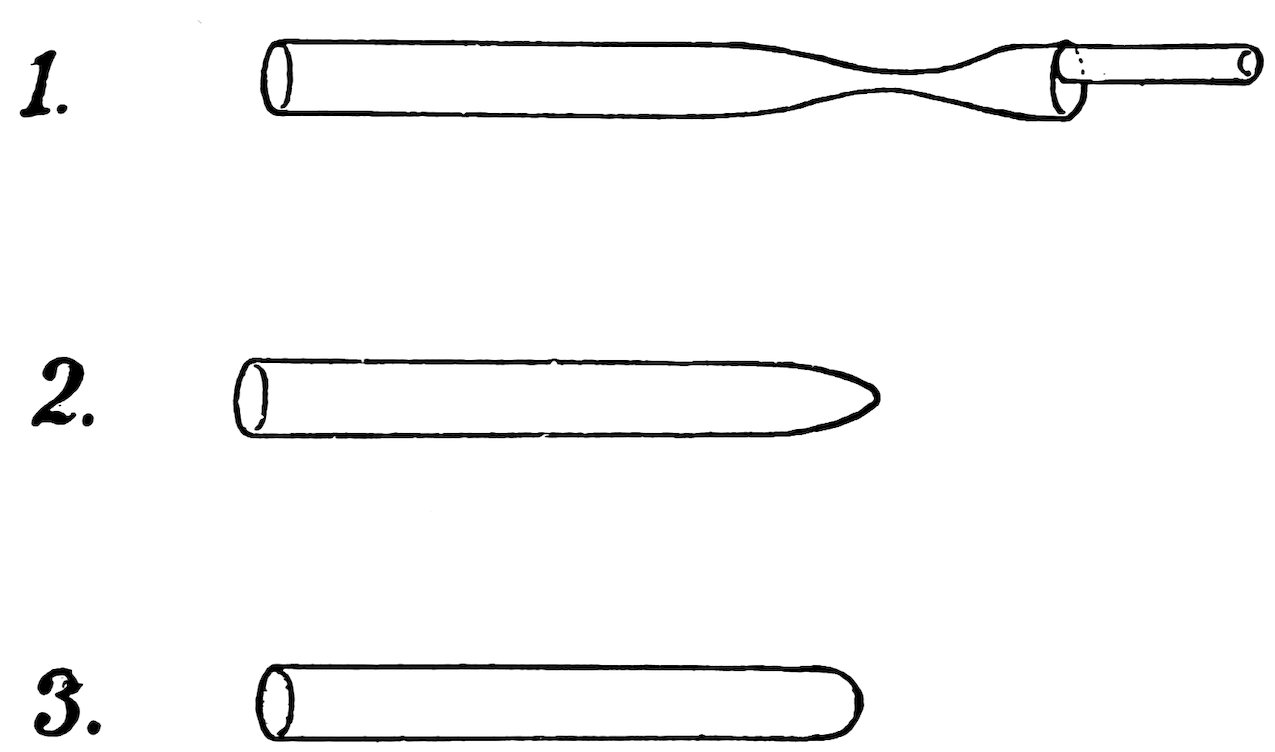
FIG. 31
CLOSING A LARGE TUBE
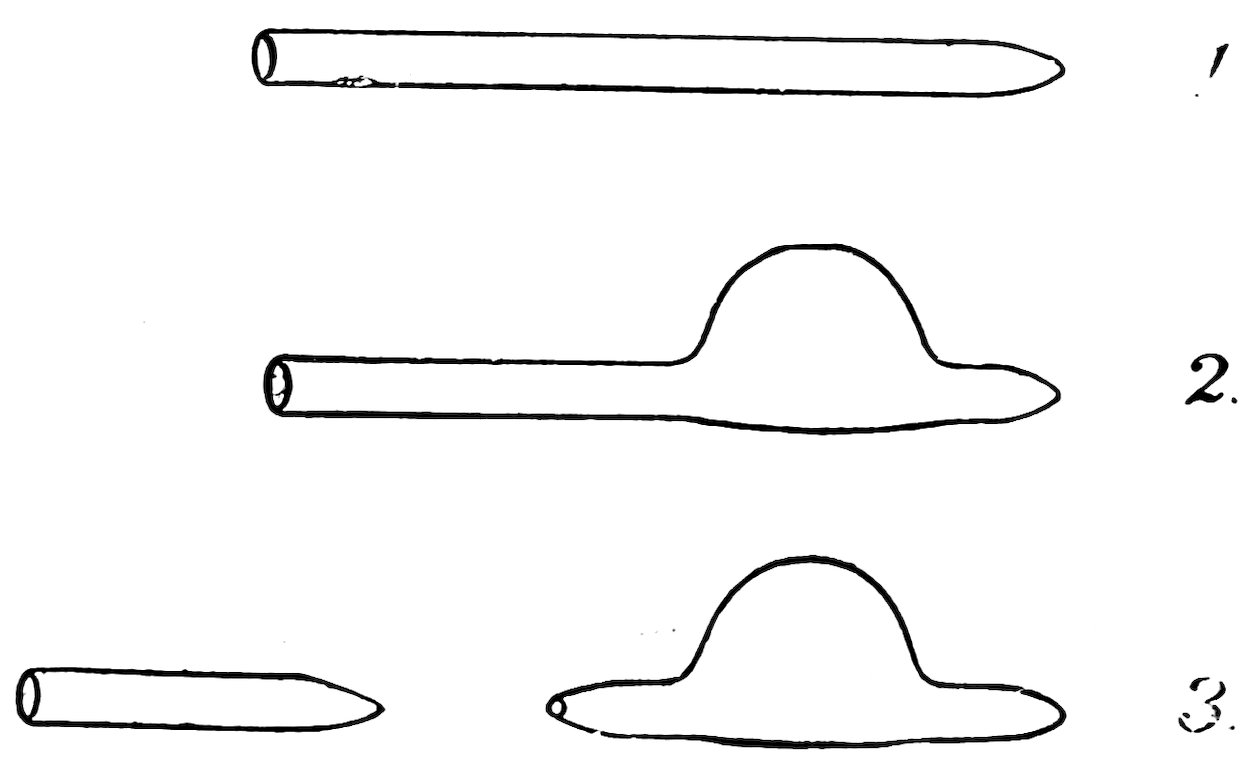
FIG. 32
MAKING A SUBMARINE
Practice the following method of closing a large tube; first with a piece of No. 4 tube, and then with a piece of No. 6: Attach a working handle to the end to be closed, heat the tube ½ inch from the end in the blowpipe flame, turn constantly, and when soft pull apart until the tube has the shape 1, Fig. 31. Heat, turn, and pull the end away to leave the tube as in 2. Heat the end and blow out until it has the shape 3. The end is now closed and the glass has about the same thickness as the remainder of the tube.
Close one end of a piece of No. 2 tubing as described above, but leave the end somewhat pointed (1, Fig. 32). Heat the tube on one side at a distance ½ inch from the end and blow a bulb about ½ inch in diameter (2). Heat the tube ¼ inch from the bulb, draw it down into a fine tube, and break off the tube, leaving a small hole in the end (3). Place the submarine in a glass of water, and if it floats it is complete.
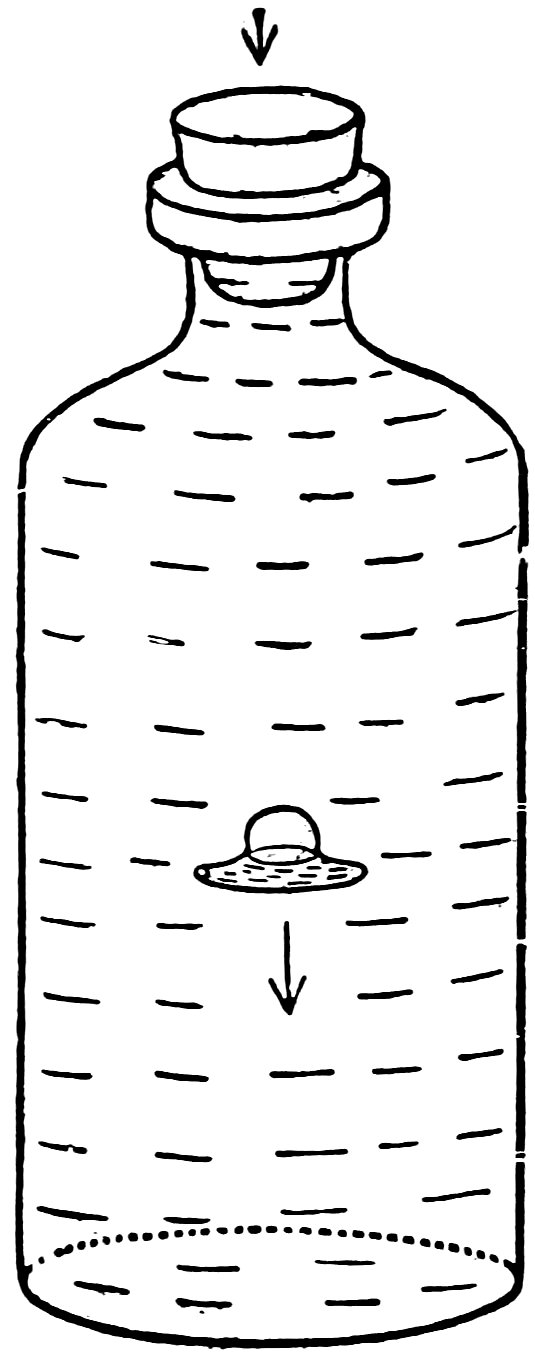
FIG. 33
THE SUBMARINE SUBMERGES
Fill a bottle to overflowing with water, insert the submarine open end down, insert the solid rubber stopper and press down hard (Fig. 33). Does the submarine submerge?
Release the stopper. Does the submarine rise and does it also move forward?
Turn the bottle on its side and release the stopper quickly. Does the submarine shoot forward at a great rate (Fig. 34)?
The submarine acts in this magical manner for the reasons given in Experiment 9. When you press the stopper in, you compress the air in the submarine and force water in until the submarine weighs more than an equal volume of water and it sinks. When you release the pressure on the stopper, the compressed air forces the water out until the submarine becomes lighter than an equal volume of water and it rises. The water rushing out through the opening exerts pressure backward on the water in the bottle and the reaction drives the submarine forward.
If your friends do not know about the little submarine, you can mystify them as follows: Tell them that submarines are 24just like other fish; namely, they lay eggs, and the little eggs hatch out after a certain number of days (of course, your friends will know that you are only joking). Pretend that you found one of these submarine eggs, hatched it out in lukewarm water, and that you have trained the baby submarine to do some simple tricks. For example, that you have trained it to submerge, rise, and attack, when you issue the commands “submerge,” “rise,” and “attack.”
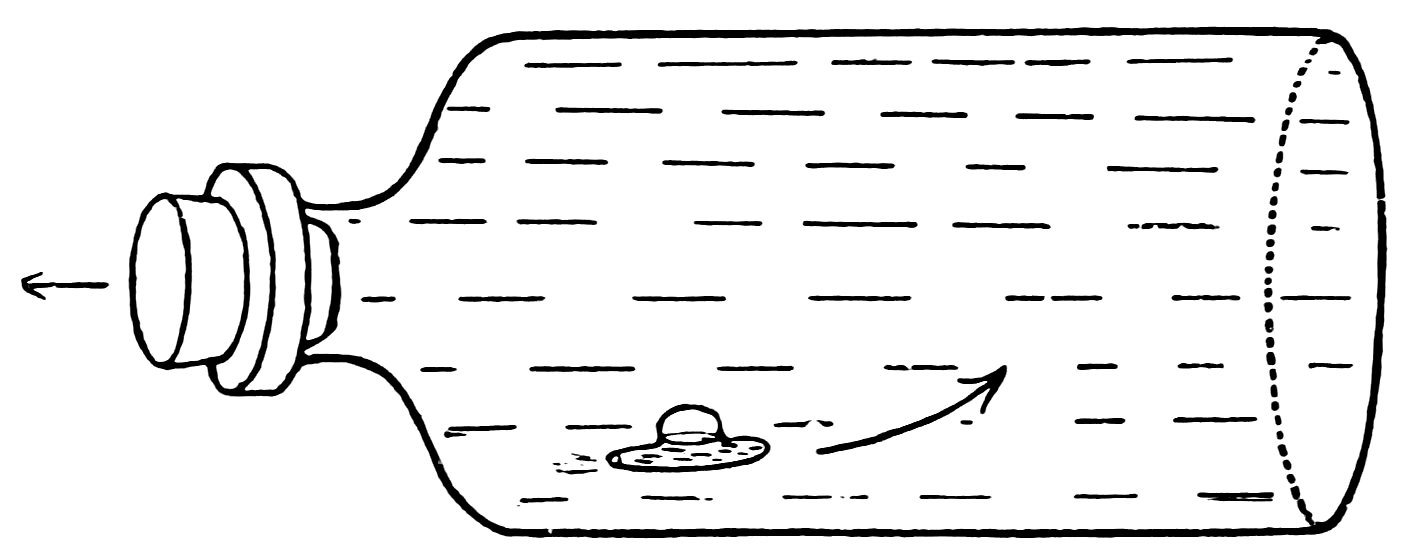
FIG. 34
THE SUBMARINE SHOOTS FORWARD
Tell them to watch the submarine carefully and to notice that it takes in water and submerges when you issue the command “submerge.” Stand the bottle on the table, issue the command “submerge” and, while your friends are watching the submarine, press down on the stopper unknown to them.
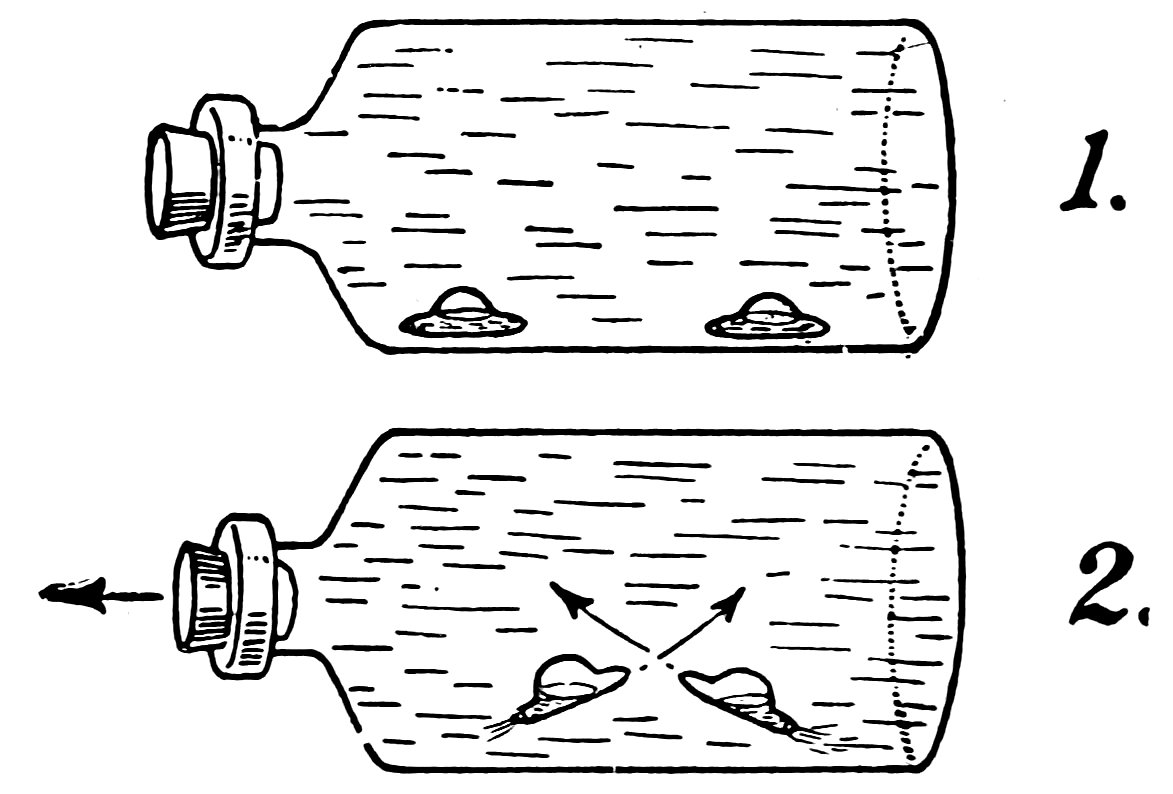
FIG. 35
A SUBMARINE BATTLE
Tell them to watch the submarine carefully again and to notice that it expels water and rises when you issue the command “rise.” Issue the command and unknown to them release the pressure on the stopper slowly.
Repeat with the command “attack” and release the pressure quickly.
Make a second submarine, place it in a large bottle 25with the first submarine, turn the bottle on its side, and make the submarines manœuver by moving the stopper in and out.
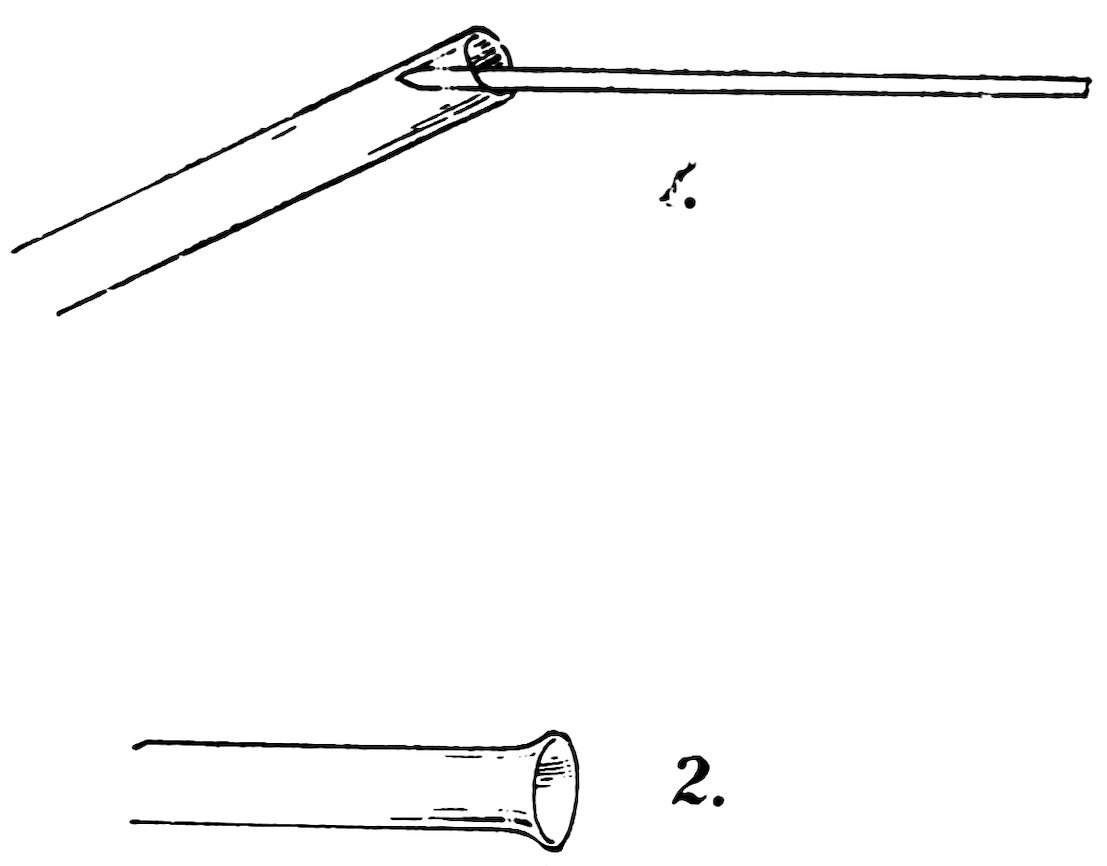
FIG. 36
FLARING A TUBE
Finally arrange them so that they are on the bottom, facing each other bow to bow, two or three inches apart (1, Fig. 35), and release the stopper quickly. Do the submarines try to ram each other (2, Fig. 35) in a most realistic manner?
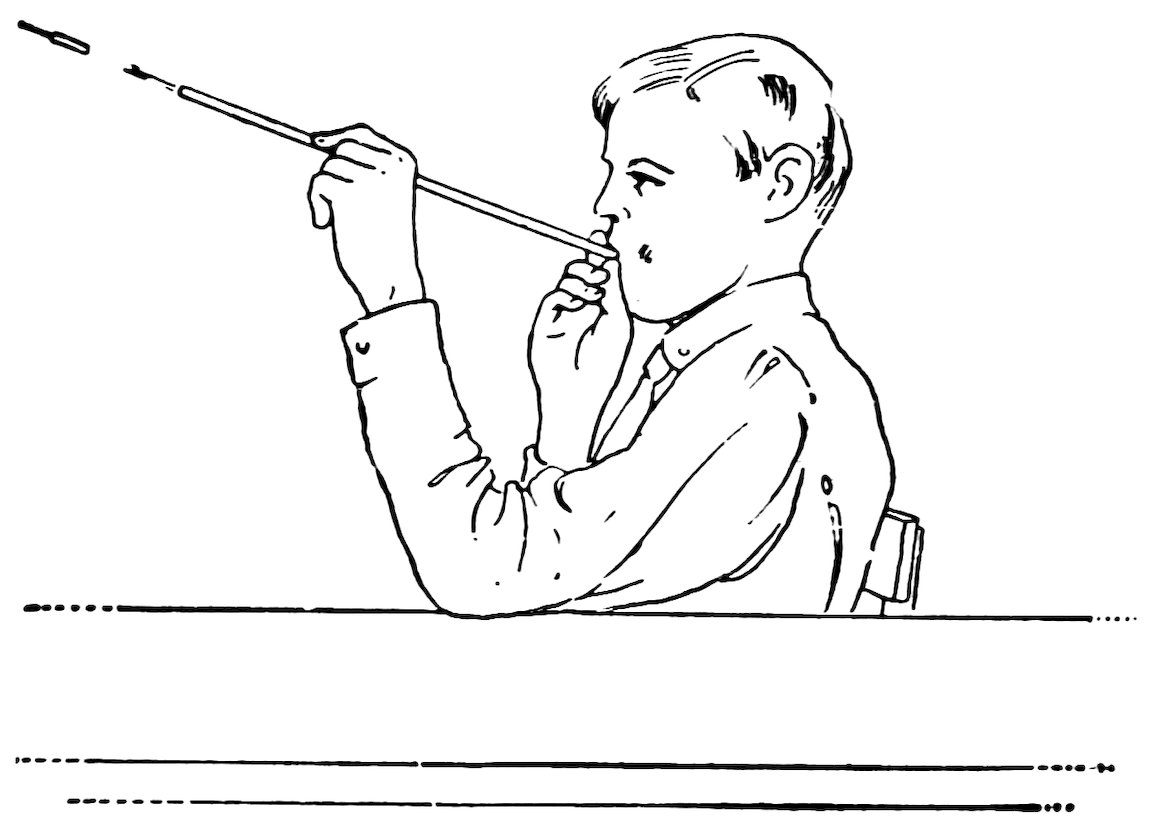
FIG. 37
AN AIR GUN
Heat the end of a piece of No. 2 tube until it is red hot, take it out of the flame, hold the flaring wire inside the end, and press outward gently while you revolve the tube (1, Fig. 36). Do you find that the end is flared out (2, Fig. 36)?
Take a full length piece of No. 4 tube and flare both ends slightly. This is the air gun (Fig. 37).
Now to make an arrow, cut off the lighting end of a match and insert a pin in the other end (Fig. 38).

FIG. 38
THE ARROW IS SHOT PIN-END FIRST
Insert this arrow in the air gun and blow it out. Does it come out with considerable speed?
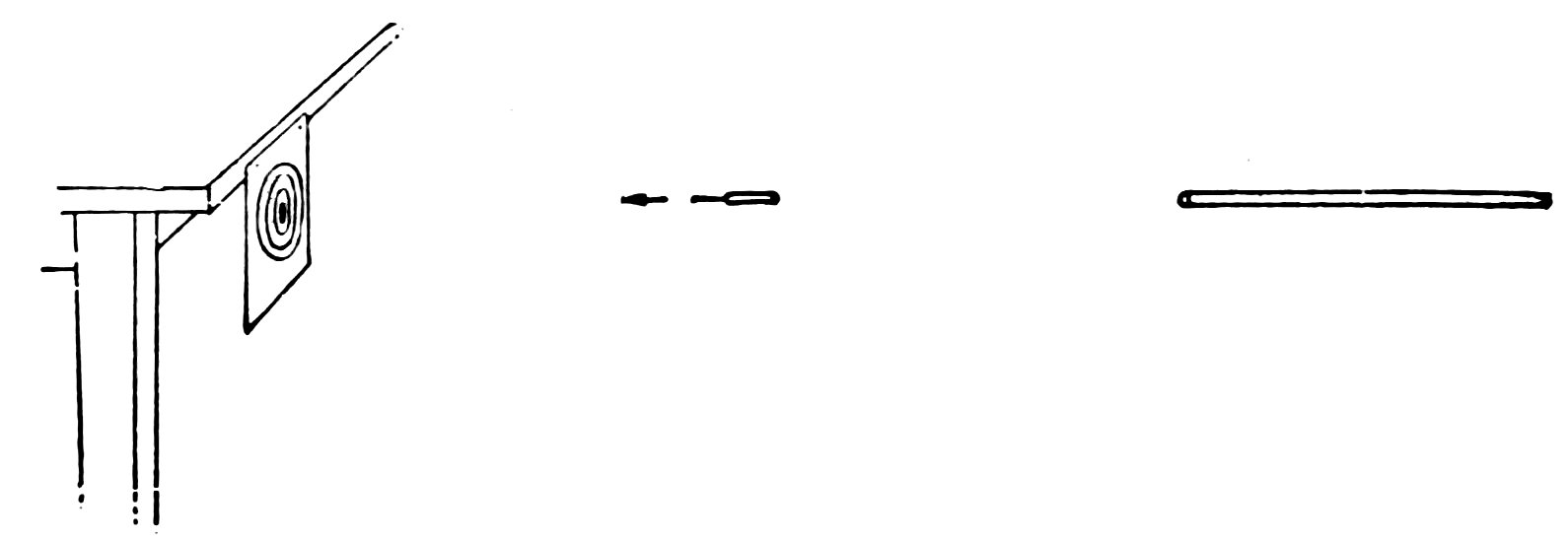
FIG. 39
A SHOOTING MATCH
Draw a target on a piece of paper and hang it up, away from the wall or at the edge of the table, where there will be space behind for the arrows to pass through. Now shoot at the target with your air gun (Fig. 39). Do you find that the arrow makes holes in the target and sometimes goes right through?
The bull’s-eye of a target is usually 1 inch in diameter, the next circle outside is 2 inches in diameter, the next 4 inches, and the outer circle 5 inches.
Get up a shooting match and keep track of the score made by each.
If the bull’s-eye is cut anywhere by the arrow, the count is 5 points; a cut anywhere inside or touching the 2-inch circle counts 4 points; anywhere inside or touching the next two circles counts 3 and 2 points respectively.
The one who makes the highest score in five shots is the winner.
It is more sanitary if each shooter has his own air gun and arrows.
Go outside and see which of you can shoot his arrow to the greatest height and to the greatest distance.
Give each contestant five shots.
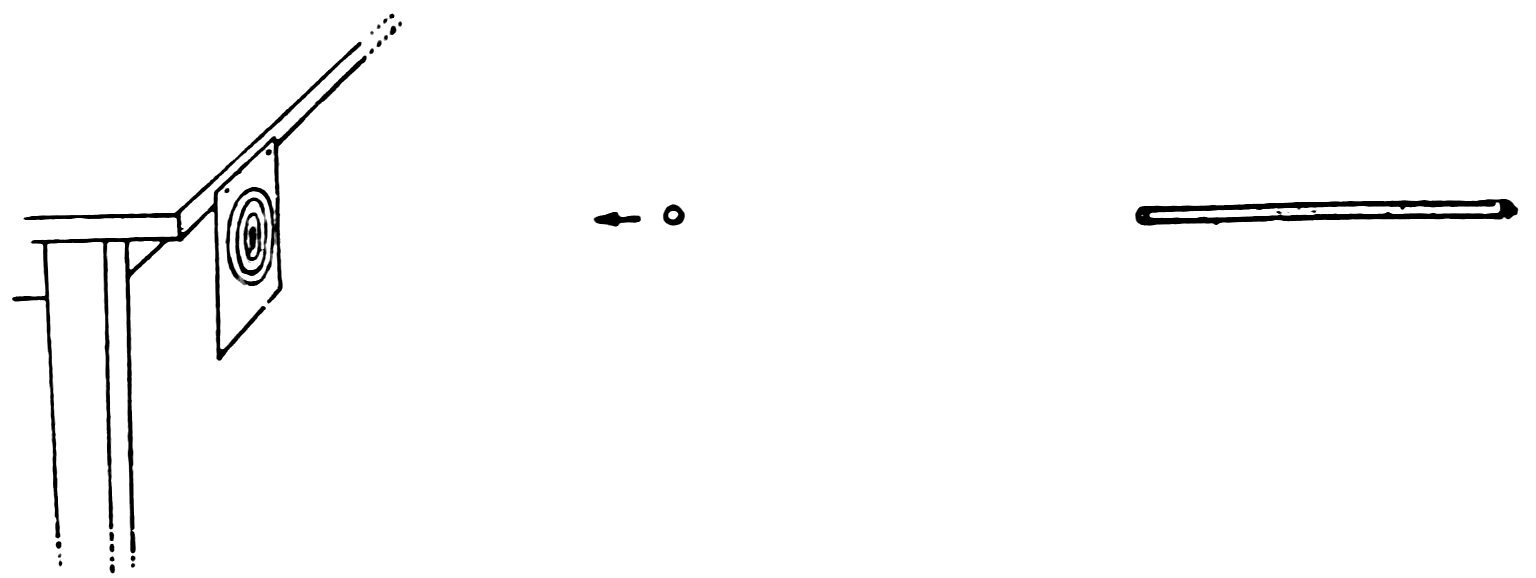
FIG. 40
THE PEA SHOOTER IN ACTION
You can make fair estimates of the heights if you shoot up beside a building or tall tree.
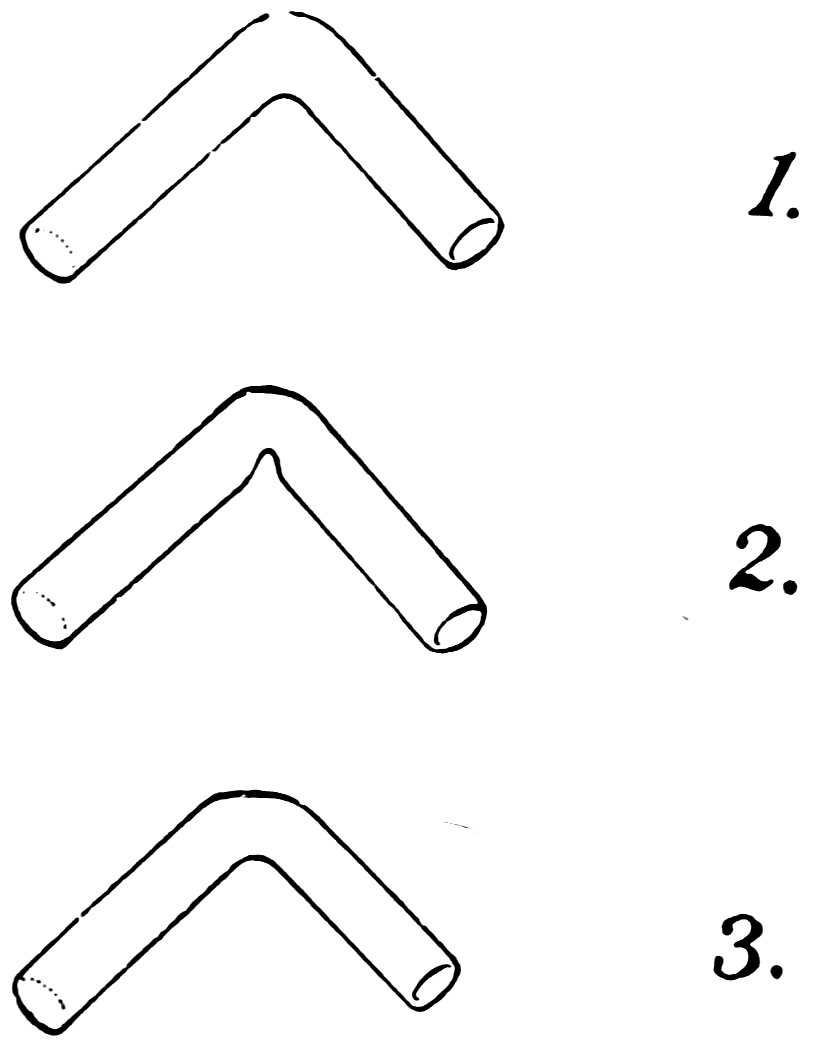
FIG. 41
BENDS
Take a full length piece of No. 6 tubing, smooth both ends and flare them out slightly. This makes an excellent pea shooter. Try it with peas. Do you find that they come out with great speed?
Make a target on a piece of paper, hang it up away from the wall or at the edge of the table, and shoot at it (Fig. 40). Do you find that the peas go right through the paper?
Arrange a match with your friends and keep track of the score as in Experiment 25.
A good bend has the same diameter in the bend as in the remainder of the tube (1, Fig. 41). It is rather difficult to make 28because the tube tends to cave in on the inside of the bend (2) or flatten on the outside (3), or both.
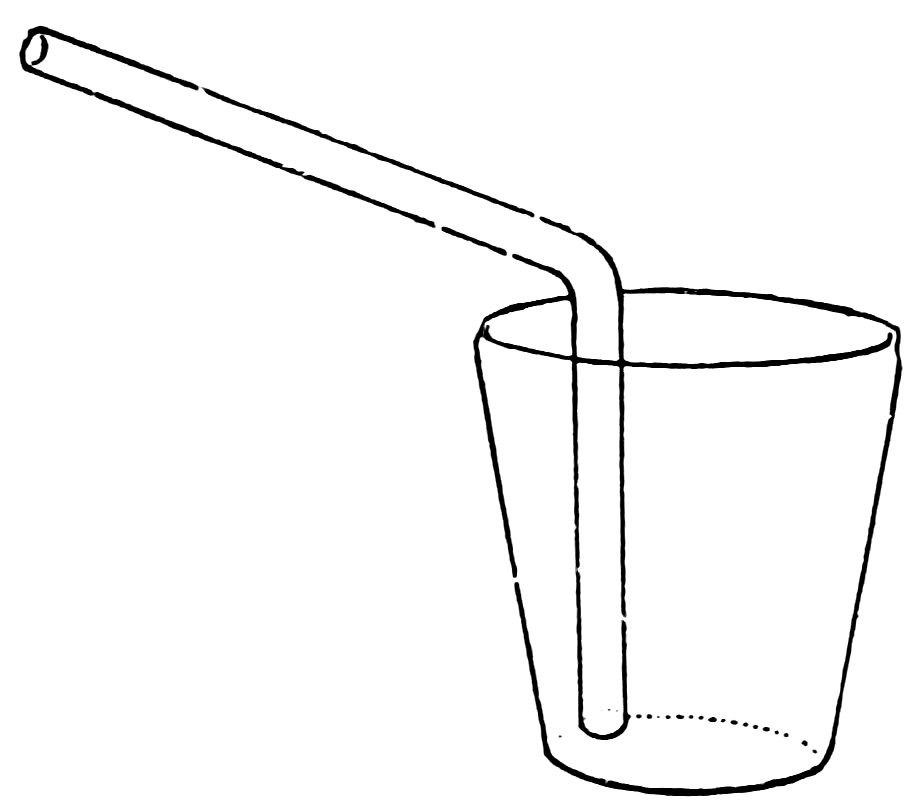
FIG. 42
A DRINKING TUBE
Make the bend as follows: Heat a piece of No. 2 tube about 2 inches from one end in the lamp flame, turn it constantly and move it back and forth endwise to heat a length of about 2½ inches. When soft, take the tube out of the flame, and bend the ends upward until the angle is 90°.
If the bend is flat on the inside or outside, close one end of the tube in the blowpipe flame, smooth the other end and allow them to cool, then heat the flat side of the bend in the blowpipe flame and blow it out slightly. This makes the diameter of the tube at the bend equal to that of the remainder of the tube. Cut off the closed end, smooth the edge, and your bend is complete.
Make bends with No. 4 tube.
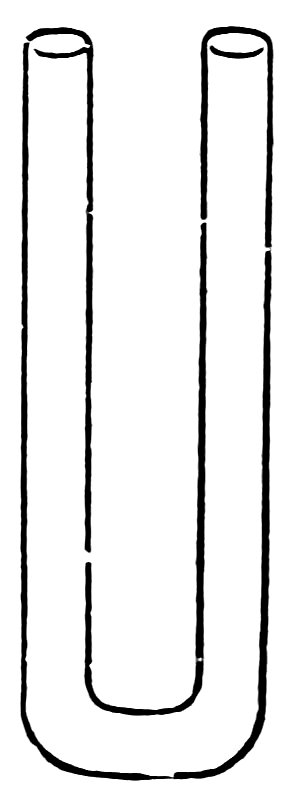
FIG. 43
A SIPHON
Many times when there is sickness in the house, it is convenient to have a glass drinking tube (Fig. 42), through which the patient can drink without raising his head.
Make such a tube from a piece of No. 4 tubing. The short arm is equal in length to the depth of the tumbler; the long arm, or mouthpiece, is about 1 inch longer than this.
Cut off a piece of No 4 tubing 8 inches long, make 29two right-angled bends about 1 inch apart at the center, smooth both ends, and your siphon is complete (Fig. 43).
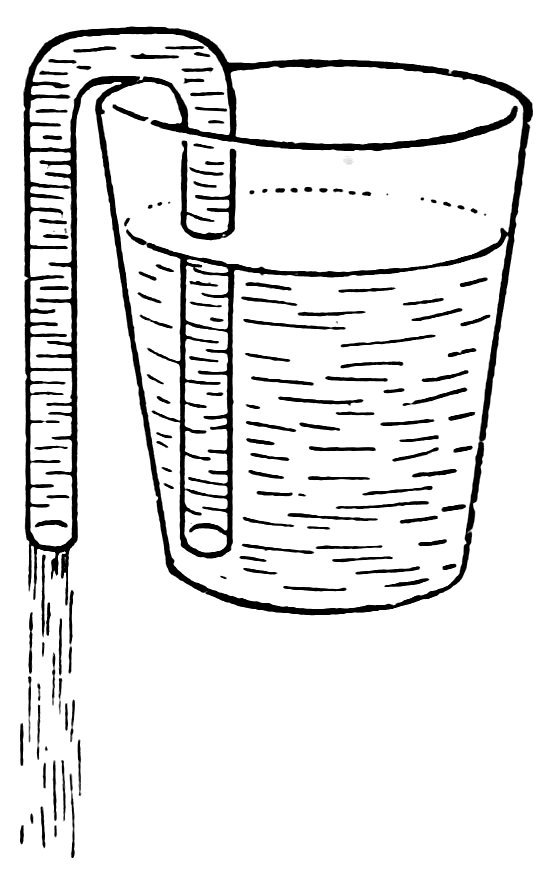
FIG. 44
A SIPHON
Put one arm of the siphon in a tumbler of water and suck air out of the other end. Does the water start running and does it continue to run in a most magical way (Fig. 44) until the water is below the end of the siphon in the tumbler?
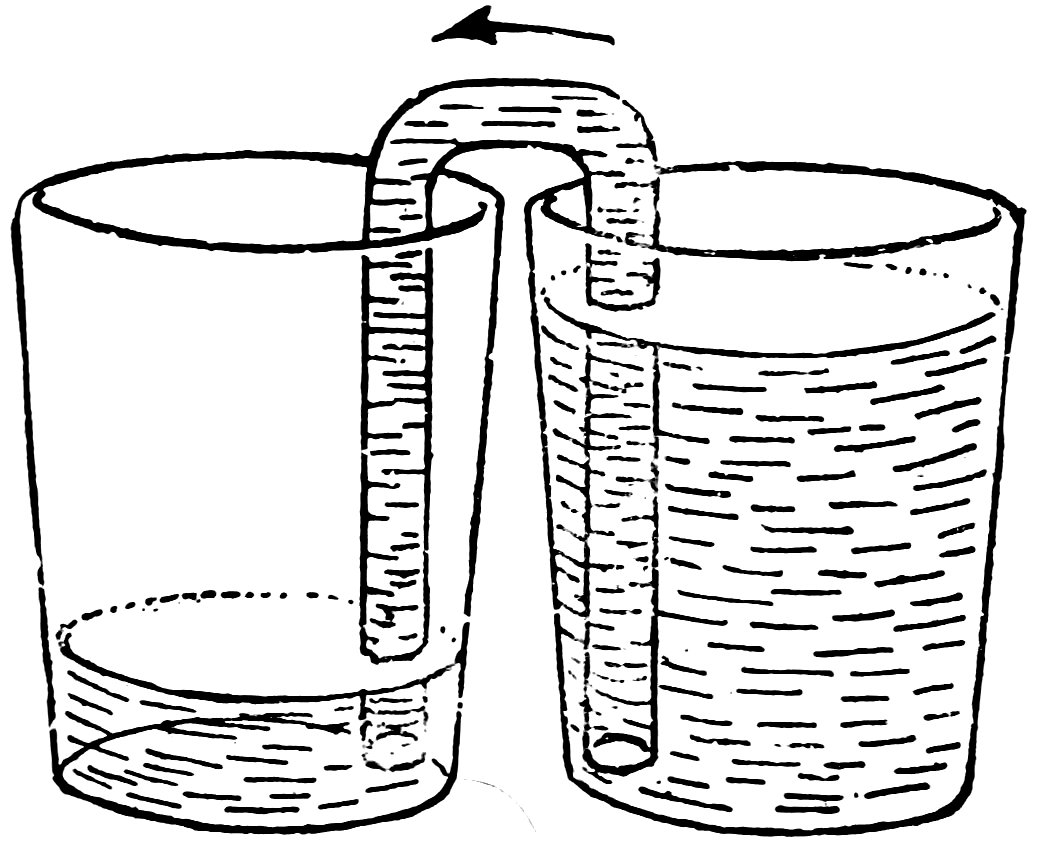
FIG. 45
FROM THE HIGH LEVEL TO THE LOW
Fill the tumbler with water again, start the water running, put the outer arm of the siphon in an empty tumbler, and stand both tumblers on the table (Fig. 45). Does the water run up one arm of the siphon and down the other into the empty tumbler? Does it stop running when the levels are the same?
Stand the first tumbler on a book. Does the water run again and stop when the levels are again the same (Fig. 46)?
Place the lower tumbler on the book and the upper tumbler on the table. Does the water now run in the opposite direction until the levels are again the same?
Raise one tumbler a foot or so above the table. Does the water run up over the edge and drop into the second? Now before the upper tumbler is empty, lower it in such a way that an arm of the siphon is in each tumbler, and raise the second tumbler. Does the water now run in the opposite direction?
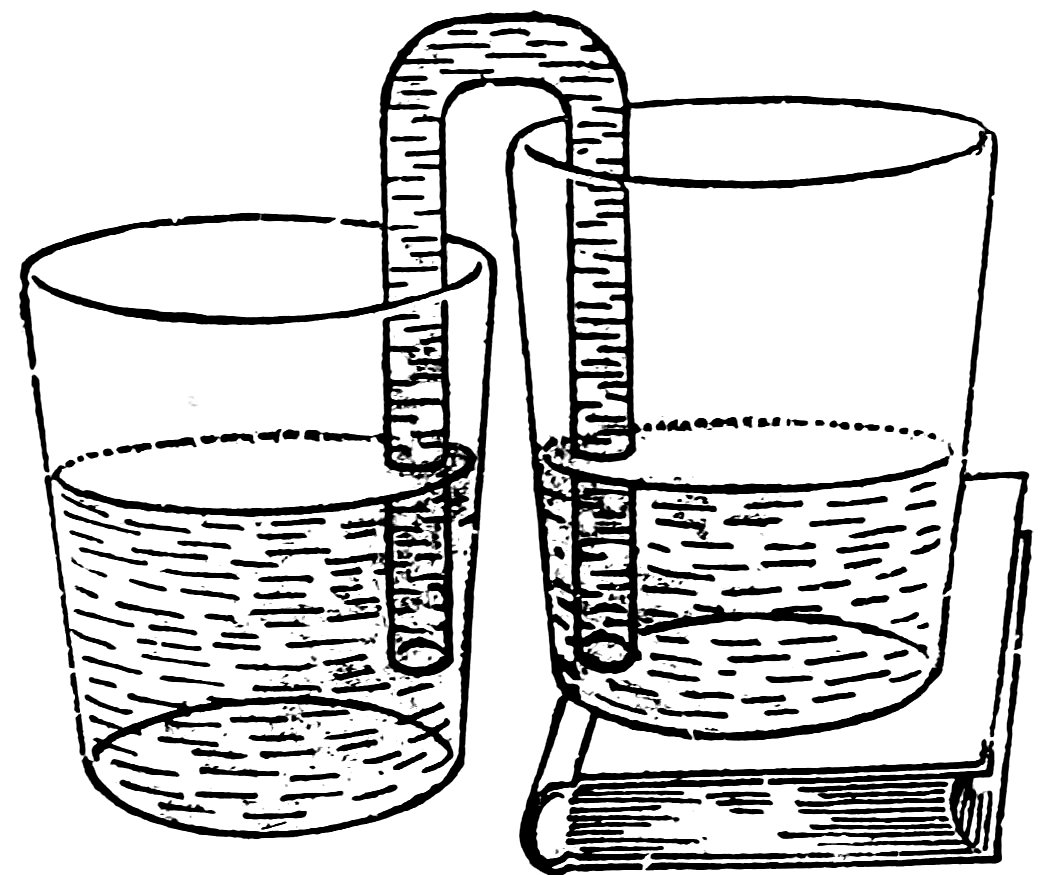
FIG. 46
THE WATER STOPS WHEN LEVELS ARE THE SAME
You boys who have the Gilbert set on “Hydraulic and Pneumatic Engineering” will know that it is the pressure of the atmosphere which causes the water to run up over the edge of the tumbler in this magical way.
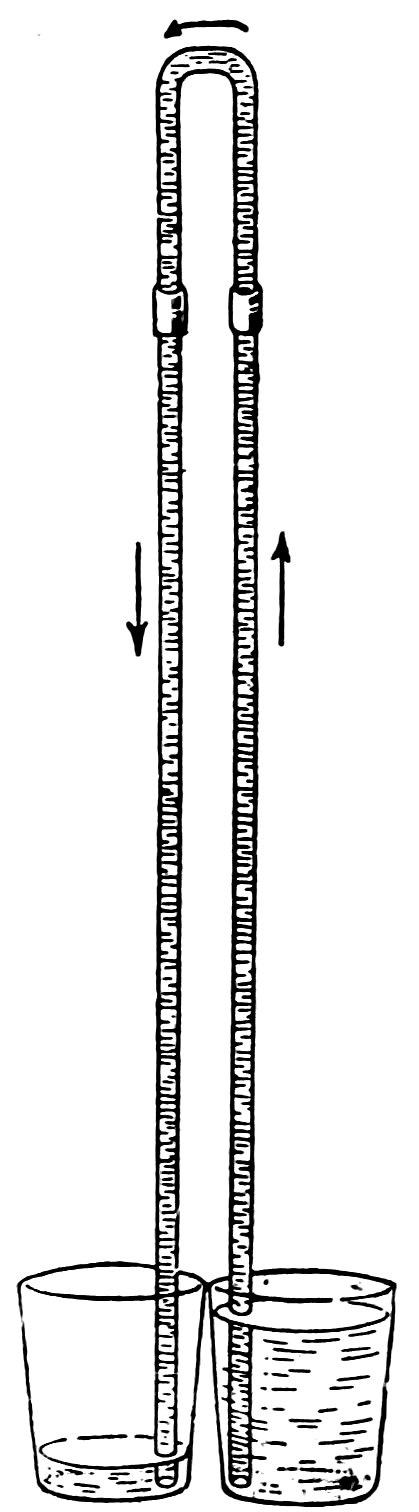
FIG. 47
SIPHONING WITH LONG TUBES
Attach a full length of No. 4 tube to each arm of the siphon, as in Fig. 47, and repeat the experiments described above.
Note: When you insert a glass tube into a rubber coupling or rubber stopper, wet the end of the glass tube and the inside of the coupling or stopper, grasp the tube near the end to be inserted, and insert with a twisting motion.
Attach a working handle to one end of a piece of No. 2 tube, heat the tube about one inch from the end in the lamp flame, turn constantly until soft, then remove from the flame, and draw it out about 3 inches. When cool, break off the thin tube, cut off the nozzle to a length of about 2½ inches, smooth the large end, and your nozzle (Fig. 48) is complete.
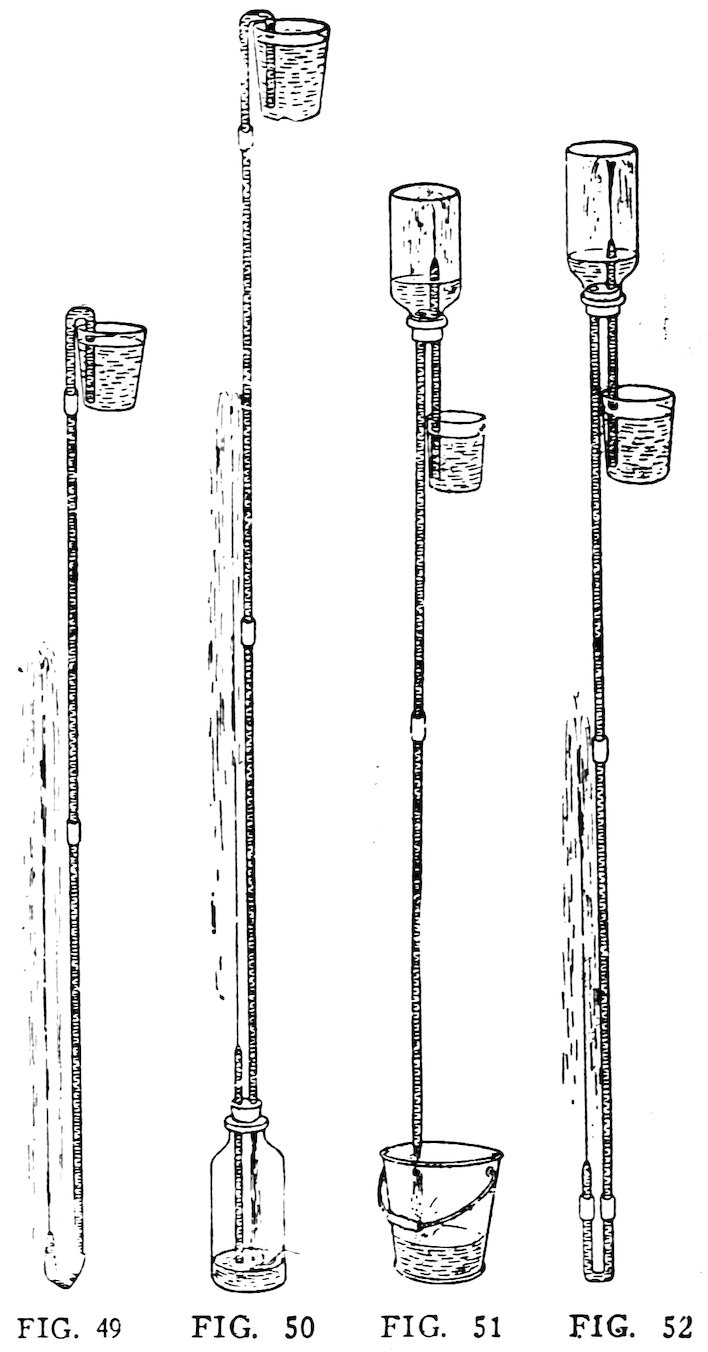
Arrange the apparatus as in Fig. 49, and 31suck air out of the nozzle. Have you made a beautiful fountain?
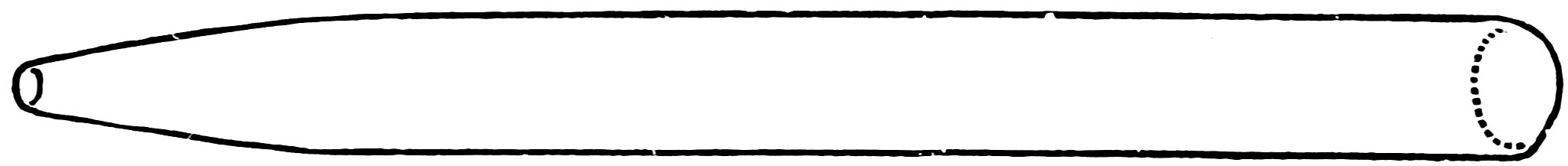
FIG. 48
A NOZZLE
YOU MAKE A NUMBER OF MAGIC FOUNTAINS
Make a nozzle 6 inches long out of No. 2 tube. Smooth the ends of the nozzle, and long tubes. Arrange the apparatus as in Fig. 50 and suck air out of the nozzle until the water runs in the siphon. Does the water squirt out of the nozzle in a magical manner?
Arrange the No. 2 apparatus as in Fig. 51, with the nozzle inside the bottle. Now to start the apparatus: Fill the bottle about quarter full of water, insert the tubes in the stopper as shown; insert the stopper into the mouth of the bottle; invert the bottle; then put the short tube in a tumbler full of water and the long tube in an empty pail or basin. Is there a magical fountain inside the bottle?
32Repeat this with a taller bottle, if you can find one to fit your two-hole stopper. Do you get a higher fountain?
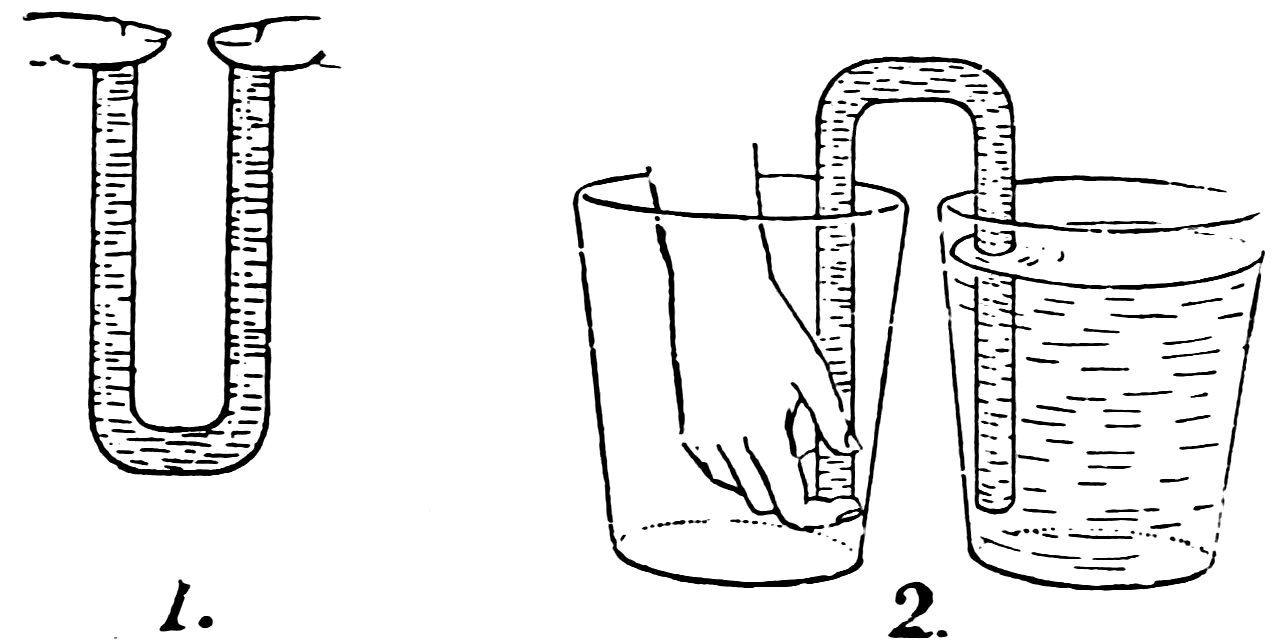
FIG. 53
STARTING A SIPHON
Make another nozzle and attach it to the apparatus used in the last experiment by means of the inverted siphon (Fig. 52). Start the experiment as described above. Do you get two fountains?
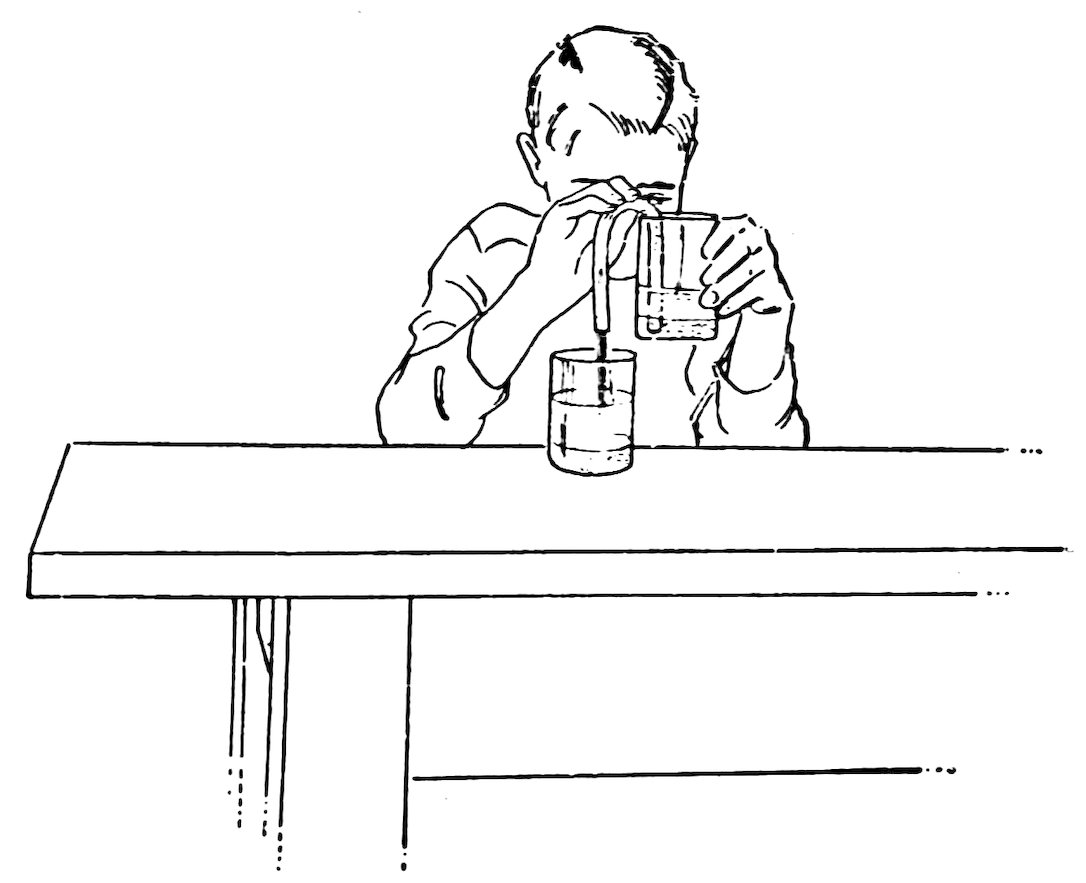
FIG. 54
SIPHONING SAND
You can start a siphon without sucking the air out of it as follows: Fill the siphon with water, put a finger over each end (1, Fig. 53), place one end in a tumbler full of water and remove the finger under water (2, Fig. 53), then remove the other finger. Does the siphon start?
In this case the water you pour into the siphon drives the air out, and this is the reason you do not need to suck the air out.
Arrange a siphon (Fig. 54), start the water flowing, and then pour sand or mud into the upper tumbler. Is the sand of mud siphoned over into the lower tumbler?
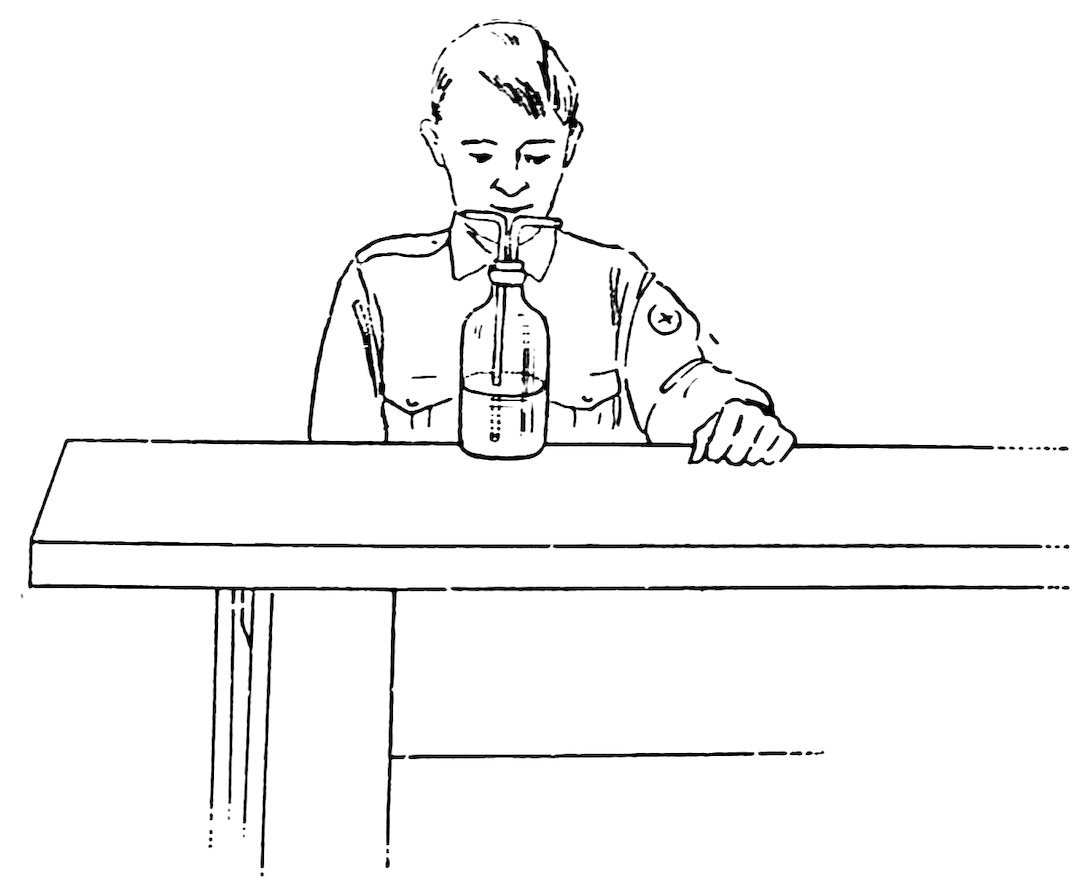
FIG. 55
A SQUIRT BOTTLE
Attach a long tube to the outer arm of the siphon and repeat the experiment. Is the sand or mud siphoned more rapidly and more thoroughly?
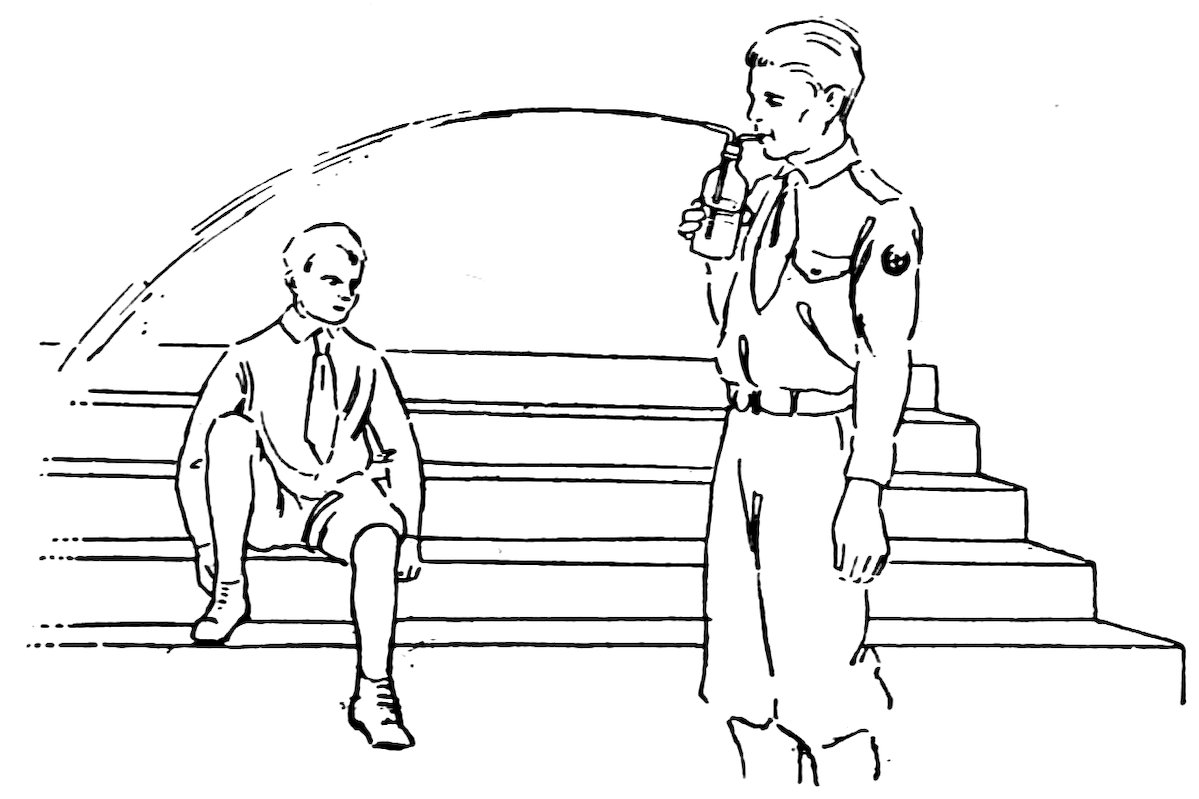
FIG. 56
SQUIRT BOTTLE IN ACTION
Make a nozzle at one end of a piece of No. 2 tubing, make a bend near the nozzle, cut off the other end at such a length that it will reach to within ¼ inch of the bottom of the bottle, smooth this end, allow it to cool, wet the tube and the two-hole stopper, shove it through one hole of the stopper, insert an elbow in the other hole, and your squirt bottle is complete (Fig. 55).
Fill the bottle with 34water, and blow through the elbow. Do you get a fine long stream from the nozzle (Fig. 56)?
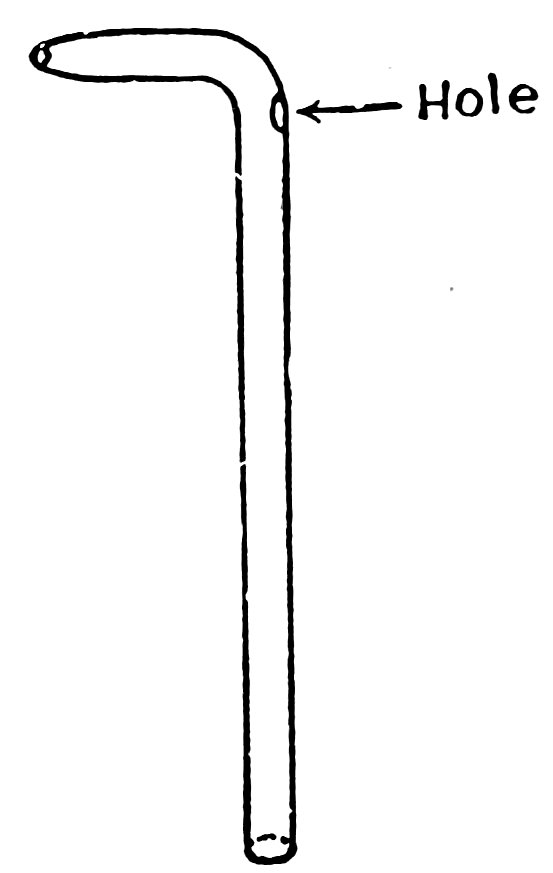
FIG. 57
TUBE FOR TRICK SQUIRT BOTTLE
You can have any amount of fun with a trick squirt bottle. It is exactly the same as the squirt bottle described in Experiment 41 except that it has a hole just below the bend (Fig. 57).
FIG. 58
MAKING A SMALL HOLE
To make the hole, make the long bent nozzle as in the last experiment, then heat the tube just below the bend in the blowpipe flame, touch a piece of glass tube to the red-hot glass (1, Fig. 58), and pull it away (2, Fig. 58). Do you find that the hot glass is pulled out into a thin pointed tube? Break off the thin tube close to the large tube, heat in the blowpipe flame until the edges are smooth and at the same level as the sides of the large tube. Flare the edges of the hole, if necessary; it should be about ⅛ inch in diameter.
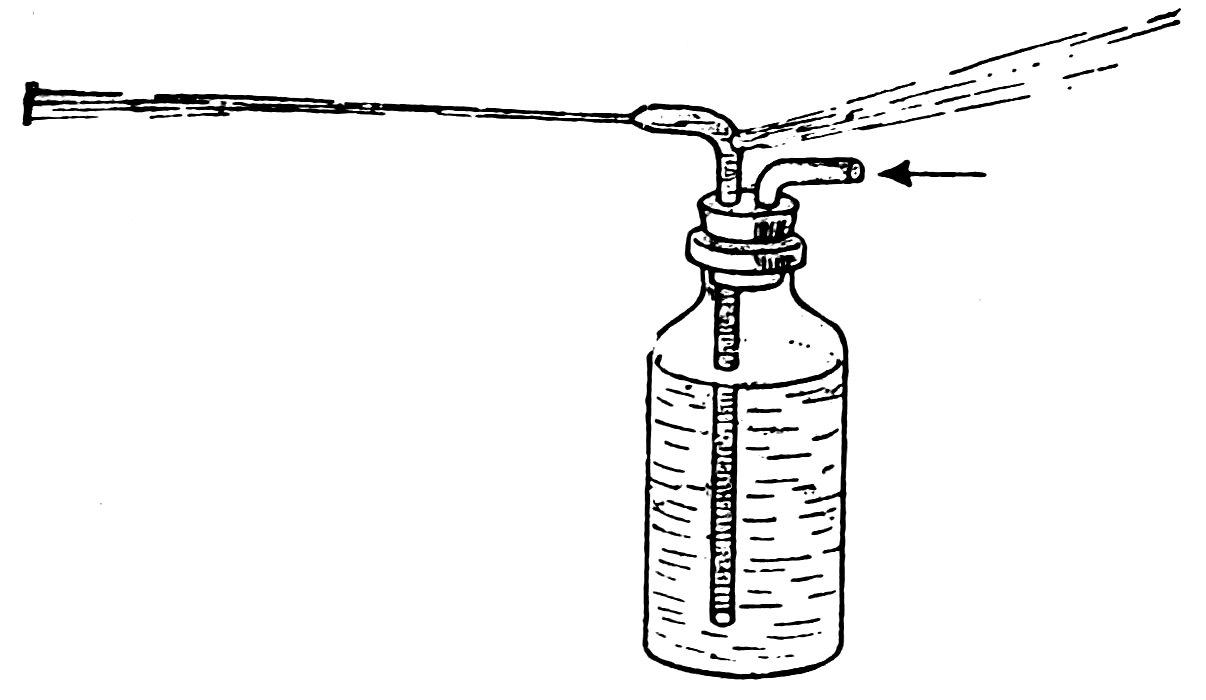
FIG. 59
TRICK SQUIRT BOTTLE
Now fill the bottle with water, and blow 35hard (Fig. 59). Do you find that one stream of water is driven into your face and another out of the nozzle?

FIG. 60
TRICK BOTTLE IN ACTION
Now to have fun with your trick bottle, show it to one friend at a time. Do not ask him to try the bottle, just go where he can see you and squirt a long stream, but unknown to him have your finger over the hole below the bend.
FIG. 61
A SIMPLE ENGINEER’S LEVEL
(From Aldous’ Physics. Courtesy of The Macmillan Company)
Your friend will just naturally want to have a try at it. So you say “All right, let’s see who can squirt the longest stream.” Tell him that all he has to do is to take a deep breath and blow as hard as he can. He will do so, with laughable results (Fig. 60).
Now together find another friend. Do not ask him to blow, but each of you blow as long a stream as you can, where 36he can see you. He will beg to be allowed to try, and finally you let him, with the same laughable results.
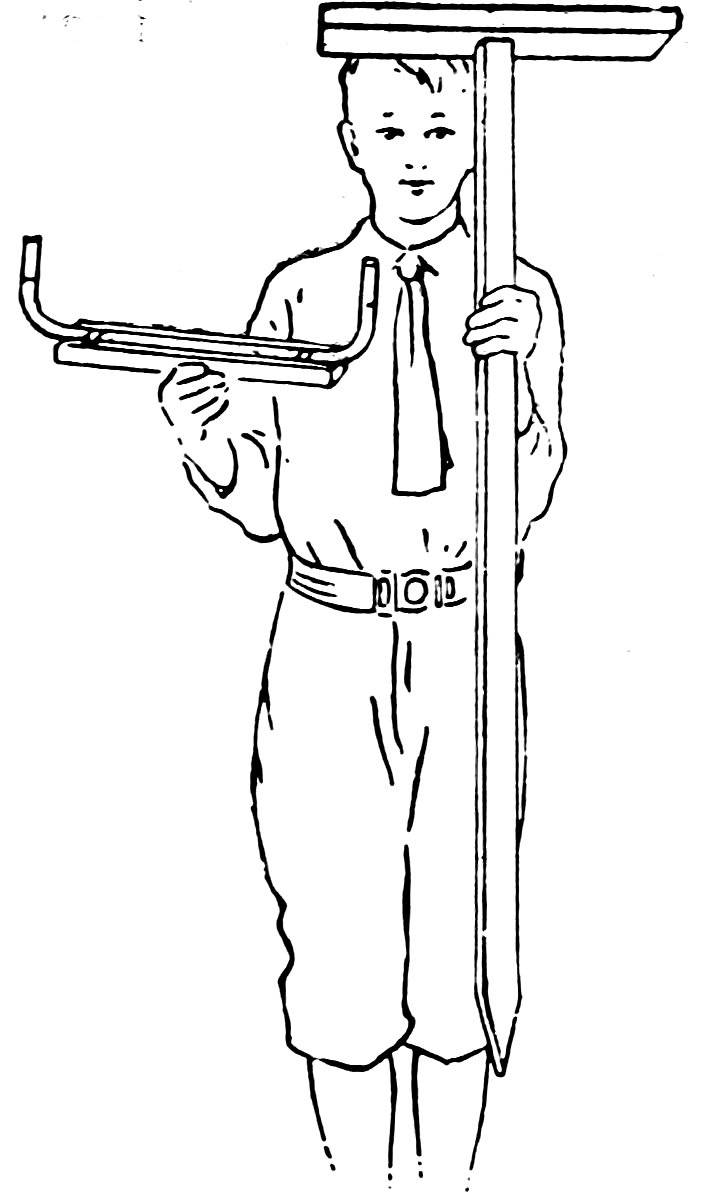
FIG. 62
ONE-LEGGED TABLE AND LEVEL
Repeat with other friends.
You can make one form of engineer’s level (Fig. 61) as follows: Take a full length of No. 6 tubing, bend it up 4 inches at each end, smooth the ends, attach it to a small board, rest the board on a one-legged table, and you have a serviceable level (Fig. 62).
Fill the tube with water, shove the pointed end of the leg into the ground and sight along the outside of the upright tubes at the level of the water surfaces. The line along which you sight is exactly horizontal, because the water surfaces are at exactly the same level.
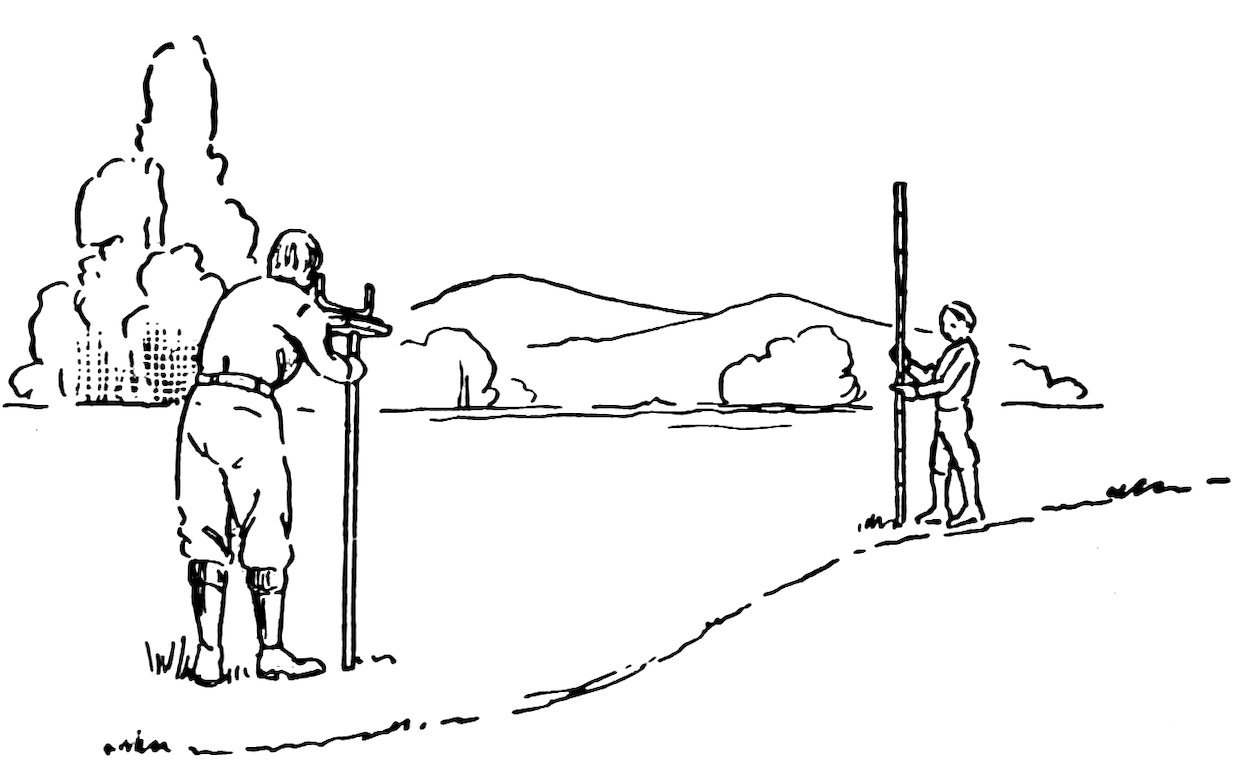
FIG. 63
HOMEMADE LEVEL IN USE
An engineer’s level is used to find the difference in level of two or more points (Fig. 63).
To practice using your level, find the difference in level of two points 100 feet apart on a road, sidewalk, or railroad.
To do this, you 37must first make what is called a leveling rod. Find a piece of wood about one or two inches square and six or more feet long, mark on it feet and inches, beginning at the bottom end, and your leveling rod is complete.
Now to find the difference in level of two points 100 feet apart, scratch a line or insert a small stake at one point, then pace off 100 feet and mark the second point. Now set up your level between the two points, ask a friend to hold the rod on the ground and upright, at the first point, sight along the water levels at the rod, and ask your friend to move his finger, or a white card, up and down until it is exactly in your line of sight. Now ask your friend to tell you exactly where his finger or card is and record this height. Let us suppose that it is 4 feet 6 inches above the ground. Now leave the level exactly where it is, ask your friend to hold the rod upright at the second point, and again sight along the water levels at the rod. Let us suppose that his finger or card is now exactly 3 feet above the ground.
The difference in level at the two points is 4 feet 6 inches minus 3 feet or 1 foot 6 inches. That is, the second point is 1½ feet above the first point or the grade is 1.5 feet in 100, or 1.5 per cent.
You can now mark a third point 100 feet beyond the second point, set up your level between the second point and third point, place the rod at the second point, then at the third point, and find their difference in level as above. If the third point is 1 foot above the second, the total rise in the 200 feet is 2½ feet; if, however, it is 1 foot below the second, the rise is 1½ minus 1 or ½ foot in the 200 feet.
You can repeat this with as many points as you please.
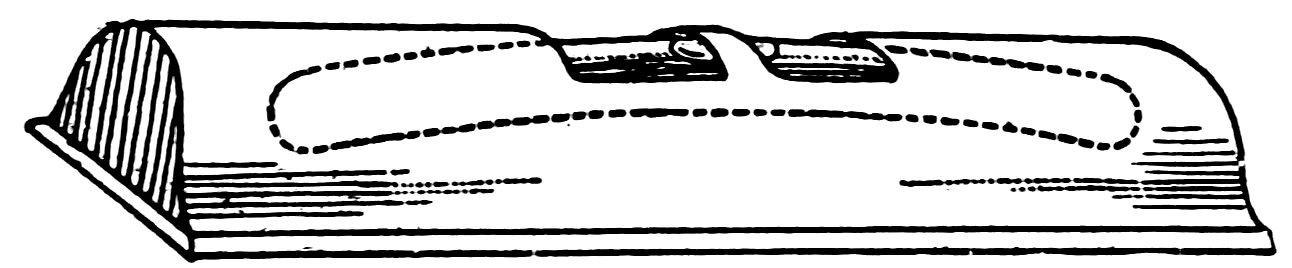
FIG. 64
A SPIRIT LEVEL
The spirit level (Fig. 64) is simply a curved glass tube filled with alcohol except for the bubble and closed at both ends. The curve of the tube is part of a circle.
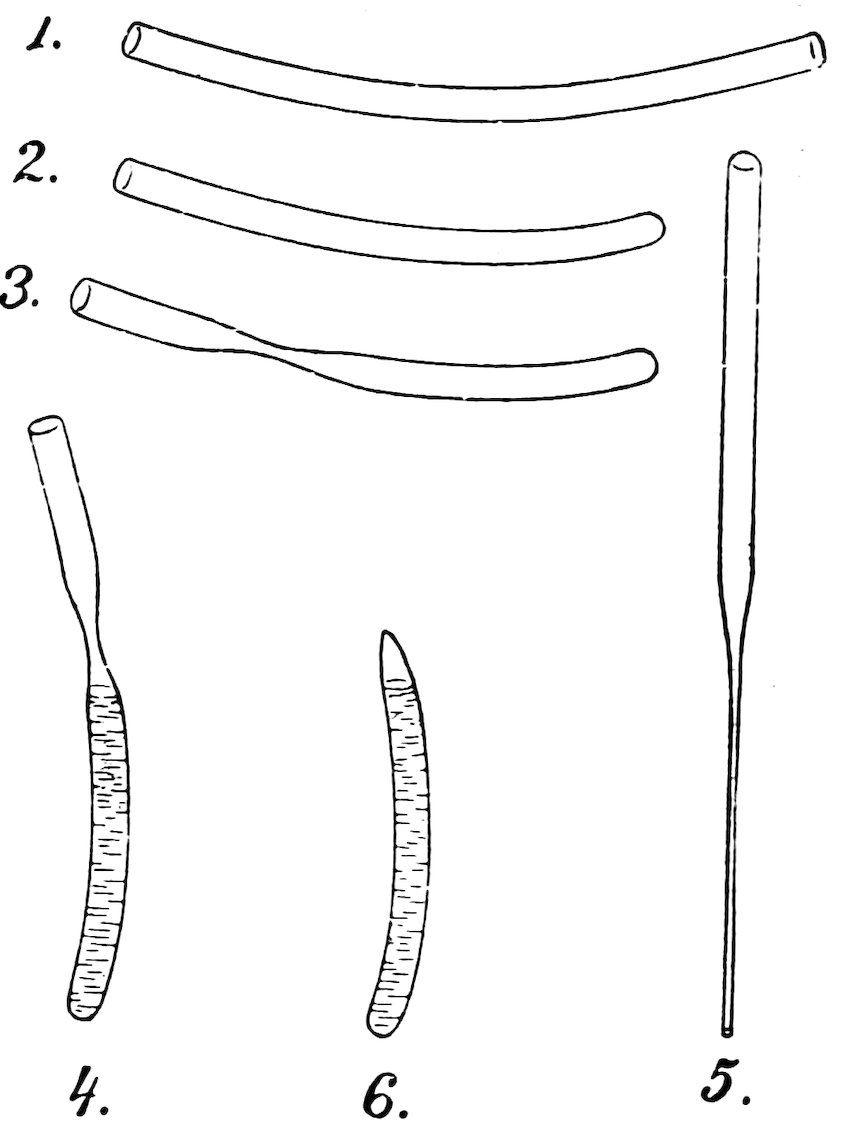
FIG. 65
MAKING A SPIRIT LEVEL
Make a spirit level as follows: Take a piece of No. 4 tube about 7 inches long, heat a space about 3 inches long in the lamp flame, turn constantly, and when soft remove from the flame, hold both ends and allow the center to sink into a slight curve (1, Fig. 65).
Let the tube cool, mark the center of the curve with ink, and make marks 2 inches from the center on each side.
Hold the tube crosswise in the lamp flame, heat at one mark, draw down the tube and close it (2).
In a similar manner draw down the tube at the other mark but do not close it (3).
Let the tube cool and fill it with alcohol to the level shown in 4. To do this easily make the pipette (5), suck alcohol into it within about 1 inch of the top, put your finger over the top, insert the lower end of the pipette to the bottom of 4, and remove your finger.
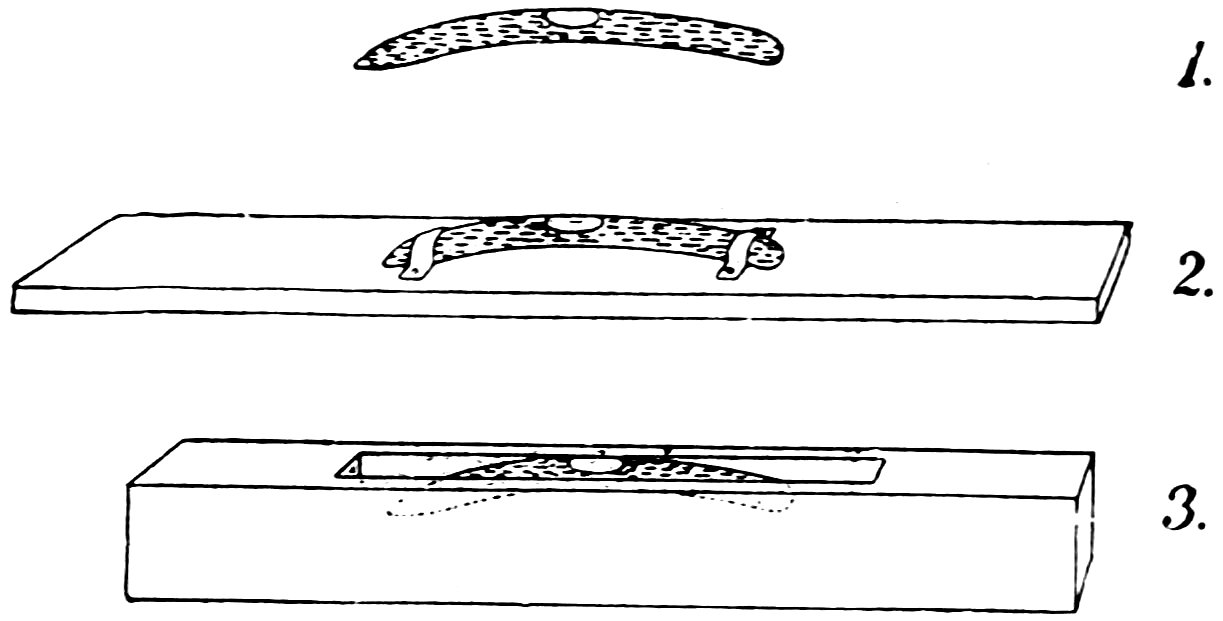
FIG. 66
MOUNTING THE SPIRIT LEVEL
Heat the small part of 4, without heating the alcohol, and close the tube (6). Now attach the level to a smooth board as 2 or 3, Fig. 66, mark the center of the bubble, and your spirit level is ready for use.
Attach a rubber coupling to the large end of one of your No. 4 nozzles, close the other end of the coupling with a glass plug, and your fountain-pen filler is made (Fig. 67).
To make the plug, close one end of a piece of No. 4 tubing, allow it to cool, cut off to a length of 1 inch and smooth the rough edges. Insert the closed end of this plug into the rubber coupling.
Practice using the filler by drawing up and shooting out water.

FIG. 67
A FOUNTAIN-PEN FILLER
Take a half length of No. 6 tube and smooth both ends in the lamp flame or blowpipe flame.
Now to make a plunger: Cut an 8½-inch length of No. 2. smooth one end, close the other end and blow a slight bulb. When cold, wet the closed end and insert it into a small wet rubber coupling.
Note: Always grasp a tube near the end when you insert it 40into a coupling or stopper, because if you hold it too far back you may break it. Insert it with a twisting motion, after wetting the end and the inside of the coupling or stopper.

FIG. 68
A SYRINGE
Wet the inside of the large tube, wet the plunger and rub it on a cake of soap to make it slippery, then try it in the large tube. If the plunger is too large, stretch the coupling lengthwise; if it is too small, crowd the coupling together lengthwise. If the bulb is too large or too small, dry it, heat in the blowpipe flame until it shrinks, and blow another.
When the plunger is made, attach a No. 4 nozzle to the No. 6 tube with a large coupling, arrange as in Fig. 68, and your syringe is made.
Fill the large tube with water and see how long a stream you can make.

FIG. 69
ANOTHER SYRINGE
Heat a piece of No. 6 in the blowpipe flame at a length of 7½ inches and draw it out into a nozzle; smooth the other end in the lamp flame. Use the same plunger as in Experiment 48, and your syringe is made (Fig. 69). Try it out with water.
Heat a piece of No. 6 tube in the blowpipe flame at a length of 7½ inches, draw it out, and close the end, then smooth the other end.
41Now to make a plunger: Heat a piece of No. 2 tube 8½ inches from one end in the lamp flame, draw it out into a nozzle, and break it off, leaving a small hole at the end of the nozzle. Smooth the other end in the lamp flame, flare it out slightly, allow it to cool, dip it into water and insert it into a small wet coupling.

FIG. 70
A THIRD SYRINGE
Now fill the large tube with water and insert the coupling plunger (Fig. 70). Do you get a fine long stream?
Use the No. 6 tube and the No. 2 plunger from Experiment 48, arrange as in Fig. 71, blow across the top, and move the plunger up and down. Do you get a most diabolical sound?
FIG. 71
THE DIABLO WHISTLE
The sound is produced by the vibration of the air column between the top of the tube and the top of the plunger. Do you find that the pitch of the note is higher the shorter the air column?
Start with the air column long and blow the note, shorten it a little and blow the next note, continue, and try 42to blow the eight notes of an octave.
FIG. 72
JOINING TWO TUBES
Try to play a tune.
Try to make the most weird sound you can.
Take a piece of No. 2 tube about 7 inches long, close one end, smooth the other, and when cool cut the tube at the middle.

FIG. 73
WORKING THE JOINT
Now join these two pieces as follows: Hold the ends opposite each other near the top of the lamp flame (Fig. 72), rotate constantly, and when nearly red hot bring the ends accurately together in the flame, press together slightly, draw out slightly, and remove from the flame.
The ends are now stuck together, but the glass is in a slight lump around the joint and if allowed to cool will crack very easily. It is necessary to work the glass back and forth to get rid of the lump 43and to make the glass uniform on both sides of the joint. Do this as follows: Heat one third of the joint in the blowpipe flame (Fig. 73), and when red hot blow a slight bulge. Now turn the joint one third, heat the next third red hot and blow a slight bulge. Repeat with the remaining third.
FIG. 74
JOINING TUBES OF DIFFERENT SIZES
Now heat the first third again until it is red hot and shrinks, then blow a slight bulge again. Repeat this with the other two thirds.
Repeat this whole operation a third time and blow just enough to leave the joint the same size as the remainder of the tube or a little larger.
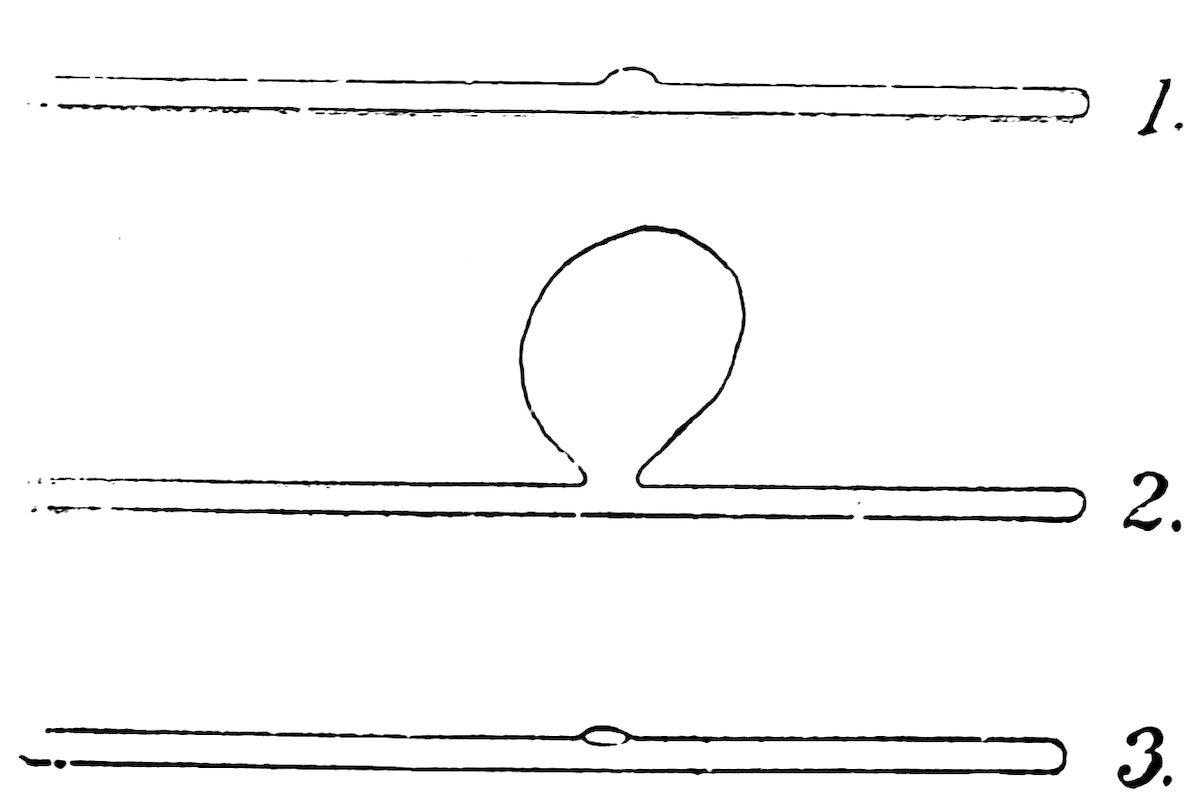
FIG. 75
MAKING A LARGE HOLE
This heating and blowing has worked the joint back and forth until the glass is fairly uniform. It makes a strong joint.
Cut off the closed end and smooth the edge.
Repeat with a piece of No. 4 tube.
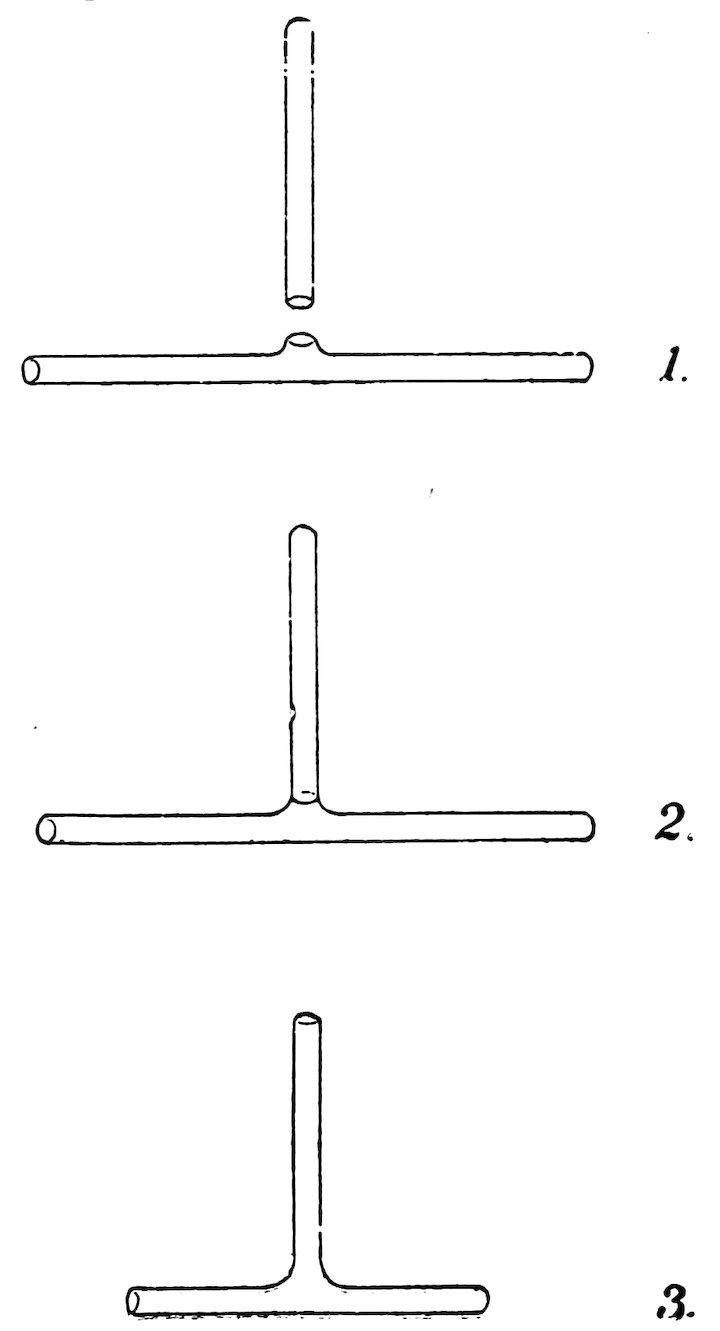
FIG. 76
MAKING A TEE
Take a piece of No. 4 tubing about 3 inches long and close one end.
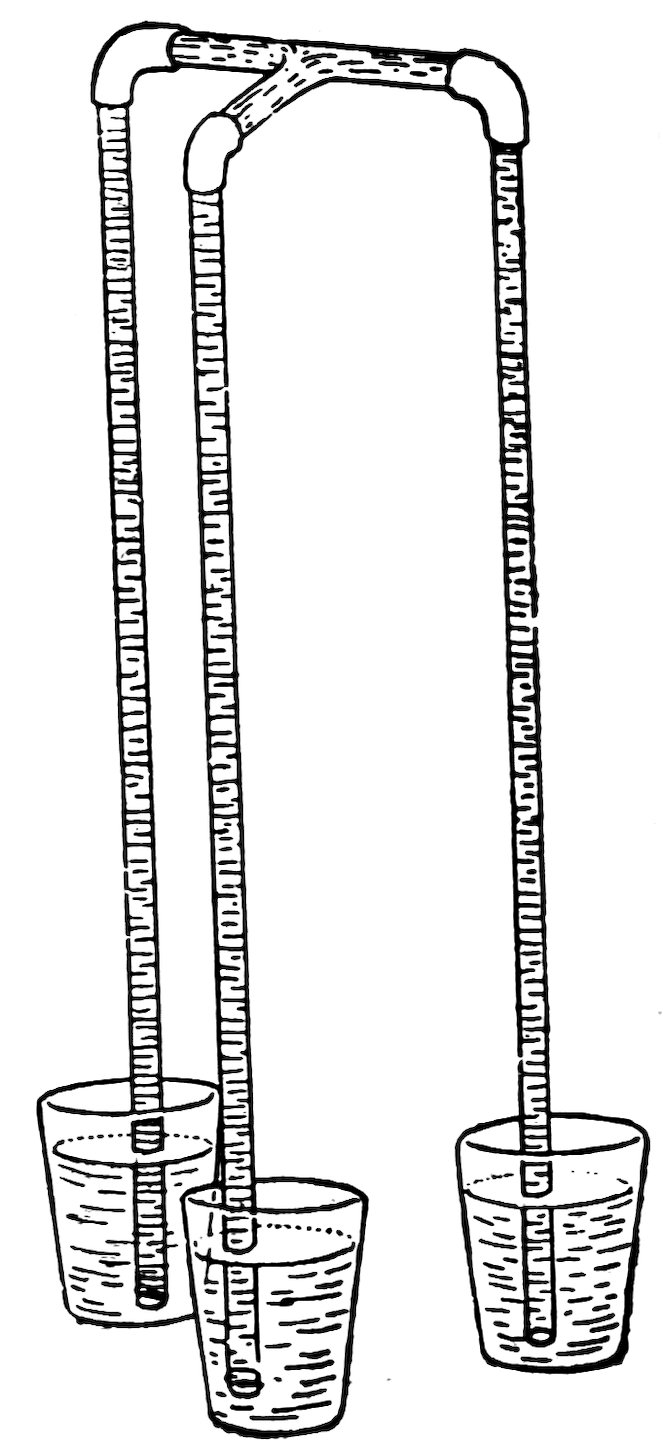
FIG. 77
THREE-ARMED SIPHON
Take a piece of No. 6 tubing, attach a handle to one end, heat the No. 6 tube in the blowpipe flame about 1 inch from this end and draw it down to smaller size.
Break the small part at a point where it is about the size of the No. 4 tube. If the hole is too large, heat the edge until it is a little too small and flare it out with the flaring tool. If the hole is too small, heat the edge and flare it out.
Now heat the ends of both tubes (Fig. 74), and join them as described in the last experiment.
45Repeat the operation of heating and blowing at least three times.
Join a No. 4 and a No. 2 tube in the same way.
Take a piece of No. 4 tube about 6 inches long, close one end, smooth the other, and allow it to cool.
FIG. 78
A REPEATING AIR GUN
Now to make a large hole in the side of this tube, proceed as follows: Heat in the blowpipe flame the point at which you wish to make the hole, and blow a slight bulge (1, Fig. 75). Then heat the top of this bulge until it is red hot over an area about equal to the size of the hole you wish to make, and blow hard to make a thin bubble (2, Fig. 75). Break away the thin glass of the bubble, smooth the edges, and the hole is made. The edge of this hole will project beyond the side of the tube (3, Fig. 75). If you wish to make the edge even with the side of the tube, heat it in the blowpipe flame until it shrinks back level with the tube.
FIG. 79
FOUR-WAY JUNCTION
Take a piece of No. 4 tube about 6 inches long, close one end, smooth the other, and allow it to cool. Take another piece 3 inches long, close one end, and allow it to cool.
Now make a hole in the side of the first tube at a point 3 inches from the closed end. Do this as described in the last experiment but leave the hole projecting beyond the side of the tube (1, Fig. 76).

FIG. 80
MAKING A Y
Now heat the edge of the hole and the end of the short piece in the lamp flame, and make a joint (2, Fig. 76) exactly as described in Experiment 53. Be particular to heat and blow all around the joint at least three times to make the glass uniform, and on the last blowing leave the joint a little larger than the tube. Cut off the closed ends, make the arms equal in length, smooth the ends, and your tee is made (3, Fig. 76).
Your first attempt may not be beautiful, but if you will repeat the heating and gentle blowing often enough, the joint will be strong, which is the main point.
Repeat until you can make a tee easily.
Make a tee with No. 2 tubing.
Your flame is hardly large enough to make a tee with No. 6 tubing.
Make a three-armed siphon as shown in Fig. 77. Put two arms in tumblers filled with water, suck air out of the third arm until the water runs, and then put it in an empty tumbler.
Stand the three tumblers on the table. Does the water run until the levels are the same?
Put one tumbler on a book. Does the water run into the other two tumblers until the levels are the same?
Return the one tumbler to the table and put the other two on 47the book. Does the water run from both tumblers to the lower tumbler until the levels are again the same?
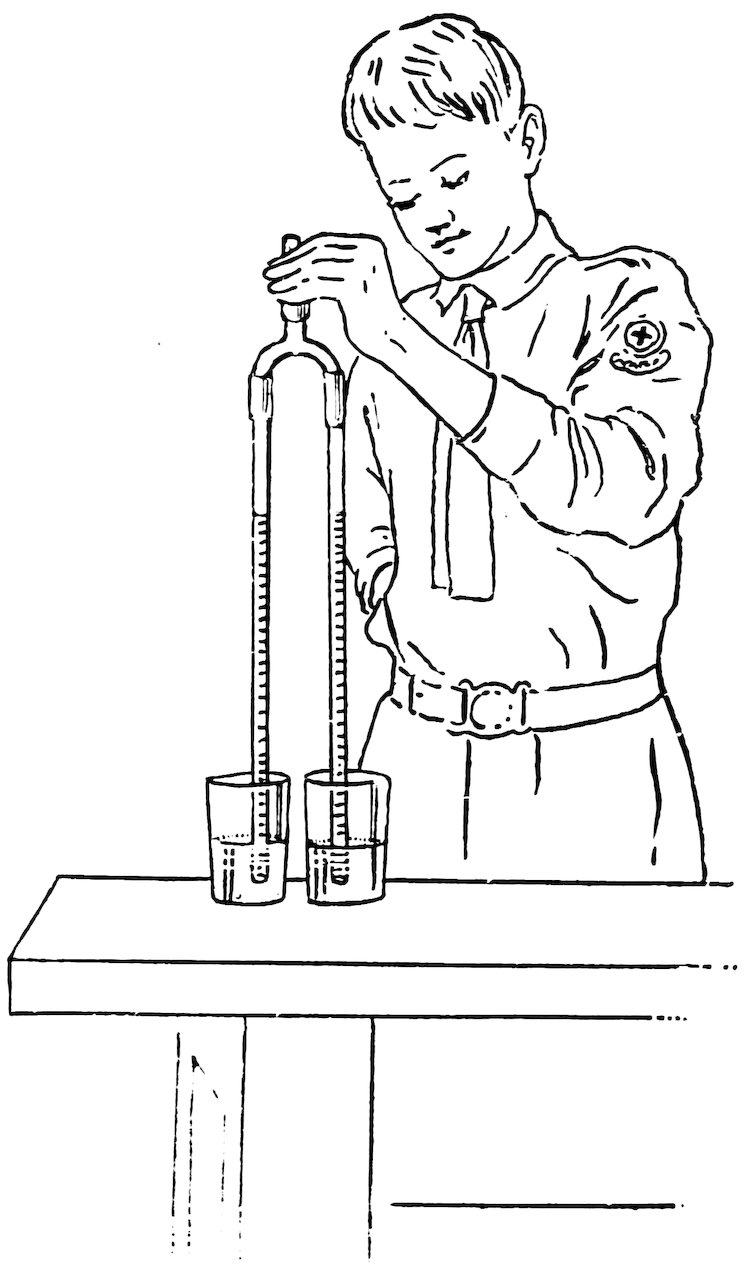
FIG. 81
BALANCING COLUMNS
Take a full length of No. 4 tubing, put a branch about 3 inches long at a point about 2 inches from one end; leave the end of the branch closed (Fig. 78). Now load the branch with shot or coarse dry sand, and your repeating air gun is ready for use.
Tilt the branch slightly above the horizontal and blow intermittently. Does your gun reload after each blow, until the ammunition is used up?
Make a tee as in Experiment 56, but do not cut off the closed ends. Now attach a fourth arm, as in Fig. 79, and heat and blow gently as before to work the glass into uniform condition. Cut off the closed arms at equal lengths, smooth the ends, and your four-way junction is made.
Make a four-arm siphon, repeat the experiments described in Experiment 57, and make others of your own.
Make a tee as in Experiment 56, then make a bend about ½ inch from the stem on each side (Fig. 80), and your Y is complete.
Arrange the apparatus as in Fig. 81, put the arms together in a glass of water, suck a little air out of the top coupling and close it with a glass plug. Do you find that the water rises to the same level in each?
Place the arms in separate tumblers filled with water to the same level and repeat. Does the water rise to the same level?
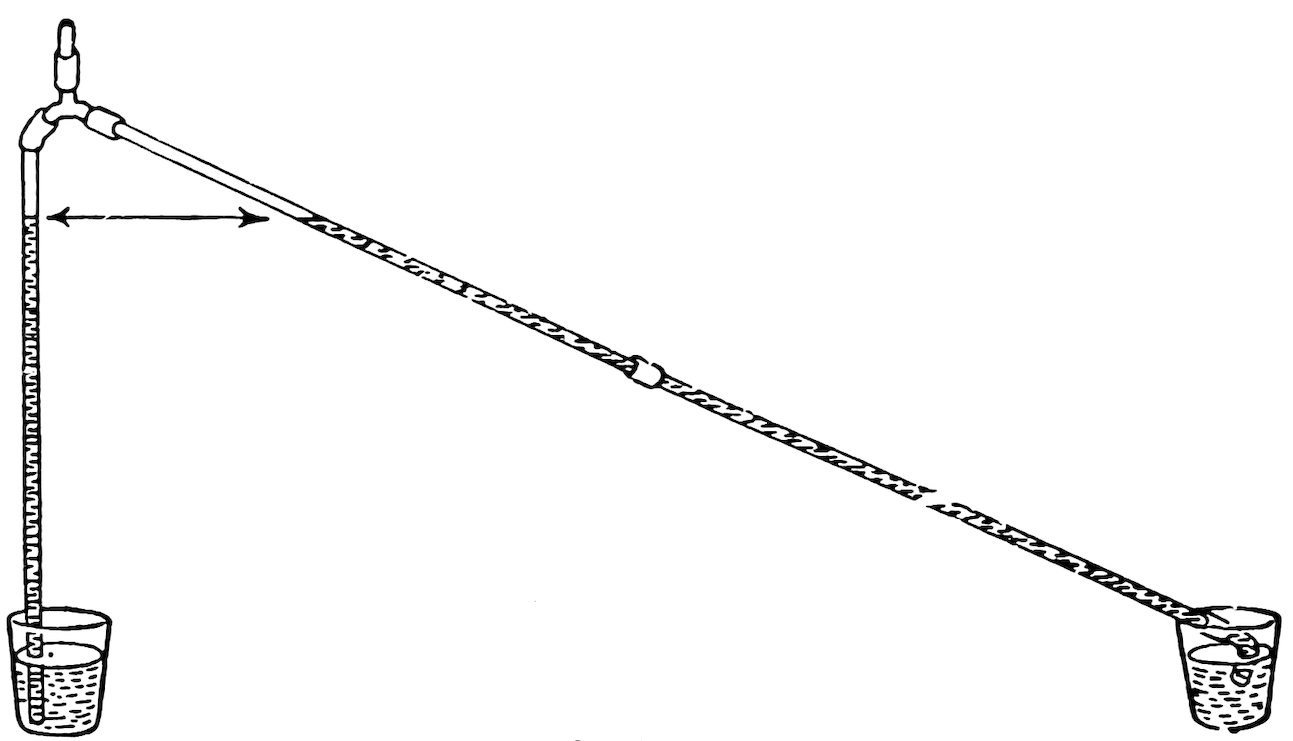
FIG. 82
THE WATER LEVELS ARE THE SAME
Add an extra length to one arm and repeat. Are the levels different but are they equal distances above the water in their respective tumblers?
Place the tumblers on the table, make one tube slanting, and repeat the experiment (Fig. 82). Are the levels again the same?
When you suck air out of the tee, you decrease the air pressure 49in the two tubes, and the atmospheric pressure on the water in the tumblers lifts the water into the tubes.
Put a large handful of salt into a tumbler partly filled with water and stir until the salt is dissolved. Now pour fresh water into another tumbler until it is at the same height as the salt water. Make the arms of equal length, put one arm in the salt water and the other in the fresh water, then suck a little air out of the top coupling and close it with a plug. Do you find that the column of salt water is shorter than the column of fresh water (1, Fig. 83)? It is shorter because salt water is heavier than fresh water.
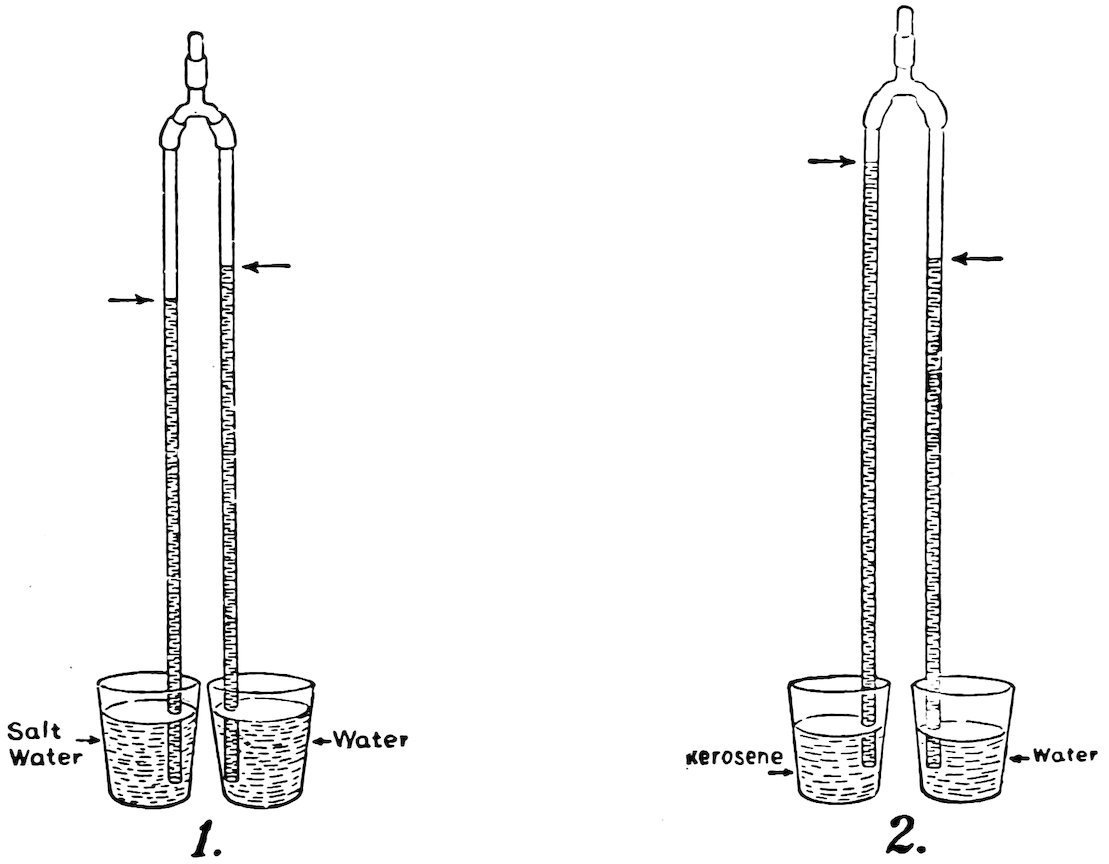
FIG. 83
UNEQUAL COLUMNS
If you have gasoline or kerosene convenient fill one tumbler half full of either, and the other tumbler half full of water, then repeat the experiment. Do you find that the column of gasoline 50or kerosene is longer than the column of water (2, Fig. 83)? It is longer because gasoline and kerosene are lighter than water.
Find a piece of thin iron or copper wire about 4 inches long, heat the end of a piece of No. 2 tubing until it is nearly closed, insert the iron or copper wire into the small hole, and heat the glass around the wire until it shrinks and grips the wire firmly (Fig. 84). The glass then serves as a handle for the wire.

FIG. 84
WIRE FUSED INTO GLASS
It is difficult to make a secure joint between iron or copper wire and glass because they both expand and contract more than glass when heated and cooled. It is easy to make a secure joint between platinum wire and glass because platinum and glass expand and contract at practically the same rate when heated and cooled. Platinum, however, is too expensive to be used for ordinary experiments.
The common glass cutter is a small very hard steel wheel mounted on a handle (Fig. 85). Practice with one on a pane of glass: place a ruler on the glass, draw the wheel along the ruler (Fig. 86) with sufficient pressure to scratch the glass, place the under side of the scratch exactly over the edge of the table, and press down on both sides.
FIG. 85
A GLASS CUTTER
FIG. 86
CUTTING A PANE OF GLASS
Place a piece of window glass flat on the table, pour a little kerosene on the spot to be bored, clasp the file near the end, press the end down hard on the spot and turn it back and forth with a gouging motion (Fig. 87). You twist the file just as you would twist an awl to force it into hard wood.
You will soon penetrate the surface; use plenty of kerosene and continue the boring until you are nearly through; then turn the plate over and start a hole on the other side to meet the one you have made.
FIG. 87
BORING A HOLE IN GLASS
Do not rush things; it will take you ten or fifteen minutes to bore through ordinary window glass.
Bore a hole in a bottle in the same way, except that the boring is all from the outside.
If the end of the file becomes dull, break off a small piece, with a pair of pliers, to expose a fresh surface.
FIG. 88
BOTTLE READY TO BE CUT IN TWO
Wind a strip of blotting-paper or wrapping paper 2 inches wide around the bottle at one side of the line along which you wish to cut. Make three or more thicknesses and then tie the paper with cord within ½ inch of the edge to be cut. Wrap another similar piece on the opposite side of the place to be cut and ³⁄₁₆ inch from the first piece (Fig. 88).
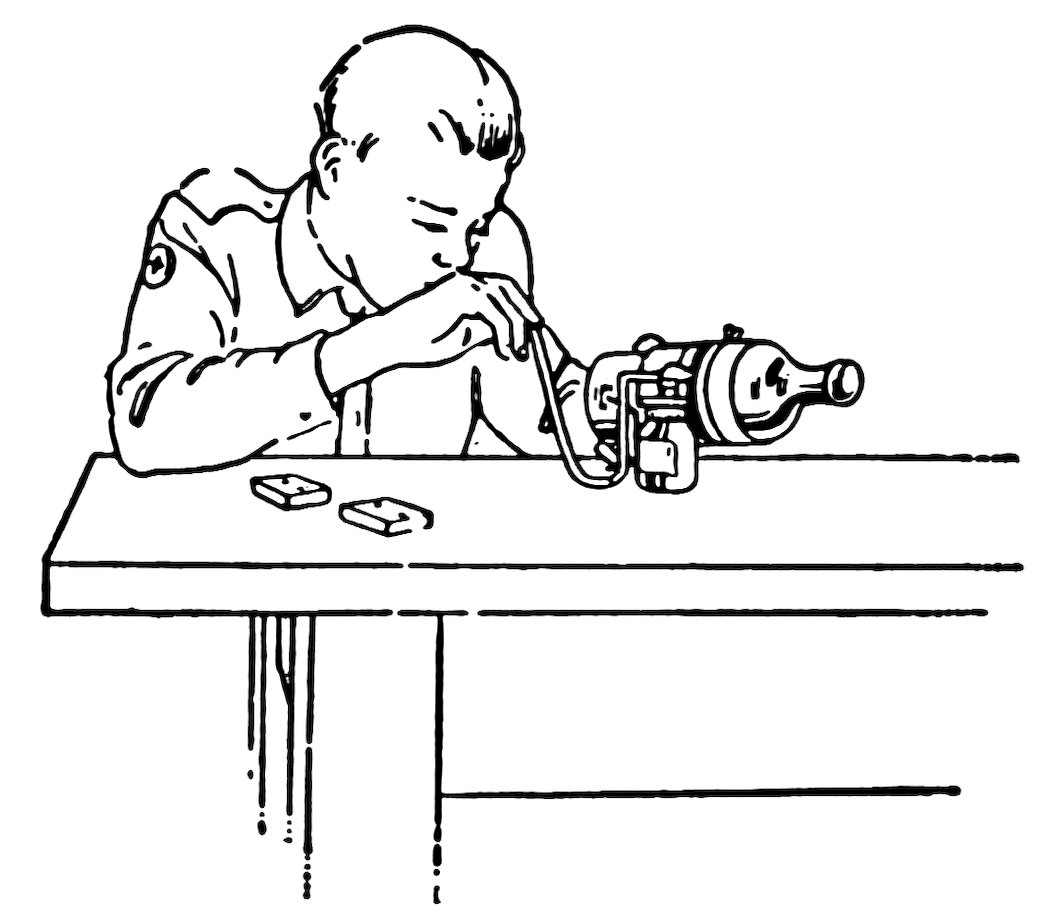
FIG. 89
HEATING THE BOTTLE
Now stand the bottle in a pail of water until the paper is thoroughly wet (about five minutes), take it out, rotate it in a horizontal position and direct the blowpipe flame against the glass between the papers (Fig. 89).
Continue this for four or five minutes, then if the bottle has not dropped apart, plunge it vertically into the pail of water.
The bottle will break into two parts along the line between the two papers (Fig. 90). If it does not do so, repeat 53the operation until it does. Smooth the rough edges outside and inside with the file. You cannot do this with the flame because the glass is too brittle.
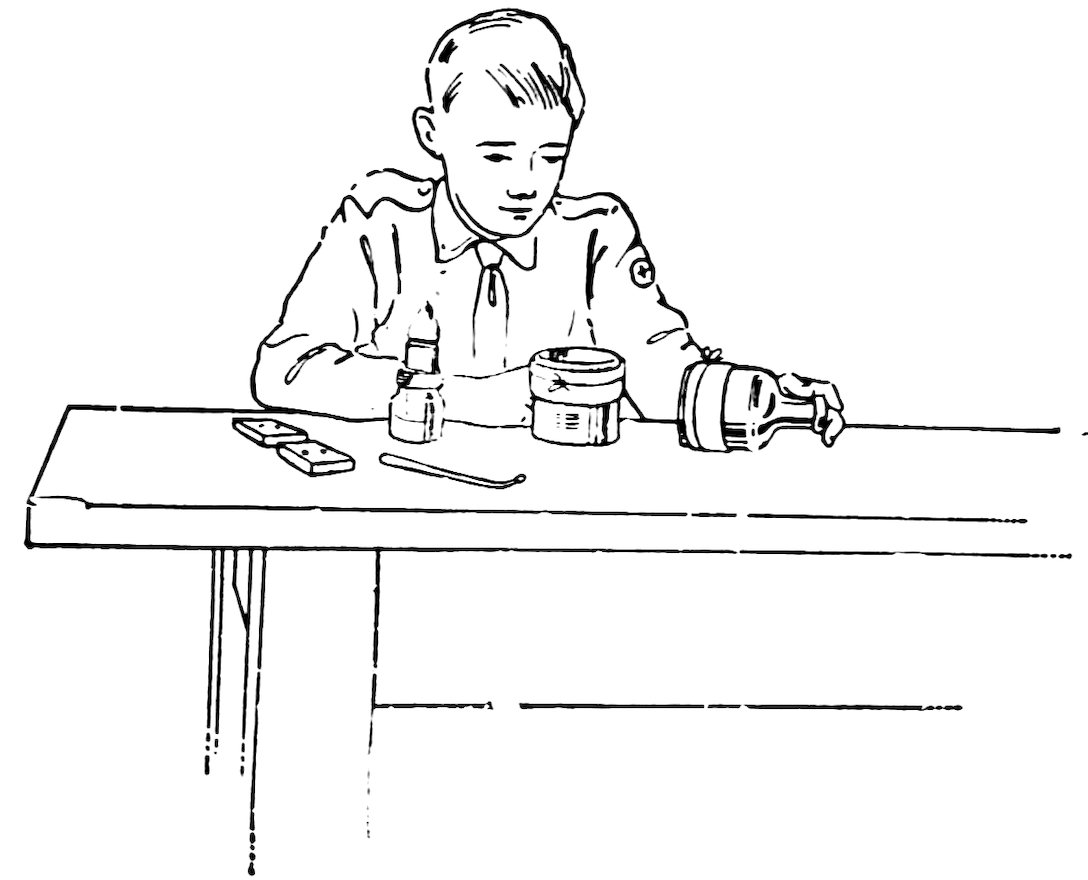
FIG. 90
THE BOTTLE CUT IN TWO
Rough edges of glass can be ground smooth by means of emery paper. For example, to smooth the edges of the glass bottle you have just cut in two, use the file for the rough work, then lay a piece of emery paper on a plate of glass, emery side up, pour a little kerosene on it and rub the rough surface on the emery with a rotary motion (Fig. 91). Finish with fine emery paper, and smooth the edges inside and out with the fine paper.

FIG. 91
SMOOTHING THE EDGES
There are two important points to remember in cementing glass: first, to get the glass clean, and second, to press the surfaces together after applying the cement, to squeeze out as much of the cement as possible, 54and to keep them pressed together until the cement is hard. To clean the glass wash it thoroughly with soap and water, rinse, and dry with a clean cloth.
FIG. 92
CEMENTING GLASS
There are many excellent glass cements on the market. Some of these are solid and are used only on hot glass; others are liquid and are used on cold or hot glass.
Cement two strips of glass together (Fig. 92) with sealing wax or solid shellac or some other solid cement as follows: Clean the glass thoroughly, place in the oven or on the stove, heat gradually until the glass just melts the cement, rub the cement over both surfaces, bring them together when the cement is fluid, press them together to squeeze out as much cement as possible, and keep them pressed together until the cement is hard.
Cement a strip of wood to a strip of glass in the same way.
Cement a strip of wood to a strip of glass with liquid glue, both wood and glass being cold. Keep them pressed together until the glue is dry, perhaps a day or two.
Boys, you can perform many magic experiments with apparatus made out of the glass tubes, rubber stoppers, and rubber unions supplied with “Experimental Glass Blowing.” We outline a number in the following pages. You can invent many more for yourselves.
Light your alcohol lamp, blow it out, and bring a lighted match 55down toward the wick from above (Fig. 93). Does the lamp light in a most magical manner before the match touches the wick?

FIG. 93
MAGIC
Repeat this with a kerosene lamp and with a candle. Do they light in the same magical manner?
When the lamp is lighted, the alcohol or kerosene turns to a gas, and it is the gas which burns; when the candle is lighted, the wax turns to an oil, the oil turns to a gas, and it is the gas which burns.
The gas rises from the wick for a short time after the flame is blown out, and it is this gas which lights when you bring the match down toward the wick.
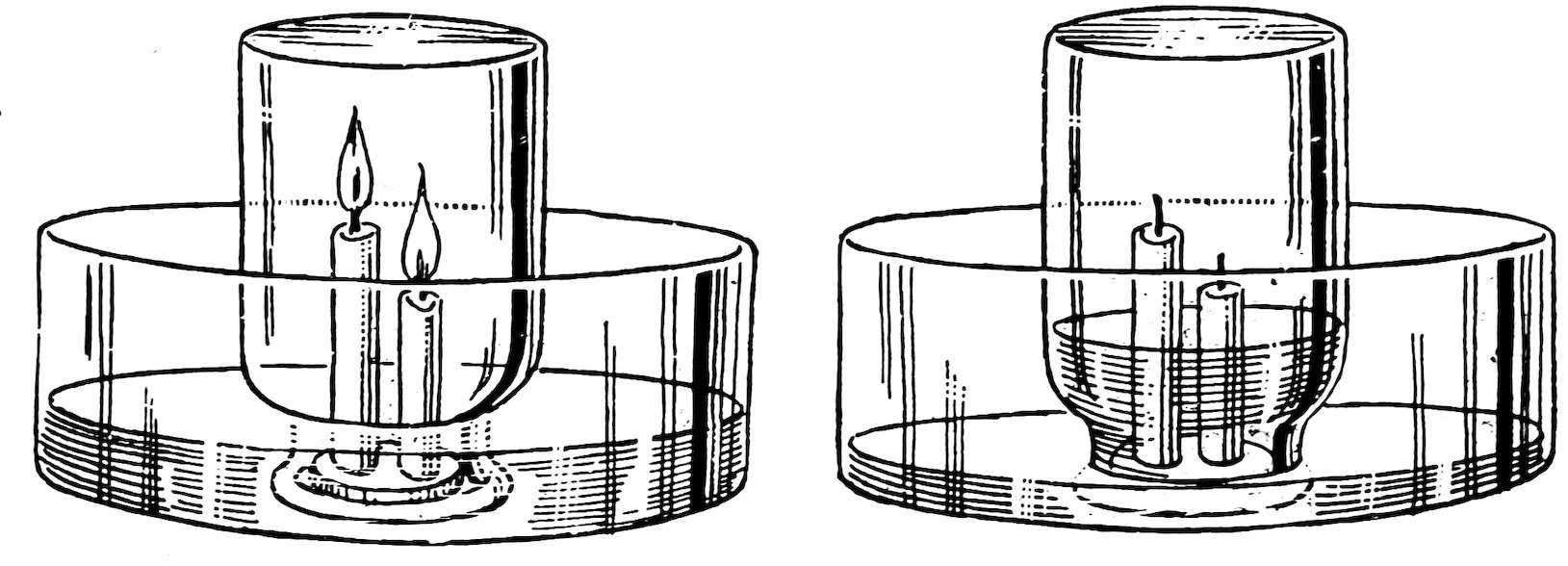
FIG. 94
THE CANDLES GO OUT AND THE WATER RISES
Drop melted candle wax on a tin can cover and attach the bottoms of two candles to the cover (Fig. 94); use one candle about 564 inches long and another about 3 inches, stand them upright in a pan of water, light them, and invert a wide-mouthed bottle over them. Does some air escape at first due to expansion, do both candles go out, the taller one first, and does the water rise until the bottle is about one-fifth full?
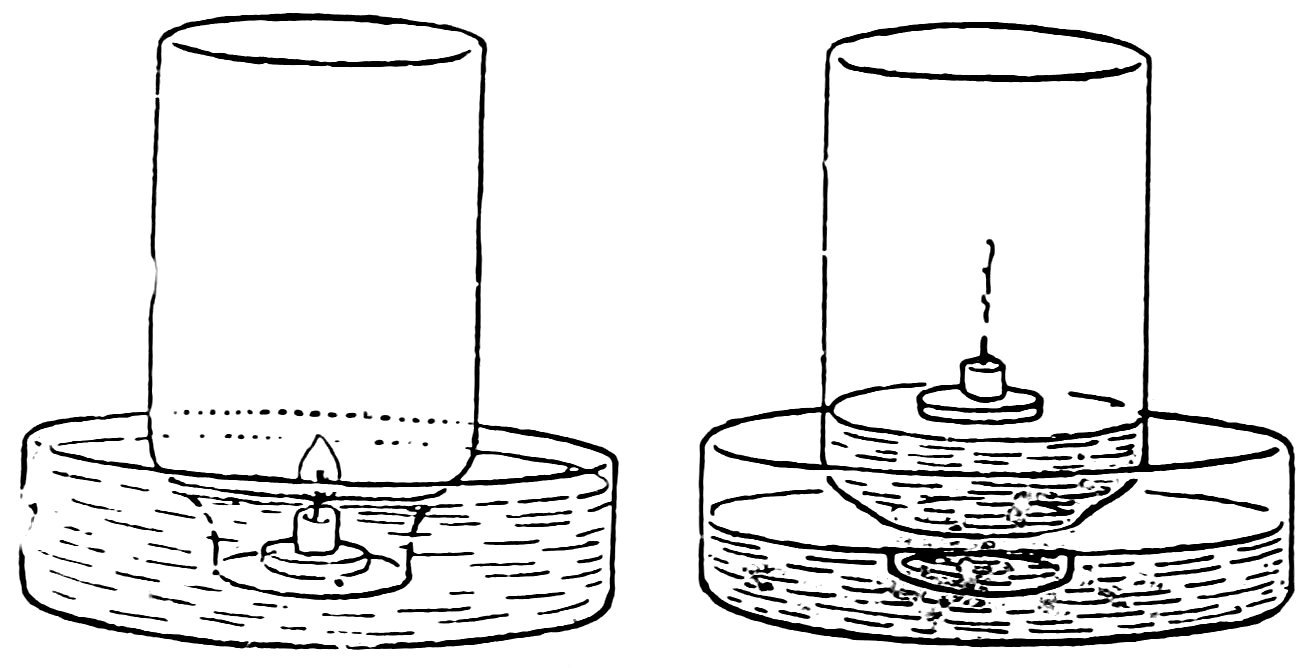
FIG. 95
THE CORK RISES
Cut a piece of candle ½ inch long, float it on a flat cork or can cover in the pan of water, light it, and invert a fresh empty bottle over it (Fig. 95). Is the result similar?
The water rises in the bottle because ⅕ of the air is used up by the burning candle. Air is ⅕ oxygen and ⅘ nitrogen. The oxygen unites with the burning gas of the candle and produces water vapor (H2O) and carbon dioxide (CO2); the nitrogen takes no part in the burning.
FIG. 96
WATER FROM FLAME
The water vapor (H2O) condenses to water on cooling and takes up very little space. The carbon dioxide remains a gas and occupies space, but this is offset by the volume of the air which escaped at first. The result is that the volume of gas at the end is about ⅕ less, and the atmospheric pressure on the water in the pan lifts water into the bottle.
The candle goes out because it must have oxygen to burn and the oxygen is used up.
It is certainly magic to produce water from fire, but you can do it easily as follows: 57Hold a clean, dry, cold tumbler over your alcohol lamp flame (Fig. 96). Does water deposit in the form of mist on the inside of the tumbler?
FIG. 97
ATMOSPHERIC PRESSURE
Repeat with fresh tumblers with the flame of a kerosene lamp and of a candle. Are the results similar?
Direct the blowpipe flame into the end of a piece of No. 2 or 4 tubing. Does water deposit in drops inside the tube about 1 inch above the end?
One of the chief constituents of alcohol, kerosene, and candle wax is hydrogen (H), and when this burns in the oxygen (O) of the air, it produces water (H2O). It is this water which condenses on the cold glass.
Arrange a No. 6 tube as in 1, Fig. 97, and suck air out at the top. Does the water run uphill into your mouth?
Hold your finger over the top and lift the tube out of the pail (2). Does the water remain in the tube? Fill a bottle with water to overflowing, insert a No. 2 tube into your one-hole stopper, insert the stopper into the mouth of the bottle (3) without admitting air below the stopper, and try to suck water out of the bottle. Do you find that you cannot do so?
FIG. 98
WATER DRIVEN UP TUBE BY ATMOSPHERE
Repeat (3) with the bottle half full of air (4). Do you find that you can now suck part of the water out of the bottle, and all of it if you admit air?
The atmosphere which surrounds the earth exerts a pressure of 15 pounds per square inch on everything at the earth’s surface. It exerts this pressure equally downward, sidewise, and upward.
It is this atmospheric pressure on the water in the pail (1) which lifts the water into the tube when you decrease the pressure on the water in the tube by sucking out air and then water.
It is this pressure upward that supports the water in 2.
The water does not rise in 3 because the atmosphere cannot exert pressure downward on the water in the bottle.
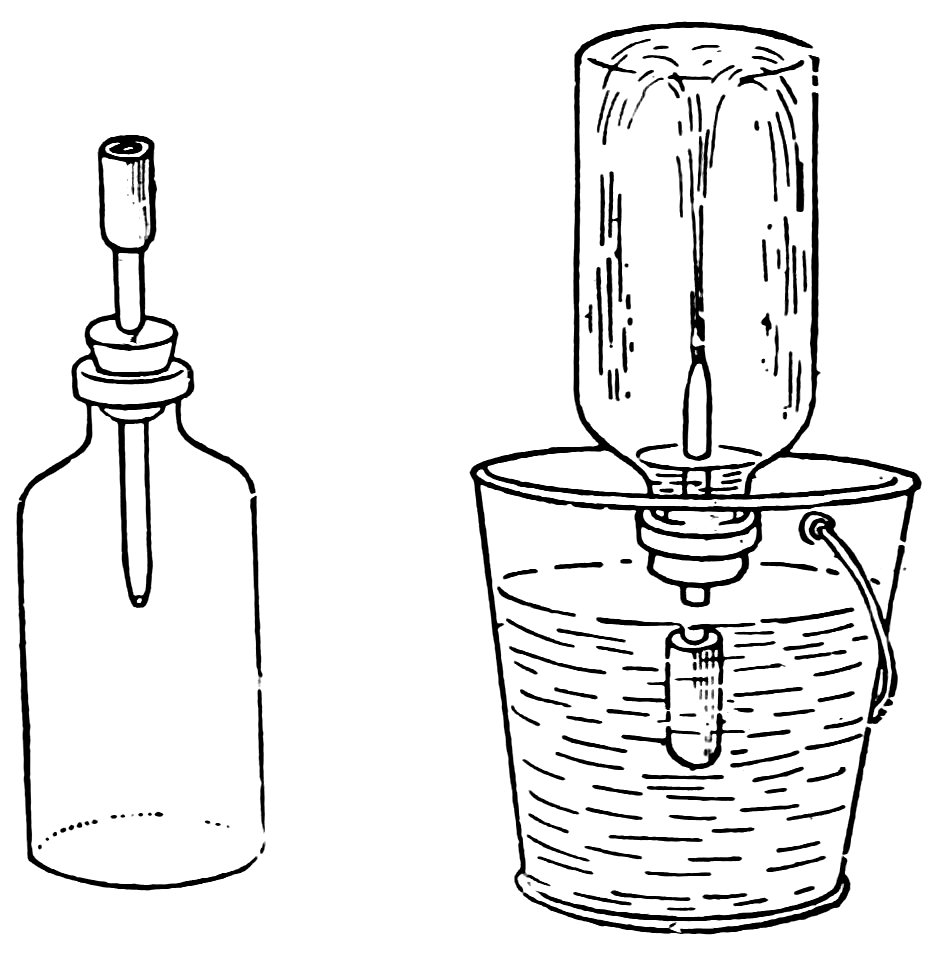
FIG. 99
A FOUNTAIN
The rise of the water in 4 is due to another fact, namely, that any gas expands when the pressure on it is decreased. When you suck air out of the tube you decrease the pressure on the water in the tube and thereby on the air in the bottle; the air then expands and lifts the water into your mouth.
With the apparatus Fig. 98 59hold your finger over the lower end of the tube, suck as much air as you can out of the tube, pinch the coupling, and remove your finger under water. Does the atmosphere drive water up the tube very rapidly and with great force?
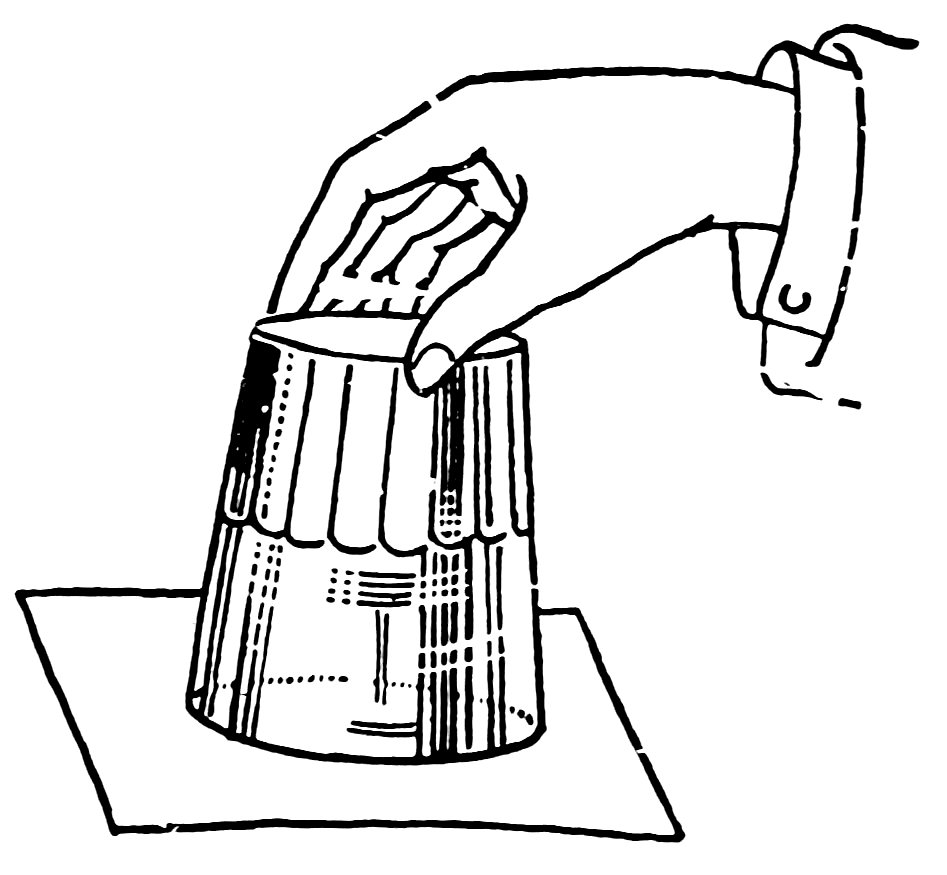
FIG. 100
MAGIC
With the apparatus Fig. 99 suck as much air as you can out of the bottle, pinch the coupling, and open it under water. Does the atmosphere lift the water into the bottle and produce a beautiful fountain?
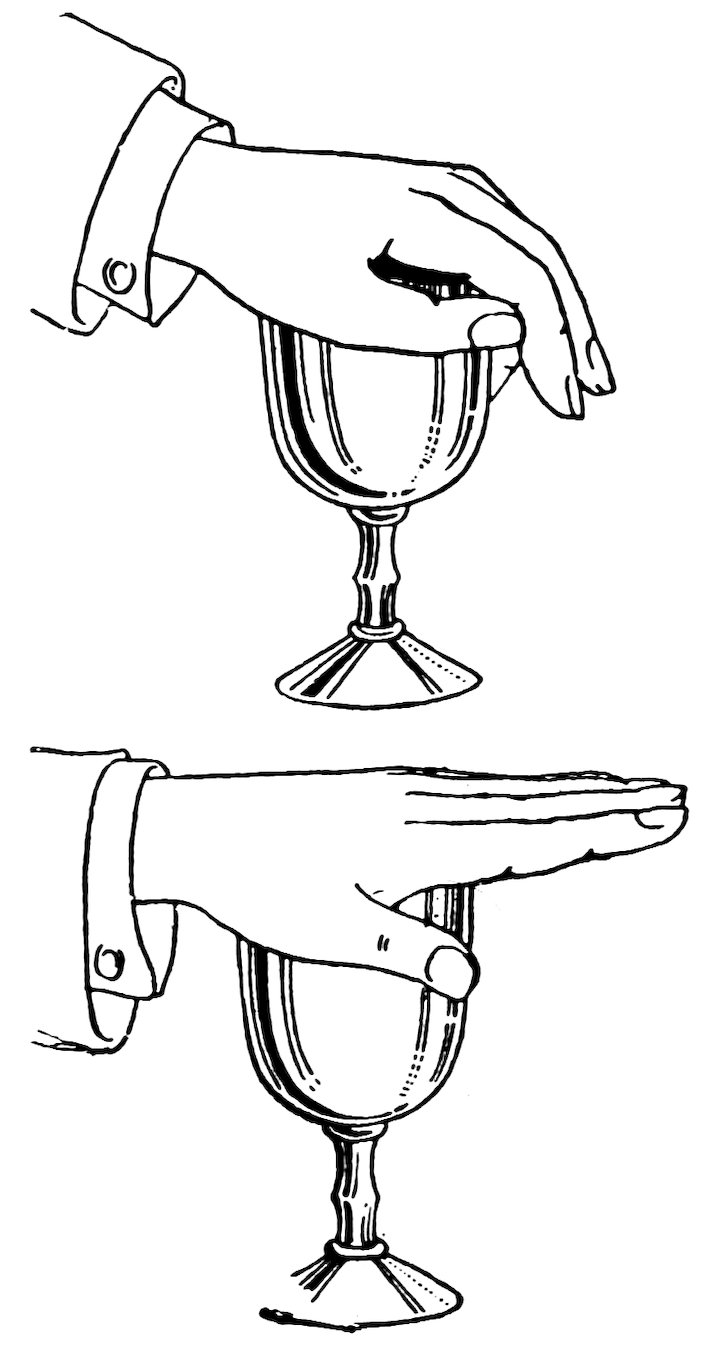
FIG. 101
MORE MAGIC
Fill a tumbler with water, cover it with a sheet of paper, hold the paper on with your hand, invert the tumbler, and remove your hand (Fig. 100). Does the atmospheric pressure upward support the paper and water?
Fill a tumbler with water, press your palm down on the top with your fingers pointing downward (Fig. 101), straighten your fingers without admitting air to the tumbler, and then lift your hand. Do you lift the tumbler of water also?
There is a partial vacuum between your hand and the water, and the 60atmospheric pressure upward and downward holds your hand and the tumbler together.
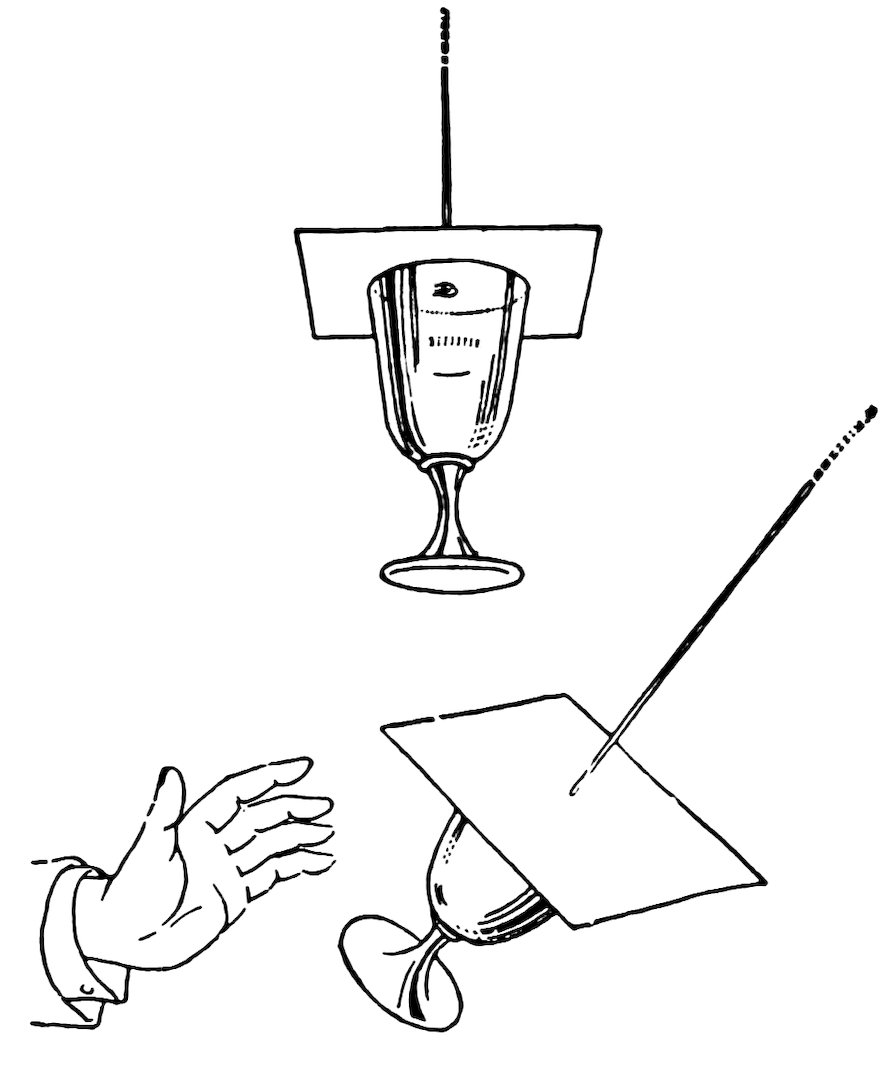
FIG. 102
TUMBLER PENDULUM
Pass a string through a small hole in a piece of cardboard, knot the end of the string, and drop melted candle wax over the hole to make it air tight.
Fill a tumbler with water, press the cardboard down on the tumbler with the palm of your hand, and lift the string. Do you also lift the tumbler (Fig. 102)?
Swing the tumbler gently as a pendulum.
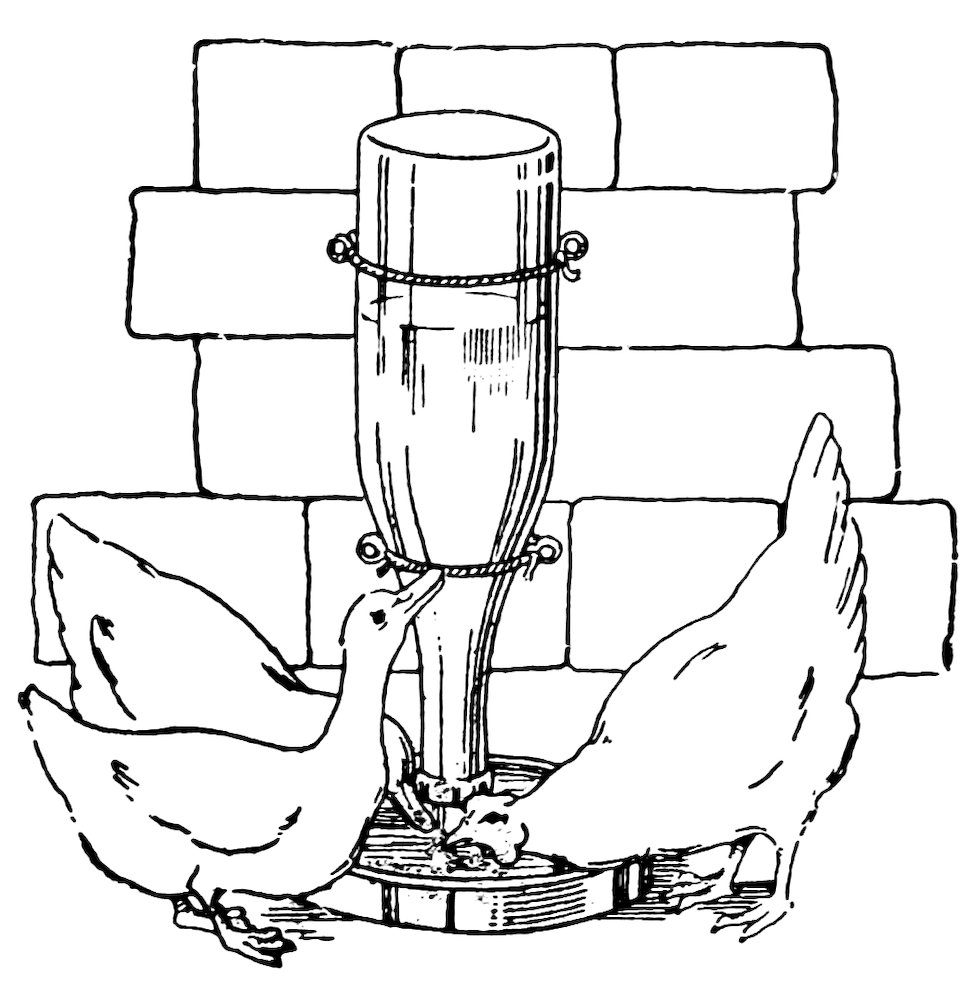
FIG. 103
POULTRY FOUNTAIN
To make the poultry fountain (Fig. 103), fill a bottle with water, hold your thumb over the mouth, invert the bottle over the pan of water, and remove your thumb under water. Does the atmospheric pressure on the water in the pan hold the water in the bottle?
Lift the bottle until the mouth is a little above the water in the pan. Does air 61enter and water run out until the mouth is again covered with water? This is what happens when the poultry, by drinking, lower the water below the mouth of the bottle.
In a poultry fountain the bottle is supported, as shown, with its mouth under water but above the bottom.
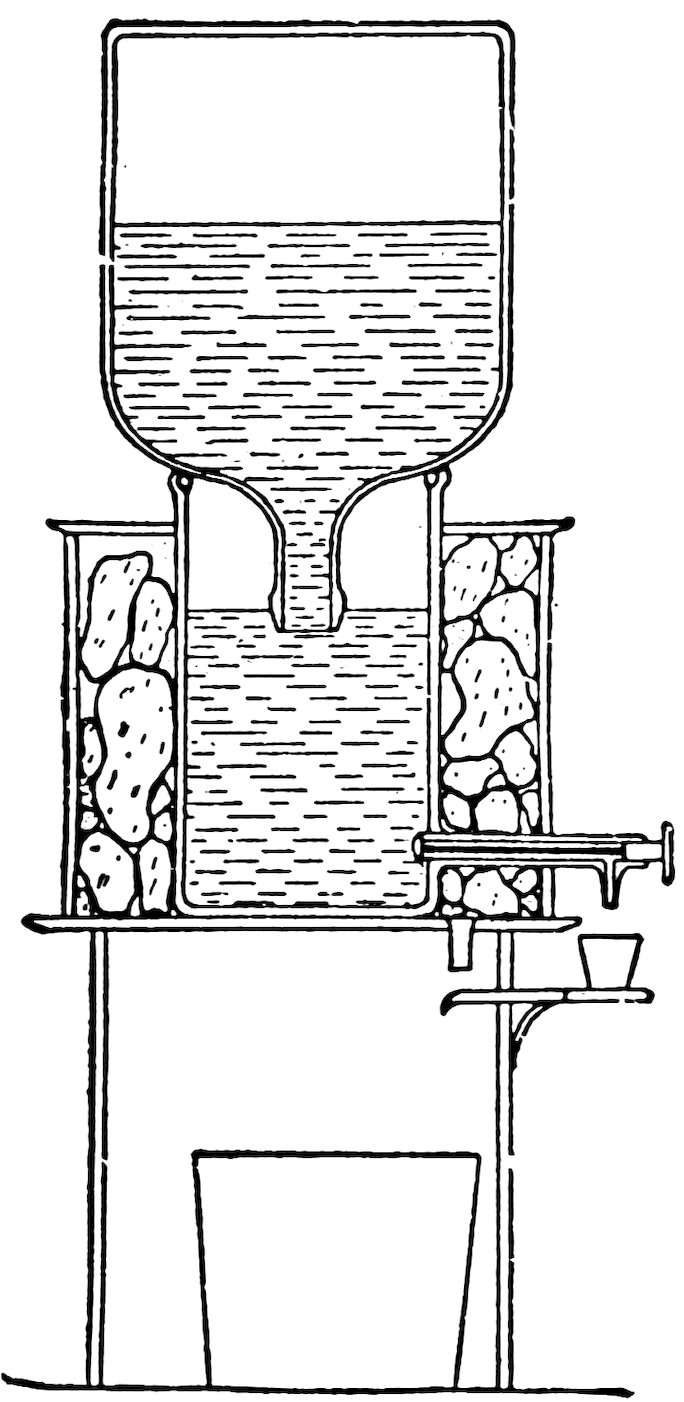
FIG. 104
A DRINKING FOUNTAIN
(From Butler’s Household Physics. Published by Whitcomb & Barrows, Boston)
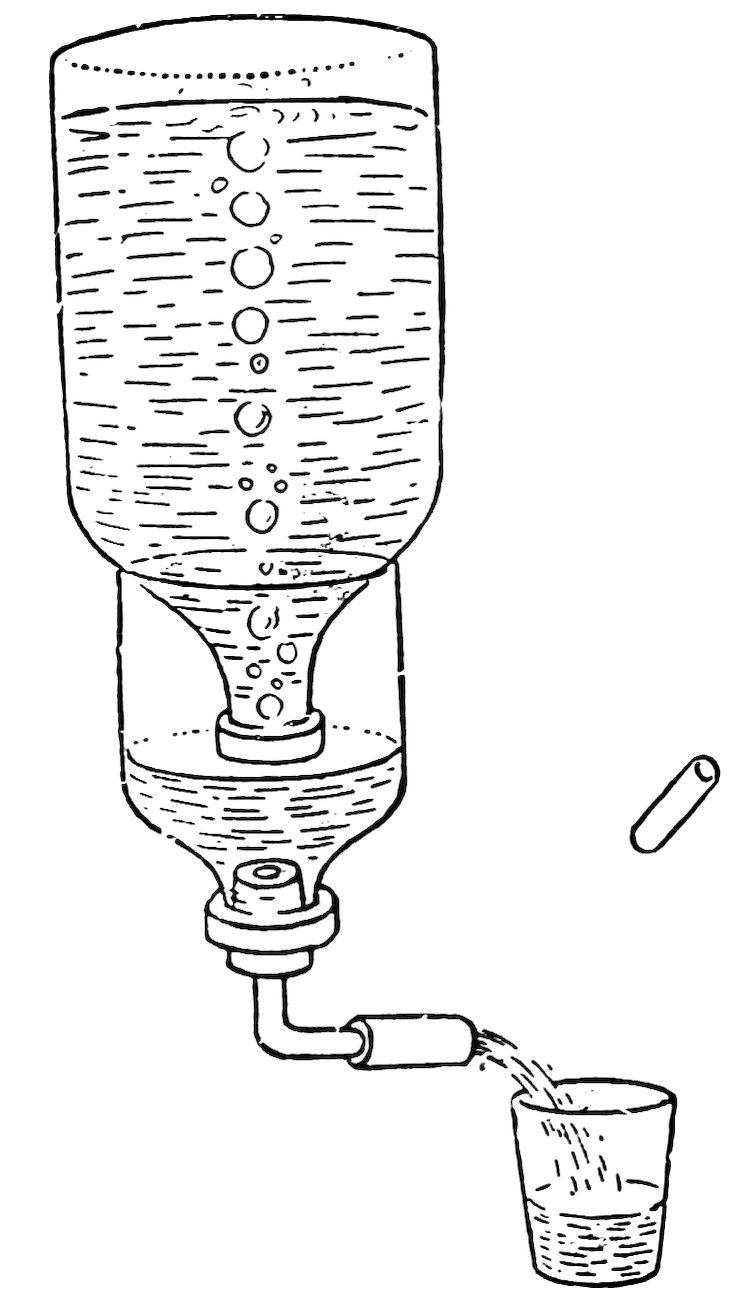
FIG. 105
HOMEMADE DRINKING FOUNTAIN
The drinking fountain (Fig. 104) is similar in principle to the poultry fountain of the last experiment. The water is held in the large inverted bottle by the atmospheric pressure on the water in the lower vessel. Air enters the bottle and water escapes from it when the level of the water in the lower vessel falls below 62the mouth of the bottle. The water is cooled by the ice surrounding the lower vessel.
Make a drinking fountain of this kind as in Fig. 105, ask a friend to hold it, remove the glass plug from the coupling, and draw a glass of water. Do you observe that air bubbles enter the inverted bottle and water flows from it only when the water level in the half bottle falls below the mouth of the inverted bottle?
Allow the water to flow continuously. Is the water level practically constant in the half bottle until the upper bottle is empty?